Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/37/61. The contractual start date was in January 2010. The draft report began editorial review in March 2015 and was accepted for publication in December 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sarah E Lamb is the chairperson of the National Institute of Health Research Health Technology Assessment programme Clinical Evaluation and Trials Board and is on the Clinical Trials Unit Standing Advisory Committee. Keith Willett declares design royalties from Zimmer Biomet, outside the submitted work, for intramedullary bone fixation implants.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Keene et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Ankle fractures are a common injury in the adult population. In high-income countries, the burden from ankle fractures placed on health-care services is substantial, as 9–17% of all fractures seen in acute care hospitals are ankle fractures. 1,2 A Scottish study reported an incidence of 132 fractures per 100,000 in men and of 112 fractures per 100,000 in women per year, with the highest incidence of 248 per 100,000 per year occurring in women between the ages of 75 and 84 years. 3 A threefold increase in the number of ankle fractures per year is predicted from 2000 to 2030 as the population ages. 4
Ankle fractures often result in ankle pain, limitation in joint range of motion,5 muscle weakness6,7 and difficulties with weight-bearing tasks such as walking and climbing stairs. 5,8 Limitations in ankle-related function are especially pronounced in the 6 months after injury, but can persist for several years. 9
Physical function outcomes after ankle fracture are generally worse when the injury occurs in older age. 9,10 The injury may contribute to a loss of independence, have a substantial impact on quality of life and have higher health and social care costs. 11–13
In this introduction chapter, we provide a background to the current management of ankle fractures, explore the issues with existing treatment options in older-aged adults, summarise the existing evidence base and outline the rationale for the Ankle Injury Management (AIM) trial and the novel close contact casting (CCC) intervention.
Management of ankle fractures
Ankle fractures can be managed conservatively or surgically, depending on the extent and pattern of bone and ligament injury. The extent/pattern of injury is determined by direction and magnitude of traumatic forces on the ankle and resilience of the musculoskeletal tissues. Ankle fractures are usually graded on radiographs using the Lauge-Hansen classification based on mechanism of injury or the Danis–Weber classification extensions developed by the AO Foundation, which are based on anatomical injury characteristics (Figure 1). 14
FIGURE 1.
Comparison of the Lauge-Hansen (left column) and the AO Foundation and Orthopaedic Trauma Association (AO/OTA) (right column) systems of ankle fracture classification.© From Oxford Textbook of Trauma and Orthopaedics edited by Bulstrode (2011) Figure 12.59.4. By permission of Oxford University Press (www.oup.com).

Stable and undisplaced fractures are typically treated conservatively in an ankle splint or cast. Displaced ankle fractures resulting in anatomical misalignment are initially managed by manipulation of the limb to restore anatomical congruence. This is termed ‘fracture reduction’, as in reduction of the deformity. The limb is then held in a cast or splint until definitive management is implemented. Usually displaced and/or unstable ankle fractures are managed with open reduction and internal fixation (ORIF) surgery to both restore and maintain congruence of the ankle mortise. 14 During ORIF, bone fragments are repositioned and held in place by plates and/or screws until healing (union) occurs.
The rationale for ORIF surgery is to improve outcomes by reducing complications from malunion such as post-traumatic osteoarthritis. 15,16 Malunion of weight-bearing joints is postulated to lead to increased contact stresses on the articular surfaces,17,18 precipitating osteoarthritis that can result in persistent symptoms and disability and potentially the need for further surgery. 19,20 However, the aetiology of post-traumatic osteoarthritis may also be a result of genetic factors and direct damage caused by the initial trauma. 21
Non-union (incomplete healing) of the fracture can be considered to be less likely when electing to treat an ankle fracture with ORIF surgery than with conservative treatment. 22 However, there are limited randomised controlled trial comparisons on whether or not non-union is more likely with conservative casting or ORIF. 23
Issues with surgery in older adults after ankle fracture
For older adults, there is greater controversy in the management of displaced and/or unstable ankle fractures. The benefits of surgery are less clear because of the substantially increased risk of complications such as infection, wound breakdown and fixation failure. 24 It is more difficult to achieve successful surgical outcomes in older people because of a higher prevalence of comorbidities resulting in lower bone density (osteoporosis), frail skin and impaired wound healing. Poor bone quality directly affects the efficacy of implant stabilisation treatment methods for the bone fracture fragments. 25 In addition, such fractures, because of the greater fragmentation and poor bone strength, tend to be less stable after repositioning, and the holding strength of fixation screws can be diminished up to 10-fold. 26 These can render fixations incompetent and prevent early joint movement and weight-bearing, the accepted advantages of the surgical fixation approach in the younger patient. Other common comorbidities in the older patient (peripheral vascular disease, chronic venous insufficiency, late-onset diabetes mellitus and/or oedema from heart failure) directly affect the lower limb skin and soft tissue tolerance of surgical wounds or traditional casts.
There are higher rates of complications in older adults after ankle ORIF. 27 In 2014, a case review of 186 patients (132 females, 71%) after ankle ORIF, which was based in the UK, found high complication rates in persons aged over 60 years. The complications included superficial surgical site infection and delayed wound healing (17/186, 9%), deep surgical site infection (13/186, 7%), failure of fixation (6/186, 3%), non-union or malunion (20/186, 11%) and additional surgery for wound debridement, removal of metalwork or revision of the fixation (20/186, 11%). 24
Operative versus casting treatment of unstable and/or displaced ankle fractures
The uncertainty in the evidence for clinical outcomes comparing surgical with non-operative treatment for ankle fractures was highlighted in a 2012 Cochrane review,23 which included 292 participants in three randomised controlled trials28–30 and one quasi-randomised controlled trial. 31 The authors concluded that there is currently insufficient evidence of the effects of surgical versus conservative treatment on outcome after ankle fracture and confirmed the need for an adequately powered clinical trial.
Current management of ankle fractures in older adults
Published research, albeit of poor quality, indicates higher rates of loss of fracture reduction or malunion (poor position at healing) and non-union (failure to heal) with traditional casting. 23 Only one previously published randomised controlled trial has studied older adults. Makwana et al. 29 randomised 43 participants aged 66 years to ORIF or a moulded below-knee plaster cast. The results of the trial indicated that participants undergoing casting were more likely to have an early loss of reduction of the fracture (8/21 casting group and 0/22 ORIF group) and lower patient-reported ankle function. Two cases of malunion and two of non-union were reported, all in the conservative treatment group, with only one of these participants reporting pain and functional limitations. The ORIF group experienced other adverse clinical events such as superficial surgical site infection and complex regional pain syndrome type 1. The results should be interpreted with caution because of the limited sample size and high risk of bias, as there was no blinding of outcome assessors, incomplete outcome data and unclear precautions regarding allocation concealment.
Many surgeons make a clinical judgement based on (1) the likely tolerance of a patient’s skin for surgical incisions and (2) the bone quality and chance of achieving implant fixation. For patients judged as higher risk for open surgery, some surgeons may select manipulation and traditional casting, assuming fewer complications but with a higher risk of malunion. As a result, ORIF is more commonly used than non-operative treatment for ankle fracture in older people.
Rationale for the Ankle Injury Management trial
A modification of the traditional casting treatment has been developed, CCC. This new casting technique has the potential of improved fracture stabilisation and lower skin damage risk compared with traditional casting. Traditional casting methods (an external support formed by an under layer of stockinette, layers of wool roll and felt, and a rigid outer layer made of plaster of Paris and/or synthetic material) can result in pressure sores on the skin. CCC is a plaster of Paris cast applied over a minimal lining and padding thickness compared with traditional casts. The cast is moulded firmly to maintain reduction of the fracture and is then reinforced with a topcoat of synthetic casting bandage. CCC can be considered a modification of ‘total-contact casting’, which has been used extensively and successfully for more than 20 years in treating leg ulcers in diabetic patients who have frail skin. 32–34
There were key stages in the development of CCC as an intervention in orthopaedic surgery. Vascular laboratory investigations as part of the single-centre pilot study for the AIM trial confirmed the potential for improved skin viability outcomes with CCC. 35 The extension of the study to a pragmatic, multicentre randomised controlled trial to assess the introduction of CCC, compared with the standard care of ORIF, in the NHS is consistent with stages of evaluation in the IDEAL (Idea, Development, Exploration, Assessment, Long-term) study recommendations for evaluation of surgical innovations. 36
The purpose of the AIM trial was to compare ORIF with CCC in the management of ankle fractures in older adults within the UK NHS. We carried out a pragmatic, equivalence randomised controlled trial with parallel economic and qualitative studies to provide an evaluation of clinical effectiveness and cost-effectiveness. The study was identified as a research priority by the orthopaedic trauma surgeon members of the UK Association for Osteosynthesis. 37
Research objectives
-
To determine if the application of CCC for unstable ankle fractures in older adults resulted in an equivalent clinical outcome compared with the standard care of open surgical reduction and internal fixation (ORIF).
-
To estimate the cost-effectiveness of the two treatments to the NHS, and the broader societal perspective, including to the individual and their family.
-
To explore the experiences of participants of the interventions and the impact of taking part in the trial.
Chapter 2 Methods
Summary of study design
This study was a pragmatic, multicentre, equivalence randomised controlled trial with parallel prospective economic evaluation. Participants were randomised to receive ORIF or CCC after admission for surgery for displaced and/or unstable ankle fractures in the trauma and orthopaedic surgery departments of 24 hospitals from 22 NHS trusts. The final study protocol has been published. 38
Settings and locations
The trial was run in the following hospitals in England and Wales:
-
Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust
-
Darlington Memorial Hospital, County Durham and Darlington NHS Foundation Trust
-
Derriford Hospital, Plymouth Hospitals NHS Trust
-
Frenchay Hospital, North Bristol NHS Trust
-
Great Western Hospital, Great Western Hospitals NHS Foundation Trust, Swindon
-
Ipswich Hospital, Ipswich Hospital NHS Trust
-
John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust
-
Leeds General Infirmary, Leeds Teaching Hospitals NHS Trust
-
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust
-
Morriston Hospital, Abertawe Bro Morgannwg University Health Board, Swansea
-
Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust
-
Newham University Hospital, Barts Health NHS Trust, London
-
Norfolk and Norwich University Hospital, Norfolk and Norwich University Hospitals NHS Foundation Trust
-
North Staffordshire Royal Infirmary, University Hospital of North Staffordshire NHS Trust, Stoke-on-Trent
-
North Tyneside General Hospital, Northumbria Healthcare NHS Foundation Trust
-
Poole Hospital, Poole Hospital NHS Foundation Trust
-
Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust, Reading
-
Royal Victoria Infirmary, Newcastle upon Tyne Hospitals NHS Foundation Trust
-
Southport and Ormskirk Hospitals, Southport & Ormskirk Hospital NHS Trust
-
Torbay Hospital, South Devon Healthcare NHS Foundation Trust
-
University Hospital Coventry, University Hospitals Coventry and Warwickshire NHS Trust
-
University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust
-
Wansbeck General Hospital, Northumbria Healthcare NHS Foundation Trust
-
Yeovil District Hospital, Yeovil District Hospital NHS Foundation Trust.
Participants
Men or women aged over 60 years with an isolated displaced and/or unstable ankle fracture who met the following eligibility criteria.
Inclusion criteria
-
Ambulatory prior to the injury – in any capacity.
-
Capable of giving informed consent.
-
Capable of adhering to postoperative instructions.
-
Resident within the catchment area of a recruiting hospital.
-
Could attend for 6 months’ follow-up.
Exclusion criteria
-
Established critical limb ischaemia.
-
Insulin-dependent diabetes mellitus.
-
Active leg ulceration.
-
Open fractures.
-
Serious concomitant disease (i.e. metastatic disease or terminal illness).
-
Clinically substantial degenerative or inflammatory arthritis in the ankle.
-
Unfit for general anaesthetic.
-
Substantial cognitive impairment (defined as a Mini Mental State Examination score of less than 16 out of 30). 39
-
Patient unwilling to give informed consent.
Participant approach and recruitment
The treating surgical team undertook the initial approach to participants. If the participant was willing, a member of the research team explained the study in more detail and checked the eligibility criteria. A Mini Mental State Examination to assess cognitive function was undertaken prior to randomisation. Potential participants were given as long a time as possible to consider participation.
Interventions
All interventions were undertaken within a hospital and conformed to the NHS standard of being performed under consultant supervision. Both study interventions were applied in theatre under general or regional anaesthesia. We recorded time to treatment and the type of anaesthesia (regional, general or both). Surgeons were advised that talar tilt or shift resulting in significant joint incongruence would be considered unacceptable.
Standard care: open surgical reduction and internal fixation
Surgeons were permitted to choose from the range of implants that are used in the UK, and complied with internationally recognised AO principles of fracture management. 40 After surgery the selection of splinting, weight-bearing and clinic follow-up was as per local practice.
Intervention: manipulation under anaesthetic in theatre and application of a close contact casting
Standardisation of the casting materials, cast design and application, and moulding technique was achieved by surgeon instruction (training session and access to training videos and documentation). The training video was available to view prior to any application if a prompt was desired, which could be accessed online (www.youtube.com/playlist?list=PL2Gg_an4nwPfIUC9RQV54Y2lbD76HiWcV). The method of closed-fracture manipulative reduction of deformity was at the discretion of individual surgeons and this falls within the common contemporary skills set of senior surgical trainees and consultants. We advised that CCC should be applied once major swelling had subsided, at a similar time to when open surgery would be considered. We also issued guidance that a consultant grade surgeon who had attended a CCC training session was required to be involved, or at least directly observe, CCC because this was a new technique being introduced into clinical practice. The use of specific moulding points and correspondingly sited pressure pads within the cast (Figure 2) aimed to prevent fracture displacement while also minimising the risk of skin damage. The locations of the moulding points were specific to the participants fracture pattern, so the points of application required to hold the fracture were the decision of the treating surgeon.
FIGURE 2.
Outline of the shapes and locations of the foam pads used in the CCC.

Fracture position was checked by monitoring radiographs in the weeks following initial CCC application, and after any reapplication of the CCC in the clinic if the cast had loosened. Such cast changes did not require anaesthesia. The protocol specified that, if during clinical follow-up there was an unacceptable loss of fracture position prior to clinical union, it was the treating surgeon’s decision whether to reapply a CCC in the outpatient clinic, remanipulate and apply CCC in the operating theatre or convert to ORIF. It was advised that participants undergoing CCC should not do more than touch weight-bearing in the initial 4 weeks after application but then build up to full weight-bearing by 6–8 weeks, but as a pragmatic trial this progression was at the surgeon’s discretion.
A member of the trial team provided training on CCC for surgeons. The training involved a presentation on the AIM trial and the rationale and guidance related to CCC. A training video was then viewed, followed by a live interactive demonstration of the cast application.
The CCC construct was formed by (1) an underlayer of bandage stockinette (e.g. bandage stockinette jersey; BSN Medical GmbH, Hamburg, Germany), (2) small self-adhesive foam pads (e.g. fleecy foam 5 mm; Hapla, Oldbury, UK) over bony prominences (tibial crest, fibular head, calcaneum and metatarsal heads) and over the moulding points on the lower leg medially and laterally, (3) two self-adhesive strips running the full length of the cast (e.g. fleecy web roll 5 cm; Hapla, Oldbury, UK) where the plaster saw would be placed to aid safe removal of the cast, (4) a single non-overlapping layer of synthetic wool roll (e.g. Soffban® Plus; BSN Medical GmbH, Hamburg, Germany), (5) plaster of Paris (e.g. Gypsona®; BSN Medical GmbH, Hamburg, Germany) and (6) a topcoat over reinforcing synthetic casting material (e.g. 3M™Soft Cast Casting Tape; 3M Health Care Ltd, Loughborough, UK).
It was possible that in some cases, after randomisation, the intervention delivered would necessarily be changed. CCC was excluded as an option outside the group randomised to CCC. However, the study protocol expected and gave instruction on the following scenarios.
-
After randomisation and allocation to ORIF, at the point of intervention with anaesthesia commenced, the temporary cast was removed and the ankle skin condition was such that the surgeon considered one or all of the necessary surgical incisions to be unsafe. An alternative treatment would be given (1) traditional plaster cast or (2) external fixation.
-
After randomisation and allocated to CCC, at the point of intervention with anaesthesia commenced, a fracture may have proved irreducible by closed manipulation. The surgeon would necessarily have proceeded to ORIF.
-
If there was an unacceptable loss of position by either treatment method prior to fracture healing, the surgeon was advised to adopt an alternative treatment approach best judged to achieve a favourable outcome.
-
A combination of bone and skin fragility and gross joint instability may have excluded either intervention. The surgeon would then apply a temporising external fixator, and definitive treatment was at the surgeon’s discretion.
-
After randomisation, there may have been a requirement to have a temporising treatment applied in theatre (manipulation and back slab cast or external fixator) until it is clinically appropriate to return to theatre to receive the allocated treatment.
Standardisation of other treatments
Each hospital followed its own antibiotic prophylaxis protocol for the type of implant insertion procedure for the ORIF group. No antibiotics were routinely administered to CCC patients in theatre. Sites were advised to follow their own local hospital policy regarding thromboprophylaxis. The postoperative management plan, including the progression of weight-bearing, was at the discretion of the individual surgeon. As a pragmatic trial, rehabilitation was not standardised for either group.
Monitoring intervention delivery
Routine data checks on theatre procedure forms facilitated monitoring of the intervention delivery as per the protocol. All sites were visited for delivery of training in the CCC intervention, as this was not part of standard care. All attending surgeons completed forms to provide details on their grade and experience of the interventions. The surgeon was then allocated a code which was required on the theatre procedure forms to monitor that the CCC interventions were being applied by those who had attended training and that a trial-trained consultant grade surgeon was involved or directly supervising. Any discrepancies in the grade of surgeon recorded at CCC training compared with their grade at primary theatre were because they had moved up a grade. Discrepancies were identified during routine data checks and sites were contacted if any occurred. Additional training was offered when there were issues regarding availability of trained surgeons.
Learning and expertise effects
It is common practice that surgeons have particular expertise in selected techniques, and for surgical teams to organise their workloads so that expertise is utilised to best effect. For each surgeon participating in the study, we collected the following information: historical experience and preferences for ORIF and casting, grade of surgeon and time since first operation on the study. Surgeon codes allocated at training were used to trace the surgeons’ activity during the trial in order to inform the learning effects analysis. However, some of the trained surgeons involved in the pilot study did not have an allocated code and were thus known exclusions at the outset.
Baseline assessments
Baseline assessments were undertaken by one of the research team following consent and prior to randomisation. None of the participants would have been ambulatory at the baseline phase, but we collected information about pre-injury mobility status using a retrospective report from the Olerud–Molander Ankle Score (OMAS)41 as well as health-related quality of life using the European Quality of Life 5-Dimensions (EQ-5D)42 and the Short Form questionnaire-12 items (SF-12). 43 Although not ideal, recall was the only method that we had for assessing pre-fracture abilities. As the recall period was relatively short (maximum of 2 weeks from injury), we did not expect problems. The type of residence in the month prior to admission was recorded, as was the level of support provided and whether or not the participants lived alone prior to the injury. Information on pre-injury mobility, medical history, smoking, alcohol intake, allergies, medication and care requirements were collected.
Outcome measures
Participants were asked to attend study assessments at 6 weeks and 6 months; those unable to were offered telephone or postal follow-up. The outcomes and time points for the study are outlined in Table 1. The primary outcome measure was the OMAS, an ankle function outcome questionnaire, at 6 months. 41 The OMAS is a rating scale from 0 (totally impaired) to 100 (completely unimpaired) based on nine items. The response to each of the nine items is assigned a score and the summation of these scores makes up the overall score. The items include pain, stiffness, swelling, stair climbing, running, jumping, squatting, supports and work/activities of daily living.
| Domain | Outcome measure | Time point | |||
|---|---|---|---|---|---|
| Baseline | Theatre | 6 weeks | 6 monthsa | ||
| Ankle function | OMAS | ✓b | ✓ | ✓ | |
| Quality of life | EQ-5D | ✓c | ✓ | ✓ | |
| SF-12 | ✓b | ✓ | ✓ | ||
| Pain | Pain items from OMAS and EQ-5D | ✓ | ✓ | ✓ | |
| Physical impairment | Ankle range of motion by goniometry (degrees) | ✓ | ✓ | ||
| Timed up and go test (seconds) | ✓ | ||||
| Radiological fracture outcomes | Ankle joint congruence and fracture healing | ✓ | ✓ | ✓ | |
| Health economics | Theatre procedure data | ✓ | |||
| Resource use questionnaire | ✓ | ✓ | |||
| Patient satisfaction | Two questions (Likert-type scale) | ✓ | ✓ | ||
Secondary outcomes included health-related quality of life measured by the EQ-5D (three levels) and SF-12 (version 1). The EQ-5D questionnaire is used to compute a health utility score that typically ranges from 0 (death) to 1 (perfect health). Negative scores can also be obtained that are reflective of a patient’s quality of life being worse than death. The SF-12 questionnaire is used to compute a mental component summary score and physical component summary score. These scores are measured on a scale of 0–100, with a lower score indicating poorer physical or mental functioning. Pain outcomes were reported and analysed using the pain subscales of the OMAS and the EQ-5D separately.
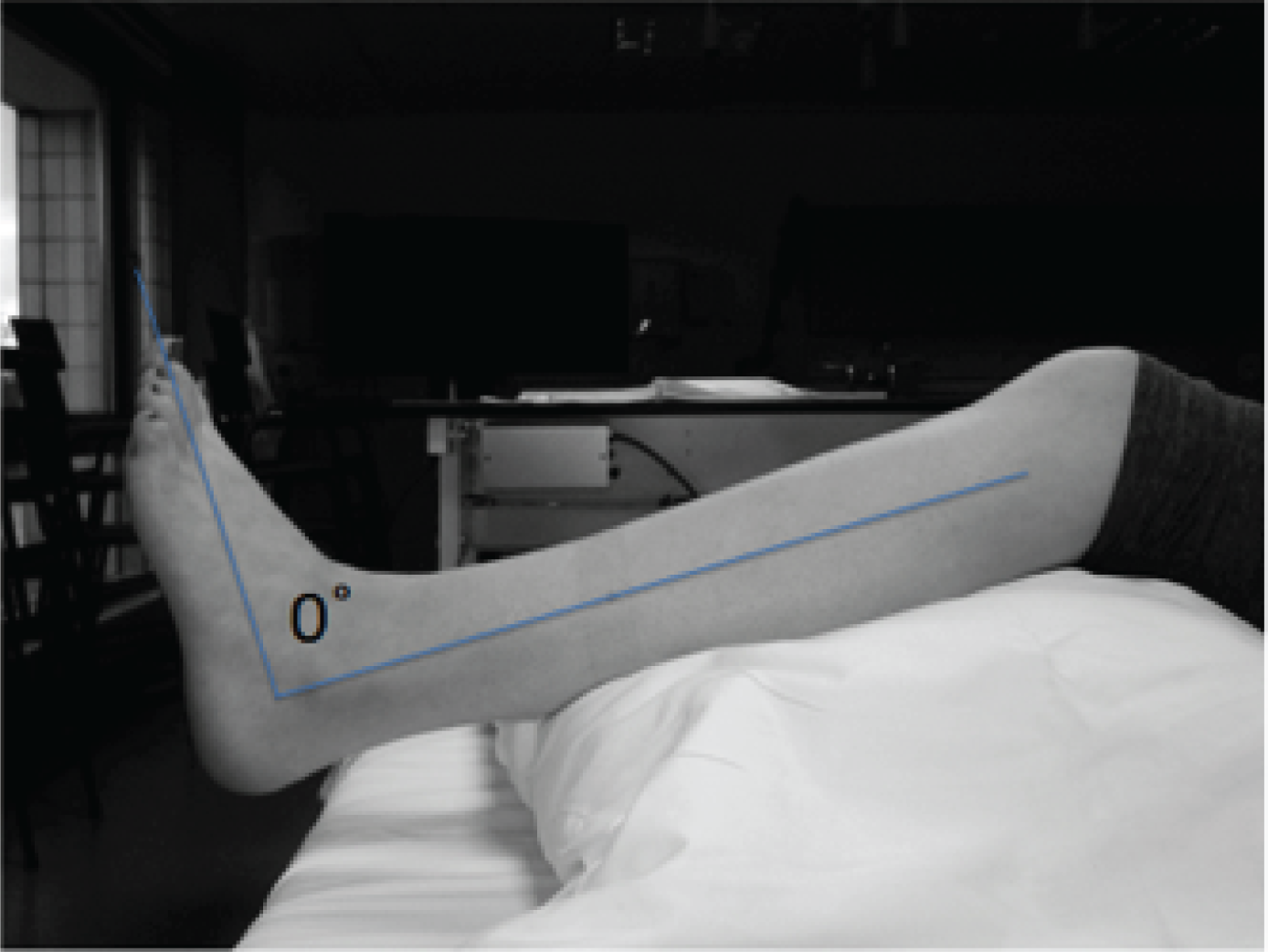
Physical impairment of ankle joint range of motion and mobility were measured. The range of joint motion was assessed at 6 weeks, when clinically appropriate, and at 6 months after randomisation. Range of motion assessments were conducted using a goniometer. 44,45 The standardised technique for the trial was outlined in an illustrated guide issued to sites. Additional training was provided when requested. Participants were positioned on a plinth in long sitting, reclined to about 45°. A support was placed under the upper part of the lower legs to flex the knee to 20–30°, to reduce tension in the triceps surae muscle complex and to lift the heels off the surface of the plinth. Ankle dorsiflexion and plantar flexion range of motion were measured in degrees from the anatomical neutral position, otherwise known as plantar grade (Figure 3). Participants with insufficient dorsiflexion to move the ankle beyond neutral were recorded as having a negative score (e.g. if the participant was 5° from reaching neutral his or her score was –5°).
FIGURE 3.
Lateral aspect of the lower leg with a line indicating plantargrade at the ankle.
For dorsiflexion and plantar flexion the goniometer was placed on the lateral aspect of the ankle; the axis of the goniometer was placed approximately 1.5 cm inferior to the lateral malleolus; the stationary arm was placed parallel to the longitudinal axis of the fibula, lining up with the fibula head; and the moveable arm was placed parallel to the longitudinal axis of the fifth metatarsal (Figure 4). For inversion and eversion, the goniometer was placed on the anterior aspect of the ankle; the axis of the goniometer was placed over the middle of the ankle joint line, the stationary arm was placed along the longitudinal axis of the tibia lining up with the tibial tuberosity; and the movable arm was placed along the second metatarsal in line with the base of the second toe (Figure 5).
FIGURE 4.
Positioning of the goniometer for the assessment of ankle dorsiflexion and plantar flexion range of motion.
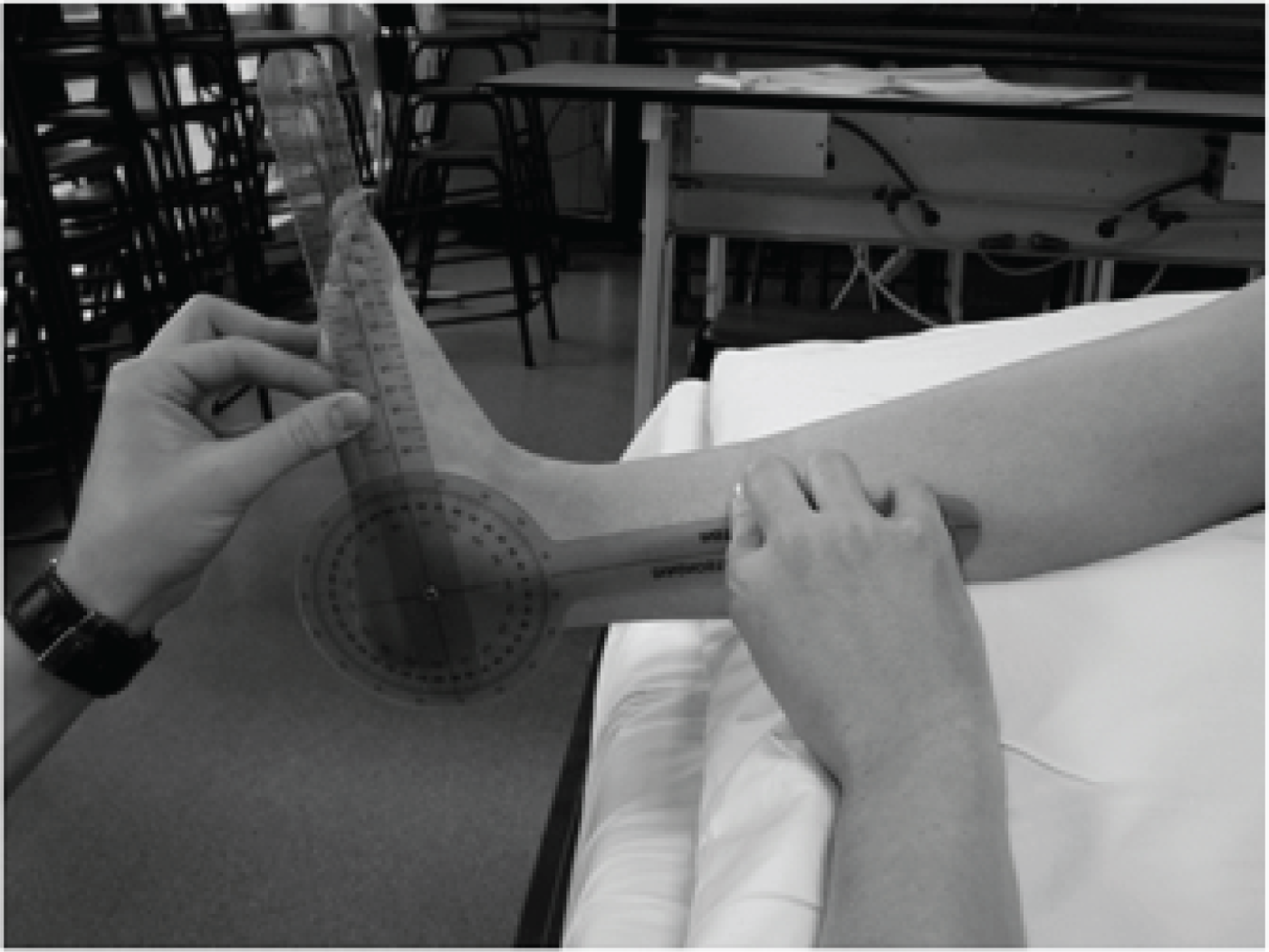
FIGURE 5.
Positioning of the goniometer for the assessment of ankle inversion and eversion range of motion.

Mobility, assessed using the timed up and go test,46 was included as an outcome at 6 months. The timed up and go test is a test specifically designed for frail older people. The test recorded the time taken to get up from a chair with armrests, walk 8.6 m (standardised distance for the AIM trial), turn at a mark on the floor, then walk back to the chair and sit down again. Participants were asked to walk safely as fast as possible and used a walking aid if they normally did so. Performance tests are a recognised standard for measuring mobility and associations with important end points including risk of falling, functional decline and institutionalisation. 47 We also noted the date that participants commenced partial weight-bearing. Patient satisfaction was measured on an ordered categorical scale.
Outcomes for the economic evaluation were the incremental quality-adjusted life-years (QALYs) and the incremental costs of CCC over ORIF. Incremental differences were the differences in totals. Total costs were the sum product of resource use with unit resource cost. Total QALYs were the integration of utility over time.
Radiological outcomes
Radiographs were taken for both groups postoperatively at 6 weeks and at 6 months. Fracture union and joint position were assessed on standard anteroposterior, ankle mortise view and lateral view radiographs using standard measures of joint congruence, fracture angulation, fibular shortening and subluxation. 48 Radiographs were assessed within a purpose built software using Matlab (The MathWorks, Natick, MA, USA). The radiographs were reviewed centrally by a trained independent assessor. Measurements and decisions regarding non-union and malunion at 6 months were also verified by two independent surgeons. Malunions were defined as a radiograph demonstrating any one or combination of showing talar subluxation > 2 mm (talar shift), excessive talar tilt (> 2°) or a diastasis (tibiofibular clear space ≥ 5 mm). Any disagreement was resolved by a radiologist.
Complications and further surgery
Expected recognised complications or harms related to the study treatments were recorded as adverse events only. Adverse events attributed to medical comorbidities or anaesthesia (part of normal care) were only recorded as adverse events and were not reported as serious adverse events. Medical problems or surgery not associated with the study interventions were not systematically collected or reported. Other fractures sustained, further ankle surgery or major illness due to the interventions were recorded. A serious adverse event was any untoward medical occurrence that was both unexpected and related to the study treatments: (1) death within 30 days of surgery, (2) death related directly to the surgical intervention at any time, (3) life- or limb-threatening complication or (4) rehospitalisation (except a hospital stay for removal of syndesmotic screws which was recorded as an adverse event).
Randomisation
Randomisation took place following screening and baseline assessments. The unit of randomisation was the individual and assignment was in a 1 : 1 allocation ratio. We used the remote 24-hour telephone randomisation service available from the University of Aberdeen.
Allocation concealment was ensured by registering participants before computer generation of the allocation code. Sequence generation was by random block size and stratified by centre and fracture pattern, using trans-/infrasyndesmotic (type A/B) and suprasyndesmotic categories (type C) as stratification factors.
Blinding
At 6 months a health professional, who was blind to treatment assignment, completed the clinical measurements and ensured completion of the study questionnaires. The presence or absence of surgical incision(s) was obscured by opaque bandage(s) applied by a research nurse/therapist prior to the participant meeting the blinded assessor. Patients unable to attend were contacted by telephone or visited at home. We undertook an analysis of the success of the blinding strategy using outcomes from questions asking blinded assessors to indicate if they believe they knew the intervention allocated and/or received.
It was not possible to blind the surgeons or participants because of the nature of the interventions. It was also not possible to blind radiograph assessors or treating surgeons as the implants, or their absence, would be apparent on the radiographs, as would the surgical scars on examination. The 6-week follow-up was conducted in the clinic by researcher, who was not blinded to treatment allocation. At 6 weeks, the researcher needed to be aware of relevant precautions and contraindications to the clinical assessment of range of ankle motion (i.e. discuss this with the surgeon to ensure fracture was stable).
Sample size
Given the paucity of data in the published literature, pilot trial data were used as a primary source to inform estimates of variance and treatment effects measured using the OMAS, and a range of secondary outcomes.
Although the original sample size estimate was based on a difference in proportions, this was modified following advice from the Data Monitoring and Ethics Committee (DMEC), as data from the pilot study showed the data to be normally distributed and that analysis based on a continuous score would be more efficient and meaningful. Parameters for the sample size were informed by data from the pilot study, known only to the study statisticians and the DMEC. We utilised a sample size calculation with one-sided testing (p = 0.05), as we were not trying to prove that the new treatment was better than the standard and, therefore, gained considerable statistical efficiency. 49,50 The power of the study was set at 80% as is conventional in clinical trials. 50
Data from the pilot study informed the sample size; the standard deviation (SD) on the operative arm was 16.2 OMAS points. An equivallence margin of ± 6 points on the OMAS yielded the final sample size of 560 in total. 49 We inflated for loss to follow-up of nearly 10%, yielding a total sample size of 620. Published estimates to inform the selection of equivalence margins using the OMAS were non-existent. Using the pilot data to calculate standardised effects sizes, the equivalence margin included small differences (< 0.37), but excluded moderate or large treatment differences. This was consistent with clinical opinion supporting a 6-point margin excluding clinically important differences in this condition gathered in an informal survey of orthopaedic surgeons. It was also consistent with published data on the minimally clinically important differences for similar scores (Foot and Ankle Score and visual analogue pain scores in acute injury) that reported minimally clinically important differences > 10 points on a 100-point scale.
Pilot study
A pilot study was conducted in the trauma service at the Oxford University Hospitals NHS Foundation Trust. We recruited 95 participants and this informed the design and established the feasibility of the multicentre phase of the study. None of the participants of the pilot study completed the full health economic evaluation questionnaire used later in the multicentre phase of the AIM trial. The independent DMEC agreed that the pilot trial design was sufficiently similar to the multicentre phase for the data from these participants to be integrated.
Statistical methods
In equivalence testing, a maximum clinical difference (ΔT) is prespecified at a level within which the two treatments can be considered not to differ in any clinically meaningful way. Therefore, the relevant null hypothesis was that a difference of greater than ΔT exists in either direction (H0: Δ ≤ –ΔT or Δ ≥ ΔT) and the trial was targeted at disproving this in favour of the alternative that no clinical difference existed (HA: –ΔT < Δ < ΔT). The US Food and Drug Administration’s regulations recommend both a treatment received (per-protocol) and an intention-to-treat (ITT) analysis, aiming to demonstrate equivalence. 51 Use of an ITT approach in a superiority trial sometimes increases the chance of falsely claiming equivalence. 52,53 ITT is conservative when trying to detect a difference, and so the opposite is true when trying to show similarity. The effect of low protocol adherence would therefore make the treatment groups appear more similar that they actually are. Initially, a per-protocol analysis was undertaken where only the patients who received their allocated treatment were analysed and those patients who did not were excluded from the analysis. Following we carried out an ITT analysis was carried out in which all randomised patients were analysed according to the treatment to which they were randomised.
Participants were excluded from the per-protocol analysis if they did not receive their allocated treatment during either their initial inpatient stay (i.e. during their primary theatre procedure or as an additional theatre procedure) or during a readmission. The protocol allowed for the eventuality of participants requiring a visit to the operating theatre for a temporary intervention prior to receiving the allocated treatment (e.g. a temporary cast, back slab or external fixation). However, if the allocated intervention was received after a clinically definitive intervention, then the participant was not deemed to have received the allocated treatment.
The result of the analysis of the primary end point could be one of the following:
-
The confidence interval (CI) for the difference between the two treatments lies entirely within the equivalence range, –ΔT to ΔT, so that equivalence may be concluded with only a small probability of error.
-
The CI covers at least some points that lie outside the equivalence range, so that differences of potential clinical importance remain a real possibility. In other words, superiority of treatment cannot be ruled out and thus equivalence cannot safely be concluded.
-
The CI is wholly outside the equivalence range (although this is likely to be rare). This is indicative of the trial being underpowered as the CI includes non-trivial effect sizes that are in favour of both arms.
As well as assessing if equivalence was demonstrated in either case, this also formed part of an additional sensitivity analysis to assess the range of potential biases that could have resulted from loss to follow-up, protocol deviations, withdrawal (and mortality). Numerical and graphical summaries of all the data were compiled, including descriptions of missing data at each level. Estimates of treatment effect were reported with 95% CIs and a figure showing CIs and margins of equivalence were also presented. Our main analytical methods were generalised linear models (GLMs), and all primary and secondary outcomes were adjusted for important baseline covariants [age, sex, centre (hospital), fracture pattern (Weber A and B or C), baseline score] to maximise precision.
The OMAS, at 6 months, was the primary outcome in this study and was compared between treatment groups as the dependent variable in a linear regression model for the primary analysis. The treatment difference was based on the estimates of the adjusted means and 95% CIs. The OMASs were also presented as an ordinal outcome in a secondary analysis using ordered logistic regression (proportional odds model). Secondary outcome measures were similarly analysed with logistic regression models being used for categorical data and linear regression models for continuous data. The EQ-5D measure was summarised both at an item level and an overall score as recommended by the EuroQol group. Time-to-event data (time from randomisation to discharge and time from randomisation to readmission) were analysed using a log-rank test. Any patients who did not experience an event at the time point of interest or who withdrew were censored. The p-value and a hazard ratio with its 95% CI from a Cox proportional hazards model were also presented. The proportional hazards assumption across treatment arms was checked graphically using a log-cumulative hazard plot. Outcomes that were not normally distributed were evaluated using a non-parametric Wilcoxon rank-sum test. A data analysis plan was agreed with the DMEC.
The success of the blinding strategy was assessed using James’ Blinding Index, which ranges from 0 (total lack of blinding) to 1 (complete blinding). 54 The learning effects were assessed by fitting a longitudinal random model. For each surgeon, operation time was ordered sequentially by date and a time variable was created. This time variable was fitted as a random effect into a longitudinal model, with the operation time as the response variable. The surgeon was also included as a random effect. Another similar model was fitted nesting surgeon within hospital and treating these as random effects. This assessed the learning effect allowing for surgeons within their hospitals.
The clinical results were analysed using Stata version 13.1 (StataCorp, College Station, TX, USA) and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Economic analyses
An evaluation of cost-effectiveness of CCC compared with ORIF was conducted as part of the AIM trial. The economic evaluation presented in this chapter was conducted for both the NHS and societal perspectives. We conducted analyses for both the per-protocol and ITT populations. Consistent with the evaluation of cost-effectiveness, the primary analysis was per protocol.
Data
Outcome data
Health outcomes were expressed as QALYs. QALYs are the length of life weighted by the health-related quality-of-life weights. Health-related quality-of-life weights were estimated by using the EQ-5D-3L instrument. In the EQ-5D-3L, respondents rate five dimensions of health: mobility, self-care, usual activities, pain/discomfort and depression/anxiety. For each dimensions, respondents rate the extent of their problem as ‘no problem’, ‘some problems’ or ‘extreme problems’. Responses are transformed into an index of general health on the interval –0.59 to 1 using an algorithm based on UK general population valuations in which a negative value corresponds to a state worse than death. 55 The interval represents the relative value of an individual’s current health state; the lower and upper bound of the interval represent death and full health, respectively. A lower utility indicates a lower preference for that health state.
Resource utilisation data
Relevant resource use prospectively collected over 6 months included resource use during hospitalisations, time spent at discharge locations, and number of health services and medications used. We collected itemised resource use for hospitalisations from administration and visit records to microcost the entire episode of hospitalisation. Hospitalisations included the index primary procedure as well as any additional procedures and rehospitalisations. Resource use items measured during each hospitalisation included implant material use (screws, plates, wires, etc.), casts, anaesthesia, theatre time and length of stay.
Time spent at discharge locations, the number of contacts with health services and the provision of medications were collected from participant questionnaires at 6 weeks and 6 months. Participants were asked to indicate the type and quantity of each resource item used for services provided by the NHS and for any private service use, whether paid out of pocket or by private insurance.
Discharge locations included community hospital days, intermediate care days and nursing home days. Health services included the number of contacts to the general practitioner (GP), nurse, physiotherapist, hospital specialist, hospital accident and emergency, psychologist, orthopaedic trauma outpatient/fracture clinic and community care centre. Medications included painkillers, anti-inflammatory medication, analgesic gel, sleeping pills and antidepressants. For medications, participants were asked to indicate the number of times the medication was prescribed.
Participants were also asked to indicate any private health-care use to inform societal resource use. In addition to the above resource uses, societal resource uses included time spent at private nursing home; private health service use in the same aforementioned services; out-of-pocket medications for the same aforementioned medications; and work days taken by both the patient and their friends or family. For out-of-pocket medications, participants indicated the number of times the medications were bought.
Unit costs
The total NHS and societal cost was calculated by multiplying resource use data by their unit costs. Unit costs included the cost of implant materials, casts, theatre time, length of stay, time spent at discharge locations, various health services, various medications and work days taken. All unit costs reported in this section are in 2014 units.
NHS health-care unit costs
Unit costs for all resources consumed and contacts with NHS health services were derived from published sources relevant to the UK. Unit costs for these items are reported in Table 2.
| Units | Mean cost (£) | Standard error (£) | Source | Distribution |
|---|---|---|---|---|
| Implant material costs | ||||
| Screw | 6 | 2 | NHS procurementa | Gamma |
| Tension band wire | 20 | 3 | NHS procurementa | Gamma |
| Antigliding plate | 17 | 3 | NHS procurementa | Gamma |
| Tubular plates | 17 | 3 | NHS procurementa | Gamma |
| Dynamic compression plate | 30 | 8 | NHS procurementa | Gamma |
| Reconstruction plate | 67 | 20 | NHS procurementa | Gamma |
| Locking plate | 114 | 29 | NHS procurementa | Gamma |
| CCC | 30 | 3 | NHS procurementa | Gamma |
| Hospitalisation costs | ||||
| Anaesthesia | 313 | 2 | NICE CG124,56 Chakladar et al.57 | Gamma |
| Theatre time, 1 hour | 975 | 87 | Information Services Division Scotland58 | Gamma |
| Inpatient time, 1 day | 276 | 59 | NHS Reference Costs 2011–2012 59 | Gamma |
| Long-term care facilities | ||||
| Community hospital time, 1 day | 194 | 19 | NHS West Sussex60 | Gamma |
| Intermediate care time, 1 day | 64 | 13 | PSSRU61 | Gamma |
| NHS nursing home time, 1 day | 98 | 10 | PSSRU61 | Gamma |
| Acute health resource use | ||||
| A&E visit | 54 | 7 | NHS Reference Costs 2011–2012 59 | Gamma |
| Physiotherapist outpatient visit | 47 | 9 | NHS Reference Costs 2011–2012 59 | Gamma |
| Hospital specialist visit | 45 | 9 | NHS Reference Costs 2011–2012 59 | Gamma |
| Psychologist visit | 62 | 12 | PSSRU61 | Gamma |
| Trauma outpatient visit | 95 | 16 | NHS Reference Costs 2011–2012 59 | Gamma |
| Hospital transport | 189 | – | National Audit Office62 | Gamma |
| Community care visit | 46 | 11 | NHS Reference Costs 2011–2012 59 | Gamma |
| Medication | ||||
| Painkillers | 1 | – | BNF63 | Determ |
| Anti-inflammatories | 3 | – | BNF63 | Determ |
| Gel | 6 | – | BNF63 | Determ |
| Sleeping pills | 21 | – | BNF63 | Determ |
| Antidepressants | 1 | – | BNF63 | Determ |
| Productivity loss, 1 day | 99 | – | ONS64 | Determ |
Unit costs for implant and cast materials were derived from NHS procurement (www.supplychain.nhs.uk). Theatre time was derived from Information Services Division Scotland,58 whose estimate of hourly theatre time was based on 123,976 hours in orthopaedic specialties across the 367 operating theatres in all 15 health boards; this was thought generalisable across the UK. General inpatient day cost was derived from NHS Reference Costs 2011–2012;59 we used the weighted average excess inpatient bed-day for all major, minor and intermediate foot procedures (HA31B, HA31C, HA32Z, HA33Z, HA34Z and HA35Z). The cost of anaesthesia was derived from a UK study measuring the anaesthesia costs in hip fracture patients,57 which was subsequently used by the National Institute for Health and Care Excellence (NICE) in its economic evaluation of hip fracture. 56
Unit costs of all health services use were derived from NHS Reference Costs 2011–201259 and the Personal Social Services Research Unit (PSSRU)’s Unit Costs of Health and Social Care 2012. 61 There were two exceptions. The day cost of a community hospital stay was derived from a study of community bed service by NHS West Sussex. 60 The cost of a hospital transport was derived from the National Audit Office’s report on NHS ambulance services. 62
Unit costs of prescriptions were derived from the British National Formulary. 63 A prescription of painkillers corresponded to a 32-tablet 500-mg pack of paracetamol; anti-inflammatories, an 84-tablet 200-mg pack of ibuprofen; gels, a 100-g tube of Ibugel™ (Dermal Laboratories, Hitchin, UK) containing 10% ibuprofen; sleeping pills, a 28-tablet 10-mg pack of temazepam; and antidepressants, a 30-capsule 20-mg pack of fluoxetine.
Societal unit costs
Societal costs included private health-care costs. The unit costs of private nursing home days, private health services and private medication use were the same for both NHS and private resource use. That is because unit costs are meant to measure not the list price but the economic cost. The economic cost is the opportunity cost of the resource. Compared with private list prices, the NHS unit costs are likely a more accurate estimation of the opportunity cost. Thus, societal unit costs were valued at the same levels as their NHS counterparts.
The productivity cost of a workday was derived assuming an average workday of 7.5 hours and an average hourly wage across all UK residents of £12.83/hour as estimated from the Office for National Statistics. 64
Analysis methods
The economic evaluation was conducted for both the NHS and societal perspectives. The evaluation was also then conducted for both the per-protocol and ITT populations. The primary analysis used the per-protocol population. The overall flow of analysis is depicted in Figure 6.
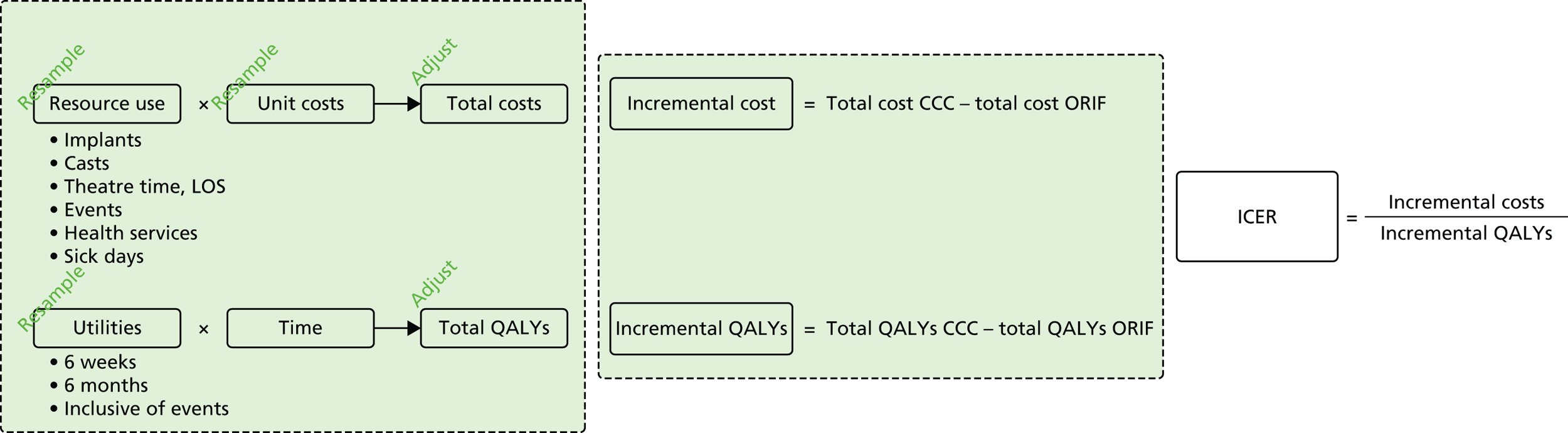
FIGURE 6.
Conceptual diagram of relevant economic inputs and their relationship to intermediate and final economic outcomes. Starting on the left are the resource use and utility information from the trial, which, when applied to their cost and time weights, give total costs and total QALYs, respectively. Incremental differences can then be calculated and synthesised into an incremental cost-effectiveness ratio, if appropriate. Sampling uncertainty, parameter uncertainty and heterogeneity are incorporated or controlled for by means of resampling/bootstrapping and adjustment via statistical modelling. ICER, incremental cost-effectiveness ratio; LOS, length of stay.
Missing data
In the case of missing data, multiple imputation with chained equations was used, in which evidence supported the conclusion that data were missing at random. Individuals who had zero information on all outcomes were removed from the analysis. This applied to individuals with no utility information at presentation, 6 weeks and 6 months.
Multiple imputation with chained equations is the dominant method for complex incomplete data across multiple variables. 65 It accounts for the process that created the missing data, preserves the relations in the data and preserves the uncertainty about these relations. 65 Potential predictors included baseline characteristics as well as all resource use and health outcome items. Downstream variables were removed as predictors of upstream variables. Multiple imputation was conducted using 40 iterations and five imputations. Density and convergence plots were inspected to confirm comparable distribution shapes to observed data and healthy convergence.
Costs and quality-adjusted life-years
Mean resource use was tabulated by category and type of health-care service for ORIF and CCC. After consideration of normality using visual inspection and Shapiro–Wilk tests, differences in resource use were estimated using the Wilcoxon’s signed-rank test, Fisher’s exact test or chi-squared tests, depending on the variable. Total NHS and societal costs for each participant were calculated by adding the cost of each resource use.
Utility weights at presentation, and at 6 weeks and 6 months after randomisation, and total QALYs are presented for the ORIF and CCC groups. After consideration of normality using visual inspection and Shapiro–Wilk tests, differences in utility and QALYs were estimated using the Wilcoxon’s signed-rank test. QALYs were estimated by integrating the length of life by the utility weights as an ‘area under the curve’. We assumed monotonic changes in utility between any two measurement points.
We presented 95% confidence limits using a bootstrap approach. Total NHS cost, total societal cost and total QALYs were resampled 10,000 times, stratified by treatment with ORIF or CCC to ensure equal sample sizes to that of the original number of participants in each group.
Total NHS and societal costs, as well as total QALYs, were estimated using a regression approach. The regression included a treatment term in addition to other relevant baseline characteristics. As total costs and QALYs were skewed (as they generally are), we used a GLM. The appropriate family and link functions were determined using the modified Park’s and Hosmer–Lemeshow tests, respectively. We used the best-fitting GLM and the generic ordinary least squares (OLS) model to present results. Baseline covariates of interest were selected by clinical reasoning, independent association with the dependent variable at the p < 0.25 level and prevalence > 10%.
The developed statistical models were then used to estimate the mean total NHS and societal costs as well as mean total QALYs using marginal prediction. Marginal prediction eliminates known covariate imbalance between the intervention groups before estimating total costs and QALYs. Any observed comparison of two groups, whether randomised or not, is likely to show imbalance in baseline covariates, regardless of statistical significance. These imbalances should be regarded as sources of bias in the estimation of mean costs and QALYs, and by extension, differences in costs and QALYs between the intervention groups. Marginal prediction effectively holds all else equal, save for the predictor of interest – treatment.
To appropriately incorporate sampling uncertainty, unit cost uncertainty and statistical model uncertainty, we conducted the entire process within a bootstrap framework. The process consisted of three steps. First, a sample was drawn from the original trial sample, stratified by treatment group. Second, unit costs were randomly drawn from their probability distributions and applied to the resource uses in the sample while QALYs were estimated as the area under the curve. Third, and finally, statistical models were assembled and marginal estimation was conducted. Thus, each of the 10,000 iterations of the bootstrap produced a new resample, a new set of unit costs and a new statistical model for marginal estimation and a new set of total NHS cost, total societal cost and total QALYs.
Lifetime extrapolation
Lifetime extrapolation or modelling may be warranted when there is reason to expect differences in long-term costs or QALYs. Factors that affect either include life expectancy, quality of life or events; however, only incremental differences in these factors would affect long-term costs or QALYs.
Life expectancy is not expected to be any different after ORIF or CCC. Quality of life and the rate of its recovery are not expected to differ in the long term. However, if there were clear differences by the end of the trial period, their extensions into the lifetime would be explored.
The AIM trial incorporated a 6-month follow-up. Under normal circumstances, clinical union takes 6 to 12 weeks. The 6-month follow-up of the AIM trial was well suited to capture failure of closed reduction or loss of reduction resulting in reoperation/rehospitalisation. Beyond 6 months, reoperation/rehospitalisation because of excess complications may be possible. However, the probability of this is likely to be very low. In an analysis of 57,183 patients with ORIF, the 6-year reoperation rate was < 1% while complications were < 2%. 27 More specifically in elderly patients, Koval et al. 66 found in 33,704 elderly patients the incidence of complications to be ≤ 2%. Importantly, there was no difference between those treated operatively and those treated non-operatively. These findings are supported by a Cochrane review that compared surgical and conservative management of ankle fractures with follow-up from 20 weeks to, on average, 7 years. 23 A randomised trial in elderly patients with a mean follow-up of 27 months also found negligible incidence beyond 3 months. 29 As published evidence of the long-term complications shows low incidence and no difference between operative and non-operative management, we did not plan to model any long-term differences in complications between ORIF and CCC. Extended follow-up of the AIM trial will provide an opportunity to investigate long-term complication rates following ORIF or CCC.
Excess implant removal because of local irritation is also possible beyond 6 months. Estimates in the literature may be biased where removal is a paid part of a surgeon’s practice. Based on the experience of the trial group, removal for irritation was estimated to be very low, at around 2–3%. We assumed the removal cost to include a theatre time and length of stay similar to that observed for an additional procedure/readmission from the trial data. This was later determined to be 1.3 theatre hours and 5 days in hospital. Thus, the total cost was £2648 per removal. After weighting by a 3% removal rate, this amounted to £79 for the population receiving implants. We attributed this removal during the index year. No impact on QALYs was modelled, as general health domain functioning is independent of whether or not hardware is removed in those experiencing pain/discomfort. 67
Incremental analysis
Using the estimated total NHS cost, total societal cost and total QALYs, we calculated the incremental differences between the CCC and ORIF groups. We presented the incremental cost-effectiveness ratio (ICER) for the NHS and societal perspectives, when appropriate. The mean ICER is the mean incremental cost divided by the mean incremental QALYs.
There are four potential combinations of incremental costs and QALYs:
-
CCC is more effective and more costly.
-
CCC is less effective and less costly.
-
CCC is more effective and less costly.
-
CCC is less effective and more costly.
These four potential combinations of incremental costs and QALYs were visualised by a cost-effectiveness plane, which is a scatterplot of incremental costs on the y-axis and incremental QALYs on the x-axis.
We used the net benefit statistic to estimate the probability that CCC is cost-effective. CCC is cost-effective when the net benefit is greater than zero. The net benefit was the incremental benefit minus the incremental cost. The incremental benefit was the incremental QALYs multiplied by the willingness to pay. There were two incremental costs: (1) the incremental total NHS cost and (2) incremental total societal cost. We estimated two net benefits for the NHS and societal perspectives.
The probability of cost-effectiveness changes as a function of the willingness to pay. Using all 10,000 bootstrap samples, we estimated the overall probability of cost-effectiveness for all willingness to pay over a common range (£0/QALY to £30,000/QALY). The probability of cost-effectiveness was plotted on a cost-effectiveness acceptability curve.
Sensitivity
The bootstrapped model already incorporates sampling uncertainty, unit cost uncertainty and statistical model uncertainty.
We explored structural uncertainty in the statistical models by employing the second best-fitted GLM as well as the generic OLS model (a GLM with Gaussian family and identity link). The entire economic evaluation was repeated for the ITT population.
Pilot patients did not have any utility information at presentation, and at 6 weeks and 6 months after randomisation. They also did not complete health economic questionnaires at 6 weeks or 6 months after randomisation. They therefore had no information valid for an economic evaluation. Patients with no valid information were excluded from the analysis. However, we explored including these patients with completely missing information after imputing their values on the basis of baseline characteristics and primary procedure resource use.
Identification of best-fit GLMs was conducted in Stata version 12 (StataCorp, College Station, TX, USA). All other statistical and economic analyses, as well as data handling, were conducted in R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Database and data processing
The return of case report forms (CRFs) to the study office was monitored daily to proactively request any documentation that was expected for arrival and to track any forms that were yet to be returned. On arrival into the study office, each CRF was checked through central monitoring systems to ensure completeness of the data and to monitor protocol compliance. These systems facilitated immediate identification of missing or contradictory data. Sites were contacted in a timely manner to resolve these queries. Data queries were reviewed on a weekly basis and followed through to completion to ensure maximum data capture. Items of missing data and the reason not obtained were logged for reference. The statistician, prior to Trial Steering Committee (TSC) and DMEC meetings, reviewed data completion and follow-up rates.
Data were single-entered into the database by study personnel. For data quality assurance, 10% of baseline, 6-week and 6-month follow-up questionnaires were randomly selected for data checking. Any discrepancies identified when checking were corrected and recorded. The data were held on Warwick Clinical Trial Unit’s SQL Server (Microsoft, Redmond, WA, USA) system that facilitated data validation, range checks and flagging of missing data.
Qualitative study
In order to explore patient experience of their treatment, recovery and what it was like to be in the trial, a purposive sample of 36 patients was interviewed between 6 and 10 weeks post treatment. An estimated sample of up to 40 patients was identified in the study protocol, but data saturation (when interviews stop revealing new data) was achieved with a sample of 36 participants. The sample covered patients from both treatments, two study sites, and a range of ages and sexes. Participants provided informed written consent. The interviews were conversational in style to allow patients to identify their experiences and the issues that concerned them. The research questions were (1) what are participants’ experiences of ankle fracture in the first 6–10 weeks after treatment? and (2) what are participants’ experiences of being in a trial? Further information on methodology and findings for the qualitative study is presented in Chapter 5.
Patient and public involvement
The study design, patient information sheets and consent forms for the main trial and qualitative substudy were discussed with the Oxford Trauma User Group who provided feedback and support for the study. The user group are former users of the Oxford Trauma Service and/or previous participants in clinical studies conducted by the Trauma Research Group based in the Kadoorie Centre for Critical Care Research and Education, all based at the John Radcliffe Hospital, Oxford, UK. A user representative was a member of the TSC and as part of this role made a suggestion for the additional secondary analysis of the primary outcome as an ordered categorical outcome, as outlined in Statistical Methods.
Ethical approval and monitoring
The Oxfordshire Research Ethics Committee A gave approval for this study (09/H0604/129). We complied with the Medical Research Council: Guidelines for Good Clinical Practice in Clinical Trials,68 and the trial was run using the standard operating procedures of Warwick University Clinical Trials Unit and the Oxford Clinical Trials Research Unit.
Trial Steering Committee
The TSC was responsible for monitoring and supervising the study. The TSC consisted of two independent members, a lay member and key members of the trial management group, in addition to clinical and methodological experts. Membership of the TSC is given in the Acknowledgements.
Data Monitoring and Ethics Committee
The DMEC was independent of the trial and was responsible for monitoring ethical, safety and data integrity. The trial statistician provided data and analyses requested by the DMEC at each of the meetings. Membership of the DMEC is given in the Acknowledgements.
Trial Management Group
A Trial Management Group was responsible for the day-to-day management of the trial, consisting of the chief investigator, coinvestigators, research fellows, research physiotherapist, statisticians, health economists, research nurse, trial manager and data co-ordinator. The role of the Trial Management Group was to monitor the conduct and ensure progress of the trial according to the study protocol and to take appropriate action to safeguard participants and the trial itself.
Reporting
The trial was reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement69 and its extensions relating to non-pharmacological,70 pragmatic71 and equivalence trials. 53 Reporting of the intervention was also based on the template for intervention description and replication (TIDieR) guidance. 72
Summary of changes to the project protocol
The changes to the project protocol are summarised in Table 3.
| Protocol version number | Date issued | Details of changes made |
|---|---|---|
| 1 | August 2009 | Original protocol |
| 2 | 18 February 2010 | Minor clarifications |
| 3 | 25 May 2010 | Removal of 10-day ASEPSIS assessment |
| Addition of EQ-5D at baseline ‘with injury’ | ||
| Addition of health economic questions at 6 weeks | ||
| 4 | 11 November 2010 | Minor clarifications |
| 5 | 16 August 2011 | Update to radiography process and also the name of service used to match, flag and trace patients |
| 6 | 10 April 2013 | Change to the extended follow-up time frame; now at least 2 years (rather than at 5 years) |
| 7 | 24 January 2014 | Statistical section updated to ensure it was in line with analysis plan. The main clarification was to confirm details relating to the sample size estimate as described in Sample size. Further clarifications to the radiograph review process were also confirmed |
Chapter 3 Clinical results
Participant flow
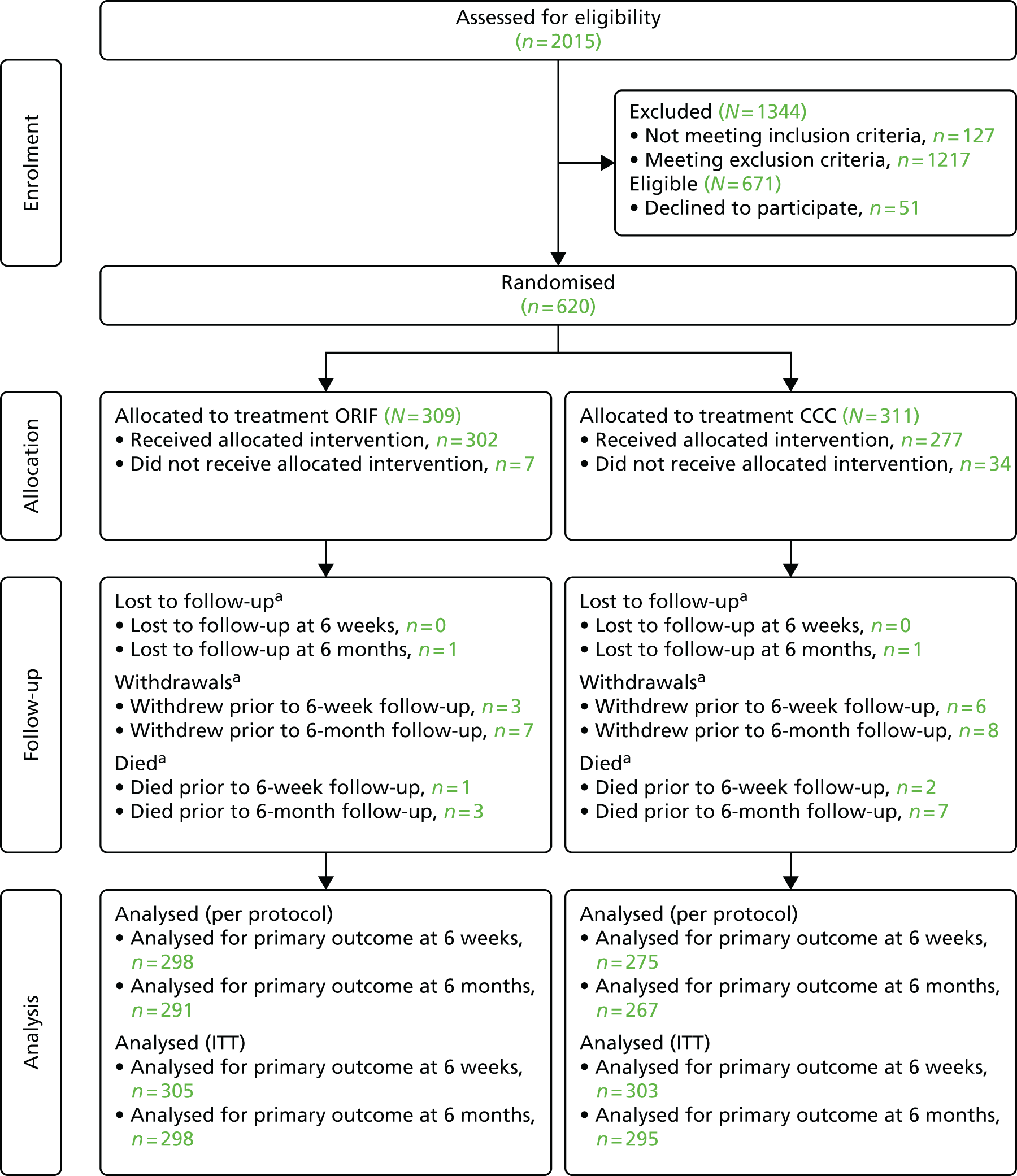
Figure 7 presents the CONSORT flow diagram summarising the different phases of the trial from the recruitment stages up to the point of final analyses. Table 4 provides details of the study flow. In summary, 620 patients were recruited and randomised. There were 309 participants randomised to ORIF, of whom 302 received their allocated treatment. There were 311 participants randomised to CCC, of whom 277 received their allocated treatment. At baseline, 618 out of 620 (99.7%) participants provided baseline data, 608 out of 620 (98.1%) participants provided data at 6 weeks and 593 out of 620 (95.6%) participants provided data at 6 months. Baseline data for two of the participants were missing as they were randomised in error, that is they were ineligible, and thus were withdrawn. Fifteen out of the 620 (2.4%) participants withdrew from the trial and 10 out of the 620 (1.6%) participants had died. At the 6-month primary end point, 558 out of 620 (90%) participants were analysed for the primary outcome in the per-protocol analysis and 593 out of 620 (96%) participants were analysed for the primary outcome in the ITT analysis. The details of each of the stages in the flow of the trial will now be described in more detail.
FIGURE 7.
The CONSORT diagram for the AIM trial. a, Number of participants (n) reported is cumulative.
| Trial participation | Participants | |
|---|---|---|
| Recruitment | All patients screened | 2015 |
| Eligible patients | 671/2015 (33.3%) | |
| Patients consented and randomised | 620/671 (92.4%) | |
| Patients declined consent | 51/671 (7.6%) | |
| Excluded patients: patients meeting inclusion criteria but also meet at least one of the exclusion criteria. Exclusion criteria include MMSE score of < 16 | 1344/2015 (66.7%) | |
| Baseline | Participants with baseline data | 618/620 (99.7%) |
| Follow-up | No follow-up data at any time point | 12/620 (1.9%) |
| Follow-up data at 6 weeks only | 15/620 (2.4%) | |
| Follow-up data at 6 months only | 0 | |
| Follow-up data at all time points | 593/620 (95.6%) | |
| Died | Participants died | 10/620 (1.6%) |
| After randomisation, but before theatre for treatment | 0 | |
| In theatre, but before starting any trial procedure | 0 | |
| During initial treatment in theatre | 0 | |
| In hospital ≤ 30 days following initial treatment in theatre | 2/620 (0.3%) | |
| In hospital > 30 days following initial treatment in theatre | 0 | |
| After initial hospital discharge ≤ 30 days after initial treatment, but before 6 weeks’ follow-up | 0 | |
| After initial hospital discharge > 30 days after initial treatment, but before 6 weeks’ follow-up | 1/620 (0.2%) | |
| After 6 weeks’ follow-up, but before 6 months’ follow-up | 7/620 (1.1%) | |
| Withdrawals | Participants withdrawn | 15/620 (2.4%) |
| After randomisation, but before initial hospital discharge | 5/620 (0.8%) | |
| After initial hospital discharge, but before 6 weeks’ follow-up | 4/620 (0.6%) | |
| After 6 weeks’ follow-up, but before 6 months’ follow-up | 6/620 (1.0%) | |
| Loss to follow-up | At 6 weeks’ follow-up | 12/620 (1.9%) |
| At 6 months’ follow-up | 27/620 (4.4%) | |
Screening for eligibility
The trial consisted of 24 hospitals (22 NHS trusts) that recruited patients over the course of the trial. The recruitment of patients took place over two time periods, the pilot study and the main study. The pilot study recruited patients from 1 May 2004 to 4 June 2010, whereas the main study recruited patients from 1 July 2010 to 1 November 2013. A total of 2015 patients were assessed for eligibility on admission to hospital. Of these patients, 1344 were excluded because they either did not meet the inclusion criteria (n = 127) or they meet one or more of the exclusion criteria (n = 1217). The remaining 671 eligible patients were asked to participate in the trial, of which 51 patients did not give consent. Thus, in total, 620 patients were randomised into the trial. A summary of key trial management processes is detailed in Appendix 1.
Randomisation
From the screening process, 671 eligible patients were approached to participate in the trial of whom 620 gave their consent and were subsequently randomised to receive ORIF (n = 309) or CCC (n = 311). This equates to an overall eligibility rate of 33.3% (671 out of 2015) and a consent rate of 92.4% (620 out of 671). Of the 620 randomised participants, 95 were from the pilot study. A summary of the recruitment across the 24 sites has been detailed in Table 5. Randomisation was balanced across the treatment groups and hospital sites, and within strata (Table 6).
| Hospital | Treatment group | Total (N = 620), n (%) | |
|---|---|---|---|
| ORIF (N = 309), n (%) | CCC (N = 311), n (%) | ||
| Addenbrooke’s Hospital | 6 (1.9) | 8 (2.6) | 14 (2.3) |
| Darlington Memorial Hospital | 2 (0.7) | 2 (0.6) | 4 (0.6) |
| Derriford Hospital | 8 (2.6) | 9 (2.9) | 17 (2.7) |
| Frenchay Hospital | 21 (6.8) | 20 (6.4) | 41 (6.6) |
| Great Western Hospital | 12 (3.9) | 10 (3.2) | 22 (3.6) |
| Ipswich Hospital | 12 (3.9) | 11 (3.5) | 23 (3.7) |
| John Radcliffe Hospital | 69 (22.3) | 68 (22.0) | 137 (22.1) |
| Leeds General Infirmary | 7 (2.3) | 9 (2.9) | 16 (2.6) |
| Leicester Royal Infirmary | 22 (7.1) | 24 (7.7) | 46 (7.4) |
| Morriston Hospital | 11 (3.6) | 11 (3.5) | 22 (3.6) |
| Musgrove Park Hospital | 15 (4.8) | 14 (4.5) | 29 (4.7) |
| Newham University Hospital | 0 | 1 (0.3) | 1 (0.2) |
| Norfolk and Norwich University Hospital | 21 (6.8) | 20 (6.4) | 41 (6.6) |
| North Tyneside General Hospital | 7 (2.3) | 7 (2.3) | 14 (2.3) |
| Poole Hospital | 8 (2.6) | 9 (2.9) | 17 (2.7) |
| Royal Berkshire Hospital | 16 (5.2) | 16 (5.1) | 32 (5.2) |
| Royal Victoria Infirmary | 15 (4.8) | 15 (4.8) | 30 (4.8) |
| Southport and Ormskirk Hospitals | 4 (1.3) | 3 (1.0) | 7 (1.1) |
| Torbay Hospital | 14 (4.5) | 13 (4.2) | 27 (4.4) |
| University Hospital, Coventry | 15 (4.8) | 15 (4.8) | 30 (4.8) |
| University Hospital of North Durham | 3 (1.0) | 4 (1.3) | 7 (1.1) |
| North Staffordshire Royal Infirmary | 2 (0.7) | 1 (0.3) | 3 (0.5) |
| Wansbeck General Hospital | 6 (1.9) | 9 (2.9) | 15 (2.4) |
| Yeovil District Hospital | 13 (4.2) | 12 (3.9) | 25 (4.0) |
| Hospital | Fracture pattern A + B | Fracture pattern C | ||
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ORIF, n (%) | CCC, n (%) | |
| Addenbrooke’s Hospital | 6 (2.2) | 7 (2.6) | 0 | 1 (2.4) |
| Darlington Memorial Hospital | 2 (0.7) | 2 (0.7) | 0 | 0 |
| Derriford Hospital | 8 (2.9) | 8 (3.0) | 0 | 1 (2.4) |
| Frenchay Hospital | 20 (7.4) | 19 (7.0) | 1 (2.7) | 1 (2.4) |
| Great Western Hospital | 10 (3.7) | 9 (3.3) | 2 (5.4) | 1 (2.4) |
| Ipswich Hospital | 11 (4.1) | 10 (3.7) | 1 (2.7) | 1 (2.4) |
| John Radcliffe Hospital | 64 (23.5) | 60 (22.3) | 5 (13.6) | 8 (19.5) |
| Leeds General Infirmary | 6 (2.2) | 7 (2.6) | 1 (2.7) | 2 (5.0) |
| Leicester Royal Infirmary | 19 (7.0) | 20 (7.4) | 3 (8.1) | 4 (9.8) |
| Morriston Hospital | 9 (3.3) | 10 (3.7) | 2 (5.4) | 1 (2.4) |
| Musgrove Park Hospital | 12 (4.4) | 12 (4.4) | 3 (8.1) | 2 (5.0) |
| Newham University Hospital | 0 | 1 (0.4) | 0 | 0 |
| Norfolk and Norwich University Hospital | 18 (6.6) | 18 (6.7) | 3 (8.1) | 2 (5.0) |
| North Tyneside General Hospital | 6 (2.2) | 6 (2.2) | 1 (2.7) | 1 (2.4) |
| Poole Hospital | 6 (2.2) | 7 (2.6) | 2 (5.4) | 2 (5.0) |
| Royal Berkshire Hospital | 15 (5.5) | 15 (5.6) | 1 (2.7) | 1 (2.4) |
| Royal Victoria Infirmary | 13 (4.8) | 12 (4.4) | 2 (5.4) | 3 (7.3) |
| Southport and Ormskirk Hospitals | 2 (0.7) | 2 (0.7) | 2 (5.4) | 1 (2.4) |
| Torbay Hospital | 11 (4.1) | 10 (3.7) | 3 (8.1) | 3 (7.3) |
| University Hospital, Coventry | 12 (4.4) | 12 (4.4) | 3 (8.1) | 3 (7.3) |
| University Hospital of North Durham | 3 (1.1) | 3 (1.1) | 0 | 1 (2.4) |
| North Staffordshire Royal Infirmary | 2 (0.7) | 1 (0.4) | 0 | 0 |
| Wansbeck General Hospital | 6 (2.2) | 8 (3.0) | 0 | 1 (2.4) |
| Yeovil District Hospital | 11 (4.1) | 11 (4.1) | 2 (5.4) | 1 (2.4) |
Treatments received
Of the 620 randomised participants, 41 (6.6%) participants did not receive their allocated treatment (data shown in Table 7). Failure to receive allocated treatment was significantly greater (p < 0.001) in the CCC arm (34/620, 5.5%), than in the ORIF arm (7/620, 1.1%). Of the 34 participants in the CCC group who did not receive their allocated treatment at their primary theatre procedure, five received a traditional plaster cast, one participant received external fixation, 17 went on to have ORIF, five did not receive treatment at all because they withdrew prior to receiving treatment and the remaining six participants had ‘other’ treatment. Of the seven participants in the ORIF group who did not received their allocated treatment at their primary theatre procedure, four participants received CCC and the remaining three received ‘other’. The reasons why participants did not receive allocated treatment are shown in Table 8.
| Treatment received if allocated treatment not received | Treatment group | Total (N = 41), n (%) | |
|---|---|---|---|
| ORIF (N = 7), n (%) | CCC (N = 34), n (%) | ||
| Traditional plaster cast | 0 | 5/34 (14.7) | 5/41 (12.2) |
| External fixation | 0 | 1/34 (2.9) | 1/41 (2.4) |
| Retrograde nail | 0 | 0 | 0 |
| ORIF | 0 | 17/34 (50.0) | 17/41 (41.5) |
| CCC | 4/7 (57.1) | 0 | 4/41 (9.8) |
| Other | 3/7 (42.9) | 6/34 (17.7) | 9/41 (21.9) |
|
1 | 0 | 1 |
|
1 | 4 | 4 |
|
– | 1 | 1 |
|
0 | 1 | 1 |
|
1 | 0 | 1 |
| Missingb | 0 | 5/34 (14.7) | 5/41 (12.2) |
| Reason did not receive allocated treatment | ORIF, number of reasons (%) | CCC, number of reasons (%) | Total, number of reasons (%) |
|---|---|---|---|
| Reason why ORIF not received if patient allocated to ORIF | |||
| Ankle too swollen for surgery | 0 | ||
| Poor skin condition for surgery | 3/9 (33.3) | ||
| Fracture blisters | 2/9 (22.2) | ||
| Poor quality or fragmented bone | 0 | ||
| Other | 4/9 (44.5) | ||
| After allocation surgeon felt suitable for conservative management | 1 | ||
| MRSA positive on routine screening | 2 | ||
| Patient refused to go to theatre | 1 | ||
| Missing | 0 | ||
| Reason why CCC not received if patient allocated to CCC | |||
| Ankle too swollen for surgery | 5/38 (13.2) | ||
| Fracture proved irreducible by closed manipulation | 10/38 (26.3) | ||
| Unable to maintain/retain reduction | 4/38 (10.5) | ||
| Other | 14/38 (36.8) | ||
| Blisters | 4 | ||
| Concern regarding adherence to non-weight-bearing | 1 | ||
| Consultant trained in CCC not available | 1 | ||
| Deemed not clinically necessary to subject patient to anaesthesia | 1 | ||
| Fracture did not need to be reduced | 1 | ||
| Fracture deemed stable | 1 | ||
| Not fit for anaesthetic | 1 | ||
| Soft-tissue damage | 1 | ||
| Did not follow CCC protocol | 3 | ||
| Missinga | 5/38 (13.2) | ||
The remainder of participants received their treatment per protocol, and this included 13 out of 298 (4.4%) participants who received a temporary treatment prior to ORIF, and 2 out of 275 (0.7%) prior to CCC. After starting treatment with the allocated CCC intervention, later loss of fracture reduction resulted in conversion to internal fixation in 52 out of 275 (18.9%) and remanipulation and CCC applied in theatre 10 out of 275 (3.6%), which were allowable events in the study protocol.
Withdrawals
Tables 9 and 10 provide summaries of those participants that withdrew or were withdrawn from the trial by the surgeon caring for them. In total, 15 out of 620 (2.4%) participants withdrew from the trial: seven from the ORIF group and eight from the CCC group. Five of the participants withdrew after randomisation but before initial hospital discharge, four withdrew after initial discharge but before the 6-week follow-up and six withdrew after the 6-week follow-up but before the 6-month follow-up. The majority of withdrawals were requested by the participant themselves. There was no evidence of an association between the withdrawal request and treatment group (p > 0.05).
| Withdrawals | Treatment group | Total, n | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| After randomisation, but before initial hospital discharge | 0 | 5 (62.5) | 5 |
| After initial hospital discharge, but before 6 weeks’ follow-up | 3 (42.9) | 1 (12.5) | 4 |
| After 6 weeks’ follow-up, but before 6 months’ follow-up | 4 (57.1) | 2 (25.0) | 6 |
| Missing | 0 | 0 | 0 |
| Withdrawal details | Treatment group | Total, n (%) | p-value | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| Patient asked to withdraw from trial | ||||
| Yes | 6 (85.7) | 5 (62.5) | 11 (73.3) | 0.569 |
| No | 1 (14.3) | 3 (37.5) | 4 (26.7) | |
| Missing | 0 | 0 | 0 | |
| Surgeon caring for patient requested for patient to be withdrawn | ||||
| Yes | 1 (14.3) | 3 (37.5) | 4 (26.7) | 0.569 |
| No | 6 (85.7) | 5 (62.5) | 11 (73.3) | |
| Missing | 0 | 0 | 0 | |
| Patient level of withdrawal | ||||
| Not stated | 5 (83.3) | 4 (80.0) | 9 (81.8) | 0.569 |
| No objection to study using data collected up to withdrawal | 1 (16.7) | 1 (20.0) | 2 (18.2) | |
| No objection to tracking overall health status via official databases (no patient contact) | 0 | 0 | 0 | |
| Objects to study using data collected up to withdrawal | 0 | 0 | 0 | |
| Objects to study tracking health status via official databases (no patient contact) | 0 | 0 | 0 | |
| Missing | 0 | 0 | 0 | |
Follow-up
A summary of the follow-up rates is presented in Tables 11 and 12. At 6-week follow-up, 608 out of 620 (98.1%) participants completed their assessment, and at 6-month follow-up 593 out of 620 (95.6%) participants completed their assessment (as shown in Table 11). The majority of the assessments were completed in hospital, with a small proportion of participants completing their assessment at home or over the telephone (see Table 12). Data collected at each of the follow-up time points were well completed. For participants who provided follow-up data, < 1% of the primary outcome data and secondary outcome data were missing at the item level.
| Follow-up time point | Treatment group | Total (N = 620), n (%) | |
|---|---|---|---|
| ORIF (N = 309), n (%) | CCC (N = 311), n (%) | ||
| Completed the 6-week follow-up assessment | 305 (98.7) | 303 (97.4) | 608 (98.1) |
| Completed the 6-month follow-up assessment | 298 (96.4) | 295 (94.9) | 593 (95.6) |
| Location follow-up completed | 6 weeks (N = 608), n (%) | 6 months (N = 593), n (%) |
|---|---|---|
| Completed assessment (in hospital) | 588 (96.7) | 572 (96.5) |
| Completed assessment (at home) | 2 (0.3) | 6 (1.0) |
| Completed assessment (over telephone) | 18 (3.0) | 15 (2.5) |
Baseline data
The baseline demographic and clinical characteristics of all randomised participants have been summarised in Tables 13 and 14. The randomised participants were, on average, around 70 years old and nearly 74% of them were female. Both groups reported similar OMASs (before injury), SF-12 mental component summary scores, SF-12 physical component summary scores and EQ-5D scores at baseline. The baseline EQ-5D score and EQ-5D visual analogue scale (VAS) score for the ‘day before injury’ and on the ‘day of assessment’ clearly suggests that the participants’ quality of life deteriorated as a result of their ankle fracture. Around 30% of all participants suffered from allergies. Most participants had either never smoked (50.7%) or were ex-smokers (40.1%). The comorbidities reported by the recruited sample are presented in Table 15. The social circumstances of all randomised participants have been summarised in Table 16. The vast majority of patients (96.9%) were admitted from their own home, most of whom were living either alone or with someone and did not use walking aids.
| Characteristic | Treatment group | Total (N = 620) | |
|---|---|---|---|
| ORIF (N = 309) | CCC (N = 311) | ||
| Age (years) | |||
| Mean | 69.8 | 71.4 | 70.6 |
| n | 309 | 311 | 620 |
| SD | 6.9 | 7.6 | 7.3 |
| Median | 69.0 | 70 | 70 |
| Minimum | 60 | 60 | 60 |
| Maximum | 89 | 96 | 96 |
| Missing | 0 | 0 | 0 |
| Sex | |||
| Male, n (%) | 82 (26.5) | 78 (25.1) | 160 (25.8) |
| Female, n (%) | 227 (73.5) | 233 (74.9) | 460 (74.2) |
| Missing, n (%) | 0 | 0 | 0 |
| Ankle fracture classification | |||
| Trans-/infrasyndesmotic, n (%) | 272 (88.0) | 270 (86.8) | 542 (87.4) |
| Infrasyndesmotic, n (%) | 37 (12.0) | 41 (13.2) | 78 (12.6) |
| Missing | 0 | 0 | 0 |
| OMAS (pre injury) | |||
| Mean | 89.8 | 87.7 | 88.7 |
| n | 309 | 309 | 618 |
| SD | 17.0 | 17.7 | 17.4 |
| Median | 100 | 95 | 100 |
| Minimum | 10 | 20 | 10 |
| Maximum | 100 | 100 | 100 |
| Missing | 0 | 0 | 0 |
| SF-12 mental component summary score (pre injury) | |||
| Mean | 53.7 | 54.5 | 54.1 |
| n | 307 | 309 | 616 |
| SD | 8.1 | 7.5 | 7.8 |
| Median | 56.5 | 56.7 | 56.6 |
| Minimum | 14.0 | 19.2 | 14.0 |
| Maximum | 66.3 | 67.7 | 67.7 |
| Missing | 2 | 0 | 2 |
| SF-12 physical component summary score (pre injury) | |||
| Mean | 51.2 | 49.6 | 50.4 |
| n | 307 | 309 | 616 |
| SD | 8.8 | 10.3 | 9.6 |
| Median | 54.2 | 53.8 | 54.1 |
| Minimum | 19.3 | 16.8 | 16.8 |
| Maximum | 67.6 | 64.7 | 67.6 |
| Missing | 2 | 0 | 2 |
| OMAS pain (pre injury) | |||
| None, n (%) | 258 (87.8) | 234 (85.7) | 492 (86.8) |
| While walking on uneven surface, n (%) | 20 (6.8) | 25 (9.1) | 45 (7.9) |
| While walking on even surface outdoors, n (%) | 9 (3.1) | 4 (1.5) | 13 (2.3) |
| While walking indoors, n (%) | 3 (1.0) | 4 (1.5) | 7 (1.2) |
| Constant and severe, n (%) | 4 (1.3) | 6 (2.2) | 10 (1.8) |
| Missing, n (%) | 0 | 0 | 0 |
| EQ-5D mobility score (day before injury) | |||
| Level 1, n (%) | 237 (76.7) | 205 (66.3) | 442 (71.5) |
| Level 2, n (%) | 41 (13.3) | 74 (24.0) | 115 (18.6) |
| Level 3, n (%) | 0 | 0 | 0 |
| Missing, n (%) | 31 (10.0) | 30 (9.7) | 61 (9.9) |
| EQ-5D self-care score (day before injury) | |||
| Level 1, n (%) | 264 (85.4) | 260 (84.1) | 524 (84.8) |
| Level 2, n (%) | 14 (4.5) | 18 (5.8) | 32 (5.2) |
| Level 3, n (%) | 0 | 1 (0.3) | 1 (0.1) |
| Missing, n (%) | 31 (10.1) | 30 (9.7) | 61 (9.9) |
| EQ-5D usual activities score (day before injury) | |||
| Level 1, n (%) | 237 (76.7) | 236 (76.4) | 473 (76.5) |
| Level 2, n (%) | 40 (12.9) | 39 (12.6) | 79 (12.8) |
| Level 3, n (%) | 1 (0.3) | 4 (1.3) | 5 (0.8) |
| Missing, n (%) | 31 (10.1) | 30 (9.7) | 61 (9.9) |
| EQ-5D pain/discomfort score (day before injury) | |||
| Level 1, n (%) | 219 (70.9) | 196 (63.4) | 415 (67.1) |
| Level 2, n (%) | 56 (18.1) | 78 (25.3) | 134 (21.7) |
| Level 3, n (%) | 3 (1.0) | 5 (1.6) | 8 (1.3) |
| Missing, n (%) | 31 (10.0) | 30 (9.7) | 61 (9.9) |
| EQ-5D anxiety/depression score (day before injury) | |||
| Level 1, n (%) | 239 (77.4) | 247 (79.9) | 486 (78.6) |
| Level 2, n (%) | 38 (12.3) | 30 (9.7) | 68 (11.0) |
| Level 3, n (%) | 1 (0.3) | 2 (0.7) | 3 (0.5) |
| Missing, n (%) | 31 (10.0) | 30 (9.7) | 61 (9.9) |
| EQ-5D score (day before injury) | |||
| Mean | 0.91 | 0.87 | 0.89 |
| n | 278 | 279 | 557 |
| SD | 0.2 | 0.2 | 0.2 |
| Median | 1 | 1 | 1 |
| Minimum | –0.02 | –0.03 | –0.03 |
| Maximum | 1 | 1 | 1 |
| Missing | 31 | 30 | 61 |
| EQ-5D VAS score (day before injury) | |||
| Mean | 83.1 | 81.4 | 82.3 |
| n | 278 | 279 | 557 |
| SD | 15.1 | 16.3 | 15.7 |
| Median | 89.5 | 85.0 | 85.0 |
| Minimum | 20 | 20 | 20 |
| Maximum | 100 | 100 | 100 |
| Missing | 31 | 30 | 61 |
| EQ-5D mobility score (on day of assessment) | |||
| Level 1, n (%) | 4 (1.3) | 3 (1.0) | 7 (1.1) |
| Level 2, n (%) | 47 (15.2) | 60 (19.4) | 107 (17.3) |
| Level 3, n (%) | 209 (67.6) | 199 (64.4) | 408 (66.0) |
| Missing, n (%) | 49 (15.9) | 47 (15.2) | 96 (15.6) |
| EQ-5D self-care score (on day of assessment) | |||
| Level 1, n (%) | 56 (18.1) | 53 (17.2) | 109 (17.6) |
| Level 2, n (%) | 162 (52.4) | 173 (56.0) | 335 (54.2) |
| Level 3, n (%) | 42 (13.6) | 36 (11.6) | 78 (12.6) |
| Missing, n (%) | 49 (15.9) | 47 (15.2) | 96 (15.5) |
| EQ-5D usual activities score (on day of assessment) | |||
| Level 1, n (%) | 9 (2.9) | 9 (2.9) | 18 (2.9) |
| Level 2, n (%) | 27 (8.7) | 32 (10.4) | 59 (9.6) |
| Level 3, n (%) | 224 (72.5) | 221 (71.5) | 445 (72.0) |
| Missing, n (%) | 49 (15.9) | 47 (15.2) | 96 (15.5) |
| EQ-5D pain/discomfort score (on day of assessment) | |||
| Level 1, n (%) | 28 (9.0) | 33 (10.7) | 61 (9.9) |
| Level 2, n (%) | 202 (65.4) | 202 (65.4) | 404 (65.4) |
| Level 3, n (%) | 30 (9.7) | 27 (8.7) | 57 (9.2) |
| Missing, n (%) | 49 (15.9) | 47 (15.2) | 96 (15.5) |
| EQ-5D anxiety/depression score (on day of assessment) | |||
| Level 1, n (%) | 133 (43.0) | 152 (49.2) | 285 (46.1) |
| Level 2, n (%) | 119 (38.5) | 100 (32.4) | 219 (35.5) |
| Level 3, n (%) | 8 (2.6) | 10 (3.2) | 18 (2.9) |
| Missing, n (%) | 49 (15.9) | 47 (15.2) | 96 (15.5) |
| EQ-5D score (on day of assessment) | |||
| Mean | 0.04 | 0.07 | 0.05 |
| n | 260 | 262 | 522 |
| SD | 0.3 | 0.3 | 0.3 |
| Median | 0.02 | 0.02 | 0.02 |
| Minimum | –0.59 | –0.59 | –0.59 |
| Maximum | 1 | 1 | 1 |
| Missing | 49 | 47 | 96 |
| EQ-5D VAS score (on day of assessment) | |||
| Mean | 57.2 | 58.6 | 57.9 |
| n | 259 | 262 | 521 |
| SD | 23.1 | 22.2 | 22.6 |
| Median | 52.0 | 60.0 | 58.0 |
| Minimum | 0 | 2 | 0 |
| Maximum | 100 | 100 | 100 |
| Missing | 50 | 47 | 97 |
| Mini-Mental State Examination score (on day of assessment) | |||
| Mean | 28.2 | 27.9 | 28.0 |
| n | 277 | 280 | 557 |
| SD | 2.1 | 2.3 | 2.2 |
| Median | 29 | 29 | 29 |
| Minimum | 17 | 17 | 17 |
| Maximum | 30 | 30 | 30 |
| Missing | 32 | 31 | 63 |
| Health status | Treatment group | Total | |
|---|---|---|---|
| ORIF | CCC | ||
| Allergies | |||
| Yes, n (%) | 88 (28.5) | 90 (29.1) | 178 (28.8) |
| No, n (%) | 221 (71.5) | 219 (70.9) | 440 (71.2) |
| Missing, n (%) | 0 | 0 | 0 |
| Smoking | |||
| Yes, n (%) | 25 (8.1) | 32 (10.4) | 57 (9.2) |
| Never, n (%) | 151 (48.9) | 162 (52.4) | 313 (50.7) |
| Ex-smoker, n (%) | 133 (43.0) | 115 (37.2) | 248 (40.1) |
| Missing, n (%) | 0 | 0 | 0 |
| If ex-smoker, how many years since stopping? | |||
| Mean | 24.2 | 26.4 | 25.2 |
| n | 133 | 115 | 248 |
| SD | 14.8 | 15.9 | 15.3 |
| Median | 25.0 | 28.0 | 25.0 |
| Minimum | 0 | 0 | 0 |
| Maximum | 60 | 70 | 70 |
| Missing | 0 | 0 | 0 |
| If yes or ex-smoker, how many smoked on average per day? | |||
| Mean | 16.0 | 14.7 | 15.4 |
| n | 155 | 146 | 301 |
| SD | 12.0 | 10.2 | 11.1 |
| Median | 15.0 | 15.0 | 15.0 |
| Minimum | 1 | 1 | 1 |
| Maximum | 80 | 60 | 80 |
| Missing | 3 | 1 | 4 |
| If yes or ex-smoker, total number of years as a smoker? | |||
| Mean | 25.1 | 25.9 | 25.5 |
| n | 133 | 129 | 262 |
| SD | 14.9 | 16.9 | 15.9 |
| Median | 25.0 | 20.0 | 24.0 |
| Minimum | 1 | 0 | 0 |
| Maximum | 60 | 65 | 65 |
| Missing | 25 | 18 | 43 |
| Number of units of alcohol in an average week | |||
| Mean | 7.4 | 6.3 | 6.8 |
| n | 308 | 306 | 614 |
| SD | 10.2 | 9.0 | 9.6 |
| Median | 4.0 | 2.0 | 2.0 |
| Minimum | 0 | 0 | 0 |
| Maximum | 60 | 50 | 60 |
| Missing | 1 | 3 | 4 |
| Comorbidity | Treatment group | Total, n (%) | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Heart disease | 38 (12.3) | 44 (14.2) | 82 (13.3) |
| Hypertension | 126 (40.8) | 140 (45.3) | 266 (43.0) |
| Asthma/COPD | 46 (14.9) | 39 (12.6) | 85 (13.8) |
| Diabetes mellitus | 31 (10.0) | 26 (8.4) | 57 (9.2) |
| Parkinson’s disease | 0 | 0 | 0 |
| Epilepsy | 4 (1.3) | 5 (1.6) | 9 (1.5) |
| Renal disease | 5 (1.6) | 7 (2.3) | 12 (1.9) |
| Liver disease | 2 (0.7) | 4 (1.3) | 6 (1.0) |
| CVA/TIA | 14 (4.5) | 21 (6.8) | 35 (5.7) |
| Peptic ulcer | 5 (1.6) | 13 (4.2) | 18 (2.9) |
| Malignancy | 37 (12.0) | 36 (11.7) | 73 (11.8) |
| DVT/PE | 10 (3.2) | 19 (6.2) | 29 (4.7) |
| Osteoarthritis | 84 (27.2) | 100 (32.4) | 184 (29.8) |
| Rheumatoid arthritis | 12 (3.9) | 14 (4.5) | 26 (4.2) |
| Depression | 35 (11.3) | 38 (12.3) | 73 (11.8) |
| Dementia | 1 (0.3) | 0 | 1 (0.2) |
| Social circumstance | Treatment group | Total, n (%) | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Admitted from | |||
| Own home | 302 (97.7) | 297 (96.0) | 599 (96.9) |
| Warden accommodation | 4 (1.3) | 4 (1.3) | 8 (1.3) |
| Residential home | 0 | 0 | 0 |
| Nursing home | 0 | 0 | 0 |
| Rehabilitation | 0 | 0 | 0 |
| Acute hospital | 0 | 2 (0.7) | 2 (0.3) |
| Community hospital | 0 | 2 (0.7) | 2 (0.3) |
| Temporary residence | 3 (1.0) | 4 (1.3) | 7 (1.1) |
| Missing | 0 | 0 | 0 |
| Home support | |||
| Lives alone | 98 (31.7) | 99 (32.0) | 197 (31.9) |
| Lives with someone | 206 (66.7) | 209 (67.6) | 415 (67.2) |
| Lives with carers | 1 (0.3) | 0 | 1 (0.2) |
| Home care package | 4 (1.3) | 1 (0.3) | 5 (0.8) |
| Institution care | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Usual walking aids | |||
| None | 271 (87.7) | 258 (83.5) | 529 (85.6) |
| One stick | 28 (9.1) | 36 (11.7) | 64 (10.4) |
| Two sticks | 4 (1.3) | 3 (1.0) | 7 (1.1) |
| Frame/rollator | 6 (1.9) | 9 (2.9) | 15 (2.4) |
| Wheelchair | 0 | 3 (1.0) | 3 (0.5) |
| Missing | 0 | 0 | 0 |
| Using appropriate aid, how far can you walk? | |||
| About house | 6 (1.9) | 14 (4.5) | 20 (3.2) |
| < 100 m | 14 (4.5) | 24 (7.8) | 38 (6.2) |
| < 0.5 mile | 28 (9.1) | 34 (11.0) | 62 (10.0) |
| > 0.5 mile | 261 (84.5) | 237 (76.7) | 498 (80.6) |
| Missing | 0 | 0 | 0 |
| What limits your mobility? | |||
| Nothing | 200 (64.7) | 175 (56.6) | 375 (60.7) |
| Pain | 28 (9.1) | 40 (12.9) | 68 (11.0) |
| Breathlessness | 28 (9.1) | 32 (10.4) | 60 (9.7) |
| Other | 53 (17.1) | 62 (20.1) | 115 (18.6) |
| Missing | 0 | 0 | 0 |
Numbers analysed
All of the results from the primary per-protocol analyses are presented from here onwards using the per-protocol sample. The ITT analyses displayed similar results and thus are not presented in this chapter (apart from the analysis of the primary outcome). The results of the ITT analyses can be found elsewhere (see Appendix 2) where the tables have been assigned the same numbering as those presented in this chapter for ease of reference. The per-protocol analyses of the 6-week data comprised data from 573 participants (n = 298 for ORIF and n = 275 for CCC) and the 6-month analyses comprised data from 558 participants (n = 291 for ORIF and n = 267 for CCC). The ITT analyses included all participants that provided follow-up assessment data. In total, 608 participants were included in the ITT analyses at the 6-week time point (n = 305 for ORIF and n = 303 for CCC) and 593 were included in the analyses at the 6-month time point (n = 298 for ORIF and n = 295 for CCC). In both the per-protocol analyses and the ITT analyses, it was assumed that the participants received the treatment to which they were initially allocated. The quality of the primary and secondary outcomes data for both the per-protocol analyses and ITT analyses have been presented in Tables 17 and 18, respectively.
| Outcome | 6 weeks (N = 573), n (%) | 6 months (N = 558), n (%) |
|---|---|---|
| Primary outcome | ||
| OMAS | 570 (99.5) | 558 (100.0) |
| Secondary outcomes | ||
| SF-12 mental component summary score | 571 (99.7) | 558 (100.0) |
| SF-12 physical component summary score | 571 (99.7) | 558 (100.0) |
| EQ-5D score | 520 (90.7) | 505 (90.5) |
| EQ-5D VAS score | 519 (90.6) | 506 (90.7) |
| Timed up and go test (6 months) | 518 (92.8) | |
| Angle of injured ankle dorsiflexion (°) | 545 (95.1) | 538 (96.4) |
| Angle of injured ankle plantar flexion (°) | 545 (95.1) | 538 (96.4) |
| Eversion of injured ankle | 529 (92.3) | 533 (95.5) |
| Inversion of injured ankle | 539 (94.1) | 538 (96.4) |
| OMAS pain score | 571 (99.7) | 558 (100.0) |
| EQ-5D pain score | 520 (90.8) | 506 (90.7) |
| Patient satisfaction | 487 (85.0) | 472 (84.6) |
| Outcome | 6 weeks (N = 608), n (%) | 6 months (N = 593), n (%) |
|---|---|---|
| Primary outcome | ||
| OMAS | 605 (99.5) | 592 (99.8) |
| Secondary outcomes | ||
| SF-12 mental component summary score | 606 (99.7) | 592 (99.8) |
| SF-12 physical component summary score | 606 (99.7) | 592 (99.8) |
| EQ-5D score | 550 (90.5) | 535 (90.2) |
| EQ-5D VAS score | 550 (90.5) | 536 (90.4) |
| Timed up and go test (6 months) | 550 (92.7) | |
| Angle of injured ankle dorsiflexion (°) | 578 (95.1) | 571 (96.3) |
| Angle of injured ankle plantar flexion (°) | 578 (95.1) | 571 (96.3) |
| Eversion of injured ankle | 562 (92.4) | 566 (95.4) |
| Inversion of injured ankle | 572 (94.1) | 571 (96.3) |
| OMAS pain score | 606 (99.7) | 592 (99.8) |
| EQ-5D pain score | 550 (90.5) | 536 (90.4) |
| Patient satisfaction | 518 (85.2) | 502 (84.7) |
Per-protocol analyses results
Primary outcome measure (per protocol)
Table 19 presents the unadjusted and adjusted per-protocol analyses for the OMASs at 6 weeks and 6 months. The 95% CIs for the difference between the two treatment groups at both 6 weeks and 6 months lie totally within the equivalence margin of ± 6 points. Therefore, there is evidence to suggest that the two treatments are equivalent. In particular, at 6 months (the primary end point), the mean treatment effect from the adjusted analyses was estimated to be –0.65 (95% CI –3.98 to 2.68) which corresponds to a standardised effect size of –0.04 (95% CI –0.23 to 0.15). Figure 8 clearly illustrates this result in which the unadjusted and adjusted 95% CIs at 6 months can be seen to lie totally within the equivalence margin. The results of the ITT analyses of the primary outcome also provided evidence that the treatments are equivalent, as shown in Table 20 and Figure 9. A plot of the OMASs over time (from pre-injury to 6-month follow-up) has been presented in Figure 10. From this plot it is evident that the ankle functionality of participants in both groups follows a similar pattern over time. The same pattern of recovery was also evident in the ITT sample.
| OMAS | Treatment group | Total | Unadjusted statistics (95% CI) | Adjusted statistics (95% CI)a | |
|---|---|---|---|---|---|
| ORIF | CCC | ||||
| 6 weeks | |||||
| Mean | 37.9 | 38.1 | 38.0 | 0.20 (–2.31 to 2.71) | 0.26 (–2.20 to 2.72) |
| n | 297 | 273 | 570 | ||
| SD | 14.7 | 15.8 | 15.2 | ||
| Median | 35 | 40 | 35 | ||
| Minimum | 0 | 0 | 0 | ||
| Maximum | 95 | 90 | 95 | ||
| Missing | 1 | 2 | 3 | ||
| 6 months | |||||
| Mean | 66.0 | 64.5 | 65.3 | –1.57 (–5.19 to 2.05) | –0.65 (–3.98 to 2.68) |
| n | 291 | 267 | 558 | ||
| SD | 21.1 | 22.4 | 21.8 | ||
| Median | 70 | 70 | 70 | ||
| Minimum | 0 | 5 | 0 | ||
| Maximum | 100 | 100 | 100 | ||
| Missing | 0 | 0 | 0 | ||
FIGURE 8.
Observed treatment difference plot at 6 months with 95% CIs (per protocol).

| OMAS | Treatment group | Total | Unadjusted statistics (95% CI) | Adjusted statistics (95% CI)a | |
|---|---|---|---|---|---|
| ORIF | CCC | ||||
| 6 weeks | |||||
| Mean | 38.0 | 38.0 | 38.0 | 0.03 (–2.41 to 2.47) | 0.12 (–2.56 to 2.79) |
| n | 304 | 301 | 605 | ||
| SD | 14.6 | 16.0 | 15.29 | ||
| Median | 35.0 | 40.0 | 35.0 | ||
| Minimum | 0 | 0 | 0 | ||
| Maximum | 95 | 90 | 95 | ||
| Missing | 1 | 2 | 3 | ||
| 6 months | |||||
| Mean | 66.0 | 64.7 | 65.3 | –1.33 (–4.85 to 2.19) | –0.17 (–3.36 to 3.02) |
| n | 298 | 294 | 592 | ||
| SD | 21.3 | 22.4 | 21.81 | ||
| Median | 70.0 | 70.0 | 70.0 | ||
| Minimum | 0 | 5 | 0 | ||
| Maximum | 100 | 100 | 100 | ||
| Missing | 0 | 1 | 1 | ||
FIGURE 9.
Observed treatment difference plot at 6 months with 95% CIs (ITT).

FIGURE 10.
Plot of mean scores and 95% CIs for the OMASs over time (per protocol).

Secondary outcome measures (per protocol)
Short Form questionnaire-12 items mental component summary subscale (per protocol)
The analyses of the SF-12 mental component summary subscale scores have been presented in Table 21. There is no evidence of a statistically significant difference between the two treatment groups at either 6 weeks or 6 months. Overall, the improvement from 6 weeks to 6 months is small. The difference between ORIF and CCC, on average, was estimated to be 0.06 (95% CI –1.64 to 1.75) at 6 weeks and –0.21 (95% CI –1.71 to 1.28) at 6 months. Participants in both groups followed a similar pattern of recovery over time, with their mental function at 6 months being similar to what it was pre-injury (Figure 11).
| Outcome | Treatment group | Unadjusted statistics (95% CI); p-value | Adjusted statistics (95% CI); p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| SF-12 mental component summary score | ||||
| 6 weeks | ||||
| Mean | 48.3 | 48.6 | 0.35 (–1.45 to 2.15); 0.700 | 0.06 (–1.64 to 1.75); 0.946 |
| n | 297 | 274 | ||
| SD | 11.3 | 10.6 | ||
| Median | 48.9 | 49.5 | ||
| Minimum | 16.5 | 19.2 | ||
| Maximum | 70.2 | 68.3 | ||
| Missing | 1 | 1 | ||
| 6 months | ||||
| Mean | 52.1 | 52.2 | 0.10 (–1.57 to 1.78); 0.902 | –0.21 (–1.71 to 1.28); 0.781 |
| n | 291 | 267 | ||
| SD | 10.3 | 9.7 | ||
| Median | 55.4 | 55.7 | ||
| Minimum | 18.9 | 19.9 | ||
| Maximum | 66.9 | 68.4 | ||
| Missing | 0 | 0 | ||
| SF-12 physical component summary score | ||||
| 6 weeks | ||||
| Mean | 33.3 | 33.4 | 0.07 (–1.02 to 1.17); 0.895 | 0.24 (–0.82 to 1.31); 0.654 |
| n | 297 | 274 | ||
| SD | 6.9 | 6.5 | ||
| Median | 32.4 | 32.7 | ||
| Minimum | 18.4 | 18.3 | ||
| Maximum | 57.1 | 54.9 | ||
| Missing | 1 | 1 | ||
| 6 months | ||||
| Mean | 45.6 | 44.0 | –1.56 (–3.28 to 0.16); 0.076 | –0.83 (–2.39 to 0.74); 0.299 |
| n | 291 | 267 | ||
| SD | 10.0 | 10.7 | ||
| Median | 47.0 | 44.5 | ||
| Minimum | 22.1 | 15.6 | ||
| Maximum | 66.2 | 64.3 | ||
| Missing | 0 | 0 | ||
| EQ-5D mobility score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 10 (3.4) | 5 (1.8) | 0.316 | – |
| Level 2, n (%) | 245 (82.2) | 221 (80.4) | ||
| Level 3, n (%) | 17 (5.7) | 22 (8.0) | ||
| Missing, n (%) | 26 (8.7) | 27 (9.8) | ||
| 6 months | ||||
| Level 1, n (%) | 134 (46.1) | 106 (39.7) | 0.333 | – |
| Level 2, n (%) | 128 (44.0) | 132 (49.4) | ||
| Level 3, n (%) | 3 (1.0) | 3 (1.1) | ||
| Missing, n (%) | 26 (8.9) | 26 (9.8) | ||
| EQ-5D self-care score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 164 (55.1) | 136 (49.5) | 0.026 | – |
| Level 2, n (%) | 107 (35.9) | 104 (37.8) | ||
| Level 3, n (%) | 1 (0.3) | 8 (2.9) | ||
| Missing, n (%) | 26 (8.7) | 27 (9.8) | ||
| 6 months | ||||
| Level 1, n (%) | 240 (82.5) | 221 (82.8) | 0.530 | – |
| Level 2, n (%) | 21 (7.2) | 19 (7.1) | ||
| Level 3, n (%) | 4 (1.4) | 1 (0.4) | ||
| Missing, n (%) | 26 (8.9) | 26 (9.7) | ||
| EQ-5D usual activities score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 10 (3.3) | 10 (3.6) | 0.037 | – |
| Level 2, n (%) | 150 (50.3) | 109 (39.6) | ||
| Level 3, n (%) | 112 (37.6) | 129 (46.9) | ||
| Missing, n (%) | 26 (8.8) | 27 (9.9) | ||
| 6 months | ||||
| Level 1, n (%) | 159 (54.6) | 125 (46.8) | 0.179 | – |
| Level 2, n (%) | 96 (33.0) | 104 (39.0) | ||
| Level 3, n (%) | 10 (3.4) | 12 (4.5) | ||
| Missing, n (%) | 26 (9.0) | 26 (9.7) | ||
| EQ-5D pain/discomfort score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 130 (43.6) | 124 (45.1) | 0.421 | – |
| Level 2, n (%) | 136 (45.6) | 122 (44.4) | ||
| Level 3, n (%) | 6 (2.0) | 2 (0.7) | ||
| Missing, n (%) | 26 (8.8) | 27 (9.8) | ||
| 6 months | ||||
| Level 1, n (%) | 118 (40.6) | 105 (39.3) | 0.589 | – |
| Level 2, n (%) | 139 (47.8) | 132 (49.5) | ||
| Level 3, n (%) | 8 (2.7) | 4 (1.5) | ||
| Missing, n (%) | 26 (8.9) | 26 (9.7) | ||
| EQ-5D anxiety/depression score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 183 (61.4) | 162 (58.9) | 0.084 | – |
| Level 2, n (%) | 84 (28.2) | 86 (31.3) | ||
| Level 3, n (%) | 5 (1.7) | 0 | ||
| Missing, n (%) | 26 (8.7) | 27 (9.8) | ||
| 6 months | ||||
| Level 1, n (%) | 199 (68.4) | 189 (70.8) | 0.706 | – |
| Level 2, n (%) | 59 (20.3) | 48 (18.0) | ||
| Level 3, n (%) | 6 (2.1) | 4 (1.5) | ||
| Missing, n (%) | 27 (9.2) | 26 (9.7) | ||
| EQ-5D score | ||||
| 6 weeks | ||||
| Mean | 0.52 | 0.48 | –0.04 (–0.08 to 0.002); 0.065 | –0.04 (–0.08 to 0.007); 0.100 |
| n | 272 | 248 | ||
| SD | 0.3 | 0.2 | ||
| Median | 0.59 | 0.49 | ||
| Minimum | –0.48 | –0.06 | ||
| Maximum | 1 | 1 | ||
| Missing | 26 | 27 | ||
| 6 months | ||||
| Mean | 0.76 | 0.76 | –0.006 (–0.05 to 0.03); 0.754 | –0.004 (–0.04 to 0.04); 0.861 |
| n | 264 | 241 | ||
| SD | 0.2 | 0.2 | ||
| Median | 0.80 | 0.78 | ||
| Minimum | –0.36 | –0.07 | ||
| Maximum | 1 | 1 | ||
| Missing | 27 | 26 | ||
| EQ-5D VAS score | ||||
| 6 weeks | ||||
| Mean | 72.8 | 72.1 | –0.67 (–3.70 to 2.36); 0.663 | –0.37 (–3.18 to 2.44); 0.796 |
| n | 272 | 247 | ||
| SD | 17.1 | 18.1 | ||
| Median | 75 | 75 | ||
| Minimum | 0 | 10 | ||
| Maximum | 100 | 100 | ||
| Missing | 26 | 28 | ||
| 6 months | ||||
| Mean | 77.5 | 77.3 | –0.16 (–3.35 to 3.03); 0.923 | –0.09 (–3.19 to 3.02); 0.956 |
| n | 265 | 241 | ||
| SD | 18.5 | 18.0 | ||
| Median | 81 | 80 | ||
| Minimum | 0 | 1 | ||
| Maximum | 100 | 100 | ||
| Missing | 26 | 26 | ||
FIGURE 11.
Plot of mean scores and 95% CIs for the SF-12 mental component summary score over time (per protocol).
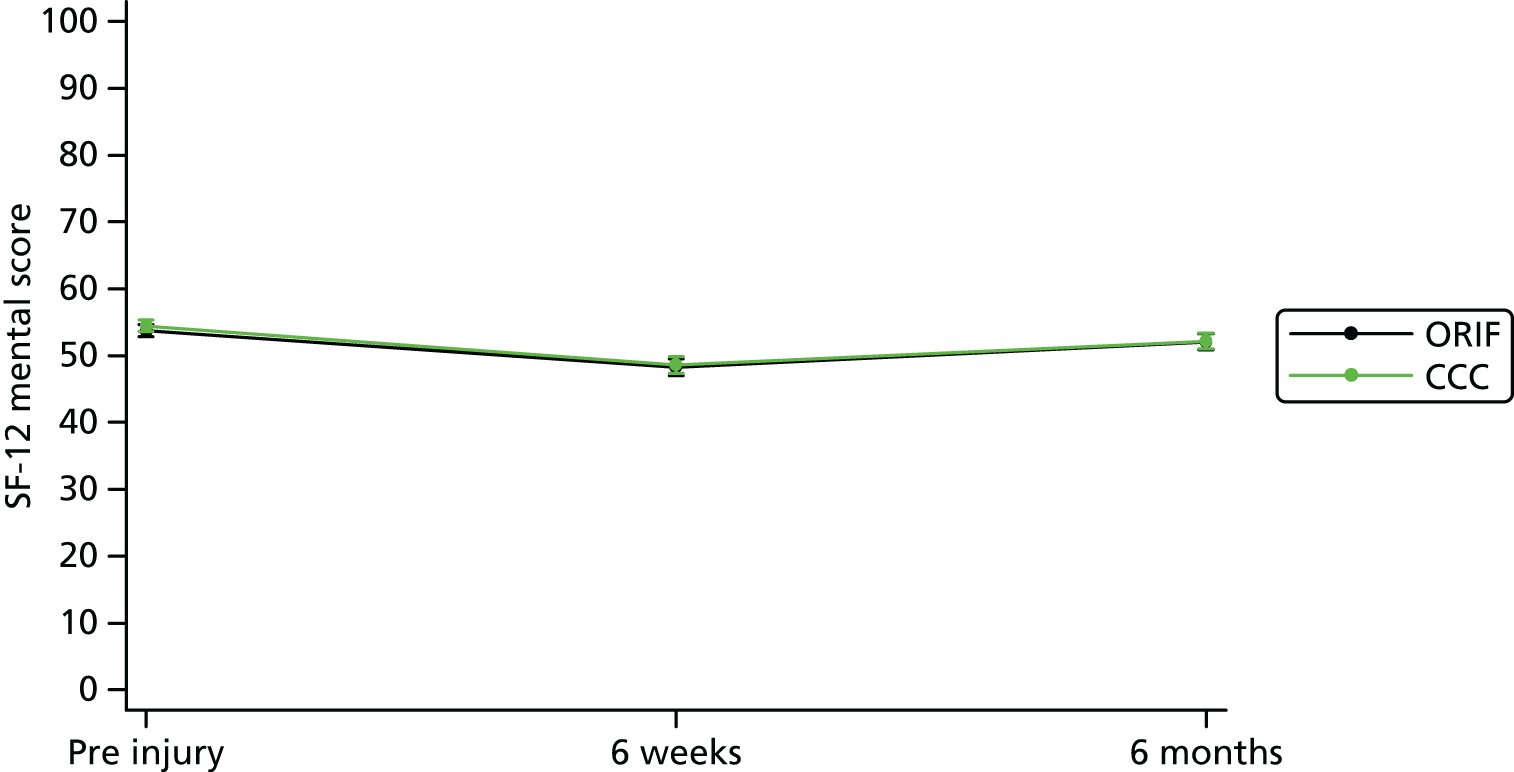
Short Form questionnaire-12 items physical component summary subscale (per protocol)
The analyses of the SF-12 physical subscale have been presented in Table 21. There is no evidence of a statistically significant difference between the two arms at either 6 weeks or 6 months. Overall, the SF-12 physical score improved from a mean score of 33.4 at 6 weeks to a mean score of around 45.0 at 6 months. The difference between ORIF and CCC on average was estimated to be 0.24 (95% CI –0.82 to 1.31) at 6 weeks and –0.83 (95% CI –2.39 to 0.74) at 6 months. Participants in both groups followed a similar pattern of recovery over time, with their physical function at 6 months being slightly less than what it was pre-injury (Figure 12).
FIGURE 12.
Plot of mean scores and 95% CIs for the SF-12 physical component summary score over time (per protocol).
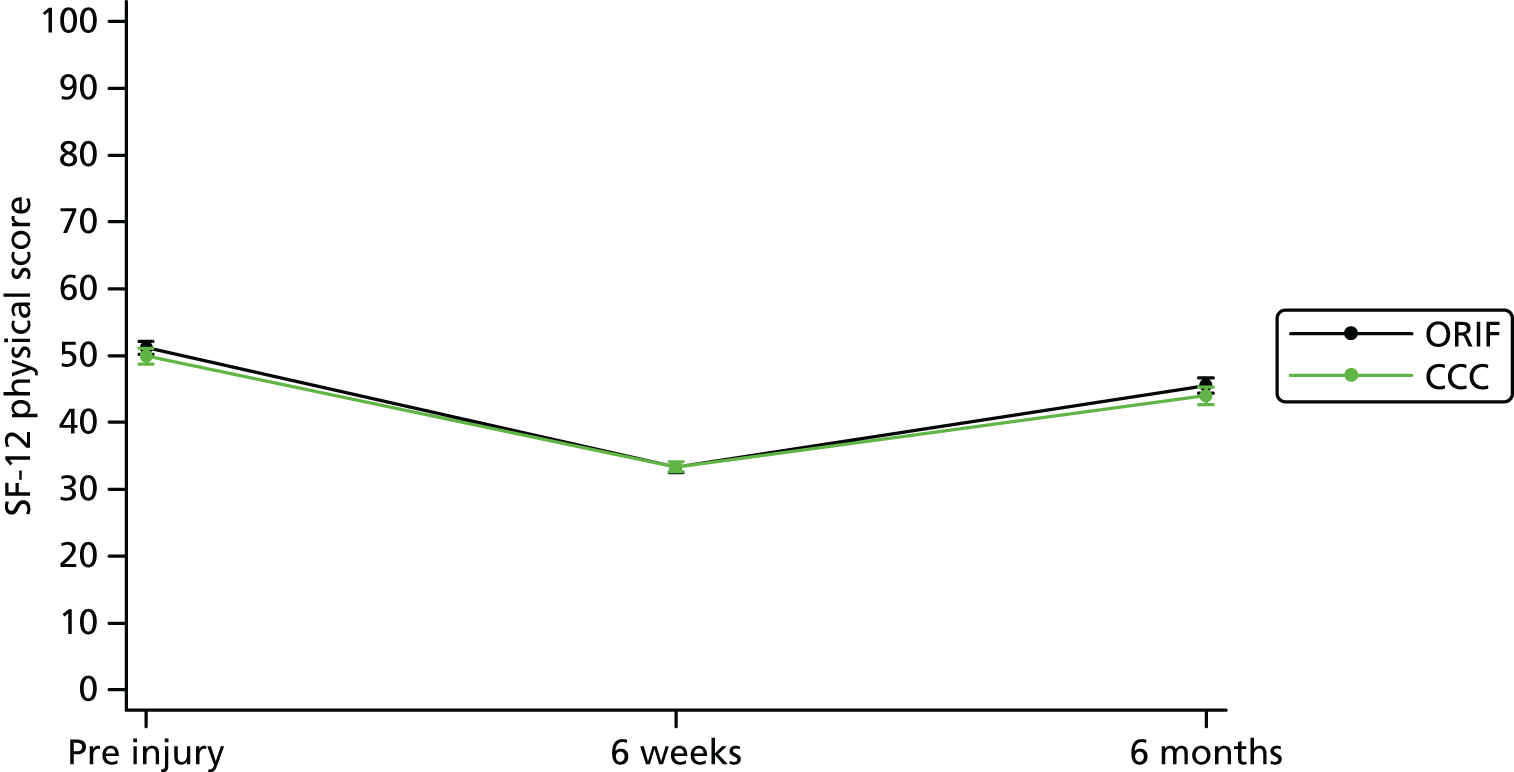
European Quality of Life 5-Dimensions score (per protocol)
The analyses of the EQ-5D score have been presented in Table 21. There is no evidence of a statistically significant difference between the two arms at both 6 weeks and 6 months. Overall, the EQ-5D score improved from a mean score of around 0.51 at 6 weeks to a mean score of 0.76 at 6 months. The difference between ORIF and CCC on average was estimated to be –0.04 (95% CI –0.08 to 0.007) at 6 weeks and –0.004 (95% CI –0.04 to 0.04) at 6 months. Participants in both groups followed a similar pattern of recovery over time, with their quality of life at 6 months being slightly less than what it was the day before their injury (Figure 13).
FIGURE 13.
Plot of mean scores and 95% CIs for the EQ-5D over time (per protocol).

European Quality of Life 5-Dimensions visual analogue scale (per protocol)
The analyses of the EQ-5D VAS score have been presented in Table 21. There is no evidence of a statistically significant difference between the two arms at both 6 weeks and 6 months. Overall, the EQ-5D VAS score improved from a mean score of around 73.0 at 6 weeks to a mean score of around 77.0 at 6 months. The difference between ORIF and CCC on average was estimated to be –0.37 (95% CI –3.18 to 2.44) at 6 weeks and –0.09 (95% CI –3.19 to 3.02) at 6 months.
Timed up and go test (per protocol)
Tables 22 and 23 present the results of the timed up and go test undertaken at the 6-month assessment. There is no evidence of a statistically significant difference in the median time between the two groups at 6 months. Most of the participants who undertook the test completed it in less than 5 minutes and did not require the use of a walking aid.
| Timed up and go test (seconds) | Treatment group | p-valuea | |
|---|---|---|---|
| ORIF | CCC | ||
| Mean | 21.3 | 22.1 | 0.079 |
| n | 276 | 242 | |
| SD | 19.2 | 13.9 | |
| Median | 18.0 | 18.4 | |
| Minimum | 6.8 | 7.8 | |
| Maximum | 224.3 | 133 | |
| Missing | 0 | 1 | |
| Additional timed up and go test details | Treatment group | p-value | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Completed in under 5 minutes | |||
| Yes | 275 (99.6) | 241 (99.2) | 0.602 |
| No | 1 (0.4) | 2 (0.8) | |
| Missing | 0 | 0 | |
| Did patient use walking aid(s)? | |||
| Yes | 26 (9.4) | 28 (11.5) | 0.394 |
| No | 207 (75.0) | 174 (71.6) | |
| Missing | 43 (15.6) | 41 (16.9) | |
| Unable to complete test because . . . | |||
| Non-weight-bearing | 0 | 0 | 0.123 |
| Declined to complete test | 1 (0.3) | 2 (0.8) | |
| Other physical limitation | 1 (0.3) | 0 | |
| Other | 13 (4.5) | 22 (8.2) | |
| Not applicable | 276 (94.9) | 243 (91.0) | |
| Missing | 0 | 0 | |
Range of ankle motion (per protocol)
Table 24 presents the results of the range of ankle motion assessments at 6 weeks and 6 months. There is evidence of a statistically significant difference of –1.4° (95% CI –2.75° to –0.08°) between the two groups for the angle of injured ankle dorsiflexion at 6 weeks, in favour of the ORIF group. However, this difference was not evident later on at 6-month follow-up, when the difference was estimated to be 0.16° (95% CI –1.59° to 1.90°).
| Range of ankle motion | Treatment group | Unadjusted statistics (95% CI); p-value | Adjusted statistics (95% CI); p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| Angle of injured ankle dorsiflexion (°) | ||||
| 6 weeks | ||||
| Mean | 5.1 | 3.7 | –1.42 (–2.94 to 0.10); 0.067 | –1.42 (–2.75 to –0.08); 0.037 |
| n | 283 | 262 | ||
| SD | 8.6 | 9.5 | ||
| Median | 5 | 4 | ||
| Missing | 15 | 13 | ||
| 6 months | ||||
| Mean | 11.9 | 11.6 | –0.31 (–2.18 to 1.57); 0.749 | 0.16 (–1.59 to 1.90); 0.861 |
| n | 282 | 256 | ||
| SD | 10.4 | 11.8 | ||
| Median | 10 | 10 | ||
| Missing | 9 | 11 | ||
| Angle of injured ankle plantar flexion (°) | ||||
| 6 weeks | ||||
| Mean | 24.5 | 22.7 | –1.81 (–4.02 to 0.40); 0.109 | –1.87 (–4.01 to 0.27); 0.087 |
| n | 283 | 262 | ||
| SD | 13.0 | 13.2 | ||
| Median | 24 | 20 | ||
| Missing | 15 | 13 | ||
| 6 months | ||||
| Mean | 33.7 | 31.1 | –2.54 (–4.81 to –0.28); 0.028 | –2.55 (–4.64 to –0.46); 0.017 |
| n | 282 | 256 | ||
| SD | 13.7 | 13.0 | ||
| Median | 35 | 30 | ||
| Missing | 9 | 11 | ||
| Eversion of injured ankle (%) | ||||
| 6 weeks | ||||
| Mean | 50.2 | 51.0 | 0.78 (–7.71 to 9.27); 0.857 | 0.56 (–7.97 to 9.10); 0.897 |
| n | 277 | 252 | ||
| SD | 40.5 | 58.1 | ||
| Median | 50 | 50 | ||
| Missing | 21 | 23 | ||
| 6 months | ||||
| Mean | 88.0 | 86.1 | –1.89 (–13.90 to 10.11); 0.757 | –1.97 (–13.83 to 9.90); 0.745 |
| n | 282 | 251 | ||
| SD | 83.1 | 52.6 | ||
| Median | 80 | 80 | ||
| Missing | 9 | 16 | ||
| Inversion of injured ankle (%) | ||||
| 6 weeks | ||||
| Mean | 47.9 | 56.6 | 8.73 (0.82 to 16.64); 0.031 | 9.02 (0.98 to 17.05); 0.028 |
| n | 281 | 258 | ||
| SD | 36.6 | 55.7 | ||
| Median | 45.5 | 50 | ||
| Missing | 17 | 17 | ||
| 6 months | ||||
| Mean | 83.4 | 83.4 | –0.05 (–9.37 to 9.27); 0.992 | –0.27 (–9.62 to 9.08); 0.955 |
| n | 282 | 256 | ||
| SD | 64.7 | 41.7 | ||
| Median | 75 | 80 | ||
| Missing | 9 | 11 | ||
| Started partial weight-bearing | ||||
| 6 weeks | ||||
| Yes, n (%) | 107 (35.9%) | 111 (40.4%) | 0.272 | |
| No, n (%) | 191 (64.1%) | 164 (59.6%) | ||
| Missing, n (%) | 0 | 0 | ||
There was no evidence of a difference between the two groups for the angle of injured ankle plantar flexion at 6 weeks with an estimated difference of –1.87° (95% CI –4.01° to 0.27°). However, there was evidence of a statistically significant difference at 6 months suggesting that those in the CCC group have, on average, a smaller range of motion of injured ankle plantar flexion of about –2.55° (95% CI –4.64° to –0.46°) than in the ORIF group.
There was no evidence of a statistically significant difference between the two groups for the eversion of the injured ankle at 6 weeks and 6 months. The difference between ORIF and CCC, on average, was estimated to be 0.56% (95% CI –7.97% to 9.10%) at 6 weeks and –1.97 (95% CI –13.83% to 9.90%) at 6 months.
There was evidence of a statistically significant difference at 6 weeks for the inversion of the injured ankle. This result suggests that those in the CCC group have on average more percentage inversion of the injured ankle at 6 weeks estimated to be 9.02% (95% CI 0.98% to 17.05%). However, by 6 months this difference diminishes such that there is no evidence of a significant difference between the groups with an estimated difference of –0.27% (95% CI –9.62% to 9.08%). The magnitude of the differences in ankle range of motion is unlikely to be clinically important in terms of ankle function.
Pain (per protocol)
Pain was assessed in this trial, using the pain items from both the EQ-5D and the OMAS questionnaires, at 6 weeks and 6 months. Table 25 presents a summary of the pain items by treatment group. For both the pain items, there is no evidence of a difference in the levels of pain reported between the two groups. In other words, the reported levels of pain are similar across both treatment groups at each of the time points.
| Pain outcomes | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-value | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| EQ-5D pain/discomfort item | ||||
| 6 weeks | ||||
| No pain/discomfort | 130 (43.6) | 124 (45.1) | 0.89 (0.63 to 1.26); 0.514 | 0.91 (0.64 to 1.29); 0.584 |
| Moderate pain/discomfort | 136 (45.6) | 122 (44.4) | ||
| Extreme pain/discomfort | 6 (2.0) | 2 (0.7) | ||
| Missing | 26 (8.8) | 27 (9.8) | ||
| 6 months | ||||
| No pain/discomfort | 118 (40.6) | 105 (39.3) | 1.01 (0.71 to 1.42); 0.969 | 1.03 (0.72 to 1.47); 0.889 |
| Moderate pain/discomfort | 139 (47.8) | 132 (49.5) | ||
| Extreme pain/discomfort | 8 (2.7) | 4 (1.5) | ||
| Missing | 26 (8.9) | 26 (9.7) | ||
| OMAS pain item | ||||
| 6 weeks | ||||
| None | 215 (72.2) | 197 (71.7) | 1.04 (0.72 to 1.49); 0.845 | 1.11 (0.76 to 1.62); 0.583 |
| While walking on uneven surface | 27 (9.1) | 19 (6.9) | ||
| While walking on even surface outdoors | 9 (3.0) | 10 (3.6) | ||
| While walking indoors | 37 (12.3) | 33 (12.0) | ||
| Constant and severe | 10 (3.4) | 14 (5.1) | ||
| Missing | 0 | 2 (0.7) | ||
| 6 months | ||||
| None | 127 (43.6) | 121 (45.3) | 0.90 (0.66 to 1.23); 0.510 | 0.94 (0.69 to 1.29); 0.711 |
| While walking on uneven surface | 88 (30.2) | 83 (31.1) | ||
| While walking on even surface outdoors | 36 (12.4) | 35 (13.1) | ||
| While walking indoors | 31 (10.7) | 19 (7.1) | ||
| Constant and severe | 9 (3.1) | 9 (3.4) | ||
| Missing | 0 | 0 | ||
Patient satisfaction (per protocol)
Table 26 summarises the patient satisfaction outcome measure by treatment group. There is no evidence of difference in the reported levels of patient satisfaction between the two treatment groups, which suggests that patient satisfaction is similar across both groups. At both 6 weeks and 6 months, around 63% of participants reported that they were ‘very satisfied’ with their treatment in both groups.
| Patient satisfaction | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-value | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| 6 weeks | ||||
| Very dissatisfied | 15 (5.0) | 12 (4.4) | 0.97 (0.65 to 1.44); 0.862 | 0.98 (0.65 to 1.48); 0.934 |
| Somewhat dissatisfied | 6 (2.1) | 9 (3.3) | ||
| Neither satisfied nor dissatisfied | 8 (2.7) | 10 (3.6) | ||
| Somewhat satisfied | 37 (12.4) | 30 (10.9) | ||
| Very satisfied | 189 (63.4) | 171 (62.2) | ||
| Missing | 43 (14.4) | 43 (15.6) | ||
| 6 months | ||||
| Very dissatisfied | 10 (3.4) | 15 (5.6) | 1.03 (0.68 to 1.56); 0.884 | 1.05 (0.68 to 1.61); 0.830 |
| Somewhat dissatisfied | 6 (2.1) | 5 (1.9) | ||
| Neither satisfied nor dissatisfied | 13 (4.5) | 8 (3.0) | ||
| Somewhat satisfied | 35 (12.0) | 27 (10.1) | ||
| Very satisfied | 184 (63.2) | 169 (63.3) | ||
| Missing | 43 (14.8) | 43 (16.1) | ||
Radiological malunion and non-union (per protocol)
Table 27 presents a summary of the different types of malunion at the 6-month follow-up. Overall, around 91% (477 out of 523) had no malunion at 6 months, 4.4% (23 out of 523) had a talar shift and 3% (16 out of 573) had a talar shift and talar tilt. Table 28 summarises radiological malunion versus no malunion at the 6-month follow-up. There is evidence of a statistically significant association between whether or not a participant has malunion and their treatment allocation at the 6-month follow-up (p ≤ 0.001). There are a higher proportion of participants in the CCC group with malunion at 6 months (15.3%) than in the ORIF group (2.9%).
| Malunion typea | Treatment group | Total (N = 523), n (%) | |
|---|---|---|---|
| ORIF (N = 274), n (%) | CCC (N = 249), n (%) | ||
| No malunion | 266 (97.1) | 211 (84.8) | 477 (91.2) |
| Talar shift | 3 (1.0) | 20 (8.0) | 23 (4.4) |
| Talar tilt | 1 (0.4) | 0 | 1 (0.2) |
| Diastasis | 0 | 0 | 0 |
| Talar shift and talar tilt | 1 (0.4) | 15 (6.0) | 16 (3.0) |
| Talar shift and diastasis | 1 (0.4) | 0 | 1 (0.2) |
| Talar tilt and diastasis | 0 | 1 (0.4) | 1 (0.2) |
| Talar shift, talar tilt and diastasis | 2 (0.7) | 2 (0.8) | 4 (0.8) |
| Missing | 24 | 26 | 50 |
| Radiological assessment | Treatment group | p-value | |
|---|---|---|---|
| ORIF (N = 274), n (%) | CCC (N = 249), n (%) | ||
| No malunion | 266 (97.1) | 211 (84.7) | < 0.001 |
| Malunion | 8 (2.9) | 38 (15.3) | |
| Missing | 24 | 26 | |
Table 29 summarises the non-union data at the 6-month follow-up. There is evidence of a statistically significant association between whether or not a participant has lateral and medial malleolus non-union and their treatment allocation. For both lateral malleolus and medial malleolus, the radiological assessments suggested that the fracture was not united for a higher proportion of participants in the CCC group than in the ORIF group.
| Radiological assessment | Treatment group | p-value | |
|---|---|---|---|
| ORIF (N = 274), n (%) | CCC (N = 248), n (%) | ||
| Lateral malleolus | |||
| Radiologically fracture not united | 0 | 8 (3.2) | 0.002 |
| Not injured/no issues with union identified | 274 (100) | 240 (96.8) | |
| Missing | 24 | 27 | |
| Medial malleolus | |||
| Radiologically fracture not united | 3 (1.1) | 18 (7.3) | < 0.001 |
| Not injured/no issues with union identified | 271 (98.9) | 230 (92.7) | |
| Missing | 24 | 27 | |
Ancillary analyses (per protocol)
Olerud–Molander Ankle Score as an ordinal outcome (per protocol)
Table 30 presents the results of the analysis when converting the OMASs into an ordinal outcome at the 6-week and 6-month follow-up. At the 6-week assessment, the majority of participants had either a ‘poor’ or a ‘fair’ outcome. By the 6-month assessment, most participants had either a ‘fair’ or a ‘good’ outcome. Both the unadjusted and adjusted analyses suggest that there is no evidence that the outcomes differ between both treatment groups; that is, both groups are equally likely to report a similar outcome.
| OMAS | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-valuea | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| 6 weeks | ||||
| Poor (0–30%) | 98 (32.9) | 96 (34.9) | 0.95 (0.68 to 1.32); 0.746 | 0.93 (0.66 to 1.31); 0.670 |
| Fair (31–60%) | 186 (62.4) | 162 (58.9) | ||
| Good (61–90%) | 12 (4.1) | 15 (5.5) | ||
| Excellent (91–100%) | 1 (0.3) | 0 | ||
| Missing | 1 (0.3) | 2 (0.7) | ||
| 6 months | ||||
| Poor (0–30%) | 18 (6.2) | 22 (8.2) | 0.89 (0.65 to 1.22); 0.484 | 0.94 (0.67 to 1.30); 0.697 |
| Fair (31–60%) | 92 (31.6) | 94 (35.3) | ||
| Good (61–90%) | 159 (54.6) | 120 (44.9) | ||
| Excellent (91–100%) | 22 (7.6) | 31 (11.6) | ||
| Missing | 0 | 0 | ||
Blinded assessments (per protocol)
Table 31 presents the analysis of the blinding strategy at the 6-month follow-up. In total, 448 of the participants had their assessor complete the 6-month follow-up question that asked if the assessor thought they knew what the actual intervention received was. James’ Blinding Index was used (range 0–1) to assess the overall blinding success at the 6-month assessments, in which a higher score reflects better blinding. The James’ Blinding Index was estimated to be 0.81 (95% CI 0.78 to 0.84), which suggests that there is evidence of adequate overall blinding at 6 months.
| Treatment received | Blinded assessors guess | Total | James’ Blinding Index (95% CI) | ||
|---|---|---|---|---|---|
| CCC | ORIF | Don’t know | |||
| CCC | 36 | 22 | 153 | 211 | 0.81 (0.78 to 0.84) |
| ORIF | 11 | 50 | 176 | 237 | |
| Total | 47 | 72 | 329 | 448 | |
Time-to-event analysis (per protocol)
There is no evidence of a difference in the time from randomisation to discharge between the two treatment arms (Table 32). However, there is evidence of a statistically significant difference in the time from randomisation to readmission between the two groups. The results of adjusted analyses suggest that those participants in receipt of CCC had a higher hazard (risk) of being readmitted than those in receipt of the ORIF treatment. The estimated hazard ratio was 2.17 (95% CI 1.16 to 4.04).
| Event | Number of participants experiencing an event, n (%) | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| Time to discharge (days) | 573 (100.0) | 0.99 (0.84 to 1.17) | 0.884 | 1.11 (0.94 to 1.32) | 0.216 |
| Time to readmission (days) | 83 (14.5) | 1.44 (0.88 to 2.34) | 0.139 | 2.17 (1.16 to 4.04) | 0.015 |
Process variables (per protocol)
The process data collected during the trial are summarised in Tables 33 and 34. In both treatment groups, the average time from injury to randomisation was 2.8 days, the time from randomisation to the primary theatre procedure was around 2.1 days and the time from injury to primary theatre procedure was around 4.9 days. There is evidence of a statistically significant difference in the time from entry into anaesthetic room to the start time in theatre, with surgery taking around 11 minutes longer in participants undergoing ORIF. Moreover, there is also evidence of a difference in the time from start to end of the primary theatre procedure, with the procedure taking around 54 minutes longer on average for ORIF. Of the additional procedures in theatre, there is significant evidence to suggest that those undergoing CCC lasted longer (10.7 days) before requiring an additional procedure than participants undergoing ORIF (4.9 days). There is evidence of a difference in the time from the primary theatre procedure to hospital discharge, with those in the CCC treatment group, on average, being discharged later. Furthermore, the mean time from hospital discharge to readmission is significantly shorter in the CCC group (45.4 days) than in the ORIF group (63.8 days), suggesting that undergoing CCC are readmitted much sooner than those undergoing ORIF. There is no evidence of a difference in the duration from randomisation and injury to the date the allocated treatment was actually received.
| Process variable | Treatment group | Total | p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| Randomisation | ||||
| Time from injury to randomisation (days) | ||||
| Mean | 2.8 | 2.8 | 2.8 | 0.978 |
| n | 298 | 275 | 573 | |
| SD | 2.7 | 2.7 | 2.7 | |
| Median | 2 | 2 | 2 | |
| IQR | 1–3.4 | 1–4 | 1–3.4 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 15 | 17 | 17 | |
| Missing | 0 | 0 | 0 | |
| Randomisation/theatre | ||||
| Time from randomisation to theatre procedure (days) | ||||
| Mean | 2.2 | 2.0 | 2.1 | 0.455 |
| n | 298 | 275 | 573 | |
| SD | 2.5 | 2.1 | 2.3 | |
| Median | 1 | 1 | 1 | |
| IQR | 0–3.4 | 0–3 | 0–3 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 17 | 9.4 | 17 | |
| Missing | 0 | 0 | 0 | |
| Treatment | ||||
| Time from injury to primary treatment (days) | ||||
| Mean | 5.0 | 4.8 | 4.9 | 0.436 |
| n | 298 | 275 | 573 | |
| SD | 3.5 | 3.2 | 3.4 | |
| Median | 4.7 | 4.4 | 4.4 | |
| IQR | 2–7 | 2–7 | 2–7 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 19 | 18 | 19 | |
| Missing | 0 | 0 | 0 | |
| Theatre | ||||
| Time from entry into anaesthetic room to start time in theatre (minutes) | ||||
| Mean | 29.5 | 18.6 | 24.2 | < 0.001 |
| n | 295 | 275 | 570 | |
| SD | 14.3 | 10.4 | 13.7 | |
| Median | 28 | 17 | 22 | |
| IQR | 19–37 | 11–24 | 15–30 | |
| Minimum | 5 | 0 | 0 | |
| Maximum | 90 | 100 | 100 | |
| Missing | 3 | 0 | 3 | |
| Time from start to end of procedure in theatre (minutes) | ||||
| Mean | 80.2 | 25.8 | 54.1 | < 0.001 |
| n | 296 | 274 | 570 | |
| SD | 29.0 | 11.9 | 35.1 | |
| Median | 79 | 24 | 44.5 | |
| IQR | 63–96 | 20–30 | 24–80 | |
| Minimum | 11 | 8 | 8 | |
| Maximum | 211 | 83 | 211 | |
| Missing | 2 | 1 | 3 | |
| Additional theatrea procedure | ||||
| Time from primary procedure to additional procedure in theatre (days) | ||||
| Mean | 4.9 | 10.7 | 8.2 | 0.008 |
| n | 14 | 19 | 33 | |
| SD | 2.1 | 11.9 | 9.5 | |
| Median | 5 | 7 | 6 | |
| IQR | 3–6 | 6–12.4 | 4–8.4 | |
| Minimum | 2 | 1 | 1 | |
| Maximum | 8.4 | 56.9 | 56.9 | |
| Missing | 0 | 0 | 0 | |
| Time from entry into anaesthetic room to start time in theatre (minutes) | ||||
| Mean | 34.3 | 27.9 | 30.6 | 0.284 |
| n | 14 | 19 | 33 | |
| SD | 12.1 | 19.3 | 16.7 | |
| Median | 35.5 | 22 | 27 | |
| IQR | 18 | 34 | 29 | |
| Minimum | 27–45 | 13–47 | 16–45 | |
| Maximum | 50 | 62 | 62 | |
| Missing | 0 | 0 | 0 | |
| Time from start to end of procedure in theatre (minutes) | ||||
| Mean | 83.9 | 70.5 | 76.2 | 0.353 |
| n | 14 | 19 | 33 | |
| SD | 33.8 | 37.3 | 35.9 | |
| Median | 80 | 77 | 80 | |
| IQR | 76–94 | 34–90 | 50–90 | |
| Minimum | 36 | 18 | 18 | |
| Maximum | 180 | 130 | 180 | |
| Missing | 0 | 0 | 0 | |
| Theatre/hospital discharge | ||||
| Time from theatre to hospital discharge (days) | ||||
| Mean | 5.2 | 5.7 | 5.4 | 0.032 |
| n | 298 | 275 | 573 | |
| SD | 5.8 | 8.4 | 7.2 | |
| Median | 3 | 3 | 3 | |
| IQR | 2–6.4 | 1–7 | 1–6.4 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 39.9 | 69.9 | 69.9 | |
| Missing | 0 | 0 | 0 | |
| Hospital discharge/hospital readmissiona | ||||
| Time from hospital discharge to readmission (days) | ||||
| Mean | 63.8 | 45.4 | 51.3 | 0.036 |
| n | 31 | 65 | 96 | |
| SD | 54.5 | 50.9 | 52.5 | |
| Median | 48.9 | 14 | 23.7 | |
| IQR | 17–105.3 | 9–62.9 | 10–92.8 | |
| Minimum | 1 | 4 | 1 | |
| Maximum | 161.2 | 174.6 | 174.6 | |
| Missing | 0 | 0 | 0 | |
| Time between events | Treatment group | p-value | |
|---|---|---|---|
| ORIF | CCC | ||
| Time from injury to allocated treatment received (days) | |||
| Mean | 5.3 | 4.8 | 0.164 |
| n | 298 | 275 | |
| SD | 3.7 | 3.3 | |
| Median | 5 | 4 | |
| IQR | 2–7 | 2–7 | |
| Minimum | 0 | 0 | |
| Maximum | 27 | 18 | |
| Missing | 0 | 0 | |
| Time from randomisation to allocated treatment received (days) | |||
| Mean | 2.4 | 2.0 | 0.264 |
| n | 298 | 275 | |
| SD | 3.0 | 2.1 | |
| Median | 1.5 | 2 | |
| IQR | 0–4 | 0–3 | |
| Minimum | 0 | 0 | |
| Maximum | 26 | 10 | |
| Missing | 0 | 0 | |
Experience and treatment preferences of surgeons (per protocol)
In total, 558 (97.4%) participants received their allocated treatment at their primary theatre procedure after randomisation. There were differences in experience level of the operating and supervising surgeon at the primary theatre procedure after randomisation in the ORIF and CCC groups (Table 35). The operating surgeon for CCC was consultant grade for approximately 85% of CCC applications. CCC was supervised by at least a consultant 97% of the time. For ORIF, approximately 39% of operating surgeons were consultants. Direct consultant supervision was in place for 76% of ORIF procedures.
| Treatment delivery information | Treatment group | Total, n (%) | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Time point when allocated treatment received | |||
| Primary theatre | 285 (95.6) | 273 (99.3) | 558 (97.4) |
| First additional theatre procedure | 12 (4.1) | 2 (0.7) | 14 (2.4) |
| Second additional theatre procedure | 0 | 0 | 0 |
| First readmission | 1 (0.3) | 0 | 1 (0.2) |
| Second readmission | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 |
| Grade of operating surgeon at primary theatre procedure if allocated treatment receiveda | |||
| FY2b | 1 (0.4) | 0 | 1 (0.2) |
| ST1c | 3 (1.0) | 0 | 3 (0.5) |
| ST2c | 5 (1.8) | 0 | 5 (0.9) |
| ST3c | 33 (11.6) | 4 (1.5) | 37 (6.7) |
| ST4c | 17 (6.0) | 4 (1.5) | 21 (3.8) |
| ST5c | 20 (7.0) | 2 (0.7) | 22 (3.9) |
| ST6c | 22 (7.7) | 2 (0.7) | 24 (4.3) |
| ST7c | 18 (6.3) | 4 (1.5) | 22 (3.9) |
| ST8c | 10 (3.5) | 1 (0.4) | 11 (2.0) |
| Staff grade | 16 (5.6) | 6 (2.2) | 22 (3.9) |
| Trust grade | 4 (1.4) | 2 (0.7) | 6 (1.1) |
| Fellow | 12 (4.2) | 6 (2.2) | 18 (3.2) |
| Consultant | 110 (38.6) | 232 (85.0) | 342 (61.3) |
| Other | 14 (4.9) | 10 (3.6) | 24 (4.3) |
| Missing | 0 | 0 | 0 |
| Grade of the most senior surgeon present at the primary theatre procedure if allocated treatment received | |||
| FY2b | 0 | 0 | 0 |
| ST1c | 0 | 0 | 0 |
| ST2c | 0 | 0 | 0 |
| ST3c | 3 (1.0) | 1 (0.4) | 4 (0.7) |
| ST4c | 6 (2.1) | 0 | 6 (1.1) |
| ST5c | 9 (3.2) | 0 | 9 (1.6) |
| ST6c | 6 (2.1) | 0 | 6 (1.1) |
| ST7c | 7 (2.5) | 2 (0.7) | 9 (1.6) |
| ST8c | 10 (3.5) | 0 | 10 (1.8) |
| Staff grade | 11 (3.9) | 1 (0.4) | 12 (2.2) |
| Trust grade | 2 (0.7) | 0 | 2 (0.4) |
| Fellow | 3 (1.0) | 0 | 3 (0.5) |
| Consultant | 218 (76.5) | 267 (97.8) | 485 (86.9) |
| Other | 10 (3.5) | 2 (0.7) | 12 (2.1) |
| Missing | 0 | 0 | 0 |
On the day of the primary theatre procedure, with treatment allocation known, the ORIF procedure was the preferred primary treatment for most surgeons in both the ORIF group (84.6%) and the CCC group (56.7%) (Table 36). In the CCC group, the CCC treatment was the preferred treatment for 27.6% of the procedures undertaken.
| Preferred treatment for patient on day of assessment | Treatment group | Total, n (%) | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Traditional plaster cast | 8 (2.7) | 31 (11.3) | 39 (6.8) |
| External fixation | 3 (1.0) | 0 | 3 (0.5) |
| Retrograde nail | 0 | 1 (0.4) | 1 (0.2) |
| ORIF | 252 (84.6) | 156 (56.7) | 408 (71.2) |
| CCC | 24 (8.0) | 76 (27.6) | 100 (17.5) |
| Other | 11 (3.7) | 11 (4.0) | 22 (3.8) |
| Missing | 0 | 0 | 0 |
Surgeon learning effects (per protocol)
We provided CCC training to 317 orthopaedic surgeons during the course of the study, of whom 154 were consultant grade (49%). Of the 275 patients randomised to the CCC intervention, details of the operating surgeon’s experience were unavailable for 20 patients, as no allocated surgeon code was reported. The allocated code was not reported for these patients because either the surgeon was from the pilot study or the procedure was conducted by a non-trained surgeon. The learning effects were therefore assessed using data from 255 patients. The number of days between CCC procedures performed by individual surgeons was very variable [mean 161 days (SD 153 days); range 0–725 days]. In total, 100 surgeons from 23 different hospitals applied a CCC during the trial. The grade of these surgeons, as reported at the CCC training, is summarised in Table 37. The number of surgeons across all the sites ranged from 1 to 9, with an average 4.7 (SD 2.3) surgeons per site. Forty-five (45%) surgeons carried out two CCC procedures, 22 (22%) surgeons carried out three CCC procedures, 19 (19%) surgeons carried out four CCC procedures, 13 (13%) surgeons carried out five or more CCC procedures and the remainder performed one CCC procedure. There was no evidence of a learning effect within the surgeons (f-test = 1.45; p = 0.087). In addition, there was no evidence of a learning effect when surgeons were assessed within hospitals (f-test = 1.44; p = 0.123). The ITT analyses gave similar results.
Adverse events (per protocol)
No serious adverse events were reporting during the trial. A breakdown of the adverse event complications reported is shown in Table 38. The number of participants who experienced an infection and/or wound problem for the surgery group was 29/298 (10%) compared with 4/275 (1%) for CCC. The participants with wound breakdown or infection in the CCC arm initially received CCC as their primary theatre procedure but later converted to ORIF either as an additional procedure in theatre or during a readmission. The number of plaster sores and pain from casts was similar between groups, but there were more plaster saw lacerations in the CCC group [5/275 (1.8%) vs. 1/298 (0.3%)]. Less common but serious complications, such as deep-vein thrombosis (DVT), pulmonary embolism (PE) and wound infections, occurred in both treatment groups but were infrequent overall.
| Complication type | Treatment group | |||||
|---|---|---|---|---|---|---|
| ORIF (n = 298) | CCC (n = 275) | |||||
| Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | |
| Intraoperative fracture | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Imperfect reduction reported | 0 | 0 | 0 | 3 (1.1) | 0 | 3 |
| Compartment syndrome | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular injury | 1 (0.3) | 0 | 1 | 1 (0.4) | 0 | 1 |
| Nerve palsy | 2 (0.7) | 0 | 2 | 2 (0.7) | 0 | 2 |
| Wound breakdown | 20 (6.7) | 1 (0.3) | 22 | 2 (0.7) | 0 | 2 |
| Infection | 7 (2.4) | 1 (0.3) | 9 | 2 (0.7) | 0 | 2 |
| Septicaemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Other clinical issue with wound (not breakdown or infection) | 6 (2.0) | 0 | 6 | 1 (0.4) | 0 | 1 |
| Clinical issue with metalwork | 4 (1.3) | 0 | 4 | 0 | 0 | 0 |
| Implant failure | 5 (1.7) | 0 | 5 | 0 | 0 | 0 |
| Non-wound skin problem | 11 (3.7) | 0 | 11 | 9 (3.3) | 0 | 9 |
| Pain from cast | 12 (4.0) | 0 | 12 | 16 (5.8) | 0 | 16 |
| Plaster sore | 13 (4.4) | 0 | 13 | 18 (6.6) | 0 | 18 |
| Plaster saw laceration | 1 (0.3) | 0 | 1 | 5 (1.8) | 0 | 5 |
| DVT | 3 (1.0) | 0 | 3 | 6 (2.2) | 0 | 6 |
| PE | 1 (0.3) | 0 | 1 | 6 (2.2) | 0 | 6 |
| Refracture of ankle | 0 | 0 | 0 | 0 | 0 | 0 |
| Fall postoperatively | 3 (1.0) | 0 | 3 | 4 (1.5) | 0 | 4 |
| Other fracture sustained | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
Additional procedures in theatre (per protocol)
A detailed breakdown of the additional procedures in theatre has been detailed in Table 39. The number of participants requiring one or more additional operating theatre procedures, for surgery-related complications, was 17 out of 298 (5.7%) in the ORIF group and 3 out of 275 (1.1%) in the CCC group.
| Complication type | Treatment group | |||||
|---|---|---|---|---|---|---|
| ORIF (n = 298) | CCC (n = 275) | |||||
| Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | |
| Remanipulation and traditional cast | 0 | 0 | 0 | 0 | 0 | 0 |
| Convert to external fixation | 0 | 0 | 0 | 0 | 0 | 0 |
| Convert to retrograde nail | 0 | 0 | 0 | 0 | 0 | 0 |
| Revision of ORIF | 3 (1.0) | 0 | 3 | 1 (0.4) | 0 | 1 |
| Wound washout | 2 (0.7) | 0 | 2 | 0 | 0 | 0 |
| Delayed wound closure | 0 | 0 | 0 | 0 | 0 | 0 |
| Wound debridement | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Incision and drainage of haematoma | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Amputation | 0 | 0 | 0 | 0 | 0 | 0 |
| Removal of syndesmotic screws | 6 (2.0) | 0 | 6 | 1 (0.4) | 0 | 1 |
| Removal of other metalwork | ||||||
| Because of infection | 1 (0.3) | 1 (0.3) | 3 | 0 | 0 | 0 |
| With washout | 0 | 0 | 0 | 1 (0.4) | 0 | 1 |
| Because of pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Because of poor position of implant | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Other reason | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
Summary of key findings
-
The results from the analyses of the primary end point allow us to conclude that the ORIF and CCC treatments are equivalent for the treatment of unstable ankle fracture in patients over 60 years.
-
The functionality of the injury ankle in terms of the range of movement was similar in both groups at 6 months.
-
The mental functioning, physical functioning and quality of life of participants in both groups follow a similar pattern of recovery up to 6 months, with their scores at 6 months being similar to their pre-injury scores.
-
Malunion and non-union of the ankle were more likely after CCC than ORIF.
-
Participants receiving CCC were more likely to be readmitted post hospital discharge; one in five had a later loss of fracture reduction resulting in conversion to ORIF or remanipulation and CCC applied in theatre.
-
Complications directly relating to ORIF, wound problems or infections, occurred in around 1 in 10 participants. Within 6 months about 1 in 20 participants who initially received ORIF required an additional surgical procedure.
Chapter 4 Health economic evaluation
Introduction
An evaluation of cost-effectiveness of CCC compared with ORIF was conducted as part of the AIM trial. The economic evaluation presented in this chapter was conducted for both the NHS and societal perspective. Consistent with the evaluation of cost-effectiveness in Chapter 3, the primary analysis was per protocol.
Results
Participant flow
Participant flow is shown in Figure 14. The per-protocol population included 573 participants. The number of participants for whom information was completely missing and who thus were ineligible for inclusion in the economic evaluation, was 53 out of 573 (9%). Therefore, there were 520 participants with valid information for an economic evaluation. This constituted the primary analysis population.
FIGURE 14.
Flow diagram of per-protocol and ITT population: withdrawals, with exclusions because of insufficient information and final analysis populations.
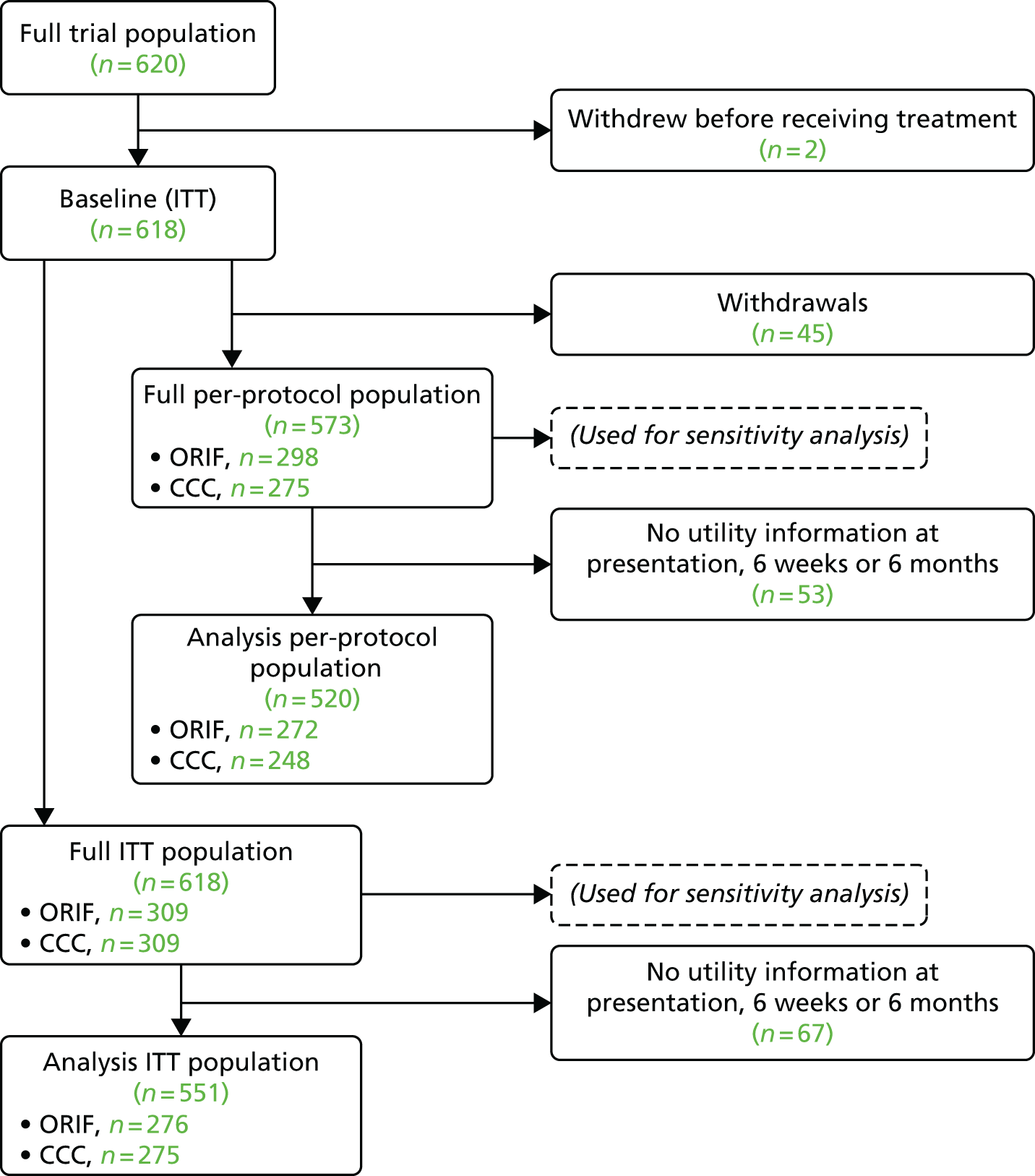
The ITT population included 618 participants. The number of participants for whom information was completely missing and who thus were ineligible for inclusion in the economic evaluation was 67 out of 618 (10%). Therefore, there were 551 participants with valid information for an economic evaluation. This constituted the population used for the ITT analysis.
A full trial per-protocol or ITT population included those with completely missing information (after multiple imputation of their entire history). This corresponded to 573 and 618 participants for the per-protocol and ITT populations, respectively. Sensitivity analyses were conducted using these populations (see Appendix 3).
Per-protocol analysis
Missing data (per protocol)
Cases included in the economic evaluation
Complete missingness was not statistically associated with intervention (odds ratio 1.13, 95% CI 0.62 to 2.09; p = 0.67; Fisher’s exact test). Missing cases also showed comparable baseline characteristics to the complete cases (see Appendix 3); therefore, we treated any remaining missingness as random.
The 53 participants in whom information was missing completely were removed and the economic evaluation continued with the 520 patients with sufficient valid information. Of the remaining participants, incomplete data remained at around 7% across most variables (see Appendix 3). Exceptions included lower missingness of utility prior to injury (0%), at 6 weeks (0%) and at 6 months (3%). Having concluded that missingness was random, we continued with multiple imputation with chained equations. 73 Multiple imputation showed healthy convergence and the density plots of imputed values followed closely to the observed values (see Appendix 3).
Baseline characteristics (per protocol)
Baseline characteristics have previously been presented in Chapter 3. It should be noted that the CCC group appeared slightly less healthy than the ORIF group. The CCC group showed lower utility prior to injury than the ORIF group (0.83 vs. 0.85; p = 0.05; Wilcoxon rank-sum test). Otherwise, the CCC group showed comparable baseline characteristics across age, sex, OMASs and a range of health conditions.
Resource use (per protocol)
Mean resource use following ORIF and CCC is presented in Table 40. Compared with the ORIF group, the CCC group shows lower theatre time during the index procedure (–54 minutes), slightly higher rates of additional procedures (+0.9%), higher rates of readmission (+14.6%) and greater cast use (+1.51). Among readmission visits, the CCC group showed greater implant material use and theatre time. Length of stay was comparable both during the index admission (8.99 vs. 9.70 days) and for any readmission (5.04 vs. 5.23 days) for the CCC and ORIF groups, respectively.
| Resource | Treatment group | Difference, mean | |
|---|---|---|---|
| ORIF, mean (SD) | CCC, mean (SD) | ||
| Index procedure | |||
| Theatre time (hours) | 1.32 (0.48) | 0.42 (0.18) | –0.90 |
| Screws | 6.65 (2.91) | 0.00 (0.00) | –6.65 |
| Antigliding plates | 0.10 (0.34) | 0.00 (0.00) | –0.10 |
| Tubular plates | 0.74 (0.60) | 0.00 (0.00) | –0.74 |
| Dynamic compression plates | 0.01 (0.10) | 0.00 (0.00) | –0.01 |
| Reconstruction plates | 0.01 (0.10) | 0.00 (0.00) | –0.01 |
| Locking plates | 0.12 (0.51) | 0.00 (0.00) | –0.12 |
| Other plates | 0.04 (0.21) | 0.00 (0.00) | –0.04 |
| Tightropes | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Wirings | 0.08 (0.33) | 0.00 (0.00) | –0.08 |
| Other implants | 0.08 (0.35) | 0.00 (0.00) | –0.08 |
| Additional procedure, n (%) | 14 (5.1) | 15 (6.0) | 0.9 |
| Theatre time (hours)a | 1.40 (0.56) | 1.27 (0.61) | –0.12 |
| Anaesthesia, n (%)a | 14 (100) | 15 (100) | 0.0 |
| Screwsa | 6.50 (2.71) | 4.73 (3.90) | –1.77 |
| Antigliding platesa | 0.29 (0.47) | 0.13 (0.35) | –0.15 |
| Tubular platesa | 0.57 (0.51) | 0.47 (0.52) | –0.10 |
| Dynamic compression platesa | 0.07 (0.27) | 0.07 (0.26) | 0.00 |
| Reconstruction platesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Locking platesa | 0.14 (0.36) | 0.13 (0.35) | –0.01 |
| Other platesa | 0.00 (0.00) | 0.07 (0.26) | 0.07 |
| Tightropesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Wiringsa | 0.07 (0.27) | 0.07 (0.26) | 0.00 |
| Other implantsa | 0.14 (0.53) | 0.00 (0.00) | –0.14 |
| Casts in plaster room | 0.00 (0.00) | 0.08 (0.34) | 0.08 |
| Casts in theatre room | 0.00 (0.06) | 1.00 (0.20) | 1.00 |
| LOS (days) | 9.70 (23.20) | 8.99 (9.55) | –0.70 |
| Readmission, n (%) | 23 (8.5) | 56 (22.6) | 14.1 |
| Theatre time (hours)a | 0.31 (0.48) | 1.03 (0.87) | 0.72 |
| Anaesthesia, n (%)a | 10 (43.5) | 41 (73.2) | 29.7 |
| Screwsa | 0.35 (1.30) | 4.59 (4.03) | 4.24 |
| Antigliding platesa | 0.00 (0.00) | 0.07 (0.32) | 0.07 |
| Tubular platesa | 0.04 (0.21) | 0.38 (0.49) | 0.33 |
| Dynamic compression platesa | 0.00 (0.00) | 0.05 (0.23) | 0.05 |
| Reconstruction platesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Locking platesa | 0.00 (0.00) | 0.16 (0.60) | 0.16 |
| Other platesa | 0.00 (0.00) | 0.04 (0.19) | 0.04 |
| Tightropesa | 0.00 (0.00) | 0.04 (0.27) | 0.04 |
| Wiringsa | 0.00 (0.00) | 0.04 (0.19) | 0.04 |
| Other implantsa | 0.00 (0.00) | 0.05 (0.30) | 0.05 |
| LOSa (days) | 5.23 (7.87) | 5.04 (9.81) | –0.19 |
| Total inclusive of 6 months | |||
| Theatre time (hours) | 1.42 (0.56) | 0.73 (0.69) | –0.69 |
| Casts | 0.07 (0.35) | 1.57 (0.91) | 1.50 |
| Hospital LOS (days) | 10.14 (23.47) | 10.13 (10.91) | –0.01 |
| Follow-up inclusive of 6 months | |||
| Care homes | |||
| Friend/family at home days | 11.70 (21.27) | 11.69 (27.56) | –0.01 |
| Community hospital days | 2.81 (14.21) | 2.34 (8.36) | –0.47 |
| Intermediate care days | 0.78 (8.93) | 1.39 (10.66) | 0.61 |
| Nursing home (NHS) days | 0.97 (7.20) | 0.39 (3.45) | –0.58 |
| Nursing home (private) days | 0.61 (6.14) | 0.34 (3.54) | –0.28 |
| Productivity | |||
| Sick days | 7.87 (24.50) | 8.40 (31.64) | 0.53 |
| Friend/family stay days | 2.78 (13.42) | 1.77 (11.89) | –1.01 |
| Work-days off by friend/family | 2.84 (9.12) | 2.64 (12.46) | –0.20 |
| Health services | |||
| GP | 0.69 (1.69) | 0.62 (1.29) | –0.07 |
| Nurse | 3.90 (11.63) | 3.65 (16.22) | –0.25 |
| Physiotherapy inpatient | 0.95 (4.14) | 1.34 (4.79) | 0.39 |
| Physiotherapy outpatient | 3.35 (4.13) | 3.17 (4.05) | –0.18 |
| Physiotherapy home | 0.63 (1.90) | 0.68 (2.10) | 0.05 |
| Hospital A&E | 0.10 (0.35) | 0.09 (0.30) | –0.01 |
| Hospital specialist | 0.92 (2.87) | 1.20 (2.12) | 0.28 |
| Psychologist | 0.01 (0.12) | 0.02 (0.23) | 0.01 |
| Trauma outpatient | 1.83 (2.08) | 2.65 (2.49) | 0.81 |
| Hospital transports | 1.23 (2.24) | 2.06 (2.98) | 0.82 |
| Community care | 5.85 (30.44) | 8.10 (35.60) | 2.25 |
| Private physiotherapy | 0.46 (2.34) | 0.22 (1.37) | –0.24 |
| Private consultant | 0.00 (0.06) | 0.00 (0.06) | 0.00 |
| Private osteopath | 0.04 (0.43) | 0.09 (0.98) | 0.05 |
| Private transports | 0.37 (2.12) | 0.28 (1.03) | –0.09 |
| Prescriptions | |||
| Painkillers | 2.28 (3.62) | 2.47 (4.11) | 0.19 |
| Anti-inflammatory | 0.43 (1.31) | 0.35 (0.84) | –0.08 |
| Gel | 0.14 (0.62) | 0.09 (0.43) | –0.05 |
| Sleeping pills | 0.05 (0.32) | 0.15 (1.40) | 0.10 |
| Antidepressants | 0.10 (0.64) | 0.27 (2.34) | 0.17 |
| Painkillers (self-buy) | 2.44 (7.05) | 2.33 (9.10) | –0.10 |
| Anti-inflammatory (self-buy) | 0.48 (1.85) | 0.32 (1.19) | –0.16 |
| Gel (self-buy) | 0.14 (0.67) | 0.13 (0.52) | –0.02 |
| Sleeping pills (self-buy) | 0.00 (0.06) | 0.00 (0.00) | 0.00 |
| Antidepressants (self-buy) | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
Over the 6 months, most of the care time was spent at home with friends/family, at an average of just under 12 days for both CCC and ORIF groups. Both groups spent around ≤ 2 days at a community hospital, nursing home or intermediate-care hospital; the CCC group had slightly shorter mean stays in the first two and slightly longer stay in the last.
Mean health service use is also presented in Table 40. Health service use was generally very low to negligible. Exceptions include modest nurse (≥ 3 visits), physiotherapy (≥ 3 visits), trauma outpatient (around two visits) and community care contacts (≥ 5 visits). The CCC group had slightly higher use in a number of health services. Medication use between the two groups was low to negligible.
Private health resource use was generally low to negligible and mean levels were comparable between ORIF and CCC. On average, patients in both groups took about 8 days off work, slightly more in the CCC group than in the ORIF group, while their family or friends took closer to 2–3 days off work, slightly less in the CCC group than in the ORIF group.
Health outcomes (per protocol)
The raw, unadjusted utilities at presentation, 6 weeks and 6 months after randomisation are presented in Table 41. At presentation, the utility in the CCC group trended higher than that in the ORIF group (0.07 vs. 0.04), although this difference was clinically small and not statistically significant (p = 0.11). Compared with the ORIF group, utility in the CCC group was comparable at 6 weeks (0.36 vs. 0.37; p = 0.15) and 6 months (0.49 vs. 0.49; p = 0.56). Total QALYs over the trial period were also comparable between the CCC and ORIF groups (0.30 vs. 0.30; p = 0.96). Figure 15 shows the mean utility scores; the QALYs are indicated by the area under the curve. QALYs were estimated from presentation time to 6 months.
| Health outcomes | Treatment group | Difference, mean (95% CI) | p-value | |
|---|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | |||
| Utility (baseline) | 0.04 (0.02 to 0.07) | 0.07 (0.04 to 0.10) | 0.03 (–0.01 to 0.07) | 0.11 |
| Utility (6 weeks) | 0.37 (0.35 to 0.39) | 0.36 (0.34 to 0.38) | –0.01 (–0.04 to 0.01) | 0.15 |
| Utility (6 months) | 0.49 (0.47 to 0.51) | 0.49 (0.47 to 0.50) | 0.00 (–0.03 to 0.03) | 0.56 |
| QALYs | 0.30 (0.28 to 0.31) | 0.30 (0.29 to 0.31) | 0.00 (–0.01 to 0.02) | 0.96 |
FIGURE 15.
Graph showing per-protocol utility (quality of life) over time (length of life). Area is the QALYs.
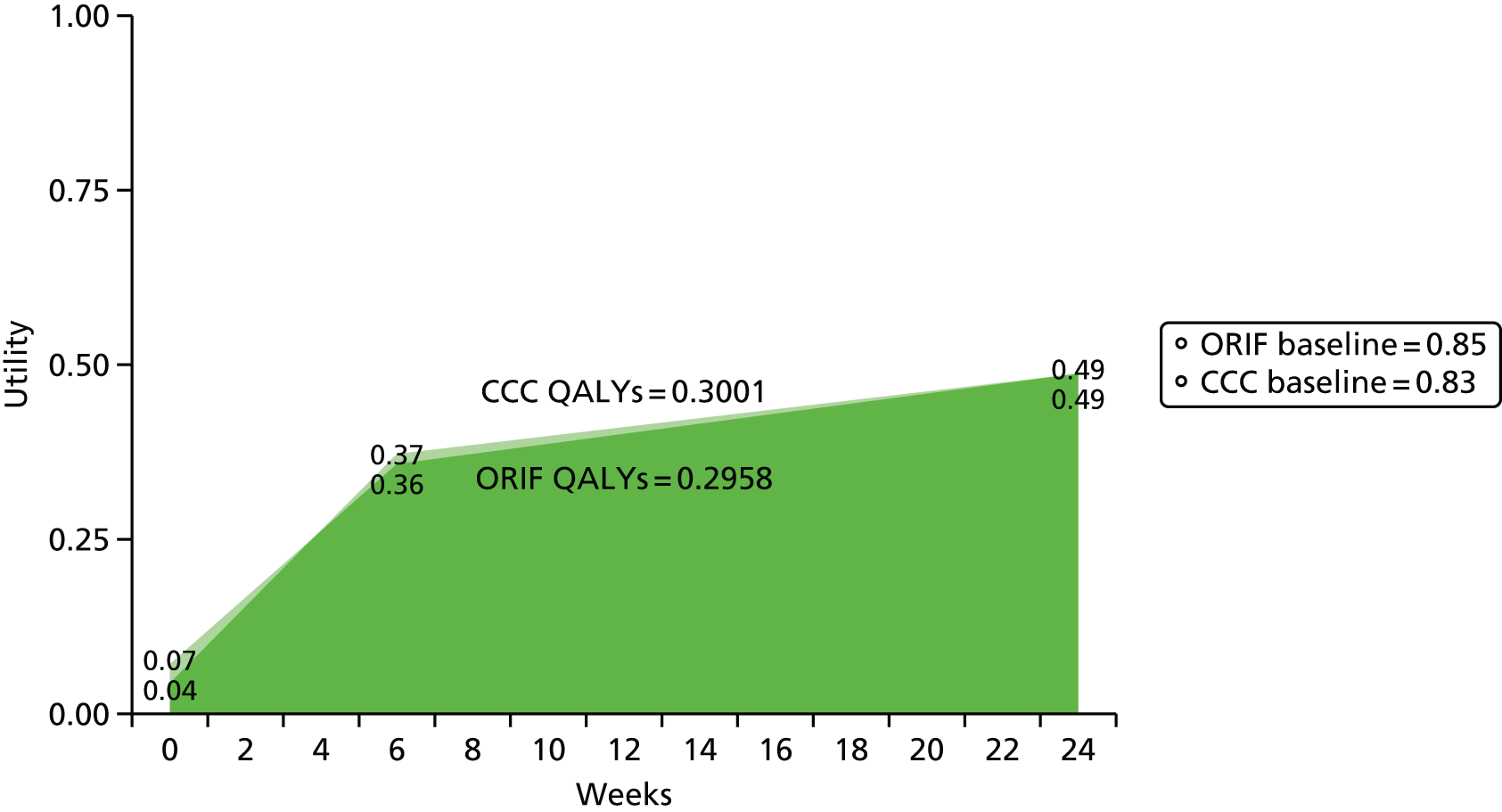
Costs (per protocol)
The mean raw, unadjusted costs of the resource uses using mean unit cost parameters for each group are presented in Table 42. Compared with the ORIF group, the CCC group had a large initial cost savings (–£1144), driven by savings in theatre time use (–£880), as well as a modest savings in care homes (–£137), that were eroded with costs in increased readmissions (+£448) and health services (+£435). Mean cost differences were negligible for additional procedure (+£8), casts (+£45) and medications (+£2).
| Resource | Treatment group | Difference, mean, £ (95% CI) | |
|---|---|---|---|
| ORIF, mean, £ (95% CI) | CCC, mean, £ (95% CI) | ||
| Index procedure | |||
| Theatre time | 1602 (1547 to 1657) | 722 (701 to 745) | –880 (–938 to –820) |
| Implant | 79 (72 to 87) | 0 (0 to 0) | –79 (–87 to –72) |
| LOS | 2667 (2134 to 3541) | 2482 (2165 to 2827) | –185 (–1120 to 493) |
| Total | 4348 (3807 to 5225) | 3204 (2886 to 3552) | –1144 (–2081 to –460) |
| Additional procedure | |||
| Theatre time | 86 (43 to 136) | 95 (48 to 147) | 8 (–60 to 77) |
| Implant | 4 (2 to 6) | 4 (2 to 7) | 0 (–3 to 4) |
| Total | 90 (45 to 142) | 99 (50 to 154) | 8 (–63 to 81) |
| Readmission | |||
| Theatre time | 37 (15 to 64) | 278 (197 to 365) | 241 (155 to 332) |
| Implant | 0 (0 to 1) | 14 (9 to 20) | 14 (9 to 20) |
| LOS | 122 (46 to 218) | 315 (170 to 518) | 193 (15 to 413) |
| Total | 159 (70 to 272) | 607 (408 to 851) | 448 (219 to 713) |
| Casts | |||
| Number | 2 (1 to 3) | 47 (43 to 50) | 45 (41 to 49) |
| Care homes | |||
| Community hospital (days) | 547 (253 to 902) | 455 (270 to 665) | –92 (–489 to 270) |
| Intermediate care (days) | 49 (2 to 129) | 88 (19 to 184) | 39 (–69 to 151) |
| Nursing home (NHS) (days) | 96 (25 to 190) | 38 (1 to 87) | –57 (–160 to 27) |
| Nursing home (private) (days) | 60 (0 to 141) | 33 (0 to 82) | –27 (–118 to 51) |
| Total | 752 (401 to 1165) | 615 (393 to 862) | –137 (–606 to 289) |
| Health services | 1,352 (1,075 to 1,670) | 1787 (1460 to 2164) | 435 (–27 to 904) |
| Health services (private) | 94 (52 to 150) | 68 (44 to 95) | –25 (–87 to 27) |
| Medications | 5 (4 to 6) | 7 (4 to 12) | 2 (–1 to 7) |
| Medications (private) | 4 (3 to 6) | 4 (3 to 5) | –1 (–3 to 1) |
| Work-days | 1058 (765 to 1381) | 1091 (695 to 1558) | 33 (–482 to 576) |
| Total societal cost | 7864 (6926 to 9027) | 7528 (6662 to 8453) | –335 (–1767 to 1005) |
| Total NHS cost | 6648 (5744 to 7777) | 6,332 (5599 to 7127) | –316 (–1661 to 919) |
Societal cost differences between the CCC and ORIF groups were negligible across private nursing homes (–£27), private health services (–£25), out-of-pocket medications (–£1) and work days (+£33).
Over the 6-month trial period, the CCC group had a mean cost savings to the NHS of –£316. The mean cost saving to society was –£335. Figure 16 presents the cost burden for the CCC and ORIF groups for the cost categories. The cost burdens, from largest to least, were the primary procedure, health services/medications, care homes, readmission, additional procedure and then casts. The figure represents the total cost burden; 360° represents around £6600. The cost savings in the CCC group is represented by the white space, where a full circle fails to fill.
FIGURE 16.
Pie chart of per-protocol NHS costs for (a) ORIF and (b) CCC, grouped by primary procedure, health services/medications, care homes, readmission, additional procedure and casts. CCC displays cost savings represented by the white space.

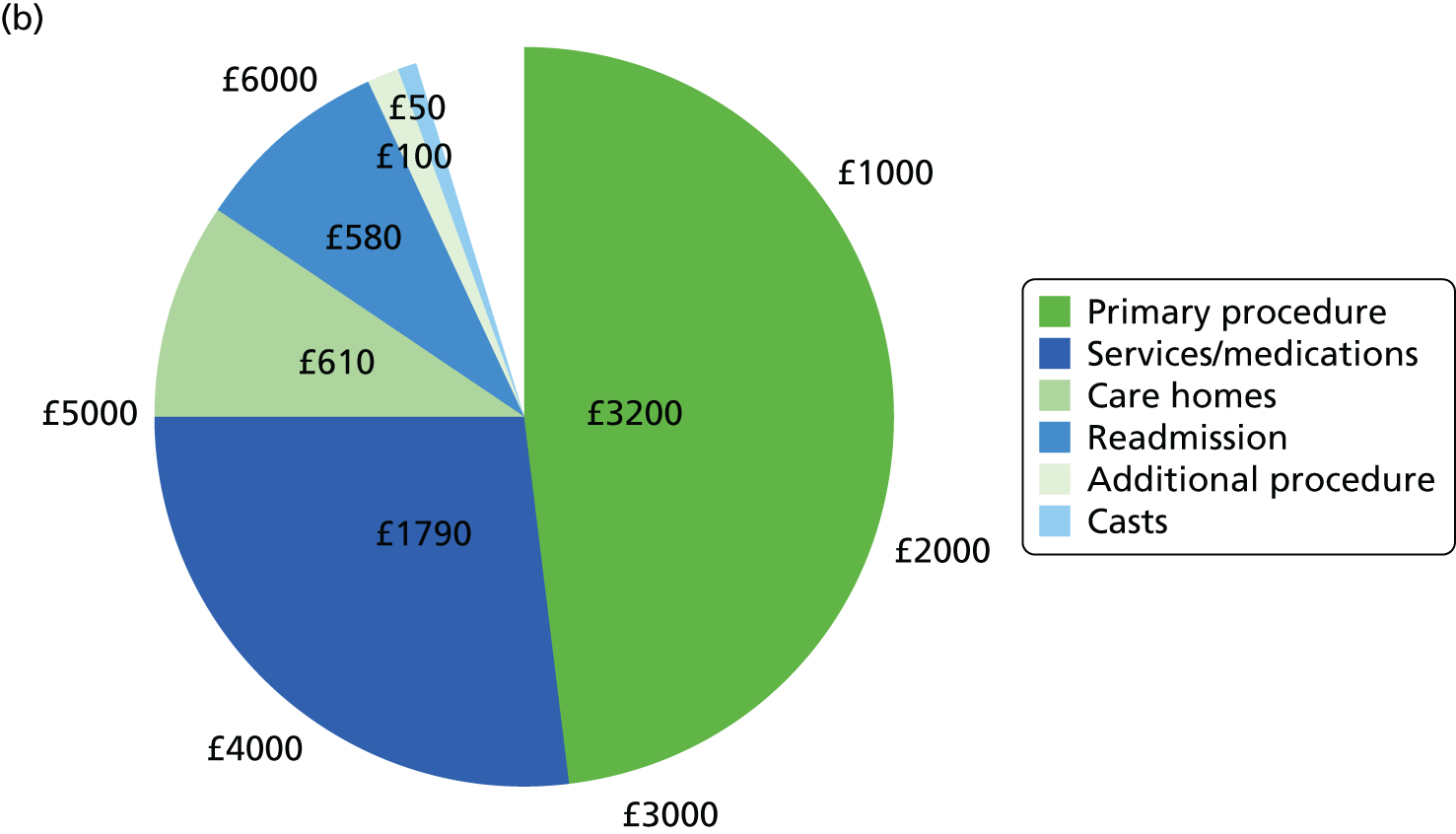
Regression models (per protocol)
Total societal and NHS costs, as well as QALYs, were obtained by fitting a GLM and adjusting for baseline characteristics. This sections details the results of the models adjusted for the predictors and the resulting estimates after marginal prediction and bootstrapping.
The GLMs dichotomised the home care support (lives alone, does not live alone), walking aids (none, one or more) and walking distance (more than half a mile, half a mile or less) because of low counts in the more severe categories. Using the Modified Park’s and Hosmer–Lemeshow tests, appropriate families and links were determined for each GLM. The gamma family and identity link were identified for total costs, whereas the Gaussian family and identity link (OLS) were identified for the total QALYs.
Regression coefficients for total NHS and societal costs are presented in Tables 43 and 44. Regression coefficients for total QALYs are presented in Table 45.
| Variable | GLM | ||
|---|---|---|---|
| Estimate | Standard error | p-value | |
| Intercept | 4269 | 3532 | 0.23 |
| CCC | –626 | 364 | 0.09 |
| Age | 92 | 33 | 0.01 |
| Female | 618 | 402 | 0.12 |
| Baseline utility | 126 | 2177 | 0.95 |
| Baseline OMAS | –53 | 22 | 0.02 |
| Hypertension | 506 | 399 | 0.21 |
| Asthma | –863 | 501 | 0.09 |
| Diabetes mellitus | 1472 | 924 | 0.11 |
| Ex-smoker | –299 | 405 | 0.46 |
| Smoker | –667 | 358 | 0.06 |
| Home support: does not live alone | –1767 | 523 | 0.001 |
| Walking aids: one or more | 2987 | 1291 | 0.02 |
| Walking distance: < 0.5 mile or worse | 2387 | 903 | 0.01 |
| Variable | GLM | ||
|---|---|---|---|
| Estimate | Standard error | p-value | |
| Intercept | 16291 | 4297 | < 0.001 |
| CCC | –637 | 459 | 0.17 |
| Age | –16 | 39 | 0.68 |
| Female | 513 | 518 | 0.32 |
| Baseline utility | –5 | 2628 | 1.00 |
| Baseline OMAS | –80 | 27 | 0.00 |
| Hypertension | 134 | 488 | 0.78 |
| Asthma | –1319 | 644 | 0.04 |
| Diabetes mellitus | 2116 | 1155 | 0.07 |
| Ex-smoker | –408 | 523 | 0.44 |
| Smoker | –1050 | 463 | 0.02 |
| Home support: does not live alone | –2468 | 645 | < 0.001 |
| Walking aids: one or more | 2696 | 1405 | 0.06 |
| Walking distance: < 0.5 mile or worse | 2798 | 1037 | 0.01 |
| Variable | Estimate | Standard error | p-value |
|---|---|---|---|
| Intercept | 0.778 | 0.064 | < 0.001 |
| CCC | –0.011 | 0.009 | 0.22 |
| Age | 0.001 | 0.001 | 0.48 |
| Female | 0.022 | 0.010 | 0.04 |
| Baseline utility | –0.106 | 0.040 | 0.01 |
| Baseline OMAS | 0.000 | 0.000 | 0.31 |
| Hypertension | –0.019 | 0.009 | 0.04 |
| Asthma | –0.006 | 0.013 | 0.68 |
| Diabetes mellitus | –0.003 | 0.016 | 0.86 |
| Ex-smoker | 0.023 | 0.011 | 0.04 |
| Smoker | –0.004 | 0.009 | 0.65 |
| Home support: does not live alone | 0.003 | 0.010 | 0.74 |
| Walking aids: one or more | 0.041 | 0.016 | 0.01 |
| Walking distance: < 0.5 mile or worse | 0.043 | 0.014 | 0.002 |
Cost-effectiveness: 6 months (per protocol)
NHS perspective
After resampling the trial population and unit costs, refitting the GLMs and using marginal estimation, mean total NHS cost and total QALYs for the CCC and ORIF groups were generated. The results of the cost-effectiveness outcomes are presented in Table 46. The mean total QALYs for CCC and ORIF were 0.30 and 0.29, respectively, while the mean total NHS costs were £6050 and £6694, respectively.
| Outcome | Treatment group | Difference, mean (95% CI) | |
|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | ||
| NHS costs (£) | 6694 (5285 to 8465) | 6050 (4711 to 7744) | –644 (–1390 to 76) |
| Societal costs (£) | 8003 (6483 to 9906) | 7320 (5815 to 9167) | –683 (–1851 to 536) |
| QALYs | 0.2928 (0.2714 to 0.3116) | 0.3034 (0.2853 to 0.3209) | 0.0106 (–0.0158 to 0.0381) |
The incremental differences favoured the CCC group. The CCC group had higher mean total QALYs (+0.01) and lower mean total NHS cost (–£644). At the mean for an ICER of £25,000/QALY, as CCC was both more effective and less costly, it dominated ORIF.
To assess the variability of the estimates, we plotted the incremental costs and incremental QALYs on the cost-effectiveness plane (Figure 17). The cost-effectiveness plane summarises all 10,000 bootstrap estimates. The cost-effectiveness plane shows that the large majority of incremental costs are negative; the incremental QALYs, however, show more uncertainty with a modest proportion being negative.
FIGURE 17.
Per-protocol cost-effectiveness plane showing 6-month incremental total NHS costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.

The variability across the range of willingness-to-pay thresholds was summarised with a cost-effectiveness acceptability curve (Figure 18). Over a common willingness to pay, CCC displayed a high probability of cost-effectiveness (> 95%).
FIGURE 18.
Per-protocol 6-month cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the NHS perspective. The dotted line presents the asymptote where the willingness to pay is infinite.
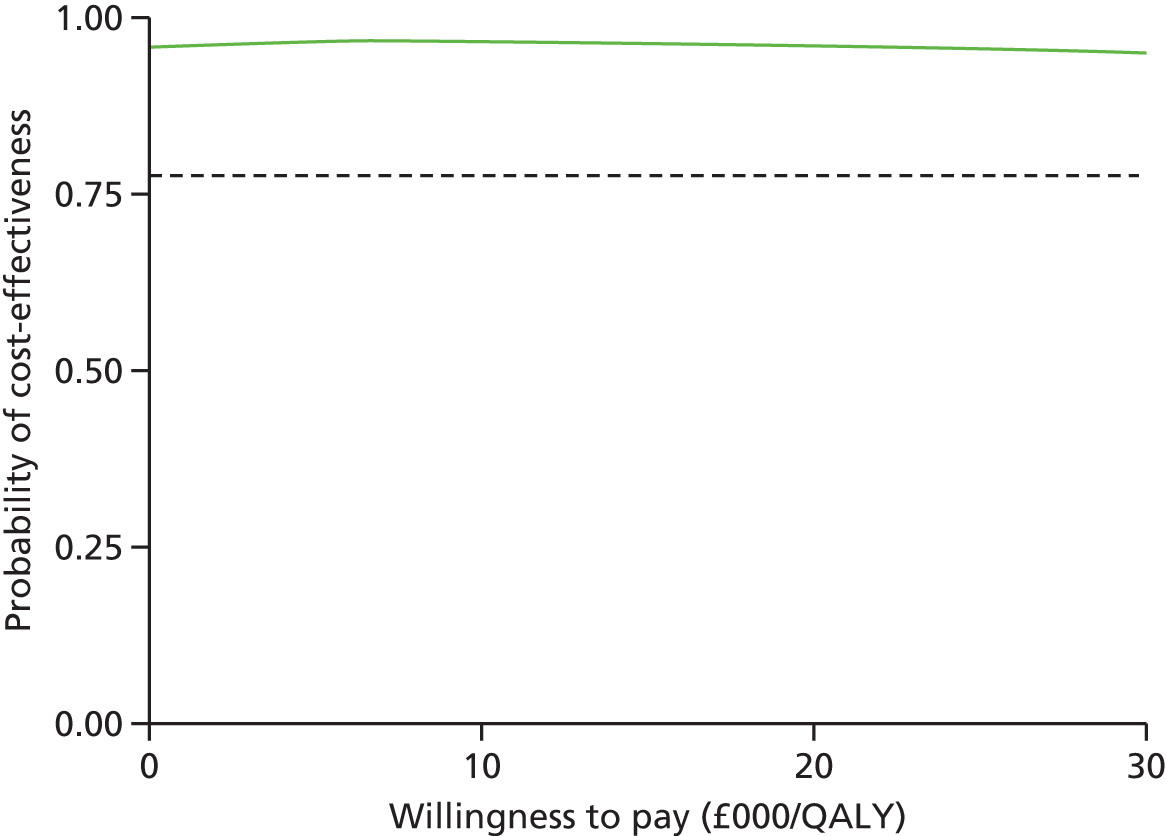
Societal perspective
The mean total QALYs remained the same for both the NHS and societal perspectives. Costs, however, differed. After resampling the trial population and unit costs; refitting the GLMs and using marginal estimation, mean total societal cost for the CCC and ORIF groups was £7320 and £8003, respectively (see Table 46).
The incremental differences favoured the CCC group. The CCC group had a lower mean total societal cost (–£683) and unchanged higher mean total QALYs (+0.01). At the mean incremental cost and QALYs for an ICER of £25,000/QALY, CCC dominated ORIF as it was both more effective and less costly.
The cost-effectiveness plane displayed modest variability in incremental costs (Figure 19). However, the large majority of incremental costs were negative. The incremental QALYs also displayed a modest proportion of negative estimates.
FIGURE 19.
Per-protocol cost-effectiveness plane showing incremental total societal costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.

Over a common willingness to pay, CCC displayed a high probability of cost-effectiveness (around 85%) (Figure 20).
FIGURE 20.
Per-protocol cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the societal perspective. The dotted line presents the asymptote where the willingness to pay is infinite.

Lifetime (per protocol)
There was no difference in QALYs between ORIF and CCC by the end of the trial period. Although return to baseline quality of life (utility) was not complete, it was nearly the same at each time point. Therefore, the QALYs, quality of life and the rate of its recovery were the same with no evidence of divergence. Based on this evidence, there was no justification for modelling long-term QALY differences between CCC and ORIF.
Implant removal may have resulted in an additional £79 to the ORIF cohort. This would result in an additional £79 in mean cost savings to the NHS and society. We did not repeat all analyses to include this as it did not affect the magnitude of incremental differences. In addition, it would be applied in the same direction (cost savings) and thus conclusions would be insensitive.
Sensitivity
Intention to treat
Results were robust to the inclusion of protocol violators. Using the ITT analysis, incremental NHS costs (–£692), incremental societal costs (–£773) and incremental QALYs (+0.01) were virtually the same to the per-protocol results. Decision certainty was also similar. Full results are provided in Appendix 3.
Imputing completely missing cases
Results were robust to the inclusion of completely missing cases (53 participants). These participants were included after imputing their entire history on the basis of baseline characteristics and index procedure resource use. Full results are provided in Appendix 3.
Ordinary least squares models in place of best-fit generalised linear model models
Ordinary least squares models of total NHS cost, total societal cost and total QALYs had little impact on the estimation of incremental costs (NHS –£613; societal –£420). CCC remained cost saving at a similar magnitude.
Discussion
An economic evaluation of CCC compared with ORIF for ankle injury management was conducted. Over the trial period, CCC showed evidence of a modest mean cost savings (around £400–700) to both the NHS and society. The cost savings were driven largely by savings in index procedure theatre time, but were eroded over time by increased readmission and health services costs. Incremental QALYs following CCC were no different from those following ORIF, with evidence leaning towards a slight advantage with CCC. Over common willingness-to-pay thresholds, the probability that CCC was cost-effective was very high (> 95% for NHS and 85% for societal perspective). Results were robust to the use of per-protocol or ITT populations, the use of standard OLS models instead of best-fitted GLM models and the inclusion of completely missing cases via multiple imputation.
The AIM trial was a randomised controlled trial. Despite randomisation, there was evidence of differences in utility prior to injury. The CCC group had a lower utility prior to injury than the ORIF group. This suggests a lower baseline quality of life. Other differences such as utility at presentation and baseline walking distance, albeit not statistically significant, represented imbalances that effect the estimation of mean total and incremental costs and QALYs.
The bootstrapped, adjusted results showed greater mean cost savings than the raw, unadjusted trial results. Without adjusting for imbalances in baseline characteristics, whether or not statistically significant, the estimated total and incremental costs can be biased. The GLM model results show that small differences in baseline characteristics can contribute to important differences in total cost. This was particularly the case for baseline walking distance and walking aids statuses, among others.
The NHS and societal perspectives showed comparable incremental differences in total cost at the mean. Uncertainty was slightly greater using the societal perspective. This led to slightly greater decision uncertainty as visualised by the cost-effectiveness acceptability curve (see Figures 18 and 20). However, incremental costs remained largely negative and the probability of cost-effectiveness for CCC remained very high.
Incremental total costs and QALYs were robust to whether the per-protocol or ITT population was used. Results with the ITT population showed cost savings to the NHS and society at similar magnitudes to the per-protocol population. The probability of cost-effectiveness of CCC was also similarly very high. The best-fit models also showed comparable results to the OLS models for the ITT population, as they did for the per-protocol population.
Incremental total costs and QALYs were also robust to whether completely missing cases were excluded or included using multiple imputation. CCC continued to show a high probability of cost-effectiveness over the common willingness to pay.
On average, CCC was followed by more rehospitalisation, because of loss of fracture reduction and conversion to ORIF, as previously shown in the clinical results. Cost increases following CCC also occurred outside hospitalisations. A substantial proportion of cost increases related to increased health services use in the follow-up period. CCC appeared to require additional health services following discharge.
We did not find justification to extrapolate trial outcomes or incorporate modelling of new outcomes to a lifetime. Utility was the same at each time point. This suggested that quality of life, its rate of recovery and QALYs were the same. There was no clinical reasoning for a difference in life expectancy between CCC and ORIF management. Implant removal because of local irritation may be an incremental cost to ORIF; however, it did not affect conclusions of incremental costs or QALYs. Given the evidence on functional assessments, quality of life, life expectancy and implant removal, there was not enough justification to model long-term differences.
Radiological evidence did identify a possible signal of increased malunion associated with CCC. Malunion has been suggested as a factor in the development of long-term osteoarthritis. 74 However, there is uncertainty regarding the rates and severity of post-traumatic osteoarthritis in relation to malunion after fracture because of a lack of clinical data. A recent review highlights the poor quality of the available evidence, with limited basic scientific knowledge and a dependence on retrospective case series. 75 We are conducting extended follow-up of the AIM trial cohort to address some of these uncertainties. When completed, this additional phase of follow-up will allow us to conduct modelling for any explicit long-term differences between ORIF and CCC regarding quality-of-life recovery, functional recovery, development of osteoarthritis or whether increased health service use with CCC is sustained or increases.
There are two other relevant cost-effectiveness studies. 76,77 Slobogean et al. 76 conducted a cost-effectiveness analysis of ORIF versus non-operative treatment for unstable ankle fractures in a Canadian setting. There are several differences in the design. Although Slobogean et al. 76 also used a trial-based evaluation, the trial randomised far fewer patients (81 vs. 620). In addition, the non-operative intervention did not include CCC. Slobogean et al. also considered a narrower perspective, only the procedure and rehospitalisations for reoperation or, in the case of the non-operative arm, secondary ORIF. In contrast, the AIM trial also included care home stay, health services, medication and productivity. Owing to their exclusion of other important resource issues, Slobogean et al. found a much larger short-term (index year) cost savings with non-operative treatment [CAD4512 (2014, GBP2718)]. This is still several times larger than just the hospitalisation costs of the present analysis. This may be because of the underlying differences in unit resource costs between Canada and the UK, as well as the use of summary unit cost estimates of different hospitalisations in contrast to the present study, which microcosted actual resource use.
Slobogean et al. 76 found mean incremental QALYs to be higher with ORIF (+0.02). They did not report uncertainty on incremental estimates or cost-effectiveness planes. However, the SDs of the total QALYs suggests imprecision nearly six times greater than the present study. Thus, incremental QALYs of +0.02 were likely highly imprecise across negative and positive space. In addition, QALYs were not similarly adjusted to baseline utility, which is important when estimating differences for cost-effectiveness (see Chapter 2). A simple plot using their Short-Form questionnaire 36-item physical component summary scores would show ORIF with consistently higher scores from time at presentation all the way through 1 year, compared with non-operative treatment. 78 This is, however, just one domain of health-related quality of life. Utility at each time point was not reported and thus it is not possible to explain the nature of the (imprecise) QALY difference in the Slobogean et al. analysis.
Slobogean et al. 76 concluded that ORIF was cost-effective in the long term (lifetime). The long-term model included only osteoarthritis and the assumed reconstruction following this event. ORIF became cost-effective because non-operative treatment gave way to osteoarthritis on the basis of higher malunion. However, osteoarthritis was modelled expressly on malunions not on ankles in which joint congruence had been achieved. In this way, it was assumed osteoarthritis could only follow malunion. However, normal anatomical alignment does not preclude osteoarthritis, neither does malunion mandate arthritis. The evidence does not support a non-significant absolute risk difference on the order of 50% between malunited and congruent ankles (50% vs. 0%), which Slobogean used. Moreover, the study which was used to derive this estimate was not a comparison of malunited and congruent ankles; it used a small sample of malunited ankles that underwent surgical reconstruction. 79 Slobogean also assumed a stable QALY benefit over the lifetime. The highly imprecise difference in QALYs at the end of the index year was simply assumed the same for all ensuing years in those without arthritic development. It is not clear that this is the most appropriate base case, given the difference was very imprecise and reasoning to justify how differences are sustained was lacking.
Michelson et al. 77 conducted a model-based evaluation of ORIF and traditional casting separately for unstable and stable ankle fractures in a US setting. There were a number of limitations. The only outcomes were infection following ORIF and osteoarthritis following either ORIF or casting; all removals, reoperations and rehospitalisations were not considered. In addition, as infection was just 1%, the driving force in the model was solely the development of arthritis. However, its cost was excluded from the analysis as well as everything outside of the index ORIF/casting hospitalisation cost. Michelson et al. estimated a 25% higher absolute risk of development of arthritis following casting than with ORIF (30% vs. 5%). However, the reasoning for this very large difference was not provided and neither was the source for this estimate. This contrasts to the evidence provided by Koval et al. 66 in 33,704 elderly patients with ankle fracture; the incidence of arthroplasty or arthrodesis (procedures assumed to be performed for osteoarthritis) was less than 1% for both operative and non-operative treatment. The Michelson et al. analysis assumed that quality of life was different in the presence of arthritis (and infection, although that branch’s effect is negligible to begin with) but otherwise equivalent between ORIF and casting. However, it is not clear the utilities employed were valid as they were not on the (0,1) range; no source was provided. The large difference in arthritis gave way to a large difference in utility and quality of life. These were applied at baseline and extended over the lifetime, this led to 0.96 greater QALYs attributed to ORIF for a 70-year-old with unstable fracture. Incremental costs for unstable fractures were not presented. Decision uncertainty was likewise not presented for unstable fractures. The Michelson et al. analysis presents evidence of low comparability because of limitations in its design and evidence base.
Summary of key findings
-
The primary economic analysis used the per-protocol population, excluding cases without any valid information.
-
There were no differences in QALYs between CCC and ORIF. However, management with CCC led to a mean cost savings greater than £600 under both NHS and societal perspectives (total £1327). The probability that CCC is cost-effective was very high (NHS > 95% and societal 85%) at common willingness-to-pay thresholds.
-
Results were robust to the use of the ITT population, OLS models in replace of best-fit GLM models and the inclusion of completely missing cases via imputation.
Chapter 5 Qualitative study findings
Introduction
A qualitative study was embedded into the AIM trial to investigate patients’ experiences of living with a fractured ankle and experiences of being in a trial. This chapter outlines the background of this element of the trial, describes the approach and methods used before presenting and discussing the findings.
Background
Researching patient experience is crucial to understanding how patients make sense of injury and recovery and how interventions impact on their daily lives. As a form of evidence, experience has utility when translating interventions into practice. 80 For example, McInnes et al. ,81 through a qualitative meta-synthesis of older people’s experience of falls interventions, demonstrated that participants had complex and varied stories with many factors influencing acceptance of risk status and preference for intervention along with a strong need to maintain control and fit interventions into everyday life. Experience evidence is also valued as an integral part of NICE guidance. Staniszewska et al. 82 from development of the Warwick Patient Experience Framework have generated quality standards around knowing the patient, tailoring care, continuity of care, participation and essential care that require consideration alongside other sources of evidence. Interventions for orthopaedic trauma are increasingly incorporating patient experience within studies of clinical effectiveness and Gooberman-Hill and Fox83 suggest this will benefit both trial design and the suitability of interventions from provider and service user perspectives.
Understanding how participants make sense of trials within their acute hospital care is important evidence for the design of future clinical trials in trauma. A study exploring participant experiences from a range of trials suggested that they take part in trials both for altruistic reasons and because of perceptions of personal benefit; however, personal benefit may take precedence. 84 Acceptance and understanding of the principles underlying trials in a clinical setting can be problematic, leading to therapeutic misconceptions. 84,85 Robinson et al. 86 identify the difficulty lay participants have viewing randomisation or equipoise as acceptable in a clinical setting in which they would prefer an interaction based on what is best for them. Other trial participants experience a sense of indifference to the interventions, particularly if they were satisfied with their treatment, and did not perceive any active harm as a consequence of the intervention. 85 Rejection of randomisation and uncertainty over outcome were reasons for declining to take part in a trial,85 as were perceptions of burden and concern over side effects of interventions. 84 How participants are informed about trials is therefore crucial. Based on their trust in clinicians, participants may pick up cues that subtly coerce them into consenting to take part in trials. 85 In this study, participants’ experience in relation to consenting to the trial and being randomised to CCC and ORIF was explored.
Current research evidence on patients’ experience of traumatic orthopaedic injury presents a broad range of emotional, physical, social and financial impacts on participants’ lives over prolonged periods of time. The experience of being hospitalised as a result of a traumatic orthopaedic injury is emotionally and physically demanding, where patients cope with feelings generated from the incident, the impact on their bodies and live with the uncertainty of full recovery. 87 Patients struggle to feel hopeful, often oscillating between hope and hopelessness as they focus on the immediate future aiming to progress their recovery and return home. 88 Evidence from patients who have experienced a broad range of injuries, the majority of whom had been hospitalised, reveals a range of experience that includes omissions in care, a struggle to cope at home with little social support, continuing pain, low emotional states, a general loss of confidence and a more cautious approach to life; physiotherapy is seen as beneficial for those who received it. 89 Patients interviewed up to 2 months after lower limb injury struggled with pain, fear and anxiety with a feeling of loss of control over their lives but had a strong desire to return to normal. 90 Trickett et al. 91 demonstrated that patients with open tibial fracture interviewed at least 15 months post injury suffered from pain, stiffness and discomfort, had not regained their normal mobility, felt fearful of many aspects of their recovery including falling, were concerned about their appearance and were anxious about their employment and finances as a result of being injured. Forsberg et al. 92 included ankle fracture in their sample of participants with surgery for lower limb fracture interviewed 1 month to 1 year after surgery. The participants identified a range of strong emotions such as anger, frustration and remorse in relation to the incident; anxiety, vulnerability and pain through the surgical period; and helplessness, fear of falling, and insecurity with use of equipment once discharged from hospital.
McPhail et al. 12 focused specifically on adults under 60 years of age from 6 to over 24 months post ankle fracture in order to develop an outcome measure. The findings suggest that participants had or were suffering from a range of impacts: reduced mobility, increased anxiety, a reduction in participation in activities and social life, disruption and sometimes change of work life, loss of earnings, weight gain and loss of ability to wear preferred shoes and some need for medication. Overall, patients experienced traumatic orthopaedic injury as a struggle to return to normal life. Evidence pertaining directly to ankle fracture is limited and there is a gap in relation to the older participant and different forms of treatment. The quality of the studies regarding description of their methodology is often limited; the studies cover a range of time frames since injury and do not take into account change in perceptions over time. This study aimed to explore older participants’ experience of ankle fracture and the acceptability of two interventions, CCC and ORIF.
Methodology
The lack of understanding around patients’ experiences of living with a fractured ankle and treatment led to the research questions.
-
What are participants’ experiences of ankle fracture in the first 6–10 weeks after treatment?
-
What are participants’ experiences of being in a clinical trial?
In order to answer these two questions a naturalistic approach drawing on the principles of Heideggerian phenomenology was utilised. Phenomenology is commonly used in health sciences to facilitate exploration of how participants come to know and understand the world through their embodied experience of what it is like to be in the world. It focuses on gaining ‘a deeper understanding of the nature of meaning in our everyday experiences’ (p. 9). 93 Todres and Wheeler94 suggest it grounds research in the reality of human experience harnessing the reflective skills of the researcher. Key tenets are (1) being in the world, (2) forestructures, (3) temporality and (4) space.
-
Being in the world is conveyed through the notion of dasein (being or presence) in which subject and object are inseparable and human beings exist within the framework of the world. Being ‘is to be shown as it is proximally and for the most part – in its average everydayness’ (pp. 16–17). 95 Research focuses on what it is like in the life-world of the other; or the ‘understandings, feelings and perceived relationships i.e. all the everyday phenomena of common experience’ (p. 3). 94
-
Forestructures reflect the notion that knowledge and meaning is founded on the social, cultural and historical context of the person. The researcher through description and interpretation uncovers what is already in existence and thus understanding leads to meaning. ‘Our investigation itself will show that the meaning of phenomenological description lies in interpretation’ (p. 61). 95 Meaning is further enhanced by a cycle of understanding, from the part to the whole, often called the hermeneutic circle by researchers. 96
-
For Heidegger, temporality is an integral part of being human as the past, present and future are part of the life world ‘time must be brought to light – and genuinely conceived – as the horizon for all understanding of being and for any way of interpreting it’ (p. 39). 95
-
How people are situated in relation to space is also important; this may be spatially but also what is brought to the fore in relation to ‘concern for’ or what merges into the background. This involves listening to what participants say to identify what is important to them in relation to the phenomena. 96 Madjar and Walton97 refer to the listening gaze as a phenomenological way of being: openness to what is unknown or taken for granted in order to develop a better understanding of the other.
Methods
The method used was unstructured interviews, a focused conversation where the interviewer keeps the question open yet focused on the phenomena and the interviewee becomes coinvestigator in the study. 93 In this study there were two questions:
-
What has it been like for you since you fractured your ankle?
-
What was it like being part of a trial?
These were followed with prompts such as: tell me what that was like?; how did that feel?; what were you thinking at that point?; what helped/did not help at that stage? Written and verbal information about the study was provided to participants and they had at least 24 hours to consider their participation. Written informed consent was obtained from the participants prior to interview.
The interviews were situated within the outpatient clinic at the 6- or 10-week clinic visit to minimise participant burden. It was considered a good time for the interview as participants’ experiences of treatment and the trial would be relatively fresh. In retrospect, the clinic environment was busy and interruptions were common. For the participant the 6-week visit involved a radiograph, consultation, plaster removal and trial questionnaires; delays to radiography, transport arriving unexpectedly and interruptions curtailed six of the interviews. However, participants in general were keen to be interviewed and pleased not to have to make a separate visit for the interviews and tolerated interruptions.
Sample
A purposive sample of 36 study participants from the main trial from two sites (Bristol and Oxford) that provided a range of ages, male and female, both treatments and a breadth of experience was obtained. From the sample 27 participants were female and nine were male; 27 were interviewed at 6 weeks and nine at 10 weeks post intervention; 24 were interviewed at Oxford and 12 at Bristol; the age range was 60–80 years, with an average and median age of 67 years; 21 received CCC and 15 received ORIF; the sample were all white, British and ethnic minorities were not represented; and the interview duration ranged from 11 to 78 minutes (average, 34 minutes; median, 33 minutes).
Analysis
The interviews were transcribed verbatim. Analysis developed an understanding of the other study participants through reading, listening and writing, focusing on the life-world, exposing what exists in the form of meaning, being alert to temporality and what is of concern to participants living with a fractured ankle. Units of meaning were identified from the words and phrases participants used, units with similar meanings were gathered together under researcher-generated categories such as ‘being vulnerable’ and then drawn together into themes such as ‘getting on with daily life’ which Van Manen93 calls the ‘structures of experience’ (p. 79). Analysis was facilitated through writing and rewriting as ‘writing teaches us what we know and in what way we know what we know’ (p. 127). 93 Constant reflection took place on the meanings and how they were similar to, or different from the theme, and in relation to the findings as a whole, drawing on the principles of the hermeneutic circle. 96 Through this process a deeper understanding of the phenomena was obtained while staying ‘true to text’ (p. 57) and the experience of the participants. 98 Data saturation was achieved in all themes. Help with organising the large amount of data was provided by NVivo version 10 (QSR International, Warrington, UK).
Rigour
Rigour in the study was demonstrated, drawing on Lincoln and Guba99 and their notion of trustworthiness. The researcher engaged with the data over a prolonged period of time; data collection took place from October 2012 to September 2013 and used verbatim quotes. A range of experiences was presented to facilitate resonance with the reader and transferability of the findings. Auditability was ascertained through clarity of the research processes. The researcher brings their own ‘being’ to the research and this influences the process of the research and interpretation of the research data: ‘Thus to work out the question of being adequately, we must make an entity – the enquirer – transparent in his own being’ (p. 27). 95 Reflection, field notes and use of memos through the research process provided ways in which the researcher made these explicit. In this study, the researcher brought her own experience of other concepts such as comfort, pain, participation and hope to the study; for example, when participants identified uncertainty in relation to healing there was a heightened awareness of their hopes and fears for the future.
Findings
The participants’ experience was conveyed through a core theme of struggling to live with a fractured ankle. This was further divided into four themes and nine categories:
-
Theme 1: suffering – categories (1) being vulnerable and (2) renegotiating roles and relationships.
-
Theme 2: getting on with daily life – categories (1) finding ways of doing things and (2) finding ways of keeping busy.
-
Theme 3: struggling to move – categories (1) living with hopping and (2) moving forward.
-
Theme 4: treatment and being in a trial – categories (1) living with a CCC, (2) living with metalwork and (3) decision-making.
Theme 1: suffering
Being vulnerable
The suddenness and unexpected nature of the fracture impacted on participants’ lives in terms of how they felt about themselves as being vulnerable and older people, what they could do, their roles and relationships. The experience was described as incapacitating, annoying, terribly frustrating, difficult, terrible, a shock, soul-destroying, unbelievably miserable, aggravating, boring and hard. Normal protective mechanisms of everyday life were removed and participants felt emotionally exposed. It was a shock for participants as they realised that they were vulnerable and dependent, were not ‘invincible’ (participant 17) and felt helpless which reminded them of their own parents:
I kept seeing myself as my parents.
Participant 12
Others felt worn out, extremely frustrated and angry that they had missed family events and holidays. Normally robust participants felt emotionally fragile and little things could tip the balance:
I just burst into tears because I so wanted to get in this bath.
Participant 21
Supportive family and thinking positively helped to contain these emotions; however, there was a sense of impending old age.
A shock. I sort of laboured under the illusion I might not be as old as I am for quite a long time and I also don’t drive and walk everywhere and I thought I was quite fit. I didn’t expect to just slip on a patch of mud and break my ankle nor did I expect when I got in here and they did a whole lot of tests that I wasn’t really quite as fit as I thought. I wasn’t ill but I just wasn’t as I thought and all of a sudden it did occur to me that I was older than I thought I was in my head, it was a bit of a surprise . . .
Participant 30
Acceptance was considered a useful way of being in order to contain emotions, and stoically getting on with it was helpful for some: ‘I’ve just accepted it and that’s it’ (participant 18), although processing anger and frustration could be a slow process: ‘It does take a while to accept it’ (participant 21). It was also seen as part of old age ‘because of being old you have to accept help in any case’ (participant 1). Ways of living with and mitigating feelings of vulnerability were keeping positive, getting back to work, drawing on inner spiritual resources and strong social connections with friends and family.
Renegotiating roles and relationships
The sudden reduction in mobility and ability to undertake daily activities in a normal fashion created a dependency on others to maintain a household where routines and responsibilities had been maintained for sometimes up to 43 years. Normal roles, sometimes related to sex, and relationships were disturbed creating a concern that relationships might not be repairable. Participants felt strongly that support was required particularly in the first few weeks:
Without it I would be really, really struggling.
Participant 25
None of the participants enjoyed dependency; some felt they got used to it, while others felt increasingly frustrated:
I hated it . . . I did the caring, people didn’t have to look after me.
Participant 21
Role reversal was frustrating as they felt they had to tell their partner what to do, ‘Always telling him that, always telling him’ (participant 8), they felt they were ‘nagging’ and had become ‘she who must be obeyed’ (participant 7). Participants attempted to minimise their demands, demonstrating an empathetic insight into the impact on their partner.
You have to be very careful I think to not say too many things at once, not make too many demands at once because it’s very hard for that person looking after you anyway to know that you’re totally dependent on them for the shopping and the cooking . . . So they’re nerve wracked I think and under a lot of pressure.
Participant 12
Relationships could become tense and volatile as both parties were stressed and anxious about the uncertainty of the future and the change in roles that reduced their freedom to act independently:
It does play havoc with your marriage.
Participant 13
To balance this, the participants encouraged their partner to maintain their normal outside activities; several went on holiday, which was important for the relationship.
Regaining independence was tricky as new boundaries were renegotiated:
As I started to get back my mobility then we started to clash a bit.
Participant 36
Negotiating intimacy within the relationship was also problematic as the body was perceived as fragile and breakable.
Yes, it’s changed his life as well as mine. And as for one’s sex life? What’s that! . . . I think he’s almost been afraid to touch me as though I have become fragile because some bones have broken. I think I’m not going to break you know, it’s just my ankle everything is absolutely fine but he’s obviously worried, he’s concerned he’s lacking in confidence about it but who knows how long it will take to repair before it all starts to feel normal again.
Participant 7
Both partners appeared to be actively undertaking forms of emotional containment in order to maintain daily life. Friends and family provided supportive functions, which alleviated the burden of dependency.
Theme 2: getting on with daily life
Finding ways of doing things
The participants found that once they got home nothing was as normal and they had to rethink how to undertake the smallest activity. This involved reframing what they thought as normal and working through activities by trial and error or by planning ahead how they could be done. Helpful equipment supplied by friends and the NHS made life easier. Being creative and using imagination to think things through could lead to a sense of achievement.
You have to start being imaginative and do things. You just have to learn how am I going to go to the toilet and bathroom. How am I going to wash my hair, how am I going to shower . . . You felt like you had achieved something if you could do it and you had thought that process through and oh I can do this, I can do that.
Participant 22
Learning through experience was crucial, as was finding ways of achieving activities while also accepting what was not possible; transporting cups of tea was a regular example of what could not be done while hopping. Occasionally, some solutions did not work so well, leading to unexpected weight-bearing incidents:
I did manage to get in the bath but the stool I was using to sit on went from under me and that’s when I put the weight on my foot.
Participant 11
If it was not straightforward some participants did not attempt to bath or shower during their 6 weeks’ non-weight-bearing. Undertaking activities in a small space where there was very little room and a walking frame created worries about a possible fall. For many there was a sheer determination to maintain household standards, ‘It’s a representation of me’ (participant 26), even though it was difficult to do and took longer than usual. Finding ways of doing things was through experience and trial and error, and creative ways were found to do what would otherwise be normal daily activities.
Finding ways of keeping busy
The participants felt that their lives had suddenly lost any sense of freedom and spontaneity. They had to adapt to new activities or bring to the fore existing activities that could be undertaken within a confined environment. Participants who could draw on skills such as sewing, embroidery or computer-based work seemed to fare better in finding suitable occupation. However, planning was required even with these activities if they were to be maintained effectively:
Really it’s things just need a little bit more thinking about, just really the lack of freedom to get in my car.
Participant 26
However, manual-based or outdoor activities were impossible to achieve, such as woodturning, cycling, swimming, walking or clay-pigeon shooting:
It just stops, it can’t be done, that ended until it could be started again.
Participant 27
Creative ways of maintaining activities, such as practising the saxophone, were found using positioning and household items. Wheelchairs were a boon and enabled participants to move around freely inside and outside the home. However, some participants struggled to find suitable occupation and felt bored and frustrated:
I’ve laid there waiting for something to happen and then you’re doing nothing all day and in the evening you think I’ve got to go to bed again now.
Participant 25
Social connections were crucial:
She got me out without too much difficulty and I pruned my clematis which was getting on my nerves and that was a very successful feeling . . . I was triumphant doing that.
Participant 32
A new way of being was required that enabled participants to adjust mentally. The participants inhabited a smaller space both geographically and mentally, but were joyful when they found they could move out of this space.
So you just put yourself in a completely different zone, I can’t describe it, it’s not like, I don’t mean like meditating or something but your life becomes very small and within that you try to control it as much as possible I think, in terms of your comfort, your entertainment, you know all that stuff . . . It is only now that I think that yes that is what I was doing, I was in a different layer of existence – a parallel universe almost. I had switched off from everything normal and if I did do anything normal, it was a bonus, like wheelchair gardening . . .
Participant 12
Mental strategies were required to reframe the present and switch off from normal activities and refocus on what was possible within a confined physical space and reduced mobility.
Theme 3: struggling to move
Living with hopping
Struggling to move was problematic for all participants. The ability to walk was taken for granted and they felt disabled and prematurely old. Hopping was considered to be a nightmare or terrifying; no one found it a good experience and, although some adapted well depending on their fitness and agility, some could not adapt. Walking frames and crutches impacted on other areas of the body, upper arms, knees, palms, the ‘good leg’, often creating more pain than the actual ankle fracture. There were many accidental weight-bearing incidents or near falls:
I was nervous and twice I had times where my foot slipped.
Participant 36
I had trouble with both my knees, the doctor called it ‘housemaid’s knee’ . . . I had to hold on to the [walking frame] and they knew I was doing it wrong and I was terrified of falling on it and I was putting all my weight on it and ended up with sore arms. I was leaning right over it, I couldn’t help it and I was going fast, I couldn’t help it. A couple of times I went to the loo and had a couple of near misses, going over.
Participant 2
The participants found crutches were not easy to use – ‘But for about three weeks after I suppose I couldn’t cope with the crutches because my balance was all out and I was giddy and I felt nauseous’ (participant 11) – but got used to them over time and used them successfully once they felt better. Frames were in general accepted as providing more security, but neither device was effective on uneven surfaces. Participants indicated that finding appropriate footwear was a serious problem:
You move your foot back and your slipper doesn’t go with you and then you lose balance. I’ve had a few scares with that.
Participant 10
Participants found managing stairs very difficult and many, if they could, didn’t attempt them; some were terrified of falling down the stairs:
You look down from the top of the stairs and you think oh my God.
Participant 31
Often participants went up the stairs on their bottom and put a chair at the top of the stairs to enable them to get from sitting to standing. Getting about the house was a struggle.
Yes that’s right. I think people have been shocked to see me and to see how I have had to cope around the house. Well when they see my sort of ladder for getting up and down the main part of the house it suddenly struck them that this is quite a difficult thing to negotiate you know, and then having to go everywhere in a wheelchair.
Participant 7
Struggling to move could be seen as ‘the struggling body’ which left them tired and lacking in energy, and they often felt low and depressed; sunshine, visitors and exercise were good for their spirits.
Moving forward
Moving forward in terms of mobility was seen as real progress, a ‘breakthrough’ (participant 7), being able to move from the walking frame to crutches felt like freedom and they could see the future unfolding:
Yes and there you are looking at yourself 3 days ago I was in the boot and now I’m in shoes.
Participant 21
The move to weight-bearing was approached with caution; participants were weary of pain, and used the walker boot for support or when they needed protection. They were unsure how their ankle would function and fearful of causing damage:
I’m weary of it, I don’t want to break it or hurt it.
Participant 3
Learning occurred through experience and trial and error, with participants working out what they could and couldn’t manage:
It did send a bit of a shooting pain up the back of my leg.
Participant 31
The Sampson boot was seen as good for support and protection but not good for stairs. The body had come to the fore and there was a greater awareness of its fragility, the ‘fragile body’. There was a sense of watchfulness:
I think from here on in I might be more watchful about what I do.
Participant 29
Removal of the cast created a sense that the foot did not belong to them any more:
It doesn’t feel part of me any more . . . it feels as if it’s had bad cramp and there’s pins and needles kicking in, it feels a bit sort of tingly.
Participant 29
There was a concern about the future outcome of the intervention in relation to their return to normal activities and exercise.
There is worry yes of course and all the time you’re slightly worried, is it ever going to be the same again . . . Yes and will I be able to run, I’m not a big deal runner but I go for little jogs along the canal but not for hours on end but I want to be able to do that and I want to be able to go walking in the mountains, how I will feel about that I don’t know but more wary I think.
Participant 36
There was a general lack of confidence, fear of falling, or ability to prevent a fall, in relation to moving forward. Moving forward was something participants wanted to do but their bodies were fragile and they were now more watchful and protective, not wishing to cause any further harm. There was a feeling that confidence would slowly grow over time and physiotherapy was mentioned as helpful.
Theme 4: treatment and being in the trial
Living with a close contact casting
Living with a CCC was seen within the overall experience of struggling to live with a fractured ankle; with personal benefits of no surgery, no scar, less risk of infection, lack of further surgery to take the metalwork out. The cast itself was generally seen as comfortable, ‘quite comfortable and I had no pain at all’ (participant 10), with some anxiety about looseness as the swelling lessened leading to extra visits to clinic:
I thought I had better tell someone that I could move around in this.
Participant 32
Participants who experienced discomfort focused on heaviness and tightness due to swelling leading to pain. Participants actively managed their legs with elevation during the day when they could to control the tightness.
The first 2 weeks as soon as I put my leg over the bed I could feel the cast filling up and it felt like something was going to explode because I had to take my foot off the ground and do it in stages but that got better and it wasn’t too bad but I try to keep a happy medium, not being upright for too long and not being horizontal for too long, just trying to keep the blood evenly distributed.
Participant 31
They endured discomfort on the basis that they hoped their ankle would heal; they did not question the strength of the ankle without metalwork but wanted to walk again. They came into clinic to have the plaster changed if the pain was overwhelming. There was uncertainty about healing; radiographs were the only tangible evidence and there was anxiety about causing further harm. The experience of having the ankle remanipulated at 2 weeks was unexpected, but the evident skill of the surgeon enabled the participants to feel positive and secure about the outcome:
I was so impressed at how they did that, to get those bits back in just by feel, that’s amazing.
Participant 35
However, confidence was undermined for participants and they had a sense of security and protection staying within the home environment.
One thing I have discovered, I’ve done less because I’ve just wanted to stay home and stay safe, that’s the thing I really felt particularly when I came back the second week and it had moved so it had to be remanipulated, which was fun . . . I couldn’t think of anything I had done to move it, it wasn’t as though I had fallen over and that did knock my confidence and I think that’s why I tended to just stay home and potter around the house and not go out. I did find it very restrictive and I think certainly in the early days I got quite depressed, sometimes the thought of 6 weeks . . . it’s not something I would ever want to do again. I think if you break your arm you can still move around. Losing the use of a leg is really, really tough I think.
Participant 35
Crossover to metalwork was an emotional time for participants, who felt that they had endured a treatment at considerable cost to themselves and their partner; they were angry, disappointed and sad. They felt they had struggled enough and now had to find the energy to face surgery and another 6 weeks of non-weight-bearing:
I’ve just got to be brave and whether you can stand up to it, can you (to husband)?
Participant 14
They questioned the trial and the care they received as they tried to make sense of what had happened:
Yes, but the point I’m trying to make here is that if the doctor said at the outset you‘ve got a 5% chance of this working.
Participant 15
Participants endured the heaviness of the CCC and actively managed the swelling, pain and discomfort; part of the uncertainty of living with CCC was not knowing if healing was occurring and the anxiety that they might further damage their ‘fragile body’.
Living with metalwork
Having surgery and metalwork was accepted as normal by participants and others in their family; often they knew someone with metalwork. Some would rather not have scars or ‘some foreign body stuck in there’ (participant 10). Concerns were that the metalwork would go rusty, break, restrict movement, cause allergic reactions or that the pins would require taking out. There was a sense of finality about metalwork that enabled participants to feel that at least the ankle was fixed and had additional strength, ‘The plate must have been better because its strengthened the bone’ (participant 3), which avoided the uncertainty of not knowing it had healed. This was set against the background of advancing age, in which time was precious.
It doesn’t really bother me, I mean if I was younger it might but I’m just so glad that people tell me it’s the best way really to get you on your feet quickly because it’s so fixed and you can’t really do damage you know following the op. Well obviously you can but you know if you follow the rules you’re pretty safe and so I was glad about that in the end, life’s too short to spend months and months and months around the house worrying about whether or not the bone has knitted properly but I’ve no idea if that is the case . . . Yes, a quick fix. The way that the surgeons you know are always saying ‘I fixed you up. You’ll be fine now. That all went very well and off you go’.
Participant 12
Both groups had pain at injury, and postsurgery pain was experienced particularly in the first 2 weeks. There were problems with controlling the pain, although some participants were surprised to have no pain at all: ‘No I wasn’t in any pain at all after the operation’ (participant 11). Both treatment groups had problems with uncontrollable shooting pains that came unexpectedly and were incapacitating and could not be controlled with medication. For some this increased in frequency on weight-bearing.
It’s not that painful when you walk on it – it’s ooh that feels a bit uncomfortable but then I’ve been woken up typically twice a night 1.30 and 4.00 o’clock is the usual time in absolute agony and that went on for about the first week after I was weight-bearing and I presume it’s because when sleeping all these healing processes are taking place . . . my goodness the pain is awful and wakes you up even though I’ve usually gone to bed with painkillers.
Participant 7
Some participants had a lot of discomfort from fracture blisters; those with a removable cast really valued the ability to get relief from discomfort and provide attention to the skin and wound:
I thought that was good because if at least I was sitting down with my foot up I could take it off.
Participant 6
Metalwork was accepted as an expected necessity and was felt to fix the ankle and be strong, but the group lived with the uncertainty of further complications.
Decision-making
Participants’ experience of the intervention was set within their broader experience of living with a fractured ankle. There was a feeling of inevitability that some sort of intervention was required, ‘I just wanted to get the whole procedure over and done with as quickly as possible . . . Just sort this ankle out and stop the pain’ (participant 22), and they trusted that professionals had the knowledge and would supply an appropriate intervention. Participants chose to join the study from a sense of altruism to improve knowledge for the future, balanced with minimum effort although some couldn’t really remember the trial:
It’s a little bit of a payback really, not much, the study doesn’t seem to have required any great effort.
Participant 24
Some were initially worried they might not get the best treatment:
I was frightened that it was something that hadn’t been tried and tested.
Participant 33
They felt that they could not really compare both arms because they had no experience of them and could go only on what they had been told. On randomisation, however, some had decided one way or the other which option they preferred and were disappointed with the outcome. There was a preference for CCC as participants had thought through this option as the ‘new’ intervention and were convinced of its value. Although they knew the intervention would be randomly assigned, the outcome did not sit well with how they felt.
Well I didn’t mind at all actually you know. And when she said you’re going to have the plates I was quite disappointed really, I thought I was going to try the different one, you know. But it’s been fine and I don’t know what the other one would have been like anyway so, so what you don’t know you can’t compare really can you? . . . No, you know when the computer decides don’t they and the computer decided ‘no, you can’t have that’. After I came around to it and then she said, no, you can’t have it, oh right.
Participant 11
There was a preference for the CCC because it avoided the need to have an operation or metalwork, avoided wounds so that there were ‘no scars or cuts’ (participant 13), reduced the risk of infection with ‘flesh-eating bugs’ (participant 33) and was better for ‘brittle bones’ (participant 35); for some it felt like a ‘lifeline’ (participant 19). ORIF was preferred because it was an intervention that participants were familiar with and that they felt was tried and tested, and there was a concern that the cast for CCC would not be close fitting enough. Participants could not always articulate why they preferred the metalwork but they felt it was right for them:
I did remember feeling quite relieved when I knew I was going to have the conventional plate and pin as opposed to the close cast and why I don’t know, I’ve got no idea why.
Participant 17
At 6 weeks, participants did not express much interest in relation to what it would be like if they had the alternative intervention. At this stage they had endured their non-weight-bearing period and were looking forward to regaining some form of mobility. Those few who experienced a crossover to metalwork did question their original treatment (see Living with close contact casting).
The participants were very pleased with the trial processes and hospital contacts because they saw the same people over time, who knew them, and they felt that they were fast tracked through the system and rarely had to wait very long. They liked the continuity, being known and having a contact telephone number, and they felt at times as if they had their own private nurse. It was easy to enter the trial, participants did not feel stressed and the demands made on them for information were minimal.
It’s been quite nice as every time I come to hospital, it’s only been four times; Kate has been there to meet me so I’ve felt like I’m somebody special. I’ve felt like an individual rather than a long line of people with long faces and a plaster on so I think it has been very positive.
Participant 26
Altruism therefore existed alongside feelings of personal benefit gained from being in a trial. In addition, despite initial feelings of disappointment in relation to the treatment arm at the time of interview, participants felt that they had had the right intervention for them.
Discussion
The findings reflect that the participants experienced the themes of suffering, getting on with daily life, struggling to move and treatment and being in the trial. The theme of suffering reflected the emotional work participants undertook in relation to feeling vulnerable and being old and to emotional containment as a way of enduring but also maintaining relationships for the future. The theme of getting on with daily life demonstrated how participants reframed their lives by being proactive, drawing on their own and others resources to recreate meaningful lives within existing limitations. The theme of struggling to move encompassed new ways of being, as participants worked hard to move their fragile bodies around a confined life space and moved forward with a heightened sense of emotional fragility. The theme of treatment and being in the trial identified the different experiences of the two treatments; how participants actively managed their symptoms and made sense of being in a trial.
Suffering
The experience of living with ankle fracture had elements that were similar to those of younger adults12 but differed because the dependency of the participants was linked to their experiences of old age and of caring for their parents and others in the past. The participants’ experience was similar to chronic conditions in which changes in appearance led to feelings of old age and unattractiveness and a reduction in capabilities led to dependency and a sense of uselessness. 100 Oakley101 suggests that ‘ageing, like illness, is primarily an experience of embodiment: a time in our lives when it’s hard to pretend that we are in any sense separate from our bodies’ (p. 111). As a consequence, participants could feel low and depressed, sometimes exacerbated by lack of sleep or pain. This is similar to the studies of hope in acute trauma care in which patients oscillate between hopefulness and hopelessness, when feeling depressed reduced their capacity for hope. 88 To mitigate these feelings participants actively focused their mind to stop worrying, used positive thinking, determinedly found ways of doing things they felt were important, kept busy, garnered social support or got back to work.
The participants lost their normal ways of being and relationships were disrupted. Embodied endurance, incorporating physical, emotional and social aspects, came to the fore as participants struggled to live within a confined geographic space, to move around and to live with pain and discomfort. Emotional endurance was evident when emotions were put on hold and participants stoically just got on with life, as identified by Morse and Penrod. 102 Their framework linked the concepts of enduring, suffering, uncertainty and hope leading to a reframing of self as people moved through the illness/injury experience. In this study these concepts were present but reflected the concept of lived experience rather than a focus solely on emotions. Acceptance as a means of enduring was a short-term strategy of being in the present in this study rather than as a result of a deep understanding of the impact of the event and a route to hope for the future as identified by Morse and Penrod. 102 In this study, suffering and enduring were apparent throughout the 6–10 weeks after treatment, were overlapping and were incorporated an embodied lived experience.
Participants and their carers undertook emotional work to manage their relationships with a view to the future. A form of containment was evident:103 ‘the ways in which emotion is experienced or avoided, managed or denied, kept in or passed on, so its effects are either mitigated or amplified’ (p. 525). Normal patterns of intimacy were disrupted by notions of a fragile body, and feelings of helplessness required processing. Physical support for daily life was required, which is also found in the demanding/dependent body that is identified by Kvigne and Kirkevold. 104 Normal relationships that were taken for granted required active management through minimising needs and supporting normal leisure activities to reduce the burden of care on the partner in order to facilitate good relationships in the long term.
Getting on with life
The participant’s sense of self in this study was disrupted by an inability to carry out normal roles and responsibilities. They used their knowledge and experience, and that of their partner, family and local community, to creatively problem-solve daily activities, gain social support and create opportunities for leaving the home environment. The suddenness and impact of the injury experience had elements of ‘biographical disruption’ and a ‘critical situation’ identified in relation to chronic illness. 105 Bury105 suggested that reflection on biographical lives and self-concept occurred along with an ability to use social and community connections to mobilise resources with a reliance on their ‘own stocks of knowledge and biographical experience’ in the light of limited medical input (pp. 173–4). Conceptual understandings in many areas since Bury105 have moved forward and further work on areas such as age, ethnicity, context and norms is still required,106 but the sense of disruption and use of personal experience and social resources is evident in this study. Being busy and sorting out new ways of being and doing was a way of getting on with life alongside finding meaningful occupation. In other areas of injury finding meaningful occupation is important for self-identity, which is constructed through interactions with others. 107 In this study those who lived alone or could not find meaningful activity struggled with getting on with life.
Struggling to move
Daily life was dominated by the struggle to move ‘the struggling body’, which was problematic for all participants. This goes beyond Kvigne and Kirkevold104 and their notions of the limited body to imply action and effort. There was a lack of skill with using walking frames and crutches, also noted by Forsberg et al. ,92 and with getting up steps and stairs; participants drew on experience and trial and error to move around. Older women who have experienced a fall note how walking aids expose their vulnerability and weakness to others yet are essential to maintaining daily life. 108 In this study, living with hopping reduced the geographical space participants could live in, in and outside the home. Reduced life space outside the home is also identified as the result of living more carefully after a fall. 108 Any support for mobility from professionals was gratefully received, although not consistent in its provision. Many participants’ skill improved as they felt better and became more mobile, but many did not improve and just waited until they could weight-bear.
Participants expressed hope as a keenness to move forward and get back to normal in a similar way to other trauma patients;88,90 however, the participants were hampered by fear, anxiety and weariness regarding weight-bearing. Loss of confidence, increased caution in general life and a fear of falling support existing evidence on patient experience in relation to trauma. 89–92 Participants’ bodies, which were previously uncontested and taken for granted, were now vulnerable and fragile, requiring monitoring and active management. This is similar to the unreliable and betraying body identified by Kvigne and Kirkevold104 in which participants did not trust their body to work consistently; this was evident in the near misses and falls in this study. Participants also felt emotionally vulnerable; a vulnerable body can be ‘in a way defenceless’ (p. 1299) and open to pain and emotional fragility. 104
Treatment and being in a trial
The lived experiences of the two treatments were similar as both groups suffered and endured the impact of ankle fracture and lived with non-weight-bearing with a cast and both lived with uncertainty regarding future function and the necessity for further interventions. The main differences were the ways in which the treatments could be experienced: (1) CCC was heavy and tight causing discomfort and pain, with swelling that had to be actively managed, healing progress that was unknown, the possibility that participants may cause damage to the ankle and strong emotions if surgery was required; and (2) ORIF was a known entity, tried and tested, which was strong and provided structural support but there was uncertainty regarding possible infection. Participants actively managed specific symptoms based on their experiential knowledge of what worked best for them using their own stocks of knowledge as identified in Bury. 105 A feature of CCC was living with the uncertainty of not knowing if healing was occurring; Oakley101 notes that ‘we have no way of knowing what our bodies are silently doing to us’ (p. 65). In contrast, those with metalwork felt some security from perceptions of their ankle as fixed. There was some concern in both groups about causing damage to their ‘fragile body’, which is also evident in women living with osteoporosis109 and women at risk of falling who actively protect themselves from further damage. 108
Although altruistic reasons for participation in the trial were expressed, other common rationales for trial participation included trust in the surgeon and personal benefits. 84,85 However, there was concern that personal benefits may link to misunderstandings of trial design84 and, in this study, there was evidence that some participants did not fully understand randomisation or had little memory of the trial. There were, at randomisation, strong feelings of preference for one treatment or the other, although, on reflection, participants felt that they had the right treatment for them. This suggests that there may be an element of ‘therapeutic misconception’ identified in other studies84,85 based on the interaction with clinicians and how participants make sense of that interaction. The participants often could not articulate a rationale for their preference or drew on experiences of family and friends, also noted by Canvin and Jacoby. 85 In order to understand this, study of the sources of knowledge that participants use to make sense of interactions with clinicians and the process of consent is required. Participants felt they could not compare treatments as they had experienced only one of them and they could not imagine what the other one was like, suggesting experiential ways of knowing are important when making sense of interventions.
Limitations
The study had four limitations:
-
One interview was undertaken at 6 weeks post injury. An interview nearer the time of consent would provide further evidence regarding the decision to participate in a clinical trial. A longitudinal study that followed patients throughout the year would provide understanding of the experience of ankle fracture over time.
-
Partners and family were not interviewed. They were often present at the interview and their lived experience would be useful additional evidence.
-
The clinic setting was not always conducive to obtaining a good interview. Some interviews were curtailed because of the intense nature of the clinic visit and unpredictability of hospital transport, but data saturation was achieved. Other ways of interviewing such as home or telephone may have been useful.
-
The sample was not ethnically diverse but reflected the population in the study. Non-western, ethnically diverse populations may have culturally different experiences.
Conclusions
The findings of this study suggest that participants require professional support for their new way of being to make it easier for them to endure this period of recovery. Supportive activities that focus on the experience of injury in relation to being old, the vulnerable and fragile body, changing relationships, skills required for mobility and support for active management of living with the prescribed treatment are required. Supportive activities would require research evidence but could focus on co-ordinated packages of care, accessible learning materials, specialist-based emotion-focused intervention, consistent allocation of helpful equipment and educational materials around lived experience for patients and staff. Further research is required to explore how this experience, in relation to biographical disruption, is processed regarding healthy ageing and future health-care experiences. A longitudinal study would provide valuable insights into the impact of injury over time and implications for rehabilitation. This study has clearly identified the similarities and differences between the lived experiences of both treatments; both of which were considered acceptable. Further exploration is required to understand how patients make decisions about preferred treatments and process the consequences of these decisions. The experience/stocks of knowledge participants draw on, the way they make sense of risks, the way they process changes in treatment because of a lack of bone healing and how the treatment choice impacts on future treatment decisions require further exploration.
Summary of key findings
-
Both treatment groups, CCC and ORIF, require support to ease, when possible, the struggle to live with a fractured ankle. Support that focuses on (1) suffering, vulnerability and challenges in relation to roles and relationships; (2) getting on with daily life, finding ways of doing things and keeping busy; and (3) struggling to move, living with hopping and moving forward.
-
Participants with CCC require support for managing pain and discomfort caused by swelling, living with uncertainty in relation to healing and concerns regarding further damage to their ankle. Those requiring further surgical intervention require help that supports their ability to endure further periods of non-weight-bearing.
-
Participants with ORIF require clarity regarding aspects around the use of metalwork and support for concerns regarding infection and further surgical interventions.
-
Further research is required to examine how patients develop preferences for treatments and process the consequences of decisions made about treatments in the light of their recovery.
Chapter 6 Discussion
This discussion chapter reviews the aims of the study and summarises the main findings before considering the internal and external validity of the trial. The interpretations and implication for clinical practice and policy are then considered, the findings of this study are compared with other studies and recommendations are made for future research.
Aim and overview of study findings
The decision to treat an ankle fracture by surgery in older adults, compared with younger adults, is complicated by a higher prevalence of comorbidities, increasing the risk of infection, surgical wound problems and inadequate fixation as a result of poor bone quality. Existing trial data are of poor quality but overall indicate that traditional casting methods may lead to a higher risk of malunion because of a loss of fracture reduction. Traditional surgical teaching equates malunion (post-traumatic joint deformity) with poor patient outcome. Therefore, surgery is usually the preferred recommended treatment, with the aim of optimising alignment and congruence (best fit) of the ankle joint, assuming this will translate into better ankle function for patients. In the AIM trial, we aimed to estimate the clinical effectiveness and cost-effectiveness of a new casting intervention, CCC, compared with ORIF surgery.
Our primary objective was to estimate if there was equivalence in patient-reported ankle function outcome between CCC and ORIF, as measured by the OMAS, at 6 months. A qualitative study of participants’ experiences of the intervention and of being in a randomised controlled trial was also embedded into the trial in order to build the context and frame of reference for interpretation of the study findings by clinicians and patients.
This study was a definitive large-scale equivalence randomised controlled trial; assessors were blinded and it was undertaken in a pragmatic way across 24 hospital orthopaedic trauma departments in the UK. The trial has demonstrated ankle function outcomes were equivalent between CCC and ORIF at 6 weeks and at the primary end point of 6 months. There were also no differences between treatments in the secondary outcomes of quality of life (mental and physical), range of ankle motion, ankle pain, mobility and patient satisfaction.
The most common treatment-related issue in the CCC arm was a loss of closed reduction. As a result about 1 in 5 patients who had CCC later returned to theatre to receive ORIF. A later conversion to ORIF was an expected and allowable event in the study protocol. Fewer participants in the ORIF group, about 1 in 20, required additional surgery within 6 months; these surgeries were usually for surgical wound- or implant-related complications, but some of which were clinically serious. At 6 months, CCC was associated with higher rates of radiological malunion and non-union.
The experiences of the treatments were similar as both groups endured the impact of ankle fracture and uncertainty regarding future function and the necessity for further interventions. However, those who had CCC experienced concern regarding healing and the possibility of further damage to their ankle. Returning to theatre for ORIF in the first 6 to 10 weeks after treatment impacted on how they felt and how they made sense of their treatment. Participants who received ORIF had concerns about the use of metalwork and the potential for infection.
Close contact casting resulted in a modest mean cost savings to the NHS and society. The cost savings were driven largely by savings in theatre time, which were partially eroded over time because of increased readmission and health services costs. Consistent with the clinical results, incremental QALYs following CCC were no different from ORIF, with evidence leaning towards a slight advantage with CCC. Over common willingness-to-pay thresholds, the probability that CCC was cost-effective was very high.
The conclusions for the clinical and health economic evaluations were consistent for the per-protocol or ITT analyses.
Internal validity and methodology
The study was powered to be a definitive equivalence trial and had lower than expected loss to follow-up. Blinding of outcome assessors and very low levels of missing data across the outcome measures strengthen the trial results. Allocation concealment was ensured through random computer allocation, stratified by site and fracture pattern, on participant registration via a remote telephone randomisation service. Analyses were preplanned and agreed by the DMEC. The consistency between the conclusions of the per protocol and ITT analyses improves confidence in the findings of the AIM trial.
The OMAS is one of the most commonly used outcome measures in ankle fracture research,23,110 but has been criticised for methodological shortcomings in its development. 111 We found no published minimal clinically important differences for the OMAS and, therefore, had to develop the sample size drawing on wider literature and in consultation with clinical experts. The OMAS has evidence supporting its construct validity and there are few alternative condition-specific measures. 112 More recently, additional evidence supporting the validity and reliability of the OMAS has been published. 113 In the context of the AIM trial, the conclusions drawn from the OMASs are supported by the high precision of estimates and the consistency with the secondary outcomes [SF-12 (mental and physical), EQ-5D and EQ-5D VAS, timed up and go test, ankle range of motion and pain].
On formal analysis the success of the outcome assessor blinding at 6 months was adequate. For the 6-week assessment the assessor was aware of relevant precautions and contraindications to the clinical assessment and so was not blinded. The 6-week results did not have a strong influence on the analysis or conclusions for the clinical and heath economic evaluations, so we feel this limitation does not detract from the overall findings.
External validity and generalisability
We consider the external validity of the AIM trial to be good for several key reasons. The study was conducted in a range of acute hospitals including major trauma centres through to smaller district general hospitals and, therefore, is representative of the range of settings for ankle fracture interventions in the UK. The CCC intervention was delivered by NHS surgical teams with relatively little training and the trial was pragmatic, which allowed other aspects of care except the intervention to continue unchanged.
Based on previous studies in the UK,1,24 the demographics of the trial participants were representative of the age and sex expected in older adults with ankle fractures, with the majority being female and approximately 70 years of age. The exclusion of patients with insulin-dependent diabetes mellitus and established peripheral vessel disease limits generalisability. However, the results of this study open up the option to investigate the applicability of CCC to patients with these diseases, as there is a high risk of harms after surgical treatment. 27,114,115
The primary end point at 6 months could be criticised as being too early for definitive assessment of outcome. A recent systematic review and meta-analysis,9 however, demonstrated that improvement of patient outcomes from all previously published studies of ankle function outcomes after ankle fractures showed that improvement plateaued by 6 months and, therefore, supports this as the definitive assessment point.
There were differences in experience level of the operating surgeon for ORIF and CCC. The operating surgeon for CCC was consultant grade for approximately 85% of procedures. For ORIF, approximately 39% of operating surgeons were consultants. CCC and ORIF procedures had high levels of consultant supervision, 97% and 76%, respectively. This difference was driven by the protocol as it was felt it was necessary to introduce the standard of direct consultant supervision for CCC because of the uncertainty of it as a new intervention and the likelihood of clinical issues early in the introduction of the technique. We did not identify a learning effect within the trial, although we might not expect to detect it given that just under half of surgeons who conducted CCC in the trial performed only two applications and that these were not necessarily conducted within a close enough time period to reinforce learning. Nevertheless, high consultant involvement is consistent with a new intervention and contemporary NHS practice, further supporting its applicability in practice without concern of unattributed inexperience or learning effects.
Interpretation and implications for clinical practice and policy
The implications of the results of this study to the orthopaedic trauma surgical community are significant and challenge what has been orthopaedic surgical dogma and teaching for several decades. For patients, who are increasingly being involved in decision-making about their treatments, these findings are an important contribution. The qualitative study within this trial elucidated the patient view that the opportunity to avoid undergoing surgery was appealing. The tight equivalence around the point estimate of the primary outcome and the almost identical patient-reported secondary outcomes strongly support the validity of the conclusion that CCC is clinically equivalent to ORIF. The previous extrapolation of witnessed outcomes for this injury in younger patients to patients 60 years of age and above, in whom there are higher incidences of poor bone quality, diminished circulation and skin resilience, now seems unjustified. The study protocol identified that both CCC and ORIF would be associated with treatment-related issues and complications, but that the type would be different for each (e.g. loss of reduction with CCC and surgical wound problems with ORIF). Such treatment-related issues and adverse events that were known to occur and therefore ‘expected’ were monitored for absolute numbers and as a proportion, and expected ranges were agreed with the DMEC. This information is now important for surgeons explaining the equivalence and for patients to understand the advantages and disadvantages of the two treatment options. In general, ORIF has the established risks of surgery and if problems occur they are usually more serious and 6% of patients require a second surgery within 6 months. Approximately 20% of patients who start off by receiving CCC later require an additional visit to theatre to undergo ORIF for unacceptable loss of position, although this may be seen as a positive outcome with 80% avoiding surgery to no detriment. Patients may value the opportunity to try to avoid surgery, in the confidence that there will be an equivalent outcome in terms of ankle function at 6 months. However, the qualitative study would suggest that support for the group that converted to ORIF would be required to help them process the change in their treatment and endure another period of non-weight-bearing.
A significant and consistent finding across the outcomes in the AIM trial was the major impairment in function and quality of life resulting from an ankle fracture, irrespective of the treatment received. This observation was supported by the qualitative study that identified how participants struggled to live with a fractured ankle. They experienced feelings of vulnerability and were challenged in their roles, relationships, daily life and in their ability to mobilise. This finding highlights the implications of ankle fracture for older adults to stakeholders in health- and social-care and policy in the UK.
There has been a deficiency in the published literature relating to the treatment of ankle fractures in older people. Surgeons recognise that they have their own preferences, formed over time from clinical and training experiences, and perhaps modified by the limited published research. Those preferences were borne out in our study by enquiry made of surgeons after randomisation. Of patients allocated ORIF, for 85% of their surgeons that would have been preferred treatment, whereas CCC was the surgeon’s preference in only 28% of those allocated to CCC. This has considerable implications for the difficulty in adoption of this study’s findings and translating them into shared decision-making with patients.
There are also implications in interpreting the results of this trial, as it can be assumed participating surgeon preferences may have influenced perseverance with CCC and willingness to redo the manipulation and cast in the presence of loss of fracture reduction. One key aspect of follow-up when using CCC, as with any conservative treatment for unstable fractures, is close monitoring of the fracture position in the first few weeks after injury with the opportunity to reapply the cast. The willingness to reapply CCC may be important. We recently conducted a laboratory investigation of the effects of reduction in lower leg swelling. We found that CCC was susceptible to a loss of ankle stabilisation as swelling resolves, highlighting the importance of monitoring and reapplication of the cast as necessary in the first few weeks after injury. 116
As a pragmatic trial, the decision to reapply CCC or convert to ORIF was left to the treating surgeon’s judgement. The decision to convert 20% of CCCs to ORIF may, we assume, have been based on the same preconceived opinions that were responsible for the preference of surgeons for ORIF, that is that they needed to achieve exact anatomical restoration of bony architecture of the ankle joint components and that implants were necessary to maintain that reduction of deformity and hold fracture fragments until healing. Those judgements have been central to surgical methods teaching for fractures involving joints for several decades. They are now challenged by this definitive study in which we move from process measures, complications rates and radiological outcomes to patient-reported outcomes in an arguably well-conducted adequately powered and valid randomised blinded trial.
The higher rates of malunion/non-union associated with CCC compared with ORIF indicate that achieving alignment may be more challenging with conservative treatment. However, the equivalence in clinical outcome challenges the importance of restoring anatomical alignment of the joint as part of ankle fracture management in older adults. There will be justifiable reservations of whether or not these differences in radiological outcomes manifest as clinical issues in the longer term. Pain and disability as a result of later post-traumatic osteoarthritis will be a concern. 20 However, the aetiology of post-traumatic osteoarthritis may also be because of genetic factors and direct damage caused by the initial trauma. 21 Lateral malleolar non-union is often thought to be more of a clinical issue than medial malleolar non-union. However, a 2012 systematic review found low-quality evidence regarding lateral malleolar non-unions, reporting these as an uncommon complication and one that can often be asymptomatic. 117 These uncertainties regarding the longer term will be addressed by the extended follow-up of the AIM trial cohort that is under way. We aim to establish if there are any differences in later ankle function, quality of life and resource use [e.g. need for ankle arthrodesis (fusion) or arthroplasty (replacement)] at least 2 years after injury.
Implications for training and implementation
The novel intervention (CCC) in this trial was successfully taught to 317 orthopaedic surgeons and the technique demonstrated to other staff (plaster nurses, technicians and operating room practitioners). Training was achieved through a short instructional video and a face-to-face demonstration. Plaster cast application is within the skill set of orthopaedic surgeons, although modern surgical practice has deprived many younger surgeons of the manual skills of fracture reduction and cast-moulding techniques of former generations. The CCC treatment method draws on elements of both the total contact cast technique32–34 for managing diabetic leg ulcers and also those traditional three-point moulding and gravity fracture-reduction techniques applicable to Potts (ankle) fractures. 118 For the CCC intervention to be adopted there will also need to be a change in the scrutiny of interpretation and surveillance of follow-up radiographs in orthopaedic fracture clinics.
Cast-related complications such as plaster sores and pain from the cast were common in both treatment arms. The most clinically serious technical casting complication was plaster saw lacerations on removal of casts, occurring in 5 out of 275 (1.8%) participants in the CCC group compared with 1 out of 298 (0.3%) in the ORIF group. It seems plausible that there may have been some technical challenges removing the CCC with the plaster saw compared with traditional casts and there are several potential reasons. The operator of the saw may have (1) not been experienced in using the saw on a minimally padded cast, (2) not known the cast was minimally padded or (3) been unaware of the location of the foam cutting strips for protecting the skin, especially if not adequately marked on the exterior of the cast. In addition, the foam cutting strips may have offered insufficient protection or were not applied during CCC application. As a pragmatic trial we did not monitor the application and removal of CCC, therefore it is recommended that when implemented in clinical practice that steps are taken locally to reduce the risk of plaster saw injuries.
When the AIM study was proposed there was concern raised regarding return to weight-bearing. The assumption was that ORIF gave patients the advantage of earlier weight-bearing compared with conservative treatment. As identified in our qualitative study, participants found coping with non-weight-bearing challenging and so an earlier return to weight-bearing may be advantageous during recovery. In the AIM study, hospitals followed their own postoperative weight-bearing protocols for ORIF and we advised no more than touch weight-bearing in the first 4 weeks after CCC. However, we found no difference in the time to start partial weight-bearing between the two interventions and so those concerns prior to the trial were not substantiated.
In addition to being of interest to the UK NHS, the findings of the AIM trial are also likely to be of interest to health services in other comparable high-income countries. The finding of clinical equivalence for a conservative treatment option for older patients with unstable ankle fracture is also important for low- and middle-income countries,119 where availability of specialist surgeons and surgical implants can be limited. 120
Comparison with other literature
A clinical trial from Canada has been published since the start of the AIM trial and subsequent to the 2012 Cochrane review, comparing ORIF with conservative treatment with a brace or cast. 78 The study included 81 participants who had an isolated, undisplaced, unstable, type B fracture of the distal fibula. Participants were younger than those in the AIM trial, aged, on average, 41 years. There were no differences in patient-reported ankle function or quality-of-life scores during the 1-year follow-up period. In the conservative treatment group, 8 out of 40 (20%) developed radiographic joint misalignment. In the operative group, 6 out of 41 (15%) experienced a surgical site infection, one of which was deep and required an additional operation to wash out the joint. Five participants in the operative treatment group also required an additional operation to remove the metalwork. The Canadian study included a health economic analysis. 76 The economic analysis was from a health service cost perspective, which estimated that within 1 year operative treatment was not cost-effective. From the lifetime horizon analysis, these conclusions were different, if it was assumed that conservative treatment results in much higher rates of post-traumatic osteoarthritis. These health economic findings cannot be easily translated to the UK NHS, as costs of rehabilitation were not included. There is also a lack of high-quality prospective research to support the rationale that conservative treatment necessarily results in higher rates of post-traumatic osteoarthritis. 21
Further research
As mentioned previously, in accordance with the IDEAL (Idea, Development, Exploration, Assessment, Long-term study) recommendations for long-term follow-up in the development of surgical innovation,36 we are conducting an extended follow-up of the AIM trial participants beyond 2 years, funded by the National Institute for Health Research Health Technology Assessment programme. The extended follow-up should be completed by November 2016. Longer-term follow-up was deemed important because of concerns from clinicians over potential later complications or additional procedures (e.g. intolerance of metalwork resulting in removal, post-traumatic osteoarthritis and possible ankle joint fusion or replacement) and their potential to impact on ankle function. The extended follow-up will also inform a longer-term projection of the cost-effectiveness analysis.
As part of the implementation of CCC in NHS practice, it is also important to examine how patients develop preferences for treatments and process the consequences of decisions made about treatments in the light of their recovery. In addition, the need to develop support strategies for patients dealing with the uncertainties faced when recovering after CCC and ORIF has been identified.
We plan to build on the findings from the AIM trial cohort. We will undertake predictive modelling of the anatomical fracture patterns, variants of radiological malunion and joint incongruence that predispose to loss of reduction with CCC (malunion or convert to ORIF). The results of the AIM trial directly challenge the assumption that the best outcomes for unstable ankle fractures can only be achieved through surgery. The equivalence of outcome with CCC still begs the question, given the overall significant impairment of function at 6 months, whether or not it is possible to identify the patients who would benefit most from one or other intervention. It is possible that certain patient or fracture injury characteristics may be associated with better outcomes or lower complication rates with one treatment. The substantial decline in function and quality of life after ankle fracture for those aged over 60 years also indicates a need to further optimise the recovery and rehabilitation of older patients.
Finally, the findings of the AIM trial, and the recent trial from Canada,78 also raise questions of whether or not CCC could provide an alternative surgery to adults under 60 years of age; further studies are now warranted.
Acknowledgements
Contributions of authors
David J Keene (Research Physiotherapist in Trauma and Rehabilitation) was clinical co-ordinator, designed the study, delivered intervention training and wrote and reviewed the report.
Dipesh Mistry (Research Fellow in Statistics) conducted the clinical quantitative analysis and wrote and reviewed the report.
Julian Nam (Research Assistant in Health Economics) conducted the health economic evaluation and wrote and reviewed the report.
Elizabeth Tutton (Senior Research Fellow in Qualitative Study) designed and conducted the qualitative study and wrote and reviewed the report.
Robert Handley (Consultant Surgeon in Orthopaedics) developed the interventions, study conception and design, and reviewed the report.
Lesley Morgan (Trial Manager in Trauma and Rehabilitation) managed and supervised the study, and wrote and reviewed the report.
Emma Roberts (Trial Co-ordinator in Trauma and Rehabilitation) co-ordinated the study and data management, and reviewed the report.
Bridget Gray (Research Nurse in Trauma) co-ordinated the pilot study, study conception and design and reviewed the report.
Andrew Briggs (Professor in Health Economics) designed and supervised the health economic evaluation, and reviewed the report.
Ranjit Lall (Senior Research Fellow in Statistics) designed the study, supervised and conducted quantitative analysis and reviewed the report.
Tim JS Chesser (Consultant Surgeon in Orthopaedics) designed the study and reviewed the report.
Ian Pallister (Professor in Orthopaedic Trauma Surgery) designed the study and reviewed the report.
Sarah E Lamb (Professor in Rehabilitation; Codirector of Clinical Trials Unit) supervised the study, developed study conception and design, and wrote and reviewed the report.
Keith Willett (Professor in Orthopaedic Trauma Surgery; National Director for Acute Episodes of Care) was chief investigator, led the funding application, study conception and design, development of interventions, provided overall study supervision, and wrote and reviewed the report.
Ankle Injury Management trial team
Chief investigator
Keith Willett.
Trial coinvestigators
Sarah E Lamb, Robert Handley, Elizabeth Tutton, Tim JS Chesser and Ian Pallister.
Trial manager
Lesley Morgan.
Research physiotherapist
David J Keene.
Trial co-ordinator and administrator
Emma Roberts.
Trial statisticians
Dipesh Mistry, Christopher Knox and Ranjit Lall.
Health economists
Julian Nam and Andrew Briggs.
Principal investigators/research fellows, nurses or therapists by hospital site in order of date starting recruitment
Bob Handley/Bridget Gray, Susanna Symonds, Louise Spoors, Vivienne Fairclough and Joseph Alsousou (John Radcliffe Hospital, Oxford).
Steve Hepple/Rebecca Fox, Ruth Halliday, Steve Barnfield and Hannah Luton (Frenchay Hospital, Bristol).
Mike Reed/Catherine Ashbrook-Raby, Maureen Armstrong, Kirstie Walker, Chris Herriott and Helen Baily (North Tyneside and Wansbeck Hospitals, Northumbria).
Andrew McAndrew/Patricia Rodrigues-Osorio, Julie Foxton, Karen Barnard, Tinashe Samakomva and Belinda Elba (Royal Berkshire Hospital).
Ben Lankester/Barbara Williams-Yesson, Rebecca Rowland and Claire Buckley (Yeovil District Hospital).
Simon Donell/Elizabeth Saunders, David Thomlinson, Jennifer Jaggar and Nicola Hunt (Norfolk and Norwich University Hospital).
Chris Roberts and Christopher Servant/Bally Purewall, Sheeba Suresh and Jenny Finch (Ipswich Hospital).
Ian Pallister/Jill Scott, Lisa Bastin and Christine Jones (Morriston Hospital, Swansea).
Andrew Kelly/Carole Chillmaid, Matthew Beebee and Keira Beacham (Musgrove Park, Taunton).
Matt Costa/Kate Dennison, Rebecca McKeown and Andrew Cuff (University Hospital, Coventry).
Andrew Gray/Jacqueline Claydon, Kelly Storey, Adam Dobson and Karen Smith (Royal Victoria Infirmary, Newcastle).
Sunny Deo/Claire Woodruffe (Great Western Hospital, Swindon).
Damian McClelland/Sarah Griffiths and Racquel Carpio (North Staffordshire Royal Infirmary, Stoke-on-Trent).
Mark Farrar/Sarah Margetts-Cooke, Katarina Kennedy, Christine Dickson, Adrian Hand and Rachel Martin (Poole Hospital).
Maneesh Bhatia/Manjit Attwal and Bianca Ngwenya (Leicester Royal Infirmary).
James Davis/Pauline Mercer, Andy Hall and Barbara Finson (Torbay Hospital).
Nicola Maffulli/Gayle Mafulli (Newham University Hospital, London).
Andrew Jennings and Graham Chuter/Glynis Rose, Jill Deane, Fiona Bezzina and Gil Horner (University Hospital of North Durham and Darlington Memorial Hospital).
Peter Giannoudis/Michalis Panteli, Suri Gudipati, Oghor Obakponovwe and Jennifer Ogden (Leeds General Infirmary).
Mark Brinsden/Claire West and Rosalyn Squire (Derriford Hospital, Plymouth).
Peter Hull/Sophie Lewis and Abigail Ford (Addenbrooke’s Hospital, Cambridge).
Eugene Toh/Margaret Marshall (Southport & Ormskirk Hospital).
Trial Steering Committee
Margot Gosney (independent chairperson), Keith Willett (chief investigator), Sarah E Lamb (grant coapplicant), Andrew Briggs (grant coapplicant and AIM Trial Health Economist), Matt Costa (orthopaedic surgeon), Dipesh Mistry and Ranjit Lall (AIM trial statisticians), Stephen Bremner (independent member – statistician), Lesley Morgan (AIM trial manager), Tim Chesser (grant coapplicant), Ian Pallister (grant coapplicant) and Rosamund Mengech (independent member – user representative).
Data Monitoring and Ethics Committee
Alan Montgomery (chairperson), David Marsh and Karen Barker.
Other acknowledgements
Special thanks to Susanna Symmonds and Bridget Gray, Research Nurses at the John Radcliffe Hospital, Oxford, who offered expertise in the development of the multicentre phase and co-ordinated the pilot phase of the study.
The system for radiological measurements was developed by Ben Van Duren who designed a bespoke software interface.
Publication
Willett K, Keene DJ, Morgan L, Gray B, Handley R, Chesser T, et al. Ankle Injury Management (AIM): design of a pragmatic multi-centre equivalence randomised controlled trial comparing close contact casting (CCC) to Open surgical Reduction and Internal Fixation (ORIF) in the treatment of unstable ankle fractures in patients over 60 years. BMC Musculoskel Disord 2014;15:79.
Data sharing statement
Requests for data sharing for secondary research purposes can be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37:691-7. http://dx.doi.org/10.1016/j.injury.2006.04.130.
- Somersalo A, Paloneva J, Kautiainen H, Lonnroos E, Heinanen M, Kiviranta I. Incidence of fractures requiring inpatient care. Acta Orthop 2014;85:525-30. http://dx.doi.org/10.3109/17453674.2014.908340.
- Court-Brown CM, McBirnie J, Wilson G. Adult ankle fractures – an increasing problem?. Acta Orthop 1998;69:43-7. http://dx.doi.org/10.3109/17453679809002355.
- Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M. Increasing number and incidence of low-trauma ankle fractures in elderly people: Finnish statistics during 1970–2000 and projections for the future. Bone 2002;31:430-3. http://dx.doi.org/10.1016/S8756-3282(02)00832-3.
- Lin CW, Moseley AM, Herbert RD, Refshauge KM. Pain and dorsiflexion range of motion predict short- and medium-term activity limitation in people receiving physiotherapy intervention after ankle fracture: an observational study. Aust J Physiother 2009;55:31-7. http://dx.doi.org/10.1016/S0004-9514(09)70058-3.
- Stevens JE, Walter GA, Okereke E, Scarborough MT, Esterhai JL, George SZ, et al. Muscle adaptations with immobilization and rehabilitation after ankle fracture. Med Sci Sports Exerc 2004;36:1695-701. http://dx.doi.org/10.1249/01.MSS.0000142407.25188.05.
- Psatha M, Wu Z, Gammie FM, Ratkevicius A, Wackerhage H, Lee JH, et al. A longitudinal MRI study of muscle atrophy during lower leg immobilization following ankle fracture. J Magn Reson Imaging 2012;35:686-95. http://dx.doi.org/10.1002/jmri.22864.
- Hancock MJ, Herbert RD, Stewart M. Prediction of outcome after ankle fracture. J Orthop Sports Phys Ther 2005;35:786-92. http://dx.doi.org/10.2519/jospt.2005.35.12.786.
- Beckenkamp PR, Lin CW, Chagpar S, Herbert RD, van der Ploeg HP, Moseley AM. Prognosis of physical function following ankle fracture: a systematic review with meta-analysis. J Orthop Sports Phys Ther 2014;44:841-51. http://dx.doi.org/10.2519/jospt.2014.5199.
- Davidovitch RI, Walsh M, Spitzer A, Egol KA. Functional outcome after operatively treated ankle fractures in the elderly. Foot Ankle Int 2009;30:728-33. http://dx.doi.org/10.3113/FAI.2009.0728.
- De Boer A, Schepers T, Panneman M, Van Beeck E, Van Lieshout E. Health care consumption and costs due to foot and ankle injuries in the Netherlands, 1986–2010. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-128.
- McPhail S, Dunstan J, Canning J, Haines T. Life impact of ankle fractures: qualitative analysis of patient and clinician experiences. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-224.
- Van Son MA, De Vries J, Roukema JA, Den Oudsten BL. Health status, health-related quality of life, and quality of life following ankle fractures: a systematic review. Injury 2013;44:1391-402. http://dx.doi.org/10.1016/j.injury.2013.02.018.
- Handley R, Gandhe A, Bulstrode CJK. Oxford Textbook of Trauma and Orthopaedics. Oxford: Oxford University Press; 2011.
- Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, et al. Articular fractures: does an anatomic reduction really change the result?. J Bone Joint Surg Am 2002;84:1259-71.
- Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, et al. Articular fractures. J Am Acad Orthop Surg 2004;12:416-23. http://dx.doi.org/10.5435/00124635-200411000-00006.
- Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am 1976;58:356-7.
- McKinley TO, Rudert MJ, Tochigi Y, Pedersen DR, Koos DC, Baer TE, et al. Incongruity-dependent changes of contact stress rates in human cadaveric ankles. J Orthop Trauma 2006;20:732-8. http://dx.doi.org/10.1097/01.bot.0000211150.00919.0e.
- Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006;20:739-44. http://dx.doi.org/10.1097/01.bot.0000246468.80635.ef.
- Horisberger M, Valderrabano V, Hintermann B. Posttraumatic ankle osteoarthritis after ankle-related fractures. J Orthop Trauma 2009;23:60-7. http://dx.doi.org/10.1097/BOT.0b013e31818915d9.
- Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma 2006;20:719-25. http://dx.doi.org/10.1097/01.bot.0000211160.05864.14.
- Stufkens SAS, van den Bekerom MPJ, Kerkhoffs GMMJ, Hintermann B, van Dijk CN. Long-term outcome after 1822 operatively treated ankle fractures: a systematic review of the literature. Injury 2011;42:119-27. http://dx.doi.org/10.1016/j.injury.2010.04.006.
- Donken CC, Al-Khateeb H, Verhofstad MH, van Laarhoven CJ. Surgical versus conservative interventions for treating ankle fractures in adults. Cochrane Database Syst Rev 2012;8. http://dx.doi.org/10.1002/14651858.cd008470.pub2.
- Zaghloul A, Haddad B, Barksfield R, Davis B. Early complications of surgery in operative treatment of ankle fractures in those over 60: a review of 186 cases. Injury 2014;45:780-3. http://dx.doi.org/10.1016/j.injury.2013.11.008.
- Litchfield JC. The treatment of unstable fractures of the ankle in the elderly. Injury 1987;18:128-32. http://dx.doi.org/10.1016/0020-1383(87)90189-6.
- Willett K, Hearn TC, Cuncins AV. Biomechanical testing of a new design for Schanz pedicle screws. J Orthop Trauma 1993;7:375-80. http://dx.doi.org/10.1097/00005131-199308000-00015.
- SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am 2009;91:1042-9. http://dx.doi.org/10.2106/JBJS.H.00653.
- Bauer M, Bergström B, Hemborg A, Sandegård J. Malleolar fractures: nonoperative versus operative treatment. A controlled study. Clin Orthop Relat Res 1985;199:17-2. http://dx.doi.org/10.1097/00003086-198510000-00004.
- Makwana NK, Bhowal B, Harper WM, Hui AW. Conservative versus operative treatment for displaced ankle fractures in patients over 55 years of age. A prospective, randomised study. J Bone Joint Surg Br 2001;83:525-9. http://dx.doi.org/10.1302/0301-620X.83B4.11522.
- Phillips WA, Schwartz HS, Keller CS, Woodward HR, Rudd WS, Spiegel PG, et al. A prospective, randomized study of the management of severe ankle fractures. J Bone Joint Surg Am 1985;67:67-78.
- Rowley DI, Norris SH, Duckworth T. A prospective trial comparing operative and manipulative treatment of ankle fractures. J Bone Joint Surg Br 1986;68:610-13.
- Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care 2001;24:1019-22. http://dx.doi.org/10.2337/diacare.24.6.1019.
- Wukich DK, Motko J. Safety of total contact casting in high-risk patients with neuropathic foot ulcers. Foot Ankle Int 2004;25:556-60. http://dx.doi.org/10.1177/107110070402500808.
- Nabuurs-Franssen MH, Sleegers R, Huijberts MS, Wijnen W, Sanders AP, Walenkamp G, et al. Total contact casting of the diabetic foot in daily practice: a prospective follow-up study. Diabetes Care 2005;28:243-7. http://dx.doi.org/10.2337/diacare.28.2.243.
- Willett KM, Gray B, Lamb S, Handa A, Handley R. The presence and pattern of vascular insufficiency in the older patient suffering an unstable ankle fracture: the relationship to skin and wound complications. Injury Extra 2009;40. http://dx.doi.org/10.1016/j.injury.2009.06.170.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. http://dx.doi.org/10.1016/S0140-6736(09)61116-8.
- Willett KM, Gray B, Moran CG, Giannoudis PV, Pallister I. Orthopaedic trauma research priority-setting exercise and development of a research network. Injury 2010;41:763-7. http://dx.doi.org/10.1016/j.injury.2010.03.026.
- Willett K, Keene DJ, Morgan L, Gray B, Handley R, Chesser T, et al. Ankle Injury Management (AIM): design of a pragmatic multi-centre equivalence randomised controlled trial comparing Close Contact Casting (CCC) to Open surgical Reduction and Internal Fixation (ORIF) in the treatment of unstable ankle fractures in patients over 60 years. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-79.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Ruedi TP, Murphy WM. AO Principles of Fracture Management. Stuttgart: Thieme; 2000.
- Olerud C, Molander H. A scoring scale for symptom evaluation after ankle fracture. Arch Orthop Trauma Surg 1984;103:190-4. http://dx.doi.org/10.1007/BF00435553.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. http://dx.doi.org/10.3109/07853890109002087.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. http://dx.doi.org/10.1016/S0895-4356(98)00109-7.
- Reese NB, Bandy WD. Joint Range of Motion and Muscle Length Testing. Philadelphia, PA: Saunders; 2002.
- Clarkson HM. Musculoskeletal Assessment: Joint Range of Motion and Manual Muscle Strength. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
- Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the ‘get-up and go’ test. Arch Phys Med Rehabil 1986;67:387-9.
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556-61. http://dx.doi.org/10.1056/NEJM199503023320902.
- Mont MA, Sedlin ED, Weiner LS, Miller AR. Postoperative radiographs as predictors of clinical outcome in unstable ankle fractures. J Orthop Trauma 1992;6:352-7. http://dx.doi.org/10.1097/00005131-199209000-00014.
- Machin D. Sample Size Tables For Clinical Studies. Oxford: Blackwell Science; 1997.
- Pocock SJ. Clinical Trials: A Practical Approach. Chichester: Wiley; 1983.
- Chow SC, Wang H. On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodyn 2001;28:155-69. http://dx.doi.org/10.1023/A:1011503032353.
- Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 2007;46:947-54. http://dx.doi.org/10.1016/j.jhep.2007.02.015.
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152-60. http://dx.doi.org/10.1001/jama.295.10.1152.
- James KE, Bloch DA, Lee KK, Kraemer HC, Fuller RK. An index for assessing blindness in a multi-centre clinical trial: disulfiram for alcohol cessation – a VA cooperative study. Stat Med 1996;15:1421-34. http://dx.doi.org/10.1002/(SICI)1097-0258(19960715)15:13<1421::AID-SIM266>3.0.CO;2-H.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Hip Fracture: The Management of Hip Fracture in Adults. London: NICE; 2011.
- Chakladar A, White S. Anaesthesia 2010;65:810-14. http://dx.doi.org/10.1111/j.1365-2044.2010.06382.x.
- Information Services Division Scotland . Theatre – Direct Cost Per Hour, by Specialty [R142X] 2012. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 13 May 2014).
- NHS Reference Costs 2011–2012. Leeds: Department of Health; 2012.
- Community Bed Review. Worthing: NHS West Sussex; 2011.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Department of Health: Transforming NHS Ambulance Services. London: NAO; 2011.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Employment Rates and Average Hourly Wage for 1994 to 2012. London: Office for National Statistics; 2012.
- Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. http://dx.doi.org/10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R.
- Koval KJ, Zhou W, Sparks MJ, Cantu RV, Hecht P, Lurie J. Complications after ankle fracture in elderly patients. Foot Ankle Int 2007;28:1249-55. http://dx.doi.org/10.3113/FAI.2007.1249.
- Brown OL, Dirschl DR, Obremskey WT. Incidence of hardware-related pain and its effect on functional outcomes after open reduction and internal fixation of ankle fractures. J Orthop Trauma 2001;15:271-4. http://dx.doi.org/10.1097/00005131-200105000-00006.
- Medical Research Council: Guidelines for Good Clinical Practice in Clinical Trials. London: Medical Research Council; 1998.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2390.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67. http://dx.doi.org/10.18637/jss.v045.i03.
- Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res 2009;467:1800-6. http://dx.doi.org/10.1007/s11999-008-0543-6.
- Giannoudis PV, Tzioupis C, Papathanassopoulos A, Obakponovwe O, Roberts C. Articular step-off and risk of post-traumatic osteoarthritis. Evidence today. Injury 2010;41:986-95. http://dx.doi.org/10.1016/j.injury.2010.08.003.
- Slobogean GP, Marra CA, Sadatsafavi M, Sanders DW. Is surgical fixation for stress-positive unstable ankle fractures cost effective? Results of a multicenter randomized control trial. J Orthop Trauma 2012;26:652-8. http://dx.doi.org/10.1097/BOT.0b013e31824aec42.
- Michelson JD. Using decision analysis to assess comparative clinical efficacy of surgical treatment of unstable ankle fractures. J Orthop Trauma 2013;27:642-8. http://dx.doi.org/10.1097/BOT.0b013e31828f9a88.
- Sanders DW, Tieszer C, Corbett B. Operative versus nonoperative treatment of unstable lateral malleolar fractures: a randomized multicenter trial. J Orthop Trauma 2012;26:129-34. http://dx.doi.org/10.1097/BOT.0b013e3182460837.
- Reidsma I, Nolte P, Marti R, Raaymakers E. Treatment of malunited fractures of the ankle: a long-term follow-up of reconstructive surgery. J Bone Joint Surg Br 2010;92:66-70. http://dx.doi.org/10.1302/0301-620X.92B1.22540.
- Rycroft-Malone J, Seers K, Titchen A, Harvey G, Kitson A, McCormack B. What counts as evidence in evidence-based practice?. J Adv Nurs 2004;47:81-90. http://dx.doi.org/10.1111/j.1365-2648.2004.03068.x.
- McInnes E, Seers K, Tutton L. Older people’s views in relation to risk of falling and need for intervention: a meta-ethnography. J Adv Nurs 2011;67:2525-36. http://dx.doi.org/10.1111/j.1365-2648.2011.05707.x.
- Staniszewska S, Bullock I, Avital L, O’Flynn N. Developing and Implementing NICE Guidance on Patient Experience 2012. http://patientexperienceportal.org/export/document/1450 (accessed 13 May 2014).
- Gooberman-Hill R, Fox R. What can qualitative approaches bring to trauma outcome research?. Injury 2011;42:321-3. http://dx.doi.org/10.1016/j.injury.2011.01.021.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. http://dx.doi.org/10.1177/1740774510392257.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7. http://dx.doi.org/10.1186/1745-6215-7-32.
- Robinson EJ, Kerr CE, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al. Lay public’s understanding of equipoise and randomisation in randomised controlled trials. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9080.
- Tutton E, Seers K, Langstaff D. Professional nursing culture on a trauma unit: experiences of patients and staff. J Adv Nurs 2008;61:145-53. http://dx.doi.org/10.1111/j.1365-2648.2007.04471.x.
- Tutton E, Seers K, Langstaff D. Hope in orthopaedic trauma: a qualitative study. Int J Nurs Stud 2012;49:872-9. http://dx.doi.org/10.1016/j.ijnurstu.2012.01.013.
- Sleney J, Christie N, Earthy S, Lyons RA, Kendrick D, Towner E. Improving recovery-Learning from patients’ experiences after injury: a qualitative study. Injury 2014;45:312-19. http://dx.doi.org/10.1016/j.injury.2012.12.025.
- Griffiths H, Jordan S. Thinking of the future and walking back to normal: an exploratory study of patients’ experiences during recovery from lower limb fracture. J Adv Nurs 1998;28:1276-88. http://dx.doi.org/10.1046/j.1365-2648.1998.00847.x.
- Trickett RW, Mudge E, Price P, Pallister I. Injury 2012;43:1071-8. http://dx.doi.org/10.1016/j.injury.2012.01.027.
- Forsberg A, Soderberg S, Engstrom A. People’s experiences of suffering a lower limb fracture and undergoing surgery. J Clin Nurs 2014;23:191-200. http://dx.doi.org/10.1111/jocn.12292.
- Van Manen M. Researching Lived Experience, Human Science for an Action Sensitive Pedagogy. Albany, NY: State University of New York Press; 1990.
- Todres L, Wheeler S. The complementarity of phenomenology, hermeneutics and existentialism as a philosophical perspective for nursing research. Int J Nurs Stud 2001;38:1-8. http://dx.doi.org/10.1016/S0020-7489(00)00047-X.
- Heidegger M. Being and Time. Oxford: Blackwell Publishing Ltd; 1962.
- Mackey S. Phenomenological nursing research: methodological insights derived from Heidegger’s interpretive phenomenology. Int J Nurs Stud 2005;42:179-86. http://dx.doi.org/10.1016/j.ijnurstu.2004.06.011.
- Madjar I, Walton J. Nursing and the Experience of Illness, Phenomenology in Practice. London: Routledge; 1999.
- Leonard V, Benner P. Interpretive Phenomenology, Embodiment, Loving and Ethics in Health and Illness. Newbury Park, CA: Sage Publications Inc.; 1994.
- Lincoln YS, Guba E. Naturalistic Inquiry. Newbury Park, CA: Sage Publications Inc.; 1985.
- Clarke LH, Griffin M. PACC Research Team . Failing bodies: body image and multiple chronic conditions in later life. Qual Health Res 2008;18:1084-95. http://dx.doi.org/10.1177/1049732308320113.
- Oakley A. Fracture, Adventures of a Broken Body. Bristol: The Policy Press; 2007.
- Morse JM, Penrod J. Linking concepts of enduring, uncertainty, suffering, and hope. Image J Nurs Sch 1999;31:145-50. http://dx.doi.org/10.1111/j.1547-5069.1999.tb00455.x.
- Lawlor D. Test of time: a case study in the functioning of social systems as a defence against anxiety: rereading 50 years on. Clin Child Psychol Psychiatry 2009;14:523-30. http://dx.doi.org/10.1177/1359104509339545.
- Kvigne K, Kirkevold M. Living with bodily strangeness: women’s experiences of their changing and unpredictable body following a stroke. Qual Health Res 2003;13:1291-310. http://dx.doi.org/10.1177/1049732303257224.
- Bury M. Chronic illness as biographical disruption. Sociol Health Ill 1982;4:167-82. http://dx.doi.org/10.1111/1467-9566.ep11339939.
- Williams S. Chronic illness as biographical disruption or biographical disruption as chronic illness? Reflections on a core concept. Sociol Health Ill 2000;22:40-67. http://dx.doi.org/10.1111/1467-9566.00191.
- Thomas EJ, Levack WM, Taylor WJ. Self-reflective meaning making in troubled times: change in self-identity after traumatic brain injury. Qual Health Res 2014;24:1033-47. http://dx.doi.org/10.1177/1049732314542809.
- Hallrup L, Albertsson D, Tops A, Dahlberg K, Grahn B. Elderly women’s experience of living with fall risk in a fragile body: a reflective lifeworld approach. Health Soc Care Community 2009;17:379-87. http://dx.doi.org/10.1111/j.1365-2524.2008.00836.x.
- Reventlow SD, Hvas L, Malterud K. Making the invisible body visible. Bone scans, osteoporosis and women’s bodily experiences. Soc Sci Med 2006;62:2720-31. http://dx.doi.org/10.1016/j.socscimed.2005.11.009.
- Keene DJ, Williamson E, Bruce J, Willett K, Lamb SE. Early ankle movement versus immobilization in the postoperative management of ankle fracture in adults: a systematic review and meta-analysis. J Orthop Sports Phys Ther 2014;44:690-701.
- Kearney RS, Achten J, Lamb SE, Plant C, Costa ML. A systematic review of patient-reported outcome measures used to assess Achilles tendon rupture management: what’s being used and should we be using it?. Br J Sports Med 2012;46:1102-9. http://dx.doi.org/10.1136/bjsports-2011-090497.
- Martin RL, Irrgang JJ. A survey of self-reported outcome instruments for the foot and ankle. J Orthop Sports Phys Ther 2007;37:72-84. http://dx.doi.org/10.2519/jospt.2007.2403.
- Nilsson GM, Eneroth M, Ekdahl CS. The Swedish version of OMAS is a reliable and valid outcome measure for patients with ankle fractures. BMC Musculoskelet Disord 2013;14. http://dx.doi.org/10.1186/1471-2474-14-109.
- Korim MT, Payne R, Bhatia M. A case–control study of surgical site infection following operative fixation of fractures of the ankle in a large UK trauma unit. Bone Joint J 2014;96-b:636-40.
- Ovaska MT, Makinen TJ, Madanat R, Huotari K, Vahlberg T, Hirvensalo E, et al. Risk factors for deep surgical site infection following operative treatment of ankle fractures. J Bone Joint Surg Am 2013;95:348-53. http://dx.doi.org/10.2106/JBJS.K.01672.
- Shipman A, Alsousou J, Keene DJ, Dyson IN, Lamb SE, Willett K, et al. Quantitative biomechanical comparison of ankle fracture casting methods. Biomed Tech 2015;60:263-7. http://dx.doi.org/10.1515/bmt-2014-0069.
- Bhadra AK, Roberts CS, Giannoudis PV. Nonunion of fibula: a systematic review. Int Orthop 2012;36:1757-65. http://dx.doi.org/10.1007/s00264-012-1556-z.
- Charnley J. The Closed Treatment of Common Fractures. Cambridge: Colt Books, in association with The John Charnley Trust; 1999.
- Spiegel DA, Gosselin RA, Coughlin RR, Joshipura M, Browner BD, Dormans JP. The burden of musculoskeletal injury in low and middle-income countries: challenges and opportunities. J Bone Joint Surg Am 2008;90:915-23. http://dx.doi.org/10.2106/JBJS.G.00637.
- Mock C, Cherian M. The global burden of musculoskeletal injuries: challenges and solutions. Clin Orthop Relat Res 2008;466:2306-16. http://dx.doi.org/10.1007/s11999-008-0416-z.
- Farrell B, Kenyon S, Shakur H. Managing clinical trials. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-78.
Appendix 1 Trial management
Many clinical trials fail to deliver because not enough attention is paid to the practicalities of delivering the project. The AIM trial recruited slightly ahead of schedule, maintaining the trajectory of recruitment (Figure 21), while also obtaining very good levels of follow-up and relatively low missing outcome data. We believe that an experienced, dedicated, trial management team who could apply the same depth of consideration as given to the scientific elements of the study, in addition to the dedication of the clinicians and researchers at the recruiting hospitals, were critical to success of the AIM trial. In this appendix we aim to provide a brief overview of the key lessons learned in the central management of the AIM trial, with the hope that sharing this information will benefit other projects, just as we have benefited from the experiences and expertise of others.
FIGURE 21.
Recruitment graph for the AIM trial. Sample size target of 620 met by 95 pilot and 525 multicentre study participants.
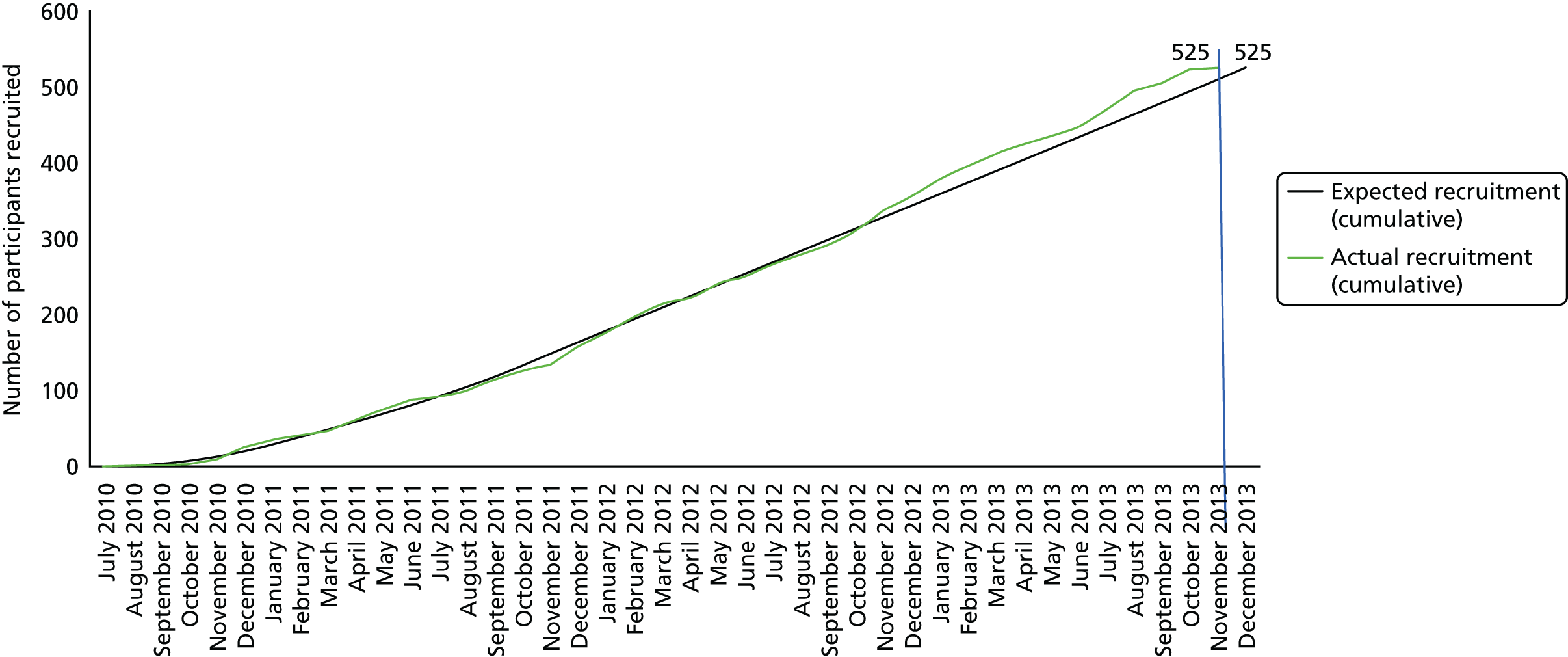
Overview of the Ankle Injury Management trial project milestones
Study start date was January 2010 and involved 20 recruiting sites around the UK at any one time. The sample size was 620 participants who were recruited from orthopaedic trauma wards/clinics. The project funding was for 5 years, with a 6-month set-up phase, 42-month recruitment phase, 6-month follow-up phase and 6-month analysis and write-up phase. The Health Technology Assessment programme recognises that good trial management is key to the success of a project and recommends all primary research projects appoint a dedicated trial manager.
During the early part of the set up phase of the study, the trial manager and chief investigator worked closely, met regularly and adopted a practical, delivery-focused approach. This emphasis on conduct took into account the likely barriers to both clinical and participant participation and helped to avoid unworkable systems and processes. The approach taken by the trial manager was to take the protocol and turn it into a living project, guided by a focus on how the processes would work best in the realities of practice. Each activity within the project was evaluated in depth by asking questions such as: ‘If I was doing that – who I would be? What would I need? How would I do it? When would I do it? How many times? Where would I do it? To whom?’
The pathway of the participant was tracked from first identification at screening, through to follow-up, to ensure all decisions made centrally by the trial team took this practical process into account. The attention to detail paid to the design of CRFs and the order of paperwork prepared for sites was always from the users’ standpoint. The approach taken is consistent with the recommendations of Farrell et al.,121 who call for trial management to be integral part of trial delivery.
Building a trial-specific multidisciplinary management team
A multidisciplinary team was funded for this study. The chief investigator was joined by an experienced senior trial manager who played a lead role in developing the roles of the research physiotherapist and the data/administrative co-ordinator based on the needs of project/protocol. The research physiotherapist role required travel throughout the UK to deliver intervention training to surgeons at collaborating sites.
In addition, research nurses based at the Kadoorie Centre for Critical Care Research and Education, in the John Radcliffe Hospital in Oxford, who led the pilot study phase, provided key guidance and support, particularly during the set-up phase of the multicentre trial.
During site recruitment, staff reacted to the needs of sites as quickly as possible, discussing any concerns raised within the team and, when necessary, held brainstorming sessions to think through all possible approaches. Central trial management staff actively participated in the sessions, were given autonomy to solve problems independently, and felt fully informed regarding key aspects of the study and the current status.
The management group, made up of coapplicants and the central trial management staff, met every 3 months to review progress. In the early stages e-mail updates were exchanged with the group and, when necessary, specific issues were discussed with relevant members.
Recruitment targets and site selection
Our target was to establish 20 sites, the process of site recruitment and management is outlined in Figure 22. In the predefined project management timetable, at least one patient per month, per site, was required to achieve target. Sites approached the trial office via two routes: (1) known collaborators (orthopaedic surgeons who were part of the Injuries and Emergencies Specialty Group or the Trauma and Orthopaedic Research Collaboration), and (2) collaborators enquiring after searching the UK Clinical Research Network Portfolio Database. Interested sites completed a site feasibility questionnaire.
FIGURE 22.
Site recruitment and management process for the AIM trial. CS, collaboration sheet.
Staff resources funded to support trial activities at sites
When the study was funded it was identified that trauma surgeons would need additional research nurse/therapist support over that available via the UK Clinical Research Network if the study was to be successful. This study involved a population aged ≥ 60 years, who had attended hospital after injury. Research nurse/therapist time with these patients was key with regard to study tasks, as busy orthopaedic trauma surgeons would need to be free to react to other emergency operating needs. In addition, participants attended for 6-week and 6-month clinic appointments at which study data were collected, one of which was a blinded assessment. The local nurse organised these visits and the relationship built with the participant helped in achieving good follow-up rates. With the knowledge that all sites were required to recruit at least one participant per month from the outset and that we had a resource in the research grant that we could offer sites which equated to a 0.25 whole-time equivalent band 6 nurse cost, close attention was paid to recruitment to ensure this resource was used wisely. The sponsors contract with the participating trust indicated that if ‘insufficient numbers of participants are being recruited by the participating site to the study’, there was the possibility of terminating the agreement. This allowed us to move resources to a new site if necessary.
Monitoring recruitment
Recruitment was monitored closely. On the first of the month a review of the previous month’s activity was carried out by the trial office team. Both recruitment and screening figures per site were investigated, and measured against expected target and figures provided by the site on the original site feasibility questionnaire.
The following recruitment documentation was circulated to site personnel each month:
-
overall recruitment graph (actual/target)
-
site-specific recruitment graphs (actual/target)
-
recruitment table listing all sites by monthly recruitment and recruitment rate against target
-
e-mail highlighting recruitment to date, highest recruiters overall and for the previous month.
As the study progressed, we also introduced a last 6 months-only league table and recruitment rate. This allowed sites who had underperformed initially to be congratulated for recent successes.
If a site had under-recruited for 2 months, the office team would contact the principal investigator and research nurse/therapist at the local site. Practicalities of recruitment were talked through in detail in an attempt to identify where the barriers to recruitment lay. A visit would be offered and/or the opportunity for the nursing staff to make contact with other nurses in ‘successful’ sites to see what had worked locally for them.
We held ‘collaborators meetings’ each year where the principal investigators and up to two further surgeons, along with two research staff were invited. In addition, in 2011 we held a researcher day in Oxford for the nursing and physiotherapy staff from our sites as a forum to share learning and problem-solve common recruitment challenges.
When it came to under-recruiting sites, we offered support as outlined above. One issue we could not resolve, however, was the lack of suitable patients presenting. If lower than expected recruitment took place over a 3-month period, and despite the best efforts of all concerned then continued, resources linked to the study were re-allocated (i.e. need to establish another site). This was the case with four of our sites:
-
In one trust we established recruitment at another hospital within the same trust to allow a wider screening pool: two geographically (approximately 20 miles) separate locations for which recruitment figures counted as one site under their trust.
-
At another trust, two hospitals who expected a large number of recruits each (geographically separate as above), accepted that recruitment was unlikely to improve and therefore agreed for one of the two nurse funding allocations to be removed and for the recruitment figures to be merged, representing the sites as a trust rather than individually.
-
At one site, we investigated establishing another hospital within their trust but this was not possible because of restructuring changes.
-
At another site, the lack of a suitable trauma hospital within their trust meant that establishing recruitment at another hospital was not possible.
For the latter two hospitals the sites were closed and new sites were established.
Screening
As mentioned, activity overall at a site was reviewed monthly which meant that screening figures were interrogated alongside recruitment figures. Screening forms, one per patient, listed the inclusion/exclusion criteria and allowed the person completing the form to select the reason why the patient had been unsuitable for the study. The forms were returned to the study office. The first check was the number of screening forms per month returned to the office, by site. Sites were contacted if no forms were returned for 2 or more months. The second check was carried out by looking at the reasons, overall and per site, why patients were not being recruited.
Data management
Sites returned CRFs to the study office using a Freepost account. All data queries were dealt with in a systematic and timely fashion to ensure missing fields could be highlighted and addressed as appropriate. Data queries related to primary outcome were dealt with within 24 hours from receipt.
Documentation and process training
Visits to sites were part of the site set-up. Practical demonstrations of the documentation pack were provided. Owing to the relatively small numbers of patients per site, it was important that the documentation was easy to understand and that the CRFs were designed taking into account the infrequency of use.
Trial promotion
A range of promotional materials were also used; some examples were:
-
A study logo was used on all documentation to allow easy identification of study materials.
-
Pens with the study logo and office telephone number were provided.
-
Removable sticky notelets were provided after a request by site research staff, so we arranged for these to be printed with the logo and key inclusion criteria.
-
Magnetic calendars were provided to act as quick tool for nurses to calculate the 6-week and 6-month follow-up appointments.
-
Newsletters were provided to sites in both hard copy and electronic formats. A large distribution list was established that included any member of staff at a site who had a role to play.
-
A website was created with the ability to update recruitment figures and the last site to recruit. This information was also circulated to the collaborators via e-mail, and allowed for constant referral to our website where fuller information about the trial could be found.
-
New simple and quick-to-read staff packs were provided, including key eligibility information and a one-page summary protocol. This was provided to sites to disseminate to new staff joining their team.
-
A consulting room poster designed for surgeons to quickly refer to the eligibility criteria was provided, which also listed their local research nurse and principal investigator details (bleeper, mobile, etc.) to allow referral if a suitable patient presented.
-
Microsoft PowerPoint® presentations (Microsoft Corporation, Redmond, WA, USA) were made, updating staff at sites with study progress.
The role of trial management
In this appendix we have highlighted some of the key trial management activities that the AIM trial team believe facilitated successful recruitment. However, it is recognised that it was the hard work and engagement of the surgeons and research nurses/therapists at the recruiting sites that made the study a success. The AIM trial team aimed to support sites in their endeavours to aid their work on the study, while also ensuring adherence with the protocol and optimising data collection and recruitment.
Appendix 2 Clinical results: intention-to-treat analysis
Intention-to-treat analyses results
The results from the ITT analyses of the primary and secondary outcomes are presented in this section of the appendix. The ITT analyses displayed similar results to that of the per-protocol analyses.
FIGURE 23.
Plot of mean scores and 95% CIs for the OMASs over time (ITT).
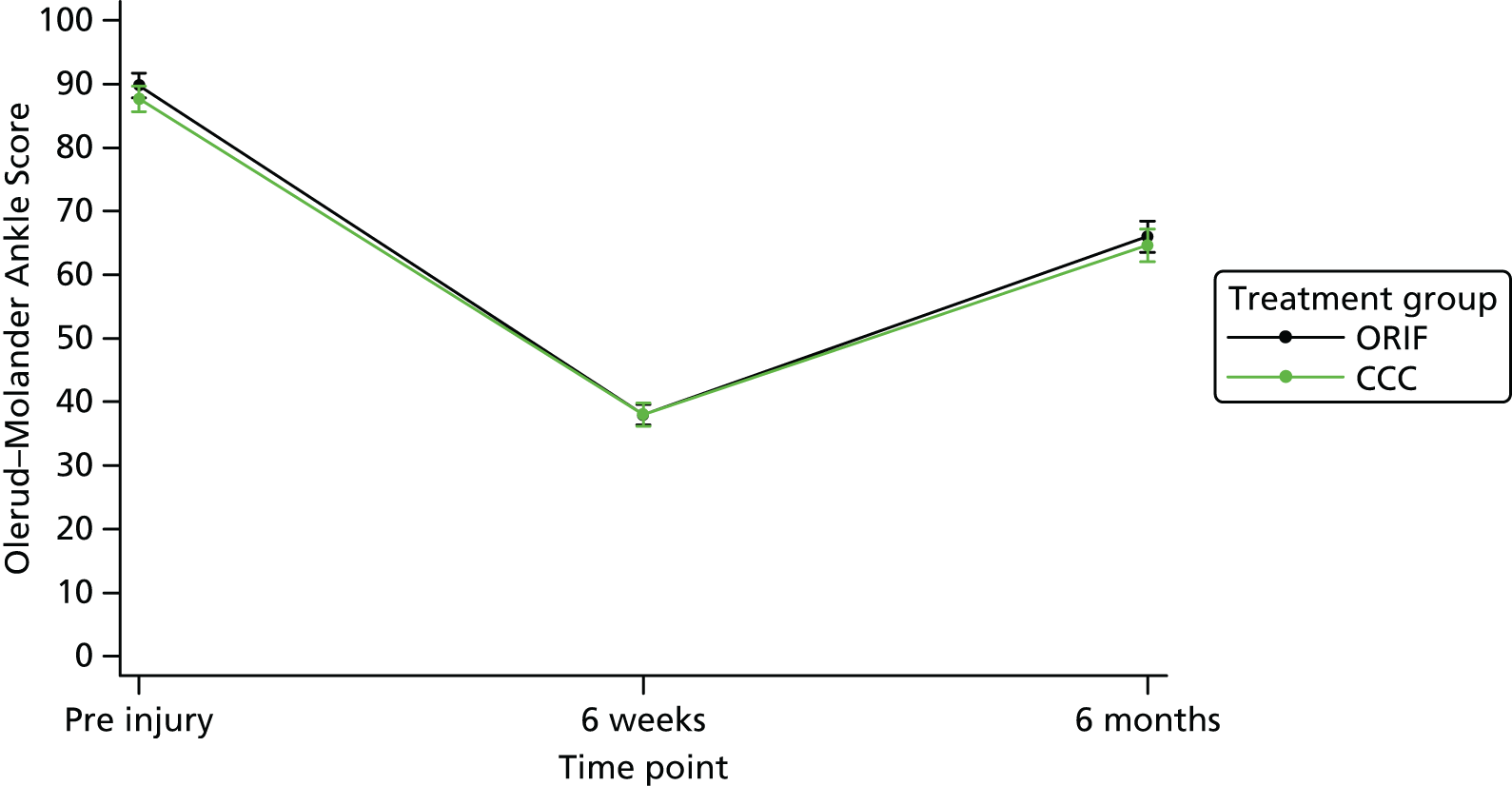
FIGURE 24.
Plot of mean scores and 95% CIs for the SF-12 mental component summary score over time (ITT).

FIGURE 25.
Plot of means and 95% CIs for the SF-12 physical component summary score over time (ITT).

FIGURE 26.
Plot of means and 95% CIs for the EQ-5D score over time (ITT).
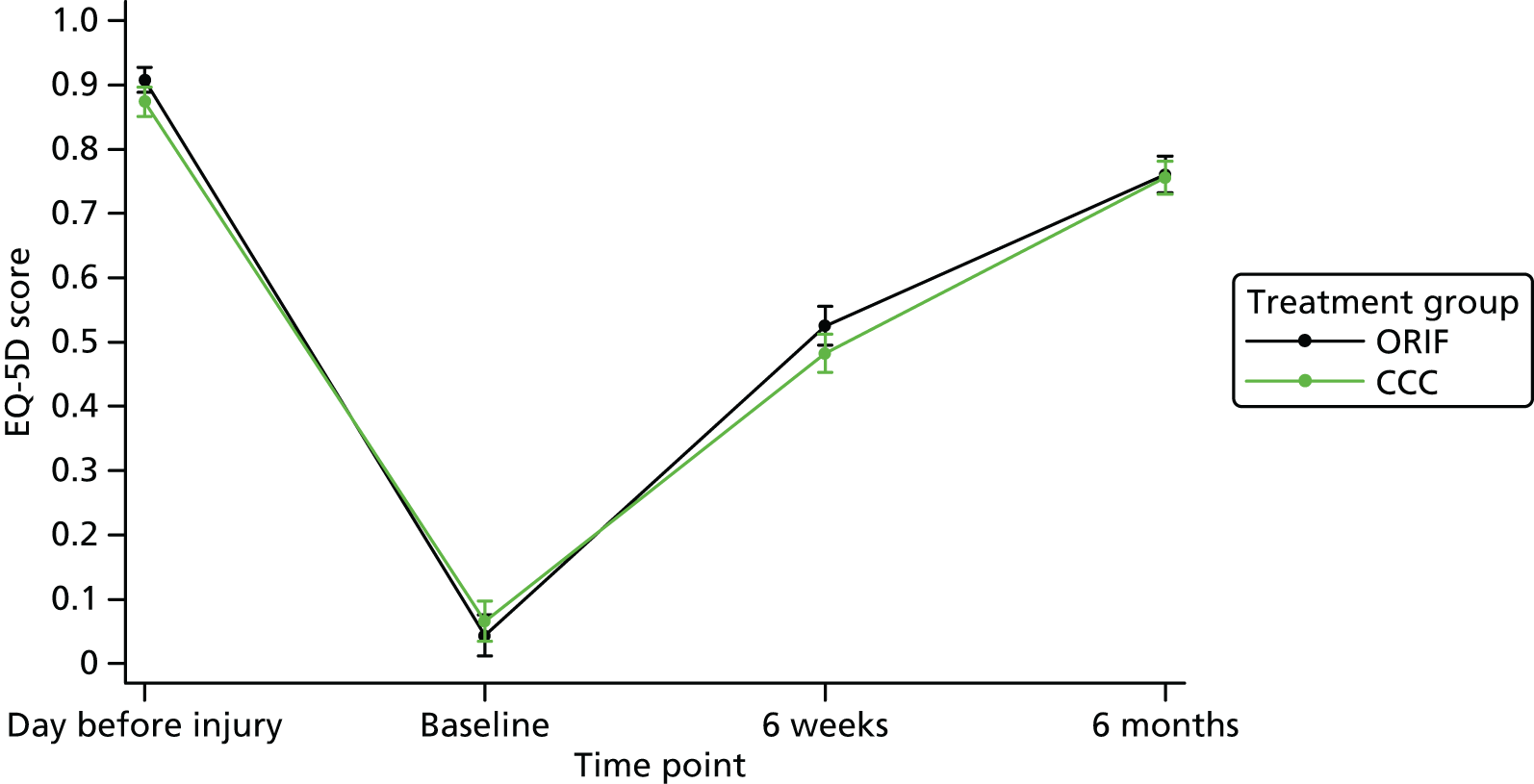
| Outcome | Treatment group | Unadjusted statistics (95% CI); p-value | Adjusted statistics (95% CI); p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| SF-12 mental component summary score | ||||
| 6 weeks | ||||
| Mean | 48.3 | 48.7 | 0.34 (–1.40 to 2.09); 0.700 | –0.14 (–1.78, 1.50); 0.864 |
| n | 304 | 302 | ||
| SD | 11.27 | 10.57 | ||
| Median | 49.1 | 49.6 | ||
| Minimum | 16.5 | 19.2 | ||
| Maximum | 70.2 | 68.3 | ||
| Missing | 1 | 1 | ||
| 6 months | ||||
| Mean | 52.0 | 52.2 | 0.21 (–1.42 to 1.83); 0.805 | –0.11 (–1.55, 1.33); 0.876 |
| n | 298 | 294 | ||
| SD | 10.47 | 9.68 | ||
| Median | 55.4 | 55.7 | ||
| Minimum | 18.9 | 19.9 | ||
| Maximum | 66.9 | 68.4 | ||
| Missing | 0 | 1 | ||
| SF-12 physical component summary score | ||||
| 6 weeks | ||||
| Mean | 33.4 | 33.3 | –0.02 (–1.10 to 1.05); 0.967 | 0.15 (–0.90 to 1.20); 0.783 |
| n | 304 | 302 | ||
| SD | 6.94 | 6.55 | ||
| Median | 32.5 | 32.7 | ||
| Minimum | 18.4 | 18.3 | ||
| Maximum | 57.1 | 54.9 | ||
| Missing | 1 | 1 | ||
| 6 months | ||||
| Mean | 45.6 | 44.2 | –1.34 (–3.00 to 0.32); 0.113 | –0.59 (–2.08 to 0.91); 0.440 |
| n | 298 | 294 | ||
| SD | 10.01 | 10.56 | ||
| Median | 46.9 | 45.4 | ||
| Minimum | 22.1 | 15.6 | ||
| Maximum | 66.2 | 64.3 | ||
| Missing | 0 | 1 | ||
| EQ-5D mobility score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 11 (3.6) | 7 (2.3) | 0.292 | |
| Level 2, n (%) | 248 (81.3) | 243 (80.2) | ||
| Level 3, n (%) | 17 (5.6) | 25 (8.3) | ||
| Missing, n (%) | 29 (9.5) | 28 (9.2) | ||
| 6 months | ||||
| Level 1, n (%) | 135 (45.3) | 120 (40.7) | 0.475 | |
| Level 2, n (%) | 131 (44.0) | 144 (48.8) | ||
| Level 3, n (%) | 3 (1.0) | 3 (1.0) | ||
| Missing, n (%) | 29 (9.7) | 28 (9.5) | ||
| EQ-5D self-care score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 167 (54.8) | 149 (49.1) | 0.028 | |
| Level 2, n (%) | 108 (35.4) | 118 (38.9) | ||
| Level 3, n (%) | 1 (0.3) | 8 (2.6) | ||
| Missing, n (%) | 29 (9.5) | 28 (9.2) | ||
| 6 months | ||||
| Level 1, n (%) | 243 (81.5) | 246 (83.4) | 0.642 | |
| Level 2, n (%) | 22 (7.5) | 19 (6.4) | ||
| Level 3, n (%) | 4 (1.3) | 2 (0.7) | ||
| Missing, n (%) | 29 (9.7) | 28 (9.5) | ||
| EQ-5D usual activities score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 11 (3.6) | 11 (3.6) | 0.023 | |
| Level 2, n (%) | 152 (49.8) | 120 (39.6) | ||
| Level 3, n (%) | 113 (37.1) | 144 (47.5) | ||
| Missing, n (%) | 29 (9.5) | 28 (9.3) | ||
| 6 months | ||||
| Level 1, n (%) | 161 (54.0) | 142 (48.1) | 0.265 | |
| Level 2, n (%) | 98 (32.9) | 111 (37.6) | ||
| Level 3, n (%) | 10 (3.4) | 14 (4.8) | ||
| Missing, n (%) | 29 (9.7) | 28 (9.5) | ||
| EQ-5D pain/discomfort score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 132 (43.3) | 137 (45.2) | 0.375 | |
| Level 2, n (%) | 137 (44.9) | 136 (44.9) | ||
| Level 3, n (%) | 6 (2.0) | 2 (0.7) | ||
| Missing, n (%) | 30 (9.8) | 28 (9.2) | ||
| 6 months | ||||
| Level 1, n (%) | 120 (40.3) | 115 (39.0) | 0.496 | |
| Level 2, n (%) | 140 (47.0) | 147 (49.8) | ||
| Level 3, n (%) | 9 (3.0) | 5 (1.7) | ||
| Missing, n (%) | 29 (9.7) | 28 (9.5) | ||
| EQ-5D anxiety/depression score | ||||
| 6 weeks | ||||
| Level 1, n (%) | 186 (61.0) | 179 (59.1) | 0.108 | |
| Level 2, n (%) | 84 (27.5) | 95 (31.4) | ||
| Level 3, n (%) | 6 (2.0) | 1 (0.3) | ||
| Missing, n (%) | 29 (9.5) | 28 (9.2) | ||
| 6 months | ||||
| Level 1, n (%) | 201 (67.4) | 210 (71.2) | 0.594 | |
| Level 2, n (%) | 61 (20.5) | 52 (17.6) | ||
| Level 3, n (%) | 6 (2.0) | 5 (1.7) | ||
| Missing, n (%) | 30 (10.1) | 28 (9.5) | ||
| EQ-5D score | ||||
| 6 weeks | ||||
| Mean | 0.53 | 0.48 | –0.04 (–0.08 to –0.001); 0.047 | –0.04 (–0.08 to 0.005); 0.088 |
| n | 275 | 275 | ||
| SD | 0.25 | 0.25 | ||
| Median | 0.59 | 0.49 | ||
| Minimum | –0.48 | –0.06 | ||
| Maximum | 1 | 1 | ||
| Missing | 30 | 28 | ||
| 6 months | ||||
| Mean | 0.76 | 0.76 | –0.004 (–0.04 to 0.03); 0.814 | –0.001 (–0.04 to 0.04); 0.968 |
| n | 268 | 267 | ||
| SD | 0.24 | 0.22 | ||
| Median | 0.80 | 0.78 | ||
| Minimum | –0.36 | –0.11 | ||
| Maximum | 1 | 1 | ||
| Missing | 30 | 28 | ||
| EQ-5D VAS score | ||||
| 6 weeks | ||||
| Mean | 72.7 | 72.0 | –0.77 (–3.73 to 2.19); 0.609 | –0.39 (–3.13 to 2.36); 0.781 |
| n | 276 | 274 | ||
| SD | 17.28 | 18.06 | ||
| Median | 75.0 | 75.0 | ||
| Minimum | 0 | 10 | ||
| Maximum | 100 | 100 | ||
| Missing | 29 | 29 | ||
| 6 months | ||||
| Mean | 77.3 | 77.4 | 0.02 (–3.09 to 3.12); 0.991 | 0.10 (–2.91 to 3.11); 0.947 |
| n | 269 | 267 | ||
| SD | 18.81 | 17.74 | ||
| Median | 81.0 | 80.0 | ||
| Minimum | 0 | 1 | ||
| Maximum | 100 | 100 | ||
| Missing | 29 | 28 | ||
| Timed up and go test (seconds) | Treatment group | p-valuea | |
|---|---|---|---|
| ORIF | CCC | ||
| Mean | 21.4 | 21.9 | 0.095 |
| n | 283 | 267 | |
| SD | 19.08 | 13.45 | |
| Median | 18.0 | 18.3 | |
| Minimum | 6.8 | 7.4 | |
| Maximum | 224.3 | 133.0 | |
| Missing | 0 | 1 | |
| Additional timed up and go walking test details | Treatment group | p-value | |
|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | ||
| Completed in under 5 minutes | |||
| Yes | 282 (99.6) | 266 (99.3) | 0.614 |
| No | 1 (0.4) | 2 (0.7) | |
| Missing | 0 | 0 | |
| Did patient use walking aid(s)? | |||
| Yes | 27 (9.5) | 32 (11.9) | 0.372 |
| No | 210 (74.2) | 194 (72.4) | |
| Missing | 46 (16.3) | 42 (15.7) | |
| Unable to complete test because of . . . | |||
| Non-weight-bearing | 0 | 1 (0.3) | 0.266 |
| Declined to complete test | 1 (0.3) | 3 (1.0) | |
| Other physical limitation | 1 (0.3) | 1 (0.3) | |
| Other | 13 (4.4) | 22 (7.5) | |
| Not applicable | 283 (95.0) | 268 (90.9) | |
| Missing | 0 | 0 | |
| Range of ankle motion | Treatment group | Unadjusted statistics (95% CI); p-value | Adjusted statistics (95% CI); p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| Angle of injured ankle dorsiflexion (°) | ||||
| 6 weeks | ||||
| Mean | 5.0 | 4.3 | –0.77 (–2.26 to 0.71); 0.306 | –0.98 (–2.27 to 0.31); 0.137 |
| n | 290 | 288 | ||
| SD | 8.68 | 9.46 | ||
| Median | 5 | 5 | ||
| Missing | 15 | 15 | ||
| 6 months | ||||
| Mean | 11.9 | 11.9 | 0.04 (–1.76 to 1.83); 0.968 | 0.28 (–1.39 to 1.94); 0.745 |
| n | 289 | 282 | ||
| SD | 10.31 | 11.49 | ||
| Median | 10 | 10 | ||
| Missing | 9 | 13 | ||
| Angle of injured ankle plantar flexion (°) | ||||
| 6 weeks | ||||
| Mean | 24.5 | 22.5 | –2.05 (–4.18 to 0.08); 0.059 | –1.95 (–4.01 to 0.11); 0.064 |
| n | 290 | 288 | ||
| SD | 12.94 | 13.11 | ||
| Median | 24 | 20 | ||
| Missing | 15 | 15 | ||
| 6 months | ||||
| Mean | 33.7 | 31.3 | –2.46 (–4.65 to –0.27); 0.028 | –2.23 (–4.25 to –0.21); 0.030 |
| n | 289 | 282 | ||
| SD | 13.68 | 12.99 | ||
| Median | 35 | 30 | ||
| Missing | 9 | 13 | ||
| Eversion of injured ankle (%) | ||||
| 6 weeks | ||||
| Mean | 49.4 | 50.2 | 0.82 (–7.26 to 8.89); 0.842 | –0.15 (–8.26 to 7.95); 0.971 |
| n | 284 | 278 | ||
| SD | 40.56 | 55.84 | ||
| Median | 50 | 50 | ||
| Missing | 21 | 25 | ||
| 6 months | ||||
| Mean | 87.6 | 86.8 | –0.79 (–12.30 to 10.71); 0.892 | –0.70 (–12.10 to 10.70); 0.904 |
| n | 289 | 277 | ||
| SD | 82.40 | 53.22 | ||
| Median | 80 | 80 | ||
| Missing | 9 | 18 | ||
| Inversion of injured ankle (%) | ||||
| 6 weeks | ||||
| Mean | 47.9 | 56.0 | 8.05 (0.51 to 15.59); 0.036 | 7.82 (0.16 to 15.47); 0.045 |
| n | 288 | 284 | ||
| SD | 36.21 | 54.00 | ||
| Median | 45.7 | 50 | ||
| Missing | 17 | 19 | ||
| 6 months | ||||
| Mean | 83.3 | 83.6 | 0.21 (–8.74 to 9.15); 0.964 | 0.42 (–8.53 to 9.37); 0.927 |
| n | 289 | 282 | ||
| SD | 64.06 | 42.29 | ||
| Median | 75 | 80 | ||
| Missing | 9 | 13 | ||
| Started partial weight-bearing | ||||
| 6 weeks | ||||
| Yes, n (%) | 110 (36.1) | 119 (39.3) | 0.414 | |
| No, n (%) | 195 (63.9) | 184 (60.7) | ||
| Missing, n (%) | 0 | 0 | ||
| Pain outcomes | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-value | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| EQ-5D pain/discomfort item | ||||
| 6 weeks | ||||
| No pain/discomfort | 132 (43.3) | 137 (45.2) | 0.90 (0.65 to 1.26); 0.555 | 0.92 (0.65 to 1.29); 0.622 |
| Moderate pain/discomfort | 137 (44.9) | 136 (44.9) | ||
| Extreme pain/discomfort | 6 (2.0) | 2 (0.7) | ||
| Missing | 30 (9.8) | 28 (9.2) | ||
| 6 months | ||||
| No pain/discomfort | 120 (40.3) | 115 (39.0) | 1.03 (0.73 to 1.44); 0.878 | 1.05 (0.74 to 1.49); 0.772 |
| Moderate pain/discomfort | 140 (47.0) | 147 (49.8) | ||
| Extreme pain/discomfort | 9 (3.0) | 5 (1.7) | ||
| Missing | 29 (9.7) | 28 (9.5) | ||
| OMAS pain item | ||||
| 6 weeks | ||||
| None | 221 (72.5) | 217 (71.6) | 1.06 (0.75 to 1.50); 0.746 | 1.13 (0.78 to 1.62); 0.519 |
| While walking on uneven surface | 28 (9.2) | 20 (6.6) | ||
| While walking on even surface outdoors | 9 (2.9) | 12 (3.9) | ||
| While walking indoors | 37 (12.1) | 37 (12.2) | ||
| Constant and severe | 10 (3.3) | 15 (5.0) | ||
| Missing | 0 | 2 (0.7) | ||
| 6 months | ||||
| None | 130 (43.6) | 137 (46.4) | 0.86 (0.64 to 1.16); 0.326 | 0.90 (0.66 to 1.22); 0.483 |
| While walking on uneven surface | 90 (30.2) | 91 (30.9) | ||
| While walking on even surface outdoors | 38 (12.8) | 35 (11.9) | ||
| While walking indoors | 31 (10.4) | 20 (6.8) | ||
| Constant and severe | 9 (3.0) | 11 (3.7) | ||
| Missing | 0 | 1 (0.3) | ||
| Patient satisfaction | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-value | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| 6 weeks | ||||
| Very dissatisfied | 16 (5.2) | 14 (4.6) | 1.01 (0.68 to 1.49); 0.976 | 1.01 (0.68 to 1.50); 0.961 |
| Somewhat dissatisfied | 6 (2.0) | 9 (3.0) | ||
| Neither satisfied nor dissatisfied | 8 (2.6) | 11 (3.6) | ||
| Somewhat satisfied | 37 (12.1) | 32 (10.6) | ||
| Very satisfied | 192 (63.0) | 193 (63.7) | ||
| Missing | 46 (15.1) | 44 (14.5) | ||
| 6 months | ||||
| Very dissatisfied | 11 (3.7) | 15 (5.1) | 1.06 (0.71 to 1.57); 0.790 | 1.05 (0.69 to 1.59); 0.814 |
| Somewhat dissatisfied | 6 (2.0) | 6 (2.0) | ||
| Neither satisfied nor dissatisfied | 13 (4.4) | 8 (2.7) | ||
| Somewhat satisfied | 36 (12.1) | 33 (11.2) | ||
| Very satisfied | 186 (62.4) | 188 (63.7) | ||
| Missing | 46 (15.4) | 45 (15.3) | ||
| Malunion typea | Treatment group | Total (N = 556), n (%) | |
|---|---|---|---|
| ORIF (N = 281), n (%) | CCC (N = 275), n (%) | ||
| No malunion | 270 (96.1) | 233 (84.7) | 503 (90.5) |
| Talar shift | 4 (1.4) | 21 (7.6) | 25 (4.5) |
| Talar tilt | 1 (0.4) | 0 | 1 (0.2) |
| Diastasis | 0 | 0 | 0 |
| Talar shift and talar tilt | 3 (1.0) | 15 (5.5) | 18 (3.2) |
| Talar shift and diastasis | 1 (0.4) | 3 (1.1) | 4 (0.7) |
| Talar tilt and diastasis | 0 | 1 (0.4) | 1 (0.2) |
| Talar shift, talar tilt and diastasis | 2 (0.7) | 2 (0.7) | 4 (0.7) |
| Missing | 28 | 36 | 64 |
| Radiological assessment | Treatment group | p-value | |
|---|---|---|---|
| ORIF (N = 281), n (%) | CCC (N = 275), n (%) | ||
| No malunion | 270 (96.1) | 233 (84.7) | < 0.001 |
| Malunion | 11 (3.9) | 42 (15.3) | |
| Missing | 28 | 36 | |
| Radiological assessment | Treatment group | p-value | |
|---|---|---|---|
| ORIF (N = 281), n (%) | CCC (N = 274), n (%) | ||
| Lateral malleolus | |||
| Radiologically fracture not united | 0 | 8 (2.9) | 0.003 |
| Not injured/no issues with union identified | 281 (100) | 266 (97.1) | |
| Missing | 28 | 37 | |
| Medial malleolus | |||
| Radiologically fracture not united | 3 (1.1) | 20 (7.3) | < 0.001 |
| Not injured/no issues with union identified | 278 (98.9) | 254 (92.7) | |
| Missing | 28 | 37 | |
| OMAS | Treatment group | Unadjusted odds ratio (95% CI); p-value | Adjusted odds ratio (95% CI); p-valuea | |
|---|---|---|---|---|
| ORIF, n (%) | CCC, n (%) | |||
| 6 weeks | ||||
| Poor (0–30%) | 98 (32.1) | 105 (34.6) | 0.94 (0.68 to 1.29); 0.686 | 0.91 (0.65 to 1.27); 0.561 |
| Fair (31–60%) | 193 (63.4) | 179 (59.1) | ||
| Good (61–90%) | 12 (3.9) | 17 (5.6) | ||
| Excellent (91–100%) | 1 (0.3) | 0 | ||
| Missing | 1 (0.3) | 2 (0.7) | ||
| 6 months | ||||
| Poor (0–30%) | 19 (6.4) | 25 (8.5) | 0.90 (0.67 to 1.23); 0.520 | 0.97 (0.70 to 1.34); 0.854 |
| Fair (31–60%) | 95 (31.9) | 101 (34.2) | ||
| Good (61–90%) | 160 (53.7) | 135 (45.8) | ||
| Excellent (91–100%) | 24 (8.0) | 33 (11.2) | ||
| Missing | 0 | 1 (0.3) | ||
| Treatment received | Blinded assessors guess | Total | James’ Blinding Index (95% CI) | ||
|---|---|---|---|---|---|
| CCC | ORIF | Don’t know | |||
| CCC | 38 | 24 | 174 | 236 | 0.82 (0.79 to 0.84) |
| ORIF | 11 | 50 | 180 | 241 | |
| Total | 49 | 74 | 354 | 477 | |
| Event | Number of participants experiencing an event, n (%) | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||
| Time to discharge (days) | 613 (98.9) | 0.97 (0.83 to 1.14) | 0.726 | 1.11 (0.94 to 1.31) | 0.230 |
| Time to readmission (days) | 89 (14.4) | 1.32 (0.82 to 2.11) | 0.249 | 2.05 (1.14 to 3.72) | 0.017 |
| Process variable | Treatment group | Total | p-value | |
|---|---|---|---|---|
| ORIF | CCC | |||
| Randomisation | ||||
| Time from injury to randomisation (days) | ||||
| Mean | 2.8 | 2.8 | 2.8 | 0.804 |
| n | 309 | 311 | 620 | |
| SD | 2.66 | 2.97 | 2.82 | |
| Median | 2 | 2 | 2 | |
| IQR | 1–4 | 1–3.4 | 1–3.7 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 15 | 28 | 28 | |
| Missing | 0 | 0 | 0 | |
| Randomisation/theatre | ||||
| Time from randomisation to theatre procedure (days) | ||||
| Mean | 2.2 | 2.1 | 2.2 | 0.789 |
| n | 309 | 306 | 615 | |
| SD | 2.55 | 2.24 | 2.40 | |
| Median | 1.0 | 2.0 | 1.4 | |
| IQR | 0–3.4 | 0–3 | 0–3 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 17 | 14.4 | 17 | |
| Missing | 0 | 5 | 5 | |
| Treatment | ||||
| Time from injury to primary treatment (days) | ||||
| Mean | 5.0 | 4.9 | 5.0 | 0.519 |
| n | 309 | 306 | 615 | |
| SD | 3.49 | 3.30 | 3.39 | |
| Median | 5.0 | 4.4 | 4.4 | |
| IQR | 2–7 | 2–7 | 2–7 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 19 | 18 | 19 | |
| Missing | 0 | 5 | 5 | |
| Theatre | ||||
| Time from entry into anaesthetic room to start time in theatre (minutes) | ||||
| Mean | 29.2 | 18.0 | 23.6 | < 0.001 |
| n | 306 | 306 | 612 | |
| SD | 14.78 | 10.74 | 14.06 | |
| Median | 27.0 | 16.0 | 21.0 | |
| IQR | 18–37 | 10–24 | 14–30 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 90 | 100 | 100 | |
| Missing | 3 | 5 | 8 | |
| Time from start to end of procedure in theatre (minutes) | ||||
| Mean | 78.9 | 30.3 | 54.7 | < 0.001 |
| n | 307 | 305 | 612 | |
| SD | 29.88 | 24.18 | 36.49 | |
| Median | 78 | 25.0 | 45.0 | |
| IQR | 60–96 | 20–32 | 24–80.5 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 211 | 216 | 216 | |
| Missing | 2 | 6 | 8 | |
| Additional theatrea procedure | ||||
| Time from primary procedure to additional procedure in theatre (days) | ||||
| Mean | 4.9 | 10.4 | 8.2 | 0.009 |
| n | 15 | 23 | 38 | |
| SD | 1.99 | 11.11 | 9.08 | |
| Median | 5.0 | 7.0 | 6.0 | |
| IQR | 3–6 | 5–12.4 | 4–9 | |
| Minimum | 2 | 1 | 1 | |
| Maximum | 8.4 | 56.9 | 56.9 | |
| Missing | 0 | 0 | 0 | |
| Time from entry into anaesthetic room to start time in theatre (minutes) | ||||
| Mean | 33.3 | 29.0 | 30.7 | 0.419 |
| n | 15 | 23 | 38 | |
| SD | 12.27 | 17.83 | 15.83 | |
| Median | 33 | 22.0 | 27.5 | |
| IQR | 20–45 | 15–42 | 19–43 | |
| Minimum | 15 | 4 | 4 | |
| Maximum | 50 | 62 | 62 | |
| Missing | 0 | 0 | 0 | |
| Time from start to end of procedure in theatre (minutes) | ||||
| Mean | 79.7 | 76.3 | 77.6 | 0.530 |
| n | 15 | 23 | 38 | |
| SD | 36.42 | 46.83 | 42.52 | |
| Median | 80.0 | 70.0 | 77.5 | |
| IQR | 56–94 | 44–90 | 50–90 | |
| Minimum | 21 | 18 | 18 | |
| Maximum | 180 | 225 | 225 | |
| Missing | 0 | 0 | 0 | |
| Theatre/hospital discharge | ||||
| Time from theatre to hospital discharge | ||||
| Mean | 5.3 | 5.9 | 5.6 | 0.021 |
| n | 309 | 304 | 613 | |
| SD | 5.84 | 9.17 | 7.68 | |
| Median | 3.0 | 2.5 | 3.0 | |
| IQR | 2–6.4 | 1–7 | 1–7 | |
| Minimum | 0 | 0 | 0 | |
| Maximum | 39.9 | 74.3 | 74.3 | |
| Missing | 0 | 7 | 7 | |
| Hospital discharge/hospital readmission | ||||
| Time from hospital discharge to readmission (days) | ||||
| Mean | 62.4 | 46.8 | 51.7 | 0.054 |
| n | 32 | 70 | 102 | |
| SD | 54.22 | 51.36 | 52.51 | |
| Median | 44.7 | 14.7 | 26.2 | |
| IQR | 17.2–101 | 9–75.9 | 10–95.3 | |
| Minimum | 1 | 4 | 1 | |
| Maximum | 161.2 | 174.6 | 174.6 | |
| Missing | 0 | 0 | 0 | |
| Time between events | Treatment group | p-value | |
|---|---|---|---|
| ORIF | CCC | ||
| Time from injury to allocated treatment received (days) | |||
| Mean | 5.2 | 4.8 | 0.186 |
| n | 302 | 277 | |
| SD | 3.73 | 3.26 | |
| Median | 5 | 4 | |
| IQR | 2–7 | 2–7 | |
| Minimum | 0 | 0 | |
| Maximum | 27 | 18 | |
| Missing | 7 | 34 | |
| Time from randomisation to allocated treatment received (days) | |||
| Mean | 2.4 | 2.0 | 0.326 |
| n | 302 | 277 | |
| SD | 2.97 | 2.10 | |
| Median | 1 | 2 | |
| IQR | 0–4 | 0–3 | |
| Minimum | 0 | 0 | |
| Maximum | 26 | 10 | |
| Missing | 7 | 34 | |
| Complication type | Treatment group | |||||
|---|---|---|---|---|---|---|
| ORIF (n = 309) | CCC (n = 311) | |||||
| Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | |
| Interoperative fracture | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Imperfect reduction reported | 0 | 0 | 0 | 4 (1.3) | 0 | 4 |
| Compartment syndrome | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular injury | 1 (0.3) | 0 | 1 | 1 (0.3) | 0 | 1 |
| Nerve palsy | 2 (0.6) | 0 | 2 | 2 (0.6) | 0 | 2 |
| Wound breakdown | 20 (6.5) | 1 (0.3) | 22 | 5 (1.6) | 0 | 5 |
| Infection | 7 (2.3) | 1 (0.3) | 9 | 2 (0.6) | 0 | 2 |
| Septicaemia | 0 | 0 | 0 | 0 | 0 | 0 |
| Other clinical issue with wound (not breakdown or infection) | 6 (1.9) | 0 | 6 | 1 (0.3) | 0 | 1 |
| Clinical issue with metalwork | 4 (1.3) | 0 | 4 | 0 | 0 | 0 |
| Implant failure | 5 (1.6) | 0 | 5 | 0 | 0 | 0 |
| Non-wound skin problem | 13 (4.2) | 0 | 13 | 12 (3.9) | 0 | 12 |
| Pain from cast | 12 (3.9) | 0 | 12 | 16 (5.1) | 0 | 16 |
| Plaster sore | 15 (4.9) | 0 | 15 | 22 (7.1) | 0 | 22 |
| Plaster saw laceration | 1 (0.3) | 0 | 1 | 5 (1.6) | 0 | 5 |
| DVT | 3 (1.0) | 0 | 3 | 6 (1.9) | 0 | 6 |
| PE | 1 (0.3) | 0 | 1 | 6 (1.9) | 0 | 6 |
| Refracture of ankle | 0 | 0 | 0 | 0 | 0 | 0 |
| Fall postoperatively | 3 (1.0) | 0 | 3 | 4 (1.3) | 0 | 4 |
| Other fracture sustained | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Poor bone quality encountered | 4 (1.3) | 0 | 4 | 0 | 0 | 0 |
| Poor skin condition encountered | 3 (1.0) | 0 | 3 | 6 (1.9) | 0 | 6 |
| Non-union | 0 | 0 | 0 | 2 (0.6) | 0 | 2 |
| Complication type | Treatment group | |||||
|---|---|---|---|---|---|---|
| ORIF (n = 309) | CCC (n = 311) | |||||
| Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | Number of participants experiencing an event once (%) | Number of participants experiencing an event twice (%) | Absolute number of events | |
| Remanipulation and traditional cast | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Convert to external fixation | 0 | 0 | 0 | 0 | 0 | 0 |
| Convert to retrograde nail | 0 | 0 | 0 | 0 | 0 | 0 |
| Revision of ORIF | 3 (1.0) | 0 | 3 | 1 (0.3) | 0 | 1 |
| Wound washout | 2 (0.7) | 0 | 2 | 1 (0.3) | 0 | 1 |
| Delayed wound closure | 0 | 0 | 0 | 0 | 0 | 0 |
| Wound debridement | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Incision and drainage of haematoma | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Amputation | 0 | 0 | 0 | 0 | 0 | 0 |
| Removal of syndesmotic screws | 6 (1.9) | 0 | 6 | 1 (0.3) | 0 | 1 |
| Removal of other metalwork | ||||||
| Because of infection | 1 (0.3) | 1 (0.3) | 3 | 0 | 0 | 0 |
| With washout | 0 | 0 | 0 | 2 (0.6) | 0 | 2 |
| Because of pain | 0 | 0 | 0 | 0 | 0 | 0 |
| Because of poor position of implant | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
| Other reason | 1 (0.3) | 0 | 1 | 0 | 0 | 0 |
Appendix 3 Health economic evaluation
Per protocol
With completely missing cases via imputation (per protocol)
We repeated the economic evaluation including patients with completely missing information. These patients were included by imputing their missing information. Results with imputed data included were comparable to the results with those data removed.
The full per-protocol population included 573 patients: 275 and 298 in the CCC and ORIF groups, respectively. Using the best-fit models, compared with ORIF, CCC had a mean cost savings to the NHS and society of £648 and £557, respectively, and comparable QALYs (difference = +0.01) (Table 63). Cost-effectiveness planes displayed the majority of incremental costs as negative while incremental QALYs were largely positive, but with a modest number of estimates being negative (Figures 27 and 28). The probability that CCC is cost-effective was very high over the range of willingness to pay for both the NHS (> 94%) and societal perspectives (> 84%) (Figures 29 and 30).
| Outcome | Treatment group | Difference, mean (95% CI) | |
|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | ||
| NHS costs (£) | 6777 (5367 to 8522) | 6129 (4796 to 7822) | –648 (–1402 to 66) |
| Societal costs (£) | 7969 (6448 to 9789) | 7412 (5935 to 9204) | –557 (–1673 to 620) |
| QALYs | 0.2949 (0.2746 to 0.3132) | 0.3037 (0.2865 to 0.3196) | 0.0088 (–0.0163 to 0.0350) |
| Variable | Complete, n (%) | Missing, n (%) | p-value |
|---|---|---|---|
| n | 520 (91) | 53 (9) | – |
| Age, years [mean (SD)] | 71 (7.13) | 68 (7.66) | 0.01 |
| OMAS [mean (SD)] | 88.5 (17.5) | 94.6 (9.9) | 0.01 |
| Female | 385 (74) | 43 (81) | 0.33 |
| Heart disease | 68 (13) | 8 (15) | 0.84 |
| Hypertension | 223 (43) | 21 (40) | 0.76 |
| Asthma | 72 (14) | 9 (17) | 0.68 |
| Diabetes mellitus | 50 (10) | 6 (11) | 0.88 |
| Epilepsy | 9 (2) | 53 (100) | 1.00 |
| Renal disease | 8 (2) | 3 (6) | 0.07 |
| Liver disease | 5 (1) | 1 (2) | 0.44 |
| CVA/TIA | 29 (6) | 2 (4) | 0.76 |
| Peptic ulcer | 14 (3) | 2 (4) | 0.65 |
| Malignancy | 62 (12) | 8 (15) | 0.65 |
| DVT/PE | 23 (4) | 4 (8) | 0.30 |
| Osteoarthritis | 154 (30) | 19 (36) | 0.43 |
| Rheumatoid arthritis | 22 (4) | 1 (2) | 0.71 |
| Smoking status | – | – | 0.49 |
| Never | 258 (50) | 31 (58) | |
| Ex-smoker | 212 (41) | 18 (34) | |
| Yes | 50 (10) | 4 (8) | |
| Home care support | – | – | 0.74 |
| Lives alone | 161 (31) | 19 (36) | |
| Lives with someone | 353 (68) | 34 (64) | |
| Lives with carers | 1 (0) | 0 (0) | |
| Home care package | 5 (1) | 0 (0) | |
| Institution care | 0 (0) | 0 (0) | |
| Walking aids | – | – | 0.38 |
| None | 446 (86) | 48 (91) | |
| One stick | 53 (10) | 4 (8) | |
| Two sticks | 5 (1) | 0 (0) | |
| Frame/rollator | 14 (3) | 0 (0) | |
| Wheelchair | 2 (0) | 1 (2) | |
| Bed-bound | 0 (0) | 0 (0) | |
| Walking distance | – | – | 0.11 |
| About house | 15 (3) | 2 (4) | |
| < 100 m | 34 (7) | 0 (0) | |
| < 0.5 mile | 53 (10) | 3 (6) | |
| > 0.5 mile | 418 (80) | 48 (91) | |
| Missing | 0 (0) | 0 (0) |
| Outcome/resource | Meana | Missing (%) |
|---|---|---|
| Utilityb | ||
| Prior to injury | 0.84 | 0 |
| At presentation | 0.06 | 7 |
| 6 weeks | 0.36 | 0 |
| 6 months | 0.49 | 3 |
| Casts | ||
| Casts in plaster room | 0.18 | 5 |
| Casts in theatre room | 0.07 | 6 |
| Sick days | ||
| Sick days | 4.78 | 6 |
| Care homes | ||
| Friend/family at home days | 8.28 | 7 |
| Community hospital days | 1.43 | 7 |
| Intermediate care days | 0.30 | 7 |
| Nursing home (NHS) days | 0.53 | 7 |
| Nursing home (private) days | 0.22 | 7 |
| Friend/family stay days | 1.41 | 7 |
| Work days off by friend/family | 2.00 | 7 |
| Health professional visits | ||
| GP | 0.29 | 7 |
| Nurse | 2.43 | 9 |
| Physiotherapy inpatient | 0.31 | 5 |
| Physiotherapy outpatient | 0.74 | 7 |
| Physiotherapy home | 0.16 | 7 |
| Hospital A&E | 0.07 | 7 |
| Hospital specialist | 0.75 | 7 |
| Psychologist | 0.01 | 7 |
| Trauma outpatient | 1.59 | 0 |
| Hospital transports | 1.09 | 7 |
| Community care | 3.18 | 7 |
| Private physiotherapy | 0.05 | 0 |
| Private consultant | 0.00 | 0 |
| Private osteopath | 0.00 | 0 |
| Private transports | 0.26 | 0 |
| Prescriptions | ||
| Painkillers | 1.53 | 7 |
| Anti-inflammatory | 0.22 | 6 |
| Gel | 0.02 | 6 |
| Sleeping pills | 0.07 | 6 |
| Antidepressants | 0.10 | 6 |
| Painkillers (self-buy) | 0.87 | 7 |
| Anti-inflammatory (self-buy) | 0.14 | 7 |
| Gel (self-buy) | 0.05 | 7 |
| Sleeping pills (self-buy) | 0.00 | 7 |
| Antidepressants (self-buy) | 0.00 | 7 |
FIGURE 27.
Sensitivity: with completely missing cases via imputation. Per-protocol cost-effectiveness plane showing incremental total NHS costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.
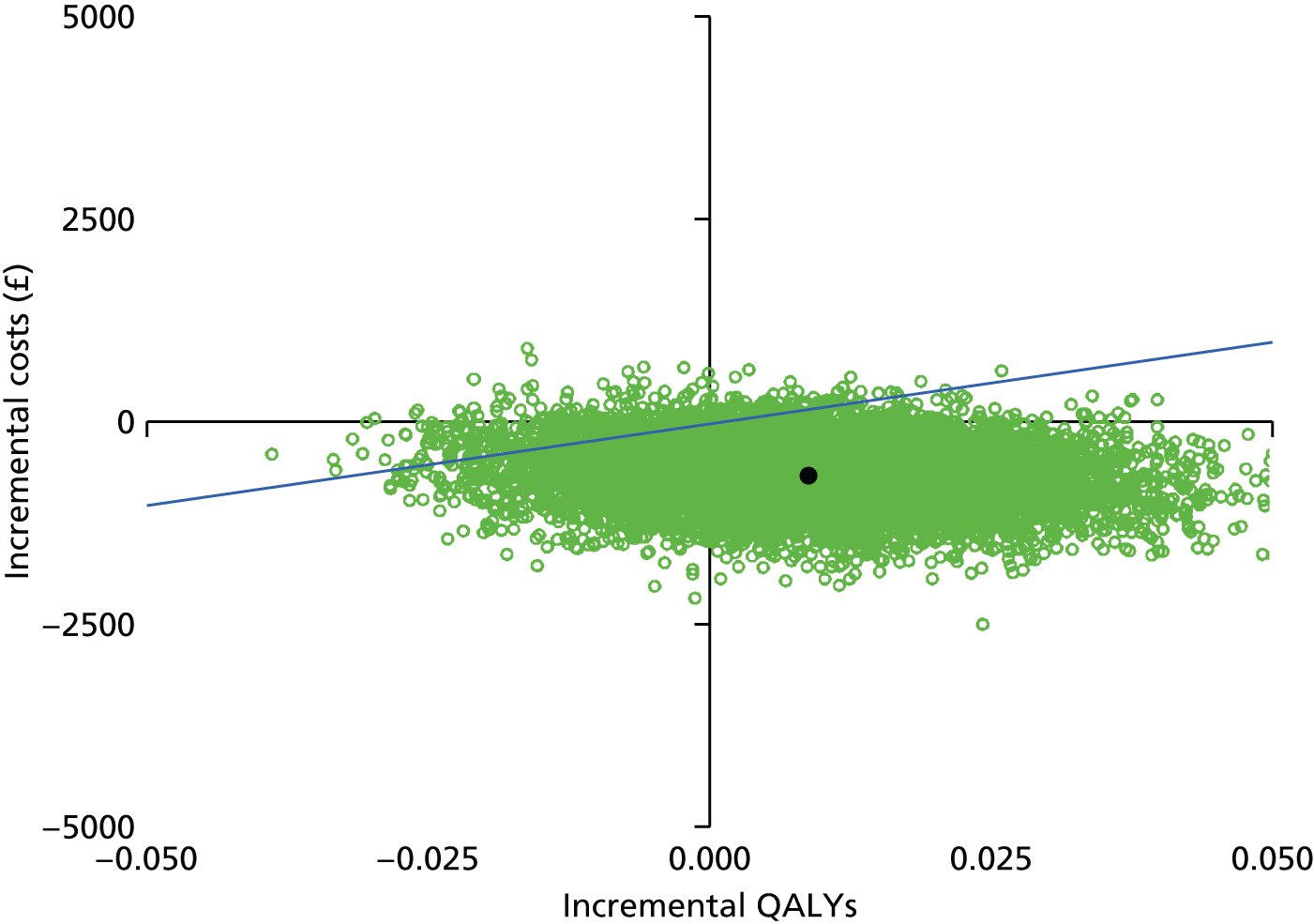
FIGURE 28.
Sensitivity: with completely missing cases via imputation. Per-protocol cost-effectiveness plane showing incremental total societal costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.

FIGURE 29.
Sensitivity: with completely missing cases via imputation. Per-protocol cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the NHS perspective. The dotted line presents the asymptote where the willingness to pay is infinite.

FIGURE 30.
Sensitivity: with completely missing cases via imputation. Per-protocol cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the societal perspective. The dotted line presents the asymptote where the willingness to pay is infinite.

Intention to treat
Cases included in the economic evaluation
The ITT population included 618 participants. The number of participants’ completely missing information invalid for an economic evaluation was 67 out of 618 (11%). Complete missingness was not statistically associated with intervention (odds ratio 1.03; 95% CI 0.62 to 1.78; p = 1.0; Fisher’s exact test). Baseline characteristics between complete cases and missing cases are presented in Table 74. Missing cases showed comparable baseline characteristics to the complete cases.
Missingness was more likely in pilot patients (odds ratio 156; p < 0.001). However, as pilot patients were not associated with intervention (odds ratio 1.03; 95% CI 0.60 to 1.78; p = 1.0; Fisher’s exact test); baseline characteristics were balanced between complete and missing cases; and missingness was not associated with intervention, we treated any remaining missingness as missing at random.
The 67 cases of completely missing information were removed and the economic evaluation continued with the 551 patients with sufficient valid information. Of the remaining participants, incomplete data remained at around 7% across most variables (see Table 75). Exceptions included lower missingness of utility prior to injury (0%), 6 weeks (0%) and 6 months (3%). Having concluded missingness was missing at random, we continued with multiple imputation with chained equations. Multiple imputation showed healthy convergence and the density plots of imputed values followed closely to the observed values (see Figures 41–44).
Baseline characteristics (intention to treat)
Baseline characteristics have previously been presented in Chapter 3. The CCC group showed lower baseline utility compared with the ORIF group (0.83 vs. 0.85; p = 0.03; Wilcoxon rank-sum test). Otherwise, the CCC group showed comparable baseline characteristics across age, sex, OMASs and a range of health conditions.
Resource use (intention to treat)
Mean resource use following ORIF and CCC is presented in Table 66. Patterns of resource use between CCC and ORIF remained similar to the per-protocol population. The CCC group showed lower theatre time during the index procedure (–49 minutes), slightly higher rate of additional procedures (+1.5%), higher rate of readmission (+13.5%) and greater cast use (+1.39). Overall length of stay was comparable (–0.25 days).
| Resource | Treatment group | Difference, mean | |
|---|---|---|---|
| ORIF, mean (SD) | CCC, mean (SD) | ||
| Index procedure | |||
| Theatre time (hours) | 1.31 (0.50) | 0.49 (0.40) | –0.81 |
| Screws | 6.56 (2.99) | 0.43 (1.83) | –6.13 |
| Antigliding plates | 0.10 (0.34) | 0.01 (0.09) | –0.09 |
| Tubular plates | 0.73 (0.60) | 0.06 (0.46) | –0.67 |
| Dynamic compression plates | 0.01 (0.10) | 0.00 (0.00) | –0.01 |
| Reconstruction plates | 0.01 (0.10) | 0.00 (0.00) | –0.01 |
| Locking plates | 0.12 (0.51) | 0.01 (0.09) | –0.11 |
| Other plates | 0.04 (0.20) | 0.00 (0.06) | –0.04 |
| Tightropes | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Wirings | 0.08 (0.33) | 0.01 (0.12) | –0.08 |
| Other implants | 0.08 (0.35) | 0.00 (0.00) | –0.08 |
| Additional procedure [n (%)] | 15 (5.4) | 19 (6.9) | 1.5 |
| Theatre time (hours)a | 1.33 (0.61) | 1.37 (0.79) | 0.04 |
| Anaesthesia [n (%)]a | 15 (100) | 19 (100) | 0.0 |
| Screwsa | 6.07 (3.10) | 4.58 (3.76) | –1.49 |
| Antigliding platesa | 0.27 (0.46) | 0.11 (0.32) | –0.16 |
| Tubular platesa | 0.53 (0.52) | 0.42 (0.51) | –0.11 |
| Dynamic compression platesa | 0.07 (0.26) | 0.05 (0.23) | –0.01 |
| Reconstruction platesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Locking platesa | 0.13 (0.35) | 0.11 (0.32) | –0.03 |
| Other platesa | 0.00 (0.00) | 0.05 (0.23) | 0.05 |
| Tightropesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Wiringsa | 0.07 (0.26) | 0.05 (0.23) | –0.01 |
| Other implantsa | 0.13 (0.52) | 0.00 (0.00) | –0.13 |
| Casts in plaster room | 0.00 (0.06) | 0.09 (0.34) | 0.08 |
| Casts in theatre room | 0.01 (0.08) | 0.91 (0.35) | 0.90 |
| LOS (days) | 9.78 (23.07) | 9.25 (10.50) | –0.53 |
| Readmission [n (%)] | 23 (8.3) | 60 (21.8) | 13.5 |
| Theatre time (hours)a | 0.30 (0.48) | 0.97 (0.88) | 0.66 |
| Anaesthesia [n (%)]a | 9 (39.1) | 42 (70.0) | 30.9 |
| Screwsa | 0.35 (1.30) | 4.28 (4.06) | 3.94 |
| Antigliding platesa | 0.00 (0.00) | 0.07 (0.31) | 0.07 |
| Tubular platesa | 0.04 (0.21) | 0.35 (0.48) | 0.31 |
| Dynamic compression platesa | 0.00 (0.00) | 0.05 (0.22) | 0.05 |
| Reconstruction platesa | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
| Locking platesa | 0.00 (0.00) | 0.15 (0.58) | 0.15 |
| Other platesa | 0.00 (0.00) | 0.03 (0.18) | 0.03 |
| Tightropesa | 0.00 (0.00) | 0.03 (0.26) | 0.03 |
| Wiringsa | 0.00 (0.00) | 0.03 (0.18) | 0.03 |
| Other implantsa | 0.00 (0.00) | 0.05 (0.29) | 0.05 |
| LOSa (days) | 5.14 (7.77) | 5.57 (10.69) | 0.43 |
| Total inclusive of 6 months | |||
| Theatre time (hours) | 1.40 (0.57) | 0.80 (0.82) | –0.61 |
| Casts | 0.07 (0.35) | 1.46 (0.96) | 1.39 |
| Hospital LOS (days) | 10.21 (23.33) | 10.47 (12.25) | 0.25 |
| Follow-up inclusive of 6 months | |||
| Care homes | |||
| Friend/family at home days | 11.81 (21.30) | 11.85 (27.00) | 0.04 |
| Community hospital days | 2.70 (13.98) | 2.95 (15.00) | 0.25 |
| Intermediate care days | 0.84 (9.44) | 1.35 (10.46) | 0.50 |
| Nursing home (NHS) days | 0.93 (7.12) | 0.51 (4.23) | –0.42 |
| Nursing home (private) days | 0.61 (6.10) | 0.61 (6.50) | 0.00 |
| Productivity | |||
| Sick days | 8.13 (24.74) | 8.20 (30.06) | 0.07 |
| Friend/family stay days | 2.61 (13.20) | 1.60 (11.31) | –1.01 |
| Work days off by friend/family | 2.99 (9.33) | 2.53 (11.88) | –0.46 |
| Health services | |||
| GP | 0.68 (1.66) | 0.60 (1.25) | –0.08 |
| Nurse | 3.86 (11.60) | 3.36 (15.09) | –0.50 |
| Physiotherapist inpatient | 0.90 (4.04) | 1.58 (7.95) | 0.68 |
| Physiotherapist outpatient | 3.33 (4.13) | 3.19 (3.98) | –0.14 |
| Physiotherapist home | 0.65 (1.92) | 0.92 (4.50) | 0.28 |
| Hospital A&E | 0.09 (0.33) | 0.11 (0.33) | 0.01 |
| Hospital specialist | 0.92 (2.86) | 1.07 (1.92) | 0.15 |
| Psychologist | 0.01 (0.12) | 0.02 (0.22) | 0.01 |
| Trauma outpatient | 1.83 (2.08) | 2.48 (2.47) | 0.65 |
| Hospital transports | 1.29 (2.28) | 1.89 (2.86) | 0.60 |
| Community care | 6.14 (30.71) | 8.85 (39.15) | 2.71 |
| Private physiotherapist | 0.46 (2.32) | 0.20 (1.30) | –0.25 |
| Private consultant | 0.00 (0.06) | 0.00 (0.06) | 0.00 |
| Private osteopath | 0.04 (0.42) | 0.08 (0.93) | 0.03 |
| Private transports | 0.37 (2.11) | 0.27 (1.01) | –0.10 |
| Prescriptions | |||
| Painkillers | 2.29 (3.49) | 2.33 (3.97) | 0.04 |
| Anti-inflammatory | 0.44 (1.31) | 0.33 (0.83) | –0.11 |
| Gel | 0.13 (0.61) | 0.12 (0.55) | –0.01 |
| Sleeping pills | 0.05 (0.32) | 0.14 (1.33) | 0.09 |
| Antidepressants | 0.10 (0.63) | 0.23 (2.22) | 0.13 |
| Painkillers (self-buy) | 2.18 (6.65) | 2.10 (8.60) | –0.08 |
| Anti-inflammatory (self-buy) | 0.47 (1.83) | 0.37 (1.55) | –0.10 |
| Gel (self-buy) | 0.15 (0.67) | 0.13 (0.51) | –0.02 |
| Sleeping pills (self-buy) | 0.00 (0.06) | 0.00 (0.00) | 0.00 |
| Antidepressants (self-buy) | 0.00 (0.00) | 0.00 (0.00) | 0.00 |
Patterns of care time between the ORIF and CCC groups were also comparable to the per-protocol population. Most of the care time was spent at homes with friends/family (≈12 days). Both groups spent around 1 or fewer days in the intermediate care home and nursing home. However, time spent in the community hospital was modestly longer at 2–3 days for each group. In addition, the CCC group spent 0.41 fewer days over the ORIF group in NHS nursing homes.
Patterns of mean health service use between the ORIF and CCC groups were also comparable to the per-protocol populations with modest nurse (≥ 3 visits), physiotherapy (≥ 3 visits), trauma outpatient (≈2 visits) and community care visits (6 to 9 visits). However, the CCC group had more visits to a community care centre compared with ORIF (9.26 vs. 6.20 days; p = 0.06). Medication use between the two groups was low to negligible.
Private health resource use was low to negligible and mean levels were comparable between ORIF and CCC. Patients in both groups took around 8 days off work, while their friends and families took just under 3 days.
Health outcomes (intention to treat)
The raw, unadjusted utilities at presentation and at 6 weeks and 6 months after randomisation are presented in Table 67. At presentation, the utility in the CCC group trended higher than the ORIF group (0.07 vs. 0.04), although this difference was not statistically significant (p = 0.10). Compared with the ORIF group, utility in the CCC group was comparable at 6 weeks (0.36 vs. 0.37; p = 0.13) and 6 months (0.48 vs. 0.49; p = 0.50). Total QALYs over the trial period were also comparable between the CCC and the ORIF groups (0.30 vs. 0.30; p = 0.88). Figure 31 shows the mean utility scores; the QALYs are indicated by the area under the curve. QALYs were estimated from presentation time to 6 months.
| Health outcomes | Treatment group | Difference, mean (95% CI) | p-value | |
|---|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | |||
| Utility (baseline) | 0.04 (0.01 to 0.07) | 0.07 (0.04 to 0.10) | 0.03 (–0.02 to 0.07) | 0.10 |
| Utility (6 weeks) | 0.37 (0.35 to 0.39) | 0.36 (0.34 to 0.37) | –0.02 (–0.04 to 0.01) | 0.12 |
| Utility (6 months) | 0.49 (0.47 to 0.51) | 0.48 (0.47 to 0.50) | 0.00 (–0.03 to 0.03) | 0.50 |
| QALYs | 0.30 (0.28 to 0.31) | 0.30 (0.29 to 0.31) | 0.00 (–0.01 to 0.02) | 0.88 |
FIGURE 31.
Graph showing ITT utility (quality of life) over time (length of life). Area is the QALYs.

Costs (intention to treat)
The mean raw, unadjusted costs of the resource uses for each group using mean unit cost parameters are presented in Table 68. Compared with the ORIF group, the CCC group had a large initial cost savings (–£1008), driven by savings in theatre time use ( –£798), which was eroded with increased readmissions (+£450) and health services (+£471). Mean cost differences were negligible for additional procedures (+£27), casts (+£41) and medications (+£2).
| Resource | Treatment group | Difference, mean, £ (95% CI) | |
|---|---|---|---|
| ORIF, mean, £ (95% CI) | CCC, mean, £ (95% CI) | ||
| Index procedure | |||
| Theatre time | 1584 (1526 to 1642) | 786 (741 to 837) | –798 (–872 to –722) |
| Implant | 77 (71 to 86) | 5 (3 to 8) | –72 (–81 to –65) |
| LOS | 2690 (2157 to 3549) | 2552 (2234 to 2919) | –138 (–1078 to 552) |
| Total | 4351 (3817 to 5218) | 3343 (3011 to 3725) | –1008 (–1963 to –304) |
| Additional procedure | |||
| Theatre time | 87 (45 to 136) | 114 (63 to 173) | 27 (–45 to 100) |
| Implant | 4 (2 to 6) | 4 (2 to 7) | 0 (–3 to 3) |
| Total | 91 (47 to 143) | 118 (65 to 179) | 27 (–48 to 103) |
| Readmission | |||
| Theatre time | 35 (13 to 60) | 254 (178 to 332) | 219 (140 to 301) |
| Implant | 0 (0 to 1) | 13 (8 to 18) | 13 (8 to 18) |
| LOS | 118 (43 to 211) | 336 (185 to 541) | 218 (40 to 437) |
| Total | 153 (66 to 263) | 603 (405 to 845) | 450 (226 to 709) |
| Casts | |||
| Number | 2 (1 to 3) | 43 (40 to 47) | 41 (38 to 45) |
| Care homes | |||
| Community hospital days | 523 (234 to 877) | 571 (284 to 958) | 49 (–418 to 537) |
| Intermediate care days | 54 (2 to 136) | 86 (20 to 175) | 32 (–73 to 137) |
| Nursing home (NHS) days | 92 (21 to 187) | 51 (11 to 105) | –41 (–146 to 48) |
| Nursing home (private) days | 59 (0 to 140) | 59 (0 to 146) | 0 (–101 to 106) |
| Total | 727 (384 to 1145) | 767 (443 to 1202) | 40 (–497 to 602) |
| Health services | 1360 (1088 to 1676) | 1830 (1413 to 2350) | 471 (–55 to 1068) |
| Health services (private) | 93 (50 to 147) | 64 (42 to 89) | –29 (–87 to 21) |
| Medications | 5 (4 to 6) | 7 (4 to 11) | 2 (–1 to 6) |
| Medications (private) | 4 (3 to 6) | 4 (3 to 5) | –1 (–2 to 1) |
| Workdays | 1097 (802 to 1,424) | 1057 (701 to 1476) | –40 (–520 to 463) |
| Total societal cost | 7885 (6933 to 8994) | 7837 (6787 to 9151) | –48 (–1599 to 1610) |
| Total NHS cost | 6631 (5720 to 7718) | 6652 (5689 to 7906) | 22 (–1460 to 1598) |
Societal cost differences between the CCC and ORIF groups were negligible across private nursing homes (£0), private health services (–£29), out-of-pocket medications (–£1) and work days (–£40).
Over the 6-month trial period, the CCC group had a slight mean cost increase to the NHS of £22 and a slight mean cost savings to society of £48. Figure 32 presents the cost burden for the CCC and ORIF groups for the cost categories. The cost burdens, from largest to least, were the primary procedure, health services/medications, care homes, readmission, additional procedure and casts. The figure represents the total cost burden; 360° represents ≈£6600. ORIF displays cost savings represented by the white space where a full circle fails to fill.
FIGURE 32.
Pie chart of ITT NHS costs for (a) ORIF and (b) CCC, grouped by primary procedure, health services/medications, care homes, readmission, additional procedure and casts.
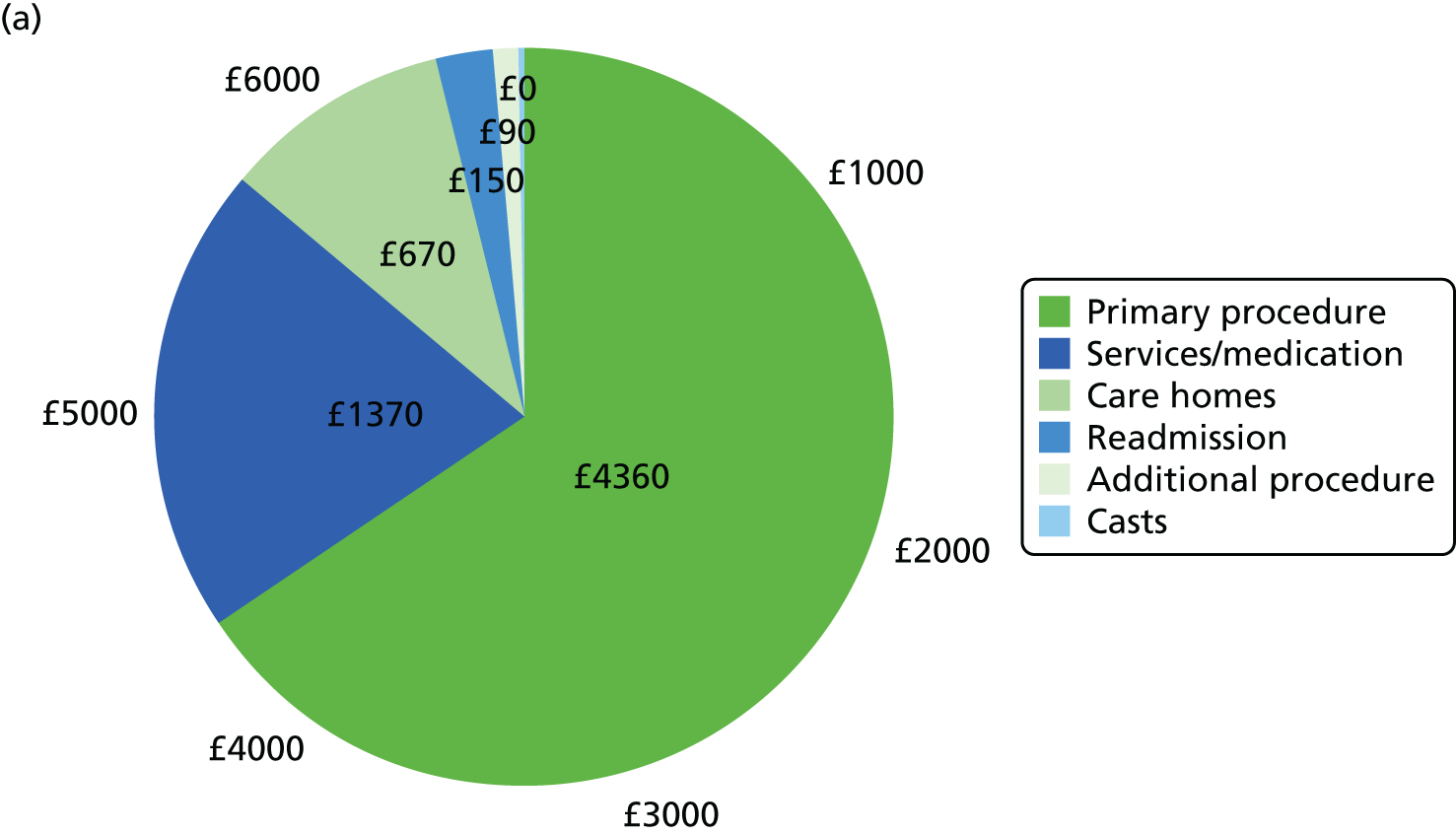

Regression models (intention to treat)
Total societal and NHS costs, as well as QALYs, were obtained by fitting a GLM and adjusting for baseline characteristics. We used the same regression models as those used for the NHS perspective.
Regression coefficients for total NHS and societal costs are presented in Tables 69 and 70. Regression coefficients for total QALYs are presented in Table 71.
| Variable | GLM | ||
|---|---|---|---|
| Estimate | Standard error | p-value | |
| Intercept | 6936 | 3695 | 0.06 |
| CCC | –689 | 378 | 0.07 |
| Age | 83 | 34 | 0.01 |
| Female | 507 | 417 | 0.22 |
| Baseline utility | –2205 | 2492 | 0.38 |
| Baseline OMAS | –53 | 23 | 0.02 |
| Hypertension | 423 | 411 | 0.30 |
| Asthma | –783 | 537 | 0.15 |
| Diabetes mellitus | 1121 | 931 | 0.23 |
| Ex-smoker | –313 | 416 | 0.45 |
| Smoker | –617 | 369 | 0.10 |
| Home support: does not live alone | –1702 | 523 | 0.001 |
| Walking aids: one or more | 3471 | 1412 | 0.01 |
| Walking distance: < 0.5 mile or worse | 2719 | 992 | 0.01 |
| Variable | GLM | ||
|---|---|---|---|
| Estimate | Standard error | p-value | |
| Intercept | 18,847 | 4385 | < 0.001 |
| CCC | –721 | 461 | 0.12 |
| Age | –18 | 39 | 0.64 |
| Female | 441 | 518 | 0.40 |
| Baseline utility | –2538 | 2908 | 0.38 |
| Baseline OMAS | –81 | 28 | 0.004 |
| Hypertension | 94 | 487 | 0.85 |
| Asthma | –1345 | 654 | 0.04 |
| Diabetes mellitus | 1673 | 1135 | 0.14 |
| Ex-smoker | –392 | 527 | 0.46 |
| Smoker | –1005 | 464 | 0.03 |
| Home support: does not live alone | –2423 | 633 | < 0.001 |
| Walking aids: one or more | 2988 | 1477 | 0.04 |
| Walking distance: < 0.5 mile or worse | 3190 | 1105 | 0.004 |
| Variable | Estimate | Standard error | p-value |
|---|---|---|---|
| Intercept | 0.765 | 0.060 | < 0.001 |
| CCC | –0.010 | 0.009 | 0.23 |
| Age | 0.001 | 0.001 | 0.44 |
| Female | 0.026 | 0.010 | 0.01 |
| Baseline utility | –0.089 | 0.037 | 0.02 |
| Baseline OMAS | –0.001 | 0.000 | 0.16 |
| Hypertension | –0.022 | 0.009 | 0.02 |
| Asthma | 0.003 | 0.013 | 0.80 |
| Diabetes mellitus | –0.005 | 0.015 | 0.72 |
| Ex-smoker | 0.019 | 0.011 | 0.09 |
| Smoker | –0.009 | 0.009 | 0.31 |
| Home support: does not live alone | 0.005 | 0.009 | 0.63 |
| Walking aids: one or more | 0.035 | 0.016 | 0.03 |
| Walking distance: < 0.5 mile or worse | 0.046 | 0.013 | 0.001 |
Cost-effectiveness: 6 months (intention to treat)
NHS perspective
Results of the cost-effectiveness outcomes are presented in Table 72. The mean total QALYs for CCC and ORIF were 0.30 and 0.29, respectively, while the mean total NHS costs were £6191 and £6883, respectively.
| Outcome | Treatment group | Difference, mean (95% CI) | |
|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | ||
| NHS costs (£) | 6883 (5400 to 8812) | 6191 (4804 to 8029) | –692 (–1438 to 24) |
| Societal costs (£) | 8233 (6645 to 10,245) | 7460 (5940 to 9382) | –773 (–1904 to 410) |
| QALYs | 0.2925 (0.2705 to 0.3120) | 0.3026 (0.2851 to 0.3195) | 0.0101 (–0.0156 to 0.0379) |
The incremental differences favoured the CCC group. The CCC group had higher mean total QALYs (+0.01) and lower mean total NHS cost (–£692). At the mean for an ICER of £25,000/QALY, as CCC was both more effective and less costly, it dominated ORIF.
To assess the variability of the estimates, we plotted the incremental costs and incremental QALYs on the cost-effectiveness plane (Figure 33). The cost-effectiveness plane summarises all 10,000 bootstrap estimates. The cost-effectiveness plane shows that the large majority of incremental costs are negative; the incremental QALYs, however, show more uncertainty with a modest proportion being negative. The majority, however, show both negative incremental costs and positive incremental QALYs.
FIGURE 33.
Intention-to-treat cost-effectiveness plane showing incremental total NHS costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.
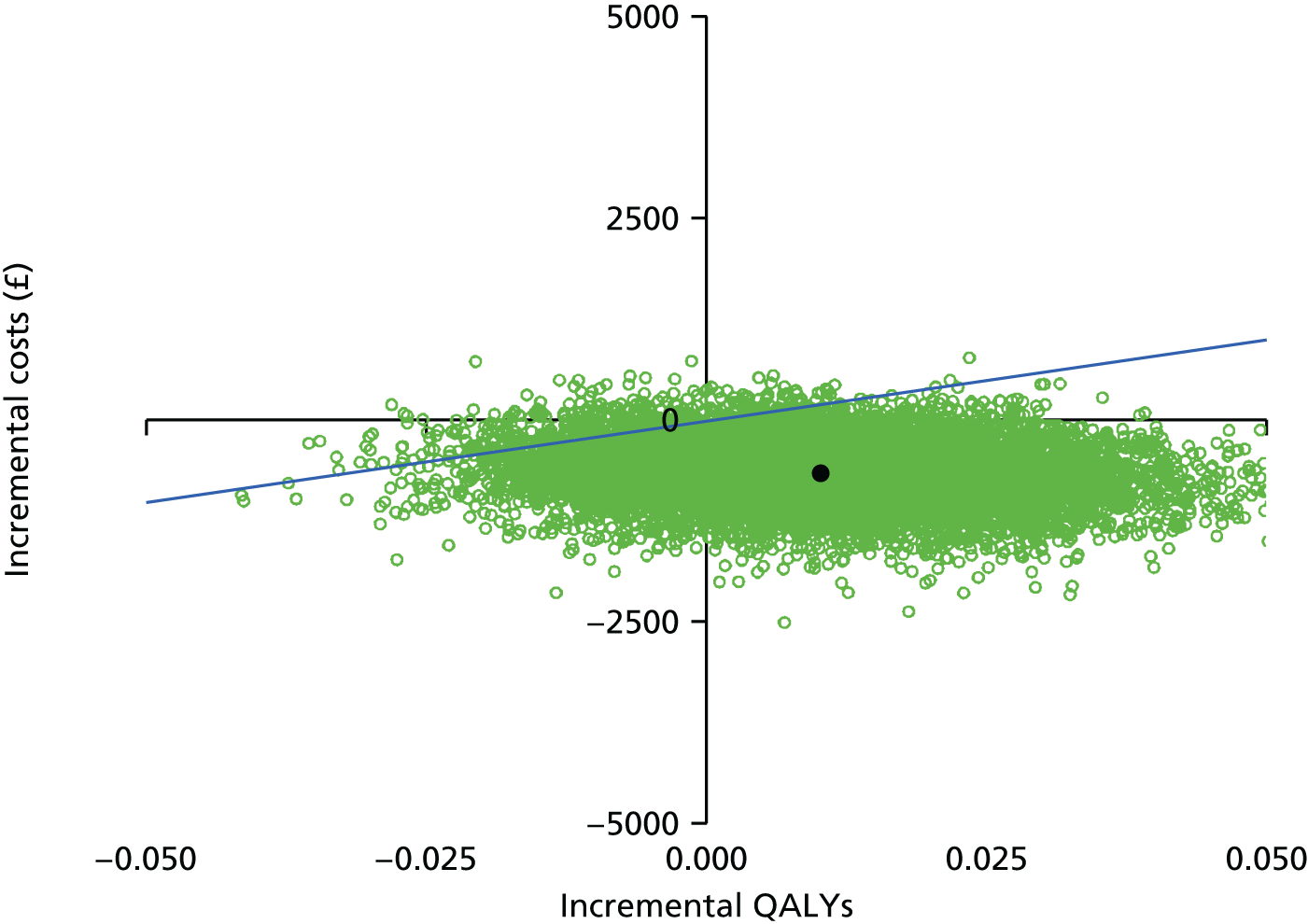
The variability across the range of willingness-to-pay thresholds was summarised with a cost-effectiveness acceptability curve (Figure 34). Over a common willingness to pay, CCC displayed a high probability of cost-effectiveness (> 96%).
FIGURE 34.
Intention-to-treat cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the NHS perspective. The dotted line presents the asymptote where the willingness to pay is infinite.
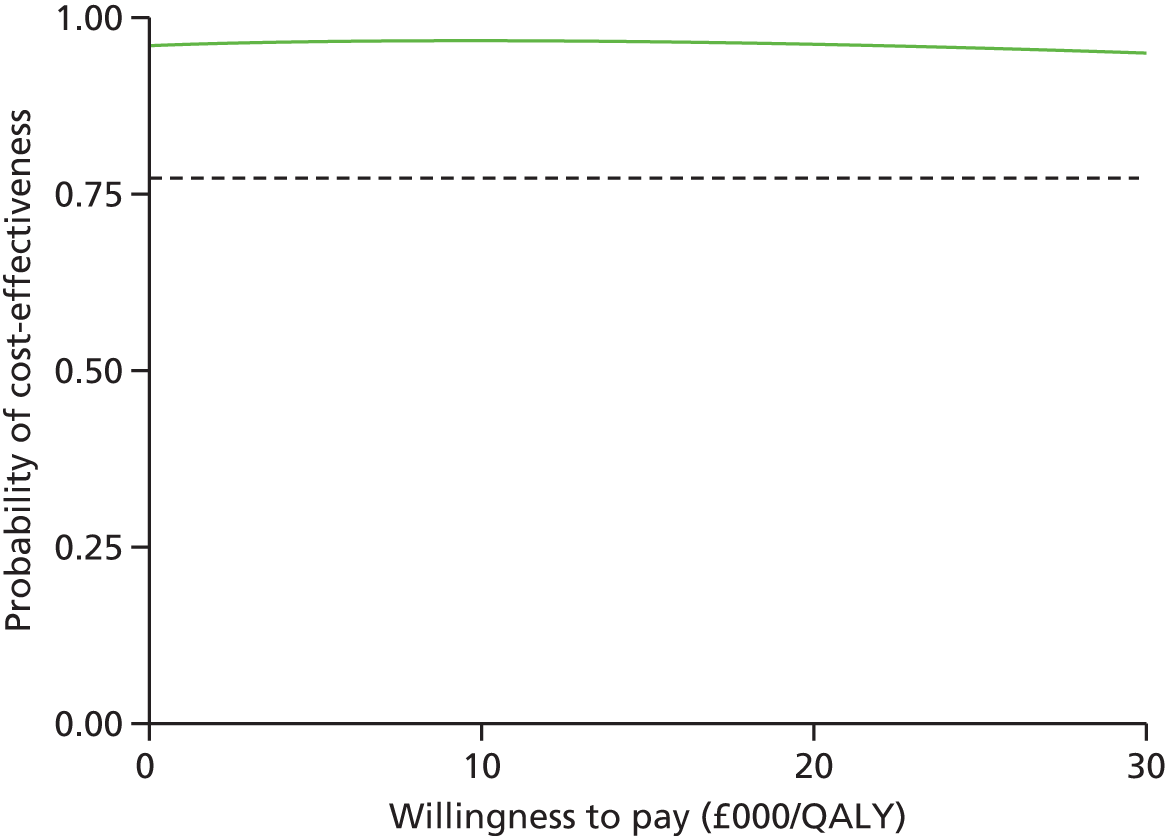
Societal perspective
The mean total QALYs remained the same for both the NHS and societal perspectives. Costs, however, differed. After resampling the trial population and unit costs, refitting the GLMs and using marginal estimation, mean total societal costs for the CCC and ORIF groups were £7460 and £8233, respectively (see Table 72).
The incremental differences favoured the CCC group. The CCC group had lower mean total societal cost (–£773) and unchanged higher mean total QALYs (+0.01). At the mean incremental cost and QALYs for an ICER of £25,000/QALY, CCC dominated ORIF, as it was both more effective and less costly.
The cost-effectiveness plane displayed modest variability in incremental costs (Figure 35). However, the large majority of incremental costs were negative. The incremental QALYs also displayed a modest proportion of negative estimates.
FIGURE 35.
Intention-to-treat cost-effectiveness plane showing incremental total societal costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.
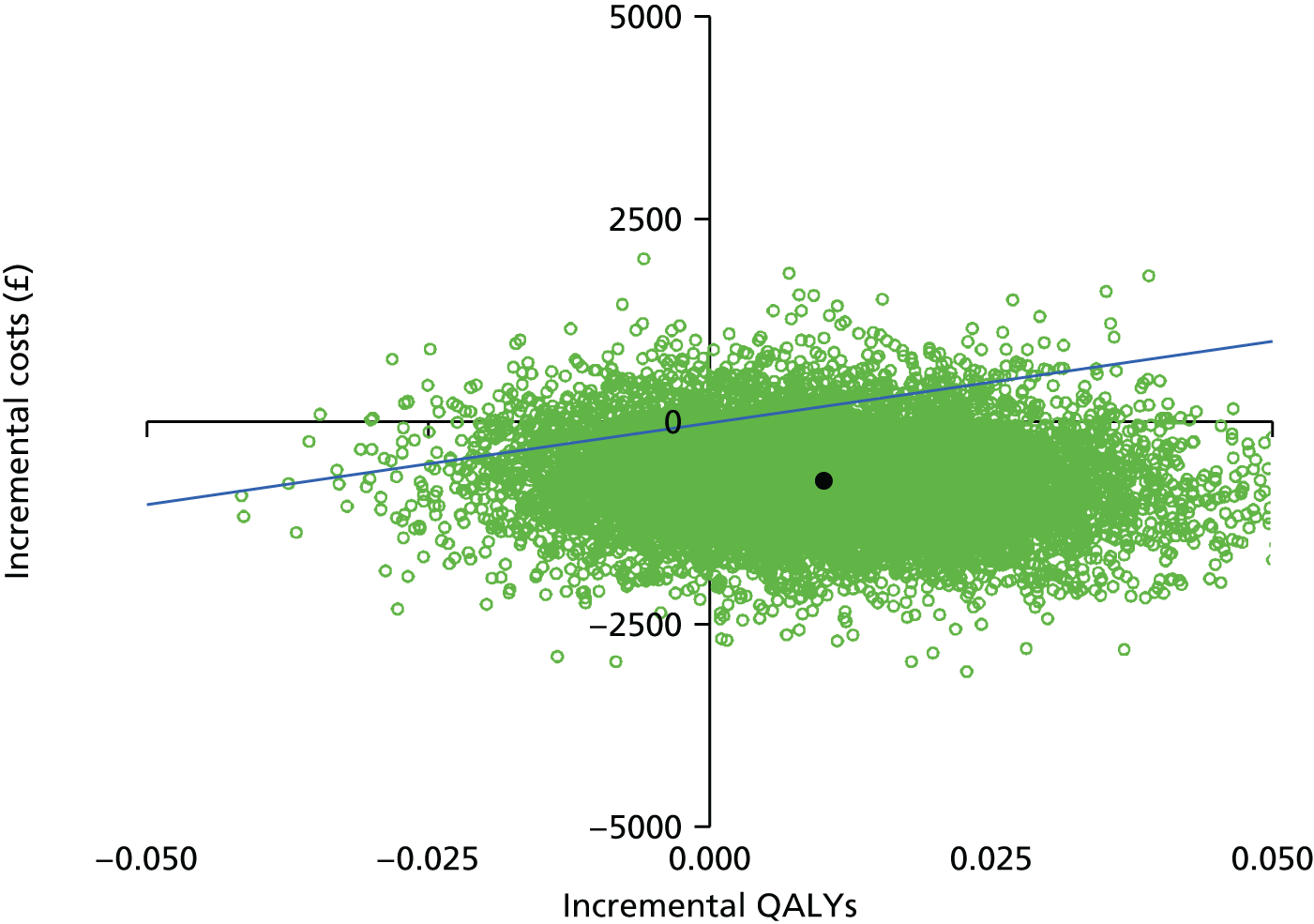
Over a common willingness to pay, CCC displayed a high probability of cost-effectiveness (> 90%) (Figure 36).
FIGURE 36.
Intention-to-treat cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the societal perspective. The dotted line presents the asymptote where the willingness to pay is infinite.

Lifetime (intention to treat)
There was no evidence to support modelling differences in the long term. Reasons were presented in the main body of the report.
Sensitivity: with completely missing cases via imputation (intention to treat)
We repeated the economic evaluation including patients with completely missing information. These patients were included by imputing their missing information. Results were comparable with the results with those patients removed. All tables and figures for the full population are provided in Tables 73–75 and Figures 37–40.
| Outcome | Treatment group | Difference, mean (95% CI) | |
|---|---|---|---|
| ORIF, mean (95% CI) | CCC, mean (95% CI) | ||
| NHS costs (£) | 6991 (5519 to 8844) | 6285 (4922 to 8009) | –707 (–1454 to 13) |
| Societal costs (£) | 8191 (6615 to 10,106) | 7517 (6034 to 9343) | –674 (–1762 to 433) |
| QALYs | 0.2938 (0.2736 to 0.3115) | 0.3027 (0.2862 to 0.3183) | 0.0089 (–0.0152 to 0.0344) |
| Variable | Complete, n (%) | Missing, n (%) | p-value |
|---|---|---|---|
| n | 551 (89) | 67 (11) | – |
| Age (years), mean (SD) | 71 (7.24) | 69 (7.82) | 0.04 |
| OMAS, mean (SD) | 88.3 (17.6) | 92.7 (14.4) | 0.01 |
| Female | 405 (74) | 54 (81) | 0.27 |
| Heart disease | 74 (13) | 8 (12) | 0.88 |
| Hypertension | 235 (43) | 31 (46) | 0.66 |
| Asthma | 75 (14) | 10 (15) | 0.91 |
| Diabetes mellitus | 51 (9) | 6 (9) | 1.00 |
| Epilepsy | 9 (2) | 67 (100) | 0.61 |
| Renal disease | 8 (1) | 4 (6) | 0.03 |
| Liver disease | 5 (1) | 1 (1) | 0.50 |
| CVA/TIA | 31 (6) | 4 (6) | 0.78 |
| Peptic ulcer | 15 (3) | 3 (4) | 0.43 |
| Malignancy | 65 (12) | 8 (12) | 1.00 |
| DVT/PE | 24 (4) | 5 (7) | 0.23 |
| Osteoarthritis | 163 (30) | 21 (31) | 0.88 |
| Rheumatoid arthritis | 24 (4) | 2 (3) | 1.00 |
| Smoking status | – | – | 0.31 |
| Never | 273 (50) | 40 (60) | |
| Ex-smoker | 226 (41) | 22 (33) | |
| Yes | 52 (9) | 5 (7) | |
| Home care support | – | – | 0.75 |
| Lives alone | 173 (31) | 24 (36) | |
| Lives with someone | 372 (68) | 43 (64) | |
| Lives with carers | 1 (0) | 0 (0) | |
| Home care package | 5 (1) | 0 (0) | |
| Institution care | 0 (0) | 0 (0) | |
| Walking aids | – | – | 0.36 |
| None | 471 (85) | 58 (87) | |
| One stick | 57 (10) | 7 (10) | |
| Two sticks | 6 (1) | 1 (1) | |
| Frame/rollator | 15 (3) | 0 (0) | |
| Wheelchair | 2 (0) | 1 (1) | |
| Bed-bound | 0 (0) | 0 (0) | |
| Walking distance | – | – | 0.31 |
| > 0.5 mile | 441 (80) | 57 (85) | |
| < 0.5 mile | 56 (10) | 6 (9) | |
| < 100 m | 37 (7) | 1 (1) | |
| About house | 17 (3) | 3 (4) | |
| Nil | 0 (0) | 0 (0) |
| Outcome/resource | Meana | Missing (%) |
|---|---|---|
| Utilityb | ||
| Prior to injury | 0.84 | 0 |
| At presentation | 0.06 | 6 |
| 6 weeks | 0.36 | 0 |
| 6 months | 0.49 | 3 |
| Casts | ||
| Casts in plaster room | 0.18 | 5 |
| Casts in theatre room | 0.07 | 6 |
| Sick days | ||
| Sick days | 4.78 | 6 |
| Care homes | ||
| Friend/family at home days | 8.05 | 7 |
| Community hospital days | 1.38 | 7 |
| Intermediate care days | 0.33 | 7 |
| Nursing home (NHS) days | 0.50 | 6 |
| Nursing home (private) days | 0.26 | 7 |
| Friend/family stay days | 1.32 | 7 |
| Work days off by friend/family | 1.96 | 7 |
| Health professional visits | ||
| GP | 0.27 | 7 |
| Nurse | 2.34 | 9 |
| Physiotherapy inpatient | 0.30 | 5 |
| Physiotherapy outpatient | 0.72 | 7 |
| Physiotherapy home | 0.17 | 7 |
| Hospital A&E | 0.07 | 7 |
| Hospital specialist | 0.73 | 7 |
| Psychologist | 0.01 | 7 |
| Trauma outpatient | 1.56 | 1 |
| Hospital transports | 1.07 | 7 |
| Community care | 3.15 | 7 |
| Private physiotherapy | 0.05 | 1 |
| Private consultant | 0.00 | 1 |
| Private osteopath | 0.00 | 1 |
| Private transports | 0.25 | 1 |
| Prescriptions | ||
| Painkillers | 1.53 | 6 |
| Anti-inflammatory | 0.22 | 6 |
| Gel | 0.02 | 6 |
| Sleeping pills | 0.07 | 6 |
| Antidepressants | 0.10 | 6 |
| Painkillers (self-buy) | 0.87 | 7 |
| Anti-inflammatory (self-buy) | 0.16 | 7 |
| Gel (self-buy) | 0.05 | 7 |
| Sleeping pills (self-buy) | 0.00 | 7 |
| Antidepressants (self-buy) | 0.00 | 7 |
FIGURE 37.
Sensitivity with completely missing cases via imputation. ITT cost-effectiveness plane showing incremental total NHS costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.

FIGURE 38.
Sensitivity with completely missing cases via imputation. ITT cost-effectiveness plane showing incremental total societal costs along y-space and incremental QALYs along x-space for 10,000 bootstrap replicates. Origin is ORIF.

FIGURE 39.
Sensitivity with completely missing cases via imputation. ITT cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the NHS perspective. The dotted line presents the asymptote where the willingness to pay is infinite.
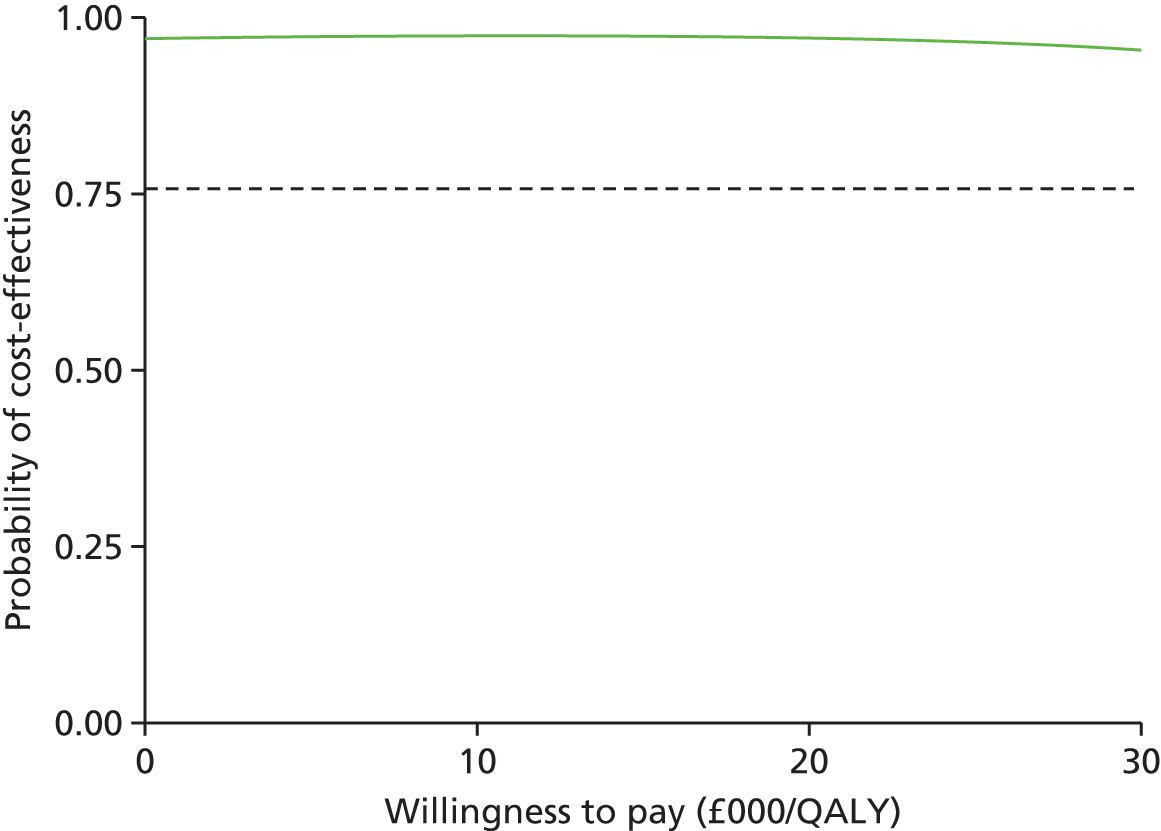
FIGURE 40.
Sensitivity with completely missing cases via imputation. ITT cost-effectiveness acceptability curve displaying the probability of cost-effectiveness (y-axis) as a function of the willingness-to-pay threshold (x-axis) under the societal perspective. The dotted line presents the asymptote where the willingness to pay is infinite.
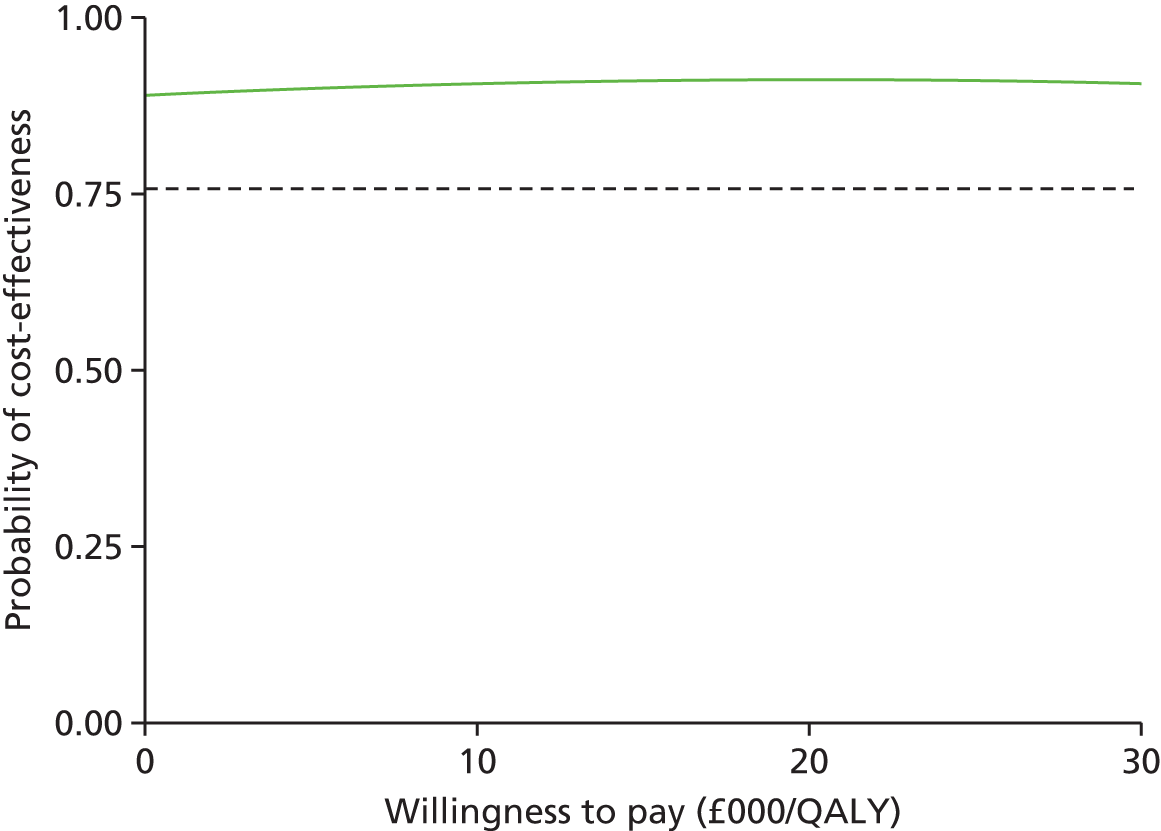
The full ITT population included 618 patients: 309 and 309 in the CCC and ORIF groups, respectively. Using the best-fit models, compared with ORIF, CCC had a mean cost savings to the NHS and to society of £707 and £674, respectively, and a mean QALY increase of 0.01 (see Table 73). Cost-effectiveness planes displayed the majority of incremental costs as negative while incremental QALYs were largely positive, but with a modest number of estimates that were negative (see Figures 37 and 38). Decision uncertainty was very high over the range of willingness to pay for both the NHS (> 95%) and societal perspectives (> 95%) (see Figures 39 and 40).
Multiple imputation with chained equations
Missing data were imputed using multiple imputation with chained equations, when appropriate, using R version 3.1.2 and the package ‘MICE’. Potential predictors included baseline characteristics as well as all resource use and health outcome items. Downstream variables were removed as predictors of upstream variables. Multiple imputation was conducted using 40 iterations and five imputations. The imputations showed healthy convergence and imputed values followed comparable distribution shapes to the observed data as displayed below for the per-protocol and ITT populations (Figures 41–44).
FIGURE 41.
Healthy convergence of the Gibbs sampler for the major health economic variables of per-protocol utilities. (a) Mean utility at presentation; (b) SD of utility at presentation; (c) mean utility at 6 months; and (d) standard utility at 6 months.

FIGURE 42.
Density plots for selected variables showing comparable distributions for the observed data and imputed data sets. (a) Utility at presentation; (b) utility at 6 months; (c) home care days; (d) community hospital days; (e) intermediate care days; (f) NHS nursing home days; (g) private nursing home days; and (h) sick days.
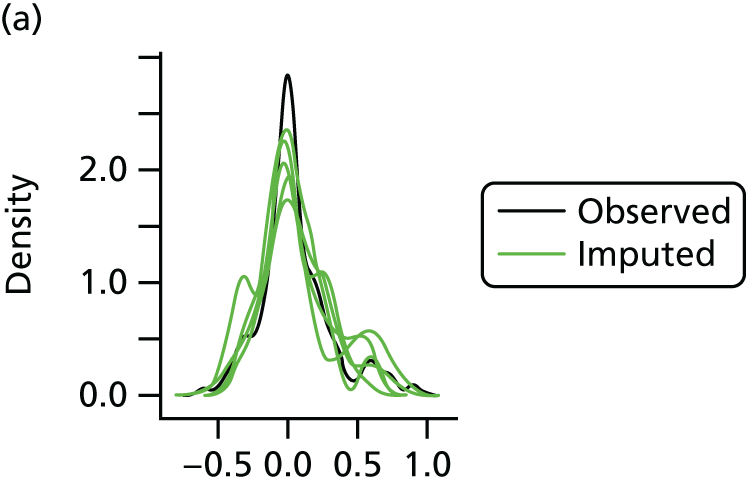
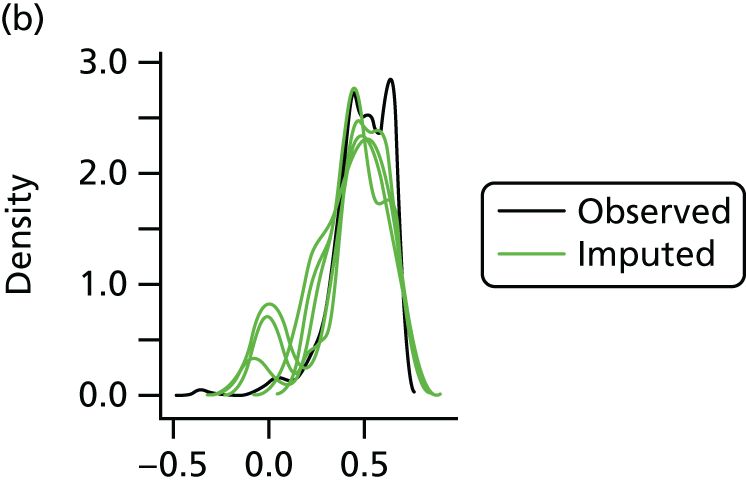


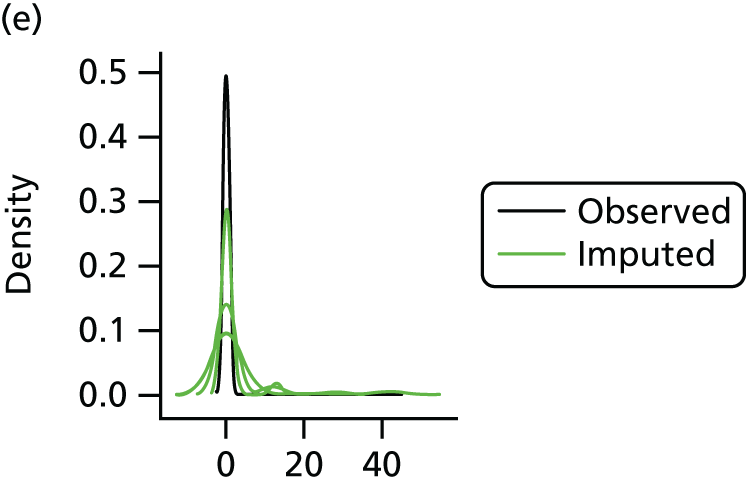
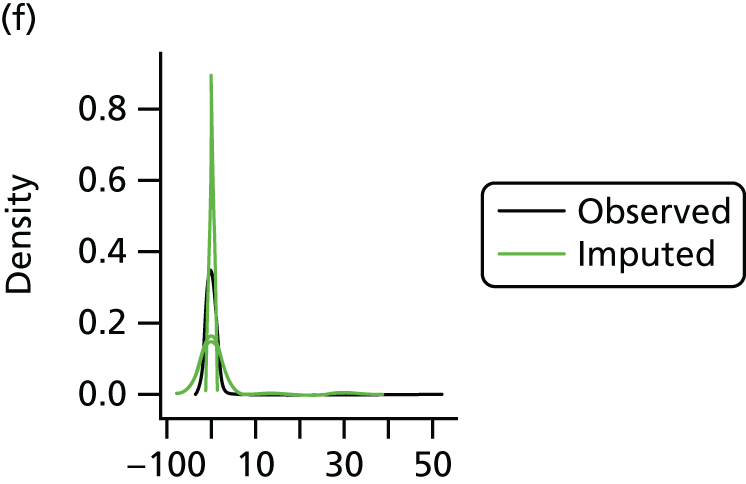

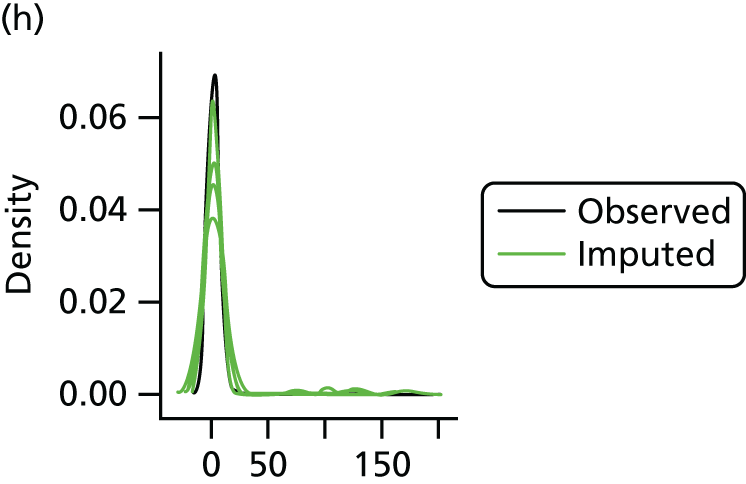
FIGURE 43.
Healthy convergence of the Gibbs sampler for the major health economic variables of ITT utilities. (a) Mean utility at presentation; (b) SD of utility at presentation; (c) mean utility at 6 months; and (d) standard utility at 6 months.
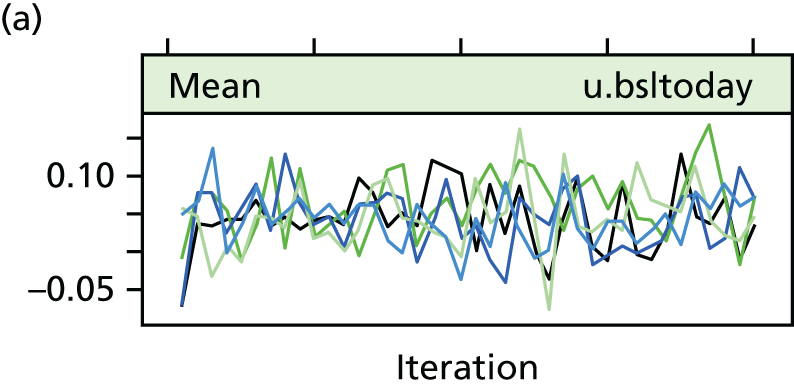

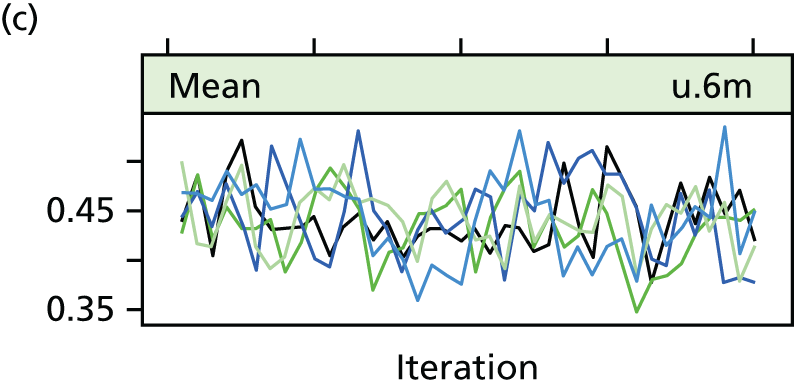

FIGURE 44.
Density plots for selected variables showing comparable distributions for the observed data and imputed data sets for the ITT population. (a) utility at presentation; (b) utility at 6 months; (c) home care days; (d) community hospital days; (e) intermediate care days; (f) NHS nursing home days; (g) private nursing home days; and (h) sick days.
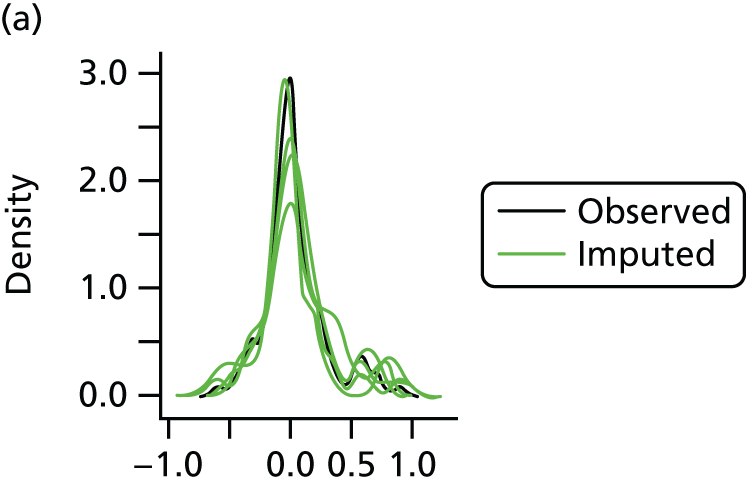
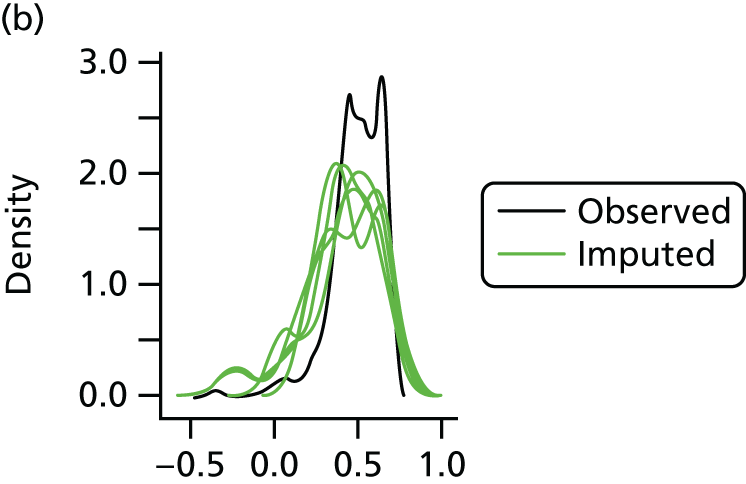
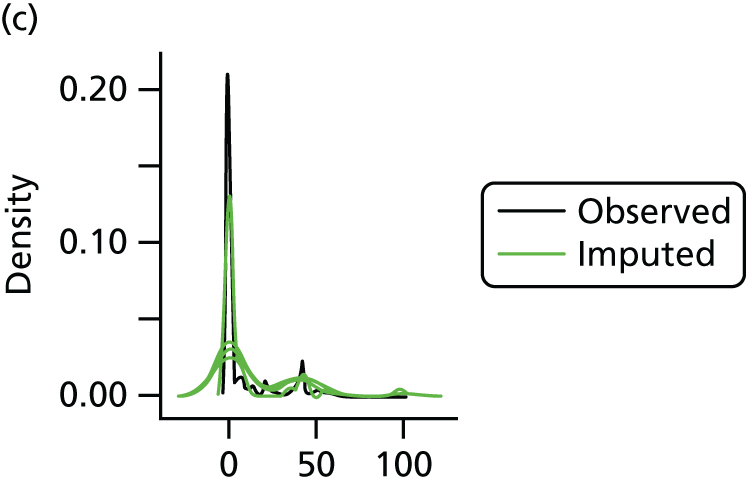

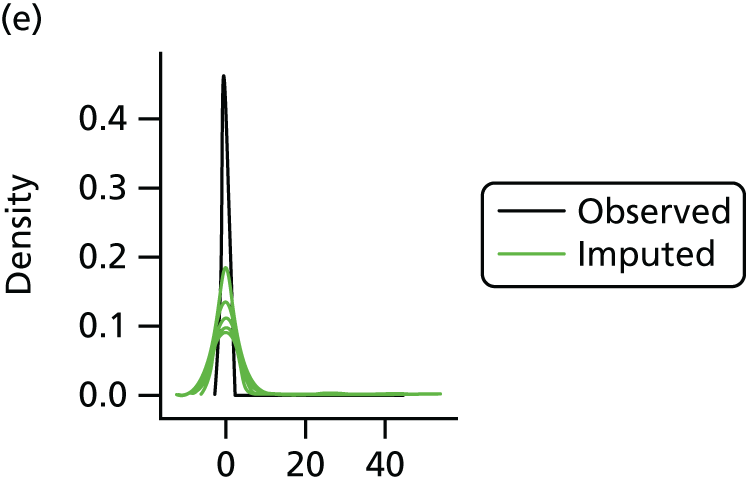
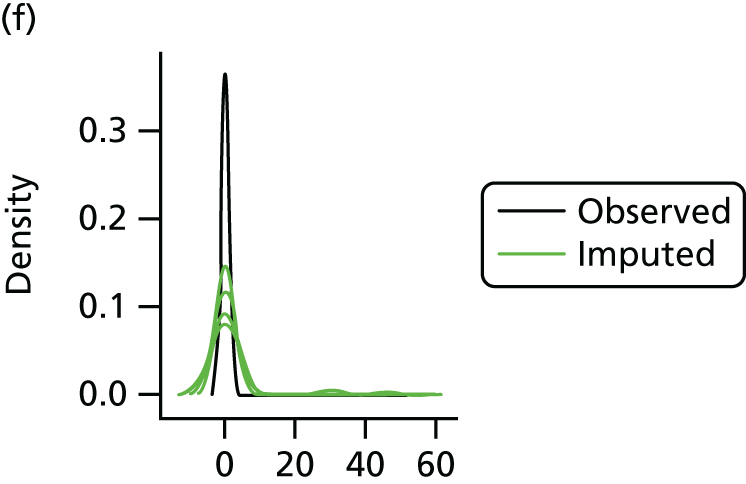


Bootstrapped statistical models
Total costs and total QALYs were adjusted using statistical modelling and predicted using marginal estimation. The entire process was bootstrapped 10,000 times to generate a distribution of adjusted, marginally estimated cost and effect pairs for ORIF and CCC.
Relevant covariates for the statistical models included age, sex, baseline utility and other baseline covariates identified by independent statistical association with the dependent variable at a p-level less than 25%. Final statistical models used a GLM framework. The appropriate family and link function were identified using the Modified Park’s Test and Pregibon Link Test, respectively (URL for the code: www.uphs.upenn.edu/dgimhsr/stat-cstanal.htm).
For costs, the gamma family was found to show the most likely fit (least likely was Gaussian) to the variance structure, while the link function was found to be the log-link. Instead of QALYs, the QALY decrement was used in order to achieve convergence. For QALYs, the Gaussian was found to show the most likely fit (least likely was inverse Gaussian), while the link function was found to be the identity link.
List of abbreviations
- AIM
- Ankle Injury Management trial
- CCC
- close contact casting
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMEC
- Data Monitoring and Ethics Committee
- DVT
- deep-vein thrombosis
- EQ-5D
- European Quality of Life 5-Dimensions
- GLM
- generalised linear model
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- OLS
- ordinary least squares
- OMAS
- Olerud–Molander Ankle Score
- ORIF
- open reduction and internal fixation
- PE
- pulmonary embolism
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale