Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/127/41. The contractual start date was in December 2011. The draft report began editorial review in September 2015 and was accepted for publication in February 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Robertson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Scientific background
Childhood obesity, both in the UK and internationally, is known to be a major public health burden. The most recent Health Survey for England found that between 1995 and 2005 obesity in children aged 2–10 years in England rose from 10% to 17% in boys and from 11% to 17% in girls. 1 However, the trend may now be reversing, with 13% of boys and 12% of girls found to be obese in 2013. 1 Analysis of general practice data found a similar trend, with the prevalence of childhood overweight and obesity stabilising from 2004 to 2013 in children aged 2–10 years. 2 This prevalence is still too high, with over one-third (33.5%) of children in year 6 (aged 10–11 years) in England classified as either overweight or obese in the 2013/14 school year. 3 This translates to over 172,000 children in year 6 alone who are overweight or obese.
Childhood overweight and obesity have been linked to a number of long-term and immediate physiological and psychological health risks,4,5 with associations between childhood body mass index (BMI) and type 2 diabetes, hypertension and coronary heart disease in adulthood. 6 This means that the prevention and management of childhood obesity is a public health priority. Effective interventions to treat children who are obese, to reduce ill health in children and to reduce the proportion of children whose obesity continues into adulthood are needed.
In 2008, there were an estimated 314–375 weight management programmes for children in operation in England at any one time. 7 These lifestyle interventions focused on diet, physical activity, behaviour change or any combination of these factors, and included programmes, courses or clubs, and online services. They could include programmes that were designed for overweight or obese children and young people, or for their parents, carers or families; designed primarily for adults but that accept, or may be used by, children and young people; or provided by the public, private or voluntary sector, in the community or in (or via) primary care organisations. Aicken et al. 7 noted that some were small local schemes, whereas others were available on a regional or national basis.
Care pathway for weight management of children and young people
Government guidelines describe a three-tier pyramid model for weight management, with three levels of service, from universal services, offered to everybody, through to specialist services, provided to those with particular needs. 8 Level 1 represents the core preventative services that all children, young people and their families should have access to, while level 2 represents targeted weight management services. The Families for Health intervention fits into level 2. This level of intervention is often aimed at children and young people with a BMI between the 91st and 98th centile, and services typically take the form of multicomponent family-based interventions, often taking place in community settings. They may also be called ‘early intervention services’. Level 3 is specialist support, offered to children with a BMI ≥ 99.6th centile, with a medical cause of obesity, significant comorbidity or complex needs. The Department of Health has recommended that overweight or obese children should be referred to appropriate weight management services. 9
Evidence for effectiveness of level 2 child obesity interventions
A Cochrane systematic review of interventions to treat obesity identified 64 randomised controlled trials (RCTs). 10 Thirty-seven studies were lifestyle interventions for children aged < 12 years (four dietary, nine physical activity and 24 behavioural). The authors concluded that it is difficult to recommend any particular intervention, but indicated that family-based lifestyle interventions combining dietary, physical activity and behavioural components can produce a significant and clinically meaningful reduction in overweight. Parental involvement was identified as useful with children aged < 12 years.
Recent UK-based trials of interventions targeting children who are obese have covered a variety of approaches. A RCT of the 9-week family-based community programme MEND (Mind, Exercise, Nutrition . . . Do it!; Mytime Active, Bromley, UK) with 116 children (aged 8–12 years) showed a between-group difference in the BMI z-score at the 6-month follow-up of –0.24 [95% confidence interval (CI) –0.34 to –0.13; p < 0.0001; n = 82] in favour of MEND over the waiting list control. 11 A further RCT compared paediatric dietitians using a one-to-one behavioural approach (5 hours) with standard dietetic care (1.5 hours) in 134 children (aged 5–11 years). 12 No significant differences in BMI z-scores were found at 6 or 12 months. A feasibility RCT that compared the community-based Watch It programme (delivered by health trainers) with a waiting list control, in 70 children and adolescents, found no significant change in BMI z-score and treatment. 13 A RCT of Epstein’s family-based behavioural treatment in a UK hospital, with 72 participants, found that there was no significant difference between the intervention and waiting list groups in BMI z-score, although both treatment and control groups showed significant reductions. 14
A National Institute for Health and Care Excellence (NICE) guideline on managing overweight and obesity among children and young people suggested that targeting both parents and children, or whole families, is effective in reducing BMI z-scores. 15 By the end of a programme, evidence on interventions involving families showed no negative effect on well-being and, in some cases, showed positive effects. Similarly, a review of the limited research on interventions focusing on parenting to treat childhood obesity shows a small positive effect on weight-related outcomes. 16
Family-based interventions rooted in behaviour theory have been shown to achieve better treatment effectiveness than those rooted in family systems theory. 17 A recent review of published studies18 investigating family-based childhood obesity interventions in the UK included 10 studies. The majority of programmes reviewed lasted 12 weeks, with only three studies providing follow-up data at 12 months or longer. Change in adiposity was a short-term benefit of participation, but there was insufficient robust evidence to suggest that this benefit was long-lasting and many studies were methodologically weak with limited internal validity. The authors concluded that there was insufficient evidence on how the inclusion of parents and the wider family may impact on the effectiveness of UK community-based weight management programmes for children and young people. 18
Evidence for cost-effectiveness of child obesity interventions
Oude Luttikhuis et al. 10 point to a paucity of cost-effectiveness studies in this area. A recent US study of families in which both children and parents were overweight or obese found that the cost per unit weight lost by parents and children was lower for family-based group treatment than for an intervention that treated the parent and child separately. 19 Evidence from seven short-term health economic analyses suggests that, in the short term, lifestyle weight management programmes will result in an increased cost to the NHS in terms of BMI z-score gains when compared with routine care. However, overall small (and in some cases non-significant) improvements in BMI z-scores can be achieved. 15 Interventions that lead to even small reductions in BMI can be cost-effective in the long term at conventional cost-effectiveness thresholds, provided that the short-term effects on BMI can be sustained into adulthood. The NICE guidance suggested that evidence from these studies is directly applicable, but there were some potentially serious limitations to the studies. 15
From randomised controlled trial to practice
Two recent studies have investigated the roll-out of an intervention tested in a RCT into the community. 20,21 Both were based on the multicomponent family-based weight management intervention, MEND, which addresses diet and physical activity through education, skills training and motivational enhancement. When delivered at scale, the rolled-out intervention resulted in the improvement of BMI and psychosocial outcomes but worked less well for some groups of children (certain ethnic groups and those from more deprived areas). This was not because of variations in uptake of the programme, which was good in deprived groups, but because of differing experiences in completing, with more disadvantaged groups being less likely to complete the programme. The intervention, therefore, had the potential to widen inequalities. The authors suggested that further research should investigate how completion rates could be improved for particular groups. 21 They felt that the intervention should be implemented in such a way as to achieve sustained impact for all groups by modifying content, training and implementation. 20
Parenting programmes
It has been argued that parenting programmes have potential to improve the mental health and well-being of children, as well as improve family relationships and benefit the community as whole. 22 A public health approach to parenting is needed to ensure that more parents benefit and that a societal-level impact is achieved. 22 The effectiveness of parenting interventions in the treatment and prevention of childhood obesity has shown a small positive effect on weight-related outcomes,16 but this approach has not been investigated in a RCT in the UK.
Development of the Families for Health intervention
‘Families for Health’ is a manualised group-based family intervention for the treatment of children aged 6–11 years who are overweight or obese, which could be an option in the care pathway as a targeted ‘early-intervention’ service. The programme was developed at Warwick University and supported by a Department of Health career development award to the principal investigator. The programme differs from other interventions for childhood obesity being used or tested in the UK by offering a greater emphasis on parenting skills, relationship skills and emotional and social development, combined with information about lifestyle. Parents and children attend separate groups, meeting mid-way for a healthy snack and activity. The groups are led by two trained facilitators.
The development and evaluation of the Families for Health programme has followed the Medical Research Council (MRC)’s framework for complex interventions. 23 A pre–post pilot study in Coventry of 27 children showed that a mean reduction in children’s BMI z-scores from baseline was sustained at 9 months (–0.21, 95% CI –0.35 to –0.07; p = 0.007) and 2 years (–0.23, 95% CI –0.42 to 0.03; p = 0.027). 24,25 There were also other health-related improvements. Interview data showed that parents found the parenting approach helpful, providing the tools for them to become ‘agents of change’ in the family. The NHS costs to deliver the programme were £517 per family or £402 per child. 25 The process evaluation of the pilot study showed the need for minor modifications to the programme including more physical activity, removal of some unnecessary material, a reduction in the number of sessions and addition of follow-up sessions. These changes to the intervention might increase effectiveness.
The materials for version 1 of this programme were developed by Candida Hunt and the University of Warwick team, including investigators in this trial (WR and SS-B). 26 Following development and evaluation of the original programme Families for Health version 1,24,25 changes were made including shortening the delivery from 12 to 10 sessions, adding two follow-up sessions and enhancing the information given to families on healthy eating and pedometers. The programme combines information on parenting skills, social and emotional development, as well as lifestyle change. The parenting aspects are based on the Nurturing Programme from Family Links (Family Links, Oxford, UK),27 and the circle time elements in the children’s programme have parallels with the Family Links Nurturing Programme for schools. 28
Delivery is group based involving 8–12 families, with children and parents attending parallel groups. The intervention is delivered in a community setting (e.g. a leisure centre or school), to enhance access and ensure adequate space and facilities for physical activity. The parents’ group covers both support with parenting skills and family lifestyle, which are integrated in the weekly sessions. The approaches include facilitated discussion, role-play, goal-setting, skill practice, a solution-focused approach and homework. The children’s programme includes a focus on healthy eating using the ‘Eatwell plate’ (Food Standards Agency, London, UK) as the basis; circle time to discuss emotional aspects of their lives and enhancing self-esteem; and physical activity aimed at increasing activity levels by participation in games, the use of pedometers and introduction to new physical activities. The parents and children meet mid-way in each session for a healthy snack and an active game. This gives facilitators an opportunity to act as role models, for example showing parents how they might reward or praise their children, and to introduce ways in which children and parents can interact at home. It also provides an opportunity for children to prepare healthy snacks and to try new foods.
The main principles underpinning the Families for Health intervention are that the parents are identified as the agents of change responsible for implementing lifestyle change in the family. 29 The parenting aspects aim to support and increase parental capacity to implement and maintain the lifestyle changes that they would like to try each week. The focus is on healthy eating (not dieting) and activity for the whole family (that is, not just for the child who is overweight), with an emphasis on children growing into their weight rather than weight loss. The programme aims to promote a sustainable, healthy approach to family-wide lifestyle change. The programme puts greater emphasis on parenting skills, relationship skills, and emotional and social development than other similar interventions, and combines this with information about lifestyle. These form a core theme throughout and are integrated alongside topics in each weekly session.
Rationale for research
Although family-based interventions for the treatment of childhood obesity have become more common, the inclusion of parenting skills within a programme is less so. Such a programme may be effective in the treatment and prevention of childhood obesity,16,24,25 and so it is important to investigate the impact on families with overweight and obese children.
This RCT provides evidence of the effectiveness and cost-effectiveness of the Families for Health programme, which emphasises parenting alongside a healthy lifestyle as an alternative approach for the treatment of obesity in 6- to 11-year-olds.
Aims and objectives
The aim of the trial was to assess the effectiveness and cost-effectiveness of the Families for Health programme delivered within the NHS.
The objectives were to:
-
assess the effectiveness of the Families for Health programme in reducing the BMI z-score in children aged 6–11 years who are overweight or obese
-
evaluate the cost-effectiveness of the Families for Health programme [expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained]
-
investigate parents’ and children’s views of the programme and their observations on approaches to maximising impact
-
investigate facilitators’ views of the programme and their observations on approaches to maximising impact.
Chapter 2 Trial design and methods
Study design
The ‘Families for Health’ trial evaluated the effectiveness of the Families for Health programme in comparison with usual care in children aged 6–11 years who were overweight or obese. The trial was a multicentre RCT with parallel economic and process evaluations. Families were randomised (1 : 1) to one of two arms: Families for Health (target 60 families) or usual care (target 60 families). Further details of the interventions are described in Treatment groups.
The primary outcome measure was the change in children’s BMI z-score at 12 months. All primary and secondary outcomes were assessed at baseline, the end of the programme (3 months) and 12 months post randomisation, to evaluate both the short- and long-term effects.
Study settings
The Families for Health study agreed collaborations with three West Midlands primary care trusts. These primary care trusts offered Families for Health as part of their care pathway for the treatment of childhood obesity, alongside their usual care. The three sites are referred to as site A, site B and site C in this report.
In sites A and C, the Families for Health intervention was run in a leisure centre (local authority) and in site B it was run in a community centre with a sports hall.
Ethical approval and research governance
Ethical approval for the study was obtained from the National Research Ethics Service (NRES) West Midlands – Coventry and Warwickshire (reference number 11/WM/0290) on 3 October 2011.
The trial was sponsored by the University of Warwick. NHS Research and Development approvals were obtained with the participating NHS trusts. The trial was conducted in accordance with the standard operating procedures of the Warwick Clinical Trials Unit. 30
A Trial Steering Committee (TSC) was convened every 6 months, comprising four independent members [chairperson, topic expert, statistician and parent (service user)] who advised on changes to the protocol, the analyses plans and oversaw the management of the trial. A Data Monitoring and Ethics Committee (DMEC) was convened annually, comprising three independent members (chairperson/topic expert, statistician and health economist). The DMEC considered any ethical issues and adverse events, and reviewed the interim analyses including BMI z-score, European Quality of Life-5 Dimensions Youth version (EQ-5D-Y) and European Quality of Life-5 Dimensions (EQ-5D) (presented blind) data.
All data were stored securely and anonymised in accordance with the Data Protection Act,31 and the trial was conducted in compliance with the principles of MRC’s Good Clinical Practice guidelines,32 the Declaration of Helsinki33 and other requirements as appropriate. All research staff were trained in good clinical practice in a paediatric setting and child protection awareness training.
Informed consent
Informed consent was obtained in three steps, giving parents and children time to consider whether or not they wished to participate. Each potential participant was given, or sent by post, information sheets about the trial (child and parent versions). After a minimum of 3 days, parents were contacted by telephone to ask whether or not they were interested in taking part in the trial and to answer any questions. A researcher then visited the parent(s) and child(ren) at their home, and obtained the parent’s written consent and the child’s written assent. All research staff were trained in informed consent, including methods for assessing competence for consent, agreement to participate and obtaining assent from children.
User involvement
Parents
A parent who had been a participant in the pilot of the Families for Health intervention was a member of the TSC. She attended seven of the eight TSC meetings and contributed to discussions on the intervention and study design.
Children
The aim for user involvement, as stated in the protocol, was met using the following approach. Claire Callens and Carly Tibbins, from the user involvement team of the West Midlands Medicines for Children’s Research Network carried out patient and public involvement consultation exercises with children from the West Midlands prior to recruitment from January to February 2012, comprising two stages: stage 1, two focus groups with hospital-based young person’s advisory groups (aged 8–18 years), which aimed to develop and pilot questions for the second stage, and also included obtaining views on the acceptability of wearing accelerometers to measure physical activity; and stage 2, consultation on the proposed intervention and the research measurements (including accelerometers) with children in the target age range for the trial (key stage 2, aged 7–11 years) from two primary schools from the areas in which the trial was to be delivered.
A specific area of inquiry in the second stage of consultation was about the types of activities children most enjoyed, which was used to inform the ‘activity tasters’ within the Families for Health intervention. Older children (years 5 and 6) completed a questionnaire and younger children (years 3 and 4) voted, by show of hands, on the options given for physical activity. Once they understood the concept of a children’s gym, all children felt that they would like to attend one to see what it was like.
Across the two stages of consultation, 35 young people aged 7–18 years were consulted on accelerometers in five focus groups. Prior to participation in the focus group discussion, 18 children were given the opportunity to wear an accelerometer (GT1M ActiGraph; ActiGraph LLC, Pensacola, FL, USA). First impressions of the acceptability of the accelerometer were often negative relating to comfort and unwanted attention from friends, indicating that this may be a difficult tool to use. Others felt that an increased attention from others would be positive. Ways to increase use by participants were suggested as presenting the accelerometer in a very positive way, include a trial wearing period and providing incentives. 34 A full report of the user involvement consultation with children is available. 35
Changes to project protocol
Some parts of the original protocol were changed, as can be seen in Table 1, which also shows where appropriate approval was obtained.
| Change to protocol | Approval | Type and date approved |
|---|---|---|
| The CSRI was developed and given ethical approval prior to piloting | NRES | Substantial amendment 1, 17 January 2012 |
| The inclusion criterion was changed from children aged 7–11 years to 6–11 years (parents of 6-year-old children were coming forward requesting to participate) | NRES/HTA programme | Amendment 2, 27 April 2012 |
| The formatting of CSRI was changed after piloting, the poster for recruitment was revised and the letter used for recruitment via the NCMP was changed | NRES | Amendment 3, 21 May 2012 |
| Interviews were originally just going to be with families in the FFH arm, but the protocol was changed to include interviews with UC families as well in order to improve comparison with UC | NRES | Substantial amendment 4, 6 August 2012 |
| HTA programme | 11 July 2012 | |
| Recruitment rates were slower than initially anticipated. The emphasis on the type of recruitment method used changed along with the study timelines as the study progressed. Approval was given for GP databases to be searched for potential overweight/obese children, who, once identified, were contacted via letter | NRES | Substantial amendment 5, 8 May 2013 |
| Addition of a seventh FFH programme in order to reach sufficient study participant numbers. This required a 9-month no-cost extension (to 31 August 2015) to the study to enable the 12-month follow-up of participants | HTA programme | Approved by HTA on 19 June 2014 |
Treatment groups
The intervention: Families for Health
Families for Health version 2 is a family-based programme aimed at the treatment of children (aged 6–11 years) who are overweight or obese. Delivery is group based, and the aim was to recruit groups of 8–12 families, with children and parents attending parallel groups. In families randomised to this arm, both parents were invited, together with all overweight and non-overweight siblings in the target age range. Where necessary, the start of a programme was delayed so that viable attendance numbers could be obtained. The programme was manualised, with detailed handbooks available to facilitators, parents and children. Groups were run on a Saturday morning or afternoon for 2.5 hours each week for 10 weeks. Follow-up sessions were planned for 1 and 3 months post intervention.
The main principles underpinning the Families for Health intervention were that the parents were identified as the agents of change responsible for implementing lifestyle change in the family. 29 A solution-focused approach was employed, focusing on solutions rather than the problem. The programme emphasises parenting skills, relationship skills, and emotional and social development, and combines this with information about lifestyle, all of which are key to implementing and maintaining behaviour change. Table 2 shows an outline of the main content of the parallel parents’ and children’s groups for the 10 weeks. The weekly topics in the Families for Health programme were broadly the same for both parents’ and children’s groups, in order to promote greater understanding and discussion at home. The parents and children met mid-way each week for a healthy snack and an active game, with an aim of introducing ways in which children and parents could interact at home.
| Week | Parents’ programme | Children’s programme |
|---|---|---|
| 1 | Let’s get started
|
Let’s get started
|
| 2 | Balancing acts
|
Balancing acts
|
| 3 | Inner power – our ally for health
|
Inner power – our health helper
|
| 4 | The question of choice
|
Our choices
|
| 5 | Health is a family affair
|
Liking ourselves
|
| 6 | Feelings – a guide to our emotional health
|
Getting to know our feelings
|
| 7 | Solutions to stress
|
Time to chill out
|
| 8 | A world of labels
|
Food detectives
|
| 9 | Taking charge
|
Living healthily
|
| 10 | A healthy family future
|
(Combined session with parents) |
The parents programme of the Families for Health programme shared some of parenting skills topics from the Nurturing Programme from Family Links,27 and included both behavioural (e.g. positive discipline, family rules) and relationship (e.g. giving praise, raising self-esteem, emotional health) approaches to parent training. The support with parenting skills were integrated with family lifestyle topics around healthy eating and physical activity in the weekly sessions. The approaches include facilitated discussion, role-play, goal-setting, skill practice, a solution-focused approach and homework.
The children’s programme included a focus on healthy eating using the Eatwell plate as the basis; circle time to discuss emotional aspects of their lives and enhancing self-esteem; and physical activity aimed to increase activity levels by participation in games, the use of pedometers and introduction to new physical activities.
Families in the intervention arm were also eligible for usual-care interventions and any usual care they received was documented.
Training and selection of facilitators: Families for Health
Four facilitators, two for the children’s group and two for the parents’ group, were required to run each Families for Health programme. Facilitators were identified from the local NHS or other services, and selected on the basis of personal attributes including empathy for families with overweight children and previous relevant experience, for example with running groups with parents and children. In site A, a formal application process was set up, whereas for sites B and C nominations were received through the leads for obesity. Professional backgrounds of facilitators included community nursing, teaching, youth work, leisure services and nutritionists.
Nineteen facilitators attended a 4-day training course in February 2012 provided by two trainers from Family Links. Trainers from Family Links were used to deliver the training because of the close association of the Families for Health programme with the Nurturing Programme from Family Links. 27,28 The training covered the content, philosophy and logistics of running the programme. For some sessions facilitators were divided up into subgroups of whether they were facilitating the children’s or parents’ group, and practised facilitating parts of the programme. Participants completed an evaluation form for each day of the training, including the level of confidence in delivering the Families for Health programme and rating the usefulness of the topics covered. Training increased facilitators’ confidence in delivering the programme and most found the training a positive and useful experience. Further details of the evaluation of the training of the facilitators are in Appendix 1.
Usual-care control group
Families assigned to the control arm were offered any usual care that was available in their area. During the duration of the study, usual care for each locality varied.
Site A had the One Body One Life (Coventry City Council, Coventry, UK) 10-week programme, which is a group-based family intervention that has been subject to published evaluation. 36 This was available throughout the study. The eligibility criteria for the One Body One Life programme was children aged 7–16 years, but was offered to the whole family if one or more member of the family was an ‘unhealthy weight’. The programme took a solution-focused approach, with the 1.5-hour sessions comprising a 45-minute physical activity workshop and a 45-minute healthy eating workshop.
Site B had Change4Life (Department of Health, London, UK) advisors who offered one-to-one support for weight management for children aged 4–13 years who were overweight or obese. Recruitment to their service was via self-referral, referral from school health or other health professionals, and via the National Child Measurement Programme (NCMP). Visits were mainly undertaken at the child’s home. This service was available for the majority of the study, though in the final few months of recruitment funding for Change4Life advisors was withdrawn and a single telephone call was instead being offered.
In site C, usual care was one of the following: (1) a weight management programme for children and young people aged 7–15 years, comprising a two-step programme, MEND and Choose It (Wolverhampton City Council, Wolverhampton, UK), focusing on taster sessions for physical activity and healthy eating. Funding for this programme was withdrawn halfway through the study and so two alternatives were offered as ‘usual care’ in site C, depending on the age of the child; (2) Weight Watchers® (Weight Watchers UK Ltd, Maidenhead, UK) for young people aged ≥ 10 years, who had to be accompanied by a parent; and (3) a referral to the school nurse for children aged 6–9 years, where children would be weighed and measured and offered advice. This type of usual care was not standard at the start of the study and, therefore, did not always occur as hoped. Table 3 gives further details of the usual-care programmes.
| Usual-care programme | Delivery | Core themes |
|---|---|---|
| One Body One Life (site A) | Parent and child group based 1.5 hours weekly for 10 weeks Delivery at school or community venue |
Healthy eating Physical activity Health checks |
| Change4Life advisor (site B) | Parent and child one to one with Change4Life advisor First session ≈ 1.5 hours, subsequent visits ≈ 45 minutes Number of visits varies according to family needs (average 5 visits) Majority of visits at home, occasionally at school or clinic |
Healthy eating (Eatwell plate; portion sizes, food labelling) Increasing physical activity Self-esteem |
| Weight management programme for parents and children (site C) | Parent and child group based 2 hours weekly for 10 weeks Delivery at community venue |
MEND programme and Choose It (focusing on taster sessions for physical activity and healthy eating) |
| Weight Watchers for children aged ≥ 10 years (site C) | Parent and child group based 1 hour weekly for 12 weeks Delivery at community venue |
Healthy eating (portion sizes) Encourage increased physical activity (pedometers for sale) |
| School nurse referral for children aged 6–9 years (site C) | One to one | Weighed and measured Offered advice |
Participants
The study recruited children aged 6–11 years who were overweight or obese and their parent(s) from the three primary care trusts.
Inclusion criteria
Families were considered for inclusion if:
-
they had at least one overweight (≥ 91st centile for BMI) or obese (≥ 98th centile for BMI) child aged 6–11 years, based on the UK 1990 BMI37
-
at least one parent or guardian and the overweight child was willing to take part.
Exclusion criteria
Families were excluded if:
-
the parent or child had an insufficient command of English and would find it difficult to participate in the group
-
the child had a metabolic or other recognised medical cause of obesity
-
the child had severe learning difficulties and/or severe behavioural problems, and would find it difficult to participate in a group-based programme.
Recruitment
The aim was to recruit 40 families from each of the three primary care trusts. At the time of recruitment, data from the NCMP 2008/938 showed an abundant pool of potential participants in just one school year (year 6) (Table 4).
| Location | Number eligible | Number measured | Participation rate (%) | Prevalence of obesity, % (95% CI) | Number of obese Year 6 children |
|---|---|---|---|---|---|
| Site A | 3477 | 3152 | 90.7 | 19.4 (18.0 to 21.3) | 611 |
| Site B | 5548 | 4584 | 82.6 | 15.1 (14.1 to 17.2) | 692 |
| Site C | 2827 | 2649 | 93.7 | 23.5 (21.9 to 25.5) | 623 |
| West Midlands SHA | 62,526 | 55,993 | 89.6 | 19.8 (19.5 to 20.8) | – |
| England | 558,633 | 497,680 | 89.1 | 18.3 (18.2 to 19.1) | – |
Both active and passive recruitment methods were used. Initially, passive recruitment included advertisements in newspapers and local radio interviews, whereas active recruitment involved letters to families with an overweight or very overweight child, who had recently been measured in the NCMP, and referrals from health-care professionals. However, recruitment was slower than planned so further recruitment strategies were employed including letters to families from the family’s general practitioner (GP) and approaches from Change4Life advisors. Flyers and posters were also sent to be displayed in schools, the community, primary care surgeries and the local hospital, and there were recruitment stands at local public events. As recruitment continued, word of mouth from families enrolled in the trial became a successful recruitment method.
Randomisation, allocation concealment and blinding
The unit of randomisation was the family. Researchers registered families after confirming eligibility and obtaining consent, and after completing the baseline data collection in order to ensure allocation concealment. The term ‘control’ was not used at any time with the families and they were told that they would be receiving one of two possible programmes, either Families for Health or usual care. After consent, the family was allocated to intervention or control by the central telephone registration and randomisation service at the Warwick Clinical Trials Unit. Randomisation was stratified by locality (site A, B or C) using a biased-coin (p = 2/3) minimisation method within each locality to ensure that approximately equal numbers of families were randomised to the Families for Health programme and control. The trial administrator (EH) assigned the participants to the interventions that they were allocated to.
The families could not be blinded to treatment allocation, but every effort was made to ensure that the research personnel involved with data collection remained blind to treatment allocation until the initial data collection was complete. Blinding was not an issue at baseline because all data collection was done before randomisation. A ‘blinding protocol’ recorded the systems in place to keep the researchers blind to treatment allocation at the 3- and 12-month follow-up visits, which included the following.
-
The family was reminded via letter and telephone call by the trial administrator that they were not to discuss their allocation with the researcher at the visit until requested.
-
The researcher took all anthropometric measurements first and then the parent(s) and child(ren) completed their self-administered questionnaires. The Client Services Receipt Inventory (CSRI) questionnaire and the interview (if being carried out) both unblinded the researcher and so these were administered last.
-
The researcher that conducted the 3-month follow-up visit (who was now unblinded) did not conduct the 12-month follow-up visit for the same family. A different researcher who was blind to treatment allocation conducted the 12-month follow-up visit.
In order to ascertain whether or not the assessors were blind to the allocated arm, an ‘unblinding log’ was kept by the trial administrator to record if a researcher was inadvertently unblinded to allocation. On 21 occasions the follow-up visits were completed by an unblinded researcher who knew the treatment allocation at the start of the research visit (e.g. because a family mentioned the name of the programme that they had been on at the start of the home visit).
Statisticians (TH and NS) were blinded for the interim analysis for the DMEC, but neither the statisticians nor health economists were blinded for the final analysis presented to the combined meeting of the TSC and DMEC. This policy was agreed by the TSC.
Data collection and management
All members of the research team were trained to use standard operating procedures for each stage of data collection. Data entry was carried out by the trial administrator and members of the research team and then checked by a different team member to minimise data entry errors. The software for data entry was bespoke, in that it was custom made and managed by the University of Warwick Clinical Trials Unit programming team.
A number of different measures were used to collect information on the impact of the intervention (see Table 5). All of these were measured in the family home at baseline, at the end of the 10-week Families for Health programme (or approximately 3 months in the usual-care arm) and at 12 months from baseline. In families with more than one eligible child who met the inclusion criteria, data were collected on all participating overweight or obese children. Research visits in the family home took, on average, between 1 and 3 hours, depending on whether they were baseline or follow-up and whether or not interviews were carried out.
Baseline assessment
All baseline visits took place between 16 March 2012 and 11 February 2014. A researcher assessed the family’s eligibility for the trial and obtained the parents’ consent and children’s assent. Parents were asked to complete a brief baseline questionnaire that included the parent’s date of birth (needed for randomisation), the child(ren)’s date of birth (age), sex and ethnic group, and primary care contact details. At the same time, parents were asked whether or not they were consulting anyone else about their child’s weight, whether the child suffered from any medical conditions or allergies, whether or not either of them was taking part in any other research project and what the main reasons were for wanting to address their child’s weight or lifestyle. Family structure was categorised into five groups: (1) two-parent family, (2) single mother, (3) single father, (4) stepfamily or (5) other. The researcher also asked how the family heard about the Families for Health programme. Subsequently, the researcher took the parent and child through all of the questionnaires described in detail below.
For families allocated to the Families for Health programme, the period between the baseline visit and the start of the intervention was, on average, 94 days, with a minimum waiting time of 1 day and a maximum waiting time of 346 days. In site A the mean waiting time was 93 days, in site B it was 73 days and in site C it was 114 days. No equivalent information was available for families allocated to usual care.
Follow-up
All 3- and 12-month follow-up visits took place between 27 July 2012 and 9 May 2014 and between 12 March 2013 and 11 March 2015, respectively. Follow-up data collection was scheduled at 3 and 12 months post randomisation. The 3-month visit had been planned to coincide with the completion of the Families for Health intervention. However, some families had to wait until there was a viable number of families to form a group (a minimum eight families) before starting the intervention. Therefore, some post-intervention follow-ups were more than 3 months after randomisation. For families receiving usual care, 3-month follow-up visits were always scheduled for 3 months post randomisation regardless of whether or not they had accessed a usual-care intervention at that point. All 12-month follow-up visits were carried out at 12 months from baseline, by a different researcher from the one who carried out the 3-month follow-up, in order the maintain blinding. Where the 3- and 12-month follow-up were too close, that is, when a family had waited so long for the intervention group to start that there would be less than 1 month between 3 and 12 months, then a combined 3- and 12-month visit was carried out. Separate one-to-one in-depth interviews with the parent and child were carried out at the 3- and 12-month follow-up, respectively, with an original plan of carrying out interviews with 24 families from each trial arm. The following table (Table 5) demonstrates the different measures used in the study, who (child or parent) this was collected from and at what time point.
| Measure | Reference | Validated | Children | Parents | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | |||
| Anthropometric | ||||||||
| Weight and height (BMI) | LMSgrowtha | – | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Waist circumference | McCarthy et al.39 | – | ✗ | ✗ | ✗ | – | – | – |
| Percentage body fat | Wells and Fewtrell40 | – | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Physical activity and diet | ||||||||
| Accelerometry (ActiGraph GT3X) | Evenson et al.41 | Evenson et al.;41 Trost et al.42 | ✗ | – | ✗ | – | – | – |
| Fruit and vegetable consumption – Day in the Life Questionnaire | Edmunds and Ziebland43 | Edmunds and Ziebland43 | ✗ | ✗ | ✗ | – | – | – |
| Family Eating and Activity Habits Questionnaire | Golan44 | Golan44 | – | – | – | ✗ | ✗ | ✗ |
| Physical and mental health | ||||||||
| Child’s quality of life – PedsQL (both child’s own and parent-proxy recording for the child) | Varni45 | Varni et al.46 (US); Upton et al.47 (UK) | ✗ | ✗ | ✗ | – | – | – |
| Warwick–Edinburgh Mental Well-Being Scale | Tennant et al.48 | Tennant et al.48 | – | – | – | ✗ | ✗ | ✗ |
| Family relationships | ||||||||
| Child–Parent Relationship Scale | Pianta49 | Pianta49 | – | – | – | ✗ | ✗ | ✗ |
| Parenting | ||||||||
| PSDQ | Robinson et al.50 | Robinson et al.50 | – | – | – | ✗ | ✗ | ✗ |
| Economic outcomes | ||||||||
| EQ-5D-Y (both child’s own and parent-proxy recordings for the child) EQ-5D health state valuation (parent’s own) |
Wille and Ravens-Sieberer51 (children); Dolan52 (parents) | Eidt-Koch et al.53 (children) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| CSRI | Beecham and Knapp54 | – | – | – | – | ✗ | ✗ | ✗ |
| Family characteristics | ||||||||
| Socioeconomic status | ONS55 | – | – | – | – | ✗ | – | – |
| Qualitative | ||||||||
| One-to-one interviews (selected) | – | – | – | ✗ | ✗ | – | ✗ | ✗ |
Outcomes
Anthropometric
Child weight and height (body mass index)
The primary outcome measure was the change in children’s BMI z-score at 12 months compared with the change in the control arm. Weight was measured using the Tanita body composition analyser (model BC-420S MA; Tanita Europe B.V., Amsterdam, the Netherlands) to the nearest 0.1 kg, taken without shoes and in light, indoor clothing. Children were asked to stand still on the scales with arms by their side. Height was measured by a Leicester stadiometer (Harlow Healthcare, South Shields, UK) to the nearest 0.1 cm, without shoes, with feet together and flat on the floor with heels touching the base of the stadiometer. Children were asked to put their arms by their side and look straight ahead, and then their head was adjusted if required so that their ear hole was aligned with the bottom of the eye socket (the Frankfurt plane). The measuring arm of the stadiometer was lowered onto the top of the head to measure height. BMI was calculated using the following equation:(1)BMI=weight (kg)height2 (m), and then was converted into standard deviation (SD) scores (z) from 1990 UK growth reference curves, giving a BMI z-score. 37 The BMI z-score is used because it takes into account children’s age and sex, and indicates how many SDs a child’s BMI is above or below the average BMI for their age and sex. This was carried out using LMSgrowth (version 2.77; Harlow Healthcare, South Shields, UK; www.healthforallchildren.com/shop-base/shop/software/lmsgrowth), which is a Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) add-in, designed to manipulate growth data using growth references based on the LMS method.
Child waist circumference
Waist circumference was measured to the nearest 0.1 cm using a Seca 200 tape (Seca, Birmingham, UK) taken at the level of the umbilicus, consistent with the method of Daniel et al. 56 Measurements were made without compressing the skin, taken while standing with feet together, arms hanging by side and looking straight ahead. Measurements were taken under clothes, unless a child was uncomfortable with this and then the measurement was taken over a thin layer of clothing. Where this was the case, a note was made at the time of data collection. Waist circumference was translated into z-scores for age and sex using 2001 reference data for British children aged 5–16 years. 39
Percentage body fat
An indirect measure of percentage body fat was made using the Tanita scales using bioelectrical impedance analysis. 40 An electrical signal (50 kHz, 800 µA) is sent through the body via the pressure-contact electrodes on which the participant stands, to get a measure of impedance (Ohms). The percentage of body fat is calculated using an in-built equation based on impedance, height, age, sex and body type.
Parent weight and height (BMI)
Height was recorded using a Leicester stadiometer and weight with Tanita scales, as with children. No waist circumference measurements were taken for parents. BMI and percentage body fat were calculated.
Physical activity and diet
Accelerometry: time spent in physical activity and intensity
All children on the trial were asked to wear an accelerometer for 7 days following the baseline and 12-month follow-up assessments in order to provide an objective measure of the amount of physical activity undertaken over a period of 7 days. Accelerometers are able to record the participant’s movement such that their physical activity level can be translated into a number of different outcomes, including total step count, bouts of physical activity at specified intensities or energy expenditure. Accelerometry data were collected using the ActiGraph GT3X, programmed to record using 15-second epochs. Data were analysed using the Actilife 6 Data Analysis Software (ActiGraph LLC, Pensacola, FL, USA).
Children, with help from parents, were asked to complete an activity diary alongside the accelerometer for 7 days. This was a pictorial ‘tick-box’ diary recording activities each hour, with a column for free-text additional comments. There was also a place to record the time the accelerometer was put on and taken off. The purpose of the diary was to aid interpretation of the accelerometer output. 57 Children and parents were provided with instructions on receiving the accelerometer about how to wear it. Children were fitted with accelerometers located at the waist at the left-hand side of the body on an elastic belt, with the open/close button (to connect accelerometer to computer) facing upwards. They were asked to wear the device under or on their clothes during all waking hours, except when undertaking water-based activities.
Data were collected for 7 consecutive days and thus included both weekdays and weekends. Owing to previous evidence suggesting that differences in activity levels exist during school time and out of school time,58 where possible accelerometers were worn during the school term, in order to keep measurements consistent across participants. If this was not possible (e.g. a child did not feel comfortable in wearing it during term time) it was worn during holiday time in order that some (rather than no) activity data were collected. In all circumstances, the 12-month follow-up data collection took place at the same time of year and consistently in either term time or holiday time. The outcome was measured at baseline and 12 months in order to minimise seasonal effects. 59 The date of wearing the accelerometer was recorded in the project management file. At the end of the 7-day period, the researcher collected the accelerometer and accompanying activity diary from the participant’s home.
Children’s fruit and vegetable consumption
Children completed a 24-hour recall using the ‘Day in the Life Questionnaire’. 43 This also asked about transport to and from school and daily activity, but the primary aim was to assess the number of portions of fruit and vegetables consumed the previous day. The questionnaire was checked and clarification sought, if necessary, with parents (e.g. orange juice: was this ‘squash’ or ‘fruit juice’?). In line with national recommendations (NHS Choices60), fruit juice could account for only one portion of fruit and vegetables per day, regardless of how much was consumed, and how often. Similarly, baked beans were counted as one portion regardless of how many times they were consumed in a single day. Composite foods (e.g. curry, pizza) that contained a unknown number portions of fruit and vegetables were excluded.
The questionnaire has been validated for measuring fruit and vegetable consumption with 7- to 9-year-olds. 43 The validation was done with four schools, comparing the questionnaire with observation by a researcher for fruit and vegetables eaten at lunchtime, achieving a modest kappa of 0.54–0.58. The reliability was assessed both by a test–retest, which was acceptable, and by inter-rater reliability of two coders assessing the portions of fruit and vegetables, with a high kappa of 0.85–0.92, indicating high agreement between coders. The questionnaire was also shown to be sensitive to change following the distribution of free fruit, confirming it as a good questionnaire to use in the current study, in order to measure any change in fruit and vegetable intake.
Family eating and activity
Eating and activity behaviour in the family was assessed using a modified version of the Family Eating and Activity Habits Questionnaire (FEAHQ) from Israel. 44 Slight modifications were made to make it suitable for use in the UK. This was principally in the list of snacks in question 5, excluding those not heard of in the UK (e.g. Chitos, Ruffles) and adding in those that were common in the UK (e.g. crisps). The questionnaire includes the following four subscales: (1) activity level (four items), to record the balance between physical activity and sedentary pastimes (e.g. television); (2) stimulus exposure (eight items), for example presence and visibility of unhealthy snacks kept at home, allowed to eat snacks/sweets without parental permission, allowed to buy own sweets; (3) eating related to hunger (four items), for example who initiates the eating in the family or behaviour if the child is not hungry; and (4) eating style/rites (13 items), for example where and with whom does eating take place and second helpings. Cronbach’s alpha as a measure of internal consistency reliability was calculated at α = 0.83 for the questionnaire overall and α = 0.82, 0.78, 0.86 and 0.88 for the subscales 1–4, respectively. 44 Parents completed the questionnaire and then summary scores were calculated for the children’s score for the four sections in accordance with the scoring instructions. Lower scores are classed as ‘better’ on each section.
Physical and mental health
Children’s quality of life
Children’s quality of life was measured using the Pediatric Quality of Life InventoryTM (PedsQL) version 4.0 (UK) for ages 8–12 years. 45 Children completed the 23-item self-report version and the parents completed the almost identical parent-proxy version about their child’s quality of life. This measures four domains of health-related quality of life: (1) physical health (eight questions), (2) emotional health (five questions), (3) social functioning (five questions) and (4) school functioning (five questions). All items are rated on a 5-point Likert scale from 0 (never a problem) to 4 (almost always a problem), which are then rescored: 0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0. Summary scores are then derived for the physical domain (eight questions), the psychosocial health domain (emotional/social/school) (15 questions) and for a total scale score (all 23 questions), with the best possible score being 100 (range 0–100).
Varni et al. 46 measured the internal consistency reliability of PedsQL using Cronbach’s alpha, with 963 children and 1629 parents in a US sample. The Cronbach’s alpha scores showed that the questionnaire had internal reliability for the total score (all 23 questions, α = 0.88 for child report, α = 0.90 for parent report), the physical health score (eight questions, α = 0.80 for child-report, α = 0.88 for parent report) and the psychosocial summary score (15 questions, α = 0.83 for child report, α = 0.86 for parent report). This study demonstrated the reliability and validity of the PedsQL version 4.0. The reliability of the UK version of the PedsQL was also assessed in a sample of 1399 children and 970 parents from south Wales, and was shown to have similar internal reliability with all subscales on both the child and parent reports, reaching α = 0.70 (minimum standard) and exceeded α = 0.90 for the total score. 47 They recommend the UK version of the PedsQL for assessment of quality of life in UK children.
Parents’ mental health
Parental mental health was measured using the Warwick–Edinburgh Mental Well-Being Scale (WEMWBS). 48 WEMWBS is a measure of mental well-being focusing entirely on positive aspects of mental health. The scale consists of 14 items, covering aspects of mental health including positive affect (feelings of optimism, cheerfulness, relaxation), satisfying interpersonal relationships and positive functioning (energy, clear thinking, self-acceptance, personal development, competence and autonomy). Individuals completing the scale are required to tick the box that best describes their experience of each statement over the past 2 weeks using a 5-point Likert scale (none of the time, rarely, some of the time, often, or all of the time). The Likert scale represents a score for each item from 1 to 5, respectively, giving a minimum score of 14 and a maximum score of 70. All items are scored positively. The overall score for the WEMWBS is calculated by totalling the scores for each item, with equal weights. Therefore, a higher WEMWBS score indicates a higher level of mental well-being. WEMWBS has shown good content validity. In addition, WEMWBS has shown high correlations with other mental health and well-being scales, and partial correlations with scales measuring overall health. 48
Family relationships
Relationship between parents and children
Assessment of the quality of the parent–child relationship was made using the Child–Parent Relationship Scale (CPRS),49 which was self-completed by parents. The CPRS assesses parents’ perceptions of their relationships with their sons and daughters. The short-form version was used, which has 15 items assessed on a 5-point Likert scale, with eight of the questions reverse scored. A mean score is derived with the best possible score being 5 (range 1–5).
The eight-item conflict subscale measures the degree to which a parent feels that his or her relationship with a particular child is characterised by negativity. In order to test the validity of the CPRS, structured interactions between parents and study children were videotaped at 54 months and 6–7 years old. The Cronbach’s alpha for maternal conflict was 0.84 at 54 months and 0.84 at 6–7 years old, whereas the Cronbach’s alpha for paternal conflict was 0.80 at 54 months and 0.78 at first grade. The seven-item closeness scale assesses the extent to which a parent feels that the relationship is characterised by warmth, affection and open communication. The Cronbach’s alpha for maternal closeness was 0.69 at 54 months and 0.64 at first grade, whereas the Cronbach’s alpha for paternal closeness was 0.72 at 54 months and 0.74 at first grade. The conflict and closeness scales of the CPRS represent two distinct domains of parent–child relationships, as evidenced by a relatively low correlation between the scales (r = 0.16).
Parenting style
Parenting style was measured using the Parenting Styles and Dimensions Questionnaire (PSDQ). 50 The PSDQ assesses how often a parent exhibits certain behaviours towards his or her child. It has 32 items contributing to three factors: (1) authoritative parenting style (measuring parent–child warmth and connection, parental use of reasoning, and inductive parenting and autonomy granting, e.g. ‘I help my child to understand the impact of behaviour by encouraging my child to talk about the consequences of his/her own actions’); (2) authoritarian parenting style (measuring physical coercion, verbal hostility and non-reasoning/punitive disciplinary practices, e.g. ‘When my child asks why he/she has to conform, I state: because I said so, or I am your parent and I want you to’); and (3) permissive parenting style (measuring parental indulgence and inconsistency, e.g. ‘I give into my child when the child causes a commotion about something’). Parents were asked to respond by indicating on a scale of 1–5 (never to always) how frequently they performed the behaviour in question. The PSDQ has been shown to demonstrate adequate reliability and validity. 50
Economic outcomes
European Quality of Life-5 Dimensions Youth version health-state valuation of the child
Children completed the descriptive system and the visual analogue scale from the EQ-5D-Y questionnaire at each visit, with the view to describing their health-related quality of life. 51,53 The descriptive system comprises the following five dimensions: (1) mobility; (2) self-care; (3) usual activities; (4) pain/discomfort; and (5) anxiety/depression. Each dimension has three levels: no problems, some problems and extreme problems. The respondent is asked to indicate their health state by ticking (or placing a cross) in the box against the most appropriate statement in each of the five dimensions. The 20-cm visual analogue scale is anchored by the descriptors ‘best possible health’ (100%) and ‘worst possible health’ (0%), with children placing a mark on how they valued their health on the day of the visit.
European Quality of Life-5 Dimensions Youth version health-state valuation of the child by parent
Parents also completed the EQ-5D-Y questionnaire describing their child’s health-related quality of life on the day of each visit. This included the same descriptive questions as those asked of the child (as detailed in European Quality of Life-5 Dimensions Youth version health-state valuation of the child). On the visual analogue scale, parents were asked how they thought their child would value their own health.
European Quality of Life-5 Dimensions health-state valuation: parent’s own
Parents completed the EQ-5D questionnaire describing their own health-related quality of life on the day of each visit. 52
Services received by child
A modified version of the CSRI was used to record hospital and community health and social services received by each child, as well as broader service utilisation (adaptation of CSRI from Beecham and Knapp54), at baseline, 3 and 12 months. The unit costs of each resource item were valued using both primary research, based on established accounting methods, and data collated from secondary national tariff sets. 61–64 Further details of the methods used are provided in Chapter 5.
Socioeconomic classification
Families’ socioeconomic classification was measured using details of parental employment. Employment status and type of employment of the mother and/or father was recorded, including their actual occupation and whether they were self-employed or an employee. This information was used to code families into eight socioeconomic classes using the The National Statistics Socio-Economic Classification. 55 As the number of families was small, this was subsequently collapsed into three classes, plus a fourth category of ‘never worked and long-term unemployment’ (Table 6).
| Eight classes | Three classes |
|---|---|
| 1. Higher managerial and professional occupations | 1. Managerial and professional occupations |
| 1.1 Large employers and higher managerial occupations | |
| 1.2 Higher professional occupations | |
| 2. Lower managerial and professional occupations | |
| 3. Intermediate occupations | 2. Intermediate occupations |
| 4. Small employees and own-account workers | |
| 5. Lower supervisory and technical occupations | 3. Routine and manual occupations |
| 6. Semiroutine occupations | |
| 7. Routine occupations | |
| 8. Never worked and long-term unemployed | Never worked and long-term unemployed |
Sample size
Original sample size justification
Power calculations assumed a residual SD in the BMI z-score of 0.22, a SD of the random family effects of 0.14, an intracluster correlation of 0.1 in the intervention groups, a two-sided significance of 5% and that 60% of participating families have one overweight or obese child and 40% have two with a within-family intracluster correlation of 0.27. Allowing for clustering effects by family and for group effects in the intervention arm, a sample size of six groups of 10 families (60 families) in the intervention arm and 60 families in the control arm gives a power of 94% to detect an intervention effect of 0.2 in BMI z-scores based on pilot study data and relevant literature. 24,65 If 30% of families were to drop out, the study retains a power of 88%. As a check, the power was calculated when no families have more than one overweight or obese child. In this case, the power is 92% or 83% if 30% of families drop out. Power values were estimated using a simulation study including 10,000 simulated trials.
Updated sample size calculation
Recruitment to the trial was slower than expected, leading to smaller groups for the Families for Health programme than planned; therefore, the addition of a seventh Families for Health programme was proposed. On 19 June 2014 this amendment to the Families for Health protocol was approved by Health Technology Assessment. The sample size calculation was updated to incorporate the additional programme and recruitment thus far (all other assumptions of the sample size calculation remained the same). A sample size of seven groups of eight families (56 families) in the intervention arm and 56 families in the control arm yields a power of 91% to detect an intervention effect of 0.2 in BMI z-scores if seven families (one with two children) were providing 12-month follow-up data (12.5% dropout) and 88% power if six families (one with two children) were providing 12-month follow-up data (25.0% dropout). If no families had more than one child, then the power would be 89% and 85% for the 12-month follow-up of seven and six families per programme, respectively.
Chapter 3 Statistical analysis methods
Analysis of accelerometer data
Accelerometer data were reduced and analysed as follows. Evenson et al. ’s41 activity count cut points for the ActiGraph for physical activity intensities (sedentary, light, moderate or vigorous) were used for the analysis, as recommended by Trost et al. 42 (Table 7). The Evenson cut point associated with moderate to vigorous physical activity in children is 2296 counts per minute.
| Time period | Cut points | |||
|---|---|---|---|---|
| Sedentary | Light | Moderate | Vigorous | |
| Per 15 seconds | 0–25 | 26–573 | 574–1002 | ≥ 1003 |
| CPM | ≤ 100 | 101–2295 | ≤ 2296 (moderate to vigorous) | |
Not all the accelerometer records were complete. A child’s record was included in the analysis if there were at least 3 complete days of data, taken as the minimum needed to obtain a reliable measurement of habitual physical activity in children, at both baseline and 12-month follow-up. A complete day was defined as one where there was ≥ 8 hours of data, after excluding periods in the day when the accelerometer appeared not to have been worn. Although 10 hours of worn time is often used, the reliability between 7 and 10 hours is not substantially different. 66 Non-wear time was identified from the data by periods of ≥ 60 minutes of consecutive zero counts, making it unlikely that the monitor was being worn.
At each time point for each child with complete records, the mean daily time spent in moderate and vigorous physical activity, sedentary time, accelerometer counts per minute and daily step count were calculated using the Actilife 6 Data Analysis Software.
Statistical methods
A comprehensive statistical analysis for the Families for Health trial was conducted with the aim of assessing the effectiveness of the Families for Health programme, in comparison with usual care, in the treatment of overweight and obesity among children aged 6–11 years. The prespecified primary outcome measure in the statistical analysis was the change in children’s BMI z-score from baseline to 12 months’ follow-up so that clinical effectiveness would be declared based on a reduction in BMI z-score relative to the comparator. Secondary outcomes in the statistical analysis fell into four categories: (1) anthropometric measures in children; (2) anthropometric measures in parents; (3) (validated) questionnaires completed by children; or (4) (validated) questionnaires completed by parents. Table 5 provides an overview of all outcomes analysed and the time points at which they were measured.
General statistical considerations
The primary outcome analysis as well as all secondary outcome analyses were conducted at the conventional (two-sided) 5% level and, corresponding to this, all presented CIs are 95% CIs. As specified in the statistical analysis plan, no formal adjustment for multiple testing among the secondary end points was used as outcomes are likely to be highly correlated so that standard adjustment techniques, such as the Bonferroni method, would be conservative. All outcome measures and child/parent characteristics were summarised by the trial allocation group and for outcome measures by follow-up period. The distribution of outcome data was investigated and transformations applied, if necessary, before performing statistical tests and modelling. The main analysis of clinical outcome data was the comparison of change from baseline between treatment groups. Using the change from baseline has a ‘normalising’ effect so that standard techniques could generally be used.
Unless otherwise stated, all analyses were performed on an intention-to-treat basis, that is all participants were analysed in the arm they were allocated to and included regardless of whether or not the treatment and follow-up schedule was complied with. This reflects the pragmatic nature of the trial and ensures that conclusions drawn from the analysis will reflect the impact of the Families for Health programme in a real-world setting.
The statistician conducting the statistical analysis was unblinded for the final statistical analysis and the analysis presented at the final DMEC/TSC meeting. The multilevel models originally proposed to be adopted in the analyses (see Primary outcome analysis) consist of two hierarchical levels in the usual-care arm and three levels in the intervention arm. For these models to be fitted, the participants’ group affiliation needed to be revealed. Unblinding was agreed to by the TSC.
The entire statistical analysis was conducted using SAS v9.4 TSL1M2 (SAS Institute Inc., Cary, NC, USA) with the exception of the sample size calculation, which was conducted using R versions 2.10 and 3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Primary outcome analysis
As indicated above, the primary outcome for the statistical analysis was the mean change in child BMI z-score after 12 months of follow-up compared between treatment arms. It was anticipated that these data would be correlated within families (if more than one child of a family participates in the trial) and within delivery groups in the Families for Health intervention arm. The analysis allowed for this clustering in order to obtain unbiased estimation of the treatment effect and its standard error (SE). 67
A three-level hierarchical mixed-effects model was proposed to be fitted in the statistical analysis plan. At the highest level of the model a random effect for delivery group was intended. Delivery group-level clustering would have been allowed for in the Families for Health arm only, as usual-care interventions varied by site, were not necessarily group based and precise details on usual-care treatment received were generally not available. As the statistical analysis showed, there was no evidence of delivery-group clustering and models comprising this random effect failed to converge (see Chapter 4, Main primary outcome analysis). The decision has therefore been made (and been approved by the TSC) to remove this effect from all hierarchical modelling and to use a two-level hierarchical mixed-effects model instead.
Correlation between measurements of children within family was allowed for in both arms.
The multilevel model was adjusted for the child-level characteristics, baseline BMI z-score and sex, and family-level variable ‘locality’ as fixed effects. Additionally, adjustment for the family-level characteristic socioeconomic status (SES) and child-level characteristic ethnicity was explored. Restricted maximum likelihood estimation was employed for estimating covariance parameters in the multilevel modelling. The Satterthwaite approximation68 was used for computing the denominator degrees of freedom for the test of a treatment effect difference between the allocation groups (and for tests of other fixed effects).
The primary analysis is a complete case analysis in the sense that if either the baseline or 12-month follow-up z-score was missing the subject had to be omitted from the analysis.
Sensitivity analyses for the primary outcome
Several sensitivity analyses were undertaken to assess the impact of areas of uncertainty surrounding the primary outcome analysis and its robustness. These involved re-estimating the treatment effect under the following scenarios: (1) conducting a per-protocol analysis in which families having participated in five or more sessions of the Families for Health programme are regarded as ‘programme completers’ (i.e. as having complied with the protocol sufficiently); and (2) (multiple) imputation of missing primary outcome data.
Three standard imputation techniques were employed to assess the sensitivity of the analysis to the missing data: first, simple regression imputation, in which missing values are imputed by predicted values from a linear regression model using the same predictors as the primary outcome analysis; second, multiple imputation methods Markov chain Monte Carlo;69 and, third, fully conditional specification regression. 70 For the two multiple imputation analyses, 200 burn-in iterations were used and estimates averaged over 100 imputed data sets. Baseline BMI z-score, age, sex and site were included as explanatory variables in the imputation models. Imputations were generated separately for each treatment group.
Subgroup analyses
To explore heterogeneity in the trial population, the following prespecified exploratory subgroup analyses were conducted with respect to the primary outcome:
-
child’s sex (male or female)
-
locality (site)
-
SES (according to The National Statistics Socio-Economic Classification’s55 four-class standard classifications)
-
parent’s BMI at baseline (normal, overweight or obese)
-
age of child at baseline (6–8 years or 9–11 years).
The difference in treatment effects by subgroups was initially assessed by interaction tests. These were performed via significance tests of interaction terms in the hierarchical model utilised for the primary outcome analysis. Variables that have been categories for subgroup analyses (e.g. age, parent BMI) were also investigated as covariates on their original scale. Separate models were then fitted for each subgroup to obtain estimates of the treatment effects within subpopulations.
Repeated measures modelling
An exploratory analysis was performed for the investigation of the difference between arms in terms of change over time in the primary outcome measure rather than a comparison between arms at either 3- or 12-month follow-up. In this analysis, the time at which follow-up data were provided was fitted as a continuous variable, accounting for the fact that the actual times varied widely (especially for the 3-month follow-up). For this purpose a repeated measures mixed model was fitted. This model was based on the aforementioned hierarchical model for the primary outcome analysis comprising the same effect (plus time). Model complexity was increased as time became the new first-level (random) factor. The same model specification, as in the primary outcome multilevel model (restricted maximum likelihood estimation), was used where possible. An unstructured covariance matrix was assumed for the correlation between measuring time points.
Analysis of baseline demographic data
Baseline demographic outcomes were obtained on a family level or on a child/parent level. Summary statistics (mean and SD for continuous variables and absolute number and percentage for categorical variables) were calculated for all participants recruited to the trial and for the two treatment groups separately. The characteristics within the two treatment groups were compared using t-tests and chi-squared tests for continuous and categorical variables, respectively. The statistical comparison of baseline characteristics was considered exploratory rather than confirmatory and ignored clustering by delivery group or family. The main intention was to identify baseline variable differences that might be deemed relevant by the main investigators and would consequently require adjustment in the multilevel models fitted in the primary and secondary outcome analyses.
Secondary outcome analyses
All secondary outcomes, including subscales of questionnaires, were summarised by trial allocation group and follow-up period using mean, SDs and 95% CIs for continuous variables and absolute number, percentages and 95% CIs for categorical variables. Statistical tests of differences for child secondary outcomes were performed using the same hierarchical mixed-effects model as the primary outcome analysis. In addition, child BMI z-score was also compared as change from baseline to 3-month follow-up and change from baseline to 12-month follow-up using independent group t-tests. Parent outcomes were compared using t-tests and chi-squared tests, as appropriate, as generally only one parent provided data and within-family correlation could not be modelled. In case parents and children provided data for the same outcome, the simpler test was used to ensure comparability. Where the analysis of parent outcomes was adjusted, hypothesis testing was done within a linear regression model and the adjustment variables provided in the respective results section.
Chapter 4 Trial results
Participant flow
In total, 115 families were recruited to the Families for Health trial, with 56 randomly allocated to the intervention arm and 59 to the usual-care arm. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram summarises families from enrolment, through eligibility screening and randomisation to the 12-month follow-up (Figure 1). Of the 194 families assessed for eligibility, 79 were excluded. Fourteen families were not eligible for a variety of reasons including the child not being overweight, being too young or having behavioural problems. Eight families did not give a reason for not wanting to take part. A further 18 families were unable to be contacted after their initial referral/interest and 39 families declined to participate. Reasons given included being unwilling to be randomised, time commitments and not being able to attend a Saturday intervention.
FIGURE 1.
The CONSORT flow diagram for the Families for Health RCT. FFH, Families for Health; UC, usual care. Adapted from Robertson W, Fleming J, Kamal A, Hamborg T, Khan KA, Griffiths F, et al. Randomised controlled trial and economic evaluation of the ‘Families for Health’ programme to reduce obesity in children [published online ahead of print 21 December 2016]. Arch Dis Child 2016. doi: 10.1136/archdischild-2016-311514. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited (see http://creativecommons.org/licenses/by/4.0/). 71

Of the 56 families allocated to Families for Health arm, 42 (75%) attended at least one session. Of the 59 families allocated to the usual-care arm, 24 (40.7%) families were known to have attended at least one session. For seven families allocated to usual care, their participation in the usual-care intervention was classified as ‘unable to determine’ (see Figure 1) because these families were lost to follow-up and their usual-care provider did not confirm their attendance.
Six families withdrew from the trial, four at 3 months and a further two at 12 months. Reasons for withdrawal were family illness, a family bereavement, child starting secondary school and feeling could manage weight on their own, child not liking study measurements being taken and family feeling the study visit was a waste of their time. One family repeatedly cancelled and rearranged their study visit time and eventually withdrew with no reason given. In addition to the withdrawal of families, some families were also lost to follow-up for unknown reasons. Table 8 provides an overview of the number of families lost at each site and time point, and the number of children retained. There was 80% retention of families at 3 months and 72% retention at 12 months, but with a lower proportion retained in the usual-care arm [Families for Health (78.6%) vs. usual care (66.1%), at 12 months] (see Figure 1).
| Time point | Treatment group | Number of families | Number of families in site A | Number of families in site B | Number of families in site C | Number of children | Number of families (%) with ≥ 2 siblingsa |
|---|---|---|---|---|---|---|---|
| Baseline | FFH | 56 | 24 | 15 | 17 | 63 | 5 (8.9) |
| UC | 59 | 26 | 16 | 17 | 65 | 6 (10.2) | |
| 3 months | FFH | 46 | 22 | 12 | 12 | 48 | 2 (4.3) |
| UC | 46 | 18 | 12 | 15 | 50 | 6 (13.0) | |
| 12 months | FFH | 44 | 19 | 11 | 14 | 45 | 1 (2.3) |
| UC | 39 | 18 | 9 | 12 | 43 | 4 (10.3) |
In total, 11 families with more than one child participating in the trial were randomised (see Table 8). This is a lower proportion of families than anticipated (see Chapter 2, Original sample size calculation). Of these 11 families, one family in the Families for Health treatment group had four participating children and the other families had two.
Baseline data
Table 9 presents a summary of key descriptive statistics at baseline comparing the randomised groups. A randomisation procedure with minimisation was used (see Chapter 2) so that the number of families, parents and children in each group were similar. With the exception of SES, where there was a significant difference between the Families for Health and usual-care groups, all other baseline characteristics were similar in both groups with no statistical differences. For the family type and child ethnicity variables, Fisher’s exact test was used as a result of there being cells with expected values < 5.
| Baseline characteristic | Treatment group | Total | p-value for between-group difference | |
|---|---|---|---|---|
| FFH | UC | |||
| Number of families (%) | 56 (48.7) | 59 (51.3) | 115 | 0.5829 |
| Number of parents/carers (%) | 64 (46.7) | 73 (53.3) | 137 | |
| Number of children (%) | 63 (49.2) | 65 (50.8) | 128 | |
| Obese | 51 (81.0) | 55 (84.6) | 106 (82.8) | |
| Overweight | 12 (19.0) | 10 (15.4) | 22 (17.2) | |
| Sex of children, n (%) | ||||
| Male | 27 (42.9) | 36 (55.4) | 63 (49.2) | 0.156 |
| Female | 36 (57.1) | 29 (44.6) | 65 (50.8) | |
| Age, years (SD) | ||||
| Mean age, child | 9.46 (1.57) | 9.43 (1.61) | 9.44 (1.59) | 0.893 |
| Mean age, parent/carer | 39.60 (5.86) | 40.59 (8.87) | 40.13 (7.60) | 0.456 |
| Family type (N = 115), n (%) | ||||
| Two-parent family | 32 (57.1) | 28 (47.5) | 60 (52.2) | 0.797 |
| Single parent (mother) | 20 (35.7) | 26 (44.1) | 46 (40.0) | |
| Single parent (father) | 0 (0) | 0 (0) | 0 (0) | |
| Stepfamily | 3 (5.4) | 4 (6.8) | 7 (6.1) | |
| Other | 1 (1.8) | 1 (1.7) | 2 (1.7) | |
| Child ethnicity (N = 128), n (%) | ||||
| White | 38 (60.3) | 41 (63.1) | 79 (61.7) | 0.712 |
| Black | 4 (6.3) | 6 (9.2) | 10 (7.8) | |
| Asian | 13 (20.6) | 9 (13.8) | 22 (17.2) | |
| Chinese | 0 (0) | 0 (0) | 0 (0) | |
| Mixed | 7 (11.1) | 9 (13.8) | 16 (12.5) | |
| Other | 1 (1.6) | 0 (0) | 1 (0.8) | |
| BMI | ||||
| Baseline child weight (kg), mean (SD) | 52.45 (14.22) | 52.41 (14.31) | 52.43 (14.21) | 0.985 |
| Baseline child BMI (kg/m2), mean (SD) | 25.79 (4.44) | 25.93 (4.32) | 25.86 (4.36) | 0.863 |
| Baseline child BMI z-score, mean (SD) | 2.69 (0.67) | 2.74 (0.70) | 2.71 (0.68) | 0.657 |
| Baseline parent/carer BMI (kg/m2), mean (SD) | 31.88 (7.30) | 32.01 (8.15) | 31.95a (7.74) | 0.923 |
| SES (N = 115), n (%) | ||||
| Class 1 (higher managerial, administrative and professional occupations) | 24 (42.9) | 15 (25.4) | 39 (33.9) | 0.038 |
| Class 2 (intermediate occupations) | 12 (21.4) | 7 (11.9) | 19 (16.5) | |
| Class 3 (routine and manual occupations) | 13 (23.2) | 23 (39.0) | 36 (31.3) | |
| Class 4 (never worked and long-term unemployed) | 7 (12.5%) | 14 (23.7%) | 21 (18.3%) | |
| Recruitment method, n (%) | ||||
| Active | 18 (41.7) | 25 (58.1) | 43 | 0.701 |
| Passive | 38 (52.8) | 34 (47.2) | 72 | |
Table 10 shows the same variables as Table 9 for families/children provided at least one child completed the 12-month follow-up. The pattern is very similar, with no significant differences between treatment groups. Comparing the differences between those who completed the 12-month follow-up and those who did not within each treatment group led to only one significant difference, which was that parents who dropped out in the usual-care group had a higher baseline BMI than those who remained (p = 0.022). There is no strong relationship between the presented baseline characteristics and likelihood of a family dropping out of the study.
| Baseline characteristic | Treatment group | Total | p-value for between-group difference | |
|---|---|---|---|---|
| FFH | UC | |||
| Number of families (%) | 44 (53.0) | 39 (47.0) | 83 | 0.9261 |
| Number of parents/carers (%) | 50 (50.5) | 49 (49.5) | 99 | |
| Number of children (%) | 45 (51.1) | 43 (48.9) | 88 | |
| Obese | 38 (84.4) | 36 (83.7) | 74 (84.1) | |
| Overweight | 7 (15.6) | 7 (16.3) | 14 (15.9) | |
| Sex, n (%) | ||||
| Boys | 19 (42.2) | 23 (53.5) | 42 (47.7) | 0.290 |
| Girls | 26 (57.8) | 20 (46.5) | 46 (52.3) | |
| Age, years (SD) | ||||
| Mean age child | 9.44 (1.47) | 9.25 (1.61) | 9.35 (1.54) | 0.557 |
| Mean age parent/carer | 40.19 (6.23) | 41.24 (8.48) | 40.71 (7.41) | 0.484 |
| Family type (N = 83), n (%) | ||||
| Two-parent family | 25 (56.8) | 22 (56.4) | 47 (56.6) | 0.999 |
| Single parent (mother) | 15 (34.1) | 14 (35.9) | 29 (34.9) | |
| Single parent (father) | 0 (0) | 0 (0) | 0 (0) | |
| Stepfamily | 3 (6.8) | 3 (7.7) | 6 (7.2) | |
| Other | 1 (2.3) | 0 (0) | 1 (1.2) | |
| Child ethnicity (N = 88), n (%) | ||||
| White | 28 (62.2) | 25 (58.1) | 53 (60.3) | 0.839 |
| Black | 2 (4.4) | 4 (9.3) | 6 (6.8) | |
| Asian | 9 (20.0) | 8 (18.6) | 17 (19.3) | |
| Chinese | 0 (0) | 0 (0) | 0 (0) | |
| Mixed | 5 (11.1) | 6 (14.0) | 11 (12.5) | |
| Other | 1 (2.2) | 0 (0) | 1 (1.1) | |
| BMI | ||||
| Baseline child BMI (kg/m2), mean (SD) | 25.92 (4.57) | 25.08 (3.57) | 25.51 (4.11) | 0.340 |
| Baseline child BMI, z-score (SD) | 2.70 (0.63) | 2.68 (0.69) | 2.71 (0.68) | 0.859 |
| Baseline parent/carer BMI (kg/m2), mean (SD) | 32.08 (7.06) | 30.24 (6.7)a | 31.16b (6.91) | 0.195 |
| SES (N = 83), n (%) | ||||
| Class 1 (higher managerial, administrative and professional occupations) | 22 (50.0) | 12 (30.8) | 34 (41.0) | 0.102 |
| Class 2 (intermediate occupations) | 8 (18.2) | 4 (10.3) | 12 (14.5) | |
| Class 3 (routine and manual occupations) | 10 (22.7) | 16 (41.0) | 26 (31.3) | |
| Class 4 (never worked and long-term unemployed) | 4 (9.1) | 7 (17.9) | 11 (13.3) | |
| Recruitment method, n (%) | ||||
| Active | 14 (41.7) | 16 (58.1) | 30 (36.1) | 0.384 |
| Passive | 30 (52.8) | 23 (47.2) | 53 (63.9) | |
Owing to the moderate sample size in this RCT, some of the original eight SES categories were sparsely populated, leading to problems when applying certain statistical methods. Therefore, families’ SES has been collapsed into four categories from the original eight-category SES according to Office for National Statistics guideline55 (see Table 6). Using these four categories, SES is significantly higher in the Families for Health arm (p = 0.038). Table 11 shows the numbers and proportions in each of the original categories. The table also shows the number of single mothers in each group. In total, 46 participating families (40.0%) were single mothers. A TSC member raised the concern that single mothers in class 1 were potentially very different from single mothers in classes 3 and 4 and an adjustment might be needed if the proportions differed substantially between arms. However, there was no statistical evidence of a difference in the SES distribution of single mothers between the Families for Health and usual-care group by Fisher’s exact test (p = 0.451).
| Feature | SES classification details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eight classes | 1.1 | 1.2 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Number (%) in class | 1 (0.87) | 8 (6.96) | 30 (26.09) | 5 (4.35) | 14 (12.17) | 20 (17.39) | 11 (9.57) | 5 (4.35) | 21 (18.26) |
| Three classes | Managerial or professional | Intermediate occupation | Routine and manual occupation | Never worked and long-term unemployed | |||||
| Number (%) in class | 39 (33.91) | 19 (16.52) | 36 (31.30) | 21 (18.26) | |||||
| Single mother FFH, n (%) | 9 (45.0) | 1 (5.0) | 4 (20.0) | 6 (30.0) | |||||
| Single mother UC, n (%) | 6 (23.1) | 2 (7.7) | 9 (34.6) | 9 (34.6) | |||||
Outcomes and estimation
Primary outcome analysis
Exploratory unadjusted analysis
The primary end point for the statistical analysis is the mean change in BMI z-score from baseline to 12 months of follow-up. The primary analysis is a complete-case analysis in the sense that if either the baseline or 12-month follow-up z-score is missing the subject was omitted from the analysis. In the subsequent section, the primary end point was compared between treatment arms using a hierarchical mixed-effect model. Before that, simple unadjusted means are compared in this section. Table 12 shows the number of children and BMI z-score means at each time point by treatment group. The change over time of these means is illustrated in Figure 2.
| Treatment group | Sample size (n) | Unadjusted mean z-score (95% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | Baseline | 3 months | 12 months | |
| FFH | 63 | 48 | 45 | 2.69 (2.52 to 2.85) | 2.62 (2.43 to 2.82) | 2.72 (2.54 to 2.89) |
| UC | 65 | 50 | 43 | 2.74 (2.57 to 2.91) | 2.65 (2.45 to 2.85) | 2.58 (2.37 to 2.79) |
FIGURE 2.
Change over time for primary outcome BMI z-score (unadjusted means and CIs). FFH, Families for Health; UC, usual care. Adapted from Robertson W, Fleming J, Kamal A, Hamborg T, Khan KA, Griffiths F, et al. Randomised controlled trial and economic evaluation of the ‘Families for Health’ programme to reduce obesity in children [published online ahead of print 21 December 2016]. Arch Dis Child 2016. doi: 10.1136/archdischild-2016-311514. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited (see http://creativecommons.org/licenses/by/4.0/). 71
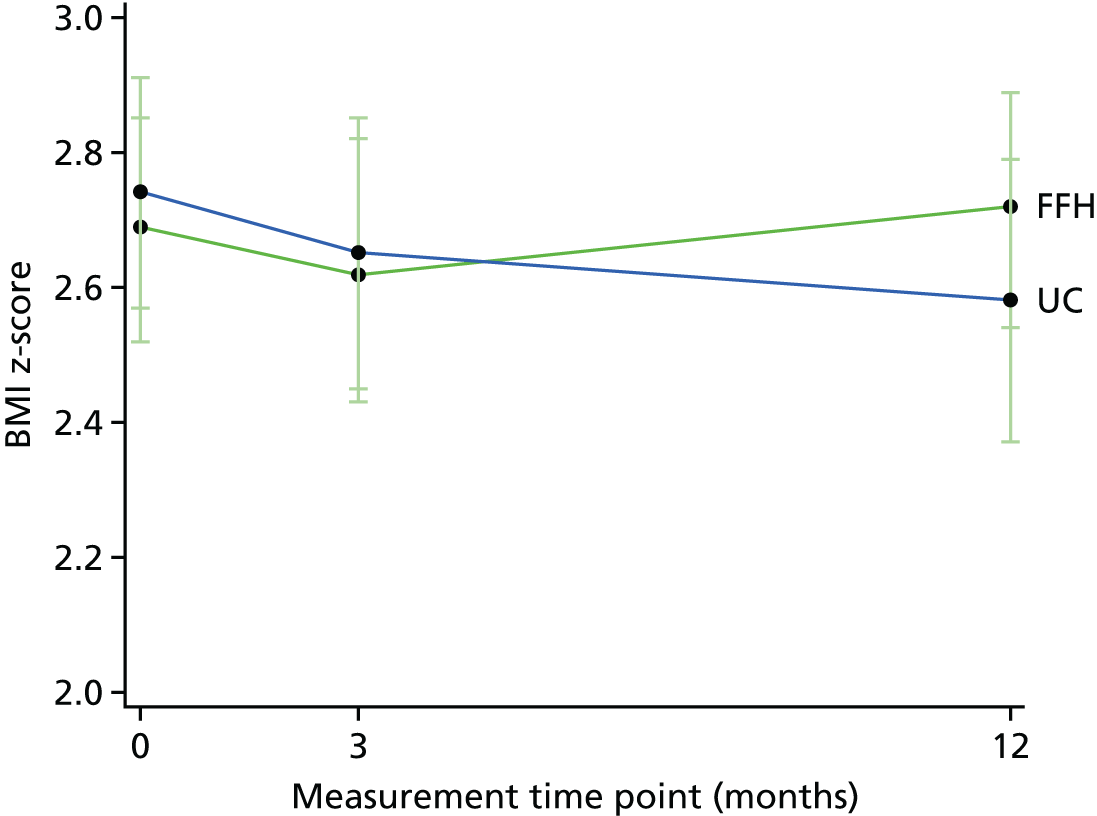
The literature suggests that a change of –0.25 in BMI z-score would be considered clinically significant. 65 Seven children (15.6%) in the Families for Health group and 10 children in the usual-care group (23.3%) achieved a reduction of ≥ 0.25. This difference is non-significant (p = 0.360). No reduction of > 0.5 was observed in the Families for Health group, whereas three children in the usual-care group had a reduction of > 0.5. The largest reduction observed was –0.746 and the largest increase was 0.895.
Table 13 presents (unadjusted) mean change from baseline estimates by treatment groups in child BMI and waist z-scores, and percentage body fat. These are called ‘longitudinal changes’ henceforth. A significant reduction from baseline to 12-month follow-up in the BMI z-score is achieved in the usual-care group with the p-value for the difference in reduction between groups being p = 0.052. The magnitude of the difference and p-value is consistent with those of the secondary (anthropometric) outcomes. Waist z-score and percentage body fat are also presented in Table 13.
| Measure | Time period | |||||
|---|---|---|---|---|---|---|
| Baseline to 3 months | Baseline to 12 months | |||||
| Treatment group | p-value | Treatment group | p-value | |||
| FFH | UC | FFH | UC | |||
| BMI z-score, mean change (SD) [95% CI] | –0.019 (0.253) [–0.093 to 0.054] | –0.042 (0.163) [–0.089 to 0.004] | 0.593 | 0.013 (0.277) [–0.070 to 0.096] –0.001 (0.264) [–0.081 to 0.079] |
–0.102 (0.268) [–0.184 to –0.019] | 0.052 0.081 |
| Waist z-score, mean change (SD) [95% CI] | –0.111 (0.276) [–0.191 to –0.031] | –0.048 (0.216) [–0.110 to 0.013] | 0.211 | 0.002 (0.372) [–0.110 to 0.113] –0.011 (0.366) [–0.122 to 0.101] |
–0.145 (0.295) [–0.235 to –0.054] | 0.045 0.065 |
| Percentage body fat, mean change (SD) [95% CI] | 0.204 (2.492) [–0.520 to 0.928] | –0.084 (1.941) [–0.636 to 0.468] | 0.524 | 0.642 (2.822) [–0.206 to 1.490] 0.480 (2.633) [–0.321 to 1.280] |
–0.900 (4.381) [–2.248 to 0.448] | 0.055 0.080 |
The Families for Health group comprised one child who gained a large amount of weight between baseline and 3-month follow-up for reasons unrelated to the intervention. Removing this child from the analysis (see Table 13, bold values) leads to larger p-values for all outcomes. However, the Families for Health RCT was specified to use an intention-to-treat analysis; therefore, this child is retained in all subsequent analyses.
Table 14 shows between-group comparisons of further anthropometric measures for children. All of them suggest a favourable development in the usual-care group compared with the Families for Health programme, albeit with small absolute differences.
| Anthropometric measure | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 48) | UC (n = 50) | FFH (n = 45) | UC (n = 43) | |
| Mean child age, years (SD)a | 9.46 (1.57) | 9.43 (1.61) | 10.01 (1.53) | 9.56 (1.65) | 10.50 (1.49) | 10.27 (1.63) |
| Mean child BMI, kg/m2 (SD)b | 25.79 (4.44) | 25.93 (4.32) | 26.18 (4.94) | 25.60 (4.24) | 27.30 (5.02) | 25.82 (3.67) |
| Mean child percentage body fat (SD) | 36.81 (6.18) | 37.46 (6.72) | 36.69 (6.63) | 36.95 (6.15) | 37.58 (6.86) | 35.71 (6.74) |
| Mean child waist circumference, cm (SD) | 86.17 (11.61) | 86.30 (11.88) | 86.44 (13.10) | 85.62 (12.19) | 90.36 (11.86) | 86.47 (10.86) |
Main primary outcome analysis (adjusted)
The primary outcome measure was the change in children’s BMI z-score from baseline to the 12-month follow-up. It was anticipated that the data would be correlated within delivery groups in the Families for Health intervention arm and within families should more than one child from a family participate in the trial. It was therefore stipulated that a three-level hierarchical mixed-effects model would be fitted for the primary outcome analysis. Furthermore, it was decided that this analysis would adjust for child-level characteristics baseline BMI z-score and sex, and the family-level variable locality (site).
Fitting a model with a random effect for the Families for Health delivery group yielded an estimate of 0 for this effect (Table 15); the model was unable to uniquely estimate any variation from one Families for Health programme to another, above and beyond the residual variance. This suggests that the responses of children from the same delivery group were no more similar than children from different groups. Several checks have been conducted to test this. The decision has therefore been made to remove this effect and proceed with a two-level hierarchical model.
| Covariance parameter | Subject | Estimate | SE | p-value |
|---|---|---|---|---|
| Intercept | FFH programme | 0 | – | – |
| Residual | 0.07441 | 0.01135 | < 0.0001 |
For the two-level model for the primary outcome the intraclass correlation estimate is:
with a 95% CI of 0.3170 to 0.6256. CI calculation is based on Bonett. 72 This suggests that 47.13% of the observed variation in the dependent variable is attributable to family-level characteristics and 52.87% is attributable to child-level traits.
Table 16 presents covariate effect estimates in the hierarchical modelling. The estimates for the prespecified primary analysis model are shown in the upper part of the table (model 1). Expert input by TSC members recommended exploring additional adjustment for child ethnicity and family SES (model 2). In both models BMI z-score was the only statistically significant predictor. We therefore continued with model 1. F-test statistic p-values for predictor variables are not shown; however, the p-value of the only significant effect is identical to the p-value in the table (because it is a continuous variable).
| Model | Effect | Estimate (SE) | p-value | 95% CI |
|---|---|---|---|---|
| Model 1 | Baseline BMI z-score | –0.108 (0.042) | 0.013 | –0.192 to –0.023 |
| Sex (male) | –0.109 (0.059) | 0.069 | –0.227 to 0.008 | |
| Site A | 0.055 (0.068) | 0.422 | –0.080 to 0.191 | |
| Site B | –0.088 (0.068) | 0.269 | –0.245 to 0.007 | |
| Model 2 | Baseline BMI z-score | –0.115 (0.047) | 0.022 | –0.213 to –0.018 |
| Sex (female) | – | – | – | |
| Sex (male) | –0.082 (0.064) | 0.199 | –0.211 to 0.045 | |
| Site C | – | – | – | |
| Site A | 0.091 (0.080) | 0.257 | –0.068 to 0.251 | |
| Site B | –0.069 (0.093) | 0.468 | –0.256 to 0.119 | |
| SES (unemployed) | – | – | – | |
| SES (managerial) | –0.027 (0.109) | 0.804 | –0.245 to 0.191 | |
| SES (intermediate) | –0.161 (0.127) | 0.211 | –0.415 to 0.093 | |
| SES (manual) | –0.050 (0.105) | 0.629 | –0.259 to 0.157 | |
| Ethnicity (white) | – | – | – | |
| Ethnicity (other) | –0.328 (0.281) | 0.246 | –0.887 to 0.231 | |
| Ethnicity (black) | –0.010 (0.145) | 0.948 | –0.298 to 0.279 | |
| Ethnicity (mixed) | 0.002 (0.092) | 0.977 | –0.183 to 0.188 | |
| Ethnicity (Asian) | 0.035 (0.086) | 0.687 | –0.137 to 0.207 |
The primary outcome analysis estimate of the between-group difference in terms of BMI z-score is 0.114 (95% CI –0.001 to 0.229), that is a non-significant difference in favour of usual-care treatment (Table 17). This estimate and its p-value are almost identical to the estimate obtained in the unadjusted analysis. Point estimates of the within-treatment group differences suggest a slightly larger reduction than in the unadjusted analysis. The different treatment effect estimates obtained for the primary outcome can be compared in Table 18. Unadjusted values are taken from Table 13, model 1 is the prespecified primary outcome analysis and model 2 is the analysis including two additional predictors. Treatment effect estimates and p-values are consistent across analyses.
| Treatment group | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|
| FFH | –0.005 (0.041) | –0.085 to 0.078 | 0.907 |
| UC | –0.118 (0.042) | –0.203 to –0.034 | 0.007 |
| Treatment effect | 0.114 (0.058) | –0.001 to 0.229 | 0.053 |
| Period | Treatment group | Estimate (SE) | 95% CI | p-value |
|---|---|---|---|---|
| Unadjusted | FFH | 0.013 (0.041) | –0.070 to 0.096 | 0.356 |
| UC | –0.102 (0.041) | –0.184 to –0.019 | 0.011 | |
| Treatment effect | 0.114 (0.058) | –0.001 to 0.230 | 0.052 | |
| Model 1 | FFH | –0.005 (0.041) | –0.085 to 0.078 | 0.907 |
| UC | –0.118 (0.042) | –0.203 to –0.034 | 0.007 | |
| Treatment effect | 0.114 (0.058) | –0.001 to 0.229 | 0.053 | |
| Model 2 | FFH | –0.068 (0.076) | –0.221 to 0.084 | 0.374 |
| UC | –0.202 (0.079) | –0.359 to –0.045 | 0.012 | |
| Treatment effect | 0.134 (0.063) | 0.008 to 0.259 | 0.037 |
Imputation analysis for primary outcome
To further investigate the consistency of the treatment effect estimates, an imputation analysis was conducted to explore the effect of the large number of missing data at 3- and 12-month follow-up. In the Families for Health group, 28.57% of 12-month primary outcome measures were missing, in the usual-care group 34.20% were missing. This difference in missing data is not significant (p = 0.520) by the chi-squared test.
Estimates presented in Table 19 for the multiple imputation models are averages over the 100 models. Treatment effect point estimates are, again, similar to the primary outcome analysis with only Markov chain Monte Carlo imputation reducing the effect size and as a result yielding a non-significant p-value.
| Imputation analysis | Treatment arm, mean change in child BMI z-score (95% CI) | Treatment effect | p-value | |
|---|---|---|---|---|
| FFH | UC | |||
| Simple regression imputation | –0.003 (–0.064 to 0.057) | –0.115 (–0.173 to –0.057) | 0.112 | 0.008 |
| FCS imputation | 0.001 (–0.069 to 0.070) | –0.194 (–0.186 to –0.040) | 0.113 | 0.0256 |
| MCMC imputation | –0.016 (–0.096 to 0.062) | –0.092 (–0.167 to –0.017) | 0.075 | 0.164 |
Baseline covariates included in the analysis were fully observed and only outcome values were missing. If the assumption is made that data are ‘missing at random’, then the primary outcome analysis, which adjusts for baseline BMI z-score (and treatment group), provides an unbiased estimate of treatment effect. In this case, multiple imputation could be regarded as unnecessary or an additional sensitivity analysis. In this scenario the simple regression imputation method also provides unbiased estimates, albeit with an underestimated variability. This effect can be seen in Table 19, in which point estimates for simple regression imputation are very similar to the primary outcome analysis, but the p-value is smaller.
Per-protocol analysis
Families that participated in five or more sessions of the Families for Health programme are regarded as ‘programme completers’ (i.e. as having complied with the protocol sufficiently). Improvements in children from these families, in terms of BMI z-score change from baseline, are compared with improvements in children from families who attended fewer than five sessions. A comparison is made based on estimates obtained from the primary analysis multilevel model (model 1). An exploratory analysis comparing families who attended nine or more sessions (essentially full attendance) with those who attended fewer sessions can be found in Appendix 2.
Figure 3 presents the number of sessions attended by families and the number of follow-up appointments completed. The mean number of Families for Health sessions attended was 5.42. For those families who provided 12 months’ data, the mean number of sessions attended was 6.96. The figure suggests no clear relationship between sessions attended and the likelihood of being lost to follow-up.
FIGURE 3.
Attendance rate (bar height) and time points at which outcome data were provided (colour/pattern) for families attending Families for Health.

Table 20 shows the difference in BMI and waist z-score between completers and non-completers. Non-completers achieved a reduction similar to the reduction observed in the usual-care group, while the mean z-score increased in the completer population. There are several potential explanations for this including (a) those who achieved a weight reduction felt that they did not need to attend further sessions; and (b) families were able to access usual care (which appears to yield a better outcome) as well and non-completers might have been more likely to have accessed usual care. Non-completers also had a (non-significantly) higher mean baseline BMI z-score (Table 21).
| Secondary outcome | Time period | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline to 3 months | Baseline to 12 months | |||||||
| Treatment group | Treatment effect | p-value | Treatment group | Treatment effect | p-value | |||
| Completer | Non-completer | Completer | Non-completer | |||||
| Mean change in child BMI z-score (95% CI) | 0.005 (–0.107 to 0.098) | –0.048 (–0.144 to 0.048) | –0.043 (–0.200 to 0.114) | 0.582 | 0.065 (–0.040 to 0.169) | –0.103 (–0.234 to 0.029) | –0.168 (–0.342 to 0.007) | 0.059 |
| Mean change in child waist z-score (95% CI) | –0.115 (–0.205 to –0.025) | –0.105 (–0.282 to 0.072) | 0.010 (–0.162 to 0.182) | 0.906 | 0.055 (–0.093 to 0.202) | –0.115 (–0.270 to 0.039) | –0.170 (–0.409 to 0.068) | 0.157 |
| Characteristic | Treatment group | |
|---|---|---|
| Completer (n = 39) | Non-completer (n = 24) | |
| Mean baseline BMI z-score (95% CI) | 2.554 (2.353 to 2.756) | 2.860 (2.597 to 3.123) |
| Mean baseline age, years (95% CI) | 9.503 (8.979 to 10.027) | 9.580 (8.995 to 10.166) |
| SES class, n (%) | ||
| 1 | 19 (48.7) | 7 (29.2) |
| 2 | 8 (20.5) | 6 (25.0) |
| 3 | 9 (23.1) | 5 (20.8) |
| 4 | 3 (7.7) | 6 (25.0) |
Repeated measures analysis
An exploratory repeated-measures analysis was conducted for the investigation of the difference between arms in terms of change over time in the primary outcome measure rather than a comparison between arms at fixed 3- or 12-month follow-up time points. For this purpose, a repeated-measures mixed model was fitted. The model is based on the hierarchical model used above. Unlike previous models, time is fitted as a continuous variable here, allowing for the fact that actual measuring time points varied considerably rather than taking place at exactly 3 and 12 months from baseline. This is illustrated in Figures 4 and 5.
FIGURE 4.
Child BMI z-scores and time points (Families for Health).
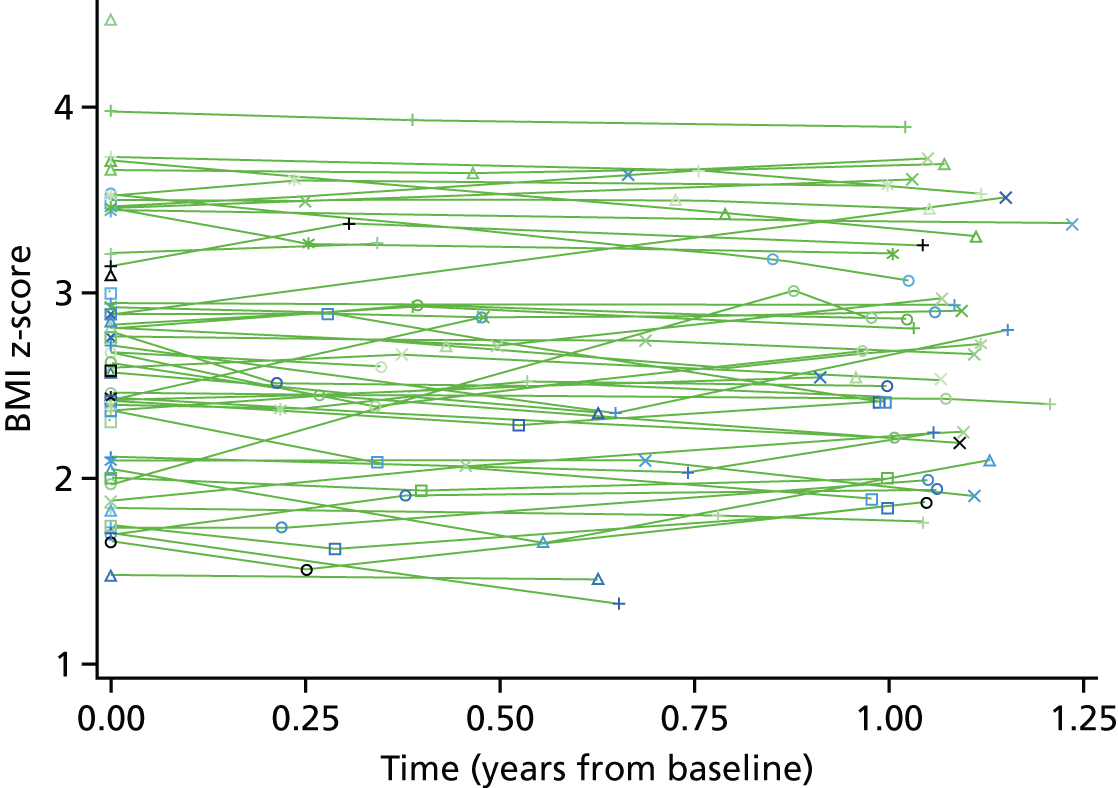
FIGURE 5.
Child BMI z-scores and time points (usual care).

Analysis of the covariance matrix for repeated observations revealed that the baseline and 12 months observation were more highly correlated than was the 3 months observations and either of the other time points. An unstructured covariance matrix leads to the best model fit and was therefore used for the mixed model.
The least squares estimates of group BMI z-scores and the treatment effect presented in Table 22 are averages over time. This includes the baseline time point, which dilutes a difference because of treatment. Had discrete time points (3 months and 12 months) been used, the differences between arms at different time points could have been estimated. However, the time by treatment interaction term in the repeated measures model is significant (see Table 23, bottom row). Consequently, it can be claimed that Families for Health and usual care have a statistically significantly different effect on children’s BMI z-score, with Families for Health yielding higher z-scores. Unlike in previous models presented in Primary outcome analysis, locality and sex are significant predictors of the outcome (Table 23). Note, however, that the outcome variable in this repeated measures model is z-score change over time [i.e. (slightly) different from ‘change from baseline to 12-month follow-up’ used in the primary outcome analysis].
| Characteristic | Time period, averaged over time (95% CI) | p-value | ||
|---|---|---|---|---|
| Treatment group | Treatment effect | |||
| FFH | UC | |||
| Mean child BMI z-score | 2.756 (2.584 to 2.928) | 2.661 (2.493 to 2.830) | 0.094 (–0.140 to 0.329) | 0.427 |
| Effect | Estimate | 95% CI | F-value | p-value |
|---|---|---|---|---|
| Intercept | 3.138 | 2.826 to 3.451 | < 0.001 | |
| Treatment group (UC) | – | – | 0.12 | 0.726 |
| Treatment group (FFH) | 0.042 | –0.195 to 0.279 | ||
| Sex (male) | – | – | 6.93 | 0.009 |
| Sex (female) | 0.312 | –0.546 to –0.08 | ||
| Site C | – | – | 3.68 | 0.028 |
| Site A | –0.378 | –0.068 to 0.251 | ||
| Site B | –0.219 | –0.256 to 0.119 | ||
| SES (unemployed) | – | – | 1.40 | 0.248 |
| SES (managerial) | –0.173 | –0.522 to 0.177 | ||
| SES (intermediate) | –0.219 | –0.622 to 0.185 | ||
| SES (manual) | 0.075 | –0.267 to 0.416 | ||
| Time | –0.102 | –0.183 to 0.188 | 2.63 | 0.108 |
| Time × treatment (UC) | – | – | 6.13 | 0.015 |
| Time × treatment (FFH) | 0.123 | 0.025 to 0.221 |
Secondary outcomes analysis
Children’s total fruit and vegetable consumption
Children completed a 24-hour food recall using the ‘Day in the Life Questionnaire’ to assess the number of portions of fruit and vegetables consumed the previous day. 43
Child-reported fruit and vegetable consumption decreased in both treatment groups from baseline to the 3- and 12-months follow-ups (Table 24). It should be noted that the changes in Table 24 are calculated for a smaller number of children than the individual means given in Table 25 because of dropouts. There was no significant difference between the Families for Health and usual-care treatment arms in the longitudinal change.
| Fruit and vegetable consumption | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Sample size (n) | |||
| Baseline | 63 | 65 | |
| Change from baseline to 3 months | 48 | 50 | |
| Change from baseline to 12 months | 45 | 42 | |
| Longitudinal changes | |||
| Change from baseline to 3 months (95% CI) | –0.375 (–1.073 to 0.323) | –0.180 (–0.826 to 0.467) | 0.681 |
| Change from baseline to 12 months (95% CI) | –0.444 (–1.162 to 0.273) | –0.195 (–0.902 to 0.511) | 0.620 |
| Fruit and vegetable consumption | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Time point average, mean (SD) | |||
| Baseline | 2.13 (2.08) | 2.43 (1.94) | |
| 3 months | 2.13 (2.03) | 2.22 (1.64) | |
| 12 months | 2.22 (1.93) | 2.29 (1.75) | |
| Longitudinal changes | |||
| Change from baseline to 3 months (SD) | –0.375 (2.402) | –0.180 (2.274) | 0.681 |
| Change from baseline to 12 months (SD) | –0.444 (2.389) | –0.195 (2.239) | 0.620 |
Children’s quality of life (Pediatric Quality of Life Inventory)
Children’s health-related quality of life is measured using the PedsQL version 4.0 (UK), for ages 8–12 years. 45 Children completed the 23-item self-report version and the parents completed the almost identical parent-proxy version about their child’s quality of life, with the same number of items.
Table 26 shows that the mean child-reported scores at baseline are 2–3 points higher in the usual-care group than in the Families for Health group. The differences between the groups are, however, not significant for any of the change-from-baseline comparisons (Table 27). Results for the parent-reported PedsQL differ from the child-reported PedsQL. Mean parent-reported scores are consistently 4–8 points lower than child-reported scores (Table 28). Table 29 shows that there were no statistically significant between-group, or within-group, differences for any of the change-from-baseline comparisons apart from the change from baseline to 12 months of the psychosocial domain in the usual care group (p = 0.0226).
| PedsQL subscale | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 48) | UC (n = 50) | FFH (n = 45) | UC (n = 42) | |
| Overall | 75.91 (72.01 to 79.81) | 78.07 (74.39 to 81.76) | 79.96 (75.74 to 84.18) | 77.90 (73.27 to 82.54) | 79.93 (75.42 to 84.43) | 79.04 (74.13 to 83.95) |
| Psychosocial | 75.00 (70.74 to 79.26) | 76.97 (72.97 to 80.97) | 79.10 (74.70 to 83.50) | 76.61 (71.48 to 81.74) | 78.85 (73.85 to 83.86) | 79.05 (73.86 to 84.24) |
| Physical | 77.54 (73.07 to 82.01) | 80.19 (76.19 to 84.18) | 81.57 (76.90 to 86.23) | 80.25 (75.59 to 84.91) | 81.94 (77.45 to 86.44) | 79.02 (74.00 to 84.04) |
| PedsQL subscale | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Overall | |||
| Change from baseline to 3 months (95% CI) | 1.411 (–1.608 to 4.431) | –2.298 (–5.043 to 0.447) | 0.070 |
| Change from baseline to 12 months (95% CI) | 2.131 (–1.830 to 6.092) | 0.254 (–3.712 to 4.220) | 0.502 |
| Psychosocial | |||
| Change from baseline to 3 months (95% CI) | 1.290 (–2.070 to 4.650) | –2.452 (–5.396 to 0.493) | 0.095 |
| Change from baseline to 12 months (95% CI) | 1.966 (–2.614 to 6.545) | 1.216 (–3.214 to 5.646) | 0.813 |
| Physical | |||
| Change from baseline to 3 months (95% CI) | 1.739 (–2.526 to 5.986) | –2.134 (–5.559 to 1.291) | 0.157 |
| Change from baseline to 12 months (95% CI) | 2.550 (–2.176 to 7.275) | –1.648 (–6.719 to 3.424) | 0.225 |
| PedsQL subscale | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 47) | UC (n = 49) | FFH (n = 45) | UC (n = 42) | |
| Overall | 72.30 (67.42 to 77.19) | 60.61 (66.58 to 74.64) | 73.72 (67.91 to 79.54) | 73.83 (68.34 to 79.31) | 76.71 (71.18 to 82.25) | 75.98 (71.19 to 80.77) |
| Psychosocial | 71.19 (66.14 to 76.24) | 69.35 (65.47 to 73.24) | 72.80 (66.82 to 78.77) | 73.77 (68.74 to 78.80) | 75.11 (69.14 to 81.08) | 75.67 (71.09 to 80.24) |
| Physical | 74.45 (64.24 to 79.66) | 72.87 (67.19 to 78.56) | 75.51 (68.53 to 82.50) | 73.93 (66.74 to 81.12) | 79.71 (73.68 to 85.74) | 76.58 (69.99 to 83.17) |
| PedsQL subscale | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Overall | |||
| Change from baseline to 3 months (95% CI) | 0.922 (–3.885 to 5.729) | 2.524 (–2.284 to 7.333) | 0.637 |
| Change from baseline to 12 months (95% CI) | 3.375 (–1.764 to 8.514) | 3.997 (–1.536 to 9.530) | 0.868 |
| Psychosocial | |||
| Change from baseline to 3 months (95% CI) | 1.449 (–3.313 to 6.211) | 3.865 (–0.421 to 8.151) | 0.449 |
| Change from baseline to 12 months (95% CI) | 3.262 (–1.975 to 8.499) | 5.350 (0.791 to 9.910) | 0.548 |
| Physical | |||
| Change from baseline to 3 months (95% CI) | –0.027 (–6.724 to 6.671) | –0.011 (–6.760 to 6.738) | 0.997 |
| Change from baseline to 12 months (95% CI) | 3.586 (–2.458 to 9.630) | 1.417 (–7.982 to 10.816) | 0.697 |
The children in this trial do seem to have lower mean PedsQL scores than ‘healthy’ children in the UK, where the mean for the overall score has been reported to be 83.9 (child reported) and 84.6 (parent reported). 47
Parent- and child-reported child quality of life (European Quality of Life-5 Dimensions Youth version)
There were no statistically significant differences in suboptimal levels of function, at any follow-up time, in either child- (Table 30) or parent-reported (Table 31) health-related quality of life, as measured by the EQ-5D-Y,51,53 between the Families for Health group and the usual-care group. Nor were there any significant between-group differences, at any follow-up time, in either children’s perception of their overall health (see Table 30) or parents’ perception of their child’s health (see Table 31) as assessed using a visual analogue scale. Finally, Table 32 shows that there was no between-group difference in either child- or parent-reported utility score over each follow-up period. The mean change in child-reported EQ-5D-Y utility score between baseline and 12 months was estimated at –0.017 (95% CI –0.098 to 0.218; p = 0.678) for the Families for Health group and –0.040 (95% CI –0.112 to 0.188; p = 0.266) for children with complete data. The mean change in parent-reported EQ-5D-Y utility score between baseline and 12 months was estimated at 0.012 (95% CI –0.041 to 0.143; p = 0.661) for the Families for Health group and 0.047 (95% CI –0.005 to 0.137; p = 0.073) for parents with complete data.
| EQ-5D-Y dimension | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | |||||||
| Treatment group | p-value | Treatment group | p-value | Treatment group | p-value | ||||
| FFH | UC | FFH | UC | FFH | UC | ||||
| Mobility, n (%) | |||||||||
| Level 1 | 56 (88.9) | 54 (83.1) | 41 (85.4) | 43 (86.0) | 37 (84.1) | 37 (90.2) | |||
| Level 2 | 5 (7.9) | 7 (10.8) | 6 (12.5) | 6 (12.0) | 7 (15.9) | 3 (7.3) | |||
| Level 3 | 2 (3.2) | 4 (6.2) | 1 (2.1) | 1 (2.0) | 0 (0.0) | 1 (2.4) | |||
| Suboptimal level of function | 7 | 11 | 0.344 | 7 | 7 | 0.934 | 7 | 4 | 0.398 |
| Self-care, n (%) | |||||||||
| Level 1 | 52 (82.5) | 59 (92.2) | 44 (91.7) | 42 (84.0) | 38 (86.4) | 39 (95.1) | |||
| Level 2 | 9 (14.3) | 5 (7.8) | 4 (8.3) | 8 (16.0) | 5 (11.4) | 1 (2.4) | |||
| Level 3 | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.3) | 1 (2.4) | |||
| Suboptimal level of function | 11 | 5 | 0.101 | 4 | 8 | 0.247 | 6 | 2 | 0.167 |
| Usual activities, n (%) | |||||||||
| Level 1 | 51 (81.0) | 55 (84.6) | 42 (87.5) | 42 (84.0) | 38 (86.4) | 35 (85.4) | |||
| Level 2 | 12 (19.1) | 10 (15.4) | 6 (12.5) | 7 (14.0) | 6 (13.6) | 6 (14.6) | |||
| Level 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) | |||
| Suboptimal level of function | 12 | 10 | 0.583 | 6 | 8 | 0.621 | 6 | 6 | 0.895 |
| Pain, n (%) | |||||||||
| Level 1 | 43 (68.3) | 46 (70.8) | 31 (64.6) | 33 (66.0) | 28 (63.6) | 24 (58.5) | |||
| Level 2 | 20 (31.8) | 17 (26.2) | 14 (29.2) | 14 (28.0) | 15 (34.1) | 16 (39.0) | |||
| Level 3 | 0 (0.0) | 2 (3.1) | 3 (6.3) | 3 (6.0) | 1 (2.3) | 1 (2.4) | |||
| Suboptimal level of function | 20 | 19 | 0.757 | 17 | 17 | 0.883 | 16 | 17 | 0.630 |
| Anxiety, n (%) | |||||||||
| Level 1 | 39 (61.9) | 44 (68.8) | 33 (68.8) | 33 (66.0) | 33 (76.7) | 27 (65.9) | |||
| Level 2 | 23 (36.5) | 15 (23.4) | 15 (31.3) | 16 (32.0) | 9 (20.9) | 10 (24.4) | |||
| Level 3 | 1 (1.6) | 5 (7.8) | 0 (0.0) | 1 (2.0) | 1 (2.3) | 4 (9.8) | |||
| Suboptimal level of function | 24 | 20 | 20 | 15 | 17 | 0.772 | 10 | 14 | 0.269 |
| Mean EQ-5D-Y VAS score (SD) | 71.95 (23.99) | 69.92 (22.60) | 0.623 | 70.72 (19.10) | 76.06 (21.45) | 0.200 | 70.22 (18.89) | 73.93 (22.67) | 0.409 |
| Mean EQ-5D-Y utility score (SD) | 0.821 (0.211) | 0.790 (0.273) | 0.469 | 0.832 (0.247) | 0.803 (0.255) | 0.567 | 0.832 (0.220) | 0.794 (0.261) | 0.471 |
| EQ-5D-Y dimension | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | |||||||
| Treatment group | p-value | Treatment group | p-value | Treatment group | p-value | ||||
| FFH | UC | FFH | UC | FFH | UC | ||||
| Mobility, n (%) | |||||||||
| Level 1 | 54 (85.7) | 52 (80.0) | 40 (87.0) | 44 (88.0) | 40 (90.9) | 38 (90.5) | |||
| Level 2 | 9 (14.3) | 12 (18.5) | 5 (10.9) | 4 (8.0) | 4 (9.1) | 4 (9.5) | |||
| Level 3 | 0 (0.0) | 1 (1.5) | 1 (2.2) | 2 (4.0) | 0 (0.0) | 0 (0.0) | |||
| Suboptimal level of function | 9 | 13 | 0.392 | 6 | 6 | 0.877 | 4 | 4 | 0.945 |
| Self-care, n (%) | |||||||||
| Level 1 | 56 (88.9) | 60 (92.3) | 40 (87.0) | 45 (90.0) | 39 (88.6) | 39 (92.9) | |||
| Level 2 | 4 (6.4) | 5 (7.7) | 5 (10.9) | 4 (8.0) | 5 (11.4) | 3 (7.1) | |||
| Level 3 | 3 (4.8) | 0 (0.0) | 1 (2.2) | 1 (2.0) | 0 (0.0) | 0 (0.0) | |||
| Suboptimal level of function | 7 | 5 | 0.507 | 6 | 5 | 0.640 | 5 | 3 | 0.501 |
| Usual activities, n (%) | |||||||||
| Level 1 | 51 (81.0) | 52 (80.0) | 39 (84.8) | 44 (88.0) | 34 (77.3) | 37 (88.1) | |||
| Level 2 | 10 (15.9) | 12 (18.5) | 7 (15.2) | 6 (12.0) | 10 (22.7) | 5 (11.9) | |||
| Level 3 | 2 (3.2) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Suboptimal level of function | 12 | 13 | 0.892 | 7 | 6 | 0.645 | 10 | 5 | 0.186 |
| Pain, n (%) | |||||||||
| Level 1 | 51 (81.0) | 47 (72.3) | 38 (82.6) | 42 (84.0) | 37 (84.1) | 34 (81.0) | |||
| Level 2 | 12 (19.1) | 17 (26.2) | 7 (15.2) | 7 (14.0) | 7 (15.9) | 8 (19.1) | |||
| Level 3 | 0 (0.0) | 1 (1.5) | 1 (2.2) | 1 (2.0) | 0 (0.0) | 0 (0.0) | |||
| Suboptimal level of function | 12 | 18 | 0.248 | 8 | 8 | 0.855 | 7 | 8 | 0.701 |
| Anxiety, n (%) | |||||||||
| Level 1 | 35 (55.6) | 39 (60.0) | 29 (63.0) | 33 (66.0) | 24 (54.6) | 25 (59.5) | |||
| Level 2 | 27 (42.9) | 23 (35.4) | 17 (37.0) | 14 (28.0) | 20 (45.5) | 15 (35.7) | |||
| Level 3 | 1 (1.6) | 3 (4.6) | 0 (0.0) | 3 (6.0) | 0 (0.0) | 2 (4.8) | |||
| Suboptimal level of function | 28 | 26 | 0.611 | 17 | 17 | 0.762 | 20 | 17 | 0.641 |
| Mean EQ-5D-Y VAS score (SD) | 75.21 (19.16) | 74.56 (20.17) | 0.853 | 80.91 (14.22) | 83.32 (14.60) | 0.421 | 80.66 (14.54) | 80.26 (16.27) | 0.905 |
| Mean EQ-5D-Y utility score (SD) | 0.850 (0.203) | 0.835 (0.236) | 0.697 | 0.857 (0.228) | 0.840 (0.261) | 0.741 | 0.876 (0.126) | 0.872 (0.194) | 0.918 |
| Change in utility score | Time period | |||||
|---|---|---|---|---|---|---|
| Baseline to 3 months | Baseline to 12 months | |||||
| Treatment group | p-value | Treatment group | p-value | |||
| FFH | UC | FFH | UC | |||
| Mean child self-reported EQ-5D-Y (SD) | –0.019 (0.237) | –0.021 (0.229) | 0.967 | –0.017 (0.265) | –0.040 (0.229) | 0.667 |
| Mean parent-reported EQ-5D-Y (SD) | –0.002 (0.171) | 0.017 (0.216) | 0.628 | 0.012 (0.173) | 0.047 (0.166) | 0.332 |
Accelerometer data (children)
For a child’s data to be included in the analysis, 3 days with 480 minutes of wear time at both baseline and 12 months was required. This resulted in pairs of baseline and 12-month follow-up data from only 46 of the 128 children in the trial (36%), 27 in the Families for Health arm and 19 in the usual-care arm.
Table 33 shows the group means of the accelerometer outcomes considered in the analysis. All four outcomes indicate a favourable development in the usual-care group. This is mirrored in the analysis of longitudinal changes (Table 34), with the point estimates indicating an improvement in all four outcomes in the usual-care group and less child physical activity in three out of four outcomes in the Families for Health arm. Owing to the small sample size in the accelerometer analysis, 95% CIs are wide and there are no significant differences between groups. The interpretation of the physical activity data must be made with caution given that paired data were available for only 36% of children.
| Outcome | Time point mean (95% CI) | |||
|---|---|---|---|---|
| Baseline | 12 months | |||
| FFH (n = 27) | UC (n = 19) | FFH (n = 27) | UC (n = 19) | |
| Minutes of moderate or vigorous activity per day | 46.96 (40.16 to 53.77) | 52.60 (40.22 to 64.98) | 43.71 (36.58 to 50.85) | 57.81 (46.82 to 68.81) |
| Minutes of sedentary behaviour per day | 454.41 (425.09 to 483.72) | 436.68 (400.52 to 472.84) | 458.65 (433.90 to 483.40) | 427.62 (384.72 to 470.52) |
| Steps per day | 8297.94 (7552.43 to 9043.46) | 8316.76 (7214.66 to 9418.87) | 8520.24 (7554.70 to 9485.79) | 9129.62 (7789.40 to 10,469.85) |
| Counts per minute | 488.93 (435.17 to 542.69) | 539.03 (439.18 to 638.87) | 462.58 (416.52 to 508.63) | 579.92 (493.92 to 665.92) |
| Outcome | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Minutes of moderate or vigorous activity per day (95% CI) | –3.249 (–9.426 to 2.927) | 5.213 (–4.971 to 15.397) | 0.125 |
| Minutes of sedentary behaviour per day (95% CI) | 4.245 (–22.003 to 30.492) | –9.061 (–56.314 to 38.192) | 0.611 |
| Steps per day (95% CI) | 222.3 (–566.0 to 1000.6) | 812.9 (–503.1 to 2128.9) | 0.400 |
| Counts per minute (95% CI) | –26.352 (–81.830 to 29.127) | 40.895 (–49.543 to 131.300) | 0.171 |
Parents’ mental well-being (Warwick–Edinburgh Mental Well-Being scale)
Parental mental health was measured using the WEMWBS. 48 The WEMWBS is a measure of mental well-being focusing entirely on positive aspects of mental health, and a higher score indicates better mental health. Tables 35 and 36 show the mean WEMWBS scores and changes from baseline by treatment group. The WEMWBS scores are, on average, higher in the Families for Health group than in the usual-care group at follow-up time points, but the difference does not reach statistical significance. Average WEMWBS scores decrease from baseline to 12 months in the usual-care group.
| WEMWBS | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Time point average, mean score (SD) | |||
| Baseline | 47.86 (8.87) | 47.74 (9.69) | |
| 3 months | 50.00 (8.43) | 48.09 (11.01) | |
| 12 months | 50.68 (10.18) | 44.98 (11.57) | |
| Longitudinal changes | |||
| Change from baseline to 3 months (SD) | 1.311 (8.562) | 0.419 (10.12) | 0.656 |
| Change from baseline to 12 months (SD) | 2.409 (8.883) | –2.167 (12.548) | 0.060 |
| WEMWBS | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Sample size (n) | |||
| Baseline | 56 | 57 | |
| Change from baseline to 3 months | 45 | 43 | |
| Change from baseline to 12 months | 44 | 36 | |
| Longitudinal changes | |||
| Change from baseline to 3 months (95% CI) | 1.311 (–1.261 to 3.883) | 0.419 (–2.693 to 3.530) | 0.656 |
| Change from baseline to 12 months (95% CI) | 2.409 (–0.291 to 5.110) | –2.167 (–6.412 to 2.079) | 0.060 |
Adjusting the comparison of WEMWBS scores between treatment groups for age and sex did not lead to any change in treatment effects or p-values for the between-group differences at 12 months (Table 37). The mean within-group differences are estimated to be slightly higher in both groups; however, neither change from baseline within a group is statistically significant, as indicated by the CIs overlapping zero in both cases.
| WEMWBS | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Sample size (n) | |||
| Baseline | 56 | 57 | |
| Change from baseline to 3 months | 45 | 43 | |
| Change from baseline to 12 months | 44 | 36 | |
| Longitudinal changes | |||
| Change from baseline to 3 months (95% CI) | 0.684 (–4.468 to 5.836) | 0.016 (–5.565 to 5.596) | 0.754 |
| Change from baseline to 12 months (95% CI) | 3.081 (–2.657 to 8.819) | –1.373 (–7.701 to 4.949) | 0.076 |
Parenting Styles and Dimensions Questionnaire
Parenting style was measured using the PSDQ. 50 The PSDQ is a 32-item, parent-report questionnaire. The 32 items form three patterns of parenting: authoritative, authoritarian and permissive. Higher scores indicate more frequent use of the described behaviour. Authoritarian and permissive parenting styles are considered to be less effective parenting styles than authoritative. Parents are classified according to their highest score in the three parenting domains.
Table 38 shows the mean score for each parenting style at each time point. The authoritative style always scored higher than the other two styles. For 53 parents in the Families for Health group, authoritative was the highest scoring parenting pattern at baseline, with the remaining three parents scoring highest on permissive (5.4%). In the usual-care group, 53 parents endorsed behaviours indicative of a primarily authoritative parenting style and six parents (10.2%) exhibited permissive styles at baseline. At the 3-month follow-up, 43 parents scored highest on authoritative style, whereas two parents (4.4%) scored highest on permissive behaviour in both treatment groups. At the 12-month follow-up, one parent (2.3%) in the Families for Health group and two parents in the usual-care group (5.3%) scored highest on permissive style. Authoritarian never emerged as the primary parenting style in this trial. Very few changes of the primary parenting style occurred over the course of the study, which is desirable as most parents are already in the preferred group (authoritative). In total, five parents scored higher for a parenting style different from their primary baseline parenting style over the course of the study: three in the Families for Health group and two in the usual-care group. Four parents changed from primarily permissive to authoritative, and one parent in the Families for Health group changed from authoritative to permissive.
| Parenting style | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 56) | UC (n = 59) | FFH (n = 45) | UC (n = 45) | FFH (n = 44) | UC (n = 38) | |
| PSDQ authoritative | 4.08 (3.92 to 4.23) | 4.02 (3.84 to 4.21) | 4.13 (3.97 to 4.29) | 4.04 (3.82 to 4.26) | 4.19 (4.04 to 4.34) | 3.97 (3.74 to 4.21) |
| PSDQ authoritarian | 1.70 (1.58 to 1.83) | 1.58 (1.47 to 1.69) | 1.60 (1.46 to 1.74) | 1.56 (1.45 to 1.66) | 1.59 (1.45 to 1.72) | 1.58 (1.46 to 1.69) |
| PSDQ permissive | 2.34 (2.14 to 2.55) | 2.28 (2.07 to 2.50) | 2.21 (1.98 to 2.43) | 2.19 (1.96 to 2.43) | 2.11 (1.90 to 2.32) | 2.09 (1.86 to 2.33) |
There were no significant differences between or within groups for authoritative, authoritarian and permissive parenting style scores over the course of the study (Table 39). Figure 6 presents the correlation of reduction in child BMI z-scores and authoritative parenting style scores at baseline and 12 months. Despite being generally regarded as the most effective parenting style, the correlation was weak (r = –0.142 and r = –0.128 for baseline and 12 months, respectively).
| Parenting style | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| PSDQ authoritative | |||
| Change from baseline to 3 months (95% CI) | 0.033 (–0.115 to 0.182) | 0.085 (–0.074 to 0.244) | 0.633 |
| Change from baseline to 12 months (95% CI) | 0.106 (–0.042 to 0.254) | 0.073 (–0.082 to 0.228) | 0.756 |
| PSDQ authoritarian | |||
| Change from baseline to 3 months (95% CI) | –0.052 (–0.184 to 0.081) | 0.011 (–0.076 to 0.097) | 0.431 |
| Change from baseline to 12 months (95% CI) | –0.072 (–0.182 to 0.039) | –0.028 (–0.084 to 0.140) | 0.204 |
| PSDQ permissive | |||
| Change from baseline to 3 months (95% CI) | –0.030 (–0.258 to 0.198) | –0.010 (–0.166 to 0.146) | 0.884 |
| Change from baseline to 12 months (95% CI) | –0.205 (–0.391 to –0.016) | –0.147 (–0.360 to 0.065) | 0.684 |
FIGURE 6.
Scatterplot showing the correlation of BMI z-score change from baseline to 12 months with an authoritative parenting style at (a) baseline; and (b) 12 months. FFH, Families for Health; UC, usual care.
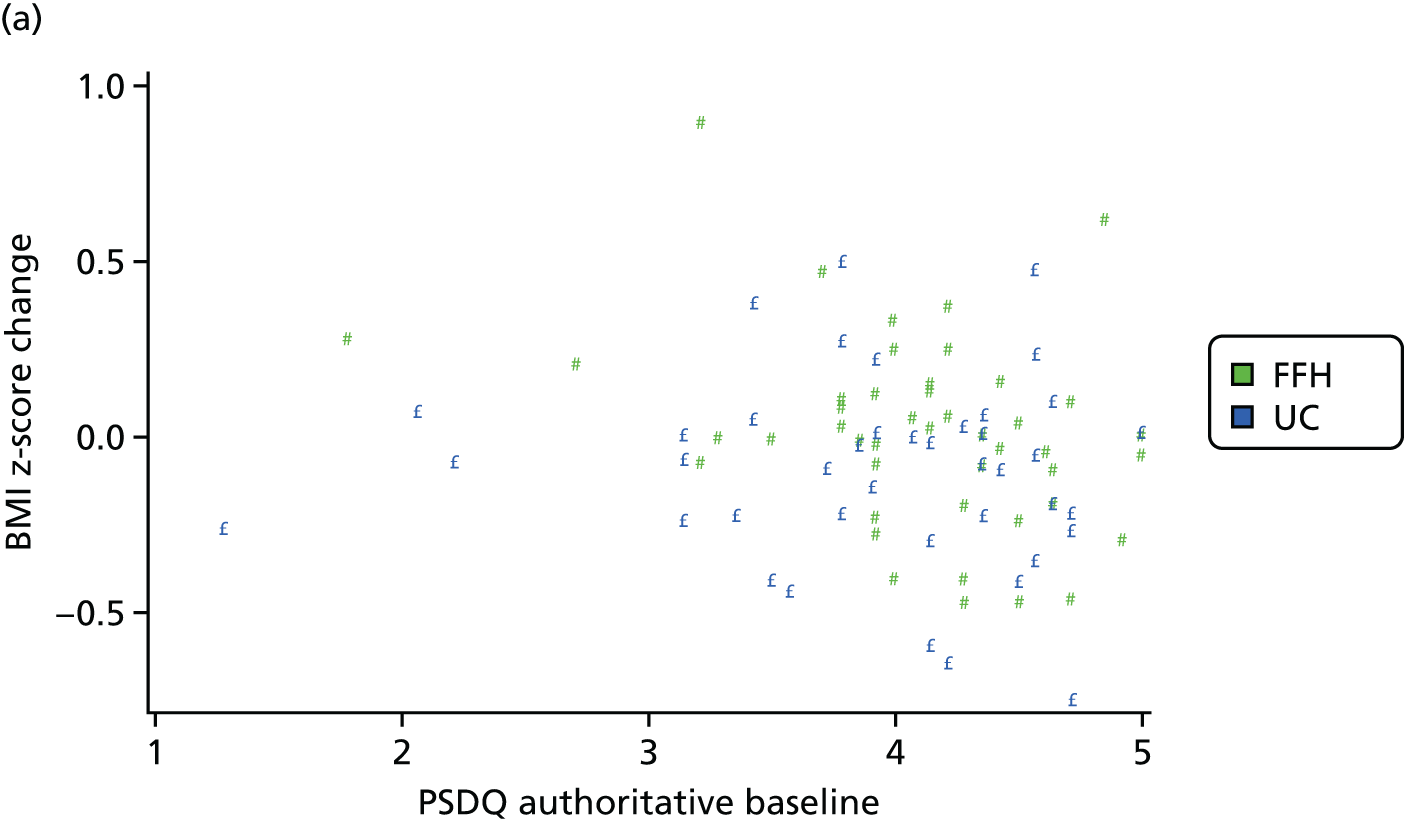

Relationship between parents and children (Child–Parent Relationship Scale)
Assesment of the quality of the parent–child relationship was made using the CPRS,49 which was self-completed by parents for each of their participating children. A mean score was derived, with the best possible score being 5 (range 1–5). Tables 40 and 41 show the results for this secondary outcome, indicating that there are no significant between- (or within-) group differences for the overall scale, or the closeness and conflicts subscales.
| CPRS subscale | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 47) | UC (n = 50) | FFH (n = 45) | UC (n = 42) | |
| Overall | 3.93 (3.77 to 4.09) | 4.11 (3.95 to 4.27) | 4.05 (3.85 to 4.24) | 4.18 (3.98 to 4.37) | 3.98 (3.77 to 4.20) | 4.12 (3.93 to 4.31) |
| Conflicts | 3.44 (3.22 to 3.66) | 3.80 (3.57 to 4.03) | 3.63 (3.36 to 3.90) | 3.81 (3.54 to 4.08) | 3.54 (3.23 to 3.85) | 3.76 (3.49 to 4.03) |
| Closeness | 4.49 (4.36 to 4.62) | 4.47 (4.30 to 4.63) | 4.53 (4.39 to 4.67) | 4.60 (4.45 to 4.75) | 4.49 (4.34 to 4.64) | 4.53 (4.36 to 4.71) |
| CPRS subscale | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Overall | |||
| Change from baseline to 3 months (95% CI) | 0.059 (–0.053 to 0.171) | 0.011 (–0.114 to 0.136) | 0.569 |
| Change from baseline to 12 months (95% CI) | 0.024 (–0.111 to 0.159) | –0.009 (–0.126 to 0.108) | 0.714 |
| Conflicts | |||
| Change from baseline to 3 months (95% CI) | 0.078 (–0.104 to 0.260) | –0.113 (–0.315 to 0.088) | 0.161 |
| Change from baseline to 12 months (95% CI) | 0.038 (–0.169 to 0.244) | –0.117 (–0.301 to 0.068) | 0.267 |
| Closeness | |||
| Change from baseline to 3 months (95% CI) | 0.045 (–0.056 to 0.145) | 0.149 (–0.041 to 0.339) | 0.331 |
| Change from baseline to 12 months (95% CI) | 0.014 (–0.130 to 0.158) | 0.106 (–0.063 to 0.275) | 0.405 |
Family eating and activity (Family Eating and Activity Habits Questionnaire)
Eating and activity behaviour in the familiy was assessed using a modified version of the FEAHQ. 44 The questionnaire comprises four subscales:
-
activity level: to record the balance between physical activity and sedentary pastimes
-
stimulus exposure: presence and visibility of unhealthy snacks kept at home, allowed to eat snack/sweets without parental permission, allowed to buy own sweets
-
eating related to hunger: who initiaties the eating in the family, behaviour if the child is not hungry
-
eating style/rites: where and with whom does eating take place, second helpings.
Parents completed the questionnaire for themselves and for the children. Higher numerical scores reflect less appropriate eating patterns in all subscales. Table 42 shows the mean scale scores for children at each time point and Table 43 shows the mean scores (median for the eating related to hunger subscale) for parents.
| FEAHQ subscale | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 47) | UC (n = 49) | FFH (n = 45) | UC (n = 42) | |
| Activity level | 8.16 (3.96 to 12.37) | 9.09 (5.59 to 12.59) | 6.91 (3.18 to 10.64) | 3.79 (0.44 to 7.14) | 7.09 (2.56 to 11.61) | 4.73 (–0.42 to 9.87) |
| Stimulus exposure | 17.33 (14.68 to 19.99) | 17.26 (14.52 to 20.00) | 14.45 (11.90 to 17.00) | 16.08 (12.68 to 19.48) | 13.78 (11.94 to 15.61) | 15.79 (12.74 to 18.84) |
| Eating related to hunger | 5.67 (5.19 to 6.14) | 5.58 (5.17 to 6.00) | 5.21 (4.67 to 5.76) | 5.57 (5.07 to 6.08) | 5.36 (4.83 to 5.88) | 5.48 (4.91 to 6.04) |
| Eating style | 16.43 (14.83 to 18.02) | 14.37 (13.03 to 15.71) | 13.85 (12.35 to 15.35) | 12.90 (11.54 to 14.26) | 14.42(12.62 to 16.22) | 13.31 (11.59 to 15.03) |
| FEAHQ subscale | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH (n = 64) | UC (n = 73) | FFH (n = 50) | UC (n = 52) | FFH (n = 50) | UC (n = 46) | |
| Activity level | 2.77 (–1.28 to 6.81) | 1.45 (–3.19 to 6.08) | 3.21 (–2.18 to 8.60) | 2.61 (–2.04 to 7.27) | 5.37 (–0.41 to 11.15) | –0.10 (–5.21 to 5.01) |
| Eating related to hungera | 0 (0 to 3) | 0 (0 to 3) | 0 (0 to 3) | 1 (0 to 3) | 0 (0 to 3) | 1 (0 to 3) |
| Eating style | 23.89 (22.22 to 25.57) | 21.33 (19.45 to 23.21) | 22.18 (20.15 to 24.20) | 21.34 (19.32 to 23.36) | 21.74 (19.51 to 23.97) | 21.24 (19.12 to 23.36) |
Changes from baseline in the child scales were not significantly different between treatment groups (Table 44). There was a significant improvement in activity level in the children in the usual-care group both from baseline to 3 months and from baseline to 12 months (see Table 44), which is in agreement with the usual-care group results in the accelerometer analysis (see Tables 33 and 34).
| FEAHQ subscale | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Activity level | |||
| Change from baseline to 3 months (95% CI) | –1.285 (–5.843 to 3.273) | –5.640 (–9.985 to –1.295) | 0.167 |
| Change from baseline to 12 months (95% CI) | –2.590 (–7.826 to 2.648) | –6.813 (–11.176 to –2.450) | 0.218 |
| Stimulus exposure | |||
| Change from baseline to 3 months (95% CI) | –1.021 (–3.804 to 1.761) | –2.102 (–5.581 to 1.377) | 0.627 |
| Change from baseline to 12 months (95% CI) | –1.690 (–4.112 to 0.734) | –2.571 (–5.820 to 0.677) | 0.662 |
| Eating related to hunger | |||
| Change from baseline to 3 months (95% CI) | –0.532 (–1.122 to 0.058) | 0.020 (–0.547 to 0.587) | 0.178 |
| Change from baseline to 12 months (95% CI) | –0.511 (–0.997 to –0.025) | –0.191 (–0.720 to 0.339) | 0.370 |
| Eating style | |||
| Change from baseline to 3 months (95% CI) | –1.681 (–3.111 to –0.250) | –1.020 (–2.258 to 0.217) | 0.483 |
| Change from baseline to 12 months (95% CI) | –0.933 (–2.229 to 0.363) | –0.429 (–2.231 to 1.374) | 0.648 |
On the parent scales, the change in activity level from baseline to 12 months was significantly different between the Families For Health group and the usual-care group (and within the usual-care group) (Table 45). Using Fisher’s exact test to compare differences in the Likert scale, the ‘eating related to hunger’ subscale yielded non-significant differences (p = 0.974 and p = 0.866, respectively).
| FEAHQ subscale | Treatment group | p-value | |
|---|---|---|---|
| FFH | UC | ||
| Activity level | |||
| Change from baseline to 3 months (95% CI) | –0.021 (–5.086 to 5.044) | –0.060 (–4.823 to 4.702) | 0.991 |
| Change from baseline to 12 months (95% CI) | 1.719 (–2.319 to 5.757) | –4.793 (–8.259 to –1.327) | 0.016 |
| Eating style | |||
| Change from baseline to 3 months (95% CI) | –1.549 (–3.310 to 0.212) | –0.038 (–1.872 to 1.797) | 0.236 |
| Change from baseline to 12 months (95% CI) | –1.840 (–3.398 to –0.282) | –0.196 (–1.533 to 1.925) | 0.081 |
Parents’ body mass index
Parents’ BMI was measured at baseline and at the 3- and 12-month follow-up assessments. Mean parent BMI measurements for each group at each time point, apart from the 12-months follow-up in the usual-care group, are > 30 kg/m2, which is the World Health Organization threshold for being classified as obese. Mean parent BMI values in Table 46 are unadjusted. The analysis of change in parent BMI in Table 47 is adjusted for parent age and sex. No statistically significant changes within or between treatment groups were observed.
| Secondary outcome | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH | UC | FFH | UC | FFH | UC | |
| BMI (kg/m2) | 31.88 (30.03 to 33.73) | 32.01 (30.08 to 33.94) | 30.20 (30.04 to 33.96) | 31.07 (29.11 to 33.03) | 31.82 (29.92 to 33.72) | 29.66 (27.67 to 31.64) |
| Secondary outcome, parent BMI | Treatment group | Treatment effect | p-value | |
|---|---|---|---|---|
| FFH | UC | |||
| Sample size (n) | ||||
| Baseline | 64 | 73 | ||
| Change from baseline to 3 months | 49 | 49 | ||
| Change from baseline to 12 months | 50 | 42 | ||
| Longitudinal changes | ||||
| Change from baseline to 3 months (95% CI) | –0.030 (–0.379 to 0.319) | 0.006 (–0.276 to 0.287) | –0.036 (–0.478 to 0.406) | 0.872 |
| Change from baseline to 12 months (95% CI) | –0.238 (–0.944 to 0.467) | –0.161 (–0.681 to 0.359) | –0.078 (–0.968 to 0.813) | 0.858 |
Parents’ body fat percentage
Mean parent per cent body fat values in Table 48 are unadjusted. The analysis of parent per cent body fat change from baseline in Table 49 is adjusted for age and sex. No significant changes within or between groups were observed. Point estimates are smaller (favourable) for all longitudinal changes in the usual-care group.
| Secondary outcome | Time point average (95% CI) | |||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 12 months | ||||
| FFH | UC | FFH | UC | FFH | UC | |
| Percentage body fat | 38.48 (36.11 to 40.85) | 39.21 (37.10 to 41.32) | 39.52 (37.27 to 41.79) | 38.72 (36.22 to 41.21) | 38.36 (36.05 to 40.67) | 36.89 (34.06 to 39.72) |
| Secondary outcome, body fat | Treatment group | Treatment effect | p-value | |
|---|---|---|---|---|
| FFH | UC | |||
| Sample size (n) | ||||
| Baseline | 64 | 73 | ||
| Change from baseline to 3 months | 47 | 49 | ||
| Change from baseline to 12 months | 46 | 41 | ||
| Longitudinal changes | ||||
| Change from baseline to 3 months (95% CI) | 0.394 (–0.268 to 1.055) | –0.182 (–0.779 to 0.416) | 0.575 (–0.303 to 1.453) | 0.197 |
| Change from baseline to 12 months (95% CI) | –0.517 (–1.527 to 0.493) | –0.695 (–1.473 to 0.083) | 0.178 (–1.102 to 1.457) | 0.779 |
Ancillary analysis
Subgroup analysis
The difference in treatment effects between subgroups was initially assessed by interaction tests. These were performed via significance tests of interaction terms in the hierarchical model used for the primary outcome analysis. Each interaction has been fitted separately in a model with the individual fixed effect of this subgroup covariate also included (if it was not already included). Finally, variables that have been categories for subgroup analyses (age, parent BMI) were also investigated as covariates on their original scale. Table 50 shows that none of the interaction effects was significant. Therefore, there is no evidence of a differential treatment effect between prespecified subgroups for any of the subgroup-generating covariates.
| Effect | F-value | p-value |
|---|---|---|
| Treatment × sex | 0.54 | 0.467 |
| Treatment × age | 0.01 | 0.983 |
| Treatment × age group | 0.35 | 0.557 |
| Treatment × parent BMI | 2.61 | 0.110 |
| Treatment × parent BMI group | 0.45 | 0.640 |
| Treatment × SES four classes | 1.07 | 0.369 |
| Treatment × locality | 0.27 | 0.761 |
The treatment effects in terms of BMI z-scores estimated separately within each subgroup are provided in Appendix 3.
Harms
No harmful effects were reported in either randomised group.
Discussion
In this chapter the data obtained from parents and children during the Families for Health RCT were analysed. Based on the statistical analysis it cannot be concluded that the Families for Health programme is effective in reducing children’s BMI z-score relative to usual care. The estimated difference in BMI z-score between the treatment groups is 0.114 (95% CI –0.001 to 0.229; p = 0.053) in favour of the usual-care group. Furthermore, the within-group analysis showed that BMI z-score was significantly reduced in the usual-care arm from baseline to 12-month follow-up (–0.118, 95% CI –0.203 to –0.034; p = 0.007), whereas there was no significant change in the Families for Health arm (–0.005, 95% CI –0.085 to 0.078; p = 0.907). This finding is consistent with the analysis of anthropometric child secondary outcomes (waist z-score, waist circumference, percentage body fat and BMI), all of which showed a more beneficial outcome in the usual-care group. This indicates a trend that children allocated to Families for Health did worse than children randomised to the usual care available locally at the three sites in terms of the management of their obesity. This trend was not observed for the parent anthropometric measures of BMI and body fat; however, neither usual care nor the Families for Health programme is intended to affect parent weight management directly.
In contrast, most self-reported parenting and well-being outcome measures analysed showed a more positive result in the Families for Health arm than in the usual-care arm, albeit without any statistically significant between-group differences. This suggests that the Families for Health programme has some impact on parenting attitudes and behaviour, but this does not translate sufficiently into a weight reduction as hypothesised. According to the PSDQ50 analysis, most parents were already classed as having a primarily authoritative parenting style (the desired style) at the start of the trial. It could be hypothesised that Families for Health programme would be more effective in families with primarily permissive or authoritarian parenting style; unfortunately, the number of families in the trial parenting in this way was too small to carry out an informative subgroup analysis.
A notable exception in the analysis of questionnaire data (showing favourable Families for Health group outcomes) was the activity level subscale in the FEAHQ. 44 This subscale showed a significant improvement from baseline to 12-month follow-up in children receiving usual care, and a markedly higher improvement than in the Families for Health arm, though the between-group difference was not significant (p = 0.167). This is consistent with an increase in physical activity according to the accelerometer data analysis. Although the sample size in the accelerometer data analysis was small, and, therefore, the statistical tests are likely to be underpowered, all point estimates suggest an increase in physical activity in the usual-care group and no effect, or even a reduction in activity, in the Families for Health group. In contrast, the PedsQL45 physical domain shows a more positive effect in the Families for Health group.
Even though the difference in the primary outcome analysis is not significant, and the 95% CI for the treatment effect is consistent with no true difference between the Families for Health programme and usual care, there is little reason to distrust the point estimate. As mentioned above, the result of the primary analysis is consistent with results of other anthropometric secondary outcomes. Furthermore, sensitivity analyses have been conducted in the form of (multiple) imputation analyses. All of those led to estimates of a similar magnitude as the primary outcome analysis. Moreover, a repeated measures model has been fitted to the data using all available data (rather than just complete cases) and allowing for the fact that actual measuring time points varied widely. This model also showed a (significant) improvement over time in the usual-care arm relative to the Families for Health programme. Some tests have been conducted to explore whether or not the observed differences might be related to dropouts (see Table 9) and no relationship has been identified. Nonetheless, the dropout rate was high and differed between groups. Therefore, the possibility that the observed differences between groups is because of dropouts rather than a true underlying difference cannot be excluded.
Chapter 5 Economic evaluation
Introduction
A prospective economic evaluation was conducted alongside the RCT with the aim of assessing the cost-effectiveness of the Families for Health programme, in comparison with usual care, in the treatment of overweight and obese children aged 6–11 years. Two main analyses of incremental cost-effectiveness were conducted. The first analysis was a cost–utility analysis calculating the incremental cost per QALY gained attributable to the Families for Health programme, whereas the second was a cost-effectiveness analysis calculating the incremental cost per unit change in BMI z-score (the primary clinical outcome).
The analyses were conducted from two perspectives: (1) a NHS and Personal Social Services perspective;73 and (2) a wider societal perspective that additionally included costs incurred by education or broader services, or by children’s families.
Methods
Measurement of resource use and costs
A comprehensive strategy was adopted to estimate the incremental costs associated with the Families for Health programme. This encompassed two broad strands of research: (1) estimation of costs associated with the experimental and usual-care programmes targeted at overweight and obese children; and (2) estimation of broader health and Personal Social Service resource inputs and broader societal resource inputs.
Costing of Families for Health and usual-care programmes
A particular focus of the economic evaluation was the assessment of the cost of delivering the Families for Health programme in community settings, including the costs of programme development, training of facilitators, staff-related expenses, and revenue and capital overheads. This primarily involved asking each of the Families for Health facilitators to complete a detailed proforma outlining the cost of delivering each Families for Health programme, including costs associated with preparation time, programme delivery time, travel, parking, refreshments and miscellaneous expenditures; these costs were subsequently converted into programme-specific estimates of cost per session per attending child using separately collected attendance data. The cost of delivering the alternative usual-care programmes [e.g. One Body One Life,36 Change4Life and Wolverhampton Inspiring and Supporting Health (WISH; Royal Wolverhampton NHS Trust, Child Healthy Lifestyle Services, Wolverhampton, UK),74 see Chapter 2, Usual-care control group] was also estimated using the same primary research methods.
Collection of broader resource-use data
Data were also collected about all health and Personal Social Service and broader societal resource inputs over the 12-month time horizon of the study (i.e. the period between randomisation and 12 months post randomisation). The main parent was asked to complete detailed resource-use questionnaires via researcher-administered interviews at baseline and at 3 and 12 months. The questionnaires administered at 3 months covered the period between baseline and 3 months post randomisation, whereas the questionnaires administered at 12 months covered the period between 3 and 12 months post randomisation. The data collected from the main parent at each time point included their child’s use of hospital care services, community-based health care, community-based social care, and medicines and drugs. Information was also collected regarding educational support, family expenditures and parental lost productivity attributable to the child’s health status, over the relevant time horizons. The use of hospital-based services included hospital inpatient admissions, hospital day-care services, paediatric outpatient department appointments, other outpatient department appointments, and visits to accident and emergency units. The use of community-based health care included contacts with GPs, dentists, opticians, dietitians, psychiatrists, psychologists and general practice nurses. It also included contacts with child development centres, child guidance units, family therapists and individual therapy or counselling sessions. The use of community-based social care included contacts with social workers and after-school activities. A list of all medications prescribed was also included in the resource-use questionnaires and items were identified for inclusion in the economic analyses. Medications were categorised by chemical entity, mode of administration, dosage and duration of use. Educational support included contacts with school nurses, educational psychologists, educational welfare officers and special education needs co-ordinators. Family expenditures accounted for changes in expenditure attributable to the child’s weight-related problems. They also covered direct non-medical costs borne by families, for example travel costs and child-care costs, associated with the study child’s use of health and social care services. Parents were asked to report the number of days they had been absent from work during the relevant time horizons as a result of the child’s weight-related problems. The resource-use questionnaires had been piloted to assess their acceptability and parents’ comprehension levels of the resource-use questions. The categories of cost included in analyses from the NHS and Personal Social Services perspective were hospital care, community-based health care, community-based social care and medication use. Analyses from a societal perspective additionally encompassed educational support, additional family expenditures, lost productivity and travel costs.
Valuation of resource use
Resource inputs were valued using a combination of primary research, based on established accounting methods, and data collated from secondary national tariff sets (NHS Reference Costs 2013/14, Unit Costs of Health and Social Care;61 £, 2013–14 prices). Inpatient admissions over this time horizon were delineated by type and duration, and valued using per-diem costs extracted from the NHS Reference Costs 2013/14. 61 Use of other hospital-based care was valued by applying unit costs extracted from national tariffs. 62 Costs for the community-based services were calculated by applying unit costs from national tariffs to resource volumes. NHS net prices per mg for the medications were obtained from the British National Formulary for Children. 63 Costs for individual children were estimated based on their reported doses and frequencies if these were available, or otherwise on an assumed daily dose based on British National Formulary for Children recommendations. 63 The costs to parents of taking time off work to care for the child(ren) were estimated by applying sex-specific median earnings data64 to occupational classifications derived from self-reported work status information. Other family-borne costs were valued using data reported by the parents as part of the follow-up resource-use questionnaires. Unit costs were inflated, where necessary, to 2013–14 prices (£) using the NHS Hospital and Community Health Services Pay and Prices Index75 or Consumer Price Index. 75 No discounting of costs or benefits was applied as the time horizon was < 12 months. Table 51 shows a list of resource valuations and their sources.
| Resource input | Unit cost (£) | Source |
|---|---|---|
| Hospital care | ||
| Inpatient admissions | Based on HRG code | NHS Reference Costs 2013/14 61 |
| A&E visits (mean) | 115.20 | aPSSRU 2011/1276 |
| Outpatient department visits (mean) | 189.00 | PSSRU 2013/1462 |
| Day hospital visits (mean) | 157.00 | PSSRU 2013/1462 |
| Consultant (per contact) | 35.00 | PSSRU 2013/1462 |
| Dietitian (per contact) | 9.25 | PSSRU 2013/1462 |
| Eye clinic (per contact) | 35.00 | PSSRU 2013/1462 |
| Occupational therapist (per contact) | 9.00 | PSSRU 2013/1462 |
| Physiotherapist (per contact) | 9.25 | PSSRU 2013/1462 |
| Psychiatrist (per contact) | 35.50 | PSSRU 2013/1462 |
| Dermatologist/skin specialist (per contact) | 48.75 | PSSRU 2013/1462 |
| Walk-in centre (mean) | 42.16 | aPSSRU 2011/1276 |
| Community-based health care | ||
| GP at surgery (per contact) | 46.00 | PSSRU 2013/1462 |
| Practice nurse (per contact) | 13.70 | PSSRU 2013/1462 |
| Dentist (per contact) | 43.30 | PSSRU 2013/1462 |
| Optician (per contact) | 35.00 | PSSRU 2013/1462 |
| Dietitian (per contact) | 9.30 | PSSRU 2013/1462 |
| Child development centre (per contact) | 42.20 | PSSRU 2013/1462 |
| Community nurse (per contact) | 16.50 | PSSRU 2013/1462 |
| Children’s centre (behaviour one to one) (per contact) | 35.50 | PSSRU 2013/1462 |
| Occupational therapist (per contact) | 9.00 | PSSRU 2013/1462 |
| Orthodontist (per contact) | 35.00 | PSSRU 2013/1462 |
| Physiotherapist (per contact) | 9.00 | PSSRU 2013/1462 |
| School nurse (per contact) | 13.69 | PSSRU 2013/1462 |
| Family therapist (per contact) | 12.50 | PSSRU 2013/1462 |
| Individual therapy (per contact) | 12.50 | PSSRU 2013/1462 |
| Psychiatrist/psychologist (per contact) | 35.50 | PSSRU 2013/1462 |
| CAMHS (mean) | 22.75 | PSSRU 2013/1462 |
| School counsellor (per contact) | 9.00 | PSSRU 2013/1462 |
| Base 25 – one-on-one counselling (per contact) | 9.00 | PSSRU 2013/1462 |
| Personal activities for young people (per contact) | 12.50 | PSSRU 2013/1462 |
| Children’s centre mental health (mean) | 35.50 | PSSRU 2013/1462 |
| Community-based social care | ||
| Social worker at home (per contact) | 19.80 | PSSRU 2013/1462 |
| Social care other (per contact) | Various | PSSRU 2013/1462 |
| Prescribed medication | Various | aBNF 201363 and NHS PCA 201377 |
| Educational support | ||
| School nurse (per contact) | 13.70 | PSSRU 2013/1462 |
| Educational psychologist (per contact) | 19.80 | PSSRU 2013/1462 |
| Educational welfare officer (per contact) | 12.50 | PSSRU 2013/1462 |
| SEN co-ordinator (per contact) | 12.50 | PSSRU 2013/1462 |
| Additional meetings with tutors (per contact) | 12.50 | PSSRU 2013/1462 |
| Other educational support (per contact) | Various | PSSRU 2013/1462 |
| Parents’ days off work | Based on derived occupational code | bONS earnings data 201264 |
Calculation of utilities and quality-adjusted life-years
The economic evaluation made use of QALYs to measure preference-based health outcomes. The health-related quality of life of the study children was assessed using the EQ-5D-Y51,53 obtained from both parents and children at baseline and 3 and 12 months after randomisation as secondary outcomes of the trial. The standard UK (York A1) tariff values52 were applied to these responses at each time point to obtain health utility scores. QALYs were calculated using linear interpolation between baseline and follow-up utility scores and form the main health outcome measure of the economic evaluation. The baseline analysis was conducted using the self-reported EQ-5D-Y data completed by the child.
Missing data
Multiple imputation was used to impute missing data and avoid biases associated with complete-case analysis. Missing data were a particular issue for costs and health utility scores collected at the 3- and 12-month follow-up time points. Multiple imputation using chained equations78 and predicted mean matching was carried out on the two main outcome measures, the BMI z-score and EQ-5D-Y self-reported by the child, as well as cost estimates, at both the 3- and 12-month follow-ups. Predicted mean matching is a semiparametric imputation approach and generally performs better than linear regression despite the similarities in method. 79 Age group, sex, SES and location were included as explanatory variables in the imputation models. In addition, the baseline BMI z-score was included as an explanatory variable in the models predicting BMI z-scores at the follow-up points; the baseline EQ-5D-Y utility score was included as an explanatory variable in the models predicting EQ-5D-Y utility scores at the follow-up points; and baseline costs were included as an explanatory variable in the models predicting costs at the follow-up points. Five imputed data sets were generated, as this is deemed sufficient to obtain valid responses. 80,81 Siblings within families (n = 24) were treated as clusters in the analyses and reflected in the multiple imputation. Twenty-three per cent (30/128) of the 3-month BMI z-scores and 31% (40/128) of the 12-month BMI z-scores were imputed. With respect to the EQ-5D-Y, 23% (30/128) of the 3-month EQ-5D-Y utility scores and 34% (44/128) of the 12-month EQ-5D-Y utility scores were imputed with the identical method of chained equations and predicted mean matching. Fifteen per cent (20/128) of the 3-month costs and 31% of the 12-month costs were also imputed.
Analyses of resource use, costs and outcome data
Resource-use items were summarised by trial allocation group and follow-up period, and differences between groups were analysed using a t-test for continuous variables and a chi-squared test for categorical variables. Mean (SE) costs by cost category and mean (SE) total costs were estimated by trial allocation group for all time periods. Total costs were estimated from both a NHS and Personal Social Services perspective, and from a broader societal perspective. Cost comparisons were carried out using Student’s t-test. Differences in mean total costs and their CIs were estimated. Non-parametric bootstrap estimates73 based on 1000 replications were also calculated for these differences in mean costs, and their CIs calculated.
In addition, bivariate regression was carried out for both costs and outcomes. These analyses explored the determinants of costs and outcomes using seemingly unrelated regression, and included the prespecified prognostic factors of trial intervention (referent: usual care), child age (referent: 6–8 years), child sex (referent: males), site (referent: site A), family SES (referent: higher managerial) and number of siblings within the trial population (referent: none).
Cost-effectiveness analyses
The main cost-effectiveness analyses were conducted for complete cases (i.e. those with complete cost and outcome data) on an intention-to-treat basis. The cost-effectiveness results were expressed primarily in terms of an incremental cost-effectiveness ratio (ICER). This was calculated as the difference in mean costs divided by the difference in mean outcomes (QALYs or change in BMI z-score between baseline and 12 months) between the trial comparators. The primary analyses took the perspective of the NHS and Personal Social Services. The non-parametric bootstrapping approach was used to determine the level of sampling uncertainty surrounding the mean ICER by generating 10,000 estimates of incremental costs and benefits. These were represented graphically on four-quadrant cost-effectiveness planes. Cost-effectiveness acceptability curves (CEACs) showing the probability that the Families for Health programme was cost-effective relative to usual care, across a range of cost-effectiveness thresholds, were also generated based on the proportion of bootstrap replicates with positive incremental net benefits. Unless otherwise stated, all statements about cost-effectiveness are based on a £20,000 per QALY gained threshold. The probability that the Families for Health programme is less costly or more effective than usual care was based on the proportion of bootstrap replicates that had negative incremental costs or positive incremental health benefits.
Secondary analyses were also conducted where the outcomes remained unchanged from the main cost-effectiveness analyses, but for the costs a wider societal perspective was taken that included broader economic costs.
Sensitivity and subgroup analyses
Several sensitivity analyses were undertaken to assess the impact of areas of uncertainty surrounding components of the economic evaluation. These involved re-estimating the main cost-effectiveness outcomes under the following scenarios: (1) conducting a per-protocol analysis where families having participated in five or more sessions of the Families for Health programme are regarded as ‘programme completers’ (i.e. as having complied with the protocol sufficiently); (2) multiple imputation of all missing cost and outcomes data; (3) parent-reported EQ-5D-Y values for the study child(ren) substituted for child self-reported values in the formulation for QALYs; and (4) incorporation of EQ-5D values reflecting the main parent’s self-reported health within calculations of overall QALYs gained.
Subgroup analyses were conducted for the main cost-effectiveness results to explore heterogeneity in the trial population. These were conducted by (1) age group (6–8 and 9–11 years); (2) sex (males or females); and (3) site (site A, B or C).
Long-term cost-effectiveness model
This study focused on the short- and medium-term costs and consequences of the Families for Health programme in the treatment of overweight and obese children in a community setting. The study protocol82 had allowed for extrapolation of costs and consequences over a longer time horizon if the results had demonstrated a difference in medium-term outcomes. This longer-term modelling would have been based on the natural history of the disease and additional evidence from the literature in the event that the trial yielded significant benefits for the Families for Health programme.
Results
Study population
A total of 128 children were randomised into the Families for Health trial, 63 to the Families for Health programme and 65 to usual care. The 128 children were recruited from 115 families (56 allocated to the Families for Health programme arm and 59 to the usual-care arm). A complete profile of resource use was collected for 94 children at 3 months post randomisation (representing 73.4% of the trial population). A complete profile of resource use was collected for 88 children at 12 months post randomisation (representing 68.8% of the trial population). A complete QALY profile between randomisation and 12 months post randomisation was available for 79 children (representing 61.7% of the trial population).
Resource use
Resource-use measures for the period 3 months pre-randomisation are summarised in Table 52 by trial allocation group. Over-the-counter medication purchases and additional meetings with tutors were significantly higher for the usual-care group compared with the Families for Health group. There were no significant differences between the trial arms across the remaining resource-use categories.
| Resource variable | Treatment group | p-valuea | |
|---|---|---|---|
| FFH (n = 63) | UC (n = 65) | ||
| Hospital care, mean (SE) | |||
| Number of inpatient admissions | 0.02 (0.02) | 0 | 0.321 |
| A&E visits | 0.02 (0.02) | 0.11 (0.04) | 0.056 |
| Outpatient department visits | 0.25 (0.08) | 0.35 (0.15) | 0.548 |
| Day hospital visits | 0.02 (0.02) | 0 | 0.321 |
| Other hospital visits | 0.03 (0.02) | 0.11 (0.11) | 0.492 |
| Community-based health-care contacts, mean (SE) | |||
| GP at surgery | 0.52 (0.11) | 0.72 (0.18) | 0.340 |
| GP at home | 0 | 0 | |
| Practice nurse | 0.05 (0.03) | 0.06 (0.03) | 0.731 |
| Dentist | 0.30 (0.06) | 0.51 (0.10) | 0.085 |
| Optician | 0.16 (0.05) | 0.20 (0.06) | 0.613 |
| Dietitian | 0.02 (0.02) | 0.08 (0.04) | 0.159 |
| Child development centre | 0.02 (0.02) | 0 | 0.321 |
| Child guidance unit | 0 | 0 | |
| General health (other) | 0.08 (0.03) | 0.06 (0.03) | 0.697 |
| Family therapist | 0 | 0 | |
| Individual therapy | 0 | 0 | |
| Psychiatrist/psychologist | 0 | 0.03 (0.02) | 0.159 |
| Counselling (other) | 0.16 (0.11) | 0 | 0.159 |
| Community-based social care contacts, mean (SE) | |||
| Social worker at office | 0.06 (0.04) | 0.38 (0.27) | 0.243 |
| Social worker at home | 0 | 0 | |
| After-school club | 0 | 0 | |
| Social care (other) | 0.14 (0.13) | 0.28 (0.18) | 0.543 |
| Medication usage, n (%) | |||
| Prescribed | 28 (44.4) | 34 (52.3) | 0.373 |
| Over the counter | 30 (47.6) | 43 (66.2) | 0.034 |
| Educational support, mean (SE) | |||
| School nurse | 0.35 (0.09) | 0.43 (0.21) | 0.718 |
| Educational psychologist | 0.03 (0.03) | 0.20 (0.16) | 0.292 |
| Educational welfare officer | 0 | 0.05 (0.03) | 0.182 |
| SEN co-ordinator | 0.52 (0.51) | 0.11 (0.06) | 0.419 |
| Additional meetings with tutors | 0 | 0.29 (0.14) | 0.0407 |
| Other educational support | 0.27 (0.21) | 0.65 (0.29) | 0.291 |
| Parents’ days off work | 0.20 (0.10) | 0.06 (0.05) | 0.210 |
Resource-use measures for the first 3 months post randomisation are summarised in Table 53 by trial allocation group. The mean (SE) number of GP contacts at surgery was 0.70 (0.11) in the Families for Health group, but was significantly lower in the usual-care group [0.28 (SE 0.07); p = 0.002]. The Families for Health group also had more contacts with social workers at home, on average, with a mean (SE) number of contacts of 0.09 (0.04) compared with none for the usual-care group (p = 0.044). There were no significant differences between the trial arms across the remaining resource-use categories. For the period 3–12 months post randomisation, there were no significant differences between the trial arms across all resource-use categories (see Appendix 4).
| Resource variable | Treatment group | p-valuea | |
|---|---|---|---|
| FFH (n = 44) | UC (n = 50) | ||
| Hospital care, mean (SE) | |||
| Number of inpatient admissions | 0 | 0 | |
| A&E visits | 0.25 (0.14) | 0.12 (0.08) | 0.412 |
| Outpatient department visits | 0.16 (0.06) | 0.18 (0.09) | 0.854 |
| Day hospital visits | 0 | 0 | |
| Other hospital visits | 0 | 0.04 (0.03) | 0.159 |
| Community-based health-care contacts, mean (SE) | |||
| GP at surgery | 0.70 (0.11) | 0.28 (0.07) | 0.002 |
| GP at home | 0 | 0 | |
| Practice nurse | 0.07 (0.04) | 0.06 (0.03) | 0.874 |
| Dentist | 0.32 (0.07) | 0.52 (0.08) | 0.066 |
| Optician | 0.05 (0.03) | 0.18 (0.06) | 0.057 |
| Dietitian | 0 | 0 | |
| Child development centre | 0 | 0.04 (0.04) | 0.322 |
| Child guidance unit | 0 | 0 | |
| General health (other) | 0.02 (0.02) | 0.04 (0.03) | 0.633 |
| Family therapist | 0 | 0 | |
| Individual therapy | 0 | 0 | |
| Psychiatrist/psychologist | 0 | 0.20 (0.20) | 0.322 |
| Counselling (other) | 0.16 (0.12) | 0.66 (0.46) | 0.298 |
| Community-based social care contacts, mean (SE) | |||
| Social worker at office | 0 | 0 | 0 |
| Social worker at home | 0.09 (0.04) | 0 | 0.044 |
| After-school club | 0 | 0 | 0 |
| Social care (other) | 0 | 0.02 (0.02) | 0.322 |
| Medication usage, n (%) | |||
| Prescribed | 19 (43.2) | 24 (48.0) | 0.640 |
| Over the counter | 21 (47.7) | 26 (52.0) | 0.679 |
| Educational support contacts, mean (SE) | |||
| School nurse | 0.34 (0.12) | 0.50 (0.17) | 0.437 |
| Educational psychologist | 0.05 (0.05) | 0.04 (0.04) | 0.928 |
| Educational welfare officer | 0 | 0 | |
| SEN co-ordinator | 0.05 (0.05) | 0.12 (0.10) | 0.505 |
| Additional meetings with tutors | 0.07 (0.05) | 0.183 | |
| Other educational support | 0.02 (0.02) | 0.40 (0.25) | 0.133 |
| Parents days off work | 0.15 (0.08) | 0.02 (0.02) | 0.116 |
Attendance rates for the Families for Health programme and a range of usual-care programmes targeted at overweight and obese children during the first 3 months post randomisation are summarised in Table 54. The mean number of contacts with the Families for Health programmes was higher than for comparator usual-care programmes. This difference may be explained, at least in part, by the sources of information for the two comparator arms. The attendance data for the Families for Health programme were based on the recorded attendance information collected as part of the trial. In contrast, attendance at comparator usual-care programmes was based on parent-reported attendance recorded as part of the resource-use questionnaires. Nevertheless, the researchers administering the resource-use questionnaires were trained to prompt parents about their children’s use of a range of services available in their locality. The attendance rates for the comparator usual-care programmes over the period 3–12 months post randomisation are presented in Appendix 5.
| Attendance | Treatment group, mean (SE) | |
|---|---|---|
| FFH | UC | |
| FFH, site A 1 | 7.5 (0.82) | NA |
| FFH, site B 1 | 5.3 (1.36) | NA |
| FFH, site C 1 | 3.9 (1.44) | NA |
| FFH, site A 2 | 6.8 (1.52) | NA |
| FFH, site C 2 | 4.8 (1.22) | NA |
| FFH, site B 2 | 3.7 (0.62) | NA |
| FFH, site A 3 | 5.2 (1.37) | NA |
| UC, One Body One Life | NA | 1.14 (0.38) |
| UC, Change4Life | NA | 2.5 (0.61) |
| UC, WISH | NA | 3.3 (1.05) |
| UC, Weight Watchers | NA | 0 |
| UC, school nurse | NA | 2.9 (1.01) |
Costing of Families for Health and comparator usual-care programmes
The costs of developing the Families for Health programme and training staff to deliver the programme were measured and valued. These costs are presented in Table 55. The costs directly attributable to the delivery of the programme encompassed the value placed on staff preparation time, programme delivery time, travel, parking, refreshments and miscellaneous expenditures. The programme delivery costs over the duration of the trial, broken down by cost category, are presented in Table 55.
| Category | Cost (£) |
|---|---|
| Cost of developing the Families for Health intervention | |
| Family links training costs | 13,137 |
| Other staff training costs | 1816 |
| Total | 14,953 |
| Cost of delivering the Families for Health programme during the trial | |
| Staff costs | 21,261 |
| Travel | 1981 |
| Subsistence | 888 |
| Venue hire | 6704 |
| Toolkit | 995 |
| Consumables | 663 |
| Total | 32,492 |
The costs of delivering the alternative usual-care programmes were also estimated using comparable primary research methods. Estimates of cost per attending child per programme session are presented for each programme in Table 56. The costs per attending child per programme session were variable among the programmes and ranged from £4.58 for the Weight Watchers programme to £95.94 for Families for Health. This is not entirely surprising because the programmes were not homogeneous in terms of their constituent components, mode or location of delivery, or staff requirements.
| Programme | Cost per attending child per programme session (£) |
|---|---|
| Families for Health | 95.94 |
| One Body One Life | 40.41 |
| Change4Life (family programme) | 83.56 |
| WISH | 70.33 |
| Weight Watchers | 4.58 |
| Change4Life (one to one) | 53.71 |
Economic costs
Economic costs for the 3-month period pre-randomisation are summarised in Appendix 6 by trial allocation group and cost category. There were no significant differences between the trial allocation groups in any cost category. Economic costs during the first 3 months post randomisation are summarised in Table 57 by trial allocation group and cost category. The mean total costs of GP visits, social worker contacts at home, overweight- and obesity-related programmes, and overall community-based social and other care were significantly higher for the Families for Health group than for the usual-care group. Mean total NHS and Personal Social Services costs, and mean total societal costs were significantly higher for the Families for Health group than for the usual-care group [£688 vs. £385 (p = 0.007) and £792 vs. £459; (p = 0.006), respectively]. For the period between 3 and 12 months post randomisation, there were no significant cost differences in any cost category between the two trial allocation groups (see Appendix 7).
| Cost category | Treatment group (£) | p-valuea | |
|---|---|---|---|
| FFH (n = 44) | UC (n = 50) | ||
| Hospital inpatient care, mean (SE) | 0.00 | 0.00 | |
| Other hospital-based care, mean (SE) | |||
| A&E | 28.79 (16.38) | 13.82 (7.81) | 0.412 |
| Outpatient departments | 30.07 (12.20) | 34.02 (17.66) | 0.854 |
| Day hospital | 0.00 | 0.00 | |
| Other | 0.00 | 4.74 (3.97) | 0.238 |
| Total other hospital care costs | 58.86 (26.00) | 52.58 (21.08) | 0.852 |
| Community-based health care, mean (SE) | |||
| GP at surgery | 28.36 (4.97) | 9.75 (3.01) | 0.002 |
| GP at home | 0.00 | 0.00 | |
| Practice nurse | 1.00 (0.65) | 0.35 (0.21) | 0.350 |
| Dentist | 11.40 (3.07) | 23.07 (5.90) | 0.084 |
| Optician | 3.18 (2.22) | 14.47 (6.35) | 0.099 |
| Dietitian | 0.00 | 0.00 | |
| Child development centre | 0.00 | 1.69 (1.69) | 0.322 |
| Child guidance unit | 0.00 | 0.00 | |
| General health other | 1.36 (1.36) | 1.17 (0.83) | 0.902 |
| Family therapist | 0.00 | 0.00 | |
| Individual therapy | 0.00 | 0.00 | |
| Psychiatrist/psychologist | 0.00 | 28.40 (28.40) | 0.322 |
| Counselling (other) | 7.15 (4.36) | 97.64 (81.37) | 0.272 |
| Total community-based health costs | 52.46 (7.78) | 176.53 (84.53) | 0.150 |
| Community-based social and other care, mean (SE) | |||
| Social worker at office | 0.00 | 0.00 | |
| Social worker at home | 1.80 (0.87) | 0.00 | 0.044 |
| After-school club | 0.00 | 0.00 | |
| Social care (other) | 0.00 | 0.09 (0.09) | 0.322 |
| Overweight- and obesity-related programmesb | 562.58 (54.35) | 127.29 (23.39) | < 0.001 |
| Total community-based social and other costs | 564.38 (54.65) | 127.38 (23.38) | < 0.001 |
| Medications, mean (SE) | |||
| Prescribed | 2.85 (0.83) | 6.42 (2.73) | 0.216 |
| Over the counter | 0.56 (0.32) | 0.36 (0.20) | 0.604 |
| Educational support, mean (SE) | |||
| School nurse | 4.62 (1.95) | 4.96 (1.67) | 0.893 |
| Educational psychologist | 0.90 (0.90) | 4.74 (4.74) | 0.429 |
| Educational welfare officer | 0.00 | 0.00 | |
| SEN co-ordinator | 1.14 (1.14) | 4.50 (3.17) | 0.322 |
| Additional meetings with tutors | 0.00 | 0.00 | |
| Other educational support | 3.02 (3.02) | 7.73 (4.61) | 0.171 |
| Total educational support costs | 9.68 (3.49) | 21.93 (10.37) | 0.268 |
| Other costs, mean (SE) | |||
| Additional family expenditures | 82.84 (21.91) | 55.40 (15.93) | 0.314 |
| Lost productivity | 16.94 (7.92) | 3.19 (3.19) | 0.113 |
| Travel costs | 3.36 (0.82) | 13.79 (6.34) | 0.109 |
| Total NHS and PSS costs, mean (SE) | 688.24 (56.08) | 384.85 (94.63) | 0.007 |
| Total societal costs, mean (SE) | 791.94 (63.47) | 457.58 (98.53) | 0.006 |
Economic costs from randomisation to 12 months post randomisation are presented by cost category in Table 58 for the Families for Health and usual-care groups. The costs are presented for complete cases (i.e. for those children for whom cost data were available at all follow-up periods). Mean total social and other non-health community care costs, total NHS and Personal Social Service costs, and total societal costs were significantly higher for the Families for Health group than for the usual-care group. There were no significant differences in mean total hospital care costs, community health-care costs, medication costs, educational support costs, family expenditures, parental lost earnings and travel costs between the trial allocation groups. Mean (SE) total NHS and Personal Social Service costs were estimated at £998 (£72) for the Families for Health group, compared with £548 (£73) for children in the usual-care group. The mean NHS and Personal Social Service cost difference was £450 (bootstrap 95% CI £249 to £650; p < 0.001). Similarly, mean (SE) total societal costs were estimated at £1311 (£100) for the Families for Health group, compared with £882 (£112) for children in the usual-care group. The mean societal cost difference was £429 (bootstrap 95% CI £110 to £713; p = 0.005).
| Cost category | Treatment group, mean £ (SE) | Mean difference,a £ (95% CI) | Bootstrapped mean difference, £ (95% CI) | p-valueb | |
|---|---|---|---|---|---|
| FFH (n = 45) | UC (n = 43) | ||||
| Hospital inpatient costs | – | – | – | – | – |
| Other hospital care costs | 158.04 (51.30) | 118.69 (41.78) | 39.36 (–92.21 to 170.92) | 39.13 (–88.74 to 172.31) | 0.554 |
| Community health-care costs | 195.14 (32.80) | 179.85 (32.72) | 15.29 (–76.80 to 107.38) | 14.44 (–85.30 to 105.04) | 0.673 |
| Social and other non-health community care costsc | 591.97 (51.44) | 125.82 (24.48) | 466.15 (352.31 to 579.99) | 462.04 (351.66 to 571.12) | < 0.001 |
| Prescribed medication costs | 15.23 (3.79) | 7.99 (2.44) | 7.24 (–1.75 to 16.22) | 6.98 (–1.15 to 16.49) | 0.113 |
| Over-the-counter medication costs | 0.28 (0.17) | 0.42 (0.23) | –0.14 (–0.71 to 0.43) | –0.14 (–0.79 to 0.36) | 0.621 |
| Educational support costs | 37.86 (18.00) | 115.48 (47.54) | –77.62 (–179.54 to 24.30) | –78.55 (–181.32 to 12.39) | 0.132 |
| Change in family expenditures | 240.18 (57.50) | 275.01 (73.58) | –34.83 (–220.66 to 150.99) | –36.20 (–212.84 to 135.48) | 0.710 |
| Lost earnings | 61.89 (23.61) | 35.16 (13.71) | 26.73 (–27.72 to 81.18) | 25.76 (–22.89 to 83.99) | 0.331 |
| Travel costs | 10.79 (1.80) | 24.01 (11.43) | –13.21 (–36.54 to 10.11) | –13.24 (–39.97 to 2.54) | 0.260 |
| Total NHS and PSS costs | 998.24 (72.16) | 547.84 (72.72) | 450.41 (246.75 to 654.08) | 444.04 (249.22 to 650.30) | < 0.001 |
| Total societal costs | 1311.38 (100.30) | 882.43 (111.75) | 428.95 (130.36 to 727.54) | 420.21 (110.33 to 712.79) | 0.005 |
Analyses of incremental costs and incremental health outcomes
A bivariate regression, in the form of a seemingly unrelated regression, was carried out with the view to estimating the incremental costs and incremental health outcomes associated with the Families for Health programme. The results of this bivariate regression are presented in Table 59. The adjusted incremental costs associated with the Families for Health programme were £483.63 when NHS and Personal Social Services costs were considered and £410.64 when societal costs were considered. These incremental costs were statistically significant at the 0.1% and 1% levels, respectively. Children from all comparator socioeconomic backgrounds incurred higher adjusted costs, regardless of study perspective, than the reference category (highly managerial). Children with siblings in the trial population incurred lower adjusted NHS and Personal Social Services costs and lower adjusted societal costs than children without siblings in the trial population, although these cost decrements were not statistically significant. Similarly, children aged 9–11 years incurred lower adjusted NHS and Personal Social Services costs and lower adjusted societal costs than children aged 6–8 years, although these cost decrements were not statistically significant. Regarding health outcome measures, the Families for Health programme was associated with a mean increment in QALYs of 0.04 (95% CI –0.04 to 0.11), but also a difference in longitudinal change in BMI z-score of 0.11 (95% CI 0.00 to 0.22). However, it should be noted that these results were based on 88 children with complete data for all covariates.
| Total costa | Costs, £ (95% CI) | |
|---|---|---|
| NHS and PSS | Societal | |
| Intervention: Family for Health | 483.63*** (270.51 to 696.74) | 410.46** (121.12 to 699.80) |
| Sex: female | –55.57 (–273.34 to 162.19) | –243.50 (–539.15 to 52.15) |
| SES | ||
| Intermediate | 165.67 (–166.31 to 497.66) | 278.75 (–171.97 to 729.48) |
| Routine | 284.16* (38.66 to 529.67) | 178.67 (–154.63 to 511.99) |
| Never worked | 179.64 (–163.21 to 522.49) | 270.47 (–195.00 to 735.95) |
| Location | ||
| Site B | 4.25 (–263.19 to 271.71) | –21.77 (–384.89 to 341.33) |
| Site C | –167.62 (–417.74 to 82.49) | –366.42* (–705.99 to –26.84) |
| Siblings: trial siblings | –179.50 (–509.63 to 150.62) | –423.34 (–871.55 to 24.85) |
| Age: 9–11 years | –41.13 (–248.98 to 166.71) | –121.92 (–404.11 to 160.26) |
| Constant | 557.93*** (255.72 to 860.14) | 1173.07*** (762.77 to 1583.37) |
| QALYs (95% CI) | BMI z-score (95% CI) | |
| Intervention: Family for Health | 0.04 (–0.04 to 0.11) | 0.11 (0.00 to 0.22) |
| Sex: female | 0.02 (–0.06 to 0.10) | –0.12* (–0.23 to 0.00) |
| SES | ||
| Intermediate | 0.07 (–0.05 to 0.19) | 0.09 (–0.08 to 0.27) |
| Routine | –0.02 (–0.10 to 0.075) | 0.02 (–0.10 to 0.15) |
| Never worked | –0.24*** (–0.36 to –0.11) | 0.03 (–0.15 to 0.21) |
| Location | ||
| Site B | –0.06 (–0.15 to 0.04) | 0.09 (–0.04 to 0.23) |
| Site C | 0.09 (–0.00 to 0.18) | 0.08 (–0.04 to 0.22) |
| Siblings: trial siblings | 0.12* (0.01 to 0.25) | –0.06 (–0.23 to 0.11) |
| Age: 9–11 years | 0.09* (0.02,0.17) | –0.03 (–0.14 to 0.07) |
| Constant | 0.72*** (0.61 to 0.83) | 0.11 (–0.04 to 0.27) |
| Observations | 88 | 88 |
Cost-effectiveness
The incremental cost-effectiveness of the Families for Health programme is shown in Table 60 for the children with complete costs and health outcomes data over the trial follow-up period, by study perspective and health outcome. When a study perspective of the NHS and Personal Social Services was adopted (i.e. that adopted for the baseline analysis) and health outcomes were measured in terms of QALYs, the average total cost was £1019 in the Families for Health group compared with £507 in the usual-care group, generating a mean incremental cost of £512. The mean incremental cost-effectiveness of the Families for Health programme was estimated at £552,175 per QALY gained. The bootstrapped mean ICERs fell in the north-east and north-west quadrants of the cost-effectiveness plane (Figure 7). The CEAC shown in Figure 7 indicates that regardless of the value of the cost-effectiveness threshold, the probability that the Families for Health programme is cost-effective does not exceed 40%. If decision-makers are willing to pay £20,000 for an additional QALY, the probability that the Families for Health programme is cost-effective is approximately 28% (see Table 60). Broadening the study perspective to that of society as a whole had little effect on these cost-effectiveness results. In particular, the mean ICER remained relatively static at £559,115 per QALY gained and the probability that the Families for Health programme is cost-effective at a £20,000 cost-effectiveness threshold remained unchanged at 28% (Table 60, Figure 8).
| Outcome measure by perspective | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH | UC | FFH | UC | |||||||||
| NHS and PSS | ||||||||||||
| Sample size (n) | 41 | 38 | 41 | 38 | ||||||||
| QALY | 1018.51 (864 to 1173) | 506.64 (361 to 652) | 511.86 (303 to 721) | 0.8315 (0.7691 to 0.8938) | 0.8305 (0.7742 to 0.8869) | 0.0009 (–0.0822 to 0.0841) | 552,175 | 51 | < 1 | 22 | 28 | 36 |
| Sample size (n) | 45 | 43 | 45 | 43 | ||||||||
| BMI z-scored | 998.25 (853 to 1144) | 547.84 (401 to 695) | 450.41 (247 to 654) | –0.0128 (–0.0961 to 0.0704) | 0.1016 (0.0191 to 0.1841) | –0.1144 (–0.0012 to –0.2301) | –3935 (UC dominates) | 2 | < 1 | < 1 | < 1 | 1 |
| Societal | ||||||||||||
| Sample size (n) | 41 | 38 | 41 | 38 | ||||||||
| QALY | 1351.09 (1136 to 1566) | 832.80 (618 to 1047) | 518.30 (219 to 818) | 0.8315 (0.7691 to 0.8938) | 0.8305 (0.7742 to 0.8869) | 0.0009 (–0.0822 to 0.0841) | 559,115 | 51 | < 1 | 22 | 28 | 35 |
| Sample size (n) | 45 | 43 | 45 | 43 | ||||||||
| BMI z-scored | 1311.38 (1109 to 1514) | 882.43 (657 to 1108) | 428.95 (131 to 727) | –0.0128 (–0.0961 to 0.0704) | 0.1016 (0.0191 to 0.1841) | –0.1144 (–0.0012 to –0.2301) | –3748 (UC dominates) | 2 | < 1 | < 1 | < 1 | 1 |
FIGURE 7.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: complete cases from a NHS and Personal Social Services perspective. Adapted from Robertson W, Fleming J, Kamal A, Hamborg T, Khan KA, Griffiths F, et al. Randomised controlled trial and economic evaluation of the ‘Families for Health’ programme to reduce obesity in children [published online ahead of print 21 December 2016]. Arch Dis Child 2016. doi: 10.1136/archdischild-2016-311514. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited (see http://creativecommons.org/licenses/by/4.0/). 71


FIGURE 8.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: complete cases from a societal perspective.
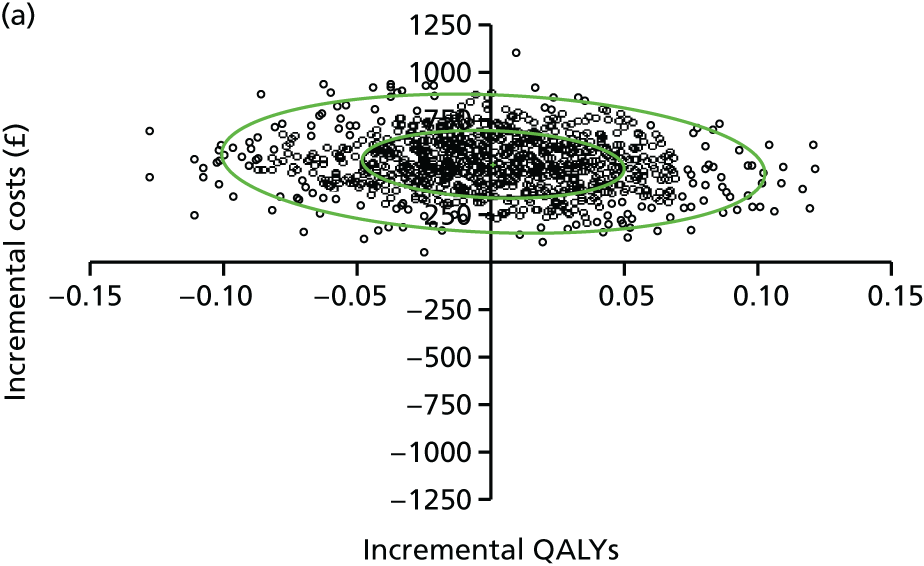
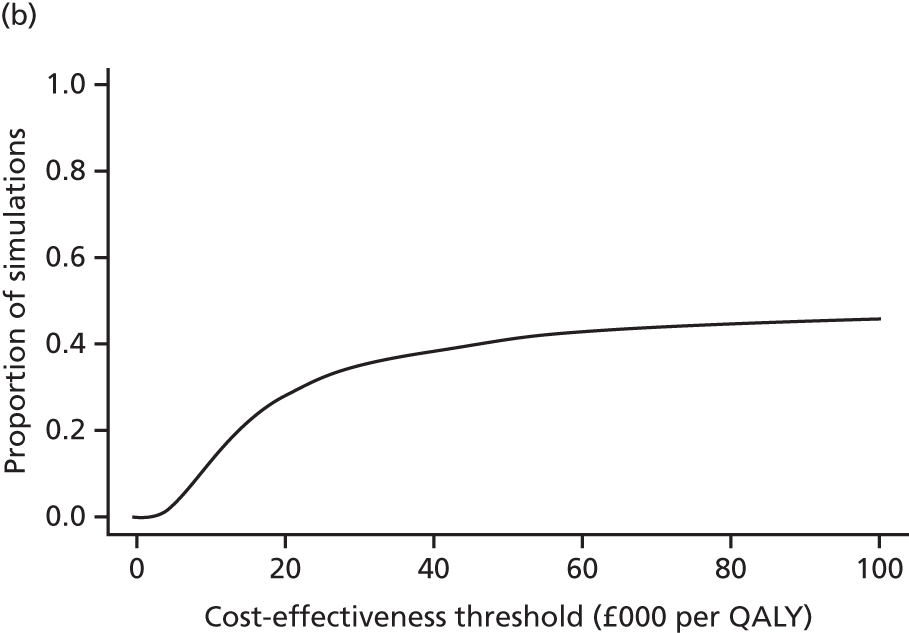
When a study perspective of the NHS and Personal Social Services was adopted and health outcomes were measured in terms of longitudinal change in BMI z-score, the average total cost was £998 in the Families for Health group (for those with complete data), compared with £548 in the usual-care group, generating a mean incremental cost of £450. The mean incremental cost-effectiveness of the Families for Health programme was estimated at –£3935 per unit change in BMI z-score. The bootstrapped mean ICERs largely fell in the north-west quadrant of the cost-effectiveness plane (Figure 9), indicating that, on average, the Families for Health programme is dominated by usual care in health economic terms. The CEAC shown in Figure 9 indicates that regardless of the value of the cost-effectiveness threshold, the probability that the Families for Health programme is cost-effective does not exceed 2% (on the basis of the BMI z-score). If decision-makers are willing to pay £20,000 per unit change in BMI z-score, the probability that the Families for Health programme is cost-effective is < 1% (see Table 60). Broadening the study perspective to that of society as a whole had little effect on these cost-effectiveness results. In particular, the mean ICER remained relatively static at –£3748 per unit change in BMI z-score and the probability that the Families for Health programme is cost-effective at a £20,000 cost-effectiveness threshold remained unchanged at < 1% (Figure 10).
FIGURE 9.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome: complete cases from a NHS and Personal Social Services perspective.


FIGURE 10.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome: complete cases from a societal perspective.

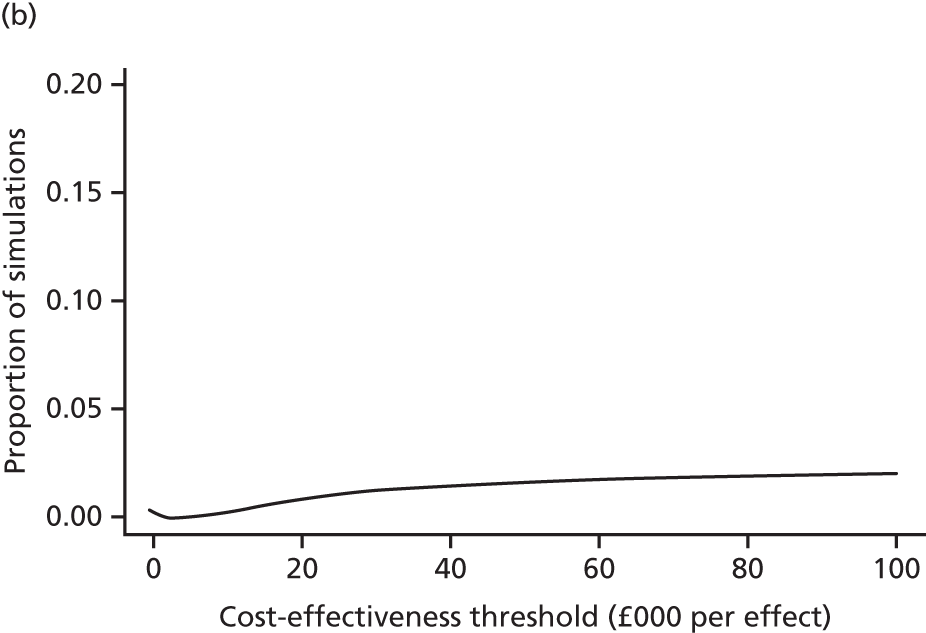
Sensitivity analyses
Several sensitivity analyses were undertaken to assess the impact of uncertainty on the cost-effectiveness results. Table 61 presents our recalculations of cost-effectiveness estimates when a per-protocol analysis was performed. These analyses defined ‘programme completers’ as families who participated in five or more sessions of the Families for Health programme and non-completers as families who participated in less than five sessions of the Families for Health programme. These analyses were also restricted to the baseline NHS and Personal Social Services perspective. Of particular note is that for programme completers, the mean incremental cost per QALY gained attributable to the Families for Health programme declined to £27,790 and the probability that the programme is cost-effective at a £20,000 cost-effectiveness threshold increased to 43%. For comparison, the mean incremental cost per QALY gained among non-completers was –£6441 (indicating that usual care is dominant in health economic terms) and the probability that the programme is cost-effective at a £20,000 cost-effectiveness threshold was 17% (Figures 11–14).
| Outcome measure by FFH completion group | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH | UC | FFH | UC | |||||||||
| QALY | ||||||||||||
| Sample size (n) | 29 | 38 | 29 | 38 | ||||||||
| FFH completersd | 1100.99 (976 to 1226) | 506.64 (361 to 652) | 594.35 (367 to 822) | 0.8519 (0.7227 to 0.9812) | 0.8305 (0.7742 to 0.8869) | 0.0214 (–0.1545 to 0.1973) | 27,790 | 69 | < 1 | 35 | 43 | 52 |
| Sample size (n) | 12 | 38 | 12 | 38 | ||||||||
| FFH non-completerse | 819.16 (418 to 1220) | 506.64 (361 to 652) | 312.52 (–41 to 666) | 0.7820 (0.5484 to 1.0156) | 0.8305 (0.7742 to 0.8869) | –0.0485 (–0.3108 to 0.2138) | –6441 (UC dominates) | 24 | 7 | 15 | 17 | 19 |
| BMI z-scoref | ||||||||||||
| Sample size (n) | 30 | 43 | 30 | 43 | ||||||||
| FFH completersd | 1102.13 (576 to 1922) | 547.84 (401 to 695) | 554.29 (352 to 756) | –0.0466 (–0.8945 to 0.4711) | 0.1016 (0.0191 to 0.1841) | –0.1482 (–0.2758 to –0.0206) | –3741 (UC dominates) | 1 | < 1 | < 1 | < 1 | < 1 |
| Sample size (n) | 15 | 43 | 15 | 43 | ||||||||
| FFH non-completerse | 790.49 (459 to 1122) | 547.84 (401 to 695) | 242.66 (–99 to 584) | 0.0546 (–0.0604 to 0.1697) | 0.1016 (0.0191 to 0.1841) | –0.0470 (–0.1932 to 0.0993) | –5168 (UC dominates) | 29 | 9 | 23 | 25 | 26 |
FIGURE 11.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome and per-protocol analyses: completers.


FIGURE 12.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome and per-protocol analyses: non-completers.


FIGURE 13.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome and per-protocol analyses: completers.


FIGURE 14.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome and per-protocol analyses: non-completers.
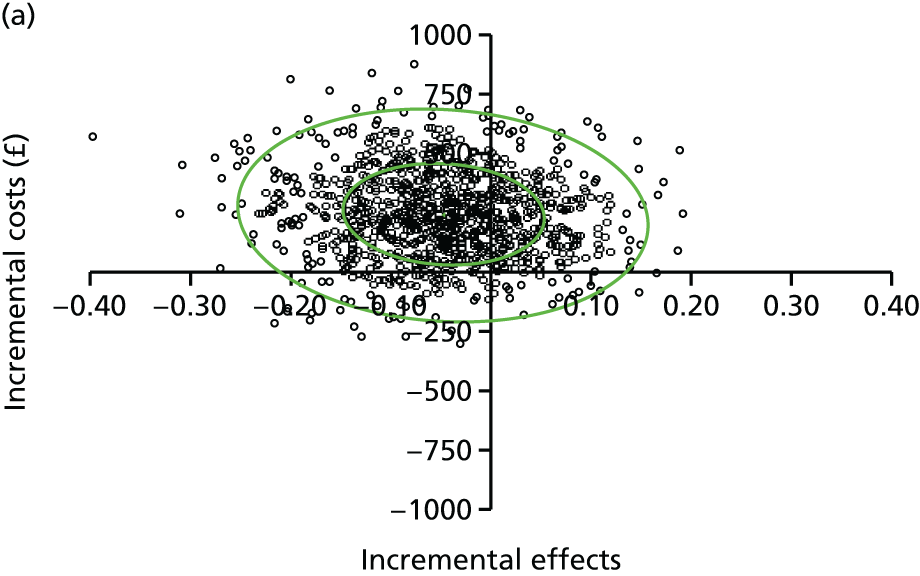
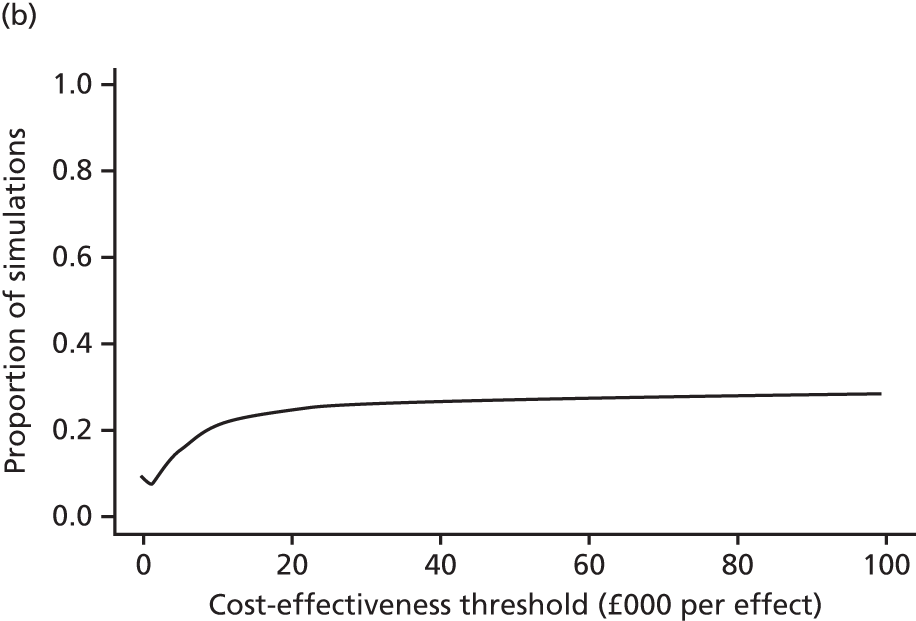
Table 62 presents our recalculations of cost-effectiveness estimates following multiple imputation of all missing cost and outcomes data (i.e. estimates are calculated for all 128 study children). Of particular note is that following multiple imputation, the mean incremental cost per QALY gained (assuming a NHS and Personal Social Services perspective) attributable to the Families for Health programme declined to £9119 and the probability that the programme is cost-effective at a £20,000 cost-effectiveness threshold increased to 67%. Similar results were observed when a societal perspective for costs was adopted. In contrast, when the BMI z-score was considered as the health outcome measure, the Families for Health programme remained dominated by usual care in health economic terms and the probability that the programme is cost-effective did not exceed 6%, regardless of the value of the cost-effectiveness threshold (Figures 15–18).
| Outcome measure by perspective | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH (n = 63) | UC (n = 65) | FFH (n = 63) | UC (n = 65) | |||||||||
| NHS and PSS | ||||||||||||
| QALY | 964.03 (817.79 to 1110.28) | 694.76 (504.84 to 884.67) | 269.27 (34.62 to 503.93) | 0.81 (0.77 to 0.86) | 0.78 (0.73 to 0.83) | 0.03 (–0.04 to 0.10) | 9118.89 | 79 | 2 | 62 | 67 | 72 |
| BMI z-scored | 964.03 (817.79 to 1110.28) | 694.76 (504.84 to 884.67) | 269.27 (34.62 to 503.93) | 0.04 (–0.33 to 0.12) | 0.12 (0.05 to 0.19) | –0.07 (–0.19 to 0.03) | –3520.99 (UC dominates) | 8 | 2 | 5 | 5 | 6 |
| Societal | ||||||||||||
| QALY | 1328.95 (1148.32 to 1509.58) | 1029.97 (798.53 to 1261.42) | 298.98 (25.79 to 572.16) | 0.81 (0.77 to 0.86) | 0.78 (0.73 to 0.83) | 0.03 (–0.04 to 0.10) | 10,124.71 | 79 | 2 | 60 | 65 | 71 |
| BMI z-scored | 1328.95 (1148.32 to 1509.58) | 1029.97 (798.53 to 1261.42) | 298.98 (25.79 to 572.16) | 0.04 (–0.33 to 0.12) | 0.12 (0.05 to 0.19) | –0.07 (–0.19 to 0.03) | –3909.35 (UC dominates) | 8 | 2 | 4 | 5 | 6 |
FIGURE 15.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: imputed analyses from a NHS and Personal Social Services perspective. The cost-effectiveness plane is based on 1000 bootstrap replicates of the data set.


FIGURE 16.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: imputed analyses from a societal perspective. The cost-effectiveness plane is based on 1000 bootstrap replicates of the data set.
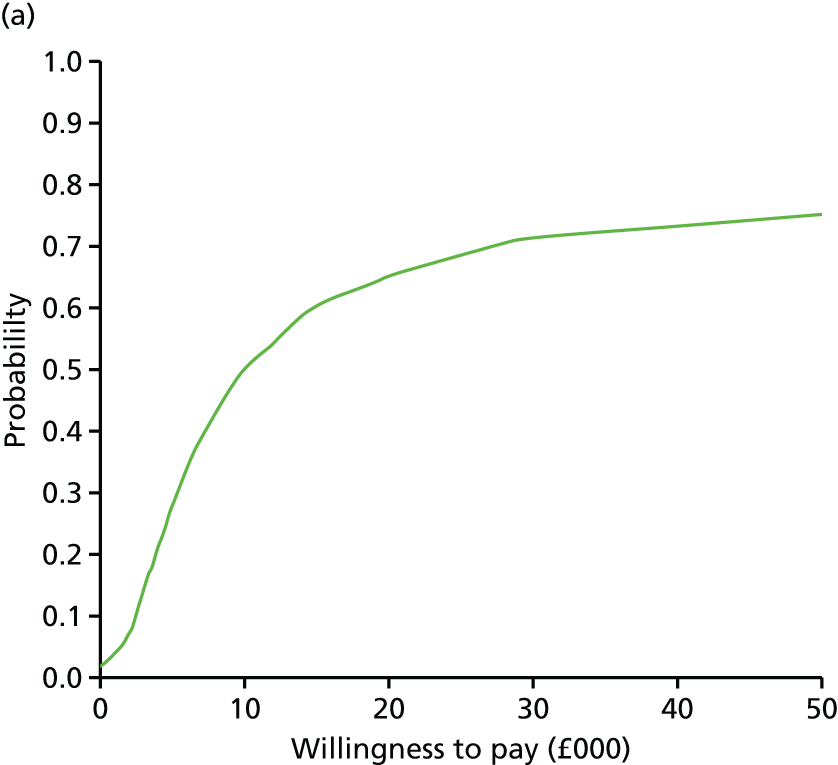
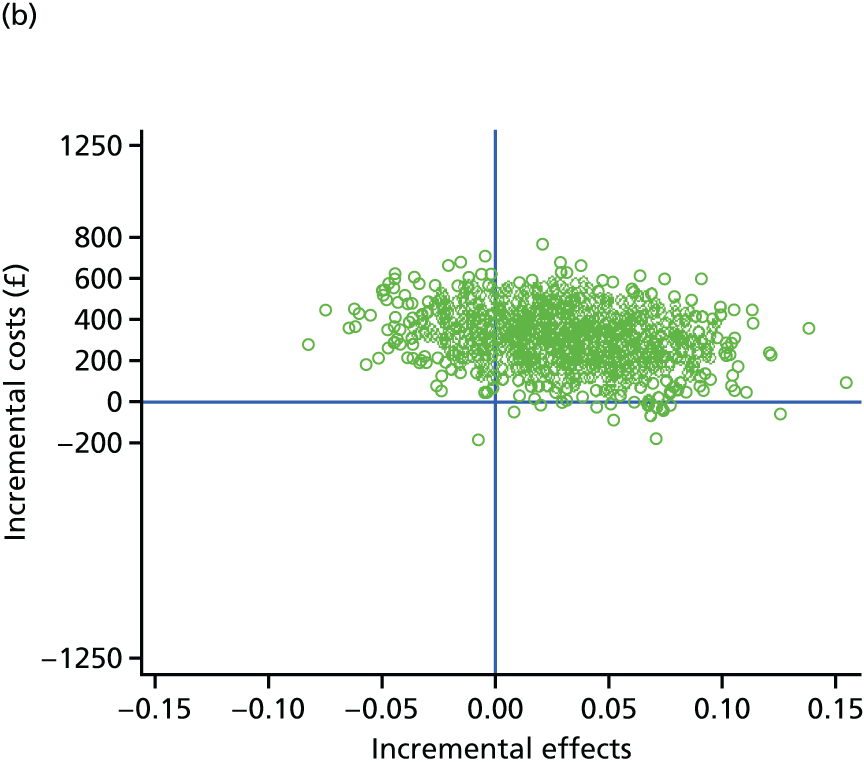
FIGURE 17.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome: imputed analyses from a NHS Personal Social Services perspective. The cost-effectiveness plane is based on 1000 bootstrap replicates of the data set.
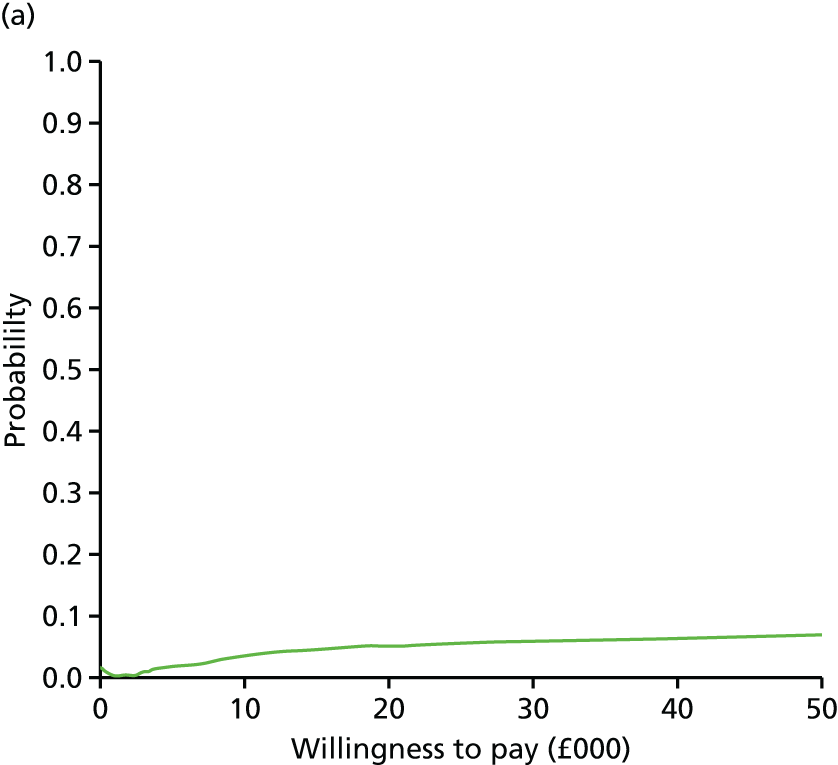
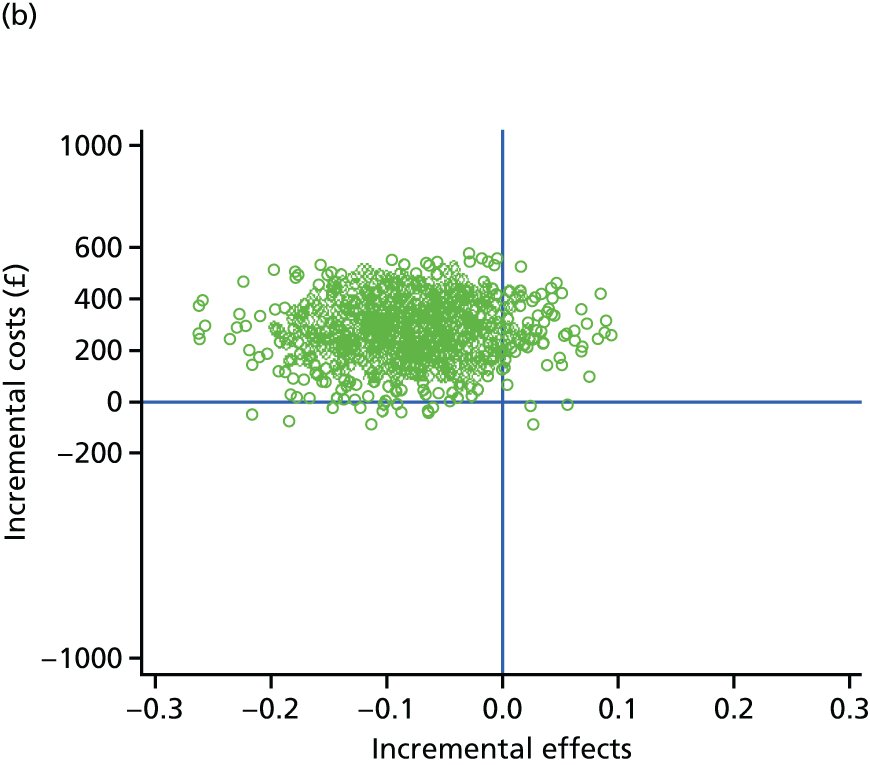
FIGURE 18.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome: imputed analyses from a societal perspective. The cost-effectiveness plane is based on 1000 bootstrap replicates of the data set.
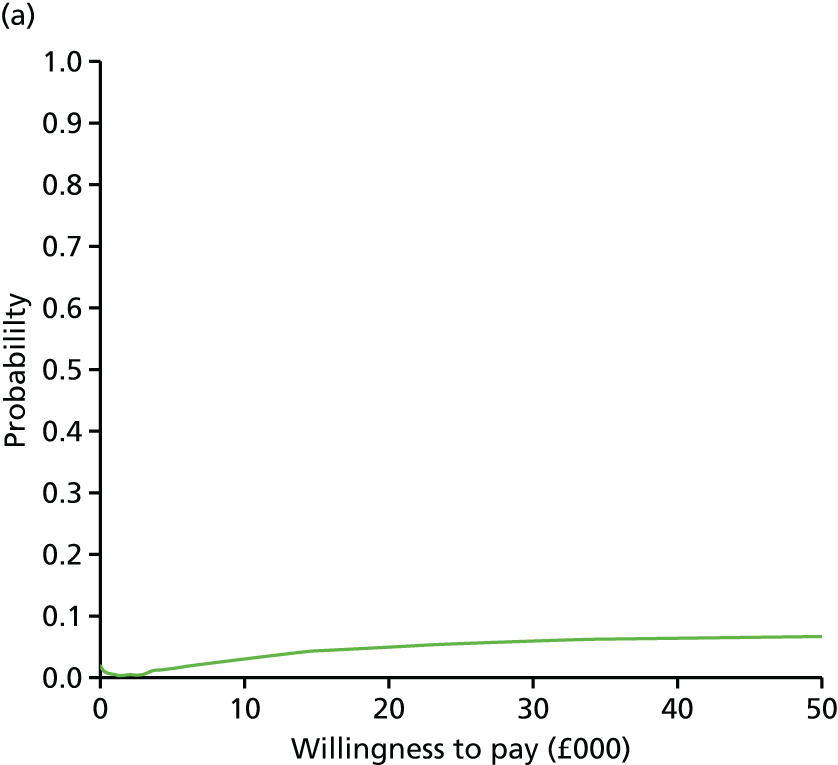

Finally, Table 63 presents our recalculations of cost-effectiveness estimates when alternative sources and inputs for EQ-5D utility values were incorporated into the analyses. Substituting parent-reported EQ-5D-Y values for the study child(ren) for child self-reported values in the formulation for QALYs removed the incremental QALY benefit associated with the Families for Health programme. The probability that the programme is cost-effective at a £20,000 cost-effectiveness threshold declined to 23% when a NHS and Personal Social Services perspective was adopted, and to 25% when a societal perspective was adopted. Furthermore, the reduction in incremental QALYs associated with the Families for Health programme increased when EQ-5D values reflecting the main parent’s self-reported health were also incorporated within calculations of overall QALYs gained. The probability that the programme is cost-effective at a £20,000 cost-effectiveness threshold declined to 2% (see Table 63 and Figures 19–22).
| Perspective | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH | UC | FFH | UC | |||||||||
| NHS and PSS | ||||||||||||
| Sample size (n) | 41 | 40 | 41 | 40 | ||||||||
| QALY (proxy EQ-5D-Y values for children) | 1028.40 (880 to 1177) | 529.11 (384 to 675) | 499.29 (296 to 703) | 0.8603 (0.7542 to 0.9664) | 0.8665 (0.7612 to 0.9719) | –0.0063 (–0.1558 to 0.1433) | –79,678 (UC dominates) | 44 | < 1 | 18 | 23 | 29 |
| Sample size (n) | 42 | 39 | 42 | 39 | ||||||||
| QALY (incorporation of EQ-5D values for parent’s health) | 1024.03 (879 to 1169) | 542.34 (395 to 689) | 481.69 (281 to 682) | 0.7453 (0.6136 to 0.8771) | 0.8143 (0.6923 to 0.9364) | –0.0690 (–0.2486 to 0.1106) | –6981 (UC dominates) | 7 | < 1 | 1 | 2 | 3 |
| Societal | ||||||||||||
| Sample size (n) | 41 | 40 | 41 | 40 | ||||||||
| QALY (proxy EQ-5D-Y values for children) | 1338.82 (1136 to 1541) | 886.65 (655 to 1119) | 452.17 (128 to 776) | 0.8603 (0.7542 to 0.9664) | 0.8665 (0.7612 to 0.9719) | –0.0063 (–0.1558 to 0.1433) | –72,159 (UC dominates) | 43 | < 1 | 21 | 25 | 31 |
| Sample size (n) | 42 | 39 | 42 | 39 | ||||||||
| QALY (incorporation of EQ-5D values for parent’s health) | 1356.77 (1156 to 1558) | 887.91 (650 to 1126) | 468.86 (144 to 793) | 0.7453 (0.6136 to 0.8771) | 0.8143 (0.6923 to 0.9364) | –0.0690 (–0.2486 to 0.1106) | –6795 (UC dominates) | 7 | < 1 | 2 | 2 | 3 |
FIGURE 19.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: proxy-reported EQ-5D-Y values, NHS and Personal Social Services perspective.
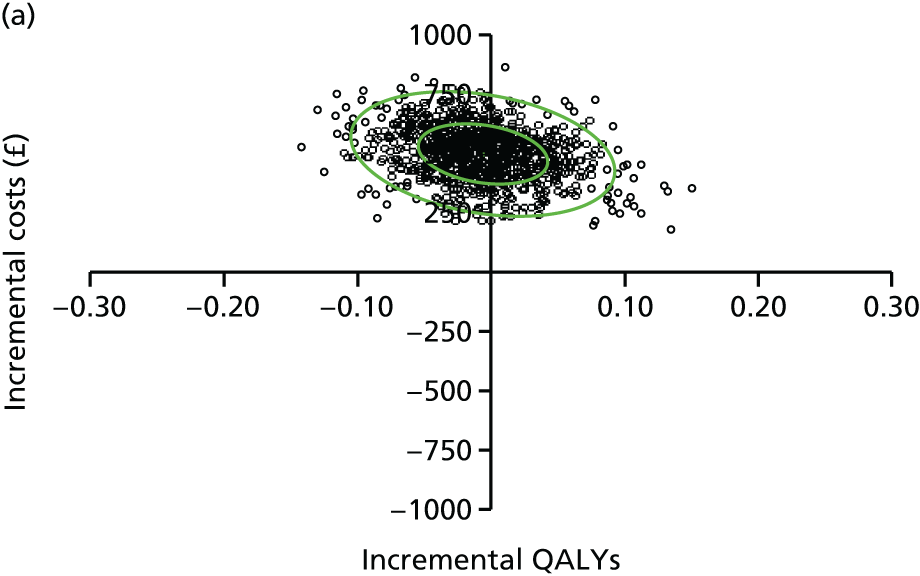
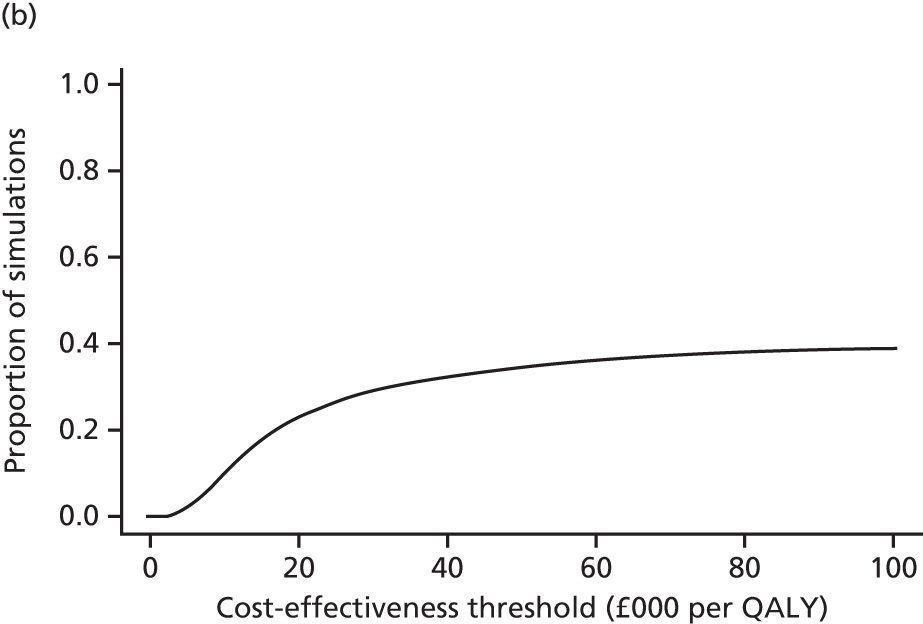
FIGURE 20.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: proxy-reported EQ-5D-Y values, societal perspective.

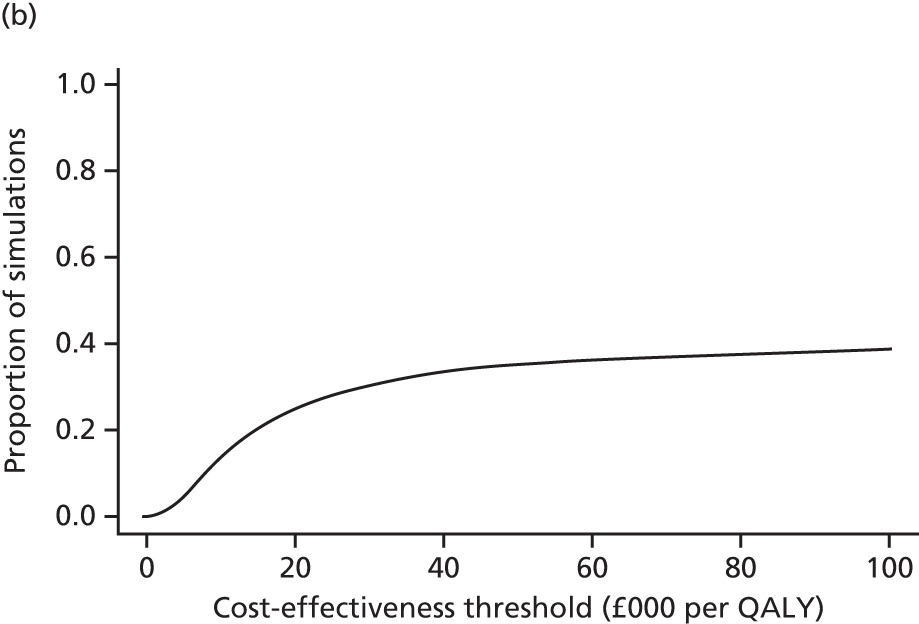
FIGURE 21.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: incorporation of EQ-5D values for parent’s health, NHS and Personal Social Services perspective.
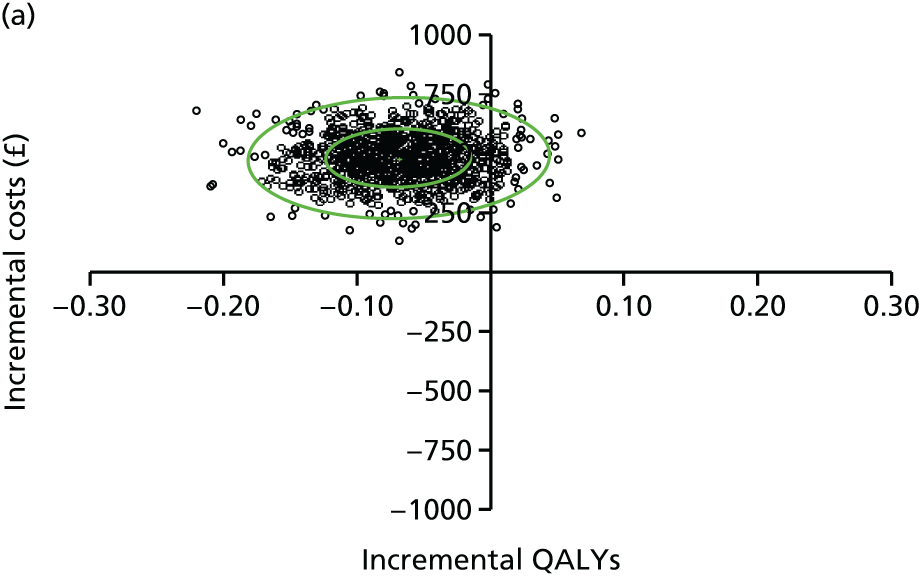

FIGURE 22.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome: incorporation of EQ-5D values for parent’s health, societal perspective.

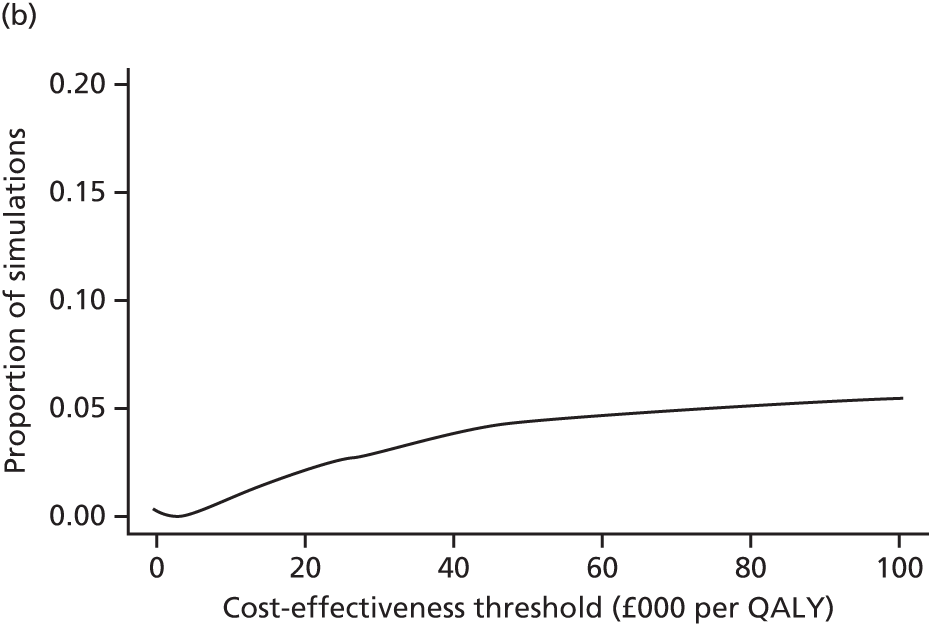
Subgroup analyses
Several subgroup analyses were conducted to explore the heterogeneity in our cost-effectiveness results. These are summarised in Appendix 8. See Table 93, which presents the cost-effectiveness results by subgroup when a NHS and Personal Social Services perspective is adopted for costs, and see Table 94 which presents the cost-effectiveness results by subgroup when a societal perspective is adopted. The subgroups considered in our analyses were (1) age group (6–8 or 9–11 years); (2) sex of child (male or female); and (3) site (site A, B or C). The cost-effectiveness results for two particular subgroups of children are worthy of comment. First, when the QALY measure was considered as the primary health outcome measure, the probability of cost-effectiveness of the Families for Health programme was notably higher for girls than for boys: 67% versus 15% at a £20,000 cost-effectiveness threshold from a NHS and Personal Social Services Perspective and 63% versus 16% at a £20,000 cost-effectiveness threshold from a societal perspective. Second, when the QALY measure was considered as the primary health outcome measure, the probability of cost-effectiveness of the Families for Health programme was notably higher in site A than in site B or C: 61% versus 11% versus 36% at a £20,000 cost-effectiveness threshold from a NHS and Personal Social Services perspective, and 64% versus 7% versus 35% at a £20,000 cost-effectiveness threshold from a societal perspective. These patterns were not replicated for the BMI z-score outcome measure.
Discussion
This chapter summarises the methods and results of the economic evaluation that was conducted as part of the Families for Health RCT. The economic evaluation was conducted in accordance with national methodological standards with a comprehensive analytical strategy adopted to handle missing data and various forms of uncertainty, including sampling, decision and methodological uncertainty. The mean incremental cost-effectiveness of the Families for Health programme was estimated at £552,175 per QALY gained, and the probability that the Families for Health programme is cost-effective at a £20,000 cost-effectiveness threshold was estimated to be approximately 28%. When health outcomes were measured in terms of longitudinal change in BMI z-score, the mean incremental cost-effectiveness of the Families for Health programme was estimated at –£3935 per unit change in BMI z-score (indicating that, on average, Families for Health is dominated by usual care in health economic terms), and the probability that the Families for Health programme is cost-effective did not exceed 2% across a range of cost-effectiveness thresholds held by decision-makers. These findings remained relatively robust to a range of sensitivity analyses, although it is noteworthy that the probability of cost-effectiveness of the Families for Health programme increased when the analyses were restricted to programme completers, and separately following multiple imputation of missing cost and health utility data. It is conceivable that family-based group interventions containing the key components of the Families for Health programme could represent a cost-effective use of public resources for the treatment of overweight status or obesity during mid-childhood, if strategies are in place to enhance programme adherence. Nevertheless, the incremental costs associated with enhancements to the programme would need to be factored into future analyses. Analyses of heterogeneity in the cost-effectiveness results revealed that the probability of cost-effectiveness of the programme was notably higher among girls and in one of the three trial sites, although this pattern was not replicated when cost-effectiveness estimates were based on the primary clinical outcome.
A number of caveats to the results of the economic evaluation should be noted. First, there was considerable stochastic uncertainty surrounding our cost-effectiveness estimates, which were addressed through the use of CEACs, and sensitivity analyses to handle uncertainty surrounding individual components of the economic evaluation. Second, a complete profile of resource utilisation, cost and health utility data over the study time horizon was available for only 79 of 128 (61.7%) children, despite intensive efforts to follow up the study children and their families. In response, multiple imputation techniques for handling missing values were applied. A third caveat is that our cost estimates are largely based on parental reports of their children’s use of health, social and broader services, and their own incremental expenditures over extended recall periods (3 and 9 months at the two follow-up points). Previous research has indicated that parents may underestimate their children’s use of some categories of services (e.g. community service utilisation) over extended recall periods. 83 If this was the case for our study, our absolute costs for some categories of services may be underestimates. Nevertheless, there is no evidence to suggest that our estimates of incremental cost-effectiveness of the Families for Health programme are biased by this concern. 84 A fourth caveat is that lost productivity in this study was measured on the basis of parents’ time off work, and this approach does not value the time losses of the non-working population, which may be relevant. Finally, in several analyses a societal perspective was applied to costs, but only health benefits accruing to the children were considered. Future research we are planning will additionally incorporate parents’ health outcomes and use a dyad approach to measuring health benefits for the family.
In conclusion, our study results indicate that the Families for Health programme is unlikely to represent a cost-effective use of resources targeted at overweight or obese children. Data from our economic evaluation can be used to inform future health economic studies in this area.
Chapter 6 Process evaluation
Introduction
This chapter reports the process evaluation of the Families for Health trial. Process evaluation is an important adjunct to the reporting of RCTs of complex interventions, and is particularly necessary in trials that involve multiple sites because the intervention may be delivered or received differently across sites. 85
Guidance from the UK MRC for the conduct of process evaluations has recommended that process evaluations should examine how the intervention was delivered (e.g. the structures, resources and processes through which intervention delivery is achieved) and what is delivered in terms of quantity and quality. 86 Although the protocol for this process evaluation82 was finalised before the MRC guidance was published, this process evaluation has similar aims. The protocol for this process evaluation was developed using Linnan and Steckler’s87 framework to address issues of recruitment, reach, dose delivered, fidelity and dose received.
Methods
Design
Table 64 shows the topics covered by the process evaluation, together with a definition of the component in the context of this trial and the associated research question.
| Component | Component definition | Associated research question |
|---|---|---|
| Recruitment | Success of methods used to approach and recruit participants | What is the best method of recruiting families? |
| Reach | Degree to which the intended population participated in the intervention | To what degree did the intended population participate in the intended interventions? |
| Dose delivered | The ‘amount’ of intervention provided by the intervention team | How much of the intervention was delivered in terms of quantity and quality? |
| Fidelity | The extent to which the intervention was delivered as planned (i.e. as prescribed in the handbook and as intended) | Was the FFH intervention delivered as intended in terms of the structures, resources and processes? |
| Dose received | Extent of engagement with the intervention by the target population | How was the intervention (FFH and UC) received by families, including which aspects (if any) of a healthier lifestyle the FFH and UC programmes enabled? |
Three members of the research team (WR, JF and AK) collected both quantitative and qualitative data from parents, children and facilitators. Table 65 shows the data used to assess each component. Interviews with families took place at baseline, and at the 3- and 12-month follow-ups. In addition, weekly feedback forms during the seven Families for Health programmes, attendance registers, focus groups with facilitators and hours worked by facilitators were also collected. Follow-up family visits were conducted by a researcher who was blinded to treatment allocation.
| Component | Data sources | Timing of data collection |
|---|---|---|
| Recruitment of participants | Record of expression of interest Parent self-report questionnaire |
Recruitment stage: baseline |
| Reach | Parent self-report questionnaire Child height and weight measurements |
Baseline |
| Dose delivered | Attendance data Facilitators focus groups Facilitator evaluation forms |
Weekly One mid-programme and one at the end of programme Weekly |
| Fidelity | Fidelity visits (FFH) Facilitators focus groups (FFH) |
3–4 sessions per programme Family registers and baseline recruitment details Facilitator training records, pay claim forms and expenses One mid-programme FG One end-of-programme FG |
| Dose received | Parent one-to-one interviews (FFH and UC) Parent end-of-session feedback form (FFH) Parent end-of-programme questionnaire (FFH) |
3 months Weekly (FFH) End of FFH programme |
Data collection methods
This section describes the data collection methods that were used solely for the process evaluation, grouped by the five components. Other data used were collected as described in Chapter 2.
Recruitment
At first contact, parents were asked to indicate how they heard about the trial. Subsequently, once recruited, parents completed a recruitment questionnaire, which included the question, ‘Where did you hear about the research study?’ with the following response options: GP, school nurse, health visitor, paediatrician, school, media, flyer/leaflet, friends/family, letter from NCMP, other. In cases where there was a discrepancy between the questionnaire response and the response given at first contact, the first contact response was used.
Reach
Data on family composition, ethnicity, sex, age of participating child(ren), employment details of parents, and height and weight measurements of parents and children were used to explore reach, and were collected as described in Chapter 2.
Population-level data for each site were obtained from 2011 census data for England and Wales,88 English Indices of Deprivation 201089 and the National Child Measurement Programme. 90 Baseline characteristics of families recruited to the Families for Health trial were compared with these data, to examine reach.
Dose delivered
Family attendance for the Families for Health intervention was recorded each week by the facilitators.
Self-reports of attendance at the 3- and 12-month follow-up visits were recorded using the CSRI. This asked families which services they had accessed (social worker, after school or homework club, Families for Health programme, usual care, other) and the number of contacts and average duration of each contact their child had with each service in the last 3 months (3-month follow-up) or 9 months (12-month follow-up). Further attendance information was obtained from the 3- and 12-month follow-up interviews, as parents were asked to confirm attendance information provided in the CSRI form.
Weekly evaluation forms with closed and open-ended questions were completed by parent and child facilitators at the end of each Families for Health session. The open-ended questions were: ‘How receptive were the group, how were the group dynamics and was everyone able to contribute?’; ‘Which topics worked well in the session?’; ‘Which topics did not work well in the session?’; ‘What do you think could improve this session?’; ‘How do you feel about today’s session?’; and ‘What are your thoughts about how you did this session?’.
One researcher (WR) conducted two focus groups with each group of Families for Health facilitators, one between weeks 3 and 6 and one after the last session of the programme. The focus group topics for the mid-programme were ‘How did the session go in relation to the session guide?’, ‘Were all the families there that you expected?’ and ‘How were the group dynamics at this stage of the programme?’. WR prompted facilitators in relation to timing issues, programme content and feedback from families. The end-of-programme schedule session topics were ‘What aspects worked and did not work on the programme and why?’, ‘What were the main facilitators and barriers to change in the families’, ‘How were the group dynamics’ and ‘Whether or not they felt the training equipped them to deliver the programme’. The mean duration of focus groups was 43 minutes (range 21–57 minutes).
Fidelity
The fidelity of delivery of the Families for Health programme was assessed by WR during visits to the Families for Health sessions, when she checked the materials and flip charts used during the Families for Health session. WR also collected facilitators’ weekly evaluation forms and data from transcripts of the facilitators’ focus groups were also used. Details of the length of each Families for Health programme (in weeks), half-term breaks, follow-up sessions delivered, number of facilitators at each session and continuity of facilitators across each programme were collected. The number of families allocated to a programme and the number of families that attended were noted, as was the number of families waiting > 3 months for a programme to start. WR/AK determined which changes to the programme affected the fidelity of delivery and which were minor adjustments.
Dose received
Parents attending a Families for Health programme completed weekly feedback forms. The feedback form asked parents to answer the questions ‘How do you feel about today’s session?’ and ‘How do you feel about the programme?’ using a 5-point Likert scale with responses ranging from ‘awful’ to ‘great’. They were asked for a written response to the questions ‘What did you find useful or enjoyable this week?’ and ‘What did you NOT find useful or enjoyable this week?’ and were given the opportunity to share further ideas or comments – ‘We would be glad to have any other ideas or comments’.
At the end of each Families for Health programme, parents were asked to complete an end-of-programme feedback form. The feedback form asked parents to answer the following questions using a 5-point Likert scale: ‘How do you feel about the programme?’ (with responses ranging from ‘awful’ to ‘great’); ‘How helpful have these parenting skills topics been?’, ‘How helpful have these healthy lifestyle physical activity topics been?’ and ‘How helpful have these healthy lifestyle food topics been?’, with responses for each topic ranging from ‘not helpful’ to ‘very helpful’; and ‘Do you think your child has enjoyed the programme?’, ‘Have you noticed any changes in your child as a result of the programme?’, ‘Do you think the programme has helped you and your child tackle his/her weight difficulty?’, ‘Do you think the programme has helped the rest of the family?’ and ‘Would you recommend the programme to other families?’, with responses ranging from ‘no’ to ‘a lot’.
One-to-one interviews were conducted with parents and children in both arms (Families for Health and usual care) of the trial by a member of the research team (JF or AK). These interviews were conducted at the 3-month follow-up visit, after the outcome measures had been collected, or at a family’s first interview after accessing a Families for Health programme. In some cases this interview took place longer than 3 months from recruitment, because the family had waited for a programme to start. All families were asked to consent to an interview at the recruitment stage, and one family declined. Consecutive sampling from those families who consented was used to ensure that representation of all Families for Health groups and of the various usual-care options, and diversity of age, ethnicity and sex of the children, family size and whether or not they completed the intervention. There were different interview schedules for parents allocated to the Families for Health programme and usual care at both 3 (see Appendix 9) and 12 months (see Appendix 10) and for children at the 3- and 12-month follow-ups (see Appendix 11). The Families for Health and usual-care parent interview schedules asked ‘Has anything changed in the life of your family/household since we first visited you?’, ‘Which intervention did you attend?’ and ‘Can you tell me a bit about what was involved?’ (usual care only), ‘How did you feel when you were approached to take part/saw or heard the advert?’, ‘What motivated you to take part?’ and ‘Why did you decide to join?’. Early in the interview parents were asked what they had got out of the programme they had attended. Parents were encouraged to tell us as much as they wanted to about this and then prompted to reflect on each aspect of the programme, such as diet and physical activity (Families for Health and usual care) and family relationships (Families for Health). Finally, the interviewer drew on the data from the parents’ end-of-programme feedback form to ask the parents to expand on their written feedback (Families for Health only). The mean duration of parent interviews was 20 minutes. Although the longest interview took 55 minutes, most (54/63) interviews lasted less than 30 minutes (range 3.5–55 minutes).
Children’s interviews included a drawing activity as well as discussion about the programme. Children were asked to draw three pictures: (1) ‘Draw a picture of your family including yourself’; (2) ‘Draw a picture of the things you liked about the usual-care activity/Families for Health activity’; and (3) ‘Draw a picture of the things you did not like about the usual-care activity/Families for Health activity’. Children were asked to explain each of the pictures they had drawn: ‘Tell me about what you have drawn?’ (pictures 1, 2 and 3); ‘Why did you like this?’ and ‘What other things did you like about the activity?’ (picture 2); and ‘Why did not you like this?’ and ‘What other things did not you like about the activity?’ (picture 3). Children had completed questionnaires during the visit and these were also used as prompts to encourage children to talk about any changes they had made to their diet, activity levels and to discuss their feelings and relationships with family and friends. The mean duration of child interviews was 14 minutes (range 2.5–35 minutes).
Data management and analysis
Quantitative data
Data were entered into Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) and SPSS version 22 (IBM SPSS Statistics, Armonk, NY, USA) and were analysed using SPSS. Numerical responses on Families for Health parent weekly evaluation forms to the questions ‘How do you feel about today’s session?’ and ‘How do you feel about the programme?’ were summed to indicate parents weekly ratings of the programme for each response option: ‘awful’, ‘bad’, ‘OK’, ‘good’ or ‘great’. Facilitators’ written responses on the weekly evaluation forms and end-of-training evaluation form were entered into Microsoft Excel and coded into categories by WR and MT.
Qualitative data
Focus groups and interviews with facilitators, parents and children were digitally recorded, subject to permission from each participant. The interviews were transcribed verbatim and all transcripts were anonymised and checked for accuracy against the initial recording. The transcripts were coded using NVivo 10 (QSR International, Warrington, UK). Coding was thematic based on the interview schedules, with the addition of emergent themes. 91 Table 66 describes the codes that were used.
| Qualitative data | Code | Definition |
|---|---|---|
| Facilitators’ focus groups | Dose delivered | The ‘amount’ of intervention provided by the intervention team Deviation from 10 sessions? Changes made to the programme content? What worked well/did not work well Comparisons with other programmes |
| Environment | Physical environment (e.g. venue) Social environment (e.g. group dynamics) |
|
| Parent interviews | Got or not got from intervention unprompted | What they got from the intervention/did not get from the intervention (using unprompted text, i.e. data which are the same for FFH and UC) |
| Child interviews | Child like or dislike about intervention unprompted | What they liked or disliked about the intervention (using unprompted text from the drawing activity for both FFH and UC) |
The qualitative team (FG, WR, MT, JF and AK) met to discuss and agree the coding framework for facilitators’ focus groups. The framework was drafted according to the process evaluation framework components. Owing to issues surrounding blinding (researchers AK and JF may have been unblinded to families by reading facilitators’ transcripts), the coding of facilitators’ focus group and interview data was carried out by WR, MT and FG.
At the early stages of coding parent and child interview data, FG, WR, JF and AK met to review the coded interviews. Initially, 16 parent and child transcripts were reviewed to identify any refinements needed to the coding scheme. FG, WR, AK and JF read four parent and child interviews each, with each transcript reviewed by two researchers. Discussions included defining the parameters of each code, for example to exercise caution when coding the way families talk about their motivations as this may steer away from their experience of the intervention, and adding new codes to the coding scheme, as required. AK and JF carried out the coding of parent and child interview data with regular meetings with FG to ensure coding reliability and to ensure any discrepancies were reconciled.
Focus group data coded at ‘Dose delivered’ and ‘Environment’ were combined. These combined data were coded at the following codes, which were identified from the focus group schedule: adjustments (e.g. content/timing issues); what facilitators thought went well or less well; the impact of group behaviour on delivery; size and composition of group; improvements; and any additional emerging themes. The data for each of these codes were extracted. Codes were then developed into potential themes and reviewed to ensure that the themes worked in relation to the coded extracts. 92 Facilitators’ weekly evaluation forms were checked to identify any additional information that challenged or supported the themes identified.
Constructed variables
Information about how parents heard about the trial was categorised into active and passive recruitment methods. Passive recruitment methods (where participants identify themselves as potential participants) included the media (e.g. adverts in newspapers and radio) and distribution of flyers. Active recruitment methods (where eligible participants are identified and targeted directly) included sending letters to families with an overweight or very overweight child who had been recently measured in the NCMP and referrals from health-care professionals.
Quality checks
Data entry for the weekly evaluation forms and parent end-of-programme feedback forms was checked against the raw data for accuracy (WR).
Interview transcripts were independently coded and at least 10% of the coded interview data ere cross-checked by another member of the research team to minimise researcher bias. The draft coding framework for facilitators’ focus groups was piloted by FG, using two focus groups. MT and WR coded the same two focus groups and discussed agreement. There was reasonable agreement that the broad coding framework worked, but there were discrepancies in relation to dose received and dose delivered. These discrepancies were discussed and resolved by defining dose delivered as facilitators’ experiences of the programme and dose received as facilitators’ perceptions of families’ experiences of the programme.
Twenty-six parent and child interview transcripts were cross-checked. JF and AK independently coded and checked 12 transcripts. JF and AK coded all 12 interview transcripts using the same codes. An additional researcher (TS) cross-checked 14 transcripts that had been coded by JF or AK. There was reasonable agreement and any discrepancies were discussed and resolved by checking the original definition of each code, which resulted in retaining the original coding (JF and AK).
Unprompted parent and child data were used to inform the following research questions:
-
What are the differences/similarities in experience of parents and children in Families for Health compared with usual-care interventions? (Dose received.)
-
Given that Families for Health has a greater emphasis on parenting skills, to what extent is this aspect of the intervention mentioned? (Dose received.)
Results
The process evaluation presented here was carried out prior to the effectiveness data being available from the trial, to avoid bias in interpretation. 85
Seven Families for Health programmes were delivered, and 255 weekly parent feedback forms and 36 parent end-of-programme feedback forms were completed (Table 67). Of the 56 families who were allocated to the intervention arm, 35 completed at least five sessions or more, all of whom completed an end-of-programme parent questionnaire. There were 120 interviews conducted with parents and children during the 3-month follow-up visit from 41 families who attended Families for Health and 21 families who attended usual care (see Table 67).
| Treatment group | Parents | Children | Facilitators | Fidelity | ||||
|---|---|---|---|---|---|---|---|---|
| Number of weekly feedback forms | Number of end-of-programme feedback forms | Parent and child 3-month interview | Parent 3-month interview | Child 3-month interview | Number of weekly evaluation forms | Number of FGs (number of participants per group) | Week fidelity visit was conducted (total number of visits) | |
| FFH | ||||||||
| Site A 1 | 46 | 7 | 7 | 7 | 22 | 2 (4, 4) | 3, 4, 6, 10 (4) | |
| Site A 2 | 40 | 4 | 7 | 7 | 7 | 2 (4, 3) | 2, 6, 9/10a (3) | |
| Site A 3 | 60 | 8 | 5 | 5 | 14 | 2 (4, 4) | 6, 10 (2) | |
| Site B 1 | 29 | 4 | 1 | 4 | 4 | 25 | 2 (1b, 3) | 2, 4, 6, 9/10a (4) |
| Site B 2 | 14 | 4 | 1 | 4 | 4 | 4 | 2 (2, 2) | 1, 4, 6, 8, 9/10a (5) |
| Site C 1 | 21 | 4 | 6 | 6 | 6 | 2 (4, 4) | 1, 4, 9/10a (3) | |
| Site C 2 | 45 | 5 | 1 | 5 | 5 | 23 | 1 (4) | 3, 8, 10 (3) |
| Total | 255 | 36 | 3 | 38 | 38 | 101 | 13 | 24 |
| UC | ||||||||
| Site A | 5 | 4 | ||||||
| Site B | 8 | 9 | ||||||
| Site C 1 | 2 | 2 | ||||||
| Site C 2 | 1 | 2 | 2 | |||||
| Site C 3 | 3 | 3 | ||||||
| Total | 1 | 20 | 20 | |||||
Some of the facilitators’ weekly evaluation forms from five of the programmes were not completed: site A, second running of the Families for Health programme (FFH2) (missing weeks 5–7 and 9–10) and third running of the Families for Health programme (FFH3) (missing weeks 9–10); site B, first running of the Families for Health programme (FFH1) and FFH2 (missing weeks 9–10 and 5–10, respectively); and site C, FFH1 (missing week 2 and weeks 5–10). Thus, there were 101 completed forms. Twelve (of a possible 14) facilitators’ focus groups and one end-of-programme telephone interview were conducted. Two mid-programme focus groups were not conducted (see Table 67).
Recruitment
There were 194 families who expressed interest in the trial and were sent further information. Of those, 115 (59.3%) went on to be recruited and randomised (Figure 23 and Table 68). Active recruitment yielded 85 potential participants, with 43 recruited; passive recruitment yielded 99 potential participants, with 72 recruited (Table 69). A higher proportion of enquires from families recruited passively resulted in recruitment to the study (72.7% vs. 50.6%; p = 0.002). The most productive active method of recruitment was a referral from a health professional, with 69 ‘expressions of interest’ resulting in 30 families being recruited to the study. Information seen at school or the GP surgery accounted for 55 enquiries and over one-third (37.4%) of final recruitment. Media-generated enquiries were few, but all nine resulted in recruitment. Further details of the recruitment to the trial via passive and active methods have been published, alongside examples of the materials. 93
FIGURE 23.
Flow chart of participant recruitment to the FFH study. FFH, Families for Health; UC, usual care.
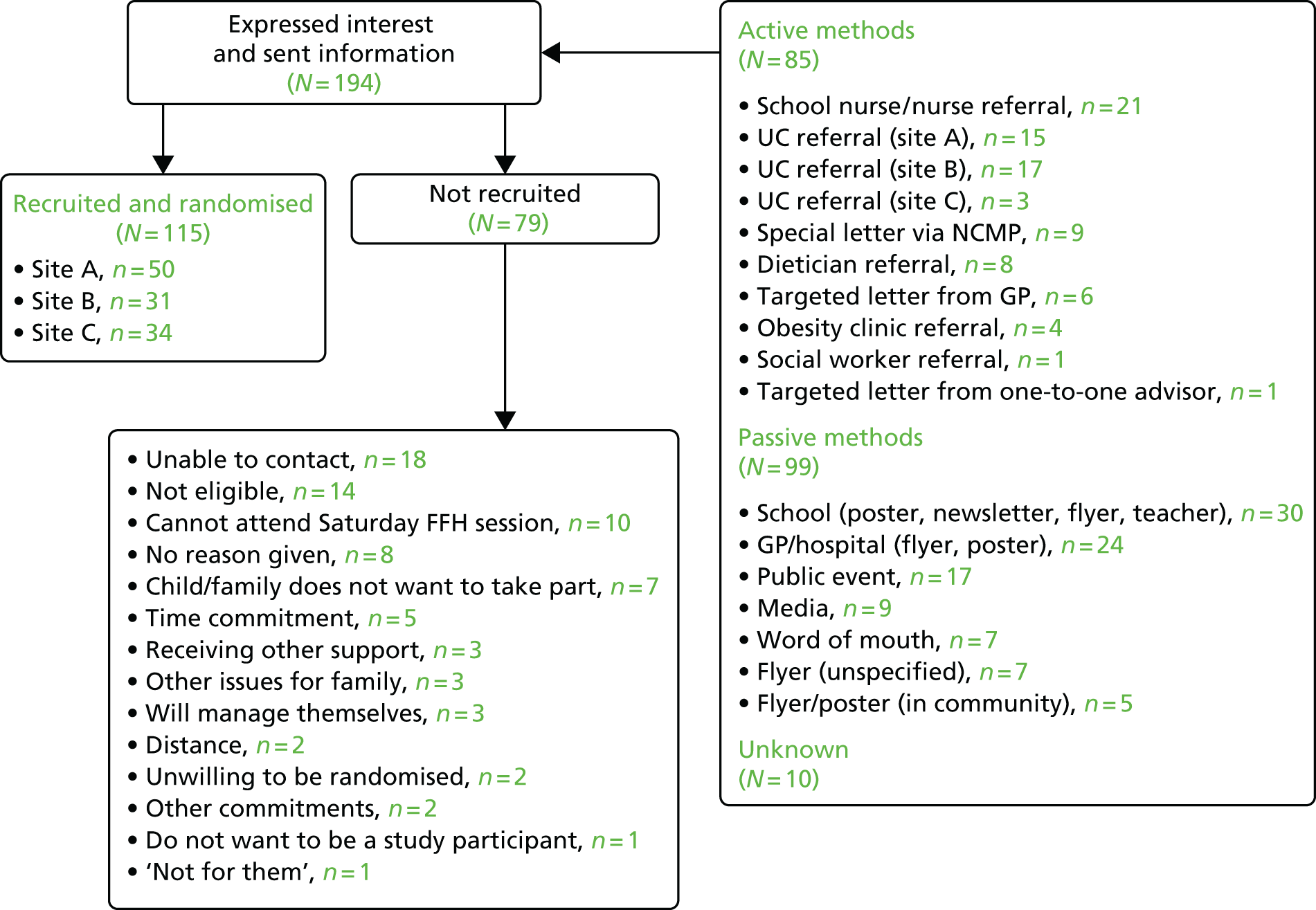
| Project months | Actual month | Numbers recruited | Key recruitment methods useda |
|---|---|---|---|
| 4–6 | March 2012–May 2012 | 19 |
|
| 7–9 | June 2012–August 2012 | 23 |
|
| 10–12 | September 2012–November 2012 | 6 |
|
| 13–15 | December 2012–February 2013 | 14 |
|
| 16–18 | March 2013–May 2013 | 14 |
|
| 19–21 | June 2013–August 2013 | 15 |
|
| 22–24 | September 2013–November 2013 | 13 |
|
| 25–27 | December 2013–February 2014 | 11 |
|
| Recruitment method | Amount/number of times | Numbers expressing interesta | Number of families recruited | % interest to recruitment |
|---|---|---|---|---|
| Active recruitment | ||||
| Referral from health professional | 65 health professionals contacted research team via telephone with family details On four occasions a researcher attended an obesity clinic where the doctor referred the family to speak to the researcher. This resulted in one referral per visit |
69 | 30 | 43.5 |
| Targeted letter from health professional | Information provided with approximately 600 NCMP letters to overweight/obese children 50 letters to children identified on GP lists 50 letters by one-to-one advisor to families on their records |
16 | 13 | 81.3 |
| Subtotal | 85 | 43 | 50.6 | |
| Passive recruitment | ||||
| School (poster, newsletter, flyer) | All primary schools in sites B and C contacted twice, site A three times (telephone call plus e-mail first time, e-mails only second/third time). Flyers sent out on request. Text for school newsletter sent to all. No record available of which were published | 30 | 24 | 80 |
| GP/hospital (poster, flyer) | Two mail outs | 25 | 19 | 76 |
| Community (poster, flyer) | Posters sent to community venues (e.g. library, children’s centres, leisure centres) across all sites – three times | 5 | 4 | 80 |
| Flyer (unspecified) | – | 6 | 2 | 33 |
| Public event | Nine events (10 days) | 17 | 9 | 52.9 |
| Media (newspaper, radio, internet) | Four radio interviews Two paid newspaper articles Five free newspaper/magazine articles Families for Health website City council website Local telegraph twitter Three Primary Care Research Network adverts in newsletter |
9 | 9 | 100 |
| Word of mouth | – | 7 | 5 | 71.4 |
| Subtotal | 99 | 72 | 72.7 | |
| Total | 184 | 115 | 62.5 | |
What were the reasons for not participating in the trial?
The most common reason for families not participating in the trial was that the research team were unable to contact the family after they expressed interest (n = 18, 22.8%). Another important reasons was that the family did not meet the inclusion criteria (n = 14, 17.7%) (see Figure 23). Some of the families (n = 10, 12.7%) were unable to attend on a Saturday when the Families for Health intervention groups were to be run.
Reach
The intended audience for this trial was families with an overweight or obese child aged 6–11 years. All participating children were overweight or obese, mostly obese (Table 70). There was no significant relationship between site and the proportion of obese and overweight children (p = 0.420), but there was a significant difference in baseline BMI z-scores, with site C having the highest mean BMI z-score [site A: mean 2.57 (SD 0.62); site B: mean 2.64 (SD 0.66); site C: mean 2.99 (SD 0.71); p = 0.010]. Asian, black and mixed ethnicity children were a higher proportion of the children recruited than would have been expected from census data for the three sites. However, the prevalence of obesity is higher for Asian, black and mixed ethnicity children in England in the NCMP. 3
| Baseline characteristics | Total |
|---|---|
| Number of families | 115 |
| Number of children | 128 |
| Family type, n (%) | |
| Two parent | 67 (58.3) |
| Single parent (mother) | 46 (40.0) |
| Single parent (father) | 0 (0) |
| Other | 2 (1.7) |
| Parents SES, n (%) | |
| Class 1 | 39 (33.9) |
| Class 2 | 19 (16.5) |
| Class 3 | 36 (31.3) |
| Never worked/long-term unemployed | 21 (18.3) |
| Children’s ethnicity, n (%) | |
| White | 79 (61.7) |
| Black | 10 (7.8) |
| Mixed | 16 (12.5) |
| Asian | 22 (17.2) |
| Other | 1 (0.8) |
| Chinese | 0 (0.0) |
| BMI | |
| Children’s mean BMI, kg/m2 (SD) | 25.86 (4.36) |
| Mean BMI z-score (SD) | 2.71 (0.68) |
| Number of overweight children (%) | 22 (17.2) |
| Number of obese children (% of total at each site) | 106 (82.8) |
| Age (years) | |
| Children’s mean age (median) | 9.44 (9.58) |
| Age range | 5.77–11.78 |
| 5a (%) | 2 (1.6) |
| 6 (%) | 10 (7.8) |
| 7 (%) | 12 (9.4) |
| 8 (%) | 25 (19.5) |
| 9 (%) | 24 (18.8) |
| 10 (%) | 29 (22.6) |
| 11 (%) | 26 (20.3) |
The families recruited to the trial approximately reflected the local population for SES. By contrast, it was clear that a higher proportion of single-parent families were recruited than would have been expected from 2011 census data88 (site A, 46% recruited vs. 9.6% in the census; site B, 25.8% vs. 6.1%; site C, 44.1% vs. 9.4%). In addition, the number of families in which the parents were unemployed was higher than expected from the census data (site A, 16% vs. 7.4%; site B, 6.5% vs. 3.4%; site C, 32.4% vs. 10%).
Dose delivered
Seven Families for Health programmes were delivered: three of them ran for the full 10 weeks and four for 9 weeks. Session cancellations were because of bad weather (two programmes), shortage of facilitators (one programme) and poor attendance (one programme).
Attendance of families
Families allocated to the Families for Health programme were more likely to have attended at least one session than those allocated to usual care (75.0% vs. 40.7%; p < 0.001) (Table 71).
| Programme | Attendance of families (n) | ||||||
|---|---|---|---|---|---|---|---|
| Did not attend | Completed at least one session | Started but later dropped out of interventiona | Completed at least five FFH sessionsa | Completed all sessions offeredb | Did not complete five sessions but did not drop outa | Unknown | |
| FFH | |||||||
| Site Ac (n = 26) | 5 | 21 | 1 | 20 | 7 | 0 | – |
| Site Bc (n = 13) | 2 | 11 | 4 | 6 | 1 | 1 | – |
| Site Cc (n = 17) | 7 | 10 | 1 | 9 | 3 | 0 | – |
| Total (%) (n = 56) | 14 (25) | 42 (75) | 6 (10.7) | 35 (62.5) | 11 (19.6) | 1 (1.8) | 0 |
| UC | |||||||
| Site Ad (n = 26) | 18 | 7 | – | – | – | – | 1 |
| Site Be (n = 16) | 7 | 8 | – | – | – | – | 1 |
| Site C groupe (n = 5) | 1 | 4 | – | – | – | – | 0 |
| Site C groupe (n = 6) | 1 | 2 | – | – | – | – | 3 |
| Site C school nursee (n = 6) | 1 | 3 | – | – | – | – | 2 |
| Total (%) (n = 59) | 28 (47.5) | 24 (40.7) | – | – | – | – | 7 (11.9) |
Adequate attendance for the Families for Health programme was defined as a family attending at least five sessions. It is not possible to define completion for the usual-care group because the number of sessions that were offered is not known for some programmes. Moreover, despite strenuous efforts, attendance data for families in the usual-care arm were available from the service provider for only one area. Two of the usual-care interventions were cancelled during the trial. Self-reported attendance is available for all but seven of the families in the usual-care arm. The other seven families either withdrew from the study or were lost to follow-up. Table 71 shows a breakdown of the attendance of families; 25% of families in the intervention arm and 47.5% of families in the usual-care arm did not attend any sessions. In the intervention arm, 62.5% of families completed at least five sessions (see Table 71).
Facilitators’ perceptions of the delivery of intervention
Both the facilitators’ completed evaluation forms and the transcriptions of the facilitators’ focus groups were used to address two questions: ‘From the perspective of the Families for Health facilitators, what components of the intervention were delivered (or not) as intended by the intervention designers?’ and ‘What group factors (if any) impacted on the delivery of the Families for Health programme?’.
Components of the intervention that were delivered as intended by facilitators
Dietary sessions were delivered as intended across all seven programmes. Snack time, shopping, marketplace, the healthy eating habits quiz, advertising and food labelling were reported to have worked well, promoting discussion and providing the opportunity for parents to share ideas and strategies:
Being able to share experiences and having the opportunity to share recipes on food that parents had made for the celebration.
Site A FFH1, week 10
The snack-time activity encouraged children to taste a range of healthier alternatives and enabled parents to observe their child eating food they do not usually eat. This activity also gave parents the opportunity to apply some of the parenting skills, such as guiding children’s choices and praising positive behaviour:
The snack time was very positive. The children really enjoyed making healthy wraps for their parents. Some children did try new fillings.
Site A FFH1, week 2
. . . I was observing parents . . . they were saying things like ‘you don’t have to eat them all you know’ and things like that . . .
Site B FFH1, week 9
. . . then it’s easier for the parents to say, ‘Oh well done, I’ve never seen you eat that before I’m really pleased,’ she had some blueberries as well and her mum said, ‘oh that’s really good’. And she hugged mum and said, you never say nice things like that to me.
Site A FFH2, week 9
Delivering programme content in a concrete manner, such as creating visual measures of a numerical value, aided parents’ and children’s understanding of food labelling:
. . . they all switched on when I explained that 6 g is a teaspoon, and then they looked and they think, oh three teaspoons, four teaspoons, it made some sense. Because what is 12 g of what, is that good, is it bad, they don’t know.
Site C FFH1, week 9
Two of the venues, sites A and C, had on-site, purpose-built gyms that families had access to as an ‘activity taster’ session, which proved successful and popular. Gym sessions guided children through a different experience of physical activity:
The [site A] gym and given children the chance to experience something new in an environment they feel is safe.
Site A FFH1, week 7
The [site A] gym, we had full attendance that day. They were all there for that . . . they absolutely loved it. It was that difference, and that confidence going through and the parents coming through to see that . . . It was taking them through and saying ‘you just pay and it costs this much and it is this many sessions and they can come as much as they like’ that was a real positive thing.
Site A FFH1, week 10
Other physical activities, such as the parachute game, badminton, volleyball, basketball and outdoor activity taster sessions, were delivered as intended across four programmes (site A, FFH1; site B, FFH1; site C, FFH1, FFH2). These sessions promoted discussion around what constitutes sufficient physical activity and that some activities do not require any equipment or resources.
The parenting skills and emotional well-being sessions were delivered, as intended, across all seven Families for Health programmes. Facilitators felt that the sessions on stress and feelings prompted helpful discussion, were relevant to parents and children and had a solution-focused approach:
Discussing feelings openly – parents comments that this helped lots.
Site C FFH2, week 6
Yes, because we were talking about you know, one of the questions obviously, do you eat around the table as a family, you know, and there was questions around that. And [Mum] was saying my daughter takes food to the bedroom, so she eats up in her bedroom. And I said, well how could you address that? And she said, well first of all she needs to come down and eat with us doesn’t she? Well she’s answered her own question, you know.
Site C FFH1, week 4
I think it gives families a new way of thinking about things as well doesn’t it? . . . It is just new information and new perceptions of just how they cope with each other and perhaps rewarding certain behaviours, giving each other praise.
Site A FFH1, week 10
In one programme, one session on feelings had to be held jointly with parents and children because of a lack of space at the venue. Facilitators reported that this combined session was particularly successful:
And didn’t that work well with the children in the room that week, because dad, a couple of dad’s said . . . ‘I’ve just realised, I don’t listen to my kids. I never ask them how they’re feeling. Might say, have you had a good day, yes, OK, see you later. I never say, how are you feeling today, and how do you feel about school and your teachers, do you like them’ . . . Because the kids were in here saying it in front of their parents . . .
Site C FFH1, week 9
Components of the intervention that were not delivered as intended by facilitators
In four out of seven programmes (site A, FFH1, FFH2; site, B FFH1; site C, FFH1) facilitators experienced difficulties delivering some of the Families for Health activities. These included games for children that were not age appropriate and activities perceived as patronising by parents:
Balancing Act felt like familiar ground to two parents.
Site A FFH2, week 1
Not sure if snacks/lunchboxes/breakfast healthy options is needed as most parents in the group seemed to know and were a bit bored with it.
Site B FFH1, week 4
Portion sizes – complaints at the lack of information relating to portion sizes throughout the day/week.
Site B FFH1, week 5
In one programme there was a particularly strong feeling that some parts of the programme were patronising:
. . . they said that they felt that the parent side of it has been written . . . what has happened is it has been written for children and they adapted the parents bit from the children’s section. They felt that it was aimed at a child’s level by doing things like the attention grabbers. They didn’t like those at all and they felt it was patronising.
Site B FFH1, week 9
The lack of continuity of facilitators at one site was felt to have had negative effects as it prevented facilitators from developing a rapport with parents and children:
And the parents were saying that as well. ‘We have had so many different people.’ And they kind of get used to the individual and their style.
Site B FFH1, week 9
. . . unfortunately the facilitating, you know, I haven’t been able to do them all and we’ve had to swap facilitators, and I think that does disrupt the, sort of the settlement of the children into the groups, in my eyes, you know.
Site B FFH2, week 9
What group factors (if any) impacted on the delivery of the Families for Health programme?
Size and composition of groups
The Families for Health programme was delivered to groups with varied levels of diversity, including children of different ages and educational needs and families from different ethnicities, financial circumstances and sex:
. . . we’ve got a Latvian, a lad from Holland, Asian, you know, really cross mixture there. But what was also obvious I think as well is the support they gave each other . . . And I think because their association, you know, they all had eating . . . bad eating habits or didn’t make necessarily positive choices, I think they could associate with each other, they didn’t feel as if they were being singled out.
Families for Health (site deliberately omitted)
Facilitators experienced difficulties delivering the Families for Health programme to children across a broad age range (6–12 years) and range of behaviour issues in four programmes (site A, FFH1, FFH3; site B, FFH1, site C, FFH1):
We had children who were six coming in. It takes them a bit longer to talk. We had two children with autism so therefore you couldn’t pressure them. And if they got on a roll and needed to talk about something, you had to sit and you could almost feel yourself going ‘Oh my God, I am going to have to rush you now’. . . . You have to let these children speak else there is no point. . . . I feel we had to let something go each week. I don’t think we ever got everything in fully . . .
Families for Health (site deliberately omitted)
Group dynamics varied and included family members with negative behaviours (site A FFH1, FFH3; site B, FFH1; site C, FFH1). Facilitators employed a range of strategies to manage dominant group members and where groups had members displaying two extremes of behaviour.
Negative, dominating individuals stifled the group and limited discussion, whereas positive characters, such as confident children, encouraged others to participate in activities:
. . . and we had really, really good laughs, positive feedback from the three families. Within seconds of this lady coming in, I’ve done it, she sat there, they basically withdrew. You could see them . . .
Site C (site deliberately omitted)
He’s quite confident and they follow his lead with the joining in, he’s not at all scared, you know, or embarrassed to sort of join in . . .
Site A FFH3, week 6
A small group size was a barrier to delivering the intervention in three out of seven programmes (site B, FFH1, FFH2; site C, FFH1). Facilitators found it difficult to deliver sessions with a small number of children and to encourage participation among quieter children. Small group sizes also restricted the number of ideas and strategies parents and children could employ to engage in a healthier lifestyle:
Struggled due to only three children
Site C FFH1, week 3
. . . do feel that it would be better if more children attended to cause more discussion.
Site B FFH1, week 3
. . . it doesn’t help in the games and things like that because a lot of it is about confidence building and getting to know other members of the group, and when there’s only two or three children it’s difficult to do that.
Site B FFH2, week 9
Fidelity
As described in Methods, a number of fidelity checks were carried out. The programmes varied in the extent to which they were delivered as prescribed (Table 72). Three Families for Health programmes (site A, FFH1, FFH2; site B, FFH1) had only very minor changes, including adapting songs and games for children so that they were age-appropriate and giving parents as well as children pedometers.
| FFH group | Delivered as prescribed in handbook? | Adjustments during programme | Examples of adjustments (quotes from facilitator focus groups) | |
|---|---|---|---|---|
| Major | Minor | |||
| Site A FFH1 | Yes | Only very minor changes (e.g. some songs too young for the group so adapted) | . . . we changed to have the puppet persona dolls rather than animals cause they are a slightly older group and I think . . . they responded much better to the dolls that were more life-like | |
| Site A FFH2 | Yes | Only very minor changes [e.g. parents given pedometers as well as children (adopted for future programmes), adapting songs to accommodate varied ages of children] | . . . we’ve got them from 6 to 13 . . . We have to up some of the language . . . it’s a lovely song for 3 to 6 but not 6 to 13 | |
| Site A FFH3 | Partial | Activity tasters spread out over the 10 weeks, not at weeks 7, 8 and 9. Some difficulty with matching the content the children and parents were doing each week | . . . we had a parent query about screen time saying, well we’ve covered it already upstairs, why have you not looked at screen time, so I’m not sure my children have got the lesson around not eating in front of the telly | |
| Site B FFH1 | Yes | Delivered as per handbook but there was an issue that parents felt that the variation in the knowledge of the group was not being addressed and that they were making slow progress/plateauing. Note: difficult to adapt manualised programme for varied ability groups | ||
| Site B FFH2 | No | Activity tasters brought forward to week 4, rather than weeks 7–9 as children and parents felt there should be more structured physical activity. Felt that this should help with attendance 2 weeks not delivered (lack of attendance) |
. . . I mean even from the start we never had enough parents to split into two groups and things, which it asks you to do at a few points in the programme | |
| Site C FFH1 | Partial | Because of small numbers, and distraction in the hall, did a few sessions combined with parents/children together. This was perceived to have worked well Minor adjustment included use of visual aids to support content delivery |
. . . I explained that 6 g is a teaspoon, and then they looked and they think, oh three teaspoons, four teaspoons, it made some sense. Because what is 12 g of what, is that good, is it bad, they don’t know . . . Visual aids work . . . And I brought along visual aids to match the sessions . . . | |
| Site C FFH2 | No | Up to week 5 in the children’s session: games substituted with organised physical activity (e.g. badminton). From week 5: Active8 (Wolverhampton City Council, Wolverhampton, UK) for children (1 hour of physical activity). Programme: ‘theory’ hour (sticking to overall content), family break, 1 hour of physical activity Gym sessions for parents (1 hour) Puppets’ role-play not age-appropriate |
. . . we kind of did everything that we needed to do for the programme but due to the games we just adapted it to the age of the child . . . | |
Partial adjustments to programme delivery included incorporating activity taster sessions across the entire programme instead of during weeks 7–9 for one Families for Health programme (site A, FFH3). In one programme the parent and child sessions were combined because of poor attendance and one programme added visual aids to support content delivery (site C, FFH1).
Two Families for Health programmes varied the way in which programme content was delivered. One programme (site C, FFH2) adapted content delivery to include a theory hour followed by a more structured physical activity hour because there was a gym available on the site. This adjustment to programme content appeared to work well:
Gym sessions have enhanced the weight loss and motivated the group lots.
Facilitator weekly evaluation form, site C, FFH2, week 6
Another programme (site B, FFH2) adjusted delivery because of poor attendance, by combining parent and child sessions and facilitating whole-group discussions instead of small-group activities. Activity taster sessions were also brought forward in this programme in the hopes of improving attendance, but this failed.
All facilitators who delivered the Families for Health programme attended the 4-day training programme, with the exception of one facilitator who joined the programme as a facilitator after the training workshop had been delivered. The training they received was perceived positively (see Appendix 1). Almost all facilitators delivered more than one Families for Health programme and facilitators’ confidence increased as they became more experienced in the delivery of Families for Health programmes (site A, FFH2, FFH3; site B, FFH1, FFH2; site C, FFH2). They found they could modify games easily and avoid issues they encountered previously:
It seemed to run a lot smoother this time than it did last time, and I think because that we had so many negatives the last time we’ve kind of turned it into a positive this time.
Site C, FFH2, week 10
The Families for Health programme is designed to be delivered in a group setting with a minimum of eight families not waiting more than 3 months to join a programme, and four facilitators (two for parents and two for children) delivering each session. This was not always achieved (Table 73). Three Families for Health programmes (site B, FFH1 and FFH2; site C, FFH2) could not be delivered as intended because of small numbers of families starting the programme and subsequent poor attendance. Two Families for Health programmes (site B, FFH1 and FFH2) lacked continuity of facilitators and one session (site B, FFH1) had to be cancelled because of a lack of facilitators.
| FFH group | Delivered as intended? | Length of main programme | Half-term break? | Number of follow-up session(s)? | Timing | Number of families randomised (attended) | Number of families waiting > 3 months for programme to start | Number and continuity of facilitators |
|---|---|---|---|---|---|---|---|---|
| Site A FFH1 | Yes. No family waiting for > 3 months; eight families started the programme | 10 weeks | Yes | 2 | Saturday morning | 8 (8) | 0 | Six facilitators in total across 10 weeks Good continuity of facilitators with little variation each week |
| Site A FFH2 | Partial. Of the eight families randomised, five families had been waiting > 3 months and six attended | 9 weeks (one week cancelled because of snow) | Yes | 1 | Saturday morning | 8 (6) | 5 | Five facilitators in total across 10 weeks Good continuity with same parent facilitators every week and little variation of child facilitators |
| Site A FFH3 | Partial. Of the 10 families randomised, four families had been waiting > 3 months and seven attended | 10 weeks | No | 1 | Saturday morning | 10 (7) | 4 | Five facilitators in total across 10 weeks Good continuity with little variation of facilitators each week |
| Site B FFH1 | No. Of the six families randomised, one family had been waiting > 3 months and five attended. Small group from the start that became less viable | 9 weeks (one week cancelled because of shortage of facilitators) | Yes | 0 | Saturday afternoon | 6 (5) | 1 | Six facilitators in total across 10 weeks Lack of continuity of parent facilitators. Lack of facilitators so had one facilitator for parents group (also because of low number of families) |
| Site B FFH2 | No. Of the seven families randomised, two families had been waiting > 3 months and six attended. Poor attendance. For 3 weeks, only one family came | 9 weeks (shortened because of lack of attendance) | No | 0 | Saturday afternoon (perceived to be an issue for attendance by facilitators) | 7 (6) | 2 | Seven facilitators in total across 10 weeks Lack of continuity in children’s facilitators |
| Site C FFH1 | Partial. Of the seven families randomised, five had been waiting > 3 months and only four families attended. Small numbers from the start. Small numbers from the start, so difficult to split into subgroups for activities | 9 weeks (one week cancelled because of snow) | No | 1 | Saturday morning | 7 (4) | 5 | Five facilitators in total across 10 weeks Good continuity of facilitators with virtually no variation each week |
| Site C FFH2 | Partial. Of the 10 families randomised, four had been waiting > 3 months and six families attended | 10 weeks | No | 1 | Saturday morning | 10 (6) | 4 | Four facilitators in total across 10 weeks Good continuity of facilitators with virtually no variation each week |
Delivering the programme as a single facilitator for the parents group for one programme that had a small number of families restricted interaction with families as the facilitator was too busy delivering the programme activity to attend to other issues, such as families that were experiencing problems, late arrivals or preparing for the next activity (site B, FFH1).
Of the 56 families that were randomised to the Families for Health intervention, 21 (37.5%) waited more than 3 months before starting a Families for Health programme (see Table 73). Other issues that were encountered included external service providers for the physical activity tasters undermining the message of the programme by rewarding children with chocolate (site B, FFH1), room booking issues at one venue, resulting in there being no suitable rooms to deliver one of the children’s sessions (site A, FFH3), shorter activity sessions because of the late arrival of the coach for one of the activity tasters (site A, FFH3) and cancelling one session because of snow (site A, FFH2; and site C, FFH1).
Only one Families for Health programme (site A, FFH1) was considered to have delivered entirely as intended (see Table 73). The programme started with eight families, there was good continuity of trained facilitators and 10 sessions were delivered.
In conclusion, although the delivery of the programmes varied, all programmes were delivered at a level that is at the top of the range of what could be expected if such an intervention were being routinely delivered by local authorities. The problems encountered were no greater than could be expected. The outcome data from each site were analysed individually, but there were no significant differences in outcome. Thus, there is no evidence to suggest that variations in adherence to the intended delivery had affected outcomes.
This was a pragmatic trial, aiming to test effectiveness in a routine setting rather than efficacy in an ideal setting and, as such, variation in delivery should be expected.
Dose received: parents
The Families for Health programme was generally well received by those families who attended. Over 90% of responses from parents rated the weekly sessions as ‘good’ or ‘great’ (Table 74). The topics that received the most positive comments were ‘Food labels: what do they mean?’, ‘Stress – and what we can do about it’ and ‘Building self-esteem’ (Table 75). At the end of the programme, parents were similarly positive, with > 80% of those who completed the end-of-programme questionnaire rating the programme as ‘good’ or ‘great’. Parents rated physical activity and food topics particularly highly. Three topics were rated as 4 or 5 by > 90% of respondents: ‘Food groups: Eatwell plate’ (90.6%), ‘Shopping: surviving at the supermarket’ (93.8%) and ‘Portion sizes’ (90.6%).
| Week | Total | How do you feel about today’s session? | How do you feel about the programme? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Awful ☹ | Bad | OK 😐 | Good | Great ☺ | % good or great | Awful ☹ | Bad | OK 😐 | Good | Great ☺ | % good or great | ||
| 1 | 36 | 0 | 0 | 2 | 17 | 17 | 94.4 | 0 | 0 | 3 | 20 | 13 | 91.7 |
| 2 | 34 | 0 | 0 | 5 | 14 | 15 | 85.3 | 0 | 0 | 4 | 15 | 15 | 88.2 |
| 3 | 32 | 0 | 0 | 2 | 16 | 14 | 93.8 | 0 | 0 | 2 | 16 | 14 | 93.8 |
| 4 | 31 | 0 | 0 | 5 | 11 | 15 | 83.9 | 0 | 0 | 1 | 16 | 14 | 96.8 |
| 5 | 31 | 0 | 0 | 4 | 17 | 10 | 87.1 | 0 | 0 | 2 | 17 | 12 | 93.5 |
| 6 | 22 | 1 | 0 | 3 | 9 | 9 | 81.8 | 0 | 1 | 1 | 7 | 13 | 90.9 |
| 7 | 25 | 0 | 0 | 0 | 10 | 14 | 100 | 0 | 0 | 0 | 10 | 15 | 100 |
| 8 | 28 | 0 | 0 | 0 | 14 | 14 | 100 | 1 | 0 | 2 | 12 | 13 | 89.3 |
| 9 | 13 | 0 | 0 | 0 | 4 | 9 | 100 | 0 | 0 | 0 | 4 | 9 | 100 |
| Total (%) | 1 (0.4) | 0 | 21 (8.4) | 112 (44.6) | 117 (46.6) | 91.2 | 1 (0.4) | 1 (0.4) | 15 (6.0) | 117 (46.4) | 118 (46.8) | 93.2 | |
| Week | Total | Main weekly topics | Times (%) mentioned as ‘useful or enjoyable’ | Times (%) mentioned as ‘not useful or enjoyable’ |
|---|---|---|---|---|
| 1 | 36 | What is health? | 3 (8) | 0 (0) |
| Balancing act 1: energy in, energy out | 2 (6) | 1 (3) | ||
| Let’s look after ourselves | 0 (0) | 0 (0) | ||
| 2 | 34 | Discipline (including setting limits and praise) | 12 (35) | 0 (0) |
| Balancing act 2: food our bodies need | 15 (44) | 1 (3) | ||
| 3 | 32 | Family guidelines and rewards | 10 (31) | 0 (0) |
| Finding our power for health (focus on physical activity) | 9 (28) | 0 (0) | ||
| 4 | 31 | Our eating habits | 12 (39) | 1 (3) |
| Children’s choices | 9 (29) | 0 (0) | ||
| 5 | 31 | How much we eat (portion sizes) | 12 (39) | 2 (7) |
| Building self-esteem | 18 (58) | 0 (0) | ||
| 6 | 22 | Thinking about feelings | 10 (46) | 1 (5) |
| Active alternatives to staring at the screen | 0 (0) | 0 (0) | ||
| 7 | 25 | Stress – and what we can do about it | 13 (52) | 0 (0) |
| Coming to our senses | 1 (4) | 0 (0) | ||
| Surviving at the supermarket | 4 (16) | 0 (0) | ||
| 8 | 28 | Food labels: what do they mean? | 21 (75) | 2 (7) |
| Labelling our children | 0 (0) | 0 (0) | ||
| 9 | 13 | From problem to solution | 4 (31) | 0 (0) |
| A healthy lifestyle or a life of diets? | 1 (8) | 0 (0) | ||
| Meeting the challenge of special occasions | 0 (0) | 0 (0) | ||
| 10 | 3a | Scaling the ladder to health | 0 (0) | 0 (0) |
| We are stars | 1 (33) | 0 (0) | ||
| Party celebration | 0 (0) | 0 (0) |
In the written responses to open-ended questions, it was clear that meeting other parents was valued by many participants. In the first week of the programme, three-quarters of parents (27/36) mentioned this as a positive aspect of the session:
Getting ideas how other parents do certain things. Tips to learn from.
Lots of thoughts and advice from others and realising your not alone!
Giving some ideas. Finding other people with the same problems.
Getting to know other parents and realising we all feel the same. Can’t wait for next week.
Nearly two-thirds (61.8%) of parents felt that the programme had helped their children tackle their weight difficulty ‘definitely’ or ‘a lot’, and a similar proportion (63.7%) felt that the programme had helped the rest of the family ‘definitely’ or ‘a lot’. When asked whether or not they had noticed changes in their child as a result of the programme, 70.6% responded ‘definitely’ or ‘a lot’.
Thematic analysis of parents’ response to the unprompted question ‘What did you get out of it?’ revealed similarities and differences in parents’ experiences of the Families for Health and usual-care programmes. Many parents talked about their experience of food topics, physical activity and gaining ‘no new experiences’ as a result of the programme. Parents attending a Families for Health programme also discussed getting support from other parents and raising their child’s awareness.
Food topics
Parents attending Families for Health identified information on food labelling as particularly useful in making informed choices, for example realising that foods they had previously thought of as being ‘healthy’ were not necessarily so and learning which foods were in fact ‘healthy’:
I found it very beneficial. Um, food portion sizes, um food value. Sometimes some of the labels and design packaging was very misleading . . . you think, oh yes that looks healthy, and then I think, no it’s not. And I don’t buy it.
F112, site C, Families for Health, eight sessions attended
Parents attending Families for Health were also positive about being introduced to new recipes and learning about correct food portion sizes:
I found out my portion sizes were just too big for children their age. So I learnt reference points and I have cut down on my portion sizes although when I did do them to exactly what they said they just looked so tiny and I was thinking, ‘my kids are never going to eat this, they are going to eat that and they’re going to be ravenous afterwards’. So I still probably give a little bit more but it is probably half of what they used to have.
F84, site B, Families for Health, five sessions attended
For parents attending two ‘usual-care’ community group-based programmes in sites A and B, there were similar discussions, with parents gaining an increased awareness of what was in food and making more informed choices over the types of food they now chose and bought:
Full-fat coke [Coca-Cola®: The Coca-Cola Company, Atlanta, GA, USA]. I drink it by the tin load. And they did a session on how much sugar was in fizzy drinks and your chocolate and stuff that I tell you what . . . I was very surprised. It was an eye opener. Admittedly, I have cut right down and I am actually on diet coke now.
F22, site A, usual care
Site B usual-care provision offered a one-to-one service whereby an advisor would advise families on how to swap meals to healthier alternatives:
You know, she [usual-care advisor] tells us about different meals, how you can swap things.
F82, site B, usual care
Physical activity
Unlike parents attending a Families for Health programme, parents attending a usual-care intervention were more likely to talk about exercise and physical activity when discussing their experience of the programme:
But yeah the exercise was good. At times he really did enjoy it, sometimes I even joined in for one or two sessions if I didn’t have [younger son] with me, yeah they played dodge ball and whatever.
F27, site C, usual care
The importance of exercise was stressed and how to be active without the need to spend money, for example going for a walk or exercising at home:
. . . and it was just about trying to be more active really from the [usual-care programme] that we got that. You know even just at home you know he could be at home here doing his running up and down and, like we’re at home and I’ll say to him, oh come on let’s do some star jumps or something.
F49, site C, usual care
Similar to being able to offer practical advice on what foods to eat, the one-to-one advisor at site B was able to give usual-care parents specific details of exercise classes that were available in the local area, such as dance classes, or exercises which families could do at home.
Nothing new
Four parents claimed to have learnt nothing new from the Families for Health programme, and a further two felt that they already knew a lot of the information they were given, but they did not necessarily want to change their behaviour:
Because the problem was never that I didn’t know what to eat, and the problem was never I couldn’t afford the right foods or, you know, because I didn’t go to the right supermarket, and that was never the issue. The issue was I just didn’t want to eat those kinds of foods and that hasn’t changed, I still don’t want to eat wheat and nuts and fruits and dried apricots and, you know, prunes.
F57, site C, Families for Health, five sessions attended
For the parents who had accessed usual care and the programme had run, no-one thought they had learnt nothing new.
Support from others
Parents attending a Families for Health programme often talked about the positive experience of meeting other parents who were in a similar situation, and being able to talk about common problems and sharing ideas:
But it was interesting, other parents saying things about their children and you think, you know, that’s what [child A]’s like, so that was interesting. It made it feel like it wasn’t just you.
F59, site B, two sessions attended
I liked when people shared ideas about what they do and I could say what I do and, you know, that was nice.
F100, site A, Families for Health, 10 sessions attended
Raising child’s awareness
Some parents spoke of how attending the Families for Health programme had made their child more aware of themselves in relation to healthy eating and exercise, and more aware of the choices they are making:
And he’s [son] been more aware of . . . conscious of foods that he’s eating and doing a little bit more exercise and stuff like that . . .
F109, site A, Families for Health, seven sessions attended
Overview of sessions
One parent described how they felt they got something from each session, and that the programme was ‘complete’:
We got a lot, didn’t we? We just got . . . it was mind boggling because it was just making you aware of the labels on foods, cooking of foods . . . Food content, just . . . I mean we went out and bought a halogen oven because we found it would be better cooking. Just portion size . . . Snacking yes, snacking was one of the things that we’ve . . . I mean like even down to praising your child, self-esteem, it was a complete . . .
F99, site C, Families for Health, six sessions attended
Parents who attended a usual-care programme did not discuss group support, raising child’s awareness or a positive overview of all sessions.
Parenting skills
A unique feature of the Families for Health intervention was the incorporation of a parenting theme. A few parents discussed how their parenting had been influenced by attending the Families for Health programme, including knowing what to say to their child and being stronger at saying no:
. . . it helped me think about [child A] a bit more what to say and what not to say around him and maybe just tweaking, bettering my parenting skills, it was really good for that.
F109, site A, Families for Health, seven sessions attended
. . . it’s made it a bit easier for me to be stronger at saying to her [daughter], no.
F26, site A, Families for Health, nine sessions attended
Parenting skills were not spontaneously discussed by families attending a usual-care intervention.
Dose received: children
Children’s unprompted comments on the Families for Health programme and usual-care intervention
Many children (17 from Families for Health and two from usual care) reported enjoying all of the programme they attended:
I just put, I enjoyed all of it and like . . . it was really fun.
F61, FFH3, site A, 10 sessions attended
I’ve written that I loved everything. I didn’t dislike anything that we done there because it was all fun and stuff, it wasn’t boring.
F47, FFH1, site C, nine sessions attended
I didn’t not like anything about the [usual-care] programme really.
F33, usual-care group, site C
Some children did not enjoy the programme they attended because of the age difference between themselves and the other children attending:
I didn’t really like anything because like I felt that I was like a bit of an odd one out because I was like the oldest and I felt a bit like enclosed and that over things because they were like all younger kids that liked the same things . . .
F79, FFH2, site B, one session attended
Well they were all a bit too young and they treated us a bit like babies . . . And because they were young they treated you like everyone was young.
F28, FFH1, site B, four sessions attended
One child felt uncomfortable because he was the only child present at a usual-care support service in site C:
. . . it was only me as a child there. They were all women really . . . and I felt uncomfortable with that. Because . . . being the only child is quite weird.
F25, usual-care group, site C
Food topics
With the exception of two usual-care services in site C (school nurse or group programme with adults), children in all programmes talked about their experience of learning new dietary information as a fun and useful activity. This included getting information on ingredients in snacks that one child described as ‘shocking’ and another child described as the ‘. . . secrets in the food’ (F8, usual-care group, site C):
Well we played the shopping game and she brought lots of food in and it is like unhealthy and healthy and me and my friend, we each had a big bag and we had to put . . . there was lots of stuff on the table and everything we thought was a good choice we had to put in the bag but you could put anything in the bag you liked and would be a good choice. And we put all the unhealthy stuff in and the healthy stuff! But that was probably the best game.
F28, FFH2, site B, four sessions attended
. . . each week there was a target sheet to do with it and we would talk about what is going wrong with our health, how we can improve it. One week we had a task we had to do . . . we were given labels of bad food products and we had, through label reading, and we looked at them and we read the amount out and we matched them to the amount of salt and sugar. And Doritos have 58 g of sugar . . . I was shocked.
F40, usual-care group, site C
Children attending a Families for Health programme had enjoyed drawing activities that required children to apply their new dietary knowledge on the creation of a healthy cereal including food labelling considerations for the packaging, shopping games and the Eatwell plate activity. Opportunities to try different food were received positively by children attending a Families for Health programme and the group-based usual-care intervention at site C. Children tried food they had never previously consumed, which one child explained resulted in healthier snack consumption:
I liked it at break time we got to try new stuff . . . Tomato juices and other juices . . . I’ve eating a lot more fruit.
F57, FFH1, site C, five sessions attended
. . . we tried like loads of fruit and stuff and vegetables that I’d never had before.
F100, FFH3, site A, 10 sessions attended
I like the [usual-care] programme because it was fun and I like meeting people and playing games and trying new food.
F33, usual-care group, site C
Making snacks and developing cooking skills were also popular in the Families for Health programme:
I enjoyed making the snacks, especially the week when we made the puff pastry pizzas. And on the last week we did like the party and stuff, everyone had to bring something and we made the pizzas and everyone loved them . . . I liked all of it, but that was the bit that I liked the most.
F61, FFH3, site A, 10 sessions attended
Because it was near lunch the children most of time got to make snacks like yoghurt and fruit and then we’d also have like a kind of fake shopping trip where you’d look at the prices and what’s inside of them. And then we’d actually taste what we’d made.
F56, FFH3, site A, seven sessions attended
Physical activity
Children who had attended a Families for Health programme or the group-based usual-care intervention at site C were more likely than other children to talk positively about physical activity. They talked positively about a range of sports activities including football, trampolining, dodgeball and gym activities:
I liked the gym . . . The equipment and how you get to like go on them.
F8, FFH1, site C, six sessions attended
I liked all the guests that came in because they were teaching us different sports and different games and I didn’t know how to play them before and then they taught us how to play them.
F26, FFH2, site A, nine sessions attended
I liked it how the children could choose what we’re going to do, all the sports . . . We did badminton, tennis, um . . . I think it’s called, and volleyball I think.
F86, FFH2, site C, 10 sessions attended
Children that attended a usual-care one-to-one support service in site B did not mention engaging in physical activities during the intervention, but were positive about the resources they received that encourage physical activity:
Well once my friend came over and [advisor] gave us this wheel thing, and when you wheel it there’s like some games that you have to play or dares, like run about or something with them. I did it with my friend and we played it outside in the garden . . . It was good.
F83, usual-care, site B
Children also talked about negative experiences of different physical activities, which mainly depended on whether they found the game easy or difficult. Children also commented not having enough time to use all of the gym equipment when this was an option. One child explained that he disliked other children, commenting on his ability to take part in the running and star jumps activity:
. . . there’s some stuff that I cannot do and there’s some stuff they cannot do. And say if I cannot do it they are sort of, ‘ah he’s too fat’ and all that to do it, and that was kind of annoying.
F27, usual-care group, site C
Balloon races . . . it is hard and not using the equipment that much . . . it was only 2 days. The first and second week that we used them and that was it really. I think the problem was the machines weren’t working properly.
F40, usual-care group, site C
There was not enough time and um we didn’t get much on the gym . . . To like do everything because there was that many stuff in the gym we could get around quick enough.
F72, FFH2, site C, three sessions attended
Children attending a Families for Health programme talked about feeling disappointed that they were unable to access the gym as they were too young, and children who did access the gym did not enjoy sharing the gym facilities with children who were not attending the Families for Health programme:
And there was one session where we were supposed to go out of the room and into the gym and we couldn’t do it because the age limit was 8–15 but some people were . . . two people were under that age, so we missed out on that one . . . We felt let down.
F56, FFH2, site A, seven sessions attended
I didn’t like the different exercise machines up in the fitness room because we had to do it with different children that we didn’t know so we had to talk to them even though we didn’t know them . . . I thought we were just going to do it with just the people in the programme but we didn’t.
F75, FFH2, site C, nine sessions attended
Social
The social aspect of making new friends was a positive experience for children attending a Families for Health programme and a group-based intervention at site C:
My friends from what I met in, the children . . . I like to have the friends because that means that I don’t have to do it on my own. And I have people to play with.
F26, FFH2, site A, nine sessions attended
Meeting new people there, friends and stuff. And having fun and playing games.
F33, usual-care group, site C
Having something to do on a Saturday . . . [would normally] . . . Sit at home, watch TV or we’d go shopping, and that’s it.
F56, FFH2, site A, seven sessions attended
Some children attending a Families for Health programme talked about feeling anxious or isolated as they did not know other group members, which limited their level of interaction:
. . . at first there was one where I felt a bit isolated because I didn’t know anyone . . . until about the third or fourth session. I did start to open up but I didn’t really talk during the sessions.
F56, FFH2, site A, seven sessions attended
I get quite shy sometimes, so I didn’t like it just myself because I got quite shy. Because if I’m alone with kids like that it’s quite scary for me.
F59, FFH2, site B, two sessions attended
Structure of sessions
Some children found some of the sessions boring, overlong and included too much talking. Children attending a Families for Health programme and the group-based usual-care intervention at site C did not enjoy the activities that involved sitting and writing, and children accessing the usual-care intervention in site B felt that too much talking was involved:
I didn’t like it because it was a bit too long . . . It’s just its 2 hours and a half, which is . . . just under half a quarter of your day wasted.
F102, FFH2, site B, four sessions attended
I got a lot bored with the [usual-care] programme because you just sit there until the end because it was too long hours, it is 3 and a half hours . . . so I’d rather stay at home, play outside or do something like that. Or go to the gym or something.
F27, usual-care group, site C
I know you have to talk like to explain stuff, but the thing is . . . it’s too much talking.
F83, usual care, site B
Current knowledge was not taken into consideration for one child accessing a usual-care intervention that involved a one-to-one advisor:
. . . it was boring . . . The lady was just going on about the same thing. And she was . . . she wasn’t really taking in that I knew the stuff already because I said that I know about the cycle thing and I could tell my Mum . . . Like I knew about this from my school . . . And I already had this [showed two resources].
F48, usual care, site B
Discussion
All Families for Health programmes were delivered broadly as planned and any deviations to programme delivery did not deviate from the intended aims of the original programme. Attendance at most Families for Health programmes was reasonable, and better than usual care in terms of the ‘did not arrive’ rate, and most of the parents and children who attended felt positive about the programme.
Families recruited to the trial broadly reflected the target population at each site, but the overall slow recruitment and the number of families who could not manage a Saturday morning session indicate some of the problems in providing any group-based intervention to families with young children.
Activities that did not work well when delivering the Families for Health programmes included games that were not age appropriate for children and activities that some parents perceived as patronising. Small group sizes, because of the difficulties in recruitment and subsequent poor attendance, were a barrier to successful programme delivery as many activities did not work well when there were not enough families.
This process evaluation revealed the importance of consistent, trained and experienced facilitators for programme delivery. A lack of continuity of facilitators had a negative impact on dose delivered, as this was disruptive for parents, children and facilitators. Beidas and Kendall94 critically reviewed studies exploring the factors involved in the training of therapists that are required to adopt and implement interventions, and found that appropriate training should emphasise the ‘underlying spirit’ of the treatment rather than solely focus on techniques. 94 Most Families for Health facilitators were positive about the programme. As facilitators became more experienced in delivering the Families for Health programmes, they became more confident and experienced fewer difficulties when delivering the programme.
This process evaluation demonstrates that parents and children attending a Families for Health programme enjoyed interactive sessions in relation to diet, which enabled children to develop cooking skills and provided parents with an opportunity to practise parenting skills learnt during the programme. Parents reported increased confidence and being stronger at saying no to their child as a result of attending the Families for Health programme, which is similar to parents’ perceptions of the Webster-Stratton parenting programme. 95 Other similarities include parents’ perception that the programme was unsuitable because nothing new was learnt and that the course would have been more effective if partners had attended the programme. Parents who attended a usual-care programme talked about food topics and physical activity positively, but did not talk about improvements in parenting skills.
There were similarities in children’s experiences of attending a Families for Health programme and community group-based usual-care services. Children talked about learning new dietary information, accessing gym facilities and making new friends as positive features of the programmes. Older children’s experiences of having younger children in Families for Health groups were negative, as games were modified to accommodate the needs of younger children. This is consistent with facilitators’ challenging experiences of delivering the programme to a varied group of children ranging from 6 to 11 years. Facilitators’ experiences in the Hunter Illawarra Kids Challenge Using Parent Support (HIKCUPS) trial suggested that future programmes should specifically cater to children of similar age or same sex. 96 Facilitators of the Families for Health programme experienced similar difficulties delivering the programme to a wide age range of children, but did not encounter difficulties with mixed-sex groups. In this trial it proved difficult to assemble groups of adequate size even with this wide range of child ages, and small group size had a negative impact on the functioning of some groups. A narrower band of child ages would have made this situation worse, but might be practical in a non-trial setting where half the number of families need to be recruited to launch each group.
In conclusion, the Families for Health programme was implemented reasonably well and pretty closely to what was planned to the intended population, and any adjustments to programme activities did not deviate widely from the original aims of the handbook activities. Facilitators’, parents’ and children’s experiences of the Families for Health programme were largely positive.
Chapter 7 Discussion and conclusions
Summary of findings
The Families for Health programme focuses on a parenting approach, designed to help parents develop their parenting skills to support lifestyle change within the family and to help children manage their weight. Within the care pathway for weight management services, Families for Health is a level 2 targeted weight management service. 8 The logic model97 shown in Figure 24 was developed prior to analysis of data, to demonstrate how the Families for Health intervention is intended to work.
FIGURE 24.
Logic model for the FFH intervention. FFH, Families for Health.
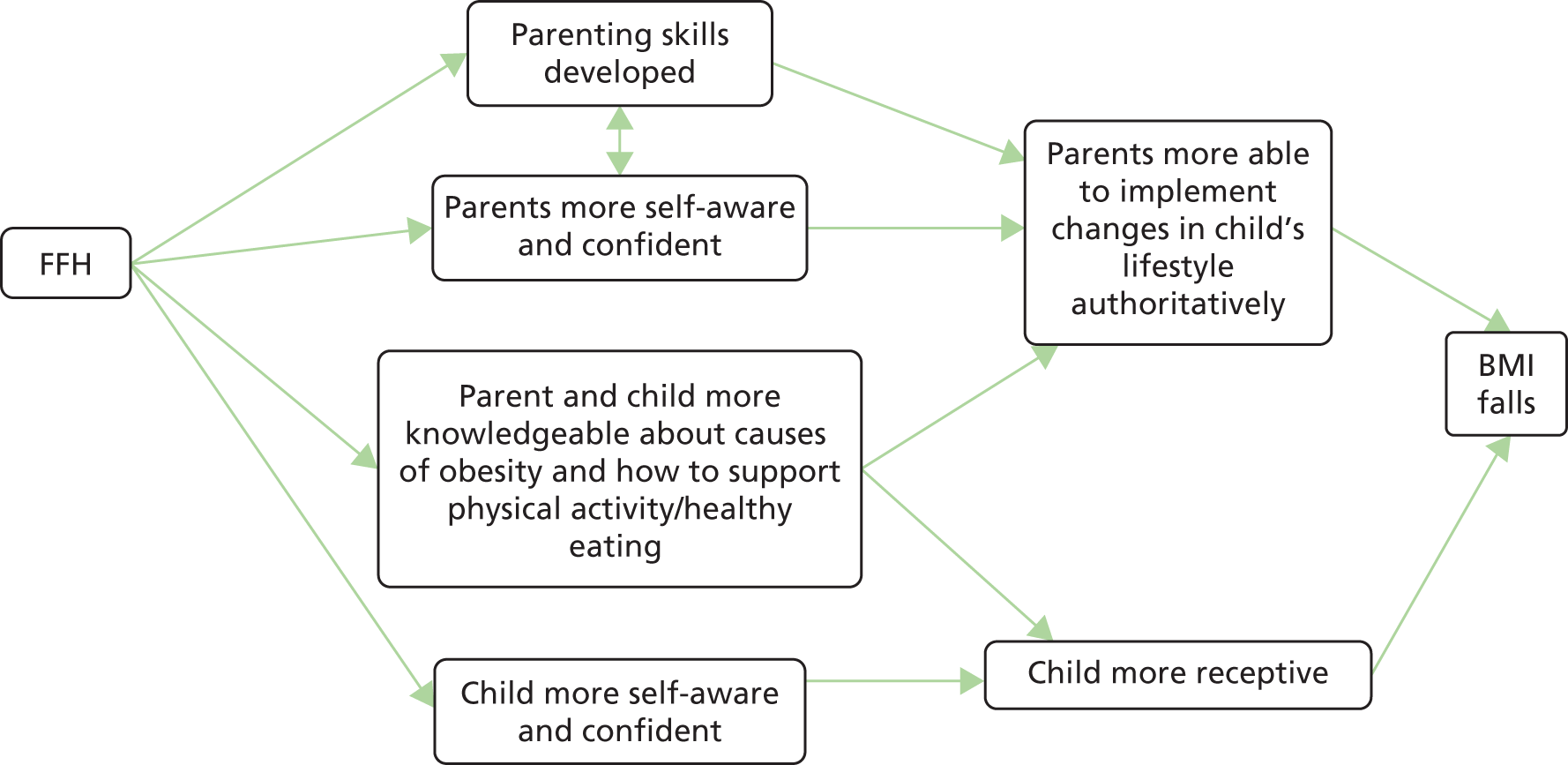
In this RCT there was no statistically significant difference in the change in BMI z-score at the 12-month follow-up between the usual-care and Families for Health arms (0.114, 95% CI –0.001 to 0.229; p = 0.053; model 1). However, the within-group analysis showed that BMI z-score was significantly reduced in the usual-care arm (–0.118, 95% CI –0.203 to –0.034; p = 0.007), whereas there was no significant change in the Families for Health arm (–0.005, 95% CI –0.085 to 0.078; p = 0.907). The measurements of waist circumference z-scores and percentage fat are consistent with the BMI findings. This indicates a trend that children allocated to the Families for Health arm did worse than children randomised to the usual care available locally at the three sites in terms of the management of their obesity.
Apart from a significant improvement in activity level in parents44 in the usual-care arm compared with the Families for Health programme, there were no other significant differences between groups for the other secondary outcome measures (objective or self-reported). Children’s quality of life, parents’ well-being and the child–parent relationship scores were all better (but not significantly different) in the Families for Health group than in the usual care group. Thus, there is some indication that the Families for Health programme may have influenced parenting and family relationships, as predicted in the logic model, although the BMI z-score change was worse than in the usual-care group.
Other studies focusing on the treatment of obesity using a parenting approach have found mixed results. Lifestyle Triple P (Positive Parenting Program®, University of Queensland, Brisbane, QLD, Australia) is a general parenting programme that is aimed at lifestyle, targeting parents of overweight and obese children, from which contrasting results have emerged. In a RCT in children aged 4–11 years in Australia, BMI z-score in the Lifestyle Triple P group was lower by –0.11 at the end of the 12-week programme and by –0.19 at 1-year follow-up, compared with a reduction of –0.01 in the wait-list control group (who then started the intervention at 12 weeks). 98 However, a study in the Netherlands of overweight and obese children aged 4–8 years found no significant intervention effects in children’s BMI z-score, waist circumference or skinfold thickness between Lifestyle Triple P and the control intervention (comprising printed material) at 4 and 12 months after baseline. 99 In general, replication studies of the Triple P suite of programmes have shown less effect than observed in the original trials,100 and in this case there was also a difference in the control intervention between these two trials, with the wait-list control trial showing more effect. A systematic review of general parenting interventions to prevent or treat childhood obesity, which included the Triple P study by West et al. 98 and the Families for Health pilot study,24 found only limited evidence but did find a small positive effect on weight-related outcomes. 16
Economic evaluation
Not only was Families for Health less effective than usual care in terms of BMI z-score, it was also significantly more costly to deliver. The increased costs were mainly because the Families for Health programme ran separate parallel groups for parents and children with two facilitators in each, whereas the group-based interventions in the usual-care arm were delivered with parents and children attending the same group. The mean incremental cost-effectiveness of Families for Health was estimated at £552,175 per QALY gained, and the probability that Families for Health is cost-effective at a £20,000 cost-effectiveness threshold is approximately 28%. When health outcomes were measured in terms of longitudinal change in BMI z-score, the mean incremental cost-effectiveness of Families for Health was estimated at –£3935 per unit change in BMI z-score (indicating that, on average, Families for Health is dominated by usual care in health economic terms) and the probability that the Families for Health programme is cost-effective does not exceed 2% across a range of cost-effectiveness thresholds. These findings leave little doubt that the Families for Health programme is not a cost-effective intervention.
Process evaluation
An uncontrolled pilot study of the Families for Health programme had demonstrated proof of principle for the programme showing a long-term reduction in BMI z-scores,24,25 so a question to be answered by the process evaluation is whether the lack of effectiveness of the Families for Health programme in this trial was because of the intervention itself or poor implementation. 86 Problems with implementation that could have impacted on effectiveness included issues with recruiting a sufficient number of families to form viable Families for Health groups (minimum of eight families) at each site. Recruitment to group-based interventions is more challenging than to one-to-one interventions and becomes doubly so in the context of a trial, as approximately half of the families recruited were randomised to the usual-care intervention. This meant that many families had to wait more than 3 months to receive the Families for Health intervention. Furthermore, some programmes were delivered with an insufficient number of families some weeks, owing to a combination of starting a group with a relatively small number recruited and low attendance, impacting on effectiveness. However, based on the quantity and quality of what was delivered, the process evaluation indicates that the intervention was implemented reasonably well, probably as well as could be expected if the programme were to be scaled up further. There were no major adaptations across the seven programmes and where activities were replaced or modified, the original aims of the handbook activity were upheld. This supports a conclusion that the Families for Health programme was delivered as planned or as well as would be possible when scaled up over three sites, and is ineffective at reducing BMI z-score.
The process evaluation indicates that the majority of families had a positive experience and found the Families for Health programme useful and enjoyable. This was particularly so in relation to food topics, with an increased knowledge and understanding of food labelling being documented. The opportunity to take part in various forms of physical activity, particularly gym sessions, was valued by both parents and children. Parents also felt that they gained a lot from meeting other parents facing similar challenges. It is important to acknowledge these aspects of the Families for Health programme that were perceived to work well. These could be built on in new interventions to tackle childhood obesity.
Our over-riding conclusion from the Families for Health trial is that the combining of support for parenting and family relationships with learning about healthy eating and physical activity in this intervention did not help families manage their children’s overweight or obesity, at least in the medium term.
Why not effective?
The Families for Health programme may have been ineffective for a number of reasons. It may be that the approach to parenting in this intervention, while perceived as offering valuable insights and skills by parents in the process evaluation, may not have helped families to manage the family diet and physical activity participation. It may be that the dietary advice based on the Eatwell plate is not adequate. Some childhood obesity authorities now recommend a zero-sugar approach rather than an attempt to reduce sugar. The latter approach may be more difficult and less effective than the former. The Families for Health programme may also have had an insufficient emphasis on physical activity.
Although the uptake of the programme varied and few families attended all the sessions, this is likely to be the reality of any roll-out of such a programme. It is not possible from the results of this trial to estimate what might have been a ‘sufficient’ dose, but there was active recruitment into this trial. It might prove very difficult in routine delivery of such an intervention to achieve any greater uptake.
It remains true that the Families for Health version 1 programme produced a result in the pilot that was not replicated in the trial. Reductions in BMI z-score of –0.18 (95% CI –0.30 to –0.05) at the end of the programme, –0.21 (95% CI –0.35 to –0.07) at the 9-month follow-up and –0.23 (95% CI –0.42 to –0.03) at the 2-year follow-up were shown. 24,25 The conclusion from the pilot was that the Families for Health intervention merited testing in a RCT. Changes between version 1 and version 2 of the Families for Health programme made between the pilot and the trial included reducing the number of sessions from 12 to 10, but adding two follow-up sessions that had been requested by parents in the pilot process evaluation. Follow-up sessions were, however, poorly attended and usually only one follow-up session was achieved by the facilitators. Other changes to the Families for Health programme between version 1 and version 2 were minor.
Families for Health is not alone as an intervention showing promise in a pilot but showing no difference at the trial phase. Other interventions in the UK on the topic of childhood obesity, physical activity and healthy eating have shown a similar pattern. For example, in the Active for Life year 5 school-based intervention the pilot study showed that it might be effective, whereas in the cluster RCT the three specified primary outcomes of physical activity, sedentary behaviour and fruit and vegetable consumption showed no difference between the intervention and control schools. 101 Furthermore, in a pilot of the ‘Family-Based Behavioural Treatment’ intervention, a reduction in BMI z-score of –0.15 (p < 0.001) was observed, whereas in the main trial, although the early change in BMI z-score was similar to the pilot within the intervention group (–0.16), there was no overall change from 0 to 12 months. 14
Speculation of possible explanations between the different results between the uncontrolled pilot and the main trial results is warranted. One possible reason is that scaling up a pilot study can attenuate the effectiveness. The intervention was delivered very well in a small pilot in site A comprising two Families for Health groups in a non-trial setting, without a control group, so families did not wait long before starting the group. 24 The two pilots were delivered by a small team of four quite experienced facilitators, one of whom was involved with the development of the intervention. Scaling up the pilot study to running the intervention across three sites in the trial, delivered by 17 facilitators mostly delivering the intervention for the first time after only 4 days’ training, may have attenuated the effect.
The piloting of the Families for Health intervention was done using a pre–post study, and maybe this was an inadequate study design to inform the decision to proceed to a full-scale trial. A pilot RCT would have been more rigorous and would have highlighted the difficulties in recruiting families and running the Families for Health intervention in a trial setting in which families had to wait longer for a viable number of families for a group to start.
The logic model for the Families for Health programme assumes capacity to improve parenting style, and then improve lifestyle. According to the PSDQ50 results, however, most parents were already mainly adopting an authoritative parenting style, maybe reflecting who enrols for these types of interventions. Given the relatively high level of optimal parenting at the start of the trial, there was perhaps less scope for change with this parenting intervention, which may be related to the lack of effect. Furthermore, some parents found the parenting aspects patronising. It could be hypothesised that the Families for Health programme would be more effective for families with primarily permissive or authoritarian parenting styles.
The Family Links Nurturing Programme is the basis for the parenting components of the Families for Health intervention. 27 A RCT of the Family Links Nurturing Programme was completed while the Families for Health trial was in progress. 102 The results have not found evidence of clinical or cost utility for the Family Links Nurturing Programme when run as a universal early-years parenting programme for the 2–4 years age group, although there were contamination issues with the trial. Thus, the parenting programme that the Families for Health programme has as its base is not itself of proven effectiveness under trial conditions. This is not necessarily a weakness of the trial methodology, but is a potential weakness of the Families for Health intervention.
The point has been made in the context of this latter Family Links Nurturing Programme trial, as well as in the context of other public health interventions, that the trial setting itself could interfere with the effectiveness of interventions, particularly those involving personal development. 103 This may have also been a factor in the current trial with families having to wait longer to access the Families for Health intervention while a viable group was formed, potentially affecting engagement. However, non-randomised designs comprising historical controls or non-randomised concurrent controls are prone to selection bias, and others advise that they should be used only when RCTs are not feasible or are unethical. 104
Another explanation that must be considered is that the positive result in the pilot study could just be regression to the mean in the uncontrolled pilot. Even if the Families for Health programme is not effective, there is a 1 in 20 chance of a positive result in the pilot, and of course it is exactly those interventions that give such a result that go on to a full RCT.
Strengths and weaknesses of the study
The strengths of the study are the randomised design and the inclusion of a comprehensive process evaluation, alongside the effectiveness and cost-effectiveness components. The economic evaluation was conducted in accordance with national methodological standards, with a comprehensive analytical strategy adopted to handle missing data and various forms of uncertainty. The process evaluation used a specified framework and mixed-methods data collection across a wide range of sources enabling triangulation of results.
A weakness of the study is the differential follow-up of the Families for Health and usual-care arms, with lower follow-up of usual care at 12 months (Families for Health 78.6% vs. usual care 66.1%). If the assumption is made that those who did worse in terms of BMI were more likely to drop out, this could have influenced the trial results by ‘improving performance’ in the control arm. Imputation analyses suggest that our results are robust to differential loss to follow-up, but cannot rule out the possibility that this influenced results to the detriment of the Families for Health programme.
A further weakness is the lack of detailed attendance data from all usual-care providers, and thus attendance data for the usual-care arm are not directly comparable with the Families for Health attendance data. Additionally, the fact that different trial sites had different usual-care provision, and in site C this changed part-way through the trial, may be a weakness. However, variation in the control group treatment is a reflection of the pragmatic nature of the trial. Rather than being a limitation, this could be viewed as an advantage as it does reflect the real world.
On reflection, greater attention should have been paid to the participants in the control arm, which may have improved follow-up rates. Furthermore, increased involvement of the research team with the usual-care providers would have given us better information on both the usual-care provision and the attendance of families, including the number of sessions attended.
A difficulty in the trial was the length of time it took to recruit families. Recruitment of the planned 120 families was intended to be completed in 12 months, but instead took 24 months, eventually recruiting 115 families. Only 40 families were required to be recruited from each of the three study sites, but even then recruitment was difficult. At the time of study recruitment, there were more than 600 children in each of the three sites in year 6 alone (aged 10–11 years) who were eligible to take part, but despite extensive active and passive recruitment methods, recruitment was problematic. The gap between receiving feedback that their child is overweight via the NCMP and subsequent behaviour change is recognised, helping to explain the disconnect between the number of potential children who were eligible and those who actually came forward to participate in the Families for Health trial. 105 As a consequence of the slow recruitment, some families had a long wait until the number of families randomised to the Families for Health arm was sufficient for their group to run. Some groups also started with a suboptimal number of families, which impacted on the delivery of the programme and group dynamics. Both of these factors could have impacted on effectiveness.
The plan was also to develop a long-term cost-effectiveness model to estimate the lifetime clinical and economic consequences of the Families for Health intervention, but this was no longer relevant when the programme was shown to be ineffective. This is a deviation from the protocol.
Recommendations for future research
Duggan et al. 106 argue for broader reporting of negative outcomes from psychological treatments, to include the negative effect of treatment on primary outcomes. In the current study, response, in terms of change in children’s BMI z-score from baseline to the 12-month follow-up, ranged from –0.746 to 0.895 across the two groups, indicating that some children experienced a clinically significant benefit,65 whereas in others treatment resulted in a negative outcome. Further exploration of these extreme cases by treatment group is warranted, analysing psychosocial variables, attendance and qualitative data to help explain the change in BMI z-score. Further exploration of the trial data is necessary to obtain an in-depth understanding of why, in some children, treatment seemed to have an adverse effect.
Further research in the UK into a parent-only intervention for the treatment of obesity is warranted. This would be much cheaper than the parallel-group model comprising separate groups for children and parents (as in the Families for Health intervention). A recent Cochrane review has indicated that parent-only interventions are as effective as treatment programmes for childhood obesity that involve both parents and children. 107 Alternatively, rather than a focus on the treatment of obesity, shifting attention to the role of parents in the prevention of obesity, alongside school-based prevention initiatives, may be worthy of future research.
Furthermore, the Foresight report in 2007108 documented the large number of determinants of obesity, many of which were beyond the control of the individual, and recognised that current interventions were not making enough impact. 108 Within this context, a whole-systems approach to tackle obesity has been advocated by Public Health England, focusing action and policy at the individual, environmental and societal levels, and across multiple sectors. 109 Family-based programmes would then be embedded in a social and fiscal environment, which would be supportive of behaviour change. Examining the effectiveness of a systems approach is a very important area of research, and needs to include the development of new evaluation methods.
Implications for public health/treatment services
In this trial usual care was shown to be effective in within-group analysis. Usual care for obesity management has often been developed and improved over time,36 and has be optimised to suit the local population, potentially explaining why no differences were found with the Families for Health intervention. Anyone developing new interventions would be advised to first carefully evaluate any local initiatives to inform local provider decisions, even if they have not been formally evaluated in a RCT. However, at least 47.5% of families did not receive the usual-care intervention, and so an alternative explanation is that informing parents of their child’s weight is as good as a structured intervention.
Childhood obesity is hard to treat. Even treatment interventions that are shown to be effective may have only a modest impact. For example, when MEND was implemented at scale, the mean change in BMI z-score was –0.18, with the authors stating that the reduction in BMI was less than the trial result (but not significantly so). 21 A concern was raised that MEND had the potential to widen ethnic and socioeconomic inequalities in the outcome of BMI. A further question is whether or not a mean change of –0.18 is making a sufficient population impact, when a clinically significant change in individuals is considered a reduction in BMI z-score, of at least –0.5, which results in a reduction in cardiovascular risk and improved insulin resistance,110,111 although others suggest a reduction of 0.25 as the minimum clinically effective change. 65 So, even when interventions work, they may have only a limited impact on the number of children who move to a healthy weight.
An alternative is to move the focus away from the individual-level determinants to the distal factors, with a focus on changing the environment, making it more conducive to healthy family choices. 112 Prevention measures may be more effective.
Conclusion
Our over-riding conclusion from the Families for Health trial is that this intervention, combining parenting skills and family relationships with learning about healthy eating and physical activity, was not effective in helping families to effectively manage the weight of overweight or obese children aged 6–11 years.
Acknowledgements
We would like to thank the following people and organisations who have supported the trial:
Bernadette Lee, Kate Reddington, John Dewsbury, Helen King, Fran Poole, Dr Adrian Phillips and Gloria Rye from the public health teams at the three sites who enabled this trial to take place in their locality.
Dr Melvyn Hillsdon (University of Exeter), the chairperson of the TSC, and the other external members: Professor Russ Jago (University of Bristol), Professor Mike Campbell (University of Sheffield) and Catherine Millard as our public representative. Professor Peymane Adab (University of Birmingham), the chairperson of the DMEC, and the other external members: Dr Sandra Hollinghurst (University of Bristol) and Ed Juszczak (Associate Professor, University of Oxford).
Katie Williams from the National Institute for Health Research (NIHR) Clinical Research Network: West Midlands and the Human Resources Department at Warwick Medical School, who supported the Research Passport and NHS Honorary Contracts process for the research team and the facilitators. West Midlands South Comprehensive Local Research network (CLRN) and West Midlands North CLRN, school nurse services, childhood obesity clinics and general practices across the three sites for their help with recruitment of families. The research and development departments at the NHS trusts for giving their approval to the study and issuing honorary contracts to the researchers and facilitators.
The Clinical Trials Unit at Warwick Medical School for its randomisation and database services. Dr Rebecca Lang, who was a Research Fellow for the first 9 months of the project.
Most importantly the dedicated facilitators of the Families for Health programme and the families who took part in the trial.
Version 2 of the Families for Health programme draws on the materials developed for the Families for Health programme version 1 (2007)26 by Candida Hunt, and the University of Warwick on funding provided by the Department of Health in England.
Contributions of authors
Dr Wendy Robertson (Associate Professor of Public Health) had overall responsibility for the study as the chief investigator, was responsible for recruitment to the trial, carried out the fieldwork with facilitators and fidelity assessment of the Families for Health programme, analysed the qualitative data and drafted sections of the final report.
Dr Joanna Fleming (Research Fellow) was responsible for recruitment to the trial, carried out the fieldwork with families, analysed the qualitative data and drafted sections of the final report.
Dr Atiya Kamal (Research Fellow) was responsible for recruitment to the trial, carried out the fieldwork with families, analysed the qualitative data and drafted sections of the final report.
Thomas Hamborg (Research Assistant, Statistics) analysed the quantitative data and drafted sections of the final report.
Kamran A Khan (Research Assistant, Health Economics) carried out the economic analysis and drafted sections of the final report.
Professor Frances Griffiths (Professor of Medicine in Society, Qualitative research) was a co-applicant on the grant application, was involved in its implementation, and oversaw the analysis of the qualitative data.
Professor Sarah Stewart-Brown (Professor of Public Health) was a co-applicant on the grant application and was involved in its implementation.
Professor Nigel Stallard (Professor of Medical Statistics) was a co-applicant on the grant application, was involved in its implementation, and oversaw the analysis of the quantitative data.
Professor Stavros Petrou (Professor of Health Economics) was a co-applicant on the grant application, was involved in its implementation, oversaw the economic analysis and drafted sections of the final report.
Dr Douglas Simkiss (Honorary Associate Clinical Professor in Child Health) was a co-applicant on the grant application and was involved in its implementation.
Dr Elizabeth Harrison (Trial Administrator) was responsible for recruitment to the trial, and the administration.
Dr Sung Wook Kim (Health Economist) carried out the economic analysis.
Professor Margaret Thorogood (Professor of Epidemiology) was a co-applicant on the grant application, was involved in its implementation, analysed the qualitative data and acted as editor.
All authors have been actively involved in the authorship of the report and approved the final manuscript.
Funding acknowledgements
Wendy Robertson is part funded by the NIHR Collaborations for Leadership in Applied Health Research and Care West Midlands initiative.
Publications
Kirby J, Tibbins C, Callens C, Lang B, Thorogood M, Tigbe W, et al. Young people’s views on accelerometer use in physical activity research: findings from a user involvement investigation. ISRN Obesity 2012;2012:948504.
Robertson W, Stewart-Brown S, Stallard N, Petrou S, Griffiths F, Thorogood M, et al. Evaluation of the effectiveness and cost-effectiveness of ‘Families for Health V2’ for the treatment of childhood obesity: study protocol for a randomized controlled trial. Trials 2013;14:81.
Fleming J, Kamal A, Harrison E, Hamborg, T, Stewart-Brown S, Thorogood M, et al. Evaluation of recruitment methods for a trial targeting childhood obesity: Families for Health randomised controlled trial. Trials 2015;16:535.
Robertson W, Fleming J, Kamal A, Hamborg T, Khan KA, Griffiths F, et al. Randomised controlled trial and economic evaluation of the ‘Families for Health’ programme to reduce obesity in children [published online ahead of print 21 December 2016]. Arch Dis Child 2016.
Data sharing statement
Owing to not obtaining specific data sharing consent from participants, there are no data that can be shared. Please contact the corresponding author for further details.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Health Survey for England . Chapter 11: Children’s BMI, Overweight and Obesity 2014. www.hscic.gov.uk/catalogue/PUB16076 (accessed September 2015).
- van Jaarsveld CHM, Gulliford MC. Childhood obesity trends from primary care electronic health records in England between 1994 and 2013: population-based cohort study. Arch Dis Child 2015;100:214-19. http://dx.doi.org/10.1136/archdischild-2014-307151.
- Health and Social Care Information Centre . National Child Measurement Programme, England, 2013 14 2014. www.hscic.gov.uk/catalogue/PUB16070 (accessed September 2015).
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: a systematic review. Int J Obes 2011;35:891-8. http://dx.doi.org/10.1038/ijo.2010.222.
- Pulgaron E. Childhood obesity: a review of increased risk for physical and psychological morbidities. Clin Ther 2013;35:A18-32. http://dx.doi.org/10.1016/j.clinthera.2012.12.014.
- Park MH, Falconer C, Viner RM, Kinra S. The impact of child obesity on morbidity and mortality in adulthood: a systematic review. Obes Rev 2012;13:985-1000. http://dx.doi.org/10.1111/j.1467-789X.2012.01015.x.
- Aicken C, Arai L, Roberts H. Schemes to Promote Healthy Weight Among Obese and Overweight Children in England. London: EPPI Centre, Social Science Research Unit; 2008.
- Department of Health . Healthy Weight, Healthy Lives: Commissioning Weight Management Services for Children and Young People 2008. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_090111.pdf (accessed September 2015).
- Healthy Child Programme: From 5 to 19 Years Old. London: Department of Health; 2009.
- Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev 2009;1. http://dx.doi.org/10.1002/14651858.cd001872.pub2.
- Sacher PM, Kolotourou M, Chadwick PM, Cole TJ, Lawson MS, Lucas A, et al. Randomized controlled trial of the MEND program: a family-based community intervention for childhood obesity. Obesity 2010;18:S62-8. http://dx.doi.org/10.1038/oby.2009.433.
- Hughes AR, Stewart L, Chapple J, McColl JH, Donaldson MD, Kelnar CJ, et al. Randomized, controlled trial of a best-practice individualized behavioural program for treatment of childhood overweight: Scottish Childhood Overweight Treatment Trial (SCOTT). Pediatrics 2008;121:3539-46. http://dx.doi.org/10.1542/peds.2007-1786.
- Bryant M, Farrin A, Christie D, Jebb SA, Cooper AR, Rudolf M. Results of a feasibility randomised controlled trial (RCT) for WATCH IT: a programme for obese children and adolescents. Clin Trials 2011;8:755-64. http://dx.doi.org/10.1177/1740774511424766.
- Croker H, Viner RM, Nicholls D, Haroun D, Chadwick O, Edwards C, et al. Family-based behavioural treatment of childhood obesity in a UK National Health Service setting: randomised controlled trial. Int J Obes 2012;36:16-2. http://dx.doi.org/10.1038/ijo.2011.182.
- NICE . Managing Overweight and Obesity Among Children and Young People: Lifestyle Management Service 2013. www.nice.org.uk/guidance/PH47 (accessed September 2015).
- Gerards SM, Sleddens EF, Dagnelie PC, de Vries NK, Kremers SP. Interventions addressing general parenting to prevent or treat childhood obesity. Int J Pediatr Obes 2011;6:e28-45. http://dx.doi.org/10.3109/17477166.2011.575147.
- Sung-Chan P, Sung YW, Zhao X, Brownson RC. Family-based models for childhood-obesity intervention: a systematic review of randomized controlled trials. Obesity Rev 2013;14:265-78. http://dx.doi.org/10.1111/obr.12000.
- Upton P, Taylor C, Erol R, Upton D. Family-based childhood obesity interventions in the UK: a systematic review of published studies. Community Pract 2014;87:25-9.
- Epstein LH, Paluch RA, Wrotniak BH, Daniel TO, Kilanowski C, Wilfley D, et al. Cost-effectiveness of family-based group treatment for child and parental obesity. Child Obes 2014;10:114-21. http://dx.doi.org/10.1089/chi.2013.0123.
- Fagg J, Chadwick P, Cole TJ, Cummins S, Goldstein H, Lewis H, et al. From trial to population: a study of a family-based community intervention for childhood overweight implemented at scale. Int J Obes 2014;38:1343-9. http://dx.doi.org/10.1038/ijo.2014.103.
- Fagg J, Cole TJ, Cummins S, Goldstein H, Morris S, Radley D, et al. After the RCT: who comes to a family-based intervention for childhood overweight and obesity when it is implemented at scale in the community?. J Epidemiol Community Health 2015;69:142-8. http://dx.doi.org/10.1136/jech-2014-204155.
- Sanders MR. Triple P-Positive parenting program as a public health approach to strengthening parenting. J Fam Psychol 2008;22:506-17. http://dx.doi.org/10.1037/0893-3200.22.3.506.
- Craig P, Dieppe P, Macintyre, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council Guidance. BMJ 2008;337:979-83. http://dx.doi.org/10.1136/bmj.a1655.
- Robertson W, Friede T, Blissett J, Rudolf MCJ, Wallis MA, Stewart-Brown S. Pilot of ‘Families for Health’: community-based family intervention for obesity. Arch Dis Child 2008;93:921-8. http://dx.doi.org/10.1136/adc.2008.139162.
- Robertson W, Thorogood M, Inglis N, Grainger C, Stewart-Brown S. Two year follow-up of the ‘Families for Health’ programme for the treatment of childhood obesity. Child Care Health Dev 2012;38:229-36. http://dx.doi.org/10.1111/j.1365-2214.2011.01237.x.
- Hunt C, Robertson W, Stewart-Brown S. Families for Health Version 1 (Handbooks for Facilitators, Parents’ Guide, Children’s Guide). Coventry: University of Warwick; 2007.
- Hunt C. The Parenting Puzzle: How to Get the Best out of Family Life. Oxford: Family Links; 2003.
- The Nurturing Programme. Classroom Handbook, Book 2, Year 1 to 4. Oxford: Family Links; 1999.
- Golan M, Crow S. Targeting parents exclusively in the treatment of childhood obesity: long-term results. Obes Res 2004;12:357-61. http://dx.doi.org/10.1038/oby.2004.45.
- Standard Operating Procedures. Coventry: Warwick Medical School, University of Warwick; 2012.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- MRC . MRC Guidelines for Good Clinical Practice in Clinical Trials 1998. www.mrc.ac.uk/documents/pdf/good-clinical-practice-in-clinical-trials/ (accessed 19 July 2016).
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4.
- Kirby J, Tibbins C, Callens C, Lang B, Thorogood M, Tigbe W, et al. Young people’s views on accelerometer use in physical activity research: findings from a user involvement investigation. ISRN Obesity 2012;2012. http://dx.doi.org/10.5402/2012/948504.
- Tibbens C, Callens C. Families for Health User Involvement Study. Birmingham: Medicines for Children Research Network, Birmingham Children’s Hospital; 2012.
- Towey M, Harrell R, Lee B. Evaluation of the ‘one body, one life’: a community-based family intervention for the prevention of obesity in children. J Obes 2011;2011. http://dx.doi.org/10.1155/2011/619643.
- Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK. Arch Dis Child 1995;73:25-9. http://dx.doi.org/10.1136/adc.73.1.25.
- Health and Social Care Information Centre . National Child Measurement Programme – England, 2008 09 School Year 2009. www.hscic.gov.uk/searchcatalogue?productid=1072&q=title%3a%22national+child+measurement+programme%22&sort=Relevance&size=10&page=1#top (accessed September 2015).
- McCarthy HD, Jarrett KV, Crawley HF. The development of waist circumference percentiles in British children aged 5–16.9 years. Eur J Clin Nutr 2001;55:902-7. http://dx.doi.org/10.1038/sj.ejcn.1601240.
- Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child 2006;91:612-17. http://dx.doi.org/10.1136/adc.2005.085522.
- Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Science 2008;26:1557-65. http://dx.doi.org/10.1080/02640410802334196.
- Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. Comparison of accelerometer cut-points for predicting activity intensity in youth. Med Sci Sports Exerc 2011;43:1360-8. http://dx.doi.org/10.1249/MSS.0b013e318206476e.
- Edmunds LD, Ziebland S. Development and validation of the Day in the Life Questionnaire (DILQ) as a measure of fruit and vegetable questionnaire for 7–9 year olds. Health Educ Res 2002;17:211-20. http://dx.doi.org/10.1093/her/17.2.211.
- Golan M. Reliability and validity of the family eating and activity habits questionnaire. Eur J Clin Nutr 1998;52:771-7. http://dx.doi.org/10.1038/sj.ejcn.1600647.
- Varni JW. PedsQL: Measurement Model for the Pediatric Quality Of Life Inventory 1998. www.pedsql.org/pedsql13.html (accessed September 2015).
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800-12. http://dx.doi.org/10.1097/00005650-200108000-00006.
- Upton P, Eiser C, Cheung I, Hutchings HA, Jenney M, Maddocks A, et al. Measurement properties of the UK-English version of the Pediatric Quality of Life Inventory 4.0 (PedsQL) generic core scales. Health Qual Life Outcomes 2005;3. http://dx.doi.org/10.1186/1477-7525-3-22.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-Being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. http://dx.doi.org/10.1186/1477-7525-5-63.
- Pianta RC. Child–Parent Relationship Scale 1992. http://curry.virginia.edu/academics/directory/Robert-c.-pianta/measures (accessed September 2015).
- Robinson CC, Mandleco B, Olsen SF, Hart CH, Perlmutter B, Toulliatos J, et al. Handbook of Family Management Techniques, Instruments and Index. Volume 3. Thousand Oaks, CA: Sage; 2001.
- Wille N, Ravens-Sieberer U. Age-Appropriateness of the EQ-5D Adult and Child-Friendly Version –Testing the Feasibility, Reliability and Validity in Children and Adolescents n.d.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Eidt-Koch D, Mittendorf T, Greiner W. Cross-sectional validity of the EQ-5D-Y as a generic health outcome instrument in children and adolescents with cystic fibrosis in Germany. BMC Pediatrics 2009;9. http://dx.doi.org/10.1186/1471-2431-9-55.
- Beecham J, Knapp M. Client Service Receipt Inventory (CSRI). Database of Instruments for Resource Use Management 1992. www.dirum.org/instruments/details/44 (accessed September 2015).
- Office for National Statistics . SOC2010 Volume 3: The National Statistics Socio-Economic Classification (NS-SEC Rebased on the SOC2010) 2010. www.ons.gov.uk/ons/guide-method/classifications/current-standard-classifications/soc2010/soc2010-volume-3-ns-sec--rebased-on-soc2010--user-manual/index.html (accessed September 2015).
- Daniel SR, Khoury PR, Morrison JA. Utility of different measures of body fat distribution in children and adolescents. Am J Epidemiol 2000;152:1179-84. http://dx.doi.org/10.1093/aje/152.12.1179.
- Robertson W, Stewart-Brown S, Wilcock E, Oldfield M, Thorogood M. Utility of accelerometers to measure physical activity in children attending an obesity treatment intervention. J Obes 2011;2011. http://dx.doi.org/10.1155/2011/398918.
- Page A, Cooper AR, Stamatakis E, Foster LJ, Crowne EC, Sabin M, et al. Physical activity patterns in nonobese and obese children assessed using minute-by-minute accelerometry. IJBNPA 2005;29:1070-6. http://dx.doi.org/10.1038/sj.ijo.0802993.
- Riddoch CJ, Mattocks C, Deere K, Saunders J, Kirkby J, Tilling K, et al. Objective measurements of levels and patterns of physical activity. Arch Dis Child 2007;92:963-9. http://dx.doi.org/10.1136/adc.2006.112136.
- NHS Choices . 5-A-Day What Counts n.d. www.nhs.uk/Livewell/5ADAY/Pages/Whatcounts.aspx (accessed September 2015).
- NHS Reference Costs 2013–2014. National Schedule of Reference Costs. London: Department of Health; 2014.
- Curtis L. Unit costs of Health and Social Care 2013. Kent: Personal Social Services Research Unit, University of Kent; 2013.
- British National Formulary for Children. London: BMJ Group, Pharmaceutical Press, and RCPCH Publications; 2013.
- Annual Survey of Hours and Earnings. London: Office for National Statistics; 2012.
- Ford AL, Hunt LP, Cooper A, Shield JPH. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health?. Arch Dis Child 2010;95:256-61. http://dx.doi.org/10.1136/adc.2009.165340.
- Penpraze V, Reilly JJ, MacLean CM, Montgomery C, Kelly LA, Paton JY, et al. Monitoring of physical activity in young children: how much is enough?. Pediatr Exerc Sci 2006;18:483-91.
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. Cary, NC: SAS Institute Inc.; 2006.
- Fai AHT, Cornelius PL. Approximate F-tests of multiple degree of freedom hypotheses in generalized least squares analyses of unbalanced split-plot experiments. J Stat Comput Simul 1996;54:363-78. http://dx.doi.org/10.1080/00949659608811740.
- Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall; 1997.
- Van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219-42. http://dx.doi.org/10.1177/0962280206074463.
- Robertson W, Fleming J, Kamal A, Hamborg T, Khan KA, Griffiths F, et al. Randomised controlled trial and economic evaluation of the ‘Families for Health’ programme to reduce obesity in children [published online ahead of print 21 December 2016]. Arch Dis Child n.d. http://dx.doi.org/10.1136/archdischild-2016-311514.
- Bonett DG. Sample size requirements for estimating intraclass correlations with desired precision. Stat Med 2002;21:1331-5. http://dx.doi.org/10.1002/sim.1108.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Royal Wolverhampton NHS Trust, Child Healthy Lifestyle Services . WISH n.d. www.royalwolverhamptonhospitals.nhs.uk/wton_city_community_services/healthy_city/child_healthy_lifestyle_servic.aspx (accessed 20 July 2016).
- Curtis L. Unit Costs of Health and Social Care 2014. Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Prescription Cost Analysis England 2013: Prescription Items Dispensed in the Community in England and Listed Alphabetically Within Chemical Entity by Therapeutic Class. London: HSCIC; 2014.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Horton NJ, Lipsitz SR. Multiple imputation in practice: comparison of software packages for regression models with missing variables. Am Stat 2001;55:244-54. http://dx.doi.org/10.1198/000313001317098266.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004.
- Van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. http://dx.doi.org/10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R.
- Robertson W, Stewart-Brown S, Stallard N, Petrou S, Griffiths F, Thorogood M, et al. Evaluation of the effectiveness and cost-effectiveness of ‘Families for Health V2’ for the treatment of childhood obesity: study protocol for a randomized controlled trial. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-81.
- Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported health care resource utilization in health economic studies. Int J Technol Assess Health Care 2002;18:705-10. http://dx.doi.org/10.1017/S026646230200051X.
- Clarke PM, Fiebig DG, Gerdtham UG. Optimal recall length in survey design. J Health Econ 2008;27:1275-84. http://dx.doi.org/10.1016/j.jhealeco.2008.05.012.
- Oakley A, Strange V, Bonnell C, Allen E, Stephenson J. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. http://dx.doi.org/10.1136/bmj.332.7538.413.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process Evaluation of Complex Interventions. UK Medical Research Council (MRC) Guidance 2014. www.ioe.ac.uk/MRC_PHSRN_Process_evaluation_guidance_final%282%29.pdf (accessed September 2015).
- Linnan L, Steckler A, Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- 2011 Census, Aggregate Data (England and Wales). London: Office for National Statistics; n.d.
- McLennan D, Barnes H, Noble M, Davies, E, Dibben C. The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Health and Social Care Information Centre . National Child Measurement Programme – England, 2011–2012 School Year 2012. www.hscic.gov.uk/catalogue/PUB09283 (accessed September 2015).
- Green J, Thorogood N. Qualitative Methods for Health Research. London: Sage; 2004.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Fleming J, Kamal A, Harrison E, Hamborg T, Stewart-Brown S, Thorogood M, et al. Evaluation of recruitment methods for a trial targeting childhood obesity: families for health randomised controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-1062-x.
- Beidas RS, Kendall PC. Training therapists in evidence-based practice: a critical review of studies from a systems-contextual perspective. Clin Psychol 2010;17:1-30. http://dx.doi.org/10.1111/j.1468-2850.2009.01187.x.
- Patterson J, Mockford C, Stewart-Brown S. Parents’ perceptions of the value of the Webster–Stratton parenting programme: a qualitative study of a general practice based initiative. Child Care Health Dev 2005;31:53-64. http://dx.doi.org/10.1111/j.1365-2214.2005.00479.x.
- Jones RA, Warren JM, Okely AD, Collins CE, Morgan PJ, Cliff DP, et al. Process evaluation of the Hunter Illawarra Kids Challenge Using Parent Support Study: a multisite randomized controlled trial for the management of childhood obesity. Health Promotion Practice 2010;11:917-27. http://dx.doi.org/10.1177/1524839908328994.
- Petersen D, Taylor EF, Peikes D. Logic Models: The Foundation to Implement, Study, and Refine Patient-Centred Medical Home Models. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- West F, Sanders MR, Cleghorn GJ, Davies PS. Randomised clinical trial of a family-based lifestyle intervention for childhood obesity involving parents as the exclusive agents of change. Behav Res Ther 2010;48:1170-9. http://dx.doi.org/10.1016/j.brat.2010.08.008.
- Gerards SM, Dagnelie PC, Gubbels JS, van Buuren S, Hamers FJ, Jansen MW, et al. The effectiveness of Lifestyle Triple P in the Netherlands: a randomized controlled trial. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0122240.
- Wilson P, Rush R, Hussey S, Puckering C, Sim F, Allely CS, et al. How evidence-based is an ‘evidence-based parenting program’? A PRISMA systematic review and meta-analysis of Triple P. BMC Med 2012;10. http://dx.doi.org/10.1186/1741-7015-10-130.
- Kipping RR, Howe LD, Jago R, Campbell R, Wells S, Chittleborough CR, et al. Effect of intervention aimed at increasing physical activity, reducing sedentary behaviour, and increasing fruit and vegetable consumption in children: Active for Life Year 5 (AFLY5) school based cluster randomised controlled trial. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g3256.
- Simkiss DE, Snooks HA, Stallard N, Kimani PK, Sewell B, Fitzsimmons D, et al. Effectiveness and cost-effectiveness of a universal parenting skills programme in deprived communities: multicentre randomised controlled trial. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002851.
- Stewart-Brown S, Anthony R, Wilson L, Winstanley S, Simkiss D, Stallard N, et al. Should randomized controlled trials be the gold standard for research on preventive interventions for children. J Child Serv 2011;6:228-35. http://dx.doi.org/10.1108/17466661111190929.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7270.
- Syrad H, Falconer C, Cooke L, Saxena S, Kessel AS, Viner R, et al. ‘Health and happiness is more important than weight’: a qualitative investigation of the views of parents receiving written feedback on their child’s weight as part of the national child measurement programme. J Hum Nutr Diet 2015;28:47-55. http://dx.doi.org/10.1111/jhn.12217.
- Duggan C, Parry G, McMurran M, Davidson K, Dennis J. The recording of adverse events from psychological treatments in clinical trials: evidence from a review of NIHR-funded trials. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-335.
- Loveman E, Al-Khudairy L, Johnson RE, Robertson W, Colquitt JL, Mead EL, et al. Parent-only interventions for childhood overweight or obesity in children aged 5 to 11 years. Cochrane Database Syst Rev 2015;12. http://dx.doi.org/10.1002/14651858.cd012008.
- Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J, et al. Foresight. Tackling Obesities: Future Choices – Project Report. London: Foresight Programme of the Government Office for Science; 2007.
- Public Health England . A Whole Systems Approach to Tackling Obesity 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/473860/Whole_system_obesity_local_authority_EOI_letter.pdf (accessed 1 June 2016).
- Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child 2004;89:419-22. http://dx.doi.org/10.1136/adc.2003.028803.
- Hunt LP, Ford A, Sabin MA, Crowne EC, Shield JPH. Clinical measure of adiposity and percentage fat loss: which measure most accurately reflects fat loss and what should we aim for?. Arch Dis Child 2007;92:399-403. http://dx.doi.org/10.1136/adc.2006.103986.
- Maziak W, Ward KD, Stockton MB. Childhood obesity: are we missing the big picture?. Obes Rev 2008;9:35-42.
Appendix 1 Evaluation from the training of the facilitators for Families for Health
Nineteen facilitators attended the 4-day training workshop in February 2012 run by Family Links. At the end of each day facilitators completed evaluation forms. They provided written responses to the following questions: ‘What thoughts/ideas have been triggered as a result of today’s training?’; ‘In what ways, if any, will today’s training enhance your professional practice?’; and ‘We would value any other comments and suggestions’. Using the 4-point rating scale: ‘not confident’, ‘unsure’, ‘quite confident’, ‘confident’, facilitators were asked to rate: ‘How confident do you feel as a Families for Health facilitator?’. Using the four-point rating scale: ‘extremely useful’, ‘useful’, ‘not very useful’, ‘pointless’, facilitators were asked to rate: ‘How useful have you found it to explore the following?’ followed by a list of topics that were covered during the training session for that particular day.
The evaluation from the training showed an increase in confidence of facilitators in delivering the programme (Table 76). The majority of the topics covered during the training were scored as either ‘useful’ or ‘extremely useful’ (Table 77).
| Day of training | Confidence level | |||
|---|---|---|---|---|
| Not confident | Unsure | Quite confident | Confident | |
| 1 | 0.0 | 5.6 | 61.1 | 33.3 |
| 2 | 0.0 | 5.3 | 57.9 | 36.8 |
| 3 | 0.0 | 6.3 | 50.0 | 43.8 |
| 4 | 0.0 | 6.7 | 33.3 | 60.0 |
| Topic | Relevance of topic | |||
|---|---|---|---|---|
| Extremely useful | Useful | Not very useful | Pointless | |
| FFH programme training | 82.4 | 17.6 | 0.0 | 0.0 |
| The programme for the parents/children you work with | 75.0 | 25.0 | 0.0 | 0.0 |
| FFH programme resources | 76.5 | 23.5 | 0.0 | 0.0 |
The written comments submitted by facilitators (n = 14) on completion of the 4-day training indicate that the training was a positive and useful experience:
I have been trained in a number of parenting and children’s programmes and I would say that this training was by far the most inspirational for me. No one has ever been able to get me drinking water, having breakfast, eating more fruit. I could go on but this training has done this in a non-judgmental way without forcing or coercion and for that I would like to thank you for your inspirational facilitating skills.
Facilitator, training day feedback
Appendix 2 Essentially full completer analysis on effectiveness
Essentially full attendance (full completer) is defined as having attended 9 or 10 Families for Health sessions. The outcomes (BMI z-score and waist circumference z-score) in these children are compared with the outcomes of children allocated to the Families for Health programme who have attended fewer than nine sessions. The same model as in the per-protocol analysis is used. In total, there were 15 children with essentially full attendance. There was no statistically significant difference between those children who attended 9 or 10 sessions and those who attended fewer.
| Period | Treatment group | Outcome, mean z-score (95% CI) | |
|---|---|---|---|
| BMI | Waist circumference | ||
| Baseline to 3 months | Full completer | 0.006 (–0.192 to 0.205) | –0.141 (–0.286 to 0.003) |
| Non-full completer | –0.038 (–0.076 to 0.001) | –0.068 (–0.122 to –0.015) | |
| Treatment effect | –0.044 (–0.162 to 0.074) | 0.073 (–0.065 to 0.211) | |
| p-value | 0.457 | 0.297 | |
| Baseline to 12 months | Full completer | 0.007 (–0.205 to 0.218) | –0.045 (–0.214 to 0.124) |
| Non-full completer | –0.053 (–0.113 to 0.008) | –0.075 (–0.156 to 0.007) | |
| Treatment effect | –0.059 (–0.277 to 0.159) | –0.030 (–0.229 to 0.170) | |
| p-value | 0.572 | 0.768 | |
Appendix 3 Subgroup analyses on effectiveness
Subgroup age (6–8 and 9–11 years)
FIGURE 25.
Baseline distribution of children’s age.

| Time point | Treatment group | Number of children | Mean child BMI z-score (SD) | Number of young children | Mean young child BMI z-score (95% CI) | Number of old children | Mean old child BMI z-score (95% CI) |
|---|---|---|---|---|---|---|---|
| Baseline | FFH | 63 | 2.69 (0.67) | 25 | 2.70 (2.36 to 3.03) | 38 | 2.68 (2.49 to 2.86) |
| UC | 65 | 2.74 (0.70) | 24 | 2.91 (2.55 to 3.27) | 41 | 2.64 (2.46 to 2.82) | |
| 3 months | FFH | 48 | 2.62 (0.67) | 20 | 2.63 (2.29 to 2.97) | 28 | 2.62 (2.37 to 2.87) |
| UC | 50 | 2.65 (0.71) | 21 | 2.80 (2.41 to 3.20) | 29 | 2.53 (2.32 to 2.75) | |
| 12 months | FFH | 45 | 2.72 (0.59) | 18 | 2.68 (2.36 to 2.99) | 27 | 2.74 (2.51 to 2.97) |
| UC | 43 | 2.58 (0.68) | 17 | 2.83 (2.46 to 3.20) | 16 | 2.41 (2.16 to 2.66) |
| Period | Treatment group | Mean young (6–8 years) child BMI z-score (95% CI) | Mean old (9–11 years) child BMI z-score (95% CI) |
|---|---|---|---|
| Baseline to 3 months | FFH | –0.008 (–0.156 to 0.140) | –0.027 (–0.106 to 0.051) |
| UC | –0.058 (–0.124 to 0.008) | –0.031 (–0.098 to 0.036) | |
| p-value | 0.523 | 0.940 | |
| Baseline to 12 months | FFH | –0.033 (–0.203 to 0.138) | 0.043 (–0.046 to 0.132) |
| UC | –0.100 (–0.235 to 0.036) | –0.103 (–0.215 to 0.009) | |
| p-value | 0.522 | 0.039 |
Subgroup sex
| Time point | Treatment group | Number of children | Mean child BMI z-score (SD) | Number of girls | Mean BMI z-score girls (95% CI) | Number of boys | Mean BMI z-score boys (95% CI) |
|---|---|---|---|---|---|---|---|
| Baseline | FFH | 63 | 2.69 (0.67) | 36 | 2.63 (2.41 to 2.85) | 27 | 2.76 (2.49 to 3.04) |
| UC | 65 | 2.74 (0.70) | 29 | 2.43 (2.19 to 2.67) | 36 | 2.99 (2.77 to 3.21) | |
| 3 months | FFH | 48 | 2.62 (0.67) | 27 | 2.55 (2.25 to 2.85) | 21 | 2.72 (2.47 to 2.97) |
| UC | 50 | 2.65 (0.71) | 25 | 2.35 (2.10 to 2.60) | 25 | 2.94 (2.66 to 3.23) | |
| 12 months | FFH | 45 | 2.72 (0.59) | 26 | 2.68 (2.41 to 2.95) | 19 | 2.77 (2.53 to 3.01) |
| UC | 43 | 2.58 (0.68) | 20 | 2.35 (2.06 to 2.63) | 23 | 2.78 (2.48 to 3.08) |
| Period | Treatment group | Mean BMI z-score girls (95% CI) | Mean BMI z-score boys (95% CI) |
|---|---|---|---|
| Baseline to 3 months | FFH | 0.005 (–0.084 to 0.094) | –0.050 (–0.181 to 0.081) |
| UC | –0.001 (–0.077 to 0.075) | –0.084 (–0.137 to –0.031) | |
| p-value | 0.922 | 0.622 | |
| Baseline to 12 months | FFH | 0.081 (–0.006 to 0.168) | –0.080 (–0.239 to 0.079) |
| UC | –0.100 (–0.191 to 0.091) | –0.146 (–0.247 to –0.046) | |
| p-value | 0.092 | 0.450 |
Subgroup parent body mass index
Children are divided into subgroups according to their parents’ BMI at baseline. Parents’ BMI is classified as underweight or healthy (BMI < 25 kg/m2), overweight (BMI ≥ 25 kg/m2 and < 30 kg/m2) or obese (BMI ≥ 30 kg/m2) according to the World Health Organization classification system. Parent A BMI was used unless parent A BMI was missing, in which case parent B BMI was used (parent B BMI used once). One child did not have a parent providing a baseline BMI.
| Time point | Treatment group | Underweight/healthy parent | Overweight parent | Obese parent | |||
|---|---|---|---|---|---|---|---|
| Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | ||
| Baseline | FFH | 10 | 2.32 (1.95 to 2.69) | 16 | 2.64 (2.32 to 2.96) | 37 | 2.80 (2.57 to 3.04) |
| UC | 8 | 2.62 (2.18 to 3.06) | 21 | 2.64 (2.38 to 2.90) | 35 | 2.83 (2.55 to 3.10) | |
| 3 months | FFH | 6 | 2.26 (1.75 to 2.76) | 15 | 2.67 (2.33 to 3.02) | 27 | 2.68 (2.39 to 2.97) |
| UC | 7 | 2.60 (2.05 to 3.15) | 17 | 2.52 (2.20 to 2.84) | 25 | 2.75 (2.42 to 3.08) | |
| 12 months | FFH | 5 | 2.39 (1.72 to 3.06) | 15 | 2.62 (2.31 to 2.93) | 25 | 2.84 (2.59 to 3.09) |
| UC | 8 | 2.40 (1.86 to 2.95) | 14 | 2.53 (2.14 to 2.92) | 20 | 2.66 (2.32 to 3.01) | |
| Time period | Treatment group | Measurement, mean BMI z-score (95% CI) | ||
|---|---|---|---|---|
| Healthy parent | Overweight parent | Obese parent | ||
| Baseline to 3 months | FFH | –0.099 (–0.264 to 0.066) | –0.049 (–0.122 to 0.221) | –0.040 (–0.132 to 0.053) |
| UC | –0.074 (–0.132 to –0.016) | –0.069 (–0.13 to 0.001) | –0.009 (–0.090 to 0.071) | |
| p-value | 0.724 | 0.187 | 0.616 | |
| Baseline to 12 months | FFH | 0.099 (–0.034 to 0.233) | 0.010 (–0.1665 to 0.186) | –0.002 (–0.118 to 0.113) |
| UC | –0.213 (–0.448 to 0.021) | –0.142 (–0.280 to –0.004) | –0.036 (–0.168 to 0.095) | |
| p-value | 0.018 | 0.161 | 0.688 | |
Subgroup locality (site)
| Time point | Treatment group | Site | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | |||||
| Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | ||
| Baseline | FFH | 28 | 2.39 (2.17 to 2.60) | 16 | 2.89 (2.52 to 3.27) | 19 | 2.95 (2.64 to 3.26) |
| UC | 28 | 2.71 (2.47 to 2.95) | 18 | 2.49 (2.18 to 2.79) | 19 | 3.02 (2.64 to 3.41) | |
| 3 months | FFH | 24 | 2.39 (2.13 to 2.64) | 11 | 2.94 (2.52 to 3.35) | 13 | 2.80 (2.37 to 3.24) |
| UC | 19 | 2.58 (2.29 to 2.86) | 14 | 2.43 (2.08 to 2.78) | 17 | 2.90 (2.46 to 3.34) | |
| 12 months | FFH | 20 | 2.53 (2.29 to 2.78) | 10 | 2.85 (2.43 to 3.27) | 15 | 2.87 (2.51 to 3.23) |
| UC | 19 | 2.58 (2.31 to 2.85) | 11 | 2.40 (1.89 to 2.91) | 13 | 2.73 (2.24 to 3.21) | |
| Site | Time period | Treatment group | Measurement, mean z-score (95% CI) | |
|---|---|---|---|---|
| BMI | Waist circumference | |||
| A | Baseline to 3 months | FFH | –0.024 (–0.156 to 0.109) | –0.160 (–0.274 to –0.046) |
| UC | –0.029 (–0.104 to 0.046) | 0.019 (–0.086 to 0.123) | ||
| p-value | 0.947 | 0.023 | ||
| Baseline to 12 months | FFH | 0.065 (–0.091 to 0.221) | 0.099 (–0.115 to 0.313) | |
| UC | –0.082 (–0.227 to 0.063) | –0.100 (–0.258 to 0.064) | ||
| p-value | 0.157 | 0.138 | ||
| B | Baseline to 3 months | FFH | 0.051 (–0.090 to 0.191) | 0.071 (–0.030 to 0.171) |
| UC | 0.016 (–0.114 to 0.147) | –0.038 (–0.136 to 0.059) | ||
| p-value | 0.701 | 0.106 | ||
| Baseline to 12 months | FFH | –0.001 (–0.180 to 0.177) | –0.108 (–0.263 to 0.047) | |
| UC | –0.115 (–0.321 to 0.091) | –0.169 (–0.339 to 0.000) | ||
| p-value | 0.366 | 0.556 | ||
| C | Baseline to 3 months | FFH | –0.069 (–0.151 to 0.120) | –0.175 (–0.367 to 0.017) |
| UC | –0.106 (–0.143 to –0.069) | –0.132 (–0.251 to –0.012) | ||
| p-value | 0.351 | 0.669 | ||
| Baseline to 12 months | FFH | –0.047 (–0.161 to 0.067) | –0.055 (–0.225 to 0.115) | |
| UC | –0.119 (–0.233 to –0.004) | –0.194 (–0.362 to –0.025) | ||
| p-value | 0.350 | 0.224 | ||
Subgroup socioeconomic status (four classes)
| Time point | Treatment group | Class | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Professional | Intermediate | Routine and manual | Unemployed | ||||||
| Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | Number of children | Mean child BMI z-score (95% CI) | ||
| Baseline | FFH | 27 | 2.70 (2.43 to 2.97) | 13 | 2.47 (2.09 to 2.85) | 14 | 2.81 (2.43 to 3.18) | 9 | 2.76 (2.20 to 3.30) |
| UC | 17 | 2.34 (2.12 to 2.55) | 8 | 2.77 (2.17 to 3.37) | 26 | 2.87 (2.56 to 3.19) | 14 | 2.96 (2.57 to 3.35) | |
| 3 months | FFH | 22 | 2.59 (2.29 to 2.89) | 9 | 2.49 (1.95 to 3.04) | 10 | 2.75 (2.31 to 3.18) | 7 | 2.74 (1.99 to 3.50) |
| UC | 16 | 2.29 (2.02 to 2.55) | 5 | 2.29 (1.46 to 3.13) | 21 | 2.88 (2.55 to 3.21) | 8 | 2.97 (2.35 to 3.60) | |
| 12 months | FFH | 22 | 2.70 (2.45 to 2.96) | 8 | 2.54 (2.05 to 3.02) | 10 | 2.72 (2.32 to 3.13) | 5 | 3.04 (2.10 to 3.97) |
| UC | 14 | 2.22 (1.88 to 2.55) | 4 | 2.54 (1.66 to 3.41) | 18 | 2.63 (2.25 to 3.01) | 7 | 3.18 (2.99 to 3.37) | |
| Class | Time period | Treatment group | Measurement, mean z-score (95% CI) | |
|---|---|---|---|---|
| BMI | Waist circumference | |||
| Managerial/professional | Baseline to 3 months | FFH | –0.038 (–0.138 to 0.062) | –0.058 (–0.164 to 0.049) |
| UC | –0.033 (–0.105 to 0.039) | –0.073 (–0.186 to 0.041) | ||
| p-value | 0.931 | 0.842 | ||
| Baseline to 12 months | FFH | 0.068 (–0.026 to 0.161) | 0.122 (–0.063 to 0.306) | |
| UC | –0.098 (–0.263 to 0.067) | –0.231 (–0.403 to –0.060) | ||
| p-value | 0.053 | 0.009 | ||
| Intermediate | Baseline to 3 months | FFH | –0.098 (–0.260 to 0.064) | –0.148 (–0.437 to 0.141) |
| UC | –0.108 (–0.406 to 0.191) | –0.039 (–0.282 to 0.204) | ||
| p-value | 0.939 | 0.561 | ||
| Baseline to 12 months | FFH | –0.165 (–0.376 to 0.046) | –0.089 (–0.390 to 0.213) | |
| UC | 0.013 (–0.243 to 0.217) | 0.010 (–0.389 to 0.410) | ||
| p-value | 0.298 | 0.637 | ||
| Routine and manual | Baseline to 3 months | FFH | 0.112 (–0.148 to 0.373) | –0.099 (–0.258 to 0.060) |
| UC | –0.021 (–0.103 to 0.061) | –0.024 (–0.127 to 0.080) | ||
| p-value | 0.295 | 0.394 | ||
| Baseline to 12 months | FFH | 0.067 (–0.209 to 0.343) | –0.094 (–0.241 to 0.053) | |
| UC | –0.112 (–0.255 to 0.032) | –0.084 (–0.240 to 0.071) | ||
| p-value | 0.175 | 0.930 | ||
| Long-term unemployed | Baseline to 3 months | FFH | –0.046 (–0.194 to 0.102) | –0.251 (–0.539 to 0.038) |
| UC | –0.076 (–0.180 to 0.027) | –0.071 (–0.268 to 0.126) | ||
| p-value | 0.689 | 0.226 | ||
| Baseline to 12 months | FFH | –0.053 (–0.391 to 0.286) | –0.190 (–0.631 to 0.251) | |
| UC | 0.100 (–0.385 to 0.119) | –0.215 (–0.443 to 0.014) | ||
| p-value | 0.627 | 0.888 | ||
Appendix 4 Resource utilisation
| Resource variable | Treatment group | p-valuea | |
|---|---|---|---|
| FFH (n = 45) | UC (n = 43) | ||
| Hospital care, mean (SE) | |||
| Number of inpatient admissions | 0 | 0 | |
| Accident and emergency visits | 0.24 (0.14) | 0.23 (0.09) | 0.944 |
| Outpatient department visits | 0.38 (0.11) | 0.26 (0.11) | 0.429 |
| Day hospital visits | 0 | 0.02 (0.02) | 0.323 |
| Other hospital visits | 0.02 (0.02) | 0.02 (0.02) | 0.974 |
| Community-based health-care contacts, mean (SE) | |||
| GP at surgery | 0.78 (0.17) | 0.81 (0.17) | 0.883 |
| GP at home | 0 | 0 | |
| Practice nurse | 0.11 (0.07) | 0.07 (0.04) | 0.618 |
| Dentist | 0.84 (0.11) | 0.95 (0.15) | 0.563 |
| Optician | 0.42 (0.09) | 0.49 (0.13) | 0.674 |
| Dietitian | 0 | 0 | |
| Child development centre | 0 | 0.09 (0.09) | 0.323 |
| Child guidance unit | 0 | 0 | |
| General health (other) | 0.20 (0.13) | 0.07 (0.07) | 0.379 |
| Family therapist | 0 | 0.28 (0.28) | 0.323 |
| Individual therapy | 0 | 0.14 (0.14) | 0.323 |
| Psychiatrist/psychologist | 0 | 0 | |
| Counselling (other) | 0.35 (0.17) | 0.02 (0.02) | 0.066 |
| Community-based social care contacts, mean (SE) | |||
| Social worker at office | 0 | 0 | |
| Social worker at home | 0.09 (0.06) | 0.02 (0.02) | 0.327 |
| After-school club | 0 | 0 | |
| Social care (other) | 0 | 0.14 (0.10) | 0.183 |
| Medication usage, n (%) | |||
| Prescribed | 23 (51.1) | 19 (44.2) | 0.516 |
| Over the counter | 24 (53.3) | 29 (67.4) | 0.176 |
| Educational support, mean (SE) | |||
| School nurse | 0.31 (0.12) | 1.19 (0.65) | 0.190 |
| Educational psychologist | 0.53 (0.53) | 0.05 (0.05) | 0.368 |
| Educational welfare officer | 0 | 0.05 (0.05) | 0.323 |
| SEN co-ordinator | 0.33 (0.27) | 2.63 (1.97) | 0.255 |
| Additional meetings with tutors | 0 | 0 | |
| Other educational support | 0.09 (0.05) | 1.72 (1.06) | 0.130 |
| Parents’ days off work | 0.49 (0.23) | 0.26 (0.11) | 0.354 |
Appendix 5 Usual-care attendance
| Programme/resource variable | Treatment group | |
|---|---|---|
| FFH (n = 45) | UC (n = 42) | |
| One Body One Life, mean contacts (SE) | NA | 0.94 (0.50) |
| Change4Life, mean contacts (SE) | NA | 0.45 (0.21) |
| WISH, mean contacts (SE) | NA | 5 (5.00) |
| Weight Watchers, mean contacts (SE) | NA | 0 |
| School nurse, mean contacts (SE) | NA | 0 |
Appendix 6 Economic costs
| Cost category | Treatment group (£) | p-valuea | |
|---|---|---|---|
| FFH (n = 63) | UC (n = 65) | ||
| Hospital inpatient care, mean (SE) | 9.90 (9.90) | 0 | 0.321 |
| Other hospital-based care, mean (SE) | |||
| Accident and emergency | 1.83 (1.83) | 12.40 (5.13) | 0.056 |
| Outpatient departments | 48.00 (14.80) | 66.88 (27.62) | 0.548 |
| Day hospital | 2.49 (2.49) | 0 | 0.321 |
| Other | 4.44 (3.12) | 7.15 (7.15) | 0.729 |
| Total other hospital care costs | 56.76 (15.89) | 86.43 (28.23) | 0.362 |
| Community-based health care, mean (SE) | |||
| GP at surgery | 21.05 (4.03) | 25.50 (5.47) | 0.514 |
| GP at home | 0 | 0 | |
| Practice nurse | 1.05 (0.62) | 0.43 (0.22) | 0.353 |
| Dentist | 15.10 (4.99) | 20.85 (4.82) | 0.409 |
| Optician | 15.74 (5.93) | 12.46 (4.95) | 0.671 |
| Dietitian | 0.29 (0.29) | 2.42 (1.35) | 0.128 |
| Child development centre | 0.17 (0.17) | 0 | 0.321 |
| Child guidance unit | 0 | 0 | |
| General health (other) | 2.41 (1.76) | 2.96 (2.23) | 0.848 |
| Family therapist | 0 | 0 | |
| Individual therapy | 0 | 0 | |
| Psychiatrist/psychologist | 0 | 4.37 (3.07) | 0.159 |
| Counselling (other) | 16.19 (14.81) | 0 | 0.279 |
| Total community-based health costs | 72.01 (17.60) | 68.99 (10.66) | 0.884 |
| Community-based social care, mean (SE) | |||
| Social worker at office | 0 | 0 | |
| Social worker at home | 1.24 (0.76) | 7.60 (5.33) | 0.243 |
| After-school club | 0 | 0 | |
| Social care (other) | 0.21 (0.15) | 33.43 (30.10) | 0.274 |
| Total community-based social costs | 1.46 (0.77) | 41.03 (30.44) | 0.198 |
| Medication, mean (SE) | |||
| Prescribed | 2.51 (0.59) | 4.01 (1.47) | 0.347 |
| Over the counter | 1.21 (0.33) | 1.25 (0.32) | 0.930 |
| Educational support, mean (SE) | |||
| School nurse | 2.43 (0.67) | 2.05 (0.80) | 0.722 |
| Educational psychologist | 0.63 (0.63) | 14.28 (12.20) | 0.268 |
| Educational welfare officer | 0 | 2.69 (1.91) | 0.163 |
| SEN co-ordinator | 5.42 (4.38) | 5.77 (3.14) | 0.949 |
| Additional meetings with tutors | 0 | 0 | |
| Other educational support | 12.57 (10.41) | 39.99 (19.21) | 0.383 |
| Total educational support costs | 21.04 (11.60) | 64.78 (26.44) | 0.133 |
| Other costs, mean (SE) | |||
| Change in family expenditure | 51.65 (14.80) | 54.08 (13.39) | 0.903 |
| Lost productivity | 23.80 (12.59) | 8.17 (6.19) | 0.268 |
| Travel costs | 4.12 (0.87) | 8.76 (3.80) | 0.238 |
| Total NHS and PSS costs, mean (SE) | 163.68 (31.49) | 265.24 (54.08) | 0.108 |
| Total societal costs, mean (SE) | 244.47 (38.33) | 337.50 (59.31) | 0.190 |
Appendix 7 Costs of care
| Cost category | Treatment group (£) | p-valuea | |
|---|---|---|---|
| FFH (n = 45) | UC (n = 43) | ||
| Hospital inpatient care, mean (SE) | 0 | 0 | |
| Other hospital-based care, mean (SE) | |||
| Accident and emergency | 28.15 (16.02) | 26.78 (10.73) | 0.944 |
| Outpatient departments | 71.40 (21.06) | 48.35 (19.98) | 0.429 |
| Day hospital | 3.65 (3.65) | 0.323 | |
| Other | 0.94 (0.94) | 0 | 0.323 |
| Total other hospital care costs | 100.49 (30.58) | 78.78 (26.34) | 0.592 |
| Community-based health care, mean (SE) | |||
| GP at surgery | 28.51 (6.10) | 29.48 (6.17) | 0.912 |
| GP at home | 0 | 0 | |
| Practice nurse | 2.26 (1.46) | 0.45 (0.29) | 0.232 |
| Dentist | 39.34 (6.56) | 41.24 (11.93) | 0.890 |
| Optician | 22.92 (5.87) | 28.22 (9.11) | 0.626 |
| Dietitian | 0 | 0 | |
| Child development centre | 0 | 3.92 (3.92) | 0.323 |
| Child guidance unit | 0 | 0 | |
| General health other | 17.68 (15.68) | 3.07 (3.07) | 0.365 |
| Family therapist | 0 | 13.95 (13.95) | 0.323 |
| Individual therapy | 0 | 3.49 (3.49) | 0.323 |
| Psychiatrist/psychologist | 0 | 0 | |
| Counselling (other) | 36.64 (18.75) | 0 | 0.071 |
| Total community-based health costs | 147.35 (30.80) | 123.82 (25.56) | 0.592 |
| Community-based social care, mean (SE) | |||
| Social worker at office | 0 | 0 | |
| Social worker at home | 1.76 (1.23) | 0.46 (0.46) | 0.327 |
| After-school club | 0 | 0 | |
| Social care (other) | 1.37 (1.18) | 0.249 | |
| FFH/UC costs | 0 | 9.72 (9.72) | 0.323 |
| Total community-based social and other costs | 1.76 (1.23) | 11.55 (9.75) | 0.325 |
| Medications, mean (SE) | |||
| Prescribed | 12.47 (3.72) | 5.44 (1.58) | 0.088 |
| Over the counter | 0 | ||
| Educational support, mean (SE) | |||
| School nurse | 3.69 (1.85) | 8.40 (3.53) | 0.242 |
| Educational psychologist | 17.56 (17.56) | 4.10 (4.10) | 0.459 |
| Educational welfare officer | 0 | 0.58 (0.58) | 0.323 |
| SEN co-ordinator | 3.33 (2.46) | 43.70 (35.53) | 0.263 |
| Additional meetings with tutors | 0 | 0 | |
| Other educational support | 3.91 (2.40) | 35.78 (24.66) | 0.205 |
| Total educational support costs | 28.49 (17.71) | 92.57 (42.92) | 0.173 |
| Other costs, mean (SE) | |||
| Change in family expenditure | 168.28 (50.45) | 214.54 (70.79) | 0.596 |
| Lost productivity | 45.32 (20.52) | 31.45 (13.41) | 0.573 |
| Travel costs | 7.60 (1.32) | 14.41 (5.87) | 0.264 |
| Total NHS and PSS costs, mean (SE) | 288.56 (46.13) | 302.45 (53.85) | 0.845 |
| Total societal costs, mean (SE) | 509.76 (75.01) | 562.86 (94.79) | 0.662 |
Appendix 8 Subgroup analyses: economic evaluation
| Outcome measure by subgroup | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH | UC | FFH | UC | |||||||||
| QALYs | ||||||||||||
| Sample size (n) | 18 | 15 | 18 | 15 | ||||||||
| Age group (6–8 years) | 1003.58 (781 to 1227) | 556.43 (336 to 777) | 447.15 (118 to 776) | 0.792 (0.7064 to 0.8776) | 0.7804 (0.6706 to 0.8903) | 0.0116 (–0.1329 to 0.156) | 38,686 | 56 | < 1 | 40 | 44 | 47 |
| Sample size (n) | 23 | 23 | 23 | 23 | ||||||||
| Age group (9–11 years) | 1030.19 (825 to 1236) | 474.17 (289 to 659) | 556.01 (279 to 833) | 0.8624 (0.7784 to 0.9463) | 0.8632 (0.8105 to 0.916) | –0.0009 (–0.1000 to 0.0983) | –647,507 (UC dominates) | 52 | < 1 | 22 | 29 | 37 |
| Sample size (n) | 18 | 18 | 18 | 18 | ||||||||
| Sex (male) | 1029.31 (836 to 1223) | 492.80 (293 to 692) | 536.5 (259 to 814) | 0.8123 (0.7101 to 0.9145) | 0.8628 (0.7693 to 0.9562) | –0.0504 (–0.1889 to 0.0880) | –10,637 (UC dominates) | 23 | < 1 | 12 | 15 | 18 |
| Sample size (n) | 23 | 20 | 23 | 20 | ||||||||
| Sex (female) | 1010.05 (787 to 1233) | 519.10 (317 to 722) | 490.95 (178 to 804) | 0.8465 (0.7726 to 0.9203) | 0.8016 (0.7414 to 0.8617) | 0.0449 (–0.0545 to 0.1443) | 10,933 | 83 | < 1 | 60 | 67 | 73 |
| Sample size (n) | 20 | 16 | 20 | 16 | ||||||||
| Site A | 1133.68 (958 to 1309) | 451.07 (244 to 658) | 682.61 (397 to 968) | 0.871 (0.8104 to 0.9316) | 0.8155 (0.7107 to 0.9202) | 0.0555 (–0.0692 to 0.1802) | 12,299 | 80 | < 1 | 53 | 61 | 67 |
| Sample size (n) | 9 | 10 | 9 | 10 | ||||||||
| Site B | 982.56 (545 to 1420) | 706.42 (366 to 1047) | 276.15 (–260 to 813) | 0.7116 (0.5763 to 0.8469) | 0.8045 (0.7139 to 0.8951) | –0.0929 (–0.25 to 0.0642) | –2972 (UC dominates) | 14 | < 1 | 9 | 11 | 12 |
| Sample size (n) | 12 | 12 | 12 | 12 | ||||||||
| Site C | 853.51 (595 to 1112) | 414.26 (221 to 608) | 439.24 (116 to 762) | 0.8555 (0.714 to 0.9971) | 0.8723 (0.8000 to 0.9447) | –0.0168 (–0.1757 to 0.1421) | –26,142 (UC dominates) | 46 | < 1 | 33 | 36 | 39 |
| BMI z-scored | ||||||||||||
| Sample size (n) | 18 | 17 | 18 | 17 | ||||||||
| Age group (6–8 years) | 1003.58 (781 to 1227) | 676.88 (424 to 930) | 326.70 (–15 to 668) | 0.0325 (–0.1259 to 0.1908) | 0.0996 (–0.0256 to 0.2248) | –0.0671 (–0.2726 to 0.1384) | –4868 (UC dominates) | 23 | < 1 | 18 | 19 | 20 |
| Sample size (n) | 27 | 26 | 27 | 26 | ||||||||
| Age group (9–11 years) | 994.70 (808 to 1181) | 463.46 (299 to 628) | 531.23 (280 to 782) | –0.043 (–0.128 to 0.0419) | 0.1029 (–0.0033 to 0.2092) | –0.146 (–0.283 to –0.0089) | –3640 (UC dominates) | 1 | < 1 | < 1 | < 1 | < 1 |
| Sample size (n) | 19 | 23 | 19 | 23 | ||||||||
| Sex (male) | 1034.87 (852 to 1218) | 572.83 (369 to 777) | 462.05 (199 to 725) | 0.0799 (–0.0685 to 0.2283) | 0.1463 (0.0512 to 0.2414) | –0.0664 (–0.2314 to 0.0986) | –6957 (UC dominates) | 22 | < 1 | 14 | 15 | 16 |
| Sample size (n) | 26 | 20 | 26 | 20 | ||||||||
| Sex (female) | 971.48 (764 to 1179) | 519.10 (317 to 722) | 452.39 (141 to 764) | –0.0806 (–0.1626 to 0.0014) | 0.0502 (–0.0821 to 0.1824) | –0.1308 (-0.2927 to 0.0311) | –3459 (UC dominates) | 6 | < 1 | 2 | 3 | 4 |
| Sample size (n) | 20 | 19 | 20 | 19 | ||||||||
| Site A | 1133.68 (958 to 1309) | 566.48 (330 to 803) | 567.20 (270 to 865) | –0.065 (–0.2112 to 0.0813) | 0.0821 (–0.0534 to 0.2176) | –0.147 (–0.3493 to 0.0552) | –3857 (UC dominates) | 8 | < 1 | 3 | 4 | 5 |
| Sample size (n) | 10 | 11 | 10 | 11 | ||||||||
| Site B | 942.87 (544 to 1342) | 688.29 (379 to 998) | 254.58 (–236 to 745) | 0.0014 (–0.1535 to 0.1563) | 0.1150 (–0.0661 to 0.2961) | –0.1136 (–0.3473 to 0.1201) | –2241 (UC dominates) | 16 | < 1 | 13 | 14 | 14 |
| Sample size (n) | 15 | 13 | 15 | 13 | ||||||||
| Site C | 854.60 (627 to 1082) | 401.74 (222 to 581) | 452.86 (150 to 756) | 0.0471 (–0.0568 to 0.1511) | 0.1187 (0.0155 to 0.222) | –0.0716 (–0.2236 to 0.0804) | –6325 (UC dominates) | 17 | < 1 | 9 | 10 | 12 |
| Outcome measures by subgroup | Mean costs, £ (95% CI) | Mean effects (95% CI) | ICER (£) | Probability FFH is | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Difference | Treatment group | Difference | More effectivea (%) | Less costlya (%) | Cost-effectivea (%)b | Cost-effectivea (%)c | Cost-effectivea (%)c | ||||
| FFH | UC | FFH | UC | |||||||||
| QALYs | ||||||||||||
| Sample size (n) | 18 | 15 | 18 | 15 | ||||||||
| Age group (6–8 years) | 1316.75 (1014 to 1620) | 935.1 (608 to 1262) | 381.65 (–85 to 848) | 0.7920 (0.7064 to 0.8776) | 0.7804 (0.6706 to 0.8903) | 0.0116 (–0.1329 to 0.156) | 33,019 | 56 | < 1 | 41 | 45 | 49 |
| Sample size (n) | 23 | 23 | 23 | 23 | ||||||||
| Age group (9–11 years) | 1377.97 (1087 to 1669) | 766.08 (496 to 1037) | 611.89 (214 to 1010) | 0.8624 (0.7784 to 0.9463) | 0.8632 (0.8105 to 0.916) | –0.0009 (–0.1000 to 0.0983) | –712,579 (UC dominates) | 52 | < 1 | 20 | 27 | 36 |
| Sample size (n) | 18 | 18 | 18 | 18 | ||||||||
| Sex (male) | 1437.59 (1107 to 1768) | 960.63 (604 to 1317) | 476.96 (–9 to 963) | 0.8123 (0.7101 to 0.9145) | 0.8628 (0.7693 to 0.9562) | –0.0504 (–0.1889 to 0.0880) | –9456 (UC dominates) | 23 | < 1 | 14 | 16 | 18 |
| Sample size (n) | 23 | 20 | 23 | 20 | ||||||||
| Sex (female) | 1283.40 (1013 to 1553) | 717.75 (491 to 944) | 565.65 (198 to 933) | 0.8465 (0.7726 to 0.9203) | 0.8016 (0.7414 to 0.8617) | 0.0449 (–0.0545 to 0.1443) | 12,596 | 83 | < 1 | 56 | 63 | 71 |
| Sample size (n) | 20 | 16 | 20 | 16 | ||||||||
| Site A | 1452.42 (1163 to 1742) | 916.32 (534 to 1299) | 536.11 (35 to 1037) | 0.871 (0.8104 to 0.9316) | 0.8155 (0.7107 to 0.9202) | 0.0555 (–0.0692, 0.1802) | 9660 | 80 | < 1 | 59 | 64 | 69 |
| Sample size (n) | 9 | 10 | 9 | 10 | ||||||||
| Site B | 1444.30 (930 to 1958) | 917.82 (532 to 1304) | 526.48 (–96 to 1149) | 0.7116 (0.5763 to 0.8469) | 0.8045 (0.7139 to 0.8951) | –0.0929 (–0.25 to 0.0642) | –5667 (UC dominates) | 14 | < 1 | 6 | 7 | 9 |
| Sample size (n) | 12 | 12 | 12 | 12 | ||||||||
| Site C | 1112.31 (757 to 1468) | 650.59 (379 to 923) | 461.72 (14 to 910) | 0.8555 (0.714 to 0.9971) | 0.8723 (0.8000 to 0.9447) | –0.0168 (–0.1757 to 0.1421) | –27,480 (UC dominates) | 46 | < 1 | 31 | 35 | 40 |
| BMI z-scored | ||||||||||||
| Sample size (n) | 18 | 17 | 18 | 17 | ||||||||
| Age group (6–8 years) | 1316.75 (1014 to 1620) | 1107.93 (709 to 1507) | 208.82 (–298 to 716) | 0.0325 (–0.1259 to 0.1908) | 0.0996 (–0.0256 to 0.2248) | –0.0671 (–0.2726 to 0.1384) | –3112 (UC dominates) | 23 | < 1 | 19 | 20 | 21 |
| Sample size (n) | 27 | 26 | 27 | 26 | ||||||||
| Age group (9–11 years) | 1307.80 (1045 to 1570) | 734.99 (494 to 976) | 572.81 (213 to 933) | –0.043 (–0.128 to 0.0419) | 0.1029 (–0.0033 to 0.2092) | –0.146 (–0.283 to –0.0089) | –3924 (UC dominates) | 1 | < 1 | < 1 | < 1 | < 1 |
| Sample size (n) | 19 | 23 | 19 | 23 | ||||||||
| Sex (male) | 1427.11 (1114 to 1740) | 1025.63 (672 to 1379) | 401.47 (–53 to 856) | 0.0799 (–0.0685 to 0.2283) | 0.1463 (0.0512 to 0.2414) | –0.0664 (–0.2314 to 0.0986) | –6045 (UC dominates) | 22 | < 1 | 15 | 17 | 18 |
| Sample size (n) | 26 | 20 | 26 | 20 | ||||||||
| Sex (female) | 1226.82 (975 to 1479) | 717.75 (491 to 944) | 509.07 (143 to 875) | –0.0806 (–0.1626 to 0.0014) | 0.0502 (–0.0821 to 0.1824) | –0.1308 (–0.2927 to 0.0311) | –3893 (UC dominates) | 6 | < 1 | 2 | 3 | 4 |
| Sample size (n) | 20 | 19 | 20 | 19 | ||||||||
| Site A | 1452.42 (1163 to 1742) | 1049.51 (638 to 1461) | 402.91 (–104 to 910) | –0.065 (–0.2112 to 0.0813) | 0.0821 (–0.0534 to 0.2176) | –0.147 (–0.3493 to 0.0552) | –2740 (UC dominates) | 8 | < 1 | 4 | 5 | 5 |
| Sample size (n) | 10 | 11 | 10 | 11 | ||||||||
| Site B | 1383.97 (909 to 1859) | 880.48 (523 to 1237) | 503.49 (–73 to 1080) | 0.0014 (–0.1535 to 0.1563) | 0.1150 (–0.0661 to 0.2961) | –0.1136 (–0.3473 to 0.1201) | –4431 (UC dominates) | 16 | < 1 | 10 | 12 | 13 |
| Sample size (n) | 15 | 13 | 15 | 13 | ||||||||
| Site C | 1074.94 (770 to 1379) | 639.89 (389 to 891) | 435.05 (23 to 847) | 0.0471 (–0.0568 to 0.1511) | 0.1187 (0.0155 to 0.222) | –0.0716 (–0.2236 to 0.0804) | –6076 (UC dominates) | 17 | < 1 | 9 | 11 | 12 |
FIGURE 26.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: age group (6–8 years) from a NHS and Personal Social Services perspective.


FIGURE 27.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: age group (9–11 years) from a NHS and Personal Social Services perspective.


FIGURE 28.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: sex (male) from a NHS and Personal Social Services perspective.
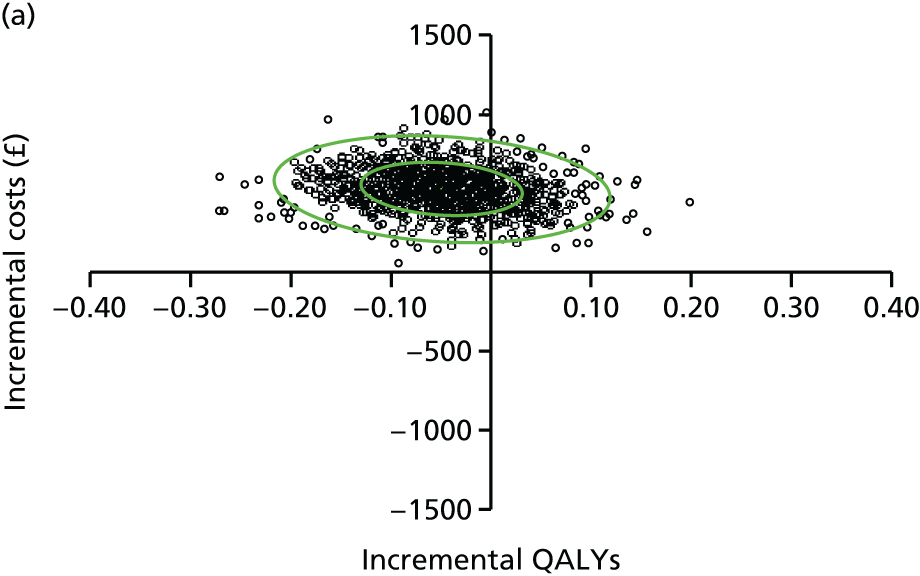
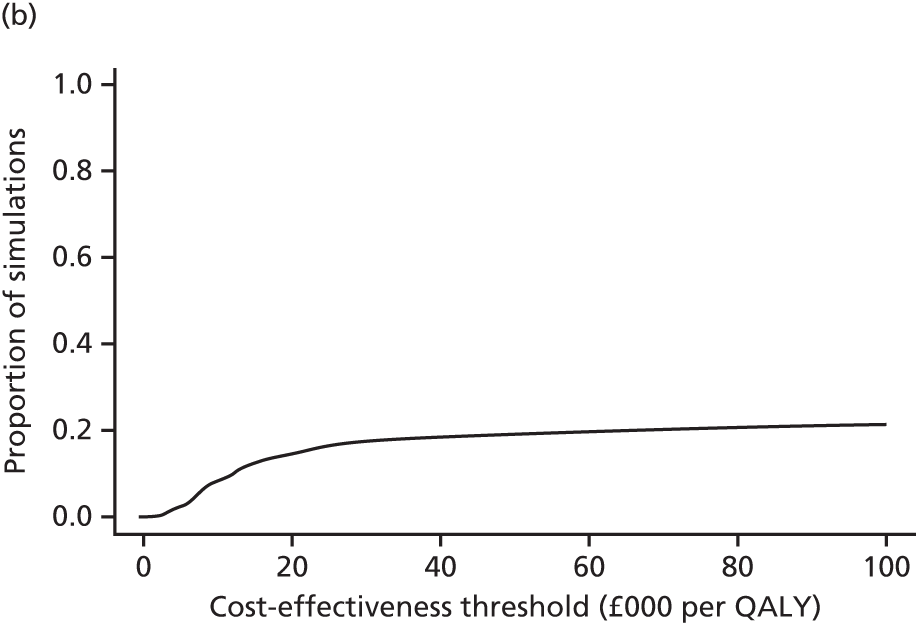
FIGURE 29.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: sex (female) from a NHS and Personal Social Services perspective.

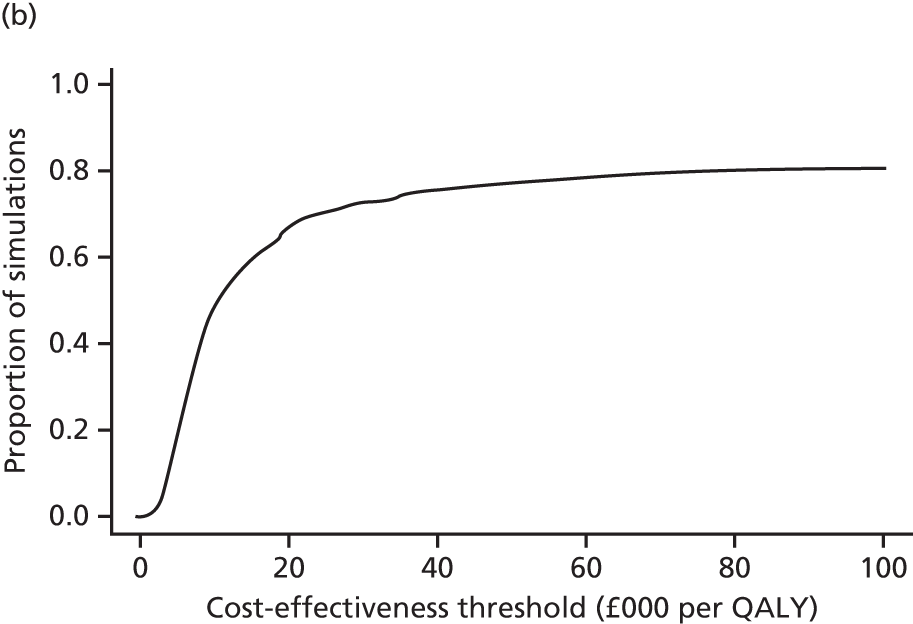
FIGURE 30.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site A from a NHS and Personal Social Services perspective.

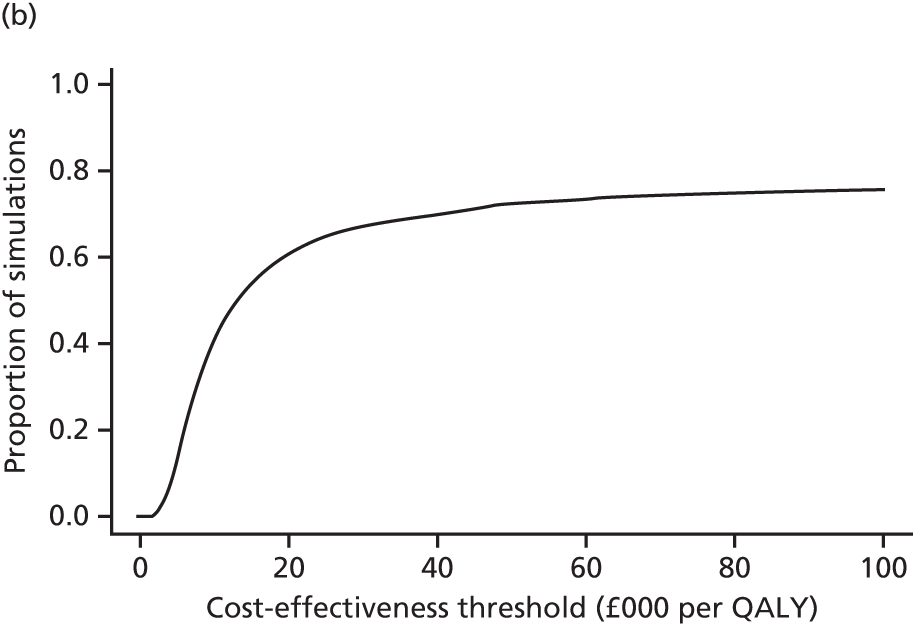
FIGURE 31.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site B from a NHS and Personal Social Services perspective.

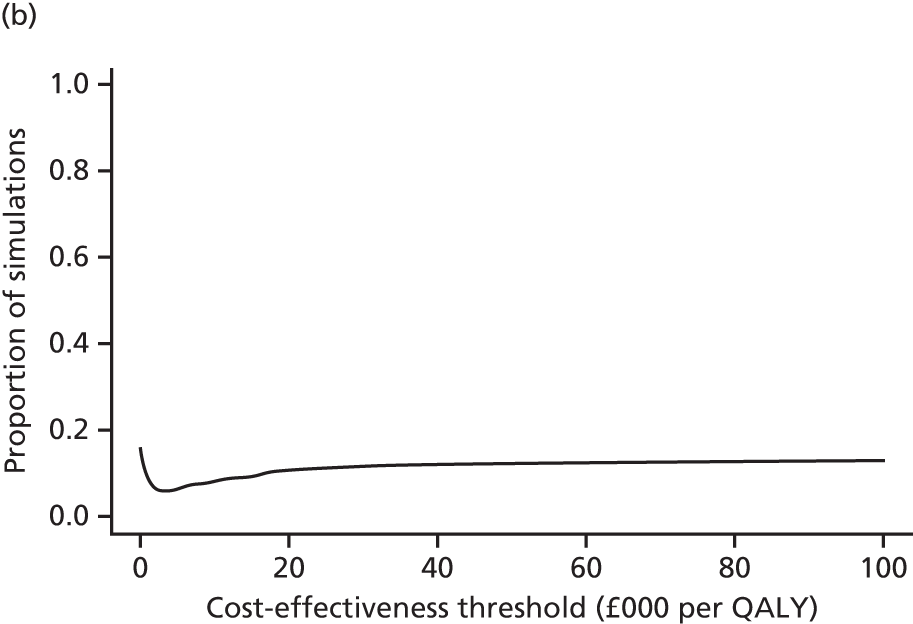
FIGURE 32.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site C from a NHS and Personal Social Services perspective.

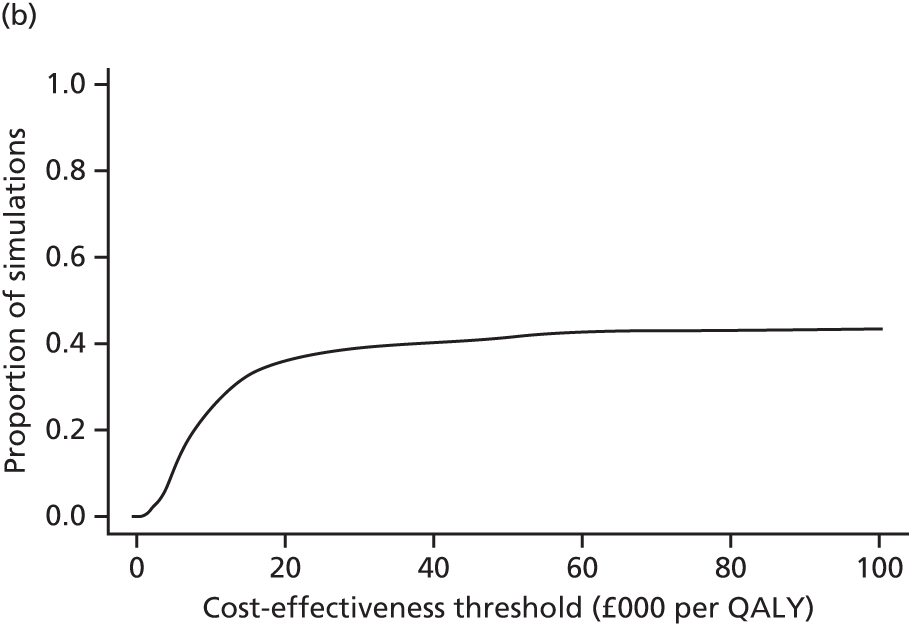
FIGURE 33.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: age group (6–8 years) from a NHS and Personal Social Services perspective.
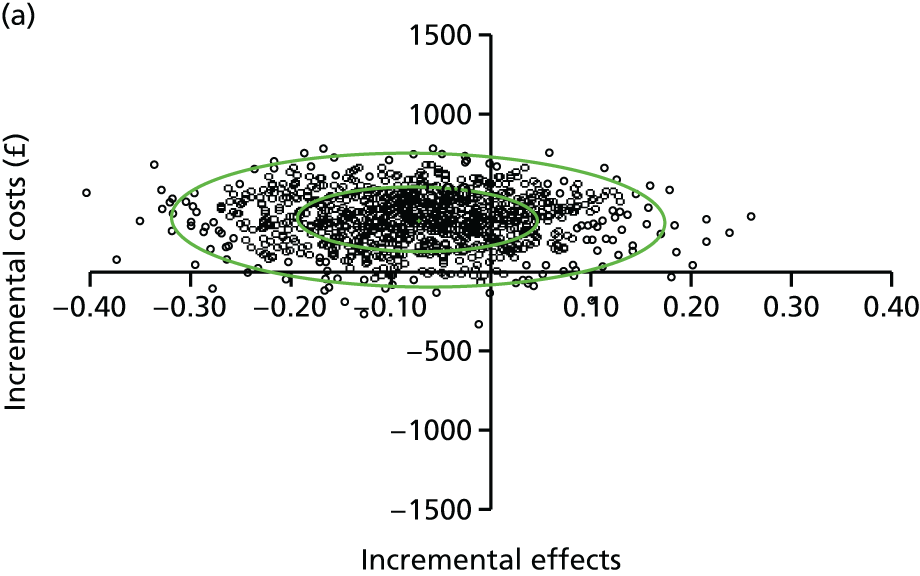
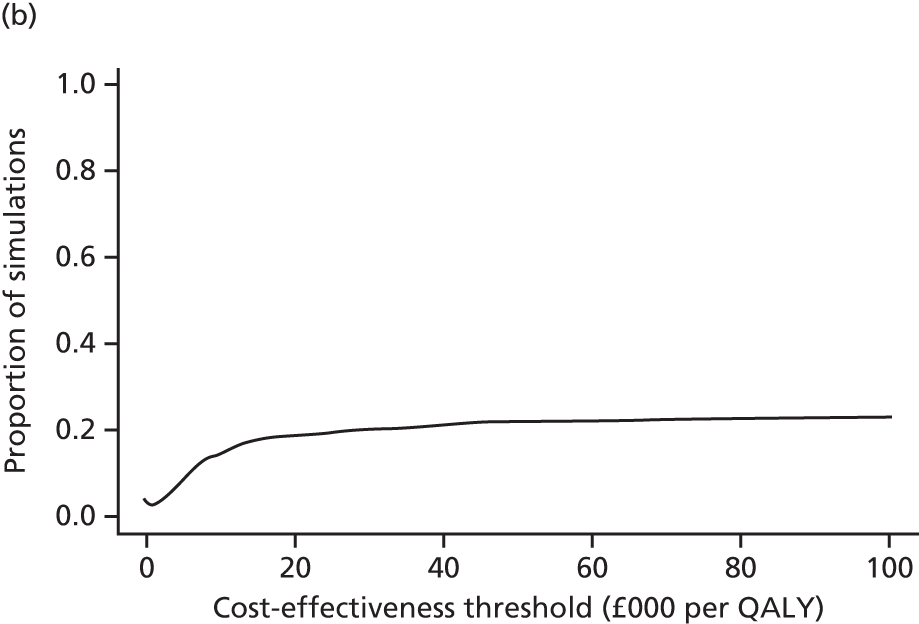
FIGURE 34.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: age group (9–11 years) from a NHS and Personal Social Services perspective.
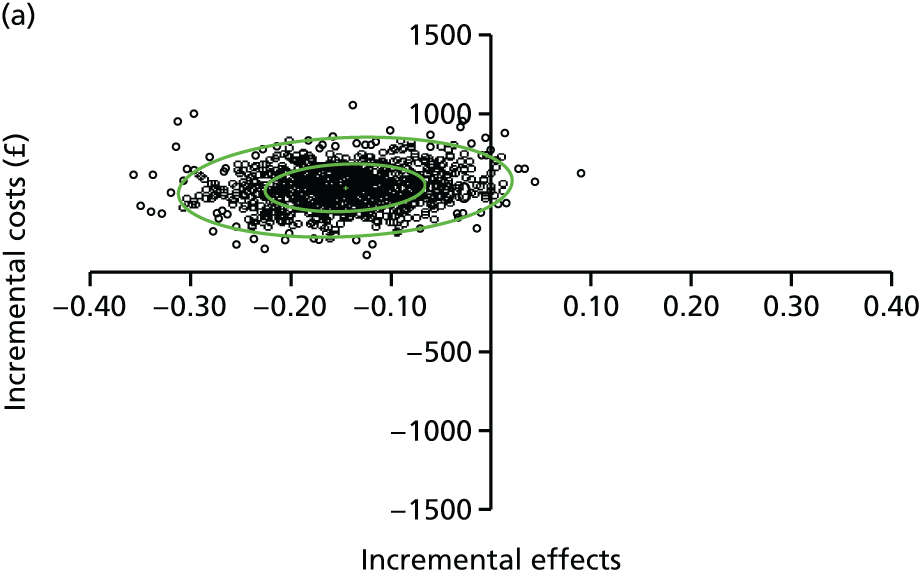
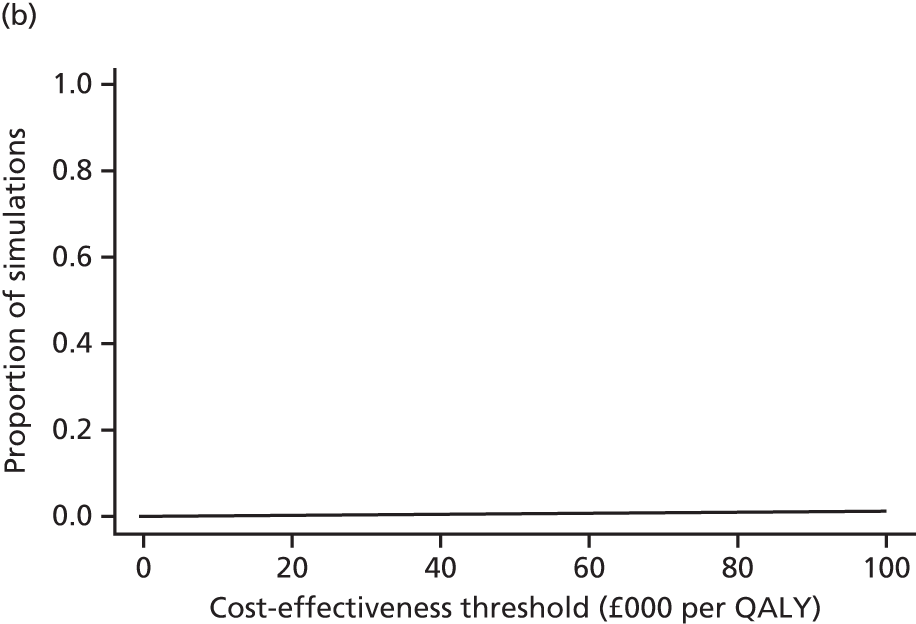
FIGURE 35.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: sex (male) from a NHS and Personal Social Services perspective.
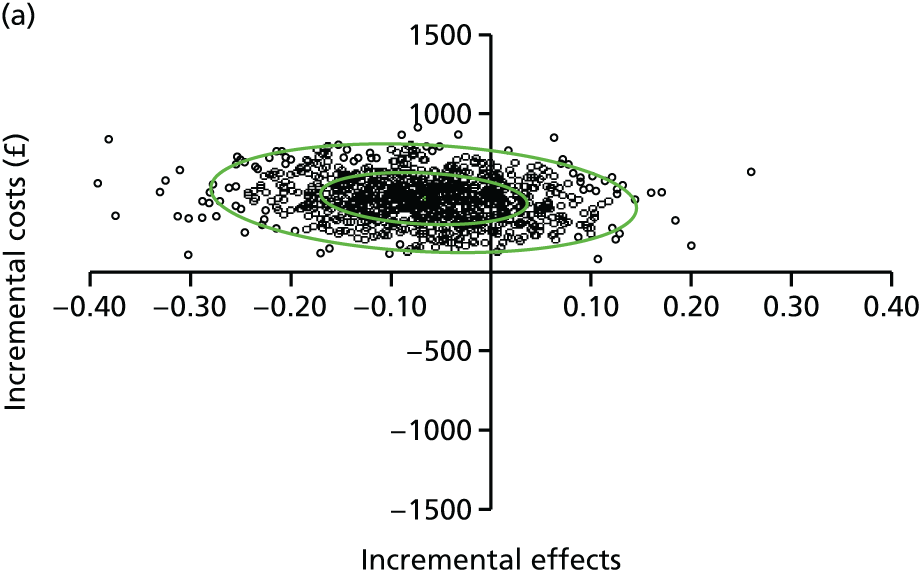
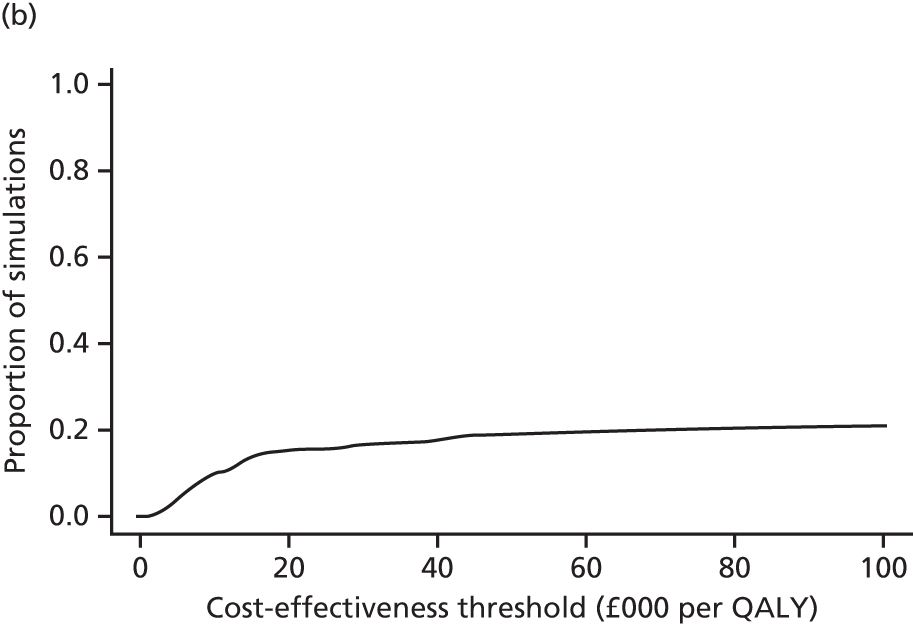
FIGURE 36.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: sex (female) from a NHS and Personal Social Services perspective.
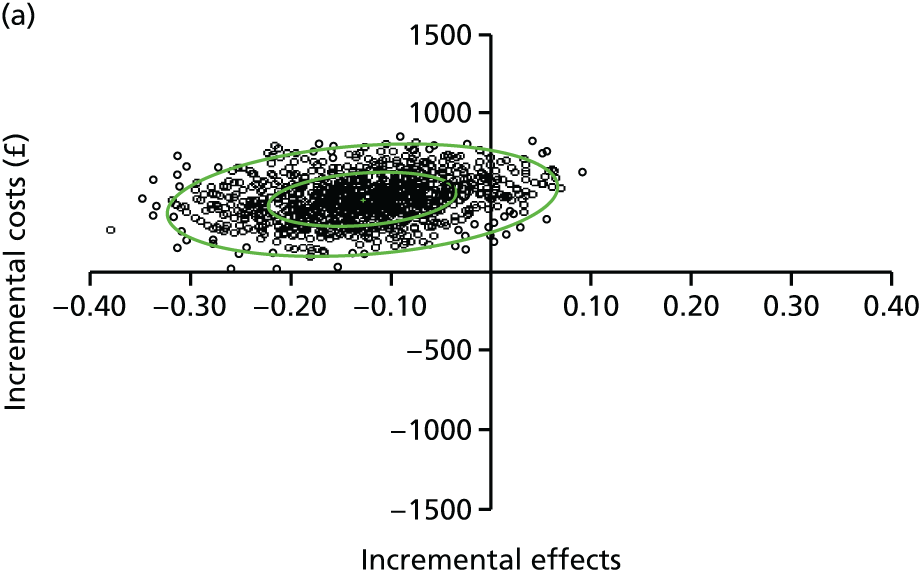

FIGURE 37.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: site A from a NHS and Personal Social Services perspective.
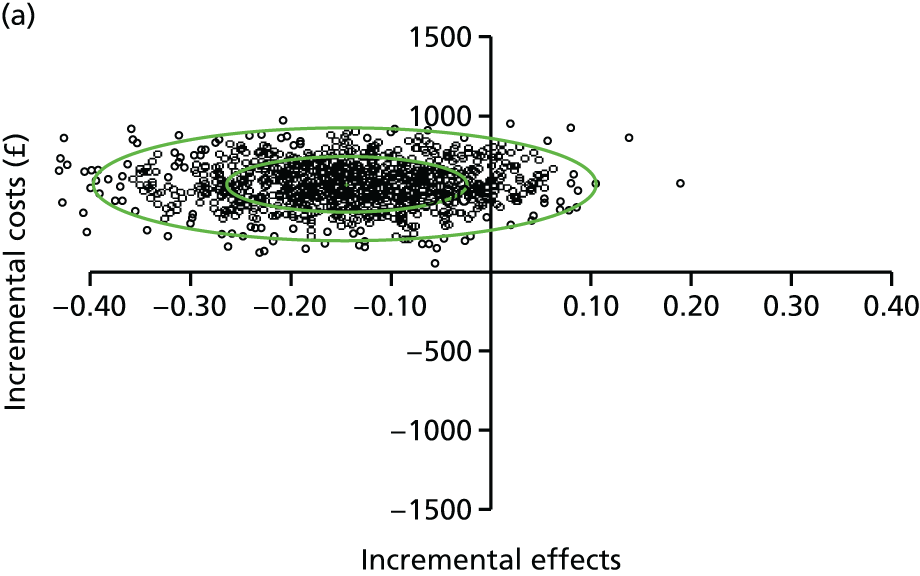

FIGURE 38.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: site B from a NHS and Personal Social Services perspective.


FIGURE 39.
(a) Cost-effectiveness plane; and (b) for BMI z-score outcome, subgroup analyses: site C from a NHS and Personal Social Services perspective.

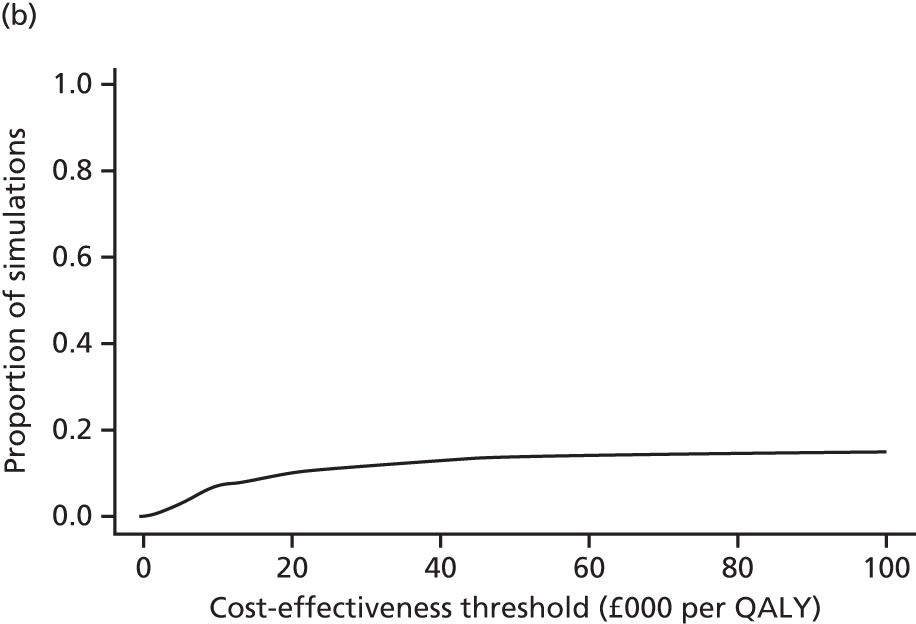
FIGURE 40.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: age group (6–8 years) from a societal perspective.

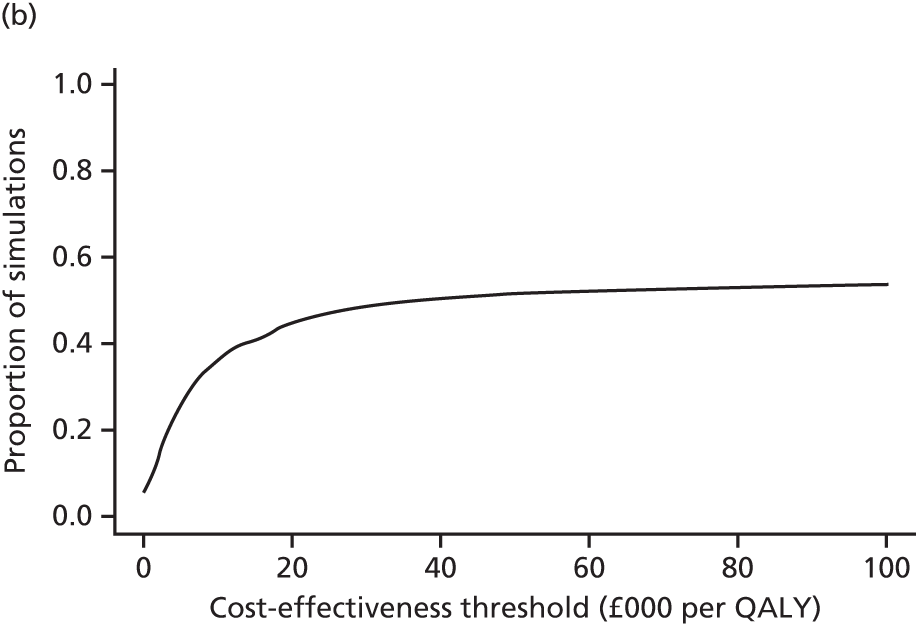
FIGURE 41.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: age group (9–11 years) from a societal perspective.
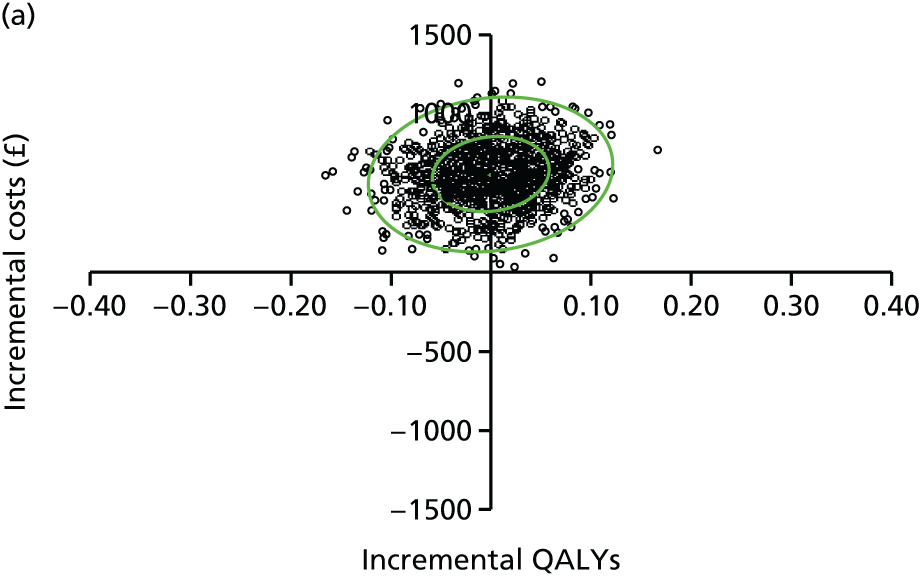
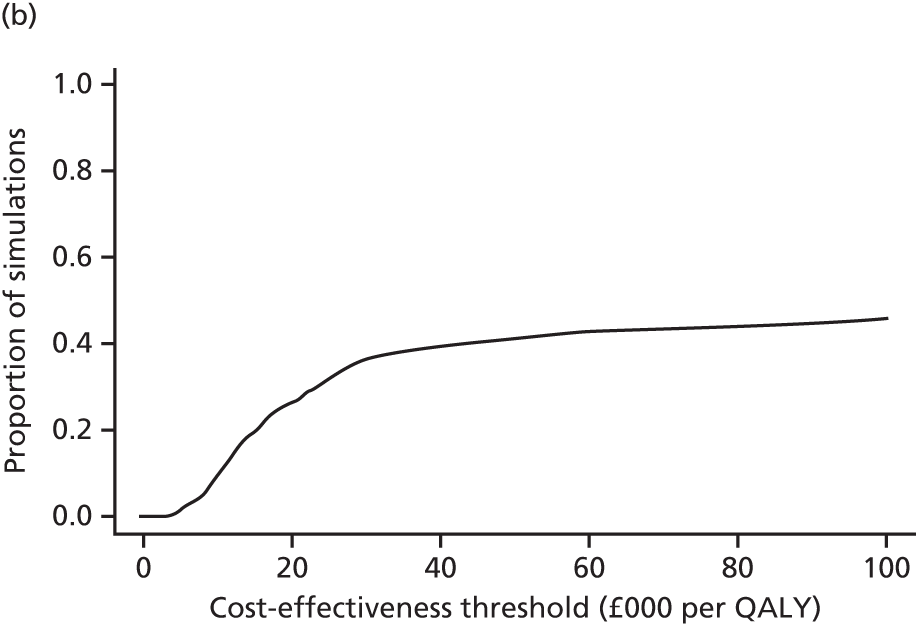
FIGURE 42.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: sex (male) from a societal perspective.


FIGURE 43.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: sex (female) from a societal perspective

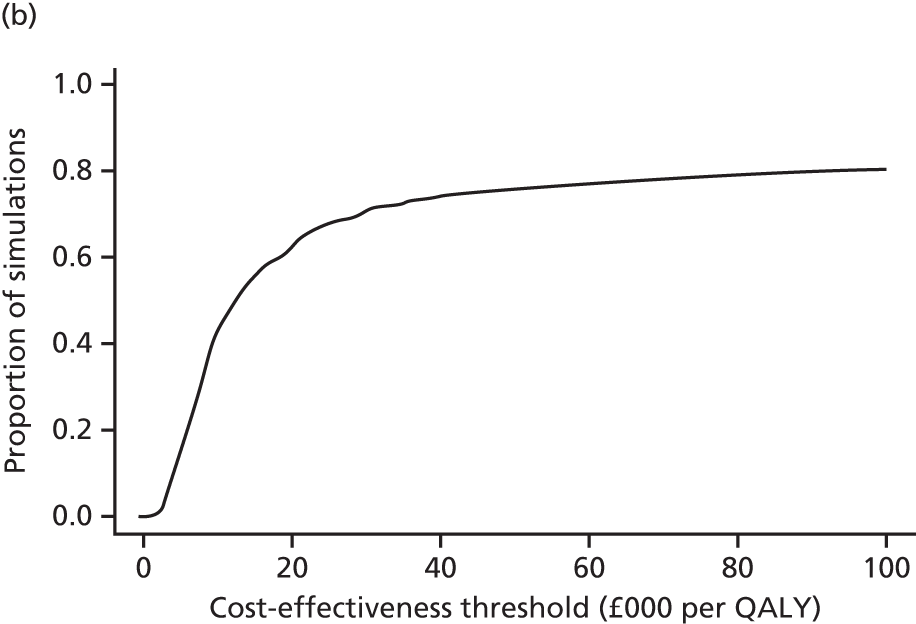
FIGURE 44.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site A from a societal perspective.
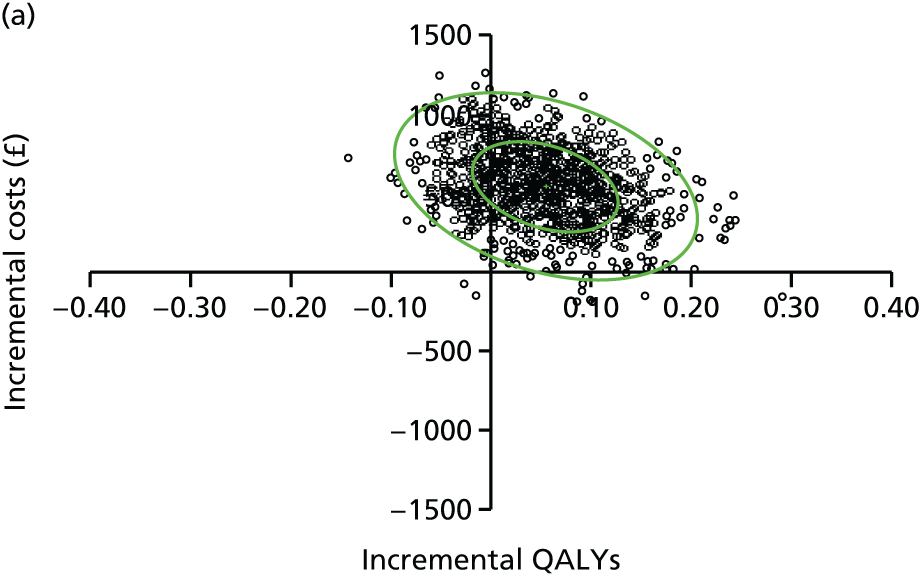

FIGURE 45.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site B from a societal perspective.
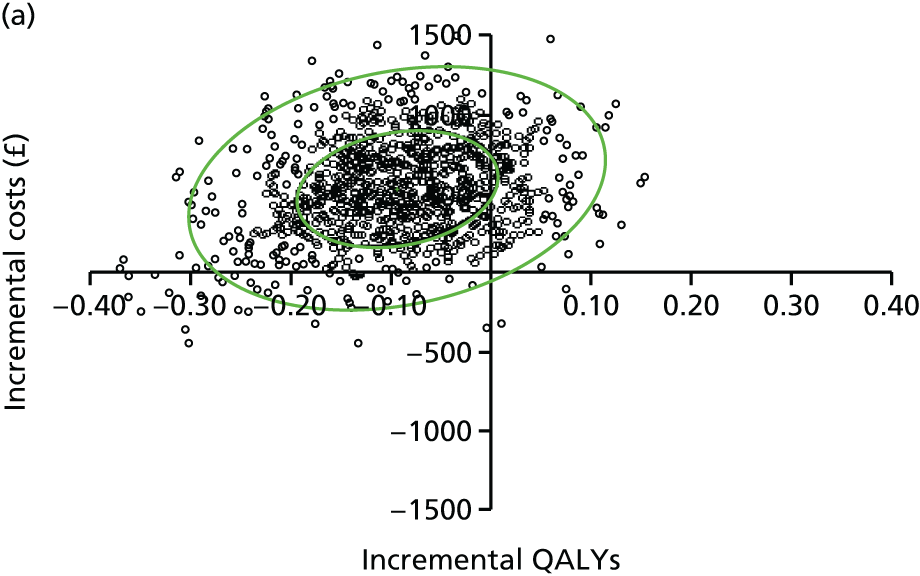

FIGURE 46.
(a) Cost-effectiveness plane; and (b) CEAC for QALY outcome, subgroup analyses: site C from a societal perspective.
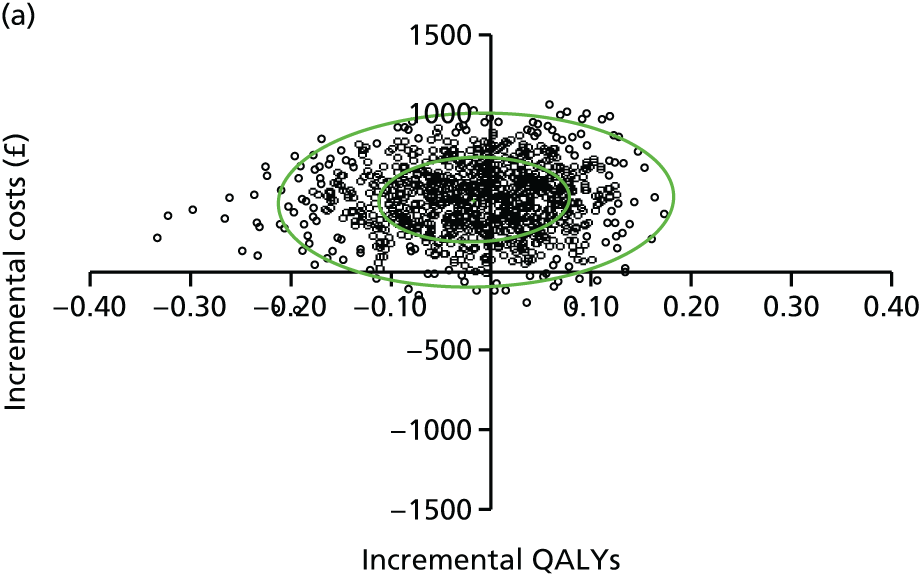
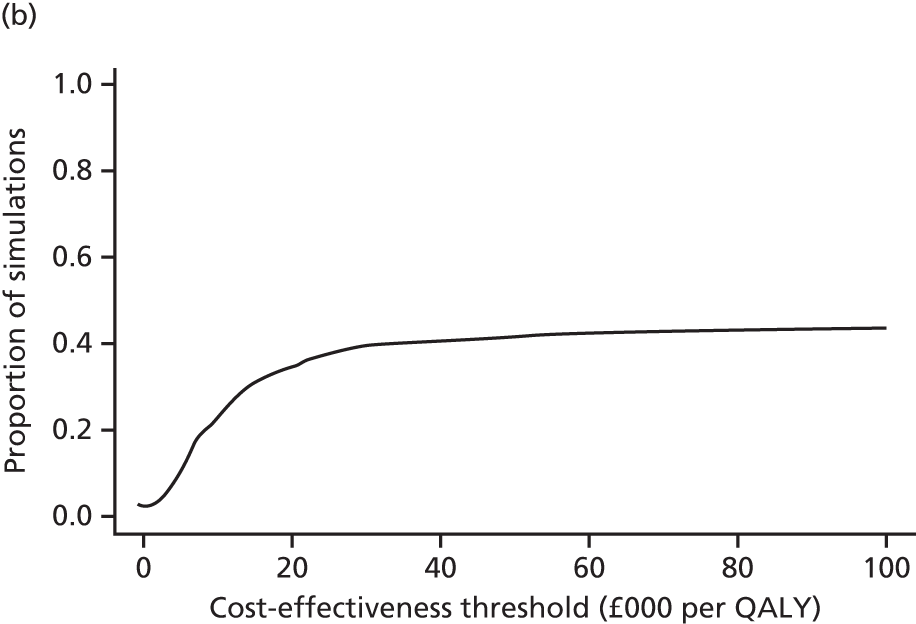
FIGURE 47.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: age group (6–8 years) from a societal perspective.
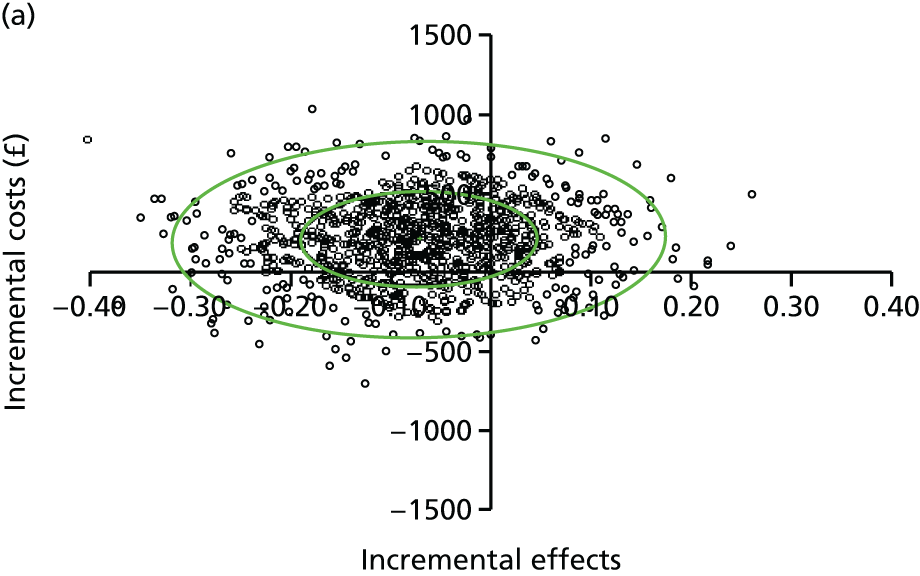
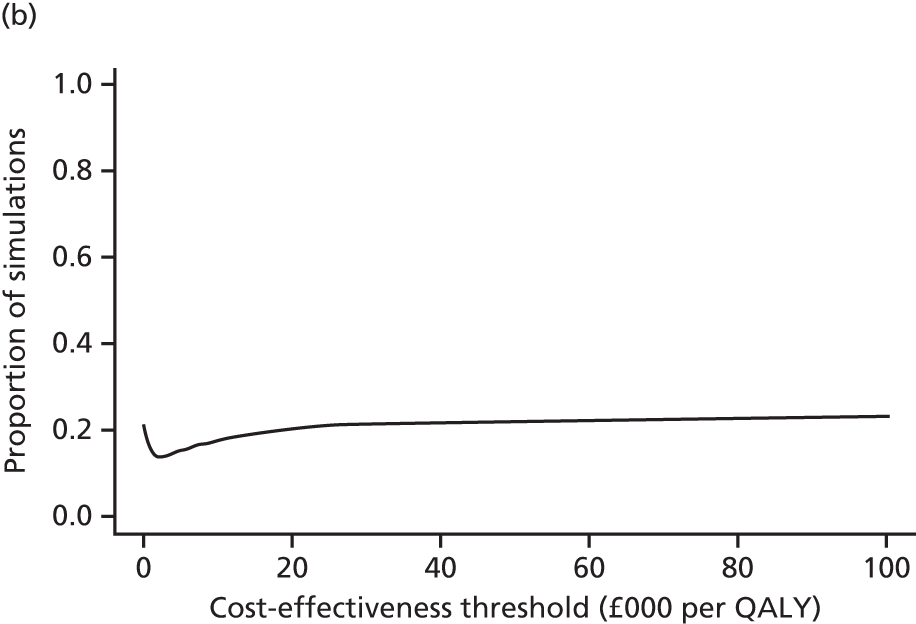
FIGURE 48.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: age group (9–11 years) from a societal perspective.

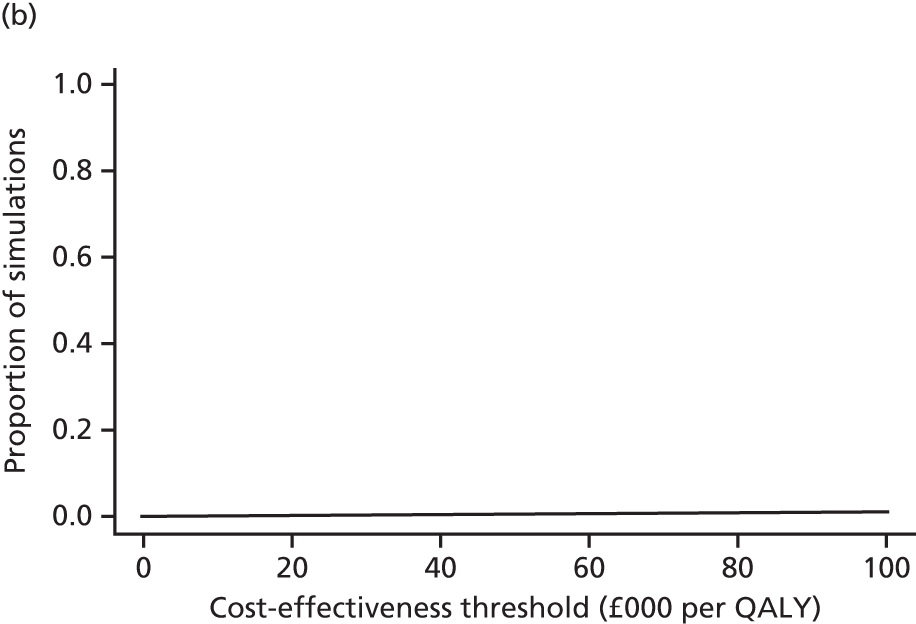
FIGURE 49.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: sex (male) from a societal perspective.


FIGURE 50.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: sex (female) from a societal perspective.
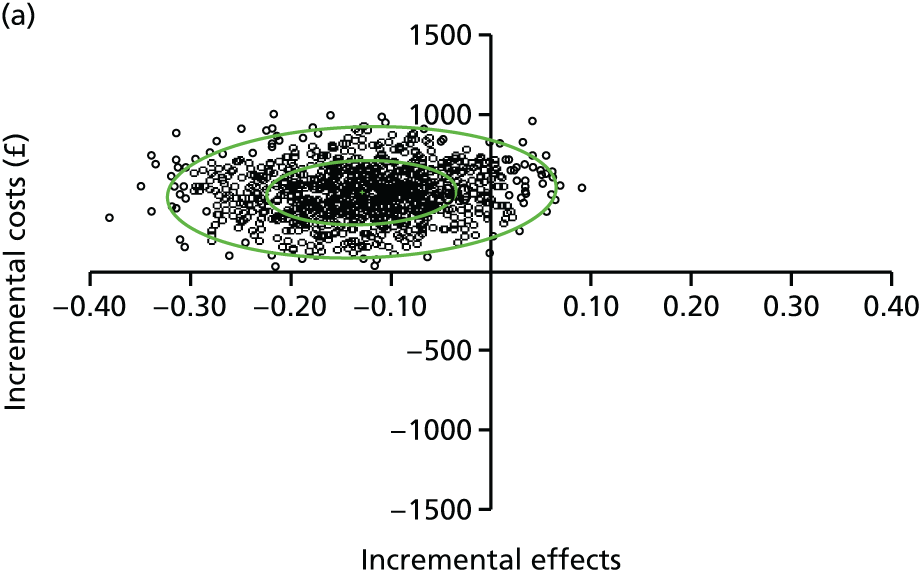

FIGURE 51.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: site A from a societal perspective.
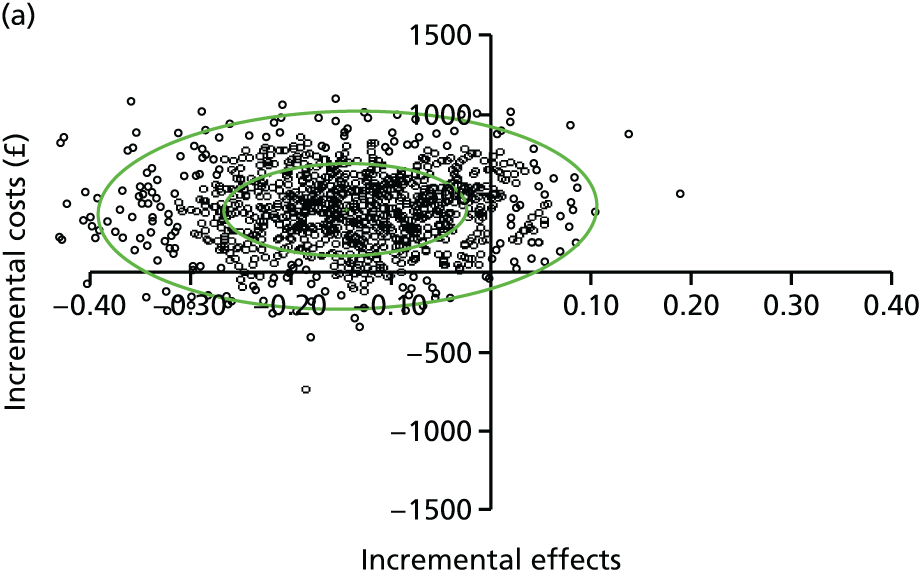

FIGURE 52.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: site B from a societal perspective.
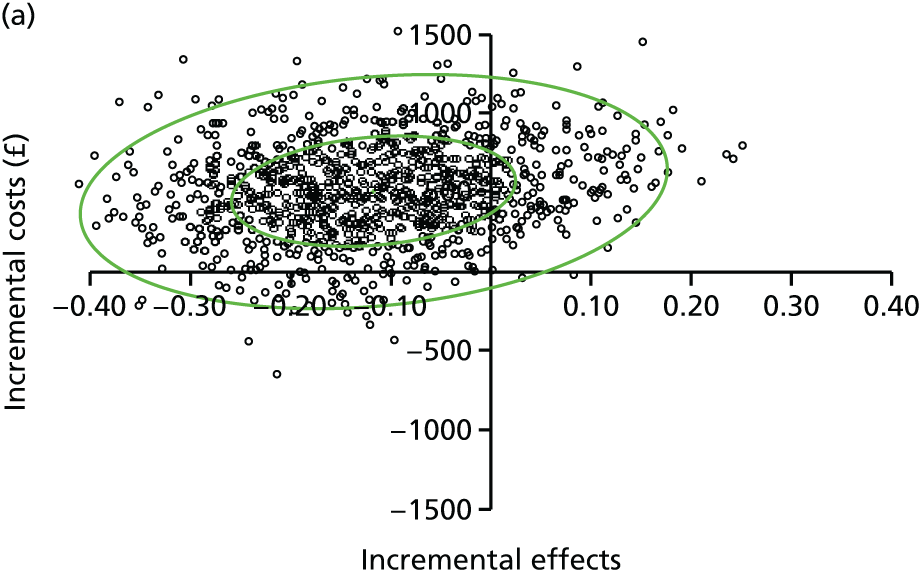

FIGURE 53.
(a) Cost-effectiveness plane; and (b) CEAC for BMI z-score outcome, subgroup analyses: site C from a societal perspective.

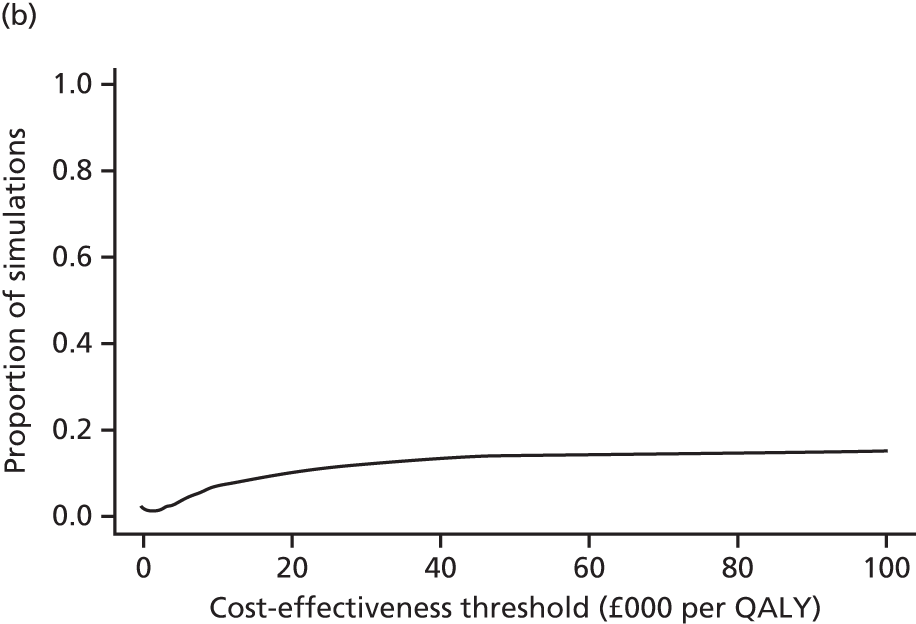
Appendix 9 Interview schedules for parents at 3-month follow-up
Appendix 10 Interview schedules for parents at 12-month follow-up
Appendix 11 Interview schedules for children
Glossary
- Data Monitoring and Ethics Committee
- An independent committee, the roles of which are to monitor the data from the trial; to safeguard the safety, rights and well-being of trial participants; to assess ethical and safety issues; and, on the basis of their assessment, to make recommendations to the Trial Steering Committee on whether or not the trial should continue.
- Logic model
- A diagrammatic representation of an intervention, describing anticipated delivery mechanisms, intervention components, the mechanisms through which an intervention is proposed to work and intended outcomes.
- Process evaluation
- An assessment conducted during the implementation of an intervention including components of whether or not it is reaching the intended participants; if the intervention is being provided and delivered as intended; and how the intervention is being received by the participants.
- Trial Steering Committee
- A committee responsible for the overall supervision and progress of a trial on behalf of the trial sponsor and trial funder. Its role is to ensure that the trial is conducted in accordance with the Guidelines for Good Clinical Practice and relevant regulatory requirements.
List of abbreviations
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CPRS
- Child–Parent Relationship Scale
- CSRI
- Client Services Receipt Inventory
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-Y
- European Quality of Life-5 Dimensions Youth version
- FEAHQ
- Family Eating and Activity Habits Questionnaire
- FFH1
- first running of the Families for Health programme
- FFH2
- second running of the Families for Health programme
- FFH3
- third running of the Families for Health programme
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- MEND
- Mind, Exercise, Nutrition . . . Do it!
- MRC
- Medical Research Council
- NCMP
- National Child Measurement Programme
- NICE
- National Institute for Health and Clinical Excellence
- NIHR
- National Institute for Health Research
- NRES
- National Research Ethics Service
- PedsQLTM
- Pediatric Quality of Life InventoryTM
- PSDQ
- Parenting Styles and Dimensions Questionnaire
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SE
- standard error
- SES
- socioeconomic status
- TSC
- Trial Steering Committee
- WEMWBS
- Warwick–Edinburgh Mental Well-Being Scale
- WISH
- Wolverhampton Inspiring and Supporting Health