Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/43/504. The contractual start date was in November 2013. The draft report began editorial review in July 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Richards reports grants from University of Exeter during the conduct of the study and is a member of the National Institute for Health Research Career Development Fellowship, Senior Research Fellowship and Transitional Research Fellowship Panel 2013 to the present.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Brabyn et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Depression
Depression is the most common mental health disorder in community settings and is estimated to become the second largest cause of global disability by 2020. 1 It is one of the most common reasons for consulting with a general practitioner (GP) and its associated personal and economic burden is considerable. 2
Psychological therapy for depression
Although antidepressants remain an important treatment option, many patients and health-care professionals would like access to psychological therapy as an alternative or adjunct to drug therapy. 3 Cognitive behaviour therapy (CBT) has emerged as the leading evidence-supported form of brief psychological therapy for people with depression. 4,5 However, demand for CBT cannot be met from existing therapist resources. 6 One promising alternative to therapist-delivered CBT is the use of self-help interventions including the provision of therapy via a computer. 7 In recent years a number of interactive computer programs have been developed that enable CBT to be delivered by a computer. The National Institute for Health and Care Excellence (NICE) guidelines for depression recommend the provision of computerised CBT (cCBT) as an initial, lower-intensity treatment for depression as part of a ‘stepped care’ approach in primary care. 5 If effective, such programmes have the potential to expand the provision of psychological therapy in primary care and may represent an efficient and effective form of care for depression. 8
For those who decide to use (or commission the provision of) cCBT there are a number of interactive internet-based products, some commercially produced and others free to use. 7 In the first category, a number of commercial products have been marketed to bodies such as the NHS. Free-to-use products comprise a range of programs that have been developed by the public sector or by research institutes. These can be accessed at no direct purchase cost to health-care providers. An example of a free-to-use cCBT program is MoodGYM (National Institute for Mental Health Research, Australian National University, Canberra, ACT, Australia), which was developed in Australia and can be accessed by patients with depression, either directly or at the suggestion of their health-care provider.
Evidence for computerised cognitive behaviour therapy
Computerised CBT represents an alternative form of therapy delivery that has the potential to enhance access to psychological care. A number of systematic reviews have been conducted studying the effectiveness of cCBT. An overall beneficial effect of cCBT has been found within trials, although there is a high level of variability in effect size between studies. An early health technology assessment review by Kaltenthaler et al. ,6 published in 2006, noted preliminary evidence of clinical effectiveness and cost-effectiveness. The authors noted the existence of internet-based free-to-use packages, such as MoodGYM, which had been evaluated in randomised trials, but also noted that a major limitation of the existing literature was that the trials had been conducted by the package developers. 8 Since this review there have been few independent evaluations of cCBT packages and the randomised literature remains dominated by developer-led studies.
Later systematic reviews have also highlighted the potential for cCBT to be effective, but have also further demonstrated variable effect sizes and substantial between-study heterogeneity. 9,10 One important source of between-study heterogeneity is the level of support that is made available to people who are offered treatment with cCBT. cCBT involves replacing the therapist with a computer, and requires the person with depression to engage with a self-help computer-based technology. Research by Waller and Gilbody11 has shown that people with depression often do not engage with cCBT, and only a minority actually complete all of the planned sessions of the computer package. This observation is consistent with a broader body of research into the uptake and effectiveness across the range of self-help interventions for depression, including bibliotherapy (self-treatment using written materials). Research in the area of self-help treatments for depression has demonstrated that entirely self-guided materials (with no professional support) are likely to be less effective than self-help technologies for which there is a level of guidance and professional support (‘guided self-help’). Unsupported self-help treatment (including unsupported computer-delivered self-help) has been shown in systematic reviews to have minimal or relatively small effect sizes. In contrast, more intensively and professionally supported treatments have generally been found in efficacy trials to have moderate effect sizes claimed to be comparable to those achieved with face-to-face therapy. To our knowledge the comparative effectiveness of minimally supported cCBT versus more intensively supported cCBT has not been directly tested in large-scale, independently conducted, head-to-head effectiveness trials. Based on indirect estimates drawn from systematic reviews of trials of cCBT the effect is therefore potentially enhanced through the provision of professional support. The magnitude of benefit associated with supported cCBT in groups of patients is, on average, larger than that with unsupported/minimally supported cCBT [pooled effect size for professionally supported therapy, Cohen’s d = 0.61, 95% confidence interval (CI) 0.45 to 0.77 vs. unsupported therapy, Cohen’s d = 0.25, 95% CI 0.14 to 0.35]. 9
Existing Health Technology Assessment programme-funded research into computerised cognitive behaviour therapy for depression: the results of the REEACT trial and the need for further research on the effectiveness of supported computerised cognitive behaviour therapy
On the basis of a UK technology appraisal by Kaltenthaler et al. 6 and the identified need for independent (non-developer-led) research into cCBT, the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial was commissioned in 2008, recruited in 2010–12 and reported in 2015. 12 The design of the REEACT trial was to compare the clinical effectiveness and cost-effectiveness of commercially developed cCBT (Beating the Blues; Ultrasis, London, UK) versus free-to-use cCBT (MoodGYM) versus usual GP care. The trial was pragmatic in design and recruited 691 participants in UK primary care. The level of support that was offered to both cCBT packages was designed to replicate current practice in NHS primary care in which professional facilitation is not routinely offered. In view of the findings of systematic reviews, and evidence of lower uptake of packages in entirely self-directed/unsupported therapies, the REEACT trial included technical telephone support. Participants were proactively offered technical support and weekly encouragement to use the computer packages, but we purposely did not augment psychological therapy over the telephone. Telephone support in the REEACT trial did not involve explanations of CBT and did not involve a review of homework or between-session tasks. The cCBT was therefore a form of supported self-help, but was not one that was guided by a clinician. The REEACT trial is, at the time of writing, the largest publicly funded, independently conducted primary care trial of cCBT.
The main finding of the REEACT trial was that for the primary outcome of depression severity at 4 months, there was no significant benefit when participants were offered technically supported cCBT in addition to usual GP care. This negative finding was true for both a free-to-use package (MoodGYM) and commercially produced cCBT (Beating the Blues). The most likely explanatory mechanism of lack of effect was poor uptake and use of computer packages by trial participants. For both cCBT packages (MoodGYM and Beating the Blues) the median number of actual computer therapy sessions that were completed by participants was between 1 and 2. The conclusion of the REEACT trial was that technically supported cCBT was clinically ineffective when added to usual GP care, and that this treatment should not routinely be offered in this form to patients with depression.
Preliminary findings from a concurrent qualitative evaluation in REEACT, and anecdotal evidence from recruiting researchers, indicated that participants were demotivated as a consequence of depression and struggled to engage with computer sessions despite appreciating the offer of technical support. Participants expressed an interest in computer packages, but identified a preference for guidance. We postulated on the basis of these findings and on the basis of emerging trial-based evidence summarised in systematic reviews (e.g. Andersson and Cuijpers9) that cCBT might show an effect but only if offered alongside a greater level of facilitation and support and designed the REEACT-2 study to run alongside, but independently of, REEACT and test this hypothesis. The REEACT-2 trial represents a follow-on trial from the REEACT trial to answer this related question, and most of the fundamental aspects of trial design (primary care setting, recruitment process and inclusion criteria) are replicated in the two trials.
Research objectives
This was a fully randomised patient trial to examine the additional benefits of telephone facilitation alongside a free-to-use computer-delivered CBT package (MoodGYM). The comparator was a minimally supported mode of delivery of the same cCBT package that replicated the mode of delivery of cCBT as offered in primary care in the NHS. The REEACT-2 study included a concurrent economic evaluation to meet the following specific aims:
-
to establish the clinical effectiveness of a telephone-facilitated cCBT package compared with minimally supported cCBT over a 1-year trial follow-up period
-
to establish the cost-effectiveness of a telephone-facilitated cCBT package compared with minimally supported cCBT over a 1-year trial follow-up period.
Chapter 2 Methods
Trial design
This study was as a multisite, pragmatic, open, two-arm, parallel-group randomised controlled trial. Participants were recruited from primary care through direct referral by their GP or a postal invitation from GP records. Participants were individually randomised to one of two arms:
-
minimally supported cCBT
-
telephone-facilitated cCBT.
Participants in both arms were given access to a free-to-use cCBT program (MoodGYM), an accompanying booklet, and a Freephone number for technical support, and continued with usual GP care. Participants in the telephone-facilitated cCBT arm were additionally allocated a telephone support worker (TSW) who provided a programme of weekly telephone calls. The programme is described in more detail in Intervention.
Participants were followed up over the course of 12 months, with data collected at 4 and 12 months post randomisation.
Approval
Ethics approval was granted by Bradford Research Ethics Committee (10/H1302/95) on 20 December 2010 and from relevant research and development committees. The trial was assigned the International Standard Randomised Controlled Trial Number (ISRCTN) ISRCTN55310481.
Trial sites
The study was conducted in four UK sites with well-established networks of practices from which to recruit. These were the Universities of York, Bristol, Sheffield and Manchester.
Participants
The study population included patients in primary care with depression or low mood as determined by a score of ≥ 10 on the Patient Health Questionnaire-9 (PHQ-9). 13 This cut-off point is known to detect clinical depression (major depression) in a UK primary care population with a sensitivity of 91.7% and a specificity of 78.3%.
The participants were recruited from a mix of rural and urban GP practices in and around Bristol, Avon, Somerset, Gloucestershire, Manchester, Sheffield and South Yorkshire, York, Humberside and East Yorkshire, Durham, Tyneside and Northumberland.
Inclusion criteria
Participants who met the following criteria were eligible to enter the study:
-
aged ≥ 18 years
-
not currently in receipt of cCBT or specialist psychological therapy
-
score of ≥ 10 overall (indicating moderate, moderately severe or severe depression) and < 3 for item 9 (measuring suicidal thoughts)14 on the PHQ-9 depression severity measure.
Both incident and prevalent cases were included. In line with the pragmatic nature of this trial reflecting usual GP care, patients were eligible to participate whether or not they were in receipt of antidepressant medication, and those with comorbid physical illness or non-psychotic functional disorders were not excluded.
Exclusion criteria
We excluded potential participants who
-
were actively suicidal as identified by the GP or as reported by item 9 on the PHQ-9
-
had been bereaved within the last year
-
had given birth within the last year
-
had a diagnosis of psychotic depression
-
had a primary diagnosis of alcohol or drug abuse
-
were not able to read and write in English.
Participant recruitment
All recruiting researchers were given training in all aspects of the trial including trial recruitment, informed consent, adverse event reporting procedures, and risk assessment and reporting procedures. Each researcher was also given a study reference manual with full instructions on all the standard procedures. Participating GPs were provided with a GP manual with details of the trial processes, a GP information leaflet, adverse event reporting guidance, information about the interventions and contact details for the trial team.
Direct referral
Practices taking part in this study were provided with patient information packs containing a cover letter, patient information sheet, copy of the consent form and a prepaid envelope addressed to the local researcher, to give to patients with depression who were receptive to participating in the trial. The GP or representative could complete and fax a referral form and patient permission-to-contact form to the study researcher who, following a consideration period of at least 2 days, then approached the patient to discuss the study in more detail and confirm eligibility and continuing interest. The study design and approvals allowed that other health-care professionals attached to practices, such as nurses or primary care mental health workers, could refer patients to the study in the same way, but in the event participants were only referred by GPs.
Participation identification from general practitioner records
General practitioner practices were also asked to conduct a search of their records to identify patients presenting with depression or low mood, and screen for potentially eligible participants. Patient information packs supplied by the research team were sent from the GP practice inviting interested patients to return a completed permission-to-contact form to the research team. A member of the study team then made contact to confirm eligibility and discuss the study in further detail.
Screening for eligibility
After receiving permission-to-contact forms, the researcher contacted the potential participant to discuss the study, answer any questions and then confirm that the patient was still interested and eligible. To assess eligibility the potential participant was asked to confirm that he or she still met the inclusion criteria, in particular those that may not have been known to the GP, such as bereavement and drug and alcohol problems. The researcher also ensured that the participant understood what participation entailed, had access to the internet and wanted to take part in the trial. Participants were then asked whether they preferred the baseline assessment to be conducted over the telephone or at a face-to-face interview.
Consenting participants
Potential participants who preferred to have baseline data collected by telephone were asked to return the signed consent form in the prepaid envelope in the information pack. Those who preferred a face-to-face interview completed the consent process at the first meeting.
All potential participants were given a full explanation of the study and the opportunity to ask any questions or discuss concerns. Researchers emphasised that participants could withdraw consent at any point and would not have to give any explanation nor would their joining the study or leaving it at any point affect their GP care. Participants were also reminded that by consenting they agreed to their GPs being informed of their participation. Written informed consent was then taken, both participant and researcher signing and dating the consent forms and each keeping a copy.
Baseline assessment
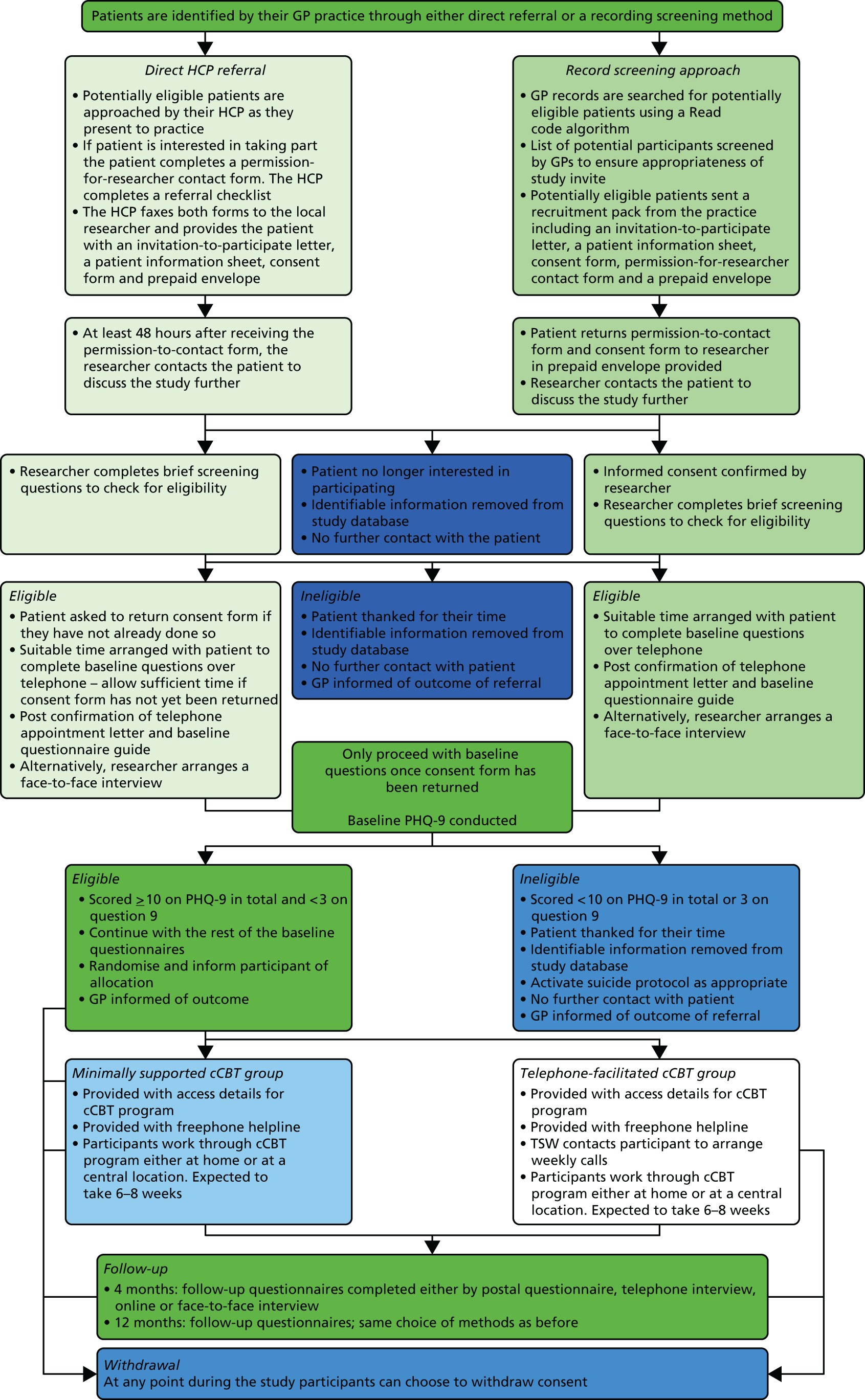
Having consented to join the trial, participants completed a series of baseline questionnaires providing biographical, health status, health state utility and service use data. The participant was then randomly allocated according to the process outlined below (Figure 1).
FIGURE 1.
Flow of recruitment to the trial. HCP, health-care professional.
Randomisation
Participants were randomly allocated in a ratio of 1 : 1 to one of two treatment conditions: (1) minimally supported cCBT or (2) telephone-facilitated cCBT. Both conditions included log-in details for a free-to-use cCBT program (MoodGYM), a booklet accompanying the program, usual GP care, and access to a free helpline for troubleshooting and general help with the program. Simple randomisation was performed using a computer-generated random number sequence. The REEACT-2 trial researchers telephoned a secure randomisation line at the York Trials Unit and were given the participant’s allocation and log-in details for the MoodGYM program. By default, randomisation was done at the face-to-face interview and the participant informed immediately and given a copy of the MoodGYM booklet. If the interview finished after the randomisation line closed, the participant was informed the next day. The researcher then informed the trial manager who allocated TSWs to participants in the supported cCBT arm.
Sample size
The REEACT-2 trial was powered on the basis of an ability to detect a between-group difference in depression severity. We sought to recruit 350 patients with depression – 175 participants per arm. The REEACT-2 trial was designed to have sufficient power to detect a Cohen’s d effect size of 0.30 with 80% power (one-sided 5% significance level) allowing for loss to follow-up of 20%, in line with our empirically based estimates from the REEACT trial. The final sample size for the two arms was 369 participants.
Intervention
In line with the template for intervention description and replication (TIDier)15 guidelines the intervention is described below using the prescribed checklist headings.
Rationale
This trial built on preliminary findings from the REEACT trial (ISRCTN91947481). In the REEACT trial, we found that participants were not always keen to engage with the cCBT programs, and they expressed a desire to receive a greater level of support. Participants were happy to receive this support over the telephone, but felt that this should reflect and emphasise the practical elements of CBT. One way to encourage engagement with self-help technologies is to offer complementary low-intensity support by telephone. 16 The REEACT-2 study was designed to examine if a programme of structured telephone facilitation would enhance engagement with the MoodGYM program and whether or not enhanced engagement would lead to better outcomes. The telephone-facilitation programme was designed to be capable of delivery by appropriately trained and supervised individuals who are not necessarily people with a professional background in mental health. The support programme was designed to be delivered after a brief structured training programme and with reference to a treatment manual.
Materials
MoodGYM is a free-to-use, internet-based, interactive CBT program for depression, developed and copyrighted at the Australian National University Centre for Mental Health Research. The online program is accompanied by a booklet with exercises and quizzes, and consists of five interactive modules released sequentially and lasting approximately 30–45 minutes and a sixth session that is predominantly consolidation and revision. Study participants were asked to aim to complete one session each week. The program provides patients with CBT techniques to overcome patterns of unhelpful thinking using cartoon characters to represent habits of thought. 17
Procedures
Experimental intervention: weekly supportive/facilitative telephone calls plus cCBT (telephone-facilitated cCBT).
Participants in the experimental group received regular (ideally weekly) telephone calls from a trained worker to offer support, guidance and encouragement.
The telephone facilitation programme comprised eight telephone calls to be completed alongside the cCBT program within the 12–14 weeks between the first contact from the TSW and the 4-month follow-up time point. The purpose of the first and longest session (30–40 minutes) was to introduce the participant to the principles of CBT and the MoodGYM program and booklet, explain the process and help the participant identify difficulties and goals, and feel confident about engaging with the intervention.
The following six sessions were between 10 and 20 minutes long and were intended to provide motivation and to help participants identify any barriers to engagement and to the achievement of their goal(s). The final session helped participants to consolidate what they had learned and discussed their next steps and, if appropriate, how they might use the program in the future. The telephone-facilitation programme was delivered according to a manual developed by Professor Karina Lovell in conjunction with the REEACT-2 trial team.
Comparator intervention: minimally supported computerised cognitive behaviour therapy
All patients in the control group were registered as users of MoodGYM and given a unique password. As with the intervention group, they were supplied with a free helpline number to ring if they had technical problems or needed advice, but they did not receive regular telephone calls. This comparator intervention replicates NHS care in most settings and represents what would happen if a patient were given the website of a cCBT package such as MoodGYM by their GP or primary care mental health worker without being offered proactive support.
Providers
The intervention was delivered by TSWs, a team of people specifically recruited to support the REEACT-2 trial and trained in the delivery of the manualised telephone facilitation intervention. The support workers were not recruited to act as psychotherapists to the participant and were not instructed to replicate or consolidate the therapeutic content of the packages, as they were not trained or instructed to act as cognitive behaviour therapists.
The professional background was mixed and included psychology graduates and people who demonstrated good interpersonal skills who had worked as counsellors, social workers, psychiatric nurses and volunteers with mental health charities (e.g. Samaritans). The telephone facilitation programme was delivered according to a manual developed for the REEACT-2 trial. Full training in delivery of the programme, including the management of information that may be troubling or indicate risk, was given to all potential TSWs and their suitability to take on the role was assessed during the training and with recorded mock facilitation sessions with an experienced TSW.
Follow-up training meetings took place during the trial on a roughly bimonthly basis and TSWs had access at all times by telephone to a supervisor with a professional background in mental health (KL, mental health nursing; DK, primary care physician; and SG, psychiatrist). Between training meetings with KL, regular contact was maintained with the trial manager via e-mail and telephone. TSWs were provided with continuing case supervision every 2 weeks from the trial manager who managed the majority of day-to-day queries. Sessions with participants were recorded (with consent) and early sessions were monitored by a senior trial principal investigator for fidelity to the manual.
Mode of delivery
Participants were given a copy of the MoodGYM booklet at the baseline interview and the MoodGYM program was generally delivered via participants’ own internet-connected computer, enabling participants to log on at their convenience. The default mode of delivery of the support programme was via the telephone. Three participants requested an alternative mode of communication (e-mail) because they felt too anxious to use the telephone.
Locations
Participants could log on to the MoodGYM application anywhere with broadband internet access. They were encouraged to make sure that they had a suitable environment for an appropriate length of time. Researchers could help participants to identify and book time at alternative locations with computer access such as public libraries or GP practices if they had no internet access at home or their home was not a suitable location for any reason.
Participants were encouraged to be at home, or somewhere comfortable and private for the telephone appointments. Whatever the location, to maintain privacy, the participant and TSW agreed a code word in the first session that the participant could use to indicate that it was not appropriate to continue with the conversation and that they would reconvene later.
When/how much
The TSWs contacted the participants at pre-arranged times to suit, as far as possible, the participant. This included some weekend and evening calls. The intervention provided eight telephone calls. The first of these was expected to last between 30 and 45 minutes, the following seven calls between 10 and 15 minutes, and the final call 20 minutes.
Tailoring
Telephone support workers worked to a manual but their conversations were not scripted. The telephone support programme could be tailored to some extent because there was some variation in the severity of participants’ depression, their availability and their willingness or ability to complete a module each week.
Adherence
Adherence by participants to the computer program was measured by requesting information from the website providing MoodGYM (hosted by the developers of MoodGYM at the Australian National University, Canberra, ACT, Australia). We obtained computer usage data on the number of times each participant logged on to the MoodGYM program and whether or not each module was 25%, 50%, 75% or 100% complete.
Telephone support workers kept records of the number and length of telephone sessions as well as detailed records of all attempts to contact the participants.
Fidelity
Fidelity to the telephone support manual was monitored in supervision discussion and from the recordings of the sessions. In keeping with the pragmatic nature of the trial there was a certain amount of variation in the delivery between TSWs and between participants. Telephone sessions were not formally scrutinised or analysed as a research activity, but the recordings have been retained for future reference.
Outcomes
Primary outcome measure
The primary outcome was depression severity and symptomatology as measured by a validated self-report measure (the PHQ-9)14 at 4 months because this is the point at which the largest between-group difference might be expected. The PHQ-9 logs the core symptoms of depression and takes the form of a questionnaire comprising nine sections.
Data were collected in one of four ways according to participant preference:
-
by telephone interview with a recruiting researcher
-
completion of a client report form (CRF) sent out by post
-
online
-
at a face-to-face interview with a recruiting researcher.
Secondary outcome measures
The secondary outcome measures comprised the PHQ-9 at 12 months, anxiety [as measured by the Generalised Anxiety Disorder Scale-7 items (GAD-7)];18 somatoform complaints [as measured by the Patient Health Questionnaire-15 (PHQ-15)];19 health state utility [as measured by the European Quality of Life-5 Dimensions (EQ-5D)];20 and service use using the adapted Client Service Receipt Inventory (CSRI)21 and Client Satisfaction Questionnaire-8 items. 22 The secondary outcome measures were recorded at 4 and 12 months. A summary of assessments and data collection time points can be found in Table 1.
| Assessment | Time point | ||
|---|---|---|---|
| Baseline | 4 months | 12 months | |
| Eligibility and consent | |||
| Eligibility | ✗ | ||
| Consent | ✗ | ||
| Background and follow-up | |||
| Personal details | ✗ | ||
| Education | ✗ | ||
| Employment status | ✗ | ✗ | |
| Marital status/living arrangements | ✗ | ||
| Previous episodes of depression | ✗ | ✗ | ✗ |
| Current antidepressant medication use | ✗ | ✗ | ✗ |
| Questionnaires | |||
| PHQ-914 | ✗ | ✗ | ✗ |
| Anxiety (GAD-7)18 | ✗ | ✗ | ✗ |
| Somatoform symptoms (PHQ-15)19 | ✗ | ✗ | ✗ |
| Health state utility (EQ-5D)20 | ✗ | ✗ | ✗ |
| Health economics/service utilisation questionnaire (adapted CSRI)21 | ✗ | ✗ | ✗ |
| Client satisfaction survey (CSQ-8)22 | ✗ | ✗ | ✗ |
| Need for affect23 | ✗ | ||
| Self-efficacy24 | ✗ | ||
Statistical analysis methods
Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) on a two-sided basis and using a 5% significance level.
Primary analysis
All outcomes were summarised descriptively by intervention group and at each time point using mean, median, standard deviation (SD), range and number of patients for continuous outcomes, and number of patients and percentage for discrete outcomes.
Patient Health Questionnaire-9 at 4 months
The primary outcome was the severity of depression as measured by the PHQ-9 at 4 months, a self-reported questionnaire. The primary analysis used the PHQ-9 score as a dichotomous outcome with a score of ≥ 10 meaning depressed and < 10 not depressed; a cut-off point of 10 has been shown to be sensitive for detecting clinical depression in UK primary care. 25 The PHQ-9 is a nine-item questionnaire that records the core symptoms of depression and gives a total score ranging from 0 to 27, with a higher score indicating more severe depression. It is categorised as follows: 0–4, none to minimal depression; 5–9, mild depression; 10–14, moderate depression; 15–19, moderately severe depression; and 20–27, severe depression. The cut-off point of 10 was used to categorise participants as depressed or not depressed at 4 and 12 months (PHQ-9 score of ≥ 10 depressed, PHQ-9 score of < 10 not depressed). To be included in the study, participants had to score of ≥ 10 on the PHQ-9 at baseline. The PHQ-9 score at each time point was calculated as the sum of all nine items. If one item was missing it was replaced with the mean of the other eight, but if two or more items were missing, then the whole questionnaire was treated as missing (using the same scoring method used in the REEACT trial).
The number and percentage of participants who were not depressed/depressed were reported at 4 and 12 months and treatment groups were compared using a chi-squared test. The missing responses were summarised and possible reasons explored by summarising and comparing baseline data between those with and without a missing outcome. The primary analysis compared minimally supported cCBT with telephone-facilitated cCBT using a logistic regression model adjusting for the baseline PHQ-9 score, age, sex, baseline GAD-7 score and treatment. Odds ratios (ORs) with 95% CIs are reported.
The primary analysis was on a complete case basis (only including those with a 4-month assessment). As some missing data were expected, sensitivity analyses were performed using imputation. A simple imputation on a worst-case scenario was used assuming that all participants with a missing outcome were still depressed. Anyone with a missing PHQ-9 score was assumed still to be depressed and have a score of ≥ 10.
Secondary analyses
Patient Health Questionnaire-9 at 12 months
The secondary analysis of the primary outcome was the same as for the primary analysis but used the PHQ-9 score of < 10/≥ 10 (not depressed/depressed) at 12 months.
Patient Health Questionnaire-9 as a continuous outcome
The PHQ-9 score was also summarised and analysed as a continuous outcome. This is summarised for each assessment time point (baseline, 4 and 12 months) using mean, SD, median and range, and the number of missing values. Plots are presented showing the mean and 95% CI at each time point.
Means and SDs at the previous assessment were summarised and compared between those with and without the subsequent PHQ-9 score missing, using t-tests, to evaluate whether or not there were differences in scores between those with and without a missing assessment (i.e. whether or not those who dropped out were more depressed).
A repeated measures mixed regression model was used to analyse the change in PHQ-9 score over time. This included all randomised participants and provides reliable estimates assuming the data are missing at random. The outcome was the PHQ-9 score at 4 and 12 months and the model included the baseline score, treatment, age, sex, baseline GAD-7 score and time. The treatment × time interaction was included to evaluate if the difference between treatments changed over time. Different covariance structures were evaluated (e.g. unstructured, compound symmetry) and the one providing the best fit was used. Residual plots were used to check model assumptions. The mean difference, 95% CI and p-values are presented for all terms in the model. Effect sizes (Cohen’s d) were calculated for the between-group differences in mean PHQ-9 score at 4 and 12 months using the difference between the means and corresponding standard errors from the mixed model. The standard errors were converted to SDs using the corresponding sample size in each treatment group.
Other secondary outcomes
The GAD-7 and PHQ-15 scores were analysed as continuous outcomes using the same repeated measures mixed models, as described for PHQ-9 above.
The number of participants taking any medication to help with their depression was summarised descriptively at each time point. cCBT use was summarised descriptively.
Adverse events
Adverse event data were summarised descriptively.
Economic methods
The primary objective of the economic analysis was to assess the relative cost-effectiveness of telephone-facilitated cCBT compared with minimally supported cCBT. The economic analysis was conducted prospectively alongside the REEACT-2 trial.
Health-related quality of life
Decisions concerning resource allocation often need to be taken across specialties and disease areas. If these decisions are to be informed by a cost-effectiveness analysis, then it is crucial that the outcome measure adopted is generic (i.e. that it has meaning outside the clinical area within which it is used). The use of a single generic measure of health benefit enables diverse health-care interventions to be compared, thus enabling broader questions of efficiency to be addressed.
In this study, the main outcome for the cost-effectiveness analysis was the quality-adjusted life-year (QALY), assessed using the EQ-5D. The EQ-5D questionnaire is a standardised generic instrument for measuring health-related quality of life (HRQoL). 20 The EQ-5D consists of five health dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has three levels of severity (no problems, moderate problems and severe problems) that generate 245 unique health states into which a patient can be classified. The EQ-5D also provides a single preference weight (also described as a utility or value) for each health state. These can be used as quality-adjustment weights to turn a profile of health states over time into QALYs. The EQ-5D has been validated in UK populations and has been used to measure HRQoL in patients with depression in primary care. 26
Resource use and cost data
Information relating to participants’ resource utilisation was obtained via patient self-report using an adapted version of the CSRI. 27 The CSRI was administered at baseline and at each follow-up. Participants were asked about their use of services in the previous 6 months (including inpatient and outpatient hospital services, community-based day services, and primary and community care contacts); and whether or not they had incurred any additional costs associated with their depression (e.g. medication or drug costs, child-care costs, travel costs). Participants were also asked to record their use of any medication to help with their depression, including medication name, dose and duration taken.
Unit costs were obtained from routinely published national literature sources, namely the British National Formulary,28 the Personal Social Services Research Unit’s Unit Costs of Health and Social Care29 and the NHS reference costs. 30 All unit costs were adjusted to 2012/13 prices using the relevant price indices. Appendix 1 provides sources and details of the unit costs.
The costs of delivering each intervention were limited to those associated with delivering telephone support, as MoodGYM is a free-to-use software package. The cost of telephone support calls was estimated based on mean duration and mean number of support calls recorded as part of the study, and assuming the support was provided by a clinical support worker (band 2). It was assumed, after consultation with our clinical advisers, that a clinical support worker or a professional in the same pay band would provide this service in the NHS, if the intervention was to be rolled out.
The unit costs estimates were then combined with the resource utilisation data to obtain a net cost per patient over the entire follow-up period for the trial. As costs were estimated over a 12-month period, no discounting was applied.
Economic analysis: statistical methods
Overview
A within-trial economic analysis was conducted to evaluate the cost-effectiveness of telephone-facilitated cCBT (MoodGYM) and minimally supported cCBT. Costs and health benefits expressed in QALYs were estimated over the 1-year follow-up. The analysis was conducted on an intention-to-treat basis from the perspective of the UK NHS and Personal Social Services. All analyses were undertaken in Stata® 12.0 (StataCorp, College Station, TX, USA).
Resource use and costs
Descriptive statistics (mean, SD, median and interquartile range) are reported for resource use and costs. The descriptive statistics for resource use presented are based on the available case data set as multiple imputation was only performed for total costs as opposed to individual resource use items. Multiple imputation by chained equations31 was performed for a total of 10 imputations, and costs were imputed at every follow-up time point (baseline, 4 and 12 months) for each resource use category. The independent variables specified in the imputation were baseline EQ-5D score, baseline costs, age, sex, anxiety level at baseline, depression level at baseline and depression duration at baseline. The descriptive statistics for resource use and costs are also reported using unadjusted estimates. Differences in mean costs (and 95% CI) between the groups were subsequently adjusted for baseline costs and additional participant covariates using regression analysis (see Cost-effectiveness analysis).
Health-related quality of life
Health-related quality of life was assessed using responses to the EQ-5D questionnaire applied at baseline and at 4 and 12 months. Missing EQ-5D scores were imputed by multiple imputation by chained equations alongside costs at the same follow-up time points (baseline, 4 months and 12 months) and specifying the same independent variables. The EQ-5D scores were used to estimate patient-specific QALYs using the area under the curve method32 and descriptive statistics were reported. Differences in QALYs (and 95% CI) between the groups were adjusted for additional participant covariates using regression analysis (see Cost-effectiveness analysis).
Cost-effectiveness analysis
Incremental estimates of total costs and QALYs were obtained through regression methods, adjusting for the following baseline characteristics: age, anxiety level, baseline depression severity, depression duration and sex. The costs and QALY estimates were also adjusted for baseline costs and baseline EQ-5D scores, respectively.
Incremental QALYs were estimated using ordinary least squares (OLS) regression, as this method has been recommended for the estimation of QALYs in economic evaluation. 33 The regression model applied for the incremental analysis of costs in the base case was a generalised linear model. 34 This type of model was preferred to an OLS model, as cost data tend to be heavily skewed and follow a non-normal distribution, which leads to violations of the OLS assumptions. For the analysis of costs, a gamma family distribution was selected. Selection of the family distribution was based on the modified Park’s test35 performed on each imputed data set and complete case data set. An identity link function was selected, thus assuming an additive effect of covariates on costs.
Cost-effectiveness was assessed by comparing the incremental costs and QALYs using standard decision rules. 36 An intervention that generates greater mean QALYs and lower mean costs can be considered dominant and, therefore, a cost-effective use of resources when compared with the alternative. When no dominance arises (i.e. one intervention is more costly and more effective than the other), the interventions can be compared by calculating the ratio between incremental costs and QALYs to establish the incremental cost-effectiveness ratio (ICER). When the ICER between two interventions is below the cost-effectiveness threshold that represents the rate at which health-care activities in the NHS (assumed to be cost-effective) generate health at the margin, then the more costly and more effective intervention is considered cost-effective. The cost-effectiveness of the interventions was assessed by comparing ICERs against a cost-effectiveness interval ranging from £20,000 to £30,000 per QALY, in line with NICE cost-effectiveness thresholds for the UK. 37
Uncertainty surrounding the decision was assessed using a probabilistic sensitivity analysis and presented through cost-effectiveness acceptability curves that graphically represent the probability of an intervention being cost-effective across a range of cost-effectiveness thresholds. The analysis was performed by simulating random draws of incremental mean costs and QALYs (n = 1000) from a multivariate normal distribution and estimating the proportion of those draws that corresponded to a cost-effective use of resources at cost-effectiveness threshold values ranging from £0 to £60,000 per additional QALY. In order to plot the cost-effectiveness acceptability curve, the variance–covariance matrices from the costs and QALYs regressions were extracted and the corresponding Cholesky decompositions calculated to parameterise multivariate normal distributions. 38 This approach is commonly used to ensure that parameters taken from a regression framework are appropriately correlated and not treated as independent when the probabilistic sensitivity analysis is performed (and cost-effectiveness acceptability curves are plotted), but it has the disadvantage of imposing normality on the sampling distribution.
Base-case and sensitivity analysis
The base-case cost-effectiveness analysis was based on a comparison of all participants receiving minimally supported cCBT (n = 187) with telephone-facilitated cCBT (n = 182), and conducted on the multiple imputed data sets. In the base-case analysis, all categories of health-care costs were included, and QALYs estimated from EQ-5D scores were considered.
A separate sensitivity analysis undertaken, which excluded all non-mental health-related hospital costs so as to assess the robustness of base-case results to alternative assumptions in terms of costs, was also considered.
Patient and public participation
Contributors with experience of depression were involved in the design of the trial and the writing of the protocol, and the chief executive of a user-led organisation was a collaborator and coapplicant. Two people with depression read the consent forms and questionnaires and commented on the experience of completing them. All research documentation was designed with patient and public input and the trial oversight committees included members with experience of mental health problems.
Chapter 3 Protocol changes
Protocol change 1: additional baseline questionnaires
Following the emergence of preliminary findings from the REEACT trial, measures to help identify possible psychosocial mechanisms that affect participants’ capacity or willingness to engage with cCBT were approved and added to the baseline CRF. They were to measure:
-
need for affect, a measure of the need for emotionally stimulating experiences, which may underlie a preference for more emotionally engaging material or interaction23
-
self-efficacy, a measure of the patient’s confidence that they can overcome typical difficulties to completing cCBT, which may underlie differences in motivation and ability to persist with the programme. 24
Protocol change 2: baseline client report form
On 29 June 2011, the Research Ethics Committee approved the addition of questions about participants’ current use of antidepressant medication. Following modification of the CRFs, participants were asked the following additional questions at baseline:
If you are currently taking any medication to help with your depression please give details:
(please include both prescription medicine and any you have bought yourself)
Name of medication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Dose . . . . . . . . . . . . . . . . . . . . . . .
Name of medication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Dose . . . . . . . . . . . . . . . . . . . . . . .
How long have you been taking the medicine(s)? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Protocol change 3: extension to the study date and sample size
In December 2012, the Research Ethics Committee approval was given to increase the trial sample size to 350 (175 participants per arm) to allow the detection of a smaller difference of 0.30 with 80% power at a level of significance of 0.05 (one sided) accounting for loss to follow-up of 20%.
Protocol change 4: primary care depression cohort
In general, studies of depression in primary care can follow the progress of participants for only a short time and little is known about the effectiveness of treatments in the longer term. Research Ethics Committee approval was granted on 14 November 2012 to invite participants to continue in the study for a further 9 years answering questions about their mood and their general health. This extra follow-up period is not funded as part of the REEACT-2 trial and is not reported here.
Chapter 4 Clinical effectiveness results
Randomisation and centre details
Four main UK sites were responsible for recruiting to the trial: the Universities of Manchester, Bristol, Sheffield and York. Table 2 shows the number of participants recruited to each arm by each site.
| Site | Recruited (n) | Trial arm | |
|---|---|---|---|
| Minimally supported cCBT (n) | Telephone-facilitated cCBT (n) | ||
| Bristol | 154 | 70 | 84 |
| York | 124 | 65 | 59 |
| Manchester | 44 | 16 | 28 |
| Sheffield | 47 | 31 | 16 |
| Total | 369 | 182 | 187 |
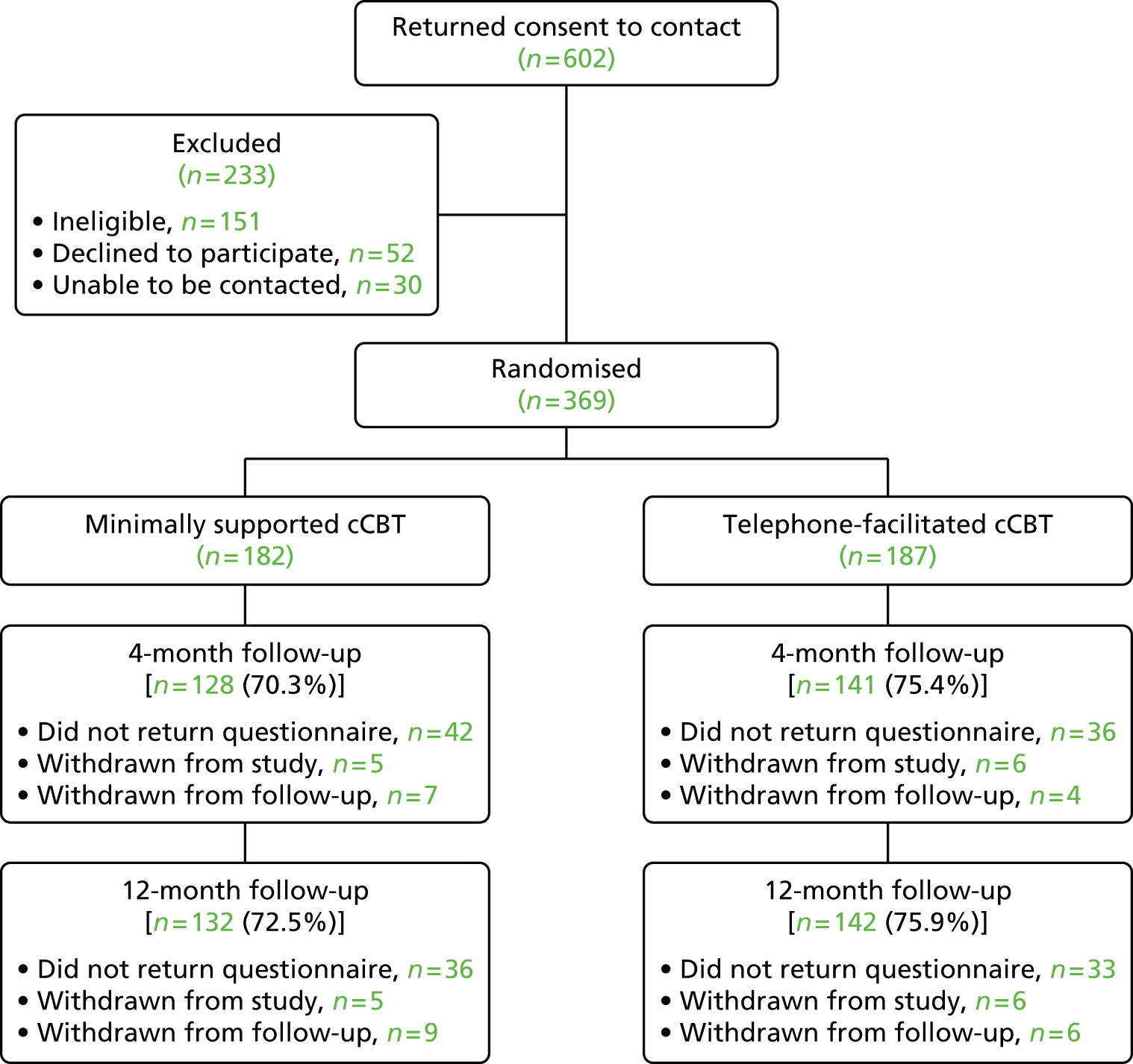
A total of 369 participants were randomised to the two-armed comparison of minimally supported cCBT with telephone-facilitated cCBT (n = 182 and n = 187, respectively). The first participant was randomised on 24 June 2011 and the last on 25 April 2013.
The rate of recruitment is shown in Figure 2. The rate had to be slowed after 3 months of rapid recruitment to accommodate the TSW workload. The flow of participants through the trial can be seen in the Consolidated Standards of Reporting Trials (CONSORT) flow chart (Figure 3).
FIGURE 2.
Cumulative recruitment graph for the REEACT-2 trial.

FIGURE 3.
The CONSORT flow diagram.
Baseline data
Participant characteristics
Baseline demographic characteristics are summarised in Tables 3–7. Continuous variables are presented as mean, SD, median and range. Categorical variables (e.g. sex) are presented as number and percentage. The number analysed and the numbers of any missing values are presented.
| Participant | Trail arm | Total (n = 369) | |
|---|---|---|---|
| Minimally supported cCBT (n = 182) | Telephone-facilitated cCBT (n = 187) | ||
| Sex, n (%) | |||
| Male | 69 (37.9) | 62 (33.2) | 131 (35.5) |
| Female | 113 (62.1) | 125 (66.8) | 238 (64.5) |
| Patient age (years) | |||
| Mean (SD) | 40.3 (13.7) | 41.0 (13.8) | 40.6 (13.8) |
| Median (range) | 40.6 (18.5–74.3) | 40.5 (18.2–77.1) | 40.6 (18.2–77.1) |
| Missing | 0 | 0 | 0 |
| Ethnicity | Trial arm | Total (N = 369), n (%) | |
|---|---|---|---|
| Minimally supported cCBT (N = 182), n (%) | Telephone-facilitated cCBT (N = 187), n (%) | ||
| White British | 173 (95.1) | 174 (93.0) | 347 (94.0) |
| Chinese | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Other | 1 (0.5) | 3 (1.6) | 4 (1.1) |
| White Irish | 2 (1.1) | 0 (0.0) | 2 (0.5) |
| Other white | 2 (1.1) | 3 (1.6) | 5 (1.4) |
| Mixed – white and black Caribbean | 0 (0.0) | 2 (1.1) | 2 (0.5) |
| Mixed – white and black African | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Mixed – white and Asian | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Other mixed | 3 (1.6) | 0 (0.0) | 3 (0.8) |
| Asian or Asian British – Indian | 0 (0.0) | 2 (1.1) | 2 (0.5) |
| Asian or Asian British – Pakistani | 0 (0.0) | 1 (0.5) | 1 (0.3) |
| Highest qualification | Trial arm | Total (N = 369), n (%) | |
|---|---|---|---|
| Minimally supported cCBT (N = 182), n (%) | Telephone-facilitated cCBT (N = 187), n (%) | ||
| Question not answered | 5 | 1 | 6 |
| GCSE/O-level | 28 (15.8) | 31 (16.7) | 59 (16.3) |
| GCE A-/AS-level or Scottish Higher | 23 (13.0) | 28 (15.1) | 51 (14.0) |
| NVQ/SVQ levels 1–3 | 22 (12.4) | 24 (12.9) | 46 (12.7) |
| GNVQ (Advanced) | 1 (0.6) | 6 (3.2) | 7 (1.9) |
| BTEC Certificate | 1 (0.6) | 2 (1.1) | 3 (0.8) |
| BTEC Diploma | 7 (4.0) | 3 (1.6) | 10 (2.8) |
| National Certificate or Diploma (ONC/OND) | 10 (5.6) | 20 (10.8) | 30 (8.3) |
| Qualified Teacher Status | 4 (2.3) | 3 (1.6) | 7 (1.9) |
| Higher Education Diploma | 6 (3.4) | 9 (4.8) | 15 (4.1) |
| Degree (first/ordinary) | 33 (18.6) | 28 (15.1) | 61 (16.8) |
| Post-graduate certificate | 6 (3.4) | 5 (2.7) | 11 (3.0) |
| Post-graduate diploma | 2 (1.1) | 2 (1.1) | 4 (1.1) |
| Master’s degree | 10 (5.6) | 8 (4.3) | 18 (5.0) |
| PhD | 1 (0.6) | 2 (1.1) | 3 (0.8) |
| Do not know/no response | 17 (9.6) | 10 (5.4) | 27 (7.4) |
| Other | 6 (3.4) | 5 (2.7) | 11 (3.0) |
| Employment status | Trial arm | Total (N = 369), n (%) | |
|---|---|---|---|
| Minimally supported cCBT (N = 182), n (%) | Telephone-facilitated cCBT (N = 187), n (%) | ||
| Baseline employment | |||
| Employed part-time | 19 (10.4) | 33 (17.7) | 52 (14.1) |
| Other | 2 (1.1) | 1 (0.5) | 3 (0.8) |
| Employed full-time | 76 (41.8) | 71 (38.2) | 147 (39.9) |
| Self-employed | 11 (6.0) | 14 (7.5) | 25 (6.8) |
| Retired | 11 (6.0) | 13 (7.0) | 24 (6.5) |
| Looking after family or home | 8 (4.4) | 8 (4.3) | 16 (4.3) |
| Not employed but seeking work | 15 (8.2) | 9 (4.8) | 24 (6.5) |
| Not employed but not seeking work because of ill health | 16 (8.8) | 20 (10.8) | 36 (9.8) |
| Not employed but not seeking work for other reasons | 1 (0.5) | 2 (1.1) | 3 (0.8) |
| Full-time student | 23 (12.6) | 15 (8.1) | 38 (10.3) |
| If employed or self-employed, off sick because of depression? | |||
| Yes | 26 (22.2) | 31 (24.8) | 57 (23.6) |
| No | 90 (76.9) | 94 (75.2) | 184 (76.0) |
| Missing | 1 (0.9) | 0 (0.0) | 1 (0.4) |
| If unemployed, for how long? | |||
| < 3 months | 3 (7.7) | 5 (10.9) | 8 (9.4) |
| 4–12 months | 10 (25.6) | 9 (19.6) | 19 (22.4) |
| 1–2 years | 9 (23.1) | 5 (10.9) | 14 (16.5) |
| 2–5 years | 6 (15.4) | 7 (15.2) | 13 (15.3) |
| > 5 years | 7 (17.9) | 10 (21.7) | 17 (20.0) |
| No response | 4 (10.3) | 10 (21.7) | 14 (16.5) |
| Most recent job | |||
| Question not answered | 10 | 5 | 15 |
| Foreman/supervisor | 18 (10.5) | 16 (8.8) | 34 (9.6) |
| Manager | 33 (19.2) | 33 (18.1) | 66 (18.6) |
| Self-employed with employees | 5 (2.9) | 5 (2.7) | 10 (2.8) |
| Self-employed without employees | 8 (4.7) | 15 (8.2) | 23 (6.5) |
| Other employee | 103 (59.9) | 109 (59.9) | 212 (59.9) |
| Never been in paid employment | 5 (2.9) | 4 (2.2) | 9 (2.5) |
| Family details | Trial arm | Total (N = 369), n (%) | |
|---|---|---|---|
| Minimally supported cCBT (N = 182), n (%) | Telephone-facilitated cCBT (N = 187), n (%) | ||
| Baseline marriage/family status | |||
| Married | 68 (37.4) | 68 (36.6) | 136 (37.0) |
| Living with a partner | 21 (11.5) | 36 (19.4) | 57 (15.5) |
| Divorced/separated | 24 (13.2) | 31 (16.7) | 55 (14.9) |
| Widowed | 5 (2.7) | 4 (2.2) | 9 (2.4) |
| Single (never married) | 63 (34.6) | 45 (24.2) | 108 (29.3) |
| Other | 1 (0.5) | 2 (1.1) | 3 (0.8) |
| If married, does your spouse live with you? | |||
| Yes | 69 (88.5) | 71 (88.8) | 140 (88.6) |
| No | 4 (5.1) | 2 (2.5) | 6 (3.8) |
| Missing | 5 (6.4) | 7 (8.8) | 12 (7.6) |
| Do you have other people living with you? | |||
| Question not answered | 1 | 1 | 2 |
| Yes | 120 (66.3) | 116 (62.4) | 236 (64.3) |
| No | 60 (33.1) | 70 (37.6) | 130 (35.4) |
| If yes, how many? | |||
| 1 | 44 (36.4) | 48 (40.0) | 92 (38.2) |
| 2 | 44 (36.4) | 32 (26.7) | 76 (31.5) |
| 3 | 18 (14.9) | 27 (22.5) | 45 (18.7) |
| 4 | 9 (7.4) | 7 (5.8) | 16 (6.6) |
| 5 | 3 (2.5) | 1 (0.8) | 4 (1.7) |
| 6 | 0 (0.0) | 1 (0.8) | 1 (0.4) |
| ≥ 7 | 2 (1.7) | 1 (0.8) | 3 (1.2) |
| Do not know/no response | 1 (0.8) | 3 (2.5) | 4 (1.7) |
| How many are < 18 years? | |||
| 0 | 60 (50.0) | 56 (47.1) | 116 (48.5) |
| 1 | 25 (20.8) | 30 (25.2) | 55 (23.0) |
| 2 | 23 (19.2) | 19 (16.0) | 42 (17.6) |
| 3 | 6 (5.0) | 12 (10.1) | 18 (7.5) |
| 4 | 3 (2.5) | 1 (0.8) | 4 (1.7) |
| ≥ 7 | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Do not know/no response | 2 (1.7) | 1 (0.8) | 3 (1.3) |
The two groups were well balanced at baseline for sex, age, ethnicity and education. The mean age of participants was 40.6 years (SD 13.8 years). The study population was mostly white British (94%) and 64.5% were female.
The minimally supported cCBT and telephone-facilitated cCBT groups were balanced at baseline for employment. The majority (61.5%) of participants were employed, and, of these, 23.6% were off work with depression at the time of their baseline assessment.
The two groups were balanced at baseline regarding marriage and family status.
Baseline clinical data
The two groups had an imbalance at baseline in the proportion of participants describing themselves as chronically depressed (11% minimally supported cCBT vs. 5.9% telephone-facilitated cCBT), but the numbers are very small.
As can be seen in Table 8, the majority of participants (70.7%) had sought help for previous episodes of depression and 84.5% of these had previously been prescribed antidepressant medication (60.4% of the trial sample), and 38.8% of participants reported taking antidepressant medication at baseline. Randomisation resulted in the groups being well balanced for all these variables.
| Episodes of depression | Trial arm | Total (N = 369), n (%) | |
|---|---|---|---|
| Minimally supported cCBT (N = 182), n (%) | Telephone-facilitated cCBT (N = 187), n (%) | ||
| Previous episodes of depression when help was sought? | |||
| Yes | 127 (69.8) | 134 (71.7) | 261 (70.7) |
| No | 53 (29.1) | 52 (27.8) | 105 (28.5) |
| Do not know | 2 (1.1) | 1 (0.5) | 3 (0.8) |
| If yes, how many episodes of treated depression? | |||
| 1 | 36 (28.3) | 46 (34.1) | 82 (31.3) |
| 2 | 25 (19.7) | 36 (26.7) | 61 (23.3) |
| 3 | 20 (15.7) | 9 (6.7) | 29 (11.1) |
| 4 | 12 (9.4) | 7 (5.2) | 19 (7.3) |
| ≥ 5 | 20 (15.7) | 29 (21.5) | 49 (18.7) |
| Chronically depressed | 14 (11.0) | 8 (5.9) | 22 (8.4) |
| If yes, prescribed antidepressants for a previous episode? | |||
| Yes | 109 (85.2) | 114 (83.8) | 223 (84.5) |
| No | 19 (14.8) | 19 (14.0) | 38 (14.4) |
| Do not know | 0 (0.0) | 3 (2.2) | 3 (1.1) |
| Currently taking medication for depression? | |||
| Yes | 71 (39.0) | 72 (38.5) | 143 (38.8) |
| No | 111 (61.0) | 115 (61.5) | 226 (61.2) |
| Seen anyone other than your GP? | 94 (74.0) | 96 (71.1) | 190 (72.5) |
| Psychiatrist | 26 (14.4) | 25 (13.4) | 51 (13.9) |
| Psychologist | 15 (8.3) | 16 (8.6) | 31 (8.5) |
| Counsellor | 65 (35.7) | 66 (35.3) | 131 (35.5) |
| Community psychiatric nurse | 10 (5.6) | 18 (9.7) | 28 (7.7) |
| Social worker | 3 (1.7) | 3 (1.6) | 6 (1.6) |
| Citizens advice bureau | 0 | 0 | 0 |
| Other statutory/voluntary agency | 3 (1.7) | 10 (5.4) | 13 (3.6) |
| Other | 12 (6.7) | 10 (5.4) | 22 (6.0) |
| Do not know | 0 (0.0) | 1 (0.5) | 1 (0.3) |
Primary outcome
Patient Health Questionnaire-9 descriptive summaries
At the 4-month follow-up, a PHQ-9 score was missing for 27% of participants who did not return the 4-month questionnaire, 25% in the telephone-facilitated cCBT group and 30% in the minimally supported cCBT group. These numbers were slightly lower at month 12, 24% in the telephone-facilitated cCBT group and 27% in the minimally supported cCBT group, as a small number of participants completed a 12-month but not a 4-month questionnaire. Scores are summarised in Table 9.
| PHQ-9 | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Month 4 | Month 12 | ||||
| Minimally supported cCBT (n = 182) | Telephone-facilitated cCBT (n = 187) | Minimally supported cCBT (n = 128) | Telephone-facilitated cCBT (n = 141) | Minimally supported cCBT (n = 132) | Telephone-facilitated cCBT (n = 142) | |
| PHQ-9 score | ||||||
| Mean (SD) | 16.4 (4.1) | 16.8 (3.9 | 10.4 (6.4) | 8.5 (6.3) | 9.2 (6.2) | 8.2 (6.4) |
| Median | 16 | 17 | 10 | 7 | 9 | 7 |
| Range | 10–25 | 10–26 | 0–27 | 0–24 | 0–25 | 0–27 |
| PHQ-9 dichotomised | ||||||
| Depressed, n (%) | 182 (100) | 187 (100) | 66 (51.6) | 51 (36.2) | 57 (43.2) | 46 (32.4) |
| Not depressed, n (%) | 0 | 0 | 62 (48.4) | 90 (63.8) | 75 (56.8) | 96 (67.6) |
| Missing, n (%) | 0 | 0 | 54 (30) | 46 (25) | 50 (27) | 45 (24) |
Participants in the telephone-facilitated cCBT group had lower mean PHQ-9 scores at month 4 (indicating a reduction in depression) with a between-group difference of 1.9 at month 4 and 1 at month 12.
Primary analyses: depressed/not depressed (Patient Health Questionnaire-9) at month 4
Primary analysis results are shown in Table 10. After 4 months, 66 (50.30%) of the 128 participants in the minimally supported cCBT group and 51 (36.2%) of the 141 in the telephone-facilitated cCBT group had a PHQ-9 score of ≥ 10.
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT | 2.050 | 1.227 to 3.424 | 0.0061 |
| Male vs. female | 0.957 | 0.559 to 1.638 | 0.8713 |
| Baseline PHQ-9 score | 0.882 | 0.819 to 0.951 | 0.0011 |
| Baseline GAD-7 score | 0.964 | 0.903 to 1.029 | 0.2654 |
| Age (years) | 1.012 | 0.994 to 1.031 | 0.1981 |
As a sensitivity analysis, an alternative scenario was also considered that assumed all participants who did not supply follow-up data remained depressed.
Both primary and sensitivity analyses of depression as a discrete outcome show a significant difference between the groups with respect to the odds of not being depressed at 4 months. For the primary analysis the OR was 2.05 (95% CI 1.23 to 3.24; p = 0.006), indicating that adding telephone support to cCBT doubled the odds of not being depressed. The baseline PHQ-9 score was also a significant predictor, indicating that a higher baseline score was related to a reduced chance of not being depressed. Table 11 shows the results of the sensitivity analysis, which included all participants and assumed that those with missing data were still depressed. The conclusion regarding the addition of telephone support was the same. In this analysis, age and baseline GAD-7 score also had statistically significant relationships with the odds of not being depressed. Older participants were more likely to not be depressed at month 4, whereas those participants with higher baseline depression and anxiety were less likely not to be depressed.
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT | 1.945 | 1.253 to 3.019 | 0.0030 |
| Male vs. female | 0.784 | 0.496 to 1.240 | 0.2977 |
| Baseline PHQ-9 score | 0.925 | 0.869 to 0.984 | 0.0137 |
| Baseline GAD-7 score | 0.938 | 0.887 to 0.991 | 0.0232 |
| Age (years) | 1.020 | 1.004 to 1.037 | 0.0132 |
The missing data were explored using univariate logistic regression models to compare baseline factors between those with and those missing a month-4 PHQ-9 score. There was no relationship between missing assessments and treatment (p = 0.274), baseline PHQ-9 score (p = 0.898), sex (p = 0.272) or taking current medication for depression (p = 0.134). However, age (OR 1.03, 95% CI 1.01 to 1.05; p = 0.002) and baseline GAD-7 score (OR 0.94, 95% CI 0.89 to 0.99; p = 0.023) were both significantly associated with the odds of missing a month-4 assessment. This indicates that older participants were more likely to have a missing assessment, and those with higher anxiety at baseline were less likely to have a missing assessment.
Secondary analyses: depressed/not depressed (Patient Health Questionnaire-9) at month 12
Tables 12 and 13 show the results of modelling the odds of being depressed or not depressed at month 12, with a graphical representation in Figure 4. Again the analysis was carried out with all available data and then with the assumption that participants whose data were missing remained depressed as a sensitivity analysis.
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT | 1.626 | 0.977 to 2.705 | 0.0615 |
| Male vs. female | 1.111 | 0.651 to 1.897 | 0.6996 |
| Baseline PHQ-9 score | 0.898 | 0.833 to 0.967 | 0.0046 |
| Baseline GAD-7 score | 0.965 | 0.904 to 1.031 | 0.2892 |
| Age (years) | 1.004 | 0.986 to 1.023 | 0.6764 |
| Effect | OR | 95% CI | p-value |
|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT | 1.613 | 1.051 to 2.474 | 0.0286 |
| Male vs. female | 0.979 | 0.627 to 1.528 | 0.9249 |
| Baseline PHQ-9 score | 0.930 | 0.875 to 0.987 | 0.0177 |
| Baseline GAD-7 score | 0.941 | 0.891 to 0.994 | 0.0290 |
| Age (years) | 1.018 | 1.002 to 1.034 | 0.0263 |
FIGURE 4.
Mean and 95% CI of PHQ-9 score at each assessment.

These results show that telephone-facilitated cCBT was better than minimally supported cCBT for increasing the chances of no longer being depressed after 12 months, but this was not statistically significant (OR 1.63, 95% CI 0.98 to 2.71; p = 0.06). However, in the sensitivity analysis, assuming participants with a missing PHQ-9 score were still depressed, telephone-facilitated cCBT was significantly better (OR 1.61, 95% CI 1.05 to 2.47; p = 0.03).
The OR was 1.61 (95% CI 1.05 to 2.47; p = 0.028) for the main analysis and 1.63 (95% CI 0.98 to 2.71; p = 0.062) for the sensitivity analysis, indicating that adding telephone support to cCBT increased the odds of no longer being depressed by approximately 60%. The baseline PHQ-9 score was also a significant predictor, indicating that a higher baseline score was related to a reduced chance of not being depressed.
Secondary analyses: repeated measures analysis of Patient Health Questionnaire-9 score
The mixed repeated measures model is reported in Tables 14 and 15. These results show a statistically significant overall effect of treatment (p = 0.025), indicating that over both follow-up assessments the addition of telephone support to cCBT reduced the PHQ-9 score by approximately –1.41 (95% CI –2.63 to –0.17). Over both groups there was also a statistically significant reduction over time (p = 0.025), indicating a general decrease in score over time. The baseline PHQ-9 score was a significant predictor of follow-up PHQ-9 score (p < 0.001) but there was no evidence of any relationship with age, sex or baseline GAD-7 score.
| Effect | Estimate | 95% CI | F-value | p-value |
|---|---|---|---|---|
| Baseline PHQ-9 score | 0.4813 | 0.3025 to 0.6600 | 28.07 | < 0.0001 |
| Baseline GAD-7 score | 0.1301 | –0.02763 to 0.2878 | 2.64 | 0.1056 |
| Age (years) | –0.0183 | –0.0622 to 0.0256 | 0.68 | 0.4119 |
| Sex | 0.4899 | –0.8039 to 1.7836 | 0.75 | 0.4567 |
| Time | Overall effect | 5.08 | 0.0250 | |
| Treatment | Overall effect | 5.05 | 0.0253 | |
| Treatment × time interaction | Overall effect | 1.94 | 0.1645 |
| Effect | Cohen’s d effect size | Estimate | 95% CI | t-value | p-value |
|---|---|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 4) | 0.324 | –1.8923 | –3.2969 to –0.4877 | 2.65 | 0.0085 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 12) | 0.155 | –0.9192 | –2.3341 to 0.4957 | 1.28 | 0.2020 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (over all assessments) | –1.4057 | –2.6336 to –0.1748 | 2.25 | 0.0253 | |
| Month 4 vs. month 12 (over all treatments) | 0.7866 | 0.0995 to 1.4737 | 2.25 | 0.0250 |
The PHQ-9 score for telephone-facilitated cCBT was significantly lower than minimally supported cCBT after 4 months (mean between-group difference –1.89, 95% CI –3.30 to –0.49; Cohen’s d = 0.324; p = 0.009), but there was no evidence of any difference after 12 months.
Generalised Anxiety Disorder Scale-7 items
The GAD-7 questionnaire was measured at baseline and at 4 and 12 months.
Secondary analyses: Generalised Anxiety Disorder Scale-7 items
The GAD-7 questionnaire was administered to assess self-reported anxiety at baseline and at 4 and 12 months. The mean score decreased in both groups during both follow-up periods.
Table 16 and Figure 5 show the mean GAD-7 scores for each group for the period for the trial. The scores in each group decreased over both time periods.
| Time point | Trial arm | Total (n = 369) | |
|---|---|---|---|
| Minimally supported cCBT (n = 182) | Telephone-facilitated cCBT (n = 187) | ||
| Baseline | |||
| Mean (SD) | 14.1 (4.4) | 14.5 (4.4) | 14.3 (4.4) |
| Median (range) | 14.0 (4.0–21.0) | 15.0 (3.0–21.0) | 15.0 (3.0–21.0) |
| Missing | 0 | 0 | 0 |
| Month 4 | |||
| Mean (SD) | 8.6 (5.4) | 7.5 (6.1) | 8.1 (5.8) |
| Median (range) | 8.0 (0.0–21.0) | 6.0 (0.0–21.0) | 7.0 (0.0–21.0) |
| Missing | 65 | 59 | 124 |
| Month 12 | |||
| Mean (SD | 8.2 (5.6) | 7.0 (5.7) | 7.6 (5.7) |
| Median (range) | 7.0 (0.0–21.0) | 5.0 (0.0–21.0) | 6.0 (0.0–21.0) |
| Missing | 59 | 56 | 115 |
FIGURE 5.
Mean and 95% CI of GAD-7 scores at each assessment.

Results are from a mixed model with repeated measures within participants to allow for within-participant correlation. An unstructured covariance matrix was used. The model analysed GAD-7 scores at months 4 and 12, and adjusted for age, sex, baseline PHQ-9 score, baseline GAD-7 score, time (as a categorical variable) and treatment.
Cohen’s d effect size was calculated as an estimate of mean difference/overall SD.
The results in Tables 17 and 18 show a statistically significant overall effect of treatment (p = 0.037), indicating that over the whole follow-up period the addition of telephone support to cCBT reduced the GAD-7 score by approximately –1.18 (95% CI –2.28 to –0.07). However, when comparing the treatments separately at month 4 and month 12, there was no statistically significant difference between them at either assessment. Baseline GAD-7 and PHQ-9 scores were significant predictors of follow-up GAD-7 score.
| Effect | Estimate | 95% CI | F-value | p-value |
|---|---|---|---|---|
| Baseline PHQ-9 score | 0.1992 | 0.0388 to 0.3596 | 5.98 | 0.0151 |
| Baseline GAD-7 score | 0.4338 | 0.2917 to 0.5759 | 36.12 | < 0.0001 |
| Age (years) | –0.0358 | –0.0750 to 0.0034 | 3.24 | 0.0730 |
| Sex | 0.5970 | –0.5812 to 1.7752 | 0.99 | 0.3194 |
| Time | Overall effect | 3.34 | 0.0690 | |
| Treatment | Overall effect | 4.42 | 0.0365 | |
| Treatment × time interaction | Overall effect | 0.02 | 0.8806 |
| Effect | Cohen’s d effect size | Estimate | 95% CI | t-value | p-value |
|---|---|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 4) | 0.236 | –1.2291 | –2.4374 to 0.1425 | 1.85 | 0.0659 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 12) | 0.166 | –1.1269 | –2.3122 to 0.1676 | 1.75 | 0.0819 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (over all assessments) | –1.1780 | –2.2813 to –0.0747 | 2.10 | 0.0365 | |
| Month 4 vs. month 12 (over all treatments) | 0.6203 | –0.0487 to 1.2894 | 1.83 | 0.0690 |
Patient Health Questionnaire-15
The PHQ-15 was administered to assess somatoform symptoms at baseline and at 4 and 12 months, and descriptive statistics are presented in Table 19 and Figure 6.
| Time point | Trial arm | Total (n = 369) | |
|---|---|---|---|
| Minimally supported cCBT (n = 182) | Telephone-facilitated cCBT (n = 187) | ||
| Baseline | |||
| Mean (SD) | 11.5 (4.8) | 11.9 (5.0) | 11.7 (4.9) |
| Median (range) | 11.0 (1.0–28.0) | 11.0 (1.0–28.0) | 11.0 (1.0–28.0) |
| Missing | 0 | 0 | 0 |
| Month 4 | |||
| Mean (SD) | 8.7 (4.7) | 8.8 (5.5) | 8.7 (5.1) |
| Median (range) | 8.0 (0.0–21.4) | 8.0 (0.0–25.0) | 8.0 (0.0–25.0) |
| Missing | 66 | 59 | 125 |
| Month 12 | |||
| Mean (SD) | 9.0 (5.1) | 8.2 (5.0) | 8.6 (5.1) |
| Median (range) | 8.0 (0.0–25.0) | 7.0 (0.0–20.0) | 8.0 (0.0–25.0) |
| Missing | 60 | 57 | 117 |
FIGURE 6.
Mean and 95% CI of PHQ-15 score at each assessment.
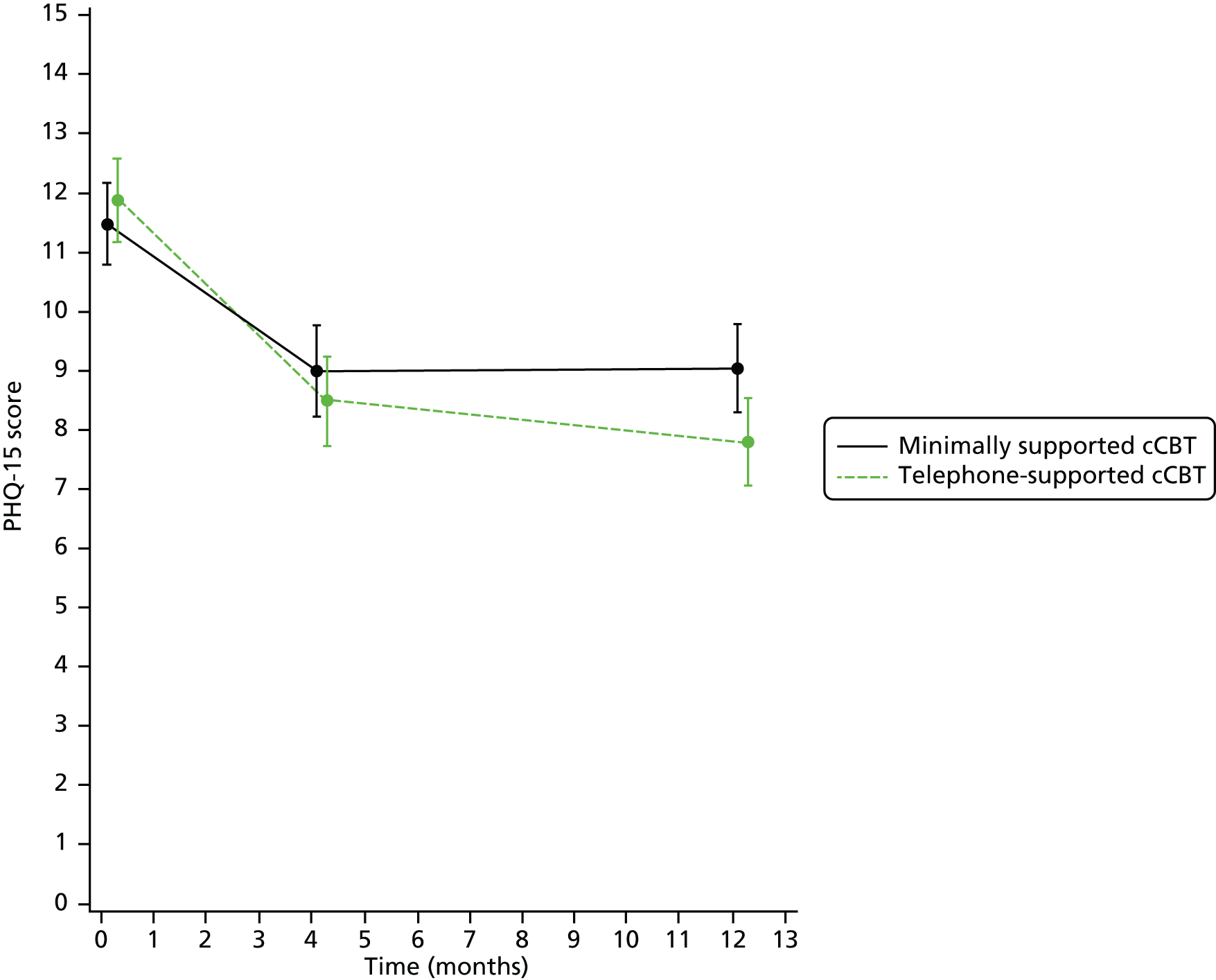
Results are from a mixed model with repeated measures within participants to allow for within-participant correlation. An unstructured covariance matrix was used. The model analysed PHQ-15 scores at months 4 and 12, and adjusted for age, sex, baseline PHQ-15 score, baseline GAD-7 score, time (as a categorical variable) and treatment.
Cohen’s d effect size was calculated as an estimate of mean difference/overall SD.
These results (Tables 20 and 21) show a borderline statistically significant overall effect of treatment (p = 0.051), indicating that over the whole follow-up period the addition of telephone support to cCBT reduced the PHQ-15 score by approximately –1.11 (95% CI –1.75 to 0.002). When comparing the treatments separately at month 4 and month 12, there was no statistically significant difference between them at month 4 but the PHQ-15 score was significantly lower with the addition of telephone facilitation at month 12 (mean difference –1.24, 95% CI –2.27 to –0.21; p = 0.018).
Baseline PHQ-15 score was the only other significant predictor of follow-up PHQ-15 score (see Table 20).
| Effect | Estimate | 95% CI | F-value | p-value |
|---|---|---|---|---|
| Baseline GAD-7 score | 0.0467 | –0.0558 to 0.1491 | 0.81 | 0.3704 |
| Baseline PHQ-15 score | 0.5800 | 0.4819 to 0.6780 | 135.66 | < 0.0001 |
| Age (years) | 0.0062 | –0.0252 to 0.0375 | 0.15 | 0.6994 |
| Sex | –0.5926 | –1.5394 to 0.3542 | 1.52 | 0.2189 |
| Time | Overall effect | 1.24 | 0.2661 | |
| Treatment | Overall effect | 3.85 | 0.0506 | |
| Treatment × time interaction | Overall effect | 1.62 | 0.2050 |
| Effect | Cohen’s d effect size | Estimate | 95% CI | t-value | p-value |
|---|---|---|---|---|---|
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 4) | 0.121 | –0.5088 | –1.5701 to 0.5526 | 0.94 | 0.3460 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (month 12) | 0.300 | –1.2410 | –2.2692 to –0.2127 | 2.38 | 0.0182 |
| Telephone-facilitated cCBT vs. minimally supported cCBT (over all assessments) | –1.1099 | –1.7521 to 0.0024 | 1.96 | 0.0506 | |
| Month 4 vs. month 12 (over all treatments) | 0.3211 | –0.2463 to 0.8884 | 1.11 | 0.2661 |
European Quality of Life-5 Dimensions
The EQ-5D is summarised descriptively at each time point showing the number and percentage of participants with each type of response (Tables 22–26).
| Time point | Trial arm | Total (N = 369), n % | |
|---|---|---|---|
| Minimally supported cCBT (N = 182) n % | Telephone-facilitated cCBT (N = 187) n % | ||
| Baseline | |||
| I have no problems in walking about | 162 (89.5) | 151 (80.7) | 313 (85.1) |
| I have some problems in walking about | 19 (10.5) | 36 (19.3) | 55 (14.9) |
| I am confined to bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 4 | |||
| I have no problems in walking about | 98 (83.8) | 103 (80.5) | 201 (82.0) |
| I have some problems in walking about | 19 (16.2) | 25 (19.5) | 44 (18.0) |
| I am confined to bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 12 | |||
| I have no problems in walking about | 101 (82.1) | 108 (82.4) | 209 (82.3) |
| I have some problems in walking about | 22 (17.9) | 23 (17.6) | 45 (17.7) |
| I am confined to bed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Time point | Trial arm | Total (N = 369), n % | |
|---|---|---|---|
| Minimally supported cCBT (N = 182) n % | Telephone-facilitated cCBT (N = 187) n % | ||
| Baseline | |||
| I have no problems with self-care | 171 (94.5) | 173 (92.5) | 344 (93.5) |
| I have some problems with self-care | 10 (5.5) | 14 (7.5) | 24 (6.5) |
| I am unable to wash or dress myself | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 4 | |||
| I have no problems with self-care | 107 (91.5) | 121 (94.5) | 228 (93.1) |
| I have some problems with self-care | 10 (8.5) | 7 (5.5) | 17 (6.9) |
| I am unable to wash or dress myself | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 12 | |||
| I have no problems with self-care | 116 (94.3) | 123 (93.9) | 239 (94.1) |
| I have some problems with self-care | 7 (5.7) | 8 (6.1) | 15 (5.9) |
| I am unable to wash or dress myself | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Time point | Trial arm | Total (N = 369), n % | |
|---|---|---|---|
| Minimally supported cCBT (N = 182) n % | Telephone-facilitated cCBT (N = 187) n % | ||
| Baseline | |||
| I have no problems with performing my usual activities | 84 (46.4) | 70 (37.4) | 154 (41.8) |
| I have some problems with performing my usual activities | 89 (49.2) | 106 (56.7) | 195 (53.0) |
| I am unable to perform my usual activities | 8 (4.4) | 11 (5.9) | 19 (5.2) |
| Month 4 | |||
| I have no problems with performing my usual activities | 68 (58.1) | 76 (59.4) | 144 (58.8) |
| I have some problems with performing my usual activities | 46 (39.3) | 47 (36.7) | 93 (38.0) |
| I am unable to perform my usual activities | 3 (2.6) | 5 (3.9) | 8 (3.3) |
| Month 12 | |||
| I have no problems with performing my usual activities | 78 (63.4) | 79 (60.3) | 157 (61.8) |
| I have some problems with performing my usual activities | 43 (35.0) | 47 (35.9) | 90 (35.4) |
| I am unable to perform my usual activities | 2 (1.6) | 5 (3.8) | 7 (2.8) |
| Time point | Trial arm | Total (N = 369), n % | |
|---|---|---|---|
| Minimally supported cCBT (N = 182) n % | Telephone-facilitated cCBT (N = 187) n % | ||
| Baseline | |||
| I have no pain or discomfort | 84 (46.4) | 84 (44.9) | 168 (45.7) |
| I have moderate pain or discomfort | 87 (48.1) | 88 (47.1) | 175 (47.6) |
| I have extreme pain or discomfort | 10 (5.5) | 15 (8.0) | 25 (6.8) |
| Month 4 | |||
| I have no pain or discomfort | 56 (47.9) | 62 (48.4) | 118 (48.2) |
| I have moderate pain or discomfort | 57 (48.7) | 60 (46.9) | 117 (47.8) |
| I have extreme pain or discomfort | 4 (3.4) | 6 (4.7) | 10 (4.1) |
| Month 12 | |||
| I have no pain or discomfort | 58 (47.2) | 67 (51.1) | 125 (49.2) |
| I have moderate pain or discomfort | 60 (48.8) | 56 (42.7) | 116 (45.7) |
| I have extreme pain or discomfort | 5 (4.1) | 8 (6.1) | 13 (5.1) |
| Time point | Trial arm | Total (N = 369), n % | |
|---|---|---|---|
| Minimally supported cCBT (N = 182) n % | Telephone-facilitated cCBT (N = 187) n % | ||
| Baseline | |||
| I am not anxious or depressed | 17 (9.4) | 9 (4.8) | 26 (7.1) |
| I am moderately anxious or depressed | 116 (64.1) | 131 (70.1) | 247 (67.1) |
| I am extremely anxious or depressed | 48 (26.5) | 47 (25.1) | 95 (25.8) |
| Month 4 | |||
| I am not anxious or depressed | 27 (22.9) | 38 (29.7) | 65 (26.4) |
| I am moderately anxious or depressed | 78 (66.1) | 74 (57.8) | 152 (61.8) |
| I am extremely anxious or depressed | 13 (11.0) | 16 (12.5) | 29 (11.8) |
| Month 12 | |||
| I am not anxious or depressed | 35 (28.5) | 57 (43.5) | 92 (36.2) |
| I am moderately anxious or depressed | 72 (58.5) | 61 (46.6) | 133 (52.4) |
| I am extremely anxious or depressed | 16 (13.0) | 13 (9.9) | 29 (11.4) |
Computerised cognitive behaviour therapy usage
MoodGYM modules were reported as 25%, 50%, 75% or 100% complete. The percentage of participants in each intervention group who completed all or part of each module is shown in Table 27. The telephone-facilitation programme has increased completion rates for each module (Figure 7).
| Proportion of module completed | Module 1 (%) | Module 2 (%) | Module 3 (%) | Module 4 (%) | Module 5 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimally supported cCBT | Telephone-facilitated cCBT | Minimally supported cCBT | Telephone-facilitated cCBT | Minimally supported cCBT | Telephone-facilitated cCBT | Minimally supported cCBT | Telephone-facilitated cCBT | Minimally supported cCBT | Telephone-facilitated cCBT | |
| Logged on but did not complete 25% | 7.1 | 5.4 | 6 | 8.1 | 1.1 | 3.2 | 1.6 | 1.1 | ||
| 25% complete | 1.6 | 2.2 | 2.2 | 3.8 | 2.7 | 2.7 | 0.5 | |||
| 50% complete | 1.6 | 2.2 | 3.8 | 3.8 | 0.5 | 4.3 | 0.5 | 1.6 | ||
| 75% complete | 2.2 | 4.8 | 0.5 | 3.8 | 0.5 | 1.1 | 0.5 | |||
| 100% complete | 45.1 | 64.5 | 29.1 | 46.2 | 17.6 | 28.5 | 14.3 | 21.5 | 10.4 | 19.4 |
FIGURE 7.
MoodGYM usage session by session.
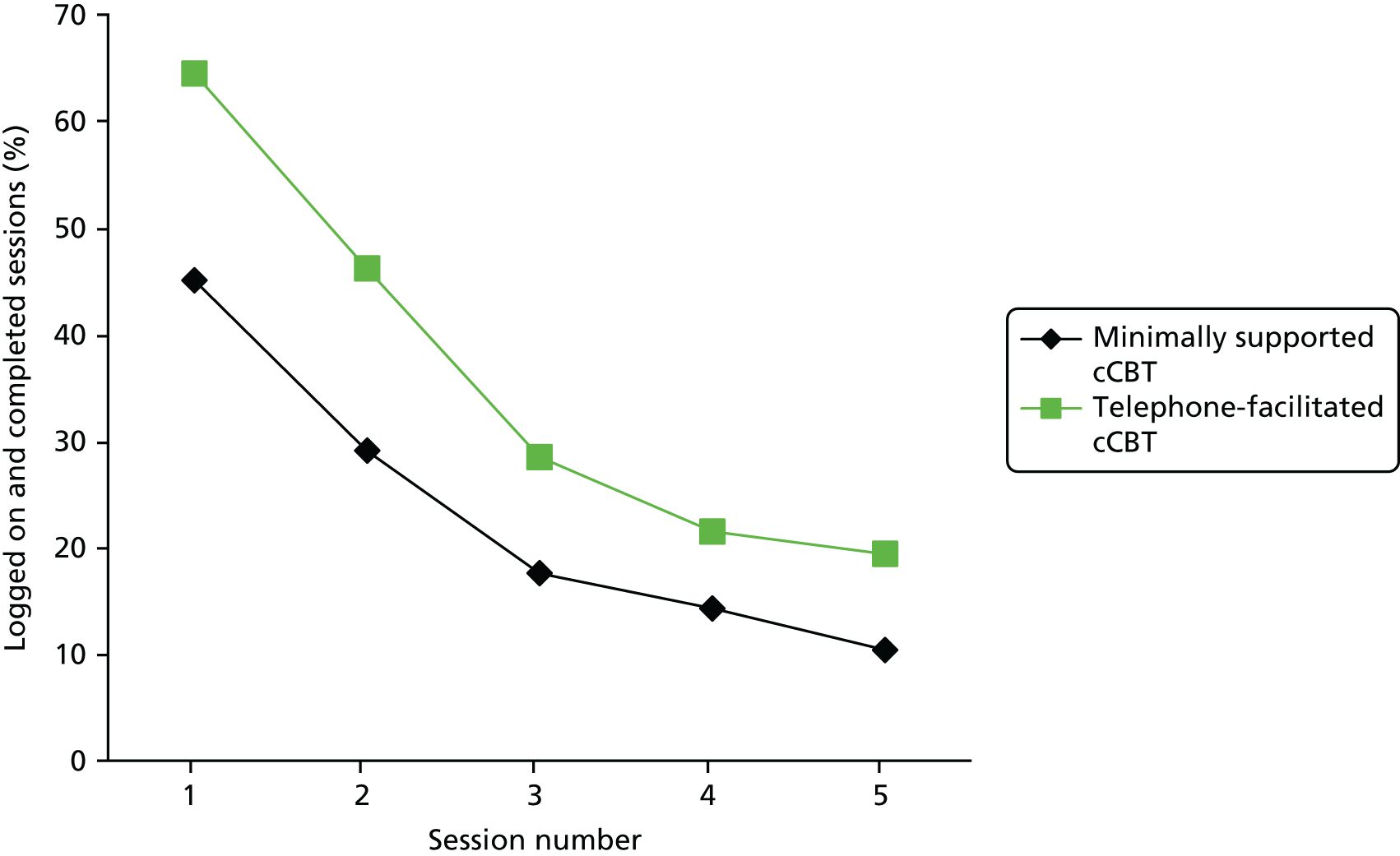
Medication to help with depression use
Participants were asked whether or not they were taking medication to help with depression. This included over-the-counter and prescribed medication. Their responses are reported in Table 28.
| Time point | Trial arm | Total, n (%) | |
|---|---|---|---|
| Minimally supported cCBT, n (%) | Telephone-facilitated cCBT, n (%) | ||
| Baseline | N = 107 | N = 111 | N = 218 |
| Yes | 71 (66.0) | 74 (66.7) | 145 (66.5) |
| No | 36 (33.6) | 37 (33.3) | 73 (33.5) |
| Month 4 | N = 128 | N = 141 | N = 269 |
| Yes | 84 (65.6) | 85 (60.3) | 169 (62.8) |
| No | 44 (34.4) | 56 (39.7) | 100 (37.2) |
| Month 12 | N = 132 | N = 142 | N = 274 |
| Yes | 70 (53.0) | 68 (47.9) | 138 (50.4) |
| No | 62 (47.0) | 74 (52.1) | 136 (49.6) |
Early participants in the trial were not asked about current medication; data collection started at participant 1151, so numbers at baseline are smaller. The proportions of participants currently taking medication for depression were similar between the two groups at baseline.
Adverse events
There were a total of 10 serious adverse events (Table 29), none of which was thought to be related to the trial. All adverse events were reviewed by the Trial Steering Committee and the Data Monitoring and Ethics Committee.
| Serious adverse events | Trial arm | Total | |
|---|---|---|---|
| Minimally supported cCBT | Telephone-facilitated cCBT | ||
| Number of events | 4 | 6 | 10 |
| Number unrelated to the trial | 4 | 6 | 10 |
| Reason for designation | |||
| Hospitalisation | 1 | 2 | 3 |
| Life-threatening | 2 | 1 | 3 |
| Death | 0 | 1 | 1 |
| Resulting in disability or incapacity | 1 | 2 | 3 |
Chapter 5 Economic evaluation results
Health-care resource use
Table 30 reports the descriptive statistics of health-care resource use by resource category during the trial follow-up.
| Health care | Unit | Trial arm | |||||
|---|---|---|---|---|---|---|---|
| Minimally supported cCBT (n = 98) | Telephone-facilitated cCBT (n = 111) | ||||||
| Mean (SD) | Median (IQR) | Use (%) | Mean (SD) | Median (IQR) | Use (%) | ||
| Community care | |||||||
| GP | Visit | 7.39 (6.43) | 5.33 (10) | 98.98 | 6.67 (4.8) | 5.33 (10) | 92.2 |
| GP home visit | Visit | 0.01 (0.67) | 0 (0) | 1.02 | 0.02 (0.15) | 0 (0) | 2.7 |
| Nurse | Visit | 1.02 (1.52) | 0 (1.33) | 48.98 | 1.44 (2.58) | 0.67 (2) | 52.25 |
| Other primary care | Visit | 0.39 (1.9) | 0 (0) | 8.16 | 2.08 (19.77) | 0 (0) | 5.41 |
| All day based services | Visit | 0.41 (2.83) | 0 (0) | 6.12 | 0.17 (1.00) | 0 (0) | 3.6 |
| Counsellor | Visit | 1.34 (3.56) | 0 (0) | 23.47 | 1.16 (3.27) | 0 (0.67) | 26.13 |
| Psychiatric nurse | Visit | 0.19 (0.90) | 0 (0) | 7.14 | 0.50 (3.39) | 0 (0) | 6.31 |
| Hospital services | |||||||
| Inpatient | |||||||
| Mental health relateda | Bed-days | 0 (0) | 0 (0) | 0.00 | 0 (0) | 0 (0) | 0.00 |
| Non-mental health relatedb | Bed-days | 0.24 (1.44) | 0 (0) | 6.12 | 0.52 (2.15) | 0 (0) | 12.61 |
| Outpatient | |||||||
| Psychiatrist | Visit | 0.43 (2.36) | 0 (0) | 7.14 | 0.03 (0.26) | 0 (0) | 1.8 |
| Clinical psychology | Visit | 0.43 (2.36) | 0 (0) | 7.14 | 0.03 (0.26) | 0 (0) | 1.8 |
| Non-mental health relatedc | Visit | 1.95 (3.67) | 0.33 (2) | 50 | 1.59 (4.07) | 0 (1.33) | 44.14 |
Full resource-use data were available for all participants at baseline, and this decreased during follow-up to 67% at 4 months and 69% at 12 months, with complete data across the trial period available for 58.1% of participants from minimally supported cCBT (n = 98) and 59.4% from telephone-facilitated cCBT (n = 111).
General practitioners were visited by nearly all participants throughout the trial (95.08%) but were, on average, more frequently attended by those in the minimally supported cCBT group (mean 7.39 visits) than those in the telephone-facilitated cCBT group (mean 6.67 visits). Hospital inpatient services (both mental health and non-mental health related) were used infrequently across each trial arm for both mental health- and non-mental health-related services. Outpatient hospital attendance for non-mental health-related illnesses was common across both minimally supported cCBT (50%) and telephone-facilitated cCBT (44%). Inpatient mental health-related hospital attendances were low across both groups. Results should be interpreted cautiously as these are unadjusted estimates from the available case data set.
Costs
Table 31 reports the mean costs from baseline to 12 months by treatment group. Mean total costs for the 12-month follow-up were £1172 for minimally supported cCBT versus £1763 for telephone-facilitated cCBT. Total costs appeared broadly similar across both trial arms for mental health-related hospital costs, GP costs, primary care costs and medication costs. However, large disparities were seen in non-mental health-related hospital costs when average costs for telephone-facilitated cCBT (£1050.53) were high with large standard errors compared with the average minimally supported cCBT cost (£561.50). These hospital costs appear to be driven by single participants who had higher costs for ongoing treatments not related to depression. The resultant total costs for these individuals could be considered as outliers. The adjusted mean differences and corresponding 95% CIs obtained through regression analysis are reported in Cost-effectiveness analysis.
| Cost category | Trial arm | |
|---|---|---|
| Minimally supported cCBT (n = 182), mean (SE) (£) | Telephone-facilitated cCBT (n = 187), mean (SE) (£) | |
| NHS costs | ||
| Hospital costs (mental health) | 109.08 (39.59) | 115.21 (39.39) |
| Hospital costs (non-mental health) | 561.50 (153.88) | 1050.53 (383.1) |
| GP costs | 381.58 (40.93) | 390.62 (44.00) |
| Other primary care | 111.43 (25.18) | 158.08 (39.33) |
| Medication costs | 8.52 (0.79) | 7.85 (0.93) |
| Intervention costs | ||
| Telephone support | – | 41.19 |
| Baseline costs | 508.71 (51.59) | 796.49 (131.26) |
| Total costs | 1172.12 (186.53) | 1763.48 (438.77) |
Health-related quality of life
Table 32 summarises the EQ-5D scores at baseline and at each follow-up period, and total QALYs over the 12-month follow-up. On average, patients in the telephone-facilitated cCBT group reported a slightly lower quality of life than the minimally supported cCBT group at baseline and 4 months, but better quality of life at 12 months.
| Time point/outcome | Trial arm | |
|---|---|---|
| Minimally supported cCBT (n = 182) | Telephone-facilitated cCBT (n = 187) | |
| Baseline | 0.620 (0.020) | 0.590 (0.021) |
| 4-month follow-up | 0.720 (0.021) | 0.699 (0.024) |
| 12-month follow-up | 0.710 (0.020) | 0.716 (0.023) |
| QALYs | 0.700 (0.016) | 0.686 (0.019) |
Mean QALYs estimated through the EQ-5D over 12 months were 0.70 for minimally supported cCBT and 0.686 for telephone-facilitated cCBT. As with the individual EQ-5D scores, the overall QALY difference appears small based on the unadjusted means.
Cost-effectiveness analysis
Base-case cost-effectiveness results
Estimates of incremental mean costs and QALYs based on the regression analysis above were used in the cost-effectiveness analysis. Table 33 presents the fully incremental cost-effectiveness estimates and probability that each intervention is cost-effective at a threshold of £20,000 and £30,000 per QALY. Telephone-facilitated cCBT appears less costly (mean cost difference £3.42) and more effective (mean QALY difference 0.0026) than minimally supported cCBT. At a cost-effectiveness threshold of £20,000 and £30,000 per QALY, the probability that telephone-facilitated cCBT is a cost-effective intervention is 0.55.
| Treatment | Incremental costsa,b | Incremental QALYsa,c | ICER | Probability cost-effective £20,000 per QALY | Probability cost-effective £30,000 per QALY |
|---|---|---|---|---|---|
| Minimally supported cCBT | 0 | 0 | Dominated | 0.45 | 0.46 |
| Telephone-facilitated cCBT | –£3.42 | 0.0026 | 0.55 | 0.55 |
Figure 8 presents the cost-effectiveness acceptability curves for each treatment. The curve indicates the probability that each intervention is the most cost-effective for a range of maximum amounts that the NHS may be willing to pay to gain an additional QALY. The curve illustrates that the probability that telephone-facilitated cCBT is cost-effective initially increases as the amount the NHS is assumed to be willing to pay for additional health gain rises, becoming fairly stable for higher cost-effectiveness thresholds (approximately ≥ £15,000 per QALY).
FIGURE 8.
Cost-effectiveness acceptability curves: base case.
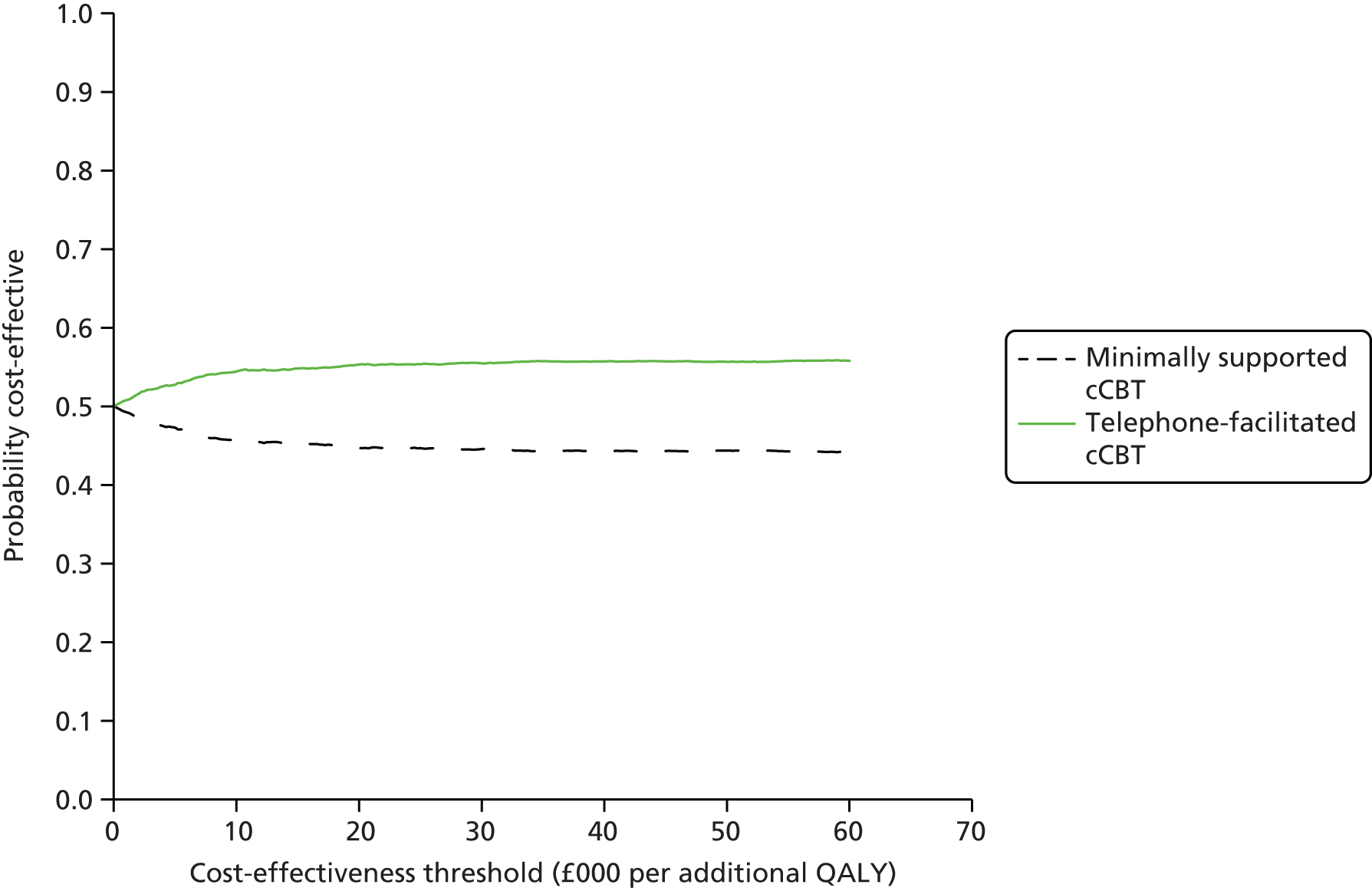
As a sensitivity analysis, an alternative costing scenario was also considered, which included the same cost categories as the base-case analysis with the exception of all non-mental health-related hospital costs. The cost-effectiveness results are reported in Table 34 and Figure 9. In contrast to previous analyses, minimally supported cCBT is no longer dominated by telephone-facilitated cCBT. The ICER for the comparison of telephone-facilitated cCBT versus minimally supported cCBT is £3596 per QALY. Since this is below the lower bound of the currently recommended NICE cost-effectiveness threshold (£20,000 per QALY), minimally supported cCBT remains the cost-effective intervention in this comparison. At a cost-effectiveness threshold of £20,000 per QALY, the probability that telephone-facilitated cCBT is the cost-effective intervention is 0.55. As for the base-case, the probability of cost-effectiveness across thresholds becomes stable at > £15,000 per QALY, with probability of cost-effectiveness for either intervention remaining equal at £20,000 and £30,000 per QALY.
| Treatment | Incremental costsa,b,c | Incremental QALYsa,d | ICER | Probability cost-effective £20,000 per QALY | Probability cost-effective £30,000 per QALY |
|---|---|---|---|---|---|
| Minimally supported cCBT | 0 | 0 | – | 0.45 | 0.45 |
| Telephone-facilitated cCBT | £9.37 | 0.0026043 | £3596.62 | 0.55 | 0.55 |
FIGURE 9.
Cost-effectiveness acceptability curves: sensitivity analysis excluding non-mental health-related hospital costs.
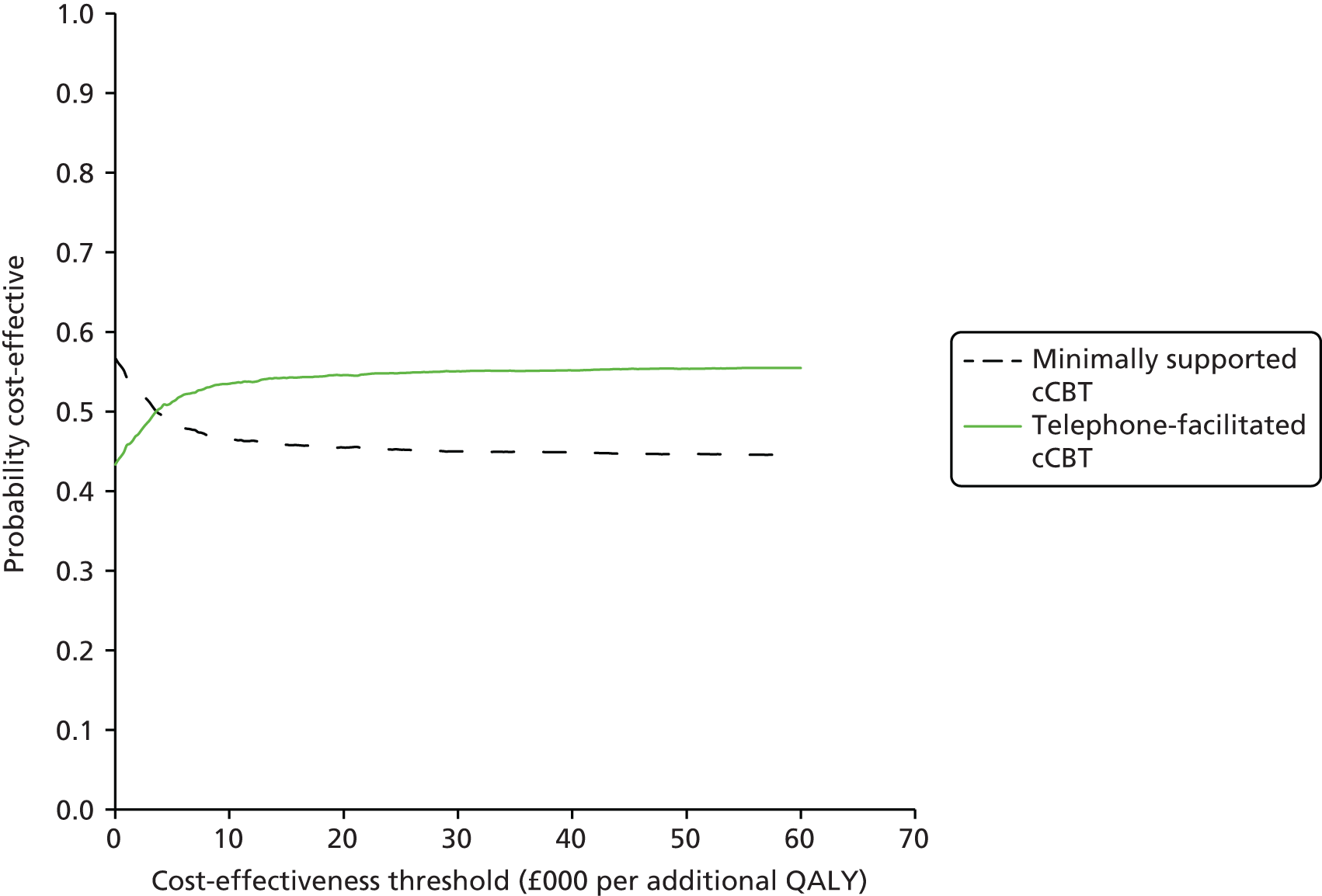
Summary
The within-trial results of the economic analysis suggest that telephone-facilitated cCBT appeared cost-effective compared with the minimally supported cCBT. In the base case, telephone-facilitated cCBT dominated minimally supported cCBT (i.e. lower mean costs and higher QALYs). Although no longer dominant, telephone-facilitated cCBT remained a cost-effective intervention in the sensitivity analyses (i.e. was well within conventional thresholds used to determine value for money in the NHS of £20,000–30,000 per QALY). However, the differences between the groups were relatively minor for both cost and QALY estimates in each of the separate analyses. Although telephone-facilitated cCBT consistently appeared the cost-effective treatment in the base-case and sensitivity analyses, the results from the probabilistic sensitivity analysis revealed high levels of uncertainty.
Chapter 6 Discussion
The REEACT-2 trial is, to our knowledge, the first head-to-head comparison of minimally supported versus telephone-facilitated cCBT in UK primary care. It answers an important question relating to the level of support required for cCBT in clinical practice in order to ensure uptake of the technology and its clinical effectiveness. The REEACT-2 trial results build on an earlier Health Technology Assessment programme-funded trial (REEACT) in which it was found that cCBT was not effective in the form that it was delivered in routine NHS care (i.e. with minimal professional support). 12 The REEACT-2 trial specifically tests the hypothesis that increasing the level of professional support offered alongside cCBT leads to a greater level of engagement with the computer technology and in turn leads to improved outcomes. The need for the REEACT-2 trial was highlighted by the negative results of the REEACT trial and the emergence of indirect evidence from systematic reviews which showed that meta-analyses of professionally supported cCBT demonstrated larger effect sizes than minimally supported cCBT. 9
Prior to the REEACT and REEACT-2 trials there had been no large-scale pragmatic trials of cCBT products in UK primary care and the REEACT trials were commissioned following an earlier technology appraisal in this area that identified the need for trials conducted independently of product developers. 6 The cCBT technology evaluated in the REEACT-2 trial (MoodGYM) was recommended in depression guidelines issued by NICE at the time of design of the REEACT-2 trial, and MoodGYM remains a NICE-endorsed treatment at the time of publication of this trial. 39
The REEACT-2 trial was unusual in comparison with earlier trial-based evaluations in that it included an extended follow-up to 12 months. Outcomes were measured across a broad range of domains, including psychological well-being and quality-of-life/health state utility. Important aspects of service utilisation were also recorded, and the trial included a concurrent economic evaluation.
The main findings of the REEACT-2 study will now be discussed in relation to (1) trial-based estimates of the clinical effectiveness of telephone facilitation of cCBT and (2) trial-based estimates of cost-effectiveness.
Trial-based estimates of the clinical effectiveness of telephone-facilitated computer-delivered cognitive behaviour therapy
The REEACT-2 trial found that when telephone facilitation was added to cCBT there were statistically significant benefits in the primary outcome of depression symptomatology and severity across the follow-up period, as measured by a commonly used tool for the identification of depression (PHQ-9). The benefit was most evident at 4 months and the magnitude of benefit was 1.9 points on the PHQ-9 scale, which equated to a small to moderate clinical effect size (Cohen’s d = 0.32). 40 By 12 months the between-group difference was attenuated and was no longer statistically significant. The odds of no longer being depressed (defined as a PHQ-9 score of < 10) at 4 months were increased twofold in the facilitated cCBT group compared with minimally supported cCBT group (OR 2.05, 95% CI 1.23 to 3.42).
Turning to the range of secondary outcomes that were collected in the REEACT-2 trial, there was evidence of statistically significant effects on the overall (including all time points) mean depression scores and anxiety scores (as measured by the GAD-7; between-group difference 1.1, 95% CI 0.1 to 2.3; p = 0.037). For somatoform complaints there was some evidence of a benefit, but the difference was not statistically significant (PHQ-15 between-group difference 1.1, 95% CI 0.0 to 1.8; p = 0.051).
When engagement with cCBT was monitored in the trial with reference to computer records, it was found that there was enhanced uptake and use of programmes. Telephone facilitation, therefore, had the anticipated effect of increasing engagement with computer-based technology. Nevertheless, very few participants (only 19%) completed all five treatment sessions. As was found in the REEACT trial, the use of computer sessions was quite low in the minimally supported treatment arm (with only 45% completing the first session) than in the telephone-facilitated group (65%).
In summary, the main finding is therefore that, for the primary outcome of depression severity and symptomatology, there was a clinically and statistically significant additional benefit across a range of psychological outcomes when participants were offered a telephone-facilitated form of computerised therapy in addition to usual GP care. This benefit was also seen in a range of secondary outcomes. Telephone facilitation resulted in additional clinical improvements when compared with minimally supported cCBT. The comparator arm represented an intervention that replicated cCBT as it is currently offered in routine NHS services, and the additional technology of telephone facilitation was therefore effective in improving clinical outcomes.
Summary of trial-based estimates of cost-effectiveness
The within-trial results of the economic analysis suggest that telephone facilitation resulted in increased quality of life (QALYs) and reduced health-care costs (i.e. it was dominant). In a more conservative sensitivity analysis, the scenario changed and telephone-facilitated cCBT was no longer dominant. However, the additional benefit in terms of QALYs was incurred at an acceptable ratio to costs. In the cost-effectiveness analysis, an ICER of £6933 per additional QALY for telephone facilitation was observed. The addition of telephone facilitation was likely to be cost-effective at a £20,000 per QALY threshold.
Although the cost-effectiveness conclusions appear sensitive to the choice costs, the magnitude of the differences between the two groups was relatively minor for both cost and QALY estimates in both the scenarios. Hence, minor differences in the assumptions can lead to different cost-effectiveness interpretations of either dominance or cost-effectiveness, and some caution should be exercised when interpreting these results. However, under no scenario was telephone facilitation cost-ineffective (> £30,000 per QALY) or dominated by minimally supported cCBT.
Discussion of main findings
The clinical results of the REEACT-2 trial are consistent with some increase in benefit that has been observed in systematic reviews of computer-mediated cCBT. 9 The addition of telephone facilitation based on a manualised support programme enhanced the effectiveness of cCBT. Minimally supported cCBT (such as is used in the NHS at present) was shown in the REEACT trial to be no more effective than usual GP care. In the REEACT-2 trial, a more intensive telephone facilitation was added to cCBT, and this resulted in statistically significant clinical benefit over and above usual GP care. This finding is important for those who deliver or commission psychological services in primary care. cCBT is a commonly advocated first-line low-intensity treatment option in UK primary care and the offer of this treatment without the provision of telephone facilitation is, on average, unlikely to be of benefit to patients. By adding a level of telephone facilitation that is structured and reinforces the content of CBT treatment sessions, engagement with the programme and clinical outcomes are, on average, improved. The magnitude of this benefit was small to moderate, and was broadly in line with other low-intensity psychological interventions for depression that are delivered in primary care. A systematic review and meta-analysis by Cuijpers et al. 41 has demonstrated that the magnitude of effect of primary care-based psychological interventions is, on average, small to moderate (pooled effect size, Cohen’s d = 0.31, 95% CI 0.17 to 0.45) and the results of the REEACT-2 trial are broadly in line with this body of research. 41 In comparison with other estimates of effect size obtained from developer-led trials, the magnitude of effect observed in REEACT-2 is smaller. It should be noted that REEACT-2 is a pragmatic trial, which recruited in primary care rather than specialist cCBT services and was conducted independently of product developers. We would therefore expect that the effect size would be smaller and more representative of the benefits expected under conditions of routine care.
To date there have been only limited cost-effectiveness data relating to psychological therapies generally and low-intensity therapies specifically. 39 In relation to cCBT there are very few economic evaluations, and those that do exist (e.g. Kaltenthaler et al. 6 and Proudfoot et al. 7) have been conducted alongside developer-led trials framed in specialist services and with larger clinical effect sizes than were observed in the REEACT and REEACT-2 trials. The REEACT and REEACT-2 trials represent the largest economic evaluations of cCBT to date, and directly examine the cost-effectiveness of cCBT from the perspective of the UK NHS. The results of the REEACT-2 trial-based economic evaluation indicate that telephone-supported cCBT is either dominant (cost saving and more effective) or cost-effective within an acceptable threshold of willingness to pay. These economic data will be of interest to decision-makers and those charged with the commissioning of services.
An important feature of the REEACT-2 trial is that we evaluated a free-to-use cCBT package that can be accessed by UK NHS patients at no direct cost. There are a number of cCBT products and packages that could be used in the NHS and that could have been trialled within the REEACT-2 trial. The rationale for choosing MoodGYM was threefold. First, there was evidence of effect from developer-led trials,8 suggesting that MoodGYM had the potential to be effective in NHS services. Second, we have shown in the REEACT trial that MoodGYM is not inferior to commercially developed cCBT products. 12 Third, the technology can be accessed at no direct cost to patients in the UK NHS and it had received cautious support in earlier technology appraisals. 6 The results of the REEACT-2 trial are therefore of relevance to decision-makers as investment or commissioning of the use of cCBT will not require purchase of commercially developed products.
There were limitations to the REEACT-2 trial. The first limitation is that we did not obtain a standardised diagnosis of depression at the point of entry to the trial. Instead, we chose a more pragmatic method that judged the presence of symptomatic depression in a more pragmatic way, according to scores on a depression severity scale (the PHQ-9). We also noted that coexisting anxiety and somatoform complaints were common. Although this might be a more heterogeneous population than that seen in efficacy studies, it could be argued that the participants in the REEACT-2 trial were more representative of people with common mental disorders seen in primary care. The level of severity of depression observed with a PHQ-9 cut-off point of ≥ 10 is in line with moderate depression and previous research has shown that this cut-off point is sensitive and specific in identifying people with clinically significant depression. The second limitation is that the level of use of cCBT was quite low and was lower than that observed in developer-led trials. It might be argued that higher levels of uptake and use might have produced larger and more clinically significant effect sizes. However, we have noted that higher levels of uptake have generally been observed in developer-led trials or studies for which there was intensive one-to-one input from a psychological therapist with the therapist present at the time of delivery of the cCBT intervention. 7 We would argue that the REEACT-2 trial represented an evaluation of a feasible model of cCBT and low-intensity support that might more readily be delivered at scale within NHS primary care psychological therapy services. The third limitation is the greater than planned level of loss to follow-up that was observed in the REEACT-2 trial. We observed that 27% of participants were lost to follow-up at 4 months and 26% of participants were lost to follow-up at 12 months. There was some evidence of differential attrition, with levels of attrition between 3% and 5% higher in the minimally supported cCBT group. The levels of loss to follow-up were, however, broadly in line with other primary care-based studies of psychological interventions (e.g. King et al. 42).
Conclusion
Computerised CBT forms a core component of stepped psychological care in the UK primary care and other health systems. We have previously found in the REEACT trial that minimally supported cCBT is clinically ineffective and cost-ineffective and confers no additional benefit over usual GP care for depression. In the REEACT-2 trial, a level of telephone-delivered facilitation was added to cCBT and modest but statistically significant improvements were found in the primary outcome of the presence of depression and other psychological symptoms. Telephone-facilitated cCBT was also cost-effective.
Implications for health care
-
In this trial for primary care patients with moderate depression, telephone-facilitated cCBT was clinically effective compared with minimally supported cCBT. Current models of care might usefully be re-examined in the light of these findings with due consideration of the level of support that should be offered alongside cCBT.
-
Minimally supported cCBT (which is routinely offered in the NHS in many services) is ineffective and our research suggests that outcomes may improve only when there is sufficient staff in place to support this technology with guidance and facilitation by telephone. This can be offered by telephone according to structured delivery manuals, and allows support to be offered at low intensity and higher volume.
-
Telephone-facilitated cCBT is likely to be cost saving or cost-effective to the NHS.
Recommendations for research
-
The uptake and use of cCBT was not as high as expected. More research is needed to understand the reasons for lower uptake, and more development is needed for cCBT products to further evolve such that they are more acceptable to people with depression. This requires further research and innovation at the human–computer interface.
-
People with depression commonly have coexisting anxiety and somatoform complaints. Although some benefits were observed in these symptoms, the cCBT materials did not specifically address these problems. Further research and development is needed to ensure that cCBT products are able to address coexisting common mental disorders within a single-treatment programme.
-
cCBT is a form of self-help. It would be useful to know how cCBT compares to other forms of guided self-help because computer-delivered therapy is not acceptable to a significant portion of patients. Large-scale pragmatic trials of treatments such as bibliotherapy or telephone-based psychological interventions are therefore needed.
-
There is a need to examine comparative effectiveness and cost-effectiveness of facilitated cCBT and traditional face-to-face therapy in head-to-head trials.
-
All effectiveness studies should be framed in primary care and conducted by researchers other than product developers.
-
Studies should include measures of absenteeism/presenteeism and be powered to examine explanatory variables.
Acknowledgements
We would especially like to thank the patients from primary care who agreed to be recruited to take part in this trial. Thanks also to members of the Primary Care Research Network, GPs, research nurses, administrative and other staff at participating GP practices, the Mental Health Research Network and the site research teams. In addition, we would like to thank the Trial Steering Committee and Data Monitoring and Ethics Committee members for overseeing the study.
We also thank Gwen Brierley, who was the trial manager at the start of the trial, and who cowrote the trial protocol and Research Ethics Committee applications; Debbie Tallon, Sarah Knowles, Anna Thake and David White who were the trial co-ordinators at the sites; the many researchers, clinical studies officers and research nurses who recruited participants to the study and collected data; the York Trials Unit for providing the randomisation service, for managing the data and conducting the analysis of the clinical data; and the team of TSWs.
We would also like to extend our gratitude to the developers of MoodGYM, in particular to Kylie Bennett and Ada Tam, for providing us with participant log-ins and usage data.
Contributions of authors
Sally Brabyn (Research Fellow) was the trial manager.
Ricardo Araya (Professor of Global Mental Health), Michael Barkham (Professor of Clinical Psychology), Peter Bower (Professor of Health Services Research), Cindy Cooper (Professor of Health Services Research and Clinical Trials), David Kessler (Senior lecturer in Primary Care and General Practitioner), Karina Lovell (Professor of Mental Health), Stephen Palmer (Professor of Health Economics), David Richards (Professor of Mental Health Services) and Simon Gilbody (Professor of Psychological Medicine and Health Services Research) were applicants and contributed to the original protocol and study design.
Ana Duarte (Research Fellow), Richard Mattock (PhD Student) and Simon Walker (Research Fellow) conducted the economic analysis.
Sarah Knowles (Research Fellow), Debbie Tallon (Trial Manager) and David White (Study Co-ordinator) were site trial co-ordinators, and collected and managed data at their sites.
Sally Brabyn and Karina Lovell trained and managed the TSWs.
The report writing team consisted of Sally Brabyn, Ana Duarte, Elizabeth Littlewood (Research Fellow), Richard Mattock, Stephen Palmer, Jodi Pervin (Trial Support Officer), Simon Walker, Gillian Worthy (Statistician) and Simon Gilbody.
Stephen Palmer oversaw the design and conduct of the economic analysis.
Gillian Worthy designed and conducted the clinical analysis.
Simon Gilbody was the chief investigator and chaired the Trial Management Group.
Contributions of collaborators
Helen Lester, Nicola Lidbetter, Mark Sculpher and David Torgerson were coapplicants and contributed to the study design. David Torgerson, Helen Lester and Nicola Lidbetter were members of the Trial Management Group.
Trial Steering Committee members
Professor Joe Reilly (Independent Chairperson), Professor of Mental Health, Durham University.
Professor Mike Slade, Professor of Health Services Research, Head of Section for Recovery, Health Service and Population Research Department, Institute of Psychiatry, King’s College London.
Professor Shirley Reynolds, Professor of Evidence-Based Psychological Therapies, University of Reading.
Professor Robbie Foy, Professor of Primary Care, Leeds Institute of Health Sciences, University of Leeds.
Dr Lina Gega, Reader in Mental Health, Northumbria University.
Mr Nick Seccombe (service user representative), informatics and governance lead, Self Help Services, Manchester. Anxiety UK, cCBT self-help services.
Data Monitoring and Ethics Committee members
Professor Mike Lucock (Independent Chairperson), Professor of Clinical Psychology, University of Huddersfield, and Associate Director of Research, South West Yorkshire Partnership NHS.
Professor Chris Roberts, Professor in Biostatistics, Centre for Biostatistics, University of Manchester.
Dr Paul Blenkiron, Consultant Psychiatrist and Honorary Senior Clinical Lecturer, Hull-York Medical School.
Publication
Gilbody S, Brabyn S, Lovell K, Kessler D, Devlin T, Smith L, et al. REEACT-2: a large scale pragmatic trial of telephone-supported computerised cognitive behaviour therapy. Br J Psychiatry 2016; in press.
Data sharing statement
Available data can be obtained from the data management group through the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- The World Health Report (2001): Mental Health: New Understanding, New Hope. Geneva: World Health Organization; 2001.
- Layard R. The case for psychological treatment centres. BMJ 2006;332:1030-2. http://dx.doi.org/10.1136/bmj.332.7548.1030.
- Roth A, Fonagy P. What Works for Whom?: A Critical Review of Psychotherapy Research. New York, NY: Guildford Press; 1996.
- A Review of Strategic Policy on NHS Psychotherapy Services in England. London: The Stationery Office; 1996.
- Lovell K, Richards D. Multiple Access Points and Levels of Entry (MAPLE): ensuring choice, accessibility and equity for CBT services. Behav Cogn Psychother 2000;28:379-91.
- Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10330.
- Proudfoot J, Goldberg D, Mann A, Everitt B, Marks I, Gray JA. Computerized, interactive, multimedia cognitive–behavioural program for anxiety and depression in general practice. Psychol Med 2003;33:217-27. http://dx.doi.org/10.1017/S0033291702007225.
- Christensen H, Griffiths KM, Jorm AF. Delivering interventions for depression by using the internet: randomised controlled trial. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.37945.566632.EE.
- Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther 2009;38:196-205. http://dx.doi.org/10.1080/16506070903318960.
- Spek V, Nyklícek I, Smits N, Cuijpers P, Riper H, Keyzer J, et al. Internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years old: a randomized controlled clinical trial. Psychol Med 2007;37:1797-806. http://dx.doi.org/10.1017/S0033291707000542.
- Waller R, Gilbody S. Barriers to the uptake of computerized cognitive behavioural therapy: a systematic review of the quantitative and qualitative evidence. Psychol Med 2009;39:705-12. http://dx.doi.org/10.1017/S0033291708004224.
- Littlewood E, Duarte A, Hewitt C, Knowles S, Palmer S, Walker S, et al. A randomised controlled trial of computerised cognitive behaviour therapy for the treatment of depression in primary care: the randomised evaluation of the effectiveness and acceptability of computerised therapy (REEACT) trial. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta191010.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann 2002;32:509-21. http://dx.doi.org/10.3928/0048-5713-20020901-06.
- Hoffman TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1687.
- Khan N, Bower P, Rogers A. Guided self-help in primary care mental health. Br J Psychiatry 2007;191:206-11. http://dx.doi.org/10.1192/bjp.bp.106.032011.
- Christensen H, Griffiths KM, Korten A. Web-based cognitive behavior therapy: analysis of site usage and changes in depression and anxiety scores. J Med Internet Res 2002;4. http://dx.doi.org/10.2196/jmir.4.1.e3.
- Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66. http://dx.doi.org/10.1097/00006842-200203000-00008.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Knapp M, Beecham J. Costing mental health services. Psychol Med 1990;20:893-908. http://dx.doi.org/10.1017/S003329170003659X.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. http://dx.doi.org/10.1016/0149-7189(79)90094-6.
- Maio GR, Esses VM. The need for affect: individual differences in the motivation to approach or avoid emotions. J Pers 2001;69:583-614. http://dx.doi.org/10.1111/1467-6494.694156.
- Bandura A, Pajares F, Urdan T. Self-Efficacy Beliefs of Adolescents. Greenwich, CT: Information Age Publishing; 2006.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE–OM. Br J Gen Pract 2007;57:650-2.
- Mann R, Gilbody S, Richards D. Putting the ‘Q’ in depression QALYs: a comparison of utility measurement using EQ-5D and SF-6D health related quality of life measures. Soc Psychiat Epidemiol 2009;44:569-78. http://dx.doi.org/10.1007/s00127-008-0463-5.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Netten A, Dennett J. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- UK National Health System . NHS Reference Costs 2012–13. National Schedules of Reference Costs: NHS Own Costs 2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed August 2014).
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care 1989;5:559-75. http://dx.doi.org/10.1017/S0266462300008461.
- Pullenayegum EM, Tarride J-E, Xie F, Goeree R, Gerstein HC, O’Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: are Tobit and CLAD models appropriate?. Value Health 2010;13:487-94. http://dx.doi.org/10.1111/j.1524-4733.2010.00695.x.
- Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. http://dx.doi.org/10.1258/1355819042250249.
- Manning WG, Mullahy J. Estimating log models: to transform or not to transform?. J Health Econ 2001;20:461-94. http://dx.doi.org/10.1016/S0167-6296(01)00086-8.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Depression in Adults: The Treatment and Management of Depression in Adults. Update – NICE Clinical Guideline 90. Manchester: National Institute for Health and Care Excellence; 2009.
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. New York, NY: Academic Press; 1988.
- Cuijpers P, van Straten A, van Schaik A, Andersson G. Psychological treatment of depression in primary care: a meta-analysis. Br J Gen Pract 2009;59:e51-60. http://dx.doi.org/10.3399/bjgp09X395139.
- King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al. Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care. Health Technol Assess 2000;4.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- UK National Health System . NHS Reference Costs 2011–12. National Schedules of Reference Costs: NHS Own Costs 2012 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/127115/NSRC01-2011-12.xls (accessed August 2014).
Appendix 1 Sources and details of unit costs
| Health-care costs | Source | Unit | Cost (£) | Extra information |
|---|---|---|---|---|
| Community care | ||||
| GP | ||||
| At surgery | PSSRU 201343 | Per hour | 230.00 | |
| At surgery | PSSRU 201343 | Per consultation | 44.85 | Average appointment = 11.7 minutes |
| At home | PSSRU 201343 | Per hour | 292.00 | |
| At home | PSSRU 201343 | Per consultation | 113.88 | Average appointment = 23.4 minutes |
| Telephone consultation | PSSRU 201343 | Per hour | 230.00 | |
| Telephone consultation | PSSRU 201343 | Per consultation | 27.22 | Average appointment = 7.2 minutes |
| Nurse | ||||
| At GP surgery | PSSRU 201343 | Per hour | 40.00 | |
| At GP surgery | PSSRU 201343 | Per consultation | 10.33 | Average appointment = 15.5 minutes |
| District nurse | PSSRU 201343 | Per hour | 48.00 | |
| District nurse | PSSRU 201343 | Per consultation | 12.40 | Average appointment = 15.5 minutes |
| Psychiatric nurse | PSSRU 201343 | Per hour | 49.00 | |
| Psychiatric nurse | PSSRU 201343 | Per consultation | 12.66 | Average appointment = 15.5 minutes |
| Other | ||||
| Counsellor | PSSRU 201343 | Per hour | 48.00 | |
| Counsellor | PSSRU 201343 | Per consultation | 44.00 | Average appointment = 55 minutes |
| Social worker | PSSRU 201343 | Per hour | 57.00 | |
| Social worker | PSSRU 201343 | Per consultation | 52.50 | Average appointment = 55 minutes |
| Occupational therapist | PSSRU 201343 | Per hour | 34.00 | |
| Occupational therapist | PSSRU 201343 | Per consultation | 17.00 | Average appointment = 30 minutes |
| Home care worker | PSSRU 201343 | Per hour | 20.00 | |
| Home care worker | PSSRU 201343 | Per consultation | 10.00 | Average appointment = 30 minutes |
| Community day care costs | ||||
| Day care centre | PSSRU 201343 | Per session | 38.00 | Assumes 1 hour per session |
| Sheltered workshop | PSSRU 201044 | Per session | 8.40 | Assumes 1 hour per session, prices uprated from 2010 |
| Medication | ||||
| Antidepressants | BNF45 | Per item | 4.48 | Assumes one prescription per month |
| Anxiolytics and hypnotics | BNF45 | Per item | 4.28 | Assumes one prescription per month |
| Propranolol | BNF45 | Per item | 9.15 | Assumes one prescription per month |
| Pregabalin | BNF45 | Per item | 64.20 | Assumes one prescription per month |
| Antipsychotics | BNF45 | Per item | 13.25 | Assumes one prescription per month |
| Hospital inpatient | ||||
| Mental health | ||||
| Acute psychiatric ward | PSSRU 201044 | Per bed-day | 333.00 | Costs uprated from 2010 |
| Psychiatric rehabilitation ward | NHS reference costs 201046 | Per bed-day | 334.01 | Activity weighted average of HRGs related to rehabilitation for psychiatric disorders. Costs uprated from 2010 |
| Psychiatric ICU | PSSRU 201044 | Per bed-day | 650.10 | Costs uprated from 2010 |
| Non-mental health | ||||
| Long-stay ward | NHS reference costs 201330 | Per bed-day | 915.92 | Activity weighted average of all HRGs for all elective/non-elective procedures |
| General medical ward | NHS reference costs 201330 | Per bed-day | 915.92 | Activity weighted average of all HRGs for all elective/non-elective procedures |
| Hospital outpatient | ||||
| Mental health | ||||
| Psychiatrist | NHS reference costs 201330 | Per admission | 221.00 | Activity weighted average of psychiatric outpatient visits |
| Clinical psychologist | NHS reference costs 201330 | Per admission | 191.00 | Activity weighted average of psychology outpatient visits |
| Non-mental health | ||||
| A&E visit | NHS reference costs 201330 | Per admission | 117.00 | Activity weighted average of all outpatient A&E visits |
| Visit (excluding A&E) | NHS reference costs 201330 | Per admission | 108.00 | Activity weighted average of all outpatient HRGs, excluding A&E |
| Day hospital | NHS reference costs 201330 | Per admission | 108.00 | Activity weighted average of all outpatient HRGs |
Appendix 2 Patient information leaflet
Appendix 3 Regression coefficients used for cost-effectiveness analysis
| Base_Total_Costs | Coefficient | Standard error | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| Telephone-facilitated cCBT | –24.5895 | 159.8417 | –0.15 | 0.878 | –338 to 288.8209 |
| age | 0.337438 | 5.123713 | 0.07 | 0.947 | –9.71153 to 10.3864 |
| gender | –110.986 | 216.7175 | –0.51 | 0.611 | –548.532 to 326.5595 |
| anxiety_bl | 1.205872 | 18.9265 | 0.06 | 0.949 | –36.0859 to 38.49764 |
| PHQ-9_S0_M0 | 63.33961 | 22.46457 | 2.82 | 0.005 | 19.21393 to 107.4653 |
| Costs_Bl | 1.623163 | 0.406401 | 3.99 | 0 | 0.817154 to 2.429172 |
| Prev_Dep_None | 110.5375 | 189.6738 | 0.58 | 0.56 | –262.502 to 483.5772 |
| Prev_Dep_Chron | 121.0653 | 240.7702 | 0.5 | 0.615 | –351.261 to 593.3917 |
| _cons | –498.323 | 413.9716 | –1.2 | 0.229 | –1310.73 to 314.0801 |
| QALY | Coefficient | Standard error | t-value | p-value | 95% CI |
|---|---|---|---|---|---|
| Telephone-facilitated cCBT | 0.003742 | 0.018268 | 0.2 | 0.838 | –0.03226 to 0.039741 |
| age | –0.00097 | 0.00064 | –1.51 | 0.133 | –0.00224 to 0.000302 |
| gender | 0.029349 | 0.020611 | 1.42 | 0.16 | –0.01198 to 0.070674 |
| anxiety_bl | –0.00286 | 0.002206 | –1.29 | 0.197 | –0.0072 to 0.001489 |
| PHQ-9_S0_M0 | –0.00376 | 0.002506 | –1.5 | 0.135 | –0.00871 to 0.001187 |
| eq5d0 | 0.488292 | 0.050897 | 9.59 | 0 | 0.385847 to 0.590738 |
| Prev_Dep_None | 0.016942 | 0.01959 | 0.86 | 0.389 | –0.02192 to 0.055807 |
| Prev_Dep_Chron | –0.08336 | 0.023284 | –3.58 | 0 | –0.12925 to –0.03747 |
| _cons | 0.531626 | 0.067896 | 7.83 | 0 | 0.397444 to 0.665807 |
List of abbreviations
- CBT
- cognitive behaviour therapy
- cCBT
- computerised cognitive behaviour therapy
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- client report form
- CSRI
- Client Service Receipt Inventory
- EQ-5D
- European Quality of Life-5 Dimensions
- GAD-7
- Generalised Anxiety Disorder Scale-7 items
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- NICE
- National Institute for Health and Care Excellence
- OLS
- ordinary least squares
- OR
- odds ratio
- PHQ-9
- Patient Health Questionnaire-9
- PHQ-15
- Patient Health Questionnaire-15
- QALY
- quality-adjusted life-year
- REEACT
- Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy
- SD
- standard deviation
- TSW
- telephone support worker