Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 13/04/001. The protocol was agreed in September 2014. The assessment report began editorial review in May 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Selby reports personal fees from Internis Pharma and non-financial support from AMGen, outside the submitted work. Neil Gittoes reports personal fees from the advisory board at Eli Lilly and Company, personal fees from the advisory board at AMGen, personal fees from speaker fees at AMGen, personal fees from speaker fees at GlaxoSmithKline, personal fees from the advisory board at ProStrakan, personal fees from advisory board at Shire, personal fees from the advisory board at Internis Pharma, personal fees from the advisory board at Consilient Health and personal fees from the advisory board at NPS Pharmaceuticals, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Davis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Osteoporosis is a disease characterised by low bone mass and structural deterioration of bone tissue, with a consequent increase in susceptibility to fragility fracture (a broken bone resulting from a fall at standing height or less). An internationally accepted definition provided by the World Health Organization (WHO) (in 1994) defines the condition as bone mineral density (BMD) 2.5 standard deviations (SDs) below peak bone mass (20-year-old healthy female average) as measured by DXA (dual-energy X-ray absorptiometry). 1 The term ‘established osteoporosis’ includes the presence of a fragility fracture. 1 Primary osteoporosis can occur in both men and women, but is most common in women after menopause, when it is termed postmenopausal osteoporosis. In contrast, secondary osteoporosis may occur in anyone as a result of medications, specifically glucocorticoids, or in the presence of particular hormonal disorders or other chronic diseases. 2
Osteoporosis was not classified as a disease until relatively recently. 3 Previously, it was considered an inevitable accompaniment of ageing. During human growth, bone formation exceeds resorption. 4 Peak bone mass is achieved by men and women in the third decade of life. 5 There then follows a period during which there is a constant turnover of bone formation when the amount of bone formed by osteoblasts approximately equals the amount resorbed by osteoclasts. 5 Both men and women lose bone after midlife, when bone resorption starts to exceed formation, and in women there is also a significant rapid loss due to menopausal hypogonadism. 6,7
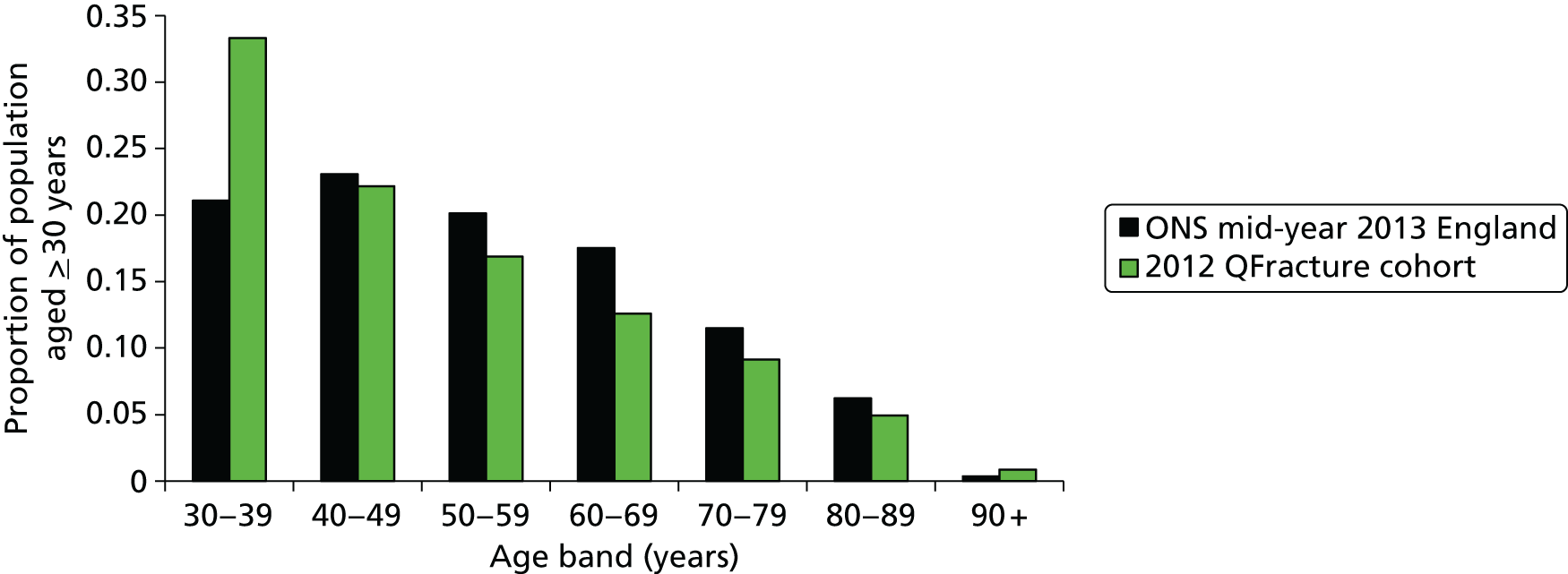
In 2010, the number of postmenopausal women living with osteoporosis in the UK, based on the definition of a BMD at least 2.5 SDs lower than that of a young healthy woman (T-score of ≤ –2.5 SDs), was predicted to increase from 1.8 million in 2010 to 2.1 million in 2020 (+16.5%). 8 The prevalence of osteoporosis in the general population of women aged ≥ 50 years was assumed to remain stable over time, at approximately 15.5%. In 2014, the reported prevalence of osteoporosis in women ranged from 9% (UK) to 15% (France and Germany) based on total hip BMD and from 16% (USA) to 38% (Japan) when spine BMD data were included. 9 Among males, prevalence ranged from 1% (UK) to 4% (Japan) based on total hip BMD and from 3% (Canada) to 8% (France, Germany, Italy, and Spain) when spine BMD data were included. 9
Fragility fractures are fractures that result from mechanical forces that would not ordinarily result in fracture, known as low-level (or ‘low-energy’) trauma. The WHO has quantified this as forces equivalent to a fall from a standing height or lower. Although osteoporosis is an important predictor of the risk of fragility fracture, 70% of fragility fractures in postmenopausal women occur in those who do not meet the criteria for osteoporosis. 10 The UK has one of the highest rates of fracture in Europe: every year 300,000 people in the UK suffer a fragility fracture, of which over 70,000 are hip fractures. 11
Impact of health problem
Significance for patients
Fractures cause significant pain, disability and loss of independence, and can be fatal. 1 Osteoporosis affects over 3 million people in the UK. 12 In the UK, 1150 people die every month following a hip fracture. 13
Significance for the NHS
In 2002, the cost to the NHS per annum was estimated to be £1.7B, with the potential to increase to £2.1B by 2020, as estimated in 2005. 14
Measurement of disease
Quantitative diagnosis in the UK relies on the assessment of BMD, usually by central DXA; BMD at the femoral neck provides the reference site. It is defined as a value for BMD of ≥ 2.5 SDs below the young female adult mean (T-score of ≤ –2.5 SDs). Severe osteoporosis (established osteoporosis) describes osteoporosis in the presence of one or more fragility fractures. 15
The National Institute for Health and Care Excellence (NICE) clinical guideline (CG) 146 (CG146) recommends the use of absolute risk of fragility fracture and recommends the use of one of two assessment tools:16 FRAX® (web version 3.9; University of Sheffield, Sheffield, UK)17 and QFracture® (QFracture-2012 open source revision 38; Clinrisk Ltd, Leeds, UK). 18,19 Both of these tools provide estimation of absolute fracture risk over a 10-year period. FRAX is intended for use in individuals aged 40–90 years and QFracture for those aged 30–99 years. The guideline recommends that assessment is indicated for all women aged > 65 years and all men aged > 75 years. 20 Above the age limit of the tools, people should be considered to be at high risk. Women aged between 50 and 65 years and men aged between 50 and 75 years should be assessed if they have additional risk factors of previous fragility fracture, current or frequent recent use of oral or systemic glucocorticoids, a known secondary cause of osteoporosis, a history of falls, a family history of hip fracture, low body mass index (BMI), smoking or weekly alcohol intake of > 14 units in women or > 21 units in men. Routine assessment of risk is not recommended for people under 50 years unless they have major risk factors. The guideline suggests that risk tools are likely to provide an underestimate of risk if the individual has previously suffered a vertebral fracture, has a very high alcohol intake, has secondary causes of osteoporosis or is receiving high-dose oral or high-dose systemic glucocorticoids. The guideline recommends that fracture risk in people under 40 years should be assessed using BMD and only in those with major risk factors such as history of multiple fragility fractures, major osteoporotic fracture or current/recent use of high-dose oral or high-dose systemic glucocorticoid therapy.
Current service provision
Clinical guidelines
Currently, related NICE guidance includes a clinical guideline for identifying women and men at risk of fracture and three technology appraisals of treatments for postmenopausal women only.
Current National Institute for Health and Care Excellence technology appraisal guidance
The NICE technology appraisal (TA) 160 (TA160; Alendronic acid, etidronic acid, Risedronic acid, Raloxifene and Strontium Ranelate for the Primary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women) guidance21 recommends alendronic acid (Fosamax® and Fosamax® Once Weekly, Merck Sharp & Dohme Ltd) as first-line treatment for the primary prevention of fragility fractures in postmenopausal women with osteoporosis who have an increased fracture risk defined by age, T-score and number of independent clinical risk factors for fracture, or indicators of low BMD. For women who cannot take alendronic acid, NICE TA16021 and TA20422 recommend risedronic acid (Actonel® and Actonel Once a Week®, Actavis), etidronic acid (no longer marketed), strontium ranelate (Protelos®, Servier Laboratories Ltd), teriparatide (Forsteo®, Eli Lilly and Company) or denosumab (Prolia®, AMGen), at specified fracture risks, defined by age, T-score and number of independent clinical risk factors for fracture. 23
The NICE TA16124 guidance recommends alendronic acid for secondary prevention of fragility fractures in postmenopausal women with confirmed osteoporosis. For women who cannot take alendronic acid, the NICE TA16124 guidance recommends risedronic acid, etidronic acid, raloxifene (Evista®, Daiichi Sankyo), strontium ranelate and teriparatide at specified fracture risks, defined by age, T-score and number of independent clinical risk factors for fracture. 23
The NICE TA20422 guidance recommends denosumab as a treatment option for the secondary prevention of osteoporotic fragility fractures only in postmenopausal women at increased risk of fractures who are unable to comply with the special instructions for administering alendronic acid and either risedronic acid or etidronic acid, or have an intolerance of, or a contraindication to, those treatments. 23
Current service cost
Hernlund et al. 25 reviewed the literature on fracture incidence and costs of fractures in the then 27 European Union (EU) countries and incorporated data into a model estimating the clinical and economic burden of osteoporotic fractures in 2010. The cost of osteoporosis, including pharmacological intervention, in the EU in 2010 was estimated at €37B. Treatment of incident fractures accounted for 66% of this cost, pharmacological prevention for 5% and long-term fracture care for 29%. Excluding the cost of pharmacological prevention, hip fractures accounted for 54% of the costs, vertebral and forearm fractures for 5% and 1%, respectively, and ‘other fractures’ for 39%. The estimated number of life-years lost in the EU because of incident fractures was approximately 26,300 in 2010. The total health burden, measured in terms of lost quality-adjusted life-years (QALYs), was estimated at 1,180,000 QALYs for the EU.
The cost of osteoporosis in 2010 in the UK (excluding the value of QALYs lost) was estimated by Hernlund et al. 25 at €103M (£88.3M in 2014 prices) for pharmacological fracture prevention, €3977M (£3410M in 2014 prices) for cost of fractures and €1328M (£1139M in 2014 prices) for long-term disability. The 2010 cost of UK osteoporosis fracture in relation to population and health-care spending was €5408M (£4637M in 2014 prices). It should be noted that the prices reported by Hernlund et al. 25 in euros have been converted to pounds sterling (2006 prices). The conversion ratio used by Hernlund et al. 25 was estimated (at 1.4065) by comparing the unit cost for nursing home stay against the cited UK-specific source data from 2006. The costs were then uplifted to 2014 prices using the hospital and community health services inflation indices from the Personal Social Services Research Unit (PSSRU)26 (290.5 for 2013/14 vs. 240.9 for 2005/6).
Variation in services and uncertainty about best practice
Existing NICE technology appraisals (TA16020 and TA16121) do not provide guidance on either ibandronic acid (Bonviva®, Roche Products Ltd) or zoledronic acid (Aclasta®, Novartis Pharmaceuticals UK Ltd). At present there is also no NICE guidance on the use of any bisphosphonate for the treatment of primary or secondary prevention in either men or people with steroid-induced osteoporosis.
Current treatment pathway
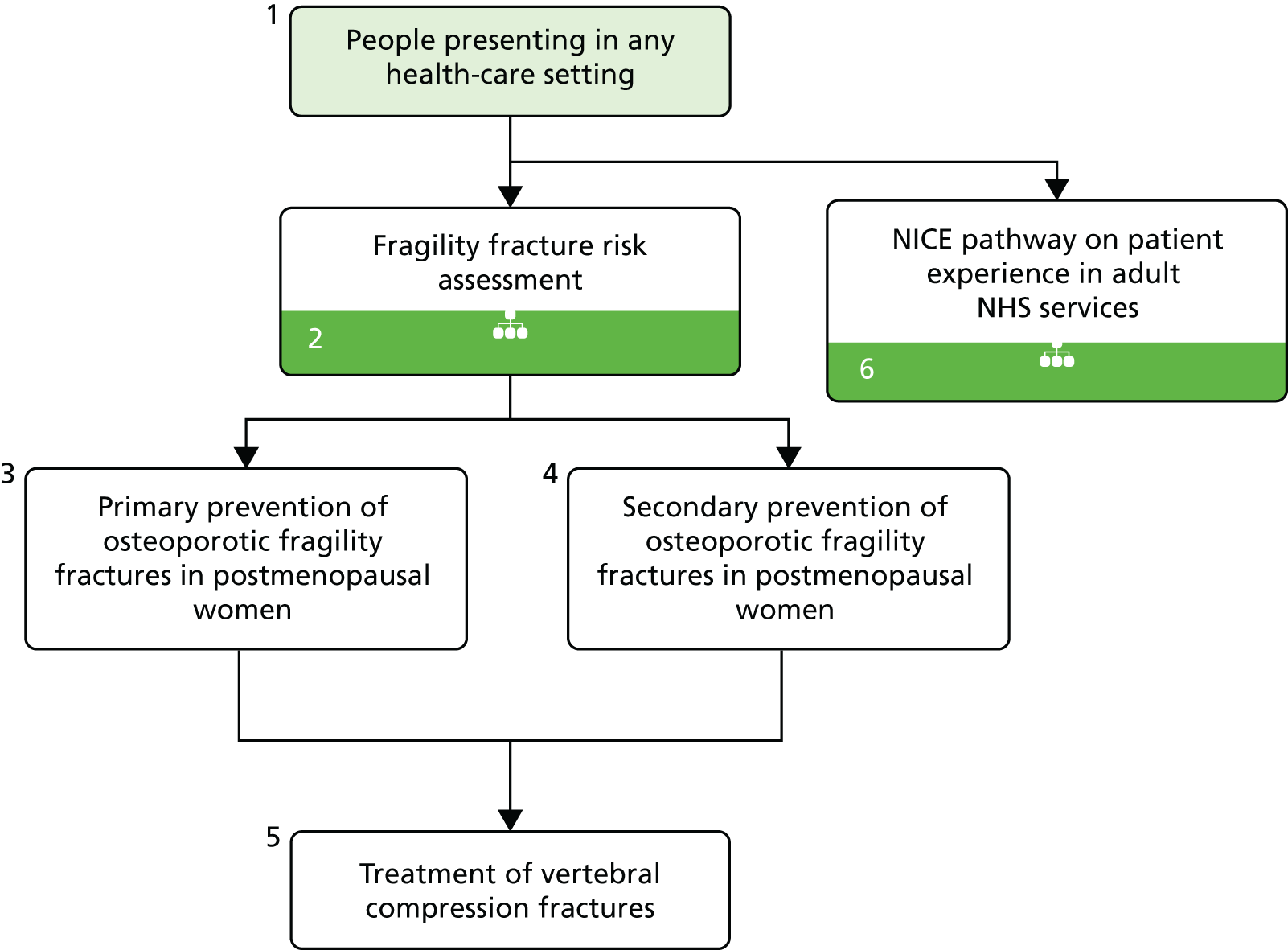
The NICE 2014 osteoporosis overview pathway27 is presented in Figure 1. This pathway covers NICE guidance on osteoporosis in adults (≥ 18 years), including assessing the risk of fragility fracture and drug treatment for the primary and secondary prevention of osteoporotic fragility fractures. 27
FIGURE 1.
Osteoporosis overview pathway. Reproduced from NICE. Osteoporosis Overview – NICE Pathway. London: NICE; 2014. 27
Current CGs recommend that fracture risk be assessed by estimating the absolute risk of fracture, whereas TAs use a defined set of risk factors to delineate people at risk. The modelling approach used in this assessment report allows intervention thresholds to be linked to absolute risk measured using the two risk assessment tools recommended in CG146,16 as specified in the scope. 23
The NICE 2014 fragility fracture risk assessment pathway28 is presented in Figure 2. This pathway covers NICE guidance on osteoporosis in adults (≥ 18 years), including assessing the risk of fragility fracture and drug treatment for the primary and secondary prevention of osteoporotic fragility fractures. 21
FIGURE 2.
Fragility fracture risk assessment pathway. Reproduced from NICE. Fragility Fracture Risk Assessment – NICE Pathway; London: NICE; 2014. 28

Description of technology under assessment
Interventions considered in the scope of this report
Five interventions will be considered within this assessment: oral alendronic acid, oral ibandronic acid, intravenous (i.v.) ibandronic acid, oral risedronic acid and i.v. zoledronic acid. These are all nitrogen-containing bisphosphonates.
Mode of action
Bisphosphonates are adsorbed onto hydroxyapatite crystals in bone. Aminobisphosphonate inhibits prenylation of proteins and leads to osteoclast apoptosis, reducing the rate of bone turnover. 29
Marketing licence and administration method
The dosages and administration routes for each treatment are summarised below (see Table 1).
Alendronic acid
Alendronic acid has a UK marketing authorisation for treating postmenopausal osteoporosis, orally once daily or weekly. The 10-mg daily dose has also has a UK marketing authorisation for treating osteoporosis in men and for preventing and treating glucocorticoid-induced osteoporosis in postmenopausal women not receiving hormone replacement therapy (HRT), orally once daily. 23
Non-proprietary alendronic acid (AAH, Accord, Actavis, Alliance Healthcare, Almus, APOTEX UK, Fannin UK, Focus, Generics (UK), Kent, Mylan UK, Phoenix Healthcare Distribution, PLIVA, Ranbaxy Laboratories, Rosemont, Somex, Sun Pharmaceuticals Industries Ltd, Teva UK, Waymade, Wockhardt UK and Zentiva N.V.) also has a UK marketing authorisation for the same indications. 23
Alendronic acid in the treatment of postmenopausal osteoporosis is administered orally: 10 mg daily or 70 mg once weekly. Treatment of osteoporosis in men is administered as 10 mg daily. Prevention and treatment of glucocorticoid-induced osteoporosis in postmenopausal women not receiving HRT is administered as 10 mg daily. Treatment is administered while sitting or standing and patients should remain seated or have stood for at least 30 minutes. 30
Ibandronic acid
Ibandronic acid has a UK marketing authorisation for treating postmenopausal osteoporosis, orally once monthly or every 3 months by i.v. injection. Non-proprietary ibandronic acid (produced by Actavis UK, Consilient Health, Mylan UK, Sun Pharmaceuticals Industries Ltd and Teva UK) also has a UK marketing authorisation for the same indications. 23
Ibandronic acid in the treatment of postmenopausal osteoporosis is administered either by mouth, 150 mg once a month, or by i.v. injection over 15–30 seconds, 3 mg every 3 months. Oral treatment is administered while sitting or standing and patients should remain seated or stand for at least 1 hour. 30
Oral and i.v. ibandronic acid are treated as separate interventions within our analysis.
Risedronic acid
Risedronic acid has a UK marketing authorisation for treating postmenopausal osteoporosis to reduce the risk of vertebral or hip fractures, orally once daily or weekly. It has a marketing authorisation for preventing osteoporosis (including glucocorticoid-induced osteoporosis) in postmenopausal women, orally once daily, and for treating osteoporosis in men at high risk of fractures, orally once weekly. Non-proprietary risedronic acid (produced by AAH, Actavis, Alliance Healthcare, Aspire, Aurobindo Pharma, Bluefish Pharmaceuticals AB, Dr Reddy’s Laboratories, Mylan UK, Phoenix Healthcare Distribution, Ranbaxy Laboratories, Sandoz, Sovereign Medical, Teva UK and Zentiva N.V.) also has a UK marketing authorisation for the same indications. 23
Risedronic acid in the treatment of postmenopausal osteoporosis to reduce the risk of vertebral or hip fractures is administered as 5 mg daily or 35 mg once weekly. For the prevention of osteoporosis (including glucocorticoid-induced osteoporosis) in postmenopausal women, treatment is administered as 5 mg daily. Treatment of osteoporosis in men at high risk of fractures is administered as 35 mg once weekly. Patients should remain seated or stand for at least 1 hour after administration. 30
Zoledronic acid
Zoledronic acid (Aclasta®, Novartis Pharmaceuticals) has a UK marketing authorisation for treating postmenopausal osteoporosis and osteoporosis in men (including glucocorticoid-induced osteoporosis in postmenopausal women and men) by i.v. infusion once a year.
Zoledronic acid in the treatment of postmenopausal osteoporosis and osteoporosis in men (including glucocorticoid-induced osteoporosis in men and postmenopausal women) is administered by i.v. infusion, 5 mg over at least 15 minutes once a year. In patients with a recent low-trauma hip fracture, the dose should be given ≥ 2 weeks following hip fracture repair. 30 Non-proprietary zoledronic acid (produced by Sun Pharmaceuticals Industries Ltd, Dr Reddy’s Laboratories and Teva UK) also has a UK marketing authorisation for the same indications. 31
Contraindications, special warnings and precautions
The summary of product characteristics (SmPC) for each intervention describes the contraindications and special warnings for bisphosphonates. 31–37
Alendronic acid
The alendronic acid 10-mg daily tablet and 70-mg weekly tablet are contraindicated in patients with abnormalities of the oesophagus or other factors that delay oesophageal emptying, such as stricture or achalasia, inability to stand or sit upright for at least 30 minutes, hypersensitivity to alendronic acid or to any of the excipients, or hypocalcaemia. Additional contraindications for the 70-mg oral solution are patients who have difficulty swallowing liquids and patients at risk of aspiration. 32,33
Special warnings and precautions for use include patients with active upper gastrointestinal (GI) problems and patients with known Barrett’s oesophagus. Patients with signs or symptoms signalling a possible oesophageal reaction should be instructed to discontinue treatment. While on treatment, patients with concomitant risk factors for osteonecrosis of the jaw (e.g. cancer, chemotherapy, radiotherapy, glucocorticoids, poor oral hygiene, periodontal disease) should avoid invasive dental procedures if possible. 32,33
Ibandronic acid
The ibandronic acid 150-mg tablet is contraindicated in patients with hypersensitivity to ibandronic acid or to any of the excipients, hypocalcaemia, abnormalities of the oesophagus that delay oesophageal emptying, such as stricture or achalasia, or inability to stand or sit upright for at least 60 minutes. The 3 mg/3 ml solution for injection every 3 months is contraindicated in patients with hypersensitivity to ibandronic acid or to any of the excipients and in patients with hypocalcaemia. 34,35
Special warnings and precautions for use include patients with existing hypocalcaemia and patients with active upper GI problems (e.g. known Barrett’s oesophagus, dysphagia, other oesophageal diseases, gastritis, duodenitis or ulcers) (oral administration). Intravenous administration may cause a transient decrease in serum calcium values. Adequate intake of calcium and vitamin D is important in all patients. Patients should be instructed to discontinue ibandronic acid and seek medical attention if they develop dysphagia, odynophagia, retrosternal pain or new or worsening heartburn. While on treatment, patients with concomitant risk factors for osteonecrosis of the jaw (e.g. cancer, chemotherapy, radiotherapy, glucocorticoids, poor oral hygiene, periodontal disease) should avoid invasive dental procedures if possible. 34,35
Risedronic acid
The risedronic acid 5-mg daily tablet and 35-mg weekly tablet are contraindicated in patients with hypersensitivity to the active substance or to any of the excipients, hypocalcaemia, or severe renal impairment (a creatinine clearance of < 30 ml/minute) and during pregnancy and lactation. 36,37
Special warnings and precautions for use include patients who have a history of oesophageal disorders that delay oesophageal transit or emptying (e.g. stricture or achalasia, patients who are unable to stay in the upright position for at least 30 minutes after taking the tablet and patients with active or recent oesophageal or upper GI problems, including known Barrett’s oesophagus). Patients should be instructed to seek timely medical attention if they develop symptoms of oesophageal irritation such as dysphagia, pain on swallowing, retrosternal pain or new or worsened heartburn. While on treatment, patients with concomitant risk factors for osteonecrosis of the jaw (e.g. cancer, chemotherapy, radiotherapy, glucocorticoids, poor oral hygiene, periodontal disease) should avoid invasive dental procedures if possible. 36,37
Zoledronic acid
A 5-mg annual infusion of zoledronic acid is contraindicated in patients with hypersensitivity to the active substance, to any bisphosphonates or to any of the excipients, patients with hypocalcaemia, patients with severe renal impairment with a creatinine clearance of < 35 ml/minute, and during pregnancy and breastfeeding. 31
Special warnings and precautions for use are required in patients with severe renal impairment (creatinine clearance < 35 ml/minute) and in those with pre-existing renal dysfunction or other risk factors, including advanced age, concomitant nephrotoxic medicinal products, concomitant diuretic therapy or dehydration occurring after administration, or with pre-existing hypocalcaemia. Adequate calcium and vitamin D intake are recommended. The incidence of post-dose symptoms occurring within the first 3 days after administration can be reduced with the administration of paracetamol (Panadol®, GlaxoSmithKline Consumer Healthcare) or ibuprofen [Nurofen, Reckitt Benckiser Healthcare (UK) Ltd]. 31
The SmPCs for each intervention also state that atypical subtrochanteric and diaphyseal femoral have been reported with bisphosphonate therapy; during bisphosphonate treatment patients should be advised to report any thigh, hip or groin pain and any patient presenting with such symptoms should be evaluated for an incomplete femur fracture. 31–37
Place in treatment pathway
Alendronic acid is recommended as first-line treatment for the primary prevention of fragility fractures in postmenopausal women with osteoporosis who have an increased fracture risk. Risedronic acid, raloxifene, strontium ranelate and teriparatide are recommended for women at specific risk of fracture who cannot take alendronic acid.
In addition to first-line treatment for the primary prevention of fragility fractures in postmenopausal women, alendronic acid is also recommended as a treatment option for the secondary prevention of osteoporotic fragility fractures in postmenopausal women who are confirmed to have osteoporosis. Risedronic acid, raloxifene, strontium ranelate and teriparatide are recommended for women at specific risk of fracture who cannot take alendronic acid. 24
Ibandronic acid and zoledronic acid do not have recommendations from NICE for the prevention of fragility fractures.
Denosumab is recommended as a treatment option for the primary prevention of osteoporotic fragility fractures only in postmenopausal women at increased risk of fracture who are unable to comply with the special instructions for administering alendronic acid and either risedronic acid or etidronic acid, or who have an intolerance of, or a contraindication to, those treatments. 22
Identification of important subgroups
The final NICE scope specified subgroups based on patient characteristics that increase the risk of fracture (those specified in NICE CG146)16 or that affect the impact of fracture on lifetime costs and outcomes. 23
Current usage in the NHS
Data from the Prescription Cost Analysis: England 201338 were analysed to determine the level of bisphosphonate usage within primary care across England in 2013. It can be seen from the data summarised in Table 1 that generic weekly alendronic acid was the most commonly prescribed preparation in primary care. Furthermore, generic prescriptions were more common than branded prescriptions across all treatments, where generic prescriptions were reported. Unlike primary care, there is no central NHS collation of information on medicines issued and used in NHS hospitals. However, a report on hospital prescribing in 201242 provides data on treatments recommended by NICE. From table 4 of the report42 it can be seen that the vast majority of prescribing for alendronic acid and risedronic acid occurred in primary care, with only 5% of the costs attributable to alendronic acid and risedronic acid prescribing occurring within secondary care. As data from Prescription Cost Analysis: England 201338 cover those medicines dispensed only in the community, and i.v. bisphosphonates are usually prescribed in secondary care, it should be noted that the figures in Table 1 will underestimate the prescribing of i.v. ibandronic acid and zoledronic acid. Data on i.v. bisphosphonates are not included in hospital prescribing data, as data were provided for individual drugs only if they had already been recommended by NICE.
| Interventions | Dosing schedule | Generic or branded | Description of preparations | List price per unit | Prescriptions in thousandsa |
|---|---|---|---|---|---|
| Alendronic acid (oral) | Daily, 10 mg | Branded | Fosamax tablets, alendronic acid (as sodium alendronate), 10 mg | 28-tablet pack = £23.12b | 0.749 |
| Generic | Tablets, alendronic acid (as sodium alendronate), 10 mg | 28-tablet pack = £2.17b | 46.605 | ||
| Weekly, 70 mg | Branded | Fosamax tablets, alendronic acid (as sodium alendronate), 70 mg | Four-tablet pack = £22.80b | 25.655 | |
| Generic | Tablets, alendronic acid (as sodium alendronate), 70 mg | Four-tablet pack = £1.01b | 7273.660 | ||
| Oral solution, sugar-free, alendronic acid (as sodium alendronate), 70 mg/100 ml | Four × 100 ml = £22.80b | 10.442 | |||
| Risedronic acid (oral) | Daily, 5 mg | Branded | Actonel tablets, risedronate sodium, 5 mg (yellow) | 28-tablet pack = £17.99b | 1.023 |
| Generic | Tablets, risedronate sodium, 5 mg | 28-tablet pack = £13.24b | 25.777 | ||
| Weekly, 35 mg | Branded | Actonel Once a Week tablets, orange, risedronate sodium, 35 mg | Four-tablet pack = £19.12b | 19.961 | |
| Generic | Tablets, risedronate sodium, 35 mg | Four-tablet pack = £1.18b | 679.026 | ||
| Ibandronic acid (oral) | Monthly, 150 mg | Branded | Bonviva tablet, 150 mg | One-tablet pack = £18.40b | 22.670 |
| Three-tablet pack = £55.21b | |||||
| Generic | Ibandronic acid tablet, 150 mg | 150-mg tablet, one-tablet pack = £1.61c | 204.006 | ||
| Ibandronic acid tablet, 50 mg | 50-mg tablet, 28-tablet pack = £10.78b | ||||
| Ibandronic acid (i.v.) | Quarterly, 3 mg | Branded | Bonviva injection, 3 mg/3 ml | 3-ml prefilled syringe = £68.64b | 0.181 |
| Generic | Ibandronic acid injection, 3-mg/3-ml prefilled syringe | 3-ml prefilled syringe = £65.20c | 0.324 | ||
| Zoledronic acid (i.v.) | Annually, 5 mg | Branded | Aclasta i.v. infusion, 5-mg/100-ml bottle | 100-ml bottle = £253.38b | 0.070 |
| Generic | i.v. infusion, zoledronic acid, 5 mg/100 ml | 100-ml bottle = £217.68d | Not reported |
Anticipated costs associated with interventions
Table 1 summarises the 2014 net costs associated with the interventions based on their list prices. 23 A list price was not available for generic zoledronic acid so the price reported in the manufacturer’s product catalogue has been included in Table 1.
Chapter 2 Definition of the decision problem
Decision problem
The aim of this assessment is to assess the clinical effectiveness and cost-effectiveness of alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid and zoledronic acid in the prevention of fragility fractures compared either with each other or with a non-active treatment.
Interventions
Five interventions will be considered within this assessment: alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid and zoledronic acid. These interventions are described in detail in Chapter 1, Description of technology under assessment.
Populations (including subgroups)
The assessment considers the following populations:
-
all women aged ≥ 65 years and men aged ≥ 75 years
-
women aged ≤ 64 years and men aged ≤ 74 years in the presence of risk factors, for example previous fragility fracture; current use or frequent recent use of oral or systemic glucocorticoids; history of falls; family history of hip fracture; other causes of secondary osteoporosis; low BMI (< 18.5 kg/m2); smoking; alcohol intake of > 14 units per week in women or > 21 units per week in men
-
women aged 64 years and men aged ≤ 74 years with low BMD (a T-score of –1 SD or more below the young adult mean).
An evaluation of the interventions in the following populations is outside the appraisal scope and will not be considered in this assessment:
-
women aged ≤ 64 years without a risk factor [see Populations (including subgroups)]
-
men aged ≤ 74 years without a risk factor [see Populations (including subgroups)].
Relevant comparators
Bisphosphonates (alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid and zoledronic acid) may be compared with each other or with a non-active agent, for example placebo.
Other bisphosphonates (e.g. etidronic acid) and other active agents (e.g. raloxifene, strontium ranelate and teriparatide) will not be considered as comparators in this assessment.
Etidronic acid is not included as a comparator as it has been discontinued by the manufacturer in the UK. Non-bisphosphonates licensed for the prevention of fragility fractures in women and men will be considered in a separate multiple technology appraisal (MTA).
Outcomes
The outcome measures to be considered included:
-
fragility fracture (fractures that result from mechanical forces that would not ordinarily result in fracture)
-
hip fracture
-
vertebral fracture (where data allow, clinical/symptomatic fractures will be reported separately from morphometric/radiographic fractures, with the latter being defined as those resulting in a ≥ 20% reduction in vertebral height)
-
all non-vertebral fracture
-
wrist fracture
-
proximal humerus fracture
-
fragility fracture at other sites
-
-
BMD at the femoral neck assessed by DXA
-
mortality
-
all cause
-
mortality following hip fracture
-
mortality following vertebral fracture
-
mortality following fracture at site other than hip or vertebral
-
-
adverse effects of treatment including but not limited to
-
upper GI symptoms
-
osteonecrosis of the jaw
-
hypocalcaemia
-
bone pain (not associated with influenza-type symptoms)
-
atypical femoral fractures
-
influenza-like symptoms including bone pain, myalgia, arthralgia, fever and rigors
-
conjunctivitis
-
atrial fibrillation
-
stroke
-
-
continuance (or persistence; proportion of people still on treatment at the end of a given period) and concordance (or compliance; proportion of prescribed doses taken during a given period)
-
health-related quality of life (HRQoL)
-
health-care resource use, for example hospitalisation, entry into long-term residential care.
Key issues
An evaluation of the interventions in the following populations is outside the appraisal scope and will not be considered in this assessment:
-
women aged ≤ 64 years without a risk factor [see Populations (including subgroups)]
-
men aged ≤ 74 years without a risk factor [see Populations (including subgroups)].
Overall aims and objectives of assessment
This assessment addresses the question ‘what is the clinical effectiveness and cost-effectiveness of alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid and zoledronic acid in the prevention of fragility fractures as compared against each other or a non-active treatment?’.
More specifically, the objectives of the assessment are to:
-
evaluate the clinical effectiveness of each intervention
-
evaluate the adverse effect profile of each intervention
-
evaluate the incremental cost-effectiveness of each intervention compared with (1) each other and (2) no active treatment
-
estimate the overall NHS budget impact in England.
Chapter 3 Assessment of clinical effectiveness
A systematic review of the literature with evidence synthesis including a network meta-analysis (NMA) was conducted in order to evaluate the clinical effectiveness and safety of alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid and zoledronic acid in the prevention of fragility fractures.
The systematic review of clinical effectiveness was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 43
Methods for reviewing effectiveness
The protocol for this review is registered with PROSPERO (CRD42013006883). 44
Identification of studies
A comprehensive search was undertaken to systematically identify clinical effectiveness literature relating to alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid, and zoledronic acid within their licensed indications for the prevention of fragility fractures. The search strategy comprised the following main elements:
-
searching of electronic databases
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers.
The following databases were searched:
-
MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE (via Ovid) from 2008 to 23 September 2014
-
EMBASE (via Ovid) from 2008 to 23 September 2014
-
Cochrane Database of Systematic Reviews (via Wiley Online Library) from 2008 to 23 September 2014
-
Database of Abstracts of Reviews of Effects (via Wiley Online Library) from 2008 to 23 September 2014
-
Cochrane Central Register of Controlled Trials (via Wiley Online Library) from 2008 to 23 September 2014
-
Health Technology Assessment Database (via Wiley Online Library) from 2008 to 23 September 2014
-
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost) from 2008 to 23 September 2014
-
Science Citation Index Expanded (via Web of Science) from 2008 to 23 September 2014
-
Conference Proceedings Citation Index – Science (via Web of Science) from 2008 to 23 September 2014
-
Bioscience Information Service (via Web of Science) from 2008 to 23 September 2014.
Existing evidence reviews20 commissioned by NICE, which included literature published up to June 2008, were assumed to have identified all papers relevant to this review published prior to 2008. Therefore, searches were limited by date from 2008 until 26 September 2014. Searches were not restricted by language or publication type. Subject headings and keywords for ‘osteoporosis’ were combined with each of the named drug interventions. The MEDLINE search strategy is presented in Appendix 1. The search was adapted for the other databases. Highly sensitive study design filters were used to retrieve clinical trials and systematic reviews on MEDLINE and other databases, where appropriate. Consultee submissions and relevant systematic reviews were also hand-searched in order to identify any further relevant clinical trials. Two clinical trials research registers (ClinicalTrials.gov and the WHO’s International Clinical Trials Registry Platform) were also searched for ongoing and recently completed research projects. Citation searches of key included studies were also undertaken using the Web of Science database. All potentially relevant citations were downloaded to Reference Manager bibliographic software (version 12.0; Thomson Reuters, Philadelphia, PA, USA) and deduplication of citation records was undertaken.
Inclusion and exclusion criteria
Inclusion criteria have been defined in line with the final scope provided by NICE23 and are outlined below.
Study selection process
The selection of eligible articles was undertaken by two reviewers (MMSJ and EG). Both reviewers sifted all downloaded citations (4117). Citations not meeting the exclusion criteria based on the title and/or abstract were excluded at the sifting stage. All potentially relevant citations were marked to be obtained at full text for further scrutiny. A check for consistency was undertaken using a Cohen’s kappa coefficient of inter-rater agreement. A high level of agreement between reviewers (0.951) was observed. Any uncertainty regarding the eligibility of potentially relevant full-text articles was resolved through discussion. Articles that were obtained as full text for screening that were subsequently excluded were recorded together with the reason for exclusion. A table of excluded studies at full text with reason is presented in Appendix 2, Table 41.
Inclusion criteria
Studies were included in the review if they met the inclusion criteria outlined below.
Interventions
Any of the following interventions were included:
-
alendronic acid (oral)
-
risedronic acid (oral)
-
ibandronic acid (oral)
-
ibandronic acid (i.v.)
-
zoledronic acid (i.v.).
Studies in which the interventions were assessed in line with licensed indications were included in the systematic review. Studies that titrated doses upwards from unlicensed to licensed doses within treatment groups during the trial period were eligible for inclusion. Studies that evaluated both licensed and unlicensed dose study groups were included where outcome data only for the licensed group could be extracted. Data reported for licensed and unlicensed doses combined (pooled study groups) were not eligible for inclusion.
With respect to ibandronic acid, the licence authorisation was supported by trials assessing the antifracture efficacy of 2.5 mg per day orally and 20 mg every other day orally (dose not licensed) compared with placebo [iBandronate Osteoporosis vertebral fracture trial in North America and Europe (BONE)45,46] and assessing non-inferiority of oral daily dosing (2.5 mg) compared with oral monthly dosing (100 mg or 150 mg) on BMD [the Monthly Oral iBandronate In LadiEs (MOBILE) trial]. 47,48 A bridging study then demonstrated superiority for the current licensed i.v. dose of 3 mg every 3 months compared with the 2.5 mg once daily oral dose in terms of BMD [the Dosing IntraVenous Administration (DIVA) trial]. 49,50 As such, these pivotal trials along with other trials comparing ibandronic acid 2.5 mg with placebo were eligible for inclusion in addition to those assessing current licensed doses.
Populations
Studies were included that evaluated women aged ≥ 65 years or men aged ≥ 75 years. Studies were included if they evaluated women aged ≤ 64 years and men aged ≤ 74 years in the presence of risk factors, for example previous fragility fracture, current use or frequent recent use of oral or systemic glucocorticoids, a history of falls, a family history of hip fracture, other causes of secondary osteoporosis, low BMI (< 18.5 kg/m2), smoking or an alcohol intake of > 14 units per week in women or > 21 units per week in men. Studies were also included if they evaluated women aged ≤ 64 years and men aged ≤ 74 years with low BMD (a T-score of –1 SD or more below the young adult mean). Studies that recruited mixed populations of men and women were also included, as were studies that recruited samples with mixed population characteristics, for example if they recruited a sample of women aged ≤ 65 years with and without risk fractures.
In studies evaluating participants with risk factors for or the presence of secondary osteoporosis [e.g. treatment with aromatase inhibitors or androgen deprivation therapy (ADT)] that did not evaluate a treatment of interest within its licensed indication, advice was sought from the clinical advisor (PS) regarding inclusion.
Comparators
Relevant comparators included interventions compared with each other. Interventions could be compared with placebo or other non-active treatments (i.e. treatment without the potential to augment bone). Studies that administered calcium and/or vitamin D to patients in both the intervention and comparator arms were included (e.g. bisphosphonate plus calcium vs. placebo plus calcium).
Outcomes
Eligible outcomes for consideration included fragility fractures, BMD at the femoral neck, mortality, adverse effects, compliance, HRQoL and health-care resource use. These are described in full in Chapter 2, Decision problem.
Study design
Randomised controlled trials (RCTs) were eligible for inclusion in the clinical effectiveness systematic review. If no RCTs were identified for an intervention, non-randomised studies were considered for inclusion. Non-randomised studies were also considered for inclusion, where necessary, as a source of additional evidence [e.g. relating to adverse events (AEs), long-term incidence of fragility fracture, treatment persistence, etc.] associated with the interventions. This evidence was considered important for demonstrating rare, catastrophic and delayed AEs of treatments along with information regarding long-term treatment continuance and concordance that are not captured by RCTs. Observational studies can provide information about how technologies function in real-world settings. For this assessment report, this evidence was summarised from existing systematic reviews.
Studies published as abstracts or conference presentations were eligible for inclusion only if sufficient details were presented to allow an assessment of the trial methodology and results to be undertaken.
Exclusion criteria
The following types of studies were excluded from the review:
-
studies in patients with normal or unspecified BMD who were not selected based on the presence of risk factors
-
studies in patients with other indications for bisphosphonate treatment, for example Paget’s disease, hypercalcaemia of malignancy, metastatic breast cancer
-
studies in which administration of interventions was not in accordance with the licensed indications
-
studies in which interventions were co-administered with any other therapy with the potential to augment bone, unless concomitant treatments are specified in the SmPC
-
systematic reviews and clinical guidelines (these were used as sources of references)
-
studies that were considered methodologically unsound in terms of study design or the method used to assess outcomes
-
studies that were published only in languages other than English
-
studies based on animal models
-
preclinical and biological studies
-
narrative reviews, editorials, opinions
-
reports published as abstracts or conference presentations only, where insufficient details were reported to allow an assessment of study quality or results.
Data abstraction strategy
Data relevant to the decision problem were extracted by two reviewers (MMSJ or EG). Data were extracted without blinding to authors or journal. A data extraction form was developed and piloted on two included trials before use on all included trials. Data relating to study arms in which the intervention treatments were administered in line with their licensed indications were extracted; data relating to the unlicensed use of the interventions were not extracted. MMSJ and EG checked at least 10% of each other’s data extraction forms. All extracted outcome data to be used in the analyses were double-checked by a third reviewer (FC). The safety data extracted were informed by the SmPCs for each product (available from www.medicines.org.uk/emc/). 31–37 The key safety issues included such items as the number of patients experiencing AEs, the number of patients withdrawing because of AEs, the number of patients experiencing upper GI tract symptoms, the number of patients with osteonecrosis of the jaw, hypocalcaemia, bone pain, atypical femoral fractures, atrial fibrillation or stroke, and the number of patients experiencing flu-like symptoms. Outcome data that were presented only in graphical format were digitised and estimated using xyExtract software (version 5.1; Wilton and Cleide Pereira da Silva, Paraiba, Brazil). Where multiple publications of the same study were identified, data extraction was undertaken on all relevant associated publications and findings were presented together with reference to their published source.
Critical appraisal strategy
The methodological quality of each included study was assessed by one reviewer (MMSJ or EG). The quality of included studies was assessed using the Cochrane Risk of Bias Tool. 51 This tool addresses specific domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. RCTs were classified as being at ‘high risk’ of attrition bias if dropout in any treatment arm was ≥ 10%. 52 In order to inform the selective reporting domain of the Cochrane Risk of Bias Tool, a judgement was made that peer-reviewed articles which reported approval of a trial protocol or a trial registration number could be considered as being at ‘low risk’ of bias for this domain. All quality assessment findings were double checked by a second reviewer (MMSJ or EG).
Methods of data synthesis
The extracted data were presented for each study both in structured tables and as a narrative description.
Methods for the estimation of efficacy using network meta-analysis
Network meta-analysis methods are described in full alongside results in Methods for the network meta-analyses, with further details provided in Appendix 3.
Supplementary meta-analyses
Where considered appropriate, secondary outcomes of interest were analysed using classical meta-analysis methods. Meta-analysis was undertaken using Cochrane Review Manager software (version 5.2, The Cochrane Collaboration, Copenhagen, Denmark). Outcomes reported as continuous data were summarised using a mean difference with 95% confidence intervals (CIs). Dichotomous outcomes were summarised as risk ratios (RRs) with associated 95% CIs. Where RCTs reported AEs in sufficient detail, these were analysed as dichotomous data. Clinical heterogeneity across RCTs (the degree to which RCTs appear different in terms of participants, intervention type, and duration and outcome type) was considered prior to data pooling. Random-effects models were applied. Effect estimates, estimated in Review Manager as z-scores, were considered statistically significant at a p-value < 0.05.
Results
Quantity and quality of the available research
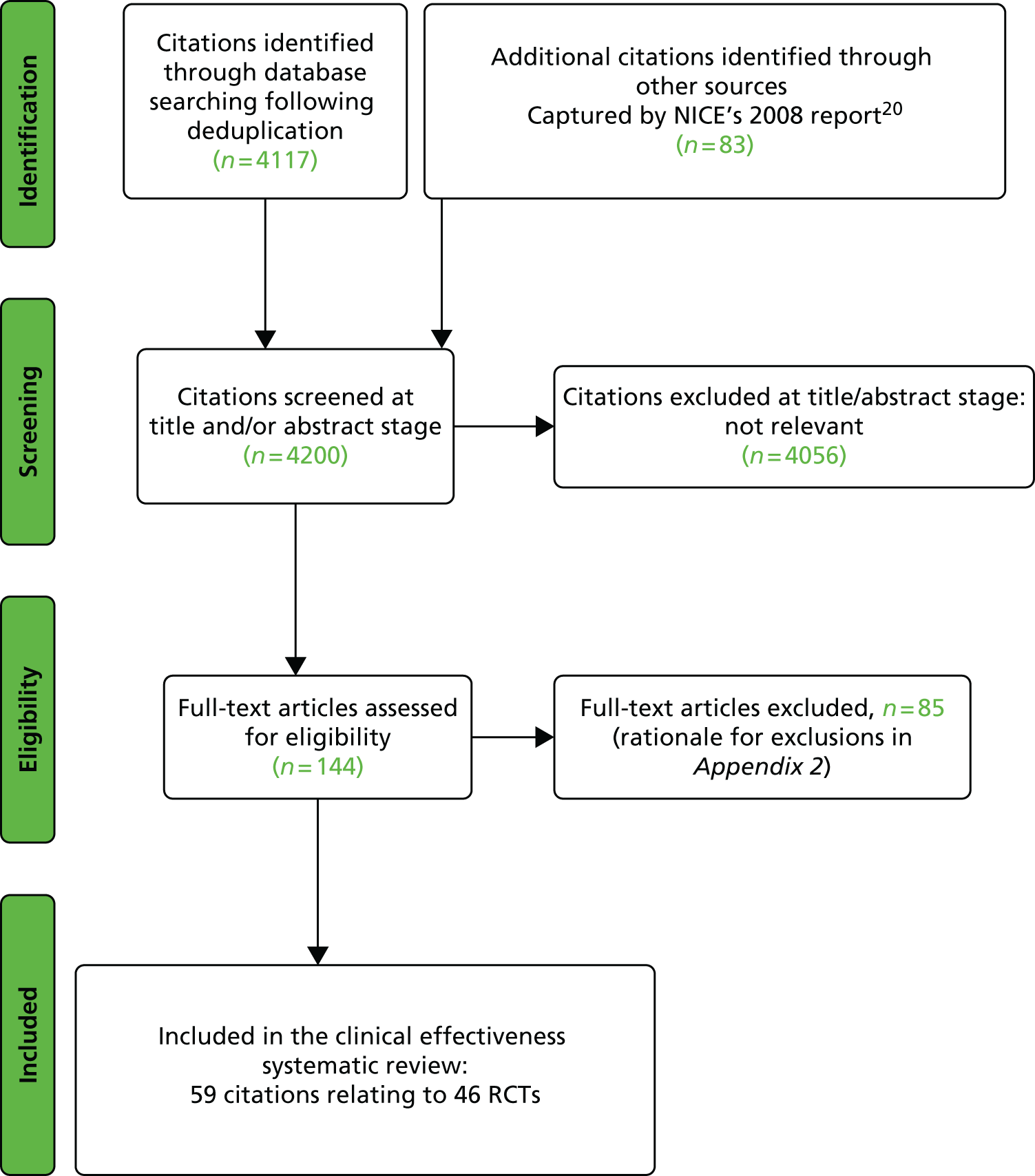
The searches described in Identification of studies identified 4117 potentially relevant citations from searches of electronic databases after removal of duplicates. A further 83 citations were identified from an existing evidence review commissioned by NICE. 20 Of these records, 4056 were excluded at the title or abstract stage. Full texts of 144 citations were obtained for scrutiny. Of these, 85 citations were excluded (excluded studies with reason for exclusion is presented in Appendix 2, Table 41). A total of 46 RCTs,45,47,49,53–95 reported across 59 citations, were included in the review.
The search process is summarised in the form of a PRISMA flow diagram96 in Figure 3.
FIGURE 3.
Flow diagram of study selection process (adapted from PRISMA): clinical effectiveness review.
The summary of the included RCTs is presented in Table 2 and the characteristics of the included RCTs are presented in Table 3.
| Treatment, number of RCTs (number of citations) | Trial (trial acronym) | Population |
|---|---|---|
| Alendronic acid vs. placebo, 17 RCTs (19 citations) | Adami et al., 199553 | Women with PMO |
| Black et al., 199655 | Women with PMO | |
| Cummings et al., 199864 | Women with PMO | |
| Bone et al., 200057 | Women with PMO | |
| Carfora et al., 199860 | Women with PMO | |
| Chesnut et al., 199561 | Women with PMO | |
| Dursun et al., 200165 | Women with PMO | |
| Greenspan et al., 200267 | Women with PMO | |
| Greenspan et al., 200368 | Women aged ≥ 65 years | |
| Ho and Kung, 200571 | Women with PMO | |
| Klotz et al., 201373 (CORAL) | Men with androgen deprivation bone loss in non-metastatic prostate cancer | |
| Liberman et al., 199576 | Women with PMO | |
| Seeman 199997 | Women with PMO | |
| Orwoll et al., 200083 | Men with OP | |
| Pols et al., 199984 (FOSIT) | Women with PMO | |
| Saag et al., 1998;91 extension of Adachi et al., 200198 | Men and women with glucocorticoid-induced OP | |
| Shilbayeh et al., 200493 | Women with PMO | |
| Smith et al., 200494 | Men and women with asthma and/or chronic obstructive airways disease | |
| Ibandronic acid (unlicensed daily oral dose) vs. placebo, one RCT (two citations) | Chesnut et al., 200445; Chesnut et al., 200546 (BONE) | Women with PMO |
| Ibandronic acid (monthly oral dose) vs. placebo, two RCTs (two citations) | Lester et al., 200874 (ARIBON) | Postmenopausal women with breast cancer |
| McClung et al., 200980 | Women with PMO | |
| Ibandronic acid dose-ranging trials (quarterly i.v. dose vs. unlicensed daily oral dose), one RCT (two citations) | Delmas et al., 2006;49 Eisman et al., 200850 (DIVA) | Women with PMO |
| Ibandronic acid dose-ranging trials (monthly oral dose vs. unlicensed daily oral dose), one RCT (two citations) | Miller et al., 2005;47 Reginster et al., 200648 (MOBILE) | Women with PMO |
| Risedronic acid vs. placebo, 12 RCTs (15 citations) | Boonen et al., 200958 | Men with OP |
| Choo et al., 201162 | Men with androgen deprivation bone loss in non-metastatic prostate cancer | |
| Cohen et al., 199963 | Men and women (≥ 1 year PM) aged 18–85 years on glucocorticoids | |
| Fogelman et al., 200066 (BMD-MN) | Women with PMO | |
| Hooper et al., 200572 | Early PM women with OP | |
| Harris et al., 199970 (VERT-NA); extension of Ste-Marie et al., 200499 | Women with PMO | |
| Reginster et al., 200085 (VERT-MN); extension of Sorensen et al., 2003100 | Women with PMO | |
| Leung et al., 200575 | Women with PMO | |
| McClung et al., 200178 | Women with PMO | |
| Reid et al., 200086 | Men and women taking glucocorticoids for ≥ 6 months | |
| Ringe et al., 2006;89 extension of Ringe et al., 2009101 | Men with OP | |
| Taxel et al., 201095 | Men aged > 55 years and within 1 month of receiving an initial injection of ADT for prostate cancer | |
| Zoledronic acid vs. placebo, four RCTs (six citations) | Black et al., 200756 (HORIZON-PFT); AEs following administration, Reid et al. 2010102 | Women with PMO |
| Lyles et al., 200777 (HORIZON-RFT); HRQoL, Adachi et al., 2011103 | Men and women ≥ 50 years of age within 90 days after surgical repair of a hip fracture | |
| Boonen et al., 201259 | Men with OP | |
| McClung et al., 200979 | Women with PMO | |
| Alendronic acid vs. ibandronic acid (monthly oral dose), one RCT (one citation) | Miller et al., 200881 (MOTION) | Women with PMO |
| Alendronic acid vs. risedronic acid, five RCTs (seven citations) | Atmaca and Gedik 200654 | Women with PMO |
| Muscoso et al., 200482 | Women with PMO | |
| Sarioglu et al., 200692 | Women with PMO | |
| Rosen et al., 200590 (FACT); extension of Bonnick et al., 2006104 | Women with PMO | |
| Reid et al., 200687 (FACTS); extension of Reid et al., 2008105 | Women with PMO | |
| Zoledronic acid vs. alendronic acid, one RCT (two citations) | Hadji et al., 2010;106 Hadji et al., 201269 (ROSE) | Women with PMO |
| Zoledronic acid vs. risedronic acid, one RCT (one citation) | Reid et al., 200988 (HORIZON) | Men and women taking glucocorticoids for ≥ 3 months and for < 3 months |
| Author, year of study publication, country, number of centres and sponsor | Inclusion and exclusion criteria | Numbers randomised and adjuvant supplements | Final follow-up and assessment time points | Primary and secondary outcomes | Fracture and BMD assessments |
|---|---|---|---|---|---|
| ALN vs. PBO | |||||
| Adami et al., 1995;53 Italy Multicentre RCT, 11 centres Sponsor NR |
Inclusion: women at least 2 years past natural menopause; the majority were aged < 65 years. Each had LS BMD which was > 2 SDs below the mean for young women. Evidence of previous vertebral fracture was not an entry criterion, and only 5% of subjects had prevalent fractures Exclusion: evidence of any secondary cause of OP, other metabolic bone disease, hyper- or hypothyroidism. Medications affecting bone metabolism |
PBO, n = 71; ALN 10 mg/day, n = 78 Adjuvant: both groups, calcium 500 mg/day |
24 months BMD assessed at 24 months |
Primary: change in the LS BMD (L1–L4) Secondary: change in the FN and trochanter spine BMD |
Fractures: not an outcome BMD: DXA (Hologic, Waltham, MA, USA; Lunar, Madison, WI, USA; Norland, WI, USA; and Sophos, Paris, France) |
| Black et al., 199755 (FIT I); USA Multicentre RCT, 11 centres Merck Research Laboratories |
Inclusion: women aged between 55 and 81 years, postmenopausal for at least 2 years, had at least one vertebral fracture and FN BMD of ≤ 0.68 g/cm2 (≤ 2 SDs below normal young adult) Exclusion: peptic ulcer disease, dyspepsia requiring treatment, abnormal renal function, major medical problems that would preclude participation, severe malabsorption syndrome, hypertension, myocardial infarction, unstable angina, disturbed thyroid or parathyroid function, use of oestrogen, calcitonin, bisphosphonates or sodium fluoride |
PBO, n = 1005; ALN 10 mg/day, n = 1022 Adjuvant: both groups, women with low calcium intake 500 mg/day of calcium supplements and 250 IU/day of vitamin D |
36 months Lateral radiographs were obtained at baseline, 24 months and 36 months |
Primary: new vertebral fractures at 3 years – a new vertebral fracture if any of the ratios of vertebral heights was more than 3 reports below the mean population norm for that vertebral level Secondary: non-vertebral fractures (hip, wrist, and others); FN, LS and TH BMD AEs |
Fractures: vertebrae were judged to be fractured by morphometric assessment using a translucent digitiser. Clinical fractures (non-spine clinical fractures, hip fractures, wrist fractures and clinical vertebral fractures, and other clinical fractures) were reported by participants and confirmed by a required written report of a radiological procedure BMD: DXA – QDR-2000 Hologic (Waltham, MA, USA) |
| Cummings et al. 199864 (FIT II); USA Multicentre RCT, 11 centres Merck Research Laboratories |
Inclusion: women aged 55–80 years; postmenopausal for at least 2 years; FN BMD of ≤ 0.68 g/cm2 (≤ 2 SDs below normal young adult) Exclusion: peptic ulcer disease, dyspepsia requiring treatment, abnormal renal function, major medical problems that would preclude participation, severe malabsorption syndrome, hypertension, myocardial infarction, unstable angina, disturbed thyroid or parathyroid function, use of oestrogen, calcitonin, bisphosphonates or sodium fluoride |
PBO: n = 2218; ALN 10 mg/day, n = 2214 Adjuvant: both groups, women with low calcium intake 500 mg/day of calcium supplements and 250 IU/day of vitamin D |
48 months Lateral radiographs were obtained at baseline and and 48 months |
Primary: clinical fractures (vertebral and non-vertebral) confirmed by radiographs at 4.2 years Secondary: change in BMD of the hip and posterior–anterior spine and whole body; AEs, from baseline in each group |
Fractures: clinical fractures were defined as one diagnosed by a physician. Self-reports of fractures were confirmed by radiographic or other tests (not described). Traumatic fractures and fractures of the face/skull were excluded Vertebral fractures were assessed by radiographs. Fracture was defined as 20% decrease in height and 4 mm decrease in vertebral height BMD: DXA – QDR-2000 (Hologic, Waltham, MA, USA) |
| Bone et al., 2000;57 countries not specified RCT, number of centres not specified Merck Research Laboratories |
Inclusion: postmenopausal osteoporotic women aged 42–82 years, with hysterectomy; BMD < 0.862 g/cm2 on at least three vertebra, LS T-score of ≤ –2.5 SDs Exclusion: metabolic bone disease, low vitamin D, oestrogen replacement therapy > 6 months, drugs that affect bone turnover, renal insufficiency, cardiac disease, upper GI disease |
PBO, n = 50; ALN 10 mg/day, n = 92 Adjuvant: both groups, 1000 mg/day of calcium |
24 months BMD assessed at 3, 6, 12, 18 and 24 months |
Primary: change BMD of the LS, at 24 months Secondary: change in BMD of the TH, FN, trochanter and total body; biochemical markers of bone turnover; fractures; AEs |
Fractures: clinical fractures recorded as AEs (assessment method NR) BMD: Hologic QDR densitometers (QDR-1000, -1000/W, -1500 or -2000; Hologic Inc., Waltham, MA, USA) |
| Carfora et al., 1998;60 Italy Single-centre RCT Sponsor NR |
Inclusion: postmenopausal women (for ≥ 5 years); aged 44–80 years; at least 2.5 SDs below the mean value in premenopausal white women Exclusion: women with other causes of OP or vitamin D deficiency, Paget’s disease, hyperparathyroidism, peptic ulcer, abnormal renal/hepatic function, abnormalities of LS |
PBO, n = 34; ALN 10 mg/day, n = 34 Adjuvant: both groups, 500 mg/day of calcium |
30 months BMD assessed every 5 months, radiography at baseline and end treatment |
Primary: change in BMD of the spine at 2.5 years Secondary: fractures; biochemical markers of bone turnover; and AEs |
Fractures: radiography of the thoracic and LS to evaluate fractures. No further details reported BMD: DXA – QD|R1000 (Hologic) |
| Chesnut et al., 1995;61 USA Multicentre RCT, seven centres Merck Research Laboratories |
Inclusion: women aged 42–75 years, at least 5 years postmenopausal, with LS BMD ≤ 0.88 g/cm2 (approximately 2 SDs below young, normal US white female mean BMD values) Exclusion: medications affecting bone metabolism were excluded, the presence of spine or hip fractures attributable to OP |
PBO, n = 31; ALN 10 mg/day, n = 30 Also evaluated ALN 5 mg/day, n = 32; 20 mg, n = 32; 40 mg/PBO, n = 32, 40 mg × 3 months then 2.5 mg × 21 months, n = 31 Adjuvant: both groups, 500 mg/day of calcium |
24 months BMD assessed every 3 months |
Primary: change in BMD of the LS, FN, TH, intertrochanter, Ward’s triangle and the forearm; bone markers; and AEs Secondary: NR |
Fractures: not an outcome BMD: DXA 1000w (Hologic Inc., Waltham, MA, USA) |
| Dursun et al., 2001;65 Turkey Single-centre RCT Sponsor NR |
Inclusion: postmenopausal women, with BMD of ≥ 2 SDs below young adult mean at either the LS or FN Exclusion: history of drug/alcohol abuse, metabolic bone disease, GI/liver disease, renal failure/calculi, glucocorticoid therapy, malignancy, disorder of calcium metabolism and LS abnormalities preventing BMD evaluation |
Calcium 1000 mg/day, n = 50 ALN 10 mg + calcium 1000 mg/day, n = 51 Also evaluated calcitonin, n = 50 |
12 months BMD and radiographic assessment at 6 and 12 months |
Primary: change in BMD of the LS, FN, trochanter and Ward’s triangle in each group at 12 months Secondary: number of factures; quality of life and pain; fractures; AEs |
Fractures: radiography of thoracic and lumbar vertebrae. A new vertebral fracture was defined as a decrease of 20% and at least 4 mm in any vertebral height BMD: DXA – model and manufacturer NR |
| Greenspan et al., 2002;67 USA Multicentre RCT, 25 centres Merck Research Laboratories |
Inclusion: ambulatory women in long-term care ≥ 65 years, LS or TH BMD T-score of ≤ –2.0 SDs Exclusion: disorders of bone mineralisation, low vitamin D, hyperthyroidism, GI disease, use of bone-active agents |
PBO, n = 164; ALN 10 mg/day, n = 163 Adjuvant: both groups, 1000 mg/day of calcium and 400 IU/day of vitamin D |
24 months BMD assessed at 6, 12, 18 and 24 months |
Primary: change in BMD of the LS, FN, hip and hip trochanter; and biochemical markers of bone turnover at 2 years Secondary: AEs including fractures |
Fractures: clinical fractures recorded as AEs (assessment method NR) BMD: DXA – Hologic (Waltham, MA, USA) |
| Greenspan et al., 2003;68 USA Single-centre RCT NIH grant NR |
Inclusion: community-dwelling women aged ≥ 65 years Exclusion: FN BMD ≥ 0.9 g/cm2 (0 SD of mean peak). Disease or drugs affecting bone metabolism |
PBO, n = 93; ALN 10 mg/day, n = 93 Adjuvant: women with low calcium intake, calcium 600 mg/day, 200 IU/day of vitamin D Both groups, vitamin D 400–800 IU/day |
36 months BMD assessed at 6, 12, 18, 24 and 36 months |
Primary: change in BMD of the hip, spine, FN, trochanter and ultradistal radius Secondary: fractures and AEs |
Fractures: fracture reduction was not a primary end point – recorded as AEs (assessment method NR) BMD: DXA – QDR4500 A (Hologic) (Bedford, MA, USA) |
| Ho and Kung 2005;71 China RCT, number of centres NR Merck Sharp & Dohme Ltd |
Inclusion: women with OP aged < 75 years, postmenopausal for > 3 years and a BMD in the LS of –2.5 SDs below local peak age Exclusion: treatment with bisphosphonates of fluorides, SERMs or oestrogen, calcitonin or any other drug that could affect bone metabolism |
Calcium 500 mg/day, n = 29; ALN 10 mg + calcium 500 mg/day, n = 29 Adjuvant: calcium 500 mg/day |
12 months BMD assessed at 3, 6 and 12 months |
Primary: change in BMD of the LS, FN and TH; bone markers; AEs Secondary: NR |
Fractures: fracture not an outcome BMD: DXA – QDR (Hologic Waltham, MA, USA) |
| Klotz et al., 201373 (CORAL); Canada Multicentre RCT, 30 centres Abbot Laboratories |
Inclusion: men with histologically confirmed prostate cancer in whom ≥ 1 year of ADT was indicated Exclusion: hypocalcaemia, abnormal renal/liver function, metabolic bone disease, bilateral hip replacement, prior treatment with bisphosphonates or therapy with glucocorticoids |
PBO, n = 102; ALN 70 mg/week, n = 84 Adjuvant: both groups, calcium 500 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS Secondary: change in BMD of the TH; changes in bone markers |
Fractures: not an outcome BMD: DXA – model NR |
| Liberman et al., 1995;76 one multicentre study was conducted in the USA and the other in Australia, Canada, Europe, Israel, Mexico, New Zealand and South America Phase III, multicentre RCT Merck Research Laboratories |
Inclusion: postmenopausal women (for at least 5 years) aged 45–80 years, with a BMD in the LS at least 2.5 SDs below the mean value of in premenopausal white women Exclusion: other disorders of BMD, abnormal hepatic function, abnormality of LS precluding assessment of BMD, history of hip fracture, and prior bisphosphonates treatment within 12 months |
PBO, n = 397; ALN 5 mg, 10 mg and 20 mg, n = 526 Adjuvant: both groups 500 mg/day of calcium |
36 months BMD and lateral spine films assessed at 12, 24 and 36 months |
Primary: new vertebral and non-vertebral fractures; change of BMD in the LS, FN, trochanter and total body, in each group at 3 years Secondary: AEs |
Fractures: the occurrence of new vertebral fractures and the progression of vertebral deformities were determined by an analysis of digitised radiographs, and loss of height was determined by sequential height measurements BMD: DXA – Hologic QDR-1000 or 1000/W (Hologic, Waltham, MA, USA), Lunar DPX-L (Lunar), or Norland XR-26 (Norland) |
| Orwoll et al., 2000;83 USA and 10 other countries Multicentre RCT, 20 centres Merck Research Laboratories |
Inclusion: men with a BMD in the FN of < 2 SDs below the mean value in normal young men and a BMD in the LS of < 1 SD below the mean, or a BMD of at least 1 SD below the mean in the FN and at least one vertebral deformity or a history of osteoporotic fracture Exclusion: secondary causes of OP, other bone diseases, vitamin D deficiency, renal disease, cardiac disease, cancer, peptic ulcer/oesophageal disease |
PBO, n = 95; ALN 10 mg/day, n = 146 Adjuvant: both groups, 1000 mg/day of calcium and 400 IU/day of vitamin D |
24 months BMD assessed at 6, 12, 18 and 24 months; radiography at 24 months |
Primary: changes in BMD of the LS (L1–L4), FN, hip and total body, between treatment groups at 2 years Secondary: incidence of vertebral fractures; biochemical markers of bone turnover; AEs |
Fractures: to detect both vertebral fractures, X-ray films were assessed. Both semiquantitative and quantitative morphometric methods were used. Non-vertebral (any site) from patient reporting confirmed by radiography BMD: DXA – Hologic, (Waltham, MA, USA) or Lunar |
| Pols et al.,199984 (FOSIT); Europe, Latin America, Australia, Canada, South Africa and China Multicentre RCT, 153 centres Merck Research Laboratories |
Inclusion: women aged ≤ 85 years, postmenopausal for ≥ 3 years with a BMD in the LS of ≥ 2 SDs below mean for postmenopausal woman 20–50% above the ideal weight Exclusion: metabolic bone disease, disturbed parathyroid/thyroid function, GI disease myocardial infarction, hypertension/angina, organ disease, treatment with bisphosphonates, fluoride, vitamin A or vitamin D |
PBO, n = 958; ALN 10 mg/day, n = 950 Adjuvant: both groups, 1000 mg/day of calcium |
12 months BMD assessed 3, 6 and 12 months |
Primary: change in BMD of the LS (L1–L4), FN, trochanter, and TH, between treatment groups at 1 year Secondary: incidence of vertebral fractures; biochemical markers of bone turnover; AEs |
Fractures: the occurrence of clinical fractures was captured through AE reporting. Documentation for each fracture comprising radiographs and/or radiology reports, hospital discharge reports with clinical diagnosis and/or confirmation by the investigator/treating physician was sought after completion of the study BMD: Hologic QDR densitometers (QDR-1000, -1000/W, –1500 or –2000 (Hologic; Waltham, MA, USA) or Lunar DPX densitometers (DPX, DPX-L or DPX-a; Lunar) |
| Saag et al., 1998;91 USA and 15 other countries Multicentre RCT, 15 centres in the USA and 22 in other countries Merck & Co. |
Inclusion: men and women aged 17–83 years, with underlying diseases requiring long-term oral glucocorticoid therapy at a daily dose of at least 7.5 mg of prednisone (Lodotra®, Napp Pharmaceuticals) or its equivalent irrespective of baseline BMD Exclusion: metabolic bone disease, a low serum vitamin D, concomitant therapy with drugs that affect bone turnover, pregnancy or lactation, renal insufficiency, severe cardiac disease and a history of recent major upper GI disease |
PBO, n = 159; ALN 10 mg/day, n = 157 Also evaluated ALN 5 mg/day, n = 161 Adjuvant: all groups, calcium 800–1000 mg/day and vitamin D 250–500 IU/day |
48 weeks BMD assessed at 4, 12, 24, 36 and 48 weeks; radiography at 48 weeks |
Primary: change in BMD of the LS from baseline to week 48 between the groups Secondary: changes in BMD of the FN, trochanter and total body; biochemical markers of bone turnover; and the incidence of new vertebral fractures |
Fractures: radiographs of the lateral lumbar and thoracic spine – semiquantitative visual assessment: grade 0, normal; grade 1, 20–25% reduction in height, 10–20% area; grade 2, 25–40% reduction in height, 20–40% area; grade 3, ≥ 40% reduction in height and area. Vertebral fractures with grades of ≥ 2 were defined as prevalent fractures, and fractures that increased in severity by at least one grade were defined as incident fractures BMD: DXA – Hologic (Waltham, MA, USA) or Lunar (Madison, WI, USA) |
| Adachi et al., 200198 (Saag et al., 199891 extension) | Patients continued to receive the double-blind study medication to which they had been randomised at the beginning of year 1 | PBO, n = 61; ALN 10 mg/day, n = 55 | 24 months | Primary: change in BMD of the LS, from baseline to week 48 between the groups Secondary: changes in BMD of the hip, FN, trochanter and total body; biochemical markers of bone turnover; and the incidence of new vertebral fractures |
|
| Shilbayeh et al., 2004;93 Jordan RCT, number of centres NR Sponsor NR |
Inclusion: menopausal or early menopausal women with OP – BMD ≥ 2.5 SDs below the young adult mean Exclusion: NR |
PBO, n = 27; ALN 10 mg/day, n = 36 Adjuvant: both groups, calcium 500 mg/day and vitamin D 10 IU/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS and FN; AEs Secondary: NR |
Fractures: not an outcome BMD: DXA – Lunar DPX-L densitometer (Lunar) |
| Smith et al., 2004;94 Australia Multicentre RCT, three centres Merck, Sharp & Dohme |
Inclusion: patients with asthma and/or chronic obstructive airways disease with the following risk factors: > 2 courses of prednisolone in the last 2 years, forced expiratory volume in 1 second < 50% predicted, any respiratory admission in the last 5 years, severely limited exercise tolerance (unable to walk > 100 m unaided), being a woman aged over 50 years and sustaining a bone fracture after the age of 40 years Exclusion: known renal disease or symptoms of dysphagia, dyspepsia, use of proton pump inhibitors or alcohol dependence or history of bilateral hip replacements |
PBO, n = 79 ALN 10 mg/day, n = 66 Adjuvant: both groups, calcium 600 mg/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS, FN and whole femur Secondary: NR |
Fractures: not an outcome BMD: DXA – Lunar (Lunar) |
| IBN vs. PBO | |||||
| Chesnut et al., 2004;45 Chesnut et al., 200546 (BONE); Europe and North America Multicentre RCT, 73 centres Hoffman-La Roche Ltd |
Inclusion: patients aged 55–80 years, ≥ 5 years postmenopausal, with 1–4 prevalent vertebral fractures (T4–L4), and with a BMD T-score of –2.0 to –5.0 SDs in at least one vertebra (L1–L4) Exclusion: upper GI disorders, a LS T-score of 5.0 SDs, > 2 vertebral fractures, disease or medication affecting bone metabolism |
PBO, n = 982; IBN 2.5 mg/day, n = 982 IBN 20 mg eod, 12 doses/month, n = 982 Adjuvant: both groups, calcium 500 mg/day and vitamin D 400 IU/day |
36 months Lateral radiography performed annually, BMD assessed every 6 months for 2 years, then annually |
Primary: new morphometric vertebral fracture Secondary: worsening fractures, clinical vertebral and osteoporotic non-vertebral fractures; change in BMD of the LS and femur; biomarkers |
Fractures: lateral radiography of the thoracic spine Diagnosis of fracture based on morphometric criteria confirmed by qualitative assessment by radiologist. Morphometric fracture – height reduction at least 20% and 4 mm decrease BMD: DXA (Hologic QDR) |
| Lester et al., 200874 (ARIBON); UK Multicentre RCT, two centres AstraZeneca and Roche |
Inclusion: postmenopausal women with a histologically confirmed diagnosis of oestrogen receptor-positive breast cancer Patients classified as osteopenic (T-scores of > –2.5 SDs and < –1.0 SD of either the LS or TH) were randomised Exclusion: menopause was induced by chemotherapy or drug therapy, concurrent administration, abnormal renal function, disorders of bone metabolism and previous bilateral hip fractures prostheses |
PBO, n = 25; IBN 150 mg/month, n = 25 Adjuvant: both groups, anastrozole (Arimidex ®, AstraZeneca UK Ltd, London, UK) 1 mg/day, calcium 500 mg/day and vitamin D 400 IU/day |
24 months BMD assessed at 12 and 24 months |
Primary: change in BMD of the LS and TH Secondary: changes in bone resorption and formation markers and AEs, including any fracture |
Fractures: recorded as AEs (assessment method NR) BMD: DXA – Lunar DPX |
| McClung et al., 2009;80 USA Multicentre RCT, 10 centres Roche |
Inclusion: postmenopausal women aged 45–60 years with a baseline mean LS BMD T-score of between –1.0 and –2.5 SDs and baseline T-score of > –2.5 SDs in the TH, trochanter and FN, with no prior vertebral fractures Exclusion: women with prevalent vertebral or low-trauma osteoporotic fractures; patients receiving treatment affecting bone metabolism |
PBO, n = 83; IBN 150 mg/month, n = 77 Adjuvant: both groups, calcium 500 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS (L2–L4) Secondary: change in BMD of the FN, TH and trochanter; change in bone resorption marker serum |
Fractures: fractures were confirmed by radiography and reported as AEs BMD: DXA – (Hologic, Bedford, MA, USA) |
| IBN-ranging trials | |||||
| Delmas et al., 200649 (DIVA); USA, Canada, Mexico, Europe, Australia and South Africa Multicentre non-inferiority RCT, 53 centres Hoffman-La Roche and GlaxoSmithKline |
Inclusion: postmenopausal women aged 55–80 years; at least 5 years since menopause with OP [mean LS (L2–L4) BMD T-score of < –2.5 to –5.0 SDs] Exclusion: prior treatment with bisphosphonates or any other drug affecting bone metabolism; upper GI disease; renal impairment |
IBN 2.5 mg/day, n = 470; IBN 2 mg/i.v. twice per month, n = 454; IBN 3 mg i.v., three times per month, n = 471 Adjuvant: all groups, calcium 500 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS (L2–L4), year 1 Secondary: change in BMD of the LS (L2–L4), year 2; change in BMD of the proximal femur; bone markers |
Fractures: clinical vertebral and non-vertebral fractures were monitored from AE reporting (all fractures were confirmed radiographically) BMD: DXA on GE Lunar and Hologic (Bedford, MA, USA) |
| Eisman et al., 200850 (DIVA); (year 2 data) | 24 months | ||||
| Miller et al., 200547 (MOBILE); involving 65 centres in the USA, Canada, Europe, Australia, South Africa, Mexico and Brazil Phase III RCT, non-inferiority study Hoffman-La Roche and GlaxoSmithKline |
Inclusion: postmenopausal women aged 55–80 years; at least 5 years since menopause with OP [mean LS (L2–L4) BMD T-score of < –2.5 and –5.0 SDs] Exclusion: patients with uncontrolled active or recurrent peptic ulcer disease were excluded. Additional exclusion criteria were a disease, disorder or therapy known to influence bone metabolism, prior treatment with bisphosphonates, fluoride treatment and renal |
IBN 2.5 mg, n = 402; IBN 50 mg two doses per month, n = 402; IBN 100 mg/month, n = 404; IBN 150 mg/month, n = 401 Adjuvant: both groups, calcium 500 mg/day plus vitamin D ≤ 400 IU |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS (L2–L4) Secondary: change in BMD of the TH, trochanter and FN |
Fractures: clinical vertebral and non-vertebral fractures were recorded as AEs BMD: DXA on GE Lunar and Hologic (Bedford, MA, USA) |
| Reginster et al., 200648 (MOBILE); (year 2 data) | 24 months | ||||
| RIS vs. PBO | |||||
| Boonen et al., 2009;58 Eastern and Western Europe, Lebanon, Australia and the USA Phase III, multicentre RCT Procter & Gamble Pharmaceuticals and Sanofi-aventis Pharmaceuticals |
Inclusion: men aged ≥ 30 years, with OP including a LS T-score of ≤ –2.5 SDs and a FN T-score of ≤ –1 SD or a LS T-score of ≤ –1 SD and a FN T-score of ≤ –2 SDs Exclusion: men with secondary OP except those with primary hypogonadism who declined testosterone replacement therapy |
PBO, n = 93; RIS 35 mg/week, n = 191 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400–500 IU/day |
24 months Radiography completed at 12 and 12 months; BMD assessed at 6, 12 and 24 months |
Primary: change in BMD of the LS at month 24 Secondary: change in BMD of the LS and proximal femur at months 6, 12, and 24; incidence of new vertebral fractures; incidence of clinical fractures (vertebral and non-vertebral) reported as AEs at months 12 and 24 |
Fractures: new vertebral fractures were determined by radiography using a semiquantitative method Clinical vertebral and non-vertebral fractures were reported as AEs BMD: DXA (Hologic, Bedford, MA, USA) |
| Choo et al., 2011;62 Canada RCT, number of centres NR AstraZeneca Pharmaceuticals |
Inclusion: non-metastatic prostate cancer patients receiving radiotherapy plus 2–3 years of androgen ablation therapy. All had LS T-scores of > –2.5 SDs | PBO, n = 52; RIS 35 mg/week, n = 52 Adjuvant: both groups, calcium and vitamin D supplements (amount NR) |
24 months BMD assessed at 12 and 24 months |
Primary: change in BMD of the LS, FN and proximal femur; and biomarkers for bone turnover | Fractures: not an outcome BMD of the LS, proximal femur and FN were measured by DXA at baseline, year 1 and year 2 |
| Cohen et al., 1999;63 USA Multicentre RCT, 28 centres Procter & Gamble/NIH |
Inclusion: men and women aged 18–85 years on glucocorticoids ≥ 7.5 mg/day within 3 months; women at least 1 year postmenopausal Exclusion: history of hyperparathyroidism, hyperthyroidism or osteomalacia, use of drugs known to affect bone metabolism |
Premenopausal women: PBO, n = 52; RIS 5 mg/day, n = 49 Postmenopausal women PBO, n = 15; RIS 5 mg/day, n = 14 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
12 months X-rays and BMD assessed at 12 months |
Primary: change in BMD of the LS, FN and femoral trochanter Secondary: fractures; biochemical markers of bone turnover; AEs |
Fractures: quantitative morphometry was used to identify prevalent (baseline) and incident (new) vertebral fractures. A new vertebral fracture was defined as a decrease of ≥ 15% (for intact vertebrae at baseline) or a decrease of ≥ 4 mm (for fractured vertebrae at baseline) BMD: DXA – Hologic (Waltham, MA, USA) or Lunar |
| Fogelman et al., 200066 (BMD-MN); France, UK, the Netherlands, Belgium and Germany Multicentre RCT, 13 centres Procter & Gamble and Sanofi-aventis |
Inclusion: women aged < 80 years, postmenopausal for at least 1 year; mean LS (L1–L4) T-score of –2 SDs Exclusion: history of hyperparathyroidism, hyperthyroidism or osteomalacia, use of drugs known to affect bone metabolism |
PBO, n = 180; RIS 5 mg/day, n = 179 Also evaluated: RIS 2.5 mg/day, n = 184 Adjuvant: both groups, calcium 1000 mg/day |
24 months BMD assessed at 6, 12, 18 and 24 months; radiography at 24 months |
Primary: incidence of vertebral and non-vertebral fractures, and percentage change of BMD of the spine Secondary: AEs; and biochemical markers of bone turnover |
Fractures: non-vertebral fractures and vertebral fractures assessed as AEs by radiography. A vertebral body was considered to be fractured if any of the vertebral height ratios fell below 3 SDs of the mean for the study population BMD: Lunar or Hologic (Waltham, MA, USA) |
| Hooper et al., 2005;72 Australia Multicentre RCT, 11 centres Procter & Gamble and Sanofi-aventis |
Inclusion: postmenopausal women for 6–36 months with a LS BMD of > –2.5 SDs (< 0.76 g/cm2) Exclusion: history of hyperparathyroidism, hyperthyroidism or osteomalacia; treatment with bone agents likely to affect bone metabolism |
PBO, n = 126; RIS 5 mg/day, n = 129 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
24 months BMD assessed at 3, 6, 12, 18 and 24 months; radiography at 24 months |
Primary: changes in BMD of the LS Secondary: change in BMD of the FN, and trochanter; incidence of vertebral and non-vertebral fractures; AEs |
Fractures: prevalence and incidence vertebral fractures assessed by morphometric analysis. An incident fracture was considered evident if anterior/middle vertebral height was ≥ 15% of normal vertebrae height BMD: Hologic (Waltham, MA, USA) or Lunar |
| Harris et al., 199970 (VERT-NA); USA Multicentre RCT, 110 centres Procter & Gamble |
Inclusion: ambulatory women no older than 85 years, ≥ 5 years since menopause, with at least one vertebral fracture at baseline Exclusion: use of drugs known to affect bone metabolism |
PBO, n = 815; RIS 5 mg/day, n = 813 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
36 months Radiography at 12, 24 and 36 months; BMD assessed every 6 months |
Primary: incidence of vertebral and non-vertebral fractures; and percentage change of BMD of the spine Secondary: AEs and biochemical markers of bone turnover |
Fractures: quantitative and semiquantitative assessment was used to assess prevalent (baseline) and incident fractures. Fracture was considered evident if anterior/middle vertebral height was ≤ 0.8 of posterior BMD: Lunar or Hologic (Waltham, MA, USA) |
| Ste-Marie et al., 200499 (VERT-NA extension) | Women who had successfully completed the original 3-year study and who had undergone baseline and month 36 iliac crest biopsies were eligible to enrol. Women continued on their assigned treatments (PBO or RIS) for an additional 2 years | PBO, n = 42; RIS 5 mg/day, n = 44 | 60 months | Primary: histological and histomorphometric assessments Secondary: change in BMD |
Fractures: recorded as AEs |
| Reginster et al., 200085 (VERT-MN); European and Australian centres Multicentre RCT, number of centres NR Procter & Gamble and Hoechst Marion Roussel |
Inclusion: ambulatory women ≤ 85 years and at least 5 years postmenopausal; had at least two radiographically confirmed vertebral fractures Exclusion: receiving treatment known to affect bone metabolism |
PBO, n = 407; RIS 5 mg/day, n = 407 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
36 months BMD assessed every 6 months, radiography every 12 months |
Primary: changes in BMD of the LS Secondary: changes in the BMD of the FN and trochanter; incidence of vertebral and non-vertebral fractures; biochemical markers of bone turnover; and AEs |
Fractures: quantitative and semiquantitative assessment was used to assess prevalent (baseline) and incident fractures. Fracture was considered evident if anterior/middle vertebral height was ≥ 15% of normal vertebrae height BMD: Lunar or Hologic (Waltham, MA, USA) |
| Sorensen et al., 2003100 (VERT-MN extension); USA Multicentre RCT, 29 centres Procter & Gamble |
Inclusion: women remained on the treatments (PBO or RIS, 5 mg daily) to which they had originally been assigned. Blinding was maintained for the patients and clinical centre personnel throughout the 5 years of study | PBO, n = 130; RIS 5 mg/day, n = 135 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
60 months | Primary: incidence of vertebral fractures Secondary: incidence of non-vertebral fractures; changes in BMD of the LS, FN, femoral trochanter and radius; biochemical markers of bone turnover; and AEs |
|
| Leung et al., 2005;75 China Multicentre RCT, four centres Aventis Pharma |
Inclusion: postmenopausal for ≥ 5 years with a spine BMD at L1–4 of < 2.5 SDs of the local peak young mean value Exclusion: any medical conditions or medication known to affect bone metabolism |
PBO, n = 34; RIS 5 mg/day, n = 31 Adjuvant: both groups, calcium 500 mg/day plus vitamin D 400 IU/day |
12 months BMD assessed at 3, 6 and 12 months |
Primary: change in BMD of the FN, LS, TH and trochanter; and bone markers Secondary: NR |
Fractures: not an outcome BMD: DXA (QDR 4500 plus Hologic, Hologic Waltham, MA, USA) |
| McClung et al., 2001;78 USA Multicentre RCT, 183 centres Procter & Gamble/Aventis Pharma |
Inclusion: women aged ≥ 70 years; low BMD of the FN with a T-score of < –4 or < –3 SDs, with at least one non-skeletal risk factor for hip fracture Exclusion: any major illness, history of another metabolic bone disease, bilateral hip fracture, recent use of drugs known to affect bone metabolism |
Women aged 70–79 years: PBO, n = 1821; RIS 2.5 mg/day, n = 1812; RIS 5 mg/day, n = 1812 Women aged ≥ 80 years: PBO, n = 1313; RIS 2.5 mg/day, n = 1281; RIS 5 mg/day, n = 1292 Adjuvant: both groups, calcium 1000 mg/day plus vitamin D ≤ 500 IU/day for women with low vitamin D |
36 months BMD assessed every 6 months |
Primary: change in BMD of the LS Secondary: change in BMD of the FN, proximal femur, trochanter, radius; vertebral fractures; biochemical markers of bone turnover; and AEs |
Fractures: radiographically confirmed hip fractures and non-vertebral osteoporotic fractures. Non-vertebral osteoporotic fractures, defined as all radiographically confirmed fractures of the wrist, leg, humerus, hip, pelvis, or clavicle BMD: DXA – Lunar or Hologic (Waltham, MA, USA) |
| Reid et al. 2000;86 UK Multicentre RCT, 23 centres Procter & Gamble and Hoechst Marion Roussel |
Inclusion: ambulatory men and women aged 18–85 years, who have taken glucocorticoids for at least 6 months Exclusion: history of hyperparathyroidism, hyperthyroidism, or osteomalacia; treatment with bone agents likely to affect bone metabolism |
PBO, n = 96; RIS 5 mg/day, n = 100 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 6 and 12 months; radiography at 12 months |
Primary: change in BMD of the LS Secondary: change in BMD of the FN, proximal femur, trochanter, radius; vertebral fractures; biochemical markers of bone turnover; and AEs |
Fractures: incident fractures were identified using quantitative morphometry defined as a reduction of ≥ 15% in vertebral height in a previously intact vertebra or a reduction of ≥ 4 mm in a previously fractured vertebra BMD: DXA – Lunar or Hologic (Waltham, MA, USA) |
| Ringe et al., 2006;89 Germany Single-centre RCT Sponsor NR |
Inclusion: men with primary or secondary OP with or without pre-existing prevalent vertebral fractures. OP was defined as a LS (BMD) T-score of ≤ –2.5 SDs and FN BMD T-score of ≤ –2.0 SDs relative to a healthy young adult male. Primary OP; secondary OP: PBO, 92 (58.2%); 66 (41.8%) RIS 5 mg/day, 94 (59.5%); 64 (40.5%) Exclusion: patients with known hypersensitivity to bisphosphonates, severe impairment of renal function, hypocalcaemia and a history of bisphosphonate or fluoride pre treatment |
PBO, n = 158; RIS, 5 mg/day, n = 158 Adjuvant: PBO with fractures, calcium 500 mg/day and alfacalcidol (One-Alpha®, LEO Pharma) 1 µg/day PBO without factures, calcium 800 mg/day and vitamin D 1000 IU/day |
12 months BMD assessments and radiography at 12 months |
Primary: change in BMD of the LS Secondary: incidence of new vertebral fractures; change in BMD of the FN and TH; change in body height; course of back pain; and the incidence of non-vertebral fractures |
Fractures: radiography of the spine. Assessment of vertebral fracture was performed using the semiquantitative technique BMD: DXA (Lunar) |
| Ringe et al., 2009;101 follow-up to Ringe et al., 200689 | PBO, n = 158; RIS 5 mg/day, n = 158 | 24 months | |||
| Taxel et al., 2010;95 USA RCT, number of centres NR Proctor and Gamble and Aventis |
Inclusion: men aged > 55 years and within a month of receiving an initial injection of ADT for prostate cancer Exclusion: metastatic bone disease, chronic kidney, GI or liver diseases, a previous cancer diagnosis, metabolic bone disorders medications that interfere with bone metabolism |
PBO, n = 20; RIS 35 mg/week, n = 20 Adjuvant: both groups, calcium 600 mg/day and vitamin D 400 IU/day |
6 months BMD assessed at 6 months |
Primary: BMD of the FN and TH Secondary: change in bone markers |
Fractures: not an outcome BMD: DXA (Lunar DXA-IQ) |
| ZOL vs. PBO | |||||
| Black et al., 200756 (HORIZON-PFT); international Multicentre RCT, number of centres NR Novartis Pharmaceuticals |
Inclusion: postmenopausal women aged 65–89 years with a FN BMD T-score of ≤ –2.5 SDs, with or without evidence of existing vertebral fracture, or a T-score of –1.5 SDs, with radiological evidence of at least two mild vertebral fractures or one moderate vertebral fracture. Use of hormone therapy, raloxifene, calcitonin (Miacalcic®, Novartis Pharmaceutical UK Ltd), tibolone (Livial®, Merck Sharp & Dohme Ltd), tamoxifen (Nolvadex®, AstraZeneca UK Ltd), dehydroepiandrosterone ipriflavone and medroxyprogesterone (Provera®, Pfizer Ltd) was allowed. Patients in stratum I (n = 6113) were not taking any OP medications at the time of randomisation, whereas patients in stratum II (n = 1652) were all taking an allowed medication Exclusion: previous use of PTH, sodium fluoride, anabolic steroids, growth hormone, glucocorticoids, or strontium |
PBO, n = 3876; ZOL 5 mg/year, n = 3889 Adjuvant: both groups, calcium 1000–1500 mg/day and vitamin D 400–1200 IU/day |
36 months Radiography at 12, 24 and 36 months in stratum I; baseline and 36 months in stratum II; BMD assessed at 6, 12, 24 and 36 months |
Primary: stratum II, vertebral fractures Strata I and II, hip fracture Secondary: any non-vertebral fracture, any clinical fracture, and clinical vertebral fracture; changes in BMD of the LS, FN and TH; changes in markers of bone resorption and formation |
Fractures: spinal lateral radiographs from vertebrae T4 to L4 were evaluated with the use of quantitative morphometry and standard methods. Incident morphometric vertebral fractures were defined as a reduction in vertebral height of at least 20% and 4 mm by quantitative morphometry, confirmed by an increase of one severity grade or more on semiquantitative analysis. Clinical fracture reports were obtained from patients at each contact. Non-vertebral fracture reports required central confirmation. Excluded were fractures of the toe, facial bone and finger and those caused by excessive trauma BMD: DXA – model NR. Measurements of BMD at the LS were obtained for a subgroup of patients |
| Reid et al., 2010102 (HORIZON-PFT) | AEs | ||||
| Lyles et al., 200777 (HORIZON-RFT); international Multicentre RCT, number of centres NR Novartis Pharmaceuticals |
Inclusion: men and women aged ≥ 50 years, within 90 days after surgical repair of a hip fracture sustained with minimal trauma; ambulatory prior to fracture Exclusion: calculated low creatinine clearance, low serum calcium concentration, active cancer, metabolic bone disease and a life expectancy of < 6 months |
PBO, n = 1062; ZOL 5 mg/year, n = 1065 Adjuvant: both groups, calcium 1000–1500 mg/day and vitamin D 800–1200 IU/day |
36 months BMD assessed every 12 months |
Primary: new clinical fractures excluding facial and digital fractures and fractures in abnormal bone (e.g. bone-containing metastases) Secondary: BMD of the non-fractured hip; new vertebral, non-vertebral and hip fractures; safety |
Fractures: lateral radiography of the chest and LS. A non-vertebral fracture (not a vertebral, facial, digital or skull fracture) was confirmed when a radiograph, a radiographic report or a medical record documented a new fracture. A new clinical vertebral fracture was defined as new or worsening back pain with a reduction in vertebral body height of 20% (grade 1) or more, as compared with baseline radiographs, or a reduction in vertebral body height of 25% (grade 2) or more if no baseline radiograph was available BMD: DXA – model NR |
| Adachi et al., 2011103 (HORIZON-RFT) | Quality of life | ||||
| Boonen et al., 2012;59 Europe, South America, Africa and Australia RCT, number of centres NR Novartis Pharmaceuticals |
Inclusion: men aged 50–85 years who had primary OP or OP associated with low testosterone levels with BMD T-score of ≤ –1.5 SDs in the TH or FN and 1–3 prevalent vertebral fractures. Men without fractures were eligible if they had a BMD T-score of ≤ –2.5 SDs in the TH, FN or LS Exclusion: four or more prevalent vertebral fractures, low serum vitamin D concentration, renal insufficiency, hypercalcaemia or hypocalcaemia, hypersensitivity to bisphosphonates, medication affecting bone metabolism |
PBO, n = 611; ZOL 5 mg/year, n = 588 Adjuvant: both groups, calcium 1000–1500 mg/day and vitamin D 800–1200 IU/day |
24 months Radiography at 12 and 24 months; BMD assessed at 6, 12 and 24 months |
Primary: proportion of men with one or more new morphometric vertebral fractures Secondary: proportion of men with one or more new morphometric vertebral fractures; one or more new moderate to severe, or new or worsening morphometric vertebral fractures; change in height; the time to first clinical fracture (vertebral or non-vertebral); change in BMD of the LS, FN and TH; bone-turnover markers; and safety |
Fractures: vertebral fractures were assessed by means of quantitative vertebral morphometry performed on lateral thoracic and LS, incident vertebral fracture was assessed by means of morphometry and defined as a reduction in vertebral height of 20% or more and 4 mm or more. Clinical fractures (vertebral and non-vertebral) were reported by participants at each visit and were verified by radiographic report or surgical notes. Only confirmed fractures were included in the analysis BMD: DXA – model NR BMD and bone markers were analysed in a subgroup of 100 or more participants |
| McClung et al., 2009;79 USA and France Multicentre RCT, 25 centres Novartis Pharmaceuticals |
Inclusion: women aged ≥ 45 years, who were postmenopausal, had a LS BMD T-score of < –1.0 SD and > –2.5 SDs and a FN T-score of > –2.5 SDs Exclusion: participants with more than one vertebral fracture or any grade 2 or 3 vertebral fracture. Participants with low vitamin D concentration, renal insufficiency, hypercalcaemia or hypocalcaemia, treatment medications affecting bone metabolism |
PBO, n = 202; ZOL 5 mg/year, n = 198 Adjuvant: both groups, calcium 500–1200 mg/day and vitamin D 400–800 IU/day |
24 months BMD assessment time points NR |
Primary: change in BMD of the LS at 12 months Secondary: change in BMD of the TH, FN, trochanter and distal radius at 12 and 24 months; and bone markers |
Fractures: not an outcome BMD: DXA Hologic or General Electric Lunar machine |
| Head to head: ALD vs. IBN | |||||
| Miller et al., 200881 (MOTION); North America, Latin America, America, Europe and South Africa Multicentre RCT, 65 centres Hoffman La-Roche Ltd and GlaxoSmithKline |
Inclusion: postmenopausal women aged between 55 and < 85 years with a LS (L2–L4) BMD T-score of between < –2.5 SD and ≥ –5.0 SD Exclusion: upper GI disease, any diseases or medications known to influence bone metabolism |
ALN 70 mg/week, n = 873; IBN 150 mg/month, n = 887 Adjuvant: both groups, calcium 500 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 12 months |
Primary: change in BMD of the LS and TH Secondary: change in BMD of the trochanter; and bone markers |
Fractures: recorded as AEs (assessment method NR) BMD: DXA – model NR |
| Head to head: ALD vs. RIS | |||||
| Atmaca and Gedick 2006;54 Turkey RCT, number of centres NR Sponsor NR |
Inclusion: late postmenopausal women with osteoporosis with a mean age of 66.3 years (range 60–85 years) and a T-score of < 2.5 SDs Exclusion: any medical conditions or medication known to affect bone metabolism |
RIS 5 mg/day, n = 14; ALN 10 mg/day, n = 14 Adjuvant: both groups, calcium 600 mg/day and vitamin D 400 IU/day |
12 months BMD assessment time point NR |
Primary: change in BMD of the FN, LS and distal radius; and bone markers Secondary: NR |
Fractures: not an outcome BMD: DXA – Hologic QDR (Waltham, MA, USA) |
| Muscoso et al., 2004;82 Italy RCT, number of centres NR Sponsor NR |
Inclusion: osteoporotic female population submitted to a treatment with antiresorption drugs Exclusion: NR |
RIS 5 mg/day, n = 1000; ALN 10 mg/day, n = 100 Other treatments were: clodronic acid (Bonefos®, Bayer plc.), n = 800 and raloxifene, n = 100 Adjuvant: all groups, calcium 1000 mg/day and vitamin D 800 IU/day |
24 months BMD assessment time point NR |
Primary: change in BMD of the LS; and fractures Secondary: NR |
Fractures: NR BMD: DXA – Lunar DPX |
| Sarioglu et al., 200692 Turkey RCT, number of centres NR Sponsor NR |
Inclusion: postmenopausal women with OP Exclusion: patients aged > 75 years and taking treatment for OP. The presence of any disease which interferes with bone metabolism, recent use of drugs known to affect bone metabolism and history of oesophagitis and peptic ulcer |
RIS 5 mg/day, n = 25; ALN 10 mg/day, n = 25 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400 IU/day |
12 months BMD assessment time point NR |
Primary: change in BMD of the hip Secondary: NR |
Fractures: not an outcome BMD: DXA – Lunar DPX |
| Rosen et al., 200590 (FACT); USA Multicentre RCT, 78 centres Merck & Co. |
Inclusion: postmenopausal women aged ≥ 40 years or ≥ 25 years if surgically menopausal. BMD T-score of ≤ –2.0 SDs in at least one of the four sites (TH, hip trochanter, FN or posterior LS) Exclusion: hypocalcaemia, hypovitaminosis D, metabolic bone disease, bisphosphonates within 1 year or bisphosphonates for ≥ 2 years within 5 years, use of PTH within 1 year, had taken oestrogen or oestrogen analogues within 6 months |
ALN 70 mg/week, n = 520; RIS 35 mg/week, n = 533 Both groups, 1000 mg/day calcium and 400 IU/day vitamin D |
12 months BMD assessed at 6 and 12 months |
Primary: change BMD of the trochanter Secondary: change in BMD of the TH, FN and LS |
Fractures: incidence of clinical fracture recorded as AEs (assessment method NR) BMD: Hologic (Waltham, MA, USA) or Lunar |
| Bonnick et al., 2006104 (FACT); extension to Rosen et al., 2005;90 USA Multicentre RCT, 72 of the original 78 centres Merck & Co. |
Inclusion: postmenopausal women aged ≥ 40 years or ≥ 25 years if surgically menopausal. BMD T-score of ≤ –2.0 SDs in at least one of the four sites (TH, hip trochanter, FN or posterior LS) Exclusion: hypocalcaemia, hypovitaminosis D, metabolic bone disease, bisphosphonates within 1 year or for ≥ 2 years within 5 years, use of PTH within 1 year, had taken oestrogen or oestrogen analogues within 6 months |
ALN 70 mg/week, n = 411; RIS 35 mg/week, n = 414 Adjuvant: both groups, 1000 mg/day calcium and 400 IU/day vitamin D |
Extension to 24 months | Primary: change of BMD in the trochanter Secondary: change in BMD of the TH, FN and LS |
Fractures: clinical fractures that occurred during the trial, regardless of association with trauma or skeletal site, were reported by investigators as clinical AEs (assessment method NR) BMD: Hologic (Waltham, MA, USA) or Lunar |
| Reid et al., 200687 (FACTS); Europe, the Americas and Asia-Pacific Multicentre RCT, 75 centres Merck & Co. |
Inclusion: postmenopausal women aged > 40 years with low bone density (–2.0 SDs below the young normal mean) at LN, FN or TH Exclusion: hypocalcaemia, hypovitaminosis D, metabolic bone diseases, use of oestrogen, oestrogen analogues, tibolone or anabolic steroids, bisphosphonates or PTH |
ALN 70 mg/week, n = 468; RIS 35 mg/week, n = 468 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400 IU/day |
12 months BMD assessed at 6 and 12 months |
Primary: change of BMD in the trochanter Secondary: change in BMD of the TH, FN and LS |
Fractures: fractures were reported as AEs whether or not they were associated with trauma and without requirements of radiographic confirmation or adjudication BMD: DXA – using Hologic (Waltham, MA, USA) or Lunar densitometers |
| Reid et al., 2008105 (FACTS); extension to Reid et al., 2006;87 72 of the original 75 international sites Merck & Co. |
Inclusion: all eligible women maintained their original randomised, blinded treatment allocation from year 1 | ALN 70 mg/week, n = 403; RIS 35 mg/week, n = 395 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400 IU/day |
24 months | ||
| Head to head: ZOL vs. ALN | |||||
| Hadji et al., 2010106 (ROSE) | Primary: quality of life and compliance | ||||
| Hadji et al., 201269 (ROSE); Germany Multicentre RCT, 95 centres Novartis Pharmaceuticals |
Inclusion: women aged 55–90 years who were considered postmenopausal with BMD T-score of ≤ –2.0 SDs of the TH or LS Exclusion: patients who had received prior therapy with bisphosphonates, PTH (Teriparatide, Forsteo®, Eli Lilly and Company Ltd), strontium ranelate, raloxifene, calcitonin, high-dose glucocorticoids, patients with a fracture within 6 months, secondary OP, primary hyperparathyroidism and patients with inappropriate blood chemistry |
ZOL 5 mg/year, n = 408; ALN 70 mg/week, n = 196 Adjuvant: both groups, calcium 1200 mg/day and vitamin D 800 IU/day |
12 months | Primary: to assess if ZOL was superior to ALN in reducing serum NTx levels Secondary: comparison of P1NP levels; safety and tolerability |
Fractures and BMD: not outcomes assessed by the trial (assessed bone markers and quality of life) |
| Head to head: ZOL vs. RIS | |||||
| Reid et al., 200988 (HORIZON); Australia, EU countries (including the UK), China and the USA Multicentre RCT, 54 centres Novartis Pharmaceuticals |
Inclusion: men and women aged 18–85 years receiving at least 7.5 mg oral prednisolone daily (or equivalent) and were expected to receive glucocorticoids for at least another 12 months Exclusion: previous treatment drugs that affect the skeleton, low serum vitamin D, history of cancer or parathyroid disease and renal impairment |
ZOL 5 mg/year: treatment n = 272; prevention, n = 144 RIS 5 mg/day: treatment, n = 273; prevention, n = 144 Adjuvant: both groups, calcium 1000 mg/day and vitamin D 400–1200 IU/day |
12 months BMD assessed at 6 and 12 months; radiography at 12 months |
Primary: change in BMD of the LS Secondary: change in BMD of the FN, TH, trochanter and distal radius; and occurrence of thoracic and lumbar vertebral fractures |
Fractures: thoracic and lumbar vertebral fractures were defined according to semiquantitative methods BMD: Hologic (Waltham, MA, USA), GE or Lunar |
Study and population characteristics of included trials
A summary of the number of RCTs and citations by treatment along with the author, trial name (where reported) and population is presented in Table 2. The trial design of the included studies including country, inclusion/exclusion criteria, treatment doses and numbers randomised, outcome assessment methods and final follow-up are presented in Table 3. Characteristics of included participants including sex, age and baseline femoral neck BMD and fractures are presented in Table 4.
| Author, year of study publication (trial acronym) and population | Characteristics | Comorbidities and associated medication | Medical history | History of fractures |
|---|---|---|---|---|
| ALN vs. PBO | ||||
| Adami et al., 199553 Women with PMO |
Male/female: 100% female Race: not reported Age (SD), years since menopause (SD):
|
None reported | Current smokers:
|
Fractures: 5% of all participants had prevalent vertebral fractures FN BMD (SD):
|
| Black et al., 199655 (FIT I) Women with PMO |
Male/female: 100% female Race:
|
None reported | Smokers:
|
Fractures: percentage with one, two or three or more:
|
| Cummings et al., 199864 (FIT II) Women with PMO |
Male/female: 100% female Race: all, Caucasian 97% Age (SD):
|
None reported | Smokers:
|
Fracture since age 45 years:
|
| Bone et al., 200057 Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Not reported |
| Carfora et al., 199860 Women with PMO |
Male/female: 100% female Race: not reported Age; years since menopause: not reported Height, weight, BMI: not reported |
None reported | Not reported | Not reported |
| Chesnut et al., 199561 Women with PMO |
Male/female: 100% female Race, all:
|
None reported | Not reported | Not reported |
| Dursun et al., 200165 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | FN BMD (SD):
|
| Greenspan et al., 200267 Women with PMO |
Male/female: 100% female
|
None reported | Not reported | Fractures: 55% had a history of fracture (type not reported) FN BMD: not reported |
| Greenspan et al., 200368 Women aged ≥ 65 years |
Male/female: 100% female Race: not reported Age (SD):
|
None reported | Not reported | Fracture since age 50 years:
|
| Ho and Kung 200571 Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Prevalent vertebral fracture:
|
| Klotz et al., 201373 (CORAL) Men with androgen deprivation bone loss in non-metastatic prostate cancer |
Male/female: 100% male Race: not reported Age (SD):
|
Gleason prostate cancer score:a
|
Years of smoking mean (SD); packs per day (SD):
|
Fractures: of the 47% who reported prior fracture, 1% had had a history of hip or vertebral fracture. Four participants in the ALN group reported a family history of osteoporotic fracture FN BMD cm3: not reported. At baseline, 63 subjects (38%) had osteopenia (25 patients treated with ALN and 38 treated with PBO) and 12 subjects (7%) had OP (three patients treated with ALN and nine treated with PBO). The remaining ITT population was considered to have normal BMD for their age |
| Liberman et al., 199576 Seeman 199997 Women with PMO |
Male/female: 100% female Race: not reported Age; years since menopause:
|
None reported | Not reported | Fractures at baseline:
|
| Orwoll et al., 200083 Men with OP |
Male/female: 100% male Race: not reported Age (SD):
|
None reported | Current smokers:
|
Fractures at baseline:
|
| Pols et al., 199984 (FOSIT) Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Fractures: not reported FN BMD:
|
| Saag et al., 1998;91 extension of Adachi 2001 et al.,98 Men and women with glucocorticoid-induced OP |
Male/female:
|
Comorbidities:
|
Not reported | Not reported |
| Shilbayeh et al., 200493 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Fractures: not reported. FN BMD (SD):
|
| Smith et al., 200494 Men and women with asthma and/or chronic obstructive airways disease |
Male/female:
Age, n (%):
|
Comorbidities: all had airways disease (asthma and/or COPD) Medications:
|
Current smokers:
|
Not reported |
| IBN vs. PBO | ||||
| Chesnut et al., 200445; Chesnut et al., 200546 (BONE) Women with PMO |
Male/female: 100% female Race: not reported Age; years since menopause:
|
Comorbidities: reports pre-existing GI disorders were similar across groups Medications: reports use of non-steroidal anti-inflammatory agents was comparable across groups |
Not reported | Vertebral fractures: one; two:
FN BMD T-score (SD):
|
| Lester et al., 200874 (ARIBON) PM women with breast cancer |
Male/female: 100% female Race: not reported Age median (range):
|
All had a histologically confirmed diagnosis of oestrogen receptor-positive breast cancer and commenced anastrozole at study entry | Not reported | Not reported |
| McClung et al., 200980 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Fractures: not reported. FN BMD (SD):
|
| IBN dose-ranging trials | ||||
| Delmas et al., 2006;49 Eisman et al., 200850 (DIVA) Women with PMO |
Male/female: 100% female Race: not reported Age; years since menopause:
|
None reported | Not reported | Fractures:
FN BMD T-score: not reported |
| Miller et al., 2005;47 Reginster et al., 200648 (MOBILE) Women with PMO |
Male/female: 100% female Race: not reported Age; years since menopause:
|
None reported | Not reported | History of previous fractures:
|
| RIS vs. PBO | ||||
| Boonen et al., 200958 Men with OP |
Male/female: 100% male Race:
|
None reported | Not reported | Fractures: not reported BMD:
|
| Choo et al., 201162 Men with androgen deprivation bone loss in non-metastatic prostate cancer |
Male/female: 100% male Race: not reported Age:
|
Comorbidities: all were non-metastatic prostate cancer patients undergoing radiotherapy Medications:
|
Not reported | Not reported |
| Cohen et al., 199963 Men and women (≥ 1 year PM) aged 18–85 years on glucocorticoids |
Male/female:
Age (SD):
|
Underlying disease requiring glucocorticoid treatment:
|
Not reported | Fractures:
|
| Fogelman et al., 200066 (BMD-MN) Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
Comorbidities: none reported Previous OP medication:
|
Not reported | Fractures:
|
| Hooper et al., 200572 Early PM women with OP |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Fractures:
|
| Harris et al., 199970 (VERT-NA); extension of Ste-Marie et al., 200499 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD)
|
None reported | Not reported | Fractures:
|
| Reginster et al., 200085 (VERT-MN); extension of Sorensen et al., 2003100 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Median (range) number of vertebral fractures:
|
| Leung et al., 200575 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Fractures: not reported FN BMD (SD):
|
| McClung et al., 200178 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
Height; weight; BMI: not reported |
None reported | Not reported | Vertebral fractures:
FN BMD T-score (SD):
|
| Reid et al., 200086 Men and women taking glucocorticoids for ≥ 6 months |
Male/female:
Age (SD):
|
Underlying disease requiring glucocorticoid treatment:
|
Not reported | Fractures:
|
| Ringe et al., 2006;89 extension Ringe et al., 2009101 Men with OP |
Male/female: 100% male Race: not reported Age (SD):
|
None reported | Not reported | One or more vertebral fracture:
FN BMD T-score (SD):
|
| Taxel et al., 201095 Men aged > 55 years and within 1 month of receiving an initial injection of ADT for prostate cancer |
Male/female: 100% male Race: not reported Age:
|
None reported | Not reported | Fractures: not reported FN BMD (SD):
|
| ZOL vs. PBO | ||||
| Black et al., 200756 (HORIZON-PFT) Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Number (%) vertebral fractures:
|
| Lyles et al., 200777 (HORIZON-RFT) Men and women 50 years of age or older within 90 days after surgical repair of a hip fracture |
Male/female:
|
Comorbidities: the most common coexisting medical conditions at baseline were hypertension, coronary artery disease, osteoarthritis, previous stroke, depression, and diabetes mellitus. n/N (%) not reported. Active tachyarrhythmia was present in 5.8% of patients in the ZOL group and in 7.5% of patients in the PBO group | Not reported | Fractures: all patients who were enrolled in the trial had undergone repair of a hip fracture FN BMD (SD):
|
| Boonen et al., 201259 Men with OP |
Male/female: 100% male Race:
|
Comorbidities: none reported OP medications used before the first infusion in the study:
|
Not reported | Number of vertebral fractures:
FN BMD T-score (SD):
|
| McClung et al., 200979 Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Fractures: not reported FN BMD (SD):
|
| Head to head: ALN vs. IBN | ||||
| Miller et al., 200881 (MOTION) Women with PMO |
Male/female: 100% female Race:
|
None reported | Not reported | Previous fractures (not described):
|
| Head to head: ALN vs. RIS | ||||
| Atmaca and Gedik, 200654 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause (SD):
|
None reported | Not reported | Fractures: not reported FN BMD (SD):
|
| Muscoso et al., 200482 Women with PMO |
Male/female: 100% female Race: not reported Age:
|
None reported | Not reported | Not reported |
| Sarioglu et al., 200692 Women with PMO |
Male/female: 100% female Race: not reported Age (SD); years since menopause
|
None reported | Not reported | Fractures:
|
| Rosen et al., 200590 (FACT); extension of Bonnick et al., 2005104 Women with PMO |
Male/female: 100% female Race:
ALN 70 mg/week: 25.2 kg/m2 (4.7 kg/m2) RIS 35 mg/week: 25.5 kg/m2 (4.5 kg/m2) |
None reported | Not reported | Fracture history of hip, spine, or wrist after age 45 years:
FN BMD T-score (SD):
|
| Reid et al., 200687 (FACTS); extension of Reid et al., 2008105 Women with PMO. |
Male/female: 100% female Race:
|
None reported | Family history of OP:
|
Fracture history (not described):
FN BMD T-score (SD):
|
| Head to head: ZOL vs. ALN | ||||
| Hadji et al., 2012;69 Hadji et al., 2010106 (ROSE) Women with PMO |
Male/female: 100% female Race:
ZOL 5 mg/year: 26.1 kg/m2 (4.12 kg/m2) ALN 70 mg/week: 26.3 kg/m2 (4.0 kg/m2) |
None reported | Current and previous smokers:
|
Fractures (not described):
|
| Head to head: ZOL vs. RIS | ||||
| Reid et al., 200988 (HORIZON) Men and women taking glucocorticoids ≥ 3 months and < 3 months |
Male/female:
Age (SD):
|
Medical disorders requiring glucocorticoid use:
|
Not reported | Fractures: not reported FN BMD/T-score: not reported |
Alendronic acid
Alendronic acid was compared with placebo in 17 RCTs reported across 19 publications. 53,55,57,60,61,64,65,67,68,71,73,76,83,84,91,93,94,97,98 Two RCTs65,71 did not include a placebo comparison, but evaluated alendronic acid combined with calcium compared with calcium alone.
Four RCTs were multicentre RCTs undertaken in the USA55,61,64,67 and six RCTs were international multicentre RCTs. 76,83,84,91,94,98 One multicentre RCT was undertaken in Italy53 and one in Canada. 73 Single-centre RCTs were undertaken in Italy,60 Turkey65 and Jordan. 93 The countries and number of participating centres was unclear for one RCT57 and the number of participating centres was unclear for one RCT undertaken in China. 71 RCT sponsor details were not reported for four RCTs. 53,60,65,93 The total numbers of participants randomised ranged from 6393 to 4432. 64 Where reported, RCTs typically excluded patients with underlying conditions or receiving medications that affect bone metabolism, and patients either with upper GI tract disorders or receiving medication for the condition.
Fourteen RCTs recruited postmenopausal women and evaluated 10 mg per day of alendronic acid. 53,55,57,60,61,64,65,67,68,71,76,84,91,93 Two of these RCTs also included an evaluation of other doses of alendronic acid not currently licensed. 76,91,98 Two of the RCTs in postmenopausal women reported that participants were switched from a 5 mg daily dose of oral alendronic acid to 10 mg per day after 24 months, spending the remaining 12 months of the RCT on 10 mg per day. 55,64 One RCT evaluated a daily dose of 10 mg of oral alendronic acid in men with osteoporosis,83 one RCT evaluated a daily 10 mg dose of oral alendronic acid in men and women (51% male) with airways disease94 and one RCT evaluated 70 mg per week of oral alendronic acid in men with ADT bone loss in non-metastatic prostate cancer. 73 One RCT, in men and women (37.4% male) with underlying diseases requiring long-term oral glucocorticoid therapy, evaluated 5 mg or 10 mg per day of oral alendronic acid (two active treatment groups) and reported fracture outcomes for both groups combined (data not used in the analysis for this assessment report). 91
Adjuvant treatment in the form of calcium alone or in combination with vitamin D was reported for all RCTs. The doses varied across the RCTs (see Table 3).
Inclusion criteria varied across the RCTs in terms of baseline BMD and T-scores (skeletal site and cut-off). Seven RCTs53,57,60,71,76,84,93 reported inclusion criteria that would identify women with osteoporosis according to the current WHO definition. 1 Two RCTs recruited women aged 55–81 years with a femoral neck BMD of ≤ 2 SDs below that of a normal young adult,55,64 an additional inclusion criterion for one of these RCTs being the presence of at least one vertebral fracture. 55 One RCT recruited women aged 42–75 years with lumbar spine BMD approximately 2 SDs below the young normal value,61 and another RCT recruited women with BMD ≥ 2 SDs below young adult mean at either lumbar spine or femoral neck. 65 One RCT recruited ambulatory women aged 65 years in long-term care with a lumbar spine or total hip BMD T-score of ≤ –2.0 SDs. 67 One RCT recruited community-dwelling women aged 65 years. 67 Femoral neck BMD above mean peak was an exclusion criterion for one RCT. 68 One RCT recruited men and women with underlying diseases requiring long-term oral glucocorticoid therapy, irrespective of baseline BMD. 91 One RCT recruited men with femoral neck and lumbar T-scores of < 2 SDs and < 1 SD below the normal for young men, or femoral neck BMD of ≤ 1 SD below the normal for young men plus vertebral deformity or fracture. 83 The RCT in men and women with airways disease included only participants with a T-score of < –2.5 SDs or z-score of < –1.0 at hip or lumbar spine. 94 The RCT in men with ADT bone loss reported that 38% of all participants had osteopenia and 7% had osteoporosis. 73
The mean age of participants was in the sixth decade (between 51 and 60 years) in two RCTs. 53,91 One RCT did not report mean age, but recruited women aged 44–73 years. 60 Another RCT not reporting mean age included participants 61–69 years. 94 In one RCT, the mean age of all included participants was 73.6 years73 and in all others the mean age of included participants was between 61 and 70 years. Seven RCTs in women reported on the number of years since menopause. 53,57,61,65,70,84,93 The mean number of years since menopause ranged from 10 to 15 years with the exception of one RCT recruiting women after hysterectomy, in which the mean number of years since menopause was 22. 57 BMI was available for 12 RCTs. 53,55,61,64,65,68,71,76,83,84,91,93 Across these RCTs, all mean BMI values were > 18.5 kg/m2. In one RCT,93 the mean BMI was > 30 kg/m2. Race of included participants was reported by eight RCTs. 55,57,61,64,67,71,84,91 One of these studies recruited 100% East Asian women. 71 Across the other RCTs the proportion of Caucasian participants was ≥ 90%. 55,57,61,64,67,84,91 Smoking status was reported by five trials,53,55,64,73,94 with four reporting that ≥ 10% of included participants were current smokers. 53,55,64,94 Mean smoking duration of 26.2 years and mean quantity of 0.98 packs per day were reported by one RCT. 73
The presence of fractures or fracture history at baseline was reported by nine RCTs. 53,55,64,67,68,71,73,76,83 One reported that 5% of all participants had vertebral fractures,53 one that 37% had vertebral fractures71 and one that 41.9% had vertebral fractures. 83 One RCT reported that 64% of participants had at least one vertebral fracture and that 14% had three or more vertebral fractures. 55 One study reported that 21% of participants had vertebral fractures and 5% had non-vertebral fractures at baseline. 76 Fifty-five per cent of participants in one RCT had a history of fracture. 67 One RCT reported that, of the 47% who reported prior fracture, 1% had a history of hip or vertebral fracture. 73 One RCT reported that 36% had experienced fractures since age 50 years68 and another RCT reported that 35% had experienced fractures since age 45 years. 64
Compliance with treatment in the form of a pill count was assessed by three RCTs. 53,55,64
Final follow-up was 12 months in six RCTs,65,71,73,84,93,94 24 months in five RCTs,53,57,61,67,83 30 months in one RCT,60 36 months in three RCTs55,68,76 and 48 months in one RCT. 64 One RCT reported an initial follow-up of 12 months,91 with an extension to 24 months. 98
The number of participants completing was not reported for two RCTs60,67 (Table 5). Overall completion rates of ≥ 90% were reported by seven RCTs53,55,64,68,71,73 (see Table 5). The highest rate of participant withdrawal was reported by Shilbayeh et al. 2004,93 with 40% of participants withdrawing overall (see Table 5).
| Author, year of study publication (trial acronym) and follow-up | Numbers completing and reasons for withdrawal | Compliance | Fracture outcomes – n/N (%) participants; reported between-group difference | FN BMD outcomes; reported between-group difference |
|---|---|---|---|---|
| ALN vs. PBO | ||||
| Adami et al., 199553 24 months |
Numbers completing: of the original 286 patients (all doses), 17 were lost to follow-up and nine withdrew consent during the study. Number of participants by group: NR Reasons for withdrawal: 13 patients discontinued treatment because of a clinical AE and two because of a laboratory AE (not described). Number of participants by group: NR |
NR | Not an outcome | Mean per cent change (SD) from baseline:
Numbers included in FN BMD analysis:
|
| Black et al., 199655 (FIT I) 36 months |
Numbers with radiograph at follow-up:
|
At closeout, 87% of those assigned to PBO and 89% of those assigned to ALN were taking study medication and 96% in each treatment group had taken at least 75% of their pills since the last clinic visit | PBO:
|
Mean per cent change (SD) from baseline (extracted from graph):
|
| Cummings et al., 199864 (FIT II) 36 months |
Numbers with radiograph at follow-up:
|
At closeout 82.5% of those assigned to PBO and 81.3% of those assigned to ALN were taking study medication and 96% in each treatment group had taken at least 75% of their pills since the last clinic visit | PBO:
|
Mean per cent change (SD) from baseline (extracted from graph):
|
| Bone et al., 200057 24 months |
Numbers completing:
|
NR | Non-vertebral fractures (e.g. foot, ankle, rib) reported as AE:
|
Mean per cent change (SD) from baseline:
|
| Carfora et al., 199860 30 months |
Numbers completing: NR Reasons for withdrawal: NR |
NR | Vertebral fractures:
|
NR |
| Chesnut et al., 199561 24 months |
Numbers completing: reports that, of 188 enrolled (PBO; ALN 10 mg, 20 mg and 5 mg), 164 (87%) completed 12 months and 154 (82%) completed 24 months. Number of participants by group: NR Reasons for withdrawal: reports that, of the 34 withdrawals, 18 were due to an AE, one to an adverse laboratory experience; five because of protocol deviations and 10 were voluntary. Number of participants by group: NR |
NR | Not an outcome | Mean per cent change (SD) from baseline:
|
| Dursun et al., 200165 12 months |
Radiographic follow-up available for:
|
NR | Vertebral fractures:
|
Mean per cent change (SD) from baseline:
|
| Greenspan et al., 200267 24 months |
Numbers completing: NR Reasons for withdrawal: NR |
NR | Clinical fractures (not described):
|
Mean per cent change (SD) from baseline (extracted from graph):
|
| Greenspan et al., 200368 36 months |
Numbers completing:
|
Participants taking 80% of medication during study:
|
Clinical fractures (not described):
|
Mean per cent change (SD) from baseline (ALN extracted from graph):
|
| Ho and Kung 200571 12 months |
Numbers completing:
|
NR | Not an outcome | Mean per cent change from baseline:
Between-group difference: p < 0.05 |
| Klotz et al., 201373 (CORAL) 12 months |
Numbers completing:
|
Reports that compliance (pill count) was similar (99% and 100%) between the two groups | AE fracture (not described):
|
Mean per cent change (SD) from baseline:
|
| Liberman et al., 199576 36 months |
Numbers completing:
|
NR | Fractures:
Between-group difference PBO vs. ALN 10 mg/day: OR 0.45 (95% CI 0.18 to 1.13) |
Mean per cent change (SD) from baseline (extracted from graph):
|
| Orwoll et al., 200083 24 months |
Numbers completing:
|
NR | Fractures:
|
Mean per cent change (SD) from baseline:
|
| Pols et al. 199984 (FOSIT) 12 months |
Numbers completing:
|
NR | Non-vertebral fractures:
|
Mean per cent change (SD) from baseline:
|
| Saag et al., 199891 48 weeks Adachi et al., 200198 24 months |
Numbers BMD data reported for 12 months:
Reasons for withdrawal 12 months:
|
NR | Fractures, 12 months:
Fractures, 24 months:
Fractures by ALN dose NR |
Mean per cent change (SD) from baseline, 12 months:
Between-group difference: p ≤ 0.001 |
| Shilbayeh et al., 200493 12 months |
Numbers completing:
|
NR | Not an outcome | Mean per cent change from baseline (SD extracted from graph):
Between-group difference: NR; p < 0.01 compared with baseline reported for ALN 10 mg/day group |
| Smith et al., 200494 12 months |
Numbers completing:
Number of participants by group: NR |
NR | Not an outcome | Change in T-score (SD):
|
| IBN vs. PBO | ||||
| Chesnut et al., 200445; Chesnut et al., 200546 (BONE) 36 months |
Numbers completing treatment:
|
Mean duration on treatment (years):
|
New vertebral:
New or worsening vertebral:
Clinical non-vertebral:
|
Not an outcome |
| Lester et al., 200874 (ARIBON) 24 months |
Numbers completing:
|
Reports that tablet compliance of the IBN was very good, with > 90% of study patients taking all of their monthly doses | Reports that no fragility fractures were reported. Three patients taking PBO (wrist, n = 1; shoulder, n = 1; rib, n = 1) experienced a traumatic fracture. Two patients taking IBN (wrist, n = 1; hip, n = 1) experienced a traumatic fracture Between-group difference: NR |
Not an outcome |
| McClung et al., 200980 12 months |
Numbers completing:
|
NR | Fracture AE:
|
Mean per cent change from baseline (SD):
|
| IBN dose-ranging trials | ||||
| Delmas et al., 200649 (DIVA) 12 months Eisman et al., 200850 24 months |
Numbers completing, 12 months:
|
12 months: reports poor compliance with the oral (n = 248) or i.v. (n = 165). Number of participants by group: NR 24 months: non-compliance with the daily regimen (≈18%), non-compliance with the i.v. regimens (≈12%) |
12 months: reports that, in total, 43 patients (3.1%) experienced clinical fractures (radiographically confirmed), including non-vertebral fractures; 13 fractures occurred in the every 2 months group, 13 fractures occurred in the every 3 months group and 17 fractures occurred in the oral treatment group. A total of 43 patients equals 3.1% and is inconsistent with safety number reported 24 months clinical osteoporotic fractures (including fractures of the vertebrae, clavicle, scapula, ribs, pelvis, sternum, humerus, forearm, femur, patella, tibia, fibula, ankle and carpus):
|
Mean per cent change from baseline (SD) extracted from graph 12 months:
Mean per cent change from baseline (SD) extracted from graph 24 months:
|
| Miller et al., 200547 Reginster et al., 200648 (MOBILE) 12 and 24 months |
Numbers completing, 12 months:
|
Reports the measures of compliance. Does not allow conclusions on differences in therapeutic adherence. Data not presented | Reports clinical fractures identified as AEs showed no statistically significant differences between the treatment arms after 1 year Clinical osteoporotic fractures recorded as AEs at 24 months:
|
Mean per cent change (SD) from baseline (extracted from graph) 12 months:
Mean per cent change (SD) from baseline (extracted from graph) 24 months:
|
| RIS vs. PBO | ||||
| Boonen et al., 200958 24 months |
Numbers completing:
|
Compliant with study drug:
|
Fractures:
|
Mean per cent change from baseline (SD) extracted from graph:
It was reported that significantly greater increases in FN BMD were observed at month 24 and end point in the RIS group compared with PBO |
| Choo et al., 201162 24 months |
Numbers included in analysis:
|
NR | Not an outcome | Percentage change from baseline (SD):
|
| Cohen et al., 199963 12 months |
Numbers completing men and women:
|
NR | Vertebral fracture:
|
Mean per cent change from baseline (SD):
|
| Fogelman et al., 200066 (BMD-MN) 24 months |
Numbers completing:
|
NR | Fractures recorded as AEs:
|
Mean per cent change from baseline (SD):
|
| Hooper et al., 200572 24 months |
Numbers completing:
|
NR | Fractures:
|
Mean per cent change (SD) from baseline (extracted from graph):
|
| Harris et al., 199970 (VERT-NA) 36 months Ste-Marie et al., 200499 60 months |
Numbers completing, 36 months:
|
55% in the PBO and 60% in the RIS 5 mg/day group completed 3 years of medication | Fractures, 36 months:
Fractures, 60 months:
|
Per cent change from baseline (SD from graph) 36 months:
Between-group difference, 60 months reported as: 4.7%, no variance estimate or p-value reported |
| Reginster et al., 200085 (VERT-MN) 36 months Sorensen et al. 2003100 60 months |
Numbers completing, 36 months:
|
NR | Fractures, 36 months:
Fractures, 60 months:
|
Mean per cent change (SD) from baseline (extracted from graph), 36 months:
Mean per cent change (SD) from baseline (mean extracted from text; SD extracted from graph), 60 months:
|
| Leung et al., 200575 12 months |
Numbers completing: NR Reasons for withdrawal: overall, 5; migration, 1; stroke, 2; gastrointestinal upset. Number of participants by group: NR |
NR | Fractures: reports that there were no symptomatic fractures in both groups during the study | Mean per cent change from baseline (SD estimated from graph):
|
| McClung et al., 200178 12 months |
Numbers completing:
|
Hip fracture, all women:
Hip fracture, aged 70–79 years:
Between-group differences reported in text: RR 0.7 (95% CI 0.4 to 1.1) Hip fracture aged 80 years:
Non-vertebral all women:
Fractures by ALN dose for all women or women 80+ years: NR |
Between-group difference in women aged 70–79 years:
|
|
| Reid et al., 200086 12 months |
Numbers completing:
|
NR | New vertebral fractures, men and women:
|
Mean per cent change (SD) from baseline:
A p-value of < 0.05 was reported for RIS 5 mg/day in postmenopausal women vs. baseline |
| Ringe et al., 200689 12 months Ringe et al., 2009101 24 months |
Numbers completing, 12 months: study reports that all 316 patients were re-examined Numbers completing, 24 months:
|
NR | New vertebral fracture, 12 months:
New vertebral fracture, 24 months:
|
Mean per cent change from baseline, 12 months:
Mean per cent change from baseline, 24 months:
Variance estimates: NR |
| Taxel et al., 201095 6 months |
|
Reports compliance with the study drug was 90–95% for all patients | Not an outcome | Mean per cent change (SD) from baseline:
|
| ZOL vs. PBO | ||||
| Black et al., 200756 (HORIZON-PFT) 36 months |
Numbers completing:
|
A total of 6260 patients (81%) received all three infusions | Fractures:
|
Mean per cent change (SD) from baseline (PBO extracted from graph): PBO: –0.04 (8.88) ZOL 5 mg/year: 5.06 (8.48) Between-group difference: 5.06% (95% CI 4.76% to 5.36%; p < 0.001) |
| Lyles et al., 200777 (HORIZON-RFT) 36 months |
Numbers completing:
|
NR | Fractures: PBO: Any new clinical, 139/1062 (13.1%) Non-vertebral, 107/1062 (10.1%) Hip, 33/1062 (3.1%) Vertebral, 39/1062 (3.7%) ZOL 5 mg/year: Any, 92/1065 (8.6%) Non-vertebral, 79/1065 (7.1%) Hip, 23/1065 (2.2%) Vertebral, 21/1065 (2.0%) Between-group difference: Any new clinical, HR 0.65 (95% CI 0.50 to 0.84); p = 0.001 Non-vertebral, 0.73 (0.55 to 0.98); 0.03 Hip, 0.70 (0.41 to 1.19); 0.18 Vertebral, 0.72 (0.56 to 0.93); 0.01 |
Mean per cent change from baseline
|
| Boonen et al., 201259 24 months |
Numbers completing: PBO: 540/611 (88.4%) ZOL 5 mg/year: 530/588 (90.1%) Reasons for withdrawal:
|
NR | One or more new morphometric vertebral fractures:
RR 0.33 (95% CI 0.16 to 07.70; p = 0.002) |
Mean per cent change from baseline (SD estimated from graph):
|
| McClung et al., 200979 24 months |
Numbers completing:
|
NR | Not an outcome | Mean per cent change from baseline (SD):
|
| Head to head | ||||
| Miller et al., 200881 (MOTION) 12 months |
Numbers completing:
|
NR | Osteoporotic fractures recorded as AEs:
|
Mean per cent change from baseline (SD):
|
| Atmaca and Gedik, 200654 12 months |
Outcomes reported for:
|
NR | Not an outcome | End of study value (SD) [% change]:
Variance estimates: NR for % change |
| Muscoso et al., 200482 24 months |
Outcomes reported for:
|
NR | Fractures:
Between-group difference: NR |
Not an outcome |
| Sarioglu et al., 200692 12 months |
Outcomes reported for:
|
NR | Fractures: the study reports that no fractures were detected throughout the study period | Mean per cent change from baseline (SD):
|
| Rosen et al., 200590 (FACT) 12 months Bonnick et al., 2005104 24 months |
Numbers completing, 12 months:
|
NR | Fractures recorded as AEs at 12 months:
Fractures recorded as AEs at 24 months:
|
Mean per cent change (SD) from baseline (extracted from graph), 12 months:
Mean per cent change (SD) from baseline (extracted from graph), 24 months:
|
| Reid et al., 200687 (FACTS) 12 months Reid et al., 2008105 24 months |
Numbers completing, 12 months:
|
NR | Fractures recorded as AEs at 12 months:
Fractures recorded as AEs at 24 months:
|
Mean per cent change (SD) from baseline (extracted from graph), 12 months:
Mean per cent change (SD) from baseline (extracted from graph), 24 months:
|
| Hadji et al., 201269 Hadji et al., 2010106 (ROSE) 12 months |
Numbers completing:
|
The study reports that 80.9% of patients were compliant with ALN therapy | Not an outcome | Not an outcome |
| Reid et al., 200988 (HORIZON) 12 months |
Numbers completing:
|
The study reports that the frequency of new vertebral fractures was n = 5 for ZOL 5 mg/year and n = 3 for RIS 5 mg/day, with no significant difference between drug groups Data by subgroup: NR |
Mean per cent change from baseline (SD):
Mean per cent change from baseline (SD):
|
|
Fractures were not assessed as an outcome in four RCTs. 53,61,71,93 Across the RCTs assessing fractures, classification of the fracture and the method of assessment was diverse (see Table 3). Five RCTs recorded fractures as AEs,57,67,68,73,84 four of which did not report details of the assessment method. 57,67,68,73 Vertebral fractures were assessed by seven RCTs. 55,60,64,65,76,83,91 All seven RCTs reported that vertebral fractures were assessed radiographically. One of the RCTs also assessed clinical fractures (non-spine clinical fractures, hip fractures, wrist fractures and clinical vertebral fractures, and other clinical fractures) reported by participants and confirmed by radiography55 and one reported that clinical fractures (clinical vertebral, hip or wrist) were assessed by participant self-reports and confirmed by radiograph. 64 One RCT reported that non-vertebral fractures were assessed from patient reporting and confirmed by radiography. 83
Femoral neck BMD assessment was reported by all but one of the RCTs. 60 Where assessed, BMD assessment was by DXA. With the exception of one RCT that did not report on DXA manufacturer65 all studies assessed BMD using DXA Hologic or Luner machines.
Ibandronic acid
Oral ibandronic acid at a dosage of 150 mg per month was evaluated against placebo in two RCTs,74,80 and 2.5 mg per day of oral ibandronic acid was evaluated against placebo in one RCT. 45 This RCT also evaluated oral ibandronic acid at a dosage of 20 mg every other day for 12 doses per month (unlicensed dose). One RCT evaluated 2.5 mg per day of oral ibandronic acid, 2 mg i.v. every 2 months (unlicensed dose) and 3 mg i.v. every 3 months (current licensed dose). 49 One RCT evaluated 2.5 mg per day, 50 mg twice per month (unlicensed dose), 100 mg per month (unlicensed dose) and 150 mg per month (current licensed dose) of oral ibandronic acid. 47 Where reported, RCTs typically excluded patients with underlying conditions or receiving medications that affect bone metabolism, and patients with upper GI tract disorders or receiving medication for these conditions.
All five RCTs were multicentre RCTs: one was undertaken in the UK,74 one in the USA,80 one in Europe and the USA,45 one in the USA, Canada, Mexico, Europe, Australia and South Africa,49 and one in the USA, Canada, Europe, Australia, South Africa, Mexico and Brazil. 47 The RCT sponsor details were reported for all five RCTs. The total numbers of participants randomised ranged from 5074 to 2946. 45
All of the RCTs recruited postmenopausal women; one recruited postmenopausal women with a histologically confirmed diagnosis of oestrogen receptor-positive breast cancer. 74
Daily adjuvant treatment in the form of 500 mg of calcium and 400 IU (international unit) of vitamin D was prescribed across all five RCTs.
Four of the RCTs45,47,49,80 reported inclusion criteria that would identify women with osteoporosis according to the current WHO definition. 1 The RCT in women with breast cancer recruited women classified as osteopenic (T-scores of > –2.5 SDs and < –1.0 SD at either the lumbar spine or total hip). 74
Four RCTs recruited participants with a mean age between 61 and 70 years. 45,47,49,74 Mean age in the other RCT was 53.6 years. 80 The mean number of years since menopause, in one RCT recruiting early postmenopausal women, was 4.2 years. 80 Mean years since menopause was 20.8 in one trial,45 18.7 in one RCT49 and 18.6 in another RCT. 47 One RCT did not report on years since menopause. 74 Mean BMI values were > 18.5 kg/m2 in all RCTs. One RCT reported a median BMI of < 30 kg/m2 in both placebo and ibandronic acid participants. 74 Race of included participants was not reported by any RCT.
The presence of fractures at baseline was reported by three RCTs:45,47,49 one in which 93% of participants had at least one vertebral fracture at baseline and 43% had two,45 one in which 42.1% had fractures at baseline49 and one in which 4.9% had fractures at baseline. 47
Compliance with treatment in the form of a pill count was assessed by one RCT. 74
Final follow-up was 12 months in two RCTs,47,80 24 months in two RCTs49,74 and 36 months in one RCT. 45 None of the RCTs reported a completion rate of ≥ 90% (see Table 5).
The highest rate of participant withdrawal was reported by the BONE trial,45 with 34% participants withdrawing overall (see Table 5).
Fractures were recorded as AEs, but the assessment method was not reported in two RCTs. 47,74 Two RCTs also assessed fractures as AEs confirmed by radiography. 49,80 The number of vertebral fractures confirmed by radiography was the primary outcome in one RCT. 45
Femoral neck BMD assessment was reported by all of the RCTs. BMD assessment was by DXA using Hologic or Lunar machines.
One of the three placebo-controlled RCTs in ibandronic acid was the pivotal 3-year BONE study, in which the antifracture efficacy of daily oral ibandronic acid 2.5 mg and intermittent oral ibandronic acid 20 mg every other day for 12 doses every 3 months (unlicensed dose) was assessed over 36 months. 45 The BONE RCT reported comparable vertebral antifracture efficacy of daily and intermittent administration, suggesting that ibandronic acid could be administered at intervals longer than daily or weekly. A further non-inferiority RCT, the MOBILE trial,47 evaluated a monthly dose of 100 mg of ibandronic acid administered as two single 50-mg doses on consecutive days; a single monthly 100-mg dose; a single monthly 150-mg dose; and a daily 2.5-mg dose (four ibandronic acid study groups). The 150-mg dose (licensed dose) produced the greatest gains in BMD compared with a daily 2.5-mg dose of ibandronic acid at 2 years (lumbar spine BMD: 6.6% vs. 5.0%, respectively; p < 0.001). 47 The DIVA study then compared the efficacy of two regimens of intermittent i.v. injections of ibandronic acid [2 mg every 2 months (unlicensed dose) and 3 mg quarterly (licensed dose)] with a daily 2.5-mg dose of oral ibandronic acid. The regimen of daily 2.5 mg oral ibandronic acid has proven antifracture efficacy. 49 At 2 years, the 2- and 3-monthly i.v. regimens produced improvements in spinal BMD (6.4% and 6.3%, respectively) that were superior to oral ibandronic acid (4.8%; p < 0.001). The MOBILE and the DIVA studies confirmed a sustained efficacy of monthly oral and quarterly i.v. regimens, respectively, over 5 years. 108,109
Risedronic acid
Risedronic acid was evaluated against placebo in 12 RCTs reported across 15 publications. 58,62,63,66,70,72,75,78,85,86,89,95,99–101
Three RCTs were multicentre RCTs undertaken in the USA. 63,70,78 One multicentre RCT was undertaken in Australia,72 one was undertaken in China75 and one was undertaken in the UK. 86 Three RCTs were international multicentre RCTs. 58,66,85 One single-centre RCT was undertaken in Germany. 89 The number of participating centres was unclear for one RCT undertaken in Canada62 and one RCT undertaken in the USA. 95 With the exception of one RCT (two publications),89,101 sponsor details were reported for all included studies. The total numbers of participants randomised ranged from 4095 to 9331. 78
Six RCTs recruited postmenopausal women and evaluated 5 mg per day of risedronic acid. 66,70,72,75,78,85 Two of these RCTs also included an evaluation of other doses of risedronic acid not currently licensed. 66,78 Both of these RCTs reported fracture outcomes for participants in the 2.5-mg and 5-mg groups combined (data not used in the analysis for this assessment report). One RCT evaluated oral risedronic acid at a dosage of 35 mg per week in men with osteoporosis58 and one RCT evaluated oral risedronic acid at 5 mg per day in men with osteoporosis. 89 Two RCTs evaluated 35 mg of oral risedronic acid per week in men with non-metastatic prostate cancer receiving ADT. 62,95 Two RCTs in men and women63,86 (32.5% male63 and 38% male,86 respectively) receiving glucocorticoids evaluated oral risedronic acid at a dosage of 5 mg per day. Where reported, RCTs typically excluded patients with underlying conditions or receiving medications that affect bone metabolism, and patients with upper GI tract disorders or receiving medication for these conditions.
Adjuvant treatment in the form of calcium alone or in combination with vitamin D was reported for all RCTs. The dosages varied across the RCTs (see Table 3).
Inclusion criteria varied across the RCTs in terms of baseline BMD and T-scores (skeletal site and cut off). Six RCTs58,66,72,75,78,89 reported inclusion criteria that would identify men and women with osteoporosis according to the current WHO definition. 1 One RCT recruited women aged ≤ 85 years with at least one vertebral fracture at baseline70 and another RCT recruited women aged ≤ 85 years with at least two radiographically confirmed vertebral fractures. 85 Baseline BMD was not an inclusion criterion for either of the two RCTs in men and women receiving glucocorticoids63,86 or the two RCTs in men with prostate cancer receiving ADT. 62,95
The mean age of participants was between 51 and 60 years in three RCTs. 72,86,89 One RCT categorised women by age into two groups: those aged 70–79 years and those aged ≥ 80 years. 78 In two RCTs, the mean age of all included participants was 71 years. 85,95 In all other RCTs the mean age of included participants was between 61 and 70 years. Five RCTs in women reported on the number of years since menopause. 66,70,72,75,85 The mean number of years since menopause was 15 years in one RCT,75 17 years in another RCT66 and ranged from 24 to 25 years in two RCTs. 70,85 The mean number of years since menopause was < 5 years in one RCT. 72 In the RCT categorising women by age into two groups (those aged 70–79 years and those ≥ 80 years), the mean number of years since menopause was 28 years and 37 years, respectively. 78 The mean years since menopause in one RCT recruiting early postmenopausal women was 3.7 years. 72 BMI was available for five RCTs. 58,66,70,75,89 Across these RCTs, all mean BMI values were > 18.5 kg/m2. Race of included participants was reported by only one of the RCTs in which proportion of Caucasian participants was 95%. 58
The presence of fractures or fracture history at baseline was reported by eight RCTs,63,66,70,72,78,85,86,89 with 20% of women in one RCT having vertebral fractures at baseline. 72 In two RCTs approximately 31% of all participants had baseline vertebral fractures,63,66 and in one RCT 35% had vertebral fractures. 86 One RCT reported that 42% had vertebral fractures at baseline78 and one that 52% had vertebral fractures. 89 In one trial, 80% of all participants had vertebral fractures at baseline. 70 One RCT reported the median number of vertebral fractures at baseline, which was three in the placebo group and four in the risedronic acid group. 85
Compliance with treatment in the form of a pill count was assessed by two RCTs. 58,95
Final follow-up was 12 months in three RCTs63,75,86 and 24 months in four RCTs. 58,62,66,72 One RCT reported a final follow-up of 6 months95 and another reported a follow-up of 36 months. 78 One RCT reported an initial follow-up of 12 months,89 with an extension to 24 months. 101 Two RCTs reported an initial follow-up of 36 months,70,85 with an extension to 60 months. 99,100
The number of participants completing the trial was not reported by three RCTs62,75,95 (see Table 5). Only one RCT reported a completion rate of ≥ 90%89 (see Table 5). The highest rate of participant withdrawal was reported by McClung et al. ,78 with 40% participants withdrawing overall (see Table 5).
Fractures were not assessed as an outcome in four RCTs. 62,75,86,95 Across the RCTs assessing fractures, classification of the fracture and the method of assessment were diverse (see Table 3). One recorded clinical fractures (non-vertebral and vertebral fractures) confirmed by radiography as AEs. 99 This was an extension to a RCT in which vertebral fractures were the primary outcome and were assessed radiographically. 70 One RCT recorded non-vertebral fractures (not described) and vertebral fractures as AEs, with vertebral fractures assessed radiographically. 66 Vertebral fractures were assessed by six other RCTs. 58,63,72,85,86,89 All six RCTs reported that vertebral fractures where assessed radiographically. One of these RCTs also assessed clinical vertebral and non-vertebral fractures reported as AEs; vertebral fractures reported as AEs included symptomatic and asymptomatic radiographically confirmed fractures. 58 One RCT assessed radiographically confirmed hip fractures and non-vertebral osteoporotic fractures; non-vertebral osteoporotic fractures were defined as all radiographically confirmed fractures of the wrist, leg, humerus, hip, pelvis or clavicle. 78
Femoral neck BMD assessment was reported by all of the RCTs. BMD assessment was by DXA using Hologic or Lunar machines.
Zoledronic acid
Zoledronic acid was evaluated against placebo in four RCTs. 56,59,77,79
All four RCTs were international multicentre RCTs. Sponsor details were reported for all trials and it was the same sponsor across the RCTs. The total number of participants randomised ranged from 40079 to 7765. 56
Two RCTs recruited postmenopausal women with osteoporosis56,79 and one recruited men with osteoporosis. 59 Across these RCTs, baseline BMD and T-scores would identify men and women with osteoporosis according to the current WHO definition. 1 One RCT recruited ambulatory men (24.5%) and women who had undergone repair of a hip fracture. 77 Baseline BMD was not an inclusion criterion for this RCT. All RCTs evaluated 5 mg per year of i.v. zoledronic acid.
Adjuvant treatment in the form of calcium in combination with vitamin D was reported for all RCTs. The dosages varied across the RCTs (see Table 3).
The mean age of participants in the Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly (HORIZON) RCTs was between 61 and 70 years. 56,77 The mean age of all participants was 66 years in one trial59 and 60 years in another trial. 79 The mean number of years since menopause was reported for only one RCT and was 11.4. 79 BMI was available for three RCTs,56,77,79 and across these RCTs, all mean BMI values were > 18.5 kg/m2. The race of included participants was reported by all four RCTs, and > 90% of the participants were Caucasian.
The presence of fractures at baseline was reported by three of the RCTs,56,59,77 one of which reported all patients who were enrolled in the RCT had undergone repair of a hip fracture. 77 One RCT reported that 28% of participants had one vertebral fracture at baseline and 35% had more than two. 56 One RCT reported that 22.1% of participants had one vertebral fracture at baseline and 10.8% had more than two. 59
An assessment method of compliance was not reported by any RCT evaluating zoledronic acid with placebo.
Final follow-up was 24 months in two RCTs59,79 and 36 months in the other two RCTs. 56,77 The proportion of participants completing each of the RCTs was 83.9%,56 71.1%,77 89.2%59 and 89.3%. 79 (see Table 5).
Fractures were assessed as an outcome in three RCTs. 56,59,77 One RCT assessed vertebral fractures from radiographs. 56 In this RCT, clinical fracture reports were also obtained from patients at each visit and non-vertebral fracture reports required central confirmation. Fractures of the toe, facial bone, finger and those caused by excessive trauma were excluded. In another RCT, non-vertebral fractures (not a vertebral, facial, digital or skull fracture) were confirmed when a radiograph, a radiographic report or a medical record documented a new fracture. 77 In this RCT, a new clinical vertebral fracture was defined as new or worsening back pain with a reduction in vertebral body height. The third RCT assessed vertebral fractures from radiographs. 59 In this RCT, clinical fractures (vertebral and non-vertebral) were reported by participants at each visit and were verified centrally by means of a radiographic report or surgical notes.
Femoral neck BMD assessment by DXA was reported by all of the RCTs. Only one RCT reported the DXA model (Hologic or Lunar machines). 79
Head to head
One RCT compared alendronic acid with monthly oral ibandronic acid (at a dose of 150 mg) in postmenopausal women;81 there was no placebo arm in this trial. This was a multicentre non-inferiority RCT conducted in North America, Latin America, Europe and South Africa, with sponsor details reported. In total, 1760 women were randomised. Mean age was 65.6 years, mean years since menopause was 18.3 years, mean BMI was 25.9 kg/m2 and race of participants was reported as 82% Caucasian. BMD inclusion criteria were based on lumbar spine [lumbar vertebrae 2–4 (L2–L4)] BMD T-score of < –2.5 and ≥ –5.0 SDs. Previous fractures (not described) were experienced by 38.2% of the alendronic acid group and 39% of the ibandronic acid group. Patients with either upper GI tract disorders or diseases affecting bone metabolism were excluded. The alendronic acid dosage was 70 mg per week and the ibandronic acid dosage was 150 mg per month. Both groups also received 500 mg of calcium and 400 IU of vitamin D per day. For compliance assessment, returned study tablets were counted. Fractures were recorded as AEs and follow-up was 12 months. Overall, 90% of participants completed the 12-month follow-up (see Table 5).
Five RCTs across seven publications compared alendronic acid with risedronic acid in postmenopausal women. 54,82,87,90,92,104,105 There was no placebo arm in any of these RCTs.
Three RCTs evaluated 10 mg per day of alendronic acid and 5 mg per day of risedronic acid. 54,82,92 Two of these RCTs were undertaken in Turkey54,92 and the other in Italy. 82 The number of participating centres and RCT sponsor details were not reported for any of the RCTs. One trial randomised 28 participants (14 in each group)54 and one randomised 50 participants (25 in each group). 92 The third trial randomised 2000 participants to treatment groups also including clodronic acid and raloxifene. In total, 1000 participants were randomised to risedronic acid and 100 to alendronic acid82 (10 : 1 randomisation ratio). All three RCTs reported osteoporosis to be an inclusion criterion, but only one reported a BMD T-score inclusion criterion. 54 Mean ages were 66 years,54 70.5 years82 and 58.8 years. 92 One RCT reported on mean years since menopause, which was 15.6 years,54 and one RCT92 reported on mean BMI, which was 27.3 kg/m2. Race was not reported by any of the three RCTs. All three RCTs prescribed a daily adjuvant of calcium and vitamin D. Fractures at baseline were not reported by two of the RCTs;54,82 however, in the other trial, approximately 10% of participants in both groups had vertebral fractures at baseline. 92 Two of the RCTs reported fracture as an outcome82,92 and one reported fracture as an AE;92 however, details of the assessment method were not reported by either RCT. Final follow-up was at 12 months in two RCTs54,92 and 24 months in the third. 82 Two of the RCTs reported 12-month femoral neck BMD assessment by DXA, using either a Hologic54 or Lunar machine. 92 None of the three RCTs reported on numbers withdrawing, but all reported that 100% of participants randomised were included in the analysis (see Table 5). Where reported, conditions or medications affecting bone metabolism were exclusion criteria, with one RCT also considering upper GI conditions as an exclusion criterion. 92
Two further RCTs undertaken by the same study group compared 70 mg per week of alendronic acid with 35 mg per week of risedronic acid in postmenopausal women. 87,90 One was undertaken as a 12-month multicentre RCT in the USA,90 with a 12-month extension to 24 months,104 and the other was a 12-month multicentre RCT across 75 centres in 27 countries in Europe, the Americas and the Asia-Pacific region,87 with a 12-month extension to 24 months. 105 Sponsor details were the same across these RCTs. The number randomised was 1053 in the US study90 and 936 in the multinational study. 87 Both RCTs recruited postmenopausal women with osteoporosis according to the current WHO definition. 1 Mean age, years since menopause and BMI was 64.5 years, 18.5 years and 25.3 kg/m2, respectively, in the US study90 and 64.1 years, 16.9 years and 25.3 kg/m2, respectively, in the international study. 87 The last RCT reported that participants with conditions or medications affecting bone metabolism were excluded. Both RCTs reported that > 90% of participants were Caucasian and both RCTs prescribed a daily adjuvant of 1000 mg of calcium and 400 IU of vitamin D.
The study undertaken in the USA reported that 12% of participants had a history of hip, spine or wrist fracture after the age of 45 years. 90 The multinational study reported that 33.7% of participants had a history of fractures (not described), and that 41% had a family history of osteoporosis. 87 Across both RCTs, clinical fractures that occurred during the trial, regardless of association with trauma or skeletal site, were reported by investigators as clinical AEs. Femoral neck BMD was assessed in both RCTs using DXA (Hologic). Both RCTs reported a completion rate of > 90% at the 12-month follow-up87,90 (see Table 5).
One RCT evaluated 5 mg once per year of i.v. zoledronic acid with 70 mg per week of alendronic acid. 69 There was no placebo arm in this trial and the sponsor was reported. In total, 604 postmenopausal women aged 55–90 years with a BMD T-score of ≤ –2.0 SDs at total hip or lumbar spine were randomised. Both groups were prescribed a daily adjuvant of 1200 mg of calcium and 800 IU of vitamin D. The mean age of participants was 67.8 years and mean BMI was 26.2 kg/m2. Of all the participants, 33% had fractures (not described) at baseline and the proportion of participants who were current or previous smokers was 22.9%. Participants with conditions affecting bone metabolism were excluded. Fractures and femoral neck BMD were not outcomes for this RCT. Quality of life was assessed using a visual analogue scale (VAS) and compliance was assessed by investigator or study personnel at each visit. 106 The triallists reported that > 90% of participants completed the 12-month follow-up (see Table 5).
One RCT reported as the HORIZON study [i.e. not the HORIZON – Pivotal Fracture Trial (HORIZON-PFT) or HORZON – Recurrent Fracture Trial (HORIZON-RFT), which compared zoledronic acid vs. placebo] recruited men and women aged 18–85 years receiving at least 7.5 mg of oral prednisolone daily (or equivalent) and who were expected to receive glucocorticoids for at least another 12 months. 88 There was no placebo arm in this trial. The RCT, which was an international multicentre RCT, categorised 416 participants receiving steroids for > 3 months as a ‘treatment’ subgroup and 417 participants receiving steroids for ≤ 3 months as a ‘prevention’ subgroup; both subgroups were randomised to receive 5 mg of i.v. zoledronic acid once annually or 5 mg per day of risedronic acid. The RCT sponsor was reported. All treatment groups were prescribed a daily adjuvant of 1200 mg of calcium and 800 IU of vitamin D. Across treatment groups, 31% were male, the mean age of all participants was 54.4 years and race was not reported. Participants with conditions or previous treatments affecting bone metabolism were excluded. Follow-up was at 12 months. Vertebral fractures were assessed by radiography and femoral neck BMD by DXA (Hologic or Lunar). EuroQol (EQ-5D) HRQoL was assessed. 110 The triallists reported that > 90% of participants completed the 12-month follow-up (see Table 5).
Quality of the available research
Of the 46 included RCTs,45,47,49,53–95 21 were considered to be at low risk of selection bias;47,49,55–57,59,64,67–70,72,73,77,79,81,87,88,90,93,94 however, the majority (25/46) of the included RCTs did not report a method of random-sequence generation and were therefore classified as being at unclear risk of selection bias. 45,53,54,58,60–63,65,66,71,74–76,78,80,82–86,89,91,92,95 A summary of all risk-of-bias criteria judgements by RCT is reported in Figure 4. A summary about each risk-of-bias item presented as percentages across all included RCTs is presented in Figure 5.
FIGURE 4.
Risk-of-bias summary: judgements about each risk-of-bias item for each included RCT.?, unclear risk of bias; +, low risk of bias, –, high risk of bias; ALN, alendronic acid; IBD, ibandronic acid; RIS, risedronic acid; ZOL, zoledronic acid.

FIGURE 5.
Risk-of-bias graph: judgements about each risk-of-bias item presented as percentages across all included RCTs.
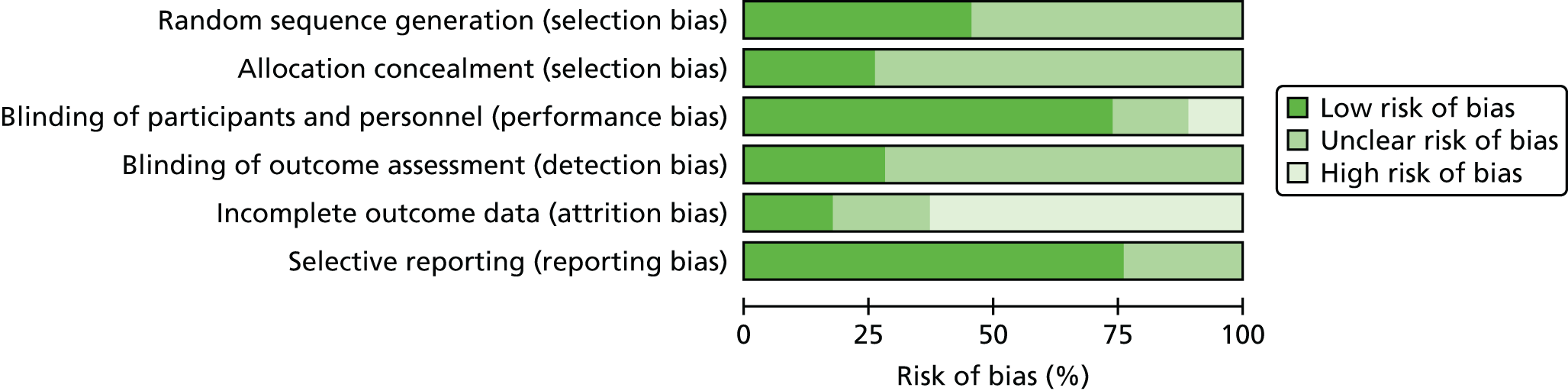
Of the 46 included RCTs, 12 reported appropriate methods for concealment of treatment allocation and were therefore judged to be at low risk of bias for this domain. 55,56,59,64,68,70,77,79,81,87,88,90 The remaining 34 RCTs did not report on allocation concealment and were therefore judged as being at unclear risk of bias for this domain.
Thirty-four of the included RCTs45,55–58,61–64,66–68,70,72–77,79–81,83–91,93–95 reported that participants and personnel were blind to treatment allocation and were therefore judged at low risk of performance bias. Five RCTs were reported as either open label or single blind and were judged as being at high risk of bias. 53,71,82,92,106 The remaining RCTs did not report on blinding and were considered to be at unclear risk of bias for this domain.
Blinding of the outcome assessment was reported by 13 RCTs,55,56,59,64,68,70,76,77,83,87–89,94 which were therefore classified as being at low risk of detection bias. The remaining RCTs were considered at unclear risk of bias for this domain.
In 29 of the RCTs,45,47,49,56–59,63,65,66,69,70,72,73,76–81,83–85,89–91,93,94,111 attrition was reported to be ≥ 10% across treatment groups and, therefore, these RCTs were judged to be at high risk of attrition bias. In eight of the included RCTs,53,55,64,68,86–88,95 attrition across treatment groups was reported as < 10% and these RCTs were judged at low risk of attrition bias. In the remaining nine RCTs,54,60–62,67,71,75,82,92 numbers withdrawing were not reported; these RCTs were therefore considered at unclear risk of bias for this domain.
Thirty-four of the included RCT reports45,55–59,63–72,74–80,83–85,87–91,93–95 contained either reference to a RCT protocol or a RCT registration number, and were therefore judged as being at low risk of selection bias. The remaining included RCTs did not contain this information and were therefore judged to be at unclear risk of bias for this domain.
Assessment of effectiveness
Outcome measures prespecified in the final protocol reported across the included RCTs are presented in Table 5.
Fracture
A total of 27 RCTs provided suitable fracture data for inclusion in the NMA reported in Results from the network meta-analyses: nine RCTs compared alendronic acid with placebo;55,57,61,64,65,67,76,83,84 two compared monthly oral ibandronic acid with placebo;74,80 one compared 2.5 mg per day of oral ibandronic acid with placebo;45 nine compared risedronic acid with placebo;58,63,66,70,72,78,85,86,89 three compared zoledronic acid with placebo;56,59,77 one compared alendronic acid with 150 mg per month of oral ibandronic acid;81 one compared alendronic acid with risedronic acid;82 and one compared zoledronic acid with risedronic acid. 88
Alendronic acid
In the Fracture Intervention Trial (FIT) I, Black et al. 55 reported a RR of 0.53 (95% CI 0.41 to 0.68) for morphometric vertebral fractures, and a relative hazard of 0.45 (95% CI 0.27 to 0.72) for clinical vertebral fractures and 0.72 (95% CI 0.58 to 0.90) for the risk of any clinical fracture at the 36-month follow-up. The relative hazards for hip fracture and wrist fracture were reported as 0.49 (95% CI 0.23 to 0.99) and 0.52 (95% CI 0.31 to 0.87), respectively. In FIT II, Cummings et al. 64 reported a RR for radiographically detected vertebral fractures at 36 months of 0.65 (95% CI 0.39 to 0.80). The relative hazard of clinical fractures (vertebral, hip or wrist) was reported as 0.64 (95% CI 0.50 to 0.82) in women with osteoporosis and 1.08 (95% CI 0.87 to 1.35) in those without osteoporosis. In the RCT by Carfora et al. ,60 vertebral fractures were reported for 8.82% of placebo participants, compared with 2.94% of alendronic acid participants. The RCT by Dursun et al. 65 reported vertebral fractures at 12 months in 40.0% of the group assigned to calcium and 31.6% in the alendronic acid combined with calcium group. The difference between treatments in these RCTs was not reported. Orwoll et al. 83 reported a significant difference between treatments at 24 months in new vertebral fractures (p = 0.02) but not non-vertebral fractures (p = 0.8) in men.
Across the RCTs assessing fractures as AEs, Bone et al. 57 reported that the difference between treatments in non-vertebral fractures (foot, ankle, rib) was not significant (p-value not reported). Greenspan et al. 67,68 reported that the difference between treatments in clinical fractures (not described) was not significant (p-values not reported). In the FOSamax International Trial, Pols et al. 84 reported a 47% risk reduction in non-vertebral fractures (95% CI 10% to 70%; p = 0.021) and, in the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) trial, Klotz et al. 73 reported no statistically significant difference in fractures (not described) between treatments (p-value 0.4395).
Two RCTs pooled fracture data from different alendronic acid dosing arms (licensed and unlicensed doses). Liberman et al. 76 reported that by 36 months participants treated with alendronic acid (5 mg, 10 mg and 20 mg groups combined) had experienced fewer fractures than those treated with placebo (vertebral fractures: RR 0.52, 95% CI 0.28 to 0.95; p = 0.03; non-vertebral fractures: RR 0.79, 95% CI 0.52 to 1.22; p-value not reported). A difference between placebo and 10 mg per day of alendronic acid was reported for this RCT as an odds ratio (OR) of 0.45 (95% CI 0.18 to 1.13; p-value not reported);97 however, numbers by group were not reported. Saag et al. 91 reported a difference in vertebral fractures at 12 months between the alendronic acid 5 mg and 10 mg groups combined and the placebo group as a RR of 0.6 (95% CI 0.1 to 4.4).
Ibandronic acid
Lester et al. ,74 in the reversal of anastrozole (ARImidex) induced bone loss with oral monthly ibandronate (BONdronat) treatment during adjuvant therapy for breast cancer (ARIBON) trial,74 reported that three patients in placebo group and two patients in the ibandronic acid group (monthly oral dose) experienced fractures as AEs. McClung et al. 80 also reported fractures as AEs, with 2% in placebo group and 3% in the ibandronic acid group (monthly oral dose) experiencing fractures. A difference between treatments was not reported by either RCT. In the BONE trial, Chesnut et al. 45 reported that the risk of new vertebral fractures at 36 months was 62% lower in the group treated with 2.5 mg of oral ibandronic acid daily than the placebo-treated group (95% CI 41% to 74%; p = 0.0001). Clinical non-vertebral fractures were experienced by 8.2% of the placebo group compared with 9.1% of the group receiving 2.5 mg per day of oral ibandronic acid. A difference between treatments was not reported. In the DIVA trial, Delmas et al. 49 reported that 43 (3.1%) participants experienced clinical fractures, including non-vertebral fractures recorded as AEs, at 12 months: 17 in the 2.5 mg per day of oral ibandronic acid group and 13 in the 3 mg i.v. every 3 months group. The corresponding numbers at the 24-month follow-up were 29 (6.2%) and 23 (4.9%). 50 Differences between treatments were not reported. In the MOBILE trial, Miller et al. 47 reported that there was no statistically significant difference between treatments in clinical fractures recorded as AEs at 12 months. At the 24-month follow-up, 24 (6.1%) participants receiving 2.5 mg per day of oral ibandronic acid and 27 (6.8%) receiving 150 mg per month had clinical fractures. 48 Differences between treatments were not reported.
Risedronic acid
Boonen et al. 58 reported no differences in new vertebral or clinical fractures (recorded as AEs) at 24 months between those treated with 35 mg per week of risedronic acid and those receiving placebo. Cohen et al. 63 found no statistically significant difference in vertebral fractures in both men and women at 12 months between those treated with 5 mg per day of risedronic acid and those receiving placebo (p = 0.072). In the RCT assessing fractures as AEs,66 14% of the placebo group experienced vertebral fractures and 9% experienced non-vertebral fractures at 24 months. Corresponding numbers in the risedronic acid 5 mg per day group were 7% and 5%, respectively. 66 A difference between treatments was not reported. The difference between treatments in new vertebral fractures or non-vertebral fractures between 5 mg per day of risedronic acid and placebo at 24 months was reported as not significant (p-value not reported) by one RCT. 72
In the Vertebral efficacy with Risedronate Therapy – North American (VERT-NA) trial, Harris et al. 70 reported a difference between treatments in favour of risedronic acid in the incidence of vertebral fractures at 36 months of 41% (95% CI 18% to 58%; p = 0.003) and in the incidence of non-vertebral fractures of 39% (95% CI 6% to 61%; p = 0.02). In the 60-month extension, fractures were recorded as AEs, the triallists reporting that AEs were similar across groups. 99 A difference between treatments for fractures was not reported. In the Vertebral Efficacy with Risedronate Therapy – MultiNational (VERT-MN) trial, Reginster et al. 85 reported a difference between treatment groups in fractures at the 36-month follow-up (vertebral fractures: RR 0.51, 95% CI 0.36 to 0.73; p < 0.001; non-vertebral fractures: RR 0.67, 95% CI 0.44 to 1.04; p = 0.063). In the extension study,100 a difference in vertebral fractures of 59% (95% CI 19% to 79%; p = 0.01) was reported. The triallists reported that fracture results observed in the study extension were consistent with those observed in the first 3 years.
In the subgroup of women aged 70–79 years, McClung et al. 78 reported a difference in hip fractures at 12 months between those treated with 5 mg per day of risedronic acid and those treated with placebo (RR 0.7, 95% CI 0.4 to 1.1). In the subgroup of women aged ≥ 80 years, hip fracture data were reported for the combined 2.5 mg risedronic acid per day group (unlicensed) and the 5 mg risedronic acid per day group and compared with data for the placebo-treated group (p = 0.35). The hip fracture results in all women were also reported for the combined 2.5 mg risedronic acid per day group and 5 mg risedronic acid per day group and compared with the placebo-treated group, risedronic acid was favoured (RR 0.7, 95% CI 0.6 to 0.9; p = 0.02).
Reid et al. 86 reported a p-value of 0.042 for the difference between treatments in vertebral fractures at 12 months across men and women for the 2.5 mg risedronic acid per day group and the 5 mg per day group combined compared with placebo. The difference between treatments for 5 mg risedronic acid per day compared with placebo was not reported. The triallists reported that the RCT was not powered to demonstrate fracture efficacy.
Ringe et al. 89 reported a significant difference between treatment groups in new vertebral fractures at 12 months in men (p = 0.028). At 24 months, the significant difference persisted (p = 0.032). 101
Zoledronic acid
In the HORIZON-PFT, Black et al. 56 reported a difference in morphometrically assessed vertebral fractures at 36 months between women treated with 5 mg of zoledronic acid annually and those treated with placebo (RR 0.30, 95% CI 0.24 to 0.38; p < 0.001). The women were not taking any osteoporosis medications at baseline (stratum I). Significant between-group differences in hip fracture, non-vertebral fractures, clinical fractures and clinical vertebral fractures in all women were also reported (p < 0.001).
In the HORIZON-RFT, Lyles et al. 77 reported a difference in any new clinical fracture at 36 months between men and women treated with 5 mg of zoledronic acid annually and those treated with placebo [hazard ratio (HR) 0.65, 95% CI 0.50 to 0.84; p = 0.001]. The difference in clinical non-vertebral fractures was reported as a HR of 0.73 (95% CI 0.55 to 0.98; p = 0.03), the difference in clinical hip fractures as a HR of 0.70 (95% CI 0.41 to 1.19; p = 0.18) and the difference in clinical wrist fractures as a HR of 0.72 (95% CI 0.56 to 0.93; p = 0.01).
Boonen et al. 59 reported a difference in the number of male participants experiencing one or more new morphometric vertebral fractures at 24 months depending on treatment group (RR 0.33, 95% CI 0.16 to 07.70; p = 0.002).
Alendronic acid versus risedronic acid
In the Monthly Oral Therapy with Ibandronate for Osteoporosis iNtervention (MOTION) trial, Miller et al. ,81 reported that, at 12 months, 18 out of 874 (2.1%) participants in the ibandronic acid group (monthly oral dose) had experienced osteoporotic fractures recorded as AEs, of which five were vertebral fractures and 14 non-vertebral, compared with 17 (comprising five vertebral and 12 non-vertebral) out of 859 (2%) participants in the alendronic acid group. A difference between treatments was not reported.
Muscoso et al. 82 reported that at 24 months there were four fractures in the risedronic acid group, compared with none in the alendronic acid group; however, it was unclear if the unit of analysis was the participant or the fracture. A difference between treatments was not reported.
In the Fosamax Actonel Comparison Trial (FACT), Rosen et al. 90 reported that at 12 months 5.0% of the alendronic acid group had an AE fracture, compared with 3.8% in the risedronic acid group. At 24 months, 8.3% of the alendronic acid group had an AE fracture, compared with 8.2% in the risedronic acid group. 104 In the FACT international Study (FACTS), Reid et al. 87 reported that at 12 months 3.6% of the alendronic acid group had an AE fracture, compared with 3.8% in the risedronic acid group. A difference between treatments was not reported. The corresponding values at 24 months105 were 5.7% and 6.3%.
Zoledronic acid versus risedronic acid
In the HORIZON trial, Reid et al. 88 reported that the frequency of new vertebral fractures was five in the zoledronic acid group and three in the risedronic acid group, with no significant difference between drug groups. Data by steroid use subgroup were not reported.
Femoral neck bone mineral density
A total of 35 RCTs provided suitable femoral neck BMD data for inclusion in the NMA reported in Results from the network meta-analyses: 12 RCTs compared alendronic acid with placebo;53,55,57,64,65,67,68,73,76,83,84,91 one compared 2.5 mg per day of oral ibandronic acid with placebo;45 one compared 150 mg per month of oral ibandronic acid with placebo;80 one compared 2.5 mg per day of oral ibandronic acid with 3 mg i.v. ibandronic acid every 3 months;49 one compared 2.5 mg per day of oral ibandronic acid with 150 mg ibandronic acid per month;47 10 compared risedronic acid with placebo;58,62,63,66,70,72,75,85,86,95 four compared zoledronic acid with placebo;56,59,77,79 three compared alendronic acid with risedronic acid;87,90,92 one compared alendronic acid with 150 mg per month of oral ibandronic acid;81 and one compared zoledronic acid with risedronic acid. 88
Alendronic acid
Statistically significant differences in femoral neck BMD between treatments for 10 mg per day of alendronic acid were reported at 48 weeks by one trial,91 at 12 months by three trials,65,71,84 at 24 months by four trials53,57,61,83 and at 36 months by three trials. 55,64,76 The variance estimates were reported as a standard error in FIT I;55 however, FIT II64 reported that the variance estimates were SDs. These triallists were contacted for confirmation of the variance estimate (Professor Dennis Black, University of California, 2015, personal communication) but no reply was received to 29 June 2016. For this assessment report it was assumed that the femoral neck BMD variance estimate was reported as standard error in both RCTs because of the sample sizes and apparent comparability of the reported values. A mean difference between treatments at 24 months of 3.4% (95% CI 2.3% to 4.4%) was reported by one RCT67 (p-value not reported). One RCT did not report the difference between treatments at 36 months (data by group presented in graphical format only)68 and one RCT reported mean per cent change from baseline compared with age-matched and young adult reference values (source not reported). 93 Significant changes from baseline were reported in the alendronic acid group (p < 0.01). One RCT reported differences between treatments in femoral neck T-scores and z-scores at 12 months,94 but no statistically significant differences between treatments were reported. One RCT assessing 70 mg per week of alendronic acid reported a mean change from baseline in femoral neck BMD at 12 months of –2.06% (SD ± 5.71%) in the placebo group, compared with 1.65% (SD ± 7.53%) in the alendronic acid group. 73 No difference between treatments was reported by this RCT. 73
Ibandronic acid
One RCT assessing 150 mg per month of ibandronic acid reported a mean change from baseline in femoral neck BMD at 12 months of –0.73% (SD ± 4.16%) in the placebo group compared with 1.09% (SD ± 2.87%) in the ibandronic acid group,80 but a between-group difference between treatments was not reported by this RCT. In the DIVA trial, Delmas et al. 49 reported a mean change from baseline at 12 months of 1.6% (SD ± 4.18%) for 2.5 mg per day of oral ibandronic acid compared with 2.3% (SD ± 3.87%) for 3 mg of i.v. ibandronic acid every 3 months. Corresponding values at 24 months were 2.01% (SD ± 5.65%) and 2.32% (SD ± 4.70%);50 differences between treatments were not reported. In the MOBILE trial, Miller et al. 47 reported a mean change in femoral neck BMD from baseline at 12 months of 1.71% (SD ± 3.68%) for 2.5 mg per day of oral ibandronic acid compared with 2.22% (SD ± 3.83%) for 150 mg per month of ibandronic acid. Corresponding values at 24 months were 1.91% (SD ± 4.45%) and 3.12% (SD ± 7.03%), respectively. 48 Between-group differences between treatments were not reported.
Risedronic acid
Statistically significant differences in femoral neck BMD between women receiving 5 mg of risedronic acid per week and those receiving placebo were found at 12 months,75 24 months,66,72 36 months70,85 and 60 months. 100 Statistically significant differences at 6 months95 and at 24 months58 were reported in men receiving 35 mg per week risedronic acid and at 12 months89 and 24 months in men receiving 5 mg per week risedronic acid compared with placebo treatment. 101 One RCT reported a p-value of 0.4670 for 35 mg per week of risedronic acid, but it was unclear whether this was compared with baseline or the placebo group. 62 One RCT reported a statistically significant difference at 12 months among men and women between those treated 5 mg per day of risedronic acid and those treated with placebo (p < 0.001);63 however, the difference between treatments was not significant when only women were considred. 63 In a subgroup of women aged 70–79 years McClung et al. 78 reported a 3.4% difference in femoral neck BMD between those treated with 5 mg per week of risedronic acid and those treated with placebo;78 data by group or a p-value were not reported. Reid et al. 86 reported a p-value < 0.05 for 5 mg/day of risedronic acid in postmenopausal women compared with baseline.
Zoledronic acid
In the HORIZON-PFT, Black et al. 56 reported a difference in femoral neck BMD between treatment groups at 36 months of 5.06% (95% CI 4.76% to 5.36%; p < 0.001). In the HORIZON-RFT trial, Lyles et al. 77 also reported a statistically significant between-group difference at 36 months (p < 0.001). Boonen et al. 59 reported a statistically significant between-group difference in men at 24 months (p < 0.05) and McClung et al. 79 reported a statistically significant between-group difference in postmenopausal women at 24 months (p < 0.001).
Alendronic acid versus ibandronic acid
In the MOTION trial, Miller et al. 81 reported a mean change in femoral neck BMD from baseline to 12 months of 2.1% (SD ± 1.77) in the 70 mg per week of alendronic acid group compared with 2.3% (± 2.12 SD) in the 150 mg per month oral ibandronic acid group; the difference between treatments was not reported.
Alendronic acid versus risedronic acid
In the RCT by Sarioglu et al. ,92 data and variance estimates by group were reported. The triallists reported that the difference in femoral neck BMD between treatments was not significant (p-value or difference between treatments not reported). In the FACT trial, Rosen et al. 90 reported that, at 12 months, the difference between treatments was 0.7% (95% CI 0.1% to 1.2%; p < 0.005) in favour of alendronic acid. The difference between treatments at 24 months104 was reported as 0.8% (95% CI 0.3% to 1.4%; p < 0.005) in favour of alendronic acid. In the FACTS trial, Reid et al. 87 reported that, at 12 months, the difference between treatments was 0.56% (95% CI 0.03% to 1.09%; p = 0.039) in favour of alendronic acid. The difference between treatments at 24 months105 was reported as 1.0% (95% CI 0.3% to 1.6%; p = 0.002) in favour of alendronic acid.
Zoledronic acid versus risedronic acid
In the HORIZON trial, Reid et al. 88 reported that, in the treatment subgroup, the difference in femoral neck BMD between treatments at 12 months was 1.06% (95% CI 0.32% to 1.79%) and the difference between treatments in the prevention subgroup was 1.33% (95% CI 0.41% to 2.25%); both were in favour of zoledronic acid.
Mortality
Details of all AEs reported for alendronic acid, ibandronic acid, risedronic acid and zoledronic acid, across all included RCTs, are presented in Appendix 4.
Nine RCTs45,55,56,58,59,64,77,81,88 reported deaths in participants treated with bisphosphonates: two RCTS compared 10 mg per day of alendronic acid with placebo;55,64 one compared 2.5 mg per day of ibandronic acid with placebo;45 one compared risedronic acid with placebo;58 four compared 5 mg per year of zoledronic acid with placebo;56,59,77,88 and one was a head-to-head comparison between alendronic acid and a monthly oral dose of ibandronic acid. 81 The frequencies of deaths in each treatment group in the included RCTs are tabulated in Appendix 4.
Alendronic acid
Two RCTs55,64 reporting AEs in postmenopausal women for 24 months55 and 48 months64 were included. Data from the two RCTs show that there were 122 deaths: 61/3236 (1.9%) in the alendronic acid group and 61/3223 (1.9%) in the placebo group (pooled RR 1.0, 95% CI 0.70 to 1.41; p = 0.98). The difference between treatments was not statistically significant (Figure 6).
FIGURE 6.
Deaths in postmenopausal women on alendronic acid compared with placebo. M–H, Mantel–Haenszel.

Ibandronic acid
The BONE trial45 compared 2.5 mg per day of oral ibandronic acid (n = 977) with placebo (n = 975) for 36 months in postmenopausal women. No association between any treatment and risk of death was found. In total, 22 deaths occurred: 11 (1.1%) in the ibandronic acid group and 10 (1.0%) in the placebo group (RR 1.1, 95% CI 0.47 to 2.5; p = 0.83). The difference between treatments was not statistically significant (Figure 7).
FIGURE 7.
Deaths in postmenopausal women on ibandronic acid compared with placebo. M–H, Mantel–Haenszel.
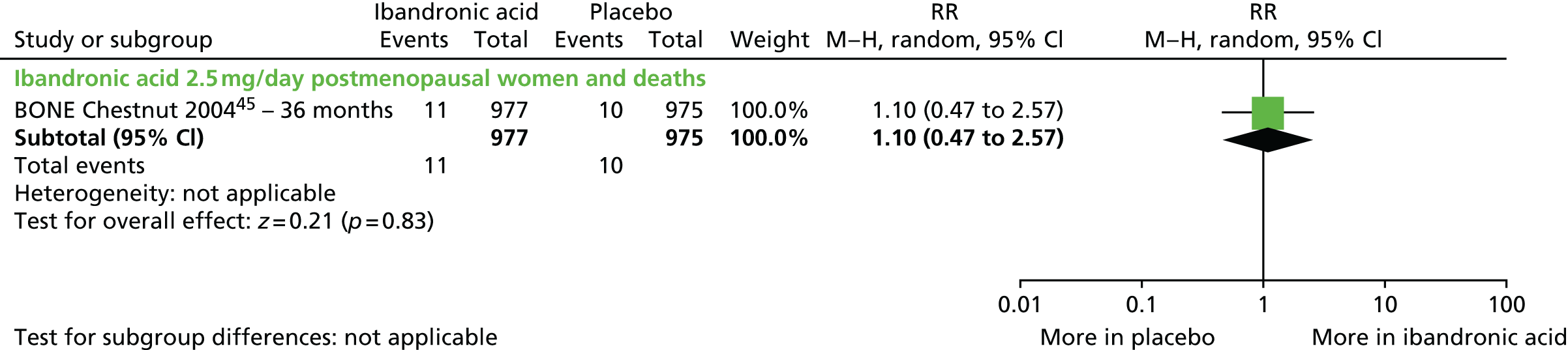
Risedronic acid
Boonen et al. 58 compared 35 mg per week of risedronic acid with placebo in osteoporotic men (risedronic acid, n = 191; placebo, n = 93). After 24 months, there were five deaths: two (1%) in the risedronic acid group and three (3%) in the placebo group (RR 0.32, 95% CI 0.06 to 1.91; p = 0.21). The difference between treatments was not statistically significant (Figure 8).
FIGURE 8.
Deaths of osteoporotic men on risedronic acid compared with placebo. M–H, Mantel–Haenszel.
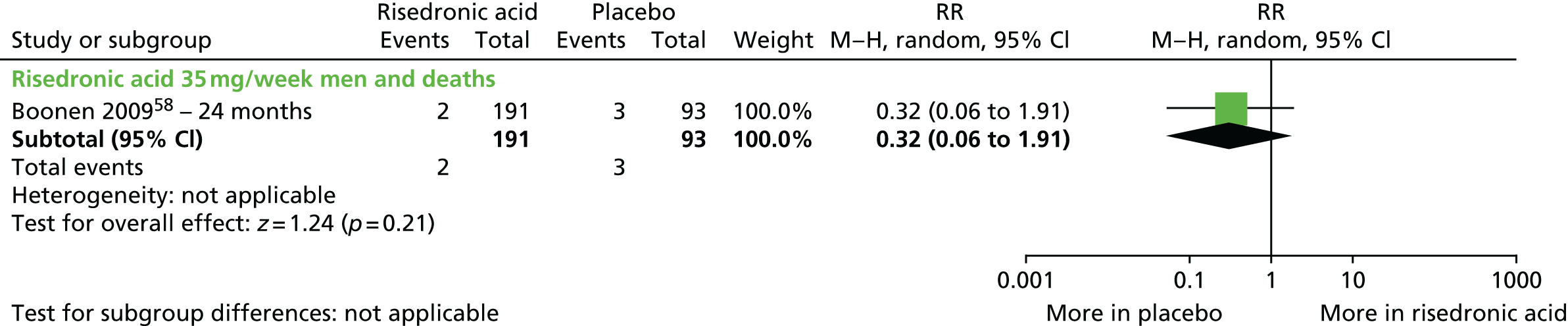
Zoledronic acid
Three RCTs reported mortality: Black et al. 56 compared 5 mg of zoledronic acid with placebo in postmenopausal women at 36 months; Boonen et al. 59 compared 5 mg of zoledronic acid with placebo in men for 36 months; and Lyles et al. 77 compared 5 mg of zoledronic acid with placebo in men and women following hip fracture at 36 months. The pooled number of deaths across these RCTs was 517, of which 246 (out of 5504; 4.5%) were in the 5 mg of zoledronic acid groups and 271 (out of 5520 participants,4.9%) were in the placebo groups (pooled RR 0.91, 95% CI 0.77 to 1.08; p = 0.28). The difference between treatments was not statistically significant; however, the difference between treatments for the HORIZON-RFT77 alone was statistically significant (p = 0.007), with a higher mortality rate in the placebo arm (Figure 9).
FIGURE 9.
Deaths of men or women on 5 mg per year of zoledronic acid compared with placebo. M–H, Mantel–Haenszel.
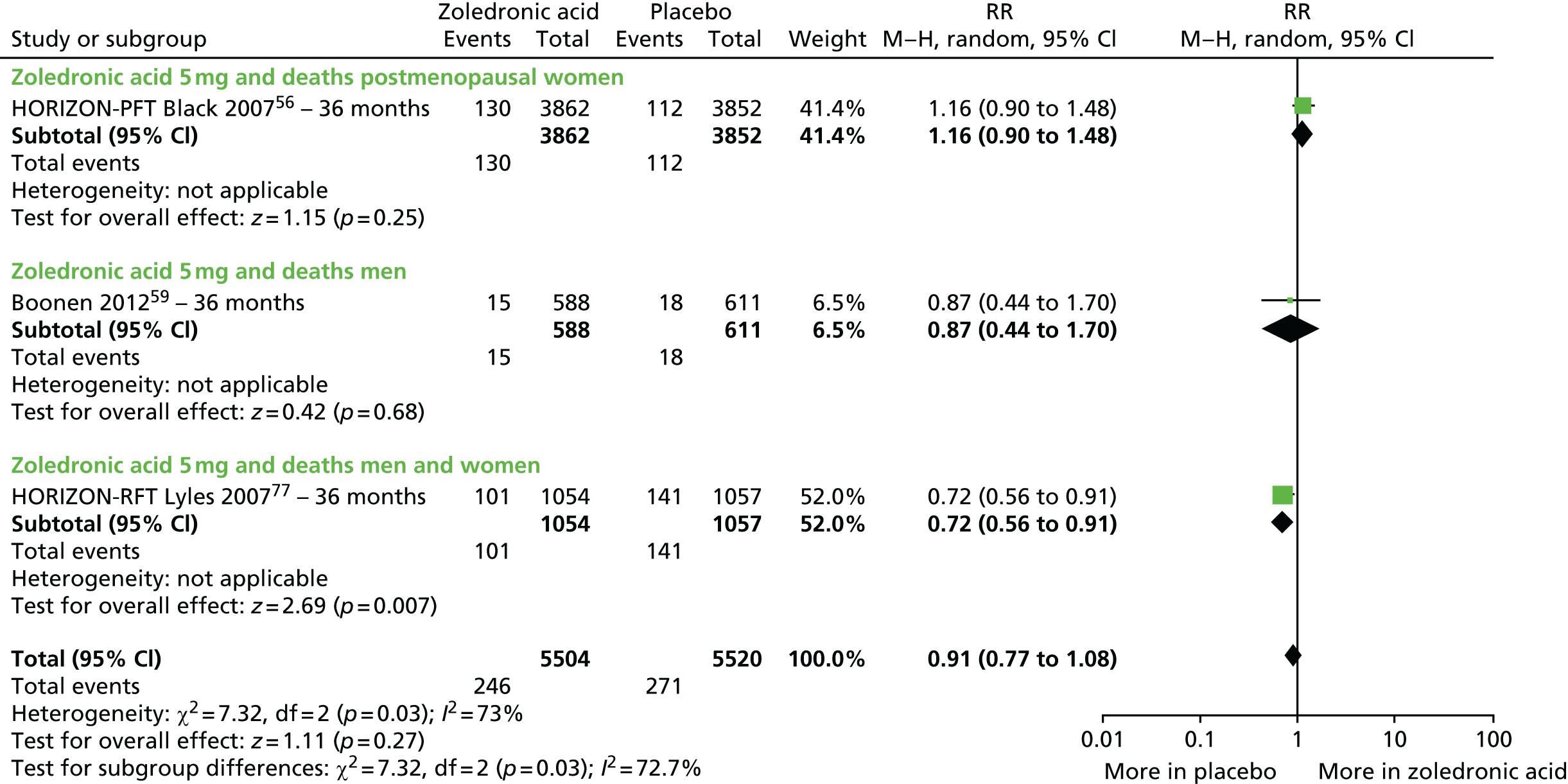
Head to head: zoledronic acid compared with risedronic acid
Reid et al. 88 compared 5 mg of zoledronic acid per year with 5 mg per day of risedronic acid for 12 months in both men and women receiving steroids and divided the participants into treatment of osteoporosis and prevention of osteoporosis subgroups. In the treatment subgroup the RR of mortality for zoledronic acid compared with risedronic acid was 0.33 (95% CI 0.04 to 3.20; p = 0.34) and in the prevention subgroup the RR of mortality was 3.06 (95% CI 0.13 to 74.57; p = 0.49). The differences between treatments were not statistically significant. A forest plot is not presented for this comparison.
Head to head: alendronic acid compared with ibandronic acid
One head-to-head RCT in postmenopausal women, comparing 70 mg per week of alendronic acid (n = 859) with 150 mg per month of oral ibandronic acid (n = 874), reported mortality at 12 months. 81 In total, six deaths were reported: two (0.2%) in the active treatment group and four (0.5%) in the placebo group (RR 0.51, 95% CI 0.09 to 2.77; p = 0.43) (Figure 10).
FIGURE 10.
Head-to-head comparison of 70 mg of alendronic acid with 150 mg of ibandronic acid in postmenopausal women and deaths. M–H, Mantel–Haenszel.
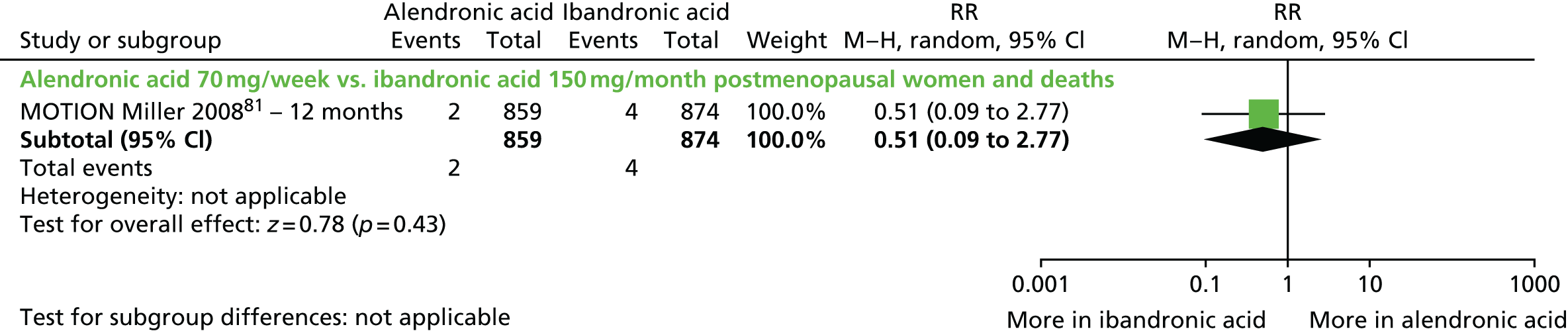
Adverse effects of treatment
Details of all AEs reported for alendronic acid, ibandronic acid, risedronic acid and zoledronic acid, across all included RCTs, are presented in Appendix 4, Table 47.
A total of 30 of the included RCTs reported AEs;20,45,55–59,64,66–70,72,76–81,83–85,88,90,91,100,102,104,105 of these, 25 reported on any AE45,55–59,66,67,69,70,72,77–81,83–85,87,88,90,91,104,105 and 19 reported on any serious AEs. 45,56–59,66,69,70,72,77,78,80,81,83–85,87,88,90 Twenty RCTs reported the number of participants withdrawing because of AEs45,55–58,64,66,69,70,72,76–78,80,83–85,87,88,90 and 20 reported data on upper GI events. 45,55–58,64,66,69,70,72,76–78,80,83–85,87,88,90 Six RCTs compared alendronic acid with placebo,55,57,64,67,83,84 six compared risedronic acid with placebo,58,66,70,72,78,85 one compared ibandronic acid (monthly oral dose) with placebo,80 one compared zoledronic acid with placebo,102 two compared alendronic acid with risedronic acid87,90 and one compared alendronic acid with zoledronic acid. 69 A total of 10 RCTs reported influenza-like symptoms,56,58,59,69,77,79–81,83,88 of which five evaluated zoledronic acid,56,59,77,79,88 one evaluated alendronic acid,83 one evaluated ibandronic acid (monthly oral dose)80 and one evaluated risedronic acid. 58 Two RCTs reporting influenza-like symptoms were head-to-head comparisons of 70 mg per week of alendronic acid with 150 mg per month of ibandronic acid 81 and 70 mg per week of alendronic acid with 5 mg per year of zoledronic acid. 69
Any adverse events, serious adverse events and withdrawals owing to adverse events
Five RCTs reported any AE associated with 10 mg of alendronic acid and placebo in postmenopausal women treated for periods ranging from 12 to 36 months. 55,57,67,84,91 Across these RCTs there were 3535 AEs; among participants on alendronic acid the incidence of AEs was 73.3% (1749/2384), compared with 76.5% (1786/2336) among those treated with placebo (pooled RR 0.98, 95% CI 0.90 to 1.06; p = 0.63). The difference between treatments was not statistically significant (Figure 11).
FIGURE 11.
Any AE in the alendronic acid group compared with placebo. M–H, Mantel–Haenszel.
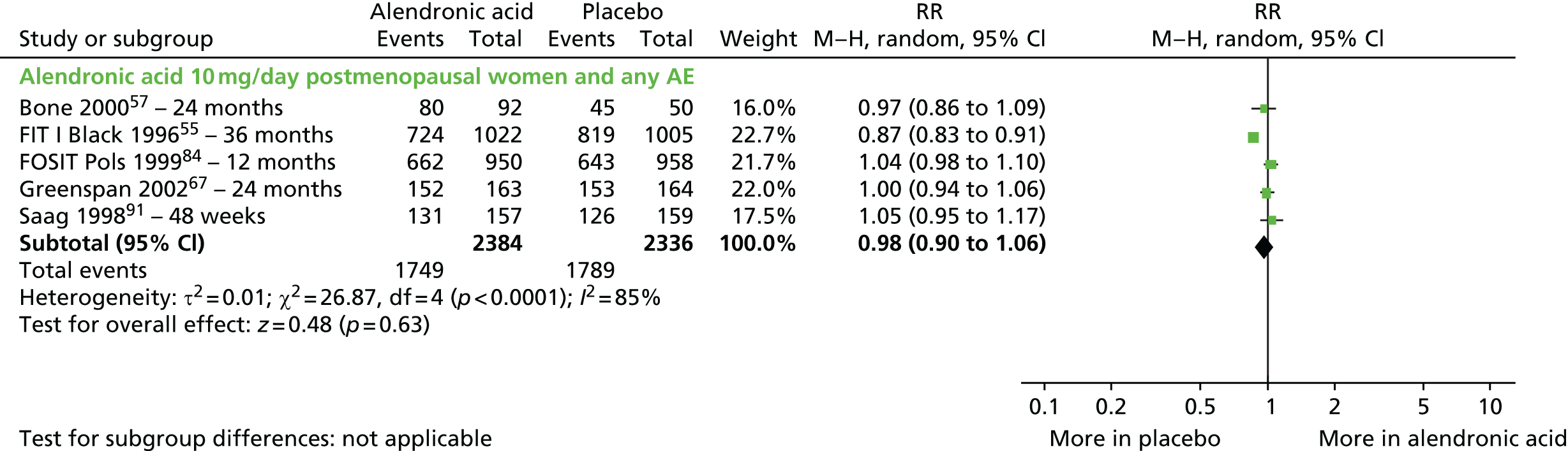
Three RCTs reported the proportion of AEs that were considered serious in postmenopausal women. 57,84,91 One reported events at 48 weeks,91 one at 12 months84 and one at 24 months. 57 One RCT in osteoporotic men reported events at 24 months. 83 Across the three RCTs in women, 205 serious AEs were observed: 103 (out of 1199 participants) in the alendronic acid groups (8.6%) and 102 (out of 1167 participants) in the placebo groups (8.7%) (pooled RR 0.96, 95% CI 0.74 to 1.25; p = 0.70). The difference between treatments was not statistically significant (Figure 12). In osteoporotic men, the number of AEs was not significantly different in different treatment groups (RR 0.80, 95% CI 0.48 to 1.32; p = 0.38). 83
FIGURE 12.
Any serious AE in the alendronic acid group compared with placebo. M–H, Mantel–Haenszel.

Seven RCTs reported on withdrawals as a result of AEs. 55,57,64,76,83,84,91 Across all RCTs the difference between treatments was not statistically significant. There were 807 withdrawals in total, and the incidence was 7.8% (376/4777) in the alendronic acid groups, compared with 8.8% (431/4882) in the placebo groups (pooled RR 0.86, 95% CI 0.73 to 1.07; p = 0.07). There was no statistically significant between-group difference across six RCTs in postmenopausal women55,57,64,84,91 ranging in duration from 48 weeks91 to 48 months. 64 There were 793 withdrawals in total and the incidence was 8.0% (372/4631) in the alendronic acid groups, compared with 8.8% (421/4787) in placebo groups (pooled RR 0.90, 95% CI 0.79 to 1.03; p = 0.13). However, in osteoporotic men, placebo treatment was associated with a significantly higher rate of withdrawals at 24 months (10/95, 10.5%) than alendronic acid (4/146, 2.7%) (RR 0.26, 95% CI 0.08 to 0.81; p = 0.02). 83 However, a statistically significant difference between treatments was not evident when RCTs were pooled by RCT duration (p = 0.68) (Figure 13).
FIGURE 13.
Withdrawals as a result of an AE in the alendronic acid group compared with placebo. M–H, Mantel–Haenszel.
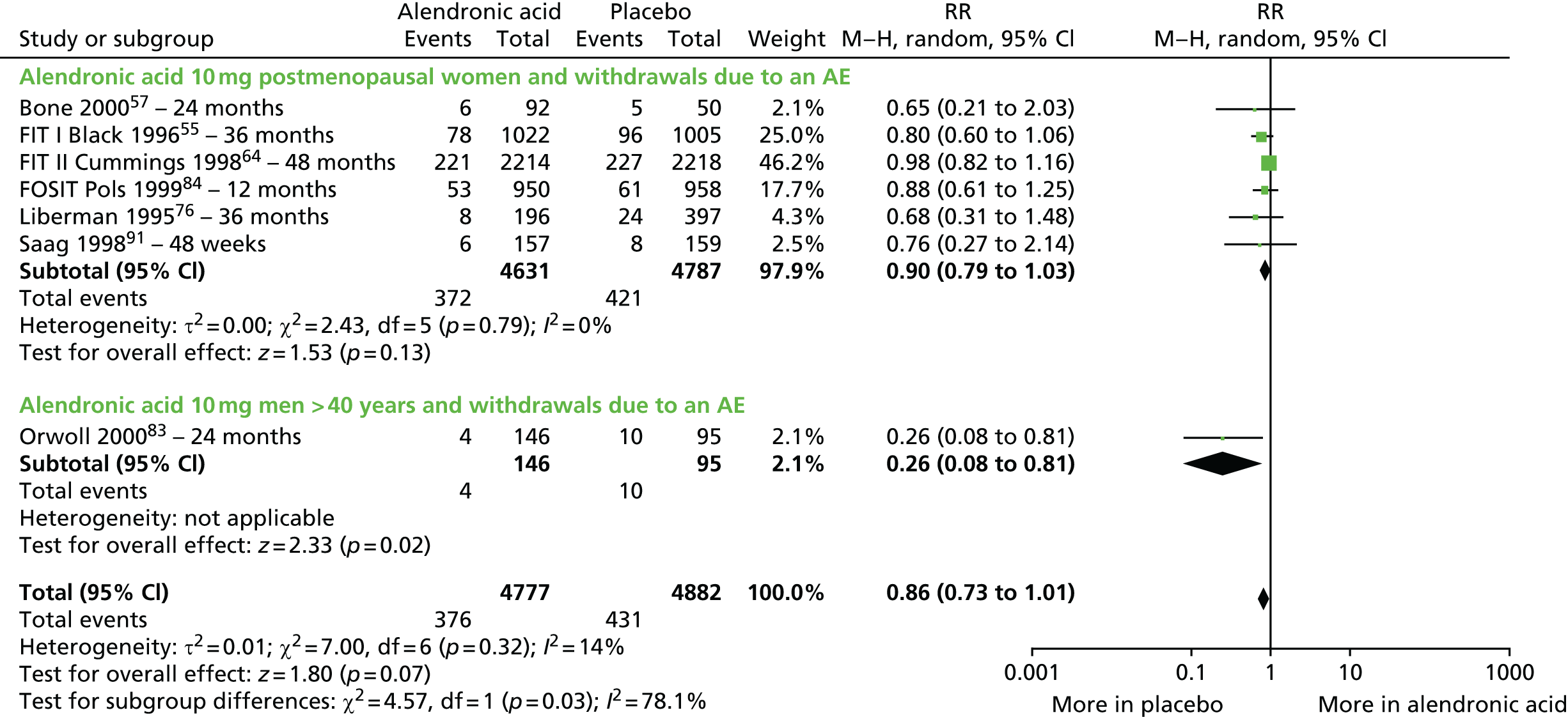
Both Chesnut et al. 45 and McClung et al. 80 reported on the number of AEs of any type in an ibandronic acid group (unlicensed 2.5 mg daily oral dose45 and licensed monthly 150 mg oral dose80) and a placebo group. Both recruited postmenopausal women and follow-up duration was 36 months and 12 months, respectively. The proportion of participants who experienced any AE did not differ by treatment group. A total of 1870 participants experienced AEs, 939 (out of 1054 participants; 89.9%) in the ibandronic acid groups and 931 (out of 1058 participants; 88.0%) in the placebo groups (pooled RR 1.01, 95% CI 0.98 to 1.04; p = 0.45), and this did not vary by dosage of ibandronic acid (p = 0.99) (Figure 14).
FIGURE 14.
Any AE in the ibandronic acid group compared with placebo. M–H, Mantel–Haenszel.

The same RCTs45,80 also reported the number of AEs that were considered serious. The difference between treatments across these trials was not statistically significant. A total of 449 participants experienced serious AEs: 237 (out of 1054 participants; 22.5%) in the ibandronic acid groups and 212 (out of 1058 participants; 20.0%) in the placebo groups (pooled RR 1.11, 95% CI 0.95 to 1.31; p = 0.20). The difference between treatments by dosage was also not statistically significant (Figure 15).
FIGURE 15.
Any serious AE in the ibandronic acid group compared with the placebo group. M–H, Mantel–Haenszel.

The same RCTs also reported the number of withdrawals as a result of AEs. 45,80 Overall, the proportion of withdrawals was similar among participants who were on ibandronic acid [17.8% (188/1054)] and those on placebo [17.6% (186/1058)] (374 AEs in total; pooled RR 1.24, 95% CI 0.56 to 2.75; p = 0.59). The difference between treatments across these RCTs was not statistically significant and results did not vary by ibandronic acid dosage (p = 0.17) (Figure 16).
FIGURE 16.
Withdrawals as a result of an AE in the ibandronic acid group compared with the placebo group. M–H, Mantel–Haenszel.
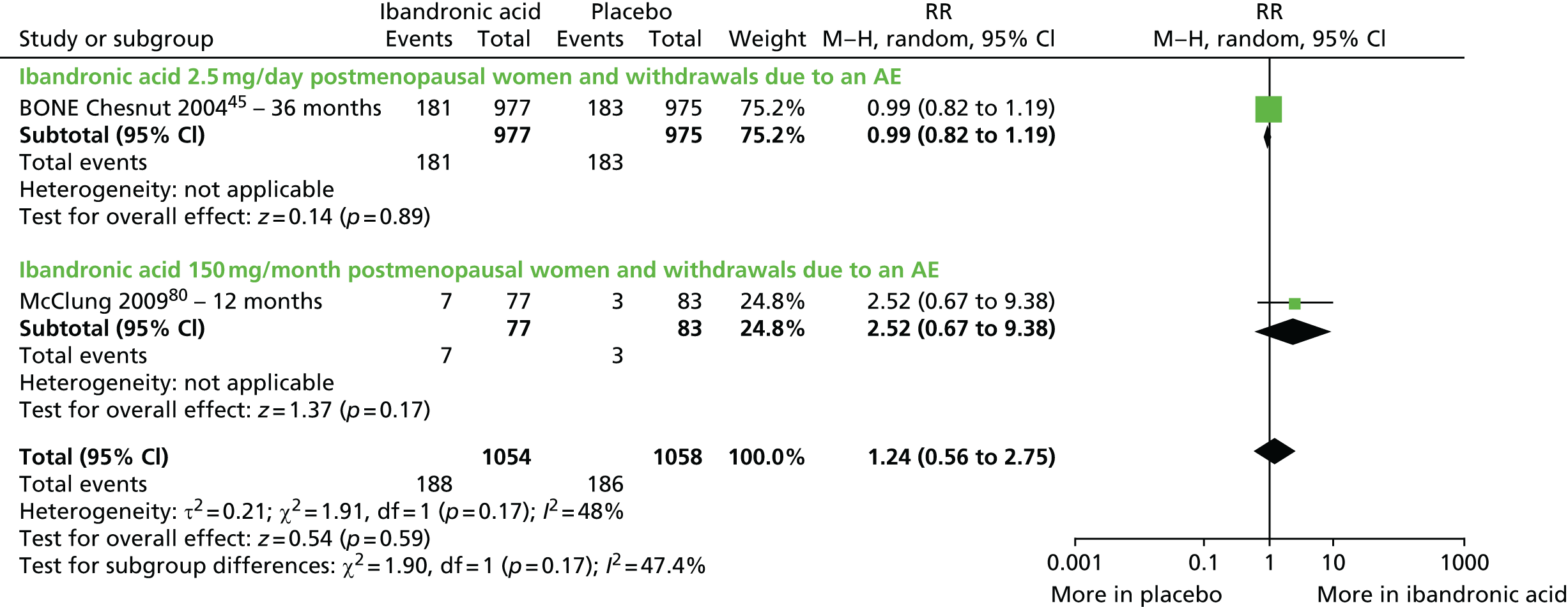
Six RCTs compared AEs in a risedronic acid-treated group and a placebo group. 58,66,70,72,78,85 Five of these were in postmenopausal women, with treatment duration ranging from 12 to 24 months,66,70,72,78,85 and one was in osteoporotic men with a follow-up at 24 months. 58 Pooled data across all six RCTs (8674 AEs) showed that the proportion of participants experiencing an AE was the same in the risedronic acid group [90.6% (4370/4821)] and the placebo group [90.5% (4304/4754)] (pooled RR 0.95, 95% CI 0.84 to 1.08; p = 0.44). The difference between treatments was not statistically significant and the results did not vary by age, sex, drug dosage (p = 0.67) or duration of follow-up (p = 0.64) (Figure 17).
FIGURE 17.
Any AE in the risedronic acid group compared with the placebo group. BMD-NA, Bone Mineral Density – North America trial; M–H, Mantel–Haenszel.
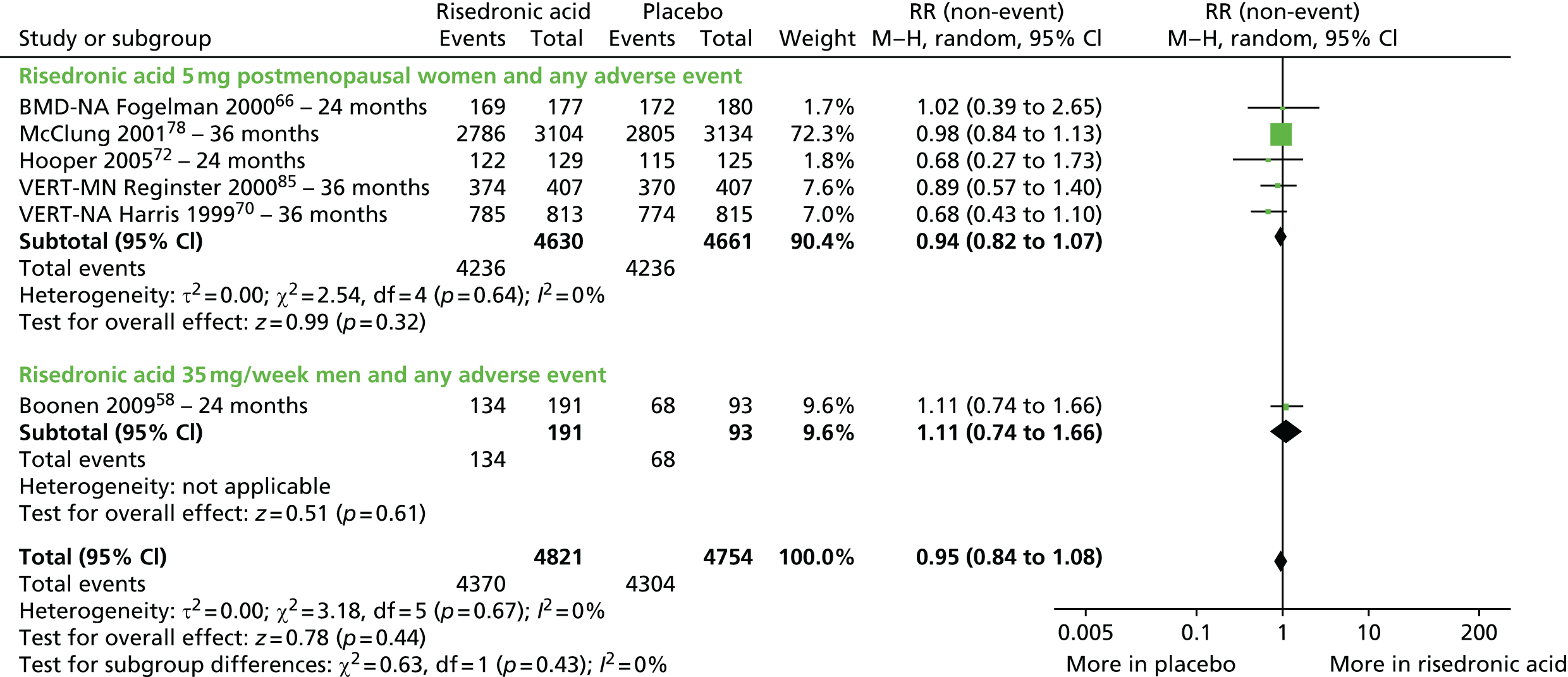
Pooled data from the same RCTs revealed that the proportions of participants experiencing serious AEs was also similar in both treatment groups; of the total of 2789 serious AEs reported, 1398 occurred in the risedronic acid group (of 4821 participants; 29.0%) and 1391 (of 4754 participants; 29.3%) in the placebo group (pooled RR 1.01, 95% CI 0.93 to 1.11; p = 0.76). The difference between treatments was not statistically significant. There were no statistically significant differences between treatments evident by age, sex or dosage (p = 0.27) or treatment duration (p = 0.18) (Figure 18).
FIGURE 18.
Any serious AE in the risedronic acid group compared with the placebo group. BMD-NA, Bone Mineral Density – North America trial; M–H, Mantel–Haenszel.
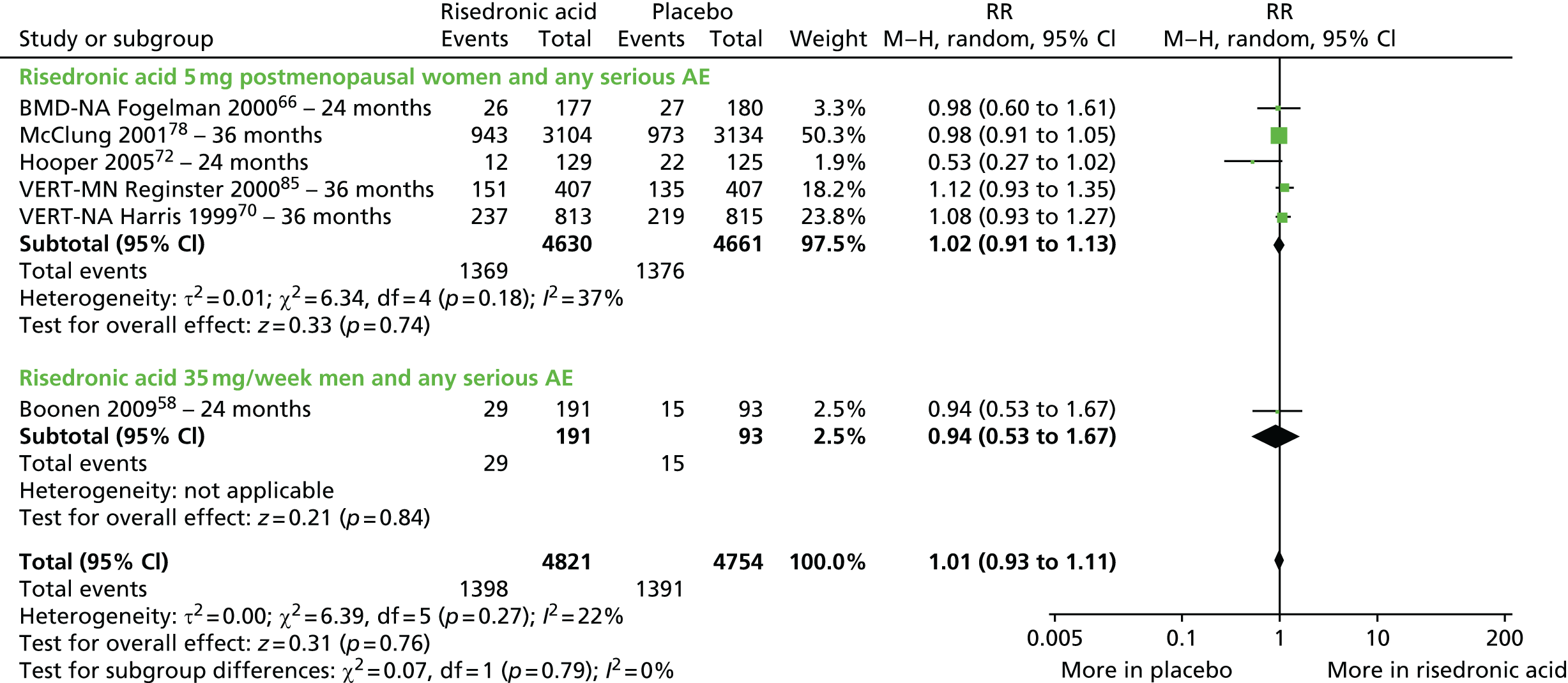
Pooled data from these six RCTs58,66,70,72,78,85 also showed that there were no statistically significant differences between treatments in withdrawals as a result of AEs [1596 withdrawals: 784 (of 4820 participants; 16.3%) in the risedronic acid group and 812 (of 4754 participants; 17.1%) in the placebo group (pooled RR 0.94, 95% CI 0.81 to 1.10; p = 0.45). However, the difference between treatments for the one RCT in osteoporotic men with follow-up at 24 months58 was statistically significant (p = 0.05) (Figure 19).
FIGURE 19.
Withdrawals as a result of an AE in the risedronic acid group compared with placebo. BMD-NA, Bone Mineral Density – North America trial; M–H, Mantel–Haenszel.

Four RCTs reported AEs for zoledronic acid compared with placebo. 56,59,77,79 Two RCTs evaluated postmenopausal women who were followed up for 3656 and 24 months,79 one evaluated men and women with hip fracture who were followed up for 36 months77 and one RCT evaluated osteoporotic men who were followed up for 36 months. 59
Pooled data across the two RCTs in postmenopausal women56,79 showed that zoledronic acid was associated with a statistically significant increase in the incidence of AEs (total 7663 AEs): the incidence was 94.5% (3861/4043) in the zoledronic acid group and 93.8% (3802/4054) in the placebo group (pooled RR 1.02, 95% CI 1.01 to 1.03; p = 0.0007). In one RCT in osteoporotic men,59 in which a total of 1000 AEs were reported, the incidence of AEs was 19% higher in the zoledronic acid group [90.8% (534 AEs in 588 participants)] than in the placebo group [76.3% (466 AEs in 611 participants) (RR 1.19, 95% CI 1.13 to 1.25; p < 0.00001]; the difference between treatments was statistically significant. Another RCT in men and women found no statistically significant difference between treatments. 77 A total of 1719 AEs effects were reported, 867 (in 1054 participants; 82.3%) in the zoledronic acid group and 852 (in 1057 participants; 80.6%) in the placebo group (RR 1.02, 95% CI 0.98 to 1.02; p = 0.33). Pooled data across all four RCTs indicated that the incidence of AEs did not differ significantly by treatment group. The total number of AEs was 10,382, and 92.5% of study participants (5262/5685) treated with zoledronic acid group experienced an AE, compared with 89.5% of placebo-treated participants (5120/5722) (pooled RR 1.06, 95% CI 1.00 to 1.13; p = 0.06) (Figure 20).
FIGURE 20.
Any AE in the zoledronic acid group compared with placebo. M–H, Mantel–Haenszel.

The number of serious AEs was reported by four RCTs. 56,59,77,88 Across these RCTs the difference between treatments was not statistically significant. There were a total of 3427 serious AEs: 1679/5504 (30.5%) in the zoledronic acid groups and 1748/5520 (32.2%) in the placebo groups (pooled RR 0.96, 95% CI 0.91 to 1.02; p = 0.16). The incidence of serious AEs did not differ by sex (p = 0.86) or by RCT duration (p = 0.68) (Figure 21).
FIGURE 21.
Any serious AE in the zoledronic acid group compared with placebo. M–H, Mantel–Haenszel.

Two RCTs reported data on withdrawals as a result of AEs. 56,77 Pooled data across these RCTs showed that the rates of withdrawal were similar in the two treatment groups. There were a total of 189 withdrawals: 101/4961 (2.0%) in the 5 mg per year of zoledronic acid group and 88/4909 (1.8%) in the placebo groups (pooled RR 1.15, 95% CI 0.86 to 1.52; p = 0.35). The difference between treatments was not statistically significant. The number of withdrawals was the same for both sexes (p = 0.12) (Figure 22).
FIGURE 22.
Withdrawals as a result of an AE in the zoledronic acid group compared with placebo. M–H, Mantel–Haenszel.

The MOTION trial81 compared 70 mg per week of alendronic acid with 150 mg per month of oral ibandronic acid in postmenopausal women for 12 months. The total number of AEs was 1291 and the proportion of participants experiencing an AE was higher in the alendronic acid group than in the ibandronic acid group [75.4% (659/859) versus 73.6% (632/874); RR 1.06, 95% CI 1.0 to 1.12; p = 0.04]; the difference between treatments was statistically significant (Figure 23).
FIGURE 23.
Head-to-head comparison of alendronic acid with ibandronic acid and any AE. M–H, Mantel–Haenszel.

Two RCTs compared 70 mg per week of alendronic acid with 35 mg per week of risedronic acid in postmenopausal women treated for 12 months. 87,90 Pooled data across these RCTs indicate that the risk of AEs was similar in both groups. The total number of AEs was 1413 and the proportion of participants experiencing an AE was 71.2% (700/983) in the alendronic acid group and 71.7% (713/995) in the risedronic acid group (pooled RR 1.0, 95% CI 0.94 to 1.05; p = 0.93); the difference between treatments was not statistically significant (Figure 24).
FIGURE 24.
Head-to-head comparison of alendronic acid with risedronic acid and any AE. M–H, Mantel–Haenszel.

The Rapid Onset and Sustained Efficacy (ROSE) trial69 compared 70 mg per week of alendronic acid with 5 mg per year of zoledronic acid. The total number of AEs was 465 and the risk of AEs was similar in the two treatment groups [74.7% (145/194) in the alendronic acid group, compared with 78.4% (320/408) in the zoledronic acid group; RR 0.95, 95% CI 0.87 to 1.05; p = 0.33]; the difference between treatments was not statistically significant (Figure 25).
FIGURE 25.
Head-to-head comparison of alendronic acid with zoledronic acid and any AE. M–H, Mantel–Haenszel.
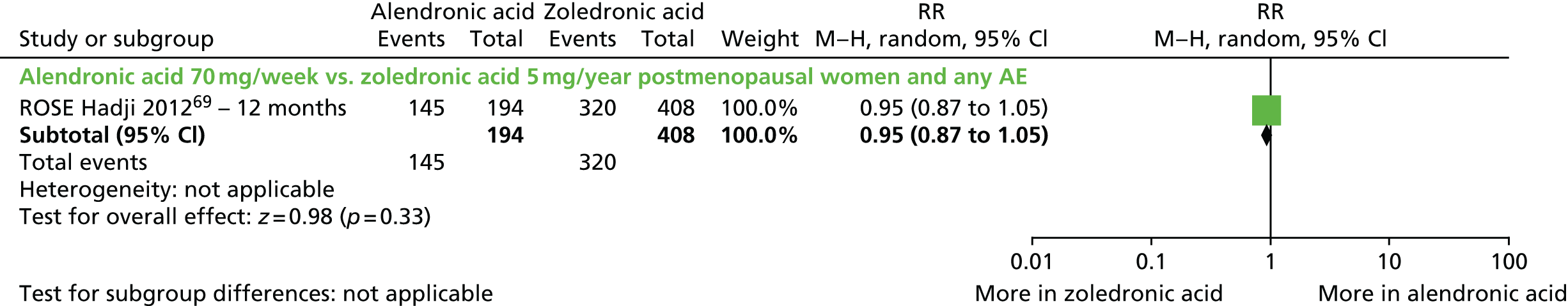
The HORIZON trial88 compared 5 mg per year of zoledronic acid with 5 mg per day of risedronic acid in both men and women receiving steroids; the participants were divided into treatment and prevention subgroups for 12 months. The difference between treatments in any AE in the treatment subgroup was a RR of 1.14 (95% CI 1.06 to 1.26; p = 0.01) and the difference between treatments in the prevention subgroup was a RR of 1.19 (95% CI 1.03 to 1.26; p = 0.01), with more AEs in the zoledronic acid groups in both cases. The differences between treatments were statistically significant. No forest plot is presented for these data.
Serious adverse events
The MOTION trial81 also reported the number of AEs experienced by participants receiving weekly alendronic acid (n = 859) or monthly oral ibandronic acid (150 mg/month) (n = 874). There were 94 serious AEs and the risk of experiencing a serious AE was similar in the two groups [4.5% (39/859) in the alendronic acid group and 6.4% (55/874) in the ibandronic acid group; RR 0.72, 95% CI 0.48 to 1.08; p = 0.11]. The difference between treatments was not statistically significant (Figure 26).
FIGURE 26.
Head-to-head comparison of alendronic acid with ibandronic acid and any serious AE. M–H, Mantel–Haenszel.

Pooled data across two RCTs87,90 indicate no statistically significant difference in the incidence of serious AEs between the two treatments [157 serious AEs; 7.0% (69/983) in the alendronic acid group and 8.8% (88/995) in the risedronic acid group; RR 0.76, 95% CI 0.35 to 1.66; p = 0.50] (Figure 27).
FIGURE 27.
Head-to-head comparison of alendronic acid with risedronic acid and any serious AE. M–H, Mantel–Haenszel.

In the ROSE trial,69 the proportion of participants experiencing a serious AE was not significantly different between the group receiving 70 mg per week of alendronic acid and the group receiving 5 mg per year of zoledronic acid [64 serious AEs: 21/194 (10.8%) in the alendronic acid group and 43/403 (10.5%) in the zoledronic acid group; RR 1.03, 95% CI 0.63 to 1.68, p = 0.92] (Figure 28).
FIGURE 28.
Head-to-head comparison of alendronic acid with zoledronic acid and any serious AE. M–H, Mantel–Haenszel.
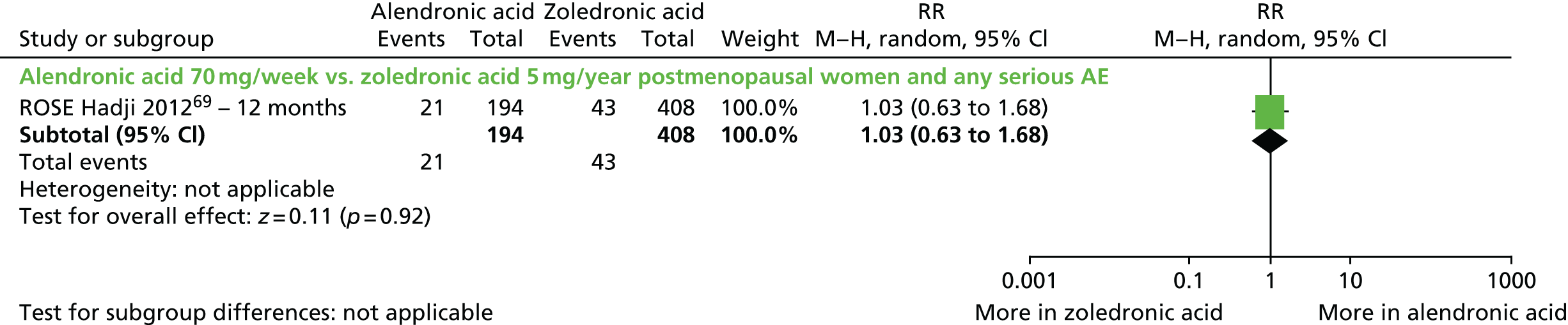
In the HORIZON trial,88 in which men and women receiving steroids were divided into treatment and prevention subgroups for 12 months, the difference between treatments in serious AEs in the treatment subgroup was a RR of 0.93 (95% CI 0.66 to 1.31; p = 0.68) and the difference between treatments in the prevention subgroup was a RR of 1.13 (95% CI 0.68 to 1.88; p = 0.64). The differences between treatments were not statistically significant. No forest plot is presented for these data.
Withdrawals as a result of adverse events
Two RCTs reported withdrawals as a result of AEs. 87,90 Pooled data across these RCTs indicate no statistically significant difference between treatments [114 withdrawals: 53/983 (5.4%) in the alendronic acid group and 61/995 (6.1%) in the risedronic acid group; pooled RR 0.88, 95% CI 0.62 to 1.26; p = 0.50] (Figure 29).
FIGURE 29.
Head-to-head comparison of alendronic acid with risedronic acid and withdrawals as a result of AEs. M–H, Mantel–Haenszel.
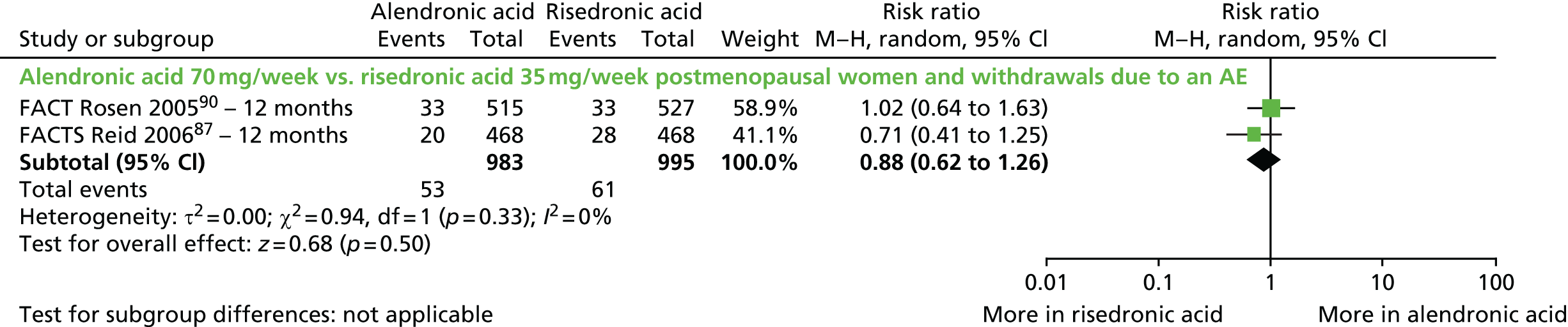
The difference between treatments in withdrawals as a result of AEs was statistically significant for one trial69 comparing 70 mg per week of alendronic acid with 5 mg per year of zoledronic acid [21 withdrawals: 19/194 (9.8%) in the alendronic acid group and (2/408 (0.5%) in the zoledronic acid group; RR 19.98, 95% CI 4.70 to 84.92; p < 0.0001] (Figure 30).
FIGURE 30.
Head-to-head comparison of alendronic acid with zoledronic acid and withdrawals as a result of AEs. M–H, Mantel–Haenszel.
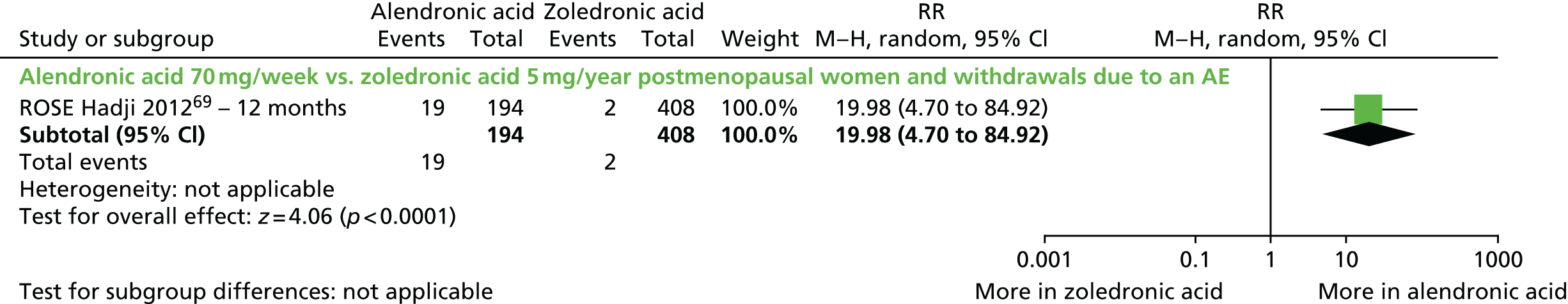
In the HORIZON trial,88 in which men and women receiving steroids were divided into treatment and prevention subgroups for 12 months, the difference between treatments in the number of withdrawals as a result of AEs was a RR of 1.00 (95% CI 0.20 to 4.93; p = 1.00) in the treatment subgroup and a RR of 2.00 (95% CI 0.51 to 7.84; p = 0.32) in the prevention subgroup; the differences between treatments were not statistically significant. No forest plot is presented for these data.
Any upper gastrointestinal adverse events
The types of upper GI events greatly varied in different RCTs. Among six RCTs55,57,64,76,83,84 that investigated alendronic acid and reported specific AEs (1738 upper GI events), abdominal pain was the most common, accounting for 32% (557/1738) of all upper GI events, followed by acid regurgitation at 17.5% (304/1738), dyspepsia at 11.2% (195/1738) and nausea at 8.1% (140/1738). Other events included peptic ulcers (i.e. oesophageal and stomach ulcers), gastritis, oesophagitis, belching, diarrhoea, dysphagia, constipation, heartburn and gastroenteritis.
In the six RCTs that administered 5 mg of risedronic acid (1076 upper GI events),66,70,72,78,85 abdominal pain was also the most common, accounting for 43.1% (464/1076) of all upper GI events, followed by dyspepsia (38.9%, 419/1076), oesophagitis (7.6%, 82/1076) and gastritis (4.0%, 43/1076). Similar results were observed in the BONE trial45 and the trial by McClung et al. ,80 in which abdominal pain and dyspepsia were the most common upper GI events, accounting for 11.4% (111/977) of upper GI events in the group receiving 5 mg ibandronic acid daily and 31.2% (24/77) in the group receiving 150 mg ibandronic acid monthly. Of the 300 upper GI events occurring in participants on 5 mg of zoledronic acid in two RCTs,88,102 nausea was the major event, with 168 reports (56.0%), followed by vomiting with 76 (25.3%), diarrhoea with 67 (22.3%), abdominal pain with 48 (16.0%) and anorexia with 45 (15.0%). However, the proportion of these upper GI events was similar in the treatment group and the placebo group, but less frequent in the zoledronic acid group. 102
Six RCTs reporting upper GI AEs evaluated 10 mg per day of alendronic acid in postmenopausal women55,57,64,67,84,91 and one investigated 10 mg per day of alendronic acid in men with osteoporosis. 83
Pooled data across all seven RCTs indicated no statistically significant difference between treatments in the incidence of upper GI AEs. There were a similar incidence of upper GI events reported in patients receiving alendronic acid (38.6%) and placebo (37.6%) when pooling data across trials (pooled RR 1.03, 95% CI 0.98 to 1.08; p = 0.30) (Figure 31). There was also no statistically significant difference between treatments according to sex (see Figure 31) or RCT duration (p = 0.83).
FIGURE 31.
Any upper GI AEs in the alendronic acid group compared with placebo. M–H, Mantel–Haenszel; UGI, upper GI.
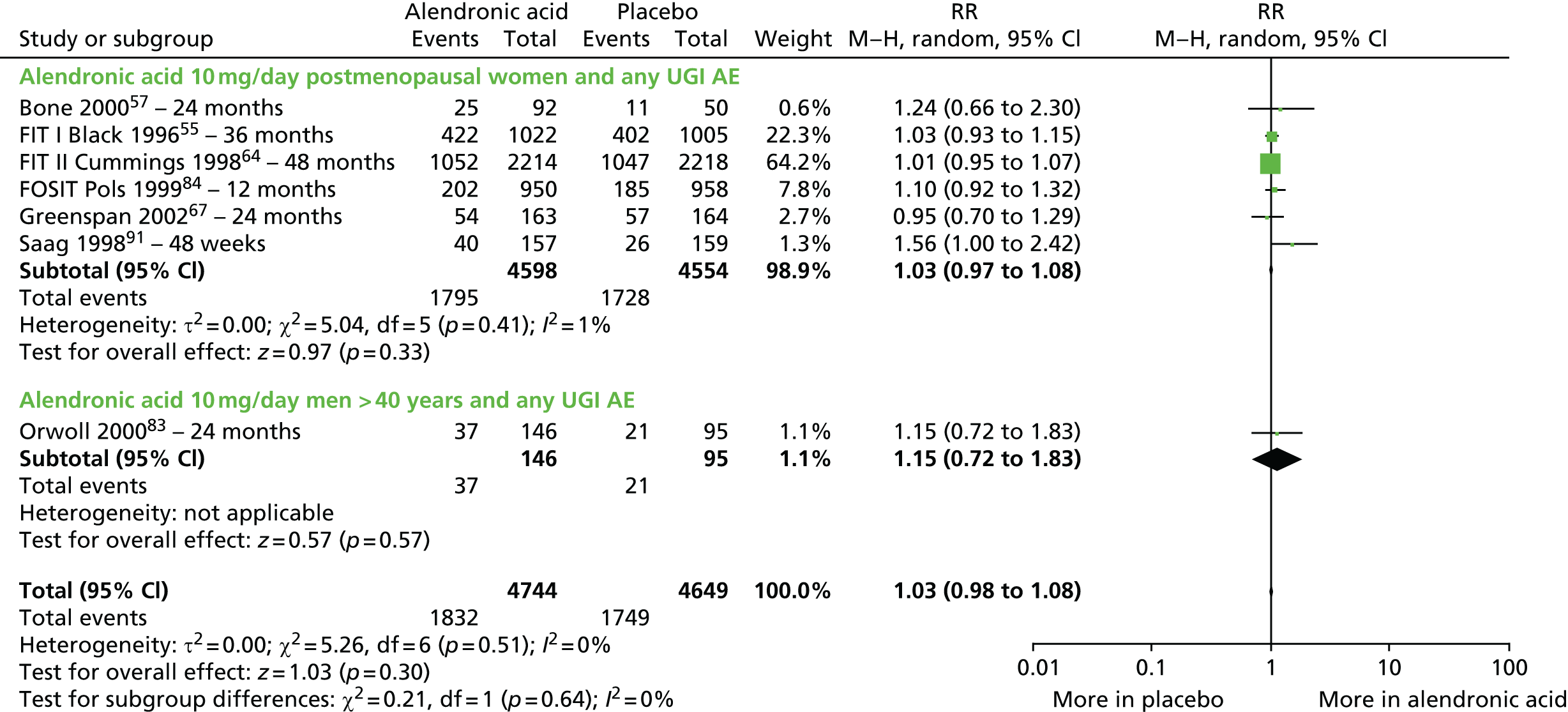
Only one trial80 using ibandronic acid reported upper GI events. The difference between treatments was not statistically significant [44 upper GI events: 24/77 (31.2%) in the ibandronic acid (monthly oral dose) group and 20/83 (24.1%) in the placebo group; RR 1.29 95% CI 0.78 to 2.15; p = 0.32] (Figure 32).
FIGURE 32.
Any upper GI AEs in the ibandronic acid group compared with placebo. M–H, Mantel–Haenszel; UGI, upper GI.
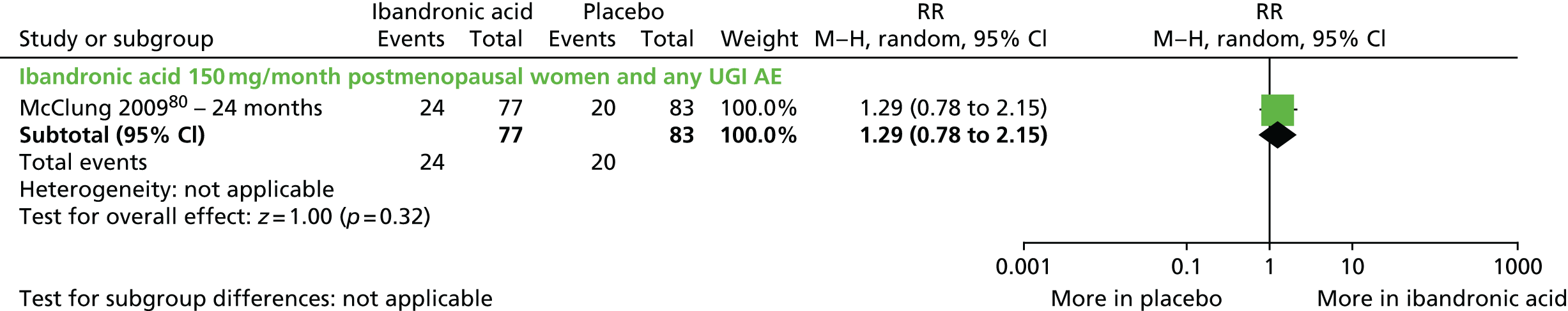
Five RCTs evaluated 5 mg per day of risedronic acid in postmenopausal women66,70,72,78,85 and one evaluated 35 mg per week of risedronic acid in osteoporotic men. 58 Pooled data across the five RCTs in postmenopausal women showed that the overall risk of upper GI AEs was similar in the two treatment groups [2150 upper GI events: 1076/4630 (23.2%) in the risedronic acid group and 1074/4661 (23.0%) in the placebo group; pooled RR 1.04, 95% CI 0.97 to 1.13; p = 0.75]. The difference between treatments was not statistically significant. Pooled results across all the six RCTs showed that there was no statistically significant difference between treatments in upper GI events in the risedronic acid treatment group or placebo [2183 upper GI events; 1092/4821 (22.7%) in the risedronic acid group and 1091/4754 (22.9%) in the placebo group; RR 0.99, 95% CI 0.87 to 1.14; p = 0.93] and this did not vary with RCT duration (p = 0.45). However, in the RCT in osteoporotic men,58 in which 33 upper GI events were reported, the risk was significantly higher (16/191, 8.4%) in the risedronic acid group than in the placebo group (19/93, 20.4%) (RR 0.46, 95% CI 0.24 to 0.87; p = 0.02) (Figure 33).
FIGURE 33.
Any upper GI AEs in the risedronic acid group compared with placebo. M–H, Mantel–Haenszel; UGI, upper GI.

Pooled data across two RCTs87,90 revealed no statistically significant difference in the number of upper GI events between the alendronic acid treatment group and the risedronic acid treatment group [411 upper GI events: 211/983 (21.5%) in the alendronic acid group and 00/995 (20.1%) in the risedronic acid group; pooled RR 1.07, 95% CI 0.90 to 1.27; p = 0.45] (Figure 34).
FIGURE 34.
Any upper GI AEs in the alendronic acid group compared with the risedronic acid group. M–H, Mantel–Haenszel; UGI, upper GI.
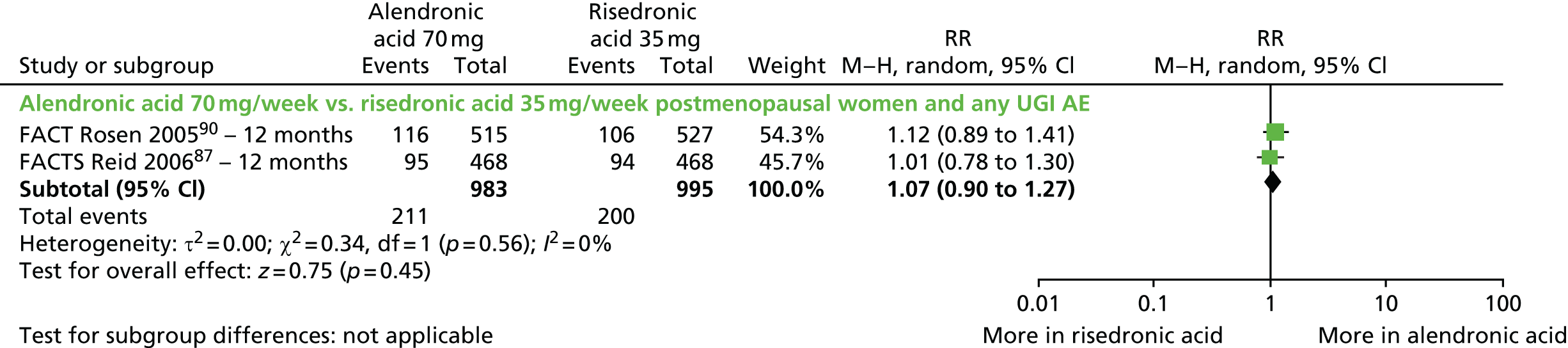
One RCT reporting upper GI events as an outcome69 found a significantly higher incidence of upper GI events in the 70 mg per week alendronic acid treatment group than in the 5 mg per year zoledronic acid group [132 upper GI events: 57/194 (29.4%) in the alendronic acid group, compared with 75/408 (18.4%) in the zoledronic acid group; RR 1.60, 95% CI 1.19 to 2.16; p = 0.002] (Figure 35).
FIGURE 35.
Any upper GI AEs in the alendronic acid group compared with the zoledronic acid group.

The HORIZON trial88 compared 5 mg per year of zoledronic acid with 5 mg per day of risedronic acid in both men and women receiving steroids and divided the participants into treatment and prevention subgroups for 12 months. The p-values for the differences between treatments in upper GI AEs reported between the treatment subgroup were as follows: upper abdominal pain, p = 0.158; abdominal pain, p = 0.16; dyspepsia, p = 0.70; nausea, p = 0.19; vomiting, p = 0.04; gastritis, p = 0.68; and gastro-oesophageal reflux, p = 0.37. The p-values for the differences between treatments reported between the prevention subgroup were as follows: upper abdominal pain, p = 1.00; abdominal pain, p = 1.00; dyspepsia, p = 0.57; nausea, p = 0.52; vomiting, p = 1.00; gastritis, p = 1.00; and gastro-oesophageal reflux, p = 0.44.
Any gastrointestinal event
In HORIZON-PFT102 the proportion of participants experiencing any GI event (abdominal pain, anorexia, diarrhoea, nausea, vomiting) in the first 3 days following i.v. administration was significantly higher in the zoledronic acid group than in the placebo group [380 GI events: 300/3862 (7.8%) in the zoledronic acid group and 80/3852 (2.1%) in the placebo group; RR 3.74, 95% CI 2.93 to 4.77; p < 0.00001] (Figure 36).
FIGURE 36.
Any GI AEs in the zoledronic acid group compared with placebo. M–H, Mantel–Haenszel; UGI, upper GI.
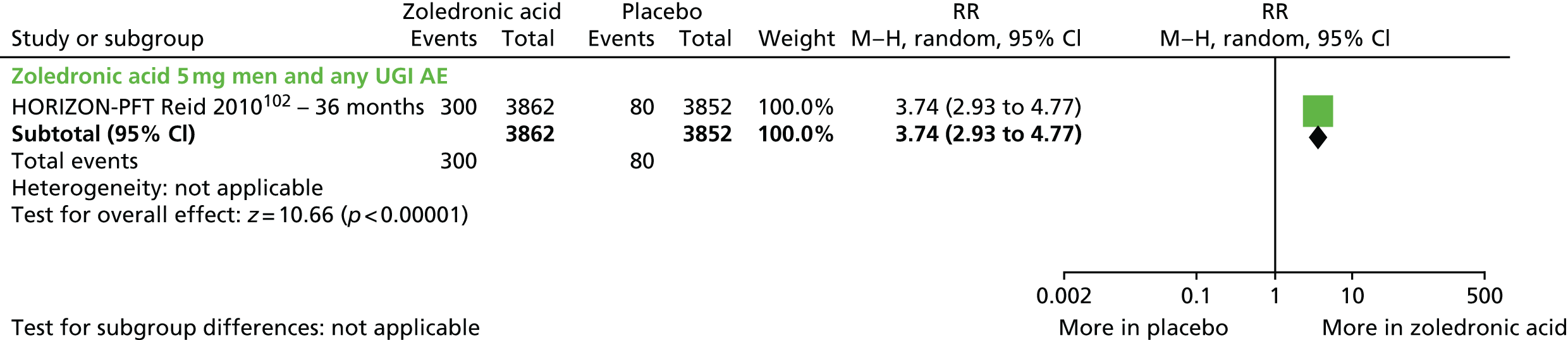
Influenza-like symptoms
The reporting of influenza-like symptoms, including upper respiratory infections, influenza, pyrexia, headache, chills, nasopharyngitis, bronchitis, pneumonia, cough and fatigue, varied across RCTs. Some RCTs reported only the occurrence of influenza-type symptoms, whereas others documented a number of potentially associated symptoms.
One RCT83 reported the incidence of influenza-like symptoms in osteoporotic men: 146 treated with alendronic acid and 95 treated with placebo. 83 Overall, 113 participants experienced influenza-like symptoms [66/146 (45.2%) in the alendronic acid group and 47/95 (49.5%) in the placebo group; RR 0.91, 95% CI 0.70 to 1.20; p = 0.51]. The difference between treatments was not statistically significant.
In the RCT by McClung et al. ,80 4.8% (4/83) of participants receiving 150 mg per month of oral ibandronic acid developed influenza-like symptoms, whereas none of the 83 (0%) participants receiving placebo developed symptoms. The difference between treatments was not statistically significant (p = 0.12).
Boonen et al. 58 reported the number of participants treated with 35 mg per week of risedronic acid or placebo who developed influenza and nasopharyngitis. The differences between treatment groups were not statistically significant. There were 15 influenza cases: 11 (among 191 participants, 5.8%) in the risedronic acid group and five (among 93 participants, 5.4%) in the placebo group (RR 1.07, 95% CI 0.38 to 2.99; p = 0.90). There were also 15 cases of nasopharyngitis: 11/191 (5.8%) in the risedronic acid group and 5/93 (5.4%) in the placebo group (RR 1.07, 95% CI 0.38 to 2.99; p = 0.90).
Five of the included RCTs using zoledronic acid reported on influenza-like symptoms. 56,59,77,79,88 Across these RCTs, zoledronic acid was associated with a significantly higher incidence of pyrexia, headache and chills than placebo. There were 1048 reports of pyrexia: 907 (in 5957 participants, 15.2%) in the zoledronic acid group and 141 (among 5866 participants, 2.4%) in the placebo group (pooled RR 4.36, 95% CI 1.91 to 9.98; p < 0.0005) (Figure 37). There were 554 cases of headache: 405 (among 4903 participants, 8.3%) in the zoledronic acid group and 149 (among 4809 participants, 3.1%) in the placebo group (pooled RR 2.14, 95% CI 1.36 to 3.39; p = 0.001) (Figure 38). There were 53 reports of chills: 44/453 (9.7%) in the zoledronic acid group and 9/346 (2.6%) in the placebo group (pooled RR 3.81, 95% CI 1.25 to 11.60, p < 0.02) (Figure 39). The incidence of pyrexia and headache significantly differed by sex (p < 0.00001 and p = 0.004, respectively).
FIGURE 37.
Pyrexia: zoledronic acid compared with placebo. M–H, Mantel–Haenszel.

FIGURE 38.
Headache: zoledronic acid compared with placebo. M–H, Mantel–Haenszel.
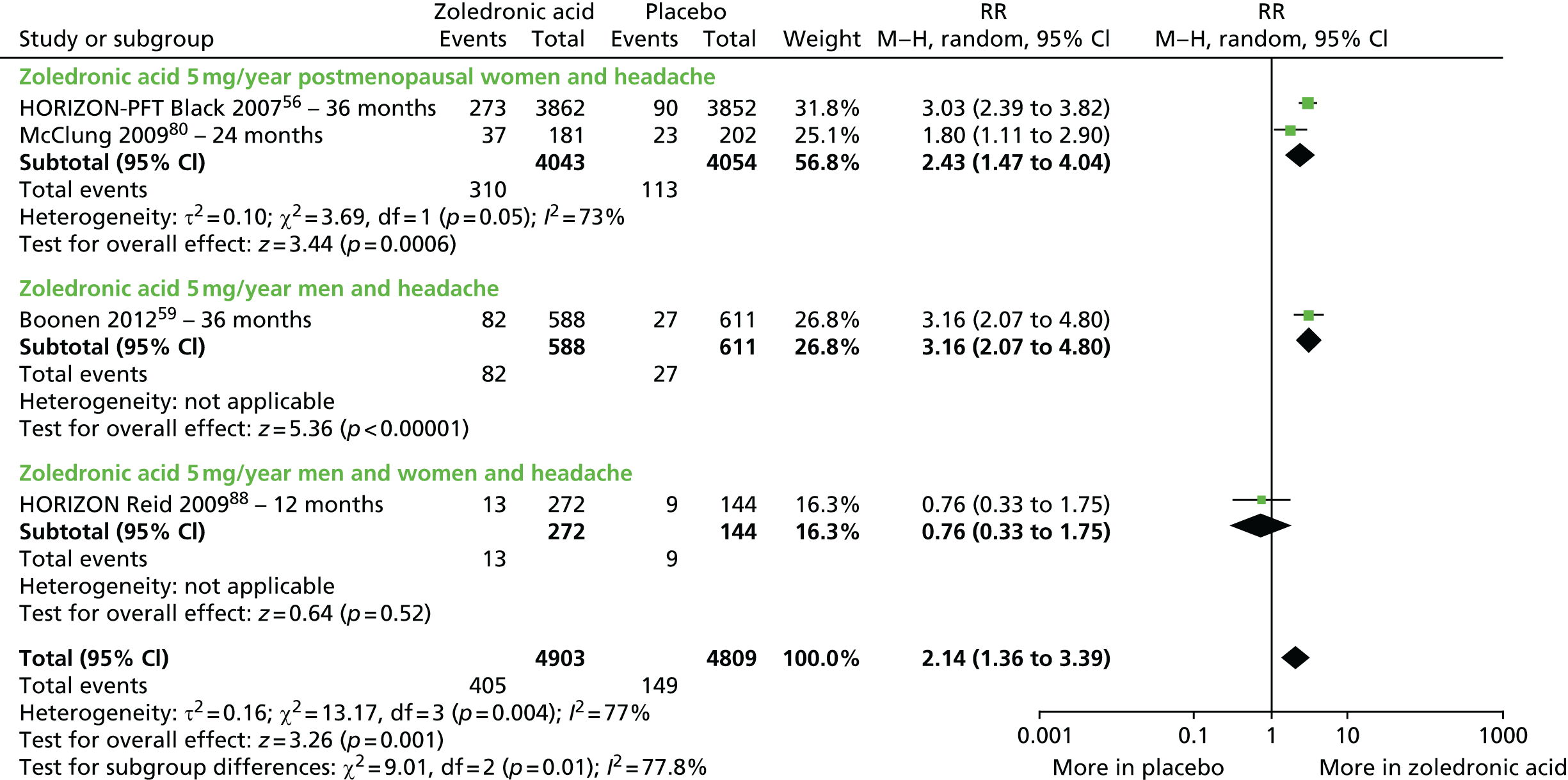
FIGURE 39.
Chills: zoledronic acid compared with placebo. M–H, Mantel–Haenszel.

In the MOTION trial,81 the incidence of neither influenza nor nasopharyngitis differed significantly between treatment groups [influenza (85 events): 36 (among 859 participants, 4.2%) in the alendronic acid group and 49 (among 874 participants, 5.6%) in the ibandronic acid group (monthly oral dose, 150 mg) (RR 0.75, 95% CI 0.49 to 1.14; p = 0.17); nasopharyngitis (92 events): 41 among 859 participants, 4.8%) in the alendronic acid group and (51 among 874 participants, 5.8%) in the ibandronic acid group (RR 0.82, 95% CI 0.55 to 1.22; p = 0.33)].
The ROSE trial69 found that 5 mg per year of zoledronic acid was associated with significantly more influenza-like symptoms than 70 mg per week of alendronic acid [137 cases: 5/194 (2.6%) in the alendronic acid group and 132/408 (32.4%) in the zoledronic acid group; RR 1.44, 95% CI 1.34 to 1.55; p < 0.00001]. It was also associated with a slight increase in pyrexia [23 cases: 2/194 (1.0%) in the alendronic acid group and 21/408 (5.2%) in the zoledronic acid group; RR 1.04, 95% CI 1.02 to 1.07; p = 0.002] and an increase in chills [26 cases: 3/194 (1.5%) in the alendronic acid group and 13/408 (3.2%) in the zoledronic acid group; RR 1.02, 95% CI 0.99 to 1.04; p = 0.19] (Figure 40).
FIGURE 40.
Influenza-like symptoms: 70 mg per week of alendronic acid compared with 5 mg per year of zoledronic acid.

The HORIZON trial88 compared 5 mg per year of zoledronic acid with 5 mg per day of risedronic acid in both men and women receiving steroids, and divided the participants into treatment and prevention subgroups for 12 months. The difference between treatments in influenza-like symptoms in the treatment subgroup was a RR of 5.02 (95% CI 1.47 to 17.14; p = 0.01) and the difference between treatments in the prevention subgroup was a RR of 10.00 (95% CI 1.30 to 77.09; p = 0.03); the differences between treatments were statistically significant (more events with zoledronic acid). No forest plot is presented for these data.
Risk of hospitalisation
Three RCTs in postmenopausal women reported on hospitalisation. 55,64,68 A total of 1850 participants were hospitalised during 36 months55,68 or 48 months of follow-up. 64 Across these RCTs there was no statistically significant difference in the risk of hospitalisation between participants receiving alendronic acid (27.9%, 928/3329) than those on placebo (27.8%, 922/3316) (pooled RR 1.01, 95% CI 0.79 to 1.28; p = 0.96) (Figure 41).
FIGURE 41.
Forest plot for hospitalisation in postmenopausal women on 10 mg per day of alendronic acid compared with placebo.
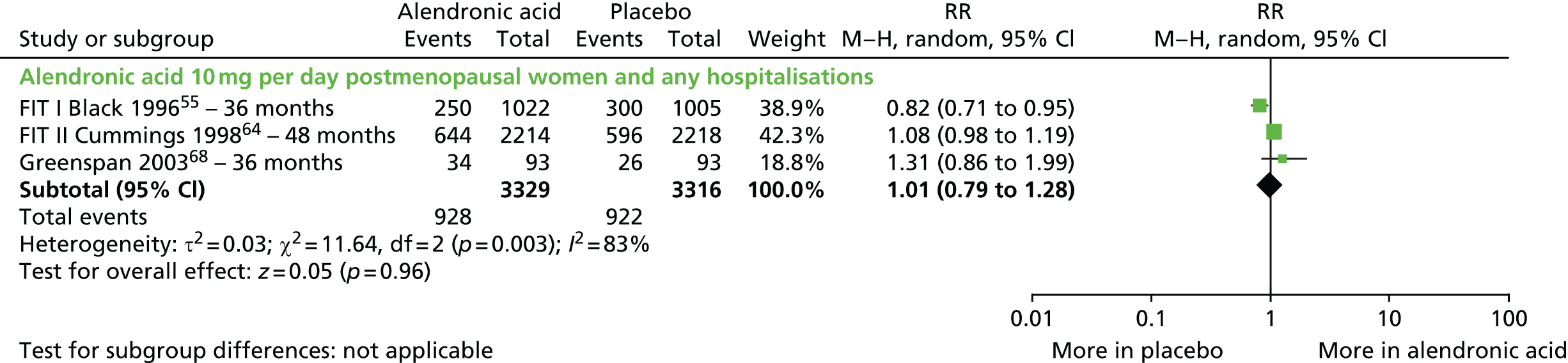
Atrial fibrillation
Atrial fibrillation was reported as an AE outcome across the two HORIZON RCTs comparing zoledronic acid with placebo56,77 and in the HORIZON RCT in men and women receiving glucocorticoids. 88 Across these RCTs no statistically significant differences between treatments were evident [HORIZON-PFT: RR 1.28 (95% CI 0.95 to 1.74; p = 0.10); HORIZON-RFT: RR 1.21 (95% CI 0.80 to 1.85; p = 0.37); HORIZON glucocorticoid – prevention group: RR 7.00 (95% CI 0.36 to 134.31; p = 0.20); HORIZON glucocorticoid – treatment group: zero events in both arms]. No forest plot is presented for these data.
Bone pain
Bone pain was reported as an AE outcome by two RCTs. 69,88
The HORIZON trial88 compared 5 mg per year of zoledronic acid with 5 mg per day of risedronic acid in both men and women receiving steroids, and divided the participants into treatment and prevention subgroups for 12 months. The difference between treatments in bone pain in the treatment subgroup was a RR of 2.61 (95% CI 0.94 to 7.22; p = 0.06). The difference between treatments was not statistically significant. There were zero events in both arms of the prevention subgroup. No forest plot is presented for these data.
The ROSE RCT69 compared 70 mg per week of alendronic acid with 5 mg per year of zoledronic acid. The difference between treatments in bone pain was a RR of 6.91 (95% CI 3.02 to 15.83; p < 0.00001). The difference between treatments was statistically significant (more events with zoledronic acid). No forest plot is presented for these data. There were zero events in both arms of the prevention subgroup.
Conjunctivitis
The HORIZON-PFT102 reported on eye inflammation as an AE in the first 3 days following administration of 5 mg per year of zoledronic acid or placebo in osteoporotic women. The difference between treatments in eye inflammation was a RR of 6.98 (95% CI 1.59 to 30.70; p = 0.01). The difference between treatments was statistically significant (more events with zoledronic acid). No forest plot is presented for these data.
Stroke
The HORIZON-RFT77 reported on stroke as an AE in men and women with history of hip fracture receiving 5 mg per year of zoledronic acid or placebo over 5 years. The difference between treatments in stroke was a RR of 1.21 (95% CI 0.80 to 1.85; p = 0.37); the difference between treatments was not statistically significant. No forest plot is presented for these data.
Osteonecrosis of the jaw
Four placebo-controlled RCTs evaluated zoledronic acid,56,59,77,79 one compared zoledronic acid with risedronic acid88 and one compared zoledronic acid with alendronic acid;69 all studies reported that no cases of spontaneous osteonecrosis were observed during the course of the RCT. The HORIZON-PFT56 reported that cases of osteonecrosis in both the zoledronic acid and placebo groups following dental surgery (one case in each group) resolved with antibiotic therapy.
Hypocalcaemia and atypical femoral fracture
None of the included RCTs reported on these AE outcomes.
Systematic review evidence for adverse events
A supplementary search in MEDLINE (via Ovid) and EMBASE (via Ovid) for systematic reviews reporting AEs of treatment was undertaken on 6 January 2015. Keywords and subheadings for AEs and safety with the drug names, and a reviews search filter were used. The MEDLINE search strategy is presented in Appendix 1. A total of 177 additional citations were identified, which were then sifted by a single reviewer (FC). Fourteen reviews summarising evidence for AEs across studies in bisphosphonates were identified. 112–125 A summary of these reviews and their findings is presented in Table 48, Appendix 5.
The review by Bobba et al. 112 evaluated the evidence from 14 studies of alendronic acid, eight studies of risedronic acid, 10 studies of ibandronic acid and nine studies in zoledronic acid. RCTs and observational studies were included. Summarising the evidence base, the reviewers reported that the rates of GI toxicity associated with alendronic acid, risedronic acid and oral ibandronic acid are similar to those associated with placebo. In addition, no significant difference in renal toxicity was evident for i.v. ibandronic acid compared with placebo; however, a decrease in renal function was evident with zoledronic acid. Osteonecrosis of the jaw was rarely described in participants receiving oral bisphosphonates, but it was more commonly reported in participants with malignancy receiving zoledronic acid. The authors concluded that the AEs associated with alendronic acid, risedronic acid and oral ibandronic acid are minimal; however, zoledronic acid may be compromised by renal toxicity. Myalgias and arthralgias were evident in the acute phase following i.v. administration.
In a review of clinical efficacy of risedronic acid for postmenopausal osteoporosis, Paget’s disease, breast cancer and participants taking glucocorticoids, Crandall113 evaluated the evidence across nine RCTs and seven clinical trials. Safety data from six RCTs of risedronic acid for any condition indicated that the safety of risedronic acid is similar to that of placebo; none of the trials found a notable rate of upper GI AEs.
In a comparative review of pivotal trials of alendronic acid and risedronic acid including a meta-analysis, Kherani et al. 114 concluded that both alendronic acid and risedronic acid result in similar rates of AEs as placebo.
In a review of clinical studies and review articles concerning the use of risedronic acid, Umland and Boyce115 observed that, although post-marketing surveillance studies reported an increase in serious or severe upper GI side effects with alendronic acid, similar findings were not evident for risedronic acid. The reviewers concluded that risedronic acid has been associated with a lower incidence of gastric ulcers than alendronic acid; however, the number of AEs associated with risedronic acid is generally similar to those observed with placebo in most clinical trials.
As part of a NICE report on adverse effects and persistence with oral bisphosphonates, Lloyd-Jones and Wilkinson116 reported that across UK prescription event monitoring studies, treatment with daily alendronic acid or risedronic acid is associated with a high level of reporting of a number of conditions in the first month of therapy, particularly those affecting the upper GI tract. There were around 30 reports of dyspepsia, the most commonly reported condition, per 1000 patient-months of exposure. However, RCTs of tolerability found no increased incidence of AEs in patients randomised to alendronic acid.
The Actavis consultee submission for this assessment reported that the patients who switched from risedronic acid to alendronic acid experienced a significant increase in the risk of GI side effects. In a retrospective cohort study evaluating anonymous medical records from 390 general practices in the UK, Ralston et al. 126 reported that the risk of developing a GI AE was higher in patients who switched to alendronic acid than in those who remained on risedronic acid (HR 1.85, 95% CI 1.26 to 2.72). The authors also reported that the risk was even greater in the subgroup of patients with a history of upper GI events (HR 3.18, 95% CI 2.79 to 3.63), but was also observed in patients with no history of GI events (HR 1.76, 95% CI 1.15 to 2.69). The authors concluded that switching patients who are stabilised on risedronic acid to alendronic acid is associated with an increased risk of GI AEs.
In a review specifically of bisphosphonate-induced osteonecrosis of the jaw, Krueger et al. 117 reviewed the evidence from 11 case reports and 26 case series studies reporting actual cases linking osteonecrosis of the jaw with bisphosphonate use, the majority of which reported on zoledronic acid. The available literature showed that i.v. bisphosphonates, especially zoledronic acid, are more likely to predispose patients to osteonecrosis of the jaw. However, in addition to bisphosphonate use, there appears to be several other factors involved in the development of osteonecrosis of the jaw. Other risk factors noted from the included studies were dental extraction or trauma to the jaw exposing part of the bone.
Van den Wyngaert et al. 118 also reviewed the evidence of an asssociation between bisphosphonates and osteonecrosis of the jaw across 22 studies based on retrospective chart reviews without a control, of which three included patients with osteoporosis. Zoledronic acid and pamidronic acid (Aredia®, Novartis Pharmaceuticals Ltd) were the main bisphosphonates covered. Of the patients included in the studies, 69.3% had undergone a dental extraction prior to the development of osteonecrosis, confirming the importance of trauma in the initiation of the disease. However, not enough evidence is available to prove a causal link.
Woo et al. 119 reviewed the evidence of a link between bisphosphonates and osteonecrosis of the jaw across 29 case reports. Zoledronic acid, aledronic acid and pamidronic acid were the main bisphosphonates covered and 94% of patients were treated with zoledronic acid or pamidronic acid or both; 85% of affected patients had multiple myeloma or metastatic breast cancer and 4% had osteoporosis. The authors concluded that the prevalence of osteonecrosis in patients with cancer is 6–10% and the prevalence in those taking alendronic acid for osteoporosis is unknown. The authors also concluded that more than half of all cases (60%) occur after dentoalveolar surgery (such as tooth extraction) to treat infections, and the remaining 40% are probably related to infection, denture trauma or other physical trauma.
Recently, Lee et al. 120 undertook a meta-analysis of 12 cohort and case–control studies evaluating oral and i.v.-administered bishphosphonates. An inclusion criterion was that the studies were carried out in non-cancer patients. The pooled effect estimate indicated that the use of bisphosphonates was associated with a significantly increased risk of jaw osteonecrosis (OR 2.32, 95% CI 1.38 to 3.91). The reviewers concluded that the use of bisphosphonates in non-cancer patients is associated with a substantial risk of jaw osteonecrosis and that patients receiving i.v. bisphosphonates are at highest risk.
Giusti et al. 121 reviewed the evidence from 39 publications reporting on women treated with a bisphosphonate in a regimen used for the prevention or treatment of osteoporosis. Twenty-seven of the publications were case series or case reports (one abstract), four were retrospective studies and one was a prospective article including three new cases. In most cases, the bisphosphonate was alendronic acid, prescribed for prevention or treatment of osteoporosis. Across the included studies, there were 58 femoral shaft fractures and 41 subtrochanteric fractures; the precise fracture site was not specified in 42 cases. Nineteen fractures were diagnosed at presentation as insufficiency fractures, with 12 of these progressing to a complete fracture. Overall, 53 (44.2%) of the 120 patients for whom data were available had a contralateral fracture (32 of which were insufficiency fractures), either concurrently with or subsequent to the initial fracture, of which 34 (64.2%) occurred in the same anatomical location as the first fracture. The authors concluded that the analysis allowed the clinical identification of patients at risk of developing atypical fractures; however, the reviewers also concluded that long-term bisphosphonate therapy is not a prerequisite for development of atypical fractures. Moreover, the use of glucocorticoids and proton pump inhibitors is an important risk factor for atypical fracture.
Recently, Gedmintas et al. 122 undertook a meta-analysis of atypical fractures reported in five case–control studies and six cohort studies. The studies were mainly carried out in women and evaluated mainly alendronic acid but also ibandronic acid, risedronic acid, zoledronic acid and other bisphosphonates. The overall pooled estimate for atypical fractures associated with bisphosphonates using data from the five case–control and six cohort studies was a RR of 1.70 (95% CI 1.22 to 2.37). Gedmintas et al. 122 concluded that there is an increased risk of atypical fracture among bisphosphonate users but that atypical fractures are rare events, even in bisphosphonate users.
Andrici et al. 123 undertook a meta-analysis of seven cohort or case–control studies investigating oral bisphosphonates and the risk of oesophageal cancer. Participants were anyone who had filed a prescription for any antiresorptive drug. The authors found a positive relationship between exposure to bisphosphonates and oesophageal cancer, with an OR of 1.74 (95% CI 1.19 to 2.55). An increased risk of oesophageal cancer was also found in the group exposed to bisphosphonates for a longer period of time. According to the authors, the results suggest a possible association between oral bisphosphonates and oesophageal cancer, and this risk is increased with a longer exposure period. An increased risk was observed for etidronic acid, but not alendronic acid.
Recently, Sun et al. 124 undertook a a meta-analysis of observational studies. Seven epidemiological studies, four cohort studies and three case–control studies, were included and, where reported, alendronic acid was the main bisphosphonate. The underlying conditions for which patients were being treated with a bisphosphonate were not reported. In the primary analysis, bisphosphonate treatment was not associated with a risk of oesophageal cancer in either the cohort studies (pooled RR 1.23, 95% CI 0.79 to 1.92) or the case–control studies (pooled OR 1.24, 95% CI 0.98 to 1.57). The authors also observed no significant increased risk of oesophageal cancer in users of alendronic acid alone across cohort studies (RR 1.08, 95% CI 0.67 to 1.75) or across case–control studies (OR 1.16, 95% CI 0.82 to 1.63). They concluded that bisphosphonate treatment is not significantly associated with an excess risk of oesophageal cancer.
Loke et al. 125 evaluated the risk of atrial fibrillation associated with biphosphonate use in patients with osteoporosis or fractures. RCTs of any biphosphonate compared with placebo and case–control and prospective or retrospective cohort studies in patients with osteoporosis that reported on the association between biphosphonate exposure and atrial fibrillation were eligible for inclusion. Interventions in the included RCTs included alendronic acid, risedronic acid and zoledronic acid. Interventions in the included case–control studies were mostly alendronic acid or etidronic acid. Across nine RCTs, biphosphonates significantly increased the risk of atrial fibrillation compared with placebo (OR 1.47, 95% CI 1.01 to 2.14). Biphosphonates did not significantly increase the risk of stroke or cardiovascular mortality (three RCTs). One case–control study found that patients with atrial fibrillation were more likely than control patients to have used biphosphonates (OR 1.86, 95% CI 1.09 to 3.15), but the second case–control study found no association. Neither study found a greater likelihood of current use of bisphosphonates among patients with atrial fibrillation. The authors concluded that bisphosphonates were associated with atrial fibrillation, but heterogeneity of the existing evidence and a paucity of information on some agents precluded any definitive conclusions with respect to risk.
Only one review reported on mortality. 116 The authors did not report an overall conclusion on this outcome, but did say that one cohort study found no difference in all-cause mortality, cancer mortality or mortality from cancer of the lung or GI tract between patients treated with risedronic acid and those treated with placebo. A non-statistically significant reduction in deaths from cardiovascular causes in the risedronic acid group was largely caused by a statistically significant reduction in stroke mortality in the combined risedronic acid groups (p = 0.015); and from one prescription event monitoring study that serious upper GI events included gastric, duodenal and peptic ulceration, gastritis, and duodenitis. However, only 9 of the 502 reported deaths for which the cause of death was established were attributed to GI causes.
The 14 reviews were published from 2001 to 2014. 112–125 One review considered any antresorptive therapy,123 10 considered any bisphosphonate therapy112,114,117–122,124,125 and three reported on AEs associated with specific bisphosphonates (two in risedronic acid113,115 and one in alendronic acid or risedronic acid116). Four reviews included evidence from both observational studies and RCTs112,116,117,125 and seven included only observational studies. 118–124 Five reviews reported on any AE,112–115 whereas nine reported on specific AEs (four in jaw osteonecrosis,117–120 two in atypical fracture,121,122 two in oesophogeal cancer123,124 and one in atrial fibrillation125). Four reviews pooled data across studies in a meta-analysis. 120,122–124
Evidence from these reviews indicates that rates of GI toxicity are similar following treatment with alendronic acid, risedronic acid, oral ibandronic acid and placebo. However, observational data suggest a high level of reporting of a number of conditions in the first month of therapy with alendronic acid or risedronic acid, particularly those affecting the upper GI tract. Zoledronic acid may be compromised by renal toxicity, and myalgia and arthralgia are evident in the acute phase following i.v. administration of the drug. Intravenous bisphosphonates, especially zoledronic acid, are more likely to predispose patients to osteonecrosis of the jaw, although absolute risk is very low. In addition to bisphosphonate use, there appears to be several other factors involved in the development of osteonecrosis of the jaw. There is an increased risk of atypical fracture among bisphosphonate users; however, events are rare and long-term bisphosphonate therapy is not a prerequisite for development of atypical fractures. Moreover, the use of glucocorticoids and proton pump inhibitors are important risk factors. Bisphosphonates are associated with serious atrial fibrillation, but heterogeneity of the existing evidence and a paucity of information on some agents preclude any definitive conclusions with respect to risk. The review evidence for the use of bisphosphonates and oesophogeal cancer is equivoval; no overlaps in this evidence either across the included reviews or with the RCT evidence base included in this assessment report were identified.
Continuance and concordance
Alendronic acid
Two trials reported that at the end of treatment (36 months) > 80% of participants were still taking study medication. 55,64 One trial reported that > 60% of participants took 80% of their study medication. 68
Ibandronic acid
The ARIBON74 trial reported that > 90% of participants took all of their monthly doses for 24 months. In the BONE trial,45 the mean duration on treatment was reported as 2.42 years in the placebo group and 2.48 years in the group receiving 2.5 mg per day of ibandronic acid.
Risedronic acid
Boonen et al. 58 reported that, at 24 months, 91% of placebo and 98% of 35 mg per week of risedronic acid participants were compliant with the study drug. In the VERT-NA trial, Harris et al. 70 reported that 55% of placebo and 60% of 5 mg per month risedronic acid groups completed 3 years of medication and Taxel et al. 95 reported that compliance with the study drug was 90–95% for all participants.
Zoledronic acid versus alendronic acid
Hadji et al. 106 reported that, in the ROSE trial, at 12 months, 80.9% of patients were compliant with alendronic acid therapy, but compliance with zoledronic acid was not reported.
Systematic review evidence for compliance and concordance
A supplementary search in MEDLINE (via Ovid) and EMBASE (via Ovid) for systematic reviews reporting on compliance and continuance was undertaken on 6 January 2015. Keywords for ‘compliance’ were combined with the named drug intervention terms and a reviews search filter. The MEDLINE search strategy is presented in Appendix 1. From this search, 57 additional citations were identified. These records were sifted by a single reviewer (MMSJ). Seven reviews were identified that summarised evidence for compliance and concordance across studies in bisphosphonates for osteoporosis and a summary of these reviews and their findings is presented in Appendix 6. 116,127–132
The review by Cramer et al. 127 included studies reporting one measure of compliance or persistence derived from administrative databases of patient demographic and prescription information. Compliance was measured as the medication possession ratio (MPR) and persistence was measured as the number of days of possession without a gap in refills, and the percentage of patients persisting with therapy for 1 year. Most of the therapies in the 14 included studies obtained were oral daily or weekly bisphosphonates (alendronic acid and risedronic acid). Studies had observation periods of mainly 12 months. The reviewers reported that the mean MPR was consistently higher for weekly therapy (0.58–0.76) than for daily therapy (0.46–0.64). Patients receiving weekly bisphosphonates exhibited better persistence (length of persistence 194–269 days; 35.7–69.7% persistent) than those receiving daily therapy (length of persistence 134–208 days; 26.1–55.7% persistent). The reviewers concluded that, although patients using weekly bisphosphonate medication follow their prescribed regimens better than those using daily therapy, overall compliance and persistence rates were suboptimal.
Imaz et al. 128 examined observational studies that prospectively analysed administrative databases of pharmacy refills for measures of persistence and compliance in patients who were prescribed either bisphosphonates (mainly alendronic acid and risedronic acid) or other anti-osteoporosis medications. Follow-up periods needed to be 1–2.5 years and compliance was to be measured by the MPR. Studies were pooled in meta-analyses, with 15 studies included in the review. The pooled persistence mean was 184.1 days (95% CI 163.9 to 204.3 days; five studies) and the pooled MPR mean was 66.9% (95% CI 63.3% to 70.5%; five studies) at the 1-year follow-up. Low compliance when compared with high compliance was significantly associated with increased overall fracture risk (RR 1.46, 95% CI 1.34 to 1.60; six studies) from 1 to 2.5 years after starting treatment. Compared with high compliance, low compliance was significantly associated with increased non-vertebral fracture risk (RR 1.16, 95% CI 1.07 to 1.26; three studies) from 1.9 to 2.2 years’ follow-up, increased hip fracture risk (RR 1.28, 95% CI 1.06 to 1.53; four studies) from 1.9 to 2.4 years’ follow-up and increased vertebral fracture risk (RR 1.43, 95% CI 1.26 to 1.63; two studies) from 2 to 2.2 years’ follow-up. The reviewers concluded that persistence and compliance were suboptimal for postmenopausal women who underwent bisphosphonate therapy for the treatment of osteoporosis.
Kothawala et al. 129 reviewed 24 observational studies assessing pharmacological drug adherence in patients with osteoporosis. Among the included studies, bisphosphonates were the most frequently assessed drug. Treatment duration ranged from 1 month to > 24 months and a higher proportion of included patients were new users. However, the types of bisphosphonates were not reported. The outcomes of interest were grouped according to standardised definitions: persistence (how long a patient received therapy after initiating treatment), compliance (how correctly, in terms of dose and frequency, patients took their medication) and adherence (a combined measure of persistence and compliance). Outcome rates were pooled in a random-effects meta-analysis. Compliance data were extracted as the percentage of patients who reported that they followed the dosing recommendations. Adherence data were extracted as the percentage of patients who achieved a predefined MPR threshold. Across seven studies the pooled refill compliance rate was 68% at both 7–12 months (95% CI 63% to 72%) and at 13–24 months (95% CI 67% to 69%). The pooled estimate from self-reported data (four studies) was 62% (95% CI 48% to 75%) of patients following the recommended instructions within 6 months of starting treatment. Across six studies, the pooled estimate of patients achieving a MPR > 66% (one study) and > 80% (five studies) ranged from 53% (95% CI 52% to 54%) for treatment lasting 1–6 months to 43% (95% CI 32% to 54%) for treatment lasting 13–24 months. The authors concluded that one-third to one-half of patients being treated with pharmacological drugs for osteoporosis did not take their medication as directed.
Lee et al. 130 reviewed 10 RCTs and observational studies. Compliance and persistence were evaluated, but data were not pooled. Studies in osteoporosis medications including alendronic acid were evaluated. These reviewers reported that adherence at 12 months was higher with weekly than with daily bisphosphonates (≥ 84% preference for weekly, MPR 60–76% vs. 46–64%; persistence 43.6–69.7% vs. 31.7–55.7%). The MPRs reported for oral bisphosphonates were 68–71% at 12 months. At 2 years, only 43% of patients had a MPR ≥ 80% for daily and weekly bisphosphonates. Observational studies (6–12 months’ duration) reported discontinuation rates of 18–22% for daily and 7% for weekly bisphosphonates. The studies suggested that patient prefer annual zoledronic acid infusions to weekly bisphosphonates (66.4–78.8% vs. 9.0–19.7%, respectively), but no data on compliance or persistence were available. The reviewers concluded that adherence is difficult to quantify and may not be exclusively influenced by the frequency of medication administration.
As part of a NICE report on AEs and persistence with oral bisphosphonates, Lloyd-Jones and Wilkinson116 reported that, across UK prescription event monitoring studies, 24.5% of patients prescribed alendronic acid by general practitioners (GPs) discontinued therapy within 1 year. The two most common reasons for stopping treatment were dyspeptic conditions (6.3%) and non-compliance (3.0%). These authors concluded that persistence might be improved by weekly rather than daily regimens.
Mikyas et al. 131 reviewed treatment adherence in studies in male osteoporosis. Eighteen retrospective or prospective observational studies were included in the analysis. The reviewers reported that the definition and measure of medication adherence varied among studies; however, adherence was measured in terms of the MPR in most studies that reported adherence. The majority of treatments were bisphosphonates, of which the majority were alendronic acid; data were not pooled. Across studies, the percentage of males adherent to bisphosphonates [MPR > 0.8] over 12 months ranged from 32% to 64%. The reviewers concluded that one-third to two-thirds of men do not adhere to bisphosphonates.
Vieira et al. 132 reviewed 27 mainly observational studies of bisphosphonates (alendronic acid, ibandronic acid, risedronic acid and zoledronic acid) covering a wide range of outcomes regarding adherence and associated factors. No data were pooled and a narrative summary of the included studies was reported. Among the included studies, the reviewers summarised evidence from one cohort study in which the proportion of days covered (described as equivalent of a MPR) was 82% with i.v. zoledronic acid and 58–62% with i.v. ibandronic acid; one cohort study in which overall compliance with oral alendronic acid, risedronic acid or ibandronic acid was 43%; one cohort study in which persistence with therapy declined from 63% at 1 year to 46% at 2 years and 12% at 9 years among patients receiving alendronic acid and risedronic acid; one RCT in which the MPR was 93–100% among women taking weekly alendronic acid or monthly ibandronic acid; and one retrospective observational study in women taking weekly (alendronic acid or risedronic acid) or monthly ibandronic acid. Patients treated with a monthly regimen were 37% less likely to be non-persistent and were more compliant, with a 5% higher absolute MPR, than women treated with weekly regimens; and one cohort study in patients taking weekly risedronic acid or weekly alendronic acid in which patients initiated on weekly oral generic alendronic acid showed a significantly lower persistence with bisphosphonate therapy than patients initiated on weekly oral branded risedronic acid and weekly oral branded alendronic acid. Across all studies, the reviewers concluded that a monthly dose is associated with better adherence than a weekly dose.
Seven reviews were identified, published between 2006 and 2014. 116,127–132 These are summarised in Appendix 6, Table 49. The majority of these reviews reported on aledronic acid and risedronic acid. One review also included studies in ibandronic acid132 and two included zoledronic acid. 130,132 The majority of reviews evaluated compliance as a MPR and persistence measured as the number of days of possession. Data were pooled across studies by three reviews. 128–130
Evidence across these reviews indicates that although patients using weekly bisphosphonate medication follow their prescribed regimens better than those using daily therapy, overall compliance and persistence rates are suboptimal for postmenopausal women receiving bisphosphonate therapy for the treatment of osteoporosis. Furthermore, one-third to one-half of patients, including men being treated with bisphosphonates for osteoporosis, did not take their medication as directed. No overlaps in this evidence either across the included reviews or with the RCT evidence base included in this assessment report were identified.
Health-related quality of life
Alendronic acid
A quality-of-life assessment was reported by one RCT65 using the Nottingham Health Profile. 133 Statistically significant improvements in all of the instrument’s domains were reported with alendronic acid. Differences between treatments with placebo were not reported.
Ibandronic acid
Health-related quality of life was not reported by any trial evaluating ibandronic acid.
Risedronic acid
Health-related quality of life was not reported by any trial evaluating risedronic acid.
Zoledronic acid
In the HORIZON-RFT trial, quality-of-life outcomes were reported by Adachi et al. 103 Quality of life was assessed at 6, 12, 24 and 36 months using the EQ-5D VAS and utility scores. 134 The authors report that, at the end of the study, mean change from baseline in EQ-5D VAS was greater (higher score better) in the zoledronic acid-treated group than in the placebo group (7.67 ± 0.56 vs. 5.42 ± 0.56; p = 0.0034). A statistically significant difference between treatments in EQ-5D VAS was also evident in the subgroup of patients experiencing clinical vertebral fractures (8.86 ± 4.91 vs. –1.69 ± 3.42; p = 0.0456), non-vertebral fractures (5.03 ± 2.48 vs. –1.07 ± 2.16; p = 0.0393) and clinical fractures (5.19 ± 2.25 vs. –0.72 ± 1.82; p = 0.0243) in favour of zoledronic acid. EQ-5D utility scores were comparable for zoledronic acid and placebo groups, but more participants in the placebo group consistently had extreme difficulty in mobility (1.74% vs. 2.13%; p = 0.6238), self-care (4.92% vs. 6.69%; p = 0.1013) and usual activities (10.28% vs. 12.91%; p = 0.0775).
Zoledronic acid versus alendronic acid
In the ROSE trial, Hadji et al. 69 assessed quality of life using the quality of life questionnaire of the European Foundation for Osteoporosis questionnaire. 135 Hadji et al. 106 reported that in the alendronic acid group only the pain domain showed a significant improvement as compared with baseline. However, across all domains the differences between the treatments were not statistically significant.
Health resource use
Alendronic acid
The FIT I55 reported that the rate of hospital admissions for fracture was 9.2% in the placebo group, compared with 6.3% in the alendronic acid groups.
No other included RCT reported any hospitalisation and service use following fracture.
Systematic review evidence for health-related quality of life
A summary of reviews of HRQoL is presented in Chapter 4, Independent economic assessment.
Methods for the network meta-analyses
A NMA was conducted for each of the four main fracture types and for femoral neck BMD. Details of the statistical methods are provided in Appendix 3.
Selection of evidence contributing to the network meta-analysis
For RCTs to be eligible for inclusion in the NMA, the interventions were required to be assessed in line with the licensing indications. RCTs that included both licensed and unlicensed dose groups were included where outcome data for the licensed group could be isolated. RCTs that reported only results pooled across RCT groups were not included.
An assumption of the NMA is that RCTs are exchangeable, that is we would be prepared to treat any patient with any one of the treatments. Strictly, the RCTs included in this evidence synthesis are not exchangeable because not all of the treatments are licensed in all patient populations but the analysis follows the agreed scope.
Two RCTs reported that participants were switched from 5 mg per day alendronic acid to 10 mg per day alendronic acid after 24 months of the 36-month trial. 55,64 A sensitivity analysis was performed to explore the impact on the results of excluding these RCTs from the analysis.
Vertebral fractures were assessed using either clinical/symptomatic (four RCTs56,77,81,89) or morphometric/radiographic (15 RCTs45,55,58–60,63–66,70,72,76,83,85,86) techniques, with two RCTs82,88 not stating the assessment method. A sensitivity analysis was performed to assess the impact on the results of including in the analysis those RCTs with only clinical assessment of fractures.
Femoral neck BMD data were presented either numerically or in graphical format. Nine RCTs55,56,64,68,72,76,85,87,90 presented results for each treatment group in graphical format but in the text presented the mean between-group differences in the percentage change in femoral neck BMD in numerical form. Two of the included RCTs45,77 reported only data on mean differences in percentage change between treatments. The remaining 24 RCTs presented sample estimates for each treatment group separately, with 20 reporting in numerical format53,57,59,62,63,65,66,70,73,75,79–81,83,84,86,88,91,95 and four graphically. 47,49,58,67 Where both formats were provided, numerical estimates were selected as the most accurate summaries of means and variances. Given potential inaccuracy and inconsistency between the numerical and graphical sample estimates, a sensitivity analysis was performed to explore the impact on the results of excluding the graphically extracted sample estimates from the analysis.
Results from the network meta-analyses
A summary of the data used in the NMA is provided in Tables 42–46 in Appendix 3. The results for each of the four fracture types are presented in Vertebral fractures, class-effects model to wrist fractures, class-effects model. Results for femoral neck BMD are presented in Femoral neck bone mineral density, class-effects model. As described earlier (see Methods for the network meta-analyses), three sensitivity analyses were undertaken. Sensitivity analysis 1 is presented below (see Sensitivity analysis 1) and assesses the robustness of the results to the inclusion of RCTs that altered dose over the study duration. Sensitivity analysis 2, which considers clinically assessed vertebral fractures, is presented in Sensitivity analysis 2. Sensitivity analysis 3 is presented in Sensitivity analysis 3, excluding RCTs for which femoral neck BMD results were provided in graphical format only. Results using the standard random-effects model are presented in Appendix 3, Figures 131–136.
Vertebral fractures, class-effects model
An NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 150 mg per month oral ibandronic acid and 2.5 mg per day oral ibandronic acid relative to placebo on the occurrence of vertebral fractures. Data were available from 21 RCTs,45,55,56,58–60,63–66,70,72,76,77,81–83,85,86,88,89 each comparing two treatments. Figure 42 presents the network of evidence for vertebral fractures.
FIGURE 42.
Vertebral fractures: network of evidence.

The network provided seven direct treatment comparisons (edges in the network diagram). For the placebo versus 2.5 mg per day oral ibandronic acid comparison there is no direct evidence. The risedronic acid versus alendronic acid comparison is contributed by one small study, with a zero count in the control arm. Three contrasts were checked for inconsistency between direct and indirect evidence. None of the comparisons showed significant evidence of inconsistency, as assessed using Bayesian p-values (Figure 43).
FIGURE 43.
Vertebral fractures: class-effects model. Assessing inconsistency using node splitting. (a) Nodes 1 and 2, placebo–risedronic acid; (b) nodes 1–4, placebo–zoledronic acid; and (c) nodes 2–4, risedronic acid–zoledronic acid.
Figure 44 presents the effects of each treatment relative to placebo, and the probabilities of treatment rankings are presented in Figure 45. The model fitted the data well, with a total residual deviance of 41.05 being close to the number of data points included in the analysis, which was 42. The deviance information criterion (DIC) was 69.28. The between-study SD was estimated to be 0.19 [95% credible interval (CrI) 0.01 to 0.49], implying mild heterogeneity in treatment effects between RCTs.
FIGURE 44.
Vertebral fractures: class-effects model – HRs and 95% credible intervals. Note that the mean effects estimates are plotted in black and predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

FIGURE 45.
Vertebral fractures: class-effects model – probability of treatment rankings. (a) Placebo, mean rank = 5.99; (b) risedronic acid, mean rank = 3.89; (c) alendronic acid, mean rank = 2.91; (d) zoledronic acid, mean rank = 2.08; (e) 150 mg per month of ibandronic acid, mean rank = 2.97; and (f) 2.5 mg per day of ibandronic acid, mean rank = 3.16.
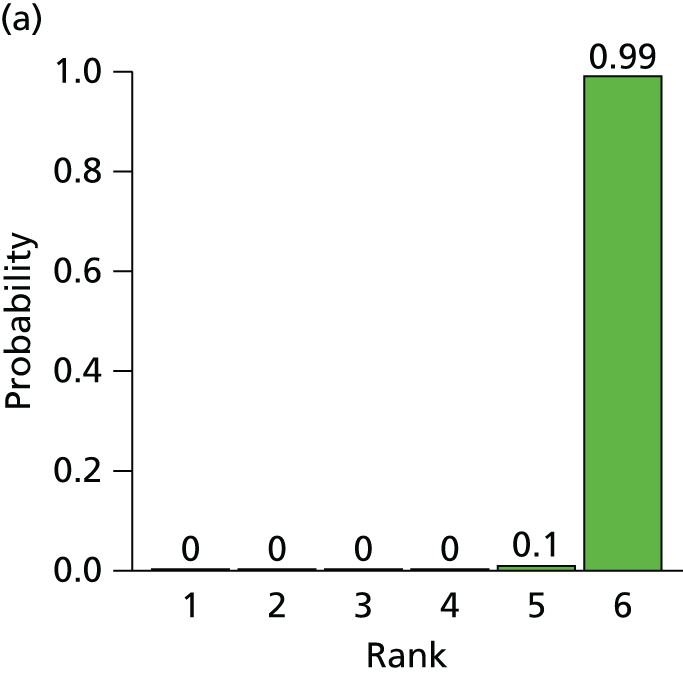


The between-treatment SD was estimated to be 0.18 (95% CrI 0.01 to 0.86), which is indicative of mild heterogeneity in treatment effects between treatments (i.e. the effects of the bisphosphonates are relatively similar) but with considerable uncertainty.
All treatments were associated with beneficial treatment effects relative to placebo, and all treatment effects were statistically significant at a conventional 5% level. Zoledronic acid was associated with the greatest effect (HR 0.41, 95% CrI 0.28 to 0.56) and was most likely to be the most effective treatment (probability of 0.44 of being the most effective). Pairwise comparisons between treatments indicated that no active treatments are significantly more effective than other active treatments. The HR for a randomly chosen study for a new bisphosphonate is 0.45 (95% CrI 0.19 to 1.12), allowing for both between-study and between-treatment heterogeneity.
The effect of baseline risk as a potential treatment effect modifier was explored using metaregression. The model fitted the data well, with a total residual deviance of 41.11 (compared with 42 data points). The between-study SD was estimated to be 0.21 (95% CrI 0.02 to 0.57) and the between-treatment SD was estimated to be 0.18 (95% CrI to 0.01 to 0.92). The between-study SD from fitting a random-effects model to the placebo baseline data was 1.23 (95% CrI 0.86 to 1.90), indicating substantial heterogeneity between RCTs. However, there was no evidence that treatment effect varied according to baseline risk, with the interaction term estimated to be 0.02 (95% CrI –0.25 to 0.22). In fact, including baseline risk did not improve the fit of the model to the data according to a comparison of DICs (70.53 vs. 69.28), and actually increased the estimate of the between-study SD of the treatment effect. Exchangeable and related treatment–specific interactions were also considered. The model did not provide a better fit to the data, with a DIC of 71.50.
Non-vertebral fractures: class-effects model
An NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 150 mg per month of oral ibandronic acid and 2.5 mg per day of oral ibandronic acid relative to placebo on the occurrence of non-vertebral fractures. Data were available from 14 RCTs,45,55–57,64,66,70,72,77,81,83–85,89 each comparing two treatments. Figure 46 presents the network of evidence for non-vertebral fractures.
FIGURE 46.
Non-vertebral fractures: network of evidence.

As the network provided no indirect evidence, an assessment of inconsistency was not performed. Figure 47 presents the effects of each treatment relative to placebo. The probabilities of treatment rankings are presented in Figure 48. The model fitted the data well, with a total residual deviance of 22.80 compared with the number of data points included in the analysis, which was 28. The DIC was 42.32. The between-study SD was estimated to be 0.08 (95% CrI 0.00 to 0.31), implying mild heterogeneity in treatment effects between RCTs.
FIGURE 47.
Non-vertebral fractures: class-effects model – HRs and 95% CrIs. Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

FIGURE 48.
Non-vertebral fractures: class-effects model – probability of treatment rankings. Note that the most efficacious = 1, and the least efficacious = 6. (a) Placebo, mean rank = 5.52; (b) risedronic acid, mean rank = 2; (c) alendronic acid, mean rank = 3.14; (d) zoledronic acid, mean rank = 2.46; (e) 150 mg per month of ibandronic acid, mean rank = 3.34; and (f) 2.5 mg per day of ibandronic acid, mean rank = 4.55.
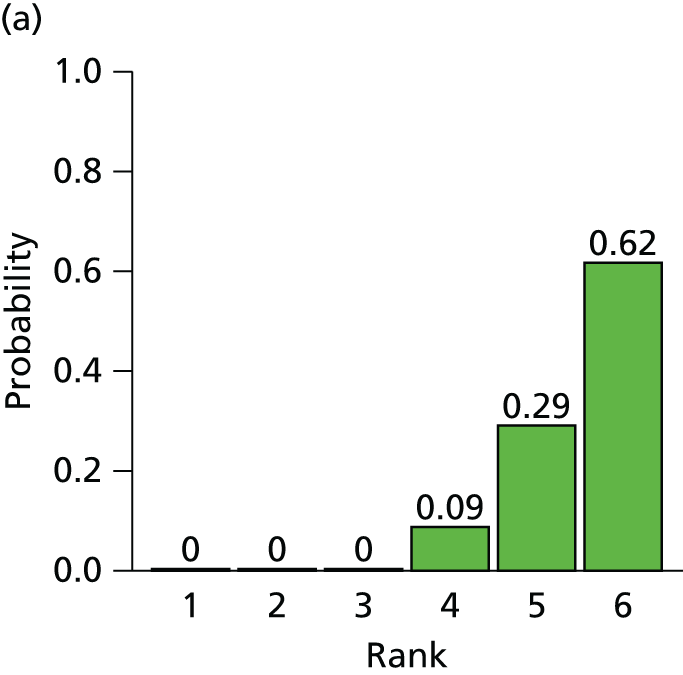
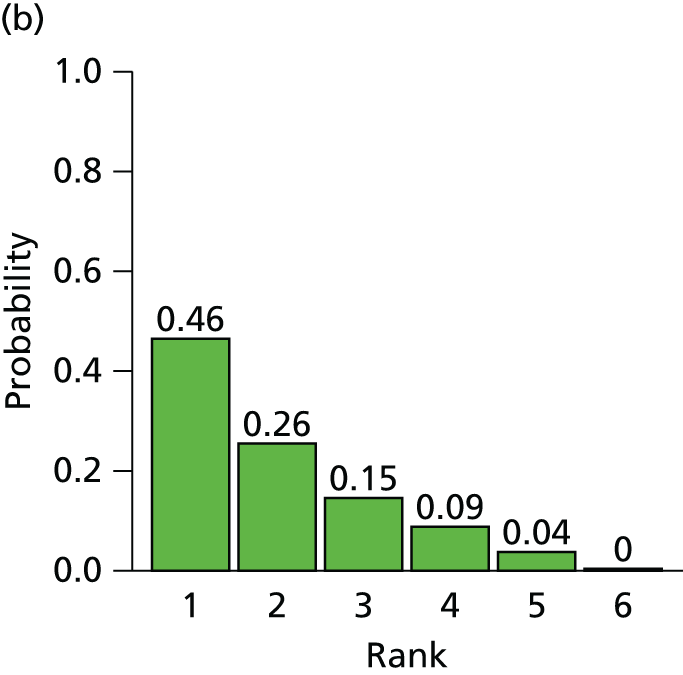



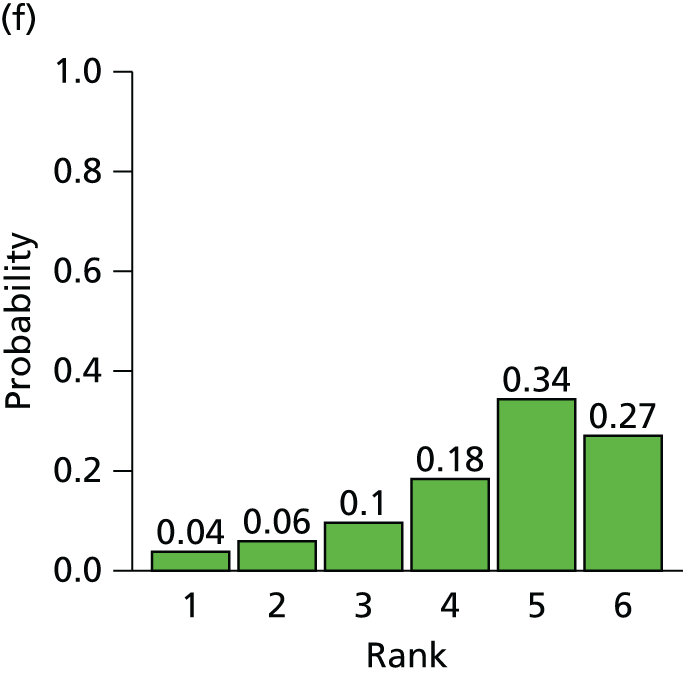
The between-treatment SD was estimated to be 0.17 (95% CrI 0.01 to 0.80), which is indicative of mild heterogeneity in treatment effects between treatments (i.e. the effects of the bisphosphonates are relatively similar) but with considerable uncertainty.
All treatments were associated with beneficial treatment effects relative to placebo, with risedronic acid, alendronic acid and zoledronic acid being statistically significant at a conventional 5% level. Risedronic acid was associated with the greatest effect (HR 0.72, 95% CrI 0.53 to 0.89) and was most likely to be the most effective treatment (with a probability of being the most effective of 0.46). No active treatments were statistically significantly more effective than other active treatment. The HR for a randomly chosen study for a new bisphosphonate is 0.79 (95% CrI 0.38 to 1.69), allowing for both between-study and between-treatment heterogeneity.
The effect of baseline risk as a potential treatment effect modifier was explored using metaregression. The model fitted the data well, with a total residual deviance of 23.65 (compared with 28 data points). The between-study SD was estimated to be 0.11 (95% CrI 0.01 to 0.37) and the between-treatment SD was estimated to be 0.17 (95% CrI 0.01 to 0.81). The between-study SD from fitting a random-effects model to the placebo baseline data was 0.48 (95% CrI 0.32 to 0.83), indicating moderate heterogeneity between RCTs. However, there was no evidence that treatment effect varied according to baseline risk, with the interaction term estimated to be –0.07 (95% CrI –0.44 to 0.22). In fact, including baseline risk did not improve the fit of the model to the data according to a comparison of DICs (44.27 vs. 44.32), and actually increased the estimate of the between-study SD of the treatment effect. Exchangeable and related treatment-specific interactions were also considered. The model did not provide a better fit to the data, with a DIC of 45.84.
Hip fractures: class-effects model
A NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid and 150 mg per month of oral ibandronic acid relative to placebo on the occurrence of hip fractures. Data were available from 10 RCTs,55,56,64,68,70,74,77,78,82,85 each comparing two treatments. Figure 49 presents the network of evidence for hip fractures.
FIGURE 49.
Hip fractures: network of evidence. Owing to the limited power of indirect evidence, assessment for inconsistency was not performed.
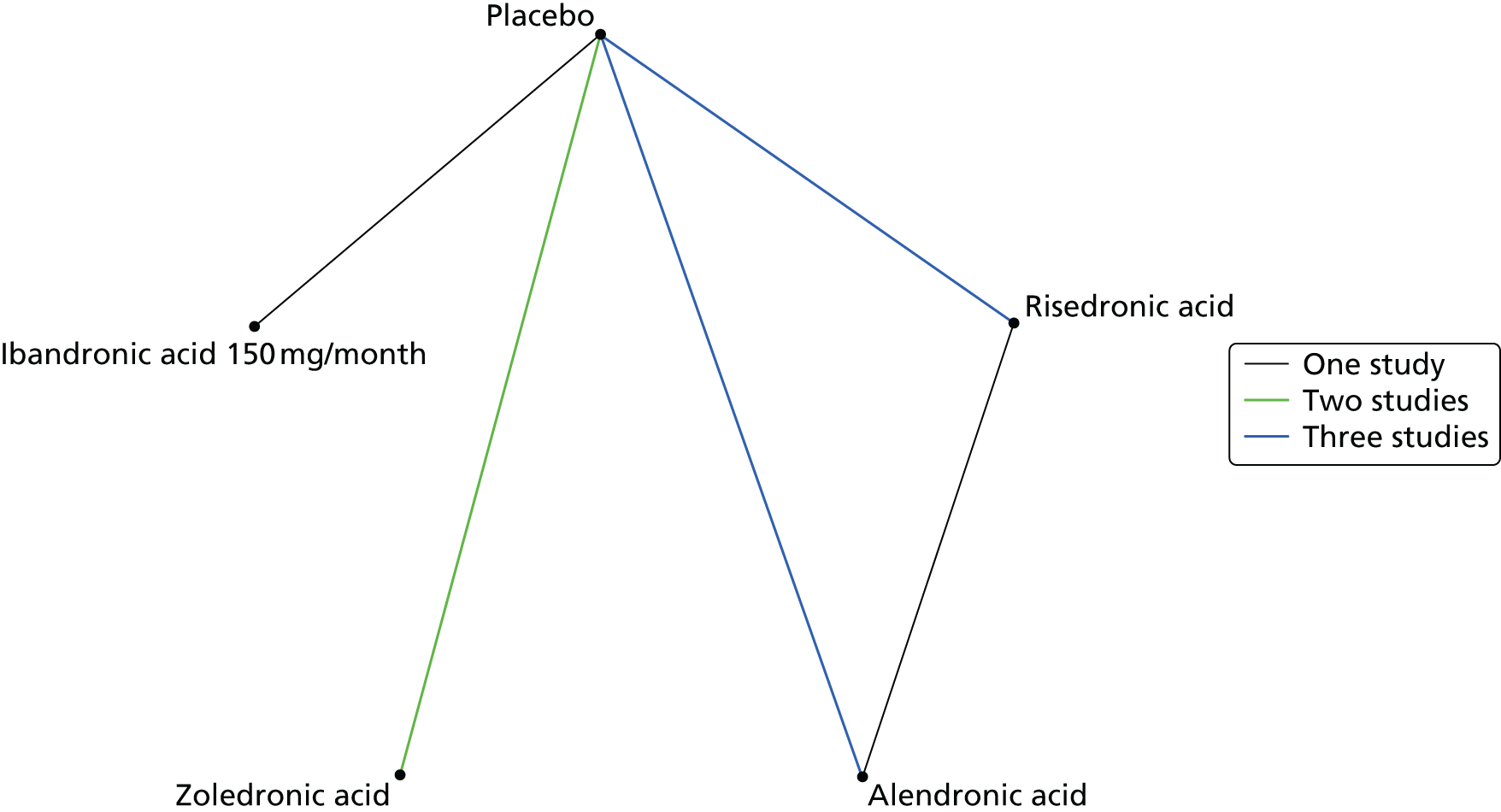
Figure 50 presents the effects of each treatment relative to placebo. The probabilities of treatment rankings are presented in Figure 51. The model fitted the data well, with a total residual deviance of 18.46 (compared with the total number of data points included in the analysis of 18). The DIC was 33.82. The between-study SD was estimated to be 0.43 (95% CrI 0.23 to 0.74), implying moderate heterogeneity in treatment effects between RCTs.
FIGURE 50.
Hip fractures: class-effects model – HRs and 95% CrIs. Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

FIGURE 51.
Hip fractures: class-effects model. Probability of treatment rankings. Note that the most efficacious = 1 and the least efficacious = 6. (a) Placebo, mean rank = 3.97; (b) risedronic acid, mean rank = 2.5; (c) alendronic acid, mean rank = 2.25; (d) zoledronic acid, mean rank = 3.34; and (e) 150 mg per month of oral ibandronic acid, mean rank = 2.95.
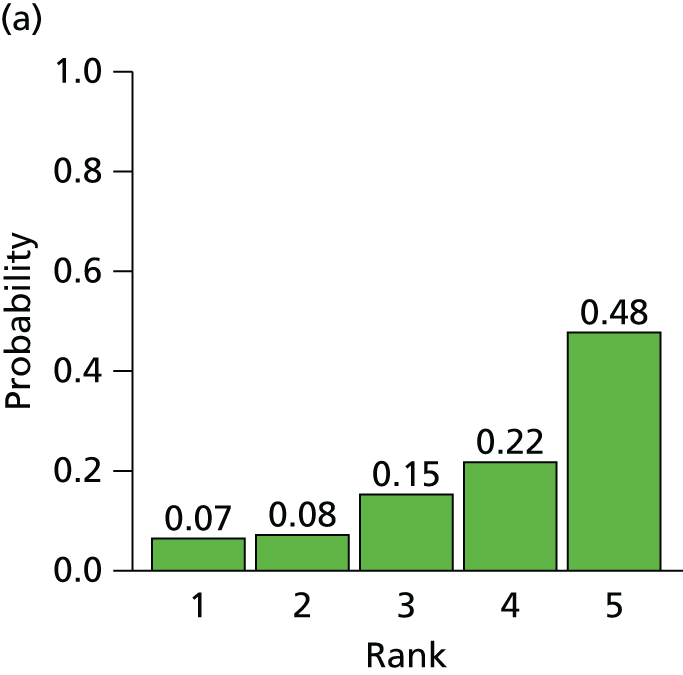
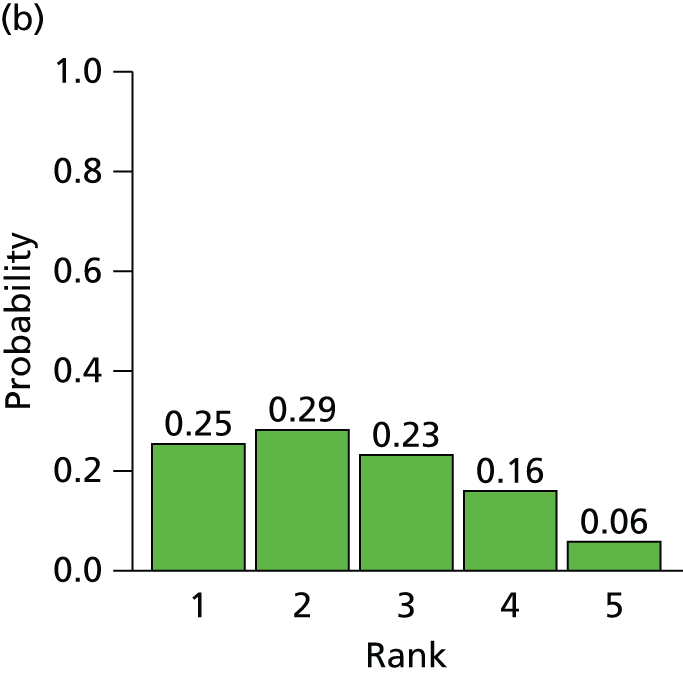
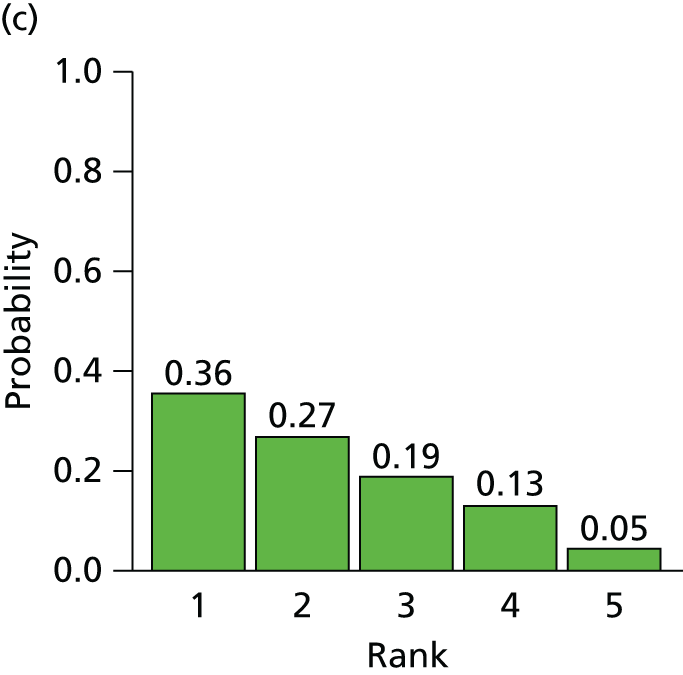
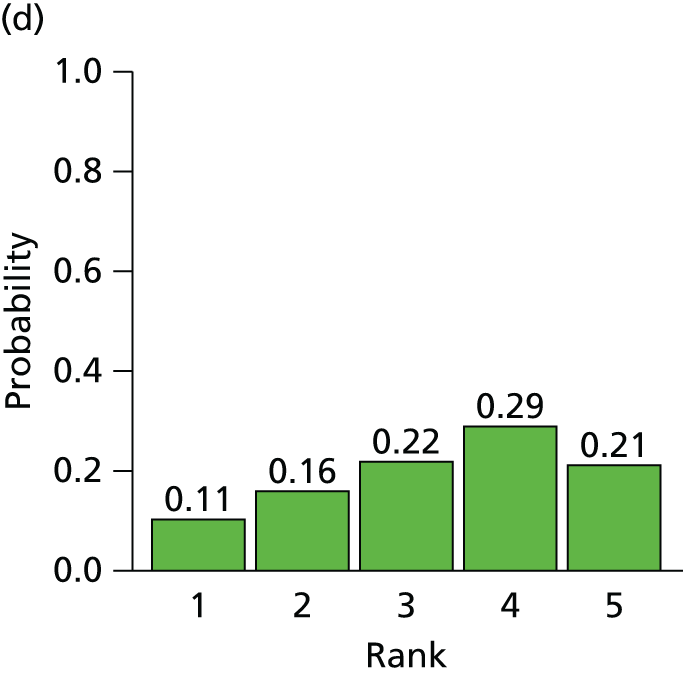

The between-treatment SD was estimated to be 0.19 (95% CrI 0.01 to 0.61), which is indicative of mild heterogeneity in treatment effects between treatments (i.e. the effects of the bisphosphonates are relatively similar) but with reasonable uncertainty.
All treatments were associated with beneficial treatment effects relative to placebo, although the treatment effects were not statistically significant at a conventional 5% level. Alendronic acid was associated with the greatest effect, with a HR of 0.79 (95% CrI 0.44 to 1.30) and was most likely to be the most effective treatment (probability 0.36 of being the most effective). The HR for a randomly chosen study for a new bisphosphonate is 0.85 (95% CrI 0.26 to 2.77).
The effect of baseline risk as a potential treatment effect modifier was explored using metaregression. For the model using standard reference priors there was evidence of poor convergence, and so weakly informative priors were used for placebo arms of two RCTs. 74,82 The model fitted the data well, with a total residual deviance of 18.78 (compared with 18 data points). The between-study SD was estimated to be 0.40 (95% CrI 0.06 to 0.75) and the between-treatment SD was estimated to be 0.19 (95% CrI 0.01 to 0.63). The between-study SD from fitting a random-effects model to the placebo baseline data was 0.46 (95% CrI 0.23 to 1.05), indicating moderate heterogeneity between RCTs. However, there was no evidence that treatment effect varied according to baseline risk, with the interaction term estimated to be 0.43 (95% CrI –0.79 to 1.67). In fact, including baseline risk did not improve the fit of the model to the data according to a comparison of DICs (33.48 vs. 33.82), and actually increased the estimate of the between-study SD of the treatment effect. Exchangeable and related treatment-specific interactions were also considered but did not provide a better fit to the data.
Wrist fractures: class-effects model
A NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid and 150 mg per month of oral ibandronic acid relative to placebo on the occurrence of wrist fractures. Data were available from seven RCTs,55,64,70,74,80,82,85 each comparing two treatments. Figure 52 presents the network of evidence for wrist fractures.
FIGURE 52.
Wrist fractures: network of evidence.

Owing to the limited indirect evidence, an assessment for inconsistency was not performed. Figure 53 presents the effects of each treatment relative to placebo. The probabilities of treatment rankings are presented in Figure 54. The model fitted the data well, with a total residual deviance of 13.32 (compared with the total number of data points included in the analysis of 12). The DIC was 23.23. The between-study SD was estimated to be 0.28 (95% CrI 0.03 to 0.66), implying mild to moderate heterogeneity in treatment effects between RCTs.
FIGURE 53.
Wrist fractures: class-effects model – HRs and 95% CrIs. Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

FIGURE 54.
Wrist fractures: class-effects model. Probability of treatment rankings. Note that the most efficacious = 1 and the least efficacious = 6. (a) Placebo, mean rank = 3.44; (b) risedronic acid, mean rank = 1.9; (c) alendronic acid, mean rank = 2.3; and (d) 150 mg per month oral ibandronic acid mean rank = 2.35.
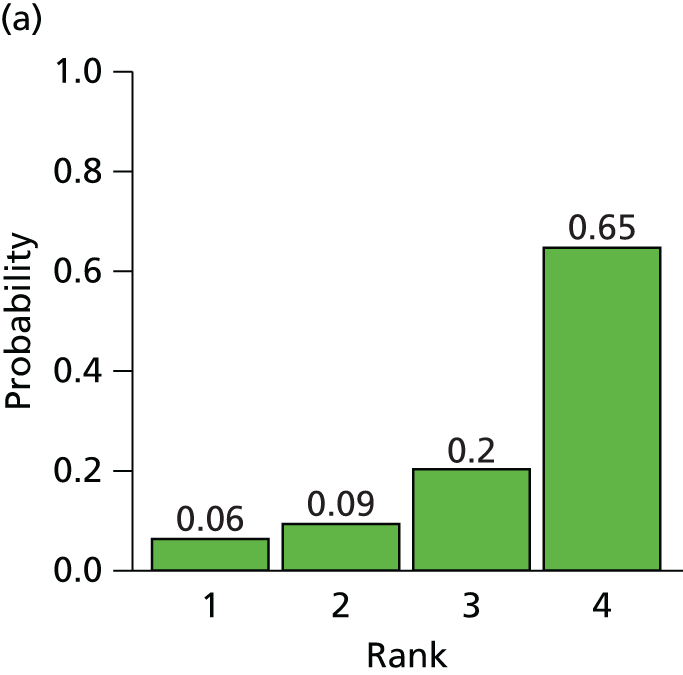
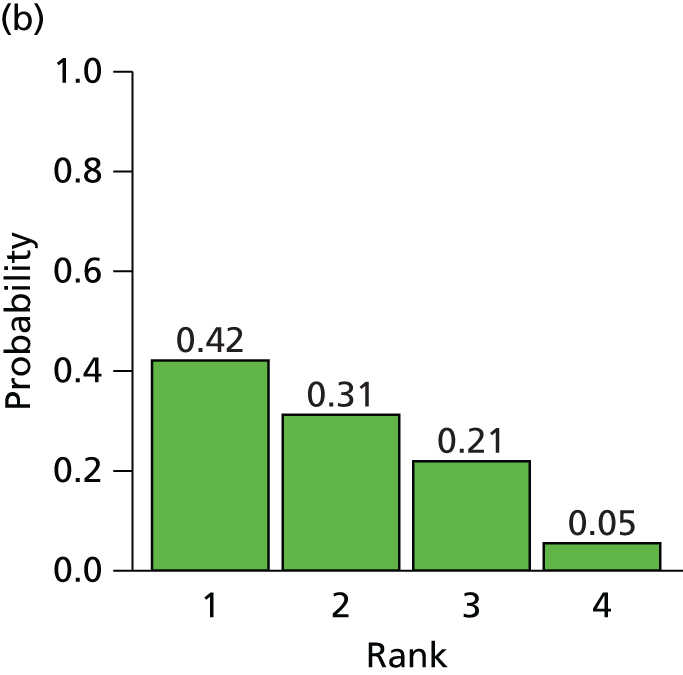


The between-treatment SD was estimated to be 0.17 (95% CrI 0.01 to 0.62), which is indicative of mild heterogeneity in treatment effects between treatments (i.e. the effects of the bisphosphonates are relatively similar) but with reasonable uncertainty.
All treatments were all associated with beneficial treatment effects relative to placebo, although the treatment effects were not statistically significant at a conventional 5% level. Risedronic acid was associated with the greatest effect, with a HR of 0.77 (95% CrI 0.39 to 1.28), and was most likely to be the most effective treatment (probability of 0.42 of being the most effective). No active treatment was statistically significantly more effective than another active treatment. The HR for a randomly chosen study for a new bisphosphonate was 0.81 (95% CrI 0.28 to 2.34).
The effect of baseline risk as a potential treatment effect modifier was explored using metaregression. For the model using standard reference priors there was evidence of poor convergence, and so weakly informative priors were used for placebo arms of two RCTs. 79,82 The model fitted the data well, with a total residual deviance of 15.21 (compared with 12 data points). The between-study SD was estimated to be 0.35 (95% CrI 0.04 to 0.75) and the between-treatment SD was estimated to be 0.17 (95% CrI 0.01 to 0.61). The between-study SD from fitting a random-effects model to the placebo baseline data was 0.44 (95% CrI 0.12 to 1.52), indicating moderate heterogeneity between RCTs. However, there was no evidence that treatment effect varies according to baseline risk, with the interaction term estimated to be –0.40 (95% CrI –2.58 to 1.38). In fact, including baseline risk did not improve the fit of the model to the data according to a comparison of DICs (25.85 vs. 23.23), and actually increased the estimate of the between-study SD of the treatment effect. Exchangeable and related treatment-specific interactions were also considered, but did not provide a better fit to the data.
Femoral neck bone mineral density: class-effects model
An NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 2.5 mg per day of oral ibandronic acid, 150 mg per month of oral ibandronic acid and 3 mg every 3 months of i.v. ibandronic acid relative to placebo, on the percentage change in femoral neck BMD. Data were available from 35 RCTs,45,47,49,53,55–59,62–68,70,72,73,75–77,79–81,83–88,90–92,95 each comparing two treatments. An assessment of inconsistency between direct and indirect evidence is presented in Figure 55. The network provided 21 direct treatment comparisons (edges in the network diagram). For 12 of these comparisons there is no direct evidence, leaving nine treatment comparisons to assess for consistency.
FIGURE 55.
Femoral neck BMD: class-effects model – assessing inconsistency using node splitting. (a) Nodes 1 and 2, placebo–alendronic acid; (b) nodes 1–3, placebo–risedronic acid; (c) nodes 1–4, placebo–zoledronic acid; (d) nodes 1–5, placebo–150 mg per month of oral ibandronic acid; (e) nodes 1–6, placebo–2.5 mg per day of oral ibandronic acid; (f) nodes 2–3, alendronic acid–risedronic acid; (g) nodes 2–5, alendronic acid–2.5 mg per day of oral ibandronic acid; (h) nodes 3–4, risedronic acid–zoledronic acid; and (i) nodes 5–6, 150 mg per month of oral ibandronic acid –2.5 mg per day of oral ibandronic acid. RE, random effects.
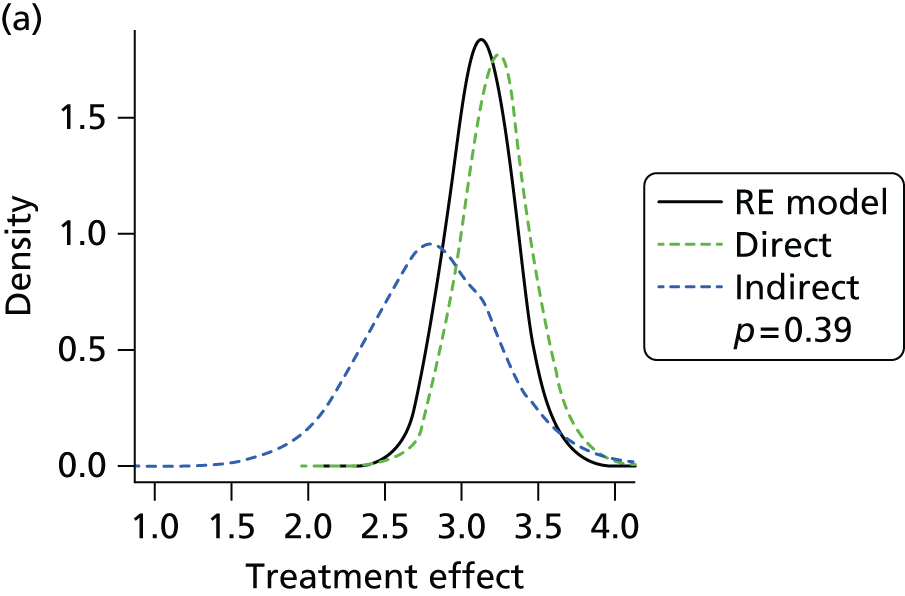
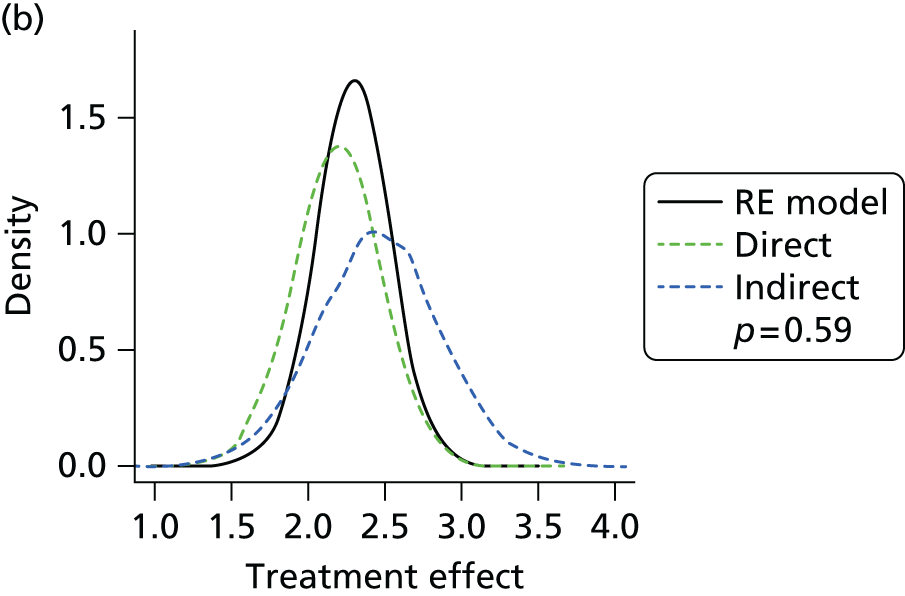
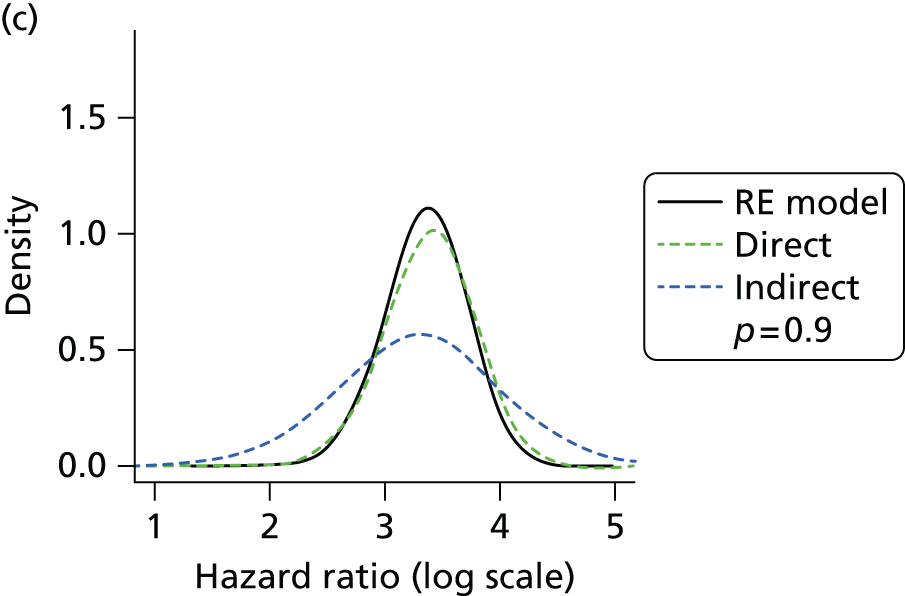

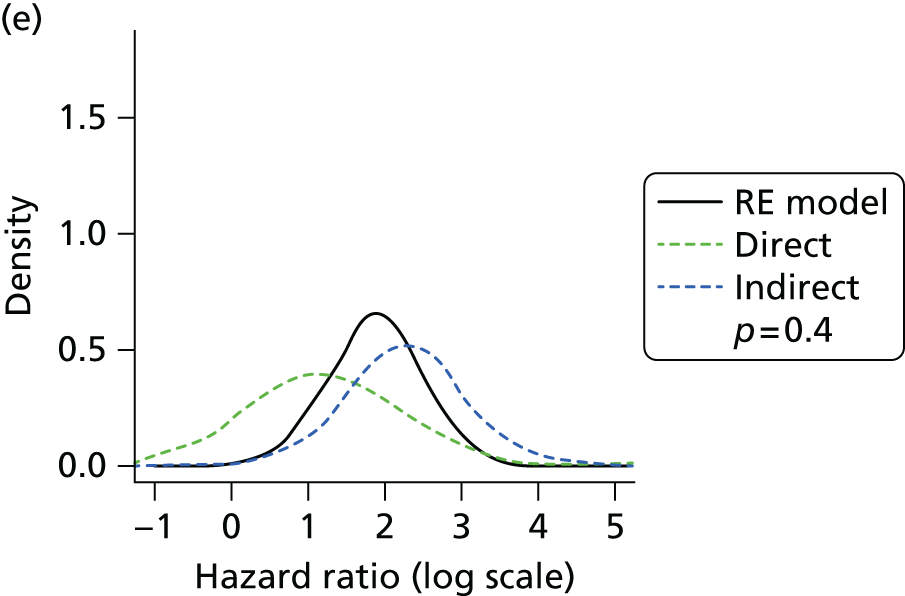
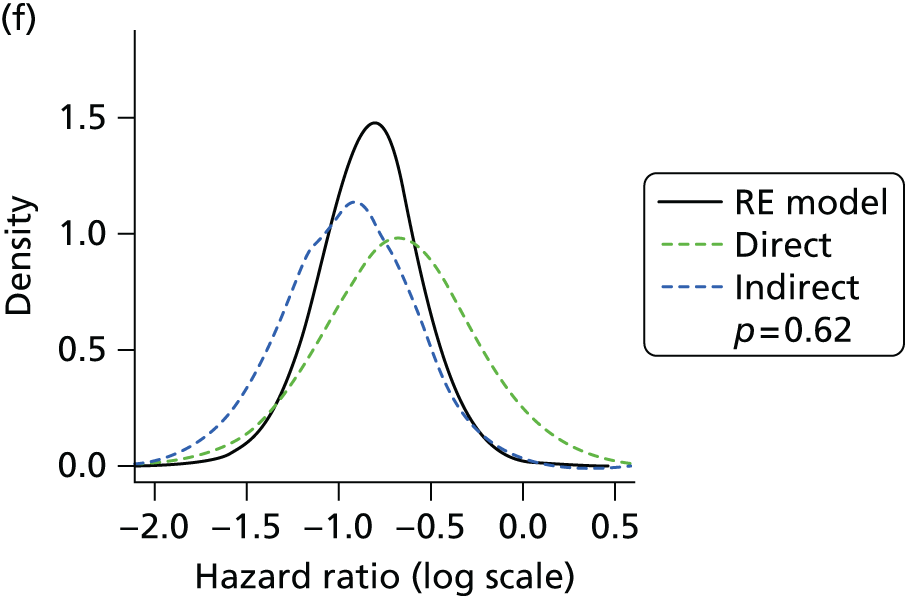
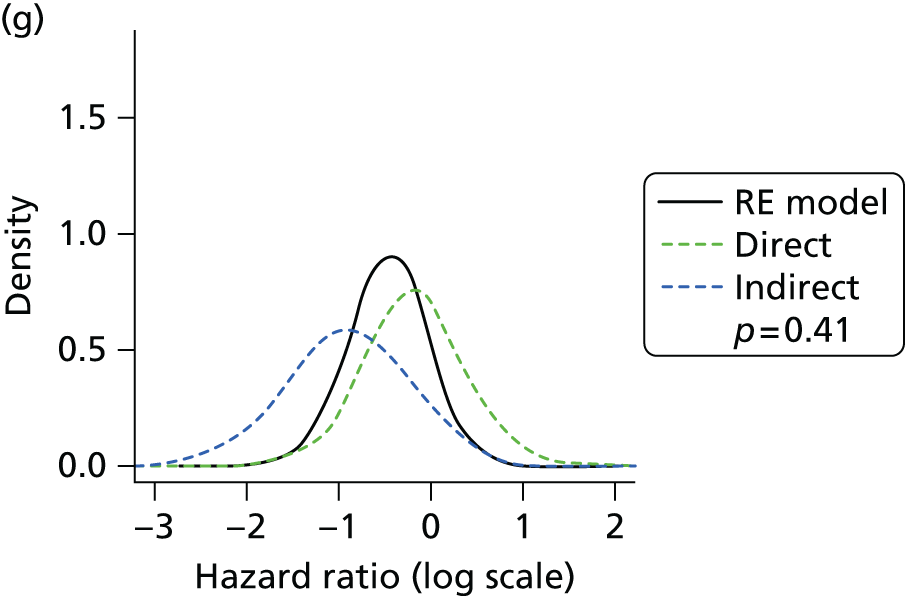
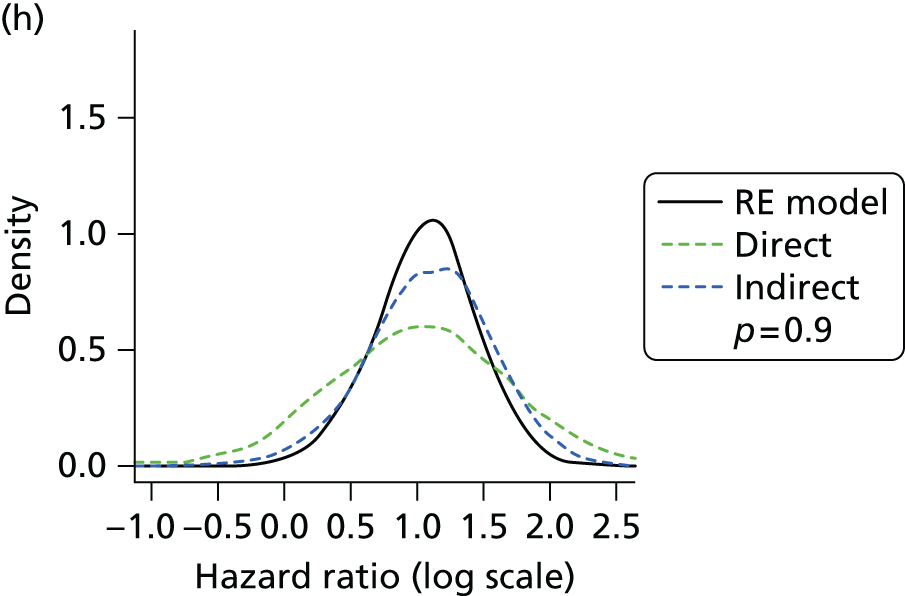
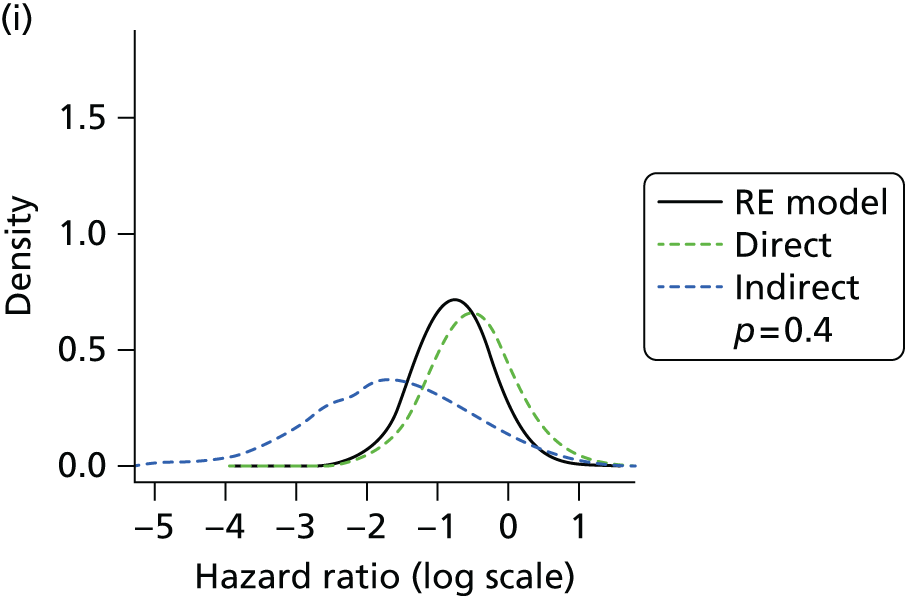
Figure 56 presents the network of evidence for femoral neck BMD. Nine RCTs55,56,64,68,72,76,85,87,90 presented summary statistics for each treatment group in graphical format while presenting the mean differences in percentage change in femoral neck BMD between treatments numerically in the text. A comparison of the numerical results and the graphically extracted results is presented in Figure 57, showing generally good but not identical correspondence between the two sample estimates.
FIGURE 56.
Bone mineral density: network of evidence.
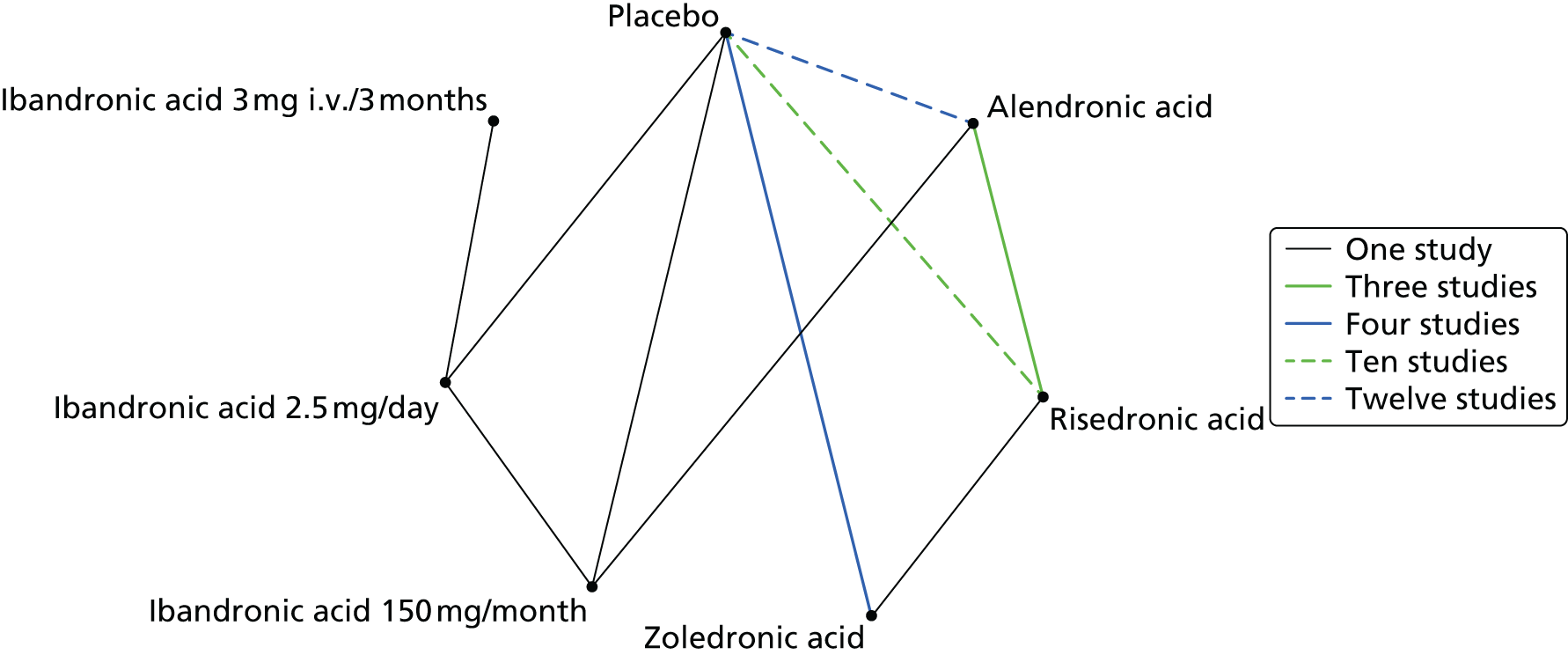
FIGURE 57.
Mean difference in percentage change in femoral neck BMD between treatments. Comparison of reported vs. computed (from graph estimates) values.
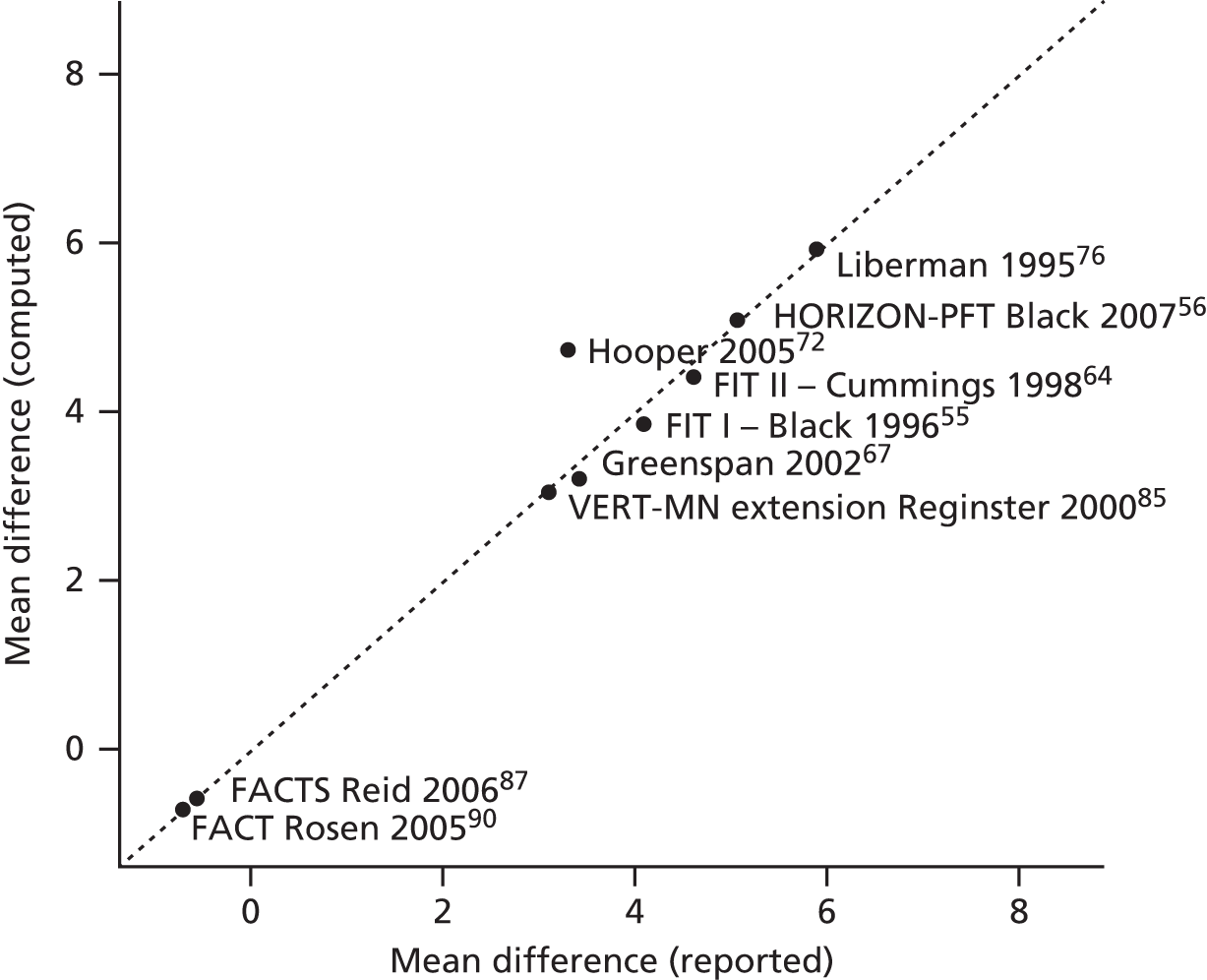
An assessment of inconsistency between direct and indirect evidence is presented in Figure 55. The network provided 21 direct treatment comparisons (edges in the network diagram). For 12 of these comparisons there is no direct evidence, leaving nine treatment comparisons to assess for consistency.
Figure 58 presents the effects of each treatment relative to placebo on percentage change in femoral neck BMD. The probabilities of treatment rankings are presented in Figure 59. The model fitted the data well, with a total residual deviance of 53.65 (compared with the number of data points included in the analysis of 59). The DIC was 96.5. The between-study SD was estimated to be 0.53 (95% CrI 0.30 to 0.86), implying moderate heterogeneity in treatment effects between RCTs.
FIGURE 58.
Femoral neck BMD: class-effects model – treatment effects and 95% CrIs. Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the left of the line favour the reference treatment (shown in green text). Treatment effects (TE) represent percentage change in BMD for a study of average duration (1.8 years).

FIGURE 59.
Femoral neck BMD: class-effects model. Probability of treatment rankings. Note that the most efficacious = 1 and the least efficacious = 6. (a) alendronic acid, mean rank = 2.13; (b) risedronic acid, mean rank = 5.14; (c) zoledronic acid, mean rank = 1.9; (d) ibandronic acid, mean rank = 3.48; (e) 2.5 mg per day of oral ibandronic acid, mean rank = 5.18; (f) 3 mg per 3 months of oral ibandronic acid, mean rank = 3.16; and (g) placebo, mean rank = 7.

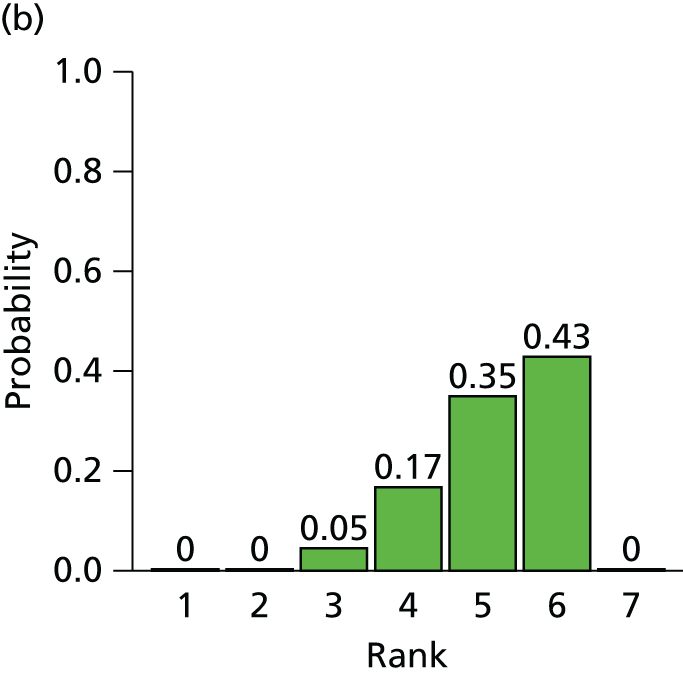

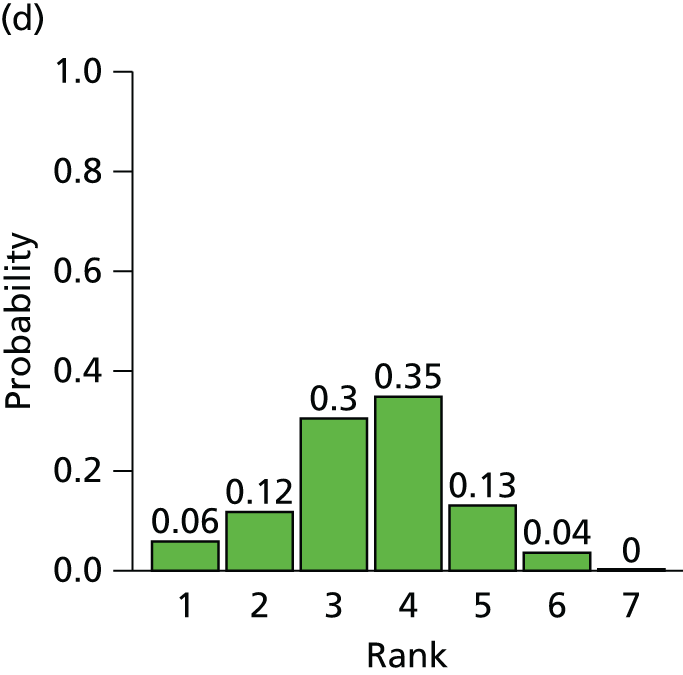
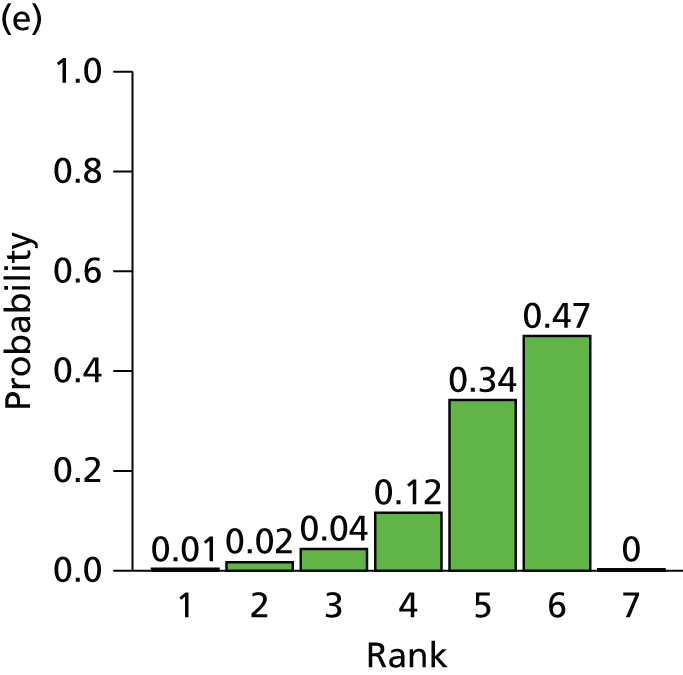


The between-treatment SD was estimated to be 0.56 (95% CrI 0.19 to 1.70), which is indicative of moderate heterogeneity in treatment effects between RCTs (i.e. the effects of the bisphosphonates are more dissimilar) but with considerable uncertainty.
The estimated interaction term for duration of study, assuming a common interaction for each treatment, was 0.89 (95% CrI 0.48 to 1.18). The estimated interaction term implies that treatment effects increase with duration of study. Exchangeable and related treatment-specific interactions were also considered. The model did not provide a better fit to the data (DIC = 97.36).
All treatments were associated with a beneficial effect relative to placebo on percentage change in femoral neck BMD, and all treatment effects were statistically significant at a conventional 5% level. Zoledronic acid was associated with the greatest effect, with a treatment effect of 3.21 (95% CrI 2.52 to 3.86), and was most likely to be the most effective treatment (a probability of 0.48 of being the most effective). The treatment effect for a randomly chosen study for a new bisphosphonate is 2.79 (95% CrI 0.72 to 4.75), allowing for both between-study and between-treatment heterogeneity.
The sample mean ages of the participants in each study ranged from 50.5 to 78.5 years, with an overall mean of 64.1 years. The effect of age as a potential treatment effect modifier was explored using metaregression. The model fitted the data well, with a total residual deviance of 53.97 (compared with 59 data points). The DIC was 97.99, suggesting that including age as a covariate in the model did not improve the model fit. The between-study SD was estimated to be 0.55 (95% CrI 0.31 to 0.88), and the between-treatment SD was estimated to be 0.56 (95% CrI 0.18 to 1.73). The interaction term for study duration in this model was 0.86 (95% CrI 0.47 to 1.25). There was no evidence that treatment effect varied according to age, with the interaction term estimated to be 0.01 (95% CrI –0.04 to 0.06). A model in which the treatment effect modifier for age was treated as separate but related (i.e. exchangeable) for each treatment was fitted, but this did not improve the model fit, with a DIC of 98.86.
Of the 35 RCTs included in the network, six RCTs58,59,62,73,83,95 included only male participants, 26 only female participants45,47,49,53,55–57,63–68,70,72,75,76,79–81,84–87,90,92 and three included both. 77,88,91 A metaregression was conducted to test for different treatment effects according to the proportion of male participants. In line with the licensing indications, interaction terms were not included for ibandronic acid treatments that are not licensed in men. The model fitted the data well, with a total residual deviance of 55.98 (compared with 59 data points). The between-study SD was estimated to be 0.51 (95% CrI 0.24 to 0.87). The between-treatment SD was estimated to be 0.45 (95% CrI 0.20 to 0.79) and the interaction term for study duration in this model was 0.81 (95% CrI 0.48 to 1.14). There was no evidence that treatment effect varied according to sex, with the interaction term estimated to be –0.79 (95% CrI –1.64 to 0.14). In fact, including sex did not improve the fit of the model to the data according to a comparison of DICs (98.24 vs. 96.5). Exchangeable and related treatment-specific interactions were also considered; the model did not provide a better fit to the data, with a DIC of 99.30.
The relationship between baseline response and treatment effect was also assessed. For the class-effects model with baseline response adjustment, there was evidence for poor convergence using standard reference priors, and so weakly informative priors were used for placebo arms of the RCTs with active treatment. The model fitted the data well, with a total residual deviance of 55.25 and DIC of 99.33. The between-study SD was estimated to be 0.51 (95% CrI 0.49 to 0.97) and the between-treatment SD was estimated to be 0.50 (95% CrI 0.19 to 1.38).
The between-study SD from fitting a random-effects model to the placebo baseline data was 1.05 (95% CrI 0.61 to 1.78). There was evidence of an interaction between baseline response and treatment effect, with the interaction term estimated to be –0.46 (95% CrI –0.76 to –0.13). Figure 57 presents the relationship between baseline response and treatment effect assuming a common interaction for each treatment. Including baseline response did not improve the fit of the model to the data according to a comparison of DICs, but did reduce the estimate of the between-study SD of the treatment effect. Exchangeable and related treatment-specific interactions were also considered. The model did not provide a better fit to the data, with a DIC of 100.43.
Sensitivity analysis 1
Sensitivity analysis 1 was conducted by excluding RCTs for which participants were switched from 5 mg per day of alendronic acid to 10 mg per day during the course of the study. 55,64 This affected the networks for vertebral and non-vertebral outcomes only.
A NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 150 mg per month of oral ibandronic acid and 2.5 mg per day of oral ibandronic acid relative to placebo on the occurrence of vertebral fractures. Data were available from 19 RCTs comparing two treatments. 45,56,58–60,63,65,66,70,72,76,77,81–83,85,86,88,89 The network of evidence is the same as that presented in Figure 42, except for the exclusion of the two alendronic acid RCTs,55,64 so that the modified network contains only four direct estimates between placebo and alendronic acid rather than six. Figure 60 presents the effects of each treatment relative to placebo. The model fitted the data well, with a total residual deviance of 36.78 (compared with the total number of data points included in the analysis of 38). The between-study SD was estimated to be 0.23 (95% CrI 0.02 to 0.59) and the between-treatment SD was estimated to be 0.20 (95% CrI 0.01 to 0.96). On exclusion of the two RCTs,55,64 a treatment effect of 0.45 (95% CrI 0.28 to 0.68) was estimated for alendronic acid. The estimated treatment effect was the same as before, but with an increase in uncertainty.
FIGURE 60.
Sensitivity 1: vertebral outcomes – class-effects model (HRs and 95% CrIs). Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).
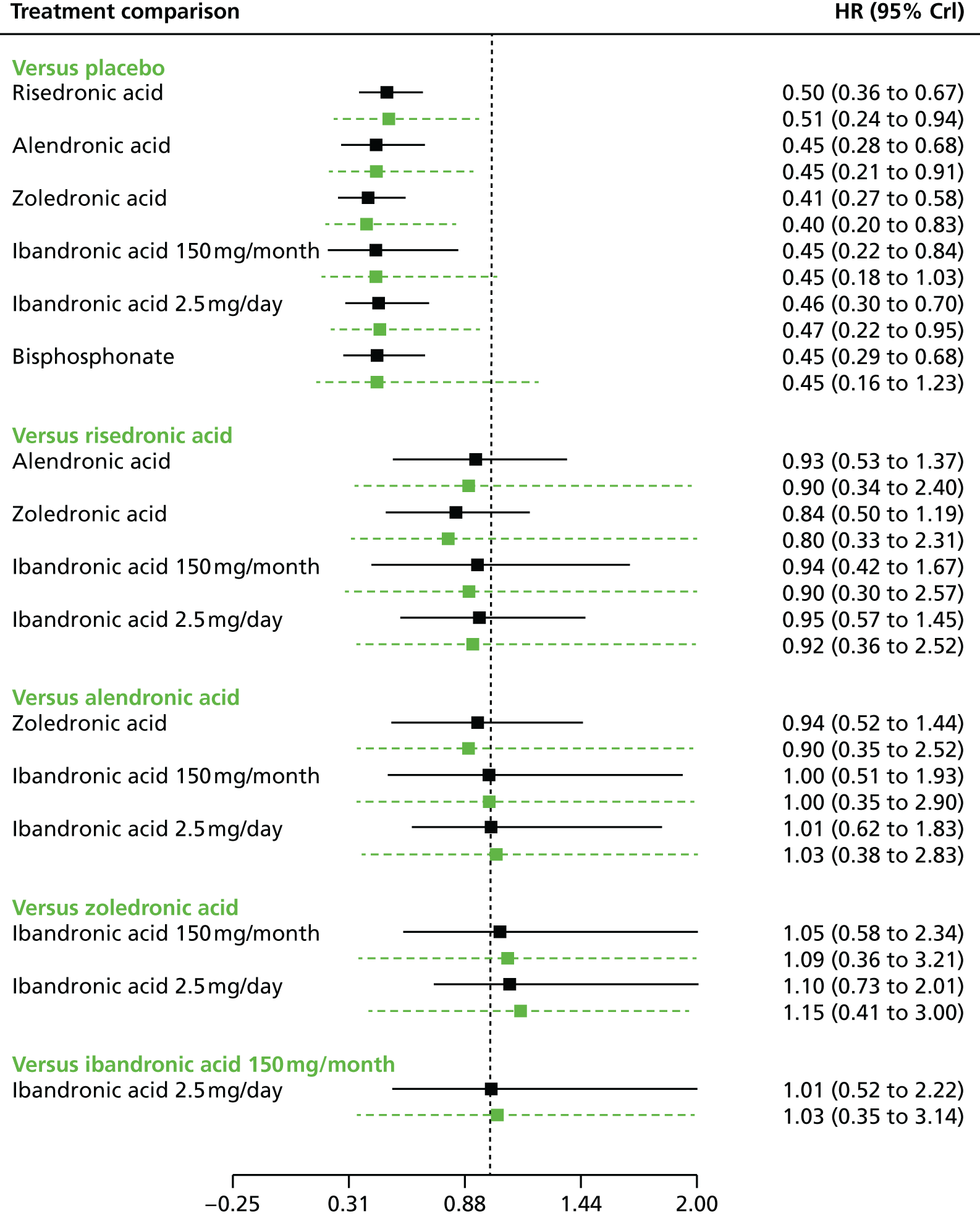
An NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 150 mg per month of oral ibandronic acid and 2.5 mg per day of oral ibandronic acid relative to placebo on the occurrence of non-vertebral fractures. Data were available from 12 RCTs comparing two treatments. 45,56,57,66,70,72,77,81,83–85,89 The network of evidence is the same as that presented in Figure 46, except for the exclusion of the two alendronic acid RCTs,55,64 so that the modified network contains only three direct estimates between placebo and alendronic acid rather than five. Figure 61 presents the effects of each treatment relative to placebo. The model fitted the data well, with a total residual deviance of 18.02 (compared with the total number of data points included in the analysis of 24). The between-study SD was estimated to be 0.10 (95% CrI 0.00 to 0.38) and the between-treatment SD was estimated to be 0.23 (95% CrI 0.01 to 1.00). On exclusion of the two RCTs,55,64 a more pronounced treatment effect of 0.68 (95% CrI 0.45 to 0.94) is observed for alendronic acid, compared with a value of 0.80 (95% CrI 0.65 to 0.94) estimated in the main analyses, Non-vertebral fractures: class-effects model, and there is an increase in uncertainty.
FIGURE 61.
Sensitivity 1: non-vertebral outcomes – class-effects model (HRs and 95% CrIs). Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).
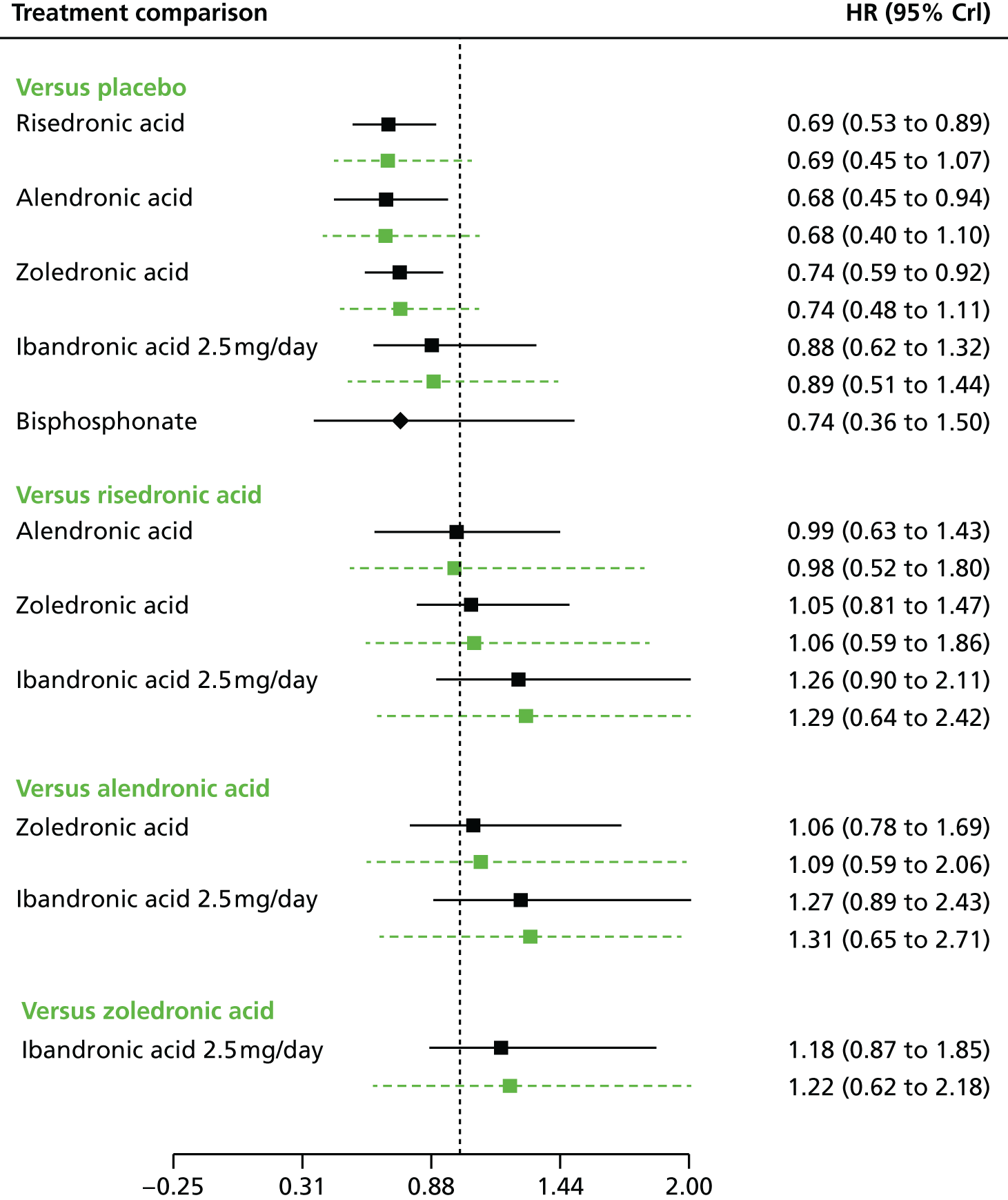
Sensitivity analysis 2
Sensitivity analysis 2 assessed vertebral fractures, including only the RCTs that used clinical/symptomatic assessment techniques. The network provides two comparisons for placebo against zoledronic acid and one comparison of placebo against risedronic acid.
Figure 62 presents the effects of each treatment relative to placebo. The model fitted the data well, with a total residual deviance of 6.32 being close to the six data points included in the analysis and a DIC of 11.68. The between-study SD was estimated to be 0.29 (95% CrI 0.02 to 0.72) and the between-treatment SD was estimated to be 0.18 (95% CrI 0.01 to 0.64). Both treatments are associated with beneficial treatment effects relative to placebo, significant at the 5% level. The HR for risedronic acid is 0.35 (95% CrI 0.17 to 0.72), compared with the HR of 0.50 (95% CrI 0.38 to 0.67) for all vertebral fractures. For zoledronic acid, the estimated HR is 0.34 (95% CrI 0.20 to 0.61), compared with 0.41 (95% CrI 0.28 to 0.56) for all vertebral fractures. No evidence was observed to suggest differential treatment effects according to assessment method.
FIGURE 62.
Sensitivity 2: clinically assessed vertebral outcomes – class-effects model (HRs and 95% CrIs).

Sensitivity analysis 3
Sensitivity analysis 3 assessed percentage change in femoral neck BMD, excluding the RCTs for which only graphically extracted results were available. 47,49,58,67 An NMA was used to compare the effects of alendronic acid, risedronic acid, zoledronic acid, 2.5 mg per day of oral ibandronic acid and 150 mg per month of oral ibandronic acid relative to placebo on the percentage change in femoral neck BMD. Data were available from 31 RCTs,45,53,55–57,59,62,66,68,70,72,73,75–77,79–81,83–88,90–92,95 each comparing two treatments. Figure 63 presents the network of evidence for femoral neck BMD.
FIGURE 63.
Sensitivity analysis 3: femoral neck BMD excluding graphically extracted results – network of evidence.

Figure 64 presents the effects of each treatment relative to placebo. The model fitted the data well, with a total residual deviance of 46.41 (compared with the number of data points included in the analysis of 55). The DIC was 81.56. The between-study SD was estimated to be 0.43 (95% CrI 0.16 to 0.77), implying moderate heterogeneity in treatment effects between RCTs. The between-treatment SD was estimated to be 0.65 (95% CrI 0.15 to 2.81). The estimated interaction term for duration of study, assuming a common interaction for each treatment, was 0.86 (95% CrI 0.55 to 1.18).
FIGURE 64.
Sensitivity analysis 3: femoral neck BMD excluding graphically extracted results – class-effects model [treatment effects (TEs) and 95% CrIs]. Note that the mean effect estimates are plotted in black; predictive effects in a new study are plotted in green beneath. Treatment effects to the left of the reference line favour the comparator treatment.

All treatments were still associated with a beneficial effect relative to placebo, and all treatment effects were statistically significant at a conventional 5% level. As in the full NMA presented in Femoral neck bone mineral density: class-effects model, zoledronic acid was associated with the greatest effect, with a treatment effect of 3.37 (95% CrI 2.69 to 3.97).
Discussion
A total of 46 RCTs were identified that provided data for the clinical effectiveness systematic review. 45,47,49,53–95 Alendronic acid was evaluated against placebo in 17 RCTs,53,55,57,60,61,64,65,67,71,73,76,83,84,91,93,94,98 while 2.5 mg per day of oral ibandronic acid was evaluated against placebo in three RCTs45,47,49 and against 3 mg per 3 months of i.v. ibandronic acid in one RCT. 49 Daily administration of 2.5 mg of oral ibandronic acid was compared with 150 mg per month oral administration in one RCT. 47 risedronic acid was compared with placebo in 12 RCTs58,62,63,66,70,72,75,78,85,86,89,95 and zoledronic acid was compared with placebo in four RCTs. 56,59,77,79 One RCT evaluated alendronic acid compared with 150 mg per month of oral ibandronic acid,81 five RCTs evaluated alendronic acid compared with risedronic acid,54,82,87,90,92 one RCT evaluated zoledronic acid compared with alendronic acid69 and one RCT evaluated zoledronic acid compared with risedronic acid. 88 The maximum trial duration was 48 months. 64
The risk of bias associated with the included RCTs was assessed using the Cochrane risk-of-bias instrument. Attrition ≥ 10% across treatment groups was evident for 29 (63%) of the included RCTs. 45,47,49,56,58,59,63,66,69,70,73,76,78–81,83–85,89,91,93,94,111 Five trials were reported as either open label or single blind, and were considered at high risk of bias of performance bias. 53,71,82,92,106 Blinded outcome assessment was reported by only 13 (28%) trials. 55,56,59,64,68,70,76,77,83,87–89,94
The outcome measures prespecified in the final NICE scope23 were addressed by the included trial evidence to varying degrees. Femoral neck BMD was the most widely reported outcome and fracture was the second most widely reported outcome. The majority of included trials reported AEs. Across the included trials there was limited reporting on outcomes of compliance (adherence and persistence), hospitalisation and service use, and quality of life.
A total of 27 RCTs provided suitable fracture data for inclusion in the fracture NMA:45,55–59,61,63–67,70,72,74,76–78,80–89 nine compared alendronic acid with placebo,55,57,61,64,65,67,76,83,84 two compared 150 mg per month of oral ibandronic acid with placebo,74,80 one compared 2.5 mg per day of oral ibandronic acid with placebo,45 nine compared risedronic acid with placebo,58,63,66,70,72,78,85,86,89 three compared zoledronic acid with placebo,56,59,77 one compared alendronic acid with risedronic acid;45 one compared 150 mg per month of oral ibandronic acid with alendronic acid82 and one compared zoledronic acid with risedronic acid. 88
A total of 35 RCTs provided suitable femoral neck BMD data for inclusion in the BMD NMA: 45,47,49,53,55–59,62–68,70,72,73,75–77,79–81,83–88,90–92,95 12 evaluated alendronic acid compared with placebo,53,55,57,64,65,67,68,73,76,83,84,91 one evaluated 2.5 mg per day of oral ibandronic acid compared with placebo;45 one evaluated 150 mg per month of oral ibandronic acid compared with placebo;80 one evaluated 2.5 mg per day of oral ibandronic acid compared with 3 mg every 3 months of i.v. ibandronic acid;49 one evaluated 2.5 mg per day of oral ibandronic acid compared with 150 mg per month of oral ibandronic acid;47 10 evaluated risedronic acid compared with placebo;58,62,63,66,70,72,75,85,86,95 four evaluated zoledronic acid compared with placebo;56,57,77,79 three evaluated alendronic acid compared with risedronic acid;56,77,79 one evaluated alendronic acid compared with 150 mg per month of oral ibandronic acid;81 and one evaluated zoledronic acid compared with risedronic acid. 88
Femoral neck BMD may be considered as a surrogate for fracture outcomes. Analysis of the femoral neck BMD data was of interest in order to confirm that the treatment effects were qualitatively the same. The analysis provided no evidence to suggest different treatment effects according to age or sex, with respect to percentage change in femoral neck BMD.
Based on the NMA, all treatments were associated with beneficial effects on each outcome measure relative to placebo. HRs for fracture varied from 0.41 to 0.92 depending on treatment and fracture site. All treatments resulted in statistically significant changes (at a conventional 5% level) in both vertebral fractures and percentage change in femoral neck BMD. Pairwise comparisons between treatments indicated that no active treatments were statistically significantly different from any other active treatment. For vertebral fractures and percentage change in femoral neck BMD, zoledronic acid had the greatest effect based on the midpoint estimates although in general the ranking of treatments varied for the different outcomes.
Assessment of vertebral fractures within the studies was based on both clinical and morphometric fractures. Ideally, the effect of assessment method would be assessed through metaregression; however, data for clinical fractures were limited. Consideration of the studies reporting clinical fractures did not provide any evidence to suggest different treatment effects according to assessment method.
The main analyses were based on a class-effects model such that the effects of each of the treatments are assumed to be related but not identical. The treatment effects estimated using the class-effects model were broadly similar qualitatively (i.e. direction of effect) and quantitatively (i.e. magnitude of effect) to those estimated using the standard random-effects model, but with the treatment effects in the class-effects model shrunk towards the overall bisphosphonate treatment effect. The qualitative effects of treatment (i.e. direction of effect) were the same for the majority of outcome types and treatments from the class effects and standard random-effects models with the exception of zoledronic acid (hip fractures), 150 mg per month of oral ibandronic acid (hip and wrist fractures) and 2.5 mg per day of oral ibandronic acid (non-vertebral fractures). Although the point estimates changed from being relative increases in effect in the standard random-effects model to relative decreases in effect in the class-effects model, there was considerable uncertainty about the true effects as reflected in the CrIs.
Non-vertebral fractures are used as a proxy for fractures of the proximal humerus, as fractures of the proximal humerus are not commonly reported. Two studies presented results for proximal humerus fractures, both considering the effects of risedronic acid against placebo. 70,85 A standard random-effects meta-analysis of these two studies provided a HR of 0.45 (95% CrI 0.13 to 1.41), which was greater than that estimated for non-vertebral fractures from the standard random-effects NMA, (HR 0.65, 95% CrI 0.47 to 0.88), and from the class-effects NMA (HR 0.71, 95% CrI 0.52 to 0.89), but with considerably more uncertainty.
There were no statistically significant differences between treatments in the incidence of upper GI events associated with any oral bisphosphonate compared with placebo when data were pooled across RCTs for each bisphosphonate. However, evidence from one RCT indicated a statistically significant risk of upper GI events in men receiving risedronic acid compared with those treated with placebo. 58 Where reported across the RCTs, treatments were prescribed in accordance with the SmPC for oral bisphosphonates to minimise gastric irritation. There was no evidence of significant differences between treatments in mortality across the RCT evidence when data were pooled by bisphosphonate. However, evidence from one RCT indicated that the proportion of men and women dying following hip fracture was significant higher in the placebo group than in the zoledronic acid group. 77 There was also no evidence of significant between-treatment differences in participants withdrawing because of AEs across the RCT evidence when data were pooled by bisphosphonate. However, in one RCT the proportion of men withdrawing because of AEs was significantly higher in the alendronic acid group than in the placebo group. 83
In agreement with the SmPC, there was evidence that zoledronic acid is associated with influenza-like symptoms . There was no statistically significant difference in the incidence of atrial fibrillation between those treated with zoledronic acid and those receiving placebo56,77 or risedronic acid. 88 There was no statistically significant difference in the incidence of bone pain between those treated with zoledronic acid and those receiving placebo88 or alendronic acid. 88 There was evidence that the risk of eye inflammation in the first 3 days following drug administration was significantly greater in those receiving zoledronic acid than in those receiving placebo. 102 Evidence from a single RCT indicated that the incidence of stroke over 36 months does not differ significantly among individuals receiving zoledronic acid and those receiving placebo. 77 All RCTs evaluating zoledronic acid reported no cases of spontaneous osteonecrosis of the jaw in any treatment group during the trial period.
Adverse events of hypocalcaemia and atypical femoral fracture were not reported as outcomes by any RCT of any bisphosphonate.
A summary of evidence from systematic reviews that include observational data indicates that alendronic acid, risedronic acid and oral ibandronic acid have similar rates of GI toxicity when compared with placebo. However, prescription event monitoring study data suggest a high level of reporting of a number of conditions in the first month of therapy with alendronic acid or risedronic acid, particularly those affecting the upper GI tract. Retrospective cohort data also suggest that switching patients who are stabilised on risedronic acid to alendronic acid is associated with an increased risk of GI AEs. Zoledronic acid may be compromised by renal toxicity, and myalgias and arthralgias are evident in the acute phase following i.v. administration. Intravenous bisphosphonates, especially zoledronic acid, are more likely to predispose patients to osteonecrosis of the jaw. However, in addition to bisphosphonate use, several other factors appear to be involved in the development of osteonecrosis of the jaw (e.g. dental trauma). There is an increased risk of atypical fracture among bisphosphonate users, but events are rare, and long-term bisphosphonate therapy might not be a prerequisite for development of atypical fractures. Moreover, the use of glucocorticoids and proton pump inhibitors is a potentially important risk factor for atypical fracture. Bisphosphonates are associated with serious atrial fibrillation, but heterogeneity of the existing evidence and a paucity of information on some agents preclude any definitive conclusions with respect to risk. The review evidence for the use of bisphosphonates and oesophogeal cancer is equivocal.
Evidence for persistence and adherence reported by RCTs was very limited. Where reported, high levels of compliance reported as a pill count were evident over the trial duration. A summary of evidence from systematic reviews including observational data indicates that, although patients using weekly bisphosphonate medication follow their prescribed regimens better than those using daily therapy, overall compliance and persistence rates are suboptimal for postmenopausal women receiving bisphosphonate therapy for the treatment of osteoporosis. Furthermore, one-third to one-half of patients, including men, being treated with bisphosphonates for osteoporosis do not take their medication as directed.
With the exception of the RCTs evaluating bisphosphonates in steroid users, the majority of RCTs included in the clinical effectiveness systematic review typically excluded people with underlying conditions that affect bone metabolism or people receiving medications that affect bone metabolism. Furthermore, people with history of, or receiving medication for, upper GI tract disorders were also excluded by the majority of included trials. Therefore, the effects of alendronic acid, ibandronic acid, risedronic acid and zoledronic acid are unknown in these populations.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Methods
The review of the published evidence surrounding the cost-effectiveness of bisphosphonates in the patient groups eligible for risk assessment within CG14616 was started by analysing the likely quantity of evidence available. A published systematic review by Müller et al. 136 included cost-effectiveness studies of screen-and-treat strategies for preventing osteoporotic fractures published between January 2006 and November 2011. Of the 24 papers included by Müller et al. ,136 22 examined the cost-effectiveness of bisphosphonates. However, only seven of these considered a UK setting. 137–143 Given the large number of published articles identified from this single systematic review, it was decided to limit the review to those papers reporting cost-effectiveness analyses for a UK setting as they would be more applicable to the decision problem defined in Chapter 2. None of the consultee submissions contained a de novo economic evaluation, so the review of existing cost-effectiveness evidence is limited to published sources.
Identification of studies
A comprehensive search was undertaken until 26 September 2014 to identify papers published in 2006 or later that evaluated the cost-effectiveness of alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid or zoledronic acid in any of the patient groups eligible for risk assessment within CG146. 16 Subject headings and keywords for ‘osteoporosis’ were combined with each of the named interventions and an economics search filter. The search strategy is provided in Appendix 1.
The following databases were searched:
-
MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE (via Ovid) 2006 to 23 September 2014
-
EMBASE (via Ovid) 2006 to 23 September 2014
-
Cochrane Database of Systematic Reviews (via Wiley Online Library) 2006 to 23 September 2014
-
Database of Abstracts of Reviews of Effects (via Wiley Online Library) 2006 to 23 September 2014
-
Health Technology Assessment Database (via Wiley Online Library) 2006 to 23 September 2014
-
NHS Economic Evaluation Database (via Wiley Online Library) 2006 to 23 September 2014
-
EconLit (via Ovid) 2006 to 23 September 2014
-
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost) 2006 to 23 September 2014
-
Science Citation Index Expanded (via Web of Science) 2006 to 23 September 2014
-
Conference Proceedings Citation Index – Science (via Web of Science) 2006 to 23 September 2014
-
Bioscience Information Service (via Web of Science) 2006 to 23 September 2014.
Published economic evaluations cited within the consultee submissions were cross-checked with those identified from the search.
Inclusions/exclusion criteria
Studies were included in the review if they reported full economic evaluations comparing alendronic acid, risedronic acid, oral ibandronic acid, i.v. ibandronic acid or zoledronic acid with each other or with no treatment. Studies were included if any of the population considered would be eligible for risk assessment within CG146. 16 For example, studies on postmenopausal women were included whether or not they specified that the women had risk factors, as those aged > 65 years would be eligible for risk assessment under CG146 even without risk factors being present. Studies that did not assess outcomes using QALYs or report the incremental cost per QALY of alternative treatment strategies were excluded. Studies that did not assess the cost-effectiveness of bisphosphonates within a UK setting were also excluded, as discussed above. Studies that assessed the cost-effectiveness of treatment with bisphosphonates at non-licensed doses were also excluded, as were studies that used bisphosphonates for other indications, such as the treatment of Paget’s disease or metastatic bone disease. Studies published prior to 2006 were excluded on the basis that the estimates of cost-effectiveness from older published studies are unlikely to be directly applicable to the decision problem outlined in the scope because of the availability of generic bisphosphonates, which has reduced the price of bisphosphonates over recent years. Studies were included only if they were reported as full papers, with conference abstracts being excluded from the review as they present insufficient detail to allow for a rigorous assessment of study quality. Studies not reported in English were also excluded.
Review methods
The results of the economic searches were sifted by title and abstract by one reviewer (AR). The full papers of studies that potentially met the inclusion criteria were retrieved for further inspection. Studies included in the systematic review were examined to determine whether or not they met the NICE reference case. 144 They were also critically appraised using the checklist published by Phillips et al. 145
Results
The study selection process is summarised in the form of a PRISMA diagram96 in Figure 65.
FIGURE 65.
Flow diagram of study selection process (adapted from PRISMA): cost-effectiveness review.

Quantity of evidence identified
The search identified 1058 unique articles, of which 1013 were excluded at the title and abstract stage. A further 37 were excluded at the full-paper stage, with the most common reasons being that they were conference abstracts presenting limited data. Table 50 in Appendix 7 provides the reasons for exclusion for those papers that were not excluded based on title or abstract. None of the consultee submissions identified any published analyses not already picked up through the systematic search.
Study characteristics
The characteristics of the included studies are summarised in Table 6. Six of the included studies137–141,146 were in postmenopausal women, with the remaining two being in populations with steroid-induced osteoporosis. 142,143
| Author, year of study publication and location | Population and interventions | Type of evaluation | Perspective | Time horizon | Cost year and cost discount rate | Cost source | Location of population and benefits discount rate | Benefits source and benefits instrument | Effectiveness data |
|---|---|---|---|---|---|---|---|---|---|
| van Staa et al., 2007143 UK |
Oral glucocorticoid users aged ≥ 40 years 5 years’ bisphosphonate treatment vs. no treatment |
Individual patient-based model | Not reported | 6 years | 2003/4 6% |
Analysis of resource allocation and standard UK reference sources | UK 1.50% |
Observational data EQ-5D |
Retrospective survey of medical notes |
| Kanis et al., 2008138 UK |
Postmenopausal women with risk factors 5 years’ alendronic acid treatment vs. no treatment |
Markov cohort model | Health care | 10 years and lifetime | Not reported 3.50% |
UK HES data combined with Swedish data | Sweden, Europe and the UK 3.50% |
Observational data EQ-5D |
Recent meta-analysis of trial results |
| van Staa et al., 2007141 UK |
Postmenopausal women 5 years’ risedronic acid/alendronic acid treatment vs. no treatment |
Individual patient-based model | Not reported | 10 years | Not reported 6% |
Analysis of resource allocation and standard UK reference sources | UK 1.50% |
See Stevenson et al.140 EQ-5D |
Retrospective survey of medical notes |
| Borgström et al., 2010137 UK |
Postmenopausal women 5 years’ risedronic acid treatment vs. no treatment |
Markov cohort model | Health care | Set according to the patient’s starting age so that the simulation ends at age 100 years | 2006 3.50% |
Standard UK and Swedish reference sources | Sweden and UK 3.50% |
Observational data EQ-5D |
Recent meta-analysis of trial results |
| Stevenson et al., 2005140 UK |
Postmenopausal women Multiple interventionsa |
Patient-level Markov model | NHS and PSS | Patients lifetime | 2001/2 6% |
Standard UK reference sources | Not reported 1.50% |
Observational data EQ-5D |
Meta-analysis conducted by authors |
| Ström et al., 2007139 UK |
Patients from the fracture intervention trial 5 years’ alendronic acid treatment vs. no treatment |
Markov cohort model | Health payer | Set according to the patient’s starting age so that the simulation ends at age 100 years | 2004 3.50% |
Standard UK reference sources, academic papers personal communication | Sweden and the UK 3.50% |
Observational data EQ-5D |
Results of the fracture intervention trial |
| Kanis et al., 2007142 UK |
Oral glucocorticoid users age ≥ 40 years 5 years’ bisphosphonate treatment vs. no treatment |
Patient-level Markov model | NHS and PSS | 10 years and lifetime | 2004/5 (drugs 2006) 6% |
Analysis of resource allocation and standard UK reference sources | Sweden 1.50% |
Observational data EQ-5D |
Meta-analysis conducted by authors |
| Borgström et al., 2006146 Australia, Germany, Japan, Spain, Sweden, the UK and the USA |
Postmenopausal women 5 years’ bisphosphonate treatment vs. no treatment |
Markov cohort model | Societal | Set according to the patient’s starting age so that the simulation ends at age 100 years | 2004 3.50% |
Standard UK reference sources and academic papers | Sweden 3.50% |
Observational data EQ-5D |
Assumption |
Three studies137–139 compared a single bisphosphonate with no treatment, one study140 compared multiple bisphosphonate strategies head to head and with no treatment, and four studies141–143,146 compared a strategy of bisphosphonates with no treatment without specifying the exact bisphosphonate used. All of the included studies assumed that treatment with bisphosphonates lasts 5 years.
Six studies137–140,142,146 used a Markov model framework, with four137–139,146 using a cohort-level modelling approach and two140,142 using a patient-level Markov simulation based on the same underlying model. The remaining two papers141,143 described an individual patient-based pharmacoeconomic model using patient-level data from two large GP record databases [General Practice Research Database (GPRD) and The Health Improvement Network].
Two studies140,142 explicitly reported using a NHS and Personal Social Services (PSS) perspective, while a further three studies137–139 reported using a health-care perspective and one reported a societal perspective. 146 The remaining two studies141,143 did not explicitly report their perspective although many of the costs used were taken from Stevenson et al. 140 which used a NHS and PSS perspective. Discounting consistent with the current NICE reference case144 (3.5% for both costs and QALYs) was applied in four of the studies,137–139,146 whereas alternative discounting at rates (6% for costs and 1.5% for QALYs) were used in the remaining four papers. 140–143 The time horizon varied from 6 years to a lifetime horizon or age of 100 years.
Evidence sources used
The study conducted by Stevenson et al. 140 was a systematic review of the literature to estimate the costs associated with osteoporotic fractures. The remaining studies used various sources including personal communication and pre-exiting literature, with two studies137,138 quoting the same source, Stevenson et al. 147
For all published cost-effectiveness studies the costs of the pharmaceutical agents were ultimately taken from the appropriate version of the British National Formulary for their cost year. The costs of case finding, BMD testing and consultations with GPs were obtained from various sources including the appropriate versions of the NHS reference costs and the Unit Costs of Health and Social Care or were assumed.
Health-related quality of life was obtained using utility multipliers for fracture states taken from the literature. The studies use different categories of fracture, with hip fracture, vertebral fracture, forearm/wrist fracture and humerus fracture being the most common. One study had the additional categories of pelvic fracture, tibia fracture, clavicle, scapula or sternum fracture and rib fracture. 142 Three studies further split hip fracture into hip fracture leading to nursing home admission and hip fracture not leading to nursing home admission. 140,141,143 Seven studies split utility multipliers for fractures into those for the year of fracture and those in subsequent years. 137–143 The remaining study split multipliers for fractures into those for the year of fracture and those in the year following fracture and those in subsequent years. 146
The National Institute for Health and Care Excellence reference case
Both studies by van Staa et al. 141,143 used data from a retrospective analysis of patient notes rather than RCT evidence, as required by the NICE reference case. 144 These authors also reported results using a 10-year time horizon rather than the lifetime horizon, which is required by the NICE reference case. The study by Borgström et al. 146 failed to meet the requirements of the NICE reference case as the RR reduction used in the study was based on an assumption involving the expected distribution of osteoporotic fractures dependent on age and the subsequent utility loss rather than on the evidence. Additionally, the study by Ström et al. 139 failed to meet the requirements of the NICE reference case by using efficacy data from a single RCT, but the study did present the results of a sensitivity analysis using data from a published meta-analysis. Two papers, by Stevenson et al. 140 and Kanis et al. ,142 which used the same underlying model but applied it in two different populations, used differential discount rates of 6% for future costs and 1.5% for future benefits rather than 3.5% for both future costs and future benefits as required by the NICE reference case. However, Kanis et al. 142 did report that using discount rates of 3.5% for both future costs and future benefits had only a minor effect on the results. In addition to the points above, none of the included studies compared all four bisphosphonates specified within the scope of this appraisal in a fully incremental analysis as required by the NICE reference case.
Quality of studies
The quality of the studies was generally good when appraised using the checklist published by Phillips et al. 145 Responses for each individual study are provided in Table 7. Five of the studies met > 50% of the checklist criteria. 137–140,142 The studies commonly performed badly on the questions related to internal and external consistency, with none of the models providing an adequate description of the quality assurance processes used to demonstrate internal validity and none demonstrating that the model had been calibrated against external data sources. All of the models assessed patient-level heterogeneity by running the model for subgroups of patients with different characteristics. However, none of the papers adequately addresses all types of uncertainty (structural, parameter, methodological). Three of the models139,140,142 assessed parameter uncertainty using analysis [probabilistic sensitivity analysis (PSA)], but in the other five cases this was either not done or not clearly reported. Only two of the studies140,142 adequately addressed the quality of the input data and there was limited discussion of the methods used to derive the utility weights applied in the model.
| Criterion | Question | Author and year of study publication | |||||||
|---|---|---|---|---|---|---|---|---|---|
| van Staa et al., 2007143 | Kanis et al., 2008138 | van Staa et al., 2007141 | Borgström et al., 2010137 | Stevenson et al., 2005 140 | Ström et al., 2007139 | Kanis et al., 2007142 | Borgström et al., 2006146 | ||
| S1: statement of decision problem/objective | Is there a clear statement of the decision problem? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the objective of the evaluation and model specified consistent with the stated decision problem? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Is the primary decision-maker specified? | No | Yes | No | Yes | Yes | No | Yes | No | |
| S2: statement of scope/perspective | Is the perspective of the model clearly stated? | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Are the model inputs consistent with the stated perspective? | NA | Yes | NA | Yes | Yes | Yes | Yes | Yes | |
| Has the scope of the model been stated and justified? | No | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| Are the outcomes of the model consistent with the perspective, scope and overall objective of the model? | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | |
| S3: rationale for structure | Is the structure of the model consistent with a coherent theory of the health condition under evaluation? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the sources of data used to develop the structure of the model specified? | No | Yes | No | Yes | Yes | Yes | Yes | No | |
| Are the causal relationships described in the model structure justified appropriately? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| S4: structural assumptions | Are the structural assumptions transparent and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the structural assumptions reasonable given the overall objective, perspective and scope of the model? | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | |
| S5: strategies/comparators | Is there a clear definition of the options under evaluation? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Have all the feasible and practical options been evaluated? | No | No | No | No | Yes | Yes | Yes | No | |
| Is there justification for the exclusion of feasible options? | No | No | No | No | NA | NA | NA | No | |
| S6: model type | Is the chosen model type appropriate given the decision problem and specified causal relationships within the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S7: time horizon | Is the time horizon of the model, the duration of treatment and the duration of treatment effect described and justified? | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| S8: disease states/pathways | Do the disease states (state transition model) or the pathways (decision tree model) reflect the underlying biological process of the disease in questions and the impact of interventions? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| S9: cycle length | Is the cycle length defined and justified in terms of the natural history of the disease? | NA | Yes | NA | Yes | Yes | Yes | Yes | No |
| D1: data identification | Are the data identification methods transparent and appropriate given the objective of the model? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Where choices have been made between data sources, are these justified appropriately? | No | Yes | No | No | Yes | Yes | Yes | No | |
| Has particular attention been paid to identifying data for the important parameters in the model? | Yes | No | Yes | Yes | Yes | Yes | Yes | No | |
| Has the quality of data been assessed appropriately? | No | No | No | No | Yes | No | Yes | No | |
| Where expert opinion has been used, are the methods described and justified? | NA | NA | NA | NA | NA | NA | NA | NA | |
| D2: pre-model data analysis | Is the data modelling methodology based on justifiable statistical and epidemiological techniques? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| D2a: baseline data | Is the choice of baseline data described and justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Are transition probabilities calculated appropriately? | NA | Unknown | NA | Unknown | Unknown | Unknown | Unknown | Unknown | |
| Has half-cycle correction been applied appropriately to both costs and outcomes? | NA | Unknown | NA | Yes | Unknown | Unknown | Unknown | Unknown | |
| If not, has the omission been justified? | NA | NA | NA | NA | NA | NA | NA | NA | |
| D2b: treatment effects | If relative treatment effects have been derived from trial data, have they been synthesised correctly using appropriate techniques? | NA | NA | NA | Yes | Yes | Yes | Yes | NA |
| Have the methods and assumptions used to extrapolate short-term results to final outcomes been documented and justified? | NA | NA | NA | NA | NA | NA | NA | NA | |
| Have alternative extrapolation assumptions been explored through sensitivity analysis? | NA | NA | NA | NA | NA | NA | NA | NA | |
| Have assumptions regarding the continuing effect of treatment once treatment is completed been documented and justified? | No | Yes | Unknown | Yes | Yes | Yes | Yes | Yes | |
| Have alternative assumptions regarding the continuing effect of treatment been explored through sensitivity analysis? | Yes | Yes | NA | Yes | No | Yes | Yes | Yes | |
| D2c: costs | Are the costs incorporated in the model justified? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Has the source of all costs been described? | No | No | No | Yes | Yes | Yes | Yes | No | |
| Have discount rates been described and justified given the target decision-maker? | NA | Yes | NA | Yes | Yes | Yes | Yes | Yes | |
| D2d: quality-of-life weights (utilities) | Are the utilities incorporated into the model appropriate? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the source of utility weights referenced? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Are the methods of derivation of the utility weights justified? | No | Yes | No | No | Yes | No | Yes | No | |
| D3: data incorporation | Have all data incorporated into the model been described and referenced in sufficient detail? | No | Yes | No | Yes | Yes | Yes | Yes | No |
| Has the use of mutually inconsistent data been justified (i.e. are the assumptions and choices appropriate)? | No | No | No | No | No | No | No | No | |
| Is the choice of data incorporation transparent? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| If data have been incorporated as distributions, has the choice of distribution for each parameter been described and justified? | No | Unknown | No | No | No | No | No | No | |
| If data have been incorporated as distribution, is it clear that second order uncertainty is reflected? | No | Yes | No | Yes | Yes | Yes | Yes | No | |
| D4: assessment of uncertainty | Have the four principal types of uncertainty been addressed? | No | No | No | No | No | No | No | No |
| If not, has the omission of particular forms of uncertainty been justified? | No | No | No | No | No | No | No | No | |
| D4a: methodological | Have the methodological uncertainties been addressed by running alternative versions of the model with different methodological assumptions? | No | No | No | No | No | No | No | No |
| D4b: structural | Is there evidence that structural uncertainties have been addressed via sensitivity analysis? | No | No | No | No | No | Yes | No | Yes |
| D4c: heterogeneity | Has heterogeneity been dealt with by running the model separately for different subgroups? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| D4d: parameter | Are the methods of assessment of parameter uncertainty appropriate? | No | Unknown | No | Unknown | Yes | Yes | Yes | No |
| If data are incorporated in the point estimates, are the ranges used for sensitivity analysis stated clearly and justified? | No | No | No | Unknown | No | Unknown | Unknown | No | |
| C1: internal consistency | Is there evidence that the mathematical logic of the model has been tested thoroughly before use? | No | No | No | No | No | No | No | No |
| C2: external consistency | Are any counterintuitive results from the model explained and justified? | No | No | No | No | No | No | No | No |
| If the model has been calibrated against independent data, have any differences been explained and justified? | No | No | No | No | No | No | No | No | |
| Have the results of the model been compared with those of previous models and any difference in results explained? | No | Yes | Yes | Yes | No | No | No | No | |
Study conclusions
All of the studies report a range of incremental cost-effectiveness ratios (ICERs) for patients with different characteristics. Patient age, BMD, the presence of prior fracture and the presence of other clinical risk factors all appear to have a significant influence on the ICER based on the included studies. The duration of treatment and the offset duration (the time over which the treatment still has an effect on fracture risk following discontinuation), as well as patient adherence to treatment, may have a lesser influence on the cost-effectiveness. Given that none of the studies used current prices for bisphosphonates and these have fallen substantially since the time these studies were published, further details on the ICERs are not reported.
Summary of existing cost-effectiveness evidence
Although a number of published studies were identified that assessed the cost-effectiveness of bisphosphonates, and the quality of those studies was generally good, none of the included studies compared all the bisphosphonate treatments specified within the scope of this appraisal in a fully incremental analysis as required by the NICE reference case. 144 Furthermore, the cost of generic formulations of bisphosphonates has fallen since these studies were conducted. The results reported by these studies were, therefore, considered to have limited applicability to the decision problem described in Chapter 2. However, these studies were used as a source of model parameters and assumptions for the independent economic assessment described in Independent economic assessment.
Independent economic assessment
Modelling rationale and overview
A de novo economic analysis was considered necessary in order to properly address the decision problem outlined in the scope, as none of the economic evaluations identified in Systematic review of existing cost-effectiveness evidence compared all five bisphosphonate treatments specified within the scope of this appraisal in a fully incremental analysis as required by the NICE reference case. 144
In the scope for this appraisal23 it was stated that this MTA would ‘develop the framework to link absolute fracture risk with intervention thresholds, based on cost effectiveness’. Therefore, in order to provide information that might inform intervention thresholds, expressed in terms of absolute risk, the aim of the de novo economic evaluation was to estimate the cost-effectiveness of the five bisphosphonates treatments compared with no treatment for patients at varying levels of absolute fracture risk. The overall population is those eligible for risk assessment under CG146,16 but it is divided into risk categories based on the estimates of fracture risk provided by the QFracture and FRAX risk assessment tools.
Discrete event simulation (DES) was used to estimate lifetime costs and QALYs for each bisphosphonate treatment strategy and a strategy of no treatment for a simulated cohort of patients with heterogeneous characteristics. The model was populated with effectiveness evidence from the systematic review and NMA described in Chapter 3. All other parameters were estimated from published sources. Evidence on the impact of fracture on HRQoL was identified from a systematic review. The published economic evaluations described in in Systematic review of existing cost-effectiveness evidence were used to identify other data sources that could be used to inform model parameters. A NHS and PSS perspective was taken and costs and benefits were discounted at 3.5% per annum. A brief summary of the modelling methodology and key data sources is provided in Table 8 alongside information of where further details can be found in Methods below.
| Model feature | Summary | Chapter and section heading |
|---|---|---|
| Decision problem | To assess the cost-effectiveness of bisphosphonates compared with no treatment at varying levels of absolute fracture risk, as defined by the FRAX and QFracture risk assessment tools | Chapter 2, Chapter 4, Modelling rationale and overview, and Chapter 4, Specifying the model population |
| Type of economic evaluation | Cost-effectiveness analysis, with benefits expressed as QALYs | Chapter 4, Modelling rationale and overview |
| Population/subgroups | The model simulates the heterogeneous patient population eligible for risk assessment under CG14616 The population is stratified into 10 risk categories and results presented for each risk category. This is done once using FRAX and once using QFracture |
Chapter 4, Specifying the model population |
| Interventions | Oral alendronic acid Oral risedronic acid Oral ibandronic acid i.v. ibandronic acid i.v. zoledronic acid |
Chapter 4, Treatment strategies |
| Comparators | No treatment | Chapter 4, Treatment strategies |
| Perspective | NHS and PSS | Chapter 4, Age- and sex-specific utility values in the absence of clinical events; and Chapter 4, Resource use and costs for bisphosphonates treatment |
| Model type | Discrete-event simulation with heterogeneous patient population | Chapter 4, Model structure |
| Model events | Clinical events are fracture, death (all-cause mortality and fracture-related mortality) and nursing home admission. There are four possible fracture events (hip, wrist, vertebral and proximal humerus), with fracture at other sites included by increasing the incidence of these events Dummy events are used to update attributes 1 year after fracture and to update the fracture risks once treatment finishes |
Chapter 4, Model structure |
| Time horizon | Lifetime (up to age of 100 years) | Chapter 4, Model structure |
| Duration of treatment | Mean duration of persistence with treatment from observational studies | Chapter 4, Treatment strategies |
| Natural history | Time to fracture is based on the estimate of absolute fracture risk for major osteoporotic fractures (hip, wrist, proximal humerus and vertebral) provided by either QFracture or FRAX, which are uplifted to include fractures at additional sites. The distribution of fractures across different sites is based on incidence data from Sweden.148 The increased risks of fracture following incident fracture are based on a published systematic review by Klotzbuecher et al.149 | Chapter 4, Estimating time to event from absolute fracture risk; Chapter 4, Incorporating the risk of fracture at other site; Chapter 4, Risk of subsequent fracture after incident fracture |
| Effectiveness | The HRs from the systematic review and NMA are applied for the duration of treatment. Some effectiveness is assumed to persist beyond treatment. A linear decline in treatment effect assumed | Chapter 4, Application of hazard ratios to incorporate treatment both during and beyond treatment period and Chapter 4, Efficacy estimates |
| AEs | Upper GI side effects for oral bisphosphonates and flu-like symptoms for i.v. bisphosphonates are included by applying one-off cost and QALY deductions in the first month of treatment | Chapter 4, Adverse event estimates |
| Mortality | All-cause mortality is based on UK life tables Fracture-related mortality is based on estimates of excess mortality attributable to hip and vertebral from a case–control study using routine data from UK general practice |
Chapter 4, Estimating time to non-fracture-related mortality to Chapter 4, Excess mortality risk at fracture sites other than hip or vertebrae |
| Utility data | Utility decrements based on EQ-5D scores pre and post fracture were obtained from a systematic review (see Health-related quality of life: review of utility values following fracture). Utility decrement for nursing home admission was based on a single study identified from the literature that used EQ-5D. Variation in baseline utility by age and sex was based on UK EQ-5D population estimates | Chapter 4, Health-related quality of life: review of utility values following fracture to Chapter 4, Age- and sex-specific utility values in the absence of clinical events |
| Resource use and unit costs | The analysis includes drug costs, administration costs and costs of fracture including those falling on primary care, secondary care and PSS Post-fracture costs were based on a case–control study that used routine data from UK general practice. Nursing home admission following hip fracture was based on a UK observational study of discharge destinations Unit costs are taken from NHS reference costs,150 PSSRU unit costs,26 the primary care national drug tariff151 and the eMIT database152 of generic drug costs in secondary care Costs are reported in pounds sterling (£) Cost year is 2014 |
Chapter 4, Risk of nursing home admission following hip fracture; Chapter 4, Risk of nursing home admission following vertebral fracture; Chapter 4, Resource use and costs for bisphosphonates treatment; and Chapter 4, Resource use and costs of fracture |
| Discounting | 3.5% per annum for both costs and QALYs | Chapter 4, Model structure |
| Sensitivity analysis | PSA was undertaken for the base-case scenario to estimate the mean costs and benefits when taking into account parameter uncertainty Structural uncertainty was assessed through scenario analysis. For most of the scenario analyses parameters were set to their mid-point values |
Chapter 4, Approach to sensitivity analysis |
Methods
Model structure
The model is a DES that simulates the clinical events occurring over the lifetimes of individual patients who are allowed to have heterogeneous characteristics. When designing the model structure, we constructed a conceptual model to explore the relationships between patient characteristics, absolute risks and cost-effectiveness, which is summarised in Figure 66 and discussed in more detail in the section Specifying the model population. Based on this conceptual model we anticipated that an unbiased estimate of the average cost-effectiveness for groups selected according to their level of absolute risk could only be obtained by calculating the mean cost-effectiveness across a population with heterogeneous characteristics. This is because we expected certain characteristics, such as age, which are not uniform across cohorts selected based on absolute risk, to have a non-linear relationship with cost-effectiveness. For example, age was expected to affect both life expectancy and the probability of a new admission to a residential care setting following fracture, both of which would alter the cost and QALY implications of fracture. Therefore, we decided to use a patient-level simulation approach in which the patient characteristics were allowed to vary stochastically in a manner that reflects our beliefs about their distribution within the general population. Having decided to use a patient-level simulation approach, we then decided that a DES approach would be more efficient than a patient-level state transition approach. This is because a DES approach updates the calculation of costs and benefits only when a patient experiences an event rather than making calculations for every model cycle. The cohort modelled included a substantial proportion of low-risk patients, as not all patients eligible for fracture risk assessment under CG14616 are at high risk of fracture. In a low-risk cohort it would be common for there to be no fracture events experienced during a patient’s lifetime. Calculating costs and QALYs every model cycle is much less efficient in low-risk populations than in high-risk populations, in which events may occur every few cycles. The main disadvantage of using a DES approach is that the risk factor tools (FRAX and QFracture), which are recommended for assessing fracture risk in CG146,16 provide estimates of the cumulative risk over a defined time frame (10 years for FRAX and 1–10 years for QFracture). In order to convert these estimates of absolute cumulative risk to time-to-event estimates, it was necessary to assume some functional form for event-free survival, and this required some additional data or assumptions regarding the hazard function.
FIGURE 66.
Relationships assumed between individual risk factors and cost-effectiveness. COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.

In general, within a DES model, patients’ experience as they progress through the model is determined by the events that occur rather than by the health states they occupy. Figure 67 shows the clinical events that can occur over a patient’s lifetime, with the arrows showing which events can occur following other events. (Note that this is not a state transition diagram, as patients do not reside in the state defined by the most recent event until the next event is experienced.) In our model, the main clinical events were fracture, death and new admission to residential care. Fractures at different sites were processed using separate fracture events for hip, wrist, and vertebral and proximal humerus. These are the sites most strongly associated with osteoporosis and these are the fracture sites included by both the QFracture and FRAX risk calculators. Fractures at additional sites (femoral shaft, humeral shaft, pelvis, scapula, clavicle, sternum, ribs, tibia and fibula) have been incorporated by increasing the incidence of these four event types rather than by adding additional competing events.
FIGURE 67.
Clinical events that can occur during a patient’s lifetime in the DES.
Separate events are shown in Figure 67 for all-cause mortality and fracture-related deaths to show that fracture-related deaths can occur only following hip and vertebral fracture. However, in practice in the model code, a single event was used to process both all-cause mortality and fracture-related deaths. If a particular fracture was sampled to be fatal, then the time to death was set equal to the time of fracture plus an additional time assumed to be 3 months. At all other times, the time to death was determined by age- and sex-specific estimates for all-cause mortality from the general population. As the data provided by the life tables allowed only the year of death to be sampled and not the exact time point, we assumed that all deaths occurred exactly 6 months through the year in which death was sampled to occur. All-cause mortality estimates were not adjusted to remove deaths following fracture and, therefore, the model may have marginally overestimated the total mortality risk.
A schematic of the model logic used to implement the DES is provided in Figure 68. In a DES, the patient’s progress is driven by a list of times at which each event has been sampled to occur. The model steps forward from one event to the next and the list of event times can be updated when events are processed, to allow the patient’s event history to affect their future progress. In a DES no changes are made to the patient’s attributes between events. Therefore, dummy events were used to ensure that certain patient attributes were updated at times other than when experiencing a clinical event (death or fracture, or new admission to residential care home). For example, dummy events were used to recalculate fracture risks at the end of treatment and at the end of the period when treatment effect is assumed to reach zero. The time between the end of treatment and the end of any remaining treatment effect is called the fall-off period. If these two events occurred prior to 5 and 10 years, respectively, then additional dummy events are scheduled for 5 and 10 years to ensure that all patients have their risk updated at these time points. Dummy events were also used to allow the patient’s health utility values to be updated 1 year after a fracture event to allow the acute (< 1 year) and chronic (> 1 year) consequences of fracture to be incorporated separately. Finally, a time horizon event was also included to process final patient outcomes for those patients who do not die before reaching the age of 100 years. The individual’s risk of fracture is updated each time a clinical event, or dummy event, occurs. The model incorporates the following structural assumptions:
-
The maximum number of hip fractures that can be experienced is limited to one per bone with an additional limit of four vertebral fractures, four rib fractures and two pelvic fractures.
-
There are no restrictions on the sequence of fractures that can be experienced.
-
Death attributable to fracture occurs 3 months after fracture (see Mortality after hip fracture), with other fracture events possible during this period but no mortality from non-fracture-related causes.
-
No further events can be experienced after death.
-
A fracture event occurring < 1 year after a previous event supersedes the dummy event used to update patient attributes 1 year after fracture, thus reducing the acute period for the earlier fracture.
-
Nursing home admission can only occur following fracture and, therefore, patients who are community dwelling at the start of the simulation do not transfer to nursing home care as they age, unless this is simulated to occur following a fracture.
FIGURE 68.
Schematic of the DES model.

Utility in the model is based on a combination of sex, age, fracture history and residential status (community dwelling or institutionalised). Every time an event occurs the patient’s utility value is updated and this utility value is used to calculate the QALYs accrued between one event and the next. Furthermore, when calculating the QALYs accrued between events an adjustment is made for age-related utility decrements over the intervening years so that the utility value applied does not remain artificially high when the time between events is long. This is done by assuming a linear fall in utility over the intervening years between events. The utility impact for each fracture type is separated into an acute utility multiplier applied in the first year after fracture and a chronic utility multiplier which is applied in all subsequent years. If more than one fracture has occurred then the chronic multiplier for each fracture is applied but no more than one acute utility multiplier is applied at any one time. A utility multiplier is also applied for institutional versus community living. Owing to the use of multipliers the absolute utility decrement for each subsequent fracture is smaller and the patient’s utility never falls to below zero. Patients who have a prior fracture (as defined by either the FRAX or QFracture risk calculators) at baseline have the chronic utility multiplier for that fracture type applied for rest of their lifetime.
Two types of costs are applied within the model to capture the consequences of fracture. Acute costs, which represent the cost of acute care such as hospitalisations, are assumed to occur at the time of the event and are applied for both fatal and non-fatal fractures. Chronic costs, which are used to represent the ongoing costs of care in the months and years after fracture, such as nursing home care or medication costs for chronic pain, are accrued gradually over the time period between events. The chronic cost is set to the maximum chronic cost for all fracture events experienced so far, with the maximum chronic cost for any individual being the cost for institutionalised patients. Drug costs are applied from the start of the simulation until the end of the treatment period and are assumed to accrue at a constant rate across time.
Death does not incur any additional costs within the model. For patients who suffer a fatal fracture, the full costs of acute care in the year following fracture are still incurred despite the reduced survival period of 3 months under the assumption that that majority of acute costs are incurred close the time of fracture.
Patients are assumed to stay in the same residential setting (community or institution) unless they experience a fracture event. So while some patients reside in an institutional setting at the start of the simulation, and this proportion is higher in older patients, no patients are simulated to move from the community into an institutional residential setting for reasons other than fracture. This may slightly overestimate the cost savings of preventing fractures, as in reality people may enter an institutional residential setting prior to a fracture occurring and, therefore, will not be at risk of incurring additional costs for residential care following fracture. However, this assumption avoids the need for regular events updating the patient’s residential status, which would reduce the computational efficiency of the DES approach.
The simulation for each individual ends when a fracture-related or non-fracture-related death occurs or when the time horizon is reached. The time horizon is set according to the patient’s starting age so that the simulation ends at age 100 years for all patients. This is because the all-cause mortality data are limited to patients aged ≤ 100 years. Costs and benefits have been discounted within the analysis at 3.5% per annum in accordance with NICE reference case. 144
As CG146 recommends that either FRAX or QFracture is used to estimate the absolute risk of fracture,16 the simulation is run once using each of these tools to estimate fracture risk. First, it is run using QFracture to estimate the absolute risk of fracture. During this run the patient characteristics are stored. The model is then rerun using the same set of patients with identical characteristics but with the absolute risk of fracture being defined by FRAX rather than QFracture. This ensures that an identical patient cohort is simulated when using either QFracture or FRAX to estimate the absolute risk of fracture. In the deterministic model, random number control is used to ensure that the random numbers used are identical when running the same patient using both FRAX and QFracture. This eliminates the possibility that results achieved using the different risk calculators are different purely through chance. The same cohort of patients is run for each treatment and for each parameter sample during the PSA. This means that the 100th patient has the same characteristics and the same set of random numbers determining their path through the model regardless of the parameter samples selected for the PSA or the treatment being simulated. 144 The DES model structure is represented in Figure 68.
Specifying the model population
The population included in the economic analysis is the whole population eligible for risk assessment within CG146. A heterogeneous population has been simulated and then stratified into risk categories based on absolute fracture risk, as predicted by either the FRAX or QFracture risk assessment tool. A heterogeneous population was simulated because we expected certain characteristics, such as age, which are not uniform across cohorts selected based on absolute risk, to have a non-linear relationship with cost-effectiveness. The population was stratified into risk categories to allow the variation in cost-effectiveness across absolute risk to be examined.
The NICE guideline on assessing the risk of fragility fracture (CG146)16 recommends that FRAX17 or QFracture18,19 should be used to assess the 10-year absolute risk of fragility fracture. Therefore, our analysis assumes that absolute fracture risk is measured using one of these two tools. (It is assumed that FRAX web version 3.9 and QFracture 2012 open-source revision 38 were used, as these were the versions available online at the time this report was prepared.) In both of these tools, absolute fracture risk is dependent on the patient’s age, sex, BMI and the presence or absence of a number of clinical risk factors. In the case of QFracture, ethnicity is also taken into account. In the case of FRAX, the patient’s BMD can also be incorporated if it is known, but CG146 recommends that BMD is measured only in patients whose absolute fracture risk falls close to a treatment threshold. Therefore, our model assumes that BMD is not known, as treatment thresholds must be defined for those without a BMD measurement for the recommendations in CG146 to be implemented. The FRAX tool estimates the individual’s 10-year absolute risk of hip fracture and their 10-year absolute risk of major osteoporotic fracture (clinical spine, hip, forearm and humerus fracture). The QFracture tool provides the absolute risk of hip and the absolute risk of major osteoporotic fracture (hip, spine, wrist or shoulder), but with the option to vary the timeframe from 1 year to 18 years (the web tool is limited to 10 years). Table 9 summarises the risk factors used by the FRAX and QFracture tools.
| Patient characteristic | Absolute fracture risk tool | |||
|---|---|---|---|---|
| FRAX17 | QFracture18,19 | |||
| Y/N | Notes | Y/N | Notes | |
| Age | Y | Y | ||
| Sex | Y | Y | ||
| BMI | Y | Y | ||
| BMD | Y | (Optional) T-score or femoral neck BMD in g/cm2 | N | |
| Ethnicity | N | Y | Categories are white or not stated, Indian, Pakistani, Bangladeshi, other Asian, black Caribbean, black African, Chinese, other | |
| Previous fracture | Y | Fragility fracture at any site in adult life | Y | Hip, wrist, spine or shoulder |
| Parental history of fracture | Y | Hip fracture in mother or father | Y | Hip fracture or osteoporosis in parent |
| Alcohol use | Y | ≥ 3 units daily | Y | Categorised as daily units of < 1, 1–2, 3–6, 7–9, > 9 |
| Smoking status | Y | Current smoking | Y | Categorised as non-smoker, ex-smoker, light smoker (< 10 cigarettes per day), moderate smoker (10–19 cigarettes per day) or heavy smoker (> 20 cigarettes per day) |
| Steroid use | Y | Currently exposed to oral glucocorticoids or past exposure > 3 months at a dosage equivalent to 5 mg per day of prednisolone | Y | Taking steroid tablets regularly |
| Rheumatoid arthritis or systemic lupus erythematosus | Y | Rheumatoid arthritis only | Y | |
| Secondary osteoporosis | Y | Any disorder strongly associated with osteoporosis. Examples given are type 1 (insulin-dependent) diabetes mellitus, osteogenesis imperfecta in adults, untreated long-standing hyperthyroidism, hypogonadism or premature menopause (< 45 years), chronic malnutrition, or malabsorption and chronic liver disease | N | Several causes of secondary osteoporosis are included as separate risk factors (see rows below) |
| Diabetes | N | Type 1 diabetes mellitus included under secondary osteoporosis | Y | Type 1 and type 2 diabetes mellitus specified separately |
| Living in nursing or care home | N | Y | ||
| History of falls | N | Y | ||
| Dementia | N | Y | ||
| Cancer | N | Y | ||
| Asthma or COPD | N | Y | ||
| Heart attack, angina, stroke or TIA (CVD) | N | Y | ||
| Chronic liver disease | N | Included under secondary osteoporosis | Y | |
| Chronic kidney disease | N | Y | ||
| Parkinson’s disease | N | Y | ||
| Malabsorption | N | Included under secondary osteoporosis | Y | For example, Crohn’s disease, ulcerative colitis, coeliac disease, steatorrhoea or blind loop syndrome |
| Endocrine problems | N | Long-standing hyperthyroidism included under secondary osteoporosis | Y | For example, thyrotoxicosis, hyperparathyroidism, Cushing’s syndrome |
| Epilepsy or taking anticonvulsants | N | Y | ||
| Taking antidepressants | N | Y | ||
| Taking oestrogen-only HRT | N | Y | ||
A particular level of absolute fracture risk, as measured by FRAX or QFracture, can be achieved in different ways by different individuals. For example, a young patient with many clinical risk factors may have the same absolute risk of fracture as an older patient who has no clinical risk factors. Although the absolute risk of fracture is likely to be an important determinant of the cost-effectiveness of treatment with bisphosphonates, other factors may affect cost-effectiveness independently of absolute fracture risk. For example, the cost and QALY consequences of fracture may be more severe in older patients, who may be more likely to die or be admitted to a nursing home following fracture. Therefore, in a group of patients who have been selected to have the same absolute fracture risk there may be variation in the cost-effectiveness of treatment. If there is a linear relationship between patient characteristics and cost-effectiveness, then it is possible to estimate the average cost-effectiveness by calculating the cost-effectiveness for a patient with average characteristics. However, previous work in this area suggests that cost-effectiveness may be non-linearly associated with patient characteristics, such as age. 153 In such cases, an unbiased estimate of the mean cost-effectiveness can be achieved by simulating a patient population with heterogeneous patient characteristics and estimating the average cost-effectiveness across that population. 154
In this analysis we have simulated a heterogeneous patient cohort that is representative of all patients eligible for risk factor assessment within CG146. 16 We have limited the population to patients > 30 years, as neither the FRAX nor the QFracture tool has been validated in patients aged < 30 years. Initially, a population of patients aged ≥ 30 years is simulated, but only those eligible for risk factor assessment with CG146 are included within the cohort used within the cost-effectiveness analysis. For example, simulated patients without clinical risk factors (any included in QFracture or FRAX) are excluded from the analysis if they are female and aged < 65 years or male and aged < 75 years, and simulated patients are also excluded if they are aged < 50 years and do not have either a prior history of fragility fracture or current steroid use. This approach of sampling the whole population and then excluding those not recommended for risk factor assessment by CG146 was necessary, as data were not available on the distribution of clinical risk factors within the specific population eligible for risk assessment under CG146.
Once the cohort eligible for risk factor assessment was defined from within the general population, we estimated FRAX and QFracture scores for each individual (where ‘score’ refers to the absolute risk of fracture over 10 years for the four main fracture sites: hip, wrist, vertebra and proximal humerus). Lifetime costs and QALYs for each patient are then estimated using the cost-effectiveness model. This step is repeated once for no treatment and once for each bisphosphonate treatment strategy. We then stratified the patients into 10 risk categories based on their absolute fracture risk and estimated the average cost-effectiveness of each bisphosphonate compared with no treatment within each risk score category. The cut-off points for each risk category have been set using deciles to ensure that a sufficient number of patients fall into each category to allow the cost-effectiveness to be estimated accurately. The stratification into risk categories is done independently for QFracture and FRAX. As there is not necessarily agreement between the risk scores calculated by these two different risk assessment tools at the patient level, the same patients may not end up in the same risk category when using different tools to define absolute risks.
In order to stochastically sample patient characteristics we needed data on the prevalence of each clinical risk factor and the distribution of continuous factors, such as age and BMI. As well as considering the prevalence of individual risk factors, it is also important to determine whether or not there are correlations between any of the patient characteristics so that the sampling process can allow for the fact that some risk factors may be more likely to occur in the same patient than in separate patients. It is difficult to fully characterise the correlation structure of all of the risk factors that go into both the QFracture and FRAX tools without access to a database containing information on all of the risk factors in a large sample of patients. However, it is most important to capture the correlations between those characteristics that are likely to be significant determinants of cost-effectiveness independently of their impact on absolute fracture risk. This is because the prevalence of these factors will determine the distribution of cost-effectiveness within groups who have the same absolute fracture risk.
We developed a conceptual model outlining which risk factors are likely to significantly impact cost-effectiveness independently of their impact on absolute fracture risk. This was based on the relationships assumed in published models in this area, advice from our clinical advisors and rapid literature searches (Table 10). A summary of this conceptual model is shown in Figure 66. Age, sex, prior fracture, steroid use and residential status were identified as risk factors thought to affect cost-effectiveness independently of absolute fracture risk. Further details on the rationale for selecting these risk factors are given in Table 10. Ethnicity, family history of fracture and BMD were excluded, as these are expected to affect cost-effectiveness solely through their impact on absolute fracture risk. Although some of the remaining risk factors included in either FRAX or QFracture (e.g. alcohol use, smoking status, comorbidities, secondary causes of osteoporosis, medications, BMI and history of falls) might be expected to affect an individual’s baseline utility, life expectancy or likelihood of living in an institutional residential setting, these relationships were felt to be too weak to include within the model without adding unnecessary complexity to the model structure. Furthermore, many of these conditions are likely to be more prevalent within older patients or those living in residential care and, therefore, their impact on utility, all-cause mortality or outcomes following a fracture may already be captured by the relationship between these variables and age or residential status. We have therefore focused on trying to capture the correlations between age, sex, steroid use, prior fracture and residential status. This was achieved by looking for age- and sex-specific estimates of steroid use, prior fracture and residential status, as these were considered to be where the most significant correlations would lie. The conceptual model was developed to allow for the possibility that different efficacy data may be applied for different sexes and for steroid- and non-steroid-induced osteoporosis, but in the final analysis efficacy evidence was pooled across all included trials reporting fracture outcomes. The potential for increased all-cause mortality in steroid users was noted at the conceptual modelling stage, but no difference in life expectancy was applied in the final model.
| Patient characteristic | Rationale |
|---|---|
| Age | Age is predictive of the following factors that affect cost-effectiveness independently of absolute fracture risk: |
| Steroid use | Efficacy data for steroid-induced osteoporosis may differ from non-steroid-induced osteoporosisa All-cause mortality may be higher in steroid users, which will affect cost-effectiveness independently of absolute fracture risk |
| Sex | Efficacy data for males and females may differa Sex is predictive of the following factors that affect cost-effectiveness independently of absolute fracture risk: |
| Prior fracture | Utility at baseline may be lower in those with significant prior fractures (e.g. hip fracture) |
| Residential status | Residential status is predictive of the following factors that affect cost-effectiveness independently of absolute fracture risk:
|
The primary data source used to characterise the patient population was the cohort used to derive the 2012 QFracture algorithm. This study used a large (n = 3,142,673) prospective cohort aged 30–100 years drawn from a large, validated primary care electronic database. 18 This study was chosen as the primary source of data on patient characteristics as it was considered to be representative of the general UK population and provided data on all of the risk factors included within the QFracture algorithm. For the majority of the clinical risk factors, we used the prevalence within the 2012 QFracture cohort and applied the same prevalence across all ages and across both sexes. These risk factors are listed in Table 11 along with the prevalence reported for the 2012 QFracture cohort. Although many of these risk factors are expected to have varying prevalence across different sexes and age groups, it was not considered necessary to capture their correlation with age or sex, as they are assumed to influence cost-effectiveness through only their impact on absolute fracture risk.
| Clinical risk factors | aPrevalence in 2012 QFracture cohort18 |
|---|---|
| Dementia | 0.6% |
| History of falls | 1.2% |
| Malabsorption | 0.5% |
| Endocrine disorders | 0.5% |
| Asthma or chronic obstructive airways disease | 7.6% |
| Any cancer | 1.9% |
| Cardiovascular disease | 5.3% |
| Epilepsy diagnosis or prescribed anticonvulsants | 1.8% |
| Chronic liver disease | 0.2% |
| Parkinson’s disease | 0.2% |
| Rheumatoid arthritis or systemic lupus erythematosus | 0.7% |
| Chronic renal disease | 0.2% |
| Type 1 diabetes mellitus | 0.3% |
| Type 2 diabetes mellitus | 2.8% |
| Parental history of osteoporosis | 0.3% |
| Unopposed HRT | 2.2% (in the female-only subgroup) |
| Any antidepressant | 7.7% |
Although data were available on the age distribution for patients within the 2012 QFracture cohort, these data were not provided separately for males and females and the age profile of the UK population is known to differ slightly by sex. 159 Therefore, sex-specific 2013 mid-year population estimates for England from the Office for National Statistics (ONS) were used to provide an empirical distribution for patient age. 159 Figure 69 shows how the proportion falling within each band compares between the ONS data and the 2012 QFracture cohort. The data appear to be reasonably well matched except that the QFracture cohort appears to have a lower proportion in the 30–39 years category. The ONS data were considered to be more representative of the population in England and, therefore, the age of each individual patient was sampled using the sex-specific ONS data.
The proportion of patients living in an institutional residential setting was estimated from the 2011 census data. 160,161 Sex-specific data were available for 5-year age bands for all people who are usual residents in communal establishments. 160 However, these 5-year estimates included people resident in other types of communal establishments such as children’s homes and prisons. Data were also available on specific types of establishments for 10-year age bands. 161 We selected data for people resident in medical and care establishments, which included NHS, local authority and other establishments both with and without nursing care. We then used the 5-year data on all communal establishments to divide up the 10-year data into 5-year age bands. These data, shown in Figure 70, were used to sample whether or not an individual was living in an institution according to their age and sex.
FIGURE 70.
Proportion living in an institutional residential setting by age band. Reproduced from Office for National Statistics. Census 2011: Communal Establishment Management and Type by Sex by Age. 2011. URL: www.nomisweb.co.uk/census/2011/dc4210ewla (accessed 28 October 2014). 161 Contains public sector information licenced under the Open Government Licence v3.0.

For steroid use, data published by van Staa et al. 162 suggest that the prevalence of current steroid use increases with age. Their estimates were based on analysis of the GPRD (which is now called Clinical Practice Research Datalink), which is a large database of GP records for UK patients. This provided a large retrospective cohort that is likely to be representative of the general population of England and Wales. Data on the prevalence of oral glucocorticoid use by sex and 10-year age bands were digitally extracted from a graph provided by van Staa et al. 162 The relationship between prevalence and age appear to follow a similar pattern for low-, medium- and high-dose users. Data were extracted for only medium-dose (2.5–7.5 mg per day) and high-dose (≥ 7.5 mg per day) steroid users as these dosages overlapped with the range specified in the FRAX fracture risk algorithm (≥ 5 mg per day). However, when these data were combined with the ONS data on the current age distribution within England to estimate the average prevalence across patients aged ≥ 30 years, this was substantially lower than the prevalence recorded in the QFracture database (0.95% vs. 2.2%). The difference may be because we did not include low-dose users from the van Staa et al. 162 estimates or that the QFracture data do not appear to relate to a specific dose of steroids. A more recent estimate of the prevalence based on UK GP records is provided by Fardet et al. 163 Although this did not provide a breakdown of the prevalence by age and sex, the overall prevalence of 0.79% for 2008 reported by Fardet et al. 163 is closer to that reported by van Staa et al. 162 than the figure reported in the QFracture database. We therefore decided to use the combined data for medium- and high-dose users provided by van Staa et al. ’s data to characterise the age and sex distribution of steroid use. Figure 71 shows age- and sex-specific prevalence estimates applied in the model for steroid use.
FIGURE 71.
Prevalence of current steroid use: data from van Staa et al. 162 combined for medium- and high-dose steroid users.

Data on the prevalence of previous fracture were taken from a meta-analysis by Kanis et al. 164 This study was selected as it provided data on the prevalence of prior fracture, reported by sex and 10-year age bands. The cohorts used to estimate the prevalence of prior fracture were the same cohorts used to estimate the impact of prior fracture on future fracture risk for the FRAX algorithm. 17 The prevalence of prior fracture is difficult to quantify as it depends on whether or not all prior fractures are included regardless of the site of fracture or the mechanism of injury. Although the definitions used varied across the multiple cohorts that informed the estimates from Kanis et al.,164 the fact that these cohorts were then used to derive the impact of prior fracture on future fracture risk provides some consistency between the definition of prior fracture used for prevalence and for risk score calculation. The prevalence reported by Kanis et al. 164 for each of the 10-year age bands, which ranged from 15% at age 30 years to 48% at age 80 years in women, is much higher than that reported within the QFracture cohort (1.9% across a cohort aged ≥ 30 years). 18,164 An alternative estimate of the prevalence of prior fracture is provided by Scholes et al. ,165 who used data collected during the Health Survey for England to estimate the prevalence of previous fracture in community-dwelling people aged > 55 years. They found that the prevalence was 49% in men and 40% in women, although these data relied on the individuals’ recall and did not distinguish between fragility fractures and those occurring in early life or associated with significant trauma. Another source of evidence that can be used to cross-check the estimates provided by Kanis et al. 164 are studies reporting the incidence of fracture by age. Prevalence can be estimated from these studies in an approximate manner by assuming that the prevalence of prior fracture at a particular age is equivalent to the cumulative incidence across all previous age bands although, under this assumption, the prevalence may be inflated by multiple fractures occurring within the same patient, if these are reported separately in the incidence data.
Data on the incidence of fracture by age and sex and the proportion of fractures that are fall related (standing fall, fall down stairs or fall from a low height) are provided by Court-Brown et al. 166 This was a prospective cohort study conducted in Scotland in 2010/11 that compared the rate of fractures presenting to the Royal Infirmary of Edinburgh with population estimates from the 2001 census to estimate incidence rates. Estimating the prevalence of fall-related fractures from these data, by assuming that it is equal to the cumulative incidence in those aged over 35 years, provides prevalence data closer to those reported by Kanis et al. 164 than those reported in the QFracture cohort. Therefore, the data presented by Kanis et al. 164 (Figure 72) were used in the model to sample the likelihood of an individual having a prior fracture. 164 A second incidence study, by van Staa et al. ,167 provides data on the incidence of fracture in England in a general practice (GPRD) cohort, which examined over 20 million person-years of follow-up. The proportion of fractures that were fall related in the study by Court-Brown et al. 166 was applied to the incidence data reported by van Staa et al. 167 to estimate the incidence of fall-related fractures in an attempt to exclude fractures related to significant trauma such as road traffic accidents. Prevalence of a prior fracture after the age of 3 years was then estimated by calculating the cumulative incidence from age 20 years, and these data are summarised in Figure 73. The prevalence estimated in younger age groups, when using this method, was lower compared with the data reported by Kanis et al. 164 This alternative estimate of the prevalence of prior fracture was applied in a sensitivity analysis to assess whether or not the cost-effectiveness of bisphosphonate treatment is sensitive to the prevalence of prior fracture in the population.
FIGURE 72.
Proportion who have had a prior fracture by sex and age band (data applied in base case). From Kanis et al. 164
Swedish estimates for the incidence of fracture at different sites across sexes and age bands were then used to estimate the cumulative prevalence of fractures at various sites up to the start age for each age band. 148 These data were used to determine the distribution of prevalent fractures across different fracture sites, as shown in Table 12. As the incidence data were presented for patients aged ≥ 50 years we have assumed that the distribution of prior fractures at ages 30–55 years is equal to the distribution of incidence of fracture from ages 50–55 years. It can be seen that as the incidence of hip fracture rises with age, the proportion of prior fractures that have occurred at the hip increases with each increasing age category.
| Fracture site | Age band (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| < 55 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | |
| Women | ||||||||
| Hip | 6 | 6 | 8 | 11 | 15 | 20 | 27 | 36 |
| Vertebral | 22 | 22 | 20 | 23 | 23 | 25 | 25 | 22 |
| Proximal humerus | 17 | 17 | 16 | 14 | 16 | 15 | 15 | 13 |
| Wrist | 56 | 56 | 55 | 52 | 46 | 40 | 34 | 29 |
| Men | ||||||||
| Hip | 10 | 10 | 14 | 18 | 23 | 29 | 36 | 44 |
| Vertebral | 48 | 48 | 41 | 41 | 35 | 36 | 35 | 32 |
| Proximal humerus | 16 | 16 | 12 | 12 | 11 | 13 | 12 | 10 |
| Wrist | 25 | 25 | 33 | 29 | 30 | 22 | 17 | 14 |
Data are available from the Health Survey for England on the average BMI for different ages and sexes. 168 These data, presented in Figure 74, show that BMI varies with age. Although BMI is not expected to affect cost-effectiveness except through its influence on absolute fracture risk, it is considered to be an important risk factor, particularly where BMD is unknown. A 2014 meta-analysis found that the relationship between BMI and fracture risk is much weaker after adjusting for BMD. 169 A significant positive correlation was also found in this study between BMI and BMD (95% CI 0.32 to 0.33, r = 0.33; p < 0.001). Given the significant correlation between these two variables and the fact that we are assuming that BMD is not available when fracture risk is first assessed, we decided to model the age variation in BMI, as this may capture some of the underlying variation in BMD with age. However, we accept this will capture only a small proportion of the association between BMD and age. We decided to use the Health Survey for England data168 to characterise the mean BMI for different age bands and sexes, as these data allow the SD to be calculated. However, they do not provide any information on the shape of the BMI distribution. We assumed that the BMI values were log-normally distributed, as we found that assuming a normal distribution overestimated the proportion falling within the underweight category. As it is the underweight group who are at particular risk of a fragility fracture, assuming a normal distribution would have overestimated population fracture risk. 169 As can be seen in Figure 75, assuming a log-normal distribution still overestimated the proportion who were underweight, but by a factor of 3 rather than 5.
FIGURE 75.
Proportion of men (adults aged ≥ 16 years) falling into different weight categories. HSE, Health Survey for England.
Treatment strategies
The model compares the following treatment strategies:
-
alendronic acid (10 mg per day or 70 mg per week)
-
risedronic acid (5 mg per day or 35 mg per week)
-
ibandronic acid (150 mg per month)
-
i.v. ibandronic acid (3 mg every 3 months)
-
zoledronic acid (5 mg/year)
-
no treatment.
We have not distinguished in the model between the daily and weekly formulations of alendronic acid and risedronic acid, as the weekly formulations for these are considered to be clinically equivalent and the effectiveness evidence has not been analysed separately for weekly and daily doses.
We assume that all patients will receive adequate supplemental calcium and vitamin D regardless of whether or not they are being treated with a bisphosphonate and, therefore, no cost is included within the model for calcium and vitamin D supplements. Patients in the no-treatment arm are assumed to receive no further treatment to reduce their fracture risk. We have not assumed any active follow-up for patients receiving either bisphosphonates or no treatment.
We assume that the intended treatment duration is 5 years for alendronic acid, risedronic acid and ibandronic acid (both oral and i.v.) and 3 years for zoledronic acid. However, not all patients persist with therapy for the intended duration, as previously discussed in Chapter 3, which describes the clinical evidence on treatment persistence. The duration of treatment in the model was therefore set to the mean duration of persistence using data from the systematic reviews described in Chapter 3. The highest-quality systematic review was considered to be that by Imaz et al. ,128 which reported that the mean duration of treatment persistence was 184 days (95% CI 164 to 204 days) for oral bisphosphonates (alendronic acid, risedronic acid and ibandronic acid). Only one of the studies included in the meta-analysis of average persistence by Imaz et al. 128 examined ibandronic acid, with the rest considering alendronic acid and risedronic acid. However, the mean duration of persistence for monthly ibandronic acid was similar to the mean duration for weekly alendronic acid and risedronic acid (98 days for ibandronic acid vs. 116 days and 113 days for alendronic acid and risedronic acid, respectively). Therefore, we decided to use the pooled estimate provided by Imaz et al. 128 for all oral bisphosphonates.
The review by Imaz et al. 128 did not provide any data on persistence in patients receiving i.v. bisphosphonate therapy. 128 However, a review by Vieira et al. 132 identified a cohort study (Curtis et al. 170) in US Medicare patients that provided estimates of the mean number of infusions received for zoledronic acid and i.v. ibandronic acid. 170 It is noted that the duration of treatment with zoledronic acid estimated by Curtis et al. 170 was considered by our clinical advisors to be low compared with their own experience of administering zoledronic acid within clinical practice. However, in the absence of an alternative estimate these data were used to estimate the mean duration of persistence with therapy for i.v. bisphosphonates. The full treatment effect was assumed to persist for 1 year after the last zoledronic acid infusion and 3 months after the last ibandronic acid infusion. Persistence data applied in the base-case model are summarised in Table 13. A sensitivity analysis in which we assumed full persistence with treatment for 3 years for zoledronic acid and 5 years for all other treatments was also examined.
| Treatment | Mean duration of persistence with treatment | SE | Source |
|---|---|---|---|
| Alendronic acid, risedronic acid and oral ibandronic acid | 184 days (0.5 years) | 10 days | Meta-analysed estimate from the Imaz et al.128 systematic review |
| i.v. ibandronic acid | 401 days (1.1 years) | 15 days | Curtis et al., 2012170 |
| Zoledronic acid | 621 days (1.7 years) | 6.5 days | Curtis et al., 2012170 |
The fall-off period was assumed to be equal to the duration of treatment for all treatments except zoledronic acid, for which a longer fall-off period was assumed. Clinical advice was that a 7-year fall-off period could be assumed for 3 years of zoledronic acid treatment. We therefore assumed an approximate fall-off period of 2.33 (= 7/3) times the treatment period for zoledronic acid.
Estimating time to event from absolute fracture risk
Time to fracture has been estimated by fitting a parametric survival function to the estimates of absolute risk provided by the QFracture algorithm. For the model using FRAX, the parametric form and shape parameter fitted to the QFracture data has been used but the rate parameter of the survival function has been adjusted to ensure that the absolute fracture risk at 10 years, predicted by the survival function, matches that predicted by the FRAX tool. Treatment effects are incorporated by applying a HR to the rate parameter, with further details on the incorporation of treatment efficacy provided in Incorporating the risk of fracture at other sites and Application of hazard ratios to incorporate treatment effects both during and beyond the treatment period.
The algorithm used by the QFracture tool to calculate the risk of fracture over varying time periods is publicly available on the QFracture website (www.qfracture.org/). This algorithm was examined and was found to have the following form:
where the parameter η is the risk-modifying factor that adjusts for patient characteristics and S0 is the underlying survival function. Different values of S0 are defined according to the time frame (t) over which risk is to be assessed. The survival model used to estimate the risk-modifying factor η is described as a Cox regression. In a Cox regression the values for S0 do not have to follow any particular parametric form. However, when the S0 values were plotted, to give the fracture-free survival for patients without any risk-modifying factors (η = 0), it was noted that they appeared to be very smooth, suggesting that it may be possible to fit a functional form to the underlying survival function. Given that the Weibull function (which includes the exponential function as a special case) and the Gompertz function are both compatible with proportional hazards assumptions, we tested both of these parametric forms to see if they were suitable.
A plot of ln(–ln(S(t))) against ln(t) was produced to see whether or not the data were consistent with a Weibull survival curve. This was done for an example patient with the following characteristics: female, aged 50 years, BMI of 24 kg/m2 and no clinical risk factors. The same plot was then produced for a patient with type 1 diabetes but no other clinical risk factors and the same age and BMI to examine the impact of clinical risk factors on the shape of the plots. From Figure 76 it can be seen that the distance between the plots is constant for these two cases, as would be expected for a proportional hazards model, but neither plot is linear over the whole time period. The plots appear to be linear over short time periods (5 or perhaps 10 years), but the Weibull curve does not appear to be appropriate over longer time frames.
FIGURE 76.
Plot to test suitability of Weibull survival curve. Patient characteristics: female, aged 50 years, BMI of 24 kg/m2, with or without type 2 diabetes.
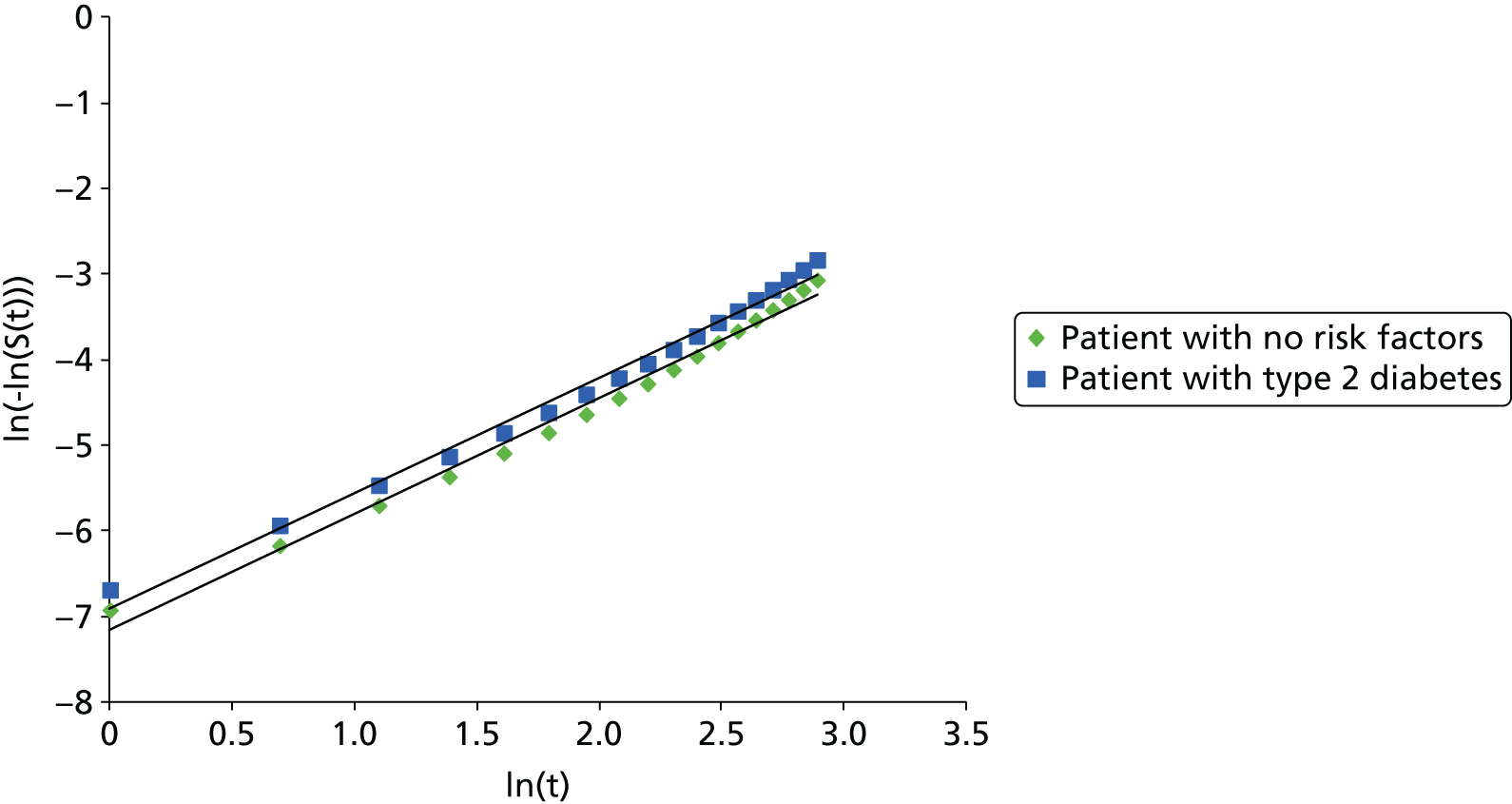
A plot of ln(hazard) against time was generated once again for a 50-year-old female with a BMI of 24 kg/m2 and either with or without type 2 diabetes as shown in Figure 77. This was found to be linear, suggesting that the underlying survival function was consistent with a Gompertz distribution. We have therefore assumed that the underlying survival function follows a Gompertz distribution and used the linear fit for the ln(hazard) function to estimate the parameters for the Gompertz distribution in patients without any risk-modifying factors (η = 0). Table 14 shows the survival parameters for the underlying Gompertz distribution in males and females for the outcomes of hip fracture-free survival and osteoporotic fracture-free survival with osteoporotic fracture defined as hip, wrist, vertebral or proximal humerus fracture.
FIGURE 77.
Plot to test suitability of Gompertz parametric form. Patient characteristics: female, aged 50 years, BMI of 24 kg/m2, with or without type 2 diabetes.
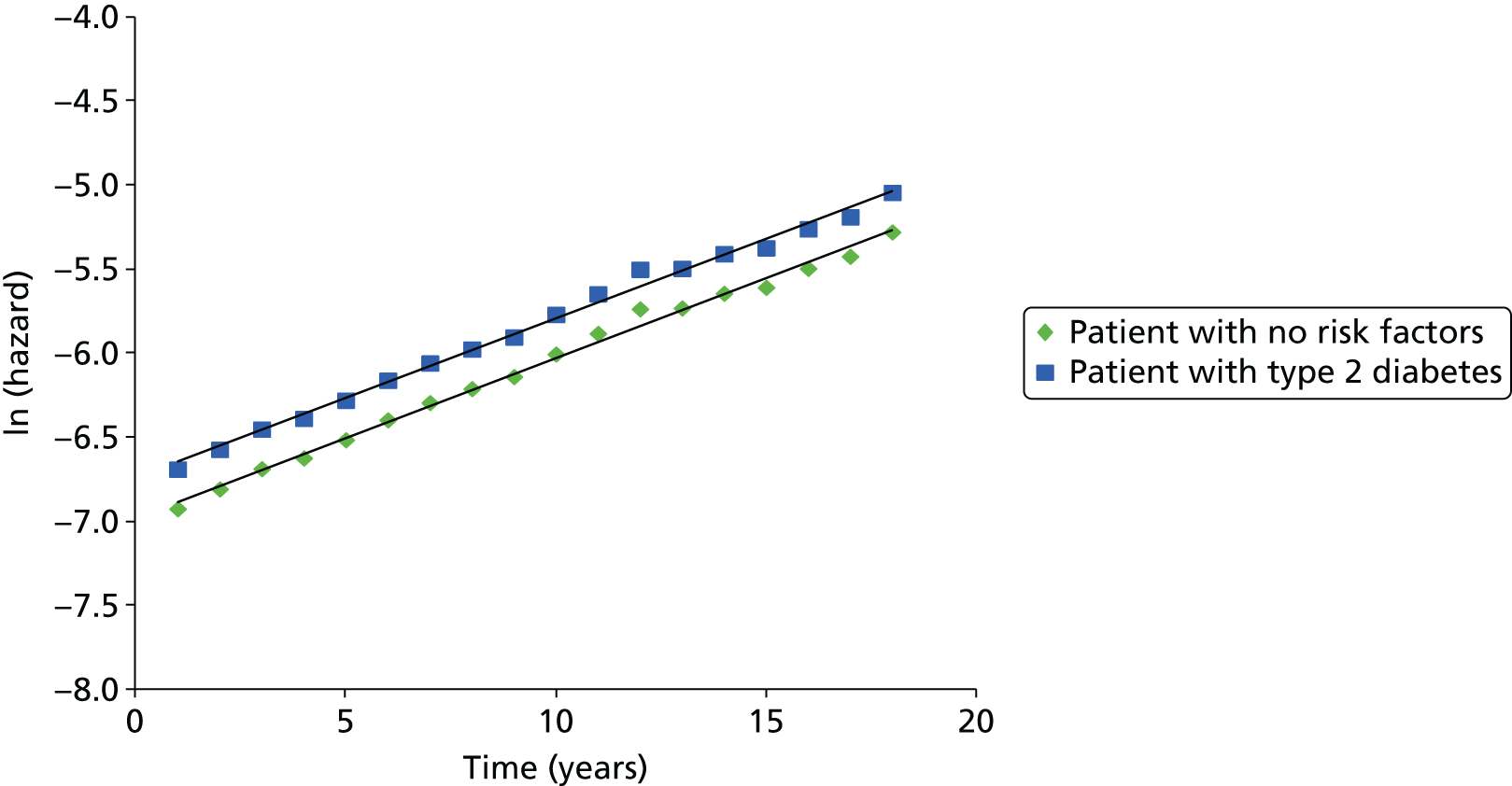
| Survival function | Sex | Alpha | Beta | R 2 |
|---|---|---|---|---|
| Osteoporotic (hip, wrist, proximal humerus or vertebral) fracture | Female | Exp(–6.9499) | 0.0947 | 0.9942 |
| Hip fracture | Female | Exp(–9.4486) | 0.1375 | 0.9963 |
| Osteoporotic (hip, wrist, proximal humerus or vertebral) fracture | Male | Exp(–8.0425) | 0.0908 | 0.9882 |
| Hip fracture | Male | Exp(–10.228) | 0.1454 | 0.9902 |
Figures 78–81 show the fit of the parametric curve against the survival data specified in the QFracture algorithm for each of these survival functions. It can be seen from the plots that the parametric curves fit the data better in the first 10 years and that the parametric curves may underestimate long-term fracture risk. Although this was noted as a limitation, the good fit up to 10 years means that the rates are sufficiently accurate during the period in which drugs are assumed to affect fracture outcomes. An underestimation of the long-term fracture risk in the period after the drug efficacy is assumed to fall to zero is likely to affect all treatment strategies equally and, therefore, is not expected to significantly bias the estimates of cost-effectiveness. We therefore assumed that the fitted Gompertz curve could be used to estimate time to fracture for patients with no risk-modifying factors.
FIGURE 78.
Gompertz fit for a female patient with no risk-modifying factors (η = 0) for the outcome of any osteoporotic fracture (hip, wrist, proximal humerus or vertebral).

FIGURE 79.
Gompertz fit for a female patient with no risk-modifying factors (η = 0) for the outcome of hip fracture.
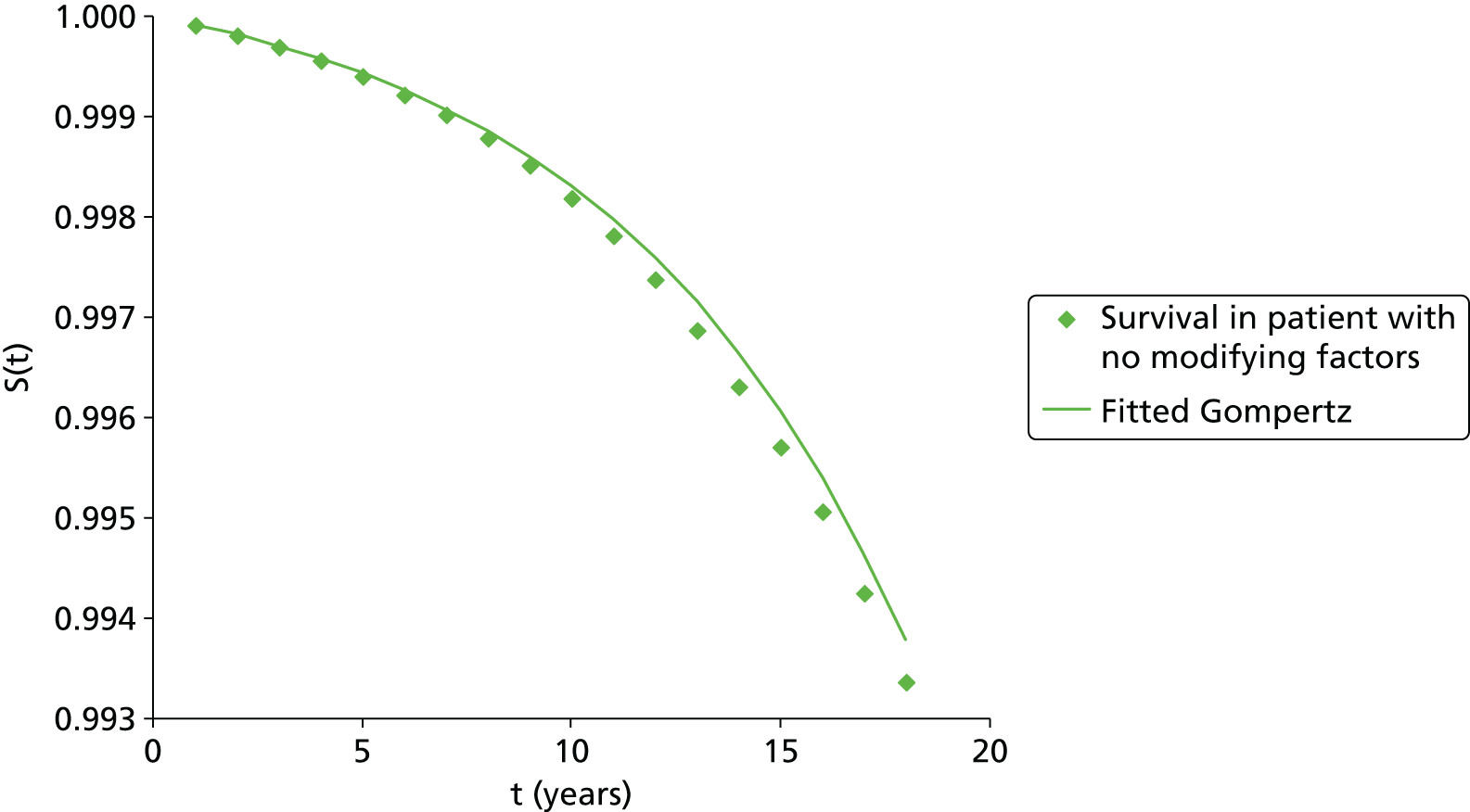
FIGURE 80.
Gompertz fit for a male patient with no risk-modifying factors (η = 0) for the outcome of any osteoporotic fracture (hip, wrist, proximal humerus or vertebral).
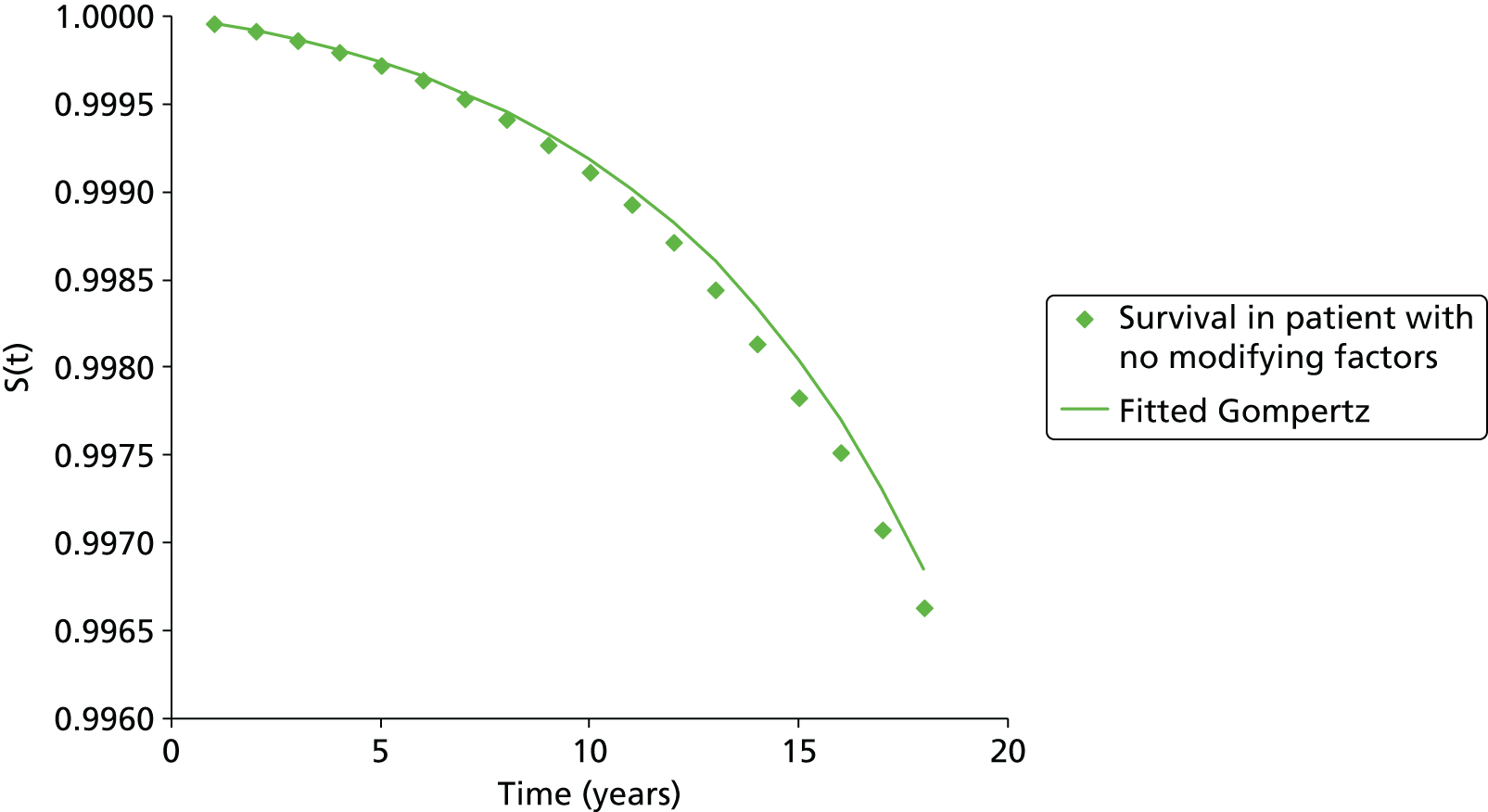
FIGURE 81.
Gompertz fit for a male patient with no risk-modifying factors (η = 0) for the outcome of hip fracture.
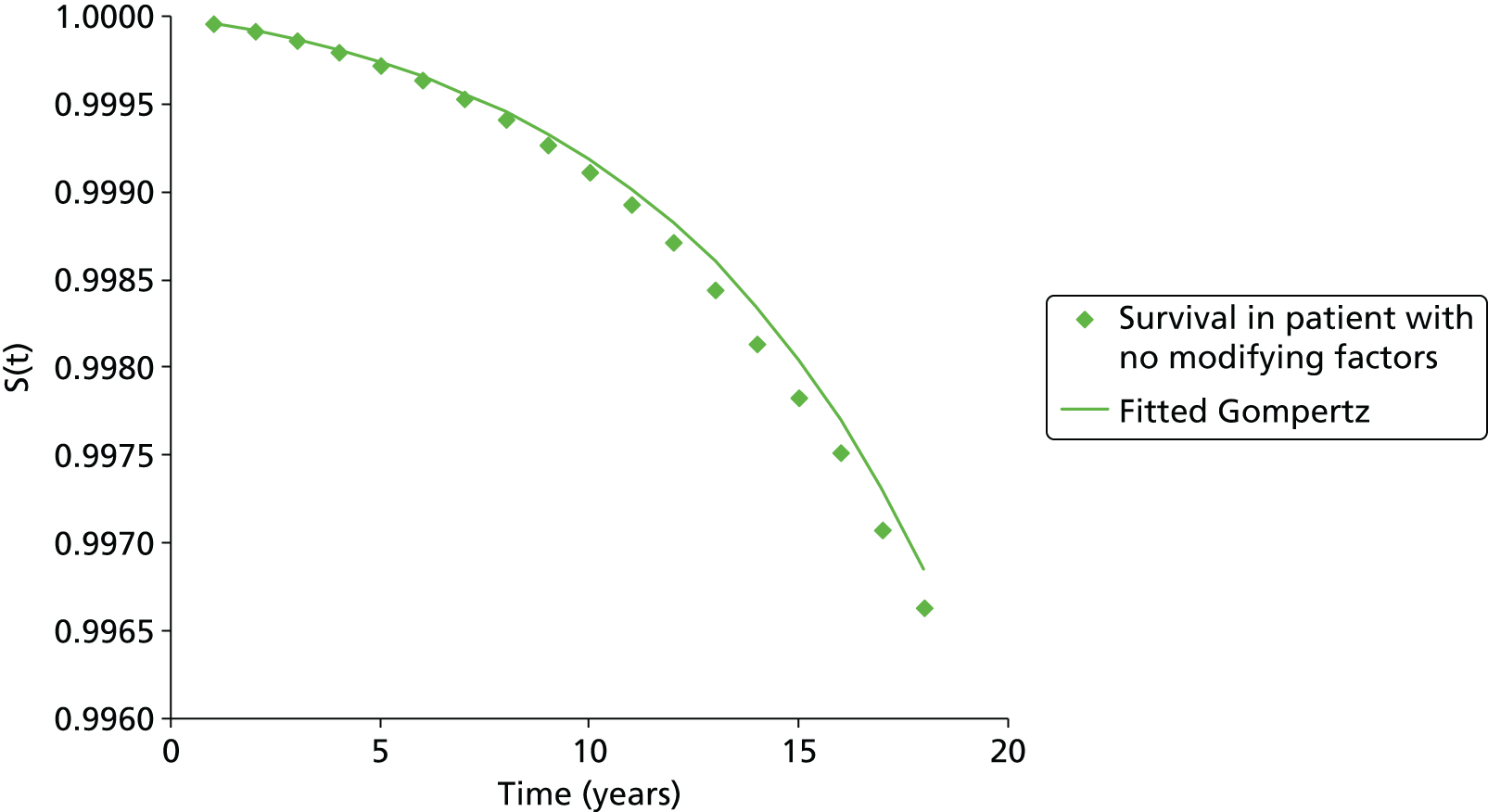
QFracture does not provide individual predictions for each of the four major osteoporotic fractures (hip, wrist, vertebral and proximal humerus). Instead, it provides an estimate of the absolute risk of fracture across all four fracture types. In order to provide an estimate of the time to fracture for each site, we multiplied the alpha parameter for the fitted Gompertz survival curve by the proportion of patients experiencing an incident fracture of that type. The proportions, shown in Table 15, were estimated from Kanis et al. 148 and provide the incidence of fractures in Sweden across different fracture sites by sex and age band.
| Fracture site | Age band (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | |
| Women | ||||||||
| Hip | 6 | 6 | 11 | 15 | 21 | 28 | 38 | 53 |
| Vertebral | 22 | 22 | 19 | 26 | 23 | 27 | 25 | 18 |
| Proximal humerus | 17 | 17 | 15 | 11 | 19 | 13 | 14 | 9 |
| Wrist | 56 | 56 | 55 | 48 | 37 | 31 | 23 | 19 |
| Men | ||||||||
| Hip | 10 | 10 | 18 | 24 | 31 | 38 | 49 | 57 |
| Vertebral | 48 | 48 | 32 | 40 | 27 | 39 | 32 | 28 |
| Proximal humerus | 16 | 16 | 8 | 11 | 10 | 16 | 9 | 7 |
| Wrist | 25 | 25 | 41 | 25 | 32 | 7 | 9 | 8 |
We used these site-specific alpha values to generate samples from the Gompertz distribution for each fracture site and plotted a survival function for time to fracture at each site. To validate this approach, of apportioning the alpha value for major osteoporotic fracture across the four sites, we calculated the time to first major osteoporotic fracture from these site-specific fracture survival curves and compared these to the survival from major osteoporotic fracture predicted by the QFracture algorithm. We found that the survival curves generated were comparable, suggesting that this method of calculating site-specific fracture curves is valid, as can be seen from Figure 82.
FIGURE 82.
Plot of survival curves for time to fracture based on 10,000 patients for each individual fracture site and for any major osteoporotic fracture.
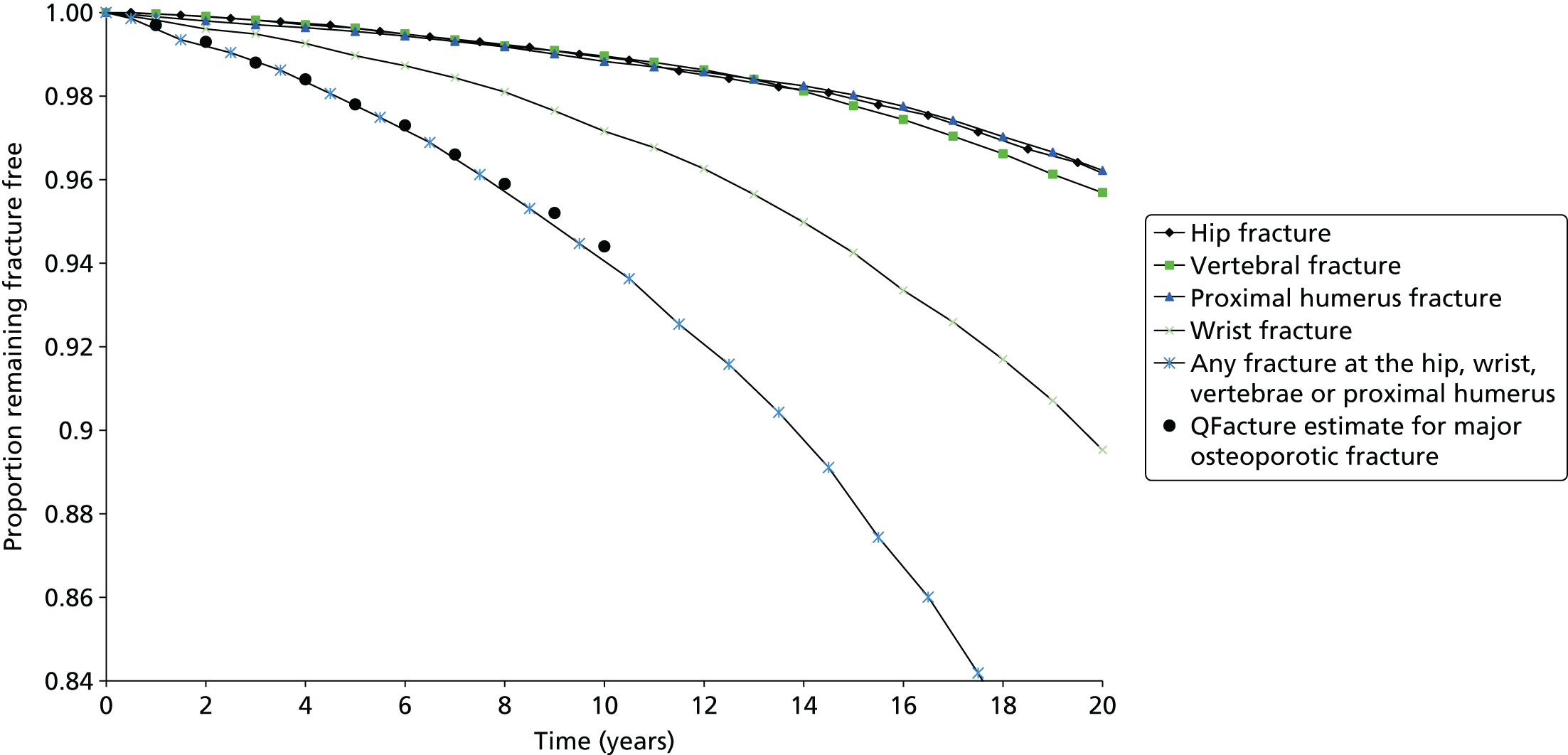
However, as can been seen from Figure 83, when we compared the hip fracture data calculated from major osteoporotic fracture to the hip fracture survival estimates provided directly from the QFracture algorithm, we found that these did not match well over longer time frames (i.e. over 5 years). This can be explained by the fact that the beta value for the hip fracture-specific Gompertz curve is higher, suggesting a faster increase over time for hip fracture than is seen over all major osteoporotic fractures. We decided to use the hip fracture survival predicted by apportioning the major osteoporotic fractures in the base-case analysis, as this would provide an estimate of major osteoporotic fracture that is consistent with the estimates from the QFracture algorithm. Furthermore, the beta value for the Gompertz function for major osteoporotic fracture is likely, in reality, to be the average of a lower value for non-hip and a higher value for hip, but as the non-hip value could not be calculated we felt it was better to use the beta value for major osteoporotic fracture and apply it to all four fracture types in the base-case analysis. A sensitivity analysis was also conducted using the hip-specific algorithm from QFracture for estimating time to hip fracture to see whether or not this had a significant impact on the cost-effectiveness.
FIGURE 83.
Comparison of survival curves from sampling directly from the Gompertz for hip fracture and from sampling hip as a proportion of the Gompertz curve for major osteoporotic fracture against the source QFracture data for hip.
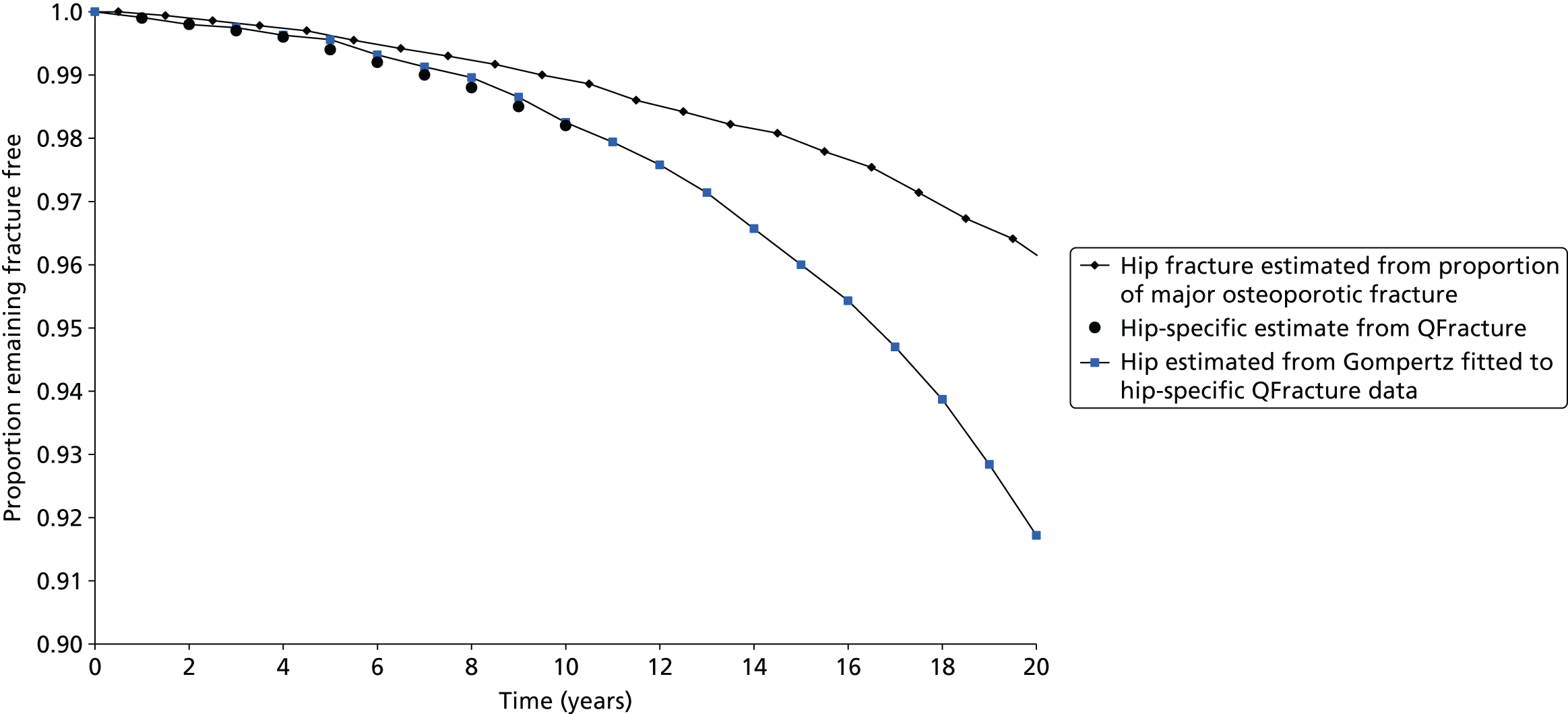
The following method was used to calculate time to event for each fracture type in the base-case analysis when assuming that patients have been assessed using the QFracture algorithm.
-
calculate the proportion, p, of major osteoporotic fractures that occur at the site of interest according to the person’s age and sex
-
calculate the risk score modifier, η, from the patient characteristics
-
select the beta for the sex-specific Gompertz survival curve
-
select HR, which incorporates any treatment effect from intervention
-
calculate alpha for the sex-specific Gompertz survival curve as follows:alpha=alpha(for η=0)×p×exp(η)×HR
-
sample time to fracture from Gompertz (alpha, beta)
A similar approach was not possible when estimating time to event using the estimates of absolute fracture risk provided by the FRAX algorithm. This is because the algorithm used to calculate absolute fracture risk within the FRAX tool is not publicly available and, therefore, it was not possible to assess whether or not survival from fracture follows a particular parametric form. Instead, we assumed the underlying shape of the survival curve for FRAX would be identical to that used in the QFracture algorithm. In effect this meant assuming a Gompertz curve is followed, which has the same beta parameter as seen in the QFracture algorithm. In doing so, we were then able to calculate the time to event for patients assessed using the FRAX tool by calculating the multiplier, Φ, which needed to be applied to the alpha value of the QFracture survival curve to provide the absolute risk of fracture at 10 years, predicted by FRAX. In doing so, we assumed that there is a constant HR between the number of events predicted by FRAX and the number predicted by QFracture across all time frames. From Equations 2–4, it can be seen that Φ can be calculated by comparing the absolute risk of fracture estimated by the two fracture risk tools.
Absolute risk at 10 years in FRAX:
Absolute risk at 10 years in QFracture:
From this we can derive that:
One of the complicating factors with this approach is that QFracture provides an estimate of fracture risk without the competing risk of mortality, whereas FRAX provides an estimate of absolute fracture risk when taking into account the competing risk of mortality. Therefore, at older ages, when the risk of mortality is higher, the FRAX algorithm will calculate lower estimates of 10-year risk than the QFracture algorithm. It was not possible to correct for this within our model, as we did not have sufficient information regarding the competing hazard of death used within the FRAX algorithm to adjust the FRAX estimates to exclude the competing risk of mortality.
Incorporating the risk of fracture at other sites
Fractures at additional sites (femoral shaft, humeral shaft, pelvis, scapula, clavicle, sternum, ribs, tibia and fibula) have been incorporated by increasing the incidence of fractures at the four main sites (hip, wrist, spine and proximal humerus). Although several of the published cost-effectiveness analyses restricted the fracture types to the four main sites,137,139,140 some of the studies incorporated fractures at additional sites45,49–106,108–143 by grouping these with one of the four main fracture sites. The decision over which fractures to group together has, in previous analyses, been justified by the expectation of similar costs and disutilities across particular groups of fractures. 171 The groupings used were consistent across the three published cost-effectiveness analyses that incorporated additional sites. 141–143
We decided to keep the groupings used in these three studies with one exception. These studies grouped pelvic fractures with hip fractures. Pelvic fractures associated with osteoporosis were considered by our clinical advisors not to be associated with an excess risk of mortality similar to that associated with hip fractures and the costs were also expected to be lower. Therefore, pelvic fractures were grouped instead with proximal humerus fractures. Therefore, the grouping of fracture sites used within our model was as follows:
-
femoral shaft grouped with hip
-
clavicle, scapula, rib and sternum grouped with wrist
-
tibia, fibula, pelvis and humeral shaft grouped with proximal humerus.
Both QFracture and FRAX use a clinical definition for vertebral fractures and, therefore, the rate of vertebral fractures predicted in our model is specific to clinical vertebral fractures. The cost and quality-of-life implications of morphometric vertebral fractures that are not clinically apparent are likely to be much smaller than for clinically apparent vertebral fractures. Therefore, we expect that excluding morphometric fractures that are not clinically apparent from the model to have a small impact on the ICER. Previous analyses by Stevenson et al. 140 (reported in appendix 15 of their monograph) suggest that the exclusion of morphometric fractures does not significantly bias the estimates of cost-effectiveness.
The multipliers applied to the rate of hip, wrist and proximal humerus fractures to incorporate the additional fractures sites were calculated based on Swedish incidence data reported by Kanis et al. 148 and are shown in Table 16. These were applied in the model to the alpha parameter for the Gompertz sampling of time to fracture. As the alpha parameter is the rate parameter for the Gompertz survival curve, a multiplier > 1 increases the risk of fracture. The data from age band 50–54 years were applied to those aged 30–50 years. The very high multiplier for wrist fractures in men is driven by a large incidence of rib fractures compared with wrist fractures in the data reported by Kanis et al. 148
| Fracture site | Age band (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85–89 | |
| Women | ||||||||
| Hip | 1.27 | 1.19 | 1.20 | 1.13 | 1.11 | 1.09 | 1.07 | 1.08 |
| Proximal humerus | 1.89 | 2.08 | 2.26 | 1.74 | 1.93 | 1.89 | 2.33 | 2.14 |
| Wrist | 1.49 | 1.57 | 1.37 | 1.70 | 1.61 | 2.23 | 2.50 | 3.56 |
| Men | ||||||||
| Hip | 1.36 | 1.36 | 1.26 | 1.18 | 1.15 | 1.09 | 1.05 | 1.05 |
| Proximal humerus | 1.52 | 1.52 | 1.84 | 1.68 | 1.67 | 1.58 | 1.78 | 2.09 |
| Wrist | 5.36 | 5.36 | 6.89 | 4.49 | 4.57 | 12.83 | 6.06 | 15.41 |
Application of hazard ratios to incorporate treatment effects both during and beyond the treatment period
As we have assumed a Gompertz underlying survival function for time to fracture, and as this is a proportional hazards model, the HR for treatment can be applied directly to the alpha parameter as described in Estimating time to event from absolute fracture risk. When taking a proportional hazards approach the treatment effect, as measured by the HR, is assumed to be constant over the entire duration of the survival curve. However, bisphosphonates are commonly given for only a few years and, therefore, we needed the model to allow for a fall-off in treatment effect after treatment is finished. For patients who complete the intended treatment period (5 years for all bisphosphonates except zoledronic acid) we have assumed a linear fall-off in HR for each year from years 5 to 10 such that the HR at 10 years is 1. For zoledronic acid, we have assumed a 3-year treatment period and a linear fall-off in treatment effect from years 3 to 10 such that the HR is 1 at year 10. This has been done by resampling the time to fracture at the end of the treatment period and applying a HR modified to account for the fall-off in treatment from years 5 to 10. The HR is modified by taking the average HR for full treatment effect and zero treatment effect. This modified HR is applied for the duration of the fall-off period. Although this linear approximation may underestimate the treatment effect in the early years after stopping and overestimate it in the later years, it should provide the correct treatment effect on average over the fall-off period. Adding more dummy events to update the HRs at more frequent intervals over the fall-off period was avoided, as it would reduce the computational efficiency of the model.
The time to fracture is resampled at the end of the fall-off period, with a HR of 1 applied thereafter. As the HR is assumed to increase over time in a Gompertz survival curve, the patient’s age is updated prior to resampling the time to fracture resulting in a new alpha value in the Gompertz function. We noted that the QFracture algorithm does not appear to be internally consistent when applied at different ages. For example, the 1-year risk of fracture in a 55-year-old is lower than the 1-year risk of fracture predicted for the fifth year in a patient aged 50 years. Given this internal inconsistency within the QFracture algorithm, our method of resampling at 5 and 10 years results in a stepped linear function for the ln(hazard) even when the HR is held constant over the whole modelled timeframe. However, this method maintains the proportional hazards assumption within each step. This can be seen in Figure 84, where the diamonds and squares show the stepped ln(hazard) function which results from resampling at 5 and 10 years when applying a constant HR of 2 or 1, respectively. It can be seen that the gap between the diamonds and squares is constant across the whole timeframe as would be expected for a proportional hazards model. Figure 85 demonstrates the additional effect of modifying the HR at 5 and 10 years to allow for reduced treatment effect during the fall-off period and no treatment effect beyond the fall-off period. It can be seen that this brings the ln(hazard) function for the treated patients (with treatment associated with a HR of 2 in this example), shown by the squares, down to match that of the no-treatment group (constant HR = 1 across all years), shown by the diamonds, from 10 years as would be expected. It should be noted that the squares and diamonds in Figure 85 do not match exactly, as the graphs are based on stochastic time-to-event estimates but we would expect them to match exactly if an infinite number of samples were used to derive the plotted points.
FIGURE 84.
Plot showing how resampling at 5 and 10 years results in a stepped ln(hazard) plot, but maintains the gap associated with the HRs.

FIGURE 85.
Plot showing the effect of adjusting the HRs to reflect falling treatment effect during the fall-off period (5–10 years) and after the fall-off period (> 10 years).

In those scenarios where we assume that patients do not persist with treatment for the full 5 years (or 3 years for zoledronic acid), we have used additional dummy events at 5 and 10 years to ensure that all patients receive an updated estimate of fracture risks at these time points.
Efficacy estimates
The HRs for fracture estimated by the systematic review and NMA described in Chapter 3 have been applied in the model. Fracture data have been synthesised using an NMA model including all studies defined by the inclusion/exclusion criteria (i.e. males and females, steroid users and non-steroid users, confirmed low BMD or BMD unknown). The resulting measure of treatment effect was a HR for the effect of each bisphosphonate relative to placebo together with an estimate of the between-study SD.
The NMA described in Chapter 3, Methods for the network meta-analyses, has been used to generate the joint predictive distribution of the HR for each treatment compared with no treatment in a new study; this acknowledges heterogeneity in the effect of each treatment depending on the characteristics of patients included in the studies. These relative treatment effects have been applied consistently across the whole modelled population within the economic analysis.
Absolute effects of treatment predicted by the economic model (e.g. number needed to treat) vary across the population because of some patients having a higher absolute risk of fracture based on either their QFracture or FRAX score.
The effect of treatment on hip fracture was estimated from studies reporting hip fracture data. The effect of treatment on vertebral fractures was estimated from studies reporting all vertebral fractures (i.e. clinical and morphometric) because not all studies (i.e. treatments) reported outcomes for clinical vertebral fracture. The effect of treatment on proximal humerus fractures was estimated using all non-vertebral fractures as a proxy because too few studies reported data for fractures specifically at the proximal humerus. Evidence on the effect of treatment on wrist fractures was available for all treatments except for zoledronic acid. The effect of zoledronic acid was estimated from the statistical model using the predictive distribution of a new bisphosphonate in a population of bisphosphonates.
The efficacy evidence from 2.5 mg per day of oral ibandronic acid has been applied to both 150 mg per month of oral ibandronic acid and 3 mg per 3 months of i.v. ibandronic acid where no alternative fracture data were available for these licensed regimens, as the monthly oral and quarterly i.v. doses were licensed based on their non-inferiority in lumbar spine BMD outcomes when compared with the daily ibandronic acid treatment regimen. 45,47,49,172,173 Where there were fracture data available for monthly oral ibandronic acid but none for quarterly i.v. ibandronic acid or daily oral ibandronic acid, we have assumed that the data from the monthly oral treatment can be applied to the i.v. treatment regimen. This was considered to be reasonable, as both the oral monthly dose and the quarterly i.v. dose were licensed based on non-inferiority compared with the daily oral dose for lumbar spine BMD outcomes. 172,173 Our own analysis of the femoral neck BMD data for these treatments would support this assumption of similar treatment effects for oral monthly ibandronic acid and quarterly i.v. ibandronic acid.
Fractures occurring at sites other than one of the four main osteoporotic fracture sites have the efficacy applied according to the site groupings previously described, that is hip fracture efficacy data are applied to other femoral fractures; wrist fracture efficacy data are applied to scapula, clavicle, rib, sternum; and all non-vertebral fracture efficacy data are applied to tibia and fibula, pelvis and humeral shaft.
The HR is assumed to be constant over the duration of the treatment period and then to decrease linearly over the fall-off period, reaching no effect by the end of the fall-off period. The linear fall-off is approximated by applying the average HR of full and zero treatment effect for the duration of the fall-off period.
The HRs applied in the base case are shown in Table 17. The median HRs estimated by the NMA were used in the deterministic analysis, and in the PSA the convergence diagnostics and output analysis samples from the NMA were used as these preserve the underlying joint distribution.
| Drug | Fracture site | |||
|---|---|---|---|---|
| Hip | Vertebral | Proximal humerus | Wrist | |
| Alendronic acid | 0.78 | 0.45 | 0.80 | 0.83 |
| Risedronic acid | 0.82 | 0.51 | 0.71 | 0.76 |
| Ibandronic acid (oral) | 0.87 | 0.45 | 0.80 | 0.83 |
| Ibandronic acid (i.v.) | 0.87 | 0.47 | 0.92 | 0.83 |
| Zoledronic acid | 0.94 | 0.41 | 0.75 | 0.81 |
Adverse event estimates
The model incorporates one-off costs and QALY decrements associated with AEs experienced in the first month of treatment. The published economic evaluations described in Chapter 4, Systematic review of existing cost-effectiveness evidence, were examined to see how they incorporated AEs. For oral bisphosphonates, the approach taken was based on the approach used by Stevenson et al. ,140 who incorporated AEs for upper GI symptoms in their analysis. For i.v. bisphosphonates the rates of flu-like symptoms were taken from the RCT evidence summarised in Chapter 3 and the quality-of-life decrement was based on an ad hoc search. The data used to incorporate AEs within the model are described in detail below and summarised in Table 18.
| AE | Oral bisphosphonates, upper GI symptoms | i.v. bisphosphonates, flu-like symptoms |
|---|---|---|
| Incidence | 3% in the first month of treatment | 14% in the first month of treatment |
| Utility decrement | 9% (i.e. a multiplier of 0.91) | 65% (i.e. a multiplier of 0.35) |
| Duration of utility decrement | 1 month | 3 days |
| QALY loss per patient experiencing AE | 0.0075 | 0.0053 |
| Resource use | GP appointment and prescription for generic ranitidine (Zantac®, GlaxoSmithKline UK, Ltd) | None |
| Cost per patient experiencing an AE | £45.00 (GP) + £1.76 (drugs) = £46.76 | None |
We have not explicitly modelled the relationship between AEs and treatment persistence, but it is expected that AEs contribute to the low levels of treatment persistence described in Treatment strategies.
Adverse events associated with bisphosphonate treatment were not consistently incorporated in economic analyses included in our review. Stevenson et al. 140 did not include any AEs in the model reported in their 2005 publication, but a later Decision Support Unit (DSU) report by Stevenson and Davis174 describes additional analyses in which AEs were included. Both Kanis et al. 138 and Borgström et al. 137 used the assumptions described in the DSU report by Stevenson et al. 174 within sensitivity analyses, but neither included AEs in their base case. The remaining published analyses139,141–143,146 did not include AEs.
Stevenson et al. 174 used data from prescription event monitoring studies identified in a systematic review by Lloyd-Jones and Wilkinson116 to determine the rate of upper GI problems in patients treated with oral bisphosphonates. In the DSU report, Stevenson et al. 174 assumed 2.35% of patients required a GP appointment and a course of H2 receptor antagonists as a result of GI AEs in the first month of therapy and 0.35% thereafter. 174 These patients were assumed to have a HRQoL decrement of 9% (utility multiplier of 0.91 from Groeneveld et al. 175) for the full month, which was described by Stevenson et al. 174 as a deliberately pessimistic assumption that aimed to counterbalance the fact that no other adverse AEs, such as nausea, had been included. Lloyd-Jones and Wilkinson116 also reported that other cohort studies found that 30% of patients starting alendronic acid may report GI AEs. A sensitivity analysis using a higher rate of AEs (24%) in the first month of alendronic acid treatment was considered by the Technology Appraisal Committee when formulating recommendations for TA16021 and TA161. 24
Our review of systematic reviews examining AEs did not identify any systematic reviews that examined GI AEs that were published more recently than the review by Lloyd-Jones and Wilkinson. 116 The prescription event monitoring studies identified by Lloyd-Jones and Wilkinson116 found a greater incidence of dyspeptic conditions in the first month of treatment with alendronic acid and risedronic acid (3%) than in later months (1%). 116 This was considered by our clinical advisors to be low compared with the rates they saw in clinical experience, which were estimated to be around 20%.
All three oral bisphosphonates were found to have similar rates of GI symptoms when compared with placebo in RCTs. Furthermore, prescription event monitoring data and data from two head-to-head RCTs suggest similar rates of GI symptoms for alendronic acid and risedronic acid. The consultee submission by Actavis176 cited a study by Ralston et al. ,126 which concluded that switching patients who are stabilised on risedronic acid to alendronic acid is associated with an increased risk of GI AEs. However, this evidence was not considered to be directly applicable to the question of whether or not AEs are more common when initiating treatment with alendronic acid or risedronic acid in patients without prior treatment with bisphosphonate. Limited data were available to assess if monthly formulations result in a lower incidence of GI symptoms than weekly formulations, but the review by Bobba et al. 112 stated that increasing the dosing interval to weekly or monthly does not appear to change the rates of GI AEs when compared with daily dosing for any of the three oral bisphosphonates. Therefore, the rates of AEs for alendronic acid from prescription event monitoring studies have been applied consistently to all oral bisphosphonates. Our clinical advisors informed us that clinical experience would suggest that upper GI symptoms are most problematic for alendronic acid, with risedronic acid being less problematic and ibandronic acid even less so owing to less frequent dosing. However, as this evidence was anecdotal, they considered it reasonable to assume equivalent AEs for the oral bisphosphonates.
In the model we applied the data on dyspeptic conditions from prescription event monitoring studies described by Lloyd-Jones and Wilkinson116 and assumed that 3% of patients starting treatment with an oral bisphosphonate experience GI symptoms requiring a GP appointment and prescription of a H2 receptor antagonist in the first month of treatment. A sensitivity analysis was also conducted examining a rate of 30% in the first month to reflect the higher rates observed in some observational studies, as described by Lloyd-Jones and Wilkinson. 116 Clinical advice was that proton pump inhibitors are usually prescribed instead of H2 receptor antagonists despite a caution in the British National Formulary regarding the potential for an increased fracture risk for proton pump inhibitors. 39 However, as generic lansoprazole (Zoton FasTab®, Pfizer) is similarly priced to generic ranitidine, we have assumed for simplicity that all patients receive a H2 receptor antagonist. Total cost per patient experiencing a GI AE was assumed to be £46.76 (£45 for GP appointment and £1.76 for generic ranitidine). 39 We have applied the same assumptions on disutility as Stevenson et al. ,140 which we calculate to be equivalent to a QALY loss of 0.0075 per patient experiencing GI symptoms. We have applied this as a fixed QALY decrement at the start of the model without adjustment for baseline health utility.
In our review of AEs, the incidence of flu-like symptoms was found to be significantly higher among patients treated with zoledronic acid than among those receiving placebo. Although none of the RCTs or observational studies reported flu-like symptoms for i.v. ibandronic acid, the SmPC for Bonronat® (Roche Products Ltd) (branded i.v. ibandronic acid) describes influenza-like symptoms that resolve after ‘a couple of hours/days’ as a common side effect, affecting up to 1 in 10 people. A study by van Hoek et al. 177 reports the utility for influenza-like illnesses as being 0.34 compared with a baseline (no flu-like symptoms) of 0.97 based on EQ-5D scores in a cohort of 655 patients with influenza-like illness. Based on these estimates, we considered that a utility multiplier of 0.35 would be reasonable for flu-like symptoms. We have assumed a disutility of 0.65 for 3 days for flu-like symptoms associated with i.v. bisphosphonates, which is equivalent to a QALY loss of 0.005. This has been applied as a fixed QALY decrement at the start of the model without adjustment for baseline utility. We took the rate of influenza-like symptoms to be the rate of pyrexia reported in the HORIZON-PFT,56 as this was the largest RCT reporting data on flu-like symptoms and pyrexia was more common than other flu-like symptoms (headache/chills). The 14% difference in pyrexia rates between zoledronic acid and placebo was applied to patients receiving either i.v. zoledronic acid or i.v. ibandronic acid. These were applied for only the first infusion to reflect the fact that these rates were measured over the whole trial period (36 months) and, therefore, applying them repeatedly would overestimate the incidence of flu-like symptoms. Furthermore, it is likely that patients who experience significant side effects are more likely to be in the group who do not persist with treatment so repeated episodes of significant disutility are unlikely.
Estimating time to non-fracture-related mortality
Sex-specific UK life tables were used to provide an empirical estimate of the likelihood of death for each year after the start of the model. 155 This was calculated based on the age of the patient. So for a patient aged 30 years, the likelihood of death (denoted by dx within the life tables) between each birthday from the age of 30 to100 years was used to estimate the empirical distribution of survival times. Similarly, for a patient aged 90 years, the likelihood of death between each birthday from age 90 to 100 years was used. This method assumes no survival beyond age 100 years, as this is the limit of the data provided in the life tables. The time horizon of the model was therefore set to equal 100 years minus the starting age, giving a variable duration modelled depending on the patient’s start age. The data used to estimate time to non-fracture-related death were not varied in the PSA.
Mortality after hip fracture
Patients experiencing hip fracture in the model have a risk of experiencing fracture-related mortality, which is applied as a one-off probability at the time of fracture. The risk is age and sex specific, and fracture-related death is assumed to occur at a fixed time following fracture rather than at a sample time in the future. All of the published cost-effectiveness analyses described in Systematic review of existing cost-effectiveness evidence included an excess of risk of mortality attributable to hip fracture. These papers and a published systematic review by Abrahamsen et al. 178 were examined to identify suitable data to include in the model.
The systematic review by Abrahamsen et al. 178 examined the relationship between hip fracture and mortality, and found that patients with hip fracture experience a high mortality rate, which is at least double that for age-matched population norms. Abrahamsen et al. 178 also noted that while the highest excess risk appears to be in the first 6 months following fracture, many of the studies they examined found an increased risk that persisted for a number of years. Age and sex were both found to be important predictors of post-fracture mortality supporting the use of age- and sex-specific estimates within our model.
Although there is clear evidence of excess mortality following hip fracture compared with general population norms, the extent to which underlying conditions contribute to the excess mortality associated with hip fracture is unclear. 178 Underlying health conditions, which may be more common in patients experiencing hip fracture than in age- and sex-matched population norms, may contribute to mortality independently of the fracture itself, confounding the relationship between fracture and mortality. Kanis et al. 179 found that 17–32% of deaths following hip fracture were causally related to fracture, whereas Parker and Anand180 estimated that 25% of deaths were directly attributable to hip fracture, with a further 42% possibly attributable to hip fracture. A study by Tosteson et al.,181 which was able to adjust for a number of prognostic factors including pre-fracture health status, found that excess mortality was limited to the first 6 months after fracture.
To populate the model, data were needed on the absolute risk of mortality following hip fracture that is directly related to the hip fracture and, therefore, potentially avoidable by treatment to prevent fractures. Age- and sex-specific estimates were sought because these are important risk-modifying factors identified in the systematic review by Abrahamsen et al. 178 UK estimates were also considered preferable, as these are more likely to be representative of the population likely to be affected by NICE guidance. Of the studies included in the review by Abrahamsen et al.,178 10 reported results for UK cohorts. 180,182–190 The majority of these studies do not report data on the absolute risk stratified by age and sex. Holt et al. 187 provide graphs of survival at 120 days for different sexes and age bands. Deakin et al. 183 provide age- but not sex-specific estimates of mortality at 30 days and 1 year rates. Parker and Anand180 provide age-specific mortality rates, but these are not reported separately for males and females. Only one study, by Roberts and Goldacre,189 provides age- and sex-specific mortality rates, and these are provided at 30, 60 and 365 days. This study used data from the Oxford record linkage study,191 which comprises anonymised abstracts of hospital statistics linked to death certificates. The population examined by Roberts and Goldacre189 was 32,590 people aged ≥ 65 years who were admitted to hospital as emergencies with fractured neck of femur between 1968 and 1998. Mortality rates were compared over six time windows between 1968 and 1998 and absolute mortality rates are provided for the cohort admitted with fractures between 1984 and 1998.
The studies included in the review of published cost-effectiveness analyses were also examined to determine the source of data used. Stevenson et al. 140 used unpublished estimates from the Anglian audit of hip fracture, which were reported for several different age bands, and adjusted these to remove those deaths not causally related to hip fracture using the data from Parker and Anand. 140,180 Ström et al. ,139 Borgström et al. 137 and Kanis et al. 142 used data from Sweden179,192,193 rather than data from the UK. van Staa et al. 141 estimated excess mortality rates from a UK database of general practice patients (GPRD, which is now called CPRD) and absolute rates are presented by age band, but this cohort was restricted to postmenopausal women. A Cox proportional hazards model was used by van Staa et al. 141 to compare 1-year mortality rates for those with fracture and controls without fracture, who were matched based on age, GP practice and calendar time. Similar methods were used in another of the included cost-effectiveness papers, by van Staa et al. ,143 which identified cases and controls from the same UK database but examined a population treated with steroids. However, this paper did not report the absolute mortality risks calculated. No additional studies were identified from the papers by Kanis et al. 138 and Borgström et al. 146
The age- and sex-specific mortality rates reported by Roberts and Goldacre189 for 1 year were much higher than the excess rates reported by van Staa et al. 141 This is to be expected because the estimates from van Staa et al. 141 are the excess mortality rates compared with age- and sex-matched controls, whereas the estimates reported by Roberts and Goldacre189 are raw mortality rates. As our aim was to include only the excess mortality associated with hip fracture in our model. The rates reported by van Staa et al. 141 were incorporated in the model for women in preference over the data from Roberts and Goldacre. 189 The excess rates in men were estimated by applying the ratio of raw events observed between men and women from Roberts and Goldacre189 to the excess rates for women from van Staa et al. 141
The excess mortality rates attributable to hip fracture that have been applied in the model are presented in Table 19. In the PSA, these rates have been varied by estimating the numbers in each category in the patient cohort used by van Staa et al. 141 by assuming that the age distribution is similar to that of the general population159 and using the estimated number with and without excess mortality to inform a beta distribution for each age band. The ratio of excess mortality rates for males versus females was not varied in the PSA.
| Age band (years) | Data for women (%)141 | Ratio of rates (male : female)189 | Estimate for males (%) |
|---|---|---|---|
| 50–59 | 2.4 | 1.63a | 3.9 |
| 60–69 | 4.4 | 1.63a | 7.2 |
| 70–79 | 7.5 | 1.75 | 13.1 |
| 80–89 | 11.4 | 1.58 | 18.1 |
| ≥ 90 | 13.6 | 1.47 | 20.0 |
Abrahamsen et al. 178 report that approximately half of all mortality associated with hip fracture occurred within 3 months and 70% occurred by 6 months. Given that Tosteson et al. 181 reported no excess mortality after 6 months following adjustment for a variety of factors, including pre-fracture functional status and comorbid conditions, we decided to assume that all deaths related to hip fracture occurred at exactly 3 months. A sensitivity analysis was conducted examining the alternative assumption that all deaths related to hip fracture occurred at exactly 1 month post fracture. Hip fractures occurring before age 50 years were assumed not to result in any excess mortality.
A systematic review by Smith et al. 157 found that the RR of death following hip fracture for those residing at home compared with those residing in an institution prior to hip fracture was 0.57 (95% CI 0.43 to 0.72) when meta-analysed across five studies including a total of 25,497 participants. To reflect the increased risk of mortality for those institutionalised prior to hip fracture, we applied a RR of 1.75 (1/0.57) to the figures in Table 19 for those residing in institutional care prior to hip fracture. This may have slightly overestimated the risk of mortality following hip fracture, as some of the patients included in the study by van Staa et al. 141 will have been institutionalised and, therefore, the risks for non-institutionalised patients should be adjusted down. However, the study by van Staa et al. 141 does not report the proportion institutionalised by age category within their sample so this adjustment was not possible. The likely bias introduced by not adjusting these figures is expected to be small, as the majority of patients within the model do not reside in institutional care (see Figure 70).
Mortality after vertebral fracture
The approach used to model mortality after vertebral fracture is similar to that used to model mortality after hip fracture. All of the papers included in the review of published cost-effectiveness analyses included some estimate of mortality following vertebral fracture within their economic evaluation. These papers were examined to determine the source data used.
The cost-effectiveness analysis by van Staa et al. 141 used estimates of mortality following clinical vertebral fracture which were derived by the authors themselves from a UK cohort of postmenopausal women identified from a database of general practice patients (GPRD). The methods used in this paper to estimate mortality after vertebral fracture were the same as those used to estimate mortality after hip fracture and have been described above in Mortality after hip fracture. Excess mortality rates are presented in this paper by age band, but are limited to women. As described previously in Systematic review of existing cost-effectiveness evidence, a second paper by van Staa et al. 143 used a similar method to estimate excess mortality after fracture in a cohort of UK patients treated with steroids but mortality rates were not reported in this second paper.
Two of the included cost-effectiveness papers137,139 reported using estimates from Oden et al. ,193 but the absolute mortality rates could not be identified from the cited paper. Kanis et al. 142 cited seven studies192,194–199 that provide data on the mortality risk after vertebral fracture. The only study to use a UK cohort, by Jalava et al. ,197 examined the impact of prevalent and incident vertebral fractures on mortality rates in patients enrolled in a RCT of clodronic acid. Jalava et al. 197 commented that the small size of this study’s cohort limited its ability to detect a mortality effect related to incident fractures with only seven deaths occurring in patients with incident vertebral fractures. 197 Kanis et al. 142 used the RR associated with prevalent vertebral fractures from the UK study by Jalava et al. 197 to determine the rate of deaths associated with vertebral fractures in their cost-effectiveness model. Data from a Swedish study by Kanis et al. 199 were used by Kanis et al. 142 to determine the percentage of deaths (28%) that were causally related to vertebral fracture and data from a second Swedish study by Johnell et al. 192 were used to justify applying the same RR for males and females. Kanis et al. 199 provide estimates of the absolute risk of mortality stratified by sex and age bands and adjusts this to account for the proportion of deaths that are causally related. Johnell et al. 192 provide estimates of excess absolute risks by sex for ages 60 and 80 years by comparing the mortality rate in those with fractures against age- and sex-matched general population controls. The remaining studies cited by Kanis et al. 142 did not provide estimates of absolute risk stratified by age and sex. No additional studies were identified from the cost-effectiveness studies by Kanis et al. ,138 Borgström et al. 146 and Stevenson et al. 140
It should be noted that not all of the studies identified agreed about the causal nature of the relationship between vertebral fractures and mortality. Several studies found no statistically significant increase in mortality rates for incident fractures after adjusting for potential confounding factors. 197,200 Those studies that found a significant relationship192,195,196,199 often did not adjust for potential confounding factors other than age and sex, although Cauley et al. 194 did find a significant increase after adjusting for six comorbidities and pre-fracture health status.
Differences between findings across studies may also be related to whether or not they considered morphometric vertebral fractures or only those coming to clinical attention, which are likely to be more severe. The study by Kanis et al. 199 considered only hospitalised vertebral fractures, which could be expected to be more severe and associated with a higher death rate than non-hospitalised clinical vertebral fractures.
Some studies used baseline radiographs to confirm that the incident fracture was in fact new and not an undiagnosed prevalent fracture,194,200 but many studies195,196,199 assumed that fractures that came to clinical attention had occurred recently. Kado et al. 198 considered only the impact of prevalent vertebral fractures on mortality. The impact of incident fractures on mortality for the same cohort were considered in a later publication by Kado et al. 200 Those studies that considered morphometric fractures may also be complicated by the potential for delay between the fracture and the time it is found on a radiograph. Kado et al. ,200 whose study relied on a single radiograph during the follow-up period to identify incident morphometric fractures, noted that some fractures might have occurred between the last radiograph and the end of follow-up, with those patients being allocated to the no-fracture group.
The data reported by van Staa et al. 141 were used in the model, as this study used a large UK cohort, adjusted for multiple confounding factors and reported the excess risk for incident clinically symptomatic vertebral fractures. Although Center et al. 195 reported higher standardised mortality rates for men than for women when considering all vertebral fractures, the differences were small when considering incident vertebral fractures alone [1.6 (95% CI 1.4 to 1.8) in women vs. 1.8 (95% CI 1.6 to 2.0) in men]. Johnell et al. 192 reported a non-significant trend for a higher RR in men than women and Kanis et al. 199 noted that the difference was not marked after taking into account sex differences in mortality within the general population. Therefore, we used the excess rates for women from van Staa et al. 141 and applied these to both men and women within our model. The timing of excess mortality attributable to vertebral fracture was less well discussed in the identified studies than for similar data for hip fracture. However, a graph of death hazard over time for both hip and vertebral fractures, presented by Kanis et al. ,199 suggests that a similar temporal pattern is seen for hip and vertebral fracture with high excess mortality in the early months. Therefore, we assumed that all mortality related to vertebral fracture occurred at 3 months, as this was the assumption used for hip fracture-related mortality.
The excess rates following vertebral fracture applied in the model are presented in Table 20.
| Age band (years) | Excess mortality because of vertebral fracture (%) |
|---|---|
| 50–59 | 2.3 |
| 60–69 | 3.5 |
| 70–79 | 5.2 |
| 80–89 | 6.7 |
| ≥ 90 | 6.6 |
In the PSA, the parameter uncertainty around these excess mortality rates has been calculated using the same method used for excess mortality following hip fracture (see Mortality after hip fracture).
Excess mortality risk at fracture sites other than hip or vertebrae
Patients experiencing fractures at sites other than the hip or vertebrae were assumed not to experience a fracture-related death. Having examined the published cost-effectiveness analyses to identify evidence on the increased risk of mortality following fractures at other sites, we decided that the evidence was not sufficient to support including this in the model.
Three of the seven papers included in our review of published cost-effectiveness analyses included an increased mortality risk for fractures at the proximal humerus. 140,142,143 Two of these studies140,142 cited the paper by Johnell et al.,192 which found an increased risk of mortality compared with age- and sex-specific matched general population estimates among patients with shoulder fracture, although the increase was not statistically significant at all ages. The third paper, by van Staa et al. ,143 used Cox proportional hazards models to assess the excess mortality in the year following hip, wrist, vertebral and proximal humerus fractures in a population treated with steroids compared with age- and sex-matched control subjects. These 1-year excess risks were incorporated in their analysis for all four fracture sites, but no data on the excess risks are presented in the paper. In a similar analysis, van Staa et al. 141 examined the excess mortality associated with hip, wrist, vertebral and proximal humerus fracture in a UK population of postmenopausal women. However, they found that the excess risk of mortality was small for fracture types other than hip or vertebral fracture and they did not include any estimates of excess mortality for wrist or proximal humerus fractures in their analysis in postmenopausal women. A study by Cauley et al. ,194 which analysed mortality rates before and after fracture using data from a RCT, found no increased risk of mortality for fractures at sites other than the hip or vertebrae after adjusting for six comorbidities and pre-fracture health status. However, a more recent paper by Piirtola et al. 201 found that the mortality rates following proximal humerus fractures were significantly increased in men but not women. Given that the evidence for an excess risk of mortality following proximal humerus fracture is not consistent across the studies we examined, we have not included any increased mortality risk for proximal humerus fractures.
Only one of the published cost-effectiveness analyses included in our literature review incorporated an increased risk of mortality for wrist fractures. 143 This paper used estimates derived by the authors of a general practice database for a cohort treated with steroids, but estimates of the excess mortality by fracture type were not provided in the paper. 143 However, two of the published analyses140,142 stated that their assumption of no increased mortality risk following wrist fractures was consistent with published surveys. 192,194–196 We have assumed no increased risk of mortality following wrist fracture in our analysis.
Stevenson et al. 140 and Kanis et al. 142 grouped fractures occurring at sites other than the hip, wrist, proximal humerus and vertebrae into one of these four fracture types. This meant that the excess mortality of hip fracture was also attributed to femoral shaft and pelvic fracture, and the excess mortality for proximal humerus fractures was also attributed to fractures of the humeral shaft, tibia and fibula. In our model, we have grouped other femoral fractures, but not pelvic fractures, with hip fractures so that the excess mortality risk associated with hip fracture is also applied to other femoral fractures. The data we have used on excess mortality following hip fracture were taken from the paper by van Staa et al. ,141 which also grouped other femoral factures with hip fractures and, therefore, the data are being used in a manner consistent with what they were intended for. In summary, our analysis allows for excess mortality following fractures at the hip, femoral shaft or vertebrae but not for any other fracture site.
Risk of nursing home admission following hip fracture
Pain, reduced physical function and lack of mobility are common outcomes after hip fracture and can lead a patient who was previously living independently to require long-term nursing care. Patients experiencing a hip fracture who are community dwelling prior to fracture are assumed in the model to be at risk of moving to a nursing home or residential care. The risk is applied as a one-off risk at the time of the fracture event. The published economic analyses described in Systematic review of existing cost-effectiveness evidence were examined to identify sources of data on this parameter and this was supplemented by a scoping search to find additional sources.
All of the published cost-effectiveness studies included in our review appeared to include some estimate of nursing home admission within their model. Two studies138,139 included in the review of published cost-effectiveness analyses cited a conference poster by Zethraeus et al.,202 which gives the proportion of patients going into long-term care in the year following hip fracture surgery in Sweden by age band. Two of the published studies140,142 used data from the East Anglian hip audit. 203 Three of the studies included in the review of published cost-effectiveness analyses137,141,143 cited a report describing the model, which was later published by Stevenson et al. 140 as their source of data on nursing home admission following hip fracture, suggesting that they too applied the data from the East Anglian hip audit. 203
As the only UK data identified from the published cost-effectiveness analyses were data from a 1999 research report,203 more recent data were sought to inform the risk within the model of patients moving from living in their own home to nursing home care after hip fracture. Age- and sex-specific data were sought, as it was believed that there may be a differential risk according to the age and sex of the patient. A scoping search identified a small number of papers addressing the issue of nursing home admission after hip fracture, of which four contained data on risk of discharge by both age and sex. 158,204–206 These papers are summarised in Table 21.
| Author and year of study publication | Location | Patient group | Observation period | Method | Outcome measure | Variables of interest |
|---|---|---|---|---|---|---|
| Osnes et al., 2004158 | Norway | Hip fracture patients aged ≥ 50 years, excluding cancers, n = 593 living respondents (235 died, 174 non-responses) | 184–584 days | Logistic regression | Discharge to nursing home | Age group:
|
| Holt et al., 2008204 | Scotland | Hip fracture patients aged 50–89 years, excluding ages ≥ 90 years, or without surgery, n = 17,357 living patients (3085 lost to follow-up) | 120 days | Logistic regression | Residence at 120 days | Age group:
|
| Deakin et al., 2008205 | England | Hip fracture patients aged ≥ 50 years, excluding bilateral, periprosthetic, road accident and pathological fractures, n = 3240 | Not stated (time to discharge) | Logistic regression | Discharge to an alternative location (to normal residence) | Age group:
|
| Nanjayan et al., 2014206 | England | Hip fracture patients aged ≥ 50 years, admitted from home, excluding no surgery, n = 1503 (133 died) | Not stated (time to discharge) | Logistic regression | Discharge to an alternative location (to home) | Age group:
|
The study by Holt et al.,204 despite covering a large sample in a UK population, was excluded on the basis that the analysis by age included only two age groups with relatively wide bounds (50–64 years and 75–89 years) and excluded patients aged 65–74 years. This was thought inadequate to assess the increasing risk of nursing home discharge with age.
We calculated approximate age- and sex-specific probabilities for discharge to a non-home location using the overall probability of discharge to institutional care and ORs for age and sex reported by the remaining three studies. 158,205,206 Studies by Osnes et al. 158 and Nanjayan et al. 206 gave similar results, but Osnes et al. 158 was thought less appropriate to the UK setting because of the potential for differences in social care structure and cultural norms regarding institutional care between the UK and Norway. Of the two UK studies, the study by Nanjayan et al. 206 was preferred because the analysis explicitly excluded those who had died before discharge and was based solely on patients who were living in their own home prior to the fracture. Both of these criteria matched the model requirements, and hence data from Nanjayan et al. 206 were used in preference to those from Deakin et al. 205 (Figure 86).
The overall rate of discharge to a non-home location (residential home, nursing home or hospitalisation) was given as 20% by Nanjayan et al. 206 Combining this with the known sex split of the cohort (71% female) and the stated ORs for each age and sex group, it was possible to derive an expected risk of non-home discharge for each age and sex group for use in the model; these are shown in Table 22. The risk of being discharged to a non-home location increases with increasing age (OR 9.09 for patients aged between 90 and 99 years whereas OR = 1 for patients of approximately 69 years) and is higher for males than for females (OR 1.67).
| Variable | ORa | Discharged from hospital to a non-home location, by age group (%) | |
|---|---|---|---|
| Female | Male | ||
| Age band (years) | |||
| 50–59 | 0.76 | 4 | 6 |
| 60–69 | 1.92 | 7 | 11 |
| 70–79 | 1.96 | 12 | 19 |
| 80–89 | 4.54 | 21 | 30 |
| 90–99 | 9.09 | 33 | 45 |
| Sex | |||
| Female | 1.00 | ||
| Male | 1.67 | ||
The risks of a new admission to an institutional residential setting after hip fracture, presented in Table 22, have been applied within the model. In the PSA, these have been varied by applying a beta distribution to the overall rate of admission to an institutional residential setting on which the rates in the individual age and sex categories is dependent (see Appendix 8, Table 53, for details on PSA distributions).
Risk of nursing home admission following vertebral fracture
In the base-case analysis we have assumed that vertebral fractures do not result in new admissions to nursing homes or residential care. The published economic evaluations identified in Systematic review of existing cost-effectiveness evidence were examined to identify sources of data on nursing home admission following fracture. Only one of the papers142 included in our review of published cost-effectiveness analyses included a rate of nursing home admission following vertebral fracture. Kanis et al. 142 incorporated data on the rate of nursing home admissions in Swedish patients from a paper by Borgström et al. ,207 which reported similar rates of patients living in ‘special living accommodation’ for hip and vertebral fractures. However, Borgström et al. 207 also noted in their discussion that, in their patient sample, the proportion hospitalised was higher than expected (72% vs. expected 10%). The study by Borgström et al. 207 recruited patients at the time of fracture and no comparison was made with matched control subjects to remove costs that may be related to comorbidities. In comparison, a study, by De Laet et al. ,208 that did compare costs in fracture patients and matched control subjects found that nursing homes costs were substantially higher for hip fracture patients than for control subjects, but were only slightly and non-significantly higher for vertebral fracture patients. However, this analysis, conducted as part of the Rotterdam study,209 included patients with a new morphometric fractures and may therefore underestimate resource use in those with clinically apparent vertebral fractures. Given the lack of consensus on the incorporation of nursing home admission rates within the published analyses and the differing data from these two studies, we decided to omit nursing home admission following vertebral fracture from our base-case model, but examined the impact of including a rate equivalent to that seen in hip fracture in a sensitivity analysis.
Risk of subsequent fracture after incident fracture
A systematic review and meta-analysis by Klotzbuecher et al. 149 has previously been used in several published economic evaluations to estimate the increased risk of fracture at various sites when a patient sustains an incident fracture within the model. 139,140,142 We conducted a citation search, using the Web of Science database, to find relevant articles published since the review by Klotzbuecher et al. ,149 on the assumption that new studies in this area would be likely to cite this published systematic review. We found 811 records of articles citing this systematic review. Given the large number of potentially relevant articles identified, we tried to establish whether or not any more recent systematic reviews had been published. The abstracts and titles of these articles were then searched separately using the free-text terms ‘review’, ‘meta-analysis’ and ‘synthesis’ to see if any of these articles provided an updated systematic review and meta-analysis similar to that presented by Klotzbuecher et al. 149 Two potential systematic reviews were identified and full texts examined. 210,211 The first, by Haentjens et al. ,210 was specifically interested in comparing whether or not the RR of hip fracture after a wrist or spine fracture differed by sex. Owing to its focus on sex differences, this study had narrower inclusion criteria and excluded many of the studies included by Klotzbuecher et al. 149 and it only included one additional recent study. 212
The second systematic review identified from our citation search, which was authored by Blank (on behalf of the FRAX Position Development Conference Members),211 identified around 20 studies published since the Klotzbuecher et al. 149 review. However, these studies are discussed narratively by Blank and no meta-analysis is provided. 211 It was not considered feasible to review and meta-analyse all of these new studies in order to update the estimates provided by Klotzbuecher et al. 149
A 2011 review by Warriner et al. ,213 which meta-analysed data from 25 studies published since the Klotzbuecher et al. 149 review, was identified opportunistically. The review by Warriner et al. 213 does not provide any details regarding the methods used to identify the studies. It also provides limited details on the studies included and does not tabulate the RRs from the individual studies prior to pooling. Therefore, it was decided that the estimates from Warriner et al. 213 should be treated with caution because of the potential for selection bias. The estimates provided by Klotzbuecher et al. 149 were used in the base-case model. These estimates were supplemented by data from Warriner et al. 213 where no estimates were provided by Klotzbuecher et al. 149 Neither meta-analysis provided data on the increased risks of fracture following proximal humerus fracture. Data on the increased risk following fracture at any site were used as a proxy for risk following fractures at the proximal humerus. Neither meta-analysis provided data on the risk of proximal humerus fracture after hip fracture, so the data on proximal humerus fracture following fracture at any site from Warriner et al. 213 were used. The data in Table 23 were applied in the model as HRs within the survival curves, used to estimate time to fracture for the base-case analysis. A sensitivity analysis has also been conducted using the estimates from Warriner et al. 213 exclusively, which are shown in Table 24.
| Location of prior fracture | Site of subsequent fracture, HR (95% CI) | |||
|---|---|---|---|---|
| Wrist | Vertebral | Hip | Proximal humerus | |
| Wrist | 3.3 (2.0 to 5.3)a | 1.7 (1.4 to 2.1)a | 1.9 (1.6 to 2.2)a | 2.5 (0.6 to 10.2)b |
| Vertebral | 1.4 (1.2 to 1.7)a | 4.4 (3.6 to 5.4)a | 2.3 (2.0 to 2.8)a | 1.6 (0.7 to 3.0)b |
| Hip | 3.0 (1.3 to 6.5)b | 2.5 (1.8 to 3.5)a | 2.3 (1.5 to 3.7)a | 2.1 (0.3 to 17.3)a |
| Proximal humerusc | 1.9 (1.3 to 2.8)a | 2.0 (1.6 to 2.4)a | 2.0 (1.9 to 2.2)a | 2.1 (0.3 to 17.3)b |
| Location of prior fracture | Site of subsequent fracture, HR (95% CI) | |||
|---|---|---|---|---|
| Wrist | Vertebral | Hip | Proximal humerus | |
| Wrist | 3.2 (1.3 to 8.1) | 2.9 (1.6 to 5.3) | 2.9 (2.0 to 4.1) | 2.5 (0.6 to 10.2) |
| Vertebral | 1.8 (1.1 to 3.2) | 4.9 (2.4 to 9.8) | 3.7 (2.3 to 5.9) | 1.6 (0.7 to 3.0) |
| Hip | 3.0 (1.3 to 6.5) | 3.6 (1.9 to 6.7) | 3.7 (2.5 to 5.3) | 2.1 (0.3 to 17.3) |
| Proximal humerusa | 2.6 (1.8 to 3.8) | 3.0 (2.2 to 4.0) | 2.4 (1.6 to 3.5) | 2.1 (0.3 to 17.3) |
The values from Klotzbuecher et al. 149 and Warriner et al. 213 are applied for the patient’s remaining lifetime once a fracture occurs. The studies included by Klotzbuecher et al. 149 in the meta-analysis had varying durations of follow-up, but generally > 1 year, so the estimates provided by Klotzbuecher et al. 149 represent the RR when averaged over all years of study follow-up. The temporal profile of increased fracture risk after an incident fracture has been studied by van Geel et al. 214 Their analysis suggests that the RR is approximately 2 when averaged over the long term, but when assessed over different time periods is much higher immediately after the first fracture, and tails off towards 1 over the next 20 years. We acknowledge that our method of applying a fixed RR over the patient’s remaining lifetime probably underestimates the increased risk in the immediate years after fracture, but it is likely to overestimate the increased risk in the long term. The alternative would be to use additional dummy events to modify the increased risk in the years after fracture, but this would reduce the computational efficiency of the model. In the PSA, the HRs in Table 23 were sampled from a log-normal distribution using standard errors calculated from the 95% CIs reported in Table 23 (see Appendix 8, Table 52, for PSA distributions).
When more than one incident fracture was sampled to occur during a patient’s lifetime, the maximum value from Table 23 has been applied for each subsequent fracture type rather than applying several multipliers concurrently. For example, if someone has had a prior wrist fracture and a prior vertebral fracture then their increased risk of vertebral fracture is 4.4, which relates to their prior history of vertebral fracture, as this is the maximum value in the vertebral column in Table 23. However, their increased risk for proximal humerus fracture would be 2.5, which relates to their prior history of wrist fracture, as this is the maximum value in the proximal humerus column.
Both QFracture and FRAX incorporate an increased risk for patients with a history of prior fracture and, therefore, those with a prior fracture at the start of the model already have an increased risk applied for prevalent fractures. This increased risk associated with fractures occurring prior to the start of the model is removed at the time of the first incident fracture and the data from Table 24 are applied instead. This is to prevent the risk being increased twice for the same patient characteristic using two different mechanisms within the model.
Health-related quality of life: review of utility values following fracture
To inform the model, data were needed on the proportionate decrease in HRQoL that occurs in the year following fracture and in subsequent years. This was then used to calculate a utility multiplier, which was applied to the pre-fracture utility value to calculate the post-fracture utility. For example, a proportionate decrease of 10% would translate into a utility multiplier of 0.9. If the patient’s prior fracture utility is 0.8, then the post-fracture utility would be 0.72. Data on the absolute HRQoL after fracture can be obtained from studies that measure HRQoL in patients who have experienced a recent fracture. However, the proportionate decrease can be obtained only if there is some estimate of pre-fracture utility. Ideally, HRQoL would be measured prospectively in a cohort of patients at risk of fracture and these patients would be followed up with HRQoL remeasured at regular intervals with the time of any incident fracture being recorded so that the correlation between HRQoL and incident fracture can be obtained after adjusting for other confounding factors. However, many studies simply recruit patients at the time of fracture and ask them to recall their pre-fracture health state, which is subject to recall bias. Other studies may compare the HRQoL in individuals who have fractured with matched controls or population norms, in which case the estimates may be confounded by differences in other factors between cases and controls.
Initially, a systematic search was conducted to identify studies reporting any measure of health utility in patients with an incident osteoporotic fracture. However, this search retrieved 3991 unique references and it was not considered feasible to sift such a large number of papers within the timescales of the NICE appraisal process. As the NICE methods guide144 states that the EQ-5D is the preferred measure of HRQoL in adults, and a recent systematic review by Peasgood et al. 215 had already demonstrated that EQ-5D data exist for the four major osteoporotic fracture sites, the search was made more specific, with the aim of identifying only those studies reporting HRQoL data measured using the EQ-5D. This more sensitive search retrieved 132 references and was sifted for relevant papers.
Studies reporting HRQoL values measured during RCTs were excluded because of the possibility that study interventions may affect HRQoL independently of their impact on fracture. In addition, studies that examined the HRQoL impact of surgical interventions to treat fracture were excluded as these were focused on comparing the impact of different surgical techniques on quality of life rather than comparing pre- and post-fracture HRQoL under usual management. Studies reporting the quality-of-life impact of prevalent fractures were excluded on the basis that there is no way of knowing how long ago the prevalent fracture was sustained and the model requires information on the quality-of-life impact in the year following fracture and in subsequent years.
Sixteen studies remained207,216–230 (summarised in Table 25), of which eight provided HRQoL for hip/fractures,216,219,220,224–227,230 eight for wrist fractures,216,217,220,221,226–228,230 10 for vertebral fractures207,216,217,220,222,226,227,229,230 and two for shoulder fractures. 217,220 Of these, two studies used non-UK utility values216,217 and two were of very specific patient cohorts,217,218 making the results of these studies less relevant to the general population at risk of fragility fracture. Cooper et al. 218 focused on women with inadequate response to therapy and Ekström et al. 219 focused on patients with subtrochanteric hip fractures only. Therefore, HRQoL values from these studies were not considered further.
| Author and year of study publication | Country | Study design | Cohort description | Sample size at baseline and % of missing data | Valuation set used for EQ-5D | Reasons for not considering some studies further |
|---|---|---|---|---|---|---|
| Hagino et al., 2009216 | Japan | Prospective cohort | Patients aged ≥ 45 years with osteoporotic hip, wrist or spine fracture | Recruited: 122 13% dropped out, excluded because of additional fractures or death |
Japanese health utility rating | Not used because not UK TTO |
| Cooper et al., 2008218 | Europe | Prospective cohort (OSSO) | PM women with osteoporosis and inadequate response to therapy | Recruited: 166 with incident fracture | UK scoring algorithm | Not used, study is a specific cohort of women with inadequate response to therapy |
| Ekström et al., 2009219 | Sweden | Prospective cohort | Patients with subtrochanteric hip fracture treated with a cephalomedullary nail | Recruited: 87 Missing: 4 months: 11% 12 months: 21% 24 months: 38% |
UK TTO | Not used, study is with patients with subtrochanteric hip fracture, which makes up a small percentage of all hip fractures |
| Calvo et al., 2011217 | Spain | Prospective cohort | PM women aged > 50 years (acute, outpatient, non-operative osteoporotic fractures only) | Recruited with HRQoL: 301 Overall: 5506 (6.5% dropped out, 6.7% excluded); HRQoL, n = 301 |
Spanish EQ-5D | Not used because not UK TTO |
| Zethraeus, 2002220 | Sweden | Prospective cohort, pilot | Patients aged ≥ 50 years with hip, spine, wrist or shoulder fractures recruited at the orthopaedic department | Recruited (response rate at 2 weeks): Hip: 533 (18%) Shoulder: 210 (25%) Wrist: 334 (42%) Spine: 172 (25%) |
UK tariff | No pre-fracture or control value reported. Used only where no other data available |
| Suzuki et al., 2008222 | Sweden | Prospective cohort | Patients aged > 40 years with acute osteoporotic spine fracture | Recruited: 147 27% lost to follow-up, died or excluded |
UK TTO | Not used because no pre-fracture or control value reported |
| Suzuki et al., 2010223 | Sweden | Prospective cohort | Patients aged > 40 years with acute osteoporotic spine fracture with or without prevalent fracture | Recruited 56 with no prevalent fracture | UK TTO | Not used because no pre-fracture or control value reported |
| Dolan et al., 1999221 | UK | Prospective cohort | Women with wrist fracture | Recruited: 50 | UK TTO | Not used because no pre-fracture or control value reported |
| Tidermark et al., 2002224 | Sweden | Prospective cohort | Patients aged ≥ 65 years with acute hip fracture and internal fixation | Recruited 90 33% died, excluded or lost to follow-up by 24 months |
UK TTO | Considered relevant |
| Tidermark et al., 2002225 | Sweden | Prospective cohort | Patients aged ≥ 65 years with acute hip fracture and internal fixation | Recruited 90 28% excluded, lost to follow-up or underwent different surgery |
UK TTO | Considered relevant |
| Ström et al., 2008226 | Sweden | Prospective cohort (KOFOR) | Patients aged ≥ 50 years with a single osteoporotic fracture of hip, spine or wrist | 684 patients survived to the 18-month follow-up | UK TTO | Considered relevant and applied in model |
| Borgström et al., 2006207 | Sweden | Prospective cohort (KOFOR) | Patients aged ≥ 50 years with a single osteoporotic fracture of hip, spine or wrist | Recruited 635 1% excluded |
UK TTO | Considered relevant |
| Borgström et al., 2013227 | International (11 countries including UK) | Prospective cohort (ICUROS) | As in KOFOR, patients within 2 (6 in USA) weeks of fracture | 2808 analysed using combined data set with KOFOR study. Results presented by country, UK not reported | UK TTO | Considered relevant |
| Lips et al., 2010228 | Europe (five centres including UK) | Prospective cohort | Ambulant patients aged 45–80 years within 14 days of wrist fracture and age-/sex-matched controls | Recruited: 105 + 74 controls 13% dropped out |
Unclear | Considered relevant |
| Roux et al., 2012230 | International (10 countries including UK) | Large prospective cohort (GLOW) | PM women with osteoporosis followed up for spine, hip and other fractures | Recruited: 1822 fractures from 51,491 women | Country-specific utilities | Considered relevant |
| Cockerill et al., 2004229 | Europe (seven countries including UK) | Population-based screening survey case–control follow-up (EVOS) | Men and women aged 50–79 years screened for spine fracture | Recruited: 121 fractures with HRQoL from 15,570 people screened | UK TTO | Considered relevant |
Four studies did not provide a pre-fracture or control utility value220–223 and these were excluded except where no other values were available.
Five of the included papers contained duplicate results,207,224–227 as both papers by Tidermark et al. 224,225 referred to the same study and the papers by Ström et al. 226 and Borgström et al. 227 referred to a single study [known as KOFOR (the costs and effects of osteoporosis-related fractures study)]. The later paper by Borgström et al. 227 was an international extension to the KOFOR study [known as ICUROS (the International Costs and Utilities Related to Osteoporotic fractures Study)] which gave HRQoL values by country but not pooled values. The Swedish cohort within ICUROS appeared to have been based on a slightly expanded version of the KOFOR sample. Of the ICUROS results, the Swedish values were thought to be the most appropriate because they were based on the largest sample of the various country-specific cohorts and they were expected to provide a good estimator of UK HRQoL values, as northern European countries have been shown to have similar values. 231
Values from eight papers207,224–230 reporting outcomes from five distinct studies were therefore compared. All studies appeared to observe similar patterns in HRQoL, with an immediate, severe drop in HRQoL associated with the acute fracture incident (where recorded), followed by a recovery to a higher HRQoL within the first 4 months, and stabilisation or slow improvement over the course of the year to 12 months. The exception to this was the Roux et al. 230 study, which was a prospective study in which utility was measured at enrolment (pre fracture) and then after 12 months, with the post-fracture values being 12-month values for patients who experienced a fracture at any time during the previous 12 months. As a result, values from the Roux et al. 230 study showed a gradual decline over a 12-month period. The advantage of this approach is that pre-fracture utilities were as measured and, therefore, not subject to recall bias. Values at 12 months should also theoretically represent an average of utility loss associated with fracture over a year, assuming all patients were surveyed at exactly 12 months. However, as a significant amount of utility loss is experienced in the first days and weeks after fracture, the results could easily be biased if patients who had recently experienced a fracture delayed completing the survey. As the study was based on self-completion postal questionnaires, it was considered possible that there may be some reporting bias in this study and, therefore, values from other studies were considered more appropriate. One of the papers by Tidermark et al. 225 did not report a HRQoL value between baseline and 4 months and, therefore, this study did not observe the severe drop in HRQoL associated with the acute fracture incident. A summary of the values reported by individual studies for utility after hip fracture, wrist fracture, vertebral fracture and shoulder fracture are presented in Tables 26–29, respectively.
| Author and year of study publication | Description of non-fracture state | Valuation of non-fracture state, mean (SD, n) | Description of fracture states valued | Value of fracture states, mean (SD, n) |
|---|---|---|---|---|
| Roux et al., 2012230 | Baseline pre fracture | 0.64 (0.34, 126) | 0–12 months post fracture (12-months post recruitment) | 0.60 (0.34, 126) |
| Ström et al., 2008226 | Pre fracture (recalled) | 0.81 (0.21, 282) | Post fracture, immediate | 0.19 (0.21, 282) |
| 4 months | 0.64 (0.26, 282) | |||
| 12 months | 0.69 (0.26, 282) | |||
| 8 months | 0.72 (0.26, 282) | |||
| Borgström et al., 2013227 | Pre fracture (recalled) | 0.80 (0.24, 355) | Post fracture, immediate | 0.18 (0.19, 355) |
| 4 months | 0.62 (0.24, 355) | |||
| Tidermark et al., 2002224 | Pre fracture (recalled) | 0.77 (NR, 90) | Post fracture at 4 months | 0.66 (NR, 42) |
| 12 months | 0.62 (NR, 42) | |||
| 24 months | 0.59 (NR, 42) | |||
| Tidermark et al., 2002225 | Pre fracture (recalled): and age-matched general population | 0.78 (0.21, 89) | Post fracture at 1 week | 0.44 (0.33, 71) |
| 4 months | 0.55 (0.37, 79) | |||
| 17 months | 0.51 (0.36, 69) | |||
| Borgström et al., 2006207 | Pre fracture (recalled) | 0.80 (0.21, 277) | Post fracture at 0–4 weeks | 0.18 (0.21, 277) |
| 4 months | 0.62 (0.30 277) | |||
| 12 months | 0.67 (0.25, 277) |
| Author and year of study publication | Description of non-fracture state | Valuation of non-fracture state, mean (SD, n) | Description of fracture states valued | Value of fracture states, mean (SD, n) |
|---|---|---|---|---|
| Lips et al., 2010228 | Age-/sex-matched controls | 0.85 (median) (NR, 73) | Post fracture, 0–14 days (baseline) | Median 0.59 |
| 6 weeks | Median 0.66 | |||
| 3 months | Median 0.76 | |||
| 6 months | Median 0.78 | |||
| 12 months | Median 0.80 | |||
| Ström et al., 2008226 | Pre fracture | 0.90 (0.18, 325) | Post fracture, immediate | 0.56 (0.28, 325) |
| 4 months | 0.83 (0.18, 325) | |||
| 12 months | 0.88 (0.23, 325) | |||
| 18 months | 0.90 (0.18, 325) | |||
| Borgström et al., 2013227 | Pre fracture (recalled) | 0.90 (0.20, 390) | Post fracture, immediate | 0.56 (0.25, 390) |
| 4 months | 0.83 (0.20, 390) | |||
| Borgström et al., 2006207 | Pre fracture (recalled) | 0.89 (0.17, 276) | Post fracture at 0–4 weeks | 0.56 (0.17, 276) |
| 4 months | 0.82 (0.17, 276) | |||
| 12 months | 0.86 (0.17, 276) |
| Author and year of study publication | Description of non-fracture state | Valuation of non-fracture state, mean (SD, n) | Description of fracture states valued | Value of fracture states, mean (SD, n) |
|---|---|---|---|---|
| Roux et al., 2012230 | Baseline pre fracture | 0.65 (0.02, 178) | 0–12 months post fracture (12 months post recruitment) | 0.58 (0.02, 178) |
| Ström et al., 2008226 | Pre fracture | 0.74 (0.24, 76) | Post fracture, immediate | 0.18 (0.27, 76) |
| 4 months | 0.49 (0.31, 76) | |||
| 12 months | 0.49 (0.31, 76) | |||
| 18 months | 0.54 (0.31, 76) | |||
| Borgström et al., 2013227 | Pre fracture (recalled) | 0.74 (0.25, 120) | Post fracture, immediate | 0.20 (0.28, 120) |
| 4 months | 0.50 (0.34, 120) | |||
| Borgström et al., 2006207 | Pre fracture (recalled) | 0.73 (0.25, 81) | Post fracture at 0–4 weeks | 0.18 (0.25, 81) |
| 4 months | 0.47 (0.34, 81) | |||
| 12 months | 0.49 (0.25, 81) | |||
| Cockerill et al., 2004229 | Age-/sex-matched controls | Incident fracture cases | 0.77 (0.19, 73) | |
| Prevalent fracture found | 0.81 (0.19, 60) | |||
| No prevalent fracture | 0.83 (0.17, 136) |
| Author and year of study publication | Description of non-fracture state | Valuation of non-fracture state | Description of fracture states valued | Value of fracture states, mean (SD, n) |
|---|---|---|---|---|
| Zethraeus et al., 2002220 | None | NR | Post fracture at 2 weeks | 0.36 (0.30, 46) |
| 6 months | 0.69 (0.25, 40) | |||
| 9 months | 0.66 (0.26, 37) | |||
| 12 months | 0.65 (0.29, 30) |
Values were plotted and a weighted average score was calculated for each fracture type. An example is shown in Figure 87 for hip fracture, for which five appropriate papers were sourced,207,224–227 relating to two studies. The weighted average score closely followed the result of the largest study (KOFOR/ICUROS) reported in the papers by Ström et al. 226 and Borgström et al. 207 Similar patterns were observed for all fracture types. The KOFOR/ICUROS study was the only study to provide pre- and post-fracture values for hip, wrist and spine fractures. It also had the largest sample size and reported similar results to other studies. Therefore, the decision was made to use values from the KOFOR/ICUROS study as the basis of the utility multipliers applied in the model. However, no study provided complete HRQoL data for shoulder fracture, so in this case values from Zethraeus et al. 220 were used, with an assumption that post-fracture HRQoL measured at 12 months represented a return to pre-fracture HRQoL levels. No studies reported pre-fracture (or control) and post-fracture values for fractures at sites other than the hip, wrist, spine or shoulder.
FIGURE 87.
Illustration of post-fracture trends in HRQoL following hip fracture taken from five papers reporting on two different studies plus a weighted average.

The average utility value in the first year following fracture has been calculated by assuming an immediate drop in HRQoL at fracture maintained for 1 month, followed by a linear improvement to 4 months and then a further linear improvement to 12 months. The utility multiplier applied in the first year post fracture was then calculated as the ratio of the average utility in the year post fracture to the baseline utility prior to fracture. The utility value observed at 12 months is assumed to persist in the long term, so the multiplier for the second and subsequent years was set to the ratio of the 12-month and pre-fracture utility value.
The data applied in the model are summarised in Table 30. The post-fracture utility values have been varied in the PSA by sampling values from a beta distribution (see Table 51, Appendix 8, for details on the distributions).
| Sample size and utility estimates | Site of subsequent fracture | |||
|---|---|---|---|---|
| Hipa | Spinea | Shoulderb | Wrista | |
| Number of patients | 282 | 76 | 38 | 325 |
| Utility index | ||||
| Pre-fracture | 0.81 | 0.74 | 0.65c | 0.90 |
| 2 weeks post | 0.19 | 0.18 | 0.36 | 0.56 |
| 4 months post | 0.64 | 0.49 | 0.58 | 0.83 |
| 12 months post | 0.69 | 0.49 | 0.65 | 0.88 |
| Annual average | 0.56 | 0.43 | 0.56 | 0.79 |
| Utility multiplier (year 1) | 0.69 | 0.57 | 0.86 | 0.88 |
| Utility multiplier (year 2 and subsequent) | 0.85 | 0.66 | 1.00 | 0.98 |
Health-related quality-of-life values for institutionalisation
The published economic analyses described in Systematic review of existing cost-effectiveness evidence were examined to identify sources of data on HRQoL for patients living in nursing home or residential care compared with those dwelling in the community. Additionally, a paper by Tidermark et al. 225 containing relevant data was opportunistically identified from the systematic review of papers reporting HRQoL following fracture.
Tidermark et al. 225 found that, in a prospective cohort study of 90 patients with hip fracture who were living independently prior to their fracture, patients with an independent living status at 4 months after fracture had significantly higher EQ-5D scores than those living in institutions (0.64 and 0.35, respectively; p < 0.05). A similar difference in mean scores (0.56 vs. 0.35) was seen at final follow-up (> 12 months after fracture with mean follow-up of 17 months), but this was no longer statistically significant. The lack of statistical significance at final follow-up may be because of the small number of patients institutionalised (seven at 4 months and eight at 17 months). We used the data from the final follow-up within our analysis to calculate a utility multiplier for nursing home admissions following fracture of 0.625. This is higher than the value of 0.4 used in four of the published analyses;140–143 however, this earlier value was based on judgement by an expert panel. 153 The remaining three137–139 published analyses did not describe the utility multiplier applied for nursing home admission. The multiplier calculated from Tidermark et al. 225 was used in our model, as this was based on EQ-5D scores valued using the UK tariff, which is consistent with the NICE reference case. 144 Tidermark et al. 225 did not report SDs for the mean EQ-5D values for institutionalised patients and patients living independently. To provide an estimate of uncertainty in the utility multiplier within the PSA, the standard error around the utility multiplier was set to give a 95% CI that coincided with no difference between these two health states, to reflect the lack of a statistically significant difference in the mean values at 17 months.
Age- and sex-specific utility values in the absence of clinical events
Utility in patients without fracture is dependent on age and sex and is based on EQ-5D data for the UK general population. 232 The age- and sex-dependent utility value applied to the period between two events is taken to be the average of the utility at the start and end of that period. This ensures that patients who do not experience any events do not stay at an artificially high level of utility, equivalent to the utility value for their age at the start of the model. The regression used to calculate utility from age and sex is as follows:
where sex is 1 for males and 0 for females, and age is in years.
A multivariate normal distribution, which takes into account the correlation between the regression coefficients, was used to sample the regression coefficients in the PSA.
Resource use and costs for bisphosphonates treatment
Drug costs for oral bisphosphonates have been taken from the The Electronic Drug Tariff,151 as it is assumed that these are prescribed in primary care. 144 zoledronic acid and i.v. ibandronic acid are assumed to be prescribed in secondary care and costs for these have therefore been taken from the electronic market information tool (eMIT) database, which reports the average cost paid by secondary care trusts for generic medicines. 144,152 It was noted by our clinical advisors that generic zoledronic acid has only recently become available and, therefore, the prices reported by the eMIT database may be higher than those currently being paid in the NHS, as the price is likely to fall after a generic preparation becomes available and the current eMIT database uses data from the 12 months prior to June 2014. Therefore, a sensitivity analysis was conducted using the price for the 4-mg preparation of zoledronic acid, which is for a different indication but has been available in generic form for a longer time. This was felt to represent a realistic lower limit for the price of the 5-mg zoledronic acid preparation.
Where there was more than one preparation available we have assumed that the lowest-cost preparation is prescribed based on the average cost for 1 year of treatment. Therefore, for alendronic acid and risedronic acid we assumed that weekly preparations are prescribed, as these had the lowest costs based on the The Electronic Drug Tariff151 costs applied in the model; these are summarised in Table 31. Drug prices are assumed to be known precisely and, therefore, have been assumed to be fixed within the PSA.
| Bisphosphonate | Items per pack and dose per item | Price per pack | Cost per annum |
|---|---|---|---|
| Alendronic acid (oral) | 4 x 70 mg | £1.13a | £14.73 |
| Risedronic acid (oral) | 4 x 35 mg | £1.26a | £16.43 |
| Ibandronic acid (oral) | 28 x 50 mg | £10.56a | £13.58 |
| Ibandronic acid (i.v.) | 1 x 3 mg/3 ml | £19.38b | £77.52 |
| Zoledronic acid (i.v.) | 1 x 5 mg/100 ml | £94.67b | £94.67 |
| Zoledronic acid (i.v.) (price used in sensitivity analysis) | 1 x 4 mg/5 ml | £5.76b | £5.76 |
Oral therapies were assumed to incur no additional costs for administration. The costs of i.v. administration of zoledronic acid and ibandronic acid have been based on NHS reference costs. 150 ibandronic acid is given by i.v. injection over 15–30 seconds. It is assumed that this is done during an outpatient endocrinology consultation at a cost of £133 (NHS reference cost £302). 150 zoledronic acid is given by i.v. infusion over a longer duration and this is assumed to be done as a day case. The reference cost for a day case delivery of a simple parenteral chemotherapy (SB12Z at £245)150 has been applied, as no alternative reference costs were identified which would cover day case admissions for the administration of a drug by infusion. The outpatient cost for the same HRG code (SB12Z) is £165, suggesting that it is classification of this activity as a day case rather than the specific nature of chemotherapy that makes this more expensive than an outpatient endocrinology appointment. It was therefore considered reasonable to apply the day case reference cost for parenteral chemotherapy as a proxy for the cost of delivering zoledronic acid owing to the longer duration of administration compared with i.v. ibandronic acid. Our clinical advisors noted that in some cases zoledronic acid is administered as an outpatient procedure and, therefore, a sensitivity analysis was conducted using the outpatient cost for both i.v. bisphosphonates. Reference costs for the administration of i.v. bisphosphonates were varied in the PSA (see Table 63, Appendix 8 for details).
Resource use and costs of fracture
The published economic analyses were searched to identify published evidence on resource use attributable to fracture. This was supplemented by ad hoc searches for UK studies published since 2006, as this was when a DSU report detailing several different methods to estimate fracture costs was published. 174
Resource use attributable to fracture was based on a UK study by Gutierrez et al. ,233,234 which used a GP database (The Health Improvement Network) to estimate resource use for those who fractured compared with matched controls. Patients were matched on age, GP practice and comorbidity score. The study was reported in two separate papers, with the first reporting the costs attributable to hip fracture233 and the second reporting the costs attributable to vertebral fracture, non-hip non-vertebral fracture and also some less detailed results for wrist and proximal humerus fracture. 234 The study examined hospitalisations, accident and emergency (A&E) visits, referrals, prescriptions and GP contacts in the year following fracture. It did not examine any costs falling within PSS, such as nursing home admission or home help. The authors also noted that they did not include rehabilitation costs, but they did estimate the total cost including rehabilitation by using estimates of rehabilitation costs from other published studies.
The difference in the percentage of patients using each type of resource between those who had fractured and matched controls was multiplied by the unit cost to get the average cost per fracture in the year following fracture. Unit costs for hospitalisations, A&E appointments and specialist referrals were based on NHS reference costs, while unit costs for social care and GP appointments were based on estimates from the PSSRU. Tables 32 and 33 show the difference in resource use between patients who fractured and their matched controls and the unit costs applied. The total first year and subsequent year costs are summarised in Table 34. Unit costs for A&E vary by fracture type as different costs were applied for admitted and non-admitted patients and these proportions vary by fracture type. Unit costs for prescriptions were calculated by dividing the difference in total prescription cost by the difference in the mean number of prescriptions using data from Gutierrez et al. 234 However, this detailed information was not available for wrist and proximal humerus fractures, so data from the broader category of non-hip non-vertebral fractures were used for wrist and proximal humerus.
| Resource use | Difference in proportion between patients with fractures and controls | |||
|---|---|---|---|---|
| Hip | Vertebrae | Proximal humerus | Wrist | |
| Hospitalisation | 0.82 | 0.23 | 0.20 | 0.17 |
| A&E | 0.14 | 0.07 | 0.15 | 0.18 |
| GP | –0.02 | 0.07 | 0.03 | 0.06 |
| Referral | 0.01 | 0.17 | 0.05 | 0.09 |
| Mean difference in number | ||||
| Prescriptions per annum | 12.34 | 22.35 | 4.61 | 4.61 |
| Home help hours per weeka | 1.57 | 2.33 | 0.12b | 0.12 |
| Unit costs for resource use | Fracture | |||
|---|---|---|---|---|
| Hip | Vertebrae | Proximal humerus | Wrist | |
| Hospitalisation | £7487 | £3846 | £5320 | £3662 |
| A&E | £92 | £85 | £85 | £84 |
| GP | £45 | £45 | £45 | £45 |
| Referral | £146 | £146 | £146 | £146 |
| Prescriptions | £9 | £15 | £15 | £15 |
| Home help per hour | £24 | £24 | £24 | £24 |
| Resource use | Fracture | |||
|---|---|---|---|---|
| Hip | Vertebrae | Proximal humerus | Wrist | |
| Costs in year of fracture | £8235 | £4173 | £1305 | £861 |
| Costs in subsequent years | £106 | £332 | £70 | £70 |
In the cost-effectiveness analysis that informed TA16021 and TA161,24 it was assumed that patients who experienced a vertebral fracture had ongoing costs in the second and subsequent years associated with the long-term prescribing of treatments to manage the chronic symptoms associated with vertebral fractures. The analysis by Gutierrez et al. 234 does not examine costs beyond the first year; however, it can be seen that for vertebral fracture, non-hip non-vertebral fractures and hip fractures, the costs of medications are fairly stable in the first and second 6 months following fracture, whereas the costs for health-care contacts such as GP appointments, referrals and A&E visits fall sharply in the second 6 months. Therefore, we decided to apply prescription costs as an ongoing cost from the time of fracture. All other costs estimated by Gutierrez et al. 234 were applied in the first year only.
In the analysis by Stevenson et al. ,140 Swedish data presented by Borgström et al. 207 were used to estimate the costs of home help. We used the same data on the average number of hours of home help following fracture, as used by Stevenson et al. ,140 but applied present-day unit costs. Home help costs are assumed to occur in only the first year after fracture and apply only to those residing in the community and not to institutionalised patients.
For patients living in an institutional residential setting we applied the cost of local authority-provided residential care for older people with the unit cost (£1100 per week) taken from the PSSRU. 26 The costs for local authority-provided care were used instead of private sector or NHS residential care, as a recent report by The King’s Fund235 states that the vast majority (78%) of residential care places are provided by local authorities. We assumed that 36% of patients self-fund their residential care based on data presented by the Care Quality Commission. 236 The annual cost falling within the NHS and PSS budget was therefore estimated at £36,608 per person in residential care per annum. In the PSA, both the resource-use estimates in Table 32 and the unit costs taken from NHS reference costs were sampled from probabilistic distributions. Those taken from PSSRU were not varied in the PSA as the PSSRU does not report a measure of variance. Further details on the distributions used in the PSA are provided in Appendix 8, Table 51.
The costs for each of the four main osteoporotic fracture sites have been applied to other sites in the same grouping (e.g. other femoral has the same cost as hip).
Approach to sensitivity analysis
A PSA has been conducted to estimate the mean cost and QALYs gained when taking into account the uncertainty in the parameter values used within the model. In general, parameters were estimated using the following distributions: gamma distributions for costs; log-normal distributions for HRs; and beta distributions for utility values and probabilities. None of the parameters used to estimate fracture risk, in the absence of treatment, was varied in the PSA. This was to ensure that a specific set of patient characteristics was consistently mapped to the same survival curve for fracture-free survival without any parameter uncertainty. The following additional parameters were not varied in the PSA: drug prices; discount rates; unit costs sourced from PSSRU; utility in the second year after proximal humerus fracture; life expectancy after fracture associated with excess mortality; unit costs for prescriptions after fracture; and proportion of self-funders for residential care. Full details on the distributions applied within the model can be found in Appendix 8, Tables 51–62.
Structural sensitivity analyses were conducted to explore whether or not the results were sensitive to different model assumptions. In the majority of cases these were conducted using the deterministic model, which does not incorporate any parameter uncertainty, owing to the significant computational time required to run the PSA. A summary of the structural sensitivity analyses conducted is provided in Table 35 alongside an indication of the model used.
| Model aspect | Assumption | Model used | |
|---|---|---|---|
| In base case | In sensitivity analysis | ||
| Interventions | Duration of treatment based on observational data on treatment persistence | Full persistence for 5 years for oral bisphosphonates and i.v. ibandronic acid, and 3 years for i.v. zoledronic acid | Model using mid-point parameters |
| Day case costs applied for zoledronic acid administration and costs generic drugs costs based on eMIT database152 for 5-mg/100-ml dose | Outpatient costs applied for zoledronic acid administration and costs generic drugs costs based on eMIT database152 for 4-mg/100-ml dose (different indication) which has been available in a generic form for longer | Model using PSA to incorporate parameter uncertainty | |
| Baseline characteristics | Prevalence of prior fracture estimated from meta-analysis by Kanis et al.164 | Prevalence of prior fracture estimated from UK incidence data166,167 | Model using mid-point parameters |
| Consequences of fracture | No admission to nursing/residential care following vertebral fracture | Rate of admission to nursing/residential care for vertebral fracture equivalent to rate for hip fracture | Model using mid-point parameters |
| Average post-fracture survival for patients with fracture-related death is 3 months | Average post-fracture survival for patients with fracture-related death is 1 month | Model using mid-point parameters | |
| Systematic review by Klotzbuecher et al.149 is used as the preferred source for estimates of the increased fracture risk following an incident fracture | Systematic review by Warriner et al.213 is used as the preferred source for estimates of the increased fracture risk following an incident fracture | Model using mid-point parameters | |
| Estimating fracture risk | Fractures occurring at additional sites (femoral shaft, clavicle, scapula, rib, sternum, tibia, fibula, pelvis and humeral shaft) are included | Only fractures at the main fracture sites (hip, wrist, proximal humerus and vertebrae) are included | Model using mid-point parameters |
| Hip fracture risks estimated as a proportion of major osteoporotic fracture (which combines hip, wrist, proximal humerus and vertebrae) | Hip fracture risks estimated using hip specific estimates from the QFracture and FRAX risk scoring tools | Model using mid-point parameters | |
| Efficacy assumptions | Fall-off time for zoledronic acid is 2.3 times treatment duration but fall-off time is equal to treatment duration for all other treatments | Fall-off time is equal to treatment duration for all treatments including zoledronic acid | Model using mid-point parameters |
| Different HRs used for oral and i.v. ibandronic acid for some fracture sites. Data from monthly oral doses used for monthly oral ibandronic acid and data from daily oral doses used for i.v. ibandronic acid where both exist | Same HRs used for oral and i.v. ibandronic acid. This is achieved by using the data from daily oral doses for both monthly oral and i.v. doses where it exists | Model using PSA to incorporate parameter uncertainty | |
| AEs | AEs included by applying one-off costs and QALY decrements in the first month of treatment in 3% of patients having oral treatment and 14% of patients having i.v. treatment | No costs or QALY decrements applied for AEs in either oral or i.v. treatment | Model using PSA to incorporate parameter uncertainty |
| AE rate for oral treatment increased to 30% | Model using PSA to incorporate parameter uncertainty | ||
Results
Patient characteristics of the simulated cohort
Summary patient characteristics are provided in Table 36 for each risk category when using both FRAX and QFracture to calculate the absolute fracture risk. It can be seen that the average age is higher in the higher-risk categories and the proportion of patients with the risk factors of prior fracture, steroid use or nursing home residency increases in the higher-risk categories. The proportion of women also appears to increase in the higher-risk categories as would be expected given that women in general have a higher risk of osteoporotic fracture than men.
| Deciles | Risk category | ||||||
|---|---|---|---|---|---|---|---|
| Mean 10-year risk (%) | Sex, % female | Age (years), mean (SD) | BMI, mean (SD) | Prior fracture (%) | Steroid use (%) | Nursing home resident (%) | |
| FRAX | |||||||
| First | 3.1 | 28 | 53 (5) | 31 (6) | 6.4 | 0.6 | 0.5 |
| Second | 4.3 | 34 | 52 (11) | 31 (5) | 39.4 | 1.3 | 0.4 |
| Third | 5.0 | 25 | 50 (13) | 29 (4) | 62.3 | 0.5 | 0.4 |
| Fourth | 5.6 | 23 | 49 (14) | 26 (4) | 73.3 | 0.5 | 0.5 |
| Fifth | 6.2 | 38 | 54 (15) | 26 (5) | 66.2 | 0.9 | 0.8 |
| Sixth | 7.3 | 43 | 61 (13) | 27 (5) | 59.5 | 1.5 | 0.9 |
| Seventh | 8.8 | 48 | 66 (10) | 28 (4) | 57.6 | 1.6 | 1.0 |
| Eighth | 10.7 | 56 | 70 (8) | 27 (4) | 57.8 | 1.8 | 1.3 |
| Ninth | 14.9 | 87 | 73 (8) | 27 (4) | 48.6 | 3.3 | 2.6 |
| Tenth | 25.1 | 99 | 81 (7) | 26 (4) | 68.9 | 4.0 | 7.6 |
| QFracture | |||||||
| First | 0.5 | 17 | 41 (8) | 30 (5) | 86.5 | 0.6 | 0.0 |
| Second | 0.7 | 13 | 46 (9) | 28 (5) | 76.8 | 0.7 | 0.1 |
| Third | 1.0 | 17 | 50 (9) | 28 (5) | 70.2 | 1.0 | 0.3 |
| Fourth | 1.4 | 27 | 55 (9) | 28 (5) | 60.7 | 1.3 | 0.4 |
| Fifth | 2.0 | 42 | 59 (9) | 28 (5) | 50.3 | 1.6 | 0.5 |
| Sixth | 2.7 | 53 | 63 (9) | 28 (5) | 41.6 | 1.7 | 0.7 |
| Seventh | 3.9 | 65 | 66 (9) | 28 (5) | 37.4 | 1.8 | 0.7 |
| Eighth | 5.5 | 75 | 70 (8) | 28 (5) | 35.1 | 2.1 | 1.1 |
| Ninth | 8.4 | 82 | 75 (7) | 27 (4) | 37.4 | 2.3 | 2.6 |
| Tenth | 16.0 | 90 | 83 (6) | 26 (4) | 45.7 | 2.8 | 9.6 |
| All | NA | 48 | 61 (15) | 28 (5) | 54.2 | 1.6 | 1.6 |
It should be noted that, in addition to there being different risk cut-off points for the risk categories when using either QFracture or FRAX scores to define absolute risk, the ranking of patients by risk within the cohort will differ between the two algorithms. It is therefore possible that patients falling into a particular risk category when using the QFracture algorithm may fall into a different risk category when using the FRAX algorithm. Figure 88 shows the distribution of 200,000 patients eligible for risk assessment under CG14616 across the QFracture and FRAX risk categories. It can be seen from Figure 88 that, although there is some agreement over the categorisation of patients across the two risk scoring algorithms, there is not perfect agreement. The correlation between the absolute risk scores was found to be 0.83 and the correlation between the risk categories based on deciles of risk score was found to be 0.76.
FIGURE 88.
Distribution of patients across FRAX and QFracture risk categories. QFracture risk categories are indexed Q1–10 and FRAX risk categories are indexed F1–10, with 1 being the lowest risk category in each case.
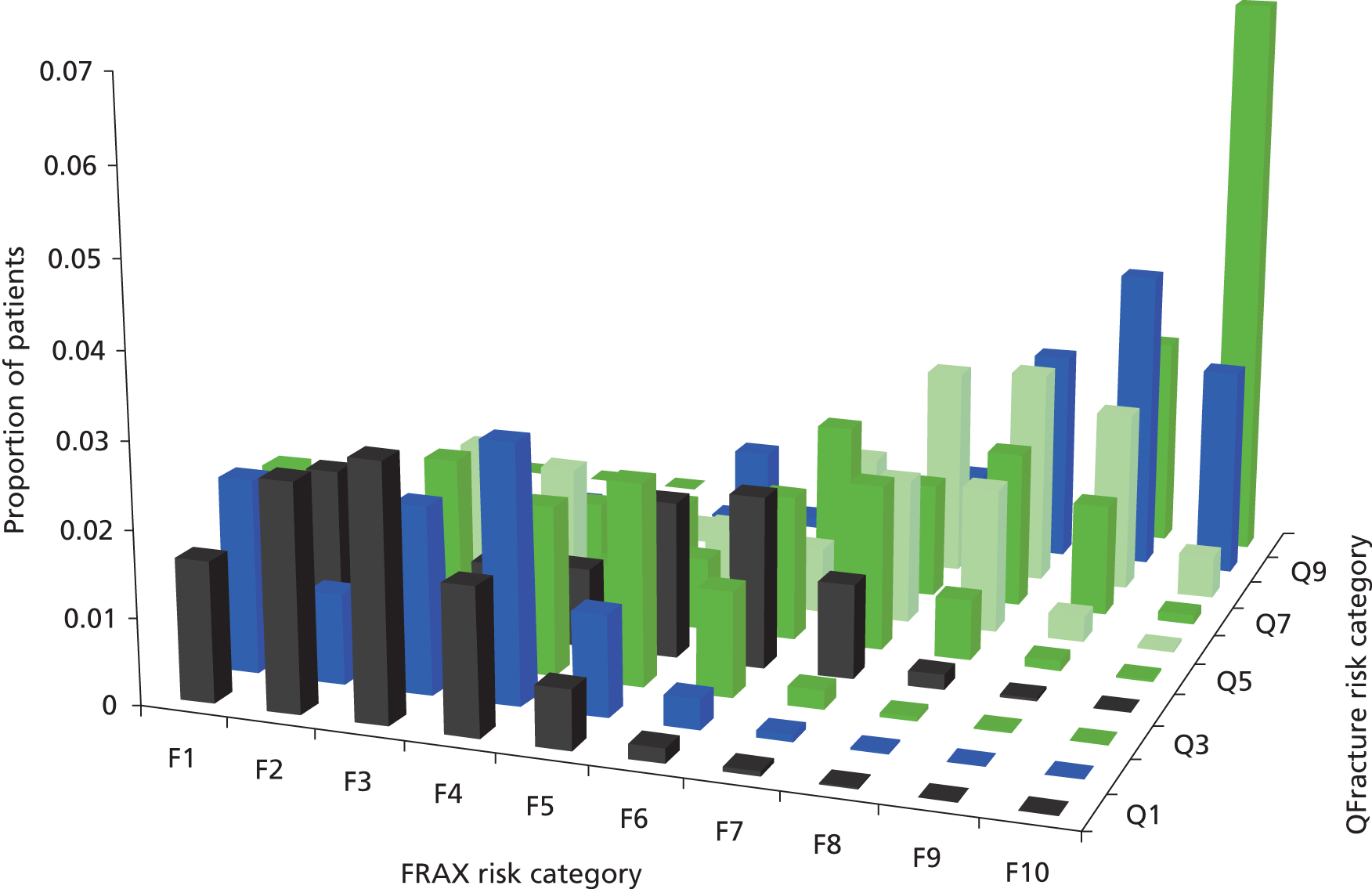
Clinical outcomes predicted by the model
Clinical outcomes for 200,000 patients are presented in Table 37 for the base-case scenario in which we have applied the mean persistence with treatment from observational data. Under these assumptions the numbers needed to treat to prevent one fracture during the first 6 months (6 months being the duration of persistence with oral bisphosphonates) is lowest for risedronic acid and highest for oral ibandronic acid. Given that it is necessary to treat around 2000 patients to prevent one fracture during the period of persistence with oral bisphosphonates treatment when using the QFracture risk score, we estimated that we would need to simulate approximately 2 million patients to obtain stable estimates of the benefits of treatment in each risk category. This is because we would expect around 1000 fractures to be prevented across a cohort of 2 million patients, with around 1% falling within the lowest risk category of QFracture. Therefore, the costs and QALY implications of treatment would be based on around 10 fractures in the lowest risk category of QFracture when using a cohort of 2 million patients.
| Treatment strategy | Fractures occurring in the first 6 months after starting treatment (the mean duration of persistence with treatment for oral bisphosphonates) | NNT to prevent one fracture occurring in the first 6 months after starting treatment | ||||
|---|---|---|---|---|---|---|
| Hip fractures (including other femoral) | Vertebral fractures | Proximal humerus fractures (including tibia and fibula) | Wrist (including all other additional sites) | All fracture sites combined | ||
| FRAX | ||||||
| No treatment | 216 | 146 | 143 | 495 | 1000 | |
| Alendronic acid | 170 | 72 | 109 | 400 | 751 | 803 |
| Risedronic acid | 175 | 80 | 98 | 360 | 713 | 697 |
| Ibandronic acid (oral) | 182 | 72 | 109 | 400 | 763 | 844 |
| Ibandronic acid (i.v.) | 182 | 75 | 130 | 400 | 787 | 939 |
| Zoledronic acid | 202 | 66 | 99 | 389 | 756 | 820 |
| QFracture | ||||||
| No treatment | 121 | 63 | 67 | 177 | 428 | 1770 |
| Alendronic acid | 99 | 19 | 52 | 145 | 315 | 1550 |
| risedronic acid | 102 | 24 | 45 | 128 | 299 | 1942 |
| ibandronic acid (oral) | 109 | 19 | 52 | 145 | 325 | 2222 |
| ibandronic acid (i.v.) | 109 | 19 | 65 | 145 | 338 | 1835 |
| Zoledronic acid | 115 | 15 | 48 | 141 | 319 | 1770 |
It can be seen from Table 37 that the number of fractures occurring in the first 6 months is higher when using the FRAX algorithm than when using the QFracture algorithm. This is because the absolute risk predicted by FRAX is higher than the absolute risk predicted by QFracture in 98% of patients.
Presentation of cost-effectiveness results
The mean costs and QALYs from the PSA are presented as the base-case results. These were considered to be preferable to estimates obtained using mid-point (mean or median) parameter inputs because we believe that there may be a non-linear relationship between parameter values and model outcomes. The data presented were obtained from a total patient population of 2 million across all 10 risk categories, with one parameter sample per patient. Therefore, approximately 200,000 patients and 200,000 parameter samples informed the estimates for each risk category.
Full results tables for the base-case scenario including an incremental analysis for each risk category for QFracture and FRAX are presented in Tables 64–73 in Appendix 9 and Tables 74–83 in Appendix 10, respectively. Results have been summarised below by plotting the incremental net benefit (INB) compared with a strategy of no treatment when assuming that a QALY is valued at £20,000. INB has been plotted instead of ICERs, as these can be difficult to interpret when the QALY gain is negative, which was the case for some treatments in some risk categories. The cost-effectiveness plane has not been presented, as a minimum of 20 graphs would be needed to present results across all 10 risk categories for both QFracture and FRAX. We used non-parametric regression to estimate the cost-effectiveness acceptability curves (CEACs). This allows variation in the costs and QALYs as a result of parameter uncertainty to be separated from variation caused by patient-level stochastic variability.
Structural sensitivity analyses have been conducted by fixing parameter values at their mid-point value. Although it would have been preferable to rerun the PSA for each structural sensitivity analysis, this was not possible within the time constraints. The PSA was rerun for the sensitivity analysis, which involved changing the HRs for treatment as we considered it important in this case to capture the underlying joint distribution for the HRs. For the sensitivity analyses on AE rates and the sensitivity analysis examining alternative treatment costs for zoledronic acid, the outputs of the base-case PSA model were adjusted, as these adjustments could be made without rerunning the PSA. For all other sensitivity analyses, the model using mid-point parameter estimates was run for 2.2 million patients.
Summary of cost-effectiveness results for the base-case scenario when using QFracture
Figure 89 summarises the cost-effectiveness results across the 10 risk categories when using QFracture to estimate absolute risk. It shows the INB, in monetary terms, when valuing a QALY at £20,000, when compared with a strategy of no treatment. Each point shows the mean INB and the mean 10-year absolute risk of fracture for one risk category for a particular bisphosphonate treatment. It can be seen that the mean INB is close to zero for all three oral bisphosphonates across the first six risk categories, which have mean absolute risks ranging from 0.5% to 2.7%; the estimates are all very close together.
FIGURE 89.
Incremental net benefit (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from QFracture.
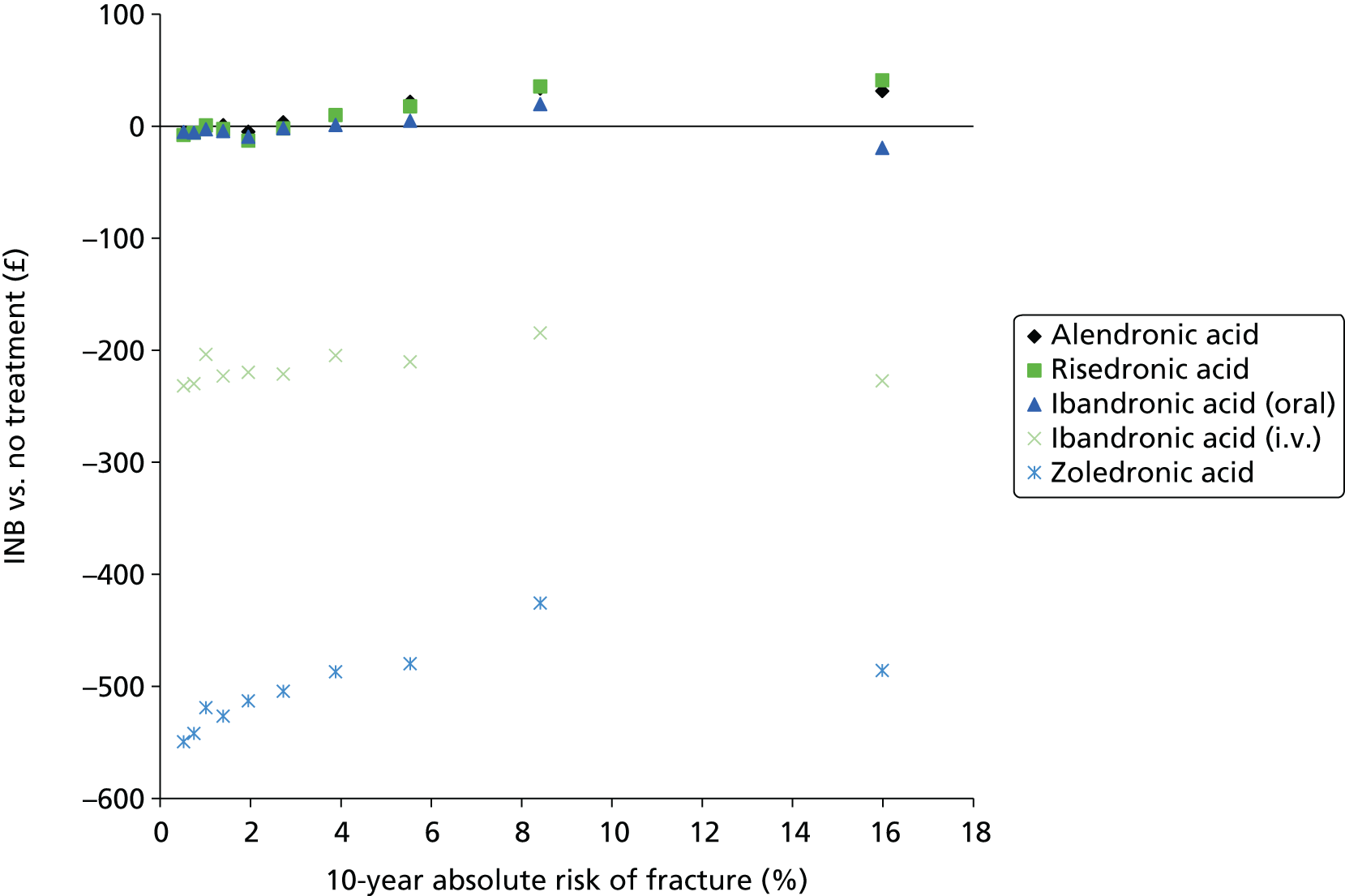
Detailed results tables providing a full incremental analysis are provided in Tables 64–73 in Appendix 9. It can be seen from these that in the third, fourth and sixth risk categories (mean absolute risks of 1.0%, 1.4% and 2.7%, respectively) at least one of the oral bisphosphonates has a positive INB, but the absolute INB is still small and close to zero. In the fifth risk category (mean absolute risk of 2%) it is below zero for all three oral bisphosphonates. The INB is positive for all three oral bisphosphonates from the seventh to the tenth risk categories (mean absolute risk of 3.9% and above). A strategy of no treatment has the maximum net benefit in the first, second and fifth risk categories (mean absolute risks of 0.5%, 0.7% and 2.0%, respectively) and when a QALY is valued at either £20,000 or £30,000 (see Tables 64–72 in Appendix 9 for INB at £30,000). In the other risk categories the treatment with maximum net benefit is always either alendronic acid or risedronic acid. Oral ibandronic acid does not fall on the cost-effectiveness frontier in any risk category when using QFracture to estimate absolute risk. The difference between oral ibandronic acid and the other two oral bisphosphonates becomes more apparent in the higher-risk categories. This is because of marginally less favourable efficacy data for oral ibandronic acid, which becomes more important as the risk increases. For the i.v. bisphosphonates the INB is negative across all 10 risk categories when valuing a QALY at either £20,000 or £30,000 (see Tables 64–73 in Appendix 9 for INB at £30,000).
The full data from the PSA for the whole population (2 million patients with one parameter sample per patient) were used in a non-parametric regression, which estimated the relationship between INB and absolute fracture risk estimated by QFracture. For each of the five treatment options, the relationship between the INB of treatment (vs. no treatment) and the absolute fracture risk was estimated using a generalised additive model. This flexible regression approach assumes that the underlying relationship between the dependent variable and the independent variable can be approximated by a cubic spline, a highly flexible function that can model any smooth relationship between two variables. The regression prediction is shown in Figure 90 with a close-up provided in Figure 91 of the lower-risk range. The results here differ from those presented in Figure 89 because the non-parametric regression method is able to average over the stochastic uncertainty associated with the individual-level patients while simultaneously estimating the relationship between INB and absolute risk. It can be seen that the INB of alendronic acid and risedronic acid increases with increasing risk. A strategy of no treatment is predicted to have the greatest net benefit for the lowest-risk patients. Table 38 summarises the thresholds over which each treatment has a positive INB compared with no treatment (when valuing a QALY at £20,000) and the range over which each treatment has the maximum INB based on the non-parametric regression. Alendronic acid is predicted to have the maximum net benefit from 1.5% and risedronic acid is predicted to have the maximum net benefit from 7.2% upwards. Oral and i.v. ibandronic acid have differing relationships with absolute risk, which may reflect the fact that different efficacy data were applied. However, the results for i.v. ibandronic acid should be treated with caution as no fracture data were available for this treatment and data from other ibandronic acid regimens were applied. It should also be noted that the regression may predict INB less well in higher-risk patients, as only 10% of the population had a risk score of > 11%. It is also important to consider the uncertainty around the INB estimates by considering the CEACs.
FIGURE 90.
Regression for INB (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from QFracture.

FIGURE 91.
Close-up of regression for INB (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from QFracture.
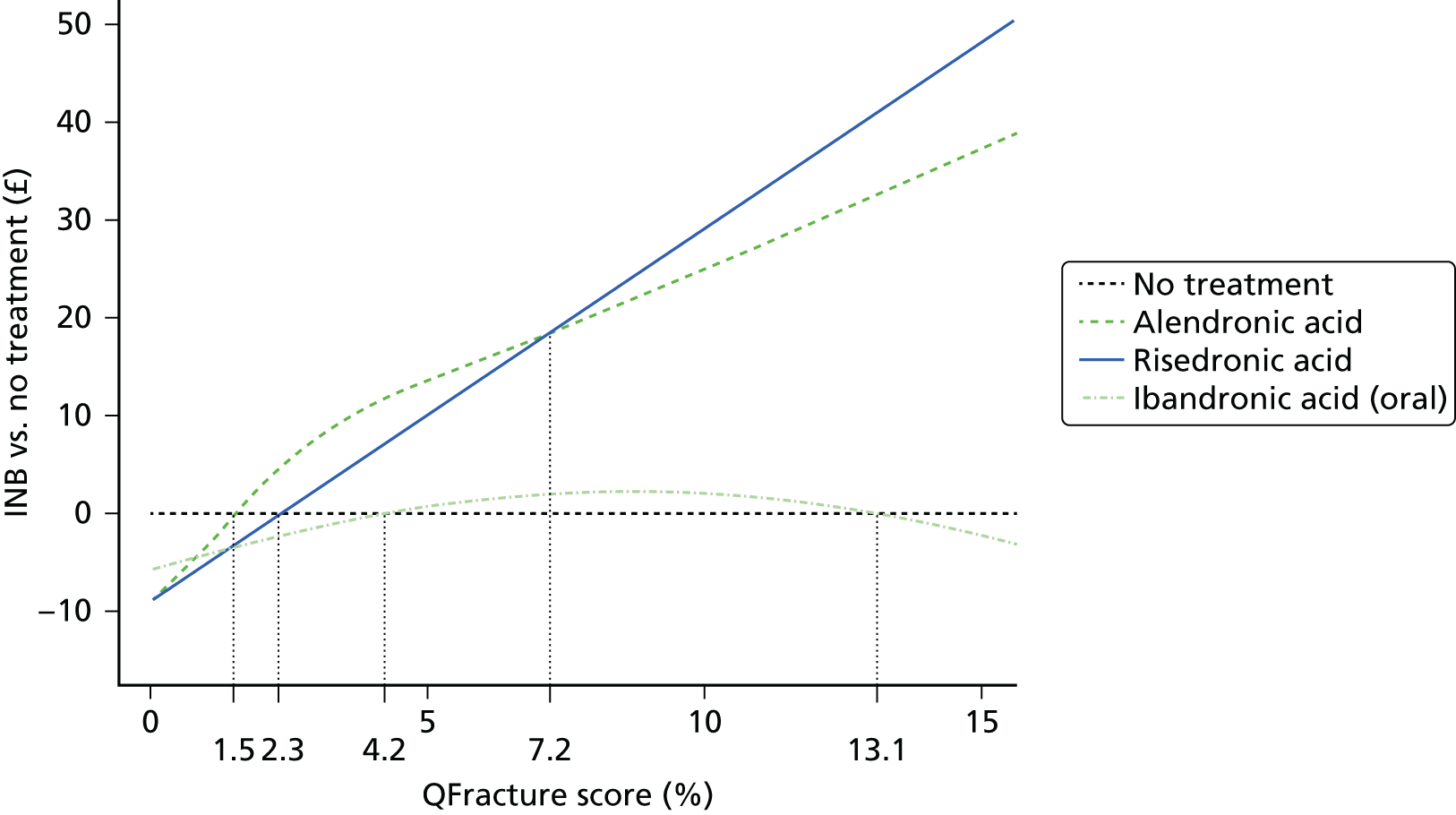
| Treatment | Range over which INB is positive compared with no treatment | Range over which INB is greater than for all other treatments |
|---|---|---|
| No treatment | NA | < 1.5% |
| Alendronic acid | > 1.5% | > 1.5 and < 7.2% |
| Risedronic acid | > 2.3% | > 7.2% |
| Ibandronic acid (oral) | > 4.2 and < 13.1% | Never |
| Ibandronic acid (i.v.) | > 75.5% | Never |
| Zoledronic acid | Never | Never |
Figures 92–101 present the CEACs for each of the risk categories when using QFracture to determine absolute risk. It can be seen that in the first and second risk categories (mean absolute risk of 0.5% and 0.7%, respectively), the no-treatment strategy has a much higher probability of being optimal, when valuing a QALY at £20,000 than any of the other strategies. However, in the third risk category (mean absolute risk of 1.0%), no treatment has the third highest probability of being most cost-effective with both risedronic acid and oral ibandronic acid having a greater probability when valuing a QALY at either £20,000 or £30,000. Although all three oral bisphosphonates have a positive INB compared with no treatment, in the seventh risk category (mean absolute risk of 3.9%) when valuing a QALY at £20,000, no treatment has a higher probability of being cost-effective than either risedronic acid or oral ibandronic acid, suggesting that there is still considerable uncertainty regarding the relative cost-effectiveness of oral bisphosphonates.
FIGURE 92.
Cost-effectiveness acceptability curve for QFracture risk category 1 (mean absolute risk of 0.5%).
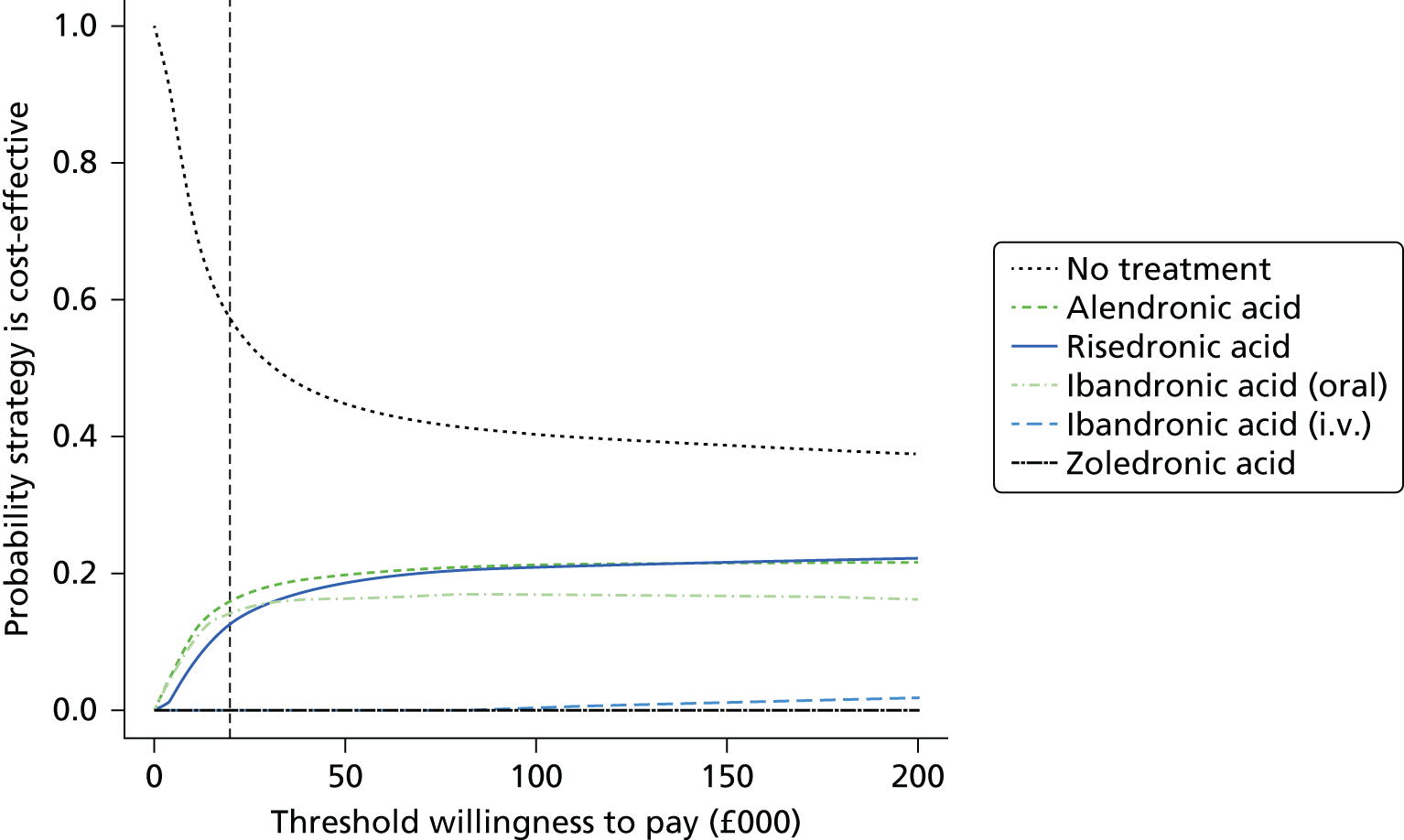
FIGURE 93.
Cost-effectiveness acceptability curve for QFracture risk category 2 (mean absolute risk of 0.7%).
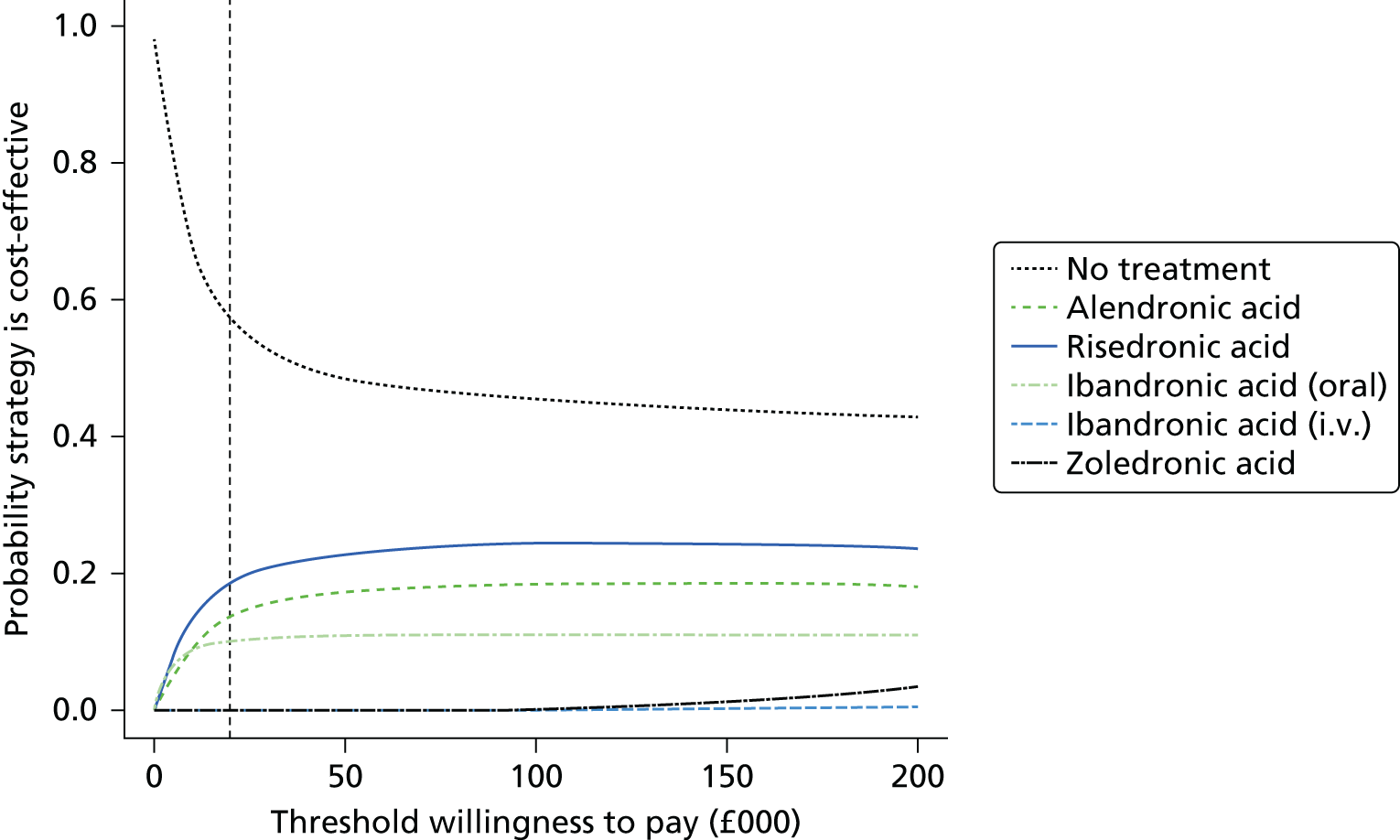
FIGURE 94.
Cost-effectiveness acceptability curve for QFracture risk category 3 (mean absolute risk of 1.0%).
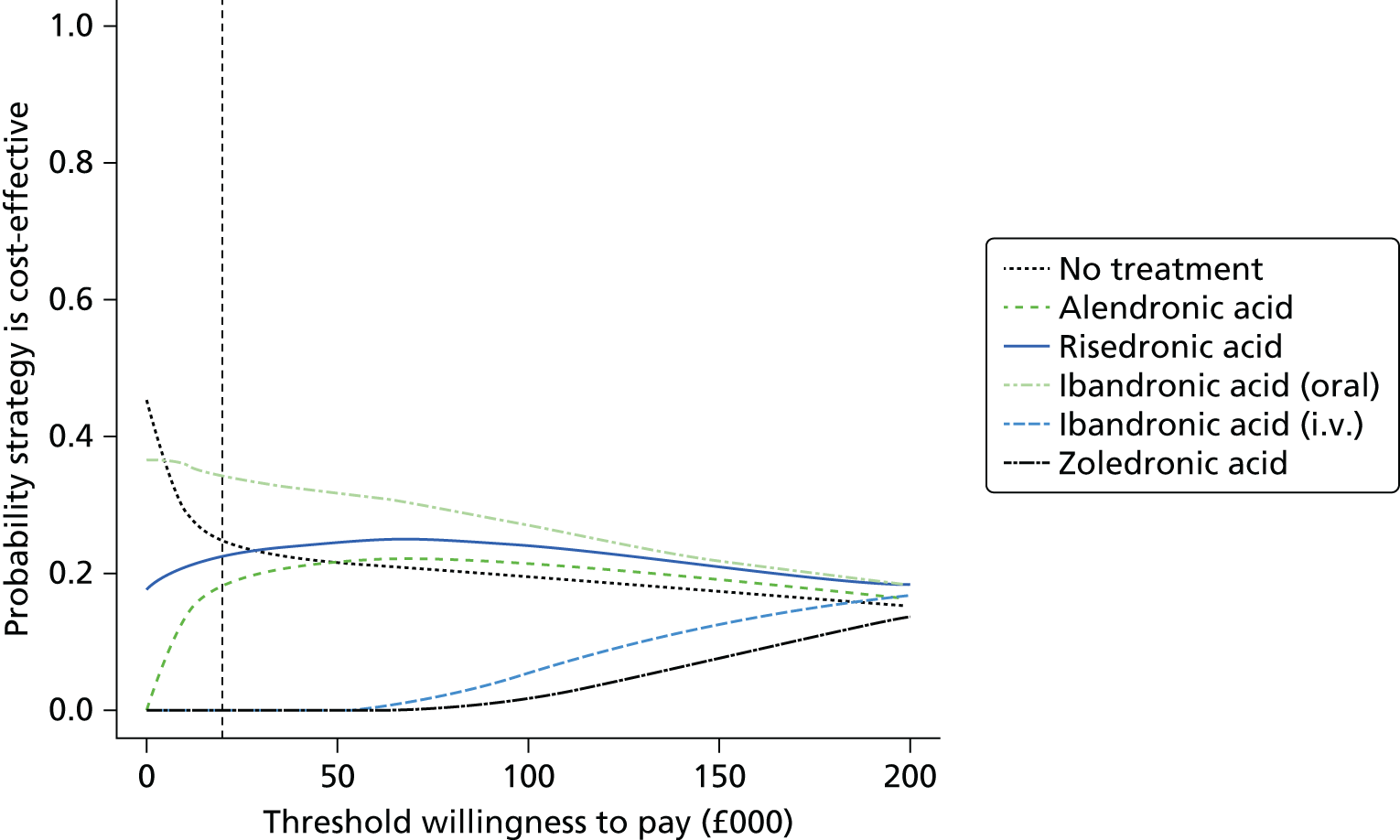
FIGURE 95.
Cost-effectiveness acceptability curve for QFracture risk category 4 (mean absolute risk of 1.4%).
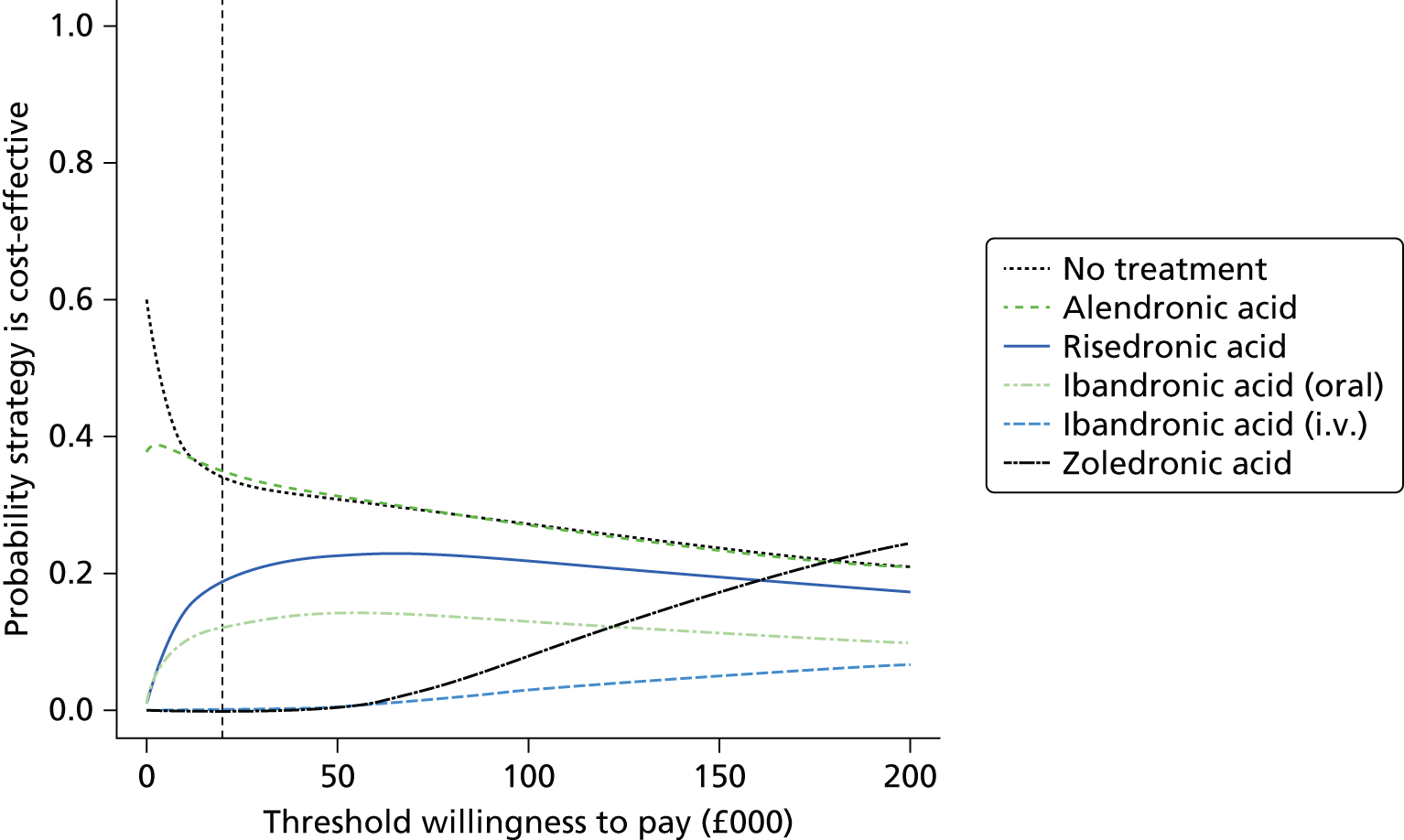
FIGURE 96.
Cost-effectiveness acceptability curve for QFracture risk category 5 (mean absolute risk of 2.0%).

FIGURE 97.
Cost-effectiveness acceptability curve for QFracture risk category 6 (mean absolute risk of 2.7%).
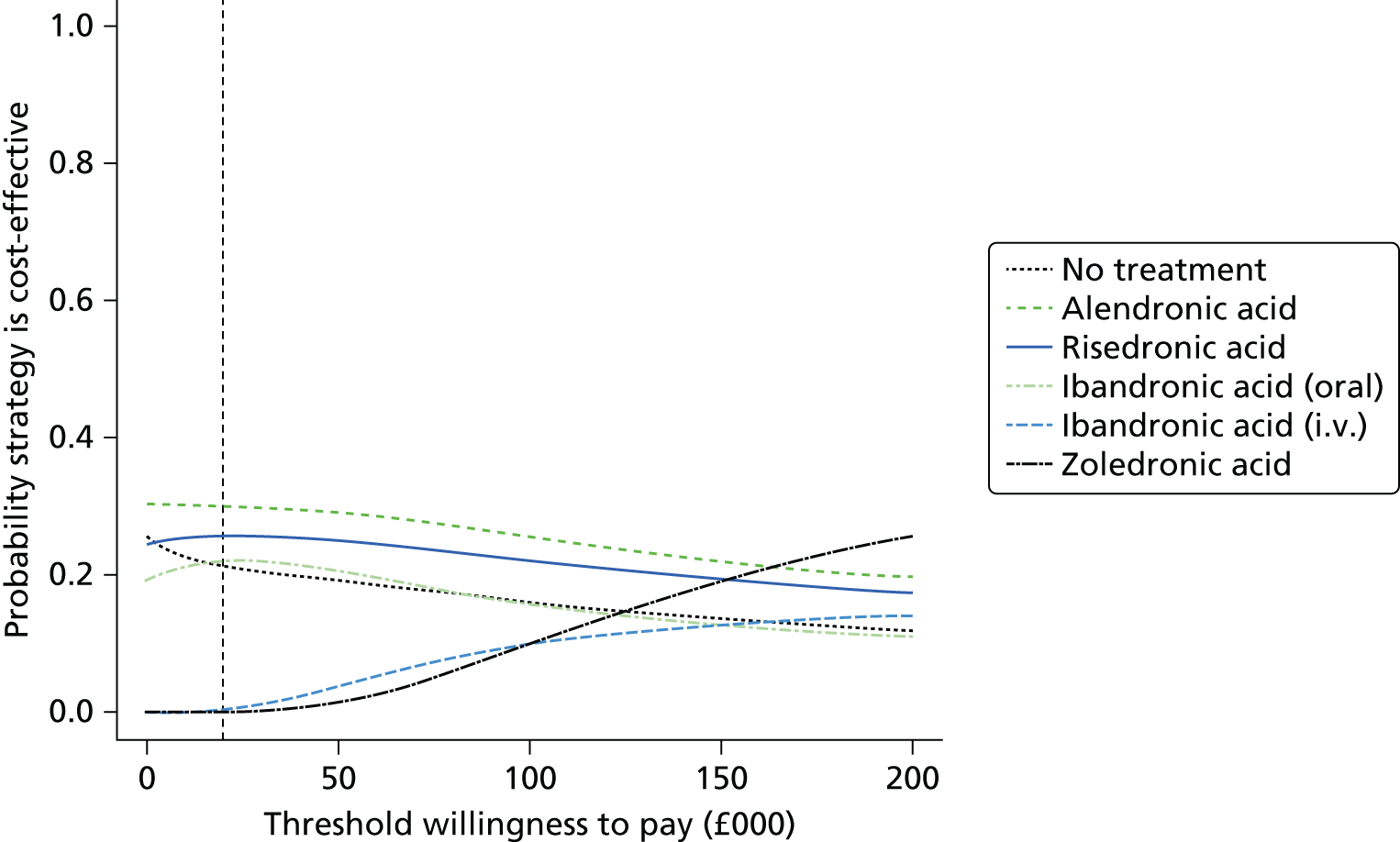
FIGURE 98.
Cost-effectiveness acceptability curve for QFracture risk category 7 (mean absolute risk of 3.9%).

FIGURE 99.
Cost-effectiveness acceptability curve for QFracture risk category 8 (mean absolute risk of 5.5%).

FIGURE 100.
Cost-effectiveness acceptability curve for QFracture risk category 9 (mean absolute risk of 8.4%).

FIGURE 101.
Cost-effectiveness acceptability curve for QFracture risk category 10 (mean absolute risk of 16.0%).
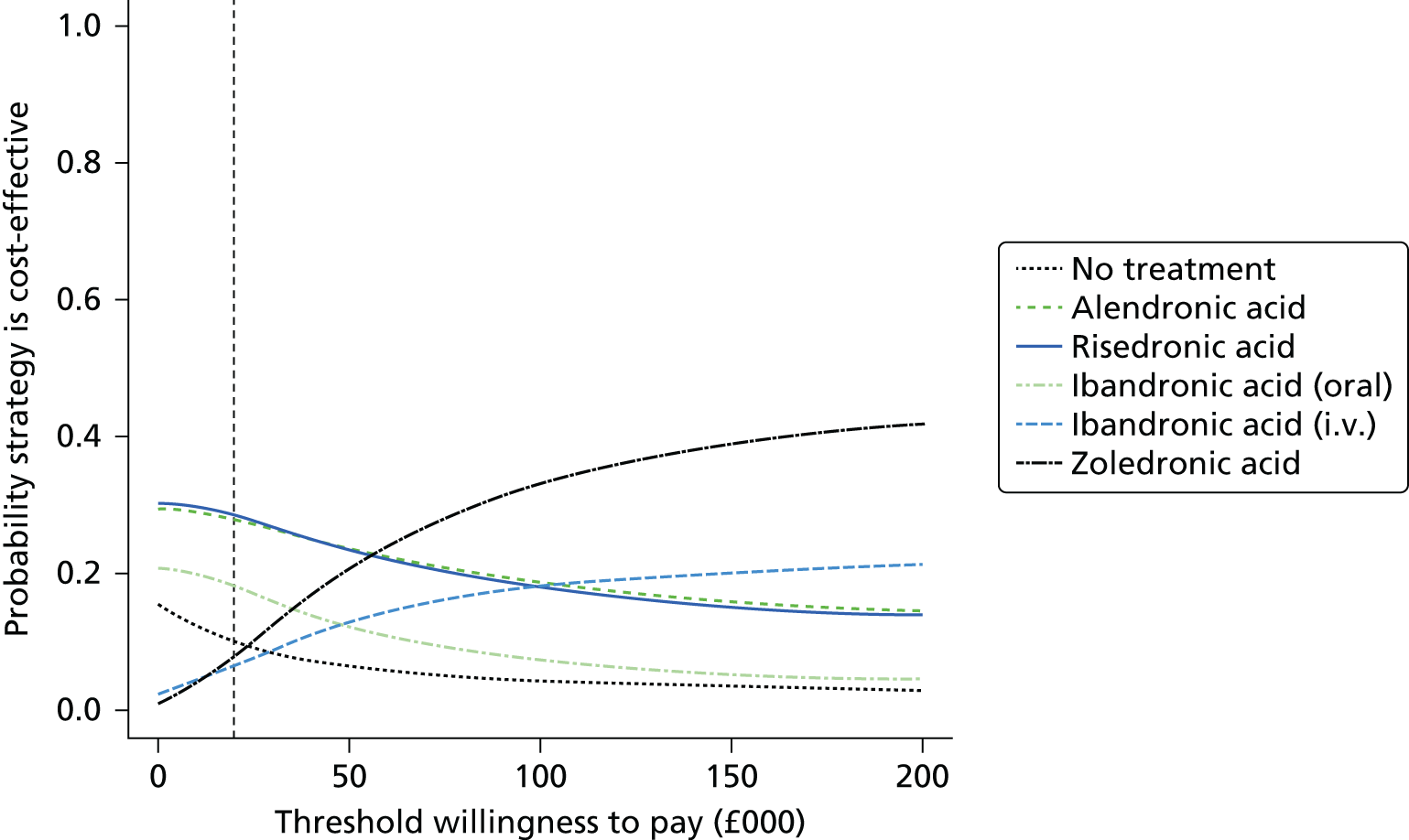
The i.v. bisphosphonates have a low probability of being optimal when valuing a QALY at £20,000, even in the highest-risk categories, although by the 10th risk category (mean absolute risk of 16.0%) they have a similar probability of being cost-effective as no treatment.
Summary of cost-effectiveness results for the base-case scenario when using FRAX
Figure 102 summarises the cost-effectiveness results across the 10 risk categories for FRAX. It shows the INB for each bisphosphonate treatment when compared with no treatment plotted against the 10-year absolute risk of fracture. Each point shows the mean INB and the mean 10-year absolute risk of fracture for one risk category when valuing a QALY at £20,000. It can be seen that the INB compared with no treatment does not have a simple relationship with absolute risk when using FRAX to define absolute risk. At first, the INB rises, but it later falls before rising again. This may reflect the differing patient characteristics across the risk categories. However, it can be seen that the mean INB compared with no treatment is above zero for all oral bisphosphonates across all 10 risk categories. The detailed results tables provided in Appendix 9 show that none of the bisphosphonates is consistently more cost-effective than the others, with all three having the highest INB (when valuing a QALY at £20,000) in at least one risk category and all three being dominated by another oral bisphosphonate in at least one risk category.
FIGURE 102.
Incremental net benefit (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from FRAX.
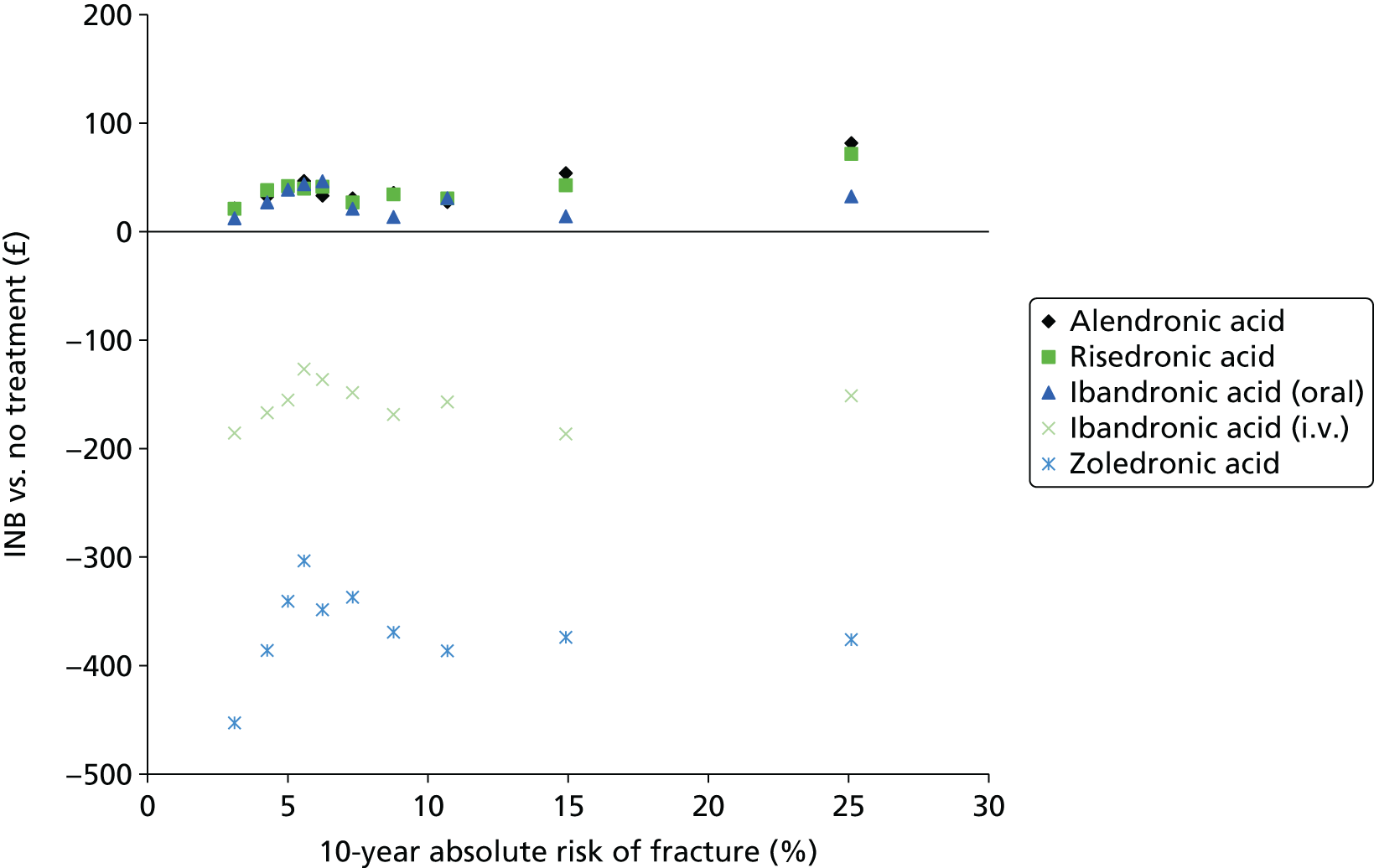
The mean INB for the two i.v. bisphosphonates is below zero across all 10 risk categories. This remains the case even when valuing a QALY at £30,000 (see Tables 74–83 in Appendix 10). Furthermore, i.v. ibandronic acid is always extendedly dominated by the other treatment strategies across all 10 risk categories for FRAX.
The full data from the PSA for the whole population (2 million patients with one parameter sample per patient) were used in a non-parametric regression, which estimated the relationship between INB and absolute fracture risk estimated by FRAX. For each of the five treatment options, the relationship between the INB of treatment (vs. no treatment) and the absolute fracture risk was estimated using a generalised additive model. This flexible regression approach assumes that the underlying relationship between the dependent variable and the independent variable can be approximated by a cubic spline, a highly flexible function that can model any smooth relationship between two variables. The regression prediction is shown in Figure 103, with a close-up shown in Figure 104 for the lower risk range. The results here differ from those presented in Figure 102 because non-parametric regression is able to average over the stochastic uncertainty associated with the individual-level patients while simultaneously estimating the relationship between INB and absolute risk. It can be seen that the INB of alendronic acid and risedronic acid increases with increasing risk. For all three oral bisphosphonates, the INB is positive compared with no treatment across the full range of absolute risk observed in the modelled population. Table 39 summarises the thresholds over which the INB of each treatment is positive compared with no treatment (when valuing a QALY at £20,000) and the range over which the INB of each treatment is maximum based on the non-parametric regression. Oral ibandronic acid is predicted to have the maximum INB up to an absolute risk level of 8.6%. Alendronic acid is predicted to have the maximum net benefit from 8.6%% to 38.5% and risedronic acid is predicted to have the maximum net benefit from 38.5% upwards. The INB compared with no treatment is negative for both the i.v. bisphosphonates across the full range of absolute risk observed in the modelled population when using FRAX to estimate absolute risk. By comparing Figure 90 with Figure 103 it can be seen that the relationship between INB and absolute risk for the i.v. bisphosphonates appears to differ when using FRAX and QFracture for patients with an absolute risk of > 20%. However, this may not reflect a true difference, as the estimates of > 11% for QFracture and > 18% for FRAX are informed by only one-tenth of the modelled population and, therefore, it is also important to consider the uncertainty in these estimates of mean INB by considering the CEACs.
FIGURE 103.
Regression for INB (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from FRAX.

FIGURE 104.
Close-up of regression for INB (when valuing QALY at £20,000) compared with no treatment against the 10-year fracture risk from FRAX.

| Treatment | Range over which INB is positive compared with no treatment | Range over which INB is greater than for all other treatments |
|---|---|---|
| No treatment | NA | Never |
| Alendronic acid | Whole range observed in modelled population | > 8.6 and < 38.5% |
| Risedronic acid | Whole range observed in modelled population | > 38.5% |
| Ibandronic acid (oral) | Whole range observed in modelled population | < 8.6% |
| Ibandronic acid (i.v.) | Never | Never |
| Zoledronic acid (i.v.) | Never | Never |
Figure 105–114 show the CEACs for the 10 FRAX risk categories. It can be seen that the strategy of no treatment has a low probability of being most cost-effective when valuing a QALY at £20,000, across all 10 risk categories. The i.v. bisphosphonates always have a lower probability of being optimal compared with no treatment or the oral bisphosphonates until risk category 8 (mean absolute risk of 10.7%) when i.v. zoledronic acid has a higher probability of being cost-effective than no treatment. In FRAX risk category 10 (mean absolute risk of 25.1%), i.v. zoledronic acid has the highest probability of being cost-effective, when valuing a QALY at £20,000 and i.v. ibandronic acid has a higher probability than oral ibandronic acid. However, it should be noted that the mean INB for both the i.v. bisphosphonates is negative in this risk category when valuing a QALY at £20,000.
FIGURE 105.
Cost-effectiveness acceptability curve for FRAX risk category 1 (mean absolute risk of 3.1%).
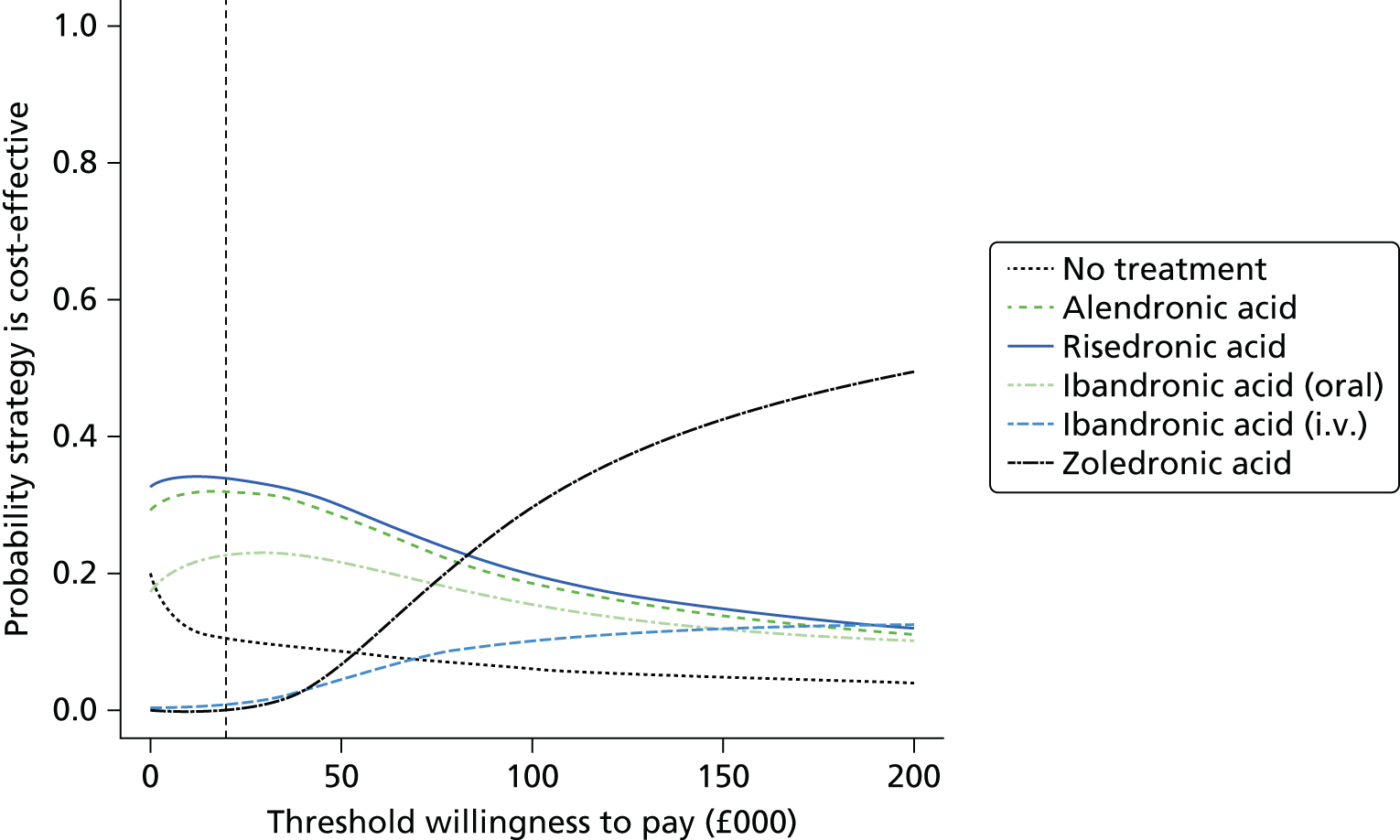
FIGURE 106.
Cost-effectiveness acceptability curve for FRAX risk category 2 (mean absolute risk of 4.3%).

FIGURE 107.
Cost-effectiveness acceptability curve for FRAX risk category 3 (mean absolute risk of 5.0%).
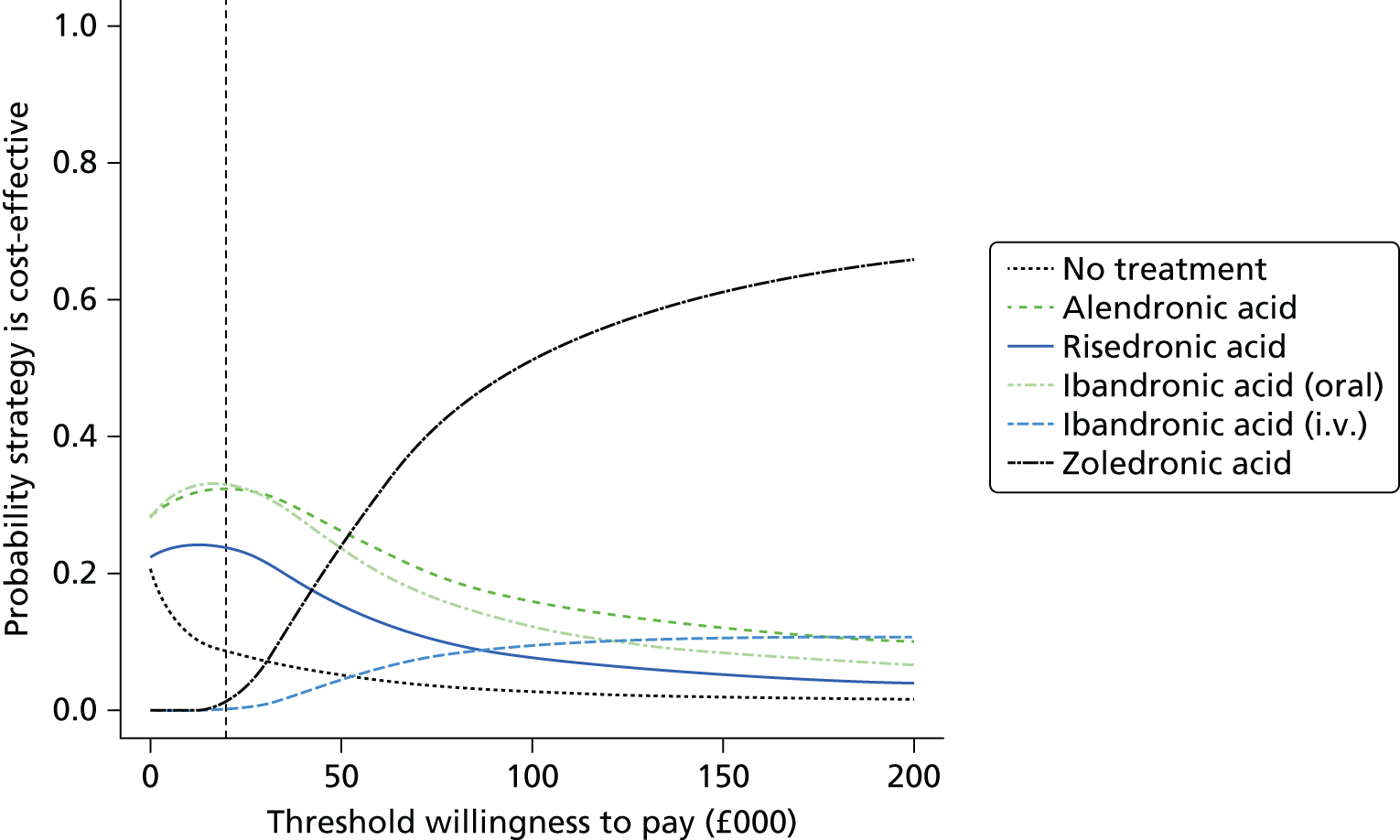
FIGURE 108.
Cost-effectiveness acceptability curve for FRAX risk category 4 (mean absolute risk of 5.6%).

FIGURE 109.
Cost-effectiveness acceptability curve for FRAX risk category 5 (mean absolute risk of 6.2%).

FIGURE 110.
Cost-effectiveness acceptability curve for FRAX risk category 6 (mean absolute risk of 7.3%).
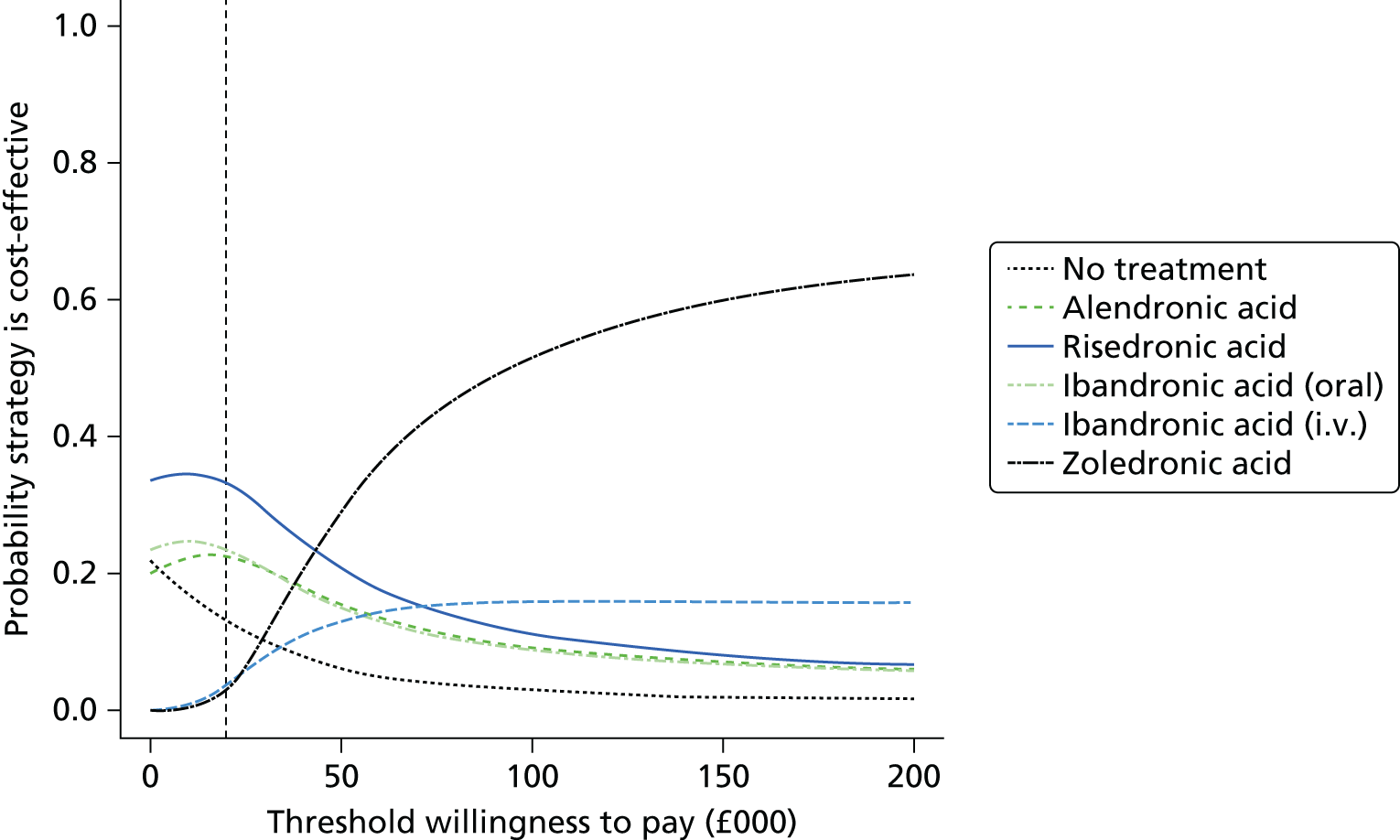
FIGURE 111.
Cost-effectiveness acceptability curve for FRAX risk category 7 (mean absolute risk of 8.8%).
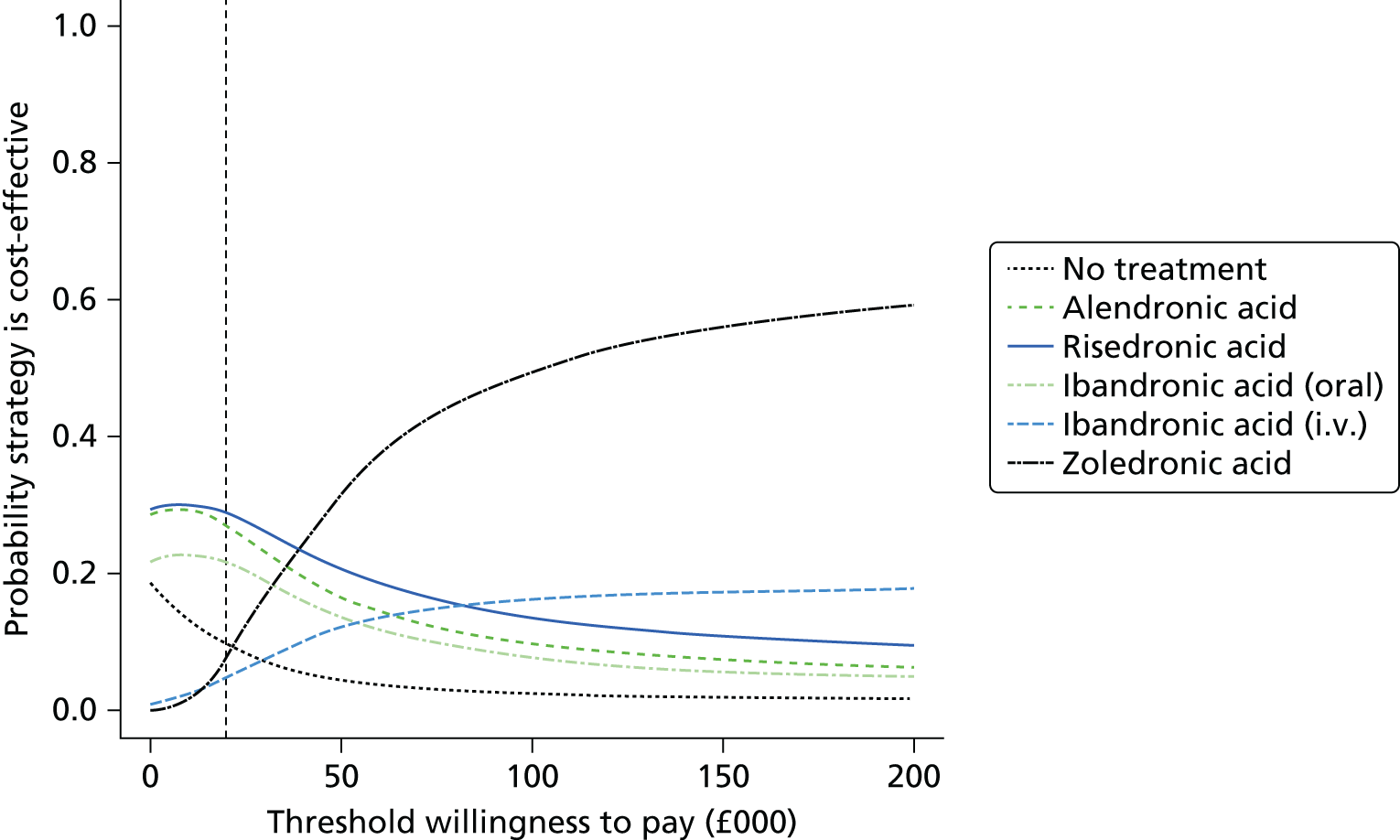
FIGURE 112.
Cost-effectiveness acceptability curve for FRAX risk category 8 (mean absolute risk of 10.7%.)
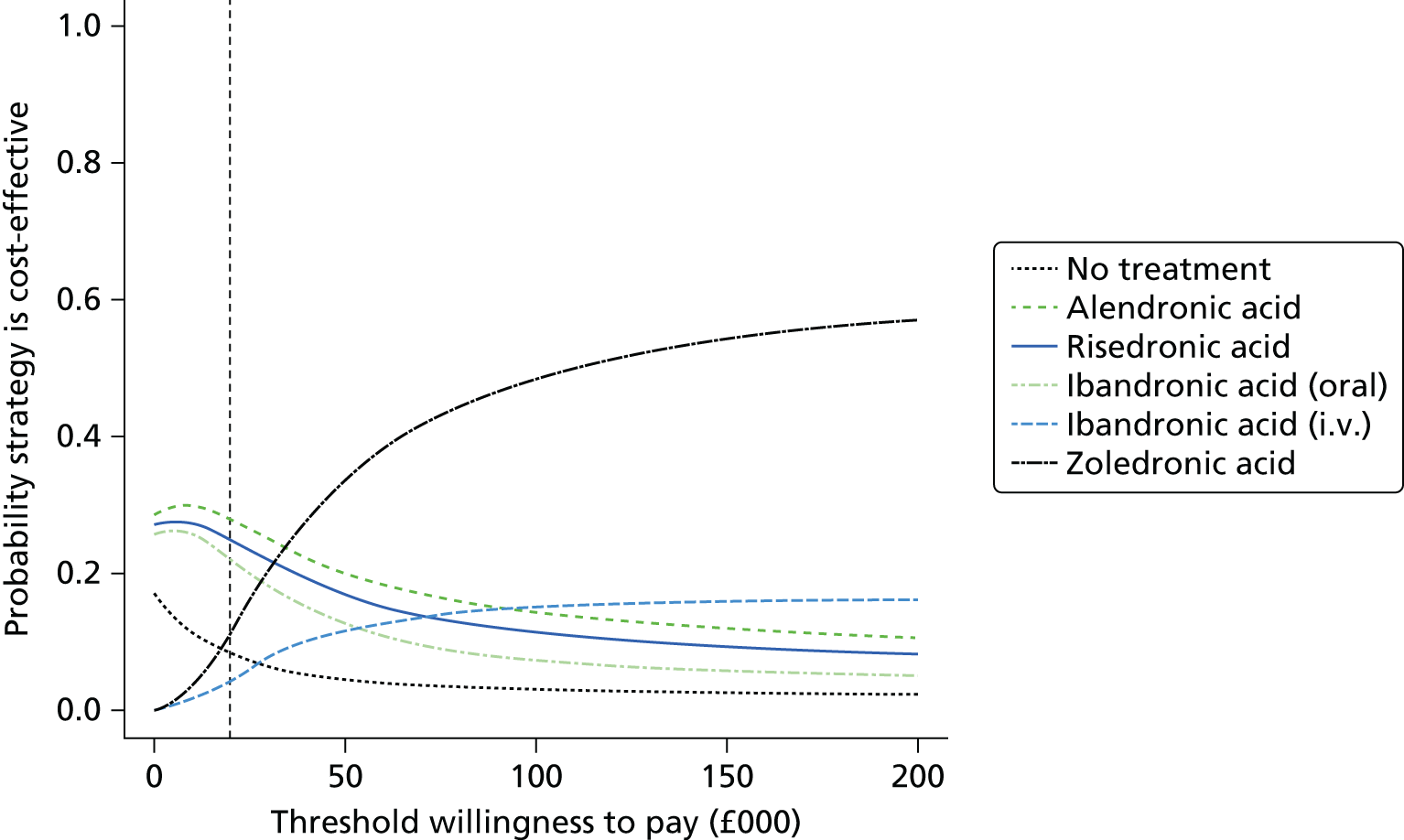
FIGURE 113.
Cost-effectiveness acceptability curve for FRAX risk category 9 (mean absolute risk of 14.9%).
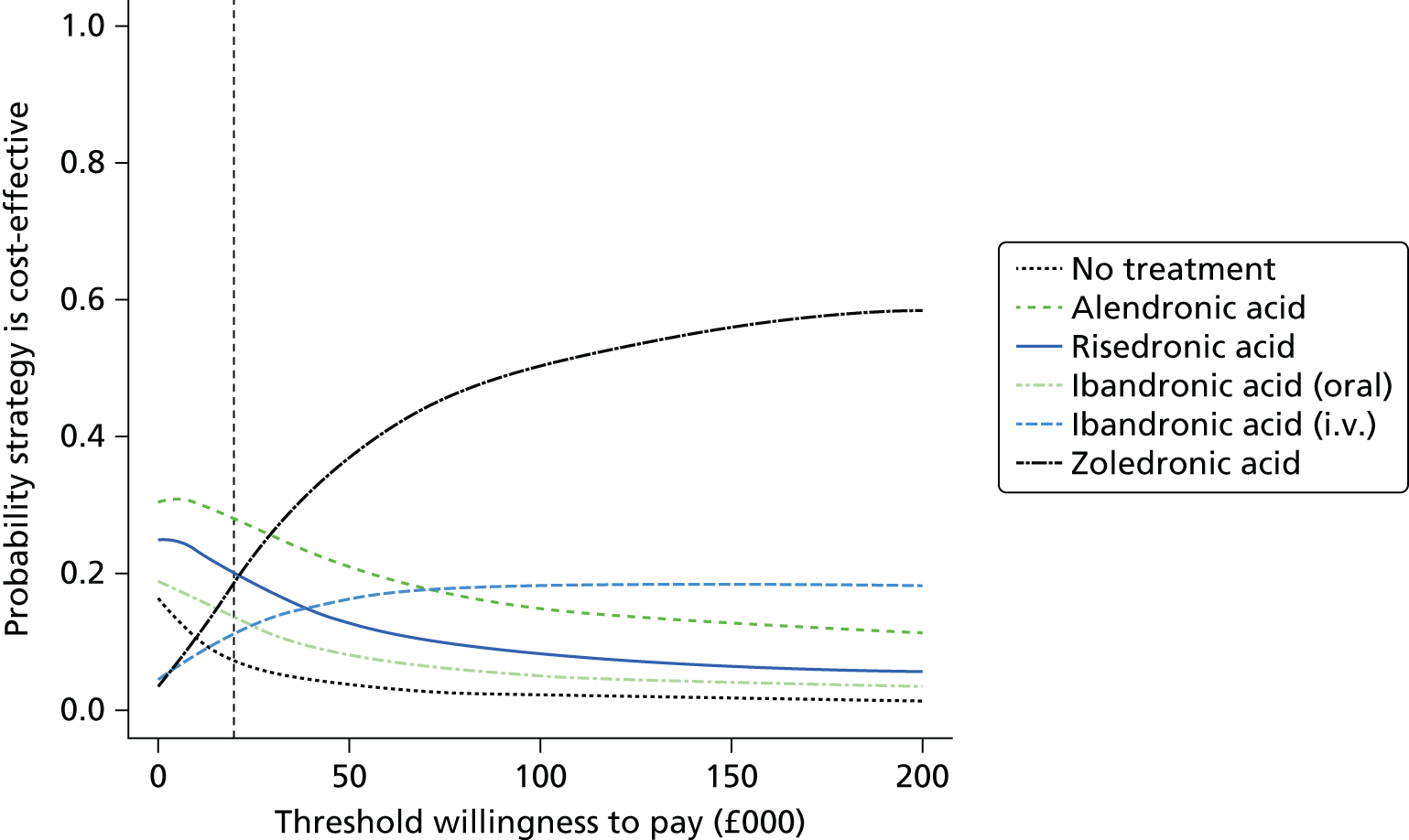
FIGURE 114.
Cost-effectiveness acceptability curve for FRAX risk category 10 (mean absolute risk of 25.1%).
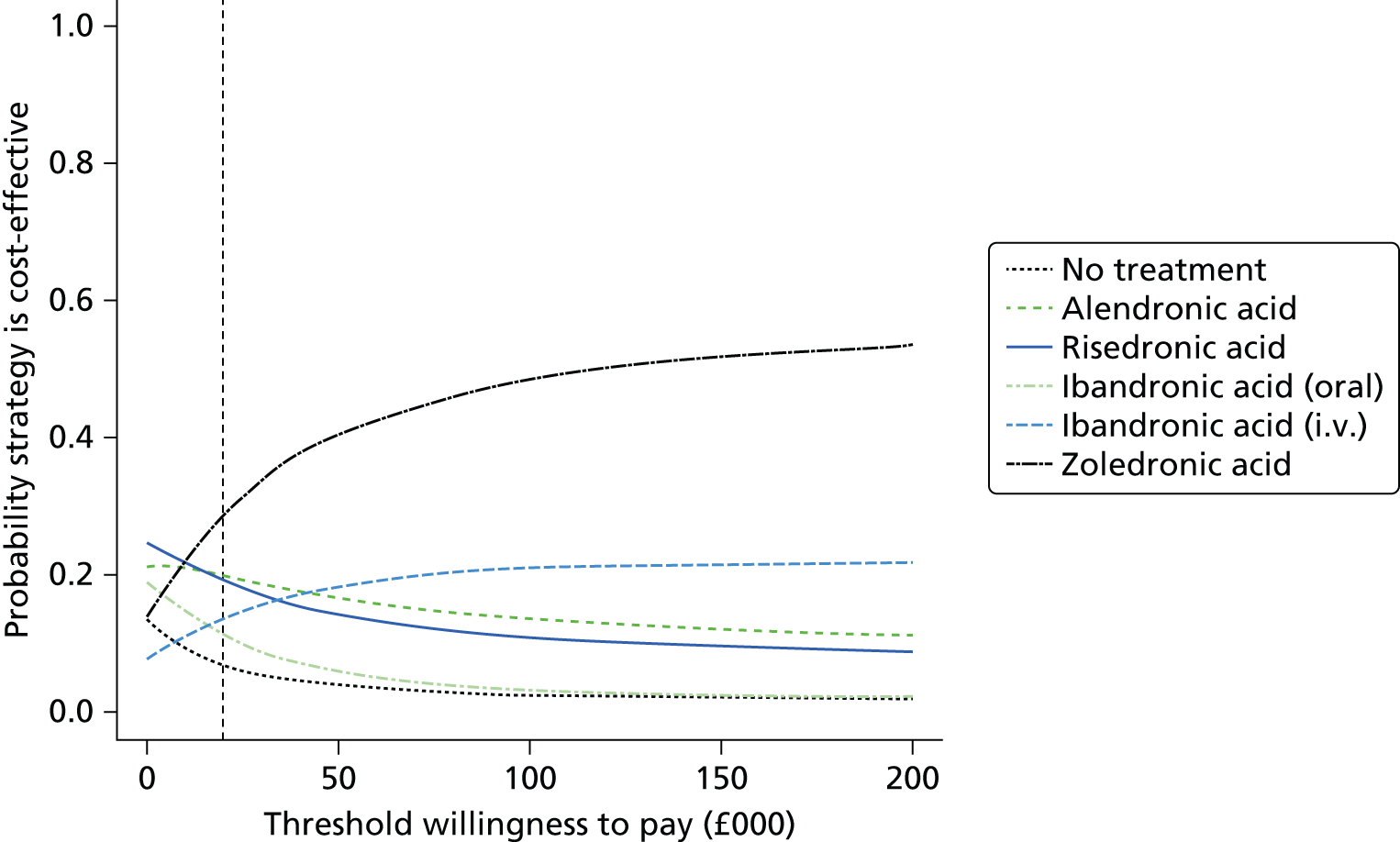
Summary of cost-effectiveness results for the base-case scenario when using FRAX
Figure 115 summarises the results from the model using mid-point parameter inputs. It shows the INB for each bisphosphonate treatment when compared with no treatment plotted against the 10-year absolute risk of fracture. The ‘F’ and ‘Q’ labels after the drug name indicate where the FRAX and QFracture algorithms have predicted the risk, respectively. The INB at the various risk levels appear to fall on a slightly higher curve when using FRAX than when using QFracture, with the difference being more pronounced for the i.v. bisphosphonates. This behaviour was also observed when examining the PSA results for QFracture and FRAX on the same plot, but the difference was slightly less pronounced (data not presented).
FIGURE 115.
Incremental net benefit for the base-case scenario when using mid-point parameter estimates. F, predicted by FRAX; Q, predicted by QFracture.
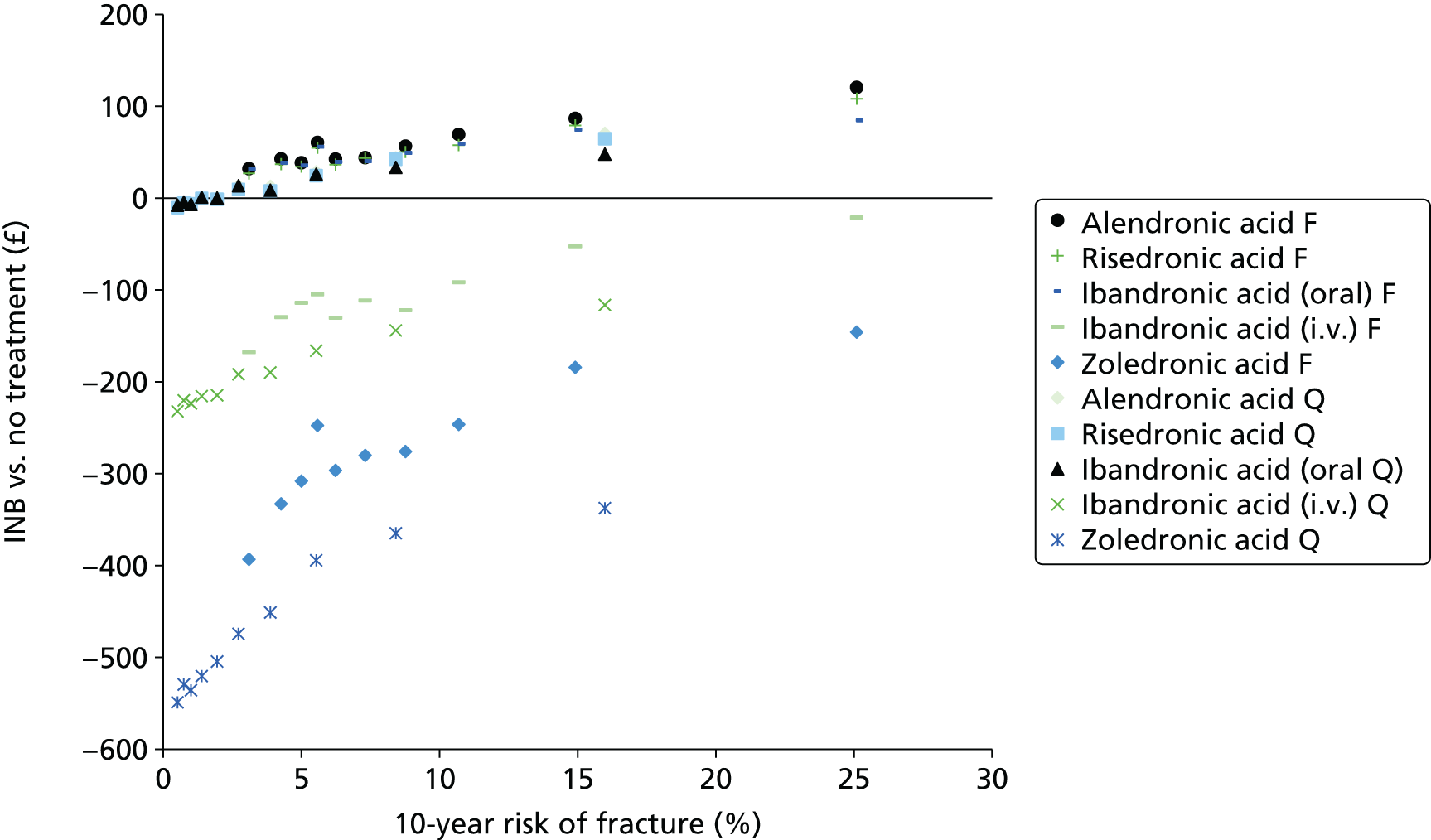
Structural sensitivity analyses
A sensitivity analysis was conducted in which we assumed that all patients would persist with treatment for the intended treatment duration (5 years for oral bisphosphonates and i.v. ibandronic acid and 3 years for zoledronic acid). In Figure 116, it can be seen that the INB is positive for oral bisphosphonates in all but the lowest risk category when using QFracture and in all risk categories when using FRAX. This is to be expected because the absolute benefits of treatment are greater when assuming that patients persist with treatment for longer. Therefore, as treatment continues the net benefit of treatment outweighs the upfront costs and disutilities associated with AEs in the first month after initiating treatment. The ICER for i.v. ibandronic acid compared with no treatment falls below £30,000 per QALY in the eighth risk category for FRAX (mean absolute risk of 10.7%) and below £20,000 per QALY in the 10th risk category of FRAX (mean absolute risk of 25.1%). For QFracture, the ICER compared with no treatment for i.v. ibandronic acid remains above £30,000 per QALY across all risk categories. For zoledronic acid, the ICER compared with no treatment does not fall below £30,000 in any risk category for either FRAX or QFracture.
FIGURE 116.
Incremental net benefit for the sensitivity analysis assuming full persistence with treatment for 3 years for zoledronic acid and 5 years for all other bisphosphonate treatments. F, predicted by FRAX; Q, predicted by QFracture.

A sensitivity analysis was conducted in which the rate of admission to a nursing home following hip fracture was applied to both hip and vertebral fractures. The results for this analysis are presented in Figure 117. The results are broadly similar to the base-case results, suggesting that our decision not to include nursing home admission following vertebral fracture within the analysis is unlikely to have significantly biased the cost-effectiveness results.
FIGURE 117.
Incremental net benefit for sensitivity analysis applying nursing home admission rates following hip fracture to vertebral fractures in addition to hip fractures. F, predicted by FRAX; Q, predicted by QFracture.

A sensitivity analysis was conducted in which we removed any fractures occurring at sites other than the four main osteoporotic fracture sites (hip, wrist, proximal humerus and vertebrae). The INBs compared with no treatment for both QFracture and FRAX are summarised in Figure 118. It can be seen that the results when using the QFracture algorithm are similar to the base case, but the results when using the FRAX algorithm have a lower INB and are more closely aligned with those for QFracture when considering risk categories with a similar mean absolute risk. The results from this structural sensitivity analysis suggests that the method used to uplift the fracture risk to incorporate fractures at additional sites may have caused the INBs of bisphosphonate treatment to be overestimated in the base-case analysis based on FRAX.
FIGURE 118.
Incremental net benefit for the sensitivity analysis excluding fractures occurring at sites other than the hip, wrist, proximal humerus and vertebrae. F, predicted by FRAX; Q, predicted by QFracture.
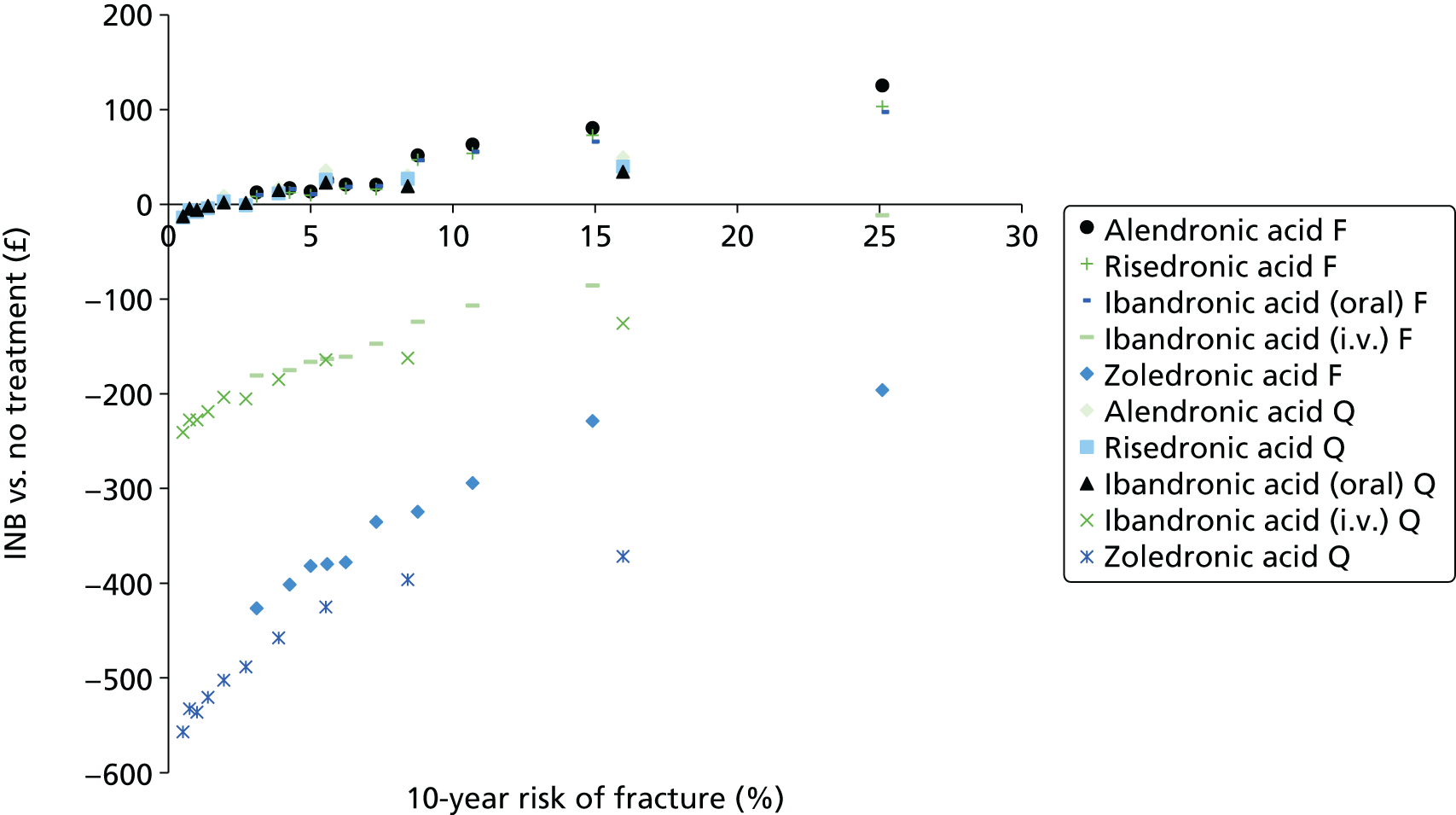
A sensitivity analysis was conducted in which the survival curves for hip fracture were based on the hip-specific absolute risk estimates from QFracture rather than a proportion of the absolute risk for the four main osteoporotic fracture sites. The results, shown in Figure 119, are broadly similar to the base case although the INB estimates for the FRAX risk categories are generally lower and fall closer to those for the QFracture categories with comparable absolute fracture risk. The INBs for all three oral bisphosphonates are negative in the first FRAX risk category (mean absolute risk of 3.1%) and the INB for risedronic acid is negative in the second FRAX risk category (mean absolute risk of 4.3%). The results of this structural sensitivity analysis suggests that the base-case scenario may have overestimated the cost-effectiveness of treatment for the FRAX risk categories because of the method used to calculate survival curves for FRAX from the data available for QFracture. The cost-effectiveness results for bisphosphonates treatment compared with no treatment may therefore be favourable to treatment when using the FRAX risk scores.
FIGURE 119.
Incremental net benefit for scenario using hip-specific estimates of absolute fracture risk. F, predicted by FRAX; Q, predicted by QFracture.
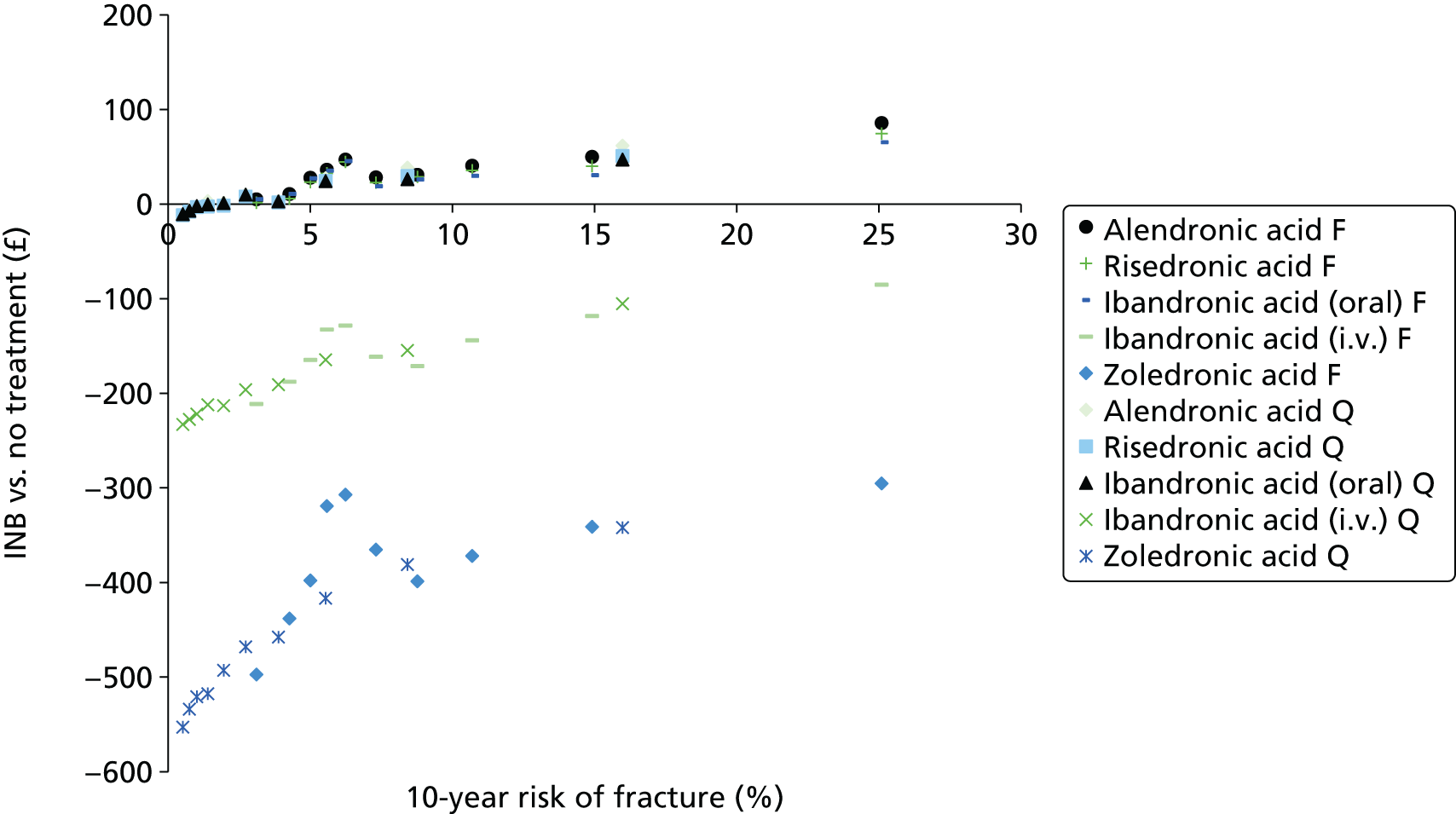
In the analysis, assuming full persistence with treatment, the duration of treatment for zoledronic acid was 3 years but the fall-off period was set to 7 years, while for the other bisphosphonates the treatment duration was 5 years and the fall-off period was 5 years. Although the assumption ensured that treatment effects fell to zero at 10 years for all drugs, when assuming full persistence, this assumption might have be favourable to zoledronic acid. In the base-case scenario in which mean persistence from observational studies was applied, the treatment duration and fall-off period for zoledronic acid were set to 1.7 years and 4 years (7/3 x 1.7), respectively. A sensitivity analysis was conducted in which the fall-off period for zoledronic acid was set equal to the treatment duration (1.7 years for both). The results are summarised in Figure 120. It can be seen that for lower-risk categories for QFracture the INB estimates for zoledronic acid do not vary smoothly suggesting that they have failed to reach a stable estimate probably owing to the limited number of fractures prevented when assuming only 1.7 years of treatment and 1.7 years of fall-off time. However, the INB for zoledronic acid versus no treatment remains below zero for all risk categories for both QFracture and FRAX, as was observed in the base-case scenario.
FIGURE 120.
Incremental net benefit for scenario in which fall-off time was set equal to treatment duration for zoledronic acid. F, predicted by FRAX; Q, predicted by QFracture.

A sensitivity analysis was conducted to examine whether or not uncertainty regarding the average survival in patients who die following a hip fracture was an important determinant of cost-effectiveness. For this analysis the average duration of survival after hip fractures associated with excess mortality was reduced from 3 months to 1 month. The results, which are summarised in Figure 121, are very close to those seen in the base-case scenario and, therefore, it can be concluded that the exact duration of survival following a hip fracture associated with excess mortality is not an important determinant of cost-effectiveness.
FIGURE 121.
Incremental net benefit when assuming that excess mortality associated with hip fractures occurs 1 month after the hip fracture. F, predicted by FRAX; Q, predicted by QFracture.
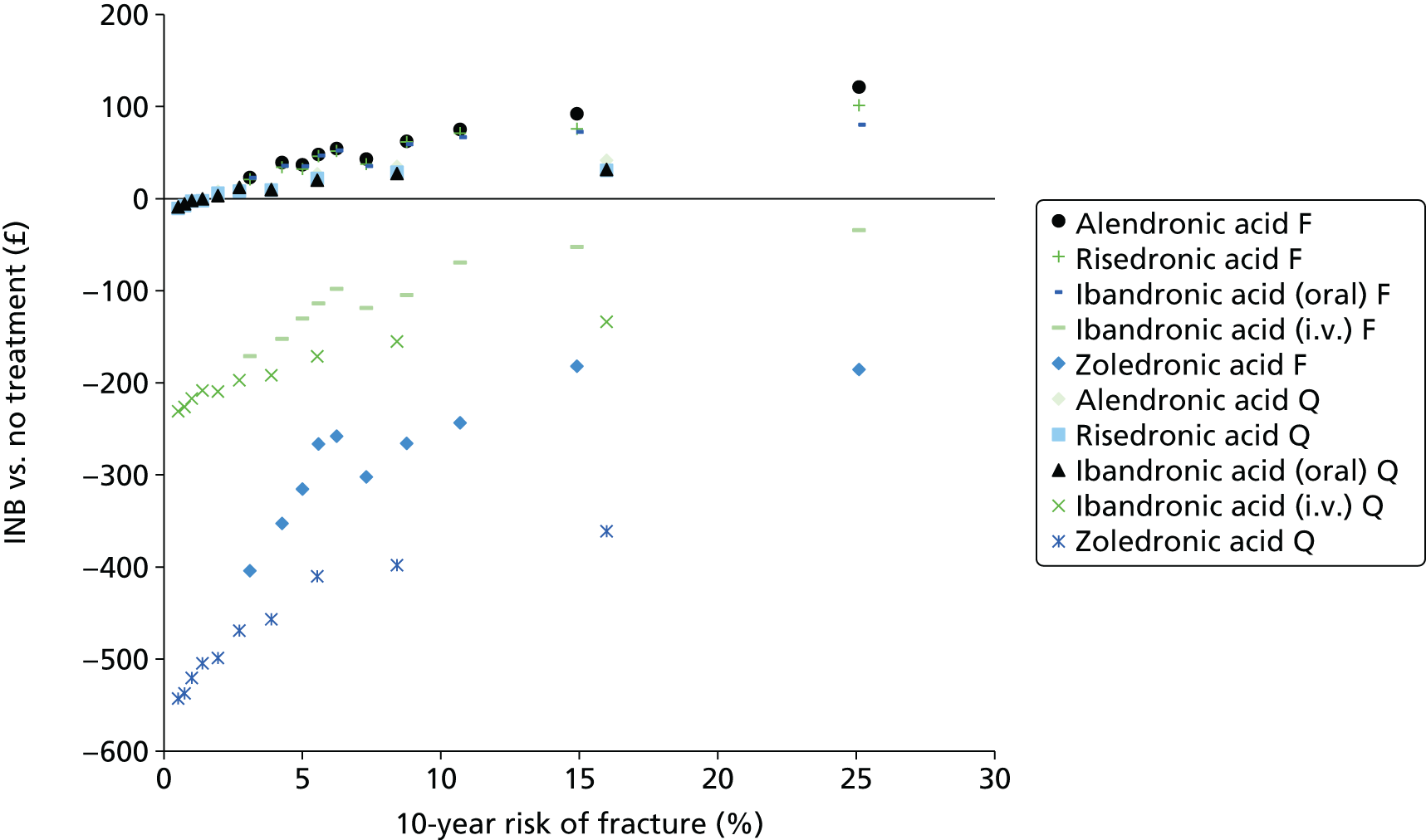
A sensitivity analysis was conducted using the more recent data on the increased risk of fracture following an incident fracture from the systematic review by Warriner et al. 213 The results, summarised in Figure 122, show marginally higher INBs for treatment compared with no treatment, which is expected because several of the HRs for increased fracture risk following an incident fracture were greater in the paper by Warriner et al. 213 than the figures presented in the paper by Klotzbuecher et al. 149 which were used in the base-case scenario. However, the results do not appear to be particularly sensitive to the choice of data source for these model parameters.
A sensitivity analysis was conducted in which the prevalence of a prior fracture at baseline was estimated from UK fracture incidence data rather than using estimates of the prevalence of a prior fracture from a meta-analysis of the cohort studies which informed the FRAX algorithm. It can be seen from Figure 123 that the results are very similar to the base-case results and, therefore, the model is not particularly sensitive to the prevalence of a prior fracture at baseline. This may be because a history of prior fracture has only a marginal impact on the individual’s utility and health resource use and the increased risk attributed to prior fracture would simply move patients between risk categories rather than making it more or less cost-effective to treat within a particular risk category.
FIGURE 123.
Incremental net benefit for sensitivity analysis using UK incidence data to estimate the prevalence of prior fracture. F, predicted by FRAX; Q, predicted by QFracture.

In the base-case analysis, data from the 150 mg per month of oral ibandronic acid regimen were applied in the model for the monthly oral dose for all four fracture sites. However, no fracture data were available for the i.v. ibandronic acid regimen. As this regimen was licensed based on a non-inferiority trial comparing it to the previously licensed 2.5 mg per day oral regimen, data from the 2.5-mg oral dose were applied to the i.v. regimen where these were available. Where these were not available, data from the 150 mg per month oral regimen were applied instead. However, this meant that different data were applied for the oral and i.v. regimen for some fracture sites (vertebral and proximal humerus). A sensitivity analysis was conducted in which the same efficacy data were applied to both the monthly oral and the quarterly i.v. ibandronic acid regimens. For vertebral and proximal humerus fractures data from the 2.5 mg per day oral ibandronic acid regimen were applied to both as both were licensed based on non-inferiority trials comparing them to the daily 2.5-mg oral dose (no longer licensed). Data for hip and wrist were unchanged as the only data available were for the 150-mg dose and these data were applied to both regimens in the base case. The efficacy data applied in the base-case and the sensitivity analysis are summarised in Table 40.
| Fracture site | Monthly oral ibandronic acid, HR (95% CI) | Quarterly i.v. ibandronic acid, HR (95% CI) |
|---|---|---|
| Base case | ||
| Hip | 0.87 (0.27 to 2.92) from monthly dosing | 0.87 (0.27 to 2.92) from monthly dosing |
| Vertebrae | 0.45 (0.21 to 0.96) from monthly dosing | 0.47 (0.25 to 0.86) from daily dosing |
| Proximal humerus | 0.80 (0.49 to 1.43) from monthly dosing | 0.92 (0.59 to 1.43) from daily dosing |
| Wrist | 0.83 (0.31 to 2.39) from monthly dosing | 0.83 (0.31 to 2.39) from monthly dosing |
| Sensitivity analysis | ||
| Hip | 0.87 (0.27 to 2.92) from monthly dosing | 0.87 (0.27 to 2.92) from monthly dosing |
| Vertebrae | 0.47 (0.25 to 0.86) from daily dosing | 0.47 (0.25 to 0.86) from daily dosing |
| Proximal humerus | 0.92 (0.59 to 1.43) from daily dosing | 0.92 (0.59 to 1.43) from daily dosing |
| Wrist | 0.83 (0.31 to 2.39) from monthly dosing | 0.83 (0.31 to 2.39) from monthly dosing |
The results for this sensitivity are summarised in Figure 124 for the QFracture risk categories and in Figure 125 for the FRAX risk categories. The estimates presented here are the mean outputs from the PSA, which incorporated the joint distribution of the HRs from the NMA. The results are very similar to the base-case analysis, suggesting that the model is not particularly sensitive to the choice of data source for the ibandronic acid HRs. This was to be expected given that the NMA did not find any strong evidence of a difference in efficacy between the monthly and daily dosing ibandronic acid regimens. It remains possible that there is a difference between fracture outcome for the monthly oral and quarterly i.v. regimens, but this could not be assessed within the NMA because no fracture outcomes were available for the quarterly i.v. regimen.
FIGURE 124.
Incremental net benefit for sensitivity analysis using the same efficacy data for oral and i.v. ibandronic acid treatments for QFracture risk categories.
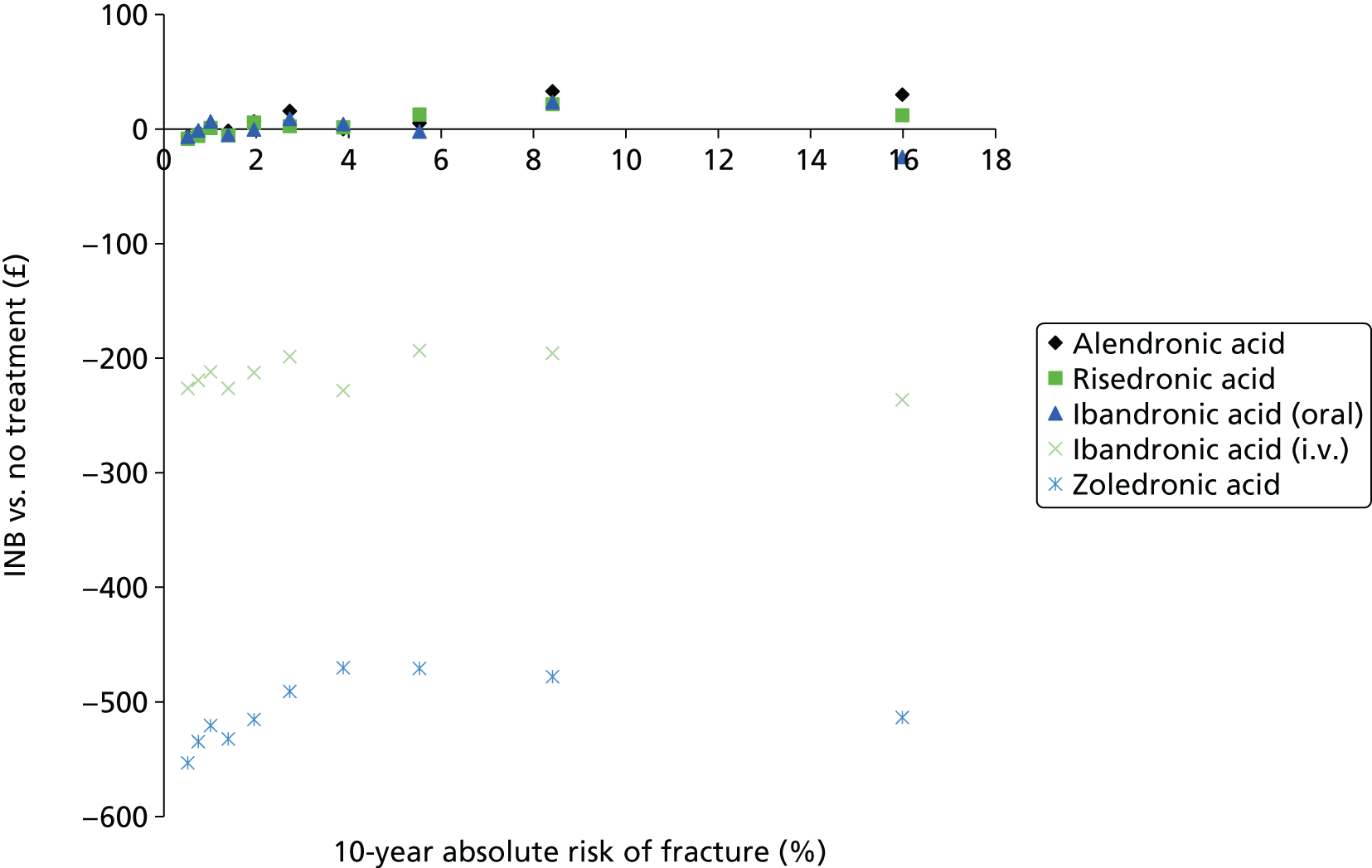
FIGURE 125.
Incremental net benefit for sensitivity analysis using the same efficacy data for oral and i.v. ibandronic acid treatments for FRAX risk categories.
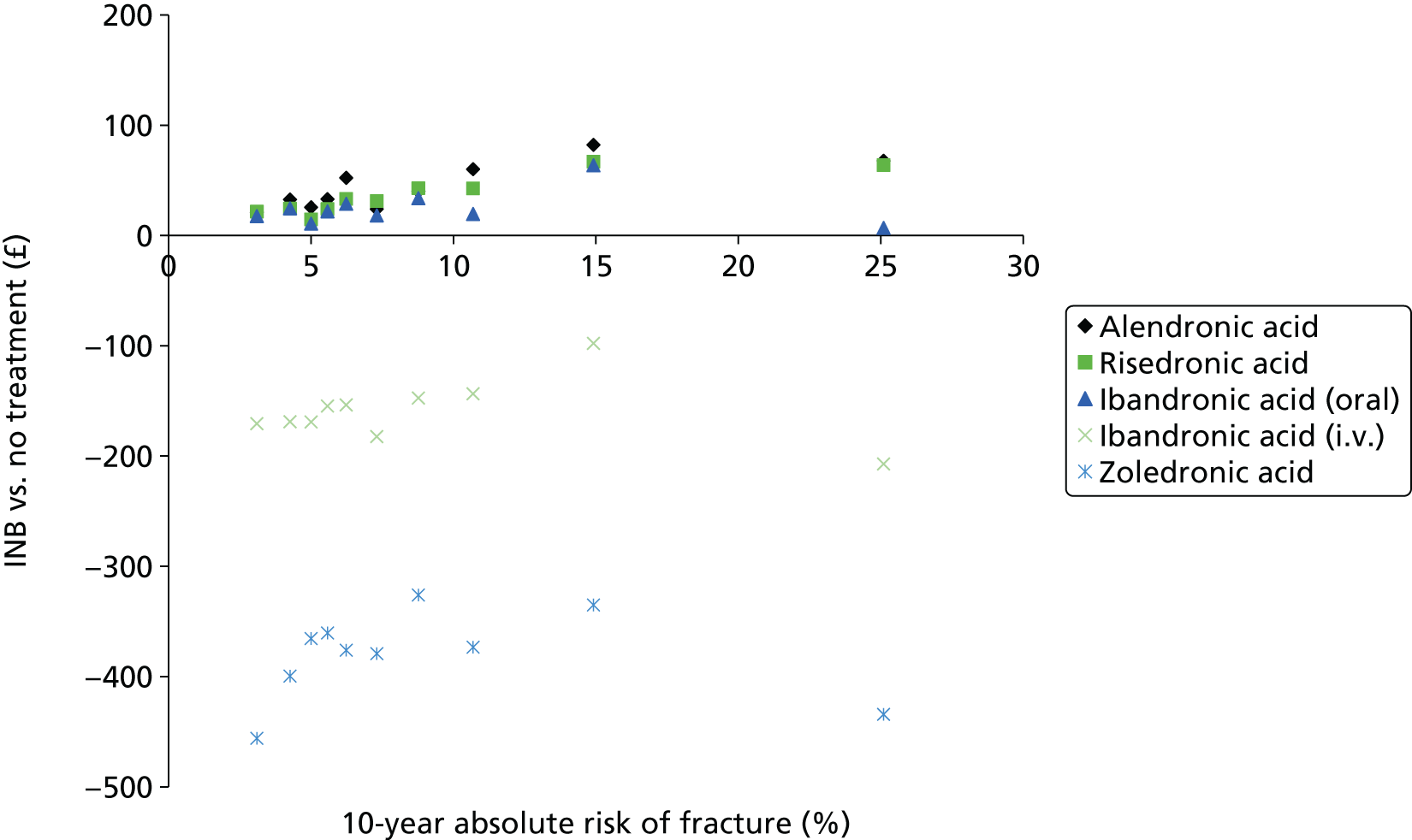
Scenario sensitivity analyses were also conducted on the costs and QALY decrements attributable to AEs. As AEs were not included as an uncertain parameter in the PSA, it was possible to adjust the PSA outputs for different assumptions regarding AEs. Figures 126 and 127 summarise the results when assuming no costs or QALY decrements attributable to AEs for the QFracture and FRAX risk categories, respectively. It can be seen that in this scenario the oral bisphosphonates are more cost-effective, with only risedronic acid having a negative INB compared with no treatment in the first QFracture risk decile (mean absolute risk of 0.5%), when valuing a QALY at £20,000. In all other risk categories the oral bisphosphonates have a positive INB, except for the fifth risk category (mean absolute risk of 2.0%), in which only alendronic acid has a positive INB. However, the results for the i.v. bisphosphonates are similar, with negative INBs compared with no treatment across all 10 risk categories for QFracture.
The results across the FRAX risk categories when assuming no costs of QALY decrements attributable to AEs were similar to the base-case scenario, with positive INBs for the oral bisphosphonates and negative INBs for the i.v. bisphosphonates when valuing a QALY at either £20,000 or £30,000.
FIGURE 126.
Incremental net benefit for sensitivity analysis assuming no costs or QALY decrements for adverse side effects for QFracture risk categories.

FIGURE 127.
Incremental net benefit for sensitivity analysis assuming no costs or QALY decrements for adverse side effects for FRAX risk categories.
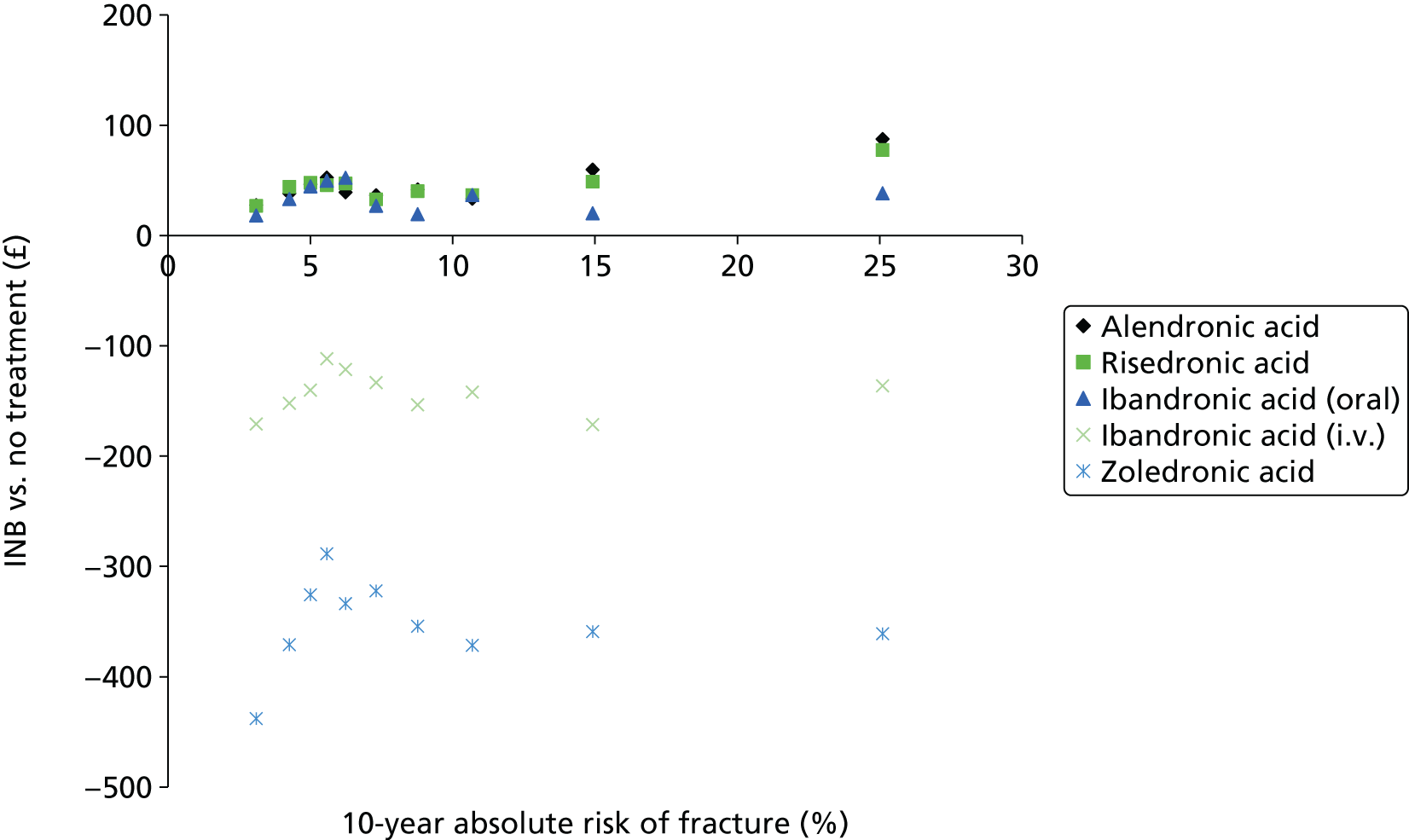
In addition, a scenario analysis was conducted in which the rate of adverse side effects for oral bisphosphonates was increased from 3% to 30%. In this scenario none of the oral bisphosphonates had a positive INB compared with no treatment across any of the QFracture risk categories when valuing a QALY at £20,000, as shown in Figure 128. The INBs remained negative for all treatments when valuing a QALY at £30,000 (data not presented).
FIGURE 128.
Incremental net benefit for sensitivity analysis assuming a 30% AE rate for oral bisphosphonates in the first month of treatment for QFracture risk categories.

The results for the FRAX risk categories when assuming an AE rate of 30% for oral bisphosphonates in the first month of treatment are shown in Figure 129. It can be seen that the INB is negative for the three oral bisphosphonates for the first eight risk categories (mean absolute risk of 10.7% and below), but is positive for alendronic acid in the ninth FRAX risk category (mean absolute risk of 14.9%) and for all three oral bisphosphonates in the 10th FRAX risk category (mean absolute risk of 25.1%).
FIGURE 129.
Incremental net benefit for sensitivity analysis assuming a 30% AE rate for oral bisphosphonates in the first month of treatment for FRAX risk categories.

Our clinical advisors were concerned that the price of zoledronic acid, which was taken from the eMIT database,152 may not be reflective of real-world prices because zoledronic acid has only recently become available in a generic format for this indication. Therefore, we conducted a sensitivity analysis using the price from eMIT for the 4-mg dose of generic zoledronic acid, which is licensed for the prevention of skeletal-related events in patients with advanced malignancies involving the bone. The average price on eMIT for the most commonly prescribed preparation of zoledronic acid for this alternative indication was £5.76. It was also noted by clinicians that zoledronic acid may be administered in some cases as an outpatient procedure rather than as a day case. Therefore, we also applied these lower administration costs in addition to the lower drug acquisition cost. This was done using the average outputs from the PSA and by assuming 1.67 doses of zoledronic acid are administered on average, with the mean number of doses estimated based on 500,000 PSA samples.
The results, when assuming these lower costs for zoledronic acid treatment, are summarised in Figure 130 for both QFracture and FRAX. It can be seen that, although the INB compared with no treatment has increased for zoledronic acid under these more favourable cost assumptions, the INB is still negative across all 10 risk categories for both QFracture and FRAX.
FIGURE 130.
Incremental net benefit for zoledronic acid when assuming a lower acquisition price and outpatient rather than day case administration costs. F, predicted by FRAX; Q, predicted by QFracture.

Discussion
In summary, when valuing a QALY at £20,000, a strategy of no treatment is predicted to have the greatest net benefit for patients with a QFracture score of < 1.5%. Alendronic acid is predicted to have the maximum net benefit from 1.5% to 7.2% and risedronic acid is predicted to have the maximum net benefit for a score of ≥ 7.2%. However, the absolute costs and QALY gains are small in patients with low absolute risk and there is considerable uncertainty regarding whether or not no treatment is the optimal strategy until the QFracture score is approximately 5.5% (the mean absolute risk for the eighth risk category for QFracture).
The mean INBs for oral bisphosphonate treatment compared with no treatment were positive across all FRAX risk categories. However, in the base-case scenario the INBs of bisphosphonate treatments compared with no treatment were generally higher for FRAX than QFracture for risk categories with similar absolute fracture risk. We would expect from the way the model is structured that the threshold for cost-effective treatment would be broadly similar across the two risk scores. The results of two structural sensitivity analyses suggest that the base-case analysis may have overestimated the INB of bisphosphonate treatment compared with no treatment. Given this possible bias in the estimates generated by the model using the FRAX risk score, and our belief that the results should be broadly similar across the two risk scores, it would be reasonable to assume that the absolute risk threshold estimated in the QFracture model could be applied to patients whose score had been calculated using either QFracture or FRAX.
Intravenous bisphosphonates had much higher ICERs than no treatment. In the highest-risk categories the ICERs for i.v. ibandronic acid and zoledronic acid compared with oral bisphosphonates were > £50,000 per QALY, even though the base-case analysis assumed longer durations of persistence with i.v. bisphosphonates than with oral bisphosphonates. Although the mean INB compared with no treatment for i.v. ibandronic acid did become positive at very high levels of absolute risk when using QFracture, the results when using FRAX went in the opposite direction. This may be because of the small number of patients and parameter samples informing the estimates at high levels of absolute risk, which makes these estimates more uncertain.
The results appeared to be broadly similar when we conducted the following structural sensitivity analyses: applying the risk of nursing home admission following hip fracture to vertebral fractures; shorter duration of survival for hip fractures associated with excess mortality; alternative data source for increased risk of fracture following incident fracture; alternative data source for prevalence of prior fracture at baseline; using the same efficacy estimates for oral and i.v. ibandronic acid; reducing the acquisition and administration costs for zoledronic acid; and reducing the fall-off period for zoledronic acid. The results were broadly similar for QFracture but slightly less favourable for FRAX for the sensitivity analysis in which fractures at additional sites were removed from the model and for the sensitivity analysis using hip specific absolute risks to estimate time to hip fracture. The results were more favourable to treatment when assuming full persistence with treatment or when assuming no AEs. The sensitivity analysis examining an AE rate of 30% in the month following initiation of oral bisphosphonate therapy showed that the cost-effectiveness of oral bisphosphonate is very sensitive to the rate of AEs experienced.
The model’s estimates of cost-effectiveness are generalisable to patients eligible for risk assessment under CG146,16 as this is the population we have simulated. However, there are some groups with secondary osteoporosis who may be considered eligible for risk assessment under CG14616 who have not been explicitly simulated within our model. Patients at increased risk of fracture after receiving hormone treatments for breast and prostate cancer have not been explicitly simulated, although patients with the more general risk factor of ‘any cancer’ have been included in the simulated cohort. Patients at increased risk of fracture following untreated premature menopause have not been simulated but the prevalence of HRT usage in female patients has been taken into account within the simulated cohort. We might expect the cost-effectiveness in these groups to be similar to groups with other secondary causes of osteoporosis that have been explicitly modelled, such as steroid-induced osteoporosis, provided the groups who have not been explicitly modelled have an increased risk of fracture and similar life expectancy to other causes of secondary osteoporosis that have been modelled.
We have applied all-cause mortality data from the UK general population to the whole modelled cohort. This may overestimate the cost-effectiveness of treating patients who have higher mortality risks because of the presence of comorbidities and, therefore, the cost-effectiveness estimates may be less generalisable to groups with lower-than-average life expectancy.
One of the strengths of the patient-level simulation approach we have used is that we have been able to simulate how the distribution of patient characteristics, such as age, varies between different risk scores and how this influences the cost-effectiveness of treatment. However, the patient-level simulation approach used required a large number of patients to be simulated because of the scarcity of events in lower-risk populations. This made it difficult to accurately measure the incremental costs and QALYs associated with treatment in the lowest-risk categories when the treatment durations were reduced to reflect real-world persistence with treatment. However, we were able to use non-parametric regression to estimate the relationship between INB and absolute risk across the whole modelled cohort when averaging over both parameter uncertainty and the stochastic uncertainty associated with patient-level simulations. This made it possible to estimate the absolute risk at which the INB crosses zero for each treatment to a more accurate level than could be achieved by simply examining the INBs for each risk category.
Fracture risk prediction within the model is based on the risk predicted over time from the QFracture algorithm, but when validating the model we identified some internal inconsistencies within QFracture, which have implications for our model. The underlying survival function applied in QFracture for patients without any risk factors incorporates a hazard that increases over time. This makes sense, as the hazard for fracture is likely to increase as the patient ages. However, the 1-year risk of fracture predicted for a patient 5 years after their 50th birthday is higher than the 1-year risk of fracture predicted in the following year for a 55-year-old. We have assumed that the data points from the earlier years of the QFracture algorithm are likely to be more reliable than points from later years, when there may have been fewer patients with follow-up in the cohort used to derive the QFracture algorithm. Hippisley-Cox and Coupland18 report that the 2012 QFracture algorithm was based on approximately 23.6 million patient-years of follow-up in approximately 3.1 million patients, suggesting that the mean duration of follow-up was around 7.6 years. We would therefore expect the model predictions to be more robust when used to estimate fracture risk over 5 years than over 10 years. Therefore, we have resampled the patient’s fracture risk every time an event occurs and at 5 and 10 years after baseline in all patients. In doing so we have ensured that we have repeatedly sampled from the early part of the survival curve, which should be less uncertain as it is based on more patients from the QFracture database. This does, however, result in some model behaviour that goes against clinical expectations in that the hazard for an individual patient may be lower in the sixth year of the model than in the fourth year despite the increase in the patient’s age. Unfortunately, there is no way to correct this internal inconsistency while using QFracture as the basis for risk prediction within the model. Introducing more frequent events to update risk at annual intervals would minimise the impact of this internal inconsistency but it would significantly reduce the computational efficiency of the model and would not remove the inconsistency altogether. However, this issue is not expected to bias the estimates of cost-effectiveness as it has an equal impact across all treatment strategies.
Several assumptions had to be made to incorporate the FRAX algorithm within the model. First, the FRAX calculator does not provide estimates of the fracture risk for different time periods. Therefore, we assumed that the shape of the survival curve for fracture-free survival would be similar in FRAX and QFracture and applied a simple ratio to the rate parameter of the QFracture survival curve to generate time-to-event estimates for the FRAX model. The ratio was calculated to ensure that the time-to-event estimates for the FRAX model generated a survival curve with the 10-year risk predicted by the FRAX model. Second, the FRAX algorithm provides the estimate of fracture risk after taking into account the competing risk of mortality, whereas the QFracture algorithm does not incorporate any competing mortality risk. Therefore, we may have underestimated the fracture risk in the FRAX model by applying our own competing mortality hazard on top of that incorporated by FRAX.
The estimates of INB versus absolute fracture risk appear to fall on a slightly higher curve for all treatments when using FRAX than when using QFracture. This difference in the INBs estimated for QFracture and FRAX risk categories with similar levels of absolute fracture risk could have occurred, in part, because of the different risk scores selecting patients with different characteristics into certain risk categories. For example, in the sixth QFracture risk category, which has a mean risk of 2.7%, the proportion of patients with a prior fracture was 41.6%, whereas in the first FRAX risk category, which has a mean risk of 3.1%, the proportion with a prior fracture was 6.4%. As those with a prior fracture have a lower starting utility, it is more cost-effective to prevent future fractures in those without a history of prior fracture. This may explain why the sixth QFracture risk category had lower estimates of INB for bisphosphonate treatment than the first FRAX risk category, despite having similar fracture risks. Furthermore, the structural sensitivity analyses conducted on hip fracture risk and the uplift for fractures at additional sites suggest that the INB of treatment with bisphosphonates compared with a strategy of no treatment may have been overestimated in the base case because of the method used to calculate the survival curve for FRAX from the survival curve for QFracture. We suspect this is because we have assumed that the proportion of major osteoporotic fractures that occur at the hip is the same across both risk tools, but in fact the proportion is lower for FRAX than QFracture in 64% of the modelled population. This suggests that the number of hip fractures may have been overestimated in the model based on FRAX in the base-case analysis.
Our population was sampled taking into account the correlation between age and sex, and the risk factors of prior fracture, steroid use and nursing home residency. The relationship between age, sex and BMI was also incorporated. However, there are likely to be other correlations within the general population that we have not captured. This may mean that the mix of patient characteristics within each decile may not perfectly reflect the mix within each risk category for the population eligible for risk assessment. However, we have tried to capture the correlations between those factors likely to affect risk independently of the absolute risk of fracture, as these have the most potential to bias the estimates of cost-effectiveness.
The model does not allow for patients to move from community living to an institutional residential setting at any time other than following a fracture, which may overestimate the impact of fractures that result in residential care in patients who would have eventually moved into residential care for other reasons. However, the model does allow for patients to live in residential care or to have experienced a prior fracture before being treated with bisphosphonates. This avoids treatment benefits being overestimated in these groups.
The decision to group fractures occurring at additional sites (scapula, clavicle, sternum, rib, pelvis, humeral shaft and femoral shaft) with one of the four main osteoporotic sites (hip, wrist, proximal humerus, vertebral) may have over- or underestimated the impact of fractures at these additional sites if these fractures have different costs and QALY implications from the ones they have been grouped with. However, evidence on the resource-use and HRQoL impactions of fractures was focused on the four main fracture sites associated with osteoporosis, making it difficult to identify site-specific evidence on the consequences of fracture for fractures occurring at other sites.
One of the key limitations of our analysis is that we have assumed that all of the bisphosphonate treatment strategies are viable options for all patients within the population. This allowed us to run the model once for the whole population eligible for risk assessment and to determine a single absolute risk threshold for cost-effective intervention with each bisphosphonate. Applying a strict interpretation of the licensed indications for each bisphosphonate would have required running the analysis multiple times for different groups who have different treatment options, which was not feasible. Although incremental analyses are usually conducted over a set of potentially interchangeable treatments, in reality it is often the case that some of the cohort of patients who are eligible for one treatment would be contraindicated for another and allowances are made for this when interpreting the cost-effectiveness results. For example, it is possible to rank the treatments in order of decreasing net benefit and treat with the next most cost-effective treatment when the optimal treatment is contraindicated.
Another limitation of our analysis is that we have assumed equal treatment effectiveness across all patients eligible for risk assessment under CG146. 16 There was no evidence of differential treatment effects with respect to sex and age. However, there was some heterogeneity in treatment effects between studies, suggesting that differential treatment effects according to study characteristics and the effect of treatment on femoral neck BMD depended on the baseline response.
The cost-effectiveness results for i.v. ibandronic acid should be treated with some caution as the fracture data used were taken from studies which used a daily dose of oral ibandronic acid. This was considered reasonable as i.v. ibandronic acid was licensed based on its non-inferiority in lumbar spine BMD outcomes when compared with the daily ibandronic acid treatment regimen. Any uncertainty regarding whether or not equivalent lumbar spine BMD outcomes do in fact translate into an equivalent reduction in fractures has not been captured within our assessment of model uncertainty.
Our estimates of the costs attributable to fracture do not include the costs of rehabilitation and may therefore underestimate the total cost. They do, however, include costs for home help and residential care which fall within the NHS and PSS perspective recommended in the methods guide. 144
The way in which the DES has been implemented only allows for one acute utility multiplier to be applied at any one time. This may mean that the utility decrement in the year following a severe fracture may be underestimated if another less severe fracture occurs within a year. This may have marginally biased the cost-effectiveness analysis against treatment with bisphosphonates by underestimating the benefits of treatments that prevent hip and vertebral fractures, which have the greatest utility impact in the year following fracture in populations with a high risk of fractures at other sites. However, two events occurring in the same year is expected to be a rare outcome, particularly in lower-risk patients, so any bias is expected to be small.
The model is sensitive to the assumptions regarding AEs, particularly in the low-risk populations, in which the mean absolute cost savings and QALY gains are small. We have included AEs for oral bisphosphonates using the rates observed in prescription event monitoring studies. However, no significant difference in upper GI AEs was found in the placebo-controlled RCTs for oral bisphosphonates. It is unclear whether or not this is because the RCT population are not representative of the real-world population, which may be more likely to experience AEs, or if the apparent increased risk in real-world cohorts is confounded by other factors which are controlled for within a RCT.
Our analysis has used the FRAX calculator for patients with unknown BMD, as CG14616 recommends that patients should receive a BMD scan only if they are close to the treatment threshold and, therefore, the majority of patients are expected to receive treatment without a BMD scan. FRAX also provides an estimate of fracture risk in patients with known BMD. It is possible that the threshold for cost-effective treatment when using the version of the FRAX calculator developed for patients with known BMD may be slightly different if BMD is correlated with patient characteristics that affect risk independently of BMD. However, to properly ascertain whether or not the treatment thresholds would be different, we would need information on the relationships between BMD and a range of other risk factors such as age, sex, prior fracture and steroid use. Including BMD within the model without information on these relationships would simply shuffle patients with similar characteristics between the different risk groups. Although information is available on the relationship between BMD and some of these risk factors, such as age and BMI,237 adding additional but incomplete information on the relationship between the various risk factors and BMD may introduce an unintended bias in the estimates of cost-effectiveness. Given that both the QFracture and FRAX algorithm have been developed for use without BMD, the correlations between the risk factors included in these risk sores and BMD is already incorporated within the calculation of fracture risk. Therefore, we decided not to run the model using the FRAX algorithm for patients with known BMD.
Although the mean INBs for treatment with oral bisphosphonates are positive at low levels of absolute risk, it is important to note that the absolute costs and benefits are small and the no-treatment strategy has a reasonable probability of being optimal until the QFracture score is above approximately 5.5% (the mean absolute risk for the eighth risk category for QFracture). It is therefore possible that patients and clinicians may not consider treatment worthwhile in the lowest-risk patients even though it may be cost-effective.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Clinical guideline 14616 provides recommendations for risk assessment for fragility fracture, including the use of DXA scans, and, therefore, we have not considered the services required to assess fragility fracture risk prior to offering treatment with bisphosphonates. We do not anticipate that any additional services would be required to offer oral bisphosphonate treatment to the population eligible for risk assessment within CG146 as these treatments are prescribed in primary care. Widespread use of zoledronic acid or i.v. ibandronic acid across the population eligible for risk assessment would be likely to result in the requirement for additional capacity in existing services to administer these treatments in secondary care.
We have conducted a simple budget impact analysis to estimate the potential impact on the NHS of changes to current prescribing patterns under certain assumptions. For the purposes of assessing the budget impact we have assumed that bisphosphonate treatment with weekly alendronic acid is offered to all patients who have a QFracture score of > 1.5%, but that uptake is gradual, with only one-fifth of the patients eligible for treatment starting treatment each year over the next 5 years. Alendronic acid has been chosen as it is neither the cheapest nor the most expensive oral bisphosphonate. The generic weekly alendronic acid preparation has been assumed to be prescribed in all patients as it is both the lowest cost and currently the most commonly prescribed treatment (see Table 1). A QFracture score of 1.5% has been chosen as the threshold for offering treatment as this was the lowest absolute risk at which the INB for any bisphosphonate compared with no treatment was positive when valuing a QALY at £20,000. The economic model simulates a population aged ≥ 30 years and selects from this population the cohort eligible for risk assessment. Therefore, it also provides an estimate of the proportion of the general population aged > 30 years who would be eligible for risk assessment. The model estimates that for every 100,000 patients who are eligible for risk assessment there are another 63,763 who are not eligible for risk assessment and, therefore, 61% of the general population are eligible. Combining this with information on the number of people aged > 30 years in England from the ONS (33.7 million)159 allows the calculation of the number of people eligible for risk assessment (20.6 million). From the characteristics of 200,000 simulated patients we have estimated that 61% of those eligible for risk assessment have a QFracture score of > 1.5%. We have assumed that the treatment duration is 6 months, as this was the treatment duration applied in the cost-effectiveness model for oral bisphosphonates based on observational data on the average persistence with treatment. Using these assumptions, the total undiscounted cost of treating the current prevalent population is estimated to be £95M over 5 years.
Data from the prescription cost analysis suggest that there are currently 8.3 million prescriptions per annum for oral bisphosphonate treatment in primary care, at an estimated cost of £10M per annum. 38 For this cost estimate we applied the cost for generic preparations for each dose to make the figures comparable with those above, where generic prescribing was assumed. Over 5 years the undiscounted cost for oral bisphosphonate treatment at the current level of prescribing is estimated to be £50M. 38
Therefore, we estimate that if all patients with a QFracture score of > 1.5% were prescribed oral bisphosphonates, this could double the current cost of bisphosphonate prescribing over the next 5 years. These estimates are provided to give an indication of the maximum cost of additional prescribing with costs likely to be lower if uptake is less than 100%. Costs would also be expected to fall once the prevalent population eligible for treatment have been treated as the numbers becoming eligible for treatment each year will be smaller than the current population who are eligible. Furthermore, some of those whom we have included in the eligible population will already have received bisphosphonate treatment, which would further reduce the numbers likely to initiate treatment in the next 5 years. Therefore, our estimates provide an upper ceiling on the expected costs.
Chapter 6 Discussion
Statement of principal findings
Principal findings: clinical effectiveness
A total of 46 RCTs were identified that provided data for the clinical effectiveness systematic review. Alendronic acid was compared with placebo in 17 RCTs. A daily dose of 2.5 mg of oral ibandronic acid (dose no longer licensed) was compared with placebo in three RCTs and with i.v. administration in one RCT. Daily administration of 2.5 mg of oral ibandronic acid was compared with 150 mg per month of oral ibandronic acid administration in one RCT. Risedronic acid was compared with placebo in 12 RCTs and zoledronic acid was compared with placebo in four RCTs. One RCT compared alendronic acid with 150 mg per month of oral ibandronic acid, five RCTs compared alendronic acid with risedronic acid, one RCT compared zoledronic acid with alendronic acid and one RCT compared zoledronic acid with risedronic acid. The maximum trial duration was 48 months.
The risk of bias associated with the included RCTs was assessed using the Cochrane risk-of-bias instrument. An attrition bias of 10% across treatment groups was evident for 29 (63%) of the included RCTs. Five trials were reported as either open label or single blind and were considered at high risk of performance bias. Blinded outcome assessment was reported by only 13 (28%) trials.
The outcome measures prespecified in the final NICE scope23 were addressed by the included trial evidence to varying degrees. Femoral neck BMD was the most widely reported outcome and fracture was the second most widely reported outcome. AEs were reported by the majority of included trials. Across the included trials there was limited reporting on outcomes of compliance (adherence and persistence), hospitalisation and service use, and quality of life.
A total of 27 RCTs provided suitable fracture data for inclusion in the fracture NMA: nine compared alendronic acid with placebo; two compared 150 mg per month of oral ibandronic acid with placebo; one compared 2.5 mg per day of oral ibandronic acid with placebo; nine compared risedronic acid with placebo; three compared zoledronic acid with placebo; one compared alendronic acid with risedronic acid; one compared 150 mg per month of oral ibandronic acid with alendronic acid; and one compared zoledronic acid with risedronic acid.
A total of 35 RCTs provided suitable femoral neck BMD data for inclusion in the BMD NMA: 12 compared alendronic acid with placebo; one compared 2.5 mg per day of oral ibandronic acid with placebo; one compared 150 mg per month of oral ibandronic acid with placebo; one compared 2.5 mg per day of oral ibandronic acid with 3 mg every 3 months of i.v. ibandronic acid; one compared 2.5 mg per day of oral ibandronic acid with 150 mg per month of oral ibandronic acid; 10 compared risedronic acid with placebo; four compared zoledronic acid with placebo; three compared alendronic acid with risedronic acid; one compared alendronic acid with 150 mg per month of oral ibandronic acid; and one compared zoledronic acid with risedronic acid.
Bone mineral density may be considered a surrogate for fracture outcomes. Analysis of the femoral neck BMD data was of interest in order to confirm the direction of treatment effects. As more studies presented data on femoral neck BMD than any of the individual fracture outcome types, the network analysis also provides more information for assessing treatment effect modifiers.
All treatments were associated with beneficial effects on fractures and femoral neck BMD relative to placebo. HRs for fractures varied from 0.41 to 0.92 depending on treatment and fracture site. For vertebral fractures and percentage change in femoral neck BMD the treatment effects were also statistically significant at a conventional 5% level for all treatments. Pairwise comparisons between treatments indicated that no active treatment was statistically significantly more effective than any other active treatment for fracture outcomes. For vertebral fractures and percentage change in femoral neck BMD, the greatest effect was for zoledronic acid, although in general the ranking of treatments varied for the different outcomes, with the treatments providing broadly similar effects. There was no evidence to suggest different treatment effects according to age or sex.
Assessment of vertebral fractures was based on both clinical and morphometric fractures. Ideally, the effect of assessment method would be assessed through metaregression; however, data for clinical fractures were limited. An analysis of the studies reporting clinical fractures did not provide any evidence to suggest differential treatment effects according to assessment method, although the evidence was limited.
The main analyses were based on a class-effects model such that the bisphosphonates are assumed to be related but not identical. The treatment effects estimated using the class-effects model were broadly similar qualitatively (i.e. direction of effect) and quantitatively (i.e. magnitude of effect) to those estimated using the standard random-effects model but with the treatments effects in the class-effects model shrunk towards the overall bisphosphonate treatment effect. The qualitative effects of treatment (i.e. direction of effect) were the same for the majority of outcome types and treatments from the class effects and standard random-effects models with the exception of zoledronic acid (hip fractures), 150 mg per month of oral ibandronic acid (hip and wrist fractures) and 2.5 mg per day of oral ibandronic acid (non-vertebral fractures). Although the point estimates changed from being relative increases in effect in the standard random-effects model to relative decreases in effect in the class-effects model, there was considerable uncertainty about the true effects as reflected in the CrIs.
Non-vertebral fractures are used as proxy for fractures of the proximal humerus, as this outcome is not commonly reported. Two studies presented results for proximal humerus fractures, both considering the effects of risedronic acid against placebo. 70,85 A random-effects meta-analysis of these two studies provided a HR of 0.45 (95% CrI 0.13 to 1.41), which was greater than that estimated for non-vertebral fractures but with considerably more uncertainty.
There were no statistically significant differences between treatments in the incidence of upper GI events associated with any oral bisphosphonate (alendronic acid, risedronic acid or ibandronic acid) compared with placebo when data were pooled across RCTs for each bisphosphonate. However, evidence from one RCT indicated a significantly higher risk of upper GI events in men receiving risedronic acid than in those receiving placebo. Where reported across the RCTs, treatments were prescribed in accordance with the SmPC for oral bisphosphonates to minimise gastric irritation. There was no evidence of significant differences between treatments in mortality across the RCT evidence when data were pooled by bisphosphonate. However, evidence from one RCT indicated that the proportion of men and women dying following hip fracture was significantly higher among those receiving placebo than among those receiving zoledronic acid. There was also no evidence of significant differences between treatments in participants withdrawing because of AEs across the RCT evidence when data were pooled by bisphosphonate. However, evidence from one RCT indicated that the proportion of men withdrawing because of AEs was significantly higher in the alendronic acid group than in the placebo group.
In agreement with the SmPC, there was evidence of influenza-like symptoms associated with zoledronic acid. There was no statistically significant difference in the incidence of atrial fibrillation associated with zoledronic acid compared with placebo (one RCT) or risedronic acid (one RCT). There was no statistically significant difference in the incidence of bone pain associated with zoledronic acid compared with placebo (one RCT) or alendronic acid (one RCT). There was evidence that the risk of eye inflammation in the first 3 days following drug administration was significantly higher for zoledronic acid than for placebo (one RCT). Single RCT evidence indicated no statistically significant difference between zoledronic acid and placebo in the incidence of stroke over 36 months. No RCT evaluating zoledronic acid reported any case of spontaneous osteonecrosis of the jaw in any treatment group during the trial period.
Adverse events of hypocalcaemia and atypical femoral fracture were not reported outcomes by any RCT of any bisphosphonate.
A summary of evidence from systematic reviews that include observational data indicates that the rates of GI toxicity associated with alendronic acid, risedronic acid and oral ibandronic acid are similar to that observed in placebo-treated participants. However, prescription event monitoring study data suggest a high level of reporting of a number of conditions in the first month of therapy with alendronic acid or risedronic acid, particularly those affecting the upper GI tract. Retrospective cohort data also suggest that switching patients who are stabilised on risedronic acid to alendronic acid is associated with an increased risk of GI AEs. Zoledronic acid may be compromised by renal toxicity, and myalgias and arthralgias are evident in the acute phase following i.v. administration. Intravenous bisphosphonates, especially zoledronic acid, are more likely to predispose patients to osteonecrosis of the jaw; however, in addition to bisphosphonate use, there appears to be several other factors involved in the development of osteonecrosis of the jaw (e.g. dental trauma). There is an increased risk of atypical fracture among bisphosphonate users; however, events are rare and long-term bisphosphonate therapy might not be a prerequisite for development of atypical fractures. Moreover, the use of glucocorticoids and proton pump inhibitors is a potentially important risk factor for atypical fracture. Bisphosphonates are associated with serious atrial fibrillation, but heterogeneity of the existing evidence and a paucity of information on some agents preclude any definitive conclusions with respect to risk. The review evidence for the use of bisphosphonates and oesophogeal cancer is equivocal.
Evidence for persistence and adherence reported by RCTs was very limited. Where reported, high levels of compliance, reported as a pill count, were evident over the trial duration. A summary of evidence from systematic reviews including observational data indicates that, although patients using weekly bisphosphonate medication follow their prescribed regimens better than those using daily therapy, overall compliance and persistence rates are suboptimal for postmenopausal women receiving bisphosphonate therapy for the treatment of osteoporosis. Furthermore, one-third to one-half of patients, including men being treated with bisphosphonates for osteoporosis, do not take their medication as directed.
With the exception of the RCTs evaluating bisphosphonates in steroid users, the majority of trials included in the clinical effectiveness systematic review typically excluded patients with underlying conditions or receiving medications that affect bone metabolism. Furthermore, patients with history of, or receiving medication for, upper GI tract disorders were also excluded by the majority of included trials. Therefore, the effects of alendronic acid, ibandronic acid, risedronic acid and zoledronic acid are unknown in these populations.
Principal findings: cost-effectiveness
The de novo economic model estimates that a strategy of no treatment is predicted to have the greatest net benefit for patients with an absolute risk of < 1.5% when using QFracture to estimate absolute risk and valuing a QALY at £20,000. Alendronic acid is predicted to have the maximum INB from 1.5% to 7.2% and risedronic acid is predicted to have the maximum INB from 7.2% upwards. However, the absolute costs and QALY gains are small in patients with low absolute risk, and the PSA suggested that there is considerable uncertainty regarding whether or not no treatment is the optimal strategy until the QFracture score is approximately 5.5% (the mean absolute risk for the eighth risk category for QFracture).
The mean INBs for oral bisphosphonate treatment (alendronic acid, risedronic acid or ibandronic acid) compared with no treatment were positive across all FRAX risk categories. An exact threshold for the absolute risk at which the INB became positive was therefore not available, but the minimum FRAX score in the modelled population was 1.2% and the lowest risk category had a mean absolute risk of 3.1%. Oral ibandronic acid is predicted to have the highest INB compared with no treatment up to 8.6%, with alendronic acid having the highest INB from 8.6% to 38.5% and risedronic acid having the maximum INB > 38.5%. The PSA suggested that there was a low probability of the no-treatment strategy being optimal across all FRAX risk categories when valuing a QALY at £20,000. However, the PSA also demonstrated that there is considerable uncertainty regarding the optimal bisphosphonate treatment with all of the oral bisphosphonates having reasonably similar probabilities of having maximum INB across most of the FRAX risk categories.
Intravenous bisphosphonates (ibandronic acid and zoledronic acid) were predicted to have lower INBs than oral bisphosphonates across all levels of absolute risk when estimated using either QFracture or FRAX. In the highest-risk categories the ICERs for i.v. ibandronic acid and zoledronic acid compared with oral bisphosphonates were consistently > £50,000 per QALY even though the base-case analysis assumed longer durations of persistence for i.v. bisphosphonates than oral bisphosphonates. Although the mean INB compared with no treatment for i.v. ibandronic acid did become positive at very high levels of absolute risk when using QFracture, the results when using FRAX went in the opposite direction. This may be because of the small number of patients and parameter samples informing the estimates at high levels of absolute risk which makes these estimates more uncertain.
The results appeared to be broadly similar across the majority of the structural sensitivity analyses which examined the application of alternative data or assumptions. The results were more favourable to treatment when assuming full persistence with treatment for the intended treatment duration (3 years for zoledronic acid and 5 years for all other bisphosphonates) or when assuming no AEs. The sensitivity analysis examining an AE rate of 30% in the month following initiation of oral bisphosphonate therapy showed that the cost-effectiveness of oral bisphosphonates is very sensitive to the rate of AEs experienced. The INBs compared with no treatment fell below zero (when valuing a QALY at £20,000) for all 10 QFracture risk categories and for all but the highest FRAX risk category when assuming an AE rate of 30% in the first month of oral bisphosphonate treatment.
The structural sensitivity analyses, which varied the way in which the fracture risk was estimated, showed results that were broadly similar for QFracture but slightly less favourable for FRAX, which brought the cost-effectiveness estimates from the QFracture and FRAX model closer together for patients with similar mean absolute risk. We would expect from the way the model is structured that the threshold for cost-effective treatment would be broadly similar across the two risk scores but in the base-case scenario the INBs of bisphosphonates compared with no treatment were higher for FRAX than QFracture for risk categories with similar absolute fracture risk. The fact that the results are similar in these particular structural sensitivity analyses suggests that the base-case analysis may have overestimated the proportion of fractures occurring at the hip for the FRAX model. We suspect this is because we have assumed that the proportion of major osteoporotic fractures which occur at the hip is the same across both risk tools but, in fact, the proportion is lower for FRAX than QFracture in 64% of the modelled population.
Some of the difference in the INBs estimated for QFracture and FRAX risk categories with similar levels of absolute fracture risk may also have occurred because of the different risk scores selecting patients with different characteristics who have different consequences of fracture into risk categories with a similar absolute fracture risk.
Strengths and limitations of the assessment
The clinical effectiveness systematic review was based on rigorous methods, with comprehensive searches for evidence, a good level of consistency between reviewers in study selection and double-checking of data extraction. A formal assessment of methodological quality of included trial was undertaken. Attrition of ≥ 10% across treatment groups was evident for 63% of the included RCTs.
Fracture data were reported for 27 (59%) of the 46 included RCTs and femoral neck BMD data were reported for 35 (76%). However, for fracture data there was variability across the included trials in the skeletal fracture site evaluated, the most frequently evaluated being vertebral fracture. In addition, femoral neck BMD was summarised in study reports as the percentage change from baseline, which is a relative measure of treatment effect and tends to have poor statistical properties. Ideally, for a continuous outcome measure assessed at baseline and post treatment, we would work with the post-treatment response adjusted for baseline in an analysis of covariance.
Network meta-analyses were used to synthesise the evidence to permit a coherent comparison of the efficacy of interventions in terms of fracture and femoral neck BMD. An assumption of the model is that the studies are exchangeable in the sense that we would be prepared to treat any patient in the population with all of the treatments. However, not all treatments are licensed in all patient populations, which means that the studies are not exchangeable, although the analysis follows the scope defined by NICE. 23
Adverse event data were widely reported and also supplemented by review evidence of observational data. However, evidence for compliance and concordance was limited in the RCT evidence base and, where reported, was reported as being assessed mainly through pill counts. Evidence for compliance and treatment persistence was limited to review evidence of observational data.
In summary, fracture, BMD and AE data were widely reported. However, these data were limited as there was variability across the included trials in the skeletal fracture site evaluated with a limited number of trials reporting data for the hip. Summary statistics for BMD were not provided by all trials and were extracted from graphical representations. Furthermore, the majority of RCTs were placebo-controlled trials with a limited number of head-to-head comparison trials.
Although the search strategy for this assessment report was comprehensive, the possibility of a publication bias cannot be discounted. A formal assessment of publication bias was not undertaken.
The majority of included trials typically excluded patients with underlying conditions or receiving medications that affect bone metabolism. Furthermore, patients with a history of or who were currently receiving medication for upper GI tract disorders were also excluded by the majority of included trials; therefore, the effects of alendronic acid, ibandronic acid, risedronic acid and zoledronic acid are unknown in these populations.
None of the consultee submissions included a de novo economic evaluation and none of the published economic evaluations compared all five bisphosphonate treatment regimens specified within the scope of this appraisal in a fully incremental analysis as required by the NICE reference case.
The patient-level simulation approach used in the Assessment Group’s model allowed the distribution of patient characteristics to differ across the risk categories providing estimates of cost-effectiveness that have taken into account the differing consequences of fracture in patients with different characteristics. However, the DES modelling approach provides a stochastic estimate of the costs and QALYs gained. We therefore needed to simulate a large number of patients to obtain stable estimates of the cost and benefits of treatment. This was particularly true in the lower-risks groups in the base-case scenario where we reduced the treatment duration to reflect evidence from observational studies on the duration of persistence with bisphosphonate treatment. In order to obtain stable estimates of the costs and QALYs at differing levels of absolute risk, we had to group the patients into broad risk categories. A full incremental analysis has been conducted for each risk category and CEACs have also been provided allowing the uncertainty in the cost-effectiveness to be assessed at different levels of absolute risk. We have also used a non-parametric regression to estimate the relationship between INB and absolute risk across the whole population eligible for risk assessment in CG146. 16 From this we have identified treatment thresholds for each treatment for both QFracture and FRAX.
The model generally adheres to the NICE reference case and fully addresses the decision problem set out in the final NICE scope. In particular, the modelling approach used allows intervention thresholds to be linked to absolute risk measured using the two risk assessment tools recommended in CG146 as specified in the scope. 23 However, in order to provide a single intervention threshold for each treatment that could be applied across the whole population, we had to assume that all of the bisphosphonate treatment strategies were viable treatment options across all patients eligible for risk assessment within CG146. This would not be true if the licensed indications for each intervention were to be strictly applied.
The de novo economic model is underpinned by a NMA across all drug options which provides a coherent synthesis of the evidence within a single model. Where appropriate and possible, systematic search methods have been used to identify evidence to inform the model’s parameters (efficacy evidence and HRQoL). However, it was not feasible to conduct a full systematic review to identify evidence to inform all model parameters and, therefore, published cost-effectiveness models and published systematic reviews were used to identify appropriate sources of evidence for some model parameters. It is possible that if we had searched systematically we may have found other more appropriate data sources for some model parameters but it is not possible to say whether or not this would have changed the model results.
The main limitations of the economic analysis relate to the assumptions required to populate the model given the data available. In particular, several assumptions were necessary to generate estimates of time to fracture for each fracture type from the estimates of absolute risk provided by the QFracture and FRAX tools.
Uncertainties
Although differential effects were found when comparing the bisphosphonates with placebo, and the effects of the bisphosphonates were generally similar, there was uncertainty about the true treatment effects and some evidence of heterogeneity in treatment effects between studies.
It is uncertain whether or not the cost-effectiveness of bisphosphonate treatment at a particular level of absolute fracture risk would be similar for patients who have been assessed using the FRAX algorithm for patients with known BMD.
Other relevant factors
Although the mean INBs for treatment with oral bisphosphonates are positive at low levels of absolute risk, it is important to note that the absolute costs and benefits are small and the no-treatment strategy has a reasonable probability of being optimal until the QFracture score is above approximately 5.5% (the mean absolute risk for the eighth risk category for QFracture). Therefore, it is possible that patients and clinicians may not consider treatment worthwhile in the lowest-risk patients even though it may be cost-effective.
Chapter 7 Conclusions
All treatments were associated with beneficial effects relative to placebo with HRs for fracture varying from 0.41 to 0.92 depending on treatment and fracture site. For vertebral fractures and percentage change in BMD, the treatment effects were also statistically significant for all treatments. For non-vertebral fractures, the treatment effects were statistically significant at a conventional 5% level for risedronic acid, alendronic acid and zoledronic acid. For the outcomes of hip fracture and wrist fracture, all treatments were associated with beneficial treatment effects relative to placebo, although the treatment effects were not statistically significant at a conventional 5% level. Pairwise comparisons between treatments indicated that no active treatment was significantly more effective than other active treatments for fracture outcomes. For vertebral fractures and percentage change in BMD, the greatest effect was for zoledronic acid, although, in general, the ranking of treatments varied for the different outcomes, with the treatments providing broadly similar effects.
For the majority of AEs reported in RCTs no significant difference was found between active treatment and placebo, suggesting that bisphosphonates are generally well tolerated in patients enrolled within clinical trials. Prescription event monitoring study data suggest a high level of reporting of a number of conditions in the first month of therapy with alendronic acid or risedronic acid, particularly those affecting the upper GI tract, suggesting that oral bisphosphonates may be less well tolerated in clinical practice. A significant difference in the incidence of influenza-like symptoms was identified from the RCTs for zoledronic acid compared with placebo, although clinical advice was that these symptoms are generally limited to the first dose and usually last only a few days.
Continuance and concordance data were limited for the RCT evidence evaluated. Supplementary review evidence of observational data indicates that, although patients using weekly bisphosphonate medication follow their prescribed regimens better than those using daily therapy, overall compliance and persistence rates are suboptimal for postmenopausal women.
The de novo economic model estimates that when using QFracture to estimate absolute risk, a strategy of no treatment is predicted to have the greatest net benefit, when valuing a QALY at £20,000, in the lowest-risk patients (QFracture absolute risk < 1.5%), with oral bisphosphonates (alendronic acid, risedronic acid, ibandronic acid) having the greatest INB at higher levels of absolute risk. However, the absolute costs and QALY gains are small in patients with low absolute risk and the PSA suggested that there is considerable uncertainty regarding whether or not no treatment is the optimal strategy until the QFracture score is approximately 5.5% (the mean absolute risk for the eighth risk category for QFracture). Therefore, it is possible that patients and clinicians may not consider treatment worthwhile in the lowest-risk patients even though it may be cost-effective.
The mean INBs compared with no treatment (when valuing a QALY at £20,000) were positive for all oral bisphosphonate treatments across all FRAX risk categories. However, in the base-case scenario the INBs of bisphosphonate treatments compared with no treatment were generally higher for FRAX than for QFracture for risk categories with similar absolute fracture risk. We would expect from the way the model is structured that the threshold for cost-effective treatment would be broadly similar across the two risk scores. The results of two structural sensitivity analyses suggest that the base-case analysis may have overestimated the INBs of treatment in the model based on FRAX because of the assumption that the proportion of major osteoporotic fractures that occur at the hip is the same for FRAX and QFracture. Given this possible bias in the estimates generated by the model using the FRAX absolute risk estimates, and our belief that the results should be broadly similar across the two risk scores, it would be reasonable to assume that the absolute risk thresholds estimated in the QFracture model could be applied to patients whose score had been calculated using either QFracture or FRAX.
The de novo economic model suggests that the cost-effectiveness of i.v. bisphosphonates (ibandronic acid and zoledronic acid) is less favourable than for oral bisphosphonates with a negative INB (when valuing a QALY at £20,000) than no treatment estimated for both i.v. bisphosphonates across all 10 risk categories for both FRAX and QFracture.
Implications for service provision
The prescribing of oral bisphosphonates in patients who have already received risk assessment under CG146 is not anticipated to have any major implications for service provision as these can be prescribed in primary care. If i.v. bisphosphonates were to be widely prescribed across the population eligible for risk assessment under CG146, it is likely that additional capacity would be required in existing services to administer these treatments in secondary care.
Suggested research priorities
Given that the cost-effectiveness results are sensitive to the assumptions regarding the rate of AEs for oral bisphosphonates, further research to quantify both the incidence of AEs and the impact of those AEs on HRQoL and treatment persistence would allow patients and clinicians to make better-informed decisions regarding the balance of costs, benefits and AEs. Although further RCTs evaluating efficacy and tolerability of bisphosphonates as well as assessing HRQoL and persistence could be recommended, data on the relationships between AEs and HRQoL alongside persistence could also be evaluated through observational study design.
We identified only a limited number of RCTs in men. There was evidence from single RCTs in men which showed a significant increase in upper GI AEs and withdrawals because of AEs than those receiving placebo. Further research to assess efficacy and tolerability of bisphosphonate treatment in men may be beneficial.
The existing economic model could be extended to include non-bisphosphonate treatments, as these are potential alternatives to bisphosphonate therapy in some patients. Non-bisphosphonates were not included as comparators in the economic model, as the scope for this NICE MTA stated that non-bisphosphonates licensed for the prevention of fragility fractures in women and men would be considered in a separate MTA. The economic model could also be extended to consider the cost-effectiveness of second-line treatments in patients who experience fractures while being treated with a bisphosphonate.
Given that an individual’s risk usually increases with age, and bisphosphonate treatment is not usually continued indefinitely, it is currently unclear whether or not treatment should be given as soon as an individual’s increased fracture risk is identified or whether treatment should be delayed so that the patient has the full benefit of treatment during the period that they are at greatest risk. The economic model could also be adapted to assess the optimal timing of bisphosphonate therapy in patients whose life expectancy exceeds the anticipated duration of treatment effect.
The economic analysis presented here focused on examining how cost-effectiveness varies with absolute fracture risk. This was done in order to meet the requirement laid out in the scope to link absolute fracture risk with intervention thresholds, based on cost-effectiveness. However, further work could be done to explore how cost-effectiveness varies across the cohort of patients at risk of fragility fracture and whether or not any factors other than absolute fracture risk could be used to select patients who can be treated cost-effectively.
Acknowledgements
We would like to thank Professor Eva Kaltenthaler [School of Health and Related Research (ScHARR), University of Sheffield] for commenting on the draft assessment report. Thanks also to Shijie Ren (ScHARR, University of Sheffield) for providing statistical analysis support and Gill Rooney (ScHARR, University of Sheffield) for providing project administration support.
Contributions of authors
Sarah Davis (Senior Lecturer, ScHARR) acted as principal investigator for this assessment.
Marrissa Martyn-St James (Research Fellow, ScHARR) undertook the clinical effectiveness systematic review.
Jean Sanderson (Research Associate, ScHARR) conducted the NMA.
John Stevens (Reader in Decision Science, ScHARR) wrote the methods of analysis/synthesis section of the protocol, co-ordinated the evidence synthesis, and reviewed and commented on the draft report.
Edward Goka (Research Associate, ScHARR) undertook the clinical effectiveness systematic review.
Sarah Davis, Andrew Rawdin (Health Economic Modeller, ScHARR) and Susi Sadler (Research Associate, ScHARR) undertook the health economic review and developed the Assessment Group’s model.
Ruth Wong (Information Specialist, ScHARR) carried out the electronic searches.
Fiona Campbell (Research Fellow, ScHARR) undertook data checking and contributed to the clinical effectiveness review.
Matt Stevenson (Professor of Health Technology Assessment, ScHARR) provided guidance on the design of the model and commented on the draft report.
Mark Strong (Clinical Senior Lecturer in Public Health, ScHARR) conducted the non-parametric regression on the model outputs.
Peter Selby (Honorary Clinical Professor of Metabolic Bone Disease, University of Manchester, Manchester Royal Infirmary) and Neil Gittoes (Consultant and Honorary Professor of Endocrinology) University of Birmingham) provided clinical advice and commented on the draft assessment report.
Publication
Sanderson J, Martyn-St James M, Stevens J, Goka E, Wong R, Campbell F, et al. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: a systematic review and network meta-analysis. Bone 2016;89:52–8.
Data sharing statement
Data can be obtained from the corresponding author subject to their being non-confidential.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group. WHO Technical Report Series, No. 843. Geneva: WHO; 1994.
- Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consens Statement. Washington, DC: United States Department of Health and Human Services; 2000.
- Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for osteoporosis. Osteoporos Int 1999;10:259-64. http://dx.doi.org/10.1007/s001980050224.
- Rizzoli R, Bonjour JP. Determinants of peak bone mass and mechanisms of bone loss. Osteoporos Int 1999;9:S17-23. http://dx.doi.org/10.1007/PL00004155.
- Bonjour JP, Theintz G, Law F, Slosman D, Rizzoli R. Peak bone mass. Osteoporosis Int 1994;4:S7-13. http://dx.doi.org/10.1007/BF01623429.
- Harris S, Dawson-Hughes B. Rates of change in bone mineral density of the spine, heel, femoral neck and radius in healthy postmenopausal women. Bone Miner 1992;17:87-95. http://dx.doi.org/10.1016/0169-6009(92)90713-N.
- Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev 1995;16:87-116. http://dx.doi.org/10.1210/edrv-16-1-87.
- Gauthier A, Kanis J, Jiang Y, Martin M, Compston J, Borgström F, et al. Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: estimations from a disease model. Arch Osteoporos 2011;6:179-88. http://dx.doi.org/10.1007/s11657-011-0063-y.
- Wade SW, Strader C, Fitzpatrick LA, Anthony M, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos 2014;9:1-10. http://dx.doi.org/10.1007/s11657-014-0182-3.
- Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 2006;17:1404-9. http://dx.doi.org/10.1007/s00198-006-0135-9.
- Marsh D, Currie C, Brown P, Cooper A, Elliott J, Griffiths R, et al. The Care of Patients with Fragility Fractures. Birmingham: British Orthopaedic Association; 2003.
- Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2011;6:59-155. http://dx.doi.org/10.1007/s11657-011-0060-1.
- National Osteoporosis Society . Facts and Figures 2009. www.nos.org.uk/page.aspx?pid=328 (accessed 14 August 2014).
- Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporosis fractures in the UK: projections for 2000–2020. J Med Econ 2005;4:51-62. http://dx.doi.org/10.3111/200104051062.
- Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, et al. Guideline for the Diagnosis and Management of Osteoporosis in Postmenopausal Women and Men from the Age of 50 years in the UK. Sheffield: University of Sheffield; 2014.
- National Institute for Health and Care Excellence (NICE) . Osteoporosis: Assessing the Risk of Fragility Fracture 2012. www.nice.org.uk/guidance/cg146 (accessed 23 September 2014).
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008;19:385-97. http://dx.doi.org/10.1007/s00198-007-0543-5.
- Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3427.
- Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFracture scores. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4229.
- National Collaborating Centre for Nursing and Supportive Care . Systematic Reviews of Clinical Effectiveness Prepared for the Guideline: ‘Osteoporosis: Assessment of Fracture Risk and the Prevention of Osteoporotic Fractures in Individuals at High Risk’ 2008. www.nice.org.uk/guidance/cg146/resources/osteoporosis-evidence-reviews2 (accessed 23 March 2015).
- National Institute for Health and Care Excellence (NICE) . Alendronate, Etidronate, Risedronate, Raloxifene and Strontium Ranelate for the Primary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women 2008. www.nice.org.uk/guidance/ta160 (accessed 22 March 2016).
- National Institute for Health and Care Excellence (NICE) . Denosumab for the Prevention of Osteoporotic Fractures in Postmenopausal Women 2010. www.nice.org.uk/guidance/ta204 (accessed 23 September 2014).
- National Institute for Health and Care Excellence (NICE) . Bisphosphonates for Preventing Osteoporotic Fragility Fractures (Including a Partial Update of NICE Technology Appraisal Guidance 160 and 161): Final Scope 2014. www.nice.org.uk/guidance/indevelopment/gid-tag462/documents (accessed 23 September 2014).
- National Institute for Health and Care Excellence (NICE) . Alendronate, Etidronate, Risedronate, Raloxifene, Strontium Ranelate and Tteriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women 2008. www.nice.org.uk/guidance/ta161 (accessed 23 March 2015).
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 2013;8:1-115.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- National Institute for Health and Care Excellence (NICE) . Osteoporosis Overview – NICE Pathway 2014. http://pathways.nice.org.uk/pathways/osteoporosis (accessed 12 November 2014).
- National Institute for Health and Care Excellence (NICE) . Fragility Fracture Risk Assessment – NICE Pathway 2014. http://pathways.nice.org.uk/pathways/osteoporosis#path+=+view%3A/pathways/osteoporosis/fragility-fracture-risk-assessment.xml&content+=+view-index&path=view%3A/pathways/osteoporosis/fragility-fracture-risk-assessment.xml&content=view-index (accessed 12 February 2016).
- Idris A, Rojas J, Greig I, Van’t Hof R, Ralston S. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 2008;82:191-20. http://dx.doi.org/10.1007/s00223-008-9104-y.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Electronic Medicines Compendium . Summary of Product Characteristics for Zoledronic Acid SUN 5 mg Solution for Infusion 2014. www.medicines.org.uk/emc/medicine/28527 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Alendronic Acid 10 mg Tablets 2014. www.medicines.org.uk/emc/medicine/28959 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Alendronic Acid 70 mg Tablets 2014. www.medicines.org.uk/emc/medicine/23733 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Ibandronic Acid 150 mg Film-Coated Tablets 2014. www.medicines.org.uk/emc/medicine/26568 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Ibandronic Acid 3 mg Solution for Injection 2014. www.medicines.org.uk/emc/medicine/27050 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Risedronate Sodium 5 mg Film-Coated Tablets 2013. www.medicines.org.uk/emc/medicine/27565 (accessed 12 November 2014).
- Electronic Medicines Compendium . Summary of Product Characteristics for Risedronate Sodium 35 mg Film-Coated Tablets 2014. www.medicines.org.uk/emc/medicine/25017 (accessed 12 November 2014).
- Prescribing and Primary Care Team Health and Social Care Information Centre . Prescription Cost Analysis: England 2013 2014. www.hscic.gov.uk/ (accessed 23 February 2015).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Haymarket Medical Media . MIMS 2015. www.mims.co.uk (accessed 7 January 2015).
- Hospira UK Ltd . Hospira Product Catalogue 2014. www.hospira.co.uk/en/products_and_services/catalog (accessed 7 January 2015).
- Hospital Prescribing: England 2012. Leeds: Health and Social Care Information Centre; 2013.
- Preferred Reporting Items for Systematic Reviews and Meta-analyses . Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2009. www.prisma-statement.org/index.htm (accessed 23 March 2015).
- Martyn-St James M. Bisphosphonates for Preventing Osteoporotic Fragility Fractures (Including a Partial Update of NICE Technology Appraisal Guidance 160 and 161): Protocol Record CRD42014014436 2013. www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42014014436 (accessed 23 October).
- Chesnut CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241-9. http://dx.doi.org/10.1359/JBMR.040325.
- Chesnut CH, Ettinger MP, Miller PD, Baylink DJ, Emkey R, Harris ST, et al. Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONE. Curr Med Res Opin 2005;21:391-40. http://dx.doi.org/10.1185/030079905X30752.
- Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE Study. J Bone Miner Res 2005;20:1315-22. http://dx.doi.org/10.1359/JBMR.050313.
- Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 2006;65:654-61. http://dx.doi.org/10.1136/ard.2005.044958.
- Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 2006;54:1838-46. http://dx.doi.org/10.1002/art.21918.
- Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008;35:488-97.
- Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Wright CC, Sim J. Intention-to-treat approach to data from randomized controlled trials: a sensitivity analysis. J Clin Epidemiol 2003;56:833-42. http://dx.doi.org/10.1016/S0895-4356(03)00155-0.
- Adami S, Passeri M, Ortolani S, Broggini M, Carratelli L, Caruso I, et al. Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone 1995;17:383-90. http://dx.doi.org/10.1016/S8756-3282(95)00262-6.
- Atmaca A, Gedik O. Effects of alendronate and risedronate on bone mineral density and bone turnover markers in late postmenopausal women with osteoporosis. Adv Ther 2006;23:842-53. http://dx.doi.org/10.1007/BF02850205.
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-41. http://dx.doi.org/10.1016/S0140-6736(96)07088-2.
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-22. http://dx.doi.org/10.1056/NEJMoa067312.
- Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, et al. Alendronate and estrogen effects in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab 2000;85:720-6. http://dx.doi.org/10.1210/jc.85.2.720.
- Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD, et al. Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 2009;24:719-25. http://dx.doi.org/10.1359/jbmr.081214.
- Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 2012;367:1714-23. http://dx.doi.org/10.1056/NEJMoa1204061.
- Carfora E, Sergio F, Bellini P. Effect of treatment of postmenopausal osteoporosis with continuous daily oral alendronate and the incidence of fractures. Gazzetta Med Ital Arch Sci Med 1998;157:105-9.
- Chesnut CH, McClung MR, Ensrud KE, Bell NH, Genant HK, Harris ST, et al. Alendronate treatment of the postmenopausal osteoporotic woman: effect of multiple dosages on bone mass and bone remodeling. Am J Med 1995;99:144-52. http://dx.doi.org/10.1016/S0002-9343(99)80134-X.
- Choo C, Lukka H, Kiss A, Danjoux C. Double-blinded, placebo-controlled randomized study evaluating the efficacy of risedronate to prevent the loss of bone mineral density in non-metastatic prostate cancer patients undergoing radiotherapy plus 2–3 years of androgen ablation therapy. Int J Radiat Oncol Biol Phys 2011;81. http://dx.doi.org/10.1016/j.ijrobp.2011.06.086.
- Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, et al. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 1999;42:2309-18. http://dx.doi.org/10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K.
- Cummings S, Black D, Thompson D, Applegate W, Barrett-Connor E, Musliner T, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 1998;280:2077-82. http://dx.doi.org/10.1001/jama.280.24.2077.
- Dursun N, Dursun E, Yalcin S. Comparison of alendronate, calcitonin and calcium treatments in postmenopausal osteoporosis. Int J Clin Pract 2001;55:505-9.
- Fogelman I, Ribot C, Smith R, Ethgen D, Sod E. Reginster for the BMD-MN Study Group . Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2000;85:1895-900.
- Greenspan SL, Schneider DL, McClung MR, Miller PD, Schnitzer TJ, Bonin R, et al. Alendronate improves bone mineral density in elderly women with osteoporosis residing in long-term care facilities: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2002;136:742-6. http://dx.doi.org/10.7326/0003-4819-136-10-200205210-00009.
- Greenspan SL, Resnick NM, Parker RA. Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA 2003;289:2525-33. http://dx.doi.org/10.1001/jama.289.19.2525.
- Hadji P, Gamerdinger D, Spieler W, Kann PH, Loeffler H, Articus K, et al. Rapid Onset and Sustained Efficacy (ROSE) study: results of a randomised, multicentre trial comparing the effect of zoledronic acid or alendronate on bone metabolism in postmenopausal women with low bone mass. Osteoporos Int 2012;23:625-33. http://dx.doi.org/10.1007/s00198-011-1583-4.
- Harris ST, Watts NB, Genant HK, McKeever C, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 1999;282:1344-52. http://dx.doi.org/10.1001/jama.282.14.1344.
- Ho A, Kung A. Efficacy and tolerability of alendronate once weekly in Asian postmenopausal osteoporotic women. Ann Pharmacother 2005;39:1428-33. http://dx.doi.org/10.1345/aph.1E580.
- Hooper MJ, Ebeling PR, Roberts AP, Graham JJ, Nicholson GC, D’Emden M, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric 2005;8:251-62. http://dx.doi.org/10.1080/13697130500118126.
- Klotz LH, McNeill IY, Kebabdjian M, Zhang L, Chin JL. Canadian Urology Research Consortium . A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur Urol 2013;63:927-35. http://dx.doi.org/10.1016/j.eururo.2012.09.007.
- Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Prevention of anastrozole-induced bone loss with monthly oral ibandronate during adjuvant aromatase inhibitor therapy for breast cancer. Clin Cancer Res 2008;14:6336-42. http://dx.doi.org/10.1158/1078-0432.CCR-07-5101.
- Leung JYY, Ho AYY, Ip TP, Lee G, Kung AWC. The efficacy and tolerability of risedronate on bone mineral density and bone turnover markers in osteoporotic Chinese women: a randomized placebo-controlled study. Bone 2005;36:358-64. http://dx.doi.org/10.1016/j.bone.2004.10.014.
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 1995;333:1437-44. http://dx.doi.org/10.1056/NEJM199511303332201.
- Lyles KW, Cólon-Emeric C, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799-809. http://dx.doi.org/10.1056/NEJMoa074941.
- McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001;344:333-40. http://dx.doi.org/10.1056/NEJM200102013440503.
- McClung M, Miller P, Recknor C, Mesenbrink P, Bucci-Rechtweg C, Benhamou CL, et al. Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet Gynecol 2009;114:999-1007. http://dx.doi.org/10.1097/AOG.0b013e3181bdce0a.
- McClung MR, Bolognese MA, Sedarati F, Recker RR, Miller PD. Efficacy and safety of monthly oral ibandronate in the prevention of postmenopausal bone loss. Bone 2009;44:418-22. http://dx.doi.org/10.1016/j.bone.2008.09.011.
- Miller PD, Epstein S, Sedarati F, Reginster JY. Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 2008;24:207-13. http://dx.doi.org/10.1185/030079908X253889.
- Muscoso E, Puglisi N, Mamazza C, Lo Giudice M, Testai M, Abbate S, et al. Antiresorption therapy and reduction in fracture susceptibility in the osteoporotic elderly patient: open study. Eur Rev Med Pharmacol Sci 2004;8:97-102.
- Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000;343:604-10. http://dx.doi.org/10.1056/NEJM200008313430902.
- Pols HA, Felsenberg D, Hanley DA, Štepán J, Muñoz-Torres M, lkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int 1999;9:461-8. http://dx.doi.org/10.1007/PL00004171.
- Reginster JY, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000;11:83-91. http://dx.doi.org/10.1007/s001980050010.
- Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. J Bone Miner Res 2000;15:1006-13. http://dx.doi.org/10.1359/jbmr.2000.15.6.1006.
- Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Weryha G, et al. Alendronic acid produces greater effects than risedronic acid on bone density and turnover in postmenopausal women with osteoporosis: results of FACTS-international. Clin Drug Invest 2006;26:63-74. http://dx.doi.org/10.2165/00044011-200626020-00002.
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2009;373:1253-63. http://dx.doi.org/10.1016/S0140-6736(09)60250-6.
- Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int 2006;26:427-31. http://dx.doi.org/10.1007/s00296-005-0004-4.
- Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 2005;20:141-51. http://dx.doi.org/10.1359/JBMR.040920.
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 1998;339:292-9. http://dx.doi.org/10.1056/NEJM199807303390502.
- Sarioglu M, Tuzun C, Unlu Z, Tikiz C, Taneli F, Uyanik BS. Comparison of the effects of alendronate and risedronate on bone mineral density and bone turnover markers in postmenopausal osteoporosis. Rheumatol Int 2006;26:195-200. http://dx.doi.org/10.1007/s00296-004-0544-z.
- Shilbayeh S, S-Zumeili A, Hilow HM. The efficacy and safety of Calidron tablets for management of osteoporosis in Jordanian women: a randomised clinical trial. Saudi Pharm J 2004;12:86-95.
- Smith BJ, Laslett LL, Pile KD, Phillips PJ, Phillipov G, Evans SM, et al. Randomized controlled trial of alendronate in airways disease and low bone mineral density. Chron Respir Dis 2004;1:131-7. http://dx.doi.org/10.1191/1479972304cd025oa.
- Taxel P, Dowsett R, Richter L, Fall P, Klepinger A, Albertsen P, et al. Risedronate prevents early bone loss and increased bone turnover in the first 6 months of luteinizing hormone-releasing hormone-agonist therapy for prostate cancer. BJU Int 2010;106:1473-6. http://dx.doi.org/10.1111/j.1464-410X.2010.09329.x.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. http://dx.doi.org/10.1016/j.ijsu.2010.02.007.
- Seeman E. The antifracture efficacy of alendronate. Int J Clin Pract Suppl 1999;101:40-5.
- Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001;44:202-11. http://dx.doi.org/10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W.
- Ste-Marie LG, Sod E, Johnson T, Chines A. Five years of treatment with risedronate and its effects on bone safety in women with postmenopausal osteoporosis. Calcif Tissue Int 2004;75:469-76. http://dx.doi.org/10.1007/s00223-004-0039-7.
- Sorensen OH, Crawford GM, Mulder H, Hosking DJ, Gennari C, Mellstrom D, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 2003;32:120-6. http://dx.doi.org/10.1016/S8756-3282(02)00946-8.
- Ringe JD, Farahmand P, Faber H, Dorst A. Sustained efficacy of risedronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int 2009;29:311-15. http://dx.doi.org/10.1007/s00296-008-0689-2.
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab 2010;95:4380-7. http://dx.doi.org/10.1210/jc.2010-0597.
- Adachi JD, Lyles KW, Colon-Emeric CS, Boonen S, Pieper CF, Mautalen C, et al. Zoledronic acid results in better health-related quality of life following hip fracture: the HORIZON-recurrent fracture trial. Osteoporos Int 2011;22:2539-49. http://dx.doi.org/10.1007/s00198-010-1514-9.
- Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SA, et al. Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 2006;91:2631-7. http://dx.doi.org/10.1210/jc.2005-2602.
- Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Marques-Neto JF, et al. A comparison of the effect of alendronate and risedronate on bone mineral density in postmenopausal women with osteoporosis: 24-month results from FACTS-International. Int J Clin Pract 2008;62:575-84. http://dx.doi.org/10.1111/j.1742-1241.2008.01704.x.
- Hadji P, Gamerdinger D, Spieler W, Kann P, Loeffler H, Articus K, et al. Rapid Onset and Sustained Efficacy (ROSE) study of zoledronic acid vs alendronate in postmenopausal women with osteoporosis: quality of life (QOL), compliance and therapy preference. J Bone Miner Res 2010;25.
- Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
- Bianchi G, Felsenberg D, Czerwienska B. Ibandronate is maintained over 5 years: the DIVA LTE study. Ann Rheum Dis 2009;63.
- Felsenberg D, Czerwienska B, Stakkestad JA, Neate C, Masanauskaite D, Reginster J-Y. Efficacy of monthly oral Ibandronate is maintained over 5 years: the MOBILE LTE study. Osteoporos Int 2009;20.
- EuroQoL-Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Lester J, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Use of monthly oral ibandronate to prevent anastrozole-induced bone loss during adjuvant treatment for breast cancer: two-year results from the ARIBON study. J Clin Oncol: ASCO Ann Meet Proc 2008;26.
- Bobba RS, Beattie K, Parkinson B, Kumbhare D, Adachi JD, . Tolerability of different dosing regimens of bisphosphonates for the treatment of osteoporosis and malignant bone disease. Drug Saf 2006;29:1133-52. http://dx.doi.org/10.2165/00002018-200629120-00005.
- Crandall C. Risedronate: a clinical review. Arch Intern Med 2001;161:353-60. http://dx.doi.org/10.1001/archinte.161.3.353.
- Kherani RB, Papaioannou A, Adachi JD. Long-term tolerability of the bisphosphonates in postmenopausal osteoporosis: a comparative review. Drug Saf 2002;25:781-90. http://dx.doi.org/10.2165/00002018-200225110-00003.
- Umland EM, Boyce EG. Risedronate: a new oral bisphosphonate. Clin Therap 2001;23:1409-21. http://dx.doi.org/10.1016/S0149-2918(01)80116-8.
- Lloyd-Jones M, Wilkinson A. Adverse Effects and Persistence with Therapy in Patients Taking Oral Alendronate, Etidronate or Risedronate: Systematic Reviews. Sheffield: University of Sheffield; 2006.
- Krueger CD, West PM, Sargent M, Lodolce AE, Pickard AS. Bisphosphonate-induced osteonecrosis of the jaw. Ann Pharmacother 2007;41:276-84. http://dx.doi.org/10.1345/aph.1H521.
- Van den Wyngaert T, Huizing MT, Vermorken JB. Bisphosphonates and osteonecrosis of the jaw: cause and effect or a post hoc fallacy?. Ann Oncol 2006;17:1197-204. http://dx.doi.org/10.1093/annonc/mdl294.
- Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006;144:753-61. http://dx.doi.org/10.7326/0003-4819-144-10-200605160-00009.
- Lee SH, Chang SS, Lee M, Chan RC, Lee CC. Risk of osteonecrosis in patients taking bisphosphonates for prevention of osteoporosis: a systematic review and meta-analysis. Osteoporos Int 2014;25:1131-9. http://dx.doi.org/10.1007/s00198-013-2575-3.
- Giusti A, Hamdy NAT, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy. A systematic review of case/case series studies. Bone 2010;47:169-80. http://dx.doi.org/10.1016/j.bone.2010.05.019.
- Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res 2013;28:1729-37. http://dx.doi.org/10.1002/jbmr.1893.
- Andrici J, Tio M, Eslick GD. Meta-analysis: oral bisphosphonates and the risk of oesophageal cancer. Aliment Pharmacol Ther 2012;36:708-16. http://dx.doi.org/10.1111/apt.12041.
- Sun K, Liu JM, Sun HX, Lu N, Ning G. Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies. Osteoporos Int 2013;24:279-86. http://dx.doi.org/10.1007/s00198-012-2158-8.
- Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf 2009;32:219-28. http://dx.doi.org/10.2165/00002018-200932030-00004.
- Ralston SH, Kou TD, Wick-Urban B, Steinbuch M, Masud T. Risk of upper gastrointestinal tract events in risedronate users switched to alendronate. Calcif Tissue Int 2010;87:298-304. http://dx.doi.org/10.1007/s00223-010-9401-0.
- Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 2007;18:1023-31. http://dx.doi.org/10.1007/s00198-006-0322-8.
- Imaz I, Zegarra P, González-Enríquez J, Rubio B, Alcazar R, Amate JM. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int 2010;21:1943-51. http://dx.doi.org/10.1007/s00198-009-1134-4.
- Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 2007;82:1493-501. http://dx.doi.org/10.1016/S0025-6196(11)61093-8.
- Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int 2011;22:741-53. http://dx.doi.org/10.1007/s00198-010-1335-x.
- Mikyas Y, Agodoa I, Yurgin N. A Systematic review of osteoporosis medication adherence and osteoporosis-related fracture costs in men. Appl Health Econ Health Policy 2014;12:267-77. http://dx.doi.org/10.1007/s40258-013-0078-1.
- Vieira HP, Leite IA, Araújo Sampaio TM, Dos Anjos de Paula J, do Nascimento Andrade A, de Abreu LC, et al. Bisphosphonates adherence for treatment of osteoporosis. Int Arch Med 2013;6.
- Klevsgård R, Fröberg B, Risberg B, Hallberg I. Nottingham Health Profile and Short-Form 36 health survey questionnaires in patients with chronic lower limb ischemia: before and after revascularization. J Vasc Surg 2002;36:310-17. http://dx.doi.org/10.1067/mva.2002.125747.
- Guyatt G, Feeny D, Patrick D. Measuring health-related quality of life. Ann Intern Med 1993;118:622-9. http://dx.doi.org/10.7326/0003-4819-118-8-199304150-00009.
- Tadic I, Vujasinovic Stupar N, Stevanovic D, Dimic A, Stamenkovic B, Stojanovic S, et al. Validation of the osteoporosis quality of life questionnaire QUALEFFO-41 for the Serbian population. Health Qual Life Outcomes 2012;10. http://dx.doi.org/10.1186/1477-7525-10-74.
- Müller D, Pulm J, Gandjour A. Cost-effectiveness of different strategies for selecting and treating individuals at increased risk of osteoporosis or osteopenia: a systematic review. Value Health 2012;15:284-98. http://dx.doi.org/10.1016/j.jval.2011.11.030.
- Borgström F, Ström O, Coelho J, Johansson H, Oden A, McCloskey EV, et al. The cost-effectiveness of risedronate in the UK for the management of osteoporosis using the FRAX. Osteoporos Int 2010;21:495-50. http://dx.doi.org/10.1007/s00198-009-0989-8.
- Kanis JA, Adams J, Borgström F, Cooper C, Jonsson B, Preedy D, et al. The cost-effectiveness of alendronate in the management of osteoporosis. Bone 2008;42:4-15. http://dx.doi.org/10.1016/j.bone.2007.10.019.
- Ström O, Borgström F, Sen SS, Boonen S, Haentjens P, Johnell O, et al. Cost-effectiveness of alendronate in the treatment of postmenopausal women in 9 European countries – an economic evaluation based on the fracture intervention trial. Osteoporos Int 2007;18:1047-61. http://dx.doi.org/10.1007/s00198-007-0349-5.
- Stevenson M, Jones ML, De NE, Brewer N, Davis S, Oakley J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9220.
- van Staa TP, Kanis JA, Geusens P, Boonen A, Leufkens HGM, Cooper C. The cost-effectiveness of bisphosphonates in postmenopausal women based on individual long-term fracture risks. Value Health 2007;10:348-57. http://dx.doi.org/10.1111/j.1524-4733.2007.00188.x.
- Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M. Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11070.
- van Staa TP, Geusens P, Zhang B, Leufkens HGM, Boonen A, Cooper C. Individual fracture risk and the cost-effectiveness of bisphosphonates in patients using oral glucocorticoids. Rheumatology 2007;46:460-6. http://dx.doi.org/10.1093/rheumatology/kel249.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Borgström F, Johnell O, Kanis JA, Jonsson B, Rehnberg C. At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 2006;17:1459-71. http://dx.doi.org/10.1007/s00198-006-0107-0.
- Stevenson M, Davis SE, Kanis J. The hospitalization costs and outpatient costs of fragility fractures. Women Health Med 2006;4:149-51. http://dx.doi.org/10.1383/wohm.2006.3.4.149.
- Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001;12:417-27. http://dx.doi.org/10.1007/s001980170112.
- Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 2000;15:721-39. http://dx.doi.org/10.1359/jbmr.2000.15.4.721.
- National Schedule of Reference Costs – Year 2013/14 – NHS Trusts and NHS Foundation Trusts. London: Department of Health; 2014.
- The Electronic Drug Tariff. London: Department of Health; 2015.
- eMit National Database. London: Department of Health; 2014.
- Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M. Treatment of established osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess 2002;6. http://dx.doi.org/10.3310/hta6290.
- Koerkamp BG, Stijnen T, Weinstein MC, Hunink MGM. The combined analysis of uncertainty and patient heterogeneity in medical decision models. Med Decis Making 2011;31:650-61. http://dx.doi.org/10.1177/0272989X10381282.
- Office for National Statistics . National Life Tables, England 2011–2013 2014. http://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014-09-25 (accessed 11 November 2014).
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht: Springer; 2014.
- Smith T, Pelpola K, Ball M, Ong A, Myint PK. Pre-operative indicators for mortality following hip fracture surgery: a systematic review and meta-analysis. Age Ageing 2014;43:464-71. http://dx.doi.org/10.1093/ageing/afu065.
- Osnes EK, Lofthus CM, Meyer HE, Falch JA, Nordsletten L, Cappelen I, et al. Consequences of hip fracture on activities of daily life and residential needs. Osteoporos Int 2004;15:567-74.
- Population Estimates by Single Year of Age and Sex for Local Authorities in the UK, Mid-2013. London: ONS; 2014.
- Office for National Statistics . Census 2011: Residence Type by Sex by Age 2011. www.nomisweb.co.uk/ (accessed 28 October 2014).
- Office for National Statistics . Census 2011: Communal Establishment Management and Type by Sex by Age 2011. www.nomisweb.co.uk/census/2011/dc4210ewla (accessed 28 October 2014).
- van Staa TP, Leufkens HGM, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM 2000;93:105-11. http://dx.doi.org/10.1093/qjmed/93.2.105.
- Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in UK over the past 20 years. Rheumatology 2011;50:1982-90. http://dx.doi.org/10.1093/rheumatology/ker017.
- Kanis JA, Johnell O, de Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004;35:375-82. http://dx.doi.org/10.1016/j.bone.2004.03.024.
- Scholes S, Panesar S, Shelton NJ, Francis RM, Mirza S, Mindell JS, et al. Epidemiology of lifetime fracture prevalence in England: a population study of adults aged 55 years and over. Age Ageing 2014;43:234-40. http://dx.doi.org/10.1093/ageing/aft167.
- Court-Brown CM, Biant L, Bugler KE, McQueen MM. Changing epidemiology of adult fractures in Scotland. Scott Med J 2014;59:30-4. http://dx.doi.org/10.1177/0036933013518148.
- van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001;29:517-22. http://dx.doi.org/10.1016/S8756-3282(01)00614-7.
- Health & Social Care Information Centre . Health Survey for England 2012: BMI Adult Trend Tables 2013. www.hscic.gov.uk/catalogue/PUB13219 (accessed 28 October 2014).
- Johansson H, Kanis JA, Oden A, McCloskey E, Chapurlat RD, Christiansen C, et al. A meta analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223-33. http://dx.doi.org/10.1002/jbmr.2017.
- Curtis JR, Yun H, Matthews R, Saag KG, Delzell E. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res 2012;64:1054-60.
- Stevenson MD, Selby PL. Modelling the cost effectiveness of interventions for osteoporosis: issues to consider. PharmacoEconomics 2014;32:735-43. http://dx.doi.org/10.1007/s40273-014-0156-8.
- European Medicines Agency . EPAR – Scientific Discussion – Variation WC500052650 2005. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion_-_Variation/human/000501/WC500052650.pdf (accessed 11 March 2015).
- European Medicines Agency . EPAR – Scientific Discussion – Variation WC500052651 2007. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion_-_Variation/human/000501/WC500052651.pdf (accessed 11 March 2015).
- Stevenson M, Davis S. Analyses of the Cost Effectiveness of Pooled Alendronate and Risedronate, Compared with Strontium Ranelate, Raloxifene, Etidronate and Teriparatide. Sheffield: University of Sheffield; 2006.
- Groeneveld PW, Lieu TA, Fendrick AM, Hurley LB, Ackerson LM, Levin TR, et al. Quality of life measurement clarifies the cost-effectiveness of Helicobacter pylori eradication in peptic ulcer disease and uninvestigated dyspepsia. Am J Gastroenterol 2001;96:338-47. http://dx.doi.org/10.1016/s0002-9270(00)02309-1.
- Prevention of Osteoporosis NICE MTA ID782 (Inc Part Review of TA160 and TA161): Actavis UK Ltd Evidence of Submission for Risedronate. Weybridge: Actavis UK Ltd; 2014.
- van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0017030.
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633-50. http://dx.doi.org/10.1007/s00198-009-0920-3.
- Kanis JA, Oden A, Johnell O, de Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone 2003;32:468-73. http://dx.doi.org/10.1016/S8756-3282(03)00061-9.
- Parker MJ, Anand JK. What is the true mortality of hip fractures?. Public Health 1991;105:443-6. http://dx.doi.org/10.1016/S0033-3506(05)80614-6.
- Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ. Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int 2007;18:1463-72. http://dx.doi.org/10.1007/s00198-007-0429-6.
- Allaf N, Lovell M. Annual review of fractured neck of femur mortality rates: is this a true picture?. Ann R Coll Surg Engl 2004;86:347-8. http://dx.doi.org/10.1308/147870804326.
- Deakin DE, Boulton C, Moran CG. Mortality and causes of death among patients with isolated limb and pelvic fractures. Injury 2007;38:312-17. http://dx.doi.org/10.1016/j.injury.2006.09.024.
- Goldacre MJ, Roberts SE, Yeates D. Mortality after admission to hospital with fractured neck of femur: database study. BMJ 2002;325:868-9. http://dx.doi.org/10.1136/bmj.325.7369.868.
- Heikkinen T, Parker M, Jalovaara P. Hip fractures in Finland and Great Britain: a comparison of patient characteristics and outcomes. Int Orthop 2001;25:349-54. http://dx.doi.org/10.1007/s002640100272.
- Holt G, Smith R, Duncan K, Finlayson DF, Gregori A. Early mortality after surgical fixation of hip fractures in the elderly. An analysis of data from the Scottish Hip Fracture Audit. J Bone Joint Surg 2008;90:1357-63. http://dx.doi.org/10.1302/0301-620X.90B10.21328.
- Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture. Evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg 2008;90:480-3. http://dx.doi.org/10.1302/0301-620X.90B4.20264.
- McColl A, Roderick P, Cooper C. Hip fracture incidence and mortality in an English region: a study using routine National Health Service data. J Public Health 1998;20:196-205. http://dx.doi.org/10.1093/oxfordjournals.pubmed.a024743.
- Roberts SE, Goldacre MJ. Time trends and demography of mortality after fractured neck of femur in an English population, 1968–98: database study. BMJ 2003;327:771-5. http://dx.doi.org/10.1136/bmj.327.7418.771.
- Wood DJ, Ions GK, Quinby JM, Gale DW, Stevens J. Factors which influence mortality after subcapital hip fracture. J Bone Joint Surg 1992;74:199-202.
- Evans JG, Seagroatt V, Goldacre MJ. Secular trends in proximal femoral fracture, Oxford record linkage study area and England 1968-86. J Epidemiol Community Health 1997;51:424-9. http://dx.doi.org/10.1136/jech.51.4.424.
- Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38-42. http://dx.doi.org/10.1007/s00198-003-1490-4.
- Oden A, Dawson A, Dere W, Johnell O, Jonsson B, Kanis JA. Lifetime risk of hip fractures is underestimated. Osteoporos Int 1998;8:599-603. http://dx.doi.org/10.1007/s001980050105.
- Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-61. http://dx.doi.org/10.1007/s001980070075.
- Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878-82. http://dx.doi.org/10.1016/S0140-6736(98)09075-8.
- Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Iton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-5.
- Jalava T, Sarna S, Pylkkänen L, Mawer B, Kanis JA, Selby P, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003;18:1254-60. http://dx.doi.org/10.1359/jbmr.2003.18.7.1254.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Arch Inter Med 1999;159:1215-20. http://dx.doi.org/10.1001/archinte.159.11.1215.
- Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108-12. http://dx.doi.org/10.1007/s00198-003-1516-y.
- Kado DM, Duong T, Stone KL, Ensrud KE, Nevitt MC, Greendale GA, et al. Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int 2003;14:589-94. http://dx.doi.org/10.1007/s00198-003-1412-5.
- Piirtola M, Vahlberg T, Löppönen M, Räihä I, Isoaho R, Kivelä SL. Fractures as predictors of excess mortality in the aged-a population-based study with a 12-year follow-up. Eur J Epidemiol 2008;23:747-55. http://dx.doi.org/10.1007/s10654-008-9289-4.
- Zethraeus N, Ström OE, Borgström F. What is the risk of institutionalization after hip fracture?. Osteoporosis Int 2006;17:S143-355.
- Todd CJ, Freeman C, Camilleri-Ferrante C, Laxton C, Murrell P, Palmer C, et al. Anglian Audit of Hip Fracture 2. Cambridge: University of Cambridge; 1999.
- Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Epidemiology and outcome after hip fracture in the under 65s. Evidence from the Scottish Hip Fracture Audit. Injury 2008;39:1175-81. http://dx.doi.org/10.1016/j.injury.2008.04.015.
- Deakin DE, Wenn RT, Moran CG. Factors influencing discharge location following hip fracture. Injury 2008;39:213-18. http://dx.doi.org/10.1016/j.injury.2007.07.012.
- Nanjayan SK, John J, Swamy G, Mitsiou K, Tambe A, Abuzakuk T. Predictors of change in ‘discharge destination’ following treatment for fracture neck of femur. Injury 2014;45:1080-4. http://dx.doi.org/10.1016/j.injury.2014.02.005.
- Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 2006;17:637-50. http://dx.doi.org/10.1007/s00198-005-0015-8.
- De Laet CEDH, van Hout BA, Burger H, Weel AEAM, Hofman A, Pols HAP. Incremental cost of medical care after hip fracture and first vertebral fracture: the Rotterdam study. Osteoporos Int 1999;10:66-72. http://dx.doi.org/10.1007/s001980050196.
- Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol 1991;7:403-22. http://dx.doi.org/10.1007/BF00145007.
- Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. J Bone Joint Surg Am 2003;85–A:1936-43.
- Blank RD. Official positions for FRAX® clinical regarding prior fractures. J Clin Densitom 2011;14:205-11. http://dx.doi.org/10.1016/j.jocd.2011.05.009.
- Ismail AA, Cockerill W, Cooper C, Finn JD, Abendroth K, Parisi G, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporos Int 2001;12:85-90. http://dx.doi.org/10.1007/s001980170138.
- Warriner AH, Patkar NM, Yun H, Delzell E. Minor, major, low-trauma, and high-trauma fractures: what are the subsequent fracture risks and how do they vary?. Curr Osteoporos Rep 2011;9:122-8. http://dx.doi.org/10.1007/s11914-011-0064-1.
- van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 2009;68:99-102. http://dx.doi.org/10.1136/ard.2008.092775.
- Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of Health State Utility Values for osteoporosis related conditions. Osteoporos Int 2009;20:853-68. http://dx.doi.org/10.1007/s00198-009-0844-y.
- Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R, et al. Sequential change in quality of life for patients with incident clinical fractures: a prospective study. Osteoporos Int 2009;20:695-702. http://dx.doi.org/10.1007/s00198-008-0761-5.
- Calvo E, Morcillo D, Foruria AM, Redondo-Santamaria E, Osorio-Picorne F, Caeiro JR, et al. Nondisplaced proximal humeral fractures: high incidence among outpatient-treated osteoporotic fractures and severe impact on upper extremity function and patient subjective health perception. J Shoulder Elbow Surg 2011;20:795-801. http://dx.doi.org/10.1016/j.jse.2010.09.008.
- Cooper C, Jakob F, Chinn C, Martin-Mola E, Fardellone P, Adami S, et al. Fracture incidence and changes in quality of life in women with an inadequate clinical outcome from osteoporosis therapy: the Observational Study of Severe Osteoporosis (OSSO). Osteoporos Int 2008;19:493-501. http://dx.doi.org/10.1007/s00198-007-0488-8.
- Ekström W, Németh G, Samnegård E, Dalen N, Tidermark J. Quality of life after a subtrochanteric fracture: a prospective cohort study on 87 elderly patients. Injury 2009;40:371-6. http://dx.doi.org/10.1016/j.injury.2008.09.010.
- Zethraeus N, Borgström F, Johnell O, Kanis J, Onnby K, Jonsson B. Costs and Quality of Life Associated With Osteoporosis Related Fractures – Results from a Swedish Survey 2002. http://econpapers.repec.org/paper/hhshastef/0512.htm (accessed 23 March 2015).
- Dolan P, Torgerson D, Kakarlapudi TK. Health-related quality of life of Colles’ fracture patients. Osteoporos Int 1999;9:196-9. http://dx.doi.org/10.1007/s001980050136.
- Suzuki N, Ogikubo O, Hansson T, Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J 2008;17:1380-90. http://dx.doi.org/10.1007/s00586-008-0753-3.
- Suzuki N, Ogikubo O, Hansson T, Suzuki N, Ogikubo O, Hansson T. Previous vertebral compression fractures add to the deterioration of the disability and quality of life after an acute compression fracture. Eur Spine J 2010;19:567-74. http://dx.doi.org/10.1007/s00586-009-1162-y.
- Tidermark J, Zethraeus N, Svensson O, Törnkvist H, Ponzer S. Quality of life related to fracture displacement among elderly patients with femoral neck fractures treated with internal fixation. J Orthop Trauma 2002;16:34-8. http://dx.doi.org/10.1097/00005131-200201000-00008.
- Tidermark J, Zethraeus N, Svensson O, Törnkvist H, Ponzer S. Femoral neck fractures in the elderly: functional outcome and quality of life according to EuroQol. Qual Life Res 2002;11:473-81. http://dx.doi.org/10.1023/A:1015632114068.
- Ström O, Borgström F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, et al. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop 2008;79:269-80. http://dx.doi.org/10.1080/17453670710015094.
- Borgström F, Lekander I, Ivergard M, Ström O, Svedbom A, Alekna V, et al. The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) – quality of life during the first 4 months after fracture. Osteoporos Int 2013;24:811-23. http://dx.doi.org/10.1007/s00198-012-2240-2.
- Lips P, Jameson K, Bianchi ML, Goemaere S, Boonen S, Reeve J, et al. Validation of the IOF quality of life questionnaire for patients with wrist fracture. Osteoporos Int 2010;21:61-70. http://dx.doi.org/10.1007/s00198-009-0946-6.
- Cockerill W, Lunt M, Silman AJ, Cooper C, Lips P, Bhalla AK, et al. Health-related quality of life and radiographic vertebral fracture. Osteoporos Int 2004;15:113-19. http://dx.doi.org/10.1007/s00198-003-1547-4.
- Roux C, Wyman A, Hooven FH, Gehlbach SH, Adachi JD, Chapurlat RD, et al. Burden of non-hip, non-vertebral fractures on quality of life in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). Osteoporos Int 2012;23:2863-71. http://dx.doi.org/10.1007/s00198-012-1935-8.
- van Schoor NM, Ewing SK, O’Neill TW, Lunt M, Smit JH, Lips P, et al. Impact of prevalent and incident vertebral fractures on utility: results from a patient-based and a population-based sample. Qual Life Res 2008;17:159-67. http://dx.doi.org/10.1007/s11136-007-9287-0.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, et al. Study of the incremental cost and clinical burden of hip fractures in postmenopausal women in the United Kingdom. J Med Econ 2011;14:99-107. http://dx.doi.org/10.3111/13696998.2010.547967.
- Gutierrez L, Roskell N, Castellsague J, Beard S, Rycroft C, Abeysinghe S, et al. Clinical burden and incremental cost of fractures in postmenopausal women in the United Kingdom. Bone 2012;51:324-31. http://dx.doi.org/10.1016/j.bone.2012.05.020.
- Humphries R. Paying for Social Care: Beyond Dilnot. London: The King’s Fund; 2013.
- The State of Health Care and Adult Social Care in England 2012/2013. London: The Stationery Office; 2013.
- Nguyen TV, Center JR, Eisman JA. Osteoporosis in elderly men and women: effects of dietary calcium, physical activity, and body mass index. J Bone Miner Res 2000;15:322-31. http://dx.doi.org/10.1359/jbmr.2000.15.2.322.
- Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Dec Making 2013;33:618-40. http://dx.doi.org/10.1177/0272989X13485157.
- Achana FA, Cooper NJ, Dias S, Lu G, Rice SJ, Kendrick D, et al. Extending methods for investigating the relationship between treatment effect and baseline risk from pairwise meta-analysis to network meta-analysis. Stat Med 2013;32:752-71. http://dx.doi.org/10.1002/sim.5539.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comp 2000;10:325-37. http://dx.doi.org/10.1023/A:1008929526011.
- Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Software 2005;12:1-16. http://dx.doi.org/10.18637/jss.v012.i03.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comp Graph Stat 1998;7:434-55.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Dec Making 2013;33:607-17. http://dx.doi.org/10.1177/0272989X12458724.
- Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B 2002;64:583-616. http://dx.doi.org/10.1111/1467-9868.00353.
Appendix 1 Literature search strategies
Search strategy in MEDLINE for the clinical effectiveness review
Date of search: 26 September 2014.
Date range: 2008 to 26 September 2014.
Search strategy
-
exp osteoporosis/
-
osteoporo$.tw.
-
bone diseases, metabolic/
-
exp Bone Density/
-
(bone adj3 densit$).tw.
-
exp fractures, bone/
-
fractures, cartilage/
-
fracture$.ti,ab.
-
(bone$ adj2 fragil$).tw.
-
bone mineral densit$.tw.
-
bone loss.tw.
-
bmd.tw.
-
or/1-12
-
(alendron$ or fosomax or fosavance).mp.
-
121268-17-5.rn.
-
(ibandron$ or boniva or bondronat or bonviva or adronil).mp.
-
114084-78-5.rn.
-
(risedron$ or actonel or atelvia or benet).mp.
-
105462-24-6.rn.
-
(zoledron$ or zometa or zomera or aclasta or reclast).mp.
-
118072-93-8.rn.
-
or/14-21
-
13 and 22
-
Randomized controlled trials as Topic/
-
Randomized controlled trial/
-
Random allocation/
-
randomized controlled trial.pt.
-
Double blind method/
-
Single blind method/
-
Clinical trial/
-
exp Clinical Trials as Topic/
-
controlled clinical trial.pt.
-
clinical trial$.pt.
-
multicenter study.pt.
-
or/24-34
-
(clinic$ adj25 trial$).ti,ab.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$ or mask$)).tw.
-
Placebos/
-
Placebo$.tw.
-
(allocated adj2 random).tw.
-
or/36-40
-
35 or 41
-
Case report.tw.
-
Letter/
-
Historical article/
-
43 or 44 or 45
-
exp Animals/
-
Humans/
-
47 not (47 and 48)
-
46 or 49
-
42 not 50
-
23 and 51
-
limit 52 to yr=“2008 –Current”
-
meta-analysis as topic/
-
(meta analy$ or metaanaly$).tw.
-
Meta-Analysis/
-
(systematic adj (review$1 or overview$1)).tw.
-
“Review Literature as Topic”/
-
or/54-58
-
(cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or cinhal or science citation index or bids or cancerlit).ab.
-
((reference adj list$) or bibliograph$ or hand-search$ or (relevant adj journals) or (manual adj search$)).ab.
-
((selection adj criteria) or (data adj extraction)).ab.
-
“review”/
-
62 and 63
-
comment/ or editorial/ or letter/
-
Animals/
-
Humans/
-
66 not (66 and 67)
-
65 or 68
-
59 or 60 or 61 or 64
-
70 not 69
-
23 and 71
-
limit 72 to yr=“2008 –Current”
Clinical Trials.gov: US National Institutes of Health (http://clinicaltrials.gov/)
Date of search: 30 September 2014.
Date range: 1 January 2008 to 30 September 2014.
Sixty-seven studies found for alendronate – received on or after 1 January 2008.
Two studies found for alendronic – received on or after 1 January 2008.
No studies found for fosomax – received on or after 1 January 2008.
Three studies found for fosavance – received on or after 1 January 2008.
Twenty-three studies found for ibandronate – received on or after 1 January 2008.
Twenty studies found for ibandronic – received on or after 1 January 2008.
Twenty-four studies found for boniva – received on or after 1 January 2008.
Twenty-three studies found for bondronat – received on or after 1 January 2008.
Twenty-four studies found for bonviva – received on or after 1 January 2008.
No studies found for adronil – received on or after 1 January 2008.
Forty-five studies found for risedronate – received on or after 1 January 2008.
Thirty-seven studies found for risedronic – received on or after 1 January 2008.
Forty-five studies found for actonel – received on or after 1 January 2008.
Forty-five studies found for atelvia – received on or after 1 January 2008.
Thirteen studies found for benet – received on or after 1 January 2008.
One hundred and ten studies found for zoledronate – received on or after 1 January 2008.
One hundred and seven studies found for zoledronic – received on or after 1 January 2008.
One hundred and ten studies found for zometa – received on or after 1 January 2008.
One study found for zomera – received on or after 1 January 2008.
One hundred and ten studies found for aclasta – received on or after 1 January 2008.
One hundred and ten studies found for reclast – received on or after 1 January 2008.
International Clinical Trials Registry Platform: World Health Organization (http://apps.who.int/trialsearch/AdvSearch.aspx)
Search strategy
Date of search: 30 September 2014.
Date range: 1 January 2008 to 30 September 2014.
Fifty-eight records for 25 trials found for alendronate or alendronic or fosomax or fosavance received on or after 1 January 2008.
Six records for five trials found for ibandronate or ibandronic received on or after 1 January 2008.
Four records for two trials found for boniva or bondronat or bonviva or adronil received on or after 1 January 2008.
Sixty-three records for 35 trials found for risedronate or risedronic or actonel or atelvia or benet received on or after 1 January 2008.
One hundred and eighteen records for 81 trials found for zoledronate or zoledronic or zometa or zomera or aclasta or reclast received on or after 1 January 2008.
Supplementary search strategy for adverse events
-
(alendron$ or fosomax or fosavance).mp.
-
121268-17-5.rn.
-
(ibandron$ or boniva or bondronat or bonviva or adronil).mp.
-
114084-78-5.rn.
-
(risedron$ or actonel or atelvia or benet).mp.
-
105462-24-6.rn.
-
(zoledron$ or zometa or zomera or aclasta or reclast).mp.
-
118072-93-8.rn.
-
or/1-8
-
(ae or to or po or co).fs.
-
(safe or safety).ti,ab.
-
side effect$.ti,ab.
-
((adverse or undesirable or harms$ or serious or toxic) adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab.
-
(toxicity or complication$ or noxious or tolerability).ti,ab.
-
or/10-14
-
9 and 15
-
MEDLINE.tw.
-
systematic review.tw.
-
meta analysis.pt.
-
or/17-19
-
16 and 20
Supplementary search strategy for compliance and concordance search
-
(alendron$ or fosomax or fosavance).mp.
-
121268-17-5.rn.
-
(ibandron$ or boniva or bondronat or bonviva or adronil).mp.
-
114084-78-5.rn.
-
(risedron$ or actonel or atelvia or benet).mp.
-
105462-24-6.rn.
-
(zoledron$ or zometa or zomera or aclasta or reclast).mp.
-
118072-93-8.rn.
-
or/1-8
-
exp Patient Compliance/
-
(complian$ or comply or adhere$ or capacitance or persistan$ or concordan$).ti,ab.
-
(noncomplian$ or nonadhere$ or nonpersistan$ or nonconcordan$).ti,ab.
-
or/10-12
-
9 and 13
-
MEDLINE.tw.
-
systematic review.tw.
-
meta analysis.pt.
-
or/15-17
-
14 and 18
Search strategy in MEDLINE for the cost-effectiveness review
-
exp osteoporosis/
-
osteoporo$.tw.
-
bone diseases, metabolic/
-
exp Bone Density/
-
(bone adj3 densit$).tw.
-
exp fractures, bone/
-
fractures, cartilage/
-
fracture$.ti,ab.
-
(bone$ adj2 fragil$).tw.
-
bone mineral densit$.tw.
-
bone loss.tw.
-
bmd.tw.
-
or/1-12
-
(alendron$ or fosomax or fosavance).mp.
-
121268-17-5.rn.
-
(ibandron$ or boniva or bondronat or bonviva or adronil).mp.
-
114084-78-5.rn.
-
(risedron$ or actonel or atelvia or benet).mp.
-
105462-24-6.rn.
-
(zoledron$ or zometa or zomera or aclasta or reclast).mp.
-
118072-93-8.rn.
-
or/14-21
-
13 and 22
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/24-44
-
23 and 45
-
limit 46 to yr=“2006 –Current”
Search strategy in MEDLINE for quality of life
The strategy was adapted from appendix 4 in Stevenson et al. 140
Search strategy
-
exp osteoporosis/
-
bone diseases, metabolic/
-
osteoporo$.tw.
-
or/1-3
-
(bone adj6 densit$).tw.
-
bone density/
-
bmd.ti,ab.
-
(bone or bones).mp.
-
exp densitometry/
-
tomography, x-ray computed/
-
densit$.tw.
-
10 and 11
-
9 or 12
-
8 and 13
-
5 or 6 or 7 or 14
-
exp fractures, bone/
-
fractures, cartilage/
-
fracture$.ti,ab.
-
or/16-18
-
15 or 19
-
4 and 20
-
(euroqol or euro qol or eq5d or eq 5d).mp.
-
21 and 22
-
limit 23 to yr=“2006 –Current”
Appendix 2 Table of excluded studies: clinical effectiveness review
| Citation | Reason for exclusion |
|---|---|
| Adachi J, Lyles K, Colon-Emeric C, Boonen S, Pieper C, Mautalen C, et al. Zoledronic acid improves health-related quality of life in patients with hip fracture: results of HORIZON-RFT. J Bone Miner Res 2010;25:S125 | Parallel publication, no additional information |
| Adachi JD, Lyles KW, Colon-Emeric CS, Boonen S, Pieper CF, Mautalen C, et al. Zoledronic acid improves health-related quality of life in patients with hip fracture: results of HORIZON-RFT. Osteoporos Int 2010;21:S151 | Parallel publication, no additional information |
| Adachi JD, Lyles KW, Boonen S, Colon-Emeric C, Hyldstrup L, Nordsletten L, et al. Subtrochanteric fractures: results from the HORIZON-recurrent fracture trial. Osteoporos Int 2010;21:S23 | Parallel publication, no additional information |
| Adachi J, Bucci-Rechtweg C, Su G, Eriksen E, Magaziner J, Lyles K, et al. Zoledronic acid improves health-related quality of life in patients with hip fracture: results of HORIZON-RFT. Osteoporos Int 2011;22:S140–2 | Parallel publication, no additional information |
| Adami S, Felsenberg D, Christiansen C, Robinson J, Lorenc RS, Mahoney P, et al. Efficacy and safety of ibandronate given by intravenous injection once every 3 months. Bone 2004;34:881–9 | Not treatment of interest, not currently licensed dose |
| Bauer D, Schwartz A, Palermo L, Cauley J, Ensrud K, Hochberg M, et al. Utility of serial BMD for fracture prediction after discontinuation of prolonged alendronate therapy: the FLEX trial. J Bone Miner Res 2010;25:S30–1 | Parallel publication, no additional information |
| Bauer DC, Schwartz A, Palermo L, Cauley J, Hochberg M, Santora A, et al. Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Interm Med 2014;174:1126–34 | Parallel publication, no additional information |
| Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. J Clin Endocrinol Metab 2000;85:4118–24 | Parallel publication, no additional information |
| Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003;349:1207–15 | Not comparator of interest |
| Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 2005;353:555–65 | Not comparator of interest |
| Black DM, Schwartz VA, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2014;296:2927–38 | Extension study, participants not in original randomised groups |
| Black DM, Eastell R, Cosman F, Man Z, Bucci-Rechtweg C, Mesenbrink P. Effect of once-yearly zoledronic acid 5 mg on ‘Super Six’ non-vertebral fractures. Bone 2009;44:S429 | Parallel publication, no additional information |
| Black DM, Seeman E, Bucci-Rechtweg C, Eastell R, Boonen S, Mesenbrink P. Zoledronic acid reduces the increased risk conferred by further fractures. Int Med J 2010;40:27 | Parallel publication, no additional information |
| Black DM, Reid IR, Boonen S, Bucci-Rechtweg C, Cauley JA, Cosman F, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27:243–54. [Erratum published in J Bone Miner Res 2012;27:2612] | Extension study, participants not in original randomised groups |
| Black DM, Eastell R, Cosman F, McLellan A, Man Z, Bucci-Rechtweg C, et al. Effect of once-yearly zoledronic acid 5 mg on ‘super six’ non-vertebral fractures. Osteoporos Int 2009;20:S281 | Parallel publication, no additional information |
| Black DM, Eastell R, Cosman F, Man Z, Bucci-Rechtweg C, Mesenbrink P. Effect of once-yearly zoledronic acid 5 mg on a sub-set of six non-vertebral fractures. J Clin Densitomet 2010;13:132 | Parallel publication, no additional information |
| Black D, Reid I, Cauley J, Boonen S, Cosman F, Leung PC, et al. The effect of 3 versus 6 years of zoledronic acid treatment in osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2010;25:S22–3 | Parallel publication, no additional information |
| Black D, Reid I, Eastell R, Buccirechtweg C, Su G, Hue TF, et al. Reduction in the risk of clinical fractures after a single dose of zoledronic acid 5 mg. Osteoporos Int 2011;22:S105–6 | Parallel publication, no additional information |
| Bone HG, Downs RW, Tucci JR, Harris ST, Weinstein RS, Licata AA, et al. Dose–response relationships for alendronate treatment in osteoporotic elderly women. J Clin Endocrinol Metab 1997;82:265–74 | Not treatment of interest, not currently licensed dose |
| Boonen S, Magaziner J, Orwig D, Lyles K, Nordsletten L, Adachi J, et al. BMD after hip fractures: response to annual i.v. zoledronic acid 5 mg. Bone 2009;44:S446 | Parallel publication, no additional information |
| Boonen S, Orwoll E, Magaziner J, Colon-Emeric C, Adachi J, Bucci-Rechtweg C, et al. Effect of once-yearly zoledronic acid in men after recent hip fracture: results from HORIZON recurrent fracture trial. J Bone Miner Res 2010;25:S471 | Parallel publication, no additional information |
| Boonen S, Black DM, Colón-Emeric CS, Eastell R, Magaziner JS, Eriksen EF, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc 2010;58:292–9 | Parallel publication, no additional information |
| Boonen S, Black DM, Colon-Emeric CS, Eastell R, Magaziner JS, Eriksen EF, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc 2010;58:292–9 | Parallel publication, no additional information |
| Boonen S, Kaufmann J-M, Orwoll E, Magaziner J, Colon-Emeric C, Adachi R, et al. Effect of once-yearly zoledronic acid in men after recent hip fracture: results from HORIZON recurrent fracture trial. Osteoporos Int 2011;22:S180 | Parallel publication, no additional information |
| Boonen S, Su G, Incera E, Orwoll E, Kaufman J-M, Reginster J-Y, et al. Antifracture efficacy and safety of once-yearly zoledronic acid 5 mg in men with osteoporosis: a prospective, randomized, controlled trial. Osteoporos Int 2011;22:S112 | Parallel publication, no additional information |
| Boonen S, Reginster J-Y, Kaufman J-M, Lippuner K, Zanchetta J, Langdahl B, et al. Efficacy of once-yearly zoledronic acid 5 mg in men with osteoporosis with different levels of serum total testosterone. Osteoporos Int 2012;23:S79–80 | Parallel publication, no additional information |
| Boonen S, Eastell R, Su G, Mesenbrink P, Cosman F, Cauley JA, et al. Time to onset of antifracture efficacy and year-by-year persistence of effect of zoledronic acid in women with osteoporosis. J Bone Miner Res 2012;27:1487–93 | Parallel publication, no additional information |
| Boonen S, Lorenc RS, Wenderoth D, Stoner KJ, Eusebio R, Orwoll ES, et al. Evidence for safety and efficacy of risedronate in men with osteoporosis over 4 years of treatment: results from the 2-year, open-label, extension study of a 2-year, randomized, double-blind, placebo-controlled study. Bone 2012;51:383–8 | Parallel publication, no additional information |
| Colon-Emeric C, Mesenbrink P, Lyles K, Pieper C, Boonen S, Delmas P, et al. Potential mediators of the reduction in mortality with zoledronic acid after hip fracture. J Bone Miner Res 2010;25:91–7 | Parallel publication, no additional information |
| Cosman F, Cauley J, Eastell R, Boonen S, Palermo L, Reid I, et al. Who is at highest risk for new vertebral fractures after 3 years of annual zoledronic acid and who should remain on treatment? Annual Meeting of the American Society for Bone and Mineral Research (ASBMR), San Diego, CA, 16–20 September 2011 | Parallel publication, no additional information |
| Delmas PD, Recker RR, Chesnut CH III, Skag A, Stakkestad JA, Emkey R, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 2004;15:792–8 | Parallel publication, no additional information |
| Devogelaer JP, Broll H, Correa-Rotter R, Cumming DC, Nagant de Deuxchaisnes C, Geusens P, et al. Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone 1996;18:141–50 | No outcomes of interest |
| Durchschlag E, Paschalis EP, Zoehrer R, Roschger P, Fratzl P, Recker R, et al. Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res 2006;21:1581–90 | No outcomes of interest |
| Eastell R, Black DM, Boonen S, Adami S, Felsenberg D, Lippuner K, et al. Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 2009;94:3215–25 | No outcomes of interest |
| Eastell R, Cosman F, Cauley JA, Boonen S, Palermo L, Reid IR, et al. After 3 years of annual zoledronic acid, who should remain on treatment? Results from the HORIZON-PFT extension study. Osteoporos Int 2012;23:S240–1 | Parallel publication, no additional information |
| Emkey R, Delmas PD, Bolognese M, Borges JL, Cosman F, Ragi-Eis S, et al. Efficacy and tolerability of once-monthly oral ibandronate (150 mg) and once-weekly oral alendronate (70 mg): additional results from the Monthly Oral Therapy With Ibandronate For Osteoporosis Intervention (MOTION) study. Clin Therap 2009;31:751–61 | Parallel publication, no additional information |
| Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone 2005;37:651–4 | Not treatment of interest, not currently licensed dose |
| Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone 2005;37:651–4 | Parallel publication, no additional information |
| Genant HK, Bucci-Rechtweg C, Bauer DC, Mesenbrink PG, Palermo L, Nusgarten L, et al. Does zoledronic acid increase risk of atypical femoral shaft fractures? Results from the HORIZON-PFT. Osteoporos Int 2010;21:S161–2 | Parallel publication, no additional information |
| Grey A, Bolland MJ, Wattie D, Horne A, Gamble G, Reid IR, et al. The antiresorptive effects of a single dose of zoledronate persist for two years: a randomized, placebo-controlled trial in osteopenic postmenopausal women. J Clin Endocrinol Metab 2009;94:538–44 | Population outside scope of appraisal, not licensed indication |
| Grey A, Bolland M, Wong S, Horne A, Gamble G, Reid IR, et al. Low-dose zoledronate in osteopenic postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 2012;97:286–92 | Population outside scope of appraisal, not licensed indication |
| Grey A, Bolland M, Mihov B, Wong S, Horne A, Gamble G, et al. Duration of antiresorptive effects of low-dose zoledronate in osteopenic postmenopausal women: a randomized, placebo-controlled trial. J Bone Miner Res 2014;29:166–72 | Population outside scope of appraisal, not licensed indication |
| Guo-ping L, Bin K, Hui Z. Effect of alendronate on bone mineral density of middle-aged and elderly patients with osteoporosis. Chin J Clin Rehabil 2005;39:186–7 | Not comparator of interest |
| Hakala M, Kroger H, Valleala H, Hienonen-Kempas T, Lehtonen-Veromaa M, Heikkinen J, et al. Once-monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: a 12-month, randomized, double-blind, placebo-controlled trial. Scand J Rheumatol 2012;41:260–6 | Population outside scope of appraisal, not licensed indication |
| Haworth CS, Sharples L, Hughes V, Elkin SL, Hodson M, Conway S, et al. Two-year multicenter, randomised, doubleblind, placebo-controlled trial assessing the effect of weekly risedronate on bone mineral density in adults with CF. Pediatr Pulmonol 2010;45:423 | Population outside scope of appraisal, not licensed indication |
| Haworth CS, Sharples L, Hughes V, Elkin SL, Hodson ME, Conway SP, et al. Multicentre trial of weekly risedronate on bone density in adults with cystic fibrosis. J Cystic Fibros 2011;10:470–6 | Population outside scope of appraisal, not licensed indication |
| Hochberg MC, Thompson DE, Black DM, Quandt SA, Cauley J, Geusens P, et al. Effect of alendronate on the age-specific incidence of symptomatic osteoporotic fractures. J Bone Miner Res 2005;20:971–6 | Parallel publication, no additional information |
| Hosking D, Chilvers CED, Christiansen C, Ravn P, Wasnich R, Ross P, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 1998;338:485–92 | Not treatment of interest, not currently licensed dose |
| Hwang JS, Chin LS, Chen JF, Yang TS, Chen PQ, Tsai KS et al. The effects of intravenous zoledronic acid in Chinese women with postmenopausal osteoporosis. J Bone Miner Metab 2011;29:328–33 | Parallel publication, no additional information |
| Hwang JS, Liou MJ, Ho C, Lin JD, Huang YY, Wang CJ, et al. The effects of weekly alendronate therapy in Taiwanese males with osteoporosis. J Bone Miner Metab 2010;28:328–33 | Population outside scope of appraisal, not licensed indication |
| Kasayama S, Fujita M, Goya K, Yamamoto H, Fujita K, Morimoto Y, et al. Effects of alendronate on bone mineral density and bone metabolic markers in postmenopausal asthmatic women treated with inhaled corticosteroids. Metabolism 2005;54:85–90 | Not treatment of interest, not currently licensed dose |
| Klotz L, McNeil I, Kebabdjian M, Zhang L, Chin J. A phase III, double-blind, randomized, parallel group, placebo-controlled study of oral aledronate, 70 mg once-a-week, for the prevention of androgen deprivation bone loss in non-metastatic prostate cancer. A Canadian urology research consortium study. J Urol 2011;185(Suppl. 1):e359 | Parallel publication, no additional information |
| Langenegger IQ, Opazo MF, Garcia AMZ. Therapeutic equivalence and adherence to treatment with ibandronate 150 mg and alendronate 70 mg in postmenopausal women of concepcion city, Chile. Actualizaciones Osteol 2011;7:175–83 | Population outside scope of appraisal, not licensed indication |
| Lindsay R, Cosman F, Lobo RA, Walsh BW, Harris ST, Reagan JE, et al. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab 1999;84:3076–81 | Not treatment of interest – combination therapy with HRT |
| Lindsay R, Cosman F, Lobo RA, Walsh BW, Harris ST, Reagan JE, et al. Addition of alendronate to ongoing hormone replacement therapy in the treatment of osteoporosis: a randomized, controlled clinical trial. J Clin Endocrinol Metab 1999;84:3076–81 | Not treatment of interest, not currently licensed dose |
| Majima T, Komatsu Y, Doi K, Takagi C, Shigemoto M, Fukao A, et al. Clinical significance of risedronate for osteoporosis in the initial treatment of male patients with Graves' disease. J Bone Miner Metab 2006;24;105–13 | Not treatment of interest, not currently licensed dose |
| McClung M, Clemmesen B, Daifotis A, Gilchrist NL, Eisman J, Weinstein RS, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis: a double-blind, randomized, controlled trial. Ann Intern Med 1998;128:253–61 | Not comparator of interest |
| McClung MR, Wasnich RD, Hosking DJ, Christiansen C, Ravn P, Wu M, et al. Prevention of postmenopausal bone loss: six-year results from the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 2004;89:4879–85 | Not treatment of interest, not currently licensed dose |
| McClung MR, Wasnich RD, Recker R, Cauley JA, Chesnut CH, Ensrud KE, et al. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res 2004;19:11–18 | No outcomes of interest |
| McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005;165:1762–8 | Not treatment of interest, not currently licensed dose |
| Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004;75:462–8 | Extension study, participants not in original randomised groups |
| Miller PD, Schnitzer T, Emkey R, Orwoll E, Rosen C, Ettinger M, et al. Weekly oral alendronic acid in male osteoporosis. Clin Drug Invest 2004;25:333–41 | Population outside scope of appraisal, not licensed indication |
| Mok CC, Tong KH, To CH, Siu YP, Ma KM. Risedronate for prevention of bone mineral density loss in patients receiving high-dose glucocorticoids: a randomized double-blind placebo-controlled trial. Osteoporos Int 2008;19:357–64 | Population outside scope of appraisal, not licensed indication |
| Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab 1998;83:396–402 | Population outside scope of appraisal, not licensed indication |
| Nakamura T, Nakano T, Ito M, Hagino H, Hashimoto J, Tobinai M, et al. Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int 2013;93:137–46 | Not treatment of interest, not currently licensed dose |
| Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, et al. Efficacy and safety of a once-yearly i.v. infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 2010;25:2239–50 | Population outside scope of appraisal, not licensed indication |
| Orwoll ES, Binkley NC, Lewiecki EM, Gruntmanis U, Fries MA, Dasic G, et al. Efficacy and safety of monthly ibandronate in men with low bone density. Bone 2010;46:970–6 | Population outside scope of appraisal, not licensed indication |
| Ravn P, Bidstrup M, Wasnich RD, Davis JW, McClung MR, Balske A, et al. Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study: a randomized, controlled trial. Ann Intern Med 1999;131:942 | Not treatment of interest, not currently licensed dose |
| Reid I, Boonen S, Black DM, Colon-Emeric C, Eastell R, Magaziner J, et al. Once-yearly treatment with zoledronic acid continues to be effective in old age. Bone 2009;44:S94 | Parallel publication, no additional information |
| Reid IR, Black DM, Eastell R, Bucci-Rechtweg C, Su G, Hue TF, et al. Reduction in the risk of clinical fractures after a single dose of zoledronic acid 5 milligrams. J Clin Endocrinol Metab 2013;98:557–63 | Parallel publication, no additional information |
| Rossini M, Gatti D, Zamberlan N, Braga V, Dorizzi R, Adami S. Long-term effects of a treatment course with oral alendronate of postmenopausal osteoporosis. J Bone Miner Res 1994;9:1833–7 | Not treatment of interest, not currently licensed dose |
| Roux C, Reid DM, Devogelaer JP, Saag K, Lau CS, Reginster JY, et al. Post hoc analysis of a single IV infusion of zoledronic acid versus daily oral risedronate on lumbar spine bone mineral density in different subgroups with glucocorticoid-induced osteoporosis. Osteoporos Int 2012;23:1083–90 | No outcomes of interest |
| Sambrook PN, Rodriguez JP, Wasnich RD, Luckey MM, Kaur A, Meng L, et al. Alendronate in the prevention of osteoporosis: 7-year follow-up. Osteoporos Int 2004;15:483–8 | Not comparator of interest |
| Sambrook PN, Silverman SL, Cauley JA, Recknor C, Olson M, Su G, et al. Health-related quality of life and treatment of postmenopausal osteoporosis: results from the HORIZON-PFT. Bone 2011;48;1298–304 | Parallel publication, no additional information |
| Schwartz AV, Bauer DC, Cummings SR, Cauley JA, Ensrud KE, Palermo L, et al. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010;25:976–82 | Parallel publication, no additional information |
| Seeman E. The antifracture efficacy of alendronate. Int J Clin Pract Suppl 1999;101:40–5 | Parallel publication, no additional information |
| Seeman E, Black D, Bucci-Rechtweg C, Eastell R, Boonen S, Mesenbrink P. Zoledronic acid substantially reduces the risk of morphometric vertebral and clinical fractures. Arthritis Rheum 2009;60:892 | Parallel publication, no additional information |
| Siris ES, Simon JA, Barton IP, McClung MR, Grauer A, Siris ES, et al. Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int 2008;19:681–6 | Parallel publication, no additional information |
| Stakkestad JA, Benevolenskaya LI, Stepan JJ, Skag A, Nordby A, Oefjord E et al. Intravenous ibandronate injections given every three months: a new treatment option to prevent bone loss in postmenopausal women. Ann Rheum Dis 2003;62:969–75 | Not treatment of interest, not currently licensed dose |
| Tee SI, Yosipovitch G, Chan YC, Chua SH, Koh ET, Chan YH, et al. Prevention of glucocorticoid-induced osteoporosis in immunobullous diseases with alendronate: a randomized, double-blind, placebo-controlled study. Arch Dermatol 2012;148:307–14 | Population outside scope of appraisal, not licensed indication |
| Thiébaud D, Burckhardt P, Kriegbaum H, Huss H, Mulder H, Juttmann JR, et al. Three monthly intravenous injections of ibandronate in the treatment of postmenopausal osteoporosis. Am J Med 1997;103:298–307 | Not treatment of interest, not currently licensed dose |
| Uchida S, Taniguchi T, Shimizu T, Kakikawa T, Okuyama K, Okaniwa M, et al. Therapeutic effects of alendronate 35 mg once weekly and 5 mg once daily in Japanese patients with osteoporosis: a double-blind, randomized study. J Bone Miner Metab 2005;23:382–8 | Not treatment of interest, not currently licensed dose |
| Wasnich RD, Bagger YZ, Hosking DJ, McClung MR, Wu M, Mantz AM, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 2004;11:622–30 | Not treatment of interest, not currently licensed dose |
| Westin JR, Thompson MA, Cataldo VD, Fayad LE, Fowler N, Fanale MA, et al. Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk 2013;13:99–105 | Not treatment of interest, not currently licensed dose |
| Yildirim K, Gureser G, Karatay S, Melikoglu MA, Ugur M, Erdal A, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskel Rehabil 2005;18:85–9 | No outcomes of interest |
Appendix 3 Network meta-analyses supplementary information
Additional details on the statistical models used for the network meta-analyses
Statistical model for the network meta-analysis of fracture outcomes
The RCTs presented data in terms of the number of individuals experiencing at least one fracture. For each fracture type, rik is defined as the number of events out of the total number of participants, nik, where the participants are receiving treatment tik in arm k of trial i. The data generation process is assumed to follow a binomial likelihood such that:
where pi,k represents the probability of an event in arm k of trial i (i = 1 . . . ns, k = 1 . . . na) after follow-up time fi. For all RCTs, the number of arms included in the analysis is 2 (i.e. na = 2) and the number of RCTs, ns, varies according to fracture type.
To account for different trial durations, an underlying Poisson process is assumed for each trial arm, so that Tik (the time until a fracture occurs in arm k of study i) follows an exponential distribution, Tik ∼ exp(λik), where λik is the event rate in arm k of study i, assumed constant over time. The probability that there are no events at time fi is given by the survivor function, P(Tik > fi) = exp(–λikfi). For each study, i, the probability of an event in arm k after follow-up time fi can be written as:
which is dependent on follow-up time. The probabilities of fracture are non-linear functions of event rates and so were modelled using the complementary log–log link function:
Here, the µi are trial-specific baselines, representing the log-hazards of fracture in the baseline treatment, which is assumed to be arm k = 1 for all trials. Note that for some trials, the baseline may be an active treatment rather than placebo. The trial-specific treatment effects, δi,1k, are log-HRs of fracture for the treatment in arm k, relative to the baseline treatment.
As described below, two different modelling strategies were considered for the treatment effects: (1) standard, independent random (treatment)-effects model; and (2) exchangeable treatment-effects model (i.e. effects model where the treatment effects are assumed to arise from a common distribution according to the class of drug). The main results are presented in Chapter 3, Results from the network meta-analyses and are based on the class-effects model for reasons discussed below, while the results for the standard independent random-effects model are provided in Results for the standard random-effects model for comparison.
Standard, independent random-effects model
The trial-specific treatment effects, δi,1k, were assumed to arise from a common population distribution with mean treatment effect relative to the reference treatment, which was defined as placebo for this analysis, such that:
where dti1tik represents the mean effect of the treatment in arm k of study i(tik) compared with the treatment in arm 1 of study i(ti1) and τ2 represents the between-study variance in treatment effects (heterogeneity), which is assumed to be the same for all treatments.
The model was completed by specifying prior distributions for the parameters. Where there were sufficient sample data, conventional reference prior distributions were used:
For both hip and wrist fracture outcomes, there were relatively few RCTs to allow Bayesian updating (i.e. estimation of parameters from the sample data alone) of the reference prior distribution for the between-study SD. When prior distributions do not represent reasonable prior beliefs then, in the absence of sufficient sample data, posterior distributions will not represent reasonable posterior beliefs. Therefore, rather than using a reference prior distribution, a weakly informative prior distribution was used for the between-study SD such that τ ∼ HN(0,0.322).
Only one RCT74 assessed the effect of ibandronic acid (monthly oral dose), relative to placebo, on hip fractures. There were no fractures in the control arm and the model was unable to converge for this parameter. A weakly informative prior distribution was used for the baseline of this study (details provided in Appendix 3), whereas reference prior distributions were used for the baselines of the remaining RCTs.
Class-effects model
Not all RCTs contributing wrist fracture data provide evidence about all bisphosphonates; in particular, there was no evidence about zoledronic acid. To allow an assessment of the uncertainty associated with zoledronic acid for inclusion in the economic model, a class-effects model was fitted from which the predictive distribution of a new intervention in the same class can be generated. This modelling approach also has the benefit of addressing data scarcity in the hip network without the need to use of a weakly informative prior for the baseline of Lester et al. 74 (as was required when fitting a standard, independent random-effects model).
A class-effects model was also fitted for all fracture types. Under a class-effects model, the trial-specific treatment effects are again assumed to be normally distributed as in Equation 8, but the mean effects of each treatment are assumed to be exchangeable and assumed to arise from a normal distribution with mean, D, and with variance τD2:
The model was completed by specifying prior distributions for the parameters.
For hip and wrist outcomes where information for some treatments was either weak or absent, a weakly informative prior was used for the between-treatment SD such that: σD2∼HN(0,0.322).
Predicting effects in new randomised controlled trials
To account for heterogeneity in the effect of treatments between RCTs, results are also presented for the predictive distributions of the effect of treatment in a new (randomly chosen) study.
From Equation 9, it follows that the study-specific population log-HR, δi,j, for study i, evaluating bisphosphonate j in reference to the control treatment can be written as:
where εij ∼ N(0,τ2). The predictive distribution for the effect of a particular bisphosphonate in a new study, δi,j, from the same class following, in a new study is:
The class-effects model also allows generation of the predictive distribution of a new, randomly chosen treatment from the same class. From Equation 13, it follows that the population log-HR for each treatment can be written as:
where ξj∼N(0,τD2). Therefore, combining Equations 16 and 18, the study-specific population log-HR, δij, for study i evaluating bisphosphonate j is:
For a new, randomly chosen, bisphosphonate, the expectation is E[δij] = E[D + ζj + ϵij] = D, with variance:
Therefore, the predictive distribution for the effect of a new, randomly chosen study from the same class is:
which accounts for both between-study, τ2, and between-treatment within class, τD2, heterogeneity for any (including a new) treatment.
It is the predictive distribution of a new treatment within the class and the predictive distribution of a new study for a new treatment within the class that we used to characterise the uncertainty about the effect of zoledronic acid for hip fractures.
Statistical model for the network meta-analysis of femoral neck bone mineral density
Data for femoral neck BMD outcomes were presented in two different formats: as the percentage change in femoral neck BMD for each treatment group or as the mean difference in the percentage change between treatment groups. Two different data generation (i.e. likelihood) models are therefore required.
Percentage change in femoral neck bone mineral density
The majority of RCTs presented data as the percentage change in femoral neck BMD, yik, and associated standard errors, seik, for arm k of trial i with study duration fi years. The data generation process is assumed to follow a normal likelihood such that:
where the population variance of the mean, seik2, is assume to be known and equal to the sample estimate. The parameters of interest, θik, are modelled using the identity link function and, to account for differing trial lengths, study duration was included as a trial-level covariate. The link function is given by:
where β11 = 0 and β1k (k = 2, . . na) are the treatment-specific interactions, describing the relationship between the effect of treatment on percentage change in femoral neck BMD and duration of study. The trial baselines, µi, represent the percentage change in femoral neck BMD from baseline in the reference arm. The treatment effects, δi,1k, represent the difference between the percentage change in the treatment group and the reference group. Assumptions about the relationship between the interaction terms are described further in the meta-regression section.
Difference between treatments in mean change in femoral neck bone mineral density
Some RCTs provided data in terms of the mean difference in percentage change in femoral neck BMD between two treatments, defined as:
together with the associated standard errors of the mean difference, νi,1k, rather than the percentage change in femoral neck BMD for individual treatments. The difference between treatments in the mean change are also assumed to be normally distributed such that:
where the population standard error of the difference, νi1k2, is assumed to be known and equal to the sample estimate. From the mean differences, no trial-specific effects of the baseline treatment can be estimated. The linear predictor is then given by
The study-specific treatment effects, δi,1k, have the same interpretation as those from the Equation 23 and thus can be combined to estimate the mean effects for each treatment, regardless of the way the data were reported.
A class-effects model was assumed such that the treatment effects of the individual bisphosphonates were assumed to be exchangeable and to arise from a normal distribution with mean, D, with variance τD2:
The model was completed by specifying prior distributions for the parameters, using conventional reference prior distributions:
-
trial-specific baseline, µi ∼ N(0,1002)
-
treatment effects relative to reference treatment, d1k ∼ N(0,1002)
-
between-study SD of treatment effects, τ ∼ U(0,100)
-
mean of related treatment effects, D ∼ N(0,1002)
-
between-treatment SD, τD ∼ U(0,100).
Meta-regression
Where appropriate, heterogeneity in treatment effects was explored by considering potential treatment effect modifiers. Meta-regression was used to test for interactions between the treatment effects and trial-level covariates, as described in Dias et al. 238
An interaction term, β, is introduced on the treatment effect by replacing:
where xi is the trial-level covariate for trial i and may represent a subgroup, continuous covariate, or baseline risk (as described in more detail in Metaregression on baseline risk/response), and β11 = 0. The regression is centred at the mean value of the covariate across the RCTs so that the interpretation of the treatment effect is as the effect at the average value of the covariate.
Different assumptions can be made about the relationship between the interaction terms for each treatment. For the main analysis, we assume a common interaction for each treatment relative to treatment 1, such that:
for k = 2, … , na. We also considered a model in which the interaction terms for each treatment were considered to be related but not identical (i.e. exchangeable) such that:
Metaregression on baseline risk/response
Baseline risk/response can be used as a proxy for differences in patient characteristics across trials that, may be modifiers of treatment effect, and so introduce a potential source of heterogeneity in the NMA. Adjustment for baseline risk/response was assessed using the method of Achana et al. 239
Dependence on baseline risk is introduced through an interaction term, so that:
where ϵi,ti1tik ∼ N(0,τ2). The updated study-specific treatment effects, δ¯i,1k, are now adjusted using the ‘true’ but unobserved baseline risk/response in the placebo arm of trial I, µip. The coefficient βti1tik represents the change in the treatment effect (e.g. log-HR or difference between treatments in mean change) per unit change in the baseline risk/response. The baseline risk/response is centred on µ¯P, the observed mean (e.g. log-HR or difference between treatments in mean change), in the placebo group, and β11 = 0.
For RCTs with an active treatment control, (ti1 ≠ P), there is no direct estimate of the placebo baseline risk/response. Under the consistency of evidence arising from the exchangeability assumption, the substitution dti1tik = dPtik – dPti1 can be made, allowing Equation 31 to be expressed as:
Although a placebo treatment may not be included in all RCTs, the assumption of exchangeability means that the treatment arms can be assumed missing at random without loss to efficacy, and the baseline risk/response in RCTs without a placebo arm can be estimated, borrowing strength from other RCTs. 239
As previously described (see Chapter 3, Methods for the network meta-analyses), some RCTs report data on the mean differences in percentage change between two treatments. Under the model described in Equations 25 and 21 study-specific effects of the baseline treatment cannot be estimated. These RCTs still contribute to the model through estimation of the treatment effects, but do not directly contribute to estimation of the slope in the meta-regression.
Statistical model for the meta-analysis of placebo baselines
To provide a suitable prior distribution for the study-specific baseline of Lester et al. ,74 a random-effects meta-analysis was performed on the placebo arms of all other studies. Again, the data generation process is assumed to follow a binomial likelihood, that is:
where pip represents the probability of an event in the placebo arm of trial i (i = . . . np). For the hip fracture network, the number of studies with placebo baseline, np, is 8. The probabilities of fracture are modelled using the complementary log–log link function:
A random-effects model is assumed, such that the trial-specific baselines are drawn from a normal distribution with common mean and variance:
To complete the model, common reference priors were assumed for the mean and variance, µi ∼ N(0,1002), and τm2∼U(0,2). The predictive distribution of a new baseline is given by
Assessing inconsistency between direct and indirect evidence
Inconsistency between direct and indirect evidence arises because of an imbalance in treatment effect modifiers across treatments comparing different pairs of treatments. Consistency of evidence was assessed using the node-splitting method of Dias et al. ,240 which separates evidence on a particular comparison into direct and indirect evidence.
In the case of fracture data, inconsistency was assessed for vertebral fractures only. For non-vertebral fractures, no indirect evidence was available. For hip and wrist fractures, an assessment of inconsistency was not performed because the direct evidence about treatment effect in the active comparator study is provided by one small study82 with no events in each baseline arm, thereby providing imprecise evidence of treatment effect.
All analyses were conducted in the freely available software package WinBUGS (MRC Biostatistics Unit, Cambridge, UK)241 and R (The R Foundation for Statistical Computing, Vienna, Austria), using the R2–WinBUGS interface package. 242 Convergence to the target posterior distributions was assessed using the Gelman–Rubin statistic, as modified by Brooks and Gelman,243 for two chains with different initial values. For all outcomes, a burn-in of 50,000 iterations of the Markov chain was used, with a further 20,000 iterations retained to estimate parameters. The NMA exhibited moderate correlation between successive iterations of the Markov chain, so were thinned by retaining every 10th sample.
Model fit was assessed using the total residual deviance, which provides an absolute measure of goodness of fit. 244 The total residual deviance can be compared with the number of independent data points to check whether or not the model provides a reasonable representation of the data. The DIC provides a relative measure of goodness of fit that penalises complexity and can be used to compare different models for the same likelihood and data. 245 Lower values of DIC are favourable, suggesting a more parsimonious model.
Data used to populate the network meta-analysis
A summary of the data used in the NMA is provided in Tables 42–46.
| Author and year of study publication (trial acronym) | Study duration (years) | Assessment method | Treatmentsa | Events | Number of participants | |||
|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | |||
| Cohen et al., 199963 | 1 | 0 | 1 | 2 | 5 | 2 | 35 | 34 |
| Fogelman et al., 200066 | 2 | 0 | 1 | 2 | 17 | 8 | 125 | 112 |
| Harris et al., 199970 (VERT-NA) | 3 | 0 | 1 | 2 | 93 | 61 | 678 | 696 |
| Reginster et al., 200085 (VERT-MN) | 3 | 0 | 1 | 2 | 89 | 53 | 346 | 344 |
| Hooper et al., 200572 | 2 | 0 | 1 | 2 | 10 | 10 | 125 | 129 |
| Reid et al., 200086 | 1 | 0 | 1 | 2 | 9 | 3 | 60 | 60 |
| Boonen et al., 200958 | 2 | 0 | 1 | 2 | 0 | 1 | 80 | 191 |
| Ringe et al., 200689 | 1 | 1 | 1 | 2 | 20 | 8 | 158 | 158 |
| Liberman et al., 199576 | 3 | 0 | 1 | 3 | 22 | 5 | 355 | 175 |
| Orwoll et al., 200083 | 2 | 0 | 1 | 3 | 7 | 1 | 94 | 146 |
| Black et al., 199655 (FIT I) | 3 | 0 | 1 | 3 | 192 | 83 | 965 | 981 |
| Cummings et al., 199864 (FIT II) | 4 | 0 | 1 | 3 | 78 | 43 | 2077 | 2057 |
| Dursun et al., 200165 | 1 | 0 | 1 | 3 | 14 | 12 | 35 | 38 |
| Carfora et al., 199860 | 2.5 | 0 | 1 | 3 | 4 | 1 | 34 | 34 |
| Boonen et al., 201259 | 2 | 0 | 1 | 4 | 28 | 9 | 574 | 533 |
| Black et al., 200756 (HORIZON-PFT) | 3 | 1 | 1 | 4 | 84 | 19 | 3861 | 3875 |
| Lyles et al., 200777 (HORIZON-RFT) | 3 | 1 | 1 | 4 | 39 | 21 | 1062 | 1065 |
| Chesnut et al., 200445 (BONE) | 3 | 0 | 1 | 6 | 93 | 46 | 975 | 977 |
| Muscoso et al., 200482 | 1 | NA | 2 | 3 | 0 | 2 | 100 | 1000 |
| Reid et al., 200988 | 1 | NA | 2 | 4 | 3 | 5 | 381 | 378 |
| Miller et al., 200881 | 1 | 1 | 3 | 5 | 5 | 5 | 859 | 874 |
| Author and year of study publication (trial acronym) | Study duration (years) | Treatmentsa | Events | Number of participants | |||
|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | ||
| Fogelman et al., 200066 | 3 | 1 | 2 | 13 | 7 | 125 | 112 |
| Harris, 199970 (VERT-NA) | 3 | 1 | 2 | 52 | 33 | 815 | 812 |
| Reginster et al., 200085 (VERT-MN) | 2 | 1 | 2 | 51 | 36 | 406 | 406 |
| Hooper et al., 200572 | 1 | 1 | 2 | 6 | 5 | 125 | 129 |
| Ringe et al., 200689 | 4 | 1 | 2 | 17 | 10 | 158 | 158 |
| Black et al., 199655 (FIT I) | 3 | 1 | 3 | 148 | 122 | 1005 | 1022 |
| Cummings et al., 199864 (FIT II) | 4 | 1 | 3 | 294 | 261 | 2077 | 2057 |
| Orwoll et al., 200083 | 2 | 1 | 3 | 5 | 6 | 94 | 146 |
| Pols et al., 199984 (FOSIT) | 1 | 1 | 3 | 37 | 19 | 958 | 950 |
| Bone et al., 200057 | 2 | 1 | 3 | 4 | 5 | 50 | 92 |
| Black et al., 200756 (HORIZON-PFT) | 0.92 | 1 | 4 | 388 | 292 | 3861 | 3875 |
| Lyles et al., 200777 (HORIZON-RFT) | 3 | 1 | 4 | 107 | 79 | 1062 | 1065 |
| Chesnut et al., 200445 (BONE) | 3 | 1 | 6 | 80 | 89 | 975 | 977 |
| Miller et al., 200881 (MOTION) | 1 | 3 | 5 | 12 | 14 | 859 | 874 |
| Author and year of study publication (trial acronym) | Study duration (years) | Treatmentsa | Events | Number of participants | |||
|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | ||
| McClung et al., 200178 | 3 | 1 | 2 | 46 | 32 | 1821 | 1812 |
| Harris et al., 199970 (VERT-NA) | 3 | 1 | 2 | 15 | 12 | 815 | 812 |
| Reginster et al., 200085 (VERT-MN) | 3 | 1 | 2 | 11 | 9 | 406 | 406 |
| Black et al., 199655 (FIT I) | 3 | 1 | 3 | 22 | 11 | 1005 | 1022 |
| Cummings et al., 199864 (FIT II) | 4 | 1 | 3 | 24 | 19 | 2218 | 2214 |
| Greenspan et al., 200267 | 2 | 1 | 3 | 4 | 2 | 164 | 163 |
| Black et al., 200756 (HORIZON-PFT) | 3 | 1 | 4 | 88 | 52 | 3861 | 3875 |
| Lyles et al., 200777 (HORIZON-RFT) | 3 | 1 | 4 | 33 | 79 | 1062 | 1065 |
| Lester et al., 200874 (ARIBON) | 2 | 1 | 5 | 0 | 1 | 19 | 21 |
| Muscoso et al., 200482 | 1 | 2 | 3 | 0 | 1 | 100 | 1000 |
| Author and year of study publication (trial acronym) | Study duration (years) | Treatmentsa | Events | Number of participants | |||
|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | ||
| Harris et al., 199970 (VERT-NA) | 3 | 1 | 2 | 22 | 14 | 815 | 812 |
| Reginster et al., 200085 (VERT-MN) | 3 | 1 | 2 | 21 | 15 | 406 | 406 |
| Black et al., 199655 (FIT I) | 3 | 1 | 3 | 41 | 22 | 1005 | 1022 |
| Cummings et al., 199864 (FIT II) | 4 | 1 | 3 | 70 | 83 | 2218 | 2214 |
| McClung et al., 200980 | 1 | 1 | 4 | 0 | 1 | 83 | 77 |
| Lester et al., 200874 (ARIBON) | 2 | 1 | 4 | 1 | 1 | 19 | 21 |
| Muscoso et al., 200482 | 1 | 2 | 3 | 0 | 1 | 100 | 1000 |
| Author and year of study publication (trial acronym) | Study duration (years) | Treatmentsa | % change in BMD | Standard error % change in BMD | Number of participants | Mean difference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | Arm 1 | Arm 2 | % change in BMD | Standard error | ||
| Adami et al., 199553 | 2 | 1 | 2 | –2.58 | 1.19 | 0.89 | 0.88 | 62 | 61 | NA | NA |
| Bone et al., 200057 | 2 | 1 | 2 | –0.6 | 2.9 | 0.60 | 0.50 | 46 | 87 | NA | NA |
| Dursun et al., 200165 | 1 | 1 | 2 | 2.33 | 3.75 | 0.73 | 1.00 | 35 | 38 | NA | NA |
| Pols et al., 199984 (FOSIT) | 1 | 1 | 2 | –0.2 | 2.3 | 0.15 | 0.15 | 884 | 863 | NA | NA |
| Greenspan et al., 200368 | 3 | 1 | 2 | –0.65 | 4.2 | 0.53 | 0.59 | 93 | 93 | NA | NA |
| Orwoll et al., 200083 | 2 | 1 | 2 | –0.1 | 2.5 | 0.50 | 0.40 | 81 | 128 | NA | NA |
| Saag et al., 199891 | 0.92 | 1 | 2 | –1.2 | 1 | 0.40 | 0.40 | 142 | 145 | NA | NA |
| Klotz et al., 201373 | 1 | 1 | 2 | –2.06 | 1.65 | 0.78 | 1.12 | 53 | 45 | NA | NA |
| Fogelman et al., 200066 | 2 | 1 | 3 | –1 | 1.3 | 0.32 | 0.44 | 180 | 175 | NA | NA |
| Harris 1999 et al.70 (VERT-NA) | 3 | 1 | 3 | –1.2 | 1.6 | 0.45 | 0.60 | 417 | 457 | NA | NA |
| Leung et al., 200575 | 1 | 1 | 3 | 1.1 | 1.8 | 0.90 | 0.70 | 34 | 31 | NA | NA |
| Cohen et al., 199963 | 1 | 1 | 3 | –2.94 | –1.04 | 0.84 | 0.94 | 36 | 34 | NA | NA |
| Reid et al., 200086 | 1 | 1 | 3 | –0.29 | 1.63 | 0.50 | 0.62 | 43 | 52 | NA | NA |
| Boonen et al., 200958 | 2 | 1 | 3 | 0.73 | 1.71 | 0.34 | 0.25 | 93 | 191 | NA | NA |
| Choo et al., 201162 | 2 | 1 | 3 | –5.56 | –2.55 | 2.92 | 2.89 | 52 | 52 | NA | NA |
| Taxel et al., 201095 | 1 | 1 | 3 | –2 | 0 | 0.61 | 0.61 | 20 | 20 | NA | NA |
| McClung et al., 200979 | 2 | 1 | 4 | –1.35 | 1.64 | 0.29 | 0.31 | 202 | 181 | NA | NA |
| Boonen et al., 201259 | 2 | 1 | 4 | 0.1 | 3.4 | 0.58 | 0.60 | 63 | 56 | NA | NA |
| McClung et al., 200980 | 1 | 1 | 5 | –0.73 | 1.09 | 0.46 | 0.33 | 83 | 77 | NA | NA |
| Sarioglu et al., 200692 | 1 | 2 | 3 | 3.7 | 2.6 | 0.96 | 0.60 | 25 | 25 | NA | NA |
| Miller et al., 200881 (MOTION) | 1 | 2 | 5 | 2.3 | 2.1 | 0.07 | 0.06 | 822 | 836 | NA | NA |
| Reid et al., 200988 (HORIZON) | 1 | 3 | 4 | 0.39 | 1.4 | 0.25 | 0.26 | 374 | 373 | NA | NA |
| Miller et al., 200547 (MOBILE) | 1 | 5 | 6 | 2.22 | 1.71 | 0.21 | 0.21 | 320 | 318 | NA | NA |
| Delmas et al., 200649 (DIVA) | 1 | 6 | 7 | 1.6 | 2.3 | 0.21 | 0.20 | 381 | 368 | NA | NA |
| Black et al., 199655 (FIT I) | 3 | 1 | 2 | –0.31 | 3.54 | 0.18 | 0.17 | 1005 | 1022 | 4.10 | 0.25 |
| Cummings et al., 199864 (FIT II) | 4 | 1 | 2 | –0.8 | 3.6 | 0.16 | 0.16 | 2218 | 2214 | 4.60 | 0.23 |
| Greenspan et al., 200267 | 2 | 1 | 2 | –0.36 | 2.84 | 0.06 | 0.35 | 164 | 163 | 3.40 | 0.50 |
| Liberman et al., 199576 | 3 | 1 | 2 | –1.28 | 4.65 | 0.30 | 0.47 | 397 | 196 | 5.90 | 0.50 |
| Hooper et al., 200572 | 2 | 1 | 3 | –2.43 | 2.29 | 0.33 | 0.20 | 125 | 125 | 3.30 | 0.27 |
| Reginster et al., 200085 (VERT-MN) | 3 | 1 | 3 | –0.97 | 2.09 | 0.37 | 0.38 | 407 | 407 | 3.10 | 0.70 |
| Lyles et al., 200777 (HORIZON-RFT) | 3 | 1 | 4 | NA | NA | NA | NA | 1062 | 1065 | 2.90 | 1.31 |
| Black et al., 200756 (HORIZON-PFT) | 3 | 1 | 4 | –0.04 | 5.06 | 0.16 | 0.15 | 3083 | 3067 | 5.06 | 0.15 |
| Chesnut et al., 200445 (BONE) | 3 | 1 | 6 | NA | NA | NA | NA | 975 | 977 | 2.20 | 0.86 |
| Rosen et al., 200590 (FACT) | 1 | 2 | 3 | 1.6 | 0.9 | 0.21 | 0.21 | 454 | 438 | –0.70 | 0.28 |
| Reid et al., 200687 (FACTS) | 1 | 2 | 3 | 2.25 | 1.67 | 0.18 | 0.18 | 424 | 430 | –0.56 | 0.27 |
Results for the standard random-effects model
Vertebral fractures: random-effects model
The model fitted the data well, with the total residual deviance of 42.17 being close to the total number of data points, 42, included in the analysis. The DIC was 72.50, suggesting a mild decline in model fit compared with the class-effects model (DIC 69.28). The between-study SD was estimated to be 0.20 (95% CrI 0.02 to 0.57), implying mild heterogeneity in treatment effects between studies.
FIGURE 131.
Vertebral fractures: random-effects model. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

Non-vertebral fractures: random-effects model
The model fitted the data well, with the total residual deviance of 22.78 being close to the total number of data points, 28, included in the analysis. The DIC was 43.47, suggesting a mild decline in model fit compared with the class-effects model (DIC 42.32). The between-study SD was estimated to be 0.08 (95% CrI 0.00 to 0.35), implying mild heterogeneity in treatment effects between studies.
FIGURE 132.
Non-vertebral fractures: random-effects model. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

Hip fractures: random-effects model
There were insufficient studies with which to estimate the between-study SD from the sample data alone and there were no events in the baseline treatment in the Lester et al. 74 study, which meant that the Markov chain did not converge. In this case, a weakly informative prior distribution was used for the between-study SD such that τi ∼ HN(0,0.322) and weakly informative prior distribution for the study-specific baseline of the Lester et al. 74 study such that µi ∼ N(–3.56,0.592); this was generated by performing a random-effects meta-analysis of the baselines from the other studies.
The model fitted the data well, with the total residual deviance of 17.73 being close to the total number of data points, 18, included in the analysis. The DIC was 33.61, suggesting little difference in model fit compared with the class-effects model (DIC 33.82). The between-study SD was estimated to be 0.44 (95% CrI 0.23 to 0.76), implying moderate heterogeneity between studies.
FIGURE 133.
Hip fractures: random-effects model. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).
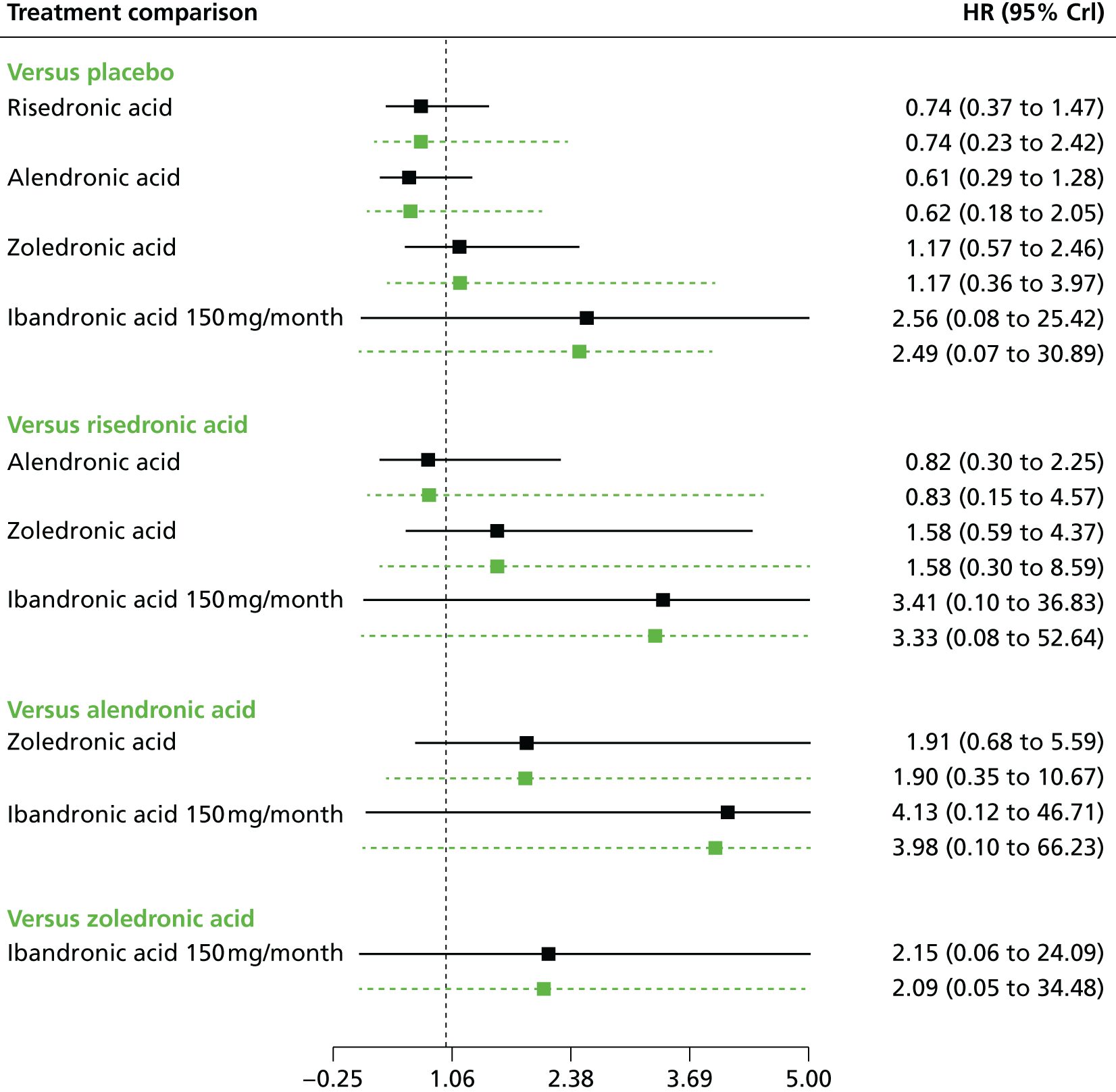
Wrist fractures: random-effects model
There were insufficient studies with which to estimate the between-study SD from the sample data alone. In this case, a weakly informative prior distribution was used for the between-study SD such that τ ∼ HN(0,0.322).
The model fitted the data well, with the total residual deviance, 13.88, being close to the total number of data points included in the analysis, 12. The DIC was 24.70, suggesting a mild decline in model fit compared with the class-effects model (DIC 23.23). The between-study SD was estimated to be 0.30 (95% CrI 0.03 to 0.71), implying mild to moderate heterogeneity between studies.
FIGURE 134.
Wrist fractures: random-effects model. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

Femoral neck bone mineral density, random-effects model
The model fitted the data well, with the total residual deviance of 55.30 being close to the total number of data points included in the analysis, 59. The DIC was 99.34, suggesting a mild decline in model fit compared with the class-effects model (DIC 96.5). The between-study SD was estimated to be 0.55 (95% CrI 0.31 to 0.88), implying moderate heterogeneity between studies.
FIGURE 135.
Femoral neck BMD: random-effects model – treatment effects (TE) and 95% Crls. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. TEs to the right of the reference line favour the comparator treatment.

Clinical vertebral fractures: random-effects model
There were insufficient studies with which to estimate the between-study SD from the sample data alone. In this case, a weakly informative prior distribution was used for the between-study SD such that τ ∼ HN(0,0.322).
The model fitted the data well, with the total residual deviance, 6.56, being close to the total number of data points included in the analysis, 6. The between-study SD was estimated to be 0.32 (95% CrI 0.03 to 0.78), which implies mild to moderate heterogeneity between studies.
FIGURE 136.
Clinical vertebral fractures: random-effects model. Note that the mean effect estimates are plotted in black and the predictive effects in a new study are plotted in green beneath. Points to the right of the line favour the reference treatment (shown in green text).

Appendix 4 Adverse events reported across included randomised controlled trials
Appendix 5 Summary of review findings of adverse events associated with bisphosphonates
Appendix 6 Summary of review findings of compliance and concordance with bisphosphonates
Appendix 7 Table of excluded studies: cost-effectiveness review
| Citation | Reason for exclusion |
|---|---|
| Jansen J, Gaugris S, Bergman G, Sen SS. P339. Cost-effectiveness of Fasavance® in the treatment and prevention of osteoporosis in the United Kingdom. Osteoporos Int 2006;17(Suppl. 1):S96 | Conference abstract |
| Liu H, Michaud K, Nayak S, Karpf DB, Owens DK, Garber AM. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Int Med 2006;166:1209–17 | Excluded interventions |
| Boonen S. Impact of treatment efficacy and dosing frequency on cost-effectiveness of bisphosphonate treatment for osteoporosis: A perspective. Curr Med Res Opin 2009;25:2335–41 | Systematic review |
| Botteman MF, Meijboom M, Foley I, Stephens JM, Chen YM, Kaura S. Cost-effectiveness of zoledronic acid in the prevention of skeletal-related events in patients with bone metastases secondary to advanced renal cell carcinoma: application to France, Germany, and the United Kingdom. Eur J Health Econ 2011;12:575–88 | Patients with renal cell carcinoma |
| Brandao CMR, Machado GPM, Acurcio FA. Pharmacoeconomic analysis of treatment strategies for osteoporosis in postmenopausal women: a systematic review. Rev Bras Reumatol 2012;52:924–37 | Systematic review |
| Cowell W, Koay A, Hunjan M. Economic analysis: ibandronate (Bonviva®) IV injection for the treatment of postmenopausal osteoporosis (PMO) in the UK. Value Health 2006;9:A380 | Conference abstract |
| Dell R, Greene D. Is osteoporosis disease management cost effective? Curr Osteoporos Rep 2010;8:49–55 | USA location |
| Fardellone P, Cortet B, Thomas T, Legrand E, Bresse X, Bisot-Locard S, et al. Cost-effectiveness simulation modeling of the compliance of 5 mg zoledronic acid once a year versus current treatments in post-menopausal osteoporosis. Value Health 2007;10:A395 | Conference abstract |
| Farquhar D, Pasquale M. Cost-effectiveness of risedronate versus ibandronate at one year: the case of the United Kingdom. J Bone Miner Res 2008;23:S212 | Conference abstract |
| Grima D, Borisov N. Cost-effectiveness of risedronate vs. generic alendronate: 1-year analysis among women 50–64 years old. J Bone Miner Res 2008;23:S212 | Conference abstract |
| Halperin M. The ethics of generics: medical and economic advantages of a generic alendronate in treating osteoporosis patients. Osteoporos Int 2006;17:S263 | Conference abstract |
| Hiligsmann M, Ethgen O, Bruyere O, Reginster J-Y. An economic evaluation of quantitative ultrasonometry as pre-screening test for the identification of patients with osteoporosis. Dis Manag Health Outcomes 2008;16:429–438 | Cost-effectiveness of a pretreatment scanning strategy |
| Hiligsmann M, Bruyere O, Ethgen O, Reginster J. Cost-effectiveness of bone densitometry screening combined with alendronate therapy for those who have osteoporosis. Value Health 2007;10:A236 | Conference abstract |
| Hiligsmann M, Kanis JA, Compston J, Cooper C, Flamion B, Bergmann P, et al. Health technology assessment in osteoporosis. Calcif Tissue Int 2013;93:1–14 | Systematic review |
| Jansen J, Gaugris S, Bergman G, Sen S. Cost-effectiveness of Fosavance® in the treatment and prevention of osteoporosis in the United Kingdom. Osteoporos Int 2006;17:S96 | Conference abstract |
| Jansen JP, Gaugris S, Bergman G, Sen SS, Jansen JP, Gaugris S, et al. Cost-effectiveness of a fixed dose combination of alendronate and cholecalciferol in the treatment and prevention of osteoporosis in the United Kingdom and the Netherlands. Curr Med Res Opin 2008;24:671–84 | Excluded interventions |
| Johnell O. Cost effectiveness of alendronate (fosamax) for the treatment of osteoporosis and prevention of fractures. PharmacoEconomics 2006;21:305–14 | Swedish location |
| Kanis J, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int 2011;22:2565–73 | Systematic review |
| Kanis JA, McCloskey EV, Jonsson B, Cooper A, Ström O, Borgström F. An evaluation of the NICE guidance for the prevention of osteoporotic fragility fractures in postmenopausal women. Arch Osteoporos 2010;5:19–48 | Excluded interventions |
| Kanis JA, McCloskey EV, Johansson H, Ström O, Borgström F, Oden A, et al. Case finding for the management of osteoporosis with FRAX–assessment and intervention thresholds for the UK. Osteoporos Int 2008;19:1395–408. [Erratum published in Osteoporos Int 2009;20:499–502] | Very limited discussion of modelling |
| Kanis JA, Adams J, Borgström F, Cooper C, Jonsson B, Preedy D, et al. Modelling cost-effectiveness in osteoporosis. Bone 2008;43:215–16 | Response to a letter published previously in the same journal |
| Logman F, Heeg B, Botteman M, Marfatia A, van Hout B. Cost-effectiveness of zoledronic acid in the prevention of fractures in postmenopausal women with early breast cancer receiving aromatase inhibitor: application to the United Kingdom. EJC Suppl 2007;5:156 | Conference abstract |
| Logman F, Heeg B, Botteman M, Kaura S, van Hout B. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in post-menopausal women with early breast cancer receiving aromatase inhibitors in the United Kingdom. Cancer Res 2009;69:S574 | Conference poster |
| Logman J, Heeg B, Botteman M, Kaura S, van Hout B. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in post-menopausal women with early-stage breast cancer receiving aromatase inhibitors in the United Kingdom. EJC Suppl 2008;6:69–70 | Conference abstract |
| Logman JF, Heeg BM, Botteman MF, Kaura S, van Hout BA, Logman JFS, et al. Economic evaluation of zoledronic acid for the prevention of osteoporotic fractures in postmenopausal women with early-stage breast cancer receiving aromatase inhibitors in the UK. Ann Oncol 2010;21:1529–36 | Excluded intervention |
| Lynch N, Earnshaw S, Graham C, Middelhoven H. Cost-effectiveness of ibandronate injection IV in the treatment of UK women with postmenopausal osteoporosis who are intolerant to oral bisphosphonates. Osteoporos Int 2007;18:S11–12 | Conference abstract |
| Lynch N, Earnshaw S, Beard S, Cowell W. Ibandronate is cost-effective in the treatment of postmenopausal osteoporosis: a comparision of bisphosphonates. Osteoporos Int 2006;17:S11 | Conference abstract |
| Lynch N, Earnshaw S, Graham C, Patroe V, Boisdron J, Middelhoven H. Ibandronate IV injection is cost-effective in the treatment of UK women with postmenopausal osteoporosis who are intolerant to oral bisphosphonates. Ann Rheum Dis 2007;66:529 | Conference abstract |
| McLellan AR, Wolowacz SE, Zimovetz EA, Beard SM, Lock S, McCrink L, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083–98 | Cost-effectiveness assessment of methods of treatment delivery with same pharmaceuticals use in both arms |
| Olson M, Brereton N, Huels J, Roberts D, Akerhurst R. Comparison of the cost-effectiveness of zoledronic acid 5 mg for the management of post-menopausal osteoporosis in the UK setting. Value Health 2007;10:A395–6 | Conference abstract |
| Rizzoli R, Akesson K, Bouxsein M, Kanis J, Napoli N, Papapoulos S, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011;22:373–90 | Systematic review |
| Rosenzweig A, Mishra R. Evaluation and management of osteoporosis and fragility fractures in the elderly. Aging Health 2009;5:833–50 | Review of osteoporosis, prevention and treatment, no economic aspect |
| Simbula S, Burchini G, Santarlasci B, Trippoli S, Messori A. Cost-effectiveness analysis of therapeutic or preventive interventions <not enough value for money>. G Itali Farmacia Clin 2008;22:86–105 | Full-text paper not in the English language |
| Stevenson MD, Oakley JE, Lloyd JM, Brennan A, Compston JE, McCloskey EV, et al. The cost-effectiveness of an RCT to establish whether 5 or 10 years of bisphosphonate treatment is the better duration for women with a prior fracture. Med Dec Making 2009;29:678–89 | Establishing optimum duration of treatment |
| Stevenson MD, Jones ML, Stevenson MD, Jones ML. The cost effectiveness of a randomized controlled trial to establish the relative efficacy of vitamin K1 compared with alendronate. Med Dec Making 2011;31:43–52 | Excluded interventions |
| Sunyecz J, Silberman C, Poston S, Earnshaw S. Cost-effectiveness of ibandronate therapy for women with postmenopausal osteoporosis with respect to nonvertebral fracture efficacy. J Bone Miner Res 2008;23:S213 | Conference abstract |
| Warde N. Prostate cancer: is fracture prevention therapy cost-effective in patients with prostate cancer treated with ADT? Nature Rev Urol 2010;7:363 | In-brief article |
Appendix 8 Parameter distributions used in the probabilistic sensitivity analysis
| Parameter description | Distribution | Mean | Standard error | Parameter 1 | Parameter 2 | Source(s) |
|---|---|---|---|---|---|---|
| Patients hospitalised | ||||||
| Following vertebral fracture | Beta | 0.40 | NA | α = 587 | β = 884 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Beta | 0.29 | NA | α = 2081 | β = 4989 | Gutierrez et al.234 |
| Following humerus fracture | Beta | 0.35 | NA | α = 894 | β = 1651 | Gutierrez et al.234 |
| Following hip fracture | Fixed | 1.00 | NA | NA | NA | Gutierrez et al.233 |
| A&E visits | ||||||
| Following vertebral fracture | Beta | 0.11 | NA | α = 171 | β = 1300 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Beta | 0.21 | NA | α = 1489 | β = 5581 | Gutierrez et al.234 |
| Following humerus fracture | Beta | 0.18 | NA | α = 469 | β = 2076 | Gutierrez et al.234 |
| Following hip fracture | Beta | 0.18 | NA | α = 442 | β = 1985 | Gutierrez et al.233 |
| GP visits | ||||||
| Following vertebral fracture | Beta | 0.97 | NA | α = 1425 | β = 46 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Beta | 0.95 | NA | α = 6689 | β = 381 | Gutierrez et al.234 |
| Following humerus fracture | Beta | 0.94 | NA | α = 2385 | β = 160 | Gutierrez et al.234 |
| Following hip fracture | Beta | 0.88 | NA | α = 2141 | β = 286 | Gutierrez et al.233 |
| Referral visits | ||||||
| Following vertebral fracture | Beta | 0.50 | NA | α = 730 | β = 741 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Beta | 0.37 | NA | α = 2623 | β = 4447 | Gutierrez et al.234 |
| Following humerus fracture | Beta | 0.34 | NA | α = 875 | β = 1670 | Gutierrez et al.234 |
| Following hip fracture | Beta | 0.33 | NA | α = 805 | β = 1622 | Gutierrez et al.233 |
| Patient deaths | ||||||
| Following vertebral fracture | Beta | 0.09 | NA | α = 131 | β = 1340 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Beta | 0.04 | NA | α = 271 | β = 6799 | Gutierrez et al.234 |
| Following humerus fracture | Beta | 0.07 | NA | α = 197 | β = 2348 | Gutierrez et al.234 |
| Following hip fracture | Beta | 0.08 | NA | α = 197 | β = 2230 | Gutierrez et al.233 |
| Patients hospitalised | ||||||
| Matched controls for vertebral fracture | Beta | 0.17 | NA | α = 245 | β = 1226 | Gutierrez et al.234 |
| Matched controls for wrist or forearm fracture | Beta | 0.13 | NA | α = 895 | β = 6175 | Gutierrez et al.234 |
| Matched controls for humerus fracture | Beta | 0.15 | NA | α = 383 | β = 2162 | Gutierrez et al.234 |
| Matched controls for hip fracture | Beta | 0.18 | NA | α = 432 | β = 1995 | Gutierrez et al.233 |
| A&E visits | ||||||
| Matched controls for vertebral fracture | Beta | 0.04 | NA | α = 64 | β = 1407 | Gutierrez et al.234 |
| Matched controls for wrist or forearm fracture | Beta | 0.03 | NA | α = 208 | β = 6862 | Gutierrez et al.234 |
| Matched controls for humerus fracture | Beta | 0.03 | NA | α = 82 | β = 2463 | Gutierrez et al.234 |
| Matched controls for hip fracture | Beta | 0.04 | NA | α = 95 | β = 2332 | Gutierrez et al.233 |
| GP visits | ||||||
| Matched controls for vertebral fracture | Beta | 0.90 | NA | α = 1319 | β = 152 | Gutierrez et al.234 |
| Matched controls for wrist or forearm fracture | Beta | 0.89 | NA | α = 6268 | β = 802 | Gutierrez et al.234 |
| Matched controls for humerus fracture | Beta | 0.91 | NA | α = 2305 | β = 240 | Gutierrez et al.234 |
| Matched controls for hip fracture | Beta | 0.91 | NA | α = 2200 | β = 227 | Gutierrez et al.233 |
| Referral visits | ||||||
| Matched controls for vertebral fracture | Beta | 0.32 | NA | α = 475 | β = 996 | Gutierrez et al.234 |
| Matched controls for wrist or forearm fracture | Beta | 0.28 | NA | α = 1988 | β = 5082 | Gutierrez et al.234 |
| Matched controls for humerus fracture | Beta | 0.29 | NA | α = 749 | β = 1796 | Gutierrez et al.234 |
| Matched controls for hip fracture | Beta | 0.32 | NA | α = 775 | β = 1652 | Gutierrez et al.233 |
| Difference in medications prescribed between patients with a previous fracture and those without | ||||||
| Following vertebral fracture | Normal | 22.35 | 2.16 | µ = 2.35 | σ = 2.16 | Gutierrez et al.234 |
| Following wrist or forearm fracture | Normal | 4.61 | 0.61 | µ = 4.61 | σ = 0.61 | Gutierrez et al.234 |
| Following humerus fracture | Normal | 4.61 | 0.61 | µ = 4.61 | σ = 0.61 | Gutierrez et al.234 |
| Following hip fracture | Normal | 12.34 | 1.72 | µ = 2.34 | σ = 1.72 | Gutierrez et al.233 |
| Utility multipliers in year of fracture | ||||||
| Hip fracture | Beta | 0.69 | 0.016 | α = 575.84 | β = 258.71 | Ström et al.226 |
| Vertebral fracture | Beta | 0.57 | 0.035 | α = 113.48 | β = 85.61 | Ström et al.226 |
| Humerus fracture | Beta | 0.86 | 0.085 | α = 13.47 | β = 2.19 | Ström et al.226 |
| Wrist or forearm fracture | Beta | 0.88 | 0.015 | α = 412.13 | β = 56.20 | Zethraeus et al.220 |
| Utility multiplier in subsequent years | ||||||
| Hip fracture | Beta | 0.85 | 0.016 | α = 422.49 | β = 74.56 | Ström et al.226 |
| Vertebral fracture | Beta | 0.66 | 0.035 | α = 120.24 | β = 61.94 | Strom et al.226 |
| Humerus fracture | Fixed | 1.00 | NA | NA | NA | Zethraeus et al. 220 |
| Wrist or forearm fracture | Beta | 0.98 | 0.015 | α = 84.39 | β = 1.72 | Ström et al.226 |
| Patient admitted to nursing home | Beta | 0.63 | 0.191 | α = 3.38 | β = 2.03 | Tidermark et al.225 |
| Life expectancy for patient suffering a fatal hip fracture | Fixed | 0.25 | NA | NA | NA | Assumption |
| RR of mortality following hip fracture for patients admitted to a nursing home | Log-normal | 0.57 | 0.074 | µ = –0.56212 | σ = 0.13150 | Smith et al.157 |
| Duration of treatment (years) | ||||||
| Alendronic acid/risedronic acid/ibandronic acid (oral) | Normal | 0.504 | 0.028 | µ = 0.504 | σ = 0.028 | Imaz et al.128 |
| ibandronic acid (i.v. preparation) | Normal | 1.100 | 0.041 | µ = 1.100 | σ = 0.041 | Curtis et al. 2012170 |
| Zoledronic acid | Normal | 1.700 | 0.018 | µ = 1.700 | σ = 0.018 | Curtis et al. 2012170 |
| Annual cost of treatment | ||||||
| Alendronic acid | Fixed | £14.73 | NA | NA | NA | Drug tariff151 |
| Risedronic acid | Fixed | £16.43 | NA | NA | NA | Drug tariff151 |
| ibandronic acid (oral preparation) | Fixed | £13.58 | NA | NA | NA | Drug tariff151 |
| ibandronic acid (i.v. preparation) | Fixed | £221.52 | NA | NA | NA | eMIT152 |
| Zoledronic acid | Fixed | £339.67 | NA | NA | NA | eMIT152 |
| Patient admitted to nursing home | Fixed | £36,608.00 | NA | NA | NA | Care Quality Commission236 and Curtis26 |
| Hours of home help per week in first 4 months after fracture | ||||||
| Hip fracture | Normal | 1.87 | 0.30 | µ = 1.87 | σ = 0.30 | Borgström et al.207 |
| Vertebral fracture | Normal | 1.88 | 0.50 | µ = 1.88 | σ = 0.50 | Borgström et al.207 |
| Humerus/wrist or forearm fracture | Normal | 0.21 | 0.10 | µ = 0.21 | σ = 0.10 | Borgström et al.207 |
| Hours of home help per week in months 8–12 following fracture | ||||||
| Hip fracture | Normal | 1.42 | 0.21 | µ = 1.42 | σ = 0.21 | Borgström et al.207 |
| Vertebral fracture | Normal | 2.56 | 0.61 | µ = 2.56 | σ = 0.61 | Borgström et al.207 |
| Humerus/wrist or forearm fracture | Normal | 0.07 | 0.04 | µ = 0.07 | σ = 0.04 | Borgström et al.207 |
| Description | Distribution | Mid-point | Standard error | Parameter 1 | Parameter 2 | Source |
|---|---|---|---|---|---|---|
| HR for future hip fracture given | ||||||
| Prior hip fracture | Log-normal | 2.3 | 0.561 | µ = 0.832909 | σ = 0.230323 | Klotzbuecher et al.149 |
| Prior vertebral fracture | Log-normal | 2.3 | 0.204 | µ = 0.832909 | σ = 0.085835 | Klotzbuecher et al.149 |
| Prior humerus fracture | Log-normal | 2.0 | 0.077 | µ = 0.693147 | σ = 0.037399 | Klotzbuecher et al.149 |
| Prior wrist/forearm fracture | Log-normal | 1.9 | 0.153 | µ = 0.641854 | σ = 0.081238 | Klotzbuecher et al.149 |
| HR for future vertebral fracture given | ||||||
| Prior hip fracture | Log-normal | 2.5 | 0.434 | µ = 0.916291 | σ = 0.169637 | Klotzbuecher et al.149 |
| Prior vertebral fracture | Log-normal | 4.4 | 0.459 | µ = 1.481605 | σ = 0.103435 | Klotzbuecher et al.149 |
| Prior humerus fracture | Log-normal | 2.0 | 0.204 | µ = 0.693147 | σ = 0.103435 | Klotzbuecher et al.149 |
| Prior wrist/forearm fracture | Log-normal | 1.7 | 0.179 | µ = 0.530628 | σ = 0.103435 | Klotzbuecher et al.149 |
| HR for future humerus fracture given | ||||||
| Prior hip fracture | Log-normal | 2.1 | 4.337 | µ = 0.741937 | σ = 1.034357 | Warriner et al.213 |
| Prior vertebral fracture | Log-normal | 1.6 | 0.587 | µ = 0.470004 | σ = 0.371247 | Warriner et al.213 |
| Prior humerus fracture | Log-normal | 2.1 | 4.337 | µ = 0.741937 | σ = 1.034357 | Klotzbuecher et al.149 |
| Prior wrist/forearm fracture | Log-normal | 2.5 | 2.449 | µ = 0.916291 | σ = 0.722759 | Warriner et al.213 |
| HR for future wrist/forearm fracture given | ||||||
| Prior hip fracture | Log-normal | 3.0 | 1.327 | µ = 1.098612 | σ = 0.410571 | Warriner et al.213 |
| Prior vertebral fracture | Log-normal | 1.4 | 0.128 | µ = 0.336472 | σ = 0.088854 | Klotzbuecher et al.149 |
| Prior humerus fracture | Log-normal | 1.9 | 0.383 | µ = 0.641854 | σ = 0.195728 | Klotzbuecher et al.149 |
| Prior wrist/forearm fracture | Log-normal | 3.3 | 0.383 | µ = 1.193922 | σ = 0.142759 | Klotzbuecher et al.149 |
| Description | Distribution | Mean | Standard error | Parameter 1 | Parameter 2 | Source |
|---|---|---|---|---|---|---|
| Female patients, age (years) | ||||||
| 30–39 | Fixed | 0.000 | NA | NA | NA | van Staa et al.141 |
| 40–49 | Fixed | 0.000 | NA | NA | NA | van Staa et al.141 |
| 50–59 | Beta | 0.024 | NA | α = 21.649 | β = 880.386 | van Staa et al.141 |
| 60–69 | Beta | 0.044 | NA | α = 109.383 | β = 2376.587 | van Staa et al.141 |
| 70–79 | Beta | 0.075 | NA | α = 301.095 | β = 3713.504 | van Staa et al.141 |
| 80–89 | Beta | 0.114 | NA | α = 433.698 | β = 3370.667 | van Staa et al.141 |
| 90–99 | Beta | 0.136 | NA | α = 139.921 | β = 888.912 | van Staa et al.143 |
| Male patients, age (years) | ||||||
| 30–39 | NA | 0.000 | NA | NA | NA | – |
| 40–49 | NA | 0.000 | NA | NA | NA | – |
| 50–59 | NA | 0.037 | NA | NA | NA | – |
| 60–69 | NA | 0.072 | NA | NA | NA | – |
| 70–79 | NA | 0.134 | NA | NA | NA | – |
| 80–89 | NA | 0.181 | NA | NA | NA | – |
| 90–99 | NA | 0.200 | NA | NA | NA | – |
| Description | Distribution | Mean | Standard error | Parameter 1 | Parameter 2 | Source |
|---|---|---|---|---|---|---|
| Overall rate of new admission to nursing home across all ages and sex | Beta | 20% | NA | α = 274 | β = 1370 | Najayan et al.206 |
| Female patients | ||||||
| Age 30–39 years | Not sampled | 0.000 | NA | NA | NA | Najayan et al.206 |
| Age 40–49 years | 0.000 | NA | NA | NA | Najayan et al.206 | |
| Age 50–59 years | 0.035 | NA | NA | NA | Najayan et al.206 | |
| Age 60–69 years | 0.064 | NA | NA | NA | Najayan et al.206 | |
| Age 70–79 years | 0.113 | NA | NA | NA | Najayan et al.206 | |
| Age 80–89 years | 0.192 | NA | NA | NA | Najayan et al.206 | |
| Age 90–99 years | 0.307 | NA | NA | NA | Najayan et al.206 | |
| Male patients | ||||||
| Age 30–39 years | Not sampled | 0.000 | NA | NA | NA | Najayan et al.206 |
| Age 40–49 years | 0.000 | NA | NA | NA | Najayan et al.206 | |
| Age 50–59 years | 0.057 | NA | NA | NA | Najayan et al.206 | |
| Age 60–69 years | 0.102 | NA | NA | NA | Najayan et al.206 | |
| Age 70–79 years | 0.175 | NA | NA | NA | Najayan et al.206 | |
| Age 80–89 years | 0.284 | NA | NA | NA | Najayan et al.206 | |
| Age 90–99 years | 0.425 | NA | NA | NA | Najayan et al.206 | |
| Description | Distribution | Mean | Standard error | Parameter 1 | Parameter 2 | Source |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Age 30–39 years | Fixed | 0.000 | NA | NA | NA | van Staa et al.141 |
| Age 40–49 years | Fixed | 0.000 | NA | NA | NA | van Staa et al.141 |
| Age 50–59 years | Beta | 0.023 | NA | α = 85.581 | β = 3635.314 | van Staa et al.141 |
| Age 60–69 years | Beta | 0.035 | NA | α = 247.105 | β = 6813.048 | van Staa et al.141 |
| Age 70–79 years | Beta | 0.052 | NA | α = 378.597 | β = 6902.117 | van Staa et al.141 |
| Age 80–89 years | Beta | 0.067 | NA | α = 285.369 | β = 3973.865 | van Staa et al.141 |
| Age 90–99 years | Beta | 0.066 | NA | α = 53.757 | β = 760.736 | van Staa et al.141 |
| Service code, currency code and description | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| T02 A, VB07Z emergency medicine, category 2 investigation with category 2 treatment patient admitted | 34,920 | 94 | £28 | Gamma | κ = 382,885.49 | θ = 0.0002 |
| T02NA, VB07Z emergency medicine, category 2 investigation with category 2 treatment patient not admitted | 24,835 | 82 | £39 | Gamma | κ = 109,477.62 | θ = 0.0007 |
| Service code, currency code and description | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| WF01B, 302 non-consultant-led outpatient appointments, first attendance, not admitted, face to face, endocrinology | 109,162 | 186.54 | 66 | Gamma | κ = 955.04 | θ = 0.20 |
| WF01 A, 302 non-consultant-led outpatient appointments, follow-up attendance, not admitted, face to face, endocrinology | 353,215 | 133.00 | 47 | Gamma | κ = 989.53 | θ = 0.13 |
| Currency code and description | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| Non-elective inpatient procedures, long stay | ||||||
| HA61B major shoulder or upper arm procedures for trauma, with cc | 951 | 7194 | 1931 | Gamma | κ = 1943.78 | θ = 3.7 |
| HA61C major shoulder or upper arm procedures for trauma, without cc | 1880 | 4305 | 1270 | Gamma | κ = 1618.63 | θ = 2.66 |
| HA62Z intermediate shoulder or upper arm procedures for trauma | 249 | 3654 | 1613 | Gamma | κ = 549.10 | θ = 6.65 |
| HA63Z minor shoulder or upper arm procedures for trauma | 611 | 2520 | 944 | Gamma | κ = 947.75 | θ = 2.66 |
| HA69Z minimal shoulder or upper arm procedures for trauma | 1 | 323 | NA | Fixed | NA | NA |
| Non-elective inpatient procedures, long stay, excess bed-day | ||||||
| HA61B major shoulder or upper arm procedures for trauma, with cc | 1,622 | 276.43 | 110 | Gamma | κ = 421.63 | θ = 0.66 |
| HA61C major shoulder or upper arm procedures for trauma, without cc | 3010 | 312.62 | 89 | Gamma | κ = 1607.77 | θ = 0.19 |
| HA62Z intermediate shoulder or upper arm procedures for trauma | 1158 | 294.37 | 114 | Gamma | κ = 380.05 | θ = 0.77 |
| HA63Z minor shoulder or upper arm procedures for trauma | 2155 | 244.89 | 86 | Gamma | κ = 800.88 | θ = 0.31 |
| Currency code and description | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| Non-elective inpatient procedures, long stay | ||||||
| HA71B major elbow or lower arm procedures for trauma, with cc | 1356 | 3835 | 1196 | Gamma | κ = 186.41 | θ = 7.27 |
| HA71C major elbow or lower arm procedures for trauma, without cc | 7494 | 2913 | 888 | Gamma | κ = 10,408.22 | θ = 0.72 |
| HA72Z intermediate elbow or lower arm procedures for trauma | 845 | 2585 | 1026 | Gamma | κ = 87.52 | θ = 9.66 |
| HA73B minor elbow or lower arm procedures for trauma, 18 years and under | 869 | 1637 | 492 | Gamma | κ = 369.14 | θ = 2.19 |
| HA73C minor elbow or lower arm procedures for trauma, 19 years and over | 963 | 1481 | 704 | Gamma | κ = 254.31 | θ = 3.79 |
| HA79Z minimal elbow or lower arm procedures for trauma | 1 | 371 | NA | Fixed | NA | NA |
| Non-elective inpatient procedures, long stay, excess bed-day | ||||||
| HA71B major elbow or lower arm procedures for trauma, with cc | 2475 | 291 | £88 | Gamma | κ = 993.96 | θ = 0.29 |
| HA71C major elbow or lower arm procedures for trauma, without cc | 3716 | 314 | £120 | Gamma | κ = 974.53 | θ = 0.32 |
| HA72Z intermediate elbow or lower arm procedures for trauma | 975 | 256 | £101 | Gamma | κ = 531.39 | θ = 0.48 |
| HA73B minor elbow or lower arm procedures for trauma, 18 years and under | 110 | 379 | £144 | Gamma | κ = 152.54 | θ = 2.48 |
| HA73C minor elbow or lower arm procedures for trauma, 19 years and over | 2703 | 265 | £93 | Gamma | κ = 943.70 | θ = 0.28 |
| Currency code and description | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| Non-elective inpatient procedures, long stay | ||||||
| HA11 A major hip procedures for trauma, category 2, with major cc | 713 | 13,408 | 4678 | Gamma | κ = 1117.05 | θ = 12.00 |
| HA11B major hip procedures for trauma, category 2, with intermediate cc | 319 | 8791 | 3503 | Gamma | κ = 680.27 | θ = 12.92 |
| HA11C major hip procedures for trauma, category 2, without cc | 773 | 7337 | 1847 | Gamma | κ = 2051.83 | θ = 3.58 |
| HA12B major hip procedures for trauma, category 1, with cc | 19,080 | 8210 | 1786 | Gamma | κ = 3064.35 | θ = 2.68 |
| HA12C major hip procedures for trauma, category 1, without cc | 9890 | 6417 | 1159 | Gamma | κ = 4507.56 | θ = 1.42 |
| HA13A intermediate hip procedures for trauma, with major cc | 10,212 | 8237 | 1997 | Gamma | κ = 2415.09 | θ = 3.41 |
| HA13B intermediate hip procedures for trauma, with intermediate cc | 5355 | 6570 | 1726 | Gamma | κ = 2057.28 | θ = 3.19 |
| HA13C intermediate hip procedures for trauma, without cc | 9673 | 5551 | 1129 | Gamma | κ = 3528.05 | θ = 1.57 |
| HA14A minor hip procedures for trauma, with major cc | 249 | 7312 | 3737 | Gamma | κ = 398.07 | θ = 18.37 |
| HA14B minor hip procedures for trauma, with intermediate cc | 216 | 4905 | 2020 | Gamma | κ = 595.70 | θ = 8.23 |
| HA14C minor hip procedures for trauma, without cc | 645 | 3939 | 1064 | Gamma | κ = 1904.04 | θ = 2.07 |
| HA19Z minimal hip procedures for trauma | 1 | 7790 | NA | Fixed | NA | NA |
| Currency code | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| Non-elective inpatient procedures, long stay, excess bed-day | ||||||
| HA11 A major hip procedures for trauma, category 2, with major cc | 1404 | 312 | 84 | Gamma | κ = 410.74 | θ = 0.76 |
| HA11B major hip procedures for trauma, category 2, with intermediate cc | 307 | 299 | 115 | Gamma | κ = 177.30 | θ = 1.69 |
| HA11C major hip procedures for trauma, category 2, without cc | 394 | 311 | 89 | Gamma | κ = 296.08 | θ = 1.05 |
| HA12B major hip procedures for trauma, category 1, with cc | 16,310 | 282 | 88 | Gamma | κ = 1376.53 | θ = 0.20 |
| HA12C major hip procedures for trauma, category 1, without cc | 4463 | 267 | 98 | Gamma | κ = 886.70 | θ = 0.30 |
| HA13A intermediate hip procedures for trauma, with major cc | 8630 | 290 | 88 | Gamma | κ = 1176.62 | θ = 0.25 |
| HA13B intermediate hip procedures for trauma, with intermediate cc | 2502 | 292 | 95 | Gamma | κ = 746.43 | θ = 0.39 |
| HA13C intermediate hip procedures for trauma, without cc | 3674 | 262 | 69 | Gamma | κ = 1715.15 | θ = 0.15 |
| HA14A minor hip procedures for trauma, with major cc | 466 | 256 | 120 | Gamma | κ = 86.67 | θ = 2.95 |
| HA14B minor hip procedures for trauma, with intermediate cc | 198 | 339 | 226 | Gamma | κ = 45.04 | θ = 7.53 |
| HA14C minor hip procedures for trauma, without cc | 962 | 317 | 159 | Gamma | κ = 232.60 | θ = 1.37 |
| Currency code | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| Non-elective inpatient procedures, long stay | ||||||
| HC20D vertebral column injury without procedure, with a cc score of ≥ 6 | 1609 | 5479 | 2858 | Gamma | κ = 543.85 | θ = 10.07 |
| HC20E vertebral column injury without procedure, with a cc score of 3–5 | 2459 | 3732 | 1648 | Gamma | κ = 758.57 | θ = 4.92 |
| HC20F vertebral column injury without procedure, with a cc score of 1–2 | 2611 | 2971 | 1136 | Gamma | κ = 1031.87 | θ = 2.88 |
| HC20G vertebral column injury without procedure, with a cc score of 0 | 1904 | 2265 | 646 | Gamma | κ = 1806.58 | θ = 1.25 |
| Non-elective inpatient procedures, long stay, excess bed-day | ||||||
| HC20D vertebral column injury without procedure, with a cc score of ≥ 6 | 2317 | 328.19 | 128 | Gamma | κ = 347.54 | θ = 0.94 |
| HC20E vertebral column injury without procedure, with a cc score of 3–5 | 3772 | 260.82 | 125 | Gamma | κ = 393.07 | θ = 0.66 |
| HC20F vertebral column injury without procedure, with a cc score of 1–2 | 2363 | 266.99 | 76 | Gamma | κ = 1171.35 | θ = 0.23 |
| HC20G vertebral column injury without procedure, with a cc score of 0 | 2047 | 282.03 | 117 | Gamma | κ = 599.23 | θ = 0.47 |
| Currency code | Number of patients treated | Mean unit cost (£) | SD (£) | Distribution | Parameter 1 | Parameter 2 |
|---|---|---|---|---|---|---|
| WF01 A, 302, non-admitted face-to-face attendance, follow-up, endocrinology | 353,215 | 133 | 114.37 | Gamma | κ = 989.48 | θ = 0.13 |
| DCRDN, SB12Z, deliver simple parenteral chemotherapy at first attendance, day case and regular day/night | 222,981 | 245 | 46.70 | Gamma | κ = 583.60 | θ = 0.42 |
Appendix 9 Base-case results from the probabilistic sensitivity analysis for QFracture
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 827.18 | 15.88153 | 0.00 | 0.00000 | NA | 316,803 | 475,619 | NA |
| Ibandronic acid (oral) | 834.63 | 15.88164 | 7.45 | 0.00011 | 67,340 | 316,798 | 475,615 | 67,340 |
| Alendronic acid | 835.01 | 15.88164 | 7.83 | 0.00011 | 68,204 | 316,798 | 475,614 | 91,325 |
| Risedronic acid | 835.96 | 15.88157 | 8.78 | 0.00004 | 219,757 | 316,795 | 475,611 | Dominated |
| Ibandronic acid (i.v.) | 1053.14 | 15.88123 | 225.96 | –0.00030 | –757,885 | 316,571 | 475,384 | Dominated |
| Zoledronic acid | 1385.41 | 15.88196 | 558.24 | 0.00043 | 1,301,875 | 316,254 | 475,073 | 1,752,783 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 1532.33 | 14.74097 | 0.00 | 0.00000 | NA | 293,287 | 440,697 | NA |
| Ibandronic acid (oral) | 1539.62 | 14.74105 | 7.29 | 0.00008 | 96,451 | 293,281 | 440,692 | Extendedly dominated |
| Alendronic acid | 1540.17 | 14.74108 | 7.84 | 0.00010 | 76,943 | 293,281 | 440,692 | Extendedly dominated |
| Risedronic acid | 1540.77 | 14.74110 | 8.44 | 0.00013 | 65,692 | 293,281 | 440,692 | 65,692 |
| Ibandronic acid (i.v.) | 1757.78 | 14.74075 | 225.45 | –0.00023 | –997,490 | 293,057 | 440,465 | Dominated |
| Zoledronic acid | 2088.19 | 14.74166 | 555.86 | 0.00068 | 813,849 | 292,745 | 440,162 | 987,243 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 2971.75 | 13.55783 | 0.00 | 0.00000 | NA | 268,185 | 403,763 | NA |
| Risedronic acid | 2977.17 | 13.55813 | 5.42 | 0.00030 | 17,906 | 268,185 | 403,767 | 17,906 |
| Alendronic acid | 2979.29 | 13.55813 | 7.54 | 0.00030 | 24,867 | 268,183 | 403,765 | Extendedly dominated |
| Ibandronic acid (oral) | 2979.64 | 13.55808 | 7.89 | 0.00025 | 31,440 | 268,182 | 403,763 | Dominated |
| Ibandronic acid (i.v.) | 3196.69 | 13.55889 | 224.94 | 0.00106 | 213,067 | 267,981 | 403,570 | 291,495 |
| Zoledronic acid | 3520.69 | 13.55932 | 548.94 | 0.00150 | 367,160 | 267,666 | 403,259 | 737,415 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 3881.90 | 12.32917 | 0.00 | 0.00000 | NA | 242,702 | 365,993 | NA |
| Alendronic acid | 3886.67 | 12.32946 | 4.77 | 0.00028 | 16,820 | 242,702 | 365,997 | 16,820 |
| Ibandronic acid (oral) | 3888.83 | 12.32930 | 6.93 | 0.00012 | 55,519 | 242,697 | 365,990 | Dominated |
| Risedronic acid | 3889.93 | 12.32945 | 8.02 | 0.00027 | 29,255 | 242,699 | 365,994 | Dominated |
| Ibandronic acid (i.v.) | 4106.75 | 12.32927 | 224.84 | 0.00009 | 2,444,347 | 242,479 | 365,771 | Dominated |
| Zoledronic acid | 4436.61 | 12.33057 | 554.71 | 0.00140 | 397,032 | 242,175 | 365,481 | 493,762 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY | Net benefit at £30,000 per QALY | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 4052.25 | 11.42224 | 0.00 | 0.00000 | NA | 224,393 | 338,615 | NA |
| Alendronic acid | 4059.38 | 11.42235 | 7.13 | 0.00010 | 68,244 | 224,388 | 338,611 | 68,244 |
| Ibandronic acid (oral) | 4060.12 | 11.42216 | 7.86 | –0.00008 | –98,972 | 224,383 | 338,605 | Dominated |
| Risedronic acid | 4065.83 | 11.42228 | 13.58 | 0.00003 | 415,596 | 224,380 | 338,602 | Dominated |
| Ibandronic acid (i.v.) | 4276.53 | 11.42247 | 224.28 | 0.00022 | 997,367 | 224,173 | 338,398 | Extendedly dominated |
| Zoledronic acid | 4604.88 | 11.42422 | 552.63 | 0.00198 | 279,227 | 223,880 | 338,122 | 290,988 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 4371.39 | 10.40268 | 0.00 | 0.00000 | NA | 203,682 | 307,709 | NA |
| Alendronic acid | 4374.47 | 10.40301 | 3.08 | 0.00032 | 9468 | 203,686 | 307,716 | 9468 |
| Risedronic acid | 4378.91 | 10.40296 | 7.52 | 0.00028 | 27,166 | 203,680 | 307,710 | Dominated |
| Ibandronic acid (oral) | 4379.07 | 10.40298 | 7.67 | 0.00029 | 26,208 | 203,680 | 307,710 | Dominated |
| Ibandronic acid (i.v.) | 4603.74 | 10.40323 | 232.35 | 0.00055 | 421,634 | 203,461 | 307,493 | Extendedly dominated |
| Zoledronic acid | 4916.96 | 10.40474 | 545.57 | 0.00206 | 265,440 | 203,178 | 307,225 | 313,498 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Risedronic acid | 4584.47 | 9.38541 | –0.57 | 0.00047 | –1213 | 183,124 | 276,978 | NA |
| Alendronic acid | 4584.52 | 9.38539 | –0.52 | 0.00045 | –1152 | 183,123 | 276,977 | Dominated |
| No treatment | 4585.04 | 9.38494 | 0.00 | 0.00000 | NA | 183,114 | 276,963 | Dominated |
| Ibandronic acid (oral) | 4590.32 | 9.38526 | 5.28 | 0.00032 | 16,705 | 183,115 | 276,967 | Dominated |
| Ibandronic acid (i.v.) | 4806.39 | 9.38577 | 221.35 | 0.00083 | 267,841 | 182,909 | 276,767 | Extendedly dominated |
| Zoledronic acid | 5136.10 | 9.38814 | 551.06 | 0.00320 | 172,324 | 182,627 | 276,508 | 202,041 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment | Net benefit at £20,000 per QALY | Net benefit at £30,000 per QALY | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost | QALYs | |||||
| Risedronic acid | 5603.84 | 8.33619 | –4.24 | 0.00067 | –6287 | 161,120 | 244,482 | NA |
| Alendronic acid | 5607.53 | 8.33657 | –0.55 | 0.00106 | –515 | 161,124 | 244,490 | 9563 |
| No treatment | 5608.08 | 8.33551 | 0.00 | 0.00000 | NA | 161,102 | 244,457 | Dominated |
| Ibandronic acid (oral) | 5616.53 | 8.33618 | 8.45 | 0.00066 | 12,715 | 161,107 | 244,469 | Dominated |
| Ibandronic acid (i.v.) | 5837.84 | 8.33648 | 229.77 | 0.00097 | 237,905 | 160,892 | 244,256 | Dominated |
| Zoledronic acid | 6157.62 | 8.33899 | 549.54 | 0.00348 | 157,893 | 160,622 | 244,012 | 227,376 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Alendronic acid | 8678.06 | 6.51525 | –10.66 | 0.00114 | –9322 | 121,627 | 186,780 | NA |
| Risedronic acid | 8680.76 | 6.51549 | –7.97 | 0.00138 | n5791 | 121,629 | 186,784 | 11,621 |
| Ibandronic acid (oral) | 8688.18 | 6.51507 | –0.54 | 0.00096 | –563 | 121,613 | 186,764 | Dominated |
| No treatment | 8688.72 | 6.51411 | 0.00 | 0.00000 | NA | 121,594 | 186,735 | Dominated |
| Ibandronic acid (i.v.) | 8902.45 | 6.51557 | 213.72 | 0.00146 | 146,407 | 121,409 | 186,565 | Extendedly dominated |
| Zoledronic acid | 9221.00 | 6.51944 | 532.28 | 0.00533 | 99,907 | 121,168 | 186,362 | 136,695 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Risedronic acid | 19,576.95 | 4.01080 | –17.24 | 0.00118 | –14,610 | 60,639 | 100,747 | NA |
| Alendronic acid | 19,587.52 | 4.01086 | –6.67 | 0.00124 | –5,392 | 60,630 | 100,738 | 188,505 |
| No treatment | 19,594.19 | 4.00962 | 0.00 | 0.00000 | NA | 60,598 | 100,695 | Dominated |
| Ibandronic acid (oral) | 19,624.63 | 4.01018 | 30.44 | 0.00055 | 54,995 | 60,579 | 100,681 | Dominated |
| Ibandronic acid (i.v.) | 19,840.81 | 4.01059 | 246.62 | 0.00096 | 255,998 | 60,371 | 100,477 | Dominated |
| Zoledronic acid | 20,137.69 | 4.01250 | 543.50 | 0.00288 | 189,028 | 60,112 | 100,237 | 335,702 |
Appendix 10 Base-case results from the probabilistic sensitivity analysis for FRAX
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 5838.92 | 13.56127 | 0.00 | 0.00000 | NA | 265,387 | 400,999 | NA |
| Alendronic acid | 5841.54 | 13.56248 | 2.62 | 0.00121 | 2175 | 265,408 | 401,033 | 2175 |
| Risedronic acid | 5842.90 | 13.56252 | 3.98 | 0.00125 | 3197 | 265,408 | 401,033 | 34,124 |
| Ibandronic acid (oral) | 5844.50 | 13.56216 | 5.57 | 0.00089 | 6268 | 265,399 | 401,020 | Dominated |
| Ibandronic acid (i.v.) | 6060.14 | 13.56305 | 221.22 | 0.00177 | 124,931 | 265,201 | 400,831 | Extendedly dominated |
| Zoledronic acid | 6394.34 | 13.56640 | 555.41 | 0.00512 | 108,395 | 264,934 | 400,598 | 141,073 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Risedronic acid | 5863.60 | 13.24259 | –10.18 | 0.00140 | –7272 | 258,988 | 391,414 | NA |
| Ibandronic acid (oral) | 5873.38 | 13.24252 | –0.40 | 0.00133 | –300 | 258,977 | 391,402 | Dominated |
| No treatment | 5873.78 | 13.24119 | 0.00 | 0.00000 | NA | 258,950 | 391,362 | Dominated |
| Alendronic acid | 5875.18 | 13.24287 | 1.40 | 0.00168 | 835 | 258,982 | 391,411 | 41,144 |
| Ibandronic acid (i.v.) | 6089.91 | 13.24364 | 216.14 | 0.00245 | 88,127 | 258,783 | 391,219 | Extendedly dominated |
| Zoledronic acid | 6401.88 | 13.24829 | 528.10 | 0.00710 | 74,347 | 258,564 | 391,047 | 97,132 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Risedronic acid | 6324.67 | 13.33625 | –6.81 | 0.00176 | –3879 | 260,400 | 393,763 | NA |
| Ibandronic acid (oral) | 6330.04 | 13.33636 | –1.44 | 0.00186 | –775 | 260,397 | 393,761 | Extendedly dominated |
| No treatment | 6331.48 | 13.33450 | 0.00 | 0.00000 | NA | 260,358 | 393,703 | Dominated |
| Alendronic acid | 6333.01 | 13.33660 | 1.53 | 0.00211 | 727 | 260,399 | 393,765 | 23,752 |
| Ibandronic acid (i.v.) | 6549.59 | 13.33764 | 218.11 | 0.00314 | 69,413 | 260,203 | 393,580 | Extendedly dominated |
| Zoledronic acid | 6854.23 | 13.34360 | 522.75 | 0.00910 | 57,436 | 260,018 | 393,454 | 74,509 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Alendronic acid | 6940.02 | 13.57697 | –3.78 | 0.00214 | –1768 | 264,599 | 400,369 | NA |
| Ibandronic acid (oral) | 6940.34 | 13.57684 | –3.47 | 0.00201 | –1726 | 264,597 | 400,365 | Dominated |
| No treatment | 6943.81 | 13.57483 | 0.00 | 0.00000 | NA | 264,553 | 400,301 | Dominated |
| Risedronic acid | 6945.84 | 13.57692 | 2.04 | 0.00208 | 978 | 264,593 | 400,362 | Dominated |
| Ibandronic acid (i.v.) | 7157.83 | 13.57920 | 214.02 | 0.00437 | 49,021 | 264,426 | 400,218 | Extendedly dominated |
| Zoledronic acid | 7474.18 | 13.58617 | 530.37 | 0.01134 | 46,776 | 264,249 | 400,111 | 58,061 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Ibandronic acid (oral) | 7466.53 | 12.32601 | –9.83 | 0.00183 | –5379 | 239,054 | 362,314 | NA |
| Risedronic acid | 7471.92 | 12.32603 | –4.44 | 0.00184 | –2406 | 239,049 | 362,309 | 329,090 |
| No treatment | 7476.36 | 12.32418 | 0.00 | 0.00000 | NA | 239,007 | 362,249 | Dominated |
| Alendronic acid | 7478.51 | 12.32595 | 2.14 | 0.00177 | 1213 | 239,041 | 362,300 | Dominated |
| Ibandronic acid (i.v.) | 7671.16 | 12.32710 | 194.80 | 0.00292 | 66,739 | 238,871 | 362,142 | Extendedly dominated |
| Zoledronic acid | 8001.50 | 12.33301 | 525.14 | 0.00882 | 59,513 | 238,659 | 361,989 | 75,873 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | |||||
| No treatment | 7616.23 | 10.59846 | 0.00 | 0.00000 | NA | 204,353 | 310,338 | NA |
| Alendronic acid | 7618.25 | 10.60009 | 2.02 | 0.00163 | 1242 | 204,384 | 310,384 | 1242 |
| Risedronic acid | 7619.22 | 10.59995 | 3.00 | 0.00149 | 2008 | 204,380 | 310,379 | Dominated |
| Ibandronic acid (oral) | 7620.80 | 10.59974 | 4.57 | 0.00128 | 3574 | 204,374 | 310,371 | Dominated |
| Ibandronic acid (i.v.) | 7833.82 | 10.60192 | 217.59 | 0.00346 | 62921 | 204,205 | 310,224 | Extendedly dominated |
| Zoledronic acid | 8138.66 | 10.60773 | 522.44 | 0.00927 | 56,383 | 204,016 | 310,093 | 68,144 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | |||||
| Alendronic acid | 7162.84 | 9.10272 | –5.67 | 0.00150 | –3766 | 174,892 | 265,919 | NA |
| Risedronic acid | 7164.94 | 9.10275 | –3.57 | 0.00154 | –2321 | 174,890 | 265,918 | 64,125 |
| No treatment | 7168.51 | 9.10121 | 0.00 | 0.00000 | NA | 174,856 | 265,868 | Dominated |
| Ibandronic acid (oral) | 7177.99 | 9.10236 | 9.48 | 0.00114 | 8295 | 174,869 | 265,893 | Dominated |
| Ibandronic acid (i.v.) | 7392.35 | 9.10398 | 223.84 | 0.00276 | 80,986 | 174,687 | 265,727 | Extendedly dominated |
| Zoledronic acid | 7702.81 | 9.10946 | 534.31 | 0.00825 | 64,770 | 174,486 | 265,581 | 80,140 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| No treatment | 7830.38 | 7.91916 | 0.00 | 0.00000 | NA | 150,553 | 229,744 | NA |
| Risedronic acid | 7833.78 | 7.92086 | 3.40 | 0.00170 | 1996 | 150,583 | 229,792 | 1996 |
| Ibandronic acid (oral) | 7836.05 | 7.92098 | 5.67 | 0.00182 | 3112 | 150,584 | 229,793 | 19,441 |
| Alendronic acid | 7839.16 | 7.92096 | 8.78 | 0.00181 | 4864 | 150,580 | 229,790 | Dominated |
| Ibandronic acid (i.v.) | 8049.13 | 7.92224 | 218.75 | 0.00308 | 70,929 | 150,396 | 229,618 | Extendedly dominated |
| Zoledronic acid | 8378.29 | 7.92722 | 547.91 | 0.00807 | 67,934 | 150,166 | 229,438 | 86,829 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Alendronic acid | 11,167.83 | 6.90026 | –7.38 | 0.00232 | –3175 | 126,837 | 195,840 | NA |
| No treatment | 11,175.20 | 6.89794 | 0.00 | 0.00000 | NA | 126,784 | 195,763 | Dominated |
| Risedronic acid | 11,176.94 | 6.90016 | 1.74 | 0.00223 | 782 | 126,826 | 195,828 | Dominated |
| Ibandronic acid (oral) | 11,195.85 | 6.89967 | 20.65 | 0.00174 | 11,891 | 126,798 | 195,794 | Dominated |
| Ibandronic acid (i.v.) | 11,430.76 | 6.90139 | 255.55 | 0.00345 | 73,995 | 126,597 | 195,611 | Extendedly dominated |
| Zoledronic acid | 11,734.98 | 6.90722 | 559.78 | 0.00929 | 60,287 | 126,409 | 195,482 | 81,469 |
| Treatment strategy | Mean outcomes (discounted) | Incremental outcomes vs. no treatment (discounted) | ICER vs. no treatment (£) | Net benefit at £20,000 per QALY (£) | Net benefit at £30,000 per QALY (£) | Incremental analysisa (£) | ||
|---|---|---|---|---|---|---|---|---|
| Cost (£) | QALYs | Cost (£) | QALYs | |||||
| Risedronic acid | 18,699.06 | 4.56088 | –27.62 | 0.00220 | –12,566 | 72,519 | 118,127 | NA |
| Alendronic acid | 18,704.64 | 4.56166 | –22.04 | 0.00297 | –7411 | 72,529 | 118,145 | 7194 |
| Ibandronic acid (oral) | 18,724.98 | 4.56022 | –1.70 | 0.00154 | –1104 | 72,479 | 118,082 | Dominated |
| No treatment | 18,726.68 | 4.55868 | 0.00 | 0.00000 | NA | 72,447 | 118,034 | Dominated |
| Ibandronic acid (i.v.) | 18,943.03 | 4.56193 | 216.35 | 0.00325 | 66,600 | 72,296 | 117,915 | Extendedly dominated |
| Zoledronic acid | 19,257.85 | 4.56644 | 531.17 | 0.00775 | 68,498 | 72,071 | 117,735 | 115,714 |
List of abbreviations
- A&E
- accident and emergency
- ADT
- androgen deprivation therapy
- AE
- adverse event
- ARIBON
- reversal of anastrozole (ARImidex) induced bone loss with oral monthly ibandronic acid (BONdronat) treatment during adjuvant therapy for breast cancer trial
- BMD
- bone mineral density
- BMI
- body mass index
- BONE
- iBandronic acid Osteoporosis vertebral fracture trial in North America and Europe
- CEAC
- cost-effectiveness acceptability curve
- CG
- clinical guideline
- CI
- confidence interval
- CrI
- credible interval
- DES
- discrete event simulation
- DIC
- deviance information criterion
- DIVA
- Dosing IntraVenous Administration trial
- DSU
- Decision Support Unit
- DXA
- dual-energy X-ray absorptiometry
- eMIT
- electronic market information tool
- EQ-5D
- EuroQol
- EU
- European Union
- FACT
- Fosamax Actonel Comparison Trial
- FACTS
- Fosamax Actonel Comparison Trial international Study
- FIT
- Fracture Intervention Trial
- GI
- gastrointestinal
- GP
- general practitioner
- GPRD
- General Practice Research Database
- HORIZON
- Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly
- HORIZON-PFT
- Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly – Pivotal Fracture Trial
- HORIZON-RFT
- Health Outcomes and Reduced Incidence with Zoledronic acid Once Yearly – Recurrent Fracture Trial
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HRT
- hormone replacement therapy
- ICER
- incremental cost-effectiveness ratio
- ICUROS
- International Costs and Utilities Related to Osteoporotic fractures Study
- INB
- incremental net benefit
- IU
- international unit
- i.v.
- intravenous
- KOFOR
- the costs and effects of osteoporosis-related fractures study
- MOBILE
- Monthly Oral ibandronic acid In LadiEs trial
- MOTION
- Monthly Oral Therapy with ibandronic acid for Osteoporosis iNtervention trial
- MPR
- medication possession ratio
- MTA
- multiple technology appraisal
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- ONS
- Office for National Statistics
- OR
- odds ratio
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROSE
- Rapid Onset and Sustained Efficacy trial
- RR
- risk ratio
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SmPC
- summary of product characteristics
- TA
- technology appraisal
- VAS
- visual analogue scale
- VERT-MN
- Vertebral Efficacy with Risedronic acid Therapy – MultiNational trial
- VERT-NA
- Vertebral Efficacy with Risedronic acid Therapy – North American trial
- WHO
- World Health Organization