Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/99/02. The contractual start date was in June 2013. The draft report began editorial review in July 2015 and was accepted for publication in January 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Farrar et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Normal pregnancy is associated with insulin resistance that is similar to that found in type 2 diabetes. Physiological resistance to insulin action during pregnancy becomes apparent in the second trimester, and insulin resistance increases progressively to term. These changes facilitate transport of glucose across the placenta to ensure normal fetal growth and development. Transfer of glucose across the placenta stimulates fetal pancreatic insulin secretion, and insulin acts as an essential growth hormone. However, if resistance to maternal insulin action becomes too pronounced then maternal hyperglycaemia occurs and gestational diabetes mellitus (GDM) may be diagnosed.
Associated risks
Gestational diabetes mellitus is associated with an increased risk of adverse perinatal outcomes, including large-for-gestational-age (LGA) birthweight (BW), macrosomia (defined as BW of > 4 kg) and Caesarean section (C-section). 1 There is also limited evidence that GDM is associated with increased risk of longer-term ill health outcomes in the mother (e.g. type 2 diabetes and cardiovascular disease)2,3 and offspring (e.g. obesity and associated cardiometabolic risk). 4,5
Recently, the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study6 examined the association between gestational fasting and post-load glucose levels in women without diabetes. These findings have been used by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) to inform their criteria to diagnose GDM. The HAPO study6 reported graded linear increases in the odds of four primary outcomes (BW of > 90th centile for gestational age, primary C-section, diagnosed neonatal hypoglycaemia and cord blood C-peptide of > 90th percentile) across the whole distribution of fasting and post-load glucose levels, illustrating no clear threshold below which there is no increase in risk. There were also graded monotonic associations with a majority of secondary outcomes, including preterm birth, shoulder dystocia and pre-eclampsia. However, there were limited numbers of South Asian (SA) women included and no SA centres. In Chapters 2 and 3 we report analyses (using similar methods to those used by the HAPO study6) using individual participant data (IPD) and data from published studies to determine the risk of adverse outcomes associated with graded increases in maternal glucose levels, in the BiB study,7 to determine the differences in risk between SA and white British (WB) women, and, in IPD and published studies, combined, for all women.
Screening
An important question regarding the diagnosis of GDM is what glucose thresholds (fasting or post load) are most clinically effective and cost-effective. Appropriate identification of women who develop GDM is essential so that treatment can be provided to reduce the associated risks. However, diagnosis is complex and there are a number of different criteria with different thresholds used internationally and nationally (Table 1). This lack of a clear threshold to signify increased risk means that somewhat arbitrary thresholds need to be used to define GDM, an issue that is similar to the diagnosis of type 2 diabetes, hypertension and dyslipidaemia [which, like GDM, are diagnoses made to indicate risk of later disease (cardiovascular disease for these exposures) that might be prevented by appropriate intervention (lifestyle change and medication)]. In Chapters 2–4 we report details of our derived thresholds using IPD and published data for diagnosing GDM and prevalences using past and current criteria.
| Criteria | Fasting | 1-hour post load | 2-hour post load | 3-hour post load |
|---|---|---|---|---|
| 75-g OGTT (plasma glucose) | ||||
| aIADPSG8 (2010), ADIPS9 (2013), WHO10 (2013) | ≥ 5.1 | ≥ 10.0 | ≥ 8.5 | – |
| aWHO11 (1999) | ≥ 6.1 | – | ≥ 7.8 | – |
| aADA12 (2006) | ≥ 5.3 | ≥ 10.0 | ≥ 8.6 | |
| aADIPS13 (1998) | ≥ 5.5 | – | ≥ 8.0 | – |
| 100-g OGTT (plasma or serum glucose) | ||||
| bACOG14/C&C | ≥ 5.3 | ≥ 10.0 | ≥ 8.6 | ≥ 7.8 |
| bNDDG15 | ≥ 5.8 | ≥ 10.6 | ≥ 9.2 | ≥ 8.0 |
| bO’Sullivan16 | ≥ 5.0 | ≥ 9.2 | ≥ 8.1 | ≥ 6.9 |
There are two main strategies to identify women with GDM: (1) universal testing, through which all women are offered a diagnostic test [usually an oral glucose tolerance test (OGTT)]; or (2) selective testing, through which those women identified as having an increased risk of developing GDM are offered a diagnostic test. The second strategy is closer to the more usual screening model described by the UK National Screening Committee (NSC). 17
Once risk is identified (in universal screening/selective testing), those at high risk (however defined) will be offered a diagnostic test (usually an OGTT) and, depending on those results, will be given advice and/or medical treatment or not. 17 Screening is therefore undertaken to (1) identify those women at greatest risk, to prevent unnecessary diagnostic testing of those women unlikely to develop GDM, and (2) reduce costs associated with universal diagnostic testing.
Several health-care agencies including the UK National Institute for Health and Care Excellence (NICE)18 recommend that pregnant women should have their risk evaluated by assessment of maternal characteristics (risk factors) (see Table 14). Those with one or more risk factor should be offered a diagnostic OGTT. Chapter 5 of this report examines the accuracy of maternal characteristics as risk factors for the identification of women who are most likely to develop GDM. We have examined the performance of maternal characteristics, first using IPD, and second using published data. Maternal risk factors for GDM include advanced maternal age, high body mass index (BMI), previous GDM, previous macrosomic infant, family history of type 2 diabetes or GDM (in a previous pregnancy), and ethnicity with a high associated prevalence of diabetes. We have chosen to focus on maternal characteristics (and not to include invasive screening tests, including blood tests) because this strategy is recommended for use by several agencies including NICE. 19
Diagnostic testing
Gestational diabetes mellitus is generally diagnosed using an OGTT. The OGTT is normally conducted in the morning following an overnight fast. A baseline plasma glucose sample is obtained; the woman then consumes a drink containing typically 75 g or 100 g of glucose and then at hourly intervals plasma glucose level is measured. The frequency of measurement depends on the glucose load and local policy. Women with an ‘elevated’ glucose level at one or two or more measurements are classified as having GDM.
There are some limitations to the OGTT as a diagnostic test, however: (1) a negative OGTT does not mean a woman will not develop GDM later in pregnancy, because as gestation progresses, insulin resistance may increase, therefore repeat glucose testing may be required; (2) glucose thresholds for diagnosis are arbitrary cut-off points and vary depending on the recommending agencies (see Table 1); and (3) the reproducibility of the OGTT is only around 75%20,21 (we have not examined the performance of the OGTT within this report).
Treatments for gestational diabetes
Treatment of GDM aims to reduce hyperglycaemia and, in doing so, reduce the risk of adverse outcomes. Diet/lifestyle modification is often used as first-line treatment; if this does not adequately reduce and control glucose levels or if glucose level is substantially elevated then pharmacological interventions [e.g. metformin (hydrochloride) (Glucophage,® Teva UK Ltd, Eastbourne, UK) and/or insulin] may also be given. Oral agents, including metformin and glibenclamide (Aurobindo Pharma – Milpharm Ltd, South Ruislip, Middlesex, UK), present a possible alternative to injected insulin and may be as effective, with the added benefit of being more acceptable to women.
Chapter 6 reports a systematic review investigating the effectiveness of different treatments for GDM to improve maternal and infant health outcomes. Meta- and network-analyses have been carried out where appropriate.
Economic evaluation
Chapter 7 details an economic evaluation of screening and diagnostic tests to identify and treat women with GDM. Current evidence on the cost-effectiveness of identifying and treating women with GDM is limited; the increasing prevalence of GDM, however, along with increasing demands on health service budgets, makes this evaluation central to the future planning of care pathways and resource allocation. We also report analyses in this chapter that examine the value of undertaking further research to understand the effects of treatments of GDM.
Chapter 2 Hyperglycaemia and the risk of adverse perinatal outcomes in South Asian and white British women: the Born in Bradford cohort
This chapter presents the methods and results of a study to determine the nature of the association between maternal pregnancy glucose levels and risk of perinatal outcomes using IPD from the Born in Bradford (BiB) study. 22 This study7 compares the associations of gestational glucose level with risk of adverse perinatal outcomes between SA and WB women (unless shown within the text sections, figures and tables are shown in the appendices and referred to within the text). A version of this chapter has been published in Farrar et al. 7 This is an Open Access article under the terms of the Creative Commons Attribution License (CC BY), which permits use, distribution and reproduction, provided the original work is properly cited (https://creativecommons.org/licenses/by/4.0/).
Introduction
Gestational diabetes increases the risk of several adverse perinatal outcomes. 1 In recent years, there has been much debate about how GDM should be diagnosed. In 2010, IADPSG recommended new thresholds for the diagnosis of the disease, which aimed to reduce obesity risk by identifying infants who were LGA, with high adiposity at birth, and who had high concentrations of cord blood C-peptide. 8 In 2013, the World Health Organization (WHO),10 whose previous criteria for diagnosing GDM have been widely used, endorsed the IADPSG criteria. The IADPSG criteria were produced with results from the HAPO study,6 which aimed to establish the association between maternal glucose concentrations that did not meet criteria for overt diabetes (pre-existing diabetes or GDM) and risk of adverse perinatal outcomes. The HAPO study6 found graded linear associations of fasting and post-load maternal glucose level with LGA, high adiposity and high concentrations of cord blood C-peptide, and similar linear associations with several other perinatal outcomes. In view of the absence of any clear threshold of glucose concentration at which risk of adverse outcomes increased, the IADPSG reached a consensus on how to calculate the new criteria. They decided that the thresholds for diagnosing GDM would be the glucose values at which the odds ratios (ORs) reached 1.75 for BW of > 90th percentile, per cent infant body fat (based on skinfolds) > 90th percentile,8 and concentration of cord C-peptide > 90th percentile. Although in most populations the application of the IADPSG criteria increases the number of women diagnosed with GDM compared with most previously used criteria (Table 2),24 they might not identify women at risk who have a high 2-hour post-load glucose result but which is still below that specified by the IADPSG criteria. 6
| Criteria | Glucose thresholds (mmol/l)a | Criteria | Coverage of use | ||
|---|---|---|---|---|---|
| Fasting | 1-hour post load | 2-hour post load | |||
| HAPO exclusion11 | 5.8 | 11.1 | 2002 | Some US cities | |
| WHO (previous)11 | 7.0 | 7.8 | 1999–2013 | Widespread globally | |
| WHO (previous, modified)23 | 6.1 | 7.8 | 1999 to current | UK | |
| NICE18 | 5.6 | 7.8 | 2015 | UK | |
| IADPSG and WHO (current)8,10 | 5.1 | 10.8 | 8.5 | 2010/2013 to present | Widespread globally |
It is unclear whether or not the association between maternal glucose level and perinatal outcomes and the IADPSG criteria for diagnosing GDM should be the same in SA women, who are at higher risk of GDM than white European women. 25 The shift in the aim of diagnosing GDM from one of identifying women at risk of type 2 diabetes to one of identifying risk of future offspring obesity is especially important for SAs, because SA women, on average, have infants of markedly lower BW and a reduced risk of LGA than white European women. 18,24 However, lower BW of SA infants masks a propensity to greater adiposity and associated cardiometabolic risk in later life. 26–32 High maternal pregnancy glucose level is an important mediator of greater birth adiposity in SA compared with white European infants. 23 Although findings of the HAPO study6 showed similar associations across different geographical centres, there were no SA centres, and too few SA participants to assess the association between maternal glycaemia and perinatal outcomes.
We aimed to establish whether or not the IADPSG criteria for diagnosis of GDM are appropriate for SA women and to assess how the prevalence of GDM varies when different criteria for its diagnosis are used in SA and WB women. Our specific objectives were to establish the nature of the association of fasting and post-load glucose levels with adverse perinatal outcomes in a large cohort of SA women and compare those findings with a similarly sized cohort of WB women; to use our results to identify appropriate thresholds for diagnosing GDM in SA and WB women; and to compare the prevalence of GDM in these two groups with different criteria. We hypothesised that the association between fasting and post-load glucose levels, and BW and infant adiposity, and the thresholds used to diagnose GDM, would differ between SA and WB women. Furthermore, we predicted that prevalence of GDM would be greater in SA women than WB women irrespective of criteria used. Our findings should inform clinical practice for diagnosing GDM.
Methods
Study design and participants
‘Born in Bradford’ is a prospective birth cohort study22 of women who delivered a live singleton baby at the Bradford Royal Infirmary, Bradford, UK. Figure 1 shows full inclusion and exclusion of women from the BiB study22 in this study. Women were excluded from all analyses if they did not complete a baseline questionnaire or the OGTT or had missing data for ethnic origin. For the main analyses of the association of gestational glucose level with perinatal outcomes and development of GDM diagnostic criteria, we excluded women who were diagnosed with GDM. GDM was defined according to modified WHO criteria operating at the time (either fasting glucose level of ≥ 6.1 mmol/l or 2-hour post-load glucose level of ≥ 7.8 mmol/l). 11,23 The cohort is broadly representative of the obstetric population in Bradford. 22 All women booked for delivery in Bradford are offered a 75-g OGTT (comprising fasting and 2-hour post-load samples) at around 26–28 weeks’ gestation, and women were recruited mainly at their OGTT appointment. At recruitment, women had their height and weight measured, completed an interviewer-administered questionnaire, and provided written consent for information to be abstracted from their medical records. Interviews were undertaken in English or in SA languages (including Urdu and Mirpuri). The analysis of glucose samples was carried out using a Siemens Advia 2400 analyser from the ADVIA® 2400 Clinical Chemistry System (Siemens Healthcare Ltd, Camberley, UK). The coefficients of variation range between 1.73% at 3.2 mmol/l and 0.64% at 19.1 mmol/l. Ethics approval was obtained from the Bradford Research Ethics Committee (07/H1302/112). All participants provided informed written consent.
FIGURE 1.
Study sample flow chart. The criteria used in the hospital in which study participants were recruited to diagnose GDM (and hence exclude them from the analyses presented here) was either fasting glucose level of ≥ 6.1 mmol/l or 2-hour post-load glucose level of ≥ 7.8 mmol/l. BRI, Bradford Royal Infirmary.
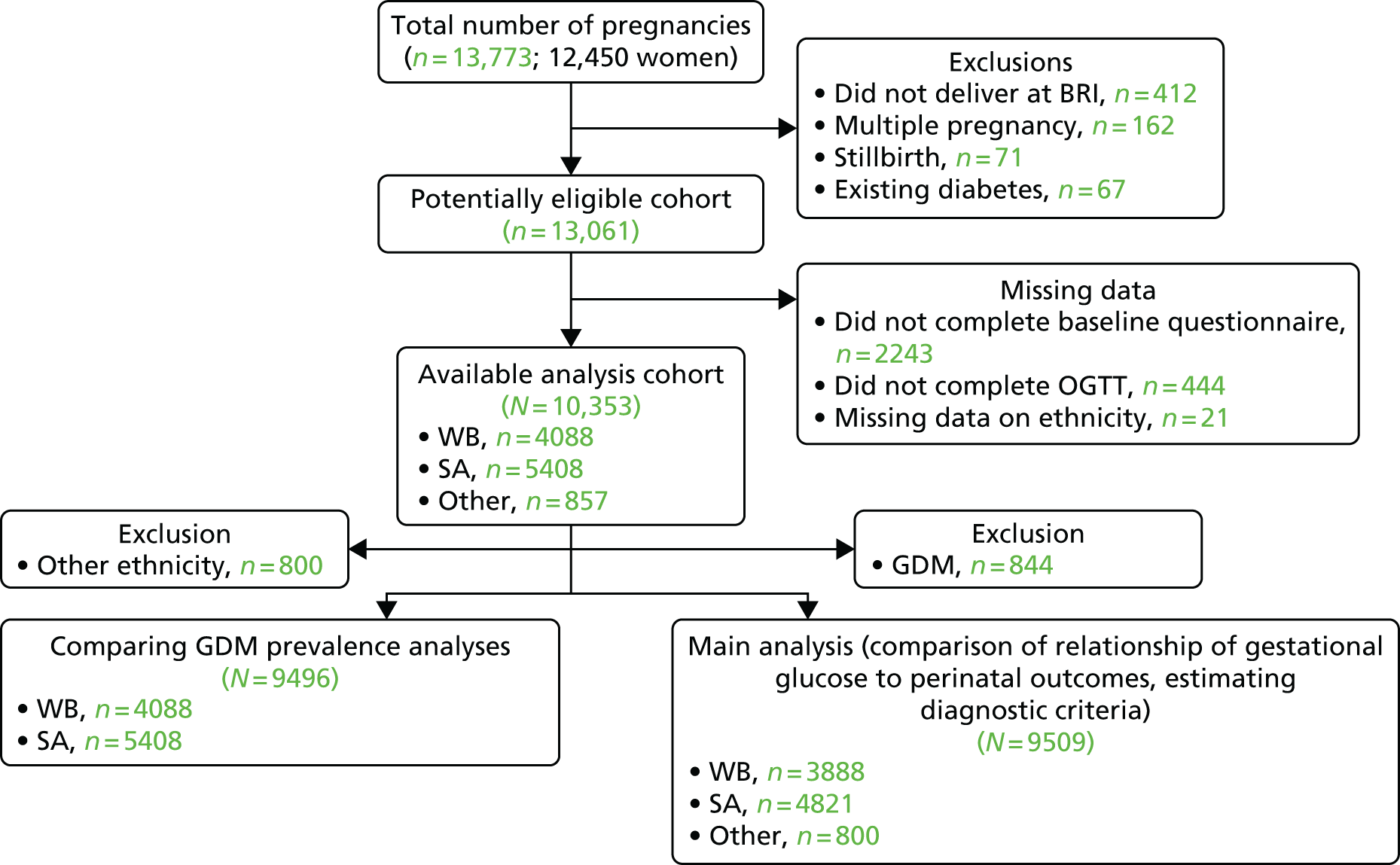
Participants completed a morning OGTT after fasting overnight. A baseline venous blood sample was taken. Participants then consumed a standard solution containing the equivalent of 75 g of anhydrous glucose over 5 minutes. After 2 hours a second sample was taken. Fasting and post-load plasma glucose assays were undertaken immediately using a glucose oxidase method. The analyses were undertaken using the Siemens Advia 2400 analyser following a standard protocol. The coefficients of variation range between 1.73% at 3.2 mmol/l and 0.64% at 19.1 mmol/l.
We assessed associations of maternal glucose concentrations with:
-
three primary outcomes – LGA (defined as BW of > 90th percentile for gestational age), infant adiposity (defined as sum of skinfolds > 90th percentile for gestational age) and C-section
-
five secondary outcomes – pre-eclampsia, preterm delivery, shoulder dystocia, instrumental vaginal delivery and admission to the neonatal unit.
These outcomes are established clinical complications of GDM, and similar to the primary and secondary outcomes in the HAPO study. 6 We did not have information about cord blood C-peptide or neonatal hypoglycaemia in our cohort. We were unable to calculate percentage body fat from skinfolds as done in the HAPO study6 because no equivalent formulae exist for SA infants; thus, we used a cut-off of > 90th percentile for the sum of skinfolds. We included C-section in our analyses as, although it is not used to predict future risk of adiposity and ill health, it is an important perinatal outcome and is associated with LGA, greater infant adiposity and increased health service costs. 33
Birthweight, mode of delivery (normal vaginal, instrumented vaginal or C-section), gestational age, pre-eclampsia, shoulder dystocia and admission to the neonatal unit were obtained from hospital records. C-section was compared with all vaginal deliveries. Pre-eclampsia was defined as new-onset proteinuria (> 300 g in 24 hours) together with blood pressure (BP) of ≥ 140/90 mmHg after 20 weeks’ gestation on more than one occasion. BWs were converted into standard deviation (SD) scores standardised for gestational age and gender relative to the UK-WHO growth standard. 34,35 Infants were then categorised as either being > 90th percentile or not. 27 The UK-WHO growth standards are based on data from six counties (USA, Norway, Oman, Brazil, India and Ghana) and describe the optimum pattern of growth for all children, rather than the prevailing pattern in the UK. 35 Skinfold thickness (triceps and subscapular) were summed and the 90th percentile was established from quantile regression using six gender–ethnic groups [combining gender and ethnic origin (WB, SA, and other)] and adjusted for parity (0, 1, 2, 3+). 36 The intra-rate and inter-rate technical error of measurements for the skinfold thicknesses were, respectively, 0.22–0.35 mm and 0.15–0.54 mm for triceps, and 0.14–0.25 mm and 0.17–0.63 mm for subscapular skinfolds. 37
Statistical analyses
Associations of fasting and post-load glucose levels with outcomes were assessed by categories, and with glucose as a continuous variable (per SD). We used multivariable logistic regression with clustered sandwich estimators38 (to account for some women in the cohort having more than one pregnancy) to assess associations of fasting and post-load glucose levels with each outcome. We followed the analytical protocol used in the HAPO study6 as closely as possible, with fasting and post-load glucose concentrations divided into seven categories (see the Table 3 footnotes for definition of categories). In order to explore any extreme threshold effects, the top two categories for fasting and post-load glucose levels included about 1% and 3% of women, respectively. Models were adjusted for gestational age at OGTT, presence or absence of family history of diabetes, family history of hypertension, previous GDM, previous macrosomia, smoking status, alcohol consumption during pregnancy, maternal age and BMI, maternal education, baby gender and parity. Models for all women were additionally adjusted for ethnic origin. Models for SA women were not adjusted for alcohol consumption during pregnancy because most reported never drinking alcohol. Additionally, preterm delivery was adjusted for squared maternal BMI because of evidence of a quadratic relationship of BMI with preterm delivery. Shoulder dystocia models were not adjusted for previous GDM because of small numbers. Ethnicity was categorised as WB, SA and other ethnicity according to UK Office for National Statistics criteria. 39 Education was equivalised to UK standard attainments, and participants were included in one of five mutually exclusive categories (< 5 GCSE equivalent, 5+ GCSE equivalent, A level equivalent, higher than A level, other). 23 Parity was categorised as 0 or ≥ 1 previous pregnancies. Smoking was categorised as never, past (not during this index pregnancy), current (during this pregnancy) and alcohol as consumed during this pregnancy or not.
Maternal BMI was calculated from height measured at the time of recruitment and from weight measured at booking antenatal clinic, which was obtained from electronic hospital records. Expected date of birth (40 weeks) was estimated from a gestation ultrasound scan at ≈ 10 weeks then, using the date of OGTT and date of birth, gestational age at OGTT and birth were calculated. Infant gender was obtained from electronic hospital records, and family history of diabetes and hypertension were abstracted from paper hospital records.
We established fasting and post-load glucose thresholds for BW of > 90th percentile and standardised sum of skinfolds of > 90th percentile that equated to an OR of 1.75, using the methods of IADPSG. 8 We estimated the ORs of these outcomes at mean glucose levels and the ORs at 0.1-mmol/l intervals across the full range of fasting and 2-hour post-load glucose levels. We then plotted this range of ORs and used the plots to estimate the thresholds of fasting, and 2-hour post-load glucose that were closest to ORs for each outcome of 1.75 in both ethnic groups. These analyses were carried out with adjustment for the same potential confounders as in all multivariable regression analyses. All analyses were undertaken separately in WB and SA women, and we tested for differences in associations by including an interaction term between glucose and ethnic origin. Because women of SA origin were mainly Pakistani, we undertook a sensitivity analysis in which we repeated analyses including only Pakistani women. To maximise statistical power and minimise bias that might occur if women with missing data were excluded from analyses, we used multivariate multiple imputation with chained equations to impute missing values40 (see Appendix 1, Table 50). We repeated all analyses with the complete data cohort for comparison.
Levels of missing data range from 0% to 32% for the different variables (see Appendix 1, Table 50), and 5056 (53%) had complete data on all variables included in any analyses. To maximise statistical power and minimise bias due to excluding those with any missing data, we used multivariate multiple imputation, with chained equations to impute missing values for covariables and outcomes for the main analyses. 40 We generated 50 imputed data sets and combined these using Rubin’s rules, using the ‘mi’ commands in Stata 13 (StataCorp LP, College Station, TX, USA). Distributions of variables from pooling of the data sets with imputed variables were similar to those for observed variables (see Appendix 1, Table 50). We repeated all analyses with the complete data cohort for comparison.
Results
Women were recruited to the BiB study22 between March 2007 and November 2010; investigators collected detailed information from 12,450 women (13,773 pregnancies resulting in 13,818 births). After exclusions, 9509 women (4821 SA and 3888 WB) were included in the main analyses looking at associations of fasting and post-load glucose levels with adverse perinatal outcomes. A total of 844 women with GDM who were excluded from main analyses were included in the analyses that compared the prevalence of GDM with different criteria. Table 3 shows characteristics of the women and infants in the eligible cohort: 51% were SA, 41% were WB and 8% were of other ethnic origin. Median fasting and post-load glucose concentrations were slightly higher in SA than WB women. WB infants were almost three times more likely than SA infants to have a BW of > 90th percentile, but the frequency of sum of skinfolds of > 90th percentile was similar in WB and SA infants. Characteristics were similar in the larger cohort of eligible women to those that were included in the main analysis cohort (see Appendix 1, Table 51).
| Outcome | N | All women: mean (SD), median (IQR) or n (%) | N | WB: mean (SD), median (IQR) or n (%) | N | SA: mean (SD), median (IQR) or n (%) | N | Other: mean (SD), median (IQR) or n (%) |
|---|---|---|---|---|---|---|---|---|
| Primary outcomes | ||||||||
| BW of > 90th percentilea | 9508 | 592 (6.2) | 3887 | 361 (9.3) | 4821 | 164 (3.4) | 800 | 67 (8.4) |
| Sum of skinfolds of > 90th percentileb | 6458 | 687 (10.6) | 2510 | 270 (10.8) | 3409 | 365 (10.7) | 539 | 52 (9.7) |
| Caesarean delivery | 9509 | 1983 (20.9) | 3888 | 870 (22.4) | 4821 | 907 (18.8) | 800 | 206 (25.8) |
| Secondary outcomes | ||||||||
| Pre-eclampsia | 9120 | 229 (2.5) | 3724 | 97 (2.6) | 4629 | 115 (2.5) | 767 | 17 (2.2) |
| Preterm delivery (< 37 weeks) | 9509 | 471 (5.0) | 3888 | 204 (5.3) | 4821 | 227 (4.7) | 8000 | 40 (5.0) |
| Shoulder dystociac | 7526 | 105 (1.4) | 3018 | 42 (1.4) | 3914 | 50 (1.3) | 594 | 13 (2.2) |
| Instrumental vaginal deliveryc | 7519 | 930 (12.4) | 3015 | 417 (13.8) | 3913 | 417 (10.7) | 591 | 96 (16.2) |
| Intensive neonatal care | 9509 | 412 (4.3) | 3888 | 166 (4.3) | 4821 | 213 (4.4) | 800 | 33 (4.1) |
| Glucose levels | ||||||||
| Fasting | 9509 | 4.4 (4.2–4.7) | 3888 | 4.3 (4.1–4.6) | 4821 | 4.5 (4.2–4.8) | 800 | 4.4 (4.1–4.6) |
| Two-hour post load | 9509 | 5.4 (4.7–6.1) | 3888 | 5.3 (4.5–6.0) | 4821 | 5.4 (4.8–6.2) | 800 | 5.3 (4.6–6.0) |
| Maternal and infant characteristics | ||||||||
| Maternal age at delivery (years) | 9509 | 27.3 (5.5) | 3888 | 26.8 (6.1) | 4821 | 27.7 (5.0) | 800 | 27.4 (5.7) |
| Aged ≥ 35 years | 1092 (11.5) | 487 (12.5) | 503 (10.4) | 102 (12.8) | ||||
| BMI (at booking) | 9073 | 25.8 (5.6) | 3708 | 26.7 (5.9) | 4596 | 25.2 (5.3) | 769 | 25.7 (5.5) |
| Obese (BMI ≥ 30 kg/m2) | 1808 (19.9) | 899 (24.2) | 768 (16.7) | 141 (18.3) | ||||
| Maternal education | 9383 | 3847 | 4755 | 781 | ||||
| < 5 GCSEs | 2024 (21.6) | 788 (20.5) | 1140 (24.0) | 96 (12.3) | ||||
| ≥ 5 GCSEs | 2954 (31.5) | 1336 (34.7) | 1453 (30.6) | 165 (21.1) | ||||
| A level | 1389 (14.8) | 652 (17.0) | 639 (13.4) | 98 (12.6) | ||||
| Higher than A level | 2402 (25.6) | 739 (19.2) | 1352 (28.4) | 311 (39.8) | ||||
| Other | 614 (6.5) | 332 (8.6) | 171 (3.6) | 111 (14.2) | ||||
| Smoking status | 9494 | 3886 | 4809 | 799 | ||||
| Never | 6518 (68.7) | 1589 (40.9) | 4428 (92.1) | 501 (62.7) | ||||
| Before pregnancy | 1359 (14.3) | 973 (25.0) | 227 (4.7) | 159 (19.9) | ||||
| In pregnancy | 1617 (17.0) | 1324 (34.1) | 154 (3.2) | 139 (17.4) | ||||
| Any alcohol during pregnancy | 9477 | 1950 (20.6) | 3875 | 1715 (44.3) | 4805 | 40 (0.8) | 797 | 195 (24.5) |
| Primiparity | 9151 | 3813 (41.7) | 3762 | 1821 (48.4) | 4623 | 1566 (33.9) | 766 | 426 (55.6) |
| Family history of diabetes | 9212 | 2313 (25.1) | 3782 | 508 (13.4) | 4660 | 1657 (35.6) | 770 | 148 (19.2) |
| Family history of hypertension | 9203 | 2519 (27.4) | 3774 | 909 (24.1) | 4654 | 1412 (30.3) | 775 | 198 (25.6) |
| Previous GDMd | 5338 | 56 (1.1) | 1941 | 19 (1.0) | 3057 | 35 (1.1) | 340 | 2 (0.6) |
| Previous macrosomia (≥ 4 kg)d | 4464 | 359 (8.0) | 1662 | 212 (12.8) | 2523 | 124 (4.9) | 279 | 23 (8.2) |
| Gestational age at OGTT (weeks) | 9509 | 26.3 (1.9) | 3888 | 26.2 (1.9) | 4821 | 26.3 (1.9) | 800 | 26.4 (1.7) |
| Gestational age at delivery (weeks) | 9509 | 39.7 (1.7) | 3888 | 39.8 (1.8) | 4821 | 39.6 (1.7) | 800 | 39.7 (1.7) |
| Male gender | 9509 | 4884 (51.4) | 3888 | 2006 (51.6) | 4821 | 2464 (51.1) | 800 | 414 (51.8) |
Associations of fasting and post-load glucose levels with primary outcomes
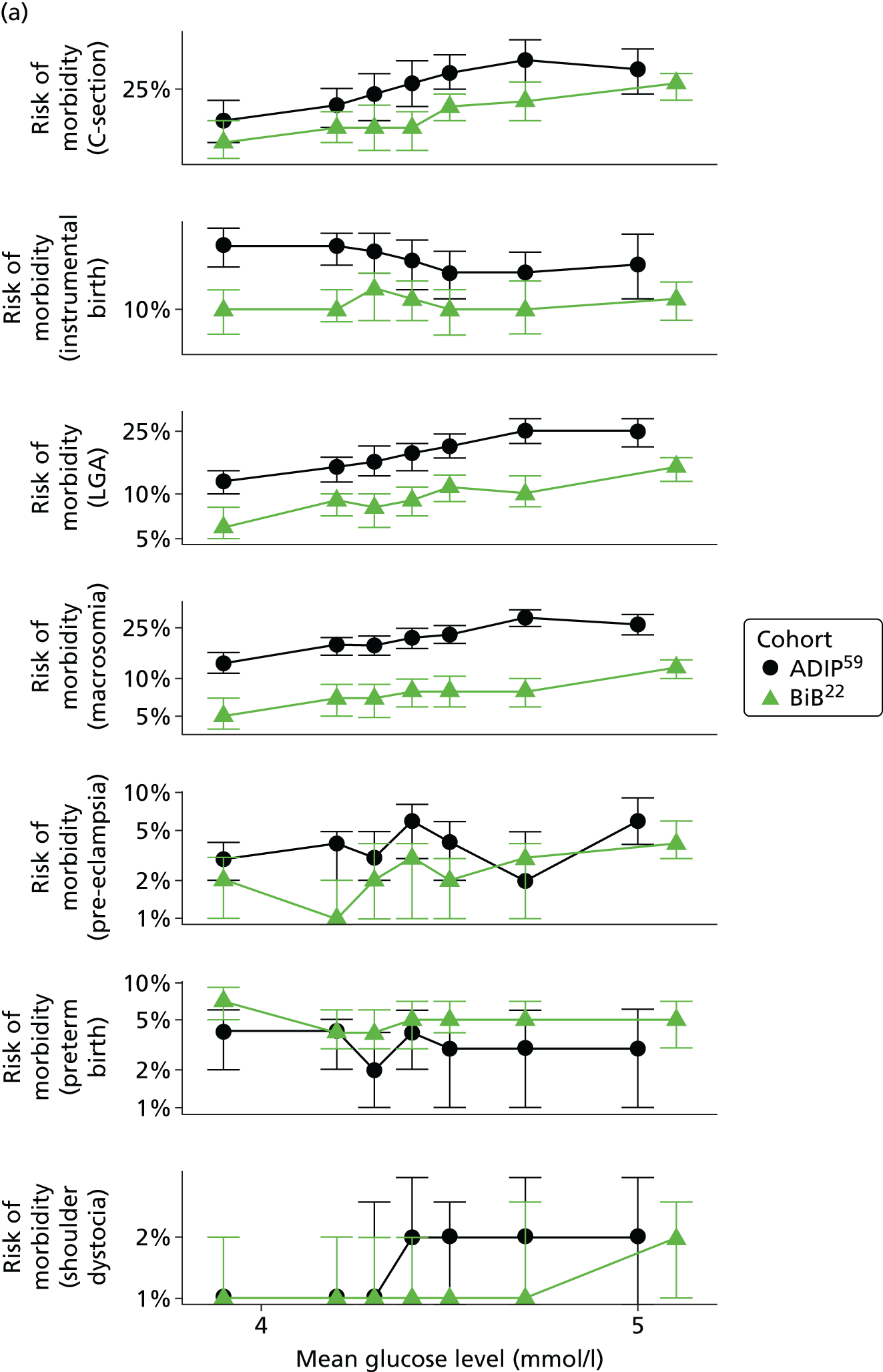
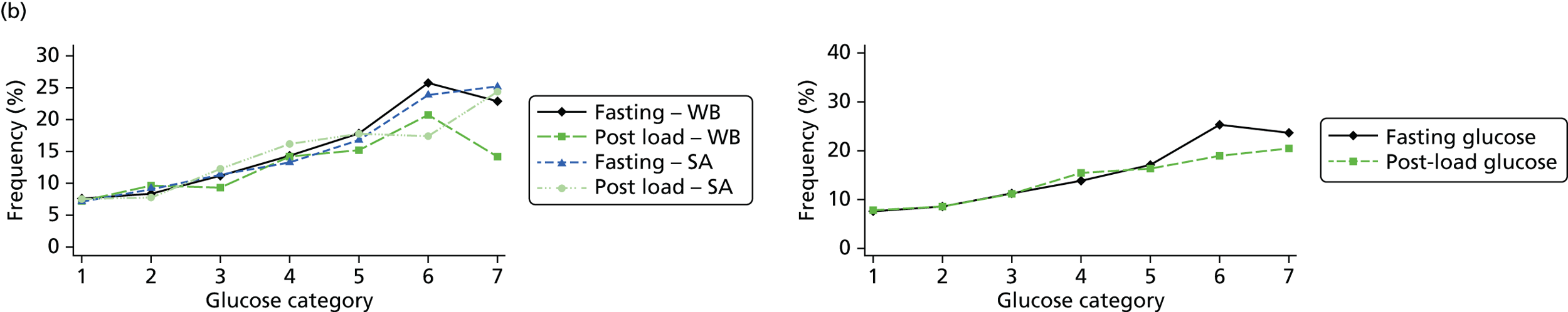
Figure 2 shows the unadjusted percentage of women in each group who had each of the three primary outcomes by categories of fasting and 2-hour post-load glucose level by ethnicity and for all women. Generally, the frequency of each of the three primary outcomes increased across the seven categories of fasting and post-load glucose levels, with no evidence of a threshold at which risk markedly increases, except for the association of fasting glucose level with C-section in SA women. The higher prevalence of BW of > 90th percentile in WB infants than in SA infants is consistent across all glucose categories. Combining data for all women (i.e. including 99% of the cohort) showed monotonic relationships of fasting and post-load glucose levels up to the sixth category (see Appendix 1, Figures 45 and 46).
FIGURE 2.
Frequency of primary outcomes across glucose categories by ethnicity: WB (n = 3888) and SA (n = 4821), and for all pregnancies (N = 9509). (a) BW of > 90th percentile; (b) sum of skinfolds > 90th percentile; (c) C-section. Glucose categories are defined as follows: FPG level: category 1, < 4.3 mmol/l; category 2, 4.3–4.4 mmol/l; category 3, 4.5–4.7 mmol/l; category 4, 4.8–4.9 mmol/l; category 5, 5.0–5.2 mmol/l; category 6, 5.3–5.6 mmol/l; category 7, 5.7–6.0 mmol/l. Post-load plasma glucose level: category 1, < 4.7 mmol/l; category 2, 4.7–5.4 mmol/l; category 3, 5.5–6.2 mmol/l; category 4, 6.3–6.6 mmol/l; category 5, 6.7–7.2 mmol/l; category 6, 7.3–7.5 mmol/l; category 7, 7.6–7.7 mmol/l. FPG, fasting plasma glucose.


Regression analyses confirmed monotonic associations of glucose level with each of the primary outcomes, in each group, without (see Appendix 1, Table 52) and with adjustment for confounders (Table 4). In view of the monotonic nature of the associations, we focused our comparisons on results with fasting or post-load glucose level as a continuous variable (per 1 SD). Although there was not strong statistical evidence of differences, the point estimates suggested stronger associations of fasting and post-load glucose levels with all three outcomes, except for those of fasting glucose level with LGA and post-load glucose level with C-section. However, there was no strong statistical evidence that the associations differed between the two groups for any primary outcome (p interaction of ≥ 0.2 for all associations).
| Outcome | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | p-interactiona | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| By fasting glucose categoryb and per 1 SD | |||||||
| BW of > 90th percentile | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.39 |
| 2 | 1.18 | 0.90 to 1.54 | 1.15 | 0.83 to 1.59 | 1.07 | 0.59 to 1.94 | |
| 3 | 1.35 | 1.04 to 1.74 | 1.38 | 1.01 to 1.90 | 1.10 | 0.65 to 1.88 | |
| 4 | 1.42 | 1.02 to 1.97 | 1.57 | 1.04 to 2.37 | 1.05 | 0.56 to 1.98 | |
| 5 | 1.90 | 1.35 to 2.67 | 1.59 | 0.97 to 2.62 | 2.12 | 1.20 to 3.76 | |
| 6 | 3.10 | 2.00 to 4.79 | 2.21 | 1.07 to 4.54 | 3.35 | 1.72 to 6.51 | |
| 7 | 2.60 | 1.35 to 5.04 | 2.09 | 0.80 to 5.48 | 3.25 | 1.29 to 8.21 | |
| Per 1 SD | 1.31 | 1.20 to 1.43 | 1.22 | 1.08 to 1.38 | 1.43 | 1.23 to 1.67 | |
| Sum of skinfolds of > 90th percentile | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.98 |
| 2 | 1.11 | 0.88 to 1.40 | 1.04 | 0.74 to 1.46 | 1.29 | 0.92 to 1.82 | |
| 3 | 1.40 | 1.14 to 1.72 | 1.35 | 0.96 to 1.88 | 1.56 | 1.15 to 2.13 | |
| 4 | 1.61 | 1.24 to 2.09 | 1.69 | 1.09 to 2.62 | 1.70 | 1.18 to 2.45 | |
| 5 | 2.02 | 1.54 to 2.64 | 2.05 | 1.26 to 3.36 | 2.15 | 1.49 to 3.10 | |
| 6 | 3.23 | 2.29 to 4.56 | 3.20 | 1.52 to 6.74 | 3.18 | 2.01 to 5.02 | |
| 7 | 2.73 | 1.53 to 4.87 | 2.71 | 0.97 to 7.58 | 3.06 | 1.44 to 6.51 | |
| Per 1 SD | 1.35 | 1.25 to 1.45 | 1.35 | 1.18 to 1.54 | 1.35 | 1.23 to 1.49 | |
| Caesarean delivery | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.47 |
| 2 | 0.98 | 0.84 to 1.13 | 1.03 | 0.83 to 1.27 | 0.99 | 0.79 to 1.24 | |
| 3 | 1.11 | 0.96 to 1.28 | 1.06 | 0.86 to 1.32 | 1.20 | 0.97 to 1.49 | |
| 4 | 1.17 | 0.97 to 1.41 | 1.11 | 0.81 to 1.51 | 1.33 | 1.03 to 1.73 | |
| 5 | 1.20 | 0.98 to 1.48 | 1.18 | 0.83 to 1.69 | 1.18 | 0.88 to 1.56 | |
| 6 | 1.14 | 0.84 to 1.55 | 1.42 | 0.83 to 2.45 | 1.02 | 0.67 to 1.56 | |
| 7 | 2.14 | 1.34 to 3.41 | 1.25 | 0.57 to 2.77 | 2.88 | 1.58 to 5.25 | |
| Per 1 SD | 1.09 | 1.03 to 1.15 | 1.06 | 0.97 to 1.16 | 1.11 | 1.02 to 1.20 | |
| By 2-hour post-load glucose categoryb and per 1 SD | |||||||
| BW of > 90th percentile | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.60 |
| 2 | 0.95 | 0.74 to 1.23 | 1.00 | 0.73 to 1.37 | 0.96 | 0.56 to 1.66 | |
| 3 | 1.08 | 0.83 to 1.39 | 0.98 | 0.71 to 1.36 | 1.04 | 0.61 to 1.76 | |
| 4 | 1.29 | 0.92 to 1.80 | 1.20 | 0.78 to 1.84 | 1.39 | 0.72 to 2.66 | |
| 5 | 1.58 | 1.14 to 2.19 | 1.18 | 0.76 to 1.82 | 2.12 | 1.15 to 3.93 | |
| 6 | 1.71 | 1.04 to 2.81 | 1.74 | 0.90 to 3.36 | 1.66 | 0.69 to 3.98 | |
| 7 | 1.29 | 0.65 to 2.60 | 1.27 | 0.50 to 3.26 | 1.64 | 0.54 to 5.05 | |
| Per 1 SD | 1.17 | 1.07 to 1.29 | 1.10 | 0.98 to 1.24 | 1.28 | 1.06 to 1.55 | |
| Sum of skinfolds of > 90th percentile | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.23 |
| 2 | 1.02 | 0.81 to 1.29 | 1.24 | 0.88 to 1.73 | 0.96 | 0.68 to 1.35 | |
| 3 | 1.32 | 1.05 to 1.65 | 1.13 | 0.78 to 1.63 | 1.51 | 1.10 to 2.07 | |
| 4 | 1.84 | 1.40 to 2.41 | 1.76 | 1.12 to 2.76 | 1.94 | 1.33 to 2.83 | |
| 5 | 1.94 | 1.47 to 2.55 | 1.79 | 1.13 to 2.82 | 2.22 | 1.52 to 3.25 | |
| 6 | 2.29 | 1.54 to 3.39 | 2.63 | 1.35 to 5.14 | 2.13 | 1.25 to 3.64 | |
| 7 | 2.53 | 1.53 to 4.17 | 1.80 | 0.68 to 4.77 | 3.13 | 1.71 to 5.74 | |
| Per 1 SD | 1.31 | 1.21 to 1.42 | 1.26 | 1.11 to 1.42 | 1.38 | 1.23 to 1.54 | |
| Caesarean delivery | |||||||
| 1 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 0.54 |
| 2 | 0.95 | 0.82 to 1.10 | 0.89 | 0.72 to 1.11 | 1.06 | 0.84 to 1.32 | |
| 3 | 1.07 | 0.92 to 1.24 | 1.09 | 0.87 to 1.37 | 1.01 | 0.80 to 1.27 | |
| 4 | 1.11 | 0.91 to 1.36 | 0.96 | 0.70 to 1.32 | 1.19 | 0.89 to 1.60 | |
| 5 | 1.00 | 0.81 to 1.23 | 1.03 | 0.76 to 1.42 | 0.97 | 0.71 to 1.33 | |
| 6 | 1.31 | 0.96 to 1.79 | 1.12 | 0.68 to 1.85 | 1.35 | 0.88 to 2.07 | |
| 7 | 1.15 | 0.76 to 1.74 | 0.86 | 0.43 to 1.72 | 1.29 | 0.72 to 2.29 | |
| Per 1 SD | 1.05 | 0.99 to 1.11 | 1.02 | 0.94 to 1.10 | 1.05 | 0.96 to 1.14 | |
Associations of fasting and post-load glucose levels with secondary outcomes
Associations with secondary outcomes were similar in the two ethnic groups [see Appendix 1, Table 53 (unadjusted) and Table 54 (confounder adjusted)]. The frequency of pre-eclampsia, shoulder dystocia and, with a weaker magnitude, instrumental delivery, also increased across each glucose category, especially with fasting glucose level (see Appendix 1, Figures 45 and 46). Neither fasting nor post-load glucose concentrations were clearly associated with preterm delivery or admission to the neonatal unit.
Criteria for diagnosing gestational diabetes mellitus
Table 5 shows the thresholds of fasting and glucose that would result in an OR of 1.75 for BW of > 90th percentile, and sum of skinfolds of > 90th percentile in each group. Fasting and post-load glucose thresholds based on the average of BW and skinfolds of > 90th percentile for all women irrespective of ethnic origin were 5.3 mmol/l and 7.5 mmol/l, respectively. Fasting glucose thresholds based on BW or the average of BW and skinfolds of > 90th percentile were higher for WB women than for SA women (see Table 5); with skinfolds of > 90th percentile alone as the outcome, the fasting glucose threshold was the same in both ethnic groups. There was no 2-hour post-load threshold that reached an OR of 1.75 for BW of > 90th percentile in either ethnic group. A threshold for sum of skinfolds of > 90th percentile was found only in SA women (see Table 5).
| Outcomes | All women (N = 10,356) | WB women (n = 4105) | SA women (n = 5445) | |||
|---|---|---|---|---|---|---|
| Fasting | 2-hour post load | Fasting | 2-hour post load | Fasting | 2-hour post load | |
| BW of > 90th percentile | 5.3 | NP | 5.6 | NP | 5.1 | NP |
| Sum skinfolds of > 90th percentile | 5.2 | 7.5 | 5.2 | NP | 5.2 | 7.2 |
| Average glucose level for both BW and sum of skinfolds of > 90th percentile | 5.3 | 7.5 | 5.4 | NP | 5.2 | 7.2 |
Table 6 shows GDM prevalence by past and present diagnostic criteria, and the criteria derived from our data. For our study criteria, we show prevalences with the same thresholds in both ethnic groups (the thresholds derived for all women) and also ethnic-specific thresholds. Prevalence of GDM was about twice as high in SA women using any criteria range (4.1–17.4%) than in WB women (1.2–8.7%) for all non-ethnic specific criteria. Prevalence was greater in both ethnic groups with the recently derived IADPSG, NICE and our criteria than the 1999 WHO criteria. Of the three recent criteria, the NICE criteria resulted in the lowest prevalences in WB women and our criteria the highest. In SA women, the NICE criteria resulted in the lowest prevalence. If we applied criteria derived in our study for all women (i.e. not taking account of ethnic origin) to the SA women, the prevalence of GDM was the same using either IADPSG/WHO or our criteria. However, when we applied our ethnic-specific criteria, prevalence in SA women was nearly three times that in WB women (see Table 6).
| Criteria | Criteria (all define GDM as the presence of having glucose levels at or above one or more of the following) | Prevalence in our study population: % (95% CI) | |||
|---|---|---|---|---|---|
| Fasting glucose | 1-hour post-load glucose | 2-hour post-load glucose | WB | SA | |
| Older, used in recent past | |||||
| Exclusion in HAPO exclusiona | 5.8 | – | 11.1 | 1.2 (0.9 to 1.5) | 4.1 (3.6 to 4.7) |
| WHO (previous)b | 7.0 | – | 7.8 | 4.7 (4.1 to 5.4) | 10.4 (9.6 to 11.2) |
| WHO (previous, modifiedc | 6.1 | – | 7.8 | 4.9 (4.3 to 5.6) | 10.8 (10.0 to 11.7) |
| Recently proposed | |||||
| NICEd | 5.6 | – | 7.8 | 5.9 (5.2 to 6.6) | 12.5 (11.7 to 13.4) |
| IADPSG/WHO (current)e | 5.1 | 10.8 | 8.5 | 7.6 (6.8 to 8.5) | 17.3 (16.3 to 18.3) |
| Our study | |||||
| Same criteria for all womenf | 5.3 | – | 7.5 | 8.7 (7.9 to 9.6) | 17.4 (16.4 to 18.4) |
| For WB | 5.4 | – | 7.5 | 8.3 (7.5 to 9.2) | – |
| For SA | 5.2 | – | 7.2 | – | 24.2 (23.1 to 25.3) |
Additional sensitivity analyses
The number of missing data ranged from 0% to 32% for the different variables (see Appendix 1, Table 50) and 5056 of the 9509 (53%) had complete data on all variables for the main analyses. Distributions of any variable with missing data were the same in the imputation data sets (see Table 4 and Appendix 1, Table 54) and for observed complete case data (see Appendix 1, Tables 56 and 57). There was no strong evidence for a quadratic curvilinear association between fasting or post-load glucose level and any of the primary or secondary outcomes (see Appendix 1, Table 55). The results of analyses restricted to Pakistani women did not differ from those presented for all SA women (see Appendix 1, Tables 58 and 59).
Discussion
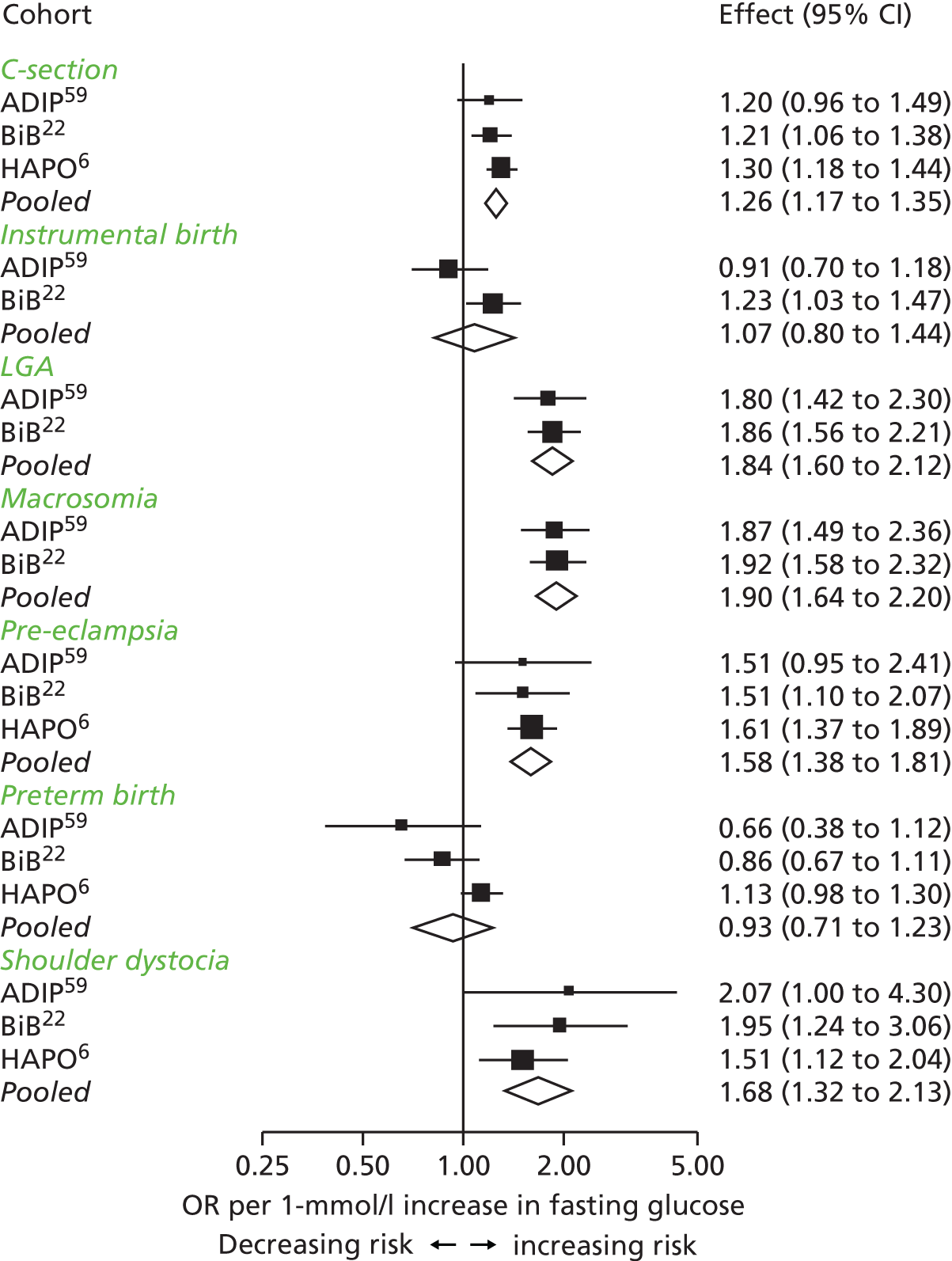
We recorded graded monotonic associations of fasting and 2-hour post-load glucose level with LGA and high adiposity (as assessed by skinfold thickness) across most of the glucose distribution in both SA and WB women. The associations of glucose level with LGA appeared stronger in SA than WB women, but there was no statistical evidence of an interaction with ethnic origin. Applying the same method as the IADPSG to our data, we estimated fasting and post-load glucose thresholds for diagnosing GDM that are lower in SA women than in WB women. For WB women, our criteria included a fasting glucose threshold that was slightly higher, and a 2-hour glucose threshold that was markedly lower, than those recommended by IADPSG and WHO. Our results support a lower threshold for both fasting and 2-hour post-load glucose level for diagnosing GDM than is currently recommended by NICE in both WB and SA women. NICE supports higher fasting glucose thresholds to those proposed by the IADPSG and WHO in WB and SA women, but lower 2-hour post-load glucose thresholds. Using existing criteria, the prevalence of GDM in our cohort was about twice as high in SA than in WB women; when we applied the ethnic-specific criteria derived from our data, the prevalence was three times higher in SA women, and identified about 25% of SA women as having GDM.
Overall patterns of associations in our study, for both primary and secondary outcomes, were similar to those seen in the HAPO study,6 especially for fasting glucose levels. 6 Because of differences between ours and the HAPO study6 in the post-load glucose threshold used to exclude women from the study cohort, our highest 2-hour post-load category (category 7) was similar to category 4 in the HAPO study. 6 As a result, for some outcomes, the linear relationship seems to flatten at the upper end of the 2-hour post-load glucose categories.
Compared with the IADPSG, who used data from the HAPO study,6 we could not identify a 2-hour post-load threshold: there was no threshold that reached an OR of 1.75 for BW of > 90th percentile, and only SA women reached a threshold for this OR for sum of skinfolds of > 90th percentile. The IADPSG consensus panel chose 1.75 to represent the lowest level of clinically important risk; a lower OR was not considered clinically important. GDM was diagnosed in our study using a lower 2-hour post-load glucose threshold than in the HAPO study;6 both studies excluded women with GDM as it would be unethical not to treat them. If we had applied the same high 2-hour post-load glucose threshold as in the HAPO study6 to diagnose GDM and to exclude women from the main analysis, we would have been more likely to identify an OR of 1.75, because women with higher glucose concentrations and greater associated risk of the primary outcomes would have been included in our analyses. The 2-hour post-load glucose level used to exclude women with GDM in the HAPO study6 was much higher than that recommended by WHO, and also by other criteria recommended at the time that the HAPO study6 began, including the Australasian Diabetes in Pregnancy Society criteria. Thus, the 2-hour post-load glucose threshold used to define GDM in the IADPSG and WHO criteria is higher than that suggested by our study (see Table 1). Because the diagnostic criteria for GDM in our study meant that we excluded women from the main analyses with a much lower post-load glucose threshold than was the case in the HAPO study,6 we had difficulty identifying a glucose threshold that reached an OR of 1.75 for sum of skinfolds of > 90th percentile in WB women. Therefore, our GDM diagnostic criteria for this group are mainly driven by results of the associations with LGA.
Consistent with other studies,42–45 we have shown that using any criteria the prevalence of GDM is greater in SA women than in WB women. When we used the same criteria for both ethnic groups, the criteria derived from our study resulted in a higher prevalence of GDM than the NICE criteria for both WB women and SA women, but broadly similar prevalences for both groups to those found with the IADPSG/WHO criteria. When we used ethnic group-specific criteria, the prevalences for WB women remained higher than the NICE criteria, but were similar to IADPSG/WHO criteria, whereas those for SA women became higher for both of these other two criteria. Our study cohort is large and well characterised. The broad consistency of our findings with the results of the HAPO study,6 and the fact that our results were unchanged when we limited the analyses in SAs to those of Pakistani origin, suggests that the results might be generalisable to all white Europeans and SAs. Some participants had missing data for some variables, but the distribution of recorded variables and those from the pooled multiple imputed data sets were similar, as were the association results. We did not collect data for 1-hour post-load glucose concentrations, which were measured in the HAPO study,6 and a 1-hour post-load glucose threshold is included in IADPSG/WHO criteria for GDM. Although the HAPO study6 found linear associations of 1-hour post-load glucose levels with adverse perinatal outcomes, none of the randomised trials that have shown the effect of treatments on adverse perinatal outcomes had used this to define GDM. Furthermore, it is unclear how many additional women in different populations are identified by this additional glucose measurement. Thus, the benefit of this additional measurement remains somewhat unclear. We do not have data for cord blood C-peptide concentrations or neonatal hypoglycaemia. High cord blood C-peptide concentrations were one of the criteria used by the IADPSG in the development of their diagnostic criteria; this additional information might have affected our results. However, the similar prevalences of GDM in WB women using the IADPSG/WHO criteria or our study criteria suggest that including these data would not have markedly changed our results.
Concerns have been raised about the increased prevalence of GDM and hence the cost to health services if the IADPSG criteria are used worldwide in place of the previously widely used 1999 WHO criteria. 25,46,47 Until the late 1990s, the main aim of diagnosing GDM was to identify women at risk of subsequent type 2 diabetes. 48 By contrast, the outcomes used to develop the IADPSG criteria, which we also used, were chosen to identify offspring at risk of future high adiposity and cardiometabolic risk. 48 Although there is evidence that GDM causes greater adiposity in offspring in later life,48,49 there is still debate about the validity of that evidence. 50 Thus, the extent to which the IADPSG or our criteria will accurately predict future adverse offspring health remains to be established. Conversely, in view of the graded association of maternal glucose concentrations with adverse perinatal outcomes, lowering the thresholds used to diagnose GDM would identify more pregnancies at risk of these outcomes. Because effective, safe, and cheap treatments are available for GDM (e.g. lifestyle advice, metformin and insulin) that reduce glucose level across its distribution and help prevent adverse perinatal outcomes,51,52 applying the IADPSG/WHO 2013 or our criteria in place of the WHO 1999 criteria, and also in place of the recently suggested NICE criteria, might improve perinatal outcomes. Because the NICE 2015 criteria recommend higher thresholds of fasting and post-load glucose levels than the IADPSG/WHO or our newly defined criteria, their use will identify fewer women who are at increased risk of adverse outcomes. 53
To conclude, our data support the use of lower fasting and post-load glucose thresholds in SA women than in WB women. They also suggest that compared with our criteria or those of the IADPSG/WHO, the criteria recommended by NICE might underestimate the prevalence of GDM, especially in SA women. The use of our ethnic-specific thresholds for diagnosing GDM in SA women, and of either our – or the IASPSG/WHO – criteria for white European women might reduce the occurrence of adverse perinatal outcomes, in particular LGA, as more at-risk women would be treated. However, the effect of applying any of the recently proposed criteria on later life adiposity and associated cardiometabolic health in offspring are unknown and require further investigation. Furthermore the effectiveness of identifying and treating women with GDM at different cost-effectiveness thresholds, together with the use of varying glucose level thresholds, is also unclear. Our comprehensive analysis detailed in Chapter 7 of this report examines this area and provides information about uncertainties and the value of further research evidence.
Chapter 3 Associations of gestational fasting and post-load glucose levels in women without existing or gestational diabetes with perinatal and longer-term outcomes: a systematic review
Introduction
This chapter presents a systematic review and meta-analyses to determine the association between graded increases in glucose level and risk of perinatal and longer-term outcomes. A version of this chapter has been published in Farrar et al. 54 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
Previous systematic reviews
To our knowledge, this is the first comprehensive systematic review and meta-analyses examining the association of gestational glucose level with risk of perinatal and longer-term outcomes. We have previously undertaken a review as part of a master’s degree dissertation. 55 In that dissertation, a systematic search was undertaken to identify studies that investigated the association between gestational glucose levels [measured using the OGTT or oral glucose challenge test (OGCT)] and adverse outcomes. The findings of that review suggested strong associations between fasting glucose categories and both LGA and macrosomia and these associations were weaker for 2-hour post-load glucose categories. However, that review included only studies that had been published up to March 2013, and we are aware of additional studies since then. Furthermore, there was no attempt to explore sources of heterogeneity between studies.
The aim in this study was to expand and update the previous search and analyses in order to determine associations between fasting and post-load glucose levels, and both perinatal and longer-term maternal and offspring outcomes. This section is reported in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 56
Methods
Search
The original (master’s dissertation) searches were undertaken in March 2013. 55 The search strategies including interfaces and search terms used, and how they were combined, are shown in Appendix 7 (see Tables 83 and 84). Although no date or language restrictions were placed on the searches, only studies with an English language title and/or an abstract were screened for inclusion. The same search strategy (as in March 2013) was used for updating this review, with repeat searches undertaken in September 2013 and October 2014. The following databases were searched: MEDLINE® and MEDLINE In-Process & Other Non-Indexed Citations,® EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) database, NHS Economic Evaluation Database (NHS EED) and the Cochrane Methodology Register (CMR).
The search results were downloaded into an EndNote (Thomson Reuters, CA, USA) library and duplicates were removed. All records (title, publication details and abstracts if available) were screened for eligibility, independently, by two reviewers. We had previously screened the records identified by the March 2013 search; however, we rescreened these again to ensure that the screening standard was high and consistent across all searches. All studies identified as potential ‘includes’ were checked by a second reviewer. Disagreements were resolved by discussion or by a third reviewer. The reference lists of all of the included studies and any related systematic reviews identified were checked for further possible inclusions.
Inclusion and exclusion criteria
All studies reporting the association of fasting or post-load glucose level (obtained from an OGTT or OGCT) with perinatal and longer-term health outcomes in mother or offspring were potentially eligible. Associations in women diagnosed with GDM, however defined, were excluded. The included studies had to have the following characteristics.
Types of studies
All published and ongoing cohort studies and control (placebo or no active treatment) arms of randomised trials were considered for inclusion.
Types of participants
Pregnant women who had undergone assessment of glucose tolerance using an OGTT or OGCT were included. Women with pre-existing diabetes and those diagnosed with GDM and treated (to reduce glycaemic levels) were excluded, by excluding either whole studies (when it was impossible to exclude those with pre-existing diabetes or treated GDM from the study results) or subgroups of studies in which those with pre-existing diabetes or treated GDM had been included, if results were presented in such a way that we were able to exclude those with pre-existing diabetes or treated GDM. For the latter, we used the within-study definition of GDM and recorded that definition. Although, a priori, we assumed that GDM would have been diagnosed differently in different studies, and our preliminary review confirmed this, it is appropriate to use the within-study definition. Excluding women with treated GDM is appropriate because the reason for excluding these women is that treatment would affect the natural association of glucose levels with adverse outcomes.
Types of tests
The OGTT, including the 75-g and 100-g tests, and the 50-g OGCT. Studies of intravenous glucose testing were excluded. Each included study had to report at least two glucose categories for comparison, following exclusion of any treated group. Diagnostic criteria and threshold for treatment differed between studies.
Types of outcomes
Outcome data had to be reported as numbers of events in each of two or more defined glucose categories, as ORs or risk ratios in each category relative to a specified baseline category, or as ORs or risk ratios per SD or per 1 mmol/l of glucose. Studies reporting only correlations were excluded. Studies had to report at least one of the following outcomes.
Perinatal maternal outcomes
C-section (elective or emergency).
Induction of labour.
Instrumental (assisted delivery) (ventouse or forceps).
Pregnancy-induced hypertension (PIH) (however defined).
Pre-eclampsia (however defined).
Perinatal infant outcomes
Macrosomia (BW of ≥ 4.0 kg).
LGA (BW of ≥ 90th percentile, or however defined).
Preterm birth (< 37 weeks’ gestation).
-
Birth injury/trauma:
-
shoulder dystocia
-
Erb’s palsy
-
fractured clavicle.
-
Admission to special care or higher-care facility.
Neonatal hypoglycaemia.
Longer-term maternal or offspring outcomes
Type 2 diabetes (offspring or mother).
Cardiovascular disease (offspring or mother).
Obesity (offspring or mother) (however defined).
Quality assessment
The risk of bias in the included published studies was assessed using a modified version of the Critical Appraisal Skills Programme57 (CASP) and Quality in Prognosis Studies (QUIPS) quality assessment tool. 58 These tools are designed to aid the assessment of observational studies of association and prediction. The following quality criteria were considered:
-
representative nature of included population
-
loss to follow-up
-
consistency of glucose measurement and outcome assessment
-
blinding of participants and medical practitioners to glucose level
-
blinding of outcome assessors to glucose level
-
selective reporting of outcomes
-
adjustment of results for key confounding variables.
Each criterion was classified as being at low, high or unclear risk of bias. One reviewer performed the quality assessment; all assessments were then checked by a second reviewer.
Data extraction
Data were extracted from each publication on the following:
-
glucose test used:
-
OGCT or OGTT
-
glucose load (50 g, 75 g or 100 g)
-
timing (fasting, 1-, 2- or 3-hour post load)
-
-
glucose levels in each defined glucose category (when reported)
-
in millimoles per litre; levels presented as milligrammes per decilitre were converted to millimoles per litre
-
-
numbers of women in each glucose category
-
for each outcome reported:
-
definition of outcome
-
number of outcome events in each glucose category
-
relative risk (RR) or OR of outcomes in each glucose category relative to baseline category (if reported)
-
RR or OR per millimole per litre of glucose or per SD of glucose (if reported)
-
-
whether women with pre-existing diabetes and GDM were excluded
-
study location
-
how GDM was defined
-
which potential confounding factors were adjusted for
-
each quality criterion.
When presented, RR or ORs adjusted for key confounding factors (such as age, BMI, parity) were extracted for each glucose category or type of glucose measure.
One reviewer performed the data extraction. A second reviewer checked the accuracy of the data extraction for all included studies, but did not independently extract data.
Contact with authors and individual participant data
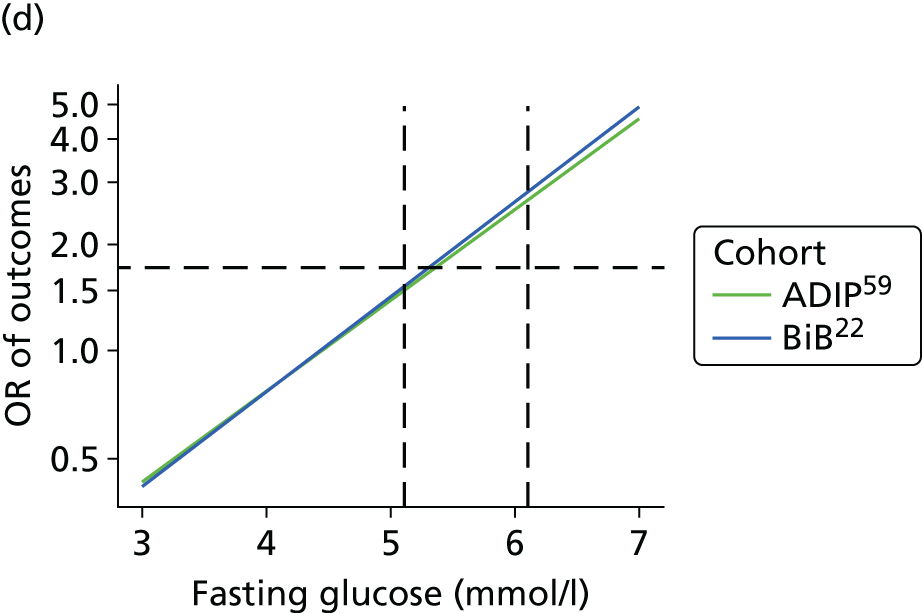
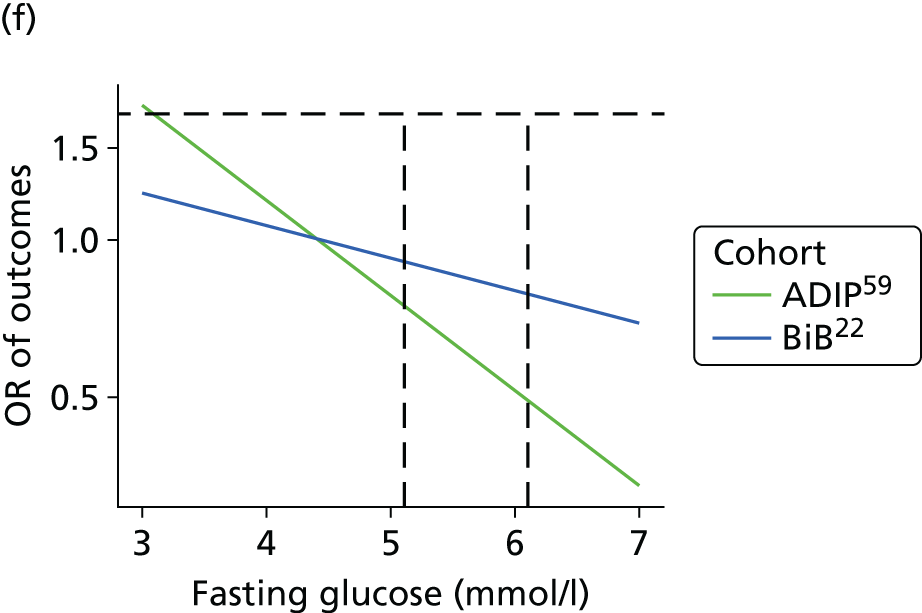
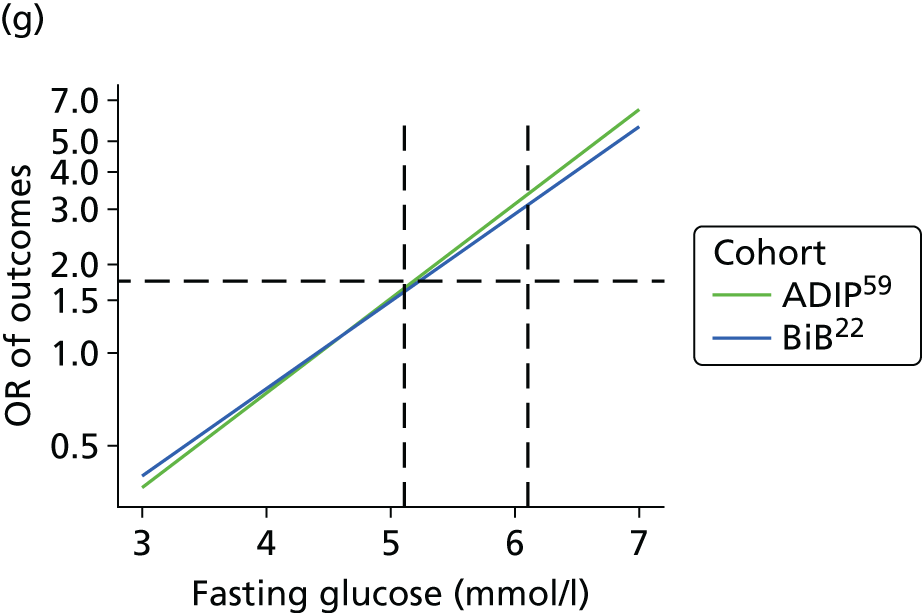
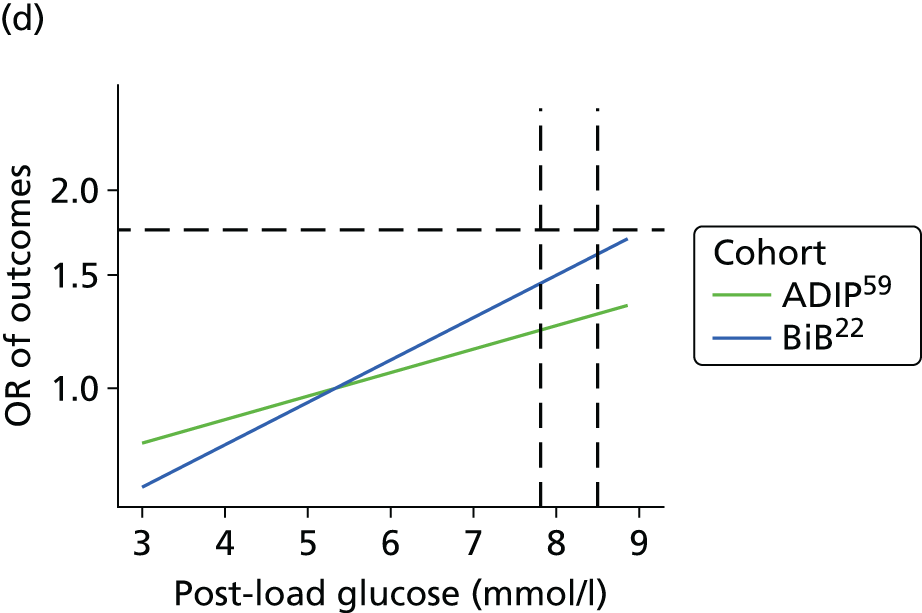
Having identified all relevant published studies that fulfilled our inclusion criteria, and, given that a goal of this project was to understand GDM within a contemporary UK population, we searched for recently recruited cohorts in the UK. We identified four eligible cohorts with IPD: two had sufficiently complete case data: the BiB study22 (data provided by John Wright, Bradford Institute for Health Research, September 2013) and the Atlantic Diabetes in Pregnancy cohort (Atlantic DIP59) (data provided by Fidelma Dunne, Department of Medicine, National University of Ireland, September 2013), one cohort had insufficient complete case data and was not included (Warwick/Coventry: P Saravanan, Warwick Medical School, 2013, personal communication60) and we were unable to secure data from one other (UK HAPO cohort6); however, we have included the published estimates from the whole HAPO cohort6 wherever possible.
Both the BiB and Atlantic DIP cohorts22,59 include fasting and 2-hour post-load glucose levels, obtained as part of a 75-g OGTT. When outcomes were not reported explicitly in the data set they were derived from available data if possible. For example, macrosomia, LGA and preterm birth were calculated from BW and gestational age data.
Statistical analyses
General approach
Statistical analyses were based on the number of women, and number of outcome events in each glucose category in each study. It should be noted that using these raw numbers means that these analyses are not adjusted for potential confounding factors. For the BiB and Atlantic DIP cohorts,22,59 glucose levels were divided into seven categories, with equal numbers of women in each category; for other published eligible studies we used whatever categories were used in the study. Studies that did not report outcomes by glucose categories were not included in these unadjusted analyses of outcome risk by glucose category. Within each glucose category we calculated the risk by dividing the number of outcome events by the total number of women in that category. With one exception,61 it was possible to do this for all of the published studies. In this one exception,61 only adjusted ORs were presented in each category (not numbers of events). For that study,61 numbers of each outcome were estimated, given the number of women in each glucose category (which was provided) and the ORs, using an exhaustive search approach to find numbers of outcomes that reproduced the reported ORs and their standard errors (SEs) as closely as possible. For each study that we were able to calculate risk per glucose category, we graphed these risks against the categories to assess the shape of the association and see if results looked generally linear (as in Chapter 2 for the BiB cohort), before modelling the identified associations and pooling results from studies. In studies that reported adjusted ORs or risk ratios for each glucose category, these results were similarly plotted to check the shape of the association and identify any divergence from results using unadjusted data.
Studies reporting odds ratios or risk ratios per standard deviation or 1 mmol/l of glucose
The aim of this analysis was to identify trends in outcomes with changes in glucose levels, so results reporting the trend as ORs per 1 mmol/l of glucose per SD in glucose level would be the preferred data. However, only the HAPO study6 reported such results, so no meta-analyses could be performed. The reported ORs per SD of glucose were converted into ORs per 1 mmol/l using the reported SDs. These were then compared with ORs obtained from the IPD cohorts.
Studies reporting three or more glucose categories
For each study the risk of each GDM-related outcome along with its 95% confidence interval (CI) was calculated for each glucose category. For each outcome, and for each study, this risk was plotted against the level of glucose in each category, to visually inspect the trends in risk with glucose level.
Studies reporting results from only two glucose groups were excluded from these analyses because they did not report sufficient data to reliably estimate median glucose levels in each glucose category.
After inspecting risk of outcome across glucose categories for as many outcomes as possible, and in as many studies as possible, we felt that it was reasonable to assume a log–linear relationship between fasting or post-load glucose levels and all outcomes. Associations of fasting or post-load glucose levels (per 1 mmol/l) were therefore modelled separately for each study, outcome and glucose test (based on timing and load), using the following logistic regression model:
where i indicates study, j glucose test (e.g. 100-g OGTT fasting level), k the outcome of interest (e.g. macrosomia) and l the glucose category. Then pijkl is the probability of having the outcome in the relevant glucose category, Gijkl is the estimated median glucose level in that category, so ɸijkl is the baseline log odds of the outcome and θijkl is the association between glucose level and outcome, in terms of the log odds of outcome per 1-mmol/l increase in glucose level.
Estimates of association between outcome and glucose level, with their 95% CI, were pooled across studies using DerSimonian and Laird random-effects meta-analysis to account for any potential heterogeneity in the trends across studies. 62 Studies were combined in meta-analyses if there were two or more studies for the specified outcome and glucose test. Heterogeneity was examined using the I2-statistic and its 95% CI. 63
To increase the number of studies and participants in each comparison, studies were pooled if they included relevant glucose levels (fasting and/or post load) from either the 75-g or 100-g OGTT. This assumes that the trends in outcome incidence with glucose level were the same for the two OGTTs. We used a ‘one-stage’ version of model 1, above, with all studies combined in a single regression model. This model includes random intercept (ɸ) and slope (θ) terms to account for heterogeneity in the baseline odds of the outcome (i.e. the odds of each outcome in the lowest glucose category) and association between glucose levels and outcome between studies.
This analysis differs slightly from that in Chapter 2, for which results were summarised as the odds per SD. In this chapter, odds per 1 mmol/l glucose are used. This is because the SD in glucose levels varies across studies and was not generally reported; using odds per 1 mmol/l glucose permits a consistent approach to analysis across studies.
For studies that reported an adjusted OR for each outcome relative to a baseline glucose category, including the two studies for which we had IPD,22,59 we examined adjusted results. The adjusted ORs with their 95% CIs were plotted against the level of glucose in each category, so that a visual inspection of trends in risk across glucose levels could be undertaken. However, we were unable to perform meta-analyses of these results because of the limited number of studies presenting adjusted ORs.
Linearity
Following a visual inspection of figures of associations and to test the validity of our assumption of a log–linear association between outcome and glucose level (based on evidence from the HAPO6 and BiB22 studies), model 1 – presented above – was fitted again with an additional glucose-squared term (i.e. a term γijklGijkl2). A statistically significant association with glucose squared would suggest a quadratic–curvilinear relationship. Therefore, a lack of evidence suggests the relationship is not likely to be quadratic and suggests, along with the visual evidence, that there is a possibility that the relationship may be linear.
Studies reporting only two glucose categories
Several studies examined associations between perinatal outcomes and glucose levels following a 50-g OGCT. The OGCT was undertaken to determine whether women should (or should not) go on to have a diagnostic OGTT. Outcomes were then compared between those who did not meet the criteria for having an OGTT with those who did meet those criteria but who were not diagnosed with GDM [i.e. two groups were compared: < 130 mg/dl vs. ≥ 130 mg/dl post-challenge glucose or (for some studies) < 140 mg/dl vs. ≥ 140 mg/dl post-challenge glucose]. 14 The glucose categories in millimoles per litre (values expressed as milligrammes per decilitre were converted to millimoles per litre) in each of these two comparisons were abstracted, as were the numbers of women in each category and the number of these with outcomes in each category.
Some studies compared outcomes in women whose glucose levels were all ‘normal’ at any OGTT time point (i.e. fasting and 1, 2 or 3 hours post load, depending on which tests was undertaken) to women who had one elevated glucose level; these comparisons were undertaken in populations using criteria that required at least two elevated levels for a diagnosis of GDM to be made.
For both of these types of study, the numbers of outcomes in each group was used to calculate ORs for outcomes. The ORs for each group (lower risk vs. higher risk) were pooled across studies for each outcome using DerSimonian and Laird random-effects meta-analysis. 62
Analyses of the individual participant data cohorts
In order to perform analyses that were not possible with published data, in particular to perform adjusted analyses, further analyses of the two IPD cohorts were performed. To maintain consistency, the statistical modelling approach used was broadly similar to that used in the analyses of published studies. To maintain a consistent approach to analyses of the two cohorts the data sets were cleaned using the same rules and analyses undertaken as described below.
The shape of the association between outcomes and glucose levels were viewed. Following this assessment and to model these associations a log–linear relationship between risk and outcome was assumed; the model was adjusted for age, BMI and ethnicity (these were the potential confounders reported across both cohorts). Women with GDM according to the WHO criteria11 were excluded from analysis, as they were offered treatment (fasting ≥ 6.1 mmol/l and/or 2-hour post-load ≥ 7.8 mmol/l). This association was modelled separately for each cohort, outcome and time of glucose measurement. Formally, the models had the form:
with parameters as in model 1, above, except that Xij is a matrix of the adjusting factors (age, BMI, ethnicity). The model was fitted using logistic regression. For each cohort outcome, for both fasting and post-load glucose model results were used to calculate the estimated odds of outcome at increasing glucose levels, and the absolute risk of an outcome at increasing glucose levels, to examine the trend in risk with glucose.
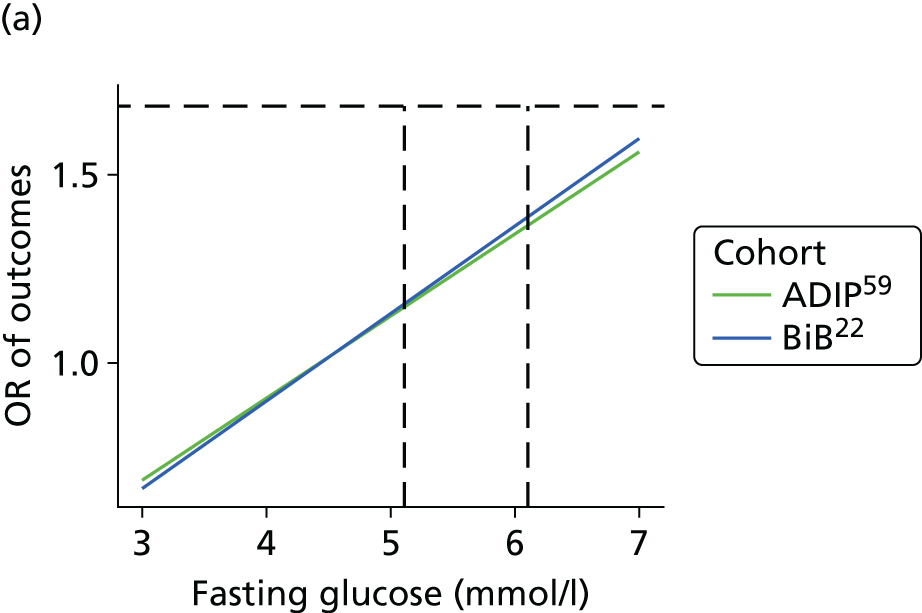
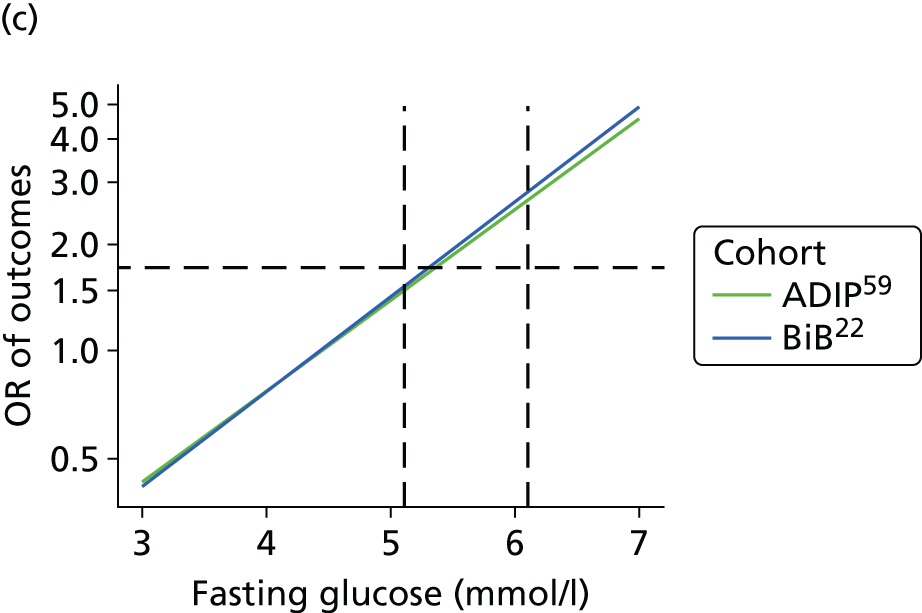
The results from model 2, above, for each cohort were used to predict the OR of having an outcome relative to the mean glucose level across the full range of fasting and post-load glucose levels. This makes the assumption that the log OR increases linearly with glucose levels.
A further ‘one-stage’ model was considered, pooling the two cohorts together in a single model. The same model structure as in model 1, above, was used, but assuming that the association between glucose level and outcome was subject to a random effect across the two cohorts, that is:
where θ is the summary association between glucose level and outcome across both cohorts and τ2 is the heterogeneity in effect. To test the validity of the assumption of a log–linear association between outcome and glucose level the model presented above was fitted again with an additional glucose-squared term.
Results
Included studies
The search from the unpublished review55 and the updated searches together identified 11,219 potentially relevant studies following removal of duplicates. After title and abstract screening 125 publications were obtained for full-text review. After full-text screening, 57 studies (see Tables 7–9) were included in the review and 37 in the meta-analysis (including the two studies22,59 for which we had IPD) (see Appendix 2, Table 62 for excluded studies with reasons). Figure 3 shows the identification of these studies.
FIGURE 3.
The search process. a, Includes 7947 from the March 2013 search, 808 from the September 2013 search, and 2464 from the October 2014 search.

Several publications reported data from the same cohort. Four of the included publications used data from the HAPO cohort,6 but reported different outcomes. One of these publications (Pettitt et al. 41) was not included in the analyses because it reported associations with outcomes in a subset of participants that had been previously reported in the whole cohort. For the remaining publications, data from the most recent and comprehensive publication for each outcome were used. Two publications61,64 used data from the same cohort: Figueroa et al. 64 examined glucose levels at OGCT and risk of adverse outcomes, whereas Landon et al. 61 examined glucose levels at OGTT and risk of adverse outcomes. Data from both publications are therefore included in analyses.
Characteristics of eligible studies are described in Tables 7–9. The studies fall into four categories: (1) 28 studies (including BiB and Atlantic DIP)6,61,64–87 reported associations between glucose levels (from OGTT or OGCT) split into three or more categories and adverse perinatal outcomes (see Table 7); (2) 20 studies88–107 reported associations between glucose levels (from OGTT or OGCT) split into two categories with adverse perinatal outcomes (see Table 8) – these studies were mostly comparisons of women with lower glucose levels at OGCT [typically < 140 mg/dl (7.8 mmol/l)] compared with women with higher glucose levels at OGCT; (3) five studies36,41,65,108,109 reported longer-term outcomes in either mother or offspring (see Table 9) (it was not possible to pool studies reporting longer-term outcomes because they were too diverse); and (4) the remaining five studies110–113 did not present numerical data that were suitable for analysis and therefore could not be included in any of the meta-analyses (see Appendix 2, Table 63). One study114 used a 75-g OGTT in a non-fasted population. As there were no other studies that had used this test in this way, and post-load glucose levels from a non-fasted group are likely to differ to those from a fasted group, we did not include results from this study in any meta-analyses.
| Study | Year | Location | Women (n) | Glucose test | Timing of test | GDM diagnosis criteria | Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | 1 hour | 2 hour | LGA | Macrosomia | Shoulder dystocia | Neonatal hypoglycaemia | Pre-eclampsia/PIH | Preterm | C-section | Induced labour | Assisted delivery | ||||||
| Aris65 | 2014 | Singapore | 1081 | 75-g OGTT | ✗ | ✗ | WHO | ✗ | |||||||||
| Atlantic DIP59 | 2015 | Ireland | 4869 | 75-g OGTT | ✗ | ✗ | WHO | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| BiB22 | 2015 | UK | 9645 | 75-g OGTT | ✗ | ✗ | WHO | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Carr66 | 2011 | USA | 25,969 | 50-g OGCT | ✗ | C&C | ✗ | ✗ | |||||||||
| Chandna67 | 2006 | Pakistan | 633 | 50-g OGCT | ✗ | Not reported | ✗ | ✗ | ✗ | ✗ | |||||||
| Cheng68 | 2007 | USA | 13,901 | 50-g OGCT | ✗ | Not reported | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Figueroa64 | 2013 | USA | 1839 | 50-g OGCT | ✗ | C&C | ✗ | ✗ | ✗ | ||||||||
| HAPO group6 | 2008 | International | 23,316 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | ✗ | ✗ | ✗ | ||||||
| HAPO group69 | 2010 | International | 21,364 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | ✗ | ||||||||
| Hillier70 | 2008 | USA | 41,450 | 50-g OGCT | ✗ | NDDG and C&C | ✗ | ||||||||||
| Jensen71 | 2001 | Denmark | 2904 | 75-g OGTT | ✗ | ✗ | Defined in paper | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Kerényi72 | 2009 | Hungary | 3787 | 75-g OGTT | ✗ | ✗ | WHO | ✗ | |||||||||
| Landon61 | 2011 | USA | 1368 | 100-g OGTT | ✗ | ✗ | ✗ | C&C | ✗ | ✗ | ✗ | ||||||
| Lao73 | 2003 | China | 2168 | 75-g OGTT | ✗ | WHO | ✗ | ✗ | ✗ | ✗ | |||||||
| Little74 | 1990 | USA | 287 | 100-g OGTT | ✗ | O’Sullivan | ✗ | ✗ | ✗ | ✗ | |||||||
| Lurie75 | 1998 | Israel | 353 | 50-g OGCT | ✗ | NDDG | ✗ | ✗ | ✗ | ||||||||
| Metzger76 [HAPO] | 2010 | International | 17,094 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | ✗ | ||||||||
| Moses77 | 1995 | Australia | 1441 | 75-g OGTT | ✗ | ADIPS | ✗ | ✗ | ✗ | ||||||||
| Ong78 | 2008 | UK | 3826 | 50-g OGCT | ✗ | Defined in paper | ✗ | ✗ | |||||||||
| Pettitt79 | 1980 | USA-Pima Indians | 811 | 75-g OGTT | ✗ | Defined in paper | ✗ | ✗ | ✗ | ||||||||
| Riskin-Mashiah80 | 2009 | Israel | 6129 | 100-g OGTT | ✗ | C&C | ✗ | ✗ | |||||||||
| Savona-Ventura81 | 2010 | Malta | 1289 | 75-g OGTT | ✗ | ✗ | Not reported | ✗ | ✗ | ||||||||
| Scholl82 | 2001 | USA | 1157 | 50-g OGCT | ✗ | Not reported | ✗ | ✗ | ✗ | ✗ | |||||||
| Sermer83 | 1995 | Canada | 3637 | 50-g OGCT/100-g OGTT | ✗ | ✗ | ✗ | NDDG | ✗ | ✗ | ✗ | ||||||
| Subramaniam84 | 2014 | USA | 56,786 | 50-g OGCT | ✗ | Not reported | ✗ | ✗ | ✗ | ||||||||
| Tallarigo85 | 1986 | Italy | 249 | 100-g OGTT | ✗ | O’Sullivan | ✗ | ✗ | ✗ | ||||||||
| Witter86 | 1988 | USA | 3897 | 50-g OGCT | ✗ | Defined in paper | ✗ | ||||||||||
| Yee87 | 2010 | USA | 13,789 | 50-g OGCT | ✗ | C&C | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Study | Year | Location | Women (n) | Glucose test used | Timing of test | Cut-off level | GDM diagnosis criteria | Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | 1 hour | 2 hour | 3 hour | LGA | Macrosomia | Shoulder dystocia | Neonatal hypoglycaemia | Pre-eclampsia | Prematurity | C-section | Induced labour | Assisted delivery | |||||||
| Aberg88 | 2001 | Sweden | 4657 | 75-g OGTT | ✗ | 7.8 mmol/l | WHO | ✗ | ✗ | ||||||||||
| Dudhbhai89 | 2006 | USA | 201 | 50-g OGCT | ✗ | 140 mg/dl | Defined in paper | ✗ | ✗ | ✗ | ✗ | ||||||||
| Forest90 | 1994 | Canada | 4314 | 100-g OGTT | ✗ | ✗ | ✗ | One abnormal value | Defined in paper | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Hedderson91 | 2003 | USA | 1956 | 50-g OGCT | ✗ | 140 mg/dl | C&C | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Herman92 | 1988 | USA | 126 | 100-g OGTT | ✗ | 140 mg/dl | Defined in paper | ✗ | ✗ | ✗ | |||||||||
| Jiménez-Moleón93 | 2002 | Spain | 1962 | 50-g OGCT | ✗ | 140 mg/dl | ADA | ✗ | ✗ | ✗ | |||||||||
| Khoshniat94 | 2002 | Iran | 1801 | 50-g OGCT | ✗ | 130 mg/dl | C&C | ✗ | |||||||||||
| Langer95 | 2005 | 50-g OGCT and 100-g OGTT | ✗ | ✗ | ✗ | ✗ | Diagnosed GDM | C&C | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Lapolla96 | 2007 | Italy | 758 | 50-g OGCT | ✗ | 140 mg/dl | Defined in paper | ✗ | ✗ | ||||||||||
| aMa97 | 2013 | USA | 436 | 50-g OGCT and 75-g OGTT | ✗ | ✗ | 120 mg/dl | ✗ | ✗ | ||||||||||
| Naylor98 | 1996 | Canada | 3778 | 50-g OGCT | ✗ | 7.8 mmol/l | C&C | ✗ | ✗ | ✗ | |||||||||
| Nord99 | 1995 | Sweden | 614 | 75-g OGTT | ✗ | 8 mmol/l | Not reported | ✗ | ✗ | ✗ | ✗ | ||||||||
| Özekinci100 | 2011 | Turkey | 212 | 50-g OGCT | ✗ | 140 mg/dl | NDDG | ✗ | ✗ | ||||||||||
| Pugh101 | 2010 | USA | 214 | 50-g OGCT | ✗ | 140 mg/dl | NDDG | ✗ | ✗ | ✗ | |||||||||
| Retnakaran102 | 2008 | Canada | 361 | 50-g OGCT | ✗ | 7.8 mmol/l | NDDG | ✗ | ✗ | ✗ | |||||||||
| Stamilio103 | 2004 | USA | 1825 | 50-g OGCT | ✗ | 135 mg/dl | NDDG | ✗ | ✗ | ✗ | ✗ | ||||||||
| Tarim104 | 2011 | Turkey | 4930 | 50-g OGCT | ✗ | 135 mg/dl | C&C | ✗ | |||||||||||
| Vambergue105 | 2000 | France | 239 | 100-g OGTT | Over all times | One abnormal value | C&C | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||
| Wang106 | 2013 | China | 7513 | 50-g OGCT and 100-g OGTT | Over all times | Number of abnormal values | NDDG | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Yogev107 | 2005 | USA | 6854 | 50-g OGCT | ✗ | 130 mg/dl | C&C | ✗ | ✗ | ✗ | ✗ | ||||||||
| Study | Year | Location | No. of children | Glucose test used | Timing of test | GDM diagnosis criteria | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Fasting | 1 hour | 2 hour | |||||||
| Aris65 | 2014 | Singapore | 1081 | 75-g OGTT | ✗ | ✗ | WHO | Percentage body fat and skinfold thickness (both > 90th centile) (perinatal) | |
| BiB22 | 2015 | UK | 6458 | 75-g OGTT | ✗ | ✗ | WHO | Skinfold thickness | |
| HAPO group36 | 2009 | International | 19,389 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | Percentage body fat and skinfold thickness (both > 90th percentile) (perinatal) |
| Hillier108 | 2007 | USA | 9439 | 50-g OGCT | ✗ | NDDG and C&C | Childhood obesity at age 5–7 years (above 85th and 95th percentiles) | ||
| Pettitt109 | 1991 | USA-Pima Indians | 552 | 75-g OGTT | ✗ | WHO | Diabetes between ages of 5 and 24 years | ||
| Pettitt41 (HAPO) | 2010 | Belfast | 1165 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | Overweight and obesity at age 2 years (above 85th and 95th percentiles) |
Quality assessment
The results of the quality assessment of the included studies are shown in Appendix 2, Table 60. In general most studies were at low risk of bias. Most studies recruited any pregnant women, without any further inclusion/exclusion criteria, so that the study population would be more representative of the whole ‘general’ obstetric population. The majority of studies were in western populations from high-income countries, with a small number from other populations, for example the Pima Indian population of Arizona. There was little loss to follow-up in most studies. Fasting and post-load glucose levels were assessed using OGCT and/or OGTT, and most outcomes were measured using standard definitions.
The main potential risk of bias was due to lack of blinding. Blinding of participants, clinicians, research staff and those assessing outcomes to glucose levels is possible; however, all staff may have been aware that glucose levels did not indicate GDM (study defined) requiring treatment. Many studies were retrospective, and in these cases it is likely that participants and medical staff were aware of the glucose levels.
Consequently, it is possible that associations of glucose levels with perinatal outcomes were ‘confounded by indication’. Women and health practitioners may have been influenced by glucose results. For example, in those women who had levels just below the thresholds for GDM diagnosis, monitoring and treatment may have differed from those whose levels were lower. Those with higher levels might have been given lifestyle advice aimed at reducing their levels, they may have been monitored more closely and there may have been a greater likelihood of intervening during labour, such as electing for C-section.
Studies generally reported all outcomes listed in their methods sections, but no single study reported data on all of our included outcomes. The studies were necessarily observational (randomisation to a given glucose level is not possible). In addition to confounding by indication, it is possible that other characteristics might have confounded the associations that we have examined. Only a minority of studies reported associations that were adjusted for what we considered to be key confounding factors (maternal age, BMI/other measure of adiposity and previous GDM).
The two cohorts for which we had IPD were judged – like most other studies described in Appendix 2, Table 60 – to be at low risk of bias. For these we had the added advantage that we were able to adjust for key confounders, although as with other studies we cannot rule out confounding by indication or residual confounding by characteristics that were unmeasured or poorly measured in those studies.
Analyses of individual participant data cohorts
The frequencies of each adverse outcome across seven fasting and 2-hour post-load glucose categories in the BiB study22 and Atlantic DIP59 are shown in Appendix 2, Figure 47. We have not included skinfolds of > 90th percentile as an outcome or any other measure of infant adiposity because it was not available in the Atlantic DIP cohort59 and in relation to the BiB study22 it has been reported in Chapter 2. For comparison we have included the HAPO study6 point estimates in the analyses presented in Appendix 2, Figures 48 and 49 (we were unable to secure IPD from the UK centres of the HAPO study6) because the results from that study6 have recently been used to develop new criteria thresholds for GDM diagnosis.
Across all categories of fasting and post-load glucose levels the frequencies of C-section, instrumental birth, LGA, macrosomia and pre-eclampsia are greater in the Atlantic DIP cohort59 than in the BiB study. 22 Preterm birth is similar in both studies, and numbers for shoulder dystocia are too few to draw conclusions.
The ORs per 1-mmol/l increase in fasting and post-load glucose levels for the BiB, Atlantic DIP and HAPO studies6,22,59 are shown in Appendix 2, Figures 48 and 49, respectively. For most outcomes the cohorts show similar results, with increases in outcome incidence as glucose levels increase, although results were not always statistically significant in the smaller Atlantic DIP cohort. 59 There are some exceptions to this. For instrumental delivery, the BiB study22 shows a positive association between outcome and glucose level, but no such association was found in the Atlantic DIP study. 59 For the Atlantic DIP study,59 risk of preterm birth reduced as fasting glucose level increased, but increased as post-load glucose level increased. This may be a chance finding and related to the low incidence of preterm birth in the Atlantic DIP study59 (3%). Associations were stronger for fasting glucose levels than 2-hour post-load glucose levels. Meta-analyses provided significant results for ORs per 1-mmol/l increases in glucose level and the majority of outcomes, with the exception of instrumental and preterm birth, for fasting glucose level.
In Appendix 2, Figures 50 and 51 show the ORs for each outcome with increasing fasting and post-load glucose categories, respectively, relative to the mean glucose level for each outcome in each of the cohorts. The dashed vertical lines show the thresholds for diagnosing GDM using the IADPSG and WHO (1999) criteria (fasting glucose levels of 5.1 mmol/l and 6.1 mmol/l; post-load glucose levels of 7.8 mmol/l and 8.5 mmol/l, respectively) and the horizontal dashed line an OR of 1.75 (the OR recommended by the IADPSG for applying GDM diagnostic thresholds).
The estimated ORs illustrated in Appendix 2, Figure 50, which are greater than the WHO fasting threshold of 6.0 mmol/l, and in Appendix 2, Figure 51, which are greater than the post-load threshold of 7.7 mmol/l, are predictions assuming that the linear trend continues at higher glucose levels (if women are not treated), and are not based on the data from the cohorts (because in both cohorts women were offered treatment if their glucose levels were greater and therefore have been excluded). For fasting glucose level, the two cohorts give similar results for C-section, LGA, macrosomia, pre-eclampsia and shoulder dystocia. For macrosomia, LGA and shoulder dystocia the IADPSG diagnostic threshold of 5.1 mmol/l corresponds reasonably closely with an OR for outcomes relative to average fasting glucose level of between 1.5 and 1.75 mmol/l. The WHO thresholds are associated with a higher OR for adverse outcome.
Trends in perinatal outcome risk with glucose levels
This section examines 28 studies presenting data on perinatal outcomes in three or more glucose categories using either an OGCT or OGTT (see Table 7). This section presents a series of figures for which the risk of a specified outcome is plotted against glucose levels. Studies are categorised according to the timing of the glucose test (fasting, 1-hour post load, 2-hour post load) and the glucose load used (50-g OGCT, 75-g OGTT, 100-g OGTT).
Appendix 2, Figure 52, shows the trend for macrosomia, and Appendix 2, Figure 53, for LGA. For both outcomes the analyses suggest the risk increases as glucose levels increase, the association seems stronger for fasting glucose level compared with post-load glucose level. The relationship appears to be linear, with no sudden increase in risk. There is considerable heterogeneity across studies in the underlying risk of macrosomia and LGA, but the trends (i.e. the slopes of the lines) appear reasonably consistent across studies. Although there are differences in the actual glucose levels according to the glucose test used, there is no evidence that the trend in risk with glucose level is different for the different glucose tests.
In Appendix 2, Figure 54 shows the trends for pre-eclampsia and increasing glucose levels; Figure 55, C-section, Figure 56 instrumental birth; and Figure 57, induction of labour and increasing glucose levels. Risk of pre-eclampsia and C-section seems to increase with increasing glucose level similarly to macrosomia and LGA. Data on assisted delivery (forceps and ventouse) and induced labour are too few to draw any meaningful conclusions.
Furthermore, in Appendix 2, Figure 58 shows the trends for shoulder dystocia; Figure 59 the trends for preterm birth; and Figure 60 the trends for neonatal hypoglycaemia. Although data for all three outcomes are limited, these outcomes do not seem to be associated with increasing glucose levels. The risk of shoulder dystocia appears to increase only at the higher levels of glucose (e.g. > 6 mmol/l at 2 hours post load), although this observation is driven primarily by one study. 61
Association between 1-mmol/l increases in fasting and post-load glucose levels and risk of adverse perinatal outcomes
This section examines the trends in adverse perinatal outcomes (see Appendix 2, Figures 52–60) and increasing glucose levels represented as ORs for each outcome per 1-mmol/l increase in glucose level, calculated using the logistic regression models described above (see Methods). Each glucose load/test is considered separately, and when there are sufficient studies for any outcome, results are combined in meta-analyses.
50-g oral glucose challenge test
Figure 4 shows the OR per 1-mmol/l increase in 1-hour post-load glucose for outcomes using the 50-g OGCT. The associations between C-section, LGA, macrosomia, neonatal hypoglycaemia, pre-eclampsia and shoulder dystocia are statistically significantly associated with 1-mmol/l increases in glucose level. Preterm birth does not seem to be associated with increases in glucose level, or does PIH/pre-eclampsia (some studies combine these two outcomes).
FIGURE 4.
Odds ratio for 1-mmol/l increases in 1-hour post-load glucose for 50-g OGCT and adverse outcomes.
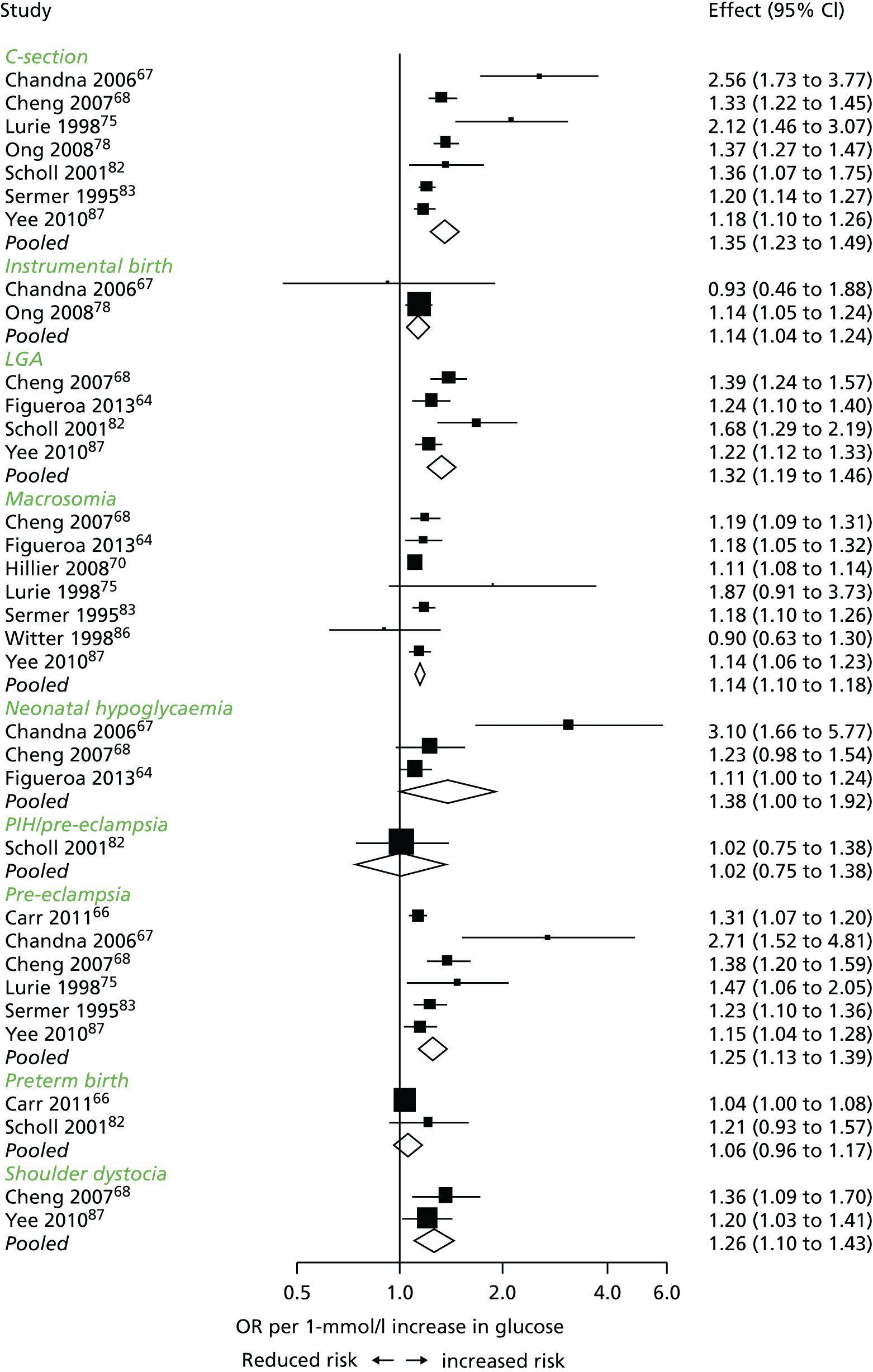
75-g oral glucose tolerance test
Figure 5 shows the OR per 1-mmol/l increase in fasting glucose for all outcomes. The risk of the majority of adverse outcomes increases with increasing fasting glucose level, with statistically significant associations for LGA, macrosomia, pre-eclampsia, C-section, induced labour and neonatal hypoglycaemia. There is no evidence of increasing odds with increasing glucose levels for shoulder dystocia or instrumental birth (forceps and ventouse). There seems to be a negative association between increasing fasting glucose levels and preterm birth, suggesting that, as glucose levels increase, the odds of a preterm birth reduces by 23% (OR 0.77, 95% CI 0.62 to 0.96).
FIGURE 5.
Odds ratio for 1-mmol/l increases in fasting glucose level for 75-g OGTT and adverse outcomes.

Appendix 2, Figure 61, shows the OR per 1-mmol/l increase in 1-hour post-load glucose for reported outcomes without meta-analysis because of the limited number of studies included. The HAPO study6 reports BW of > 90th percentile and Pettitt et al. 41 report LGA; however, Pettitt et al. report results for a subset of participants from the whole HAPO study. 6 The analyses suggest that the 2-hour post-load glucose (Figure 6) associations are weaker than the fasting glucose associations (see Figure 5), and the statistically significant associations between increasing fasting glucose and reduced odds of preterm birth and increased odds of C-section are lost.
FIGURE 6.
Odds ratio for 1-mmol/l increases in 2-hour post-load glucose level for 75-g OGTT and adverse outcomes.
100-g oral glucose challenge test
Figure 7 shows the OR per 1-mmol/l increase in fasting glucose for all outcomes using the 100-g OGTT, and Figure 8 shows the results for 2-hour post-load glucose without meta-analysis because of the limited number of studies. Only one study61 reported 1-hour 100-g results for outcomes including cord C-peptide and LGA. One study80 performed the OGTT at 9 weeks rather than at the more usual 26–28 weeks. Fasting results (the only levels reported by this study80) seem consistent with the fasting glucose results reported at the more conventional 26–28 weeks. 83
FIGURE 7.
Odds ratio for 1-mmol/l increases in fasting glucose for 100-g OGTT and adverse outcomes.

FIGURE 8.
Odds ratio for 1-mmol/l increases in 2-hour post-load glucose for 100-g OGTT and adverse outcomes.
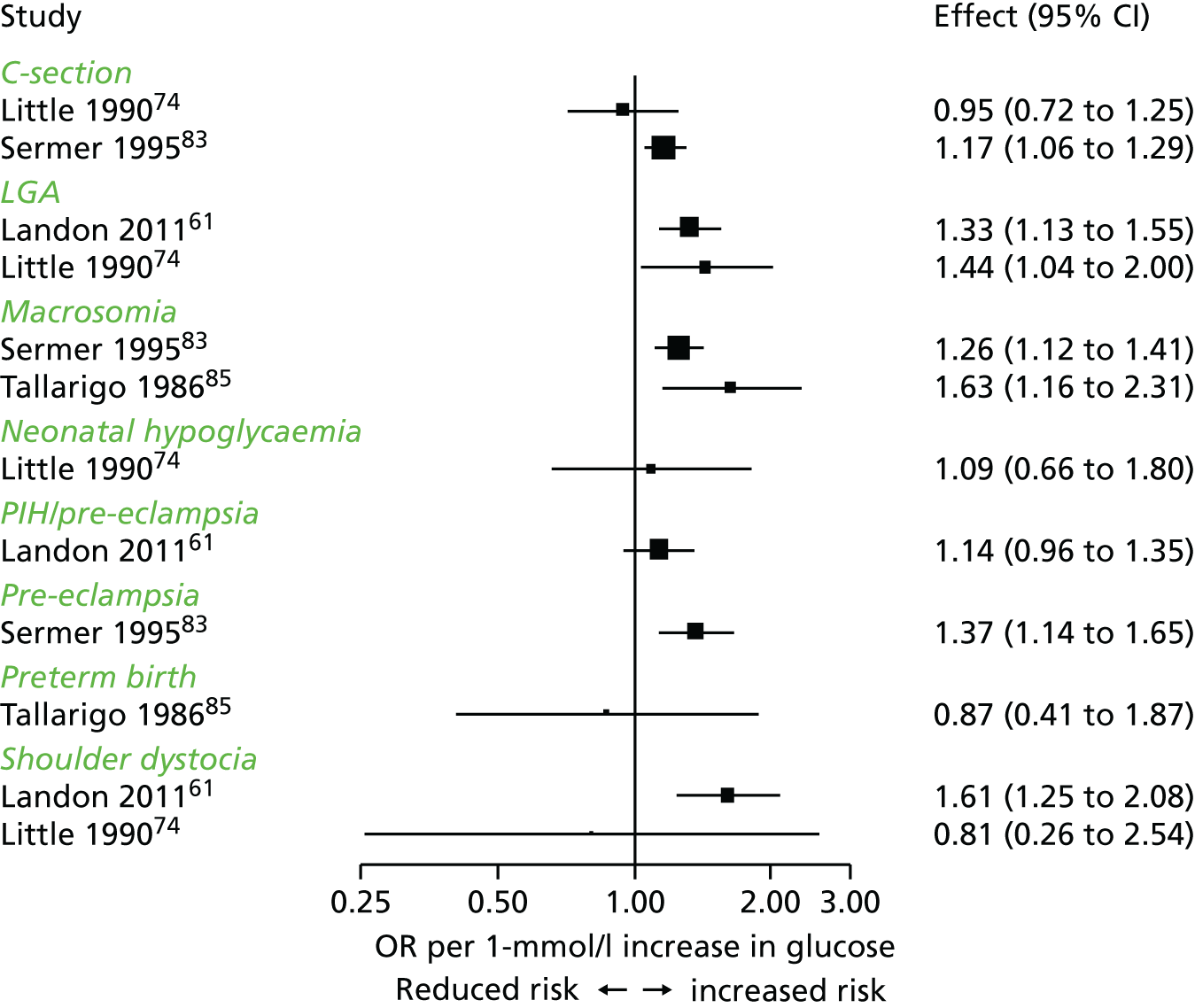
It is difficult to draw conclusions from the results reported by these studies, given the limited data, but the findings are similar to the post-load associations of the 75-g test results. Fasting glucose and associated outcomes should not be affected by the subsequent glucose load; however, differences in glucose load and subsequent post-load glucose associations may be.
Combining 75-g and 100-g glucose test results
To increase the number of studies and participants included in the comparisons, we combined the results for the 75-g and 100-g OGTTs. We therefore assumed that the association between outcomes and increases in glucose were the same for both tests. The results of meta-analyses combining these tests are shown in Figure 9.
FIGURE 9.
Combined 75-g and 100-g OGTT fasting glucose, 1-hour glucose levels, 2-hour glucose levels and adverse outcomes.

Combined results (75-g and 100-g OGTT) are similar to those for the 75-g OGTT alone. For fasting glucose there are statistically significant increases in risk of C-section, LGA, macrosomia, pre-eclampsia, neonatal hypoglycaemia, induction (of labour) and shoulder dystocia. Glucose levels do not appear to be associated with preterm birth or assisted (instrumental) delivery. The increase in odds can be substantial, with a more than doubling in odds per 1-mmol/l increase in fasting glucose level for pre-eclampsia and for LGA.
The results for the 2-hour post-load glucose levels are weaker than fasting associations, although CIs are narrower, particularly for preterm birth and pre-eclampsia, The association of increasing 2-hour post-load glucose levels with C-section just misses significance (OR 1.10, 95% CI 0.96 to 1.25).
The associations are weaker when the 75-g and 100-g test results are combined compared with the 75-g test results alone, reflecting the weaker associations when the 100-g test is administered.
Testing the linearity assumption
We viewed the shape of the associations between the log odds of outcomes and increasing glucose levels or categories for fasting and 2-hour OGTT (combining 75-g and 100-g tests) and for the 1-hour OGCT, for each outcome, these associations appeared generally linear. We tested the assumption of a linear association by including a squared term for the glucose levels in the regression models (see Methods). If the associations were quadratic curvilinear (rather than linear) we would expect there to be a statistically significant association between outcome risk and the square of the glucose level. The results of this analysis for all outcomes for the fasting and 2-hour 75-g OGTT and the OGCT are presented in Appendix 2, Table 61. Data were too limited to repeat this analysis for the 100-g OGTT alone.
The small number of studies limits the ability to draw conclusions; however, for the majority of outcomes, the association between outcome and the square of glucose was not statistically significant – it is therefore reasonable that, given the visual evidence (see Appendix 2, Figures 52–60), the association between glucose levels and outcomes is linear. There were, however, statistically significant curvilinear associations between fasting and 2-hour post-load glucose levels (75-g OGTT) and 1-hour post-load glucose level (50-g OGCT) and PIH/pre-eclampsia, but two of these associations were negative, suggesting that the odds of pre-eclampsia ‘levels off’ slightly at higher glucose levels rather than continuing to increase, and one association (fasting glucose, 75-g OGTT) was positive. This inconsistency in direction suggests that these may be chance findings, and therefore caution is advised when considering these results. There was a positive association with fasting glucose (75-g OGTT) and neonatal hypoglycaemia and preterm birth, but few studies were included, and, again, these results should be interpreted with caution (see Appendix 2, Table 61).
Analyses of adjusted odds ratios
Of the included studies, 106,61,64,66,68–70,72,80,82 reported ORs for outcomes adjusted for potential confounding factors such as maternal BMI and previous GDM. This section presents these ORs relative to the baseline glucose category for macrosomia64,70,80 LGA6,61,64,72,82 C-section6,68,80,82 and pre-eclampsia66,68,69 which were the only outcomes reported across studies.
Appendix 2, Figure 62, shows the results for macrosomia; Figure 63, LGA; Figure 64, C-section; and Figure 65, pre-eclampsia. The limited data make drawing conclusions difficult; however, there seems to be a general trend of increasing odds (when results are adjusted for selected potentially confounding variables) of each adverse perinatal outcome with each 1-mmol/l increase in glucose level. Compared with post-load glucose level, fasting is more strongly associated with odds of a perinatal outcome. For example the odds of LGA doubles with each 1-mmol/l increase in fasting glucose level, whereas the odds of LGA doubles with each 3 mmol/l increase in 2-hour post-load glucose level.
Meta-analysis of studies with two oral glucose challenge test or oral glucose tolerance test categories
Fifteen studies reported associations between two glucose categories and adverse perinatal outcomes. The characteristics of these studies are presented in Table 8. Ten of these studies examined associations between glucose levels following a 50-g OGCT and perinatal outcomes. Outcomes were compared between those who did not meet the criteria for having an OGTT to those who did meet those criteria, but who were not diagnosed with GDM, that is, two groups were compared [< 130 mg/dl vs. ≥ 130 mg/dl post challenge glucose level or (for some studies) < 140 mg/dl vs. ≥ 140 mg/dl post challenge glucose level]. Three studies compared lower levels of glucose with higher levels 2 hours after a 75-g or 100-g OGTT, whereas two other studies compared women with no elevated glucose levels at any time following an OGTT, with women with one elevated glucose level. For all of these comparisons, the numbers of outcomes in the two groups were used to calculate ORs for outcomes comparing one group with a perceived lower risk with another group with a perceived higher risk.
The results of these meta-analyses are shown in Figures 10 and 11. Women in the group with higher glucose levels following an OGCT have a statistically significant increased risk of C-section, LGA, macrosomia and pre-eclampsia than women with lower glucose levels. Other outcomes were reported in only one or two studies; therefore, these associations are less clear (see Figure 10).
FIGURE 10.
Meta-analysis for ORs of outcomes comparing those with OGCT negative results with those with OGCT positive results.
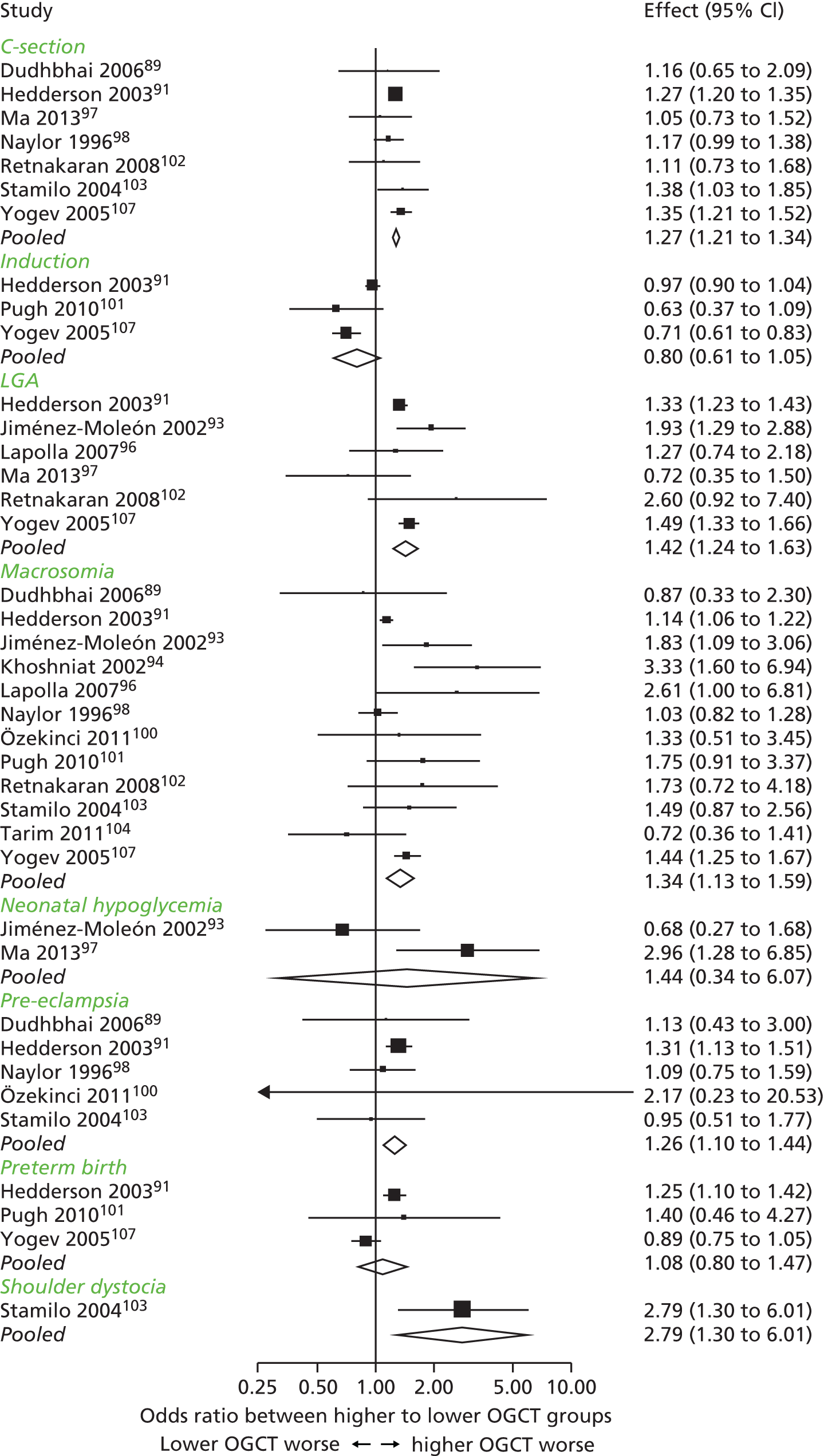
FIGURE 11.
Meta-analysis for ORs of outcomes comparing those with one OGTT elevated glucose level with those with no elevated OGTT glucose levels.
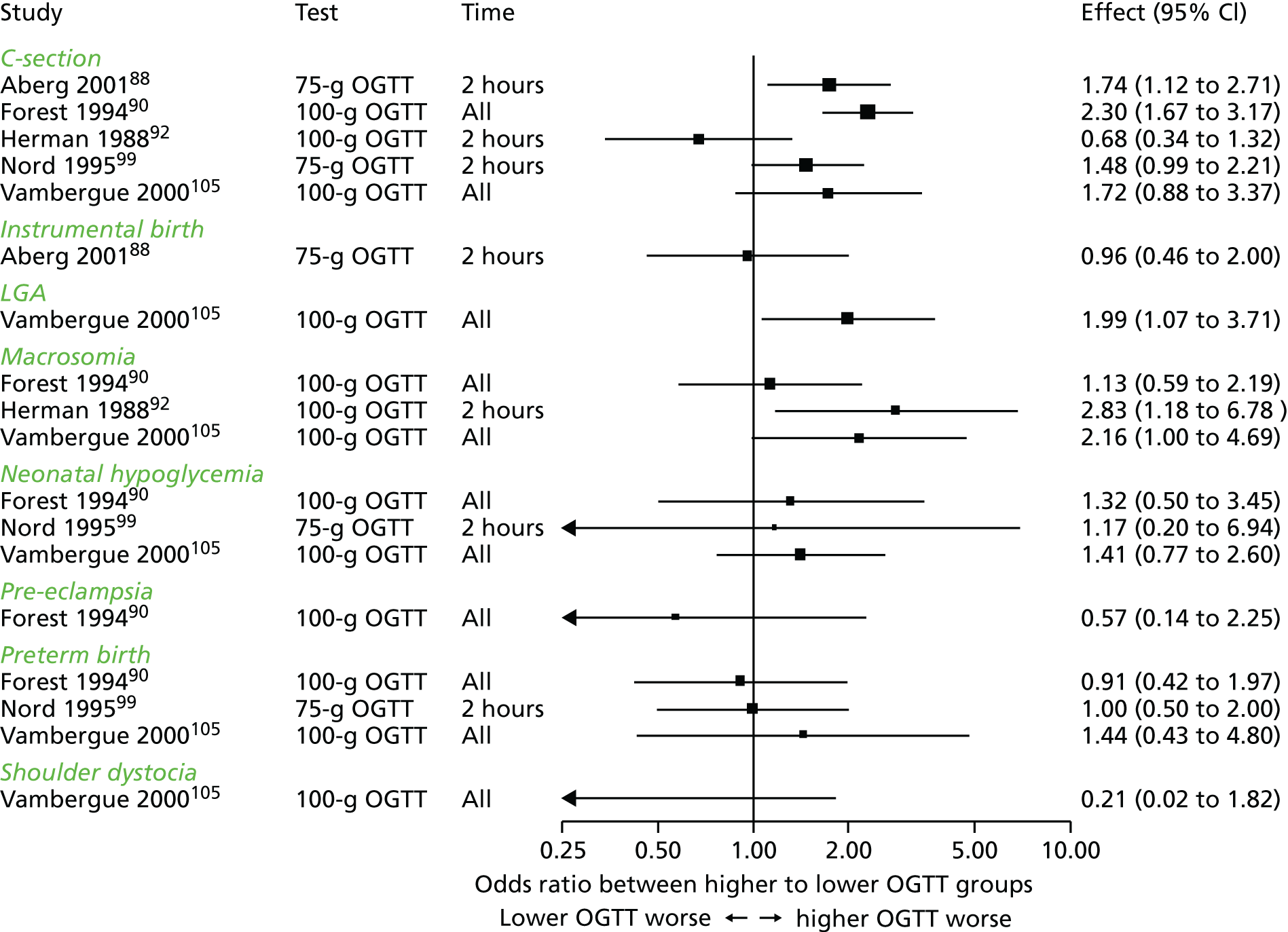
Results from studies comparing lower levels of glucose with higher levels at 2 hours following a 75-g or 100-g OGTT, and results from studies comparing women with no elevated glucose levels at any time after an OGTT to women with one elevated glucose level, were not pooled in meta-analyses. This was because of the differences in the glucose tests used and the timings of the glucose measurements. Generally, however, there was a suggestion that women with one elevated glucose level at OGTT are at higher risk of C-section and macrosomia than women with no elevated glucose levels, although the CIs are wide and often include the null value. There was no evidence of a difference between groups in the odds of preterm birth.
Studies of longer-term and anthropometric outcomes
Six studies22,36,41,65,108,109 reported longer-term and/or anthropometric outcomes, either measures of adiposity or incidence of diabetes. The characteristics of these studies are presented in Table 9.
Three studies7,36,65 (see Chapter 2) reported neonatal obesity (skinfold thickness and percentage body fat of > 90th centile), one study41 reported obesity at age 2 year and one study108 reported obesity at age 5–7 years. One study,109 in Pima Indians, reported longer-term incidence of diabetes in offspring.
The associations of glucose levels with each outcome for each study are shown in Figure 12. Data from one study115 were presented only as ORs of association, so this study could not be included in this figure.
FIGURE 12.
Associations between glucose level and longer-term and anthropometric outcomes: risk of morbidity. Glucose level measured at (a) fasting; (b) 1 hour; and (c) 2 hours post load. Colour indicates the category of morbidity. ● = results for 50-g OGCT; ▲ = results for 75-g OGTT.

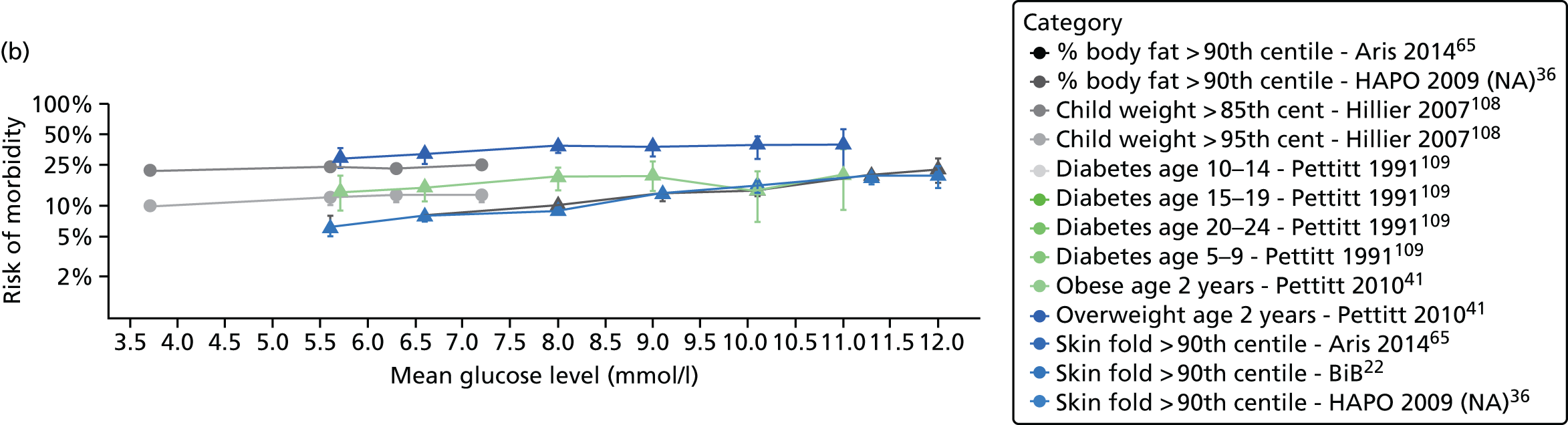
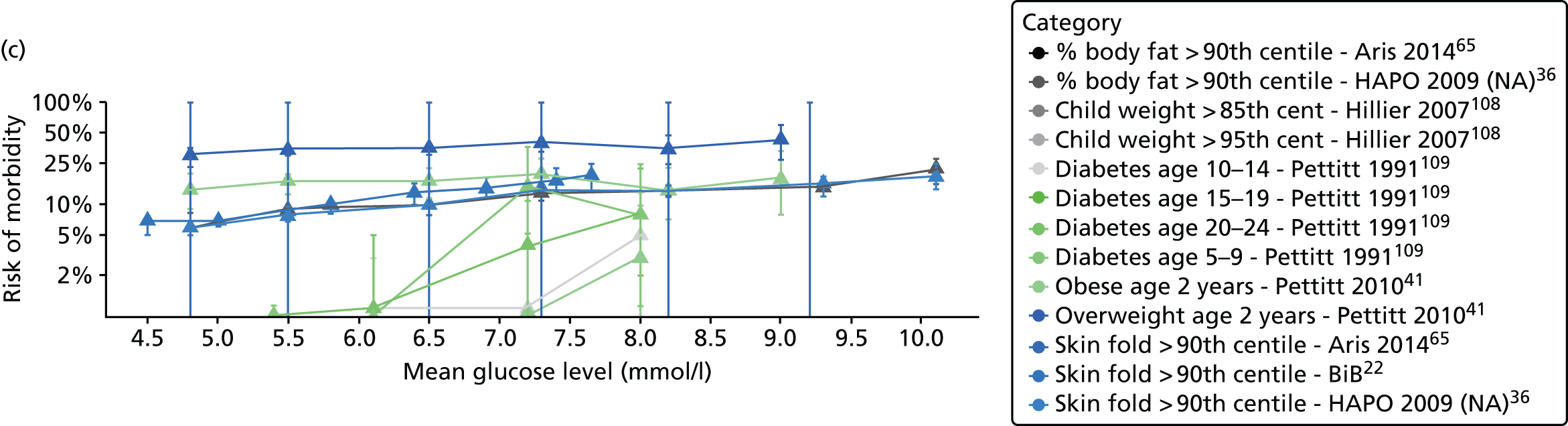
Data are too few to perform a meta-analysis; however, the HAPO36 (2009) model II results (model II, adjusted for age, BMI, BMI2, height, mean arterial BP, gestational age at OGTT, smoking, alcohol use, hospitalisation prior to delivery, and any family history of diabetes) suggest a strong association between glucose levels and sum of skinfolds (flank, triceps and subscapular) of > 90th centile (fasting OR per SD 1.39, 95% CI, 1.33 to 1.47; 1-hour post-load glucose level OR 1.42, 95% CI 1.35 to1.49); 2-hour post-load glucose level OR 1.36, 95% CI 1.30 to 1.43) and body fat of > 90th centile (fasting OR 1.35, 95% CI 1.28 to 1.42; 1-hour post-load glucose level OR 1.44, 95% CI 1.37 to 1.52; 2-hour post-load glucose level OR 1.35, 95% CI 1.29 to 1.42). Aris et al. 65 and the BiB study7 (see Chapter 2) report a linear association between skinfold thickness or body fat percentage of > 90th percentile with increasing glucose levels (fasting and 2-hour post load).
There is limited evidence of an association between increasing maternal glucose levels and longer-term child obesity and overweight. Hillier et al. 108 report the risk of childhood obesity and overweight is greater for infants of women with the highest OGCT glucose levels than for those with the lowest, and that risk is greater for infants of women with GDM than infants of women without GDM (child weight > 95th centile, OR 1.82, 95% CI 1.15 to 2.88). Pettitt et al. 41 found no evidence of an association between glucose levels and overweight and obesity at the age of 2 years. 41
Pettitt et al. 109 reported a strong association between increasing maternal glucose level and increased risk of offspring diabetes, but this was in an unusual population (Pima Indians) therefore the results may not generalise to European populations.
Other identified studies not included in the meta-analyses
Several studies could not be included in the analyses: one study110 reported the association between fasting, 1-hour and 2-hour OGTT glucose levels and outcomes only as an OR per SD. The study110 reports statistically significant increases in risk of LGA, C-section, preterm birth, shoulder dystocia and gestational hypertension with increasing glucose levels.
One study111 examined the incidence of type 2 diabetes in Pima Indians (similarly to Pettitt et al. ,109 and possibly for the same cohort, so is excluded) and concluded that diabetes incidence in offspring increases as maternal glucose levels increase, the 2-hour post-load OGTT glucose level was used, diabetes was recorded at ages from 10 to 25 years.
One study71 reported preliminary results, so it was excluded because it was superseded by a later publication included in the analyses. 112 The earlier study71 reported 2-hour post-load 75-g OGTT glucose levels and incidence of LGA, shoulder dystocia, C-section and preterm birth.
One study114 reported a significant association between the 2-hour post-load glucose level following a 75-g non-fasted OGCT and macrosomia. The study114 was excluded because the 75-g OGCT is a glucose load not normally used in a non-fasted state and there were no other studies using this test in this way.
One study113 compared the risk of outcomes in women without elevated glucose levels to those with a single elevated glucose level at either 1, 2 or 3 hours following a 100-g OGTT. The study113 reported that, compared with women without an elevated glucose level, women with one elevated glucose level were at higher risk of adverse outcomes including C-section, pre-eclampsia and neonatal hypoglycaemia. The associations were stronger for women with an elevated glucose level at 1-hour post-load compared with 2 or 3 hours. There were no women with an elevated fasting glucose level and normal post-load glucose level.
Discussion
We identified 57 eligible studies examining the association between maternal glucose levels at OGTT and OGCT and risk of adverse maternal and infant outcomes. The data reported by five studies71,110,111,113,114 were insufficient to allow inclusion in any meta-analyses.
Studies examining the association between three or more graded increases in glucose level and risk of perinatal adverse outcomes
There was a positive association between LGA, macrosomia, C-section plus pre-eclampsia and increasing glucose levels. There was evidence for an increase in risk in shoulder dystocia, neonatal hypoglycaemia, instrumental (assisted) birth and induced labour, although data were more limited for these outcomes. There was no evidence of an association between preterm birth and increasing maternal glucose levels. The associations, in terms of OR per 1-mmol/l increase in glucose level, were stronger for fasting glucose levels than post-load glucose levels.
Fewer studies examining glucose levels and adverse outcomes used the 100-g OGTT than the 75-g OGTT; however, our findings suggest that associations are similar for the two tests. Equally, the post-load glucose results for the 1-hour 50-g OGCT were broadly consistent with the post-load glucose results of the 75-g and 100-g OGTT. We combined the fasting results from the 75-g and 100-g OGTT together and the results from the 2-hour post-load 75-g and 100-g OGTT. Combining results for fasting glucose measurements is reasonable, because fasting glucose levels will not be affected by subsequent glucose load. The validity of combining results for post-load glucose level is more uncertain, as it assumes that the association of risk between adverse outcomes and glucose level is independent of the test’s glucose load.
Our analysis generally suggests the odds of an adverse outcome increases linearly with glucose levels. Therefore, there appears to be a continuum of risk across glucose levels, with no sudden increase in risk at any glucose level, suggesting that there is no glucose level below which there is no increased risk, and no clear glucose threshold that can distinguish between women at low risk from those at a substantially increased risk of having an adverse outcome.
We excluded women from our analysis with diagnosed GDM, however defined, because these women would have been offered treatment and treatment to reduce hyperglycaemia will influence the natural association between glucose level and outcome risk (see Chapter 2).
Most of the analyses were based on unadjusted raw numbers of women with outcomes in different categories. The risks may therefore be over or underestimated because of potential confounding. For example, the risk of macrosomia is recognised as being higher for obese women than ‘normal’ weight women irrespective of glucose levels. 116 In the few studies presenting adjusted analyses to correct for possible confounding, there was evidence that risk of adverse outcomes increased (similarly to unadjusted analyses) as glucose levels increased, and we have also shown this using the BiB study data,22 as described in Chapter 2. However, we cannot rule out confounding by indication or residual confounding by characteristics that were unmeasured or poorly measured in studies.
Studies examining the association between graded increases in glucose level and risk of longer-term adverse outcomes
There were few studies investigating longer-term outcomes for the mother or infant. Two studies41,108 examined glucose levels and risk of offspring obesity and reported variable results. One study109 reported an association with diabetes in childhood; however, the women were Pima Indians, who are at greater risk of diabetes, and therefore these results may not generalise to a European population.
Studies examining the association between two categories of glucose level and risk of adverse outcomes
Several studies present glucose levels at OGCT divided into two categories (typically women considered to have OGCT positive results vs. OGCT negative results). Our analyses showed that elevated OGCT levels (those indicating an OGTT should be offered) compared with ‘normal’ OGCT results were associated with an increased risk of C-section, LGA, macrosomia and pre-eclampsia, suggesting that this test does identify women at increased risk of these adverse outcomes. A limited number of studies presented results for the OGTT with just two glucose groups, but data were too few to draw any firm conclusions.
Strengths and limitations
We identified a large number of high-quality studies examining associations between maternal glucose levels and adverse perinatal outcomes; most studies included > 1000 women. Studies tended to report similar adverse outcomes and therefore we were able to pool estimates in meta-analyses. We were able to examine (because they were available and reported) a variety of outcomes including macrosomia, LGA, C-section, pre-eclampsia, neonatal hypoglycaemia and shoulder dystocia, across the whole glucose spectrum.
Included studies were necessarily observational (randomisation to a given glucose level is not possible), therefore it is conceivable that other characteristics might have confounded the associations that we have examined and may be responsible for the results we present.
Unfortunately, five studies71,110,111,113,114 did not include sufficient data or information to allow inclusion in the meta-analyses and therefore our estimates may have been different if these studies were able to be included. However, as most studies suggest a positive relationship between increasing glucose level and adverse outcome, the inclusion of these studies would be unlikely to change results.
Five studies36,41,65,108,109 examined maternal glucose level and longer-term outcomes, although they investigated diabetes and adiposity, the timing of the measurements and methods of assessment varied, preventing pooling of estimates. Data for the 100-g OGTT, and particularly the 1-hour post-load measure, were more limited than the 75-g OGTT, therefore less confidence should be placed on these results.
Conclusion
Our meta-analyses suggest an increasing risk for the majority of reported adverse perinatal outcomes including macrosomia, LGA, C-section, pre-eclampsia, neonatal hypoglycaemia and shoulder dystocia, across the whole spectrum of glucose levels. Associations between risk of an outcome and graded increases in glucose level seem to apply to all glucose loads (50-g, 75-g and 100-g) and at all measurement times (fasting, and 1-hour and 2-hour post load), although the strength of these associations varies. Associations were stronger for fasting glucose levels than post-load glucose levels and for the 75-g OGTT compared with the 100-g OGTT.
Chapter 4 Prevalence of gestational diabetes in the UK and Republic of Ireland: a systematic review
Introduction
Prevalence of GDM is influenced by (1) population characteristics, for example Asian or Middle Eastern ethnicity and obesity;44,117–120 (2) criteria used for GDM diagnosis, because lower glucose level thresholds will identify greater numbers of women with GDM;121–123 and (3) screening and testing strategy, because the application of universal – rather than selective – glucose tolerance testing leads to greater numbers of women tested, leading to increased numbers identified. 124
Prevalence of GDM is increasing alongside rising levels of obesity and inactivity, which can increase insulin resistance,125 mirroring the increasing rate of type 2 diabetes in the non-pregnant population.
The shift from identifying women at future risk of type 2 diabetes, to trying to predict risk of perinatal and longer-term ill-health outcomes in the infants of women who have had GDM, has prompted changes to diagnostic criteria. Criteria with lower thresholds will identify more women at risk, thus increasing prevalence and if treatment strategies remain unchanged, costs will increase. However, providing treatment to more women may reduce the risk of perinatal and longer-term ill health, potentially saving money for the UK NHS (and the individual). Chapter 7 details a cost-effectiveness analysis that examines alternative identification and treatment strategies.
We have estimated the prevalence of GDM using different criteria for WB and SA women in the BiB cohort,22 described in Chapter 2 of this report. In this chapter, however, we report a systematic review to determine the prevalence of GDM in the UK and Irish obstetric population, using identified and eligible published reports. We also derive and compare estimates from three IPD cohorts (including that of the BiB study22). This section is reported in accordance with PRISMA guidelines. 56
Methods
Search strategy
Searches were undertaken in July 2014 in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, the Maternity and Infant Care database and CENTRAL. No date restrictions were applied to the searches; citations were restricted to English language only (see Appendix 7, Table 84).
Title and abstract screening and full-text screening were performed in duplicate by two reviewers with disagreements resolved by consensus or by a third reviewer.
Three cohort studies were eligible and provided data at the individual participant level:
-
the BiB study22 (John Wright, Bradford Institute for Health Research, September 2013)
-
the Atlantic DIP study59 from the Irish Atlantic seaboard (Fidelma Dunne, Department of Medicine, National University of Ireland, September 2013)
-
the Warwick/Coventry cohort,60 unpublished data from Warwick Hospital, George Eliot Hospital, Nuneaton and University Hospital Coventry (Ponnusamy Saravanan, Warwick Medical School, September 2013).
Because IPD were available from an Irish cohort (Atlantic DIP59), we have considered prevalence of GDM in the UK and Ireland together.
Inclusion/exclusion criteria
This review sought to identify all cohorts of pregnant women in whole, or in part, in the UK or Republic of Ireland who were assessed for GDM.
The included studies had to have the following characteristics.
Population
Pregnant women from the UK or Republic of Ireland without pre-existing diabetes.
Diagnostic test
All women had to receive an OGTT (75 g or 100 g) in pregnancy to diagnose GDM using recognised diagnostic criteria, or with criteria reported in the paper.
Outcomes
Studies had to report numbers of women, with and without GDM, according to the diagnostic test used or the prevalence of GDM.
Study design
All published, unpublished and ongoing observational cohort studies, or cross-sectional studies reporting data for women resident in the UK or Republic of Ireland. Only studies published in English were included.
When multiple publications reported prevalence estimates for the same cohort of women only the most recent and comprehensive publication was included.
Quality assessment
We assessed the characteristics of all of the publication/study criteria (including the population, location and publication year) that were used to diagnose GDM and derive prevalence estimates.
Data extraction
The following data were extracted from each publication:
-
year of publication
-
location of the study
-
details of the population characteristics, for example ethnicity, age, BMI distribution (if reported)
-
details of the OGTT methods and diagnostic criteria used
-
total number of women with and without GDM, or the prevalence of GDM
-
prevalence of GDM in participant subgroups, such as ethnic group or BMI group.
For the IPD, the prevalence of GDM was calculated, based on the reported OGTT glucose measurements, with GDM diagnosed according to a range of diagnostic criteria as described earlier in Table 1. Prevalence was also calculated by ethnic group (white, SA or ‘Other’) and by age categories using the modified WHO 1999 criteria11 (fasting glucose level of ≥ 6.1 mmol/l and 2-hour post-load glucose level of ≥ 7.8 mmol/l).
Synthesis methods
Prevalences of GDM, along with their 95% CI, were estimated from the data for each study. These prevalence estimates are shown on forest plots. Studies were categorised by GDM diagnostic criteria and year of publication, in order to investigate the effect of these factors (see Figures 14 and 15).
Meta-analyses of the prevalence data were considered, but not performed because of the heterogeneity across the studies, particularly the diversity of diagnostic criteria used to diagnose GDM.
Results
The database searches identified 1591 references for checking (1196 following deduplication). After title and abstract screening, 92 publications were retrieved for full-text screening (17 of which were potentially relevant for the systematic review on risk factors and so kept for that review). The main reasons for exclusion were that the study was published only as a conference abstract and data reported were insufficient, or the study did not include a UK or Irish population. The full list of excluded citations with reasons is contained in Appendix 3, Table 64.
Of the 92 publications, 12 were potentially eligible for inclusion. We also identified three cohorts with IPD (the Atlantic DIP study,59 Warwick/Coventry60 and the BiB study22), reporting GDM prevalence for a UK or Irish cohort. 42,44,118,126–134 After data extraction, two publications128,134 were excluded because they reported data from the same cohort. One additional paper (on the HAPO cohort6) was included, having been identified for another review undertaken as part of this project (see Chapter 3). 131 One publication135 was excluded because it reported prevalence for the Atlantic DIP cohort59 for which IPD were available. After including the IPD cohorts, a total of 13 studies with 16 cohorts of women (see Table 10) defined either by criteria used to define GDM or by location (for multisite studies) were included. Full details of the identification process are presented in Figure 13.
FIGURE 13.
The search process.
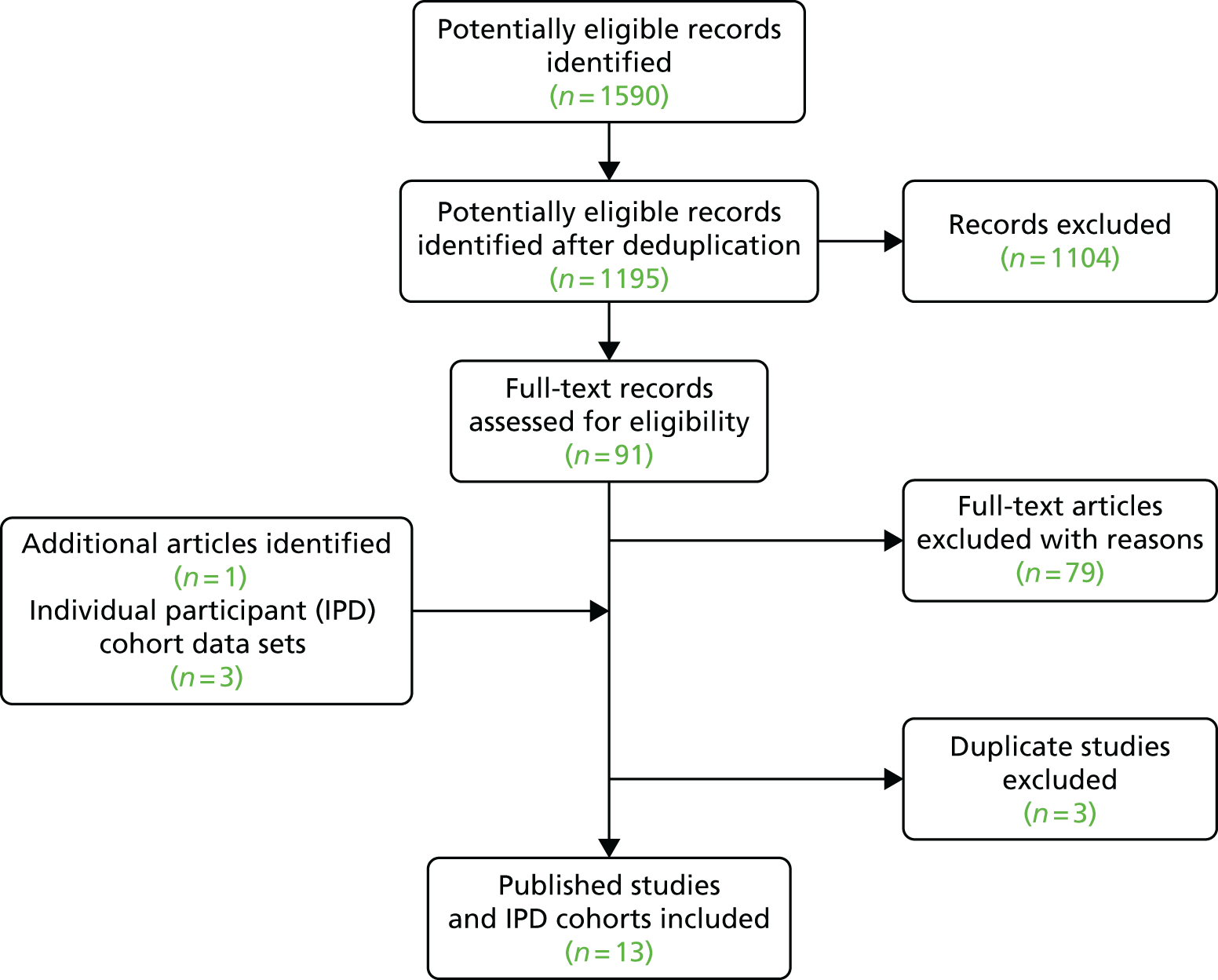
Quality assessment and included studies
A summary of GDM diagnostic criteria are presented in the introduction to this report (see Table 1). Table 10 summarises the 10 published studies42,44,118,127–129,131–133 and the three IPD cohorts included in this review.
| Study | Publication year | Location | GDM diagnostic criteria | No. of women | No. with GDM | Prevalence of GDM (%) |
|---|---|---|---|---|---|---|
| Ali126 | 2013 | Dublin | NDDG | 1375 | 139 | 10.1 |
| IADPSG | 1679 | 221 | 13.2 | |||
| Atlantic DIP59 | IPD | Ireland | WHO 1999b | 6105 | 622 | 10.2 |
| BiB22 | IPD | Bradford | WHO 1999b | 10,432 | 850 | 8.1 |
| Warwick/Coventry60c | IPD | Warwick/Coventry | WHO 1999b | 6569 | 570 | 8.7 |
| Dornhost44 | 1992 | London (St Mary’s) | Reported in paperd | 11,035 | 170 | 1.5 |
| Gregory127 | 1998 | Cambridge | WHO 1980b | 3316 | 67 | 2.0 |
| Griffin133 | 2000 | Dublin | NDDG | 1299 | 35 | 2.7 |
| Janghorbani128 | 2006 | Plymouth | WHO 1980b | 4942 | 90 | 1.8 |
| Khalifeh129 | 2014 | Dublin | WHO 1999b | 68,494 | 888 | 1.2 |
| Dublin | WHO 1999b | 112,138 | 2016 | 1.8 | ||
| Koukkou118 | 1995 | London – St Thomas’ | EASDe | 6887 | 136 | 2.0 |
| Makgoba42 | 2012 | London – St Mary’s | Variedf | 174,320 | 1688 | 1.0 |
| Sacks131 | 2012 | Manchester | IADPSG | 2376 | 577 | 24.3 |
| Belfast | IADPSG | 1671 | 286 | 17.1 | ||
| Samanta132 | 1989 | Leicester | WHO 1980 | 12,005 | 128 | 1.1 |
Prevalence of gestational diabetes mellitus by year the study was undertaken and gestational diabetes mellitus criteria used
Figure 14 shows prevalence by year and GDM criteria used by each study. Using data from the three IPD cohorts we calculated GDM prevalence according to the most commonly used GDM diagnostic criteria presented in Table 1; 1-hour post-load glucose levels (75-g OGTT) were not available for the BiB,22 Atlantic DIP59 and Warwick/Coventry cohorts,60 therefore prevalences may be underestimated for criteria that include a 1-hour glucose level [American Diabetes Association (ADA), IADPSG, NDDG (National Diabetes Data Group)]. These prevalence estimates are shown in Figure 15. The Atlantic DIP study59 has higher prevalence estimates for all diagnostic criteria. NDDG criteria are the most conservative, having the highest glucose thresholds. The WHO 1980, WHO 1999, ADA and Australasian Diabetes in Pregnancy Society (ADIPS) criteria produce similar prevalence estimates, despite their different glucose threshold criteria. The IADPSG criteria give the highest prevalence estimates for the IPD cohorts, similarly to published estimates, as a result of the lower fasting glucose threshold.
FIGURE 14.
Prevalence of GDM by year the study was undertaken and GDM criteria used. DPSG (EASD), Diabetic Pregnancy Study Group (of the European Association for the Study of Diabetes).

FIGURE 15.
Estimated prevalence according to different GDM criteria in the IPD cohorts. See Table 1 for criteria thresholds.
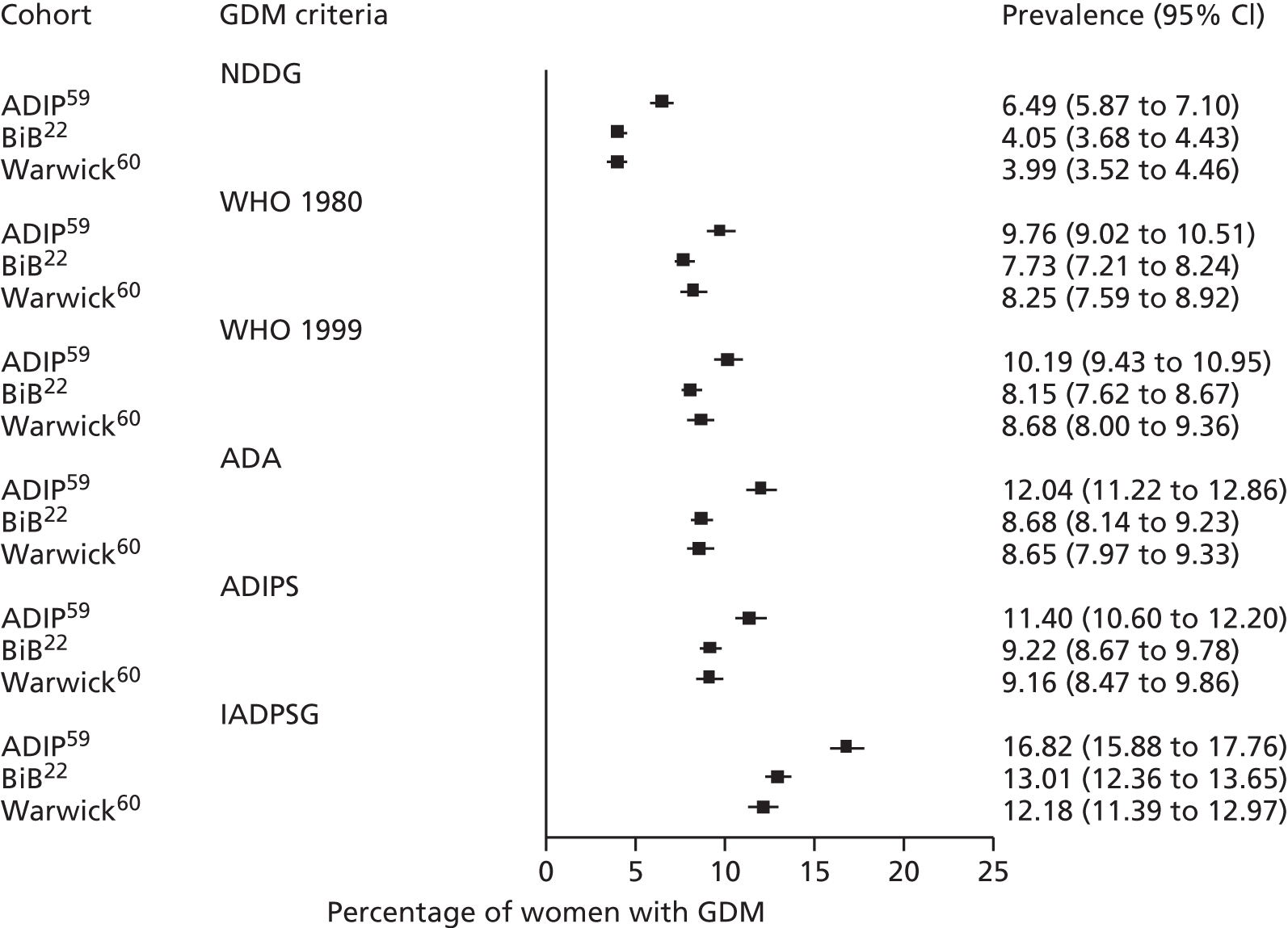
Prevalence of gestational diabetes mellitus by ethnicity
Two published studies44,118 report prevalence of GDM by ethnicity (Table 11). Both of these studies44,118 were undertaken when recommended criteria thresholds were higher (1992 and 1995) than those now suggested by the IADPSG and consequently report lower GDM prevalence than would be expected today. Both studies,44,118 however, report differing GDM prevalence by ethnicity, with women of Asian and SA origin having the highest rates. Koukkou et al. 118 do not provide more information on the origin of the Asian women in their study (they were recruited from an inner-city London hospital), so they could be of any number of Asian ethnicities.
| Study | Year | Prevalence: percentage (n) | ||||
|---|---|---|---|---|---|---|
| White European | African/Afro-Caribbean | South-East Asian | SA | Asian | ||
| Dornhorst44 | 1992 | 0.4 (6279) | 1.5 (1953) | 3.5 (386) | 4.4 (1159) | – |
| Koukkou118 | 1995 | 1.2 (315) | 2.7 (300) | – | – | 5.8 (49) |
Prevalence of GDM by ethnicity was calculated using the three IPD cohorts. These data are summarised in Table 12.
| Cohort | White | SA | Other |
|---|---|---|---|
| Atlantic DIP59 | 8.6 (6.1 to 11.1) [481/5613] | 39.1 (28 to 50) [77/197] | 21.7 (12 to 32) [64/295] |
| BiB22 | 4.9 (1.9 to 7.9) [201/4105] | 10.8 (8.1 to 13.4) [512/4745] | 8.7 (4.0 to 13.4) [137/1582] |
| Warwick/Coventry60 | 8.1 (5.2 to 11.0) [336/4167] | 10.8 (5.1 to 16.5) [113/1046] | 8.9 (3.8 to 14.0) [121/1356] |
Prevalence of gestational diabetes mellitus by age
The published studies provided insufficient data to estimate prevalence by age, but we have been able to calculate estimates using the IPD cohorts. The results are summarised in Table 13. GDM prevalence appears to increase as age category increases in all three cohorts. A logistic regression confirmed this, with a statistically significant increase in odds of GDM of 1.08, 95% CI 1.08 to 1.10 per year.
| Cohort | Age (years) group | |||||
|---|---|---|---|---|---|---|
| < 20 | 20–25 | 25–30 | 30–35 | 35–40 | > 40 | |
| Atlantic DIP59 | 5.0 (0 to 23) [6/119] | 4.0 (0 to 13) [19/472] | 8.7 (3 to 14) [103/1179] | 12.6 (8 to 17) [234/1858] | 15.8 (11 to 21) [195/1238] | 21.3 (10 to 32) [51/240] |
| BiB22 | 3.4 (0 to 9) [36/1050] | 4.0 (1 to 8) [121/2989] | 8.0 (5 to 11) [269/3346] | 12.3 (8 to 16) [250/2028] | 17.2 (11 to 23) [153/888] | 16.0 (0 to 31) [21/131] |
| Warwick/Coventry60 | 4.4 (0 to 14) [16/364] | 5.2 (0 to 11) [65/1245] | 7.6 (3 to 12) [151/1976] | 10.0 (6 to 14) [177/1771] | 12.5 (7 to 18) [122/974] | 16.3 (5 to 28) [39/239] |
Prevalence of gestational diabetes mellitus by timing of oral glucose tolerance test
The BiB22 IPD included information on the timing of the OGTT, in terms of gestational age.
We examined results (numbers in parenthesis) by the following gestational age categories (in weeks plus days): < 25 (438), 25–25 plus 6 days (1733), 26–26 plus 6 days (5695), 27–27 plus 6 days (1133), 28–28 plus 6 days (529), 29–29 plus 6 days (276), 30–30 plus 6 days (263) and ≥ 31 (364). A logistic regression analysis found no evidence that the prevalence of GDM changed according to the timing of the test (OR 1.00, 95% CI 0.96 to 1.04).
Discussion
Studies in this review demonstrate a wide range of GDM prevalences. The differences in prevalence are partly explained by the differing criteria and thresholds used to diagnose GDM. Prior to 2010, the WHO criteria11 were used widely in the UK and Ireland, and GDM prevalence was consistently estimated at between 1% and 3% across cohorts. Since 2010, however, variation in estimates are wider (8–24%). The IADPSG criteria8 (published in 2010 and used in several later studies) produced the highest prevalences because of their lower (than previous criteria) fasting glucose threshold. Given the linear monotonic association across the whole spectrum of glucose levels and adverse outcomes, using lower thresholds (as recommended by the IADPSG) will increase the number of women identified who are at increased risk of an adverse outcome. Treatment aims to reduce glucose levels with the goal of reducing the associated increased risks. Treatment trials,51,52 however, have used diagnostic criteria with higher glucose level thresholds than those recommended by the IADPSG and now endorsed by the WHO (or those derived using the BiB data,22 detailed in Chapter 2), therefore the degree to which treatments will improve outcomes for women identified by these criteria using lower glucose level thresholds is unknown.
Several criteria recommend that women have their risk of GDM evaluated either by assessment of maternal characteristics/risk factors (including ethnicity and weight) or by administration of the 50-g OGCT, those that are classified as ‘high risk’ are offered diagnostic testing usually using the OGTT. Some criteria (including the IADPSG), however, recommend universal testing. Criteria recommending that all women are offered testing, rather than only ‘high-risk’ women, will increase the prevalence of GDM irrespective of glucose level thresholds used. 124
Differing population characteristics explain some of the diversity in prevalence estimates. In the BiB study,22 GDM prevalence in SA women was two- to threefold greater than in WB women (see Tables 4 and 12). Other characteristics also influence prevalence, including advanced maternal age or increasing maternal weight. We have shown that timing of OGTT does not seem to influence prevalence of GDM, however we had few women undergoing OGTT below 25 or above 30 weeks’ gestation. Women who are tested outside the usual 26–28 week range may have specific high-risk status, including previous GDM or symptoms/clinical indications such as polyhydramnios or ultrasound indication of a LGA fetus, therefore the population characteristics of studies with a wider range of OGTT timings should be examined carefully.
Strengths and limitations
We identified 13 studies22,42,44,59,60,118,126–129,131–133 undertaken over 25 years in varied areas of England and Ireland. We were able to demonstrate how prevalence changed over these 25 years and how participant characteristics and criteria influence prevalence. The studies were large, all included > 1000 women and all reported their inclusion and GDM criteria. Our IPD provided valuable information that was not available from published estimates, and showed that, even in contemporary cohorts, GDM prevalence can vary considerably between groups with varying maternal characteristics, including ethnicity.
Few published studies included populations at high risk of GDM because of their ethnicity therefore the inclusion of the BiB cohort22 is extremely valuable. Estimates of prevalence for SA women in the Atlantic DIP cohort59 are uncertain because there were few women of SA ethnicity in that cohort, and even fewer with diagnosed GDM. We undertook several subgroup comparisons; however, these results should be interpreted cautiously given that the studies were not designed or powered to detect differences in prevalence across subgroups. The prevalence of GDM in WB women in the BiB study22 is lower (5%) than that in the Atlantic DIP59 (9%) and the Warwick/Coventry60 (8%) cohorts, even although all used the same diagnostic criteria. The Atlantic DIP study59 (like the BiB study22) universally offered an OGTT, whereas the Warwick/Coventry studies60 selectively tested their population, but both cohorts59,60 had similar and higher GDM prevalence in their white populations than the BiB study,22 and it is unclear why this is so.
We did not identify any eligible studies that included Scottish or Welsh cohorts, therefore, although we intended to present data on UK prevalence of GDM, our data represent England, Northern Ireland and the Republic of Ireland (as we were able to include the Atlantic DIP cohort59).
Conclusions
The prevalence of GDM is increasing in the UK; the offer of an OGTT to all women, the lowering of diagnostic thresholds, and increases in the proportion of women at risk, either because of their ethnicity or increasing weight or age, are all contributing factors (which is examined in the Chapter 5). Within a narrow gestational time frame we have demonstrated that timing of OGTT does not seem to influence prevalence; however, we had few women tested at < 25 or > 31 weeks of gestation and therefore caution should be taken when interpreting these findings. We showed that populations of older women or women whose ethnicity conveys a high risk of diabetes will have higher GDM prevalence.
Chapter 5 Maternal characteristics (risk factors) to identify women at increased risk of gestational diabetes: a systematic review
Introduction
In this chapter we first examine maternal characteristics/risk factor screening performance using IPD from the BiB22 and Atlantic DIP59 studies. Second, we report the findings from a systematic review of published literature on maternal characteristics/risk factor screening for GDM. Within this review we investigate whether or not multiple risk factor screening strategies represent a useful approach to screening for GDM. We examine the degree to which these approaches detect cases of GDM and whether or not they reduce the number of OGTTs performed.
Screening identifies apparently healthy women who are at increased risk of having or developing GDM. Once a woman is identified as having an increased risk she can be given information and advice and further tests. Treatment can be started following a definitive diagnosis of GDM (usually using the OGTT). Screening is therefore undertaken to (1) identify those women at greatest risk in order to prevent unnecessary testing of those women who are unlikely to develop GDM and (2) reduce the costs associated with universal diagnostic testing.
Diagnostic testing can be undertaken in either the whole obstetric population, by offering all women an OGTT (universal testing), or in a selected population, by offering an OGTT to only those women at increased risk of developing GDM (selective testing).
Screening options
There are two ‘screening’ methods generally used to identify women who are at increased risk of developing GDM; (1) the 50-g OGCT, which is similar to the OGTT, but does not require an overnight fast: one plasma glucose level is obtained 1 hour following the consumption of a 50-g glucose drink – women with a positive test (above a predefined glucose level) are offered an OGTT; and (2) maternal characteristics/risk factor assessment, which involves the assessment of maternal characteristics to identify increased risk of GDM: family history of diabetes; being of an ethnicity with a high prevalence of diabetes; previous history of having a macrosomic infant or GDM; or BMI of ≥ 30 kg/m2 are risk factors recommended for use by NICE18 – when one or more risk factors are identified then NICE recommends that an OGTT is offered.
Diagnostic testing
Gestational diabetes mellitus is generally diagnosed using an OGTT. The OGTT is normally conducted in the morning following an overnight fast. A baseline plasma glucose sample is obtained, the woman then consumes a drink containing, typically, 75 g or 100 g of glucose, and then at hourly intervals plasma glucose is measured. The frequency of measurement depends on the glucose load and local policy. Women with an ‘elevated’ glucose level at one or more measurements are classified as having GDM.
There are some limitations to the OGTT as a diagnostic test: (1) a negative OGTT result does not mean a woman will not develop GDM later in pregnancy – because, as gestation progresses, insulin resistance may increase, repeat glucose testing therefore may be required; (2) the linear positive graded association across the whole spectrum of maternal glucose and risk of adverse outcomes has made the identification of clear diagnostic glucose level thresholds for GDM difficult;6,7 and (3) the reproducibility of the OGTT is around only 75%. 20,21
The accuracy of a screening or diagnostic test relates to its ability to distinguish between those with the condition (GDM) and those who do not have the condition; this can be presented in terms of a test’s sensitivity and specificity, predictive values, likelihood ratios, and the area under the receiver operating characteristic (ROC) curve.
Several health-care agencies, including NICE and the ADIPS (see Table 14), have recommended that pregnant women should have their risk of GDM evaluated by assessment of maternal characteristics/risk factors. Those with one or more maternal characteristics/risk factors should be offered a diagnostic OGTT (or alternative test: see Table 14, ADA entry).
Table 14 shows a selection of the risk factors recommended for use to guide diagnostic testing.
| Agency | Nature of screening strategy |
|---|---|
| NICE (UK) 201518 | Offer OGTT only to women with at least one of:
|
| ADA 2014136 | Testing at first antenatal visit should be undertaken to identify undiagnosed type 2 diabetes (universal OGTT testing is recommended at 24–28 weeks) in all pregnant women who are overweight (BMI ≥ 25 kg/m2) and have additional risk factors:
|
| ADIPS 20139 | Women who are from a high-risk ethnic background or have a BMI of 25–35 kg/m2 as their only risk factor should be considered as ‘moderate risk’, and should initially be screened with either a random or a fasting glucose test in early pregnancy, followed by an OGTT if clinically indicated. ADIPS suggests that the thresholds for further action are not clear at present and clinical judgement should be exercised Women at ‘high risk’ of GDM (one high-risk factor or two moderate risk factors) should be offered a 75-g OGTT, with venous plasma samples taken: fasting, 1 hour and 2 hours, at the first opportunity after conception Women at moderate or high risk with normal glucose should be offered an OGTT at 24–28 weeks Moderate risk factors for GDM
|
Recommended risk factors vary considerably; however, all strategies suggest that early pregnancy (first trimester) screening and testing for GDM or previously undiagnosed type 2 diabetes in those women classified as being at particularly high risk. NICE recommends that first trimester testing is offered to women who have had previous GDM, whereas the ADA and ADIPS recommend early testing of women with a variety of risk factors. Of these three institutions, the ADA recommends that all of those not identified as having GDM (or previously undiagnosed diabetes) in the first trimester should be tested in the third trimester. 136 NICE and ADIPS recommend universal risk factor screening and selective OGTT in the third trimester. 9,18
Risk factor screening: individual participant data cohorts
Methods
Two cohorts with IPD were eligible and agreed to share their data:
-
The BiB cohort (John Wright, Bradford Institute for Health Research, September 2013) At the time of recruitment to the BiB study,22 all women planning to give birth at the Bradford Royal Infirmary were offered a 75-g OGTT (irrespective of risk factors). The WHO 199911 (modified) criteria were used to diagnose GDM (fasting glucose level of ≥ 6.1 mmol/l, 2-hour post-load glucose level of ≥ 7.8 mmol/l).
-
The Atlantic DIP Cohort59 (Fidelma Dunne, Department of Medicine, National University of Ireland, September 2013) Women at participating hospitals in the south-west of Ireland were all offered a 75-g OGTT (irrespective of risk factors) and, as with the BiB study,22 the WHO 1999 (modified) criteria11 were used to diagnose GDM (fasting glucose level of ≥ 6.1 mmol/l, 2-hour post-load glucose level of ≥ 7.8 mmol/l).
An OGTT was offered to all women in both cohorts. Uptake of the offer varied between the two cohorts [63% (the BiB study22) vs. 58% (the Atlantic DIP study59)]. 124,137
We have examined the following characteristics because they are associated with a greater risk of GDM development, and their use as indicators for OGTT is recommended by institutions including NICE18 and the ADA. 136
-
age
-
obesity, measured by BMI
-
parity (multiparous vs. primiparous)
-
ethnicity (white, SA or other)
-
family history of diabetes
-
GDM in previous pregnancy
-
macrosomic baby (≥ 4 kg) in previous pregnancy.
Age was examined yearly from 20 to 40 years and BMI at every 1.0-kg/m2 unit increase from 15.0 to 40.0 kg/m2. For age and BMI combined, however, we present results for age ≥ 25 years and ≥ 30 years and BMI of ≥ 25 kg/m2 and ≥ 30 kg/m2.
Ethnicity was coded as white, SA or other [the majority of women were either of white European (in the BiB22 and Atlantic DIP59 studies) or SA ethnicity (the BiB22 study)] and parity was coded as primiparous (first pregnancy) or multiparous (second or subsequent pregnancy).
Statistical analyses
For each risk factor the following were calculated with their SEs and 95% CIs:
-
Sensitivity:
-
The proportion of women with GDM who had the risk factor (i.e. proportion of GDM cases correctly identified by the test).
-
-
Specificity:
-
The proportion of women without GDM who did not have the risk factor.
-
-
Positive rate:
-
The proportion of women with the risk factor (i.e. proportion who would be offered an OGTT).
-
Most existing GDM screening guidelines recommend offering an OGTT to any woman who has at least one risk factor from a set of risk factors (see Table 14). To investigate the screening potential of this approach we considered the risk factors in the list above, with age ≥ 25 years or ≥ 30 years, and ≥ BMI 25 kg/m2 or ≥ 30 kg/m2. For each of the 287 possible combinations of these risk factors we calculated whether or not each woman had at least one of the risk factors, and then estimated the sensitivity, specificity and positive rate associated with having one or more risk factors.
From this set of 287 possible combinations of risk factors we removed those that were ‘dominated’ by others. A screening test is dominated if there is at least one other ‘test’ with both higher sensitivity and specificity, which would be preferred to the dominated test. Sensitivity and positive rate for the remaining non-dominated tests were plotted in ROC space.
We examined screening based on a predicted risk of GDM, similar to screening strategies used to identify those at risk of cardiovascular disease. 138 A logistic regression model was fitted to the data from both cohorts, regressing GDM incidence against the risk factors. The resulting log ORs from this regression model were used to calculate a predicted risk of GDM for each woman in the data set. The sensitivity and positive rate for predicting GDM at each percentage point of risk from 1% to 80% was calculated and plotted in ROC space. The same analyses were conducted on the separate and pooled data sets for comparison.
Results
Risk factor sensitivities, specificities and positive rates
A total of 14,103 women (Atlantic DIP59 4164/6105, BiB22 9939/10,432) with complete data on all risk factors were included. Table 15 presents performance characteristics (sensitivities, specificities and positive rates) for predicting GDM using the presence of a maternal characteristic/risk factor by cohort.
| Risk factor | Cohort | |||||
|---|---|---|---|---|---|---|
| BiB22 | Atlantic DIP59 | |||||
| Sensitivitya (%) | Specificitya (%) | Positive rate (%) | Sensitivityb (%) | Specificityb (%) | Positive rate (%) | |
| Age ≥ 25 years | 85.5 | 33.7 | 67.7 | 96.5 | 11.8 | 89.0 |
| Age ≥ 30 years | 57.0 | 66.6 | 35.1 | 80.2 | 36.0 | 65.7 |
| BMI ≥ 25 kg/m2 | 69.8 | 51.2 | 50.3 | 85.6 | 39.1 | 63.4 |
| BMI ≥ 30 kg/m2 | 37.0 | 80.0 | 21.3 | 55.2 | 76.0 | 27.2 |
| Ethnicity: non-white | 76.3 | 40.6 | 60.6 | 24.1 | 93.3 | 8.5 |
| Multiparity | 42.4 | 68.6 | 29.9 | 30.0 | 73.1 | 26.3 |
| Family history of diabetes | 38.9 | 74.4 | 25.7 | 31.4 | 71.2 | 28.5 |
| Previous GDMc | 6.0 | 99.3 | 1.0 | – | – | – |
| Previous macrosomiac | 7.9 | 86.3 | 4.8 | – | – | – |
Only age and BMI achieve a sensitivity of > 50% in both cohorts (i.e. detect more than half of all GDM cases).
Risk factors as predictors for gestational diabetes mellitus
We next consider risk factor screening, by which women are offered an OGTT if they have at least one positive maternal characteristic/risk factor among a set. The results for these analyses are provided in Figure 16, which shows the percentage of GDM cases identified (sensitivity) against the percentage of women offered an OGTT (positive rate) for all of the sets of risk factors that were not ‘dominated’ by others.
Figure 16 shows that in order to identify 80% of women with GDM (80% sensitivity) then approximately 60% of women would have to be offered an OGTT (40% specificity). To identify 90% of women with GDM, about 70% of women would need to be offered an OGTT, and for 95%, 80% of women need to be offered an OGTT. Therefore, most women would need to be tested to identify the majority of women with GDM using risk factors to identify those at higher risk. Figure 16 shows that strategies that identify one risk factor or one out of two risk factors seem to detect < 60% of GDM cases. To detect ≥ 90% of GDM cases requires that at least three or four risk factors are considered (in Figure 16, ‘Number of risk factors’ refers to the following: 1 = one risk factor; 2 = at least one risk factor out of two; 3 = at least one risk factor out of three; and 4 = at least one risk factor out of four).
Both cohorts have similar estimates of sensitivity and specificity across the range of possible risk factor screening strategies, that is, the points in Figure 16 all lie on approximately the same curve for both cohorts. Importantly, however, the risk factors used to achieve, for example, a sensitivity of 90% differs between cohorts. Table 16 provides examples of risk factors, included in screening strategies, with sensitivity of between 90% and 95% (so they detect almost all cases of GDM) for the two cohorts, separately and combined. Age and BMI are the most commonly occurring risk factors, with family history of diabetes, prior GDM and non-white ethnicity also common. In the BiB cohort22 offering an OGTT to anyone either aged ≥ 25 years or with a BMI of ≥ 30 kg/m2 detects 92% of all GDM cases (because the majority of women in the BiB cohort are aged > 25 years of age or have a BMI of > 30 kg/m2), including other factors, does not substantially increase the detection rate, but there are few women left to test and only an 8% increase was needed to achieve a 100% detection rate.
| Risk factors included | Sensitivity | Specificity | Positive rate |
|---|---|---|---|
| BiB cohort22 | |||
| Aged ≥ 25 years, BMI ≥ 30 kg/m2 | 90.4 | 28.7 | 72.7 |
| Aged ≥ 25 years, BMI ≥ 30 kg/m2, prior GDM | 90.4 | 28.6 | 72.8 |
| Aged ≥ 25 years, BMI ≥ 30 kg/m2, diabetes | 91.6 | 23.2 | 77.7 |
| Aged ≥ 25 years, BMI ≥ 30 kg/m2, diabetes, prior GDM | 91.6 | 23.1 | 77.7 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, non-white | 94.3 | 21.3 | 79.8 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, non-white, prior GDM | 94.3 | 21.3 | 79.9 |
| Aged ≥ 25 years, BMI ≥ 25 kg/m2, diabetes | 94.4 | 16.9 | 83.8 |
| Aged ≥ 25 years, BMI ≥ 25 kg/m2, diabetes, prior GDM | 90.4 | 28.7 | 72.7 |
| Atlantic DIP cohort59 | |||
| BMI ≥ 25 kg/m2, non-white | 90.1 | 36.8 | 66.0 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2 | 90.8 | 28.6 | 73.4 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, non-white | 93.9 | 26.0 | 76.0 |
| Cohorts combined | |||
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, diabetes | 90.0 | 24.6 | 76.4 |
| Aged ≥ 30 years, BMI ≥ 25 kg/m2, diabetes, prior GDM | 90.3 | 24.6 | 76.5 |
| BMI ≥ 25 kg/m2, non-white | 92.0 | 24.0 | 77.3 |
| BMI ≥ 25 kg/m2, non-white, prior GDM | 92.1 | 24.0 | 77.3 |
| Aged ≥ 25 years, BMI ≥ 30 kg/m2 | 93.2 | 23.3 | 78.0 |
| Aged ≥ 25 years, BMI ≥ 30 kg/m2, prior GDM | 93.2 | 23.3 | 78.1 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, non-white | 94.1 | 22.7 | 78.7 |
| Aged ≥ 30 years, BMI ≥ 30 kg/m2, non-white, prior GDM | 94.1 | 22.7 | 78.7 |
| Aged ≥ 25 years, BMI ≥ 25 kg/m2 | 95.9 | 16.5 | 84.5 |
| Aged ≥ 25 years, BMI ≥ 25 kg/m2 prior GDM | 95.9 | 16.5 | 84.5 |
Risk prediction models
The association between each risk factor and GDM, in terms of the OR, is shown in Table 17. All risk factors, except multiparity, were statistically significantly associated with GDM. The results were generally consistent across the two cohorts. Having GDM in a previous pregnancy is the most dominant risk factor, associated with a five-fold increase in the odds of GDM development in the current pregnancy. Non-white ethnicity is also a strong indicator of risk. Multiparity was associated with lower risk (see Table 17).
| Risk factor | BiB22 | Atlantic DIP59 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age (per year) | 1.09 | 1.08 to 1.1 | 1.10 | 1.07 to 1.12 |
| BMI (per kg/m2) | 1.06 | 1.05 to 1.08 | 1.13 | 1.11 to 1.15 |
| Ethnicity (non-white) | 2.32 | 1.90 to 2.83 | 5.16 | 3.85 to 6.91 |
| Multiparity | 0.89 | 0.73 to 1.08 | 0.74 | 0.58 to 0.96 |
| Family history of diabetes | 1.36 | 1.14 to 1.63 | 1.42 | 1.17 to 1.80 |
| Previous macrosomia | 1.54 | 1.12 to 2.13 | – | – |
| Previous GDM | 5.90 | 3.78 to 9.22 | – | – |
The ROC curve of sensitivity against positive rate using predicted risk to screen for GDM is shown in Figure 17 for both the BiB22 and Atlantic DIP59 cohorts. Both cohorts provide similar results. To detect 90% of GDM cases based on a risk model requires that 70% of pregnant women undergo an OGTT.
FIGURE 17.
Sensitivity and positive rate when using a risk prediction model to predict GDM.

Figure 18 compares the screening performance of the risk prediction model with screening performance based on age alone and based on oral glucose tolerance testing of anyone with at least one risk factor using data from the BiB cohort22 only. Figure 18 shows that using a risk prediction model to screen for GDM generally provides improved performance compared with screening based on counting positive risk factors alone. This is because the sensitivity is generally higher at all specificities and positive rates, so the number of women who would be offered an OGTT could be reduced while detecting the same number of GDM cases. However, at high specificities (where most GDM cases are detected) there is little performance difference between using a risk prediction model and counting risk factors.
FIGURE 18.
Screening performance using risk prediction compared with having one positive risk factor, or using age alone.

Risk factor screening: a systematic review
We have undertaken a systematic review to identify studies examining risk factors to identify women with GDM and have conducted analyses where appropriate. This section is reported in accordance with PRISMA guidelines. 56
Methods
Search strategy
Searches were undertaken in June 2014 in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, Maternity and Infant Care database and CENTRAL (see Appendix 7, Table 85). No date or other restrictions were applied to the searches, however, because of logistical constraints the results were restricted to English language only. In addition to database searches, reference checking of included journal articles and related systematic reviews was undertaken. Title and abstract screening and then full-text screening was performed in duplicate by two reviewers, with disagreements resolved by consensus or by a third reviewer.
Inclusion/exclusion criteria
This review took a broad approach to identifying publications related to risk factors for GDM by seeking to identify any study that measured the association or predictive value of the following risk factors:
-
age
-
obesity and/or BMI
-
ethnicity (where applicable to the UK)
-
parity
-
previous GDM, macrosomia or other GDM-related morbidity
-
family history of diabetes.
Only risk factors that were likely to be recorded in medical records without the need for further measurement were considered. Specifically, OGCT, fasting plasma glucose (FPG), vitamin D and genetic factors were excluded.
The included studies had to have the following characteristics.
Population
Pregnant women without pre-existing diabetes.
Screening test
Any risk factor listed above was eligible.
Diagnostic test
All women had to receive a diagnostic test (usually 75-g or 100-g OGTT) to diagnose GDM by recognised diagnostic criteria, or with criteria reported in the paper.
Outcomes
Numbers of women with and without GDM, according to the results of the diagnostic test (usually OGTT). Studies had to report numbers of women with each risk factor, or the sensitivity and specificity (screening performance) of the risk factor to identify GDM, or data from which those statistics could be calculated.
Study design
All published, unpublished and ongoing observational studies, cohort studies, case–control studies or cross-sectional studies. Only studies published in English were considered.
Individual participant data cohorts (the BiB22 and Atlantic DIP59 studies) were not eligible for inclusion because these cohorts did not use maternal characteristics/risk factors to identify high-risk women, but offered all women an OGTT.
Studies reporting only ethnicity outside the UK were excluded to focus on ethnicity risk relevant only to the UK population. Studies not reporting on at least one of the risk factors listed above were excluded.
Quality assessment
No formal quality assessment process was planned or undertaken for this review because of the lack of any validated quality assessment tool for screening studies, and the diversity of type of study included.
Data extraction
The following data were extracted from each publication:
-
year of publication
-
location in which study was performed
-
details of the population, such as ethnicity, age, BMI distribution (if the study was not performed on the general population of pregnant women)
-
details of the diagnostic criteria used
-
details of the maternal characteristics/risk factors and cut-off levels applied to risk factors if appropriate
-
total number of women with and without GDM
-
number of women with and without GDM according to diagnostic test results
-
screening performance statistics (sensitivity and specificity, if reported).
Synthesis methods
The following screening performance statistics were calculated from the data presented for each study:
-
sensitivity (proportion of GDM cases correctly identified as high risk by screening)
-
specificity (proportion of women without GDM correctly identified as low risk)
-
positive rate (proportion of women who would be offered an OGTT).
These statistics were plotted across studies in ROC space by plotting detection rate against positive rate. The general performance of risk factor screening was then summarised and the conclusions of each study considered.
Meta-analysis methods for pooling of screening studies [such as the hierarchical summary receiver operator curves (HSROC) model] were considered, but not performed because of the considerable diversity across studies in terms of screening strategies and included risk factors.
Results
Included studies
The database searches identified 5867 citations (3140 after deduplication). After title and abstract screening 181 publications were retrieved for full-text screening; 47 of these were excluded (see Appendix 4, Table 65). Ninety-seven studies reported associations between risk factors and GDM incidence, but did not consider multiple risk factor screening, and eight studies examined the effect of screening based on a single risk factor (although some studies reported more than one risk factor, they were not considered in combination). Because the analysis in the first section of this chapter suggests that single risk factor screening is not the most efficient strategy, these studies have not been included and they are not considered further. Five publications were identified through reference checking of related reviews and eight from other searches conducted for this report.
One hundred and thirty-four studies reported the association between maternal characteristics/risk factors and GDM, and 29 of these reported data on risk factors, 24 of which had sufficient data to allow inclusion in the analyses. Details of the identification process are presented in Figure 19.
FIGURE 19.
The search process.

Quality assessment and risk of bias
All included studies were observational, consisting of a mix of prospective and retrospective cohort studies. All studies used an OGTT to diagnose GDM, and all specified the diagnostic criteria used. Criteria varied between studies therefore there are differences in the thresholds used to define GDM. As discussed in Chapters 2–4 of this report; different criteria thresholds can influence GDM prevalence. All of the risk factors examined in this review are simple observable maternal characteristics/risk factors; the assessment of whether or not a risk factor is present therefore is unlikely to be subject to substantial measurement or reporting error or bias.
Studies were diverse in their included populations (see Table 18). This heterogeneity limits the ability to draw conclusions across studies and generalise findings.
Studies of multiple risk factor screening
Of the 29 included studies,93,122,139–165 24 provided sufficient data for screening performance (sensitivity, specificity) to be calculated; these 24 studies are summarised in Table 18. The studies were conducted in a variety of countries and used different criteria for diagnosing GDM; therefore different studies will produce different GDM prevalences. Six studies93,122,139,143,146,157 assessed the screening performance of existing guideline recommendations [NICE, ADA, American College of Obstetricians and Gynecologists (ACOG), ADIPS, Irish, French]. Seven studies93,122,139,142,143,146,157 counted the number of risk factors for each woman. Six studies140,141,149,151,155,164 used a risk prediction model or a risk score to determine the results of risk factor screening and five studies148,150,152,158,165 examined various risk factors.
| Study | Year | Country | GDM diagnosis criterion | Total women | No. with GDM | Risk factor screening strategy |
|---|---|---|---|---|---|---|
| Avalos122 | 2013 | Ireland | IADPSG | 5500 | 681 | Irish guideline recommendations |
| Caliskan142 | 2004 | Turkey | NDDG | 422 | 14 | Number of risk factors |
| Cosson143 | 2013 | France | WHO | 18,755 | 2710 | French guideline recommendations |
| Cypryk144 | 2008 | Poland | WHO | 2180 | 510 | Number of risk factors |
| Danilenko-Dixon146 | 1999 | USA | NDDG | 18,504 | 564 | ADA guideline recommendations |
| Jensen145 | 2003 | Denmark | DPSG | 2992a | 83 | Number of risk factors |
| Jiménez-Moleón93 | 2002 | Spain | NDDG | 1962 | 65 | ADA and ACOG guideline recommendations |
| Marquette147 | 1985 | USA | C&C | 434 | 12 | Number of risk factors |
| Moses148 | 1998 | Australia | ADIPS | 2907 | 183 | Age, BMI, ethnicity |
| Nanda149 | 2011 | UK | WHO | 11,464 | 297 | Risk model |
| Naylor164 | 1997 | US | NDDG or C&C | 1571 | 69 | Risk score |
| Ostlund150 | 2003 | Sweden | WHO | 3616 | 61 | ‘Traditional risk factors’ |
| Phaloprakam151 | 2009 | Thailand | C&C | 469 | 127 | Risk score |
| Pintaudi152 | 2014 | Italy | IADPSG | 1015 | 113 | ‘Standard risk factors’ |
| Sacks153 | 1987 | USA | ADA | 4116 | 138 | Number of risk factors |
| Savona-Ventura165 | 2013 | Mediterranean | ADA | 1368 | 119 | Based on age, BMI and diastolic BP |
| Shamsuddin154 | 2001 | Malaysia | OGTT levels reported | 768 | 191 | Number of risk factors |
| Shirazian155 | 2009 | Iran | ADA | 924 | 68 | Risk score |
| Sunsaneevithayakul156 | 2003 | Thailand | Not reported | 9325 | 235 | Number of risk factors |
| Teh157 | 2011 | Australia | ADIPS | 2426 | 250 | NICE, ADA and ADIPS guideline recommendations |
| Van Leeuwen141 (A) | 2010 | Netherlands | OGTT/GCT levels reported | 995 | 24 | Risk model |
| Van Leeuwen140 (B) | 2009 | Netherlands | WHO | 1266 | 47 | Risk score |
| Williams158 | 1999 | US | NDDG | 25,118 | 148 | Based on age, BMI, ethnicity, family history |
| Yang139 | 2002 | China | WHO | 9471 | 171 | ADA guideline |
Figure 20 shows the estimates of sensitivity and positive rate for the studies in Table 18 plotted against each other in ROC space. In Figure 20 (see also Figure 21) the shape of the points indicates the type of screening method used (● = existing guidelines; ▲ = counting numbers of risk factors; + = use of a risk prediction model or score; and ▪ = other methods).
FIGURE 20.
Screening performance (sensitivity and positive rate) for the included studies.
Figure 21 presents the results for studies93,122,139,143,146,157 reporting the performance of current screening guidelines. The vertical lines here show the 95% CIs for sensitivity and positive rate.
FIGURE 21.
Screening performance of existing risk factor screening guidelines.
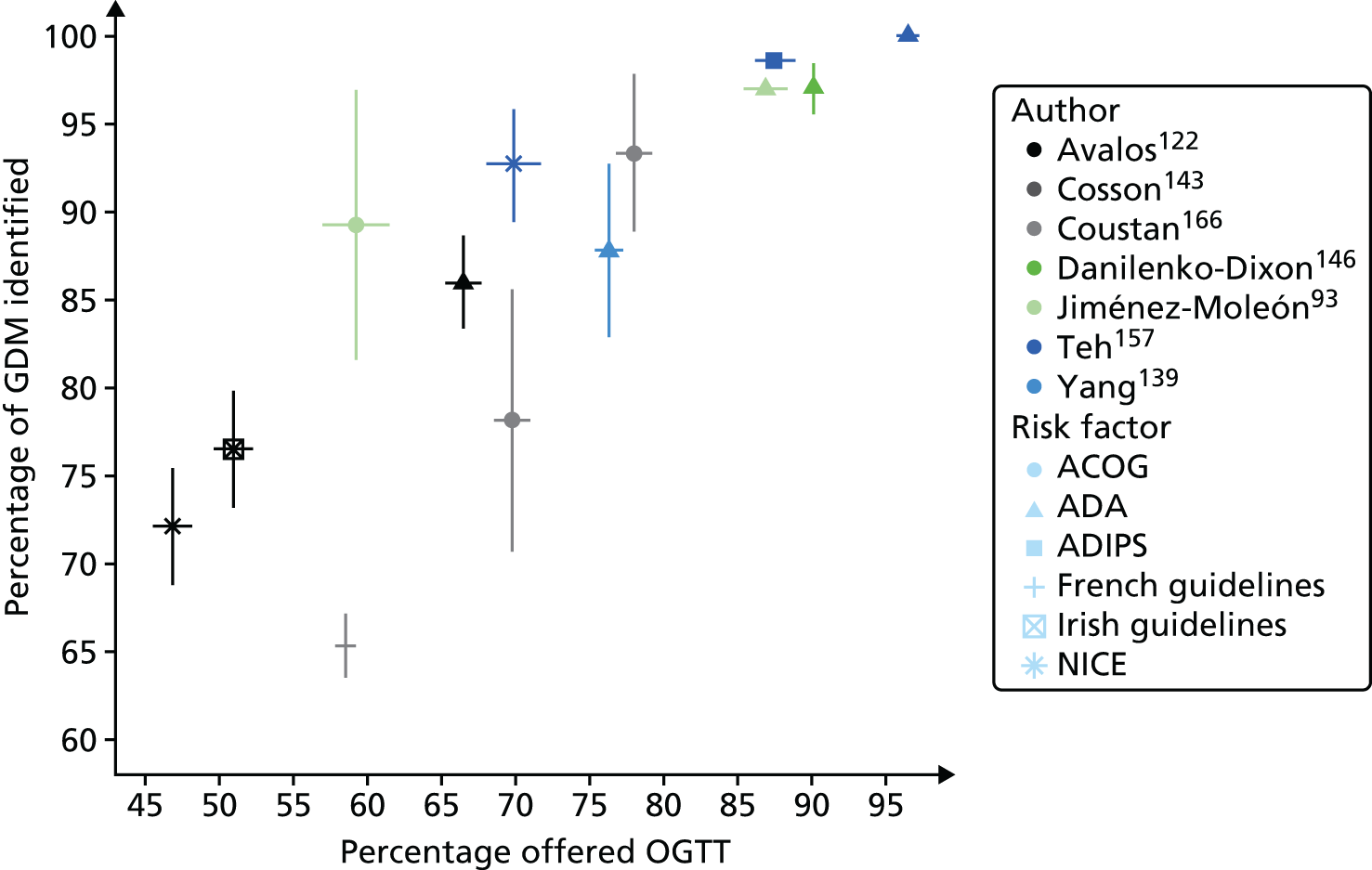
Figure 22 shows the sensitivity and specificity for those studies140,141,149,151,155,164 evaluating risk prediction models or risk scores. Each study has multiple points because the studies reported results at various levels of risk. Results are reasonably consistent across studies, with all points lying approximately on a common ROC curve, suggesting that no specific risk scoring method is superior to another. Increasing sensitivity reduces specificity, for example to achieve a sensitivity of 80%, specificity is approximately 45%; to achieve a sensitivity of 90%, the specificity is approximately 35%. So to identify greater numbers of women with GDM requires offering an OGTT to increasing numbers.
FIGURE 22.
Screening performance of risk prediction or scoring models.

Conclusions reported by the study authors
We examined the conclusions drawn by the authors for each included paper to determine whether or not the study authors recommended maternal characteristics/risk factor (selective) testing or universal testing (with OGTT). Appendix 4, Table 66, presents a summary of the conclusions for all included studies. This includes the 24 studies included in the analyses above and the five remaining studies that did not have extractable data. The conclusions of the study authors are varied, with 11 favouring universal diagnostic testing and 10 supporting some form of maternal characteristic/risk factor screening (universal screening and selective testing). Eight of the study authors made no firm recommendations. Of those studies that investigated current screening guideline recommendations (eight studies), seven did not recommend risk factor screening, three favoured universal diagnostic testing and four were undecided.
Studies without extractable data
Five studies159–163 considered multiple risk factor screening, but reported insufficient data to be included. These studies are briefly described below.
-
Corcoy et al. 159 (2004, Spain) examined the presence of multiple ‘low’ risk factors (e.g. ethnicity and BMI of < 25 kg/m2) in a general pregnancy cohort and in a cohort of women with GDM. Women with GDM were less likely to have known ‘low’ risk factors for GDM than women with GDM (7% vs. 1.3%). Although selective screening seems to reliably identify low-risk women, only 7% of women in this population would not require screening.
-
Cosson et al. 163 (2006, France) compared two different strategies in different time periods for identifying GDM: selective and universal testing using a 75-g OGTT. Risk factors were reported by year and adverse outcomes were reported for the women with GDM by year, but not for the whole population.
-
Crete and Anaste160 (2012, USA) examined age, BMI, ethnicity, family history of diabetes and prior GDM, and previous macrosomic infant, and reported that age 30–34 years and BMI of > 30 kg/m2 doubled the risk of GDM; risk increased fourfold in those with previous GDM. Women who were both older and heavier had a higher risk than women with a single risk factor, but not significantly so.
-
Davey and Hamblin161 (2001, Australia) compared risk factor prevalence of women with GDM to those without, and presented percentages and ORs for these two groups.
-
Göbl et al. 162 (2012, Austria) presented results for risk screening in combination with FPG, and concluded that the combination may be useful in screening for GDM.
Discussion
Risk factor screening aims to identify as many ‘at risk’ women as possible so that diagnostic testing can be offered to those most likely to test positive, while preventing unnecessary testing in those least likely to test positive. A screening test with high sensitivity and specificity is therefore beneficial. Screening for GDM based on risk factors can take a variety of forms, including offering an OGTT to women with just one risk factor important in that population, or with one or more risk factors out of a set, which is the approach recommended by several institutions providing guidance, including NICE,19 or by calculating a predicted risk or risk score. This review identified 29 eligible studies, 93,122,139–165 which were methodologically diverse. We were unable to demonstrate superiority of any one strategy over another.
Certain risk factors are increasing in the pregnant population (e.g. obesity and advanced maternal age) therefore in the future it may be that the majority of women will have at least one risk factor and fulfil many criteria for diagnostic testing. Risk factor screening in such a population would require most women to be tested, but a proportion of women with hyperglycaemia/GDM would still be missed. Screening women for risk factors makes additional demands on consultation time and risk factors may not be recognised; however, even although they may be relatively easy to identify. Generally, the risk factor screening strategies examined in this review use only maternal characteristics; occasionally, however, as in the Nanda et al. study149 biochemical markers are included; these may increase screening complexity and costs, without necessarily improving case detection rates.
Performance of risk factors
We found that all of the risk factors we examined, excluding multiparity, were associated with increased odds of GDM. Multiparity does not seem to be linked to increased insulin resistance during pregnancy, but is associated with GDM through the mediation of progressive ageing and weight gain. 167 The BiB study22 has relatively fewer older women included – 11.5% aged ≥ 35 years at delivery (see Tables 3 and 13) – therefore for the BiB population this mediated effect seems to have been lost, although we have not examined this formally. Regardless of the method used, risk factors to identify GDM generally have poor screening performance. Unfortunately, if risk factor sensitivity is high, specificity is low; therefore, to identify > 50% of GDM cases, > 50% of women need to be offered an OGTT; to identify 80% of women with GDM, around 60% need to be offered an OGTT; and to identify 90% of women, around 70% need to be offered an OGTT. These numbers may vary, depending on the prevalence of GDM in the population, but our analyses using IPD suggests not significantly so. Our analyses using IPD also suggests that offering an OGTT to everyone aged > 25 years will identify 86% of GDM cases, but nearly 68% of women will receive the test. Although 68% is a considerable proportion of women to test, this strategy would avoid testing in 32% of the population and this may equate to a considerable cost saving. Our results were consistent across the IPD cohorts and the included published studies. Risk factor screening could therefore avoid the need for an OGTT in 20–30% of women at lowest risk of GDM. Using this strategy would lead to some women with GDM not being identified; these women would therefore not benefit from treatment. Considering other risk factors, other than age or BMI category, such as previous macrosomia or family history of diabetes, adds little value, because their addition does not seem to identify additional GDM cases.
The magnitude of risk associated with GDM in a subsequent pregnancy following a pregnancy complicated by GDM suggests that all women with a previous pregnancy complicated by GDM should be offered an OGTT. This would not increase the number of women being offered an OGTT substantially because the prevalence of this risk factor is relatively low, although numbers are increasing. Our results suggest that the use of age or BMI category to identify those at increased risk is as effective as using multiple risk factors and the use of this latter strategy may overcomplicate the screening process. Offering all women an OGTT may avoid missed cases through selection; however, the uptake of a universal offer of OGTT is between 63% and 75% depending on population and, therefore, a universal offer will also miss cases. 124,137,150 Furthermore, offering an OGTT to all women may unnecessarily ‘medicalise’ some pregnancies at relatively lower risk, and this may adversely affect the woman’s experience and possibly increase the risk of medical interventions such as induction of labour and C-section.
Performance of different guideline recommendations
Analysis examining the performance of guideline recommendations was unable to demonstrate superiority of one set of recommendations over another, although the number of included studies was few. Screening performance of recommendations varies across studies. For example, if the ADA guideline recommendations are used to selectively screen in the third trimester they would identify between 86% and 100% of GDM cases, but between 66% and 96% of women would be offered an OGTT (depending on population characteristics). How recommendations are implemented in practice will also affect performance. For example, the ADA recommend selective screening using risk factors in the first trimester, but suggest that an OGTT should be offered in the third trimester to all women not identified as having diabetes in the first trimester. Therefore, all women are offered an OGTT at some point in pregnancy. Guideline performance varies, some identifying more cases than others. Those with higher case identification generally require that greater numbers of women are tested. Depending on the guideline used, the strategy adopted and the population characteristics, detection can be anything from 65% to 100% of GDM cases.
Performance of risk prediction models
We presented a model in Table 16 that could be used to calculate the odds of having GDM for any woman provided her characteristics are known (i.e. age, BMI, family history of diabetes). These estimated odds can be converted into an estimated risk of GDM. This risk could be used to screen for GDM, by offering an OGTT to any woman with a risk score above a prespecified threshold, for example 5%. Using a low risk cut-off will identify more GDM cases, but will also lead to more women having an OGTT. A higher threshold will reduce the number of OGTTs performed, but will identify fewer GDM cases. Of the 10 studies that recommended risk factor screening, six were studies140,141,149,151,155,164 that proposed a new risk prediction model or scoring system (using risk factors). However, our analysis using IPD suggests that using a risk algorithm or prediction score does not substantially improve performance compared with identifying one or two risk factors, and is similar to using multiple risk factors (over one or two risk factors). The extra complexity of the risk prediction model may therefore complicate screening while adding little to performance.
Conclusions
Our analyses suggest that no single method of risk factor screening is better overall. Risk factor screening based on having one or more risk factors and methods based on risk prediction or scoring performed similarly, suggesting that if risk factor screening is to be used, the simpler approach of offering an OGTT if at least one risk factor is present may be preferable and this is the recommended approach of several institutions including NICE. 18
The potential benefits of offering universal testing must be weighed against any adverse effects and costs. Taken in this context the most efficient method of identifying women with GDM is likely to differ between populations. For high-risk populations in which the majority of women have a risk factor, especially a BMI of > 30 kg/m2 or advanced maternal age, universal testing may be most beneficial. For a young population of women with few risk factors, selective testing may be best; the use of risk factors in this population could be used to identify those at low risk who do not need testing and those remaining would be therefore offered an OGTT.
Chapter 6 Treatments for gestational diabetes: a systematic review
Introduction
As discussed throughout this report GDM is associated with an increased risk of several important perinatal adverse outcomes, including C-section and macrosomia (BW of > 4 kg) and there is growing evidence that longer-term health of both mother and infant may also be adversely affected.
Treatment of GDM aims to control hyperglycaemia, which, in turn, aims to reduce the risk of adverse outcomes. Diet and lifestyle modification may be used as first-line treatment and if partly or wholly unsuccessful, or where women have substantially elevated glucose level at diagnosis, pharmaceutical interventions (metformin, glibenclamide and/or insulin) may also be given. Certain oral hypoglycaemic agents including metformin and glibenclamide present a possible alternative to injected insulin and may be as effective with the added benefit of being more acceptable to women.
This chapter reports a systematic review investigating the effectiveness of treatments for GDM to improve maternal and infant health outcomes.
Methods
Search strategy
This review updates five existing systematic reviews of treatments for GDM: Alwan et al. (Cochrane review),168 Hartling et al. (Annals of Internal Medicine 2013),1 Horvath et al. (BMJ 2010),169 Falavigna (Diabetes, Research and Clinical Practice 2012)170 and Gui et al. (PLOS ONE). 171 The search strategies of all five reviews1,168–171 included randomised controlled trials (RCTs), one review also included observational studies which were ineligible in our review and therefore these studies were not considered. 1 One review compared the effects of metformin with insulin only,171 the four remaining reviews compared any treatment for GDM. It is likely that search strategies and assessment of eligibility differed between the reviews (these details are not published); however, the reviews seemed to take a broad approach to potential inclusion (RCTs, women with GDM and any treatments) and generally excluded and included the same trials, although there were slight variation when multiple publications of the same trial were identified.
The search strategies (see Appendix 7, Table 86) were designed to identify records of RCTs added to search sources since the most recent search date of the review by Alwan et al. 168 (July 2011). Strategies were developed using a combination of subject indexing terms and free-text search terms in the title and abstract fields, to identify relevant trials related to GDM and impaired glucose tolerance (IGT) in pregnancy. Where database functionality allowed, results were limited to records added to the database since 2011, using appropriate fields such as the entry date field in MEDLINE. Searches were first conducted in September 2013 and updated in October 2014 using the same search strategies. The databases searched were MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations, EMBASE, and CENTRAL. Results of the searches were downloaded into EndNote X7 bibliographic management software and duplicate records were removed using several algorithms. In addition to database searches, reference checking of included journal articles and related systematic reviews were undertaken. For full details of all database search strategies, including interfaces used, search dates and result numbers, see Appendix 7, Table 86.
All trials included in the existing systematic reviews were obtained. Title and abstract screening and then full-text screening were performed by two reviewers with disagreements resolved by consensus, or by a third reviewer.
Inclusion/exclusion criteria
This review identified RCTs in which a treatment designed to lower blood glucose in women with GDM was examined in comparison with routine or standard antenatal care or an alternative treatment designed to lower blood glucose. The other inclusion criteria were as follows.
Population
Pregnant women diagnosed with GDM or IGT using any threshold definition and women with pre-existing diabetes were excluded.
Intervention
The treatment could be any one or more of the following:
-
insulin
-
metformin
-
glibenclamide
-
dietary advice and diet modification with or without additional lifestyle modification (e.g. exercise) or monitoring
-
any combination of the above.
Comparator
The comparison group could receive ‘standard/routine obstetric care’ (however defined by the trial) or any of the above treatments.
Outcomes
Trials had to report incidence of adverse outcomes, for example RRs, ORs or mean differences (MDs) for outcomes compared across treatment groups for at least one of the following, which could be defined variously by the trials:
-
gestational age at birth
-
BW
-
macrosomia (BW of ≥ 4 kg)
-
LGA (BW of > 90th centile)
-
shoulder dystocia
-
preterm birth < 37 weeks’ gestation)
-
neonatal hypoglycaemia
-
admission to neonatal intensive care unit (NICU)
-
C-section (elective or emergency)
-
pre-eclampsia
-
PIH
-
induced labour
-
instrumental birth (forceps or vacuum/ventouse)
-
Apgar score at 5 minutes
-
negative treatment effects (e.g. gastrointestinal upset, well-being).
Trials
Only RCTs were eligible. Blinding of clinicians or researchers (to the intervention) or those assessing outcome data was not part of the inclusion criteria. Conference abstracts of RCTs and letters to journals were eligible for inclusion if they reported sufficient information.
Quality assessment
The risk of bias of the included trials was assessed using the Cochrane risk of bias tool,172 which considers the following characteristics:
-
sequence generation
-
allocation concealment
-
blinding of participants and medical staff to treatment allocation
-
blinding of the assessors of outcomes to the treatment allocation
-
completeness of outcome reporting (e.g. loss to follow-up)
-
selective reporting of outcomes
-
Other sources of bias (not addressed by the above domains).
Each criterion was classified as being at low, high or unclear risk of bias. One reviewer performed the quality assessment, which was checked by a second.
Data extraction
Data were extracted from each publication on the following:
-
maternal age at randomisation
-
gestational age at randomisation and/or oral glucose tolerance testing
-
ethnicity
-
BMI
-
what test, and diagnostic criteria were used to diagnose GDM
-
details of treatment and control used.
Statistical data for each reported outcome were extracted, when reported:
-
numbers of women in treatment and control groups
-
numbers of morbidities in each group
-
OR or RR for comparison between groups (with 95% CIs)
-
mean and SD for the outcome in each group.
One reviewer performed the data extraction, which was checked by a second reviewer.
Synthesis methods
Meta-analyses
For the statistical analysis, the included trials were divided into the following categories according to the included treatments:
-
insulin vs. metformin
-
insulin vs. glibenclamide
-
metformin vs. glibenclamide
-
diet or dietary advice and or lifestyle vs. pharmacological (glibenclamide, metformin or insulin) treatment
-
diet or dietary advice and/or glucose monitoring and/or insulin use vs. routine antenatal care.
The results of trials comparing different types of insulin and different types of diet were not pooled because of their diversity and were reviewed narratively.
For dichotomous outcomes the RR for each outcome comparing each trial arm, with its 95% CI, was calculated from the numbers of women with the outcome. For continuous outcomes the MD between trial arms, with its 95% CI, was calculated from the mean and SD of the outcome.
For each outcome, and within each of the four treatment categories listed above, RRs or mean differences were pooled in random-effects DerSimonian and Laird meta-analyses. Heterogeneity was assessed using Higgins I2-statistic. Subgroup analyses were performed to investigate differences across varying definitions of GDM.
Network meta-analysis
This review examines a number of different treatments for GDM. Rather than comparing just two treatments, as in the meta-analyses undertaken in previous reviews, network meta-analysis was used to combine information across multiple treatments simultaneously. Information on the effectiveness of a treatment can be obtained directly, for example by comparing glibenclamide and metformin in those trials that included these treatments, or indirectly, for example by examining the effects of insulin compared with metformin, and insulin compared with glibenclamide in order to compare glibenclamide and metformin. Network meta-analysis combines this direct and indirect evidence to improve the estimation of the effectiveness of treatments. 173 Formally, analyses were conducted for each dichotomous outcome using a Bayesian approach, based on the models originally created by Lu and Ades,174 using the OpenBUGS software: www.openbugs.net/w/FrontPage (last accessed January 2015). Each model generated a comparison between treatments, expressed as an OR and a probability that each treatment was the best treatment to reduce the incidence of the outcome.
Network meta-analysis was performed to compare insulin versus metformin versus glibenclamide.
Results
Existing reviews
This review updates five existing systematic reviews (see Chapter 5, Methods). Here we present a short summary of the existing reviews.
Alwan et al. 2009
This Cochrane review168 included RCTs examining any treatment for GDM, including diet and lifestyle modification and drug treatments (such as metformin and insulin) in addition to routine antenatal care, against any treatment or routine care. A range of outcomes were considered, including all of those included in our review. The Cochrane risk of bias tool was used to assess trial quality and trials were synthesised in meta-analyses. Database searches were most recently performed in 2011; these trials are still awaiting classification. This Cochrane treatments review168 has now been divided into separate intervention comparisons and these separate reviews are now being conducted.
The current review, however, includes eight trials, involving 1418 women, of which five are included in our review. This Cochrane review concluded that treatments, including dietary advice and insulin were effective in lowering the incidence of a range of outcomes (pre-eclampsia, macrosomia, LGA and shoulder dystocia) compared with routine antenatal care. Induction of labour was more common in the treated group than in those having only routine antenatal care. The review found evidence of a reduction in the risk of C-section for women receiving oral hypoglycaemic agents compared with insulin. The review suggested that conclusions may change when the review is updated to incorporate the 29 citations awaiting classification. Ten of the trials awaiting classification have been included in our review and are indicated by the solid black triangles in Table 14.
Hartling et al. 2013
Five RCTs involving 2945 women and six observational studies involving 3110 women were included in this review1 comparing diet and lifestyle modification, glucose monitoring and insulin as needed to routine antenatal care. Database searches were performed in 2012. All five trials were included in our update review (see Table 14). The Cochrane risk of bias tool was used to assess trial quality, and trials were synthesised in meta-analyses. The review found that treatment lowered the risk of pre-eclampsia, shoulder dystocia and macrosomia, but data were too limited to be confident about the effects on gestational weight gain and longer-term health outcomes.
Horvath et al. 2010
Five trials were included, involving 2999 women, comparing a specific treatment, which included diet and lifestyle modification and insulin as needed to routine antenatal care. All five trials were included in our review (see Table 14). This review169 also included 13 observational studies (not included in our review) comparing more intensive specific treatment to less-intensive specific treatment; this comparison was not considered in our review. Database searches were performed in 2009. The Cochrane risk of bias tool was used to assess trial quality, and trials were synthesised in meta-analyses. The review found that treatment was effective in lowering the risk of shoulder dystocia and LGA.
Falavigna et al. 2012
Seven trials were included involving 3157 women, comparing diet and lifestyle modification and insulin as needed to routine antenatal care. Six of these trials were included in our review (see Table 14). Database searches were performed in 2012. The Cochrane risk of bias tool was used to assess trial quality and trials were synthesised in meta-analyses. The review170 reported that treatment lowered the risk of LGA, macrosomia, pre-eclampsia and shoulder dystocia.
Gui et al. 2013
Five trials, involving 1270 women, were included comparing metformin to insulin; all were included in our review (see Table 14). Database searches were performed in 2012. The Cochrane risk of bias tool was used to assess trial quality and trials were synthesised in meta-analyses. The review171 found that gestational weight gain and gestational age at birth were lower and PIH occurred significantly less often, but preterm birth occurred more often for women who were treated with metformin than in those treated with insulin.
Included trials
Trial publications from the five identified systematic reviews1,168–171 were obtained for screening. The two searches in September 2013 and October 2014 identified 6450 citations (2985 and 3555 citations, respectively). Following deduplication of titles (and abstracts where available), 3645 citations were reviewed (including citations of trials included in the previous reviews). Of these, 158 were judged potentially eligible based on title and abstract. After obtaining the full text and assessing eligibility, 48 trials were included (46 in the meta-analyses: two trials reported insufficient data to allow inclusion) and 110 were excluded (see Appendix 5, Table 67). The full details of the search process are presented in the flow chart (Figure 23).
FIGURE 23.
Flow chart of the search process.

Of the included trials, 23175–195 compared drug treatments: 10 trials175–183,196 compared metformin with insulin, eight trials184–191 compared glibenclamide with insulin, two trials192,193 compared glibenclamide with metformin, one trial compared a metformin–glibenclamide combination with insulin195 and one trial194 compared glibenclamide in addition to diet therapy with placebo in addition to diet therapy. Ten trials51,52,197–203 compared combinations of diet modification, glucose monitoring and insulin use to routine obstetric care. Five trials204–208 compared different insulin formulations. Of the remaining nine trials,200–202,209–214 five trials201,202,211–213 were comparisons of different diets. One trial200 compared types of dietary education, one trial214 compared diet to insulin, one trial210 compared exercise to insulin, and one trial209 compared exercise to diet. None of these nine trials200–202,209–214 was included in any meta-analysis because of trial diversity. The included trials are summarised in Table 19.
| First author | Year | Recruitment location | Population | BMI, kg/m2 | Age: year (mean or range) | Gestational age (weeks) | Glucose test and/or criteria used | Intervention group | Control group | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbassi-Ghanavati194 | 2014 | USA | 395 | 24-30 | NDDG | Glyburidea | Placebo | BW | ||
| Anjalaksh184 | 2007 | India | 23 | 22–25 | 24–27 | 22 | WHO | Glibenclamide (n = 10) | Insulin (n = 13) | BW |
| Ardilouze214 | 2014 | Canada | 63 | 31 | Not reported | Metformin/glyburidea | Insulin | Glycaemic control Mode of birth BW Gestational age |
||
| Asemi215 | 2014 | Iran | 52 | 29–31 | 31–32 | 26 | ADA | DASHb | Control | BW C-section Need for insulin Macrosomia Polyhydramnios Gestational age at birth |
| Balaji195 | 2012 | India | 323 | ≤ 35 | 20–30 | 12 to 28 | 75-g OGTT | Analogue insulin Aspart BIAsp 30 (n = 163) | Human insulin Aspart BHI 30 (n = 160, 157 completed) | C-section Gestational age at birth Preterm birth Pre-eclampsia Stillbirth LGA Apgar score at 5 minutes |
| Bertini185 | 2005 | Brazil | 70 | 25–27.5 | 28–31 | 11–33 weeks | 75-g OGTT | Insulin (rapid acting human) (n = 27) | Glyburidea (5–20 mg) (n = 24), 5 insulin Acarbose (50–300 mg) (n = 19), 8 insulin |
Gestational age at birth Apgar score at 5 minutes LGA > 90th percentile BW Special care C-section |
| Bevier 202 | 1999 | USA | 103 | 26–27 | 1-hour OGCT | Low-calorie diet with glucose monitoring (n = 35), 1 insulin | Routine care (n = 48), 4 insulin | Gestational age at birth Induced labour Assisted birth C-section Shoulder dystocia Apgar score at 5 minutes BW Macrosomia > 4kg Pre-eclampsia |
||
| Bo216 | 2014 | Italy | 200 | 75-g OGTT, criteria not reported | Combination of either diet and exercise plus behavioural recommendations | Compared with different combination of either diet and exercise plus behavioural recommendations | Maternal weight BMI Glycaemic control Cholesterol Insulin |
|||
| Bonomo203 | 2005 | 300 | 23 | 31 | 24–28 | 100-g OGTT C&C | Standard treatment or diet and monitoring | Control | Glycaemic control Gestational age BW Macrosomia LGA SGA Ponderal index Hypoglycaemia Hyperbilirubinaemia Admission to NICU |
|
| Bung204 | 1991 | USA | 41 | 32 | 31–32 | 30.3 weeks average | Fasting glucose 5.88–7.22 mm | Exercise (supervised exercise bike use) (n = 21), 4 insulin | Insulin (n = 20) | Gestational age at birth Vacuum/forceps C-section BW Macrosomia Hypoglycaemia Premature labour Gestational age at birth |
| Cao217 | 2012 | China | 275 | 26.56 and 27.21 | 30 | 30 | unclear | Comprehensive individual education (n = 127) | Standard group education (n = 148) | Macrosomia > 4 kg BW Respiratory distress Stillbirth Neonatal death Preterm birth Congenital malformation Admission to neonatal nursery Gestational age at birth Induced labour C-section Pre-eclampsia Insulin needed |
| Crowther51 | 2005 | Australia and UK | 1000 | Median 26.8 and 26 | 30.5 | 24–34 (IQR 28–30) | 75-g OGTT WHO 1999 | Dietary advice, monitoring, insulin as needed (n = 490) | Routine care (n = 510) | Perinatal complication Stillbirth Neonatal death Shoulder dystocia Admission to neonatal nursery Induction C-section (elective or emergency) BW LGA > 90th percentile Macrosomia > 4 kg Apgar score at 5 minutes Hypoglycaemia Respiratory distress Gestational age at birth Pre-eclampsia |
| Cypryk209 | 2007 | Poland | 30 | 28.7 | Unclear | High-carbohydrate diet | Low-carbohydrate diet | Gestational age at birth C-section BW Macrosomia > 4 kg Apgar score at 5 minutes |
||
| Deveer197 | 2013 | Turkey | 100 | 28 | 30 | High 50-g GCT (not full GDM) | Individualised diet advice (n = 50) | Routine care (n = 50) | BW Gestational age at birth LGA Macrosomia (> 4 kg) C-section Preterm Neonatal intensive care admission Pre-eclampsia |
|
| Di Cianni210 | 2007 | Italy | 96 | 27.5 weeks | Unclear | Insulin aspart, (n = 31), 16 bed-time NPH insulin | Insulin lispro (n = 33), 18 bed-time NPH insulin Insulin (human regular) (n = 32) 23 bed-time NPH insulin |
Gestational age at birth BW Macrosomia Hypoglycaemia |
||
| Elnour200 | 2006 | United Arab Emirates | 180 | 30.9 | 8–19 weeks | Unclear | Best treatment, advice, monitoring (n = 108) | Routine care (n = 72) | Hyperglycaemia Pre-eclampsia/toxaemia Preterm birth Eclampsia C-section Post-partum haemorrhage Neonatal hypoglycaemia Respiratory distress Macrosomia LGA Pre-term birth Shoulder dystocia Congenital abnormality Occasional insulin Daily insulin |
|
| Garrner201 | 1997 | Canada | 299 | 30 | 24–32 | 75-g OGTT/study specific criteria | Diet, monitoring, insulin (n = 149) | Routine care (n = 150) | BW Macrosomia (> 4 kg) Neonatal hypoglycaemia C-section Gestational age at birth Stillbirth, neonatal deaths or congenital abnormalities Birth trauma |
|
| Hague176 | 2003 | Australia | 30 | 37.9 and 39.5 | 34.1 and 33.7 | 27.6 and 25.8 | ADIPS | Metformin | Insulin | BW Macrosomia Cord glucose Cord C-peptide Neonatal intravenous dextrose Jaundice |
| Hassan175 | 2012 | Pakistan | 150 | 29 | 30 | 20–35 | 75-g OGTT/WHO | Insulin (n = 75) | Metformin, (n = 75), 18 insulin | Induced labour C-section BW Macrosomia Apgar score at 5 minutes Neonatal intensive care admission Neonatal hypoglycaemia Respiratory distress |
| Ijäs177 | 2010 | Finland | 100 | 30–31 | 31–32 | 30 | 75-g OGTT | Insulin (protaphan and humalog) (n = 50) | Metformin, (n = 50), 16 insulin (protaphan/humalog) | Gestational age at birth BW Macrosomia LGA Apgar score at 5 minutes Neonatal intensive care admission Neonatal hypoglycaemia Induced labour Vacuum extraction C-section |
| Jovanovic218 | 1999 | USA | 42 (95% Hispanic, 5% white) | 31.5–33.3 | 30–34 | 25–27 | C&C (NDDG) 100-g OGTT | Insulin lispro (humalog) (n = 19) | Insulin, regular human (humulin) (n = 23) | C-section Gestational age at birth BW Apgar score at 5 minutes Neonatal hypoglycaemia Macrosomia |
| Kjos205 | 2001 | USA | 98 | 31.2 and 33.8 | 30–31 | 26.9 | Unclear | Insulin if AC > 70% or FPG > 120 (n = 49) | Insulin (n = 49) | Gestational age at birth BW Macrosomia LGA Neonatal morbidity Neonatal hypoglycaemia |
| Lain186 | 2009 | USA | 99 (≈5% black) | 30.9–33.4 | 31–32 | 30.6–30.8 | 1-hour OGTT | Insulin (n = 50) | Glyburidea 2.5 mg (n = 49) | Gestational age at birth BW Neonatal BMI LGA Macrosomia |
| Landon52 | 2009 | USA | 958 (57% Hispanic, 25% white, 11% black, 5% Asian) | 30 | 28–29 | 28 | 100-g OGTT | Diet intervention, Monitoring, Insulin if needed, (n = 485) | Routine care (n = 473) | Gestational age at birth Composite neonatal morbidity Neonatal hypoglycaemia Birth trauma BW Macrosomia LGA Pre-term birth Neonatal intensive care admission Respiratory distress Induced labour C-section Shoulder dystocia Pre-eclampsia or gestational hypertension |
| Langer187 | 2000 | USA | 404 (83% Hispanic, 12% white, 5% black) | 29–30 | 24–25 | OGTT | Insulin (n = 203) | Glyburidea (n = 201) | Gestational age at birth LGA BW Macrosomia Neonatal hypoglycaemia Neonatal intensive care admission Congenital abnormality Neonatal death Stillbirth Pre-eclampsia C-section |
|
| Li198 | 1987 | Hong Kong | 158 | 27–28 | 31 | 75-g OGTT positive on NDDG, negative on WHO | Diet 30–35 cal/kg and monitored blood sugar levels (n = 85) | Routine care (n = 73) | Gestational age at birth BW macrosomia LGA Apgar score of < 4 at 5 minutes Induction labour C-section Birth trauma Neonatal hypoglycaemia Perinatal death Congenital abnormality |
|
| Louie219 | 2011 | Australia | 99 (≈58% Asian, 36% white, 6% other) | 24 | 33 (range 26–42) | 26 (range 20–32) | 75-g OGTT, ADIPS | Low-GI diet (n = 50) | High-fibre, moderate-GI diet (n = 49) | Gestational age at birth BW LGA Macrosomia Emergency C-section |
| Mesdaghinia178 | 2013 | Iran | 200 | 27–28 | 30 (range 18–45) | 27.9–28.9 | OGTT load not reported | Insulin (NPH or regular) (n = 100) | Metformin (n = 100) Note: 22 excluded as needed insulin control |
BW Macrosomia LGA Jaundice Neonatal hypoglycaemia Respiratory distress Neonatal intensive care admission Shoulder dystocia Apgar score of < 7 at 5 minutes Pre-term birth |
| Moore179 | 2007 | USA | 63 (49% African American, 44% native American, 6% white) | 35–39 average | 27 | 27–28 | 100-g OGTT ADA | Insulin (n = 31) | Metformin (n = 32) | BW Macrosomia > 4.5 kg Apgar score at 5 minutes Neonatal intensive care admission Neonatal hypoglycaemia Respiratory distress Gestational age at birth C-section Shoulder dystocia Post-partum haemorrhage Neonatal hypoglycaemia Intrauterine fetal death |
| Moore192 | 2010 | New Mexico | 149 (89% Hispanic, 3% native American, 7% white, 1% African American) | 32 | 29–31 | 27–29 | 100-g OGTT C&C | Glyburidea 2.5 mg (n = 74) | Metformin 500 mg (n = 75) | Gestational age at birth BW Macrosomia Neonatal intensive care admission Neonatal hypoglycaemia Maternal hypoglycaemia Pre-eclampsia Shoulder dystocia C-section Apgar score of < 7 at 5 minutes |
| Moreno-Castilla206 | 2013 | Spain | 152 (97% white) | 25–26 | 32 (range 18–45) | 30 | OGCT Spanish criteria | Low-carbohydrate diet (n = 76) | Control diet (n = 76) | Gestational age at birth Insulin use Maternal hypertension C-section LGA Macrosomia Neonatal hypoglycaemia |
| Mukhopadhyay188 | 2012 | India | 60 | 23 | 26 | 27–28 | 75-g OGTT WHO | Glibenclamide 2.5 mg (n = 30) | Insulin (n = 30) | Gestational age at birth BW LGA Neonatal hypoglycaemia Congenital abnormality Fetal death Neonatal intensive care due to respiratory distress syndrome Macrosomia |
| Nachum207 | 1999 | Israel | 392 (56% Jewish) | 27 | 31–33 | 26 | 100-g OGTT NDDG | Insulin twice daily, with GDM (n = 136), with pre-GDM (n = 60) | Insulin four times, with GDM (n = 138); with preGDM (n = 58) | Gestational age at birth Maternal hypoglycaemia C-section Pregnancy induced hypertension BW Congenital abnormality LGA Macrosomia Apgar score at 5 minutes Neonatal hypoglycaemia Birth trauma Perinatal mortality |
| Niromanesh196 | 2012 | Iran | 160 | 27–28 | 30–31 | 20–34 | 100-g OGTT C&C | Metformin 500 mg 2 × day (n = 80) | Insulin (NPH) (n = 80) | Gestational age at birth Pre-eclampsia PIH Abruption Pre-labour, preterm rupture of membranes Shoulder dystocia C-section Emergency C-section Preterm birth BW LGA Macrosomia Apgar score at 5 minutes Neonatal intensive care admission Neonatal hypoglycaemia Birth defect |
| Ogunyemi189 | 2007 | USA | 97 (80% Hispanic, 15% African American) | 30–32 | Mean 24.6–28.1 | Unclear | Insulin (n = 49) | Glyburidea (n = 48) | Maternal hypoglycaemia Gestational age at birth C-section BW Neonatal hypoglycaemia Birth defects |
|
| O’Sullivan211 | 1966 | USA | 615 | 100-g OGTT study- specific criteria | Diet and insulin (n = 308) | Routine care (n = 307) | Macrosomia Congenital anomaly Prematurity Stillbirth Neonatal death |
|||
| Rae212 | 2000 | Australia | 124 | 38 | 30 | 28 | 75-g OGTT study-specific criteria | Low-calorie diabetic diet (n = 66) | Normal diabetic diet (n = 58) | Pre-eclampsia Induction Assisted birth Shoulder dystocia Gestational age at birth BW Macrosomia LGA |
| Rowan180 | 2008 | Australia and New Zealand | 751 (47% white) | 32 | 33 (range 18–45) | 30 (range 20–33) | 75-g OGTT ADIPS | Metformin 500 mg (n = 373) | Insulin (n = 378) | Respiratory distress Birth trauma Apgar score of < 7 at 5 minutes Preterm birth Neonatal intensive care admission Gestational age at birth BW LGA Maternal hypoglycaemia Pre-eclampsia Post-partum diabetes Maternal serious adverse events Stillbirth Congenital abnormalities Infection |
| Silva190 | 2007 | Brazil | 68 | > 18 | 11–33 | 75-g OGTT criteria not reported | Glibenclamide (n = 36) | Human insulin (n = 32) | Glycaemic control BW Macrosomia LGA Apgar score at 5 minutes Neonatal glucose, C-section |
|
| Silva193 | 2012 | Brazil | 200 | 28.6 | 31–32 | 26 (11–33) | 75-g OGTT WHO | Metformin (n = 104) | Glyburidea (n = 96) | C-section Gestational age at birth BW LGA Apgar score at 5 minutes Neonatal hypoglycaemia Neonatal intensive care admission Death |
| Spaulonci181 | 2013 | Brazil | 94 | 31–32 | 31–32 | 32 | 100-g OGTT ADA | Metformin 1700 mg (n = 47) | Insulin (human NPH) (n = 47) | Gestational age at birth BW Macrosomia Neonatal hypoglycaemia Respiratory distress Apgar score at 5 minutes LGA |
| Tempe191 | 2013 | India | 64 | 26–27 | 100-g OGTT C&C | Glyburidea 2.5 mg (n = 32) | Insulin (n = 32) | Stillbirth Neonatal hypoglycaemia Macrosomia Fetal distress Preterm birth Neonatal intensive care admission Maternal infection Pre-eclampsia Gestational age at birth BW |
||
| Tertti182 | 2013 | Finland | 217 | 28–29 | 32 | 30 | 100-g OGTT, Finnish criteria | Metformin 500 mg (n = 110) | Insulin (n = 107) | Pre-eclampsia PIH Gestational age at birth Induced labour Assisted birth C-section BW LGA Macrosomia > 4 kg Macrosomia Preterm birth Apgar score at 5 minutes Neonatal intensive care admission Neonatal hypoglycaemia |
| Thompson208 | 1990 | USA | 108 (13 excluded < 6 weeks) | 26 diet 27 diet and insulin |
100-g OGTT trial specific criteria | Diet and insulin (NPH/regular) (n = 45) | Diet only (n = 50) | C-section Gestational age at birth BW Macrosomia Shoulder dystocia Neonatal hypoglycaemia Perinatal death |
||
| Yang199 | 2003 | China | 150 | Unclear | Diet/exercise advice (n = 95) | Routine care (n = 55) | Fetal distress Macrosomia Shoulder dystocia Neonatal Infection Neonatal intensive care admission Instrumental birth Febrile morbidities Post-partum haemorrhage Mode of birth Neonatal hypoglycaemia |
|||
| Zinnat183 | 2013 | Bangladesh | 450 | Unclear | Metformin (n = 225) | Insulin (n = 225) | C-section Neonatal hypoglycaemia |
The trials included women with GDM who were diagnosed following a 75-g or 100-g OGTT, a variety of threshold criteria were used, including the Carpenter and Coustan criteria (C&C) or those of the NDDA,14 WHO,11 ADA136 and local guidelines (criteria were specified in the publications). 177,182 Inclusion criteria for women in the dietary modification trials were more varied. Some of these trials included women with a positive OGCT, but negative OGTT (therefore not diagnosed as GDM by usual criteria); some included women with ‘mild or borderline’ GDM (usually defined as having fasting glucose below the threshold, but post-load glucose above the threshold for diagnosing GDM) and women defined as having ‘IGT’ (although current diagnostic criteria would consider these women to have GDM).
Quality assessment
The quality assessment results are provided in Appendix 5, Table 68. In general, reporting of many aspects of trial quality was poor. The randomisation procedure was rarely described, and so its quality could often not be assessed, although all trials were described in the publications as being ‘randomised’ trials. Blinding of participants and medical staff was generally not reported, but as most of these trials include some administration of insulin, it is probable that most clinicians could not be blinded. Most trials had reasonably complete outcome data and loss to follow-up was low. Most trials presented results for all their prespecified outcomes; therefore selective reporting was assessed as minimal. The large number of possible outcomes, however, means that the possibility that some trials collected data on outcomes, but did not report them, cannot be ruled out.
Trials comparing metformin and insulin
There were 10 trials that compared metformin with insulin. 175–183,196 In general, women were eligible if they were unable to achieve glycaemic control with dietary and lifestyle modification. Therefore, there is the possibility that those included may have more severe insulin resistance or may be less compliant or find adhering to lifestyle interventions more difficult than those requiring only dietary intervention outside of a trial. The specific criteria for the addition of insulin are not reported in most trials, although some trials do report that supplemental insulin was prescribed if glycaemic control was not achieved by participants receiving metformin. It is possible that thresholds for what is defined as ‘good’ control differed between trials and between sites in multicentre trials. Included trials are summarised in Table 20. Criteria for diagnosing GDM vary across trials as does the screening strategy used; these differences should be considered when interpreting the results from the meta-analyses. Meta-analyses are shown by outcome; not all trials report each outcome.
| Reference | Year | Location | Population | Criteria used to diagnose GDM | Screening strategya |
|---|---|---|---|---|---|
| Hague176 | 2003 | Australia | 30 | ADIPS | Risk based |
| Hassan175 | 2012 | Pakistan | 150 | WHO | 50-g OGCT |
| Ijäs177 | 2010 | Finland | 100 | Reported in paperb | Risk based |
| Mesdaghinia178 | 2013 | Iran | 200 | ADA | 50-g OGCT |
| Moore179 | 2007 | USA | 63 | NDDG | 50-g OGCT |
| Niromanesh196 | 2012 | Iran | 160 | C&C | 50-g OGCT |
| Rowan180 | 2008 | Australia/NZ | 751 | ADIPS | Risk based |
| Spaulonci181 | 2013 | Brazil | 94 | ADA | Universal OGTT |
| Tertti182 | 2013 | Finland | 217 | Finnish criteriac (changed during trial) | Risk-based screening then OGTT or universal OGTT testing |
| Zinnat183 | 2013 | Bangladesh | 450 | Not reportedd | Not reportedd |
Because of the large volume of treatments and outcome comparisons, we have generally combined trials and presented pooled estimates in forest plots. We have provided an example of one outcome (usually macrosomia) and treatment comparison by individual trials (results across trials of all outcomes are available from the contact author on request).
Figure 24 shows the results of the meta-analysis of trials comparing metformin and insulin treatment and risk of macrosomia. There was no evidence of heterogeneity in this analysis (I2 = 2%).
FIGURE 24.
Metformin vs. insulin: macrosomia.
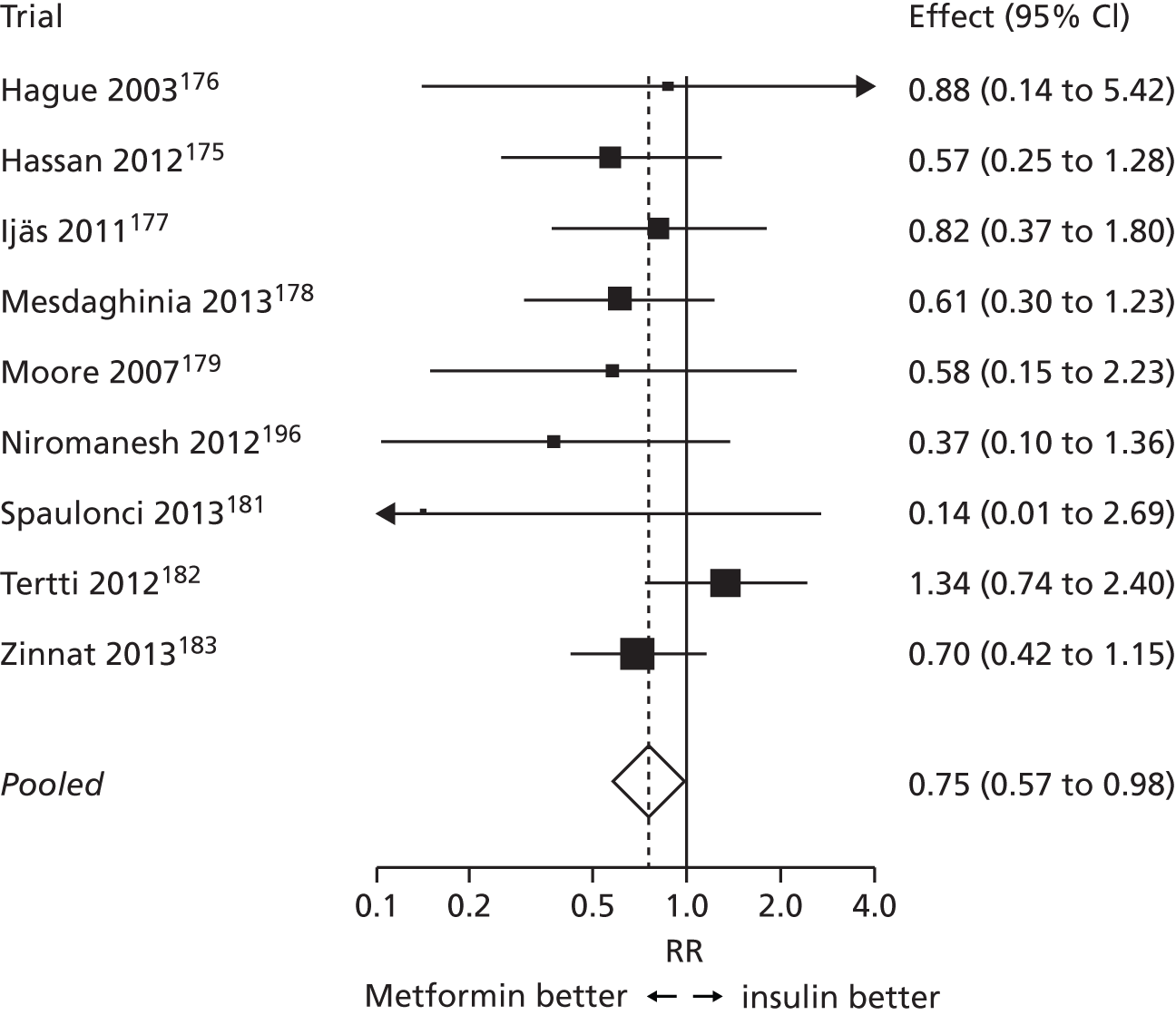
Figure 25 shows the results of the meta-analysis of trials for all dichotomous perinatal outcomes reported. There is a significantly reduced risk of macrosomia, neonatal hypoglycaemia and PIH (although for PIH this is marginal) for women given metformin compared with those who were given insulin. There is a suggestion that those who were given metformin rather than insulin are less likely to develop pre-eclampsia or have a baby admitted to the NICU, although results were not statistically significant. Metformin use was associated with a statistically significantly increased risk of instrumental birth (vaginal delivery by forceps or ventouse) compared with insulin use.
FIGURE 25.
Metformin vs. insulin: dichotomous outcomes.
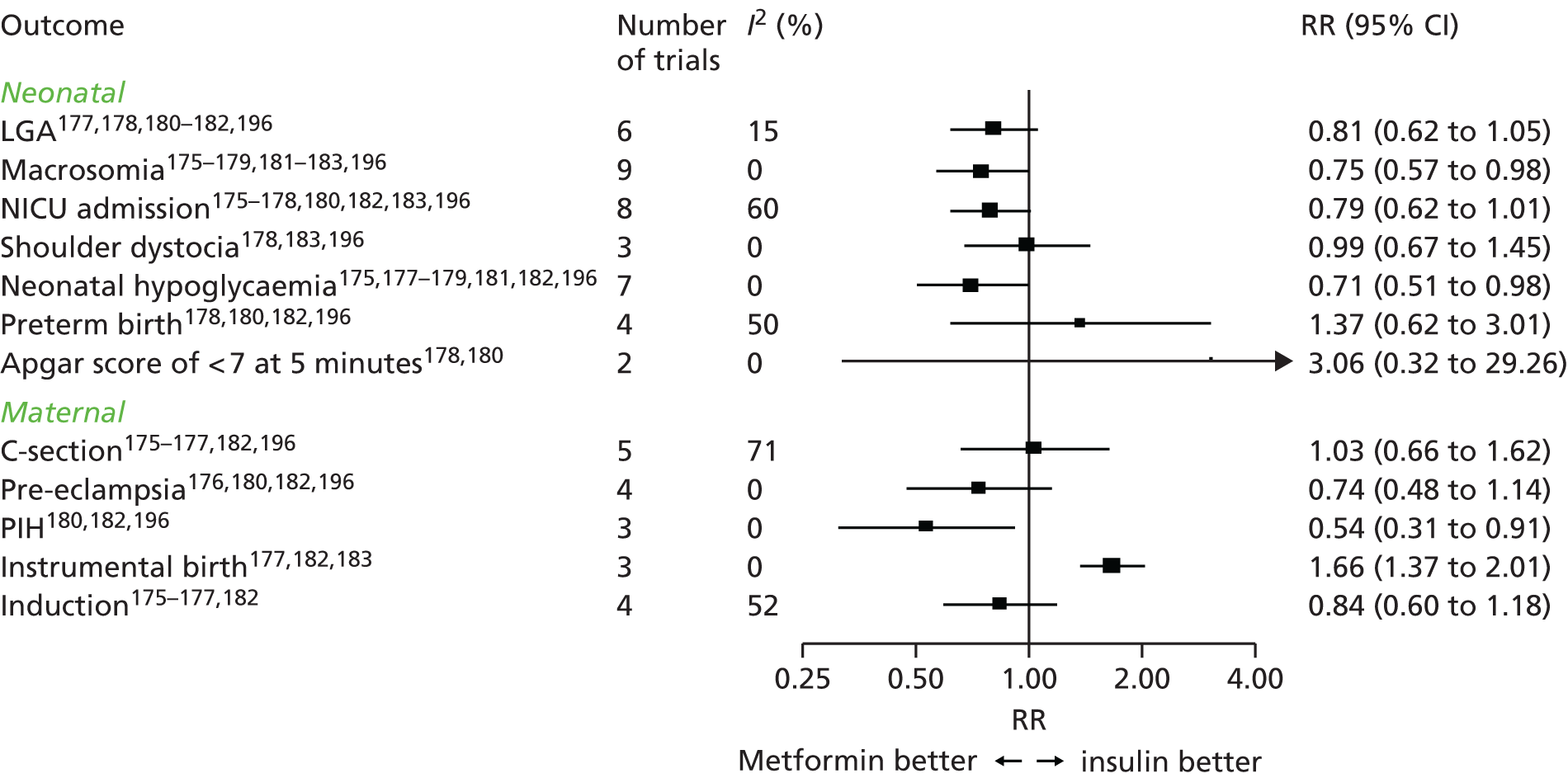
Heterogeneity varied across these analyses but was generally low to moderate (I2 = 0–60%); however, trials reporting C-section had high heterogeneity (I2 = 71%).
Figure 26 shows the results of the meta-analysis of trials for all continuous outcomes: gestational age at birth, BW and 5-minute Apgar score for metformin vs. insulin treatment. All outcomes were similar between the two groups. Heterogeneity across trials was low.
FIGURE 26.
Metformin vs. insulin: continuous outcomes. GA, gestational age.
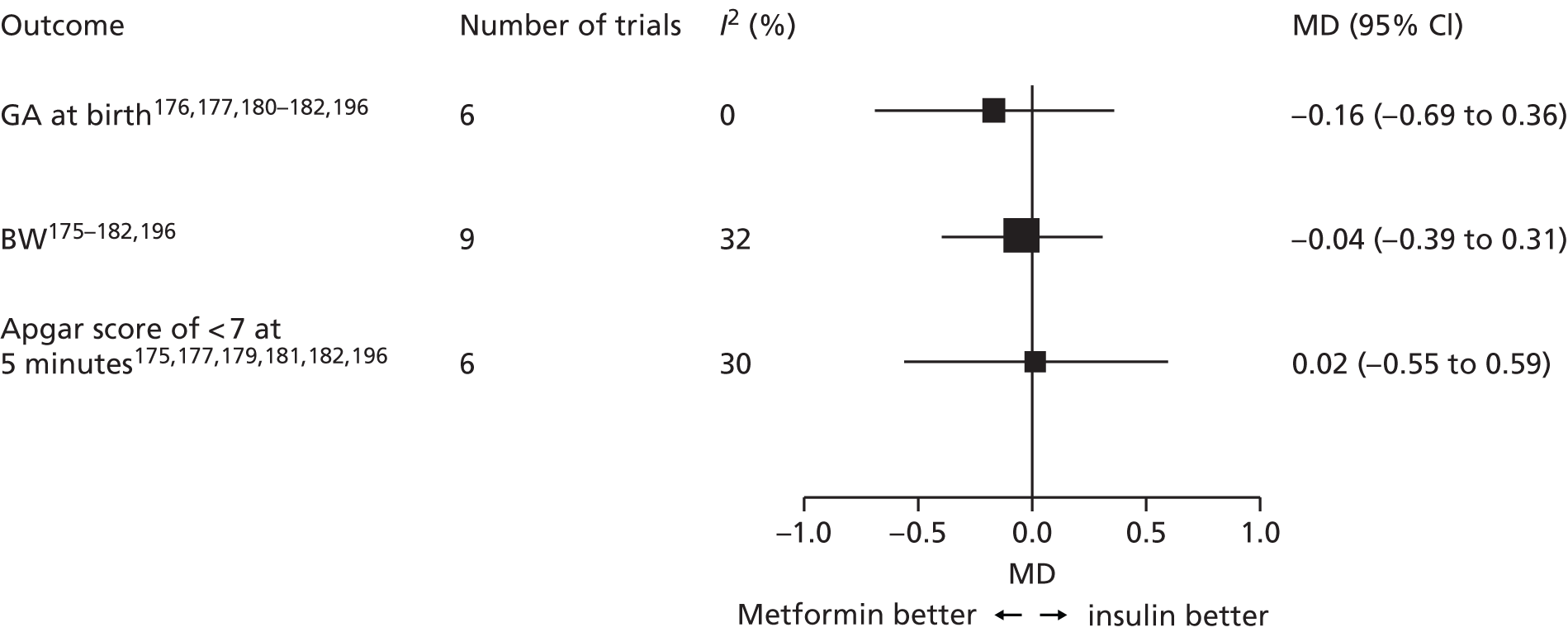
Trials comparing glibenclamide and insulin
Eight trials compared glibenclamide to insulin. 184–191 Details of these trials are given in Table 21.
| Reference | Year | Location | Population | Criteria used to diagnose GDM | Screening strategya |
|---|---|---|---|---|---|
| Anjalakshi184 | 2007 | India | 23 | WHO | Universal OGTT |
| Bertini185 | 2005 | Brazil | 70 | WHO | Not reported |
| Lain186 | 2009 | USA | 99 | ADA – C&C | 50-g OGCT then OGTT |
| Langer187 | 2000 | USA | 404 | C&C | 50-g OGCT then OGTT |
| Mukhopadhyay188 | 2012 | India | 60 | WHO | Universal OGTT (2-hour post load > 7.7 mmol/l only) |
| Ogunyemi189 | 2007 | USA | 97 | Not reported | Not reported |
| Silva190 | 2007 | Brazil | 68 | WHO | Universal OGTT |
| Tempe191 | 2013 | India | 64 | C&C | 50-g OGCT then OGTT |
Figure 27 shows the results of the meta-analysis of trials for all dichotomous perinatal outcomes for glibenclamide compared with insulin treatment. The analysis suggests that insulin is more effective in reducing the odds of an adverse outcome compared with glibenclamide (LGA, macrosomia, neonatal hyperglycaemia and pre-eclampsia) or has similar effects (NICU admission, preterm birth and C-section); however, the increases were not statistically significant and the CIs were wide.
FIGURE 27.
Glibenclamide vs. insulin: dichotomous outcomes.
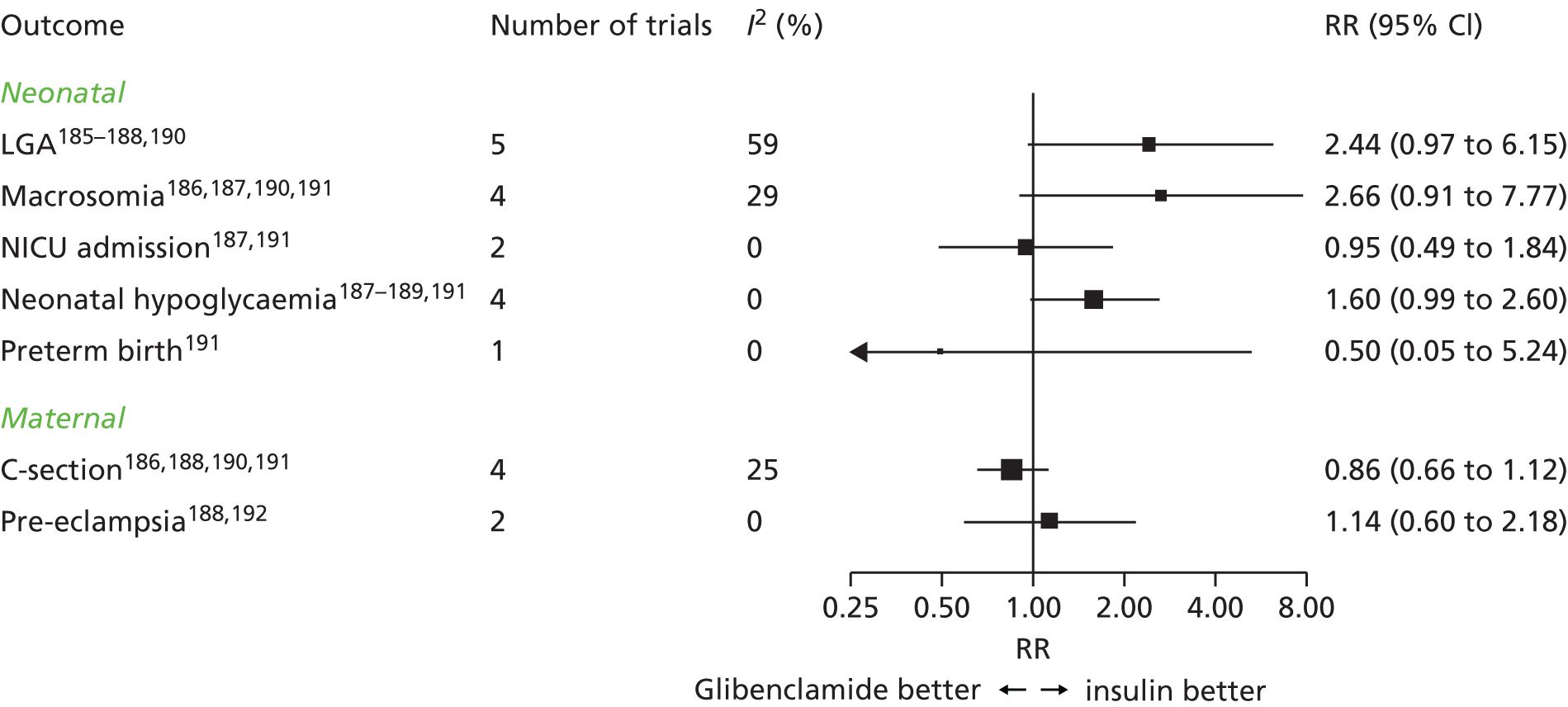
Figure 28 shows the results of the meta-analysis of trials for all continuous outcomes – gestational age at birth, BW and Apgar score at 5 minutes – for glibenclamide vs. insulin treatment. Infants whose mothers were given glibenclamide were generally born heavier than infants of mothers given insulin (approximately 120 g). Gestational age at birth and Apgar score at 5 minutes were both similar between the two groups.
FIGURE 28.
Glibenclamide vs. insulin: continuous outcomes. GA, gestational age.
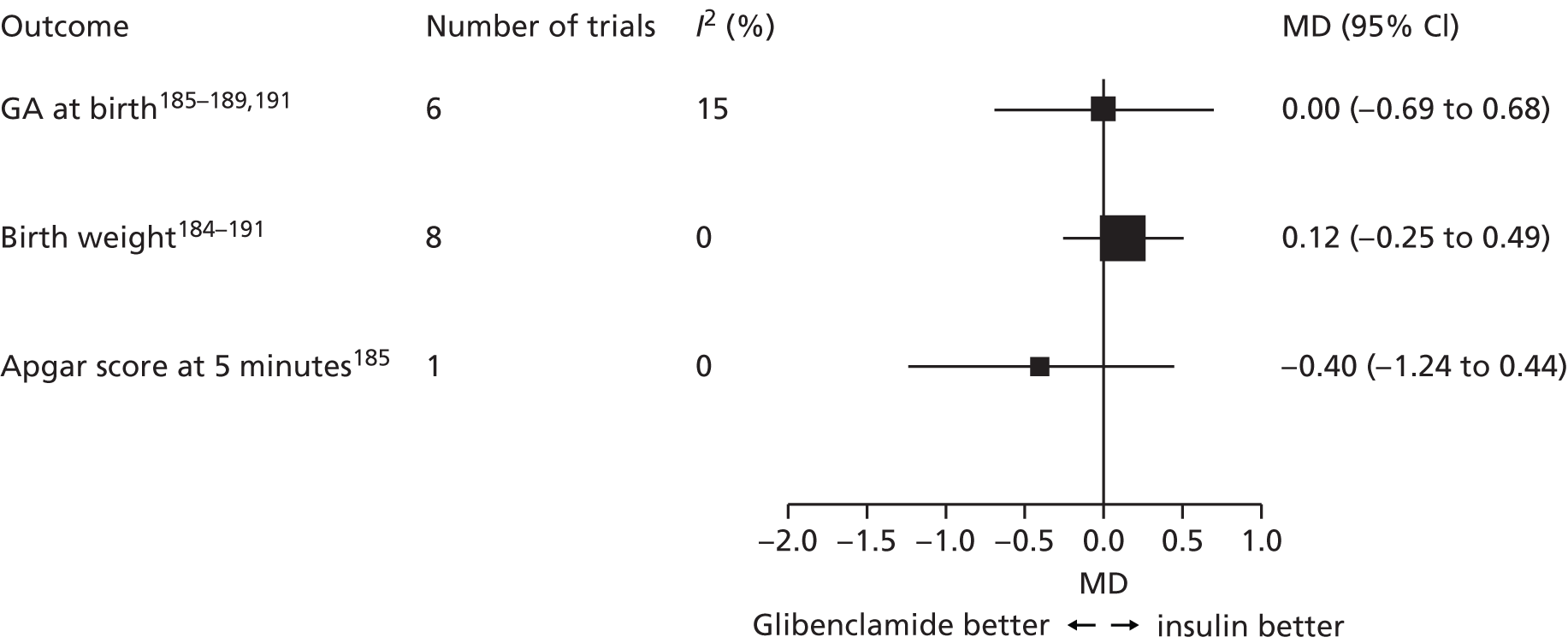
Trials comparing glibenclamide and metformin
Two trials directly compared metformin to glibenclamide (Table 22). 192,213
| Reference | Year | Location | Population | Criteria used to diagnose GDM | Screening strategya |
|---|---|---|---|---|---|
| Moore192 | 2010 | USA | 149 | C&C | 50-g OGCT then OGTT |
| Silva193 | 2012 | Brazil | 200 | WHO | Not reported |
Figure 29 shows the risk of dichotomous outcomes for glibenclamide treatment compared with metformin treatment, and Figure 30 shows the risk of continuous outcomes for glibenclamide treatment compared with metformin treatment. Given the limited number of trials in this analysis with few women (349) included, it is difficult to draw meaningful conclusions. For several outcomes, only one trial reported data, and, even when data from two trials were combined, the results were generally non-significant, with wide CIs.
FIGURE 29.
Glibenclamide vs. metformin: dichotomous outcomes.
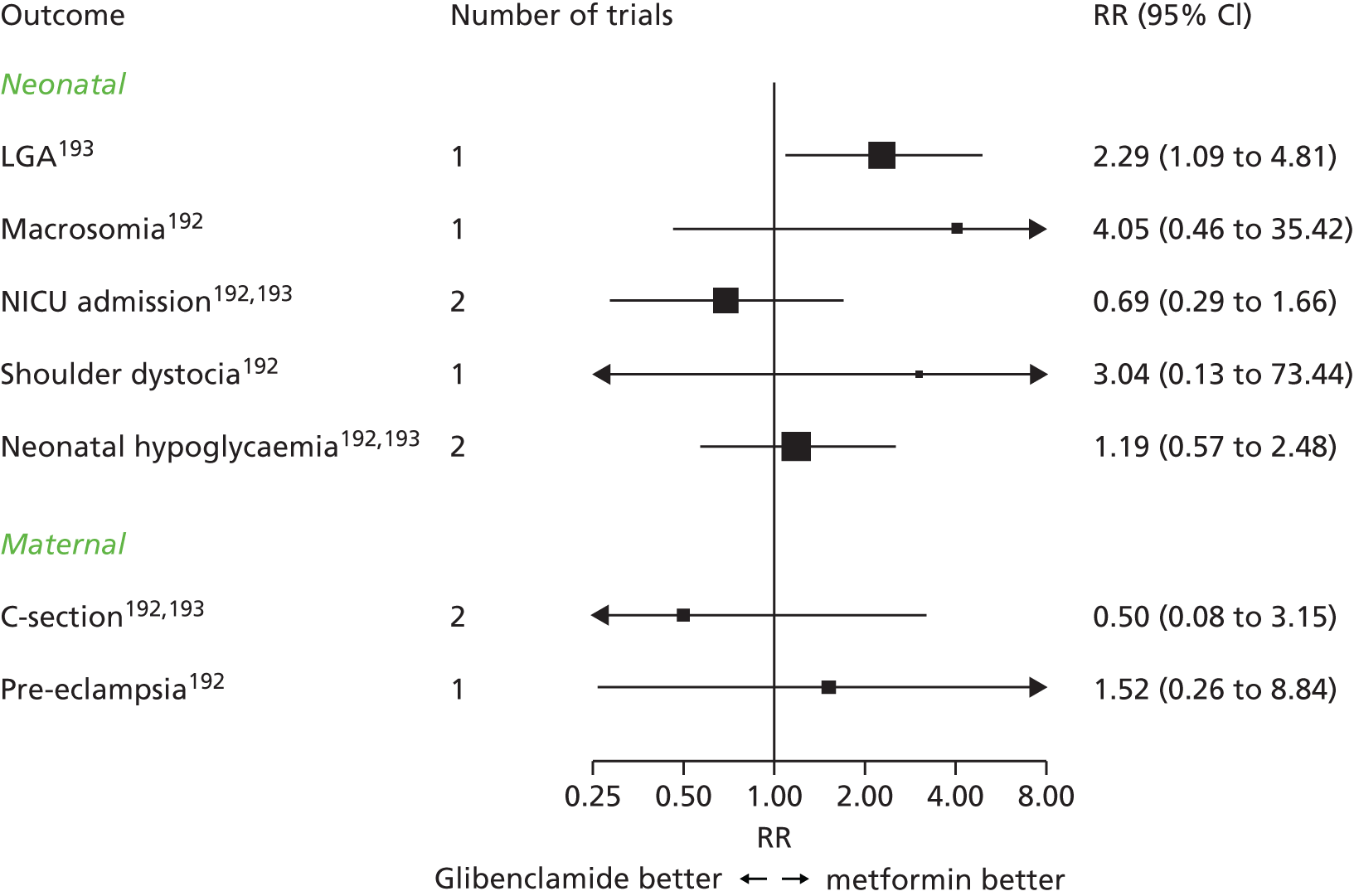
FIGURE 30.
Glibenclamide vs. metformin: continuous outcomes. GA, gestational age.
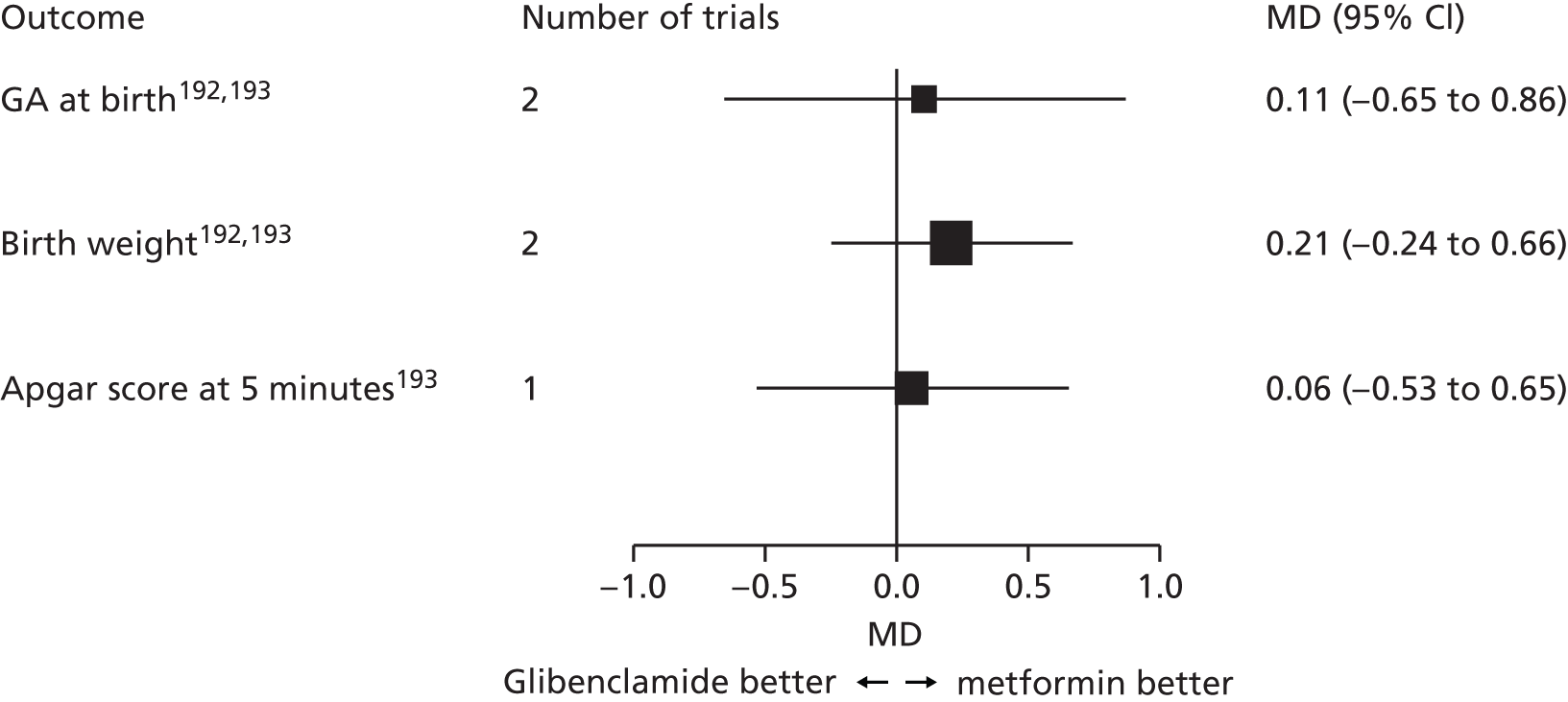
Network meta-analysis comparing glibenclamide, insulin and metformin
For the network analysis, all trials comparing one pharmacological treatment with another have been included: metformin, glibenclamide and insulin (Figure 31). Only dichotomous outcomes reported in at least two glibenclamide trials were included to ensure there were sufficient trials in each network analysis to produce meaningful results. Separate network analyses were performed for each outcome; their results are shown in Figure 32.
FIGURE 31.
Network meta-analyses, relationship of comparisons.

FIGURE 32.
Network meta-analysis comparing metformin, glibenclamide and insulin. First better – treatment listed first in the outcome column is superior; second better – treatment listed second in the outcome column is superior.
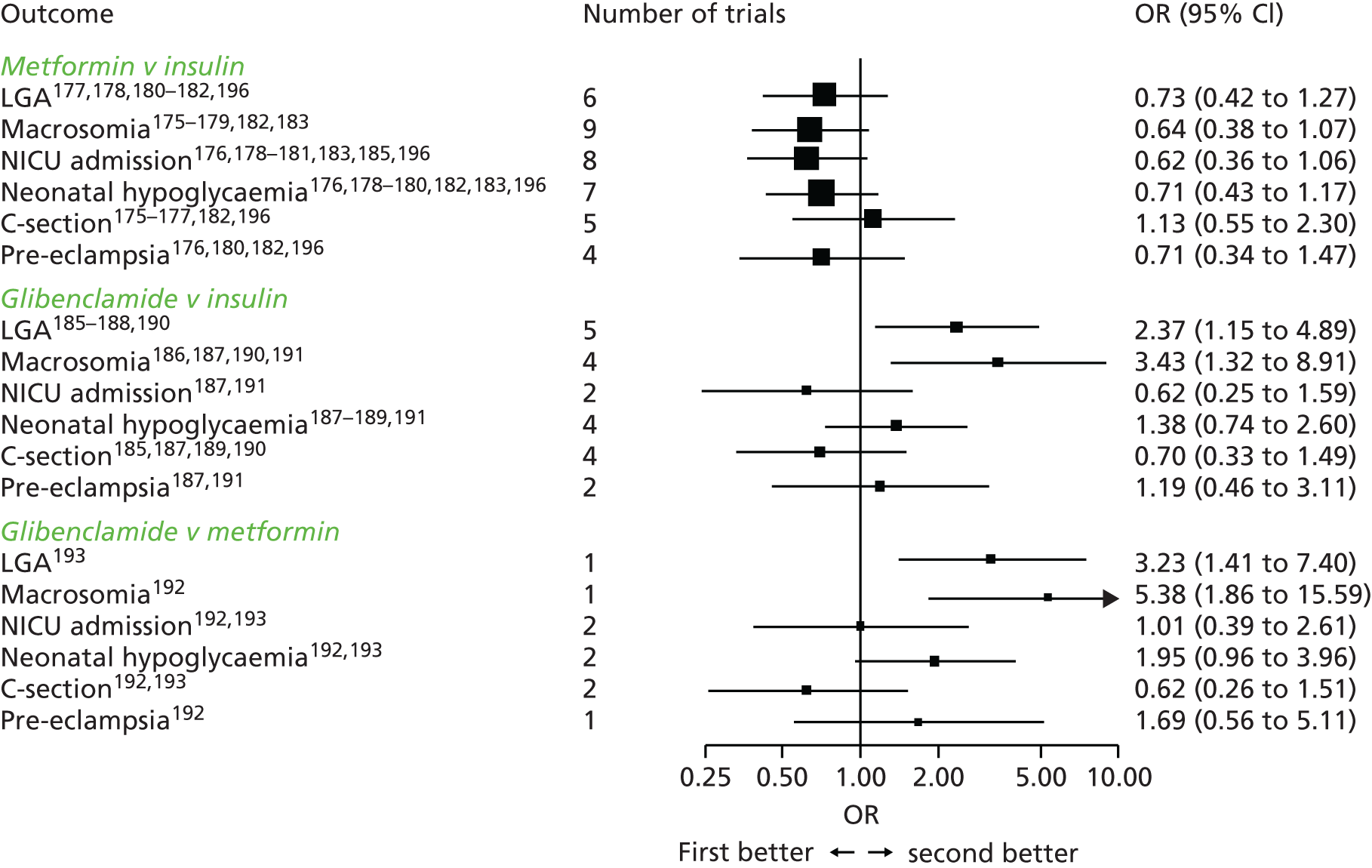
The network analyses suggest that, compared with insulin, metformin reduces the risk of all reported outcomes with the exception of C-section. However, comparisons include the null value, and the CIs are wide and include either an increase or a decrease in risk.
Glibenclamide compared with insulin or metformin treatment seems to increase the risk of macrosomia and LGA. There is no evidence of a difference between glibenclamide and insulin or glibenclamide and metformin treatment for the remaining outcomes. These results are similar to the results from the direct meta-analyses. The loss of estimate precision (and statistical significance) for comparisons in the network meta-analyses compared with the direct (head-to-head) meta-analyses may be a result of the increased heterogeneity within group comparisons across trials.
Table 23 shows the estimated probability of the effectiveness of each treatment at reducing the risk of each outcome, derived from the network meta-analysis. This analysis suggests that for all of the outcomes, with the exception of C-section, metformin is most likely to be the most effective treatment, and for most outcomes the probability that metformin is most effective is reasonably high.
| Outcome | Treatment | ||
|---|---|---|---|
| Insulin | Metformin | Glibenclamide | |
| LGA | 17.2 | 82.7 | 0.1 |
| Macrosomia | 3.5 | 96.4 | 0 |
| NICU admission | 1.4 | 50.3 | 48.3 |
| Neonatal hypoglycaemia | 7.9 | 89.5 | 2.7 |
| C-section | 12.6 | 9.4 | 78 |
| Pre-eclampsia | 11.5 | 74.6 | 13.9 |
Insulin does not ever seem to be the best treatment, with very low probabilities of being best for each outcome. However, it should be noted that supplemental insulin was often added to treatment with metformin in the trials that report management of blood glucose when glycaemic control was not achieved with diet, lifestyle and metformin. This analysis therefore suggests metformin is superior (in terms of its ability to influence the risk of associated adverse outcomes) to insulin and glibenclamide as a first-line treatment, rather than a standalone treatment.
Other trials comparing metformin and glibenclamide
Two trials194,214 (Table 24) could not be included in any meta-analysis because there was insufficient information reported, the trials investigated the effectiveness of metformin or glibenclamide. Both reports194,214 were available only as conference abstracts.
| Reference | Year | Location | No. | Experimental group | Control group | Criteria used to diagnose GDM |
|---|---|---|---|---|---|---|
| Abbassi-Ghanavanti194 | 2014 | USA | 395 | Glyburidea with diet | Placebo with diet | NDDG |
| Ardilouze214 | 2014 | Canada | 63 | Metformin with glyburidea | Insulin | Not reported |
The placebo controlled trial of diet with glibenclamide194 reported that glibenclamide treatment had no additional influence on macrosomia or LGA risk over diet modification alone. Ardilouze et al. 214 reported that the infants of mothers using the metformin–glibenclamide combination of half maximum doses had more neonatal hypoglycaemia episodes than infants of mothers using insulin; however, the number of women included were small. Other outcome rates reported were similar between groups.
Trials comparing different insulin preparations
Five trials compared different insulin preparations:195,205,207,210,218 three compared analogue to human insulin,195,210,218 one different numbers of doses per day,207 and one gave insulin only to women with the most elevated glucose levels. 205 Details of these trials are given in Table 25.
| Reference | Year | Location | No. | Experimental insulin | Control insulin | Criteria used to diagnose GDM |
|---|---|---|---|---|---|---|
| Balaji195 | 2012 | India | 323 | Analogue insulin (Aspart BIAsp) | Human insulin | WHO |
| Di Cianni210 | 2007 | Italy | 96 | Analogue (Aspart) or lispro | Human insulin | C&C |
| Jovanovic218 | 1999 | USA | 42 | Lispro (humalog) | Human insulin | C&C |
| Kjos205 | 2001 | USA | 98 | Insulin if fetal abdominal circumference > 70th centile and/or FPG > 6.7 | Insulin irrespective of fetal growth or glucose levels | FPG 5.8–6.7 mmol/l |
| Nachum207 | 1999 | Israel | 392 | Four times daily administration | Twice-daily administration | NDDG |
The differences in the composition of the insulin preparations used by the trials precluded their inclusion in a meta-analysis. As an alternative, summary results for each trial and each outcome are presented in forest plots, without pooling across trials. Figure 33 shows the effects of the differing insulin preparations on dichotomous outcomes, and Figure 34 shows the effect on continuous outcomes. For the majority of outcomes, there are no statistically significant differences in the effectiveness of different insulin preparations.
FIGURE 33.
The effect of different insulin preparation on dichotomous outcomes.
FIGURE 34.
The effect of different insulin preparation on continuous outcomes. GA, gestational age.
Trials comparing different types of diet modification
Ten trials51,52,196–198,217–221 compared diet modification or advice, possibly alongside glucose monitoring and insulin use (although this was often not reported) to routine antenatal care (usually no specific diet modification or insulin treatment) (Table 26).
| Reference | Year | Location | No. included | Criteria used to diagnose GDM | Screening strategy | Insulin use in diet group |
|---|---|---|---|---|---|---|
| Bevier202 | 1999 | USA | 103 | Positive OGCT, negative OGTT | Universal 50-g OGCT only | If needed |
| Bonomo203 | 2005 | Italy | 300 | Positive OGCT, negative OGTT | Risk factors and 50-g OGCT | Not reported |
| Crowther51 | 2005 | UK/Australia | 1000 | WHO 199911 | Risk factors or 50-g OGCT | If needed |
| Deveer197 | 2013 | Turkey | 100 | ACOG-positive OGCT, negative OGTT | Universal 50-g OGCT and OGTT | Not reported |
| Elnour200 | 2006 | UAE | 180 | Not reporteda | Not reported | If needed |
| Garner201 | 1997 | Canada | 299 | bHatem 1988221 | Universal 75-g OGCT and selective OGTT test result | If needed |
| Landon52 | 2009 | USA | 958 | ADA 200612 | Universal 50-g OGCT and selective OGTT | If needed |
| Li198 | 1987 | Hong Kong | 158 | NDDG 197915 | Risk factors and selective OGTT | Not reported |
| O’Sullivan211 | 1966 | USA | 615 | O’Sullivan 196416 | OGCT or risk factors | Only in treated group |
| Yang199 | 2003 | China | 150 | WHO 1998222 | Universal OGCT and selective OGTT | If needed |
In seven trials, insulin was reported as being used if required; three trials197,198,203 did not report insulin use. Two trials220 reported secondary analyses of previously published trials;52,211 both trials220 are therefore excluded from the analyses.
Across the included trials, the dietary interventions included specific diets, individualised advice from a dietitian, or more general advice (the compositions of the dietary interventions were generally well reported by trials). Two of the included reports were secondary analyses of data from the Crowther trial,51 one223 of which was a secondary analysis, examining longer-term infant (4- to 5-year-olds) obesity risk (BMI z-scores) and so was excluded from our meta-analyses.
For the meta-analysis the varying forms of dietary modification and advice were assumed to be equivalent, and any potential differences in insulin use were not considered. The forest plot for the meta-analysis of trials reporting macrosomia as an outcome is presented in Figure 35 (results across trials of all other outcomes are available from the authors on request). This analysis suggests that diet modification halves the incidence of macrosomia compared with routine care (whatever that care may be). The three trials that did not report insulin use report similar results to those trials reporting that supplemental insulin was used when required. Heterogeneity was moderate (I2 = 32%), driven by the two small trials with extreme outcomes. 197,202
FIGURE 35.
The effect of diet modification on macrosomia incidence.
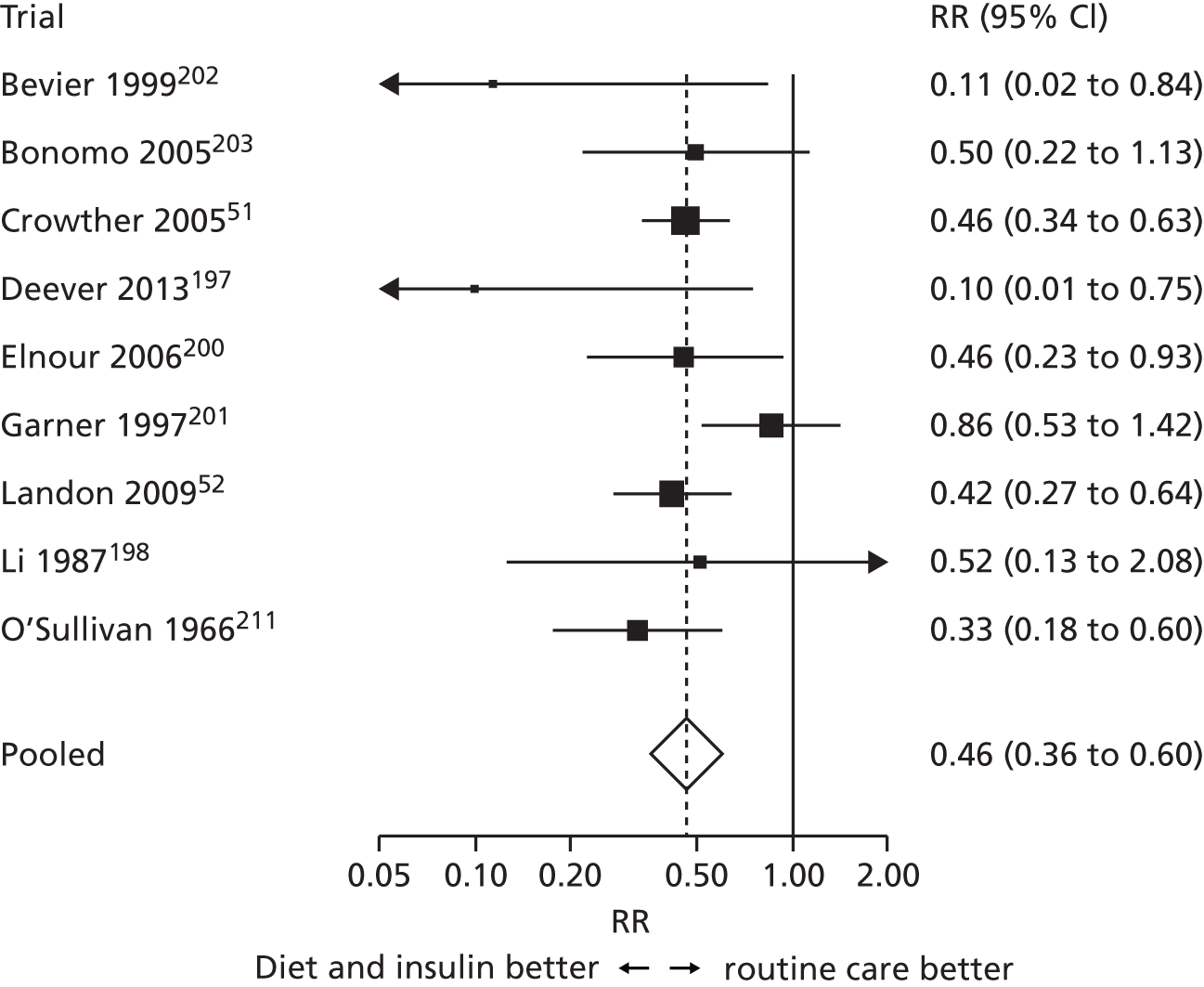
Figure 36 shows the risk of dichotomous outcomes and diet modification compared with routine care, and Figure 37 shows the risk of continuous outcomes and diet modification compared with routine care. Diet modification seems to reduce the risk of LGA, macrosomia, shoulder dystocia and pre-eclampsia by around 50%, with a more modest 15% reduction in the incidence of C-section compared with routine care. Diet modification compared with routine care also seems to reduce BW by approximately 120 g. There is no evidence that diet modification reduces the incidence of neonatal intensive care admission, neonatal hypoglycaemia, induced labour, preterm birth or Apgar score at 5 minutes. Heterogeneity varied across outcomes, with I2 ranging from 0% to 67%.
FIGURE 36.
The effect of diet modification on dichotomous outcomes.
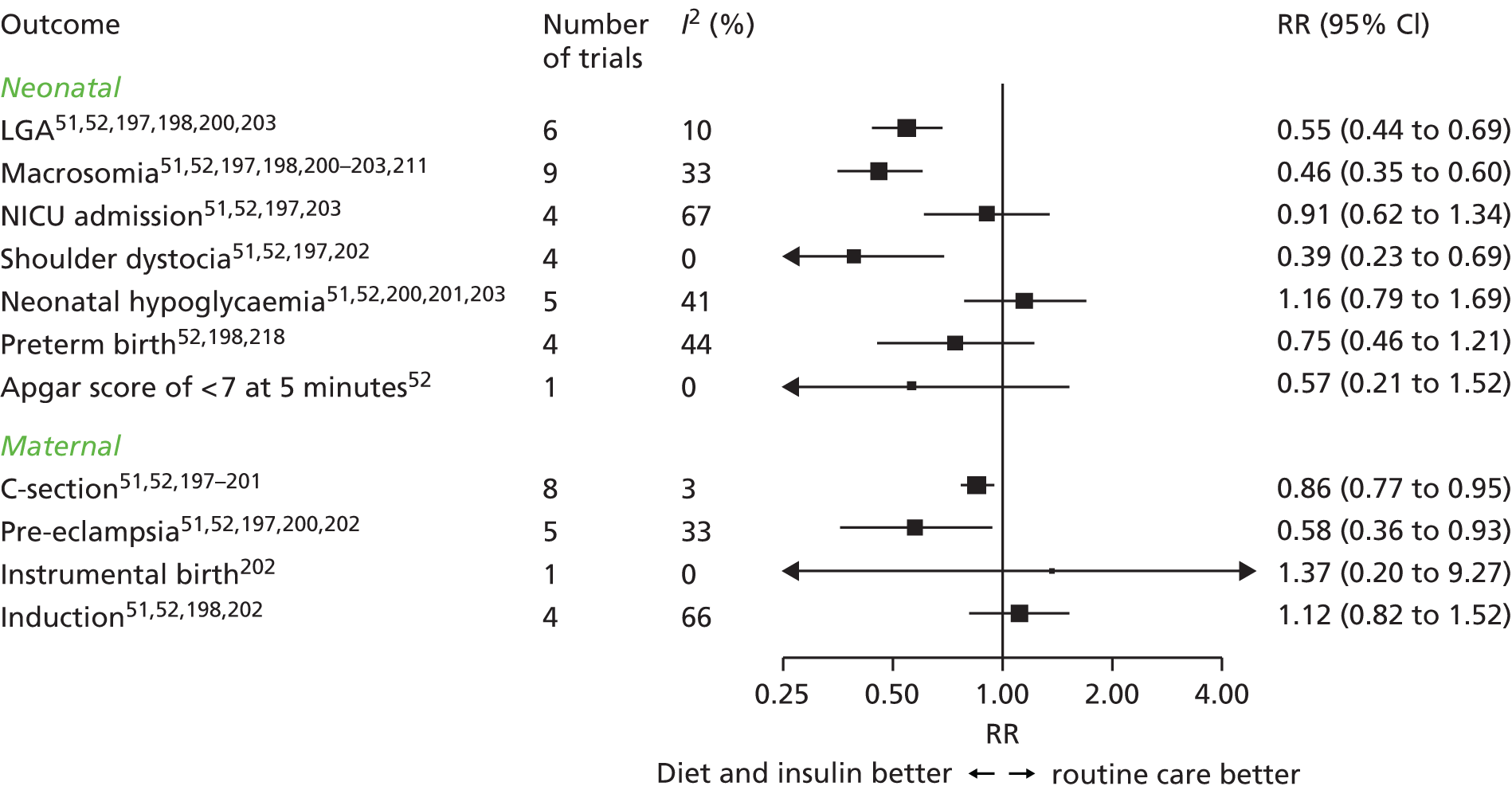
FIGURE 37.
The effect of diet modification on continuous outcomes. GA, gestational age.

Subgroup analyses: diet modification trials by definition of gestation diabetes mellitus
Unlike the trials evaluating pharmacological treatments (which tended to use recommended tests, criteria and thresholds for diagnosing GDM) the tests, criteria and thresholds used to define a comparison group’s ‘GDM status’ varied considerably across the 10 trials included in the meta-analyses of diet modification trials. These differences are briefly summarised in Table 27.
| Reference | Year | Criteria or test used to diagnose GDM | Thresholds |
|---|---|---|---|
| Bevier202 | 1999 | Positive OGCT | Positive OGCT and negative OGTT |
| Bonomo203 | 2005 | Positive OGCT | Positive OGCT and negative OGTT |
| Crowther51 | 2005 | WHO | Mild GDM fasting < 7.8 mmol/l and 2-hour post load 7.8–11.1 mmol/l |
| Deveer197 | 2013 | Positive OGCT | Positive OGCT and negative OGTT |
| Elnour200 | 2006 | Not reported | ‘GDM’ diagnosed ≤ 20 weeks’ gestation |
| Garner201 | 1997 | ‘Hatem’ trial specific | GDM fasting > 7.5 mmol/l 2-hour > 9.6 mmol/l |
| Landon52 | 2009 | ADA | Mild GDM fasting < 5.3 mmol/l and two or more of: 2-hour > 10.0 mmol/l and 2-hour > 8.6 mmol/l, 3-hour > 7.8 mmol/l |
| Li198 | 1987 | WHO | IGT fasting < 7.9 mmol/l and 2-hour post load 7.8–11.1 mmol/l |
| O’Sullivan211 | 1966 | O’Sullivan | GDM fasting > 6.1 mmol/l 1-hour> 9.4 mmol/l, 2-hour> 6.7 mmol/l, 3-hour > 6.1 mmol/l |
| Yang199 | 2003 | WHO 1998 | Fasting ≥ 7.0 mmol/l and 2-hour post load 7.8–11.1 mmol/l |
Three trials included women with a positive OGCT and a negative OGTT; therefore, they did not meet any criteria for current GDM diagnoses. Some early trials, for example Li (1987) classify women as having IGT or mild GDM. Today, however, IGT and ‘mild’ GDM are viewed within the same spectrum of hyperglycaemia and are therefore usually classified as GDM.
In order to investigate the impact of these potentially different populations we performed subgroup analyses (irrespective of glucose load and glucose thresholds used), dividing the trials into four categories:
-
GDM Women had GDM according to a ‘standard’ diagnostic criteria, e.g. ADA, WHO or C&C.
-
Mild GDM Women described as having mild GDM.
-
IGT Women described as having IGT.
-
Negative OGTT Women had positive OGCT but negative OGTT.
Figure 38 shows the effect of diet modification on risk of macrosomia, with trials grouped by glucose level/severity classification [most severe first (GDM) down to positive OGCT, negative OGTT]. Although data within each group are limited, there is no evidence that the effect of diet modification varies substantially according to the glucose level/severity classification. Figure 39 summarises the results for the dichotomous outcomes LGA, macrosomia, C-section and pre-eclampsia, the outcomes where there is strongest evidence of a benefit of diet modification. Again, there is no evidence of any treatment effect differences between trials, although, again, data are limited.
FIGURE 38.
Impact of diet modification on macrosomia, by degree of glucose intolerance [GDM, IGT, mild GDM or (positive OGCT) negative OGTT].
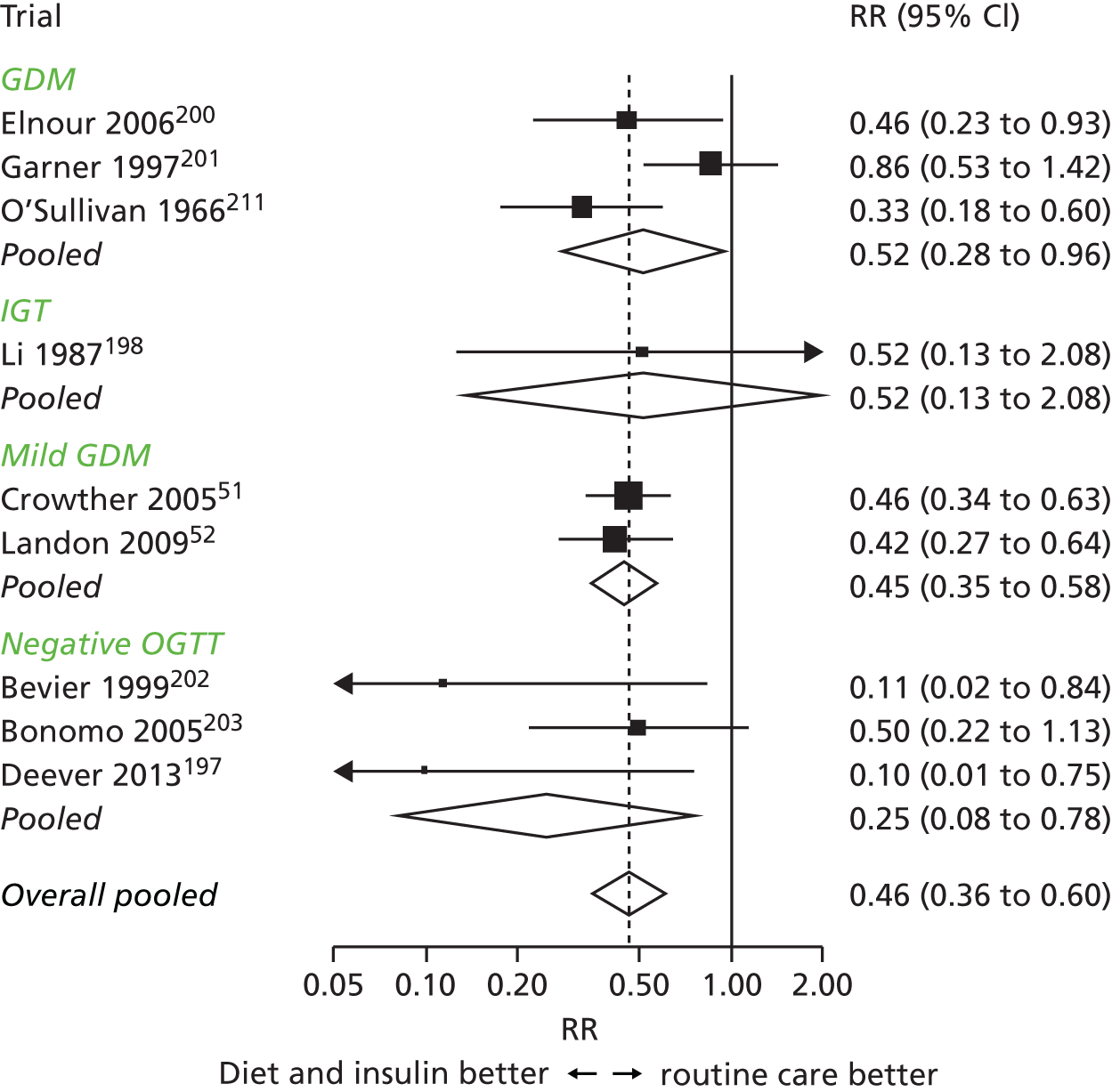
FIGURE 39.
Impact of diet modification on dichotomous outcomes, by degree of glucose intolerance [GDM, IGT, mild GDM or (positive OGCT) negative OGTT].

Because women with ‘mild’ GDM and IGT may both be categorised as having GDM under current diagnostic guidelines we combined these groups with those described as having GDM in a further subgroup analysis. We compared this new GDM group to women without GDM (those with elevated glucose at OGCT and with a subsequently ‘normal’ OGTT). The results of this subgroup analysis are shown in Figure 40. Diet modification (with insulin if needed), irrespective of the severity of the hyperglycaemia identified or the test used to identify it, seems to be effective in reducing the risk of adverse perinatal outcomes.
FIGURE 40.
Impact of diet modification comparing women with GDM to those with only a positive OGCT and negative OGTT.

‘Other’ diet and exercise trials
Nine trials204,206,208,209,212,215–217,219 were too methodologically diverse to allow pooling of data. Six trials206,209,212,215,217,219 compared two different types of diets, one trial204 compared exercise to insulin use, one trial compared diet and insulin to diet alone, and one compared exercise with diet216 (Table 28).
| Author | Year | Location | No. included | Experimental group | Control group | Criteria used to diagnose GDM |
|---|---|---|---|---|---|---|
| Asemi215 | 2014 | Iran | 52 | ‘DASH’ diet (high fruit, veg, wholegrain and dairy) | Control diet | ADA |
| Bo216 | 2014 | Italy | 200 | Diet and exercise | Diet only | Not reported |
| Bung204 | 1991 | USA | 41 | Exercise bike use | Insulin | Not reported |
| Cao217 | 2012 | China | 275 | Individual diet education | Standard group diet education | Not reported |
| Cypryk209 | 2007 | Poland | 30 | High-carbohydrate diet | Low carbohydrate diet | WHO |
| Louie219 | 2011 | Australia | 99 | Low-GI diet | High fibre moderate GI diet | ADIPS |
| Moreno-Castilla206 | 2013 | Spain | 152 | Low-carbohydrate diet | Control diet | NDDG |
| Rae212 | 2000 | Australia | 124 | Low-calorie diabetic diet | Standard diabetic diet | OGTT fasting > 5.5 mmol/l or 2-hour > 7.9 mmol/l (glucose load not reported) |
| Thompson208 | 1990 | USA | 108 | Diet and insulin | Diet only | NDDG |
As commented on above, these trials compared very different interventions (diet, exercise and insulin preparations), therefore meta-analysis has not been undertaken; instead, summary results for each trial and each outcome are presented in forest plots (without pooling across trials).
Figure 41 shows the effect of the differing diets, by trial, on the risk of dichotomous outcomes, and Figure 42 shows the effect of the differing diets, by trial, on the risk of continuous outcomes. Results are varied; however, there is no evidence that any one particular type of diet improves all outcomes reported. See Table 28 for information on type of intervention and participant numbers.
FIGURE 41.
Effect of diet interventions on dichotomous outcomes.

FIGURE 42.
Effect of diet interventions on continuous outcomes. GA, gestational age.
In the three remaining trials,204,208,216 a mixture of interventions was evaluated. Bo et al. 216 gave the same diet to four groups of women; the first group also received diet recommendations, the second group was advised to walk briskly for 20 minutes each day, the third group received behavioural dietary recommendations and the fourth group received both of the second and third groups’ interventions. Bo et al. 216 reported that exercise reduced postprandial glucose levels and a composite measure of maternal and neonatal complications, whereas behavioural interventions had no effect. Bung et al. 204 compared exercise and diet to insulin and diet alone in a group of women who had ‘failed’ to achieve ‘normal’ glucose levels on diet alone within a week of GDM diagnosis. Bung et al. 204 reported no differences between groups in outcomes; however, macrosomia rate was doubled in the group receiving insulin (two vs. four), which seems contrary to results expected; numbers in the trial were small (34), however, possibly accounting for the results. Thompson et al. 208 compared diet with insulin to diet alone, reporting that the group that was given diet advice with insulin had infants with lower BW and incidence of macrosomia compared with the group that was given diet advice alone. Group rates of C-section, shoulder dystocia and neonatal hypoglycaemia were similar.
Discussion
Pharmacological treatments
For women requiring pharmacological intervention to reduce hyperglycaemia (often when diet alone had proved ineffective), metformin as first-line treatment seems to be at least as effective as insulin in reducing the risk of adverse perinatal outcomes, and metformin and insulin seem to be more effective than glibenclamide.
Although the treatment effects of metformin and insulin were similar, there was a trend for metformin to perform better, or at least no worse, than insulin for most outcomes reported. For example, metformin reduces the risk of macrosomia by 25% compared with insulin. Although not statistically significant, there was some evidence that treatment with metformin may be associated with a greater risk of preterm birth and an Apgar score of < 7 at 5 minutes compared with insulin treatment. Glibenclamide, performed less well than insulin for several outcomes, for example the infants of mothers who were given glibenclamide were, on average, 120 g heavier at birth compared with the infants of mothers who were given insulin; the risk of LGA and macrosomia is also greater for those given glibenclamide compared with insulin, but not significantly so. The network meta-analysis confirmed the direct trial meta-analyses findings and suggests that there is a high probability that metformin is the most effective treatment, compared with insulin or glibenclamide, for reducing the risk of most adverse perinatal outcomes examined within this review.
Metformin, in addition to performing well, may also be preferred by women, as it is administered orally and can be stored at room temperature as opposed to insulin, which requires subcutaneous injection and refrigerated storage. Metformin is associated with gastrointestinal upset, however, which may affect compliance; unfortunately, few trials report side effects or participant satisfaction, quality of life or well-being, which should be examined by future trials.
Dietary modification
Dietary modification generally reduces the risk of most reported perinatal outcomes compared with ‘usual or routine’ antenatal care. For example, the risk of macrosomia is halved (RR 0.46, 95% CI 0.36 to 0.60) and the risk of C-section is reduced by 14% (RR 0.86, 95% CI 0.77 to 0.95) with dietary modification compared with usual/routine antenatal care. If trials are adequately powered and methodologically robust (particularly in terms of adequacy of randomisation) it is reasonable to accept that diet modification does improve adverse outcome rates compared with routine care (without special diet modification); however, risk of bias was generally high or unclear, suggesting poor trial quality with respect to these indicators.
There was no evidence that the effect of diet modification varied according to the types of women (or severity of hyperglycaemia) included in the trials. For LGA, macrosomia and C-section, diet modification seemed to be equally effective in women with a negative OGTT (usually following a positive OGCT) compared with women with diagnosed GDM or ‘mild’ GDM. This finding suggests that modifying the diet of women who do not have GDM (as currently defined) may be as effective in reducing risks as in women with diagnosed GDM. This is supported by trials of diet and lifestyle modification that have been undertaken in women who do not have GDM. 224 However, compliance with diet advice may be less (because women may be less amenable to change if they do not believe themselves at risk) and thus any beneficial effects may be reduced. The finding that dietary modification can reduce the risk of most adverse outcomes in women with lower glucose levels (below those currently diagnostic of GDM at the time the trials were conducted) is important given the recommendations of the IADPSG and our analyses findings (detailed in Chapter 2), which suggest that lower thresholds are required to identify the majority of infants at risk of LGA and high adiposity at birth. As we have explained in Chapter 2, these outcomes (LGA and high adiposity at birth) are associated with increased obesity and cardiometabolic risk. It is assumed that treatment of GDM, however, will reduce the risk of these longer-term outcomes, although that remains to be substantiated by large RCTs with longer-term follow-up.
Although our meta-analyses suggest that diet modification is effective in reducing the risk of the majority of reported adverse perinatal outcomes compared with routine antenatal care, nine trials could not be included because of differences between comparisons and interventions (see Table 28). These trials often included small numbers reducing the reliability of their results, for example although Asemi et al. 215 reported that their ‘Dietary Approaches to Stop Hypertension’ (DASH) diet significantly lowered BW, reduced macrosomia, C-section rate and need for insulin compared with a control diet; only 52 women were included, and head circumference and ponderal index were also significantly reduced in the infants of women consuming the DASH diet compared with control diet, suggesting a detrimental effect. The remaining trials showed varying results by outcome (see Figures 41 and 42).
Analogue and human insulin
Although the number of trials was limited, the analyses suggest that analogue and human insulin are equally effective.
Frequency of insulin administration
One trial207 investigated frequency of insulin administration and reported a statistically significant reduction in neonatal hypoglycaemia when insulin was administered four times daily in comparison with twice daily. However, the number of women included were few (274) and, consequently, there were few events (one in the four-times administration group; eight in the two-times administration group).
We found that few trials included in this review reported negative treatment effects and it is possible that negative outcomes such as gastrointestinal upset associated with metformin use may reduce compliance and treatment effects.
Conclusions
Treatment of GDM with diet and lifestyle and pharmacological interventions seems to reduce the risk of most reported perinatal adverse outcomes. Diet modification alone seems to reduce the risk of adverse outcomes even in women with glucose levels below those currently diagnostic of GDM. Given the graded linear association between glucose levels and adverse outcomes (reported in Chapters 2 and 3) and findings from a systematic review of trials in non-GDM populations,224 the provision of dietary advice for all pregnant women (irrespective of their glucose levels at OGTT) may be beneficial in terms of reducing the risk of adverse outcomes across the whole glucose spectrum. Dietary advice, however, may be costly, especially if specialist advice is provided above the dietary advice that could be given by obstetricians and midwives during ‘routine’ antenatal appointments. It is also possible that women who view themselves as ‘normal’ may be less compliant with dietary advice than women who are aware that they have GDM and who appreciate that dietary modification may improve their and their infant’s health outcomes and reduce their need for supplementary pharmacological intervention, especially insulin. Women requiring pharmacological intervention in addition to diet and lifestyle, however, may also be more insulin resistant or their insulin resistance may be more refractory than women who require only diet and lifestyle advice.
Supplemental metformin in addition to diet and lifestyle modification (if required to normalise glucose levels) is as effective as insulin and therefore should be the first-line pharmacological treatment of choice, as it is at least as effective as insulin and may be preferred by women because it does not require injection, although it should be remembered that trials generally used insulin in the metformin group if hyperglycaemia was not ‘well’ controlled. The results of this review provide reassurance, however, that a ‘step-up’ approach of first providing dietary and lifestyle advice then adding supplementary metformin or insulin if glucose levels are not adequately controlled is a reasonable and effective approach to take.
Chapter 7 Economic evaluation of screening and diagnostic tests to identify and treat women with gestational diabetes
Introduction
Existing evidence indicates that hyperglycaemia in pregnancy is associated with a range of adverse perinatal and longer-term health outcomes. Chapters 2 and 3 of this report present evidence for a continuum of risk between adverse perinatal outcomes and increasing blood glucose levels (at OGTT), with no clear threshold below which there is no increased risk. A range of different glucose thresholds has been proposed, above which women are categorised as having GDM and thus identified to receive treatment to reduce hyperglycaemia and risk of adverse health outcomes. Diagnostic glucose thresholds for GDM have been informed by the level of excess risk of adverse health outcomes without consideration of the impact of diagnosis on health outcomes. In this chapter we evaluate the impact of treatment at alternative glucose thresholds in order to determine the threshold at which it is most cost-effective to intervene. We also consider the most cost-effective way of identifying a cohort of pregnant women with IGT for which treatment may be beneficial. Alternative options for identifying women for treatment include maternal characteristic/risk factor screening and blood glucose tests (OGCT).
Rates of hyperglycaemia will vary with gestational age because insulin resistance increases as pregnancy progresses. Lowering the gestational age at which hyperglycaemia is determined allows for earlier intervention but would be expected to detect fewer cases. Therefore, earlier detection would reduce exposure to hyperglycaemia in some pregnancies at the expense of increased exposure in others. Repeated testing for hyperglycaemia would minimise the number of missed cases, but at increased cost to the health service. Consequently, the number of potential alternative strategies for identifying women with hyperglycaemia is large and depends on the type and timing of screening, the type and timing of the diagnostic test, the number of screening and/or diagnostic tests offered, and the threshold for initiating treatment.
Treatment to reduce the risk of the adverse health outcomes associated with hyperglycaemia during pregnancy can be initiated on the basis of increased risk determined by screening and/or the results of a diagnostic test. Women who screen positive can be provided with information, advice and further tests. The benefits of screening may therefore include the impact of lifestyle advice on the risk of adverse health outcomes, the incentivising effect of being identified as high risk in persuading women to undergo further diagnostic testing and/or treatment, and a reduction in the number of diagnostic tests in women who are at low risk of hyperglycaemia. Women who test positive can be provided with lifestyle advice and, as necessary, pharmacological treatments such as metformin or insulin to reduce the risks associated with hyperglycaemia.
Methods
Overview
A decision tree model was developed to evaluate the cost-effectiveness of alternative strategies of combined screening, diagnosis and treatment of hyperglycaemia during pregnancy in the UK for a time horizon of 3 months in the base-case analysis. The best-performing strategy is identified by backward induction. At the first step the best-performing diagnostic glucose thresholds are identified (defining the best-performing diagnostic strategy). The second step is a full incremental comparison of all strategies composed variously of screening, diagnosis and treatment, but for which the diagnostic glucose thresholds are set at those identified at the first stage. Results are expressed in terms of costs and quality-adjusted life-years (QALYs). The perspective of the analysis is that of NHS and personal social services. The key modelling assumptions in the economic analysis are listed in Appendix 6, Table 69.
Cost-effectiveness is reflected in the model using the metric of net health benefit (NHB). The NHB of each strategy is the value of the incremental health benefits (ΔE) minus the health benefits forgone as a result of the increased costs (ΔC). Increased costs represent health costs because within a constrained budget any additional funds can be obtained only by reducing provision of other health-care activities. The rate at which displaced health-care activities generate health can be used to determine a cost-effectiveness threshold (k). A cost-effective intervention is one that generates more health per pound spent than the activities it displaces. The cost-effectiveness threshold can be used to convert monetary costs into health costs, or correspondingly to convert health gains to monetary gains. By this method a cost-effective intervention is simply one that has higher net benefits than alternative activities. The model estimates net monetary benefits (NMBs), according to equation 1:
The cost-effectiveness threshold utilised in the model to estimate net benefits was k = £20,000 per additional QALY, which corresponds to the lower bound of the threshold range currently used by NICE. 225 We also explored the impact of using alternative values of £13,000 and £30,000 per QALY. The latter relates to the upper bound of NICE cost-effectiveness range,225 and the former is based on recent research that was the first to use NHS routine data to provide an empirical estimate of the cost per QALY of the current NHS activities that would be displaced to release resources for new activities. 226
Screening, diagnosis and treatment of hyperglycaemia in pregnancy
Risk factor screening for hyperglycaemia in pregnancy
As described in Chapter 5, it is possible to identify pregnant women who are at increased risk of the adverse health outcomes associated with hyperglycaemia based on their characteristics. These ‘risk factors’ can be used in isolation or in combination to form risk factor screening strategies. For example, NICE recommends that pregnant women are assessed for risk of GDM18 and diagnostic testing is offered to all women who have one or more of the following risk factors:
-
BMI of ≥ 30 kg/m2
-
previous macrosomic baby
-
previous GDM
-
family history of diabetes
-
family minority ethnic origin with a high prevalence of diabetes.
The review presented in Chapter 5 compared alternative risk factor screening strategies composed of several different maternal characteristics. These maternal risk factors include advanced maternal age, high BMI, diagnosis of GDM in a previous pregnancy, previous macrosomic infant, multiparity, family history of diabetes and ethnicity associated with higher GDM prevalence than those of white European origin (namely SA, black or Middle Eastern origin). Two levels of risk were specified for two of the risk factors: maternal BMI (≥ 25 or ≥ 30 kg/m2) and maternal age (≥ 25 or ≥ 30 years). The number of possible risk factor screening strategies is large if all of the possible subsets of seven factors are considered (128), with further variations because of two levels for two of the factors (a further 128 unique strategies) and if a screen positive is defined by the presence of more than one factor. In order to reduce the number of risk factor screening strategies modelled we chose to focus only on strategies for which screen positivity required the presence of at least one characteristic (e.g. BMI ≥ 30 kg/m2 or previous GDM) rather than those that require the presence of all characteristics (e.g. BMI ≥ 30 kg/m2 and previous GDM). We evaluated the sensitivity and specificity of all such strategies over a range of diagnostic thresholds and excluded those strategies that were dominated in terms of sensitivity and specificity for all diagnostic thresholds (i.e. less sensitive and no more specific, or less specific and no more sensitive than one or more other strategies). This left 68 non-dominated risk factor screening strategies, to which the risk factor screening strategy utilised by NICE18 was added, giving a total of 69 modelled risk factor screening strategies. The list of included risk factor screening strategies can be found in Appendix 6, Table 70.
Blood-based tests for hyperglycaemia in pregnancy
There are a number of blood tests that can be administered to measure blood glucose levels. The level of blood glucose that would be regarded as normal depends on the interval between the blood sample and the last ingestion of glucose, and the amount of glucose ingested. The amount of glucose ingested prior to the sample being taken can be standardised by providing a glucose load and/or by asking the woman to fast prior to testing. FPG is typically assessed by obtaining a blood sample after an overnight fast of approximately 12 hours. Post-load glucose response can be measured, with increasing standardisation, on the basis of (1) a random plasma glucose (RPG) sample in which there is no control over the timing or amount of prior glucose ingestion; (2) a plasma glucose sample obtained after the woman has ingested a set glucose load; or (3) a plasma glucose sample obtained after the woman has fasted overnight and then ingested a set glucose load. RPG can be assessed with no preparation or wait time. The OGCT provides a post-load measure of plasma glucose 1 hour after ingestion of a set glucose load, typically 50 g. The OGTT provides both a fasting glucose level and one or more post-load glucose levels taken at fixed intervals after ingestion of a set glucose load, typically 75 g or 100 g. Traditionally in the UK, only the OGTT has been used for diagnosis of GDM. The distinction between screening and diagnosis is in how the results of the different tests influence the subsequent care pathway for individuals. Following a positive screening test women are not yet regarded as having the condition (in this case GDM) and may be offered further testing and/or preventative interventions. When such tests are administered for the purposes of screening, the thresholds are often set towards high sensitivity. The OGCT is typically provided in this manner, in which those who screen positive go on to receive an OGTT. Following a positive diagnostic test (OGTT), women are regarded as having GDM and can be provided with treatment without further testing.
The review presented in Chapter 3 included one blood-based screening test: the 1-hour 50-g OGCT. This test requires women to ingest a 50-g glucose load and a sample of blood is collected at 1 hour following ingestion. Women whose 1-hour blood glucose values are equal to or above a predetermined screening threshold are identified as being at a higher risk of GDM than women below the threshold, and are offered a diagnostic test. The screening threshold value is usually set between 7.2 and 7.8 mmol/l. 227 The OGCT was not administered to women in the BiB22 or Atlantic DIP59 cohorts, but was included in the model as an alternative to risk factor screening strategies.
The commonly used diagnostic test of choice is an OGTT. In practice, two alternative glucose loads are utilised: 75 g and 100 g. At present, there is a range of criteria for determining the presence of GDM on the basis of exceeding thresholds for the post-load glucose levels variously in combination with thresholds for the fasting glucose levels (see Chapters 2 and 3). Although the fasting levels should be comparable between the 75-g and 100-g tests, the post-load measures may not be directly comparable because of the difference in glucose load and any differences in timing of assessment. Although we do not directly compare the 75-g and 100-g tests, the review presented in Chapter 3 indicates that the trends in outcome incidence with graded increases in glucose level are similar for the two diagnostic test loads, but that the associations were weaker for the 100-g OGTT than the 75-g test. In general the 75-g OGTT will be less costly than the 100-g OGTT, as it can be administered with only two (or three) blood samples over a 2-hour interval compared with up to four samples over a 3-hour interval for the 100-g test. Ingestion of the glucose load can be unpleasant and may induce vomiting in some women, which would preclude completion of the test. Acceptance and completion of the test may be more favourable with the 75-g glucose load compared with the 100-g load, as the load is less. 228
The diagnostic test modelled in our economic analysis is the 2-hour 75-g OGTT administered between 26 and 28 weeks’ gestation. This matches that used in the BiB22 and Atlantic DIP59 cohorts, from which we had access to IPD, and corresponds to current practice in the UK. 18 Diagnosis based on this test relies on dual glycaemic thresholds, with the 2-hour post-load glucose threshold identifying additional women who would be considered normoglycaemic on the basis of their fasting blood glucose levels alone. We varied the glucose threshold in increments of 0.1 mmol/l between 5.0 and 7.5 mmol/l for fasting glucose and between 5.5 and 10 mmol/l for post-load glucose, and thereafter in increments of 0.5 mmol/l up to a final limit equal to 11.1 mmol/l (the threshold at which overt diabetes is diagnosed). By assuming that the post-load glucose threshold should be at least 0.5 mmol/l higher than the corresponding fasting glucose threshold, this provided 969 alternative dual glycaemic thresholds. The first step of the economic analysis compares these 969 potential dual glycaemic thresholds to identify the fasting and post-load glucose levels that would provide the greatest expected NHB compared with all other possible fasting and post-load glucose levels. This best-performing diagnostic glucose threshold is then set within the full set of alternative screening, diagnosis and treatment strategies in order (at the second step of the economic analysis) to identify the cost-effective strategy.
Treatment of hyperglycaemia
We sought to define treatment to be reflective of current practice for women diagnosed with GDM in the UK. This comprises provision of dietary and lifestyle advice then adding supplementary metformin or insulin if glucose levels are not adequately controlled. The effectiveness of treatment with dietary and lifestyle interventions, which can be provided in the absence of any blood glucose measures, was also modelled.
Intervention strategies for the identification and treatment of hyperglycaemia in pregnancy
The sections above have described how we determined which screening strategies and which diagnostic tests and glucose thresholds to include in the model. The full set of alternatives compared in the model are variously composed of screening, diagnostic tests and treatment, and are outlined below. The set of intervention strategies include:
-
No screening/testing or treatment.
-
Screen only Screening followed by dietary and lifestyle advice for those who screen positive (maternal characteristic/risk factor screening strategies, as outlined in Chapter 5, and 1-hour 50-g OGCT).
-
Universal diagnostic test Diagnostic test followed by dietary and lifestyle advice with pharmacological treatment as required for those who exceed either of the set fasting and post-load glucose levels (where the best-performing glucose thresholds are identified as outlined above in Blood-based tests for hypoglycaemia in pregnancy).
-
Screen and diagnostic test Screening followed by diagnostic test in those who screen positive, with dietary and lifestyle advice and pharmacological treatment as required for those who exceed either of the set fasting and post-load glucose thresholds (risk factor screening strategies combined with diagnostic test using best-performing glucose threshold).
Additional screen, diagnosis and treatment strategies
In addition to the base-case set of combined screening, diagnostic and treatment strategies, we included some additional scenario analyses to explore the use of an alternative diagnostic test to the OGTT and to explore the sensitivity of the results to alternative treatment options for women diagnosed with GDM.
A further screen and diagnostic test strategy that incorporated the use of a biochemical screening test – the 1-hour 50-g OGCT – was included but with the OGTT based on the diagnostic glucose threshold utilised in the source trial. This diagnostic glucose threshold differs from the best-performing glucose threshold identified in the model because we did not have sufficient information on how the sensitivity and specificity of the OGCT would vary with alternative diagnostic glucose thresholds to those used in the source trial. In other words, we were unable to combine screening with the OGCT with a subsequent diagnostic test, using the best-performing diagnostic glucose threshold.
The FPG requires only one measurement of blood glucose with no wait time, and so may be more convenient than the OGTT. We included an exploratory analysis to assess the utility of FPG as a diagnostic test, although current clinical practice does not recommend the FPG alone. As there is a fasting blood glucose component of the 75-g OGTT reported in the BiB data,22 these fasting glucose values obtained with the OGTT in the BiB study22 were used to model the FPG test performance.
We did not seek to directly compare alternative treatment options for women diagnosed with GDM. However, in a sensitivity analysis (see Treatment costs), we considered the cost implications of replacing supplementary insulin use (in addition to diet and lifestyle) with metformin, as the results of the review presented in Chapter 6 indicate that metformin seems to be at least as effective and possibly superior to insulin, and is potentially more acceptable to women who are inadequately controlled by lifestyle modification alone. The base-case analysis incorporated treatment only to reduce the risk of immediate perinatal outcomes associated with hyperglycaemia. In a secondary analysis we explored the impact of early treatment and prevention of type 2 diabetes among women who experience hyperglycaemia in pregnancy.
Further sensitivity analysis was conducted to explore the potential impact of alternative assumptions in terms of treatment effectiveness, costs and uptake of diagnostic tests.
Decision-analytic model
Screening, diagnosis and treatment
A decision-analytic model was developed to characterise the risk of adverse perinatal and longer-term maternal health outcomes as a function of blood glucose levels. The outcomes considered were those associated with hyperglycaemia during pregnancy, which impacted on any maternal or neonatal health-related quality of life (HRQL), survival or health-care resource use. The choice of perinatal outcomes was informed by analysis of IPD from the BiB22 and Atlantic DIP59 cohorts, and supplemented with additional outcomes identified in previous reviews of screening and treatment for GDM. 18 The choice of longer-term maternal health outcomes was informed by existing evidence.
Pregnant women enter the model depicted in Figure 43, and a decision is made on whether to screen them or not. If screening is undertaken, the cohort is divided into two groups: those who screen positive (S+) and those who screen negative (S–). This is followed by a decision regarding whether or not to offer a diagnostic test to women with positive screening tests. Women who screen negative will not be offered any treatment or further testing. If a diagnostic test is provided to women who screen positive, those who have both screened and tested positive go on to receive treatment (S+T+). Those who screen positive but test negative are not offered any further testing or treatment (S+T–). If screening is not undertaken then all women may still be offered a diagnostic test, with subsequent treatment for those who test positive (T+). Women who test negative (T–) would not be offered any further testing or treatment. We assume that if women are not screened or tested then treatment would not be provided. It is assumed that treatment in the absence of blood glucose measurement can include dietary and lifestyle interventions, but will exclude pharmacological interventions, such as insulin and metformin. In other words, treatment is offered to those who screen positive (S+) and OGTT is not offered before receiving dietary and lifestyle interventions, which differs to the treatment that can be provided in those who test positive [with an OGTT (S+T+ and T+)].
FIGURE 43.
Model structure. NNU, neonatal unit.
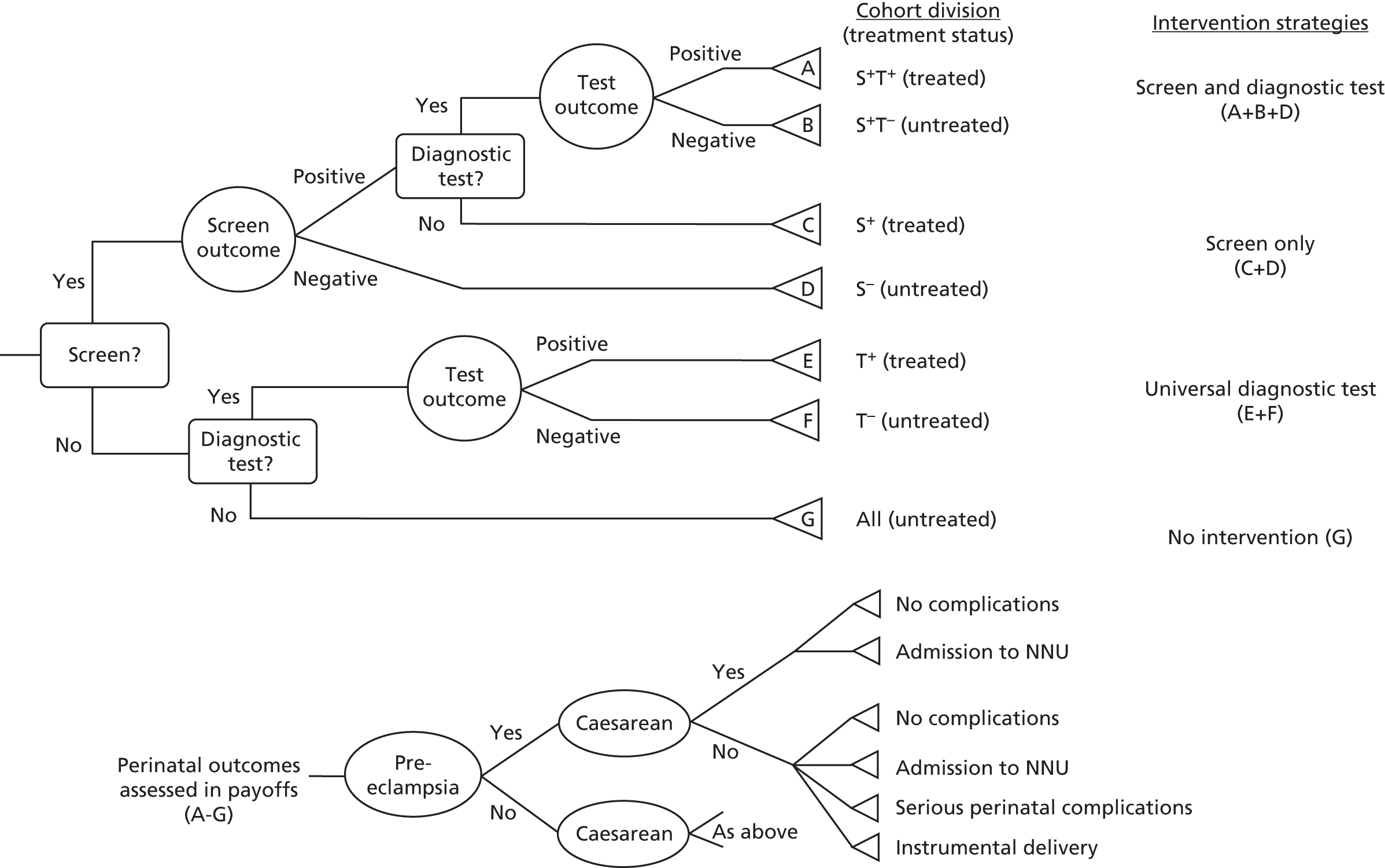
Adverse perinatal outcomes
The adverse perinatal outcomes included in the model identified from the BiB and Atlantic DIP cohorts22,59 include:
-
pre-eclampsia
-
C-section
-
shoulder dystocia
-
instrumental delivery
-
induction of labour
-
admission to a neonatal care unit
-
macrosomia.
The recently updated NICE guideline for diabetes in pregnancy18 identified two further adverse health outcomes: birth trauma and neonatal death. We follow the assumption used within those guidelines that the rate of birth trauma and neonatal death is proportional to the rate of shoulder dystocia (see Risk models for the prediction of adverse perinatal outcomes). For simplicity of presentation, these three outcomes are combined in the composite outcome ‘serious perinatal complications’ within the decision tree.
The model structure allows for the fact that the probability of C-section differs between women with and without pre-eclampsia. Similarly, the probability of admission to a neonatal care unit was affected by the occurrence of C-section and pre-eclampsia. It was assumed that shoulder dystocia and instrumental delivery would not occur in women who underwent C-section. This is illustrated by the subtree depicted in Figure 43. Model assumptions are listed in Appendix 6, Table 69.
For simplicity of representation, the tree (see Figure 43) does not show induction of labour, although the probability of this adverse outcome is incorporated into the model for all women in a similar way to that of pre-eclampsia, that is, it is not affected by the presence of any other outcomes. The models and data sources used to determine the risk of outcomes are presented in more detail below (see Baseline probabilities of perinatal outcomes).
In the base-case analysis we include only immediate perinatal and maternal adverse outcomes. In a secondary analysis the model incorporates longer-term maternal outcomes among women who are treated for hyperglycaemia in pregnancy. Women with GDM have a sevenfold higher risk of developing type 2 diabetes later in life compared with women who were normoglycaemic during pregnancy. 2,229 Diagnosing women with GDM can therefore identify a cohort of women who are at high risk of future type 2 diabetes. Women diagnosed with GDM are routinely invited for blood glucose assessment post partum. A proportion of women who present as hyperglycaemic during pregnancy will be found to have persistent glucose intolerance and subsequently diagnosed with type 2 diabetes post partum. In these women, appropriate treatment can begin immediately. Continued monitoring of women identified with hyperglycaemia in pregnancy allows for the earlier identification, treatment and possible prevention of type 2 diabetes. The longer-term maternal outcomes included in the model are:
-
prevalence of undiagnosed overt type 2 diabetes
-
incidence of type 2 diabetes associated with prior GDM.
Hypothetical cohorts of pregnant women move through the model to estimate the overall impact on health outcomes and costs associated with each strategy. Mean levels of FPG, post-load plasma glucose and risk factors are estimated for the possible subdivision of the cohort (all women, T+, T–, S+, S–, S+T+, S+T–). Adverse perinatal health outcomes are reflected in the base case in terms of their risk and the decrements in HRQL associated with them. Treatment has the effect of reducing the risk of those outcomes. Thus, the least effective strategy will be associated with the largest overall health loss in terms of QALYs, and the most effective strategy will be associated with the least health loss. Further to this, any antenatal maternal health gains from treatment are characterised in terms of maternal HRQL (see Health-related quality of life for further details).
Costs included in the model are those associated with tests (screening and diagnostic), adverse perinatal outcomes, and the costs of treatment for GDM. Both health outcomes and costs are calculated for the period from the beginning of the third trimester of pregnancy until the infant’s birth, that is, for the duration of the time horizon. The benefits and costs of early treatment of type 2 diabetes and preventative measures to reduce the probability of developing type 2 diabetes in later life were also included in the model as one-off benefit and cost for the purposes of the secondary analysis.
Evaluating the decision tree
At each decision node, the decision between alternative branches is taken so as to maximise NHB (screening/no screening; diagnosis/no diagnosis; treatment/no treatment) and the best-performing overall strategy is defined by backwards induction (i.e. working backwards through the tree) such that NMB is maximised over the combined set of alternatives (see Intervention strategies for the identification and treatment of hyperglycaemia in pregnancy). The method of backward induction means that we first identify the best-performing dual glycaemic threshold to initiate treatment, that is, the fasting and post-load glucose levels that would provide the maximum NHB among the full range of possible dual glycaemic thresholds, given the observed results of an OGTT. The performance of alternative screening and test strategies is then evaluated on the basis that subsequent testing and treatment is determined by this best-performing glucose threshold.
Data used to populate the model
In the following sections, we describe how the risk models for adverse perinatal outcomes were estimated based on IPD, and how perinatal and maternal longer-term outcomes were implemented in the model based on previously published evidence. We also present the parameter values for treatment effects, uptake of diagnostic tests, health benefits and costs that were applied in the base case and sensitivity analysis.
Baseline probabilities of perinatal outcomes
Risk models for the prediction of adverse perinatal outcomes
The risk models estimated in Chapter 3 show a log–linear relationship between both fasting and post-load glucose measures and the risk of a range of perinatal outcomes. The economic model incorporates the baseline risks of adverse outcomes that were considered to have an impact on maternal or neonatal HRQL or associated costs, and on data collected in the BiB and Atlantic DIP22,59 data sets. We further included in the base case the risk of macrosomia (BW of ≥ 4.5 kg), although no cost or HRQL impact was included for this outcome. Macrosomia may be associated with longer-term outcomes, such as childhood obesity, diabetes and metabolic disorders in later life. 4,5,230 The aim was to build the economic model with the flexibility to link to further longer-term adverse outcomes and potentially to extend the model once follow-up data on the children from the BiB cohort22 are available. The perinatal outcomes included in the model were:
-
pre-eclampsia
-
C-section
-
labour induction
-
serious perinatal complication, including: shoulder dystocia, birth trauma and neonatal death (and/or stillbirth)
-
admission to NICU
-
instrumental delivery
-
macrosomia.
The risk models estimated in Chapter 3 were the basis for the risk models applied in the economic model. The risk models were adapted in order to reflect interdependence between outcomes as depicted by the model structure, and to combine both fasting and post-load glucose measures into a single model. The inclusion of the fasting and post-load glucose measurements into a single risk model allows the exploration of the impact on adverse outcomes of varying the dual diagnostic threshold. The potential interdependence between outcomes (e.g. between pre-eclampsia and C-section) was incorporated by including as independent covariables in the risk model for each adverse outcome any outcomes that precede it in the decision tree structure (see Figure 43). For example, the probability of C-section was estimated by including the occurrence of pre-eclampsia as an independent covariable in the risk model. The risk model for admission to the NICU included the occurrences of C-section and pre-eclampsia as independent covariables. Shoulder dystocia and instrumental delivery were assumed to be mutually exclusive from C-section, and therefore the probabilities of shoulder dystocia and instrumental delivery outcomes were estimated among women who did not undergo C-section.
The data set used to estimate the probabilities of adverse perinatal outcomes in untreated women comprised women in the BiB and Atlantic DIP22,59 data sets who were not considered eligible to receive treatment for GDM (i.e. those with blood glucose levels of < 6.1 mmol/l at fasting, and < 7.8 mmol/l 2 hours after a 75-g OGTT test). Multiple imputation by chained equations (MICE) was used to handle missing data in outcomes and covariables for each of the data sets, prior to combining them. This method replaces missing values with multiple imputed values based on observed characteristics, and thus assumes that the pattern and values of the missing data are dependent on observable characteristics alone. 231 The main advantage of this method is that it is less likely to yield biased and inefficient estimates than complete case analysis, while incorporating the uncertainty associated with the imputation method in the estimates that replace the missing values. 40
Distributions of variables from the pooling of the data sets with imputed variables were similar to those for observed variables. Two outcomes were considered to be inadequately captured within the Atlantic DIP59 data set such that the preferred estimation sample was limited to women from BiB cohort. Induction of labour was not recorded in Atlantic DIP;59 and the level of missingness for instrumental delivery in the Atlantic DIP59 data set (approximately 25%) was considered too high to be adequately addressed with the application of MICE. The output of the logistic regressions for each risk model used to predict perinatal outcomes is reported in Appendix 6, Table 70.
In order to capture the additional adverse outcomes of neonatal death and birth trauma among women identified with GDM it was assumed that the probability of these outcomes would be proportional to the rate of shoulder dystocia alone. This follows the assumption used in NICE updated guideline for diabetes in pregnancy18 and a previous cost-effectiveness study identified in the guideline,232 in which a composite outcome of serious perinatal complications was defined to include shoulder dystocia, neonatal death and birth trauma. The guideline reported data from two RCTs, selected by Round et al.,232 which evaluated the impact of treatment for GDM on perinatal complications: the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial51 and the study by Landon et al. 52 These two trials51,52 had been selected from a meta-analysis of five trials on the effects of treatment for GDM,169 in which Round et al. 232 considered that the remaining three trials did not use adequate randomisation methods. The number of events in both treated and untreated (routine care) arms was pooled across the two trials to estimate the total number of fatal events (neonatal and stillbirth), birth trauma and shoulder dystocia. Birth trauma was defined as brachial plexus palsy or clavicular, humeral or skull fracture in Landon et al. ,52 whereas the ACHOIS trial51 reported only bone fracture and nerve palsy. All of these events were assumed equivalent and aggregated as a category defined as birth trauma in the NICE guideline. 18 Table 29 details the number of events from each trial and the pooled number of events used in the NICE guideline model to estimate the relative proportion of each outcome (death, shoulder dystocia and birth trauma) to the total composite number of serious perinatal complications.
| Outcomes | ACHOIS 200551 (n) | Landon 200952 (n) | Pooled (n) | Relative frequency | ||
|---|---|---|---|---|---|---|
| Routine care | Treatment | Routine care | Treatment | |||
| Death | 5 | 0 | 0 | 0 | 5 | 0.076 |
| Shoulder dystocia | 16 | 7 | 18 | 7 | 48 | 0.727 |
| Birth trauma | 4 | 0 | 6 | 3 | 13 | 0.197 |
| Serious perinatal complications | 25 | 7 | 24 | 10 | 66 | 1.00 |
A multiplier of 1.37 was applied to the baseline risk of shoulder dystocia estimated in the BiB and Atlantic DIP22,59 data to adjust it to the risk of serious perinatal complications. This follows the way in which this outcome was modelled in the NICE updated guideline for diabetes in pregnancy. 18 The multiplier is the inverse of 0.73, that is, the inverse of the proportion of all serious perinatal complications that corresponded to shoulder dystocia (48/66) pooled from the two RCTs. 51,52 The use of pooled data from the two trials51,52 assumes that the two populations are equivalent despite the differences in diagnostic test glucose load between the trials (100 g in Landon et al. ,52 75 g in Crowther et al. 51), and the relative frequency of each individual component of the serious perinatal complications outcome is similar in both trials.
Prevalence of undiagnosed overt maternal type 2 diabetes
The prevalence of undiagnosed overt type 2 diabetes among women who were diagnosed with hyperglycaemia in pregnancy was assumed to be 11%, based on a study233 that measured the rate of type 2 diabetes among women who would be diagnosed with GDM based on the 1999 WHO criteria. 11 We have sourced the prevalence parameter from this study233 because the data were collected from an obstetric population attending the same NHS Trust as those in the BiB cohort (the Bradford Teaching Hospitals Trust) and, therefore, it is likely to reflect the characteristics of the BiB study22 population. This estimate is in line with estimates of between 7% and 11.6% reported in other studies that have been identified to inform rates of uptake of post-partum follow-up (see Uptake of screening, diagnosis and treatment). 127,234 A further study235 was identified, which reported a much lower estimate of prevalence of type 2 diabetes detected post partum (2.4%). However, this study235 was conducted in a population where 86% of women were Caucasian and was, therefore potentially less reflective of the BiB cohort. 235
Incidence of type 2 diabetes among women with a history of gestational diabetes mellitus
Women with GDM have a sevenfold increase in the risk of developing type 2 diabetes later in life compared with women who were normoglycaemic during pregnancy. 2,229 The estimated incidence of type 2 diabetes among women with a history of GDM was taken from women who were randomised to receive placebo in the Diabetes Prevention Program Outcome Study (DPPOS). 236,237 Women recruited to the DPPOS236,237 were on average 12 years post pregnancy. A history of GDM was associated with an additional 10-year incidence of 14.8% (64.7% cumulative incidence in women with a self-reported history of GDM compared with 49.9% in women without a history of GDM). The benefit of this approach to estimating the risk of type 2 diabetes is that it allows us to isolate the proportion of type 2 diabetes that could be predicted only on the basis of diagnosing GDM (by controlling for the correlated risk factors of IGT and obesity). The DPPOS236,237 was conducted in a US setting in obese women with IGT (defined as fasting glucose between 5.2 and 7.0 mmol/l). 236,237 Consequently, we assumed that this increased risk of type 2 diabetes applied to only the proportion of women who test positive for GDM and have a BMI of ≥ 30 kg/m2.
Treatment effects
The impact of treatment on perinatal outcomes was incorporated by means of a RR reduction, as reported in the systematic review and meta-analysis in Chapter 6. The impact of treatments on the risks of longer-term adverse maternal health outcomes were informed by literature and included in exploratory analysis. Where the available evidence related outcome risks to diagnosis of GDM and not blood glucose level, additional assumptions were required as to the impact of altering the diagnostic glucose threshold. These assumptions are reported in detail in Appendix 6, Table 69.
In the model, the diagnostic glucose threshold for treatment determines the proportion of women who are treated for GDM in each strategy, and therefore the proportion of women whose baseline risk of adverse outcomes will be modified by applying a RR to estimate the ‘treated’ probability of each perinatal outcome. The estimates of RR applied in the model for the adverse outcomes were sourced from the treatment review (see Chapter 6). The base-case analysis used the meta-analysis that compares diet modification or advice – accompanied by glucose monitoring and insulin use in some women – to routine antenatal care. This treatment ‘bundle’ was selected as it more closely reflects the current practice for the treatment of GDM in the UK, that is, diet and exercise modification as first-line treatment followed by pharmacological therapy (metformin and/or insulin) if first-line therapy is unsuccessful. 18 Although the meta-analysis of trials of diet modification did not include metformin, it was assumed, nevertheless, that the effectiveness of the treatment ‘bundle’ would not change by replacing insulin with metformin in a proportion of the women treated for GDM. This assumption was based on the review presented in Chapter 6, which suggests that the effects of metformin and insulin were generally comparable and that there was a trend for metformin to perform better, or at least no worse, than insulin for all adverse outcomes reported, with the exception of assisted/instrumental delivery.
The base-case analysis assumes that all of those who test positive (S+T+ or T+) will undergo treatment. As treatment effects are derived from an intention-to-treat analysis, it is assumed that compliance and adherence will be reflected within the RR estimates. As the fasting and post-load glucose levels above which treatment is offered are varied in the model, an assumption is required as to whether or not the magnitude of the relative treatment effect will remain constant regardless of the mean glucose levels in the treated groups. Subgroup analysis by definition of GDM (see Chapter 6) did not find evidence that the effect of diet modification varied according to the population included in the trials in terms of levels of glucose (GDM, mild GDM, IGT or women who screened positive with OGCT, but tested negative with OGTT). In the base case, the relative treatment effect on adverse perinatal outcomes is assumed constant, regardless of the fasting and post-load glucose thresholds above which treatment is initiated (although it should be noted that the baseline risk of those outcomes, and thus the absolute risk reduction offered by treatment, is adjusted with those thresholds). Although data collected on BiB and Atlantic DIP22,59 refer to admissions to neonatal care (in which intensive care is also included), data that were specific to admissions to NICU were not available. As NICU is the outcome for which the treatment affect is reported in Chapter 6, we assumed that the treatment effect on neonatal admissions would be equivalent to that for NICU. Table 30 shows the base-case estimates of the RR for each adverse outcome in the model with treatment.
| Adverse perinatal outcome | RR | SE log RR |
|---|---|---|
| NICU | 0.91 | 0.197 |
| Shoulder dystocia | 0.39 | 0.280 |
| C-section | 0.86 | 0.054 |
| Pre-eclampsia | 0.58 | 0.242 |
| Labour induction | 1.12 | 0.157 |
| Instrumental delivery | 1.37 | 0.979 |
| Macrosomia | 0.46 | 0.130 |
As the trials included in the meta-analyses had some variation in terms of treatment delivered, and uncertainty over the length of time insulin was used, a scenario analysis was also conducted with alternative RR estimates as used in a previous study232 and applied in the NICE updated guideline for diabetes in pregnancy. 18 Round et al. 232 pooled the results of two high-quality trials51,52 that allowed treatment with insulin in addition to diet modification to estimate treatment effects for each outcome. These trials51,52 are a subset of the trials included in the treatment review (see Chapter 6, Trials comparing different types of diet modification). The estimated RRs used in the scenario analysis are reported in Table 31.
| Adverse perinatal outcome | RR | SE log RR | Source |
|---|---|---|---|
| NICUa | 0.77 | 0.194 | Landon52 |
| Shoulder dystocia | 0.41 | 0.314 | Crowther,51 Landon52 |
| C-section | 0.88 | 0.068 | Crowther,51 Landon52 |
| Pre-eclampsiab | 0.46 | 0.345 | Landon52 |
| Labour induction | 1.17 | 0.069 | Landon52 |
| Instrumental deliveryc | 1.37 | 0.979 | Diet modification meta-analysis (see Chapter 6) |
| Macrosomiad | 0.47 | 0.161 | Crowther51 |
The only adverse effect of treatment which is considered sufficiently important to impact significantly on costs or outcomes was hypoglycaemia. We incorporated this adverse effect following the same approach utilised in the NICE updated guidelines to estimate a probability of severe hypoglycaemia. The probability of hypoglycaemia for women treated with insulin was sourced from a trial that compared the effectiveness of insulin and glibenclamide in GDM, and corresponded to 0.202. 187 In the NICE updated guideline it was assumed that there was a 0.05 probability of severe hypoglycaemia in those women treated for GDM who developed hypoglycaemia as an adverse effect. 18 In our analysis we applied the same probability of severe hypoglycaemia (0.05 × 0.202 = 0.010) to women who received treatment with insulin, but assumed that metformin would not be associated with severe hypoglycaemia.
It was assumed that treatment in the absence of blood glucose testing would not include pharmacological interventions. The treatment effects on adverse perinatal outcomes applied to women who are treated without undergoing a blood glucose test (i.e. S+ in the screen-only strategies) are sourced from a review on the effects of dietary and lifestyle interventions on obstetric outcomes. 224
The Thangaratinam review224 (Table 32) does not report estimates for instrumental delivery or macrosomia. For the latter, this is not an issue, as macrosomia does not impact on costs or health benefits in the current model set up (see Baseline probabilites of perinatal outcomes). For instrumental delivery we assume that it had the same treatment effect as the base-case treatment (i.e. RR = 1.37).
| Perinatal outcome | RR | SE log RR |
|---|---|---|
| NICU | 1.00 | 0.146 |
| Shoulder dystocia | 0.39 | 0.295 |
| C-section | 0.93 | 0.044 |
| Pre-eclampsia | 0.74 | 0.109 |
| Labour induction | 1.12 | 0.059 |
| Instrumental delivery | – | – |
| Macrosomia | – | – |
Treatment for longer-term maternal outcomes
Women who are diagnosed with GDM are routinely invited for blood glucose assessment post partum. A proportion of women who present with hyperglycaemia during pregnancy will be found to have persistent glucose intolerance post partum, and may be diagnosed with type 2 diabetes. In these women, appropriate treatment can begin immediately. The benefits of early treatment of type 2 diabetes will depend on whether or not those women would have been identified with type 2 diabetes in the absence of pregnancy screening and, if they would have been identified, at what point in time. We were unable to directly model the benefit of early detection of type 2 diabetes among pregnant women. Instead, the potential benefits of the early detection of undiagnosed type 2 diabetes were informed by a study238 that evaluated the cost-effectiveness of screening for type 2 diabetes. We used estimates of the QALY gain and incremental costs associated with a one-off screening strategy for type 2 diabetes in order to estimate a NHB for those women diagnosed with GDM who are found to have type 2 diabetes at 6 weeks’ post-partum follow-up. It is important to emphasise that the population in the Gillies study238 is different from the group for which they are being applied here (model cohort included both men and women, and the base-case estimated cost and QALYs for screening at 45 years old). This estimate is intended to explore the potential impact of early detection and is incorporated in only a secondary analysis.
Diagnosing women with hyperglycaemia in pregnancy identifies a cohort of women at high risk of future type 2 diabetes. Continued monitoring of these women post partum allows for the early identification, treatment and possible prevention of type 2 diabetes. The rate of type 2 diabetes that could be potentially avoided among women diagnosed with hyperglycaemia in pregnancy was determined by combining the additional risk of developing type 2 diabetes that is associated with previous GDM with evidence for the reduction in risk from a preventative treatment package that could be offered to the proportion of women that are followed up post partum. This preventative treatment package was defined in terms of the intensive lifestyle intervention (ILS) utilised in the Diabetes Prevention Program (DPP) study236,237 and DPPOS,239 which was associated with a RR of 0.352 for incidence of type 2 diabetes. The increased incidence of type 2 diabetes attributable to testing positive for hyperglycaemia in pregnancy would be expected to vary with the diagnostic threshold. However, the estimates for this RR were available based on diagnosis of GDM determined at a fixed threshold. These longer-term maternal benefits of prevention are applicable only to the proportion of women who test positive and have a BMI of ≥ 30 kg/m2. As the rate of obesity among women who test positive does vary with the diagnostic threshold, this allows the longer-terms benefits to be a function of the alternative diagnostic thresholds.
Uptake of screening, diagnosis and treatment
The effects of screening and treatment were further reduced in the model by assumptions about rates of uptake. Uptake of screening and diagnostic tests has been found to be an important area of uncertainty in previous NICE guidance,240 although it was not incorporated in the economic model in the updated guideline. 18 In our model, we evaluate uptake at four points relating to the offering of screening and diagnostic tests and treatment.
Uptake of:
-
screening
-
diagnostic test
-
post-partum blood glucose tests
-
preventative interventions post partum.
In all cases we assume that uptake is not a function of the population characteristics. There is evidence to suggest that uptake of screening varies with the type of test. 228 In the economic analysis we focus on risk factor screening strategies and consider scenarios for screening with OGCT. We assume that uptake of risk factor screening (assessment of maternal characteristics) can be 100%, as it can easily be integrated in current routine antenatal care. The performance of risk factor screening strategies may be affected if some factors are difficult to determine in practice (e.g. family history of diabetes).
Available evidence also suggests that uptake of diagnostic tests is higher in a population identified by screening as high risk of GDM than in an unscreened population (and therefore universally tested). 124,228 We assume that uptake of OGTT is 63% in an unscreened population and 89% in a risk factor-screened population. That is, risk factor screening increases uptake of the diagnostic test by > 30%. The estimate for the uptake of universal OGTT is sourced from a study124 based on routine hospital data from Bradford in the period between 2008 and 2010. The same study124 also provides an estimate of 99% for the uptake of OGTT on a risk factor-screened group corresponding to an earlier period (2004–6). Based on the advice of the study authors, we considered that this latter estimate could be artificially high, as uptake may have been increased by more intensive pursuit of participants with telephone reminders that may not reflect current practice across the UK. Thus, in the base case we averaged the uptake estimates in risk factor-screened populations from two UK studies,124,235 resulting in an estimated uptake of approximately 90%. We explore the impact of these assumptions on diagnostic uptake in a sensitivity analysis, in which we simultaneously apply a higher uptake of diagnostic estimate for an unscreened population and a lower estimate for a risk factor-screened population. The alternative diagnostic uptake estimates are sourced from an unscreened population (73%),150 and from a risk factor-screened population (80%),235 and correspond to more extreme estimates of the parameters found in the literature. This scenario analysis aims to test if the model is sensitive to smaller improvements in uptake of diagnostic tests in a screened population in comparison with an unscreened one.
Post-partum follow-up may also be influenced by the type of screening. This assumes that, conditional on diagnosis of GDM, the experience of having been risk factor screened prior to diagnosis influences uptake of further screening. Gregory et al. 127 reported an uptake of follow-up for women with GDM who were identified with universal OGTT of 52%. For women who were previously screened by risk factors, a higher estimate of follow-up uptake was identified (83%) from a retrospective study233 based on routine hospital data collected in Bradford. We assumed that 52% of women who were never screened attend for glucose testing at 6-week postnatal follow-up. We assumed that 83% of women who were screened for being at high risk of having GDM would attend for glucose testing at 6-week postnatal follow-up. Alternative scenarios were undertaken with estimates from a retrospective US study241 of women with GDM, which indicated that 38% would have any type of glucose testing post partum and 23% would be screened with an appropriate test (FPG or OGCT). As it is not clear whether or not women in the study241 were screened or not for GDM, the estimates were applied to a set of strategies with and without selective screening.
Test characteristics of screening
The presence of maternal risk factors in the hypothetical cohort and the mean fasting and post-load glucose measures for each cohort (all, T–, T+, S–, S+T+, S+T–) are estimated over the range of alternative dual glycaemic thresholds using the BiB study22 IPD. In other words, the sensitivity and specificity of the alternative risk factor screening strategies was estimated directly using the BiB cohort22 data.
A screening strategy based on providing all women with a 1-hour 50-g OGCT was explored by applying estimates of test performance (sensitivity and specificity compared to the 2-hour 75-g OGTT) sourced from the literature. 242 The study242 used to inform test performance in the model was identified in the NICE guidance and applied in their economic model. 18 The reported sensitivity (80%) and specificity (43%) of the OGCT correspond to the diagnostic threshold used in the study,242 which was a fasting glucose level of ≥ 7.0 mmol/l or a post-load glucose level of ≥ 7.8 mmol/l. These estimates of sensitivity and specificity were combined with the proportion of women that would be identified as having GDM (T+) based on BiB data22 at this same diagnostic threshold. We assumed that true positives (S+T+) would have the same mean blood glucose levels as women who tested positive (T+) and that false positives (S+T–) would have the same mean blood glucose levels as those who test negative (T–). Similarly, the mean blood glucose levels for those who screen negative was estimated by assuming that true negatives would have the same mean blood glucose levels as those who test negative, and false negatives would have the same mean blood glucose level as those who test positive. Table 33 summarises the data from South Asian et al. ,242 alongside the proportion of women in the model (S+, S–, S+T+ and S+T–) that was estimated based on that study.
| Economic model | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reported (n) | Calculated proportion | |||||||
| Parameter | ||||||||
| T+ | T– | T+ | S+T+ | S+T– | S+ | S– | T– | |
| S+ | 134 | 414 | 0.08 | 0.06 | 0.26 | 0.32 | 0.68 | 0.92 |
| S– | 34 | 309 | – | – | – | – | – | – |
| Mean fasting glucose | 5.24 | 5.24 | 4.46 | 4.65 | 4.54 | 4.47 | ||
| Mean post-load glucose | 9.12 | 9.12 | 5.39 | 6.30 | 5.71 | 5.39 | ||
Health-related quality of life
The health benefits of the alternative strategies are summarised in terms of mean QALY by adjusting the period of time spent in alternative health states by the HRQL (also referred to as utility value) that is associated with that health state. In the model, we estimated the QALY loss associated with the occurrence of adverse outcomes, the QALY gains from treatment of GDM and from ILS to reduce the risk of future maternal type 2 diabetes.
Health-related quality of life loss from adverse perinatal outcomes
The NICE updated guideline18 for diabetes in pregnancy included QALY loss only from serious perinatal complications in the economic model. As we considered that other perinatal outcomes would also imply HRQL loss, we sourced QALY loss estimates for those outcomes from NICE clinical guidelines, as described throughout this subsection.
The perinatal outcomes for which a QALY loss was applied in the model were:
-
pre-eclampsia
-
C-section
-
serious perinatal complications
-
instrumental delivery.
Pre-eclampsia
The NICE hypertension in pregnancy guideline243 assumed that the QALY loss from pre-eclampsia could be attributed to severe complications of pre-eclampsia alone, and calculated that the weekly QALY loss from these would correspond to 0.019. The time spent in this health state was assumed to be 2 weeks, the maximum number of weeks that women would spend in treatment for the severe complications. 243 This 2-week QALY loss from severe complications of pre-eclampsia is multiplied by the probability of developing severe complications conditional on having pre-eclampsia as sourced from the hypertension in pregnancy guideline. 243 The resultant QALY loss associated with pre-eclampsia applied in the model was calculated as a total loss of 0.00456 QALYs.
Caesarean section
The estimate of QALY loss from C-section was adapted from the NICE clinical guideline on C-section,33 and consisted of the difference between the expected HRQL in women with adverse outcomes from C-section compared with that for a vaginal delivery. This weighted average of HRQL for each mode of delivery is calculated by multiplying the utility loss of individual adverse outcomes, namely maternal death, hysterectomy, hypoxic–ischaemic encephalopathy and urinary incontinence, by the risk of those outcomes depending on the mode of delivery. In the C-section guideline, the weighted average also included neonatal death, which in our model is accounted for through the QALY loss associated with serious perinatal complications, and thus was excluded from these calculations. This yields a QALY loss of approximately 0.0017 for C-section. If neonatal death had been included in the calculation the QALY loss associated with C-section would have been approximately 0.030. Table 34 summarises the calculation of QALY loss from C-section.
| Outcome | QALY loss | Vaginal delivery | C-section | ||
|---|---|---|---|---|---|
| Risk | Weighted QALY loss | Risk | Weighted QALY loss | ||
| Maternal deatha | 24.8 | 0.00002 | 0.000496 | 0 | 0 |
| Hysterectomy | 9.79 | 0.00016 | 0.0015664 | 0.00058 | 0.0056782 |
| Hypoxic–ischaemic encephalopathy | 4.43 | 0.00234 | 0.0103662 | 0.00191 | 0.0084613 |
| Total | – | – | 0.0124286 | – | 0.0141395 |
| Difference | – | – | – | – | 0.0017109 |
Adverse perinatal complications
As reported above (see Adverse perinatal outcomes), the composite outcome of serious perinatal complications includes shoulder dystocia, birth trauma and neonatal death (including stillbirths). In the model, we applied the estimate of QALY loss from the NICE updated guideline for serious perinatal complications. 18 This estimate is a weighted average of the QALY loss from shoulder dystocia, birth trauma and neonatal death, for which the weights correspond to the relative frequency of each individual outcome from the Crowther and Landon trials. 51,52 The QALY loss for shoulder dystocia in the NICE guidelines18 includes the utility decrement from brachial plexus injury, adjusted for the proportion of neonates that suffer the complication of shoulder dystocia, and the average time until the complication is resolved. As in the NICE guideline,18 the QALY loss associated with birth trauma was assumed to be the same as for shoulder dystocia. The QALY loss from neonatal death was approximated as the discounted QALY (at a rate of 3.5%) from a life expectancy of 80 years lived in perfect health. Table 35 shows the QALY loss, and relative frequency by adverse outcome, from serious perinatal complications.
| Outcome | QALY loss | Relative frequency51,52 |
|---|---|---|
| Shoulder dystocia | 0.179 | 0.727 |
| Birth trauma | 0.179 | 0.197 |
| Neonatal death | 25a | 0.076 |
| Weighted QALY loss | 2.05 |
Instrumental delivery
The estimate of QALY loss from instrumental delivery was adapted from the NICE clinical guideline on C-section,33 and calculated similarly to the QALY loss from C-section. Thus it is the difference in QALY loss between instrumental delivery and vaginal delivery, for which the QALY loss for each mode of delivery is a weighted average of the QALY loss from urinary incontinence (permanent) multiplied by the risk of this outcome for each mode of delivery. It was assumed that the only outcome with impact on HRQL, and which occurs at a different rate depending on whether the delivery is assisted or not, is maternal urinary incontinence . The calculation yields a QALY loss of approximately 0.053 for instrumental delivery. Table 36 summarises the calculation of QALY loss from instrumental delivery.
| Outcome | QALY loss | Vaginal delivery | Instrumental delivery | ||
|---|---|---|---|---|---|
| Risk | Weighted QALY loss | Risk | Weighted QALY loss | ||
| Urinary incontinence | 2.77 | 0.199 | 0.55123 | 0.218 | 0.60386 |
| Difference | – | – | – | – | 0.05263 |
Health-related quality of life gains from treatment of maternal hyperglycaemia
The ACHOIS trial51 collected HRQL data for women during pregnancy and in the post-partum period, according to whether they were treated or untreated for GDM. This suggests that women who are treated for hyperglycaemia experience direct improvements in HRQL prior to giving birth. The estimates from the trial are shown in Table 37.
| Pregnancy | QALYs | SE |
|---|---|---|
| Treated | 0.72 | 0.03 |
| Untreated | 0.70 | 0.02 |
We assumed that these differences in HRQL would be applied for the duration of treatment during pregnancy (i.e. last 3 months of pregnancy) and so we time adjusted the estimates (by multiplying each by 0.25). The QALY gain from GDM treatment was estimated by subtracting the time-adjusted HRQL when untreated from the time-adjusted HRQL when treated, which resulted in a QALY gain of 0.0050. This assumes that maternal HRQL is not related to glucose levels – only to whether or not the women are treated.
Health-related quality of life gains from the prevention of maternal type 2 diabetes
We assumed that women who go on to develop type 2 diabetes that is related to their GDM would do so on average 15 years after pregnancy, and would, on average, experience 10.5 years of asymptomatic diabetes before progressing to symptomatic diabetes. 244 The life expectancy of women who developed diabetes was assumed to be 69 years if untreated, whereas non-diabetic women were assumed to have 80 years of life expectancy. 244 Age- and gender-adjusted lifetime QALYs after pregnancy245 were estimated, applying a 3.5% annual discount rate in accordance to current NICE guidance. 225 The utility loss of having asymptomatic and symptomatic diabetes was sourced from a UK catalogue of EQ-5D (European Quality of Life-5 Dimensions) estimated disutilities,246 and applied in the calculation of lifetime QALYs for diabetic women. The QALY gain from intervening to prevent diabetes was applied as the difference between the lifetime post-pregnancy QALYs of a healthy woman (21.17) and a diabetic woman (19.08), multiplied by the reduction in RR of developing diabetes given treatment with the ILS, and corresponded to QALY loss of approximately 0.20. The probability of developing diabetes or intolerance to glucose being attributable to having experienced a GDM pregnancy (0.148), and the RR reduction from delivering the ILS (0.352) were sourced from Aroda et al. 239 The utility gain was applied in the model to the proportion of women with GDM who were treated with the ILS, which consisted of those with a BMI > 30 kg/m2 who were treated for hyperglycaemia in pregnancy, and who subsequently attended the 6-week follow-up and accepted treatment with the ILS.
Health-related quality of life loss from severe hypoglycaemia
The average utility loss associated with severe hypoglycaemia (an adverse event of treatment with insulin) is small, and, therefore, was assumed to be negligible given the short duration of this event.
Resource use and costs
Resource use and costs applied in the model include treatment and test-related costs, as well those associated with the consumption of health resources resulting from adverse perinatal outcomes. The majority of costs are based on the 2013 price year, the exceptions being drugs, insulin, needles, lancets, test strips, glucose solution and laboratory costs (all 2014 prices). No discount rate was applied to costs that were assumed to occur within 12 months of testing (screening and/or diagnosis), that is, all costs except those related to the ILS. Future costs that were assumed to occur beyond 12 months of testing were discounted at a 3.5% annual rate, in accordance with current NICE guidance. 225
Screening and diagnostic testing costs
The cost of diagnosing with a 75-g OGTT 2-hour test was based on the NICE updated guideline. 18 This cost included the costs associated with time spent by a specialised nurse (band 6) to explain the test and inform the participant of the test result (5 minutes) and the time needed by a health assistant to obtain participant consent, prepare the glucose solution and collect blood samples (20 minutes), as well as the costs associated with laboratory work and the glucose solution. 18 The costs of the screening test were also included in the economic model, namely for the 50-g OGCT 1-hour test and FPG test. As the NICE updated guideline for diabetes in pregnancy18 did not include these tests in their main analysis, it was assumed that both tests would imply the same laboratory costs and nurse time as the 2-hour 75-g OGTT. Furthermore, it was assumed that the 1-hour 50-g OGCT would also imply the same health assistant time and same preparation of glucose solution as the 2-hour 75-g OGTT. The FPG does not require the ingestion of a glucose solution, and therefore this cost was not included, and the time spent by the health assistant was assumed to be 10 minutes. Table 38 shows the resource use and unit costs applied in the model for the costs associated with blood-based glucose tests.
| Resource | Unit cost | Source of unit costs | Comments |
|---|---|---|---|
| 2-hour 75-g OGTT: £22.06 per test | |||
| Nurse, band 6: 5 minutes | £49.00 per hour | PSSRU 2013247 | Duration based on assumption of GDG;18 time to explain the test, and inform the participant of result |
| Health-care assistant, band 3: 20 minutes | £25.00 per hour | aPSSRU 2013247 | Duration based on assumption of GDG;18 time needed to obtain participant consent, prepare the glucose solution and collect blood samples |
| Laboratory | £8.00 | NICE guideline18 | From a NHS hospital trust personal communication18 |
| Glucose solution, 200 ml | £1.64 | BNF248 | aPolycal® |
| 1-hour 50-g OGCT: £22.06 per test | |||
| Nurse, band 6: 5 minutes | £49.00 per hour | PSSRU 2013247 | Assumed to be the same as for 2-hour 75-g OGTT |
| Health-care assistant, band 3: 20 minutes | £25.00 per hour | bPSSRU 2013247 | Assumed to be the same as for 2-hour 75-g OGTT |
| Laboratory | £8.00 | NICE guideline18 | Assumed to be the same as for 2-hour 75-g OGTT |
| Glucose solution, one bottle 200 ml | £1.64 | BNF248 | Assumed to be the same as for 2-hour 75-g OGTT |
| FPG: £20.42 per test | |||
| Nurse, band 6: 5 minutes | £49.00 per hour | PSSRU 2013247 | Assumed to be the same as for 75-g OGTT, two hours |
| Health-care assistant, band 3: 10 minutes | £25.00 per hour | aPSSRU 2013247 | Assumed to be the same as the for the 2-hour 75-g OGTT |
The cost of testing with 2-hour 75-g OGTT and 1-hour 50-g OGCT was £22.06 per test, and the cost of testing with FPG was £20.42 per test. Our clinical advisors considered that the underlying assumptions to the cost calculation for the three tests, as well as the resulting cost estimates, were plausible.
No additional cost of screening activities based on risk factors was included in the model. This was because it was assumed that this type of screening would occur within one of the routine antenatal visits, and therefore was not associated with any additional costs.
Adverse perinatal outcomes costs
The costs associated with adverse perinatal outcomes were estimated based on the frequency of these outcomes as predicted by the model, and applying the costs used in the NICE updated guideline. 18 We reviewed the sources of unit costs applied in the NICE updated guideline, and considered them to be consistent and in accordance with recommended costing approaches. 225 All costs related with the birth were calculated as an incremental cost above the cost of a vaginal delivery according to the NHS reference costs schedule. 225 Although the economic model in the NICE updated guideline18 did not include a cost for instrumental delivery, we considered that it should be included, and a unit cost was calculated for instrumental delivery based on costs in excess of those for a vaginal delivery. As described above (see Baseline probabilities of perinatal outcomes), although the outcomes ‘birth trauma’ and ‘neonatal death’ were not collected in the available data (BiB and Atlantic DIP22,59 data sets), they were included in the model via the estimated relationship between their relative frequency compared with shoulder dystocia, obtained by pooling the event rates in two. 51,52 The pooling of these event rates also allowed estimation of the relative weights for these three outcomes in the cost composition of serious perinatal complications (see Health-related quality of life). The unit cost for serious perinatal complications was thus calculated in the NICE updated guideline18 as a weighted average of the unit costs of shoulder dystocia, birth trauma and neonatal death. 18 As mentioned above (see Treatment effects). Admission to neonatal care unit was the adverse perinatal outcome reported in the BiB22 and Atlantic DIP59 studies, and therefore that which was included in the model. The unit cost estimate applied in the model to this adverse outcome corresponds with cost of admission to NICU, which is likely to be an overestimation of the cost. Table 39 summarises the costs associated with adverse perinatal outcomes that are included in the model.
| Outcome | Cost (£) | Source | Comments |
|---|---|---|---|
| Admission to NNU | 1118 | NHS reference costs 2012–13225 | Currency code XA01Z (Neonatal Critical Care, Intensive Care, Total HRGs) |
| Induction of labour | 329 | NHS reference costs 2012–13225 | Costs over and above those incurred in a normal vaginal delivery, in woman with a non-elective long stay admission and with CC score 0 (currency code NZ30C, Obstetrics) Currency code NZ31C (Epidural or Induction) |
| C-section | 884 | NHS reference costs 2012–13225 | Costs over above those incurred in a normal vaginal delivery, in woman with a non-elective long stay admission and with CC score 0 (currency code NZ30C, Obstetrics) Currency code NZ50C (Planned C-Section, Obstetrics) |
| Shoulder dystocia | 1256 | NHS reference costs 2012–13225 | Currency code PB02Z (Minor neonatal diagnoses, Neonatology, non-elective long stay admission and with CC score 0) |
| Neonatal death | 767 | NHS reference costs 2005–6249 | Currency code PB02Z (Neonatal death) Uprated to 2012–13 prices using the HCHS index247 |
| Birth trauma | 1256 | NHS reference costs 2012–13225 | Currency code PB02Z (Minor neonatal diagnoses, Neonatology, non-elective long stay admission and with CC score 0) |
| Serious perinatal complications | 1219 | Calculated | Weighted average of the unit costs of shoulder dystocia, neonatal death and birth trauma |
| Pre-eclampsia | 4656 | NICE hypertension in pregnancy guideline243 | Uprated to 2012–13 prices using the HCHS index247 |
| Instrumental birth | 1086 | NHS reference costs 2012–13225 | Costs over and above those incurred in a normal vaginal delivery, in woman with a non-elective long stay admission and with CC score 0 (currency code NZ30C, Obstetrics) Currency code NZ40C (Assisted Delivery with CC score 0, Obstetrics) |
Treatment costs
The majority of treatment costs applied in the model are based on the NICE ‘Diabetes in pregnancy’ guideline,18 and include the costs of self-monitoring glucose levels, hypoglycaemic therapy, insulin therapy instruction, dietary instruction and assessment, and additional antenatal care.
Treatment was assumed to have a duration of 90 days, and to consist of diet as first line, as in the NICE guideline. 18 The NICE guideline18 did not include the cost of metformin in the treatment costs, for those women whose glycaemia is not controlled with the first-line of treatment. We assumed in the base case that 27% of women would continue solely on diet throughout the treatment duration, whereas the remaining women would transition to metformin (35%) or insulin therapy (28%) after the first 10 days of diet. The proportion of women for which treatment consisted of diet alone was based on the proportion of women with GDM on each treatment component until the end of pregnancy, averaged across four NHS Hospital trusts, as reported in the NICE updated guideline. 18 Our clinical advisors considered that the averaged proportion of women in each type of treatment was reflective of current UK practice. Two alternative assumptions regarding the relative proportion of each treatment type was applied in a sensitivity analysis. First, we sourced the proportion estimates for the treatment types from the NHS hospital trust (of the four reported in the guideline) that reported less frequent use of insulin (scenario 3: 11% insulin, 100% metformin, 47% diet and activity advice). Second, we assumed that women would not receive insulin therapy (scenario 4: 64% metformin, 100% diet and activity advice), which was part of an extreme low-cost scenario.
The cost of providing dietary advice was applied to all treated women, and consisted of the cost of 15 minutes of a nurse (band 6) and 30 minutes of a dietitian (band 5). The NICE guideline18 assumed that a nurse band 7 would deliver the service alongside a dietitian. However, according to our clinical advisors, diet advice was more likely to be delivered by a band 6 health professional, more specifically a band 6 midwife. We assumed that the cost would be similar as to the service being provided a nurse (band 6). The unit cost of a dietitian (band 5) was adjusted so as to reflect cost per hour of patient contact rather than cost per hour. This was done by multiplying the unit cost per hour estimate for dietitian (band 5) by the ratio between the cost per hour of patient contact and cost per hour of a nurse (grade 6) (£119/£49). The resulting cost per patient hour for a dietitian (band 5) was £85. 247 This adjustment was required as the Personal Social Services Research Unit (PSSRU) cost schedule247 reports only the cost per hour for dietitians. In scenario 4, it was assumed that dietary and exercise advice was delivered to a group of 12 women.
The cost of insulin treatment included the cost of insulin use instruction and treating severe hyperglycaemia caused by insulin. Insulin instruction was assumed to be delivered by a midwife band 6 and have a duration of 45 minutes. The NICE updated guideline18 assumed that the instruction would be delivered by a nurse band 7, but for the same reason as described above for dietary advice, we applied the unit cost for a nurse band 6. 247 In scenario 4, it was assumed that insulin use instruction was delivered to a group of 12 women. The cost of the use of insulin included the cost of 20 units per day of rapid-acting insulin and 10 units per day of intermediate insulin and needles required for four injections of insulin per day, over 80 days of treatment. The unit cost of treating severe hyperglycaemia corresponded with the cost of an ambulance service and a weighted average of the unit costs for the health-care resource groups related to the treatment of diabetes with hypoglycaemic disorders from the NHS reference. 225
The instruction on blood glucose self-monitoring (BGSM) was assumed to be delivered by a midwife (band 6) – and not by a band 7 nurse as in the guideline (for the same reasons described above for the delivery of dietary advice) –and have a duration of 30 minutes. All women who were treated for GDM were assumed to receive BGSM instruction and to test themselves four times a day during the 90 days of treatment. Women who were treated with insulin did three additional tests per day for 80 days. The cost of the BGSM test included the test strips and lancets. In scenario 4, it was assumed that BGSM instruction was delivered to a group of 12 women.
The costs of metformin corresponded to the cost of taking a dosage of 850 mg three times a day, which is the recommended dosage according to the British National Formulary (BNF) for 80 days. 248
We also included the cost of additional antenatal care for women with GDM, including three standard antenatal ultrasound scans and three antenatal appointments. These were costed by applying unit costs from the NHS reference costs schedule. 225 All durations of contacts with NHS staff were sourced from the NICE ‘Diabetes in pregnancy’ updated guideline,18 and were considered to be reflective of NHS practice according to our clinical advisors. Our clinical advisors also considered that resource-use assumptions in terms of drug dosages, treatment of severe hyperglycaemia, test consumables, staff required to deliver services and composition of additional antenatal care were reflective of current NHS practice. The costs reported here differ to those reported in the NICE guideline18 after we corrected values that did not match the cited source.
Resource use, unit costs and respective sources are summarised in Table 40 for each cost category, and the updated costs for base-case treatment bundle (35% metformin, 28% insulin) are displayed. The total cost of treatment accrues to £935 per woman with GDM in the base case.
| Resource use | Unit cost (£) | Source of unit costs | Comments |
|---|---|---|---|
| Dietary instruction and assessment: for all women treated for GDM | |||
| Midwife, band 6: 15 minutes | 119.00 per patient hour | PSSRU, 2013247 | Assessment duration based on assumption of GDG Nurse team leader |
| Dietitian, band 5: 30 minutes | 85.00 per patient hour | PSSRU, 2013247 | Instruction duration based on assumption of GDG Hour of patient contact was calculated assuming the same mathematical relationship as between the cost per hour and cost per patient hour for a nurse team leader (band 6) |
| Insulin instruction and use: for 28% of all women treated for GDM and for 80 days of treatment | |||
| Midwife, band 6: 45 minutes | 119.00 per patient hour | PSSRU247 | Instruction duration based on assumption of GDG |
| Rapid-acting insulin (aspart): 20 units per day | 0.02 | BNF248 | Novo Rapid® (insulin aspart; Novo Nordisk A/S, Bagsværd, Denmark) |
| Intermediate insulin (isophane): 10 units per day | 0.01 | BNF248 | Insuman® (regular insulin; Sanofi UK, Guildford, UK) |
| Needles: four per day | 0.10 | NHS Drug Tariff250 | BD Micro-Fine™ Ultra 4-mm/32-gauge (syringe; BD Medical, Oxford, UK) |
| Treatment of severe hypoglycaemia | 629.00 | NHS reference costs225 | Cost of an ambulance (Currency code ASS02) and a weighted average of A&E costs for diabetes with hypoglycaemic disorders (Currency code KB01C, KB01D, KB01E and KB01F) |
| Metformin use: for 35% of all women treated for GDM and for 80 days of treatment | |||
| Metformin 500 mg | 0.01 per tablet | BNF248 | Non-proprietary, 84 tablets presentation |
| BGSM instruction and testing: for all women treated for GDM | |||
| Nurse band 6: 30 minutes | 119.00 per patient hour | PSSRU247 | Assessment duration based on assumption of GDG |
| Lancets: | |||
| Four per day if on diet only Seven per day if on insulin therapy |
0.03 | NHS Drug Tariff250 | BD Micro-Fine+ 0.20-mm/33-gauge (syringe; BD Medical, Oxford, UK) |
| Test strips: | |||
| 4 per day if on diet only 7 per day if on insulin therapy |
0.20 | BNF248 | Accu-Check™ Active (blood glucose monitor; Roche Diagnostics Ltd, Burgess Hill, UK) |
| Additional antenatal care: for all women treated for GDM | |||
| Three antenatal standard ultrasounds | 130.00 | NHS reference costs225 | Code NZ21Z – Procedures in Outpatients, Obstetrics, Antenatal Standard ultrasound |
| Three antenatal appointments | 89.00 | NHS reference costs225 | Code WF01A- Obstetrics, non-consultant led, non-admittance, follow-up appointment |
The cost composition for the base-case treatment ‘bundle’ (28% insulin, 35% metformin, 100% diet and advice) and two alternative costing scenarios described above in this section (1) 11% insulin, 42% metformin, 100% diet and advice; and (2) 64% metformin, 100% diet and advice) is detailed in Table 41.
| Cost category | Base case | Scenario three | Scenario four | |||
|---|---|---|---|---|---|---|
| Treated women (%) | Cost per woman (£) | Treated women (%) | Cost per woman (£) | Treated women (%) | Cost per woman (£) | |
| Dietary instruction and assessment | 100 | 72 | 100 | 72 | 100 | 6 |
| SMBG instruction and testing | 100 | 142 | 100 | 142 | 100 | 88 |
| More intensive antenatal care | 100 | 657 | 100 | 657 | 100 | 657 |
| Insulin instruction and use | 28 | 160 | 11 | 160 | 0 | – |
| Additional SMBG for women on insulin | 28 | 55 | 11 | 55 | 0 | – |
| Metformin use | 35 | 3 | 42 | 3 | 64 | 3 |
| Total cost of treatment | 935 | 897 | 753 | |||
Costs of intensive lifestyle intervention for prevention of type 2 diabetes
A cost estimate was also included for the delivery of the ILS to 36% of the GDM treated women in the base case, that is, the proportion of those who had a BMI of ≥ 30 kg/m2 in the BiB study22 (see Scenario analysis: maternal longer-term outcomes). The ILS consisted of 16 individual sessions of dietary and exercise advice, lasting 1 hour, and delivered over 1 year. This initial delivery of the intervention followed by a maintenance phase started approximately 3 years after the initial advice session, which consisted of up to 12 quarterly 1-hour group sessions. 251 It was assumed that the sessions were delivered by a dietitian (band 5) and that the groups in the maintenance phase were composed of 10 individuals. It was further assumed that the group sessions would continue to be delivered until the end of life in the model.
Although an estimate of direct medical costs (hospital stays, emergency room, urgent care, outpatient services and telephone calls to health-care providers) of ILS was available in the literature, this was not included, as the study251 was set in the USA, and was therefore unlikely to be reflective of UK practice. Excluding these costs is likely to be conservative, as, in the follow-up study of DPP, the ILS was found to accrue less direct medical costs than placebo (US$26,810 vs. US$29,007). 251
All ILS costs were discounted at a 3.5% annual rate. The total discounted cost of the ILS intervention accrued to £3585 per woman treated for GDM. Unit costs and resource use for the ILS intervention are displayed in Table 42.
| Resource use | Unit cost (£) | Source of unit costs | Comments |
|---|---|---|---|
| ILS initial phase | |||
| Dietitian, band 5: 60 minutes | 35.00 per hour | PSSRU254 | Individual sessions |
| ILS maintenance phase | |||
| Dietitian, band 5: 60 minutes | 35.00 per hour | PSSRU254 | Group session for 10 individuals |
Net benefit of early detection of diabetes
We identified a study238 that assessed the cost-effectiveness of screening for type 2 diabetes. We used estimates of the QALY gain and incremental costs associated with screening and treating for type 2 diabetes at age 45 years in a population at risk as a proxy for the potential NHB for those women diagnosed with GDM who are found to have type 2 diabetes at 6-week post-partum follow-up. Although we recognise that the population in the Gillies et al. study238 is different from the group considered here, this scenario allows the exploration of the inclusion of a potential longer-term benefit, but we were unable to model this directly for a population of pregnant women. The study238 estimates a discounted incremental cost of £587, a discounted incremental QALY gain of 0.03, and a corresponding incremental cost-effectiveness ratio (ICER) of £14,150 for a one-off screening strategy in a white population with an underlying prevalence of 5% type 2 diabetes. The reported mean costs and QALYs did not match the ICER, and so we utilised an incremental costs of £425. The study238 reports undiscounted scenario analyses, which suggest that the ICER for the screening strategy is similar between populations, with underlying prevalence of type 2 diabetes of 5% and 10%, the latter value being similar to the estimated underlying prevalence of 11% type 2 diabetes at 6 weeks post partum among women diagnosed with GDM. The study238 estimates that the incremental costs of screening a SA population are approximately 52% higher than those for a white population, and that incremental QALYs are 83% higher for a SA population than a white population. We therefore adjusted the incremental costs and QALY gain of early detection according to the proportion of women of SA ethnicity in the BiB cohort. 22 The model parameters are summarised in Table 43.
| Parameter | Estimate (95% credible interval) | Source | Comments |
|---|---|---|---|
| Additional cost associated with detecting and treating diabetes post partum | 558 (61 to 1525) | Gillies 2008238 | Time horizon 50 years Discounted at 3.5% Uprated to 2013 price year |
| QALYs gain associated with detecting and treating diabetes post partum | 0.05 (–0.03 to 0.14) | Gillies 2008238 | Time horizon 50 years Discounted at 3.5% |
Sensitivity and scenario analysis
Sensitivity analysis was conducted so as to characterise uncertainty at different levels in the economic analysis. The methods used to conduct sensitivity analysis in the model are described in this section.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was performed to quantify and incorporate into results the joint uncertainty of the model input parameters. Probability distributions were specified for the model parameters so as to reflect uncertainty in the mean estimates. 252,253 The selection of probability distributions for each parameter was based on well-established literature recommendations254 and is reported alongside the parameter point estimates for the base-case in the model (see Appendix 6, Table 73). Monte Carlo simulation was used to propagate uncertainty in input parameters through the model, by sampling from each parameter’s distribution and estimating the corresponding expected costs and QALYs for each alternative strategy. The Monte Carlo simulation was performed for 5000 iterations, and mean values for the model outputs were estimated as the average across the iterations. The results reported are based on the mean values of expected costs and QALYs across simulations, and are therefore probabilistic, in accordance with current NICE guidance. 225 The probability that each strategy would represent the most cost-effective strategy was estimated by the proportion of the 5000 simulations in which it would be regarded as having the maximum NHB.
We calculated the gain in net benefits that could be achieved if all of the parameter uncertainty were eliminated from the model. This is the expected value of perfect information (EVPI), which forms an upper bound for the value of further research. It was estimated by evaluating the model using 5000 random possible input sets, determined by the probability distributions assigned to each of the inputs in order to generate a distribution of 5000 possible total costs and QALYs for each strategy. The EVPI is the difference between the average of the maximum net benefit that could be achieved within each of the 5000 simulations and the expected net benefits of the best-performing strategy (i.e. the net benefits expected to be achieved if the cost-effective strategy is determined and implemented based on current information). The structure of the decision model is such that the best-performing diagnostic glucose threshold is estimated first, and then the best-performing strategy with respect to do nothing, test and treat, screen and treat, or screen test and treat, is calculated at the predetermined best-performing diagnostic glucose threshold. This poses a challenge for calculating the EVPI, as the uncertainty in the best preforming diagnostic glucose threshold is not propagated through to the decision uncertainty between alternative strategies.
The value of further research around individual input parameters was estimated using the Sheffield Accelerated Value of Information (SAVI) software, release version 2.0.10: 2015-09-24 (University of Sheffield, Sheffield, UK). 123 This provides an estimate of the gain in NHBs that could be achieved if the uncertainty were eliminated from each individual parameter in the model, and can indicate where additional research would be most valuable.
For the main analysis the best-performing diagnostic glucose threshold was calculated by evaluating the model with each input set to its mean value (i.e. deterministically). A probabilistic evaluation of the best-performing diagnostic glucose threshold would have required more computing time, which would have restricted the number of scenario and subgroup analyses feasible. However, for these EVPI calculations we evaluated the best-performing diagnostic glucose threshold using 5000 random possible input parameter sets for the base-case analysis and for a single cost-effectiveness threshold in the scenario incorporating maternal longer-term outcomes. We evaluated the value of further research for cost-effectiveness thresholds of £13,000, £20,000 and £30,000 per QALY for the base-case results, and for a cost-effectiveness threshold of £20,000 per QALY for the scenario analysis with maternal longer-term outcomes included. Consequently, the net benefits associated with the best-performing diagnostic strategy may differ between the EVPI calculations and the base-case results.
Scenario analysis
Scenario analysis was conducted when assumptions underlying the base-case analysis were varied. The aim of this sensitivity analyses was to assess the robustness of base-case results to alternative assumptions in terms of costs, treatment effectiveness, uptake of screening and diagnostic tests, and inclusion of potential longer-term maternal outcomes. The scenario analysis has been described throughout this report. Table 44 illustrates which elements were varied in each scenario analysis.
| Scenario | Element | Base case | Variation for the sensitivity analysis |
|---|---|---|---|
| 1 | Longer-term outcomes | No longer-term outcomes are included in the analysis | Includes costs and QALY gains from early detection of maternal type 2 diabetes at post-partum follow-up, and of prevention of type 2 diabetes later in maternal life by delivering an intensive lifestyle intervention to women who were treated for GDM |
| 2 | Treatment effectiveness | RR of GDM treated was sourced from the diet modification meta-analysis (see Chapter 6) | RR of GDM treated was sourced from NICE previous guidance18 |
| 3 | Cost of treatment for GDM | Cost of treatment reflects the treatment ‘bundle’ in which the proportion on each treatment is:
|
Cost of treatment reflects the treatment ‘bundle’ in which the proportion on each treatment is:
|
| 4 | Cost of treatment for GDM | Cost of treatment reflects the treatment ‘bundle’ in which the proportion on each treatment is:
|
Cost of treatment reflects the treatment ‘bundle’ in which the proportion on each treatment is:
|
| 5 | Uptake of diagnostic test | Uptake of diagnostic test is:
|
Uptake of diagnostic test is:
|
Subgroup analysis
In Chapter 2, it was highlighted that GDM prevalence is higher in SA women than WB women across the different diagnostic criteria (see Table 6) and this is due to differing population characteristics. To explore whether or not this differing characteristics would impact on the cost-effective strategy identified in the model, the BiB study data22 were divided in two subgroups, and the costs and QALYs of the alternative intervention strategies were evaluated in each population separately. The first subgroup included all SA women, as well as of all other ethnicities excluding WB. The rationale for including the category ‘Other’ in the subgroup was that higher prevalence of GDM can also be found in ethnicities such as black Caribbean and Middle Eastern, which are likely to be captured under this category. The second subgroup corresponded to WB women. The analysis was conducted by repeating the base-case analysis and scenario 1 (inclusion of longer-term outcomes) using only the individual patient data for each subgroup. The average baseline characteristics by subgroup are shown in Appendix 6, Table 74.
Results
The alternative screening and diagnostic intervention strategies are evaluated on the 10,353 women in the BiB data set22 in order to determine the cohort characteristics for the decision model. This includes the proportion that would screen positive, the proportion that would test positive, the mean fasting and post-load glucose levels and the risk factors for each subdivision of the cohort. For example, Table 45 shows the cohort characteristics for each subdivision of the cohort if the NICE risk factor screening strategy is applied and a diagnostic threshold of 6.1 mmol/l for fasting blood glucose and 7.8 mmol/l for post-load blood glucose is used.
| Characteristics | All | S+T+ | S+T– | S+ | S– | T+ | T– |
|---|---|---|---|---|---|---|---|
| Proportion of cohort (%) | 100 | 7.5 | 70.0 | 77.6 | 22.4 | 8.2 | 91.8 |
| Mean fasting blood glucose (mmol/l) | 4.52 | 5.36 | 4.50 | 4.58 | 4.34 | 5.28 | 4.46 |
| Mean post-load blood glucose (mmol/l) | 5.68 | 9.05 | 5.47 | 5.82 | 5.27 | 9.00 | 5.39 |
| Mother’s age | 27.6 | 30.8 | 27.7 | 28.0 | 26.1 | 30.6 | 27.3 |
| BMI | 26 | 29 | 27 | 27 | 24 | 28 | 26 |
| Previous GDM | 0.01 | 0.06 | 0.01 | 0.01 | 0 | 0.06 | 0.01 |
| Previous macrosomia | 0.05 | 0.10 | 0.06 | 0.07 | 0 | 0.09 | 0.04 |
| SA | 0.52 | 0.78 | 0.69 | 0.70 | 0 | 0.70 | 0.51 |
| White | 0.39 | 0.15 | 0.20 | 0.19 | 1 | 0.24 | 0.41 |
| Other ethnicity | 0.08 | 0.08 | 0.11 | 0.11 | 0 | 0.07 | 0.08 |
| Nulliparous | 0.41 | 0.28 | 0.35 | 0.35 | 0.58 | 0.31 | 0.40 |
| One child | 0.29 | 0.22 | 0.28 | 0.28 | 0.26 | 0.23 | 0.28 |
| Two children | 0.17 | 0.20 | 0.18 | 0.18 | 0.09 | 0.19 | 0.16 |
| Three or more children | 0.13 | 0.26 | 0.15 | 0.16 | 0.04 | 0.24 | 0.12 |
| Family history of diabetes | 0.26 | 0.43 | 0.33 | 0.34 | 0 | 0.38 | 0.24 |
In the BiB data set,22 81 individuals (0.78%) had fasting or post-load blood glucose measures of ≥ 11.1 mmol/l. In the remainder, the fasting glucose measure varied between 3.0 and 9.4 mmol/l, and the post-load glucose measure varied between 1.6 and 11.0 mmol/l. Women who had fasting glucose measures of > 9.5 mmol/l all had a post-load glucose measurement of ≥ 11.1 mmol/l, and hence 9.5 mmol/l forms an effective upper bound for the fasting glucose threshold in this cohort.
Without treatment, the model predicts a cost per pregnant woman of £466 and an expected QALY loss of –0.036 as a result of adverse perinatal outcomes. These are the cost and QALYs estimated for the ‘no screening/testing or treatment’ strategy. Among the whole cohort of pregnant women, and without any intervention for hyperglycaemia, the model would predict 2% to have pre-eclampsia, 16.6% would have induction of labour, 20.2% would be expected to have C-section and 7.1% would require instrumental delivery. Immediate birth outcomes would include 4.1% with admission to a neonatal unit and 1.5% serious perinatal complications.
In the following sections we build up the results, first considering the best-performing strategy of each type. We start with the best-performing diagnostic glucose threshold (see Best-performing diagnostic threshold). We then consider the best-performing screen-only strategy (see Screen-only strategies), through which no one is provided with a diagnostic test and diet, and lifestyle modification advice is provided to women on the basis of screening positive. Next (see Screen and test strategies) we consider the best-performing screen and test strategy in which women are first subject to screening, with those who screen positive offered a diagnostic test using cut-offs determined by the best-performing fasting and post-load glucose levels. Women who have screened positive, and in whom blood glucose levels exceed either of the best-performing cut-off values, are offered diet and lifestyle modification advice, followed by pharmacological therapy as required. Finally, we report the results of the full incremental analysis (see Full incremental analysis) in order to determine which is the most cost-effective intervention strategy for the screening, diagnosis and treatment of GDM.
Best-performing diagnostic threshold
Base-case results
The best-performing diagnostic glucose threshold was estimated by evaluating the costs and QALYs associated with a universal diagnostic test strategy for all of the 969 potential dual glucose thresholds, and identifying the fasting and post-load blood glucose levels at which the NHB (and equivalently NMB) would be maximised. The best-performing diagnostic glucose threshold will differ according to the cost-effectiveness threshold, because NHBs are calculated by dividing through the costs by the cost-effectiveness threshold and subtracting them from the QALYs. That is to say, the fasting and blood-glucose diagnostic thresholds at which NHBs are maximised depend on the cost-effectiveness threshold. The model predicts that the cost per pregnant woman is increased and QALY losses are decreased as the fasting and post-load glucose thresholds are decreased from their maximum values. In other words, as fasting and post-load glucose cut-offs for diagnosis are lowered, and a larger proportion of women are diagnosed with GDM, the cost per pregnant woman increases, but the QALY losses are reduced, that is, lower diagnostic glucose threshold values are more costly and more effective than higher threshold values. The incremental difference in expected costs and QALYs is very small for every 1-mmol/l increment in diagnostic thresholds. In the base-case analysis that includes only short-term health outcomes, the QALYs vary between a maximum of –0.0274 (lowest threshold: fasting 5.0 mmol/l, post-load 5.5 mmol/l) and a minimum –0.036 (highest threshold: fasting 9.5 mmol/l and post-load 11.1 mmol/l). Costs vary between £784 and £491 per pregnant woman. The costs and QALYs for the £20,000 per QALY cost-effectiveness thresholds for the base case are shown via heat maps in Appendix 6, Figures 66 and 67.
Using a cost-effectiveness threshold of £20,000 per QALY, the best-performing diagnostic glucose threshold is to treat women identified with a post-load glucose level that exceeds 10.0 mmol/l. NHBs cannot be improved further by treating any additional women on the basis of fasting glucose levels. If the cost-effectiveness threshold is increased to £30,000 per QALY, the best-performing diagnostic glucose threshold is 5.2 mmol/l for fasting glucose and 8.8. mmol/l for post-load glucose. Table 46 and Figure 44 show the relationship between the cost-effectiveness threshold and the best-performing fasting glucose and post-load glucose levels at which to treat.
| Cost-effectiveness threshold (£) | Fasting glucose, mmol/l | Post-load glucose, mmol/l | Proportion T+ | Costs (£) | QALYs |
|---|---|---|---|---|---|
| 18,000 | 9.5 | 11.1 | 0.008 | 491 | –0.036 |
| 19,000 | 9.5 | 10 | 0.015 | 495 | –0.036 |
| 20,000 | 9.5 | 10 | 0.015 | 495 | –0.036 |
| 21,000 | 9 | 9.5 | 0.020 | 498 | –0.036 |
| 22,000 | 8.5 | 9.2 | 0.025 | 501 | –0.035 |
| 23,000 | 8.5 | 9.1 | 0.028 | 502 | –0.035 |
| 24,000 | 8 | 8.8 | 0.033 | 505 | –0.035 |
| 25,000 | 8 | 8.5 | 0.043 | 510 | –0.035 |
| 26,000 | 8 | 8.5 | 0.043 | 510 | –0.035 |
| 27,000 | 5.4 | 11.1 | 0.058 | 518 | –0.035 |
| 28,000 | 5.3 | 9.8 | 0.072 | 526 | –0.034 |
| 29,000 | 5.3 | 8.8 | 0.082 | 532 | –0.034 |
| 30,000 | 5.2 | 8.8 | 0.102 | 543 | –0.034 |
| 31,000 | 5.2 | 8.1 | 0.122 | 554 | –0.034 |
| 32,000 | 5.1 | 8.2 | 0.138 | 562 | –0.033 |
| 33,000 | 5.2 | 7.2 | 0.177 | 584 | –0.033 |
| 34,000 | 5 | 7.2 | 0.222 | 609 | –0.032 |
| 35,000 | 5 | 7.2 | 0.222 | 609 | –0.032 |
| 36,000 | 5 | 6.6 | 0.287 | 645 | –0.031 |
| 37,000 | 5 | 6.6 | 0.287 | 645 | –0.031 |
| 38,000 | 5 | 6.2 | 0.356 | 683 | –0.030 |
| 39,000 | 5 | 6.1 | 0.377 | 695 | –0.030 |
| 40,000 | 5 | 6 | 0.401 | 708 | –0.029 |
| 41,000 | 5 | 5.7 | 0.479 | 752 | –0.028 |
| 42,000 | 5 | 5.6 | 0.508 | 768 | –0.028 |
| 43,000 | 5 | 5.5 | 0.536 | 784 | –0.027 |
FIGURE 44.
Best-performing diagnostic glucose threshold and mean blood glucose levels among those diagnosed.
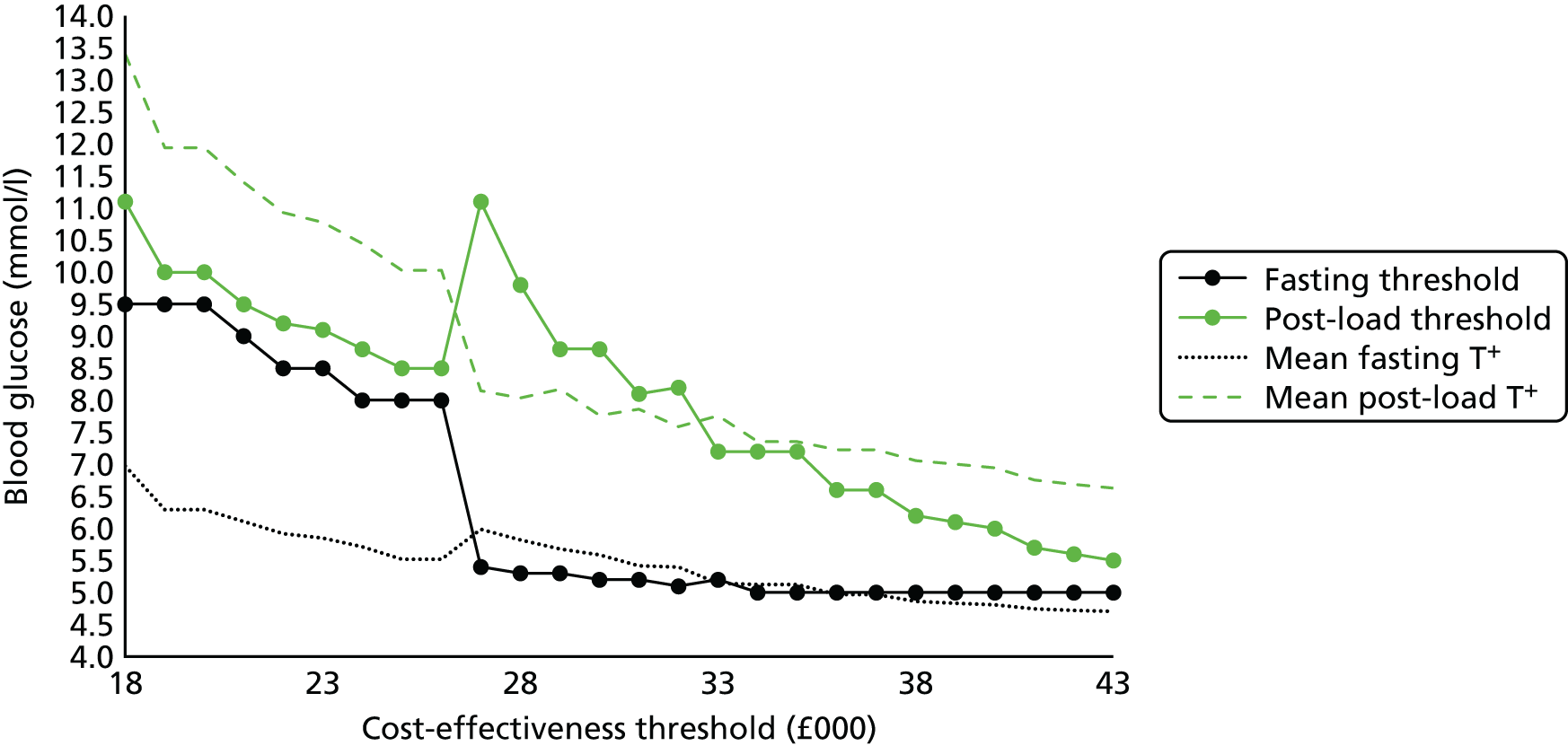
Below a cost-effectiveness threshold of £18,000 per QALY it is not cost-effective to diagnose women as having GDM. As the cost-effectiveness threshold increases from £18,000 to £26,000, the best-performing fasting and post-load glucose thresholds at which treatment would commence fall. Once the cost-effectiveness threshold reaches £27,000 there is a switch, and the best-performing fasting glucose level drops to 5.4 mmol/l while the post-load glucose level returns to the maximum of 11.1 mmol/l. The best-performing fasting and post-load diagnostic thresholds then both reduce as the cost-effectiveness threshold increases, until they reach the minimum bounds tested of 5.0 mmol/l for fasting glucose and 5.5 mmol/l for post-load glucose levels.
Although Figure 44 identifies a particular best-performing threshold, the results indicate that there are ranges of diagnostic thresholds that would be associated with very similar costs and health outcomes. Compared with the base-case best-performing threshold, the diagnosis and treatment of women – based on the criteria utilised in the BiB study,22 that is, 6.1 mmol/l for fasting glucose and 7.8 mmol/l for post load glucose – would increase costs by £36 per pregnant woman and increase QALYs by 0.001. Differences between this threshold and the best-performing diagnostic glucose threshold are small, but the criteria used in the BiB study22 are predicted to provide lower NHBs than ‘no screening/testing or treatment’ and lower NHBs than the best-performing diagnostic glucose threshold.
At a cost-effectiveness threshold of £20,000 per QALY, the model predicts that for a universal diagnostic test strategy using the best-performing diagnostic glucose threshold, the cost per pregnant woman is £495 and the expected QALY loss is –0.036 as a result of adverse perinatal outcomes. At the diagnostic thresholds of fasting glucose 9.5 mmol/l and post-load glucose 10.0 mmol/l, 1.5% of women would be diagnosed with GDM and offered treatment. Treatment reduces pre-eclampsia, C-section, admission to neonatal unit and serious perinatal complications, but increases instrumental delivery and induction. With only 1.5% of women offered treatment, and uptake at 63%, the differences in perinatal outcomes are indistinguishable when figures are rounded to one decimal place. The QALY difference between a universal diagnostic test strategy using the best-performing diagnostic glucose threshold compared with ‘no screening/testing or treatment’ is 0.00 per pregnant woman, and the incremental cost is £28, resulting in a large ICER. Among the 1.5% of women who were diagnosed with GDM at this best-performing threshold, the QALY gain is estimated to be 0.006 (0.00009/0.015), and this would need to be increased to 0.09 in order for NHBs to exceed those of ‘no screening/testing or treatment’.
At a cost-effectiveness threshold of £30,000 per QALY, the best-performing diagnostic thresholds are 5.2 mmol/l for fasting blood glucose and 8.8 mmol/l for post-load glucose. Using these values, 10.2% of women would be diagnosed with GDM. The model predicts a cost per pregnant woman of £543 and QALYs of –0.034. The rate of perinatal outcomes is 1.9% pre-eclampsia, 20.2% C-section (small reduction), 4.1% admission to the neonatal unit (NNU), 7.7% instrumental delivery, 1.4% serious perinatal complications and 17.0% induction of labour. The cost per QALY gained with universal diagnostic test compared with ‘no screening/testing or treatment’ is £80 per 0.002 QALYs, giving an ICER of £45,000. Even with the best-performing diagnostic threshold, the NHBs of a universal diagnostic test strategy do not exceed those of ‘no screening/testing or treatment’.
Scenario analysis: maternal longer-term outcomes
If longer-term health outcomes are included in the model, the expected costs and QALYs, given fasting and post-load glucose levels, are increased compared with the base-case analysis. When the QALY gains and additional costs associated with the treatment and prevention of type 2 diabetes are incorporated, the model predicts expected QALY per pregnant woman of between a maximum of –0.015 (lowest threshold: fasting 5.0 mmol/l, post-load 5.5 mmol/l) and a minimum of –0.036 (highest threshold: fasting 9.5 mmol/l and post-load 11.1 mmol/l). The corresponding expected cost per pregnant woman varies between £971 and £495 per pregnant woman. Table 47 shows the relationship between the best-performing diagnostic glucose thresholds and the cost-effectiveness threshold. With the inclusion of longer-term outcomes, the best-performing diagnostic glucose threshold is lower than the base-case analysis for cost-effectiveness thresholds in the range of £18,000–43,000.
| Cost-effectiveness threshold (£) | Fasting glucose (mmol/l) | Post-load glucose (mmol/l) | Proportion T+ | Costs | QALYs | Proportion obese T+ |
|---|---|---|---|---|---|---|
| 17,000 | 9.5 | 11.1 | 0.008 | 495 | –0.036 | 0.45 |
| 18,000 | 9 | 9.5 | 0.020 | 507 | –0.035 | 0.45 |
| 19,000 | 8.5 | 9.1 | 0.028 | 514 | –0.035 | 0.43 |
| 20,000 | 5.4 | 11.1 | 0.058 | 545 | –0.033 | 0.46 |
| 21,000 | 5.2 | 9.9 | 0.092 | 578 | –0.031 | 0.42 |
| 22,000 | 5 | 8.3 | 0.167 | 647 | –0.028 | 0.37 |
| 23,000 | 5 | 7.2 | 0.222 | 695 | –0.026 | 0.33 |
| 24,000 | 5 | 6.2 | 0.356 | 816 | –0.021 | 0.30 |
| 25,000 | 5 | 5.6 | 0.508 | 947 | –0.016 | 0.27 |
| 26,000 | 5 | 5.5 | 0.536 | 971 | –0.015 | 0.26 |
At a cost-effectiveness threshold of £20,000 per QALY, the model predicts that for a universal diagnostic test strategy using the best-performing diagnostic glucose threshold, the cost per pregnant woman is £545 and the expected QALY loss is –0.033 resulting from adverse perinatal outcomes. Compared with ‘no screening/testing or treatment’, the cost per QALY gained is £81 per 0.003 QALYs, giving an ICER of £29,752. Among the 5.8% of women diagnosed with GDM the QALY gain is estimated to be 0.05. This QALY gain would have to be increased to 0.07 in order for the NHBs to exceed those associated with ‘no screening/testing or treatment’. For the scenario incorporating longer-term maternal outcomes, the NHB of a universal diagnostic test strategy using the best-performing diagnostic glucose threshold would exceed the NHB of ‘no screening/testing or treatment’ at cost-effectiveness thresholds of > £24,000. However, in order to determine whether or not a universal diagnostic test strategy is cost-effective it is necessary to compare with the full range of alternative strategies. The results of this full incremental analysis are shown below (see Full incremental analysis).
Scenario analysis: fasting plasma glucose test
At a cost-effectiveness threshold of £20,000 per QALY, the best-performing fasting glucose level is 11.1 mmol/l, suggesting that it is not cost-effective to diagnose women with GDM. If the cost-effectiveness threshold is increased to £30,000 per QALY, the best-performing fasting blood glucose level at which to commence treatment is 5.2 mmol/l. At this fasting blood glucose threshold, 8.8% of women would be diagnosed with GDM and the expected cost per pregnant woman is £532, with associated QALY loss of 0.034. The mean fasting blood and post-load glucose measures among women in the BiB study,22 who would be diagnosed with GDM on the basis of a FPG test with a threshold of 5.2 mmol/l, are 5.73 mmol/l and 7.48 mmol/l. If the post-load measure is taken into account with a threshold set at 8.8 mmol/l, as indicated in Table 46, a further 1.4% of women would be diagnosed with GDM. and the mean fasting blood glucose levels would reduce to 5.59 mmol/l, whereas the mean post-load glucose level would increase to 7.76 mmol/l. Compared with the OGTT, the use of FPG as a diagnostic test appears to offer similar health outcomes, but at a lower cost. The expected difference in expected QALYs is very small but negative, indicating that the FPG is not dominant (i.e. not less costly and more effective) compared with the OGTT.
Screen-only strategies
At a cost-effectiveness threshold of £20,000 per QALY, the screen-only strategy associated with the highest NHB compared with all screen-only strategies is to offer treatment to women who have experienced GDM in a previous pregnancy. In the BiB data set22 only 1% of women would screen positive on this basis. The expected cost per pregnant woman of providing diet and lifestyle modification to women with prior GDM is £484 and the expected QALY loss is 0.036. The QALYs are higher than those expected with ‘no screening/testing or treatment’ or a universal ‘test and treat’ strategy, but the differences are very small. The cost per pregnant woman is estimated to be £484, and hence a screen-only strategy would provide similar QALY outcomes, but at a lower cost than a universal diagnostic test strategy (–£12) and at a higher cost than a ‘no screening/testing or treatment’ strategy (£18). The ICER for a screen-only strategy compared with a ‘no screening/testing or treatment’ strategy is £77,574, and the QALY gain per woman with previous GDM is estimated to be 0.02. The estimated QALY gain per woman with previous GDM would have to be increased to 0.08 in order for the NHBs of this screen-only strategy to exceed those of a ‘no screening/testing or treatment’ strategy.
Scenario analysis: maternal longer-term outcomes
If maternal longer-term outcomes are incorporated in the model, the best-performing screen-only strategy remains to screen women on the basis of prior GDM. The expected cost per pregnant woman is increased to £495 and the QALY loss is reduced to 0.035. Screen-only remains cheaper than universal diagnostic test, but is no longer more effective. When the cost-effectiveness threshold is increased to £30,000 per QALY, the best-performing screen-only strategy is to offer treatment to any woman based on maternal age ≥ 25, BMI ≥ 25kg/m2 and non-white ethnicity. Using these criteria 92% of women would be expected to screen positive and the expected cost per pregnant woman would be £1,920 with a QALY gain of 0.029. In this scenario the maternal longer-term QALY gains from the early treatment and prevention of type 2 diabetes exceed the QALY losses from perinatal outcomes. For full incremental results (see Full Incremental analysis).
Scenario analysis: screen only with oral glucose challenge test
With a 1-hour 50-g OGCT and threshold of 7.2 mmol/l for post-load glucose the model estimates that 32% of women would screen positive. The expected cost per pregnant woman would be £769 and the QALY losses would be –0.034. This represents an additional cost of £285 and a QALY gain of 0.002 compared with the best-performing risk factor screening strategy, giving an ICER of £161,271.
Screen and test strategies
At a cost-effectiveness threshold of £20,000 per QALY, the best-performing screen and diagnostic test strategy among all possible screen and diagnostic test strategies is to offer OGTT only to women with prior GDM. The model predicts an expected cost per pregnant woman of £478 and a QALY loss of 0.036. Hence ‘screen and diagnostic test’ offers similar QALY gains to the other intervention strategies, but at a lower cost than ‘screen only’ or ‘universal diagnostic test’ and an incremental cost of £11 per pregnant woman compared with ‘no screening/testing or treatment’. In general, screen and diagnostic test strategies incur lower costs and QALYs than the commensurate ‘screen-only’ strategy as fewer women are offered treatment, the cost of which exceeds the cost of the test.
Scenario analysis: longer-term outcomes
When maternal longer-term outcomes are incorporated in the model, the best-performing screen and diagnostic test strategy is unchanged; however, the expected cost per pregnant woman is increased to £482. When maternal longer-term outcomes are incorporated and the cost-effectiveness threshold is increased to £30,000 per QALY, the best-performing screen and diagnostic test strategy is to offer OGTT to women based on maternal age of ≥ 25 years, BMI of ≥ 25 kg/m2 and non-white ethnicity. For complete incremental results, see the following section.
Full incremental analysis
A full incremental analysis is used to compare all of the intervention strategies. The universal diagnostic test strategy is defined using the best-performing diagnostic threshold identified above (see Screening, diagnosis and treatment). The set of risk factor screening strategies was explained above (see Screening, diagnosis and treatment of hyperglycaemia in pregnancy). Combining the best-performing diagnostic threshold (identified in Best-performing diagnostic threshold) with the alternative screening strategies provides the full set of screen and diagnostic test interventions. This results in 140 possible alternative strategies (‘no screening/testing or treatment’, ‘universal diagnostic test’, 69 ‘risk factor screening’ strategies and 69 ‘screen and diagnostic test’ strategies). For brevity, the results tables show only the best-performing strategy from each type of intervention. Cost-effectiveness results for the base case are summarised in Table 48, and reported for all remaining non-dominated strategies in Appendix 6, Tables 75–79.
| Identification strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 477 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0356 | –947 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 491 | –0.0359 | –959 | |||||
| £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0359 | –1197 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0356 | –1197 | ||||||
| Diagnostic | 9.5 | 10 | 0.015 | 0.985 | 495 | –0.0357 | –1210 | |||||
| £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 5.2 | 8.8 | 0.005 | 0.006 | 0.011 | 0.989 | 480 | –0.0358 | –1554 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0356 | –1553 | ||||||
| Diagnostic | 5.2 | 8.8 | 0.102 | 0.898 | 543 | –0.0339 | –1560 | |||||
At a cost-effectiveness threshold of £20,000 it is not cost-effective to identify women for treatment for hyperglycaemia. This remained the case when the cost-effectiveness threshold was increased to £30,000.
Scenario analysis: full incremental
For all scenario analysis, the cost-effective strategy at a £20,000 cost-effectiveness threshold is ’no screening/testing or treatment’, despite the variations in results that we highlight in the following paragraphs. The results of the four scenario analyses performed at a cost-effectiveness threshold of £20,000 per QALY are presented in Table 49 (alongside the base-case analysis results to facilitate the comparison).
| Identification strategy | Risk factor | Fasting glucose (mmol/l) | Post-load glucose (mmol/l) | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0359 | –1197 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0356 | –1197 | ||||||
| Diagnostic | 9.5 | 10 | 0.015 | 0.985 | 495 | –0.0357 | –1210 | |||||
| Scenario 1: inclusion of maternal long term | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 5.4 | 11.1 | 0.003 | 0.007 | 0.011 | 0.989 | 482 | –0.0357 | –1195 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 495 | –0.0349 | –1194 | ||||||
| Diagnostic | 5.4 | 11.1 | 0.058 | 0.942 | 545 | –0.0330 | –1206 | |||||
| Scenario 2: alternative diagnostic uptake estimates | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 477 | –0.0359 | –1197 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 483 | –0.0357 | –1197 | ||||||
| Diagnostic | 9.5 | 10 | 0.015 | 0.985 | 500 | –0.0357 | –1210 | |||||
| Scenario 3: alternative treatment ‘bundle’ with lower use of insulin | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9 | 9.5 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0359 | –1196 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0356 | –1197 | ||||||
| Diagnostic | 9 | 9.5 | 0.020 | 0.980 | 498 | –0.0357 | –1211 | |||||
| Scenario 4: minimum cost of treatment | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 8 | 8.5 | 0.003 | 0.007 | 0.011 | 0.989 | 478 | –0.0359 | –1196 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 483 | –0.0356 | –1196 | ||||||
| Diagnostic | 8 | 8.5 | 0.043 | 0.957 | 507 | –0.0352 | –1210 | |||||
| Scenario 5: alternative treatment effect estimates | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0360 | –1197 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 484 | –0.0357 | –1197 | ||||||
| Diagnostic | 9.5 | 10 | 0.015 | 0.985 | 495 | –0.0358 | –1210 | |||||
For scenarios 2 and 5, the best-performing diagnostic glucose threshold remained that of the base case, that is, fasting glucose level of ≥ 9.5 mmol/l and post-load glucose level ≥ 10.0 mmol/l. However, in scenario 1, which included maternal longer-term outcomes, the best-performing diagnostic glucose threshold was lowered for fasting glucose (5.4 mmol/l), but increased for post-load glucose (11.1 mmol/l) comparison. The inclusion of maternal longer-term outcomes will increase the costs (545 vs. £495) and the effectiveness (–0.0350 vs. –0.0357) of the universal diagnostic test strategy compared with the base case, with more women being treated in the scenario.
In scenario 3, for which an alternative treatment ‘bundle’ with a smaller proportion of women on insulin than the base case (11% vs. 28%), the best-performing diagnostic glucose threshold was reduced to fasting glucose ≥ 9.0 mmol/l and post-load glucose ≥ 9.5 mmol/l. The reduction of the cost of treatment for pregnant woman from £935 (base case) to £897 translates into more women (2.0% vs. 1.5%) being treated in scenario 3 for the universal diagnostic strategy for a small increase in cost compared with the base-case equivalent strategy (£3). When the cost of treatment is further reduced in scenario 4 with insulin not being offered, and all advice and instruction activities being delivered as group sessions for 12 women, the best-performing diagnostic glucose threshold was further reduced to fasting glucose level of ≥ 8.0 mmol/l and post-load glucose level of ≥ 8.5 mmol/l. Similarly to scenario 3, the reduction in treatment cost (from £935 to £753) will translate into more women being treated in scenario 4 (4.3% vs. 1.5%) for the universal diagnostic strategy for a small increase in cost compared with the base-case equivalent strategy (£12).
To explore the individual impact of removing the cost of insulin and delivering advice and instruction to groups of 12 (reducing the cost of treatment to £874 and £791, respectively) scenario 4 was initially run separately for each element of cost reductions. The results were broadly consistent with the base-case at £13,000 and £20,000 per QALY for these sub-scenarios. In scenario 4, and at a cost-effectiveness threshold of £30,000 per QALY, the best-performing strategy is to screen, based on a maternal age of ≥ 25 years, BMI of ≥ 25kg/m2 and non-white ethnicity, and offer treatment to women who test positive on the 2-hour 75-g OGTT (fasting glucose level of ≥ 5.0 mmol/l and/or post-load glucose level of ≥ 6.4 mmol/l), which corresponds to treating 24% of the population at an expected cost of £647 per woman and minus 0.295 expected QALYs. When only the cost reduction of delivering instruction and advice in groups of 12 women is included in the scenario analysis, the same strategy emerges as ‘best performing’ at a cost-effectiveness threshold of £30,000 per QALY, although at a slightly higher diagnostic threshold for post-load glucose level (6.9 mmol/l). When only the cost of insulin therapy is removed from the cost of treatment, ‘no screening/testing or treatment’ remains the best-performing strategy at cost-effectiveness thresholds of £13,000, £20,000 and £30,000 per QALY. This implies that cost reductions by restructuring the delivery of advice and instruction regarding treatment may improve cost-effectiveness of this type of intervention strategy compared with ‘no screening/testing or treatment’.
The best-performing screening criterion at a cost-effectiveness threshold of £20,000 per QALY in the ‘screen-only’ and ‘screen and test’ strategies is previous GDM for all scenarios and the same as for the base case.
In scenario 2, changes in the uptake of diagnostic test following or selective risk factor screening (–10%) and the uptake of a universal diagnostic test (+11%) did not result in considerable changes to results compared with the base case. Costs were slightly reduced for the ‘screen and test’ strategy (£1) and increased for the universal diagnostic strategy (£5), with no visible changes in outcomes. These changes in uptake of diagnostic test reduce the difference in terms of proportion of women treated when comparing ‘screen and test’ with the universal diagnostic test. In the base case, ‘screen and test’ increased the rate of treatment compared with the universal diagnostic test by 15%, whereas in scenario 2 the corresponding increase is only 11%. A further extreme case scenario in which all types of uptake were set to 100% did not alter the best-performing intervention strategy (results available from the corresponding author on request).
It is only with the inclusion of maternal longer-term health outcomes (scenario 1) and at cost-effectiveness thresholds of > £24,000 per QALY that NHBs are improved by intervening. At a cost-effectiveness threshold of £30,000 per QALY, the best-performing intervention strategy is to offer treatment to women based on maternal age ≥ 25 years, BMI of ≥ 25 kg/m2 and non-white ethnicity. This would entail treating 92% of pregnant women at an expected cost of £1921 per woman and a QALY gain of 0.0295, largely attributable to the benefits of early treatment and prevention of type 2 diabetes. Adding a diagnostic test to those that screened positive would reduce the proportion offered treatment to 34%, and result in an expected cost per pregnant woman of £1035 and an associated QALY loss of –0.009. ‘Screen and diagnostic test’ would be associated with higher NHBs than ‘no screening/testing or treatment’ or universal diagnostic test, but lower NHBs compared with ‘screen only’ testing.
The base-case results were robust to the remaining scenarios at all evaluated cost-effectiveness thresholds (£13,000, £20,000 and £30,000 per QALY).
Subgroup analysis
We evaluated the results separately in a population of WB ethnicity and in a population of SA and ‘Other’ ethnicity (see Appendix 6, Tables 79–81). Among the SA and ‘Other’ subgroup, the mean fasting blood glucose level was 4.60 mmol/l and the mean post-load glucose level was measured at 5.83 mmol/l. We had observations of 6265 participants with SA or ‘Other’ ethnicity in the BiB study,22 of which 75 (1.2%) had fasting or post-load glucose measures of > 11.1 mmol/l. The remainder had fasting blood glucose levels in the range 3–9.4 mmol/l and post-load blood glucose measurements in the range of 2.2–11 mmol/l. The model predicts that 2% would experience pre-eclampsia, 19.5% would undergo C-section and 15.5% would receive induction of labour. Immediate birth outcomes would be predicted to include 4.2% admission to neonatal unit, 6.4% instrumental delivery and 1.6% serious perinatal complications. In the subgroup of WB ethnicity, the mean fasting glucose level was 4.41 mmol/l and the mean post-load glucose level was 5.44 mmol/l. There were 4088 women of WB ethnicity in the BiB data set22 that was used for the analysis, of whom only six (0.2%) had fasting or post-load glucose measures of > 11.1 mmol/l. The remainder had blood glucose levels in the range of 3–7.6 mmol/l for fasting glucose and 1.6–10.9 mmol/l for post-load glucose. In this group, the upper bound for the fasting threshold is 7.6 mmol/l, and values of > 7.6 mmol/l suggest that no-one be diagnosed with GDM on the basis of fasting glucose. The model would predict that 1.8% of white women would experience pre-eclampsia, 21.4% would undergo C-section and 18.3% would receive induction of labour. In this subgroup, immediate birth outcomes would include 4.1% admission to neonatal unit, 8.3% instrumental delivery and 1.5% serious perinatal complications.
The results indicate that the best-performing diagnostic glucose threshold would be similar in a SA cohort compared with the overall cohort, but that cost per pregnant woman would be increased and QALY losses reduced for every intervention strategy. At a cost-effectiveness threshold of £20,000 per QALY, the best-performing diagnostic glucose threshold is the same as that found in the base case, that is, 9.5 mmol/l for fasting blood glucose level and 10.0 mmol/l for post-load glucose level. Using this threshold, 2.3% of SA woman would be diagnosed as having GDM. The expected cost per pregnant woman of a universal diagnostic test strategy is increased to £499 and the QALY loss reduced to –0.035. This is compared with an expected cost of £454 associated with ‘no screening/testing or treatment’ and a QALY loss of –0.036.
In a population of white ethnicity, the best-performing diagnostic glucose threshold is altered, compared with the base case, from 9.5 mmol/l to 8.0 mmol/l for fasting glucose level, and from 10.0 to 11.1 mmol/l for post-load glucose level. However, this effectively forms the upper bound for the observed glucose levels, indicating that very few women (0.2%) would be diagnosed with GDM. At these fasting and post-load glucose levels, the cost per woman of a universal diagnostic test strategy is predicted at £508, with an associated QALY loss of 0.036. In the base-case analysis, the best-performing diagnostic glucose thresholds remain at these upper bounds, even if the cost-effectiveness threshold is raised to £30,000 per QALY.
Scenario analysis: maternal longer-term outcomes
When longer-term outcomes are included it does become cost-effective to intervene at a threshold of £20,000 for a cohort of SA women. The best-performing intervention strategy is to screen women on the basis of BMI of > 25 kg/m2 and to treat all women who screen positive. In the BiB cohort,22 48% of women of SA or ‘Other’ ethnicity had BMI of ≥ 25 kg/m2, and the mean glucose measures in those that would screen positive would be a fasting glucose level of 4.79 mmol/l and post-load glucose level of 6.74. The expected cost per woman would be £1306, but QALY losses from perinatal outcomes would be outweighed by large gains from the early treatment and prevention of type 2 diabetes, resulting in an expected QALY gain per pregnant woman of 0.007. The benefits of the early detection of type 2 diabetes were modelled as higher in a SA population. The benefits of the ILS to prevent the development of type 2 diabetes were adjusted for the proportion of women that had a BMI of ≥ 30 kg/m2, which was higher among the SA and ‘Other’ subgroup than the overall cohort. Although the best-performing diagnostic glucose threshold would be 5.2 mmol/l for fasting glucose and 11.1 mmol/l for post-load glucose, the NHBs of a universal diagnostic test strategy (12% diagnosed; expected cost £595 and QALYs –0.031) and the best-performing screen and test strategy (3% diagnosed; expected cost £521 and expected QALYs –0.033) are still lower than the best-performing ‘screen only’ strategy, but ‘screen and test’ would improve NHBs compared with ‘no screening/testing or treatment’. When the cost-effectiveness threshold increases to £30,000 per QALY it is optimal to intervene with a screen-only strategy on the basis of maternal age of ≥ 25 years, BMI of ≥ 25 kg/m2 and family history of diabetes. This would increase the proportion of women who screen positive and are treated to 87% of the cohort, with an expected cost per pregnant woman of £1833 and a QALY gain of 0.03.
Value of information analysis
In the base-case analysis for a cost-effectiveness threshold of £20,000 per QALY, there does not appear to be value in further research. There is uncertainty as to the best-performing diagnostic glucose threshold, but the expected net benefit that could be achieved in the absence of this uncertainty (–£1205) is still less than that expected with ‘no screening/testing or treatment’ based on current information. This would seem to indicate that there is no value in research to reduce uncertainty in the best-performing diagnostic glucose threshold.
At a cost-effectiveness threshold of £20,000 per QALY, the expected net benefit that could be achieved in the absence of uncertainty regarding the best-performing diagnostic glucose threshold (–£1205) is, again, less than that expected with ‘no screening/testing or treatment’ based on current information. In the full incremental analysis and using the best-performing diagnostic glucose threshold, the likelihood that a ‘no screening/testing or treatment’ strategy is cost-effective is 0.98 (EVPI £1 per decision), and the probability that a universal diagnostic test strategy is cost-effective is 0.02. The parameters predominantly associated with decision uncertainty are the fasting and post-load glucose levels among women who would test negative at the best-performing threshold (expected value of partial perfect information for each £0.01 per person).
At a cost-effectiveness threshold of £30,000 per QALY, the expected net benefit that could be achieved in the absence of uncertainty regarding the best-performing diagnostic glucose threshold does exceed that associated with a ‘no screening/testing or treatment’ strategy. This suggests that the probability that ‘no screening/testing or treatment’ is cost-effective could be lower than that evaluated at a fixed diagnostic threshold (0.51), and that the value of further research could potentially be even higher than the estimated £55 EVPI per decision. If this £55 is multiplied by 700,000255 to represent the number of pregnancies in England in a 1-year period, this would suggest a very high upper bound for the population value of research (£38.5M). The other strategies associated with a probability of > 1% that could be the most cost-effective are ‘screen and treat’ based on BMI of ≥ 25 kg/m2 (0.08); ‘screen and treat’ based on BMI of ≥ 30 kg/m2 and previous GDM (0.06); ‘screen, test and treat’ based on maternal age of ≥ 30 years and BMI of ≥ 25 kg/m2 (0.02); ‘screen and treat’ based on maternal age of ≥ 25 years, BMI of ≥ 25 kg/m2 and non-white ethnicity (0.21); and to ‘screen and treat’ based on BMI of ≥ 25 kg/m2 and previous GDM (0.02). The inputs that contribute most to the decision uncertainty (EVPI > £1 per decision) include the maternal utility gain from GDM treatment (£16), the effect of treatment (applied only to women who test positive on OGTT) on risk of instrumental delivery (£9), and the effect of diet and exercise (applied to women who are identified and treated based on screening alone applied to the screen and treat strategies) on the risk of shoulder dystocia (£2).
In the scenario analysis with maternal longer-term outcomes included and at a cost-effectiveness threshold of £20,000 per QALY, there is again a high value to further research. Using the best-performing diagnostic glucose threshold, the likelihood that a ‘no screen/test or treat’ strategy is cost-effective is 0.87 and the EVPI is £252 per decision. If this is multiplied by 700,000 to represent the number of pregnancies in England in a 1-year period, this would suggest a very high population value of research. The value of further research is estimated to be high because there is uncertainty surrounding the benefits attributed to longer-term outcomes, and in some scenarios these convey large additional net benefits to all women who exceed the given diagnostic threshold. Two other strategies associated with a probability of > 1%, which may be the most cost-effective, are ‘screen and treat’ based on a BMI of ≥ 25 kg/m2 (0.0124) and ‘screen and treat’ based on maternal age of ≥ 25 years, a BMI of ≥ 25 kg/m2 and non-white ethnicity (0.0956). The parameter that contributes most to the decision uncertainty is the QALY gain associated with early detection of maternal type 2 diabetes at post-partum follow-up (EVPI £196). The other parameters that are associated with EVPI of > £1 per decision include the costs associated with the early detection of maternal type 2 diabetes at post-partum follow-up (£58), the maternal utility gain from GDM treatment (£17), the effect of treatment (applied only to women who test positive on OGTT) on risk of instrumental delivery (£20) and the effect of diet and exercise (applied to women who are identified and treated based on screening alone applied to the screen and treat strategies) on the risk of shoulder dystocia (£4).
Discussion
We had access to large IPD sets that allowed us to specify risk models for immediate perinatal outcomes based on the glucose measurements obtained from a 2-hour 75-g OGTT given at between 26 and 28 weeks’ gestation. The effects of treatment on perinatal outcomes were estimated in meta-analyses. We combined these risk models in a decision-analytic model with evidence from the wider literature in order to model the cost-effectiveness of alternative screen, diagnostic and treatment strategies. The costs and QALYs were sourced from the wider literature, with key reference to sources used in the recent NICE guideline. 18
The base-case analysis indicates that it is not cost-effective to identify women for treatment for hyperglycaemia at a cost-effectiveness threshold of £20,000 per QALY. Although treatment reduces the risk of some adverse outcomes, it increases the risk of others and the overall QALY gains from treatment compared with ‘no screening/testing or treatment’ are not sufficient to justify the increased costs. The treatment costs are driven largely by more intensive antenatal care, and drug costs are low such that switching from insulin to metformin use has little impact on the conclusions, because antenatal surveillance remains the same for both drugs. However, if pharmacological intervention, additional to routine antenatal care is provided with no, or limited, additional surveillance, costs may be similar to those for women without GDM who receive only routine care, but the risk of adverse perinatal outcomes may be reduced. The costs of diagnostic testing could also be reduced by utilising a FPG in place of the full OGTT. Our analysis suggests that the FPG could result in similar benefits to the OGTT, but at a reduced cost. However, the cost savings are not sufficient to make intervention cost-effective, even at a cost-effectiveness threshold of £30,000 per QALY.
Our results are broadly in line with those of the recent NICE update,18 which estimated ICERs in excess of £20,000 for diagnosis at commonly used glucose thresholds. The NICE guideline18 provided estimates of cost-effectiveness in two data sets: HAPO (four centres: Belfast and Manchester in the UK and Brisbane and Newcastle in Australia) and Norwich. In the Norwich data set they estimated that ‘no treatment’ (equivalent to ‘no screening/testing or treatment’ in our model) was cost-effective, with the next-best alternative of diagnosis based on WHO 1999 criteria providing an ICER of £35,000 per QALY. In the HAPO (four centres) data set the next best alternative to ‘no treatment’ was a fasting glucose level of 5.5 mmol/l and a post-load glucose level of 8.5 mmol/l, associated with an ICER of £28,103 per QALY. The NICE guideline18 did not perform a full incremental analysis of alternative screen and test strategies and screen-only approaches. However, NICE did compare diagnosis at commonly used thresholds following NICE risk factor screening in the HAPO (four centres) data set, and found that treating above a fasting glucose level of 5.6 mmol/l or a post-load glucose level of 8.5 mmol/l was associated with an ICER of £23,902 compared with ‘no treatment’. The reported ICERs in the NICE guideline were based on deterministic analysis and, as such, may be subject to bias if the cost-effectiveness model is non-linear. However, a probabilistic analysis was conducted that estimated the probability that each strategy was cost-effective. When risk factor screening was not considered, ‘no treatment’ had 99.8% (at a cost-effectiveness threshold £20,000 per QALY) and 82.7% (at a cost-effectiveness threshold of £30,000 per QALY) probability of being the most cost-effective. Applying NICE risk factor screening, then at a cost-effectiveness threshold of £20,000 per QALY, ‘no treatment’ had 82.5% probability of being the most cost-effective compared with 9.4% for diagnosis at a fasting glucose level of > 5.6 mmol/l or post-load glucose level of > 8.5 mmol/l. These respective probabilities for a cost-effectiveness threshold of £30,000 were 40.1% and 6.2% (the strategy with the highest probability of being cost-effective of 41.0% was, in fact, diagnosis using the WHO criteria following NICE risk factor screening).
Intervention is likely to appear comparatively less cost-effective in our analysis because, in contrast with the economic evaluation for the NICE guideline, we include a cost for instrumental delivery, the risk of which is increased by treatment. Intervention strategies may also appear more cost-effective in the HAPO (four centres) based on higher baseline risk of GDM and of adverse perinatal outcomes given the reported mean BMI of 29 kg/m2 compared with 26 kg/m2 in the BiB study. 22 However, the proportion of women of white ethnicity was higher in HAPO (four centres), which would be associated with lower baseline risk of GDM.
The updated NICE guideline18 recommendations do not draw on the reported cost-effectiveness analysis, instead suggesting a fasting glucose level of 5.6 mmol/l and a post-load glucose level of 7.8 mmol/l, which were not found to be cost-effective at a threshold of £30,000 per QALY in both our and their analyses. The guideline group considered that the fasting threshold of 7.0 mmol/l in the WHO 1999 criteria was too high, based on the fact that they had observed that the treatment trials utilised lower fasting glucose thresholds for inclusion. Furthermore, they expressed concern that the regression models underpinning the economic analysis in the NICE guideline did not incorporate fasting blood glucose levels, as this covariate had been dropped in the stepwise selection process. We did not use a stepwise selection process, and our risk models for adverse perinatal outcomes all include both fasting and post-load glucose levels. This further supports the view that although intervention at lower glucose thresholds does improve health outcomes, the resources required result in the displacement of greater health outcomes elsewhere in the NHS. We identified the best-performing diagnostic glucose threshold, but differences between similar thresholds were small. However, if clinicians use a lower diagnostic glucose threshold than that suggested by the model then the result will be a greater volume of women being treated, and hence an increase in the absolute volume of resources required and, correspondingly, an increase in the absolute amount of health displaced elsewhere in the NHS.
In a scenario analysis, we also included the longer-term impact of diagnosing women with GDM in terms of the early treatment of type 2 diabetes and the provision of further interventions to prevent the onset of type 2 diabetes post partum. Although it is not cost-effective to treat GDM on its own, in combination with early detection post partum and/or prevention of type 2 diabetes it could be cost-effective, particularly in a population with a higher risk of type 2 diabetes in later life, for example a SA population. The generalisability and sustainability of diabetes prevention programme effects, however, have been questioned recently. 256
At a cost-effectiveness threshold of £20,000 per QALY and with maternal longer-term outcomes included, the best-performing strategy for a SA cohort is to screen on the basis of BMI of ≥ 25 kg/m2 and then to treat all women. Because evidence related to longer-term infant outcomes is limited, and the generalisability and sustainability of diabetes prevention programmes are not clear, the results of the scenario analyses incorporating longer-term outcomes should be interpreted with caution. The benefits of early treatment of type 2 diabetes were based on a trial that considered a mixed-gender population, with underlying prevalence rates of 5% and 10%, and we did not adjust the estimated benefits as we altered the diagnostic threshold used in the model. The benefits of the ILS intervention was adjusted with the diagnostic threshold in terms of the proportion of women with BMI of ≥ 30 kg/m2 among those who would test positive. We assumed that treatment in the absence of a blood glucose test could not include metformin and insulin, and we applied no lower bound for the glucose level at which treatment benefits would cease to apply. In this strategy, the benefits of the diet and lifestyle intervention are assumed to extend to the 48% of women who would screen positive. If this intervention strategy is considered to be unrealistic, the next-best strategy would be ‘no screening/testing or treatment’.
Strengths and limitations
This study is, to the best of our knowledge, the first cost-effectiveness analysis that selects diagnostic glucose thresholds on the basis of cost-effectiveness and NHBs within the NHS (rather than excess risk of adverse outcomes), and the first to provide a direct simultaneous comparison of alternative intervention strategies that are composed of all of the combinations of screening, diagnostics and treatment for GDM. Furthermore, data from a large UK obstetric cohort (n = 10,353) were used to model women’s characteristics and glucose levels, while two data sets combining data from the BiB and Atlantic DIP22,59 untreated population (n = 14,368) informed the majority of adverse perinatal outcomes. Both of these sources of data contributed to the high quality of the analysis. Importantly, this study explored the potential impact of longer-term maternal outcomes on the cost-effectiveness of the competing strategies, and allowed us to identify effects on longer-term outcomes as an important topic for future research.
Nevertheless, there are limitations to our study, which mostly relate to areas of uncertainty and/or evidence gaps. One of the key findings of our study is that unless the costs of treatment are reduced considerably or there is evidence that the net benefit from longer-term outcomes would offset the costs of testing and treating then ‘no screening/testing or treatment’ is the cost-effective intervention at the considered range of cost-effectiveness thresholds. Although we found evidence suggesting that women with previous GDM are at a higher risk of developing glucose intolerance, and, ultimately, type 2 diabetes later in life compared with women without GDM in their pregnancy, we did not find evidence that the decrease in risk was mediated via treatment for GDM. Thus in the model, treatment for GDM does not modify the risk of type 2 diabetes. Instead, it is assumed that women who have had GDM will be identified as being at a higher risk of type 2 diabetes and that a preventative intervention will be delivered to those with IGT (proxied by having a BMI of ≥ 30kg/m2) to decrease this risk. The first issue here is whether or not the proxy for IGT is appropriate, but a more important issue is the inclusion of the ILS intervention in the model; we should also consider other interventions that could be offered to prevent type 2 diabetes and which do not require identification of GDM. In this sense, we can consider that the comparison is incomplete.
The other component of maternal longer-term outcomes included in scenario analysis was early detection of type 2 diabetes during the post-partum follow-up. The net benefit attributed to this outcome was sourced from a study238 in a previously undiagnosed older population (45 years old) that included males and females, and was exclusively of white ethnicity. The characteristics of the population in this study are considerably different from those of the obstetric population in the model, and, therefore, it is likely that the size of the benefit of screening for type 2 diabetes is different from what it would be for the cohort in the model. In the absence of evidence in an obstetric population, this was the best estimate that we could apply within the time constraints, but identifying the size of this benefit for women with GDM would be important to resolve the uncertainty around the size of the benefits of improving longer-term maternal outcomes. Furthermore, the benefits obtained by screening women identified with GDM were not compared with alternative policies to screen for the detection of type 2 diabetes.
It is important to notice that the longer-term maternal outcomes included in scenario analysis were not linked to varying diagnostic thresholds and mediated only through obesity levels. As the diagnostic threshold for GDM is lowered, it would be expected that the risk of type 2 diabetes in the post-partum period and later in maternal life will change in women identified with GDM.
This study did not include longer-term outcomes for the offspring of pregnant women diagnosed with GDM because of the paucity of evidence that would link GDM and treatment of GDM to changes in longer-term outcomes such as obesity and metabolic syndrome in the offspring. Nevertheless, the infants in the BiB cohort22 will continue to be followed up, so it is possible that as more data become available in the future it may be used to overcome this limitation.
Although IPD from a large cohort (n = 10,353) were used to inform population characteristics and glucose level, this constrains the model to the range of observed characteristics and glucose measurements in the BiB cohort. 22 We attempted to model glucose levels as a function of maternal characteristics, but we did not identify a statistical model that adequately fitted the data, especially at the tails of the glucose distribution. The use of more complex statistical models may overcome this limitation, making the decision-analysis model more flexible and more generalisable. Given that the model is currently constrained to the characteristics of the BiB cohort,22 it was not possible to present separate subgroup analyses for other ethnicities also at higher risk of GDM, such as Afro-Caribbean and Middle Eastern women.
Finally, the treatment effect estimates applied in the model were sourced from pooled RCT data, and may not be directly generalisable to (1) the GDM obstetric population in the model, as study entry criteria are in general more restrictive than for observational studies and (2) treatment at more extreme GDM diagnostic thresholds outside of the range that was used for diagnosis in the RCTs. Nevertheless, these were best treatment effectiveness estimates that were available to inform the model, despite this caveat.
Implications for future research
Value of information analysis may identify areas for which an investment in further research could provide better value. At cost-effectiveness thresholds ranging from £13,000 to £20,000 per QALY there is uncertainty surrounding the best-performing diagnostic glucose threshold. However, the expected net benefit that could be achieved in the absence of uncertainty regarding the best-performing diagnostic glucose threshold is less than that expected with ‘no screening/testing or treatment’ based on current information, suggesting that it would not be worth conducting further research. At a cost-effectiveness threshold of £30,000 per QALY, three parameters were identified as contributing the most for decision uncertainty: (1) maternal utility gain from GDM treatment (population EVPI £11M); (2) the effect of treatment on risk of instrumental delivery (population EVPI £6M); and (3) the effect of diet and exercise on the risk of shoulder dystocia (population EVPI £1M).
Once maternal longer-term outcomes are included in the value of information analysis, there is a high value to further research at a cost-effectiveness threshold of £20,000 per QALY. The EVPI (accounting for the 700,000 pregnancies per year in England and Wales) at this cost-effectiveness threshold suggests that it would be worth investing up to £38.5M to resolve uncertainty at this level, as this represents the potential opportunity cost of taking the wrong decision. Thus, the upper bound for investing in research that would provide more accurate and reliable data on the cost-effectiveness of identifying and treating GDM (including longer-term outcomes) is £38.5M. The EVPI estimates allow prioritisation of the research by focusing on areas for which resolving uncertainty would be of greater value. The parameter that contributes most to the decision uncertainty is the QALY gain associated with early detection of maternal type 2 diabetes at post-partum follow-up, with an EVPI of £196 per pregnancy and £134M for the population. The parameter with the second highest EVPI was the costs associated with early detection of maternal type 2 diabetes at post-partum follow-up (population EVPI £41M). However, as described above (see Strengths and limitations), these uncertain benefits pertain to the screening for type 2 diabetes in a high-risk population that is identified on the basis of GDM. The cost-effectiveness analysis here does not compare alternative screening programmes for type 2 diabetes.
Although this aspect could not be captured by the value of information analysis, as this is not an area of uncertainty but rather a question of how service delivery can be more efficiently organised, research into less expensive ways of delivering treatment for GDM is another potential area of interest. The results suggest that the cost-effectiveness of screening, testing and treating strategies can be improved considerably by delivering dietary advice and insulin instruction in large groups, rather than individually. It is, however, worth emphasising that, even with this reduction in cost, these strategies would not be cost-effective at a £20,000 per QALY, but only at £30,000 per QALY and when including longer-term maternal outcomes. The use of the higher £30,000 cost-effectiveness threshold (NICE) can be applied in circumstances in which there is little uncertainty and when there are significant health benefits that have not been captured within the economic analysis. We did seek to capture longer-term health benefits within the model, but they are estimated with considerable uncertainty, suggesting that a cost-effectiveness threshold of £30,000 may not be applicable.
Conclusion
The evidence of the effects of identifying and treating women with GDM in terms of the reduction in adverse perinatal outcomes is not sufficient to justify the cost of treatment at a cost-effectiveness threshold of £20,000 per QALY. However, if longer-term outcomes are included in the model (although evidence is limited) and costs of providing GDM treatment are reduced by more efficiently deploying existing resources then it may be cost-effective to intervene in populations with a high prevalence of glucose intolerance. The considerable uncertainty surrounding the potential size of longer-term benefits suggests that at a cost-effectiveness threshold of £20,000 per QALY it would be worth conducting additional research on the HRQL gains and costs of early detection of maternal type 2 diabetes. Furthermore, there are important evidence gaps regarding offspring longer-term outcomes and data that allow linking longer-term outcomes to varying diagnostic thresholds.
Chapter 8 Conclusions
Conclusions, implications and recommendations for future research
Previously the aim of diagnosing GDM has been to reduce the risk of perinatal complications through the treatment of hyperglycaemia and to identify women at risk of developing future type 2 diabetes. 166,257 Newly proposed criteria, however, seek to identify infants at risk of future obesity through its association with LGA, high infant adiposity and high cord blood C-peptide levels. 8 The shift in the aim of diagnosing GDM is particularly important for SAs, as their infants, in comparison with white Europeans, have markedly lower BW and reduced risk of LGA, but this lower BW masks a propensity to greater adiposity and associated cardiometabolic risk. Our analysis using the BiB study IPD123 detailed in Chapter 2 suggests that to capture the majority of those infants at risk of LGA and/or high adiposity at birth (by the methods used by the IADPSG), glucose thresholds at OGTT used to diagnose GDM would need to be lowered (compared with previous threshold criteria,11 and more in line with the new IADPSG criteria8). Lowering glucose thresholds in this way will increase the proportion of women at risk of important adverse perinatal outcomes: one in 12 WB women and one in four SA women will be diagnosed based on estimates using the BiB study IPD123 (see Chapters 2 and 4). As there are effective, safe and relatively cheap treatments for GDM (lifestyle advice, metformin and insulin), which reduce glucose levels across its distribution and help prevent adverse perinatal outcomes (see Chapter 6), applying lower threshold criteria may importantly improve perinatal outcomes. However, there is limited evidence from observation studies regarding the strength of the association between maternal glucose levels and longer-term outcomes (maternal and infant obesity and diabetes) (see Chapter 3) and there are no treatment trials examining treatment effects and the risk of future adverse outcomes, including infant obesity (see Chapter 6). Therefore, the degree to which new criteria will influence perinatal and longer-term outcomes is unclear. This has resulted in concern that lowering glucose thresholds will increase GDM prevalence and associated costs without evidence of benefit. 258
The increased identification of women resulting from lowering glucose thresholds has resource implications for the NHS in terms of antenatal services (OGTTs, treatments, induction of labour), intrapartum care (C-section) and postnatal services (infant care needs, 6-week screening for type 2 diabetes). There are also resource implications for primary care, in terms of the increased numbers of women requiring yearly screening for type 2 diabetes. Our economic analysis suggests that the benefits of identifying and treating women with GDM in terms of the reduction in risk of perinatal outcomes are not sufficient to justify the cost of treatment at a cost-effectiveness threshold of £20,000 per QALY. This finding may seem surprising, given that our analyses suggest that lower glucose levels are required to identify the majority of infants (using the methods of the IADPSG) at increased risk of future obesity (see Chapter 2) and that our treatments for GDM review (see Chapter 6) show statistically significant reduction in risks of most adverse perinatal outcomes if treatment to reduce maternal glucose is provided (compared with routine care). There are, however, several factors that are contributing to the findings of the economic evaluation. The higher cost perinatal outcomes associated with GDM, such as shoulder dystocia and neonatal unit admission, occur relatively infrequently and the reduction in these costs from treatment is therefore small (even although costs associated with one shoulder dystocia or one neonatal unit admission may be substantial). The more frequent adverse outcomes, such as C-section, are less costly. Moreover, some adverse outcomes are increased in treated women, including induction of labour, and, as discussed above, there is uncertainty about the effects of treating hyperglycaemia (at any glucose level) on longer-term maternal and infant outcomes; therefore, the use of a higher QALY threshold may not be appropriate.
Because there is uncertainty about GDM treatment effects on longer-term maternal and infant health, it is unclear if interventions outside pregnancy (e.g. obesity and diabetes prevention programmes) would convey greater gains compared with interventions delivered during pregnancy. However, there is little evidence on best timing of interventions outside pregnancy or what would constitute an effective obesity or diabetes prevention intervention.
We attempted to characterise longer-term effects in our economic model; however, we were unable to link them directly to alternative diagnostic thresholds. The magnitude of effects (both positive and negative) for the longer-term outcomes related to different diagnostic thresholds and approaches to the identification of women at risk (e.g. timing or diagnostic test) for treatment would seem key in determining whether or not it is cost-effective to intervene in pregnancy and therefore this should be examined. Also research examining the effectiveness of diabetes prevention programmes for women who have had GDM would help quantify the potential effects of identifying women with GDM, especially in light of recent concerns questioning the effectiveness of diabetes prevention programmes for the general at-risk population. 256
The cost-effectiveness analysis presented here suggests that the health gained by addressing GDM is comparatively lower than that generated by other NHS activities. As NHS funding is unlikely to be increased to address rising GDM prevalence, cheaper and/or more efficient ways of identifying GDM, and new and innovative methods of providing care, are required. Although our systematic review (see Chapter 6) found that the ‘step-up approach’ to treating GDM with diet first and glucose monitoring, with supplemental metformin or insulin if needed, is effective in reducing risks, trials investigating different packages of care or approaches to care are needed.
Treatment trials have not generally reported negative effects such as medicalisation, anxiety or drug side effects; this information would help to fully understand the effectiveness of treatments and the influence of non-compliance.
Our analysis detailed in Chapter 5 suggests that the assessment of maternal characteristics (e.g. ethnicity with a high prevalence of diabetes or previous macrosomic infant), currently recommended by NICE18 to identify high-risk women for diagnostic testing, in whatever form, performs poorly, because a large proportion of women need to be offered an OGTT, and it is likely that some women with hyperglycaemia would not be identified (low specificity) and therefore would not benefit from treatment. The identification of women who are at low risk of developing GDM and do not require an OGTT may be advantageous in some populations, however, and may prevent testing in at least 30%.
There is a balance between costs and improved perinatal and longer-term health impacts from the application of different diagnostic criteria and treatments. We found that at a cost-effectiveness threshold of £20,000 per QALY it is not cost-effective to identify women for treatment for hyperglycaemia, even in the scenario in which longer-term outcomes are incorporated into the model. It is only with the inclusion of longer-term health outcomes and at cost-effectiveness thresholds of > £24,000 per QALY (which is above the £20,000-per-QALY threshold recommended when there are uncertainties regarding treatment effectiveness) that NHBs are improved by intervening. Given the uncertainty surrounding the estimation of longer-term outcomes, and that only when these are incorporated into our economic model are health benefits improved, further research in this area (longer-term health effects) would be useful. Research examining the performance (sensitivity, specificity) and influence on outcomes of different methods to identify women at risk (screening tests) and those with GDM (diagnostic tests) are needed. The influence of alternative diagnostic thresholds and different treatment approaches on outcomes also require investigation.
Acknowledgements
We would like to thank the women who agreed to take part in the studies included in this report. We are grateful also to all the health professionals and researchers who have made this research possible.
Thank you to Ponnusamy Saravanan and Hema Venkataraman for providing the Warwick and Coventry data that are included in our prevalence estimates.
We would also like to thank Julie Glanville and Mick Arber of the Yorkshire Health Economics Consortium, who carried out the systematic review searches.
All of the authors made substantial contributions to progressing the research and interpretation of data; all authors were involved in the drafting of the manuscript or revising it critically for important intellectual content and all approved the final version to be published.
Contributions of authors
Diane Farrar (Senior research fellow, Bradford Institute for Health Research) contributed to the design of the study described in Chapter 2 and the research protocols for Chapters 3–6; advised on the analyses for Chapters 3–6 and drafted results, discussions and assessed studies for inclusion in the systematic reviews; and commented on the design and results of the economic model, analyses and results. Drafted Chapter 8.
Mark Simmonds (Research fellow, Centre for Reviews and Dissemination, University of York) contributed to the design of the research protocols for Chapters 3–6; assessed studies for inclusion in the systematic reviews and performed the analyses; and drafted results and discussions for Chapters 3–8.
Susan Griffin (Senior research fellow, Health Economics, University of York) contributed to the design and building of the economic model, and the writing of the economics chapter (see Chapter 7), and commented on all chapters.
Ana Duarte (Research fellow, Health Economics, University of York) contributed to the design and building of the economic model, and the writing of the economics chapter (see Chapter 7), and commented on all chapters.
Debbie A Lawlor (Professor of Epidemiology, MRC Integrative Epidemiology Unit, University of Bristol) contributed to the design of the study described in Chapter 2 and developed the analysis protocol; provided statistical advice for Chapters 3–6; contributed to the writing of the report; and commented on the design and results of the economic model.
Mark Sculpher (Professor of Health Economics, University of York) contributed to the design and building of the economic model, the writing of the economics chapter and commented on all chapters.
Lesley Fairley (Senior statistician, Bradford Institute for Health Research, Bradford Teaching Hospitals) developed the analysis protocol for Chapter 2 and performed the analyses; contributed to the writing of that chapter and commented on the remaining chapters.
Su Golder (Research fellow, Department of Health Sciences, University of York) assessed studies for inclusion in the systematic reviews and contributed to the writing of the associated chapters.
Derek Tuffnell (Professor of Obstetrics and Gynaecology, Bradford Teaching Hospitals) provided clinical interpretation of results; commented on the design of the economic model, analyses and results; and contributed to the writing of the report.
Martin Bland (Professor of Health Statistics, Department of Health Sciences, University of York) provided statistical advice for Chapters 3–6 and commented on all of the chapters.
Fidelma Dunne (Professor of Medicine, Galway Diabetes Research Centre and School of Medicine, National University of Ireland) provided Atlantic DIP data and clinical interpretation of results, and commented on all chapters.
Donald Whitelaw (Consultant diabetologist, Department of Diabetes & Endocrinology, Bradford Teaching Hospitals) provided clinical interpretation of results and commented on all of the chapters.
John Wright (Director of Research, Bradford Institute for Health Research, Bradford Teaching Hospitals) provided BiB data; a public health and global interpretation of results, and commented on all chapters.
Trevor A Sheldon (Professor of Health Services Research and Policy, Hull York Medical School) contributed to the design of the research protocols; advised on the analyses for Chapters 2–7; commented on the design of the economic model, analyses and results; and contributed to the writing of the report.
Publications
Farrar D, Fairley L, Santorelli G, Tuffnell D, Sheldon TA, Wright J, et al. Association between hyperglycaemia and adverse perinatal outcomes in South Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabet Endocrinol 2015;3:795–804.
Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 2016;13:354.
Data sharing statement
Published data can be accessed via relevant journal and or authors. BiB IPD can be requested via the BiB project website (www.borninbradford.nhs.uk/contact-us/) and Atlantic-Dip/Warwick/Coventry from the relevant authors.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013;159:123-9. http://dx.doi.org/10.7326/0003-4819-159-2-201307160-00661.
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773-9. http://dx.doi.org/10.1016/S0140-637609)60731-5.
- Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008;31:1668-9. http://dx.doi.org/10.2337/dc08-0706.
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290-6. http://dx.doi.org/10.1542/peds.2004-1808.
- Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010;53:89-97. http://dx.doi.org/10.1007/s00125-009-1560-z.
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002. http://dx.doi.org/10.1056/NEJMoa0707943.
- Farrar D, Fairley L, Santorelli G, Tuffnell D, Sheldon TA, Wright J, et al. Association between hyperglycaemia and adverse perinatal outcomes in South Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabet Endocrinol 2015;3:795-804.
- International Association of Diabetes and Pregnancy Study Groups Consensus panel . International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010;33:676-82. http://dx.doi.org/10.2337/dc09-1848.
- Nankervis A, McIntyre H, Moses R, Ross GP, Calloway L, Porter C, et al. ADIPS Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia 2013. http://adips.org/downloads/ADIPSConsensusGuidelinesGDM-030513VersionACCEPTEDFINALpdf (accessed 3 September 2015).
- Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: WHO; 2013.
- Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: WHO; 1999.
- American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2006;29:43-8.
- Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D. Gestational diabetes mellitus – management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust 1998;169:93-7.
- American College of Obstetricians and Gynecologists . Practice Bulletin Clinical Management Guidelines for Obstetricians – Gynecologists. Obstet Gynecol Clin North Am 2013;122:406-16.
- National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57. http://dx.doi.org/10.2337/diab.28.12.1039.
- O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13:278-85.
- National Screening Committee . What Is Screening? n.d. www.screening.nhs.uk/ (accessed May 2015).
- Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal period. London: NICE; 2015.
- Diabetes in Pregnancy. Management of Diabetes and Its Complications from Preconception to the Postnatal Period. London: NICE; 2015.
- Catalano PM, Avallone DA, Drago NM, Amini SB. Reproducibility of the oral glucose tolerance test in pregnant women. Am J Obstet Gynecol 1993;169:874-81. http://dx.doi.org/10.1016/0002-9378(93)90019-F.
- Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol 1991;164:564-8. http://dx.doi.org/10.1016/S0002-9378(11)80021-9.
- Wright J, Small N, Raynor P, Tuffnell D, Bhopal R, Cameron N. Cohort profile: the Born in Bradford multi-ethnic family cohort study. Int J Epidemiol 2012;4:1-14.
- Lawlor DA, West J, Fairley L, Nelson SM, Bhopal RS, Tuffnell D, et al. Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother-offspring pairs: findings from a prospective pregnancy cohort. Diabetologia 2014;57:2492-500. http://dx.doi.org/10.1007/s00125-014-3386-6.
- Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Diagnostic thresholds for gestational diabetes and their impact on pregnancy outcomes: a systematic review. Diabet Med 2014;31:319-31. http://dx.doi.org/10.1111/dme.12357.
- Jenum AK, Mørkrid K, Sletner L, Vangen S, Vange S, Torper JL, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol 2012;166:317-24. http://dx.doi.org/10.1530/EJE-11-0866.
- Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab 2002;87:5575-80. http://dx.doi.org/10.1210/jc.2002-020434.
- West J, Lawlor DA, Fairley L, Bhopal R, Cameron N, McKinney PA, et al. UK-born Pakistani-origin infants are relatively more adipose than white British infants: findings from 8704 mother-offspring pairs in the Born-in-Bradford prospective birth cohort. J Epidemiol Community Health 2013;67:544-51. http://dx.doi.org/10.1136/jech-2012-201891.
- West J, Wright J, Fairley L, Sattar N, Whincup P, Lawlor DA. Do ethnic differences in cord blood leptin levels differ by birthweight category? Findings from the Born in Bradford cohort study. Int J Epidemiol 2014;43:249-54. http://dx.doi.org/10.1093/ije/dyt225.
- Yajnik CS, Joglekar CV, Lubree HG, Rege SS, Naik SS, Bhat DS, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia 2008;51:39-46. http://dx.doi.org/10.1007/s00125-007-0847-1.
- Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord 2003;27:173-80. http://dx.doi.org/10.1038/sj.ijo.802219.
- Krishnaveni GV, Hill JC, Veena SR, Leary SD, Saperia J, Chachyamma KJ, et al. Truncal adiposity is present at birth and in early childhood in South Indian children. Indian Pediatr 2005;42:527-38.
- Bansal N, Ayoola OO, Gemmell I, Vyas A, Koudsi A, Oldroyd J, et al. Effects of early growth on blood pressure of infants of British European and South Asian origin at one year of age: the Manchester children’s growth and vascular health study. J Hypertens 2008;26:412-18. http://dx.doi.org/10.1097/HJH.0b013e3282f3168e.
- Caesarean Section. London: NICE; 2011.
- Royal College of Paediatrics and Child Health (RCPCH) . UK-WHO Growth Charts 2014. www.rcpch.ac.uk/improving-child-health/public-health/uk-who-growth-charts/uk-who-growth-charts-0-18-years (accessed 3 September 2015).
- Wright CM, Williams AF, Elliman D, Bedford H, Birks E, Butler G, et al. Using the new UK-WHO growth charts. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1140.
- HAPO Study Cooperative Research Group . The hyperglycaemia and adverse outcome (HAPO) study: associations with neonatal anthropometrics. Diabetes 2009;58:453-9. http://dx.doi.org/10.2337/db08-1112.
- West J, Manchester B, Wright J, Lawlor DA, Waiblinger D. Reliability of routine clinical measurements of neonatal circumferences and research measurements of neonatal skinfold thicknesses: findings from the Born in Bradford study. Paediatr Perinat Epidemiol 2011;25:164-71. http://dx.doi.org/10.1111/j.1365-3016.2010.01181.x.
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56:645-6. http://dx.doi.org/10.1111/j.0006-341X.2000.00645.x.
- Ethnic Group Statistics: A Guide for the Collection and Classification of Ethnicity Data. London: ONS; 2013.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Pettitt DJ, McKenna S, McLaughlin C, Patterson CC, Hadden DR, McCance DR. Maternal glucose at 28 weeks of gestation is not associated with obesity in 2-year-old offspring: the Belfast Hyperglycemia and Adverse Pregnancy Outcome (HAPO) family study. Diabetes Care 2010;33:1219-23. http://dx.doi.org/10.2337/dc09-2384.
- Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG 2012;119:276-82. http://dx.doi.org/10.1111/j.1471-0528.2011.03156.x.
- Mukerji G, Chiu M, Shah BR. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia 2012;55:2148-53. http://dx.doi.org/10.1007/s00125-012-2549-6.
- Dornhorst A, Paterson CM, Nicholls JS, Wadsworth J, Chiu DC, Elkeles RS, et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med 1992;9:820-5. http://dx.doi.org/10.1111/j.1464-5491.1992.tb01900.x.
- Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 2014;103:176-85. http://dx.doi.org/10.1016/j.diabres.2013.11.003.
- Holt RI, Coleman MA, McCance DR. The implications of the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria for gestational diabetes. Diabet Med 2011;28:382-5. http://dx.doi.org/10.1111/j.1464-5491.2011.03236.x.
- Moses RG. New consensus criteria for GDM: problem solved or a Pandora’s box?. Diabetes Care 2010;33:690-1. http://dx.doi.org/10.2337/dc09-2306.
- Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition: an old hypothesis with new importance?. Int J Epidemiol 2013;42:7-29. http://dx.doi.org/10.1093/ije/dys209.
- Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity – a determinant of perinatal and offspring outcomes?. Nat Rev Endocrinol 2012;8:679-88. http://dx.doi.org/10.1038/nrendo.2012.176.
- Donovan LE, Cundy T. Does exposure to hyperglycaemia in utero increase the risk of obesity and diabetes in the offspring? A critical reappraisal. Diabet Med 2015;32:295-304. http://dx.doi.org/10.1111/dme.12625.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-86. http://dx.doi.org/10.1056/NEJMoa042973.
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339-48. http://dx.doi.org/10.1056/NEJMoa0902430.
- Meek CL, Lewis HB, Patient C, Murphy HR, Simmons D. Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia 2015;58:2003-12. http://dx.doi.org/10.1007/s00125-015-3647-z.
- Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 2016;13.
- Van Overveld L. Risk of Adverse Outcomes and the Association With Increases in Maternal Glucose Levels 2013.
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) . PRISMA Statement n.d. www.prisma-statement.org (accessed June 2015).
- Critical Appraisal Skills Programme (CASP). 2014 . CASP Checklists n.d. www.casp-uk.net/#!casp-tools-checklists/c18f8 (accessed July 2014).
- Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280-6. http://dx.doi.org/10.7326/0003-4819-158-4-201302190-00009.
- Dunne F. Atlantic Diabetes in Pregnancy cohort. Personal Communication 2014.
- Saravanan P. Warwick/Coventry individual participant data. Personal Communication 2013.
- Landon MB, Mele L, Spong CY, Carpenter MW, Ramin SM, Casey B, et al. The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol 2011;117:218-24. http://dx.doi.org/10.1097/AOG.0b013e318203ebe0.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Figueroa D, Landon MB, Mele L, Spong CY, Ramin SM, Casey B, et al. Relationship between 1-hour glucose challenge test results and perinatal outcomes. Obstet Gynecol 2013;121:1241-7. http://dx.doi.org/10.1097/AOG.0b013e31829277f5.
- Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, et al. Effect of maternal glycemia on neonatal adiposity in a multiethnic Asian birth cohort. J Clin Endocrinol Metab 2014;99:240-7. http://dx.doi.org/10.1210/jc.2013-2738.
- Carr DB, Newton KM, Utzschneider KM, Faulenbach MV, Kahn SE, Easterling TR, et al. Gestational diabetes or lesser degrees of glucose intolerance and risk of preeclampsia. Hypertens Pregnancy 2011;30:153-63. http://dx.doi.org/10.3109/10641950903115012.
- Chandna A, Zuberi LM, Munim S. Threshold values for the glucose challenge test in pregnancy. Int J Gynaecol Obstet 2006;94:119-20. http://dx.doi.org/10.1016/j.ijgo.2006.04.010.
- Cheng YW, McLaughlin GB, Esakoff TF, Block-Kurbisch I, Caughey AB. Glucose challenge test: screening threshold for gestational diabetes mellitus and associated outcomes. J Matern Fetal Neonatal Med 2007;20:903-8. http://dx.doi.org/10.1080/14767050701739384.
- Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 2010;202:255.e1-7. http://dx.doi.org/10.1016/j.ajog.2010.01.024.
- Hillier TA, Pedula KL, Vesco KK, Schmidt MM, Mullen JA, LeBlanc ES, et al. Excess gestational weight gain: modifying fetal macrosomia risk associated with maternal glucose. Obstet Gynecol 2008;112:1007-14. http://dx.doi.org/10.1097/AOG.0b013e31818a9779.
- Jensen DM, Damm P, Sørensen B, Mølsted-Pedersen L, Westergaard JG, Klebe J, et al. Clinical impact of mild carbohydrate intolerance in pregnancy: a study of 2904 nondiabetic Danish women with risk factors for gestational diabetes mellitus. Am J Obstet Gynecol 2001;185:413-19. http://dx.doi.org/10.1067/mob.2001.115864.
- Kerényi Z, Tamás G, Kivimäki M, Péterfalvi A, Madarász E, Bosnyák Z, et al. Maternal glycemia and risk of large-for-gestational-age babies in a population-based screening. Diabetes Care 2009;32:2200-5. http://dx.doi.org/10.2337/dc09-1088.
- Lao TT, Ho LF. Does maternal glucose intolerance affect the length of gestation in singleton pregnancies?. J Soc Gynecol Investig 2003;10:366-71. http://dx.doi.org/10.1016/S1071-5576(03)00115-1.
- Little RR, McKenzie EM, Shyken JM, Winkelmann SE, Ramsey LM, Madsen RW, et al. Lack of relationship between glucose tolerance and complications of pregnancy in nondiabetic women. Diabetes Care 1990;13:483-7. http://dx.doi.org/10.2337/diacare.13.5.483.
- Lurie S, Levy R, Weiss R, Boultin G, Hagay ZJ. Low values on 50 gram glucose challenge test or oral 100 gram glucose tolerance test are associated with good perinatal outcome. J Obstet Gynaecol 1998;18:451-4. http://dx.doi.org/10.1080/01443619866778.
- Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics 2010;126:e1545-52. http://dx.doi.org/10.1542/peds.2009-2257.
- Moses RG, Calvert D. Pregnancy outcomes in women without gestational diabetes mellitus related to the maternal glucose level. Is there a continuum of risk?. Diabetes Care 1995;18:1527-33. http://dx.doi.org/10.2337/diacare.18.12.1527.
- Ong KK, Diderholm B, Salzano G, Wingate D, Hughes IA, MacDougall J, et al. Pregnancy insulin, glucose, and BMI contribute to birth outcomes in nondiabetic mothers. Diabetes Care 2008;31:2193-7. http://dx.doi.org/10.2337/dc08-1111.
- Pettitt DJ, Knowler WC, Baird HR, Bennett PH. Gestational diabetes: infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in the Pima Indians. Diabetes Care 1980;3:458-64. http://dx.doi.org/10.2337/diacare.3.3.458.
- Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 2009;32:1639-43. http://dx.doi.org/10.2337/dc09-0688.
- Savona-Ventura C, Craus J, Vella K, Grima S. Lowest threshold values for the 75g oral glucose tolerance test in pregnancy. Malta Med J 2010;22:18-20.
- Scholl TO, Sowers M, Chen X, Lenders C. Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol 2001;154:514-20. http://dx.doi.org/10.1093/aje/154.6.514.
- Sermer M, Naylor CD, Gare DJ, . Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes: the Toronto tri-hospital gestational diabetes project. Am J Obstet Gynecol 1995;173:146-56. http://dx.doi.org/10.1016/0002-9378(95)90183-3.
- Subramaniam A, Jauk V, Tita A, Harper L. Interaction between maternal obesity and 1-hour glucose challenge test results on maternal and perinatal outcomes. Am J Obs Gynecol 2014;210. http://dx.doi.org/10.1016/j.ajog.2013.10.279.
- Tallarigo L, Giampietro O, Penno G, Miccoli R, Gregori G, Navalesi R. Relation of glucose tolerance to complications of pregnancy in nondiabetic women. N Engl J Med 1986;315:989-92. http://dx.doi.org/10.1056/NEJM198610163151603.
- Witter FR, Niebyl JR. Abnormal glucose screening in pregnancy in patients with normal oral glucose tolerance tests as a screening test for fetal macrosomia. Int J Gynaecol Obstet 1988;27:181-4. http://dx.doi.org/10.1016/0020-7292(88)90005-7.
- Yee LM, Cheng YW, Liddell J, Block-Kurbisch I, Caughey AB. 50-Gram glucose challenge test: is it indicative of outcomes in women without gestational diabetes mellitus?. J Matern Fetal Neonatal Med 2011;24:1102-6. http://dx.doi.org/10.3109/14767058.2010.546450.
- Aberg A, Rydhstroem H, Frid A. Impaired glucose tolerance associated with adverse pregnancy outcome: a population-based study in southern Sweden. Am J Obstet Gynecol 2001;184:77-83. http://dx.doi.org/10.1067/mob.2001.108085.
- Dudhbhai M, Lim L, Bombard A, Juliard K, Meenakshi B, Trachelenberg Y, et al. Characteristics of patients with abnormal glucose challenge test and normal oral glucose tolerance test results: comparison with normal and gestational diabetic patients. Am J Obstet Gynecol 2006;194:e42-5. http://dx.doi.org/10.1016/j.ajog.2005.11.031.
- Forest JC, Massé J, Garrido-Russo M. Glucose tolerance test during pregnancy: the significance of one abnormal value. Clin Biochem 1994;27:299-304. http://dx.doi.org/10.1016/0009-9120(94)00029-8.
- Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-6. http://dx.doi.org/10.1097/00006250-200310000-00030.
- Herman G, Raimondi B. Glucose tolerance, fetal growth, and pregnancy complications in normal women. Am J Perinatol 1988;5:168-71. http://dx.doi.org/10.1055/s-2007-999679.
- Jiménez-Moleón JJ, Bueno-Cavanillas A, Luna-Del-Castillo JD, Garciá-Martín M, Lardelli-Claret P, Gálvez-Vargas R. Prevalence of gestational diabetes mellitus: variations related to screening strategy used. Eur J Endocrinol 2002;146:831-7. http://dx.doi.org/10.1530/eje.0.1460831.
- Khoshniat Nikoo M, Garshasbi A, Amini S, Pasandi F, Peimani M, Larijani B. Relationship between maternal glucose intolerance and fasting plasma glucose with macrosomia during pregnancy. Iran J Diabet Lipid Disorders 2010;9:1-7.
- Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005;192:989-97. http://dx.doi.org/10.1016/j.ajog.2004.11.039.
- Lapolla A, Dalfrà MG, Bonomo M, Castiglioni MT, Di Cianni G, Masin M, et al. Can plasma glucose and HbA1c predict fetal growth in mothers with different glucose tolerance levels?. Diabetes Res Clin Pract 2007;77:465-70. http://dx.doi.org/10.1016/j.diabres.2007.01.022.
- Ma KK, Mele L, Landon MB, Spong CY, Ramin SM, Casey B, et al. The obstetric and neonatal implications of a low value on the 50-g glucose screening test. Am J Perinatol 2013;30:715-22. http://dx.doi.org/10.1055/s-0032-1331027.
- Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA 1996;275:1165-70. http://dx.doi.org/10.1001/jama.1996.03530390031030.
- Nord E, Hanson U, Persson B. Blood glucose limits in the diagnosis of impaired glucose tolerance during pregnancy. Relation to morbidity. Acta Obstet Gynecol Scand 1995;74:589-93. http://dx.doi.org/10.3109/00016349509013467.
- Özekinci M, Mendilcioğlu İ, İnel M, Simşek M, Gülkesen KH. Abnormal one-hour 50-gram glucose challenge test and perinatal outcomes. Turkiye Klinikleri J Med Sci 2011;31:1211-17. http://dx.doi.org/10.5336/medsci.2010-21971.
- Pugh SK, Poole AT, Hill JB, Magann EF, Chauhan SP, Morrison JC. Abnormal 1 hour glucose challenge test followed by a normal 3 hour glucose tolerance test: does it identify adverse pregnancy outcome?. J Miss State Med Assoc 2010;51:3-6.
- Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care 2008;31:1275-81. http://dx.doi.org/10.2337/dc08-0126.
- Stamilio DM, Olsen T, Ratcliffe S, Sehdev HM, Macones GA. False-positive 1-hour glucose challenge test and adverse perinatal outcomes. Obstet Gynecol 2004;103:148-56. http://dx.doi.org/10.1097/01.AOG.0000109220.24211.BD.
- Tarim E, Cok T. Macrosomia prediction using different maternal and fetal parameters in women with 50 g glucose challenge test between 130 and 140 mg/dl. Arch Gynecol Obstet 2011;284:1081-5. http://dx.doi.org/10.1007/s00404-010-1797-2.
- Vambergue A, Nuttens MC, Verier-Mine O, Dognin C, Cappoen JP, Fontaine P. Is mild gestational hyperglycaemia associated with maternal and neonatal complications? The Diagest Study. Diabet Med 2000;17:203-8. http://dx.doi.org/10.1046/j.1464-5491.2000.00237.x.
- Wang P, Lu MC, Yan YH. Abnormal glucose tolerance is associated with preterm labor and increased neonatal complications in Taiwanese women. Taiwan J Obstet Gynecol 2013;52:479-84. http://dx.doi.org/10.1016/j.tjog.2013.10.005.
- Yogev Y, Langer O, Xenakis EM, Rosenn B. The association between glucose challenge test, obesity and pregnancy outcome in 6390 non-diabetic women. J Matern Fetal Neonatal Med 2005;17:29-34. http://dx.doi.org/10.1080/14767050400028766.
- Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287-92. http://dx.doi.org/10.2337/dc06-2361.
- Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes 1991;40:126-30. http://dx.doi.org/10.2337/diab.40.2.S126.
- Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 2010;33:2524-30. http://dx.doi.org/10.2337/dc10-1445.
- Franks PW, Looker HC, Kobes S, Touger L, Tataranni PA, Hanson RL, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 2006;55:460-5. http://dx.doi.org/10.2337/diabetes.55.02.06.db05-0823.
- Jensen DM, Korsholm L, Ovesen P, Beck-Nielsen H, Mølsted-Pedersen L, Damm P. Adverse pregnancy outcome in women with mild glucose intolerance: is there a clinically meaningful threshold value for glucose?. Acta Obstet Gynecol Scand 2008;87:59-62. http://dx.doi.org/10.1080/00016340701823975.
- Kim HS, Chang KH, Yang JI, Yang SC, Lee HJ, Ryu HS. Clinical outcomes of pregnancy with one elevated glucose tolerance test value. Int J Gynaecol Obstet 2002;78:131-8. http://dx.doi.org/10.1016/S0020-7292(02)00129-7.
- Khan KS, Syed AH, Hashmi FA, Rizvi JH. Relationship of fetal macrosomia to a 75g glucose challenge test in nondiabetic pregnant women. Aust N Z J Obstet Gynaecol 1994;34:24-7. http://dx.doi.org/10.1111/j.1479-828X.1994.tb01033.x.
- Atia HC, Koren Y, Weintraub AY, Novack L, Sheiner E. Is a value of over 200 mg/dL in the oral glucose tolerance test, a marker of severity in patients with gestational diabetes mellitus?. J Matern Fetal Neonatal Med 2013;26:1259-62. http://dx.doi.org/10.3109/14767058.2013.777421.
- Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 2013;381:476-83. http://dx.doi.org/10.1016/S0140-673612)61605-5.
- Retnakaran R, Hanley AJ, Connelly PW, Sermer M, Zinman B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: a comparison of Asian, South Asian, and Caucasian women. J Clin Endocrinol Metab 2006;91:93-7. http://dx.doi.org/10.1210/jc.2005-1253.
- Koukkou E, Taub N, Jackson P, Metcalfe G, Cameron M, Lowy C. Difference in prevalence of gestational diabetes and perinatal outcome in an innercity multiethnic London population. Eur J Obstet Gynecol Reprod Biol 1995;59:153-7. http://dx.doi.org/10.1016/0028-2243(95)02043-R.
- Bryant M, Santorelli G, Lawlor DA, Farrar D, Tuffnell D, Bhopal R, et al. A comparison of South Asian specific and established BMI thresholds for determining obesity prevalence in pregnancy and predicting pregnancy complications: findings from the Born in Bradford cohort. Int J Obes 2014;38:444-50. http://dx.doi.org/10.1038/ijo.2013.117.
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070-6. http://dx.doi.org/10.2337/dc06-2559a.
- Cundy T. Proposed new diagnostic criteria for gestational diabetes: a pause for thought?. Diabet Med 2012;29:176-80.
- Avalos GE, Owens LA, Dunne F. ATLANTIC DIP Collaborators . Applying current screening tools for gestational diabetes mellitus to a European population: is it time for change?. Diabetes Care 2013;36:3040-4. http://dx.doi.org/10.2337/dc12-2669.
- Strong M, Oakley J, Brennan A. Estimating multi-parameter partial Expected Value of Perfect Information from a probabilistic sensitivity analysis sample: a non-parametric regression approach. Med Decis Making 2014;34:311-26. http://dx.doi.org/10.1177/0272989X13505910.
- Farrar D, Fairley L, Wright J, Tuffnell D, Whitelaw D, Lawlor DA. Evaluation of the impact of universal testing for gestational diabetes mellitus on maternal and neonatal health outcomes: a retrospective analysis. BMC Pregnancy Childbirth 2014;14. http://dx.doi.org/10.1186/1471-2393-14-317.
- Di Cianni G, Miccoli R, Volpe L, Lencioni C, Del Prato S. Intermediate metabolism in normal pregnancy and in gestational diabetes. Diabetes Metab Res Rev 2003;19:259-70. http://dx.doi.org/10.1002/dmrr.390.
- Ali FM, Farah N, O’Dwyer V, O’Connor C, Kennelly MM, Turner MJ. The impact of new national guidelines on screening for gestational diabetes mellitus. Ir Med J 2013;106:57-9.
- Gregory R, Swinn RA, Wareham N, Curling V, Dalton KJ, Edwards OM, et al. An audit of a comprehensive screening programme for diabetes in pregnancy. Pract Diabetes Int 1998;15:45-8. http://dx.doi.org/10.1002/pdi.1960150208.
- Janghorbani M, Stenhouse E, Jones RB, Millward A. Gestational diabetes mellitus in Plymouth, U.K.: prevalence, seasonal variation and associated factors. J Reprod Med 2006;51:128-34.
- Khalifeh A, Breathnach F, Coulter-Smith S, Robson M, Fitzpatrick C, Malone F. Changing trends in diabetes mellitus in pregnancy. J Obstet Gynaecol 2014;34:135-7. http://dx.doi.org/10.3109/01443615.2013.830596.
- O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne FP. Atlantic DIP collaborators . Atlantic DIP: the prevalence and consequences of gestational diabetes in Ireland. Ir Med J 2012;105:13-5.
- Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526-8. http://dx.doi.org/10.2337/dc11-1641.
- Samanta A, Burden ML, Burden AC, Jones GR. Glucose tolerance during pregnancy in Asian women. Diabetes Res Clin Pract 1989;7:127-35. http://dx.doi.org/10.1016/0168-8227(89)90103-4.
- Griffin ME, Coffey M, Johnson H, Scanlon P, Foley M, Stronge J, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000;17:26-32. http://dx.doi.org/10.1046/j.1464-5491.2000.00214.x.
- Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Hum Reprod Update 2000;15:2433-7. http://dx.doi.org/10.1093/humrep/15.11.2433.
- O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 2011;54:1670-5. http://dx.doi.org/10.1007/s00125-011-2150-4.
- American Diabetes Association (ADA) . Standards of medical care in diabetes – 2014. Diabetes Care 2014;37:14-80. http://dx.doi.org/10.2337/dc14-s014.
- Gillespie P, O’Neill C, Avalos G, O’Reilly M, Dunne F. ATLANTIC DIP Collaborators . The cost of universal screening for gestational diabetes mellitus in Ireland. Diabet Med 2011;28:912-18. http://dx.doi.org/10.1111/j.1464-5491.2011.03293.x.
- Simmonds MC, Wald NJ. Risk estimation versus screening performance: a comparison of six risk algorithms for cardiovascular disease. J Med Screen 2012;19:201-5. http://dx.doi.org/10.1258/jms.2012.012076.
- Yang X, Hsu-Hage B, Yu L, Simmons D. Selective screening for gestational diabetes in Chinese women. Diabetes Care 2002;25. http://dx.doi.org/10.2337/diacare.25.4.796.
- Van Leeuwen M, Opmeer BC, Zweers EJ, van Ballegooie E, ter Brugge HG, de Valk HW, et al. External validation of a clinical scoring system for the risk of gestational diabetes mellitus. Diabetes Res Clin Pract 2009;85:96-101. http://dx.doi.org/10.1016/j.diabres.2009.04.025.
- Van Leeuwen M, Opmeer BC, Zweers EJ, van Ballegooie E, ter Brugge HG, de Valk HW, et al. Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG 2010;117:69-75. http://dx.doi.org/10.1111/j.1471-0528.2009.02425.x.
- Caliskan E, Kayikcioglu F, Oztürk N, Koc S, Haberal A. A population-based risk factor scoring will decrease unnecessary testing for the diagnosis of gestational diabetes mellitus. Acta Obstet Gynecol Scand 2004;83:524-30. http://dx.doi.org/10.1111/j.0001-6349.2004.00389.x.
- Cosson E, Benbara A, Pharisien I, Nguyen MT, Revaux A, Lormeau B, et al. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 2013;36:598-603. http://dx.doi.org/10.2337/dc12-1428.
- Cypryk K, Szymczak W, Czupryniak L, Sobczak M, Lewiński A. Gestational diabetes mellitus: an analysis of risk factors. Endokrynol Pol 2008;59:393-7.
- Jensen DM, Mølsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Ovesen P, Damm P. Screening for gestational diabetes mellitus by a model based on risk indicators: a prospective study. Am J Obstet Gynecol 2003;189:1383-8. http://dx.doi.org/10.1067/S0002-9378(03)00601-X.
- Danilenko-Dixon DR, Van Winter JT, Nelson RL, Ogburn PL. Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol 1999;181:798-802. http://dx.doi.org/10.1016/S0002-9378(99)70304-2.
- Marquette GP, Klein VR, Niebyl JR. Efficacy of screening for gestational diabetes. Am J Perinatol 1985;2:7-9. http://dx.doi.org/10.1055/s-2007-999901.
- Moses RG, Moses J, Davis WS. Gestational diabetes: do lean young Caucasian women need to be tested?. Diabetes Care 1998;21:1803-6. http://dx.doi.org/10.2337/diacare.21.11.1803.
- Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn 2011;31:135-41. http://dx.doi.org/10.1002/pd.2636.
- Ostlund I, Hanson U. Occurrence of gestational diabetes mellitus and the value of different screening indicators for the oral glucose tolerance test. Acta Obstet Gynecol Scand 2003;82:103-8. http://dx.doi.org/10.1080/j.1600-0412.2003.00001.x.
- Phaloprakarn C, Tangjitgamol S, Manusirivithaya S. A risk score for selective screening for gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2009;145:71-5. http://dx.doi.org/10.1016/j.ejogrb.2009.04.016.
- Pintaudi B, Di Vieste G, Corrado F, Lucisano G, Pellegrini F, Giunta L, et al. Improvement of selective screening strategy for gestational diabetes through a more accurate definition of high-risk groups. Eur J Endocrinol 2014;170:87-93. http://dx.doi.org/10.1530/EJE-13-0759.
- Sacks DA, Abu-Fadil S, Karten GJ, Forsythe AB, Hackett JR. Screening for gestational diabetes with the one-hour 50-g glucose test. Obstet Gynecol 1987;70:89-93.
- Shamsuddin K, Mahdy ZA, Siti Rafiaah I, Jamil MA, Rahimah MD. Risk factor screening for abnormal glucose tolerance in pregnancy. Int J Gynaecol Obstet 2001;75:27-32. http://dx.doi.org/10.1016/S0020-7292(01)00468-4.
- Shirazian N, Emdadi R, Mahboubi M, Motevallian A, Fazel-Sarjuei Z, Sedighpour N, et al. Screening for gestational diabetes: usefulness of clinical risk factors. Arch Gynecol Obstet 2009;280:933-7. http://dx.doi.org/10.1007/s00404-009-1027-y.
- Sunsaneevithayakul P, Boriboohirunsarn D, Sutanthavibul A, Ruangvutilert P, Kanokpongsakdi S, Singkiratana D, et al. Risk factor-based selective screening program for gestational diabetes mellitus in Siriraj Hospital: result from clinical practice guideline. J Med Assoc Thai 2003;86:708-14.
- Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51:26-30. http://dx.doi.org/10.1111/j.1479-828X.2011.01292.x.
- Williams CB, Iqbal S, Zawacki CM, Yu D, Brown MB, Herman WH. Effect of selective screening for gestational diabetes. Diabetes Care 1999;22:418-21. http://dx.doi.org/10.2337/diacare.22.3.418.
- Corcoy R, García-Patterson A, Pau E, Pascual E, Altirriba O, Adelantado JM, et al. Is selective screening for gestational diabetes mellitus worthwhile everywhere?. Acta Diabetol 2004;41:154-7. http://dx.doi.org/10.1007/s00592-004-0159-6.
- Crete JE, Anasti JN. Diagnosis of gestational diabetes mellitus: can we avoid the glucose challenge test?. J Am Assoc Nurse Pract 2013;25:329-33. http://dx.doi.org/10.1111/j.1745-7599.2012.00792.x.
- Davey RX, Hamblin PS. Selective versus universal screening for gestational diabetes mellitus: an evaluation of predictive risk factors. Med J Aust 2001;174:118-21.
- Göbl CS, Bozkurt L, Rivic P, Schernthaner G, Weitgasser R, Pacini G, et al. A two-step screening algorithm including fasting plasma glucose measurement and a risk estimation model is an accurate strategy for detecting gestational diabetes mellitus. Diabetologia 2012;55:3173-81. http://dx.doi.org/10.1007/s00125-012-2726-7.
- Cosson E, Benchimol M, Carbillon L, Pharisien I, Pariès J, Valensi P, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabet Metab 2006;32:140-6. http://dx.doi.org/10.1016/S1262-3636(07)70260-4.
- Naylor CD, Sermer M, Chen E, Farine D. Selective screening for gestational diabetes mellitus. Toronto Trihospital Gestational Diabetes Project Investigators. N Engl J Med 1997;337:1591-6. http://dx.doi.org/10.1056/NEJM199711273372204.
- Savona-Ventura C, Vassallo J, Marre M, Karamanos BG. MGSD-GDM study group . A composite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int J Gynaecol Obstet 2013;120:240-4. http://dx.doi.org/10.1016/j.ijgo.2012.10.016.
- Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768-73. http://dx.doi.org/10.1016/0002-9378(82)90349-0.
- Seghieri G, De Bellis A, Anichini R, Alviggi L, Franconi F, Breschi MC. Does parity increase insulin resistance during pregnancy?. Diabet Med 2005;22:1574-80. http://dx.doi.org/10.1111/j.1464-5491.2005.01693.x.
- Alwan N, Tuffnell D, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.cd003395.pub2.
- Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, et al. Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1395.
- Falavigna M, Prestes I, Schmidt MI, Duncan BB, Colagiuri S, Roglic G. Impact of gestational diabetes mellitus screening strategies on perinatal outcomes: a simulation study. Diabetes Res Clin Pract 2013;99:358-65. http://dx.doi.org/10.1016/j.diabres.2012.12.009.
- Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0064585.
- The Cochrane Collaboration . The Cochrane Collaboration’s Tool for Assessing Risk of Bias n.d. http://ohgcochrane.org/sites/ohgcochraneorg/files/uploads/Risk%20of%20bias%20assessment%20tool.pdf (accessed January 2014).
- Bafeta A, Trinquart L, Seror R, Ravaud P. Reporting of results from network meta-analyses: methodological systematic review. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1741.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Hassan JA, Karim N, Sheikh Z. Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci 2012;28:384-9.
- Hague WM, Davoren PM, Oliver J, Rowan J. Contraindications to use of metformin. Metformin may be useful in gestational diabetes. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7392.762/a.
- Ijäs H, Vääräsmäki M, Morin-Papunen L, Keravuo R, Ebeling T, Saarela T, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 2011;118:880-5. http://dx.doi.org/10.1111/j.1471-0528.2010.02763.x.
- Mesdaghinia E, Samimi M, Homaei Z, Saberi F, Moosavi SG, Yaribakht M. Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med 2013;4:327-33.
- Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med 2007;52:1011-15.
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. MiG Trial Investigators . Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358:2003-15. http://dx.doi.org/10.1056/NEJMoa0707193.
- Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol 2013;209:34.e1-7. http://dx.doi.org/10.1016/j.ajog.2013.03.022.
- Tertti K, Ekblad U, Koskinen P, Vahlberg T, Ronnemaa T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab 2013;15:246-51. http://dx.doi.org/10.1111/dom.12017.
- Zinnat ANS, Shareen S, Rahman S. Can metformin be used in place of insulin for the treatment of GDM for low resource countries?. BJOG 2013;120.
- Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract 2007;76:474-5. http://dx.doi.org/10.1016/j.diabres.2006.09.031.
- Bertini AM, Silva JC, Taborda W, Becker F, Lemos Bebber FR, Zucco Viesi JM, et al. Perinatal outcomes and the use of oral hypoglycemic agents. J Perinat Med 2005;33:519-23. http://dx.doi.org/10.1515/JPM.2005.092.
- Lain KY, Garabedian MJ, Daftary A, Jeyabalan A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared with insulin. Am J Obstet Gynecol 2009;200:501.e1-6. http://dx.doi.org/10.1016/j.ajog.2009.02.038.
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000;343:1134-8. http://dx.doi.org/10.1056/NEJM200010193431601.
- Mukhopadhyay P, Bag TS, Kyal A, Saha DP, Khalid N. Oral hypoglycemic glibenclamide: can it be a substitute to insulin in the management of gestational diabetes mellitus? A comparative study. J South Asian Feder Obstet Gynae 2012;4:28-31. http://dx.doi.org/10.5005/jp-journals-10006-1167.
- Ogunyemi D, Jesse M, Davidson M. Comparison of glyburide versus insulin in management of gestational diabetes mellitus. Endocr Pract 2007;13:427-8. http://dx.doi.org/10.4158/EP.13.4.427.
- Silva JC, Bertini AM, Taborda W, Becker F, Bebber FR, Aquim GM, et al. Glibenclamide in the treatment for gestational diabetes mellitus in a compared study to insulin. Arq Bras Endocrinol Metabol 2007;51:541-6. http://dx.doi.org/10.1590/S0004-27302007000400007.
- Tempe A, Mayanglambam RD. Glyburide as treatment option for gestational diabetes mellitus. J Obstet Gynaecol Res 2013;39:1147-52. http://dx.doi.org/10.1111/jog.12042.
- Moore LE, Clokey D, Rappaport VJ, Curet LB. Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstet Gynecol 2010;115:55-9. http://dx.doi.org/10.1097/AOG.0b013e3181c52132.
- Silva JC, Fachin DR, Coral ML, Bertini AM. Perinatal impact of the use of metformin and glyburide for the treatment of gestational diabetes mellitus. J Perinat Med 2012;40:225-8. http://dx.doi.org/10.1515/jpm-2011-0175.
- Abbassi-Ghanavati M, Casey B, Shivvers S, Tudela C, McIntire D, Leveno K. Randomized trial of glyburide plus diet compared to placebo plus diet in women with gestational diabetes. Am J Obs Gynecol 2014;210.
- Balaji V, Balaji MS, Alexander C, Srinivasan A, Suganthi SR, Thiyagarajah A, et al. Premixed insulin aspart 30 (BIAsp 30) versus premixed human insulin 30 (BHI 30) in gestational diabetes mellitus: a randomized open-label controlled study. Gynecol Endocrinol 2012;28:529-32. http://dx.doi.org/10.3109/09513590.2011.650661.
- Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract 2012;98:422-9. http://dx.doi.org/10.1016/j.diabres.2012.09.031.
- Deveer R, Deveer M, Akbaba E, Engin-Üstün Y, Aydoğan P, Celikkaya H, et al. The effect of diet on pregnancy outcomes among pregnant with abnormal glucose challenge test. Eur Rev Med Pharmacol Sci 2013;17:1258-61.
- Li DF, Wong VC, O’Hoy KM, Yeung CY, Ma HK. Is treatment needed for mild impairment of glucose tolerance in pregnancy? A randomized controlled trial. Br J Obstet Gynaecol 1987;94:851-4. http://dx.doi.org/10.1111/j.1471-0528.1987.tb03753.x.
- Yang X, Hsu-Hage B, Dong L, Shao P, Wang H, Tian H, et al. Intensive diabetes management may improve pregnancy: outcomes in Chinese gravidas with impaired glucose tolerance. Diabetes Care 2003;26:254-5. http://dx.doi.org/10.2337/diacare.26.1.254.
- Elnour AA, Mugammar IT, Jaber T, Revel T, McElnay JC. Pharmaceutical care of patients with gestational diabetes mellitus. J Eval Clin Prac 2008;14:131-40. http://dx.doi.org/10.1111/j.1365-2753.2007.00819.x.
- Garner P, Okun N, Keely E, Wells G, Perkins S, Sylvain J, et al. A randomized controlled trial of strict glycemic control and tertiary level obstetric care versus routine obstetric care in the management of gestational diabetes: a pilot study. Am J Obstet Gynecol 1997;177:190-5. http://dx.doi.org/10.1016/S0002-9378(97)70461-7.
- Bevier WC, Fischer R, Jovanovic L. Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. Am J Perinatol 1999;16:269-75. http://dx.doi.org/10.1055/s-2007-993871.
- Bonomo M, Corica D, Mion E, Gonçalves D, Motta G, Merati R, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med 2005;22:1536-41. http://dx.doi.org/10.1111/j.1464-5491.2005.01690.x.
- Bung P, Artal R, Khodiguian N, Kjos S. Exercise in gestational diabetes. An optional therapeutic approach?. Diabetes 1991;40:182-5. http://dx.doi.org/10.2337/diab.40.2.S182.
- Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001;24:1904-10. http://dx.doi.org/10.2337/diacare.24.11.1904.
- Moreno-Castilla C, Hernandez M, Bergua M, Alvarez MC, Arce MA, Rodriguez K, et al. Low-carbohydrate diet for the treatment of gestational diabetes mellitus: a randomized controlled trial. Diabetes Care 2013;36:2233-8. http://dx.doi.org/10.2337/dc12-2714.
- Nachum Z, Ben-Shlomo I, Weiner E, Shalev E. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy: randomised controlled trial. BMJ 1999;319:1223-7. http://dx.doi.org/10.1136/bmj.319.7219.1223.
- Thompson DJ, Porter KB, Gunnells DJ, Wagner PC, Spinnato JA. Prophylactic insulin in the management of gestational diabetes. Obstet Gynecol 1990;75:960-4.
- Cypryk K, Kamińska P, Kosiński M, Pertyńska-Marczewska M, Lewiński A. A comparison of the effectiveness, tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol 2007;58:314-19.
- Di Cianni G, Volpe L, Ghio A, Lencioni C, Cuccuru I, Benzi L, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes mellitus treated with lispro or aspart insulin: comparison with regular insulin. Diabetes Care 2007;30. http://dx.doi.org/10.2337/dc06-2586.
- O’Sullivan JB, Gellis SS, Dandrow RV, Tenney BO. The potential diabetic and her treatment in pregnancy. Obstet Gynecol 1966;27:683-9.
- Rae A, Bond D, Evans S, North F, Roberman B, Walters B. A randomised controlled trial of dietary energy restriction in the management of obese women with gestational diabetes. Aust N Z J Obstet Gynaecol 2000;40:416-22. http://dx.doi.org/10.1111/j.1479-828X.2000.tb01172.x.
- Silva JC, Pacheco C, Bizato J, de Souza BV, Ribeiro TE, Bertini AM. Metformin compared with glyburide for the management of gestational diabetes. Int J Gynaecol Obstet 2010;111:37-40. http://dx.doi.org/10.1016/j.ijgo.2010.04.028.
- Ardilouze J-L, Ménard J, Hivert M-F. Gestational diabetes mellitus: a randomized study comparing insulin therapy to a combination of half-maximal dosages of metformin and glyburide. Can J Diab 2014;38. http://dx.doi.org/10.1016/j.jcjd.2014.07.064.
- Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr 2014;68:490-5. http://dx.doi.org/10.1038/ejcn.2013.296.
- Bo S, Rosato R, Ciccone G, Canil S, Gambino R, Poala CB, et al. Simple lifestyle recommendations and the outcomes of gestational diabetes. A 2×2 factorial randomized trial. Diabetes Obes Metab 2014;16:1032-5. http://dx.doi.org/10.1111/dom.12289.
- Cao X, Wang Z, Yang C, Mo X, Xiu L, Li Y, et al. Comprehensive intensive therapy for Chinese gestational diabetes benefits both newborns and mothers. Diabetes Technol Ther 2012;14:1002-7. http://dx.doi.org/10.1089/dia.2012.0142.
- Jovanovic L, Ilic S, Pettitt DJ, Hugo K, Gutierrez M, Bowsher RR, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care 1999;22:1422-7. http://dx.doi.org/10.2337/diacare.22.9.1422.
- Louie JCY, Markovic TP, Perera N, Foote D, Petocz P, Ross GP, et al. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011;34:2341-6. http://dx.doi.org/10.2337/dc11-0985.
- Bahado-Singh RO, Mele L, Landon MB, Ramin SM, Carpenter MW, Casey B, et al. Fetal male gender and the benefits of treatment of mild gestational diabetes mellitus. Am J Obstet Gynecol 2012;206:422.e1-5. http://dx.doi.org/10.1016/j.ajog.2012.03.015.
- Hatem M, Anthony F, Hogston P, Rowe DJ, Dennis KJ. Reference values for 75g oral glucose tolerance test in pregnancy. Br Med J 1988;296:676-8. http://dx.doi.org/10.1136/bmj.296.6623.676.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. http://dx.doi.org/10.1002/SICI)1096-9136199807)15:7<539::AID-DIA668>3.0.CO;2-S.
- Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964-8. http://dx.doi.org/10.2337/dc09-1810.
- Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2088.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19140.
- Van Leeuwen M, Louwerse MD, Opmeer BC, Limpens J, Serlie MJ, Reitsma JB, et al. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. BJOG 2012;119:393-401. http://dx.doi.org/10.1111/j.1471-0528.2011.03254.x.
- Waugh NR, Shyangdan D, Taylor-Phillips S, Suri G, Hall B. Screening for type 2 diabetes: a short report for the National Screening Committee. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17350.
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862-8. http://dx.doi.org/10.2337/diacare.25.10.1862.
- Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011;123:258-65. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.980169.
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Round JA, Jacklin P, Fraser RB, Hughes RG, Mugglestone MA, Holt RI. Screening for gestational diabetes mellitus: cost-utility of different screening strategies based on a woman’s individual risk of disease. Diabetologia 2011;54:256-63. http://dx.doi.org/10.1007/s00125-010-1881-y.
- McClean S, Farrar D, Kelly CA, Tuffnell DJ, Whitelaw DC. The importance of postpartum glucose tolerance testing after pregnancies complicated by gestational diabetes. Diabet Med 2010;27:650-4. http://dx.doi.org/10.1111/j.1464-5491.2010.03001.x.
- Ward R, Lennard-Jones HE, Scott EM. How good is the postpartum follow-up of women with gestational diabetes: are NICE clinical recommendations being adhered to?. Diabetic Med 2015;32:177-8.
- Holt RI, Goddard JR, Clarke P, Coleman MA. A postnatal fasting plasma glucose is useful in determining which women with gestational diabetes should undergo a postnatal oral glucose tolerance test. Diabet Med 2003;20:594-8. http://dx.doi.org/10.1046/j.1464-5491.2003.00974.x.
- Ratner RE. Diabetes Prevention Program Research . An update on the Diabetes Prevention Program. Endocr Pract 2006;12:20-4. http://dx.doi.org/10.4158/EP.12.S1.20.
- Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774-9. http://dx.doi.org/10.1210/jc.2008-0772.
- Gillies CL, Lambert PC, Abrams KR, Sutton AJ, Cooper NJ, Hsu RT, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5. http://dx.doi.org/10.1136/bmj.39545.585289.25.
- Aroda VR, Christophi CA, Edelstein SL, Zhang P, Herman WH, Barrett-Conner E, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: The Diabetes Prevention Program Outcomes Study 10-Year Follow-Up. J Clin Endocrinol Metab 2015;100:1646-53. http://dx.doi.org/10.1210/jc.2014-3761.
- Diabetes in Pregnancy: Management of Diabetes and Its Complications from Pre-conception to the Postnatal Period. London: NICE; 2008.
- Kim C, Tabaei BP, Burke R, McEwen LN, Lash RW, Johnson SL, et al. Missed opportunities for type 2 diabetes mellitus screening among women with a history of gestational diabetes mellitus. Am J Public Health 2006;96:1643-8. http://dx.doi.org/10.2105/AJPH.2005.065722.
- Seshiah V, Balaji V, Balaji MS, Sekar A, Sanjeevi C, Green A. One step procedure for screening and diagnosis of gestational diabetes mellitus. Diabetes 2005;126.
- Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy. London: National Collaborating Centre for Women’s & Children’s Health (NCC-WCH); 2010.
- Werner EF, Pettker CM, Zuckerwise L, Reel M, Funai EF, Henderson J, et al. Screening for gestational diabetes mellitus: are the criteria proposed by the international association of the Diabetes and Pregnancy Study Groups cost-effective?. Diabetes Care 2012;35:529-35. http://dx.doi.org/10.2337/dc11-1643.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4. http://dx.doi.org/10.1177/0272989X11401031.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- NHS Reference Costs 2005–2006. London: DH; 2006.
- National Health Service England and Wales . Electronic Drug Tariff – July 2014 2014. www.ppa.org.uk/edt/July_2014/mindex.htm (accessed 19 November 2014).
- Diabetes Prevention Program Research Group . The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723-30. http://dx.doi.org/10.2337/dc11-1468.
- Briggs A, Mark Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making 1985;5:157-77. http://dx.doi.org/10.1177/0272989X8500500205.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Office for National Statistics (ONS) . Births in England and Wales, 2013–14. 2015. www.ons.gov.uk/ons/dcp171778_371129pdf (accessed 3 September 2015).
- Barry E, Roberts S, Finer S, Vijayaraghavan S, Greenhalgh T. Time to question the NHS diabetes prevention programme. BMJ 2015;351. http://dx.doi.org/10.1136/bmj.h4717.
- O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol 1973;116:895-900.
- Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1567.
- Balaji V, Balaji MS, Alexander C, Ashalata S, Sheela Suganthi R, Suresh S, et al. Premixed insulin aspart 30 (Biasp 30) vs. premixed human insulin 30 (BHI 30) in gestational diabetes mellitus – a pilot study. J Assoc Physicians India 2010;58:99-101.
- Department of Health (DH) . National Reference Costs 2012–13 2013. www.gov.uk/government/uploads/system/uploads/attachment_data/file/261154/nhs_reference_costs_2012-13_acc.pdf (accessed December 2014).
Appendix 1 Tables and figures for Chapter 2
| Variables | Level/unit | No. (%) with missing data | Complete case | Multiple imputationa |
|---|---|---|---|---|
| BW | Mean (SE) SD score | 1 | (–0.37 0.01) | (–0.37 0.01) |
| Sum of skinfolds | Mean (SE) | 3051 (32.1) | 9.82 (0.03) | 9.75 (0.02) |
| Pre-eclampsia | % | 389 (4.1) | 2.5 | 2.5 |
| Instrumental vaginal deliverya | % | 7 (0.1) | 12.4 | 12.4 |
| Maternal BMI | Mean (SE) | 436 (4.6) | 25.8 (0.06) | 25.9 (0.06) |
| Maternal education | % 5 + GCSE equivalent | 126 (1.3) | 31.5 | 31.5 |
| % higher than A-level equivalent | 25.6 | 25.6 | ||
| Smoking | % | 15 (0.2) | 17.0 | 17.0 |
| Alcohol | % | 36 (0.4) | 20.6 | 20.6 |
| Parity | % primiparious | 358 (3.8) | 41.7 | 41.4 |
| Family history of diabetes | % | 297 (3.1) | 25.1 | 25.1 |
| Family history of hypertension | % | 306 (3.2) | 27.4 | 27.4 |
| Previous macrosomia | % | 874 (16.4) | 4.5 | 4.8 |
| Variable | Category/statistic | n with observed data from included | Included in study maximum (N = 9509) | n with observed data from the potentially eligible | Potentially eligiblea maximum (N = 12,044) |
|---|---|---|---|---|---|
| Ethnicity | WB | 9509 | 3888 (40.9%) | 9929 | 4067 (41.0%) |
| SA | 4821 (50.7%) | 5015 (50.5%) | |||
| Other | 800 (8.4%) | 847 (8.5%) | |||
| Maternal age | Mean (SD) | 9509 | 27.3 (5.5) | 12,044 | 27.2 (5.5) |
| Maternal booking BMI | Mean (SD) | 9073 | 25.8 (5.6) | 9469 | 25.8 (5.6) |
| BW | Mean (SD) | 9505 | 3253 (548) | 12,044 | 3238 (552) |
| Sum of skinfolds | Mean (SD) | 6458 | 9.82 (2.02) | 8238 | 9.82 (2.04) |
| C-section | No | 9509 | 7526 (79.1%) | 12,044 | 9458 (78.5%) |
| Yes | 1983 (20.9%) | 2586 (21.5%) |
| Outcomes by fasting glucose categorya and per 1 SD | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Primary outcomes | ||||||
| BW of > 90th centile | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.23 | 0.95 to 1.58 | 1.31 | 0.95 to 1.79 | 1.15 | 0.66 to 2.02 |
| 3 | 1.48 | 1.16 to 1.88 | 1.84 | 1.36 to 2.48 | 1.34 | 0.81 to 2.21 |
| 4 | 1.62 | 1.19 to 2.20 | 2.22 | 1.50 to 3.29 | 1.60 | 0.88 to 2.90 |
| 5 | 2.16 | 1.59 to 2.94 | 2.47 | 1.57 to 3.86 | 3.26 | 1.91 to 5.54 |
| 6 | 3.48 | 2.36 to 5.13 | 3.92 | 1.98 to 7.75 | 5.71 | 3.15 to 10.34 |
| 7 | 3.37 | 1.83 to 6.24 | 3.77 | 1.50 to 9.50 | 5.99 | 2.49 to 14.44 |
| Per 1 SD | 1.37 | 1.27 to 1.49 | 1.44 | 1.29 to 1.60 | 1.67 | 1.45 to 1.92 |
| Sum of skinfolds of > 90th centile | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.16 | 0.93 to 1.46 | 1.11 | 0.79 to 1.56 | 1.34 | 0.96 to 1.88 |
| 3 | 1.57 | 1.28 to 1.93 | 1.55 | 1.12 to 2.14 | 1.72 | 1.27 to 2.32 |
| 4 | 1.97 | 1.53 to 2.53 | 2.04 | 1.34 to 3.10 | 2.04 | 1.43 to 2.90 |
| 5 | 2.55 | 1.97 to 3.30 | 2.62 | 1.63 to 4.20 | 2.67 | 1.88 to 3.80 |
| 6 | 4.20 | 3.01 to 5.87 | 4.20 | 2.10 to 8.38 | 4.15 | 2.67 to 6.45 |
| 7 | 3.82 | 2.19 to 6.67 | 3.57 | 1.34 to 9.48 | 4.41 | 2.16 to 8.98 |
| Per 1 SD | 1.47 | 1.37 to 1.57 | 1.46 | 1.29 to 1.65 | 1.47 | 1.34 to 1.62 |
| Caesarean delivery | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.03 | 0.90 to 1.19 | 1.12 | 0.91 to 1.37 | 1.04 | 0.83 to 1.30 |
| 3 | 1.25 | 1.09 to 1.43 | 1.34 | 1.09 to 1.64 | 1.32 | 1.08 to 1.62 |
| 4 | 1.41 | 1.18 to 1.69 | 1.60 | 1.20 to 2.13 | 1.56 | 1.21 to 1.99 |
| 5 | 1.49 | 1.22 to 1.81 | 1.77 | 1.26 to 2.47 | 1.41 | 1.07 to 1.85 |
| 6 | 1.51 | 1.14 to 2.01 | 2.54 | 1.50 to 4.29 | 1.27 | 0.86 to 1.87 |
| 7 | 2.63 | 1.68 to 4.10 | 1.88 | 0.85 to 4.19 | 3.43 | 1.95 to 6.03 |
| Per 1 SD | 1.18 | 1.12 to 1.24 | 1.24 | 1.15 to 1.35 | 1.18 | 1.10 to 1.26 |
| Outcome by 2-hour post-load glucose categorya and per 1 SD | ||||||
| BW of > 90th percentile | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.99 | 0.78 to 1.27 | 1.13 | 0.84 to 1.53 | 1.09 | 0.64 to 1.86 |
| 3 | 1.22 | 0.96 to 1.56 | 1.25 | 0.92 to 1.70 | 1.40 | 0.83 to 2.34 |
| 4 | 1.54 | 1.13 to 2.11 | 1.60 | 1.07 to 2.41 | 2.06 | 1.12 to 3.78 |
| 5 | 1.95 | 1.45 to 2.64 | 1.77 | 1.18 to 2.66 | 2.98 | 1.68 to 5.28 |
| 6 | 2.12 | 1.37 to 3.29 | 2.72 | 1.54 to 4.82 | 2.30 | 1.01 to 5.26 |
| 7 | 1.50 | 0.79 to 2.85 | 1.66 | 0.69 to 4.01 | 2.54 | 0.94 to 6.89 |
| Per 1 SD | 1.26 | 1.15 to 1.38 | 1.25 | 1.12 to 1.40 | 1.46 | 1.23 to 1.72 |
| Sum of skinfolds > 90th percentile | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.09 | 0.87 to 1.38 | 1.31 | 0.94 to 1.83 | 1.01 | 0.72 to 1.41 |
| 3 | 1.49 | 1.19 to 1.86 | 1.27 | 0.89 to 1.82 | 1.68 | 1.23 to 2.29 |
| 4 | 2.16 | 1.66 to 2.81 | 2.03 | 1.31 to 3.16 | 2.30 | 1.60 to 3.31 |
| 5 | 2.32 | 1.77 to 3.03 | 2.21 | 1.41 to 3.46 | 2.56 | 1.78 to 3.68 |
| 6 | 2.79 | 1.91 to 4.08 | 3.21 | 1.74 to 5.89 | 2.50 | 1.48 to 4.23 |
| 7 | 3.06 | 1.88 to 4.98 | 1.99 | 0.76 to 5.21 | 3.83 | 2.13 to 6.88 |
| Per 1 SD | 1.40 | 1.29 to 1.51 | 1.34 | 1.18 to 1.51 | 1.47 | 1.32 to 1.63 |
| Caesarean delivery | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.02 | 0.89 to 1.17 | 0.99 | 0.80 to 1.22 | 1.14 | 0.92 to 1.41 |
| 3 | 1.23 | 1.07 to 1.42 | 1.32 | 1.07 to 1.63 | 1.15 | 0.92 to 1.43 |
| 4 | 1.35 | 1.12 to 1.64 | 1.23 | 0.91 to 1.66 | 1.45 | 1.10 to 1.92 |
| 5 | 1.29 | 1.06 to 1.57 | 1.53 | 1.14 to 2.06 | 1.17 | 0.87 to 1.57 |
| 6 | 1.79 | 1.34 to 2.39 | 2.00 | 1.26 to 3.15 | 1.66 | 1.11 to 2.49 |
| 7 | 1.48 | 1.00 to 2.20 | 1.26 | 0.65 to 2.45 | 1.60 | 0.94 to 2.73 |
| Per 1 SD | 1.14 | 1.09 to 1.20 | 1.15 | 1.07 to 1.25 | 1.12 | 1.04 to 1.21 |
| Outcome by fasting glucose categorya and per 1 SD | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Pre-eclampsia | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.45 | 0.98 to 2.14 | 1.39 | 0.79 to 2.44 | 1.46 | 0.83 to 2.56 |
| 3 | 1.33 | 0.90 to 1.95 | 1.22 | 0.67 to 2.20 | 1.24 | 0.72 to 2.15 |
| 4 | 2.00 | 1.28 to 3.15 | 2.66 | 1.37 to 5.15 | 1.54 | 0.81 to 2.92 |
| 5 | 2.29 | 1.41 to 3.71 | 3.52 | 1.69 to 7.36 | 1.35 | 0.65 to 2.79 |
| 6 | 2.69 | 1.42 to 5.09 | 1.86 | 0.43 to 8.03 | 2.14 | 0.91 to 5.03 |
| 7 | 3.23 | 1.27 to 8.23 | 2.03 | 0.27 to 15.46 | 3.71 | 1.26 to 10.97 |
| Per 1 SD | 1.31 | 1.15 to 1.48 | 1.38 | 1.13 to 1.69 | 1.19 | 1.00 to 1.43 |
| Preterm delivery | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.84 | 0.65 to 1.08 | 0.74 | 0.50 to 1.09 | 0.96 | 0.66 to 1.41 |
| 3 | 0.94 | 0.74 to 1.20 | 0.91 | 0.63 to 1.32 | 0.97 | 0.68 to 1.40 |
| 4 | 0.78 | 0.55 to 1.12 | 0.96 | 0.56 to 1.64 | 0.70 | 0.42 to 1.18 |
| 5 | 1.01 | 0.70 to 1.45 | 1.06 | 0.57 to 1.97 | 1.02 | 0.63 to 1.67 |
| 6 | 0.74 | 0.40 to 1.38 | 0.85 | 0.26 to 2.78 | 0.62 | 0.27 to 1.46 |
| 7 | 1.65 | 0.79 to 3.48 | 0.59 | 0.08 to 4.37 | 2.10 | 0.87 to 5.08 |
| Per 1 SD | 0.93 | 0.84 to 1.03 | 0.91 | 0.77 to 1.08 | 0.93 | 0.80 to 1.08 |
| Shoulder dystociab | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.89 | 0.51 to 1.56 | 0.48 | 0.17 to 1.30 | 1.15 | 0.50 to 2.61 |
| 3 | 0.87 | 0.50 to 1.53 | 1.06 | 0.48 to 2.33 | 0.90 | 0.39 to 2.08 |
| 4 | 1.80 | 0.98 to 3.30 | 1.59 | 0.58 to 4.37 | 1.43 | 0.56 to 3.66 |
| 5 | 1.33 | 0.63 to 2.81 | 1.52 | 0.44 to 5.26 | 1.50 | 0.56 to 4.02 |
| 6 | 2.32 | 0.96 to 5.63 | 3.86 | 0.86 to 17.34 | 1.16 | 0.26 to 5.25 |
| 7 | 2.70 | 0.63 to 11.57 | – | – | 5.05 | 1.09 to 23.46 |
| Per 1 SD | 1.26 | 1.04 to 1.52 | 1.26 | 0.90 to 1.74 | 1.21 | 0.91 to 1.59 |
| Instrumental vaginal deliveryb | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.09 | 0.90 to 1.31 | 1.03 | 0.79 to 1.34 | 0.99 | 0.74 to 1.33 |
| 3 | 1.03 | 0.85 to 1.24 | 0.97 | 0.73 to 1.28 | 1.06 | 0.80 to 1.40 |
| 4 | 1.01 | 0.78 to 1.30 | 0.95 | 0.62 to 1.45 | 1.05 | 0.73 to 1.51 |
| 5 | 1.18 | 0.90 to 1.56 | 1.47 | 0.93 to 2.32 | 1.22 | 0.84 to 1.77 |
| 6 | 0.98 | 0.63 to 1.52 | 0.96 | 0.37 to 2.50 | 1.09 | 0.63 to 1.86 |
| 7 | 1.33 | 0.64 to 2.73 | 1.59 | 0.52 to 4.80 | 1.37 | 0.52 to 3.59 |
| Per 1 SD | 1.02 | 0.95 to 1.09 | 1.05 | 0.94 to 1.18 | 1.04 | 0.93 to 1.15 |
| Admission to neonatal unit | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.88 | 0.67 to 1.17 | 0.85 | 0.57 to 1.28 | 0.91 | 0.60 to 1.39 |
| 3 | 0.94 | 0.72 to 1.22 | 0.75 | 0.49 to 1.16 | 1.09 | 0.74 to 1.59 |
| 4 | 1.15 | 0.81 to 1.61 | 1.12 | 0.64 to 1.96 | 1.20 | 0.75 to 1.91 |
| 5 | 1.14 | 0.78 to 1.66 | 0.64 | 0.27 to 1.49 | 1.45 | 0.90 to 2.34 |
| 6 | 1.26 | 0.73 to 2.18 | 2.66 | 1.16 to 6.07 | 0.88 | 0.39 to 1.96 |
| 7 | 0.97 | 0.35 to 2.69 | – | – | 1.19 | 0.36 to 3.94 |
| Per 1 SD | 1.00 | 0.90 to 1.11 | 0.94 | 0.78 to 1.14 | 1.03 | 0.89 to 1.18 |
| Outcome by 2-hour post-load glucose categorya and per 1 SD | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | |||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Pre-eclampsia | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.16 | 0.78 to 1.74 | 1.79 | 0.95 to 3.39 | 0.80 | 0.47 to 1.37 |
| 3 | 1.58 | 1.07 to 2.33 | 2.28 | 1.22 to 4.29 | 0.97 | 0.57 to 1.64 |
| 4 | 1.65 | 1.00 to 2.71 | 2.85 | 1.32 to 6.13 | 0.99 | 0.49 to 1.99 |
| 5 | 1.86 | 1.13 to 3.05 | 3.03 | 1.40 to 6.53 | 0.95 | 0.46 to 1.99 |
| 6 | 1.51 | 0.67 to 3.40 | 2.37 | 0.67 to 8.32 | 1.05 | 0.36 to 3.07 |
| 7 | 2.78 | 1.22 to 6.31 | 1.65 | 0.21 to 12.77 | 2.30 | 0.86 to 6.15 |
| Per 1 SD | 1.26 | 1.11 to 1.43 | 1.36 | 1.14 to 1.64 | 1.10 | 0.91 to 1.34 |
| Preterm delivery | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.99 | 0.77 to 1.28 | 1.00 | 0.69 to 1.44 | 1.06 | 0.72 to 1.56 |
| 3 | 0.95 | 0.72 to 1.24 | 0.93 | 0.62 to 1.38 | 0.97 | 0.65 to 1.45 |
| 4 | 1.14 | 0.80 to 1.62 | 1.14 | 0.67 to 1.93 | 1.26 | 0.76 to 2.09 |
| 5 | 0.99 | 0.68 to 1.44 | 0.81 | 0.44 to 1.50 | 1.08 | 0.63 to 1.84 |
| 6 | 1.43 | 0.86 to 2.40 | 1.02 | 0.40 to 2.60 | 1.81 | 0.93 to 3.51 |
| 7 | 0.83 | 0.36 to 1.93 | 1.99 | 0.76 to 5.19 | 0.25 | 0.03 to 1.82 |
| Per 1 SD | 1.04 | 0.94 to 1.14 | 1.02 | 0.89 to 1.17 | 1.05 | 0.91 to 1.20 |
| Shoulder dystociab | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.00 | 0.55 to 1.83 | 1.14 | 0.50 to 2.60 | 1.19 | 0.39 to 3.65 |
| 3 | 1.68 | 0.96 to 2.95 | 1.20 | 0.51 to 2.85 | 3.19 | 1.19 to 8.59 |
| 4 | 1.86 | 0.90 to 3.82 | 1.71 | 0.59 to 4.97 | 2.49 | 0.72 to 8.66 |
| 5 | 1.74 | 0.83 to 3.65 | 0.76 | 0.17 to 3.46 | 3.94 | 1.28 to 12.13 |
| 6 | 1.58 | 0.47 to 5.37 | 1.33 | 0.17 to 10.51 | 3.01 | 0.58 to 15.68 |
| 7 | 3.67 | 1.23 to 10.93 | 2.20 | 0.28 to 17.49 | 8.09 | 1.89 to 34.62 |
| Per 1 SD | 1.36 | 1.12 to 1.66 | 1.16 | 0.86 to 1.55 | 1.72 | 1.27 to 2.31 |
| Instrumental vaginal deliveryb | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.86 | 0.71 to 1.04 | 0.95 | 0.72 to 1.25 | 0.84 | 0.63 to 1.12 |
| 3 | 0.98 | 0.81 to 1.19 | 1.06 | 0.80 to 1.42 | 0.96 | 0.72 to 1.29 |
| 4 | 1.15 | 0.89 to 1.50 | 1.18 | 0.80 to 1.76 | 1.25 | 0.86 to 1.83 |
| 5 | 1.05 | 0.81 to 1.38 | 1.26 | 0.84 to 1.90 | 0.99 | 0.66 to 1.47 |
| 6 | 1.35 | 0.90 to 2.05 | 2.09 | 1.13 to 3.85 | 1.23 | 0.68 to 2.20 |
| 7 | 1.12 | 0.64 to 1.97 | 0.77 | 0.27 to 2.20 | 1.46 | 0.72 to 2.96 |
| Per 1 SD | 1.07 | 0.99 to 1.14 | 1.11 | 1.00 to 1.23 | 1.06 | 0.95 to 1.18 |
| Admission to neonatal unit | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.89 | 0.68 to 1.16 | 1.12 | 0.75 to 1.66 | 0.70 | 0.47 to 1.04 |
| 3 | 0.85 | 0.64 to 1.13 | 0.81 | 0.52 to 1.27 | 0.79 | 0.53 to 1.16 |
| 4 | 0.94 | 0.64 to 1.38 | 0.85 | 0.45 to 1.62 | 0.87 | 0.52 to 1.46 |
| 5 | 0.84 | 0.56 to 1.25 | 0.83 | 0.42 to 1.61 | 0.85 | 0.50 to 1.44 |
| 6 | 0.55 | 0.25 to 1.20 | 0.97 | 0.34 to 2.76 | 0.35 | 0.11 to 1.14 |
| 7 | 1.48 | 0.76 to 2.90 | 1.38 | 0.42 to 4.60 | 1.31 | 0.54 to 3.13 |
| Per 1 SD | 0.98 | 0.88 to 1.08 | 0.97 | 0.83 to 1.12 | 0.98 | 0.85 to 1.13 |
| Outcome by fasting glucose categorya and per 1 SD | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | p-interactionb | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Pre-eclampsia | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.62 |
| 2 | 1.37 | 0.92 to 2.04 | 1.28 | 0.72 to 2.27 | 1.38 | 0.78 to 2.46 | |
| 3 | 1.17 | 0.78 to 1.75 | 1.08 | 0.58 to 1.99 | 1.07 | 0.60 to 1.91 | |
| 4 | 1.57 | 0.97 to 2.53 | 1.88 | 0.94 to 3.75 | 1.28 | 0.65 to 2.50 | |
| 5 | 1.88 | 1.11 to 3.19 | 2.65 | 1.18 to 5.93 | 1.16 | 0.53 to 2.52 | |
| 6 | 1.99 | 1.02 to 3.87 | 1.16 | 0.31 to 4.39 | 1.55 | 0.63 to 3.82 | |
| 7 | 2.60 | 0.97 to 6.97 | 1.65 | 0.19 to 14.58 | 2.76 | 0.86 to 8.91 | |
| Per 1 SD | 1.20 | 1.04 to 1.38 | 1.24 | 0.98 to 1.55 | 1.10 | 0.90 to 1.33 | |
| Preterm delivery | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.72 |
| 2 | 0.86 | 0.66 to 1.12 | 0.76 | 0.52 to 1.12 | 0.97 | 0.66 to 1.42 | |
| 3 | 1.02 | 0.79 to 1.31 | 1.04 | 0.71 to 1.51 | 1.02 | 0.71 to 1.47 | |
| 4 | 0.86 | 0.60 to 1.25 | 1.15 | 0.67 to 1.99 | 0.74 | 0.44 to 1.24 | |
| 5 | 1.15 | 0.79 to 1.67 | 1.30 | 0.69 to 2.45 | 1.09 | 0.66 to 1.81 | |
| 6 | 0.83 | 0.43 to 1.58 | 1.05 | 0.31 to 3.51 | 0.68 | 0.28 to 1.66 | |
| 7 | 2.12 | 0.98 to 4.57 | 0.82 | 0.10 to 6.33 | 2.30 | 0.91 to 5.82 | |
| Per 1 SD | 0.96 | 0.86 to 1.08 | 0.98 | 0.82 to 1.17 | 0.95 | 0.81 to 1.10 | |
| Shoulder dystociac | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.52 |
| 2 | 0.88 | 0.50 to 1.54 | 0.43 | 0.15 to 1.17 | 1.18 | 0.52 to 2.72 | |
| 3 | 0.87 | 0.49 to 1.53 | 0.90 | 0.41 to 1.96 | 0.92 | 0.39 to 2.21 | |
| 4 | 1.69 | 0.90 to 3.16 | 1.26 | 0.47 to 3.35 | 1.40 | 0.53 to 3.65 | |
| 5 | 1.19 | 0.55 to 2.58 | 1.13 | 0.33 to 3.87 | 1.38 | 0.49 to 3.86 | |
| 6 | 2.01 | 0.79 to 5.12 | 2.85 | 0.69 to 11.72 | 0.98 | 0.21 to 4.64 | |
| 7 | 2.56 | 0.59 to 11.10 | – | – | 4.49 | 0.92 to 21.87 | |
| Per 1 SD | 1.22 | 1.00 to 1.49 | 1.13 | 0.82 to 1.56 | 1.17 | 0.88 to 1.55 | |
| Instrumental vaginal deliveryc | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.87 |
| 2 | 1.16 | 0.96 to 1.42 | 1.08 | 0.81 to 1.43 | 1.06 | 0.77 to 1.45 | |
| 3 | 1.15 | 0.94 to 1.40 | 1.01 | 0.75 to 1.36 | 1.18 | 0.87 to 1.60 | |
| 4 | 1.18 | 0.89 to 1.57 | 0.93 | 0.59 to 1.47 | 1.30 | 0.87 to 1.93 | |
| 5 | 1.53 | 1.12 to 2.08 | 1.61 | 0.97 to 2.68 | 1.54 | 1.02 to 2.33 | |
| 6 | 1.27 | 0.78 to 2.06 | 0.94 | 0.33 to 2.69 | 1.43 | 0.78 to 2.62 | |
| 7 | 2.21 | 0.92 to 5.29 | 2.11 | 0.51 to 8.77 | 2.44 | 0.78 to 7.68 | |
| Per 1 SD | 1.11 | 1.02 to 1.20 | 1.07 | 0.94 to 1.22 | 1.13 | 1.00 to 1.27 | |
| Intensive neonatal care | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.13 |
| 2 | 0.90 | 0.68 to 1.19 | 0.87 | 0.58 to 1.30 | 0.93 | 0.61 to 1.43 | |
| 3 | 0.96 | 0.73 to 1.25 | 0.78 | 0.50 to 1.20 | 1.15 | 0.79 to 1.68 | |
| 4 | 1.17 | 0.82 to 1.66 | 1.17 | 0.67 to 2.03 | 1.29 | 0.80 to 2.08 | |
| 5 | 1.6 | 0.79 to 1.70 | 0.64 | 0.27 to 1.50 | 1.58 | 0.97 to 2.56 | |
| 6 | 1.23 | 0.70 to 2.17 | 2.47 | 1.06 to 5.75 | 0.96 | 0.42 to 2.21 | |
| 7 | 1.04 | 0.37 to 2.91 | – | – | 1.33 | 0.40 to 4.45 | |
| Per 1 SD | 1.00 | 0.89 to 1.12 | 0.93 | 0.77 to 1.13 | 1.05 | 0.91 to 1.22 | |
| Outcome by 2-hour post-load glucose categorya and per 1 SD | All women (N = 9509) | WB (n = 3888) | SA (n = 4821) | p-interactionb | |||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Pre-eclampsia | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.33 |
| 2 | 0.99 | 0.65 to 1.49 | 1.56 | 0.82 to 2.98 | 0.64 | 0.37 to 1.12 | |
| 3 | 1.27 | 0.84 to 1.90 | 1.77 | 0.92 to 3.37 | 0.78 | 0.45 to 1.36 | |
| 4 | 1.21 | 0.72 to 2.06 | 2.29 | 1.03 to 5.08 | 0.66 | 0.32 to 1.36 | |
| 5 | 1.34 | 0.80 to 2.25 | 2.02 | 0.92 to 4.43 | 0.66 | 0.31 to 1.43 | |
| 6 | 1.03 | 0.44 to 2.41 | 1.43 | 0.38 to 5.32 | 0.70 | 0.23 to 2.12 | |
| 7 | 2.13 | 0.91 to 4.97 | 1.09 | 0.14 to 8.74 | 1.77 | 0.65 to 4.83 | |
| Per 1 SD | 1.13 | 0.99 to 1.30 | 1.19 | 0.99 to 1.45 | 0.98 | 0.80 to 1.21 | |
| Preterm delivery | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.61 |
| 2 | 1.02 | 0.78 to 1.32 | 1.07 | 0.73 to 1.56 | 1.02 | 0.69 to 1.51 | |
| 3 | 1.00 | 0.76 to 1.31 | 1.02 | 0.68 to 1.54 | 0.96 | 0.64 to 1.44 | |
| 4 | 1.21 | 0.84 to 1.73 | 1.25 | 0.73 to 2.14 | 1.26 | 0.75 to 2.11 | |
| 5 | 1.09 | 0.75 to 1.58 | 0.96 | 0.52 to 1.78 | 1.14 | 0.67 to 1.94 | |
| 6 | 1.58 | 0.93 to 2.68 | 1.25 | 0.48 to 3.25 | 1.87 | 0.95 to 3.68 | |
| 7 | 0.90 | 0.39 to 2.09 | 2.13 | 0.81 to 5.60 | 0.28 | 0.04 to 2.09 | |
| Per 1 SD | 1.07 | 0.97 to 1.18 | 1.08 | 0.93 to 1.24 | 1.07 | 0.93 to 1.24 | |
| Shoulder dystociac | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.33 |
| 2 | 0.98 | 0.53 to 1.79 | 1.13 | 0.49 to 2.57 | 1.10 | 0.36 to 3.39 | |
| 3 | 1.61 | 0.90 to 2.88 | 1.07 | 0.44 to 2.61 | 3.02 | 1.09 to 8.35 | |
| 4 | 1.74 | 0.82 to 3.69 | 1.39 | 0.45 to 4.27 | 2.36 | 0.64 to 8.65 | |
| 5 | 1.57 | 0.74 to 3.35 | 0.53 | 0.12 to 2.34 | 3.80 | 1.21 to 11.97 | |
| 6 | 1.37 | 0.39 to 4.83 | 0.82 | 0.09 to 7.55 | 2.73 | 0.53 to 14.03 | |
| 7 | 3.47 | 1.15 to 10.50 | 1.81 | 0.26 to 12.67 | 9.05 | 2.00 to 40.91 | |
| Per 1 SD | 1.33 | 1.08 to 1.64 | 1.05 | 0.79 to 1.40 | 1.75 | 1.27 to 2.41 | |
| Instrumental vaginal deliveryc | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.53 |
| 2 | 0.81 | 0.67 to 1.00 | 0.90 | 0.67 to 1.21 | 0.75 | 0.55 to 1.03 | |
| 3 | 0.96 | 0.78 to 1.18 | 0.98 | 0.72 to 1.33 | 0.94 | 0.69 to 1.30 | |
| 4 | 1.15 | 0.86 to 1.53 | 1.09 | 0.71 to 1.68 | 1.35 | 0.88 to 2.06 | |
| 5 | 1.01 | 0.74 to 1.36 | 1.14 | 0.71 to 1.84 | 0.97 | 0.62 to 1.50 | |
| 6 | 1.46 | 0.94 to 2.25 | 1.66 | 0.86 to 3.18 | 1.49 | 0.81 to 2.73 | |
| 7 | 1.00 | 0.54 to 1.85 | 0,49 | 0.16 to 1.50 | 1.54 | 0.71 to 3.34 | |
| Per 1 SD | 1.07 | 0.99 to 1.15 | 1.05 | 0.94 to 1.18 | 1.10 | 0.98 to 1.24 | |
| Intensive neonatal care | |||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 0.43 |
| 2 | 0.88 | 0.67 to 1.15 | 1.16 | 0.78 to 1.72 | 0.68 | 0.46 to 1.01 | |
| 3 | 0.84 | 0.63 to 1.10 | 0.83 | 0.53 to 1.30 | 0.78 | 0.53 to 1.15 | |
| 4 | 0.90 | 0.62 to 1.33 | 0.83 | 0.44 to 1.59 | 0.87 | 0.51 to 1.46 | |
| 5 | 0.83 | 0.55 to 1.24 | 0.85 | 0.44 to 1.66 | 0.87 | 0.51 to 1.49 | |
| 6 | 0.52 | 0.24 to 1.15 | 1.03 | 0.36 to 2.93 | 0.35 | 0.11 to 1.17 | |
| 7 | 1.44 | 0.73 to 2.86 | 1.27 | 0.37 to 4.40 | 1.42 | 0.58 to 3.48 | |
| Per 1 SD | 0.97 | 0.88 to 1.08 | 0.97 | 0.84 to 1.13 | 0.99 | 0.86 to 1.15 | |
| Outcomes | Fasting | Post load | ||
|---|---|---|---|---|
| OR (95% CI) for 1 SD increase | OR (95% CI) for glucose squared | OR (95% CI) for 1 SD increase | OR (95% CI) for glucose squared | |
| Primary | ||||
| BW of > 90th centile | 1.22 (1.08 to 1.38) | 1.04 (0.96 to 1.13) | 1.10 (0.98 to 1.23) | 1.04 (0.96 to 1.13) |
| Sum of skinfolds of > 90th centile | 1.35 (1.18 to 1.54) | 1.04 (0.95 to 1.14) | 1.25 (1.11 to 1.41) | 1.04 (0.95 to 1.15) |
| Caesarean delivery | 1.06 (0.97 to 1.16) | 1.00 (0.95 to 1.06) | 1.02 (0.94 to 1.10) | 1.00 (0.95 to 1.07) |
| Secondary | ||||
| Pre-eclampsia | 1.24 (0.98 to 1.57) | 0.88 (0.75 to 1.04) | 1.26 (0.99 to 1.60) | 0.86 (0.72 to 1.02) |
| Preterm delivery | 0.98 (0.82 to 1.17) | 0.99 (0.90 to 1.09) | 1.08 (0.93 to 1.24) | 1.00 (0.90 to 1.11) |
| Shoulder dystociaa | 1.14 (0.82 to 1.59) | 0.92 (0.73 to 1.17) | 1.05 (0.76 to 1.44) | 0.93 (0.73 to 1.18) |
| Instrumental deliverya | 1.07 (0.94 to 1.22) | 0.99 (0.91 to 1.07) | 1.05 (0.94 to 1.18) | 0.99 (0.91 to 1.08) |
| Intensive neonatal care | 0.94 (0.77 to 1.13) | 0.99 (0.87 to 1.12) | 0.97 (0.83 to 1.13) | 0.98 (0.86 to 1.12) |
| Outcome by glucose categorya and per 1 SD | All women | WB | SA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Primary outcomes | ||||||||||||
| BW of > 90th percentile | n = 9508 | n = 7620 | n = 3887 | n = 3215 | n = 4821 | n = 3720 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.23 | 0.95 to 1.58 | 1.29 | 0.96 to 1.72 | 1.31 | 0.95 to 1.79 | 1.35 | 0.95 to 1.91 | 1.15 | 0.66 to 2.02 | 0.93 | 0.48 to 1.83 |
| 3 | 1.48 | 1.16 to 1.88 | 1.34 | 1.01 to 1.79 | 1.84 | 1.36 to 2.48 | 1.37 | 0.97 to 1.95 | 1.34 | 0.81 to 2.21 | 1.05 | 0.57 to 1.95 |
| 4 | 1.62 | 1.19 to 2.20 | 1.33 | 0.90 to 1.95 | 2.22 | 1.50 to 3.29 | 1.33 | 0.82 to 2.18 | 1.60 | 0.88 to 2.90 | 0.93 | 0.44 to 1.99 |
| 5 | 2.16 | 1.59 to 2.94 | 1.99 | 1.36 to 2.92 | 2.47 | 1.57 to 3.86 | 1.56 | 0.88 to 2.75 | 3.26 | 1.91 to 5.54 | 2.24 | 1.18 to 4.24 |
| 6 | 3.48 | 2.36 to 5.13 | 3.24 | 1.99 to 5.26 | 3.92 | 1.98 to 7.75 | 2.34 | 1.08 to 5.05 | 5.71 | 3.15 to 10.34 | 3.34 | 1.57 to 7.08 |
| 7 | 3.37 | 1.83 to 6.24 | 2.11 | 0.98 to 4.54 | 3.77 | 1.50 to 9.50 | 1.98 | 0.73 to 5.38 | 5.99 | 2.49 to 14.44 | 2.30 | 0.70 to 7.58 |
| Per 1 SD | 1.37 | 1.27 to 1.49 | 1.27 | 1.15 to 1.41 | 1.44 | 1.29 to 1.60 | 1.17 | 1.02 to 1.34 | 1.67 | 1.45 to 1.92 | 1.41 | 1.18 to 1.68 |
| Sum of skinfolds of > 90th percentile | n = 6458 | n = 5294 | n = 2510 | n = 2101 | n = 3409 | n = 2724 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.05 | 0.83 to 1.32 | 0.95 | 0.73 to 1.24 | 1.03 | 0.71 to 1.48 | 0.96 | 0.64 to 1.44 | 1.21 | 0.86 to 1.69 | 1.06 | 0.72 to 1.56 |
| 3 | 1.59 | 1.29 to 1.96 | 1.54 | 1.21 to 1.97 | 1.53 | 1.09 to 2.15 | 1.50 | 1.01 to 2.22 | 1.70 | 1.26 to 2.30 | 1.60 | 1.14 to 2.26 |
| 4 | 1.76 | 1.34 to 2.32 | 1.47 | 1.07 to 2.02 | 1.90 | 1.20 to 3.00 | 1.84 | 1.09 to 3.09 | 1.79 | 1.24 to 2.59 | 1.32 | 0.85 to 2.04 |
| 5 | 2.61 | 1.99 to 3.42 | 2.05 | 1.49 to 2.82 | 2.40 | 1.45 to 3.98 | 2.23 | 1.24 to 4.01 | 2.88 | 2.01 to 4.12 | 1.98 | 1.30 to 3.03 |
| 6 | 4.29 | 3.01 to 6.12 | 3.53 | 2.36 to 5.28 | 4.38 | 2.16 to 8.89 | 3.65 | 1.50 to 8.91 | 4.09 | 2.59 to 6.48 | 3.27 | 1.94 to 5.48 |
| 7 | 3.70 | 2.03 to 6.75 | 2.92 | 1.45 to 5.85 | 3.91 | 1.37 to 11.14 | 3.06 | 0.91 to 10.27 | 3.56 | 1.64 to 7.72 | 2.82 | 1.07 to 7.40 |
| Per 1 SD | 1.46 | 1.36 to 1.58 | 1.37 | 1.26 to 1.49 | 1.44 | 1.27 to 1.64 | 1.39 | 1.19 to 1.62 | 1.47 | 1.33 to 1.62 | 1.35 | 1.20 to 1.51 |
| Caesarean delivery | n = 9509 | n = 7621 | n = 3888 | n = 3216 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.03 | 0.90 to 1.19 | 0.95 | 0.81 to 1.12 | 1.12 | 0.91 to 1.37 | 1.03 | 0.82 to 1.30 | 1.04 | 0.83 to 1.30 | 0.90 | 0.70 to 1.16 |
| 3 | 1.25 | 1.09 to 1.43 | 1.10 | 0.94 to 1.29 | 1.34 | 1.09 to 1.64 | 1.11 | 0.88 to 1.40 | 1.32 | 1.08 to 1.62 | 1.10 | 0.87 to 1.39 |
| 4 | 1.41 | 1.18 to 1.69 | 1.14 | 0.93 to 1.41 | 1.60 | 1.20 to 2.13 | 1.07 | 0.76 to 1.50 | 1.56 | 1.21 to 1.99 | 1.27 | 0.95 to 1.69 |
| 5 | 1.49 | 1.22 to 1.81 | 1.19 | 0.94 to 1.50 | 1.77 | 1.26 to 2.47 | 1.18 | 0.79 to 1.76 | 1.41 | 1.07 to 1.85 | 1.11 | 0.81 to 1.53 |
| 6 | 1.51 | 1.14 to 2.01 | 0.97 | 0.68 to 1.40 | 2.54 | 1.50 to 4.29 | 1.11 | 0.58 to 2.13 | 1.27 | 0.86 to 1.87 | 0.90 | 0.55 to 1.46 |
| 7 | 2.63 | 1.68 to 4.10 | 2.53 | 1.54 to 4.14 | 1.88 | 0.85 to 4.19 | 1.52 | 0.65 to 3.55 | 3.43 | 1.95 to 6.03 | 3.44 | 1.80 to 6.58 |
| Per 1 SD | 1.18 | 1.12 to 1.24 | 1.08 | 1.01 to 1.15 | 1.24 | 1.15 to 1.35 | 1.06 | 0.96 to 1.17 | 1.18 | 1.10 to 1.26 | 1.09 | 1.00 to 1.19 |
| Secondary outcomes | ||||||||||||
| Pre-eclampsia | n = 9120 | n = 7407 | n = 3724 | n = 3135 | n = 4629 | n = 3610 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.49 | 1.01 to 2.20 | 1.32 | 0.87 to 2.00 | 1.43 | 0.81 to 2.52 | 1.36 | 0.75 to 2.48 | 1.48 | 0.84 to 2.60 | 1.19 | 0.65 to 2.16 |
| 3 | 1.35 | 0.91 to 1.99 | 1.14 | 0.74 to 1.77 | 1.23 | 0.68 to 2.24 | 1.16 | 0.61 to 2.21 | 1.26 | 0.73 to 2.18 | 0.88 | 0.48 to 1.63 |
| 4 | 1.99 | 1.27 to 3.12 | 1.51 | 0.90 to 2.51 | 2.70 | 1.40 to 5.23 | 1.93 | 0.94 to 3.97 | 1.47 | 0.78 to 2.80 | 1.07 | 0.51 to 2.24 |
| 5 | 2.37 | 1.46 to 3.84 | 1.85 | 1.06 to 3.23 | 3.64 | 1.74 to 7.63 | 2.54 | 1.09 to 5.94 | 1.37 | 0.66 to 2.83 | 1.01 | 0.44 to 2.34 |
| 6 | 2.68 | 1.41 to 5.08 | 1.47 | 0.67 to 3.23 | 1.88 | 0.44 to 8.14 | 1.28 | 0.34 to 4.86 | 2.07 | 0.88 to 4.89 | 0.96 | 0.32 to 2.91 |
| 7 | 3.30 | 1.29 to 8.39 | 3.02 | 1.09 to 8.32 | 1.99 | 0.26 to 15.20 | 1.47 | 0.16 to 13.70 | 3.81 | 1.29 to 11.25 | 3.74 | 1.10 to 12.68 |
| Per 1 SD | 1.31 | 1.16 to 1.48 | 1.19 | 1.02 to 1.38 | 1.38 | 1.13 to 1.69 | 1.22 | 0.97 to 1.53 | 1.19 | 0.99 to 1.42 | 1.07 | 0.85 to 1.34 |
| Preterm delivery | n = 9509 | n = 7621 | n = 3888 | n = 3198 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.84 | 0.65 to 1.08 | 0.82 | 0.62 to 1.09 | 0.74 | 0.50 to 1.09 | 0.77 | 0.51 to 1.18 | 0.96 | 0.66 to 1.41 | 0.91 | 0.60 to 1.39 |
| 3 | 0.94 | 0.74 to 1.20 | 1.04 | 0.79 to 1.37 | 0.91 | 0.63 to 1.32 | 1.04 | 0.69 to 1.57 | 0.97 | 0.68 to 1.40 | 1.04 | 0.70 to 1.55 |
| 4 | 0.78 | 0.55 to 1.12 | 0.89 | 0.59 to 1.33 | 0.96 | 0.56 to 1.64 | 1.19 | 0.65 to 2.21 | 0.70 | 0.42 to 1.18 | 0.76 | 0.43 to 1.34 |
| 5 | 1.01 | 0.70 to 1.45 | 1.29 | 0.86 to 1.94 | 1.06 | 0.57 to 1.97 | 1.40 | 0.69 to 2.83 | 1.02 | 0.63 to 1.67 | 1.20 | 0.70 to 2.06 |
| 6 | 0.74 | 0.40 to 1.38 | 0.75 | 0.36 to 1.58 | 0.85 | 0.26 to 2.78 | 0.86 | 0.20 to 3.69 | 0.62 | 0.27 to 1.46 | 0.56 | 0.20 to 1.59 |
| 7 | 1.65 | 0.79 to 3.48 | 2.30 | 1.01 to 5.25 | 0.59 | 0.08 to 4.37 | 1.09 | 0.14 to 8.72 | 2.10 | 0.87 to 5.08 | 2.52 | 0.92 to 6.93 |
| Per 1 SD | 0.93 | 0.84 to 1.03 | 0.99 | 0.87 to 1.11 | 0.91 | 0.77 to 1.08 | 1.00 | 0.82 to 1.22 | 0.93 | 0.80 to 1.08 | 0.94 | 0.80 to 1.11 |
| Shoulder dystocia | n = 7526 | n = 6040 | n = 2998 | n = 2496 | n = 3914 | n = 3015 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.89 | 0.51 to 1.56 | 0.98 | 0.52 to 1.86 | 0.48 | 0.17 to 1.30 | 0.52 | 0.16 to 1.65 | 1.15 | 0.50 to 2.61 | 1.09 | 0.44 to 2.70 |
| 3 | 0.87 | 0.50 to 1.53 | 1.05 | 0.55 to 1.99 | 1.06 | 0.48 to 2.33 | 1.36 | 0.59 to 3.17 | 0.90 | 0.39 to 2.08 | 0.77 | 0.29 to 2.08 |
| 4 | 1.80 | 0.98 to 3.30 | 2.17 | 1.09 to 4.32 | 1.59 | 0.58 to 4.37 | 1.90 | 0.67 to 5.44 | 1.43 | 0.56 to 3.66 | 1.46 | 0.54 to 3.97 |
| 5 | 1.33 | 0.63 to 2.81 | 1.00 | 0.37 to 2.73 | 1.52 | 0.44 to 5.26 | 1.19 | 0.24 to 5.75 | 1.50 | 0.56 to 4.02 | 0.90 | 0.24 to 3.34 |
| 6 | 2.32 | 0.96 to 5.63 | 3.00 | 1.15 to 7.84 | 3.86 | 0.86 to 17.34 | 3.59 | 0.81 to 15.83 | 1.16 | 0.26 to 5.25 | 1.34 | 0.28 to 6.53 |
| 7 | 2.70 | 0.63 to 11.57 | 2.08 | 0.27 to 16.12 | – | – | – | – | 5.05 | 1.09 to 23.46 | 3.57 | 0.43 to 30.02 |
| Per 1 SD | 1.26 | 1.04 to 1.52 | 1.28 | 1.03 to 1.60 | 1.26 | 0.90 to 1.74 | 1.27 | 0.91 to 1.77 | 1.21 | 0.91 to 1.59 | 1.12 | 0.81 to 1.56 |
| Instrumental vaginal delivery | n = 7519 | n = 6034 | n = 3015 | n = 2509 | n = 3913 | n = 3043 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.09 | 0.90 to 1.31 | 1.22 | 0.99 to 1.50 | 1.03 | 0.78 to 1.34 | 1.10 | 0.82 to 1.48 | 0.99 | 0.74 to 1.33 | 1.08 | 0.76 to 1.52 |
| 3 | 1.02 | 0.85 to 1.24 | 1.17 | 0.94 to 1.46 | 0.97 | 0.73 to 1.28 | 1.04 | 0.76 to 1.43 | 1.05 | 0.79 to 1.40 | 1.23 | 0.88 to 1.71 |
| 4 | 1.01 | 0.78 to 1.30 | 1.30 | 0.96 to 1.75 | 0.95 | 0.62 to 1.45 | 1.05 | 0.65 to 1.69 | 1.05 | 0.73 to 1.51 | 1.41 | 0.92 to 2.16 |
| 5 | 1.18 | 0.90 to 1.55 | 1.63 | 1.17 to 2.28 | 1.46 | 0.92 to 2.31 | 1.83 | 1.08 to 3.12 | 1.22 | 0.84 to 1.77 | 1.58 | 0.99 to 2.51 |
| 6 | 0.98 | 0.63 to 1.52 | 1.21 | 0.72 to 2.06 | 0.96 | 0.37 to 2.50 | 1.02 | 0.35 to 2.94 | 1.09 | 0.63 to 1.86 | 1.30 | 0.67 to 2.53 |
| 7 | 1.33 | 0.64 to 2.73 | 1.77 | 0.64 to 4.92 | 1.59 | 0.52 to 4.80 | 0.98 | 0.17 to 5.58 | 1.37 | 0.52 to 3.59 | 3.14 | 0.89 to 11.12 |
| Per 1 SD | 1.02 | 0.95 to 1.09 | 1.11 | 1.01 to 1.21 | 1.05 | 0.94 to 1.18 | 1.08 | 0.95 to 1.24 | 1.04 | 0.93 to 1.15 | 1.12 | 0.98 to 1.28 |
| Admission to neonatal unit | n = 9509 | n = 7621 | n = 3859 | n = 3174 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.88 | 0.67 to 1.17 | 0.90 | 0.66 to 1.21 | 0.85 | 0.57 to 1.28 | 0.89 | 0.58 to 1.37 | 0.91 | 0.60 to 1.39 | 0.95 | 0.60 to 1.49 |
| 3 | 0.94 | 0.72 to 1.22 | 0.89 | 0.66 to 1.20 | 0.75 | 0.49 to 1.16 | 0.77 | 0.48 to 1.23 | 1.09 | 0.74 to 1.59 | 1.09 | 0.72 to 1.65 |
| 4 | 1.15 | 0.81 to 1.61 | 0.97 | 0.65 to 1.46 | 1.12 | 0.64 to 1.96 | 1.12 | 0.60 to 2.12 | 1.20 | 0.75 to 1.91 | 1.03 | 0.60 to 1.78 |
| 5 | 1.14 | 0.78 to 1.66 | 1.29 | 0.86 to 1.94 | 0.64 | 0.27 to 1.49 | 0.68 | 0.26 to 1.72 | 1.45 | 0.90 to 2.34 | 1.77 | 1.07 to 2.93 |
| 6 | 1.26 | 0.73 to 2.18 | 1.09 | 0.57 to 2.10 | 2.66 | 1.16 to 6.07 | 1.90 | 0.65 to 5.60 | 0.88 | 0.39 to 1.96 | 0.94 | 0.39 to 2.25 |
| 7 | 0.97 | 0.35 to 2.69 | 0.91 | 0.28 to 2.93 | – | – | – | – | 1.19 | 0.36 to 3.94 | 1.05 | 0.25 to 4.49 |
| Per 1 SD | 1.00 | 0.90 to 1.11 | 0.97 | 0.86 to 1.10 | 0.94 | 0.78 to 1.14 | 0.91 | 0.74 to 1.12 | 1.03 | 0.89 to 1.18 | 1.02 | 0.87 to 1.20 |
| Outcome by glucose categorya and per 1 SD | All women | WB | SA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Primary outcomes | ||||||||||||
| BW of > 90th percentile | n = 9508 | n = 7620 | n = 3887 | n = 3215 | n = 4821 | n = 3720 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.99 | 0.78 to 1.27 | 0.85 | 0.64 to 1.14 | 1.13 | 0.84 to 1.53 | 0.79 | 0.55 to 1.12 | 1.09 | 0.64 to 1.86 | 1.07 | 0.55 to 2.08 |
| 3 | 1.22 | 0.96 to 1.56 | 0.99 | 0.74 to 1.33 | 1.25 | 0.92 to 1.70 | 0.83 | 0.58 to 1.19 | 1.40 | 0.83 to 2.34 | 1.27 | 0.68 to 2.40 |
| 4 | 1.54 | 1.13 to 2.11 | 1.19 | 0.82 to 1.74 | 1.61 | 1.07 to 2.42 | 0.98 | 0.60 to 1.58 | 2.06 | 1.12 to 3.78 | 1.74 | 0.81 to 3.70 |
| 5 | 1.95 | 1.45 to 2.64 | 1.45 | 1.01 to 2.09 | 1.77 | 1.18 to 2.66 | 1.07 | 0.67 to 1.71 | 2.98 | 1.68 to 5.28 | 2.15 | 1.02 to 4.54 |
| 6 | 2.12 | 1.37 to 3.29 | 1.82 | 1.08 to 3.07 | 2.72 | 1.54 to 4.82 | 1.78 | 0.91 to 3.47 | 2.30 | 1.01 to 5.26 | 2.60 | 1.02 to 6.62 |
| 7 | 1.50 | 0.79 to 2.85 | 1.31 | 0.60 to 2.85 | 1.66 | 0.69 to 4.01 | 0.85 | 0.27 to 2.68 | 2.54 | 0.94 to 6.89 | 2.59 | 0.82 to 8.19 |
| Per 1 SD | 1.26 | 1.15 to 1.38 | 1.15 | 1.03 to 1.29 | 1.25 | 1.12 to 1.40 | 1.06 | 0.93 to 1.22 | 1.46 | 1.23 to 1.72 | 1.35 | 1.08 to 1.68 |
| Sum of skinfolds of > 90th percentile | n = 6458 | n = 5294 | n = 2510 | n = 2101 | n = 3409 | n = 2724 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.02 | 0.81 to 1.29 | 1.05 | 0.80 to 1.37 | 1.31 | 0.91 to 1.89 | 1.32 | 0.88 to 1.98 | 0.91 | 0.65 to 1.29 | 0.94 | 0.63 to 1.40 |
| 3 | 1.44 | 1.15 to 1.81 | 1.35 | 1.04 to 1.76 | 1.16 | 0.79 to 1.70 | 0.98 | 0.63 to 1.52 | 1.65 | 1.20 to 2.28 | 1.63 | 1.12 to 2.36 |
| 4 | 2.26 | 1.72 to 2.97 | 1.97 | 1.44 to 2.70 | 2.09 | 1.32 to 3.29 | 1.96 | 1.17 to 3.26 | 2.50 | 1.71 to 3.64 | 2.15 | 1.38 to 3.34 |
| 5 | 2.21 | 1.67 to 2.92 | 1.89 | 1.37 to 2.61 | 2.36 | 1.49 to 3.74 | 2.13 | 1.27 to 3.58 | 2.41 | 1.65 to 3.53 | 2.08 | 1.32 to 3.29 |
| 6 | 2.91 | 1.98 to 4.29 | 2.34 | 1.48 to 3.69 | 3.54 | 1.84 to 6.82 | 3.42 | 1.59 to 7.36 | 2.68 | 1.59 to 4.50 | 2.01 | 1.08 to 3.75 |
| 7 | 3.04 | 1.87 to 4.94 | 2.62 | 1.51 to 4.55 | 1.96 | 0.73 to 5.26 | 2.01 | 0.68 to 5.90 | 3.81 | 2.09 to 6.94 | 3.24 | 1.62 to 6.47 |
| Per 1 SD | 1.41 | 1.31 to 1.53 | 1.31 | 1.20 to 1.44 | 1.36 | 1.20 to 1.55 | 1.31 | 1.13 to 1.52 | 1.49 | 1.34 to 1.66 | 1.36 | 1.20 to 1.55 |
| Caesarean delivery | n = 9509 | n = 7621 | n = 3888 | n = 3216 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.02 | 0.89 to 1.17 | 0.94 | 0.80 to 1.10 | 0.99 | 0.80 to 1.22 | 0.87 | 0.68 to 1.10 | 1.14 | 0.92 to 1.41 | 1.09 | 0.85 to 1.39 |
| 3 | 1.23 | 1.07 to 1.42 | 1.04 | 0.88 to 1.22 | 1.32 | 1.07 to 1.63 | 1.07 | 0.84 to 1.36 | 1.15 | 0.92 to 1.43 | 1.05 | 0.81 to 1.35 |
| 4 | 1.35 | 1.12 to 1.64 | 1.15 | 0.92 to 1.43 | 1.23 | 0.91 to 1.66 | 0.88 | 0.62 to 1.25 | 1.45 | 1.10 to 1.92 | 1.36 | 0.98 to 1.89 |
| 5 | 1.29 | 1.06 to 1.57 | 1.03 | 0.82 to 1.29 | 1.53 | 1.14 to 2.06 | 1.02 | 0.72 to 1.43 | 1.17 | 0.87 to 1.57 | 1.07 | 0.76 to 1.51 |
| 6 | 1.79 | 1.34 to 2.39 | 1.30 | 0.92 to 1.83 | 2.00 | 1.26 to 3.15 | 0.96 | 0.55 to 1.69 | 1.66 | 1.11 to 2.49 | 1.57 | 0.99 to 2.48 |
| 7 | 1.48 | 1.00 to 2.20 | 1.04 | 0.65 to 1.66 | 1.26 | 0.65 to 2.45 | 0.62 | 0.27 to 1.42 | 1.60 | 0.94 to 2.73 | 1.36 | 0.74 to 2.53 |
| Per 1 SD | 1.14 | 1.09 to 1.20 | 1.05 | 0.99 to 1.12 | 1.15 | 1.07 to 1.25 | 1.00 | 0.91 to 1.09 | 1.12 | 1.04 to 1.21 | 1.09 | 1.00 to 1.19 |
| Secondary outcomes | ||||||||||||
| Pre-eclampsia | n = 9120 | n = 7407 | n = 3724 | n = 3135 | n = 4629 | n = 3610 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.15 | 0.77 to 1.73 | 0.92 | 0.59 to 1.43 | 1.83 | 0.97 to 3.49 | 1.62 | 0.81 to 3.22 | 0.77 | 0.45 to 1.32 | 0.54 | 0.30 to 0.96 |
| 3 | 1.60 | 1.09 to 2.37 | 1.25 | 0.81 to 1.93 | 2.34 | 1.24 to 4.41 | 2.02 | 1.02 to 4.01 | 0.96 | 0.57 to 1.63 | 0.67 | 0.37 to 1.21 |
| 4 | 1.67 | 1.01 to 2.75 | 1.36 | 0.78 to 2.37 | 2.96 | 1.37 to 6.40 | 3.03 | 1.35 to 6.81 | 0.97 | 0.48 to 1.94 | 0.61 | 0.28 to 1.32 |
| 5 | 1.84 | 1.12 to 3.02 | 1.38 | 0.80 to 2.38 | 3.06 | 1.42 to 6.61 | 2.31 | 1.01 to 5.28 | 0.89 | 0.43 to 1.87 | 0.56 | 0.24 to 1.29 |
| 6 | 1.52 | 0.68 to 3.42 | 1.10 | 0.44 to 2.76 | 2.40 | 0.68 to 8.44 | 1.87 | 0.50 to 7.08 | 1.04 | 0.36 to 3.02 | 0.63 | 0.17 to 2.27 |
| 7 | 2.85 | 1.26 to 6.47 | 1.67 | 0.61 to 4.52 | 1.58 | 0.20 to 12.23 | 1.21 | 0.15 to 10.01 | 2.35 | 0.88 to 6.29 | 1.06 | 0.31 to 3.58 |
| Per 1 SD | 1.27 | 1.11 to 1.44 | 1.14 | 0.98 to 1.33 | 1.37 | 1.14 to 1.64 | 1.27 | 1.04 to 1.55 | 1.10 | 0.90 to 1.34 | 0.92 | 0.73 to 1.16 |
| Preterm delivery | n = 9509 | n = 7621 | n = 3888 | n = 3198 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.99 | 0.77 to 1.28 | 0.96 | 0.72 to 1.27 | 1.00 | 0.69 to 1.44 | 1.03 | 0.68 to 1.54 | 1.06 | 0.72 to 1.56 | 0.93 | 0.61 to 1.43 |
| 3 | 0.95 | 0.72 to 1.24 | 0.94 | 0.70 to 1.27 | 0.93 | 0.62 to 1.38 | 0.94 | 0.60 to 1.47 | 0.97 | 0.65 to 1.45 | 0.91 | 0.58 to 1.42 |
| 4 | 1.14 | 0.80 to 1.62 | 1.23 | 0.84 to 1.81 | 1.14 | 0.67 to 1.93 | 1.12 | 0.62 to 2.04 | 1.26 | 0.76 to 2.09 | 1.33 | 0.77 to 2.29 |
| 5 | 0.99 | 0.68 to 1.44 | 1.12 | 0.75 to 1.67 | 0.81 | 0.44 to 1.50 | 0.93 | 0.48 to 1.81 | 1.08 | 0.63 to 1.84 | 1.21 | 0.68 to 2.15 |
| 6 | 1.43 | 0.86 to 2.40 | 1.66 | 0.95 to 2.90 | 1.02 | 0.40 to 2.60 | 1.14 | 0.39 to 3.29 | 1.81 | 0.93 to 3.51 | 2.02 | 0.99 to 4.10 |
| 7 | 0.83 | 0.36 to 1.93 | 1.08 | 0.46 to 2.56 | 1.99 | 0.76 to 5.19 | 2.64 | 0.97 to 7.18 | 0.25 | 0.03 to 1.82 | 0.31 | 0.04 to 2.39 |
| Per 1 SD | 1.04 | 0.94 to 1.14 | 1.09 | 0.98 to 1.21 | 1.02 | 0.89 to 1.17 | 1.06 | 0.91 to 1.24 | 1.05 | 0.91 to 1.20 | 1.11 | 0.95 to 1.31 |
| Shoulder dystocia | n = 7526 | n = 6040 | n = 3018 | n = 2512 | n = 3914 | n = 3015 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 1.00 | 0.55 to 1.83 | 1.06 | 0.54 to 2.10 | 1.14 | 0.50 to 2.60 | 1.10 | 0.45 to 2.70 | 1.19 | 0.39 to 3.65 | 1.06 | 0.29 to 3.81 |
| 3 | 1.68 | 0.96 to 2.95 | 1.49 | 0.76 to 2.90 | 1.20 | 0.51 to 2.85 | 0.84 | 0.30 to 2.33 | 3.19 | 1.19 to 8.59 | 2.63 | 0.85 to 8.09 |
| 4 | 1.86 | 0.90 to 3.82 | 1.88 | 0.81 to 4.37 | 1.71 | 0.59 to 4.97 | 1.24 | 0.37 to 4.17 | 2.49 | 0.72 to 8.66 | 3.09 | 0.79 to 12.05 |
| 5 | 1.74 | 0.83 to 3.65 | 1.62 | 0.69 to 3.81 | 0.76 | 0.17 to 3.46 | 0.56 | 0.12 to 2.57 | 3.94 | 1.28 to 12.13 | 3.59 | 0.98 to 13.09 |
| 6 | 1.58 | 0.47 to 5.37 | 1.76 | 0.48 to 6.45 | 1.33 | 0.17 to 10.51 | 0.77 | 0.08 to 7.47 | 3.01 | 0.58 to 15.68 | 3.93 | 0.71 to 21.77 |
| 7 | 3.67 | 1.23 to 10.93 | 3.25 | 0.91 to 11.57 | 2.20 | 0.28 to 17.49 | 1.77 | 0.24 to 12.96 | 8.09 | 1.89 to 34.62 | 6.64 | 1.14 to 38.68 |
| Per 1 SD | 1.36 | 1.12 to 1.66 | 1.32 | 1.04 to 1.68 | 1.16 | 0.86 to 1.55 | 1.02 | 0.75 to 1.39 | 1.72 | 1.27 to 2.31 | 1.73 | 1.19 to 2.51 |
| Instrumental vaginal delivery | n = 7519 | n = 6034 | n = 3015 | n = 2509 | n = 3913 | n = 3043 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.86 | 0.71 to 1.04 | 0.81 | 0.66 to 1.01 | 0.95 | 0.72 to 1.25 | 0.99 | 0.73 to 1.35 | 0.84 | 0.63 to 1.12 | 0.67 | 0.48 to 0.95 |
| 3 | 0.98 | 0.81 to 1.19 | 0.93 | 0.75 to 1.16 | 1.06 | 0.80 to 1.42 | 0.99 | 0.71 to 1.38 | 0.96 | 0.72 to 1.29 | 0.90 | 0.64 to 1.27 |
| 4 | 1.15 | 0.89 to 1.49 | 1.05 | 0.77 to 1.43 | 1.18 | 0.79 to 1.76 | 1.02 | 0.64 to 1.60 | 1.25 | 0.86 to 1.83 | 1.23 | 0.78 to 1.94 |
| 5 | 1.05 | 0.81 to 1.38 | 0.84 | 0.61 to 1.17 | 1.26 | 0.84 to 1.90 | 1.12 | 0.68 to 1.83 | 0.99 | 0.66 to 1.47 | 0.66 | 0.40 to 1.09 |
| 6 | 1.35 | 0.90 to 2.05 | 1.46 | 0.92 to 2.33 | 2.09 | 1.13 to 3.85 | 1.54 | 0.77 to 3.08 | 1.23 | 0.68 to 2.20 | 1.58 | 0.83 to 3.01 |
| 7 | 1.12 | 0.64 to 1.97 | 0.87 | 0.46 to 1.66 | 0.77 | 0.27 to 2.20 | 0.51 | 0.16 to 1.60 | 1.46 | 0.72 to 2.96 | 1.36 | 0.61 to 3.01 |
| Per 1 SD | 1.06 | 0.99 to 1.14 | 1.02 | 0.94 to 1.10 | 1.11 | 1.00 to 1.23 | 1.03 | 0.92 to 1.16 | 1.06 | 0.95 to 1.18 | 1.03 | 0.91 to 1.18 |
| Admission to neonatal unit | n = 9509 | n = 7621 | n = 3888 | n = 3198 | n = 4821 | n = 3755 | ||||||
| 1 reference | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| 2 | 0.89 | 0.68 to 1.16 | 0.80 | 0.60 to 1.08 | 1.12 | 0.75 to 1.66 | 1.19 | 0.77 to 1.82 | 0.70 | 0.47 to 1.04 | 0.54 | 0.35 to 0.84 |
| 3 | 0.85 | 0.64 to 1.13 | 0.78 | 0.58 to 1.06 | 0.81 | 0.52 to 1.27 | 0.86 | 0.53 to 1.39 | 0.79 | 0.53 to 1.16 | 0.72 | 0.47 to 1.09 |
| 4 | 0.94 | 0.64 to 1.38 | 0.92 | 0.61 to 1.38 | 0.85 | 0.45 to 1.62 | 0.92 | 0.47 to 1.81 | 0.87 | 0.52 to 1.46 | 0.88 | 0.51 to 1.52 |
| 5 | 0.84 | 0.56 to 1.25 | 0.82 | 0.53 to 1.26 | 0.83 | 0.42 to 1.61 | 0.73 | 0.34 to 1.57 | 0.85 | 0.50 to 1.44 | 0.90 | 0.52 to 1.56 |
| 6 | 0.55 | 0.25 to 1.20 | 0.43 | 0.17 to 1.08 | 0.97 | 0.34 to 2.76 | 0.91 | 0.28 to 3.01 | 0.35 | 0.11 to 1.14 | 0.25 | 0.06 to 1.06 |
| 7 | 1.48 | 0.76 to 2.90 | 1.15 | 0.51 to 2.57 | 1.38 | 0.42 to 4.60 | 1.10 | 0.25 to 4.91 | 1.31 | 0.54 to 3.13 | 1.20 | 0.45 to 3.19 |
| Per 1 SD | 0.98 | 0.88 to 1.08 | 0.95 | 0.85 to 1.06 | 0.97 | 0.83 to 1.12 | 0.95 | 0.81 to 1.11 | 0.98 | 0.85 to 1.13 | 0.99 | 0.84 to 1.17 |
| Outcome by fasting glucose categorya and per 1 SD | Unadjusted | Confounder adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Primary outcomes | ||||
| BW of > 90th centile | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.35 | 0.75 to 2.41 | 1.31 | 0.71 to 2.41 |
| 3 | 1.45 | 0.85 to 2.46 | 1.22 | 0.69 to 2.14 |
| 4 | 1.86 | 1.01 to 3.45 | 1.26 | 0.65 to 2.43 |
| 5 | 3.71 | 2.12 to 6.49 | 2.45 | 1.34 to 4.46 |
| 6 | 6.59 | 3.53 to 12.31 | 3.82 | 1.89 to 7.70 |
| 7 | 6.68 | 2.72 to 16.42 | 3.77 | 1.47 to 9.66 |
| Per 1 SD fasting glucose | 1.70 | 1.48 to 1.96 | 1.45 | 1.24 to 1.70 |
| Sum of skinfolds of > 90th centile | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.36 | 0.96 to 1.93 | 1.31 | 0.92 to 1.88 |
| 3 | 1.78 | 1.30 to 2.45 | 1.60 | 1.15 to 2.22 |
| 4 | 2.09 | 1.43 to 3.04 | 1.76 | 1.19 to 2.59 |
| 5 | 2.60 | 1.78 to 3.80 | 2.05 | 1.38 to 3.05 |
| 6 | 4.07 | 2.54 to 6.53 | 3.02 | 1.85 to 4.92 |
| 7 | 4.21 | 1.97 to 8.99 | 2.90 | 1.29 to 6.51 |
| Per 1 SD fasting glucose | 1.45 | 1.31 to 1.60 | 1.33 | 1.19 to 1.48 |
| Caesarean delivery | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.05 | 0.83 to 1.34 | 0.99 | 0.78 to 1.27 |
| 3 | 1.30 | 1.04 to 1.63 | 1.17 | 0.93 to 1.47 |
| 4 | 1.59 | 1.22 to 2.07 | 1.37 | 1.04 to 1.81 |
| 5 | 1.46 | 1.09 to 1.95 | 1.22 | 0.90 to 1.65 |
| 6 | 1.28 | 0.83 to 1.95 | 1.01 | 0.64 to 1.60 |
| 7 | 3.23 | 1.77 to 5.90 | 2.64 | 1.39 to 5.04 |
| Per 1 SD fasting glucose | 1.18 | 1.10 to 1.28 | 1.11 | 1.02 to 1.21 |
| Outcome by 2-hour post-load glucose categorya and per 1 SD | ||||
| BW of > 90th centile | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.25 | 0.71 to 2.20 | 1.11 | 0.62 to 1.96 |
| 3 | 1.59 | 0.92 to 2.74 | 1.21 | 0.70 to 2.12 |
| 4 | 2.44 | 1.30 to 4.58 | 1.64 | 0.83 to 3.22 |
| 5 | 3.22 | 1.76 to 5.91 | 2.25 | 1.18 to 4.30 |
| 6 | 2.40 | 0.99 to 5.83 | 1.77 | 0.69 to 4.54 |
| 7 | 2.93 | 1.06 to 8.08 | 1.97 | 0.63 to 6.11 |
| Per 1 SD fasting glucose | 1.48 | 1.25 to 1.75 | 1.30 | 1.08 to 1.58 |
| Sum of skinfolds > 90th centile | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.99 | 0.69 to 1.41 | 0.94 | 0.65 to 1.34 |
| 3 | 1.60 | 1.16 to 2.23 | 1.45 | 1.04 to 2.01 |
| 4 | 2.34 | 1.60 to 3.43 | 1.96 | 1.32 to 2.90 |
| 5 | 2.58 | 1.77 to 3.76 | 2.21 | 1.50 to 3.27 |
| 6 | 2.40 | 1.37 to 4.22 | 2.03 | 1.14 to 3.61 |
| 7 | 3.24 | 1.70 to 6.16 | 2.64 | 1.35 to 5.14 |
| Per 1 SD fasting glucose | 1.45 | 1.30 to 1.62 | 1.36 | 1.21 to 1.53 |
| Caesarean delivery | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.22 | 0.96 to 1.54 | 1.16 | 0.91 to 1.48 |
| 3 | 1.19 | 0.94 to 1.51 | 1.07 | 0.84 to 1.37 |
| 4 | 1.55 | 1.15 to 2.09 | 1.31 | 0.95 to 1.79 |
| 5 | 1.26 | 0.92 to 1.72 | 1.04 | 0.75 to 1.45 |
| 6 | 1.66 | 1.07 to 2.59 | 1.41 | 0.88 to 2.26 |
| 7 | 1.50 | 0.85 to 2.65 | 1.15 | 0.63 to 2.10 |
| Per 1 SD fasting glucose | 1.13 | 1.04 to 1.23 | 1.06 | 0.97 to 1.15 |
| Outcome by fasting glucose categorya and per 1 SD | Unadjusted | Confounder adjusted | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Primary outcomes | ||||
| Pre-eclampsia | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.41 | 0.78 to 2.55 | 1.31 | 0.72 to 2.41 |
| 3 | 1.24 | 0.70 to 2.19 | 1.06 | 0.58 to 1.92 |
| 4 | 1.57 | 0.81 to 3.04 | 1.25 | 0.62 to 2.52 |
| 5 | 1.48 | 0.71 to 3.11 | 1.18 | 0.53 to 2.63 |
| 6 | 1.40 | 0.48 to 4.08 | 0.99 | 0.34 to 2.89 |
| 7 | 3.86 | 1.30 to 11.51 | 2.70 | 0.78 to 9.38 |
| Per 1 SD fasting glucose | 1.16 | 0.96 to 1.41 | 1.06 | 0.86 to 1.29 |
| Premature delivery | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.01 | 0.66 to 1.53 | 1.01 | 0.66 to 1.54 |
| 3 | 1.04 | 0.70 to 1.54 | 1.09 | 0.73 to 1.62 |
| 4 | 0.70 | 0.39 to 1.24 | 0.72 | 0.40 to 1.29 |
| 5 | 1.18 | 0.70 to 1.99 | 1.25 | 0.73 to 2.14 |
| 6 | 0.67 | 0.26 to 1.70 | 0.72 | 0.27 to 1.93 |
| 7 | 2.52 | 1.03 to 6.18 | 2.74 | 1.08 to 6.97 |
| Per 1 SD fasting glucose | 0.98 | 0.84 to 1.15 | 1.00 | 0.85 to 1.18 |
| Shoulder dystociab | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.13 | 0.48 to 2.67 | 1.16 | 0.49 to 2.78 |
| 3 | 0.66 | 0.26 to 1.72 | 0.69 | 0.26 to 1.85 |
| 4 | 1.33 | 0.49 to 3.60 | 1.31 | 0.47 to 3.61 |
| 5 | 1.08 | 0.34 to 3.43 | 1.04 | 0.33 to 3.27 |
| 6 | 1.29 | 0.28 to 5.89 | 1.06 | 0.22 to 5.21 |
| 7 | 5.05 | 1.07 to 23.74 | 4.44 | 0.86 to 22.82 |
| Per 1 SD fasting glucose | 1.17 | 0.85 to 1.62 | 1.14 | 0.82 to 1.58 |
| Instrumental vaginal deliveryb | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.95 | 0.68 to 1.31 | 0.99 | 0.70 to 1.40 |
| 3 | 1.03 | 0.75 to 1.40 | 1.11 | 0.79 to 1.55 |
| 4 | 1.15 | 0.78 to 1.69 | 1.33 | 0.87 to 2.03 |
| 5 | 1.03 | 0.67 to 1.58 | 1.25 | 0.78 to 2.01 |
| 6 | 1.29 | 0.73 to 2.28 | 1.77 | 0.93 to 3.37 |
| 7 | 1.55 | 0.58 to 4.11 | 2.45 | 0.78 to 7.74 |
| Per 1 SD fasting glucose | 1.05 | 0.94 to 1.18 | 1.14 | 1.00 to 1.30 |
| Intensive neonatal care | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.87 | 0.55 to 1.38 | 0.87 | 0.55 to 1.39 |
| 3 | 1.09 | 0.73 to 1.64 | 1.13 | 0.76 to 1.69 |
| 4 | 1.24 | 0.75 to 2.05 | 1.29 | 0.77 to 2.15 |
| 5 | 1.45 | 0.86 to 2.43 | 1.52 | 0.90 to 2.57 |
| 6 | 1.06 | 0.47 to 2.39 | 1.11 | 0.47 to 2.59 |
| 7 | 1.33 | 0.40 to 4.42 | 1.38 | 0.41 to 4.63 |
| Per 1 SD fasting glucose | 1.06 | 0.91 to 1.24 | 1.07 | 0.92 to 1.26 |
| Outcomes by 2-hour post-load glucose categorya and by 1 SD | Unadjusted | Confounder adjusted | ||
| OR | 95% CI | OR | 95% CI | |
| Secondary outcomes | ||||
| Pre-eclampsia | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.87 | 0.49 to 1.53 | 0.75 | 0.42 to 1.34 |
| 3 | 0.97 | 0.55 to 1.71 | 0.79 | 0.44 to 1.43 |
| 4 | 1.15 | 0.57 to 2.35 | 0.77 | 0.36 to 1.62 |
| 5 | 1.10 | 0.52 to 2.33 | 0.78 | 0.35 to 1.71 |
| 6 | 0.93 | 0.27 to 3.16 | 0.64 | 0.18 to 2.25 |
| 7 | 2.08 | 0.70 to 6.19 | 1.63 | 0.53 to 4.99 |
| Per 1 SD fasting glucose | 1.13 | 0.92 to 1.38 | 1.00 | 0.81 to 1.24 |
| Premature delivery | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.07 | 0.70 to 1.62 | 1.05 | 0.69 to 1.60 |
| 3 | 1.03 | 0.67 to 1.58 | 1.01 | 0.65 to 1.56 |
| 4 | 1.19 | 0.68 to 2.07 | 1.17 | 0.67 to 2.06 |
| 5 | 0.89 | 0.48 to 1.64 | 0.91 | 0.49 to 1.67 |
| 6 | 1.61 | 0.76 to 3.42 | 1.64 | 0.76 to 3.53 |
| 7 | – | |||
| Per 1 SD fasting glucose | 1.00 | 0.86 to 1.15 | 1.00 | 0.86 to 1.17 |
| Shoulder dystociab | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 1.24 | 0.40 to 3.80 | 1.12 | 0.36 to 3.46 |
| 3 | 2.39 | 0.86 to 6.68 | 2.29 | 0.79 to 6.65 |
| 4 | 2.07 | 0.55 to 7.75 | 2.01 | 0.50 to 8.04 |
| 5 | 3.53 | 1.11 to 11.22 | 3.45 | 1.04 to 11.51 |
| 6 | 3.13 | 0.60 to 16.36 | 3.05 | 0.59 to 15.73 |
| 7 | 5.23 | 0.99 to 27.58 | 6.18 | 1.09 to 34.96 |
| Per 1 SD fasting glucose | 1.58 | 1.14 to 2.20 | 1.63 | 1.14 to 2.34 |
| Instrumental vaginal deliveryb | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.81 | 0.58 to 1.12 | 0.79 | 0.56 to 1.12 |
| 3 | 1.02 | 0.74 to 1.40 | 1.08 | 0.76 to 1.52 |
| 4 | 1.32 | 0.87 to 2.00 | 1.56 | 0.99 to 2.47 |
| 5 | 1.05 | 0.68 to 1.62 | 1.07 | 0.67 to 1.73 |
| 6 | 1.37 | 0.73 to 2.57 | 1.82 | 0.94 to 3.52 |
| 7 | 1.81 | 0.88 to 3.71 | 1.93 | 0.87 to 4.26 |
| Per 1 SD fasting glucose | 1.10 | 0.98 to 1.24 | 1.16 | 1.02 to 1.32 |
| Intensive neonatal care | ||||
| 1 reference | 1.00 | – | 1.00 | – |
| 2 | 0.79 | 0.52 to 1.19 | 0.77 | 0.50 to 1.17 |
| 3 | 0.78 | 0.51 to 1.19 | 0.76 | 0.50 to 1.16 |
| 4 | 0.90 | 0.51 to 1.57 | 0.88 | 0.50 to 1.54 |
| 5 | 0.80 | 0.45 to 1.44 | 0.79 | 0.44 to 1.41 |
| 6 | 0.43 | 0.13 to 1.41 | 0.43 | 0.13 to 1.42 |
| 7 | 1.27 | 0.49 to 3.30 | 1.28 | 0.49 to 3.36 |
| Per 1 SD fasting glucose | 0.99 | 0.85 to 1.15 | 0.98 | 0.85 to 1.15 |
FIGURE 45.
Frequency of secondary outcomes across glucose categories by ethnicity: WB, n = 3888; and SA, n = 4821. (a) Pre-eclampsia; (b) premature delivery; (c) shoulder dystocia; (d) instrumental vaginal delivery; and (e) admission to neonatal unit. Glucose categories are defined as follows: FPG level – category 1, < 4.3 mmol/l; category 2, 4.3–4.4 mmol/l; category 3, 4.5–4.7 mmol/l; category 4, 4.8–4.9 mmol/l; category 5, 5.0–5.2 mmol/l; category 6, 5.3–5.6 mmol/l; category 7, 5.7–6.0 mmol/l. Post-load plasma glucose level – category 1, < 4.7 mmol/l; category 2, 4.7–5.4 mmol/l; category 3, 5.5–6.2 mmol/l; category 4, 6.3–6.6 mmol/l; category 5, 6.7–7.2 mmol/l; category 6, 7.3–7.5 mmol/l; category 7, 7.6–7.7 mmol/l. For plots of shoulder dystocia and instrumental vaginal delivery, women who had a C-section are excluded, therefore n = 3018 for WB and n = 3914 for SA.
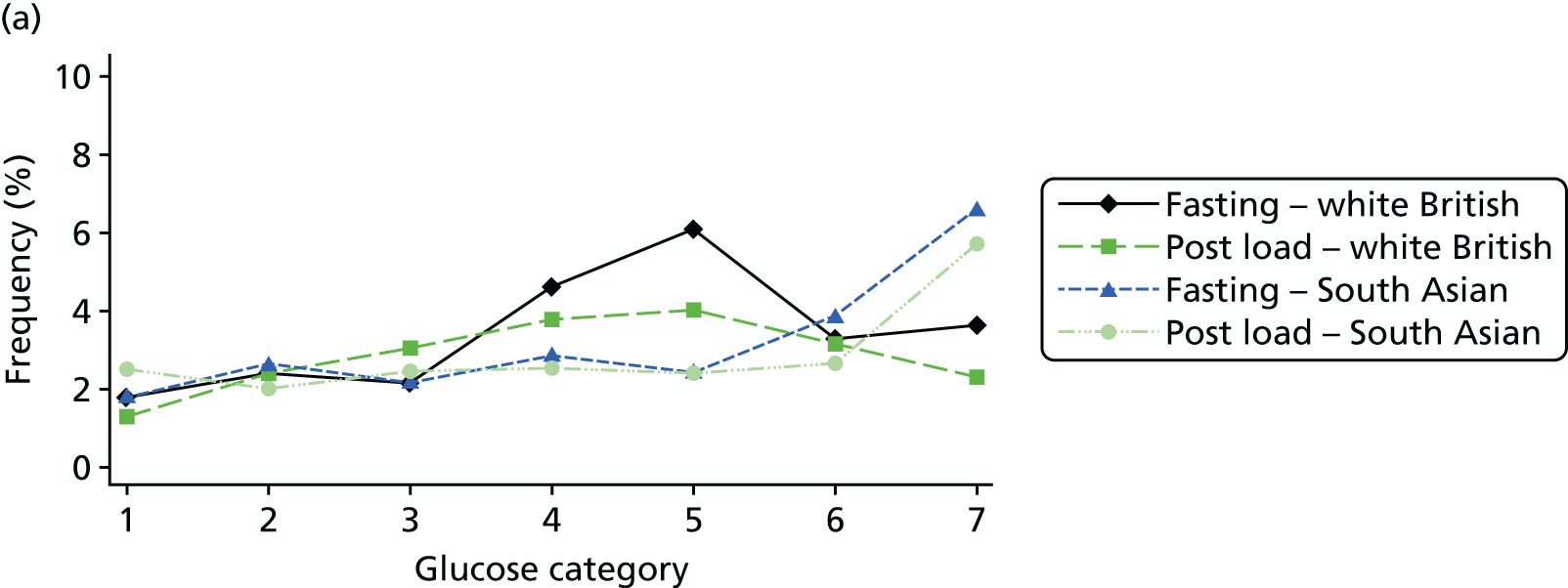

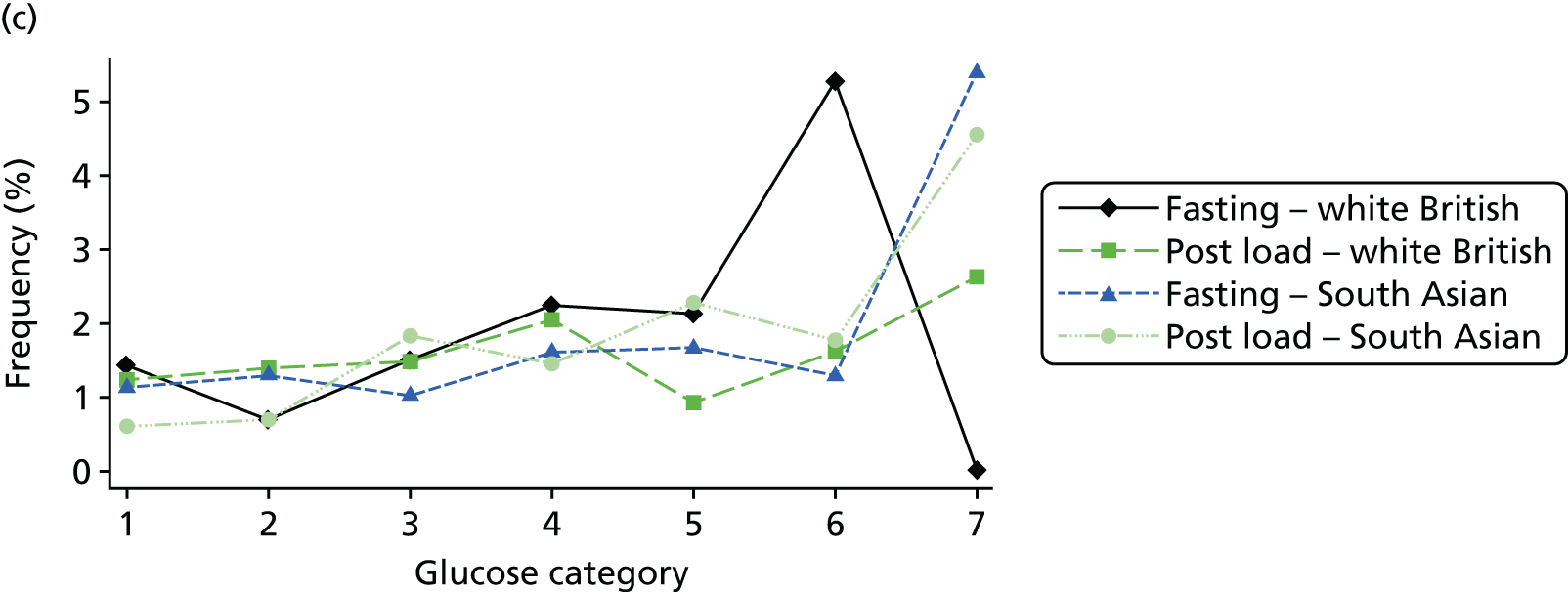


FIGURE 46.
Frequency of secondary outcomes across glucose categories for all pregnancies (N = 9509). (a) Pre-eclampsia; (b) premature delivery; (c) shoulder dystocia; (d) instrumental vaginal delivery; and (e) admission to neonatal unit. Vaginal births only for shoulder dystocia and instrumental delivery. Glucose categories are defined as follows: FPG level–category 1, < 4.3 mmol/l; category 2, 4.3–4.4 mmol/l; category 3, 4.5–4.7 mmol/l; category 4, 4.8–4.9 mmol/l; category 5, 5.0–5.2 mmol/l; category 6, 5.3–5.6 mmol/l; category 7, 5.7–6.0 mmol/l. Post-load plasma glucose level–category 1, < 4.7 mmol/l; category 2, 4.7–5.4 mmol/l; category 3, 5.5–6.2 mmol/l; category 4, 6.3–6.6 mmol/l; category 5, 6.7–7.2 mmol/l; category 6, 7.3–7.5 mmol/l; category 7, 7.6–7.7 mmol/l. For plots of shoulder dystocia and instrumental vaginal delivery, women who had a C-section are excluded, therefore n = 3018 for WB and n = 3914 for SA.
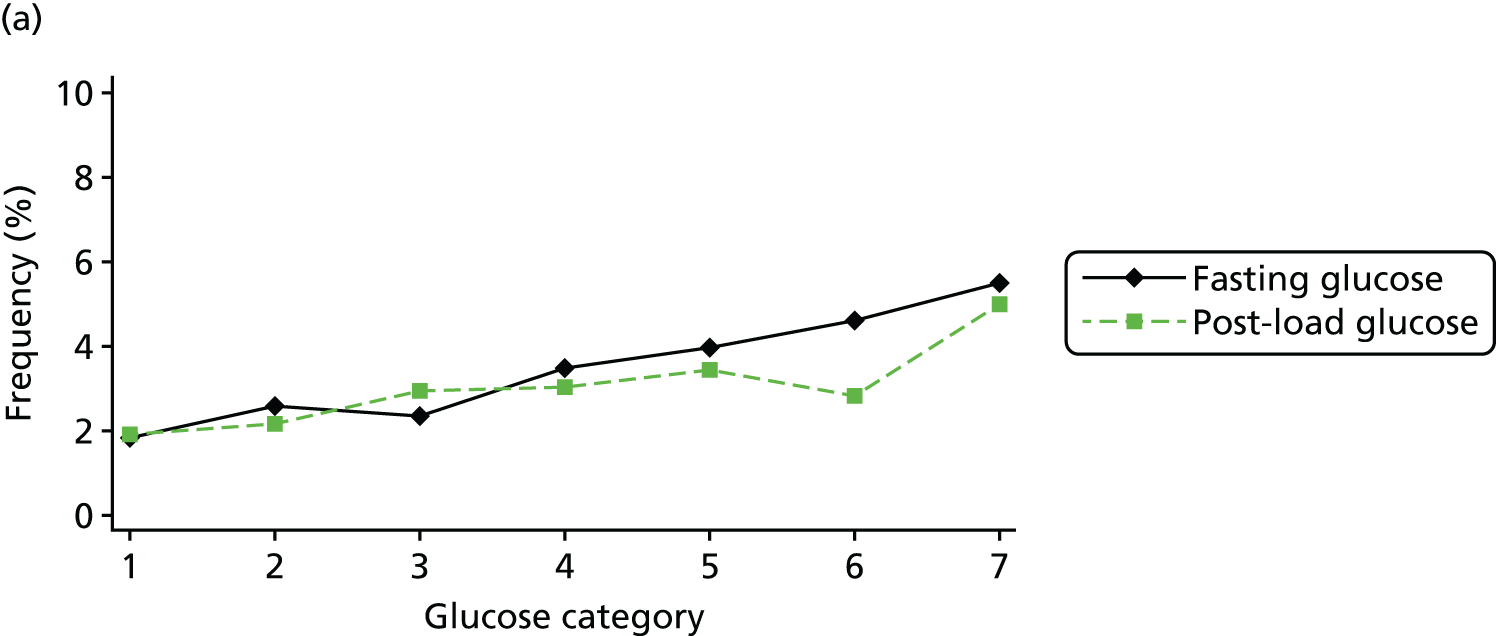
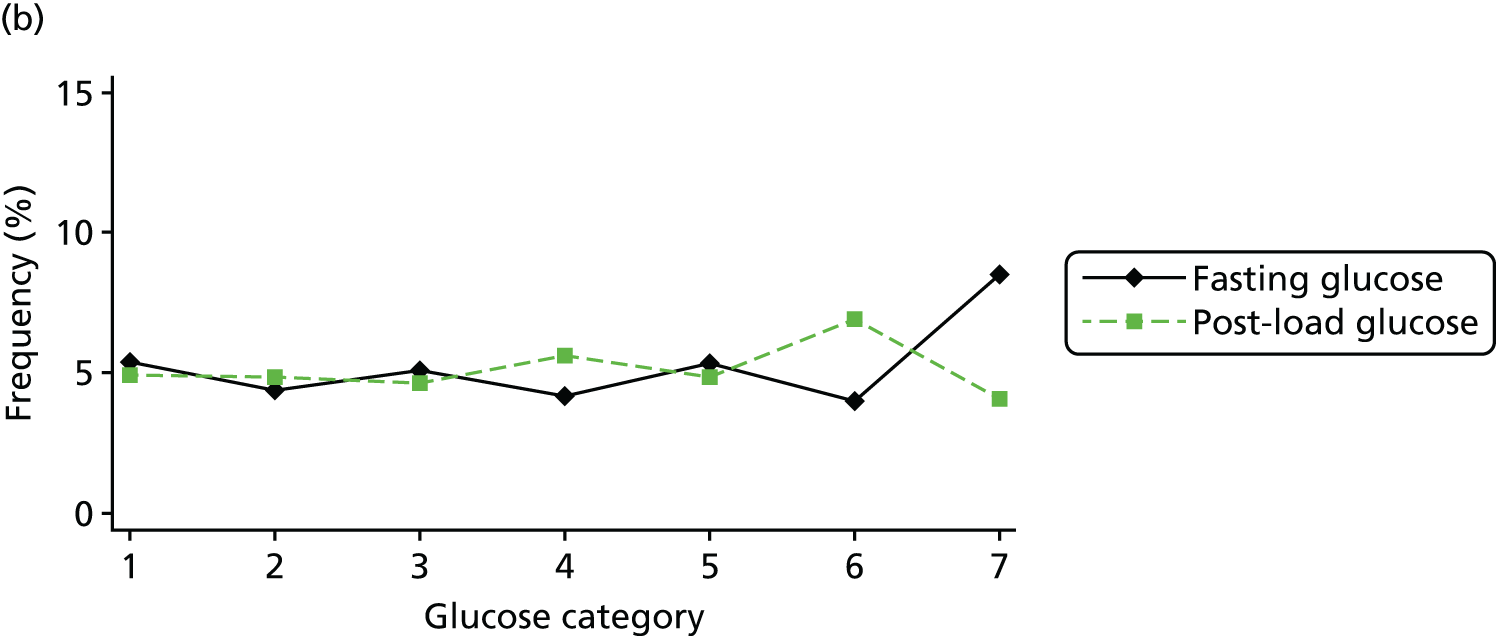
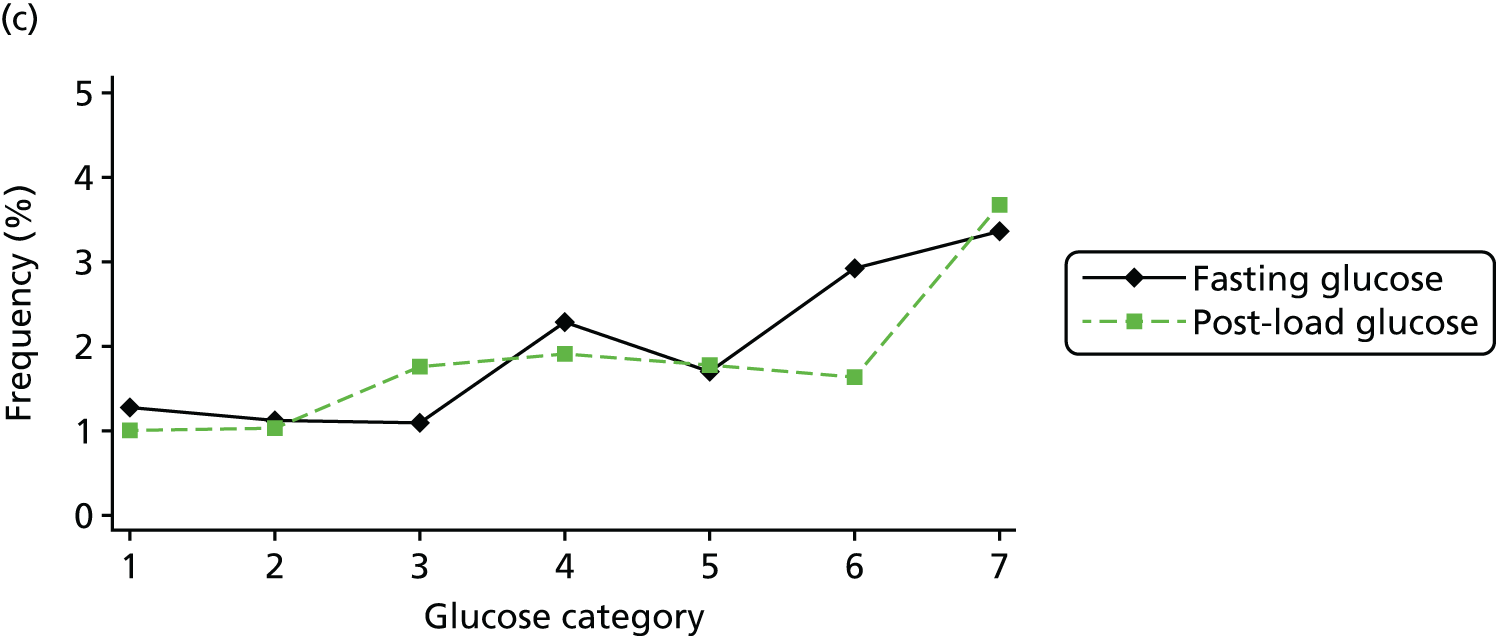

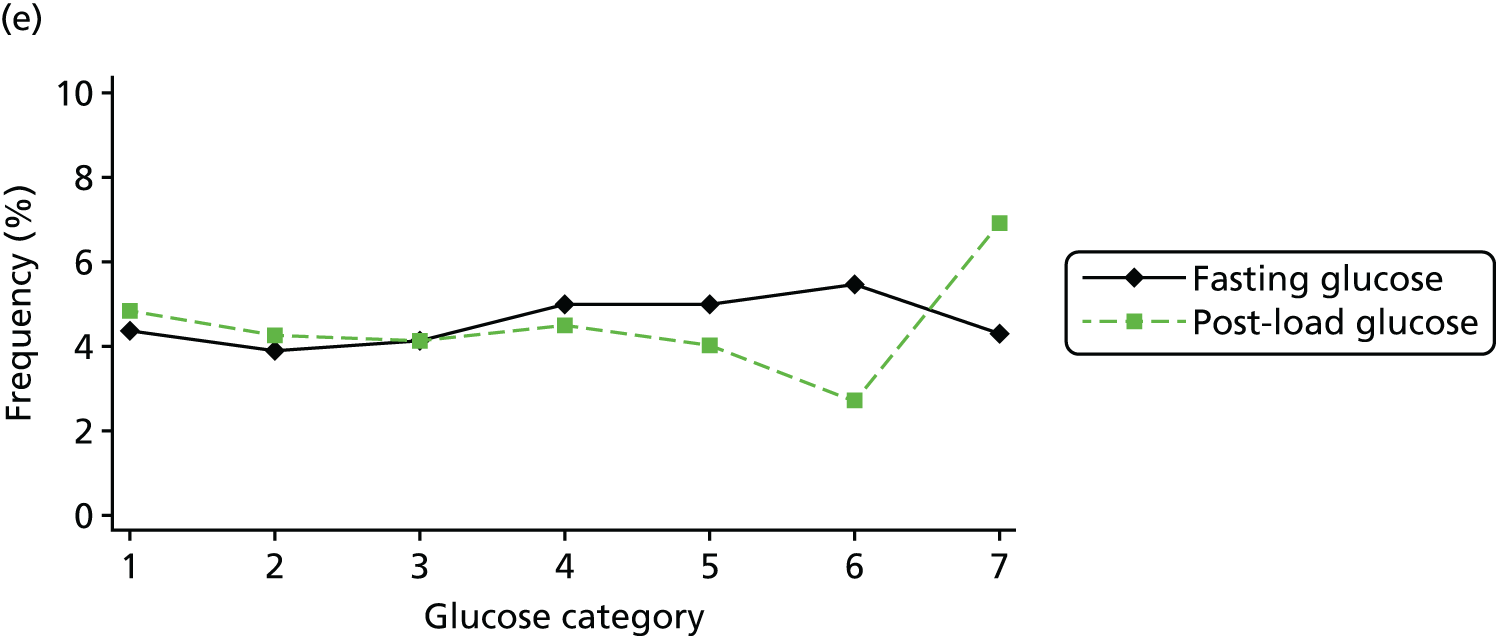
Appendix 2 Tables and figures for Chapter 3
| Study | Publication year | Prospective or retrospective | Representative population | Loss to follow-up | Measurement of: | ‘Blinding’ of: | Selective reporting | Adjusted results presented | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Consistent glucose | Consistent outcome | Glucose measurements | Outcomes | |||||||
| Aberg88 | 2001 | R case–control | Low | Low | Low | Low | Unclear | Unclear | Low | High |
| Aris65 | 2014 | P | Low | Low | Low | Low | Unclear | Unclear | Low | Low |
| Atlantic DIP59 | – | P | Low | Low | Low | Low | Low | Low | Low | Low |
| BiB22 | – | P | Low | Low | Low | Low | Low | Low | Low | Low |
| Black110 | 2010 | R | Low | Low | Low | Low | High | High | Low | Low |
| Carr66 | 2011 | R | Low/moderate | Low | Low | Low | High | High | Low | Low |
| Chadna67 | 2006 | R | Unclear | Low | Unclear | Unclear | High | High | Unclear | High |
| Cheng68 | 2007 | R | Low | Low | Low | Unclear | High | High | Unclear | Low |
| Dudhbhai89 | 2006 | R | Low | Low | Low | Low | High | High | Low | High |
| Figueroa64 | 2013 | Secondary RCT | Low (but subset of trial) | Low | Low | Low | Unclear | Unclear | Low | Low |
| Forest90 | 1994 | R | Low | Low | Low | Low | High | High | Low | High |
| Franks111 | 2006 | P | High (Pima Indian) | High | Low | Unclear | Unclear | Unclear | Low | Limited adjustment |
| HAPO36 | 2009 | P | Low | Low | Low | Low | Low | Low | Low | Low |
| HAPO6 | 2008 | P | Low | Low | Low | Low | Low | Low | Low | Low |
| HAPO69 | 2010 | P | Low | Low | Low | Low | Low | Low | Low | Low |
| Hedderson91 | 2003 | R | Low | Low | Low | Low | High | High | Low | High |
| Herman92 | 1988 | R | Low | Low | Low | Unclear | High | High | High | High |
| Hillier108 | 2007 | Unclear | Low | Low | Low | Low | High | High | Low | Unclear |
| Hillier70 | 2008 | Unclear | Low | Low | Low | Unclear | Unclear | Unclear | Unclear | Low |
| Jensen71 | 2001 | R | High (higher-risk group) | Low | Low | Low | High | High | Low | High |
| Jensen112 | 2008 | R | High (higher-risk group) | Low | Low | Low | High | High | Low | High |
| Jiménez-Moleón93 | 2002 | R | Low | Low | Low | Low | High | High | Low | High |
| Kerényi72 | 2009 | Unclear | Low | Low | Low | Low | Unclear | Unclear | Unclear | Low |
| Khan114 | 1994 | R | Unclear/high-risk (Pakistani population) | Low | Low | Low | High | High | Unclear | High |
| Khoshniat94 | 2010 | P | Unclear (Iranian) | Low | Low | Unclear | Unclear | Unclear | Unclear | High |
| Landon61 | 2011 | Secondary analyses of RCT data | Low (but subset of trial) | Low | Low | Low | Unclear | Low | Low | Low |
| Langer95 | 2005 | P case–control | Low | Unclear | Low | Low | High | High | Low | High |
| Lapolla96 | 2007 | P | Low | High | Low | Low | Unclear | Unclear | Low | High |
| Lao73 | 2003 | R | Low (Chinese) | Low | Low | Low | High | High | Low | High |
| Little74 | 1990 | P | Low | Low | Low | Unclear | Unclear | Unclear | Unclear | High |
| Lurie75 | 1998 | P | Low | Low | Low | Low | Unclear | Low | Low | High |
| Ma97 | 2013 | P | Low | Unclear | Low | Low | High | Low | Unclear | High |
| Metzger76 | 2010 | P | Low | Low | Low | Low | Low | Low | Low | Low |
| Moses77 | 1995 | P | Low | Unclear | Low | Low | Unclear | Unclear | Low | High |
| Naylor98 | 1996 | P | Low | Low | Low | Unclear | Low | Unclear | Unclear | High |
| Nord99 | 1995 | Unclear | Unclear | Low | Low | Unclear | Unclear | Unclear | Unclear | High |
| Ong78 | 2008 | R | Low | Low | Low | Unclear | High | High | Unclear | High |
| Özekinci100 | 2011 | R | Low | Low | Low | Low | High | High | Unclear | High |
| Pettitt79 | 1980 | P | High (Pima Indian) | Low | Low | Low | Unclear | Unclear | Unclear | High |
| Pettitt109 | 1991 | P | High (Pima Indian) | Unclear | Low | Low | Unclear | Unclear | Low | Limited adjustment |
| Pettitt41 | 2010 | P | Low | Low | Low | Low | Unclear | Unclear | Low | Low |
| Pugh101 | 2010 | P (matched) | Low | Low | Low | Unclear | Unclear | Unclear | Unclear | Low |
| Retnakaran102 | 2008 | P | Low | Low | Low | Low | Unclear | Unclear | Low | High |
| Riskin-Mashiah80 | 2009 | R | Low | Low | Low | Low | High | High | Low | Limited adjustment |
| Savona-Ventura81 | 2010 | R | Low | Low | Low | Unclear | High | High | Unclear | High |
| Scholl82 | 2001 | P | Low | Low | Low | Low | Unclear | Unclear | Low | Low |
| Sermer83 | 1995 | P | Low | Low | Low | Low | Low | Low | Low | High |
| Stamilo103 | 2004 | R | Low | Low | Low | Low | High | High | Low | Low |
| Subramaniam 84 | 2014 | R | Low | Low | Low | Unclear | High | High | Unclear | Low |
| Tallarigo85 | 1986 | Unclear | Low | Low | Low | Low | Unclear | Unclear | Low | High |
| Tarim104 | 2011 | R | Low | Low | Low | Low | High | High | Unclear | High |
| Vambergue105 | 2000 | P case–control | Low | Low | Low | Low | Unclear | Unclear | Low | High |
| Wang106 | 2013 | R | Low | Low | Low | Low | High | High | Low | Low |
| Witter86 | 1988 | R | Low, but young age group | Low | Low | Low | High | High | Low | High |
| Yee87 | 2011 | R | Low | Low | Low | Low | High | High | Low | Low |
| Yogev107 | 2005 | P | Low | Low | Low | Low | Unclear | Unclear | Low | High |
| Outcomea | No. of studies | Log OR of glucose squared | 95% CI | p-value |
|---|---|---|---|---|
| Fasting 75-g OGTT | ||||
| C-section | 6 | –0.115 | –0.25 to 0.02 | 0.1 |
| Induction | 3 | –0.197 | –0.52 to 0.13 | 0.23 |
| Instrumental birth | 3 | 0.107 | –0.21 to 0.42 | 0.5 |
| LGA | 7 | –0.02 | –0.16 to 0.12 | 0.77 |
| Macrosomia | 6 | –0.18 | –0.39 to 0.03 | 0.09 |
| Neonatal hypoglycaemia | 2 | 0.29 | 0.05 to 0.53 | 0.02 |
| PIH/pre-eclampsia | 3 | 0.461 | 0.03 to 0.9 | 0.04 |
| Pre-eclampsia | 4 | –0.005 | –0.28 to 0.27 | 0.97 |
| Preterm birth | 3 | 0.577 | 0.09 to 1.07 | 0.02 |
| Shoulder dystocia | 4 | –0.142 | –1.06 to 0.78 | 0.76 |
| 2-hour 75-g OGTT | ||||
| C-section | 9 | –0.016 | –0.03 to 0.00 | 0.06 |
| Induction | 3 | 0.006 | –0.04 to 0.05 | 0.81 |
| Instrumental birth | 4 | –0.01 | –0.05 to 0.03 | 0.65 |
| LGA | 11 | 0.004 | –0.01 to 0.02 | 0.67 |
| Macrosomia | 7 | 0.006 | –0.03 to 0.05 | 0.77 |
| Neonatal hypoglycaemia | 3 | 0.002 | –0.02 to 0.03 | 0.91 |
| PIH/pre-eclampsia | 3 | 0.02 | –0.07 to 0.11 | 0.67 |
| Pre-eclampsia | 4 | –0.026 | –0.05 to 0.00 | 0.05 |
| Preterm birth | 6 | 0.009 | –0.05 to 0.07 | 0.78 |
| Shoulder dystocia | 5 | –0.067 | –0.19 to 0.06 | 0.29 |
| 50-g OGCT | ||||
| C-section | 7 | –0.029 | –0.07 to 0.01 | 0.18 |
| Instrumental birth | 2 | –0.008 | –0.08 to 0.07 | 0.84 |
| LGA | 4 | –0.044 | –0.1 to 0.01 | 0.11 |
| Macrosomia | 7 | –0.004 | –0.02 to 0.02 | 0.69 |
| Neonatal hypoglycaemia | 3 | 0.047 | –0.18 to 0.27 | 0.68 |
| Pre-eclampsia | 6 | –0.082 | –0.15 to –0.02 | 0.01 |
| Preterm birth | 2 | 0.021 | –0.05 to 0.09 | 0.55 |
| Shoulder dystocia | 2 | –0.113 | –0.25 to 0.03 | 0.12 |
| Article | Reason for exclusion | |
|---|---|---|
| 1 | Abdel-Wareth LO, Kumari AS, Haq A, Bakir A, Sainudeen A, Sedaghatian MR, et al. An evaluation of the latest ADA criteria for screening and diagnosing gestational diabetes at a tertiary care hospital in the United Arab Emirates. Int J Diabetes Metab 2006;14:55–60 | Results by diagnostic criteria not by glucose levels |
| 2 | Abell DA, Beischer NA, Wood C. Routine testing for gestational diabetes, pregnancy hypoglycemia and fetal growth retardation, and results of treatment. J Perinatal Med 1976;4:197–212 | Comparison of women with GDM and diabetes prior to commencement of pregnancy. Treatment was administered including admission to hospital, dietary control and insulin therapy |
| 3 | Abell DA. The significance of abnormal glucose tolerance in pregnancy. Aust N Z J Obstet Gynaecol 1978;18:17–20 | Glucose levels were analysed in three different groups; hypoglycaemia, normoglycaemia, and hyperglycaemia. Glucose levels are poorly defined |
| 4 | Abell DA. The significance of abnormal glucose tolerance (hyperglycaemia and hypoglycaemia) in pregnancy. Br J Obstet Gynaecol 1979;86:214–21 | Glucose levels were analysed in three different groups; hypoglycaemia, normoglycaemia, and hyperglycaemia. Glucose levels are poorly defined |
| 5 | Anazawa S, Kitamura S, Matsuoka K. Diabetologic and obstetric analysis of abnormal glucose tolerance during pregnancy. [Japanese]. J Japan Diabetes Soc 1985;28:747–53 | Not in English |
| 6 | Anderberg E, Kallen K, Berntorp K. The impact of gestational diabetes mellitus on pregnancy outcome comparing different cut-off criteria for abnormal glucose tolerance. Acta Obstet Gynecol Scand 2010;89:1532–7 | Only one group without GDM, comparison of women with GDM with women without GDM |
| 7 | Anthony R, Ikomi A, Khan R, Angala P, Kiss S. Clinical outcomes in pregnant women newly reclassified as gestational diabetes (GDM) using IADPSG criteria. 16th Annual Conference of the British Maternal and Fetal Medicine Society; April; Dublin, Ireland: Archives of Disease in Childhood: Fetal and Neonatal Edition; 2013 | Conference abstract: no relevant data |
| 8 | Atia HC, Koren Y, Weintraub AY, Novack L, Sheiner E. Is a value of over 200mg/dL in the oral glucose tolerance test, a marker of severity in patients with gestational diabetes mellitus? J Matern Fetal Neonatal Med 2013;26:1259–62 | Comparison of women with GDM with at least one value over 200 mg/dl in the glucose tolerance test and those women with GDM without any value 200 mg/dl Treatment consisted of diet control |
| 9 | Basu A, Bhatti N, Lee BC. Pregnancy outcome in women with gestational diabetes: results of a four-year audit. Practical Diabetes 2012;29:237–42 | Treatment study |
| 10 | Basu A, Parghi S. Pregnancy outcome in women with pregestational diabetes mellitus at a district general hospital in Australia. Practical Diabetes 2012;29:372–7 | Treatment study |
| 11 | Black MH, Sacks DA, Li X, Lawrence JM. Examining the thresholds for diagnosing gestational diabetes mellitus (GDM): How many adverse outcomes will be missed? Diabetes 2014;63:A361 | Conference abstract: no relevant data |
| 12 | Breschi MC, Seghieri G, Bartolomei G, Gironi A, Baldi S, Ferrannini E. Relation of birthweight to maternal plasma glucose and insulin concentrations during normal pregnancy. Diabetologia 1993;36:1315–21 | Study does not present the relation between any relevant adverse outcomes and categorical or continuous glucose levels Study represents a BW regression analysis only |
| 13 | Bush NC, Chandler-Laney PC, Rouse DJ, Granger WM, Oster RA, Gower BA. Higher maternal gestational glucose concentration is associated with lower offspring insulin sensitivity and altered beta-cell function. J Clin Endocrinol Metab 2011;96:E803–9 | No relevant outcomes |
| 14 | Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A1c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab 2005;90:3225–9 | Results not presented by glucose level and no relevant outcomes |
| 15 | Damm P, Kühl C, Bertelsen A, Mølsted-Pedersen L. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol 1992;167:607–16 | Comparison of women with GDM and controls Women with GDM were treated with diet alone, oral hypoglycaemic agents or insulin therapy Study also does not present categorical or continuous glucose levels |
| 16 | Darling AM, Liu E, Aboud S, Urassa W, Spiegelman D, Fawzi W. Maternal hyperglycemia and adverse pregnancy outcomes in Dar es Salaam, Tanzania. Int J Gynecol Obstet 2014;125:22–7 | Ineligible glucose test (RFG, repeat fasting glucose) |
| 17 | Di Cianni G, Lencioni C, Volpe L, Ghio A, Cuccuru I, Pellegrini G, et al. C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diabetes Metab Res Rev 2007;23:135–40 | Comparison of women with GDM and controls |
| 18 | Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol 2007;47:307–13 | Glucose levels were analysed in three different groups, control, mild GDM and GDM A treatment package of dietary modification, blood glucose monitoring and insulin therapy were offered to those with mild GDM or GDM |
| 19 | Dong L, Liu E, Guo J, Pan L, Li B, Leng J, et al. Relationship between maternal fasting glucose levels at 4-12 gestational weeks and offspring growth and development in early infancy. Diabetes Res Clin Pract 2013;102:210–7 | Ineligible glucose test (FPG at 4–12 weeks) |
| 20 | Farmer G, Russell G, Hamilton-Nicol DR, Ogenbede HO, Ross IS, Pearson DW, et al. The influence of maternal glucose metabolism on fetal growth, development and outcome in 917 singleton pregnancies in nondiabetic women. Diabetologia 1988;31:134–41 | Results not presented in glucose categories. Presented by the following categories; summed plasma glucose, insulin response, FPG and indices of glucose disposal |
| 21 | Godbout A, Chastang N, Laubies A, Golmard JL, Vauthier-Brouzes D, Jacqueminet S, et al. Gestational diabetes: increasing therapeutic glucose level in pregnant women without risk factors of fetal overweight. 70th Scientific Sessions of the American Diabetes Association; 2010; Orlando, FL, USA: Diabetes | Conference abstract: ineligible glucose test (FBG, PPBG) |
| 22 | Godwin M, Muirhead M, Huynh J, Helt B, Grimmer J. Prevalence of gestational diabetes mellitus among Swampy Cree women in Moose Factory, James Bay. CMAJ 1999;16:1299–302 | Comparison of women with and without GDM only |
| 23 | Gui J, Li A, Su X, Feng L. Association between hyperglycemia in middle and late pregnancy and maternal-fetal outcomes: a retrospective study. BMC Pregnancy Childbirth 2014;14:34 | All women had GDM or DM, and a large proportion of women in each group received treatment |
| 24 | Heerey AM, Carmody L, Kirwan B, Dunne FP, Egan M. ATLANTIC DIP: Are the IADPSG criteria for GDM missing women who would previously have been identified with GDM using WHO criteria? Diabetes 2013;62:A376–7 | Conference abstract Only one non-GDM group reported |
| 25 | Herman G, Raimondi B. Glucose tolerance, fetal growth, and pregnancy complications in normal women. Am J Perinatol 1988;5:168–71 | Only one non-GDM group reported (by current definition) |
| 26 | Herrera K, Brustman L, Foroutan J, Suffecool K, Scarpelli S, Rosenn B. Does the number of abnormal values on the one step 2 hour (GTT) correlate with the severity of gestational diabetes? Reproductive Sci 2013;1:246A | Conference abstract Examines outcomes by timing of glucose abnormality |
| 27 | Heude B, Thiébaugeorges O, Goua V, Forhan A, Kaminski M, Foliguet B, et al. Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Maternal Child Health J 2012;16:355–64 | No results by glucose category, no relevant outcomes by glucose levels |
| 28 | Hill JC, Krishnaveni GV, Annamma I, Leary SD, Fall CH. Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstet Gynecol Scand 2005;84:159–65 | Comparison of women with and without GDM only |
| 29 | Hiramatsu Y, Masuyama H, Mizutani Y, Kudo T, Oguni N, Oguni Y. Heavy-for-date infants: their backgrounds and relationship with gestational diabetes. J Obstet Gynaecol Res 2000;26:193–8 | Study does not present the relation between any relevant adverse outcomes and categorical or continuous glucose levels Study represents a BW graphical correlation analysis |
| 30 | Jensen DM, Damm P, Sørensen B, Mølsted-Pedersen L, Westergaard JG, Korsholm L, et al. Proposed diagnostic thresholds for gestational diabetes mellitus according to a 75-g oral glucose tolerance test. Maternal and perinatal outcomes in 3260 Danish women. Diabet Med 2003;20:51–7 | Duplicate cohort of Jensen 200171 but without more limited data presentation |
| 31 | Kaufmann RC, McBride P, Amankwah KS, Huffman DG. The effect of minor degrees of glucose intolerance on the incidence of neonatal macrosomia. Obstet Gynecol 1992;80:97–101 | Comparison of women with and without GDM, women with GDM are likely to have been treated The glucose levels in the control group are not reported |
| 32 | Kaymak O, Iskender CT, Ustunyurt E, Yildiz Y, Doganay M, Danisman N. Retrospective evaluation of perinatal outcome in women with mild gestational hyperglycemia. J Obstet Gynaecol Res 2011;37:986–91 | The number of untreated groups is unclear It appears that there is only one group without GDM |
| 33 | Khan MS, Kinsley BT, Daly S, McCarthy A. Increased birthweight and shoulder dystocia with fasting plasma glucose (FPG) levels between 5.1 and 5.7 mmol/l in screening for gestational diabetes mellitus (GDM). Ir J Med Sci 2011;180:S507 | Abstract only Comparison of GDM definitions |
| 34 | Kim S, Min WK, Chun S, Lee W, Chung HJ, Lee PR, et al. Quantitative risk estimation for large for gestational age using the area under the 100-g oral glucose tolerance test curve. J Clin Lab Analysis 2009;23:231–7 | No results by glucose category |
| 35 | Korucuoglu U, Biri A, Turkyilmaz E, Doga Yildirim F, Ilhan M, et al. Glycemic levels with glucose loading test during pregnancy and its association with maternal and perinatal outcomes. Diabetes Res Clin Pract 2008;80:69–74 | GDM in groups 2, 3, 4 and 5 possible |
| 36 | Langhoff-Roos J, Wibell L, Gebre-Medhin M, Lindmark G. Maternal glucose metabolism and infant birthweight: a study in healthy pregnant women. Diabetes Res 1988;8:165–70 | Used an intravenous glucose test |
| 37 | Lapolla A, Dalfra M, Ragazzi E, De Cata A, Masin M, Bonsembiante B, et al. Analysis of pregnancies after new IADPSG recommendation. Diabetologia 2010;53:S9–10 | Conference abstract for 38 |
| 38 | Lapolla A, Dalfra MG, Ragazzi E, De Cata AP, Fedele D. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria. retrospective study on pregnancy outcome. Diabet Med 2011;28:1074–7 | Only one non-GDM group reported |
| 39 | Lauszus FF, Paludan J, Klebe JG. Birthweight in women with potential gestational diabetes mellitus: an effect of obesity rather than glucose intolerance? Acta Obstet Gynecol Scand 1999;78:520–5 | Two groups glucose levels not defined, results not presented by glucose levels |
| 40 | Leikin EL, Jenkins JH, Pomerantz GA, Klein L. Abnormal glucose screening tests in pregnancy: a risk factor for fetal macrosomia. Obstet Gynecol 1987;69:570–3 | Women with abnormal glucose screening tests and either one or no abnormal values on their glucose tolerance test were compared to women with normal glucose screening tests |
| 41 | Leung TW, Lao TT. Placental size and large-for-gestational-age infants in women with abnormal glucose tolerance in pregnancy. Diabet Med 2000;17:48–52 | No results by glucose category, results by LGA, AGA and SGA |
| 42 | Liao S, Mei J, Song W, Liu Y, Tan YD, Chi S, et al. The impact of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) fasting glucose diagnostic criterion on the prevalence and outcomes of gestational diabetes mellitus in Han Chinese women. Diabet Med 2014;31:341–51 | Compares different diagnostic criteria, groups diagnosed with GDM |
| 43 | Lin CH, Wen SF, Wu YH, Huang MJ. Using the 100-g oral glucose tolerance test to predict fetal and maternal outcomes in women with gestational diabetes mellitus. Chang Gung Med J 2009;32:283–9 | Comparison of women with GDM and controls Women with GDM were given medical nutrition counselling, monitoring of blood glucose and, in some cases, insulin therapy |
| 44 | Liu J, Leng J, Tang C, Liu G, Hay J, Wang J, et al. Maternal glucose level and body mass index measured at gestational diabetes mellitus screening and the risk of macrosomia: results from a perinatal cohort study. BMJ Open 2014;4:e004538 | Does not exclude women with positive OGTT (and treated), therefore the association would be biased |
| 45 | Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012;35:574–80 | Does not present results by glucose categories only present data by maternal A1C |
| 46 | Macafee CAJ, Beischer NA, Willis MM, Wood C. The correlation of fetal, placental and maternal weight with glucose tolerance. Aust N Z J Obstet Gynaecol 1974;14:88–94 | Study does not present categorical or continuous glucose levels Data presented in terms of average glucose level according to outcome |
| 47 | Mello G, Parretti E, Mecacci F, Lucchetti R, Lagazio C, Pratesi M, et al. Risk factors for fetal macrosomia: the importance of a positive oral glucose challenge test. Eur J Endocrinol 1997;137:27–33 | Results not presented by glucose level or categories |
| 48 | Mello G, Parretti E, Cioni R, Lucchetti R, Carignani L, Martini E, et al. The 75-gram glucose load in pregnancy: relation between glucose levels and anthropometric characteristics of infants born to women with normal glucose metabolism. Diabetes Care 2003;26:1206–10 | Study does not present the relation between any relevant adverse outcomes and categorical or continuous glucose levels Study presents complicated glucose testing with a single OR |
| 49 | Metzger BE, Lowe LP, Dyer AR, Trimble ER, Persson B, Oats JJN, et al. The Hyperglycemia & Adverse Pregnancy Outcome (HAPO) Study: Perinatal Outcome In Pregnancies with GDM and Fasting Plasma Glucose (FPG) < 4.4 mmol/l. 70th Scientific Sessions of the American Diabetes Association Orlando, FL, USA, 2010 | Conference abstract Only one non-GDM group reported |
| 50 | Nasrat AA, Augensen K, Abushal M, Shalhoub JT. The outcome of pregnancy following untreated impaired glucose tolerance. Int J Gynecol Obstet 1994;47:1–6 | Only one non-GDM group (reported by current definition) |
| 51 | Negrato CA, Jovanovic L, Tambascia MA, Calderon M, Geloneze B, Dias A, et al. Mild gestational hyperglycaemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab Res Rev 2008;24:324–31 | Results not presented by glucose level, metabolic syndrome only |
| 52 | Nobile de Santis MS, Taricco E, Radaelli T, Spada E, Rigano S, Ferrazzi E, et al. Growth of fetal lean mass and fetal fat mass in gestational diabetes. Ultrasound Obstet Gynecol 2010;36:328–37 | Comparison of women with GDM and controls |
| 53 | Nordin NM, Wei JW, Naing NN, Symonds EM. Comparison of maternal-fetal outcomes in gestational diabetes and lesser degrees of glucose intolerance. J Obstet Gynaecol Res 2006;32:107–14 | Comparison of women with and without GDM plus impaired glucose Those women with GDM/impaired glucose were treated with insulin therapy or diet control The glucose levels in the control group were not reported |
| 54 | Okada T, Iwashina M, Kasatani T, Kanno H, Yoshie M, Morikawa K, et al. Clinical outcomes of pregnancies complicated with and treated for gestational diabetes mellitus: Consequences of screening under the IADPSG criteria. Diabetol Int 2013;4:186–9 | Only one non-GDM group reported |
| 55 | Peters CJ, Kayemba-Kays S, Geary MPP, Hindmarsh PC. Blood glucose in multiparous women influences offspring birth size but not size at 2 years of age. J Clin Endocrinol Metab 2013;98:4916–22 | No relevant data (only correlations) |
| 56 | Peters CJ, Kayemba-Kays S, Geary MP, Hindmarsh PC. Blood glucose in multiparous women influences offspring birth size but not size at 2 years of age. Diabetes 2013;62:A360 | Conference abstract: no relevant data |
| 57 | Pettitt DJ, Bennett PH, Knowler WC. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy. Long-term effects on obesity and glucose tolerance in the offspring. Diabetes 1985;34(Suppl. 2):119–22 | Duplicate cohort of Pettitt 198079 but without more limited data presentation |
| 58 | Saleh J, Machado L, Razvi Z. 2-Hour post-load serum glucose levels and maternal blood pressure as independent predictors of birthweight in ‘appropriate for gestational age’ neonates in healthy nondiabetic pregnancies. Biomed Res Int 2013:757459 | Did not report on required outcomes |
| 59 | Savona-Ventura C, Chircop M. Significant thresholds for the 75-g oral glucose tolerance test in pregnancy. J Diabetes Complications 2008;22:178–80 | Comparison of women with and without GDM plus impaired glucose Those women with GDM/impaired glucose are likely to have been treated The glucose levels in the control group were not reported |
| 60 | Stuebe A. Is there a threshold OGTT value for predicting adverse neonatal outcome? Am J Obstet Gynecol 2011;204(Suppl. 1):216 | Conference abstract: no relevant data |
| 61 | Ouzilleau C, Roy MA, Leblanc L, Carpentier A, Maheux P. An observational study comparing 2-hour 75-g oral glucose tolerance with fasting plasma glucose in pregnant women: both poorly predictive of birthweight. CMAJ 2003;168:403–9 | No relevant outcomes, only gives average BW percentiles (via box-and-whisker plots) Pregnant women received ‘minimal’ treatment when diagnosed with GDM |
| 62 | Phillipou G. The 1-h 50-g glucose challenge does not predict large-for-gestational-age infants. Diabet Med 1992;9:81–3 | Study does not present categorical or continuous glucose levels Data presented in terms of average glucose level according to BW percentile |
| 63 | Sacks DA, Abu-Fadil S, Karten GJ, Forsythe AB, Hackett JR. Screening for gestational diabetes with the one-hour 50-g glucose test. Obstet Gynecol 1987;70:89–93 | Study of prediction of GDM |
| 64 | Sacks DA, Greenspoon JS, Abu-Fadil S, Henry HM, Wolde-Tsadik G, Yao JF. Toward universal criteria for gestational diabetes: the 75-gram glucose tolerance test in pregnancy. Am J Obstet Gynecol 1995;172:607–14 | Study does not present the relation between any relevant adverse outcomes and categorical or continuous glucose levels Study presents ROC curves and ORs and graphical presentation of percentage with outcome as increasing plasma glucose levels |
| 65 | Schrader HM, Jovanovic-Peterson L, Bevier WC, Peterson CM. Fasting plasma glucose and glycosylated plasma protein at 24 to 28 weeks of gestation predict macrosomia in the general obstetric population. Am J Perinatol 1995;12:247–51 | Study does not present the relation between any relevant adverse outcomes and categorical or continuous glucose levels Study represents a BW graphical correlation analysis |
| 66 | Verma A, Mitchell BF, Demianczuk N, Flowerdew G, Okun NB. Relationship between plasma glucose levels in glucose-intolerant women and newborn macrosomia. J Matern Fetal Med 1997;6:187–93 | Comparison of women with GDM and ‘GCT positive and OGCT negative’ 26% of the women with GDM were treated with insulin therapy Case–control study presenting data by outcome rather than glucose category |
| 67 | Willman SP, Leveno KJ, Guzick DS, Williams ML, Whalley PJ. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol 1986;154:470–5 | Study includes only diabetic women, all of whom are treated with insulin therapy |
| 68 | Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med 2009;14:77–84 | Narrative review |
| 69 | Yogev, Chen, Hod, Coustan, Oats, McIntyre, Metzger, Lowe, Dyer, Dooley, Trimble, McCance, Hadden, Persson, Rogers; Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 2010;202:255.e1–7 | Comparison of women with differing severity of GDM The women received treatment of diet or diet and insulin |
| 70 | Zhang C, Martin K, Bowers K, Liu A, Bao W, Vaag A, et al. Fasting glucose levels during pregnancy and long-term childhood growth in the offspring. Am J Obstet Gynecol 2014;1:S44 | Conference abstract: no relevant data |
| First author | Year | Location | No. of women | Glucose test used | Timing of test | GDM diagnosis criteria | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fasting | 1 hour | 2 hour | LGA | Macrosomia | Shoulder dystocia | Pre-eclampsia/PIH | Preterm birth | C-section | ||||||
| Black110 | 2010 | USA (California) | 8711 | 75-g OGTT | ✗ | ✗ | ✗ | Defined in paper | ✗ | ✗ | ✗ | ✗ | ||
| Franks111 | 2006 | USA Arizona (Pima Indians) | 911 | Unclear | ✗ | WHO | Type 2 diabetes | |||||||
| Jensen112 | 2008 | Denmark | 2885 | 75-g OGTT | ✗ | Defined in paper | ✗ | ✗ | ✗ | ✗ | ||||
| Khan114 | 1994 | Pakistan | 1278 | 75-g OGCT | ✗ | Defined in paper | ✗ | ✗ | ||||||
| Kim113 | 2002 | South Korea | 5019 | 100-g OGTT | All times | NDDG | ✗ | ✗ | ||||||
FIGURE 52.
Frequency of macrosomia across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
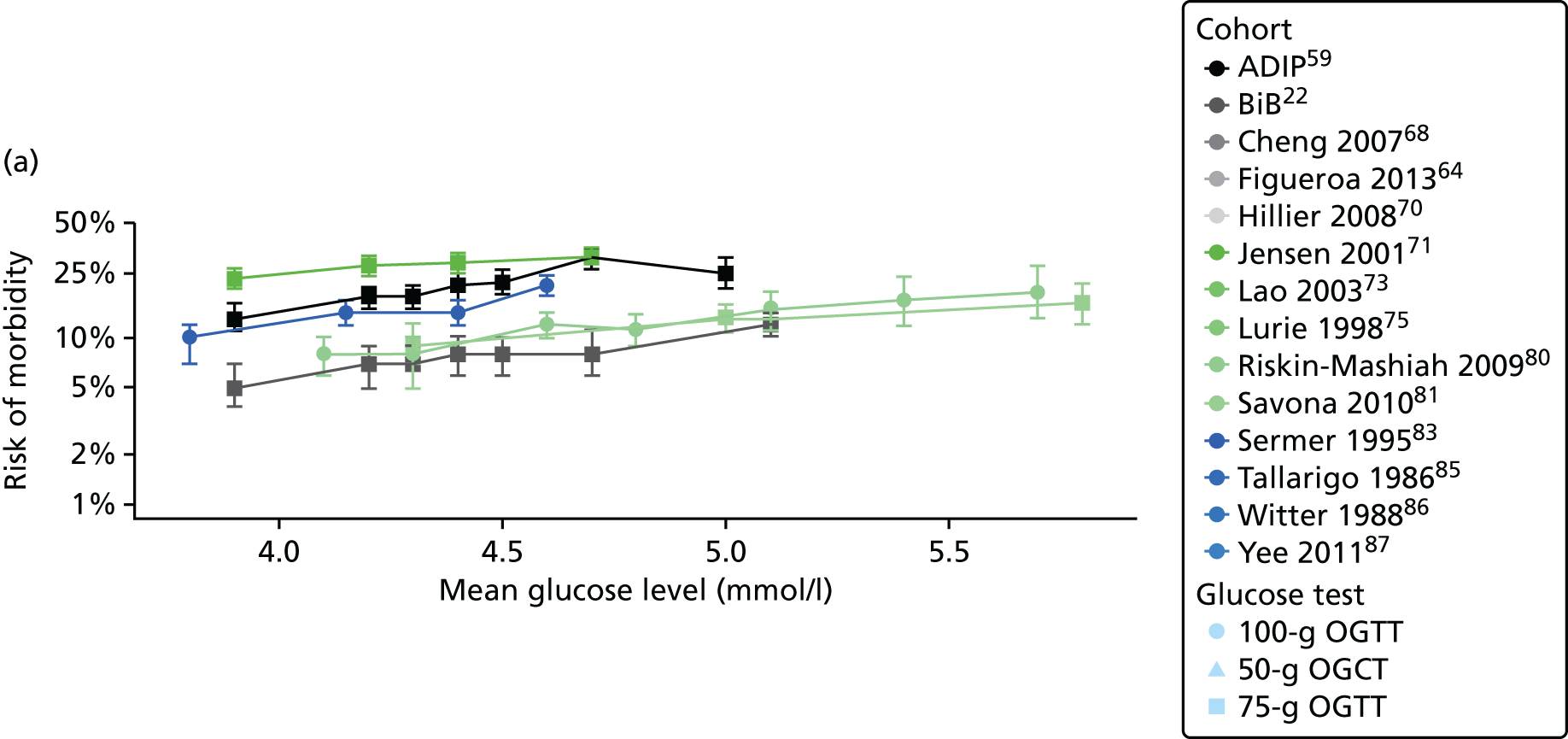

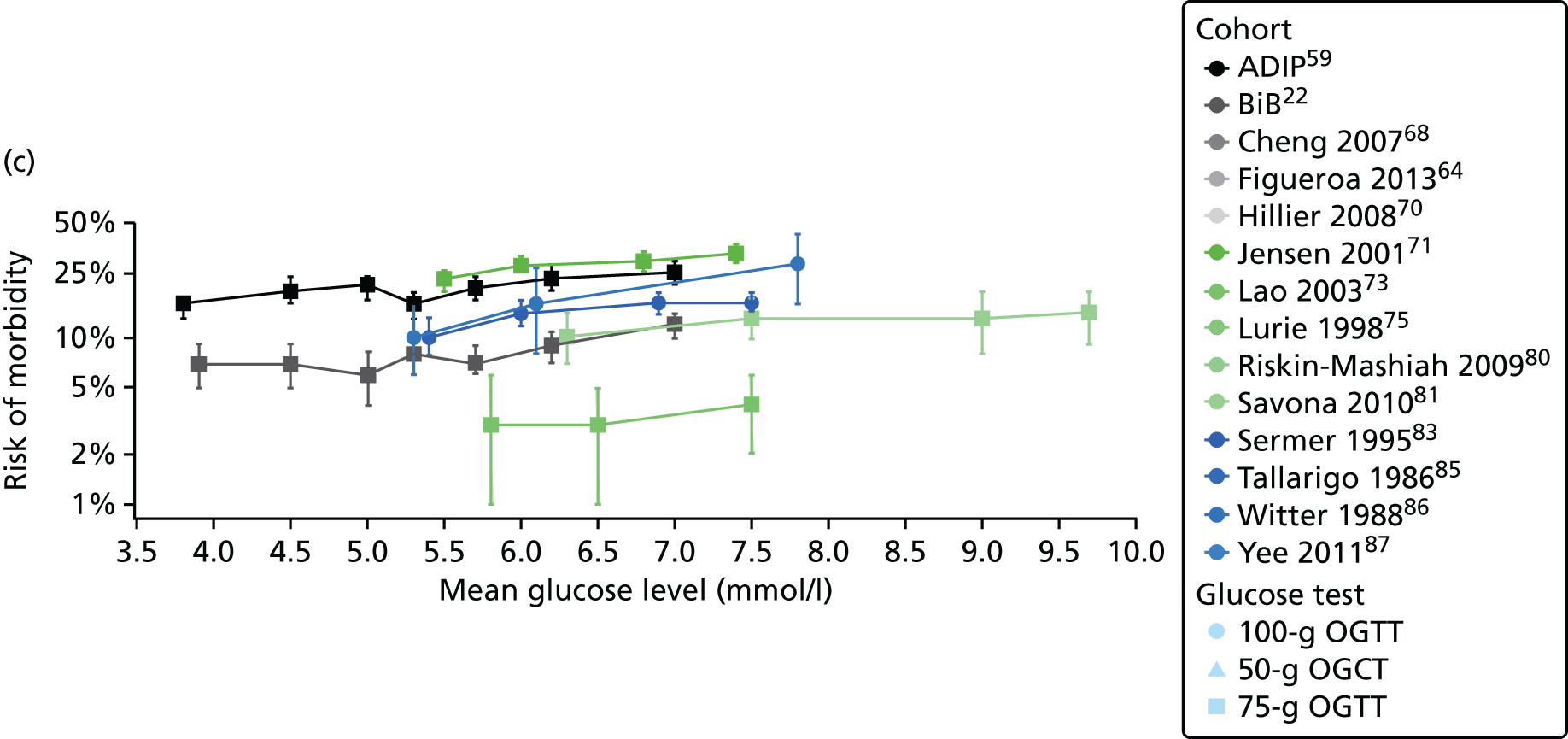
FIGURE 53.
Frequency of LGA across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
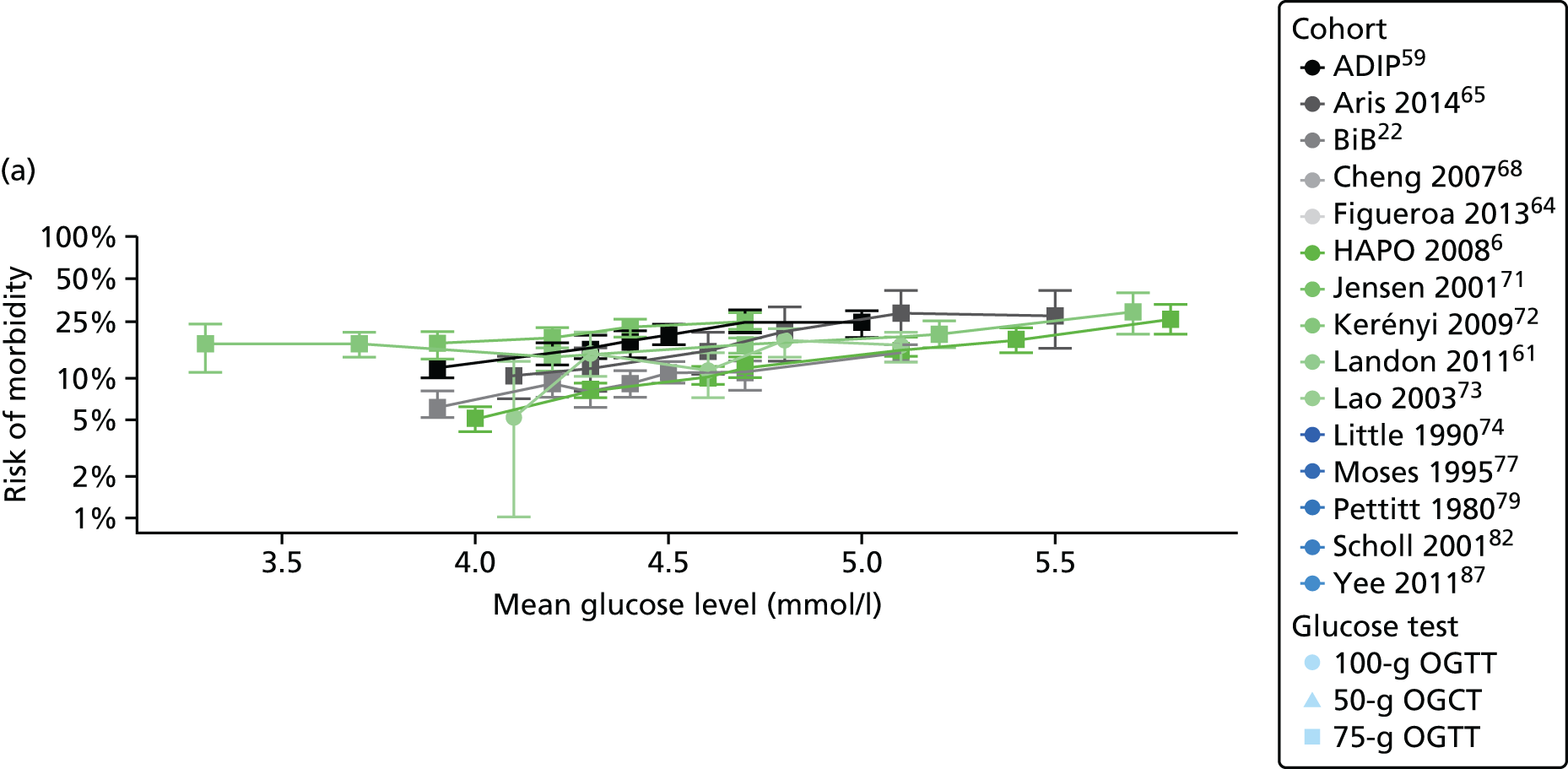

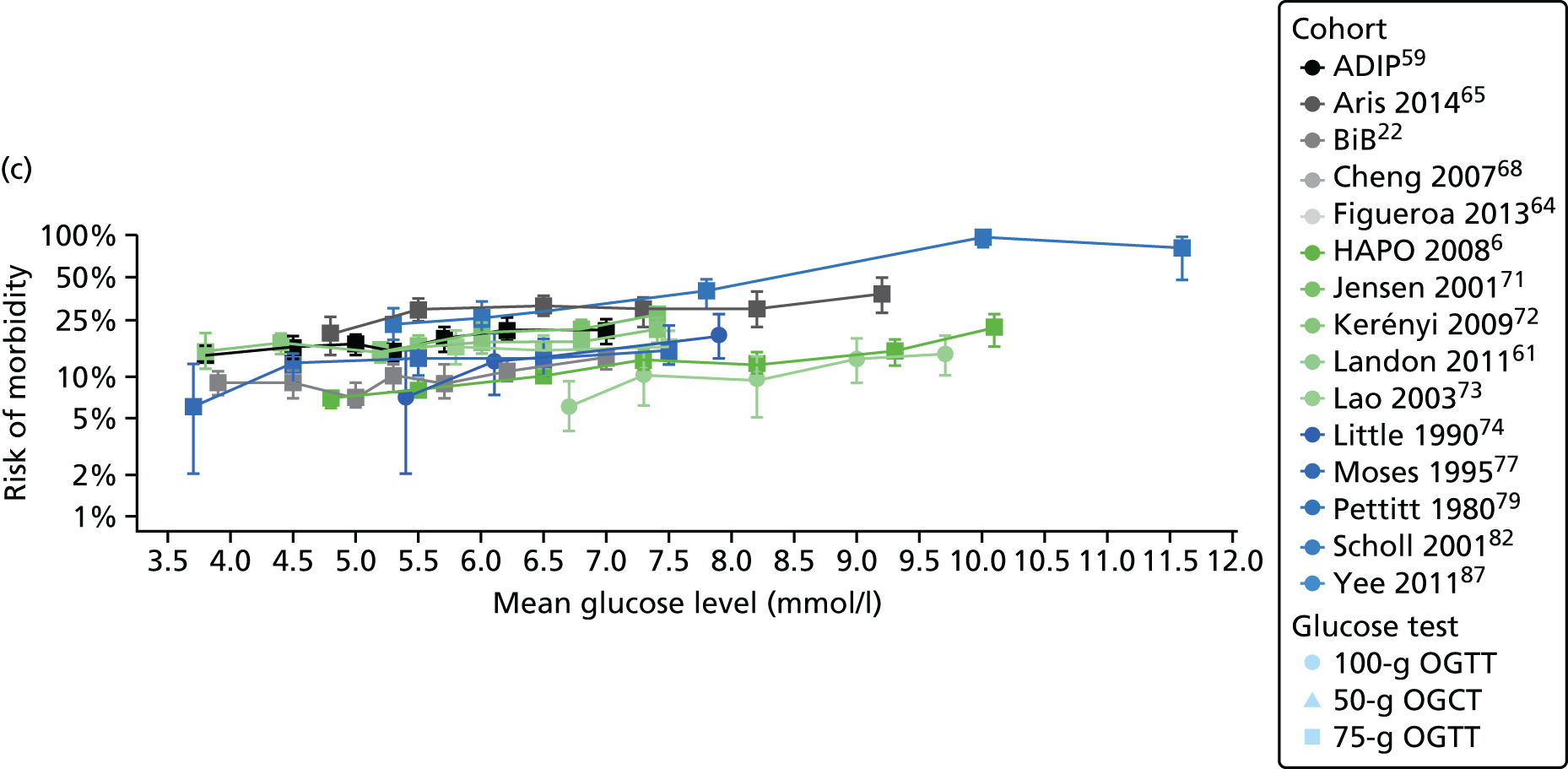
FIGURE 54.
Frequency of pre-eclampsia across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
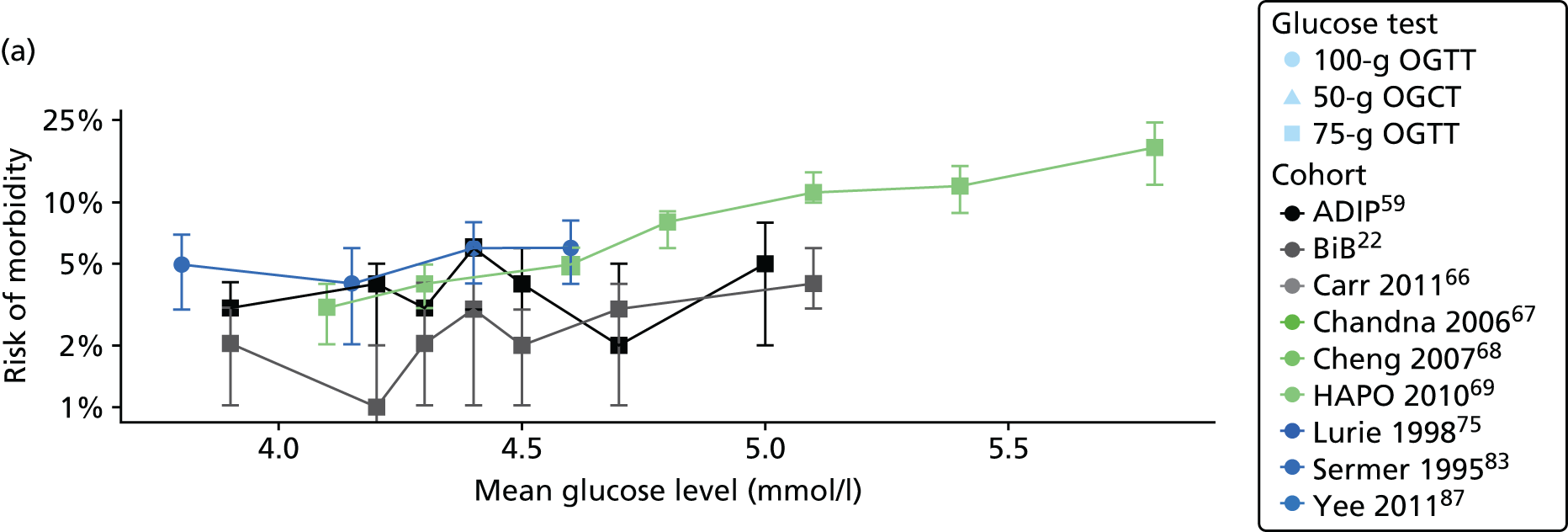


FIGURE 55.
Frequency of C-section across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.

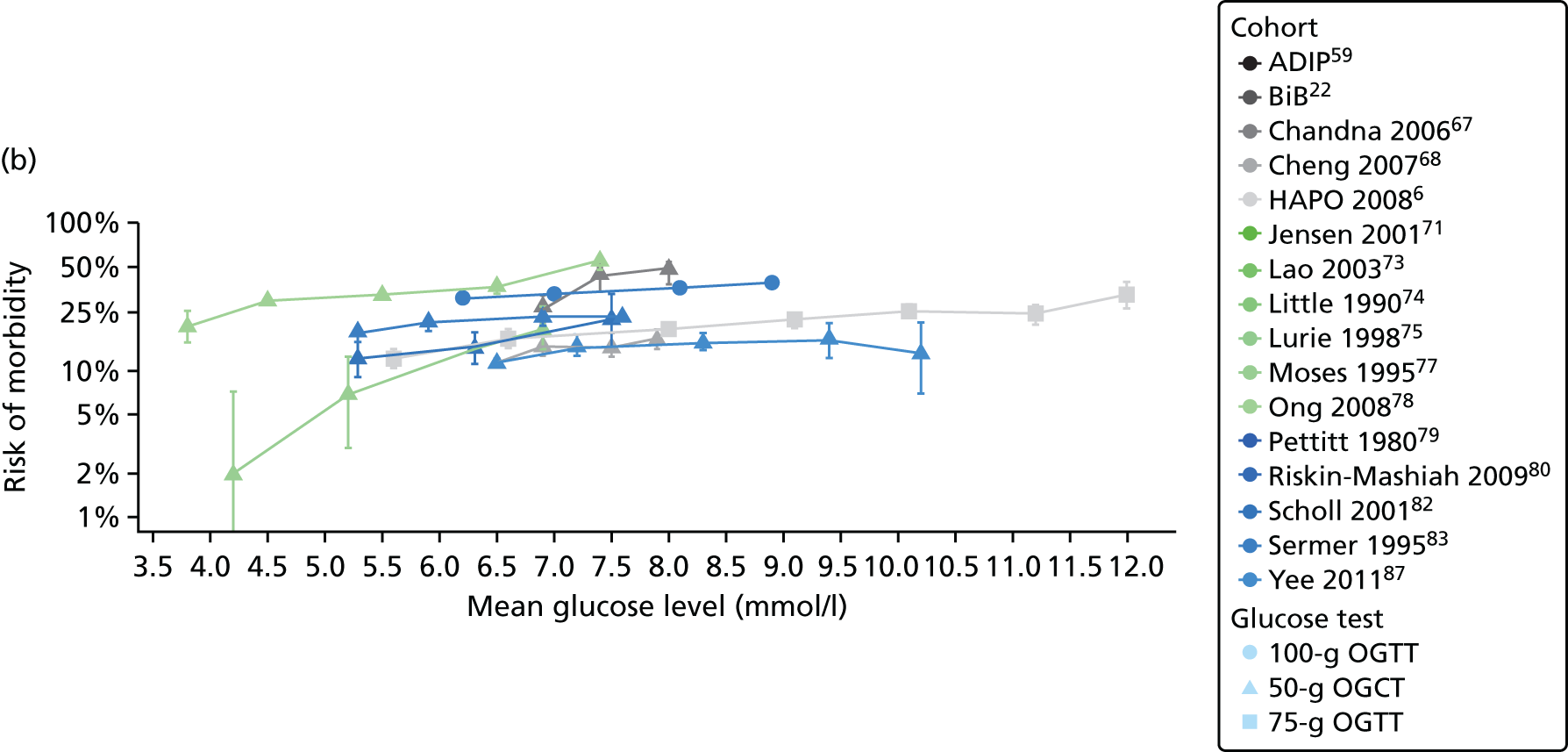
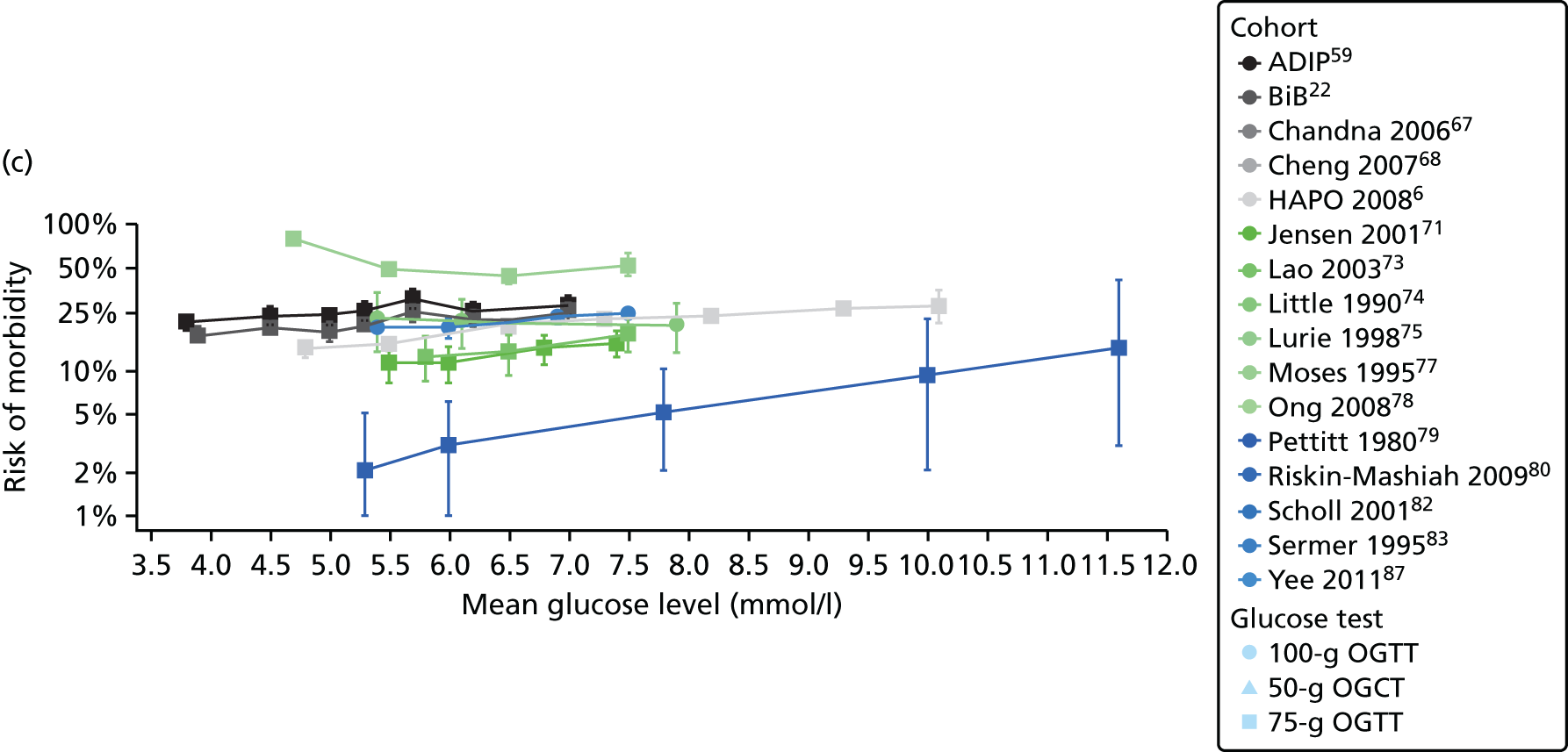
FIGURE 56.
Frequency of instrumental birth across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
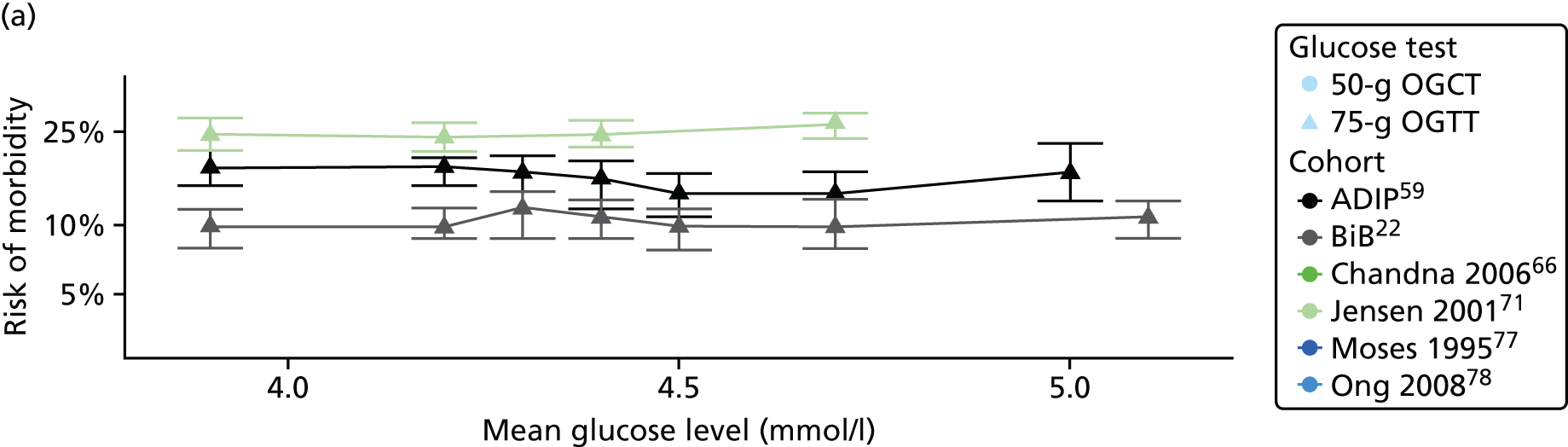
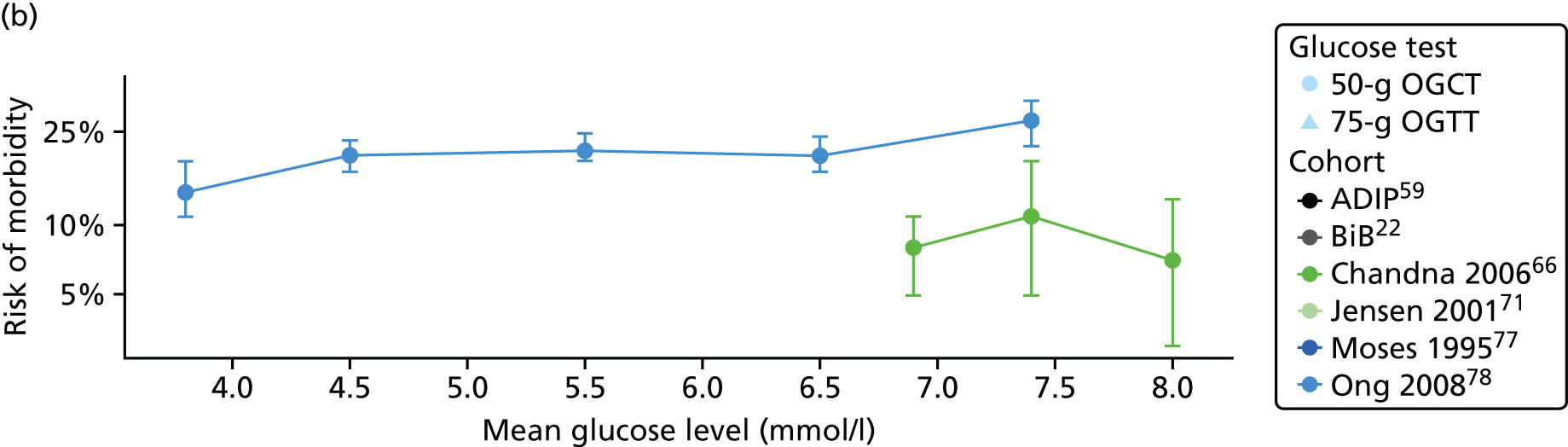
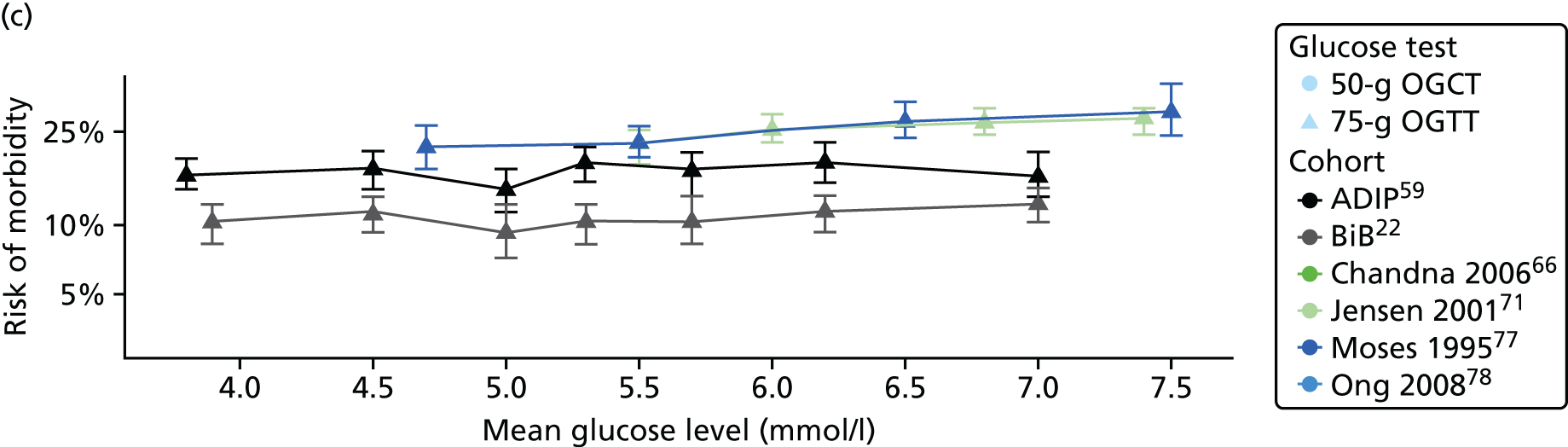
FIGURE 57.
Frequency of induction of labour across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
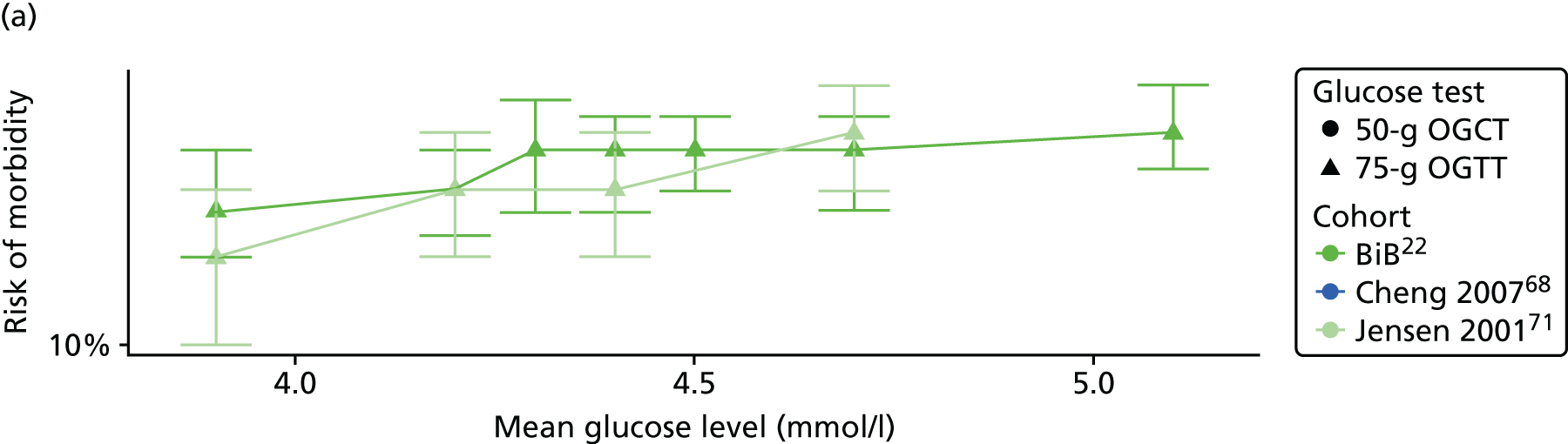

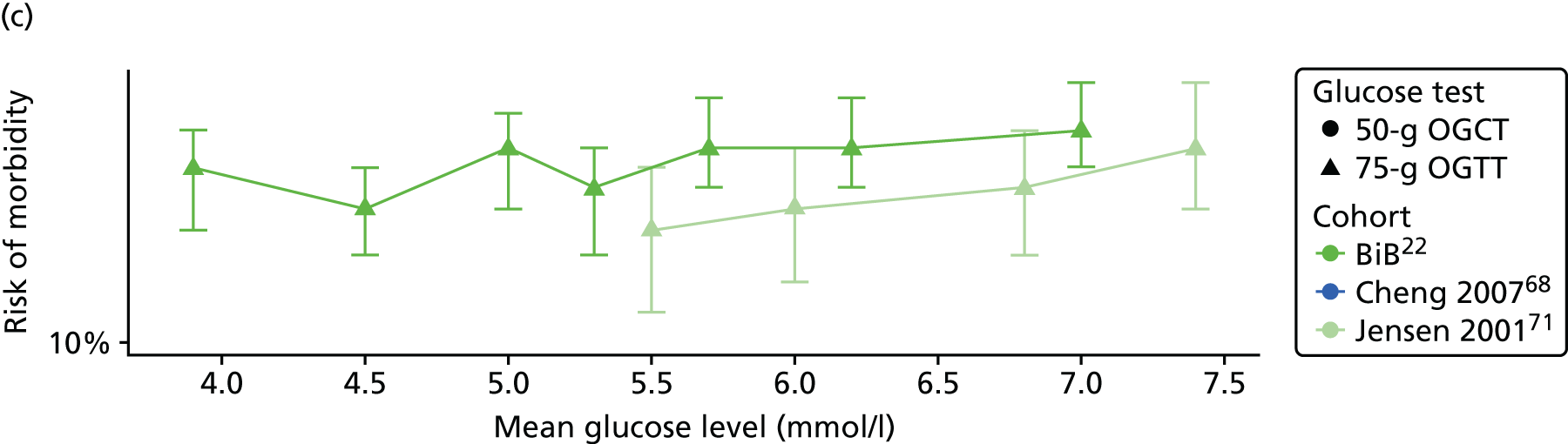
FIGURE 58.
Frequency of shoulder dystocia across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
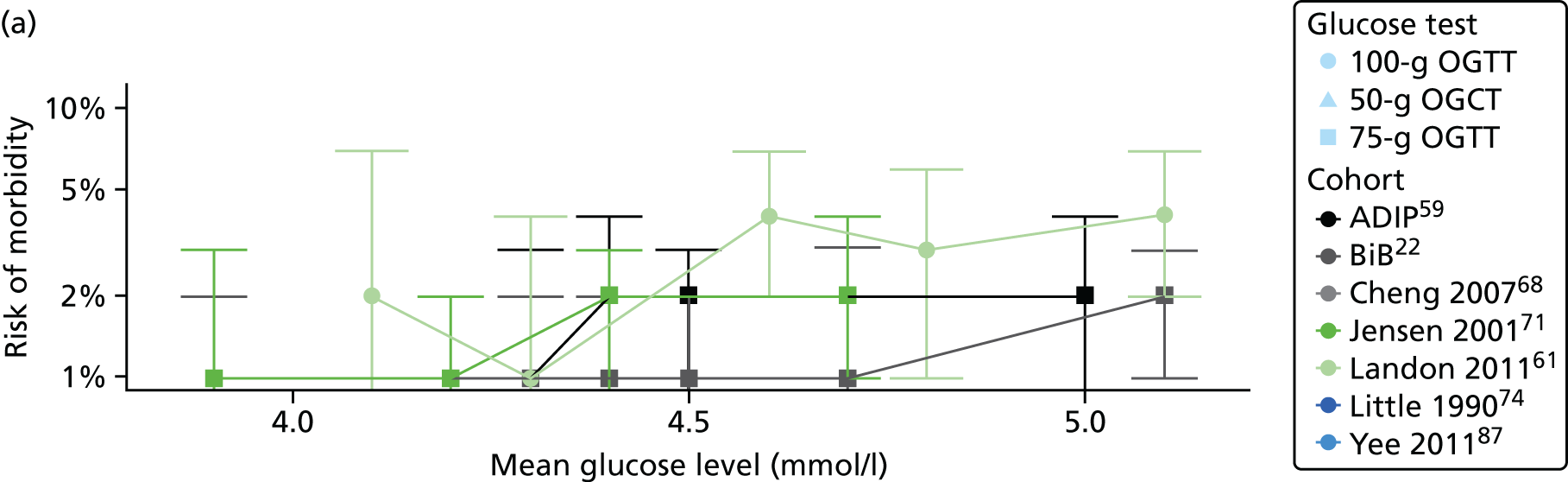
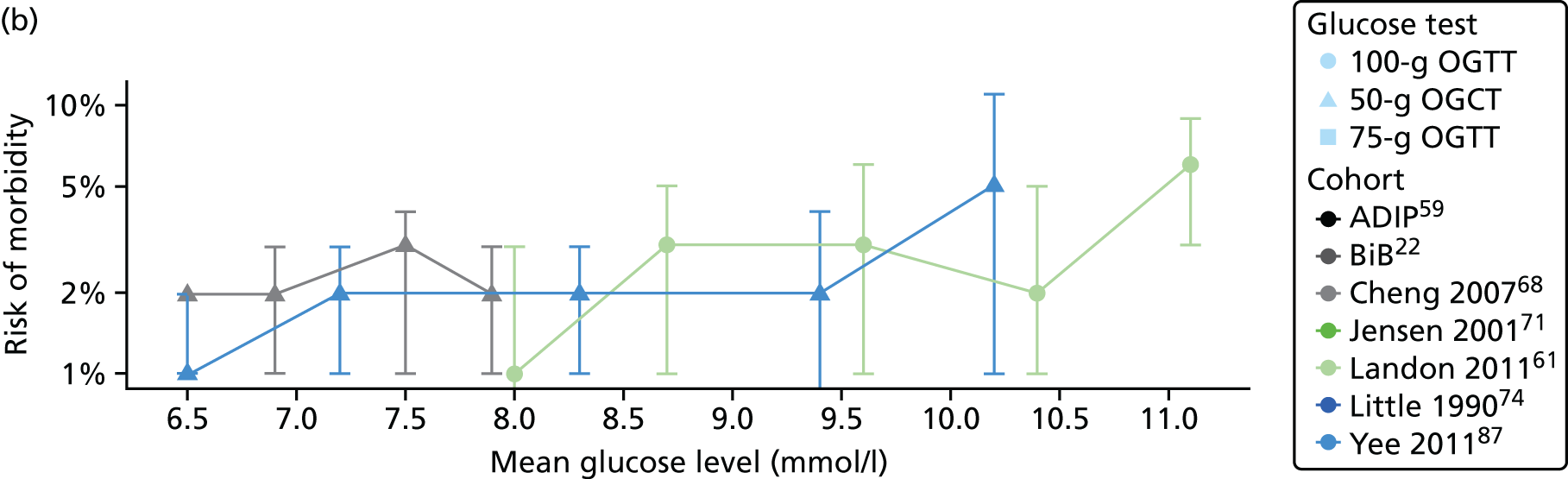

FIGURE 59.
Frequency of preterm birth across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.
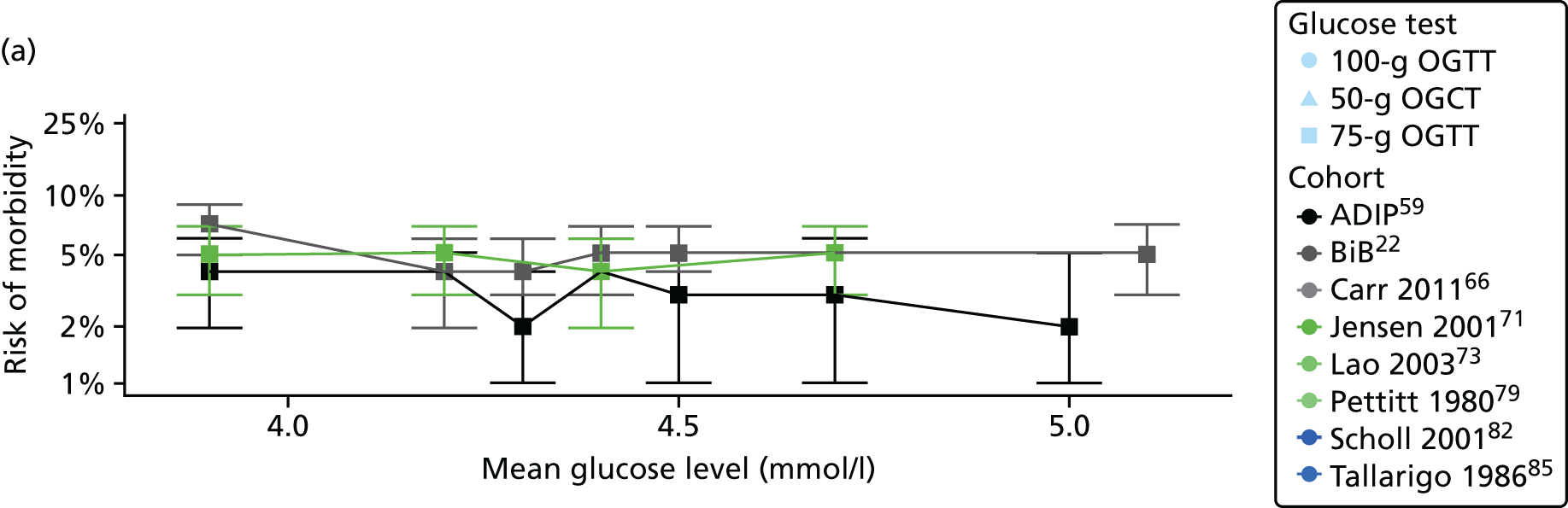
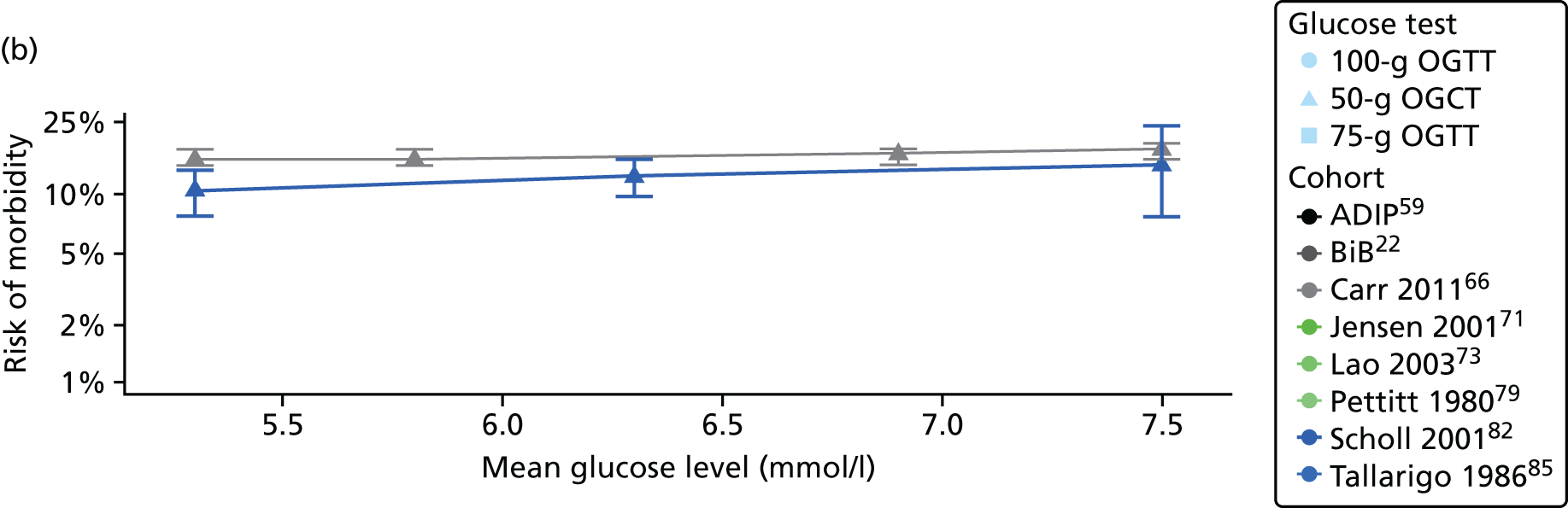

FIGURE 60.
Frequency of neonatal hypoglycaemia across glucose categories by study. (a) Fasting; (b) 1 hour; and (c) 2 hours.


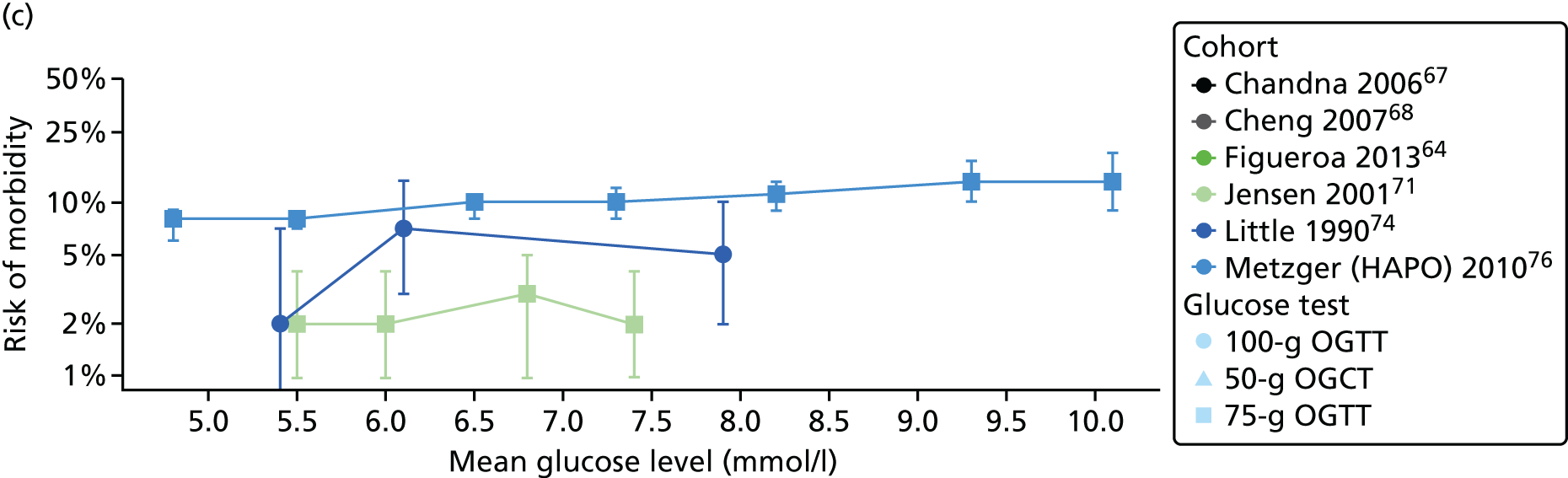
FIGURE 61.
Odds ratio for 1-mmol/l increases in 1-hour post-load glucose for 75-g OGTT and reported perinatal outcomes.

FIGURE 62.
Adjusted ORs across categories of fasting and post-load glucose levels and macrosomia. (a) Fasting; and (b) 1 hour.

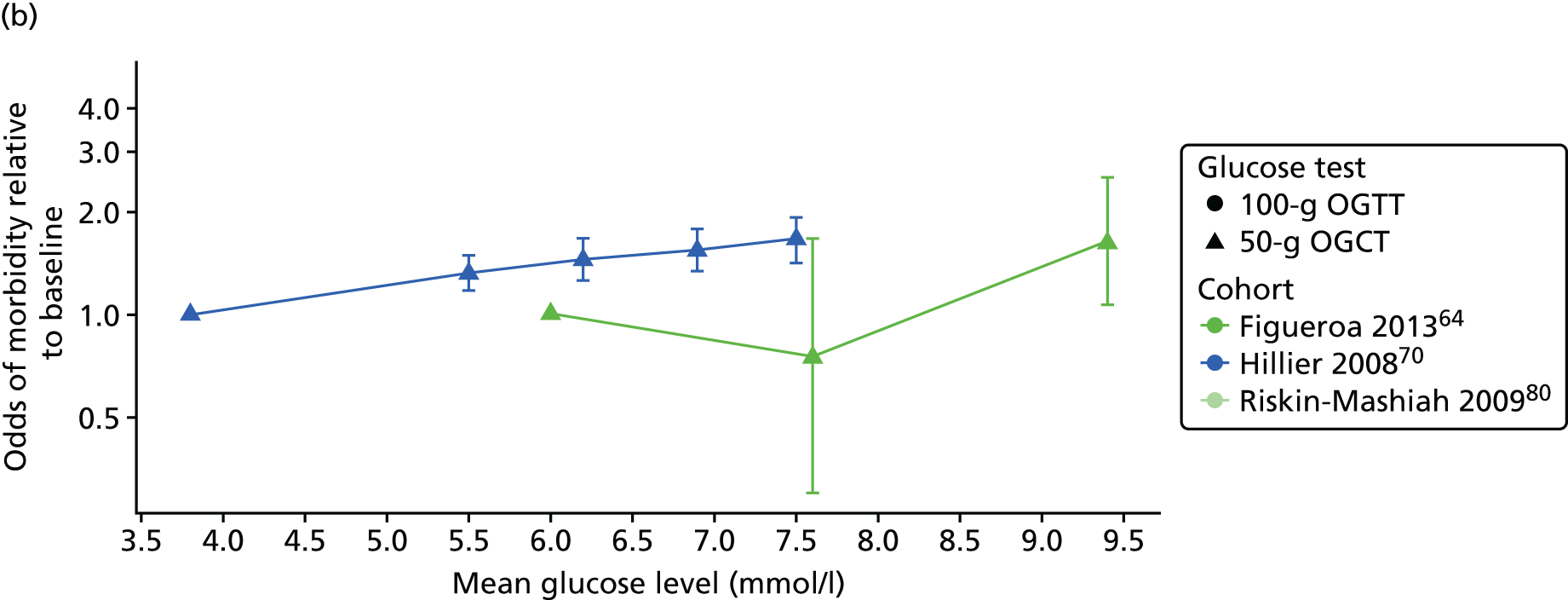
FIGURE 63.
Adjusted ORs across categories of fasting and post-load glucose levels and LGA. (a) Fasting; (b) 1 hour; and (c) 2 hours.
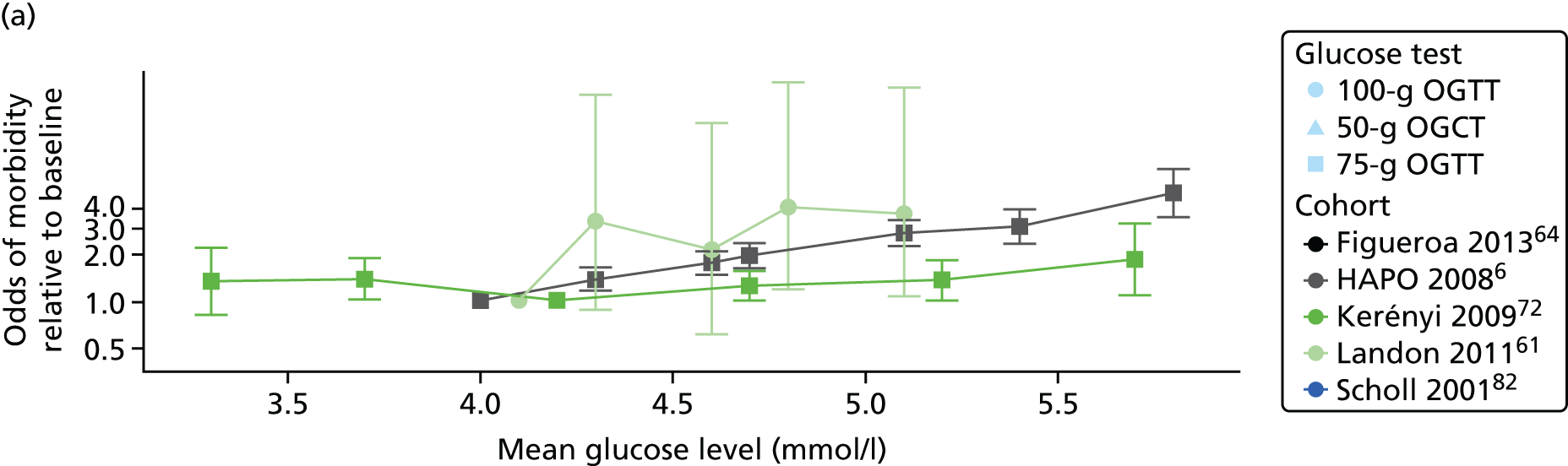

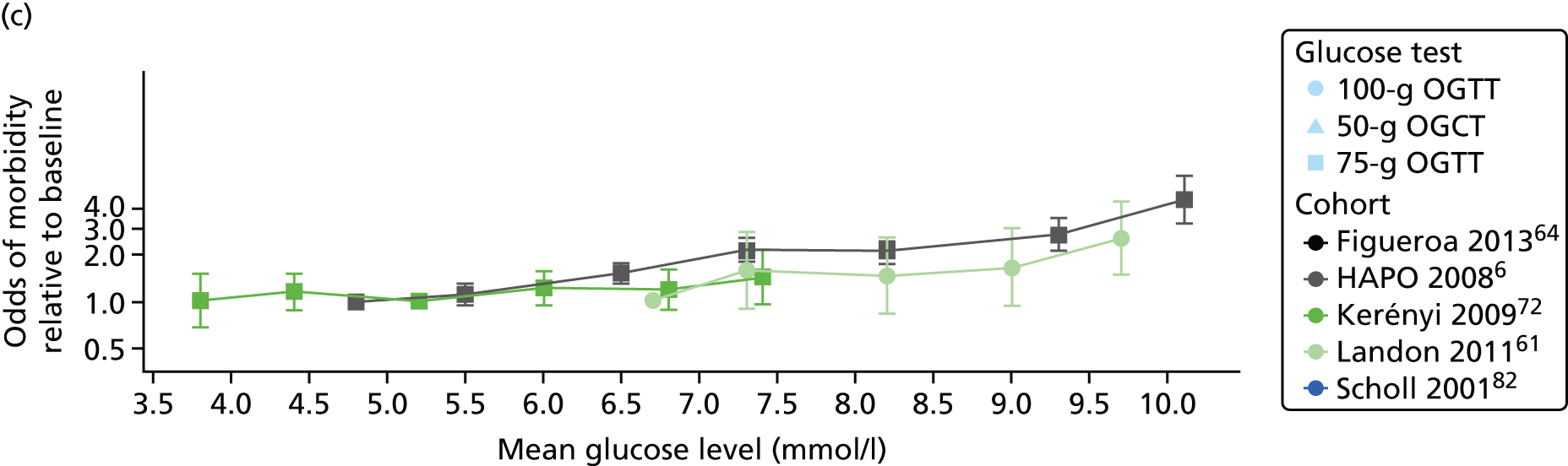
FIGURE 64.
Adjusted ORs across categories of fasting and post-load glucose levels and C-section. (a) Fasting; (b) 1 hour; and (c) 2 hours.



FIGURE 65.
Adjusted ORs across categories of fasting and post-load glucose levels and pre-eclampsia. (a) Fasting; (b) 1 hour; and (c) 2 hours.
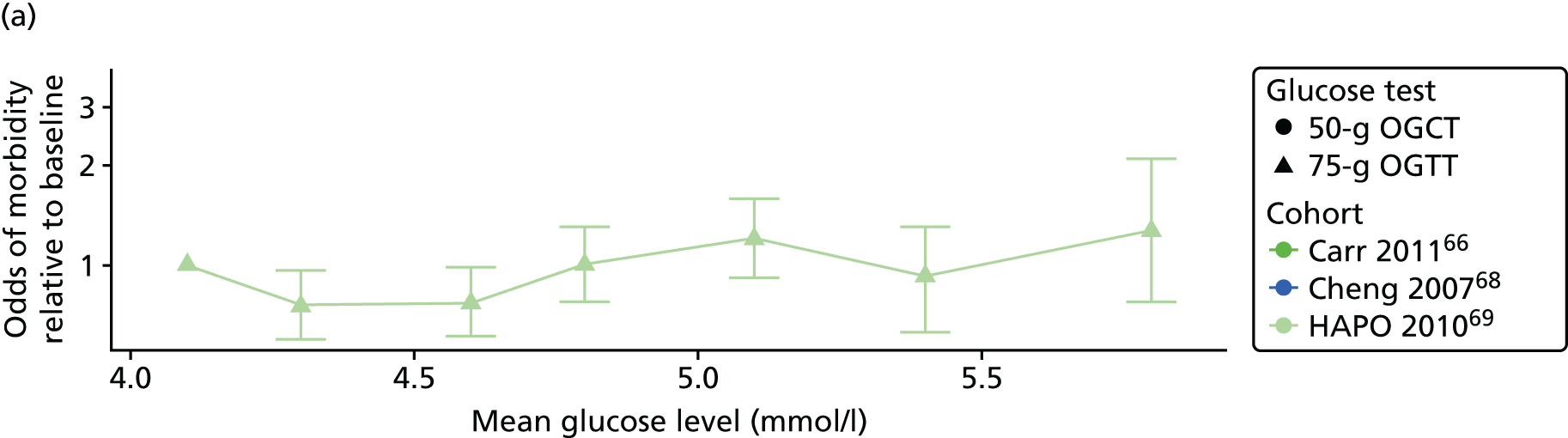


Appendix 3 Tables for Chapter 4
| Excluded study | Reason |
|---|---|
| Agarwal MM, Dhatt GS, Punnose J, Koster G. Gestational diabetes in a high-risk population: Using the fasting plasma glucose to simplify the diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol 2005;120:39–44 | Non-UK, United Arab Emirates and FPG performance |
| Ajala O, Stenhouse E, Shaw N, Carr S, Millward A. Cardiovascular risk following diagnosis of gestational diabetes: Diabetes in Pregnancy Mother Baby Study 3. Diabet Med 2011;28:173 | Conference abstract and incidence of type 2 diabetes after GDM |
| Akhtar S, Ramanathan R, Ewins DL, Goenka N, Davies J, Joseph F. The impact of the new International Association of Diabetes and Pregnancy Study Groups criteria for gestational diabetes on glycaemic management. Diabet Med 2012;29:66 | Conference abstract and impact of diagnostic criteria; no usable data |
| Al-Ramli W, Dennedy MC, Avalos G, Dunne F. Gestational weight gain and pregnancy outcomes in women with gestational diabetes mellitus. Ir J Med Sci 2012;181:S350–1 | Conference abstract, gestational weight gain |
| Anonymous. Number of women with gestational diabetes underestimated. Medilexicon 2010 | Not available at the British Library Review of multiple countries (estimated at 16% in nine countries) |
| Anthony R, Angala P, Ikomi A, Khan R, Kiss S. Resource implications of converting from a WHO/ADA hybrid to IADPSG criteria for diagnosing GDM in a UK University Hospital. Arch Dis Childhood Fetal Neonatal Ed 2013;98(Suppl. 1):A35 | Conference abstract; impact of diagnostic criteria |
| Avalos G, Owens L, Dunne F. Applying current screening tools for gestational diabetes mellitus to a European population: Is it time for change? Ir J Med Sci 2012;181:S346 | Conference abstract, full paper already obtained |
| Avalos GE, Owens L, Dunne F. How many women with gestational diabetes mellitus are missed if selective screening strategies are used? Diabetologia 2012;55:S446 | Conference abstract, full paper already obtained |
| Avalos GE, Owens LA, Dunne F. Applying current screening tools for gestational diabetes mellitus to a European population: Is it time for change? Diabetes Care 2013;36:3040–4 | Conference abstract |
| Baci Y, Ustuner I, Keskin HL, Ersoy R, Avsar AF. Effect of maternal obesity and weight gain on gestational diabetes mellitus. Gynecol Endocrinol 2013;292:133–6 | Considered for risk factors review |
| Beischer NA, Oats JN, Henry OA, Sheedy MT, Walstab JE. Incidence and severity of gestational diabetes mellitus according to country of birth in women living in Australia. Diabetes 1991;40(Suppl. 2):35–8 | Australia |
| Bell R, Hayes L, Crowder D, Bilous M, Lewis-Barned N, Brandon H, et al. Outcome of pregnancies complicated by gestational diabetes: a multi-centre study from the North East of England. Arch Dis Childhood Fetal Neonatal Ed 2010;95:Fa97 | Conference abstract |
| Bell R, Hayes L, Lewis-Barned N, Bilous M, Brandon H, Pearson S, et al. Diagnosis, treatment and outcome of gestational diabetes: a multi-centre study in north-east England (NorGES). Diabet Med 2010;1:15 | Conference abstract, describes characteristics of women with GDM |
| Bertolotto A, Volpe L, Calianno A, Pugliese MC, Lencioni C, Resi V, et al. Physical activity and dietary habits during pregnancy: Effects on glucose tolerance. J Maternal Fetal Neonatal Med 2010;23:1310–14 | Italy |
| Brite J, Shiroma EJ, Bowers K, Yeung E, Laughon SK, Grewal JG, et al. Height and the risk of gestational diabetes: Variations by race/ethnicity. Diabet Med 2014;313:332–40 | Considered for risk factors review; USA |
| Bryant M, Santorelli G, Lawlor DA, et al. A comparison of South Asian specific and established BMI thresholds for determining obesity prevalence in pregnancy and predicting pregnancy complications: Findings from the Born in Bradford cohort [published online ahead of print July 20 2013). Int J Obes | BiB cohort |
| Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: Prevalence, current screening practice and barriers to screening. A review. Diabet Med 2012;29:844–54 | Review – checked references |
| Buhling KJ, Elze L, Henrich W, Starr E, Stein U, Siebert G, et al. The usefulness of glycosuria and the influence of maternal blood pressure in screening for gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2004;113:145–8 | Dipstick analysis, Germany |
| Cairnduff V, Hill AJ, Sinclair M, Patterson C, McCance DR. Relationship between maternal BMI, nutrient intakes and glycaemic control in third trimester of pregnancy. Proc Nutrition Soc 2012;71:E53 | Conference abstract; glycaemic control |
| Chandy E, Rolph N, O’Donnell J, Scott J, Wilson J, Herlihy O. A review of oral glucose tolerance tests at the Borders General Hospital. Pract Diabetes 2012;29:358–60a | Compares oral glucose tests, no suitable GDM data |
| Chico A, Lopez-Rodo V, Rodriguez-Vaca D, Novials A. Features and outcome of pregnancies complicated by impaired glucose tolerance and gestational diabetes diagnosed using different criteria in a Spanish population. Diabetes Res Clin Pract 2005;68:141–6 | Compares criteria; Spain |
| Coolen JC, Verhaeghe J. Physiology and clinical value of glycosuria after a glucose challenge during pregnancy. Eur J Obstet Gynecol Reprod Biol 2010;150:132–6 | Compares tests; Belgium |
| Crowe C, Noctor E, Carmody LA, Wickham B, Avalos G, Gaffney G, et al. ATLANTIC DIP: The prevalence of pre-diabetes/type 2 diabetes in an Irish population with gestational diabetes mellitus 1-5 years post index pregnancy. Ir J Med Sci 2011;180:S483–4 | Conference listing |
| Crowe C, Noctor E, Carmody LA, Wickham B, Avalos G, Gaffney G, et al. ATLANTIC DIP: The prevalence of pre-diabetes/type 2 diabetes in an Irish population with gestational diabetes mellitus 1-5 years post index pregnancy. BMC Proceedings 2012;6(Suppl. 4):O35 | Conference abstract |
| Cullinan J, Gillespie P, Owens L, Avalos G, Dunne FP, ATLANTIC DIP collaborators. Is there a socioeconomic gradient in the prevalence of gestational diabetes mellitus? Ir Med J 2012;105(Suppl. 5):21–3 | ATLANTIC DIP,59 only data on prevalence is reference to paper already obtained |
| Davenport MH, Campbell MK, Mottola MF. Increased incidence of glucose disorders during pregnancy is not explained by pre-pregnancy obesity in London, Canada. BMC Pregnancy Childbirth 2010;10:85 | Considered for risk factors review; Canada |
| Denison FC, Norwood P, Bhattacharya S, Duffy A, Mahmood T, Morris C, et al. Association between maternal body mass index during pregnancy, short-term morbidity, and increased health service costs: a population-based study. BJOG 2014;121:72–81; discussion 2 | No data on GDM |
| Di Cianni G, Volpe L, Lencioni C, Miccoli R, Cuccuru I, Ghio A, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract 2003;62:131–7 | Considered for risk factors review. Italy |
| Fadl HE, Ostlund IKM, Magnuson AFK, Hanson USB. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med 2010;27:436–41 | Sweden |
| Forbes S, Reynolds RM, Patrick AW, Denison F, Norman JE. Implications of new Scottish Intercollegiate Guidelines Network (SIGN) criteria on diagnosis of gestational diabetes in a severely obese pregnant population. Diabet Med 2011;28:23 | Conference abstract, severely obese women only |
| Fox NS, Roman AS, Saltzman DH, Klauser CK, Rebarber A. Obesity and adverse pregnancy outcomes in twin pregnancies. J Maternal Fetal Neonatal Med 2014;27:355–9 | USA |
| Gayle C, Germain S, Marsh MS, Rajasingham D, Carroll P, Brackenridge A, et al. Management of gestational diabetes using the World Health Organisation (WHO) criteria in a diabetes antenatal clinic benefit women compared to routine care based on European Association for the Study of Diabetes (EASD) criteria. A comparison of treatment based on an oral glucose tolerance test 2-hour blood glucose 7.8 - 8.9 mmol/l. Diabet Med 2010;1:35 | Conference abstract, no GDM data |
| Gillespie P, O’Neill C, Avalos G, Dunne FP, ATLANTIC DIP Collaborators. New estimates of the costs of universal screening for gestational diabetes mellitus in Ireland. Ir Med J 2012;105(Suppl. 5):15–18 | Costs, have AD prevalence elsewhere ATLANTIC DIP |
| Gillespie P, O’Neill C, Avalos G, et al. The cost of universal screening for gestational diabetes mellitus in Ireland. Diabet Med 2011;28:912–18 | Costs |
| Gillespie P, O’Neill C, Cullinan J, Dunne F. The effect of Gestational Diabetes Mellitus (GDM) on maternity care and costs in Ireland. Diabetologia 2012;55:S449 | Conference abstract; no GDM data |
| Hall C, Going A, Moutter S, Thynne AD, Salloum M, Sengupta S, et al. Implications of the HAPO study on diagnosis of gestational diabetes in existing patients screened during pregnancy with a glucose tolerance test, 2009-2010. Diabet Med 2011;28:174 | Conference abstract; no GDM data |
| Healy GM, Vellinga A, Carmody L, Avalos G, Mustafa E, Khalil S, et al. Atlantic DIP: Universal vs. Selective Screening for Gestational Diabetes (GDM). Diabetes 2012;61:A641 | Conference abstract of Atlantic DIP cohort |
| Hieronimus S, Le Meaux JP. Relevance of gestational diabetes mellitus screening and comparison of selective with universal strategies. Diabetes Metab 2010;36(Pt 2):575–86 | Considered for risk factors review; review of screening |
| Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational diabetes mellitus: Results from a survey of country prevalence and practices. J Maternal Fetal Neonatal Med 2012;25:600–10 | Survey only reporting NICE guidelines |
| Kavvoura FK, Graham D, Crowley R, Simpson H, Street P, Elsheikh M. Diabetes antenatal care at a large district general hospital: an audit from 1997 to 2010. Diabet Med 2012;29:153 | Conference abstract; insufficient data on prevalence |
| Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: Incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract 2005;69:279–86 | Considered for risk factors review; Iran |
| Kim S, Nakai H, Okabe K, Nohira T, Yoneyama K. Recurrence of gestational diabetes mellitus: rates and risk factors from initial GDM and one abnormal GTT value. Diabetes Res Clin Pract 2006;71:75-81 | Japan |
| Kong M, Meakin L, Donley P, Gregory R, Scudamore I. Evidence of an ‘epidemic’ of gestational diabetes 1995–2008: Implications for service delivery. Diabet Med 2010;1:168 | Conference abstract; Insufficient data on prevalence |
| Kousta E, Lawrence NJ, Penny A, Millauer BA, Robinson S, Johnston DG, et al. Women with a history of gestational diabetes of European and South Asian origin are shorter than women with normal glucose tolerance in pregnancy. Diabet Med 2000;17:792–7 | Only data on women with previous GDM |
| Lacey A, Roche J, Wheatley T. Screening for gestational diabetes: are NICE risk factors adequate? Diabet Med 2011;28:182–3 | Conference abstract; Insufficient data on prevalence |
| Lappin SM, Watt P, Traub AI, Tharma S, Courtney H, McCance DR. Audit of risk factors for Gestational Diabetes (GDM) in women diagnosed by a universal screening programme. Diabet Med 2010;1:173–4 | conference abstract |
| Lohse N, Marseille E, Kahn JG. Development of a model to assess the cost-effectiveness of gestational diabetes mellitus screening and lifestyle change for the prevention of type 2 diabetes mellitus. Int J Gynecol Obstet 2011;115(Suppl. 1):20–5 | Not UK cost |
| Lowy C, Beard RW, Goldschmidt J. The UK diabetic pregnancy survey. Acta Endocrinol Suppl (Copenh) 1986;277:86–9 | Survey of GDM population |
| Maitland RA, Barr S, Briley A, Seed P, Poston L. Incidence of gestational diabetes in an obese population using the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria in the UK Pregnancies Better Eating and Activity Trial (UPBEAT) pilot study. Diabet Med 2012;29:152 | Obese population only, small RCT conference abstract |
| Maitland RA, Patel N, Rajasingham D, Brackenridge A. Trends in gestational diabetes (GDM) prevalence over three years within a high risk inner city population attending Guy’s and St Thomas’ NHS Foundation Trust (GSTFT). Diabet Med 2013;30:166 | Conference abstract |
| Mannan S, Ikomi A, Khan R, Kiss S. Implementation of the new international guidelines in a UK university hospital: Predicted versus actual consequences. BJOG 2014;121:151 | Conference abstract |
| Mansell A, Gouveia C, Braggins F, Claydon A, Nobeebux A, Joseph T, et al. Early screening for gestational diabetes is essential to detect undiagnosed impaired glucose tolerance and Type 2 diabetes in a high risk, ethnically-diverse population. Diabet Med 2009;26:117–18 | No data on prevalence conference abstract |
| Marseille E, Lohse N, Jiwani A, Hod M, Seshiah V, Yajnik CS, et al. The cost-effectiveness of gestational diabetes screening including prevention of type 2 diabetes: application of a new model in India and Israel. J Maternal Fetal Neonatal Med 2013;26:802–10 | Cost; India and Israel |
| Most O, Langer O. Gestational diabetes: Maternal weight gain in relation to fetal growth, treatment modality, BMI and glycemic control. J Maternal Fetal Neonatal Med 2012;25:2458–63 | Study of women with GDM |
| Munigoti SP, Davies R, Peters J. Impact of adopting the IADPSG criteria for diagnosing gestational diabetes. Diabet Med 2011;28:170 | Conference abstract; high-risk patients only |
| Myagerimath R, Albert S, Nwosu EC. Outcome of glucose tolerance test in a district general hospital. BJOG 2013;120:134 | Conference abstract |
| Nijjar SK, Hunt KF, Rogers H, Smith C, Gayle CM, Marsh MS, et al. Clinical outcomes of patients with gestational diabetes mellitus who do not have typical risk factors. Diabetologia 2011;54:S479–S80 | Conference abstract; all women had GDM |
| Noctor E, Crowe C, Avalos G, Carmody L, Wickham B, O’Shea P, et al. ATLANTIC DIP: Index pregnancy factors associated with progression to pre-diabetes/diabetes up to 5 years post gestational diabetes in the west of Ireland. Diabetes 2012;61:A343 | Conference abstract; abstract of Atlantic DIP cohort |
| Noctor E, Crowe C, Carmody LA, Wickham B, Avalos G, Gaffney G, et al. ATLANTIC DIP: The prevalence of pre-diabetes/diabetes up to 5 years post partum in women with previous gestational diabetes along the Atlantic coast. Diabetologia 2012;55:S442 | Conference abstract; abstract of Atlantic DIP cohort |
| O’Higgins AC, Dunne FP, Lee B, Smith D, Turner MJ. A national survey of implementation of guidelines of screening for gestational diabetes mellitus. BJOG 2013;120:470 | Conference abstract; insufficient data on prevalence |
| O’Sullivan EP, Avalos G, O’Reilly MW, Dennedy C, Dunne F. ATLANTIC DIP: Prevalence and implications of abnormal glucose tolerance in pregnancy in Ireland. Diabetologia 2010;53:S10 | Conference abstract, Abstract of Atlantic DIP cohort |
| Ozumba BC, Obi SN, Oli JM. Diabetes mellitus in pregnancy in an African population. Int J Gynecol Obstet 2004;84:114–19 | Considered for risk factors review; Nigeria |
| Perovic M, Garalejic E, Gojnic M, Arsic B, Pantic I, Bojovic DJ, et al. Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus. J Maternal Fetal Neonatal Med 2012;25:1348–53 | High-risk population only |
| Poncet B, Touzet S, Rocher L, Berland M, Orgiazzi J, Colin C. Cost-effectiveness analysis of gestational diabetes mellitus screening in France. Eur J Obstet Gynecol Reprod Biol 2002;103:122–9 | France; cost analysis |
| Rajab KE, Issa AA, Hasan ZA, Rajab E, Jaradat AA. Incidence of gestational diabetes mellitus in Bahrain from 2002 to 2010. Int J Gynaecol Obstet 2012;117:74–7 | Considered for risk factors review. Bahrain |
| Rees G, Bennett SJ, Colleypriest O, Ellis L, Porter JM, Stenhouse E. The prevalence of overweight and obesity in early pregnancy and the incidence of gestational diabetes. Diabet Med 2010;1:172 | Conference abstract; overweight women only |
| Sayeed MA, Mahtab H, Khanam PA, Begum R, Banu A, Khan AKA. Diabetes and hypertension in pregnancy in a rural community of Bangladesh: a population-based study. Diabet Med 2005;22:1267–71 | Considered for risk factors review; Bangladesh |
| Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG 2013;120:932–9 | No GDM prevalence data |
| Sella T, Shalev V, Elchalal U, Chovel-Sella A, Chodick G. Screening for gestational diabetes in the 21st century: a population-based cohort study in Israel. J Maternal Fetal Neonatal Med 2013;26:412–16 | Israel |
| Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;104:353–7 | Review |
| Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51:26–30 | Considered for risk factors review; Australia |
| Thorpe LE, Berger D, Ellis JA, et al. Trends and racial/ethnic disparities in gestational diabetes among pregnant women in New York City, 1990-2001. Am J Public Health 2005;95:1536–9 | Considered for risk factors review; USA |
| Van Leeuwen M, Opmeer BC, Yilmaz Y, Limpens J, Serlie MJ, Mol BWJ. Accuracy of the random glucose test as screening test for gestational diabetes mellitus: a systematic review. Eur J Obstet Gynecol Reprod Biol 2011;154:130–5 | Review |
| Wilson N, Ashawesh K, Smith S, Anwar A. The cost of screening for gestational diabetes mellitus. J Med Screening 2008;15:213 | Cost; no GDM prevalence data |
| Yang H, Wei Y, Gao X, Xu X, Fan L, He J, et al. Risk factors for gestational diabetes mellitus in Chinese women - A prospective study of 16 286 pregnant women in China. Diabet Med 2009;26:1099–104 | Considered for risk factors review. Chinese |
| Yapa M, Simmons D. Screening for gestational diabetes mellitus in a multiethnic population in New Zealand. Diabetes Res Clin Pract 2000;48:217–23 | New Zealand |
| Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract 2004;66:139–45 | Considered for risk factors review; India |
| Zhang F, Dong L, Zhang CP, Li B, Wen J, Gao W, et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabet Med 2011;28:652–7 | Considered for risk factors review; Chinese |
| Zhong Y, Lin PJ, Winn A, Cohen JT, Neumann PJ. A systematic review of cost-utility analyses in diabetes. Value Health 2013;16:A166 | Conference abstract; review; no GDM data |
Appendix 4 Tables for Chapter 5
| Reference | Reason for exclusion |
|---|---|
| Ahkter J, Qureshi R, Rahim F, Moosvi S, Rehman A, Jabbar A, et al. Diabetes in pregnancy in Pakistani women: prevalence and complications in an indigenous South Asian community. Diabet Med 1996;13:189–91 | States percentage of women with GDM with risk factors only, no other group |
| Bouzari Z, Yazdani S, Samakosh MA, Mohammadnetaj M, Emamimeybodi S. Prevalence of gestational diabetes and its risk factors in pregnant women referred to health centers of Babol, IRAN, from September 2010 to March 2012. Iranian J Obstet Gynecol Infert 2013;164:6–13 | Not in English |
| Branchtein L, Schmidt MI, Matos MC, Yamashita YT, Pousada JM, Duncan BB. Short stature and gestational diabetes in Brazil. Brazilian Gestational Diabetes Study Group. Diabetologia 43:848–51 | Reported height only |
| Branchtein L, Schmidt MI, Mengue SS, Reichelt AJ, Matos MC, Duncan BB. Waist circumference and waist-to-hip ratio are related to gestational glucose tolerance. Diabetes Care 1997;20:509–11 | Waist circumference and waist to hip ratio only |
| Brisson D, Perron P, Guay SP, Gaudet D, Bouchard L. The ‘hypertriglyceridemic waist’ phenotype and glucose intolerance in pregnancy. CMAJ 2010;182:E722–5 | Waist girth only, no GDM measure |
| Bryant M, Santorelli G, Lawlor DA, Farrar D, Tuffnell D, Bhopal R, et al. A comparison of South Asian specific and established BMI thresholds for determining obesity prevalence in pregnancy and predicting pregnancy complications: Findings from the Born in Bradford cohort. Int J Obesity 2014;38:444–50 | Based on the BiB cohort; already have original raw data |
| Bunthalarath S, Sunsaneevithayakul P, Boriboohirunsarn D. Risk factors for early diagnosis of gestational diabetes mellitus. J Med Assoc Thai 2004;87(Suppl. 3):50–3 | Early vs. late diagnosis of GDM |
| Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070–6 | Review |
| Chung JH, Melsop KA, Gilbert WM, Caughey AB, Walker CK, Main EK. Increasing pre-pregnancy body mass index is predictive of a progressive escalation in adverse pregnancy outcomes. J Mat Fetal Neonat Med 2012;25:1635–9 | No suitable data: GDM by obesity level, prevalence and outcomes study |
| Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Jaber Y, Banu I, et al. The diagnostic and prognostic performance of a selective screening strategy for gestational diabetes mellitus according to ethnicity in Europe. J Clin Endocrinol Metab 2014;99:996–1005 | Ethnicity only, same cohort as 37 |
| Dahanayaka NJ, Agampodi SB, Ranasinghe OR, Jayaweera PM, Wickramasinghe WA, Adhikari AN, et al. Inadequacy of the risk factor based approach to detect gestational diabetes mellitus. Ceylon Med J 2012;571:5-9 | No comparison between women with and without GDM |
| Detsch JCM, de Almeida ACR, Bortolini LGC, Nascimento DJ, Oliveira, Jr, FC, Rea RR. Markers of diagnosis and treatment in 924 pregnancies with gestational diabetes mellitus. Arq Bras Endocrinol Metabol 2011;55:389–98 | Not in English |
| Dooley SL, Metzger BE, Cho NH. Gestational diabetes mellitus. Influence of race on disease prevalence and perinatal outcome in a U.S. population. Diabetes 1991;40(Suppl. 2):25–9 | Ethnicity only, not UK |
| Edwards L, Hellerstedt W, Alton I, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstet Gynecol 1996;87:389–94 | Gestational weight gain only |
| Esakoff TF, Cheng YW, Caughey AB. Screening for gestational diabetes: different cut-offs for different ethnicities? Am J Obstet Gynecol 2005;193(Pt 2):1040–4 | Ethnicity only, not UK |
| Ezimokhai M, Joseph A, Bradley-Watson P. Audit of pregnancies complicated by diabetes from one center five years apart with selective versus universal screening. Ann N Y Acad Sci 2006;1084:132–40 | Ethnicity only, not UK |
| Foster-Powell KA, Cheung NW. Recurrence of gestational diabetes. Aust N Z J Obstet Gynaecol 1998;38:384–7 | Factors for recurrence of GDM only |
| Gregory R, Swinn RA, Wareham N, Curling V, Dalton KJ, Edwards OM, et al. An audit of a comprehensive screening programme for diabetes in pregnancy. Prac Diabet Int 1998;15:45–8 | No risk factors considered, audit only |
| Guttorm E. Practical screening for diabetes mellitus in pregnant women. Acta Endocrinol 1974;75(Suppl. 18):11–24 | Not explicitly GDM |
| Harder T, Franke K, Kohlhoff R, Plagemann A. Maternal and paternal family history of diabetes in women with gestational diabetes or insulin-dependent diabetes mellitus type I. Gynecol Obstet Invest 2001;51:160–4 | Only one risk factor (maternal family history of diabetes) considered |
| Hayes L, Bilous R, Bilous M, Brandon H, Crowder D, Emmerson C, et al. Universal screening to identify gestational diabetes: a multi-centre study in the North of England. Diabetes Res Clin Pract 2013;10:e74–7 | Women with GDM only |
| Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol 2010;24:441–8 | Ethnicity only, non UK |
| Helton MR, Arndt J, Kebede M, King M. Do low-risk prenatal patients really need a screening glucose challenge test? J Family Pract 1997;44:556–61 | No data on risk factors |
| Kim HS, Chang KH, Yang JI, Yang SC, Lee HJ, Ryu HS. Clinical outcomes of pregnancy with one elevated glucose tolerance test value. Int J Gynaecol Obstet 2002;78:131–8 | IGT rather than GDM |
| Lamberg S, Raitanen J, Rissanen P, Luoto R. Prevalence and regional differences of gestational diabetes mellitus and oral glucose tolerance tests in Finland. Eur J Public Health 2012;22:278–80 | Insufficient risk factor data, GDM prevalence only |
| McGuire V, Rauh MJ, Mueller BA, Hickock D. The risk of diabetes in a subsequent pregnancy associated with prior history of gestational diabetes or macrosomic infant. Paediatr Perinat Epidemiol 1996;10:64–72 | insufficient data on risks |
| Neelakandan R, Shankar Sethu P. Early universal screening for gestational diabetes mellitus. J Clin Diagnostic Res 2014;8:OC12–14 | Insufficient data on risks, prevalence only |
| Pedersen ML, Jacobsen JL, Jorgensen ME. Prevalence of gestational diabetes mellitus among women born in Greenland: measuring the effectiveness of the current screening procedure. Int J Circumpolar Health 2010;69:352–60 | No risk factor data |
| Pertot T, Molyneaux L, Tan K, Ross GP, Yue DK, Wong J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care 2011;34:2214–6 | Predicting insulin need, not GDM |
| Poyhonen-Alho MK, Teramo KA, Kaaja RJ, Hiilesmaa VK. 50 gram oral glucose challenge test combined with risk factor-based screening for gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2005;121:34–7 | Comparison of GCT and risk factors |
| Puavilai G, Kheesukapan P, Chanprasertyotin S, Chantraraprasert S, Suwanvilaikorn S, Nitiyanant W, et al. Random capillary plasma glucose measurement in the screening of diabetes mellitus in high-risk subjects in Thailand. Diabetes Res Clin Pract 2001;51:125–31 | Not GDM |
| Ray R, Heng BH, Lim C, Ling SL. Gestational diabetes in Singaporean women: use of the glucose challenge test as a screening test and identification of high risk factors. Ann Acad Med Singapore 1996;25:504–8 | GCT results rather than GDM |
| Retnakaran R, Connelly PW, Sermer M, Zinman B, Hanley AJ. The impact of family history of diabetes on risk factors for gestational diabetes. Clin Endocrinol 2007;67:754–60 | Insufficient data on risks |
| Rizvi JH, Rasul S, Malik S, Rehamatuallh A, Khan MA. Experience with screening for abnormal glucose tolerance in pregnancy: maternal and perinatal outcome. Asia Oceania J Obstet Gynaecol 1992;18:99–105 | No risk factor data |
| Salih S, Tedd H, Gillmer M. Screening for gestational diabetes mellitus in an indigenous Melanesian population on the islands of Vanuatu. J Obstet Gynaecol 2009;29:98–100 | GCT results only, not GDM |
| Samuel A, Simhan HN. Clinical indications for abnormal early gestational 50-g glucose tolerance testing. Am J Perinatol 2011;28:485–8 | Examining early testing, not GDM risk |
| Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995-2003. BJOG 2008;115:969–78 | Ethnicity only, non UK |
| Savona-Ventura C, Azzopardi J, Sant R. Risk factors for gestational diabetes mellitus in the Maltese population: a population based study. Int J Risk Safety Med 2000;13:1–7 | Only single risk factors considered |
| Sepe SJ, Connell FA, Geiss LS, Teutsch SM. Gestational diabetes. Incidence, maternal characteristics, and perinatal outcome. Diabetes 1985;34(Suppl. 2):13–16 | Insufficient risk factor data, incidence and outcomes only |
| Spong CY, Guillermo L, Kuboshige J, Cabalum T. Recurrence of gestational diabetes mellitus: identification of risk factors. Am J Perinatol 1998;15:29–33 | Recurrence of GDM |
| Sumeksri P, Wongyai S, Aimpun P. Prevalence of gestational diabetes mellitus (GDM) in pregnant women aged 30 to 34 years old at Phramongkutklao Hospital. J Med Assoc Thai 2006;89(Suppl. 4):94–9 | Used a different set of risk factors, combined risk factors into two categories: positive or negative only |
| Tan PC, Ling LP, Omar SZ. Screening for gestational diabetes at antenatal booking in a Malaysian university hospital: the role of risk factors and threshold value for the 50-g glucose challenge test. Aust N Z J Obstet Gynaecol 2007;47:191–7 | Compares GCT to OGTT; limited risk factor data |
| Theriault S, Forest JC, Masse J, Giguere Y. Validation of early risk-prediction models for gestational diabetes based on clinical characteristics. Diabetes Res Clin Pract 2014;103:419–25 | Secondary review of risk prediction models |
| Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 2009;10:194–203 | Review, no relevant data |
| Volpe L, Di Cianni G, Bottone P, Orsini P, Murru S, Casadidio I, et al. Gestational diabetes: clinical characteristics and birthweight. Ann Ist Super Sanita 1997;33:407–10 | No risk factor data presented. Outcome data |
| Wein P, Dong ZG, Beischer NA, Sheedy MT. Factors predictive of recurrent gestational diabetes diagnosed before 24 weeks’ gestation. Am J Perinatol 1995;12:352–6 | Recurrence of GDM |
| Young C, Kuehl TJ, Sulak PJ, Allen SR. Gestational diabetes screening in subsequent pregnancies of previously healthy patients. Am J Obstet Gynecol 2000;182:1024–6 | No relevant data |
| First author | Year | Screening method | Conclusions of the study authors | Favours risk factor screening? |
|---|---|---|---|---|
| Avalos122 | 2013 | ADA, NICE, Irish guideline recommendations | Strong case for universal screening; however, if selective screening adopted, the ADA guideline recommendations would have highest diagnosis rate, with lowest proportion of missed cases | Undecided |
| Caliskan142 | 2004 | Number of risk factors | Population based scoring system decreases unnecessary testing, but still diagnoses ≥ 85% of cases | Yes |
| Corcoy159 | 2004 | Various risk factors | Depending on the population selective screening is reliable at identifying women at low risk but unnecessarily complicated, as only 7% of women were at low risk on all factors | No |
| Cosson143 | 2013 | French guideline recommendations | One-third of women with GDM would be missed if selectively screened; do not support use of current French guideline recommendations | No |
| Cosson163 | 2006 | Number of risk factors | Universal rather than selective screening may improve outcomes | No |
| Crete160 | 2013 | Age, BMI, prior GDM | Risk factor screening to avoid need for glucose challenge testing, may increase glucose tolerance testing and costs | No |
| Cypryk144 | 2008 | Number of risk factors | Use of risk factors does not reliably identify those at risk of GDM, therefore all pregnant women should undergo laboratory screening | No |
| Danilenko-Dixon146 | 1999 | ADA guideline recommendations | Adherence ADA guideline recommendations would reduce number of screens by only 10% while increasing complexity | No |
| Davey161 | 2001 | ADA and ADIPS guideline recommendations | Selective screening can reduce need for testing with negligible loss of diagnostic accuracy | Yes |
| Göbl162 | 2012 | Risk factors with FPG | Risk factor screening with fasting plasma glucose is accurate but needs further evaluation | Undecided |
| Jensen145 | 2003 | Number of risk factors | Risk factor screening had similar performance to OGCT or the fasting plasma glucose test; using a risk based model could avoid OGTT in two-thirds of women | Yes |
| Jiménez-Moleón93 | 2002 | ADA and ACOG guideline recommendations | ADA guideline recommendations have similar disadvantages to other selective screening criteria, without apparent benefit | No |
| Marquette147 | 1985 | Number of risk factors | Testing only women over ≥ age 24 years would reduce costs with reasonable sensitivity in this population, but 10 of the 12 women with GDM were ≥ 24 years old | No |
| Moses148 | 1998 | Age, BMI ethnicity | GDM was diagnosed in 2.8% of low-risk women (Caucasian, < 25 years of age and < 25 kg/m2 BMI); not testing lower-risk women requires further evaluation, but selective testing requires testing 80% of this population and will miss 10% of cases | No |
| Nanda149 | 2011 | Risk model | First trimester screening for GDM is possible using a combination of maternal characteristics and biomarkers | Yes |
| Naylor164 | 1997 | Risk score | Consideration of women’s clinical characteristics can allow efficient selective screening | Yes |
| Ostlund150 | 2003 | Family history (of diabetes), obesity, prior macrosomic infant > 4500 g) or prior GDM | Using risk factors as an indicator to perform an OGTT gives a low sensitivity to detect GDM | No |
| Phaloprakam151 | 2009 | Risk score | The risk score is reliable for identifying women likely to have an abnormal OGCT | Yes |
| Pintaudi152 | 2014 | Number of risk factors | Selective screening reduces the number screened but 25% of women with GDM without risk factors will be missed | Undecided |
| Sacks153 | 1987 | Number of risk factors | Risk factor screening may enhance GDM detection, but criteria thresholds may prevent the identification of a proportion of cases | Undecided |
| Savona-Ventura165 | 2013 | Risk factors and fasting plasma glucose | Risk factor screening with fasting plasma glucose may be used in place of universal glucose tolerance testing in centres facing health-cost pressures | Undecided |
| Shamsuddin154 | 2001 | Number of risk factors | Universal screening appears to be the most reliable method of diagnosing GDM | No |
| Shirazian155 | 2009 | Risk score | Risk factor screening does not miss a substantial number of GDM cases | Yes |
| Sunsaneevithayakul156 | 2003 | Number of risk factors | Risk factor screening is appropriate | Yes |
| Teh157 | 2011 | NICE, ADA and ADIPS guideline recommendations | Selective screening criteria afford varied performance characteristics, with generally reasonable sensitivity but poor specificity with no benefit over universal screening. Costs and population characteristics should be considered, however | Undecided |
| Van Leeuwen141 | 2010 | Risk model | The clinical prediction model performed poorly, but the selective screening strategy is satisfactory and as accurate as universal screening with an OGCT | Yes |
| Van Leeuwen140 | 2009 | Risk score | Risk score has moderate discriminative capacity but appears clinically useful | Yes |
| Williams158 | 1999 | ADA guideline recommendations | ADA guideline recommendations will ensure 90% of women are screened and will miss only 4% of cases | Undecided |
| Yang139 | 2002 | ADA and WHO guideline recommendations | As this population are ‘high risk due to ethnicity’, different cut points or risk factors are required if screening is to be useful | Undecided |
Appendix 5 Tables for Chapter 6
| No. | Reference | Reason |
|---|---|---|
| 1 | Afaghi A, Ghanei L, Ziaee A. Effect of low glycemic load diet with and without wheat bran on glucose control in gestational diabetes mellitus: a randomized trial. Indian J Endocrinol Metab 2013;17:689–92 | No eligible outcomes, measured the reduction in women requiring insulin |
| 2 | Ainuddin J. Metformin: a safe alternative to insulin therapy in gestational diabetes. Int J Gynecol Obstet 2012;119:S270 | Conference abstract only, no data |
| 3 | Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract 2007;76:474–5 | Letter, only one outcome |
| 4 | Ardilouze JL, Menard J, Perron P, Houde G, Moutquin JM, Hivert MF, et al. Gestational diabetes mellitus: the first prospective randomised study of metformin-glyburide vs insulin. Diabetologia 2014;1:S449–50 | Duplicate of Ardilouze 2014214 |
| 5 | Arshad R, Karim N, Hasan JA. Effects of insulin on placental, fetal and maternal outcomes in gestational diabetes mellitus. Pak J Med Sci 2014;30:240–4 | Not randomised: two groups had different glucose levels |
| 6 | Asemi Z, Samimi M, Tabassi Z, Sabihi S-S, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 2013;29:619–24 | No relevant outcomes |
| 7 | Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr 2013;109:2024–30 | Duplicate of 6; no eligible outcomes measured tolerance and lipid glucose profiles |
| 8 | Athukorala C, Crowther CA, Willson K; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Women with gestational diabetes mellitus in the ACHOIS trial: risk factors for shoulder dystocia. Aust N Z J Obstet Gynaecol 2007;47:37–41 | ACHOIS secondary analysis; risk factors for shoulder dystocia |
| 9 | Avery MD, Leon AS, Kopher RA. Effects of a partially home-based exercise program for women with gestational diabetes. Obstet Gynecol 1997;1:10–15 | Exercise only, no relevant outcomes |
| 10 | Bahado-Singh RO, Mele L, Landon MB, et al. Fetal male gender and the benefits of treatment of mild gestational diabetes mellitus. Am J Obstet Gynecol 2012;206:422.e1–5 | Secondary analysis of Landon 200952 |
| 11 | Balaji V, Balaji MS, Alexander C, Ashalata S, Sheela Suganthi R, Suresh S, et al. Premixed insulin aspart 30Biasp(30) vs. premixed human insulin 30BHI(30) in gestational diabetes mellitus: a pilot study. J Assoc Phys India 2010;58:99–101 | Pilot of Balaji 2012195 |
| 12 | Balaji V, Balaji MS, Alexander C, Ashalata S, Suganthi RS, Suresh S, et al. Premixed insulin aspart 30 (Biasp 30) vs premixed human insulin 30 (BHi30) in gestational diabetes mellitus â?’ a pilot study. J Asso Phys Ind 2010;58:96–7 | Duplicate of 11259 |
| 13 | Bambicini JT, Soares VCM, Zanetti MRD, Torloni MR, Ribeiro MC, Mattar R. Effects of aerobic and resistance exercises on glycemic levels of patients with gestational diabetes: Pilot study. Int J Gynecol Obstet 2012;119:S603 | Conference abstract only; no eligible outcomes |
| 14 | Bancroft K, Tuffnell DJ, Mason GC, Rogerson LJ, Mansfield MA. Randomised controlled pilot study of the management of gestational impaired glucose tolerance. BJOG 2000;107:959–63 | Monitoring only |
| 15 | Barakat R, Perales M, Bacchi M, Coteron J, Refoyo I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot 2014;29:2–8 | Health promotion exercise |
| 16 | Barrett HL, Dekker Nitert M, Jones L, O’Rourke P, Lust K, Gatford KL, et al. Determinants of maternal triglycerides in women with gestational diabetes mellitus in the Metformin in Gestational Diabetes (MiG) study. Diabetes Care 2013;36:1941–6 | Subgroup analysis; no relevant outcomes |
| 17 | Barrett HL, Gatford KL, Houda CM, De Blasio MJ, McIntyre HD, Callaway LK, et al. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the Metformin in Gestational Diabetes (MiG) trial: responses to maternal metformin versus insulin treatment. Diabet Care 2013;36:529–36 | Subsequent analysis of data from an included trial, perinatal outcomes already reported in primary trial publication |
| 18 | Battin MR, Wouldes T, Buksh M, Rowan J. Neurodevelopmental outcome at 24-months in children following a randomized trial of metformin versus insulin treatment for gestational diabetes (MiG trial). J Paediat Child Health 2013;49:21 | No relevant outcomes |
| 19 | Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes Metab 2004;30:237–44 | Monitoring; no relevant outcomes |
| 20 | Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol 2004:1:188–93 | No relevant outcomes; need for insulin only |
| 21 | Bung P, Bung C, Artal R, Khodiguian N, Fallenstein F, Spätling L. Therapeutic exercise for insulin-requiring gestational diabetics: effects on the fetus: results of a randomized prospective longitudinal study. J Perinat Med 1993;21:125–37 | Duplicate of Bung 1991204 and no relevant data |
| 22 | Clarke P, Coleman MA, Holt RI. Alternative site self blood glucose testing is preferred by women with gestational diabetes. Diabetes Technol Ther 2005;7:604–8 | No relevant outcomes |
| 23 | Coiner J, Rowe M, DeVente J. The treatment of diabetes in pregnancy; metformin vs glyburide and insulin–biomedical evidence of fetopathy. Am J Obstet Gynecol 2014;1:S148 | Conference abstract: no relevant outcomes; not explicitly in GDM |
| 24 | Cordua S, Secher AL, Ringholm L, Damm P, Mathiesen ER. Real-time continuous glucose monitoring during labour and delivery in women with Type 1 diabetes: observations from a randomized controlled trial. Diabet Med 2013;30:1374–81 | Monitoring only |
| 25 | Cordua S, Secher AL, Ringholm L, Damm P, Mathiesen ER. Real-time continuous glucose monitoring during delivery in women with type 1 diabetes. Diabetes Technol Ther 2013;15:A73 | Glucose monitoring trial and duplicate of 23 |
| 26 | Corrado F, D’Anna R, Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet Med 2011;8:972–5 | No relevant outcomes |
| 27 | Cortez J, Tarsa M, Agent S, Chmait R, Moore T. Randomized controlled trial of acarbose vs. placebo in the treatment of gestational diabetes. Am J Obstet Gynecol 2006;6(Suppl. 1):S149 | Abstract, insufficient data |
| 28 | Coustan DR, Lewis SB. Insulin therapy for gestational diabetes. Obstet Gynecol 1978;51:306–10 | Presentation of outcome data not compatible |
| 29 | Coustan D. Treating mild gestational diabetes yields benefits with little or no evidence of harms. Evid Based Med 2014;19:88 | Commentary on another review |
| 30 | Crowther CA, Hague WM, Middleton PF, Baghurst PA, McPhee AJ, Tran TS, et al. The IDEAL study: investigation of dietary advice and lifestyle for women with borderline gestational diabetes: a randomised controlled trial - study protocol. BMC Pregnancy Childbirth 2012;12:106 | Protocol only |
| 31 | Dalfra MG, Nicolucci A, Lapolla A, TISG. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. J Telemed Telecare 2009;15:238–42 | Telemedicine |
| 32 | de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol 2010;203:556.e1–6 | No relevant outcomes |
| 33 | Ding G, Liang P, Peng Y, Pang Y, Zheng Y. Evaluation of Continuous Glucose Monitoring (CGM) on gestational diabetes mellitus in China. Diabetes 2012;61:A588 | Monitoring only |
| 34 | Durnwald CP, Mele L, Spong CY, Ramin SM, Varner MW, Rouse DJ, et al. Glycemic characteristics and neonatal outcomes of women treated for mild gestational diabetes. Obstet Gynecol 2011;117:819–27 | Secondary analysis of Langdon 2008, association between post-prandial glucose and outcomes |
| 35 | Ehrlich S. Physical activity and gestational weight gain (GWG) in women with GDM. Diabetes 2013;62:A18–19 | No relevant outcomes |
| 36 | Ehrlich SF, Hedderson MM, Feng J, Crites Y, Quesenberry CP, Ferrara A. Lifestyle intervention improves postpartum fasting glucose levels in women with gestational diabetes. Diabetes 2014;63:A95 | No relevant outcomes |
| 37 | Ehrlich SF, Hedderson MM, Quesenberry CP, Jr, Feng J, Brown SD, Crites Y, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med 2014;31:862–7 | No relevant outcomes |
| 38 | Ferrara A. Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial). ClinicalTrials.gov. URL: http://clinicaltrials.gov/ (accessed 20 April 2015) | Ongoing trial |
| 39 | Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry Jr CP, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34:1519–25 | No relevant outcomes |
| 40 | Ford FA, Bruce CB, Fraser RB. Preliminary report of a randomised trial of dietary advice in women with mild abnormalities of glucose tolerance in pregnancy | Unpublished work cited in Alwan review168 |
| 41 | Garcia-Patterson A, Martin E, Ubeda J, Maria MA, de Leiva A, Corcoy R. Evaluation of light exercise in the treatment of gestational diabetes. Diabetes Care 2001;24:2006–7 | No relevant outcomes |
| 42 | Gatford KL, Houda CM, Lu ZX, Coat S, Baghurst PA, Owens JA, et al. Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: responses to maternal metformin compared with insulin treatment. Diabetes Obes Metab 2013;15:660–7 | |
| 43 | Gillen LJ, Tapsell LC. Advice that includes food sources of unsaturated fat supports future risk management of gestational diabetes mellitus. J Am Diet Assoc 2004;104:1863–7 | No relevant outcomes |
| 44 | Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–8 | Secondary analysis of Crowther 200551 |
| 45 | Graham G, Johnson EB, Johnson A, Anderson R, Devine P. Cinnamon for glycemic control in gestational diabetes: a randomized double-blind placebo controlled pilot study. Am J Obstet Gynecol 2005;193:S91 | No relevant outcomes |
| 46 | Grant SM, Wolever TM, O’Connor DL, Nisenbaum R, Josse RG. Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Res Clin Pract 2011;91:15–22 | No relevant outcomes |
| 47 | Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLOS ONE 2013;8:e64585 | Systematic review |
| 47 | Han S, Crowther CA, Middleton P, Heatley E. Different types of dietary advice for women with gestational diabetes mellitus. Cochrane Database Syst Rev 2013;3:CD009275 | Systematic review on dietary advice for women |
| 48 | Han S, Crowther CA, Middleton PF, Tran T, Zhang Y. Women with pregnancy hyperglycaemia: How well are lifestyle information booklets used? J Paediatr Child Health 2013;49:93–4 | Use of information lifestyle booklet |
| 49 | Han S, Heatley E, Middleton P, Crowther CA. Different types of dietary advice for women with gestational diabetes mellitus: a Cochrane review. J Paediatr Child Health 2012;48:114 | Abstract on Cochrane Review on dietary advice |
| 50 | Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013;159:123–9 | Systematic review |
| 51 | Hashmi F, Malik A, Sheikh L, Ismail H. Effectiveness of metformin versus insulin for treating diabetes in pregnancy: a retrospective cohort study to compare maternal and perinatal outcomes. BJOG 2012;119:95 | No relevant outcomes |
| 52 | Hernandez TL, Vanpelt RE, Krause MA, Reece MS, Donahoo WT, Mande A, et al. Higher carbohydrate vs. Higher fat diet in gestational diabetes: a randomized study. Diabetes 2012;61:A50 | No relevant outcomes and a crossover study |
| 53 | Hernandez TL, Anderson MA, Vanpelt RE, Reece MS, Reynolds R, De La Houssaye B, et al. Women with gestational diabetes randomized to a low-carbohydrate/ higher fat diet demonstrate greater insulin resistance and infant adiposity. Diabetes 2013;62:A18 | No relevant outcomes |
| 54 | Hernandez TL, Van Pelt RE, Anderson MA, Daniels LJ, West NA, Donahoo WT, et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care 2014;37:1254–62 | No relevant outcomes and a crossover study |
| 55 | Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. Am J Perinatol 2013;30:483–90 | Includes pre-existing diabetics |
| 56 | Homko CJ, Deeb LC, Rohrbacher K, Mulla W, Mastrogiannis D, Gaughan J, et al. Impact of a telemedicine system with automated reminders on outcomes in women with gestational diabetes mellitus. Diabetes Technol Ther 2012;14:624–9 | Telemedicine |
| 57 | Homko CJ, Santamore WP, Whiteman V, Bower M, Berger P, Geifman-Holtzman O, et al. Use of an internet-based telemedicine system to manage underserved women with gestational diabetes mellitus. Diabetes Technol Ther 2007;9:297–306 | Telemedicine |
| 58 | Homko CJ, Sivan E, Reece EA. The impact of self-monitoring of blood glucose on self-efficacy and pregnancy outcomes in women with diet-controlled gestational diabetes. Diabetes Educ 2002;28:435–43 | Monitoring |
| 59 | Hopp H, Vollert W, Ragosch V, Novak A, Weitzel HK, Glöckner E, et al. Indication and results of insulin therapy for gestational diabetes mellitus. J Perinat Med 1996;24:521–30 | No relevant data; this trial compared outcomes rates associated with amniotic fluid insulin concentration and mean blood glucose levels |
| 60 | Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, et al. Effects of treatment in women with gestational diabetes mellitus: Systematic review and meta-analysis. BMJ 2010;340:796 | Systematic review |
| 61 | Hutchinson A, Haugabrook C, Long L, Mason L, Kipikasa J, Adair D. A comparison between glyburide/metformin and insulin for gestational diabetes. Am J Obstet Gynecol 2008;199:S200 | No relevant outcomes |
| 62 | Ibrahim MI, Hamdy A, Shafik A, Taha S, Anwar M, Faris M. The role of adding metformin in insulin-resistant diabetic pregnant women: a randomized controlled trial. Archives Gynecol Obstet 2014;289:959–65 | Included women with pre-pregnancy diabetes |
| 63 | Jovanovic L, Gutierrez M, Peterson CM. Chromium supplementation for women with gestational diabetes mellitus. J Trace Elem Exp Med 1999;12:91–7 | Chromium supplementation; no relevant outcomes |
| 64 | Jovanovic L, Howard C, Pettitt D, Zisser H, Ospina P. Insulin aspart vs. regular human insulin in basal/bolus therapy for patients with gestational diabetes mellitus: safety and efficacy. Diabetologia 2005;48:A317 | No relevant outcomes |
| 65 | Jovanovic-Peterson L, Durak EP, Peterson CM. Randomized trial of diet versus diet plus cardiovascular conditioning on glucose levels in gestational diabetes. Am J Obstet Gynecol 1989;161:415–19 | No relevant outcomes |
| 66 | Jovanovic-Peterson L, Sparks S, Palmer JP, Peterson CM. Jet-injected insulin is associated with decreased antibody production and postprandial glucose variability when compared with needle-injected insulin in gestational diabetic women. Diabetes care 1993;16:1479–84 | No relevant outcomes |
| 67 | Joy S, Roman A, Rebarber A, Fox N, Istwan N, Rhea D, et al. Is risk for gestational diabetes modifiable once an obese woman is pregnant? Am J Obstet Gynecol 2012;1:S132 | No relevant outcomes |
| 68 | Kaveh M, Kiani A, Salehi M, Amouei S. Impact of education on nutrition and exercise on the level of knowledge and metabolic control indicators (FBS & PPBS) of gestational diabetes mellitus (GDM) patients. Iranian J Endocrinol Met 2012;13:442–9 | Non-English |
| 69 | Kavitha N, De S, Kanagasabai S. Oral hypoglycaemic agents in pregnancy. J Obstet Gynecol India 2013;63:82–7 | Review |
| 70 | Keely EJ, Malcolm JC, Hadjiyannakis S, Gaboury I, Lough G, Lawson ML. Prevalence of metabolic markers of insulin resistance in offspring of gestational diabetes pregnancies. Pediatr Diabetes 2008;9:53–9 | Follow-up study |
| 71 | Khin MO, Vatish M, Gates S, Saravanan P. Evaluation of metformin in gestational diabetes: Systematic review and metaanalysis. Diabet Med 2013;30:12 | Abstract of systematic review |
| 72 | Klebanoff M. Treatment of gestational diabetes (GDM), weight gain and perinatal outcome-marginal structural model (MSM) analysis. Am J Epidemiol 2011;173:S41 | Secondary analysis of Landon 2009;52 no relevant outcomes |
| 73 | Knopp RH, Magee MS, Raisys V, Benedetti T, Bonet B. Hypocaloric diets and ketogenesis in the management of obese gestational diabetic women. J Am Coll Nutr 1991:649–67 | No relevant outcomes |
| 74 | Lain K, Garabedian M, Daftary A, Jeyabalan A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared to insulin. 2008:S34 | No relevant outcomes |
| 75 | Landon MB, Thom E, Spong CY, Carpenter M, Mele L, Johnson F, et al. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network randomized clinical trial in progress: standard therapy versus no therapy for mild gestational diabetes. Diabetes Care 2007;30:S194–9 | Trial in progress |
| 76 | Landon MB. A prospective multicenter randomized treatment trial of mild gestational diabetes (GDM). Am J Obstet Gynecol 2008;196:S2 | Abstract of Landon 200952 |
| 77 | Landon MB, Thom E, Spong CY, Gabbe SG, Leindecker S, Johnson F, et al. A planned randomized clinical trial of treatment for mild gestational diabetes mellitus. J Matern Fetal Neonatal Med 2002:4:226–31 | Planned trial |
| 78 | Landon M. Mild gestational diabetes mellitus (GDM) treatment and long term child health. Am J Obstet Gynecol 2014;210:S408–9 | Follow-up of included trial; no relevant outcomes |
| 79 | Langer O, Anyaegbunam A, Brustman L, Divon M. Management of women with one abnormal oral glucose tolerance test value reduces adverse outcome in pregnancy. Am J Obstet Gynecol 1989;161:593–9 | Ineligible intervention |
| 80 | Langer O, Conway D, Berkus M, Xenakis EMJ. Oral hypoglycaemic agent is comparable to insulin in GDM management. Am J Obstet Gynecol 1999;1(Pt 2):S6 | Preliminary for a subsequently published trial |
| 81 | Langer O, Rodriguez DA, Xenakis EM, McFarland MB, Berkus MD, Arrendondo F. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol 1994;4:1036–46; discussion 46–7 | No relevant outcomes |
| 82 | Langer O, Yogev Y, Xenakis EMJ, Rosenn B. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. Am J Obstet Gynecol 2005;1:134–9 | Secondary analysis |
| 83 | Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol 2005:6:1768–76 | Outcomes by BMI |
| 84 | Lauszus FF, Rasmussen OW, Henriksen JE, Klebe JG, Jensen L, Lauszus KS, et al. Effect of a high monounsaturated fatty acid diet on blood pressure and glucose metabolism in women with gestational diabetes mellitus. Eur J Clin Nutr 2001:6:436–43 | No relevant outcomes |
| 85 | Lepercq J, Lin J, Hall GC, Wang E, Dain M-P, Riddle MC, et al. Meta-analysis of maternal and neonatal outcomes associated with the use of insulin glargine versus NPH insulin during pregnancy. Obstet Gynecol Int 2012:649070 | Meta-analysis not based on RCTs |
| 86 | Lesser KB, Gruppuso PA, Terry RB, Carpenter MW. Exercise fails to improve postprandial glycemic excursion in women with gestational diabetes. J Matern Fetal Med 1996;4:211–17 | No relevant outcomes |
| 87 | Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes 1990;2:234–40 | No relevant outcomes |
| 88 | Mahdian M, Behrashi M, Aliasgharzadeh A. Effects of zinc supplementation on glycemic control and complications of gestational diabetes. Pakistan J Med Sci 2011;27:1203–6 | Ineligible treatment |
| 89 | Martinez P, Abdulhaj Martinez M, Andres Nunez P, Garcia Leon P, Lopez Sanchez EJ, Gonzalez Ramirez AR. A randomized study comparing metformin and insulin in the treatment of gestational diabetes mellitus. Interim results. J Maternal Fetal Neonatal Med 2010;S1:381 | No relevant outcome data |
| 90 | Maso G, Alberico S, Wiesenfeld U, Ronfani L, Erenbourg A, Hadar E, et al. GINEXMAL RCT: Induction of labour versus expectant management in gestational diabetes pregnancies. BMC Pregnancy Childbirth 2011;11:31 | Protocol |
| 91 | Maslovitz S, Shenhav M, Bibi G, Pauzner D, Many A. Insulin combined with metformin for glucose control of diabetes during pregnancy. Am J Obstet Gynecol 2012;1:S132–3 | Retrospective trial; abstract only |
| 92 | Mathews JE, Biswas B, Samuel P, Jana AK, Muliyil JP, Mathai M. Retrospective cohort study comparing neonatal outcomes of women treated with glyburide or insulin in gestational diabetes: a 5-year experience in a South Indian teaching hospital. Indian J Med Sci 2011;65:476–81 | Not an RCT |
| 93 | Mendelson SG, McNeese-Smith D, Koniak-Griffin D, Nyamathi A, Lu MC. A community-based parish nurse intervention program for Mexican American women with gestational diabetes. J Obstet Gynecol Neonatal Nurs 2008;4:415–25 | No relevant outcomes |
| 94 | Middleton PF, Collins CT, Crowther CA, Flenady V, Makrides M, Rumbold A, et al. Dietary influences on diabetes in pregnancy: a systematic review. J Paediatr Child Health 2011;47:40 | Systematic review |
| 95 | Moore L, Clokey D, Curet L. A randomized controlled trial of metformin and glyburide in gestational diabetes. Am J Obstet Gynecol 2008;6(Suppl. 1):34 | Abstract of Moore 2010192 |
| 96 | Moore L, Clokey D, Robinson A. A randomized trial of metformin compared to glyburide in the treatment of gestational diabetes. Am J Obstet Gynecol 2005;6(Suppl.):92 | Abstract of Moore 2010192 |
| 97 | Moses RG, Barker M, Winter M, Petocz P, Brand-Miller JC. Can a low-glycemic index diet reduce the need for insulin in gestational diabetes mellitus? A randomized trial. Diabetes Care 2009:6:996–1000 | No relevant outcomes |
| 98 | Moss JR, Crowther CA, Hiller JE, McPhee AJ, Jeffries WS, Willson KJ. Costs and consequences of treatment of gestational diabetes mellitus – evaluation from the ACHOIS randomised trial. J Paediatr Child Health 2007;43(Suppl. 1):A28–9 | Economics paper |
| 99 | Moss JR, Crowther CA, Hiller JE, Willson KJ, Robinson JS. Costs and consequences of treatment for mild gestational diabetes mellitus – evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth 2007;7:27 | Economics paper |
| 100 | Ney D, Hollingsworth DR, Cousins L. Decreased insulin requirement and improved control of diabetes in pregnant women given a high-carbohydrate, high-fiber, low-fat diet. Diabetes Care 1982;5:529–33 | No relevant outcomes |
| 101 | Nolan CJ. Improved glucose tolerance in gestational diabetic women on a low fat, high unrefined carbohydrate diet. Aust N Z J Obstet Gynaecol 1984;3:174–7 | No relevant outcomes |
| 102 | Nor Azlin MI, Nor NA, Sufian SS, Mustafa N, Jamil MA, Kamaruddin NA. Comparative study of two insulin regimes in pregnancy complicated by diabetes mellitus. Acta Obstet Gynecol Scand 2007;4:407–8 | No relevant outcomes |
| 103 | O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Medical treatment of the gestational diabetic. Obstet Gynecol 1974;43:817–21 | Secondary analysis of O’Sullivan 1966211 |
| 104 | O’Sullivan JB, Mahan CM. Insulin treatment and high risk groups. Diabetes Care 1980;3:482–5 | No relevant outcomes; secondary analysis of O’Sullivan 1966211 |
| 105 | Page RC, Harnden KE, Walravens NK, Onslow C, Sutton P, Levy JC, et al. ‘Healthy living’ and sulphonylurea therapy have different effects on glucose tolerance and risk factors for vascular disease in subjects with impaired glucose tolerance. Q J Med 1993;3:145–54 | Not GDM |
| 106 | Perichart-Perera O, Balas-Nakash M, Rodriguez-Cano A, Legorreta-Legorreta J, Parra-Covarrubias A, Vadillo-Ortega F. Low glycemic index carbohydrates versus all types of carbohydrates for treating diabetes in pregnancy: a randomized clinical trial to evaluate the effect of glycemic control. Int J Endocrinol 2012:296017 | No relevant outcomes |
| 107 | Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009;35:418–21 | Not GDM |
| 108 | Pettitt DJ, Ospina P, Howard C, Zisser H, Jovanovic L. Efficacy, safety and lack of immunogenicity of insulin aspart compared with regular human insulin for women with gestational diabetes mellitus. Diabet Med 2007;10:1129–35 | No relevant outcome data |
| 109 | Pirc LK, Owens JA, Crowther CA, Willson K, Blasio MJ, Robinson JS. Mild gestational diabetes in pregnancy and the adipoinsular axis in infants born to mothers in the ACHOIS randomised controlled trial. BMC Pediatrics 2007;7:18 | No relevant outcomes |
| 110 | Reader D, Splett P, Gunderson EP. Impact of gestational diabetes mellitus nutrition practice guidelines implemented by registered dietitians on pregnancy outcomes. J Am Diet Assoc 2006;106:1426–33 | Nutrition guidelines |
| 111 | Reece EA, Hagay Z, Gay LJ, O’Connor T, DeGennaro N, Homko CJ. A randomized clinical trial of a fiber-enriched diabetic diet vs. the standard American Diabetes Association-recommended diet in the management of diabetes mellitus in pregnancy. J Maternal Fetal Invest 1995;1:8–12 | No relevant outcome data |
| 112 | Rosales L, Morales F, Stuardo P, Marquez J, Barria M, Martinovic C. Metabolic profile in diet treated and glibenclamide treated gestational diabetes. J Perinat Med 2011;39 | No relevant outcome data |
| 113 | Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care 2011;34:2279–84 | No relevant outcomes |
| 114 | Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care 2010;1:9–16 | Secondary analysis of Rowan 2008180 (MiG); no relevant outcomes |
| 115 | Schaefer-Graf UM, Kjos SL, Fauzan OH, Buhling KJ, Siebert G, Buhrer C. A randomized trial evaluating a predominately fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 2004;27:297–302 | Ineligible intervention |
| 116 | Silva JC, Pacheco C, Bizato J, Souza BV, Ribeiro TE, Bertini AM. Metformin compared with glyburide for the management of gestational diabetes. Int J Gynaecol Obstet 2010;1:37–40 | Preliminary publication of Silva 2010213 (114) |
| 117 | Macrosomia: results and preventions strategies. Rev Bras Ginecol Obstet 2005;8:461–6 | Preliminary results of Silva and non-English |
| 118 | Singh S, Mahajan S, Aswani R, Trader B, Hale S, Mullarky L. Outcome of an Appalachian pregnant diabetics managed @perinatal diabetes center (PDC) on modified ADA diet calorie, carbohydrate (CHO) (restricted and on either conventional insulin therapy regular/NPH) or analog insulin novolog (glargine or detemir) therapy. 70th Scientific Sessions of the American Diabetes Association Orlando, FL, USA, 2010 | Retrospective observational cohort review |
| 119 | Sugiyama T, Hiramatsu Y, Sagawa N, Yaegashi N. A retrospective multi-institutional study of the treatment of mild gestational diabetes in Japan. 73rd Scientific Sessions of the American Diabetes Association July; Chicago, IL, USA, 2013, A362 | Not an RCT |
| 120 | Sugiyama T, Metoki H, Hamada H, Nishigori H, Saito M, Yaegashi N, et al. A retrospective multi-institutional study of treatment for mild gestational diabetes in Japan. Diabetes Res Clin Pract 2014;103:412–18 | Not an RCT and duplicate of 118 |
| 121 | Tertti, K, Laine K, Ekblad U, Rinne V, Rönnemaa T. The degree of fetal metformin exposure does not influence fetal outcome in gestational diabetes mellitus. Acta Diabetologica 2014;51:731–8 | No relevant outcomes |
| 122 | Todorova K, Palaveev O, Petkova VB, Stefanova M, Dimitrova Z. A pharmacoeconomical model for choice of a treatment for pregnant women with gestational diabetes. Acta Diabetologica 2007;3:144–8 | Not RCT |
| 123 | Tuuli M, Caughey A, Odibo A, Macones G, Cahill A. Glyburide versus insulin for management of gestational diabetes: a systematic review and meta-analysis. Am J Obstet Gynecol 2012;1:S170–1 | Abstract of a systematic review |
| 124 | Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med 1995;333:1237–41 | Monitoring trial |
| 125 | Yang X, Hsu-Hage BH, Dong L, Zhang H, Zhang C, Zhang Y. Postpartum glucose intolerance in Chinese women with gestational diabetes. Diabet Med 2003;20:687–9 | Letter linked to Yang 2003199 |
| 126 | Zanganeh M. The comparative study of therapeutic effects of insulin and glibenclamide in the gestational diabetes mellitus. Iranian Registry of Clinical Trials. URL: www.irct.ir (accessed April 2015) | No published data |
| Author | Year | Included in a previous review | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessments | Completeness of outcome data | Selective reporting |
|---|---|---|---|---|---|---|---|---|
| Abbassi-Ghanavati194 | 2014 | – | Unclear | Low risk | Unclear | Low risk | High risk | Unclear |
| Anjalakshi184 | 2007 | – | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear |
| Ardilouze214 | 2014 | – | Unclear | Unclear | Unclear | Unclear | Unclear | High risk |
| Asemi215 | 2014 | – | Low risk | Unclear | High risk | High risk | Low risk | Low risk |
| Balaji195 | 2012 | a | Unclear | Unclear | High risk | High risk | Low risk | Low risk |
| Bertini185 | 2005 | Alwan168 | Low risk | Low risk | High risk | High risk | Low risk | Low risk |
| Bevier202 | 1999 | Hartling1 | Unclear | Unclear | High risk | High risk | High risk | Low risk |
| Bo216 | 2014 | – | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Bonomo203 | 2005 | Hartling,1 Horvath169 | Unclear | Unclear | High risk | High risk | Low risk | Unclear |
| Bung204 | 1991 | – | Unclear | Unclear | High risk | High risk | High risk | Unclear |
| Cao217 | 2012 | – | Unclear | Unclear | High risk | Unclear | High risk | Low risk |
| Crowther51 | 2005 | Alwan,168 Falavigna,170 Hartling,1 Horvath169 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Cypryk209 | 2007 | – | Unclear | High risk | Unclear | Unclear | Low risk | High risk |
| Deveer197 | 2013 | – | High risk | High risk | High risk | High risk | Low risk | Low risk |
| Di Cianni210 | 2007 | – | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Elnour200 | 2008 | a | Unclear | High risk | High risk | High risk | High risk | Low risk |
| Garner201 | 1997 | Falavigna,170 Hartling1 | Low risk | High risk | High risk | High risk | Low risk | Low risk |
| Hague176 | 2003 | Alwan168 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Hassan175 | 2012 | – | High risk | High risk | Unclear | Unclear | Low risk | Low risk |
| Ijäs177 | 2010 | aGui171 | Low risk | Low risk | High risk | High risk | Low risk | Low risk |
| Jovanovic218 | 1999 | – | Low risk | Unclear | High risk | High risk | Low risk | Low risk |
| Kjos205 | 2001 | – | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| Lain186 | 2009 | a | Low risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Landon52 | 2009 | Hartling,1 Falavigna,170aHorvath169 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Langer187 | 2000 | aAlwan168 | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Li198 | 1987 | Falavigna170 | High risk | Unclear | High risk | Unclear | Low risk | Low risk |
| Louie219 | 2011 | – | Low risk | Low risk | Low risk | Unclear | Low risk | High risk |
| Mesdaghinia178 | 2013 | – | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Moore179 | 2007 | Gui171 | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Moore192 | 2010 | a | Low risk | Low risk | High risk | High risk | Low risk | Low risk |
| Moreno-Castilla206 | 2013 | – | Unclear | Low risk | High risk | Unclear | Low risk | Low risk |
| Mukhopadhyay188 | 2012 | – | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Nachum207 | 1999 | – | Low risk | Low risk | High risk | Unclear | Low risk | Low risk |
| Niromanesh196 | 2012 | Gui171 | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk |
| Ogunyemi189 | 2007 | – | Low risk | Unclear | Unclear | Unclear | Low risk | Unclear |
| O’Sullivan211 | 1966 | Falavigna170 | Unclear | Unclear | High risk | High risk | Unclear | Unclear |
| Rae212 | 2000 | – | Unclear | Unclear | Low risk | Unclear | Low risk | High risk |
| Rowan180 | 2008 | aGui171 | Low risk | Unclear | High risk | High risk | Low risk | Low risk |
| Silva193 | 2012 | – | Low risk | Unclear | High risk | High risk | Low risk | Low risk |
| Silva190 | 2007 | a | Unclear | Low risk | High risk | High risk | Low risk | Low risk |
| Spaulonci181 | 2013 | – | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Tempe191 | 2013 | – | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk |
| Tertti182 | 2013 | Gui171 | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk |
| Thompson208 | 1990 | – | Low risk | Unclear | High risk | Unclear | High risk | Low risk |
| Yang199 | 2003 | – | Unclear | Unclear | High risk | Unclear | High risk | Unclear |
| Zinnat183 | 2013 | – | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear |
Appendix 6 Tables and figures for Chapter 7
| Key aspects | Approach | Assumptions/Justification | Section in Chapter 7 |
|---|---|---|---|
| Model | Cost-effectiveness (cost–utility) analysis using a decision tree | – | Decision-analytic model |
| Population | Obstetric population | – | |
| Time horizon | Three months (third pregnancy trimester) |
|
Adverse perinatal outcomes |
| Comparators | (a) No intervention (b) Screen only: screening followed by dietary and lifestyle advice for those who screen positive (c) Universal diagnostic test: diagnostic test followed by dietary and lifestyle advice with pharmacological treatment as required for those who exceed a diagnostic threshold (d) Screen and diagnostic test: screening followed by diagnostic test in those who screen positive, with dietary and lifestyle advice and pharmacological treatment as required for those who exceed a diagnostic threshold (screening strategies combined with the test using best-performing diagnostic threshold) |
– | Additional screen, diagnosis and treatment strategies |
| Subgroups |
|
||
| Screening and diagnostic |
|
Blood-based tests for hypoglycaemia in pregnancy | |
| Clinical outcomes | Perinatal adverse outcomes with impact on costs and/or HRQL that were available in the BiB22 and Atlantic DIP59 cohorts, or could be modelled by including external data: pre-eclampsia; CS; shoulder dystocia; instrumental delivery; induction of labour; admission to a neonatal care unit; macrosomia; neonatal death; and birth trauma |
|
Adverse perinatal outcomes |
|
|||
| Baseline risk models were estimated in a combined data set with BiB and Atlantic DIP31,59 data |
|
Baseline probabilities of perinatal outcomes | |
| Missing data on outcome models was handled with MICE |
|
||
| Maternal prevalence of undiagnosed overt type 2 diabetes and incidence if type 2 associated to prior GDM are included in scenario analysis | Incidence of type 2 diabetes among women with a history of gestational diabetes mellitus | ||
|
|||
| Treatment effectiveness | RR of adverse perinatal outcomes on treated vs. untreated women with GDM |
|
Treatment effects |
|
|||
|
|||
|
|||
| Uptake | Model includes uptake estimates on screening, diagnostic, treatment and post-partum follow-up |
|
Uptake of screening diagnosis and treatment |
|
|||
|
|||
|
|||
| HRQL | QALY loss from adverse perinatal outcomes (pre-eclampsia, CS, shoulder dystocia, instrumental delivery, neonatal death, and birth trauma) |
|
Health-related quality of life loss from adverse perinatal outcomes |
|
|||
|
|||
| QALY gain from treatment of hyperglycaemia |
|
||
| QALY gain from prevention of type 2 diabetes QALY gain from early detection of overt diabetes at post-partum follow-up |
|
Health-related quality of life gains from the prevention of maternal type 2 diabetes
Net benefit of early detection of diabetes |
|
|
|||
| Adverse events | Hypoglycaemia resulting from treatment for GDM |
|
Treatment effects |
|
|||
| Resource use and costs | Resource use and costs categories in the model include blood-based tests, adverse perinatal outcomes, treatment of hyperglycaemia, prevention of type 2 diabetes and early detection of overt diabetes |
|
Resource use and costs |
| Discount rates | Annual rate of 3.5% for costs early detection of overt diabetes post partum and HRQL gains and losses realised after post-partum period | No discount rate was applied to costs which were assumed to occur within 12 months of testing (screening and/or diagnosis) in accordance with current guidance225 | Resource use and costs |
| Sensitivity analysis | Sensitivity analysis includes probabilistic sensitivity analysis and scenario analysis. The scenarios assessed in the analysis include:
|
– | Sensitivity and scenario analysis |
| Strategy | Screen positive determined on the basis of at least one factor: risk factor criteria | ||||||
|---|---|---|---|---|---|---|---|
| Previous GDM | Previous macrosomia | BMI (kg/m2) ≥ | Multiparous | Maternal age ≥ | Non-white ethnicity | Family history of diabetes | |
| 1 | ✗ | ||||||
| 2 | ✗ | ||||||
| 3 | 30 | ||||||
| 4 | ✗ | ||||||
| 5 | 30 | ||||||
| 6 | 25 | ||||||
| 7 | ✗ | ||||||
| 8 | 25 | ||||||
| 9 | ✗ | ✗ | |||||
| 10 | ✗ | 30 | |||||
| 11 | ✗ | ✗ | |||||
| 12 | ✗ | ✗ | |||||
| 13 | ✗ | 30 | |||||
| 14 | 30 | ✗ | |||||
| 15 | 30 | 30 | |||||
| 16 | 25 | 30 | |||||
| 17 | ✗ | 25 | |||||
| 18 | 30 | ✗ | |||||
| 19 | 30 | 25 | |||||
| 20 | 25 | ✗ | |||||
| 21 | 25 | ✗ | |||||
| 22 | 25 | 25 | |||||
| 23 | ✗ | 30 | ✗ | ||||
| 24 | ✗ | 30 | 30 | ||||
| 25 | ✗ | 30 | ✗ | ||||
| 26 | 30 | ✗ | 30 | ||||
| 27 | 30 | 30 | ✗ | ||||
| 28 | ✗ | 25 | 30 | ||||
| 29 | ✗ | 30 | ✗ | ||||
| 30 | 25 | 30 | ✗ | ||||
| 31 | ✗ | 30 | 25 | ||||
| 32 | 30 | 25 | ✗ | ||||
| 33 | 30 | 30 | ✗ | ||||
| 34 | 25 | 25 | ✗ | ||||
| 35 | ✗ | 25 | ✗ | ||||
| 36 | 25 | 30 | ✗ | ||||
| 37 | 30 | 25 | ✗ | ||||
| 38 | 25 | 25 | ✗ | ||||
| 39 | 30 | ✗ | ✗ | ||||
| 40 | 30 | ✗ | ✗ | ||||
| 41 | ✗ | 30 | ✗ | 30 | |||
| 42 | ✗ | 30 | 30 | ✗ | |||
| 43 | ✗ | 25 | 30 | ✗ | |||
| 44 | ✗ | 30 | 25 | ✗ | |||
| 45 | ✗ | 30 | 30 | ✗ | |||
| 46 | ✗ | 25 | 25 | ✗ | |||
| 47 | ✗ | 25 | ✗ | ✗ | |||
| 48 | ✗ | 25 | 30 | ✗ | |||
| 49 | ✗ | 30 | 25 | ✗ | |||
| 50 | 30 | ✗ | ✗ | ✗ | |||
| 51 | ✗ | ||||||
| 52 | ✗ | 25 | |||||
| 53 | 25 | ✗ | |||||
| 54 | ✗ | 25 | ✗ | ||||
| 55 | 30 | ✗ | |||||
| 56 | ✗ | 30 | ✗ | ||||
| 57 | ✗ | 30 | ✗ | ✗ | |||
| 58 | 30 | ✗ | ✗ | ||||
| 59 | ✗ | 30 | ✗ | ✗ | |||
| 60 | 30 | 30 | ✗ | ✗ | |||
| 61 | ✗ | 30 | 30 | ✗ | ✗ | ||
| 62 | 25 | ✗ | ✗ | ||||
| 63 | ✗ | 25 | ✗ | ✗ | |||
| 64 | 30 | ✗ | |||||
| 65 | ✗ | 30 | ✗ | ||||
| 66 | ✗ | 25 | ✗ | ||||
| 67 | 30 | ✗ | ✗ | ||||
| 68 | ✗ | 30 | ✗ | ✗ | |||
| 69 | ✗ | ✗ | 30 | ✗ | ✗ | ||
| Strategy | Criteria |
|---|---|
| 1 | Previous GDM pregnancy |
| 2 | Previous macrosomic baby |
| 3 | BMI ≥ 30 kg/m2 |
| 4 | Multiparous |
| 5 | Maternal age ≥ 30 years |
| 6 | Maternal age ≥ 25 years |
| 7 | Non-white ethnicity |
| 8 | BMI ≥ 25 kg/m2 |
| 9 | Previous GDM pregnancy or previous macrosomic baby |
| 10 | BMI ≥ 30 kg/m2 or previous GDM pregnancy |
| 11 | Family history of diabetes mellitus or previous GDM pregnancy |
| 12 | Multiparous or previous GDM pregnancy |
| 13 | Maternal age ≥ 30 years or previous GDM pregnancy |
| 14 | BMI ≥ 30 kg/m2 or family history of diabetes |
| 15 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 |
| 16 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 |
| 17 | Maternal age ≥ 25 years or previous GDM pregnancy |
| 18 | BMI ≥ 30 kg/m2 or non-white ethnicity |
| 19 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 |
| 20 | Maternal age ≥ 25 years or non-white ethnicity |
| 21 | BMI ≥ 25 kg/m2 or non-white ethnicity |
| 22 | Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 |
| 23 | BMI ≥ 30 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy |
| 24 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or previous GDM pregnancy |
| 25 | Maternal age ≥ 30 years or family history of diabetes mellitus or previous GDM pregnancy |
| 26 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or multiparous or |
| 27 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or family history of diabetes |
| 28 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or previous GDM pregnancy |
| 29 | BMI ≥ 30 kg/m2 or non-white ethnicity or previous GDM pregnancy |
| 30 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 family history of diabetes |
| 31 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or previous GDM pregnancy |
| 32 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or family history of diabetes |
| 33 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or non-white ethnicity |
| 34 | Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or family history of diabetes |
| 35 | Maternal age ≥ 25 years or non-white ethnicity or previous GDM pregnancy |
| 36 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or non-white ethnicity |
| 37 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or non-white ethnicity |
| 38 | Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or non-white ethnicity |
| 39 | BMI ≥ 30 kg/m2 or non-white ethnicity or multiparous |
| 40 | BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes |
| 41 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or multiparous or previous GDM pregnancy |
| 42 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or family history of diabetes or previous GDM pregnancy |
| 43 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or previous GDM pregnancy |
| 44 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy |
| 45 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or non-white ethnicity or previous GDM pregnancy |
| 46 | Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or family history of diabetes or previous GDM pregnancy |
| 47 | Maternal age ≥ 25 years or Non-white ethnicity or family history of diabetes or previous GDM pregnancy |
| 48 | Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or non-white ethnicity or previous GDM pregnancy |
| 49 | Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or non-white ethnicity or previous GDM pregnancy |
| 50 | BMI ≥ 30 kg/m2 or non-white ethnicity or multiparous or family history of diabetes |
| 51 | Family history of diabetes |
| 52 | BMI ≥ 25 kg/m2 or previous GDM pregnancy |
| 53 | BMI ≥ 25 kg/m2 or family history of diabetes |
| 54 | BMI ≥ 25 kg/m2 or family history of diabetes or previous GDM pregnancy |
| 55 | Maternal age ≥ 30 years or non-white ethnicity |
| 56 | Maternal age ≥ 30 years or non-white ethnicity or previous GDM pregnancy |
| 57 | BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes or previous GDM pregnancy |
| 58 | Maternal age ≥ 30 years or non-white ethnicity or family history of diabetes |
| 59 | Maternal age ≥ 30 years or non-white ethnicity or family history of diabetes or previous GDM pregnancy |
| 60 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes |
| 61 | Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes or previous GDM pregnancy |
| 62 | BMI ≥ 25 kg/m2 or non-white ethnicity or family history of diabetes |
| 63 | BMI ≥ 25 kg/m2 or non-white ethnicity or family history of diabetes or previous GDM pregnancy |
| 64 | BMI ≥ 30 kg/m2 or Multiparous |
| 65 | BMI ≥ 30 kg/m2 or multiparous or previous GDM pregnancy |
| 66 | BMI ≥ 25 kg/m2 or non-white ethnicity or previous GDM pregnancy |
| 67 | BMI ≥ 30 kg/m2 or multiparous or family history of diabetes |
| 68 | BMI ≥ 30 kg/m2 or multiparous or family history of diabetes or previous GDM pregnancy |
| 69 | NICE criteria |
| Covariable | Adverse perinatal outcome: OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Pre-eclampsia | C-section | Shoulder dystocia | NNUa | Instrumental delivery | Induction of labour | Macrosomia | |
| Fasting glucose | 1.370* (1.027 to 1.827) | 1.186** (1.054 to 1.335) | 1.627* (1.074 to 2.466) | 1.019 (0.821 to 1.266) | 1.206 (0.997 to 1.458) | 1.115 (0.963 to 1.291) | 1.864*** (1.603 to 2.168) |
| 2-hour glucose | 1.068 (0.967 to 1.180) | 1.024 (0.982 to 1.068) | 1.222* (1.035 to 1.443) | 1.027 (0.954 to 1.106) | 1.046 (0.975 to 1.122) | 1.012 (0.957 to 1.071) | 1.057* (1.001 to 1.116) |
| Ethnicity SA | 1.049 (0.760 to 1.448) | 0.991 (0.873 to 1.125) | 0.745 (0.467 to 1.189) | 1.216 (0.967 to 1.530) | 0.946 (0.794 to 1.127) | 0.885 (0.764 to 1.024) | 0.234*** (0.194 to 0.283) |
| Ethnicity other | 0.970 (0.619 to 1.522) | 1.240* (1.046 to 1.470) | 1.541 (0.881 to 2.695) | 0.975 (0.704 to 1.351) | 0.940 (0.739 to 1.196) | 1.003 (0.821 to 1.225) | 0.784* (0.626 to 0.982) |
| One pregnancy | 0.353*** (0.273 to 0.455) | 0.694*** (0.629 to 0.766) | 0.966 (0.656 to 1.424) | 0.650*** (0.540 to 0.784) | 0.226*** (0.188 to 0.272) | 0.473*** (0.410 to 0.546) | 1.579*** (1.387 to 1.798) |
| Two pregnancies | 0.182*** (0.120 to 0.276) | 0.486*** (0.425 to 0.554) | 1.382 (0.878 to 2.177) | 0.681** (0.534 to 0.869) | 0.0999*** (0.0728 to 0.137) | 0.502*** (0.419 to 0.603) | 1.757*** (1.498 to 2.062) |
| Three or more pregnancies | 0.240*** (0.159 to 0.361) | 0.333*** (0.281 to 0.394) | 0.840 (0.465 to 1.519) | 0.869 (0.661 to 1.141) | 0.0471*** (0.0292 to 0.0759) | 0.534*** (0.432 to 0.660) | 1.935*** (1.599 to 2.340) |
| Gestational age | 1.012 (0.971 to 1.054) | 0.981 (0.961 to 1.002) | 1.037 (0.957 to 1.122) | 0.995 (0.963 to 1.028) | 1.004 (0.966 to 1.044) | 1.000 (0.971 to 1.031) | 0.986 (0.962 to 1.010) |
| Family history diabetes | 1.009 (0.797 to 1.276) | 1.083 (0.984 to 1.193) | 1.067 (0.747 to 1.525) | 1.094 (0.928 to 1.290) | 1.036 (0.875 to 1.226) | 1.059 (0.926 to 1.212) | 1.035 (0.916 to 1.170) |
| Past smoker | 0.593** (0.413 to 0.852) | 1.013 (0.890 to 1.153) | 1.069 (0.664 to 1.720) | 1.115 (0.884 to 1.407) | 0.894 (0.726 to 1.102) | 1.113 (0.944 to 1.312) | 1.118 (0.959 to 1.303) |
| Smoker during pregnancy | 0.510*** (0.343 to 0.759) | 1.185* (1.032 to 1.362) | 0.731 (0.414 to 1.290) | 1.459** 1.162 to 1.830) | 0.867 (0.701 to 1.073) | 0.982 (0.828 to 1.165) | 0.546*** (0.452 to 0.660) |
| Maternal age | 1.020 (0.999 to 1.042) | 1.073*** (1.063 to 1.082) | 0.985 (0.952 to 1.019) | 0.993 (0.978 to 1.008) | 1.050*** (1.036 to 1.065) | 1.014 (0.932 to 1.102) | 1.003 (0.993 to 1.014) |
| Maternal age squared | – | – | – | – | – | 1.000 (0.999 to 1.001) | – |
| Previous macrosomia | – | – | – | – | 1.221 (0.732 to 2.037) | 1.063 (0.800 to 1.412) | – |
| Previous GDM | – | – | – | – | 0.840 (0.191 to 3.700) | 0.564 (0.241 to 1.317) | – |
| Maternal BMI | 1.089*** (1.071 to 1.108) | 1.089** (1.030 to 1.151) | 1.020 (0.990 to 1.052) | 0.991 (0.974 to 1.008) | 0.982* (0.969 to 0.996) | 1.041*** (1.031 to 1.052) | 1.194*** (1.105 to 1.291) |
| Maternal BMI squared | – | 1.000 0.999 to 1.000) | – | – | – | – | 0.998*** (0.997 to 0.999) |
| Pre-eclampsia | – | 2.666*** (2.152 to 3.302) | 1.362 (0.536 to 3.458) | 3.389*** (2.617 to 4.389) | 1.150 (0.791 to 1.674) | – | – |
| C-section | – | – | – | 2.820*** (2.418 to 3.290) | – | – | – |
| Atlantic DIP59 | 1.233 (0.932 to 1.632) | 0.902 (0.802 to 1.015) | 1.202 (0.764 to 1.893) | 2.335*** (1.901 to 2.869) | – | – | 1.737*** (1.514 to 1.992) |
| Variable | Value | Distribution | Source |
|---|---|---|---|
| Uptake | |||
| Universal OGTT | 62.83% | Beta α = 11,516 β = 6182 |
Farrar 2014124 |
| Selective RF OGTT | 89.66% Average A (99.05%) and B (80.26%) |
Beta (A) α = 1151 β = 11 |
Farrar 2014124 |
| Beta (B) α = 122 β = 30 |
Holt 2003235 | ||
| 6 weeks’ follow-up for universally OGTT | 52.24% | Beta α = 35 β = 32 |
Gregory 1998127 |
| 6 weeks’ follow-up for selective RF OGTT | 82.84% | Beta α = 985 β = 204 |
McClean 2010233 |
| Preventative treatment with ILS | 57.50% | Deterministic | DPPOS 2012251 |
| Probabilities | |||
| Perinatal outcomes | Logistic regression parameters | Log-normal | BiB22 and Atlantic DIP59 data |
| Hypoglycaemia, given treatment with insulin | 0.2020 | Beta α = 41 β = 162 |
Langer 2000187 |
| Severe hypoglycaemia, given hypoglycaemia | 0.05 | Deterministic | Assumption Diabetes in pregnancy, NICE guideline 201518 |
| Incidence of post-partum type 2 diabetes mellitus | 0.1107 | Beta α = 109 β = 876 |
McClean 2010233 |
| 10-year risk of developing type 2 diabetes mellitus, given GDM | 0.1480 | Calculated | Aroda 2015239 |
| BMI ≥ 20 kg/m2, given GDM | Variable across diagnostic thresholds | Beta | BiB data22 |
| Treatment effect | |||
| NICU | 0.91 | Log-normal (LNSE) = 0.197 |
Treatment review, Chapter 5 |
| Shoulder dystocia | 0.39 | Log-normal (LNSE) = 0.280 |
|
| C-section | 0.86 | Log normal (LNSE) = 0.054 |
|
| Pre-eclampsia | 0.58 | Log normal (LNSE) = 0.242 |
|
| Induction | 1.12 | Log normal (LNSE) = 0.157 |
|
| Instrumental | 1.37 | Log normal (LNSE) = 0.979 |
|
| Macrosomia | 0.46 | Log normal (LNSE) = 0.130 |
|
| Prevention of type 2 diabetes mellitus | 0.3520 | – | Aroda 2015239 |
| HRQL (utilities) | |||
| C-section | –0.0017 | Deterministic | C-section, NICE guideline 201133 |
| Pre-eclampsia | –0.0046 | Deterministic | Hypertension in pregnancy, NICE guideline 2007243 |
| Instrumental birth | –0.0526 | Deterministic | C-section, NICE guideline 201133 |
| Serious perinatal complications | –2.0594 | Deterministic | Diabetes in pregnancy, NICE guideline 2015240 Calculated |
| Maternal during pregnancy untreated | 0.1750 | Beta SE = 0.02 |
Crowther 200551 |
| Maternal during pregnancy treated | 0.1800 | Beta SE = 0.03 |
|
| Maternal post partum, untreated | 0.1975 | Beta SE = 0.02 |
|
| Maternal post partum, treated | 0.2000 | Beta SE = 0.02 |
|
| Early treatment of type 2 diabetes mellitus QALY gain | 0.045 | Normal S = 0.046 |
Gillies 2008238 |
| Prevention of type 2 diabetes mellitus with ILS | 0.20 | – | Calculated |
| Type 2 diabetes mellitus without complications | –0.0621 | Gamma SE = 0.0038 |
Sullivan 2011246 |
| Type 2 diabetes mellitus with complications | –0.0565 | Gamma SE = 0.0181 |
|
| Women in general UK population, years | – | – | |
| 25–34 | 0.93 | Beta SE = 0.00729325 |
Kind 1999245 |
| 35–44 | 0.91 | Beta SE = 0.008588975 |
|
| 45–54 | 0.85 | Beta SE = 0.014075771 |
|
| 55–64 | 0.81 | Beta SE = 0.015320647 |
|
| 65–74 | 0.78 | Beta SE = 0.015504342 |
|
| ≥ 75 | 0.71 | Beta SE = 0.018811791 |
|
| Costs (£) | |||
| Pre-eclampsia | 4656.00 | Deterministic | Hypertension in pregnancy, NICE guideline 2007243 |
| C-section | 884.00 | Gamma SE = 86.00 |
Diabetes in pregnancy, NICE guideline 2015240 NHS reference costs 2012–13260 |
| Induction | 329.00 | Gamma SE = 72.00 |
|
| NICU | 1118.00 | Gamma SE = 35.00 |
|
| Shoulder dystocia | 1256.00 | Gamma SE = 125.00 |
|
| Birth trauma | 1256.00 | Gamma SE = 125.00 |
|
| Neonatal death | 767.00 | Gamma SE = 39.00 |
Diabetes in pregnancy, NICE guideline 2015240 NHS reference costs 2005–6249 |
| Serious perinatal complications | 1221.77 | – | Weighted average of shoulder dystocia, birth trauma and neonatal death Calculated |
| Instrumental birth | 1086.00 | Deterministic | NHS reference costs 2012–13260 Calculated |
| Treatment for GDM | 934.66 | Deterministic | Calculated |
| Diagnostic with OGTT | 22.06 | Deterministic | Calculated |
| RF screening | 0.00 | Deterministic | Assumption |
| Screening with OCGT | 22.06 | Deterministic | Calculated |
| Screening with FPG | 20.42 | Deterministic | Calculated |
| Prevention of type 2 diabetes mellitus with ILS | 3585.17 | Deterministic | Calculated |
| Treatment of early-type DM | 558.07 | Gamma SE = 478.58 |
Gillies 2008238 |
| Severe hypoglycaemia | 629.00 | Deterministic | Diabetes in pregnancy, NICE guideline 2015240 NHS reference costs 2012–13260 |
| Characteristics | Base case, n = 10,353 | Subgroup | |
|---|---|---|---|
| SA and other, n = 6265 | WB, n = 4088 | ||
| Gestational age, weeks | 26.29 | 26.32 | 26.24 |
| Maternal age, years | 27.58 | 27.97 | 26.95 |
| BMI, kg/m2 | 26.05 | 25.56 | 26.81 |
| Previous GDM | 0.01 | 0.01 | 0.01 |
| Previous macrosomic baby | 0.05 | 0.04 | 0.07 |
| Ethnicity, SA | 0.52 | 0.86 | 0 |
| Ethnicity, white | 0.39 | 0 | 1 |
| Ethnicity, other | 0.08 | 0.14 | 0 |
| No previous pregnancy | 0.41 | 0.36 | 0.48 |
| One previous pregnancy | 0.29 | 0.27 | 0.317 |
| Two previous pregnancies | 0.16 | 0.19 | 0.13 |
| Three or more previous pregnancies | 0.13 | 0.18 | 0.07 |
| Family history of diabetes mellitus | 0.25 | 0.34 | 0.13 |
| Never smoker | 0.70 | 0.88 | 0.41 |
| Past smoker | 0.16 | 0.07 | 0.25 |
| Smoker during pregnancy | 0.14 | 0.05 | 0.34 |
| Alcohol in pregnancy | 0.20 | 0.04 | 0.44 |
| Fasting glucose, mmol/l | 4.52 | 4.60 | 4.41 |
| Post-load glucose, mmol/l | 5.68 | 5.83 | 5.44 |
| Screening strategy | S+T+ | S+T– | S+ | S– | Cost (£) | QALY | NMB (£) |
|---|---|---|---|---|---|---|---|
| Multiparous or previous GDM pregnancy | 0.005 | 0.294 | 0.30 | 0.70 | 480.92 | –0.0360 | –1201.75 |
| Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 | 0.013 | 0.466 | 0.48 | 0.52 | 500.06 | –0.0355 | –1210.23 |
| Previous macrosomic baby | 0.05 | 0.95 | 526.72 | –0.0350 | –1227.14 | ||
| Previous GDM pregnancy or previous macrosomic baby | 0.06 | 0.94 | 533.86 | –0.0347 | –1228.80 | ||
| BMI ≥ 30 kg/m2 | 0.21 | 0.79 | 633.92 | –0.0311 | –1256.01 | ||
| BMI ≥ 30 kg/m2 or previous GDM pregnancy | 0.22 | 0.78 | 638.64 | –0.0310 | –1257.85 | ||
| Family history of diabetes mellitus or previous GDM pregnancy | 0.26 | 0.74 | 676.20 | –0.0308 | –1291.88 | ||
| Multiparous or previous GDM pregnancy | 0.30 | 0.70 | 700.55 | –0.0306 | –1313.52 | ||
| Maternal age ≥ 30 years | 0.35 | 0.65 | 745.91 | –0.0295 | –1335.90 | ||
| Maternal age ≥ 30 years or previous GDM pregnancy | 0.36 | 0.64 | 748.51 | –0.0294 | –1336.40 | ||
| BMI ≥ 30 kg/m2 or family history of diabetes mellitus | 0.41 | 0.59 | 788.88 | –0.0276 | –1340.29 | ||
| BMI ≥ 30 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy | 0.42 | 0.58 | 791.51 | –0.0275 | –1341.31 | ||
| BMI ≥ 30 kg/m2 or multiparous | 0.45 | 0.55 | 813.69 | –0.0272 | –1356.89 | ||
| BMI ≥ 30 kg/m2 or multiparous or previous GDM pregnancy | 0.46 | 0.54 | 817.33 | –0.0270 | –1358.13 | ||
| Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 | 0.48 | 0.52 | 840.75 | –0.0265 | –1371.52 | ||
| Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or previous GDM pregnancy | 0.48 | 0.52 | 842.32 | –0.0265 | –1372.00 | ||
| BMI ≥ 25 kg/m2 | 0.50 | 0.50 | 857.99 | –0.0256 | –1369.57 | ||
| BMI ≥ 25 kg/m2 or previous GDM pregnancy | 0.51 | 0.49 | 859.94 | –0.0255 | –1370.68 | ||
| BMI ≥ 30 kg/m2 or multiparous or family history of diabetes mellitus | 0.60 | 0.40 | 927.21 | –0.0246 | –1418.64 | ||
| BMI ≥ 30 kg/m2 or multiparous or family history of diabetes mellitus or previous GDM pregnancy | 0.60 | 0.40 | 929.11 | –0.0245 | –1419.43 | ||
| Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or family history of diabetes mellitus | 0.60 | 0.40 | 935.98 | –0.0245 | –1425.55 | ||
| Maternal age ≥ 30 years or BMI ≥ 30 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy | 0.60 | 0.40 | 936.80 | –0.0244 | –1425.68 | ||
| BMI ≥ 25 kg/m2 or family history of diabetes mellitus | 0.62 | 0.38 | 950.27 | –0.0237 | –1424.84 | ||
| BMI ≥ 25 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy | 0.62 | 0.38 | 951.58 | –0.0237 | –1425.52 | ||
| Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 | 0.65 | 0.35 | 971.29 | –0.0233 | –1438.10 | ||
| Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or previous GDM pregnancy | 0.65 | 0.35 | 972.16 | –0.0233 | –1438.51 | ||
| BMI ≥ 30 kg/m2 or non-white ethnicity | 0.71 | 0.29 | 1024.46 | –0.0226 | –1477.45 | ||
| BMI ≥ 30 kg/m2 or non-white ethnicity or previous GDM pregnancy | 0.71 | 0.29 | 1025.48 | –0.0226 | –1477.98 | ||
| Maternal age ≥ 30 years or BMI 25 kg/m2 family history of diabetes mellitus | 0.72 | 0.28 | 1032.92 | –0.0222 | –1476.16 | ||
| Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or previous GDM pregnancy | 0.72 | 0.28 | 1033.37 | –0.0221 | –1476.30 | ||
| BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes mellitus | 0.75 | 0.25 | 1053.95 | –0.0220 | –1493.38 | ||
| BMI ≥ 30 kg/m2 or non-white ethnicity or family history of diabetes mellitus or previous GDM pregnancy | 0.75 | 0.25 | 1054.82 | –0.0220 | –1493.84 | ||
| NICE criteria | 0.78 | 0.22 | 1076.47 | –0.0216 | –1507.95 | ||
| Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or family history of diabetes mellitus | 0.79 | 0.21 | 1083.72 | –0.0214 | –1510.84 | ||
| Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy | 0.79 | 0.21 | 1083.95 | –0.0213 | –1510.92 | ||
| BMI ≥ 30 kg/m2 or non-white ethnicity or multiparous | 0.81 | 0.19 | 1097.35 | –0.0211 | –1519.45 | ||
| Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 | 0.81 | 0.19 | 1102.07 | –0.0208 | –1518.73 | ||
| BMI ≥ 25 kg/m2 or non-white ethnicity | 0.83 | 0.17 | 1111.87 | –0.0207 | –1524.93 | ||
| BMI ≥ 25 kg/m2 or non-white ethnicity or previous GDM pregnancy | 0.83 | 0.17 | 1112.42 | –0.0206 | –1525.21 | ||
| BMI ≥ 25 kg/m2 or non-white ethnicity or family history of diabetes mellitus | 0.85 | 0.15 | 1128.27 | –0.0203 | –1534.23 | ||
| BMI ≥ 25 kg/m2 or non-white ethnicity or family history of diabetes mellitus or previous GDM pregnancy | 0.85 | 0.15 | 1128.74 | –0.0203 | –1534.50 | ||
| Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or family history of diabetes mellitus | 0.85 | 0.15 | 1129.36 | –0.0203 | –1534.96 | ||
| Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or family history of diabetes mellitus or previous GDM pregnancy or | 0.85 | 0.15 | 1129.52 | –0.0203 | –1535.02 | ||
| Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or non-white ethnicity | 0.87 | 0.13 | 1151.69 | –0.0199 | –1550.61 | ||
| Maternal age ≥ 30 years or BMI ≥ 25 kg/m2 or non-white ethnicity or previous GDM pregnancy | 0.87 | 0.13 | 1151.84 | –0.0199 | –1550.67 | ||
| Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or non-white ethnicity | 0.88 | 0.12 | 1159.06 | –0.0199 | –1557.37 | ||
| Maternal age ≥ 25 years or BMI ≥ 30 kg/m2 or non-white ethnicity or previous GDM pregnancy | 0.88 | 0.12 | 1159.37 | –0.0199 | –1557.48 | ||
| Maternal age ≥ 25 years or BMI ≥ 25 kg/m2 or non-white ethnicity | 0.92 | 0.08 | 1187.95 | –0.0192 | –1572.49 |
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario 1: Inclusion of longer-term outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 478 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 495 | –0.0349 | –949 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 495 | –0.0357 | –959 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 5.4 | 11.1 | 0.003 | 0.007 | 0.011 | 0.989 | 482 | –0.0357 | –1195 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 495 | –0.0349 | –1194 | ||||||
| Diagnostic | 5.4 | 11.1 | 0.058 | 0.942 | 545 | –0.0330 | –1206 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, non-white | 5 | 5.5 | 0.339 | 0.580 | 0.919 | 0.081 | 1035 | –0.0088 | –1297 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, non-white | 0.919 | 0.081 | 1921 | 0.0295 | –1037 | ||||||
| Diagnostic | 5 | 5.5 | 0.536 | 0.464 | 971 | –0.0147 | –1412 | |||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario 2: Alternative uptake of diagnostic test estimates | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 477 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 483 | –0.0357 | –947 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 496 | –0.0360 | –963 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 477 | –0.0359 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | ||||||||||
| Screening RF | 0.011 | 0.989 | 483 | –0.0357 | ||||||||
| Diagnostic | pGDM | 9.5 | 10 | 0.015 | 0.985 | 500 | –0.0357 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | BMI 30 kg/m2 | 5.2 | 8.8 | 0.041 | 0.171 | 0.212 | 0.788 | 510 | –0.0347 | –1551 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 483 | –0.0357 | –1553 | ||||||
| Diagnostic | 5.2 | 8.8 | 0.102 | 0.898 | 556 | –0.0336 | –1563 | |||||
| Scenario 2: Inclusion of longer-term maternal outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 478 | –0.0359 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 493 | –0.0350 | –948 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 500 | –0.0357 | –964 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | BMI ≥ 30 kg/m2 | 5.4 | 11.1 | 0.027 | 0.185 | 0.212 | 0.788 | 527 | –0.0334 | –1195 | ||
| No Scr/Tst or Treat | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | BMI ≥ 30 kg/m2 pGDM | 0.219 | 0.781 | 827 | –0.0181 | –1188 | ||||||
| Diagnostic | 5.4 | 11.1 | 0.058 | 0.942 | 559 | –0.0325 | –1210 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, non-white | 5 | 5.5 | 0.339 | 0.580 | 0.919 | 0.081 | 975 | –0.0116 | –1323 | ||
| No Scr/Tst or Treat | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, non-white | 0.919 | 0.081 | 1768 | 0.0226 | –1089 | ||||||
| Diagnostic | 5 | 5.5 | 0.536 | 0.464 | 1057 | –0.0111 | –1390 | |||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario 3: Lowest insulin use | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 477 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.989 | 0.011 | 484 | –0.0356 | –947 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 491 | –0.0359 | –959 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9 | 9.5 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0359 | –1196 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1184 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 484 | –0.0356 | –1197 | ||||||
| Diagnostic | 9 | 9.5 | 0.020 | 0.980 | 498 | –0.0357 | –1211 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 5.1 | 8.3 | 0.005 | 0.005 | 0.011 | 0.989 | 480 | –0.0358 | –1554 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 484 | –0.0356 | –1553 | ||||||
| Diagnostic | 5.1 | 8.3 | 0.134 | 0.866 | 557 | –0.0333 | –1558 | |||||
| Scenario 3: Inclusion of longer-term maternal outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 478 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 495 | –0.0349 | –949 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 495 | –0.0357 | –959 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 5.3 | 10 | 0.004 | 0.007 | 0.011 | 0.989 | 483 | –0.0356 | –1195 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 495 | –0.0349 | –1194 | ||||||
| Diagnostic | 5.3 | 10 | 0.072 | 0.928 | 557 | –0.0324 | –1205 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, non-white | 5 | 5.5 | 0.339 | 0.580 | 0.919 | 0.081 | 1023 | –0.0088 | –1286 | ||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | |||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, non-white | 0.919 | 0.081 | 1921 | 0.0295 | –1037 | ||||||
| Diagnostic | 5 | 5.5 | 0.536 | 0.464 | 959 | –0.0147 | –1399 | |||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario 4: Minimum costs | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 477 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –933 | |||||||||
| Screening RF | Previous GDM | 0.989 | 0.011 | 483 | –0.0356 | –946 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 490 | –0.0360 | –958 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Previous GDM | 8 | 8.5 | 0.003 | 0.007 | 0.011 | 0.989 | 478 | –0.0359 | –1196 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1184 | |||||||||
| Screening RF | Previous GDM | 0.011 | 0.989 | 483 | –0.0356 | –1196 | ||||||
| Diagnostic | 8 | 8.5 | 0.043 | 0.957 | 507 | –0.0352 | –1210 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, non-white | 5 | 6.4 | 0.237 | 0.682 | 0.919 | 0.081 | 647 | –0.0295 | –1530 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1543 | |||||||||
| Screening RF | BMI 30 kg/m2, pGDM | 0.219 | 0.781 | 615 | –0.0310 | –1544 | ||||||
| Diagnostic | 5 | 6.4 | 0.318 | 0.682 | 637 | –0.0309 | –1564 | |||||
| Scenario 4: Inclusion of longer-term maternal outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 478 | –0.0360 | –945 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –933 | |||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 494 | –0.0349 | –948 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 494 | –0.0358 | –959 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 30 years, BMI 25 kg/m2, pGDM | 5 | 7.2 | 0.157 | 0.491 | 0.648 | 0.352 | 733 | –0.0223 | –1180 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1184 | |||||||||
| Screening RF | BMI ≥ 30 kg/m2, pGDM | 0.219 | 0.781 | 810 | –0.0182 | –1175 | ||||||
| Diagnostic | 5 | 7.2 | 0.222 | 0.778 | 678 | –0.0266 | –1209 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years BMI 25 kg/m2, non-white | 5 | 5.5 | 0.339 | 0.580 | 0.919 | 0.081 | 979 | –0.0088 | –1242 | ||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1543 | |||||||||
| Screening RF | Maternal age 25 years BMI 25 kg/m2, non-white | 0.919 | 0.081 | 1821 | 0.0295 | –937 | ||||||
| Diagnostic | 5 | 5.5 | 0.536 | 0.464 | 930 | –0.0157 | –1400 | |||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario 5: NICE treatment effectiveness estimates | |||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | |||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 477 | –0.0360 | –945 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | ||||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 484 | –0.0357 | –947 | |||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 491 | –0.0360 | –959 | ||||||
| Cost-effectiveness threshold = £20,000 per QALY | |||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 10 | 0.002 | 0.009 | 0.011 | 0.989 | 478 | –0.0360 | –1197 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | ||||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 484 | –0.0357 | –1197 | |||||||
| Diagnostic | 9.5 | 10 | 0.015 | 0.985 | 495 | –0.0358 | –1210 | ||||||
| Cost-effectiveness threshold = £30,000 per QALY | |||||||||||||
| Screening RF + diagnostic | pGDM | 5.2 | 9.2 | 0.004 | 0.006 | 0.011 | 0.989 | 480 | –0.0358 | –1555 | |||
| No Scr/Tst or Treatment | 466 | –0.0359 | –1543 | ||||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 484 | –0.0357 | –1553 | |||||||
| Diagnostic | 5.2 | 9.2 | 0.097 | 0.903 | 542 | –0.0343 | –1570 | ||||||
| Scenario 5: Inclusion of longer-term outcomes | |||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | |||||||||||||
| Screening RF + diagnostic | pGDM | 9.5 | 11.1 | 0.001 | 0.010 | 0.011 | 0.989 | 478 | –0.0360 | –945 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | –933 | ||||||||||
| Screening RF | pGDM | 0.011 | 0.989 | 495 | –0.0349 | –949 | |||||||
| Diagnostic | 9.5 | 11.1 | 0.008 | 0.992 | 495 | –0.0357 | –959 | ||||||
| Cost-effectiveness threshold = £20,000 per QALY | |||||||||||||
| Screening RF + diagnostic | pGDM | 5.4 | 11.1 | 0.003 | 0.007 | 0.011 | 0.989 | 482 | –0.0357 | –1195 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1184 | ||||||||||
| Screening RF | BMI ≥ 30 kg/m2 | 0.212 | 0.788 | 856 | –0.0168 | –1192 | |||||||
| Diagnostic | 5.4 | 11.1 | 0.058 | 0.942 | 544 | –0.0331 | –1206 | ||||||
| Cost-effectiveness threshold = £30,000 per QALY | |||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, non-white | 5 | 5.5 | 0.339 | 0.580 | 0.919 | 0.081 | 1031 | –0.0090 | –1300 | |||
| No Scr/Tst or Treatment | 467 | –0.0359 | –1543 | ||||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, non-white | 0.919 | 0.081 | 1909 | 0.0291 | –1036 | |||||||
| Diagnostic | 5 | 5.5 | 0.536 | 0.464 | 965 | –0.0149 | –1413 | ||||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | multipar | 9.5 | 11.1 | 0.003 | 0.271 | 0.274 | 0.726 | 464 | –0.0364 | –938 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –924 | |||||||||
| Screening RF | pmacro | 0.038 | 0.962 | 507 | –0.0355 | –969 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.012 | 0.988 | 483 | –0.0363 | –954 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Multipar | 9.5 | 10 | 0.004 | 0.270 | 0.274 | 0.726 | 466 | –0.0363 | –1192 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –1177 | |||||||||
| Screening RF | pmacro | 0.038 | 0.962 | 507 | –0.0355 | –1217 | ||||||
| Diagnostic | 9.5 | 10 | 0.023 | 0.977 | 488 | –0.0360 | –1208 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | BMI 30 kg/m2 | 5.2 | 8.2 | 0.052 | 0.134 | 0.186 | 0.814 | 514 | –0.0346 | –1552 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –1538 | |||||||||
| Screening RF | BMI 30 kg/m2 | 0.186 | 0.814 | 602 | –0.0318 | –1554 | ||||||
| Diagnostic | 5.2 | 8.2 | 0.150 | 0.850 | 561 | –0.0335 | –1566 | |||||
| South Asian and ‘other’: inclusion of longer-term maternal outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | multipar | 9.5 | 11.1 | 0.003 | 0.271 | 0.274 | 0.726 | 468 | –0.0362 | –938 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –924 | |||||||||
| Screening RF | pmacro | 0.038 | 0.962 | 549 | –0.0327 | –974 | ||||||
| Diagnostic | 9.5 | 11.1 | 0.012 | 0.988 | 488 | –0.0359 | –955 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | multipar pGDM | 5.2 | 11.1 | 0.032 | 0.252 | 0.284 | 0.716 | 521 | –0.0333 | –1186 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –1177 | |||||||||
| Screening RF | BMI 25 kg/m2 | 0.478 | 0.522 | 1306 | 0.0069 | –1168 | ||||||
| Diagnostic | 5.2 | 11.1 | 0.117 | 0.883 | 595 | –0.0305 | –1205 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, diabetes | 5 | 5.5 | 0.344 | 0.524 | 0.868 | 0.132 | 1032 | –0.0066 | –1230 | ||
| No Scr/Tst or Treatment | 454 | –0.0361 | –1538 | |||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, diabetes | 0.868 | 0.132 | 1833 | 0.0302 | –926 | ||||||
| Diagnostic | 5 | 5.5 | 0.572 | 0.428 | 1014 | –0.0132 | –1410 | |||||
| Strategy | Risk factor | Fasting glucose, mmol/l | Post-load glucose, mmol/l | S+T+ | S+T– | S+ | S– | T+ | T– | E(costs) (£) | E(QALYs) | NMB (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostica | Diabetes | 8 | 11.1 | 0.000 | 0.133 | 0.134 | 0.866 | 499 | –0.0356 | –962 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –950 | |||||||||
| Screening RF | pmacro | 0.065 | 0.935 | 557 | –0.0344 | –1004 | ||||||
| Diagnostic | 8 | 11.1 | 0.001 | 0.999 | 507 | –0.0357 | –972 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostica | Diab | 8 | 11.1 | 0.000 | 0.133 | 0.134 | 0.866 | 499 | –0.0356 | –1211 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –1199 | |||||||||
| Screening RF | pmacro | 0.065 | 0.935 | 557 | –0.0344 | –1245 | ||||||
| Diagnostic | 8 | 11.1 | 0.001 | 0.999 | 507 | –0.0357 | –1222 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostica | Diab | 8 | 11.1 | 0.000 | 0.133 | 0.134 | 0.866 | 499 | –0.0356 | –1211 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –1199 | |||||||||
| Screening RF | pmacro | 0.065 | 0.935 | 557 | –0.0344 | –1245 | ||||||
| Diagnostic | 8 | 11.1 | 0.001 | 0.999 | 507 | –0.0357 | –1222 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| 5.3 | 9.2 | 0.009 | 0.125 | 0.134 | 0.866 | 506 | –0.0354 | –1567 | ||||
| 488 | –0.0355 | –1555 | ||||||||||
| 0.065 | 0.935 | 557 | –0.0344 | –1589 | ||||||||
| 5.3 | 9.2 | 0.037 | 0.963 | 529 | –0.0349 | –1577 | ||||||
| White British: inclusion of longer-term maternal outcomes | ||||||||||||
| Cost-effectiveness threshold = £13,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Diabetes | 8 | 11.1 | 0.000 | 0.133 | 0.134 | 0.866 | 499 | –0.0356 | –962 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –950 | |||||||||
| Screening RF | pmacro | 0.065 | 0.935 | 627 | –0.0303 | –1022 | ||||||
| Diagnostic | 8 | 11.1 | 0.001 | 0.999 | 508 | –0.0357 | –972 | |||||
| Cost-effectiveness threshold = £20,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Diabetes | 6.5 | 11.1 | 0.000 | 0.133 | 0.134 | 0.866 | 499 | –0.0356 | –1211 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –1199 | |||||||||
| Screening RF | pmacro | 0.065 | 0.935 | 627 | –0.0303 | –1234 | ||||||
| Diagnostic | 6.5 | 11.1 | 0.003 | 0.997 | 510 | –0.0355 | –1220 | |||||
| Cost-effectiveness threshold = £30,000 per QALY | ||||||||||||
| Screening RF + diagnostic | Maternal age 25 years, BMI 25 kg/m2, diabetes, pGDM | 5 | 5.5 | 0.299 | 0.513 | 0.812 | 0.188 | 1080 | –0.0095 | –1364 | ||
| No Scr/Tst or Treatment | 488 | –0.0355 | –1555 | |||||||||
| Screening RF | Maternal age 25 years, BMI 25 kg/m2, diabetes, pGDM | 0.812 | 0.188 | 2012 | 0.0293 | –1132 | ||||||
| Diagnostic | 5 | 5.5 | 0.480 | 0.520 | 1025 | –0.0158 | –1499 | |||||
FIGURE 66.
Heat map for costs of treatment by diagnostic threshold.
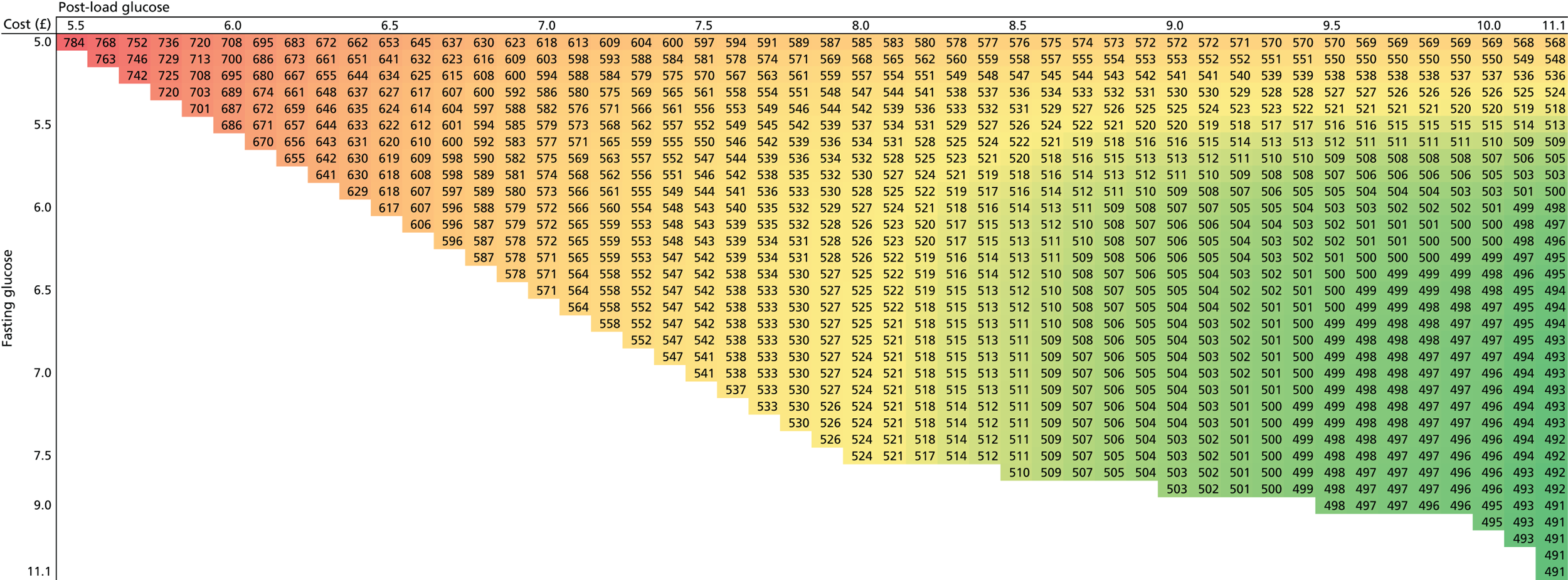
FIGURE 67.
Heat map for QALYs from treatment by diagnostic threshold.
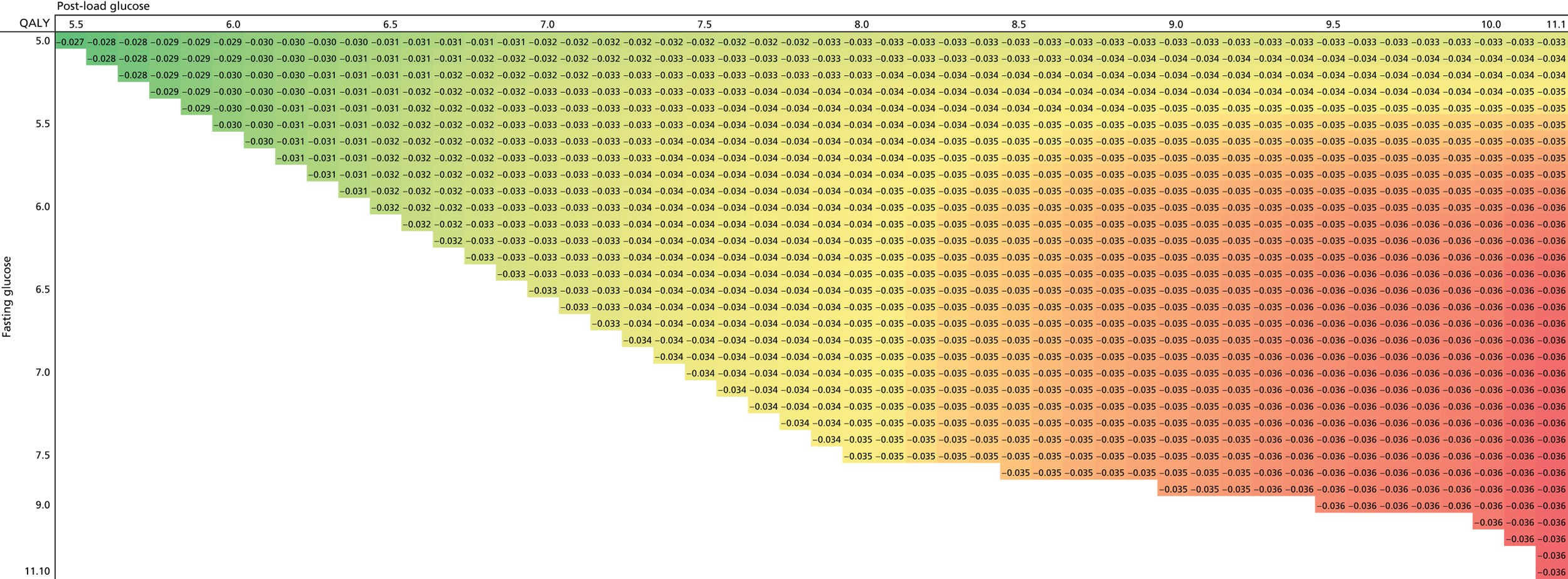
Appendix 7 Search strategies
The databases and information sources searched in September 2013 and October 2014
| Database/information source | Interface/URL |
|---|---|
| MEDLINE® and MEDLINE In-Process & Other Non-Indexed Citations | OvidSP |
| EMBASE | OvidSP |
| CINAHL Plus | EBSCOhost |
| CENTRAL | The Cochrane Library/Wiley Interscience |
| CDSR | The Cochrane Library/Wiley Interscience |
| DARE | The Cochrane Library/Wiley Interscience |
| HTA database | The Cochrane Library/Wiley Interscience |
| NHS EED | The Cochrane Library/Wiley Interscience |
| Cochrane Methodology Register | The Cochrane Library/Wiley Interscience |
| Records identified | |
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 4217 |
| EMBASE | 7873 |
| CINAHL Plus | 1097 |
| CENTRAL | 165 |
| CDSR | 41 |
| DARE | 6 |
| HTA database | 1 |
| NHS EED | 1 |
| Cochrane Methodology Register | 0 |
| TOTAL | 13,401 |
| TOTAL after deduplication | 808 |
A. September 2013 search strategies:
Source: MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE 1946 to present
Interface/URL: OvidSP.
Search date: 16 September 2013.
Retrieved records: 4217.
Search strategy
-
(pregnancy adj4 diabetes).ti,ab. (3811)
-
(gestational adj4 diabetes).ti,ab. (7253)
-
exp DIABETES, GESTATIONAL/ (6899)
-
gdm.ti,ab. (2828)
-
(glucose adj4 (pregnan* or gestation* or natal or maternal)).ti,ab. (3314)
-
1 or 2 or 3 or 4 or 5 (13,904)
-
macrosomia.ti,ab. (2142)
-
exp FETAL MACROSOMIA/ (1747)
-
7 or 8 (2949)
-
exp BIRTH INJURIES/ (4780)
-
((perinatal or labor or labour or birth) adj4 trauma).ti,ab. (1297)
-
((perinatal or labor or labour or birth) adj4 injur*).ti,ab. (2385)
-
((perinatal or labor or labour or birth) adj4 complication*1).ti,ab. (4130)
-
exp OBSTETRIC LABOR COMPLICATIONS/ (50,946)
-
*DYSTOCIA/ (1845)
-
(shoulder adj4 dystocia).ti,ab. (959)
-
(fracture*1 adj4 clavicle*1).ti,ab. (1123)
-
(fracture*1 adj4 humerus).ti,ab. (3203)
-
(fracture*1 adj4 shoulder*1).ti,ab. (709)
-
(fracture*1 adj4 arm*1).ti,ab. (437)
-
“erb* palsy”.ti,ab. (168)
-
neuropath*.ti,ab. (93,706)
-
exp BRACHIAL PLEXUS NEUROPATHIES/ (2692)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (160,801)
-
(preeclampsia or pre-eclampsia).ti,ab. (18,840)
-
exp PRE-ECLAMPSIA/ (23,296)
-
25 or 26 (29,671)
-
(heart adj4 (disorder*1 or disease*1)).ti,ab. (135,343)
-
(cardiovascular adj4 (disorder*1 or disease*1)).ti,ab. (111,102)
-
(cardiac adj4 (disorder*1 or disease*1)).ti,ab. (25,664)
-
exp CARDIOVASCULAR DISEASES/ (1,866,094)
-
exp HEART DISEASES/ (887,858)
-
28 or 29 or 30 or 31 or 32 (1,938,013)
-
exp HYPOGLYCEMIA/ (21,601)
-
hypoglyc*.ti,ab. (40,106)
-
34 or 35 (46,491)
-
exp DIABETES MELLITUS, TYPE 2/ (86,196)
-
((“type 2” or “type AND two” or “type II”) adj4 diabet*).ti,ab. (80,227)
-
37 or 38 (113,317)
-
exp OBESITY/ (140,385)
-
(obesity or obese or bmi or “body mass” or overweight).ti,ab. (282,204)
-
40 or 41 (312,183)
-
9 or 24 or 27 or 33 or 36 or 39 or 42 (2,436,744)
-
(offspring or son*1 or daughter*1 or child or children or pediatric*1 or paediatric*1).ti,ab. (1,102,950)
-
exp CHILD OF IMPAIRED PARENTS/ (4216)
-
exp CHILD/ (1,547,523)
-
(maternal or mother*2).ti,ab. (275,418)
-
exp MOTHERS/ (26,220)
-
44 or 45 or 46 or 47 or 48 (2,141,917)
-
43 and 49 (259,112)
-
6 and 50 (4434)
-
51 not (animals/ not humans/) (4217)
Source: EMBASE 1974 to 13 September 2013
Interface/URL: OvidSP.
Search date: 16 September 2013.
Retrieved records: 7873.
Search strategy
-
(pregnancy adj4 diabetes).ti,ab. (5134)
-
(gestational adj4 diabetes).ti,ab. (10,165)
-
exp DIABETES, GESTATIONAL/ (19,158)
-
gdm.ti,ab. (4151)
-
(glucose adj4 (pregnan* or gestation* or natal or maternal)).ti,ab. (4075)
-
1 or 2 or 3 or 4 or 5 (24,428)
-
macrosomia.ti,ab. (3031)
-
exp FETAL MACROSOMIA/ (3632)
-
7 or 8 (4445)
-
exp BIRTH INJURIES/ (5609)
-
((perinatal or labor or labour or birth) adj4 trauma).ti,ab. (1703)
-
((perinatal or labor or labour or birth) adj4 injur*).ti,ab. (2912)
-
((perinatal or labor or labour or birth) adj4 complication*1).ti,ab. (5268)
-
exp OBSTETRIC LABOR COMPLICATIONS/ (131,242)
-
exp SHOULDER DYSTOCIA/ (962)
-
(shoulder adj4 dystocia).ti,ab. (1388)
-
(fracture*1 adj4 clavicle*1).ti,ab. (1321)
-
(fracture*1 adj4 humerus).ti,ab. (3994)
-
(fracture*1 adj4 shoulder*1).ti,ab. (883)
-
(fracture*1 adj4 arm*1).ti,ab. (529)
-
erb* palsy.ti,ab. (219)
-
neuropath*.ti,ab. (118,816)
-
exp BRACHIAL PLEXUS NEUROPATHIES/ (1613)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (267,112)
-
(preeclampsia or pre-eclampsia).ti,ab. (25,457)
-
exp PRE-ECLAMPSIA/ (35,429)
-
25 or 26 (39,026)
-
(heart adj4 (disorder*1 or disease*1)).ti,ab. (174,985)
-
(cardiovascular adj4 (disorder*1 or disease*1)).ti,ab. (141,764)
-
(cardiac adj4 (disorder*1 or disease*1)).ti,ab. (34,098)
-
exp CARDIOVASCULAR DISEASES/ (2,920,008)
-
exp HEART DISEASES/ (1,306,554)
-
28 or 29 or 30 or 31 or 32 (2,968,279)
-
exp HYPOGLYCEMIA/ (51,492)
-
hypoglyc*.ti,ab. (53,319)
-
34 or 35 (72,756)
-
exp DIABETES MELLITUS, TYPE 2/ (132,330)
-
((“type 2” or “type two” or “type II”) adj4 diabet*).ti,ab. (107,631)
-
37 or 38 (158,436)
-
exp OBESITY/ (275,568)
-
(obesity or obese or bmi or “body mass” or overweight).ti,ab. (372,903)
-
40 or 41 (451,582)
-
9 or 24 or 27 or 33 or 36 or 39 or 42 (3,621,049)
-
(offspring or son*1 or daughter*1 or child or children or pediatric*1 or paediatric*1).ti,ab. (1,363,229)
-
exp CHILD OF IMPAIRED PARENTS/ (1,789,089)
-
exp CHILD/ (1,789,089)
-
(maternal or mother*2).ti,ab. (323,775)
-
exp MOTHERS/ (80,799)
-
44 or 45 or 46 or 47 or 48 (2,503,254)
-
43 and 49 (401,545)
-
6 and 50 (7873)
Source: CINAHL Plus
Interface/URL: EBSCOhost.
Search date: 17 September 2013.
Retrieved records: 1097.
Search strategy
S55 S51 not S54 (1097)
S54 S52 not S53 (44,999)
S53 (MH “Human”) (1,095,475)
S52 (MH “Animals”) (49,408)
S51 S6 AND S50 (1103)
S50 S43 AND S49 (51,638)
S49 S44 OR S45 OR S46 OR S47 OR S48 (474,583)
S48 (MH “Mothers+”) (19,731)
S47 TI (maternal or mother*) or AB (maternal or mother*) (52,862)
S46 (MH “Child+”) (379,811)
S45 (MH “Children of Impaired Parents+”) (1460)
S44 TI (offspring or son or sons or daughter* OR child OR children OR pediatric* OR paediatric*) or AB (offspring or son or sons or daughter* OR child OR children OR pediatric* OR paediatric*) (232,624)
S43 S9 OR S24 OR S27 OR S33 OR S36 OR S39 OR S42 (431,186)
S42 S40 OR S41 (76,702)
S41 TI (obesity or obese or bmi or “body mass” or overweight) or AB (obesity or obese or bmi or “body mass” or overweight) (57,664)
S40 (MH “Obesity+”) (48,791)
S39 S37 OR S38 (35,582)
S38 TI ((“type 2” or “type two” or “type II”) N4 diabet*) or AB ((“type 2” or “type two” or “type II”) N4 diabet*) (21,280)
S37 (MH “Diabetes Mellitus, Type 2”) (31,041)
S36 S34 OR S35 (8255)
S35 TI (hypoglyc*) or AB (hypoglyc*) (5766)
S34 (MH “Hypoglycemia+”) (5158)
S33 S28 OR S29 OR S30 OR S31 OR S32 (324,646)
S32 (MH “Heart Diseases+”) (150,895)
S31 (MH “Cardiovascular Diseases+”) (312,990)
S30 TI (cardiac N4 (disorder* or disease*)) or AB (cardiac N4 (disorder* or disease*)) (3433)
S29 TI (cardiovascular N4 (disorder* or disease*)) or AB (cardiovascular N4 (disorder* or disease*)) (19,387)
S28 TI (heart N4 (disorder* or disease*)) or AB (heart N4 (disorder* or disease*)) (21,170)
S27 S25 OR S26 (4403)
S26 (MH “Pre-Eclampsia+”) (3600)
S25 TI (preeclampsia or pre-eclampsia) or AB (preeclampsia or pre-eclampsia) (3124)
S24 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 (20,330)
S23 (MH “Brachial Plexus Neuropathies+”) (603)
S22 TI (neuropath*) or AB (neuropath*) (11,432)
S21 TI (erb* palsy) or AB (erb* palsy) (54)
S20 TI (fracture* N4 arm*) or AB (fracture* N4 arm*) (97)
S19 TI (fracture* N4 shoulder*) or AB (fracture* N4 shoulder*) (179)
S18 TI (fracture* N4 humerus) or AB (fracture* N4 humerus) (644)
S17 TI (fracture* N4 clavicle*) or AB (fracture* N4 clavicle*) (326)
S16 TI (shoulder N4 dystocia) or AB (shoulder N4 dystocia) (378)
S15 (MH “Shoulder Dystocia”) (329)
S14 ((MH “Labor Complications+”) (5672)
S13 TI ((perinatal or labor or labour or birth) N4 complication*) or AB ((perinatal or labor or labour or birth) N4 complication*) (721)
S12 TI ((perinatal or labor or labour or birth) N4 injur*) or AB ((perinatal or labor or labour or birth) N4 injur*) (407)
S11 TI ((perinatal or labor or labour or birth) N4 trauma) or AB ((perinatal or labor or labour or birth) N4 trauma) (276)
S10 (MH “Birth Injuries+”) (868)
S9 S7 OR S8 (686)
S8 (MH “Fetal Macrosomia”) (510)
S7 TI (macrosomia) OR AB (macrosomia) (387)
S6 S1 OR S2 OR S3 OR S4 OR S5 (3944)
S5 TI (glucose N4 (pregnan* or gestation* or natal or maternal)) or AB (glucose N4 (pregnan* or gestation* or natal or maternal)) (551)
S4 TI (gdm) or AB (gdm) (715)
S3 (MH “Diabetes Mellitus, Gestational”) (2870)
S2 TI (gestational N4 diabetes) or AB (gestational N4 diabetes) (2343)
S1 TI (pregnancy N4 diabetes) or AB (pregnancy N4 diabetes) (905)
Source: Cochrane Central Register of Controlled Trials, Issue 8 of 12, August 2013
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 165.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Trials (165)
Source: Cochrane Database of Systematic Reviews, Issue 9 of 12, September 2013
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 41.
Search strategy
#1 ((pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Cochrane Reviews (Reviews and Protocols) (41)
Source: Database of Abstracts of Reviews of Effects, Issue 3 of 4, July 2013
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 6.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Other Reviews (6)
Source: Health Technology Assessment database, Issue 3 of 4, July 2013
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 1.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Technology Assessments (1)
Source: NHS Economic Evaluation Database (issue number not given)
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 1.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Economic Evaluations (1)
Source: Cochrane Methodology Register, Issue 3 of 4, July 2012
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 18 September 2013.
Retrieved records: 0.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (635)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (483)
#3 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#4 #1 or #2 or #3 (875)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (108)
#6 MeSH descriptor: [Fetal Macrosomia] explode all trees (61)
#7 #5 or #6 (108)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1003)
#9 MeSH descriptor: [Birth Injuries] explode all trees (33)
#10 MeSH descriptor: [Obstetric Labor Complications] explode all trees (2143)
#11 MeSH descriptor: [Dystocia] explode all trees (77)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (323)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (3785)
#14 MeSH descriptor: [Brachial Plexus Neuropathies] explode all trees (44)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (6670)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1008)
#17 MeSH descriptor: [Pre-Eclampsia] explode all trees (558)
#18 #16 or #17 (1008)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (12,623)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (9318)
#21 MeSH descriptor: [Heart Diseases] explode all trees (34,478)
#22 MeSH descriptor: [Cardiovascular Diseases] explode all trees (69,430)
#23 #19 or #20 or #21 or #22 (77,250)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (6540)
#25 MeSH descriptor: [Hypoglycemia] explode all trees (1070)
#26 #24 or #25 (6543)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (6726)
#28 MeSH descriptor: [Diabetes Mellitus, Type 2] explode all trees (7868)
#29 #27 or #28 (9566)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (18,800)
#31 MeSH descriptor: [Obesity] explode all trees (6607)
#32 #30 or #31 (18,817)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (107,317)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (73,610)
#35 MeSH descriptor: [Child] explode all trees (99)
#36 MeSH descriptor: [Child of Impaired Parents] explode all trees (109)
#37 MeSH descriptor: [Mothers] explode all trees (847)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (10,448)
#39 #34 or #35 or #36 or #37 or #38 (80,319)
#40 #33 and #39 (6929)
#41 #4 and #40 in Methods Studies (0)
| Database/information source | Records identified |
|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 4622 |
| EMBASE | 9726 |
| CINAHL | 1261 |
| CENTRAL | 256 |
| CDSR | 42 |
| DARE | 7 |
| HTA database | 1 |
| NHS EED | 1 |
| Cochrane Methodology Register | 0 |
| TOTAL | 15,916 |
| TOTAL after deduplication | 2464 |
B. October 2014 search strategies:
Source: MEDLINE In-Process & Other Non-Indexed Citations and MEDLINE 1946 to present
Interface/URL: OvidSP.
Search date: 20 October 2014.
Retrieved records: 4622.
Search strategy
-
(pregnancy adj4 diabetes).ti,ab. (4082)
-
(gestational adj4 diabetes).ti,ab. (8108)
-
exp DIABETES, GESTATIONAL/ (7439)
-
gdm.ti,ab. (3272)
-
(glucose adj4 (pregnan* or gestation* or natal or maternal)).ti,ab. (3469)
-
1 or 2 or 3 or 4 or 5 (15,075)
-
macrosomia.ti,ab. (2314)
-
exp FETAL MACROSOMIA/ (1826)
-
7 or 8 (3157)
-
exp BIRTH INJURIES/ (4937)
-
((perinatal or labor or labour or birth) adj4 trauma).ti,ab. (1355)
-
((perinatal or labor or labour or birth) adj4 injur*).ti,ab. (2542)
-
((perinatal or labor or labour or birth) adj4 complication*1).ti,ab. (4372)
-
exp OBSTETRIC LABOR COMPLICATIONS/ (53,369)
-
*DYSTOCIA/ (1902)
-
(shoulder adj4 dystocia).ti,ab. (1021)
-
(fracture*1 adj4 clavicle*1).ti,ab. (1218)
-
(fracture*1 adj4 humerus).ti,ab. (3451)
-
(fracture*1 adj4 shoulder*1).ti,ab. (753)
-
(fracture*1 adj4 arm*1).ti,ab. (454)
-
“erb* palsy”.ti,ab. (185)
-
neuropath*.ti,ab. (97,784)
-
exp BRACHIAL PLEXUS NEUROPATHIES/ (2817)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (168,258)
-
(preeclampsia or pre-eclampsia).ti,ab. (20,669)
-
exp PRE-ECLAMPSIA/ (24,509)
-
25 or 26 (31,679)
-
(heart adj4 (disorder*1 or disease*1)).ti,ab. (142,562)
-
(cardiovascular adj4 (disorder*1 or disease*1)).ti,ab. (119,950)
-
(cardiac adj4 (disorder*1 or disease*1)).ti,ab. (26,958)
-
exp CARDIOVASCULAR DISEASES/ (1,944,605)
-
exp HEART DISEASES/ (922,916)
-
28 or 29 or 30 or 31 or 32 (2,024,083)
-
exp HYPOGLYCEMIA/ (22,500)
-
hypoglyc*.ti,ab. (42,033)
-
34 or 35 (48,692)
-
exp DIABETES MELLITUS, TYPE 2/ (90,640)
-
((“type 2” or “type AND two” or “type II”) adj4 diabet*).ti,ab. (87,156)
-
37 or 38 (121,847)
-
exp OBESITY/ (152,662)
-
(obesity or obese or bmi or “body mass” or overweight).ti,ab. (311,123)
-
40 or 41 (343,012)
-
9 or 24 or 27 or 33 or 36 or 39 or 42 (2,561,831)
-
(offspring or son*1 or daughter*1 or child or children or pediatric*1 or paediatric*1).ti,ab. (1,177,569)
-
exp CHILD OF IMPAIRED PARENTS/ (4392)
-
exp CHILD/ (1,595,153)
-
(maternal or mother*2).ti,ab. (288,181)
-
exp MOTHERS/ (27,857)
-
44 or 45 or 46 or 47 or 48 (2,246,955)
-
43 and 49 (274,768)
-
6 and 50 (4840)
-
51 not (animals/ not humans/) (4622)
Source: EMBASE 1974 to 17 October 2014
Interface/URL: OvidSP.
Search date: 20 October 2014.
Retrieved records: 9726.
Search strategy
-
(pregnancy adj4 diabetes).ti,ab. (5533)
-
(gestational adj4 diabetes).ti,ab. (11,687)
-
exp DIABETES, GESTATIONAL/ (20,744)
-
gdm.ti,ab. (5092)
-
(glucose adj4 (pregnan* or gestation* or natal or maternal)).ti,ab. (4355)
-
1 or 2 or 3 or 4 or 5 (26,356)
-
macrosomia.ti,ab. (3278)
-
exp FETAL MACROSOMIA/ (4036)
-
7 or 8 (4784)
-
exp BIRTH INJURIES/ (5520)
-
((perinatal or labor or labour or birth) adj4 trauma).ti,ab. (1758)
-
((perinatal or labor or labour or birth) adj4 injur*).ti,ab. (3012)
-
((perinatal or labor or labour or birth) adj4 complication*1).ti,ab. (5608)
-
exp OBSTETRIC LABOR COMPLICATIONS/ (136,143)
-
exp SHOULDER DYSTOCIA/ (1155)
-
(shoulder adj4 dystocia).ti,ab. (1473)
-
(fracture*1 adj4 clavicle*1).ti,ab. (1389)
-
(fracture*1 adj4 humerus).ti,ab. (4150)
-
(fracture*1 adj4 shoulder*1).ti,ab. (948)
-
(fracture*1 adj4 arm*1).ti,ab. (545)
-
erb* palsy.ti,ab. (232)
-
neuropath*.ti,ab. (125,149)
-
exp BRACHIAL PLEXUS NEUROPATHIES/ (1479)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (278,656)
-
(preeclampsia or pre-eclampsia).ti,ab. (27,629)
-
exp PRE-ECLAMPSIA/ (37,874)
-
25 or 26 (41,348)
-
(heart adj4 (disorder*1 or disease*1)).ti,ab. (180,301)
-
(cardiovascular adj4 (disorder*1 or disease*1)).ti,ab. (154,633)
-
(cardiac adj4 (disorder*1 or disease*1)).ti,ab. (35,636)
-
exp CARDIOVASCULAR DISEASES/ (3,031,448)
-
exp HEART DISEASES/ (1,365,227)
-
28 or 29 or 30 or 31 or 32 (3,080,008)
-
exp HYPOGLYCEMIA/ (56,246)
-
hypoglyc*.ti,ab. (57,381)
-
34 or 35 (77,892)
-
exp DIABETES MELLITUS, TYPE 2/ (147,853)
-
((“type 2” or “type two” or “type II”) adj4 diabet*).ti,ab. (121,802)
-
37 or 38 (174,880)
-
exp OBESITY/ (307,381)
-
(obesity or obese or bmi or “body mass” or overweight).ti,ab. (421,534)
-
40 or 41 (506,838)
-
9 or 24 or 27 or 33 or 36 or 39 or 42 (3,782,413)
-
(offspring or son*1 or daughter*1 or child or children or pediatric*1 or paediatric*1).ti,ab. (1,423,835)
-
exp CHILD OF IMPAIRED PARENTS/ (2,113,745)
-
exp CHILD/ (2,113,745)
-
(maternal or mother*2).ti,ab. (337,918)
-
exp MOTHERS/ (90,011)
-
44 or 45 or 46 or 47 or 48 (2,803,538)
-
43 and 49 (482,980)
-
6 and 50 (9726)
Source: CINAHL Plus
Interface/URL: EBSCOhost.
Search date: 20 October 2014.
Retrieved records: 1261.
Search strategy
S55 S51 not S54 (1261)
S54 S52 not S53 (51,441)
S53 (MH “Human”) (1,248,660)
S52 (MH “Animals”) (56,845)
S51 S6 AND S50 (1269)
S50 S43 AND S49 (57,843)
S49 S44 OR S45 OR S46 OR S47 OR S48 (520,392)
S48 (MH “Mothers+”) (21,782)
S47 TI (maternal or mother*) or AB (maternal or mother*) (58,083)
S46 (MH “Child+”) (415,087)
S45 (MH “Children of Impaired Parents+”) (1570)
S44 TI (offspring or son or sons or daughter* OR child OR children OR pediatric* OR paediatric*) or AB (offspring or son or sons or daughter* OR child OR children OR pediatric* OR paediatric*) (255,886)
S43 S9 OR S24 OR S27 OR S33 OR S36 OR S39 OR S42 (479,375)
S42 S40 OR S41 (87,332)
S41 TI (obesity or obese or bmi or “body mass” or overweight) or AB (obesity or obese or bmi or “body mass” or overweight) (65,840)
S40 (MH “Obesity+”) (55,658)
S39 S37 OR S38 (39,817)
S38 TI ((“type 2” or “type two” or “type II”) N4 diabet*) or AB ((“type 2” or “type two” or “type II”) N4 diabet*) (23,906)
S37 (MH “Diabetes Mellitus, Type 2”) (34,699)
S36 S34 OR S35 (9166)
S35 TI (hypoglyc*) or AB (hypoglyc*) (6420)
S34 (MH “Hypoglycemia+”) (5725)
S33 (S28 OR S29 OR S30 OR S31 OR S32 (358,605)
S32 (MH “Heart Diseases+”) (165,936)
S31 (MH “Cardiovascular Diseases+”) (345,747)
S30 TI (cardiac N4 (disorder* or disease*)) or AB (cardiac N4 (disorder* or disease*)) (3743)
S29 TI (cardiovascular N4 (disorder* or disease*)) or AB (cardiovascular N4 (disorder* or disease*)) (21,477)
S28 TI (heart N4 (disorder* or disease*)) or AB (heart N4 (disorder* or disease*)) (22,833)
S27 S25 OR S26 (5004)
S26 (MH “Pre-Eclampsia+”) (4037)
S25 TI (preeclampsia or pre-eclampsia) or AB (preeclampsia or pre-eclampsia) (3555)
S24 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 (22,645)
S23 (MH “Brachial Plexus Neuropathies+”) (693)
S22 TI (neuropath*) or AB (neuropath*) (12,777)
S21 TI (erb* palsy) or AB (erb* palsy) (58)
S20 TI (fracture* N4 arm*) or AB (fracture* N4 arm*) (106)
S19 TI (fracture* N4 shoulder*) or AB (fracture* N4 shoulder*) (197)
S18 TI (fracture* N4 humerus) or AB (fracture* N4 humerus) (748)
S17 TI (fracture* N4 clavicle*) or AB (fracture* N4 clavicle*) (367)
S16 TI (shoulder N4 dystocia) or AB (shoulder N4 dystocia) (413)
S15 (MH “Shoulder Dystocia”) (338)
S14 (MH “Labor Complications+”) (6280)
S13 TI ((perinatal or labor or labour or birth) N4 complication*) or AB ((perinatal or labor or labour or birth) N4 complication*) (806)
S12 TI ((perinatal or labor or labour or birth) N4 injur*) or AB ((perinatal or labor or labour or birth) N4 injur*) (437)
S11 TI ((perinatal or labor or labour or birth) N4 trauma) or AB ((perinatal or labor or labour or birth) N4 trauma) (300)
S10 (MH “Birth Injuries+”) (903)
S9 S7 OR S8 (769)
S8 (MH “Fetal Macrosomia”) (552)
S7 TI (macrosomia) OR AB (macrosomia) (446)
S6 S1 OR S2 OR S3 OR S4 OR S5 (4523)
S5 TI (glucose N4 (pregnan* or gestation* or natal or maternal)) or AB (glucose N4 (pregnan* or gestation* or natal or maternal)) (608)
S4 TI (gdm) or AB (gdm) (838)
S3 (MH “Diabetes Mellitus, Gestational”) (3293)
S2 TI (gestational N4 diabetes) or AB (gestational N4 diabetes) (2729)
S1 TI (pregnancy N4 diabetes) or AB (pregnancy N4 diabetes) (1018)
Source: Cochrane Central Register of Controlled Trials, Issue 9 of 12, September 2014
Interface/URL: The Cochrane Library/Wiley Interscience
Search date: 20 October 2014
Retrieved records: 256
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Trials (256)
Source: Cochrane Database of Systematic Reviews, Issue 10 of 12, October 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 20 October 2014.
Retrieved records: 42.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Cochrane Reviews (Reviews and Protocols) (42)
Source: Database of Abstracts of Reviews of Effects, Issue 3 of 4, July 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 20 October 2014.
Retrieved records: 7.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Other Reviews (7)
Source: Health Technology Assessment database, Issue 3 of 4, July 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 20 October 2014.
Retrieved records: 1.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Technology Assessments (1)
Source: NHS Economic Evaluation Database, Issue 3 of 4, July 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 20 October 2014.
Retrieved records: 1.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Economic Evaluations (1)
Source: Cochrane Methodology Register, Issue 3 of 4, July 2012
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 20 October 2014.
Retrieved records: 0.
Search strategy
#1 (pregnancy near diabetes):ti,ab,kw or (gestational near diabetes):ti,ab,kw or “GDM”:ti,ab,kw or “gestational diabetes”:ti,ab,kw or (pregnancy diabetes):ti,ab,kw (Word variations have been searched) (840)
#2 (glucose near (pregnan* or gestation* or natal or maternal)) (588)
#3 [mh “Diabetes, Gestational”] (352)
#4 #1 or #2 or #3 (1107)
#5 Macrosomia:ti,ab,kw (Word variations have been searched) (137)
#6 [mh “Fetal Macrosomia”] (67)
#7 #5 or #6 (137)
#8 ((perinatal or labor or labour or birth) near trauma):ti,ab,kw or ((perinatal or labor or labour or birth) near injur*):ti,ab,kw or ((perinatal or labor or labour or birth) near complication*):ti,ab,kw (Word variations have been searched) (1146)
#9 [mh “Birth Injuries”] (33)
#10 [mh “Obstetric Labor Complications”] (2336)
#11 [mh Dystocia] (88)
#12 (shoulder near dystocia):ti,ab,kw or (fracture* near clavicle*):ti,ab,kw or (fracture* near humerus):ti,ab,kw or (fracture* near shoulder*):ti,ab,kw or (fracture* near arm*):ti,ab,kw (Word variations have been searched) (441)
#13 (“erb* palsy”):ti,ab,kw or (neuropath*):ti,ab,kw (Word variations have been searched) (4623)
#14 [mh “Brachial Plexus Neuropathies”] (47)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (7923)
#16 “preeclampsia”:ti,ab,kw or “pre-eclampsia”:ti,ab,kw (Word variations have been searched) (1181)
#17 [mh Pre-Eclampsia] (613)
#18 #16 or #17 (1181)
#19 heart near disease*:ti,ab,kw or heart near disorder*:ti,ab,kw or cardiac near disease*:ti,ab,kw or cardiac near disorder*:ti,ab,kw (Word variations have been searched) (14,511)
#20 cardiovascular near disease*:ti,ab,kw or cardiovascular near disorder*:ti,ab,kw (Word variations have been searched) (11,691)
#21 [mh “Heart Diseases”] (37,370)
#22 [mh “Cardiovascular Diseases”] (75,564)
#23 #19 or #20 or #21 or #22 (85,943)
#24 hypoglyc*:ti,ab,kw (Word variations have been searched) (7938)
#25 [mh Hypoglycemia] (1176)
#26 #24 or #25 (7941)
#27 “type 2 diabetes mellitus”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw or “type 2 diabetes”:ti,ab,kw or “type two diabetes”:ti,ab,kw or “type II diabetes mellitus”:ti,ab,kw (Word variations have been searched) (9096)
#28 [mh “Diabetes Mellitus, Type 2”] (8928)
#29 #27 or #28 (12,190)
#30 “obese”:ti,ab,kw or “body mass”:ti,ab,kw or obesit*:ti,ab,kw or “overweigh*”:ti,ab,kw or “body mass index”:ti,ab,kw (Word variations have been searched) (24,839)
#31 [mh Obesity] (7607)
#32 #30 or #31 (24,858)
#33 #7 or #15 or #18 or #23 or #26 or #29 or #32 (124,396)
#34 “offspring”:ti,ab,kw or “daughter”:ti,ab,kw or “son”:ti,ab,kw or “Child”:ti,ab,kw or “paediatric” or “pediatric”:ti,ab,kw (Word variations have been searched) (84,655)
#35 [mh Child] (116)
#36 [mh “Child of Impaired Parents”] (120)
#37 [mh Mothers] (990)
#38 mother*:ti,ab,kw or “maternal”:ti,ab,kw (Word variations have been searched) (12,225)
#39 #34 or #35 or #36 or #37 or #38 (92,429)
#40 #33 and #39 (8640)
#41 #4 and #40 in Methods Studies (0)
Searches were carried out for Chapter 4 on 16 July 2014
| Database/information source | Interface/URL | Records identified |
|---|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | OvidSP | 409 |
| EMBASE | OvidSP | 1034 |
| Maternity and Infant Care | OvidSP | 116 |
| Incidence and Prevalence Database (IPD) | Free version | 32 |
| TOTAL | 1591 | |
| TOTAL after deduplication | 1196 |
Source: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE <1946 to present>
Interface/URL: OvidSP.
Search date: 16 July 2014.
Retrieved records: 409.
Search strategy
-
exp great britain/ (303,828)
-
Ireland/ (13,048)
-
(“united king*” or uk or “U.K.” or “UK.” or “U.K” or britain).ti,ab. (106,466)
-
(british or english or scottish or welsh or irish).ti,ab. (121,608)
-
(scotland or ireland).ti,ab. (78,711)
-
eire.ti,ab. (175)
-
(england not “new england”).ti,ab. (30,291)
-
(wales not “new south wales”).ti,ab. (11,198)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).ti,ab. (74,398)
-
((london adj2 ontario) or (london adj on) or new london).ti,ab. (647)
-
(manchester adj3 (USA or massach*)).ti,ab. (8)
-
(newcastle adj4 (australia* or “new south wales” or nsw)).ti,ab. (249)
-
(liverpool adj4 (australia* or “new south wales” or nsw)).ti,ab. (16)
-
or/10-13 (920)
-
9 not 14 (73,478)
-
(or/1-8) or 15 (567,708)
-
exp diabetes, gestational/ (7009)
-
(gestation$ adj4 diabet$).ti,ab. (8072)
-
gdm.ti,ab. (3046)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (3402)
-
exp Hyperglycemia/ (26,451)
-
exp Pregnancy/ (714,470)
-
21 and 22 (1575)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (894)
-
17 or 18 or 19 or 20 or 23 or 24 (13,372)
-
16 and 25 (557)
-
exp Epidemiology/ (21,538)
-
exp Epidemiologic Studies/ (1,664,350)
-
exp Incidence/ (177,085)
-
exp Prevalence/ (192,691)
-
(incidence or prevalence or occur* or frequenc* or proportion* or rate* or number* or percent*).ti,ab. (5,612,742)
-
or/27-31 (6,466,375)
-
26 and 32 (412)
-
limit 33 to english language (409)
Source: EMBASE <1974 to 2014 Week 28>
Interface/URL: OvidSP.
Search date: 16 July 2014.
Retrieved records: 1034.
Search strategy
-
United Kingdom/ (329,209)
-
Ireland/ (20,079)
-
(“united king*” or uk or “U.K.” or “UK.” or “U.K” or britain).ti,ab. (189,361)
-
(british or english or scottish or welsh or irish).ti,ab. (173,545)
-
(scotland or ireland).ti,ab. (160,575)
-
eire.ti,ab. (206)
-
(england not “new england”).ti,ab. (35,698)
-
(wales not “new south wales”).ti,ab. (13,659)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).ti,ab. (156,936)
-
((london adj2 ontario) or (london adj on) or new london).ti,ab. (784)
-
(manchester adj3 (USA or massach*)).ti,ab. (9)
-
(newcastle adj4 (australia* or “new south wales” or nsw)).ti,ab. (311)
-
(liverpool adj4 (australia* or “new south wales” or nsw)).ti,ab. (26)
-
or/10-13 (1130)
-
9 not 14 (155,806)
-
(or/1-8) or 15 (831,515)
-
exp pregnancy diabetes mellitus/ (20,129)
-
(gestation$ adj4 diabet$).ti,ab. (11,810)
-
gdm.ti,ab. (4778)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (4298)
-
hyperglycemia/ (61,078)
-
exp Pregnancy/ (589,193)
-
21 and 22 (1647)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (1171)
-
17 or 18 or 19 or 20 or 23 or 24 (25,055)
-
16 and 25 (1738)
-
Epidemiology/ (179,438)
-
epidemiological data/ or epidemiological monitoring/ (24,516)
-
incidence/ or familial incidence/ or standardized incidence ratio/ (213,226)
-
Prevalence/ (381,394)
-
(incidence or prevalence or occur* or frequenc* or proportion* or rate* or number* or percent*).ti,ab. (6,756,057)
-
or/27-31 (6,980,639)
-
26 and 32 (1040)
-
limit 33 to english language (1034)
Source: Maternity and Infant Care <1971 to June 2014>
Interface/URL: OvidSP.
Search date: 16 July 2014.
Retrieved records: 116.
Search strategy
-
(Great Britain or United Kingdom or England or Wales or Scotland or Northern Ireland).de. (10,228)
-
Ireland.de. (437)
-
(“united king*” or uk or “U.K.” or “UK.” or “U.K” or britain).ti,ab. (9318)
-
(british or english or scottish or welsh or irish).ti,ab. (4652)
-
(scotland or ireland).ti,ab. (2350)
-
eire.ti,ab. (13)
-
(england not “new england”).ti,ab. (3895)
-
(wales not “new south wales”).ti,ab. (2025)
-
(london or manchester or birmingham or leeds or sheffield or liverpool or newcastle or edinburgh or glasgow or cardiff or oxford or bristol).ti,ab. (6179)
-
((london adj2 ontario) or (london adj on) or new london).ti,ab. (65)
-
(manchester adj3 (USA or massach*)).ti,ab. (0)
-
(newcastle adj4 (australia* or “new south wales” or nsw)).ti,ab. (11)
-
(liverpool adj4 (australia* or “new south wales” or nsw)).ti,ab. (1)
-
or/10-13 (77)
-
9 not 14 (6102)
-
(or/1-8) or 15 (26,375)
-
(Gestational diabetes or Diabetes - gestational).de. (1191)
-
(gestation$ adj4 diabet$).ti,ab. (2730)
-
gdm.ti,ab. (993)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (811)
-
Hyperglycemia.de. (1)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (180)
-
17 or 18 or 19 or 20 or 21 or 22 (3251)
-
16 and 23 (171)
-
Epidemiology.de. (128)
-
(incidence or prevalence or occur* or frequenc* or proportion* or rate* or number* or percent*).ti,ab. (76,295)
-
25 or 26 (76,373)
-
24 and 27 (116)
-
limit 28 to english language [Limit not valid; records were retained] (116)
Source: Incidence and Prevalence Database
Interface/URL: free Internet version on Dialog.
Search date: 16 July 2014.
Retrieved records: 32.
Search strategy
A restricted search of this database was carried out because of its prohibitive cost. Thirty-two records were retrieved when searches were undertaken for ‘Gestational diabetes’ or ‘gdm’ in the title.
Searches for Chapter 5 were carried out on 6 June 2014
| Resource | Interface/URL | Records identified |
|---|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | OvidSP | 2429 |
| EMBASE | OvidSP | 2289 |
| Maternity and Infant Care | OvidSP | 884 |
| CENTRAL | The Cochrane Library/Wiley Interscience | 265 |
| TOTAL | 5867 | |
| TOTAL after deduplication | 3140 |
Source: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE <1946 to present>
Interface/URL: OvidSP.
Search date: 6 June 2014.
Retrieved records: 2429.
Search strategy
-
risk/ (98,061)
-
risk factors/ (563,466)
-
risk$.tw. (1,321,838)
-
related.tw. (1,576,712)
-
relationship.tw. (681,818)
-
rates.tw. (688,903)
-
difference$.tw. (1,634,999)
-
prevalence.tw. (382,911)
-
associated factors.tw. (8200)
-
predict$.tw. (943,185)
-
or/1-10 (5547,109)
-
exp overweight/ (142,733)
-
(obese or obesity).tw. (175,969)
-
(overweight or over weight).tw. (38,413)
-
body mass index/ (80,128)
-
BMI.tw. (75,276)
-
body mass index.tw. (101,816)
-
exp Ethnic Groups/ (112,019)
-
(ethnicity or ethnic or multiethnic$ or race).tw. (133,942)
-
(caucasian$ or asian$ or spanish or mexican$ or hispanic$ or afrocaribbean$ or african$ or caribbean$).tw.)
-
(237,285)
-
(middle eastern or bangladeshi$ or pakistani$).tw. (6067)
-
maternal age/ (16,131)
-
age.tw. (1,482,617)
-
(pregnan$ adj2 late$ adj2 life).tw. (41)
-
older.tw. (263,039)
-
over 35.tw. (2427)
-
over 25.tw. (3623)
-
over 30.tw. (10,584)
-
(previous adj3 (gdm or diabet$)).tw. (2130)
-
(prior adj3 (gdm or diabet$)).tw. (1392)
-
(history adj3 (gdm or diabet$)).tw. (7728)
-
(family adj3 (gdm or diabet$)).tw. (3296)
-
(relative adj3 (gdm or diabet$)).tw. (1141)
-
family history.tw. (42,205)
-
prior history.tw. (3983)
-
previous history.tw. (7267)
-
((prior or previous or history) adj2 macrosomia).tw. (48)
-
((prior or previous or history) adj2 macrosomic).tw. (25)
-
((prior or previous or history) adj2 LGA).tw. (5)
-
((prior or previous or history) adj2 large gestational age).tw. (0)
-
((prior or previous or history) adj2 large for gestational age).tw. (1)
-
((prior or previous or history) adj2 large bab$).tw. (3)
-
((prior or previous or history) adj2 large infant$).tw. (2)
-
parity.tw. (21,039)
-
parity/ (20,499)
-
risk factor$.ti. (73,764)
-
or/12-46 (2,152,353)
-
11 and 47 (1,297,220)
-
exp diabetes, gestational/ (6917)
-
(gestation$ adj4 diabet$).tw. (7955)
-
gdm.tw. (2973)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).tw.)
-
(3380)
-
exp Hyperglycemia/ (26,216)
-
exp Pregnancy/ (711,357)
-
53 and 54 (1565)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).tw. (884)
-
or/49-52,55-56 (13,211)
-
48 and 57 (5062)
-
Mass Screening/ (81,803)
-
screen$.ti. (116,932)
-
screen$.ab. /freq=2 (122,782)
-
Glucose Tolerance Test/ (29,299)
-
Blood glucose/an (47,809)
-
(glucose adj3 (test$ or measur$ or assess$ or evaluat$)).tw. (36,335)
-
((glucose adj2 tolerance) or gtt or ogtt).tw. (33,566)
-
((glucose adj2 challeng$) or gct or ogct).tw. (4698)
-
(fasting adj2 glucose).tw. (24,915)
-
or/59-67 (332,758)
-
Diagnosis/ (16,639)
-
Prenatal Diagnosis/ (31,200)
-
exp Diagnostic errors/ (94,398)
-
Diagnosis, Differential/ (379,054)
-
diagnos$.ti. (450,817)
-
diagnos$.ab. /freq=2 (536,082)
-
(di or du).fs. (2,229,471)
-
exp “Sensitivity and Specificity”/ (416,076)
-
(sensitivity or specificity).tw. (725,114)
-
((pre-test or pretest) adj probabilit$).tw. (1402)
-
((post-test or posttest) adj probabilit$).tw. (738)
-
(predictive adj3 value$).tw. (70,018)
-
(false positiv$ or false negativ$).tw. (56,289)
-
observer variation$.tw. (959)
-
roc curve$.tw. (14,358)
-
(likelihood adj3 ratio$).tw. (9220)
-
accurac$.tw. (232,990)
-
detection.tw. (591,376)
-
or/69-86 (3,919,528)
-
68 or 87 (4,099,746)
-
58 and 88 (2801)
-
animals/ not humans/ (3,855,883)
-
(editorial or case reports or news or letter or comment).pt. (2,995,254)
-
89 not (90 or 91) (2665)
-
limit 92 to english language (2429)
Source: EMBASE <1974 to 2014 Week 22>
Interface/URL: OvidSP.
Search date: 6 June 2014.
Retrieved records: 2289.
Search strategy
-
high risk infant/ or high risk patient/ or high risk population/ or high risk pregnancy/ or low risk population/or population risk/ (159,235)
-
risk factor/ (609,961)
-
risk$.tw. (1,729,570)
-
related.tw. (1,894,574)
-
relationship.tw. (820,833)
-
rates.tw. (844,892)
-
difference$.tw. (2,006,843)
-
prevalence.tw. (488,098)
-
associated factors.tw. (10,057)
-
predict$.tw. (1,155,249)
-
or/1-10 (6,731,793)
-
exp obesity/ (292,645)
-
(obese or obesity).tw. (235,953)
-
(overweight or over weight).tw. (52,865)
-
body mass/ (185,645)
-
BMI.tw. (133,729)
-
body mass index.tw. (131,385)
-
exp “ethnic and racial groups”/ (333,104)
-
(ethnicity or ethnic or multiethnic$ or race).tw. (167,278)
-
(caucasian$ or asian$ or spanish or mexican$ or hispanic$ or afrocaribbean$ or african$ or caribbean$).tw.)
-
(302,094)
-
(middle eastern or bangladeshi$ or pakistani$).tw. (7602)
-
maternal age/ (22,047)
-
age.tw. (2,013,347)
-
(pregnan$ adj2 late$ adj2 life).tw. (52)
-
older.tw. (329,442)
-
over 35.tw. (3025)
-
over 25.tw. (4644)
-
over 30.tw. (13,528)
-
(previous adj3 (gdm or diabet$)).tw. (3297)
-
(prior adj3 (gdm or diabet$)).tw. (2294)
-
(history adj3 (gdm or diabet$)).tw. (11,881)
-
(family adj3 (gdm or diabet$)).tw. (4461)
-
(relative adj3 (gdm or diabet$)).tw. (1363)
-
family history.tw. (60,508)
-
prior history.tw. (6224)
-
previous history.tw. (10,741)
-
((prior or previous or history) adj2 macrosomia).tw. (78)
-
((prior or previous or history) adj2 macrosomic).tw. (41)
-
((prior or previous or history) adj2 LGA).tw. (9)
-
((prior or previous or history) adj2 large gestational age).tw. (0)
-
((prior or previous or history) adj2 large for gestational age).tw. (2)
-
((prior or previous or history) adj2 large bab$).tw. (7)
-
((prior or previous or history) adj2 large infant$).tw. (2)
-
parity.tw. (24,255)
-
parity/ (23,009)
-
risk factor$.ti. (93,554)
-
or/12-46 (2,962,447)
-
11 and 47 (1,790,792)
-
exp *pregnancy diabetes mellitus/ (11,726)
-
(gestation$ adj4 diabet$).tw. (11,561)
-
gdm.tw. (4660)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).tw.)
-
(4254)
-
*hyperglycemia/ (16,323)
-
exp *pregnancy/ (160,365)
-
53 and 54 (224)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).tw. (1161)
-
or/49-52,55-56 (19,285)
-
48 and 57 (7226)
-
exp *screening/ (136,180)
-
screen$.ti. (144,840)
-
screen$.ab. /freq=2 (161,674)
-
Glucose Tolerance Test/ (21,678)
-
Blood glucose/an (16,029)
-
(glucose adj3 (test$ or measur$ or assess$ or evaluat$)).tw. (48,149)
-
((glucose adj2 tolerance) or gtt or ogtt).tw. (44,930)
-
((glucose adj2 challeng$) or gct or ogct).tw. (6122)
-
(fasting adj2 glucose).tw. (36,533)
-
or/59-67 (424,217)
-
*diagnosis/ (50,263)
-
*prenatal diagnosis/ (23,505)
-
*differential diagnosis/ (11,262)
-
exp *diagnostic error/ (6128)
-
*diagnostic accuracy/ (4749)
-
diagnos$.ti. (540,893)
-
diagnos$.ab. /freq=2 (750,292)
-
di.fs. (2,549,486)
-
*”sensitivity and specificity”/ (697)
-
(sensitivity or specificity).tw. (844,233)
-
((pre-test or pretest) adj probabilit$).tw. (2063)
-
((post-test or posttest) adj probabilit$).tw. (874)
-
(predictive adj3 value$).tw. (92,314)
-
(false positiv$ or false negativ$).tw. (69,384)
-
observer variation$.tw. (1211)
-
roc curve$.tw. (23,111)
-
(likelihood adj3 ratio$).tw. (11,254)
-
accurac$.tw. (275,387)
-
detection.tw. (703,313)
-
or/69-87 (4,429,688)
-
68 or 88 (4,688,631)
-
58 and 89 (3792)
-
(animal experiment/ or animal model/ or animal tissue/ or nonhuman/) not exp human/ (3,776,825)
-
(editorial or letter).pt. or case report.ti. or (conference abstract or conference paper or conference proceeding or conference review).pt. (3,666,517)
-
90 not (91 or 92) (2587)
-
limit 93 to english language (2289)
Source: Maternity and Infant Care <1971 to April 2014>
Interface/URL: OvidSP.
Search date: 6 June 2014.
Retrieved records: 884.
Search strategy
-
risk$.tw. (47,816)
-
related.tw. (16,447)
-
relationship.tw. (9690)
-
rates.tw. (16,144)
-
difference$.tw. (22,927)
-
prevalence.tw. (8332)
-
associated factors.tw. (234)
-
predict$.tw. (14,779)
-
or/1-8 (91,159)
-
obesity.de. (1257)
-
(obese or obesity).tw. (3186)
-
(overweight or over weight).tw. (1220)
-
body mass index.de. (812)
-
BMI.tw. (1988)
-
body mass index.tw. (3064)
-
Ethnic Groups.de. (2291)
-
(ethnicity or ethnic or multiethnic$ or race).tw. (6778)
-
(caucasian$ or asian$ or spanish or mexican$ or hispanic$ or afrocaribbean$ or african$ or caribbean$).tw. (5455)
-
(middle eastern or bangladeshi$ or pakistani$).tw. (316)
-
age.tw. (39,774)
-
(pregnan$ adj2 late$ adj2 life).tw. (15)
-
older.tw. (3367)
-
over 35.tw. (148)
-
over 25.tw. (61)
-
over 30.tw. (171)
-
(previous adj3 (gdm or diabet$)).tw. (110)
-
(prior adj3 (gdm or diabet$)).tw. (61)
-
(history adj3 (gdm or diabet$)).tw. (222)
-
(family adj3 (gdm or diabet$)).tw. (83)
-
(relative adj3 (gdm or diabet$)).tw. (36)
-
family history.tw. (724)
-
prior history.tw. (83)
-
previous history.tw. (209)
-
((prior or previous or history) adj2 macrosomia).tw. (23)
-
((prior or previous or history) adj2 macrosomic).tw. (14)
-
((prior or previous or history) adj2 LGA).tw. (4)
-
((prior or previous or history) adj2 large gestational age).tw. (0)
-
((prior or previous or history) adj2 large for gestational age).tw. (1)
-
((prior or previous or history) adj2 large bab$).tw. (5)
-
((prior or previous or history) adj2 large infant$).tw. (1)
-
parity.tw. (4622)
-
parity.de. (527)
-
risk factor$.ti. (2244)
-
or/10-43 (51,430)
-
9 and 44 (36,837)
-
Diabetes - gestational.de. (1181)
-
(gestation$ adj4 diabet$).tw. (2863)
-
gdm.tw. (965)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).tw. (801)
-
Hyperglycaemia.de. (119)
-
(Pregnancy complications or Pregnancy).de. (57,381)
-
50 and 51 (74)
-
((hyperglycemi$ or hyperglycaemi$) adj5 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or)
-
ante-natal$ or maternal$)).tw. (195)
-
or/46-49,52-53 (3225)
-
45 and 54 (1637)
-
Screening.de. (5491)
-
screen$.tw. (15,289)
-
Mass screening.de. (705)
-
Prenatal diagnosis.de. (4440)
-
diagnos$.tw. (26,426)
-
(“Sensitivity and specificity” or “Predictive value of tests”).de. (2526)
-
Glucose tolerance test.de. (238)
-
(glucose adj3 (test$ or measur$ or assess$ or evaluat$)).tw. (1073)
-
((glucose adj2 tolerance) or gtt or ogtt).tw. (934)
-
((glucose adj2 challeng$) or gct or ogct).tw. (226)
-
(fasting adj2 glucose).tw. (380)
-
((pre-test or pretest) adj probabilit$).tw. (18)
-
((post-test or posttest) adj probabilit$).tw. (24)
-
(predictive adj3 value$).tw. (4212)
-
(false positiv$ or false negativ$).tw. (1660)
-
observer variation$.tw. (143)
-
roc curve$.tw. (356)
-
(likelihood adj3 ratio$).tw. (492)
-
accurac$.tw. (2774)
-
detection.tw. (4826)
-
or/56-75 (40,118)
-
55 and 76 (889)
-
(correspondance or editorial or case report or case study or news or news release or news item or letter or commentary).pt. (30,045)
-
77 not 78 (884)
Source: Cochrane Central Register of Controlled Trials, Issue 6 of 12, June 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 6 June 2014.
Retrieved records: 265.
Search strategy
#1 MeSH descriptor: [Diabetes, Gestational] explode all trees
#2 (gestation* near/4 diabet*)
#3 gdm
#4 (glucose near/4 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*))
#5 MeSH descriptor: [Hyperglycemia] explode all trees
#6 MeSH descriptor: [Pregnancy] explode all trees
#7 #5 and #6
#8 ((hyperglycemi* or hyperglycaemi*) near/5 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*))
#9 #1 or #2 or #3 or #4 or #7 or #8
#10 MeSH descriptor: [Risk] this term only
#11 MeSH descriptor: [Risk Factors] this term only
#12 (related or relationship or rates or difference* or prevalence or associated or predict*)
#13 #10 or #11 or #12
#14 #9 and #13
#15 MeSH descriptor: [Mass Screening] this term only
#16 MeSH descriptor: [Blood Glucose] this term only
#17 glucose or screen*
#18 MeSH descriptor: [Diagnosis] this term only
#19 MeSH descriptor: [Prenatal Diagnosis] this term only
#20 MeSH descriptor: [Diagnostic Errors] explode all trees
#21 MeSH descriptor: [Diagnosis, Differential] this term only
#22 diagnos* or sensitivity or specificity or pre-test or pretest or post-test or posttest or predictive near/4 value* or false positive* or false negative* or observer variation* or roc curve* or likelihood near/4 ratio or accuracy* or detection
#23 MeSH descriptor: [Sensitivity and Specificity] explode all trees
#24 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #14 and #24
#26 MeSH descriptor: [Overweight] explode all trees
#27 obesity or obese or over weight or overweight or BMI or body mass index
#28 MeSH descriptor: [Body Mass Index] this term only
#29 MeSH descriptor: [Ethnic Groups] explode all trees
#30 ethnicity or ethnic or multi-ethnic* or race or Caucasian* or Asian* or Spanish or Mexican* or Hispanic* or afrocaribbean or African or Caribbean or middle eastern or Bangladeshi* or Pakistani*
#31 MeSH descriptor: [Maternal Age] this term only
#32 age or late near/2 life or older or over or previous or prior or history or family or relative or parity
#33 MeSH descriptor: [Parity] this term only
#34 #26 or #27 or #18 or #29 or #30 or #31 or #32 or #33
#35 #25 and #34
The September 2013 search for Chapter 6 identified 2895 records: 2226 records remained after deduplication; the October 2014 update search identified 3555 records
| Database/information source | Interface/URL | |
|---|---|---|
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | OvidSP | |
| EMBASE | OvidSP | |
| CENTRAL | The Cochrane Library/Wiley Interscience | |
| Records identified in the original 2013 searches | Records identified in the 2014 update searches | |
| MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations | 864 | 940 |
| EMBASE | 1420 | 1813 |
| CENTRAL | 611 | 802 |
| TOTAL | 2895 | 3555 |
| TOTAL after deduplication | 2226 | 1419 |
Search strategies (September 2013)
Source: Cochrane Central Register of Controlled Trials, Issue 8 of 12, August 2013
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 11 September 2013.
Retrieved records: 611.
Search strategy
#1 MeSH descriptor: [Diabetes, Gestational] explode all trees (293)
#2 (gestation* near/4 diabet*) (563)
#3 gdm (142)
#4 (glucose near/4 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*)) (394)
#5 #1 or #2 or #3 or #4 (785)
#6 MeSH descriptor: [Glucose Intolerance] this term only (413)
#7 MeSH descriptor: [Glucose Tolerance Test] this term only (1483)
#8 IGT (355)
#9 ((impair* or reduced) near/2 glucose) (1506)
#10 (glucose next (tolerance* or intolerance*)) (3335)
#11 (gtt or ogtt) (657)
#12 MeSH descriptor: [Prediabetic State] this term only (118)
#13 (prediabet* or pre-diabet*) (234)
#14 MeSH descriptor: [Insulin Resistance] explode all trees (2574)
#15 (metabolic next syndrome* or syndrome* next x or borderline next diabet*) (1521)
#16 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (6512)
#17 MeSH descriptor: [Pregnancy] explode all trees (5409)
#18 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*) (31,548)
#19 #17 or #18 (31,663)
#20 #16 and #19 (514)
#21 #5 or #20 in Trials (611)
Source: MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations 1946 to present
Interface/URL: OvidSP.
Search date: 12 September 2013.
Retrieved records: 864.
Search strategy
-
exp diabetes, gestational/ (6899)
-
(gestation$ adj4 diabet$).ti,ab. (7705)
-
gdm.ti,ab. (2826)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (3366)
-
or/1-4 (12,010)
-
Glucose Intolerance/ (6367)
-
Glucose Tolerance Test/ (29,619)
-
IGT.ti,ab. (3628)
-
((impair$ or reduced) adj2 glucose).ti,ab. (17,221)
-
(glucose adj (tolerance$ or intolerance$)).ti,ab. (36,342)
-
(gtt or ogtt).ti,ab. (6783)
-
Prediabetic State/ (3657)
-
(prediabet$ or pre-diabet$).ti,ab. (4581)
-
exp Insulin Resistance/ (54,897)
-
(metabolic syndrome$ or syndrome$ x or borderline diabet$).ti,ab. (29,541)
-
or/6-15 (114,795)
-
exp Pregnancy/ (713,514)
-
(pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$).ti,ab. (572,627)
-
or/17-18 (903,336)
-
16 and 19 (8640)
-
5 or 20 (16,584)
-
randomized controlled trial.pt. (385,372)
-
controlled clinical trial.pt. (89,166)
-
random$.ti,ab. (725,429)
-
placebo.ti,ab. (166,003)
-
drug therapy.fs. (1,750,436)
-
trial.ti,ab. (374,230)
-
groups.ab. (1,352,980)
-
or/22-28 (3,543,468)
-
21 and 29 (4978)
-
(2012$ or 2013$ or 2014$).ed,dc,dp,ep,vd,yr. (2,303,896)
-
30 and 31 (956)
-
animals/ not humans/ (3,937,252)
-
32 not 33 (864)
Source: EMBASE 1974 to 11 September 2013
Interface/URL: OvidSP.
Search date: 12 September 2013.
Retrieved records: 1420.
Search strategy
-
exp pregnancy diabetes mellitus/ (19,149)
-
(gestation$ adj4 diabet$).ti,ab. (10,764)
-
gdm.ti,ab. (4148)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (4143)
-
or/1-4 (22,936)
-
impaired glucose tolerance/ (16,945)
-
glucose intolerance/ (10,830)
-
exp glucose tolerance test/ (41,669)
-
IGT.ti,ab. (5232)
-
((impair$ or reduced) adj2 glucose).ti,ab. (21,989)
-
(glucose adj (tolerance$ or intolerance$)).ti,ab. (46,496)
-
(gtt or ogtt).ti,ab. (10,650)
-
(prediabet$ or pre-diabet$).ti,ab. (6237)
-
insulin resistance/ (73,020)
-
metabolic syndrome X/ (43,215)
-
(metabolic syndrome$ or syndrome$ x or borderline diabet$).ti,ab. (41,778)
-
or/6-16 (175,188)
-
exp pregnancy/ (618,511)
-
(pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$).ti,ab. (679,071)
-
or/18-19 (931,013)
-
17 and 20 (12,193)
-
5 or 21 (28,523)
-
randomized controlled trial/ (358,223)
-
“randomized controlled trial (topic)”/ (38,557)
-
crossover procedure/ (38,413)
-
double blind procedure/ (120,036)
-
single blind procedure/ (18,230)
-
random$.ti,ab. (856,059)
-
factorial$.ti,ab. (22,209)
-
(crossover$ or cross-over$).ti,ab. (70,187)
-
placebo$.ti,ab. (200,440)
-
doubl$ blind$.ti,ab. (146,751)
-
singl$ blind$.ti,ab. (14,162)
-
assign$.ti,ab. (235,266)
-
allocat$.ti,ab. (80,922)
-
volunteer$.ti,ab. (179,039)
-
trial.ti,ab. (455,704)
-
groups.ab. (1,662,182)
-
or/23-38 (2,878,357)
-
22 and 39 (6177)
-
(2012$ or 2013$ or 2014$).em,dp,yr. (2,472,974)
-
40 and 41 (1503)
-
(animal experiment/ or animal model/ or animal tissue/ or nonhuman/) not exp human/ (3,657,804)
-
42 not 43 (1420)
Update search strategies: October 2014
Source: Cochrane Central Register of Controlled Trials, Issue 9 of 12, September 2014
Interface/URL: The Cochrane Library/Wiley Interscience.
Search date: 14 October 2014.
Retrieved records: 802.
Search strategy
#1 [mh “Diabetes, Gestational”] (352)
#2 (gestation* near/4 diabet*) (726)
#3 gdm (230)
#4 (glucose near/4 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*)) (484)
#5 #1 or #2 or #3 or #4 (979)
#6 [mh ^”Glucose Intolerance”] (472)
#7 [mh ^”Glucose Tolerance Test”] (1593)
#8 IGT (434)
#9 ((impair* or reduced) near/2 glucose) (1905)
#10 (glucose next (tolerance* or intolerance*)) (3987)
#11 (gtt or ogtt) (778)
#12 [mh ^”Prediabetic State”] (161)
#13 (prediabet* or pre-diabet*) (351)
#14 [mh “Insulin Resistance”] (3093)
#15 (metabolic next syndrome* or syndrome* next x or borderline next diabet*) (2249)
#16 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 (8217)
#17 [mh Pregnancy] (5804)
#18 (pregnan* or gestation* or prenatal* or antenatal* or pre-natal* or ante-natal* or maternal*) (35,893)
#19 #17 or #18 (36,019)
#20 #16 and #19 (631)
#21 #5 or #20 in Trials (802)
Source: Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946 to present
Interface/URL: OvidSP.
Search date: 14 October 2013.
Retrieved records: 940.
Search strategy
-
exp diabetes, gestational/ (7433)
-
(gestation$ adj4 diabet$).ti,ab. (8559)
-
gdm.ti,ab. (3263)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (3520)
-
or/1-4 (13,077)
-
Glucose Intolerance/ (6655)
-
Glucose Tolerance Test/ (30,420)
-
IGT.ti,ab. (3868)
-
((impair$ or reduced) adj2 glucose).ti,ab. (18,095)
-
(glucose adj (tolerance$ or intolerance$)).ti,ab. (38,304)
-
(gtt or ogtt).ti,ab. (7294)
-
Prediabetic State/ (3939)
-
(prediabet$ or pre-diabet$).ti,ab. (5048)
-
exp Insulin Resistance/ (58,654)
-
(metabolic syndrome$ or syndrome$ x or borderline diabet$).ti,ab. (32,817)
-
or/6-15 (122,777)
-
exp Pregnancy/ (733,700)
-
(pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$).ti,ab. (596,979)
-
or/17-18 (936,507)
-
16 and 19 (9233)
-
5 or 20 (17,945)
-
randomized controlled trial.pt. (396,977)
-
controlled clinical trial.pt. (90,468)
-
random$.ti,ab. (758,589)
-
placebo.ti,ab. (167,219)
-
drug therapy.fs. (1,773,912)
-
trial.ti,ab. (390,474)
-
groups.ab. (1,427,636)
-
or/22-28 (3,672,104)
-
21 and 29 (5452)
-
(2013$ or 2014$ or 2015$).ed,dc,dp,ep,vd,yr. (2,338,649)
-
30 and 31 (1033)
-
animals/ not humans/ (3,981,381)
-
32 not 33 (940)
Source: EMBASE 1974 to 2014 October 13
Interface/URL: OvidSP.
Search date: 14 October 2014.
Retrieved records: 1813.
Search strategy
-
exp pregnancy diabetes mellitus/ (20,732)
-
(gestation$ adj4 diabet$).ti,ab. (12,311)
-
gdm.ti,ab. (5089)
-
(glucose adj4 (pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$)).ti,ab. (4425)
-
or/1-4 (24,743)
-
impaired glucose tolerance/ (19,159)
-
glucose intolerance/ (11,688)
-
exp glucose tolerance test/ (43,801)
-
IGT.ti,ab. (5676)
-
((impair$ or reduced) adj2 glucose).ti,ab. (23,596)
-
(glucose adj (tolerance$ or intolerance$)).ti,ab. (49,570)
-
(gtt or ogtt).ti,ab. (12,028)
-
(prediabet$ or pre-diabet$).ti,ab. (7337)
-
insulin resistance/ (81,011)
-
metabolic syndrome X/ (49,311)
-
(metabolic syndrome$ or syndrome$ x or borderline diabet$).ti,ab. (47,074)
-
or/6-16 (191,349)
-
exp pregnancy/ (595,884)
-
(pregnan$ or gestation$ or prenatal$ or antenatal$ or pre-natal$ or ante-natal$ or maternal$).ti,ab. (700,285)
-
or/18-19 (935,882)
-
17 and 20 (13,412)
-
5 or 21 (30,882)
-
randomized controlled trial/ (353,710)
-
“randomized controlled trial (topic)”/ (59,334)
-
crossover procedure/ (40,369)
-
double blind procedure/ (118,207)
-
single blind procedure/ (18,906)
-
random$.ti,ab. (918,458)
-
factorial$.ti,ab. (24,012)
-
(crossover$ or cross-over$).ti,ab. (72,507)
-
placebo$.ti,ab. (208,414)
-
doubl$ blind$.ti,ab. (150,493)
-
singl$ blind$.ti,ab. (14,995)
-
assign$.ti,ab. (247,571)
-
allocat$.ti,ab. (87,329)
-
volunteer$.ti,ab. (184,812)
-
trial.ti,ab. (489,194)
-
groups.ab. (1,777,499)
-
or/23-38 (3,059,904)
-
22 and 39 (7133)
-
(2013$ or 2014$ or 2015$).em,dp,yr. (2,778,898)
-
40 and 41 (1913)
-
(animal experiment/ or animal model/ or animal tissue/ or nonhuman/) not exp human/ (3,834,022)
-
42 not 43 (1813)
List of abbreviations
- ACHOIS
- Australian Carbohydrate Intolerance Study in Pregnant Women
- ACOG
- American College of Obstetricians and Gynecologists
- ADA
- American Diabetes Association
- ADIPS
- Australasian Diabetes in Pregnancy Society
- Atlantic DIP
- Atlantic Diabetes in Pregnancy
- BGSM
- blood glucose self-monitoring
- BiB
- Born in Bradford
- BMI
- body mass index
- BNF
- British National Formulary
- BP
- blood pressure
- BW
- birthweight
- C&C
- Carpenter and Coustan
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- C-section
- Caesarean section
- DARE
- Database of Abstracts of Reviews of Effects
- DPP
- Diabetes Prevention Program (study)
- DPPOS
- Diabetes Prevention Program Outcomes Study
- EVPI
- expected value of perfect information
- FPG
- fasting plasma glucose
- GDM
- gestational diabetes mellitus
- HAPO
- Hyperglycemia and Adverse Pregnancy Outcomes (study)
- HCHS
- Hospital and Community Health Services
- HRQL
- health-related quality of life
- HTA
- Health Technology Assessment
- IADPSG
- International Association of Diabetes in Pregnancy Study Groups
- ICER
- incremental cost-effectiveness ratio
- IGT
- impaired glucose tolerance
- ILS
- intensive lifestyle intervention
- IPD
- individual participant data
- LGA
- large for gestational age
- MD
- mean difference
- MICE
- multiple imputation by chained equations
- NDDG
- National Diabetes Data Group
- NHB
- net health benefit
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NMB
- net monetary benefit
- NNU
- neonatal unit
- NSC
- National Screening Committee
- OGCT
- oral glucose challenge test
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- PIH
- pregnancy-induced hypertension
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic (curve)
- RPG
- random plasma glucose
- RR
- relative risk
- S–
- screen negative
- S+
- screen positive
- S+T+
- screen positive and test positive on diagnostic
- S+T–
- screen positive, but test negative on diagnostic
- SA
- South Asian
- SAVI
- Sheffield accelerated value of information
- SD
- standard deviation
- SE
- standard error
- T–
- diagnostic test negative
- T+
- diagnostic test positive
- WB
- white British
- WHO
- World Health Organization