Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/01/10. The contractual start date was in January 2013. The draft report began editorial review in February 2016 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jennifer Campbell, Rachael Williams and Robin May are employees of Clinical Practice Research Datalink who received payment from the University of Sheffield during the conduct of the study and funding from multiple organisations outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Julious et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Asthma episodes and deaths are known to be seasonal. 1 A number of reports have shown peaks in asthma episodes in school-aged children associated with the return to school following the summer vacation. 2–10 These studies mainly report hospital admissions, although one study has reported peaks both in hospital admissions and in other medical contacts. 10
Children returning to school are exposed to a variety of novel respiratory insults, including allergens and viruses, at a time of changing climactic conditions. It has previously been shown that viral infection and allergen exposure in allergen-sensitised asthmatics are associated with an increased risk of hospital admission for acute asthma. The same study demonstrated the protective effect of inhaled corticosteroids on acute asthma exacerbations in a paediatric asthma population. 11
In previous research by members of our team, a random sample of approximately 75,000 school-aged (5–16 years) children from England, Wales and Scotland, with a medical diagnosis of asthma, were obtained from general practices within the General Practice Research Database [now the Clinical Practice Research Datalink (CPRD)12] to investigate the seasonal effect of asthma in a primary care setting. Age- (within 2 years) and sex-matched control patients (i.e. no asthma diagnosis) from the same practices were also sampled for comparison. 13
This investigation confirmed the increase in unscheduled medical contacts in children with asthma throughout the year, and a regression analysis showed that children with asthma were approximately twice as likely as control children to have an unscheduled medical contact with their doctor around the time of return to school in September. If children with asthma were at a constant increased risk of medical contacts throughout the year, Figure 1 would show a random scatter of the residuals in England. 13 However, around the time of return to school there is a pronounced positive increase in the value of residuals. A similar pattern was observed in Scotland but with an earlier peak, which can be attributed to school term starting 2 weeks earlier in Scotland than in England. These analyses indicate that, at this time, there is a greater than expected increase in the number of unscheduled contacts by children with asthma compared with control children.
FIGURE 1.
Mean residuals for excess medical contacts for children with asthma for over control children in England. The vertical lines represent, from left to right, 1 September, 1 January and 1 April.
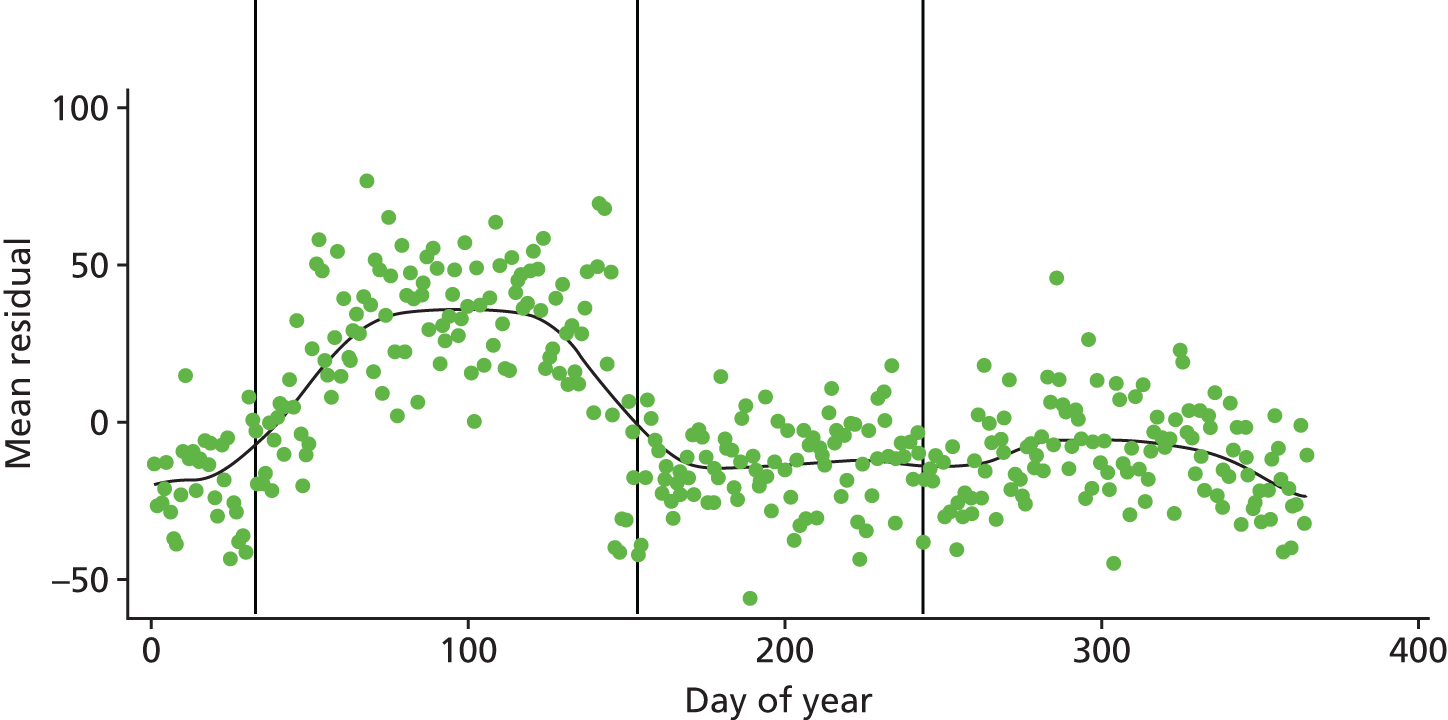
We suggest that July and August are periods of reduced viral exposure (owing to reduced contact with other children because of the holidays) and reduced pollen (antigen) exposure for children with asthma.
The reduced exposures could be an explanation for the observed drop in prescriptions for inhaled steroids we found in August immediately preceding the return to school, with 25% fewer prescriptions in August than in July or September (Figure 2, taken from Julious et al. 13) This drop in prescriptions precedes the viral challenge of a return to school. We further showed that patients who received a prescription for inhaled corticosteroids had, on average, 0.14 fewer contacts than those who did not receive an August prescription [England: 95% confidence interval (CI) 0.12 to 0.16 fewer contacts per patient; p < 0.001; Scotland: 95% CI 0.10 to 0.18 fewer contacts per patient; p < 0.001].
FIGURE 2.
Average number of inhaled steroid prescriptions by month for (a) England and (b) Scotland.
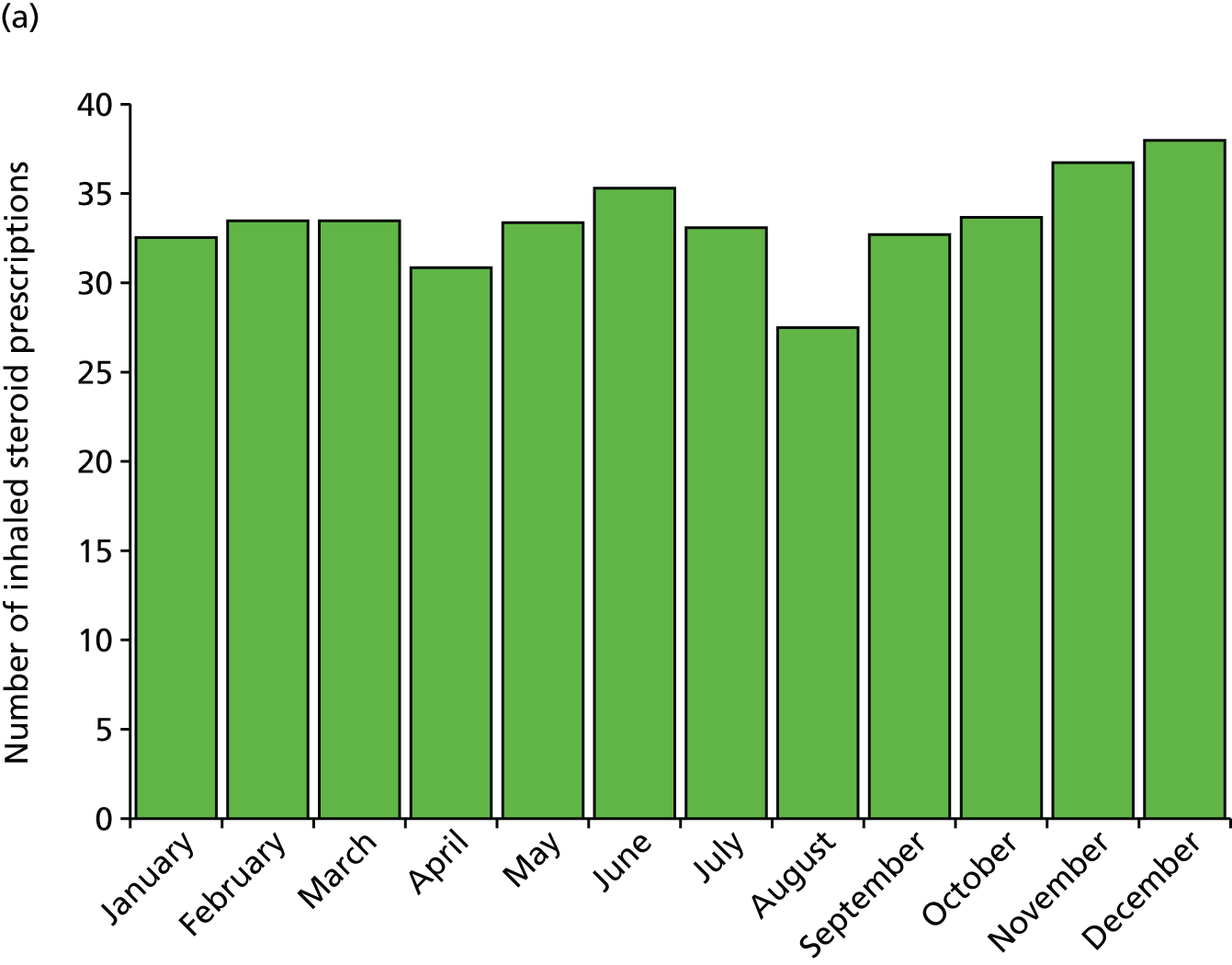
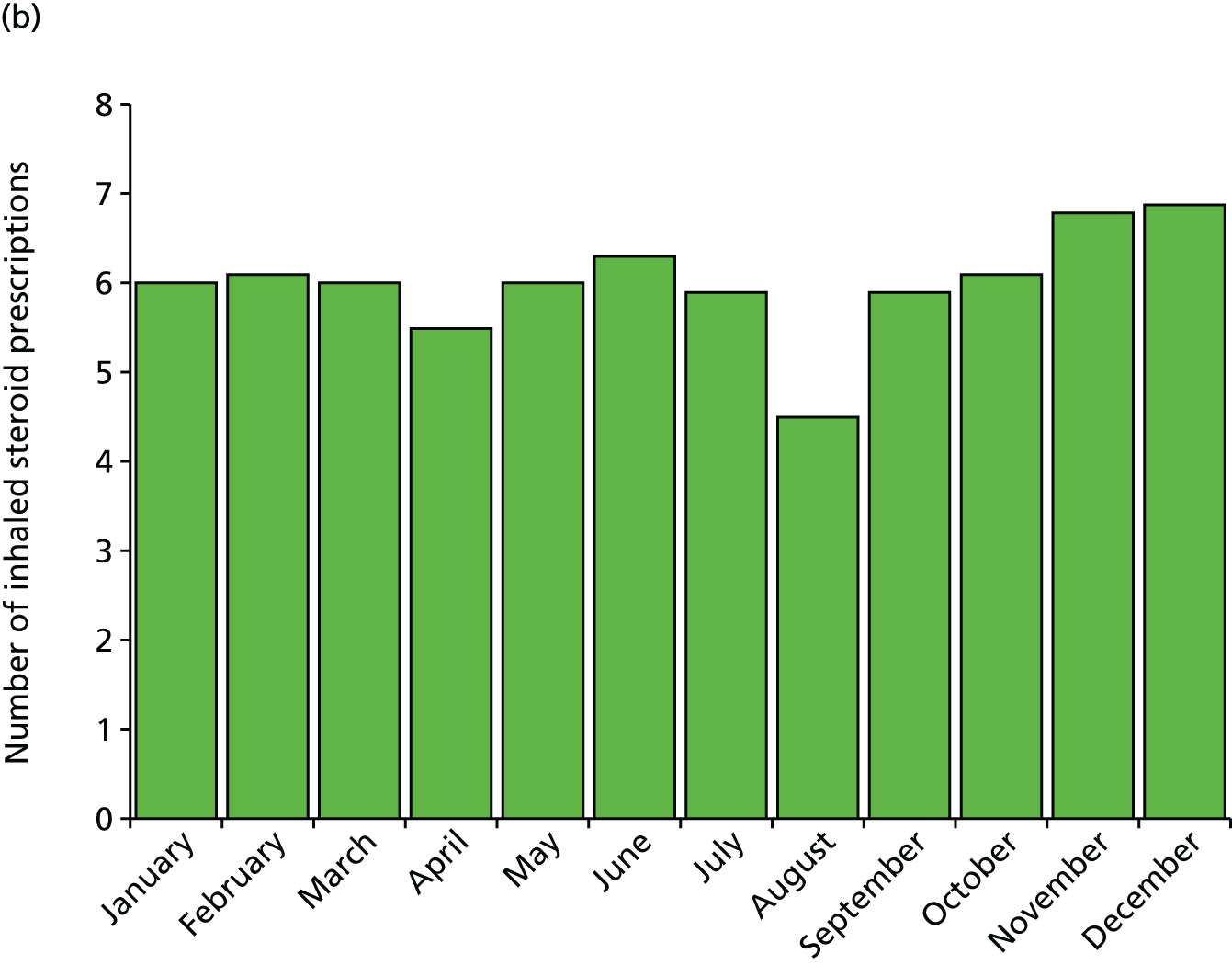
To interpret the figure of 0.14, imagine a hypothetical cohort of 200 children with asthma on inhaled corticosteroid, of whom 100 receive an August prescription and 100 do not. If the 100 patients with a prescription make a total of 50 unscheduled medical visits (0.5 mean visits/patient), then 64 unscheduled medical contacts would be made by those not receiving a prescription (0.64 mean visits/patient; difference 0.14). Therefore, for every 100 children with asthma on inhaled corticosteroids not receiving a prescription in August, there is an excess of 14 unscheduled medical contacts.
It is therefore possible that children who stop taking or reduce their inhaled corticosteroids over the summer months, and/or who run low on other medications and fail to restart them before the return to school, render themselves more vulnerable to an acute asthma exacerbation and unscheduled medical contacts.
The cost to the NHS of unplanned medical contacts is £36 for a general practitioner (GP) surgery contact, £121 for a GP home visit,14 £59–142 for an emergency department contact if not admitted and £74–249 if admitted, and £385 for a non-elective short stay for asthma without complications. 15 The intervention/letter therefore has the potential to benefit the health and quality of life of children with asthma while also improving the effectiveness of NHS services by reducing NHS use in one of the busiest months of the year.
Research aims and objectives
The aim of the study was to assess if a NHS-delivered public health intervention (a letter sent from the GP to parents/carers of school-aged children with asthma) can reduce the number of unscheduled medical contacts after the school return.
The primary objective of the study was to assess whether or not the intervention reduces the September peak in unscheduled medical contacts.
Chapter 2 Methods
This report is concordant with the Consolidated Standards of Reporting Trials (CONSORT) statement extension for cluster randomised trials. 16
Ethics approval and research governance
Ethics approval for the study was given by South Yorkshire Research Ethics Committee on 25 October 2012 (reference number 12/YH/04). NHS permissions to conduct the study were obtained for all the primary care trusts in England and health boards in Wales.
The trial was registered with the International Standard Randomised Controlled Trial Number ISRCTN03000938.
Trial design
The study was a cluster randomised trial17 to assess if a letter sent by a GP to the parents/carers of school-aged children with asthma, reminding them to take their medication, reduces the number of unscheduled medical contacts after return to school following summer holiday in September. The unit of cluster was general practices; site recruitment commenced in January 2013, with the intervention being delivered during the week commencing 29 July 2013. Data for the trial were collected via the CPRD.
Clinical Practice Research Datalink
The CPRD GOLD is the world’s largest validated computerised database of anonymised longitudinal medical records for primary care. 18 Records are derived from GP software systems and contain complete prescribing and coded diagnostic and clinical information, as well as information on tests requested, laboratory results and referrals made at or following on from each consultation. 19
The CPRD is thus able to capture all medical contacts, from prescription request through to out-of-hours contacts, along with the reason for the contact. This therefore negated the need to request this information from the individual GP practices.
Settings and locations where the data were collected
The setting was primary care, with the unit of cluster being general practices. Site eligibility required practices to be using the Vision IT software [INPS (In Practice Systems), London, UK] and be part of CPRD. Site recruitment was conducted by CPRD and the National Institute for Health Research (NIHR) Primary Care Research Network.
Clinical Practice Research Datalink recruitment
Practice recruitment was carried out predominantly by CPRD. A practice recruitment pack, consisting of a detailed study information sheet and an expression of interest (EoI) form, was sent to all 433 practices contributing to CPRD in England and Wales at the time of recruitment. This was sent by post to the preferred contact at the practice as specified in CPRD’s records for the practice. Non-responding practices were sent a reminder e-mail, followed by a second reminder e-mail and then final reminders by e-mail and post. In addition to this, some practices were contacted by telephone, either by CPRD or by members of the study team at the Sheffield Clinical Trials Research Unit (CTRU).
Practices that wanted to take part in the study, or to decline participation, returned the completed EoI form, confirming or updating as necessary the information about the practice held by CPRD. Responses were tracked by CPRD to ensure practices that had expressed interest or declined to participate were not contacted again. The EoIs were then forwarded to the study team to contact practices and complete site set-up.
The details of the recruitment processes and contacts required to enrol GP practices have been published. 20
National Institute for Health Research Primary Care Research Network
The Primary Care Research Network also advertised and invited recruitment to the trial. Eligibility criteria for general practices were that they were using Vision IT software and agree to be signed up to CPRD if they were not already. Completed EoIs were returned to the study team for follow-up and site set-up.
Site set-up
The study team contacted interested practices to complete site set-up; this was done via telephone or Skype™ (Microsoft Corporation, Redmond, WA, USA). Once set-up was complete and verbal consent obtained from the practice to participate, practice details were forwarded to the study statistician for randomisation. See Randomisation and blinding for details of randomisation.
Practices randomised to intervention group were sent GP packs that included the intervention letter template, which was added to practice headed paper, with procedures on confirming patient eligibility and instructions on the process and timing for delivery of the intervention via DocMail (www.cfhdocmail.com/; accessed 18 January 2016; CFH Docmail Ltd, Radstock, UK).
Practices randomised to the control group were to continue with care as usual; no other activity was required.
Participants and eligibility criteria
Participants were school-aged children with asthma, aged between 4 and 16 years, who were registered with a GP.
Inclusion criteria
Children were eligible if they:
-
were aged between 4 and 16 years on 1 September 2013
-
had a coded diagnosis of asthma
-
had been prescribed asthma medication in the 12-month period from March 2012 to March 2013.
Exclusion criteria
Children were excluded if they:
-
were aged 4 years or under on 1 September 2013 or 16 years or over on 31 August 2013
-
were not considered appropriate for this intervention by their GP
-
had asthma but were not receiving asthma medication
-
had co-existing neoplastic disease.
The CPRD identified eligible participants based on pre-agreed diagnostic codes for asthma and the inclusion/exclusion criteria, which were subsequently screened by the GP to confirm inclusion. The list of diagnostic codes used by CPRD used for patient identification is available on the study Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term (PLEASANT) website: www.sheffield.ac.uk/scharr/sections/dts/ctru/pleasant/index (date last accessed 31 May 2016). When writing this report it became apparent that there was a discrepancy between the code list provided by the study team and the code list used by CPRD. The impact of this on the data extraction is discussed in Changes to the data collection, data extraction and methods for allocation of data after the trial commenced, with reasons.
Trial intervention
Sites were randomly allocated to:
-
intervention group: sending out the letter
-
control group: standard care (no letter).
For the intervention, a letter sent from a GP to the parents/carers of children with asthma reminding them to maintain their children’s medication and collect a prescription if they are running low. It also advised that, should their child have stopped their medication, it should be resumed as soon as possible (see Appendix 1).
The letter template was developed based on standard letters already used in general practice. The wording of the letter had input from the study team, which includes a GP, health psychologist and consultant respiratory paediatrician, and was also discussed in detail at two patient and public events that included school-aged children with asthma and their parents. 21
The intervention letters were sent out during the week commencing 29 July 2013 to obviate the distraction of planning for family holidays and yet left enough time for parents and children to renew prescriptions and gain benefit from the medication. The timing of the letter was decided following discussion with the patient and public involvement (PPI) group. 21
Postal procedures
Practices were encouraged to use the DocMail service for sending the letters, which was done via a website to secure servers. This was done, first, to reduce practice burden and, second, to allow monitoring to confirm that the letters had been sent, the number of letters sent, and on which dates.
Practices that preferred not to use this method, and to arrange the posting themselves, were asked to confirm that the letters had been posted, the dates and the numbers sent.
Outcomes
The effectiveness of the intervention was assessed on the basis of prescription uptake prior to the school term and medical contacts thereafter. Analyses of medical contacts were defined in four overlapping time intervals:
-
September 2013 (the primary study period)
-
September to December 2013 (the extended study period)
-
September 2013 to August 2014 (the 12-month study period)
-
September 2014 (the echo substudy).
The primary study period spans from 1 to 30 September 2013, as this was the period when, prior to the start of the study, the intervention was considered most likely be able to demonstrate an impact. The extended study period was from 1 September to 31 December 2013, since asthma-related appointments are more frequent in these months. The full follow-up period was 12 calendar months from 1 September 2013 to 31 August 2014. There was also an echo (or follow-on) study period in September 2014 to see if the effect from September 2013 was maintained when there was no actual study intervention.
Prescription uptake and scheduled medical contacts were evaluated during three periods:
-
August 2013
-
August 2013 to July 2014
-
August 2014 (the echo substudy).
The health economic analyses were based on a 12-month period from 1 August 2013 to 31 July 2014. The period starts 1 month earlier than the evaluation of medical contacts in order to incorporate the cost associated with delivering the intervention including any increase in prescriptions or medical contacts in response to the intervention that occurred during August 2013.
Primary outcome
The primary outcome measure was the proportion of patients who had an unscheduled medical contact in September 2013. The primary analysis population was the intention-to-treat (ITT) population among children aged between 5 and 16 years, but was repeated for other subgroups (see Analysis population for more details).
Secondary outcomes
The following outcomes were evaluated:
-
medical contacts during the primary study period (September 2013):
-
unscheduled medical contacts:
-
– the proportion of patients who had an unscheduled medical contact
-
– the number of unscheduled medical contacts.
-
-
unscheduled medical contact associated with a respiratory diagnosis:
-
– the proportion of patients who had an unscheduled medical contact associated with a respiratory diagnosis
-
– the number of unscheduled medical contacts associated with a respiratory diagnosis.
-
-
any medical contacts (scheduled or unscheduled):
-
– the proportion of patients who had any medical contact
-
– the number of medical contacts.
-
-
-
medical contacts during the extended study period (September to December 2013):
-
– as for September 2013.
-
-
medical contacts during the 12-month study period (September 2013 to August 2014):
-
unscheduled medical contacts:
-
– the proportion of patients who had an unscheduled medical contact
-
– the number of unscheduled medical contacts
-
– the time to first unscheduled medical contact.
-
-
unscheduled medical contact associated with a respiratory diagnosis:
-
– the proportion of patients who had an unscheduled medical contact associated with a respiratory diagnosis
-
– the number of unscheduled medical contacts associated with a respiratory diagnosis
-
– the time to first unscheduled medical contact associated with a respiratory diagnosis.
-
-
any medical contacts (scheduled or unscheduled):
-
– the proportion of patients who had any medical contact
-
– the number of medical contacts
-
– the time to first medical contact.
-
-
-
medical contacts during the echo period (September 2014):
-
– as for September 2013.
-
-
prescriptions and scheduled contacts in the month of August 2013:
-
– the proportion of patients who had a scheduled medical contact (e.g. asthma review)
-
– the number of prescriptions for preventative medications.
-
-
scheduled contacts in the 12-month period from August 2013 to July 2014:
-
– the proportion of patients who had a scheduled medical contact (e.g. asthma review).
-
-
prescriptions and scheduled contacts in the month of August 2014:
-
– as for August 2013.
-
Changes to trial outcomes after the trial commenced, with reasons
An additional secondary outcome has been added to include data up to September 2014 to evaluate whether or not there was a carry-over effect into the following year. If the intervention were to increase prescription uptake and reduce unscheduled medical contacts in the original study period, it would be of interest to know whether or not the effect was repeated in the subsequent year without the need for a repeat intervention. As routinely collected data were used throughout the study, there was no cost associated with this extension. Therefore, the follow-up period was extended by 1 month. This was agreed by the Trial Steering Committee (TSC) and NIHR Health Technology Assessment (HTA) programme as a non-cost extension to the trial (see Appendix 2).
The non-cost extension also facilitated a survey of the GP practices that were in the intervention arm of the study to inform the health economic evaluation of the study by asking questions on resource use in the sending of the intervention letter. 22
A second change concerned the analysis of adherence to medication. The planned statistical analysis defined the medical possession ratio as the total number of days of prescriptions in the last year. During the study it became clear that the CPRD data did not hold sufficient data to enable this analysis. The amount of medication dispensed (e.g. the number of inhalers) is captured usually, but the amount of medication required (e.g. the number of puffs an individual is required to take) is not. This information is required to calculate the medical possession ratio. For this reason, our planned analyses of adherence were not possible.
Data collection, data extraction and methods for allocation of data
Data were extracted from the December 2013 CPRD GOLD database build. Patients who were between 4 and 16 years of age on 1 September 2013 and currently registered at a participating practice were considered for inclusion. A Read Code list, supplied by the study team at the Sheffield CTRU, was applied to identify all patients who had a diagnosis code for asthma. The population was then limited to patients who had been prescribed an asthma medication in the 12-month period of March 2012 to March 2013 using a Multilex code list. Patients who had a Read Code indicating neoplastic disease were excluded. All data for the included patients were extracted from the database. In order to protect the identity of included patients, the patient and practice identification numbers (IDs) were pseudonymised by the research team at CPRD before the data were delivered to the CTRU. Read Code lists used for the data extraction are available on request and are also on the website of the PLEASANT study at the following address: www.sheffield.ac.uk/scharr/sections/dts/ctru/pleasant/index (accessed 31 May 2016).
Subsequent data extractions were done for the same patients on the April 2014, June 2014 and January 2015 builds in order to increase the length of follow-up.
Data handling
The primary data source was anonymised, and GP and NHS contacts extracted by the CPRD and forwarded to the CTRU via a password-protected zip file.
Every NHS service contact is coded by the general practice and captured within the practice database. These codes, which include diagnostic, consultation, prescription and test result codes, were used to enable allocation to either scheduled or unscheduled contact. This allocation was carried out by presenting a summary of the consultation codes, medcode descriptions and other tables used (as described in Methods for allocation of data to scheduled/unscheduled contacts) to an independent GP adjudication panel comprising three GPs. The GP adjudication panel reviewed the data and confirmed assumptions to use in order to code contacts as scheduled, unscheduled or not applicable (not relevant). Detail and definitions used for this coding are provided in the following section (see Methods for allocation of data to scheduled/unscheduled contacts).
The age for inclusion in the study is age as of 1 September 2013. Day of birth is missing for all children in the data set so there is no patient-identifiable information. For day of birth, the value ‘15’ was used. If month of birth was also missing the value ‘September’ was used. The latter assumption was used because data provided by CPRD have been checked for meeting the eligibility criteria, and hence subjects with a missing month will be eligible with this month. All children with a missing month were born in the years 1997, 1998 and 1999. Thus assuming September, October, November or December for the missing months ensures that all subjects born in 1997 are included in the analysis.
Note that the data were provided by CPRD as anonymised to prevent the identification of the children and the practices in the study.
Given CPRD anonymisation of the practices, it was not possible to reconstruct the full disposition of some children. For example, in the present report, 5917 children are allocated to the intervention arm and 6262 to the control arm in 141 practices, which is based on the data extracted from the database for the analysis. In a different source (the original practice size listing used for the randomisation), 5907 subjects are allocated to the intervention and 6431 to the control in 142 practices (including one practice, with a size of 99, that subsequently withdrew its consent after randomisation – see Recruitment and participant flow). Owing to the blinding, it was not possible to fully describe the discrepancy in numbers of children.
A similar issue is found with subject allocation to the per-protocol (PP) population. Although it is possible to ascertain whether or not a subject is included in the PP population, because of blinding it was not possible to fully link to the reason (e.g. ‘letter not sent’ or ‘letter sent late’) without a little interpolation.
Detailed data management processes are set out in a data management plan. Data will be retained in accordance with the Data Protection Act 199823 and CTRU data management standard operating procedures (SOPs).
Methods for allocation of data to scheduled/unscheduled contacts
A scheduled contact was defined as any contact that is part of the planned care for the patient, for example an asthma review, a medical review, repeat prescription or immunisation. An unscheduled contact was defined as any contact not part of the patient’s care plan that is either patient initiated or a result of illness.
To ensure that the allocation of scheduled and unscheduled contacts was robust, a GP Adjudication Panel comprising three independent GPs attended meetings to review the data blind to treatment. The GP Adjudication Panel reviewed the unique terms (17% of the unique terms were reviewed, which accounted for 90% of the data). During these meetings the GP Adjudication Panel devised the assumptions (i.e. rules) used to allocate to scheduled, unscheduled or not applicable (irrelevant) contacts. These assumptions were documented (see Appendix 3) and approved by the GP Adjudication Panel.
All types of ‘consultation’ are recorded within the data that CPRD provides; each consultation was considered a medical contact. Not all consultations are considered relevant to the study. One ‘consultation’ in the consultation table was considered one contact. All consultation data supplied are taken into account for the study, not just those that are asthma related. Only consultations that happened on or after 1 August were included.
Assumptions used to code records as scheduled, unscheduled or not applicable were based on a GP Adjudication Panel review of the clinical, immunisation, therapy, referral, and test and consultation data.
The ‘medcode description’ from the clinical data was used first, as it was felt that this table gave most description about the reason for the consultation. If contact type could not be determined by the ‘medcode description’, then clinical consultation was referenced.
Following the GP Adjudication Panel review of the medcode descriptions and clinical consultation types, in which over 90% of the data were reviewed (17% of the unique terms), clinical records were identified to be marked as scheduled (these include asthma annual review terms and other obvious types of planned appointments), unscheduled (e.g. examinations, emergency appointments), not applicable or unknown.
The clinical data contain more than one record per consultation and the same consultation ID can have more than one clinical contact type. For these clinical records, we assumed that unscheduled takes precedence (i.e. they are likely to have come in for an unscheduled visit but had a scheduled ‘type’ of procedure at the same time). Of all the contacts coded, a small proportion (2.27%, 10,011 of 440,429) could have been defined as both, based on the assumptions made about the clinical data.
Consultation data marked as unknown, based on the clinical data, as well as consultation data that did not link to clinical data, are coded based on immunisation, therapy, referral, and test and consultation data. If at least one match was found in the immunisation record, then the data were coded as scheduled. If at least one match was found with therapy (medication) data, then the data were coded as unscheduled. If the data matched with the test data as part of the routine asthma review, then they were coded as scheduled. If they matched with test data of peak expiratory flow rate, then they were coded as unknown. Otherwise, a match with test data was coded as unscheduled.
Finally, contacts were coded on the consultation type in the consultation table, where follow-up/routine visits, repeat issue and medicine management were coded as scheduled; consultation types that indicated an emergency visit were coded as unscheduled; administrative-type consultation types were coded as not applicable; and those that were unclear, for example clinic, surgery consultation and other, were coded as unscheduled because of the likelihood that most scheduled consultation types would be clearly recorded. The process of allocation is detailed in Appendix 3.
Changes to the data collection, data extraction and methods for allocation of data after the trial commenced, with reasons
When reviewing the code lists used by CPRD to identify patients, after the study was completed and it was being reported, it became apparent that there was a discrepancy between the codes provided by the study team and the codes used by CPRD. The missing codes included some asthma, neoplasm and medication codes.
The clinicians on the Trial Management Group (TMG) reviewed the discrepancies between the codes used by CPRD and those provided by the study team. With reference to the omitted asthma codes, the clinical view was the codes were secondary codes and so there was no concern that children with asthma would have been missed. For the omitted neoplasm codes, the clinical view was that there was no concern as the codes were usually for adults and not children. However, there was a concern with the medication codes, which had been on the original code list but which were omitted from the CPRD extraction. This is of importance, as one of the inclusion criteria for the study was asthma medication prescribed in the previous 12 months. The clinical view was this may have resulted in fewer children having been identified as eligible for the trial than should have been if the correct, full list of product codes had been used. The views of the TMG, which were subsequently endorsed by the TSC, was that a further data extraction should be undertaken by the CPRD to quantify the effect of using the correct medication code list compared with the one used in error.
The CPRD undertook a review to identify the source of the problem and identified human error that was not picked up in their quality assurance. The CPRD has amended its documentation and quality assurance procedures to ensure that this type of human error is more readily identified and corrected at the time of the mistake.
In response to the request by the TSC, the set of product codes supplied by the study team was used by CPRD to identify those children with asthma who had received medication for asthma in the previous 12 months. This was done to estimate the magnitude of the impact upon the eligible patients. The CPRD conducted a post hoc analysis identifying children aged between 4 and 16 years, and still registered with a study practice, on 12 March 2013 using the April 2013 build of the CPRD GOLD database, utilising both lists of product codes. This date and database were chosen as they were the most recent versions documented to have been used for the identification of eligible patients for the trial. Owing to the human error, it is not possible to exactly extract the same data set as used in the PLEASANT study. However, it would give an estimate of the relative effect on the same size.
Using the CPRD list of medical codes, 10,753 children were identified, compared with 11,273 children identified using the full list of medical codes. This equates to an estimated 5% of children with asthma who potentially could have been in the trial but who were not. Thus, although this error has been identified, it was unlikely to have a major impact upon the trial.
The error is unfortunate, but CPRD has introduced procedures to prevent such an error from happening again.
Sample size
From previous research in the CPRD practice population, 30% of school-aged children with asthma had at least one unscheduled medical contact during the month of September. 13 We postulated that the intervention may reduce the number of children who have unscheduled medical contacts from 30% to 25% (i.e. an absolute reduction of 5%). The average practice size in the CPRD is 8294. Thus, we expected c. 100 school-aged children with asthma per practice (based on 12% of a practice being school-aged children and 11% of school-aged children having asthma). Therefore, to detect a difference of 5% with 90% power and two-sided significance level of 5%, and with an intraclass correlation (ICC) of 0.03 to account for clustering, we required 70 practices per arm. The sample size of 140 practices would equate to approximately 14,000 school-aged children with asthma.
Ukoumunne et al. 24 give estimates of ICCs for patients with respiratory symptoms in general practice. Based on the work of Ukoumunne et al., an ICC of 0.03 is a conservative estimate. The power of the study for ICCs of 0.01, 0.02, 0.03, 0.04 and 0.05 was 99.4%, 96.0%, 90.0%, 83.1% and 76.2%, respectively.
As a further sensitivity analysis, we investigated the effect of practices not sending out the letter as planned. Suppose 10 practices failed to send out the letter, these would still be included in the primary analysis under the ITT principle. However, the effect that could be observed would be reduced to 4.3%. Under the sample assumptions (ICC = 0.03, etc.), the power for the same sample size is reduced to 79.3%. This is a little under 80%, but it does demonstrate reasonable robustness to at least one deviation in the planned design.
Randomisation and blinding
Randomisation was at cluster (general practice) level, and was stratified by size of general practice (i.e. the ‘list size’), to ensure that there was an equal sample size, in terms of number of school-aged children with asthma, in each arm of the trial. The randomisation sequence was generated by the main trial statistician based within the CTRU, and allocation concealment was ensured by restricting access to the two CTRU statisticians. The randomisation was undertaken by a statistician within the CTRU, in line with a study-specific randomisation plan. Once practices had agreed to participate, their identifier and list size were forwarded to the trial statisticians for randomisation to one of the two groups (intervention or usual care). The allocation was subsequently revealed to the study manager and research assistant.
The study team were unblinded throughout the study, but had no access to data until after a statistical analysis plan was developed, and had no influence on data capture. The GP Adjudication Panels did not have access to the randomisation group when reviewing the data.
Statistical methods
Analysis populations
Each of the outcomes listed in Outcomes were evaluated on each of the four subpopulations:
-
children aged 5–16 years (the primary analysis population)
-
children aged under 5 years
-
children aged 5–16 years with a prescription for steroid inhalers
-
children aged under 5 years with a prescription for steroid inhalers.
The choice of the 5–16 years age group as the primary analysis population is a result of the difficulty associated with making a diagnosis of asthma among children below this age. 25,26 Patients aged 4–5 years were analysed separately to those aged 5–16 years, as the diagnosis of asthma is more controversial in the former age group; it is often not practical to measure variable airway obstruction below the age of 5 years, making diagnosis of asthma difficult. 25,26 The impact of the intervention in patients under 5 years will be compared with that seen in the main analysis to assess whether or not the intervention appears to benefit younger children. Additional analyses were restricted specifically to children who had received a prescription for steroid inhalers in the previous year, again undertaken separately for children aged 5–16 years and under 5 years.
Analyses of effectiveness were performed on both ITT and PP bases, with the ITT being primary. The health economic analyses were based on the PP population. ITT analyses included all practices for which data were obtained by study period. The PP analyses were the subset of children in the ITT analyses to whom the intervention was delivered as intended by the protocol. The two criteria for exclusion from PP analyses were:
-
Practices that did not send intervention letters as requested. In such cases, the entire practice data were excluded from the PP analyses.
-
Individual children who were not sent the intervention letter. GPs were given discretion to withhold the letter from any children they believed were unsuitable. In such cases, the individual was excluded from the PP analyses.
Analytical methods
The proportion of children having an unscheduled medical contact was analysed separately for each time period using logistic regression in which the covariates were the individual’s age, sex, number of contacts the previous September and the trial arm (intervention or control) as fixed effects, and the design/cluster effect of general practice as a random effect. The proportion of children having a prescription within each time period was analysed in the same manner.
Both the number of unscheduled medical contacts made in each period by the children and the number of prescriptions ordered within a time period were analysed using a random-effects negative binomial model in which the same covariates as above were included.
Within each time period, the time to first medical contact was defined as the number of days from the start of school term to the date of first contact. If no contact was made in the period, the time was censored at the last date within the period. Analyses were conducted using Cox proportional hazards regression using a random-effects (or ‘shared frailty’) model to account for the clustering within each practice. The same covariates were used (i.e. age, sex, number of contacts the previous September and trial arm).
It should be noted that there was information on death status (no deaths were observed), but no information about the movement outside GPs or region.
Full details of the analyses are in the statistical analysis plan (see Appendix 4).
Patient and public involvement
Patient and public involvement throughout the trial
Patient and public involvement during the conduct of the study had two components: (1) PPI consultations, held in September 2012 and October 2015, that involved children with asthma and their parents; and (2) two parents were invited to be independent members of the TSC.
Patient and public involvement consultation events
The September 2012 PPI consultation21 event was used to:
-
Remind attendees of the purpose of the study and give feedback on outcome of the HTA programme application.
-
Discuss the adherence of the children to their medication over the school holidays and subsequently since they have been back at school.
-
Present the GP letter (trial intervention) that was discussed at a pre-funding PPI event in January 2011, to show how it has changed as a result of the previous consultation and to invite further discussion on how the wording of the letter could be improved.
-
Invite comment on the design and end points to be used in the study and, in particular, on what, from their perspective, is a scheduled and unscheduled contact.
-
Discuss plans for PPI throughout the study and invite interested parents onto the TSC.
-
Discuss the ethics application and the rationale of the research team for how the ethics of the study are being addressed.
-
Invite comment on the lay summary of the research ethics committee.
-
Invite opinions on the study logo and the website.
The October 2015 PPI consultation27 was used to:
-
recap the reason for the trial
-
discuss the findings and any implications
-
obtain advice from the children and parents on the interpretation of the findings
-
give children and parents an opportunity to discuss how the findings should best be disseminated
-
provide details of the next steps for the research
-
consider any future research.
For attending the consultation events, each child was given a £20 gift voucher and parents were paid travel expenses. Refreshments were also provided. 28
Patient and public involvement members of the Trial Steering Committee
Two parents of children with asthma were invited onto the TSC. Payment for time was offered at a rate of £50 per meeting, plus travel expenses. A glossary of key research terms used in the study was provided and the study’s PPI lead was available to meet with the parent members of the TSC before or after each meeting to discuss the agenda items and any issues of concern.
Trial oversight
Two committees were established to govern the conduct of the trial: the TMG and TSC.
All committees are governed by Sheffield CTRU SOPs. The TMG comprised the principal investigator, co-investigators and key staff within the CTRU. The role of the TMG was to implement all parts of the trial.
The TSC comprised an independent chairperson (GP), two independent members (academic GP and statistician), two lay members (parents of children with asthma), the principal investigator and key staff within the CTRU (as non-voting members). The role of the TSC was to provide supervision of the protocol; a statistical analysis plan; and to provide advice on, and monitor, the progress of the trial.
Safety assessments
The trial intervention aimed to optimise usual asthma care and improve adherence to medications already prescribed by the GP, thus reducing the potential exacerbation of asthma following return to school in September. Therefore, involvement in the trial was not expected to result in any adverse or serious adverse events arising from participation.
Any asthma complications relating to the health of the child were expected to be picked up by their GP or out-of-hours service and managed as per usual care. On advice from the TSC, no formal reporting procedures for adverse events or serious adverse events were put in place.
Practices randomised to the intervention were provided with a short template to report any incidents that they felt were related to the conduct of the trial.
Chapter 3 Trial results
Recruitment and participant flow
In total, 142 GP surgeries agreed to take part in the study (Figure 3). 20 Of these, one (a control group practice with 99 children with asthma) withdrew consent after the start of the study for the data to be extracted and stored by the CPRD (independent of the study); this practice was excluded from all analyses. In total, 70 practices (comprising 5917 individuals) were randomised to the intervention (letter) group and 71 practices (6262 individuals) to the control group.
FIGURE 3.
Participant recruitment curve.

Baseline characteristics
The descriptive statistics (age, sex and surgery size) of the 12,179 subjects included are given in Tables 1 and 2. Summaries reported are stratified by intervention type and overall.
| Statistic | Group | Total (n = 12,179) | |
|---|---|---|---|
| Intervention (letter) (n = 5917) | Control (no letter) (n = 6262) | ||
| Sex, n (%) | |||
| Male | 3505 (59.24) | 3749 (59.87) | 7254 (59.56) |
| Female | 2412 (40.76) | 2513 (40.13) | 4925 (40.44) |
| Age (years) | |||
| Mean (SD) | 10.51 (3.29) | 10.55 (3.30) | 10.53 (3.30) |
| Median (IQR) | 10.80 (7.88–15.97) | 10.89 (7.80–15.97) | 10.89 (7.80–15.97) |
| Range | 4.05–15.97 | 4.05–15.97 | 4.05–15.97 |
| Statistic | Practice group | Total (n = 141) | |
|---|---|---|---|
| Intervention (letter) (n = 70) | Control (no letter) (n = 71) | ||
| Sample size (n) | |||
| Mean (SD) | 85 (44) | 88 (SD 64) | 86 (55) |
| Median (IQR) | 80 (49–114) | 75 (41–107) | 76 (45–113) |
| Range | 4–209 | 10–293 | 4–293 |
An analysis has been undertaken on practice recruitment into the trial. For the practices recruited through CPRD, it was found that there was little difference in terms of the size of the practice. 20 It was also found that practices that have been involved in more research were more likely to be in the PLEASANT study, and that the more studies the practice had previously participated in, the greater the likelihood of entering the trial.
Number of participants and analysis subsets
Analyses were conducted using outcome data from four overlapping time periods and one baseline period. For each period, analyses were based only on practices that contributed data to the entirety of that period. In other words, if practices stopped submitting data to the CPRD before the end of a given follow-up period, they were excluded from all analyses for that time period.
Figure 4 shows the flow of subjects from the overall population (aged 4–16 years) to the main cohort (aged 5–16 years). Of the 456 practices invited, 433 were through the CPRD and 23 were through the primary care research network and joined the CPRD. 20
FIGURE 4.
The CONSORT diagram of the number of GP surgeries and individuals in the PLEASANT study. It is not a mistake that there are zero GP exclusions in the arm that did not send letters, as it is impossible for the GPs to exclude individuals from receiving letters when no one in that arm is receiving letters. a, In comparison to those in the experimental arm (n = 5917); b, in comparison to those in the control arm (n = 6262); and c, these figures include withdrawals and patients aged 4 years.

Table 3 provides the number of practices and the number of individuals aged 5–16 years (the primary analysis population) included for each time period.
| Time period | Group | |||
|---|---|---|---|---|
| Intervention | Control | |||
| Practices (n) | Individuals 5–16 years (n) | Practices (n) | Individuals 5–16 years (n) | |
| Prescription uptake and scheduled medical contacts | ||||
| August 2013 | 68 | 5305 | 69 | 5586 |
| August 2013–July 2014 | 58 | 4541 | 54 | 4549 |
| August 2014 | 58 | 4541 | 54 | 4549 |
| All medical contacts | ||||
| September 2013 (primary study period) | 68 | 5305 | 69 | 5586 |
| September–December 2013 (extended study period) | 65 | 5097 | 67 | 5384 |
| September 2013–August 2014 (12-month study period) | 58 | 4541 | 54 | 4549 |
| September 2014 (echo substudy) | 57 | 4411 | 53 | 4438 |
Adherence to protocol
Of the 70 intervention practices, two did not send letters to any of the patients identified and four sent the intervention out late, on 6, 8, 12 and 23 August. In addition, GPs were given discretion to withhold the letter from any children they believed were unsuitable candidates; among the remaining 64 practices (5222 individuals), letters were not sent to 786 children. These individuals were included in the primary ITT analyses but were excluded from the PP analyses.
Outcomes and estimation
Primary outcome
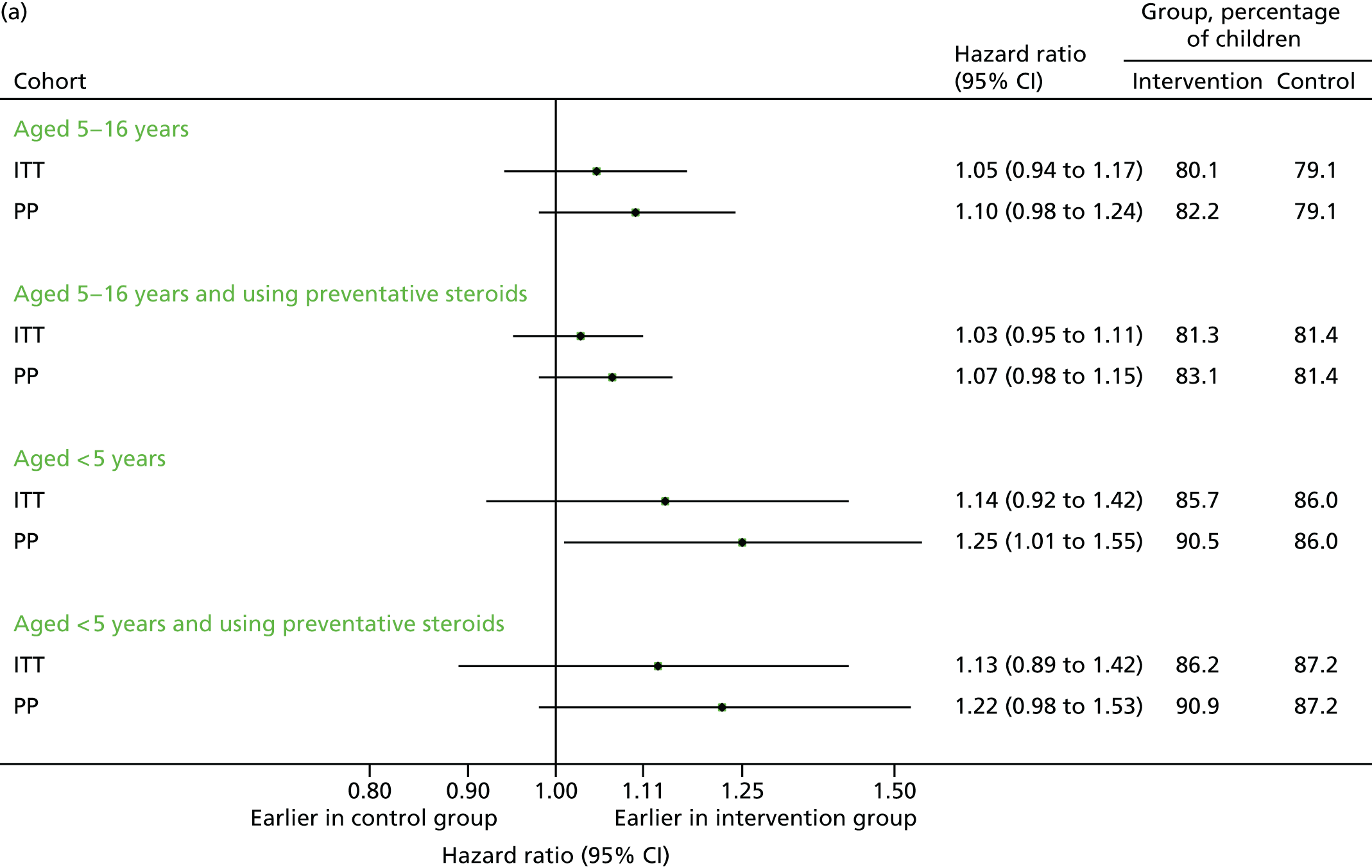
Unscheduled medical contacts in September 2013
The proportion of individuals with at least one unscheduled contact is summarised for each of the four populations (including the aforementioned primary analysis population) in Figure 5. Overall, 2399 individuals (45.2%) in the intervention arm had at least one unscheduled medical contact, compared with 2441 (43.7%) in the control arm [adjusted odds ratio (OR) 1.09, 95% CI 0.96 to 1.25]. The actual number of contacts was similar in the two groups, but there were 81 unscheduled contacts per 100 children in each arm [adjusted incidence rate ratio (IRR) 1.02, 95% CI 0.94 to 1.12]. Restricting the analyses to the PP population gave a similar (but slightly greater) increase in the effect sizes.
FIGURE 5.
Unscheduled medical contacts in September 2013. (a) Number of children with one or more unscheduled contact; and (b) mean number of unscheduled contacts per child.
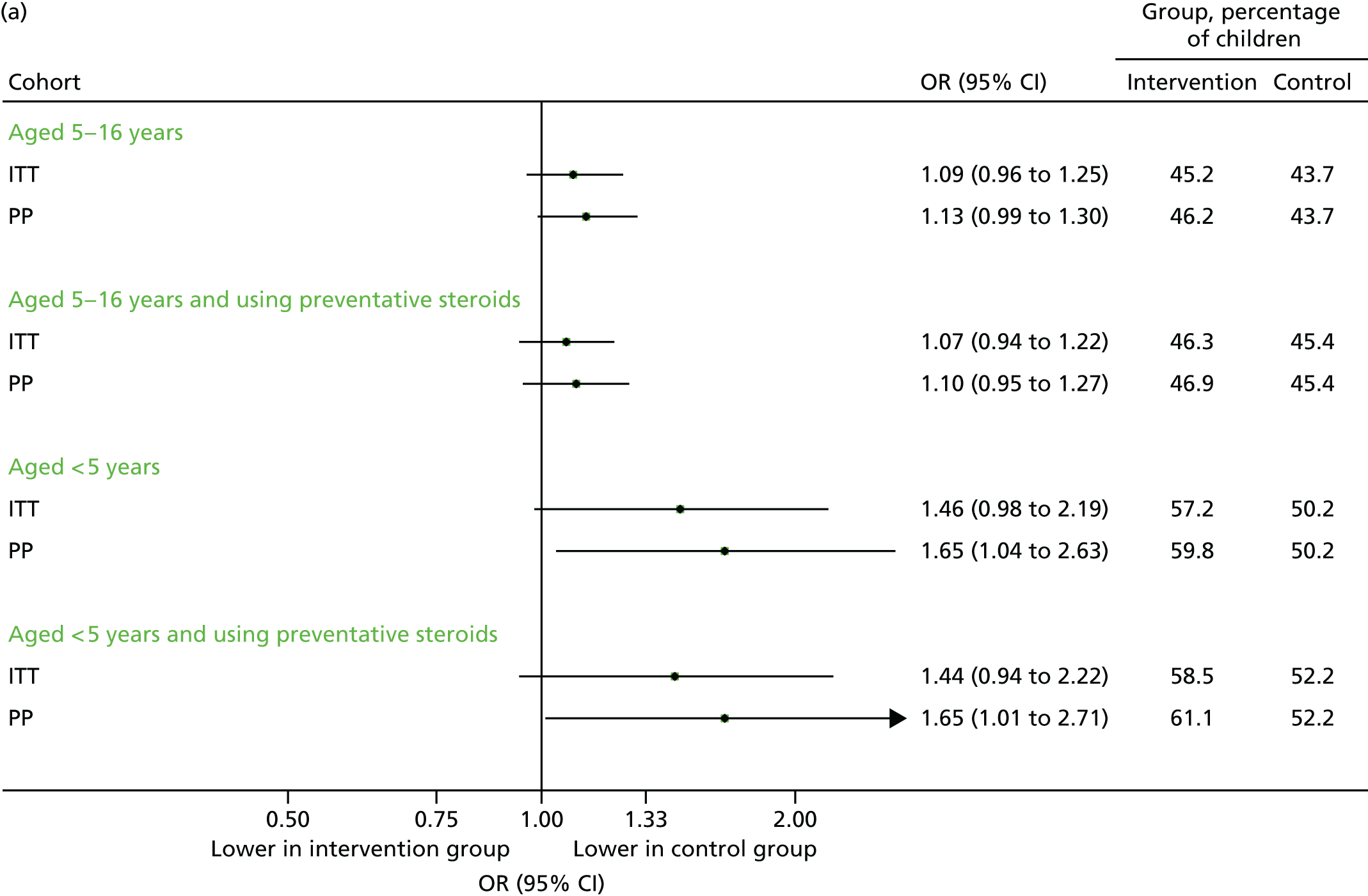

The ICC for the primary analysis was 2.6%, which was consistent with the ICC used for the sample size calculation. 29
Similar results were observed for 5- to 16-year-old children who had been prescribed preventative steroids. Among children aged under 5 years, the differences were larger, and of borderline statistical significance, with the intervention being associated with more unscheduled visits for all subgroups. In all cases the effect among the PP population was greater than that observed in the ITT population.
The percentage of children aged 5–16 years who required one or more unscheduled contact between August and December 2013 is given in Table 4. The most immediate feature is the excess unscheduled contacts in August 2013, which is out of keeping both with the following months and with the equivalent figures in the previous year. Overall, the proportion of children making an unscheduled contact was higher in 2012 than in 2013, but only August (and to a lesser extent, September) 2013 showed a pronounced difference between the groups.
| Month | 2012 (preceding year) | 2013 (intervention year) | ||
|---|---|---|---|---|
| Intervention (%) | Control (%) | Intervention (%) | Control (%) | |
| August | 41.8 | 41.0 | 41.1 | 34.4 |
| September | 47.4 | 48.2 | 45.2 | 43.7 |
| October | 51.4 | 50.0 | 44.5 | 45.8 |
| November | 51.5 | 50.1 | 43.7 | 44.3 |
| December | 49.0 | 49.1 | 42.2 | 41.8 |
To further investigate the effect observed in Table 4, the analysis of unscheduled contacts by month was repeated (Table 5), but only for children who received preventative medication. The effects are similar to those observed in Table 4.
| Month | 2012 (preceding year) | 2013 (intervention year) | ||
|---|---|---|---|---|
| Intervention (%) | Control (%) | Intervention (%) | Control (%) | |
| August | 41.9 | 41.3 | 39.0 | 34.1 |
| September | 47.8 | 48.9 | 43.2 | 41.5 |
| October | 52.0 | 52.0 | 42.7 | 43.5 |
| November | 52.1 | 50.9 | 41.5 | 40.5 |
| December | 49.9 | 50.0 | 39.9 | 38.5 |
In both Tables 4 and 5, after the initial increase in unscheduled contacts associated with the intervention in August and September there is a fall. The fact that there seems to be a reduction in contacts after September will be discussed further in Chapter 3, Contacts in the extended post-intervention phase (September to December 2013) and in Health economic methods.
In Scheduled visits and steroid prescriptions in August 2013, in the analysis of prescription data it will be highlighted how the intervention caused an increase in the proportion of prescriptions being collected. A likely explanation for the possible increase in August and September in the letter group is patients who have not collected a prescription in a while needing to see their GP before a new prescription could be given. This could be caused by patients wishing to see their GP or by the GP requiring an appointment with the patient before a prescription is given. The excess observed in August/September would therefore be, for some patients, a level of planned care that would then be reflected in the subsequent reductions in following months. To investigate this we have the results in Table 6.
| Month | Time period (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| August–October 2012 | November 2012–January 2013 | February–April 2012 | May–July 2013 | |||||
| Intervention (n = 385) | Control (n = 385) | Intervention (n = 645) | Control (n = 670) | Intervention (n = 738) | Control (n = 813) | Intervention (n = 1927) | Control (n = 2004) | |
| August | 29.2 | 24.9 | 30.4 | 28.9 | 38.5 | 34.5 | 51.6 | 45.5 |
| September | 35.5 | 30.8 | 35.9 | 37.3 | 42.1 | 39.7 | 55.2 | 54.3 |
| October | 31.3 | 33.6 | 33.5 | 41.2 | 37.4 | 41.8 | 58.6 | 56.4 |
| November | 33.4 | 24.2 | 33.0 | 36.7 | 40.0 | 37.7 | 54.1 | 53.0 |
| December | 30.8 | 25.6 | 34.3 | 34.1 | 36.0 | 36.5 | 53.2 | 50.3 |
The results in Table 6 are the same data as in Table 5 but broken down by when a patient last collected a prescription. There is little evidence of a difference in terms of the excess in unscheduled contacts in the letter arm in August. For children who last collected a prescription within the previous 3 months, 51.6% in the letter arm had an unscheduled contact in August, compared with 45.5% in the control arm, therefore 6.1% more children in the letter arm had a scheduled contact. For children who had collected a prescription within the previous 3–6 months, 38.5% in the letter arm had an unscheduled contact, compared with 34.5% in the control arm, which represents a 4.0% excess.
In September there does seem to be a difference in the proportion of children having an unscheduled contact according to when they last collected a prescription. If a prescription had been collected within 3 months, then in the letter arm 55.2% of children had an unscheduled contact in September, which is comparable to the 54.3% in the control arm. Conversely, if it was 3–6 months since the last prescription was collected, 42.1% of children had an unscheduled contact in the intervention arm, compared with just 39.7% in the control arm, which is an increase of 2.4%. Therefore, the effect in September seems to be greatest in children who had not collected a prescription recently.
One important thing to note is that when making the assessment of unscheduled contacts, we cannot determine whether the unscheduled contact is at the request of the patient or of the GP. This factor is important in the interpretation of the results in Tables 4–6.
Secondary outcomes
Scheduled visits and steroid prescriptions in August 2013
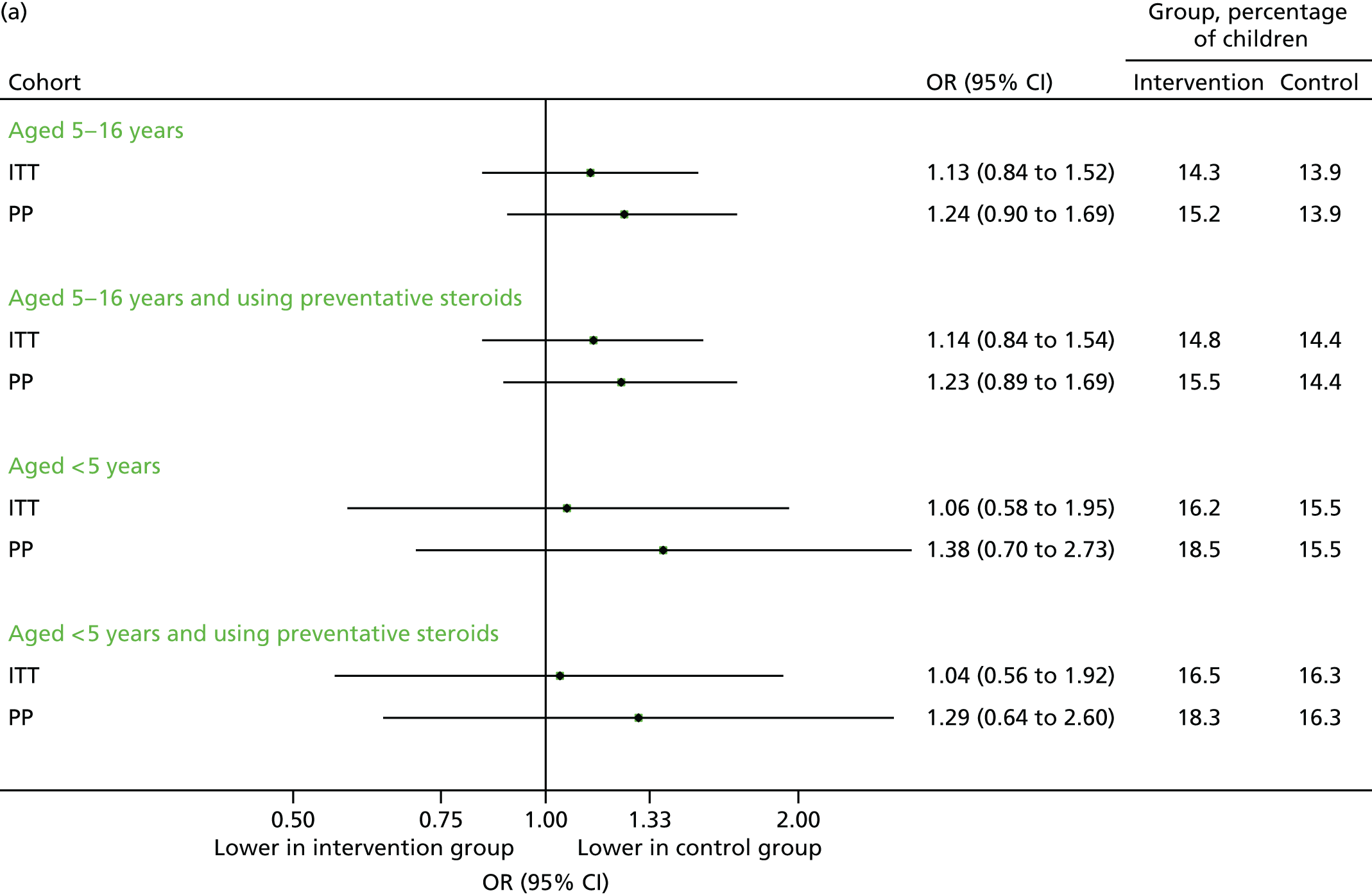
An objective of the PLEASANT study was that the intervention would increase the proportion of children who had a prescription in August 2013, which would follow through to an increase in medication usage and, thereby, a reduction in unscheduled medical contacts. Although the latter was not evident from the data in Primary outcome, and adherence could not be assessed, the intervention (letter) was associated with an increased uptake of prescriptions in the month of August 2013. Among children aged 5–16 years, 876 (16.5%) requested at least one prescription, compared with 703 (12.6%) in the control group (adjusted OR 1.43, 95% CI 1.24 to 1.64); the total number of prescriptions was also higher (adjusted IRR 1.31, 95% CI 1.17 to 1.48). These findings are displayed graphically in Figure 6.
FIGURE 6.
Uptake of steroid inhaler prescriptions, August 2013. (a) Number of children with one or more prescription; and (b) mean number of prescriptions.

Scheduled contacts made in August 2013 are displayed in Figure 7. The percentage of children with a scheduled medical contact was higher in the intervention group compared with the control group (see Figure 7a), but this was not statistically significant. The actual number of scheduled contacts were significantly increased in the intervention group (see Figure 7b).
FIGURE 7.
Scheduled medical contacts in August 2013. (a) Number of children with one or more scheduled contact; and (b) mean number of scheduled contacts per child.
Unscheduled medical contacts associated with respiratory diagnosis in September 2013
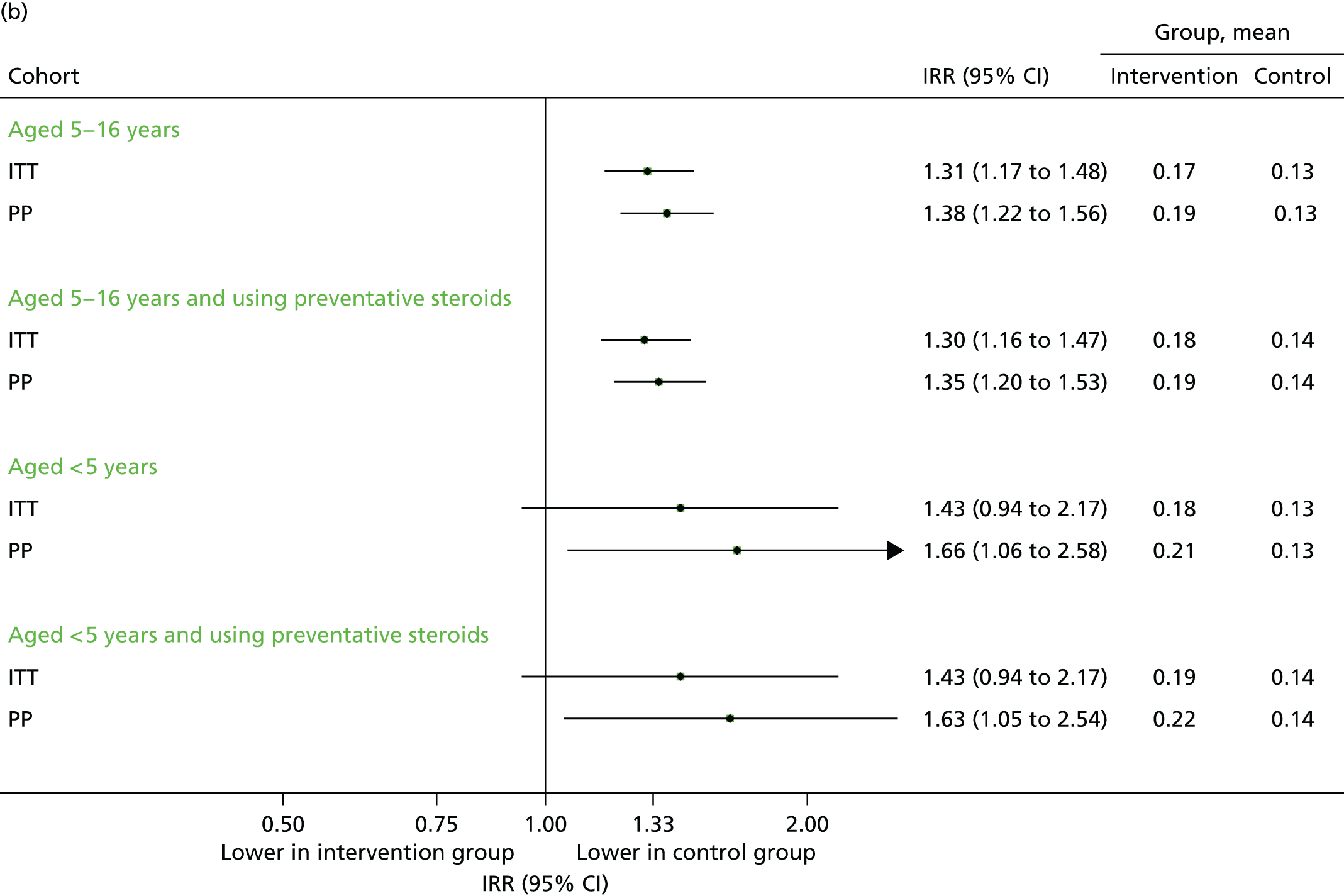
Unscheduled respiratory-related medical contacts are important, as these are the most sensitive outcome for determining whether or not the intervention is preventing episodes of asthma exacerbation. In absolute terms, however, these contribute only a small fraction of the total attendances (Table 7). Among the primary ITT analysis population of children aged between 5–16 years, a total of 513 subjects experienced at least one unscheduled respiratory-related contact across all practices, with slightly higher uptake in the intervention group. In the intervention group, 279 (5.3%) required at least one unscheduled respiratory related contact compared with 234 (4.2%) in the control group (adjusted OR 1.30, 95% CI 1.03 to 1.66). The percentages for other subgroups are presented in Figure 8a, and the total number in Figure 8b.
| Allocation | Total, n | Contact | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Respiratory-related | ||||||||
| Relevant (n) | Scheduled, n (%) | Unscheduled, n (%) | Unclassified (n) | Relevant (n) | Scheduled, n (%) | Unscheduled, n (%) | Unclassified (n) | ||
| September 2013 | |||||||||
| Letter (n = 5305) | 7480 | 5585 | 1306 (23.38) | 4279 (76.62) | 1895 | 682 | 374 (54.84) | 308 (45.16) | 34 |
| No letter (n = 5586) | 8400 | 6126 | 1591 (25.97) | 4535 (74.03) | 2274 | 748 | 492 (65.78) | 256 (34.22) | 39 |
| September–December 2013 | |||||||||
| Letter (n = 5097) | 30,084 | 21,981 | 5745 (26.14) | 16,236 (73.86) | 8103 | 2833 | 1674 (59.09) | 1159 (40.91) | 181 |
| No letter (n = 5384) | 32,138 | 24,368 | 6504 (26.69) | 17,864 (73.31) | 7770 | 3027 | 1901 (62.80) | 1126 (37.20) | 151 |
| September 2013–August 2014 | |||||||||
| Letter (n = 4541) | 71,126 | 52,330 | 11,089 (21.19) | 41,241 (78.81) | 18,796 | 6492 | 3909 (60.21) | 2583 (39.79) | 642 |
| No letter (n = 4549) | 72,175 | 54,962 | 12,344 (22.46) | 42,618 (77.54) | 17,213 | 6546 | 4134 (63.15) | 2412 (36.85) | 414 |
| September 2014 | |||||||||
| Letter (n = 4411) | 6191 | 4590 | 1079 (23.51) | 3511 (76.49) | 1601 | 447 | 255 (57.05) | 192 (42.95) | 32 |
| No letter (n = 4438) | 6267 | 4629 | 1144 (24.71) | 3485 (75.29) | 1638 | 486 | 300 (61.73) | 186 (38.27) | 20 |
FIGURE 8.
Unscheduled respiratory-related medical contacts in September 2013. (a) Number of children with one or more unscheduled respiratory-related contact; and (b) mean number of unscheduled respiratory-related contacts per child.


All medical contacts (scheduled and unscheduled) in September 2013
The total number of medical contacts (excluding those deemed irrelevant) is presented in Figure 9. In contrast to previous analyses, children in the intervention arm had fewer contacts, although, again, none of the comparisons was statistically significant. Among children aged 5–16 years, 57.8% of the intervention arm participants made one or more contact, compared with 58.4% of those in the control arm (adjusted OR 0.99, 95% CI 0.80 to 1.22). The total number of appointments per child was 1.05 in the intervention arm, compared with 1.10 in the control group (adjusted IRR 0.97, 95% CI 0.87 to 1.07). Similar findings were observed for children on preventative steroids. By contrast, an increase in contacts was observed among children under 5 years.
FIGURE 9.
Total medical contacts in September 2013. (a) Number of children with one or more contacts; and (b) mean number of contacts per child.


Contacts in the extended post-intervention phase (September–December 2013)
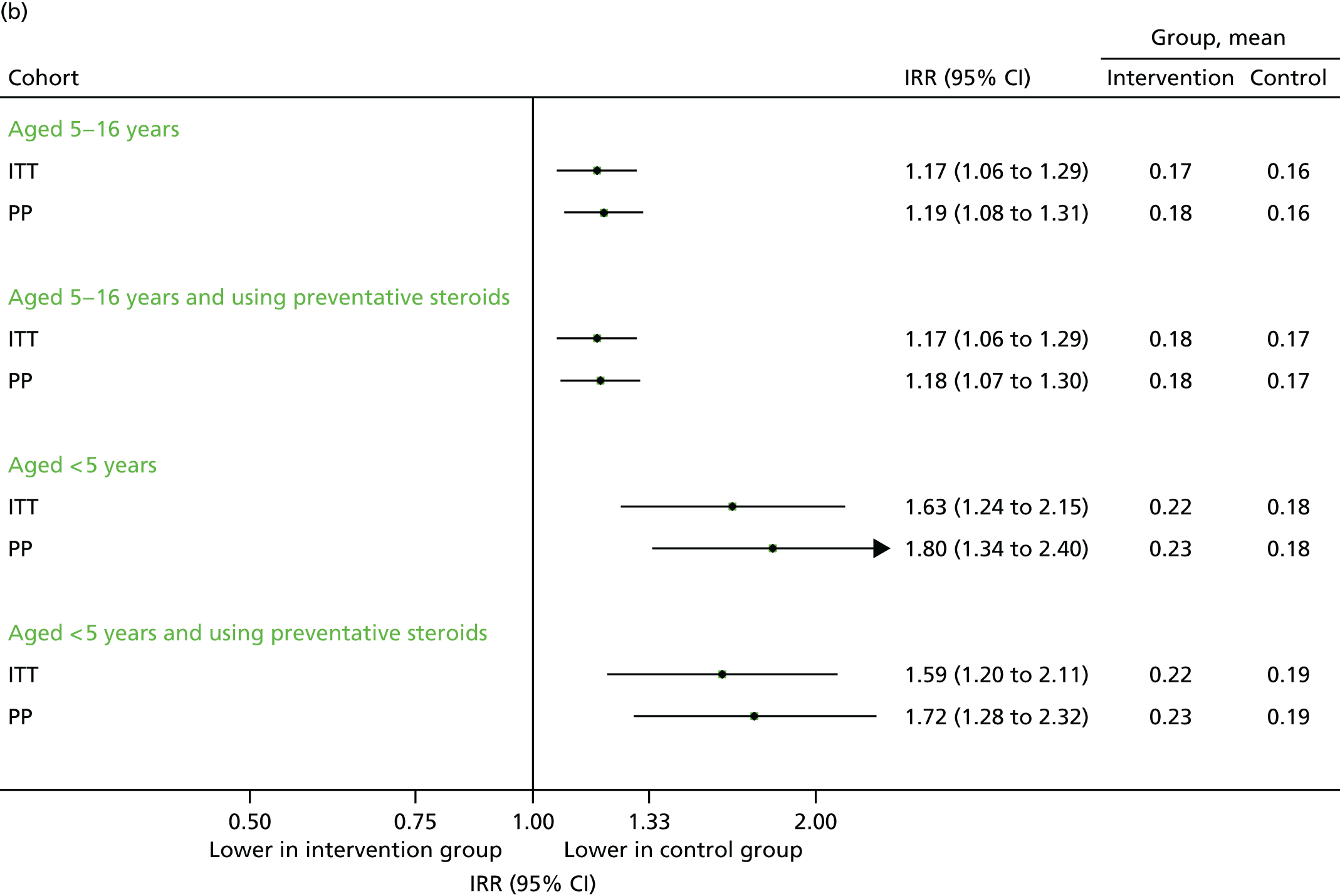
Data on medical contacts in the extended study period (September–December 2013) were available for 65 of the original 70 intervention practices and 67 of the 71 control practices. The results are presented in Figures 10–12. In some subgroups, a statistically significant excess of contacts was observed in the intervention group, although it should be noted that this could be because of the multiple outcomes and hypotheses tested.
FIGURE 10.
Unscheduled medical contacts in the period September–December 2013. (a) Number of children with one or more unscheduled contact; and (b) mean number of unscheduled contacts per child.
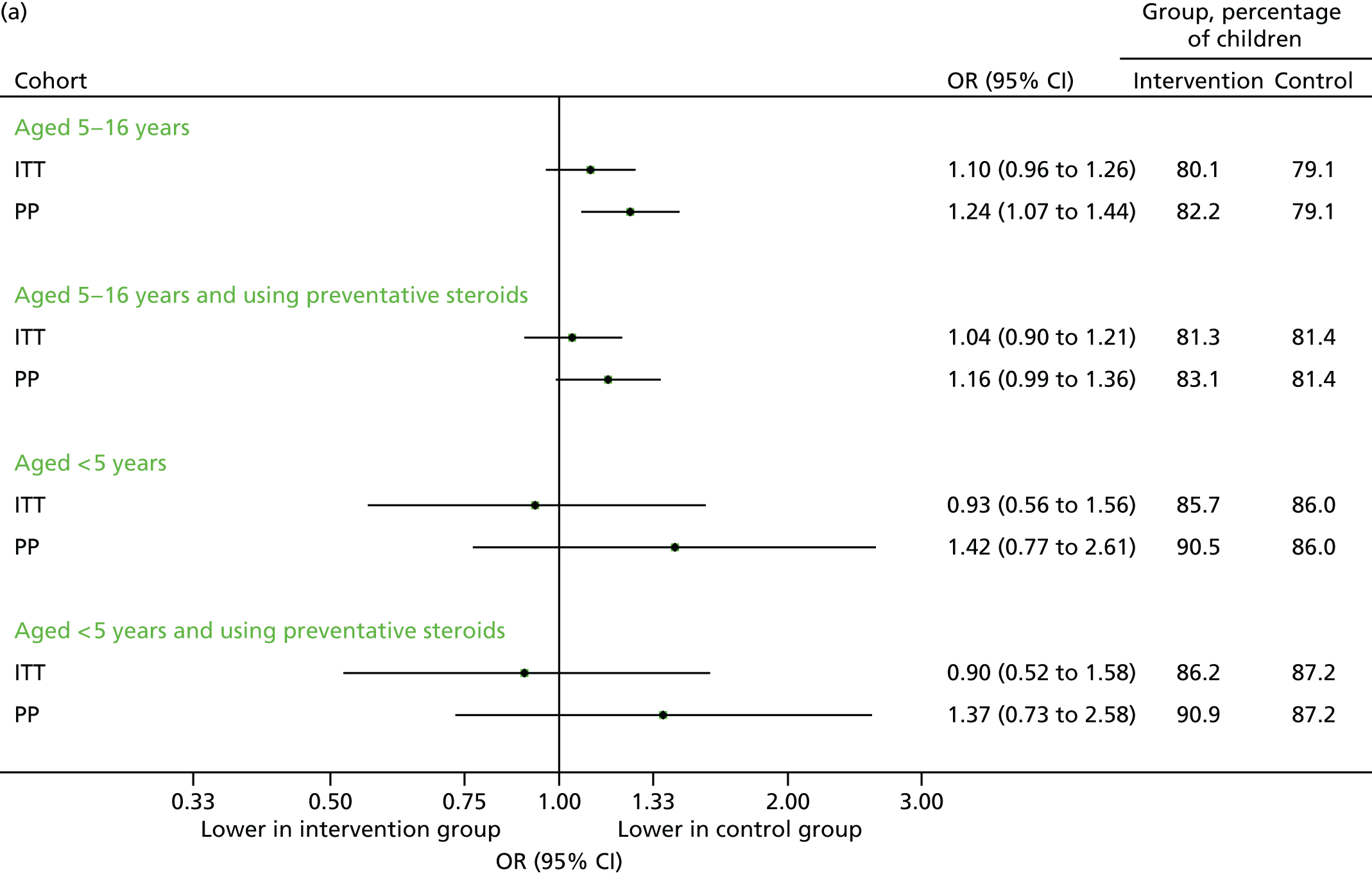
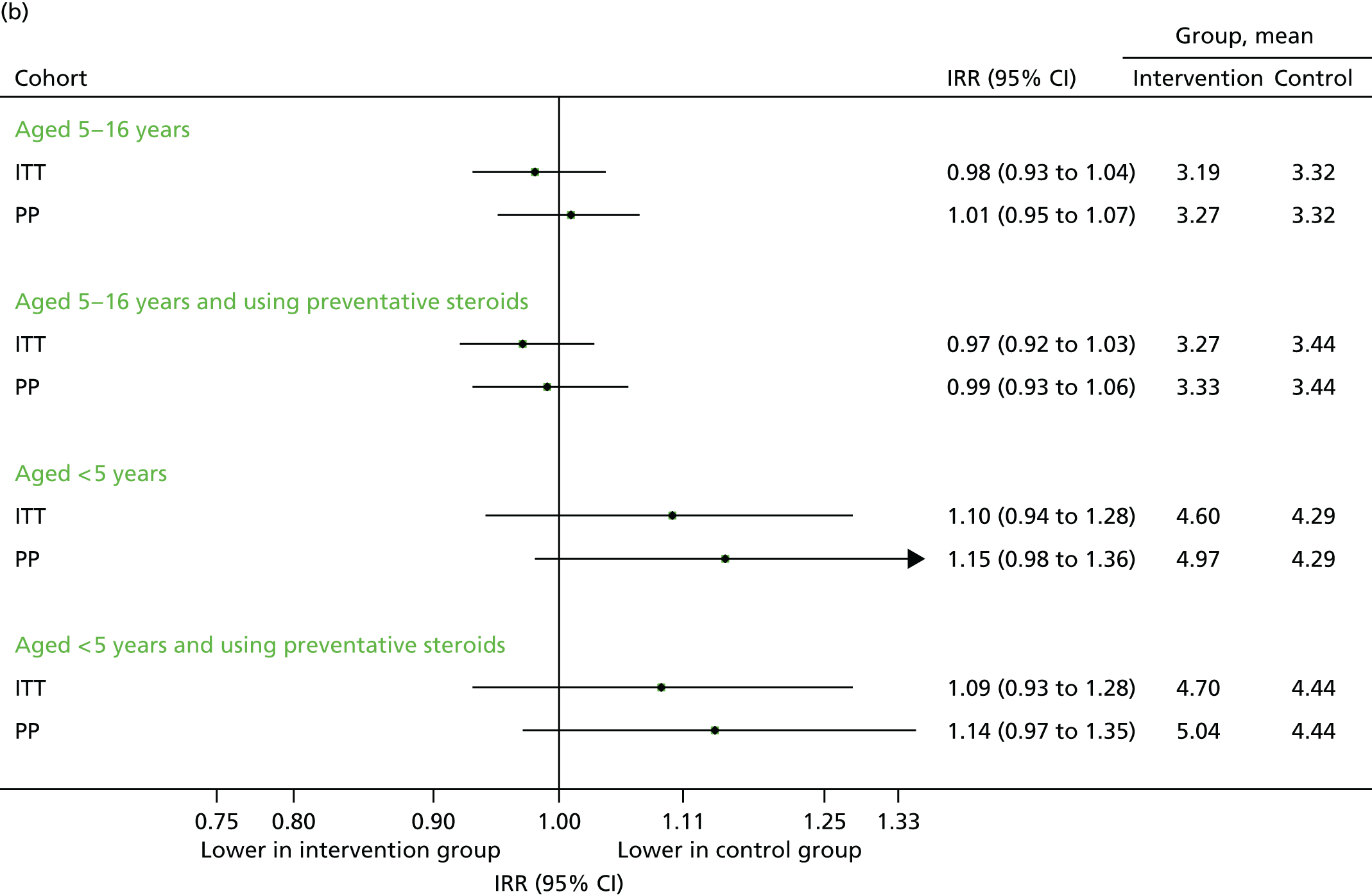
FIGURE 11.
Unscheduled medical contacts associated with a respiratory diagnosis in the period September–December 2013. (a) Number of children with one or more unscheduled respiratory-related contact; and (b) mean number of unscheduled respiratory-related contacts per child.

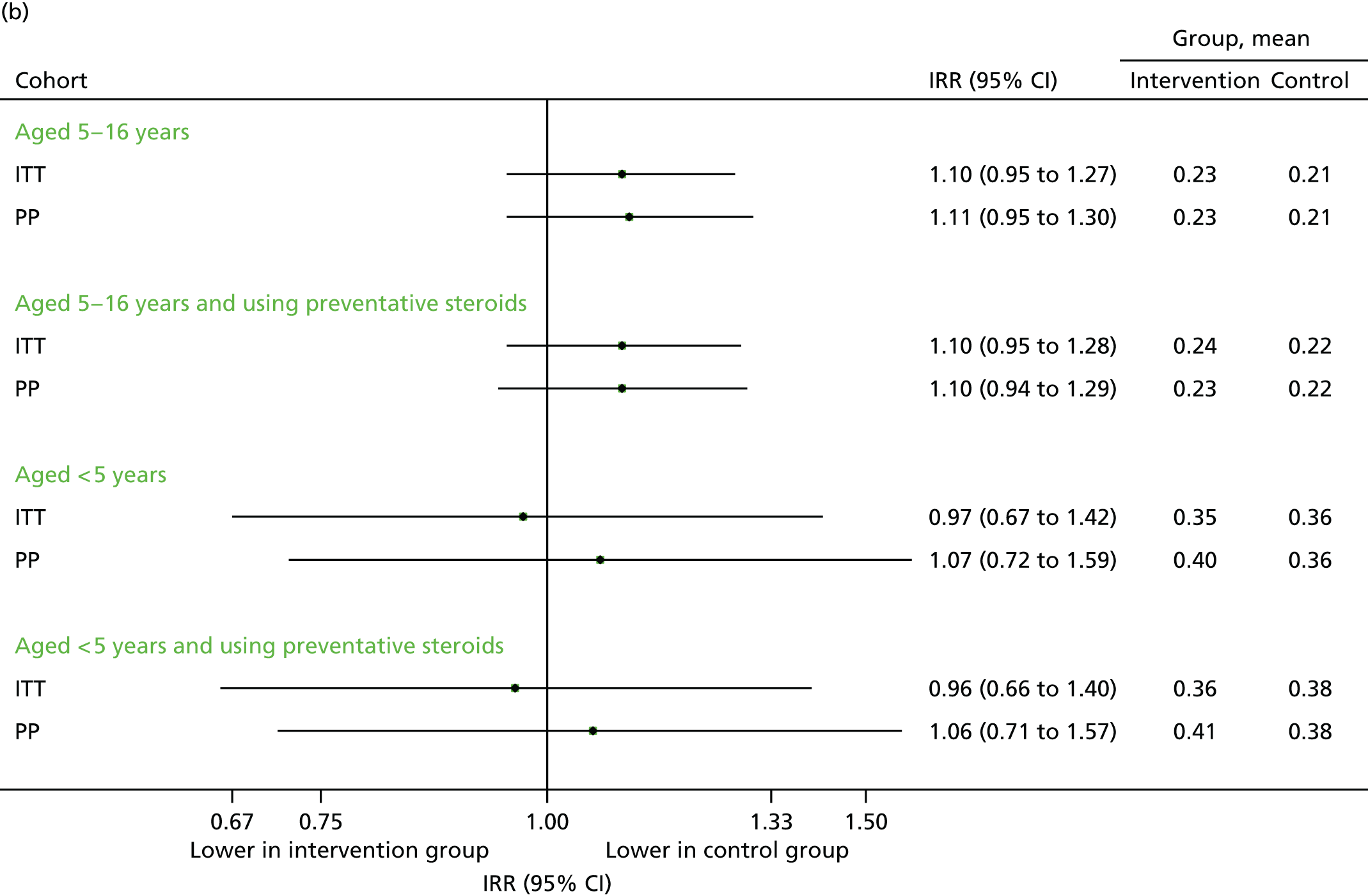
FIGURE 12.
All medical contacts in September–December 2013. (a) Number of children with one or more contact; and (b) mean number of contacts per child.

The total number of unscheduled contacts is of particular relevance in the period September to December 2013. This includes the months (October to December) when the intervention arm seemed to reduce the number of contacts. It is this time interval that forms part of the health economic analysis, which analyses the total number of contacts rather than the percentage of children who required one.
The total number of contacts declined over the period from September to December. Although unscheduled respiratory-related contacts demonstrated a slight increase, the proportion of children aged 5–16 years requiring any medical contact remained higher in the intervention arm (although not statistically significant) for unscheduled contacts (see Figure 10a), unscheduled respiratory contacts (see Figure 11a) and all contacts (see Figure 12a). The overall number of contacts and the number of unscheduled was slightly reduced in the intervention arm (see Figures 10b and 12b) for children aged 5–16 years, but not those aged under 5 years, for whom the number of unscheduled respiratory contacts was also higher (see Figure 11b). However, these differences were, generally, not statistically significant.
Contacts over 12 months (September 2013–August 2014)
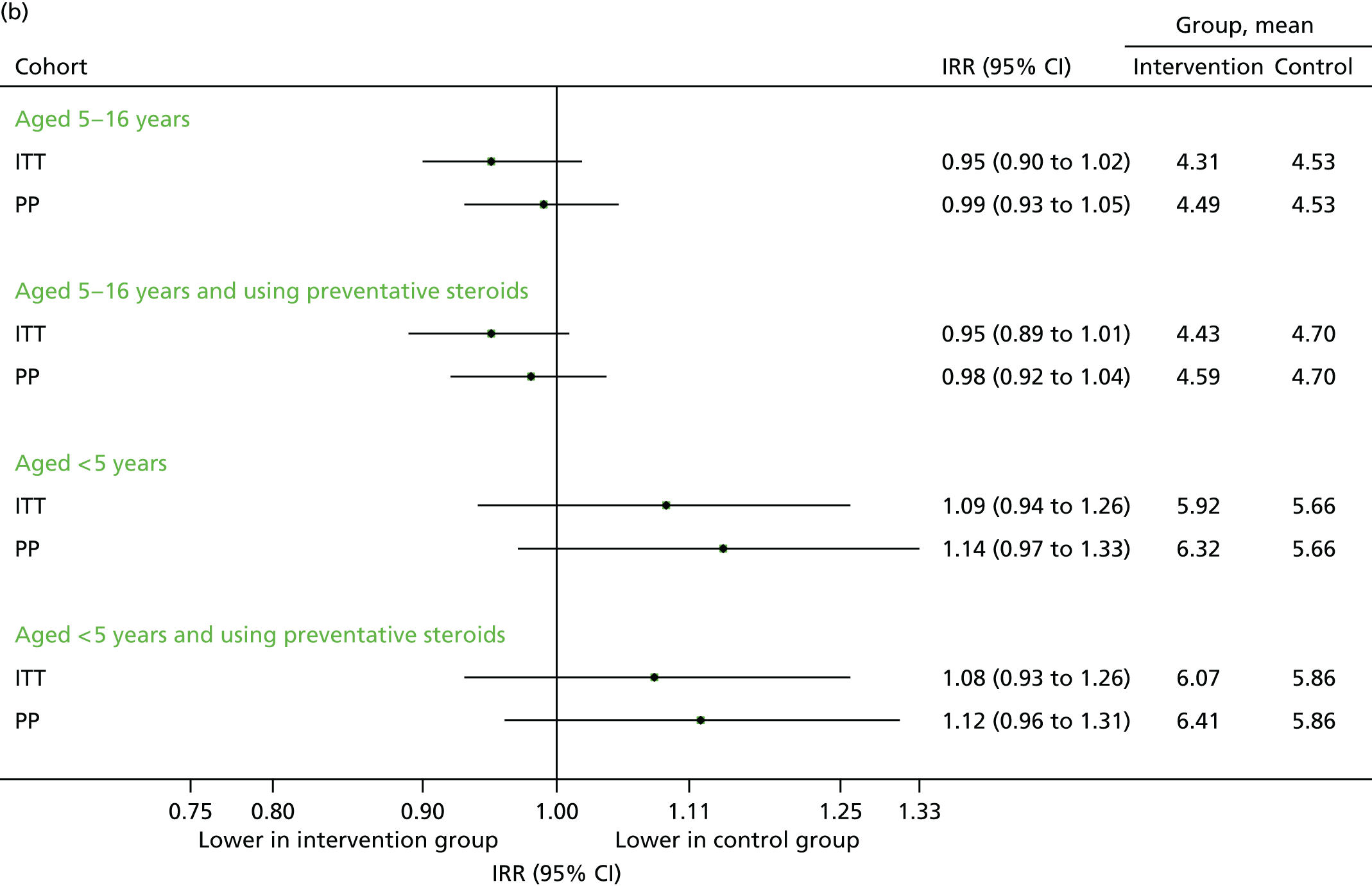
Data on medical contacts in September 2013–August 2014 were available for 58 intervention practices and 54 control practices. The results are presented in Figures 13–15. The differences in percentages between the intervention and control groups were generally modest and not statistically significant on the ITT population, and differed according to the subgroups. For the primary population (the ITT among 5- to 16-year-olds), the number of unscheduled contacts was similar (see Figure 13) and respiratory contacts remained higher (see Figure 14), but overall, contacts were reduced (see Figure 15). The total number of contacts over the 12-month period was 11.5 per child in intervention group, compared with 12.1 in the control group, which equated to a 5% reduction overall (adjusted IRR 0.95, 95% CI 0.91 to 0.99). This analysis is particularly relevant to the economic analyses in the following section, which primarily considered the overall difference in resource costs between the groups and was largely based on this time period.
FIGURE 13.
Unscheduled medical contacts from September 2013 to August 2014. (a) Number of children with one or more unscheduled contact; and (b) mean number of unscheduled contacts per child.
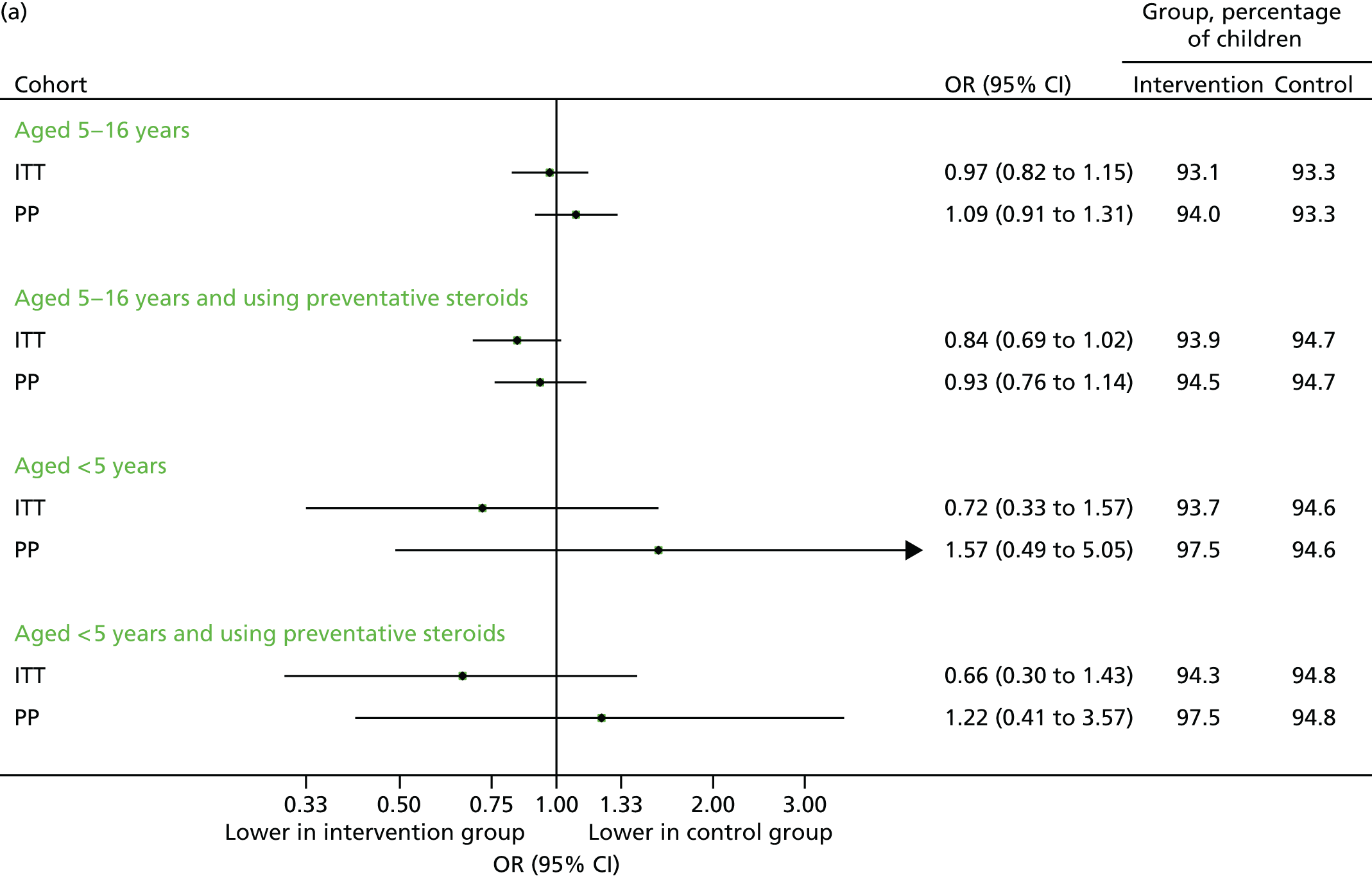

FIGURE 14.
Unscheduled medical contacts associated with a respiratory diagnosis from September 2013 to August 2014. (a) Number of children with one or more unscheduled respiratory contact; and (b) mean number of unscheduled respiratory contacts per child.

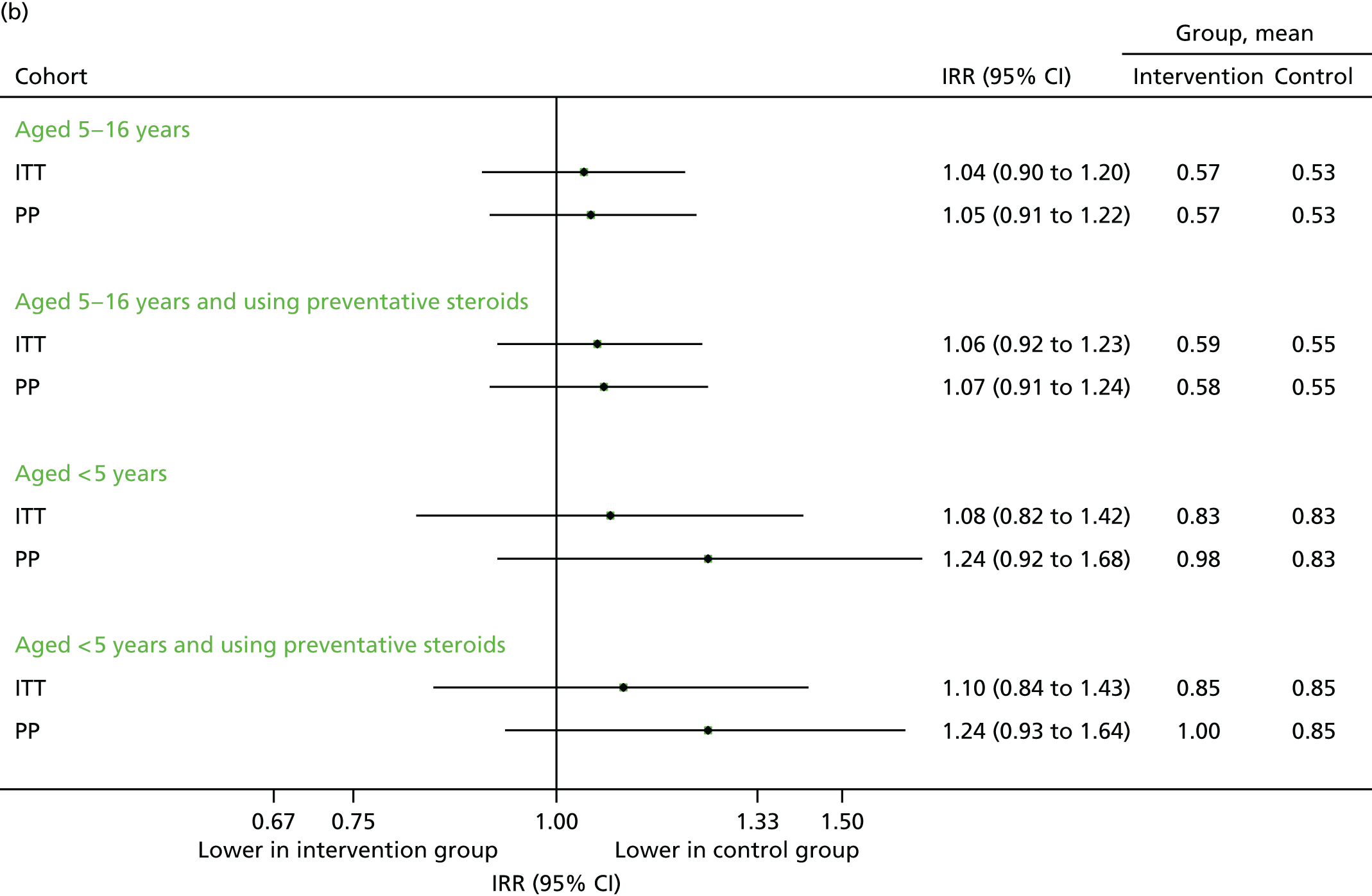
FIGURE 15.
All medical contacts from September 2013 to August 2014. (a) Number of children with one or more contact; and (b) mean number of contacts per child.


Echo substudy
The protocol was amended to include additional outcomes for the subsequent year. We refer to this as the ‘echo substudy’, the rationale of which was to assess whether or not any immediate intervention effect in 2013 was echoed the following year. A total of 110 practices (57 intervention and 53 control) contributed data to this time period.
In a survey of the practices in the intervention it was found that, of those that responded, 54% (13 out of 24 responding practices) had sent out the intervention again in 2014. 22 This would also contribute to an echo effect
Steroid prescriptions and scheduled contacts in the echo substudy (August 2014)
Although the increase in prescriptions found in 2013 was not as marked in 2014, the under-fives subgroup (i.e. children who were now aged under 6 years) did demonstrate an increase overall in terms of prescription uptake. These findings are displayed graphically in Figure 16. Unexpectedly, the proportion of children making at least one scheduled contact was lower in the intervention arm (Figure 17a). However, this association disappeared when evaluating the total number of scheduled medical contacts (see Figure 17b).
FIGURE 16.
Uptake of steroid inhaler prescriptions in August 2014. (a) Number of children with one or more prescriptions; and (b) mean number of prescriptions.
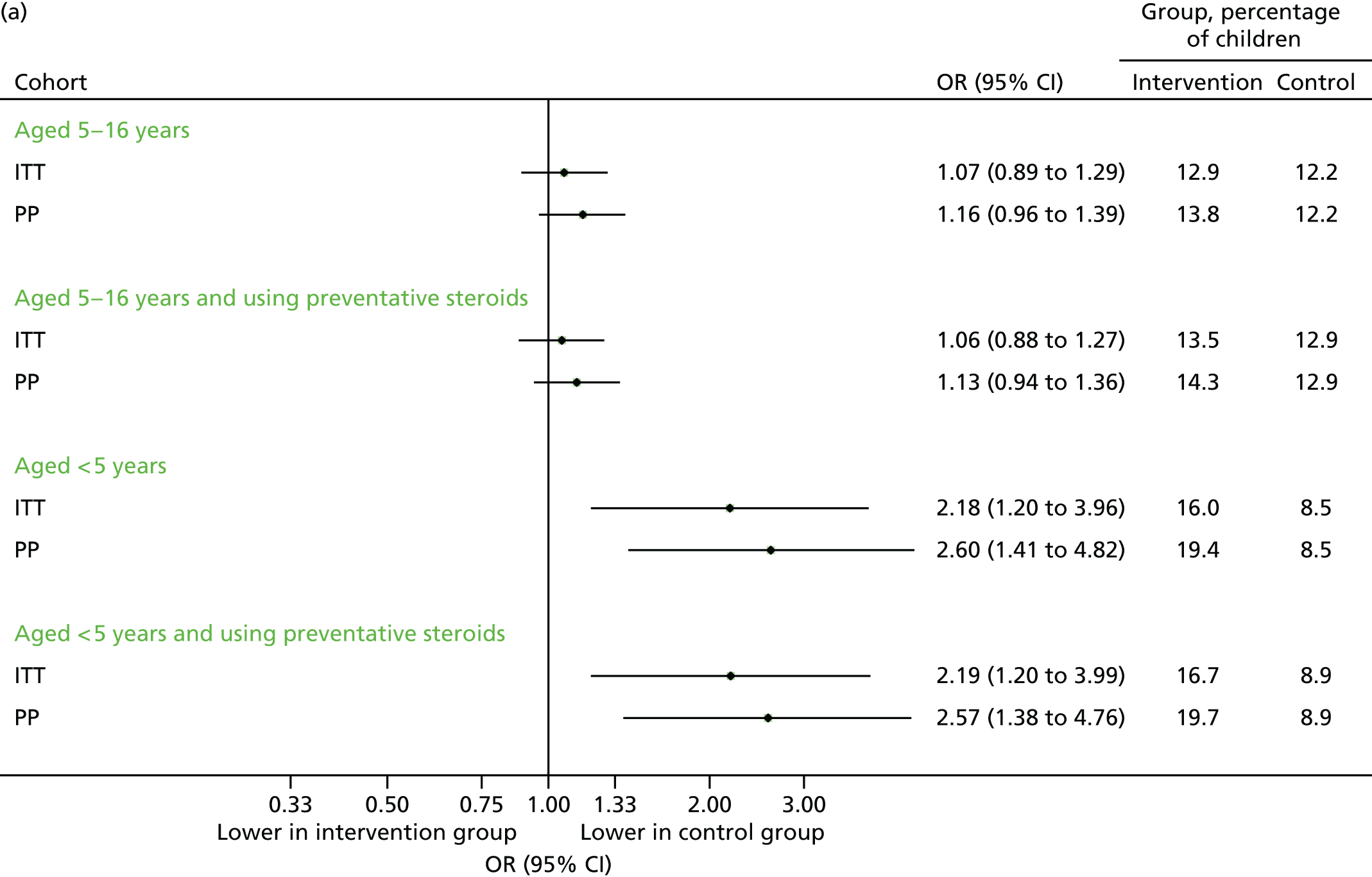
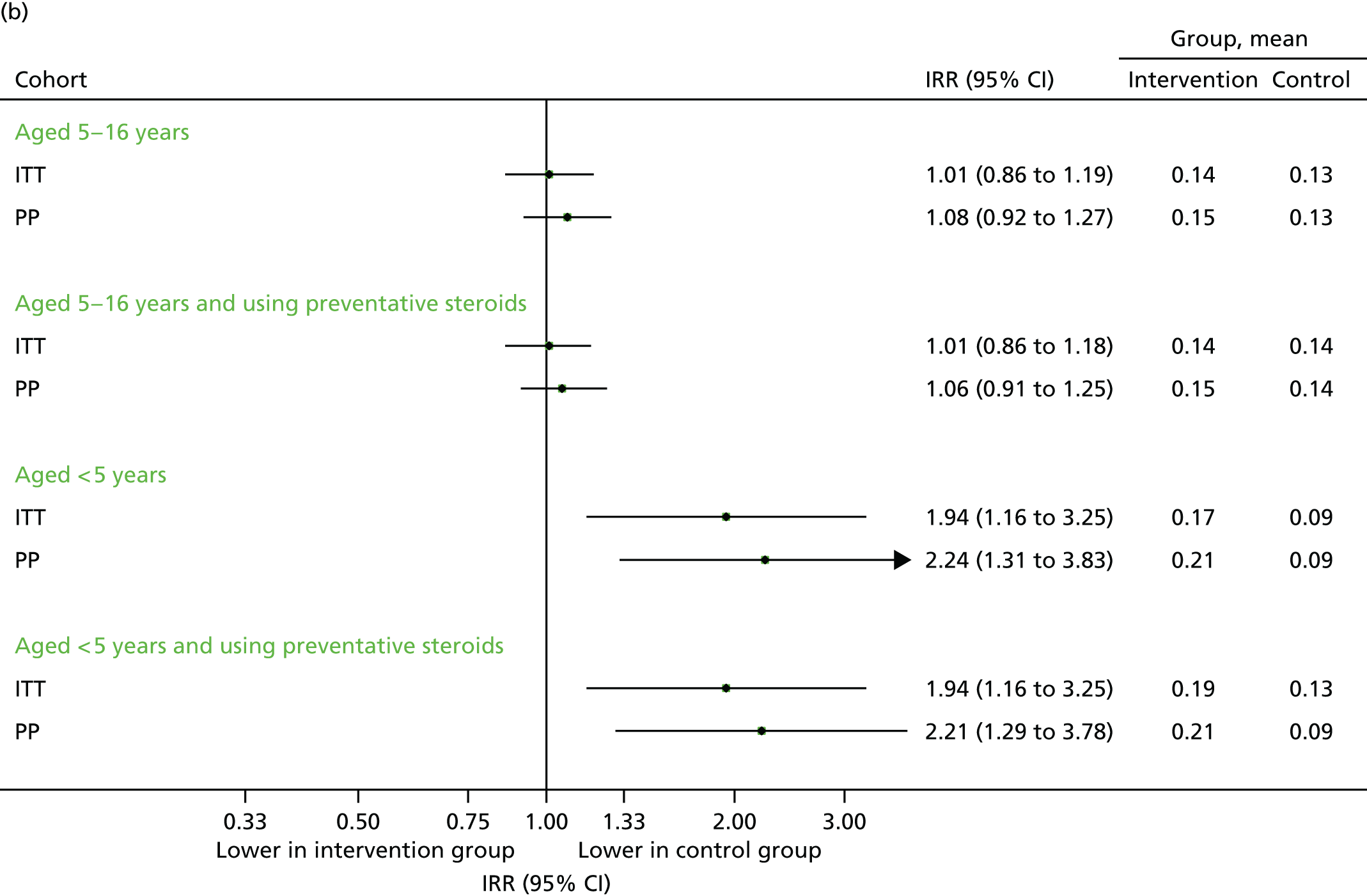
FIGURE 17.
Scheduled contacts in August 2014. (a) Number of children with one or more contact; and (b) number of contacts per child.
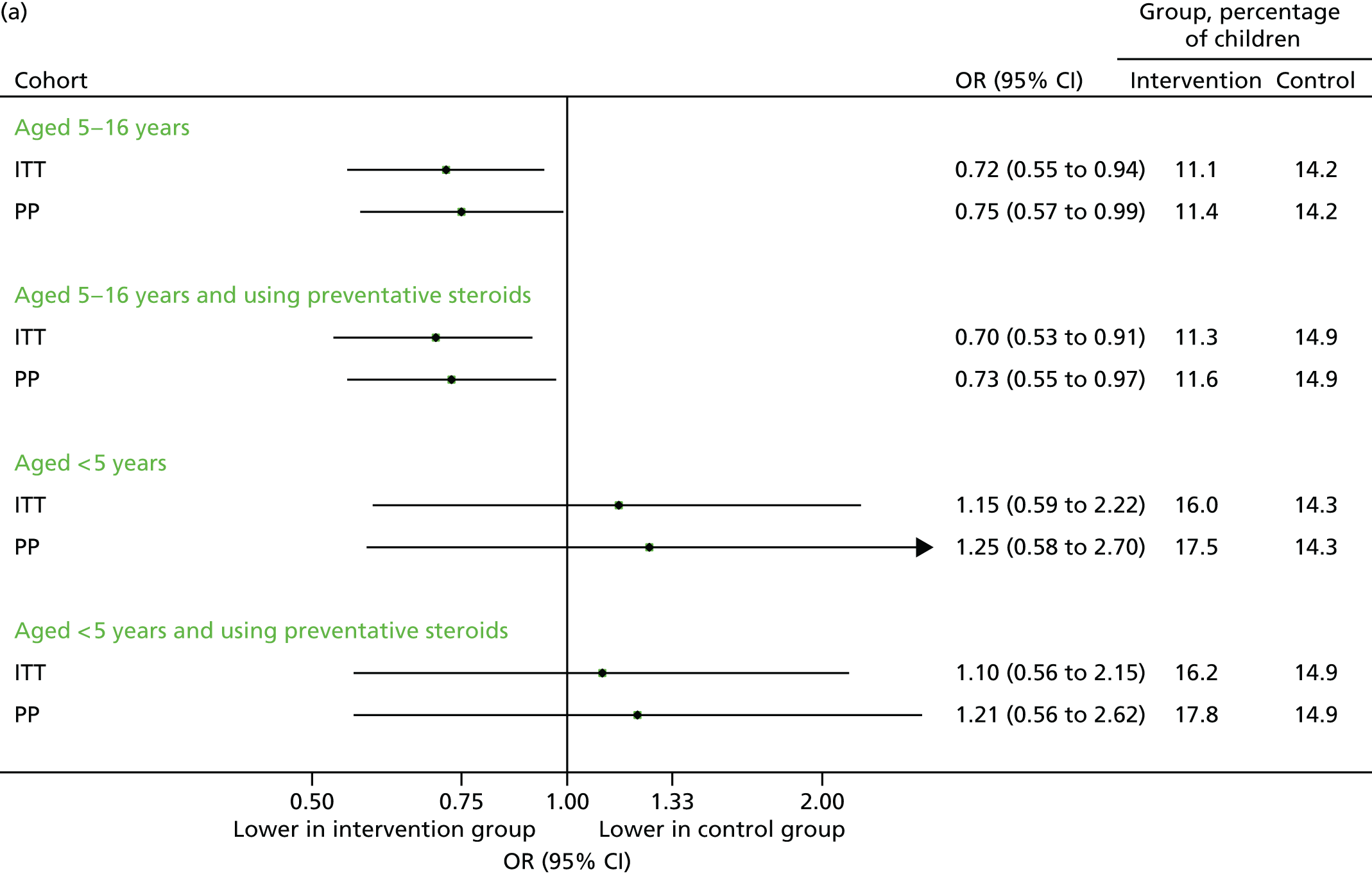
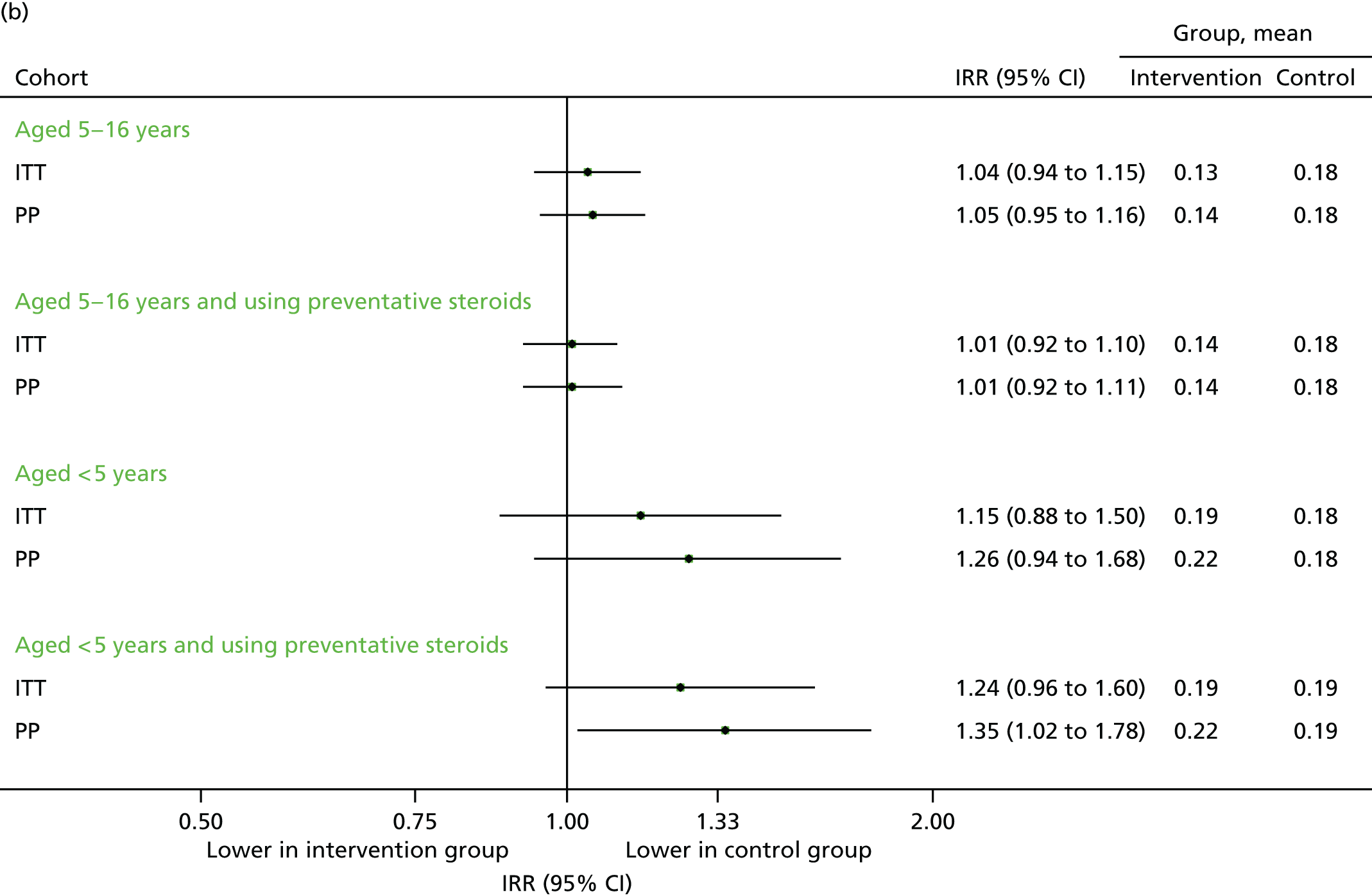
Contacts in the echo substudy (September 2014)
The findings were similar to those of September 2013. Unscheduled contacts, unscheduled respiratory-related contacts and scheduled contacts were all marginally higher in the intervention group (Figures 18–20), although the size of the difference was more modest than that observed in the previous year.
FIGURE 18.
Unscheduled medical contacts in September 2014. (a) Number of children with one or more unscheduled contact; and (b) mean number of unscheduled contacts per child.
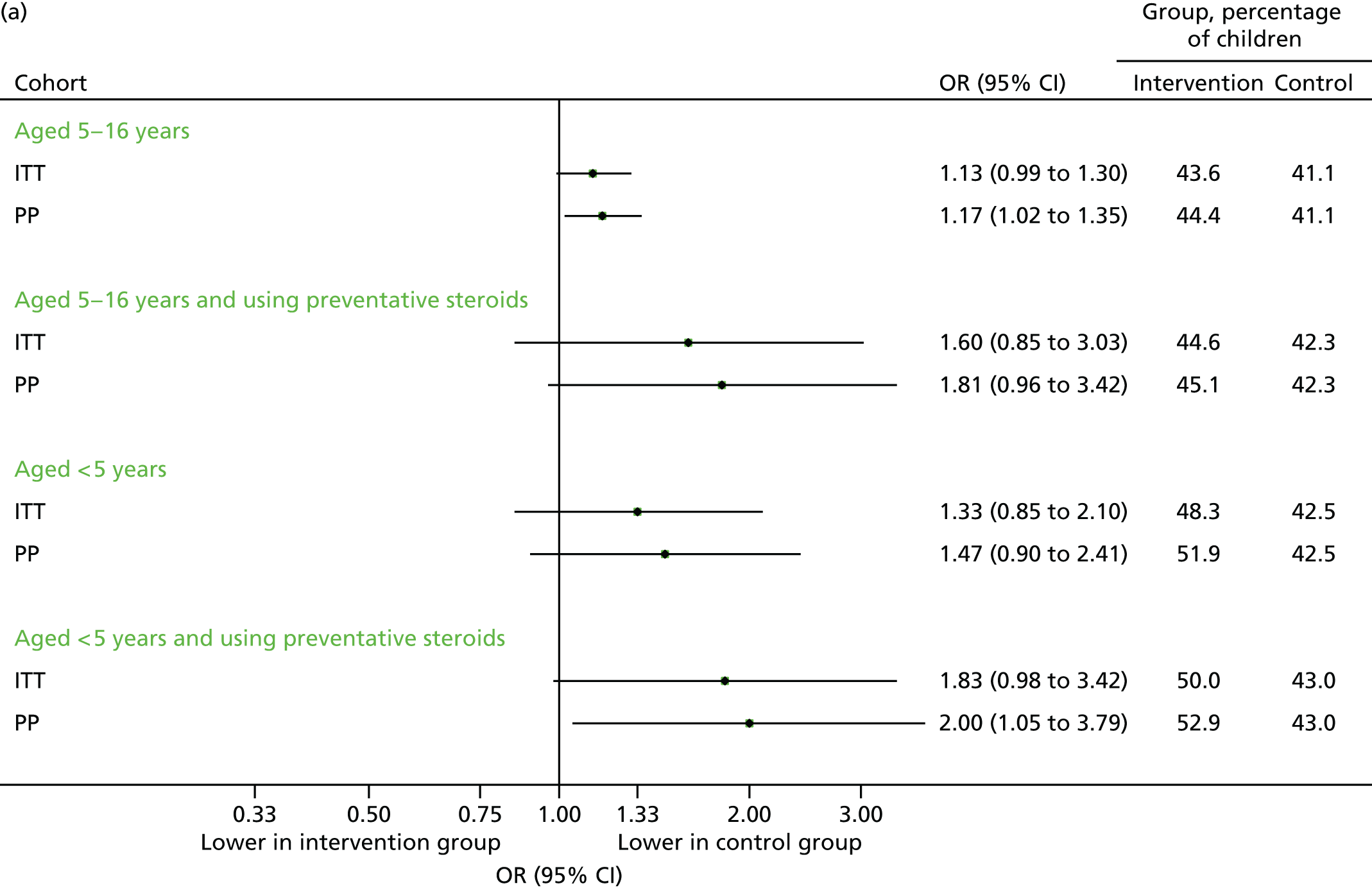
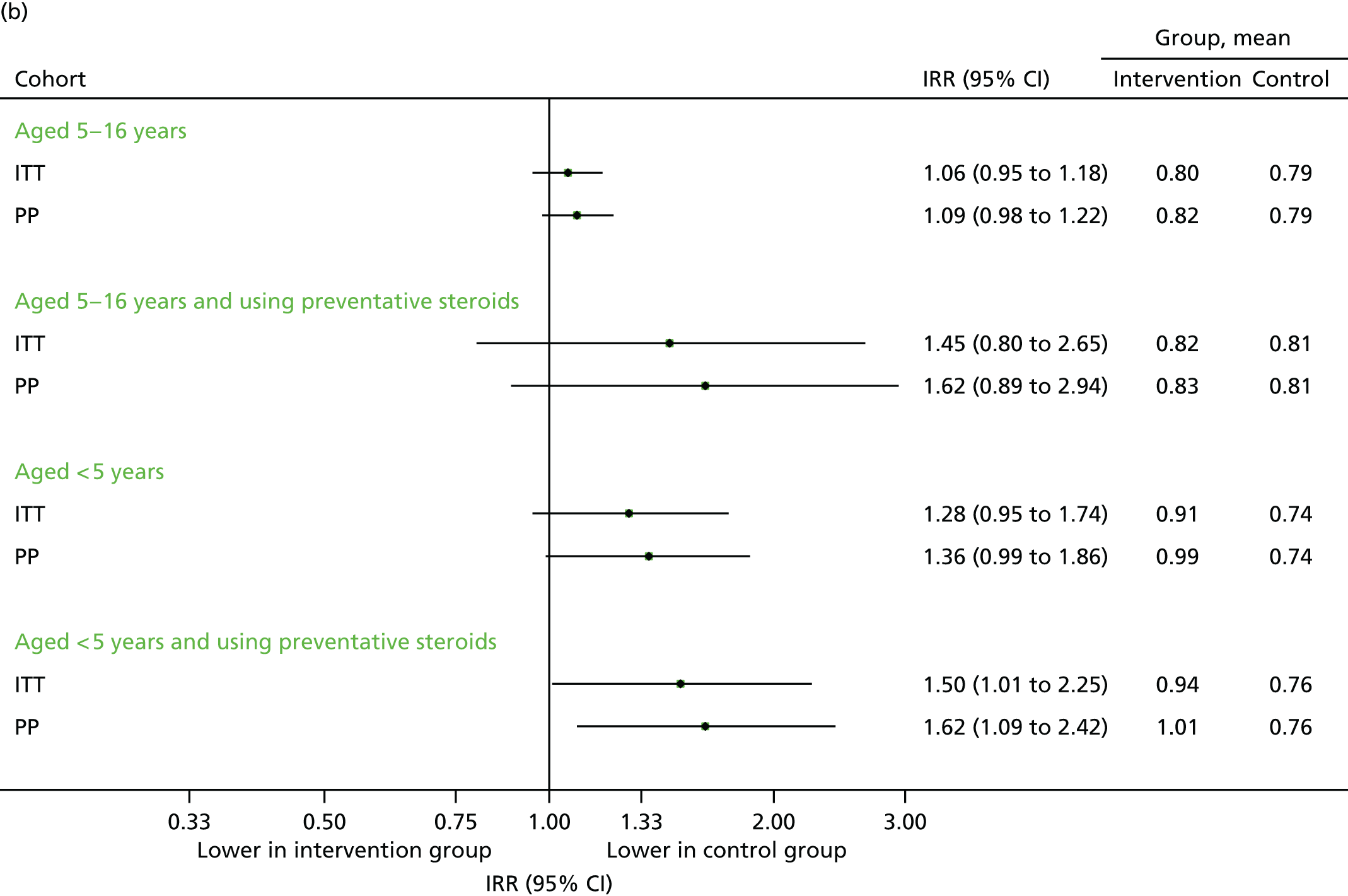
FIGURE 19.
Unscheduled respiratory-related medical contacts in September 2014. (a) Number of children with one or more unscheduled contact; and (b) mean number of unscheduled contacts per child.
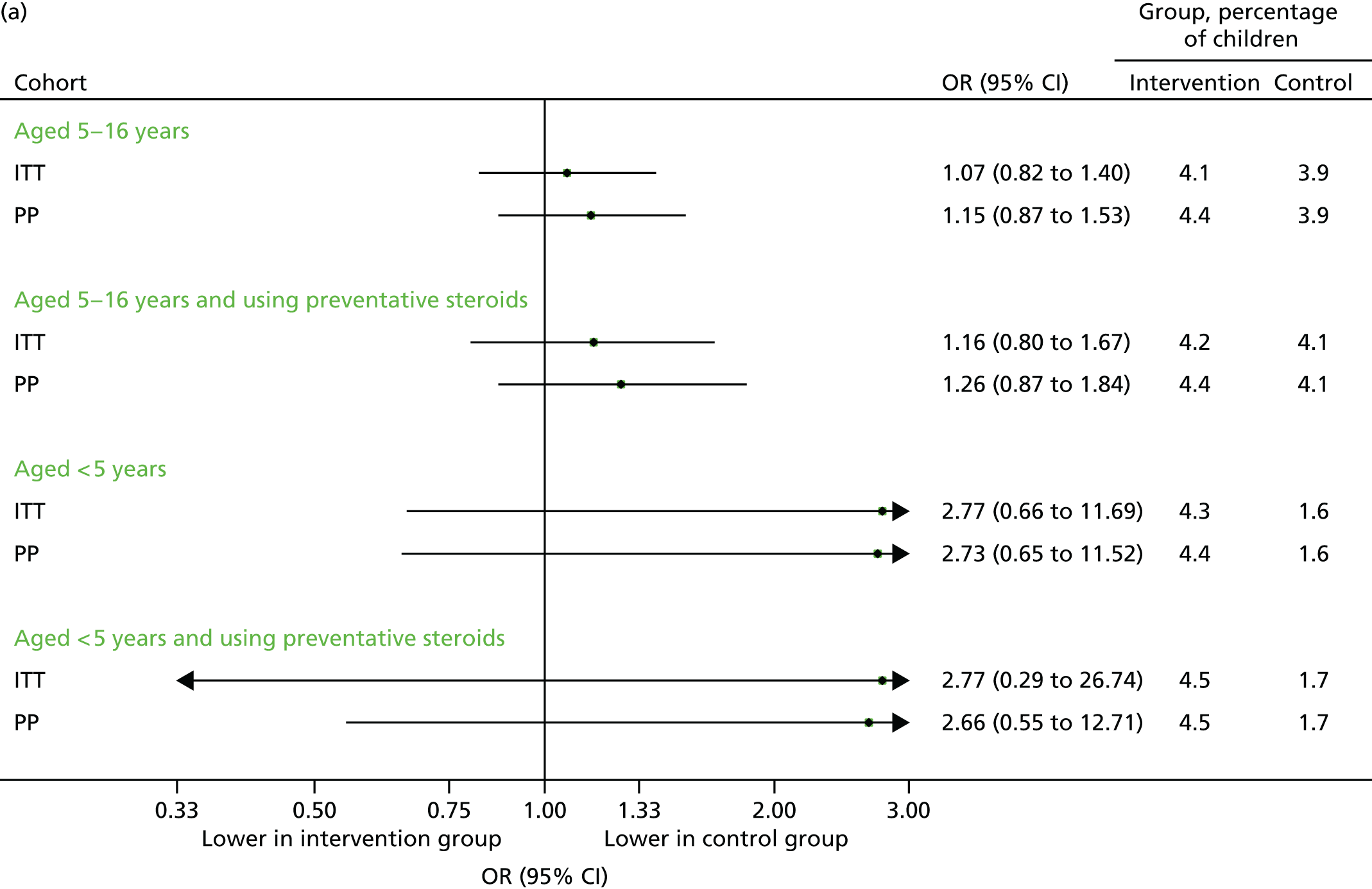
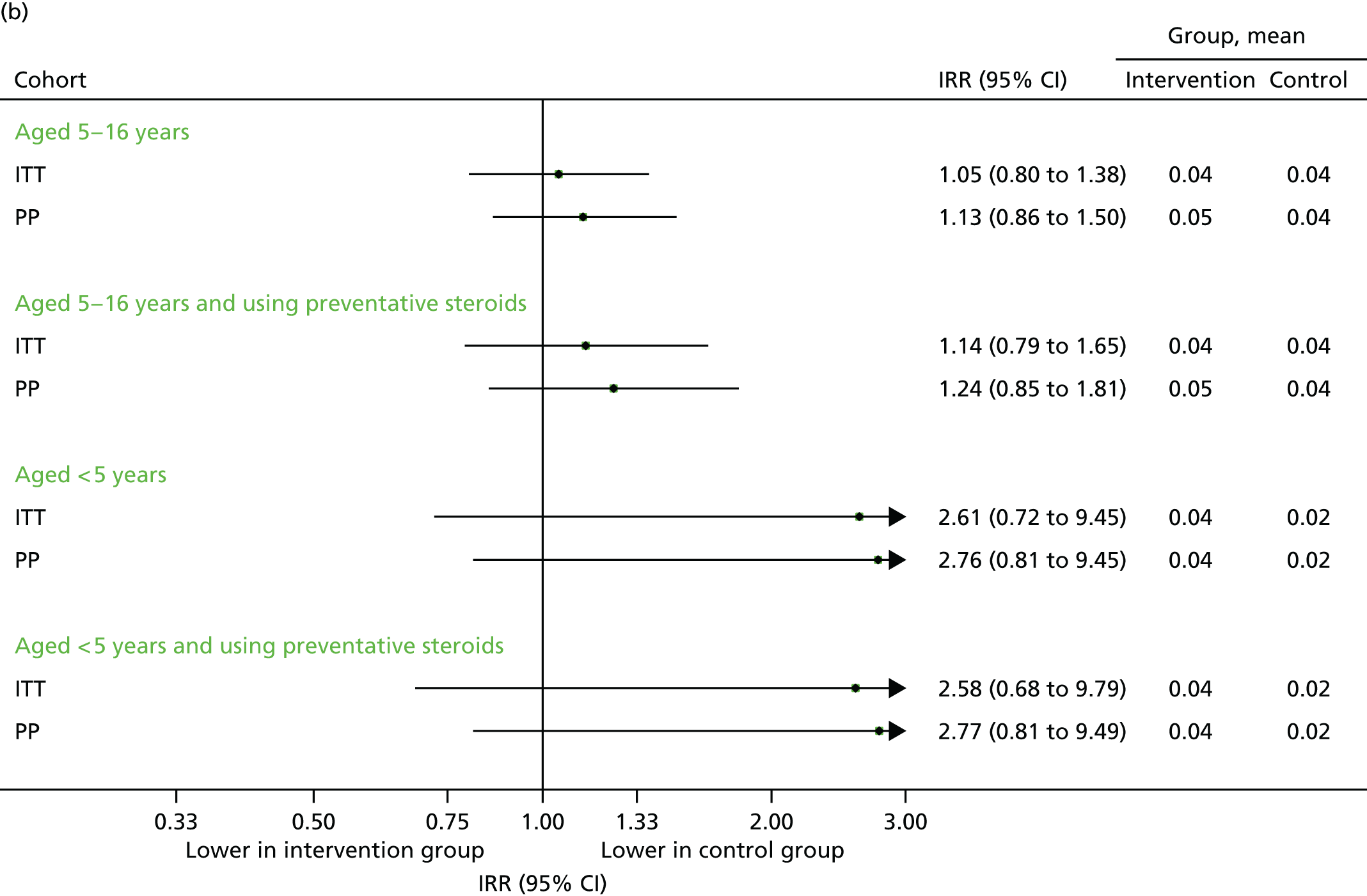
FIGURE 20.
All medical contacts in September 2014. (a) Number of children with one or more contact; and (b) mean number of contacts per child.

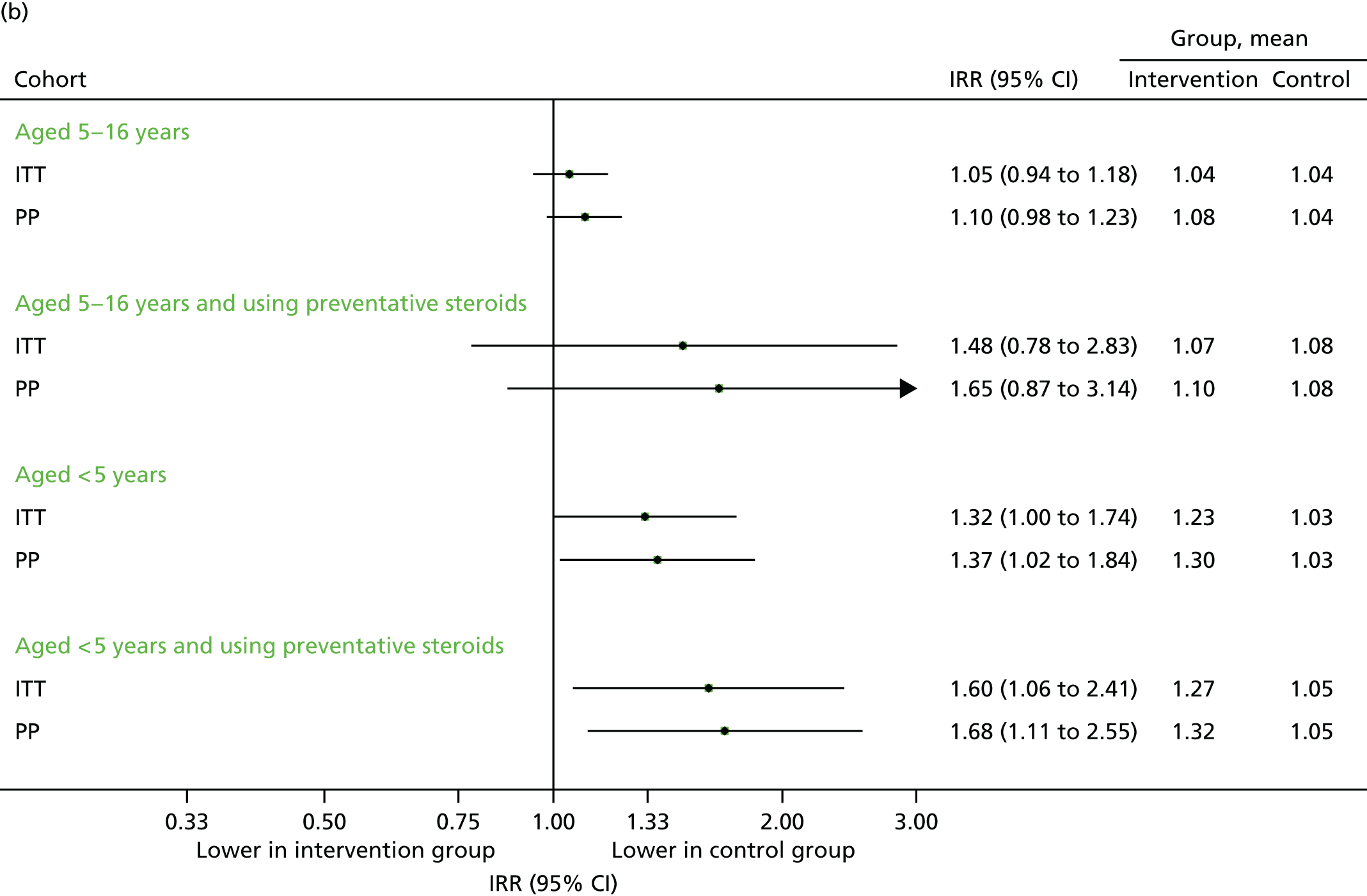
Time to first unscheduled contact
The time to first unscheduled and respiratory-related unscheduled contacts are presented in Figure 21 (September 2013) and Figure 22 (September–December 2013). Consistent with the number of contacts as previously demonstrated, the intervention group tended to make their first contact earlier than the control group. As the majority of contacts were unscheduled, the time to first contact of any type was similar to the time to first unscheduled contact.
FIGURE 21.
Time to first contact in September 2013. (a) First unscheduled contact; and (b) first respiratory-related unscheduled contact.
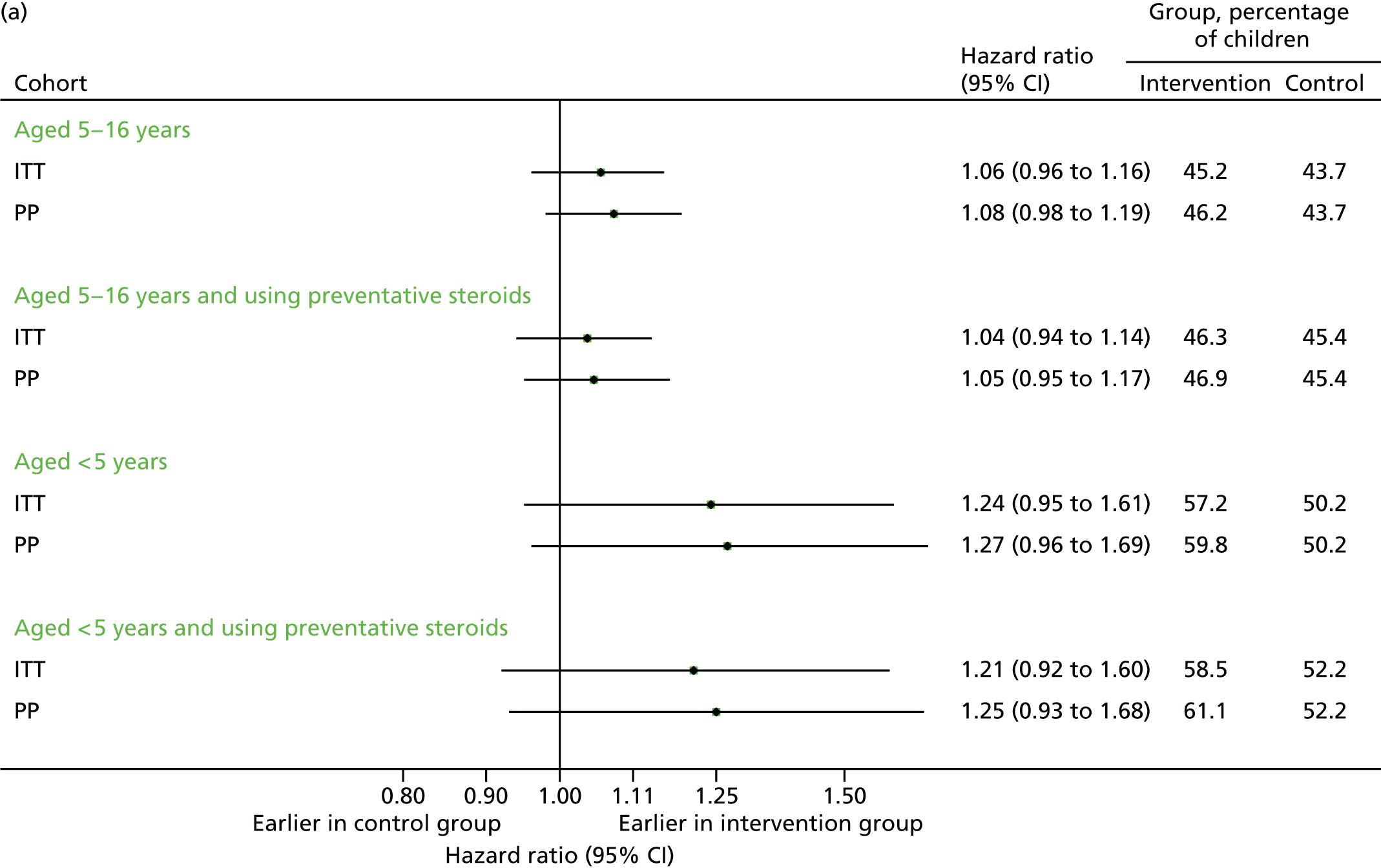
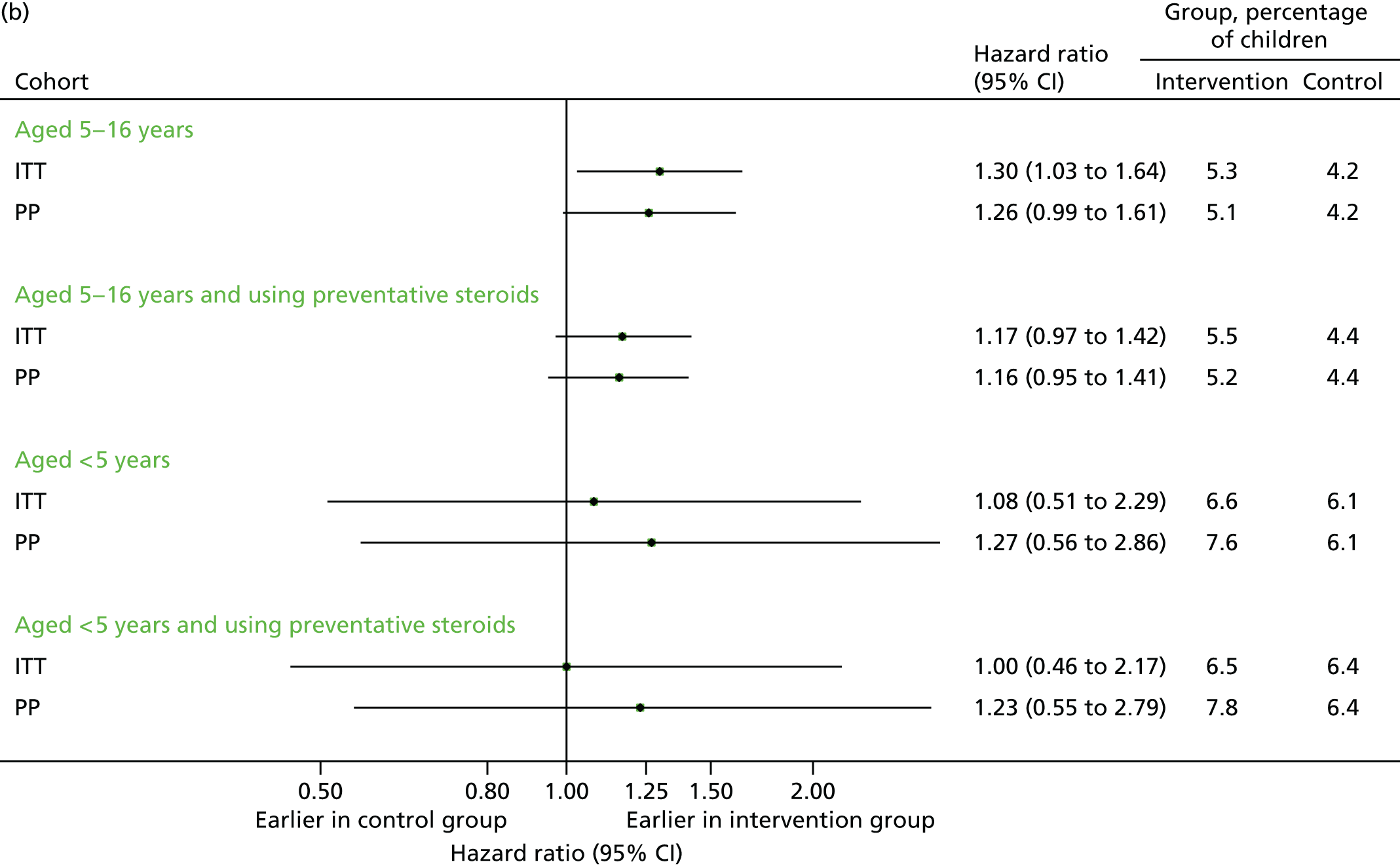
FIGURE 22.
Time to first contact in September–December 2013. (a) First unscheduled contact; and (b) first respiratory-related unscheduled contact.

Chapter 4 Health economics
Health economic methods
Background
Asthma exacerbations in school-aged children have the potential to result in a reduction in health-related quality of life (HRQoL) for the child and additional NHS resource use in either primary or secondary care if the child presents with symptoms of an acute exacerbation. As the aim of the letter is to reduce the incidence of asthma exacerbations associated with the new school term, the intervention has the potential to result in both an improvement in HRQoL for children and cost savings for the NHS. There is, however, an initial upfront cost of delivering the letter intervention, and those resources could be used to implement other initiatives within the NHS. It was therefore important to consider not only whether the intervention is clinically effective for the individual patient, but also whether or not adopting the letter intervention across the NHS would be a cost-effective use of resources.
Overview
An economic evaluation was undertaken to compare the incremental cost per quality-adjusted life-year (QALY) of the reminder letter compared with standard care. The population for the economic evaluation was defined as school-aged children with asthma who are registered with a GP in England or Wales, and therefore the analysis was based on the PLEASANT study population. As the primary outcome for the PLEASANT study was restricted to those children aged 5–16 years, we have used this age subgroup for the base-case cost-effectiveness analysis, and have done a subgroup analysis looking at children aged 4 years. The perspective of the analysis was that of the NHS and Personal Social Services (PSS), although, as the intervention is not expected to have any impact on PSS resource use, no costs for PSS were included in the analysis. Both primary and secondary care NHS costs were included. We considered the benefits, measured in QALYs, for the individual children included in the study and did not include any benefits falling on parents, carers or other family members. The time horizon was 1 year from the intervention and, therefore, no discounting was applied.
Unscheduled and scheduled contacts in the year following intervention were included in the economic analysis to capture any change in health-care resource use in response to the letter. Prescriptions in the year following the intervention for asthma medications used in the management of chronic asthma and asthma medications used in the treatment of acute exacerbations were included to establish if the cost of prescriptions had increased in response to the letter intervention. It was considered necessary to include costs in the year following intervention rather than just in the first 4 months to distinguish between an increase in the number of scheduled contacts and a change in the timing of the scheduled contacts.
The cost of the letter intervention was included for intervention practices with no cost included for practices in the control arm, as standard care was assumed to be the same in both intervention and control practices.
Measure of effectiveness for economic evaluation
Owing to the design of the trial, no data were collected directly from patients and, therefore, it was not possible to directly determine the number, severity or duration of any acute asthma exacerbations associated with the new school term. It was therefore necessary to estimate the number of asthma exacerbations experienced from the routine data collected by the CPRD. As a single exacerbation may be associated with more than one unscheduled contact, we needed to define the number of exacerbations based on the pattern of unscheduled contacts. To do this, we split the 4-month follow-up period into 1-week intervals, and assumed that the patient was having an exacerbation in any week that included an unscheduled contact of any type. The intervention was assumed not to have any impact on exacerbations after December.
Resource use
Data on the number and type of medical contacts in the intervention and control arms were collected through the CPRD. As patients may present with multiple problems at a single contact, and the reason for the contact is not always accurately coded, we have not restricted our analysis to respiratory-related contacts. Although not all scheduled contacts in children with asthma will be related to their asthma management, it is reasonable to expect that the number of contacts for other reasons will not differ between the trial arms. We have therefore assumed in our analysis that any difference in the number of scheduled or unscheduled contacts between the intervention and comparator arms is related to the intervention.
For primary care contacts, the staff mix and duration of staff contact for each type of primary care contact was estimated by clinical experts (clinicians on the TMG). For some unscheduled surgery visits and emergency consultations, the estimates of resource use were stratified according to the severity of acute exacerbation. The percentage having moderate, severe or life-threatening exacerbations was based on clinical opinion and is shown in Table 8. The resource use estimates for primary care contacts are summarised in Tables 9 and 10.
| Setting | Exacerbation type | Percentage |
|---|---|---|
| GP surgery | Moderate asthma | 70 |
| Severe asthma | 25 | |
| Life-threatening asthma | 5 | |
| Emergency department | Moderate asthma | 20 |
| Severe asthma | 50 | |
| Life-threatening asthma | 30 | |
| Hospital | Severe asthma | 60 |
| Life-threatening asthma | 40 |
| Scenario for exacerbation type | Staff (ratio) | Duration (minutes) | Costs | Weighted average of provider costa | Proportion of cases presented at surgery | |
|---|---|---|---|---|---|---|
| GP | Nurse | |||||
| (1) Moderate asthma | ||||||
| Contact with GP | PN or GP (20 : 80) | 15 | £58.50 | £13.25 | £49.45 | 0.70 |
| (2) Severe asthma | ||||||
| Cost components | 0.25 | |||||
| Seeing and diagnosing patients | GP | 5 | £19.50 | £19.50 | ||
| Administration of medications | PN or GP (10 : 1) | 15 | £58.50 | £13.25 | £17.36 | |
| Monitoring patient | PN or GP (10 : 1) | 5 | £19.50 | £4.42 | £5.79 | |
| Total cost | £42.65 | |||||
| (3) Life-threatening asthma | ||||||
| Cost components | ||||||
| Stabilise and monitor patient until ambulance arrives | PN and GP | 5 | £19.50 | £4.42 | £23.92 | 0.05 |
| Unit cost of an unscheduled visit | £46.47b | |||||
| Scenario for exacerbation type | Staff (ratio) | Range of duration (minutes) | Average duration (minutes) | Costs | Weighted average of provider cost | ||
|---|---|---|---|---|---|---|---|
| GPa | Nurseb | Administratorc | |||||
| Unscheduled clinic review | PN | 15–30 | 22.5 | – | £19.88 | – | £19.88 |
| Acute visit | GP | 10–15 | 12.5 | £48.75 | – | – | £48.75 |
| Third-party consultationd | NA | – | – | – | – | – | £212.00 |
| Unscheduled home visit | GP | 15–30 | 22.5 | £87.75 | – | – | £87.75 |
| Unscheduled phone consultation | PN : GP (10 : 1) | – | 5 | £19.50 | £4.42 | – | £5.79 |
| Scheduled phone consultation | PN : GP (10 : 1) | – | 2 | £7.80 | £1.77 | – | £2.32 |
| Scheduled surgery consultation | PN : GP (2 : 8) | 10–15 | 12.5 | £48.75 | £11.04 | – | £41.21 |
| Scheduled clinic review | PN | – | 30 | £26.50 | – | – | – |
| Medication management | PN : GP (25 : 75) | – | GP, 2; PN, 5 | £7.80 | £4.42 | – | £6.95 |
| Administration | Administrative staff | – | 0.5 | – | – | £0.11 | £0.11 |
| Results recording | Administrative staff | – | 0.5 | – | – | £0.11 | £0.11 |
Data were also obtained from the CPRD on the number of prescriptions for medications used in the management of chronic asthma and for medications used to treat acute exacerbations. A list of relevant drugs was prepared in consultation with clinical experts, and these are shown in Table 11. The list of antibiotics was restricted to those commonly used to treat respiratory infections associated with asthma exacerbations in children. Although some drugs are used in the management of both chronic and acute symptoms, they have been included in Table 11 under their primary use, but this has no implication for the cost-effectiveness analysis, as drugs used for both indications are included in the total cost.
| Drug class | Drug or unique combination of drugs | Average cost per prescriptiona (unit cost × mean number of units) |
|---|---|---|
| Drugs used primarily in the management of chronic asthmab | ||
| Inhaled beta-2 agonist | Salbutamolb | £2.33 |
| Salmeterol | £34.66 | |
| Formoterol fumarate | £27.16 | |
| Terbutaline | £8.64 | |
| Inhaled corticosteroids | Beclometasone dipropionate | £6.71 |
| Budesonide | £14.29 | |
| Fluticasone | £9.32 | |
| Leukotriene receptor antagonists | Montelukastc | £4.85 |
| Zafirlukast | £20.80 | |
| Theophylline | Modified-release oral theophylline (aminophylline/theophylline) | £5.42 |
| Cromoglicic acid and related therapy | Sodium cromoglycate | Not prescribed within the data set |
| Nedocromil sodium | £34.94 | |
| Combination inhalers | Beclometasone dipropionate/formoterol fumarate dihydrate | £32.25 |
| Budesonide/formoterol fumarate dihydrate | £44.27 | |
| Fluticasone propionate/formoterol fumarate | £30.48 | |
| Fluticasone propionate/salmeterol xinafoate | £32.64 | |
| Drugs used primarily in the management of acute asthma exacerbations | ||
| Antimuscarinic bronchodilators | Ipratropium bromide | £6.43 |
| Oral corticosteroidsd | Oral prednisolone | £26.68 |
| Drugs used in the treatment of respiratory infections associated with asthma exacerbations | ||
| Broad-spectrum penicillins | Amoxicillin | £1.40 |
| Co-amoxiclav | £4.85 | |
| Macrolides | Clarithromycin | £12.95 |
| Erythromycin | £7.61 | |
| Cephalosporins | Cefaclor | £5.68 |
| Cefradine | Not prescribed within the data set | |
| Cefalexin | £2.22 | |
Information on the staff time required to deliver the letter intervention was based on the survey of participating practices,22 which included questions regarding the staff members involved and the duration of time required to complete the various tasks necessary to deliver the letter intervention across the eligible population within a single practice. Resource-use data from the survey are summarised in Table 12. The survey was undertaken as part of a no-cost extension of the trial (see Appendix 2).
| Activity, cost or staff member | Staff | Average cost | |||||
|---|---|---|---|---|---|---|---|
| Practice manager | Administrative staff | GP | Practice nurse | Research nurse | Per practicea | Per patienta | |
| Unit cost per hour for different staff membersb | £30.42 | £14.15 | £80.00 | £0.42 | £30.42 | – | – |
| Resource use and average cost per task | |||||||
| Database search | |||||||
| Time per practice (minutes) | 40 | 29 | 38 | – | – | – | – |
| Percentage of staff involved (%) | 38 | 46 | 17 | – | – | – | – |
| Weighted mean cost across staff | £19.03 | £0.22 | |||||
| Checklist generated by search | |||||||
| Mean time per 10 patients (minutes) | 6 | 9 | 6 | 7 | 10 | – | – |
| Percentage of staff involved (%) | 30 | 13 | 39 | 13 | 4 | – | – |
| Weighted mean cost across staff | £44.31 | £0.51 | |||||
| Mail-out process | |||||||
| Mail-out by DocMail | |||||||
| Time per practice (minutes) | 36 | 26 | – | – | – | – | – |
| Percentage of staff involved (%) | 47 | 53 | – | – | – | – | – |
| Weighted mean cost across staff types | £11.92 | £0.14 | |||||
| Mail-out by other process | |||||||
| Mean time per 10 patients (minutes) | 15 | 13 | – | – | – | – | – |
| Percentage of staff involved (%) | 14 | 86 | – | – | – | – | – |
| Weighted mean cost across staff | £31.21 | £0.36 | |||||
| DocMail cost per letter/cost of postage plus materials for other mail-out process | £33.69 | £0.39 | |||||
| Average across DocMail and other mail-out processes (67% DocMail and 33% other) | £18.34 | £0.21 | |||||
| Total | £115.38 | £1.34 | |||||
Unit costs
For primary care contacts, the unit costs for scheduled and unscheduled patient contacts were taken from Unit Costs of Health and Social Care 2014,30 and these are summarised in Tables 9 and 10. The Department of Health’s reference costs32 were used for secondary care contacts, and these are summarised in Tables 13 and 14. Drug costs were taken from the British National Formulary for Children August 2015. 33 The average cost per prescription (see Table 11) was calculated by combining data on the pack size (e.g. inhaler containing 200 doses), the number of doses prescribed (e.g. 200 inhalations or one inhaler) and the unit cost for each item. When more than 10 different preparations of the same drug were prescribed across the whole data set, we have applied the average unit cost, weighted by frequency, from the 10 most commonly prescribed preparations. Unit costs for the staff time associated with delivering the intervention were based on the national costing template from the NIHR Primary Care Research Network’s Clinical Research Network Industry Costing Template. 31 Unit costs for materials and postage were based on commercial costs for DocMail. 34 The total average cost per patient was estimated by combining data on resource use, with the unit costs as shown in Table 12.
| Exacerbation type | Code | Healthcare Resource Group | Investigations | Treatment | Unit costa |
|---|---|---|---|---|---|
| Moderate asthma presenting to emergency department | VB09Z | Emergency Medicine, category 1 investigation with category 1–2 treatment (type 1 non-admitted) | None | i.v. cannula, guidance advice, inhalers, oral prednisolone | £102 |
| Severe asthma presenting to emergency department | VB06Z | Emergency medicine, category 1 investigation with category 3–4 treatment (type 1 non-admitted) | Capillary blood gas | Administration of drug via spacer or nebuliser, supplemental oxygen, oral prednisolone | £128 |
| Life-threatening asthma presenting to emergency department | VB43Z | Emergency medicine, category 2 investigation with category 4 treatment (type 1 non-admitted) | Capillary blood gas, chest radiography | Nebulisation, guidance advice, vital signs monitoring, radiograph review, CPAP, supplemental oxygen, administration of infusion or subcutaneous drug | £224 |
| Weighted average for all emergency department attendances | £152 | ||||
| Reference cost details | Frequencya | Unit costb |
|---|---|---|
| PD12C: Non-elective short stay: paediatric asthma or wheezing, with a CC score of 0 | 15,159 | £559 |
| PD12B: Non-elective short stay: paediatric asthma or wheezing, with a CC score of 1–3 | 8390 | £579 |
| PD12A: Non-elective short stay: paediatric asthma or wheezing, with a CC score of ≥ 4 | 392 | £572 |
| Weighted average across all non-elective short-stay admissions | £566 | |
Health outcomes
Owing to the design of the trial, no data were collected directly from patients and, therefore, it was not possible to obtain direct estimates of health utility for the enrolled patients. Instead, these were obtained indirectly from the estimated number of asthma exacerbations. Children experiencing an exacerbation were assumed to have a utility decrement for the week of exacerbation, which was defined as any week containing one or more unscheduled contacts.
A systematic review of HRQoL data in children with asthma was conducted to identify appropriate sources of data for patients with and without an exacerbation. Selection of utility data to use in the economic analysis was based on (1) quality of the study, (2) the relevance of utility data to the population and health states in the PLEASANT study and (3) the extent to which the measurement method was in accordance with the National Institute for Health and Care Excellence (NICE) reference case.
The systematic review found that there was a lack of relevant and high-quality data on the impact of exacerbations on HRQoL for school-aged children. Many of the studies in children did not estimate the impact of exacerbation or used a less suitable measure of HRQoL, such as direct valuation or subjective mapping from disease states to HRQoL. However, data were available for adults on the impact of exacerbations estimated using NICE’s preferred instrument, the European Quality of Life-5 Dimensions (EQ-5D). 35 We considered that the estimates for exacerbations in adults may underestimate the degree of utility loss in children with a severe or life-threatening acute exacerbation during the period of hospitalisation, so alternative estimates that were considered to provide an upper limit on the utility decrement attributable to exacerbation in children were explored in a sensitivity analysis. The systematic review and rationale for selection of model inputs is further detailed in Appendix 5 and the data applied in the model are summarised in Table 15.
| Health state | Health utility value | Description of state from source study | Measurement | Source |
|---|---|---|---|---|
| Base-case scenario | ||||
| No exacerbation | 0.96 (SD 0.07) | Average baseline utility across children (n = 27) aged 7–18 years with GINA severity stage I to III receiving standard outpatient care in the Netherlands as part of the control arm of a RCT | EQ-5D child version (filled out by parent for children aged < 12 years). UK adult TTO valuation set | Willems et al.36 |
| Exacerbation not requiring hospitalisation (including those managed in the emergency department) | –0.10 relative to no exacerbation | Adult patients enrolled in a prospective observational study who have moderate or severe asthma (BTS/SIGN stage 4/5) at baseline and who have experienced one exacerbation requiring oral steroid treatment (without hospitalisation) in the previous 4 weeks (n = 22) | EQ-5D UK adult valuation set | LLoyd et al.37 |
| Exacerbation requiring hospitalisation | –0.20 relative to no exacerbation | Adult patients enrolled in a prospective observational study who have moderate or severe asthma (BTS/SIGN stage 4/5) at baseline who have experienced one exacerbation requiring hospitalisation in the previous 4 weeks (n = 5) | EQ-5D UK adult valuation set | LLoyd et al.37 |
| Sensitivity analysis | ||||
| No exacerbation | As per base case | As per base case | As per base case | As per base case |
| Any exacerbation | –0.216 relative to no exacerbation | Patients aged over 12 years (including adults) enrolled in the GOAL study38 who experienced an exacerbation (defined as deterioration in asthma requiring treatment with an oral corticosteroid, or an emergency department visit or hospitalisation) | AQLQ values mapped to EQ-5D (valuation set not stated) | Briggs et al.38 |
The QALYs accrued per patient in the 4 months following the intervention (from 1 September to 31 December) were estimated using the area under the curve method. We assumed that the intervention would have no impact on QALYs gained outside this 4-month period. We have also assumed that the intervention had no impact on mortality.
Analysis
The health economics analysis was restricted to the PP patient group for whom CPRD data were available over the period of analysis (1 August 2012 to 31 July 2014). The PP patient group was used, as these are the patients who actually received the intervention and for whom resource use information was available within the CPRD for the time period of interest and, therefore, this group better predicts the costs and benefits of implementing the intervention in clinical practice. The average cost per patient was calculated by combining the resource use estimates with unit costs. This resource use is based on all ‘tasks’ recorded in the CPRD data set, which could be face-to-face contacts (surgery visits), non-face-to-face contacts (e.g. telephone calls) or general administrative tasks (i.e. recording letters sent to the surgery). In addition to reporting the average cost per patient, we have also reported the breakdown of costs of intervention costs, scheduled contacts, unscheduled contacts, prescriptions for chronic asthma management and treatment of acute exacerbations. This breakdown is reported by mean value and distribution statistics [for number of tasks, the mean, standard deviation (SD) and range are reported; for costs, the mean, 95% CIs, median and range are reported]. The mean number of acute exacerbations per patient has been reported in addition to the average QALYs accrued per patient. A statistically significant difference in resource use or costs was assessed by using the t-test assuming unequal variance (because of the unequal sample sizes between the letter intervention and no-letter groups). Statistical significance is defined as a p-value below 0.05 when describing statistical significance associated with 95% CIs for the outcomes of the economic evaluation. We have defined statistical significance as p < 0.1 when assessing baseline imbalances.
Quality-adjusted life-years were calculated for the 4-month post-intervention time period, and costs were calculated for 1 year post intervention. Utilities were assigned using a Markov assumption, whereby the 4-month post-intervention time period was split into 17 1-week time periods and one 3-day period (accounting for the 122 days from 1 September to 31 December). It was assumed that, if an unscheduled contact occurred within any particular week, a utility decrement for exacerbation was applied dependent on the type of contact (see Table 16). The utility decrement for an exacerbation resulting in hospitalisation overruled a non-hospital-related exacerbation utility value such that the most severe utility decrement for a given exacerbation week was applied to the whole week. QALYs were calculated using the area under the curve method39 based on the assumed exacerbation time period using Equation 1:
where u is the utility score, i denotes an individual and t is time so that at baseline t = 0. For each group j [j = 0 for the no-letter group (control) and j = 1 for the letter group (intervention)], the consecutive time measures are added, averaged and then rescaled (δ) for the percentage of a year that t and t – 1 cover, which in this case was 0.019 (7 days in a week divided by 365.25 days in year) for the 17 1-week time periods and 0.008 (3 days divided by 365.25 days in year) for the last 3 days in December. The total QALYs (Q) for the 4-month estimation period (T) was the summation of the utility values at all 18 time points starting at t = 1, the first week period, such that
Patient costs were adjusted by 1-year baseline costs [baseline adjusted (BA)] using bootstrapped ordinary least squares regression models (1000 replications) with 1-year baseline costs and intervention group as covariates in the model, as recommended by van Asselt et al. 40 Non-parametric bootstrapped estimation was employed for unadjusted patient costs and QALYs, also using 1000 replications. Clustering at the practice level with random effects was accounted for in the bootstrapped analysis. Unadjusted (observed) and adjusted (BA, accounting for baseline costs) results are reported for mean and incremental values as well as the bootstrapped standard error (SE), and bias corrected and accelerated (BCa) 95% CIs41 for all post-bootstrap estimations. For the BA mean cost estimations (not the BA incremental results), the reported SE are the delta method SEs, which are appropriate for adjusted/transformed cost approximations,42 and normal 95% CIs. The main structural uncertainty analyses in reference to sensitivity analysis and subgroup analysis are summarised in Table 16.
| Model aspect varied | Base-case scenario | Sensitivity scenarios | Rationale |
|---|---|---|---|
| Unit cost for contact types defined as ‘other’ | Unit cost of £0.11, assuming that ‘other’ are undefined administrative tasks | Pooled weighted unit cost of £45.58 based on the recorded resource use for all contacts and associated unit costs excluding ‘other’ tasks | It is uncertain if these ‘other’ consultation types are administrative |
| Duration of period used to define exacerbation | 1 week | 3 days 2 weeks | The average duration of symptoms for an exacerbation is uncertain |
| Utility values for exacerbation | –0.1 for non-hospital and –0.2 for hospitalisation following exacerbation | –0.216 relative to no exacerbation | The utility decrement relative to no exacerbation is uncertain |
| Type of contacts included | All contacts regardless of whether or not they are respiratory related | Respiratory-related contacts | Contacts coded as respiratory related are more likely to be affected by the intervention but a large proportion of contacts could not be coded as respiratory or non-respiratory related |
| QALY and cost estimation period | QALYs estimated for 4 months and costs for 1 year post intervention | QALY estimated for 4 months and costs for 5 months post intervention | To assess the shorter-term (5-month) cost implications of the intervention |
| Age of population receiving intervention | Children aged 5–16 years | Children aged < 5 years | To assess the cost-effectiveness of the intervention for children aged < 5 years |
Health economic results
Descriptive statistics of number of exacerbations, resource use and unit costs
A total of 8190 patients (letter group, n = 3641; no-letter group, n = 4549) were defined as being a part of the PP patient group and had data available over the analysis period of 1 August 2012 until 31 July 2014; it is these 8190 patients who were the focus of the health economic analysis. A summary of the number of exacerbations per patient, as well as resource use and associated costs per patient by classified resource use type (i.e. scheduled or unscheduled), prescription costs and overall costs, is presented in Table 17. A detailed summary of patient resource use and associated costs by task, as reported in the CPRD data set (reported in alphabetical order based on the task type), is presented in Table 18 (A–H; i.e. ‘acute visit’ to ‘hospital admission’) Table 19 (L–O; i.e. ‘letters from outpatient’ to ‘out-of-hours practice’) and Table 20 (R–W; i.e. ‘radiology result’ to ‘walk-in centre’).
| Variable and statistics | Variable type | Group | Significant difference (p-value) | |
|---|---|---|---|---|
| Intervention | Control | |||
| Mean number of exacerbations,a mean (SD), range | Number of exacerbations when exacerbation period was 1 week | 2.50 (2.19), 0–14 | 2.41 (2.19), 0–16 | 0.078 |
| Number of tasks,b mean (SD), range | Scheduled tasks | 2.60 (2.72), 0–22 | 2.69 (2.85), 0–30 | 0.130 |
| Unscheduled tasks | 9.39 (8.32), 0–73 | 9.36 (9.22), 0–101 | 0.867 | |
| ‘Not relevant’ tasks | 4.17 (4.79), 0–45 | 3.75 (4.73), 0–60 | < 0.001 | |
| Total number of tasksc | 16.16 (13.30), 0–120 | 15.80 (14.42), 0–163 | 0.249 | |
| Mean costs, mean (95% CI), median (range) | Scheduled tasks | £169 (£158 to £181), £27 (£0–3857) | £173 (£163 to £183), £41 (£0–3839) | 0.623 |
| Unscheduled tasks | £266 (£255 to 277), £146 (£0–4661) | £283 (£272 to 294), £186 (£0–8010) | 0.030 | |
| ‘Not relevant’ tasks | £204 (£191 to £217), £1 (£0–£6149) | £205 (£193 to £218), £1 (£0–£7675) | 0.915 | |
| Total task costd | £639 (£612 to £667), £342 (£0–£8829) | £662 (£636 to £688), £358 (£0–13,411) | 0.251 | |
| Prescriptions | £55 (£52 to £59), £20 (£0–849) | £49 (£47 to £52), £16 (£0–789) | 0.003 | |
| Total task and prescription coste | £695 (£666 to £723), £402 (£0–8921) | £711 (£684 to £738), £412 (£0–13,484) | 0.420 | |
| Intervention | £1.34 | £0 | – | |
| Overall resource use costf | £696 (£668 to 3725), £403 (£1–8922) | £711 (£684 to £738), £412 (£0–13,484) | 0.460 | |
| Resource use (n = 8190) | Group | Statistical significant difference in resource use (and costsa) between intervention groups (p-value) | |||||
|---|---|---|---|---|---|---|---|
| Intervention (n = 3641) | Control (n = 4549) | ||||||
| Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (95% CI; median) | Mean cost per resource user (95% CI; median, range) | Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (95% CI; median) | Mean cost per resource user (95% CI; median, range) | ||
| Acute visit | 7 (0.19), 1 (0, 1–1) | £0.09 (£0.02 to £0.16; £0) | £48.75 (£48.75 to £48.75; £48.75, £48.75–48.75) | 13 (0.29), 1 (0, 1–1) | £0.14 (£0.06 to £0.21; £0) | £48.75 (£48.75 to £48.75; £48.75, £48.75–48.75) | 0.384 |
| Administration | 3078 (84.54), 4.7 (4.35, 1–52) | £0.44 (£0.42 to £0.45; £0.33) | £0.52 (£0.50 to £0.53; £0.39, £0.11–5.72) | 3565 (78.37), 4.25 (4.19, 1–86) | £0.37 (£0.35 to £0.38; 0.22) | £0.47 (£0.45 to £0.48; £0.33, £0.11–9.46) | < 0.001 |
| Casualty attendance | 51 (1.4), 1.33 (0.77, 1–4) | £2.84 (£1.95 to £3.73; £0) | £202.67 (£169.92 to £235.41; £152.00, £152.00–608.00) | 6 (0.13), 1.5 (0.55, 1–2) | £0.30 (£0.05 to £0.55; £0) | £228.00 (£140.63 to £315.37; £228.00, £152.00–304.00) | < 0.001 |
| Children’s home visit | 0 (0), . (.,.–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 1 (0.02), 1 (., 1–1) | £0.02 (–£0.02 to £0.06; £0) | £87.75 (.–.; £87.75; £87.75–87.75) | 0.317 |
| Clinic | 804 (22.08), 2.24 (1.73, 1–22) | £9.83 (£9.03 to £10.62; £0) | £44.51 (£42.13 to £46.89; £39.76, £19.88–£437.36) | 851 (18.71), 1.9 (1.44, 1–16) | £7.07 (£6.51 to £7.63; £0) | £37.77 (£35.85 to £39.70; £19.88, £19.88–318.08) | < 0.001 |
| Co-op surgery consultation | 0 (0), . (.,.–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 40 (0.88), 1.48 (0.85, 1–5) | £2.75 (£1.77 to £3.73; £0) | £312.70 (£255.28 to £370.12; £212.00, £212.00–1060.00) | < 0.001 |
| Co-op telephone advice | 0 (0), . (.,.–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 34 (0.75), 1.41 (0.92, 1–5) | £0.06 (£0.04 to £0.09; £0) | £8.17 (£6.31 to £10.04; £5.79, £5.79–28.95) | < 0.001 |
| Discharge details | 56 (1.54), 2.2 (4.02, 1–31) | £0 (£0 to £0.01; £0) | £0.24 (£0.12 to £0.36; £0.11, £0.11–3.41) | 90 (1.98), 1.63 (1.13, 1–6) | £0 (£0 to £0; £0) | £0.18 (£0.15 to £0.21; £0.11, £0.11–0.66) | 0.886 |
| Emergency consultation | 160 (4.39), 1.63 (1.22, 1–8) | £3.33 (£2.7 to £3.97; £0) | £75.8 (£66.94 to £84.67; £46.47, £46.47–371.76) | 243 (5.34), 1.7 (1.34, 1–11) | £4.22 (£3.55 to £4.88; £0) | £78.98 (£71.11 to £86.85; £46.47, £46.47–511.17) | 0.058 |
| Follow-up/routine visit | 0 (0), . (.,.–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 6 (0.13), 1.5 (0.84, 1–3) | £0.08 (£0.01 to £0.16; £0) | £63.57 (£28.61 to £98.52; £46.47, £41.21–123.63) | 0.029 (0.027) |
| Home visit | 10 (0.27), 1.1 (0.32, 1–2) | £0.27 (£0.09 to £0.44; £0) | £96.53 (£76.67 to £116.38; £87.75, £87.75–175.5) | 12 (0.26), 1.92 (1.73, 1–6) | £0.44 (£0.11 to £0.78; £0) | £168.19 (£71.74 to £264.63; £87.75, £87.75–526.5) | 0.348 |
| Hospital admission | 10 (0.27), 2 (1.49, 1–5) | £3.11 (£0.75 to £5.47; £0) | £1132 (£528.42 to £1735.58; £566, £566–2830) | 0 (0), . (., .–.) | £0 (£0 to £0; £0) | (.–.; .,. –.) | 0.01 |
| Resource use (n = 8190) | Group | Statistical significant difference in resource use (and costsa) between intervention groups (p-value) | |||||
|---|---|---|---|---|---|---|---|
| Intervention (n = 3641) | Control (n = 4549) | ||||||
| Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (95% CI; median) | Mean cost per resource user (95% CI; median, range) | Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (£) (95% CI; median) | Mean cost per resource user (£) (95% CI; median, range) | ||
| Letter from outpatients | 168 (4.61), 1.96 (2.08, 1–15) | £0.01 (£0.01 to £0.01; £0) | £0.22 (£0.18 to £0.25; £0.11, £0.11–1.65) | 122 (2.68), 1.45 (0.98, 1–9) | £0 (£0 to £0.01; £0) | £0.16 (£0.14 to £0.18; £0.11, £0.11–0.99) | < 0.001 |
| Mail from patient | 10 (0.27), 2.8 (1.69, 1–5) | £0 (£0 to £0; £0) | £0.31 (£0.18 to £0.44; £0.28, £0.11–0.55) | 3 (0.07), 1 (0, 1–1) | £0 (£0 to £0; £0) | £0.11 (£0.11 to £0.11; £0.11, £0.11–0.11) | 0.013 |
| Mail to patient | 1098 (30.16), 1.83 (1.18, 1–7) | £0.06 (£0.06 to £0.06; £0) | £0.20 (£0.19 to £0.21; £0.11, £0.11–0.77) | 1362 (29.94), 1.94 (1.41, 1–15) | £0.06 (£0.06 to £0.07; £0) | £0.21 (£0.20 to £0.22; £0.11, £0.11–1.65) | 0.253 |
| Medicine management | 250 (6.87), 1.42 (0.88, 1–6) | £0.68 (£0.58 to £0.77; £0) | £9.87 (£9.11 to £10.63; £6.95, £6.95–41.7) | 363 (7.98), 2.24 (2.42, 1–20) | £1.24 (£1.06 to £1.43; £0) | £15.58 (£13.85 to £17.32; £6.95, £6.95–139) | < 0.001 |
| Minor injury service | 3 (0.08), 1 (0, 1–1) | £0.04 (–£0.01 to £0.08; £0) | £46.47 (£46.47 to £46.47; £46.47, £46.47–£46.47) | 3 (0.07), 1 (0, 1–1) | £0.03 (£0 to £0.07; £0) | £46.47 (£46.47 to £46.47; £46.47, £46.47–46.47) | 0.787 |
| NHS Direct report | 0 (0), . (., .–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 1 (0.02), 1 (., 1–1) | £0 (£0 to £0; £0) | £0.11 (.–.; £0.11, £0.11–0.11) | 0.317 |
| Other | 1946 (53.45), 4.05 (4.5, 1–44) | £0.24 (£0.22 to £0.25; 0.11) | £0.45 (£0.42 to £0.47; £0.33, £0.11–4.84) | 2053 (45.13), 3.39 (3.81, 1–35) | £0.17 (£0.16 to £0.18; 0) | £0.37 (£0.35 to £0.39; £0.22, £0.11–3.85) | < 0.001 |
| Out-of-hours non-practice | 424 (11.65), 1.68 (1.17, 1–10) | £9.07 (£8.06 to £10.08; £0) | £77.92 (£72.75 to £83.10; £46.47, £46.47–464.70) | 419 (9.21), 1.65 (1.31, 1–14) | £7.08 (£6.24 to £7.92; £0) | £76.86 (£71.02 to £82.69; £46.47, £46.47–650.58) | 0.003 |
| Out-of-hours practice | 0 (0), . (., .–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | 9 (0.2), 1.22 (0.67, 1–3) | £0.11 (£0.03 to £0.19; £0) | £56.8 (£32.98 to £80.61; £46.47, £46.47–139.41) | 0.008 |
| Resource use (n = 8190) | Group | Significant difference in resource use (and costsa) between intervention groups (p-value) | |||||
|---|---|---|---|---|---|---|---|
| Intervention (n = 3641) | Control (n = 4549) | ||||||
| Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (95% CI; median) | Mean cost per resource user (95% CI; median, range) | Number of resource users (% study group), mean number of tasks per resource user (SD, range) | Mean cost per patient (95% CI; median) | Mean cost per resource user (95% CI, median; range) | ||
| Radiology result | 1 (0.03), 1 (., 1–1) | £0 (£0 to £0; £0) | £0.11 (.–.; £0.11, £0.11–0.11) | 0 (0), . (., .–.) | £0 (£0 to £0; £0) | £0 (£0 to £0; £0, £0–0) | 0.317 |
| Referral letter | 30 (0.82), 1.23 (0.63, 1–3) | £0 (£0 to £0; £0) | £0.14 (£0.11 to £0.16; £0.11, £0.11–0.33) | 5 (0.11), 1 (0, 1–1) | £0 (£0 to £0; £0) | £0.11 (£0.11 to £0.11; £0.11, £0.11–0.11) | < 0.001 |
| Repeat issue | 1786 (49.05), 4.23 (3.95, 1–30) | £0.23 (£0.22 to £0.24; £0) | £0.47 (£0.45 to £0.49; £0.33, £0.11–3.30) | 2293 (50.41), 4.28 (4.51, 1–53) | £0.24 (£0.23 to £0.25; £0.11) | £0.47 (£0.45 to £0.49; £0.33, £0.11–5.83) | 0.307 |
| Residential home visit | 2 (0.05), 1 (0, 1–1) | £0.05 (–£0.02 to £0.12; £0) | £87.75 (£87.75 to £87.75; £87.75, £87.75–87.75) | 4 (0.09), 1 (0, 1–1) | £0.08 (£0 to £0.15; £0) | £87.75 (£87.75 to £87.75; £87.75, £87.75–87.75) | 0.574 |
| Results recording | 656 (18.02), 2.88 (2.79, 1–28) | £0.06 (£0.05 to £0.06; £0) | £0.32 (£0.29 to £0.34; £0.22, £0.11–3.08) | 839 (18.44), 3.02 (3.43, 1–38) | £0.06 (£0.06 to £0.07; £0) | £0.33 (£0.31 to £0.36; £0.22, £0.11–4.18) | 0.324 |
| Surgery consultation | 2948 (80.97), 3.95 (3.45, 1–35) | £145.96 (£140.79 to £151.13; £92.94) | £180.28 (£174.56 to £186.00; £139.41, £41.21–1615.93) | 3762 (82.7), 4.62 (4.61, 1–70) | £174.21 (£168.15 to £180.27; £128.89) | £210.66 (£203.89 to £217.43; £139.41, £41.21–3247.64) | < 0.001 |
| Telephone call from a patient | 239 (6.56), 1.42 (1.07, 1–10) | £0.51 (£0.43 to £0.59; £0) | £7.76 (£7.05 to £8.47; £5.79, £2.32–50.96) | 450 (9.89), 1.7 (1.7, 1–17) | £0.92 (£0.80 to £1.04; £0) | £9.3 (£8.43 to £10.17; £5.79, £2.32–94.96) | < 0.001 |
| Telephone call to a patient | 321 (8.82), 1.66 (1.56, 1–14) | £0.76 (£0.65 to £0.87; £0) | £8.62 (£7.74 to £9.5; £5.79, £2.32–74.12) | 663 (14.57), 2.01 (2.86, 1–52) | £1.54 (£1.34 to £1.74; £0) | £10.56 (£9.38 to £11.75; £5.79, £2.32–276.79) | < 0.001 |
| Third-party consultation | 2118 (58.17), 3.73 (3.92, 1–40) | £460.04 (£435.87 to £484.21; £212) | £790.85 (£755.45 to £826.24; £424.00, £212–8480) | 2599 (57.13), 3.79 (4.12, 1–53) | £458.72 (£436.31 to £481.13; £212.00) | £802.89 (£769.27 to £836.51; £424, £212–11,236) | 0.937 |
| Triage | 359 (9.86), 2.33 (2.25, 1–21) | £1.27 (£1.09 to £1.45; £0) | £12.92 (£11.61 to £14.22; £5.79, £2.32–121.59) | 640 (14.07), 2.27 (1.92, 1–18) | £1.76 (£1.59 to £1.93; £0) | £12.51 (£11.70–£13.31; £8.11, £2.32–90.34) | < 0.001 |
| Twilight visit | 1 (0.03), 1 (., 1–1) | £0.01 (–£0.01 to £0.04; £0) | £46.47 (.–.; £46.47, £46.47–46.47) | 1 (0.02), 1 (., 1–1) | £0.01 (–£0.01 to £0.03; £0) | £46.47 (.–.; £46.47, £46.47–46.47) | 0.876 |
| Walk-in centre | 28 (0.77), 1.46 (0.88, 1–5) | £0.52 (£0.30 to £0.75; £0) | £68.05 (£52.17 to £83.92; £46.47, £46.47–232.35) | 0 (0), . (.,.–.) | £0 (£0 to £0; £0) | . (.–.; ., .–.) | < 0.001 |
The estimated number of exacerbations for the 4-month QALY estimation period (that period for which a utility decrement was attached to these exacerbations) is dependent on the assumed period of exacerbation, which was 1 week for the base-case analysis (the period of exacerbation was varied to 3 days or 2 weeks as part of the sensitivity analysis). The mean number of exacerbations per patient, as presented in Table 17, was not statistically significantly different between the letter and no-letter groups for this 4-month period (2.50 vs. 2.41, respectively; p = 0.078). These results suggest that over the 4-month period, on average, patients in the letter group spent 2.5 weeks in an exacerbated state and the patients in the no-letter group spent just under 2.5 (2.41) weeks in an exacerbated state.
The resource use and associated cost results, as presented in Table 17, suggest that those patients in the no-letter (control) group had more scheduled tasks over the 12-month post-intervention period than the letter (intervention) group (2.69 vs. 2.60 tasks, respectively; p = 0.130), but the difference was not statistically significant. The cost for scheduled tasks was also higher on average for the no-letter group, but was not statistically significantly different (£169 for letter vs. £173 for no letter; p = 0.623). The reverse was true for those tasks that were classified as ‘not relevant’. These ‘not relevant’ tasks were not classified as either ‘scheduled’ or ‘unscheduled’ but were included in the economic analysis as part of the resource use and cost estimations. The letter group had statistically significant more ‘not relevant’ tasks than the no-letter group (mean number of tasks of 4.17 for letter vs. 3.75 for no letter; p < 0.001), but again this did not translate into statistically significant different costs associated with these tasks (£204 for letter vs. £205 for no letter; p = 0.915). There was no statistically significant difference in the number of unscheduled tasks between groups (p = 0.867). There was a statistically significant lower mean cost for unscheduled tasks in the letter group (£266 for letter vs. £283 for no letter; p = 0.030). There was no statistically significant difference between the letter and no-letter groups in either the overall number of tasks (p = 0.249) or associated costs (p = 0.251).
The results presented in Tables 18–20 suggest that there were some statistically significant (p < 0.001) differences in the types of tasks and associated costs that defined the patients’ resource use over the 12-month post-intervention time period between the letter and no-letter groups. For example, the no-letter group had a statistically significant lower number of casualty attendances [51 (1.40%) people from the letter group had a casualty attendance, compared with 6 (0.13%) from the no-letter group]. This resulted in a statistically significant mean higher cost of £2.84 vs. £0.30 for the letter compared with the no-letter group (see Table 18). Tasks such as ‘co-op surgery consultation’, ‘co-op telephone advice’ and ‘follow-up/routine visit’ were mainly associated with the no-letter group, with no recorded entry of these tasks for the letter group (see Table 18). Surgery consultations occurred statistically significantly more often in the no-letter group, which translated into a statistically significantly higher mean cost per patient associated with these tasks of £174 for the no-letter group compared with £146 for the letter group (see Table 20).
Tasks recorded as ‘other’ occurred statistically significantly more often in the letter group than in the no-letter group, with 53.5% of the letter group having a recorded ‘other’ task, compared with 45.1% in the no-letter group. Among patients for whom an ‘other’ task was recorded (defined as ‘resource users’), the number of tasks defined as ‘other’ was higher in the letter group than in the no-letter group (on average, 4.1 tasks per patient in the letter group compared with 3.4 tasks per patient in the no-letter group). This resulted in a statistically significant higher mean cost of £0.24 for the letter group compared with £0.17 for the no-letter group. Although this difference is very small when a unit cost of £0.11 is assigned to this ‘other’ task, larger unit costs being assigned to ‘other’ tasks could have an impact on the cost-effectiveness of the letter group, as tasks classified as ‘other’ occur significantly more often for this group (this aspect has been assessed as part of the sensitivity analysis; see Table 16).
The costs associated with prescriptions were statistically significantly different (p = 0.003) between groups, with the mean cost for prescriptions being approximately £55 for the letter group and £49 for the no-letter group, for the 12-month post-intervention period.
Although there appears to be a pattern of resource use and associated costs between the letter and no-letter group, this did not translate into significantly different costs between the groups as a whole (see Table 17).
Mean and incremental costs and quality-adjusted life-years from main, adjusted and sensitivity analyses
The mean, SE and 95% CI for costs and QALYs by intervention (letter) and control group (no letter) are presented in Table 21; the incremental results (mean difference for the intervention group minus control group and distribution statistics) are presented in Table 22. BA results are presented alongside the unadjusted results. It should be noted that there was a statistically significant difference (p < 0.1) in overall costs at baseline (12 months before intervention) between the trial arms (these baseline results are presented in Appendix 10, Table 40).
| Analysis | Letter (yes/no) | Cost | QALYs | ||||
|---|---|---|---|---|---|---|---|
| Mean | SE | 95% CIa | Mean | SE | 95% CIa | ||
| Main | Yes | £696.24 | £23.11 | £649.31 to £740.98 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £710.98 | £20.81 | £670.07 to £752.73 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| BA main | Yes | £684.39 | £17.08 | £650.93 to £717.86 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £720.46 | £12.33 | £696.29 to £744.64 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| Sensitivity analysis: ‘other’ unit cost | |||||||
| Cost | Yes | £794.72 | £27.19 | £747.51 to £853.65 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £780.53 | £24.07 | £733.49 to £826.41 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| BA cost | Yes | £770.99 | £18.74 | £734.26 to £807.72 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £799.52 | £13.91 | £772.26 to £826.79 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| Sensitivity analysis: duration of exacerbation | |||||||
| 3 days | Yes | £696.24 | £23.11 | £649.31 to £740.98 | 0.31843 | 0.00006 | 0.31830 to 0.31855 |
| No | £710.98 | £20.81 | £670.07 to £752.73 | 0.31848 | 0.00006 | 0.31835 to 0.31858 | |
| BA 3 days | Yes | £684.39 | £17.08 | £650.93 to £717.86 | 0.31843 | 0.00006 | 0.31830 to 0.31855 |
| No | £720.46 | £12.33 | £696.29 to £744.64 | 0.31848 | 0.00006 | 0.31835 to 0.31858 | |
| 2 weeks | Yes | £696.24 | £23.11 | £649.31 to £740.98 | 0.31236 | 0.00022 | 0.31190 to 0.31276 |
| No | £710.98 | £20.81 | £670.07 to £752.73 | 0.31270 | 0.00020 | 0.31226 to 0.31304 | |
| BA 2 weeks | Yes | £684.39 | £17.08 | £650.93 to £717.86 | 0.31236 | 0.00022 | 0.31190 to 0.31276 |
| No | £720.46 | £12.33 | £696.29 to £744.64 | 0.31270 | 0.00020 | 0.31226 to 0.31304 | |
| Sensitivity analysis: utility of exacerbation | |||||||
| Utility | Yes | £696.24 | £23.11 | £649.31 to £740.98 | 0.31048 | 0.00028 | 0.30989 to 0.31100 |
| No | £710.98 | £20.81 | £670.07 to £752.73 | 0.31083 | 0.00025 | 0.31028 to 0.31126 | |
| BA utility | Yes | £684.39 | £17.08 | £650.93 to £717.86 | 0.31048 | 0.00028 | 0.30989 to 0.31100 |
| No | £720.46 | £12.33 | £696.29 to £744.64 | 0.31083 | 0.00025 | 0.31028 to 0.31126 | |
| Sensitivity analysis: type of contacts | |||||||
| Respiratory | Yes | £123.17 | £4.98 | £114.10 to £133.39 | 0.31999 | 0.00004 | 0.31992 to 0.32006 |
| No | £120.76 | £6.87 | £108.90 to £136.88 | 0.32007 | 0.00003 | 0.32001 to 0.32013 | |
| BA respiratory | Yes | £119.02 | £3.17 | £112.80 to £125.23 | 0.31999 | 0.00004 | 0.31992 to 0.32006 |
| No | £124.08 | £3.17 | £114.42 to £133.74 | 0.32007 | 0.00003 | 0.32001 to 0.32013 | |
| Sensitivity analysis: cost estimation period | |||||||
| 5 months | Yes | £322.70 | £9.64 | £303.47 to £341.75 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £318.96 | £11.15 | £298.17 to £340.84 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| BA 5 months | Yes | £317.17 | £7.03 | £303.40 to £330.94 | 0.31594 | 0.00013 | 0.31567 to 0.31619 |
| No | £323.38 | £7.13 | £309.40 to £337.35 | 0.31611 | 0.00012 | 0.31585 to 0.31631 | |
| Subgroup analysis: under-fives | |||||||
| Under-fives | Yes | £1006.21 | £120.73 | £798.09 to £1289.15 | 0.31397 | 0.00049 | 0.31285 to 0.31485 |
| No | £809.30 | £53.23 | £722.15 to £932.29 | 0.31500 | 0.00038 | 0.31407 to 0.31561 | |
| BA under-fives | Yes | £906.71 | £67.80 | £773.82 to £1039.59 | 0.31397 | 0.00049 | 0.31285 to 0.31485 |
| No | £871.01 | £50.85 | £771.35 to £970.67 | 0.31500 | 0.00038 | 0.31407 to 0.31561 | |
| Analysis (1 – 0)a | Cost | QALYs | ||||
|---|---|---|---|---|---|---|
| Difference in means | SE | BCa 95% CI | Difference in means | SE | BCa 95% CI | |
| Main | –£14.74 | £31.25 | –£75.86 to £45.19 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| BA main | –£36.07 | £21.10 | –£77.11 to £9.67 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| Sensitivity analysis: ‘other’ unit cost | ||||||
| Cost | £14.19 | £36.86 | –£56.22 to £95.34 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| BA cost | –£28.53 | £23.64 | –£72.74 to £20.18 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| Sensitivity analysis: duration of exacerbation | ||||||
| 3 days | –£14.74 | £31.25 | –£75.86 to £45.19 | –0.00005 | 0.00009 | –0.00022 to 0.00012 |
| BA 3 days | –£36.07 | £21.10 | –£77.11 to £9.67 | –0.00005 | 0.00009 | –0.00022 to 0.00012 |
| 2 weeks | –£14.74 | £31.25 | –£75.86 to £45.19 | –0.00034 | 0.00030 | –0.00093 to 0.00025 |
| BA 2 weeks | –£36.07 | £21.10 | –£77.11 to £9.67 | –0.00034 | 0.00030 | –0.00093 to 0.00025 |
| Sensitivity analysis: utility of exacerbation | ||||||
| Utility | –£14.74 | £31.25 | –£75.86 to £45.19 | –0.00035 | 0.00038 | –0.00109 to 0.00039 |
| BA utility | –£36.07 | £21.10 | –£77.11 to £9.67 | –0.00035 | 0.00038 | –0.00109 to 0.00039 |
| Sensitivity analysis: type of contacts | ||||||
| Respiratory | £2.41 | £8.65 | –£17.58 to £17.26 | –0.00008 | 0.00005 | –0.00017 to 0.00001 |
| BA respiratory | –£5.06 | £5.98 | –£18.51 to £5.87 | –0.00008 | 0.00005 | –0.00017 to 0.00001 |
| Sensitivity analysis: cost estimation period | ||||||
| 5 months | £3.74 | £14.78 | –£25.68 to £32.47 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| BA 5 months | –£6.21 | £10.04 | –£25.73 to £14.54 | –0.00017 | 0.00018 | –0.00051 to 0.00018 |
| Subgroup analysis: under-fives | ||||||
| Under-fives | £196.91 | £132.94 | –£47.60 to £466.99 | –0.00102 | 0.00062 | –0.00221 to 0.00020 |
| BA under-fives | £35.69 | £85.30 | –£137.40 to £195.31 | –0.00102 | 0.00062 | –0.00221 to 0.00020 |
For the main unadjusted analysis, the mean observed cost and number of QALYs gained was £696.24 and 0.31594, respectively, in the letter group and £710.98 and 0.31611, respectively, in the no-letter group. For the BA main analysis, the adjusted mean costs were £684.39 and £720.46 for the letter and no-letter groups, respectively. From these results, it can been seen that the baseline adjustment had an important effect on the cost results. The influence of the baseline adjustment on the cost results is discussed further in Strengths and weaknesses of the economic analysis.
As presented in Table 22 for the main analysis, the incremental QALY loss for the letter group was 0.00017, with a cost saving of £14.74 or £36.07 for the unadjusted and adjusted cost analysis, respectively. These mean point estimates were surrounded by large amounts of uncertainty. For example, the uncertainty around the incremental adjusted main analysis of a mean cost saving of £36.07 was a SE of £21.10 and 95% CI ranging from a cost saving of £77.11 up to an additional cost of £9.67. For the main analysis (QALY loss of 0.00017), the SE for the incremental QALY was 0.00018 and the 95% CI ranged from a QALY loss of 0.00051 up to a QALY gain of 0.00018.
In the sensitivity analyses using BA costs, the letter was always shown to result in a mean cost saving, although the difference never achieved statistical significance. However, in the sensitivity analyses using the observed unadjusted costs, the letter resulted in an additional cost on average, albeit still non-statistically significant, in all of the sensitivity analyses in which costs were affected. For the under-fives subgroup analysis, a cost increase was identified in the unadjusted and adjusted analyses of £196.11 and £35.69 per person, respectively. In general, for the adjusted analysis, the letter group was shown to be cost-saving at the mean point estimate for the main and sensitivity analysis.
Across the main, sensitivity and subgroup analyses, a QALY loss was observed for the mean point estimates ranging from a QALY loss of 0.00005 when the period of exacerbation was reduced from 1 week to 3 days up to 0.00102 in the under-fives subgroup analysis. All incremental and mean point QALY estimates were surrounded by large amounts of uncertainty, as presented in Tables 21 and 22.
In general, the letter intervention was found to be cost-saving, but less effective than no letter at the mean point estimate. However, all QALY and costs estimates were surrounded by large amounts of uncertainty. In order to give these results some context based on a hypothetical population of 100,000 people, the mean point estimates from the adjusted main analysis suggest that by implementing the letter intervention, 17 QALYs would have to be traded against a cost saving of £3,607,000 by decision-makers if the letter was implemented for 1 year, but with 95% confidence that the intervention may result in a loss of up to 51 QALYs or a gain of up to 18 QALYs and in a cost saving of up to £7,711,000 or an additional cost of up to £967,000.
Summary of key results from the cost-effectiveness, sensitivity and subgroup analyses
The cost-effectiveness analysis found that there is considerable uncertainty regarding the impact of the letter intervention on both benefits to patients and costs falling on the NHS. Although the adjusted base-case analysis showed a mean cost saving of £36.07 per patient and a mean QALY loss of 0.00017, the CIs around each were wide and did not demonstrate significantly significant differences. The differences in costs and QALYs from the bootstrapped analysis can also be visually interpreted from the cost-effectiveness planes for the unadjusted and adjusted main analyses as presented in Figures 23 and 24, respectively. While the intervention was cost-saving in 96.3% of samples, it also resulted in a QALY loss in 82.9% of samples (Table 23 and Figure 24). Overall, the intervention was found to be cost-effective in 93.8% of samples when valuing a QALY at £20,000 (see Table 23 and the cost-effectiveness acceptability curve for the unadjusted and adjusted main analysis in Figure 25).
FIGURE 23.
Cost-effectiveness plane for the letter intervention vs. no letter from the main analysis.

FIGURE 24.
Cost-effectiveness plane for the letter intervention vs. no letter from the BA main analysis.
| Analysis (1–0)a | Mean (SE) ICERb [£/QALY] | ICERs by cost-effectiveness plane quadrant (%) | Percentages of cost-effectiveness at λ < willingness to pay (%) | ||||
|---|---|---|---|---|---|---|---|
| South-east | South-west | North-east | North-west | λ < £0 | λ < £20,000 | ||
| Main | 88,733 (114,126) | 14.6 | 52.7 | 2.5 | 30.2 | 67.3 | 63.1 |
| BA main | 597,103 (187,787) | 17.0 | 79.3 | 0.1 | 3.6 | 96.3 | 93.8 |
| Sensitivity analysis: ‘other’ unit cost | |||||||
| Cost | Dominated | 11.6 | 26.6 | 5.5 | 56.3 | 38.2 | 34.6 |
| BA cost | 171,716 (167,195) | 16.7 | 73.5 | 0.4 | 9.4 | 90.2 | 86.5 |
| Sensitivity analysis: duration of exacerbation | |||||||
| 3 days | 279,489 (288,070) | 23.5 | 43.8 | 4.3 | 28.4 | 67.3 | 66.0 |
| BA 3 days | 683,777 (314,408) | 27.6 | 68.7 | 0.2 | 3.5 | 96.3 | 95.5 |
| 2 weeks | 43,121 (112,808) | 11.6 | 55.7 | 1.8 | 30.9 | 67.3 | 59.9 |
| BA 2 weeks | 105,496 (156,183) | 13.3 | 83.0 | 0.1 | 3.6 | 96.3 | 90.3 |
| Sensitivity analysis: utility of exacerbation | |||||||
| Utility | 41,607 (53,125) | 14.9 | 52.4 | 2.6 | 30.1 | 67.3 | 59.9 |
| BA utility | 101,793 (91,714) | 17.4 | 78.9 | 0.1 | 3.6 | 96.3 | 89.8 |
| Sensitivity analysis: type of contacts | |||||||
| Respiratory | Dominated | 2.0 | 35.5 | 1.9 | 60.6 | 37.5 | 32.5 |
| BA respiratory | 65,020 (22,731) | 3.4 | 76.2 | 0.5 | 19.9 | 79.6 | 70.7 |
| Sensitivity analysis: cost estimation period | |||||||
| 5 months | Dominated | 11.7 | 29.3 | 5.4 | 53.6 | 41.0 | 34.2 |
| BA 5 months | 37,358 (45,190) | 16.1 | 58.9 | 1.0 | 24.0 | 75.0 | 62.4 |
| Subgroup analysis: under-fives | |||||||
| Under-fives | Dominated | 1.4 | 4.2 | 2.6 | 91.8 | 5.6 | 4.5 |
| BA under-fives | Dominated | 2.8 | 30.6 | 1.2 | 65.4 | 33.4 | 26.3 |
FIGURE 25.
Cost-effectiveness acceptability curve for the letter intervention vs. no letter. Note that this graph demonstrates the probability of cost-effectiveness at a range of decision-maker ceiling willingness-to-pay values for the letter intervention from the main analysis (unadjusted) and the baseline cost-adjusted main analysis.
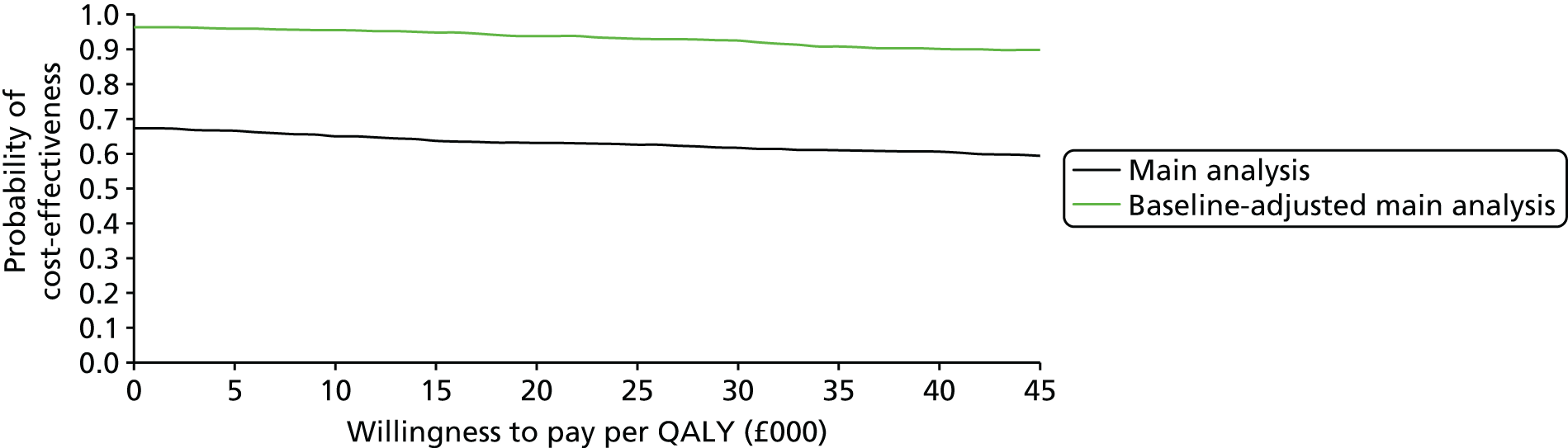
The very small QALY loss means that the incremental cost-effectiveness ratio (ICER) is very large for all the analyses. For example, for the adjusted main analysis, the ICER based on the mean point estimates was £597,103 (SE from the bootstrapped estimations: £187,787) per QALY, which is the ICER for the cost savings per QALY forgone, rather than the slightly more common cost per QALY gained associated with reported ICERs. The intervention was dominated (the letter intervention was more costly and less effective than no letter at the mean point estimate) when the ‘other’ unit cost was assigned a pooled weighted cost, when accounting for only ‘respiratory’-related contacts, when restricting the analysis to 5 months after intervention, and in the under-fives subgroup analysis; however, this was for the unadjusted analysis and the intervention was not dominated in the adjusted analysis except in the subgroup analysis. No ICER could be defined as dominant (a scenario in which the letter intervention would be cost-saving and more effective at the mean point estimate) as a result of this analysis. These ICERs are suggestive of an intervention that would be generally deemed not cost-effective if effectiveness (QALY gain) was important for decision-makers.
The sensitivity analyses showed that the cost-effectiveness results were sensitive to the assumptions regarding the costing of ‘other’ contacts, the duration and utility decrement assigned to a period of exacerbation, the types of contact included in the analysis, as well as the period of cost-estimation and whether or not the focus was on 5- to 16-year-olds or under-5-year-olds. The probability of cost-effectiveness in the adjusted analysis for the 5- to 16-year-olds, however, generally remained above 62% at a willingness-to-pay (WTP) threshold (λ) of £20,000 and above 75% when focused on the cost savings (rather than effectiveness; WTP threshold of £0) of the intervention. The probability of cost-effectiveness in the adjusted analysis for the under 5 years of age was 26.3% (λ < £20,000) or 33.4% (λ < £0). It should also be noted, however, that none of the sensitivity analyses demonstrated a significant difference in QALYs or costs, so the overall conclusion is that there is considerable uncertainty around the cost-effectiveness of the letter intervention.
Although there appears to be some discordance between the cost-effectiveness results and the trial’s primary clinical outcome of proportion of children having an unscheduled contact in September, the cost-effectiveness results are consistent with the mean contacts per child endpoints, particularly over the wider time interval.
Chapter 5 Discussion
Main findings
Previous work has shown an increase in the number of unscheduled medical contacts by children in autumn months (September to December), which may be a result of the start of the new school term. 13 By sending a letter in July to remind children (and their parents) of the importance of using their inhaler, it was hypothesised that the increase may be averted. More specifically, the hope was that a reminder letter would lead to a greater uptake of inhaler prescriptions in August, that this, in turn, would also lead to an increased adherence and, finally, that fewer unscheduled medical appointments would be required.
There is evidence of an impact on the first part of this pathway, as the intervention group demonstrated a higher uptake of prescriptions in August 2013. There was also an increase in scheduled contacts in the same month in this group. The data are not available to confirm actual medicine use (as quantified by the medicine possession ratio), and so it is unclear whether or not the increased uptake also translated into increased use.
The primary end point was unscheduled medical contacts in September 2013, which coincided with the start of the new school term. There was no evidence of a reduction in the intervention group, but the finding of a greater number of unscheduled medical contacts (albeit not statistically significant) is unexpected. We can offer three potential explanations for this.
First, a repeat prescription request may not be dispensed without a review in situations in which the child has not received a prescription for several months, or in which the parent wishes to discuss the advantages and/or disadvantages of recommencing treatment that the child has stopped some months before. This in turn may be classified as an unscheduled contact in the coding algorithm that we used to define contacts as unscheduled. The evidence to support this is the large increase in unscheduled contacts in the intervention group in August (relative to the control group). In addition, it seems the longer the time since a patient last collected a prescription, the higher the likelihood of an unscheduled contact in September in the intervention group. This implies that patients with more troublesome asthma may be more likely to have collected a prescription recently; conversely, patients who have not collected a prescription recently may have stopped their preventative medication and may be seeing their GP to check whether or not it is still necessary.
Second, the letter may have acted inadvertently as a trigger to contact the practice in relation to an unrelated medical issue they had been meaning to discuss, which may increase the number of contacts in the short term.
Third, and finally, the data collected at the time may be equivocal in the coding of the contact, leading us to incorrectly adjudicate certain contacts as unscheduled when the contact was scheduled, a limitation of routine data that we will return to in the next section; this is a factor that is important for this intervention, which did increase scheduled contacts in the first instance.
Despite the increase in unscheduled contacts in September, both the total number of contacts per child (i.e. scheduled plus unscheduled) and unscheduled contacts were lower in the intervention group than in the control over the extended study period (September–December 2013) and the full year (September 2013–August 2014). Although the effects were not statistically significantly, the minimal cost associated with the intervention meant that the intervention was found to have a high probability of being cost-saving overall. The economic analysis (which used data over a 12-month period from August 2013 to July 2014) estimated a mean cost saving across the base case of £36.07 per child and 96.3% probability that the intervention is cost-saving. By contrast, the cost-effectiveness results are suggestive of an intervention that would be generally deemed not cost-effective if effectiveness (QALY gain) was important for decision-makers, as it also resulted in a QALY loss in 82.9% of samples and a mean loss of 0.00017 QALYs.
The small effects observed could be because of the strength of the link between prescription uptake and unscheduled medical contacts. Around 5–6% more children received a prescription for asthma medication in August 2013, a difference that, while substantial, may not be sufficient to achieve the postulated 5% reduction in unscheduled medical contacts that the study was planned to detect. On the other hand, it could be that the increased prescription uptake could have reduced the severity (and days off school), which we could not detect from routine health-care data.
Strengths and weaknesses
Strengths and weaknesses of the trial
The primary strength of the trial is its simple and generalisable design, incorporating an intervention that could be delivered within general practices with minimal cost. The intervention comprised a short letter, delivered at the start of the school summer holidays, reminding children of the importance of adhering to their asthma medication. The trial demonstrated that doing so increased the number of children both requesting a repeat asthma prescription and having a scheduled medical contact (such as an asthma review) in August without an associated increase in cost. Nevertheless, as noted above [see Secondary outcomes, Contacts over 12 months (September 2013 to August 2014)], there was (at best) limited evidence that this translated into an overall reduction of medical contacts.
We believe the risk of methodological bias is low in this study. The designation of contacts as ‘scheduled’, ‘unscheduled’ and ‘irrelevant’ was based on an independent adjudication panel comprising experienced GPs who were blinded to treatment group.
The main limitations of the trial were those imposed by the use of routinely collected data as the sole data source. The issues with using routine data are fairly obvious; data that are collected primarily as a record of medical care may not contain the information needed for a subsequent research question. For the outcomes evaluated within this trial, there was considerable uncertainty around the adjudication of some of the contacts as scheduled, unscheduled or irrelevant. Many of the contacts were coded ‘other’ or otherwise ambiguous, and the GP Adjudication Panel advised that this probably reflected the limited time available to GPs when summarising the appointment. There were 34,947 such contacts in the data set, out of 440,429 contacts (109,352 of which were deemed irrelevant). Even if adjudication of all contacts was feasible, however, some of more detailed fields are withheld as per CPRD policy, with the (understandable) reason being to safeguard potentially identifiable details contained therein. As a general issue, this highlights the tension between research ethics and individual rights, which arises in the use of routine data for research. On the advice of the GP Adjudication Panel, these contacts were coded as unscheduled.
We note that the use of routine data was a strength in some regards. First and foremost, using routine data substantially reduced the cost of the trial and allowed us to study a relatively large cohort of children. It is also the only appropriate way to assess the impact of a population-level intervention. If patients were recruited to a trial of the effect of receiving (or not) a letter, the very process of recruitment would have been an intervention. It should also be noted that this is the first large-scale trial of its type using routine CPRD data as the sole source data: issues that arose in the course of this trial may be abated on future trials as researchers and data providers become more familiar with the practical considerations involved in the process of data collation, transfer, recoding and analysis. Some specific examples encountered here are that practices may withdraw from being with CPRD; that prespecified coding for contacts are often not used; and that some fields are withheld for reasons of data confidentiality. The extent of these had not been appreciated or accounted for at the design stage of this trial.
The push for the use of routine data in clinical evaluation seems likely to continue, and it is important that researchers have appropriate expectations of what is, and what is not, realistic and achievable, when using these repositories. Practices leaving the CPRD is a particular issue for studies that have a long-term follow-up as the primary analysis.
Over the course of the study period, 28 practices stopped contributing data to the CPRD as a result of switching practice computer systems. At the time of the conclusion of the study period, the CPRD was only able to collect research-usable data from practices using the Vision IT computer system. It should be noted, however, that the CPRD is working towards being able to collect research data from GP practices using computer systems other than Vision. Once this work is complete, it should be the case that practices switching from one computer system to another will be able to continue their participation in trials and studies.
In retrospect, qualitative interviews with key stakeholders, including practice nurses and GPs, as well as a larger group of children with asthma, may have added a different dimension in both the development and (suggested) implementation of the intervention.
Strengths and weaknesses of the economic analysis
As with the clinical evaluation, the main limitation of the economic analysis is that it relied solely on routine data available within the CPRD database. We therefore had to infer the number of exacerbations experienced by patients, as well as the duration and severity of those exacerbations, from data on health-care resource use, which required several assumptions. For example, we assumed that any week including one or more unscheduled health care contacts was an exacerbation week. Under this assumption, two unscheduled contacts occurring 2 days apart may count as 1 or 2 weeks of exacerbation depending on whether or not they fall within the same calendar week. This adds further uncertainty to the QALY estimates that is not quantified within the CIs provided by the bootstrap analysis.
The use in the study of routine data also meant that we had to rely on published estimates for the impact of asthma exacerbations on children rather than measuring HRQoL in the patients themselves. This was problematic, as there was a lack of relevant and high-quality data on the impact of exacerbations on HRQoL for school-aged children. As a result, we decided to use data from adults in the base-case analysis, but this may not accurately reflect the impact of exacerbations on HRQoL in children, as their experiences of asthma may differ from those of adults.
Although the CPRD provides comprehensive data on resource use for the costing analysis, a number of assumptions were needed to classify all the health-care contacts as either scheduled or unscheduled. We also had difficulty classifying contacts as respiratory related or not, with a large proportion (38%) remaining unclassified. For this reason, in the costing analysis we included all contacts, whether they could be classified as respiratory related or not. As the intervention is not expected to have any effect on non-respiratory-related contacts, our analysis assumes that any difference between the intervention and comparator arms was a result of a change in respiratory-related contacts. The inclusion of these unrelated contacts in the costing analysis is likely to have made it harder to detect whether or not there was a true difference in respiratory-related contacts. We also found that a significant proportion of contacts (10%) were coded as consultation type ‘other’, which does not provide a clear indication of the activity involved. We therefore had to make an assumption regarding the type of activity that might be coded this way. Our sensitivity analysis found that making alternative assumptions regarding contacts coded as ‘other’ made some difference to the likelihood that the intervention was cost-effective, further supporting our conclusions that the cost-effectiveness of the letter intervention remains uncertain.
The data recorded in the CPRD on the duration of the consultation and the staff members involved in each consultation were not considered to be robust enough to use for calculating unit costs. Therefore, in calculating the unit costs for different types of consultation, we had to make assumptions using advice from our clinical experts regarding the likely staff mix and duration of contact. We also had to make assumptions regarding the likely severity of asthma exacerbations presenting in primary and secondary care.
The costing analysis for prescriptions was also problematic, as many of the drugs used in the management of asthma are available as a large number of different preparations, each with a unique product code. For example, for salbutamol inhalers alone, 17 unique products were prescribed within the data set. To keep the prescription cost analysis manageable, we estimated the cost per prescription for the 10 most commonly prescribed products for each drug. However, this approximation is not expected to have significantly biased the cost-effectiveness analysis because the absolute cost of most products prescribed in the management of asthma is low. We did find that the cost of prescriptions was significantly higher in the year overall for the letter arm, but this was more than offset by cost savings for other activity, resulting in a statistically non-significant cost-saving for letter compared with no letter overall.
The analysis takes an NHS and PSS perspective for costs and considers the benefits falling on patients themselves. Although this is the perspective preferred by NICE,35 it excludes the broader impact of asthma exacerbations on the children themselves, such as reduced educational opportunities due to missed schooling. It also excludes any impact on parents and carers, such as time off work when children are not fit enough to attend school.
A strength of this analysis was the ability to control for baseline costs in the BA economic analysis, which is an important aspect for consideration based on the allocation of patients (and their associated resource use patterns) to the control and intervention arms of the trial. Although it was shown in Table 17 that there was no statistically significant difference in overall resource use or costs between the trial arms for the 12 months post intervention, there was a statistically significant (p < 0.1) difference in overall costs at baseline (12 months before intervention) between the trial arms (these baseline results are presented in Appendix 10, Table 40). There are four reasons why the baseline resource use and costs are important for the BA economic analysis results and their interpretation:
-
A strong predictor of future resource use is past resource use, and it may be more difficult to influence the resource use habits of high resource users (who are also referred to as frequent attenders in the empirical literature) and, therefore, the patients in the letter group may have been less influenced by the intervention than those in the no-letter group may have been if they were allocated to receive the letter.
-
The letter intervention was allocated to a group of higher resource users, and this is not accounted for in the unadjusted economic analysis, which accounts only for the incremental difference in costs at the 12-month follow-up. Therefore, the costs of the letter group were already naturally higher in the letter group, which has an effect on the assessment of the incremental 12-month follow-up costs in this economic evaluation.
-
In relation to points 1 and 2, high resource users are, by nature, able to have larger changes in resource use and costs than low resource users, which will influence the incremental difference between groups when focusing on incremental differences at follow-up (in terms of costs) if these high resource users are allocated more to one arm of the trial than the other (e.g. reducing the resource use patterns of healthier patients with low resource use using an intervention will potentially have a much smaller change than the potential change if the intervention successfully altered the resource use patterns of unhealthier patients who are high resource users).
-
The higher resource use and costs could actually have been a result of improved (or just more) resource use recording at the practices allocated to the letter intervention group (note that allocation was at the practice level, rather than the patient level), which would have resulted in artificially higher costs and resource use in the letter group.
For the purpose of discussion, it is unclear which of the aforementioned points may have contributed to the statistically significantly higher resource use and costs for the letter group at baseline; however, whatever the reason for the difference in costs and resource use between the letter and no-letter group at baseline and post intervention, this aspect was controlled for in the BA economic analysis. Therefore, there is reason to consider that the results from the BA economic analysis may be a better representation of the potential economic benefit of the letter intervention than the unadjusted (observed) economic analysis. In this report, both results have been presented in order to allow decision-makers to account for both sets of results when judging the cost-effectiveness of this intervention; however, without the BA results, the intervention would seem less cost-effective than it actually may be in practice.
Patient and public involvement input on the trial results
The children and parents fed back that they felt that the saving per individual as a result of the intervention was an important finding from the study. 27 Their feedback was that this should be emphasised more in the reporting of the result. We had the children and parents involved throughout, but this input was from October 2015.
A key point that came from the feedback was the question of whether the results would have been different if the study had been conducted over a 3-year period rather than 1 year. The parents felt that if the letter was something they expected each summer it could help in their planning for the start of the school year.
The trial in context: other studies and differences in results
We have not identified any other studies that have examined the economic benefits of a simple postal intervention in asthma patients and, therefore, it is difficult to compare our results with those of existing published studies. Yong and Shafie43 have published a systematic review that looked more broadly at non-pharmacological interventions aiming to improve asthma management. The interventions included by Yong and Shafie varied from educational and self-management interventions to environmental interventions. While the PLEASANT study intervention letter could be considered a simple form of patient education, the educational interventions included by Yong and Shafie were all more intensive, and the population was not restricted to school-aged children, making comparisons difficult. However, the broader evidence reviewed by Yong and Shafie suggests that non-pharmacological interventions that aim to improve individuals’ management of their asthma have the potential to be cost-effective.
Meaning of the study and implications for clinicians or policy-makers
The intervention in the PLEASANT study caused an increase in prescription collection in August, as well as an increase in scheduled medical contacts. It also had the effect of increasing medical contacts in August and September. After September, there was evidence of a fall in medical contacts, which, although not statistically significant, did follow through in the economic analysis to give a high probability of the intervention being cost-saving.
The increase in prescriptions and scheduled contacts in August could lead to individual GP practices wishing to implement the intervention. Evidence from the trial suggests that this would not increase overall costs associated with the asthma management, and may improve scheduled care. However, the evidence from the PLEASANT study is not sufficient to support a general recommendation for all GP practices.
Recommendations for future research
An objective of the study was to increase the take-up of prescriptions in August, as well as the adherence of children with asthma to taking their medications. The study was not able to assess the latter aspect.
Using routine data has many advantages in terms of trial efficiency in assessing public health or population-level interventions or in the assessment of proven interventions in real-world settings. Our analysis of the PLEASANT study data set suggests that further work is required to determine how to assess adherence using such data.
A suggested refinement for future trials using routine data could be the inclusion of a prompt for clinicians to answer study-related questions for patients in the study. For example, if patients enrolled in the study were to be given a specific study code, then clinicians, when having a consultation with such patients, could be automatically presented with a template for reporting key data during the study period. For the PLEASANT study, this would involve asking the clinician one simple question: whether the appointment was scheduled or not (and perhaps, second, if this was a respiratory-related consultation).
An additional point would be to emphasise to clinicians the importance of ensuring that the routinely collected patient data needed for the trial are complete, for example, in the PLEASANT study, prescription data to assess adherence. This could be done through a system prompt.
An investigation of the intervention on emergency contacts (such as out of hours, walk-in centres and emergency departments) would be of interest.
Future research in assessing interventions to improve adherence in school-aged children with asthma could include additional qualitative interviews with key stakeholders such as practice nurses, GPs and a wider group of children with asthma.
A study estimating the impact of asthma exacerbations on school-aged children, using a preference-based measure of HRQoL that has been validated for use in children, would be useful to inform future cost-effectiveness analyses.
Chapter 6 Conclusions
The intervention did not reduce unscheduled care in September, which was the primary end point. However, the intervention succeeded in increasing the proportion of children who collected a prescription in August, along with the proportion of children who had scheduled contacts in the same month.
Over a wider time interval, there is weak evidence that the intervention may have reduced medical contacts. This is reflected in the health economic evaluation, which, overall, showed that the intervention had a high probability of giving a cost saving. However, there was no associated increase in QALYs.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following: Hilary Pinnock (Reader, University of Edinburgh) for her advice; Gerry McCann and Zaynah Gurreebun (CPRD) for their contribution to site recruitment and Tjeer Van Staa (CPRD) for his advice on CPRD; Cara Mooney and David White (University of Sheffield) for support with study set-up, site recruitment, site set-up and site close-down; and Dan Beever and Helen Wakefield for administrative and clerical support.
We would like to especially thank the GP Adjudication Panel: Dr Mark Boon (Conisbrough Group Practice), Dr Karen Forshaw (Bentley Surgery) and Dr Julie Hackney (The Avenue Surgery) for their advice, steer and valued contributions.
We offer special thanks to the members of our oversight committee (TSC): Dr Steve Holmes (Independent Chairperson, GP), Professor Andrew Wilson (Independent Academic GP, University of Leicester), Dr Martyn Lewis (Independent Statistician, Keele University), Zaiada Bibi (Independent Parent Representative) and Camilla Mills (Independent Parent Representative).
The PLEASANT Research Team
Writing Group: Professor Steven A Julious, Dr Michelle J Horspool, Neil Shephard, Mike Bradburn, Dr Amanda Loban, Sarah Davis, Professor Cindy L Cooper, Dr Matthew Franklin (University of Sheffield), Robin May, Rachael Williams and Jennifer Campbell (CPRD).
TMG: Professor Steven A Julious, Dr Michelle J Horspool, Neil Shephard, Dr Amanda Loban, Cara Mooney, David White, Professor Paul Norman, Sarah Davis (University of Sheffield), Dr Jonathan Boote (University of Hertfordshire), Professor W Henry Smithson (University of Cork), Dr Heather Elphick (Sheffield Children’s Hospital), Mr Robin May, Rachael Williams and Jennifer Campbell (CPRD).
TSC: Dr Steve Holmes (Independent Chair, GP), Professor Andrew Wilson (Independent Academic GP, University of Leicester), Dr Martyn Lewis (Independent Statistician, Keele University), Camilla Mills (Independent Parent Representative), Professor Steven A Julious (Chief Investigator, University of Sheffield), Dr Michelle Horspool (Trial Manager, University of Sheffield), Mike Bradburn (Senior Statistician, University of Sheffield) and Dr Amanda Loban (Data Manager, University of Sheffield).
Co-applicants: Professor Steven A Julious, Dr Michelle J Horspool, Professor Paul Norman, Sarah Davis, Professor Cindy L Cooper (University of Sheffield), Dr Jonathan Boote (University of Hertfordshire), Professor W Henry Smithson (University of Cork) and Dr Heather Elphick (Sheffield Children’s Hospital).
GP Adjudication Panel: Dr Mark Boon (Conisbrough Group Practice), Dr Julie Hackney (Avenue Medical Centre) and Dr Karen Forshaw (Bentley Surgery).
Contributions of authors
Steven A Julious (Professor of Medical Statistics), Michelle J Horspool (Trial Manager), Sarah Davis (Health Economist), Mike Bradburn (Senior Statistician), Amanda Loban (Data Manager), Matthew Franklin (Health Economist), Wei Sun Kua (Health Economist), Robin May (CPRD), Jennifer Campbell (CPRD) and Rachael Williams (CPRD) together produced the first draft of the report.
The following conceived of or designed the work: Steven A Julious (Professor of Medical Statistics), Michelle J Horspool (Trial Manager), Sarah Davis (Health Economist), Paul Norman (Professor of Health Psychology), Cindy L Cooper (Sheffield CTRU Director), W Henry Smithson (Professor of Primary Care), Jonathan Boote (PPI Lead) and Heather Elphick (Consultant Respiratory Paediatrician).
The following were involved in the acquisition of data for the work: Jennifer Campbell (CPRD) and Rachael Williams (CPRD).
The following were involved in the interpretation of data for the work: Steven A Julious (Professor of Medical Statistics), Michelle J Horspool (Trial Manager), Neil Shephard (Medical Statistician), W Henry Smithson (Professor of Primary Care), Amanda Loban (Data Manager) and Saleema Rex (Data Analyst).
The following were involved in the analysis of data: Neil Shephard (Medical Statistician), Sarah Davis (Health Economist), Mike Bradburn (Senior Statistician), Matthew Franklin (Health Economist) and Oscar Bortolami (Medical Statistician).
The following drafted the monograph: Steven A Julious (Professor of Medical Statistics), Michelle J Horspool, (Trial Manager), Sarah Davis (Health Economist), Mike Bradburn (Senior Statistician), Amanda Loban (Data Manager), Matthew Franklin (Health Economist), Wei Sun Kua (Health Economist), Robin May (CPRD) and Jennifer Campbell (CPRD).
The following reviewed the work critically for important intellectual content: Steven A Julious (Professor of Medical Statistics), Michelle J Horspool, (Trial Manager), Sarah Davis (Health Economist), Mike Bradburn (Senior Statistician), Paul Norman (Professor of Health Psychology), Neil Shephard (Medical Statistician), Cindy L Cooper (CTRU Director), W Henry Smithson (Professor of Primary Care), Heather Elphick (Consultant Respiratory Paediatrician), Amanda Loban (Data Manager), Matthew Franklin (Health Economist) Robin May (CPRD) and Jennifer Campbell (CPRD).
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Journal articles and reports
Boote J, Julious S, Horspool M, Elphick H, Smithson WH, Norman P. PPI in the PLEASANT trial: involving children with asthma and their parents in designing an intervention for a randomised controlled trial based within primary care. Prim Health Care Res Dev 2016;17:536–48.
Hatfield I, Julious SA, Davis S, Horspool M, Norman P and Mooney C. An Assessment of the Resources Used by General Practices in the Intervention Arm of the PLEASANT Study in Sending out the Intervention. ScHARR Report Series No: 30. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield; 2015.
Horspool MJ, Julious SA, Boote J, Bradburn MJ, Cooper CL, Davis S, et al. Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term (PLEASANT): study protocol for a cluster randomised control trial. BMC Trials 2013;14:297.
Horspool MJ, Julious SA, Mooney C, May R, Sully B, Smithson WH. Preventing and Lessening Exacerbations of Asthma in School-aged children Associated with a New Term (PLEASANT): recruiting primary care research sites – the PLEASANT experience. NPJ Prim Care Respir Med 2015;25:15066.
Conference presentations
Horspool MJ. Recruiting Primary Care Research Sites – The PLEASANT Experience. Invited presentation at Primary Care Respiratory Society UK, Hinkley, September 2014.
Horspool MJ, Julious SA. Recruiting Primary Care Research Sites – The PLEASANT Experience. Presented at International Primary Care Respiratory Group, Athens, May 2014.
Horspool MJ, Julious SA. Preventing and Lessening Exacerbations of Asthma in School age children Associated with a New Term: PLEASANT. Clinical Trials Methodology Conference, Edinburgh, November 2013.
Patient and public involvement reports
Julious SA, Lai J, Boote J, Elphick H, Smithson WH. A Report of a Patient and Public Involvement Consultation Event with Children with Asthma and their Parents to Review a Proposal for a Primary Care Intervention around the September School Return. ScHARR Report Series No: 25. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield; 2011.
Julious SA, Horspool MJ, Boote J, Smithson WH. PLEASANT TRIAL: 2nd Consultation Event with Children with Asthma and their Parents. ScHARR Report Series No: 28. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield: 2012.
Simpson RM, Julious SA, Mooney CD and Norman P. PLEASANT TRIAL: 3rd CONSULTATION Event with Children with Asthma and their Parents. ScHARR Report Series No: 32. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield; 2015.
Data sharing statement
Access to patient-level data is provided by the CPRD for health research purposes and is dependent on approval of a study protocol by the Medicines and Healthcare products Regulatory Agency Independent Scientific Advisory Committee. More information on the Independent Scientific Advisory Committee and the protocol submission process can be found at www.cprd.com/isac (date accessed 30 May 2016).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Campbell MJ, Holgate ST, Johnston SL. Trends in asthma mortality. BMJ 1997;315. http://dx.doi.org/10.1136/bmj.315.7114.1012.
- Storr J, Lenney W. School holidays and admissions with asthma. Arch Dis Child 1989;64:103-7. http://dx.doi.org/10.1136/adc.64.1.103.
- Grech V, Balzan M, Distefano S. Paediatric wheezy admissions at and around school holiday periods. Malta Med J 2004;16:23-6.
- Kimes D, Levine E, Timmins S, Weiss SR, Bollinger ME, Blaisdell C. Temporal dynamics of emergency department and hospital admissions of pediatric asthmatics. Environ Res 2004;94:7-17. http://dx.doi.org/10.1016/S0013-9351(03)00046-X.
- Kimbell-Dunn M, Pearce N, Beasley R. Seasonal variation in asthma hospitalizations and death rates in New Zealand. Respirology 2000;5:241-6. http://dx.doi.org/10.1046/j.1440-1843.2000.00255.x.
- Gergen PJ, Mitchell H, Lynn H. Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner-City Asthma Study (NCICAS). J Pediatr 2002;141:631-6. http://dx.doi.org/10.1067/mpd.2002.127510.
- Harju T, Keistinen T, Tuuponen T, Kivelä SL. Seasonal variation in childhood asthma hospitalisations in Finland, 1972–92. Eur J Pediatr 1997;156:436-9. http://dx.doi.org/10.1007/s004310050632.
- Julious SA, Osman LM, Jiwa M. Increases in asthma hospital admissions associated with the end of the summer vacation for school-age children with asthma in two cities from England and Scotland. Public Health 2007;121:482-4. http://dx.doi.org/10.1016/j.puhe.2006.11.011.
- Silverman RA, Stevenson L, Hastings HM. Age-related seasonal patterns of emergency department visits for acute asthma in an urban environment. Ann Emerg Med 2003;42:577-86. http://dx.doi.org/10.1067/S0196-0644(03)00410-4.
- Fleming DM, Cross KW, Sunderland R, Ross AM. Comparison of the seasonal patterns of asthma identified in general practitioner episodes, hospital admissions, and deaths. Thorax 2000;55:662-5. http://dx.doi.org/10.1136/thorax.55.8.662.
- Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax 2006;61:376-82. http://dx.doi.org/10.1136/thx.2005.042523.
- Gulliford MC, van Staa T, McDermott L, Dregan A, McCann G, Ashworth M, et al. Cluster randomised trial in the General Practice Research Database: 1. Electronic decision support to reduce antibiotic prescribing in primary care (eCRT study). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-115.
- Julious SA, Campbell MJ, Bianchi SM, Murray-Thomas T. Seasonality of medical contacts in school-aged children with asthma: association with school holidays. Public Health 2011;125:769-76. http://dx.doi.org/10.1016/j.puhe.2011.08.005.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- 2010–11 Reference Costs Publication. London: Department of Health; 2011.
- Campbell MK, Elbourne DR, Altman DG. CONSORT group . CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. http://dx.doi.org/10.1136/bmj.328.7441.702.
- Horspool MJ, Julious SA, Boote J, Bradburn MJ, Cooper CL, Davis S, et al. Preventing and lessening exacerbations of asthma in school-age children associated with a new term (PLEASANT): study protocol for a cluster randomised control trial. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-297.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK primary care data resource. Ther Adv Drug Saf 2012;3:89-9. http://dx.doi.org/10.1177/2042098611435911.
- Tate AR, Beloff N, Al-Radwan B, Wickson J, Puri S, Williams T, et al. Exploiting the potential of large databases of electronic health records for research using rapid search algorithms and an intuitive query interface. J Am Med Inform Assoc 2014;21:292-8. http://dx.doi.org/10.1136/amiajnl-2013-001847.
- Horspool MJ, Julious SA, Mooney C, May R, Sully B, Smithson WH. Preventing and Lessening Exacerbations of Asthma in School-aged children Associated with a New Term (PLEASANT): recruiting primary care research sites – the PLEASANT experience. NPJ Prim Care Respir Med 2015;25. http://dx.doi.org/10.1038/npjpcrm.2015.66.
- Julious SA, Horspool MJ, Boote J. PLEASANT Trial: 2nd Consultation Event with Children with Asthma and their Parents. Sheffield: University of Sheffield; 2012.
- Hatfield I, Julious SA, Davis S, Horspool MJ, Norman P, Mooney C. An Assessment of the Resources used by General Practices in the Intervention Arm of the PLEASANT Study in Sending out the Intervention. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield; 2015.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technol Assess 1999;3.
- British Thoracic Society/Scottish Intercollegiate Guideline . British Guideline on the Management of Asthma: A National Clinical Guideline 2012. www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2012 (accessed 5 December 2016).
- Bush A. Diagnosis of asthma in children under five. Prim Care Respir J 2007;16:7-15. http://dx.doi.org/10.3132/pcrj.2007.00001.
- Simpson RM, Julious SA, Mooney C, Norman P. PLEASANT TRIAL: 3rd Consultation Event with Children with Asthma and their Parents. Sheffield: School of Health and Related Research (ScHARR), University of Sheffield; 2015.
- Boote J, Julious S, Horspool M, Elphick H, Smithson WH, Norman P. PPI in the PLEASANT trial: involving children with asthma and their parents in designing an intervention for a randomised controlled trial based within primary care. Prim Health Care Res Dev 2016;17:536-48. http://dx.doi.org/10.1017/S1463423616000025.
- Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials 2012;33:869-80. http://dx.doi.org/10.1016/j.cct.2012.05.004.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Clinical Research Network Industry Costing Template. London: National Institute for Health Research Clinical Research Network; 2015.
- National Schedule of Reference Costs 2013–14: NHS Trusts and NHS Foundation Trusts. London: Department of Health; 2014.
- British National Formulary for Children August 2015. London: BMJ Group and Pharmaceutical Press; 2015.
- DocMail . DocMail Online Price List 2015. www.docmail.co.uk (accessed 25 July 2016).
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Willems DC, Joore MA, Hendriks JJ, Wouters EF, Severens JL. Cost-effectiveness of a nurse-led telemonitoring intervention based on peak expiratory flow measurements in asthmatics: results of a randomised controlled trial. Cost Eff Resour Alloc 2007;5. http://dx.doi.org/10.1186/1478-7547-5-10.
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7. http://dx.doi.org/10.3132/pcrj.2007.00002.
- Briggs AH, Bousquet J, Wallace MV, Busse WW, Clark TJ, Pedersen SE, et al. GOAL Investigators Group . Cost-effectiveness of asthma control: an economic appraisal of the GOAL study. Allergy 2006;61:531-6. http://dx.doi.org/10.1111/j.1398-9995.2006.01038.x.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. http://dx.doi.org/10.1007/s40273-014-0247-6.
- van Asselt AD, van Mastrigt GA, Dirksen CD, Arntz A, Severens JL, Kessels AG. How to deal with cost differences at baseline. PharmacoEconomics 2009;27:519-28. http://dx.doi.org/10.2165/00019053-200927060-00007.
- Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171-85. http://dx.doi.org/10.1080/01621459.1987.10478410.
- Oehlert GW. A note on the delta method. Am Stat 1992;46.
- Yong YV, Shafie AA. Economic evaluation of enhanced asthma management: a systematic review. Value Health 2013;16. http://dx.doi.org/10.1016/j.jval.2013.08.312.
- Stevens K. Valuation of the Child Health Utility Index 9D (CHU9D). Sheffield: University of Sheffield; 2010.
- Papaioannou D, Brazier JE, Paisley S. The Identification, Review and Synthesis of Health State Utility Values from the Literature. Sheffield: University of Sheffield; 2011.
- Powell C, Kolamunnage-Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. MAGNEsium Trial In Children (MAGNETIC): a randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol Assess 2013;17.
- Price D, Musgrave S, Wilson E, Sims E, Shepstone L, Blyth A, et al. A pragmatic single-blind randomised controlled trial and economic evaluation of the use of leukotriene receptor antagonists in primary care at steps 2 and 3 of the national asthma guidelines (ELEVATE study). Health Technol Assess 2011;15.
- Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009;103:1633-42. http://dx.doi.org/10.1016/j.rmed.2009.06.014.
- Chiou CF, Weaver MR, Bell MA, Lee TA, Krieger JW. Development of the multi-attribute Pediatric Asthma Health Outcome Measure (PAHOM). Int J Qual Health Care 2005;17:23-30. http://dx.doi.org/10.1093/intqhc/mzh086.
- Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. PharmacoEconomics 1999;15:369-76. http://dx.doi.org/10.2165/00019053-199915040-00004.
- Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Ferrie PJ. Minimum skills required by children to complete health-related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J 1997;10:2285-94. http://dx.doi.org/10.1183/09031936.97.10102285.
- Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17520.
- Doull I, Price D, Thomas M, Hawkins N, Stamuli E, Tabberer M, et al. Cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhaler in chronic asthma. Curr Med Res Opin 2007;23:1147-59. http://dx.doi.org/10.1185/030079907X187982.
- Rodríguez-Martínez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Cost-utility analysis of the inhaled steroids available in a developing country for the management of pediatric patients with persistent asthma. J Asthma 2013;50:410-18. http://dx.doi.org/10.3109/02770903.2013.767909.
- Carroll AE, Downs SM. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr 2009;155:21-5.e1.
- Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 2007;62:149-53. http://dx.doi.org/10.1111/j.1398-9995.2006.01310.x.
- Gerald JK, Grad R, Bailey WC, Gerald LB. Cost-effectiveness of school-based asthma screening in an urban setting. J Allergy Clin Immunol 2010;125:643-50.e1.
- Brazier J, Ratcliffe J, Solomon J, Tsuchiya A. Measuring and valuing health benefits for economic valuation. PharmacoEconomics 2007;25. http://dx.doi.org/10.2165/00019053-200725040-00007.
- Cheung K, Oemar M, Oppe M, Rabin R. User Guide: BASIC Information on How to Use EQ-5D. Rotterdam: EuroQol Research Foundation; 2009.
- Brazier JE, Rowen D. Alternatives to EQ-5D for Generating Health State Utility Values. Sheffield: University of Sheffield; 2011.
- Steuten L, Palmer S, Vrijhoef B, van Merode F, Spreeuwenberg C, Severens H. Cost-utility of a disease management program for patients with asthma. Int J Technol Assess Health Care 2007;23:184-91. http://dx.doi.org/10.1017/S0266462307070298.
- Tsuchiya A, Brazier JE, McColl E, Parkin D. Deriving Preference-Based Condition-Specific Instruments: Converting AQLQ into EQ-5D Indices. Sheffield: University of Sheffield; 2002.
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy 2004;59:701-8. http://dx.doi.org/10.1111/j.1398-9995.2004.00533.x.
- Eccles M, Grimshaw J, Steen N, Parkin D, Purves I, McColl E, et al. The design and analysis of a randomized controlled trial to evaluate computerized decision support in primary care: the COGENT study. Fam Pract 2000;17:180-6. http://dx.doi.org/10.1093/fampra/17.2.180.
- Price D, Brown RE, Lloyd A. Burden of poorly controlled asthma for patients and society in the UK. Prim Care Respir J 2004;13.
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. GOAL Investigators Group . Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med 2004;170:836-44. http://dx.doi.org/10.1164/rccm.200401-033OC.
Appendix 1 Trial intervention
GP letterhead
<Address line 1>
<Address line 2>
<Address line 3>
<Address line 4>
<Insert Date>
Dear Parent
Please read this important letter regarding your child’s asthma
It is really important that your child continues to take their asthma medication during the summer holidays. Returning to school is a time when asthma can get worse and make children and young people with asthma poorly. This may be due to contact with infections at the start of the new school year.
To reduce the chances of getting poorly when they return to school, your child should continue to take their asthma medication as prescribed by their GP or practice nurse. If your child has stopped taking their medication over the summer holidays it is important to start it again as soon as possible. If they are short of medication, or you are not sure of the proper dose, please get in touch with the practice.
Yours sincerely
<Name of Doctor>
Appendix 2 Changes to protocol
| Changes to protocol | Outcome | Research ethics committee approval date | Approved by |
|---|---|---|---|
| Protocol version 2 (14 May 2015): this version included an additional secondary outcome to include data up to September 2014, to see if the effect from September 2013 is maintained when there is no study intervention, thus extending the follow-up period by 1 month (see Chapter 2, Changes to the data collection, data extraction and methods for allocation of data after the trial commenced, with reasons) | Agreed as a 2-month, non-cost contact variation by the HTA programme on 2 February 2015 | 25 May 2014 | National Research Ethics Service Committee Yorkshire & The Humber – South Yorkshire |
Appendix 3 Data management process: allocation of medical contacts and follow-up data
All types of ‘consultation’ are recorded within the data that the CPRD provides. For the purpose of this study, each consultation is considered a medical contact, but not all consultations are considered relevant to the study. According to the protocol, a scheduled contact is any contact that is part of the planned care for the patient, for example an asthma review, a medical review, repeat prescription or immunisation. An unscheduled contact is any unplanned contact that is either patient initiated or is a result of illness.
Details of how this has been applied and other assumptions to propose the allocation of medical contacts as ‘scheduled’ and ‘unscheduled’ are described in this appendix.
Data received from the Clinical Practice Research Datalink
Initial test data set received 10 May 2013.
First baseline data set received 19 December 2013.
Second baseline data set received 3 February 2014.
Third baseline data set received 13 July 2014.
Baseline and follow-up data set received 19 January 2015.
Clinical Practice Research Datalink data
Data from the consultation, clinical, immunisation, test, referral and therapy tables from the CPRD Gold Data dictionary were used.
Overview
Figure 26 shows a very broad overview of how the data have been processed and the number of records. Full details of assumptions are now described.
FIGURE 26.
Decision tree showing an overview of how medical contacts have been allocated.
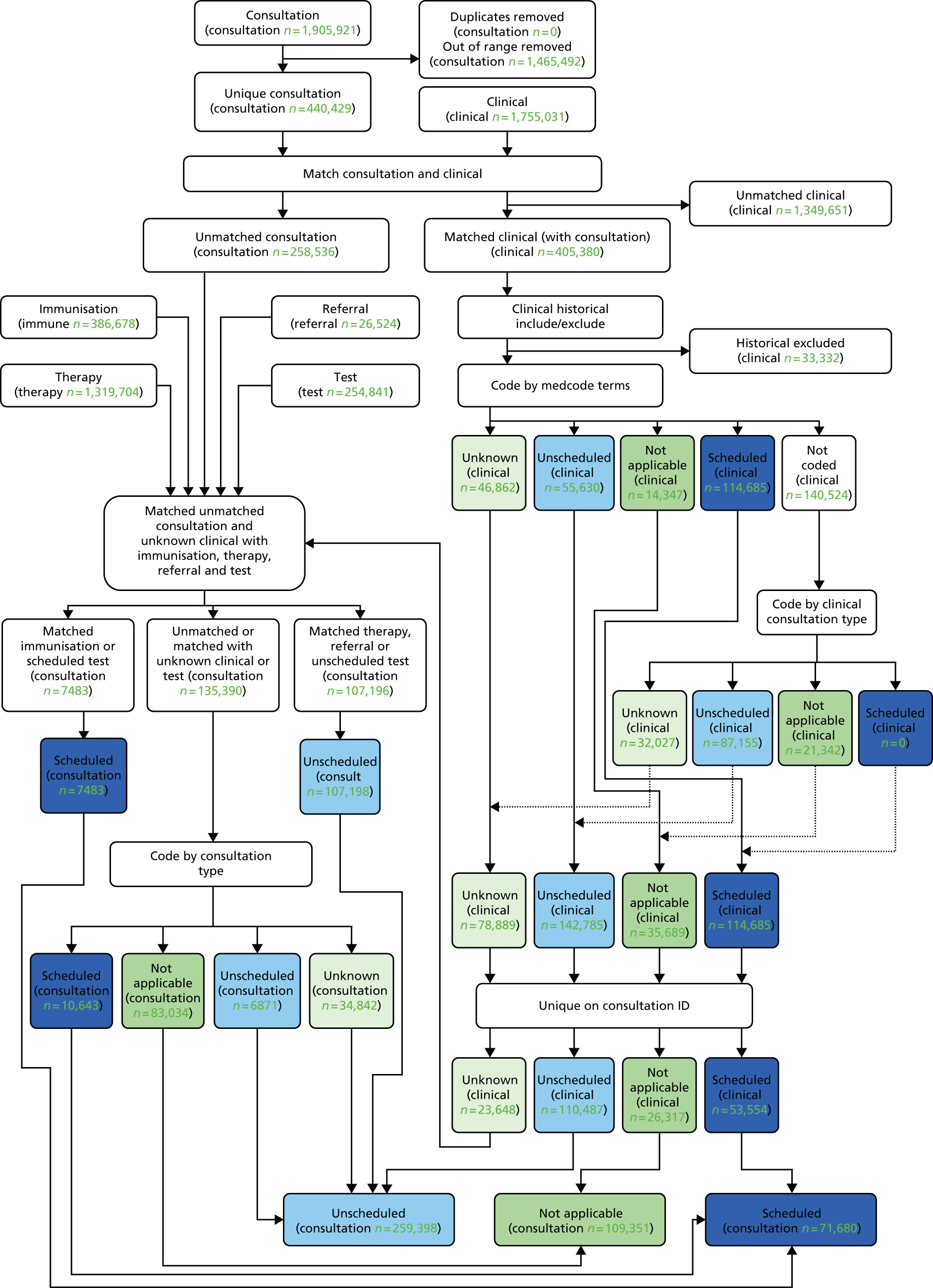
General assumptions
One ‘consultation’ (based on the combination of patient ID, practice ID and consultation ID) in the consultation table is considered one contact.
All consultation data supplied, not just those that are asthma related, are taken into account for the study.
Only consultations that happened on or after 1 August 2012 are included.
Assumptions used to code records as scheduled or unscheduled (contact type) are based on clinical, immunisation, therapy, referral, test and consultation data.
Records with unmatched event dates
Each consultation record is supplied with an event date; this event date does not always match the event date recorded for that consultation within the other tables. This is most likely a result of information entered into the database historically. Those contacts within the clinical table were included if they were relevant and unlikely to be duplicated (see Inclusion of ‘unmatched/historical’ data section for more details). All immunisation, therapy, referral and test records that did not match the event date supplied in the consultation data were excluded.
Clinical data
Clinical data are linked to consultation data, and all matched records are included.
Inclusion of ‘unmatched/historical’ data
If the event date does not match but the clinical event date is within the dates of interest (i.e. from 1 August 2012 to 30 September 2014), then those records that are both relevant and unlikely to be duplicated are included. The decision on which records to include was made by the GP Adjudication Panel after reviewing the most common unique terms (10% of the terms, which covered 88% of the data). The most common 10 terms and the decision of whether or not to include them is shown in Table 25 for information; rules based on this review were used to decide whether or not to include the 12% of data not reviewed, for example ‘if it contains seen it is relevant and unlikely to be duplicated’.
| Medcode_desc | Records | Flag |
|---|---|---|
| Seen in paediatric clinic | 5214 | Relevant and unlikely to be duplicated |
| Seen in hospital casualty | 4863 | Relevant and unlikely to be duplicated |
| Letter received | 2901 | Irrelevant |
| Letter encounter | 2654 | Irrelevant |
| Administration | 2167 | Irrelevant |
| Seen in orthopaedic clinic | 1699 | Relevant and unlikely to be duplicated |
| Letter from consultant | 1587 | Likely to be duplicated |
| Letter from specialist | 1488 | Likely to be duplicated |
| Discharge summary | 1479 | Likely to be duplicated |
| Asthma | 1398 | Likely to be duplicated |
Summary of coding using clinical data
The records included are used to determine scheduled or unscheduled contacts based on ‘medcode description’; the clinical data references Pegasus medical data using the field ‘medcode’ to get ‘medcode description’. If contact types cannot be determined by ‘medcode description’, then clinical consultation type ‘constype’ (consultation type) is referenced.
Following GP Adjudication Panel review of the medcode descriptions, in which over 90% of the data were reviewed (17% of the unique terms), clinical records to be marked as scheduled, unscheduled, not applicable or unknown were identified based on terms (see Boxes 1–4 and Tables 26 and 27 for examples; full details are available on request). Based on this review, rules to apply to the data were also determined (see Table 26). Finally, decisions on how to code the remaining records based on the clinical consultation type were made (see Table 27).
Administration of medication under patient group direction.
Administration of medication under patient-specific direction.
Antimalarial drug prophylaxis.
Asthma annual review.
Asthma causes daytime symptoms once or twice per month.
Asthma causes daytime symptoms once or twice per week.
Asthma causes daytime symptoms most days.
Asthma causes night-time symptoms once or twice per month.
Asthma causes symptoms most nights.
Asthma causes night waking.
Asthma control questionnaire.
Abdomen examined – NAD.
Abdominal examination – NAD.
Breast examination.
CVS examination.
CVS examined – NAD.
Ear examination – normal.
Emergency appointment.
Examination of cardiovascular system.
Examination of digestive system.
Examination of abdomen.
[V]Healthy person accompanying sick person.
Advice to GP to change patient medication.
Did not attend – no reason.
Discharged from hospital.
Discharged from accident and emergency.
Discharged from inpatient care.
Drug not collected – no reason.
Employment milestones.
Failed encounter.
Failed encounter – message left on answer machine.
[V]Issue of repeat prescription.
Advice.
Asthma.
Asthma medication review.
Asthma monitoring due.
Asthma NOS.
Bronchial asthma.
Change in asthma management plan.
Clinical management plan agreed.
Family history.
| Medcode description | Rule |
|---|---|
| Letter rule | If contains ‘letter’ then scheduled, unless it contains ‘referral’; in that case not applicable |
| Pain/inflamed/sore rule | If contains ‘pain’, ‘inflamed’ or ‘sore’, then unscheduled |
| Asthma trigger rule | If contains ‘asthma trigger’ then scheduled, unless it contains ‘infection’; then unscheduled |
| Immunisation/vaccination rule | If does not contain ‘letter’ and contains ‘immunisation’, ‘immunisats’, ‘imm’, ‘vacc’ or ‘vaccination’, then scheduled, unless it contains ‘flu’, ‘Influenza’, ‘advice’, ‘not consent’, ‘declined’ or ‘requires’; in that case ‘unknown’ |
| Exam/examination rule | If contains ‘exam’ or ‘examination’, then unscheduled, unless it contains ‘chest’, ‘respiratory’, ‘lung’, ‘breath’, ‘foot’ or ‘eye’; then scheduled |
| Asthma management plan rule | If contains ‘asthma management plan’ then scheduled, unless it also contains ‘change’ or ‘step up’; in that case unknown |
| Referral rule | If contains ‘referral’ then not applicable, unless it contains ‘a and e’, ‘accident and emergency’ or ‘admission’; in that case unscheduled |
| Blank rule | If blank, then unscheduled |
| DNA rule | If contains ‘did not attend’ or ‘DNA’, then not applicable |
| Failed encounter rule | If contains ‘failed encounter’, then not applicable |
| Lloyd George rule | If contains ‘Lloyd George’, then not applicable |
| Seen rule | If contains ‘seen’, scheduled, unless it contains ‘accident’, ‘emergency’, ‘out of hours’, ‘GP’, ‘A&E’, ‘rota’, ‘primary care centre’, ‘patient call’, ‘triage’, ‘co-op’, ‘coop’, ‘co op’, ‘walk in’, ‘ooh’, ‘injury’, ‘on call’, ‘home visit’, ‘urgent’, ‘surgery’, ‘doctor’, in which case unscheduled |
| Out-of-hours rule | If contains ‘out of hours’, then unscheduled |
| Emergency rule | If contains ‘emergency’, then unscheduled |
| Discharge report | If contains ‘discharge report’, then unscheduled |
| Clinical consultation type | Code as |
|---|---|
| Administration | Not applicable |
| Symptom | Unscheduled |
| Diagnosis | Unscheduled |
| Intervention | Unscheduled |
| Management | Unscheduled |
| Presenting complaint | Unscheduled |
| Examination | Unknown |
Conflicting clinical contact types
Clinical data contain more than one record per consultation. In some cases, the same consultation ID can have more than one clinical contact type. For these clinical records, we assume that unscheduled takes precedence (i.e. they are likely to have come in for an unscheduled visit but had a scheduled ‘type’ of procedure at the same time) over all other contact types; that scheduled takes precedence over not applicable and unknown; and that unknown takes precedence over not applicable.
Clinical to consultation
The code assigned in accordance with clinical contact type as described above is linked to the consultation data.
Consultation data marked as ‘unknown’ based on the clinical data as well as consultation data that did not link to clinical data are coded based on immunisation, therapy, referral, test and consultation data as described in the following sections.
Immunisation data
Uncoded consultation data are matched against immunisation data; if at least one match is found in the immunisation record, then these are marked as ‘scheduled’.
Therapy
Those that do not match with immunisation data are linked to medication data. If at least one match is found, they are marked as ‘unscheduled’.
Referral
Those that do not match with immunisation or therapy data are linked to referral data. If at least one match is found, they are marked as ‘unscheduled’.
Test
Those that are still uncoded are linked to test. If linked to test it is coded as either ‘scheduled’, ‘unknown’ or ‘unscheduled’ based on a review of the data. In general, if the test is part of the routine asthma review, then it is coded as ‘scheduled’; if it is testing peak expiratory flow rate then it is coded as ‘unknown’. Otherwise, it is coded as ‘unscheduled’ (full details are available on request).
When a consultation links to more than one record, the same rules of precedence apply as outlined in the Conflicting clinical contact types section.
Consultation data
For consultation data that are still ‘unknown’, ‘unlinked’ and, therefore, uncoded, consultation type is used to determine whether it is scheduled, unscheduled or not applicable (see Table 28 for a summary; full details are available on request). In this way, all contacts will now be coded as either ‘scheduled’, ‘unscheduled’ or ‘not applicable’.
| Consultation type | Coded as |
|---|---|
| Follow-up/routine visit | Scheduled |
| Repeat issue | |
| Community clinic | |
| Medicine management | |
| If the consultation is about out-of-hours visits, telephone calls, acute visits, inpatient/hospital admission, accident and emergency attendance, triage, home/hotel visit, walk-in centre, co-op surgery or injury | Unscheduled |
| Clinic | Unscheduled (it is assumed that most scheduled consultation types will be clearly recorded) |
| Surgery consultation | |
| Other | |
| If the consultation type is ‘Data not entered’, or if it is about correspondence, reports, administration, nursing/residential home visits, test results or non-consultation data | Not applicable |
Emergency contacts
In addition to coding as scheduled, unscheduled and not applicable, some consultation types from the consultation table were coded as emergency (Table 29).
| Code | Consultation type | Relevant medical contact? | Unscheduled/scheduled | Emergency |
|---|---|---|---|---|
| 2 | Night visit, deputising service | Yes | Unscheduled | Yes |
| 4 | Night visit, local rota | Yes | Unscheduled | Yes |
| 6 | Night visit, practice | Yes | Unscheduled | Yes |
| 7 | Out of hours, practice | Yes | Unscheduled | Yes |
| 8 | Out of hours, non-practice | Yes | Unscheduled | Yes |
| 11 | Acute visit | Yes | Unscheduled | Yes |
| 18 | Emergency consultation | Yes | Unscheduled | Yes |
| 20 | Casualty attendance | Yes | Unscheduled | Yes |
| 23 | Hospital admission | Yes | Unscheduled | Yes |
| 24 | Children’s home visit | Yes | Unscheduled | Yes |
| 27 | Home visit | Yes | Unscheduled | Yes |
| 28 | Hotel visit | Yes | Unscheduled | Yes |
| 32 | Twilight visit | Yes | Unscheduled | Yes |
| 34 | Walk-in centre | Yes | Unscheduled | Yes |
| 37 | Co-op home visit | Yes | Unscheduled | Yes |
| 50 | Night visit | Yes | Unscheduled | Yes |
Appendix 4 Statistical analysis plan
Appendix 5 Systematic review of health-related quality of life data to inform health economic analysis
This review aimed to identify preference-based utility values for asthma day-to-day symptoms (baseline utility) and asthma exacerbation.
Scoping
A scoping search was conducted to establish the likely quantity and relevance of published literature. This was done by searching MEDLINE and The Cochrane Library (Cochrane Database of Systematic Review, HTA database and NHS Economic Evaluation Database) using a limited number of population terms in addition to a search filter for quality of life. It was found that there was a lack of utility data derived from EQ-5D in children with asthma. Although EQ-5D is the preferred outcome measure, the standard version of EQ-5D is not designed to be used with children. EQ-5D-Youth is available for children and adolescents, but there is not yet a validated UK tariff. In view of this, the NICE reference case states to use other validated preference-based measures developed for children, but does not specify the preferred quality-of-life instrument. 35 Therefore, a broad approach was taken in the search to identify utility values derived from EQ-5D, as well as other preference-based measures. EQ-5D values estimated from mapping studies were also considered.
Search strategy
Search terms
Both free text and medical subject headings (MeSH) pertaining to children, asthma and asthma exacerbation were used in the search (see Appendix 6). The InterTASC Information Specialists’ Sub-Group (ISSG) search filter was used to filter studies that report HRQoL (see Appendix 7). The filter was adapted to include a newly developed preference-based measure for children, Child Health Utility Index 9D,44 as well as other preference-based measures in asthmatic children, such as Asthma Symptom Utility Index. Full search terms for this review are presented Appendices 6 and 7.
Search limit
The search was not limited by language, publication type, publication dates or study design, with the aim of increasing sensitivity.
Sources searched
The following clinical and economic databases were searched:
-
MEDLINE (via Ovid) (In-Process & Other Non-Indexed Citations) (1946 to 5 July 2014)
-
The Cochrane Library (includes Cochrane Database of Systematic Review, NHS Economic Evaluation Database and HTA database) (up to 5 July 2014)
-
EMBASE (1974 to 5 July 2014)
-
EconLit (1886 to 5 July 2014)
-
School of Health and Related Research (ScHARR) Health Utilities Database (up to 5 July 2014).
In addition to the electronic database search, reference lists of the retrieved papers were screened for relevant papers.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for the review are summarised in Table 30. Systematic reviews and protocols were not included, but were used to identify relevant papers. Modelling studies were examined to determine the source of utility values used. Modelling studies that described utility data not reported elsewhere were included in the review.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population |
|
|
|
|
|
| Intervention |
|
|
| Outcomes |
|
|
| Publication type |
|
|
| Language/others |
|
|
Selection of studies
In the first stage of study selection, titles and abstracts of the searched results were screened against the inclusion/exclusion criteria. Full articles were assessed if titles and abstracts were unclear. All studies identified at titles and abstracts were further screened at full text. The studies were screened by a single reviewer.
Quality assessment
Quality assessment of articles in this review followed the criteria (sample size, number loss at follow-up and handling of missing data) recommended by Papaioannou et al. 45 in the Decision Support Unit Technical Support Document on the identification, review and synthesis of health state utility values from the literature.
Data extraction
Data extracted comprised characteristics of study population, study design and details of outcome measurements (descriptive system, tariff used, method of valuation, time of measurement, mean utility data and other relevant measures).
Selection of utility data for use in the economic analysis
Selection of utility data to use in the economic analysis was based on (1) quality of the study; (2) the relevance of utility data to the population and health states in the PLEASANT study; and (3) the extent to which the measurement method was in accordance with the NICE reference case.
Results of systematic review of health-related quality-of-life data
A total of 927 studies were retrieved from the database search and reference tracking. After removal of duplicates, 683 studies were screened at titles and abstract. A total of 659 studies were excluded at this stage (see Appendix 8). Subsequently, 24 papers were screened at full text and 10 papers were excluded, with reasons given in Appendix 9. Finally, 14 papers were included in this review. Figure 27 shows the search process of this review.
FIGURE 27.
Flow diagram of search process. EQ-VAS, EuroQol Visual Analogue Scale.

Study characteristics for the included studies are summarised in Table 31. The study populations are summarised in Table 32 and methods used to measure HRQoL are summarised in Table 33. Details regarding study quality are provided in Table 34. Details regarding the suitability of the studies for use in the economic model, based on the criteria described above, are provided in Table 35.
| Number | First author, year | Country | Study design | Total participants | Duration | Intervention | Control | Primary outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Willems et al., 200736 | The Netherlands | Economic evaluation alongside a RCT | 109 (56 aged under 18 years) | 1 year | Nurse-led telemonitoring | Usual care | Cost per QALY |
| 2 | Powell et al., 201346 | UK | Multicentre, double-blind RCT | 508 children | 1 month | Nebulised magnesium sulphate | Usual care | Asthma severity score 1 hour after treatment |
| 3 | Price et al., 201147 | UK | Single-blind RCT, pragmatic | 687 (mixed age group, 12–80 years) | 2 years | Leukotriene receptor antagonist (step 2), as above plus ICS (step 3) | ICS (step 2), ICS + LABA (step 3) | Changes in Mini-AQLQ |
| 4 | Brusselle et al., 200948 | Belgium | Cohort | 158 (mixed age group) | 1 year | Omalizumab | N/A (single-arm study) | Clinical effectiveness (asthma symptoms, lung function, HRQoL) and safety of omalizumab |
| 5 | Chiou et al., 200549 | USA | Outcome measure was used in the baseline assessment of a RCT | Utility measurement was performed on a sample of 72 children from the RCT | Baseline utility measurement | Environmental intervention | Placebo | HRQoL |
| 6 | Mittmann et al., 199950 | Canada | Cross-sectional survey | 17,626 household residents of which 229 had asthma | Cross-sectional | None | None | HRQoL |
| 7 | Juniper et al., 199751 | Canada | Cohort | 52 children | 9 weeks | None | None | Validity of outcome measures in children |
| 8 | Norman et al., 201352 | UK | Decision model, EQ-5D data from the EXALT study used for day-to-day symptoms. Literature-based estimate used for exacerbation | EXALT: 404 (mixed age group) | EXALT: 36 weeks | Omalizumab and usual care | Usual care | Cost per QALY |
| 9 | Briggs et al., 200638 | Multinational | Economic evaluation of GOAL (multinational, double-blind RCT), CSM data from the GOAL study were mapped onto EQ-5D | GOAL: 3416 (mixed age group) | GOAL: 1 year, model as weekly event | Salmeterol/fluticasone | Fluticasone | Cost per QALY |
| 10 | Doull et al., 200753 | Multinational | Decision model, CSM data from GOAL (multinational, double-blind, RCT) were mapped onto EQ-5D | GOAL: 3416 (mixed age group) | GOAL: 1 year, model as weekly event | Salmeterol/fluticasone | Fluticasone | Cost per QALY |
| 11 | Rodríguez-Martínez et al., 201354 | Spain | Decision model (Markov), utility values were derived from a utility valuation survey | 76 parents were involved in the survey | Utility measured at one time point | Budesonide, fluticasone, ciclesonide | Beclomethasone dipropionate | Cost per QALY |
| 12 | Carroll and Downs, 200955 | USA | Cross-sectional | 4016 parents, each valued 3 of 29 health states (≈415 valuations per health state) | Duration of recruitment: 2 years. HRQoL measurement was performed at a time point | None | None | Utility values |
| 13 | Brown et al., 200756 | Multinational | Decision model (Markov), CSM data from the ETOPA study (open-label trial) were mapped onto EQ-5D | ETOPA: 312 (mixed age group) | 1 year | Omalizumab and BSC | BSC | Cost per QALY |
| 14 | Gerald et al., 201057 | USA | Decision model (decision tree and Markov) | Utility data based on study by Chiou et al.49 | Time horizon: 1 year, cycle length: 1 day | Four school-based asthma screening strategies | Status quo | Cost per QALY |
| First author, year | Disease type | Severity/stage | Age (years) | Male sex | Ethnicity |
|---|---|---|---|---|---|
| Willems et al., 200736 | Mild to moderate asthma managed in outpatient care | GINA stages I–III, mean FEV1% predicted for children: 96.5 (SD 8.4) for intervention and 99.4 (SD 11.3) for control | 7–18 strata. Intervention, mean 10.57 (SD 2.1); control, mean 10.85 (SD 2.3) | Intervention, 72.4%; control, 55.6% | Not reported |
| Powell et al., 201346 | Acute asthma | Severe acute asthma (BTS/SIGN definition) | Median 4.0 (IQR 3.0–7.0), range 2–16 | 58% | Not reported |
| Price et al., 201147 | Poorly controlled asthma at BTS/SIGN step 2 or 3 | ACQ ≥ 1 or Mini-AQLQ ≤ 6 | Step 2, mean 44.74 (SD 16.49); step 3, mean 50.02 (SD 15.93); range 12–80 | Step 2, 162 (49.7%); step 3, 136 (37.7%) | 98% Caucasian |
| Brusselle et al., 200948 | Poorly controlled severe persistent allergic asthma on ICS/LABA (GINA definition) | Mean FEV1% < 80% predicted, day and night symptoms, ≥ two exacerbations (requiring systematic steroid, emergency department or hospitalisations) in past 2 years | Mean 48.17 (SD 17.18), range 12–83 | 73 (46.2%) | 94.9% Caucasian |
| Chiou et al., 200549 | Diagnosed asthma | Mild to severe | Age group: 7–8, 37.5%; 9–10, 34.7%, 11–12, 27.8% | Not reported | White (15.3%), Asian (40.3%) and African American (29.2%) |
| Mittmann et al., 199950 | Asthma generally | Not reported | Not reported. 10.5% (n = 1847) of total respondents were under 19 years | 8058 (45.7%), but data were not stratified to age | Caucasian |
| Juniper et al., 199751 | Symptomatic asthma | Mean FEV1% predicted: 85 ± 16.6. No previous exacerbation in past 2 weeks | Mean 12 (SD 3.1), range 7–17 | 30 (57.7%) | Majority Caucasian |
| Norman et al., 201352 | Poorly controlled severe persistent allergic asthma on high-dose ICS and LABA with > one severe exacerbation in previous year and FEV1 < 80% predicted | BTS/SIGN ≥ step 4 EXALT: FEV1 40–80% predicted. > one severe exacerbation within previous year | EXALT: mean across both arms 44.7, range 12–75 (only five patients under 18 years) | EXALT: 141 (35.2%) | Not reported |
| Briggs et al., 200638 | Diagnosed asthma (≥ 6 months), no use of LABA or oral β2-agonists in previous 2 weeks | Uncontrolled, mean FEV1% predicted: ranged from 76 to 79 | SFC: stratum 1, mean 36.1 (SD 15.6); stratum 2, mean 40.4 (SD 16.4), stratum 3; mean 44.1 (SD 15.9) | 42% | Not reported |
| FC: stratum 1, mean 36.4 (SD 15.6); stratum 2, mean 40.3 (SD 16.6); stratum 3, mean 42.7 (SD 15.7) | |||||
| Doull et al., 200753 | Diagnosed asthma (≥ 6 months), no use of LABA or oral β2-agonists in previous 2 weeks | Uncontrolled, mean FEV1% predicted: ranged from 76 to 79 | SFC: stratum 1, mean 36.1 (SD 15.6); stratum 2, mean 40.4 (SD 16.4); stratum 3, mean 44.1 (SD 15.9) | 42% | Not reported |
| FC: stratum 1, mean 36.4 (SD 15.6); stratum 2, mean 40.3 (SD 16.6); stratum 3, mean 42.7 (SD 15.7) | |||||
| Rodríguez-Martínez et al., 201354 | Persistent asthma | Mild to moderate | Not reported | Not reported | Caucasian |
| Carroll and Downs, 200955 | Persistent asthma | Mild to severe | Not reported | 1982 (49%) (sex of parent’s child) | African American (48%) and Caucasian (47%) |
| Brown et al., 200756 | Poorly controlled severe persistent allergic asthma despite high-dose ICS and LABA | Subgroup of severe patients from ETOPA included | For whole ETOPA trial: omalizumab, mean 37.5 (range 12–73); best supportive care, mean 39.3 (range 12–71) | For whole ETOPA trial: omalizumab and best supportive care, 58 (28.2%); best supportive care 34 (32.1%) | Caucasian |
| Gerald et al., 201057 | Asthma symptom-free day, symptom days, exacerbation recovery days, emergency department visits, and hospitalisation days | Intermittent, mild, moderate, severe | Utility data based on study by Chiou et al.49 | Utility data based on study by Chiou et al.49 | Utility data based on study by Chiou et al.49 |
| First author, year | Descriptive system | Type | Descriptive measure filled by | Population in valuation | Valuation method | When HRQoL data were obtained | Utility estimates, mean (SD) | Other HRQoL measures |
|---|---|---|---|---|---|---|---|---|
| Willems et al., 200736 | EQ-5D (child version) | Generic | Carer (age < 12 years), ≥ 12 years by patient | Adult UK tariff | TTO | Baseline, 4 months, 8 months and 12 months | 7–18 years strata at baseline: usual care, 0.96 (0.07); telemonitoring, 0.92 (0.20) | PAQLQ |
| Powell et al., 201346 | EQ-5D | Generic | Carer of children age between 5 and 16 years | Adult UK tariff | TTO | 1 month post exacerbation | Exacerbation: 0.52 (based on mean ASS of 5.8 mapped to EQ-5D 22222) | PedsQL™ |
| 1 month: magnesium group, 0.86 (0.04); standard care, 0.88 (0.04) | ||||||||
| Price et al., 201147 | EQ-5D | Generic | Patient | Adult UK tariff | TTO | 2 months and 2 years | Step 2 at baseline: intervention, 0.795 (0.245); control 0.830 (0.195) | Mini AQLQ, asthma control questionnaire |
| Step 3 baseline: intervention, 0.780 (0.237); control 0.772 (0.234) | ||||||||
| Brusselle et al., 200948 | EQ-5D | Generic | Patient | Belgian tariff | VAS | Baseline, 52 weeks | At baseline: 0.54 (0.24) | AQLQ |
| Chiou et al., 200549 | PAHOM | Population-specific measure | Patient | Adults valuing for children | VAS, SG | Single time point | General asthma (VAS 0.7, converted SG 0.83) | None |
| Mittmann et al., 199950 | HUI-3 | Generic | Participant was interviewed by telephone or in person | HUI-2 (Canada algorithm) 293 parents of school children | VAS, SG | Single time point | 12–19 years: 0.90 (0.12) | None |
| Juniper et al., 199751 | HUI (interviewer version) | Generic | Children | HUI-2 (Canada algorithm) 293 parents of school children | VAS, SG | Baseline, week 5 and week 9 | At baseline: 0.89 (SD 0.09) (range 0.67–1.00) | PAQLQ, feeling thermometer, direct valuation via SG |
| Norman et al., 201352 | EQ-5D | Generic | Patients | Not stated | Not stated | 31 weeks | 31 weeks: standard care, 0.719 (0.026); omalizumab, 0.767 (0.02) | AQLQ |
| Briggs et al., 200638 | Mapped EQ-5D from AQLQ | Mapping of CSM to EQ-5D | Patient | Valuation population not reported | Valuation method not reported | Baseline, 12, 24, 36 and 52 weeks | Totally controlled: 0.946 (SE 0.011) | AQLQ |
| Well controlled: 0.900 (SE 0.011) | ||||||||
| Not well controlled: 0.842 (SE 0.011) | ||||||||
| Exacerbation: 0.729 (SE 0.013) | ||||||||
| Doull et al., 200753 | Mapped EQ-5D from AQLQ | Mapping of CSM to EQ-5D | Patient | Valuation population not reported | Valuation method not reported | Baseline, 12, 24, 36, and 52 weeks | Symptom free: 0.97 (0.014) | AQLQ |
| With symptoms: 0.85 (0.015) | ||||||||
| Rodríguez-Martínez et al., 201354 | Direct valuation using vignettes | Direct valuation | N/A | Parents | SG | Single time point | No symptoms, 0.989; symptom no exacerbation, 0.705; and asthma exacerbation (0.275) | None |
| Carroll and Downs, 200955 | Direct valuation using vignettes | Direct valuation | N/A | Parents | TTO, SG | Single time point | SG: mild intermittent, 0.91 (0.18); mild persistent, 0.90 (0.18); moderate persistent, 0.88 (0.18); severe persistent asthma, 0.83 (0.21); 10-day hospitalisation, 0.94 (0.14) | None |
| TTO: mild intermittent, mild persistent, 0.91 (0.17); moderate persistent, 0.91 (0.15); severe persistent asthma, 0.85 (0.20); 10-day hospitalisation, 0.95 (0.15) | ||||||||
| Brown et al., 200756 | Mapped EQ-5D from mini AQLQ | Mapping of CSM to EQ-5D | Patient | UK adult tariff for EQ-5D | TTO for EQ-5D | Baseline and 52 weeks | Daily symptoms, baseline: best supportive care, 0.62; omalizumab, 0.58 | Mini AQLQ |
| Daily symptoms, week 52: best supportive care, 0.65; omalizumab, 0.82 | ||||||||
| Gerald et al., 201057 | PAHOM | Population-specific measure | N/A (utility data for modelled states were estimated by averaging utility values of PAHOM states) | PAHOM: adults valuing for children | VAS, SG | N/A | Asthma symptom-free day, 1.0 (range 0.98–1.0); symptomatic, 0.90 (range 0.84–0.96); recovery, 0.70 (range 0.64–0.76); emergency department, 0.43 (range 0.37–0.049); hospitalisation, 0.06 (range 0.01–0.11) | None |
| First author, year | Sample size | Number loss at follow-up | Methods of handling missing data |
|---|---|---|---|
| Willems et al., 200736 | 109 (mixed age group) | 7/109 (four children) | Data imputation by using mean for baseline score, interpolation between scores and last value carried forward |
| Powell et al., 201346 | 508 children | Postal survey response rate: 45%. 228 completed PedsQL™. 89 patients aged over 5 years completed EQ-5D questionnaires (46 in magnesium arm and 43 in placebo) | Multiple imputation by chained equations was used to impute missing data. In under-fives the EQ-5D scores were estimated by mapping from the PedsQL™ scores. EQ-5D scores at time of exacerbation were mapped subjectively from ASSs |
| Price et al., 201147 | 687 (mixed age group) | Step 2: 20/326 excluded post randomisation, 13/306 loss to follow-up, but 300/306 had some data post randomisation | Complete data in 218/683 patients (32%), data available for ≥ 10 out of 13 items: 514/683 (75%). 19% missing visit 2 EQ-5D data. Complete-case analysis presented. In addition, imputed case presented using Rubin’s multiple imputation |
| Step 3: 9/361 excluded post randomisation, 12/352 were lost to follow-up, but 350/352 had some data post randomisation | |||
| Brusselle et al., 200948 | 158 (mixed age group) | Only 126 of 158 patients had baseline EQ-5D values and only 67 had EQ-5D data at 1 year | Not reported |
| Chiou et al., 200549 | 72 children | Not applicable | Not reported |
| Mittmann et al., 199950 | 17,626 household residents, of whom 229 had asthma | Not relevant as cross-sectional data | Not reported |
| Juniper et al., 199751 | 52 children | None | Complete data sets provided for all patients |
| Norman et al., 201352 | EXALT: 404 (mixed age group) | EQ-5D scores available for 318 (79%) at 31 weeks | Not reported |
| Briggs et al., 200638 | GOAL: 3416 (mixed age group) | 526 withdrawals, including 111 lost to follow-up. Reasons were adverse events, withdrawal of consent, protocol violation, ineligible for study, data that could not be analysed (n = 117) | Not reported |
| Doull et al., 200753 | GOAL: 3416 (mixed age group) | 526 withdrawals, including 111 lost to follow-up. Reasons were adverse events, withdrawal of consent, protocol violation, ineligible for study, data that could not be analysed (n = 117) | Not reported |
| Rodríguez-Martínez et al., 201354 | 76 parents | Not reported | Not reported |
| Carroll and Downs, 200955 | 4016 parents, 29 diseases | Not reported | Not reported |
| Brown et al., 200756 | ETOPA: 312 (mixed age group) | Not reported | Imputation method for patient prematurely withdrawn. Event with zero duration was assigned if patient did not experience any event after 7 days of discontinuation |
| Gerald et al., 201057 | Utility data based on study by Chiou et al.49 | Utility data based on study by Chiou et al.49 | Utility data based on study by Chiou et al.49 |
| First author, year | Relevance of population | Relevance of health states | Instrument | Measured from | Tariff | Valuation method | Applicability issues |
|---|---|---|---|---|---|---|---|
| Willems et al., 200736 | Stratified into adults and children | Baseline utility for mild to moderate asthma patients | EQ-5D | Carer or children (≥ 12 years) | Adult UK tariff | TTO | EQ-5D from non-UK population |
| Powell et al., 201346 | Young children | Utility of severe acute asthma and post exacerbation | EQ-5D for post exacerbation. For acute exacerbation, EQ-5D states were mapped to ASSs | Carer or children (≥ 5 years) | Adult UK tariff | TTO | EQ-5D are preferred, but subjective mapping was used to estimate EQ-5D from ASS during exacerbation |
| Price et al., 201147 | Mixed age (above 12 years, mean age of 44.7 years in step 2, 50 years in step 3 | Baseline utility (uncontrolled asthma) by intervention arm, utility changes due to intervention | EQ-5D | Patient | Adult UK tariff | TTO | Utility decrement for exacerbations not reported |
| Brusselle et al., 200948 | Population is constrained to patients with severe asthma of long duration, older population (mean age 48 years), allergic and on maintenance steroids | Baseline utility of population with uncontrolled severe allergic asthma | EQ-5D | Patient | Belgian tariff | VAS | Utility decrement for exacerbations not reported. None UK. Tariff VAS not TTO |
| Chiou et al., 200549 | Children with diagnosed asthma of at least mild persistent severity | Utility of asthma generally, score stratified by severity | PAHOM | Children | Adult preference | VAS, SG (converted from VAS) | Utility decrement for exacerbations not reported |
| Mittmann et al., 199950 | Stratified by age 12–19 years | Utility of asthma generally | HUI-3 | Patient | HUI-2 (Canadian algorithm) | VAS, SG | Utility decrement for exacerbations not reported |
| Juniper et al., 199751 | Children population, symptomatic asthma, with no exacerbation in past 2 weeks, FEV1 > 80% predicted | Baseline utility in general asthma | HUI | Children | HUI-2 (Canadian algorithm) | VAS, SG | Utility decrement for exacerbations not reported |
| Norman et al., 201352 | Poorly controlled severe persistent allergic asthma | Utility of day to day symptoms (not exacerbation) | EQ-5D | Patients | Not stated | Not stated | Utility decrement for exacerbations not derived from this study (literature-based estimates used) |
| Briggs et al., 200638 | Mean age > 30 years, mean FEV1 < 80% predicted, utility adjusted in regression to UK population, population treated with inhaled fluticasone or salmeterol/fluticasone | Relevant health states: totally controlled, well controlled, not well controlled, without exacerbation and exacerbation | Mapped EQ-5D from AQLQ | Patient | Not reported | Not reported | Used an unpublished mapping algorithm and insufficient details reported to assess validity mapping method |
| Doull et al., 200753 | Mean age > 30, mean FEV1 < 80% predicted, utility adjusted in regression to UK population, population treated with inhaled fluticasone or salmeterol/fluticasone | Health states were less relevant than those used by Briggs et al.38 as the exacerbation state was combined with other symptomatic states | Mapped EQ-5D from AQLQ | Patient | Not reported | Not reported | Used an unpublished mapping algorithm and insufficient details reported to assess validity mapping method |
| Rodríguez-Martínez et al., 201354 | Parents answering for children | Health states were no symptoms, suboptimal control, no exacerbation and asthma exacerbation | Direct valuation | Parents | No | SG | Direct valuation of clinical vignettes does not meet the NICE reference case |
| Carroll and Downs, 200955 | Carer valuing for children aged between 0 and 18 years | Utility data for different asthma severity | Direct valuation | Parents | No | TTO, SG | Direct valuation of clinical vignettes does not meet the NICE reference case |
| Brown et al., 200756 | Poorly controlled allergic, severe asthma with mean age of 37.5–39.3 years | Utility for day to day symptoms at baseline and 1 year | Mapped EQ-5D from mini-AQLQ | Patient | UK adult tariff for EQ-5D | TTO for EQ-5D | Utility decrement for exacerbations not derived from this study (literature-based estimates used) |
| Gerald et al., 201057 | Cohort of school children with asthma | Reported health states related to asthma exacerbations | PAHOM | Estimated based on children’s characteristics | PAHOM derived from adult preferences | VAS, SG (SG converted from VAS) | Health states were subjectively mapped to PAHOM state |
Six studies included UK patients,38,46,47,52,53,56 three of which were multinational studies. 38,53,56 Three papers were from the USA,49,55,57 two were based in Canada50,51 and one each was from the Netherlands,36 Belgium48 and Spain. 54 Only the studies by Juniper et al. ,51 Chiou et al. 49 and Powell et al. 46 directly measured HRQoL in populations confined to children. Chiou et al. 49 recruited children aged between 7 and 12 years with diagnosed asthma of at least mild persistent severity, while Juniper et al. 51 studied children with symptomatic asthma with a mean age of 12 years (range 7–17 years) and Powell et al. 46 included children aged between 2 and 16 years with acute severe asthma. Two studies, by Rodríguez-Martínez et al. 54 and Carroll and Downs,55 elicited preferences from parents regarding health states in children. Other studies comprised populations with mixed age groups. Of these, the studies by Mittmann et al. 50 and Willems et al. 36 presented HRQoL data stratified by age.
The populations in the included studies differed in asthma severity and characteristics. Five studies measured HRQoL using EQ-5D. 36,46–48,52 Other studies used outcome measurements, such as the Pediatric Asthma Health Outcome Measure (PAHOM) (n = 2) and Health Utilities Index Mark 2 (HUI-2) (n = 1) and Mark 3 (HUI-3) (n = 1). Direct valuation using vignettes was used in two studies. This review also included three modelling studies,38,53,56 which estimated EQ-5D data from mapping exercises.
The EQ-5D is a generic preference-based measure in which the descriptive systems consist of five dimensions: mobility, depression/anxiety, self-care, usual activities, pain and discomfort. Each dimension has three levels of severity, and this gives rise to 243 possible health states described by the EQ-5D. In the UK, scoring of EQ-5D was based on time trade-off (TTO) in a representative sample of 2997 adults administered using York Measurement and Valuation of Health TTO protocol. Public preferences were obtained for 43 health states and regression was used to model data for the remaining health states. Utility score from the algorithm was anchored at ‘1’ for perfect health and ‘0’ for a state equivalent to death. 58
Willems et al. ,36 Price et al. 47 and Powell et al. 46 were randomised controlled trials (RCTs) that elicited an EQ-5D index score using UK preferences, whereas the EQ-5D score in a cohort study by Brusselle et al. 48 was based on the Belgian tariff. Norman et al. 52 was a modelling study that used EQ-5D data collected from the Evaluate Xolair for Asthma as Leading Treatment (EXALT) trial. The tariff used in the EXALT study is not described by Norman et al. ,52 but the data are described as being consistent with the NICE reference case, suggesting that the UK TTO valuation set was used.
In the MAGNEsium Trial In Children (MAGNETIC), Powell et al. 46 included a population of children (n = 508) with severe acute exacerbations, as defined by British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN). The MAGNETIC was a prospective, double-blind, multicentre RCT in the UK, designed to compare efficacy of nebulised magnesium sulphate with usual care. EQ-5D and Paediatric Quality of Life Inventory (PedsQL™) postal questionnaires were collected at 1 month post exacerbation. EQ-5D data were obtained for children aged ≥ 5 years and were filled out by parents as proxy, while PedsQL™ were obtained for all children and were self-completed if children were over 5 years. Respondents were asked to recall events in the previous 4 weeks while filling out the outcome measures. The adult UK tariff was applied to EQ-5D to obtain utility value for each child. Utility values for patients under 5 years were estimated through mapping between EQ-5D and PedsQL™. In this study, baseline EQ-5D data during exacerbation were not collected for ethical reasons. Therefore, asthma symptom scores (ASSs) at exacerbation were mapped to EQ-5D based on experts’ opinions. The expert team comprised a paediatric consultant and two respiratory nurses who routinely treated asthmatic paediatric patients. An EQ-5D health state of 11111 was assigned to ASSs of 1–3 in the base case, while ASSs of 4–6 and 7–9 were mapped to EQ-5D health states of 22222 and 33333, respectively. In our opinion, the subjective nature of this mapping between ASS and EQ-5D was considered to make the EQ-5D scores estimated at the time of exacerbation very uncertain. Furthermore, these data would be relevant only to the subgroup of patients who have severe acute exacerbations requiring treatment in secondary care, as this was the population recruited into the MAGNETIC. This study was blinded to patients, health-care providers and outcome analysts. Therefore, it had low risk of performance and detection bias. However, the study was subjected to risk of attrition bias due to the low response rate of EQ-5D questionnaires. The authors addressed this limitation by using a mapping function to estimate EQ-5D data for those with PedsQL™ data. This was based on the subset of patients for whom both PedQL and EQ-5D data were available. Following mapping estimations, a total of 218 EQ-5D data were available for analysis for the outcome 1 month after exacerbation.
Price et al. 47 included patients in the UK aged between 12 and 80 years with poorly controlled asthma at BTS/SIGN treatment step 2 or 3. The mean age of patients was 44.74 years (SD 16.49 years) at step 2 and 50.02 years (SD 15.93 years) at step 3. In step 2 patients, a leukotriene receptor antagonist was compared with inhaled corticosteroid. In step 3 patients who were already receiving inhaled corticosteroid, a leukotriene receptor antagonist was compared with a long-acting β2-agonist. EQ-5D data were directly measured from patients and were presented by treatment steps and interventions at baseline, 2 months and 2 years. Utility values were estimated using UK preferences. This RCT had a high retention rate, with 5–10% loss to follow-up. A large proportion (75%) of patients presented with less than four missing data, and missing data were handled using multiple imputation. This single-blind RCT (n = 687) was robust, with large sample size, low risk of attrition bias and measured outcomes with EQ-5D. However, utility data presented were not stratified by age nor related to asthma exacerbations. Therefore, these data lack applicability to the PLEASANT trial and the health states modelled.
Willems et al. 36 used UK preferences to estimate utility scores for asthmatic patients in the Netherlands. Populations comprised adults (n = 53) and children (n = 56) with mild to moderate asthma [Global Initiative for Asthma (GINA) states I–III]. EQ-5D questionnaires were filled by carers for children under 12 years and self-completed for those aged ≥ 12 years. There were only four children lost to follow-up, and various imputation techniques were applied. Missing baseline scores were imputed with mean scores. Quality of life scores at baseline (usual care 0.96, nurse monitoring 0.92) were consistent with the good lung function of the study’s population [mean forced expiratory volume in the first second (FEV1) above 90% predicted]. However, these results were elicited from a non-UK population, but did use a UK valuation set. Willems et al. 36 did not examine the utility decrement in exacerbation.
Brusselle et al. 48 conducted a 1-year cohort study (n = 158) to determine the efficacy and safety of omalizumab by looking at changes from baseline in a single-arm study. The mean age of the population studied was 48.17 years (SD 17.18 years) and age ranged from 12 to 83 years. Patients included had poorly controlled severe allergic asthma (FEV1 < 80% predicted) and past history of exacerbations. The Belgian tariff was applied to the collected EQ-5D data at baseline and 1 year. Only 126 of 158 patients had baseline EQ-5D values, and only 67 had EQ-5D data at 1 year. Handling of missing data, however, was not reported. This tariff was obtained from public preferences in Belgium using the visual analogue scale (VAS) valuation method. 59 However, valuation using VAS is not a choice-based method. In the UK, NICE expressed a preference of using TTO as the valuation method, and, in the absence of TTO, other choice-based methods such as subgroup analysis are preferred over VAS. 60 Therefore, utility data estimated from this study do not meet the NICE requirement of using a choice-based valuation method.
Two USA-based studies, by Chiou et al. 49 and Gerald et al. ,57 used PAHOM, an asthma-specific preference-based measure designed for children. It consists of a descriptive system with three dimensions: symptoms, emotions and activity. The symptoms dimension is classified to three levels of severity, while emotions and activity are dichotomous choices to indicate presence or absence of problems. Unlike EQ-5D with a recall period of 1 day, respondents are asked to describe health states for the past 7 days using PAHOM. The utility value of a health state is calculated as the average utility values over 7 days. Preference weights for PAHOM were elicited from 114 adults in Seattle, WA, USA, who responded for children. Subgroup analysis and VAS were used to value health states. As not all health states were valued using subgroup analysis, because of cognitive burden, VAS values were transformed into subgroup analysis values using relative risk attitude equation. 49
Chiou et al. 49 measured utility value in 72 children (aged 7–12 years) with diagnosed asthma of at least mild persistent severity as 0.83 (converted subgroup analysis value). Chiou et al. 49 also reported mean VAS and subgroup analysis values for patients according to asthma severity, with subgroup analysis values of 0.79 for mild or no symptoms, 0.70 for moderate and 0.28 for severe. A limitation of this study was the small sample size, which may have affected the accuracy and validity of results, particularly for the estimates stratified by severity. Values stratified by presence or absence of exacerbation were not reported.
Gerald et al. 57 performed a modelling study on different screening strategies for asthma. A decision tree and Markov models for a cohort of children were constructed. The Markov model consists of five health states: asthma symptom-free day, symptom days, exacerbation recovery days, emergency department visits and hospitalisation days. The utility value for each health state was derived using PAHOM. PAHOM states were allocated to the modelled health states. When several PAHOM states could describe a modelled health state, utility values of the relevant states were averaged to estimate a single utility value. For example, three or four PAHOM states were thought to characterise ‘symptom days’ in the model. The utility values of these states were averaged to derive utility value for ‘symptom days’. The authors highlighted that this approach may fail to capture valuation of ‘symptom days’ accurately. In our opinion, the subjective nature of this mapping from modelled health states to PAHOM states reduces the robustness of these utility estimates. In addition, a general concern regarding PAHOM was that this measure was not validated for its psychometric properties. Furthermore, validation of the relative risk attitude equation used to derive subgroup analysis values was not performed. 49
Two Canadian-based observational studies used the Health Utilities Index (HUI) as an outcome measure. Juniper et al. 51 studied the minimum skills required by children to complete outcome measurements unassisted. The Paediatric Asthma Quality of Life Questionnaire, Feeling Thermometer, HUI and direct valuation were administered to 52 children aged 7–17 years (mean 12 years) with symptomatic asthma (mean FEV1 85% predicted). The HUI-2 Canadian tariff was applied to obtain utility value. The mean HUI baseline value for asthma was reported as 0.89 (SD 0.09).
The six-dimensional version of HUI-2 is a common generic outcome measure in children. Each dimension has 3–5 levels, allowing 8000 unique health states to be defined. The HUI-2 tariff was estimated from a sample of 293 parents of school children in Ontario, Canada. Valuations were performed using VAS and three health states were valued with VAS and subgroup analysis. A power function was then derived to map VAS values to subgroup analysis values, and multiattribute utility theory was used to derive the valuation functions. 58
Mittmann et al. 50 conducted a cross-sectional study to measure HRQoL of 20 chronic diseases. The HUI-3 was administered through interview to 17,626 household residents (≥ 12 years) in Canada. HUI-3 is an adapted version of HUI-2 with additional dimensions and levels. HUI-3 weights were elicited from a random sample of adults (n = 504) in Ontario, Canada. In this study, however, the HUI-2 scoring algorithm was used for HUI-3 data. The mean HUI score reported for children (aged 12–19 years) with asthma was similar to that reported by Juniper et al. 51
In measuring and valuing children’s health, NICE is less clear on the preferred instrument, but advises use of a standardised and validated preference-based measure designed for children. Although HUI is an example of an instrument that meets the mentioned criteria, the HUI data from these studies may not be valid, as the study designs lack rigour. First, the small sample size (n = 52) recruited by Juniper et al. 51 may introduce inaccuracy to the results. Second, HUI-3 data were inappropriately scored in the study by Mittmann et al. 50 and utility scores estimated were deemed to be provisional by the authors. Furthermore, neither of these studies reported the utility decrement attributable to asthma exacerbation.
Four modelling studies performed mapping to estimate EQ-5D values. Brown et al. 56 and Norman et al. 52 constructed Markov models to evaluate the cost-effectiveness of omalizumab in addition to standard care. Norman et al. 52 used EQ-5D scores measured in the EXALT study for day-to-day asthma symptoms. The EXALT study was an open-label RCT, comprising 404 patients in the UK (age range from 12 to 75 years) with poorly controlled severe allergic asthma (FEV1< 80% predicted). Utility for day-to-day symptoms (by treatment arm) was estimated from EQ-5D scores recorded in the EXALT study.
Norman et al. 52 also conducted a systematic review of HRQoL literature to identify HRQoL data of relevance to both adult and paediatric populations. In their base-case analysis they used data from Lloyd et al. ,37 a study conducted in an adult population that provides estimates of the health utility decrement (loss) associated with exacerbations requiring oral steroid treatment and exacerbations requiring hospitalisation. The decrement was measured by comparing baseline EQ-5D values with those reported at 4 weeks for patients who did and did not experience exacerbations during that 4-week period. They cited another study by Steuten et al. ,61 which also provided utility values for exacerbations in an adult population. However, this study collected data at 3- to 6-month intervals, which could make it harder to detect the relationship between short-term exacerbations and health utility than the 4-week interval used by Lloyd et al. 37
Brown et al. 56 used a published algorithm by Tsuchiya et al. 62 to map the mini-Asthma Quality of Life Questionnaire (AQLQ) scores from the ETOPA trial onto the EQ-5D. The ETOPA trial was a multinational open-label trial that recruited 312 patients aged between 12 and 73 years (mean > 35 years) with poorly controlled allergic asthma (mean FEV1 < 73% predicted). 63 (Note that Brown et al. 56 used data from the subgroup of ETOPA patients with severe disease, but baseline characteristics are not described for this subgroup, so Table 32 provides characteristics for the ETOPA trial as a whole.) The AQLQ scores were mapped to EQ-5D for patients separated by disease state and responder status. The mapping algorithm used by Brown et al. 56 was derived from a RCT of 3000 adults in the UK with a wide range of asthma. 62 In the RCT used to generate the mapping algorithm, both EQ-5D and AQLQ were collected. 64 Domains in EQ-5D were found to overlap with those in AQLQ, with correlations between 0.56 and 0.65. Six main mapping models and two supplementary models were derived using the regression method and were validated using an external data set. However, these mapping functions were associated with large marginal errors, and should be considered only as second best to direct elicitation of EQ-5D data. 58
In the economic modelling study by Brown et al. ,56 literature-based estimates were used to model the decrement associated with exacerbations, as the authors stated that the ETOPA trial collected insufficient patient quality-of-life data during exacerbations. The literature-based estimates cited by Brown et al. 56 appear to be from an earlier publication65 of the study by Lloyd et al. 37
The modelling studies by Briggs et al. 38 and Doull et al. 53 mapped AQLQ scores from the 52-week Gaining Optimal Asthma ControL (GOAL) trial onto EQ-5D values. The GOAL study was a multinational double-blind RCT designed to evaluate efficacy of a combination of fluticasone/salmeterol compared with fluticasone in terms of asthma control. The GOAL study comprised 3416 patients (mean age > 35 years; range 12–80 years) with uncontrolled asthma (mean FEV1 < 80% predicted) from 44 countries. 66 Asthma control in the GOAL trial was classified as totally controlled, well controlled, not well controlled or exacerbation requiring oral steroid or secondary care by Briggs et al. 38 using the GINA definition. As the GOAL trial collected only AQLQ data, a mapping function obtained through personal communication with Macran and Kind (no further details of this communication provided by Briggs et al. 38) was used to transform AQLQ scores to EQ-5D values. Subsequently, the utility value for each asthma control health state was derived using regression. In the regression model, a UK indicator was added as a dummy variable to adjust for a UK specific-population. The dependent variable was the utility value, whereas asthma control and the UK indicator were the independent variables. Both independent variables were found to be significant predictors of quality of life. The quality-of-life data from this study are of relevance to the PLEASANT trial. However, the mapping function used in the analysis by Briggs et al. 38 was inadequately described by the authors, and a published article providing more details could not be identified from searches. Therefore, an assessment of mapping performance was not possible.
Doull et al. 53 adapted the analysis by Briggs et al. ,38 and reclassified asthma control to ‘symptom free’ and ‘with symptoms’. Totally controlled asthma was classified as ‘symptom free’, while other states were classified as ‘with symptoms’. The weekly utility in the ‘with symptom’ state was equivalent to the weighted average of the weekly utility in well controlled, not well controlled and exacerbation health states from Briggs et al. 38 Regression was used to estimate the relationship between asthma control and quality of life, when quality of life was obtained by mapping AQLQ scores to EQ-5D. Asthma control and the UK indicator were entered into the model as the independent variables, while weekly utility was entered as the dependent variable. Subsequently, utility for the ‘with symptoms’ and the ‘symptoms-free’ health states were estimated from the regression coefficients. As utility data in this study were adapted from Briggs et al. ,38 which mapped AQLQ scores to EQ-5D using the mapping function by Macran and Kind, the validity of mapped data was likewise not assessable.
The method used in Carroll and Downs55 and Rodríguez-Martínez et al. 54 involved valuation of hypothetical health states by parents. Parents were asked to value health states described in vignettes by imagining their children affected by those states. Descriptions in vignettes, however, differed across studies. Rodríguez-Martínez et al. 54 developed asthma-specific vignettes based on PAHOM,49 and these were validated by expert opinions, whereas Carroll and Downs55 developed general descriptions of 29 health states with the inclusion of time as a factor. Rodriguez-Martinez et al. 54 requested parents (n = 76) to value vignettes using subgroup analysis, while Carroll and Downs55 used subgroup analysis and TTO methods in a sample of 4016 parents (Note that each parent valued only three of a potential 29 states providing around 415 values per state.) Neither study constructed vignettes based on rigorous methods such as a focus group. The lack of standardised descriptive systems of vignettes and different valuation methods also resulted in a lack of comparability of results between studies. In addition, vignettes are limited to specific descriptions of a condition, and may not fully reflect all experiences of a patient. Therefore, vignettes do not meet the NICE reference case and are considered of little value in economic evaluation. 60 In view of the various limitations associated with vignettes, utility values from Carroll and Downs55 and Rodríguez-Martínez et al. 54 were not considered suitable for use in the PLEASANT study economic analysis.
Health state utility values used in the analysis
The utility values used in the economic evaluation by Briggs et al. 38 appear to be particularly relevant to our proposed model structure, as they are reported for relevant health states, including an exacerbation state, and have been estimated from a trial population that included some children. However, the mapping algorithm used to convert from the condition-specific HRQoL measure (AQLQ) to the EQ-5D utility score is not from a published source, and is not described in detail, making it difficult to assess its validity. However, if the values reported by Briggs et al. 38 are taken at face value, they provide an estimate of the utility loss for exacerbation versus total asthma control of –0.216 (SE 0.007). It is possible that some patients do not have total asthma control in the absence of an exacerbation and the difference between the utility values for the exacerbation state and the not well controlled states is smaller, at –0.112. The data from Briggs et al. 38 suggest that the utility decrement for exacerbation in the average patient is likely to fall in the range –0.112 to – 0.216. The utility decrements provided by Lloyd et al. 37 from an adult population are –0.1 and –0.2 for exacerbations requiring oral steroids and exacerbations requiring hospitalisation, respectively. It therefore appears that there is reasonable agreement between the values reported by Briggs et al. 38 and Lloyd et al. 37
We accept that the estimates provided by Briggs et al. 38 and Lloyd et al. 37 probably underestimate the degree of utility loss in children with a severe or life-threatening acute exacerbation during the period of hospitalisation. This is because the utility values were not measured during the acute exacerbation period itself. In the MAGNETIC, which estimated utility scores in children attending EDs with severe acute asthma, the utility was estimated to be reduced from a baseline of 0.88 to 0.516 during the initial acute period, giving a utility decrement of 0.364. However, in the MAGNETIC, this more severe utility decrement was only applied until hospital discharge, with the average length of hospital stay being 1 day. If we apply a decrement of 0.364 for 1 day and assume a loss of 0.2 in the remaining 6 days, the average utility loss over the whole week of exacerbation (–0.22) would be similar to that reported by Briggs et al. 38
Given the uncertainty regarding the mapping algorithm used by Briggs et al. ,38 and the previous use of data from Lloyd et al. 37 in a number of published economic evaluations, we decided to use the data from Lloyd et al. 37 in the base-case analysis. The data from Briggs et al. 38 have been explored in a sensitivity analysis using the difference between the total control state and the exacerbation state (–0.216) to estimate the quality-of-life decrement from exacerbations. This sensitivity analysis is considered to provide an upper limit on the utility decrement attributable to exacerbation.
For patients without an exacerbation, we have taken the baseline utility score for the control arm of the study by Willems et al. ,36 as this provides an estimate based on the child version of the EQ-5D valued using the adult UK TTO valuation set. The population was Dutch children aged 7–18 years with a GINA severity stage I–III receiving standard outpatient care. The value applied to patients without an exacerbation will affect the calculation of absolute QALYs in each trial arm, but does not affect the estimation of incremental QALY gain that goes into the cost-effectiveness ratio. Therefore, the selection of this data source is less critical than that used to determine the decrement attributable to exacerbations. The data that have been applied in the model are summarised in Health outcomes.
Appendix 6 Full search strategy
| Search database | Search terms |
|---|---|
| MEDLINE/EMBASE |
|
| The Cochrane Library (Cochrane Database of Systematic Review, HTA and the NHS Economic Evaluation Database) |
|
| School of Health and Related Research (ScHARR) Health Utilities Database |
|
| EconLit |
|
Appendix 7 Quality-of-life filter
| Source/database | Filter |
|---|---|
| Original quality of life (ISSG): MEDLINE/EMBASE |
|
| (A) Adapted quality of life (ISSG): MEDLINE/EMBASE |
|
| (B) Adapted quality of life (ISSG): Cochrane. QOL FILTER – 4 July 2014 |
|
Appendix 8 Reasons for exclusion at titles and abstracts
| Reasons | Number of studies excluded |
|---|---|
| Aged ≥ 18 years | 175 |
| Did not publish utility data | 197 |
| Non-asthma population | 87 |
| Non-English papers | 8 |
| Non-preference-based/non-utility measure | 158 |
| Publication types | 34 |
| Total | 659 |
Appendix 9 Reasons for exclusion at full texts
| Study | Reasons for exclusion |
|---|---|
| Janse A, Sinnema G, Uiterwaal C, Kimpen J, Gemke R. Quality of life in chronic illness: perceptions of parents and paediatricians. Arch Dis Child 2005;90:486–91 | Used HUI-3, but did not report utility data. Results were presented as percentage similarity in outcome measurements between physician and parents |
| Mo F, Choi BC, Li FC, Merrick J. Using Health Utility Index (HUI) for measuring the impact on health-related quality of Life (HRQL) among individuals with chronic diseases. Sci World J 2004;4:746–57 | Used HUI-3, but did not report utility data. Results were presented as graphical differences of quality of life between diseases |
| Willems DC, Joore MA, Nieman FH, Severens JL, Wouters EF, Hendriks JJ. Using EQ-5D in children with asthma, rheumatic disorders, diabetes, and speech/language and/or hearing disorders. Int J Technol Assess Health Care 2009;25:391–9 | Used EQ-5D, but did not report utility data. Results were presented as EQ-5D interclass coefficients and Spearman coefficients between outcome measures |
| Burstrom K, Svartengren M, Egmar AC. Testing a Swedish child-friendly pilot version of the EQ-5D instrument – initial results. Eur J Public Health 2011;21:178–83 | Used direct valuation using EQ-VAS as outcome measure (non-preference based) in Swedish children |
| Finnell SM, Carroll AE, Downs SM. Application of classic utilities to published pediatric cost-utility studies. Acad Pediatr 2012;12:219–28 | Utility data were obtained from an included study by Carroll and Downs55 |
| Brodtkorb TH, Zetterstrom O, Tinghog G. Cost-effectiveness of clean air administered to the breathing zone in allergic asthma. Clin Respir J 2010;4:104–10 | Utility data were presented as utility changes associated with intervention |
| Meadows A, Kaambwa B, Novielli N, Huissoon A, Fry-Smith A, Meads C, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess 2013;17(27) | |
| Smith LJ, Holbrook JT, Wise R, Blumenthal M, Dozor AJ, Mastronarde J, et al. Dietary intake of soy genistein is associated with lung function in patients with asthma. J Asthma 2004;41:833–43 | |
| Wilson EC, Price D, Musgrave SD, Sims EJ, Shepstone L, Murdoch J, et al. Cost effectiveness of leukotriene receptor antagonists versus long-acting beta-2 agonists as add-on therapy to inhaled corticosteroids for asthma: a pragmatic trial. PharmacoEconomics 2010;28:597–608 | |
| Wilson EC, Sims EJ, Musgrave SD, Shepstone L, Blyth A, Murdoch J, et al. Cost effectiveness of leukotriene receptor antagonists versus inhaled corticosteroids for initial asthma controller therapy: a pragmatic trial. PharmacoEconomics 2010;28:585–95 |
Appendix 10 Baseline (12 months pre intervention) and post-intervention (12 months) resource use and costs per patient
| Statistics | Resource-use type | Baseline (12 months pre intervention) | Post intervention (12 months) | ||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Significant difference (p-value) | Intervention | Control | Significant differences (p-value) | ||
| Mean number of tasks,a SD (range) | Scheduled tasks | 2.77 (2.63, 0–29) | 2.74 (2.69, 0–36) | 0.583 | 2.60 (2.72, 0–22) | 2.69 (2.85, 0–30) | 0.130 |
| Unscheduled tasks | 10.53 (8.03, 0–79) | 10.44 (8.67, 0–127) | 0.610 | 9.39 (8.32, 0–73) | 9.36 (9.22, 0–101) | 0.867 | |
| ‘Not relevant’ tasks | 4.31 (4.48, 0–52) | 3.97 (4.54, 0–60) | < 0.001 | 4.17 (4.79, 0–45) | 3.75 (4.73, 0–60) | < 0.001 | |
| Total tasksb | 17.61 (12.47, 0–115) | 17.14 (13.38, 0–168) | 0.102 | 16.16 (13.3, 0–120) | 15.8 (14.42, 0–163) | 0.249 | |
| Mean costs (95% CI; median, range) | Scheduled tasks | £178 (£167 to £190; £41, £0–3871) | £160 (£150 to £169; £41, £0–5554) | 0.015 | £169 (£158 to £181; £27, £0–3857) | £173 (£163 to £183; £41, £0–3839) | 0.623 |
| Unscheduled tasks | £305 (£295 to £316; £208, £0–3181) | £315 (£305 to £326; £212, £0–4027) | 0.198 | £266 (£255 to £277; £146, £0–4661) | £283 (£272 to £294; £186, £0–8010) | 0.030 | |
| ‘Not relevant’ tasks | £215 (£202 to £228; £1, £0–5727) | £197 (£186 to £209; £1, £0–4111) | 0.045 | £204 (£191 to £217; £1, £0–6149) | £205 (£193 to £218; £1, £0–£7675) | 0.915 | |
| Total costc | £699 (£672 to £725; £435, £0–8597) | £672 (£649 to £696; £408, £0–8919) | 0.147 | £639 (£612 to £667; £342, £0–8829) | £662 (£636 to £688; £358, £0–13,411) | 0.251 | |
| Prescriptions | £62 (£59 to £66; £27, £0–1141) | £55 (£52 to £57; £20, £0–808) | < 0.001 | £55 (£52 to £59; £20, £0–849) | £49 (£47 to 52, £16; £0–789) | 0.003 | |
| Total task and prescription costd | £761 (£734 to £789; £498, £0–8622) | £727 (£703 to £751; £468, £0–8997) | 0.069 | £695 (£666 to £723; £402, £0–8921) | £711 (£684 to £738; £412, £0–13484) | 0.420 | |
List of abbreviations
- AQLQ
- Asthma Quality of Life Questionnaire
- ASS
- asthma symptom score
- BA
- baseline adjusted
- BCa
- bias corrected and accelerated
- BTS
- British Thoracic Society
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPRD
- Clinical Practice Research Datalink
- CTRU
- Clinical Trials Research Unit
- EoI
- expression of interest
- EQ-5D
- European Quality of Life-5 Dimensions
- EXALT
- Evaluate Xolair for Asthma as Leading Treatment
- FEV1
- forced expiratory volume in the first second
- GINA
- Global Initiative for Asthma
- GOAL
- Gaining Optimal Asthma ControL
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- HUI-2
- Health Utilities Index Mark 2
- HUI-3
- Health Utilities Index Mark 3
- ICC
- intraclass correlation
- ICER
- incremental cost-effectiveness ratio
- ID
- identification number
- IRR
- incidence rate ratio
- ISSG
- Information Specialists’ Sub-Group
- ITT
- intention to treat
- MAGNETIC
- MAGNEsium Trial In Children
- MeSH
- medical subject heading
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- PAHOM
- Pediatric Asthma Health Outcome Measure
- PedsQL
- Pediatric Quality of Life Inventory
- PLEASANT
- Preventing and Lessening Exacerbations of Asthma in School-age children Associated with a New Term
- PP
- per protocol
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SIGN
- Scottish Intercollegiate Guidelines Network
- SOP
- standard operating procedure
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TTO
- time trade-off
- VAS
- visual analogue scale
- WTP
- willingness to pay