Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/129/61. The contractual start date was in May 2013. The draft report began editorial review in January 2016 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by McDermott et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Burden of disease
Cardiovascular disease (CVD) accounts for more than one-quarter of all deaths in the UK, with about 155,000 deaths per year. 1 Diabetes mellitus is increasing in frequency and now affects around 6.2% of the UK population2 and the importance of early detection and treatment of chronic kidney disease is increasingly recognised. 3,4 Dementia shares many risk factors for CVD5 and might be reduced through more effective cardiovascular prevention. The British Heart Foundation estimates that the cost to the UK of premature death, lost productivity, hospital treatment and prescriptions relating to CVD is approximately £19B each year. Health-care costs alone may account for £8B per year. 1 The cost of informal care for people with CVD in the UK was around £3.8B in 2009. 6
Inequalities and cardiovascular risk
There are substantial social inequalities in the distribution of CVD. During 2001–3, CVD mortality was 2.8 times higher for men in routine occupations than for men in higher managerial and professional occupations; for women, mortality was 3.8 times higher for routine occupations than for managerial and professional occupations. 6 Mortality from CVDs has been declining but gains in life expectancy have not been equally shared by all groups; deprived communities have generally shown smaller mortality reductions than more affluent areas. There is also ethnic patterning of risk, with diabetes mellitus being more frequent in people of African, Caribbean and South Asian origins, stroke being more frequent among those of African origin and coronary heart disease being more frequent among those of African origin.
The major risk factors for CVD are well characterised. An analysis of the burden of disease for the UK in 20107 revealed that smoking, high blood pressure, overweight and obesity, low levels of physical activity, poor diet and elevated cholesterol accounted for the highest proportion of burden of disease measured in disability-adjusted life-years.
The NHS Health Check programme
The NHS Health Check programme is a cardiovascular risk assessment programme, which was introduced by the Department of Health in 2009. 8 The programme aims to identify people who are at increased risk of heart disease, stroke, diabetes mellitus or chronic kidney disease, with the intention of delivering individualised interventions to reduce risk, and enable treatment of people with established disease. The Department of Health estimated that the NHS Health Check programme could potentially prevent 2000 deaths and 9500 non-fatal myocardial infarctions and strokes each year. 8 Maximising the uptake of health checks across all groups is important in realising this aim and ensuring that the programme does not perpetuate existing health inequalities.
Programme implementation
The NHS Health Check programme has been rolled out across England. 9 The first full year of the programme began in April 2011, but in many areas the programme was initiated from April 2010 or before. From 2011/12, the NHS Health Check programme aimed to enrol 90% of the eligible population into a 5-yearly cycle of call–recall through the participation of all primary care organisations (PCOs). PCOs invite 18% of their eligible cohort each year, with about 1.8 million individuals receiving an invitation to a health check in 2011/12. 9 Since 2013, the NHS Health Check programme has been a responsibility of local authorities,10 supported by Public Health England (PHE), the National Institute for Health and Care Excellence (NICE) and the Local Government Association. Implementation is through commissioned services from NHS clinical commissioning groups and other providers. Implementation of health checks is a key indicator in the Public Health Outcomes Framework in domain 2, health improvement. 11
Eligibility for the NHS Health Check programme
Adults aged 40–74 years are eligible to be offered health checks. People who have previously been diagnosed with clinical disease (including ischaemic heart disease, heart failure, atrial fibrillation, stroke or transient ischaemic attack, diabetes mellitus, chronic kidney disease, peripheral vascular disease) are excluded, as are people being treated for increased vascular risk (including those with hypertension or hypercholesterolaemia or who are being treated with antihypertensive drugs or statins). 1,6
Health check process
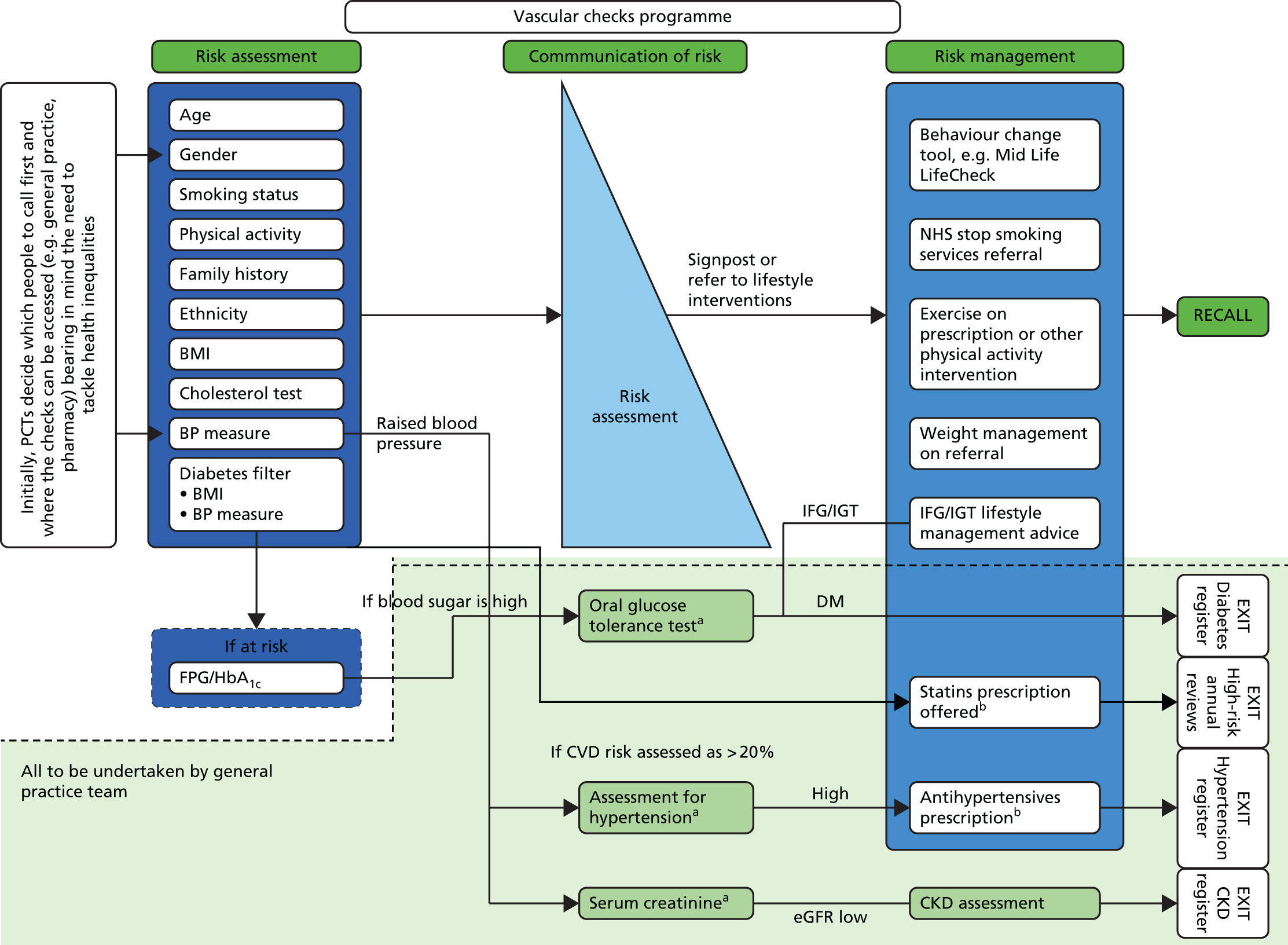
Each NHS health check consists of recording personal history, including age, ethnic group, smoking status, family history, assessment of physical activity, dietary quality including fruit and vegetable and salt intake, body mass index (BMI), blood pressure, smoking status, renal function, lipid levels and blood glucose when indicated. The patient’s risk of developing CVD is then calculated using a CVD risk score calculator, which may include the Joint British Societies (JBS)12 calculator or the QRISK®2 score. 13 During the period of this trial, the JBS calculator was mandated by the Health Check programme in the study area. The cardiovascular risk assessment implemented as part of the health check is used to inform graded intervention. 14 Individuals whose risk of a cardiovascular event is > 20% over 10 years are classified as ‘high risk’ and exit the Health Check programme to enter a high-risk register with a designated care pathway. Individuals who are identified as having clinical disease, such as diabetes mellitus or atrial fibrillation, also enter appropriate care pathways based in primary care. Other individuals, especially those with a risk of a cardiovascular event of 10–20%, are offered advice on reducing risk, or maintaining low risk, primarily through lifestyle advice. The individual elements of the health check intervention follow recognised and evidenced-based clinical pathways approved by NICE to improve outcomes for individual patients15 (Figure 1).
FIGURE 1.
Schematic diagram of the health check. Reproduced from Putting Prevention First. NHS Health Check: Vascular Risk Assessment. Best Practice Guidance. 8 Contains public sector information licensed under the Open Government Licence v3.0. BP, blood pressure; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; PCT, primary care trust. a, People recalled to separate appointments for diagnosis; b, professionals with suitable patient information and prescribing rights.
Evidence of effectiveness
It is beyond the scope of this report to review the evidence for or against a national programme of health checks, nor do we aim to review the criteria to be satisfied that a programme of health checks is effective or discuss the appropriate balance between ‘population’ and ‘high-risk’ strategies for disease prevention. The purpose of this research was to evaluate methods for improving the delivery of an established policy of health checks.
Randomised controlled trials (RCTs) from earlier decades, when arguably fewer effective interventions were routinely available, are not supportive of cardiovascular risk screening. 16 In a Cochrane systematic review, Krogsbøll et al. 16 identified 16 trials of health checks in unselected adults, with 155,899 participants and 11,940 deaths. Risk ratios for total and cardiovascular mortality were 0.99 [95% confidence interval (CI) 0.95 to 1.03] and 1.03 (95% CI 0.91 to 1.17), respectively. The review concluded that general health checks were unlikely to be effective. Twelve of the 16 studies were from 1982 or before and this represents an important limitation because older studies were less likely to use current methods of risk assessment and risk management. However, more recent studies, including the Oxford and Collaborators Health CHECK (OXCHECK)17 and British Family Heart18 studies, suggest that health checks in primary care are unlikely to provide a cost-effective approach to cardiovascular prevention. Other arguments19 against the use of health checks in their current form include the inefficiency of using predictive risk scores to allocate treatments,20 the small effects resulting from risk factor intervention and the high costs of the programme. 19
The Health Check programme is consistent with the vision, proposed in the Wanless report,21 of a health service that ‘invest[s] in reducing demand by enhancing the promotion of good health and disease prevention’ (p. 3), with ‘health services evolving from dealing with acute problems through more effective control of chronic conditions to promoting the maintenance of good health’ (p. 10). This is even more relevant at the present time with growing problems of obesity, pre-diabetes, alcohol and physical inactivity. The Health Check programme offers opportunities to address health problems of obesity, initiate diabetes prevention interventions and identify lifestyle concerns, as well as detecting other conditions including atrial fibrillation or cognitive decline. There may be insufficient evidence to reach clear conclusions concerning the value of the Health Check programme. There is evidence for the effectiveness of the individual interventions that may be delivered through health checks, as summarised in guidance from NICE,3,15,22,23 but the question remains whether the Health Check programme can be used to improve implementation of this guidance into practice.
Economic modelling for cost-effectiveness estimates
The case for implementing health checks was made through health economic modelling. The Department of Health’s economic model24 provided evidence to show that the costs of the NHS Health Check programme would be between £180M and £243M per year (2008 costs). The cost of the health checks was estimated to be about £40M, with additional treatment costs accounting for the remainder. Health benefits were estimated to be substantial and the intervention was judged to be cost-effective, with a cost of < £3000 per quality-adjusted life-year. 24
The economic model incorporated a range of assumptions concerning population engagement with the programme and the likely effectiveness of interventions in the context of the present quality of care. The present research focuses on one key assumption, the uptake of health checks. The Department of Health model assumed that overall uptake of the health check would be about 75%; 70% of individuals were assumed to always attend, 15% might never attend and 15% might have a 33% probability of attending. These assumptions were informed by data on the uptake of the national breast screening programme. Women are generally more likely than men to seek help and engage with health services.
Uptake of the NHS Health Check programme
At the time that this trial was initiated in 2012, early indications were that uptake of health checks was lower than anticipated. Dalton et al. 25 reported a 45% uptake of health checks in west London. This was consistent with experience in south London, where uptake was running at < 40%. In national data for English PCOs in the third quarter of 2011/12,9 the median uptake was 52%, with values in different PCOs ranging from 0% to 100%, with an interquartile range (IQR) of 35–67%. Only 30 (20%) out of 151 PCOs had an uptake of ≥ 75%. 9 At that stage of the implementation of the NHS Health Check programme, 80% of PCOs were reporting that uptake of the checks was lower than expected based on the health economic model. In more recent cumulative data for 2013–15, it was reported that 48.4% of people offered a NHS health check received a check. Uptake ranged from 21% to 100% in different local authority areas, with 143 out of 161 (89%) local authorities failing to achieve the lowered target of 66% uptake (Figure 2).
Evaluations of the roll-out of health checks
Local evaluations have confirmed a pattern of low uptake of health checks. Dalton et al. 25 reported on the uptake of health checks in Ealing, a deprived and culturally diverse setting in London, with an estimated uptake of 44.8%. Attendance was found to be significantly lower among younger patients and smokers, consistent with the later findings of Artac et al. 27 Uptake was significantly higher for those with a South Asian background (53.0%) or a mixed ethnic background (57.8%), those with hypertension and those from smaller general practices. 28
A 3-year observational cohort study conducted by Robson et al. 29 evaluated the implementation of the NHS Health Check programme among general practices in East London serving an ethnically diverse and socially disadvantaged population. Coverage in this period ranged from 33.9% in the first year to 73.4% in year 3. Older people were more likely to attend than younger people. Attendance was similar across deprivation quintiles and was in accordance with population distributions of black African/Caribbean, South Asian and white ethnic groups. One in 10 attendees had a high CVD risk (≥ 20% 10-year risk). In the two PCOs stratifying risk, 14.3% and 9.4% of attendees had a high CVD risk compared with 8.6% in the PCO using an unselected invitation strategy. 29
In a previously reported study,30 which is discussed further in Chapter 7, we evaluated some of the influences on an individual’s decision to take up the offer of a health check. We identified several issues that contribute to the low uptake of checks. There is a lack of public awareness of the Health Check programme. This may have arisen from the decision not to mount a national communications campaign to promote the Health Check programme from the outset. Beliefs about susceptibility may influence uptake if individuals believe that they have a healthy lifestyle or are free from symptoms and do not have other chronic conditions. Practical difficulties of gaining access to appointments for a blood test or a health check may represent a significant barrier to accessing a check for patients at some practices. Finally, receiving advice to change lifestyle behaviours may be unwelcome to some patients. This is consistent with the low uptake of health checks observed among smokers. 31
Evidence regarding effective interventions to increase the uptake of health checks or screening
Health checks have similarities with other population-based screening programmes. Both invite individuals who believe themselves to be healthy to undergo a risk assessment procedure that may result in the attendee discovering that they have a serious health problem or are at high risk of a serious health problem in the future. The literature on interventions to increase screening uptake may reasonably be applied to the uptake of health checks.
Interventions to increase uptake can have a number of foci: changes to the method of delivering the programme, different patterns of invitation letters and reminders or provision of additional information at the time of invitation. Camilloni et al. 32 recently updated the landmark review by Jepson et al. ,33 focusing on methods to increase uptake of screening for breast, cervical or colorectal cancer. The review suggested that postal reminders in addition to the initial invitation could significantly increase uptake, whereas the evidence for telephone reminders was largely favourable, although some primary studies did not demonstrate significant effects. The review identified one trial which found that patients were more likely to attend if they were invited by telephone rather than by letter. In the context of health checks, an observational study found significantly higher uptake at practices using telephone or verbal health check invitations, either singly or in combination with letters. 34 Telephone or face-to-face invitations to promote health check uptake may be difficult to implement on a large scale, as would be required for the national Health Check programme.
In the cancer screening literature, providing a stated appointment time led to significantly higher uptake of screening than open appointments for cervical [relative risk (RR) 1.49, 95% CI 1.27 to 1.75] and breast (RR 1.26, 95% CI 1.02 to 1.75) cancer screening. 32 Kumar et al. 35 compared health check uptake by patients offered only a booked appointment with uptake by patients offered a choice of a booked appointment or attending a drop-in clinic. Uptake rates for the two groups separately are not reported, but the authors suggested that use of drop-in clinics may be less costly. Norman et al. 36 found a 70% attendance rate for an invitation letter with a given appointment time compared with 37% for an open letter invite.
Evidence of factors that may be associated with improved uptake of health checks was reviewed by Cooper and Dugdill. 37 They identified from existing studies, deemed transferable to a UK context, the key factors influencing the uptake of health screening, including demographic, social, cultural and psychological influences. Demographic and cultural factors that affect uptake may not be modifiable, whereas psychological factors may be more amenable to change. In cancer screening, the impact of providing additional information, such as leaflets or pamphlets, was mixed. 32 The reviewers noted that the interventions assessed were perhaps not entirely comparable. Different health conditions, and different types of screening test [e.g. the self-completed faecal occult blood test (FOBT) for colorectal cancer vs. attending a mammography appointment at a hospital], may additionally account for variation in results. We next consider interventions targeting psychological factors to increase health check uptake.
One useful intervention may be providing invitees with planning prompts, asking them to form concrete plans about when, where or how to perform a behaviour. Such prompts can help motivated individuals be more likely to act on their intentions. Sallis et al. (cited in Perry et al. 38) found that an enhanced health check invitation letter, which included a tear-off slip for individuals to write the date and time of their health check, led to a 33% uptake of health checks in Medway, compared with 29% uptake for individuals receiving the original, control invitation letter. Although this is a small absolute increase, it was achieved using minimal extra resource. Planning prompts are not always effective. For example, they have failed to increase the uptake of colorectal cancer screening among first-time invitees39 or antenatal screening. 40 In field settings, a substantial proportion of participants asked to do so may not record a plan. 41 Moreover, plan formation has been shown to be most effective for individuals who are already motivated to perform the behaviour,42 for example those who accepted a previous round of colorectal cancer screening. 43
An alternative brief intervention that may be useful to increase uptake was demonstrated by Conner et al. ,44 who reported a study conducted in one general practice in 1991. Sending a preliminary questionnaire prior to inviting individuals for a health check enhanced uptake, with 68.3% of the intervention group having a health check compared with 53.5% of the control (no questionnaire) group. This increase in uptake was attributed to the question–behaviour effect (QBE), a phenomenon in which asking questions about people’s views on a behaviour or their current behaviour increases the likelihood that individuals will later perform that behaviour. The potential of the QBE to increase uptake was also demonstrated in a study focusing on cervical cancer screening uptake. 45 Uptake increased from 21% in the control group to 26% in the two experimental questionnaire groups, with those in one of the groups being asked to complete additional questions about whether or not they anticipated regretting not being screened. Among individuals who returned the questionnaire in these two experimental groups, those who had been asked about anticipated regret had a higher screening uptake (65%) than those in the other questionnaire group (44%). Taken together, these two studies suggest that the QBE might be a useful intervention to increase NHS health check uptake. Subsequent to the initiation of the present study, a further trial reported no significant QBE on the uptake of colorectal cancer screening via a FOBT in Scotland. 46
How does the question–behaviour effect work?
Psychologists have long been aware that asking questions about a behaviour may change the respondent’s future behaviour. 47 A number of mechanisms have been proposed for the operation of the QBE. 48 The attitude accessibility account suggests that asking people to report their attitudes or intentions for a behaviour makes the attitude about that behaviour more accessible in memory. The increased accessibility makes it more likely that a person will perform the behaviour (e.g. have a health check) when the opportunity arises.
A second explanation concerns cognitive dissonance. Cognitive dissonance is a mental state that occurs when a person’s behaviour is not consistent with his or her beliefs about how he or she should behave. Experiencing cognitive dissonance is uncomfortable and so individuals are motivated to try to reduce it. In terms of the QBE, cognitive dissonance can arise when completing a questionnaire leads individuals to realise that their current or past actions are incompatible with their beliefs about how they should act. To reduce the cognitive dissonance aroused by completing a questionnaire, people may subsequently change their behaviour to be more in line with their beliefs.
A final explanation for the QBE concerns behavioural simulation and processing fluency. In this account, the QBE is driven by questioning, leading individuals to form behavioural scripts, that is, mental representations of how to carry out that behaviour. These scripts are stored in memory and can be reactivated when the individual encounters an opportunity to perform the relevant behaviour. This reactivation of the mental representation makes it seem easier for the person to perform that behaviour than it would otherwise, which then increases the likelihood that he or she proceeds to perform the behaviour in question.
Recent systematic review and other evidence regarding the question–behaviour effect
The QBE has been tested in a wide range of behaviours, including not only health-related behaviours but also consumer and prosocial behaviours. Dholakia et al. 49 provided an overview of the literature but did not subject it to meta-analysis. Subsequent to the initiation of this project, two systematic reviews of the QBE with meta-analyses have been published. 50,51 The two differ in their inclusion criteria and aims and so both are discussed here.
Wood et al. 50 reviewed literature published up to March 2013. They set out to examine the impact of asking intention or self-prediction (i.e. rating the likelihood that one will perform a behaviour) questions on subsequent behaviour. Intentions are a key component of the theory of planned behaviour (TPB),52 a psychological model which states that behaviour is determined by an individual’s behavioural intention and perceived behavioural control (PBC). Intentions reflect the individual’s motivation to engage in a particular behaviour. PBC is very similar to the concept of self-efficacy, concerning the extent to which people perceive that they have control and ability to engage in the behaviour. Intentions, in turn, are determined by an individual’s attitude towards the behaviour (whether the outcomes of performing the behaviour are considered positive or negative), subjective norms (e.g. perceptions of whether or not others think one should engage in a behaviour) and PBC. Therefore, the review by Wood et al. 50 examined whether including questionnaire items relating to other TPB variables, such as attitudes, subjective norms or PBC, or including additional questionnaire items about anticipated regret altered the effect of intention or self-prediction questions on behaviour. This review also attempted to explore the mechanisms by which the QBE might operate by examining the effect of different study characteristics on effect sizes. The measure of effect size used was the standardised mean difference, Cohen’s d.
Overall, this review included 116 tests of the QBE. It found a small, statistically significant positive effect on behaviour of asking intention or self-prediction questions (pooled effect size, d+ = 0.24, 95% CI 0.18 to 0.30). There was evidence of publication bias, with a disproportionate concentration of studies with larger effect sizes and larger standard errors. There was also evidence of significant heterogeneity in effect sizes. The effects for health (d+ = 0.29), consumer (d+ = 0.34) and prosocial (d+ = 0.19) behaviours were significantly larger than those for risky or undesirable behaviours (d+ = –0.05, 95% CI –0.23 to 0.13). Larger effect sizes were observed for behaviours that the reviewers rated as easier to perform and more socially desirable.
A number of methodological factors were also significantly related to observed effect sizes. In particular, smaller effects were observed for studies conducted in field rather than laboratory settings (d+ = 0.17 vs. d+ = 0.38). The longer the time interval between answering questions and the measurement of the behaviour, the smaller the effect size tended to be. Providing an incentive for study participation was associated with a larger effect size (d+ = 0.36) than not doing so (d+ = 0.19).
The types of question asked also influenced the observed effects on behaviour. Studies that asked only self-prediction questions (‘How likely is it that you will . . .’) reported significantly larger effects (d+ = 0.29) than studies that asked a mix of self-prediction and intention questions (d+ = 0.14). Measuring TPB constructs other than intentions did not significantly influence effect sizes. Asking anticipated regret items was associated with smaller effects on behaviour (d+ = 0.08) than not doing so (d+ = 0.26).
In contrast to Wood et al. ’s50 review, that of Rodrigues et al. 51 focused only on studies published up to December 2012 examining the QBE on health behaviours. Also, in contrast to Wood et al. ’s50 focus on intention and self-prediction questions, Rodrigues et al. 51 included studies that measured cognitions, behaviours or a mix of cognitions and behaviours, as long as the effects of the questionnaire condition(s) were contrasted with the effects of a no-measurement condition. The review also aimed to examine the impact of study methodological features on observed effects.
The results were based on 38 papers reporting 41 studies of the QBE. The overall effect size was statistically significant but small [standardised mean difference (SMD) 0.09, 95% CI 0.04 to 0.13], with moderate heterogeneity. Again, there was significant evidence of publication bias. Moreover, there was considerable risk of bias when assessed using the Cochrane Collaboration tool. 53 There was no significant effect of risk of bias on observed effects. Effect sizes varied by behaviour type, with the largest for physical activity (SMD 0.20) and the smallest for drinking (SMD 0.04). Most relevant to health checks, the SMD for screening was 0.06 (95% CI 0.003 to 0.12). The review concluded by calling for future QBE trials to be preregistered, to focus on reducing risk of bias and to provide detailed descriptions of the procedures in each trial arm.
Financial incentives to increase questionnaire return rates
Financial incentives for questionnaire return are known to increase response rates. A systematic review including 94 trials with a pooled total of 160,004 participants found that the odds of returning a postal questionnaire were considerably increased if a financial incentive was offered [odds ratio (OR) 1.87, 95% CI 1.73 to 2.04]. 54 As the QBE is greater among individuals who return a questionnaire,44 incentivising questionnaire return may increase the size of any effect of a questionnaire on uptake of health checks. A meta-analysis of 85,671 participants in 88 randomised trials of financial incentives to increase response rates for mailed questionnaires reported that there was a significant increase in response rates for incentives up to the value of $5. 55 There is strong evidence to suggest that the offer of a financial incentive may increase the rate of return of the QBE questionnaire.
What is the potential impact of the question–behaviour effect on socioeconomic inequalities in uptake?
Death rates from coronary heart disease are highest in areas of greatest deprivation,6 so considering socioeconomic inequalities in the evaluation of any intervention to increase the uptake of NHS health checks is important. Although evidence suggests that enhanced invitation methods such as a QBE-based questionnaire increase the uptake of screening and the performance of health-related behaviours, we do not know their impact on NHS Health Check, a relatively new programme. Theoretical arguments suggest that uptake inequality might be either reduced or increased.
One argument is that it may be more difficult for those experiencing higher levels of deprivation to convert their positive attitudes and intentions with regard to health checks into action. The QBE may increase the cognitive accessibility of attitudes towards the behaviour, thereby increasing the likelihood that the behaviour is performed. This increased cognitive accessibility may make it easier for people experiencing more socioeconomic deprivation to find an opportunity to act on their intentions, thereby increasing health check uptake.
Another argument is that the strength of the QBE is affected by individuals’ beliefs about the behaviour in question, being stronger for individuals who hold positive attitudes to, and intentions for, the behaviour. 44 The extent to which any socioeconomic inequality in health check uptake may be the result of more socioeconomically deprived individuals having more negative views of health checks than less deprived individuals is unclear. If socioeconomic deprivation is associated with fewer perceived benefits of and greater perceived barriers to uptake, as it is for cancer screening, then an intervention using the QBE may increase uptake inequality. 56
How might offering an incentive for questionnaire return affect the social patterning of responses to the question–behaviour effect?
The offer of a financial incentive may increase questionnaire return rates only among those with already positive attitudes towards health checks, in which case it would result in increased uptake. If the offer of a financial incentive increases questionnaire return rates among those with less positive attitudes, the incentive is likely to have less of an impact on uptake. There is little research examining how and if incentives influence uptake of screening differentially across different levels of deprivation. 57 The offer of a financial incentive may be most attractive for individuals who are experiencing deprivation and so may increase the strength of the QBE on health check uptake particularly in individuals from deprived backgrounds.
According to a cognitive dissonance explanation of the QBE an incentive may backfire and not result in an increase in attendance. This argument suggests that having an incentive gives respondents a reason for completing questions and reduces the cognitive dissonance that might be experienced. It is the dissonance that drives the behaviour and removing it reduces the impact on behaviour. Such an effect has been suggested in studies in progress on bowel cancer screening and cervical cancer screening (Professor Mark Conner, University of Leeds, 2015, personal communication). It will be important to consider inequalities in uptake in any investigation of the impact of the QBE, with or without the provision of an incentive for questionnaire return, on the uptake of health checks.
Will informed choice be evaluated?
The concept of informed choice has received considerable attention in the context of the offer of screening tests and is relevant here even though NHS health checks are offered as a clinical service, not as a screening programme. Marteau et al. 58 defined an informed choice as an action ‘based on relevant knowledge, consistent with the decision-maker’s values’. Marteau and Kinmonth59 argued that, in the context of cardiovascular screening, participation on the basis of informed choice might encourage the participation of individuals who were more motivated to reduce their level of risk. This might have the unwanted consequence of increasing inequalities in cardiovascular risk. 59 These hypotheses were not supported by the results of a study of diabetes mellitus screening in which individuals’ knowledge, and the receipt of an invitation that promoted informed choice, were only weakly associated with screening attendance. 60 In the context of the present study, we believe that it will not be feasible to evaluate whether the uptake of NHS health checks is adequately informed. Distributing questionnaires to assess informed choice in the no-questionnaire control condition would obviously contaminate the control condition. Including the questionnaire items to measure knowledge of health checks and their potential outcomes, which would be required to assess informed choice, would increase questionnaire length and potentially dilute the QBE in those allocated to questionnaire conditions. Questions of informed choice and NHS health checks are left for a future study.
Uptake patterns
As previously explained, the NHS Health Check programme in England aims to identify people at risk of developing preventable illness, including heart disease, stroke, diabetes mellitus and kidney disease. Any individual between the ages of 40 and 74 years without an existing chronic condition should be invited for a health check once every 5 years.
Initial modelling of the cost-effectiveness of the programme was based on a 75% uptake. Since implementation, uptake of the health checks remains below the national target. Research on NHS health checks has identified some patterns in uptake that are often observed in screening programmes. These include lower uptake rates in men, people at the younger end of the target age range and people with better health profiles. 37 Associations between deprivation and uptake have been less consistent. Higher deprivation has been linked with lower uptake,61 which is consistent with evidence from other screening programmes,16 whereas some studies have reported higher uptake in more deprived areas27 or no relationship. 25 In the same trial,25 the proportion of health checks and demographic characteristics were compared between patients who received a postal invitation and those whose health checks were performed opportunistically.
Research objectives
The aim of this research was to determine whether enhanced invitation methods, using the QBE, lead to increased uptake of NHS health checks. The project aimed to rapidly implement a RCT to generate evidence in the short term to inform decision-making in the NHS.
The specific objective of this research was to implement a RCT using individual participants who are eligible for NHS health checks as the unit of allocation. The trial compared the effects of (1) standard invitation only, (2) a QBE questionnaire followed by a standard invitation 1 week later and (3) a QBE questionnaire with an offer of a retail voucher as an incentive for questionnaire completion followed by a standard invitation 1 week later.
The intervention effect was evaluated using the primary outcome of whether or not each individual completed their NHS health check within 182 days (6 months) of the standard invitation being sent.
The research also evaluated the feasibility of a rapid trial using electronic health records, with an automated randomisation procedure embedded into the Health Check programme management information system.
We also conducted a cohort study of all health checks conducted during the study period at general practices participating in the trial with the aim of comparing the characteristics of participants receiving invited health checks with the characteristics of participants receiving ‘opportunistic’ health checks.
We also conducted a qualitative interview study with the aim of evaluating the views of health-care professionals and patients concerning the uptake of health checks to identify factors that influence uptake and response to the trial interventions.
Context
This research was conducted in the inner London boroughs of Lambeth and Lewisham. The age structure of the resident population at the 2011 census is shown in Figure 3. It can be seen that both boroughs have a strikingly young population, with a low proportion of older adults. Table 1 shows the distribution of the population in the age range 40–74 years, which is the group eligible for NHS health checks. This age range accounts for only about 30% of the total population in these boroughs, with only some 7% or 8% of the total population being in the age range 60–74 years.
| Age group (years) | Lambeth | Lewisham | ||
|---|---|---|---|---|
| Population | % | Population | % | |
| ≤ 40 | 199,929 | 66.0 | 170,288 | 61.7 |
| 40–44 | 23,088 | 7.6 | 21,919 | 7.9 |
| 45–49 | 20,897 | 6.9 | 20,481 | 7.4 |
| 50–54 | 15,601 | 5.1 | 15,750 | 5.7 |
| 55–59 | 11,372 | 3.8 | 11,523 | 4.2 |
| 60–64 | 9012 | 3.0 | 9789 | 3.5 |
| 65–69 | 6623 | 2.2 | 7284 | 2.6 |
| 70–74 | 5994 | 2.0 | 6357 | 2.3 |
| ≥ 75 | 10,570 | 3.5 | 12,494 | 4.5 |
| Total | 303,086 | 275,885 | ||
At study initiation, Lambeth was the 22nd most deprived local authority in England and Lewisham was the 26th most deprived. 64 Both boroughs have large ethnic minority populations, with black African and black Caribbean groups being the largest minority groups (Table 2). The black African population generally has a higher proportion with a university education than the local white population.
| Ethnicity | Lambeth | Lewisham | London | England | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | Frequency | % | |
| White | 173,025 | 57 | 147,686 | 54 | 4,887,435 | 60 | 45,281,142 | 85 |
| Mixed | 23,160 | 8 | 20,472 | 7 | 405,279 | 5 | 1,192,879 | 2 |
| Asian | 20,938 | 7 | 25,534 | 9 | 1,511,546 | 18 | 4,143,403 | 8 |
| Black African | 35,187 | 12 | 32,025 | 12 | 573,931 | 7 | 977,741 | 2 |
| Black Caribbean/black other | 43,355 | 14 | 42,917 | 16 | 514,709 | 6 | 868,873 | 2 |
| Other | 7421 | 2 | 7251 | 3 | 281,041 | 3 | 548,418 | 1 |
| Total | 303,086 | 100 | 275,885 | 100 | 8,173,941 | 100 | 53,012,456 | 100 |
The Health Check programme in the two boroughs utilises a call–recall system. This is operated through the offices of the primary care shared services but is supported by a private sector company called Quality Medical Solutions (QMS) Ltd (Bournemouth, UK). QMS has developed a software system, known as Health Check Focus, that links the general practices in the boroughs to primary care shared services to enable invitations to health checks to be organised. Standard invitations are sent out using a slightly modified version of the national invitation template. The operation of this system is discussed further in Chapter 2. Patients who receive a standard invitation to a health check are offered a choice of making an appointment with their general practice or attending for a check at a local pharmacy. In general, a two-stage process is followed, with a blood test followed by a health check appointment. Most general practices offer opportunistic health checks in addition to any health checks carried out in patients who respond to a standard invitation letter. Both boroughs also commission outreach teams to conduct health checks opportunistically among high-risk groups. About 25% of health checks in Lewisham and 20% in Lambeth are conducted by third-party providers, with pharmacy-based checks accounting for the majority and outreach teams accounting for < 5% of all health checks. Results of third-party checks are communicated to general practices through the Health Check Focus software. The ratio of invited to opportunistic health checks is discussed further in Chapter 6.
Chapter 2 Methods
The protocol for the trial has been reported previously. 65 The trial was registered on 21 March 2013 (trial registration number ISRCTN42856343).
Trial design
This was a three-arm superiority RCT with equal allocation to each arm (Figure 4). The trial interventions consisted of (1) standard invitation only to a NHS health check, (2) a QBE questionnaire followed by a standard invitation and (3) a QBE questionnaire and offer of a financial incentive to complete the questionnaire followed by a standard invitation. Participants in all three trial arms received a reminder letter at 3 months following the initial invitation.
Setting
The trial was conducted in two London boroughs, Lambeth and Lewisham, which are described briefly in Chapter 1. Both boroughs are typical of areas that are in need of intervention to increase the number of individuals attending for their NHS health check; uptake is below the national average (48%). In 2012/13, 31% of individuals invited for a health check in one borough attended and 45% in the other.
General practice recruitment
General practices in the two participating boroughs were eligible to participate in the trial. Each practice participated in the trial for a minimum of 12 months to allow for seasonal variation in uptake of health checks.
General practice recruitment was facilitated by the National Institute for Health Research (NIHR) South London Clinical Research Network, which advertised the trial to its general practices and offered reimbursement of costs for participation. In addition, practices were recruited into the trial based on existing working relationships either with members of the research team or with the borough NHS Health Check programme co-ordinators. A non-probability sampling strategy was used because conducting the trial required a significant level of access to, and co-operation from, general practices to utilise the general practice electronic health record system and participating practices were necessarily volunteers. To evaluate selection bias, general practices that participated in the trial were compared with all general practices in the two boroughs with respect to general practice list size, area deprivation [Indices of Multiple Deprivation (IMD) 2010 score66], proportion of ethnic minorities and achievement of Quality and Outcomes Framework (QOF) targets, both overall and for public health. Although the method of general practice selection might be associated with patients’ uptake of health checks, it was not considered likely that general practice selection would be associated with patients’ propensity to respond to the study interventions.
Individual participant recruitment
Patients were included in the trial only if the senior partner at the practice provided written informed consent. All participants in the consented practices who were eligible to be invited for an NHS health check were included in the trial. There were no exclusion criteria for trial recruitment.
A cross-borough call–recall system is used to recruit patients into the NHS Health Check programme. The call–recall system is commissioned by the boroughs and is implemented by the primary care shared services team, working in association with the commercial information technology company, QMS. QMS has developed bespoke software (Health Check Focus) that is used in the management of the Health Check programme. All general practices in the two boroughs utilise Health Check Focus software.
Invitations to the programme are issued through a monthly cycle. This begins with the ‘harvesting’ of eligible patients from general practice information systems. All general practices in the two boroughs use EMIS (EMIS Health, Cambridge, UK) electronic health records software. Eligibility for the NHS Health Check programme is initially defined on the basis of age and comorbidity. Individuals who are registered with general practices in Lambeth and Lewisham form the initial population. Participants are eligible for invitation for a NHS health check if they are aged from 40 to 74 years. Participants are ineligible if they already have a defined comorbidity, including CVD, or treated risk states. Participants are excluded if they have diagnosed ischaemic heart disease, heart failure, atrial fibrillation, stroke or transient ischaemic attack, diabetes mellitus, chronic kidney disease or peripheral vascular disease. Participants are also excluded if they have diagnosed hypertension or hypercholesterolaemia or are being treated with antihypertensive drugs or statins.
General practice data are uploaded into Health Check Focus on a monthly basis and bespoke software is used to remove ineligible participants on the basis of Read codes that identify patients already included on CVD registers. An initial pre-notification list (PNL) is prepared by QMS and sent to general practices for review. Any participants whom the general practice considers should not be invited (e.g. they have died, are terminally ill or have left the practice) are excluded. The exclusions are confirmed by practice staff prior to patient invitations being sent each month by checking the records of patients included in the PNL. Only a very small number of patients are removed at the PNL stage. The final list of participants eligible for invitation is then forwarded to primary care shared services by the 21st of each month and standard NHS Health Check programme invitation letters are then sent out on the 28th of each month. A commercial mailing house is used to send the invitation letters.
Recruitment and randomisation
To conduct the trial, we negotiated with the borough teams, QMS, the primary care shared services team and general practices to introduce modifications into the standard NHS Health Check programme invitation process. Our aim was to introduce an automated recruitment and randomisation procedure into the standard invitation process, through modifications to QMS Health Check Focus software. At the start of the trial the feasibility, reliability and likely time scale for such a modification were unknown. We considered that reliance on an unproven recruitment and randomisation procedure might carry a significant risk to the successful conduct of the trial. We therefore developed an alternative method of recruitment and randomisation that could be implemented through visits to general practices. The trial was delivered through the use of these two different recruitment and randomisation procedures, which will be referred to as the ‘automated’ and ‘in-practice’ recruitment methods, respectively.
In-practice method for recruitment and randomisation
For the in-practice method of allocation, members of the research team visited each practice monthly to access the practice-approved PNL. Participants included in the approved PNL were allocated to the three trial arms using previously prepared randomisation lists. Each month, the trial statistician drew up randomisation lists, stratified by general practice. As all patients within a practice were assigned simultaneously, participants were allocated to intervention arms in a ratio of 1 : 1 : 1 by means of a computer-generated randomisation list stratified by general practice and month using permuted blocks of three. Randomisation lists were generated using the Stata command ‘ralloc’ in Stata 12 (StataCorp LP, College Station, TX, USA). The randomisation list was applied to the approved PNL by the trial researcher, who assigned the trial arm in the existing order of the approved PNL. Practice staff responsible for preparing the approved PNL never had access to the randomisation list for the practice. This process was considered to provide adequate concealment of the allocation procedure.
Automated method for recruitment and randomisation
For general practices assigned to the automated method for recruitment and allocation, randomisation was performed automatically using a randomisation procedure programmed into QMS Health Check Focus software, used to manage the Health Check programme. Randomisation lists were generated using a bespoke algorithm embedded within the QMS software. Simple randomisation, stratified by practice and month, was employed. Participants were automatically assigned a study identification number and group allocation when the PNL was electronically approved by the general practice.
Pilot study
During the first 2 months of use of the automated recruitment method, a review of the trial data showed that the correct allocation ratio was not being achieved. Further adjustment to the software was therefore made. The first 2 months were therefore considered to act as a pilot study and data from these months were excluded from the main trial analysis.
Once randomisation was completed, the PNL list, with trial arm allocations included, was forwarded to the offices of the primary care shared services team. Here, trial research staff arranged for intervention materials to be sent to participants, as outlined in the following section. Initially, an in-house mailing procedure was used; later, we used the services of the commercial mailing house, Docmail (Radstock, UK). Table 3 displays the procedure for mailing invitations during the trial.
| Procedure | Standard practice | Standard invitation | QBE questionnaire | QBE questionnaire and incentive |
|---|---|---|---|---|
| PNL list sent to practice | 21st day of month | 21st day of month | 21st day of month | 21st day of month |
| Randomisation completed by 28th day of month | Randomisation completed by 28th day of month | Randomisation completed by 28th day of month | ||
| QBE questionnaire and covering letter sent on 28th day of month | QBE questionnaire and covering letter offering incentive sent on 28th day of month | |||
| Standard invitation letter sent to participants | 28th day of month | Standard invitation letter delayed by 7 days | Standard invitation letter delayed by 7 days | Standard invitation letter delayed by 7 days |
Intervention rationale and development
The original study by Conner et al. ,44 which suggested that the QBE may promote health check uptake, employed a questionnaire that assessed constructs from the TPB in relation to health check attendance. The questionnaire employed as the intervention in the present study was based on the same theory. It was also decided to add items assessing anticipated regret because a previous QBE study45 found that, among participants who returned completed questionnaires, those who completed a TPB with anticipated regret questionnaire had a significantly higher cervical cancer screening attendance rate (65.1%) than those who received a TPB-only questionnaire (44%).
The questionnaire employed in the previous study using the QBE to promote health check uptake44 included 23 items, each in relation to health check attendance: eight measuring attitudes, nine measuring subjective norms, three measuring PBC and three assessing intentions. The study by Conner et al. 44 was conducted in a single general practice in rural England. In contrast, the present trial was implemented in two London boroughs ranked among the most deprived local authorities in England. 62 Given the known relationship between socioeconomic deprivation and low levels of literacy,67 there were concerns that employing a questionnaire of similar length in these boroughs would deter questionnaire completion, inhibiting the operation of the QBE. A decision was made to reduce the questionnaire length for use in the present study so that it contained two items for each psychological concept specified by the TPB and for anticipated regret, giving a total of 10 items.
For all questionnaire items, wording was based as much as possible on items that had been employed in previously successful QBE studies. When a number of possible items were available for assessing a construct, the choice was guided by considering which options had the best readability. One of the intentions items was asked in the interrogative form, that is, as a self-posed question (‘Will I . . .?), because a previous study found that the QBE was stronger when intentions items were asked in the interrogative. 68 All items were rated on a 7-point scale with labelled end points. The questionnaire items were printed in 16pt font in an effort to maximise legibility in line with ‘clear print’ standards. 67
The questionnaire was tested with six individuals attending for a NHS health check at a local community event. Most completed it within 5 minutes and made favourable comments on the layout and font size. However, respondents did not use the full range of response options, with responses instead clustered around the labelled end points. Another version of the questionnaire was developed that had all response options labelled. Further pilot testing with five people in the target age range for a health check found that this format was associated with respondents using a wider range of the response options. Comments were made on the repetitiveness of some items and a suggestion was made to reduce the number of questions. At this stage the questionnaire was circulated for feedback to members of the project management team, including local general practitioners (GPs) and NHS Health Check programme leaders. It was agreed that the questionnaire was too long as the items covered three A5 sides in a booklet. The study team decided to have only one item for each of the two concepts that were thought to be less central to the operation of the QBE, namely subjective norms and PBC. The PBC item retained reflected perceived confidence and so might also be referred to as self-efficacy.
Table 4 presents the intervention questionnaire items in order. The full version of the questionnaire began with an example of how to record one’s response to a question, about a different topic (television viewing). Appendix 1 provides a fully formatted version of the questionnaire, as received by participants, including this example item. The leaflet had a Flesch reading ease score of 80.1 and a Flesch–Kincaid grade level of 5.9, suggesting that it was accessible to people with the reading ability of an 11-year-old.
| Construct | Item stem | Response options | Previously used to promote |
|---|---|---|---|
| Intentions | I intend to go for a health check in the next few weeks | Strongly disagree to strongly agree | Health check uptake44 |
| Attitudes | For me, going for a health check in the next few weeks would be . . . | Very bad to very good | Health check uptake44 |
| Anticipated regret | If I did not go for a health check in the next few weeks, I would feel regret | Strongly disagree to strongly agree | Cervical cancer screening uptake45 |
| Intentions | Will I go for a health check in the next few weeks? | Definitely no to definitely yes | Physical activity68 |
| Anticipated regret | If I did not go for a health check in the next few weeks, I would later wish I had | Strongly disagree to strongly agree | Cervical cancer screening uptake45 |
| Attitudes | For me, going for a health check in the next few weeks would be . . . | Very worrying to very reassuring | Health check uptake44 |
| PBC (self-efficacy) | I’m confident I can go for a health check in the next few weeks | Strongly disagree to strongly agree | Health check uptake44 |
| Subjective norms | People who are important to me would . . . . . . of me going for a health check in the next few weeks | Completely disapprove to completely approve | Flu vaccination uptake44 |
Patient and public involvement
In addition to patient involvement in questionnaire development, as outlined in the previous section, patient feedback was also obtained on the use of an incentive, the study invitation letter and the protocol. The six patients interviewed during questionnaire development in addition to three members of the public who met the target age range for a health check were questioned regarding the acceptability of the incentive for questionnaire completion and the trial invitation letter (both of which were considered acceptable). In addition, members of the Trial Steering Committee (TSC), which included stakeholders (NHS Lambeth and Lewisham Health Check programme and CVD managers) and patient representatives, discussed and provided feedback on the study documents and the protocol, which led to general acceptability and some minor amendments being made before documents were sent out.
Justification of the incentive
Strong evidence suggests that financial incentives for questionnaire return increase response rates. A systematic review including 94 trials with a pooled total of 160,004 participants found that the odds of returning a postal questionnaire were almost doubled if a financial incentive was offered. 54 As the QBE is greater among individuals who return a questionnaire,44 incentivising questionnaire return may increase the size of any effect of distributing a questionnaire on uptake of the NHS health check. A meta-analysis of 85,671 participants in 88 randomised trials of financial incentives to increase response rates for mailed questionnaires reported a significant increase in response rates for incentives up to the value of $5. 55 It was decided to offer participants in one arm of the trial the incentive of a £5 gift voucher to complete and return the questionnaire. The gift voucher scheme employed (Love2shop) provided vouchers that could be exchanged at a wide variety of shops and so was intended to appeal to as many participants as possible.
Details of the interventions received in each trial arm
Participants in the standard invitation trial arm received the standard invitation letter for a NHS health check, sent from the primary care shared services team that organises health check invitations. This consisted of a single-page letter from the participants’ GP inviting them to make an appointment at their general practice to receive a health check or to visit a local participating pharmacy. Participants also received an information sheet. Individuals were sent a reminder letter if they did not attend for a health check within 12 weeks of their first invitation.
Participants in the QBE questionnaire trial arm were sent the QBE questionnaire with a prepaid return envelope and covering letter (see Appendix 2) 7 days before they were sent the standard NHS health check invitation letter and information sheet. They were also sent a reminder letter at 12 weeks if appropriate.
Participants in the QBE questionnaire and incentive trial arm were sent the QBE questionnaire with a prepaid return envelope and covering letter (see Appendix 3) 7 days before they were sent the standard NHS health check invitation letter and information sheet (plus a reminder letter at 12 weeks if appropriate). The covering letter in this trial arm offered the £5 retail voucher as an incentive to return the questionnaire.
Languages other than English
In the study areas, the London boroughs of Lambeth and Lewisham, ethnic minority groups account for a high proportion of the population. The major ethnic groups are of black African and African Caribbean origins and are English speaking. There are significant groups of European origin who speak languages other than English, but these groups are generally literate in English.
Standard invitation letters were sent in English. A single sentence was included on the reverse side of the letter, translated into 11 different languages, offering translated versions of the invitation letter and leaflet if required. Up to the study start date, no translations had been requested (primary care shared services, 10 September 2012, personal communication). In view of this, we sent the QBE questionnaire and covering letter in English only and included a telephone number to call on the final page of the questionnaire if a translation was required. In a randomised study any differences in literacy between the two arms will occur at random.
Sample size
It was important to detect even modest increments in screening uptake between trial arms because in a public health programme small effects may yield substantial benefits across the population at risk. We made the statistically conservative assumption that the underlying proportion of people invited who actually receive a health check is about 50%. If there are 4263 participants in each trial arm, with 12,789 in total, this will provide > 90% power to detect a difference in uptake of health checks between each active treatment arm and the standard intervention arm of at least 4%. These calculations are based on a 5% significance level using a Bonferroni correction for three comparisons (i.e. 0.0167). Calculations were performed in Stata 12. As present rates of health check uptake are < 50% in the study area, slightly greater power may be realised in the study. There were no planned interim analyses and no stopping guidelines as this trial had a low risk of adverse events.
In the study by Conner et al. 44 47% of intervention group participants returned the QBE questionnaire and health check uptake was 78% among participants who completed the questionnaire, compared with 60% among non-completers and 54% in control participants. Based on experience in the study area in London, we expected that the response rate to the QBE questionnaire would be about 40%. 69
The anticipated flow of patients through the intervention trial arms is shown in Table 5. If the QBE effect was restricted to questionnaire returners, then to achieve an overall increment in health check uptake of 4% we expected that uptake would have to be 10% higher in questionnaire returners than non-returners. Conner et al. 44 found that health check uptake was 24% higher in questionnaire returners than in control participants. In this study there was also a modest increment in uptake in questionnaire non-returners.
| Invited participants | QBE questionnaire | Health check uptake by QBE return | Overall health check uptake |
|---|---|---|---|
| 1000 | 400 return questionnaire | 240 receive health check | 540 receive health check in total |
| 600 do not return questionnaire | 300 receive health check |
Blinding
Participants’ GPs provided consent to their participation in the trial and so participants were not overtly aware that there were other trial arms. However, participants in the QBE questionnaire and QBE questionnaire and incentive groups received a postal intervention and thus could not be blinded to their trial arm allocation. Members of the study team were blind to participants’ details during trial arm allocation and were blind to group allocation during extraction of participant data and outcomes from general practice records.
Duration of the treatment period
Participants were followed up for a minimum of 6 months after the first NHS health check invitation was sent.
Outcome data collection
No data were collected directly from patients. Data were extracted from electronic health records into a spreadsheet and were then transferred to a database for statistical analysis.
The primary outcome was uptake of a NHS health check within 182 days (6 months) of receiving the standard invitation letter. Secondary outcomes included uptake of a NHS health check 91 days (3 months) post standard invitation and time elapsed between participants receiving the standard invitation and uptake of a health check.
Outcome data were extracted from participant electronic health records by members of the research team using nationally specified Read codes to record completion of NHS health checks (Table 6). Participants frequently had more than one code recorded to identify the completion of a health check. The codes 8BAg (NHS health check completed) and 38B1 (vascular disease risk assessment) were often co-recorded. At the time of data extraction, participants’ postcodes were linked to the IMD 2010 score66 as a marker of deprivation. Data for gender, year of birth and practice-recorded ethnicity were also extracted. Data were extracted in a single batch for each practice between 1 June 2015 and 2 July 2015.
| Read code | Read term |
|---|---|
| Codes for completion of the NHS health check | |
| 8BAg | NHS health check completed |
| 8BAg0 | NHS health check completed by third party |
| 38B1 | Vascular disease risk assessment |
| 38B10 | CVD risk assessment by a third party |
| 9OhA | CVD risk assessment carried out |
| Codes for CVD risk score recording | |
| 38DF | QRISK CVD 10-year risk score |
| 38DP | QRISK2 CVD 10-year risk score |
| 662k | JBS CVD risk < 10% over next 10 years |
| 662l | JBS CVD risk 10–20% over next 10 years |
| 662m | JBS CVD risk > 20% up to 30% over next 10 years |
| 662n | JBS CVD risk > 30% over next 10 years |
| 38DR | Framingham 1991 CVD 10-year risk score |
Analysis of the data extracted on health check completion revealed a high proportion of health checks completed in non-trial participants. To investigate this observation further, we subsequently collected data for CVD risk scores (see Table 6) and BMI for registered patients either who had a health check recorded during the study period or who were trial participants. Data were extracted from general practice systems between 17 August 2015 and 2 September 2015. Because there may have been changes to practice populations and health check recording between the first and second data extraction, the evaluation of case mix for invited compared with opportunistic checks was treated as a separate analysis from the trial analysis. At the time of the study, the JBS risk score calculator12 was mandated by the NHS Health Check programme locally. Values for the QRisk2 score13 were utilised if the JBS score was not recorded.
Reliability and data checking
Extensive checks were performed to ensure that the recruitment and randomisation procedures were working as intended:
-
We evaluated the recording of completed health checks during the course of the trial and found that health checks in non-trial patients outnumbered health checks in trial patients by a ratio of about 2 : 1.
-
We checked the details, including name and NHS number, of participants included in the approved PNL against general practice records. These checks identified a small number of discrepancies in NHS numbers on the PNL list prepared through QMS Health Check Focus. This was drawn to the attention of QMS who corrected the problem and there was no further recurrence.
-
We conducted checks that confirmed that standard invitation letters were sent to the correct patients identified on the approved PNL list for practices using both the in-practice and the automated recruitment methods.
-
During the trial, we checked the recording of completed health checks for a 10% sample of patients in 1 month using general practice records. This check revealed 100% accuracy of recording of the completion or non-completion of health checks.
At the protocol stage we envisaged using outcome data extracted centrally for the NHS Health Check programme through the health check management information system, QMS Health Check Focus. During the conduct of the trial, we decided that higher-quality data could be obtained by using data extracted by trial staff from general practice systems. Following the completion of the trial, we evaluated the reliability of the trial data against data extracted from the health check management information system. We evaluated the number of patients invited for health checks during the trial period at each practice. We also evaluated the number of completed health checks in invited and non-invited patients at each practice. Completed health checks were divided into those delivered by the general practice and those delivered by third-party providers, including pharmacies and outreach services.
Before analysis, basic checks to view incomplete or inconsistent data were performed. These included assessment of missing data, data outside the expected range and other inconsistencies between variables. When any inconsistencies were found, data were double-checked and corrected if necessary or set to missing otherwise. All changes were documented.
Data analysis plan: data description
A detailed statistical analysis plan was drawn up by the study statistician, principal investigator and study psychologist prior to completion of participant follow-up and compilation of the study data set. Recruitment and flow of participants through the study was depicted using a Consolidated Standards of Reporting Trials (CONSORT) flow chart.
Baseline comparability of randomised groups
Baseline descriptive variables of participants were summarised by treatment arm. Mean and standard deviation (SD) or median (range, IQR) were reported for continuous outcomes, whereas frequencies and proportions were reported for categorical outcomes. Data available for analysis included participant age, gender and ethnicity and deprivation quintile. Participants were divided into the age groups 40–59 years and ≥ 60 years for analysis. Practice-recorded data for ethnicity were recoded to six categories based on the 2011 census question as shown in Table 7. It should be noted that general practice ethnicity recording often includes non-standard terminology, including details of nationality or religion. IMD 2010 scores66 at the lower super output area level were mapped to quintiles of score for England. No significance testing of differences in baseline values was undertaken.
| Ethnicity | Included categories |
|---|---|
| ‘White’ | White; English/Welsh/Scottish/Northern Irish/British |
| White; Irish | |
| White; gypsy or Irish traveller | |
| White; other white | |
| ‘Black’ | Black/African/Caribbean/black British; African |
| Black/African/Caribbean/black British; Caribbean | |
| Black/African/Caribbean/black British; other black | |
| ‘Asian’ | Asian/Asian British; Indian |
| Asian/Asian British; Pakistani | |
| Asian/Asian British; Bangladeshi | |
| Asian/Asian British; Chinese | |
| Asian/Asian British; other Asian | |
| ‘Mixed’ | Mixed/multiple ethnic groups; white and black Caribbean |
| Mixed/multiple ethnic groups; white and black African | |
| Mixed/multiple ethnic groups; white and Asian | |
| Mixed/multiple ethnic groups; other mixed | |
| ‘Other’ | Other ethnic group; Arab |
| Other ethnic group; any other ethnic group | |
| ‘Missing’ | Not recorded |
Data analysis plan: inferential analysis
Analysis of the primary outcome
Uptake of a NHS health check was defined as having taken place if any of the codes shown in Table 6 were recorded within 6 months of the randomisation date.
The primary analysis was an intention-to-treat analysis including all participants who were randomised regardless of whether the intervention was subsequently received. Patients who died or left the practice prior to the 6-month follow-up period were included in the analysis using the status at their last recorded follow-up.
The distribution in time of health checks following randomisation was evaluated in a time-to-event framework. This allowed us to evaluate the impact of interventions on the primary outcome over time up to 6 months’ follow-up. To visualise the cumulative proportion of participants having a health check by trial arm, a Kaplan–Meier curve was plotted for each trial arm.
The number and proportion of health checks were tabulated by practice and between-practice variation was quantified by estimating the intraclass correlation coefficient using analysis of variance separately for each trial arm.
To adjust for practice effect, a marginal model using the method of generalised estimating equations (GEEs) was implemented. As the number of practices was relatively small, we used an exchangeable correlation structure with model-based variance estimates. 70 As the primary interest was to estimate absolute differences in NHS health check uptake, rather than in relative measures such as ORs, the GEE model was implemented using the binomial family and an identity link. In addition to treatment arm the model included the stratification variables month of invitation and year. The associated p-values for treatment arm were assessed at the 1.67% significance level to allow for three comparisons: standard invitation with QBE, standard invitation with QBE and incentive and QBE with QBE and incentive. Estimates were also obtained using the same methods for subgroups of gender, age group, ethnic group and deprivation quintile. Forest plots were constructed using the ‘forestplot’ package in the R program (The R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analysis of the primary outcome
The model estimates from the primary analysis were compared using different estimation methods. The GEE model was compared with a general linear model (GLM) with and without the use of robust standard errors and the results tabulated.
A meta-analysis was used to examine the impact of recruitment and randomisation procedures by comparing automated and in-practice methods for each of the three trial arm comparisons in turn. A forest plot was used to visualise the intervention estimates for the difference in uptake between arms at practice level. Heterogeneity in estimates was assessed using the I2 statistic. 71 In the absence of heterogeneity, estimates were combined by use of a fixed-effects model.
Analysis of secondary outcomes
Time to health check uptake
Time to health check uptake was calculated as the time between the invitation date and the date of the recorded health check or 6-month follow-up, whichever came first. Patients who died or who left the practice were treated as a censored observation at the last date registered. A time to health check curve was plotted by treatment arm using the method of Kaplan–Meier. 72
Inequalities
The distribution of the primary outcome was evaluated by subgroups of gender, age (5- and 10-year age groups), ethnicity (white, mixed, black Caribbean, black African, black other, other and not known) and deprivation (using deciles of the distribution of IMD 2010 scores for England66). The intervention effect was estimated for each subgroup and displayed by means of a forest plot. The impact of participant characteristics on uptake was further examined by undertaking adjusted analyses using the primary analysis model including treatment arm, month of invitation, year, age, gender and deprivation quintile. Fully adjusted analyses were conducted using a logit link, to estimate adjusted ORs.
Analysis of questionnaire responses
To explore whether or not individuals who completed the questionnaire were more likely to subsequently attend a health check and to assess whether or not offering an incentive for return differed across deprivation quintile, we fitted a marginal model with a binomial family and identity link using the method of GEEs. Covariates in the model included the stratification variable month of invitation, year, questionnaire return (yes/no), treatment arm, deprivation quintile and trial arm by deprivation quintile interaction. A complier average causal effect (CACE) analysis was performed to estimate the effect of the intervention on health check uptake in ‘compliers’. Compliers were defined as participants who returned a QBE questionnaire in either of the intervention arms, or who ‘would have’ returned a questionnaire if they were in the standard invitation arm. This analysis followed the approach laid out in Dunn et al. 73 As no participants were lost to follow-up we were able to estimate the intervention effect without taking into account the missing data mechanism. An estimate of the standard error for the statistic was obtained through bootstrapping.
Responses to questionnaire items were tabulated using means (SDs) of the seven-category scale. Pairwise correlations of relevant questionnaire items were evaluated prior to constructing scale scores for further use in the analyses. The correlations of these constructs with each other were evaluated. Analysis of variance was employed to evaluate differences in responses between the two questionnaire trial arms. The association of each construct with health check uptake was evaluated in a logistic model, with the construct fitted as a linear predictor. Robust standard errors were estimated. Finally, each construct was dichotomised at the median and the association of highly positive responses, compared with less positive responses, with health check uptake was evaluated in a logistic model.
Evaluation of the study as a rapid trial and analysis of the randomisation methods
The trial was commissioned and evaluated as a ‘rapid trial’. We evaluated the delivery of key milestones such as the recruitment rate over time. We also compared the in-practice and automated methods of recruitment. The number and proportion of participants with the primary outcome were tabulated by practice. Between-practice variation was quantified by estimating the intraclass correlation coefficient using analysis of variance separately for each trial arm. A fixed-effects meta-analysis was conducted, using aggregated data for each general practice as observations. Randomisation method (in-practice or automated) was used as a subgroup. Heterogeneity was evaluated using I2 and tau2 statistics. The ‘meta’ package in the R program was use for analysis.
Statistical considerations
Missing outcome data
All participants who did not have a health check visit recorded were assumed not to have attended one. We cannot exclude the possibility that patients attended for a check but that this was not recorded. As NHS health checks are a key indicator in the Public Health Outcomes Framework, and practices receive remuneration for performing and recording health checks, it was anticipated that a high proportion of all completed health checks would be recorded. The number of missing baseline characteristics was reported by treatment arm. Baseline variables were not included in the primary analysis and no imputation for these variables was undertaken. In secondary analyses participants with missing baseline values were included in the analysis by use of a missing indicator variable.
Software for statistical analysis
Stata 13 was used for data description and the main inferential analysis.
Economic evaluation
As neither intervention was found to be effective, a cost-effectiveness analysis was not pursued.
Cohort study
A retrospective cohort study was conducted of all health checks completed at trial practices during the study period. The trial study period ran from 1 July 2013 to 30 June 2015. During the study period, the total number of NHS health checks recorded at trial practices, after their start date in the trial, was 6184, including 2280 in trial participants and 3904 in non-trial participants. We then extracted data for cardiovascular risk scores and BMI for participants with health checks recorded. Cardiovascular risk score data were obtained for 5359 participants, including 2246 out of 2280 (99%) trial participants with health checks recorded and 3113 out of 3904 (80%) non-trial participants with health checks recorded. The 3113 out of 5359 (58%) cardiovascular risk assessments in non-trial participants either were performed opportunistically or were carried out in patients invited before their general practice joined the trial. Health checks were classified into ‘invited’ – those performed following a standard invitation letter to a trial participant – and ‘opportunistic’ – those that did not follow a standard invitation during the trial. The relative contribution of invited and opportunistic health checks to overall health check uptake was estimated. We also compared the case mix of invited and opportunistic checks in terms of age group, gender, ethnic group and deprivation quintile. We also compared BMI category and CVD risk score estimates between invited and opportunistic checks. Among the opportunistic checks, 1363 out of 3113 (44%) were completed within 6 months of the practice start date in the trial and might potentially have resulted from invitations before the practice entered the trial. Sensitivity analyses were performed to evaluate the impact of possible misclassification on the results of the study.
Process evaluation and qualitative study
Themes and objectives
This evaluation assessed the structure, process and outcomes of the Health Check programme. Structure evaluates the provision of facilities and services for effective delivery of the programme. Process elements evaluate how well the programme was delivered to patients and outcome evaluation assesses the outputs from the programme. The purpose of this evaluation was to assess the NHS Health Check programme carried out across 18 general practices in the London boroughs of Lambeth and Lewisham.
As part of this evaluation, practice staff’s views and attitudes in relation to the workings of the programme, benefits and weaknesses, influences on uptake and how improvements, if any, could be made were explored. Hence, this evaluation deals solely with an assessment of the medical process at the ‘patient–physician’ level. It focuses on the process of the NHS Health Check programme delivery, rather than the findings or indeed the effects and outcomes of such a programme.
Delivery of the NHS Health Check programme: practice staff and programme lead perspectives
A questionnaire delivered to practice staff included qualitative elements to capture their views on the overall organisation and delivery of the NHS Health Check programme within their repsective practices. In addition, the two public health programme leads for the NHS Health Check programme in the Lewisham and Lambeth boroughs were asked for their thoughts on the future of this programme.
In total, 22 practice staff members from 17 practices involved in delivering the Health Check programme and two programme leads at commisioning level were interviewed using a semistructured questionnaire. These interviews were conducted over the telephone and face to face; 20 were recorded and transcribed and for two handwritten notes were made during the interviews.
Interviews with general practice and Health Check programme staff
General practice staff involved in the implementation of health checks from the 18 practices across Lambeth and Lewisham included in the trial were invited to be interviewed using a semistructured interview schedule. The interview explored attitudes towards the NHS Health Check programme and towards our RCT to promote uptake of health checks, interviews were conducted face to face or over the telephone according to preference. Data were analysed using a thematic content analysis. Themes and illustrative quotes were discussed and agreed between three members of the research team.
Content analysis of participant free-text responses
Participants in the intervention arms of the trial were invited to provide comments about the research in a free-text box at the end of the QBE questionnaire. A simple content analysis was conducted to categorise the comments under emerging themes. Themes were agreed between two researchers and the comments were grouped together under these themes in a Microsoft Excel® spreadsheet (2013; Microsoft Corporation, Redmond, WA, USA).
Patient interview study
We conducted a qualitative interview study with non-trial participants in the target population in south-east London. The aim was to explore the influences on attendance for a NHS health check among people recently invited to receive one. Semistructured interviews were conducted with a purposive sample recruited according to age, gender and attendance or non-attendance for the health check.
Ethical arrangements
The study received ethical approval from the National Research Ethics Service Committee London, London Bridge (reference no. 13/LO/01/97). The main ethical issue raised by the study concerned consent to participation. It was not feasible to obtain individual participant consent for randomisation. Obtaining consent, through a postal invitation, from individual participants before entering the study would have led to a sample that was likely to be highly biased with respect to the propensity to return a questionnaire or to participate in a health check. For this reason we obtained consent from the senior partner at each general practice that participated in the study. This approach is commonly used in cluster RCTs. Obtaining consent from an individual who has a stewardship role in respect of a group of individual participants was judged to be acceptable by the Medical Research Council in its recommendations on ethical issues in cluster randomisation. 41 The approach was also applicable to the present study in which individual participant randomisation was to be used. The research also accessed individual participant data on whether or not a health check was completed and, for completed checks, the results of the check; however, we had access only to fully anonymised data.
Research governance
King’s College London was the sponsor of the study. There was a TSC with an independent chair and two independent members. Stakeholder representatives, including managers and GPs, who are responsible for the implementation of the NHS Health Check programme in Lambeth and Lewisham were also members of the TSC. There was also a patient member of the TSC.
Chapter 3 Results 1: main trial results
The following chapters of the report present the main findings of this research. The results are organised into five chapters. The first chapter addresses general practice and participant recruitment. This is followed by a presentation of the main results of the trial. The second chapter analyses data from the QBE questionnaire to evaluate potential mechanisms of effect. The third chapter compares health checks conducted in response to invitations through the population-based call–recall system with those conducted opportunistically by health check providers. The fourth chapter presents qualitative data from interviews with health-care professionals and patients that aid interpretation of the findings of this research. The final chapter evaluates the research as a rapid trial using electronic health records, presenting a comparison of the fully automated and in-practice randomisation methods.
Recruitment of general practices and participants
General practice recruitment
In total, 18 general practices were recruited into the trial. Of these, 12 were selected for the in-practice recruitment method and six were selected for the automated recruitment method. A 2 : 1 ratio was implemented to minimise risk to the trial because of the unknown difficulties of implementing the automatic procedure.
At one practice using the manual method, recruitment started 2 months later than initially intended because of access issues. Three general practices using automated recruitment completed only 11 months in the trial because the recruitment target was reached.
Table 8 shows a comparison of selected measures between general practices included in the trial and other practices in the two boroughs. Trial practices tended to have larger list sizes than non-trial practices but achievement of QOF targets was generally similar between trial and non-trial practices. Trial practices showed similar levels of deprivation66 and a similar proportion of non-white participants to the entire populations of the two boroughs. Evaluation of practices using the automated recruitment method showed that these had slightly higher list sizes and slightly higher deprivation scores but a lower proportion of ethnic minorities than practices using the in-practice recruitment method.
| Characteristic | Non-trial practices | Trial practices | |
|---|---|---|---|
| In-practice recruitment | Automated recruitment | ||
| Lambeth (n) | 39 | 6 | 3 |
| Lewisham (n) | 32 | 6 | 3 |
| List size 2014–15 (n) | 6554 (4851–9348) | 8093 (6179–12,568) | 11,269 (7115–14,404) |
| IMD 2010 score | Lewisham, 31.0; Lambeth, 31.2 | 30.2 (23.8–35.1) | 34.6 (30.7–39.5) |
| Ethnic minorities (%) | Lewisham, 46.4; Lambeth, 42.9 | 47.3 (43.7–50.9) | 42.5 (40.9–44.1) |
| Overall QOF achievement (%) | 95.7 (92.4–97.3) | 95.6 (90.5–98.5) | 94.3 (92.7–95.3) |
| Clinical QOF achievement (%) | 95.1 (91.5–96.8) | 94.4 (89.6–98.1) | 94.7 (92.4–95.7) |
| Public health QOF achievement (%) | 98.5 (93.8–100) | 99.4 (93.1–100) | 91.5 (89.8–96.6) |
Individual participant recruitment
Figure 5 shows the flow of participants through the trial. Between July 2013 and December 2014, 12,702 participants were recruited but 21 participants were found to be registered with non-trial practices and were excluded and second records for 38 participants were found to be duplicated after their first allocation and these were also excluded, leaving 12,643 participants (Tables 9 and 10). In total, 184 participants were included in the pilot of the automated randomisation procedure and were not eligible for the trial, leaving 12,459 participants randomised in the trial. The median number of participants per site was 711, with a range of 189–1220 (Table 11).
FIGURE 5.
Flow of individuals throughout the study from identification to randomisation and follow-up at 6 months.
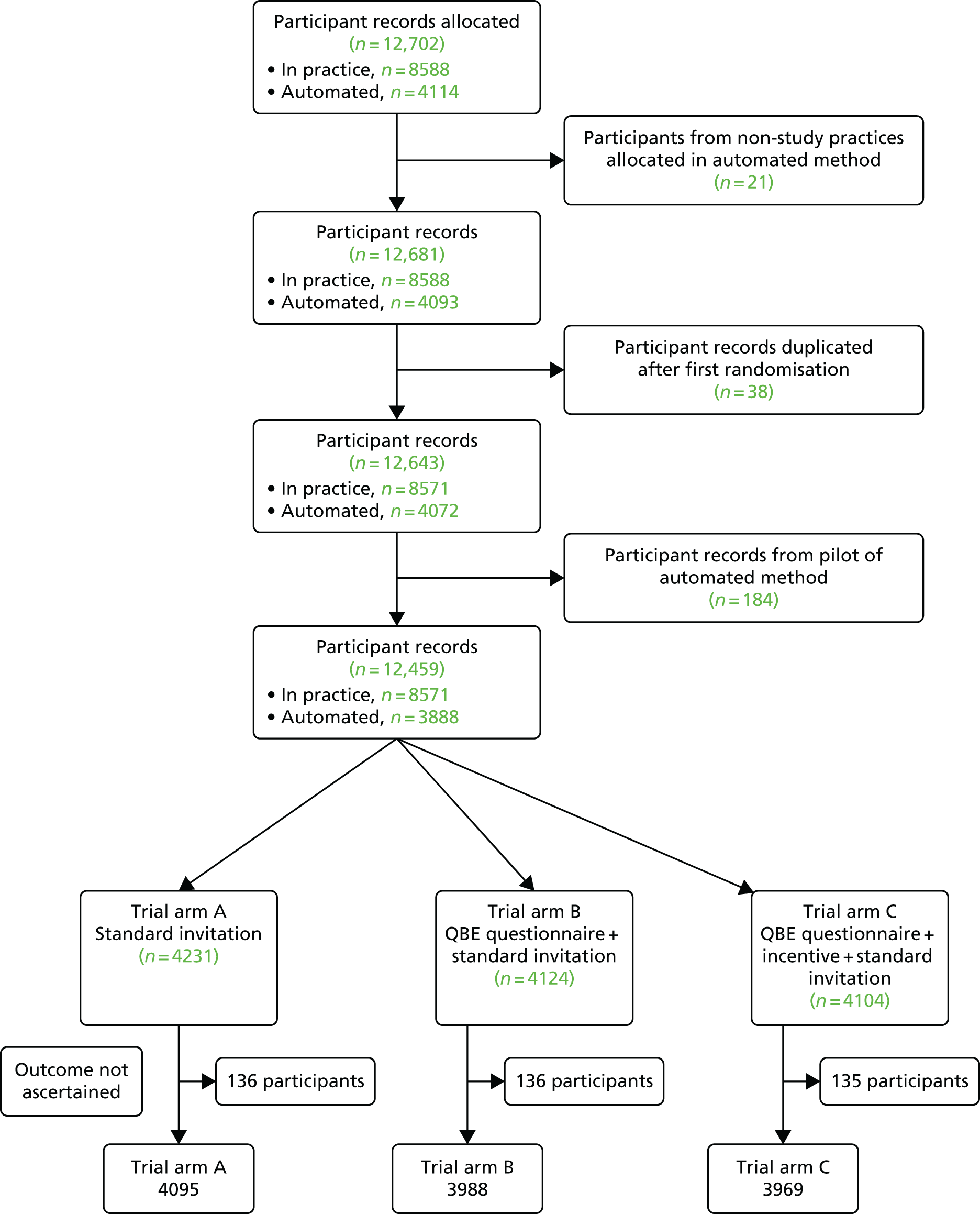
| Month | Practice | Number per month | Cumulative total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| 1 | 82 | 21 | 103 | 103 | ||||||||||
| 2 | 87 | 46 | 92 | 37 | 67 | 329 | 432 | |||||||
| 3 | 104 | 54 | 53 | 35 | 89 | 29 | 364 | 796 | ||||||
| 4 | 57 | 39 | 59 | 51 | 75 | 33 | 314 | 1110 | ||||||
| 5 | 81 | 38 | 71 | 35 | 59 | 24 | 308 | 1418 | ||||||
| 6 | 75 | 39 | 75 | 58 | 77 | 27 | 56 | 0 | 60 | 467 | 1885 | |||
| 7 | 88 | 46 | 57 | 44 | 76 | 19 | 57 | 0 | 40 | 38 | 27 | 10 | 502 | 2387 |
| 8 | 82 | 56 | 77 | 46 | 69 | 32 | 54 | 31 | 71 | 51 | 36 | 17 | 622 | 3009 |
| 9 | 86 | 42 | 55 | 49 | 76 | 20 | 55 | 41 | 67 | 37 | 29 | 9 | 566 | 3575 |
| 10 | 122 | 39 | 78 | 48 | 73 | 28 | 64 | 33 | 83 | 43 | 19 | 13 | 643 | 4218 |
| 11 | 90 | 27 | 65 | 47 | 56 | 21 | 56 | 35 | 79 | 30 | 28 | 17 | 551 | 4769 |
| 12 | 73 | 25 | 78 | 41 | 66 | 21 | 49 | 26 | 52 | 36 | 32 | 4 | 503 | 5272 |
| 13 | 95 | 45 | 52 | 49 | 64 | 26 | 42 | 31 | 40 | 31 | 26 | 18 | 519 | 5791 |
| 14 | 40 | 37 | 77 | 37 | 56 | 23 | 66 | 30 | 68 | 37 | 28 | 30 | 529 | 6320 |
| 15 | 38 | 61 | 64 | 34 | 58 | 19 | 48 | 31 | 80 | 40 | 19 | 17 | 509 | 6829 |
| 16 | 83 | 44 | 75 | 44 | 76 | 25 | 38 | 42 | 47 | 40 | 25 | 18 | 557 | 7386 |
| 17 | 0 | 22 | 68 | 16 | 62 | 20 | 47 | 36 | 52 | 24 | 27 | 31 | 405 | 7791 |
| 18 | 133 | 46 | 70 | 25 | 121 | 35 | 94 | 41 | 70 | 92 | 48 | 5 | 780 | 8571 |
| Total | 1416 | 727 | 1166 | 696 | 1220 | 402 | 726 | 377 | 809 | 499 | 344 | 189 | 8571 | |
| Month | Practice | Number per month | Cumulative total | |||||
|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | 17 | 18 | |||
| 1 | ||||||||
| 2 | ||||||||
| 3 | ||||||||
| 4 | 39 | 39 | 39 | |||||
| 5 | 20 | 53 | 72 | 145 | 184 | |||
| 6 | 55 | 91 | 16 | 162 | 346 | |||
| 7 | 43 | 84 | 101 | 228 | 574 | |||
| 8 | 28 | 82 | 103 | 75 | 21 | 38 | 347 | 921 |
| 9 | 37 | 75 | 93 | 74 | 22 | 29 | 330 | 1251 |
| 10 | 33 | 67 | 100 | 71 | 21 | 34 | 326 | 1577 |
| 11 | 38 | 73 | 70 | 71 | 14 | 35 | 301 | 1878 |
| 12 | 37 | 108 | 84 | 67 | 26 | 34 | 356 | 2234 |
| 13 | 32 | 86 | 78 | 73 | 18 | 29 | 316 | 2550 |
| 14 | 35 | 80 | 81 | 74 | 19 | 36 | 325 | 2875 |
| 15 | 33 | 68 | 72 | 77 | 24 | 32 | 306 | 3181 |
| 16 | 44 | 65 | 68 | 57 | 14 | 26 | 274 | 3455 |
| 17 | 15 | 81 | 34 | 22 | 17 | 25 | 194 | 3649 |
| 18 | 74 | 136 | 78 | 71 | 22 | 42 | 423 | 4072 |
| Total | 563 | 1149 | 1050 | 732 | 218 | 360 | 4072 | |
| Practice | Frequencya | % |
|---|---|---|
| 12 | 189 | 1.5 |
| 17 | 218 | 1.7 |
| 11 | 344 | 2.8 |
| 18 | 360 | 2.9 |
| 8 | 377 | 3.0 |
| 6 | 402 | 3.2 |
| 10 | 499 | 4.0 |
| 13 | 504 | 4.0 |
| 4 | 696 | 5.6 |
| 7 | 726 | 5.8 |
| 2 | 727 | 5.8 |
| 16 | 732 | 5.9 |
| 9 | 809 | 6.5 |
| 15 | 978 | 7.8 |
| 14 | 1096 | 8.8 |
| 3 | 1166 | 9.4 |
| 5 | 1220 | 9.8 |
| 1 | 1416 | 11.4 |
| Total | 12,459 | 100 |
Participant characteristics collected at the time of outcome data extraction included age, gender, ethnic group and deprivation category (Table 12). There was a higher proportion of men (52%) than women (44%) invited. This was confirmed in data from the Heath Checks programme management information system, which showed that, in the 2014/15 financial year, 52% of invited patients in Lewisham were men and 56% of invited participants in Lambeth were men. This reflects the demographic distribution of the registered population in this area. The median age of participants, based on recorded year of birth and year of randomisation, was 45 (IQR 40 to 54) years. Calculation of age from year of birth may lead to misclassification by 1 year, but there were nine participants randomised whose ages were < 39 years and two aged > 75 years who were nevertheless included in analyses. Trial participants showed generally high levels of deprivation, with 29.5% in the most deprived quintile for England, 51.2% in the second most deprived quintile and none in the least deprived quintile for England. In total, 35.9% of participants were white, 19.4% were black and 19.5% were of mixed ethnicity. All participant characteristics were evenly balanced across the three trial arms.
| Characteristic | Standard invitation (n = 4231) | QBE questionnaire (n = 4124) | QBE questionnaire + incentive (n = 4104) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Gender | Female | 1857 | 43.9 | 1783 | 43.2 | 1809 | 44.1 |
| Male | 2211 | 52.3 | 2180 | 52.9 | 2135 | 52.0 | |
| Missing | 163 | 3.9 | 161 | 3.9 | 160 | 3.9 | |
| Age (years) | Median and IQR | 46 | 40 to 54 | 45 | 40 to 54 | 45 | 40 to 54 |
| Age group (years) | 40–59 | 3501 | 82.8 | 3431 | 83.2 | 3414 | 83.2 |
| 60–74 | 567 | 13.4 | 532 | 12.9 | 530 | 12.9 | |
| Missing | 163 | 3.9 | 161 | 3.9 | 160 | 3.9 | |
| Ethnicity | White | 1502 | 35.5 | 1477 | 35.8 | 1489 | 36.3 |
| Black | 797 | 18.8 | 822 | 19.9 | 813 | 19.8 | |
| Asian | 197 | 4.7 | 224 | 5.4 | 248 | 6.0 | |
| Mixed | 861 | 20.4 | 806 | 19.5 | 769 | 18.7 | |
| Other | 98 | 2.3 | 99 | 2.4 | 95 | 2.3 | |
| Missing | 776 | 18.3 | 696 | 16.9 | 690 | 16.8 | |
| IMD quintile | Most deprived | 1214 | 28.7 | 1224 | 29.7 | 1225 | 29.9 |
| 4 | 2183 | 51.6 | 2128 | 51.6 | 2068 | 50.4 | |
| 3 | 365 | 8.6 | 367 | 8.9 | 381 | 9.3 | |
| 2 | 11 | 0.3 | 16 | 0.4 | 14 | 0.3 | |
| Least deprived | 0 | 0 | 0 | ||||
| Missing | 458 | 10.8 | 389 | 9.4 | 416 | 10.1 | |
Trial results
Missing outcome data
Data for the primary outcome were extracted from general practice electronic health records. At the time of data extraction it was not possible to search for outcome data for a small number of participants. Research and information governance regulations required that all patient-identifying records remained at general practices. Spreadsheets documenting recruitment and randomisation for the in-practice randomisation method were therefore stored on practice systems. At the time of data extraction, 6 months after the last participant had been recruited, a small number of spreadsheets were found to be missing. Possible reasons for the spreadsheets being missing included their not being saved, their being saved in the wrong location or their being inadvertently deleted during the 6-month follow-up period. It is also possible that in certain months a procedure might have been performed remotely at the offices of primary care shared services and data stored there, but because of reorganisation and relocation of the shared services function during 2015 it was not possible to investigate this possibility. Details of the missing outcome data are provided in Table 13. The 407 participants with missing outcome data amounted to 3.3% of all trial participants and 4.8% of participants recruited through the in-practice recruitment method. These participants were excluded from trial analyses, leaving 12,052 participants for further analysis (see Figure 5).
| Practice | Month | Number of participants |
|---|---|---|
| 3 | 14 | 77 |
| 4 | 18 | 25 |
| 5 | 14 | 56 |
| 7 | 9 and 17 | 102 |
| 8 | 17 and 18 | 77 |
| 10 | 16 | 40 |
| 11 | 16 | 25 |
| 12 | 18 | 5 |
| Total | 407 |
Primary and subgroup analyses
The primary analysis was undertaken on all participants for whom we had extracted outcome data (n = 12,052). The unadjusted proportions of participants recorded as attending a health check within 6 months of the first invitation are shown in Table 14. In total, 590 out of 4095 (14.41%) participants in the standard invitation arm, 630 out of 3988 (15.80%) participants in the QBE questionnaire arm and 629 out of 3969 (15.85%) participants in the QBE questionnaire and incentive arm attended a health check. The primary analysis model estimated the difference in uptake between the standard invitation arm and the QBE questionnaire arm to be 1.4% (95% CI –0.1 to 3.0%; p = 0.070), indicating a slight increase in health check uptake in the intervention arm. Similarly, the difference in uptake between the standard invitation arm and the QBE questionnaire and incentive trial arm was estimated to be 1.5% (95% CI –0.0 to 3.1%; p = 0.054). The two intervention arms were found to have a similar uptake, with an estimated difference of –0.01% (95% CI –1.59% to 1.58%; p = 0.995). Overall variation in health check uptake by calendar month was not significant (p = 0.239) but the highest uptake was for January invitations (18.2%) and the lowest was for June invitations (12.9%).
| Trial arm | Number of participants | Number (%) of checks within 6 months | Difference in uptake (95% CI) (%)a | p-valueb |
|---|---|---|---|---|
| Standard invitation | 4095 | 590 (14.41) | – | |
| QBE questionnaire | 3988 | 630 (15.80) | 1.43 (–0.12 to 2.97) | 0.070 |
| QBE questionnaire and incentive | 3969 | 629 (15.85) | 1.52 (–0.03 to 3.07) | 0.054 |
| Total | 12,052 | 1849 (15.34) |
The results of the adjusted analysis, which yielded marginally more precise results, are presented in Table 15. These results demonstrate that health check uptake was about 4% higher in women than in men and about 5% higher in people aged ≥ 60 years than in younger adults. Health check uptake was also higher in participants of black, Asian or mixed ethnicity than in the local white population. There was also evidence that health check uptake tended to be higher as level of deprivation decreased. Fitting IMD quintile as a continuous variable revealed an increase in the odds of health check uptake of 1.10 (95% CI 1.01 to 2.21; p = 0.035) per quintile decrease in deprivation.
| Characteristic | Category | Uptake of health checks at 6 months after randomisation | |||||
|---|---|---|---|---|---|---|---|
| N | n | % | ORa | 95% CI | p-valueb | ||
| Trial arm | Standard invitation | 4095 | 590 | 14 | Reference | ||
| QBE questionnaire | 3988 | 630 | 16 | 1.13 | 1.00 to 1.27 | 0.042 | |
| QBE questionnaire and incentive | 3969 | 629 | 16 | 1.13 | 1.02 to 1.26 | 0.018 | |
| Gender | Female | 5449 | 992 | 18 | Reference | ||
| Male | 6526 | 857 | 13 | 0.74 | 0.69 to 0.80 | < 0.001 | |
| Missing | 77 | 0 | 0 | – | |||
| Age group (years) | 40–59 | 10,346 | 1530 | 15 | Reference | ||
| 60–74 | 1629 | 319 | 20 | 1.43 | 1.20 to 1.71 | < 0.001 | |
| Missing | 77 | 0 | 0 | – | |||
| Ethnicity | White | 4537 | 501 | 11 | Reference | ||
| Black | 2457 | 497 | 20 | 2.15 | 1.86 to 2.49 | < 0.001 | |
| Asian | 680 | 138 | 20 | 2.03 | 1.63 to 2.67 | < 0.001 | |
| Mixed | 2471 | 673 | 27 | 3.09 | 2.07 to 4.62 | < 0.001 | |
| Other | 295 | 38 | 13 | 1.28 | 0.88 to 1.85 | 0.194 | |
| Missing | 1755 | 36 | 2 | 0.15 | 0.07 to 0.34 | < 0.001 | |
| IMD quintile | Most deprived | 3663 | 550 | 15 | Reference | ||
| 4 | 6379 | 993 | 16 | 1.08 | 0.95 to 1.22 | 0.215 | |
| 3 | 1113 | 193 | 17 | 1.15 | 0.95 to 1.39 | 0.156 | |
| 2 | 41 | 13 | 32 | 2.78 | 1.87 to 4.12 | < 0.001 | |
| Missing | 856 | 100 | 12 | ||||
Figures 6–8 display intervention effect estimates by subgroup. It can be seen that the estimated difference in proportion of uptake between arms was consistent with the overall intervention effect for all subgroups except for the subgroup of men. Men had a greater estimated difference in uptake for both intervention arms compared with standard invitation [difference 2.88% (95% CI 0.91% to 4.84%; p = 0.004) between standard invitation and QBE questionnaire and 2.29% (95% CI 0.33% to 4.24%; p = 0.022) between standard invitation and QBE questionnaire plus incentive].
FIGURE 6.
Uptake of health checks within 6 months of randomisation for the standard invitation and QBE questionnaire trial arms by subgroup.
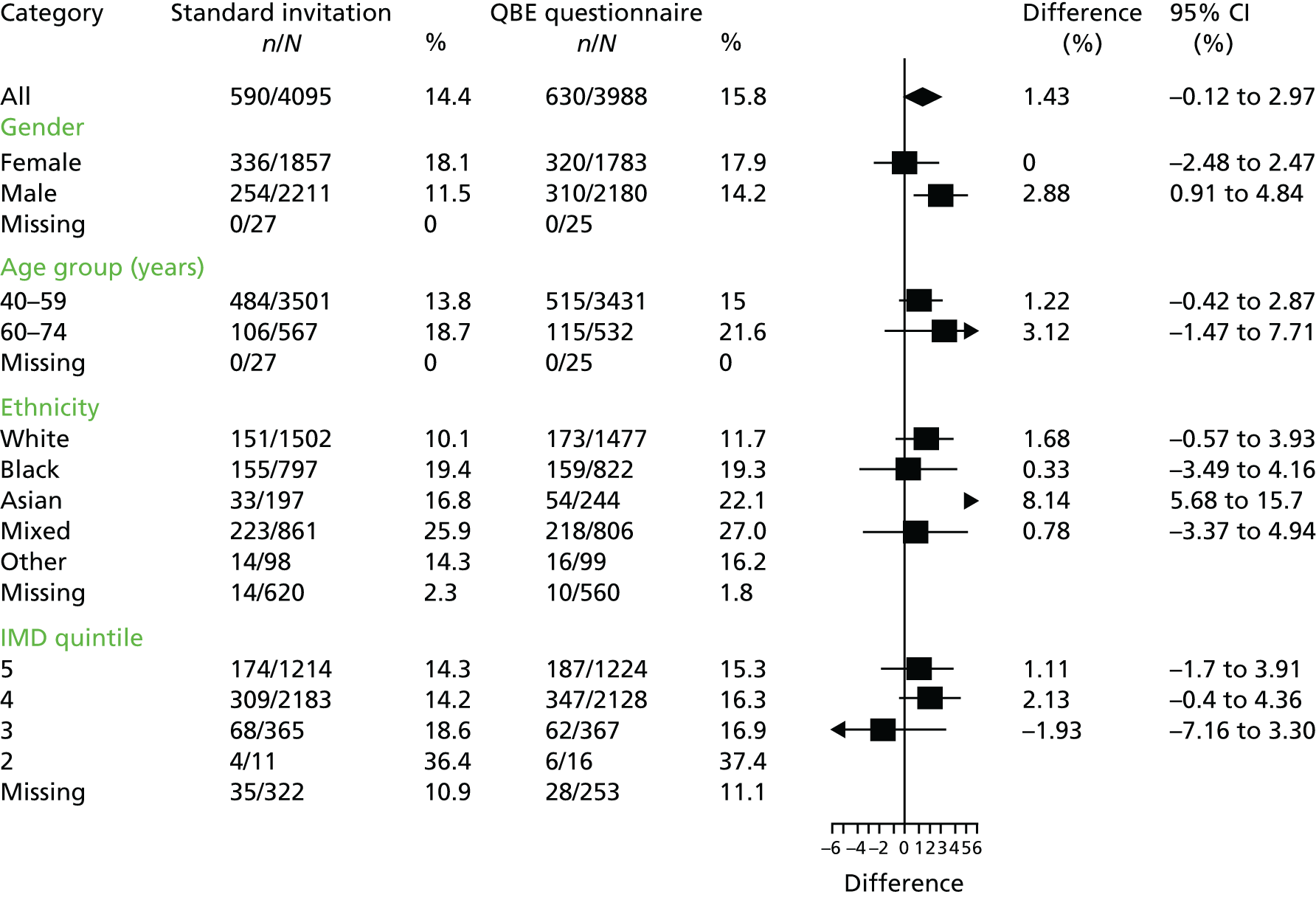
FIGURE 7.
Uptake of health checks within 6 months of randomisation for the standard invitation and QBE questionnaire with incentive trial arms by subgroup.
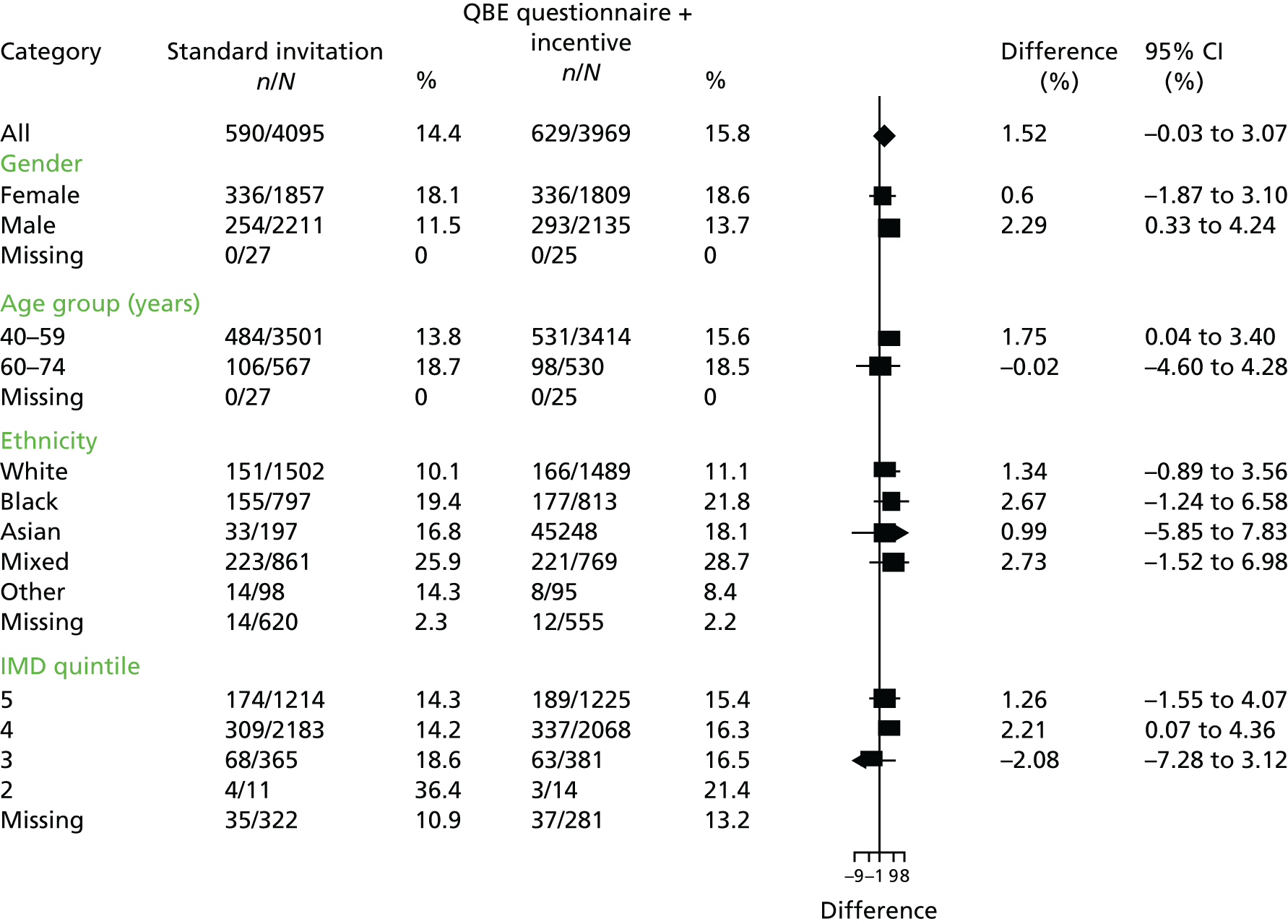
FIGURE 8.
Uptake of health checks within 6 months of invitation for the QBE questionnaire and QBE questionnaire with incentive trial arms by subgroup.

Secondary analyses
Time to health check
In total, 2280 health checks were recorded for trial participants, including 42 from the pilot study; therefore, a total of 2238 health checks were recorded in eligible patients during the entire period of the trial. There were 1849 health checks within 6 months of the randomisation date, of which 1126 were within 3 months of the randomisation date. In total, 388 health checks were completed > 6 months after the randomisation date, with the latest being recorded 538 days following randomisation. One health check conducted on the date of randomisation was not considered to result from trial invitation. A total of 319 out of 2280 (14%) health checks were recorded with codes indicating that the health check was completed by a third-party provider. A Kaplan–Meier plot displaying the cumulative proportion recorded as attending a NHS health check by time since randomisation is shown in Figure 9. There is no apparent difference between the curves for the two intervention trial arms but evidence of a small increase in uptake between the intervention curves and standard care over time. A reminder letter was sent to non-responders at 3 months after the first invitation.
FIGURE 9.
Proportion of participants in each trial arm recorded as attending a NHS health check by time since first invitation.

Analysis in a time-to-event framework showed that the adjusted hazard ratio between the standard invitation arm and the QBE questionnaire arm indicated a non-significant relative increase in health check attendance across time of 11% [HR 1.11, 95% CI 0.99 to 1.24; p = 0.072); similarly, the adjusted HR between the standard invitation arm and the QBE questionnaire with incentive arm was 1.11 (95% CI 0.99 to 1.25; p = 0.062).
The intraclass correlation coefficient for the primary outcome by practice was 0.007 (95% CI 0.001 to 0.013). Values for each trial arm were as follows: standard invitation 0.003 (95% CI 0.000 to 0.009); QBE questionnaire 0.005 (95% CI 0.000 to 0.012); and QBE questionnaire and incentive 0.008 (95% CI 0.000 to 0.0175). Table 16 presents a comparison of the risk difference estimates obtained using different estimation methods. Estimates obtained using either the GEE approach or a GLM were consistent, whereas use of robust standard errors yielded narrower CIs and smaller p-values with either estimation method, but this did not lead to any difference in interpretation.
| Method | QBE questionnaire – standard invitation | QBE questionnaire and incentive – standard invitation | ||
|---|---|---|---|---|
| Risk difference (95% CI) | p-value | Risk difference (95% CI) | p-value | |
| GEE | 1.43 (–0.12 to 2.97) | 0.070 | 1.52 (–0.03 to 3.07) | 0.054 |
| GEE, robust SE | 1.42 (0.01 to 2.75) | 0.035 | 1.52 (0.20 to 2.84) | 0.024 |
| GLM | 1.39 (–0.17 to 2.95) | 0.080 | 1.48 (–0.09 to 3.04) | 0.065 |
| GLM, robust SE | 1.39 (0.06 to 2.73) | 0.041 | 1.48 (0.20 to 2.75) | 0.023 |
Health check uptake at 3 months following the invitation was 353 out of 4095 (8.6%) in the standard invitation arm, 386 out of 3988 (9.7%) in the QBE questionnaire arm and 387 out of 3969 (9.8%) in the QBE questionnaire and incentive arm. Risk differences were 1.04 (95% CI –0.21 to 2.28; p = 0.103) for the QBE questionnaire arm compared with the standard invitation arm and 1.11 (–0.14 to 2.36; p = 0.083) for the QBE questionnaire and incentive arm compared with the standard invitation arm.
Chapter 4 Results 2: question–behaviour effect questionnaire responses
Question–behaviour effect questionnaire return
The QBE questionnaire was sent to 3988 participants in the QBE questionnaire arm and 3969 participants in the QBE questionnaire plus incentive arm. Questionnaire return rates are presented in Table 17. The questionnaire return rate was slightly higher, by an adjusted estimate of 1.42% (95% CI –0.4% to 3.26%; p = 0.132), in the arm that included an incentive for completing the questionnaire.
| Questionnaire return | Trial arm | |||
|---|---|---|---|---|
| QBE questionnaire | QBE questionnaire and incentive | |||
| n | % | n | % | |
| Not returned | 3071 | 77.0 | 2995 | 75.5 |
| Returned | 917 | 23.0 | 974 | 24.5 |
| Total | 3988 | 3969 | ||
Health check uptake was higher in participants who returned the QBE questionnaire. In the two intervention trial arms (QBE questionnaire and QBE questionnaire and incentive), 32.5% and 32.8% of participants who returned the QBE questionnaire subsequently attended a health check, respectively. A per-protocol analysis estimated an increase in uptake of 17.9% for the QBE questionnaire and 18.3% for the QBE questionnaire and incentive compared with standard care (Table 18).
| Trial arm | Number of participants | Number of checks within 6 months (%) | Difference in uptake (95% CI)a | p-valueb |
|---|---|---|---|---|
| Standard invitation | 4095 | 590 (14.4) | – | |
| QBE questionnaire | 917 | 298 (32.5) | 17.9 (14.7 to 21.1) | < 0.001 |
| QBE questionnaire and incentive | 974 | 319 (32.8) | 18.3 (15.2 to 21.5) | < 0.001 |
These estimates may be biased because a minority of randomised participants are included in the intervention trial arms but all participants are included in the control trial arm. To obtain an improved estimate of the effect of completing and returning the QBE questionnaire, a CACE analysis was performed73 (Table 19). This analysis compared the average uptake of participants who returned the questionnaire in the intervention trial arms with the average uptake in the standard care arm of participants who were expected to return the questionnaire had the QBE questionnaire been sent to them. 73 As there was no loss to follow-up, it was possible to estimate the intervention effect without taking into account the missing data mechanism. The CACE analysis estimated the health check uptake to be 6.0% greater in the QBE questionnaire arm compared with the standard invitation arm (95% CI 0.8% to 11.3%; p = 0.024) and 5.9% greater in the QBE questionnaire and incentive arm compared with the standard invitation arm (95% CI 0.8% to 10.9%; p = 0.022) (see Table 19).
| Trial arm | Number of compliers (%); uptake (%) | Number of non-compliers (%); uptake (%) | Total (%); uptake (%) |
|---|---|---|---|
| Standard invitation | NK; NK | NK; NK | 4095 (100); 14.4 |
| QBE questionnaire | 917 (23.0); 32.5 | 3071 (77.0); 10.8 | 3988 (100); 15.8 |
| QBE questionnaire and incentive | 974 (24.5); 32.8 | 2995 (75.5); 10.4 | 3969 (100); 15.9 |
Table 20 presents an analysis of variables associated with return of the QBE questionnaire. The offer of an incentive for questionnaire return did not lead to significantly higher rates of return but there was demographic variation among questionnaire respondents and non-respondents. Men were significantly less likely than women to return the questionnaire. Individuals for whom gender information was missing were significantly more likely to return the questionnaire than were women. Compared with white participants, black participants and those for whom ethnic group data were missing were significantly less likely to return a questionnaire. In contrast, those whose ethnic group was described as mixed were more likely than white participants to return the questionnaire. There was an impact of socioeconomic deprivation on questionnaire return. Individuals living in the middle 20% and second least deprived 20% of areas were more likely to return a questionnaire than individuals living in the most deprived quintile.
| Characteristic | Return of QBE questionnaire | ||||||
|---|---|---|---|---|---|---|---|
| N | n | % | ORa | 95% CI | p-value | ||
| Trial arm | QBE questionnaire | 3988 | 917 | 23.0 | Reference | ||
| QBE questionnaire and incentive | 3969 | 974 | 24.5 | 1.09 | 0.98 to 1.22 | 0.105 | |
| Gender | Female | 3592 | 962 | 26.8 | Reference | ||
| Male | 4315 | 915 | 21.2 | 0.76 | 0.69 to 0.85 | < 0.001 | |
| Missing | 50 | 14 | 28.0 | 1.93 | 1.01 to 3.71 | 0.047 | |
| Age group (years) | 40–59 | 6845 | 1539 | 22.5 | Reference | ||
| 60–74 | 1062 | 338 | 31.8 | 1.56 | 1.39 to 1.75 | < 0.001 | |
| Missing | 50 | 14 | 28.0 | – | |||
| Ethnicity | White | 2966 | 722 | 24.3 | Reference | ||
| Black | 1635 | 314 | 19.2 | 0.76 | 0.65 to 0.90 | 0.001 | |
| Asian | 472 | 115 | 24.4 | 1.02 | 0.78 to 1.34 | 0.890 | |
| Mixed | 1575 | 526 | 33.4 | 1.54 | 1.34 to 1.77 | < 0.001 | |
| Other | 194 | 37 | 19.1 | 0.77 | 0.53 to 1.13 | 0.181 | |
| Missing | 1115 | 177 | 15.9 | 0.59 | 0.44 to 0.78 | < 0.001 | |
| IMD quintile | Most deprived | 2449 | 519 | 21.2 | Reference | ||
| 4 | 4196 | 1020 | 24.3 | 1.16 | 0.97 to 1.40 | 0.111 | |
| 3 | 748 | 206 | 27.5 | 1.31 | 1.04 to 1.65 | 0.024 | |
| 2 | 30 | 14 | 46.7 | 3.08 | 1.74 to 5.45 | < 0.001 | |
| Missing | 534 | 132 | 24.7 | 1.13 | 0.88 to 1.43 | 0.321 | |
Table 21 presents the mean (SD) questionnaire item responses by trial arm. These responses reveal that, among questionnaire respondents, beliefs about having a health check were largely positive, with no apparent difference between trial arms. Table 22 presents correlation coefficients for inter-item correlations. Correlations between items tapping the same constructs were assessed. The items tapping ‘intentions’ (questions 1 and 4) were well correlated and so an ‘intentions’ score for use in further analysis was constructed from the mean of these two items for each participant. The items tapping ‘anticipated regret’ (questions 3 and 5) were also correlated and so an ‘anticipated regret’ score was calculated as the mean of the scores for these two items. The items tapping ‘attitudes’ (questions 2 and 6) were correlated (r = 0.525) but it was decided to use these as separate scores in the analysis because question 2 reflects instrumental attitudes, whereas question 6 reflects affective attitudes. Table 23 presents the correlations between the resulting questionnaire constructs.
| Questionnaire item | Construct | Total | QBE questionnaire | QBE questionnaire and incentive | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Q1. I intend to go for a health check in the next few weeks | Intentions | 1884 | 5.69 | 1.53 | 912 | 5.71 | 1.55 | 972 | 5.68 | 1.52 |
| Q2. For me, going for a health check in the next few weeks would be . . . (very bad to very good) | Attitudes: instrumental | 1888 | 6.17 | 1.02 | 916 | 6.17 | 1.02 | 972 | 6.16 | 1.01 |
| Q3. If I did not go for a health check in the next few weeks, I would feel regret | Anticipated regret | 1884 | 5.23 | 1.62 | 912 | 5.28 | 1.61 | 972 | 5.18 | 1.63 |
| Q4. Will I go for a health check in the next few weeks? | Intentions | 1885 | 6.03 | 1.39 | 913 | 6.02 | 1.36 | 972 | 6.03 | 1.42 |
| Q5. If I did not go for a health check in the next few weeks, I would later wish I had | Anticipated regret | 1873 | 5.57 | 1.46 | 909 | 5.59 | 1.44 | 964 | 5.54 | 1.49 |
| Q6. For me, going for a health check in the next few weeks would be . . . (very worrying to very reassuring) | Attitudes: affective | 1881 | 5.69 | 1.24 | 911 | 5.70 | 1.24 | 970 | 5.68 | 1.25 |
| Q7. I’m confident I can go for a health check in the next few week | PBC | 1883 | 5.85 | 1.23 | 913 | 5.86 | 1.19 | 970 | 5.83 | 1.26 |
| Q8. People who are important to me would . . . | Subjective norms | 1888 | 6.24 | 0.98 | 914 | 6.26 | 0.96 | 973 | 6.22 | 1.00 |
| Questionnaire item | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 |
|---|---|---|---|---|---|---|---|
| Q1. I intend to go for a health check in the next few weeks | – | ||||||
| Q2. For me, going for a health check in the next few weeks would be . . . (very bad to very good) | 0.527 | – | |||||
| Q3. If I did not go for a health check in the next few weeks, I would feel regret | 0.468 | 0.504 | – | ||||
| Q4. Will I go for a health check in the next few weeks? | 0.685 | 0.621 | 0.536 | – | |||
| Q5. If I did not go for a health check in the next few weeks, I would later wish I had | 0.502 | 0.573 | 0.715 | 0.600 | – | ||
| Q6. For me, going for a health check in the next few weeks would be . . . (very worrying to very reassuring) | 0.349 | 0.525 | 0.397 | 0.442 | 0.446 | – | |
| Q7. I’m confident I can go for a health check in the next few week | 0.562 | 0.575 | 0.479 | 0.704 | 0.537 | 0.499 | – |
| Q8. People who are important to me would . . . | 0.377 | 0.552 | 0.437 | 0.502 | 0.508 | 0.415 | 0.497 |
| Construct | Intentions | Attitude: instrumental | Anticipated regret | Attitude: affective | PBC |
|---|---|---|---|---|---|
| Intentions | – | ||||
| Attitude: instrumental | 0.624 | – | |||
| Anticipated regret | 0.619 | 0.582 | – | ||
| Attitude: affective | 0.430 | 0.525 | 0.458 | – | |
| PBC | 0.685 | 0.575 | 0.549 | 0.499 | – |
| Subjective norms | 0.476 | 0.552 | 0.510 | 0.415 | 0.497 |
Table 24 presents analysis of the variance of the questionnaire constructs by trial arm. There was no evidence that mean questionnaire construct scores differed by trial arm. Therefore, the suggestion that people in the non-incentivised condition may hold more positive beliefs about health checks is not supported.
| Construct | Number of observations | F-statistic | p-value |
|---|---|---|---|
| Intentions | 1878 | 0.03 | 0.871 |
| Attitude: instrumental | 1888 | 0.02 | 0.886 |
| Anticipated regret | 1872 | 1.30 | 0.254 |
| Attitude: affective | 1881 | 0.18 | 0.675 |
| PBC | 1883 | 0.32 | 0.569 |
| Subjective norms | 1887 | 0.85 | 0.355 |
Table 25 presents univariate ORs associating questionnaire responses with health check uptake. ORs represent the increase in odds of health check uptake per unit increase in score on each questionnaire construct. Each of the constructs predicted uptake, with participants with higher intentions, more positive attitudes, higher levels of PBC, perceiving greater social approval for having a check and anticipating more regret for not having a check being more likely to have a health check. The largest OR was for the effect of intentions, followed by those for subjective norms, PBC and instrumental attitudes. Anticipating more regret and affective attitudes were the weakest predictors, but still had significant effects.
| Construct | ORa | 95% CI | p-value |
|---|---|---|---|
| Intentions | 1.35 | 1.25 to 1.47 | < 0.001 |
| Attitude: instrumental | 1.25 | 1.16 to 1.36 | < 0.001 |
| Anticipated regret | 1.13 | 1.08 to 1.19 | < 0.001 |
| Attitude: affective | 1.17 | 1.09 to 1.25 | < 0.001 |
| PBC | 1.26 | 1.16 to 1.36 | < 0.001 |
| Subjective norms | 1.26 | 1.13 to 1.41 | < 0.001 |
In Table 26 questionnaire constructs were dichotomised at the median score, which was 6 for each construct. Odds of health check uptake were contrasted for those with highly positive responses (score of 6 or 7) and those with less positive responses (score ≤ 5). For each of the questionnaire constructs, participants expressing positive responses showed a higher uptake of health checks than participants expressing negative responses. ORs were greatest for intentions, subjective norms and PBC.
| Construct | Response | Attended health check | p-value | |||
|---|---|---|---|---|---|---|
| n/N | % | ORa | 95% CI | |||
| Intentions | More positive response | 500/1362 | 36.7 | 2.05 | 1.72 to 2.44 | < 0.001 |
| Less positive response | 114/516 | 22.1 | ||||
| Attitude: instrumental | More positive response | 545/1595 | 34.2 | 1.59 | 1.20 to 2.11 | 0.001 |
| Less positive response | 72/293 | 24.6 | ||||
| Anticipated regret | More positive response | 369/1039 | 35.5 | 1.33 | 1.16 to 1.52 | < 0.001 |
| Less positive response | 244/833 | 29.3 | ||||
| Attitude: affective | More positive response | 468/1318 | 35.5 | 1.54 | 1.25 to 1.91 | < 0.001 |
| Less positive response | 148/563 | 26.3 | ||||
| PBC | More positive response | 515/1462 | 35.2 | 1.70 | 1.35 to 2.14 | < 0.001 |
| Less positive response | 102/421 | 24.2 | ||||
| Subjective norms | More positive response | 562/1645 | 34.2 | 1.76 | 1.34 to 2.32 | < 0.001 |
| Less positive response | 55/242 | 22.7 | ||||
Chapter 5 Results 3: evaluation of automated allocation at source in the context of a rapid trial
This trial was commissioned as a ‘rapid trial’ with the aim of providing evidence for clinical and policy decision-making within short times cales. This chapter evaluates the extent to which the trial achieved its design objectives.
A timeline for the major activities of the trial is shown in Table 27. The award letter for the trial was dated 1 November 2012 and the contract for the trial was signed off in February 2013. The start date for the project, when staff came into post, was 1 May 2013. By this time research ethics committee and NHS research and development approvals had already been obtained. The first participants were recruited into the trial on 28 July 2013, 3 months ahead of the intended schedule. Recruitment of general practices to the study was completed in the first quarter of 2014 and participant recruitment into the trial continued until December 2014.
| Activity | 2012 | 2013 | 2014 | 2015 | 2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | |
| Award letter | ||||||||||||||
| Contract signed off | ||||||||||||||
| Research ethics approval | ||||||||||||||
| NHS research and development approval | ||||||||||||||
| Staff in post | ||||||||||||||
| First participants recruited | ||||||||||||||
| Develop automated allocation | ||||||||||||||
| Practice recruitment | ||||||||||||||
| Participant recruitment | ||||||||||||||
| Participant follow-up for 6 months | ||||||||||||||
| Data checking | ||||||||||||||
| Qualitative interviews | ||||||||||||||
| Ascertain primary outcome | ||||||||||||||
| Data analysis and report writing | ||||||||||||||
| Report submission | ||||||||||||||
Figure 10 illustrates the predicted and observed recruitment into the trial. Observed recruitment began earlier than predicted, but was at a slightly slower rate. Recruitment was completed over 18 months rather than the 12 months predicted. The recruitment rate could have been increased by drawing more general practices into the trial, but we aimed to recruit from all general practices over a 12-month period and this consideration influenced our decision to accept only a slightly longer recruitment period. The overall rate of recruitment was 702 participants per month over an 18-month period. Subsequent to the completion of recruitment, we followed up participants for 6 months to ascertain the primary outcome. We also collected data to evaluate the outcome of the health check. Data collection was completed in October 2015. The final report was submitted in January 2016.
FIGURE 10.
Projected and observed recruitment by month from trial start.
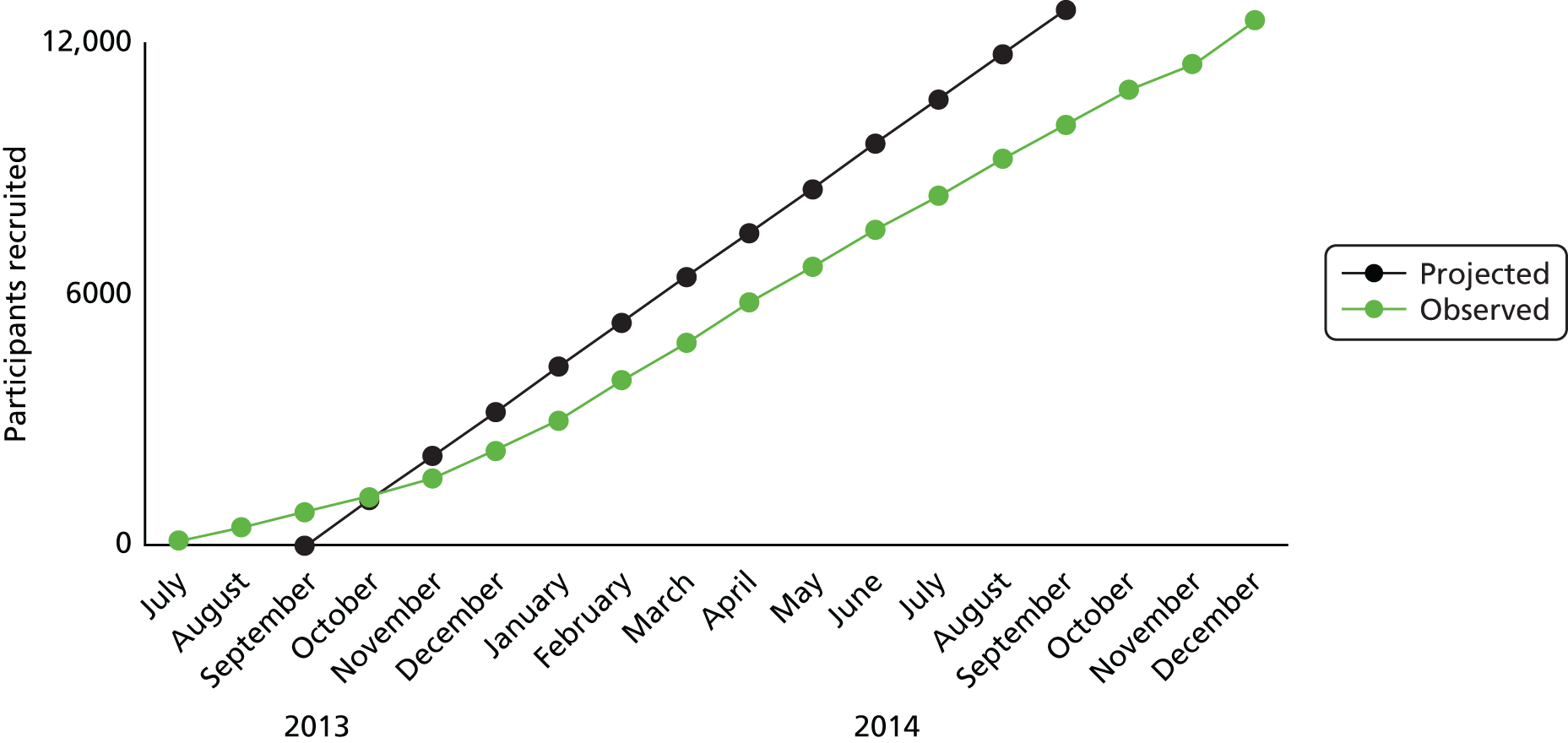
The use of electronic health records from primary care for participant recruitment, randomisation and outcome evaluation contributed to the speed of the study and efficiency of the study design. The study was methodologically innovative in developing a fully automated technique for recruitment and randomisation that was embedded into the management information system that was used to manage the health check invitations. Table 28 presents a comparison of the strengths and limitations of the fully automated and in-practice methods of recruitment and randomisation.
| Variable | In-practice method | Automated method |
|---|---|---|
| Time to start | Sooner (3 months) | Later (7 months) |
| Randomisation design | In-house | In-house/third party |
| Randomisation conduct | In-house | Third party |
| Randomisation record | Full | Partial |
| Labour intensive | Monthly general practice visits over 18 months | No requirement for practice visits |
| Outcome data | Extracted at general practice visits | Extracted at general practice visits |
| Missing data | Present for 10/178 (6%) practice months | 0/72 practice months |
| Trial outcomes | Generally consistent | Generally consistent |
The in-practice system required only limited development work and was ready to be started within 3 months of the trial start date. Responsibility for design of the randomisation procedure and the conduct of randomisation were retained ‘in-house’ at King’s College London. A full record of randomisation was retained. However, the in-practice method was labour intensive requiring the study team to conduct monthly visits to practices, with records for trial participants being stored on practice systems for the duration of the trial. As already noted, spreadsheets for 10 practice months were found to be missing at the time of outcome data collection.
The automated system for recruitment and randomisation required a longer development period as well as a 2-month pilot study. The design of the randomisation was in part determined by the third-party provider and this resulted in the choice to use simple randomisation, stratified by practice and month, rather than block randomisation. It was not possible for the research team to fully audit and document the randomisation process. However, records for trial participants were stored centrally at the offices of the primary care shared services team and records for all participants were successfully retrieved at the end of the trial.
An analysis to examine whether practice or method of randomisation had any impact on the primary trial outcome did not suggest a difference in uptake. Forest plots that display the intervention effect by practice and randomisation method are presented in Figure 11 for the standard invitation and QBE questionnaire trial arms, Figure 12 for the standard invitation and QBE questionnaire and incentive trial arms and Figure 13 for the QBE questionnaire and QBE questionnaire and incentive trial arms. Between-practice variation was found to be low. There was no evidence of heterogeneity either between general practices within allocation method or between allocation methods.
FIGURE 11.
Forest plot displaying the intervention effect (risk difference) by practice and randomisation method for the comparison between standard care and the QBE questionnaire. RD, risk difference; W(fixed), weight in the fixed-effects meta-analysis.

FIGURE 12.
Forest plot displaying the intervention effect (risk difference) by practice and randomisation method for the comparison between standard care and the QBE questionnaire plus incentive. RD, risk difference; W(fixed), weight in the fixed-effects meta-analysis.

FIGURE 13.
Forest plot displaying the intervention effect (risk difference) by practice and randomisation method for the comparison between the QBE questionnaire and the QBE questionnaire plus incentive. RD, risk difference; W(fixed), weight in the fixed-effects meta-analysis.

Chapter 6 Results 4: cohort study of case mix for invited and opportunistic NHS health checks
During the trial study period, from 1 July 2013 to 30 June 2015, the total number of NHS health checks recorded at trial practices, after their start date in the trial, was 6184, including 2280 in trial participants and 3904 in non-trial participants. We extracted data for cardiovascular risk scores and BMI for participants with health checks recorded. Cardiovascular risk score data were obtained for 5359 participants, including 2246 out of 2280 (99%) trial participants with health checks recorded and 3113 out of 3904 (80%) non-trial participants with health checks recorded. The proportion of health checks in non-trial participants with a CVD risk score recorded ranged from 33% to 93% at different general practices. These findings suggest that primary care staff may be less likely to record a CVD risk score when they complete an opportunistic health check. The 3113 cardiovascular risk assessments in non-trial participants [out of 5359 participants with cardiovascular risk score data (58%)] either were performed opportunistically or were carried out in patients invited before their general practice joined the trial. These will be referred to as ‘opportunistic health checks’. Among the opportunistic checks were 1363 (44%) that were completed within 6 months of the practice start date in the trial, which might potentially have resulted from invitations sent before the practice entered the trial. Sensitivity analyses were performed to evaluate the impact of possible misclassification on the results of the study.
To confirm the reliability of these findings, routinely collected monitoring data were obtained from the health check management information system employed by the two boroughs. The routine data differ from trial data because they are reported for follow-up to 31 December 2014 and not to the end of 6 months’ follow-up in June 2015, as was completed for the trial.
Table 29 presents routinely collected and trial data for the numbers of participants invited for health checks at trial general practices during the study period. There is generally close agreement between the management information and trial data, with < 2% discrepancy between the two data sources for 11 practices. More substantial differences were observed for two practices (numbers 9 and 15). Reasons for these discrepancies were not clear, although management information system data were extracted 12 months after the completion of trial recruitment and we have observed previously that data from the management information system may show minor changes over time. The mean difference in number of participants identified by the two data sources was 0 (95% CI –34 to 33; p = 0.984). In the context of routinely collected health service data, the overall level of agreement between the two sources of data was considered to be good.
| Practice | Start date | End date | Months | Invited (MIS data) | Invited (trial data) | Difference |
|---|---|---|---|---|---|---|
| 1 | 1 July 2013 | 31 December 2014 | 18 | 1429 | 1416 | 13 |
| 2 | 1 July 2013 | 31 December 2014 | 18 | 738 | 727 | 11 |
| 3 | 1 August 2013 | 31 December 2014 | 17 | 1144 | 1166 | –22 |
| 4 | 1 August 2013 | 31 December 2014 | 17 | 696 | 696 | 0 |
| 5 | 1 August 2013 | 31 December 2014 | 17 | 1211 | 1220 | –9 |
| 6 | 1 September 2013 | 31 December 2014 | 16 | 403 | 402 | 1 |
| 7 | 1 December 2013 | 31 December 2014 | 13 | 723 | 726 | –3 |
| 8 | 1 December 2013 | 31 December 2014 | 13 | 356 | 377 | –21 |
| 9 | 1 December 2013 | 31 December 2014 | 13 | 621 | 809 | –188 |
| 10 | 1 January 2014 | 31 December 2014 | 12 | 524 | 499 | 25 |
| 11 | 1 January 2014 | 31 December 2014 | 12 | 374 | 344 | 30 |
| 12 | 1 January 2014 | 31 December 2014 | 12 | 160 | 189 | –29 |
| 13 | 1 December 2013 | 31 December 2014 | 13 | 451 | 504 | –53 |
| 14 | 1 December 2013 | 31 December 2014 | 13 | 1156 | 1096 | 60 |
| 15 | 1 December 2013 | 31 December 2014 | 13 | 1156 | 978 | 178 |
| 16 | 1 February 2014 | 31 December 2014 | 11 | 732 | 732 | 0 |
| 17 | 1 February 2014 | 31 December 2014 | 11 | 219 | 218 | 1 |
| 18 | 1 February 2014 | 31 December 2014 | 11 | 360 | 360 | 0 |
| 12,453 | 12,459 | Mean 0 (95% CI –34 to 33); p = 0.984 |
Table 30 presents routinely collected data from the management information system for the general practices included in the trial. During the period identified, 12,453 patients were recorded as being sent an invitation for a health check. Among invited patients, 1206 health checks were carried out at general practices and 484 health checks were carried out by third-party providers (including pharmacies and outreach teams), giving a total of 1690 ‘invited’ health checks. Among non-invited patients, 1285 health checks were carried out at general practices and 337 were carried out at third-party providers. In the management information system data, the term ‘invited’ refers to health checks completed within 6 months of an invitation. The 1622 health checks classified as ‘not invited’ accounted for 49.0% of all health checks carried out during the reporting period. The proportion of not invited health checks ranged from 26.9% to 78.7% at different general practices. These routinely collected data confirm the finding from the trial data that a high proportion of health checks are not performed within 6 months of an invitation issued through the call–recall system. Many of these non-invited health checks may be performed opportunistically in general practices and pharmacies and through the activities of outreach teams, who are commissioned by the boroughs to conduct checks in high-risk and underserved groups of the local population. Health checks by third-party providers accounted for 24.8% (821/3312) of all health checks in the reporting period. Data were therefore collected to compare case mix and outcomes for trial participants who received invited checks and those who received non-invited or opportunistic checks.
| Practice | Start date | End date | Eligible | Sent invitation | Completed health checks | Total no. of checks | Total not invited | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General practice | Third party | |||||||||||
| n | % | Invited | Not invited | Invited | Not invited | n | % of all checks | |||||
| 1 | 1 July 2013 | 31 December 2014 | 3374 | 1429 | 42 | 122 | 75 | 23 | 30 | 250 | 105 | 42.0 |
| 2 | 1 July 2013 | 31 December 2014 | 1612 | 738 | 46 | 94 | 197 | 5 | 3 | 299 | 200 | 66.9 |
| 3 | 1 August 2013 | 31 December 2014 | 2871 | 1144 | 40 | 115 | 111 | 76 | 68 | 370 | 179 | 48.4 |
| 4 | 1 August 2013 | 31 December 2014 | 2050 | 696 | 34 | 71 | 39 | 19 | 16 | 145 | 55 | 37.9 |
| 5 | 1 August 2013 | 31 December 2014 | 3258 | 1211 | 37 | 129 | 91 | 76 | 48 | 344 | 139 | 40.4 |
| 6 | 1 September 2013 | 31 December 2014 | 1017 | 403 | 40 | 36 | 24 | 29 | 8 | 97 | 32 | 33.0 |
| 7 | 1 December 2013 | 31 December 2014 | 2250 | 723 | 32 | 62 | 72 | 16 | 7 | 157 | 79 | 50.3 |
| 8 | 1 December 2013 | 31 December 2014 | 1251 | 356 | 28 | 39 | 11 | 10 | 7 | 67 | 18 | 26.9 |
| 9 | 1 December 2013 | 31 December 2014 | 2068 | 621 | 30 | 64 | 61 | 30 | 25 | 180 | 86 | 47.8 |
| 10 | 1 January 2014 | 31 December 2014 | 1674 | 524 | 31 | 41 | 42 | 43 | 36 | 162 | 78 | 48.1 |
| 11 | 1 January 2014 | 31 December 2014 | 1259 | 374 | 30 | 11 | 19 | 14 | 13 | 57 | 32 | 56.1 |
| 12 | 1 January 2014 | 31 December 2014 | 860 | 160 | 19 | 27 | 106 | 2 | 1 | 136 | 107 | 78.7 |
| 13 | 1 December 2013 | 31 December 2014 | 1671 | 451 | 27 | 29 | 25 | 3 | 4 | 61 | 29 | 47.5 |
| 14 | 1 November 2013 | 31 December 2014 | 3463 | 1156 | 33 | 117 | 162 | 72 | 28 | 379 | 190 | 50.1 |
| 15 | 1 December 2013 | 31 December 2014 | 3129 | 1156 | 37 | 75 | 46 | 26 | 19 | 166 | 65 | 39.2 |
| 16 | 1 February 2014 | 31 December 2014 | 2907 | 732 | 25 | 66 | 61 | 11 | 9 | 147 | 70 | 47.5 |
| 17 | 1 February 2014 | 31 December 2014 | 923 | 219 | 24 | 16 | 6 | 20 | 11 | 53 | 17 | 32.1 |
| 18 | 1 February 2014 | 31 December 2014 | 2094 | 360 | 17 | 92 | 137 | 9 | 4 | 242 | 141 | 58.3 |
| Total | 37,731 | 12,453 | 33.0 | 1206 | 1285 | 484 | 337 | 3312 | 1622 | 49.0 | ||
Table 31 presents data for the distribution of invited and opportunistic health checks by case mix variables including gender, age group, ethnicity and deprivation quintile. Adjusted ORs were adjusted for each variable shown. There was no gender difference in the proportion of opportunistic checks. Opportunistic checks were more frequent in younger participants, with invited checks being more frequent in the older age group. There was some evidence for a trend in the distribution of health checks by deprivation quintile, with opportunistic checks being more frequent in the most deprived quintile and invited checks being more frequent in less deprived quintiles. However, evaluation of a possible linear trend showed an OR of 0.92 (95% CI 0.82 to 1.03; p = 0.137) for unit decrease in deprivation quintile from most to least deprived.
| Characteristic | Invited health checks | Opportunistic health checks | OR (95% CI)a | p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| All | 2246 | 42 | 3113 | 58 | |||
| Gender | Female | 1210 | 42 | 1671 | 58 | – | |
| Male | 1036 | 42 | 1442 | 58 | 1.01 (0.90 to 1.14) | 0.868 | |
| Age group (years) | 40–59 | 1880 | 41 | 2703 | 59 | – | |
| 60–74 | 366 | 47 | 410 | 53 | 0.82 (0.67 to 1.00) | 0.050 | |
| Ethnicity | White | 533 | 40 | 803 | 60 | – | |
| Black | 551 | 39 | 875 | 61 | 1.03 (0.90 to 1.18) | 0.642 | |
| Asian | 143 | 40 | 217 | 60 | 1.00 (0.84 to 1.18) | 0.994 | |
| Mixed | 789 | 45 | 953 | 55 | 0.82 (0.68 to 0.98) | 0.030 | |
| Other | 59 | 46 | 70 | 54 | 0.77 (0.52 to 1.13) | 0.182 | |
| Missing | 171 | 47 | 195 | 53 | 0.77 (0.47 to 1.25) | 0.287 | |
| IMD quintile | Most deprived | 695 | 40 | 1028 | 60 | – | |
| 4 | 1197 | 42 | 1646 | 58 | 0.96 (0.82 to 1.12) | 0.582 | |
| 3 | 235 | 45 | 290 | 55 | 0.89 (0.67 to 1.19) | 0.420 | |
| 2 | 15 | 88 | 2 | 12 | 0.10 (0.04 to 0.26) | < 0.001 | |
| Missing | 104 | 41 | 147 | 59 | 0.96 (0.78 to 1.17) | 0.666 | |
Table 32 presents the proportion of checks with a CVD risk score of ≥ 10% by source of health check. Overall, 17.0% of invited health checks and 22.2% of opportunistic health checks were associated with a CVD risk score of ≥ 10%. The relative odds of an elevated CVD risk for opportunistic checks compared with invited checks was 1.70 (95% CI 1.45 to 1.99; p < 0.001). Higher proportions with an increased CVD risk were consistently observed across subgroups of gender, age and ethnicity. In the most deprived quintile, 15.3% of invited checks and 22.4% of opportunistic checks were associated with an elevated CVD risk (OR 1.94, 95% CI 1.37 to 2.74; p < 0.001). In the third quintile of deprivation (the highest for which estimation was feasible), similar proportions had an elevated CVD risk (OR 1.10, 95% CI 0.80 to 1.51; p = 0.572). These results show that opportunistic checks tend to be targeted towards individuals with a higher CVD risk and greater levels of deprivation.
| Characteristic | Invited health checks | Opportunistic health checks | Relative odds of 10% CVD risk if check is opportunistic (95% CI)a | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | ||||
| All | 382 | 2246 | 17.0 | 692 | 3113 | 22.2 | 1.70 (1.45 to 1.99) | < 0.001 | |
| Gender | Female | 106 | 1210 | 8.8 | 216 | 1671 | 12.9 | 1.85 (1.36 to 2.53) | < 0.001 |
| Male | 276 | 1036 | 26.6 | 476 | 1442 | 33.0 | 1.63 (1.33 to 2.00) | < 0.001 | |
| Age group (years) | 40–59 | 206 | 1880 | 11.0 | 448 | 2703 | 16.6 | 1.66 (1.37 to 2.00) | < 0.001 |
| 60–74 | 176 | 366 | 48.1 | 244 | 410 | 59.5 | 1.85 (1.33 to 2.59) | < 0.001 | |
| Ethnicity | White | 96 | 533 | 18.0 | 179 | 803 | 22.3 | 1.49 (1.17 to 1.89) | 0.001 |
| Black | 70 | 551 | 12.7 | 150 | 875 | 17.1 | 1.74 (1.37 to 2.21) | < 0.001 | |
| Asian | 25 | 143 | 17.5 | 47 | 217 | 21.7 | 1.66 (1.03 to 2.69) | 0.037 | |
| Mixed | 146 | 789 | 18.5 | 254 | 953 | 26.7 | 1.92 (1.53 to 2.42) | < 0.001 | |
| Other | 13 | 59 | 22.0 | 13 | 70 | 18.6 | 1.18 (0.42 to 3.30) | 0.752 | |
| Missing | 32 | 171 | 18.7 | 49 | 195 | 25.1 | 1.62 (1.15 to 2.28) | 0.005 | |
| IMD quintile | Most deprived | 106 | 695 | 15.3 | 230 | 1028 | 22.4 | 1.94 (1.37 to 2.74) | < 0.001 |
| 4 | 202 | 1197 | 16.9 | 365 | 1646 | 22.2 | 1.68 (1.39 to 2.04) | < 0.001 | |
| 3 | 48 | 235 | 20.4 | 60 | 290 | 20.7 | 1.10 (0.80 to 1.51) | 0.572 | |
| 2 | 5 | 15 | 33.3 | 0 | 2 | 0 | – | ||
| Missing | 21 | 104 | 20.2 | 37 | 147 | 25.2 | – | ||
As a sensitivity analysis, we omitted all health checks completed within 6 months of the practice joining the study, as these might have been invited in an earlier period. The adjusted OR for a ≥ 10% CVD risk associated with opportunistic checks was then 1.40 (95% CI 1.14 to 1.73; p = 0.002), based on 3996 observations. We evaluated the effect of omitting health checks in invited participants that were completed > 6 months after the invitation, as these might have been opportunistic checks. The adjusted OR was then 1.65 (95% CI 1.40 to 1.94; p < 0.001), based on 4983 observations. We conclude that the reported association is robust to varying the case definition for an opportunistic check. We also evaluated whether or not lower ascertainment of risk scores for opportunistic health checks might have influenced the findings. At nine practices where a CVD risk score was obtained for > 85% of opportunistic health checks, the OR was 1.77 (95% CI 1.45 to 2.16; p < 0.001); at nine practices where a CVD risk score was obtained for ≤ 85% of opportunistic health checks the OR was 1.70 (95% CI 1.45 to 1.99; p < 0.001). This finding does not suggest that the results could be explained by differential underascertainment of CVD risk scores.
Table 33 presents equivalent data for the proportions of participants with a ≥ 20% CVD risk score. Overall, 5.0% of invited checks and 6.3% of opportunistic checks were associated with a ≥ 20% CVD risk. The OR was 1.46 (95% CI 1.12 to 1.91; p = 0.005). ORs were generally consistent across subgroups but power was necessarily lower because of the smaller numbers of individuals with a higher CVD risk score.
| Characteristic | Invited health checks | Opportunistic health checks | Relative odds of 20% CVD risk if check is opportunistic (95% CI)a | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | ||||
| All | 113 | 2246 | 5.0 | 196 | 3113 | 6.3 | 1.46 (1.12 to 1.91) | 0.005 | |
| Gender | Female | 24 | 1210 | 2.0 | 46 | 1671 | 2.8 | 1.48 (1.03 to 2.13) | 0.036 |
| Male | 89 | 1036 | 8.6 | 150 | 1442 | 10.4 | 1.48 (1.07 to 2.04) | 0.019 | |
| Age group (years) | 40–59 | 54 | 1880 | 2.9 | 99 | 2703 | 3.7 | 1.27 (0.90 to 1.80) | 0.166 |
| 60–74 | 59 | 366 | 16.1 | 97 | 410 | 23.7 | 1.78 (1.16 to 2.73) | 0.008 | |
| Ethnicity | White | 39 | 533 | 7.3 | 54 | 803 | 6.7 | 0.96 (0.56 to 1.66) | 0.891 |
| Black | 20 | 551 | 3.6 | 39 | 875 | 4.5 | 1.45 (0.83 to 2.53) | 0.194 | |
| Asian | 5 | 143 | 3.5 | 11 | 217 | 5.1 | 2.00 (0.78 to 5.10) | 0.148 | |
| Mixed | 35 | 789 | 4.4 | 70 | 953 | 7.3 | 1.87 (1.21 to 2.91) | 0.005 | |
| Other | 6 | 59 | 10.2 | 5 | 70 | 7.1 | 0.86 (0.31 to 2.40) | 0.775 | |
| Missing | 8 | 171 | 4.7 | 17 | 195 | 8.7 | 2.68 (0.96 to 7.50) | 0.060 | |
| IMD quintile | Most deprived | 30 | 695 | 4.3 | 72 | 1028 | 7.0 | 1.99 (1.18 to 3.38) | 0.010 |
| 4 | 59 | 1197 | 4.9 | 96 | 1646 | 5.8 | 1.35 (0.92 to 1.98) | 0.124 | |
| 3 | 15 | 235 | 6.4 | 15 | 290 | 5.2 | 0.99 (0.43 to 2.27) | 0.985 | |
| 2 | 0 | 15 | 0 | 0 | 2 | 0 | – | ||
| Missing | 9 | 104 | 8.7 | 13 | 147 | 8.8 | |||
Table 34 presents the proportions of participants who were overweight or obese by source of health check. Overall, 55.7% of participants receiving invited checks and 58.8% of participants receiving opportunistic checks were identified as being overweight or obese (OR 1.15, 1.04 to 1.28; p = 0.008). This distinction was greater for women, with 52.8% of women receiving invited checks and 58.0% of women receiving opportunistic checks, being overweight or obese (OR 1.25, 1.07 to 1.47; p = 0.005). Higher proportions with overweight and obesity also tended to be observed among younger participants and white participants who received opportunistic health checks. There were 189 health checks with missing values for BMI but associations were similar if missing values were either omitted or combined with the normal weight category for reference.
| Characteristic | Invited health checks | Opportunistic health checks | ORb (95% CI) | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | % | n | N | % | ||||
| All | 1252 | 2246 | 55.7 | 1831 | 3113 | 58.8 | 1.13 (1.03 to 1.24) | 0.008 | |
| Gender | Female | 639 | 1210 | 52.8 | 969 | 1671 | 58.0 | 1.23 (1.05 to 1.45) | 0.005 |
| Male | 613 | 1036 | 59.2 | 862 | 1442 | 59.8 | 1.03 (0.89 to 1.19) | 0.521 | |
| Age group (years) | 40–59 | 1057 | 1880 | 56.2 | 1625 | 2703 | 60.1 | 1.16 (1.09 to 1.25) | < 0.001 |
| 60–74 | 195 | 366 | 53.3 | 206 | 410 | 50.2 | 0.97 (0.64 to 1.47) | 0.861 | |
| Ethnicity | White | 268 | 533 | 50.3 | 439 | 803 | 54.7 | 1.25 (1.04 to 1.50) | 0.007 |
| Black | 387 | 551 | 70.2 | 626 | 875 | 71.5 | 1.05 (0.82 to 1.34) | 0.687 | |
| Asian | 54 | 143 | 37.8 | 104 | 217 | 47.9 | 1.52 (0.88 to 2.62) | 0.228 | |
| Mixed | 406 | 789 | 51.5 | 501 | 953 | 52.6 | 1.07 (0.89 to 1.27) | 0.299 | |
| Other | 41 | 59 | 69.5 | 42 | 70 | 60.0 | 0.51 (0.24 to 1.09) | 0.040 | |
| Missing | 96 | 171 | 56.1 | 119 | 195 | 61.0 | 1.24 (0.74 to 2.07) | 0.349 | |
| IMD quintile | Most deprived | 421 | 695 | 60.6 | 645 | 1028 | 62.7 | 1.11 (0.90 to 1.37) | 0.291 |
| 4 | 650 | 1197 | 54.3 | 946 | 1646 | 57.5 | 1.16 (1.04 to 1.30) | 0.005 | |
| 3 | 121 | 235 | 51.5 | 162 | 290 | 55.9 | 1.20 (0.78 to 1.84) | 0.363 | |
| 2 | 6 | 15 | 40.0 | 0 | 2 | 0 | – | ||
| Missing | 54 | 104 | 51.9 | 78 | 147 | 53.1 | – | ||
Chapter 7 Results 5: process evaluation and qualitative study
Alongside the RCT, we undertook evaluations of processes that might have an impact on the outcome of the trial. Such evaluations are useful to assess the fidelity and quality of implementation, clarify causal mechanisms and identify contextual factors associated with variation in outcomes. 74 This may provide insights to aid implementation of an intervention or suggest how an intervention might be refined for better effect.
We did not need to assess the fidelity of our intervention as this was a written intervention and was in the same format and delivered in the same way to each participant randomised to receive it. We examined attitudes of practice staff and programme leads engaged in delivering the health check in case these influenced implementation of our intervention in any way. Free-text responses by participants at the end of the questionnaire (intervention) were also analysed to assess participant views towards either receiving our intervention or the health check itself. Finally, an in-depth qualitative interview study of individuals invited to receive a health check was conducted in the same boroughs as the trial, exploring influences on uptake of the health check that might help to explain participant behaviour in relation to our intervention to promote uptake.
There were therefore three aspects to our evaluation of process, which were analysed qualitatively:
-
qualitative interview study with general practice staff
-
content analysis of trial participant responses to an open-ended question
-
qualitative interview study with general practice patients.
Interviews with general practice staff
Twenty-two general practice staff from 17 general practices and two public health leads responsible for implementing health checks in Lambeth and Lewisham were interviewed. Participants included practice managers (52%), nurses/health-care assistants (9%), administrators (30%) and public health leads (9%). Twenty interviews were recorded and transcribed and handwritten notes were made during two interviews and included in the analysis. Responses were categorised according to three main themes: attitudes towards health checks; attitudes towards service delivery of the health checks; and attitudes towards the RCT.
Attitudes towards health checks
In general, staff expressed the view that health checks were beneficial but that the programme did have strengths and weaknesses:
It’s a good way to try and prevent illness and long term or serious conditions developing in the future.
P9 practice manager
I think it’s good that we have a health check programme in place and we’re all for supporting that. Healthier people mean less people queuing up in the hospitals.
P11 practice manager
Specific benefits that were highlighted included optimism that informing people of their risk might lead to positive behaviour change:
They can help people to improve their health and understand risks of disease and lifestyle. Patients can understand their risks and the way it influences their health. It’s good for the practice as potential health issues can be detected early and prevention of these can begin.
P3 nurse
Patients will come in for them so that’s good as they get an extra check. Main benefit of them seems to be that people will be made aware of how they are at risk and change what they are eating etc.
P2 administrator
Reservations about the programme included doubts about the long-term benefits of conducting health checks and about the costs of implementation, including staff resources:
The Health Check programme seems like a good idea. Not really sure about how good they will be in the long term, it might just be one of those things that people forget about . . . Health checks are good to get people thinking about their health but whether or not they do anything about this is another issue. I’m not convinced they will result in behaviour change or health improvement – they just tell you what you already know, which is that you’re not healthy.
P1 administrator
I think the theory behind the programme is very laudable, but in terms of the amount of resource it take up within a general practice, and what it picks up, it doesn’t seem to be what I would consider sort of value for money.
P10 practice manager
It’s very time-consuming. It’s lengthy to deliver sometimes and a lot of the people that come for it have got an actual problem that they want to discuss.
P17 nurse
Attitudes towards service delivery of the health checks
When asked more specifically about the implementation of the health checks in their practices many staff felt that it worked well, with clear communication channels among the staff who were involved in the delivery of the health checks:
Yes patients ring in, the health checks are done by a nurse. There are three of us. We work alternate shifts. So there’s a nurse here eight ‘til eight, Mondays to Fridays. And they can see any nurse, any day, any time for anything. But there’s not a specific clinic.
P17 health-care assistant
The patients are seen by a nurse. And we issue blood forms for patients. And once they’ve had their blood tests, we ask them to then come in to book an appointment with the nurse. It is usually a longer appointment, a 30-minute appointment that’s booked with the nurse for them to go through all the tests and all the check-ups. And all the staff in reception of how to book the health check, also whether the patient needs to have the blood test beforehand. And that is what we’ve done. The are checked and if any patient is not eligible for whatever reason, it is then marked as such and the lists are returned.
P13 practice manager
Problems identified by some staff included concerns that uptake was low in their particular practice and that sometimes when patients are referred for blood tests they fail to have these carried out so the health check is not completed:
Not working well in the practice – more checks being done by third parties . . . we [practice] need to identify ways of being more proactive.
P8 practice manager
Well you send them to [hospital A] to get their bloods done and they don’t come back.
P8 practice manager
All general practices appeared to offer health checks opportunistically, that is, to patients attending the practice for another, unrelated reason rather than them being invited through the Health Check programme:
Patients can ring up and book one, they don’t have to have invitation letter, or might have one if seeing GP about something else or joining the practice.
P2 health check administrator
Yes, we encourage as many people to have one as possible. We checked that we were able to do this and got permission from the Lambeth team and we can get paid for each one we do whether they were invited or not.
P4 health check administrator
Routinely when patients register and they are between 40–74 we will then invite them in ourselves and also opportunistically when patients come up to the desk they will be asked verbally to have a health check.
P3 practice manager
Yes a lot of opportunistic invites as the patients come into the practice also by doctors and we have posters in the surgery.
P3 practice manager
A large proportion of people receiving the health check may therefore not be those who receive a written invitation to receive one. As well as receiving a health check opportunistically at their general practice, individuals may obtain checks at local pharmacies and community events, through outreach teams, without having to wait to be invited through the Health Check programme. Information from the health check in these cases is sent through to the relevant general practice, where it is added to the database and coded as a completed health check:
All coded relevantly as NHS health check completed by pharmacist or community-based nurse or anything like that, of if it’s done on site, obviously we have our own relevant code. Yes it’s coded accordingly . . . Yes, we get them same, through the software, the QMS practice software, we get confirmations of ‘completed health checks’ coming through, which we process and obviously note all the information down on the patient’s file.
P15 health check administrator
Attitudes towards the randomised controlled trial
The general practices were, for the most part, unaware of and unaffected by the process of running our RCT. Identification and randomisation of eligible participants was mostly conducted automatically and remotely from the practices. Those practices assigned to the in-practice method, whereby trial staff required access to general practice computers and software, reported a few problems with accessing the relevant list of patients eligible for a health check but this did not appear to be a major barrier to the smooth running of the trial:
The trial was easy to run but difficult to check the PNL.
P3 practice nurse
There was no challenge at all running study at practice. Just making sure the staff member who deals with health checks was in office.
P1 administrator
It was good for the practice to have a trial running here.
P2 administrator
Staff did not report the impression that uptake had increased during the trial study period:
So I wouldn’t assume that we’ve had a lot of uptake from even what you’ve done, to be honest.
P12 practice manager
Conclusions
Although health-care staff appeared broadly to support the idea of a Health Check programme, they did express reservations about how effective it was likely to be. Their reservations were mainly centred on the uptake of the health check and about the likelihood of individuals responding to information about their cardiovascular risk in a meaningful way, that is, by changing their behaviour and adopting a healthier lifestyle.
It is interesting to note that many of the health checks are conducted opportunistically and not just delivered to individuals who have received a written invitation to receive one. Health checks delivered opportunistically are added to the overall figures for uptake in the programme. Our trial targeted only people receiving the written invitation for a health check, which may be a small proportion of the total number actually completing the health check.
Despite the ambivalence of some general practice staff about the Health Check programme, it is unlikely that this affected the smooth running of our trial. Most of the participants were identified and randomised remotely and practices included in the ‘manual’ method did not report many problems with this, nor did trial researchers report significant barriers to obtaining the information and support that they needed to complete the randomisation procedures at these practices.
Content analysis of participant responses
The total number of participants returning the QBE questionnaire was 1956 across the two intervention trial arms. Of those returning a questionnaire, 648 (33%) had made comments in the free-text box. It was possible to categorise comments according to whether they related to the health check itself or to the QBE intervention. There were also some miscellaneous responses that did not fit these two themes.
Views about the health check
Participants expressed their views about the health check. In general, health checks were viewed positively and the preventative aspect of the health check was appreciated:
I would like an annual health check. I think they are a very good idea and would probably be cost-effective in helping people to live healthier lives.
This is very important because this will help early detection and treatment of an illness or conditions. I’m very pleased with this, thanks.
Some respondents expressed more negative views about the health check, querying the value for money of conducting them. Despite returning a questionnaire to the research team such individuals may have felt ambivalent about attending for their health check:
The government is wasting its money on public health programmes when it is the arrogant, incompetent compassionless behaviour of NHS employees at every level that deters people from listening or bothering to go.
Waste of money the NHS needs for other things.
Others appreciated the value of a health check and may have expressed the intention to have one but may not have attended because they anticipated that it would be difficult to obtain an appointment at an appropriate time:
I’m 100% supportive of health checks but have two small children and not much support so depends on childcare arrangements. Will make every effort to attend.
Just starting a new job so as much as I would like to, it could be dependent on timing/availability.
I have been unable to get an appointment with the GP of my choice, since the end of September and he is not available until the New Year.
A few concerns were expressed about the consequences of having the health check and the implications for their health:
After having a health check waiting for your result is worrying.
I am looking forward to it. Hopefully I will be healthy. I am very worried.
A lack of awareness of the Health Check programme was apparent and receiving our questionnaire ahead of the invitation to attend raised some queries about what the health check might involve:
Are they free? How do you go about getting a health check?
I could answer this [questionnaire] more accurately if I knew more detail about the health check, e.g. does it include a mammogram?
The invitation letter would have arrived within days of the questionnaire, with comprehensive information about the health check, but these comments raise the question of whether or not it might be beneficial to improve general knowledge about cardiovascular health checks and what they entail ahead of inviting people to attend for one.
Views about the question–behaviour effect intervention (questionnaire)
Participants commented on the content and style of the questionnaire. Although these participants clearly returned their questionnaires, it is possible that the issues they raise affected other potential participants who failed to complete or post their questionnaires back to us. It is also possible that comments reflect polarised views not held by non-respondents; perhaps only those who felt strongly in either direction were motivated to return the questionnaire:
This is the most absurd questionnaire I have completed in some time.
I can’t tell you anything except that this is a bizarre form and would certainly put me off going.
Feel it is patronising and if I didn’t have some intelligence to make a decision I would be more than likely put off from going.
Questionnaire is poorly written and in my case completely irrelevant.
There was a suggestion that the questionnaire items may not have been understood as intended:
I’m curious about your research methodology, one question about TV but nothing about whether the distance/ease of transport/opening hours affect decision making, particularly as these are key for me.
The questionnaire is rather repetitive and question 5 is slightly misleading, you should take out the dots! It depends on whether you offer reasonable appointments outside working hours for those who work (i.e. the majority in London).
Although the intervention had been developed and tested for acceptability within the target population ahead of the trial, these comments suggest that the questionnaire may have benefited from further refinement. Some participants may have felt that the questionnaire did not let them express their full range of views about health checks. This is a challenge for QBE interventions which attempt to address salient beliefs that will promote the behaviour of interest.
Conclusions
Although only one-third of the participants who returned the questionnaire responded with comments in the free-text box, these participants raised some interesting issues in relation to attendance for a health check. These included the views of some individuals that such checks should not be a priority in an overstretched NHS and do not represent good value for money. Even among those who felt that health checks were positive and beneficial there was a perception that having a health check at a time that was convenient would be a challenge or just arranging the appointment to have one might be difficult. It is possible that our intervention lacked the potency to overcome these particular barriers to attendance. It is clear that some participants in the trial expressed the intention to attend for a health check but then failed to do so, suggesting that some changes in the system of delivering the checks may be needed to overcome the intention–behaviour gap in these cases.
Our intervention was modified from a QBE questionnaire developed for use in a study conducted outside London. Although we tested and simplified our version of the intervention with the target population for our trial and obtained feedback from relevant health professionals and service providers, the questionnaire may have benefited from further piloting for use with the population in south-east London. It is unclear whether further refinement might have improved the response rate to the questionnaire or impacted on attendance for the health check.
Patient interview study
Twenty-seven non-trial participants were included in the analysis. Five themes emerged from the data relating to views towards having the health check: (1) awareness and expectations of the health check, (2) beliefs about susceptibility to CVD and eligibility for a health check, (3) civic responsibility, (4) practical barriers to attending and (5) beliefs about the consequences of having a health check. These themes are illustrated briefly below. Full details and results of the study are published elsewhere. 30 The quotations that follow in this section have been reproduced with permission from Influences on individuals’ decisions to take up the offer of a health check: a qualitative study. Burgess C, Wright AJ, Forster AS, Dodhia H, Miller J, Fuller F, et al. Health Expectations volume 18, issue 6. 30 Copyright © 2015 John Wiley & Sons Ltd under the terms of the Creative Commons Attribution License (CC BY) which allows users to copy, distribute and transmit an article, adapt the article and make commercial use of the article. The CC BY license permits commercial and non-commercial re-use of an open access article, as long as the author is properly attributed.
Participants were generally unaware of the Health Check programme and did not appreciate that it is designed specifically to assess the risk of CVD. Only three of those interviewed reported having heard of health checks prior to being invited; two had seen a promotional poster and one participant’s spouse had already been invited.
Lack of awareness emerged as a general theme across both those who accepted and those who declined to have a health check. It may be that a lack of clarity and understanding of what the health check involved had discouraged attendance. People may need more specific information about what is involved in a health check to inform their decision-making about attendance.
It appeared that the decision to take up the offer of a health check or not was influenced to some extent by perceived personal risk of CVD. There was evidence that a family history of stroke or heart attack affected personal risk perceptions; attendance might be encouraged in those with a family history and discouraged in those without:
family history is obviously, you know, a huge determinant of various things. OK not completely conclusive, but you know, law of averages, I thought I’m probably OK. So it just slipped and then I never took up on it.
ID22, female aged 62 years, did not attend30
It was not always clear to those invited why they had been selected to receive a health check when they felt well and enjoyed a healthy lifestyle:
if it’s something that I need to do and something I need to be aware of [I’d do it] but unless you’re really dying or feeling unwell, you’re not really going to bother with it,
ID25, female aged 57 years, did not attend30
Individuals expressed a need to understand why they had been selected for assessment when they were currently feeling well or perceived themselves as living a healthy lifestyle.
A sense of duty, not only to friends and family but also to the health-care system, encouraged attendance in some cases, as did taking advantage of a free service when it is offered:
I wasn’t sure with cuts to funding whether or not this is the sort of thing that will be continuing in the future. So the thought was to make the most of it as soon as possible, I might not have the opportunity or I’ll have to pay for it going forward.
ID14, male aged 40 years, attended30
Conversely, others felt that they should not burden the doctor or NHS unnecessarily by diverting time and resources away from people who were actually unwell:
I mean there’s no point in doing that if it’s, you know, using up people’s precious time and resources if it’s not necessary.
ID23, female aged 56 years, did not attend30
The data illustrated a complex relationship between individuals and the NHS health-care system. In particular, some people seemed to express a sense of personal responsibility towards making the best use of NHS resources. This led to them questioning whether or not undergoing a cardiovascular risk assessment was justifiable in their case, particularly if they were not currently experiencing symptoms.
Obtaining an appointment for a health check at a convenient time was reported as an obstacle to attendance for some of those who worked normal office hours or whose income was directly proportional to hours worked:
It’s very difficult for me to [go to the appointment] and hold on to a nine-to-five job. It means I have to take personal time off from my employer to do this. They don’t give you an option where you can go in the evening. I would have to take it off as annual leave, and do it in my own personal time.
ID25, female aged 57 years, did not attend30
Those who reported few practical problems in attending for a health check tended to live within walking distance to their general practice and were more likely to be retired or employed in part-time work.
Some individuals who did have their health check nevertheless reported initial difficulties obtaining an appointment at their general practice, which was discouraging:
I remember ringing the surgery and the receptionist said ‘There is a tremendous waiting list for this’. She said ‘I’ll tell the nurse’ and I never heard anything. Then when I got the next [reminder] letter I rang up and they did give me an appointment.
ID 11, female aged 66 years, attended30
Non-attendance was also sometimes linked to a belief that it might be better not to know that one might have an undiagnosed condition or be at risk of developing one. Furthermore, people who suspect that their risk could be high might avoid having this confirmed with a health check, particularly if they would also receive unwelcome lifestyle change advice:
I didn’t want to find out I had more medical problems, I have epilepsy. And I don’t need a doctor to tell me I need to stop smoking and lose weight.
ID01, male aged 46 years, did not attend30
Does it actually help you to have knowledge, or not? That’s kind of an interesting thing, isn’t it, because it can just make you more anxious and the thing about health checks is its sort of fine if everything is fine. And if it’s not fine, are people prepared enough for what they might feel?
ID23, female aged 56 years, did not attend30
Conclusions
The findings from the patient interview study suggest that there are a number of factors that may affect uptake of a cardiovascular risk check. Many of the findings resonate with the comments of our trial participants in the free-text responses to the questionnaire. For example, those who were working full-time may have expressed the intention to attend but then found that it was not possible to arrange a convenient appointment time. The findings also suggest that some people found it difficult to arrange an appointment at their general practice or that no-one got back to them after they requested an appointment. Again, these individuals may have held positive attitudes towards having a health check and fully intended to have one, but then found it challenging to fit into their busy lives.
Chapter 8 Discussion
This chapter summarises the main findings from this research, makes comparisons with other studies, discusses the limitations of the research and finally makes recommendations for future research. The main lessons learned from this research are summarised in Box 1.
-
Trial data revealed a low uptake of health checks in response to the standard invitation letter of 15% in the first 6 months following invitation.
-
Uptake of health checks following a standard invitation letter was associated with greater age, female gender, lower levels of deprivation and non-white ethnicity.
-
There was no evidence that an invitation method employing the QBE was associated with a useful increase in the uptake of health checks.
-
There was no evidence that the offer of a financial incentive was associated with an increased response to the QBE questionnaire.
-
Effects were generally consistent across subgroups of gender, ethnicity and deprivation quintile but there was weak evidence of a greater effect in men than in women.
-
Participants who returned the QBE questionnaire were substantially more likely to attend for a health check.
-
Participants with highly positive responses to the QBE questionnaire items were more likely to attend for a health check than those with less positive responses.
-
These findings suggest that, in the subgroup of participants who responded to the QBE questionnaire, uptake of health checks was predicted by psychological constructs represented in the questionnaire.
-
This was a rapid trial. Participant recruitment was initiated within 3 months of the study start. Data collection, with 6 months’ follow-up of > 12,000 participants, was completed within 2.5 years of the study start.
-
The trial demonstrated the feasibility of an automated randomisation procedure programmed into health service programme management software, which enabled randomisation of 100% of eligible participants over a 1-year period.
-
Qualitative interviews with general practice staff confirmed that trial general practices were active in offering opportunistic health checks.
-
During the study period, nearly 6 out of 10 health checks were completed either > 6 months after the invitation or in participants who did not receive invitations through the population-based call–recall system. These are referred to as ‘opportunistic health checks’.
-
Opportunistic health checks showed a similar distribution to invited health checks with respect to gender, age group and ethnicity but were associated with greater deprivation.
-
Health checks conducted opportunistically were more likely to reveal elevated cardiovascular risk scores or overweight and obesity than health checks conducted following a standard invitation.
-
General practice staff endorsed the concept of preventative medical intervention but expressed concerns regarding the opportunity costs of conducting health checks and their possible lack of effectiveness in promoting behaviour change.
-
Patient interviews and free-text questionnaire responses revealed a lack of public understanding of the Health Check programme. Patients often welcomed the idea of a health check but were concerned about burdening services when they were well. They also experienced difficulty gaining access to appointments at times that were convenient to them.
Invitation methods for health checks and the question–behaviour effect
The primary objective of the study was to evaluate the effectiveness of an enhanced invitation method, based on the QBE, with or without the offer of a financial incentive to return the QBE questionnaire, at increasing the uptake of health checks. 65 The research provided no evidence that an invitation method based on the QBE could be associated with an increase in health check uptake that could be of clinical or public health importance. There was weak evidence, which did not reach the prespecified level of statistical significance, of a small increase in uptake, which amounted to < 2%. There was also weak evidence that this effect might be greater in men than in women but effects were otherwise similar across subgroups of age, ethnicity and deprivation. There was no evidence that the offer of a financial incentive to return the QBE questionnaire might increase the level of response to this invitation method.
Health check uptake was higher among individuals who returned the QBE questionnaire. The CACE analysis provided an intervention effect estimate for comparable groups, finding an approximate 6% increase in uptake among those who returned the questionnaire. Positive responses to the QBE questionnaire items were associated with greater uptake of the offer of a health check, with the strongest association being observed for the ‘intentions’ construct. The association of health check uptake with QBE questionnaire return might be explained in several different ways. This might be interpreted as evidence of the QBE at work, with stronger evidence of an effect among those who responded to the QBE intervention. A second alternative is that return of the QBE questionnaire, and attendance for a health check, are both predicted by similar underlying psychological factors. These might include, for example, attaching high importance to health. A third explanation could be that questionnaire returners and health check attendees share certain demographic characteristics that might make these behaviours easier to perform. The similarity of demographic factors predicting both health check uptake and questionnaire return, including older age and lower levels of deprivation, might lend support to these second two explanations.
How do the results compare with those of other studies of the question–behaviour effect?
This trial did not provide evidence that the QBE could make a contribution to increasing health check uptake. This is in contrast to earlier study findings regarding the positive impact of the QBE on health check uptake. 44 The previous study used a longer questionnaire. It is possible that the reduction in the number of items or slight changes in the constructs tapped could explain the difference in effects. However, a recent systematic review did not find that the number of questionnaire items affected the magnitude of the observed QBE. 50 The earlier study on health check uptake was conducted > 20 years ago. 44 Changes in pressures and resource constraints on general practice may have made it more difficult for participants in the present study to act on their invitation to have a health check. The present study also sampled participants from a wider range of socioeconomic and ethnic backgrounds, which may have also led to the difference in findings.
This is one of the largest trials conducted using the QBE and the results add to the weight of evidence against a quantitatively important impact of an intervention based on the QBE. 50,51 A recent trial of the impact of the QBE on colorectal cancer screening uptake46 also found no benefit of the intervention. Indeed, the effect of QBE interventions on uptake in our trial (OR 1.12/1.13) was highly consistent with the effect of the QBE on screening uptake reported in a recent review of the effects of the QBE on health behaviours51 (d = 0.06 can be converted to an OR of 1.1175). In contrast, a recent review that focused solely on the effects of asking intention or self-prediction questions to invoke the QBE found a larger overall pooled effect size. 50 This review included studies from both field and laboratory settings and a wider range of behaviours, rather than being restricted to studies focusing on health behaviours.
A number of trials of the QBE have been found to have a considerable risk of bias. 51 Although one review found no evidence that risk of bias significantly moderated the observed effects of the QBE on health behaviours,51 an ongoing review suggests that, when tests of the QBE are restricted to those with a lower risk of bias, the size of the observed effect is diminished. 76
The nature of the question–behaviour effect intervention used in this trial
Question–behaviour effect interventions have used a wide variety of questionnaire items50,51 and it is reasonable to question whether or not the questionnaire used in this trial shared the features of those used in the most effective QBE interventions. The trial QBE intervention was developed before the recent publication of two systematic reviews of the effects of the QBE. One of these reviews50 suggested that two aspects of our questionnaire, including anticipated regret questions and having a mixture of intention and self-prediction questions instead of just self-prediction questions, may have been associated with smaller effects on behaviour. A future study could evaluate the effects of a redesigned questionnaire, focusing on self-prediction items and omitting anticipated regret, on health check uptake.
The primary purpose of this study was not to test the psychological predictors of health check uptake. The questionnaire functioned as an intervention, increasing the accessibility of certain beliefs among questionnaire completers and so making them more likely to act on a health check invitation. The questionnaire was not intended to provide exhaustive measurement of TPB constructs and anticipated regret in relation to health check uptake. There is no evidence that the QBE requires the questionnaire employed to be reliable and valid. A recent meta-analysis did not find that the number of questionnaire items employed significantly moderated the magnitude of the QBE. 50 The review also noted that the vast majority of studies focused on measuring intention and self-prediction, so our measurement approach is consistent with extant QBE literature. The questionnaire leaflet as a whole had a Flesch reading ease score of 80.1 and a Flesch–Kincaid grade level of 5.9. This means that it is accessible to people with the reading ability of an 11-year-old. It is possible that the reduction in the number of items or a slight change in the ways that constructs were tapped could be explanations for the different effects in this study compared with those in previous studies. However, we find the alternative explanations for the differences in our findings, namely changes in pressures on general practice over the intervening 20-year period and the wider sociodemographic range of our participants, more plausible.
The free-text responses on a small proportion of returned questionnaires suggested that some participants felt that the questionnaire did not tap all of their key views on health check uptake, with participants particularly noting that there were no items asking about various barriers to health checks, such as appointment availability and timing. The attitude accessibility account of the QBE argues for omitting such items, as they would make perceived barriers to having a check more salient, thus being likely to deter uptake when invitation letters were received. This is at odds with the QBE questionnaire being distributed under the guise of an attempt to explore invitees’ views about health checks.
Previous studies and the present results suggest that the QBE best increases behaviour in participants with a positive reaction to the behaviour. Among questionnaire respondents, views about health checks were largely positive but only a small proportion of study participants returned the questionnaire. Questionnaire non-respondents may have had less positive views about health checks. Combining the QBE with a motivational intervention to increase positive intentions and attitudes might better support health check uptake. 77 One recent systematic review50 also revealed several factors associated with smaller effects of the QBE that may be less amenable to change in the context of promoting health check uptake. In particular, field studies were associated with smaller effects than laboratory studies and the effect was attenuated when there was a longer interval between the questionnaire and the opportunity to perform the behaviour and the behaviour was more difficult. The qualitative analyses suggest that having a health check may have been regarded as difficult by a notable proportion of participants.
How did offering a financial incentive affect the impact of the question–behaviour effect intervention?
This study was predicated on the idea that patients would be more likely to return a questionnaire if they are given a financial incentive, as there is considerable evidence to support the positive, although modest, effects of incentivising postal questionnaire return. 55 Return of the questionnaire was expected to be associated with improved health check uptake, as a previous trial44 found that the magnitude of the QBE is greatest in participants who complete the questionnaire. By incentivising questionnaire return, the hypothesis was that those in the incentive arm would have an even greater health check uptake rate than those receiving the questionnaire alone. The research showed that a financial incentive was not effective at increasing questionnaire return in this area as a means to potentially increase the QBE. There were concerns that, in certain circumstances, a higher questionnaire return rate would not necessarily lead to greater health check uptake in incentive arm participants. First, the magnitude of the QBE is largest for individuals with positive beliefs about the behaviour in question. If the incentive led to more participants with negative views about health checks completing the questionnaire than in the non-incentivised QBE arm, then the increased return rate may not be associated with increased health check uptake. A further alternative is that any cognitive dissonance effect of completing a questionnaire is undermined by an incentive. Participants might attribute the fact that they filled in the questionnaire to the incentive and therefore not experience any dissonance if they fail to have a health check, even though they said that they would do so in the questionnaire.
This trial showed that offering a conditional financial incentive for participants to complete a questionnaire made no difference to the rate of questionnaire return or the likelihood of patients taking part in the Health Check programme. There was no evidence that the incentive differentially affected response rates for participants with different levels of socioeconomic deprivation. The lack of effect of the incentive on health check uptake was consistent across subgroups of age, gender, ethnicity and deprivation. The randomised design, large sample size and the fact that the study took place in a natural setting all lend themselves to supporting the strength of these findings. There was no evidence that the offer of a financial incentive encouraged individuals who had less positive beliefs about health checks to complete and return the questionnaire. However, given that the provision of the incentive did not lead to a higher rate of questionnaire return, this finding must be interpreted with caution.
The present trial found no evidence that offering incentives enhanced the magnitude of the QBE effect on health check uptake. There was no evidence that response to the incentive, in terms of either questionnaire return or health check uptake, was moderated by socioeconomic deprivation. Because the offer of an incentive in the present study failed to increase questionnaire return rates, it is not possible to ascertain whether or not a greater response rate in the incentive arm was associated with less positive beliefs about health checks. The finding of no effect of an incentive on questionnaire return contrasts with the results of a recent trial of the QBE’s effects on FOBT uptake (Professor Mark Conner, personal communication), in which a financial incentive and a Post-it® note (Post-it® Brand, 3M United Kingdom PLC, Bracknell, UK) thank you message increased questionnaire return rates in an older population than was studied here. However, the incentive and thank you message did not lead to increased rates of the behaviour. Both of these findings contrast with those of a systematic review,50 which found a larger QBE effect in studies in which participants were incentivised. Participant incentives were more likely to be offered in laboratory studies with immediate measurement of the behaviour, both study features that were also associated with larger QBE effects. It was not possible to disentangle the effects of these three factors.
There are a number of possible explanations for the lack of effect of the incentive on questionnaire return in the trial. These relate to the nature of the incentive, participant motivation and research project-related factors. The incentive was offered conditional on questionnaire return. However, larger benefits of incentives with regard to response rates in postal surveys have been found for unconditional incentives, that is, when the incentive is provided at the time that the survey is sent to participants rather than on its return. However, unconditional incentives may not be feasible when targeting large groups of patients, such as in the Health Check programme, and may not be deemed acceptable by either patients or commissioners. It is possible that the value of the incentive offered, £5, was not considered sufficiently motivating to increase questionnaire return and a higher-value incentive might have been effective. However, this value is identical to the incentive offered in the trial of the QBE on FOBT uptake, which found that the incentive enhanced response rates (Professor Mark Conner, personal communication). A further possibility is that participants mistakenly viewed the incentive as being paid from NHS funds. Given the qualitative findings that individuals were concerned about not wasting NHS resources, it may be that some participants were reluctant to claim their incentive payment by returning the questionnaire, feeling that this was not a good use of the NHS’s limited funds. This study is limited by the scope of the type of financial incentive offered. Our findings could be specific to the value of the incentive offer, although existing literature would suggest that a higher value of incentive would not necessarily change the results. 57
Research suggests that individuals are motivated to respond to surveys for three main types of reason: altruism (e.g. believing that the research is important, wanting to help society or the researchers), egoistic responses (e.g. enjoying surveys, interested in or would benefit from the results) and survey characteristics (e.g. interest in the topic, length of survey). 78 When surveys offer incentives, one frequently given ‘egoistic’ reason for participation is to receive the payment. However, the incentive may be insufficient to create a response if interest in the topic is low. It may be that interest in health checks among participants was so low that even the offer of an incentive was insufficient to motivate questionnaire return for many. Another possibility is that the offer of an incentive ‘crowded out’ motivation to return the questionnaire among individuals who would have otherwise done so for altruistic reasons. There is evidence that tangible rewards undermine intrinsic motivation for a wide range of simple tasks, when individuals are already motivated to perform the behaviour. 79 Indeed, some free-text responses on returned questionnaires included statements such as ‘no need to send me the voucher’, suggesting that some participants were keen to express that they had taken part for more altruistic reasons. The reduction in response rate as a result of the incentive causing motivational crowding out may have been countered by an increase in response rate among less intrinsically motivated individuals, leading to the finding of no significant difference in response rates between the QBE questionnaire arm and the QBE questionnaire and incentive arm.
There may also be factors specific to this particular research project that diminished the impact of incentives on questionnaire return. Participants may have felt that they lacked a prior relationship with the signatories and senders of the invitation letter and so may have been uncertain that the promise of an incentive would be honoured. A second issue was that the participant questionnaires were linked to health check data through study identification numbers. Participants may have been unclear how they would be reimbursed for returning an apparently anonymous survey. Some chose to provide their name and address in the free-text box on the questionnaire, whereas others directly questioned how they would be sent a voucher if the research team did not know who they were. It may be important for the cover letter in any future QBE studies using conditional incentives to explain to participants how returned questionnaires can be linked to individuals’ contact details to enable incentive distribution. A third issue, also hinted at by a few free-text comments, is that some participants may have misunderstood the incentive as being offered for having a health check, rather than returning the questionnaire. It might have been informative to speak to non-responders about why the offer of a voucher did not lead them to return the questionnaire, but this was not feasible in the context of this research. Incentivising the health check itself rather than return of the questionnaire might have also altered the results but this was a different research question from that asked in this study.
Cost-effectiveness
We did not complete a cost-effectiveness analysis alongside the trial because there was no evidence that the trial intervention was effective. This research raises a question concerning the cost-effectiveness of issuing standard invitation letters for health checks through the population-based call–recall system, when uptake within 6 months is low. It was beyond the scope of this research to compare the effectiveness and cost-effectiveness of opportunistic compared with population-based invitation methods for health checks, but this could be pursued through future research.
Rapid trials using electronic health records
This trial was conducted as a rapid trial using electronic health records. The conduct of trials using electronic health records as a means for increasing the speed and efficiency with which trials can be completed is a topic of growing importance. 80 We have previously conducted cluster RCTs using primary care electronic health records, with the Clinical Practice Research Datalink (CPRD)81 as the data source. These cluster trials were in implementation research focusing on primary care management and appropriate prescribing for respiratory infections82 and stroke secondary prevention. 83 Van Staa et al. 84 reported on two pilot clinical trials to evaluate statin prescribing for CVD prevention and antibiotic prescribing for chronic obstructive pulmonary disease. Such trials are sometimes referred to as ‘point-of-care’ trials because recruitment, randomisation and outcome evaluation are all conducted in the context of routine service delivery settings. 85 The CPRD clinical trials demonstrated the feasibility of conducting pragmatic drug trials using electronic health records but identified several difficulties that would need to be overcome before these could be implemented on a wider scale. These difficulties especially related to the issue of obtaining informed consent in the context of routine primary care consultations. The present trial did not require individual participant consent. Individual randomisation was conducted at scale across 18 general practices. It was possible to randomise > 12,000 individual participants in this trial. The study was methodologically innovative in incorporating and demonstrating the feasibility of an automated system for randomisation into the software system that was used to manage invitations to the Health Check programme. This approach to trial conduct might now be pursued in other contexts.
Uptake of health checks
The research yielded important insights into the processes underlying health check uptake. The level of uptake of health checks within 6 months of a standard invitation letter was much lower than initially anticipated, being close to 15% overall. Nationally reported data for health check uptake in England, London, Lambeth and Lewisham are presented in Table 35. 9 The results show that health check uptake is presently < 50% at the national level. Returns for London show slightly lower uptake rates than for England. Data for the two boroughs, where the present trial was conducted, show lower uptake rates than for London as a whole, with lower uptake in Lambeth than in Lewisham. Uptake for 2015–16 appears to be lower than for the period 2013–16. In the present trial, uptake of 15% was observed in the first 6 months following a standard invitation letter. Checks undertaken > 6 months after the invitation letter and non-invited checks outnumbered checks occurring within 6 months of the invitation letter by a ratio of 2 : 1. As the numerator for nationally reported rates includes both invited and non-invited health checks, we conclude that the data reported in this trial are consistent with nationally reported data for health check uptake.
| Area | Uptake 2013–present (%) | Uptake 2015–16 (%) | Uptake last quarter: July–September 2015 (%) |
|---|---|---|---|
| England | 48.15 | 45.19 | 45.81 |
| London | 46.79 | 42.20 | 46.24 |
| Lambeth | 26.36 | 15.07 | 17.66 |
| Lewisham | 41.47 | 39.96 | 38.68 |
This research was conducted in inner London in a context that has several distinctive features. This is a very deprived area and the majority of the trial sample lived in areas that were in the two most deprived quintiles of deprivation score for England. The local population has a very young age distribution and this has important implications for the Health Check programme in terms of the low proportion of older adults among the population invited for health checks in this area and the low proportion with a substantially elevated CVD risk (≥ 20%). Both the overall level of deprivation and the age distribution of the population might have contributed to a low overall uptake of health checks, although the two characteristics are differently associated with CVD risk.
Only a minority of the local population was classified as being of ‘white’ ethnicity, with a high proportion being of black Caribbean or black African origins. These groups are generally at greater risk of diabetes mellitus and hypertension and their complications. These ethnic minority populations often have higher educational attainment than the local white population and this might contribute to the higher health check uptake among these ethnic minority groups in this area. In addition, newer migrants to the UK often have experience of fee-for-service health systems, which may increase their motivation to take up the offer of health services that are free at the point of use. 30
Conclusions from qualitative studies
Qualitative evaluations suggested some ambivalence towards the NHS Health Check programme among both health professionals and the general public. Reservations about the programme tended to focus on value for money and the resources required for programme implementation at a time when the NHS is under financial pressure. Most patients expressed positive views about having their health checked and recognised the value of preventative medical interventions. Some expressed scepticism about being able to obtain an appointment at their general practice, especially at a time that is convenient to them, given other priorities such as work and caring responsibilities. They may be prepared to forgo these priorities when they are feeling ill but not when they currently feel healthy, as may be the case when they receive the standard invitation for a health check. Patients may have limited motivation for preventative health care, especially in deprived populations. However, they may not object to being offered a health check opportunistically when they attend the general practice for another reason. Rather than focusing interventions on individual patients to increase health check uptake, it may be more effective to focus on service delivery factors to improve accessibility and the ease with which people can obtain a health check. Patients commented on the difficulty of accessing appointments for blood tests and health checks and the difficulty of prioritising these when they did not feel in need of a doctor. In the context of significant obstacles to taking up the offer of a health check, it is understandable that an intervention grounded in behavioural science theory that targeted the individual patient behavioural response to receiving a standard invitation letter might not be effective.
Opportunistic compared with invited health checks
This research showed that invited health checks, conducted within 6 months following receipt of a standard invitation letter, represented a minority of completed health checks in this area. Data collected for the trial revealed that nearly six out of 10 completed checks either were carried out > 6 months after an invitation or were performed opportunistically. This finding was confirmed through analysis of routinely collected data from the health check management information system. Qualitative interview results confirmed that most general practices offered opportunistic health checks. This may reflect practices recognising the barriers to attending invited health checks in their patient populations suggested by the qualitative analysis and so offering health checks in a manner that is more feasible for patients. There was considerable variation among practices in the extent to which opportunistic health checks were pursued, with variation in the proportion of opportunistic health checks, and late responders to invitations, among practices. An opportunistic approach to offering health checks might be effective because the offer of a health check is made at a time when the general practice is ready to complete it and at a time when the patient is already engaged in accessing health-care services to meet their health needs. Data collected for the trial showed that the case mix of patients attending for opportunistic checks was generally similar to that of patients attending for invited checks in terms of age, gender and ethnicity, although the proportion of opportunistic checks appeared to be slightly higher in deprived populations. Importantly, the yield from opportunistic checks, in terms of elevated cardiovascular risk and overweight and obesity, was higher than for invited health checks. This suggests that general practices generally target the offer of a health check to higher-risk sections of their registered population.
Strengths and limitations of this research
This study had several strengths. The trial was conducted in a large number of general practices and the large target sample size for the trial was achieved. The study had sufficient power to detect small increases in uptake of health checks. Two different methods for recruitment and randomisation were used and these gave consistent results. We evaluated the reliability of the trial results against routinely collected data from the health check management information system. This comparison showed that the number of participants invited was consistent between the two data sources. The high proportion of patients receiving non-invited checks was also consistent in trial data and in routinely collected data. We conducted qualitative research, including interviews with health-care staff and patients, and analysed free-text responses from returned questionnaires. These qualitative data confirmed our interpretation that there are significant obstacles to the uptake of standard health check invitations, but that most practices are active in offering opportunistic health checks.
The trial was conducted in a deprived area of inner London with a young population age distribution and a high proportion of ethnic minority groups. This research does not exclude the possibility that an enhanced invitation method based on the QBE might be more effective in more affluent areas with older populations. However, trial subgroup analyses did not suggest any possible important difference in effect according to subgroups of age or deprivation quintile.
The conduct of the trial depended on using primary care electronic health records, linked to the proprietary health check management information system, to recruit participants, arrange for the delivery of the intervention materials and ascertain trial outcomes. We conducted extensive checks to ensure that these processes were being implemented as intended. Nevertheless, there was evidence of discrepancies arising in the health check management information system. In the automated recruitment trial arm, a small number of participants who were registered at non-trial general practices were initially included. These individuals had possibly moved to a new general practice. In addition, a small number of participants were identified as eligible for a health check more than once during the trial study period. Both groups of discrepant participants were excluded at the time of analysis. The number of discrepancies recorded was too small to have an influence on the overall results of the study. We used Read codes recorded in electronic health records to ascertain trial outcomes. It is possible that general practices might use non-standard Read codes to identify health checks. However, we searched for the Read codes that are mandated by the Health Check programme, which are used for reimbursement of practices for completed health checks. The same Read codes are used by the information system to manage the Health Check programme. As noted earlier, data from the trial were generally consistent with data obtained from the health check management information system. It is noted that there may be variation between practices in use of Read codes for recording information about patients and that recording systems are not always up to date. However, any data discrepancies should be equally distributed between trial arms and should not have a differential effect. Any data discrepancy large enough to mask a clinically important effect should have been detected through our data checks, but none was identified.
Research recommendations
Research recommendations will be discussed in relation to the NHS Health Check programme, the QBE and financial incentives and methods for conducting rapid trials using electronic health records.
NHS Health Check programme
Public Health England has already developed research recommendations to support the NHS Health Check programme. 86 These identify four general areas of importance including delivery and implementation, outcomes, cost-effectiveness and health inequalities. The research conducted for the present trial mainly focused on the first of these areas, with a more limited relevance to the question of health inequalities.
-
We endorse the PHE suggestion that further research should address optimal methods of recruitment of patients for NHS health checks. Our research raises a question concerning the value of a population-based call–recall system, when uptake of health checks within 6 months is low. Opportunistic checks might sometimes be effective at reaching more deprived populations and those at higher risk.
-
We also endorse the PHE recommendation for further research into the organisation and delivery of health checks. In particular, our research identifies barriers to access in terms of time costs to patients and difficulties of obtaining access to appointments at convenient times and locations as key determinants of health check uptake.
-
This research draws attention to the young age distribution and high proportion of ethnic minorities in this urban population. In this population, the proportion with a ≥ 20% risk of CVD is comparatively low, with implications for the efficiency of the Health Check programme. In areas with younger and more ethnically diverse populations there may be a case for tailoring health checks to address relevant health problems, including diabetes mellitus, obesity, hypertension and risk of chronic kidney disease. This will enable a link with diabetes prevention initiatives. Research is needed to identify the most effective and efficient ways of achieving this.
The question–behaviour effect
Our findings offer no support for use of the QBE in increasing attendance at NHS health checks. Our findings suggest that the QBE may have limited use as an intervention in public health.
Future research should:
-
evaluate the effects of a redesigned questionnaire focusing on self-prediction items and omitting anticipated regret
-
assess whether or not combining the QBE with a motivational intervention to increase positive intentions and attitudes might better support health check uptake
-
investigate whether or not alternative documentation sent to participants would result in similar levels of attendance.
Methods for conducting rapid trials using electronic health records
We have shown that it is feasible to conduct a rapid RCT, with automated randomisation, using electronic health records. Future research should:
-
identify a wider range of health service and public health settings in which automated randomisation may be used
-
identify a wider range of interventions that can be delivered remotely at low cost
-
address issues of governance and informed consent in the context of large simple trials.
Acknowledgements
The trial was funded by the NIHR Health Technology Assessment programme (reference number 11/129/61).
Caroline Burgess and Alice Forster were also supported by the Guy’s and St Thomas’ Charity (grant number G100702). Martin Gulliford was supported by the NIHR Biomedical Research Centre at Guy’s and St Thomas’ Hospitals. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
We thank all of the general practices and individuals participating in this trial, the staff of the central service that co-ordinates health check invitations in the two trial boroughs and the members of our TSC.
Contribution of authors
Lisa McDermott designed and tested the intervention questionnaire, planned the conduct of the trial, implemented the randomisation lists and conducted data collection and data management for the trial.
Alison J Wright designed the study, designed and tested the intervention questionnaire, provided additional data management and data analysis support and drafted the report.
Victoria Cornelius designed the study, planned the conduct of the trial, prepared the randomisation lists, wrote the statistical analysis plan and conducted the analysis and drafted the report.
Caroline Burgess designed the study, designed and tested the intervention questionnaire and planned the conduct of the trial.
Alice S Forster designed the study, designed and tested the intervention questionnaire, planned the conduct of the trial, implemented the randomisation lists and conducted data collection and data management for the trial.
Mark Ashworth planned the conduct of the trial and assisted with data collection and data management.
Bernadette Khoshaba conducted data collection and data management for the trial, provided additional data analysis support and drafted the report.
Philippa Clery conducted data collection and data management for the trial.
Frances Fuller planned the conduct of the trial and assisted with data collection and data management.
Jane Miller planned the conduct of the trial and assisted with data collection and data management.
Hiten Dodhia planned the conduct of the trial and assisted with data collection and data management.
Caroline Rudisill conducted the economic evaluation.
Mark T Conner designed the study and designed and tested the intervention questionnaire.
Martin C Gulliford designed the study, planned the conduct of the trial, provided additional data management and data analysis support and drafted the report.
All authors reviewed and commented on the draft report. Martin C Gulliford is guarantor.
Data sharing statement
All available data can be obtained by contacting the corresponding author; the study team will retain exclusive use until the publication of major outputs.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cardiovascular Disease Statistics. UK Factsheet. London: British Heart Foundation; 2014.
- Diabetes Prevalence 2014. London: Diabetes UK; 2015.
- Chronic Kidney Disease in Adults: Assessment and Management. London: NICE; 2014.
- Aitken GR, Roderick PJ, Fraser S, Mindell JS, O’Donoghue D, Day J, et al. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005480.
- Dregan A, Stewart R, Gulliford MC. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013;42:338-45. http://dx.doi.org/10.1093/ageing/afs166.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation; 2012.
- Murray CJL, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381:997-1020. http://dx.doi.org/10.1016/S0140-6736(13)60355-4.
- Putting Prevention First. NHS Health Check: Vascular Risk Assessment. Best Practice Guidance. London: Department of Health; 2009.
- Health Check Uptake Data. 2016.
- The Local Authorities (Public Health Functions and Entry to Premises by Local Healthwatch Representatives) Regulations No. 351. Regulation 4. London: The Stationery Office; 2013.
- Healthy Lives, Healthy People: Transparency in Outcomes. Proposals for a Public Health Outcomes Framework. London: Department of Health; 2010.
- JBS3 Board . Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100:ii1-67. http://dx.doi.org/10.1136/heartjnl-2014-305693.
- Hippisley-Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c6624.
- Caley M, Chohan P, Hooper J, Wright N. The impact of NHS health checks on the prevalence of disease in general practices: a controlled study. Br J Gen Pract 2014;64:e516-21. http://dx.doi.org/10.3399/bjgp14X681013.
- Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification. London: NICE; 2014.
- Krogsbøll LT, Jorgensen KJ, Gronhoj Larsen C, Gotzsche PC. General health checks in adults for reducing morbidity and mortality from disease: Cochrane systematic review and meta-analysis. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7191.
- Imperial Cancer Research Fund OXCHECK Study Group . The effectiveness of health checks conducted by nurses in primary care: final results from the OXCHECK study. BMJ 1995;310:1099-104. http://dx.doi.org/10.1136/bmj.310.6987.1099.
- British Family Heart Study Group . Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of the British Family Heart Study. BMJ 1994;308:313-20. http://dx.doi.org/10.1136/bmj.308.6924.313.
- Capewell S, McCartney M, Holland W. Invited debate: NHS Health Checks – a naked emperor?. J Public Health 2015;37:187-92. http://dx.doi.org/10.1093/pubmed/fdv063.
- van Staa T-P, Smeeth L, Ng ES-W, Goldacre B, Gulliford M. The efficiency of cardiovascular risk assessment: do the right patients get statin treatment?. Heart 2013;99:1597-602. http://dx.doi.org/10.1136/heartjnl-2013-303698.
- Wanless D. Securing Good Health for the Whole Population. Final Report. London: HMSO; 2004.
- Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults. London: NICE; 2014.
- Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: NICE; 2008.
- Economic Modelling For Vascular Checks. London: Department of Health; 2008.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. http://dx.doi.org/10.1093/pubmed/fdr034.
- NHS . NHS Health Check n.d. www.healthcheck.nhs.uk (accessed November 2016).
- Artac M, Dalton AR, Majeed A, Car J, Huckvale K, Millett C. Uptake of the NHS Health Check programme in an urban setting. Fam Pract 2013;30:426-35. http://dx.doi.org/10.1093/fampra/cmt002.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Implementation of the NHS Health Checks programme: baseline assessment of risk factor recording in an urban culturally diverse setting. Fam Pract 2011;28:34-40. http://dx.doi.org/10.1093/fampra/cmq068.
- Robson J, Dostal I, Madurasinghe V, Sheikh A, Hull S, Boomla K, et al. The NHS Health Check programme: implementation in east London 2009–2011. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2015-007578.
- Burgess C, Wright AJ, Forster AS, Dodhia H, Miller J, Fuller F, et al. Influences on individuals’ decisions to take up the offer of a health check: a qualitative study. Health Expect 2015;18:2437-48. http://dx.doi.org/10.1111/hex.12212.
- Forster AS, Burgess C, Dodhia H, Fuller F, Miller J, McDermott L, et al. Do health checks improve risk factor detection in primary care? Matched cohort study using electronic health records. J Public Health 2015;fdv119:1-8. http://dx.doi.org/10.1093/pubmed/fdv119.
- Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-464.
- Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J. The determinants of screening uptake and interventions for increasing uptake: a systematic review. Health Technol Assess 2000;4.
- Gidlow C, Ellis N, Randall J, Cowap L, Smith G, Iqbal Z, et al. Method of invitation and geographical proximity as predictors of NHS Health Check uptake. J Public Health 2015;37:195-201. http://dx.doi.org/10.1093/pubmed/fdu092.
- Kumar J, Chambers R, Mawby Y, Leese C, Iqbal Z, Picariello L, et al. Delivering more with less? Making the NHS Health Check work in financially hard times: real time learning from Stoke-on-Trent. Qual Prim Care 2011;19:193-9.
- Norman P, Conner MT, Willits DG, Bailey DR, Hood DHJ, Coysh HL. Health checks in general practice: a comparison of two invitation letters. Br J Gen Pract 1991;41:432-3.
- Cooper AM, Dugdill L. Evidence of Improved Uptake of Health Checks: Rapid Review. Salford: University of Salford; 2014.
- Perry C, Krishna C, Damesick D, Hobden S, Volpe L. Behavioural Insights in Health Care: Nudging to Reduce Inefficiency and Waste. London: Health Foundation; 2015.
- Lo SH, Good A, Sheeran P, Baio G, Rainbow S, Vart G, et al. Preformulated implementation intentions to promote colorectal cancer screening: a cluster-randomized trial. Health Psychol 2014;33:998-1002. http://dx.doi.org/10.1037/a0033507.
- Dormandy E, Bryan S, Gulliford MC, Roberts TE, Ades AE, Calnan M, et al. Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14200.
- Michie S, Dormandy E, Marteau TM. Increasing screening uptake amongst those intending to be screened: the use of action plans. Patient Educ Couns 2004;55:218-22. http://dx.doi.org/10.1016/j.pec.2003.09.005.
- Prestwich A, Kellar I. How can the impact of implementation intentions as a behaviour change intervention be improved?. Eur Rev Appl Psychol 2014;64:35-41. http://dx.doi.org/10.1016/j.erap.2010.03.003.
- Neter E, Stein N, Barnett-Griness O, Rennert G, Hagoel L. From the bench to public health: population-level implementation intentions in colorectal cancer screening. Am J Prev Med 2014;46:273-80. http://dx.doi.org/10.1016/j.amepre.2013.11.008.
- Conner M, Godin G, Norman P, Sheeran P. Using the question–behavior effect to promote disease prevention behaviors: two randomized controlled trials. Health Psychol 2011;30:300-9. http://dx.doi.org/10.1037/a0023036.
- Sandberg T, Conner M. A mere measurement effect for anticipated regret: impacts on cervical screening attendance. Br J Soc Psychol 2009;48:221-36. http://dx.doi.org/10.1348/014466608X347001.
- O’Carroll RE, Chambers JA, Brownlee L, Libby G, Steele RJC. Anticipated regret to increase uptake of colorectal cancer screening (ARTICS): a randomised controlled trial. Soc Sci Med 2015;142:118-27. http://dx.doi.org/10.1016/j.socscimed.2015.07.026.
- Sherman SJ. On the self-erasing nature of errors of prediction. J Pers Soc Psychol 1980;39:211-21. http://dx.doi.org/10.1037/0022-3514.39.2.211.
- Wood C, Conner M, Sandberg T, Godin G, Sheeran P. Why does asking questions change health behaviours? The mediating role of attitude accessibility. Psychol Health 2014;29:390-404. http://dx.doi.org/10.1080/08870446.2013.858343.
- Dholakia UM, Malhotra NK. Review of Marketing Research. Bingley: Emerald; 2010.
- Wood C, Conner M, Miles E, Sandberg T, Taylor N, Godin G, et al. The impact of asking intention or self-prediction questions on subsequent behavior: a meta-analysis. Pers Soc Psychol Rev 2016;20:245-68. http://dx.doi.org/10.1177/1088868315592334.
- Rodrigues AM, O’Brien N, French DP, Glidewell L, Sniehotta FF. The question–behavior effect: genuine effect or spurious phenomenon? A systematic review of randomized controlled trials with meta-analyses. Health Psychol 2015;34:61-78. http://dx.doi.org/10.1037/hea0000104.
- Ajzen I. Theories of cognitive self-regulation. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. http://dx.doi.org/10.1016/0749-5978(91)90020-T.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Edwards PJ, Roberts I, Clarke MJ, Diguiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.mr000008.pub4.
- Edwards P, Cooper R, Roberts I, Frost C. Meta-analysis of randomised trials of monetary incentives and response to mailed questionnaires. J Epidemiol Community Health 2005;59:987-99. http://dx.doi.org/10.1136/jech.2005.034397.
- von Wagner C, Good A, Whitaker KL, Wardle J. Psychosocial determinants of socioeconomic inequalities in cancer screening participation: a conceptual framework. Epidemiol Rev 2011;33:135-47. http://dx.doi.org/10.1093/epirev/mxq018.
- Dolan P, Rudisill C. The effect of financial incentives on chlamydia testing rates: evidence from a randomized experiment. Soc Sci Med 2014;105:140-8. http://dx.doi.org/10.1016/j.socscimed.2013.11.018.
- Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect 2001;4:99-108. http://dx.doi.org/10.1046/j.1369-6513.2001.00140.x.
- Marteau TM, Kinmonth AL. Screening for cardiovascular risk: public health imperative or matter for individual informed choice?. BMJ 2002;325:78-80. http://dx.doi.org/10.1136/bmj.325.7355.78.
- Mann E, Kellar I, Sutton S, Kinmonth AL, Hankins M, Griffin S, et al. Impact of informed-choice invitations on diabetes screening knowledge, attitude and intentions: an analogue study. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-768.
- Cochrane T, Gidlow CJ, Kumar J, Mawby Y, Iqbal Z, Chambers RM. Cross-sectional review of the response and treatment uptake from the NHS Health Checks programme in Stoke on Trent. J Public Health 2013;35:92-8. http://dx.doi.org/10.1093/pubmed/fds088.
- Office for National Statistics . National Statistics 2015. www.neighbourhood.statistics.gov.uk/dissemination/ (accessed 20 September 2016).
- Demographic Data. 2016.
- Department for Communities and Local Government . English Indices of Deprivation 2015 2015. www.gov.uk/government/statistics/English-indices-of-deprivation-2015 (accessed 20 September 2016).
- Forster AS, Burgess C, McDermott L, Wright AJ, Dodhia H, Conner M, et al. Enhanced invitation methods to increase uptake of NHS health checks: study protocol for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-342.
- Department for Communities and Local Government . English Indices of Deprivation 2010 2011. www.gov.uk/government/statistics/English-indices-of-deprivation-2010 (accessed 20 September 2016).
- Sensory Trust . Accessible Information – Clear and Large Print Sensory Trust Information Sheet n.d. www.sensorytrust.org.uk/resources/connect/infosheet_clearlargeprint.pdf (accessed 18 January 2016).
- Godin G, Belanger-Gravel A, Vezina-Im LA, Amireault S, Bilodeau A. Question–behaviour effect: a randomised controlled trial of asking intention in the interrogative or declarative form. Psychol Health 2012;27:1086-99. http://dx.doi.org/10.1080/08870446.2012.671617.
- Cowie L, Morgan M, Gulliford M. Handwritten ‘post-it’ notes, questionnaire formats and response to a postal questionnaire survey. Int J Epidemiol 2011;40:254-5. http://dx.doi.org/10.1093/ije/dyq043.
- Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome – when, why, and how?. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-20.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. http://dx.doi.org/10.1002/sim.1186.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. http://dx.doi.org/10.1080/01621459.1958.10501452.
- Dunn G, Maracy M, Dowrick C, Ayuso-Mateos JL, Dalgard OS, Page H, et al. Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry 2003;183:323-31. http://dx.doi.org/10.1192/bjp.183.4.323.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 2000;19:3127-31. http://dx.doi.org/10.1002/1097-0258(20001130)19:22%3C3127::AID-SIM784%3E3.0.CO;2-M.
- Wilding S, Conner MT, Lawton RJ, Prestwich AJ. Can Answering Questions Change Behaviour? A Systematic Review and Meta-Regression n.d. www.ehps.net/ehp/index.php/contents/article/view/1093 (accessed 20 September 2016).
- Ayres K, Conner M, Prestwich A, Hurling R, Cobain M, Lawton R, et al. Exploring the question–behaviour effect: randomized controlled trial of motivational and question–behaviour interventions. Br J Health Psychol 2013;18:31-44. http://dx.doi.org/10.1111/j.2044-8287.2012.02075.x.
- Singer E, Ye C. The use and effects of incentives in surveys. Ann Am Acad Pol Soc Sci 2013;645:112-41. http://dx.doi.org/10.1177/0002716212458082.
- Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? comparing behaviors studied in psychological and economic literatures. Health Psychol 2013;32:950-7. http://dx.doi.org/10.1037/a0032727.
- van Staa TP, Goldacre B, Gulliford M, Cassell J, Pirmohamed M, Taweel A, et al. Pragmatic randomised trials using routine electronic health records: putting them to the test. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e55.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. http://dx.doi.org/10.1093/ije/dyv098.
- Gulliford M, van Staa T, Dregan A, McDermott L, McCann G, Asworth M, et al. Utilising electronic health records for intervention research. Cluster randomised trial to reduce antibiotic prescribing in primary care (eCRT study). Ann Fam Med 2014;4:244-51.
- Dregan A, van Staa TP, McDermott L, McCann G, Ashworth M, Charlton J, et al. Point-of-care cluster randomized trial in stroke secondary prevention using electronic health records. Stroke 2014;45:2066-71. http://dx.doi.org/10.1161/STROKEAHA.114.005713.
- van Staa TP, Dyson L, McCann G, Padmanabhan S, Belatri R, Goldacre B, et al. The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations of two exemplar trials. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18430.
- Fiore LD, Brophy M, Ferguson RE, D’Avolio L, Hermos JA, Lew RA, et al. A point-of-care clinical trial comparing insulin administered using a sliding scale versus a weight-based regimen. Clin Trials 2011;8:183-95. http://dx.doi.org/10.1177/1740774511398368.
- NHS Health Check Programme: Priorities for Research. London: Public Health England; 2014.
Appendix 1 Trial question–behaviour effect questionnaire
Appendix 2 Covering letter: question–behaviour effect questionnaire trial arm
Dear [Title, Surname],
Over the next few weeks you will be receiving an invitation to a free Health Check offered by the NHS.
We want to find out how people decide whether to accept the invitation to have a NHS Health Check. We would be grateful if you would complete the questions in the booklet that came with this letter and return it as soon as possible in the prepaid envelope.
We will not tell anyone else what answers you give us. Your personal details will only be seen by the people involved in your health care.
Yours sincerely,
Clinical Lead,
Lewisham NHS Health Check programme
Appendix 3 Covering letter: question–behaviour effect questionnaire and incentive trial arm
Dear [Title, Surname],
Over the next few weeks you will be receiving an invitation to a free Health Check offered by the NHS.
We want to find out how people decide whether to accept the invitation to have a NHS Health Check. We would be grateful if you would complete the questions in the booklet that came with this letter and return it as soon as possible in the prepaid envelope.
We will not tell anyone else what answers you give us. Your personal details will only be seen by the people involved in your health care.
If you complete and return the survey we will send you a £5 ‘Love to Shop’ voucher.
Yours sincerely,
Clinical Lead,
Lewisham NHS Health Check programme
Appendix 4 Interview guide: health check trial – general practice staff
Aim
To identify organisational factors within general practices that may have influenced the uptake of NHS health checks and implementation of the enhanced invitation methods trial.
Participants
Participants were practice management staff, health check procedure managers/administrators and health-care staff involved in the delivery of NHS health checks.
The following table indicates the justification aims of each interview question.
| Question | Prompts | Topic | Aim of question | |
|---|---|---|---|---|
| 1 | Please could you briefly outline your role in the practice and delivery of the NHS health checks? | Monthly tasks, etc. | Introduction question | Role of participant |
| 2 | Could you briefly describe your views on the NHS Health Check programme? |
|
Health check attitude | Staff understanding and views of NHS health checks |
| 3 | What do you think are the main benefits of the NHS Health Check programme? |
|
Health check attitude | Are staff willing to support health check bookings and the trial? |
| 4 | What do you feel are the main weaknesses of the NHS Health Check programme? |
|
Health check attitude | What are the possible barriers to staff support for health check bookings and the trial? |
| 5 | Could you briefly describe the way in which the programme works within your practice? | PNL/booking slots/staff responsible | Uptake influences | What are the possible procedural barriers to patients receiving health checks? |
| 6 | How is information about NHS health checks communicated within the practice? | Reception staff/staff responsible | Uptake influences | Could untrained staff be providing inadequate information to patients about health checks and booking checks? |
| 7 | How easy is it to find time/ensure that the PNL list is checked each month? | Does this ever get missed? | Uptake influences | Are ineligible patients invited for health checks and included in the trial? |
| 8 | What difficulties are there in booking slots for health checks? | Uptake influences | Can patients easily book appointments for health checks? | |
| 9 | Are health checks offered to patients who have not been invited to a health check? | How common is this? | Uptake influences | Are opportunistic checks regularly conducted? |
| 10 | How do you know if a patient has a health check in a pharmacy or community team? | Uptake influences | Are practices aware of external health checks conducted on their patient population? | |
| 11 | If a patient has a health check in a pharmacy or community team, how are these data recorded into their practice notes? | Any problems with this? | Uptake influences | Are all external health checks recorded by general practices? |
| 12 | What difficulties do you have in delivering NHS health checks? | Staff available/trained to carry out health checks? | Uptake influences | Are there any difficulties in delivering health checks that may reduce uptake? |
| 13 | What were that main challenges involved in running the trial at your practice | Trial implementation | Perceived challenges of trial | |
| 14 | Were there any benefits of the trial taking place at your practice? | Trial implementation | Perceived benefits of trial | |
| 15 | Do you have any other comments you would like to add about the trial of the Health Check programme? | Closing question | Additional comments | |
List of abbreviations
- BMI
- body mass index
- CACE
- complier average causal effect
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CVD
- cardiovascular disease
- FOBT
- faecal occult blood test
- GEE
- generalised estimating equation
- GLM
- general linear model
- GP
- general practitioner
- IMD
- Indices of Multiple Deprivation
- IQR
- interquartile range
- JBS
- Joint British Societies
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PBC
- perceived behavioural control
- PCO
- primary care organisation
- PHE
- Public Health England
- PNL
- pre-notification list
- QBE
- question–behaviour effect
- QMS
- Quality Medical Solutions
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SMD
- standardised mean difference
- TPB
- theory of planned behaviour
- TSC
- Trial Steering Committee