Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/127/34. The contractual start date was in December 2011. The draft report began editorial review in September 2015 and was accepted for publication in April 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sandra Eldridge is a member of the Health Technology Assessment clinical trials board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by McRobbie et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Recent estimates show that over one-third of the world’s adult population is overweight or obese, which is equivalent to more than 2.1 billion people globally. 1 By 2030 the proportion of adults who are overweight or obese is expected to rise to > 40%. 1 In terms of health risk, the World Health Organization estimates that around 2.8 million deaths per year, worldwide, are directly attributable to excess body weight or obesity. 2
In England, the proportion of men who are classified as overweight [body mass index (BMI) of ≥ 25 kg/m2)] has increased from 58% to 67% in the last decade, with a similar magnitude increase (49% to 57%) in women. Current rates of obesity (BMI of ≥ 30 kg/m2) are 26% and 24% for men and women, respectively. 3 However, modelling has indicated that these rates could rise to 60% and 50% in men and women, respectively, by 2050.
Ill health resulting from obesity is responsible for approximately 10% of morbidity and mortality in the UK. 4 A summary of illnesses associated with high BMI is shown in Box 1. Weight loss has been shown to improve many of these illnesses6 and reduce all-cause mortality. 7
-
Type 2 diabetes mellitus.
-
Abnormal blood lipids (e.g. increased low-density lipoprotein).
-
Cardiovascular disease (e.g. high blood pressure, stroke, myocardial infarction, congestive heart failure).
-
Obstructive sleep apnoea.
-
Cancer (e.g. cancers of the endometrium, breast, colon and gallbladder).
-
Reproductive disorders (e.g. ovulatory dysfunction).
-
Osteoarthritis.
-
Liver and gall bladder disease (e.g. fatty liver and gallstones).
Source: adapted from Reducing Obesity: Future Choices. 5
In 2002, the direct annual health-care costs associated with the treatment of obesity were around £1B. In 2007, the costs were estimated to have increased to £4.2B, and they are predicted to increase further, to £10B, by 2050. 5 The costs to society are far greater. Obesity currently accounts for 3–8% of health costs in different parts of Europe,8 with the overall impact on health-care costs estimated to range from €59B (direct) to €118B–236B (indirect), because obesity is linked to a range of comorbidities. In the UK, obesity is second to smoking in terms of economic loss, costing the country around £45B in 2012. 9 This equates to 3% of gross domestic product.
Obesity has links to health inequalities, and the proportion of obese people is particularly high in the lower socioeconomic groups. 3 There are also ethnic differences; for example, the highest obesity rates are reported in African-Caribbean and Irish men. 3 Rates are also high in Bangladeshi women, with 17% classified as having a BMI of ≥ 30 kg/m2. This proportion rises to 50% when obesity is defined using the waist-to-hip ratio. 10
Given the high prevalence of obesity, there is a need to investigate the effectiveness of simple, pragmatic and cost-effective interventions that have the ability to reach the large number of obese and overweight individuals in the UK.
Weight management strategies
In 2011, the Department of Health published a policy paper that called for efforts to reduce the proportion of adults with excess weight by 2020. 11 A range of strategies will be required if the UK is to reduce its obesity rates. 9 These strategies include personal health-care interventions, such as weight management programmes and weight loss medicines, as well as education and environmental changes.
The menu of evidence-based interventions currently available for people unable to lose weight on their own is relatively limited. A stepped-care approach is currently the recommended approach for weight management depending on the severity of the patient’s obesity. Current pharmacological treatments have modest effects that can be beneficial but are likely to be lost once the medication is stopped. 12 Surgical interventions are more successful but are currently expensive and unsuitable for large-scale use, and are usually indicated for the morbidly obese or those with coexisting conditions. 13,14 Dietary interventions on their own have only modest effects15 and brief routine interventions within primary care have generally reported disappointing results. 16
More intensive behavioural interventions generate a small but sustainable weight loss,17 which can engender significant and clinically worthwhile long-term health benefits. 18 Despite the fact that some of the initial weight lost is regained, interventions that lead to at least a 5% reduction in body weight can lead to health improvements (e.g. a decreased risk of type 2 diabetes mellitus). 19–22
Obesity is a chronic condition that requires lifelong management, as weight is often regained, but achieving changes in behaviour is challenging. 23 Weight management in overweight individuals who seek help normally requires changes to their habitual lifestyle, which are difficult to implement and maintain without specialist input, structure and support. 24,25 The National Institute for Health and Care Excellence (NICE) guidance on Managing Overweight and Obesity in Adults recommends multicomponent interventions as the treatment of choice (Box 2). 26 These interventions should include behaviour change techniques to increase people’s physical activity levels or decrease inactivity, improve eating behaviour and the quality of the person’s diet, and reduce energy intake. Several systematic reviews have demonstrated that the combination of diet, exercise and behavioural approaches are effective management strategies. 27 However, few studies have specifically targeted primary care patients.
-
They are multicomponent (i.e. they address dietary intake, physical activity levels and behaviour change).
-
They are developed by a multidisciplinary team.
-
They focus on lifelong lifestyle change and the prevention of future weight gain.
-
They last at least 3 months, and sessions are offered at least weekly or fortnightly and include a ‘weigh-in’ at each session.
-
Achievable goals for weight loss are agreed for different stages.
-
Specific dietary targets are agreed (e.g. for a clear energy intake or for a specific reduction in energy intake), tailored to individual needs and goals.
-
Discussions take place about how to reduce sedentary behaviour and the type of physical activities that can easily be integrated into everyday life and maintained in the long term.
-
Programmes are tailored to support the needs of different groups.
-
Weight, indicators of behaviour change and participants’ personal goals are monitored throughout the programme.
-
A respectful, non-judgemental approach is adopted.
-
They foster independence and self-management (including self-monitoring).
-
Opportunities for ongoing support once the programme or referral period has ended are discussed.
-
The importance of maintaining new dietary habits and increased physical activity levels in the long term to prevent weight regain is stressed and strategies to overcome any difficulties in maintaining the new behaviours are discussed.
-
They encourage dietary habits that will support weight maintenance and are sustainable in the long term.
-
They promote ways of being more physically active and less sedentary that are sustainable in the long term (e.g. walking). The wider benefits of physical activity should also be emphasised.
Source: adapted from NICE guidance on Managing Overweight and Obesity in Adults. 26
Intensive weight management programmes can make considerable demands on staff expertise and budgets, and they also face the challenge of participant retention. The programmes usually include, as one of their core active ingredients, assignment and monitoring of tasks. These are difficult to implement for most participants and the participant dropout is usually large. 28
Primary care interventions
Similar to smoking cessation, primary care has the potential to play a key role in helping overweight and obese people to achieve a healthy weight because of its unique role in the health-care system.
General practice is potentially an ideal location for running weight management services. People trust the advice of their general practitioner (GP) team, their GP practice is often local and convenient, and large GP practices now have multidisciplinary teams and physical space to operate weight management services. Some patients, especially those from ethnic minority groups, may be less likely to use commercial providers. 29–31 GPs have also been incentivised via the Quality and Outcomes Framework to maintain a register of patients (aged ≥ 16 years) with a BMI of ≥ 30 kg/m2 as part of routine care. 32
Current guidelines recommend that primary care physicians in England should identify people with obesity and offer clinical management, although few options for treatment exist. In 2013, the Royal College of Physicians published a report on how the NHS should adapt to deal with the rising rates of obesity. 33 This report highlighted the role of GPs and the practice team, recommending that GPs should deal with excess weight and obesity as an important risk factor for non-communicable diseases. Although most obesity management in the UK takes place in primary care, the approach is not co-ordinated or consistent. 34
Despite the advantages of targeting obesity in primary care, the effectiveness of interventions in this setting has not been widely evaluated. Where studies have been conducted, the results can be disappointing (Box 3). 34–36 If research evaluations showed little impact then real-life impact is likely to be worse.
A total of 740 obese adults were randomised to one of six weight loss interventions or a control group. Three were commercial programmes [Weight Watchers® (New York, NY, USA), Slimming World (Alfreton, UK) and Rosemary Conley (Markfield, UK)], one was a NHS group-based programme (Size Down) and the last two were delivered either by GPs or pharmacists who had received weight loss advice training from dietitians. GP and pharmacist interventions were no better than the minimal intervention control, achieving a weight loss of 1 kg at 1 year.
Jebb et al. (2011)36In a trial that compared the effects of a referral to Weight Watchers with weight loss advice from a primary care professional at the local GP practice, weight loss at 12 months was significantly lower in participants in the GP arm than in those who used Weight Watchers (1.6 kg vs. 4.0 kg).
POWER-UP trial (2011)37A US primary care study randomised 390 obese patients to usual care (quarterly GP visits during which 5–7 minutes was devoted to reviewing the patients’ weight change), brief lifestyle counselling (quarterly visits plus 10- to 15-minute appointments with a health-care assistant) or enhanced brief lifestyle counselling [monthly visits supplemented by participants’ choice from orlistat, sibutramine (Meridia; Abbott Laboratories, Chicago, IL, USA) or meal replacements]. At the 12-month follow-up the weight loss in the three groups was 2.3 kg, 3.4 kg and 7.1 kg, respectively, with only the weight loss in the enhanced condition significantly greater than in the other groups.
Appel et al. (2011)38In this randomised controlled trial, participants from six primary care practices were randomly assigned to weight loss advice delivered by telephone, internet and e-mail (remote support), in-person support during individual and group sessions (in-person support) and to a self-directed weight loss programme (control group). At 2 years, weight loss was similar in the groups that received in-person support (5.1 kg) and remote support (4.5 kg), and significantly greater than in the control group.
CAMWEL (2012)23Participants were recruited in 23 general practices in a borough in London, UK. A total of 381 adults were randomised to the control group (usual weight management advice from the GP) or to the intervention condition (a structured one-to-one programme delivered in 14 visits over 12 months). At the 12-month follow-up, the difference in mean weight change between the intervention and control groups was not statistically significant (2.39 vs. 1.31 kg).
Think Health! (2012)39Two interventions were tested in five primary care practices in the USA. A total of 261 participants were randomised to either usual care (four visits with a primary care provider over 1 year) or usual care supplemented by monthly lifestyle coaching provided by administrative staff. At 1 year, there was no significant difference in weight loss between the groups (1.61 kg in the intervention group and 0.62 kg in the control group).
The Counterweight Programme (2005, 2008)40,41This has been promoted as a programme that could be implemented in primary care, and some results from a prospective cohort study have been reported. Practice nurses provided nine treatment sessions and mean weight loss of < 3 kg at 12 months was reported. However, this weight loss was was achieved in the 45% of enrolled patients who attended for follow-up. Without accounting for those lost to follow-up, and in the absence of a control group, the efficacy of the programme is difficult to appraise.
Several recent systematic reviews have suggested that weight loss interventions in primary care yield small reductions in weight that are not likely to be clinically significant. 42,43 In the USA, a recent review concluded that obesity treatment delivered in primary care has limited effectiveness. 44
One strategy that has shown modest effectiveness is primary care referral to evidence-based commercial programmes for weight loss treatment. 36
The NICE guidelines recommend that GP practices raise the issue of weight loss with overweight patients and refer them to weight management services, where these exist. 26 These guidelines also recommend referring people to a group rather than an individual programme if they express no preference because, on average, group programmes tend to be more cost-effective.
In the field of health behaviour modification, group approaches can dramatically reduce the costs of treatments and increase their reach. 45 They may also have potential to improve participant retention. Social support has been associated with positive change in a number of areas, including weight management. 46 Some potentially useful pointers can be derived from the field of smoking cessation, which shares a number of key features with weight management. Interaction-oriented groups have been shown to improve attendance and participant retention,47 mutual linking of individual tasks improves treatment compliance and short-term outcome,48 and on a national scale group treatments seem to be yielding results superior to individual treatment. 49 Current group weight management programmes usually have a strongly didactic focus, with limited efforts to utilise social support and to link the progress of individual participants. It is likely that the mutual support-oriented group approach, which has proved useful in smoking cessation, can be used here as well.
Several types of such programmes have been commissioned by local councils, but their efficacy is generally not known. With previous evidence suggesting that obesity interventions in primary care have had little impact, there is a need for evidence-based public domain weight management programmes that are clinically effective and cost-effective, and readily accessible and attractive for patients from diverse ethnic and socioeconomic backgrounds.
Trial objectives
The trial objective was to determine whether or not a promising task-based weight management programme [Weight Action Programme (WAP)] targeting underprivileged groups has a long-term effect that is over and above the effect of a ‘best practice’ weight management intervention provided in primary care by practice nurses.
Primary objective
To determine whether or not the WAP can generate better-sustained weight loss over 12 months in overweight adults than a best practice intervention delivered by nurses in general practice.
Secondary objective
To determine the cost-effectiveness [in terms of costs of interventions and quality-adjusted life-years (QALYs) derived from the European Quality of Life-5 Dimensions-5 Levels (EQ-5D-5L) questionnaire] of the two interventions.
Chapter 2 Methods
Overview of trial design
We conducted a randomised controlled trial (RCT) between 2012 and 2015 in two NHS general practices. Eligible adults were recruited primarily from these practices, supplemented by wider advertising, and randomised to the intervention or control arm in the ratio of 2 : 1 (WAP arm to nurse arm). Participants in the WAP and nurse arms started treatment within 1 week after randomisation. All participants were invited to attend 6- and 12-month follow-up appointments. To maximise retention, home and work visits were conducted for those unable to attend the follow-up appointments.
Changes to trial design
We initially intended to randomise 116 participants to the control arm and 214 to the intervention arm. However, we realised that for statistical and logistical reasons it was simpler to randomise 110 and 220 to the nurse and WAP arms, respectively. This change did not affect the total sample size (n = 330) and made little difference to the power. This major amendment was submitted and approved by the trial sponsor and ethics committee before the trial commenced recruitment. Protocol amendments are summarised in Table 1.
| Version | Date | Summary |
|---|---|---|
| 1.0 | 5 December 2011 | Original protocol |
| 2.0 | 17 May 2012 | Change in randomisation procedure (conducted by the Sheffield Clinical Trials Unit rather than a PCTU statistician); clarification of the primary and secondary outcomes; and procedures relating to confidentiality and quality assurance processes clarified |
| 3.0 | 22 January 2015 | See Change from planned analysis, clinic address updated |
Participants
Inclusion criteria
Participants were eligible if they were aged ≥ 18 years, wanted to lose weight and had a BMI of ≥ 30 kg/m2 or ≥ 28 kg/m2 plus comorbidities.
Exclusion criteria
Those who were unable to read/write/understand English, had a BMI of > 45 kg/m2, had lost > 5% of their body weight in the previous 6 months, were pregnant, were taking psychiatric medications, were not registered with a GP in the local areas or were involved in a current research project were excluded.
The decision to exclude participants on psychiatric medication, including antidepressants, was based on the fact that these medications can have a significant effect on weight, and that psychiatric illness often makes follow-up and adherence to long-term programmes more difficult. We did not exclude people with a history of psychiatric illness if they were no longer taking psychiatric medication.
We did not exclude any other comorbidities to ensure that the study addressed NHS needs and that the results are generalisable. Clients who were unable to exercise were not excluded as both the nurse and the WAP interventions are multimodal and do not rely solely on exercise.
Recruitment
Box 4 lists the strategies that we used to recruit participants.
Posters/flyers (Figure 1) in reception area and consultation rooms; adverts on GP practice website and boards; text and letter mailshots to potential participants identified via GP database searches; GP fax/telephone referrals (Figure 2); ‘comments’ box on GP reception allowing potential participants to express their interest; newsletters for practice patients and staff providing feedback on current participants; and attending regular clinical meetings to ensure GP staff were aware of the purpose of the study and how to refer onto it. Several GP practices throughout Tower Hamlets, Hackney and the City were contacted.
Publicity in media NewspapersLocal papers: East End Life (on 4 February 2013, 3 June 2013 and 14 October 2013).
Other Community venuesPosters and leaflets were distributed in various community venues throughout Tower Hamlets, including the Osmani Trust. Stalls were also held at various health promotion events in the Tower Hamlets area.
Workplace venuesPosters and leaflets were distributed in various locations throughout Queen Mary University and the Royal London Hospital.
FIGURE 1.
Leaflets (a) and posters (b) used to promote the study.


FIGURE 2.
General practitioner fax referral form.

Recruitment commenced in September 2012 with the first participant enrolled at the Barkantine Practice, London, UK, on 24 September 2012. The first participant was screened at the Lawson Practice, London, UK, on 18 October 2012.
We encountered some difficulties in recruiting our sample in the short time frame that we had set. These are discussed in Chapter 5, Recruitment barriers and facilitators.
All publicity (except GP fax referrals) invited potential participants to contact the study team by telephone. A researcher would explain the study, assess interest and eligibility, and invite the potential participant to attend the initial screening session.
Setting
We wrote to all practices in the two boroughs with a brief explanation of the study and an invitation to contact the study team if interested in participating as a host site. The chairperson of the Trial Steering Committee helped facilitate site identification in Hackney.
The interventions were delivered in two GP practices, one in the London borough of Tower Hamlets and one in Hackney. Recruitment of participants was primarily from these two practices, but participants were also referred from four other neighbouring practices to facilitate recruitment. Hackney is ranked as the most deprived borough in England and Tower Hamlets is ranked third. 50
In the London borough of Tower Hamlets it is estimated that 47% [95% confidence interval (CI) 42.3% to 52.1%] of adults are classified as overweight or obese. In Hackney, the figure is similar (49%, 95% CI 43.7% to 53.7%).
The Barkantine Practice is a large GP practice and walk-in centre in Tower Hamlets. It has approximately 18,000 patients on its list. The practice staff comprises 13 doctors, two nurse practitioners, three nurses, three health-care assistants, two health visitors and administrative staff.
The Lawson Practice is a large GP practice in the centre of Hackney. It has approximately 13,000 patients on its list. The practice staff comprises 13 doctors, one nurse practitioner, one nurse, three health-care assistants, two health visitors and administrative staff.
Study procedures
Screening procedures
Participants were either invited to telephone the study team if recruited by posters or leaflets, or telephoned by the study team if referred by GP fax referral.
At the initial screening telephone call, a good clinical practice (GCP)-trained member of staff provided the participant with information on the study. If interested, participants were screened for eligibility over the telephone. Eligible participants were booked onto the next available screening session and were posted or e-mailed the participant information sheet, baseline questionnaire and letter of invitation in advance of this. Participants who were not eligible but still interested in losing weight were either offered the option to attend the standard care clinic or advised to visit their GP for further advice on weight management.
Informed consent procedures
Participants were provided with detailed trial information and allowed sufficient time (at least 24 hours) to consider whether or not they wanted to participate in the trial. All participants were provided with a participant information sheet with more details of the study.
All participants provided written informed consent at the baseline (first) screening session, prior to being randomised to the study arms.
Written informed consent was obtained by an appropriately GCP-trained member of staff delegated by the investigator as documented on the site delegation log, prior to any participation/study-specific procedures.
Randomisation procedures
If eligible, participants were invited to attend the randomisation session a few days later. At this session, participants completed further questionnaires and had their weight, waist circumference and blood pressure recorded. They were then randomised (see Randomisation for more detail of randomisation procedure) to the WAP or nurse (weight loss intervention from a trained GP practice nurse) arms. The first session of the WAP and the nurse intervention were provided within 7 and 14 days of the randomisation session. Table 2 summarises the main purpose of the study visits. All visits were held face to face.
| Visit number | Time point | Top-level tasks |
|---|---|---|
| 1 | Week –1 | Screening |
| 2 | Week 0 | Randomisationa |
| 3–10 | Weeks 1–8 | WAP – eight weekly sessions Nurse – four fortnightly sessions |
| 11–20 | Months 3–12 | WAP – 10 monthly follow-up sessions Nurse – 6- and 12-month follow-up sessions only |
Interventions
Practice nurse intervention
We standardised the nurse intervention to ensure that participants received a consistent standard of care. The nurse intervention was modelled on the best practice intervention in primary care, derived from discussions with GPs and practice nurses, and incorporating national guidelines at the time51 and NHS materials (Your Weight, Your Health: Raising the Issue of Weight in Adults52).
In 2011, when we were designing this project, we conducted a survey of weight management interventions in a range of general practice surgeries. GPs typically provided brief advice followed by referral to a practice nurse. A minority of practices used dietitians but this was slowly being phased out. Some practice nurses had received 1-day training in weight management and provided one-off sessions or sessions with a degree of follow-up, either optional or scheduled, over 2–8 weeks. In about half of the practices, the nurses also referred patients to local community-based physical activity programmes.
We modelled the nurse intervention on the more intensive end of the spectrum, which is still routinely practicable across GP surgeries. Participants received weight management intervention from a practice nurse who had been given training in the study procedures by the research team. The nurses provided the intervention in four sessions delivered over 8 weeks.
The intervention included advice on (1) diet (instructions on understanding food groups, food labels and calories; eat at least five portions of a variety of fruit and vegetables each day in place of foods higher in fat and calories; eat breakfast; watch the portion size of meals and snacks; and replace high-calorie food with healthier options); and (2) activity (make enjoyable physical activities part of everyday life; minimise sedentary activities; build activity into the working day; and take up one of the local exercise opportunities). Table 3 shows a summary of the control intervention. Each session lasted up to 30 minutes. Participants received information about local exercise provision and ‘exercise on prescription’, and received relevant vouchers and referrals. This advice was supported with written materials. Participants received a:
-
Drink Swap: How to Cut Down on Calories in Drinks without Having to Say ‘No’ leaflet53
-
Portion Swap: How Smaller Plates and Portions Help Prevent us Eating too Many Calories leaflet54
-
Snack Swap: How to stay Healthy Without Giving Up all Snacks leaflet55
-
Walk 4 Life: Tips to Get Walking Every Day leaflet56
-
The Eatwell Plate leaflet57
-
5 a Day: What Counts? leaflet58
-
Food Labels leaflet (created by the Health and Lifestyle Unit, Wolfson Institute of Preventive Medicine, London, UK – available on request)
-
calorie guide (created by the Health and Lifestyle Unit, Wolfson Institute of Preventive Medicine, London, UK – available on request)
-
exercise guide (created by the Health and Lifestyle Unit, Wolfson Institute of Preventive Medicine, London, UK – available on request).
| Session | Content description |
|---|---|
| 1 | Introduction, dietary advice (food labels, 5 a day, easy switches), opportunities for exercise and information on orlistat |
| 2 | Discuss progress, provide encouragement |
| 3 | Discuss progress, provide encouragement |
| 4 | Discuss progress, provide encouragement and discuss plans for continuing |
Where appropriate, participants were given an information sheet about orlistat (based on the information provided on the NHS Choices website59) and advised to see their GP if they wished to use it as part of their weight loss programme.
Participants’ weight was recorded at all treatment and follow-up visits. Participants were not restricted from using any other weight loss intervention (including pharmacological treatment if their GP agreed it was appropriate) during the study. They were, however, asked to report on the use of such interventions during the study period.
Weight Action Programme group intervention
The WAP is a multimodal health behaviour modification intervention developed at the Wolfson Institute of Preventive Medicine via extensive client feedback and piloting with underprivileged groups since 2002. The programme is a multicomponent service utilising evidence-based behaviour change techniques in the context of group support targeted to individual needs that aims to provide participants with tools to lose weight and to maintain a long-term healthy lifestyle.
The evidence-based strategies and contents include:
-
self-regulation through the use of (1) food diaries to monitor caloric intake; (2) self-monitoring of weight; and (3) goal-setting and contingent reinforcement
-
motivational components incorporating the standard elements of cognitive–behavioural interventions aimed at encouraging and improving self-efficacy, facilitated by a range of concrete and verifiable tasks agreed individually with each participant (e.g. participants agree incremental pedometer targets)
-
fostering a non-judgemental support network strengthened by shared experience, outcome expectations, positive reinforcement and information on coping with lapses and long-term support
-
dietary advice, information on healthy eating and caloric content of food, cue management, provision of opportunities for exercise and close monitoring of exercise levels.
Participants commit to implementing each of a series of concrete and verifiable tasks for at least 1 week (see Box 8 for a full description). They can drop the task after that if they find it unhelpful.
Another innovative feature of the programme consists of the use of group-oriented interventions aiming to increase participant retention, involvement and adherence to weekly tasks. For example, ‘buddy pairs’ of participants are made responsible for each other’s completion of the weekly task and weight loss of 1 lb (0.45 kg) between the pair. The group format also makes the programme more cost-efficient. Facilitator-led group support creates an environment in which participants can discuss their progress, identify patterns of behaviour and develop coping strategies to facilitate weight loss and maintenance.
The programme was initially implemented within NHS Tower Hamlets, and then modified in the light of participants’ feedback to make it suitable for underprivileged groups, including ethnic minorities. Where information is imparted, it is mostly in a pictorial and easily understandable format.
The WAP has been evaluated in two pilot studies of 162 overweight adults (mean BMI of 35 kg/m2) from multiethnic areas of high deprivation. 60 The average weight loss was 2.8 kg at the end of treatment and 4.5 kg at the 3-month follow-up (with 24% of participants attending follow-up losing ≥ 5% of their body weight). Limited promotion via GP practices and local adverts generated a large volume of interest. The client retention was at least as good as in comparable programmes conducted in research settings with more traditional clients (59% completed the 6-week treatment) and the programme received very high approval ratings. Clients also demonstrated significant improvements in knowledge of healthy eating, and in their exercise levels, as measured by pedometer monitoring. Clients considered the group support essential in helping them to stick to their tasks and to lose weight. 60 In its current form, the WAP also includes information on orlistat.
The version of WAP used in the trial comprised eight weekly sessions, followed by monthly follow-up visits lasting up to 1 hour each. The content of the programme is summarised in Table 4. The target weight loss was 1 lb (0.45 kg) per week. Two advisors conducted the WAP sessions in groups of 10–20 participants.
| Session | Content description and key tasks |
|---|---|
| 1 | Content: introductions, explanation of the course and setting positive and accurate expectations Tasks: wear pedometer and record steps daily, keep a food diary on at least 3 days, monitor ‘screen time’ and do not make any changes yet |
| 2 | Content: understanding calories Tasks: pedometer reading to reach agreed level and food diary to include calories |
| 3 | Content: 5 a day, orlistat and triggers for overeating Tasks: pedometer reading to reach agreed level, 5 a day and obtain orlistat from GP if interested and eligible |
| 4 | Content: exercise Tasks: pedometer reading to reach agreed level and 2 × 10–30 minutes of exercise/moderate-intensity activity |
| 5 | Content: awareness of unnecessary eating, ‘buddy’ up participants and importance of regular weigh-ins Tasks: pedometer reading to reach agreed level, 3 × 20–30 minutes of exercise, ‘say no’ to unnecessary eating and monitor weight |
| 6 | Content: calories recap and monitor hunger Tasks: pedometer reading to reach agreed level, 3 × 30 minutes of moderate-intensity activity, ‘say no’ to unnecessary eating and monitor weight |
| 7 | Content: avoiding triggers to eating and easy switches Tasks: pedometer reading to reach agreed level, 3 × 30 minutes of moderate-intensity activity, ‘say no’ to unnecessary eating, monitor weight and easy switches |
| 8 | Content: recap of 8-week course, feedback and discuss plans for continuing Tasks: pedometer reading to reach agreed level, 3 × 30 minutes of moderate-intensity activity, ‘say no’ to unnecessary eating and monitor weight |
| 9–18 | Content: maintenance sessions, monitor progress and reinstate interventions as needed Tasks: pedometer reading to reach agreed level, 3 × 30 minutes of moderate-intensity activity, ‘say no’ to unnecessary eating and monitor weight |
Participants were provided with an Oregon pedometer PE980 (Oregon Scientific, Tualatin, OR, USA).
As in the control intervention, participants were not barred from using any other weight loss intervention (including pharmacological treatment from their GP). They also received information about local exercise provision and where ‘exercise on prescription’ was available, they received relevant vouchers and referrals. Participants were asked to report on the use of such interventions during the study period.
To help improve replication and further evidence synthesis, Table 5 summarises the content of the WAP according to the CALO-RE (Coventry, Aberdeen, London – Refined) taxonomy of behaviour change techniques for changing physical activity and healthy eating behaviours. 61
| Behaviour change techniques61 | Session number | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9–18 | |
| Providing information on consequences of behaviour in general | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Providing information on consequences of behaviour to the individual | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Providing information about others’ approval | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Providing normative information about others’ behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Goal-setting (behaviour) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Goal-setting (outcome) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Action-planning | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Barrier identification/problem-solving | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Setting graded tasks | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Prompting review of behavioural goals | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prompting review of outcome goals | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prompting rewards contingent on effort or progress towards behaviour | ✗ | ✗ | ✗ | ✗ | |||||
| Providing rewards contingent on successful behaviour | ✗ | ✗ | ✗ | ✗ | |||||
| Shaping | |||||||||
| Prompting generalisation of a target behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prompting self-monitoring of behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prompting self-monitoring of behavioural outcome | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Prompting focus on past success | ✗ | ✗ | |||||||
| Providing feedback on performance | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Providing information on where and when to perform the behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Providing instruction on how to perform the behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Modeling demonstrating the behaviour | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Teaching to use prompts/cues | |||||||||
| Environmental restructuring | ✗ | ✗ | |||||||
| Agreeing behavioural contract | ✗ | ✗ | ✗ | ✗ | |||||
| Prompting practice | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Use of follow-up prompts | |||||||||
| Facilitating social comparison | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Planning social support/social change | ✗ | ✗ | ✗ | ✗ | ✗ | ||||
| Prompting identification as role model/position advocate | ✗ | ||||||||
| Prompting anticipated regret | |||||||||
| Fear arousal | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Prompting self-talk | ✗ | ✗ | |||||||
| Prompting use of imagery | |||||||||
| Relapse prevention/coping planning | ✗ | ✗ | |||||||
| Stress management/emotional control training | ✗ | ||||||||
| Motivational interviewing | |||||||||
| Time management | |||||||||
| General communication skills training | ✗ | ✗ | |||||||
| Stimulating anticipation of future rewards | |||||||||
Staff training
All staff delivering the WAP were trained by shadowing Professor Hajek or Professor McRobbie delivering the programme, and were supervised and mentored when delivering the WAP themselves.
Monitoring of intervention fidelity
For the WAP intervention, the chief investigator (Professor McRobbie) attended five sessions led by each advisor (two in the early phase of the trial and then quarterly) and formally checked the conduct of the session against the counselling protocol to provide feedback to the advisors and record fidelity of the intervention. Professor McRobbie also attended five sessions of the control intervention (two in the early phase of the trial and then quarterly) and checked the conduct of the session formally against the counselling protocol to provide feedback to the nurse and record fidelity of the intervention. Professor Hajek attended one session at each practice and provided feedback.
Outcomes
Primary outcome
The primary outcome is the change in weight (in kg) at 12 months post randomisation.
Secondary outcomes
We recorded the following secondary outcomes:
-
change in weight (in kg) at 1, 2 and 6 months post randomisation
-
change in BMI at 1, 2, 6 and 12 months post randomisation [BMI is calculated as weight (in kg) divided by the square of height (in metres); the height measured at screening was used for each follow-up assessment]
-
change in waist circumference (in cm) at 2, 6 and 12 months post randomisation
-
change in systolic blood pressure (in mmHg) at 2, 6 and 12 months post randomisation
-
change in diastolic blood pressure (in mmHg) at 2, 6 and 12 months post randomisation
-
change in the Food Craving Inventory score (frequency domain) at 1, 2, 6 and 12 months post randomisation
-
change in the Food Craving Inventory score (strength domain) at 1, 2, 6 and 12 months post randomisation
-
change in Food Knowledge Assessment Questionnaire score at 2, 6 and 12 months post randomisation
-
change in the Three-Factor Eating Questionnaire score (cognitive restraint domain) at 2, 6 and 12 months post randomisation
-
change in the Three-Factor Eating Questionnaire score (uncontrolled eating domain) at 2, 6 and 12 months post randomisation
-
change in the Three-Factor Eating Questionnaire score (emotional eating domain) at 2, 6 and 12 months post randomisation
-
change in the International Physical Activity Questionnaire score [metabolic-equivalent (MET) minutes/week domain] at 2, 6 and 12 months post randomisation
-
change in the International Physical Activity Questionnaire score (sitting domain) at 2, 6 and 12 months post randomisation
-
proportion of participants losing 5% of body weight at 2, 6 and 12 months post randomisation
-
proportion of participants losing 10% of body weight at 2, 6 and 12 months post randomisation.
Measurements
Baseline
The following variables were collected at baseline:
-
Demographics: includes age, sex, ethnicity, employment and level of education.
-
Health and lifestyle: includes smoking status, alcohol consumption and general health.
-
Weight loss history: includes number of past weight loss attempts, methods used, most weight ever lost and regular monitoring of weight.
-
Concurrent medications: all current medications are recorded.
-
Height and weight: measured in centimetres and kilograms respectively, BMI was calculated from these. Height was measured, without shoes, on a Seca 2013 portable stadiometer (Seca, Birmingham, UK). Weight was measured on an Omron HBF 400 Body Fat Monitor and Scale (Omron Healthcare UK Ltd, Milton Keynes, UK), with participants wearing light clothing and no shoes. Accuracy was ensured by calibration against standard weights.
-
Waist circumference: measured in centimetres.
-
Blood pressure: resting blood pressure recorded using an Omron 705IT BP monitor (Omron Healthcare UK Ltd, Milton Keynes, UK) using an appropriately sized cuff.
The following questionnaires were also administered at baseline:
-
International Physical Activity Questionnaire62
-
Food Craving Inventory63
-
Three-Factor Eating Questionnaire 64
-
EQ-5D-5L questionnaire65
-
Use of Health Services Questionnaire (see Appendix 1).
We also administered a picture-based food knowledge assessment at baseline and at follow-up. This was developed by the Health and Lifestyle Unit, Queen Mary University of London, to measure basic knowledge of caloric content of different food groups.
Scoring details for the Food Craving Inventory, the Food Knowledge Assessment Questionnaire, the Three-Factor Eating Questionnaire and the International Physical Activity Questionnaire are available in Appendix 2.
Timing of measurements
The study sessions are summarised in measurement schedule shown in Table 6.
| Measure | Time | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week –1 | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | Month 8 | Month 9 | Month 10 | Month 11 | Month 12 | |
| Informed consent | ✓ | |||||||||||||||||||
| Demographics | ✓ | |||||||||||||||||||
| Weight loss history | ✓ | |||||||||||||||||||
| Height | ✓ | |||||||||||||||||||
| Weighta | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Waist circumference | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Randomisation | ✓ | |||||||||||||||||||
| Comorbidities | ✓ | |||||||||||||||||||
| Concurrent medications | ✓ | ✓ | ✓ | |||||||||||||||||
| Pedometer useb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| International Physical Activity Questionnaire62 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Food diary useb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Weekly tasksb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Food knowledge assessment | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| Food Craving Inventory63 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| Three-Factor Eating Questionnaire 64 | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||
| EQ-5D-5L65 | ✓ | ✓ | ✓ | |||||||||||||||||
| Use of Health Services Questionnaire | ✓ | ✓ | ✓ | |||||||||||||||||
| Participant feedback | ✓ | ✓ | ✓ | |||||||||||||||||
| AEs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓b | ✓ | ✓b | ✓ | ✓b | ✓ | ✓b | ✓ | ✓b | ✓ | ||
Adverse events
We used the sponsor’s definition of an adverse event (AE), defined as any untoward medical occurrence in a subject to whom the intervention has been administered, including occurrences that are not necessarily caused by or related to the intervention.
At every visit all participants were asked whether or not they had experienced any AEs since their last contact with the research team.
All AEs were categorised by a member of the research team, blinded to treatment group, according to their severity and whether or not they were related to participation in the Peer-Support Weight Action Programme (SWAP). When possible, serious adverse events (SAEs) and any AEs for which relatedness to participation in SWAP was not clear were followed up by a telephone call to the participant. AEs that occurred before the baseline measurement period were not recorded.
Serious adverse events
A SAE was defined as an adverse event meeting at least one of the following criteria:
-
fatal
-
life-threatening
-
necessitating inpatient hospitalisation or prolongation of existing hospitalisation
-
resulting in persistent or significant disability/incapacity
-
a congenital anomaly/birth defect
-
otherwise considered medically significant by the investigator.
Any SAEs were reported immediately to the chief investigator, the sponsor and the Research Ethics Committee. A report of all SAEs was provided at every Trial Management Committee and Trial Steering Committee meeting.
Follow up
The following variables were collected during follow-up visits: weight, waist circumference, blood pressure, International Physical Activity Questionnaire,62 Food Knowledge Assessment, Food Craving Inventory, Three-Factor Eating Questionnaire,64 EQ-5D-5L,65 Use of Health Services Questionnaire (see Appendix 1), AEs, participant feedback (see Appendix 3) and use of any concomitant weight loss treatment.
In the intervention arm, the following were collected during the 8-week intervention phase: pedometer use, food diary use and adherence to weekly tasks (e.g. increase in fruit and vegetable intake, increase in exercise, monitoring television and computer use).
The 6- and 12-month follow-ups
The 6- and 12-month follow-up sessions for both arms were held at each GP practice. To maximise retention at each follow-up, participants were (1) telephoned 3–4 weeks prior to the visit to schedule a suitable time and explain the importance of attending; (2) sent a confirmation letter/e-mail 1–2 weeks before the scheduled visit; (3) sent a text reminder on the day of the appointment; (4) offered a home/work visit if attending the GP practice was difficult; and (5) offered £10 as a contribution towards travel expenses. The sample size calculation assumed that 50% of participants would be lost to follow-up at 12 months; however, the study team implemented a range of strategies to ensure that as many participants as possible completed the final follow-up (Box 5).
The implementation of multiple follow-up routes (e.g. telephone, text, letter, e-mail).
Calls made to participant at different times of the day (e.g. early mornings/late evenings).
All contact attempts documented, so study team could quickly assess which route to try next.
Involving staff affiliated with the study team (i.e. not involved in the intervention) to make contact with participants to invite them to attend for follow-up so as not to put participant off if they speak to the researcher involved in leading the intervention.
Flexible appointments offered (e.g. evening/weekend/home visits).
Stressing the importance to participants at end of treatment to attend for follow-up, even if they feel that they have gained weight.
Potential strategies for future researchConsider ways of keeping participants ‘involved’ in the study, even if they stop attending from an early stage (e.g. newsletters/interim texts/e-mails).
Send feedback forms to those participants who did not attend to better capture reasons for drop out.
Consider detailing in protocol plans to contact GP to capture data collected at surgery where participants do not attend follow-up.
Consider the use of using self-captured data.
Incentivise follow-up.
Weight, BMI, waist circumference and blood pressure outcomes were measured by researchers who were blind to treatment arm. These researchers were affiliated with the trial team, but were involved only in collecting outcomes during follow-up and had no role in providing the intervention, and no contact with patients other than while collecting follow-up measurements.
Data management
Data collection
All data were collected in the paper clinical record form, questionnaires, and on participant diaries and task cards. All data were kept in accordance with GCP and data protection requirements. 66
Data entry
Data were entered into Oracle Database version 11 (Oracle Corporation UK Ltd, London, UK), an online database hosted at the Barts Cancer Centre. The electronic data capture forms are web based and built using Java, with data validation in JavaScript (Java framework Struts 2; Oracle Corporation UK Ltd, London, UK).
Data quality
When recruitment and follow-up were completed, the study team cleaned the data. Source data verification was also conducted by taking a random sample of 10% of case report forms. A member of the quality assurance (QA) team (based at the Pragmatic Clinical Trials Unit; PCTU) compared all written entries with those entered onto the main study database. The prespecified data quality target was a ≤ 2% discrepancy rate between entries in the case report form and the electronic database, which was met.
Process measures
The process measures included attendance throughout the programme, duration of involvement in the programme (time to dropout), results of knowledge tests, participant feedback on components of treatment at 2, 6 and 12 months (e.g. weekly tasks, new information, group discussion, buddy system), and use of concomitant treatments. Some of these process measures were available only in the WAP arm.
Sample size determination
A clinically significant effect can be achieved with 3–5 kg of weight loss in obese people. 67 We assumed that the WAP would increase weight loss by 2.6 kg compared with usual care (WAP 3 kg vs. nurse 0.4 kg) among participants available for follow-up at 1 year, and that there would be no difference in weight loss between treatment arms among participants not available for follow-up. Assuming that 50% of participants in both treatment arms were available for follow-up at 1 year, the difference in weight loss between arms would be 1.3 kg (WAP 1.5 kg vs. usual care 0.2 kg). Assuming a standard deviation (SD) of 3 in both treatment arms, and a 5% two-sided significance level, we would require 112 participants in each arm to detect this mean difference with 90% power. Our estimate of 50% loss to follow-up is conservative and based on international experience in this field and existing data from similar underprivileged and highly mobile populations and interventions.
To account for potential clustering effects due to group treatment in the WAP arm, assuming a mean cluster size of 18 and an intracluster correlation coefficient of 0.05, a total of 208 individuals will be required in the WAP arm. The same power can be achieved with 108 in the nurse arm and 216 in the WAP arm, which we increased to 110 in the nurse arm and 220 in the WAP arm to give an allocation ratio between the two arms (2 : 1) that can be expressed in whole numbers. Thus, we required a total of 330 individuals for the entire study.
Randomisation
After providing written, informed consent, eligible participants were randomised in a 2 : 1 ratio (WAP to nurse) using permuted blocks with randomly varying sizes of 18, 21 and 24, stratified by study site (Barkantine or Lawson). Randomisation was conducted using an internet-based application produced by the Sheffield Clinical Trials Unit, University of Sheffield. The randomisation sequence was generated by a statistician from epiGenesys, a wholly owned subsidiary of the University of Sheffield (www.epigenesys.org.uk/).
The study staff randomising the participant accessed the randomisation program remotely when the patient was with them, entering their ID number into the program. The ID number was specific to study site. No other information was entered, as there were no other stratification factors. The allocation was immediately provided by the program.
Investigators randomising participants were unaware of the allocation until after they performed the randomisation (allocation concealment), but were then unblinded after the randomisation had been performed. Researchers who collected measurements at 6 and 12 months’ follow-up were blinded to treatment allocation.
Treatment masking (blinding)
Participants and study staff providing the interventions and collecting data at the 1- and 2-month follow-up were not blinded. However, the study staff collecting the measurements (including weight, BMI, waist circumference and blood pressure) at the 6- and 12-month follow-up were blinded to treatment allocation.
All members of the trial team remained blinded to outcome data, summarised according to treatment arm until the statistical analysis plan was signed off.
Statistical methods
Change from planned analysis
In version 1.0 of the trial protocol we specified that we would use a baseline observation carried forward (BOCF) approach for dealing with patients with missing weight data during follow-up. This approach assumes that all those who were lost to follow-up returned to their exact baseline weight. Although this approach has been commonly used in other RCTs, it is problematic because it will provide biased estimates of the treatment effect when this assumption is incorrect (i.e. when participants do not return to their exact baseline weight when they fail to show up to their 6- or 12-month appointment). 68 In addition, BOCF will often lead to an inflated type I error (false-positive) rate as it tends to underestimate the standard error for the treatment effect (as a result of ignoring the within-patient variability in weight when imputing using BOCF). 68 This is particularly problematic in the SWAP trial, as it is unlikely that all participants who are lost to follow-up will return to their baseline weight; in many cases, we would expect them to gain weight. Cross-sectional and prospective cohort studies show that individuals gain weight over time, with an average weight gain per year of 0.5–1 kg. 69
We therefore decided to use a mixed-effects linear regression model for the primary analysis. This analysis method provides unbiased estimates of treatment effect and correct type I error rates, provided that the data are missing at random. That is, the probability that a participant is lost to follow-up depends on their previous weight measurements (e.g. their weight at baseline and 6 months if they are lost to follow-up at 12 months) and baseline patient characteristics. 70
This strategy of analysis has been widely recommended in the presence of missing outcome data. 68 The decision to change analysis methods was made before we had any access to the trial data or ongoing trial results and, therefore, there was no risk of bias associated with this decision.
The statistical analysis plan is provided in Appendix 4.
General analysis principles
All analyses were performed using intention-to-treat principles, meaning that all participants with at least one recorded outcome during follow-up were included in the analysis, and participants were analysed according to the treatment group to which they were randomised. 71 More information on which participants were included in each analysis is available in Missing data for outcomes. All p-values are two-sided, and the significance level was set at 5%.
All analyses accounted for clustering by group in the WAP arm and clustering by nurse in the nurse arm. 72,73 Each participant has been defined as belonging to a cluster, by which group they belonged to if they were in the intervention arm and by which nurse they were treated by if they were in the control arm. This variable has been included as a random intercept in a mixed-effects regression model. This analysis assumes that the intraclass correlation coefficient is the same between groups in the intervention arm as it is between nurses in the control arm. The Kenward–Roger degree of freedom correction was used for all linear mixed-effects models. 74
All analyses were adjusted for baseline weight, age, sex, ethnicity (white British, white other, black, Asian, mixed or other), smoking status (smoker vs. non-smoker) and GP practice (Lawson vs. Barkantine) as covariates in a regression model. 75–77 Outcomes that were measured at baseline were also adjusted for the value of the outcome at baseline (this includes weight, BMI, waist circumference, systolic and diastolic blood pressure, Food Craving Inventory, Food Knowledge Assessment, Three-Factor Eating Questionnaire, and International Physical Activity Questionnaire). Continuous covariates (baseline weight and age) were assumed to have a linear association with outcome. Binary and categorical covariates (sex, ethnicity, smoking status and GP practice) were included in the regression model using indicator (dummy) variables. Missing baseline data were accounted for using mean imputation. 78
Missing data for outcomes
For outcomes that are measured at multiple time points during follow-up, we based our analysis strategy on that proposed by White et al. 71 To deal with incomplete data (i.e. when patients have missing data at one of the follow-up time points) we:
-
attempted to follow up all randomised patients even if they withdrew from the study
-
performed a main analysis of all observed data that are valid under a plausible assumption about the missing data
-
performed sensitivity analyses to explore the effect of departures from the assumptions made in the main analysis
-
accounted for all randomised participants, at least in the sensitivity analyses.
In the analyses we:
-
Included all patients with at least one post-randomisation assessment (i.e. if they have recorded data for at least one follow-up time point) in the analysis. This allows data from patients who dropped out before 12 months to contribute to the treatment effect estimate at 12 months (e.g. patients with recorded data at 1 month but who dropped out after that would still contribute towards the 12-month analysis).
-
Used a mixed-effects model adjusted for baseline covariates, which assumes that the data are missing at random (i.e. they are missing based on their observed outcome at other time points, and other patient characteristics, Box 6).
-
Performed sensitivity analyses under other missing data assumptions (e.g. that patients who were lost to follow-up gained more weight than patients who remained in the trial).
Data are assumed to be missing completely at random if being lost to follow-up (LTFU) is not dependent on any baseline covariates or outcomes. This would be the wrong assumption to make about missing data in this study, as LTFU is highly likely to be related to outcome (i.e. those not losing weight are more likely to drop out).
Missing at randomData are assumed to be missing at random if LTFU is dependent on observed data, including those data collected during follow-up (e.g. those who have not lost weight at early follow-up points are more likely to be lost to follow-up later). Missing at random is a reasonable assumption for missing data in this trial.
Missing not at randomData are assumed to be missing not at random if LTFU is dependent on both observed and unobserved outcomes. Missing not at random is a reasonable assumption for missing data in this trial.
Analysis of primary outcome
The primary outcome (change in weight at 12 months post randomisation) was analysed using a mixed-effects linear regression model. The model included change in weight at 1, 2, 6 and 12 months as outcomes.
The model included a random intercept for ‘cluster’ (group or nurse, depending on treatment arm). The correlation between observations at different time points from the same patient (1, 2, 6 and 12 months) was modelled using an unstructured correlation structure. The model was estimated using restricted maximum likelihood. Treatment arm, time point (month 1, 2, 6 or 12) and the interaction between treatment arm and time point were included in the model as fixed factors. Time point was included as an indicator variable. The covariates listed in General analysis principles were also included in the model as fixed factors.
This analysis approach meant that any participant who had a recorded weight for at least one follow-up session (at either 1, 2, 6 or 12 months) was included in the analysis for the primary outcome. So, for example, a participant who lost 0.5 kg at 1 month but had no further weight measurements at 2, 6 or 12 months would still be included in the primary analysis, and would contribute towards the estimated treatment effect at 12 months. Their 12-month weight would be estimated based on their weight at 1 month, their treatment group and their baseline factors, such as baseline weight, age, sex, ethnicity, smoking status and GP practice. This analysis approach provides unbiased estimates of treatment effect provided the reason the participant’s weight data at 2, 6 and 12 months are missing is based upon their observed weight at 1 month or their baseline characteristics (e.g. participants with lower weight loss at 1 month are more likely to be lost to follow-up at 12 months). 79
Sensitivity analyses for primary outcome
Missing data
We performed two sensitivity analyses to assess the robustness of our primary analysis to different assumptions regarding the missing data. These sensitivity analyses were performed only for the primary outcome (change in weight at 12 months):
-
a complete-case analysis, where only patients with recorded data at 12 months are included
-
an analysis that assumes data missing at 12 months is missing not at random (Box 7 and see Figure 6).
Where we assumed data to be missing not at random we used the following formula:(1)Δ=ΔCC+Y1P1−Y2P2.
-
Δ is the treatment effect under the missing not at random scenario.
-
ΔCC is the treatment effect from a complete-case analysis.
-
Y1 and Y2 are the assumed mean change in weight at 12 months for participants with missing 12-month weight data in treatment groups 1 and 2, respectively.
-
P1 and P2 are the proportion of participants with missing weight data at 12 months in groups 1 and 2, respectively.
-
Groups 1 and 2 represent the intervention and control groups, respectively.
The standard error for Δ is assumed to be approximately equal to the standard error for ΔCC.
Y2 was varied between –10, –5, –2.5, 0, 2.5, 5 and 10. Negative values indicate that the participant lost weight at 12 months, positive values indicate that they gained weight and a value of 0 indicates that there was no change from baseline. For each value of Y2, Y1 was set to Y2 – 5, Y2 and Y2 + 5.
For example, for Y2 = 10, this would indicate an assumption that patients in treatment arm 2 (the control arm) who were lost to follow-up at 12 months, had gained 10 kg, on average, at 12 months. Y1 would vary between 5, 10 and 15, indicating the assumption that patients in treatment arm 1 (the intervention arm) who were lost to follow-up had gained 5 kg, on average, at 12 months (5 kg less than those in the control arm), 10 kg (the same amount as those in the control arm) or 15 kg (5 kg more than those in the control arm).
Cross-sectional and prospective cohort studies show that individuals gain weight over time, with an average weight gain per year of 0.5–1 kg. 69 Therefore, those lost to follow-up are unlikely to gain > 5 kg in 1 year.
Participants who became pregnant or had bariatric surgery during follow-up
We performed a sensitivity analysis to assess the impact on results of patients who became pregnant or underwent bariatric surgery during follow-up. Patients who became pregnant or underwent bariatric surgery during follow-up were excluded from the analysis. This analysis was performed using the same methods as for the primary analysis.
Analyses of secondary outcomes
Change in weight at 1, 2 and 6 months were included as outcomes in the same analysis model as change in weight at 12 months.
The analyses for change in BMI, waist circumference, systolic and diastolic blood pressure, and the Food Craving Inventory, Food Knowledge Assessment and Three-Factor Eating Questionnaires all used the same method of analysis as the primary outcome, with the exception of which baseline covariates were included in the analysis. These differences are summarised in Table 7.
| Outcome | Difference to analysis method for primary outcome |
|---|---|
| Change in BMI at 1, 2, 6 and 12 months | Baseline BMI was included as a covariate in the regression model and baseline weight was not |
| Change in waist circumference at 2, 6 and 12 months | Baseline waist circumference was included as a covariate in the regression model and baseline weight was not |
| Change in systolic blood pressure at 2, 6 and 12 months | Baseline systolic blood pressure was also included as a covariate in the regression model |
| Change in diastolic blood pressure at 2, 6 and 12 months | Baseline diastolic blood pressure was also included as a covariate in the regression model |
| Change in Food Craving Inventory (frequency domain) at 1, 2, 6 and 12 monthsa | The baseline Food Craving Inventory (frequency domain) score was also included as a covariate in the regression model |
| Change in Food Craving Inventory (strength domain) at 1, 2, 6 and 12 monthsa | The baseline Food Craving Inventory (strength domain) score was also included as a covariate in the regression model |
| Change in Food Knowledge Assessment at 2, 6 and 12 months | The baseline Food Knowledge Assessment score was also included as a covariate in the regression model |
| Change in Three-Factor Eating Questionnaire (cognitive restraint domain) at 2, 6 and 12 months | The baseline Three-Factor Eating Questionnaire (cognitive restraint domain) score was included as a covariate |
| Change in Three-Factor Eating Questionnaire (uncontrolled eating domain) at 2, 6 and 12 months | The baseline Three-Factor Eating Questionnaire (uncontrolled eating domain) score was included as a covariate |
| Change in Three-Factor Eating Questionnaire (emotional eating domain) at 2, 6 and 12 months | The baseline Three-Factor Eating Questionnaire (emotional eating domain) score was included as a covariate |
| Change in International Physical Activity Questionnaire (MET-minutes/week domain) at 2, 6 and 12 months | The baseline MET-minutes/week domain score was included as a covariate |
| Change in International Physical Activity Questionnaire (sitting domain) at 2, 6 and 12 months | The baseline sitting domain score was included as a covariate |
The analysis of the proportion of patients losing 5% of their body weight used a mixed-effects logistic regression model. The model included as outcomes whether or not participants had lost 5% of their body weight at 2, 6 and 12 months. The model included three levels. The top level included a random intercept for ‘cluster’ (group or nurse, depending on treatment arm). The second level included a random intercept for patient and a random slope for time point. The third level included patient’s visit (i.e. whether it was the patient’s 2-, 6- or 12-month visit). Treatment arm, time point and the interaction between treatment arm and time point were included in the model as fixed factors. The fixed effect for time point was included as an indicator variable. This analysis adjusted for the same baseline covariates as that of the primary outcome.
The proportion of patients losing 10% of their body weight was analysed separately at 6 and 12 months (the analysis at 2 months was not performed because of the small number of events at this time point). The analysis at 12 months used a mixed-effects logistic regression model, with a random intercept for ‘cluster’ and adjusted for the same baseline covariates as in the analysis of the primary outcome. The analysis at 6 months also used a mixed-effects logistic regression model, but adjusted only for baseline weight (as the model did not converge when the other covariates were included).
Subgroup analyses
No subgroup analyses were performed.
Other data summaries
Data summaries are also provided for:
-
number of participants on both treatment arms who used orlistat
-
weight change at 12 months in participants who used orlistat versus those who did not
-
participant feedback (mean and SD, number and per cent) in both treatment arms at 2, 6 and 12 months.
Statistical software
All analyses were implemented in Stata version 14 (StataCorp LP, College Station, TX, USA).
Departures from the statistical analysis plan
-
Sensitivity analysis: patients who became pregnant or had bariatric surgery.
-
The statistical analysis plan stated that for this sensitivity analysis patients who became pregnant or had gastric surgery would be excluded from the point at which they had surgery or became pregnant (i.e. their follow-up data from before pregnancy or surgery would be included in the analysis). However, the date of pregnancy was unavailable for a number of patients. Therefore, this analysis completely excluded patients who became pregnant or had gastric surgery.
-
-
Secondary outcome: proportion of patients losing ≥ 10% of their body weight.
-
The statistical analysis plan stated that this outcome would be analysed in the same way as the proportion of patients losing ≥ 5% of their body weight (i.e. using a three-level mixed-effects logistic regression model). However, this analysis model did not reach convergence. We therefore tried to refit the model after removing the random slope for time point, but the model still did not reach convergence. We therefore analysed this outcome separately at 6 and 12 months (i.e. separate logistic regression models were used at each time point); we did not analyse this outcome at 2 months because of the small number of events. The analysis at 12 months was performed using a mixed-effects logistic regression model with a random intercept for cluster and adjusted for the same baseline covariates as the analysis of the primary outcome. However, this model did not converge for the 6-month analysis; we therefore removed all baseline covariates from the model except for baseline weight.
-
Ongoing public and patient involvement
The Trial Steering Committee included the lay member Julie Griffiths, an ex-service user who, in addition to providing general feedback to the study team regarding the progress of the study, contributed to the redrafting of the recruitment and follow-up strategies, providing invaluable suggestions to improve participant retention based on her previous experience of participating in the WAP. For example, Julie suggested that in telephone conversations with participants who failed to attend follow-up appointments, it would be helpful if the study team reasserted the importance of attending. Julie was also key in assisting the study team with the design of the study to help ensure that the delivery of the intervention and control conditions was as practicable as possible. Study documents, including questionnaires, information sheets and invitation letters, were reviewed by Julie, who provided useful feedback.
Two participants (both in the intervention condition) attended several general practice meetings with a member of the study team to encourage regular referral onto the study. Participants presented their first-hand experience of taking part in the WAP, presenting the advantages and challenges faced, providing practice staff with direct feedback from active participants. Several general practice staff members informed the study team that such presentations were not only useful in helping them to remember to offer the study to their patients but also reassuring, as they felt more comfortable offering the study to their patients upon hearing the honest feedback from participants.
Ex-service users are involved in regular panels held at the Health and Lifestyle Research Unit, during which potential study ideas are discussed as well as ways to improve the WAP in its current format.
Quality control and quality assurance
The PCTU was responsible for monitoring and audit of the study. The PCTU QA manager drafted a monitoring/audit plan prior to study initiation, which consisted of a combination of remote and on-site monitoring. A risk assessment of the study was conducted by the PCTU QA manager and chief investigator, which informed the frequency of monitoring and audit visits.
Approvals
This study was sponsored by the Joint Research Management Office, Queen Mary University of London, and received ethics approval from the London – Central Ethics Committee on 3 February 2012 (reference number 12/LO/0122).
Trial committees
Members of the Trial Steering Committee and Trial Management Committee are shown in Table 8.
| Committee | |
|---|---|
| Trial Steering | Trial Management |
| Dr Vicky Hobart (chairperson, public health consultant) | Professor Hayden McRobbie |
| Dr Simon Coppack (consultant physician) | Professor Peter Hajek |
| Dr Clare Grace (obesity research dietitian) | Dr Katie Myers Smith |
| Professor Luke Vale (health economist) | Mrs Sarah Snuggs |
| Dr John Stapleton (independent statistician) | Dr Amanda Bunten (City and Hackney PCT) |
| Professor Hayden McRobbie (chief investigator) | Mr Mike Waring (data manager) |
| Professor Peter Hajek (co-investigator) | Mrs Anitha Manivannan (QA manager) |
| Ms Julie Griffiths (lay member and service user) | Professor Sandra Eldridge |
| Dr Brennan Kahan (trial statistician) | |
| Miss Sarrah Peerbux | |
Chapter 3 Results
Participant flow
Figure 3 shows participant flow through the trial.
FIGURE 3.
Consolidated Standards of Reporting Trials diagram.

Losses and exclusions
Of the 1018 people registering an interest during the recruitment period, 435 were excluded from the trial (55 decided against participation, 389 were not eligible and 16 could not be randomised as the sample size target had been reached). Of the 389 participants excluded from taking part, 87 participants (22%) were excluded because they were taking psychiatric medication (Table 9). A total of 221 participants were randomly allocated to the intervention group and 109 to the control group.
| Reason for exclusion | Number of participants |
|---|---|
| BMI of < 30 kg/m2 or < 28 kg/m2 plus comorbidities | 58 |
| BMI of > 45 kg/m2 | 6 |
| Lost > 5% of their body weight in the previous 6 months | 66 |
| Currently pregnant | 14 |
| Taking psychiatric medications | 87 |
| Not registered with a GP in the local areas | 21 |
| Involved in a current research project | 8 |
| Aged < 18 years | 5 |
| Unable to commit to the sessions/unavailable for follow-up | 54 |
| Othera | 23 |
Recruitment
It was originally planned to recruit approximately 30 participants per month over a 12-month period starting in October 2011. However, because of delays in research and development (R&D) approvals and contracting, the project plan was revised to start recruitment in July 2012, recruiting 40 participants per month over a 9-month period.
Despite this revised timetable, the start of recruitment was delayed until September 2012 and was slower than anticipated (Figure 4). This led us to further revise our strategy and timetable, extending recruitment until January 2014 (Figure 5). The barriers to recruitment, and our strategies to remedy this, are discussed in Chapter 5.
FIGURE 4.
Initial recruitment targets.

FIGURE 5.
Revised recruitment targets.
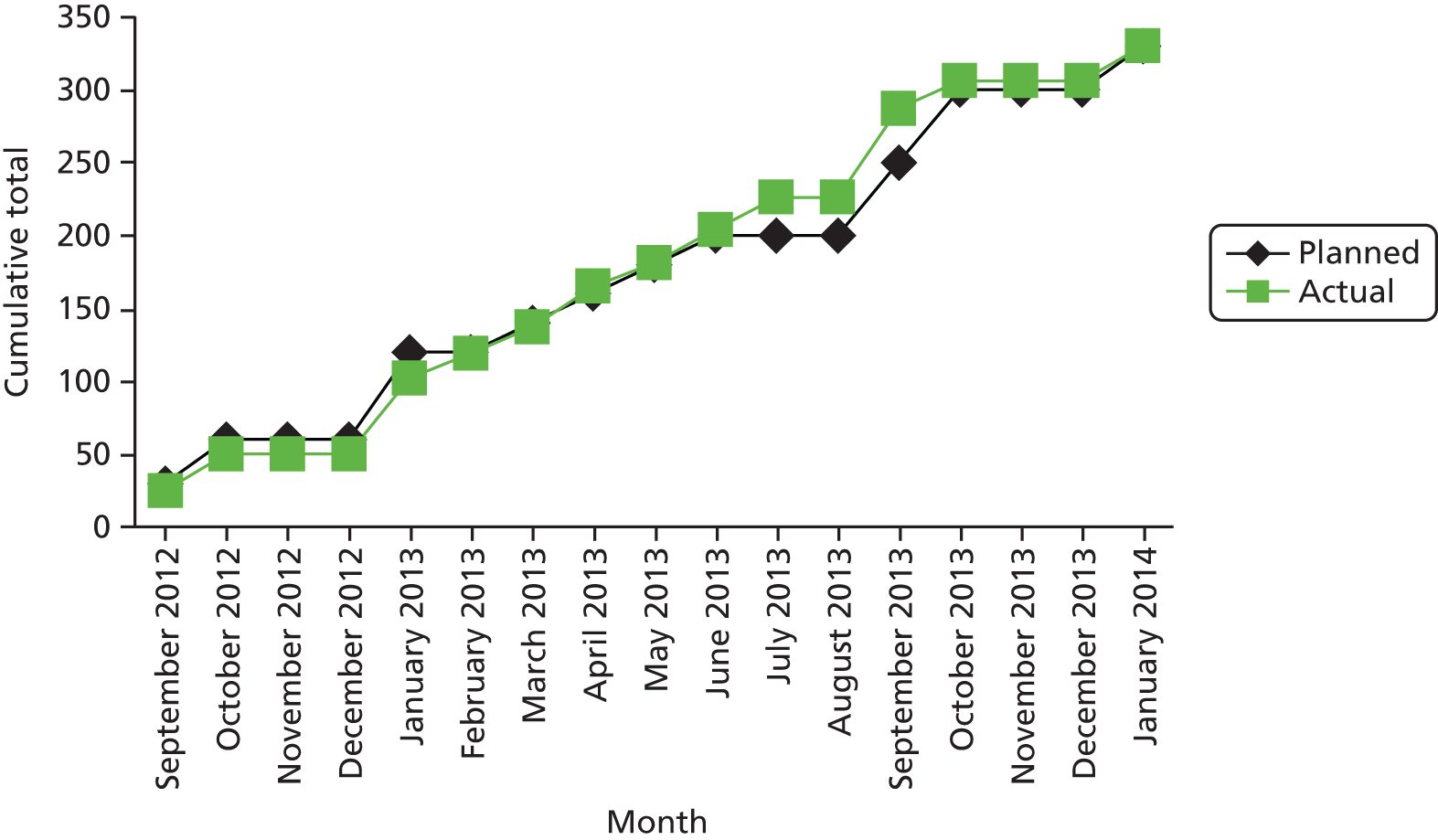
The were a total of 15 waves of recruitment, eight at the Barkantine Practice and seven at the Lawson Practice (Table 10).
| Clinic | Number of participants | |||||
|---|---|---|---|---|---|---|
| Booked for a screening visit | Who attended a screening visit | Who attended a randomisation visit | Randomised | Randomised to the WAP | Randomised to the nurse arm | |
| Barkantine wave 1 | 34 | 29 | 25 | 25 | 16 | 9 |
| Barkantine wave 2 | 49 | 31 | 29 | 29 | 18 | 11 |
| Barkantine wave 3 | 39 | 21 | 17 | 17 | 13 | 4 |
| Barkantine wave 4 | 51 | 34 | 28 | 28 | 19 | 9 |
| Barkantine wave 5 | 48 | 31 | 25 | 23 | 17 | 6 |
| Barkantine wave 6 | 61 | 40 | 33 | 33 | 21 | 12 |
| Barkantine wave 7 | 37 | 23 | 19 | 19 | 13 | 6 |
| Barkantine wave 8a | 35 | 24 | 20 | 4 | 3 | 1 |
| Lawson wave 1 | 41 | 31 | 25 | 25 | 15 | 10 |
| Lawson wave 2 | 50 | 28 | 23 | 23 | 16 | 7 |
| Lawson wave 3 | 42 | 23 | 19 | 18 | 13 | 5 |
| Lawson wave 4 | 28 | 19 | 16 | 16 | 10 | 6 |
| Lawson wave 5 | 37 | 26 | 22 | 22 | 14 | 8 |
| Lawson wave 6 | 57 | 32 | 28 | 28 | 19 | 9 |
| Lawson wave 7 | 35 | 24 | 20 | 20 | 14 | 6 |
| Total | 644 | 416 | 349 | 330 | 221 | 109 |
Four practice nurses were allocated a median of 24 participants [interquartile range (IQR) 17–38 participants]. The 15 groups contained a median of 15 participants (IQR 13–18 participants). Of the intervention group, 120 (54%) were from the Barkantine Practice and 101 (46%) from the Lawson Practice (Table 11). The Barkantine nurses saw 58 (53%) control group participants and the Lawson nurse saw 51 (47%) participants.
| Clusters | Arm | |
|---|---|---|
| Nurse (n = 109) | WAP (n = 221) | |
| Number of nurses/groups | 4 | 15 |
| Number of participants per nurse/group, median (IQR) | 24 (17–38) | 15 (13–18) |
Retention was good for weight management programmes, with 96% of the WAP participants and 90% of the nurse participants attending at least one of the treatment sessions. Seventy-nine per cent of participants in the WAP arm attended at least half of the prescribed sessions, compared with 69% in the nurse arm.
The first 6-month follow-ups were conducted in March 2013. The first 12-month follow-ups were conducted in September 2013 and the final 12-month follow-up was conducted in February 2015.
Overall, 70% of participants completed the 12-month follow-up, with the follow-up rates being slightly higher in the nurse arm than in the WAP arm (Table 12).
| Follow-up | Arm, n (%) | |
|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |
| 1 month | 74 (68) | 164 (74) |
| 2 months | 62 (57) | 144 (65) |
| 6 months | 70 (64) | 141 (64) |
| 12 months | 83 (76) | 149 (67) |
Follow-up rates were similar at the two study sites (Table 13).
| Site | Number of participants randomised | Attended follow-up, n (%) | ||
|---|---|---|---|---|
| Nurse | WAP | Total | ||
| Barkantine | 178 | 45 (76) | 79 (66) | 124 (70) |
| Lawson | 152 | 38 (75) | 70 (69) | 108 (71) |
| Total | 330 | 83 (76) | 149 (67) | 232 (70) |
Baseline data
Table 14 shows demographics of participants. The majority (72%) of participants were women, as is fairly typical of weight management programmes. Participants were middle-aged (mean age 45 in the nurse arm and 47 years in the WAP).
| Variable | Arm | Missing data (number in nurse arm, number in WAP arm) | |
|---|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | ||
| Age (years), mean (SD) | 45.1 (14.2) | 46.6 (15.0) | 0, 0 |
| Female, n (%) | 75 (69) | 161 (73) | 0, 0 |
| Marital status, n (%) | 0, 0 | ||
| Single | 40 (37) | 72 (33) | – |
| Separated or divorced | 13 (12) | 33 (15) | – |
| Married or living with partner | 49 (45) | 92 (42) | – |
| Other | 7 (6) | 24 (11) | – |
| Ethnicity, n (%) | 1, 3 | ||
| White British | 46 (43) | 85 (39) | – |
| White other | 11 (10) | 27 (12) | – |
| Black | 26 (24) | 53 (24) | – |
| Asian | 16 (15) | 27 (12) | – |
| Mixed | 1 (1) | 11 (5) | – |
| Other | 8 (7) | 15 (7) | – |
| Educational qualification, n (%) | 1, 0 | ||
| None | 15 (14) | 36 (16) | – |
| GCSE or equivalent | 25 (23) | 49 (22) | – |
| A-Level or equivalent | 12 (11) | 30 (14) | – |
| Degree or equivalent | 41 (38) | 71 (32) | – |
| Other | 15 (14) | 35 (16) | – |
| Employment status, n (%) | 0, 0 | ||
| In paid employment | 57 (52) | 103 (47) | – |
| Unemployed | 18 (17) | 38 (17) | – |
| Looking after the home | 6 (6) | 15 (7) | – |
| Retired | 17 (16) | 34 (15) | – |
| Full-time student | 3 (3) | 10 (5) | – |
| Other | 8 (7) | 21 (10) | – |
| Entitled to free prescriptions, n (%) | 0, 0 | ||
| Yes | 62 (57) | 133 (60) | – |
| No | 45 (41) | 81 (37) | – |
| Not known | 2 (2) | 7 (3) | – |
| Baseline comorbidities, n (%) | |||
| Heart disease | 6 (6) | 21 (10) | 1, 1 |
| Diabetes mellitus | 9 (8) | 21 (10) | 0, 0 |
| At least one comorbidity | 66 (61) | 135 (61) | 0, 0 |
| Current smoker, n (%) | 18 (17) | 35 (16) | 0, 0 |
| Units of alcohol consumed per week, mean (SD) | 7.6 (11.7) | 7.2 (10.3) | 55, 104 |
Almost half (48%) of participants were from black and ethnic minorities, which reflects the population of the study setting. Forty per cent were white British and 12% were classified as ‘white other’. Approximately one-third of participants were single and 43% were married or living with their partner. Three measures (entitlement to free prescriptions, employment status and educational qualification) provided an indication of the socioeconomic status of participants. Overall, 59% were entitled to free prescriptions, 51% had no higher education and 52% did not have paid employment.
On average, alcohol consumption was within recommended limits. Sixteen per cent of participants reported that they were current smokers.
Baseline data related to weight, physical activity and eating behaviours are shown in Table 15. The mean weight of both groups was > 95 kg (98.3 kg in the nurse arm and 95.5 kg in the WAP arm). The average BMI for both groups was > 35 kg/m2, indicating that participants were obese. Approximately one-third of participants reported being overweight or obese as children and one-third reported having an overweight or obese mother. Fewer participants (16%) reported that their father was overweight or obese.
| Variable | Arm | Missing data (number in nurse arm, number in WAP arm) | |
|---|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | ||
| Weight (kg), mean (SD) | 98.3 (16.6) | 95.5 (15.8) | 0, 0 |
| BMI (kg/m2), mean (SD) | 35.7 (4.3) | 35.0 (4.2) | 0, 0 |
| BMI categories (kg/m2), n (%) | |||
| 25–29.9 | 9 (8) | 16 (7) | – |
| 30–34.9 | 44 (40) | 112 (51) | – |
| 35–39.9 | 35 (32) | 60 (27) | – |
| 40–45 | 21 (19) | 33 (15) | – |
| Waist circumference (cm), mean (SD) | 114.2 (10.1) | 113.4 (10.7) | 0, 0 |
| Systolic blood pressure (mmHg), mean (SD) | 134.8 (15.9) | 134.5 (16.7) | 0, 0 |
| Diastolic blood pressure (mmHg), mean (SD) | 80.6 (8.6) | 81.3 (10.5) | 1, 0 |
| Food Craving Inventory score, mean (SD) | |||
| Frequency domain | 9.6 (3.8) | 9.1 (3.8) | 0, 0 |
| Strength domain | 8.9 (4.0) | 8.5 (3.9) | 0, 1 |
| Food Knowledge Assessment Questionnaire score, mean (SD) | 6.6 (1.7) | 6.6 (1.7) | 0, 0 |
| Three-Factor Eating Questionnaire score, mean (SD) | |||
| Cognitive restraint domain | 2.3 (0.6) | 2.4 (0.6) | 1, 2 |
| Uncontrolled eating domain | 2.2 (0.7) | 2.2 (0.7) | 4, 3 |
| Emotional eating domain | 2.5 (0.9) | 2.6 (1.0) | 1, 2 |
| International Physical Activity Questionnaire, mean (SD) | |||
| MET-minutes/week domain | 1815 (2355) | 1919 (2508) | 12, 28 |
| Sitting domain | 391 (197) | 382 (219) | 13, 38 |
| Overweight or obese as a child, n (%) | 36 (33) | 84 (38) | 0, 0 |
| Mother overweight or obese, n (%) | 30 (30) | 78 (39) | 9, 22 |
| Father overweight or obese, n (%) | 19 (21) | 34 (18) | 20, 29 |
| Number of previous attempts at weight loss, median (IQR) | 3 (2–5) | 3 (1–5) | 8, 12 |
| Greatest previous amount of weight loss (kg), median (IQR) | 9.3 (5.0–19.1) | 10.9 (6.0–19.1) | 9, 20 |
The median number of previous weight loss attempts was three, with participants claiming to have lost a significant amount of weight in past attempts (median amount of weight lost was 9.3 kg and 10.9 kg in the nurse and WAP arms, respectively).
Although participants reported sitting for approximately 6.5 hours per day, self-reported baseline levels of physical activity were high in both the nurse and WAP arms (mean 1815 and 1919 MET-minutes per week, respectively).
Numbers analysed
As specified in the statistical analysis plan, we followed the recommended guidance for applying intention-to-treat principles in the presence of missing data,68 and have included all participants with at least one follow-up measurement (e.g. who had their weight recorded at at least one of their 1-, 2-, 6- or 12-month visits) in the analysis of the primary outcome.
The numbers of participants available for analysis of each of the clinical outcomes are shown in Table 16. For the primary outcome, weight loss at 12 months, we used data from participants who provided weight measurements at least once during follow-up. Therefore, data were available for 88% (n = 291) of participants, with almost identical rates in both arms (89% nurse, 88% WAP). For the secondary measures, data available at each follow-up point were used; therefore,12-month data for the other clinical outcomes were available for 76% of participants in the nurse arm and 67% of participants in the WAP arm.
| Variable | Arm, n (%) | |
|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |
| Weight/BMI | ||
| 1 month | 74 (68) | 157 (71) |
| 2 months | 62 (57) | 140 (63) |
| 6 months | 70 (64) | 141 (64) |
| 12 months | 83 (76) | 149 (67) |
| Measured at least once during follow-up | 97 (89) | 194 (88) |
| Number of measurements per patient | ||
| 0 | 12 (11) | 27 (12) |
| 1 | 13 (12) | 26 (12) |
| 2 | 23 (21) | 39 (18) |
| 3 | 14 (13) | 33 (15) |
| 4 | 47 (43) | 96 (43) |
| Waist circumference | ||
| 2 months | 60 (55) | 140 (63) |
| 6 months | 70 (64) | 141 (64) |
| 12 months | 83 (76) | 149 (67) |
| Measured at least once during follow-up | 92 (84) | 182 (82) |
| Number of measurements per patient | ||
| 0 | 17 (16) | 39 (18) |
| 1 | 18 (17) | 36 (16) |
| 2 | 27 (25) | 44 (20) |
| 3 | 47 (43) | 102 (46) |
| Blood pressure | ||
| 2 months | 60 (55) | 140 (63) |
| 6 months | 70 (64) | 141 (64) |
| 12 months | 83 (76) | 149 (67) |
| Measured at least once during follow-up | 92 (84) | 182 (82) |
| Number of measurements per patient | ||
| 0 | 17 (16) | 39 (18) |
| 1 | 18 (17) | 36 (16) |
| 2 | 27 (25) | 44 (20) |
| 3 | 47 (43) | 102 (46) |
| Lost 5% or 10% of body weight | ||
| 2 months | 62 (57) | 140 (63) |
| 6 months | 70 (64) | 141 (64) |
| 12 months | 83 (76) | 149 (67) |
| Measured at least once during follow-up | 92 (84) | 182 (82) |
| Number of measurements per patient | ||
| 0 | 17 (16) | 39 (18) |
| 1 | 18 (17) | 36 (16) |
| 2 | 25 (23) | 44 (20) |
| 3 | 49 (45) | 102 (46) |
Table 17 shows the numbers of participants available for analysis of questionnaire data.
| Questionnaire | Arm, n (%) | |
|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |
| Food Knowledge Assessment | ||
| 2 months | 47 (43) | 144 (65) |
| 6 months | 70 (64) | 140 (63) |
| 12 months | 83 (76) | 147 (67) |
| Measured at least once during follow-up | 90 (83) | 180 (81) |
| Number of measurements per patient | ||
| 0 | 19 (17) | 41 (19) |
| 1 | 18 (17) | 33 (15) |
| 2 | 34 (31) | 43 (19) |
| 3 | 38 (35) | 104 (47) |
| Food Craving Inventory frequency domain | ||
| 1 month | 66 (61) | 161 (73) |
| 2 months | 61 (56) | 143 (65) |
| 6 months | 70 (64) | 140 (63) |
| 12 months | 83 (76) | 147 (67) |
| Measured at least once during follow-up | 97 (89) | 192 (87) |
| Number of measurements per patient | ||
| 0 | 12 (11) | 29 (13) |
| 1 | 14 (13) | 24 (11) |
| 2 | 22 (20) | 36 (16) |
| 3 | 22 (20) | 33 (15) |
| 4 | 39 (36) | 99 (45) |
| Food Craving Inventory strength domain | ||
| 1 month | 63 (58) | 160 (72) |
| 2 months | 57 (52) | 140 (63) |
| 6 months | 70 (64) | 140 (63) |
| 12 months | 83 (76) | 146 (66) |
| Measured at least once during follow-up | 97 (89) | 192 (87) |
| Number of measurements per patient | ||
| 0 | 12 (11) | 29 (13) |
| 1 | 15 (14) | 25 (11) |
| 2 | 24 (22) | 36 (16) |
| 3 | 22 (20) | 35 (16) |
| 4 | 36 (33) | 96 (43) |
| Three-Factor Eating cognitive restraint domain | ||
| 2 months | 62 (57) | 140 (63) |
| 6 months | 69 (63) | 140 (63) |
| 12 months | 83 (76) | 146 (66) |
| Measured at least once during follow-up | 91 (83) | 179 (81) |
| Number of measurements per patient | ||
| 0 | 18 (17) | 42 (19) |
| 1 | 18 (17) | 32 (14) |
| 2 | 23 (21) | 47 (21) |
| 3 | 50 (46) | 100 (45) |
| Three-Factor Eating uncontrolled eating domain | ||
| 2 months | 59 (54) | 140 (63) |
| 6 months | 69 (63) | 138 (62) |
| 12 months | 80 (73) | 147 (67) |
| Measured at least once during follow-up | 90 (83) | 179 (81) |
| Number of measurements per patient | ||
| 0 | 19 (17) | 42 (19) |
| 1 | 18 (17) | 33 (15) |
| 2 | 26 (24) | 46 (21) |
| 3 | 46 (42) | 100 (45) |
| Three Factor Eating emotional eating domain | ||
| 2 months | 63 (58) | 142 (64) |
| 6 months | 70 (64) | 140 (63) |
| 12 months | 83 (76) | 146 (66) |
| Measured at least once during follow-up | 92 (84) | 180 (81) |
| Number of measurements per patient | ||
| 0 | 17 (16) | 41 (19) |
| 1 | 18 (17) | 33 (15) |
| 2 | 24 (22) | 46 (21) |
| 3 | 50 (46) | 101 (46) |
| International Physical Activity Questionnaire MET-minutes/week domain | ||
| 2 months | 53 (49) | 113 (51) |
| 6 months | 58 (53) | 118 (53) |
| 12 months | 72 (66) | 123 (56) |
| Measured at least once during follow-up | 87 (80) | 161 (73) |
| Number of measurements per patient | ||
| 0 | 22 (20) | 60 (27) |
| 1 | 24 (22) | 34 (15) |
| 2 | 30 (28) | 61 (28) |
| 3 | 33 (30) | 66 (30) |
| International Physical Activity Questionnaire sitting domain | ||
| 2 months | 49 (45) | 114 (52) |
| 6 months | 59 (54) | 116 (52) |
| 12 months | 62 (57) | 113 (51) |
| Measured at least once during follow-up | 78 (72) | 159 (72) |
| Number of measurements per patient | ||
| 0 | 31 (28) | 62 (28) |
| 1 | 18 (17) | 36 (16) |
| 2 | 28 (26) | 62 (28) |
| 3 | 32 (29) | 61 (28) |
Primary outcome: weight at 12 months
A total of 291 participants (nurse, n = 97; WAP, n = 194) were included in the model and so contributed data to the estimated treatment effect at each time point. At 12 months, participants in the WAP arm had lost more weight (4.2 kg) than those in the nurse arm (2.3 kg). This 1.9-kg difference was statistically significant (95% CI –3.7 kg to –0.1 kg; p = 0.04). Except at 1 month post randomisation, participants in the WAP arm lost significantly greater amounts of weight than those the nurse arm (Table 18).
| Time point | Arm, mean (SD)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse (n = 109) | WAP (n = 221) | |||
| 1 month | –1.0 (1.6) | –1.0 (1.7) | –0.1 (–0.6 to 0.5) | 0.81 |
| 2 months | –2.2 (2.6) | –3.2 (2.7) | –1.0 (–1.7 to –0.3) | 0.009 |
| 6 months | –2.1 (4.3) | –5.0 (5.4) | –2.5 (–3.8 to –1.2) | < 0.001 |
| 12 months | –2.3 (6.6) | –4.2 (7.3) | –1.9 (–3.7 to –0.1) | 0.04 |
Sensitivity analyses on primary outcome
We performed two sensitivity analyses to assess the robustness of our primary analysis to different assumptions regarding the missing data.
Figure 6 shows the results for different missing not at random assumptions. These scenarios assume that participants were lost to follow-up because of their weight, and that those lost to follow-up (non-responders) had different weight values at 12 months than those who provide data (responders). The values along the x-axis represent the mean change in weight from baseline for non-responders in the nurse arm (usual care). Then, each colour (black, green or blue) represents the mean change in weight for non-responders in the WAP arm (black indicates that non-responders in the WAP arm lost, on average, 5 kg more at 12 months than non-responders in the nurse arm; green indicates that they lost the same amount; and blue indicates that WAP non-responders gained 5 kg more, on average, than the nurse arm non-responders). The points and bars in the graph indicate the treatment effect and 95% CI for each scenario.
FIGURE 6.
Treatment effect and 95% CI for change in weight at 12 months under the assumption that non-responders are missing not at random. UC, usual care.
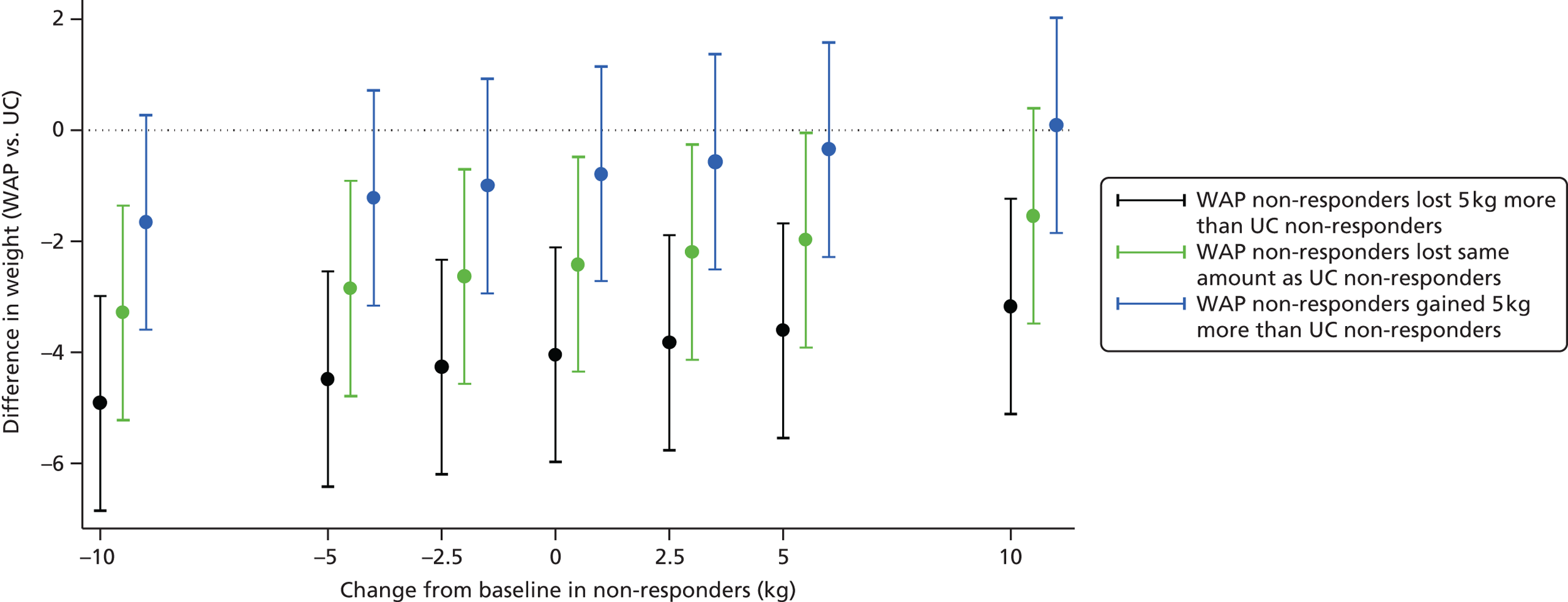
The outcome here is that results are not materially affected unless we assume that non-responders (patients who were lost to follow-up at 12 months) in the WAP arm gained more weight than non-responders in the nurse arm, or if we assume that non-responders in both arms gained a large amount of weight (i.e. 10 kg). Under the assumption that, on average, non-responders in both arms had no change from their baseline weight, the results are unaffected (difference –2.4 kg, 95% CI –4.3 to –0.5 kg).
Table 19 shows the results of the two other sensitivity analyses. One is the complete-case analysis, where only patients with recorded data at 12 months are included, and the other excludes women who fell pregnant and participants who had bariatric surgery, after randomisation. The treatment effect is not substantially altered.
| Sensitivity analysis | Arm, mean (SD) | Treatment effect (95% CI) | |
|---|---|---|---|
| Nurse | WAP | ||
| Complete-case analysis (n = 232) | –2.3 (6.6) | –4.2 (7.3) | –2.4 (–4.9 to 0.1) |
| Excluding patients who had bariatric surgery or became pregnant during follow-upa (n = 221) | –2.1 (5.7) | –4.2 (7.3) | –2.1 (–3.9 to –0.4) |
Secondary outcomes
Changes in body mass index
As expected, change in BMI followed change in weight, with participants in the WAP arm showing a greater reduction in BMI than those in the nurse arm (Table 20).
| Time point | Arm, mean (SD)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse (n = 109) | WAP (n = 221) | |||
| 1 month | –0.4 (0.6) | –0.4 (0.6) | 0.0 (–0.2 to 0.2) | 0.73 |
| 2 months | –0.8 (0.9) | –1.2 (1.0) | –0.4 (–0.6 to –0.1) | 0.005 |
| 6 months | –0.7 (1.5) | –1.8 (1.9) | –0.9 (–1.4 to –0.5) | < 0.001 |
| 12 months | –0.8 (2.3) | –1.5 (2.6) | –0.7 (–1.3 to 0.0) | 0.04 |
Changes in waist circumference
Table 21 shows that participants in the nurse arm had a greater reduction in waist circumference than those in the WAP arm at the end of treatment (–7.7 vs. –3.9 cm; p = 0.001), but this was reversed at 6-month follow-up (–1.5 vs. –5.0 cm; p = 0.004). By 12 months, the difference had narrowed (–2.0 vs. –4.1 cm) and the difference was no longer significant (p = 0.07).
| Time point | Arm, mean (SD)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse (n = 109) | WAP (n = 221) | |||
| 2 months | –7.7 (7.3) | –3.9 (4.9) | 3.9 (2.0 to 5.7) | 0.001 |
| 6 months | –1.5 (6.2) | –5.0 (6.7) | –3.1 (–5.1 to –1.2) | 0.004 |
| 12 months | –2.0 (7.3) | –4.1 (7.9) | –2.0 (–4.1 to 0.2) | 0.07 |
Proportion of participants losing at least 5% or 10% of their baseline body weight
At the 12-month follow-up the proportion of participants who had lost at least 5% of their body weight was significantly greater in the WAP arm than in the nurse arm (41% vs. 27%; p = 0.004). Similarly, the proportion of participants who lost 10% of their baseline body weight was higher in the WAP arm than in the nurse arm (participants in the WAP arm were twice as likely as those in the nurse arm to have lost 10% of their weight), but the difference was not significant (Table 22).
| Time point | Arm, n (%)a | Odds ratio (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |||
| Participants losing 5% of their body weight | ||||
| 2 months | 10 (16) | 32 (23) | 2.41 (0.69 to 8.46) | 0.17 |
| 6 months | 14 (20) | 65 (46) | 31.60 (6.52 to 153.18) | < 0.001 |
| 12 months | 22 (27) | 61 (41) | 14.61 (2.32 to 91.96) | 0.004 |
| Participants losing 10% of their body weight | ||||
| 2 months | 0 (0) | 2 (1) | –c | – |
| 6 months | 3 (4) | 26 (18) | 5.10 (1.48 to 17.56) | 0.01 |
| 12 months | 7 (8) | 25 (17) | 2.50 (0.99 to 6.32) | 0.05 |
Changes in blood pressure
The only significant change in blood pressure between the arms was a greater drop in systolic blood pressure in the nurse arm at the end of treatment (–9.6 mmHg vs. –2.1 mmHg; p = 0.02). At the 12-month follow-up there was no significant difference in blood pressure between participants in the nurse and WAP arms (Table 23).
| Time point | Arm, mean (SD)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse (n = 109) | WAP (n = 221) | |||
| Change in systolic blood pressure (mmHg) | ||||
| 2 months | –9.6 (14.4) | –2.1 (13.7) | 5.6 (1.0 to 10.3) | 0.02 |
| 6 months | –5.1 (13.0) | –5.1 (14.6) | 0.4 (–4.4 to 5.1) | 0.88 |
| 12 months | –3.5 (16.0) | –2.8 (15.0) | 0.6 (–4.3 to 5.4) | 0.81 |
| Change in diastolic blood pressure (mmHg) | ||||
| 2 months | –0.6 (8.9) | –1.5 (7.7) | –0.3 (–3.0 to 2.4) | 0.81 |
| 6 months | –2.0 (9.2) | –3.6 (8.4) | –0.9 (–3.8 to 1.9) | 0.51 |
| 12 months | –0.4 (10.2) | –1.7 (9.1) | –0.7 (–3.6 to 2.1) | 0.59 |
Changes in food knowledge
Participants in the WAP arm showed a significant increase in their knowledge of the calorie content of foods compared with participants in the nurse arm at the end of treatment and at the 6-month follow-up (Table 24). By the 12-month follow-up this effect had disappeared.
| Time point | Arm, mean (SD)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse | WAP | |||
| Change in Food Knowledge Assessment Questionnaire score | ||||
| 2 months | 0.1 (1.7) | 1.1 (1.7) | 1.1 (0.6 to 1.6) | < 0.001 |
| 6 months | 0.2 (2.0) | 0.8 (1.7) | 0.5 (0.1 to 1.0) | 0.03 |
| 12 months | 0.4 (1.9) | 0.6 (2.0) | 0.1 (–0.3 to 0.6) | 0.61 |
| Change in Food Craving Inventory score (frequency domain) | ||||
| 1 month | –2.2 (3.8) | –2.1 (3.6) | –0.3 (–1.4 to 0.7) | 0.53 |
| 2 months | –1.8 (3.8) | –2.0 (3.8) | –0.6 (–1.7 to 0.5) | 0.25 |
| 6 months | –1.2 (4.1) | –1.7 (3.7) | –0.8 (–1.9 to 0.3) | 0.13 |
| 12 months | –0.9 (3.7) | –1.5 (4.0) | –0.8 (–1.9 to 0.2) | 0.12 |
| Change in Food Craving Inventory score (strength domain) | ||||
| 1 month | –2.3 (4.4) | –2.0 (3.4) | 0.1 (–0.9 to 1.1) | 0.85 |
| 2 months | –2.2 (4.0) | –1.7 (3.9) | –0.1 (–1.1 to 0.9) | 0.85 |
| 6 months | –1.2 (3.7) | –1.3 (4.0) | –0.3 (–1.4 to 0.7) | 0.48 |
| 12 months | –1.4 (3.8) | –1.3 (4.2) | –0.2 (–1.2 to 0.9) | 0.75 |
| Change in Three-Factor Eating Questionnaire score (cognitive restraint domain) | ||||
| 2 months | 0.2 (0.6) | 0.4 (0.6) | 0.2 (0.0 to 0.3) | 0.05 |
| 6 months | 0.2 (0.5) | 0.4 (0.7) | 0.1 (0.0 to 0.3) | 0.07 |
| 12 months | 0.2 (0.6) | 0.3 (0.6) | 0.1 (0.0 to 0.3) | 0.10 |
| Change in Three-Factor Eating Questionnaire score (uncontrolled eating domain) | ||||
| 2 months | –0.2 (0.5) | –0.1 (0.5) | 0.1 (–0.1 to 0.2) | 0.35 |
| 6 months | –0.2 (0.5) | –0.2 (0.5) | 0.0 (–0.1 to 0.1) | 0.93 |
| 12 months | –0.3 (0.6) | –0.2 (0.6) | 0.0 (–0.1 to 0.2) | 0.66 |
| Change in Three-Factor Eating Questionnaire score (emotional eating domain) | ||||
| 2 months | –0.3 (0.8) | –0.2 (0.7) | 0.1 (–0.1 to 0.3) | 0.32 |
| 6 months | –0.3 (0.7) | –0.2 (0.7) | 0.1 (–0.2 to 0.3) | 0.59 |
| 12 months | –0.3 (0.7) | –0.2 (0.7) | 0.1 (–0.1 to 0.2) | 0.54 |
Changes in food craving
All participants showed a decrease in the frequency and strength of food craving at the 1-, 2-, 6- and 12-month follow-up points (see Table 24). There were no significant differences between the groups.
Changes in Three-Factor Eating Questionnaires
Changes in the scores of the domains of the Three-Factor Eating Questionnaire were minimal (see Table 24). Participants in both arms showed a small increase in cognitive restraint scores, but the increase was greater in the WAP arm than in the nurse arm at the end of treatment (0.4 vs. 0.2; p = 0.05). Participants, on average, showed small decreases in uncontrolled and emotional eating scores. There were no significant differences in the changes between study arms.
Changes in levels of physical activity
Participants in both arms increased their levels of physical activity above baseline across the duration of the study to the same extent (Table 25) (818 MET-minutes/week vs. 264 MET-minutes/week; p = 0.09).
| Time point | Arm, median (IQR)a | Treatment effect (95% CI)b | p-value | |
|---|---|---|---|---|
| Nurse | WAP | |||
| MET-minutes/week | ||||
| 2 months | 264 (–347 to 1030) | 818 (0 to 2517) | 923 (–167 to 2014) | 0.09 |
| 6 months | 336 (–240 to 1644) | 415 (–258 to 1584) | –441 (–1380 to 497) | 0.33 |
| 12 months | 215 (–763 to 1589) | 359 (–385 to 1750) | 613 (–312 to 1537) | 0.18 |
| Minutes spent sitting/day | ||||
| 2 months | –60 (–120 to 0) | –60 (–150 to 60) | –12 (–93 to 69) | 0.77 |
| 6 months | 0 (–90 to 30) | –60 (–150 to 0) | –5 (–71 to 61) | 0.87 |
| 12 months | –60 (–120 to 60) | 0 (–120 to 60) | 19 (–51 to 89) | 0.57 |
Participants reported reducing their sitting time by 1 hour at the end of treatment, but no significant differences between groups was observed.
Adverse events
Table 26 provides a summary of all AEs. There were more AEs in the WAP group, although this difference was not statistically significant (WAP arm 11% vs. nurse arm 6%; odds ratio 2.19, 95% CI 0.86 to 5.58; p = 0.10).
| AEs | Arm, n | |
|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |
| Number of AEsa | 8 | 45 |
| Number of patients with at least one AE | 6 | 25 |
| Number of AEs per patient | ||
| 0 | 103 | 196 |
| 1 | 5 | 16 |
| 2 | 0 | 5 |
| 3 | 1 | 1 |
| 4 | 0 | 0 |
| 5 | 0 | 2 |
| 6 | 0 | 1 |
| Number of SAEs | 0 | 3 |
| Systems affected by AE | ||
| Gastrointestinal | 5 | 21 |
| Nervous system | 0 | 7 |
| General disorders | 0 | 6 |
| Musculoskeletal and connective tissue | 0 | 6 |
| Psychiatric | 0 | 2 |
| Respiratory, thoracic and mediastinal | 1 | 1 |
| Infections and infestations | 2 | 0 |
| Blood and lymphatic | 0 | 1 |
| Skin and subcutaneous tissue | 0 | 1 |
| AE category | ||
| Arthralgia | 0 | 4 |
| Bloating | 0 | 2 |
| Bruising | 0 | 1 |
| Constipation | 1 | 2 |
| Diarrhoea | 1 | 6 |
| Dizziness | 0 | 4 |
| Dry skin | 0 | 1 |
| Flatulence | 0 | 2 |
| Flu-like symptoms | 0 | 6 |
| Generalised muscle weakness | 0 | 1 |
| Headache | 0 | 2 |
| Haemorrhoids | 1 | 0 |
| Insomnia | 0 | 2 |
| Lung infection | 2 | 0 |
| Memory impairment | 0 | 1 |
| Myalgia | 0 | 1 |
| Steatorrhoea | 0 | 7 |
| Stomach pain | 1 | 1 |
| Voice alteration | 0 | 1 |
| Vomiting | 1 | 1 |
| Wheezing | 1 | 0 |
Three SAEs were reported (shortness of breath, myalgia and gastrointestinal complaints) and resulted in participants being hospitalised overnight. These were all in the WAP arm and were not related to study procedures.
Chapter 4 Economics evaluation methods and results
Overview
Obesity-related illness is responsible for about 10% of morbidity and mortality in the UK and costs the NHS about £7B annually. 80 In order to estimate the cost-effectiveness of the WAP (intervention group) versus nurse-led weight management (representing usual care), a within-trial cost–utility analysis was undertaken. The costs were estimated from a NHS and Social Services perspective. 81 To inform the estimation of QALYs, participants completed the EQ-5D-5L questionnaire. As already mentioned (see Chapter 2, Overview of trial design), participants were randomised to treatment groups prior to receiving a weight loss intervention (when baseline data were also collected). The impact of treatment was measured by following up participants at 6 and 12 months post randomisation.
Methods
An incremental cost-effectiveness analysis was conducted to estimate the cost per QALY of the WAP over and above the best practice nurse-led intervention.
Valuation of resource use
The NHS health-care costs were estimated using UK unit costs applied from national sources such as NHS Reference Costs82 and the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2013. 83
The costs in each study arm were calculated, including the time spent by health-care professionals delivering care, equipment and materials used in the interventions, and overhead costs. Patients completed a service-use questionnaire to record their use of NHS resources including hospital and primary care services [see Appendix 4 (Appendix 1: Timing of data collection)].
All costs were valued in pounds sterling, according to the price year representing the mid-point of the trial (2012/13). Any costs occurring in prior price years were inflated using the Hospital and Community Health Services pay and prices index. 83 As the trial follow-up was 12 months post randomisation, no discounting will be required. Quantities of services were multiplied by the relevant unit costs to estimate total cost.
Outcome measures
Health-related quality of life was measured using the EQ-5D-5L84 at baseline, and at the 6- and 12-month follow-up in the SWAP trial, and forms the primary cost-effectiveness end point, following NICE guidance. 85
Responses were then converted to utility scores (a scale where death is equal to 0 and full health is equal to 1) using the population-based EQ-5D-5L value sets study86 and preliminary reported results. 87
Quality-adjusted life-years for patients receiving the WAP and usual care were derived from utility scores using the area under the curve method over the follow-up period. 88 This enables cost–utility analysis expressing the value of the WAP as the incremental cost per QALY.
Cost-effectiveness analysis
The incremental cost-effectiveness ratio (ICER) was calculated using the formula below where Δ represents change, C represents the costs, E represents the effects and I and C refer to the intervention and control, respectively:
Incremental costs of the WAP intervention over and above routine care were calculated and combined with the incremental effectiveness to compute the ICER. In order to allow for the skewness typically encountered with cost data, both costs and outcomes were bootstrapped (using 10,000 replications) and the data used to construct cost-effectiveness acceptability curves to show the probability that the WAP is a more cost-effective intervention than routine care.
Base-case analysis makes three key assumptions regarding the cost of the WAP intervention: (1) the prior history of health-care use should not influence results; (2) that group sessions are conducted by a band 5 (hospital dietitian); and (3) that the cost of the WAP assumes attendance of 15 participants for all sessions. These three assumptions are subject to sensitivity analysis (further explanation is provided in Results).
Results
Resource utilisation and costs
Weight Management Programme intervention
The resources required to deliver the WAP intervention consisted of two research health psychologists per session delivering group sessions over eight weekly group sessions. As reported in Table 11, the mean group size per session was 15 participants (IQR 13–18 participants), thus indicating a staff-to-participant ratio of 2 : 15 in the base-case estimate cost of the intervention. In the trial, the WAP intervention was delivered by two health research psychologists (grade 6). However, for the base-case analysis, staff costs were based on a NHS band 5 hospital dietitian (see Table 28). Further details of the within-research costs are provided in Appendix 5, Table 41.
Further to the staff costs of running the WAP sessions, equipment costs (including pedometers, materials, digital scales, blood pressure monitors, batteries, measuring tapes, stationery and venue) are included in the cost of the intervention (for full details see Appendix 4: Appendix 5 – Costs).
Following the initial 8-week course, 10 further monthly group sessions were provided during the maintenance sessions; again these were assumed to take place with a staff-to-participant ratio of 2 : 15.
Each group session lasted 2 hours and for the direct contact per group session both intervention staff further required 1 hour for pre-session preparation (preparing materials, photocopying, scheduling text messages) and 2 hours post session (checking/filing forms, contacting participants for missing information, following up non-attenders).
Based on the above information, and assuming a constant group size of 15, the cost per participant is calculated to be £10.33 per session. It was further assumed that a participant prescribed the WAP would receive eight initial treatment sessions (£82.64) and a further 10 sessions in the maintenance phase (£103.30) and would account for a proportion of equipment costs (£8.69). This indicates that the cost per participant in the WAP was £194.63.
Nurse intervention (control)
The control group intervention was based on best usual care, consisting of four sessions lasting 1 hour each delivered over 8 weeks by a practice nurse. The cost of the control intervention also includes equipment costs (materials, digital scales, blood pressure monitors, batteries, measuring tape, stationery and venue). The cost per participant of the nurse intervention is £180.90. For further details of costs related to nurse intervention, see Appendix 2, Table 42.
In addition to the direct cost of providing weight management interventions, the economic analysis considers consequences of intervention on wider NHS resources. Table 27 provides observations of the resource utilisation (by study group), as measured using the resource-use questionnaire in four categories. The first category indicated contacts with general practice and community nursing services. The second widens the perspectives to consider contacts with social services. The third considers contact with psychiatric services (both hospital and community). The fourth, and final, category considers other in-hospital services, NHS Direct, paramedics and prescriptions. All statistics on resource use are reported as mean and SD, based on the final available sample for the complete-case cost-effectiveness analysis (WAP arm, n = 116; nurse arm, n = 63).
| Type of service | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||||||||
| WAP arm | Nurse arm | WAP arm | Nurse arm | WAP arm | Nurse arm | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| General practice and community nursing services | ||||||||||||
| GP (surgery) | 2.696 | 2.381 | 3.097 | 3.308 | 1.904 | 2.675 | 1.887 | 2.057 | 1.53 | 2.019 | 1.871 | 1.806 |
| GP (home) | 0.009 | 0.093 | 0.081 | 0.635 | 0.009 | 0.093 | 0 | 0 | 0.035 | 0.263 | 0 | 0 |
| GP (telephone) | 0.583 | 1.026 | 1.097 | 2.133 | 0.513 | 1.165 | 0.581 | 0.915 | 0.383 | 0.96 | 0.645 | 1.319 |
| Nurse (surgery) | 1.078 | 1.434 | 1.113 | 1.45 | 0.565 | 1.01 | 0.774 | 1.453 | 0.591 | 1.059 | 0.532 | 1.004 |
| Nurse (home) | 0 | 0 | 0.661 | 5.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0.016 | 0.127 |
| Counsellor (surgery) | 0.148 | 1.086 | 0.629 | 4.331 | 0.052 | 0.292 | 0.129 | 0.64 | 0.348 | 2.347 | 0.097 | 0.469 |
| Other practice contactsa | 0.104 | 0.502 | 0.033 | 0.256 | 0.052 | 0.346 | 0.048 | 0.216 | 0.035 | 0.227 | 0.177 | 1.274 |
| Social services | ||||||||||||
| Social worker | 0.052 | 0.475 | 0 | 0 | 0.07 | 0.588 | 0 | 0 | 0.052 | 0.346 | 0.016 | 0.127 |
| Home help | 1.67 | 15.806 | 1.968 | 15.494 | 0 | 0 | 0 | 0 | 0.209 | 2.238 | 0 | 0 |
| Care assistant | 0 | 0 | 0 | 0 | 0.73 | 7.833 | 0 | 0 | 0.887 | 9.324 | 0.032 | 0.254 |
| Day centre (visit) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other social service contactsa | 0.017 | 0.187 | 0.161 | 1.27 | 0.07 | 0.434 | 0 | 0 | 0.017 | 0.187 | 0.194 | 1.524 |
| Psychiatric hospital and community services | ||||||||||||
| Psychiatrist (hospital) | 0.035 | 0.227 | 0.129 | 1.016 | 0.026 | 0.208 | 0.129 | 1.016 | 0.009 | 0.093 | 0.048 | 0.381 |
| Psychiatrist (home) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychologist | 0.009 | 0.093 | 0.161 | 1.27 | 0.009 | 0.093 | 0.161 | 1.27 | 0.009 | 0.093 | 0.403 | 2.036 |
| Psychiatric nurse | 0.017 | 0.131 | 0 | 0 | 0.009 | 0.093 | 0 | 0 | 0.009 | 0.093 | 0 | 0 |
| Other psychiatric service contactsa | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 2.702 | 0.209 | 1.899 | 0 | 0 |
| Other services | ||||||||||||
| Hospital (day case) | 0.043 | 0.205 | 0.952 | 6.109 | 0.017 | 0.131 | 0.048 | 0.216 | 0.061 | 0.305 | 0.032 | 0.254 |
| Hospital (A&E) | 0.165 | 0.494 | 0.323 | 0.647 | 0.104 | 0.36 | 0.129 | 0.527 | 0.13 | 0.45 | 0.065 | 0.248 |
| Hospital (outpatient) | 0.443 | 1.371 | 0.71 | 1.712 | 0.522 | 1.629 | 0.419 | 0.967 | 0.487 | 1.334 | 0.419 | 0.95 |
| Hospital (inpatient) | 0.096 | 0.577 | 0.113 | 0.483 | 0.026 | 0.208 | 0.081 | 0.635 | 0.017 | 0.131 | 0.016 | 0.127 |
| Other hospital contactsa | 0.165 | 0.794 | 0.129 | 0.461 | 0.061 | 0.404 | 0.032 | 0.178 | 0.07 | 0.508 | 0.113 | 0.483 |
| NHS Direct | 0.096 | 0.418 | 0.081 | 0.329 | 0.009 | 0.093 | 0.048 | 0.216 | 0.061 | 0.483 | 0 | 0 |
| Ambulance or paramedic | 0.043 | 0.244 | 0.097 | 0.433 | 0.026 | 0.16 | 0.081 | 0.417 | 0.07 | 0.472 | 0.016 | 0.127 |
| Prescriptions received (6 months) | 2.991 | 5.905 | 4.048 | 6.759 | 2.757 | 4.26 | 2.29 | 3.659 | 2.252 | 3.541 | 2.613 | 4.451 |
Examining the profile of resource use at baseline, there are small differences evident between the two study groups across the four categories. In the category General practice and community nursing services (see Table 27), across all subcategories rates of service contact was higher in the nurse arm. Social work and psychiatric services others displays a pattern of large magnitudes of service use in the nurse arm compared with the WAP arm. These patterns may indicate potential selection bias. Differences in service use are most commonly explained by individuals’ health status (at baseline) or by differences in the age distribution between groups. Baseline health-state utilities are presented later in this chapter (see Table 30). Differences in age distribution by study group are presented in Figure 7. Observations would suggest there exists some potentially influential difference in the age distributions between groups and, therefore, is it important that cost-effectiveness analysis controls for age when explaining both costs and outcomes.
FIGURE 7.
Age distribution by study arm.

Follow-up data were collected at 6 and 12 months and these visits represent NHS contacts following the intervention. The most frequently reported service contact in the post-intervention period was contact with a GP in the surgery. The mean number of contacts by group at 6 months was 1.90 (SD 2.675) in the WAP arm and 1.89 (SD 2.057) in the nurse arm. At 12 months, the mean number of contact in the WAP arm was 1.53 (SD 2.019) and in the nurse arm was 1.87 (SD 1.806). Observing this pattern over time may suggest that the nurse arm shows reduced service use at 6 months (compared with the WAP arm), but by 12 months the mean number of GP surgery visits was lowest in the WAP group (potentially attributable to an effect of the available maintenance phase for up to 12 months with the WAP arm).
At baseline, the mean number of nurse contacts within the surgery setting was comparable between the WAP (1.08, SD 1.434) and nurse arms (1.11, SD 1.45), and the magnitude of variance was the same in both trial arms. At the 6-month follow-up, the mean number of service contacts in the WAP arm (0.56, SD 1.01) was lower than in the nurse arm (0.77, SD 1.453), and variance was also reduced. This trend seems to have been sustained to 12 months in the WAP arm (0.59, SD 1.059); however, by this time, the mean number of nurse visits in the nurse arm (0.53, SD 1.004) was comparable to that in the WAP arm.
The home help variable demonstrates a significant amount of variance from baseline in both the WAP (1.67, SD 15.806) and nurse arms (1.97, SD 15.494). A small number (n = 3) of influential outliers reported very high levels of home help (ranging between 24 and 168 visits from home help during the 6-month period).
The mean number of calls to GP at baseline was lower in the WAP arm (0.58, SD 1.026) than in the nurse arm (1.10, SD 2.133). By 12 months, the average number of calls decreased in both the nurse arm (0.64, SD 1.319) and the WAP arm (0.38, SD 0.96); similar to surgery contact, this may be attributed to the maintenance phase within the WAP.
To assign a monetary value to the above resource consequences, Table 28 presents the identified units per item of resource use. Table 29 multiplies the quantity of resource use by the unit cost to obtain cost consequences to the NHS.
| Item | Unit cost (£) | References | Price year |
|---|---|---|---|
| Interventions | |||
| WAP session | 10 | PSSRU 2013:83 two hospital dietitians (band 5). Attendance: 15 participants per session. Indirect time per session: 3 hours | 2012–13 |
| Practice weight loss | 44 | PSSRU 2013:83 nurse (GP practice), per hour of face-to-face contact, | 2012–13 |
| General practice and community nursing services | |||
| GP (surgery) | 45 | PSSRU 2013:83 ‘per-patient contact lasting 11.7 minutes’ | 2012–13 |
| GP (home) | 114 | PSSRU 2013:83 ‘per out-of-surgery visit lasting 23.4 minutes’ | 2012–13 |
| GP (telephone) | 27 | PSSRU 2013:83 ‘per telephone consultation lasting 7.1 minutes’ | 2012–13 |
| Nurse (surgery) | 13 | PSSRU 2013:83 £52 per hour. Face-to-face contact, duration of contact 15.5 minutes | 2012–13 |
| Nurse (home) | 60 | PSSRU 2013:83 community nurse (includes district nursing sister, district nurse). Cost per hour of home visiting | 2012–13 |
| Counsellor (surgery) | 63 | PSSRU 2013:83 counselling services in primary medical care. Cost per hour of client contact | 2012–13 |
| Social services | |||
| Social worker | 159 | PSSRU 2013:83 social worker (adult services). Cost per hour of face-to-face contact | 2012–13 |
| Home help | 24 | PSSRU 2013:83 home care worker. Cost per hour (weekday) | 2012–13 |
| Care assistant | 30 | PSSRU 2013:83 senior ‘home care worker’. Cost per hour (weekday) | 2012–13 |
| Day centre (visit) | 38 | PSSRU 2013:83 local authority social services day care for people with mental health problems. Cost per user session | 2012–13 |
| Psychiatric services | |||
| Psychiatrist (hospital) | 261 | PSSRU 2013:83 consultant – psychiatric. Cost per hour (face-to-face contact) | 2012–13 |
| Psychiatrist (home) | 261 | PSSRU 2013:83 consultant – psychiatric. Cost per hour (face-to-face contact) | 2012–13 |
| Psychologist | 134 | PSSRU 2013:83 clinical psychologist. Cost per hour of client contact | 2012–13 |
| Psychiatric nurse | 65 | PSSRU 2013:83 nurse (mental health). Cost per hour of face-to-face contact | 2012–13 |
| Other services | |||
| Hospital (day case) | 697 | PSSRU 2013:83 Day cases HRG data (weighted average of all stays) | 2012–13 |
| Hospital (A&E) | 956 | PSSRU 2013:83 ‘outpatient and A&E (all service users)’ | 2012–13 |
| Hospital (outpatient) | 135 | PSSRU 2013:83 outpatient procedures (weighted average of all outpatient procedures) | 2012–13 |
| Hospital (inpatient) | 598 | PSSRU 2013:83 non-elective inpatient stays (short stays) | 2012–13 |
| NHS Direct | 52 | PSSRU 2013:83 £52 per hour of telephone contact with nurse specialist | 2012–13 |
| Ambulance/paramedic | 221 | National Audit Office 201189 (£213.5 mid-point of cost per incidence in range £176–251). Applied annual rate of 3.5% | 2012–13 |
| Prescriptions | 44.64 | PSSRU 2013:83 prescription costs per consultation (net ingredient cost) | 2012–13 |
| Type of service | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||||||||
| WAP | Nurse | WAP | Nurse | WAP | Nurse | |||||||
| Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | Mean (£) | SD (£) | |
| General practice and community nursing services | ||||||||||||
| GP (surgery) | 121 | 107 | 139 | 148 | 86 | 120 | 84 | 92 | 69 | 90 | 83 | 81 |
| GP (home) | 1 | 11 | 9 | 72 | 1 | 11 | 0 | 0 | 4 | 30 | 0 | 0 |
| GP (telephone) | 16 | 28 | 29 | 57 | 14 | 31 | 15 | 25 | 10 | 26 | 17 | 35 |
| Nurse (surgery) | 15 | 19 | 15 | 19 | 8 | 15 | 10 | 19 | 8 | 14 | 7 | 13 |
| Nurse (home) | 0 | 0 | 7 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Counsellor (surgery) | 8 | 56 | 39 | 271 | 6 | 48 | 7 | 39 | 22 | 148 | 12 | 73 |
| Other practice contacts | 1 | 8 | 0 | 0 | 1 | 9 | 0 | 0 | 1 | 7 | 1 | 5 |
| Social services | ||||||||||||
| Social worker | 3 | 27 | 0 | 0 | 10 | 74 | 0 | 0 | 8 | 55 | 0 | 3 |
| Home help | 79 | 755 | 46 | 369 | 0 | 0 | 0 | 0 | 15 | 160 | 0 | 0 |
| Care assistant | 0 | 0 | 0 | 0 | 43 | 468 | 0 | 0 | 104 | 1114 | 0 | 4 |
| Day centre (visit) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other social service contacts | 1 | 8 | 0 | 0 | 1 | 9 | 0 | 0 | 1 | 7 | 1 | 5 |
| Psychiatric hospital and community services | ||||||||||||
| Psychiatrist (hospital) | 9 | 59 | 33 | 263 | 7 | 54 | 33 | 263 | 2 | 24 | 12 | 99 |
| Psychiatrist (home) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychologist | 1 | 12 | 21 | 169 | 1 | 12 | 21 | 169 | 1 | 12 | 51 | 270 |
| Psychiatric nurse | 1 | 7 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Other psychiatric service contacts | 2 | 14 | 0 | 0 | 2 | 14 | 0 | 0 | 1 | 8 | 1 | 8 |
| Other Services | ||||||||||||
| Hospital (day case) | 30 | 142 | 653 | 4225 | 12 | 91 | 33 | 150 | 42 | 211 | 22 | 176 |
| Hospital (A&E) | 157 | 471 | 303 | 615 | 99 | 342 | 121 | 500 | 124 | 428 | 61 | 235 |
| Hospital (outpatient) | 64 | 190 | 94 | 230 | 76 | 226 | 56 | 130 | 69 | 182 | 56 | 127 |
| Hospital (inpatient) | 57 | 344 | 66 | 286 | 15 | 124 | 47 | 377 | 10 | 78 | 9 | 75 |
| Other hospital contacts | 9 | 89 | 0 | 0 | 9 | 89 | 0 | 0 | 1 | 4 | 2 | 12 |
| NHS Direct | 1 | 4 | 1 | 7 | 0 | 2 | 1 | 7 | 1 | 12 | 0 | 0 |
| Ambulance or paramedic | 10 | 54 | 21 | 95 | 6 | 35 | 18 | 91 | 15 | 104 | 4 | 28 |
| Prescriptions received (6 months) | 134 | 264 | 178 | 300 | 127 | 193 | 101 | 163 | 104 | 162 | 117 | 197 |
| Total cost (at time point) | 716 | 1219 | 1681 | 4411 | 523 | 957 | 548 | 933 | 613 | 1485 | 455 | 576 |
The main variable to inform the relative cost consequences of intervention and to inform cost-effectiveness analysis is the total cost. First, it should be noted that, at baseline, the mean total cost in the nurse arm (£1681, SD £4411) is effectively double the cost in the WAP arm; hence there may exist significant differences in the overall resource requirement between the two study groups.
To conduct the cost-effectiveness analysis, only cost consequence after intervention (i.e. at the 6- and 12-month follow-ups) would be incorporated within a base-case cost-effectiveness. However, the assumption that retrospective differences in total costs may not be of relevance raises important questions regarding the validity of the base-case estimate on value for money; implications of potential differences (as indicated by baseline total costs) are explored using a sensitivity analysis (see Sensitivity analysis 1).
Outcomes
Health-related quality of life using the EQ-5D-5L is measured to enable the estimation of QALYs. Table 30 presents the EQ-5D-5L mean (SD) utility scores at each time point and the group QALY.
| Arm | Time point | QALY | ||
|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||
| WAP (intervention) | 0.849 (0.153) | 0.868 (0.157) | 0.868 (0.173) | 0.404 (0.079) |
| Nurse (control) | 0.838 (0.148) | 0.838 (0.178) | 0.841 (0.171) | 0.389 (0.072) |
The difference in baseline utility scores between groups is 0.011, indicating that we should control for baseline utility. The unadjusted QALY for the WAP arm is 0.404 (SD 0.079) and in the nurse arm is 0.389 (SD 0.072), indicting that the unadjusted incremental QALY (i.e. the difference between the WAP and nurse arm) is 0.015.
Cost-effectiveness analysis: base-case analysis
To inform the base-case estimation of cost-effectiveness, a seemingly unrelated regression is utilised to jointly explain the change in total cost and QALYs (Table 31). As outlined above, group differences were observed in both group baseline utility and age distributions, and hence it is necessary to control for age and baseline utility in the regression analysis. This provides adjusted estimates of the effect of the WAP on the incremental QALY and incremental total cost.
| Coefficients | Total costs (£) (95% CI) | QALY (95% CI) |
|---|---|---|
| WAP | 80 (–505 to 667) | 0.0104 (–0.0015 to 0.0224)* |
| Age | 33 (14 to 52)*** | –0.0004 (–0.0008 to 0.000)* |
| Baseline utility | – | 0.4228 (0.3836 to 0.4619)*** |
| Constant | 2191 (1804 to 2578)*** | 0.280 (0.267 to 0.290)*** |
Adjusting for age, the WAP is associated with a mean incremental total cost of £80 (95% CI –£505 to £667). This suggests no significant difference in cost between the two arms of the trial. The level of significance on the age coefficient confirms the importance to control for group differences in age distribution.
Controlling for age and baseline, intervention with the WAP with an adjusted mean incremental QALY is 0.0104 (95% CI –0.0015 to 0.0224; p = 0.060). The estimated magnitude of the change in QALY indicates that there remains some uncertainty in the health gains from the WAP.
The following formula shows the calculation of the adjusted ICER based on the estimated mean incremental total cost and QALY is:
An ICER of £7742 per QALY would suggest that the WAP would represent a cost-effective intervention with respect to the cost per QALY threshold of £20,000, as used by NICE. However, assuming that a decision-maker may be risk averse, it is also important to consider uncertainty in the ICER.
We can demonstrate the uncertainty surrounding the ICER (and to address skewness resulting from the count nature of cost data) by initially bootstrapping the cost and outcome data using 10,000 bootstrap replications. Figure 8 presents the results of the bootstraps on both a cost-effectiveness plane and cost-effectiveness acceptability curve. Presenting the result of the bootstrap on the cost-effectiveness plane helps to illustrate uncertainty in probabilistic terms as they relate to decisions related to each quadrant. The first observation is the uncertainty in whether the WAP will cost more or less than best usual care, and bootstrap results suggest a 38% probability that the WAP will cost less than usual care, of which 36% also have a positive health gain. Despite the low level of significance surrounding the adjusted mean incremental QALY, the results suggest a 96% probability of a positive health gain from the WAP. However, the most probable scenario is that the WAP will be more effective and will, overall, cost more to the NHS (60%).
FIGURE 8.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b).

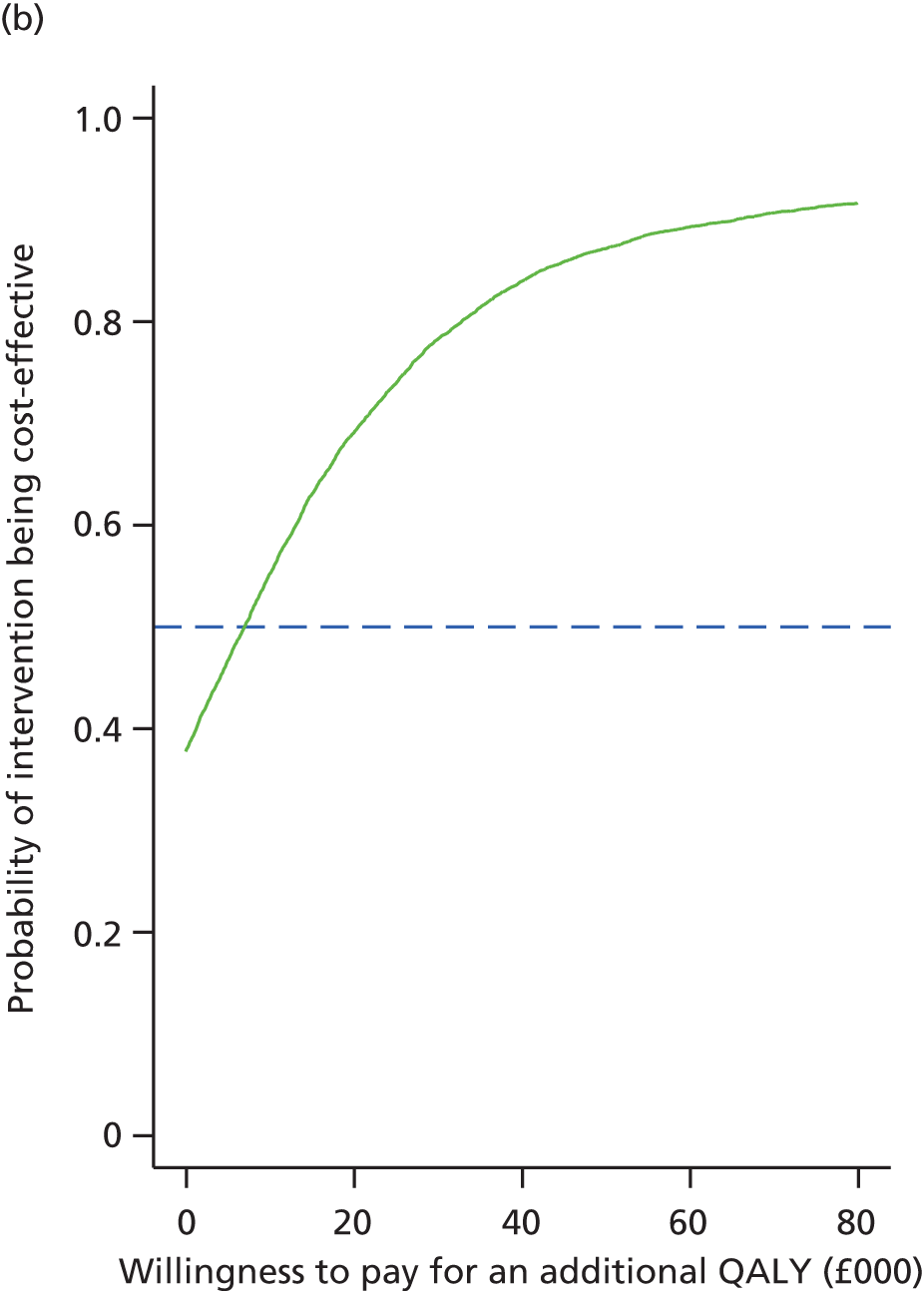
To further explore the decision in the context of uncertainty, bootstrap results are utilised to form a cost-effectiveness acceptability curve. This plot demonstrates how the probability that an intervention is cost-effective increases as the decision-makers willingness to pay increases. First, it can be observed from the cost-effectiveness acceptability curve that should a decision-maker not be willing to pay any more than usual care, there is a 0.38 probability that the WAP will be cost-effective. Decision-makers must make decisions in the context of uncertainty. The probabilities that the WAP falls within the NICE willingness-to-pay threshold of £20,000–30,000 are p(ICER < 20,000/QALY) = 0.6826 and p(ICER < 30,000/QALY) = 0.7746.
Given the available information (in addition to base-case required assumptions), the base-case cost-effectiveness analysis suggests that the WAP may represent value for money to the NHS.
To explore the implication of underlying assumptions, the following three sensitivity analyses aim to explore the implications of assumptions of the base-case findings.
Sensitivity analysis 1
The first assumption was that the two populations have a difference in profiles of health service use in the 6 months preceding randomisation and, controlling for baseline total costs, will influence estimates of cost-effectiveness. Table 32 repeats the base-case seemingly unrelated regression alongside a second equation in which the regression of total cost controls for baseline total cost.
| Variables | Regression coefficients (95% CI) | |
|---|---|---|
| Base case | Controlling for baseline total cost | |
| Total costs | ||
| WAP | 60 (–532 to 653) | 244 (–338 to 826) |
| Age | 33 (14 to 52) | 28 (9 to 46) |
| Baseline total costs | – | 0.1804 (0.0809 to 0.2798) |
| Constant | –337 (–1337 to 664) | –397 (–1367 to 572) |
| QALY | ||
| WAP | 0.0115 (–0.0005 to 0.0235) | 0.0115 (–0.0005 to 0.0235) |
| Age | 0.4293 (0.3897 to 0.469) | 0.4285 (0.3889 to 0.4681) |
| Baseline utility | –0.0003 (–0.0007 to 0.0000) | –0.0004 (–0.0007 to 0.0000) |
| Constant | 0.0446 (0.0016 to 0.0877) | 0.0454 (0.0024 to 0.0884) |
| Statistics | ||
| n | 177 | 177 |
| Correlation matrix of residuals: QALY to total costs | –0.0611 | –0.0855 |
| Breusch–Pagan test of independence | 0.660 (p = 0.4165) | 1.293 (p = 0.2555) |
The results suggest that future health-care use (i.e. cost consequences over the 12-month follow-up period) is related to use in the previous 6 months and controlling for this may improve the estimation of the treatment effect given potential heterogeneity in the available complete-case sample. The effect of the WAP on total cost (having controlled for baseline total costs) compared with the base-case assumption suggests that the incremental costs related to the WAP may increase. This finding may suggest that there is merit in obtaining further evidence on the treatment effect of the WAP (e.g. additional trials or future meta-analysis).
Sensitivity analysis 2
Sensitivity analysis was repeated on the assumption that a band 5 hospital dietitian is the correct competency level and associated pay scale should the WAP be deployed in the NHS. To examine the implication, Table 33 presents the following scenario, which examines the implications of varying NHS band on cost-effectiveness estimates (assuming that treatment effect is not related to NHS band).
| NHS staff band | Salary (£) | Per hour (£) | Session cost (£) | ICER (£) | p(ICER < 20,000) | p(ICER < 30,000) |
|---|---|---|---|---|---|---|
| 3a | 18,264 | 24 | 8 | 3726 | 0.7285 | 0.8037 |
| 4a | 21,122 | 27 | 9 | 5450 | 0.7104 | 0.7926 |
| 5 (base case)b | 23,441 | 31 | 10 | 7742 | 0.6826 | 0.7746 |
| 6c | 31,752 | 42 | 14 | 14,068 | 0.5962 | 0.7081 |
In the current trial the intervention was delivered by two grade 6 research psychologists (equivalent to band 6) and it may be argued that a dose–response relationship was evident in that greater effectiveness was a consequence of the higher level of training. The cost of someone on band 6 is £42 per hour, compared with £31 per hour for someone on band 5; adjusting most to band 6 hourly rates shows a substantial change in ICER [ICER(band 6) = £14,068 vs. ICER(band 5) = £7742]. As there is uncertainty around cost-effectiveness estimates, this illustrates an important parameter should a decision rule such as ‘approval with research’ be considered. 90
It may also be the case that potential cost savings could be realised should the NHS utilise NHS staff below band 5. However, this analysis suggests that there is limited added value in going below band 5 [ICER(band4) = £5450 or ICER(band 3) = £3726].
Sensitivity analysis 3
The final sensitivity analysis considered the effect of the assumption that group size is assumed static (i.e. a mean group size is 15). Proportions attending sessions during the intervention and maintenance phase (see Figure 9) are utilised to estimate overall session attendance (based on the assumption that each group aims to obtain 20 participants at onset). Table 34 presents results comparing the base case to adjusted estimates based on the observed data.
| Scenario | Mean number attending | Session cost (£) | ICER (£) | p(ICER < 20,000) | p(ICER < 30,000) | |
|---|---|---|---|---|---|---|
| Session | Maintenance | |||||
| Base case | 15 | 15 | 10 | 7742 | 0.6826 | 0.7746 |
| Observed | 14.7 | 6.96 | 20 | 24,935 | 0.4339 | 0.5755 |
The results suggest that the mean number of attendances per session is 14.7 during the intervention period and 6.96 in the maintenance phase. Specifically, low attendance during maintenance doubles the attendance cost per session, from £10 (base case) to £20 (based on observed proportions). This is because group interventions are subject to decreasing average cost functions when cost is plotted against attendance. This may substantially elevate the ICER and reduces the probability of falling within the NICE reimbursement threshold. Should the WAP be implemented, commissioners may wish to consider incentive structures based on providers ensuring adherence in the longer term.
Summary of within-trial cost-effectiveness findings
-
The total cost of the WAP is £195 per person (or £10 per group session attended), compared with £176 for best usual care.
-
Controlling for baseline utility and age, the incremental QALY gain is 0.0104 (95% CI –0.0015 to 0.0224; p = 0.088).
-
The mean incremental total cost was not significantly different from the cost of best practice, nurse-led usual care £80 (95% CI –505 to 667; p = 0.787).
-
In the base case, the ICER is estimated at £7742 per QALY, with a probability that the WAP is the most cost-effective intervention of 68.26% when a QALY is valued at £20,000 and of 77.46% when a QALY is valued at £30,000.
Chapter 5 Process evaluation: methods and results
Introduction
This chapter explores the processes involved in the study, from recruitment to delivery of treatment, follow-up and participant satisfaction.
The focus in this section is on exploring the many components of the WAP. We do not explore in any detail the components of the nurse-based intervention, but provide a summary of participant feedback on the overall helpfulness of the programme.
The WAP is a multicomponent programme that includes a range of concrete and verifiable tasks agreed individually with each participant (Box 8). Tasks varied in their ease to complete and the additional resources that they required (e.g. regular weighing required participants to have access to scales).
Participants were provided with an Oregon PE980 pedometer at the first session and received a demonstration on how to use it. Participants were advised to wear the pedometer all day, every day, as they went about their usual activities and to make a note on their task card of the number of steps displayed on the pedometer at the end of every evening.
After the first ‘baseline’ week, WAP facilitators assigned the participant with a daily step count target, which was increased until an agreed level was reached. Opportunities to help achieve the step count target were discussed (e.g. getting off at a bus stop earlier and walking the rest of the journey).
All participants were informed of the recommendations to walk 10,000 steps per day and those who were able to achieve this were encouraged to do so.
Television/screen time useDuring the first week, participants were advised to monitor their ‘screen time’ (i.e. the number of hours spent watching television or using the computer for leisure purposes) and to write down the amount of time spent engaged in screen-time activities on their task card each day. During the second week, participants were asked to continue monitoring and those who identified spending > 4 hours per day engaged in screen time activities were asked to reduce this by half.
Food diary useDuring the first week, participants were advised to keep a food diary (paper copies provide by the study team) for at least 3 days, and write down everything that was consumed (both food and drink), without changing their usual eating habits. Participants were advised against keeping the food diary retrospectively and were instead advised to write items down as they ate. Participants were advised to tick when they had completed the task on their task card. From week 3 onwards, the task of keeping a food diary was optional.
Counted caloriesAt the second session, participants were introduced to ‘calorie counting’, taught how to read food labels and provided with a calorie booklet and directed to a range of resources, including MyFitnessPal (MyFitnessPal Inc., San Francisco, CA, USA). Participants were provided with an individual daily calorie plan (using the Harris–Benedict equation) and were asked to keep a food diary and to count calories. Participants were advised to tick when they had completed the task on their task card.
5 a dayAt the third session, participants were introduced to the 5-a-day task (to consume five portions of fruits or vegetables a day) and provided with a leaflet providing examples of how to achieve this. The 5-a-day task remained a task throughout the programme. Participants were advised to tick when they had completed the task on their task card.
ExerciseAt the fourth session, participants were introduced to the importance of regular physical activity and were set the task of conducting two short bouts of moderate-intensity activity (10–20 minutes in length). Participants were provided with information on opportunities for exercise in their local areas. The frequency and length of the exercise was increased gradually until participants were able to achieve at least three bouts lasting 30 minutes each, with the goal of five 30-minute bouts per week. Participants were advised to tick when they had completed the task on their task card.
No junkAt the fifth session, participants were advised to monitor their hunger and say no to junk/unnecessary eating. Participants were advised to tick when they had completed the task on their task card.
ScalesAt the fifth session, the importance of regular weigh-ins was discussed and participants were advised to buy a set of scales for their home if they did not already have a set. Participants were given the task to weigh themselves at least once a week, at the same time of day. Participants were advised to tick when they had completed the task on their task card.
Removed triggers/avoiding temptationsAt the seventh session, the importance of removing triggers from home and work environments was discussed. Participants were encouraged to identify opportunities to do this and given the task of removing triggers from sight on at least one occasion over the next week. Participants were also advised to replace any tempting foods on display with healthier alternatives (e.g. a fruit bowl instead of a biscuit tin). Participants were advised to tick when they had completed the task on their task card.
Food swaps/easy switchesAt the seventh session, participants were asked to identify any ‘food swaps’ (adoption of healthier alternatives) that they had made while attending the WAP. Participants were then given a leaflet that provided further examples of food swaps and asked to think about any more swaps that could be made.
Thinking about reasons for overeatingAt the third session, participants were provided with a list of common reasons why people overeat (e.g. boredom, stress) and were asked to choose the two main reasons that applied to them. A group discussion followed, allowing participants to explain their choice and provide examples of instances where overeating occurs. Participants were then asked to identify alternative behaviours (other than overeating) that could be conducted (e.g. if eating because food is there, the participant identified that food could be moved out of sight).
BuddyingIntroduced at the fifth session, the task of buddying was introduced, in which participants were paired up and had the option to place small bets/pledges (of monetary value). If the pair lost 1 lb per week between them, the pair would have the option to either have their money back or roll it over to the following week. If the pair failed to lose 1 lb per week between them, they would lose their money (which would be donated to charity). Participants were also given ‘buddy cards’ with the contact details of their buddy and encouraged to contact their buddy at least once each week (via telephone, text or e-mail).
Hunger thoughtsAt the second session, participants were asked to monitor their hunger and to ask themselves if they were hungry before they ate. If participants decided that they were not hungry, they were advised not to eat. Participants were asked to report back on their experience of this at the third session.
Food recallAt the eighth session, participants were asked to think about their last meal every time they were about to eat to see if this would influence how much they ate at their next meal.
The WAP also includes monthly ‘maintenance’ sessions that aim to improve participant motivation, allowing participants to discuss the challenges they have faced since the last session, and to anticipate challenges of the month ahead. Owing to the flexible nature of the monthly sessions, participants were able to elect what they would like to discuss at the next session. For example, one group requested that they go over calorie counting and take the calorie test again. Participants were encouraged to make changes to their diet and physical activity that they could sustain, while the importance of self-monitoring was reasserted at each session. In instances where weight gain had occurred, participants were asked to identify the strategies that had helped to facilitate weight loss in the past and to consider adopting these again (e.g. returning to keeping a food diary and monitoring the effect of this on weight loss). The monthly sessions also allowed for discussions around learning how to deal with setbacks.
Methods
We followed the process evaluation recommendations in the Standard Evaluation Framework for Weight Management Interventions91 for weight management interventions.
We collected data on the number of participants responding to the various recruitment strategies, the number invited to attend screening and the number enrolled into the study. We also summarised some of the problems we encountered during the study set-up and recruitment phases.
Participant attendance was recorded at each session.
At each session, participants were given a task card detailing their pedometer target (from session 2) and tasks for the week ahead. At the start of the following session, the task cards were collected so that we could measure adherence to each task.
At the end of the treatment (8 weeks) participants completed an anonymous feedback questionnaire that asked them to rate the helpfulness of the programme and how likely they would be to recommend it to others (both scored out of 10). Participants were also provided with a list of the various components of the WAP (e.g. keeping a food diary) and asked to rate the helpfulness of each aspect and how likely they were to continue with each aspect. Participants were also required to choose the three main aspects that they found most useful. Participants were able to rate the convenience of the programme in terms of location and timing, and suggest alternative timings if preferred. Finally, all participants were invited to offer any advice or suggestions they might have on how to improve the programme. Participants were required to complete the same questionnaires at the 6- and 12-month follow-ups.
People who dropped out of treatment were called, and if reached were asked their reasons for dropping out.
For the nurse arm, participant attendance was recorded and participants were asked to provide feedback via a questionnaire that asked them to rate the helpfulness of the programme and how likely they would be to recommend it to others. Participants were also provided with a list of the three main aspects of the nurse arm and asked to rate the helpfulness of each aspect before choosing the aspect they found the most useful. Participants were able to rate the convenience of the appointments in terms of location and timing and suggest alternative timings if preferred. Finally, all participants were invited to offer any advice or suggestions they might have on how to improve the programme. Participants were required to complete the same questionnaires at the 6- and 12-month follow-ups.
Analysis
We summarised the data on attendance and adherence to each of the tasks. Participant feedback on the helpfulness of the components of both the WAP and nurse interventions was also summarised. The mean scores of overall helpfulness of the programmes and how likely participants would be to recommend it to others were compared between arms. Descriptive feedback from participants, where available, was themed.
Results
Study set-up
Recruitment of practice nurses
It was initially decided to have just one nurse at each of the two GP sites who would provide the nurse-based intervention for all participants. The practices were agreeable to this and allocated a nurse to the task. Senior study staff trained the nurses to deliver the nurse-based intervention and carry out the study procedures (measurements, questionnaires, etc.). We did, however, encounter some minor problems that we had not anticipated, but were able to find solutions for these (Table 35).
| Problem | Description | Solution(s) |
|---|---|---|
| GCP training | The study sponsor requires all research staff to be GCP trained. Although the sponsor offers GCP training, free of charge, the nurse could not attend this during normal practice hours | Nurses completed GCP training online |
| Demand on nurse time | Concerns were raised by both practices over the amount of nurse involvement the study required | An additional nurse was trained at one practice, so workload could be shared. Appropriate spacing of recruitment waves was necessary so that nurses were not overloaded with appointments |
| Nurse turnover | Two nurses left one practice during the study period | Identification and training of additional nurses |
Recruitment
Our primary avenue for recruitment was via GP practices. However, posters were displayed in a small range of other community venues and workplaces.
We asked GP practices to query their patient database for potentially eligible participants. Owing to data protection issues, practices had to perform all database queries and printing of letters in-house. The processing of the letters (i.e. folding, stuffing envelopes and franking) took considerable time and effort. Later, practices offered to send text messages instead to their patients who were potentially eligible for participation. This method was significantly easier and less costly, although it relies on people having an up-to-date mobile phone number. We sent out approximately 3800 letters and 6500 text messages.
The most frequently reported route into the study was via GP text and mailshots to potential participants (Figure 9).
FIGURE 9.
Number of volunteers contacting the study by source.
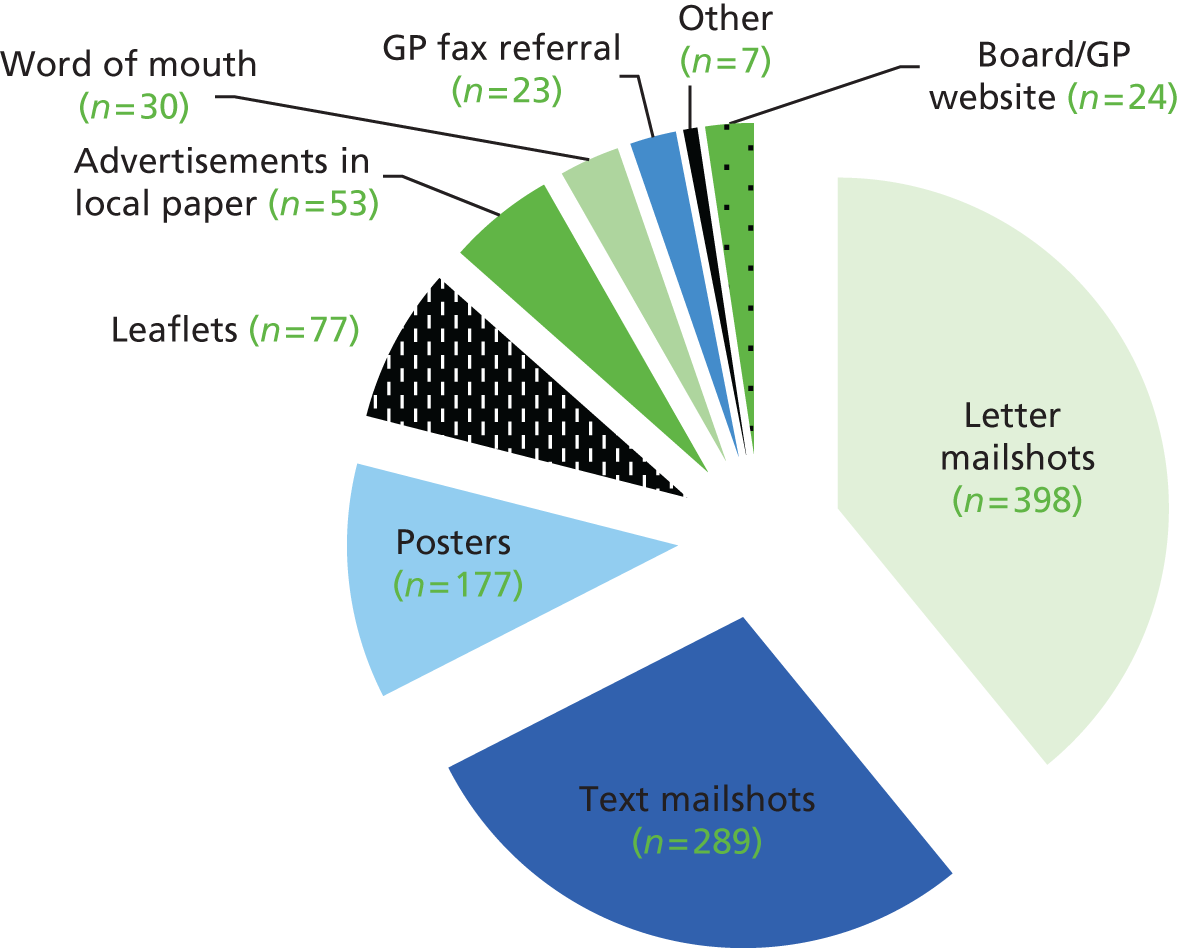
Recruitment barriers and facilitators
It was originally planned to recruit approximately 30 participants per month over a 12-month period starting in October 2011. However because of delays in R&D approvals and contracting, the project plan was revised to start recruitment in July 2012, recruiting 40 participants per month over a 9-month period.
Despite this revised timetable the start of recruitment was delayed until September 2012 and was slower than anticipated (see Chapter 3, Recruitment).
One of the barriers to recruitment was our reliance on the GP team completing referral forms that we asked the practice to fax to us. To try to increase the profile of the study, the research team attended GP team meetings on a fortnightly basis to remind GP staff of how to refer. We had participants from our early waves who wanted to share their success with their GP practice and so attended some of these meetings. However, although staff were interested and hugely supportive, we did not see an increase in GP referrals.
Plans for improving recruitment were discussed during the Trial Management Committee and Trial Steering Committee meetings. The recruitment targets were revised in February 2013 (see Chapter 3, Recruitment), extending the recruitment period to January 2014, adding an additional 10 months to the recruitment period and extending the 12-month follow-up to January 2015. These changes were presented at a Health Technology Assessment monitoring meeting in July 2013 and a 6-month no-cost extension was approved to enable these changes.
The recruitment strategy was redrafted in November 2012 to include mobile phone text mailshots, advertising on websites and boards in practices, producing newsletters on study progress to staff and GP patients, and holding stalls to advertise the study to potential participants.
It was also agreed that participants could be recruited from neighbouring practices. This, however, was not straightforward. Practice managers proved difficult to get hold of and often did not return calls. The process was extremely time-consuming, requiring study staff to explain the study and answer any questions posed. In the end, 15 surgeries were contacted via telephone, letters and e-mails, nine replied expressing an interest and four participated, referring 290 participants to the study. We also needed to find a process by which participants from outside the practice could attend for their weight management appointments without mistakenly being turned away because they were not registered with the practice.
Learning points
Owing to the steadily increasing burden of research regulation, R&D delays are increasingly common and are now practically a norm. Research timetables need to include contingency time of several months for unexpected bureaucratic delays.
We had also initially overestimated our ability to recruit from primary care, despite initial assurances that the practices would be able to do this. We had based our assumptions that the most would be GP fax referrals on our work in smoking cessation as the majority of referrals to smoking cessation from GPs come in this way. However, this system has been in place for approximately 10 years.
In terms of recruitment from the additional practices, we found benefit in working with network managers (where available) rather than individual practice managers. The network managers are often in charge of a number of surgeries in their local area partnership and can co-ordinate letter/text mail-outs for these GP surgeries.
In hindsight it would have been wise to employ a broad range of recruitment strategies from the beginning, instead of a stepwise approach.
Screening sessions
Our experience in clinical practice with running smoking cessation and weight management clinics is that approximately half of people invited to attend the first session do not attend. We therefore double-booked all appointments for the first session. We did, however, have a lower did-not-attend rate (35%) than expected, which meant that some screening sessions were busy and participants were required to wait for up to 30 minutes longer than usual.
Weight Action Programme groups
Over the course of the study a total of 15 groups were run. The size of the groups ranged from 10 to 21 participants. Table 36 shows the times and days of the week that these groups were run. A greater number of evening clinics (17.30–18.30 hours) were offered, as this tended to be when most people were able to attend. We tried running one clinic between 14.00 and 15.00 hours, but this was not repeated as it clashed with collecting children from school.
| WAP group | Day of week | Session time | Group size |
|---|---|---|---|
| Barkantine group 1 | Monday | 12.30–13.30 | 16 |
| Barkantine group 2 | Monday | 17.30–18.30 | 18 |
| Barkantine group 3 | Tuesday | 14.00–15.00 | 13 |
| Barkantine group 4 | Tuesday | 17.30–18.30 | 19 |
| Barkantine group 5 | Tuesday | 17.30–18.30 | 17 |
| Barkantine group 6 | Tuesday | 17.30–18.30 | 21 |
| Barkantine group 7 | Tuesday | 12.30–13.30 | 13 |
| Barkantine group 8 | Tuesday | 17.30–18.30 | 19 |
| Lawson group 1 | Thursday | 17.00–18.00 | 15 |
| Lawson group 2 | Wednesday | 17.30–18.30 | 16 |
| Lawson group 3 | Wednesday | 11.30–12.30 | 13 |
| Lawson group 4 | Wednesday | 17.30–18.30 | 10 |
| Lawson group 5 | Wednesday | 11.30–12.30 | 14 |
| Lawson group 6 | Wednesday | 17.30–18.30 | 19 |
| Lawson group 7 | Wednesday | 11.30–12.30 | 14 |
Participant attendance at treatment sessions
More than two-thirds of participants in both study arms completed at least half of all treatments sessions (Table 37). Session attendance generally declined over time (Table 38 and Figure 10).
| Attendance | Arm | |
|---|---|---|
| Nurse (N = 109) | WAP (N = 221) | |
| Attended at least one session, n (%) | 98 (90) | 213 (96) |
| Attended half or more of the sessions,a n (%) | 75 (69) | 175 (79) |
| Number of sessionsa attended per participant, median (IQR) | 3 (1–4) | 7 (5–8) |
| Attendance and task | Session, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Attendance | 202 (91) | 193 (87) | 174 (79) | 164 (74) | 153 (69) | 140 (63) | 133 (60) | 144 (65) |
| Completed task card | 170 | 144 | 147 | 126 | 111 | 117 | 98 | 47 |
| Pedometer use | 146 (86) | 128 (89) | 132 (90) | 115 (91) | 103 (93) | 108 (92) | 84 (86) | 31 (66) |
| Television/screen time | 149 (88) | 116 (81) | – | – | – | – | – | – |
| Food diary use | 131 (77) | – | – | – | – | – | – | – |
| Counted calories | – | 94 (65) | – | – | – | – | – | – |
| 5 a day | – | – | 88 (60) | 74 (59) | 69 (62) | 76 (65) | 62 (63) | 34 (72) |
| Exercise | – | – | – | 84 (67) | 69 (62) | 73 (62) | 60 (61) | 30 (64) |
| No junk | – | – | – | – | 68 (61) | 68 (58) | 65 (66) | 28 (60) |
| Scales | – | – | – | – | 55 (50) | 63 (54) | 56 (57) | 27 (57) |
| Removed triggers | – | – | – | – | – | – | 6 (6) | – |
FIGURE 10.
Proportion of participants in the nurse arm attending each session. Sessions 1–4: fortnightly treatment sessions over 8 weeks.
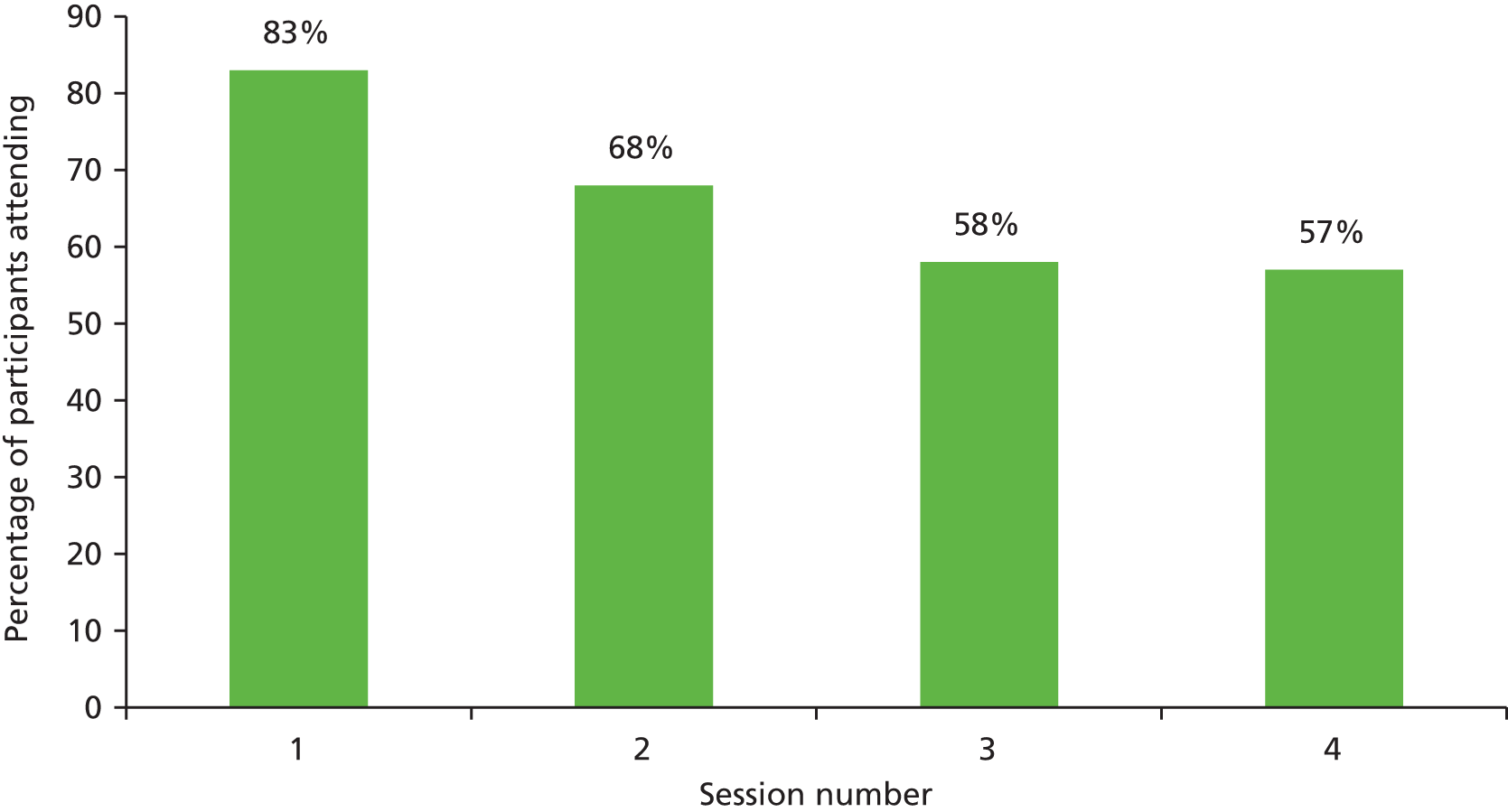
Not everyone who dropped out of the WAP provided a reason for doing so. Among those who did, inability to attend because of inconvenient times was the most common reason (Figure 11). Although participants were informed of the clinic times at the very first contact, it was often because life circumstances had changed (e.g. changes in work rota or child care).
FIGURE 11.
Reasons given for dropping out of the WAP treatment (n = 19).

Participant attendance at maintenance sessions
Attendance at each session is shown in Figure 12. Participation declined over time, with only around one in five participants attending maintenance sessions in the last 6 months. The maintenance sessions were held as ‘open sessions’, with participants at different stages of the intervention attending the same maintenance session.
FIGURE 12.
Proportion of participants in the WAP arm attending each session. Sessions 1–8: weekly treatment sessions; sessions 9–18: monthly maintenance sessions.

Follow-up rates
We implemented a number of strategies for minimising loss to follow-up (Box 9). Follow-up rates were higher than predicted (70% vs. 50% predicted at 12 months). There was no difference in follow-up rate by study site, but the proportion of participants followed up at 12 months was greater in the nurse arm (76%) than in the WAP arm (67%).
Stress the importance of attending at the end of treatment, even if participants feel that they have gained weight.
Multiple follow-up routes (telephone, GP practice, text, letter, e-mail).
Flexible appointments offered (evening/weekend/home visits). Calls made to participant at different times of the day (early mornings/late evenings).
All contact attempts documented so study team could quickly assess which route to try next.
Involving staff affiliated with the study team (i.e. not involved in the intervention) to make contact with participants to invite them to attend for follow-up so as not to put participant off if they speak to the researcher involved in leading the intervention.
Participant adherence to Weight Action Programme tasks
Of all tasks, pedometer use was the most likely to be adhered to, with close to 90% of participants who handed in task cards reporting to use these devices daily. Approximately half of participants weighed themselves regularly (50–57%) and around 60% adhered to the 5-a-day task. Removal of triggers to eat was the least used tool (6%).
Use of orlistat
During the planning stage we were alerted to the fact that people were offered orlistat as part of standard care. We therefore included information about orlistat at the third WAP session. All participants were given an information sheet about orlistat. Among those who expressed an interest in using orlistat, eligibility was checked by study staff, to prevent participants making an unnecessary visit to their GP. Those who were eligible were advised to make an appointment with their GP to obtain a prescription and bring the medication back to the session the following week, during which participants were given a recap on how to use orlistat.
All eligible participants were prescribed a 1-month course of orlistat in the first instance, with continued prescriptions contingent on weight loss, as per NICE guidelines. At subsequent sessions, participants were asked about their orlistat usage.
At total of 75 (23%) participants opted to use orlistat as part of their weight loss attempt. Participants in the WAP arm were significantly more likely to use orlistat than participants in the nurse arm (31% vs. 6%; odds ratio 6.50, 95% CI 2.78 to 15.59; p < 0.001). Weight loss at 12 months was greater in those who used orlistat (mean –5.4 kg, SD 8.1 kg) than in those who did not (mean –2.9 kg, SD 6.6 kg), with the difference (–2.5 kg) being statistically significant (95% CI –4.5 to –0.4 kg; p = 0.02).
Participant feedback
Participants in both arms provided feedback on the helpfulness of the weight loss intervention they received at the end of treatment (Table 39) and at the 12-month follow-up (Table 40). Ratings of helpfulness in losing weight were high in both arms, but significantly greater from participants who used the WAP (9.1 vs. 8.0; p < 0.001). The WAP participants were also more likely to recommend the programme to others (9.3 vs. 8.1; p < 0.001). Ratings were only slightly lower at the 12-month follow-up, but remained significantly greater in the WAP arm (see Table 40).
| Question | Arm, meana (SD) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Nurse (n = 48) | WAP (n = 129) | |||
| How helpful was the programme? | 8.0 (2.1) | 9.1 (1.4) | 1.1 (0.6 to 1.6) | < 0.001 |
| Would you recommend the programme to others? | 8.1 (2.2) | 9.3 (1.3) | 1.2 (0.6 to 1.7) | < 0.001 |
| Question | Arm, meana (SD) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Nurse (n = 48) | WAP (n = 129) | |||
| How helpful was the programme? | 7.2 (2.9) | 8.4 (2.3) | 1.3 (0.5 to 2.0) | 0.001 |
| Would you recommend the programme to others?b | 7.8 (2.7) | 8.8 (2.2) | 1.0 (0.3 to 1.7) | 0.004 |
Participants also ranked their top three most useful aspects from each of the treatment programmes (nurse and WAP). Few participants (n = 5) in the nurse arm responded, but at the end of treatment all highly ranked advice from the nurse.
Of the respondents in the WAP arm, the component most frequently ranked in the ‘top 3′ at the end of treatment was ‘monitoring with a pedometer’ (30%; Figure 13). This was followed by ‘having weight regularly monitored’ (14%) and ‘coming to group sessions’ (13%). ‘Avoiding temptation’, ‘leaflets provided’, ‘exercise programmes’ and ‘buddy system’ were not seen as particularly helpful.
FIGURE 13.
Percentage of participants rating each component of the WAP treatment in their top three most useful.
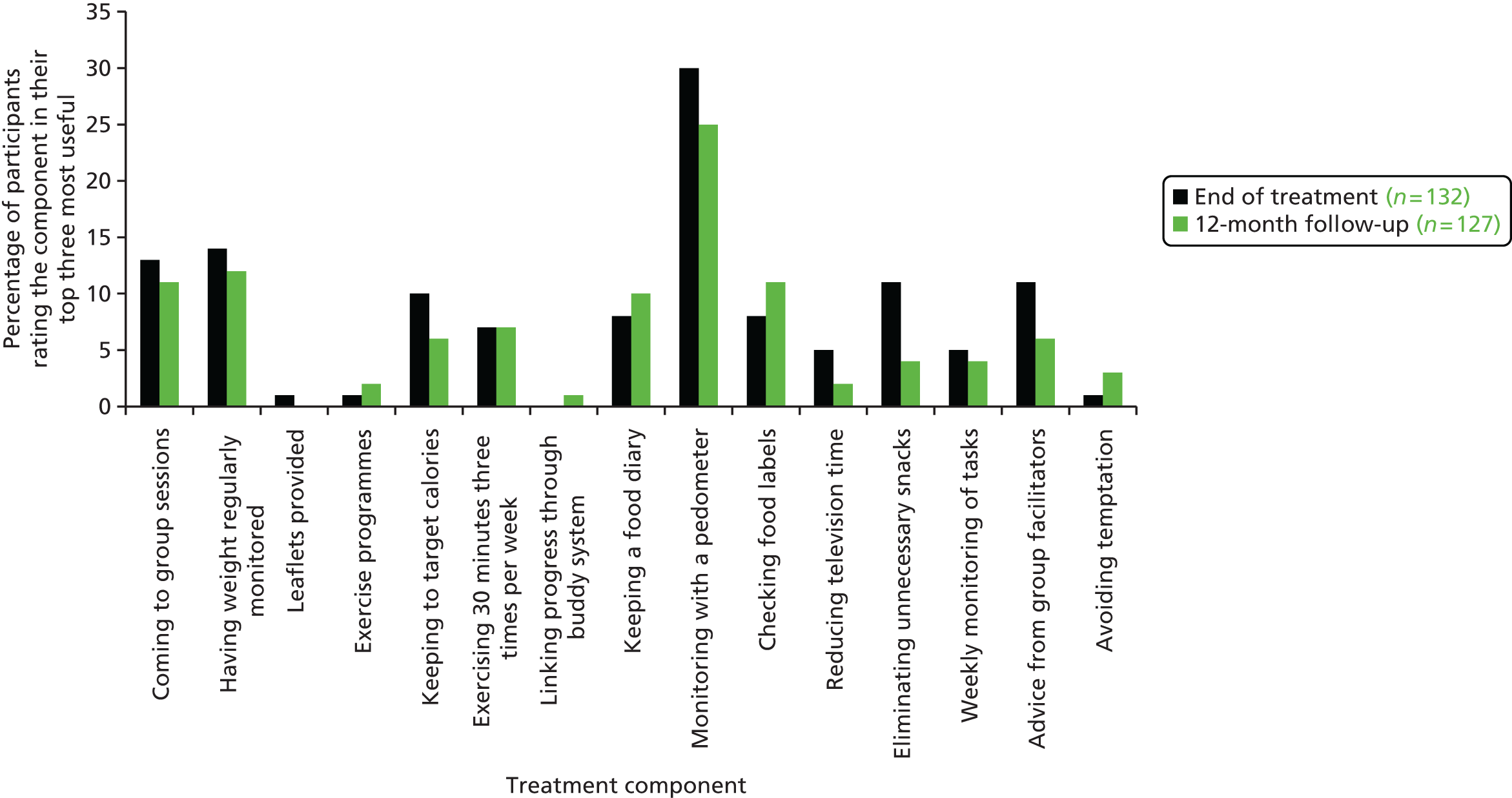
In their feedback, some participants provided written comments about what they liked about the WAP. These comments were collapsed into three themes.
(1) Group-based treatment format
There are a number of advantages of running a group-based treatment programme. Participants get to learn from others’ experience. This is often vicarious learning, but also includes learning of a normative experience. Groups also provide support, encouragement and motivation from others. This is type of support is often more relevant when it comes from peers going through the same experience, as opposed to a trained facilitator:
Group aspects were good and raised my awareness.
It was good hearing other participants’ experiences during sessions.
I’ve found it helpful and although know how to lose weight; it’s good to have support and encouragement.
(2) Specific tasks
In general, participants appreciated the different tasks they were given. The pedometer/walking task was well liked. The information and tasks provided on calories and food was also noted as helpful:
The information on calories for food, discussions on eating times, the pedometer were all very useful tools as were the report backs when we had to measure results and discuss. The messages and info have made me more aware and altered my habits.
The programme was very helpful, helped me with my walking and taught me to count calories.
(3) General comments
The ratings of helpfulness of the programme were high, which is reflected in these general comments:
The programme has been very interesting and for most people, effective.
Staff were lovely and helpful and I’m grateful for the opportunity of attending the programme.
Participants were also given the opportunity to comment on how the WAP could be improved. These comments also fell into three main themes.
(1) Group-based treatment format
To some extent the success of group-based treatment relies on an element of group pressure, that is, participants are accountable to each other for meeting their targets and losing weight. Two participants commented that this element could have been strengthened in their groups:
In the group we are too nice to each other – we should be more encouraging and tougher on each other about keeping to targets. I would rather I was more accountable for not losing weight – maybe a tougher GP for people who need a bit more pressure.
It may be different in the different groups of people, but I did feel that a lot of the people already knew the basics. Might be better to focus on areas of motivation rather than just weekly ‘confessions’. Maybe more team time to make you feel closer to the group and more accountable for your results.
One component of the WAP is to ask people to pair up (or buddy up) with others (see Box 8). The purpose of this task was to foster the experience of social support outside of the weekly sessions in the hope that this would encourage motivation and self-efficacy. This type of task is used in smoking cessation groups where buddies work together to remain abstinent from smoking, and generally it works well. 92,93 However, it did not rate highly with participants overall (see Figure 13) and was specifically mentioned by one participant:
It was good hearing other participants’ experiences during sessions but I really didn’t like/want to participate in the ‘buddy’ scheme.
(2) Specific tasks
Three comments related to two specific tasks. One concerned the need for more advice on choosing healthy food:
Maybe more advice on food labelling and healthy food.
In general, the advice on calories and choosing healthy options was well received. Although many people had a good general knowledge of calories and could determine which food options were more calorific than others, there was, anecdotally, some confusion over food consumption and weight loss. For example, in one of the groups a participant who did not have a particularly healthy diet lost weight by eating smaller portions of his usual foods. Some participants could not understand how weight loss was possible when he was eating ‘unhealthy’ foods.
The other two comments concerned exercise and the programme’s instructions about how to exercise. The WAP does not give specific advice on the type of exercise to do and how this might be tailored to individual need. Instead it provides goals and general information on the types of exercise that counts as moderate intensity and options for structured exercise programmes in the local areas:
Health and safety issues/advice before exercising, explanation regarding vigorous vs. moderate exercise, invite local exercise group leaders to attend one session to advertise what is available.
I think for me, with my disability it was difficult to engage with some of the activities recommended.
(3) Clinic times
Although we tried to offer a range of different options for clinic times, some participants still found the groups difficult to attend:
I would have loved if the class was a little later, possibly 6, as I had to leave work early to attend which was not too convenient. The sessions were helpful and informative but felt a little bit too long at times. Perhaps would have been good to do the programme in the summer.
I’ve enjoyed the programme. I would prefer if the sessions were held at 6 p.m. as allow time for me to attend straight from work.
It’s always difficult to find a regular time to be available between work. A longer period of sessions would have helped me only because I was away a lot.
If you can do some at the weekends.
Chapter 6 Discussion
Key results
The WAP helped people lose almost 2 kg more, on average, than the nurse-based intervention at 12 months. This difference was statistically significant and was robust across different sensitivity analyses. In the WAP arm, 41% of participants lost at least 5% of their baseline body weight, compared with 27% in the nurse arm (p < 0.001). At the end of treatment both arms rated the weight loss programme they received as very helpful and were likely to recommend the programme to others.
The health economic analysis showed that that the cost of the WAP is £195 per participant (or £10 per group session attended), compared with £176 per participant for best usual care. Controlling for baseline utility and age, the incremental QALY gain is 0.0104 (95% CI –0.0015 to 0.0224; p = 0.088). Mean incremental total cost was not significantly different from the cost of best practice nurse-led usual care at £80 (95% CI –£505 to £667; p = 0.787).
In the base case, the ICER is estimated at £7742 per QALY, with a probability that the WAP is the most cost-effective intervention of 68.26% when a QALY is valued at £20,000 and of 77.46% when a QALY is valued at £30,000. With respect to the explicit decision threshold stipulated by NICE, these results would suggest that the WAP is likely to represent value for money to the NHS.
Study limitations
There are several limitations of the trial worth noting. Although we exceeded our expected retention rates, we were unable to measure weight in 30% of participants at 1 year. High attrition rates in trials of weight loss are well recognised and estimated to range between 30% and 60%. 94 Missing data pose problems for weight loss and most other behaviour change trials. A traditional approach has been to use the last observation carried forward, but as people often stay in treatment while they are doing well and drop out when they put on weight, and most initially successful dieters regain at least some of their initial weight loss during the follow-up period, this approach is likely to overestimate treatment effects, and in controlled trials this generates noise that may obscure real treatment effects. We used a mixed-effects model approach that is currently the preferred approach, although it does not completely resolve the problems associated with missing data. It is reassuring that the various sensitivity analyses confirmed the main result.
We were unable to blind staff taking measurements of weight, waist circumference and blood pressure to participant allocation throughout the treatment phase, but staff collecting these measurements at the key 6- and 12-month follow-ups were blind to participant allocation.
Participants were recruited from six general practices in two London boroughs. This reduces the generalisability of the results somewhat, although there is little reason to assume that patients in other parts of London or the country would be markedly different. We also only provided treatment at two sites; in reality, not all GP practices would have the facilities to run the WAP and referring patients to neighbouring GPs would be the most likely solution.
Participants in the WAP arm had more treatment contact time than the nurse arm. This could, in theory, generate better follow-up rates via greater participant involvement and thus generate a potential bias. This fortunately did not happen: follow-up rates were slightly better in the nurse arm than in the WAP arm.
The trial attracted mostly women (72%). This is the norm in weight management research and clinical practice. 35,37–39 As obesity is no less prevalent in men than in women, there is a need to explore factors that would make such programmes more attractive to men.
Obesity management guidelines typically consider 5% weight loss to be clinically meaningful, and behavioural intervention can help a proportion of clients to achieve this. However, there is an increasing acceptance among weight management experts that a weight loss target of 5% is no longer sufficient for many of the patients being treated in primary care. Currently, the only proven life-transforming treatment for patients with severe obesity is bariatric surgery. Lifestyle modification programmes of the type we evaluated, however, can improve weight, health and fitness in people who have not reached morbid obesity levels. Such programmes may also stop further weight gain in people who would reach morbid obesity in future, though evidence for this is lacking to date.
One of the strengths of the trial was its inclusiveness. There were few exclusion criteria. Many weight management studies recruit primarily middle-class clients, which limits generalisability to clinical populations. Our trial enrolled participants from a diverse range of ethnic and socioeconomic backgrounds. Only 62% had completed a high school education, compared with > 90% reported in similar trials,38,39 and participants were also less likely to be employed (48%) than those in other studies. 38,39
Interpreting study findings
When interpreting the main finding, it is important to note that the positive result was not an artefact of the control group doing poorly. The nurse intervention did better than expected,27 so the benefit seen with the WAP was not the result of having an inferior comparator.
Participants in the WAP group had a greater reduction in waist circumference at the 6- and 12-month follow-ups, although the difference did not reach statistical significance at 12 months. At the end of treatment, participants in the nurse arm had a significantly greater reduction in waist circumference, but achieved less weight loss. These contradictory findings may have been related to errors in measurement of waist circumference, which are a well-recognised problem,95,96 and related to site of measurement, time since last meal and phase of respiration. 97 Nurses were trained to measure waist circumference at the end of treatment, but at baseline and other follow-up points the study team measured this. Similar to other studies,37,38 we did not find any significant change in blood pressure. In a meta-analysis of data from 34 trials, systolic blood pressure did not change with weight loss in 18 trials and diastolic blood pressure did not change with weight loss in 21 trials. 98
A greater proportion of participants in the WAP arm than in the nurse arm reported AEs, although this difference was not statistically significant. This may have been a consequence of the fact that participants in the WAP arm were asked about AEs more frequently, and so there is potential for recall bias.
The WAP relies primarily on the group format, focusing on encouraging attendance and adherence to programme tasks. There remains the question, however, of the different effects of different tasks. The study was not set up to allow dismantling of the WAP effects and determining which parts of the programme were responsible for its effect. However, the WAP does not rely on any one specific task or advice. The key innovative element of the WAP is that it encourages participants to try a range of strategies, insisting that each new behaviour is not just considered but practically implemented for at least 1 week. After that participants can decide whether or not to carry on with it. Unlike most other approaches that insist on adherence to some core recommendation, the expectation here is that none of the tasks will be adopted by 100% of the participants, but that there is a sufficient variety to allow as many participants as possible to find and adopt one or more strategies that work for them. Seeing other group members adopting such strategies and benefiting from them may increase willingness to try and maintain such new behaviours as well. The use of pedometers appeared to be a task that was liked by the majority of participants.
Orlistat use provides another good illustration of the group effect. Participants in the WAP arm were more likely to use orlistat and orlistat use contributed to weight loss. Most clients react to the offer of orlistat with uncertainty, mostly related to the drug’s unpleasant side effects. The group format seems to have provided additional encouragement and reassurance via social learning. In most groups there would be one or two people who had benefited from orlistat in the past, or are benefiting from it during treatment, and report this at the WAP sessions, which encourages others to consider using the medication.
Interpretation in relation to other studies
The WAP group programme surpassed the effects of nurse intervention and enabled > 40% of participants to lose ≥ 5% of their body weight and maintain this over 1 year. This tallies with previous findings showing the popularity and efficacy of group programmes for weight loss. 35,38
The WAP differed from the nurse intervention in two procedural variables: contact frequency and group format. Some studies suggest that more frequent contact promotes better weight loss,35,36,99,100 but a recent meta-analysis found no evidence of this. 101 The advantages of group support are the more likely explanation of our finding, but both elements may have contributed.
The finding generates the obvious question of whether or not, and how, such a treatment could be disseminated on a larger scale.
The reason for relying on practice nurses to help primary care patients lose weight is largely pragmatic. Most people see their GP at least once per year and obese patients often present with obesity-related problems. GPs are thus uniquely placed to trigger weight loss attempts. However, apart from offering a prescription for orlistat (which without further support is likely to have only limited effects) GPs do not have the tools, training or time to engage in weight management treatments. A referral to a practice nurse is the obvious solution. Our earlier survey showed that practice nurses would mostly provide one-off advice, sometimes suggesting to patients to arrange proactively further sessions if they want to. This is likely to generate less weight loss than the relatively intensive and well-structured intervention used in our trial and it may not be the best use of the precious primary care time.
There is a close parallel with how stop-smoking interventions used to be delivered prior to the establishment of the NHS Stop Smoking Service (SSS) in 1999. GPs were prescribing nicotine replacement treatments and referring smokers to practice nurses for behavioural support. This was time-consuming and therefore expensive, and had limited efficacy. The idea behind the SSS was to take this burden and expense away from primary care. GPs were expected to simply refer smokers to trained full-time advisors who provide a more effective, intensive multisession treatment.
In theory, the WAP could help to translate some of the most useful features of the SSS into the new tier 2 and tier 3 weight management services that are currently being set up across the country.
The initial model of stop-smoking services assumed that smokers would be treated in groups, as this is by far the most cost-efficient approach, and there is growing evidence that it is also more effective. 49,102,103 In practice, however, recruitment of smokers into the service proved difficult, and only a few services have large enough throughput to be able to run groups. This is not an issue in weight management where the interest in treatment is much greater and group approaches are much more widespread.
Another important feature of the SSS was that the service included compulsory objective and standard monitoring of its throughput and outcome. This generated data essential for service evaluation and improvements, and for establishing service standards. The services also had access to standard training and were mandated to provide evidence-based treatments.
Weight management services have followed a different trajectory. Primary care remains the key source of weight management advice. In addition to this, public health services, which are now placed with local councils rather than the NHS, are now responsible for commissioning tier 2 and tier 3 weight management services. These should be based on evidence, but unlike the SSS there has been a relative lack of clear guidance. Commercial providers are being commissioned with no request to provide evidence of their outcomes and no mandated monitoring of weight loss achieved. It is likely that much of the investment will generate limited benefits, if any.
To be successful, any healthy lifestyle programme must be able to be integrated into existing GP practice systems and convenient to patients. 104 We found that practices were very willing to refer patients, but in practice few were referred. This, in part, may have initially been because of the referral mechanism we asked them to use that was outside their normal systems. Other factors that may have contributed to low referral are lack of time, limited understanding about weight management and fear of damaging the relationship with the patient by raising a potentially sensitive topic. 105 This finding is supported by a recent study. 106 Of the 91,413 overweight and obese patient records analysed, 90% had no weight management intervention recorded, and 59% of patients with morbid obesity had no intervention recorded.
Sending letters or text messages to patients who met inclusion criteria generated a lot of interest, with little effort from busy practice staff. The WAP groups were run in meeting rooms within the practice at times when these were available. Running this outside of usual practice hours (e.g. 17.30–18.30 hours) ensured that space was more likely to be available and was more convenient for participants. Running WAP clinics within GP practices was also convenient for participants.
Cost-effectiveness
Obesity-related illness costs the NHS about £7B annually through the increased likelihood of mortality and morbidity. The economic analysis in this report finds the WAP group intervention to be the cost-effective option with respect to best routine care. The cost of providing the WAP is similar to nurse-led routine care, however, the group format allows for a longer-term treatment phase with sustained maintenance. Undoubtedly, the structured approach to long-term weight management is associated with the observed improvement in health-related quality of life. On aggregate, the results of cost-effectiveness analysis fall below explicit reimbursement thresholds (£7742 per QALY), suggesting that the WAP represents good value for money.
A programme like the WAP seems suitable for adoption in such services for several reasons. There is evidence of its efficacy: it is based on an inclusive pragmatic trial; its group format with 15–20 participants per group means that it is much more cost-efficient than approaches requiring individual contact; it is based on multiple strategies to allow participants to identify those suitable for their individual needs, which means that it can incorporate and roll out new methods, techniques and medications as they are discovered; it includes standard monitoring of objective outcomes that could be collated across services; and it is easy to teach and disseminate.
Given that a commitment was already made to fund tier 2 and 3 services for people seeking help with weight management at each individual borough in the country, there now exist structures and funding that could incorporate the WAP on a large scale. In theory, Public Health England and NICE could consider how best to encourage these services to consider the results of this trial before this large investment settles with the current mixture of programmes with dubious rationale and efficacy, and with no standard outcome checks.
Further research
Although we report on 12-month data, which give a sound indication of the effectiveness of weight loss programmes, ongoing follow-up of this study cohort would enable investigation of whether or not the WAP is able to support weight loss in the long term.
The WAP treatment programme is delivered over 8 weeks, with ongoing maintenance sessions. NICE guidelines51 recommend that weight management programmes should be 12 weeks in length. With demands on staff and patient time in addition to financial restraints, research is needed on the added benefit, if any, of longer programmes.
Overall, individual-level non-surgical interventions for obesity tend not to be highly effective, and more research on obesity prevention through community-level interventions may be required.
Like other studies in this field, a minority of participants were men. Given slightly higher rates of obesity in men compared with women, research is needed into factors that would make weight loss programmes more attractive to men.
Further research may explore incentive structures based on providers ensuring patient adherence to the WAP over the course of treatment and within a continuous maintenance phase.
Finally, the efficacy of the WAP delivered through electronic media should be investigated. Some of the components of the WAP have already been modified for delivery via mobile text messages and websites,107 and tested in a feasibility study. 108 Further work is needed on how the group-based aspect of treatment can be utilised, perhaps using existing social media applications [e.g. Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) and Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com)].
Chapter 7 Conclusions
The group-based WAP intervention delivered in a general practice setting was more effective at helping obese patients lose weight at 1 year than weight loss advice delivered by a practice nurse.
The WAP intervention was also more cost-effective than nurse-based treatment, although both would be deemed highly cost-effective based on the current NICE recommendations. However, as the WAP is delivered in a group format, it is a more cost-efficient way of treating patients.
The WAP can be relatively easily implemented within primary care. It can be delivered by auxiliary staff, such as health trainers, with just 2 days of training and with relatively little specialist or costly equipment.
Future research should focus on longer-term follow-up, how to make weight management programmes more attractive to men and explore whether or not the programme, or parts of the programme, could be delivered via electronic media. Further work should confirm its effectiveness when implemented outside the setting of a RCT. As with most weight management studies, further attention needs to be given to increasing retention rates.
Acknowledgements
We would like to acknowledge the following groups and individuals:
Our Trial Steering Committee members, in particular the chairperson Dr Vicky Hobart and our lay member Julie Griffiths.
All the people who participated in the study.
Our National Institute for Health Research project managers, Alexa Cross and Hazel Church.
Staff at the Barkantine Practice, including Mostafa Farook (practice manager), Shahzad Firoz (assistant practice manager) and Dr Stuart Bingham (lead GP).
Staff at the Lawson Practice, including Lauren Stephenson (practice manager) and Dr Deborah Colvin (lead GP).
Staff at the North Central London Research Consortium who were involved in awarding NHS support costs.
Dr Dunja Przulj, Miss Celine Homsey, Miss Elizabeth Rock, Miss Anna Phillips, Miss Rebecca Anderson, Miss Kirsten McAlpine, Mr Ronnie Troughton, Miss Danielle Cornwall, Miss Dawn Lindsay and Miss Lizzie Carter-Fox from the Health and Lifestyle Research Unit, Queen Mary University of London, who collected data at follow-up points and contributed to data entry.
Mike Waring, data manager from the PCTU, who was responsible for the set-up and monitoring of the database.
Professor Cliona Ni Mhurchu, National Institute for Health Innovation, University of Auckland, New Zealand, who provided feedback on the initial grant application and project plan.
We are grateful to the four practice nurses who provided the intervention in the nurse arm. They are Alexandra Truelove, Maria Marco, Abbie McFarlane and Barbara Curran.
Contributions of authors
Dr Hayden McRobbie (Professor of Public Health Interventions) co-wrote the original grant application, co-developed the intervention, co-designed the trial, co-wrote the statistical analysis plan, trained staff, interpreted the findings and led the drafting of this report. He was the chief investigator of the trial and was a grant holder on this project.
Professor Peter Hajek (Professor of Clinical Psychology) co-wrote the grant application, co-developed the intervention, co-designed the trial, trained staff, helped interpret the study findings and contributed to the drafting of this report. He was a grant holder on this project.
Miss Sarrah Peerbux (Research Health Psychologist) co-ordinated the study between October 2013 and February 2015, delivered the intervention, contributed to data collection, co-wrote the statistical analysis plan, helped interpret the study findings and contributed to the drafting of this report.
Dr Brennan C Kahan (Lecturer in Medical Statistics) co-wrote the statistical analysis plan, undertook the statistical analyses and contributed to writing this report.
Professor Sandra Eldridge (Professor of Biostatistics) contributed to the original grant application, advised on statistical methods and analyses, advised on study governance and management and contributed to writing this report. She was a grant holder on this project.
Dr Dominic Trépel (Senior Lecturer in Health Economics) conducted the health economic analysis in the report and wrote Chapter 4.
Dr Steve Parrott (Reader in Health Economics) contributed to the initial research proposal, the health economics analysis plan, and supervised and contributed to the writing of the health economic analysis.
Professor Chris Griffiths (Professor of Primary Care) contributed to the original grant application, advised on standard weight management practice in primary care, advised on engagement with general practice and contributed to writing this report. He was a grant holder on this project.
Sarah Snuggs (Research Health Psychologist) was involved in the study set-up, recruitment and delivering the WAP treatment. She also contributed to writing this report.
Dr Katie Myers Smith (Research Fellow) co-wrote the original grant application, co-developed the intervention, co-designed the trial, trained staff, interpreted the findings and led the drafting of this report. She co-ordinated the study between September 2012 and October 2013, led the approvals process and governance aspects, delivered the intervention, contributed to data collection and helped to interpret the study findings. She was a grant holder on this project.
Data sharing statement
Data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. http://dx.doi.org/10.1016/S0140-6736(14)60460-8.
- Global Status Report on Noncommunicable Diseases. Geneva: World Health Organization; 2010.
- Statistics on Obesity, Physical Activity and Diet – England, 2015. Leeds: Health and Social Care Information Centre; 2015.
- Rayner M, Scarborough P. The burden of food related ill health in the UK. J Epidemiol Community Health 2005;59:1054-7. http://dx.doi.org/10.1136/jech.2005.036491.
- Reducing Obesity: Future Choices. London: Government Office for Science; 2007.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8210.
- Poobalan AS, Aucott LS, Smith WC, Avenell A, Jung R, Broom J. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007;8:503-13. http://dx.doi.org/10.1111/j.1467-789X.2007.00393.x.
- Stubbs RJ, Morris L, Pallister C, Horgan G, Lavin JH. Weight outcomes audit in 1.3 million adults during their first 3 months’ attendance in a commercial weight management programme. BMC Public Health 2015;15. http://dx.doi.org/10.1186/s12889-015-2225-0.
- Dobbs R, Sawers C, Thompson F, Manyika J, Woetzel J, Child P, et al. Overcoming Obesity: An Initial Economic Analysis 2014. www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity (accessed 26 July 2015).
- Obesity and Ethnicity. London: National Obesity Observatory; 2011.
- Healthy Lives, Healthy People: A Call to Action on Obesity in England. London: Department of Health; 2011.
- Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes Relat Metab Disord 2003;27:1437-46. http://dx.doi.org/10.1038/sj.ijo.0802475.
- Colquitt J, Clegg A, Loveman E, Royle P, Sidhu MK. Surgery for morbid obesity. Cochrane Database Syst Rev 2005;4. http://dx.doi.org/10.1002/14651858.cd003641.pub2.
- Martin LF, Smits GJ, Greenstein RJ. Treating morbid obesity with laparoscopic adjustable gastric banding. Am J Surg 2007;194:333-43. http://dx.doi.org/10.1016/j.amjsurg.2007.03.002.
- Sacerdote C, Fiorini L, Rosato R, Audenino M, Valpreda M, Vineis P. Randomized controlled trial: effect of nutritional counselling in general practice. Int J Epidemiol 2006;35:409-15. http://dx.doi.org/10.1093/ije/dyi170.
- Jain A. Treating obesity in individuals and populations. BMJ 2005;331:1387-90. http://dx.doi.org/10.1136/bmj.331.7529.1387.
- Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.cd003818.pub2.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-40. http://dx.doi.org/10.1056/NEJMoa012512.
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481-6. http://dx.doi.org/10.2337/dc10-2415.
- Fayh AP, Lopes AL, da Silva AM, Reischak-Oliveira A, Friedman R. Effects of 5% weight loss through diet or diet plus exercise on cardiovascular parameters of obese: a randomized clinical trial. Eur J Nutr 2013;52:1443-50. http://dx.doi.org/10.1007/s00394-012-0450-1.
- Gaal LFV, Mertens IL, Ballaux D. What is the relationship between risk factor reduction and degree of weight loss?. Eur Heart J Suppl 2005;7:21-6. http://dx.doi.org/10.1093/eurheartj/sui082.
- Blackburn G. Effect of degree of weight loss on health benefits. Obes Res 1995;3:211-16. http://dx.doi.org/10.1002/j.1550-8528.1995.tb00466.x.
- Nanchahal K, Power T, Holdsworth E, Hession M, Sorhaindo A, Griffiths U, et al. A pragmatic randomised controlled trial in primary care of the Camden Weight Loss (CAMWEL) programme. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000793.
- Jehn ML, Patt MR, Appel LJ, Miller ER. One year follow-up of overweight and obese hypertensive adults following intensive lifestyle therapy. J Hum Nutr Diet 2006;19:349-54. http://dx.doi.org/10.1111/j.1365-277X.2006.00711.x.
- Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792-8. http://dx.doi.org/10.1001/jama.289.14.1792.
- Managing Overweight and Obesity in Adults. London: National Institute for Health and Care Excellence; 2014.
- Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA 2014;312:1779-91. http://dx.doi.org/10.1001/jama.2014.14173.
- Brownell KD, Wadden TA. Etiology and treatment of obesity: understanding a serious, prevalent, and refractory disorder. J Consult Clin Psychol 1992;60:505-17. http://dx.doi.org/10.1037/0022-006X.60.4.505.
- Card A. Health Needs Assessment: Community-Based Weight Management Services and the Black and Minority Ethnic Community in Dartford Borough. Dartford: Dartford Borough Council; 2011.
- Murphree D. Patient attitudes toward physician treatment of obesity. J Fam Pract 1994;38:45-8.
- Tsai AG, Wadden TA, Pillitteri JL, Sembower MA, Gerlach KK, Kyle TK, et al. Disparities by ethnicity and socioeconomic status in the use of weight loss treatments. J Natl Med Assoc 2009;101:62-70. http://dx.doi.org/10.1016/S0027-9684(15)30813-0.
- Quality and Outcomes Framework (QOF) – 2013-14. London: Health and Social Care Information Centre; 2014.
- Action on Obesity: Comprehensive Care for All. London: Royal College of Physicians; 2013.
- Moore H, Summerbell CD, Greenwood DC, Tovey P, Griffiths J, Henderson M, et al. Improving management of obesity in primary care: cluster randomised trial. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7423.1085.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten Up randomised controlled trial. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d6500.
- Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485-92. http://dx.doi.org/10.1016/S0140-6736(11)61344-5.
- Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011;365:1969-79. http://dx.doi.org/10.1056/NEJMoa1109220.
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959-68. http://dx.doi.org/10.1056/NEJMoa1108660.
- Kumanyika SK, Fassbender JE, Sarwer DB, Phipps E, Allison KC, Localio R, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity 2012;20:1249-57. http://dx.doi.org/10.1038/oby.2011.329.
- McQuigg M, Brown J, Broom J, Laws RA, Reckless JP, Noble PA, et al. Empowering primary care to tackle the obesity epidemic: the Counterweight Programme. Eur J Clin Nutr 2005;59:93-100. http://dx.doi.org/10.1038/sj.ejcn.1602180.
- Counterweight Project Team . Evaluation of the Counterweight Programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract 2008;58:548-54. http://dx.doi.org/10.3399/bjgp08X319710.
- Booth HP, Prevost TA, Wright AJ, Gulliford MC. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract 2014;31:643-53. http://dx.doi.org/10.1093/fampra/cmu064.
- Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P. Behavioural Weight Management Review Group . Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obes Rev 2014;15:920-32. http://dx.doi.org/10.1111/obr.12220.
- Ard J. Obesity in the US: what is the best role for primary care?. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.g7846.
- Hajek P, Ayers S. Cambridge Handbook of Psychology, Health and Medicine. Cambridge: Cambridge University Press; 2007.
- Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol 1999;67:132-8. http://dx.doi.org/10.1037/0022-006X.67.1.132.
- Hajek P. Withdrawal-oriented therapy for smokers. Br J Addict 1989;84:591-8. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03474.x.
- West R, Edwards M, Hajek P. A randomized controlled trial of a “buddy” system to improve success at giving up smoking in general practice. Addiction 1998;93:1007-11. http://dx.doi.org/10.1046/j.1360-0443.1998.93710075.x.
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;31:1650-60. http://dx.doi.org/10.1016/j.addbeh.2005.12.014.
- Indices of Deprivation 2010. London: Department for Communities and Local Government; 2010.
- PH53 Overweight and Obese Adults – Lifestyle Weight Management: Guidance. London: National Institute for Health and Care Excellence; 2014.
- Your Weight, Your Health: Raising the Issue of Weight in Adults. London: Department of Health; 2006.
- Drink Swap: How to Cut Down on Calories in Drinks without Having to Say ‘No’. London: Department of Health; 2010.
- Portion Swap: How Smaller Plates and Portions Help Prevent us Eating too Many Calories. London: Department of Health; 2010.
- Snack Swap: How to stay Healthy Without Giving Up all Snacks. London: Department of Health; 2010.
- Walk 4 Life: Tips to Get Walking Every Day. London: Department of Health; 2010.
- The Eatwell Plate. London: Public Health England; 2013.
- NHS Choices . 5 a Day: What Counts? 2015. www.nhs.uk/Livewell/5ADAY/Pages/Whatcounts.aspx (accessed 2 August 2015).
- NHS Choices . Information Specific To: Orlistat 120mg Capsules When Used in Obesity 2014. www.nhs.uk/medicine-guides/pages/MedicineOverview.aspx?condition=Obesity&medicine=orlistat&preparation (accessed 2 August 2015).
- Hajek P, Humphrey K, McRobbie H. Using group support to complement a task-based weight management programme in multi-ethnic localities of high deprivation. Patient Educ Couns 2010;80:135-7. http://dx.doi.org/10.1016/j.pec.2009.10.017.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. http://dx.doi.org/10.1080/08870446.2010.540664.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. http://dx.doi.org/10.1249/01.MSS.0000078924.61453.FB.
- White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res 2002;10:107-14. http://dx.doi.org/10.1038/oby.2002.17.
- de Lauzon B, Romon M, Deschamps V, Lafay L, Borys J-M, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr 2004;134:2372-80.
- EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument. Rotterdam: The Euroqol Group; 2013.
- Barts Health NHS Trust . Good Clinical Practice n.d. http://bartshealth.nhs.uk/research/governance/compliance-with-the-regulations/good-clinical-practice/ (accessed 27 July 2016).
- Yanovski SZ, Bain RP, Williamson DF. Report of a National Institutes of Health – Centers for Disease Control and Prevention workshop on the feasibility of conducting a randomized clinical trial to estimate the long-term health effects of intentional weight loss in obese persons. Am J Clin Nutr 1999;69:366-72.
- Lane P. Handling drop-out in longitudinal clinical trials: a comparison of the LOCF and MMRM approaches. Pharm Stat 2008;7:93-106. http://dx.doi.org/10.1002/pst.267.
- Hutfless S, Gudzune KA, Maruthur N, Wilson RF, Bleich SN, Lau BD, et al. Strategies to prevent weight gain in adults: a systematic review. Am J Prev Med 2013;45:e41-51. http://dx.doi.org/10.1016/j.amepre.2013.07.013.
- Wood AM, White IR, Hillsdon M, Carpenter J. Comparison of imputation and modelling methods in the analysis of a physical activity trial with missing outcomes. Int J Epidemiol 2005;34:89-9. http://dx.doi.org/10.1093/ije/dyh297.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- Kahan BC, Morris TP. Assessing potential sources of clustering in individually randomised trials. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-58.
- Pals SL, Murray DM, Alfano CM, Shadish WR, Hannan PJ, Baker WL. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health 2008;98:1418-24. http://dx.doi.org/10.2105/AJPH.2007.127027.
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983-97. http://dx.doi.org/10.2307/2533558.
- Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med 2012;31:328-40. http://dx.doi.org/10.1002/sim.4431.
- Turner EL, Perel P, Clayton T, Edwards P, Hernández AV, Roberts I, et al. Covariate adjustment increased power in randomized controlled trials: an example in traumatic brain injury. J Clin Epidemiol 2012;65:474-81. http://dx.doi.org/10.1016/j.jclinepi.2011.08.012.
- Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-139.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. http://dx.doi.org/10.1002/sim.1981.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2012.
- McCormick B, Stone I. Corporate Analytical Team . Economic costs of obesity and the case for government intervention. Obes Rev 2007;8:161-4. http://dx.doi.org/10.1111/j.1467-789X.2007.00337.x.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- NHS Reference Costs 2011–12. London: Department of Health; 2012.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. http://dx.doi.org/10.1007/s11136-012-0322-4.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/article/pmg9/chapter/foreword (accessed 4 September 2015).
- Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health 2014;17:445-53. http://dx.doi.org/10.1016/j.jval.2014.04.002.
- Devlin N, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England 2016.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Hughes G. Transforming NHS ambulance services. Emerg Med J 2011;28.
- Claxton K, Palmer S, Longworth L, Bojke L, Griffin S, McKenna C, et al. Informing a decision framework for when NICE should recommend the use of health technologies only in the context of an appropriately designed programme of evidence development. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16460.
- Roberts K, Cavill N, Rutter H. Standard Evaluation Framework for Weight Management Interventions. London: Public Health England; 2009.
- May S, West R, Hajek P, McEwen A, McRobbie H. Randomized controlled trial of a social support (‘buddy’) intervention for smoking cessation. Patient Educ Couns 2006;64:235-41. http://dx.doi.org/10.1016/j.pec.2006.02.008.
- May S, West R, Hajek P, McEwen A, McRobbie H. Social support and success at stopping smoking. J Smok Cessat 2007;2:47-53. http://dx.doi.org/10.1375/jsc.2.2.47.
- Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes 2005;29:1153-67. http://dx.doi.org/10.1038/sj.ijo.0802982.
- Sebo P, Haller D, Pechère-Bertschi A, Bovier P, Herrmann F. Accuracy of doctors’ anthropometric measurements in general practice. Swiss Med Wkly 2015;145. http://dx.doi.org/10.4414/smw.2015.14115.
- Verweij LM, Terwee CB, Proper KI, Hulshof CT, van Mechelen W. Measurement error of waist circumference: gaps in knowledge. Public Health Nutr 2013;16:281-8. http://dx.doi.org/10.1017/S1368980012002741.
- Agarwal SK, Misra A, Aggarwal P, Bardia A, Goel R, Vikram NK, et al. Waist circumference measurement by site, posture, respiratory phase, and meal time: implications for methodology. Obesity 2009;17:1056-61. http://dx.doi.org/10.1038/oby.2008.635.
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42:878-84. http://dx.doi.org/10.1161/01.HYP.0000094221.86888.AE.
- Finley CE, Barlow CE, Greenway FL, Rock CL, Rolls BJ, Blair SN. Retention rates and weight loss in a commercial weight loss program. Int J Obes 2007;31:292-8. http://dx.doi.org/10.1038/sj.ijo.0803395.
- Stubbs RJ, Brogelli DJ, Pallister CJ, Whybrow S, Avery AJ, Lavin JH. Attendance and weight outcomes in 4754 adults referred over 6 months to a primary care/commercial weight management partnership scheme. Clin Obes 2012;2:6-14. http://dx.doi.org/10.1111/j.1758-8111.2012.00040.x.
- Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P. Behavioural Weight Management Review Group . Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta-analysis and meta-regression. Obes Rev 2014;15:598-609. http://dx.doi.org/10.1111/obr.12165.
- Brose LS, West R, McDermott MS, Fidler JA, Croghan E, McEwen A. What makes for an effective stop-smoking service?. Thorax 2011;66:924-6. http://dx.doi.org/10.1136/thoraxjnl-2011-200251.
- Judge K, Bauld L, Chesterman J, Ferguson J. The English smoking treatment services: short-term outcomes. Addiction 2005;100:46-58. http://dx.doi.org/10.1111/j.1360-0443.2005.01027.x.
- Bennett WL, Gudzune KA, Appel LJ, Clark JM. Insights from the POWER practice-based weight loss trial: a focus group study on the PCP’s role in weight management. J Gen Intern Med 2014;29:50-8. http://dx.doi.org/10.1007/s11606-013-2562-6.
- Blackburn M, Stathi A, Keogh E, Eccleston C. Raising the topic of weight in general practice: perspectives of GPs and primary care nurses. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2015-008546.
- Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2014-006642.
- Waterlander W, Whittaker R, McRobbie H, Dorey E, Ball K, Maddison R, et al. Development of an evidence-based mHealth weight management program using a formative research process. JMIR Mhealth Uhealth 2014;2. http://dx.doi.org/10.2196/mhealth.2850.
- Ni Mhurchu C, Whittaker R, McRobbie H, Ball K, Crawford D, Michie J, et al. Feasibility, acceptability and potential effectiveness of a mobile health (mHealth) weight management programme for New Zealand adults. BMC Obes 2014;1. http://dx.doi.org/10.1186/2052-9538-1-10.
Appendix 1 Use of health services questionnaire
Appendix 2 Questionnaire scoring
Food Knowledge Assessment score
The Food Knowledge Assessment score is scored on an 11-point scale (range 0–10), with higher scores indicating more knowledge. It contains 10 questions and each question is scored either 0 or 1. The overall score is calculated by summing the scores of the individual questions.
The scores for the individual questions are shown in Table 41. Each question has four possible answers (a, b, c or d); the table indicates which of the four answers results in a score of 1 (all other answers result in a score of 0).
| Question | Score = 1, if answer is: |
|---|---|
| 1 | A |
| 2 | A |
| 3 | C |
| 4 | B |
| 5 | D |
| 6 | B |
| 7 | C |
| 8 | B |
| 9 | B |
| 10 | A |
Food Craving Inventory score
Each of the five food types (fatty foods, carbohydrates and starches, sweet foods, savoury snacks and fruit) is assigned a score from 0 to 5 on both frequency and urge of craving. The frequency domain is then calculated by summing the scores of the individual questions related to frequency; the strength domain is calculated in a similar manner. The overall scores from both domains range from 0 to 25, with higher scores indicating more frequent or stronger urges.
International Physical Activity Questionnaire
Metabolic-equivalent minutes/week domain
This score represents the total MET-minutes/week, and is expressed on a continuous scale with a minimum score of 0. It is calculated as:
Sitting domain
This score represents the number of minutes per day spent sitting. It is calculated directly from question 4.
Three-Factor Eating Questionnaire
The Three-Factor Eating Questionnaire contains 18 questions, each of which is scored from 1 to 4, with higher values indicating a higher level of the behaviour. Domain scores (cognitive restraint, uncontrolled eating and emotional eating) are calculated as the mean of all the questions within a domain.
Table 42 indicates which questions are included in which domain.
| Domain | Questions included in domain |
|---|---|
| Cognitive restraint | 2, 11, 12, 15, 16, 18 |
| Uncontrolled eating | 1, 4, 5, 7–9, 13, 14, 17 |
| Emotional eating | 3, 6, 10 |
Table 43 indicates how each question is scored.
| Question | Scoring system |
|---|---|
| 1–13 | Definitely true = 4 Mostly true = 3 Mostly false = 2 Definitely false = 1 |
| 14 | Almost always = 4 Often between meals = 3 Sometimes between meals = 2 Only at meal times = 1 |
| 15 | Almost always = 4 Usually = 3 Seldom = 2 Almost never = 1 |
| 16 | Very likely = 4 Moderately likely = 3 Slightly likely = 2 Unlikely = 1 |
| 17 | At least once a week likely = 4 Sometimes likely = 3 Rarely likely = 2 Never = 1 |
| 18 | Answer 7–8 = 4 Answer 5–6 = 3 Answer 3–4 = 2 Answer 1–2 = 1 |
Appendix 3 Participant feedback questionnaire
Appendix 4 Statistical analysis plan
List of abbreviations
- AE
- adverse event
- BMI
- body mass index
- BOCF
- baseline observation carried forward
- CI
- confidence interval
- EQ-5D-5L
- European Quality of Life-5 Dimensions-5 Levels
- GCP
- good clinical practice
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MET
- metabolic equivalent
- NICE
- National Institute for Health and Care Excellence
- PCTU
- Pragmatic Clinical Trials Unit
- QA
- quality assurance
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SSS
- Stop Smoking Service
- SWAP
- Peer-Support Weight Action Programme
- WAP
- Weight Action Programme