Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/127/19. The contractual start date was in January 2012. The draft report began editorial review in January 2016 and was accepted for publication in August 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Little is Editor-in-Chief of the Programme Grants for Applied Research journal and a member of the National Institute for Health Research (NIHR) Journals Library Board. James Raftery is a member of the Health Technology Assessment (HTA) and Efficacy and Mechanism Evaluation editorial boards and a member of the NIHR Journals Library Editorial Group and was previously Director of the Wessex Institute and Head of NETSCC. Barrie Margetts states that since retiring from the University of Southampton (in September 2014) he has undertaken paid consultancies for the World Bank, UNICEF and the World Health Organization. Up until December 2015 he was an unpaid trustee of the international charity ‘Riders for Health’ and up until May 2015 he was unpaid president of the World Public Health Nutrition Association. Lucy Yardley is a member of the NIHR Public Health Research Funding Board and the HTA Efficient Study Designs Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Little et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Scientific background
Obesity is a major threat to public health,1,2 the prevalence has risen sharply3 and the vast majority of obese patients are managed in primary care. 4 For some time systematic reviews,4 including those from the National Institute for Health and Care Excellence (NICE),5 have advocated a dietary and physical activity intervention supported by intensive behavioural techniques. The NICE economic model6 estimated that if trial results from other settings could be extrapolated to the skills mix, resource availability and setting of primary care, then intervention is likely to be cost-effective. The trials identified in the NICE review5 mostly had expert lifestyle and behavioural input, and intensively followed up patients (on average 13 times per year during the first 12 months). 5
An average practice with three general practitioners (GPs) and 5500 patients will have > 1300 obese patients. There is a demand from obese patients for primary care to provide longitudinal programmes of personalised support, but dropout from intensive face-to-face programmes is typically high and most practice staff have neither the training nor the time to implement intensive obesity management programmes based on one-to-one counselling, or even group counselling, to cope with such numbers. 7 The problem will become worse as the obesity epidemic progresses. Therefore, it is not feasible to apply current NICE recommendations in primary care because the high-level dietetic expertise required is not available and it is impossible to free up enough staff time for counselling and follow-up, particularly given the progressively resource-constrained environment in primary care. A research priority identified by NICE in 20065 was to develop and assess interventions in primary care, and also to assess the scope for less resource-intensive interventions in a primary care setting.
A review of studies in primary care8 using strict criteria for inclusion (e.g. excluding those with > 30% attrition at 12 months, which is common in studies of obesity and so may not be an appropriate exclusion) also concluded that there was very little evidence of studies using appropriately intensive behavioural counselling in primary care and suggested the need for using trained interventionists.
Rationale for this research
An alternative, or addition, to a cadre of highly trained interventionists is to harness the capacity of the internet to help support behaviour change.
A systematic review of internet-based behavioural health-related interventions9 concluded that web-based interventions were effective for supporting behaviour change, although with considerable heterogeneity, and that interventions employing multiple theory-based techniques achieved the best results.
Heterogeneity in the effectiveness of internet interventions may be, in part, due to the level of support, with a trend towards better outcomes in interventions with additional personal support,10,11 so it remains very unclear how much facilitation is needed for effective weight reduction, whether face-to-face support is necessary and/or whether briefer remote support could suffice. It is also unclear whether such interventions work better than using novel but simple written materials that do not emphasise cutting out or cutting down favourite foods, but instead promote healthy food swaps and increased fruit and vegetable consumption, which we have shown aid modest weight reduction. 12
Development of the intervention
Key elements of the content of the website
-
Choice of diet: low calorie versus protein-sparing low carbohydrate. The NICE review,5 using the previous National Institute for Health Research (NIHR)’s Health Technology Assessment review’s terminology,4 documented that ‘protein-sparing’ low-carbohydrate diets are likely to be as effective for weight loss as a low-calorie diet with no major disadvantage for major cardiovascular risk factors. However, a major concern with low-carbohydrate diets is the limiting of fruit and vegetable consumption, which is likely to be harmful to health13–15 and is discouraged by NICE guidance. 5 We developed and piloted a modified version of a low-carbohydrate diet that encourages high fruit and vegetable consumption, is compatible with longer-term healthy eating and achieved similar weight loss to a low-calorie diet in piloting. In piloting, our patients fed back a strong request that some choice of diets should be allowed and also that when initiating a low-carbohydrate diet, a 20-g induction phase is unrealistic and difficult to comply with.
-
Physical activity. NICE documented that physical activity (defined as at least 2–2.5 hours of moderate-intensity physical activity per week), in addition to diet (600 kcal/deficit or low fat), is likely to be more effective than diet alone (a difference of –1.95 kg, 95% CI –3.22 to –0.68 kg) at 12 months. This is supported by other systematic reviews of weight loss and maintenance. 16 There is debate about the level of physical activity required for maintenance of weight; the average time spent in moderate-intensity physical activity in the systematically reviewed randomised trials is 180–200 minutes per week (i.e. about 35 minutes per day),17 but higher levels of activity were needed in the observational studies. 17 We proposed offering pedometers to help initiate and maintain physical activity, supported by systematic review evidence which demonstrates that pedometers help increase physical activity by 26.9%, particularly if a step goal is used, and significantly decrease body mass index (BMI) [by 0.38 kg/m2, 95% confidence interval (CI) 0.05 to 0.72 kg/m218].
-
Behavioural component. A combination of diet and cognitive–behavioural therapy probably increases weight loss (–7.66 kg; 95% CI –11.96 to –3.36 kg) compared with diet alone. 5 The cognitive–behavioural approach we have developed is in line with NICE guidance and draws on existing theory and evidence. The approach emphasises that forming ‘healthy habits’ that can be maintained long term rather than following prescriptive, complex or intrusive eating plans that are not sustainable. 19,20
Initial development of the prototype intervention
Research for Patient Benefit funded the initial development of the intervention. Using the person-centred approach,21 we developed an internet site to help support nurse-led weight loss in primary care – the Positive Online Weight Reduction (POWeR) programme. The Research for Patient Benefit-funded website was a prototype version and we demonstrated that this helped to support weight loss in a feasibility study. 22 Participants chose a low-calorie or a low-carbohydrate diet, and either a walking plan (with a supplied pedometer) or a self-selected mixture of physical activities, emphasising sustainable habits. The format was a set of 12 weekly sessions, teaching active cognitive and behavioural self-regulation techniques (‘POWeR tools’), with evidence for their effectiveness and examples of success (‘POWeR stories’). A food diary in session 1 helped to identify foods to omit or replace and a goal-setting tool obliged patients to choose goals from preset choices (e.g. avoid ‘red’ foods, eat ‘amber foods’ only once a day, reduce main meal portion sizes by 25%, avoid all high-calorie/carbohydrate snacks or drinks). At subsequent sessions, goals were reviewed with feedback (e.g. positive feedback if successful, advice on overcoming barriers if unsuccessful). Session 2 covered getting support and session 3 covered physical activity. This was followed by a choice of weekly sessions covering cravings, relapse, increasing physical activity, emotional eating, eating when busy, environment restructuring, alcoholic and non-alcoholic drinks, and eating out.
Development of the full intervention
Following the success of the feasibility study, the prototype version of the POWeR programme was developed further as POWeR+ – the development of which was finalised during the current grant from NIHR’s Health Technology Assessment programme.
The POWeR+ web-based intervention
POWeR+ is a theory- and evidence-based intervention that is designed to teach patients self-regulation and cognitive–behavioural techniques and that aims to help them to form sustainable eating and physical activity habits for long-term weight management. POWeR+ was developed, as with POWeR, using the person-based approach to maximise acceptability, feasibility and engagement. 22–25 We finally created a series of 24 web-based sessions lasting up to 6 months with novel content, links to external content and e-mail reminders to encourage patients to continue to use the website weekly to track their weight, set and review eating and physical activity goals, and receive personalised advice. After entering their weight and whether or not they had achieved the goals they had set themselves the previous week, patients receive tailored feedback giving encouragement if maintaining weight loss (e.g. reminders of health benefits accrued) and meeting goals. Weight gain and failing to meet goals triggers automated personalised advice, such as appropriate goal-setting and planning, boosting motivation, overcoming difficulties and recovering from lapses (see Appendix 1).
Having developed the internet-based intervention, we chose to trial it in comparison with an active control group – a brief approach using brief written materials to support food swaps and increased fruit and vegetable consumption. We have previously shown this to control weight well, achieving in the order of a 2% reduction in weight compared with providing a simple advice booklet. 12
Objectives
Compared with a brief intervention promoting brief written materials to support food swaps and increased fruit and vegetable consumption, we report the incremental effectiveness of (1) an internet-based behavioural intervention (POWeR+) with face-to-face support as needed (POWeR+F) and (2) an internet behavioural intervention with remote support (POWeR+R).
Chapter 2 Methods
Study design
This was an individually randomised parallel-group study.
The control group received a brief but active intervention; the rationale was to minimise the pressure to cut down favourite foods, but instead to swap less healthy foods for healthier choices (the swap sheet) or to increase healthy foods (fruit and vegetables sheet). It was chosen for its simplicity to provide brief advice, which we have previously shown to control weight well. 12
We chose an individually randomised design because, with structured advice in each group, we have been able to minimise contamination in previous trials and an individually randomised design has the additional advantage of minimising cluster effects.
This was a very pragmatic trial to provide the most realistic estimates of effectiveness, so participants were not constrained from pursuing other activities in any study group (e.g. attending commercial slimming clubs).
Trial registration and ethics
The trial was registered on 16 March 2012 [International Standard Randomised Controlled Trial Number (ISRCTN) 21244703] and ethics approval was given by the National Research Ethics Service Committee South Central Southampton B First Multicentre Research Ethics Committee (approved 19 December 2011; reference number 11/SC/0455).
Participant inclusion and exclusion criteria
Patients with a BMI of ≥ 30 kg/m2 (or ≥ 28 kg/m2 with additional risk factors of hypertension, diabetes mellitus or hypercholesterolaemia) documented in GP case records4 were eligible.
Patients were excluded if they had current major mental problems, such as psychosis, or were very ill (e.g. severe left ventricular failure), that is, they had difficulty completing outcomes, were unable to change diet, were pregnant or breastfeeding, or had a perceived inability to walk 100 m (physical activity difficult).
Recruitment: invitation of patients/recruitment
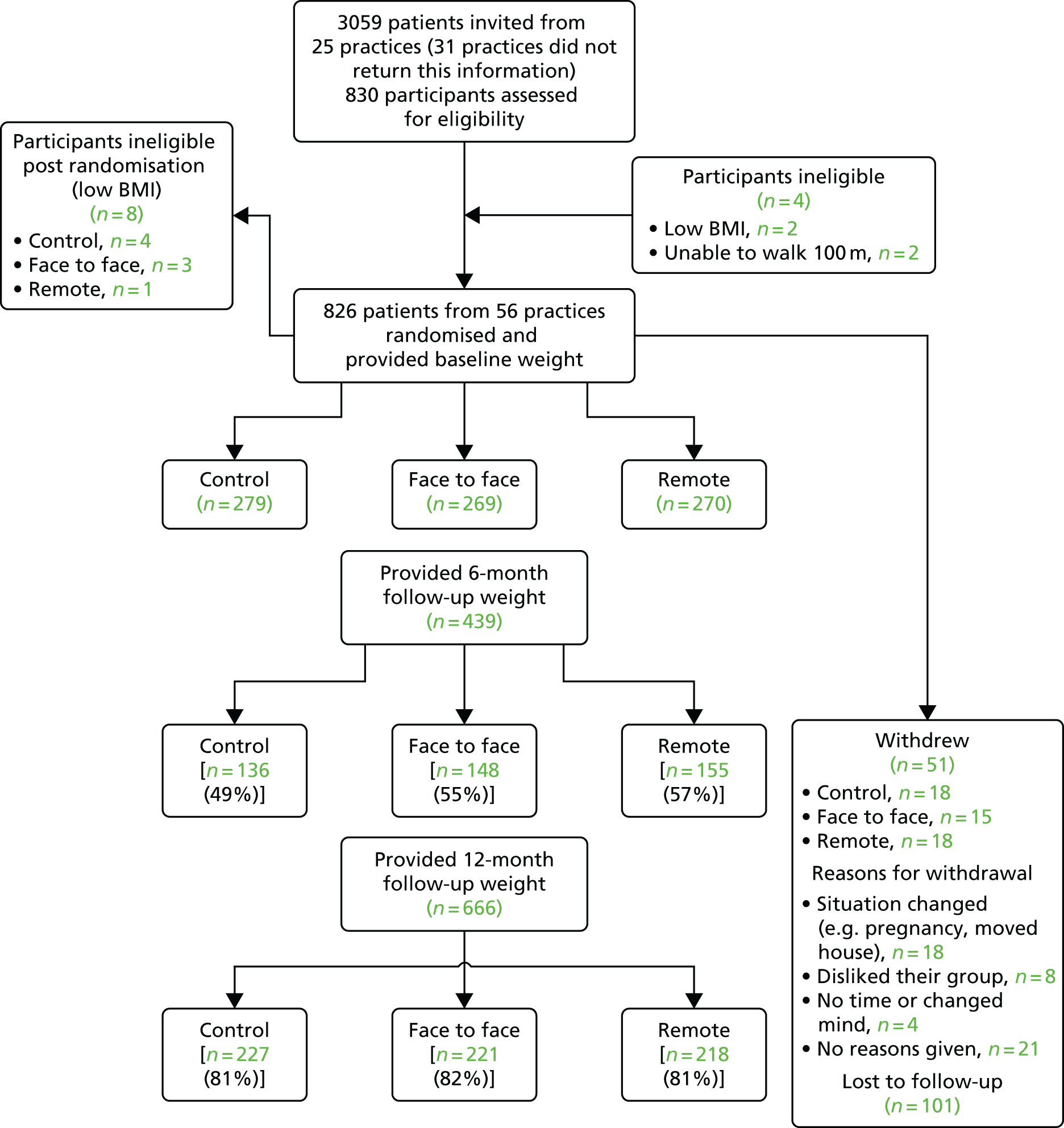
General practices in the south of England (recruited around the centres of Southampton and Oxford) identified participants from their electronic records. Up to 100 patients from each practice were randomly chosen and invited, by letter, to a screening appointment to confirm eligibility. Patients could also be referred opportunistically when seen in clinics run by practice nurses or doctors (Figure 1).
FIGURE 1.
The CONsolidated Standards Of Reporting Trials (CONSORT) flow diagram: flow of participants in the trial. This figure has been reproduced from Little et al. 26 under the terms of the CC BY 4.0 licence (https://creativecommons.org/licenses/by/4.0/).
Informed consent
During the screening appointment, the study was explained and informed written consent was taken. Participants were given details of how to log in and register with the LifeGuide website (www.lifeguideonline.org/), POWeR+.
Randomisation/group allocation
On registering with the study website, participants were presented with baseline questionnaires, on completion of which they were automatically randomised using computer-based random numbers by the website to one of three feasible interventions in primary care:
-
Control group: evidence-based advice and simple materials to support behaviour change, and follow-up. Those randomised to the control group were taken to pages of the POWeR+ website containing two brief, structured, printable pages of advice about a healthy diet (healthy food swaps and NHS 5-a-day leaflet27). These materials had previously been developed by the Institute of Food Research, and had been trialled by us in primary care, resulting in modest weight loss (around 2%) compared with a generic advice booklet. 12 To enhance retention in the control group, participants were informed that this intervention had been shown to support weight loss. For follow-up, nurses arranged brief 5- to 10-minute appointments with sufficient time to measure weight at 6 months and 12 months, but not to provide extensive counselling.
-
POWeR+F: web intervention with face-to-face appointments for nurse support. The rationale for this intervention was to provide automated behavioural counselling with just three scheduled (and four optional) face-to-face nurse support sessions, thus requiring substantially less health professional skill and time than the evidence-based lifestyle interventions documented in the NICE review,5 and, hence, much easier to implement in the NHS. In addition to 6-monthly weighing, as in the control group, participants had three scheduled face-to-face appointments in the first 3 months and then up to four more during the next 3 months, if needed. Weight gain on two consecutive logins triggered an automated e-mail to the nurse advising that the patient required further support. Patients could also request additional support.
-
POWeR+R: web intervention with remote support. The rationale here was to test whether or not even briefer professional support for the web intervention could be effective. Patients could access the same web-based intervention as in the face-to-face group. In addition to 6-monthly weighing, as in the control group, participants had three scheduled telephone or e-mail contacts and up to two optional telephone/e-mail contacts during the first 6 months (triggered by weight gain or patient request as in the face-to-face group). This level of support was confirmed as acceptable and helpful during the development and piloting stages.
Data collection and management
Baseline assessment
Participants had appointments with the practice nurse at baseline to record baseline measures and to provide the questionnaire to participants.
Follow-up
Participants were mailed the questionnaire again at 6 months and 1 year with two further mailings to non-responders.
Practice nurses saw individuals for a follow-up appointment to measure weight at 6 months and participants also had a visit by a nurse researcher blinded to the group to perform a weight measurement at 12 months. Where a visit to obtain a blinded weight measurement was not possible, we used practice nurses’ recorded weights and, where that was not possible, we used participants’ reported weight.
Notes review
During the available follow-up time all patients’ notes were reviewed to document consultations, returns, time to return, reasons for returns, complications, NHS resource use and any subsequent referrals.
Measures
Weight reduction averaged over a 12-month period, measured lightly clothed, without shoes, where possible at the same time each day, and using automated Tanita digital scales (Tanita Corporation of America Inc., Arlington Heights, IL, USA), was the primary outcome.
A secondary weight outcome was the proportion of participants maintaining a 5% weight loss or loss of around 4–5 kg . This is very likely to be important clinically (a reduction of 3–5 kg has a significant impact on the incidence of diabetes mellitus28,29) and it allows direct comparison with a previous UK trial from primary care settings,30 which published its results after our study commenced.
Secondary outcomes
-
Physical activity: the validated Godin leisure-time physical activity questionnaire. 31
-
Food and drink consumption: using the brief Food Frequency Questionnaires for major food groups as well as alcohol.
-
Indices of the metabolic syndrome:32 three out of five of elevated waist circumference (> 94 cm in males, 80 cm in females), triglyceride concentration of ≥ 1.7 mmol/l, reduced high-density lipoprotein cholesterol (< 1.00 mmol/l in males and < 1.3 mmol/l in females), elevated blood pressure (BP) (systolic BP of > 130 mmHg or diastolic BP of > 85 mmHg, or treatment of high BP) or elevated fasting glucose (≥ 5.6 mmol/l). We also proposed exploring the use of a continuous outcome based on the mean z-score of each component of the syndrome for each individual (or mean rank as appropriate), supported by empirical evidence of a close factor structure among the variables. 33
-
Waist: nurses measured the waist midway between the lower ribs and the iliac crest34 as well as height to allow estimation of BMI. Fat mass was measured using Tanita scales.
-
BP: measured three times (after 5 minutes) using a validated OMRON BP monitor (OMRON Healthcare UK Ltd, Milton Keynes, UK).
-
Serum measures: liver function tests, serum cholesterol/high- and low-density lipoprotein/triglycerides, glucose, glycated haemoglobin (HbA1c) and ferritin [important to measure because of reduced cereal consumption (a key source of iron) in the low-carbohydrate diet].
-
-
Patient enablement: we used a modified version of the enablement scale. 35
-
EuroQol-5 Dimensions (EQ-5D): the EQ-5D provided a measure of quality of life for economic analysis.
-
Self-reported behavioural adherence: website usage (frequency and duration of logins, options selected and all data inputted).
-
Usage of other weight management activities: perceptions of nurse support. 36
-
Habits: a modified self-reported habit index measured physical activity and dietary habits. 37
-
Intention to change behaviour: based on the theory of planned behaviour components (attitudes, social norms, perceived behavioural control). 38
-
Other measures: sociodemographic data (age, gender, education, internet experience and social deprivation indices based on postcode) were also recorded.
-
Sample size
We wished to compare each of the intervention groups primarily with the control group, but also potentially with each other, so we allowed for an alpha of 0.017 (i.e. 0.05/3). For the primary outcome (weight) we estimated that a standardised effect size of 0.33 [equivalent to a 2–3 kg difference, assuming a standard deviation (SD) of change of 6.5–7.5 kg36,39] and 80% power required 174 patients per group with complete data, or 654 patients in total allowing for a 20% loss to follow-up. Following liaison with both the funder (NIHR’s Health Technology Assessment programme) and the Trial Steering Committee, the power calculation was revised to allow for modest clustering at practice level if significant clustering was found; we assumed, on average, a minimum of 18 patients per practice to achieve 15 patients at follow-up, of whom roughly five or six were in each of the two intervention groups at follow-up, assuming five participants per group in each practice, for an intracluster correlation coefficient of 0.05 {i.e. a design effect of 1.2 = 1 + [(5 – 1) × 0.05]}. This resulted in a minimum of 654 × 1.2 = 785 patients. This was a conservative calculation because we made no allowance for repeated measures.
Statistical analysis
Primary analysis
A mixed multivariate regression model was chosen to estimate the average impact over 12 months and to enable data to be used from anyone who had 6- or 12-month data, controlling for weight at baseline, gender, age, smoking, diabetes mellitus, medications (including orlistat used at baseline), any comorbidities, deprivation (Indices of Multiple Deprivation40) and any clustering by practice.
Missing data
The primary analysis was the intention-to-treat analysis using both measured and reported weights, and imputing data by multiple imputation. Secondary, less conservative, analyses were of complete cases and also using measured weights only.
Potential clustering effects
Although the trial was individually randomised, we controlled for any clustering by practice in the analysis. In the repeated measures model, after controlling for baseline weight, the intracluster correlation coefficient at the practice level was 0.01 (95% CI 0.003 to 0.09) and at the participant level was 0.74 (95% CI 0.69 to 0.78).
Patient and public involvement
Weight Concern was a partner in this project, and the study team requested named individuals to be part of the team. Weight Concern preferred, instead, to provide a panel-based input, arguing cogently that a more balanced input would be provided. The input was indeed very helpful, particularly during the development of the intervention. Nevertheless, in retrospect, patient and public involvement input would still have been strengthened by engaging named individuals to contribute, perhaps sourced through additional channels.
Subgroup analyses
We explored estimates of effect in subgroups according to baseline waist measurement (high vs. low waist circumference) and the presence of metabolic syndrome. We assessed outcome (weight, reported behaviour change, measured behaviour change) according to website usage and to the type of diet chosen by patients.
Other analyses
We assessed the relationship between weight changes compared with the changes in metabolic variables.
Changes to the protocol
Based on the advice of the Trial Steering Committee we increased the sample size to allow for clustering, as necessary. A repeated measures analysis of variance for the principal continuous outcome (i.e. weight) was originally proposed but could be undertaken only in those who completed both the 6- and 12-month follow-up, thus unnecessarily losing data; therefore, the analysis was changed to mixed multivariable regression modelling. We originally planned for one-third of the sample to be randomised to wear activity monitors to allow for more precise estimation of physical activity, but the logistics of organising this subsample at a time of intensive attempts to achieve high follow-up for the primary outcome became too difficult. Similarly, piloting suggested that intensive follow-up for anything but the primary outcome would result in participants dropping out, so all the effort of follow-up concentrated on achieving as high a rate of follow-up for the primary outcome as possible. Although the original protocol justified the sample size based on a clinically important weight reduction, the proportion maintaining a clinically important weight loss was not originally specified. We rectified this omission by specifying a secondary weight outcome as the proportion maintaining a 5% weight loss, or around 4- to 5-kg weight loss. This is important clinically (a reduction of 3–5 kg significantly reduces the incidence of diabetes mellitus28,29) and it allows a direct comparison with the previous UK trial from primary care settings,30 which published its results after our study commenced.
Compliance with intervention
Compliance with the use of the POWeR+ intervention using the LifeGuide software version 1.0.7.30 (University of Southampton, Southampton, UK) was documented automatically by the website, which recorded the number of sessions completed and the time taken.
Chapter 3 Trial results
Practice recruitment
Fifty-six general practices were recruited in the south of England around the main study centres (Southampton and Oxford).
Participant recruitment
A total of 818 eligible individuals were recruited and randomised between January 2013 and March 2014.
Baseline comparability
Table 1 shows the baseline characteristics of the groups and shows that groups are mostly well balanced.
| Characteristic | Group | ||
|---|---|---|---|
| Control: brief verbal and online healthy eating advice | POWeR+F: access to website and brief face-to-face support | POWeR+R: access to website and brief remote support | |
| Female, n/N (%) | 185/279 (66.31) | 175/269 (65.06) | 160/269 (59.48) |
| Age (years), mean (SD) | 52.69 (13.25) | 53.70 (13.21) | 54.74 (12.95) |
| Smoker, n/N (%) | 24/279 (8.6) | 21/269 (7.81) | 25/269 (9.29) |
| Diabetes mellitus, n/N (%) | 48/279 (17.20) | 46/268 (17.16) | 42/270 (15.56) |
| Orlistat use, n/N (%) | 3/270 (1.11) | 5/262 (1.91) | 5/266 (1.88) |
| Comorbid condition, n/N (%) | 48/281 (17.08) | 55/269 (20.45) | 55/272 (20.22) |
| Deprivation score,40 mean (SD) | 14.32 (10.45) | 13.73 (10.28) | 13.29 (10.17) |
| Weight (kg), mean (SD) | 104.38 (21.11) | 102.40 (16.87) | 102.93 (18.26) |
| BMI (kg/m2), mean (SD) | 37.10 (5.97) | 36.66 (5.36) | 36.28 (5.65) |
Losses to follow-up and missing data
Of the 818 individuals, 439 had a weight recorded at the 6-month follow-up and 666 had a weight recorded at the 12-month follow-up. Of the 666, 510 (76.6%) were blinded weights, 28 (4.2%) were unblinded weights and 128 (19.2%) were reported weights. The reported weights were similar in each group (control, n = 40; POWeR+F, n = 48; POWeR+R, n = 40).
Primary outcome
Mean weight reduction
Tables 2 and 3 show the mean weights at baseline and follow-up. The complete data suggest that the control group maintained a weight loss of nearly 3 kg at both 6 and 12 months, although the imputed data suggest less weight loss at 6 months (imputed weight 6 months, 103.16 kg; 12 months, 102.17 kg). Compared with the control group, for both the complete cases (Tables 4 and 5) and for the imputed analysis (Table 6), there was a significant additional reduction in mean weight averaged over 12 months in the POWeR+ groups. The primary imputed analysis documented that the POWeR+F group achieved an estimated additional 1.5-kg reduction over the 12-month period (95% CI –2.4 to –0.6 kg; p = 0.001) and the POWeR+R group an additional 1.3-kg reduction (95% CI –2.3 to –0.3 kg; p = 0.005). The secondary analysis of only the complete cases over 12 months was slightly less conservative, with a greater weight reduction compared with the control group (POWeR+F: –1.78 kg, 95% CI –2.8 to –0.8 kg; POWeR+R: –1.6 kg, 95% CI –2.6 to –0.6 kg) (see Table 5). Considering individual time points, the mean weight reduction was approximately 2 kg at 6 months compared with the control group in both POWeR+ groups, but was smaller by 12 months (approximately a 0.5-kg difference).
| Group | Weight (kg), mean (SD); n | ||
|---|---|---|---|
| Time point | |||
| Baseline | 6 months | 12 months | |
| Control | 104.38 (21.11); 279 | 101.91 (19.35); 136 | 101.73 (19.57); 227 |
| POWeR+F | 102.40 (16.87); 269 | 97.55 (15.99); 148 | 98.56 (15.95); 221 |
| POWeR+R | 102.93 (18.26); 270 | 98.30 (18.34); 155 | 99.72 (18.88); 218 |
| Group | Weight (kg), mean (SD) | |
|---|---|---|
| Time point | ||
| 6 months | 12 months | |
| Control | 101.86 (19.95) | 101.02 (18.26) |
| POWeR+F | 98.58 (17.65) | 98.19 (16.10) |
| POWeR+R | 97.18 (16.67) | 99.02 (18.13) |
| Group | Time point, difference (95% CI); p-value | Over study period (repeated measures), difference (95% CI); p-value | |
|---|---|---|---|
| 6 months | 12 months | ||
| POWeR+F | –2.90 (–4.09 to –1.71); < 0.001 | –0.63 (–1.92 to 0.66); 0.340 | –1.56 (–2.47 to –0.65); 0.001 |
| POWeR+R | –2.42 (–3.59 to –1.24); < 0.001 | –0.63 (–1.93 to 0.67); 0.342 | –1.30 (–2.21 to –0.39); 0.005 |
| Group | Time point, estimated difference (95% CI); p-value | Over study period (repeated measures), estimated difference (95% CI); p-value | |
|---|---|---|---|
| 6 months | 12 months | ||
| POWeR+F | –3.20 (–5.00 to –1.39); 0.001 | –1.02 (–2.27 to 0.22); 0.107 | –1.78 (–2.81 to –0.76); 0.001 |
| POWeR+R | –3.22 (–5.03 to 1.41); < 0.001 | –0.96 (–2.21 to 0.29); 0.134 | –1.60 (–2.63 to 0.57); 0.002 |
| Group | Time point; , difference (95% CI); p-value | Over study period (repeated measures), difference (95% CI); p-value | |
|---|---|---|---|
| 6 months | 12 months | ||
| POWeR+F | –2.54 (–3.66 to –1.42); < 0.001 | –0.37 (–1.66 to 0.92); 0.566 | –1.49 (–2.41 to –0.58); 0.001 |
| POWeR+R | –1.97 (–3.18 to –0.76); 0.002 | –0.58 (–1.88 to 0.72); 0.375 | –1.27 (–2.19 to –0.34); 0.007 |
Clinically important weight reduction
By 12 months, using data from complete cases, 18.5% (42/227) of the control group had maintained a ≥ 5% reduction in weight and 20.8% from the imputed data. The proportion maintaining clinically valuable weight loss for POWeR+F, estimated from the imputed data, had waned a little between 6 and 12 months (36.8% vs. 29.2%), but was similar for POWeR+R (33.7% vs. 32.4%) (Table 7).
| Group | Proportion losing ≥ 5% of baseline weight: complete cases, n/N (%) | Risk ratio of achieving weight loss of ≥ 5% of baseline weight compared with control group: complete cases (95% CI); p-value | Proportion losing ≥ 5% of baseline weight: imputed data (%) | Risk ratio of achieving weight loss of ≥ 5% of baseline weight compared with control group: imputed data (95% CI); p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |
| Control | 16/136 (11.8) | 42/227 (18.5) | 1.00 | 1.00 | 15.9 | 20.8 | 1.00 | 1.00 |
| POWeR+F | 59/148 (39.9) | 62/221 (28.1) | 3.42 (2.10 to 5.56); < 0.001 | 1.46 (1.02 to 2.08); 0.036 | 36.8 | 29.2 | 3.10 (1.85 to 5.18); < 0.001 | 1.56 (0.96 to 2.51); 0.070 |
| POWeR+R | 55/155 (35.5) | 69/218 (31.7) | 3.02 (1.89 to 4.83); < 0.001 | 1.67 (1.17 to 2.37); 0.004 | 33.7 | 32.4 | 2.64 (1.60 to 4.36); < 0.001 | 1.82 (1.21 to 2.74); 0.004 |
Adherence and the impact of adherence on weight reduction
Of the 539 participants randomised to the POWeR+ intervention groups, 524 started the first session and 404 completed all three core sessions (196 of the POWeR+R group and 208 of the POWeR+F group). Participants completed an average of 10.97 (SD 12.65; range 0–52) weight and goal reviews; the average was 10.16 (SD 11.92) in the POWeR+F group and 11.85 (SD 13.38) in the POWeR+R group. The median number of nurse contacts was four (range 0–7) in both intervention groups, with a median of two face-to-face, one telephone and one e-mail contacts in the POWeR+F group, and a median of one telephone call and three e-mails in the POWeR+R group. There was around a 2.0- to 2.5-kg difference in weight reduction for those who completed more than the first basic stage of the programme (Table 8). There was no evidence of a significant difference in weight loss according to the type of diet chosen (Table 9).
| Sessions completed | Proportion of the sample, n/N (%) | Difference compared with those who completed stage 1 onlya (95% CI); p-value | ||
|---|---|---|---|---|
| 6 months | 12 months | Over study period (repeated measures) | ||
| Did not complete stage 1 | 434/839 (51.73)b | 0.74 (–1.21 to 2.69); 0.457 | 0.58 (–1.22 to 2.38); 0.528 | 0.87 (–0.47 to 2.00); 0.204 |
| Completed stage 1 only | 179/839 (21.33) | REF | REF | REF |
| Completed at least one session from stage 2 | 226/839 (26.94) | –2.21 (–3.50 to –0.93); < 0.001 | –2.53 (–4.05 to –1.00); 0.001 | –2.50 (–3.54 to –1.46); < 0.001 |
| Diet | Weight (kg), mean (SD) | ||
|---|---|---|---|
| Time point | |||
| Baseline | 6 months | 12 months | |
| Low calorie | 102.68 (17.35) | 98.12 (17.00) | 99.25 (17.65) |
| Low carbohydrate | 102.57 (18.00) | 97.30 (17.86) | 98.37 (17.46) |
Other activities
For those returning the final questionnaire, almost half the people in the control group (47.1%; 64/136) were doing another activity to help lose weight as opposed to 37.2% (51/137) in the POWeR+F group and only 26.7% (40/150) in the POWeR+R group (Table 10).
| Activities | Group, n/N (%) | ||
|---|---|---|---|
| Control | POWeR+F | POWeR+R | |
| Take part in regular activity long enough to work up a sweat | |||
| Rarely | 50/135 (37.0) | 38/134 (28.4) | 39/146 (26.7) |
| Sometimes | 57/135 (42.2) | 69/134 (51.5) | 77/146 (52.7) |
| Often | 28/135 (20.7) | 27/134 (20.2) | 30/146 (20.6) |
| Take part in another weight-loss activity | 64/136 (47.1) | 51/137 (37.2) | 40/150 (26.7) |
| Of these, the reported activities were: | |||
| Weight Watchers® (Weight Watchers International, Inc., New York, NY, USA)/Slimming World (Slimming World, Alfreton, UK) (or similar) meetings | 23/136 (16.9) | 22/137 (16.1) | 14/150 (9.3) |
| Another weight management website | 0/135 (0.0) | 4/136 (2.9) | 4/150 (2.7) |
| Telephone application | 13/136 (9.6) | 8/136 (5.9) | 10/149 (6.7) |
| Weight-loss pills | 5/136 (3.7) | 4/137 (2.9) | 2/150 (1.3) |
| Health trainer programme | 4/136 (2.9) | 2/136 (1.5) | 3/148 (2.0) |
| Exercise referral scheme | 4/136 (2.9) | 7/137 (5.1) | 4/148 (2.7) |
| Another weight-loss scheme | 8/136 (5.9) | 13/136 (9.6) | 8/149 (5.4) |
| Any other weight management method | 22/97 (22.7) | 13/87 (14.9) | 8/103 (7.8) |
Secondary outcomes
A range of secondary outcomes is shown in Tables 11–22. Owing to the priority to obtain follow-up for weights, and the clear feedback from piloting that pressurising participants to have blood taken was offputting, achieving high follow-up for blood samples was necessarily a secondary priority for the trial team and so a minority of participants had follow-up blood measurements. As a result, even though estimates use multiple imputation, the results for blood samples must be interpreted cautiously. What results are available suggest a generally positive direction of outcomes in the POWeR+ groups (i.e. raised high-density lipoprotein cholesterol, lower aspartate transaminase and alanine transaminase levels).
| Group | Median physical activity score (IQR) | Difference compared with control group (95% CI); p-value |
|---|---|---|
| Control | 20 (11–33) | |
| POWeR+F | 25 (12–41) | 2.49 (–2.72 to 7.70); 0.348 |
| POWeR+R | 21 (13–45) | –0.90 (–5.97 to 4.17); 0.728 |
| Group | Liver function test (units/l), crude mean (SD) | |||||
|---|---|---|---|---|---|---|
| ALT (n = 329) | AST (n = 303) | Gamma-GT (n = 319) | ||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Control | 30.17 (16.62) | 30.23 (15.33) | 30.99 (14.26) | 29.38 (10.96) | 39.18 (35.20) | 38.65 (31.01) |
| POWeR+F | 31.41 (17.48) | 27.26 (14.06) | 31.80 (13.79) | 27.15 (9.36) | 43.76 (46.55) | 35.40 (26.10) |
| POWeR+R | 31.26 (17.57) | 27.68 (16.97) | 30.73 (13.69) | 27.80 (9.27) | 38.04 (30.85) | 40.19 (40.47) |
| Group | Liver function test (units/l), estimated difference (95% CI); p-value | ||
|---|---|---|---|
| ALT | AST | Gamma-GT | |
| POWeR+F | –3.01 (–6.59 to 0.57); 0.093 | –2.21 (–4.77 to 0.35); 0.087 | –3.06 (–9.87 to 3.74); 0.356 |
| POWeR+R | –2.72 (–6.08 to 0.64); 0.107 | –2.02 (–4.38 to 0.34); 0.090 | 2.03 (–5.24 to 9.32); 0.568 |
| Group | Measurement, crude mean (SD) | |||||
|---|---|---|---|---|---|---|
| Glucose, mmol/l (n = 338) | HbA1c, mmol/l (n = 330) | Ferritin, µg/l (n = 362) | ||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Control | 5.68 (1.88) | 5.88 (1.94) | 40.49 (10.42) | 41.09 (28.68) | N/A | 95.73 (94.77) |
| POWeR+F | 5.91 (2.53) | 5.83 (2.67) | 42.00 (13.09) | 38.78 (11.39) | N/A | 95.47 (84.16) |
| POWeR+R | 5.64 (1.93) | 5.64 (1.99) | 40.07 (11.60) | 38.74 (12.79) | N/A | 103.78 (76.24) |
| Group | Measurement, estimated difference (95% CI); p-value | ||
|---|---|---|---|
| Glucose (mmol/l) | HbA1c (mmol/l) | Ferritin (µg/l) | |
| POWeR+F | –0.20 (–0.66 to 0.26); 0.375 | –3.15 (–8.01 to 1.72); 0.192 | –4.69 (–23.78 to 14.40); 0.616 |
| POWeR+R | –0.32 (–0.79 to 0.15); 0.176 | –2.96 (–7.74 to 1.83); 0.213 | 5.86 (–12.63 to 24.36); 0.516 |
| Group | Cholesterol (mmol/l), crude mean (SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 346) | HDL (n = 348) | LDL (n = 218) | Triglycerides (n = 286) | |||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Control | 5.30 (1.24) | 5.05 (1.26) | 1.43 (0.37) | 1.37 (0.32) | 3.12 (1.03) | 2.97 (0.98) | 1.74 (0.96) | 1.72 (0.82) |
| POWeR+F | 5.19 (1.19) | 5.18 (1.18) | 1.40 (0.33) | 1.47 (0.35) | 3.02 (0.96) | 2.94 (0.98) | 1.72 (0.85) | 1.75 (1.04) |
| POWeR+R | 5.35 (1.33) | 5.33 (1.20) | 1.41 (0.36) | 1.50 (0.38) | 3.11 (1.05) | 3.08 (1.10) | 1.89 (1.59) | 1.78 (1.49) |
| Group | Cholesterol (mmol/l), estimated difference (95% CI); p-value | |||
|---|---|---|---|---|
| Total | HDL | LDL | Triglycerides | |
| POWeR+F | 0.11 (–0.92 to 0.31); 0.270 | 0.08 (0.02 to 0.15); 0.010 | 0.01 (–0.19 to 0.22); 0.894 | 0.03 (–0.28 to 0.34); 0.826 |
| POWeR+R | 0.14 (–0.08 to 0.35); 0.204 | 0.10 (0.03 to 0.17); 0.006 | 0.06 (–0.16 to 0.28); 0.572 | 0.01 (–0.30 to 0.32); 0.937 |
| Group | Measurement, crude mean (SD) | |||||
|---|---|---|---|---|---|---|
| Body fat percentage (n = 454) | SBP (mmHg) (n = 494) | DBP (mmHg) (n = 494) | ||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Control | 43.59 (7.08) | 42.71 (7.20) | 133.78 (17.84) | 133.11 (18.61) | 81.17 (10.64) | 79.10 (10.79) |
| POWeR+F | 43.86 (8.00) | 41.40 (8.81) | 132.28 (15.81) | 133.31 (15.70) | 80.97 (9.37) | 79.89 (9.20) |
| POWeR+R | 42.18 (7.79) | 40.53 (8.42) | 134.17 (17.00) | 132.11 (17.08) | 80.71 (10.00) | 78.92 (9.71) |
| Group | Measurement, estimated difference (95% CI); p-value | ||
|---|---|---|---|
| Body fat percentage | SBP (mmHg) | DBP (mmHg) | |
| POWeR+F | –0.96 (–1.83 to –0.08); 0.033 | 0.05 (–2.76 to 2.86); 0.973 | 0.67 (–1.12 to 2.46); 0.461 |
| POWeR+R | –0.53 (–1.47 to 0.42); 0.274 | –2.72 (–5.56 to 0.12); 0.061 | 0.02 (–1.64 to 1.68); 0.979 |
| Food | Group, crude mean (SD) | |||||
|---|---|---|---|---|---|---|
| Control | POWeR+F | POWeR+R | ||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Sweets | 1.14 (1.33) | 0.89 (0.87) | 1.39 (1.66) | 0.71 (0.98) | 1.24 (1.61) | 0.86 (1.32) |
| Cereals | 1.92 (1.17) | 1.67 (0.98) | 2.02 (1.26) | 1.45 (0.97) | 1.97 (1.43) | 1.40 (0.90) |
| Fatty foods | 2.72 (1.85) | 2.15 (1.67) | 2.86 (2.01) | 2.06 (1.45) | 2.65 (1.83) | 1.97 (1.32) |
| Salty snacks | 0.33 (0.62) | 0.19 (0.31) | 0.33 (0.54) | 0.16 (0.23) | 0.31 (0.54) | 0.15 (0.22) |
| Sweet drinks | 0.49 (1.20) | 0.22 (0.59) | 0.38 (0.88) | 0.11 (0.30) | 0.48 (1.17) | 0.24 (0.63) |
| Fruit and vegetables | 3.70 (2.28) | 4.63 (2.70) | 4.02 (2.57) | 4.46 (2.55) | 3.85 (2.22) | 4.63 (2.40) |
| Low-fat dairy | 1.29 (1.44) | 1.23 (1.36) | 1.64 (1.51) | 1.45 (1.53) | 1.43 (1.64) | 1.53 (1.54) |
| Ratio of high- to low-fat foods | 2.66 (8.27) | 1.54 (8.00) | 1.52 (6.41) | 1.43 (5.53) | 2.39 (7.74) | 1.39 (4.59) |
| Group | Portions of food, estimated difference (95% CI); p-value | ||||||
|---|---|---|---|---|---|---|---|
| Sweets | Cereals | Fatty foods | Salty snacks | Sweet drinks | Fruit and vegetables | Low-fat dairy | |
| POWeR+F | –0.18 (–0.37 to 0.02); 0.072 | –0.21 (–0.44 to 0.02); 0.072 | –0.06 (–0.40 to 0.28); 0.722 | –0.03 (–0.09 to 0.03); 0.357 | –0.09 (–0.22 to 0.04); 0.165 | –0.04 (–0.67 to 0.58); 0.888 | 0.14 (–0.21 to 0.48); 0.432 |
| POWeR+R | –0.13 (–0.32 to 0.06); 0.161 | –0.23 (–0.46 to 0.01); 0.057 | –0.19 (–0.52 to 0.15); 0.270 | –0.04 (–0.09 to 0.02); 0.203 | 0.02 (–0.10 to 0.14); 0.769 | 0.17 (–0.43 to 0.76); 0.570 | 0.27 (–0.09 to 0.64); 0.138 |
| Group | Mean item score (SD) | Difference in PEI score compared with control group (95% CI); p-value | |
|---|---|---|---|
| Baseline | 12 months | ||
| Control | 3.19 (1.27) | 3.23 (1.57) | |
| POWeR+F | 3.42 (1.19) | 4.10 (1.28) | 0.70 (0.39 to 1.01); < 0.001 |
| POWeR+R | 3.31 (1.26) | 3.85 (1.35) | 0.54 (0.24 to 0.85); < 0.001 |
Fat mass and BP were recorded in the majority of participants at follow-up (in both cases > 450 individuals) and, although there were no consistent changes in BP, fat mass reduced slightly in both POWeR+ groups (see Tables 18 and 19). Participants in the control group reported healthy changes in many areas of food consumption – with modest changes in sweets, fatty foods, and fruit and vegetables, and in the ratio of high- to low-fat foods. In comparison with the control group, in the POWeR+ groups there were no significant changes but there were trends in the reduction in sweets and cereals, and increasing low-fat dairy foods. Participants felt significantly more enabled to manage their weight problem in the POWeR+ groups (see Table 22).
Subgroups
Metabolic syndrome
There was evidence of an interaction with the presence of metabolic syndrome (Table 23). The interaction term for those having metabolic syndrome at 6 months was 3.04 (95% CI 0.33 to 5.74; p = 0.028) for the POWeR+F group and 1.26 (95% CI –1.37 to 3.88; p = 0.348) for the POWeR+R group (i.e. POWeR+F was more effective in those not having metabolic syndrome but only in the short term). At 12 months, the interaction terms were 0.58 (95% CI –2.42 to 3.57; p = 0.707) for the POWeR+F group and 0.45 (95% CI –2.51 to 3.41; p = 0.765) for the POWeR+R group.
| Group | Difference in weight (kg) compared with the control group (95% CI); p-value | |||||
|---|---|---|---|---|---|---|
| No metabolic syndrome | Metabolic syndrome | |||||
| 6 months | 12 months | Over study period (repeated measures) | 6 months | 12 months | Over study period (repeated measures) | |
| POWeR+F | –3.79 (–5.51 to –2.07); < 0.001 | –0.42 (–2.62 to 1.77); 0.705 | –1.87 (–3.38 to –0.36); 0.015 | –1.49 (–3.29 to 0.32); 0.107 | 0.05 (–1.88 to 1.97); 0.963 | –0.48 (–1.89 to 0.92); 0.500 |
| POWeR+R | –2.69 (–4.40 to –0.98); 0.002 | –0.57 (–2.75 to 1.61); 0.609 | –1.46 (–2.97 to 0.04); 0.056 | –2.04 (–3.74 to –0.34); 0.019 | –0.19 (–2.06 to 1.68); 0.844 | –1.01 (–2.35 to 0.34); 0.143 |
Waist
We originally specified high waist measurements as a possible subgroup, but because none of the trial cohort had low waist measurements this was not possible. We have instead explored whether or not there is an interaction in those with above and below median waist measurements. There was no evidence of a significant interaction at either 6 or 12 months. The interaction term at 6 months for those with an above-median waist circumference was 0.99 (95% CI –1.46 to 3.44; p = 0.472) for the POWeR+F group and 0.23 (95% CI –2.21 to 2.67; p = 0.853) for the POWeR+R group. At 12 months, the interaction term was 0.51 (95% CI –2.12 to 3.13; p = 0.706) for the POWeR+F group and 1.69 (95% CI –1.00 to 4.37; p = 0.218) for the POWeR+R group.
Harms
No harms were reported.
Chapter 4 Economic evaluation
This section presents both a cost-effectiveness (cost per kilogram lost) and a cost–utility [cost per quality-adjusted life-year (QALY)] analysis. Results were compared with the threshold suggested by NICE of £100 per kilogram lost if maintained over the long term. 6
The economic analysis alongside this trial was influenced by aspects of the trial, including its size (> 800 patients randomised), the low levels of research contacts with patients made feasible using predominantly web-based support as opposed to person-based support, the involvement of 56 different GP practices, each with its own nurse support, and reliance on practice case notes (mostly electronic) for service use and on short, focused patient reports. The number, but not the duration, of contacts with practice nurses was recorded. As a result, the costing of the interventions had to rely on assumptions, which are tested in a sensitivity analysis.
Methods
The costing comprised two elements: one measuring the intervention cost alone, based on the cost of internet plus that of nurse support; and the other measuring total cost, that is, the cost of the intervention plus differences in the cost of NHS services. The perspective was that of the NHS and Personal Social Services. The time horizon was 12 months. The implications of different cost scenarios were explored.
The main cost of the intervention was the nurse support time comprising face-to-face consultations, telephone and e-mail contact. The cost of nurse support was based on trial data that documented the number of contacts by type (face to face and by telephone and e-mail, all with the practice nurse). Face-to-face contacts linked to the trial interventions were planned to be brief and last half as long as normal visits; hence, these were assumed to cost 50% of standard practice nurse consultations. The time taken for telephone contacts relative to face-to-face visits was based on the split for GPs between face-to-face and other contacts provided in Personal Social Services Research Unit (PSSRU) unit costs. 41 Telephone contacts were shorter than most telephone consultations as no new diagnosis and management plan was being instituted; instead, patients were given brief support and signposted back to the POWeR+ resources, as appropriate. The time taken for e-mail contacts was put at 50% of that of telephone contacts and, because e-mail contacts are normally quick, this may be an overestimate. These assumptions were tested in sensitivity analyses. Nurse costs were costed using national costs per consultation for a practice nurse. 41 The unit costs used in costing the intervention are shown in Table 24.
| Service | Unit cost (£) | Source |
|---|---|---|
| GP in surgery | 46 | PSSRU41 |
| GP at home | 234 | PSSRU41 |
| Practice nurse in surgery | 14 | PSSRU41 |
| Practice nurse at home | 53 | PSSRU41 |
| GP telephone | 28 | PSSRU41 |
| Practice nurse telephone | 14 | PSSRU41 |
| Out-of-hours clinic | 95 | PSSRU41 |
| Walk-in clinic | 135 | PSSRU41 |
| Hospital day case | 468 | NHS Reference Costs 2013–2014 42 |
| Outpatient | 135 | PSSRU41 |
| A&E | 135 | PSSRU41 |
| Intervention costs | ||
| Nurse support: face-to-face contact, weight related | 7a | PSSRU41 |
| Nurse support: telephone, weight related | 3.5b | PSSRU41 |
| Nurse support: e-mail, weight related | 1.75c | PSSRU41 |
| Cost of provision of website per user | 1.00 | Trial estimate based on costs incurred |
The cost of the intervention comprised the cost of the website and the support offered to users. The cost of providing a web intervention that could be used widely would be spread among many thousands of individuals. We therefore put the cost of the website at £1 per person, based on the cost of providing and maintaining the website, but this may overestimate its cost, were it to be made widely available.
The brief verbal intervention and supporting advice sheets in the control group were not costed separately, but were assumed to be included in primary care consultations. Resource use data were extracted from GP case notes 12 months after recruitment – covering medication, primary care visits, outpatient consultant, accident and emergency attendance and hospital admission. Intervention-specific resource use was recorded by study nurses.
The costing of medications was limited to those plausibly associated with obesity, that is, medications for diabetes mellitus, BP, clotting/antiplatelet, musculoskeletal problems (back, hip and knee pain), and lipid lowering and weight management. The names of these medications, dosage and days of use were recorded. Prices were the listed pack price, the cost to the NHS. For drugs started before the trial or for which no end date was recorded, we assumed the duration of use to be that of the trial (12 months). For all other obesity-related medications, if no duration of use data were available, we assumed the recommended duration from the starting date. The unit costs of medications were those in the British National Formulary in 2013–14 prices. 43
Consultations in primary care, walk-in centres, and accident and emergency departments and outpatient attendances were recorded and priced using PSSRU 2013/14 unit costs41 (see Table 24).
Data on all hospital admissions were also extracted. We used Healthcare Resource Group cost per episode linked to cause of admission. 43 Data on these unit costs and those for medications are available on request.
The outcomes were weight lost and QALYs. Data on weight were collected at 6 and 12 months, when patients were weighed by the practice nurse. Health-related quality of life was measured by the EQ-5D, completed by patients at baseline and at 6 and 12 months. The EQ-5D scores were expressed as utilities using the national tariff, with interpolation using area under the curve.
Missing data were imputed using multiple imputation, as in the clinical analyses in Chapter 3. Bootstrapping was applied to the imputed data to estimate costs, weights, QALYs and incremental cost-effectiveness ratios (ICERs). Weights were analysed by repeated measure analyses. Estimates of the differences in QALYs were adjusted by baseline EQ-5D scores. Pooled results of the bootstrapped analyses were used to generate the 95% CIs around the estimates. In line with the approach that matches the effectiveness analysis (where a reduction is indicated by a negative value), where costs are reduced we have also indicated this with a negative value (in particular, the negative values for ICERs indicate that there was an estimated reduction in costs for every kilogram of weight lost).
The base case for the cost per weight lost analysis was that for the imputed case based on repeated measures analyses, linked to the statistical analysis report in Chapter 3, chosen in view of the low completion of the EQ-5D (< 50%). We also present the cost per QALY analysis based on imputed cases, but given the extent of missing data caution should be used in interpreting such results.
Sensitivity analyses were conducted on complete cases, the cost per percentage achieving weight loss of > 5% from baseline and without hospital admission costs.
Results
The pattern of use of the nurse support in the two intervention groups is shown in Table 25. Approximately 70% of those in each intervention group had at least one contact [POWeR+F, 72% (193/269); POWeR+R, 67% (181/270)]. Contacts could be face-to-face, e-mail or telephone contacts. As expected, the mean face-to-face contacts were highest in the POWeR+F group at 1.50 per patient, but a few also occurred in the POWeR+R group (mean 0.10). The mean total number of contacts was slightly higher in the POWeR+F group, at 3.23, than in the POWeR+R group at 2.85.
| Group | Contacts | Complete cases, mean (95% CI); n | Intention to treat, mean (95% CI) |
|---|---|---|---|
| POWeR+F (n = 269) | Face to face | 2.33 (2.17 to 2.49); 173 | 1.50 (1.33 to 1.67) |
| 2.13 (1.91 to 2.35); 116 | 0.92 (0.76 to 1.08) | ||
| Telephone | 1.82 (1.6 to 2.03); 120 | 0.81 (0.67 to 0.95) | |
| Total | 4.50 (4.25 to 4.74); 193 | 3.23 (2.93 to 3.53) | |
| POWeR+R (n = 270) | Face to face | 1.56 (1.13 to 1.98); 18 | 0.10 (0.05 to 0.16) |
| 3.13 (2.94 to 3.32); 173 | 2.00 (1.79 to 2.22) | ||
| Telephone | 1.62 (1.47 to 1.77); 124 | 0.74 (0.63 to 0.86) | |
| Total | 4.25 (4.05 to 4.46); 181 | 2.85 (2.58 to 3.13) |
Total costs
The total unadjusted cost by group (Table 26) put the cost per patient (including ‘nurse support’ in each intervention arm) at £398 (95% CI £296 to £500) in the control group; this was slightly higher at £401 (95% CI £296 to £506) with POWeR+F but lower in the POWeR+R group at £349 (95% CI £266 to £432). These cost differences were not statistically significant.
| Cost use categories | Mean (95% CI) for those using service; n | Mean for all (95% CI) |
|---|---|---|
| Control group (n = 279) | ||
| Cost (£) | ||
| All costs | 428 (320 to 537); 259 | 398 (296 to 500) |
| Medication | 271 (191 to 350); 119 | 116 (78 to 153) |
| GP consultation | 71.69 (60.22 to 83.16); 142 | 36.49 (29 to 44) |
| Outpatient attendance | 548 (388 to 709); 49 | 96.29 (60 to 133) |
| Hospital admission | 2569 (1158 to 39,805); 11 | 101 (26 to 177) |
| Number of cases | ||
| Medication usage | 3.23 (2.84 to 3.62); 119 | 1.38 (1.13 to 1.63) |
| GP consultation | 2.68 (2.33 to 3.03); 142 | 1.37 (1.13 to 1.60) |
| Outpatient attendance | 4.06 (2.87 to 5.25); 49 | 0.71 (0.44 to 0.99) |
| Hospital admission | 1 (1 to 1); 11 | 0.04 (0.02 to 0.06) |
| POWeR+F (n = 269) | ||
| Cost (£) | ||
| All costs | 431 (319 to 543); 250 | 401 (296 to 506) |
| Medication | 191 (150 to 232); 140 | 99.6 (75 to 124) |
| GP consultation | 82.77 (67 to 97); 148 | 45.54 (36 to 55) |
| Outpatient attendance | 534 (373 to 695); 47 | 93.35 (57 to 130) |
| Hospital admission | 3986 (1840 to 6132); 7 | 104 (17 to 191) |
| Nurse support | 21.68 (20.59 to 22.77); 193 | 15.56 (14 to 17) |
| Number of cases | ||
| Medication usage | 2.84 (2.55 to 3.13); 140 | 1.48 (1.25 to 1.70) |
| GP consultation | 2.74 (2.37 to 3.11); 149 | 1.52 (1.26 to 1.78) |
| Outpatient attendance | 3.96 (2.76 to 5.15); 47 | 0.69 (0.42 to 0.96) |
| Hospital admission | 1.43 (0.70 to 2.16); 7 | 0.04 (0.01 to 0.07) |
| Nurse support | 4.5 (4.25 to 4.74); 193 | 3.23 (2.93 to 3.53) |
| POWeR+R (n = 270) | ||
| Cost (£) | ||
| All costs | 386 (296 to 477); 244 | 349 (266 to 432) |
| Medication | 215 (150 to 279); 122 | 96.99 (65 to 128) |
| GP consultation | 62.97 (53 to 73); 126 | 29.39 (23 to 35) |
| Outpatient attendance | 530 (375 to 684); 52 | 102 (64 to 140) |
| Hospital admission | 2794 (997 to 4591); 6 | 62.09 (5 to 119) |
| Nurse support | 11.92 (11.22 to 12.62); 181 | 7.99 (7.17 to 8.81) |
| Number of cases | ||
| Medication usage | 3.17 (2.80 to 3.55); 122 | 1.43 (1.18 to 1.69) |
| GP consultation | 2.55 (2.20 to 2.91); 127 | 1.20 (0.97 to 1.43) |
| Outpatient attendance | 3.92 (2.78 to 5.07); 52 | 0.76 (0.47 to 1.04) |
| Hospital admission | 1.33 (0.48 to 2.19); 6 | 0.03 (0.00 to 0.06) |
| Nurse support | 4.25 (4.05 to 4.46); 181 | 2.85 (2.58 to 3.13) |
The pattern of service use by arm was fairly similar, as expected. The main costs in each group were those of medications, at around one-quarter of the total. The mean frequency of GP consultations was similar, at around 1.44 per person, and varied little between groups. Hospital admissions were rare (11, 7 and 6 in the control, POWeR+F and POWeR+R groups, respectively), but, given their high unit cost, they accounted for around one-quarter of the total mean cost in the control and POWeR+F groups and only 18% in the POWeR+R group. Although the number of patient admissions was similar in both intervention groups, the lower total cost in the POWeR+R group was mainly because participants in this group incurred less cost.
The mean cost per patient of nursing support in the POWeR+F group was £15.56, and in the POWeR+R group was £7.99. Adding the estimated cost of the web raised these to £16.56 and £8.99, respectively. The cost results estimated by bootstrapping using imputed cases (Table 27) remained largely unchanged, as expected. The main total costs based on the bootstrapping method shows the same pattern as above, with £424 (95% CI £338 to £515), £447 (95% CI £358 to £544) and £388 (95% CI £315 to £468) for the control, POWeR+F and POWeR+R groups, respectively. Compared with the control group, POWeR+F had slightly higher mean costs at £23 (95% CI –£105 to £152) and POWeR+R had lower costs at –£36 (95% CI –£154 to £81). None of these differences was statistically significant.
| Group | Costs (£), mean (95% CI) | |
|---|---|---|
| Total | Incremental | |
| Control | 424 (338 to 515) | |
| POWeR+F | 447 (358 to 544) | 23 (–105 to 152) |
| POWeR+R | 388 (315 to 468) | –36 (–154 to 81) |
EuroQol-5 Dimensions score and quality-adjusted life-years
EuroQol-5 Dimensions forms were completed by almost all at baseline, but completion fell to around 50% at 12 months, a figure that varied only slightly by arm (Table 28). Quality of life, as measured by the EQ-5D, was similar in both intervention groups at baseline (POWeR+F, 0.824; POWeR+R, 0.816) and a little higher than in the control arm (0.785). Both intervention groups showed a slight decline in quality of life over time, whereas that of the control group improved.
| Group | Time point | Mean EQ-5D score for complete cases (95% CI) | Number completing | % completion |
|---|---|---|---|---|
| Control (n = 279) | Baseline | 0.785 (0.757 to 0.812) | 274 | 98 |
| 6 months | 0.799 (0.764 to 0.835) | 168 | 60 | |
| 12 months | 0.783 (0.742 to 0.824) | 134 | 48 | |
| POWeR+F (n = 269) | Baseline | 0.824 (0.804 to 0.845) | 267 | 99 |
| 6 months | 0.823 (0.791 to 0.854) | 169 | 63 | |
| 12 months | 0.820 (0.786 to 0.855) | 139 | 52 | |
| POWeR+R (n = 270) | Baseline | 0.816 (0.790 to 0.842) | 266 | 99 |
| 6 months | 0.822 (0.787 to 0.857) | 177 | 66 | |
| 12 months | 0.811 (0.770 to 0.851) | 151 | 56 |
Quality-adjusted life-years over 12 months, based on complete cases (< 50%), are reported in Table 29. Compared with the control group (0.780), the crude QALY estimates for both POWeR+F and POWeR+R were slightly higher (0.825 and 0.820, respectively). Bootstrapped estimates based on imputed data (Table 30) indicated a similar pattern.
| Group | Mean QALYs | 95% CI |
|---|---|---|
| Control (n = 119) | 0.78 | 0.74 to 0.82 |
| POWeR+F (n = 122) | 0.83 | 0.80 to 0.86 |
| POWeR+R (n = 135) | 0.82 | 0.78 to 0.86 |
| Group | Mean QALYs | 95% CI |
|---|---|---|
| Control (n = 279) | 0.79 | 0.77 to 0.82 |
| POWeR+F (n = 269) | 0.82 | 0.80 to 0.84 |
| POWeR+R (n = 270) | 0.81 | 0.78 to 0.83 |
Incremental cost-effectiveness
The incremental cost per kilogram lost for POWeR+F versus the control group (Table 31) was £18 (95% CI –£129 to £195), and for POWeR+R versus control it was –£25 (95% CI –£268 to £157). The point estimate for the latter indicated that POWeR+R dominated the control group as it was more effective and cost less, but the 95% CIs of this estimate range from negative to positive. Almost identical results, in terms of both sign and magnitude, were obtained when the complete cases were used (Table 32). The different ICERs in Tables 31 and 32 are a result of the effects of bootstrapping. The lack of difference between these sets of results is attributable to the low level of missing data for this outcome.
| Group | Mean difference in costs (£) (95% CI) | Mean weight lost (kg) (95% CI) | Incremental cost (£) per kilogram of weight lost (95% CI) |
|---|---|---|---|
| POWeR+F vs. control | 23 (–105 to 152) | 1.49 (0.58 to 2.41) | 18 (–129 to 195) |
| POWeR+R vs. control | –36 (–153 to 81) | 1.27 (0.34 to 2.19) | –25 (–268 to 157) |
| Group | Mean difference in costs (£) (95% CI) | Mean weight lost (kg) (95% CI) | Incremental cost (£) per kilogram of weight lost (95% CI) |
|---|---|---|---|
| POWeR+F vs. control | 0.48 (–129 to 135) | 1.56 (0.65 to 2.47) | 0.31 (–198 to 207) |
| POWeR+R vs. control | –43 (–161 to 74) | 1.30 (0.39 to 2.21) | –32.74 (–7 to 19,090) |
The incremental cost per person achieving a ≥ 5% weight loss (Table 33) for POWeR+F versus the control group was £312 (95% CI –£2481 to £3294) and for POWeR+R versus the control group was –£208 (95% CI –£2134 to £992). The point estimate of the latter was dominant but, again, with wide CIs showing great uncertainty.
| Group | Costs (£), mean (95% CI) | Percentage maintaining weight loss of at least 5% from baseline, mean (95% CI) | Incremental weight loss (%), mean (95% CI) | ICER (weight loss %), mean (95% CI) | |
|---|---|---|---|---|---|
| Total | Incremental | ||||
| Control | 424 (338 to 515) | 21 (17 to 26) | |||
| POWeR+F | 447 (358 to 544) | 23 (–105 to 152) | 29 (24 to 34) | 7.3 (0.9 to 13.7) | 312 (–2481 to 3294) |
| POWeR+R | 388 (315 to 468) | –36 (–154 to 81) | 32 (27 to 37) | 10.3 (3.7 to 16.9) | –208 (–2134 to 992) |
Incremental cost per quality-adjusted life-year gained
Table 34 provides a similar analysis to Table 31 but using QALYs based on imputed cases. After adjustment for baseline EQ-5D scores, the difference in QALY increments for the POWeR+F group compared with the control group was –0.007 (95% CI –0.030 to 0.014), and for the POWeR+R group was –0.012 (95% CI –0.032 to 0.008). The ICERs, based on imputed data and adjusted for baseline (see Table 34), led to the cost per incremental QALY for POWeR+F versus control of £1204 (95% CI –£35,636 to £38,404) and for POWeR+R versus control of –£966 (95% CI –£26,621 to £27,765). Small differences in QALYs (close to zero) resulted in wide CIs, which ranged in each instance from negative to positive.
| Group | Costs (£), mean (95% CI) | QALYs | |||
|---|---|---|---|---|---|
| Total | Incremental | Mean (95% CI) (calculated based on area under the curve) | Incremental, adjusted baseline EQ-5D scores (95% CI) | ICER (£), mean (95% CI) | |
| Control | 424 (338 to 515) | 0.792 (0.769 to 0.815) | |||
| POWeR+F | 447 (358 to 544) | 23 (–105 to 152) | 0.818 (0.798 to 0.840) | –0.007 (–0.030 to 0.014) | 1203 (–35,636 to 38,403) |
| POWeR+R | 388 (315 to 468) | –36 (–154 to 81) | 0.807 (0.783 to 0.831) | –0.012 (–0.032 to 0.008) | –966 (–26,621 to 27,765) |
Cost-effectiveness acceptability curves
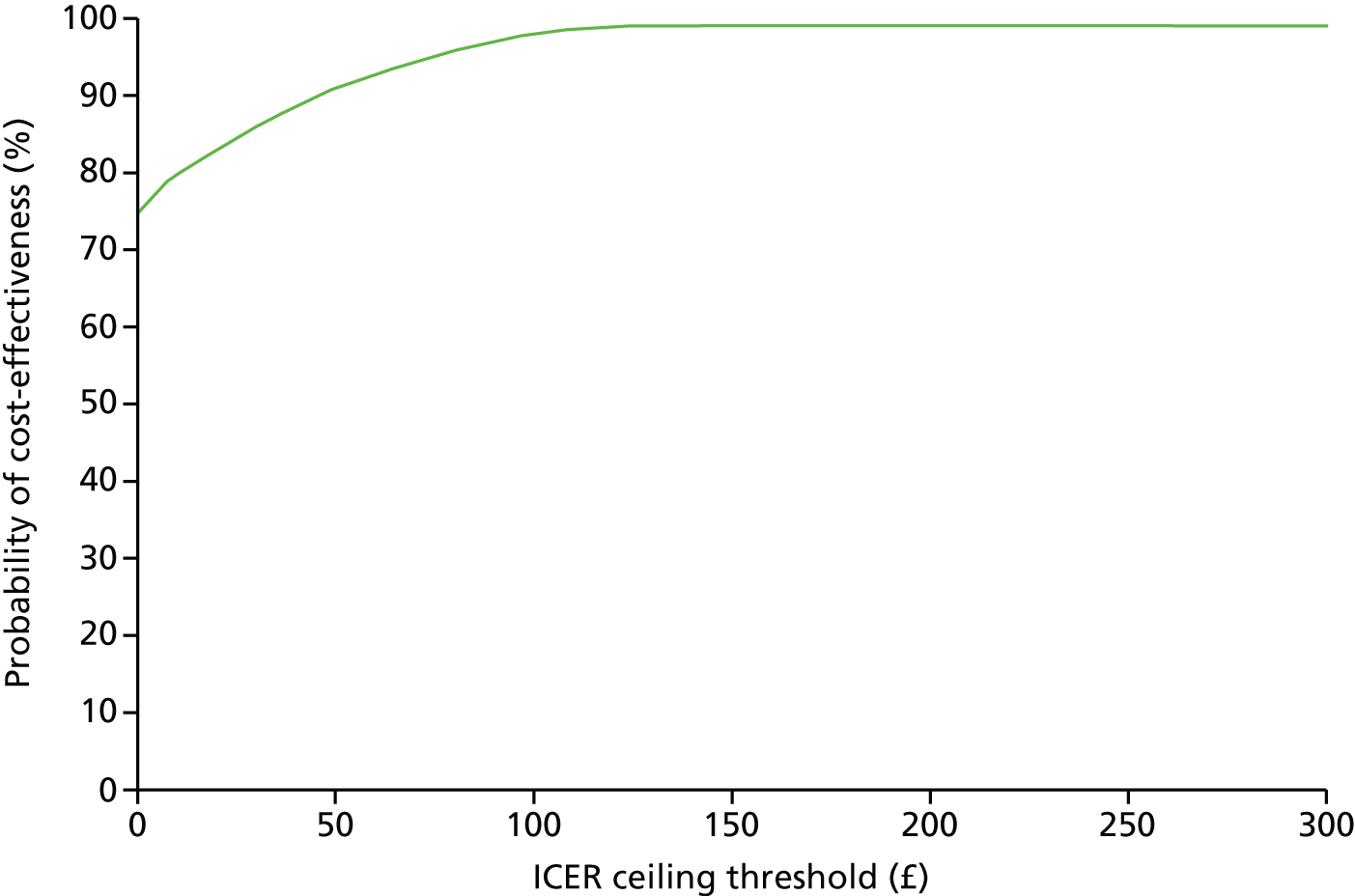
The cost-effectiveness acceptability curves (Figures 2 and 3) for weight loss show the probability of each intervention being cost-effective compared with the control group at various of levels of willingness to pay per kilogram lost, including NICE’s suggested willingness-to-pay threshold of £100 per kilogram lost. Compared with the control group, this puts the probability of being cost-effective at 88% and a 98% for the POWeR+F and the POWeR+R groups, respectively.
FIGURE 2.
Cost-effectiveness acceptability curve of POWeR+F compared with control group based on average weight loss (kg) from baseline during 12 months.
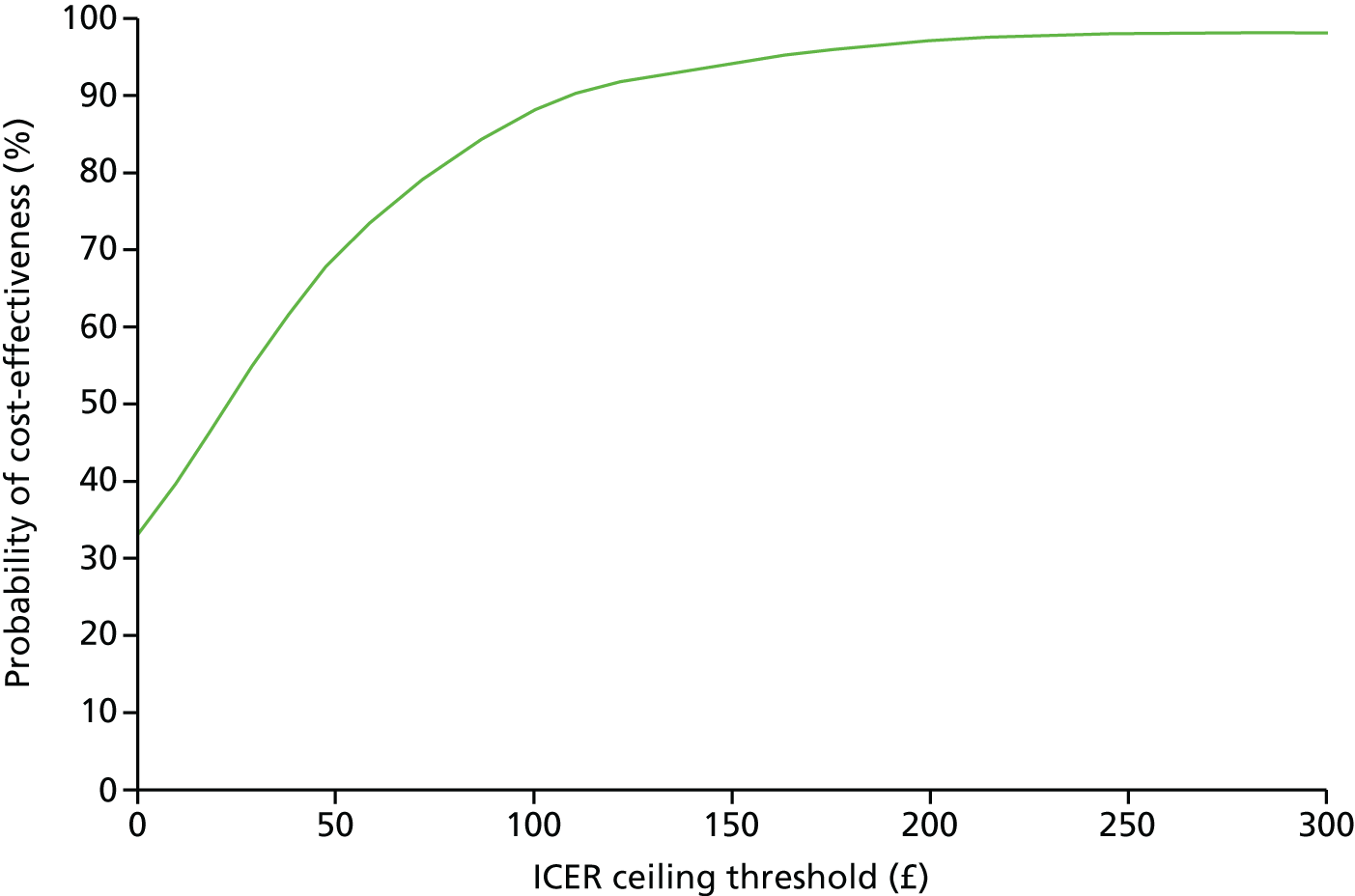
FIGURE 3.
Cost-effectiveness acceptability curve of POWeR+R compared with control group based on average weight loss (kg) from baseline during 12 months.
The incremental cost per QALY analysis is complicated by the negative QALY increments for both interventions versus control, which is unreliable as a result of missing data. For POWeR+F, the control group was dominant, as it also cost less. For POWeR+R, which cost less than the control group, the ICER was positive as a result of the division of two negative values. Given the uncertainty of these results, with half of the EQ-5D values missing, cost-effectiveness acceptability curves are not reported.
Sensitivity analyses
Given the importance of the cost of each intervention, two issues were explored: one about differential costing of the two interventions and the other about excluding hospital costs.
To recapitulate, the pattern of use of face-to-face, e-mail and telephone contacts by intervention arm (see Table 25) put the total mean number of contacts at 4.50 for POWeR+F and at 4.25 for POWeR+R. The mean nurse support cost per person was £21.68 and £11.92 in the POWeR+F and POWeR+R groups, respectively. As noted in Methods, face-to-face contacts were costed at half the national average, telephone calls at half and e-mails at one-quarter of that for face-to-face contacts. Therefore, how sensitive are the intervention costs to changes in these assumptions?
Increasing the cost of face-to-face contacts in the POWeR+R group would make little difference, as the mean number of such contacts was only 0.10 (see Table 25). Similarly, as the mean number of telephone calls was almost the same in each group (mean of 0.81 and 0.74 in the POWeR+F and POWeR+R groups, respectively), adjusting their unit cost would make little difference to the difference in cost between interventions.
The main difference between the two groups was the use of e-mails, with a mean number of 0.92 in the POWeR+F and 2.0 in the POWeR+R groups (see Table 25). The mean cost in the POWeR+R group would rise to that of the POWeR+F group only if e-mails cost the same as face-to-face contacts; this, however, seems highly unlikely. The only indication we have of the relative costs of face-to-face and telephone contacts is that in PSSRU, which is in turn based on the 2011 GP Worklife Survey. 44 This did not include e-mail contacts, which remain relatively uncommon between general practices and patients in the UK. More work is required on costing such contacts. However, it seems inescapable that e-mail contacts cost considerably less than face-to-face contacts, with the cost of telephone calls somewhere in between. The key point, besides both interventions being of very low cost, is that the mean cost of POWeR+R was less than the cost of POWeR+F.
Given that the inclusion of the costs of NHS services led to the POWeR+R group having the lowest mean cost per patient, in turn caused mainly by very few hospital admissions, an exploration of the cost-effectiveness based on exclusion of hospital costs was carried out.
When the cost of hospitalisations only was excluded, both interventions had a lower cost than the control group, which, combined with the same weight reductions (see Table 31), resulted in dominance for both over the control group.
Conclusions
Overall, both interventions were cost-effective in terms of weight loss, but less so in terms of incremental cost per QALY. This was the case for our base-case analyses of cost per kilogram lost and per QALY, and showed little variation in other analyses. The former result is more robust for several reasons, including the relationship between QALYs and weight loss and the level of missing EQ-5D data. The cost per kilogram lost is highly likely to be below NICE’s threshold of £100 per kilogram lost, but this conclusion is limited by our lack of data on the maintenance of weight loss beyond 12 months.
Chapter 5 Qualitative study with health professionals
This study presents a qualitative process evaluation that explored health-care professionals’ (HCPs’) perceptions of delivering remote and face-to-face support to patients using POWeR+. Process evaluations are recommended in national guidance,45 and can help to identify how health care is implemented: the likely mechanisms through which an intervention produces an effect and important factors within the health-care context that might influence the delivery and functioning of an intervention. We were particularly interested in understanding what it was like for HCPs to provide support for patients using POWeR+, as this could tell us more about whether or not the intervention might be feasible to implement in primary care, and could highlight any modifications that would be important to ensure its success.
Design
We used semistructured qualitative interviews to gain a rich, in-depth understanding of HCPs’ experiences of supporting patients using POWeR+.
POWeR+
POWeR+ consists of two parts: one for patients and one for the HCPs who supported both intervention groups. The development of the patient website is explained more fully elsewhere21,25,46 but, in brief, it provided patients with a choice of two eating plans (low calorie or low carbohydrate) and two physical activity plans (a walking plan, with free pedometer, or a plan for any other physical activity). POWeR+ aimed to help patients to develop self-regulation skills by promoting weekly weighing, weekly reviews of eating and physical activity goals, as well as by providing cognitive–behavioural techniques in weekly online sessions.
The HCP website provided brief information about POWeR+ and how to provide support to patients (this information was also given on paper in HCP study files). The website also allowed HCPs to view patients’ recorded weekly weight and goals, and to send patients support e-mails.
In the first version of POWeR22 we gave HCPs access to detailed information about the patient website, hoping that practitioners would therefore be able to give advice that was consistent with POWeR. However, HCPs reported that they lacked the time to look at this information and usage analysis revealed that very few had looked at these support pages. Taking a person-based approach,21 we redesigned the HCP support for POWeR+ based on practitioners’ feedback. In POWeR+, practitioners were asked not to give advice to patients; instead, all advice came from POWeR+ and the practitioners simply provided a supportive relationship to promote adherence.
We developed the Congratulate, Ask, Remind (CARe) approach to facilitate a non-directive supportive relationship, which would be easy to deliver and would fit with practitioners’ busy schedules. In addition to being developed using a person-based approach, the CARe approach is also based on self-determination theory. 47 The CARe approach aims to provide an autonomy-supportive relationship, which can raise patients’ autonomous motivation for behaviour change by promoting feelings of autonomy, competence (feeling effective) and relatedness (understood and cared for by others). 48 Autonomy-supportive relationships with HCPs predict better weight-loss outcomes,45,48 as well as predicting outcomes in a range of other health conditions. 49 The theorised mechanisms of the CARe approach are explained in Table 35.
| Guidance given to practitioners about CARe | Theoretical basis |
|---|---|
Congratulate the patient on any use of POWeR+ programme:
|
Praise was focused on the process of behaviour change (e.g. ‘great job on sticking to your goals’, or ’well done on losing weight’) rather than focused on the person as a whole (e.g. ‘you’re great at losing weight’). Process-focused praise can enhance autonomous motivation,49,50 as well as feelings of competence and relatedness.50 Praise was also informational (‘That’s great that you have logged on and had a look at POWeR+’) rather than controlling (‘Well done you have logged on to POWeR+, as you should’), which also supports autonomy.49,51 Participants who had not engaged with behaviour change were not pressured, as minimising pressure supports autonomy48 |
Ask the patient if they have any questions or concerns about making lifestyle changes, and then:
|
Asking about potential barriers and exploring possible solutions with patients can build more autonomous motivation.52 It could also help patients to feel understood and cared for, and so enhance relatedness. In this case, emphasis was put on discussing the patients’ (rather than HCP’s) ideas of possible solutions to challenges, to help build their feelings of competence and to help them to rely on themselves, rather than the HCP, for solutions. If patients were struggling to lose weight, then HCPs could suggest that patients self-monitored their dietary intake more closely for a short period of time to understand where they might need to make changes, to help build feelings of competence |
Remind the patient about future support from you:
|
HCPs monitoring patients’ progress online could potentially enhance external, rather than autonomous, motivation. However, minimising pressure can help support,48 probably negating some of this effect. This was achieved by mentioning monitoring only in the context of telling patients that they could access more support if they wanted to. Providing choice (in this case about whether or when to receive additional support) also helps to support autonomy.48 Offering the opportunity for additional support might also enhance feelings of relatedness |
Sampling and recruitment
The 54 HCPs who supported POWeR+ were nurses (n = 53) or health-care assistants (n = 1), and the majority were female (n = 53). All HCPs were sent a study invitation letter, information sheet and consent form. Nineteen female nurses expressed an interest in taking part and 13 were interviewed by telephone. No significant new themes emerged with later interviews, implying that saturation had been achieved. 53 Practitioners who chose not to participate noted that they did not have time.
Data collection
The telephone interviews were conducted between April and June 2014 by LP, a health psychology Master of Science student who was given training in qualitative interviewing and analysis. LP had no prior relationship with any of the HCPs interviewed. HCPs who agreed to participate posted a signed consent form to the researcher prior to the interview.
Before each interview, LP explained that she was a postgraduate student, with no previous involvement in the design or evaluation of POWeR+. The interviews explored HCPs’ experiences of providing support for POWeR+ participants, their experiences of using the POWeR+ website and of the study procedures. All interviews were audio-recorded and lasted between 23 and 46 minutes.
Analysis
All interviews were transcribed verbatim and imported into NVivo 10 (QSR International, Warrington, UK), to allow systematic comparisons to be made across the data set. An inductive thematic analysis54 was carried out, augmented with procedures from grounded theory. 55 First, the researchers familiarised themselves with the data. The interviews were then coded and a coding manual was created. This coding manual was continually updated to reflect the ongoing analysis. Constant comparison was used to ensure that codes were being used consistently and reflected the data. 55 Codes that identified similar aspects of the data were clustered together into themes. Inter-rater agreement on all the final codes and themes was agreed with ES (a post-doctoral researcher), LY (a health psychologist) and LP. Deviant case analysis was used to ensure that perspectives that diverged from dominant trends were not overlooked.
Results
Four themes were identified: HCPs’ perceptions and use of POWeR+, supporting patients in their use of POWeR+, the impact of POWeR+ and comparisons to existing weight management services. These are discussed in detail below.
Health-care professionals’ perceptions and use of POWeR+
Health-care professionals reported finding the POWeR website straightforward and easy to use:
It was, you know, easy to use. I didn’t have any problems using it.
HCP-13
I found it quite easy to sort of navigate around and you know, dip in and out of. Yeah, no I found it quite, quite user friendly, certainly.
HCP-15
Practitioners’ views about the content of POWeR+ varied; some were very positive, describing the website as ‘very good. It’s very comprehensive’ (HCP-09), whereas others felt that it was adequate: ‘basic I would think, but quite satisfactory’ (HCP-12).
One feature of POWeR+ that HCPs found particularly valuable was the e-mail prompts, which reminded HCPs of key actions that they needed to take, for example contacting patients for support appointments. Practitioners viewed these as very helpful in their busy work environments:
It’s useful to have the e-mails just to flag up and I used to leave them in my inbox just as a reminder until the patient you know had been dealt with.
HCP-15
A few practitioners voiced frustration that they could not see the information given to patients on the POWeR+ website. They felt that seeing this information would have allowed them to understand more fully what patients were referring to during consultations. However, some did acknowledge that their time was very limited and, therefore, it might have been challenging to view this additional information:
I don’t know what they’re reading . . . they don’t understand that and I find that difficult. It would be easier to talk to them about it if we knew what they were looking at.
HCP-08
There is no time to sort of browse, so the things I’m saying to you that may well have been on there and I didn’t actually get round to looking at them.
HCP-05
A few practitioners found it challenging to avoid giving patients specific advice, as they were used to directing patients to what they thought was the best solution and worried that patients might expect this, rather than the non-directive support used in the CARe approach:
Just to give general advice and nothing specific. So, that was hard, yeah, compared to my job where you’re doing, yeah, the opposite all the time. So, that was a little bit difficult and I think perhaps a little frustrating for some of the patients, that they didn’t get more out of me.
HCP-19
I’m not allowed to sort of, what’s the word, guide them too much am I, so, from that point of view so all I can sort of do is point them in the direction of the website, you know, and it, sometimes you just want to say something and you can’t, so I find that quite difficult.
HCP-07
Some HCPs suggested that it would have been good for the patients to have been able to leave comments for the practitioner to see:
All I got to see was their goals and any weights they've logged in . . . if they’d made any comments about how they’d had a particularly good week or bad week or anything like that, might’ve been quite interesting, just to have sort of been able to see that as well.
HCP-18
Supporting patients in their use of POWeR+
Health-care professionals supported patients in both the face-to-face and remote support groups. HCPs enjoyed providing face-to-face care, and some expressed a preference for this rather than providing remote support:
Ideally I’d like everybody to be in a face-to-face group really. That was the most satisfying for us as research nurses I think, because as we are practice nurses as well we work with people all the time so we’re not so used to communicating online with people, and I feel you get far more out of a conversation if you’re actually looking at someone.
HCP-05
Some HCPs felt they got the best response from participants in the face-to-face support group:
Those that were in the remote group I felt really had to have a lot more willpower just to keep going really, and with faceless e-mails coming, but those that were in the face-to-face groups did really well.
HCP-05
However, some practitioners did sometimes struggle to get patients to attend appointments.
Views about remote support were mixed: on the one hand some nurses found face-to-face support more enjoyable, but on the other they understood that remote support might be more practical for some patients:
I suppose the face to face is nicer from the relationship you get with the patients, but on the other hand the e-mail can be perfectly efficient and a lot of people lead very busy lives, they don’t want to be coming in.
HCP-06
It appeared that some HCPs did not view remote support as support at all; one nurse noted that patients in the remote support group would ‘probably (be) disappointed that they didn’t get any support’ (HCP-12).
Some HCPs reported that remote support was difficult, as patients were not always available by telephone. Equally, patients did not always reply to the supportive e-mails, meaning that nurses did not know whether or not their support was helping:
I didn’t go about phoning because that was just too difficult ‘cause when you’re in the surgery they might not be there . . . so it’s actually easier for people to e-mail them.
HCP-08
I wasn’t sure whether the person had got my e-mail maybe, or if there was no feedback, so if they hadn’t logged on or something, then I had no means of knowing if they were interested any more.
HCP-13
The impact of POWeR+
Participating in the POWeR trial was reported to impact on practices in a variety of ways. A small number of nurses reported that POWeR+ raised their awareness of the damaging impacts of obesity on physical and mental health, as well as how much improvement weight loss could make to health conditions:
We’ve had one lady who’s gone from being hypertensive on two therapies to now on no medication at all . . . that was a huge sort of wake-up call to me actually how much difference you could make to someone’s life.
HCP-09
The availability of POWeR+ was occasionally viewed as a useful opportunity to help other nurses and GPs to broach the topic of being overweight with patients:
[Practice staff] were quite keen to put people my way and I think sort of GPs and nurses found it a useful place to send people because weight loss is quite a difficult subject sometimes, it can be, um, can be quite hard to either broach or work through so I think it was useful that they had something that was on offer.
HCP-04
There were mixed views on the impact that providing support had on practitioners’ time. HCPs from research-active or flexible working practices felt that supporting patients with POWeR+ had only a little impact on practice time. For HCPs who did not have time allocated specifically to research, managing the logistics of providing support for patients could be challenging. Booking time out of their diaries to provide support for POWeR+ patients was reported as a good solution:
For the first few weeks I did feel I was possibly a little bit behind . . . Obviously, seeing the patients is fine ‘cause they were booked into my clinic so I had that time, but it was the follow-up over phone calls or e-mails. So once I realised that and I could book the time then I managed to sort of keep on top of it much more.
HCP-18
Comparisons to existing weight management services
Health-care professionals drew comparisons between POWeR+ and what was available to patients through their practice or externally in the form of slimming clubs and groups. Many practitioners discussed how their practice offered a fairly limited service for people who wanted to lose weight. POWeR+ was, therefore, seen as a useful alternative:
We don’t do specific clinics. Some people just come in for, to be weighed. I don’t give them specific advice really, it’s just to come in and get weighed and have a little chat.
HCP-12
We need a standard response, and I think having the POWeR tools means we’re all singing from the same hymn sheet.
HCP-09
Some HCPs did report that they could refer patients to external weight management programmes. Others reported that ordinarily they would allow patients to come into the practice to weigh themselves, but that formal support was not available. One HCP mentioned that routine weighing had stopped for cost reasons:
We did offer patients regular sort of weigh-ins so to speak. If they wanted to they could come back every couple of weeks or a month but the practice has since then decided that we wouldn’t be offering this service because it was, this will sound terrible, a waste of money.
HCP-05
Chapter 6 Qualitative process interviews with patients
Methods
Participants
Telephone interviews were conducted with 31 POWeR+ programme users between August 2013 and June 2014. Purposive sampling was applied to ensure broadly similar representation of males and females from both the remote and face-to-face support groups, as well as a mixture of high (at least four sessions completed) and low (fewer than four sessions completed) intervention users. A total of 133 trial patients were invited to take part in an interview, 111 of whom were randomly sampled; the remaining 22 were purposively selected because of their ‘low user’ status. Each potential participant was sent an information sheet, consent form and response sheet by post. Those who returned completed consent forms and response sheets were contacted by a member of the study team to arrange an interview. Ethics approval was granted within the main trial and all participants were made aware that participation was both confidential and voluntary.
Procedure
Semistructured telephone interviews were conducted with 14 remote support and 17 face-to-face support patients. Interviews explored patients’ expectations of POWeR+, experiences of the POWeR+ programme, experiences of using the POWeR+ website and experiences of nurse support. Interviews were audio-recorded and lasted between 30 and 80 minutes. Thirty-one participants were felt to be sufficient to capture a range of views from varied demographics, with no new themes emerging from later interviews.
Data analysis
Interviews were transcribed verbatim and identifiable data were removed. Inductive thematic analysis of each transcript was carried out,54,56 supported by the use of NVivo 10.0 software, and coded into emerging categories or themes, which represented frequent patterns of meaning within the data set. Deviant case analysis was used to ensure that perspectives that diverged from dominant trends were not overlooked. 57 Analysis was iterative, with ES and RD independently reading and rereading each transcript. The initial coding structure was revised to develop a coding manual based on consensus and inter-rater agreement between three members of the research team, with an audit trail maintained throughout. The final breakdown of coded themes can be found in Table 36.
| Subtheme | Content |
|---|---|
| Theme 1: reactions to the POWeR+ website content and function | |
| Website perceptions | Participants’ views on overall appearance, design, layout and navigation of the website |
| Participants’ views on acceptability of information contained within the website | |
| Participants’ views on interaction and engagement with the website | |
| User views on the optional links for more information contained in the website | |
| Accountability | Participants’ desires to be able to account for themselves |
| Non-judgemental | Participants’ views on feeling ‘judged’ |
| Computer confidence | Participants’ views on their computer confidence |
| Views towards POWeR+ eating plan | User acceptance of eating plan content and format |
| Views towards POWeR+ physical activity plans | Users’ views of the POWeR+ physical activity advice |
| Views towards goal-setting | Users’ views of POWeR+ goal-setting |
| General views towards goal-setting | Users’ general perceptions of goal-setting |
| Theme 2: changes to thinking and lifestyle | |
| Awareness raising | Participants’ accounts of how POWeR+ raised their awareness of food and physical activity requirements |
| Changed habits | Participants’ descriptions of how they have changed eating and exercise habits as a result of participating in POWeR+ |
| Changes to eating | Users’ accounts of changes made to their eating as a result of the eating plan advice in POWeR+ |
| Changes to physical activity | Users’ accounts of changes made to their physical activity as a result of the advice from POWeR+ |
| Psychological outcomes | Participants’ accounts of how POWeR+ made them feel better about themselves |
| Focused thinking | Participants’ accounts of how POWeR+ helped them to focus on their weight change efforts |
| Theme 3: motivators of and barriers to change | |
| Motivated to change | Participants’ accounts of being motivated to change |
| Pedometers | Positive and negative reflections of the POWeR+-issued pedometer |
| General barriers to change | Participants’ accounts of life factors that acted as barriers to change |
| Barriers to adopting physical activity changes | Participants’ accounts of health and injury issues that restricted physical activity |
| Overcoming barriers | Participants’ accounts of overcoming perceived barriers |
| Theme 4: using POWeR+ with and without support | |
| Nurse support | Users’ perceptions of level of support |
| Users’ perception of support provider | |
| Outcome of nurse support | |
| Difficulties accessing nurse for support | |
| Need for human contact | Users’ views on the need for support |
| Comparison of POWeR+ to slimming clubs | Users’ comparisons of POWeR+ to slimming clubs |
| Usage patterns and reasons for usage | Accounts of usage frequency and patterns |
Results
Fifteen (48%) women and 16 (52%) men aged between 45 and 88 years (mean 61 years) took part in individual telephone interviews. Full demographic details are shown in Table 37.
| Characteristic | Group | |
|---|---|---|
| POWeR+R (n) | POWeR+F (n) | |
| Gender (male/female) | 5/9 | 11/6 |
| Age (< 65 years/65+ years) | 7/7 | 14/3 |
| Usage (low/high) | 3/11 | 2/15 |
Four themes were identified: reactions to the POWeR+ website content and function, changes to thinking and lifestyle, motivators of and barriers to change, and using POWeR+ with and without support (see Table 36).
Theme 1: reactions to POWeR+ website content and function
Website perceptions
The majority of participants reported positive perceptions towards the POWeR+ website, largely based on ease of use and accessibility. They perceived the design and layout to be logical, yet also found the content interesting and informative. Even participants who felt that their information technology skillset may be lacking reported feeling positively about the website:
It’s quite easy to use and I’m not that computer savvy and I found it quite easy to get into the system once I had sorted things out, so it was quite good.
Male, aged 60 years, face-to-face support, high user, 37A04
Other participants were more critical, reporting that they would prefer if the website allowed them to account for any health conditions that had restricted them from doing what POWeR+ suggested, their absence from the website as a result of holidays or illness, or their struggle to lose weight. For several participants, importance was placed on being able to explain their behaviour:
There is nowhere you can say well I am sorry I am going to be putting on weight, I am going on holiday, but I thought that might be useful if you could have something that you could explain why things are going as they are going.
And what would you like to be done with these comments?
It is just to let the person that is doing the survey like yourself, you can think well that weight has gone up that week, and she hasn’t been on for 2 weeks and it would just be letting you know why.
Female, aged 59 years, remote support, high user, 14A06
A small number of participants mentioned already being familiar with the information and discussed the design and content of the website, perceiving it to be less engaging because of what they felt was a somewhat condescending tone.
Views towards eating plans
Participants held contrasting views of the POWeR+ eating plan format. Those who viewed the plan more positively reported finding the plan both helpful and straightforward, describing the flexibility and variety of the format as beneficial:
What I like about it is it gives you a list of green and amber and red, so what you can eat on the green list and what to avoid and so on, which I found quite good, I mean I have tried every diet you can think of, and the problem is that eventually you put the weight back on, and I think this is a more sensible way of doing it, because you can eat normally.
Female, aged 66 years, remote support, high user, 39A06
In contrast, a small number of participants suggested ways in which they felt that the plan could be improved, for example by making it more prescriptive with specific menus to follow. However, there was acknowledgement that prescribed meal plans may not be as beneficial in the long term:
I think it [having a set meal plan to follow] would have been to a certain extent easier at the beginning, but I don’t think it would of actually adjusted my attitudes and thinking which it [POWeR+] has done. So I think that is the bonus that the actual programme is designed to change your attitudes to foods, your attitudes to eating.
Male, aged 64 years, face-to-face support, high user, 07A02
Views towards physical activity plans
About one-third of participants spoke positively about the physical activity plans, perceiving them to be straightforward and clear. In particular, participants reported liking the way that targets were provided for them to alter and work towards, and that the plans prompted them to reflect on personal physical activity by providing ideas about how to increase activity levels.
Many participants were more critical of the plans, with most negative discussion centred on the way POWeR+ was set up to record their daily and weekly exercise. Specific critiques included finding daily exercise recording too time-consuming, feeling that age and physical activity abilities were not accounted for, and not having the option to record activities that were not listed.
Goal-setting
Some participants spoke about goal-setting, reporting that setting regular goals and receiving the feedback from the goal review process was beneficial as it provided encouragement to keep progressing:
It is always down to you, and doing POWeR+ has shown that to me, it’s down to you, and the discipline of writing it down has been excellent, that works very well, writing a plan and knowing what you are doing, it’s very good.
Female, aged 66 years, remote support, high user, 01A16
In contrast, others found the process to be repetitive and unnecessary, although only one participant reported that the compulsory goal-setting would deter them from completing the session content:
You have got to write down what your goals this week but I’m not prepared to do that, you see, I don’t want to do all that, so you can’t just skip that section, unless you fill that section in you can’t get to the next section.
Male, aged 62 years, face-to-face support, high user, 05A01
Theme 2: changes to thinking and lifestyle
Some participants discussed the impact that POWeR+ eating plans had exerted on their awareness about personal food choices.
They reported a greater awareness of calorie and carbohydrate content as well as portion size, and felt more confident in their ability to continue implementing this knowledge beyond the trial:
I shall take it [the eating plan] with me because I shall know what I can and can’t eat because that will be really embedded hopefully by then.
Male, aged 64 years, face-to-face support, high user, 07A02
Specific changes reported by participants included a change in eating habits, such as cutting out certain foods, looking at the nutritional content of the food before buying it, eating less or adding breakfast into their daily routine.
Theme 3: motivators and barriers to change
A small number of participants reflected on their level of motivation before, during and as a result of POWeR+. Several discussed their need to lose weight as a criterion for surgery and, thus, had long-term motivation to reduce their weight. A few others spoke about being in the ‘right frame of mind’ when introduced to POWeR+ or explained that they had already begun to make changes and had hoped that POWeR+ would give them the motivation to continue.
A number of participants described how the use of a pedometer as part of the trial had motivated them to alter physical activity habits by increasing exercise in order to meet specific goals. Participant accounts included discussions of increasing how much walking they did on a day-to-day basis because they were wearing and recording the data from the pedometer. In addition, several participants reported feeling disappointed if they had not met their daily step target.
The most commonly discussed barrier to change was related to physical activity, with about half of participants reporting a medical condition or physical problem/injury that restricted their engagement in physical activities. Some participants felt unable to work around these perceived barriers, whereas others reported having found alternative options to accommodate their reduced mobility:
I’ve had a knackered knee for many years, so I can’t necessarily go out and sprint down the road, so what I’ve done is and what I know I can do and I enjoy doing is and it’s been effective is I will walk and I tend to walk most evenings I had been walking most evenings.
Male, aged 60 years, face-to-face support, high user, 13A16
Theme 4: using POWeR+ with and without support
User perceptions of support
Participants, including those who felt that they had received minimal support, provided positive accounts of their nurses, describing them as helpful and supportive:
She was great. I feel she’s probably someone I would like to have a cup of coffee with, you know. She was very good and very encouraging.
Female, aged 64 years, face-to-face support, low user, 05A17
The nurse was very nice, very efficient, and very encouraging, when I said I have lost all this weight, she was, ‘you should be pleased with yourself’, and very encouraging, it is nice to hear when you’ve got someone on your side.
Male, aged 67 years, remote support, high user, 07A18
Face-to-face versus remote support
Some participants within each support group reported feeling positive about the type of support that they had received. Many in the face-to-face group liked the encouragement that came from these meetings:
There was face-to-face [sic] meetings built into the programme, that was nice because it was someone else who was there, who wasn’t if you like part of the family, or whatever, which was supportive and encouraging.
Male, aged 64 years, face-to-face support, high user, 02A13
Similarly, there were those who were happy to have received remote support:
I was quite glad I got the remote one, because I don’t really want someone coming in and telling me what to eat each week because I have done enough diets to know.
Female, aged 52 years, remote support, high user, 38A06
For some remote users, knowing that the support was available if they wanted it appeared to be sufficient, whereas others who wanted greater support felt that it would have helped to keep them motivated.
Conversely, other participants, regardless of allocated support group, reported feeling a lack of support because of what they perceived to be infrequent nurse contact. For a small number, this was despite making deliberate attempts to arrange support. It would appear, when comparing support records kept by practice nurses with participant accounts, that the lack of support may be linked to participants’ differing expectations and preferences about support levels.
Need for human contact
Related to perceived support, a proportion of participants discussed their need for greater personal contact, with some suggesting that personal rather than remote contact would increase their motivation:
Everything that was on [the website] was positive, but I need more pushing in the right direction, more support than what I did by just doing it on the computer.
Female, aged 48 years, face-to-face support, low user, 10A21
Again, these views were shared by participants in both support groups.
Comparison to slimming clubs
When discussing the support provided by POWeR+, many compared it with their experiences of slimming clubs. For some, slimming clubs were seen as preferable because of greater perceived support and social interaction:
Whereas you’re in an environment with people that are there, like a slimming clubs, or other people that’s encouraging you face to face, then it gives you more incentive than what it would personally for myself on a computer.
Female, aged 48 years, face-to-face support, low user, 10A21
In contrast, others preferred POWeR+ because it was free to use and there was no guarantee that the outcome of a slimming club would be any better:
I could have been paying, what, me [sic] fiver a week or whatever at the fat club and who’s to say the results would have been any better?
Male, aged 65 years, remote support, low user, 28A12
For others it was a matter of convenience, as logging into POWeR+ was easier than attending slimming club meetings. Finally, for two participants, the prospect of going and being weighed at a slimming club was perceived as intrusive and embarrassing:
Being able to do it online and having the support, rather than having to leave your own home to get weighed in front of other people . . .
Female, aged 49 years, face-to-face support, high user, 04A05
It’s not as intrusive as going to a class.
Female, aged 55 years, face-to-face support, high user, 06A14
POWeR+ usage
All participants provided accounts of how often, and under what circumstances, they used POWeR+. Regular and occasional users alike described logging into POWeR+ to seek specific information to help them with any issues that they were experiencing:
I went on every week to do my weigh in and then I would use it if and when I felt that I needed to get some information that I wasn’t sure about.
Male, aged 49 years, remote support, low user, 04A05
A number of reasons were given for decreased usage, with several participants explaining that they felt as though they had got what they needed from the programme and had settled into a routine, perhaps only logging in on occasion to remind themselves of specific information:
I think I’d gotten into a routine of losing roundabout a pound a week and was happy with doing that, and so I thought I didn’t really think to use it a lot.
Female, aged 64 years, face-to-face support, low user, 05A17
You’ve got the weekly thing that you go on the e-mails and then, after a while, you get into a pattern and you think, I don’t need this as much as I used to and I’m quite happy to carry on doing what I’m doing and see where we go.
Male, aged 65 years, remote support group, low user, 28A12
Others reported not being in the right frame of mind to use the programme, being discouraged by the perceived lack of nurse support or feeling that certain sections were no longer relevant based on little or no changes to their goals:
There’s nothing more they can really add to me apart from do I want to stay on the same diet or do I want to change it, do I want to change my goals? At the moment I’m happy with my goals, so hence the reason I’m just keeping them, and I’m doing the same thing and I’m losing weight.
Male, aged 60 years, face-to-face support, high user, 13A16
Chapter 7 Discussion
Summary of main findings
As far as we are aware, this is one of the few studies in primary care that has compared three feasible briefer behavioural interventions using primary care staff for support to manage obesity. It demonstrates that some participants managed in primary care can achieve clinically important weight loss over a 12-month period by combining simple written materials with occasional brief nurse follow-up, but that significantly more will maintain clinically important weight reduction with the POWeR+ behavioural internet programme and brief follow-up.
Strengths and limitations
The individuals who take part in trials are likely to be a relatively well-motivated group, but this is no different from other trials in obese populations, and this is also the intended target group for which intervention is most likely to be helpful. The study was large and pragmatic, mimicking the everyday conditions in primary care settings, which had the disadvantage that the control group were not closely controlled and so they undertook other activities to lose weight. This may have reduced the estimates of benefit from the internet intervention, but this is also a strength as estimates are more realistic – this is precisely what would happen in practice. The data also suggest that when controlling for such other activities there was a modest change to the estimates.
Participants with obesity in primary care settings are notoriously difficult to follow up, and we were not able to record weights in nearly 20% of individuals at 12 months. The estimates from the complete-case analysis, and the analysis using recorded weights only, are therefore both likely to provide overestimates of effectiveness; hence our primary analysis was the more conservative analysis using the imputed data. However, in practice, multiple imputation modified the estimates only slightly, which suggests that attrition bias was not a major issue. The qualitative study of health professionals used a rigorous approach to thematic analysis, involving constant comparison, deviant case analysis and multiple analysts. Another strength was that HCPs’ interview data could be triangulated with patient interviews, as well as engagement and weight-loss outcomes from the POWeR+ trial, providing a fuller understanding of the data. A weakness of this study was that only 19 practitioners volunteered to take part in this study, out of the 54 who had taken part in the original trial; the views of those who took part may not be the same as those who did not and could either over-represent or under-represent positive experiences of POWeR+. The larger qualitative study of participants may also not have been representative of the whole cohort, but in both qualitative studies there was a suitable range of participant characteristics and saturation of themes was reached, so it seems unlikely that major issues were missed. A significant limitation is that, although the intervention finished at 6 months and we were able to assess maintenance at 12 months, there was no longer-term follow-up beyond 1 year. This is relevant not only for clinical effectiveness, but also because the NICE modelling suggests that cost-effectiveness is sensitive to the duration of clinical effectiveness. The major concentration on weight outcomes meant that there was much less good coverage of the other outcomes (e.g. cholesterol, glucose, etc.).
A strength of the study was that Weight Concern was a partner in this project and chose to provide very helpful panel-based input, particularly during the development of the intervention. Although this was well justified, the project would have been strengthened by seeking additional patient and public involvement engagement from other named individuals, perhaps sourced through additional channels.
Effectiveness: main findings in the context of existing literature
We confirmed our previous findings12 that a brief intervention, using the novel approach of promoting the use of healthy food-swap sheets and with brief follow-up for weighing, can be useful. In this study, this strategy helped around 20% of participants to achieve a clinically important loss in weight. Many patients in primary care do not get such simple, positive interventions, so we have probably underestimated the effectiveness of POWeR+ in routine practice. As discussed above, the effectiveness in the control group may also be, in part, due to motivated individuals who want to lose weight, and will do other activities to help themselves (which about 50% did). In contrast, fewer individuals offered the POWeR+ intervention undertook other activities.
The systematic reviews of interventions both in other settings and primary care suggest that both intensive dietetic behavioural counselling and intensive follow-up are necessary to achieve effective weight reduction. 5,8,11 In contrast, we found that for a behavioural internet intervention, such as POWeR+, very restricted face-to-face and remote follow-up is effective (median of four contacts, comprising 6-monthly weighing, brief telephone calls and e-mails). The weight loss achieved with POWeR+ compares favourably with other internet-based interventions, which on average10 (albeit with high heterogeneity) have led to only short-term weight loss, of < 1 kg compared with no treatment controls or < 2 kg if combined with face-to-face support (in a review based mainly on studies of motivated volunteer samples). Better weight management results using an internet-based programme (around 5 kg) have been achieved only with much more intensive human support (weekly 20-minute contact for 12 weeks, and then monthly up to 24-month follow-up). Encouragingly, the weight loss achieved by POWeR+ was comparable to the best-performing interventions10 evaluated in a primary care setting, including that achieved by face-to-face commercial programmes,30 but much less costly. The difference in mean weight loss of the intervention groups when compared with the control group at the 12-month follow-up was smaller than the difference compared with the control group at 6 months, so, although clinically important reductions in weight were maintained from 6 to 12 months, particularly in the POWeR+R group, maintenance beyond 1 year is unclear.
It is difficult to be sure that physical activity did not mediate intervention effectiveness because reported leisure time physical activity is a relatively crude measure of physical activity. Nevertheless, the biases are such that over-reporting of physical activity is more likely in the intervention groups, so it seems likely that physical activity changed little. There was also little increase in the use of drugs, such as orlistat, for managing weight. Thus, most of the effect of the intervention observed is probably a result of better dietary management, which suggests that more effective engagement of participants for both physical activity and appropriate drug treatment could result in significantly greater weight loss.
Health economic analyses
The total cost per kilogram for both interventions compared with control was low relative to NICE’s suggested benchmark of £100. It was also well below the cost per kilogram loss in a recent systematic review of commercial weight-loss programmes:53 among the five most effective interventions, average cost per kilogram of weight lost ranged from US$155 (95% CI US$110 to US$218) for Weight Watchers® (Weight Watchers International, Inc., New York, NY, USA) to US$546 (95% CI US$390 to US$736) for orlistat. Economic analysis of the Counterweight Programme® (Counterweight Ltd; www.counterweight.org/Programmes),58 in which the intervention cost £59 per patient and achieved 3 kg of weight loss, and which modelled only impacts on diabetes mellitus, cardiovascular disease and colon cancer, demonstrated that this level of weight reduction is likely to be very cost-effective, even allowing for weight gain following the end of the programme. The likelihood of both POWeR+F and POWeR+R groups being cost-effective at the NICE threshold was high, well over 80% (88% and 98%, respectively). A similar figure applied to the probability of POWeR+R being cost-effective at this threshold relative to POWeR+F.
This pattern of result is robust for several reasons: it held regardless of whether costing was restricted to the intervention cost or extended to total cost, the results are based on data that are largely complete and the directions of change were consistent at 6 and 12 months.
What is less expected was the finding that the POWeR+R group was more cost-effective than the POWeR+F group. This was because it achieved similar changes in weight loss (depending on the measure), but at lower cost. Its lower intervention cost was as expected, but the finding of a greater reduction in total cost was not expected. The difference in total costs was not, however, statistically significant, but the cost of the POWeR+R intervention was shown in sensitivity analysis to be highly likely to be less than that in POWeR+F. The difference is mainly because of the lower cost of e-mails compared with face-to-face contacts.
The cost per QALY results are more difficult to interpret, mainly because both intervention groups recorded small (not statistically significant) QALY losses relative to the control group. This is despite both losing more weight than the control group. The QALY losses combined with inevitably higher intervention costs in both intervention arms means that the control would dominate in an intervention cost analysis. When total costs were used, one intervention cost more, and the other cost less than the intervention.
These incremental cost per QALY results are highly uncertain for several reasons: the decreases in QALYs from baseline linked to greater weight loss may or may not be plausible; the high proportion of missing data precluded more detailed exploration of the POWeR+ trial results; and the incremental cost per QALY values were unstable, varying by type of analysis.
In summarising the economic data, both interventions deliver a low cost per kilogram lost, as judged by NICE’s suggested threshold of £100 per kilogram lost. This is the case regardless of whether intervention or total costs are used. The probability of each intervention being cost-effective compared with the control was > 80%, with a similar figure applying to POWeR+R being more cost-effective than POWeR+F. This result reflects the combination of statistically significant differences in weight loss but not in total cost. Incremental cost per QALY results also indicated both interventions were cost-effective by NICE standards, but the results were highly uncertain. Comparison of the two interventions generally showed an advantage for the POWeR+R group as a result of its lower cost, however defined.
Even if weight loss had been identical in the POWeR+ remote group at all stages when compared with the active control group, there was no evidence that health service costs increased. Furthermore, individuals also apparently gained utility in feeling more enabled to manage their weight, and they undertook fewer other weight-loss activities, suggesting that societal costs were probably lower in the POWeR+ remote group.
Qualitative health-care professional study
Very few studies have previously looked at HCPs’ perceptions of providing support for online weight-loss interventions. Two focus group studies have explored physicians’ perceptions of providing support for patients using an online intervention. 59,60 However, in both these studies the physicians provided infrequent support to patients (every 3–6 months), whereas a coach provided patients with weekly intensive support. Coaches were not interviewed in these studies, meaning that only the perceptions of physicians who provided relatively sparse support were explored. Still, these studies usefully show that some physicians perceive that they lack the skills to support patients losing weight59 and that others use dubious motivational strategies, such as expressing frustration to patients who are not making progress. 60 These findings suggest that a more structured approach to providing support, such as the CARe approach, may be a useful tool for practitioners wanting to support patients using online weight-loss interventions. The wider literature examining HCPs’ experiences of providing support for an online intervention in chronic health conditions tends to be of slightly lower quality, as a result of very small samples of practitioners (n = 4 or 5). This literature suggests that HCPs see a lack of time as a key challenge to providing support,61,62 which is consistent with the findings of our study and represents a challenge to implementing digital interventions into health-care settings.
Health-care professionals found it feasible and acceptable to support patients using POWeR+. As POWeR+ has been shown to be effective and can be provided at low cost, it may serve as a useful tool for the treatment of obesity in primary care and public health. Digital interventions are rising in popularity and additional human support is likely to maximise the effectiveness of these interventions. The CARe approach shows promise as a model to guide human support for digital weight-loss interventions, and may be a useful model to guide human support in digital interventions more broadly.
Qualitative study with patients
Overall, the POWeR+ website was viewed positively and as a trustworthy source of information relating to weight loss, particularly when compared with other sources of information. Confidence in the accuracy of the information was further enhanced by delivering the programme through participants’ usual GP practices. Participants appreciated the flexibility offered by an online intervention and were complimentary about the formatting and design of both the eating and exercise plans, although suggestions for improvement included the provision of structured meal plans and example recipes. Participants provided mixed views regarding the setting of short-term, realistic goals on a weekly basis, with some finding it beneficial to make and review their goals regularly, but others perceiving this to be an unnecessary inconvenience. Nurse support was also perceived positively by all participants, even those who reported feeling that the level of support received was low. Nurse records kept during the trial show that participants all received a regular amount of support in line with the trial guideline; however, there was an indication that participants did not always perceive remote support, particularly by e-mail, as a form of support. The findings suggest that participants would prefer support to include the chance to speak with a nurse, or at the very least to know that someone was available for them to talk to as required.
Interpretation of results
Some participants in primary care maintain clinically important weight loss over a 12-month period by promoting simple written materials without expert dietetic support, but significantly more will achieve clinically important weight reduction with a behavioural internet programme and brief remote follow-up, and resource use is not likely to increase. HCPs find POWeR+ both feasible and acceptable to use in daily practice.
Implications for health care
Practices could feasibly consider implementing a weight management approach with internet-based support for behaviour change (such as POWeR+R) among their obese patients, because it likely to be more effective and more efficient than brief interventions using simple dietary advice.
Future research implications
-
Many individuals did not continue using the POWeR+ website and, given the evidence that those who did lost rather more weight, a research priority is to identify clinically effective and cost-effective ways of continuing to engage individuals.
-
Given the utility of POWeR+ with brief support in primary care, the question then arises of what public health benefit might accrue from using POWeR+ in community settings and what magnitude and nature of support is necessary, such as a central facilitator or pharmacy support, in order to achieve effective weight control.
-
Very few participants were engaged in using drug management of their weight problems despite this being intended as part of the package, and few individuals increased physical activity; an implementation study to develop and trial a complex intervention to address both these issues is warranted.
-
Future research to test the effectiveness, acceptability and feasibility of the CARe approach as a method for providing human support for digital interventions in a range of health conditions is warranted.
Acknowledgements
Richard Hobbs is part supported by the National Institute for Health Research (NIHR) School for Primary Care Research, NIHR Oxford Biomedical Research Centre (BRC), and NIHR Collaborations for Leadership in Applied Health Research Oxford. Christopher D Byrne is part funded by the Southampton NIHR BRC. The funder had no role in day-to-day management of the project, nor in the analysis or write up of the report.
We are grateful to all the patients and HCPs who have contributed their time and effort, and provided helpful insights to make POWeR+ possible. We are grateful to the Trial Steering Committee for their support and advice throughout the study. We are grateful to the following people for providing technological support for POWeR+ and for assistance with the development: Jin Zhang, Matthew Taylor, Mark Weal and Rosie Jones. We are grateful to David Turner (University of East Anglia) who helped to develop the protocol for funding and to Catherine Brant for help in developing the complex intervention.
We are grateful to Weight Concern for their input into the original application and in the development of the intervention.
Contributions of authors
Paul Little (GP and Professor of Primary Care Research) had the original idea for the protocol, led the protocol development and the funding application, supervised the running of the lead study centre and co-ordination of centres, contributed to the analysis and led the drafting of the report.
Beth Stuart (Study Statistician) developed the analysis protocol, performed the quantitative analysis and contributed to the drafting of the report.
FD Richard Hobbs (GP and Professor of Primary Care) developed the protocol for funding, contributed to the management of the study, supervised the Oxford study centre and contributed to the drafting of the paper.
Jo Kelly (Senior Trial Manager) developed the protocol, provided day-to-day overall management of the study, co-ordinated recruitment in the lead study centre and undertook co-ordination of another centre, and commented on drafts of the report.
Emily R Smith (Senior Trial Manager) developed the protocol, provided day-to-day overall management of the study, co-ordinated recruitment in the lead study centre and undertook co-ordination of another centre, and commented on drafts of the report.
Katherine J Bradbury (Health Psychologist) contributed to protocol development, was responsible for the day-to-day development and piloting of the intervention, and commented on drafts of the report.
Stephanie Hughes (Health Psychologist) contributed to protocol development, was responsible for the day-to-day development and piloting of the intervention, and commented on drafts of the report.
Peter WF Smith (Professor of Statistics) developed the analysis protocol, performed the quantitative analysis and contributed to the drafting of the report.
Michael V Moore (GP and Professor in Primary Care) developed the protocol for funding, contributed to the management of the study, and contributed to the analysis and to the drafting of the report.
Mike EJ Lean [Professor of Human Nutrition (Medicine)] developed the protocol for funding, contributed to the management of the study and contributed to the drafting of the report.
Barrie M Margetts (Professor of Public Health Nutrition) developed the protocol for funding, contributed to the management of the study, led the analysis of the food frequency data and contributed to the drafting of the report.
Christopher D Byrne (Professor of Endocrinology and Metabolism) developed the protocol for funding, contributed to the management of the study and contributed to the drafting of the report.
Simon Griffin (Professor of General Practice) developed the protocol for funding, contributed to the management of the study and contributed to the drafting of the report.
Mina Davoudianfar (Trial Manager) co-ordinated the Oxford study centre.
Julie Hooper (Trial Administrator) provided co-ordination and data management for the Southampton study centre.
Guiqing Yao (Health Economist) developed the protocol for analysis of the notes review data and contributed to the drafting of the report.
Shihua Zhu (Health Economist) developed the protocol for analysis of the notes review data and contributed to the drafting of the report.
James Raftery (Professor of Health Economics) developed the protocol for funding, contributed to the management of the study, supervised the analysis of resource use data, developed the protocol for analysis of the notes review data and contributed to the drafting of the report.
Lucy Yardley (Professor of Health Psychology) developed the protocol and funding application, led the development of the intervention, contributed to daily supervision of website issues, contributed to broader study management and contributed to drafting the report.
Publication
Little P, Stuart B, Hobbs FDR, Kelly J, Smith E, Bradbury K, et al. An internet-based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel-group, randomised controlled trial. Lancet Diabet Endocrinol 2016;4:821–8.
Data sharing statement
There are no further data available.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Troiano RP, Frongillo EA, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord 1996;20:63-75.
- The National Heart Lung and Blood Institute. Clinical Guidelines on the Identification and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 1998.
- Health Survey for England 2014. London: National Health Service Information Centre; 2015.
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8210.
- NICE . Obesity Full Guideline 2015. http://guidance.nice.org.uk/CG43/guidance (accessed 20 May 2016).
- NICE . Maintaining a Healthy Weight and Preventing Excess Weight Gain in Children and Adults. Cost Effectiveness Considerations from a Population Modelling Viewpoint 2014. www.nice.org.uk/guidance/ng7/evidence/report-1-cost-effectiveness-considerations-from-a-population-modelling-viewpoint-8735005 (accessed 20 May 2016).
- Brown I, Thompson J, Tod A, Jones G. Primary care support for tackling obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract 2006;56:666-72.
- Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA 2014;312:1779-91. http://dx.doi.org/10.1001/jama.2014.14173.
- Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res 2010;12. http://dx.doi.org/10.2196/jmir.1376.
- Kodama S, Saito K, Tanaka S, Horikawa C, Fujiwara K, Hirasawa R, et al. Effect of web-based lifestyle modification on weight control: a meta-analysis. Int J Obes 2012;36:675-85. http://dx.doi.org/10.1038/ijo.2011.121.
- Neve M, Morgan PJ, Jones PR, Collins CE. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: a systematic review with meta-analysis. Obes Rev 2010;11:306-21. http://dx.doi.org/10.1111/j.1467-789X.2009.00646.x.
- Little P, Kelly J, Barnett J, Dorward M, Margetts B, Warm D. Randomised controlled factorial trial of dietary advice for patients with a single high blood pressure reading in primary care. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.38037.435972.EE.
- John JH, Ziebland S, Yudkin P, Roe LS, Neil HA. Oxford Fruit and Vegetable Study Group . Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet 2002;359:1969-74. https://doi.org/10.1016/S0140-6736(02)98858-6.
- Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr 2000;72:922-8.
- Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr 2002;76:93-9.
- Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes 2005;29:1168-74. http://dx.doi.org/10.1038/sj.ijo.0803015.
- Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain – a systematic review. Obes Rev 2000;1:95-111. https://doi.org/10.1046/j.1467-789x.2000.00016.x.
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007;298:2296-304. http://dx.doi.org/10.1001/jama.298.19.2296.
- Anhøj J, Jensen AH. Using the internet for life style changes in diet and physical activity: a feasibility study. J Med Internet Res 2004;6. https://doi.org/10.2196/jmir.6.3.e28.
- Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare’s search for effective obesity treatments: diets are not the answer. Am Psychol 2007;62:220-33. http://dx.doi.org/10.1037/0003-066X.62.3.220.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. http://dx.doi.org/10.2196/jmir.4055.
- Yardley L, Ware LJ, Smith ER, Williams S, Bradbury KJ, Arden-Close EJ, et al. Randomised controlled feasibility trial of a web-based weight management intervention with nurse support for obese patients in primary care. Int J Behav Nutr Phys Act 2014;11. http://dx.doi.org/10.1186/1479-5868-11-67.
- Bradbury K, Dennison L, Little P, Yardley L. Using mixed methods to develop and evaluate an online weight management intervention. Br J Health Psychol 2015;20:45-5. http://dx.doi.org/10.1111/bjhp.12125.
- Ware LJ, Williams S, Bradbury K, Brant C, Little P, Hobbs FD, et al. Exploring weight loss services in primary care and staff views on using a web-based programme. Inform Prim Care 2012;20:283-8.
- Yardley L, Williams S, Bradbury K, Garip G, Renouf S, Ware L, et al. Integrating user perspectives into the development of a web-based weight management intervention. Clin Obes 2012;2:132-41. http://dx.doi.org/10.1111/cob.12001.
- Little P, Stuart B, Hobbs FDR, Kelly J, Smith E, Bradbury K, et al. An internet-based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel-group, randomised controlled trial. Lancet Diabet Endocrinol 2016;4:821-8. https://doi.org/10.1016/S2213-8587(16)30099-7.
- NHS . 5 A DAY: What’s It All About? n.d. www.nhs.uk/Livewell/5ADAY/Documents/Downloads/5%20A%20DAY%20z%20card.pdf (accessed January 2017).
- Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50. http://dx.doi.org/10.1056/NEJM200105033441801.
- Knowler W, Barrett-Connor E, Fowler S, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Eng J Med 2002;346:393-40. https://doi.org/10.1056/NEJMoa012512.
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: LightenUp randomised controlled trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d6500.
- Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 1993;25:81-9. https://doi.org/10.1249/00005768-199301000-00012.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.192644.
- Shen BJ, Todaro JF, Niaura R, McCaffery JM, Zhang J, Spiro A, et al. Are metabolic risk factors one unified syndrome? Modelling the structure of the metabolic syndrome X. Am J Epidemiol 2003;157:701-11. https://doi.org/10.1093/aje/kwg045.
- Han TS, Lean ME. Self-reported waist circumference compared with the ‘Waist Watcher’ tape-measure to identify individuals at increased health risk through intra-abdominal fat accumulation. Br J Nutr 1998;80:81-8. https://doi.org/10.1017/S0007114598001809.
- Little P, Lewith G, Webley F, Evans M, Beattie A, Middleton K, et al. Randomised controlled trial of Alexander technique lessons, exercise, and massage (ATEAM) for chronic and recurrent back pain. BMJ 2008;337. https://doi.org/10.1136/bmj.a884.
- Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar H, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998;352:167-72. https://doi.org/10.1016/S0140-6736(97)11509-4.
- Verplanken B, Orbell S. Reflections on past behaviour: a self-report index of habit strength. J Appl Soc Psychol 2015;33:1313-30. https://doi.org/10.1111/j.1559-1816.2003.tb01951.x.
- Armitage CJ, Conner M. Efficacy of the theory of planned behaviour: a meta-analytic review. Br J Soc Psychol 2001;40:471-99. https://doi.org/10.1348/014466601164939.
- Rothert K, Strecher VJ, Doyle LA, Caplan WM, Joyce JS, Jimison HB, et al. Web-based weight management programs in an integrated health care setting: a randomized, controlled trial. Obesity 2006;14:266-72. https://doi.org/10.1038/oby.2006.34.
- Department for Communities and Local Government . The English Indices of Deprivation 2010 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf (accessed 20 December 2016).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Department of Health . NHS Reference Costs 2013–2014 2015. www.gov.uk/government/publications/nhsreference-costs-2013-to-2014 (accessed June 2015).
- Joint Formulary Committee . British National Formulary 2015. www.bnf.org/bnf/ (accessed May 2015).
- Hann M, Santos R, Sutton M, Gravelle H, Sibbald B. Sixth National GP Worklife Survey. Manchester: National Primary Care Research and Development Centre; 2011.
- Gorin AA, Powers TA, Koestner R, Wing RR, Raynor HA. Autonomy support, self-regulation, and weight loss. Health Psychol 2014;33:332-9. http://dx.doi.org/10.1037/a0032586.
- Arden-Close EJ, Smith E, Bradbury K, Morrison L, Dennison L, Michaelides D, et al. A visualization tool to analyse usage of web-based interventions: the example of Positive Online Weight Reduction (POWeR). JMIR Hum Factors 2015;2. http://dx.doi.org/10.2196/humanfactors.4310.
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55:68-7. https://doi.org/10.1037/0003-066X.55.1.68.
- Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol 1996;70:115-26. https://doi.org/10.1037/0022-3514.70.1.115.
- Henderlong J, Lepper MR. The effects of praise on children’s intrinsic motivation: a review and synthesis. Psychol Bull 2002;128:774-95. https://doi.org/10.1037/0033-2909.128.5.774.
- Coatsworth JD, Conroy DE. The effects of autonomy-supportive coaching, need satisfaction, and self-perceptions on initiative and identity in youth swimmers. Dev Psychol 2009;45:320-8. http://dx.doi.org/10.1037/a0014027.
- Ryan R, Mims V, Koestner R. Relation of reward contingency and interpersonal context to intrinsic motivation: a review and test using cognitive evaluation theory. J Person Soc Psychol 1983;45:736-50. https://doi.org/10.1037/0022-3514.45.4.736.
- Ryan R, Patrick H, Deci E, Williams G. Facilitating health behaviour change and its maintenance: interventions based on self-determination theory. Eur Health Psychol 2008;10:2-5.
- Finkelstein EA, Kruger E. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity 2014;22:1942-51. http://dx.doi.org/10.1002/oby.20824.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine; 1967.
- Joffe H, Yardley L, Marks D, Yardley L. Research Methods for Clinical and Health Psychology. London: Sage; 2004.
- Yardley L, Smith J. Qualitative Psychology. London: Sage; 2008.
- Trueman P, Haynes SM, Felicity Lyons G, Louise McCombie E, McQuigg MS, Mongia S, et al. Long-term cost-effectiveness of weight management in primary care. Int J Clin Pract 2010;64:775-83. http://dx.doi.org/10.1111/j.1742-1241.2010.02349.x.
- Bennett WL, Gudzune KA, Appel LJ, Clark JM. Insights from the POWER practice-based weight loss trial: a focus group study on the PCP’s role in weight management. J Gen Intern Med 2014;29:50-8. http://dx.doi.org/10.1007/s11606-013-2562-6.
- Gudzune KA, Clark JM, Appel LJ, Bennett WL. Primary care providers’ communication with patients during weight counselling: a focus group study. Patient Educ Couns 2012;89:152-7. http://dx.doi.org/10.1016/j.pec.2012.06.033.
- Fairbrother P, Ure J, Hanley J, McCloughan L, Denvir M, Sheikh A, et al. Telescot programme team . Telemonitoring for chronic heart failure: the views of patients and healthcare professionals – a qualitative study. J Clin Nurs 2014;23:132-44. http://dx.doi.org/10.1111/jocn.12137.
- Seto E, Leonard KJ, Masino C, Cafazzo JA, Barnsley J, Ross HJ. Attitudes of heart failure patients and health care providers towards mobile phone-based remote monitoring. J Med Internet Res 2010;12. http://dx.doi.org/10.2196/jmir.1627.
Appendix 1 Intervention description: POWeR+
The text in this appendix has been reproduced from Little et al. 26 under the terms of the CC BY 4.0 licence (https://creativecommons.org/licenses/by/4.0/).
POWeR+ is an automated online intervention to support weight management over 1 year. It was adapted from an earlier version (POWeR), which was extensively trialled and shown to improve weight loss. Development was informed by the person-based approach, which employs an iterative process of in-depth qualitative user testing and a theoretical basis involving the principles of self-determination theory and techniques from cognitive–behavioural theory.
POWeR+ was designed to provide support for sustainable long-term self-management of weight. Based on our early piloting it was clear that patients wanted choice and that this would increase engagement; patients therefore choose either a low-calorie eating plan (a reduction of around 600 calories a day) or a low-carbohydrate eating plan (a carbohydrate limit of 50 g a day). Users can base their eating plan on a traffic light system that categorises foods into those that could be eaten freely (‘green’), in moderation (‘orange’) or very sparingly (‘red’). The low-carbohydrate eating plan categorises most vegetables and some fruits as ‘green’ and categorises only very high-sugar and starchy foods as ‘red’, and is therefore compatible with a sustainable healthy diet. Patients are also encouraged to increase their physical activity levels by choosing either a walking plan (in which case they can request a pedometer) or a self-selected mixture of other physical activities.
POWeR+ focuses principally on fostering users’ self-regulation skills for autonomously self-managing their weight rather than providing detailed dietetic advice. Throughout POWeR+, users are taught active cognitive and behavioural self-regulation techniques (‘POWeR tools’) to overcome problems such as low motivation, confidence or relapse. Evidence is provided for the effectiveness of these techniques and examples given of how others have successfully used them (‘POWeR stories’). POWeR+ emphasises forming healthy eating and physical activity habits that should become non-intrusive and require little effort to sustain. POWeR+ is tailored by gender, using language and ‘POWeR stories’ designed to appeal to both men and women.
The intervention was developed based on behavioural theory and evidence from existing successful interventions, with very extensive iterative qualitative piloting with a wide range of users from the target population to check usability, accessibility and acceptability, and to elicit and respond to user views. Aspects of POWeR+ that were modified from the original prototype POWeR intervention included use of game-based techniques (i.e. sessions grouped into stages through which the user advances), addition of extensive novel content (extra sessions to maintain interest for a longer period), shorter sessions (to enhance engagement), and improvements to usability and navigation.
Overview of the four stages of POWeR+
Stage 1: users are tunnelled (i.e. they cannot proceed to a session until they have completed the previous session) through three core sessions introducing them to POWeR+ and guiding them through the process of setting and reviewing weekly goals (Figure 4). There are three components to the goal review:
-
recording weight
-
choosing an eating plan from:
-
a low-calorie diet
-
a low-carbohydrate diet
-
-
choosing a physical activity plan:
-
increasing walking using a provided pedometer and recording the weekly step count
-
choosing any other physical activity and setting weekly goals.
-
FIGURE 4.
Overview of weekly goal review. This figure has been reproduced from Little et al. 26 under the terms of the CC BY 4.0 licence (https://creativecommons.org/licenses/by/4.0/).
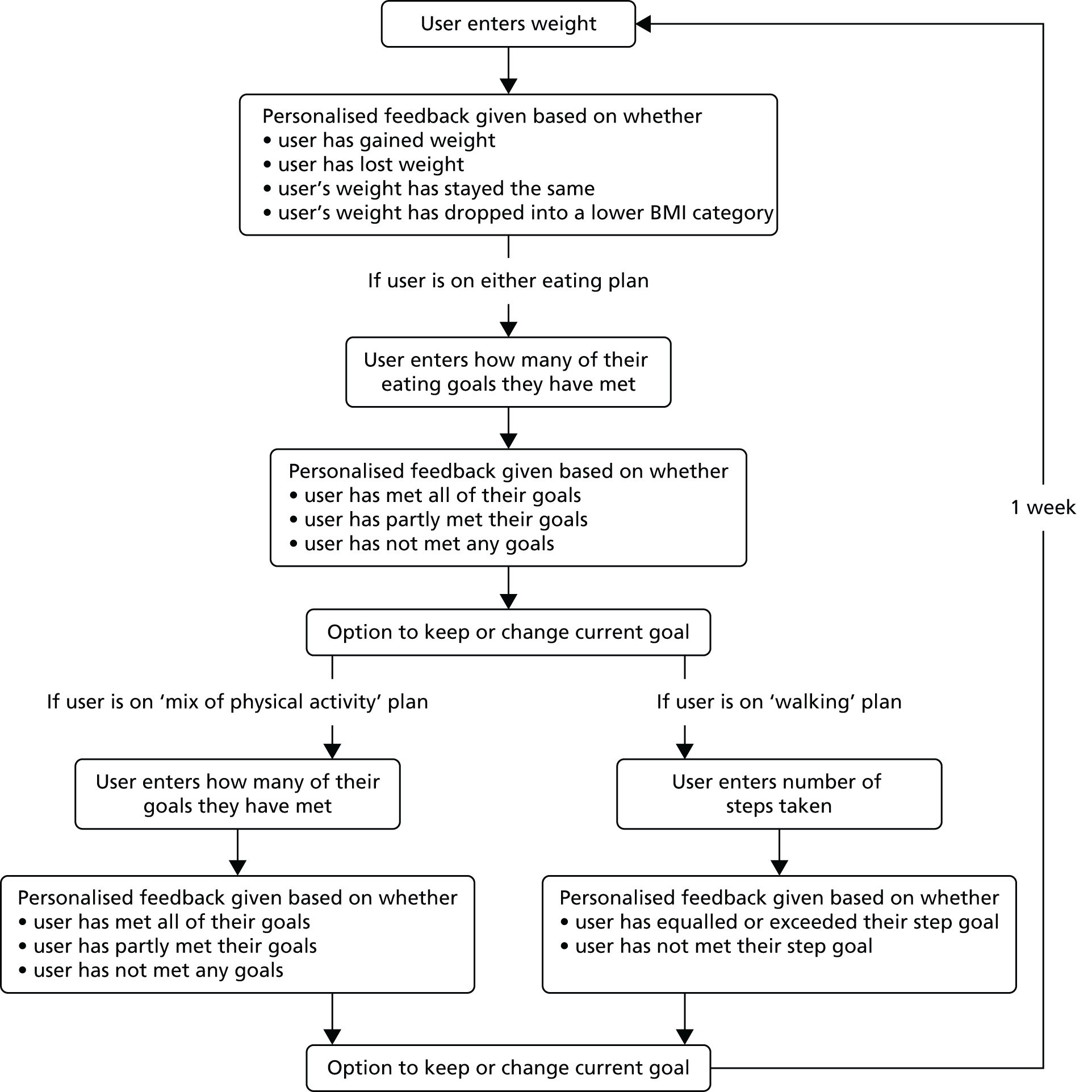
Participants may opt to change their plans after their weekly goal review at any point during the intervention.
Stage 2: users have free-choice access (i.e. they can access the sessions in any order) of sessions 4–10. Goals can now be reviewed weekly and users can view their progress on a graph. If a user misses 1 week of goal review, they may still review their goals the following week. After all sessions are completed or a period of 7 weeks has passed since the stage was started, stage 3 sessions become available.
Stage 3: users have free-choice access to sessions 11–17 and continue to complete weekly goal reviews. After all sessions are completed or a period of 7 weeks has passed since the stage was started, stage 4 sessions become available.
Stage 4: users have free-choice access to sessions 18–25 and continue to complete weekly goal reviews.
Throughout the intervention, weekly e-mails are sent to remind users to complete their goal review. Set periods of inactivity at various points during the intervention also trigger e-mails prompting users to return to either complete sessions or complete their goal review.
| Session number | Description |
|---|---|
| Stage 1 (user is tunnelled through these sessions) | |
| 1 part 1 | Introduction to POWeR+ |
| 1 part 2 | Introduction to goal-setting and weekly weighing |
| 2 | Getting support from other people |
| 3 | Physical activity |
| Stage 2 (free-choice sessions, these are unlocked once user completes session 3) | |
| 4 | Setting the strongest plans: mini session |
| 5 | Controlling your cravings |
| 6 | Stretching your physical activity |
| 7 | Setting up your environment to help you lose weight |
| 8 | Dealing with slip-ups |
| 9 | How to break bad habits: mini session |
| 10 | How to develop your new healthy identity: mini session |
| Stage 3 (free-choice sessions unlocked once user completes stage 2 or after 7 weeks) | |
| 11 | Eating when times are tough |
| 12 | Eating out |
| 13 | Being drink aware |
| 14 | Take on a physical activity challenge: mini session |
| 15 | A quiz to show you which tricky situations you are best at dealing with and which you still need to work on: mini session |
| 16 | Winning against temptations: mini session |
| 17 | When losing weight gets hard: mini session |
| Stage 4 (free-choice sessions unlocked once user completes stage 3 or after 7 weeks) | |
| 18 | Using mindfulness to help you lose weight |
| 19 | Getting support from your family: mini session |
| 20 | Have you made your changes into habits? Take this quiz to find out: mini session |
| 21 | Make new habits easier by linking them to things you already do: mini session |
| 22 | A quick, easy tool to stop stress spoiling your weight loss: mini session |
| 23 | Busy lives |
| 24 | Maintaining your weight loss (we recommend that you complete this session last) |
| 25 | Successful celebrations (available only from the ‘tools’ page) |
List of abbreviations
- BMI
- body mass index
- BP
- blood pressure
- CARe
- Congratulate, Ask, Remind
- CI
- confidence interval
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HCP
- health-care professional
- ICER
- incremental cost-effectiveness ratio
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- POWeR+
- Positive Online Weight Reduction
- POWeR+F
- Positive Online Weight Reduction – face-to-face support
- POWeR+R
- Positive Online Weight Reduction – remote support
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- SD
- standard deviation