Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/19/04. The contractual start date was in September 2010. The draft report began editorial review in November 2014 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Lewis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Depression in older adults
Depression accounts for the greatest burden of disease among all mental health conditions and is expected to become the second most common of all general health problems by 2020. 1 Projected demographic changes mean that population strategies to tackle depression will increasingly have to address the specific needs of older adults. 2 Depression often occurs alongside long-term physical health conditions3 and/or cognitive impairment, and it is more prevalent in people who live alone in social isolation. All these factors tend to disproportionately affect the older adult population. Among older adults, a clinical diagnosis of a major depressive disorder is the strongest predictor for impaired quality of life. 4 Indeed, beyond personal suffering and family disruption, depression worsens the outcomes of many medical disorders and promotes disability. 5 In 2009, the National Institute for Health and Care Excellence (NICE) published guidelines that acknowledged the coexistence of physical health problems and depression. 6,7 Furthermore, it was recognised that the impairments in quality of life associated with depression are comparable to those associated with major physical illness. 4
In 2006, as part of the primary care framework, Quality and Outcomes Framework indicator DEP1 (Depression Indicator 1) was introduced to encourage screening for depression among individuals with a diagnosis of either coronary heart disease and/or diabetes. 8 This was achieved using two standard screening questions. 9 If depression was detected, evidence-supported guidance advocated the prescription of antidepressant drugs and appropriate provision of psychological care. 6,7,10 However, this indicator was retired in 2013 as part of the 2013/14 General Medical Services contract changes. 11 Irrespective of these recent Quality and Outcomes Framework changes, the focus to date has been on identifying and treating those with more severe depressive syndromes as set down in classificatory systems such as the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)12 (major depressive disorder) or the International Statistical Classification of Diseases and Related Health Problems, 10th Revision13 (moderate/severe depressive disorder). 14
Subthreshold depression
Less attention has been paid to those with mild disorders/subthreshold depression or those who give positive responses to screening questions but who do not have sufficient levels of depressive symptoms to meet diagnostic criteria. 10 A large cross-sectional study conducted in 20 countries4 showed that even relatively minor levels of depression are associated with a significant decrement in all quality-of-life domains and with a pattern of negative attitudes towards ageing. Subthreshold depression is also a clear risk factor for progression and the development of more severe depressive syndromes. 15 The focus of the CollAborative care and active surveillance for Screen-Positive EldeRs with subthreshold depression (CASPER) study was on a population of screen-positive subthreshold older adults.
Rationale for the CASPER trial
Guidance issued by NICE in 20097 supported screening and case finding for depression in adults with a chronic physical health problem. The recommendation was intended to enable primary care providers to identify and treat those with severe depressive syndromes. However, the screening programme also identified those with subthreshold depression, yet there was no clear evidence-based guidance regarding treatment for this patient group. Although NICE guidelines6,7 for adults with a chronic physical health problem acknowledged that patients with subthreshold depression need to be provided for, there was no evidence of what works for this group. Yet the case for screening for depression among older adults was clear, given that a substantial proportion of that age group who have a depressive syndrome go unrecognised and untreated. 10 Indeed, the Chief Medical Officer’s 2013 annual report,16 which focused on mental health, highlighted that depression is poorly detected in primary care and that around 10–20% of people aged ≥ 65 years have depression. Given that the proportion of older people with subthreshold depression is likely to be far greater than the proportion with case-level depression, the rationale for the CASPER trial is strong.
Collaborative care: an organisational model of providing care
The vast majority of depression in older adults is managed entirely in primary care without recourse to specialist mental health services. 2,10 Although a range of individual treatments have been shown to be effective in the management of clinical depression in older adults, including antidepressants and psychosocial interventions,10 a repeated observation among those with depression has been the failure to integrate these effective elements of care into routine primary care services. 17 Additionally, the implementation of any form of care will require a strategy that both is of low intensity and can be offered within primary care. 18
In recent years an organisational model of care has been introduced called collaborative care. 19 Collaborative care borrows much from chronic disease management and ensures the delivery of effective forms of treatment (such as pharmacotherapy and/or brief psychological therapy) through augmenting the role of non-medical specialists in primary care. Collaborative care is a model whereby the non-medical specialists, or case managers in this case, form a close collaboration with the participant and others involved in his or her care. The case manager acts as a conduit for the passage of information between all individuals involved and supports the participant to enable effective discussion of important issues. Case managers provide information and help participants to access appropriate services such as social care and voluntary sector services.
The ubiquity of depression in primary care settings along with the poor integration and co-ordination of care has led to the development and increased use of this model of care. In a recent Cochrane Collaboration review20 of 79 randomised controlled trials (RCTs) (24,308 participants), clear and robust evidence of the effectiveness of collaborative care was shown. It improved depression outcomes in both the short term and the medium term. Moreover, there was evidence to suggest that collaborative care can be cost-effective by reducing health-care utilisation and improving overall quality of life. 21 However, the greater proportion of studies related to working-age adults. A relative lack of any evidence for older adults was identified, which resulted in a call for further research on collaborative care among that age group. One important exception was the evidence provided by the US Improving Mood – Promoting Access to Collaborative Treatment study of the effectiveness of collaborative care for older adults. 22
The IMPACT study was conducted by Unützer and colleagues22 among those aged > 60 years with case-level clinical depression. The main finding was that at 12 months almost half of the participants in the intervention group were at least 50% improved in depression severity from baseline compared with only one in five of those receiving usual care. The only UK trial of collaborative care in older adults showed some positive results but focused on more severe depression, with participants meeting a diagnostic threshold. 23 A smaller US trial has used a collaborative-care model in low-severity depression (DSM-IV minor depression and dysthymia) and has shown good clinical improvements at 12 months in the collaborative-care group compared with the usual-care group [50% reduction in depressive symptoms: odds ratio (OR) 5.21, 95% confidence interval (CI) 2.01 to 13.49]. 24 Most recently, the CollAborative DEpression Trial (CADET) has shown that collaborative care improves depression outcomes in a UK primary care population but this is for case-level not subthreshold depression. 25
In addition to the provision of collaborative care, the studies also provide information and support to enable participants to undertake brief psychological therapies, in this case behavioural activation. Behavioural activation for this trial was adapted from the behavioural activation intervention delivered in the CADET. 25 Low-intensity psychological interventions such as behavioural activation may benefit individuals experiencing depressive symptoms. Behavioural activation focuses on addressing the behavioural deficits common among those with depression, reintroducing positive reinforcement and reducing avoidance. Such interventions aim to manipulate the behavioural consequence of a trigger (environmental or cognitive), rather than directly interpret or restructure cognitions. 26 Behavioural activation is about helping patients to ‘act their way out’ of depression rather than waiting until they are ready to ‘think their way out’. Helping people to identify and reintroduce valued activities that they have stopped doing, or ones that they would like to take up, is an important component. The effectiveness of this psychological approach is now well demonstrated. 27 Behavioural activation can be readily delivered by a trained case manager either over the telephone or face to face for those who experience difficulty using or accessing telephone-based therapy. 28
Limitations of previous trials
The major limitations of previous trials are twofold. First, preceding trials have focused on or included participants with above-threshold depression and have not looked exclusively at subthreshold depression. Second, a key component of collaborative care is ‘medication management’ – encouragement of compliance and guideline-concordant prescription of antidepressants – but antidepressants are not indicated in those with subthreshold depression. 6,7,10 It was from this context that the need to find an intervention appropriate for older adults with subthreshold depression was recognised and the National Institute for Health Research Health Technology Assessment (HTA) programme commissioned the CASPER trial. We proposed to measure the clinical effectiveness and cost-effectiveness of using collaborative care in older adults with subthreshold depression in response to a lack of evidence on its benefit to the older population in UK primary care.
Identifying depressive symptoms and validating measures of depression
The two tools that we selected for screening and measuring depression were in regular use in primary care at the time that this study was designed. These included the Whooley questions,9 an ultra-brief two-item depression screen that asks about depressed mood and lack of interest/pleasure in activities, and the Patient Health Questionnaire-9 items (PHQ-9),29 used to measure depression severity once a diagnosis of depression has been made. These two tools were adopted in primary care to fulfil Quality and Outcomes Framework objectives30 in operation when the study was designed, although both have subsequently been retired (Whooley questions)/replaced (PHQ-9). 11 Before commencing the trial, we acknowledged that there was a lack of evidence for either tool in older adults or in identifying subthreshold rather than case-level depression. Indeed, the Whooley questions had been validated only against above-threshold depression in working-age adults31 and/or in non-primary care populations;9 they had not been validated in older adults in UK primary care or against subthreshold depression. Moreover, little was known about the ability of either instrument to identify and measure levels of subthreshold depression.
Further uncertainty related to what should be offered to patients who screened positive but who, following diagnostic assessment, did not meet the threshold for case-level depression. At present, no treatment is offered, yet it seems likely that individuals with subthreshold depression have substantial decrements in their quality of life4 and are at increased risk of developing more severe depressive disorders in the future. 15
In summary, it is currently estimated that in the UK around 10–20% of people aged ≥ 65 years have depression. 16 This underlines the need for the CASPER trial to provide evidence on the clinical effectiveness and cost-effectiveness of delivering collaborative care to older adults.
Chapter 2 Research objectives
Screening for depression, collaborative care and a low-intensity psychological intervention represent a complex intervention. Given that there is currently insufficient evidence and experience with regard to identifying subthreshold depression in older adults, we needed to refine the collaborative-care intervention to tailor it to this particular patient group. We chose to include only those aged ≥ 75 years for the pilot phase to ensure that the sample was representative of ‘older’ older adults. We were concerned that the relatively small pilot sample size would have been overpopulated by ‘younger’ older adults, those aged between 65 and 74 years. This group would have been less likely to inform us of the challenges associated with delivering the intervention to older people, which we sought to identify before the main trial commenced. Although there is no clear definition on the age of older adults, more developed countries use ≥ 65 years as their marker. 32 To reflect this, we changed the inclusion criterion for the main trial to include those aged ≥ 65 years (see Chapter 4), which is more in line with definitions of older adults from the developed world – an age associated with retirement and changing roles. We therefore had two sets of objectives: the first set (objectives 1–4) related to the pilot trial; the second set (objectives 5 and 6) related to the ‘definitive RCT’, which was seamlessly adopted following successful completion of the first set of objectives.
Pilot study
-
To develop a low-intensity collaborative-care intervention based on evidence-supported models of care for those aged ≥ 75 years with screen-positive subthreshold depression who represent ‘older’ older adults.
-
To establish the acceptability and uptake of this service by ‘older’ older adults with screen-positive subthreshold depression in primary care.
-
To test the feasibility of conducting a successful trial of a low-intensity intervention of collaborative care for adults aged ≥ 75 years with screen-positive subthreshold depression.
-
To validate the Whooley questions9 as a screening tool for depression in a UK ‘older’ older adult population.
Main study (the CASPER trial)
-
To establish the clinical effectiveness of a low-intensity intervention of collaborative care for older adults aged ≥ 65 years with screen-positive subthreshold depression.
-
To examine the cost-effectiveness of a low-intensity intervention of collaborative care for older adults aged ≥ 65 years with screen-positive subthreshold depression across a range of health and social care costs.
Chapter 3 Methods
Trial design
We conducted a pragmatic, multicentred, two-arm, parallel, open RCT. Participants with subthreshold depression were individually randomised (1 : 1) to receive either collaborative care or usual general practitioner (GP) care.
Approvals obtained
This study was approved by the NHS Leeds East Research Ethics Committee (REC) on 28 September 2010 (reference number 10/H1306/61). Research management and governance approval was obtained for each trial centre thereafter (see Appendix 1). This trial was assigned the number ISRCTNO2202951.
Trial centres
Four centres in the north of England were selected as trial sites: York centre (core study centre) covering the cities of York, Harrogate and Hull and the surrounding areas; Leeds centre and the surrounding area; Durham centre and the surrounding area; and Newcastle upon Tyne centre, including Northumberland and North Tyneside. Each centre was responsible for co-ordinating the recruitment of participants into the study (trial and epidemiological cohort).
Duration of follow-up
All participants were followed up at 4 and 12 months.
Participant eligibility
Inclusion criteria
Those for whom the following criteria applied were eligible for inclusion in the trial:
-
aged ≥ 75 years during the pilot phase or ≥ 65 years during the main trial (see Chapter 4)
-
identified by a GP practice as being able to take part in collaborative care.
Exclusion criteria
Potential participants were excluded if identified by a primary care clinician as:
-
having a known alcohol dependency (as recorded on GP records)
-
experiencing psychotic symptoms (as recorded on GP records)
-
having any known comorbidity that would, in the GP’s opinion, make entry to the trial inadvisable (e.g. recent evidence of suicidal risk/self-harm, significant cognitive impairment)
-
being affected by other factors that would make an invitation to participate in the trial inappropriate (e.g. recent bereavement, terminal malignancy).
Sample size
We estimated that to detect a minimum effect size of 0.3 (with 80% power and a two-sided 5% significance level) would require 352 patients, 176 in each arm. Although this was an individually randomised trial, there may have been potential clustering at the level of each collaborative-care case manager, hence we needed to inflate the sample size to account for this. Based on an intracluster correlation coefficient (ICC) of 0.02 and a projected caseload size of 20, the design effect would be 1.38 {1 + [(20 – 1) × 0.02]}, which meant that we required 486 patients or 243 in each group. Allowing for a potential loss to follow-up of 26%, the final sample size needed to be at least 658 patients or 329 in each group.
Epidemiological cohort
The CASPER study was originally designed to assemble an epidemiological cohort of people aged ≥ 75 years; after the pilot phase the age threshold was reduced to include adults aged ≥ 65 years (see Chapter 4). Through our broad inclusion criteria we successfully recruited a total of 4668 patients aged ≥ 65 years into the CASPER cohort, from which we identified those eligible to participate in the CASPER trial, a trial of collaborative care in older adults with subthreshold depression. The reasoning behind this strategy was twofold: first, to enable us to recruit an adequate number of potential participants who would subsequently be identified as suffering from subthreshold depression, as we believed that this would not necessarily be recorded in GP records; second, to establish an epidemiological cohort of older adults who could be followed up and who would help inform the knowledge base around the health and well-being of older adults. This type of study design is termed a cohort multiple randomised controlled trial. 33
Recruitment into the trial
Recruitment of all participants into the trial took place through primary care. GP practices agreed to participate after a member of the study team had introduced the trial to them and provided the practice with written information followed by a face-to-face visit to explain the study and what participation would involve. Patients were identified by a computer search and were then invited to participate in the study by their GP practice, who posted an invitation pack to all of their eligible patients. The packs included an invitation letter (see Appendix 2.1) signed from the GP practice, a consent form (see Appendix 2.2), a decline form (see Appendix 2.3), a participant information sheet (see Appendix 2.4), a background information sheet (see Appendix 2.2) and a prepaid return envelope addressed to the core study centre. No patient-identifiable data were available to the study teams until a patient returned his or her consent form. Although it had been stated in the grant application that we would recruit participants directly from residential care as well as from primary care, this proved unnecessary; we were able to access all patients living in residential care through their GP practices. In total, 4% of the CASPER trial lived in residential care.
Consenting participants
During the consent stage potential participants were asked to complete the Whooley questions,9 a two-item depression screening/case-finding tool. These questions were asked at two different time points – on the background information sheet at invitation and in the baseline questionnaire – both times as self-reports. At the consent stage, participants were informed about the opportunity of participating in other related studies (e.g. qualitative studies); they were asked to indicate if they agreed to be approached in the future by ticking a box on the consent form (see Chapter 4). All participants who consented to take part in the CASPER study at this stage became part of the CASPER cohort. Participants did not become part of the CASPER trial until they had been subsequently assessed for suitability by completing a standardised diagnostic interview and randomised.
Baseline assessment
On receipt of written consent from participants, baseline data were collected through a self-report questionnaire. All participants who returned a completed consent form to the core study centre were sent a baseline questionnaire (see Appendix 3). Participants were asked to respond to the Whooley questions9 for a second time and to provide self-report medication data. They were also asked to complete a range of health surveys consisting of the PHQ-9,34 a measure of depression severity using a nine-item depression scale in reference to how a respondent has been feeling over the past 2 weeks; the Short Form questionnaire-12 items (SF-12),35 a measure of health-related quality of life; the European Quality of Life-5 Dimensions (EQ-5D),36 a standardised measure of health state utility, designed primarily for self-completion by respondents; the Generalised Anxiety Disorder seven-item scale (GAD-7),37 a severity measure of generalised anxiety used to gauge anxiety in the past 2 weeks; the Patient Health Questionnaire-15 items (PHQ-15),38 a measure of somatic complaints using a 15-item scale in reference to the last month; and the Connor–Davidson Resilience Scale two-item version (CD-RISC 2),39 used to measure an individual’s resilience and ability to bounce back.
Randomisation
Randomisation was carried out by the York Trials Unit Randomisation Service [see www.yorkrand.com/ (accessed 16 September 2015)]. Participants were automatically randomised into the trial on a 1 : 1 basis to either the intervention group or the control group following the completion of a diagnostic interview. All diagnostic interviews were conducted by a trained researcher from the study team over the telephone. The major depressive episode module of the Mini International Neuropsychiatric Interview (MINI)14 was used to ascertain the presence or absence of core depressive symptoms. This allowed potential recruits to be identified as having major depressive disorder (five or more symptoms), subthreshold depression (two to four symptoms) or no depression (one symptom) (Box 1). 14,40,41 All participants diagnosed with subthreshold depression were randomised to either the intervention arm or the control arm.
Depressed mood.
Loss of interest.
Other symptomsSubstantial changes in weight/appetite.
Change in sleep patterns.
Change in energy levels.
Movement slowing down or speeding up.
Feeling guilty or worthless.
Unable to make decisions.
Thinking about death or suicide.
Once participants had been randomised they were sent a letter informing them of the outcome of their diagnostic interview; if identified as having subthreshold depression they were informed of their group allocation, either collaborative care or usual care. Each participant’s GP was also sent a letter informing him or her that the named patient was eligible to take part in the CASPER trial because of the outcome (subthreshold depression) of the diagnostic interview (MINI) and specifying which arm of the trial he or she had been randomised to.
Ineligible participants
For all those participants whose outcome was not subthreshold depression (either non-depressed or major depressive disorder), a letter was sent informing them that they were ineligible for the CASPER trial but that they would remain in the CASPER epidemiological cohort and continue to be followed up by questionnaire. Their GPs were also informed of this.
Whooley questions validation
One of the main aims during the pilot phase of the study was to validate the two-item screening tool known as the Whooley questions,9 as this instrument had not been validated in the UK among an older adult population. The validation, published in 2015 in the Journal of Affective Disorders,42 was carried out during the pilot phase of the study in which all participants who consented and completed a baseline questionnaire were eligible for a diagnostic interview. Eligibility was not affected by how a participant scored on the Whooley questions – even those who scored negatively at both consent and/or baseline were eligible for a diagnostic interview. The validation showed that the Whooley questions performed with high sensitivity (94.3%, 95% CI 80.8% to 99.3%) and modest specificity (62.7%, 95% CI 59.0% to 66.2%). They proved effective at ruling out depression in older adults: a negative response to both questions was 99.6% likely to be a true negative. However, they were responsible for a high rate of false positives, which creates added burden for GPs, who have to conduct further investigations on patients who screen positive, many of whom turn out not to have depression.
Amendment to those eligible for randomisation
The decision to make recruitment to the fully powered trial more targeted had been taken at the design stage of the CASPER trial [see original protocol National Institute for Health Research Evaluation, Trials and Studies (NETS) website: www.nets.nihr.ac.uk/projects/hta/081904 (accessed 24 September 2015)]. This decision was supported by the validation of the Whooley questions, which provided evidence of a negative response being effective at ruling out depression. The randomisation process changed once the pilot was completed. For the main trial, only those participants who responded positively to at least one of the two Whooley questions, at either consent or baseline, were eligible for a diagnostic interview using the MINI. This amendment was necessary to increase the capacity for recruitment of subthreshold participants into the trial. All participants who responded negatively to both the Whooley questions at both time points were informed that they were not eligible for the CASPER trial but would remain in the CASPER study (epidemiological cohort) and would be followed up at 4 and 12 months using the same questionnaires as those used for the trial participants.
Trial interventions
Control group
Participants in the control group were allocated to receive usual GP care. They received no additional care to the usual primary care management of subthreshold depression offered by their GP, in line with NICE depression guidance as implemented by their GP and local service provision. 6,7
Intervention group
Participants in the intervention group were allocated to receive a low-intensity programme of collaborative care using behavioural activation, designed specifically for those aged ≥ 65 years with subthreshold depression. Collaborative care was delivered by a case manager [a primary care mental health worker/Improving Access to Psychological Therapies (IAPT) worker] for an intended 8–10 weeks. This took place alongside participants’ usual GP care. The defining feature of collaborative care is a collaboration of expertise to help support the participant: the case manager works alongside the participant, sharing any relevant information with the GP and a mental health specialist (psychiatrist or psychologist). The case manager is a cohesive link between the participant and other professionals involved in his or her care. For example, if a case manager deemed a participant’s depressive symptoms to have deteriorated (moving from subthreshold to above-threshold depression), he or she informed the GP to optimise the management of the patient’s condition.
Collaborative care in the CASPER trial included telephone support, symptom monitoring and active surveillance, facilitated by a computerised case management system [Patient Case Management Information System (PC-MIS); see www.york.ac.uk/healthsciences/pc-mis (accessed 16 September 2015)], and low-intensity psychosocial management (behavioural activation). Participants randomised to the collaborative-care intervention group were contacted by a case manager within a week of their randomisation to arrange their first session of collaborative care. This was carried out face to face, usually at their home unless they preferred an alternative venue. After this initial meeting subsequent sessions were carried out on a weekly basis by telephone unless the participant had sensory impairments or preferred to have face-to-face visits. Case managers worked collaboratively with participants, liaising with GPs and other health professionals involved in their care. They contacted them to discuss issues relating to participants’ mental and physical health, both in routine updates and when any concerns were identified. Case managers worked with participants to identify problems and agree goals for the intervention. They also worked with participants to identify and subsequently provide information about other services that may be helpful, such as voluntary and statutory sector organisations and services.
Refinement of collaborative care/behavioural activation
The delivery of collaborative care and behavioural activation had been established in working-age adults and an appropriate training package and manual already existed. 28 However, these had not been tailored for use with older adults diagnosed with subthreshold depression. Before the study began, necessary changes were made to both the training package and the manual (see following sections) to account for differences that may exist in the older adult population. Further refinement was made following the pilot phase, based on feedback from participants to case managers.
Adaptations to language and content
Adaptations were made to the information gathered at the initial assessment. Older adults are more likely to experience long-term health problems and a reduced level of functioning, with their psychological status often closely linked to their physical functioning. 43 Additional questions regarding health conditions and their impact were added to the standard assessment format. However, case managers were reminded to use a person-centred approach and not let preconceptions about the level of functioning of older adults influence their information gathering. Liaison with health professionals involved in treating participants’ long-term health conditions was encouraged to promote a depth of understanding of these issues. Depression in older adults is associated with impaired social support44 and therefore additional questions regarding social contacts and family were added. The risk assessment (see Appendix 4) was also adapted to enquire about past passive and past active suicide ideation as well as current plans and preparations, as past suicidality is a risk factor for current suicidal behaviour. 45
Information in the manual was tailored to meet the needs of older adults. Age-appropriate examples were used, such as bereavement and loss of role, to facilitate engagement and make it easier to relate to. The psychoeducation material given to participants was also modified to include information about depressive symptoms specifically in older adulthood. As depression is associated with cognitive impairment in older people,46 a larger font and increased space for writing was introduced. In addition, when individuals displayed mild cognitive impairment, simpler language was used and the steps in each session were reduced along with homework. Questions were also added to help case managers assess participants’ understanding of the treatment principles.
Functional equivalence and the Keeping Well Plan
Further adaptations were made in response to participant feedback during the pilot phase. Case managers were made aware of the importance of helping patients to identify functionally equivalent activities. Case managers then suggested that adding a section in the participant pack on functional equivalence would help them to engage participants with this subject more effectively. Consequently, a section was added to the Keeping Well Plan, prompting participants to identify functionally equivalent activities that might replace enjoyable or rewarding activities that they were no longer undertaking. It became apparent during the pilot phase that a significant number of participants with subthreshold depression were active and able to carry out the activities that they wished to undertake; they did not wish to add anything else to their usual activities. Behavioural activation is a behavioural treatment which involves the monitoring and scheduling of activities that are being avoided. Therefore, working through behavioural activation was not something that these active participants wanted to do. A step-down option was added to the case managers’ manual that bypassed activity scheduling and allowed more time to focus on the Keeping Well Plan. Further details of the adaptations made can be found in Pasterfield et al. 47
Participant follow-up
All participants in the CASPER trial were followed up with questionnaires at 4 months (see Appendix 5) and 12 months (see Appendix 6). Post-randomisation questionnaires were posted to participants from the York Trials Unit, along with a pre-addressed prepaid envelope. Participants could complete the questionnaires manually and return them by post to the York Trials Unit, or they could complete the questionnaire online; an instruction sheet explaining how to log onto the CASPER website and complete the process was included with each questionnaire. Reminder letters were sent by post to any participants who at 2 weeks had not returned their questionnaire. Telephone follow-up by one of the study team’s researchers was conducted for any participants who had not returned a questionnaire, to enable the primary outcome measure (PHQ-9) to be completed at the very least.
Trial completion and exit
Participants were deemed to have exited the trial when they:
-
withdrew consent (wished to exit the trial with no further contact for follow-up or objective data)
-
had been in the trial for 12 months post randomisation
-
had reached the end of the trial
-
died
-
moved GP practice to one not participating in the CASPER study
-
had another reason to exit according to clinical judgement from a health professional.
Withdrawals
Withdrawal could occur at any point during the study at the request of the participant. If a participant indicated that he or she wished to withdraw from the study, a researcher would speak to the participant to clarify to what extent he or she wished to withdraw (withdrawal from the intervention, from follow-up or from all aspects of the study). When withdrawal was only from the intervention, follow-up data continued to be collected. Data were retained for all participants up to the date of withdrawal, unless they specifically requested that their details be removed.
Objective data
Once all of a GP practice’s participants had completed their follow-up, objective data were collected for each trial participant. Objective data consisted of details on participants’ prescribed medication and the number of contacts that they had with their GP practice during their time in the trial. The only exception was for those participants who had withdrawn in full, thereby withdrawing consent to access their medical records. Objective data were collected from GP practices by request from the core study centre. A spreadsheet template was emailed to the key contact of each GP practice, which included each of the practice’s trial participants’ identification codes along with pre-written frozen headings; no identifiable data were included. Also listed were the search dates for each participant, from the date that they were randomised until either the date that they completed the study 12 months later or the date that they had died if that was the case. Data were still collected on participants who had withdrawn from treatment or follow-up as they had provided us with consent to access their health records for the 12 months that they would have been in the study. All objective data were collected by e-mail and this method was approved by the University of York’s data protection team on the basis that no identifiable data were transferred; patients were identified by GP practices using the administration code assigned to them at the recruitment stage.
Suicide protocol
A small but elevated risk of suicide and self-harm was inherent in the population studied, who had all been identified as having subthreshold depression. All participants (both usual care and collaborative care) were subject to usual GP care. GPs were responsible for the day-to-day management of subthreshold depression. GPs were accountable for all treatment and management decisions, including prescribing, referral and assessment of risk. This arrangement was made clear to all clinicians and GP practices who agreed to participate in the study. The pragmatic nature of the CASPER trial meant that we did not seek to influence this arrangement. However, we did follow good clinical practice by monitoring for suicide risk during all of our encounters with participants. When a patient expressed a risk through thoughts of suicide or self-harm, we followed the study-specific procedure for suicide risk (see Appendices 7.1 and 7.2).
Patient and public involvement in research
The CASPER trial was informed by the involvement of users of mental health services and carers throughout the research period. An advisory group was established at the end of the pilot phase to review the materials used in the study. This consisted of a number of older adults, some of whom had mental health conditions, along with a carer representative. This group provided valuable insights into the relevance and readability of the study documentation. In the future we plan to engage with patient and public involvement in our dissemination strategies to guide on how best to share the findings.
Further studies
Following validation of the Whooley questions during the CASPER pilot phase42 it was acknowledged that a sizeable number of participants (approximately 5%) were identified with case-level depression after completing the diagnostic interview. The CASPER Plus trial was born out of an aspiration to be able to offer collaborative care and behavioural activation to older adults with more severe depression [see www.trialsjournal.com/content/15/1/451 (accessed 16 September 2015)].
Clinical effectiveness
Primary outcome
The primary end point for the trial was patient-reported depression severity as measured by the PHQ-9 at 4 months’ follow-up. Each item is scored between 0 and 3 and thus PHQ-9 scores can range from 0 to 27, with higher scores indicating greater depression. Total scores from 0 (not depressed) to 27 (severely depressed) were calculated based on the nine PHQ-9 items. These data were collected via self-report on the follow-up questionnaire. Any participants who did not return a completed questionnaire were sent a reminder; those participants who did not respond were telephoned by one of the study team’s researchers to ask them to complete the PHQ-9 over the telephone. If one or two items were missing, missing items were replaced with the mean of the remaining items.
The PHQ-9 data were collected at baseline and randomisation as well as at 4 and 12 months’ follow-up. Scores at baseline and randomisation are reported in Baseline characteristics. When analyses were adjusted for initial PHQ-9 score, the score at randomisation was used. The primary end point for the CASPER trial was at 4 months’ follow-up. A standard effect size of 0.3 for mean PHQ-9 difference was sought between treatment arms, equivalent to a PHQ-9 score difference of approximately 1.35 points, assuming a standard deviation (SD) of 4.5. The standard effect size of 0.3 is of moderate size for psychological interventions and in line with collaborative care effects observed in other studies. Cohen48 classifies a standard effect size of 0.3 as a small to medium effect size and this is in line with NICE guidelines for depression,6 in which a similar grading of clinical significance is adopted.
Secondary outcomes
The secondary outcome measures used were:
-
depression severity and symptomatology at 12 months (PHQ-9)
-
binary depression severity at 4 and 12 months (PHQ-9) using scores of ≥ 10 to designate moderate depression caseness
-
quality of life at 4 and 12 months (SF-12 and EQ-5D)
-
psychological anxiety at 4 and 12 months (GAD-7)
-
mental health medication at 4 and 12 months
-
mortality at 4 and 12 months.
Short Form questionnaire-12 items
The SF-12 is a generic health status measure and a short form of the Short Form questionnaire-36 items health survey. It consists of 12 questions measuring eight domains (physical, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) rated over the past month. Questions have three or five response categories and responses are summarised into physical and mental component summary (PCS and MCS) scores. The PCS and MCS scores range from 0 (lowest level of health) to 100 (highest level of health) and were designed to have a mean score of 50 in a representative sample of the US population. Thus, scores > 50 represent above average health status and vice versa.
European Quality of Life-5 Dimensions
The EQ-5D is a standardised measure of current health status developed by the EuroQol Group for clinical and economic appraisal. The EQ-5D consists of five questions, each assessing a different quality-of-life dimension (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each dimension is rated on three levels: ‘no problems’ (score = 1), ‘some problems’ (score = 2) and ‘extreme problems’ (score = 3). A weighted summary index can be derived to give a score between 1 (perfect health) and 0 (death). For the purpose of the clinical effectiveness analysis, only scores for the individual dimensions were utilised. The summary index was analysed as part of the cost–utility analysis.
Generalised Anxiety Disorder seven-item scale
The GAD-7 is a brief measure of symptoms of anxiety based on diagnostic criteria described in the DSM-IV. It consists of seven questions and is calculated by assigning scores of 0, 1, 2 and 3 to the response categories of ‘not at all’, ‘several days’, ‘more than half the days’ and ‘nearly every day’ respectively. The GAD-7 total score for the seven items ranges from 0 to 21. Scores of 5, 10 and 15 represent cut-off points for mild, moderate and severe anxiety respectively.
Mental health medication
Medication data were captured by self-report on the follow-up questionnaires. Participants indicated prescribed medication by selecting from a list of 10 antidepressants as well as listing any other medications that they had been prescribed. Independent objectively collected medication data from GP records were incorporated in the economic analysis.
Mortality data
A data linkage service was established with the Health and Social Care Information Centre to provide regular updates from the Office for National Statistics mortality data on any trial participants who had died while in the study.
Members of the research team recorded any identified deaths on the study management database.
Other patient questionnaire data collected
Patient Health Questionnaire-15 items
The PHQ-15 is a 15-item physical health problems questionnaire. Each health issue is rated as 0 (‘not bothered’), 1 (‘bothered a little’) or 2 (‘bothered a lot’). The total score ranges from 0 to 30, with higher scores indicating worse symptom severity. Scores of 5, 10 and 15 have been used as cut-offs for low, medium and high symptom severity. Item 4 of the PHQ-15 (menstrual problems) was not deemed relevant for the elderly patient population in the CASPER trial and was omitted from all questionnaires. Therefore, the total possible PHQ-15 score was 28.
Connor–Davidson Resilience Scale two-item version
The CD-RISC 2 is a two-item short form of the full Connor–Davidson Resilience Scale (CD-RISC 25). It is a psychological resilience measure with specific items for bounce back from adversity and adaptability to change. The two items are scored from 0–4, resulting in a total score of 0–8, with a higher score indicating greater resilience.
Adverse events
The CASPER study was a non-clinical trial of an investigational medicinal product and was therefore not subject to any additional restrictions. Decisions regarding prescription of medications were made by the participants in conjunction with their GP; participation in the study had no bearing on this process. If participants asked a member of the CASPER study team for an opinion on medication issues, they were strongly encouraged to seek advice from their GP.
The study recorded details of all serious adverse events (SAEs); any judged to have been related to the study were required to be reported to the main REC under the terms of the Standard Operating Procedures for RECs. 49 In the context of the CASPER study’s older adult population the occurrence of many of the SAEs was expected; unscheduled hospitalisations, life-threatening conditions, incapacitating illnesses and deaths were not perceived as unexpected events and therefore they would be reported as SAEs only if they appeared to be related to an aspect of taking part in the study (e.g. participation in treatment, completion of follow-up questionnaires, participation in qualitative substudies or participation in telephone contact).
When a SAE was identified, the Trial Manager was informed by e-mail using a participant’s trial identification number, not any identifiable data. The Trial Manager then informed the Chief Investigator and two members of the Trial Management Group (TMG), who jointly decided if the event should be reported to the REC as a SAE. A SAE form was completed and a copy filed securely at the core study centre. Any unexpected SAEs that were also judged to have been related were to be reported to the REC within 15 days of the Chief Investigator becoming aware of them. In the CASPER study none of the SAEs appeared to have been related to the trial.
The occurrence of adverse events during the trial was monitored by an independent Data Monitoring and Ethics Committee (DMEC) and the Trial Steering Committee (TSC). The DMEC/TSC would have seen immediately all SAEs thought to be treatment related.
Data collection schedule
An overview of the time points at which trial data were collected is presented in Table 1.
| Data collection | Invitation | Baseline | Diagnostic interview/randomisation | 4-month follow-up | 12-month follow-up |
|---|---|---|---|---|---|
| Consent/decline | • | ||||
| Demographic questionnaire | • | ||||
| Whooley questionnaire | • | • | |||
| Physical health problems | • | ||||
| Falls questions | • | ||||
| PHQ-9 | •a | •b | • | • | |
| SF-12 | • | • | • | ||
| EQ-5D | • | • | • | ||
| GAD-7 | • | • | • | ||
| PHQ-15 | • | • | • | ||
| CD-RISC 2 | • | • | • | ||
| Medication questionnaire | • | • | • | ||
| MINI | • | ||||
| Economic evaluation | • | • | • | ||
| Objective medication data | • | • | • |
Statistical assumptions
The CASPER trial was powered to detect a standard effect size of 0.3 for PHQ-9 depression severity between treatment arms, assuming 80% power, 5% two-sided significance, an ICC of 0.02 (based on a caseload of 20 patients) and 26% loss to follow-up (a revised figure following interim data review). The total sample size required based on these criteria was 658 patients (329 in each arm). Participants were randomised 1 : 1 using simple randomisation to either collaborative care or usual care.
Participants, care deliverers and the study team were not blinded to treatment allocation. However, allocations were concealed (group A and group B) for interim study reports, for example for the purpose of independent data monitoring reporting. The trial statistician responsible for the final statistical analysis was kept blind to group allocation until the primary analysis had been completed.
All analyses were conducted on an intention-to-treat basis, using a two-sided statistical significance level of 0.05 unless otherwise stated. All statistical analyses followed a prespecified statistical analysis plan.
Economic analysis
Economic analysis took the form of a cost-effectiveness analysis and, in line with NICE guidance,50,51 adopted the perspective of the health and personal social services. The aim of the analysis was to estimate the value for money of providing collaborative care compared with usual care. The time horizon for the analysis was 12 months from the date of randomisation; therefore, costs and quality-adjusted life-years (QALYs) were not discounted. The analysis was conducted in Stata version 13 (StataCorp LP, College Station, TX, USA).
Quality-adjusted life-years were estimated using the European Quality of Life-5 Dimensions three-level version questionnaire to enable comparisons to be made across different health interventions and provide extra information for decision-makers. QALYs were estimated by measuring the area under the curve,52 which joins baseline and follow-up EQ-5D utility scores derived from population-based values.
A base-case cost of collaborative care was estimated based on the case managers’ training manual, which describes the treatment protocol (manual available on request). Participants’ health-care resource use during the study was assessed to indicate the total cost of health care during the treatment and follow-up periods. Various methods of collecting resource use data were initially considered (e.g. self-report questionnaires and medical records checks). Objective data were obtained from GP practices giving information on (1) participants’ contacts with GPs (appointments, home visits or telephone consultations), (2) participants’ contacts with practice nurses (appointments or telephone consultations) and (3) prescriptions. Given the sample age (≥ 75 years for the pilot phase and ≥ 65 years for the main trial), additional ‘self-report questions’ were not added to limit the overall questionnaire burden. National unit costs were applied to the quantities of resources utilised. 53
For decision analysis, the costs of the intervention, health-care use and changes in QALYs in the RCT were combined to calculate the incremental cost-effectiveness ratio (ICER) using the following formula:
where C is the costs and E is the effects (as QALYs) in the intervention (I) or control (C) group.
To estimate the joint distributions of costs and QALYs, non-parametric bootstrapping was conducted on the observed data. 54 This non-parametric bootstrap resampling technique allows us to assess uncertainty in the ICER. 55 First, the results of the bootstrapped costs and QALYs were presented on the cost-effectiveness plane. Confidence ellipses illustrate the uncertainty in the joint distribution of costs and QALYs on the cost-effectiveness plane; this shows the probability space (50%, 75% and 95% respectively) within which we are confident the true ICER is found.
To further evaluate the joint distributions of costs and benefits, a cost-effectiveness acceptability curve (CEAC) was generated. 56 The CEAC illustrates how the probability that collaborative care will be cost-effective changes as decision-makers’ willingness to pay increases. According to NICE, the willingness to pay for an additional QALY ranges between £20,000 and £30,000; the CEAC indicates the probability that collaborative care is within this range.
Participants’ take-up of collaborative care was recorded during sessions by case managers. This allowed deterministic sensitivity analysis of the potential variation in direct costs of the intervention to be carried out. Over the course of treatment, for each contact with participants, the case managers recorded information on the duration of the contact and how this took place. This information was used to adjust the expected cost of collaborative care when patient, case manager and supervisors agreed to deviate from the manualised intervention. The results were expressed on a CEAC and the adjusted probabilities of falling within the NICE range of willingness to pay were presented.
Chapter 4 Protocol changes
The following changes were made to the original protocol after it was initially approved by the REC on 28 September 2010 [see NETS website: www.nets.nihr.ac.uk/projects/hta/081904 (accessed 24 September 2015)].
Consent process
In the original protocol the consent form contained a section titled Other research studies; participants were asked to tick the opt-out box if they did not wish to receive information about other studies. In response to comments from the REC, the opt-out box was removed and replaced with a ‘yes/no’ box format before permissions were granted.
Inclusion and exclusion criteria
In the original protocol the inclusion criteria specified that participants must be aged ≥ 75 years. This was to ensure that we recruited enough ‘older’ older adults into our pilot phase to inform us of the challenges associated with delivering the intervention to older people. However, once we had completed the pilot phase and further tailored the intervention to meet the needs of older adults, we changed the inclusion criterion for age from ≥ 75 years to ≥ 65 years. This was to bring recruitment into line with the typical age used to define older adults in more developed countries and to increase the rate of recruitment into the main trial.
Participant documentation
In the original protocol it was stated that a MIND leaflet on depression would be sent to participants with their allocation letter post randomisation. However, following some negative comments from participants, who described the MIND leaflet as inappropriate for this age group, alternative leaflets were reviewed by the CASPER trial’s TMG. The MIND leaflet How to improve your mental wellbeing was replaced with a more age-appropriate leaflet produced jointly by Age Concern and Help the Aged, entitled Down, but not out factsheet: What is depression?. We also stopped sending leaflets on depression to non-depressed cohort participants as it was deemed superfluous.
New site recruitment
As outlined in the original CASPER protocol our intention was to increase the number of recruiting sites for Phase III of the study to ensure that the trial met its recruitment target. We therefore introduced Newcastle upon Tyne as a fourth site to ensure that we met our recruitment target. The trusts involved were the NHS North Tyne, Newcastle and North Tyneside Primary Care Trusts, the Northumberland Care Trust and the Northumberland, Tyne and Wear NHS Foundation Trust.
Extension of recruitment
Our original sample size calculations for the CASPER trial were based on an attrition rate of 10%. In reality we experienced a higher than anticipated dropout rate, particularly among participants in the ‘collaborative care’ arm of the trial. In response, we extended our recruitment target from 540 to 658 to maintain 80% power. We actually went on to recruit a total of 705 participants, which would result in 83% estimated power.
Chapter 5 Clinical results
Recruitment and follow-up
Recruitment and flow of participants through the trial
Participants were recruited into the CASPER trial between June 2011 and July 2013 from four UK sites and their surrounding areas in the north of England: York, Leeds, Durham and Newcastle upon Tyne. A total of 32 GP practices consented to screen their practice lists and identify patients who met the initial inclusion criterion: aged ≥ 75 years during the pilot phase and ≥ 65 years during the main trial (see Chapter 4). The exclusion criteria consisted of any known alcohol dependency and/or psychotic symptoms as recorded on GP records, any known adverse comorbidities or any other factors that resulted in GPs deeming it inadvisable to invite patients, such as a recent bereavement.
We were obliged to follow a broad recruitment strategy because we would not have been able to accurately identify patients with subthreshold depression using Read codes from GP records. Therefore, GP practices conducted searches with broad inclusion criteria, which meant that we invited nearly all patients aged ≥ 65 years, except for the few who met the exclusion criteria. This explains why we recruited such a large number of participants into the CASPER cohort and why the number who made it into the CASPER trial with subthreshold depression was far smaller.
A total of 37,134 patients were identified by GP practices between June 2011 and July 2013 and invited by letter to take part in the CASPER study. Of 6693 patients who consented, 4259 were excluded and 2434 patients were assessed for eligibility by diagnostic interview. Based on the diagnostic interview, 705 (29%) patients were identified to have subthreshold depression and were randomised into the CASPER trial. Of those 705 participants randomised, 344 were allocated to collaborative care and 361 to usual care. The remaining patients were classified either as being below the threshold for depression (n = 1558, 64%) or as suffering from a major depressive disorder (n = 171, 7%); they became part of the epidemiological cohort. The randomised number of 705 participants exceeded the planned sample size of 658 participants. The flow of participants is illustrated in a Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 1.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. IQR, interquartile range; max., maximum; min., minimum.

Trial completion and trial exit
Participants were able to withdraw from the study at any point. They were offered the options of withdrawing from the intervention only, from questionnaire follow-up or from all aspects of the study. Data were retained for all participants up to the date of withdrawal, unless they specifically requested that their details be removed. The total number of withdrawals by trial arm is given in Table 2.
| Time | Type of withdrawal | Collaborative care (n = 344) | Usual care (n = 361) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| By 4 months’ follow-up | Withdrawal from treatment | 82 | 23.8 | – | – |
| Withdrawal from follow-up | 16 | 4.7 | 0 | 0.0 | |
| Full withdrawal | 27 | 7.8 | 2 | 0.6 | |
| Died | 1 | 0.3 | 4 | 1.1 | |
| By 12 months’ follow-up | Withdrawal from treatment | 95 | 27.6 | – | – |
| Withdrawal from follow-up | 25 | 7.3 | 4 | 1.1 | |
| Full withdrawal | 37 | 10.8 | 5 | 1.4 | |
| Died | 5 | 1.5 | 18 | 5.0 | |
A total of 28% of participants in the collaborative-care arm withdrew from treatment and the number of full and partial withdrawals was seven times greater in this group (n = 62) than in the usual-care group (n = 9). A larger number of patients in the usual-care arm died during the course of the trial (18 vs. 5).
When reasons for withdrawal were provided by the participants, these were documented in the study management database. Following completion of the trial, reasons were grouped into common categories and these are listed in Tables 3–5 for the different types of follow-up.
| Reason for withdrawal | Collaborative care (n = 95 withdrawn) | Usual care (n = 0 withdrawn) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Too busy | 14 | 14.7 | – | – |
| Carer – no time | 6 | 6.3 | – | – |
| Does not wish to engage | 21 | 22.1 | – | – |
| Not low in mood | 9 | 9.5 | – | – |
| Invasive | 6 | 6.3 | – | – |
| Ill health | 11 | 11.6 | – | – |
| Cognitive impairment | 4 | 4.2 | – | – |
| Physical disabilities | 1 | 1.1 | – | – |
| Recent bereavement | 2 | 2.1 | – | – |
| Receiving support from others | 3 | 3.2 | – | – |
| Literacy problems | 1 | 1.1 | – | – |
| Unable to contact | 2 | 2.1 | – | – |
| Unknown | 15 | 15.8 | – | – |
| Total | 95 | 100.0 | – | – |
| Reason for withdrawal | Collaborative care (n = 25 withdrawn) | Usual care (n = 4 withdrawn) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Too busy | 4 | 16.0 | 0 | 0.0 |
| Does not wish to engage | 4 | 16.0 | 0 | 0.0 |
| Not low in mood | 3 | 12.0 | 0 | 0.0 |
| Invasive | 2 | 8.0 | 0 | 0.0 |
| Ill health | 3 | 12.0 | 0 | 0.0 |
| Receiving support from others | 1 | 4.0 | 0 | 0.0 |
| Causing marital disharmony | 1 | 4.0 | 0 | 0.0 |
| Unknown | 7 | 28.0 | 4 | 100.0 |
| Total | 25 | 100.0 | 4 | 100.0 |
| Reason for withdrawal | Collaborative care (n = 37 withdrawn) | Usual care (n = 5 withdrawn) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Too busy | 2 | 5.4 | 0 | 0.0 |
| Carer – no time | 0 | 0.0 | 0 | 0.0 |
| Does not wish to engage | 8 | 21.6 | 1 | 20.0 |
| Not low in mood | 5 | 13.5 | 1 | 20.0 |
| Invasive | 6 | 16.2 | 1 | 20.0 |
| Ill health | 4 | 10.8 | 0 | 0.0 |
| Cognitive impairment | 1 | 2.7 | 0 | 0.0 |
| Physical disabilities | 1 | 2.7 | 0 | 0.0 |
| Recent bereavement | 0 | 0.0 | 1 | 20.0 |
| Receiving support from others | 1 | 2.7 | 0 | 0.0 |
| Literacy problems | 0 | 0.0 | 0 | 0.0 |
| Unable to contact | 1 | 2.7 | 0 | 0.0 |
| Unknown | 8 | 21.6 | 1 | 20.0 |
| Total | 37 | 100.0 | 5 | 100.0 |
The final trial sample size calculation allowed for losses to follow-up of 26% at the primary end point at 4 months. The primary outcome (PHQ-9 depression severity) was available for 586 patients at that point, equating to an actual loss to follow-up of 16.9% (23.8% in the collaborative-care arm and 10.2% in the usual-care arm).
Timing of follow-up
Different patients in the CASPER trial were followed up according to two different time schedules. Follow-up times (4 and 12 months) were initially calculated from baseline (n = 165 randomised participants, 23%), with randomisation expected shortly after. As the volume of participant throughput increased, diagnostic interview and randomisation occurred only sometime after baseline data collection; therefore, follow-up times for participants later on in the trial were calculated from the time of randomisation as the more appropriate reference point (n = 540 randomised participants, 77%). A sensitivity analysis was carried out to assess the impact of having adopted two different reference points (see Table 22).
Table 6 illustrates the average delay between baseline and randomisation, with a median delay of 2 weeks and considerable variability. Table 6 also shows that, for those patients whose follow-up reference time was at baseline, their 4-month follow-up could occur much sooner after (and for four cases before) randomisation.
| Time interval | Statistics | Follow-up reference at baseline (n = 165) | Follow-up reference at randomisation (n = 540) | Total (n = 705) |
|---|---|---|---|---|
| Days from return of baseline questionnaire to randomisation | n | 165 | 540 | 705 |
| Mean (SD) | 23 (28.9) | 28 (34.1) | 27 (33.0) | |
| Median | 12 | 14 | 14 | |
| Min., max. | 0, 180 | 0, 214 | 0, 214 | |
| Days from randomisation to 4-month questionnaire being sent | n | 131 | 448 | 579 |
| Mean (SD) | 87 (34.4) | 134 (21.7) | 124 (32.0) | |
| Median | 96 | 125 | 124 | |
| Min., max. | –74, 162 | 120, 231 | –74, 231 |
Response types and response times
Participants had the option to complete questionnaires at baseline and follow-up on paper or online. Only a small proportion of questionnaires were completed online, which reduced over time [2.7% at baseline (3.8% collaborative-care group, 1.7% usual-care group), 1.9% at 4 months’ follow-up (2.6% collaborative-care group, 1.2% usual-care group) and 1.0% at 12 months’ follow-up (1.7% collaborative-care group, 0.4% usual-care group). Patients in the collaborative-care arm were slightly more likely to use the online option.
The average response times to questionnaires are detailed in Table 7 by trial arm for data for which completion dates were provided by participants or dates of return were logged on the trial database. Participants generally completed questionnaires within a week and returned them within 2 weeks. There were no differences in response time between treatment arms.
| Time interval (months) | Interval from date questionnaire sent | Statistics | Collaborative care (n = 344) | Usual care (n = 361) |
|---|---|---|---|---|
| 4 | Days to complete questionnaire | n | 242 | 298 |
| Mean (SD) | 8.7 (12.41) | 8.5 (13.47) | ||
| Median | 4 | 5 | ||
| Min., max. | 0, 93 | 0, 93 | ||
| Days to return questionnaire | n | 256 | 319 | |
| Mean (SD) | 15.8 (13.70) | 15.2 (13.65) | ||
| Median | 11.5 | 11 | ||
| Min., max. | 0, 93 | 0, 101 | ||
| 12 | Days to complete questionnaire | n | 221 | 262 |
| Mean (SD) | 6.7 (9.73) | 6.5 (7.61) | ||
| Median | 4 | 4 | ||
| Min., max. | 0, 81 | 0, 70 | ||
| Days to return questionnaire | n | 232 | 279 | |
| Mean (SD) | 13.8 (12.27) | 13.1 (9.86) | ||
| Median | 11 | 10 | ||
| Min., max. | 0, 103 | 0, 88 |
The intervention: collaborative care
Collaborative care was offered to all patients in the intervention arm. The intervention was delivered by 18 case mangers (case load of 19.1 randomised patients and 16.2 patients who received any treatment per case manager). Further details on the case load of each individual case manager are given as part of the practitioner analysis in Adjusting for clustering by case manager.
An overview of received treatments is provided in the CONSORT diagram in Figure 1 and further details are presented in Tables 8 and 9. Of 344 randomised patients, 85% had at least one collaborative-care session and 46% completed the treatment. Participants received on average six sessions over 7–8 weeks, of which two were delivered face to face and four were delivered by telephone. The average session duration was around half an hour. When reasons for not wanting to receive any collaborative care were recorded by case managers in the notes, being too busy and not being low in mood were the most frequent responses (Table 10).
| Collaborative care received | Patients randomised to collaborative care (n = 344) | |
|---|---|---|
| n | % | |
| Did not start treatment | 53 | 15.4 |
| Started treatment | 291 | 84.6 |
| Started but did not complete treatment | 134 | 39.0 |
| Completed treatment | 157 | 45.6 |
| Characteristic | Patients who received some collaborative care (n = 291) | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | Median | Min. | Max. | |
| Days from randomisation to first session | 291 | 23.8 | 13.73 | 21 | 0 | 96 |
| Number of sessions received | 291 | 6.0 | 3.06 | 7 | 1 | 15 |
| Face to face | 291 | 1.7 | 1.65 | 1 | 0 | 10 |
| By telephone | 291 | 4.3 | 3.10 | 5 | 0 | 14 |
| Average length of session (minutes) | 291 | 34.5 | 14.12 | 30 | 10 | 95 |
| Days from first to last session | 291 | 52.9 | 37.7 | 55 | 0 | 200 |
| Reason | Patients who received no collaborative care (n = 53) | |
|---|---|---|
| n | % | |
| Too busy | 8 | 15.1 |
| Carer – no time | 4 | 7.5 |
| Does not wish to engage | 5 | 9.4 |
| Not low in mood | 8 | 15.1 |
| Invasive | 5 | 9.4 |
| Ill health | 4 | 7.5 |
| Cognitive impairment | 1 | 1.9 |
| Physical disabilities | 1 | 1.9 |
| Receiving support from others | 2 | 3.8 |
| Causing marital disharmony | 1 | 1.9 |
| Unable to contact | 4 | 7.5 |
| Unknown | 10 | 18.9 |
Baseline characteristics
Characteristics at consent, baseline and diagnostic interview (point of randomisation) for randomised participants and participants included in the primary analysis [‘as analysed’ population: patients with a valid PHQ-9 score at 4 or 12 months’ follow-up and valid covariate data (PHQ-9 score at randomisation and baseline SF-12 PCS score)] are presented in Tables 11–13 respectively.
| Characteristic | As randomised, n (%) | As analysed, n (%)a | ||
|---|---|---|---|---|
| Collaborative care (n = 344) | Usual care (n = 361) | Collaborative care (n = 274) | Usual care (n = 327) | |
| Age at consent (years) | ||||
| n (%) | 344 (100.0) | 361 (100.0) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 77.1 (7.08) | 77.5 (7.18) | 76.6 (7.21) | 77.4 (7.13) |
| Median (min., max.) | 77 (65, 96) | 78 (64, 93) | 77 (65, 93) | 78 (64, 93) |
| Sex | ||||
| Male | 159 (46.2) | 139 (38.5) | 122 (44.5) | 123 (37.6) |
| Female | 185 (53.8) | 222 (61.5) | 152 (55.5) | 204 (62.4) |
| Educated beyond 16 years | 180 (52.3) | 186 (51.5) | 146 (53.3) | 168 (51.4) |
| Degree or equivalent professional qualification | 115 (33.4) | 106 (29.4) | 95 (34.7) | 96 (29.4) |
| Smoker | 16 (4.7) | 29 (8.0) | 12 (4.4) | 25 (7.6) |
| Three or more alcohol units per day | 32 (9.3) | 21 (5.8) | 26 (9.5) | 16 (4.9) |
| Ethnicity | ||||
| White | 340 (98.8) | 358 (99.2) | 271 (98.9) | 324 (99.1) |
| Asian or Asian British | 2 (0.6) | 0 (0.0) | 2 (0.7) | 0 (0.0) |
| Black or black British | 0 (0.0) | 2 (0.6) | 0 (0.0) | 2 (0.6) |
| Other | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Fallen in the last 12 months | ||||
| Yes | 110 (32.0) | 142 (39.3) | 89 (32.5) | 131 (40.1) |
| No | 224 (65.1) | 212 (58.7) | 176 (64.2) | 190 (58.1) |
| Cannot recall | 8 (2.3) | 5 (1.4) | 8 (2.9) | 4 (1.2) |
| If fallen, how many times | ||||
| n (%) | 105 (30.5) | 139 (38.5) | 85 (31.0) | 128 (39.1) |
| Mean (SD) | 2.9 (4.91) | 2.2 (1.71) | 3.1 (5.40) | 2.2 (1.76) |
| Median (min., max.) | 2 (1, 40) | 2 (1, 14) | 2 (1, 40) | 2 (1, 14) |
| Health problems | ||||
| Diabetes | 55 (16.0) | 66 (18.3) | 43 (15.7) | 64 (19.6) |
| Osteoporosis | 33 (9.6) | 42 (11.6) | 27 (9.9) | 40 (12.2) |
| High blood pressure | 157 (45.6) | 174 (48.2) | 131 (47.8) | 160 (48.9) |
| Rheumatoid arthritis | 38 (11.0) | 57 (15.8) | 31 (11.3) | 53 (16.2) |
| Osteoarthritis | 98 (28.5) | 114 (31.6) | 81 (29.6) | 106 (32.4) |
| Stroke | 28 (8.1) | 31 (8.6) | 22 (8.0) | 27 (8.3) |
| Cancer | 49 (14.2) | 37 (10.2) | 38 (13.9) | 34 (10.4) |
| Respiratory conditions | 65 (18.9) | 81 (22.4) | 51 (18.6) | 73 (22.3) |
| Eye condition | 130 (37.8) | 136 (37.7) | 98 (35.8) | 117 (35.8) |
| Heart disease | 88 (25.6) | 86 (23.8) | 66 (24.1) | 75 (22.9) |
| Other | 74 (21.5) | 74 (20.5) | 64 (23.4) | 65 (19.9) |
| Whooley question: Over the past month have you been bothered by feeling down, depressed or hopeless? | ||||
| Yes | 233 (67.7) | 238 (65.9) | 192 (70.1) | 218 (66.7) |
| No | 111 (32.3) | 123 (34.1) | 82 (29.9) | 109 (33.3) |
| Whooley question: Over the past month have you been bothered by having little or no interest or pleasure in doing things? | ||||
| Yes | 198 (57.6) | 199 (55.1) | 161 (58.8) | 176 (53.8) |
| No | 146 (42.4) | 162 (44.9) | 113 (41.2) | 151 (46.2) |
| Characteristic | As randomised, n (%) | As analysed, n (%) | ||
|---|---|---|---|---|
| Collaborative care (n = 344) | Usual care (n = 361) | Collaborative care (n = 274) | Usual care (n = 327) | |
| PHQ-9 score | ||||
| n (%) | 340 (98.8) | 358 (99.2) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 7.8 (4.71) | 7.8 (4.64) | 7.6 (4.32) | 7.6 (4.55) |
| Median (min., max.) | 7 (0, 27) | 7 (0, 25) | 7 (0, 27) | 7 (0, 25) |
| PHQ-9 grouping | ||||
| No depression | 96 (27.9) | 90 (24.9) | 74 (27.0) | 89 (27.2) |
| Mild depression | 137 (39.8) | 155 (42.9) | 118 (43.1) | 138 (42.2) |
| Moderate depression | 76 (22.1) | 85 (23.5) | 61 (22.3) | 77 (23.5) |
| Moderately severe depression | 23 (6.7) | 21 (5.8) | 18 (6.6) | 18 (5.5) |
| Severe depression | 8 (2.3) | 7 (1.9) | 3 (1.1) | 5 (1.5) |
| PHQ-15 score | ||||
| n (%) | 339 (98.5) | 356 (98.6) | 274 (100.0) | 326 (99.7) |
| Mean (SD) | 9.1 (4.12) | 9.5 (3.94) | 9.1 (4.17) | 9.4 (3.93) |
| Median (min., max.) | 9 (0, 25) | 9 (0, 20) | 9 (0, 25) | 9 (0, 20) |
| SF-12 PCS score | ||||
| n (%) | 337 (98.0) | 356 (98.6) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 38.0 (13.37) | 36.5 (13.02) | 38.5 (13.15) | 36.6 (13.11) |
| Median (min., max.) | 37.5 (4.6, 69.9) | 35.1 (5.7, 66.6) | 38.1 (4.6, 69.9) | 35.0 (5.7, 66.6) |
| SF-12 MCS score | ||||
| n (%) | 337 (98.0) | 356 (98.6) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 44.3 (10.96) | 45.1 (10.02) | 44.5 (10.97) | 45.2 (10.04) |
| Median (min., max.) | 44.9 (12.5, 66.0) | 46.3 (9.6, 67.0) | 45.1 (12.5, 66.0) | 46.5 (9.6, 67.0) |
| GAD-7 score | ||||
| n (%) | 340 (98.8) | 358 (99.2) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 5.7 (4.82) | 5.7 (4.45) | 5.5 (4.58) | 5.6 (4.38) |
| Median (min., max.) | 5 (0, 21) | 5 (0, 21) | 4.5 (0, 21) | 5 (0, 21) |
| EQ-5D | ||||
| Mobility | ||||
| No problems | 129 (37.5) | 115 (31.9) | 110 (40.1) | 106 (32.4) |
| Some problems | 210 (61.0) | 241 (66.8) | 164 (59.9) | 220 (67.3) |
| Confined to bed | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.3) |
| Self-care | ||||
| No problems | 287 (83.4) | 292 (80.9) | 234 (85.4) | 270 (82.6) |
| Some problems | 48 (14.0) | 62 (17.2) | 38 (13.9) | 54 (16.5) |
| Unable to wash/dress | 3 (0.9) | 2 (0.6) | 2 (0.7) | 2 (0.6) |
| Usual activities | ||||
| No problems | 136 (39.5) | 124 (34.3) | 115 (42.0) | 115 (35.2) |
| Some problems | 189 (54.9) | 209 (57.9) | 148 (54.0) | 192 (58.7) |
| Unable to perform | 13 (3.8) | 23 (6.4) | 11 (4.0) | 20 (6.1) |
| Pain/discomfort | ||||
| No pain | 76 (22.1) | 54 (15.0) | 64 (23.4) | 45 (13.8) |
| Moderate pain | 224 (65.1) | 262 (72.6) | 185 (67.5) | 245 (74.9) |
| Extreme pain | 39 (11.3) | 40 (11.1) | 25 (9.1) | 37 (11.3) |
| Anxiety/depression | ||||
| Not anxious/depressed | 132 (38.4) | 133 (36.8) | 103 (37.6) | 121 (37.0) |
| Moderately anxious/depressed | 193 (56.1) | 211 (58.4) | 161 (58.8) | 196 (59.9) |
| Extremely anxious/depressed | 12 (3.5) | 11 (3.0) | 9 (3.3) | 9 (2.8) |
| Prescribed antidepressants | 35 (10.2) | 51 (14.1) | 29 (10.6) | 46 (14.1) |
| Whooley question: Over the past month have you been bothered by feeling down, depressed or hopeless? | ||||
| Yes | 254 (73.8) | 258 (71.5) | 210 (76.6) | 235 (71.9) |
| No | 88 (25.6) | 103 (28.5) | 64 (23.4) | 92 (28.1) |
| Whooley question: Over the past month have you been bothered by having little or no interest or pleasure in doing things? | ||||
| Yes | 192 (55.8) | 209 (57.9) | 160 (58.4) | 188 (57.5) |
| No | 150 (43.6) | 152 (42.1) | 114 (41.6) | 139 (42.5) |
| Characteristic | As randomised, n (%) | As analysed, n (%) | ||
|---|---|---|---|---|
| Collaborative care (n = 344) | Usual care (n = 361) | Collaborative care (n = 274) | Usual care (n = 327) | |
| PHQ-9 score | ||||
| n (%) | 344 (100.0) | 361 (100.0) | 274 (100.0) | 327 (100.0) |
| Mean (SD) | 7.5 (4.29) | 7.6 (4.21) | 7.6 (4.24) | 7.6 (4.23) |
| Median (min., max.) | 7 (0, 23) | 7 (0, 20) | 7 (0, 23) | 7 (0, 20) |
| PHQ-9 grouping | ||||
| No depression | 90 (26.2) | 92 (25.5) | 69 (25.2) | 84 (25.7) |
| Mild depression | 152 (44.2) | 159 (44.0) | 124 (45.3) | 143 (43.7) |
| Moderate depression | 80 (23.3) | 87 (24.1) | 64 (23.4) | 79 (24.2) |
| Moderately severe depression | 18 (5.2) | 22 (6.1) | 14 (5.1) | 20 (6.1) |
| Severe depression | 4 (1.2) | 1 (0.3) | 3 (1.1) | 1 (0.3) |
| From MINI: Were you ever depressed or down, most of the day, nearly every day for 2 weeks? | ||||
| Yes | 148 (43.0) | 159 (44.0) | 115 (42.0) | 146 (44.6) |
| No | 196 (57.0) | 202 (56.0) | 159 (58.0) | 181 (55.4) |
| From MINI: For the past 2 weeks, were you depressed or down, most of the day, nearly every day? | ||||
| Yes | 40 (11.6) | 39 (10.8) | 32 (11.7) | 38 (11.6) |
| No | 304 (88.4) | 322 (89.2) | 242 (88.3) | 289 (88.4) |
| From MINI: Were you ever much less interested in most things or much less able to enjoy things you used to enjoy most of the time for 2 weeks? | ||||
| Yes | 164 (47.7) | 173 (47.9) | 131 (47.8) | 155 (47.4) |
| No | 180 (52.3) | 188 (52.1) | 143 (52.2) | 172 (52.6) |
| From MINI: In the past 2 weeks, were you much less interested in most things or much less able to enjoy the things you used to enjoy, most of the time? | ||||
| Yes | 68 (19.8) | 86 (23.8) | 55 (20.1) | 78 (23.9) |
| No | 276 (80.2) | 275 (76.2) | 219 (79.9) | 249 (76.1) |
Primary outcome results
A summary of the primary outcome results is provided in Box 2.
-
Valid PHQ-9 outcome data were available for 83% of patients at 4 months and 74% of patients at 12 months. Attribution was substantially greater in the collaborative care arm (see Table 14).
-
The primary analysis model revealed significantly greater average improvements in PHQ-9 score in favour of collaborative care: 1.31 score points (p < 0.001) at 4 months’ follow-up and 1.33 score points (p = 0.001) at 12 months’ follow-up (see Table 17 and Figure 3).
-
The results remained robust following secondary analyses including adjustment for case manager clustering, adjustment for covariates predictive of the outcome at 4 months or adjustment for covariates predictive of missing data at 4 months (see Table 25).
-
There was a greater reduction in moderately to severely depressed cases in the collaborative-care arm than in the usual-care arm, which was statistically significant at 12 months’ follow-up (16% vs. 28%) but not at 4 months’ follow-up (17% vs. 24%) (see Table 26 and Figure 4).
Descriptives
Complete PHQ-9 responses were available for all participants at randomisation. At follow-up, 495 patients (70%) had valid PHQ-9 scores at both follow-up times, 115 patients (16%) had a valid PHQ-9 score at 4 months or 12 months only, and for 95 patients (13%) no PHQ-9 scores were available at follow-up.
The extent of, and reasons for, missing PHQ-9 scores at each time point are detailed in Table 14 by trial arm. Although there were fewer valid PHQ-9 responses in the collaborative-care arm at both follow-up points, this was mainly because of a higher dropout rate in this group. Of all attempted PHQ-9 completions, only one questionnaire had an insufficient number of questions completed to allow scoring (one patient in the usual-care arm completed only the first five questions at 4 months’ follow-up). The total number of times that a particular PHQ-9 item was missing for any attempted completions is presented in Table 15. Earlier questionnaire items (items 1, 2 and 4) tended to be missed more frequently, but no meaningful pattern emerged.
| Time point | Response type | Collaborative care (n = 344) | Usual care (n = 361) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Randomisation | Valid PHQ-9 response | 344 | 100.0 | 361 | 100.0 |
| No missing items | 344 | 100.0 | 361 | 100.0 | |
| One missing item | 0 | 0.0 | 0 | 0.0 | |
| Two missing items | 0 | 0.0 | 0 | 0.0 | |
| 4 months | Valid PHQ-9 response | 262 | 76.2 | 324 | 89.8 |
| No missing items | 260 | 75.6 | 319 | 88.4 | |
| One missing item | 2 | 0.6 | 5 | 1.4 | |
| Two missing items | 0 | 0.0 | 0 | 0.0 | |
| No valid PHQ-9 response | 82 | 23.8 | 37 | 10.2 | |
| Three to eight missing items | 0 | 0.0 | 1 | 0.3 | |
| All items missing | 3 | 0.9 | 2 | 0.6 | |
| Questionnaire not returned | 35 | 10.2 | 28 | 7.8 | |
| Withdrawn | 43 | 12.5 | 2 | 0.6 | |
| Died | 1 | 0.3 | 4 | 1.1 | |
| 12 months | Valid PHQ-9 response | 235 | 68.3 | 284 | 78.7 |
| No missing items | 234 | 68.0 | 275 | 76.2 | |
| One missing item | 1 | 0.3 | 8 | 2.2 | |
| Two missing items | 0 | 0.0 | 1 | 0.3 | |
| No valid PHQ-9 response | 109 | 31.7 | 77 | 21.3 | |
| Three to eight missing items | 0 | 0.0 | 0 | 0.0 | |
| All items missing | 1 | 0.3 | 0 | 0.0 | |
| Questionnaire not returned | 41 | 11.9 | 50 | 13.9 | |
| Withdrawn | 62 | 18.0 | 9 | 2.5 | |
| Died | 5 | 1.5 | 18 | 5.0 | |
| PHQ-9 item | Wording | Number of times missing |
|---|---|---|
| 1 | Little interest or pleasure in doing things | 5 |
| 2 | Feeling down, depressed or hopeless | 4 |
| 3 | Trouble falling or staying asleep or sleeping too much | 2 |
| 4 | Feeling tired or having little energy | 4 |
| 5 | Poor appetite or overeating | 1 |
| 6 | Feeling bad about yourself or that you are a failure or have let yourself or your family down | 2 |
| 7 | Trouble concentrating on things, such as reading the newspaper or watching television | 1 |
| 8 | Moving or speaking so slowly that other people could have noticed. Or the opposite – being so fidgety or restless that you have been moving around a lot more than usual | 2 |
| 9 | Thoughts that you would be better off dead or of hurting yourself in some way | 1 |
Score distribution
Figure 2 illustrates the distribution of PHQ-9 scores for each treatment group over time. At randomisation, scores were distributed normally with a slight right skew, which became more pronounced at 4 and 12 months’ follow-up as patients in both arms improved. A floor effect can be observed at the follow-up time points as a larger number of participants reported no or very mild depression on the PHQ-9 scale.
FIGURE 2.
Distribution of PHQ-9 scores by treatment group. (a) Randomisation; (b) 4-month follow-up; and (c) 12-month follow-up.
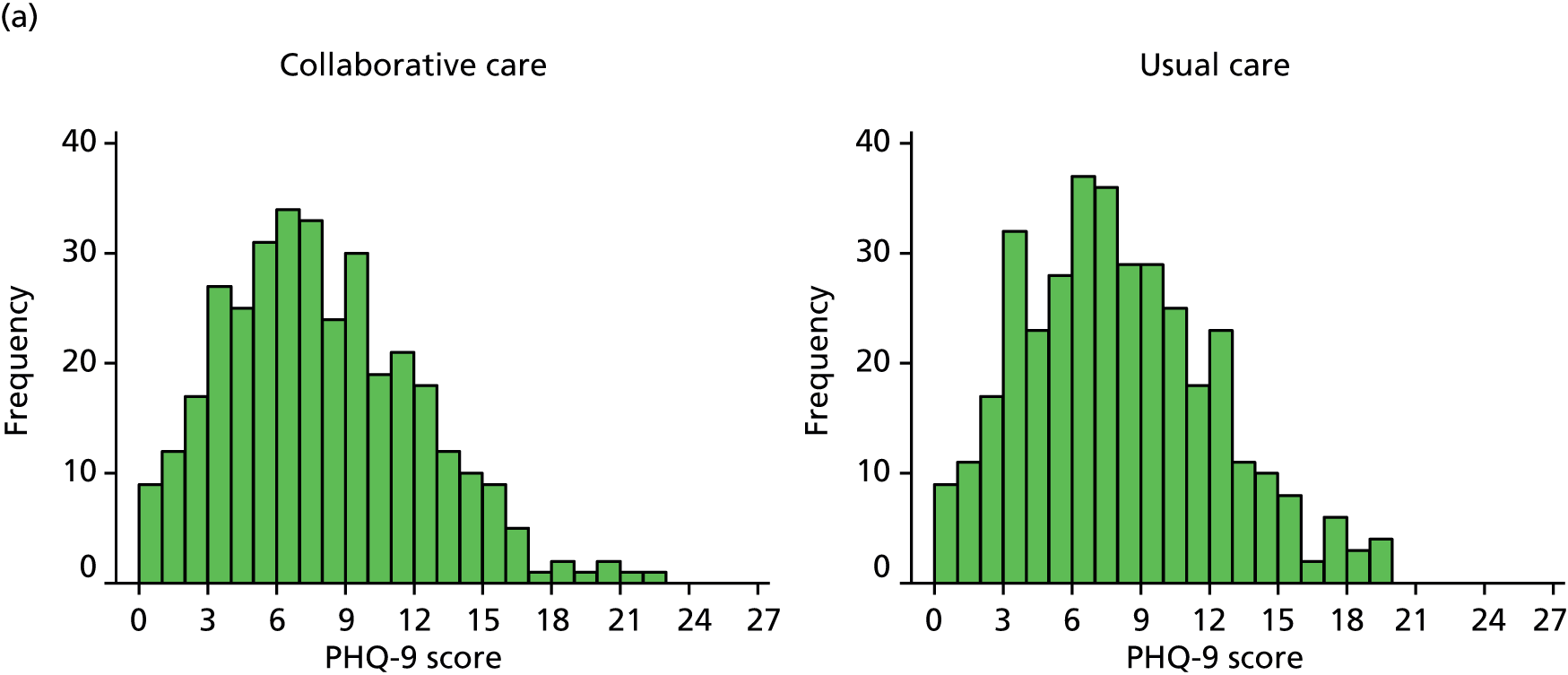
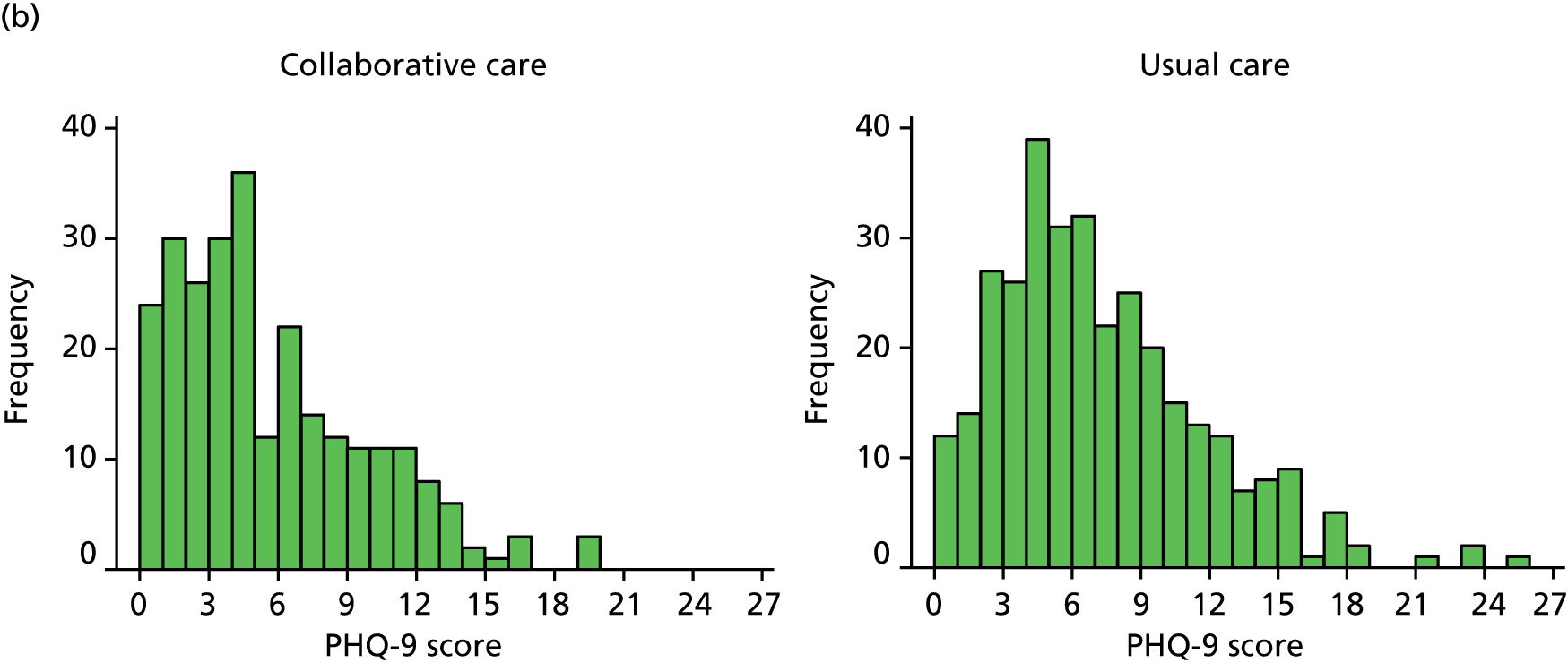
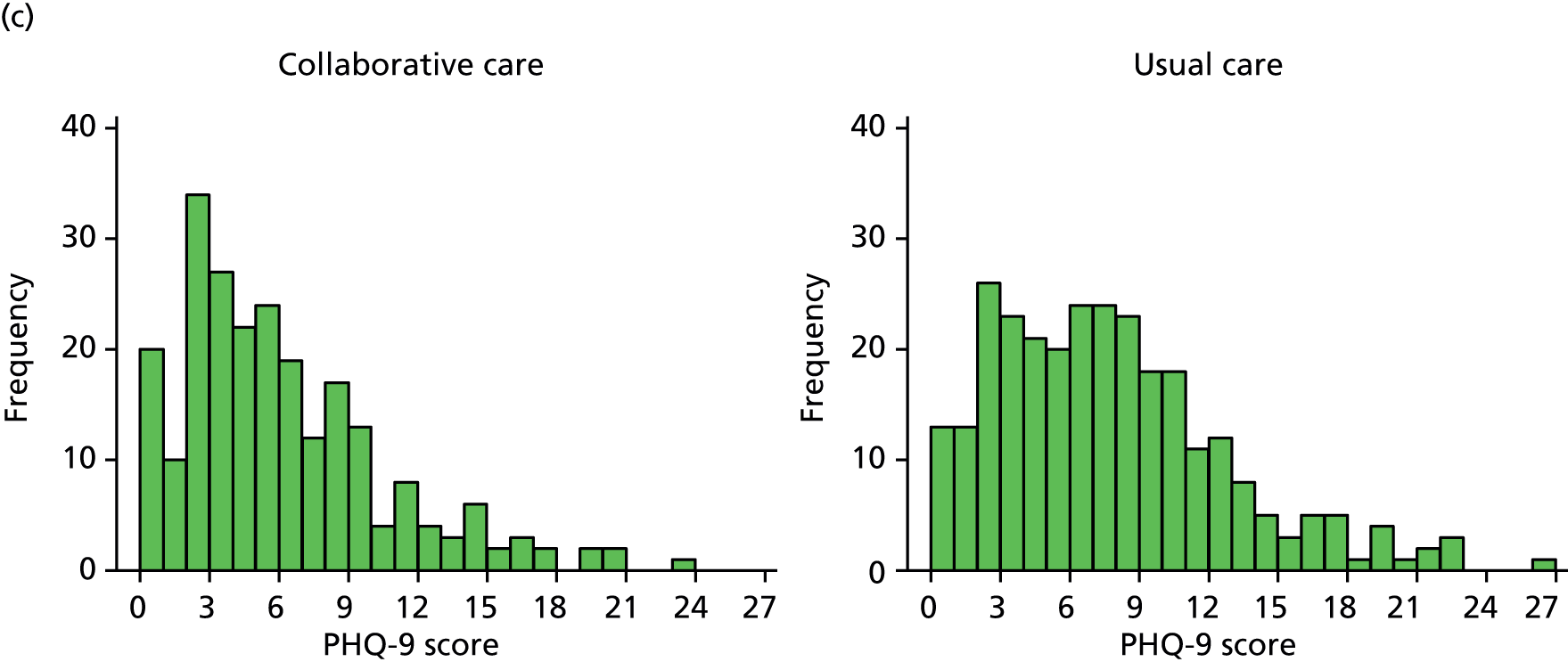
Unadjusted summary statistics
Summary statistics of the raw PHQ-9 scores are given in Table 16 and are illustrated in Figure 3. Average depression severity as measured by the PHQ-9 was around 7.5 score points at randomisation, which is classed as mild depression. Scores improved for both arms between randomisation and 4 months’ follow-up, more so for the collaborative-care arm (to a score of 5.2) than for the usual-care group (to a score of 6.8). At 12 months, average depression scores increased again by approximately half a PHQ-9 score point, but the difference between trial arms was maintained.
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Randomisation, n (%) | 344 (100) | 361 (100) | 705 (100) |
| Mean (SD) | 7.5 (4.29) | 7.6 (4.21) | 7.5 (4.24) |
| Median (min., max.) | 7 (0, 23) | 7 (0, 20) | 7 (0, 23) |
| 4 months, n (%) | 262 (76.2) | 324 (89.8) | 586 (83.1) |
| Mean (SD) | 5.2 (4.17) | 6.8 (4.50) | 6.0 (4.42) |
| Median (min., max.) | 4 (0, 20) | 6 (0, 26) | 5 (0, 26) |
| 12 months, n (%) | 235 (68.3) | 284 (78.7) | 519 (73.6) |
| Mean (SD) | 5.7 (4.50) | 7.2 (5.01) | 6.5 (4.84) |
| Median (min., max.) | 5 (0, 24) | 7 (0, 27) | 6 (0, 27) |
FIGURE 3.
Unadjusted mean PHQ-9 scores (with 95% CIs).
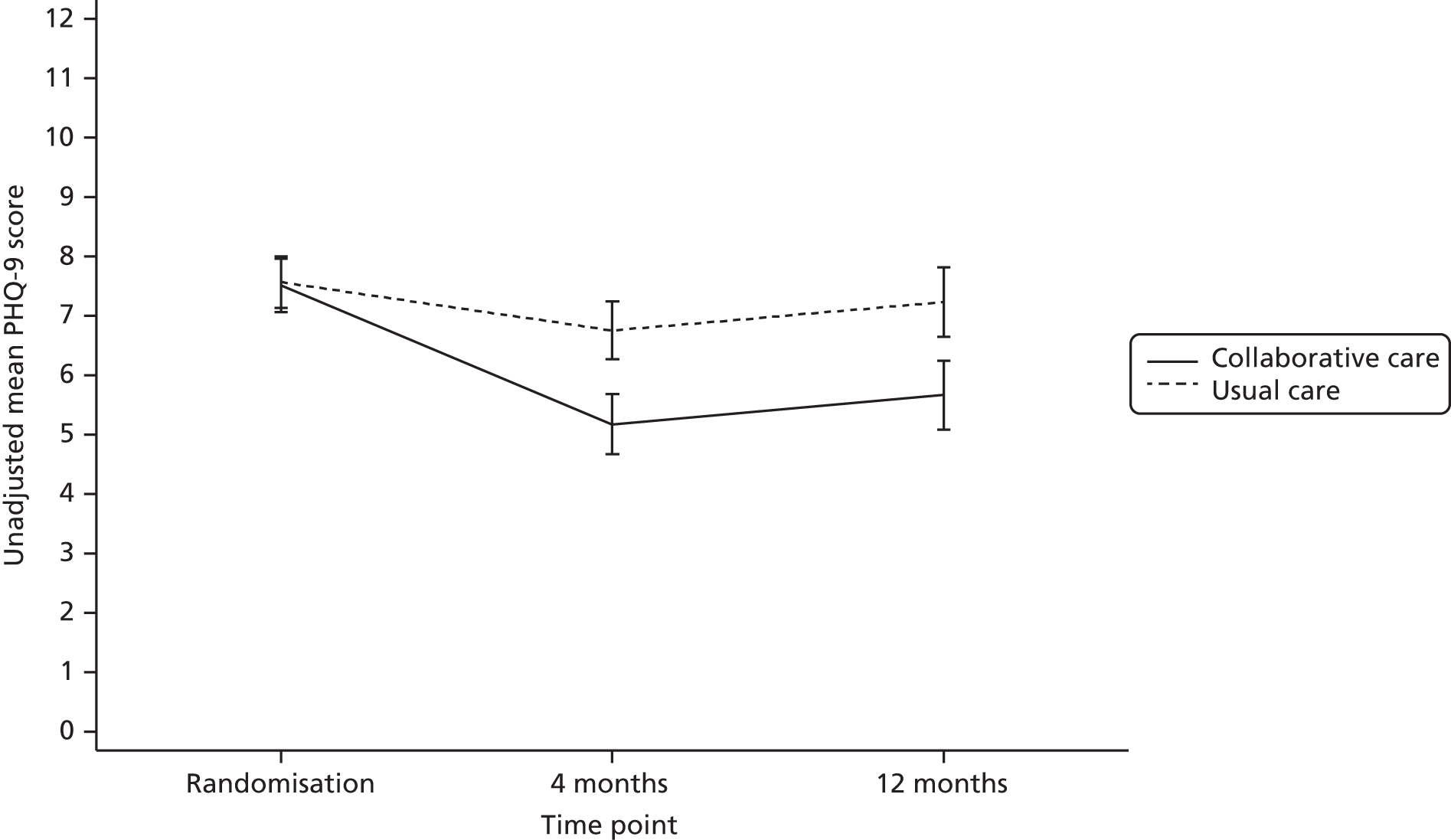
Primary analysis
The primary outcome was analysed using a covariance pattern linear mixed model using PHQ-9 score at 4 and 12 months as the outcome. The model included as fixed effects time, treatment group and time × treatment group interaction, adjusting for PHQ-9 depression at randomisation and physical/functional limitations as measured by the baseline SF-12 PCS score. Patients were included in the analysis if they had a valid PHQ-9 score at either 4 or 12 months’ follow-up and complete covariate data. Patients were analysed as part of the group to which they had been randomised (intention to treat).
The correlation of observations within patients over time was modelled by a covariance structure to describe the random effects. Different types of available covariance structures were investigated for this model (unstructured, independent, exchangeable, autoregressive and exponential). The model did not converge for the autoregressive covariance pattern after 1500 iterations. Of the remaining patterns, the unstructured covariance structure (estimating the two residual variances and covariance over time separately) displayed the lowest and therefore best-fitting log-likelihood values and emerged as the significantly better-fitting model when comparing nested models by chi-square test. Therefore, the unstructured covariance pattern was selected.
Diagnostics of model fit showed an acceptable distribution of standard residuals with slight non-normality at the higher end of the distribution because of the right skew of PHQ-9 scores. There was uniform variance between predicted and actual residuals, and no transformation of PHQ-9 scores was carried out for the analysis.
Adjusted PHQ-9 mean scores and group differences for the primary analysis model as specified above are presented in Table 17. The analysis revealed significant differences between trial arms at each of the follow-up time points in favour of collaborative care: 1.31 score points (95% CI 0.67 to 1.95 score points; p < 0.001) for the primary end point at 4 months’ follow-up and 1.33 score points (95% CI 0.55 to 2.10 score points; p = 0.001) at 12 months’ follow-up. Using the overall residual SD (4.35), the score difference at 4 months equates to a standard effect size of 0.30, the exact value for which the trial was powered.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4a | 274 | 5.36 | 4.89 to 5.83 | 327 | 6.67 | 6.24 to 7.10 | 1.31 | 0.67 to 1.95 | < 0.001 |
| 12 | 274 | 5.93 | 5.35 to 6.50 | 327 | 7.25 | 6.73 to 7.77 | 1.33 | 0.55 to 2.10 | 0.001 |
Secondary analyses
Adjusting for clustering by case manager
It was anticipated in the planning and sample size calculation for this trial that collaborative-care case managers would work with an average case load of 20 patients and the clustering of outcomes within case managers was expected to be described by an ICC of 0.02. In total, there were 18 case managers (five in York, four in Leeds, eight in Durham and one in Newcastle upon Tyne) for a total of 344 participants in the collaborative-care arm, that is, an average case load of 19.1 randomised patients per case manager. Case loads varied considerably between 1 and 84 patients.
The average ICC for clustering within case managers was found to be lower than expected (ICC 0.0069, 95% CI 0.0000 to 0.0644, for PHQ-9 scores at 4 months; ICC 0.0072, 95% CI 0.0000 to 0.0676, for PHQ-9 scores at 12 months).
To quantify the impact of the grouping by case managers with respect to the primary outcome, case manager identifiers were included as a random effect in the primary linear mixed analysis model, nested within treatment arm. Participants in the usual-care arm were coded as their own case managers for the purpose of the analysis and the covariance structure was estimated separately for each treatment arm to account for the differences in variability for the random effect.
Adjusted PHQ-9 mean scores and group differences for this analysis are provided in Table 18. Group differences remained significant in favour of collaborative care at 4 and 12 months’ follow-up (a difference of 1.21 score points at both times). Thus, accounting for the clustering by case manager reduced the size of the treatment effect only slightly compared with the primary analysis by approximately 0.1 score points. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented later in Table 26.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 | 274 | 5.46 | 4.80 to 6.11 | 327 | 6.67 | 6.23 to 7.11 | 1.21 | 0.42 to 1.99 | 0.003 |
| 12 | 274 | 6.03 | 5.30 to 6.76 | 327 | 7.24 | 6.71 to 7.78 | 1.21 | 0.31 to 2.12 | 0.008 |
Adjusting for covariates predictive of Patient Health Questionnaire-9 items score at 4 months
The primary analysis was adjusted for PHQ-9 depression at randomisation and baseline physical limitations (SF-12 PCS score). To identify any other relevant covariates of depression severity at follow-up, a number of selected demographic variables and baseline measures were used as predictors of PHQ-9 depression at 4 months in individual regressions followed by a combined regression to avoid issues of multicollinearity, using a non-conservative significance level of p < 0.10 at each stage. All analyses were adjusted for PHQ-9 score at randomisation.
Considered predictors were age, sex, an indicator of whether or not any selected antidepressants had been prescribed at baseline, a history of depression [as measured by two questions in the MINI at randomisation: (1) whether or not patients had ever been consistently depressed for a minimum of 2 weeks and (2) whether patients had ever experienced a lack of interest or enjoyment for a minimum of 2 weeks], baseline anxiety (as measured by the GAD-7) and baseline physical functioning (as measured by the PHQ-15).
Results of the individual regressions and the summary regression are given in Table 19. Positive coefficients indicate increased depression at 4 months for higher values of the predictor variable (or for the condition specified in Table 19 for categorical variables). Initial identified predictors following individual regressions were prescribed medication, a history of depression (both indicators) and baseline GAD-7 and PHQ-15 scores. Higher levels of anxiety, physical problems and a greater likelihood of being described antidepressants and having a history of depression were associated with higher PHQ-9 depression severity at 4 months. Of these predictors, prescribed antidepressants, GAD-7 score and PHQ-15 score remained significant in a summary regression and were included in the primary analysis mixed model. Age and sex were not significant predictors of PHQ-9 score.
| Characteristic | Coefficient | Standard error | p-valuea |
|---|---|---|---|
| Individual linear regressions | |||
| Age | 0.02 | 0.024 | 0.340 |
| Sex (being female) | –0.01 | 0.340 | 0.973 |
| Prescribed antidepressants (yes) | 1.40 | 0.513 | 0.007 |
| MINI: history of feeling depressed (yes) | 1.05 | 0.335 | 0.002 |
| MINI: history of lack of interest/enjoyment (yes) | 1.02 | 0.333 | 0.002 |
| Baseline GAD-7 score | 0.27 | 0.037 | < 0.001 |
| Baseline PHQ-15 score | 0.24 | 0.042 | < 0.001 |
| Summary linear regression | |||
| Prescribed antidepressants (yes) | 0.85 | 0.040 | 0.086 |
| MINI: history of feeling depressed (yes) | 0.30 | 0.370 | 0.420 |
| MINI: history of lack of interest/enjoyment (yes) | 0.59 | 0.361 | 0.103 |
| Baseline GAD-7 score | 0.21 | 0.040 | < 0.001 |
| Baseline PHQ-15 score | 0.17 | 0.043 | < 0.001 |
Adjusted PHQ-9 mean scores and group differences for the primary analysis mixed-effects model (additionally adjusting for prescribed antidepressants, GAD-7 score and PHQ-15 score at baseline) are given in Table 20. Group differences remained significantly in favour of collaborative care at 4 and 12 months’ follow-up (a difference of 1.20 score points at both times). Thus, accounting for additional predictors of the primary outcome reduced the size of the treatment effect only slightly compared with the primary analysis (by approximately 0.1 score points). An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented later in Table 26.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI) | p-value | |
| 4 | 269 | 5.77 | 5.20 to 6.33 | 322 | 6.97 | 6.46 to 7.48 | 1.20 | 0.59 to 1.82 | < 0.001 |
| 12 | 269 | 6.31 | 5.67 to 6.95 | 322 | 7.51 | 6.93 to 8.09 | 1.20 | 0.46 to 1.93 | 0.001 |
Adjusting for covariates predictive of non-response at 4 months
There was no valid PHQ-9 response at the primary end point of 4 months’ follow-up for 23.8% (n = 82) of patients in the collaborative-care arm and 10.2% (n = 37) of patients in the usual-care arm. Baseline characteristics of the randomised and analysed patient populations have already been presented in Baseline characteristics. To investigate the impact of missing data on the treatment effect, any baseline predictors of non-response at 4 months’ follow-up (no valid PHQ-9 score) were identified by individual logistic regressions and a summary logistic regression using p < 0.10 and included as covariates in the primary analysis model.
Considered predictors were age, sex, an indicator of whether or not any selected antidepressants had been prescribed at baseline, a history of depression (as measured by two questions of the MINI at randomisation), depression at randomisation (PHQ-9 score), baseline mental well-being (SF-12 MCS score), baseline anxiety (GAD-7 score) and baseline physical functioning (PHQ-15 score and SF-12 PCS score).
Results of the individual and summary regressions are presented in Table 21. ORs > 1 indicate a greater likelihood of non-response at 4 months for higher values of the predictor variable (or for the condition specified in Table 21 for categorical variables). Initial identified predictors were age and SF-12 PCS and MCS scores. Older participants and participants with reduced mental or physical functioning were more likely to be missing a PHQ-9 response at 4 months. Of these predictors, age and the SF-12 MCS score remained significant in a summary regression and were included in the primary analysis mixed model. Sex and measures of baseline depression and depression history did not significantly predict non-response.
| Characteristic | OR | Standard error | p-valuea |
|---|---|---|---|
| Individual regressions | |||
| Age | 1.04 | 0.015 | 0.013 |
| Sex (being female) | 0.82 | 0.167 | 0.339 |
| Baseline GAD-7 score | 1.03 | 0.022 | 0.198 |
| Baseline SF-12 MCS score | 0.98 | 0.009 | 0.054 |
| Baseline SF-12 PCS score | 0.99 | 0.008 | 0.071 |
| Baseline PHQ-15 score | 1.01 | 0.025 | 0.656 |
| Randomisation PHQ-9 score | 0.98 | 0.024 | 0.475 |
| Prescribed antidepressants (yes) | 1.15 | 0.342 | 0.640 |
| MINI: history of feeling depressed (yes) | 0.82 | 0.181 | 0.372 |
| MINI: history of lack of interest/enjoyment (yes) | 0.84 | 0.170 | 0.380 |
| Summary regression | |||
| Age | 1.03 | 0.016 | 0.050 |
| Baseline SF-12 MCS score | 0.97 | 0.010 | 0.011 |
| Baseline SF-12 PCS score | 0.99 | 0.009 | 0.111 |
Adjusted PHQ-9 mean scores and group differences for the primary analysis mixed-effects model (additionally adjusting for age and baseline SF-12 MCS scores) are given in Table 22. Group differences remained significant in favour of collaborative care at 4 and 12 months’ follow-up (differences of 1.36 and 1.34 score points, respectively). Thus, accounting for predictors of non-response did not affect the size of the treatment effect. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented later in Table 25.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 | 274 | 5.31 | 4.86 to 5.76 | 327 | 6.67 | 6.27 to 7.08 | 1.36 | 0.75 to 1.97 | < 0.001 |
| 12 | 274 | 5.88 | 5.34 to 6.43 | 327 | 7.22 | 6.73 to 7.72 | 1.34 | 0.60 to 2.07 | < 0.001 |
Sensitivity analysis for patients whose follow-up was timed from randomisation only
Patients in the CASPER trial were followed up according to two different time regimes. Follow-up times were initially calculated from baseline (for 165 patients) but, as there was an increasing delay between baseline and diagnostic interview and randomisation for many participants for logistic reasons, follow-up times for participants later on in the trial were calculated from the time of randomisation (for 540 patients).
As a result, the sequence of randomisation, treatment and follow-up (in particular the time between scheduled treatment and follow-up) was consistent only for the later follow-up regime. An unplanned sensitivity analysis aimed to reveal any impact on the treatment effect of collaborative care by repeating the primary mixed-effects analysis for the subgroup of patients whose follow-up was timed from randomisation only (n = 540). Although for many patients not in this subgroup randomisation occurred shortly after baseline, this sensitivity population was selected as the most conservative analysis choice.
Descriptive statistics for the subsample are presented in Table 23 and the estimates of the analysis model are given in Table 24. Group differences remained significant in favour of collaborative care at 4 and 12 months’ follow-up (a difference of 1.24 and 1.34 score points, respectively). Thus, compared with the primary analysis, the treatment effect at 4 months was slightly smaller and the treatment effect at 12 months was slightly larger using the subsample of patients with the more standard follow-up regime in relation to treatment. An overall comparison of treatment effects for PHQ-9 depression severity from different analyses is presented in Table 25.
| Time point | Collaborative care (n = 269) | Usual care (n = 271) | Total (n = 540) |
|---|---|---|---|
| Randomisation, n (%) | 269 (100) | 271 (100) | 540 (100) |
| Mean (SD) | 7.5 (4.29) | 7.8 (4.32) | 7.8 (4.34) |
| Median (min., max.) | 7 (0, 23) | 7 (0, 20) | 7 (0, 23) |
| 4 months, n (%) | 207 (77.0) | 246 (90.8) | 453 (83.9) |
| Mean (SD) | 5.5 (4.26) | 7.0 (4.56) | 6.4 (4.48) |
| Median (min., max.) | 4 (0, 20) | 6 (0, 26) | 6 (0, 26) |
| 12 months, n (%) | 181 (67.3) | 210 (77.5) | 391 (72.4) |
| Mean (SD) | 6.0 (4.68) | 7.7 (4.86) | 6.9 (4.84) |
| Median (min., max.) | 5 (0, 24) | 7 (0, 22) | 6 (0, 24) |
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 | 219 | 5.70 | 5.17 to 6.24 | 249 | 6.94 | 6.45 to 7.44 | 1.24 | 0.51 to 1.97 | 0.001 |
| 12 | 219 | 6.29 | 5.64 to 6.94 | 249 | 7.63 | 7.03 to 8.23 | 1.34 | 0.46 to 2.22 | 0.003 |
Summary of Patient Health Questionnaire-9 items analysis models
Table 25 provides an overview of group means and treatment effect estimates from the primary analysis and secondary analyses of depression severity at 4 and 12 months as measured by PHQ-9 score. Unadjusted means are presented for reference. Adjusted average estimates of group differences from the different analyses ranged from 1.20 to 1.36 score points in favour of collaborative care (equivalent to a standard effect size of 0.28–0.31).
| Analysis | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| Unadjusted means | |||||||||
| 4 months | 262 | 5.17 | 4.67 to 5.68 | 324 | 6.75 | 6.26 to 7.24 | 1.58 | – | – |
| 12 months | 235 | 5.67 | 5.09 to 6.24 | 284 | 7.23 | 6.65 to 7.82 | 1.57 | – | – |
| Primary analysisa | |||||||||
| 4 monthsb | 274 | 5.36 | 4.89 to 5.83 | 327 | 6.67 | 6.24 to 7.10 | 1.31 | 0.67 to 1.95 | < 0.001 |
| 12 months | 274 | 5.93 | 5.35 to 6.50 | 327 | 7.25 | 6.73 to 7.77 | 1.33 | 0.55 to 2.10 | 0.001 |
| Analysis adjusted for clustering by case managerc | |||||||||
| 4 months | 274 | 5.46 | 4.80 to 6.11 | 327 | 6.67 | 6.23 to 7.11 | 1.21 | 0.42 to 1.99 | 0.003 |
| 12 months | 274 | 6.03 | 5.30 to 6.76 | 327 | 7.24 | 6.71 to 7.78 | 1.21 | 0.31 to 2.12 | 0.008 |
| Analysis adjusted for additional covariates predictive of PHQ-9 score at 4 monthsd | |||||||||
| 4 months | 269 | 5.77 | 5.20 to 6.33 | 322 | 6.97 | 6.46 to 7.48 | 1.20 | 0.59 to 1.82 | < 0.001 |
| 12 months | 269 | 6.31 | 5.67 to 6.95 | 322 | 7.51 | 6.93 to 8.09 | 1.20 | 0.46 to 1.93 | 0.001 |
| Analysis adjusted for covariates predictive of non-response at 4 monthse | |||||||||
| 4 months | 274 | 5.31 | 4.86 to 5.76 | 327 | 6.67 | 6.27 to 7.08 | 1.36 | 0.75 to 1.97 | < 0.001 |
| 12 months | 274 | 5.88 | 5.34 to 6.43 | 327 | 7.22 | 6.73 to 7.72 | 1.34 | 0.60 to 2.07 | < 0.001 |
| Sensitivity analysis for patients whose follow-up was timed from randomisation onlyf | |||||||||
| 4 months | 219 | 5.70 | 5.17 to 6.24 | 249 | 6.94 | 6.45 to 7.44 | 1.24 | 0.51 to 1.97 | 0.001 |
| 12 months | 219 | 6.29 | 5.64 to 6.94 | 249 | 7.63 | 7.03 to 8.23 | 1.34 | 0.46 to 2.22 | 0.003 |
Binary Patient Health Questionnaire-9 items outcome
Using a cut-off of ≥ 10 PHQ-9 score points, Table 26 presents the numbers and percentages of moderately to severely depressed participants at randomisation and follow-up by treatment arm. This is illustrated in Figure 4. Approximately 30% of randomised CASPER participants were depressed at randomisation. At 4 months’ follow-up, this percentage decreased to 17% in the collaborative-care arm and was maintained to 12 months. In contrast, in the usual-care arm the percentage of moderately to severely depressed participants decreased to only 24% by 4 months’ follow-up and increased again to 28% by 12 months’ follow-up.
| Time point | Collaborative care | Usual care | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Total | % | n | Total | % | n | Total | % | |
| Randomisation | 102 | 344 | 29.7 | 110 | 361 | 30.5 | 212 | 705 | 30.1 |
| 4 months | 45 | 262 | 17.2 | 76 | 324 | 23.5 | 121 | 586 | 20.6 |
| 12 months | 37 | 235 | 15.7 | 79 | 284 | 27.8 | 116 | 519 | 22.4 |
FIGURE 4.
Unadjusted percentage of patients (with 95% CIs) with moderate to severe depression.

It was planned to analyse the data by logistic mixed-effects modelling, including moderate to severe PHQ-9 depression (yes or no) at 4 and 12 months as the outcome, predicted by treatment group, time (4 and 12 months), group × time interaction, depression severity at randomisation (PHQ-9 score), baseline physical functioning (SF-12 PCS score) and the additionally identified covariates of PHQ-9 depression (prescribed antidepressants at baseline, GAD-7 anxiety and PHQ-15 physical functioning; see Adjusting for covariates predictive of non-response at 4 months). The correlation of outcomes by the same patient was to be modelled by a suitable covariance structure.
However, such a model did not converge in Stata and data were instead analysed by individual logistic regressions at each follow-up time point using the same predictors as above except for the variable for time. The resulting treatment effect estimates are presented in Table 27.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | n | OR | 95% CI | OR | 95% CI | p-value | |
| 4 | 255 | 0.32 | 0.19 to 0.45 | 315 | 0.43 | 0.27 to 0.58 | 1.35 | 0.85 to 2.16 | 0.205 |
| 12 | 228 | 0.27 | 0.16 to 0.39 | 275 | 0.54 | 0.34 to 0.75 | 1.98 | 1.21 to 3.25 | 0.007 |
The relatively greater reduction in moderately to severely depressed cases seen in the collaborative-care arm compared with the usual-care arm was not statistically significant at 4 months’ follow-up (OR 1.35, 95% CI 0.85 to 2.16; p = 0.205) but did reach statistical significance at 12 months’ follow-up (OR 1.98, 95% CI 1.21 to 3.25; p = 0.007).
Secondary outcomes
A summary of the secondary outcomes is provided in Box 3.
-
Statistically significant between-group differences in favour of collaborative care were observed for:
-
Other beneficial effects of collaborative care were seen for:
-
No statistically significant treatment group differences were observed for prescribed antidepressants (see Figure 13 and Table 37).
-
A comparable number of SAEs occurred in both trial arms (see Tables 41 and 42).
-
Greater mortality rates were observed in the usual-care arm (see Table 38), a finding attributed to chance.
Descriptive statistics for secondary outcomes are generally presented in tabular form and also in graphical form where appropriate.
For continuous outcomes, any planned statistical analyses were conducted using the same covariance pattern linear mixed model as described for the primary analysis, adjusting for baseline PHQ-9 score and SF-12 PCS score (see Primary analysis). The models used the secondary outcome in question as the dependent variable and further included any baseline assessment of the outcome as well as the additionally identified significant predictors of the primary outcome depression [baseline anxiety (GAD-7), physical functioning (PHQ-15) and prescribed antidepressants; see Adjusting for covariates predictive of Patient Health Questionnaire-9 items score at 4 months] as covariates. Estimates of the effect of the intervention were derived and are presented for each follow-up time point. Any other planned analyses for different types of outcome are described in the relevant section for each measure.
Quality of life
Quality of life was assessed by patient report on the physical and mental component scales of the SF-12 (PCS and MCS) as well as the five dimensions of the EQ-5D.
Short Form questionnaire-12 items physical component summary score
The SF-12 PCS score ranges from 0 to 100, with higher scores indicating better functioning. Unadjusted means for physical functioning are presented in Table 28 and Figure 5, and the results of the formal statistical analysis by mixed modelling are given in Table 29. The figures indicate that physical functioning was below the average adult physical health status (score of < 50) for participants throughout the trial period, as would be expected in an elderly population. Patients in the usual-care arm maintained physical functioning scores at approximately 35–36 score points over follow-up, whereas patients in the collaborative-care arm improved on average to up to 40 points over follow-up. The differences in physical functioning were statistically significant based on the mixed-effects analysis at 4 months (mean score difference 2.83, 95% CI 1.62 to 4.03; p < 0.001) and 12 months (mean score difference 1.67, 95% CI 0.27 to 3.06; p = 0.020).
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Baseline, n (%) | 337 (98.0) | 356 (98.6) | 693 (98.3) |
| Mean (SD) | 38.0 (13.37) | 36.5 (13.02) | 37.2 (13.20) |
| Median (min., max.) | 37.5 (4.6, 69.9) | 35.1 (5.7, 66.6) | 36.4 (4.6, 69.9) |
| 4 months, n (%) | 254 (73.8) | 315 (87.3) | 569 (80.7) |
| Mean (SD) | 40.0 (12.39) | 35.4 (12.96) | 37.5 (12.90) |
| Median (min., max.) | 40.6 (10.5, 66.0) | 34.9 (10.0, 69.7) | 38.2 (10.0, 69.7) |
| 12 months, n (%) | 226 (65.7) | 276 (76.5) | 502 (71.2) |
| Mean (SD) | 38.8 (13.11) | 35.4 (12.73) | 36.9 (13.00) |
| Median (min., max.) | 39.2 (4.6, 69.6) | 34.9 (7.1, 64.5) | 36.5 (4.6, 69.6) |
FIGURE 5.
Unadjusted mean SF-12 PCS scores (with 95% CIs).

| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 | 263 | 38.8 | 37.7 to 39.9 | 316 | 36.0 | 35.0 to 37.0 | –2.83 | –4.03 to –1.62 | < 0.001 |
| 12 | 263 | 37.8 | 36.6 to 39.0 | 316 | 36.1 | 35.0 to 37.2 | –1.67 | –3.06 to –0.27 | 0.020 |
Short Form questionnaire-12 items mental component summary score
The SF-12 MCS score ranges from 0 to 100, with higher scores indicating better functioning. Unadjusted means for psychological functioning are presented in Table 30 and Figure 6 and the results of the formal statistical analysis by mixed modelling are given in Table 31.
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Baseline, n (%) | 337 (98.0) | 356 (98.6) | 693 (98.3) |
| Mean (SD) | 44.3 (10.96) | 45.1 (10.02) | 44.7 (10.49) |
| Median (min., max.) | 44.9 (12.5, 66.0) | 46.3 (9.6, 67.0) | 45.6 (9.6, 67.0) |
| 4 months, n (%) | 254 (73.8) | 315 (87.3) | 569 (80.7) |
| Mean (SD) | 48.7 (10.89) | 46.6 (9.82) | 47.5 (10.36) |
| Median (min., max.) | 50.3 (15.1, 68.8) | 47.3 (7.5, 66.9) | 48.6 (7.5, 68.8) |
| 12 months, n (%) | 226 (65.7) | 276 (76.5) | 502 (71.2) |
| Mean (SD) | 48.2 (10.11) | 45.6 (10.55) | 46.7 (10.43) |
| Median (min., max.) | 48.5 (17.5, 72.8) | 46.6 (13.9, 66.9) | 47.2 (13.9, 72.8) |
FIGURE 6.
Unadjusted mean SF-12 MCS scores (with 95% CIs).
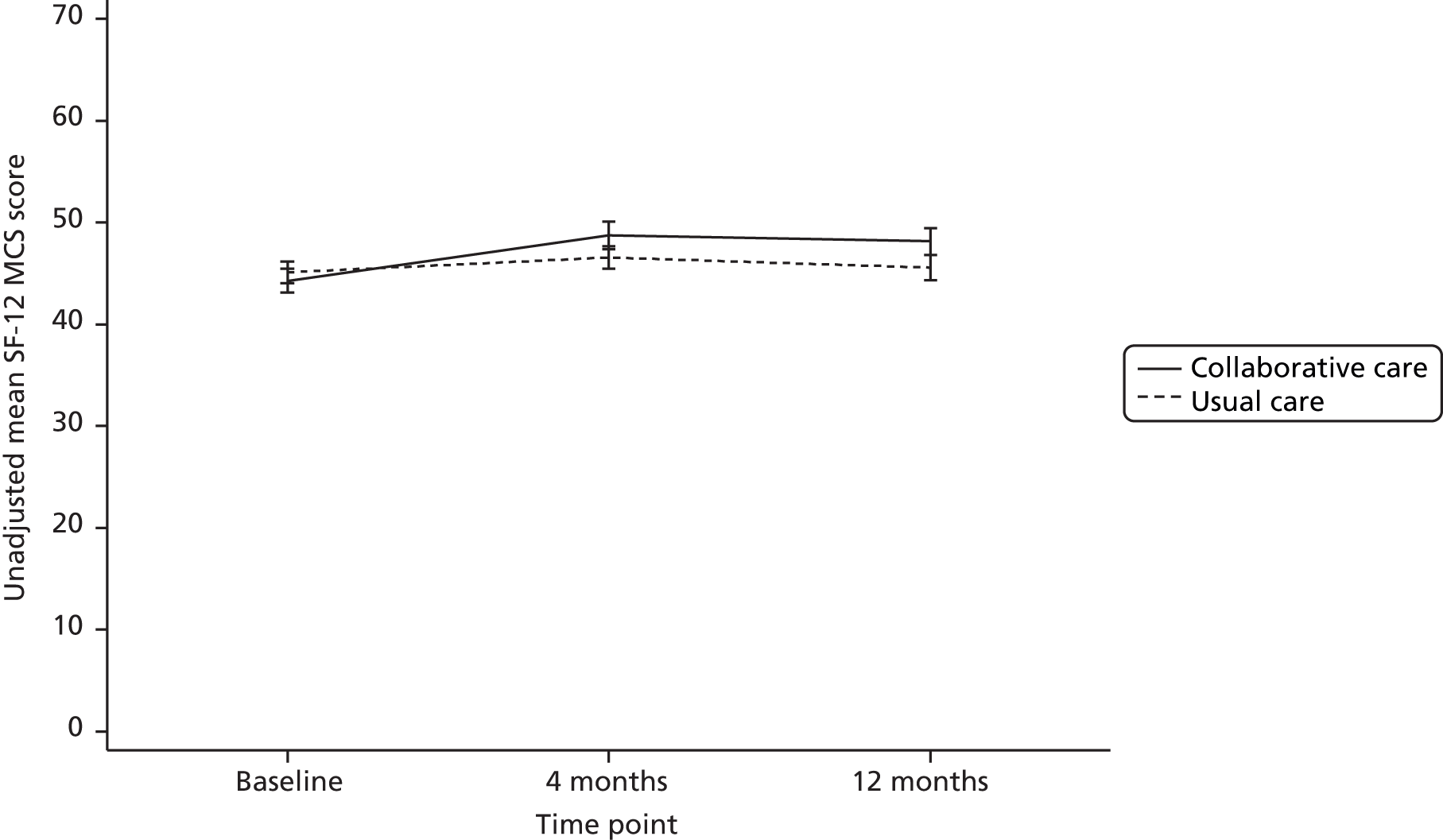
| Time | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 months | 263 | 47.6 | 46.3 to 48.9 | 316 | 45.7 | 44.6 to 46.9 | –1.88 | –3.29 to –0.47 | 0.009 |
| 12 months | 263 | 46.8 | 45.4 to 48.1 | 316 | 44.6 | 43.4 to 45.9 | –2.15 | –3.70 to –0.59 | 0.007 |
The figures indicate that mental functioning was just below the average adult mental health status (score of < 50) for participants throughout the trial period. Patients in the usual-care arm maintained physical functioning scores at approximately 45–46 score points over follow-up, whereas patients in the collaborative-care arm improved on average to up to 48 points over the follow-up. The differences in mental functioning were statistically significant based on the mixed-effects analysis at 4 months (mean score difference 1.88, 95% CI 0.47 to 3.29; p = 0.009) and 12 months (mean score difference 2.15, 95% CI 0.59 to 3.70; p = 0.007).
European Quality of Life-5 Dimensions
The EQ-5D measures quality of life on five dimensions – mobility, self-care, usual activities, pain/discomfort and anxiety/depression – with participants given three response options to indicate their level of problems for each dimension. The weighted summary index derived from these dimensions is summarised and formally analysed as part of the CASPER cost–utility analysis in the economic evaluation (see Chapter 6). For the purpose of exploring differences in quality of life between treatment arms, the frequencies of responses for each category in each dimension are presented descriptively in Table 32 and illustrated in Figures 7–11.
| EQ-5D dimension | Time | Severitya | Collaborative care | Usual care | ||||
|---|---|---|---|---|---|---|---|---|
| Total (N) | n | % | Total (N) | n | % | |||
| Mobility | Baseline | Level 1 | 339 | 129 | 38.1 | 357 | 115 | 32.2 |
| Level 2 | 210 | 61.9 | 241 | 67.5 | ||||
| Level 3 | 0 | 0.0 | 1 | 0.3 | ||||
| 4 months | Level 1 | 254 | 117 | 46.1 | 316 | 98 | 31.0 | |
| Level 2 | 137 | 53.9 | 218 | 69.0 | ||||
| Level 3 | 0 | 0.0 | 0 | 0.0 | ||||
| 12 months | Level 1 | 231 | 98 | 42.4 | 279 | 83 | 29.7 | |
| Level 2 | 133 | 57.6 | 194 | 69.5 | ||||
| Level 3 | 0 | 0.0 | 2 | 0.7 | ||||
| Self-care | Baseline | Level 1 | 338 | 287 | 84.9 | 356 | 292 | 82.0 |
| Level 2 | 48 | 14.2 | 62 | 17.4 | ||||
| Level 3 | 3 | 0.9 | 2 | 0.6 | ||||
| 4 months | Level 1 | 250 | 215 | 86.0 | 315 | 253 | 80.3 | |
| Level 2 | 34 | 13.6 | 61 | 19.4 | ||||
| Level 3 | 1 | 0.4 | 1 | 0.3 | ||||
| 12 months | Level 1 | 231 | 191 | 82.7 | 279 | 225 | 80.6 | |
| Level 2 | 39 | 16.9 | 51 | 18.3 | ||||
| Level 3 | 1 | 0.4 | 3 | 1.1 | ||||
| Usual activities | Baseline | Level 1 | 338 | 136 | 40.2 | 356 | 124 | 34.8 |
| Level 2 | 189 | 55.9 | 209 | 58.7 | ||||
| Level 3 | 13 | 3.8 | 23 | 6.5 | ||||
| 4 months | Level 1 | 253 | 123 | 48.6 | 314 | 108 | 34.4 | |
| Level 2 | 116 | 45.8 | 187 | 59.6 | ||||
| Level 3 | 14 | 5.5 | 19 | 6.1 | ||||
| 12 months | Level 1 | 231 | 112 | 48.5 | 280 | 89 | 31.8 | |
| Level 2 | 108 | 46.8 | 165 | 58.9 | ||||
| Level 3 | 11 | 4.8 | 26 | 9.3 | ||||
| Pain/discomfort | Baseline | Level 1 | 339 | 76 | 22.4 | 356 | 54 | 15.2 |
| Level 2 | 224 | 66.1 | 262 | 73.6 | ||||
| Level 3 | 39 | 11.5 | 40 | 11.2 | ||||
| 4 months | Level 1 | 253 | 69 | 27.3 | 317 | 45 | 14.2 | |
| Level 2 | 166 | 65.6 | 239 | 75.4 | ||||
| Level 3 | 18 | 7.1 | 33 | 10.4 | ||||
| 12 months | Level 1 | 230 | 63 | 27.4 | 280 | 43 | 15.4 | |
| Level 2 | 147 | 63.9 | 200 | 71.4 | ||||
| Level 3 | 20 | 8.7 | 37 | 13.2 | ||||
| Anxiety/depression | Baseline | Level 1 | 337 | 132 | 39.2 | 355 | 133 | 37.5 |
| Level 2 | 193 | 57.3 | 211 | 59.4 | ||||
| Level 3 | 12 | 3.6 | 11 | 3.1 | ||||
| 4 months | Level 1 | 251 | 151 | 60.2 | 313 | 141 | 45.0 | |
| Level 2 | 95 | 37.8 | 163 | 52.1 | ||||
| Level 3 | 5 | 2.0 | 9 | 2.9 | ||||
| 12 months | Level 1 | 231 | 123 | 53.2 | 280 | 117 | 41.8 | |
| Level 2 | 104 | 45.0 | 152 | 54.3 | ||||
| Level 3 | 4 | 1.7 | 11 | 3.9 | ||||
FIGURE 7.
European Quality of Life-5 Dimensions: mobility – percentage of patients in each severity category.
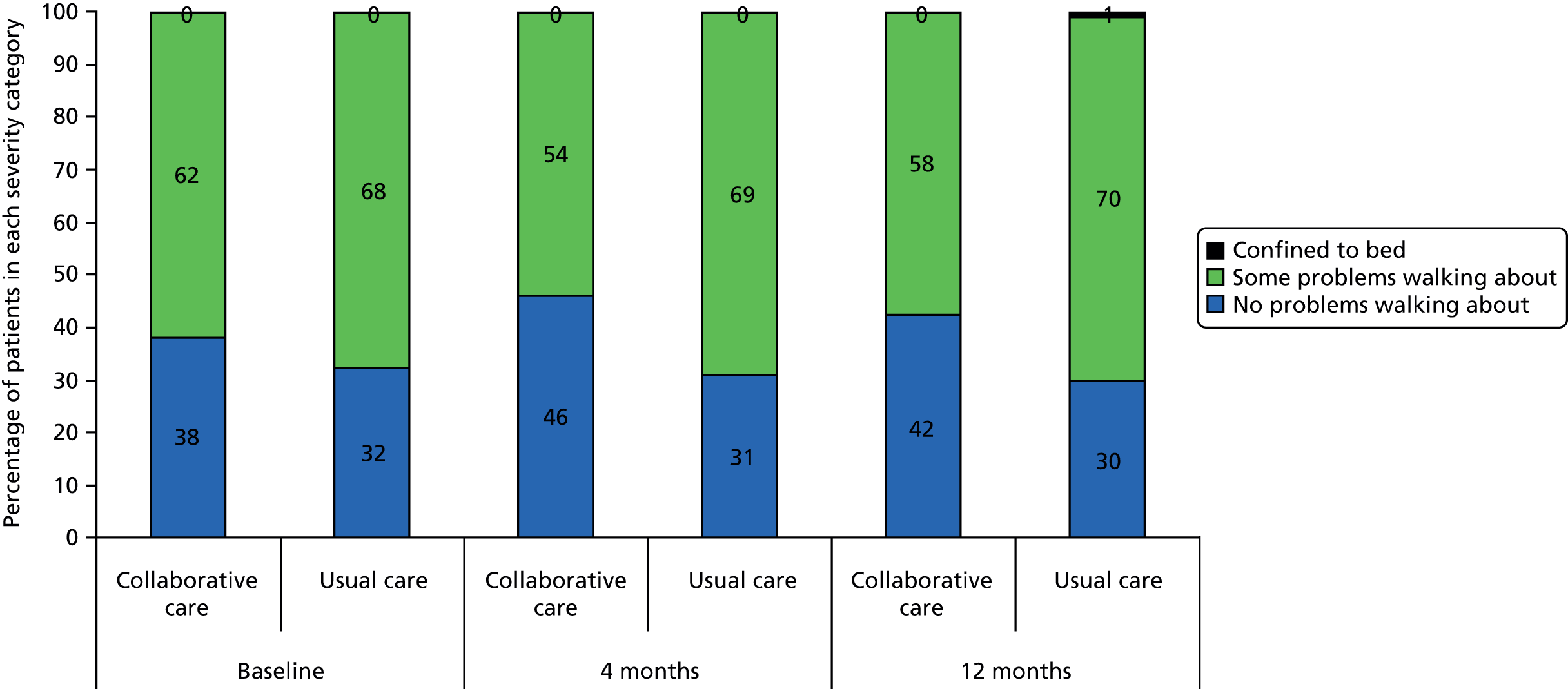
FIGURE 8.
European Quality of Life-5 Dimensions: self-care – percentage of patients in each severity category.

FIGURE 9.
European Quality of Life-5 Dimensions: usual activities – percentage of patients in each severity category.
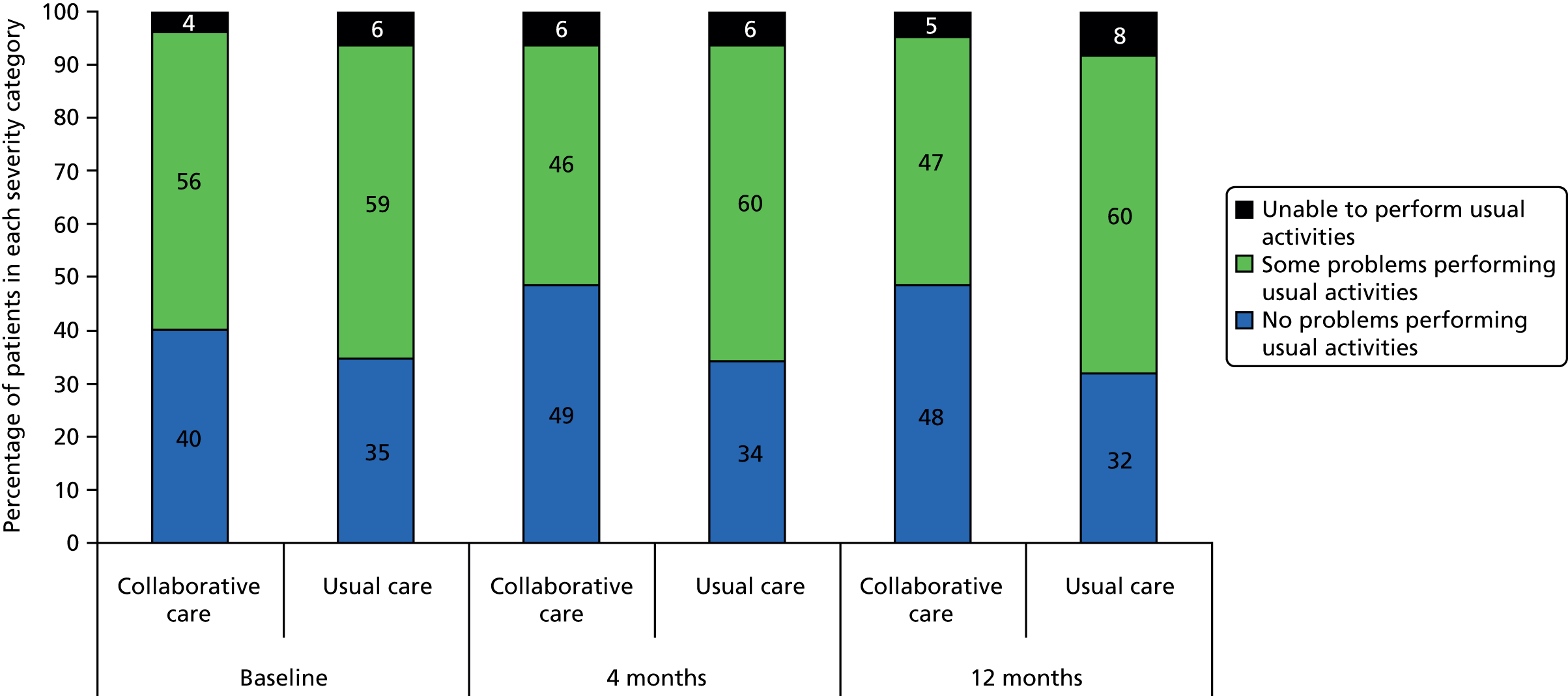
FIGURE 10.
European Quality of Life-5 Dimensions: pain/discomfort – percentage of patients in each severity category.
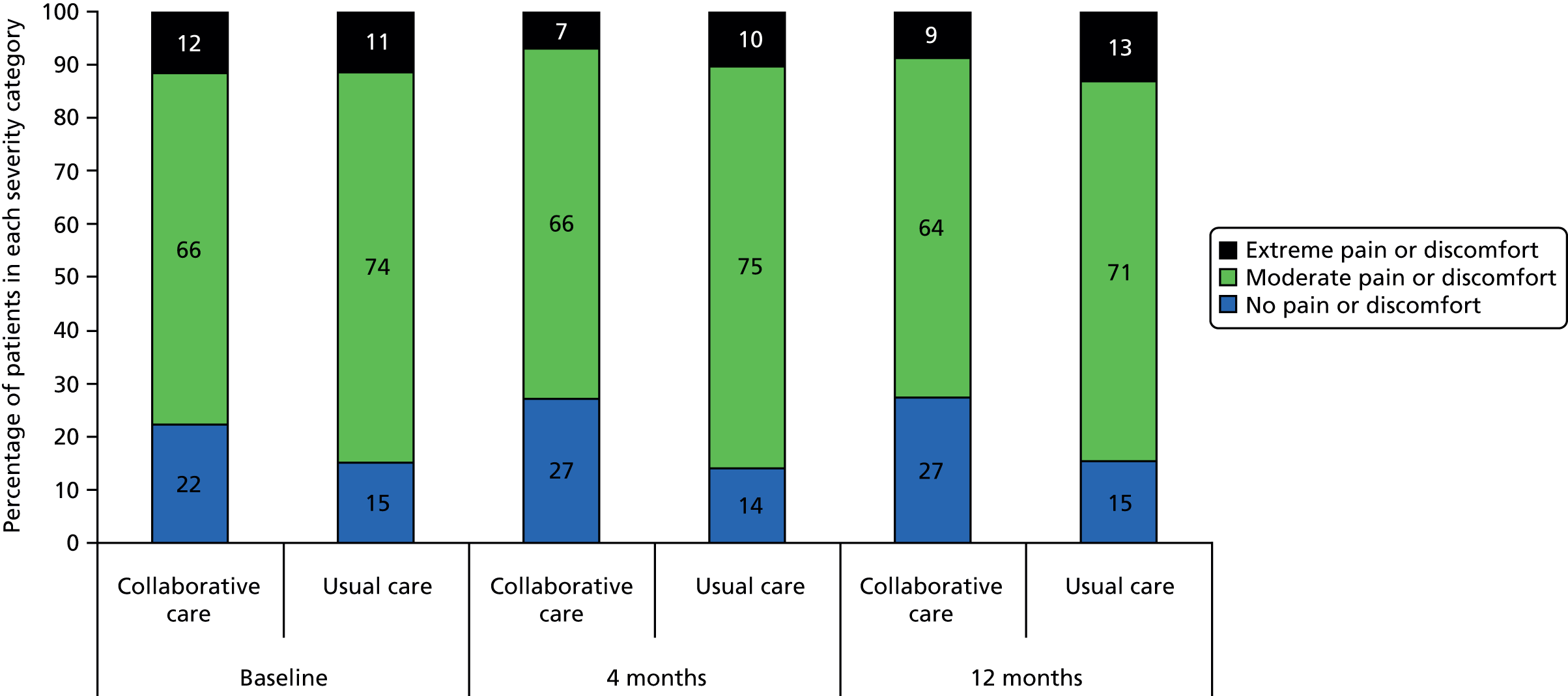
FIGURE 11.
European Quality of Life-5 Dimensions: anxiety/depression – percentage of patients in each severity category.

The majority of CASPER participants indicated no problems or some problems in each of the EQ-5D dimensions, with few patients having severe difficulties. The most frequent use of the severe category was in the pain/discomfort dimension. The greatest treatment group differences were seen for anxiety/depression, with the number of non-depressed patients in the collaborative-care arm increasing from 39% to 60% at 4 months’ follow-up compared with a lower recovery rate from 37% to 45% in the usual-care arm. Relatively greater improvements in favour of the intervention arm were also seen for mobility, usual activities and pain/discomfort, although in each case the number of patients with no difficulties at baseline was already 5–7% higher in the collaborative-care group. Any differences between treatment arms were generally maintained between 4 and 12 months. There were no evident group differences in the self-care dimension.
Psychological anxiety
The GAD-7 is a brief measure of symptoms of anxiety with a score range of 0–21, with higher scores indicating more severe anxiety. Unadjusted means for psychological anxiety based on the GAD-7 are presented in Table 33 and Figure 12 and the results of the formal statistical analysis by mixed modelling are given in Table 34.
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Baseline, n (%) | 340 (98.8) | 358 (99.2) | 698 (99.0) |
| Mean (SD) | 5.7 (4.82) | 5.7 (4.45) | 5.7 (4.63) |
| Median (min., max.) | 5 (0, 21) | 5 (0, 21) | 5 (0, 21) |
| 4 months, n (%) | 254 (73.8) | 314 (87.0) | 568 (80.6) |
| Mean (SD) | 3.8 (4.06) | 5.1 (4.36) | 4.5 (4.27) |
| Median (min., max.) | 3 (0, 18) | 4 (0, 21) | 4 (0, 21) |
| 12 months, n (%) | 230 (66.9) | 279 (77.3) | 509 (72.2) |
| Mean (SD) | 3.8 (3.96) | 5.2 (4.47) | 4.6 (4.30) |
| Median (min., max.) | 3 (0, 20) | 5 (0, 21) | 4 (0, 21) |
FIGURE 12.
Unadjusted mean GAD-7 scores (with 95% CIs).
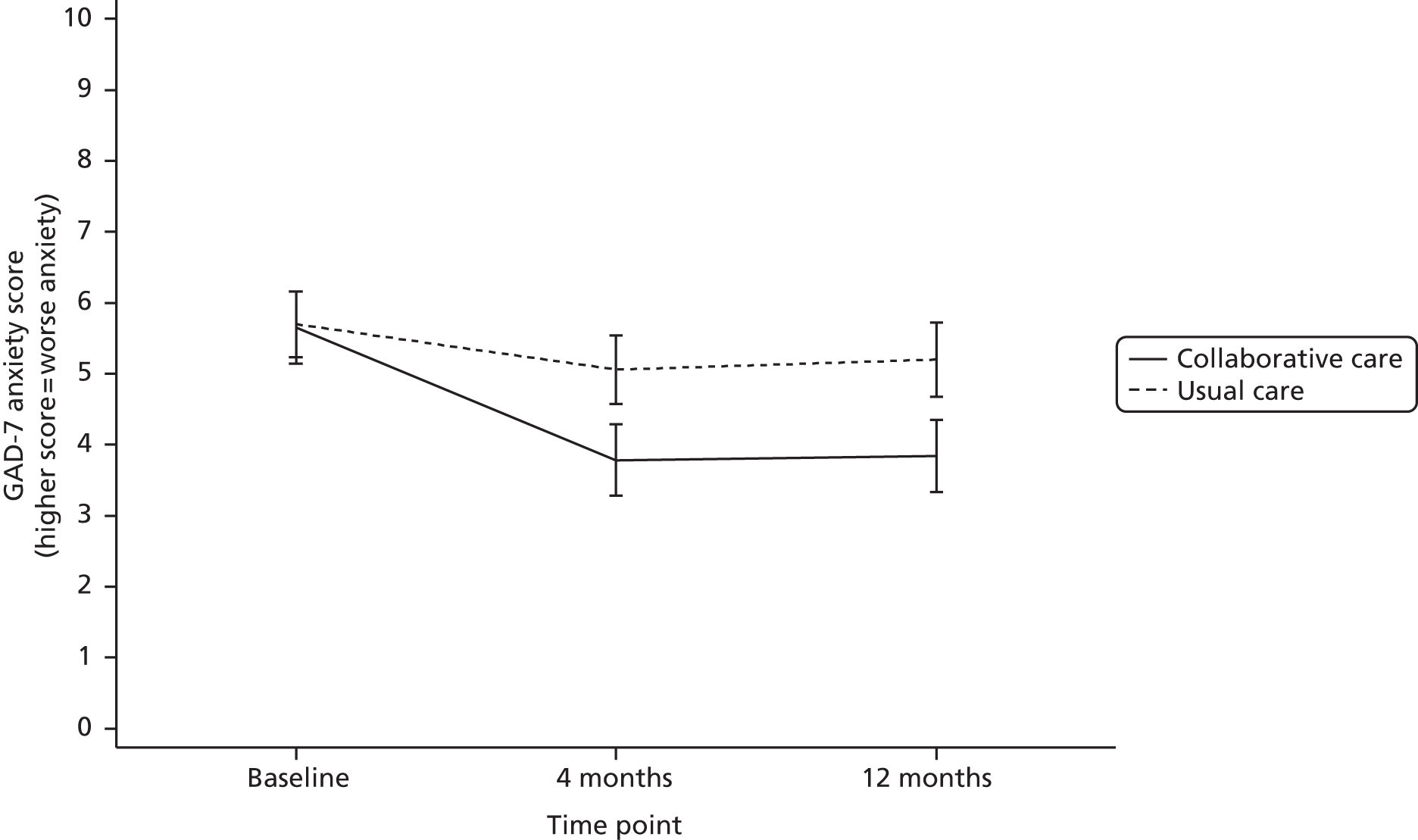
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean | 95% CI | p-value | |
| 4 | 264 | 4.05 | 3.54 to 4.55 | 315 | 5.13 | 4.67 to 5.59 | 1.08 | 0.52 to 1.64 | < 0.001 |
| 12 | 264 | 4.18 | 3.66 to 4.71 | 315 | 5.20 | 4.72 to 5.67 | 1.01 | 0.42 to 1.61 | 0.001 |
The figures indicate that anxiety was, on average, at a mild level (scores between 5 and 9) for all participants at baseline. Both treatment groups improved anxiety levels at 4 months’ follow-up, which was maintained up to 12 months; however, patients in the usual-care arm remained just above the cut-off for mild anxiety (a score of 5) whereas collaborative-care patients improved to be below that level, with an average score of around 4. The differences in psychological anxiety were statistically significant based on the mixed-effects analysis at 4 months (mean score difference 1.08, 95% CI 0.52 to 1.64; p < 0.001) and 12 months (mean score difference 1.01, 95% CI 0.42 to 1.61; p = 0.001).
Antidepressants
Patients indicated on questionnaires whether or not they were currently prescribed any of a list of 10 antidepressants. Table 35 shows the frequencies of prescriptions by trial arm. Overall, patients were prescribed between none and three of the selected drugs (only one patient indicated three and this was at a single time point). Citalopram was the most commonly prescribed antidepressant.
| Antidepressant | Collaborative care | Usual care | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | |
| Dosulepin | 0 | 0 | 0 | 5 | 3 | 4 | 5 | 3 | 4 |
| Sertraline | 2 | 3 | 4 | 3 | 6 | 8 | 5 | 9 | 12 |
| Venlafaxine | 2 | 0 | 1 | 4 | 3 | 1 | 6 | 3 | 2 |
| Lofepramine | 1 | 0 | 0 | 4 | 2 | 3 | 5 | 2 | 3 |
| Fluoxetine | 8 | 2 | 3 | 6 | 3 | 4 | 14 | 5 | 7 |
| Duloxetine | 3 | 2 | 1 | 1 | 1 | 0 | 4 | 3 | 1 |
| Citalopram | 13 | 14 | 9 | 22 | 23 | 22 | 35 | 37 | 31 |
| Paroxetine | 2 | 2 | 2 | 3 | 3 | 3 | 5 | 5 | 5 |
| Trazodone | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 3 | 2 |
| Mirtazapine | 5 | 5 | 2 | 3 | 1 | 2 | 8 | 6 | 4 |
| Total drugs | 37 | 29 | 23 | 53 | 47 | 48 | 90 | 76 | 71 |
| Total patientsa | 35 | 26 | 23 | 51 | 46 | 44 | 86 | 72 | 67 |
A binary variable was created to indicate whether patients had been prescribed any of the listed antidepressants or not. The variable was assumed to be missing if the participant indicated ‘Don’t know’ and none of the drugs had been selected.
Table 36 presents the numbers and percentages of patients on antidepressants at baseline and follow-up by treatment arm. This is illustrated in Figure 13. Approximately 10% of patients in the collaborative-care arm were prescribed antidepressants at baseline compared with 14% in the usual-care arm. This baseline imbalance (although CIs overlapped) was maintained at each of the two follow-up time points, with little change over time evident for either patient group.
| Time | Collaborative care | Usual care | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Total | % | n | Total | % | n | Total | % | |
| Baseline | 35 | 338 | 10.4 | 51 | 357 | 14.3 | 86 | 695 | 12.4 |
| 4 months | 26 | 264 | 9.8 | 46 | 321 | 14.3 | 72 | 585 | 12.3 |
| 12 months | 23 | 234 | 9.8 | 44 | 281 | 15.7 | 67 | 515 | 13.0 |
FIGURE 13.
Unadjusted percentage of patients (with 95% CIs) who were prescribed antidepressants.
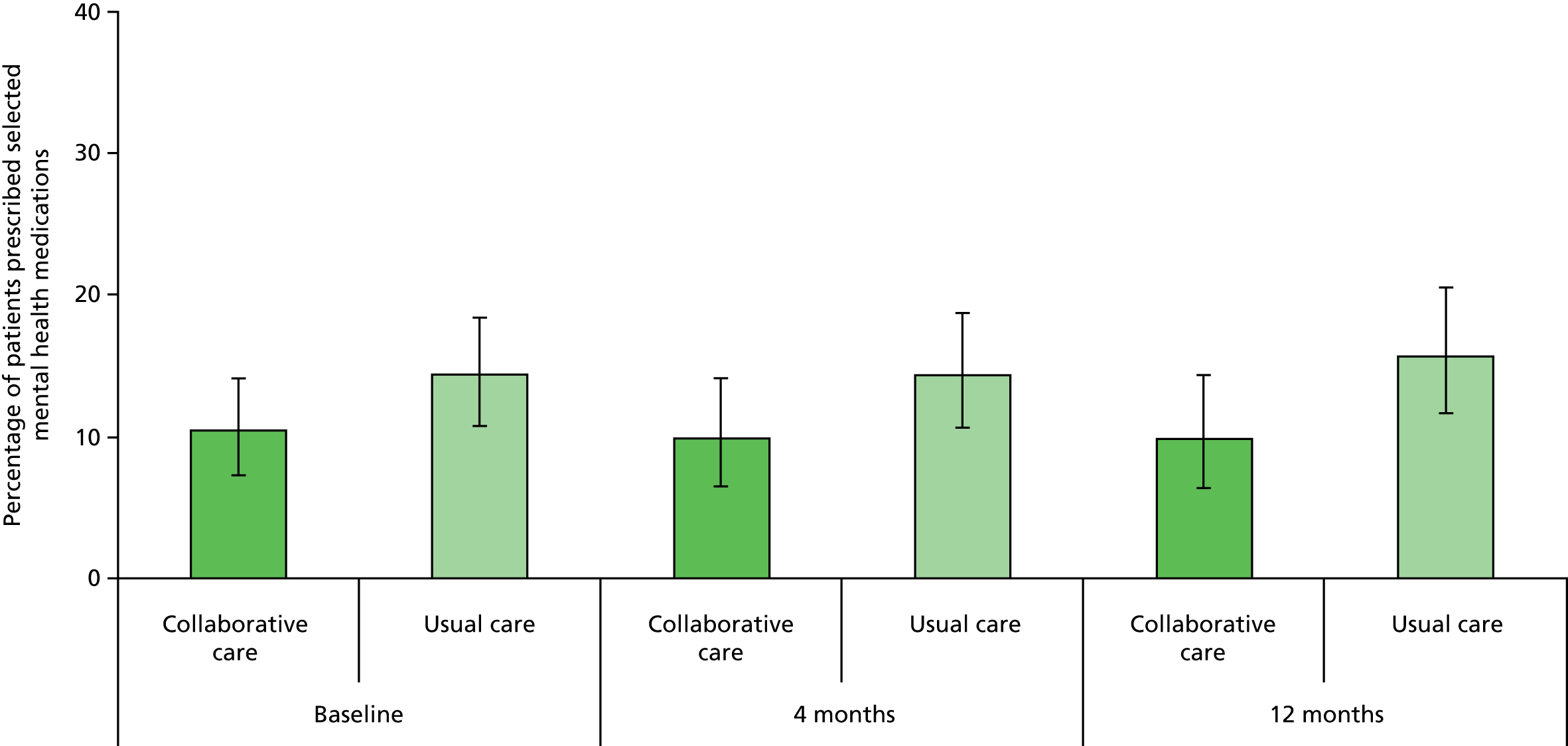
It was planned to analysed the data using a logistic mixed-effects model including prescribed medication (yes or no) at 4 and 12 months as the outcome, predicted by treatment group, time (4 and 12 months), group × time interaction, prescribed antidepressants at baseline, depression severity at randomisation (PHQ-9 score), baseline anxiety (GAD-7 score) and baseline physical functioning (SF-12 PCS score). The correlation of outcomes by the same patient was to be modelled by a suitable covariance structure.
Such a model did not converge in Stata, however, and data were instead analysed by individual logistic regressions at each follow-up time point using the same predictors as above except for the variable for time. The resulting treatment effect estimates are presented in Table 37.
| Time point (months) | Collaborative care | Usual care | Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95% CI | n | OR | 95% CI | OR | 95% CI | p-value | |
| 4 | 256 | 1.12 | –0.25 to 2.50 | 313 | 2.22 | –0.59 to 5.04 | 1.98 | 0.76 to 5.19 | 0.165 |
| 12 | 228 | 0.65 | 0.03 to 1.27 | 272 | 0.90 | 0.12 to 1.69 | 1.39 | 0.57 to 3.41 | 0.469 |
The relative odds of being prescribed antidepressants were higher in the usual-care arm than in the collaborative-care arm; however the differences were not statistically significant at 4 months’ follow-up (OR 1.98, 95% CI 0.76 to 5.19; p = 0.165) or 12 months’ follow-up (OR 1.39, 95% CI 0.57 to 3.41; p = 0.469).
Mortality
A total of 23 participants died within their 12-month follow-up period, five in the collaborative-care arm (1.5% of randomised patients) and 18 in the usual-care arm (5.0% of randomised patients). Causes of death are summarised in Table 38. A chi-square test revealed that the difference in mortality rate between treatment arms was statistically significant (χ2 = 6.97, degrees of freedom = 1, p = 0.008).
| Allocation | Cause of death |
|---|---|
| Collaborative care | Bowel cancer |
| Myocardial infarction | |
| Participant fell, was admitted to hospital and subsequently died. Inquest into death ongoing | |
| Ischaemic heart disease | |
| Glioblastoma | |
| Usual care | Pulmonary oedema, right-sided heart failure, chronic kidney disease |
| Myocardial infarction | |
| Pulmonary embolism | |
| Haemopericardium, systemic atherosclerosis, congestive cardiac failure | |
| Mantle cell lymphoma | |
| Metastatic oesophageal carcinoma | |
| Septic shock multiorgan failure, mediastinitis, emergency aortic dissection | |
| Myocardial fibrosis, myocardial ischaemia | |
| Aspiration pneumonia, chronic obstructive pulmonary disease, Parkinson’s disease | |
| Coronary heart disease | |
| Ruptured aortic aneurysm | |
| Small bowel obstruction | |
| Pneumonia, chronic obstructive pulmonary disease, ischaemic heart disease | |
| Acute exacerbation of chronic obstructive pulmonary disease | |
| Pneumonia, congestive cardiac failure, atrial fibrillation and ischaemic heart disease | |
| Myocardial infarction | |
| Cause unknown | |
| Cause unknown |
All deaths were further recorded as SAEs (see Adverse events) and their potential relatedness to the trial treatment was assessed as part of the processing of adverse events. In total, 81% of deaths were categorised as being unrelated to treatment and 18% as unlikely to be related to treatment. The DMEC noted the marked imbalance in mortality rate between treatment arms and assessed that causes of death could not be reasonably attributed to either the intervention or the control treatment. The difference was therefore treated as a chance result.
Other collected data
Additional questionnaire data that were collected but which did not formally constitute secondary outcomes of the CASPER trial were physical symptom severity (PHQ-15 score) and psychological resilience (CD-RISC 2 score). They are presented descriptively in the following sections.
Patient Health Questionnaire-15 items
The PHQ-15 is a measure of physical health problems. In this study it had a score range of 0–28 (usual maximum is 30), as a question regarding menstrual problems was removed for the elderly CASPER patient population.
The data are described by summary statistics in Table 39 and are illustrated in Figure 14. Physical health problems as measured by the PHQ-15 did not change over time for usual-care patients, with a score of around 9 (medium symptom severity) at baseline and 4 and 12 months’ follow-up; however, symptoms improved for collaborative-care patients over follow-up, especially at 4 months. Symptoms for which the greatest improvements were observed were pain in arms, legs or joints; dizziness; shortness of breath; constipation, loose bowels or diarrhoea; and trouble sleeping.
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Baseline, n (%) | 339 (98.5) | 356 (98.6) | 695 (98.6) |
| Mean (SD) | 9.1 (4.12) | 9.5 (3.94) | 9.3 (4.03) |
| Median (min., max.) | 9.0 (0.0, 25.0) | 9 (0, 20) | 9 (0, 25) |
| 4 months, n (%) | 254 (73.8) | 314 (87.0) | 568 (80.6) |
| Mean (SD) | 7.4 (3.99) | 9.1 (4.28) | 8.4 (4.24) |
| Median (min., max.) | 7 (0, 20) | 9 (0, 22) | 8 (0, 22) |
| 12 months, n (%) | 227 (66.0) | 275 (76.2) | 502 (71.2) |
| Mean (SD) | 8.1 (4.03) | 9.2 (4.53) | 8.7 (4.34) |
| Median (min., max.) | 8 (0, 20) | 9 (0, 22) | 8 (0, 22) |
FIGURE 14.
Unadjusted mean PHQ-15 scores (with 95% CIs).
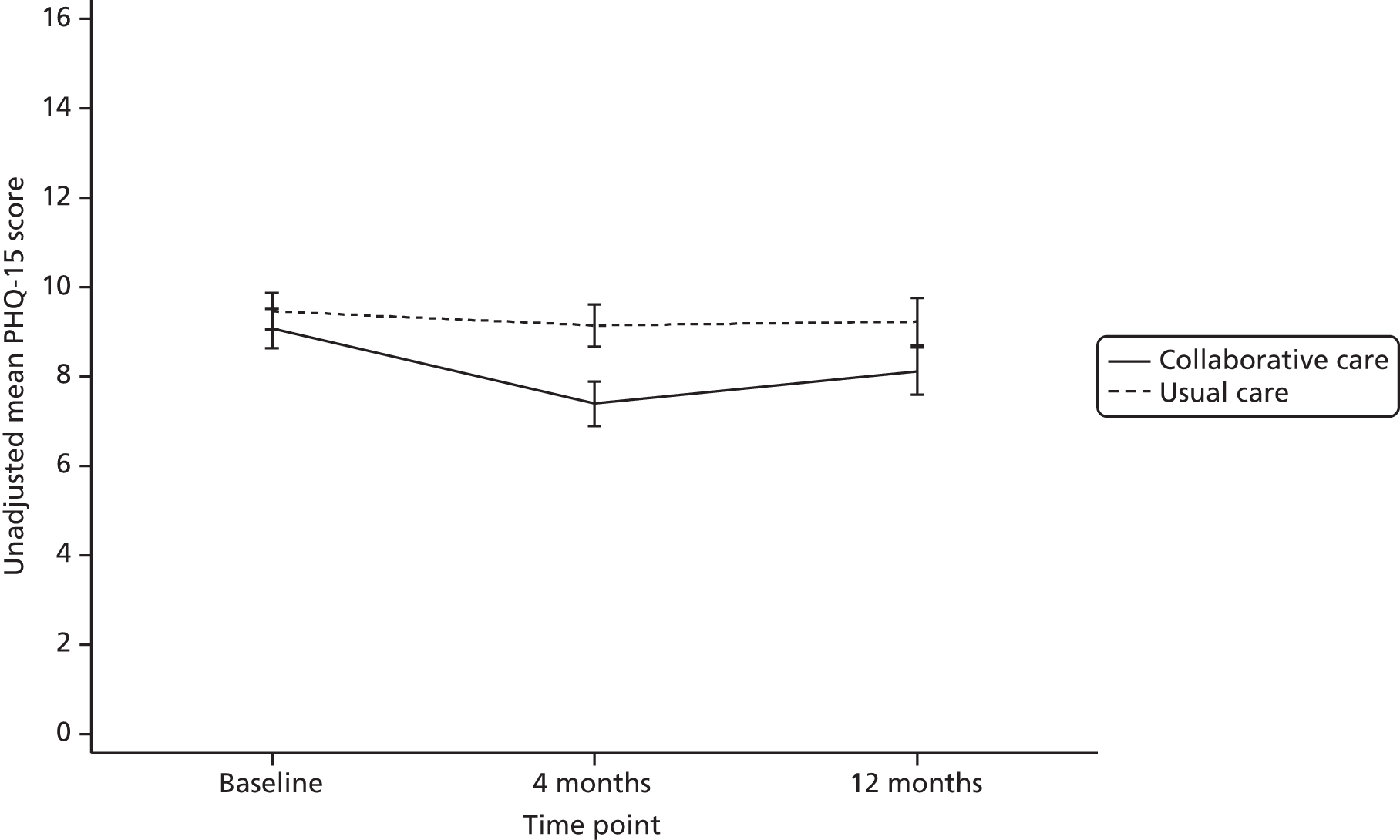
Connor–Davidson Resilience Scale two-item version
The two-item CD-RISC 2 resilience measure has a score range of 0–8, with a higher score indicating greater psychological resilience. The CD-RISC 2 data are described by summary statistics in Table 40 and are illustrated in Figure 15. Average resilience at baseline was just under 6 score points and remained constant over the 12 months of follow-up for patients in the usual-care group. Patients in the collaborative-care group improved their average resilience to > 6 score points and the difference between the treatment groups was most pronounced at 4 months’ follow-up.
| Time point | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Baseline, n (%) | 339 (98.5) | 358 (99.2) | 697 (98.9) |
| Mean (SD) | 5.8 (1.76) | 5.8 (1.67) | 5.8 (1.72) |
| Median (min., max.) | 6 (0, 8) | 6 (0, 8) | 6 (0, 8) |
| 4 months, n (%) | 253 (73.5) | 313 (86.7) | 566 (80.3) |
| Mean (SD) | 6.2 (1.71) | 5.7 (1.71) | 5.9 (1.73) |
| Median (min., max.) | 6 (0, 8) | 6 (0, 8) | 6 (0, 8) |
| 12 months, n (%) | 225 (65.4) | 277 (76.7) | 502 (71.2) |
| Mean (SD) | 6.1 (1.71) | 5.7 (1.77) | 5.9 (1.75) |
| Median (min., max.) | 6 (0, 8) | 6 (0, 8) | 6 (0, 8) |
FIGURE 15.
Unadjusted mean CD-RISC 2 scores (with 95% CIs).

Adverse events
A total of 81 SAEs including deaths were identified for CASPER participants over the 12-month follow-up period, 37 events in 33 patients in the collaborative-care arm and 44 events in 43 patients in the usual-care arm (Table 41). The maximum number of SAEs was two, and the average number of SAEs experienced per CASPER participant was 0.11 in the collaborative-care arm and 0.12 in the usual-care arm.
| SAE summary measure | Collaborative care (n = 344) | Usual care (n = 361) | Total (n = 705) |
|---|---|---|---|
| Total number of adverse events | 37 | 44 | 81 |
| Number of patients with any adverse event | 33 | 43 | 76 |
| Percentage of patients with any adverse event | 9.6 | 11.9 | 10.8 |
| Average number of events per patient | |||
| Mean | 0.11 | 0.12 | 0.11 |
| SD | 0.35 | 0.34 | 0.34 |
| Min., max. | 0, 2 | 0, 2 | 0, 2 |
The majority of SAEs (93%) were assessed as being unrelated to the intervention and the remaining SAEs were unlikely to be related. A breakdown of these figures by trial arm as well as the type and nature of the events are presented in Table 42. The majority of events were unscheduled hospitalisations, with cardiovascular events being the most likely reason for admissions. Causes of death are further detailed in Mortality.
| SAE classification | Collaborative care (n = 37 events) | Usual care (n = 44 events) | Total (n = 81 events) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Relatedness to the intervention | ||||||
| Unrelated | 35 | 94.6 | 40 | 90.9 | 75 | 92.6 |
| Unlikely to be related | 2 | 5.4 | 4 | 9.1 | 6 | 7.4 |
| Possibly related | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Probably related | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Definitely related | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Type | ||||||
| Unscheduled hospitalisation | 32 | 86.5 | 24 | 54.5 | 56 | 69.1 |
| Other medically important condition | 0 | 0.0 | 2 | 4.5 | 2 | 2.5 |
| Death | 5 | 13.5 | 18 | 40.9 | 23 | 28.4 |
| Nature of adverse event | ||||||
| Cancer | 2 | 5.4 | 5 | 11.4 | 7 | 8.6 |
| Cardiovascular | 16 | 43.2 | 23 | 52.3 | 39 | 48.1 |
| Infection | 0 | 0.0 | 4 | 9.1 | 4 | 4.9 |
| Acute infection | 5 | 13.5 | 3 | 6.8 | 8 | 9.9 |
| Injury from falls | 6 | 16.2 | 1 | 2.3 | 7 | 8.6 |
| Injury from falls/acute infection | 0 | 0.0 | 1 | 2.3 | 1 | 1.2 |
| Miscellaneous | 8 | 21.6 | 5 | 11.4 | 13 | 16.0 |
| Unknown | 0 | 0.0 | 2 | 4.5 | 2 | 2.5 |
The relatively low rate of SAEs for this population is a reflection of the strict criteria used to define an adverse event: unscheduled hospitalisation, medically important condition or death. A broader definition would have resulted in an overwhelming incidence of adverse events for a population group of this age.
Summary of the clinical effectiveness analysis
The primary analysis was a mixed model of PHQ-9 depression severity, adjusting for baseline PHQ-9 depression at randomisation and baseline SF-12 physical functioning. Model estimates at the primary end point of 4 months revealed a statistically significant effect in favour of collaborative care (mean difference 1.31 score points, 95% CI 0.67 to 1.95; p < 0.001). The difference equates to a standard effect size of 0.30, for which the trial was powered. Treatment differences were maintained at 12 months’ follow-up (mean difference 1.33 score points, 95% CI 0.55 to 2.10; p = 0.001).
Secondary analyses revealed that the treatment effect remained statistically significant at both follow-up time points when adjusting for clustering by case managers, additional predictors of depression severity (physical symptom severity, anxiety and prescribed medication) and predictors of non-response (age and mental functioning). All mean group differences ranged between 1.20 and 1.36 score points (all p < 0.010). The relatively greater reduction in moderately to severely depressed cases (PHQ-9 score of ≥ 10) seen for collaborative-care patients was statistically significant at 12 months’ follow-up (p = 0.007) but not at 4 months’ follow-up (p = 0.205).
Collaborative-care patients also had more favourable secondary outcomes at 4 and 12 months’ follow-up: improved SF-12 physical functioning (p < 0.001 and p = 0.020 at 4 and 12 months’ follow-up, respectively), improved SF-12 mental functioning (p = 0.009 and p = 0.007, respectively) and reduced GAD-7 psychological anxiety (p < 0.001 and p = 0.001, respectively). Descriptive statistics further showed fewer PHQ-15 physical symptoms, better CD-RISC 2 psychological resilience and improved functioning on the EQ-5D domains of anxiety/depression, pain/discomfort, mobility and usual activities for patients in the collaborative-care arm. The treatment did not affect the secondary outcome of prescription of antidepressants at 4 months’ (p = 0.165) or 12 months’ (p = 0.469) follow-up.
A comparable number of SAEs occurred in the two trial arms (collaborative care 37 events, usual care 44 events). A greater number of patients died in the usual-care arm (18 compared with five in the collaborative-care arm; χ2 = 6.97, degrees of freedom = 1, p = 0.008).
Chapter 6 Economic results
Response rates
For the base-case analysis, the number of complete cases was primarily determined by the number of individuals who completed all five items of the EQ-5D at all three time points (baseline, 4 months and 12 months) followed by GP practices being able to provide a retrospective record of the individual resource use in the 12-month period since randomisation.
Table 43 presents the response rates for the EQ-5D over the three time points of the study. At randomisation, the majority of participants completed all five items of the EQ-5D (98% in both the collaborative-care group and the usual-care group).
| Response type | Baseline | 4 months | 12 months | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Collaborative care | Usual care | Collaborative care | Usual care | Collaborative care | Usual care | Collaborative care | Usual care | |
| EQ-5D questionnaire | ||||||||
| No missing items | 336 | 354 | 249 | 310 | 230 | 278 | 206 | 260 |
| One missing item | 2 | 2 | 3 | 4 | – | – | n/a | n/a |
| Two missing items | 1 | 0 | 1 | 3 | – | – | n/a | n/a |
| Three missing items | 0 | 1 | – | – | – | n/a | n/a | |
| Four missing items | – | – | 1 | 0 | – | – | n/a | n/a |
| All items missing | 5 | 4 | 11 | 10 | 5 | 4 | n/a | n/a |
| General reasons for non-responseb | ||||||||
| Questionnaire not returned | – | – | 35 | 28 | 41 | 50 | 41 | 50 |
| Withdrawal | – | – | 43 | 2 | 62 | 9 | 62 | 9 |
| Died | – | – | 1 | 4 | 5 | 18 | 5 | 18 |
As discussed in Chapter 5, the most common reasons for non-response were unreturned questionnaires, withdrawal from the study or death of the participant. As the CASPER trial is based on the intention-to-treat population, economic analysis categorises ‘withdrawal from treatment’ as session 1 not being recorded as taking place following randomisation. The potential implications of missing data are explored further (following presentation of the base case) using sensitivity analysis. Health-care use was measured by collecting information from participants’ primary care practices (see Chapter 3, Objective data). This indicated the number of GP appointments, GP home visits, GP telephone consultations, practice nurse appointments and telephone consultations by the practice nurse.
Practices provided their consent to retrospectively audit patients’ records to provide individual-level information on the health-care resource use of patients for exactly 12 months since the date of randomisation. This process yielded highly complete individual-level data on participants’ rates of contact with primary care services (overall completion rate 99.57%). For participants who completed study questionnaires at all time points, resource use information was missing for only two out of 466 (0.43%), and these isolated cases were solely because the participants changed practice during the study period and their former practices no longer had access to their records.
Following randomisation, when offered collaborative care, a further 18 participants declined to receive the programme of care before their first session contact; however, they did complete the study questionnaires and we obtained full resource use information from GP records. The final number of participants available for cost-effectiveness analysis using complete cases was therefore 448.
Resource use and costs
Collaborative care: required resources and associated costs
Case managers were psychological wellbeing practitioners (PWPs) employed at NHS Band 5 level. After recruitment to provide collaborative care as part of the CASPER trial, case managers all received training. In total, there were three training events covering four regions of the study (i.e. Durham, Leeds, Newcastle upon Tyne and York), each consisting of 2 consecutive days of training. The number of attendees per training event varied and efforts were made to provide training in a manner which ensured that the overall costs of travel and accommodation were minimal.
During the training, PWPs were orientated to the case managers’ manual, outlining the overall principles of collaborative care and a ‘session by session overview’ of what case managers aimed to achieve with patients. The training courses for case managers were predominantly provided by two trainers; these trainers subsequently also supervised case managers during implementation of the collaborative-care programme.
The manual stipulated that the programme of treatment should consist of ‘8–10 mainly telephone contacts with occasional face-to-face contacts over a period of 12 weeks’. In terms of the expectation for each session, it further stated that ‘contacts last 45 minutes for session one and 20–30 minutes for each subsequent contact’. The first session was generally held face to face and took place at a participant’s home, a GP surgery or another community venue.
Case managers also received weekly supervision from a designated supervisor. The schedule of supervision followed a standardised agenda whereby for each patient there was a weekly discussion, with case managers preparing feedback on each case to discuss with their supervisor. Supervisors were responsible for providing support to case managers on the process of collaborative care, medication management and specific psychological interventions. On average, each patient contact was discussed between the case manager and the supervisor for approximately 5 minutes.
Case managers provided participant-specific feedback to GPs. In the first instance, case managers worked with and advised participants’ GPs on their care. During treatment case managers would provide a letter to update GPs on participants’ progress and, when appropriate, whether or not GPs might consider further treatment. At the end of the programme, case managers also sent a participant-specific summary report to the GPs. Supervisors were available to advise case managers on next steps and consultations with GPs. Three letters were prepared and sent over the 12 weeks, requiring approximately 30 minutes’ administration per letter.
Case managers were also charged with a duty of care to engage outside agencies (e.g. social services or in response to safeguarding issues) in situations where they became aware of abuse. However, the client group had a generally low level of need in this respect and this additional responsibility was not generally required.
To estimate personnel costs required to provide collaborative care (as intended within the manual), estimates of NHS unit costs were derived from national reference costs53 (Table 44).
| Item | Unit cost (£) | Referencea |
|---|---|---|
| PWP (Band 5): per hourb | 39 | Nurse (mental health) |
| PWP (Band 5): patient-related workb | 52 | Nurse (mental health) |
| PWP (Band 5): face-to-face contactb | 74 | Nurse (mental health) |
| PWP (Band 6): supervisionb | 49 | Nurse team leader |
| GP appointment | 45 | ‘Per patient contact lasting 11.7 minutes’ |
| GP home visit | 114 | ‘Per out of surgery visit lasting 23.4 minutes’ |
| GP telephone consultation | 27 | ‘Per telephone consultation lasting 7.1 minutes’ |
| Practice nurse appointment | 13.43 | £52 per hour – face-to-face contact, duration of contact 15.5 minutes |
| Practice nurse telephone consultation | 6.15 | £52 per hour – assumed similar time as GP (7.1 minutes) |
Table 45 summarises the resources required over the 12-week programme of collaborative care and indicates our estimate of the direct cost for the base-case analysis. Without further information from implementation, the direct cost of collaborative care (based on the previous estimation within the manual) was estimated as £494.73. The base-case estimate is naive of the actual levels of collaborative care required by individuals and observed variation was explored in sensitivity analyses.
| Item | Frequency | Duration | Total quantity | Cost (£) | Description |
|---|---|---|---|---|---|
| Training case managers | |||||
| Case managers attending | 16 case managers | 13 hours | 208 | 8112a | 2 days, 6.5 hours each |
| Supervision of course | Two trainers, three sessions | 13 hours | 96 hours | 4704b | 2 days, 6.5 hours each |
| Manual | One manual per case manager | – | 16 | 80 | Printing |
| Travel and accommodation | For two trainers × two sessions | 1 night | 4 nights | 600 | Sessions in Durham and Leeds |
| Subtotal (total cost of training) | 13,496 | Cost to train all case mangers | |||
| Subtotal (total cost of training per participant) | 39.23 | 344 allocated to the programme | |||
| Collaborative care | |||||
| Session 1 | One per patient | 45 minutes | 45 minutes | 55.5 | Assumed by home visitc |
| Sessions 2–10 | Median of nine per patient | 30 minutes | 4.5 hours | 234 | Assumed by telephoned |
| Supervisions | One per week (12 weeks) | 5 minutes | 1 hour | 88 | 1 hour over 12 weeksa,b |
| GP communication | Three letters | 30 minutes | 1.5 hours | 78 | Patient-related workd |
| Engaging outside agencies | 0 | 0 | 0 | 0 | Not required in the CASPER trial |
| Subtotal (total cost of intervention per participant | 455.50 | ||||
| Total cost (training + intervention) | 494.73 | Cost for base-case analysis | |||
Consequences for health care by treatment group
Table 46 presents information collected from primary care practices on each patient, comparing summary statistics of those who accessed collaborative care and those who underwent usual care. In all cases (except GP telephone consultations), we observed that contact rates were lower in the collaborative-care group than in the usual-care group. Using a simple t-test indicated that the mean contact rates for GP home visits, practice nurse appointments and nurse telephone consultations displayed significant levels of difference.
| Categories of resource use (contacts) | Intervention | Usual care | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | n | Mean | SD | Min. | Max. | n | |
| GP: appointment | 5.546 | 5.267 | 0 | 35 | 271 | 6.060 | 5.261 | 0 | 39 | 350 |
| GP: home visit | 0.428 | 1.812 | 0 | 24 | 271 | 0.616 | 1.672 | 0 | 15 | 349 |
| GP: telephone consultation | 1.934 | 3.371 | 0 | 27 | 271 | 1.814 | 2.927 | 0 | 26 | 349 |
| Practice nurse: appointment | 3.576 | 4.670 | 0 | 35 | 271 | 4.206 | 5.600 | 0 | 55 | 349 |
| Practice nurse: telephone consultation | 0.100 | 0.473 | 0 | 5 | 271 | 0.192 | 0.695 | 0 | 6 | 349 |
This resource utilisation data have highly skewed distributions (similar to most count data) and also have the feature of a significant number of zeros (representing the large proportion of people who do not use any services). In robustly estimating the marginal effect of treatment assignment on each resource, specific analytical procedures are required. 57 Zero-inflated negative binomial regression58 was utilised to model the count variables while accommodating the excessive number of zeros. This approach found that treatment assignment was most strongly associated with differences in the number of nurse appointments and nurse telephone contacts (see Appendix 8 for full details). The results suggest that, relative to usual care, the rate of nurse appointments was 15% less (p = 0.072) and that of nurse telephone consultations was 47% less (p = 0.062) in the collaborative-care group.
Cost–consequences and total costs
By assigning unit costs (as presented in Table 44) to resource use, the associated individual-level costs of health-care contacts were estimated and means calculated by treatment group (Table 47). Excluding the cost of collaborative care, the average cost of health-care utilisation was £51.01 less in the collaborative-care group than in the usual-care group, suggesting an average cost offsetting of 10% off the base-case cost of collaborative care (£494.73).
| Categories of cost | Intervention (£) | Usual care (£) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | n | Mean | SD | Min. | Max. | n | |
| Collaborative care | 494.73 | 0.00 | 494.73 | 494.73 | 292 | 0.00 | 0.00 | 0.00 | 0.00 | 361 |
| GP: appointment | 249.58 | 237.00 | 0.00 | 1575.00 | 271 | 272.70 | 236.75 | 0.00 | 1755.00 | 350 |
| GP: home visit | 48.80 | 206.55 | 0.00 | 2736.00 | 271 | 70.23 | 190.58 | 0.00 | 1710.00 | 349 |
| GP: telephone consultation | 52.21 | 91.03 | 0.00 | 729.00 | 271 | 48.97 | 79.02 | 0.00 | 702.00 | 349 |
| Practice nurse: appointment | 48.03 | 62.73 | 0.00 | 470.17 | 271 | 56.50 | 75.23 | 0.00 | 738.83 | 349 |
| Practice nurse: telephone consultation | 0.61 | 2.91 | 0.00 | 30.77 | 271 | 1.18 | 4.28 | 0.00 | 36.92 | 349 |
| Total | 893.96 | 391.37 | 494.73 | 4004.73 | 271 | 450.24 | 393.24 | 0.00 | 2511.50 | 349 |
Overall, the mean total cost in the collaborative-care group was £893.96 (95% CI £847.15 to £940.76) and the mean total cost in the usual-care group was £450.24 (95% CI £408.84 to £491.64). The implication is that the unadjusted incremental cost of collaborative care was £443.72 (95% bootstrapped CI £381.321 to £506.1115; n = 620) or the cost specifically for the sample available for the base-case cost-effectiveness analysis was £420.93 (95% bootstrapped CI £347.73 to £494.14; n = 448).
Health benefits
Health state utility by time point
The utility score for each participant was estimated from the responses to the EQ-5D at baseline, 4 months and 12 months. Table 48 presents a summary of the unadjusted utility scores by time point and treatment group.
| Treatment group | Mean | SD | Median | Min. | Max. | n |
|---|---|---|---|---|---|---|
| Collaborative care | ||||||
| Baseline utility | 0.633 | 0.248 | 0.691 | –0.184 | 1 | 285 |
| 4-month utility | 0.691 | 0.251 | 0.725 | –0.184 | 1 | 229 |
| 12-month utility | 0.672 | 0.246 | 0.725 | –0.016 | 1 | 211 |
| Usual care | ||||||
| Baseline utility | 0.598 | 0.254 | 0.656 | –0.239 | 1 | 354 |
| 4-month utility | 0.607 | 0.242 | 0.689 | –0.239 | 1 | 310 |
| 12-month utility | 0.583 | 0.278 | 0.656 | –0.331 | 1 | 278 |
Comparing utility scores at baseline, the treatment groups display a difference which suggests that the estimate of cost-effectiveness should control for baseline utility.
Quality-adjusted life-years
Quality-adjusted life-years were estimated by summing the time-weighted averages of the utility scores between each time point. Table 49 compares the summary statistics of unadjusted QALYs by treatment group.
| Treatment group | Mean | SD | Median | Min. | Max. | n |
|---|---|---|---|---|---|---|
| Collaborative care | 0.756 | 0.246 | 0.762 | –0.029 | 1.436 | 188 |
| Usual care | 0.660 | 0.247 | 0.718 | –0.269 | 1.131 | 260 |
This suggests that the difference between group means (i.e. unadjusted incremental QALYs) is 0.096 gained from collaborative care compared with usual care. However, this difference does not account for the between-group difference in baseline utility scores. To adjust for baseline utility, we applied ordinary least squares regression to explain QALYs, controlling for treatment group and baseline utility. Table 50 presents the outputs of the ordinary least squares regression.
| Variables | Coefficient | SE | t | p > t | 95% CI |
|---|---|---|---|---|---|
| Baseline utility | 0.823 | 0.030 | 26.99 | < 0.001 | 0.763 to 0.882 |
| Collaborative care | 0.044 | 0.015 | 2.97 | 0.003 | 0.015 to 0.072 |
| Constant | 0.168 | 0.021 | 8.20 | < 0.001 | 0.128 to 0.209 |
Examining regression coefficients (and their respective level of significance), collaborative care significantly increases QALYs by 0.044 (p = 0.003) when controlling for baseline utility.
Cost-effectiveness and uncertainty
The adjusted base-case analysis demonstrated that randomised participants who were referred to collaborative care to treat subthreshold levels of depression showed, on average, a small but statically significant increase in QALYs over the 12-month period, with a marginally higher cost. Examining QALYs as the outcome, the mean cost per incremental QALY was £9633. If, based on this ICER, the NHS decided to recommend treatment of subthreshold levels of depression, collaborative care would represent value for money in terms of improving individuals’ health status within explicit willingness-to-pay thresholds. 51
To characterise the uncertainty in the ICER, non-parametric bootstrapping of the difference in costs and QALYs was used to generate 10,000 replications. Figure 16 provides a graphical representation on the cost-effectiveness plane of the uncertainty surrounding the mean difference in costs and QALYs. This shows that the majority of the replications fall within the north-east quadrant (99.76%), which implies (with reference to usual care) that collaborative care will add both costs and health benefits. Uncertainty in the ICER implies that the probability that collaborative care is cost-effective will vary as a decision-maker’s willingness to pay increases. Figure 17 provides confidence ellipses illustrating the degrees of dispersion not directly apparent from the scatter of bootstrap replicates.
FIGURE 16.
Cost-effectiveness plane (controlling for baseline utility).

FIGURE 17.
Confidence ellipse controlling for baseline utility.
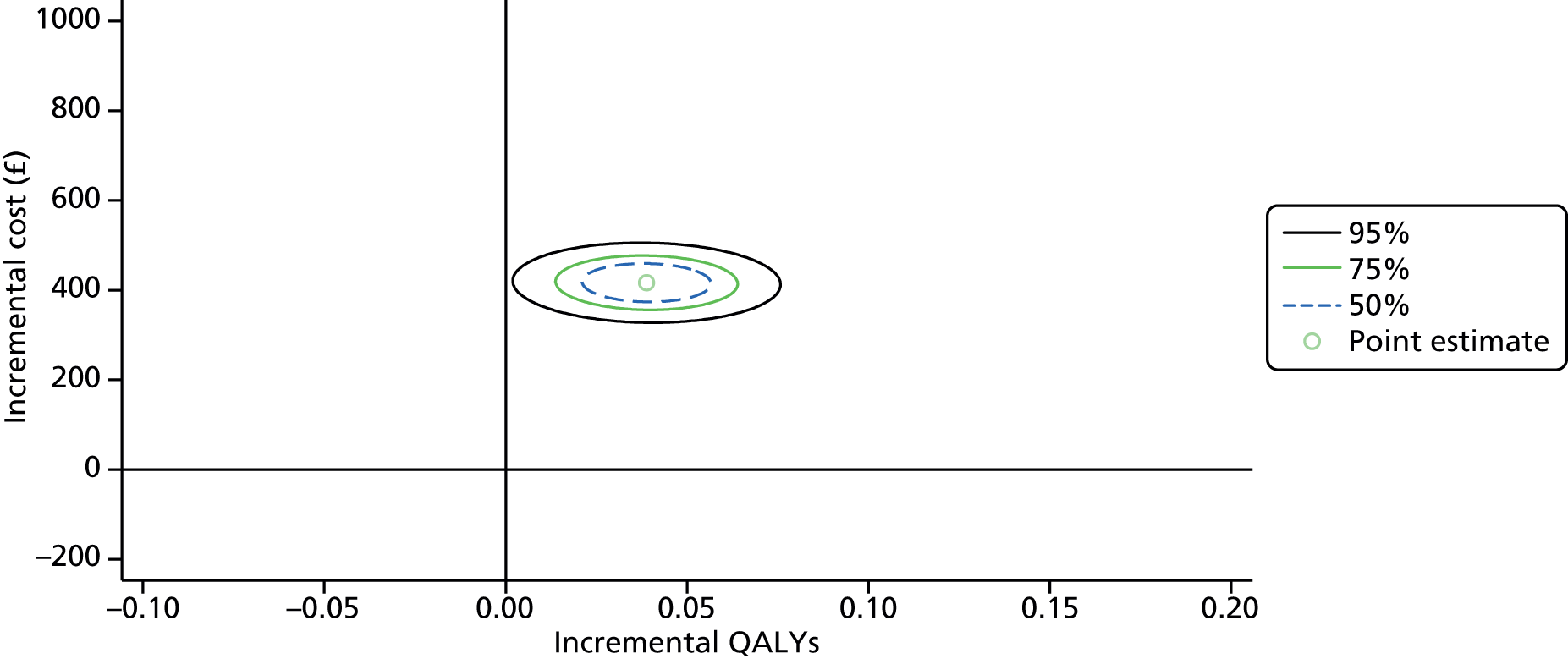
To explore the probability that the ICER for collaborative care falls below the stated willingness-to-pay threshold, Figure 18 presents the CEAC. This suggests that the probability that the ICER for collaborative care is < £20,000 per QALY is 0.9239 and < £30,000 per QALY is 0.9735.
FIGURE 18.
Cost-effectiveness acceptability curve for cost per QALY analysis.
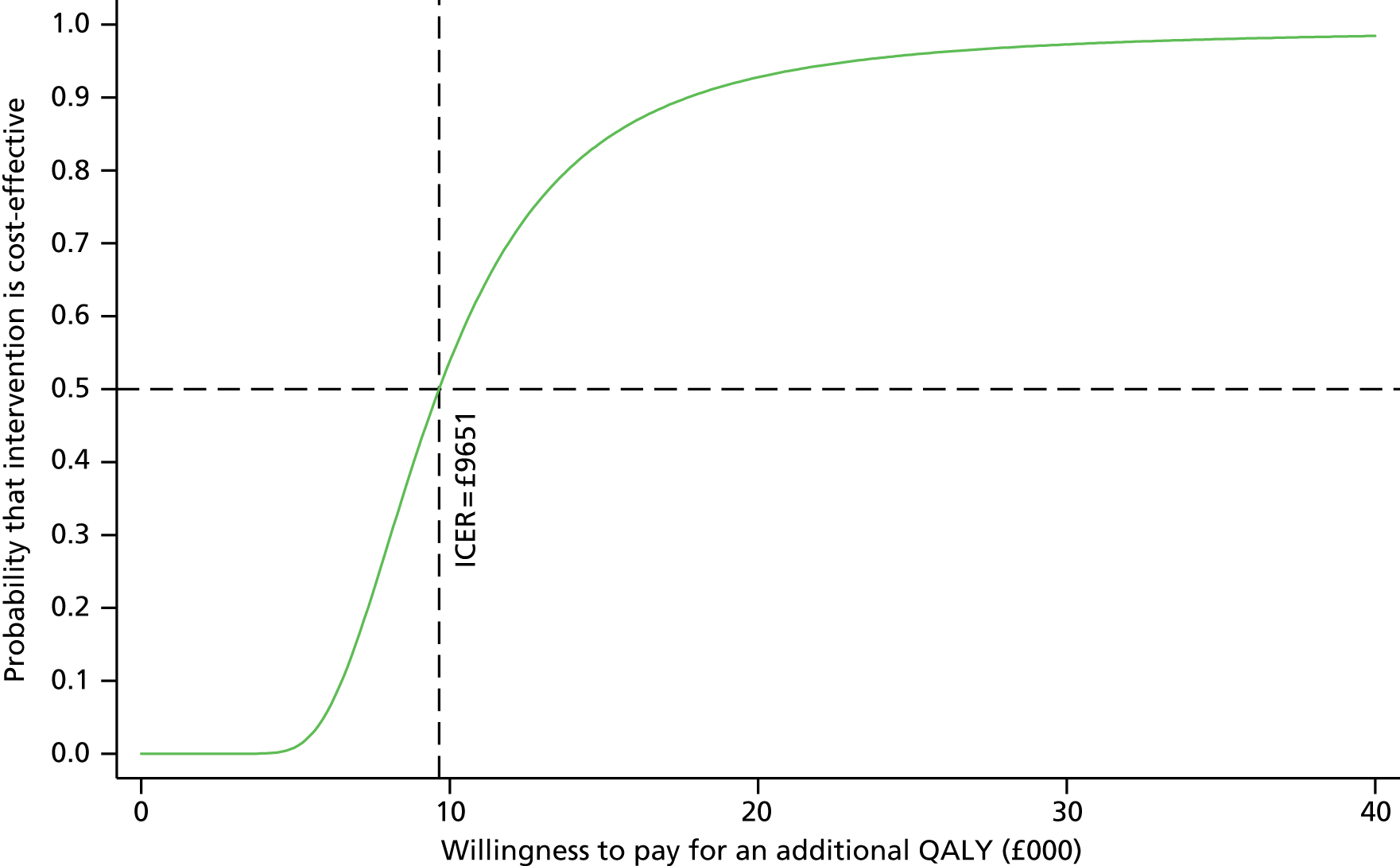
Sensitivity analysis
Ex post adjustments of the cost of collaborative care
The base-case analysis of the cost of collaborative care assumed that all participants received the full course of treatment (i.e. 8–10 sessions) whereas for usual care it was assumed that participants received no extra services beyond contacts within primary care. To provide a more realistic insight into how collaborative care might be implemented in real-world NHS settings and how demand varies with regard to individuals’ specific levels of need, all case managers were asked to log their activities with patients on PC-MIS, which has been designed for IAPT.
The number and duration of participant contacts with their case manager were contemporaneously logged on PC-MIS. It was noted whether these contacts occurred face to face or by telephone. Figure 19 summarises the distribution in the number of contacts. The total number of registered sessions was highly variable, with a modal number of eight sessions. Furthermore, a bimodal distribution seems apparent, suggesting that there may exist a certain subgroup of participants who were more likely to withdraw during the early stage of care. Information on referrals to IAPT services was not available within the control group.
FIGURE 19.
Number of sessions of collaborative care.
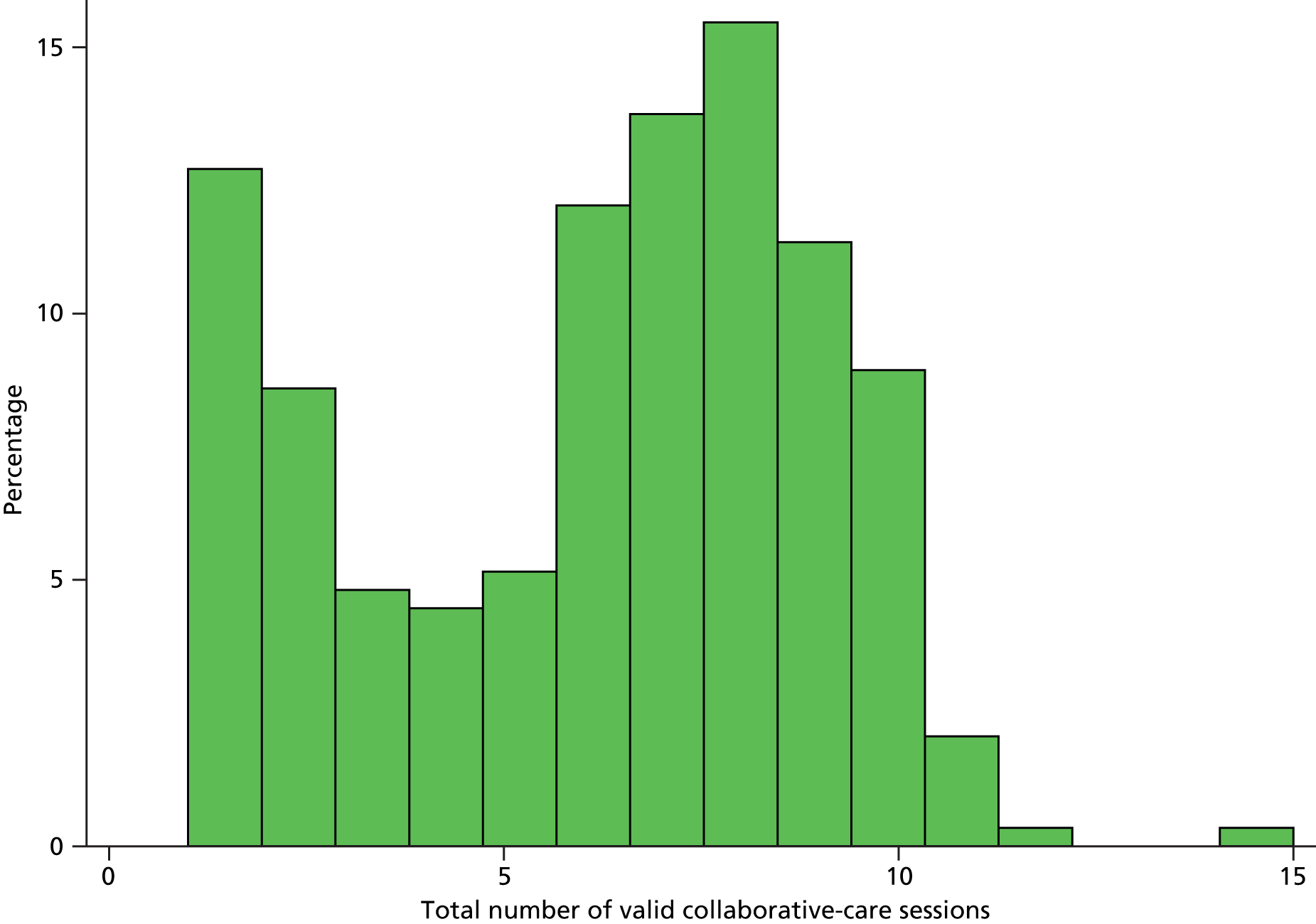
Accounting for variation in the numbers of contacts, the duration of contacts and how the contacts took place (i.e. face to face or by telephone), Table 51 provides adjusted ex post costs of collaborative care.
| Session | Type of contact (%) | Mean duration (minutes) | Mean cost (£) | Poisson exact (95% CI) (£) | n | |
|---|---|---|---|---|---|---|
| Face to face | Telephone | |||||
| 1 | 97.59 | 2.41 | 63 | 76.25 | 75.02 to 77.50 | 190 |
| 2 | 15.35 | 84.65 | 31 | 25.54 | 24.83 to 26.28 | 188 |
| 3 | 13.1 | 86.9 | 29 | 22.95 | 22.27 to 23.64 | 190 |
| 4 | 14.88 | 85.12 | 29 | 21.98 | 21.32 to 22.66 | 189 |
| 5 | 15.84 | 84.16 | 29 | 21.13 | 20.48 to 21.79 | 190 |
| 6 | 16.04 | 83.96 | 29 | 19.63 | 19.00 to 20.27 | 188 |
| 7a | 11.84 | 87.5 | 28 | 15.82 | 15.26 to 16.40 | 190 |
| 8 | 9.82 | 90.18 | 28 | 11.60 | 11.12 to 12.09 | 189 |
| 9 | 11.94 | 88.06 | 28 | 6.74 | 6.38 to 7.12 | 189 |
| 10 | 20.59 | 79.41 | 29 | 3.29 | 3.04 to 3.56 | 190 |
| 11 | 37.5 | 62.5 | 35 | 0.74 | 0.62 to 0.87 | 190 |
| Total cost | 223.70 | 221.55 to 225.87 | 184 | |||
Examining real-time resource use for collaborative care as delivered in the context of this trial demonstrated an average cost of £223.70 (95% CI £221.55 to £225.87).
The cost-effectiveness analysis was repeated to account for the costs implied by the PC-MIS data and to summarise the findings. Figure 20 reproduces the CEAC, adjusting for the ex post costs of collaborative care. The updated ICER of £3328 per QALY suggests that collaborative care requires fewer resources than expected and may prove significantly more cost-effective if implemented.
FIGURE 20.
Cost-effectiveness acceptability curve using ex post costs of collaborative care.
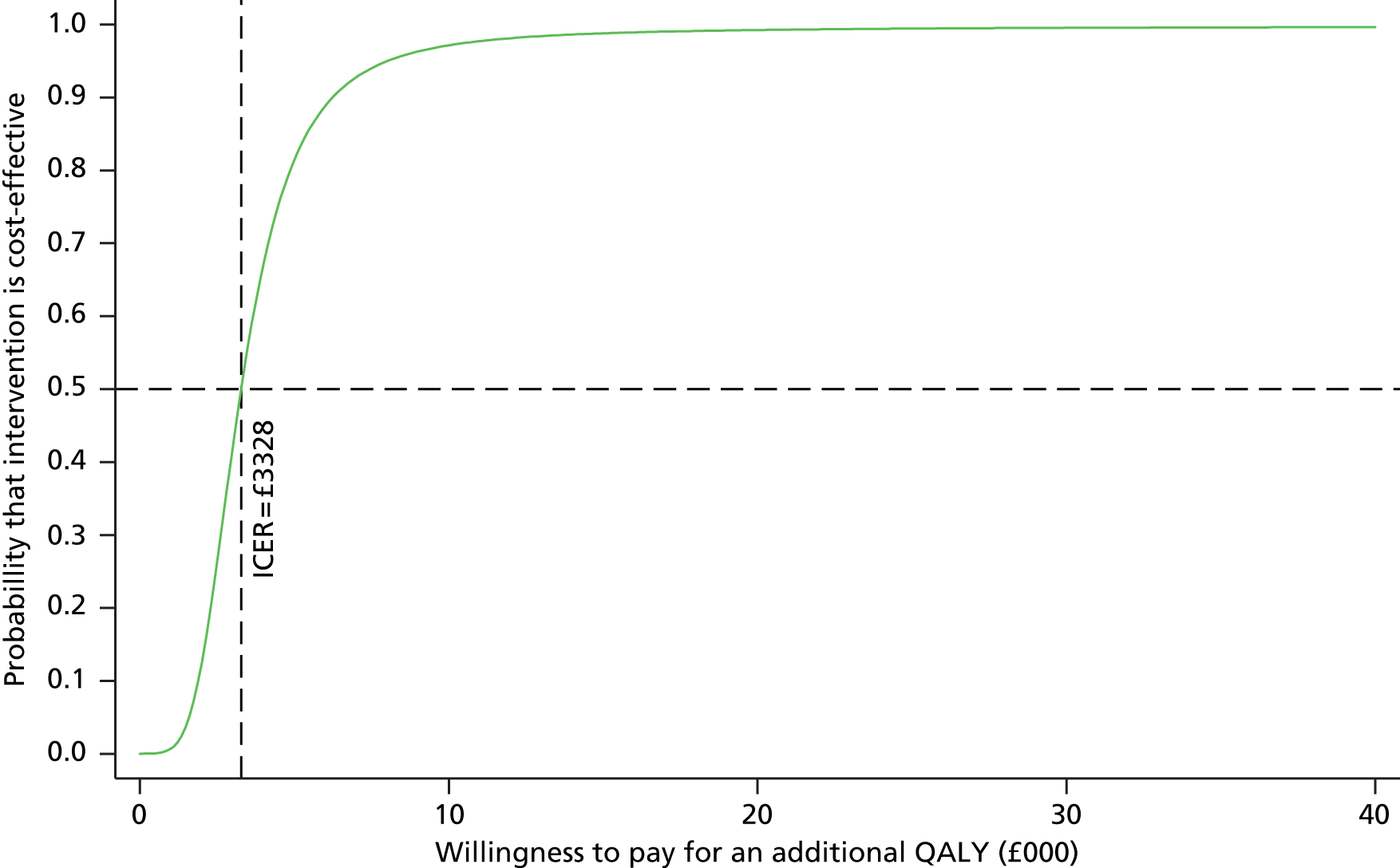
This sensitivity analysis incorporates information that would be known only once a programme had been implemented but demonstrates that, should collaborative care be recommended by the NHS, the programme has the scope to become efficient through adjusting the resources required within any given context. However, the reason why participants’ service needs varied has so far not been established and requires further investigation.
Summary of within-trial cost-effectiveness findings
-
Providing collaborative care was estimated to cost £494.73 more per participant than usual care. This estimate accounts for the costs of training case managers, their expected rate of patient contacts (following a manual) and the cost of a standardised agenda case manager.
-
On average, participants allocated to collaborative care displayed significantly higher QALYs than those allocated to the control group (annual difference in adjusted QALYs of 0.044, 95% bias-corrected CI 0.015 to 0.072; p = 0.003).
-
The base-case cost-effectiveness analysis found that the ICER was £9633 per QALY. Accounting for uncertainty in the ICER on a CEAC suggests that the probability that the ICER for collaborative care is < £20,000 per QALY is 0.9239 and the probability that the ICER for collaborative care is < £30,000 per QALY is 0.9735.
-
The record of case managers’ contacts with participants exhibits a bimodal distribution, which suggests that, in certain situations, participants discontinue service shortly after their initial contact. Accounting for the true observed contact rate, sensitivity analysis suggests that the mean direct cost of collaborative care is £223.70 (95% CI £210.98 to £236.42) and the associated ICER is £3328 per QALY.
Chapter 7 Qualitative findings
Aims
The concurrent qualitative study explores the experience of receiving and delivering the collaborative-care intervention. In particular, it considers the barriers to and facilitators of intervention delivery and the perceived utility of the intervention, from the perspective of both service users and service providers. An in-depth appreciation of these issues is essential for the future successful implementation of collaborative care in this population group.
Following the findings from the main CASPER RCT (see Chapter 5), which indicate that the collaborative-care intervention was effective in improving depression symptomatology and reducing the proportion of people with case-level depression in the medium term, but also that the intervention was associated with an observed attrition rate of 28%, we present the qualitative data to provide insights into:
-
possible reasons for withdrawal from the intervention
-
aspects of the intervention that the participants found useful
-
lessons that could be learned in terms of delivery of the intervention to be considered during implementation.
Methods
Design
A semistructured interview study was used to gather in-depth information on the trial participants’ experiences of receiving the collaborative-care intervention alongside the case managers’ experiences of delivering the intervention. Interviews were conducted with participants in the trial (at the end of the intervention period) and case managers delivering the intervention during the pilot phase of the RCT.
Sampling
The sampling followed a pragmatic and purposive strategy. Initially, because of slow recruitment into the pilot phase of the trial, all eligible trial participants were approached for participation in the qualitative study. The second half of the sample was recruited by using a purposive sampling strategy to gain a varied sample of pilot trial participants representing the wider population of older people in terms of age, sex, education, mental health scores, trial arm and those who had withdrawn from the intervention. This was with the aim of achieving maximum variation59 to represent the views of the whole range of individuals who could potentially be offered the intervention, rather than to consider any similarities or differences between any subgroups of the sample by broad characteristics (e.g. age and sex). Previous studies have indicated that a sample of approximately 40 trial participants would be sufficient to address these aims from the point of view of service users. 60 All case managers working in York and Leeds at the time of the pilot phase were recruited for interview by invitation through the local trial co-ordinators.
Sample
For the participant interviews, 39 older people were recruited during the pilot phase of the trial (Table 52). Of the 39 interviewees, five were in the usual-care group and another five had been selected for interview specifically because they had withdrawn from the intervention but had consented to remain in the trial. These interviews were to provide information on participants’ motivations for participation and reasons for withdrawal.
| Sex | Age (years) | ||||
|---|---|---|---|---|---|
| 75–79 | 80–84 | 85–89 | 90+ | All | |
| Female | 9 | 8 | 6 | 1 | 24 |
| Male | 10 | 2 | 2 | 1 | 15 |
| All | 19 | 10 | 8 | 2 | 39 |
Case managers in York were based at the university whereas case managers in Leeds were based in the community mental health team. All 18 case managers were female and aged between 27 and 50 years. All case managers had been trained as NHS PWPs as part of the IAPT initiative. They each had several years’ experience of delivering low-intensity psychological interventions. In addition, two of the case managers had been involved in training PWPs. They were also involved in training case managers for the CASPER trial and in their supervision.
Consent
All CASPER trial participants were eligible for participation in the qualitative study. Following sampling, study participants were approached by letter, which contained an information sheet and two consent forms. In accordance with ethical guidelines (see Chapter 3, Approvals obtained), informed consent was gained by the researcher before the commencement of the interview and after the aim of the interview was explained and the participant had an opportunity to ask questions about the study. Anonymity and confidentiality of participants’ personal information were assured by the researcher.
Case managers were invited to take part in the interviews. They received an invitation, information sheet and consent forms in the post. This was followed up by a telephone call. Case managers were given an opportunity to ask questions about the study and were assured anonymity and confidentiality. Similar to the process for trial participants, consent was subsequently obtained at the time of interview.
Data collection
The semistructured interviews with participants were carried out in the home between January and September 2012 and on average lasted for 90–120 minutes; they were conducted using a topic guide (see Appendix 10). All interviews were audio recorded, transcribed and anonymised before data analysis.
The semistructured interviews with case managers were carried out in 2011 and 2012 in a private room on university premises (York) or on premises where the community mental health team was based (Leeds). The interviews lasted between 60 and 90 minutes and were conducted using a topic guide (see Appendix 9).
Data analysis
Initial thematic analysis61 was carried out using the qualitative data analysis software package ATLAS.ti 6 (Scientific Software Development GmbH, Berlin). 62 An initial coding framework was developed based on a priori themes relating to issues included in the topic guide while allowing for emergent themes. Descriptive coding was conducted, following familiarisation with the data, by the main qualitative researcher on the project (FZ), informed by regular discussion with the qualitative supervisors (KS and JA). Subsequently, initial codes were refined to address the aims of the qualitative study outlined earlier, following the analysis of the main trial. A constant comparison method63 was used to check and compare across the data set and to establish appropriate analytical categories. This also ensured that any additional categories were added to reflect as many of the nuances or outlier views in the data as possible, taking into consideration the participants’ wider contexts. Anonymised participant IDs are used for the reporting of results; those starting ‘CM’ indicate a quote from a case manager.
Our method of analysis was particularly suited to the project as specific questions were being addressed through the qualitative data. The main researcher (FZ) was immersed in the data by listening to the recordings and reading transcripts to list key ideas and recurrent themes. Memos were used during the initial stages of analysis to provide a visible ‘audit trail’ as the analysis moved from ‘raw’ data to the production of findings. Although the main qualitative researcher was responsible for the descriptive coding of the data, analysis was discussed with the wider qualitative team (KS, JA and PK).
To promote quality, the following strategies were used: description of the participants to provide context (credibility and transferability), transparency of the research process (transferability), evidence of consistency using multiple examples from data (dependability) and engagement of the wider research team with interim findings (confirmability). 64 In addition, a reflexive approach was taken to data analysis, with the main interviewer (FZ) being an academic research fellow from a nursing background with research experience in the topic of ageing but with no previous knowledge or experience of collaborative care or RCTs. The other members of the qualitative team (KS, PK and JA) all have academic research backgrounds (KS also has a nursing background) with no previous knowledge or experience of collaborative care. This placed the qualitative research team in a very neutral position with regard to any prior expectations relating to the study intervention.
Findings
Lack of engagement with the intervention
The perceived utility of the intervention, in terms of mental well-being, was directly related to the level of engagement with the intervention. This was observed for the patients themselves and articulated by the case managers:
I suppose it depends really how much they want to sort of engage with it. You know, that’s, that’s the thing that I’ve noticed. I mean if somebody, if, if you’ve got a participant that really wasn’t, you know, motivated and they didn’t really sort of know what else they could do, I could see that, you know, helping them, you know, with suggestions and things would be helpful, but then, there again it’s, it’s how much they want to engage in doing these things, isn’t it. You know, each individual would be different.
CMQ2
A significant proportion of participants felt that they could not relate to the intervention being tested. There were a number of possible reasons for this, as discussed in the following sections.
Lack of symptoms
Participants rejected the ‘diagnosis’ of low mood/subclinical depression:
I just lost interest [in the intervention] . . . Now I, I don’t, as I say, I told the other lady that, err, I wasn’t interested anymore and that’s about it. I’ve just lost interest now . . . And, erm, I don’t want to keep being reminded that I’m, err, depressed and things like that when I’m not! It just, it just gets up my back a bit. That’s the sort of little thing that makes me fly off the handle!
Q11657
There remains a stigma attached to mental ill health among this population; participants demonstrated learned attitudes and behaviours that admonish depressed people to ‘just get on with it’:
Withdraw after the first session, yeah. Err, had no idea why I was here to see him, agreed to the appointment [laughs], spent about one and a half hours with him! [laughs] And then he decided he wanted to withdraw. And, erm, yeah he really struggled to understand the idea, couldn’t understand how anybody, why somebody would become depressed. Couldn’t understand xxxx’s example AT ALL! ‘Well he needs to get on and do something! I can’t understand why people would become depressed!’ [laughs]. ‘As long as I have my family I’m fine!’, and all this stuff, so yeah, he was, I think he was quite annoyed that I’d come to see him! [laughs].
CMQ8
Participants had an active life
Given that a significant component of the intervention related to the older person’s activities, those who deemed themselves to have an active life found it difficult to understand the relevance of the content of the intervention:
I mean some of the . . . lady, this lady, she was like, you know, erm, . . . ’I can’t, you’d be better off doing this with somebody who, who wasn’t active and I don’t see how I can help to answer this question because I am so active, so nothing’s gonna change and’, and like it’s hard for people to understand that they’ve kind of ticked these boxes so they’re appropriate, but they can’t connect with that. That is a, that’s been the major problem for me.
CMQ7
Difficulty with intervention tasks
Others, particularly those participants with low levels of education, described feeling uncomfortable with a writing task and felt unable to express their feelings in this way. Keeping a detailed diary of activities and scoring related ‘moods’ was sufficiently difficult for some to give this as a reason for withdrawal:
So when you did [the diary] at the time, what did you think about it? I mean did you enjoy doing it?
Well no, I never enjoy writing ‘cos I have such a job to understand, I struggle with words.
Oh, OK.
Thinking of what to put and thinking of how to spell it.
. . . and then I’ve had some people like, just . . . put so much effort and time into it, and really reflected and I’ve been astounded! But we’re all different aren’t we? We’re all different and we, you know, it’s like I told you they’re very well educated people, generally, so their engagement has been different. I’ve had . . . academics and their engagement has been very intense, I’ve had business men and their engagement has been a bit more flippant. . . . Yeah, they’ve, it depends on how, I mean that, that’s just them as, as individuals isn’t it? You know, how they tackle everything in their lives.
CMQ2
For some people, the difficulty lay in opening up to someone about potential problems or feelings, linked to the stigma of doing so:
Lets go back to talking about your participation in CASPER. So you said earlier, when I came in, that you were very reluctant at first [to participate in the intervention]?
Because of my reluctance to admit to anyone else how I feel, because I thought it made me less of a man to, to have these problems [depression]. I didn’t when, err, when I got the sort of the first documentation saying, you know, I thought, ‘Mmm, well I may be useful in my old age! I might as well’. Erm, and I would be interested just to see what the study was about. But it was when you have to start admitting what you see as weaknesses and failings, it, it’s not so easy to do that.
Phew, I think with some people they’re scared, they’re scared to . . . open up. They don’t open up, maybe they’re very private people.
Q11206
The intervention required participants to write down their activities and reflections and some found this task difficult because of physical problems, for example restricted eyesight or hand problems:
No, I don’t love, no. ‘Cos, you know, I have to write . . . heavy, yeah, yeah. I can’t see. I couldn’t, I couldn’t, err, . . . write small things, you know. Like when they say, you know, ‘Don’t let it go out of this box’, I, I can’t. I have to write large letters and, and, and, and, and . . . you know, really heavy, yeah. And I’m heavy handed.
Q11227M
For others the tasks were simply too demanding and they did not have the energy to complete them because of physical ill health:
. . . and then perhaps a couple of people who have been more physically unwell and that’s affected engagement, just through literally not having the energy, having a lot of hospital appointments, with one particular guy being in hospital.
CMQ7
Raised awareness
Many of the respondents highlighted that one of the consequences of the intervention was to raise awareness of how they were coping and/or feeling about their lives. This process of self-analysis and reflection had different consequences for different participants (which will be discussed further in Positive aspects of the intervention). For example, one participant who was interviewed withdrew from the intervention because she felt that the task of self-analysis of moods damaged her own coping strategy. She felt that keeping a diary and scoring her moods made her aware that she was less happy than she thought she was and therefore felt the intervention to be damaging:
Because . . . instead of just doing things because you want to do them and because you feel you ought to do them, erm, I, I, I have to analyse everything that I did in this diary thing, and I kept thinking, you know, ‘Was I happy doing that or wasn’t I?’, and, and I started off by being quite positive and then I gradually thought, ‘No I’m not happy, no, no, I’m not. I’m not nearly as happy as I thought I was’. I was kidding myself that I was much happier than I was. And that didn’t help me, because even if I’m, my way of coping is to kid myself that I’m happy, as long as it works it doesn’t matter how you cope does it? So that was when I started questioning whether this analysing myself the whole time was a good idea. And I decided it wasn’t.
Q10243F
However, in contrast, for some participants the raised awareness and reappraisal led them to conclude that they were quite happy and content with their own lives and that they did not actually need any help to become more active. As a result of this positive appraisal some participants withdrew from the intervention as they felt that they did not need it:
Yes [CM], yes [CM]. Erm, . . . she . . ., whether it was her intention or not I don’t know, . . . but she was seen to be working on, wanting to get me, get me up and going, moving around. Well, as I say, I’m quite happy in me own little pig sty . . . I’m quite happy. As I say, . . . if I feel like dusting I’ll dust. If I feel like putting the vacuum cleaner on I’ll do it. I don’t have to have a, a cause or a reason. If I feel like doing it I’ll do it, if I don’t, well hard luck!
Q11300M
Pragmatic intervention delivery
For some, it was issues with the practical delivery of the intervention that caused problems with engagement. Although in many cases the intervention was delivered over the intended period of 8–10 weekly sessions, there were a few participants for whom those weekly sessions had been disrupted for various reasons (e.g. case manager illness). This meant that there were often longer than anticipated gaps between sessions, resulting in some participants disengaging from the intervention. The data suggest that even when participants continued their sessions the distractions and gaps affected their ability to successfully engage with the intervention:
Yeah, [I don’t know] whether, whether, whether I can go on with it. . . . How much longer is it?
Err, how many session have you had? Do you know? Have you had about four or five?
Well you came, then she came here, and, and I’ve had two telephone conversations. Because, yes, because she was, she was off, she was ill and then, erm, . . . this time something happened . . . could have been me, I had to ring up because I was doing something I didn’t expect to do, yes, and then she, she’s ringing tomorrow actually, because she was off for a few days, wasn’t she or something? So the, tomorrow will be the third of her [calls]. . . . I’m not sure that it’s a good idea to go on, I don’t know. Unless it can, unless it’s any help. But I think I’ve said an awful lot already! [laughs] I don’t know if I’ve got a lot more to say really.
Some case managers were concerned that a lack of engagement with the intervention was related to their own lack of gravitas with the client group, which they often linked to a disparity in age:
So I think sometimes maybe they think I’m from the university, ‘cos they see a young person, they hear the word university, they think you’re from the university so
Mmm. Do you explain to people? What your position is?
Yeah, yeah, yeah, I think that . . . that helps. Erm, And like with one lady, erm, who actually was my . . . I think most successful in terms of the feedback she gave, that she benefited, I remember at the home visit she was saying, ‘Oh yes, I’ve been referred to your service before and I saw a young girl and I just thought, you know, you’re not going to be able to help me’, and then I was thinking, ‘Oh is that, you know, are you going to have that same opinion of me?’, and she, ‘cos she did make reference to my age. ‘You are so young’, and . . ., erm
Mmm. Do you think that that’s an issue, working with older people, the age?
Possibly, possibly. They might have a . . . a view of . . ., they might, I mean I think it . . . maybe not even with older people but with anybody like sometimes people need an authority figure to tell, you could, I could tell them exactly the same thing as, you know, the psychiatrist, but because it’s kind of coming from somebody else, the psychiatrist could tell somebody about relaxation, I could tell them about relaxation but they’ll take it far more seriously from a psychiatrist. So sometimes I think age can get in the way with how much people will, are willing to kind of take the message seriously. Erm, but I think that’s the same with . . . old and, erm [young people].
So you didn’t think, I think the age gap might have, might have been an issue, do you think?
Yeah, I think it definitely was. Well the ones that er, the ones that completed, no, but the ones that didn’t, I think it probably was a factor, for me anyway.
One or more of the five issues identified here led some participants to withdraw from the intervention part-way through (or indeed never start). On the other hand, there was a subgroup of participants for whom some of these issues were equally relevant yet who went on to complete the intervention, but with limited capacity to benefit. They appeared to have motivations for completion other than the perception that this was likely to impact on their mental health:
I mean obviously sometimes there might be things that are happening that, like [the supervisor], she says, ‘You don’t know what they might be getting out of it’, and . . . but I’m, I think for a couple of people at least they seem to be so closed minded to it that I literally don’t think they’re getting anything. She [the supervisor] says, ‘Well he keeps doing it . . . so he must be getting something out of it’, but maybe sometimes just people like to see things through. They start something so they finish it, you know.
CMQ7
Case managers expressed difficulties with attempting to deliver the intervention when such engagement was lacking:
I’ve really enjoyed meeting the, the, err, the older people and going to their homes, I like doing the home visits and they’ve been lovely. But it’s just sometimes when, once you get into it you’re thinking, ‘Well . . . they . . . they’re kind of doing it for the wrong reasons’, and that’s impeding their engagement. That’s just the only problem.
So do you find, did you find it quite hard work to keep people engaged?
You keep, keep on having to have the same conversation, ‘I don’t, why I’m not sure why I’m doing this and how this is helping’, and then you think, yeah you just try and give them the same . . ., it’s more or less the same answers and . . . like you know, this helping the university to answer this question and . . . but . . . if they don’t see themselves as having subthreshold depression how can they see . . . themselves as helping to answer the question of . . .
These participants willingly and diligently carried out the various tasks involved in the intervention without always really understanding clearly the intended purpose of those tasks, but reported other benefits.
Did you, did you find you learned anything from taking part in this? Anything new?
Well no, no, err, . . . I enjoyed taking part in it.
What did you enjoy?
Well, err, what do you call her [case manager]? She, she more or less asked me the questions and I, I answered ‘em like, but, err, I aren’t one for, for a lot of theory. You know. Like pen pusher types. Err, if I can answer it by word of mouth it makes it easier for me. So, but yes, . . ., if I, if I could answer the question I would do, like, you know.
Several participants mentioned having particularly enjoyed talking to the case manager and having the case manager’s company for a time. They also reported that participation in the study gave them ‘an interest’ or ‘something to do’ for a few weeks. Participation in the trial for this group is thus seen mainly as an interesting activity that relieves the routine, loneliness or boredom of everyday life without any expectation of a personal benefit in terms of mental well-being:
I just wanted to ask you whether you remember any of the work that you did with [case manager]?
Erm, . . . no not really, you know I’ve sort of, that has gone from my, my mind. Erm, all I can really remember is that she is a CHARMING girl. And, and I just loved her coming.
Is there anything else you enjoyed about taking part?
Oh yeah, mmm, mmm, oh it didn’t bother me, it’s something to do. I mean I’m on me own, so I’ve got company else, that’s the way I look at it, like, so, but, err, this is it.
Because some participants engaged in the research as ‘something to do’, some case managers found it difficult to keep them focused on the tasks required by the intervention:
Yeah, I think it was more having someone to talk to. Cos quite a lot of mine [participants] were quite lonely and isolated. So you’d find it quite difficult to get them off the phone sometime. Or like not engage with just general conversation with them, which I don’t mind doing, but [laughs] you know, it’s quite, quite difficult to just be like ‘Right, let’s move on’.
CMQ10
Although these participants gained satisfaction from having taken part in the intervention, and enjoyed talking to an outsider (the case manager), they failed to see the experience as being aimed at helping them; rather, they were hoping to help other people:
But I enjoyed doing it. I mean it’s something I never thought I would do.
Is there anything else you found helpful apart from that? . . . Or anything else you got out of it for yourself?
It helped discussing your, what was wrong with you, you know, from, to an outsider. And . . . like personal things as well, maybe that you wouldn’t tell anybody else, you know . . . Because you stop and think, ‘Well you’re telling what’s happened in your life’, and you don’t particularly like [that] . . . But . . . like I say, no more things than other people do but, err, . . . maybe ease, it may ease your mind that you’ve talked to somebody about things that have maybe been at the back of your head for a while.
Mmm . . . but it also makes you feel uncomfortable, that you’ve talked about these things?
Yeah, maybe . . . But I suppose it’s got to be done, hasn’t it really? So that you, it helps other people maybe in years to come like, you know.
As we have seen, for some there was little engagement with the reason for the intervention and/or the intervention tasks. These participants either withdrew or ‘went through the motions’ of completing the study for a number of possible reasons, as outlined above. For these participants, although they might have gained some benefits from taking part in the intervention, they did not perceive that this process would impact on their mental health. However, this experience was not universal; others who were able to see the relevance of the intervention indicated a great capacity for benefit and this will be discussed in the following section.
Positive aspects of the intervention
Many participants had really positive experiences of the intervention and could see direct benefits to their mental well-being. According to individual participants, these came in several forms. As a case manager commented:
I think for some people it’s been an eye-opener, and for some it’s been a confirmation, that what they’re doing is right, and that they’re OK. And some have been like, you know, a light bulb has turned on.
CMQ2
Raised awareness
As discussed earlier, many of the respondents highlighted that one of the consequences of the intervention was to raise awareness of how they were coping and/or feeling about their lives. In some cases, this had genuinely positive effects on the participants concerned:
‘Cos I suppose that the ideas are very kind of common sense but perhaps it’s this going through a more kind of formal process of like analysing what you do, how you feel, how they relate to each other, erm, and then sort of . . ., yeah writing things down, I guess people say that that’s helpful to . . . reflect and then think about what they might like to change, little things. Yeah. So it gets people to, yeah, it gets people to think. Erm, and yeah, just make little alterations and . . . yeah.
CMQ7
For many of these participants, this raised awareness was accompanied by a positive reappraisal of their own lives:
Well, erm, I didn’t realise before I started to fill those [diaries] in, how much I did. I really thought, ‘Oh no I don’t seem to do much. I do spend time in the house but it’s mainly evenings’, and I didn’t realise until I wrote down the things that I did, that I am more active than I thought.
Q10628
Several participants spoke about how the intervention had made them feel that they were more fortunate than others, in spite of their own often difficult lives and poor health. This positive social comparison led participants to a new appraisal of their own lives:
And do you think, do you feel you’ve got anything out of taking part in this?
Yes I do. I think it makes you look back and you realise, well you’re not quite so badly off as some people, and when you hear people complain and groan in the morning about things, and you think, ‘I don’t have that, I don’t have that problem’, you know . . . There’s always people worse off than yourself, wherever you look!
Because of being more aware of their situation, some participants reported that they were now more able to identify the problems to prevent or to address feeling low:
Do you think it will have a lasting effect, a lasting impact? . . . Do you think?
Yes I think it will because I think it has made me think and I think it’s made me aware of where my problems lie, and that I’ve got to . . . a, be a little bit more sort of . . . independent, you know, not sort of . . . not look upon the times when I see my family as the most pleasurable, the only really pleasurable things I do.
[Reading] ‘How will I spot symptoms of low mood?’ Watching, wanting to withdraw, yes, feeling sorry for myself, weeping, indecision. That’s a big thing that I notice . . . when I become REALLY, I can be indecisive but when I become REALLY indecisive and can’t make up my mind about things; I don’t know what I want to do, you know, all that sort of thing. Or losing concentration. I can’t read a book. Not interested in anything. Don’t want to watch television. It’s all awful! That sort of thing. It’s triggers, I know that they’re signs that . . . But I, it’s something that I . . . must, I, I think it’s just writing it down that you become aware that you, you know, it is something that you, you know.
Q10513F
The intervention created a ‘valid’ space in which to spend time reflecting, which might otherwise be regarded as self-indulgent:
Yeah I thought, well I, I think maybe I have when I think about it and read through it I think, yeah, I think, mmm, you know, like, you think about yourself, I mean I don’t think I ever would have thought, ‘Well, you know, sit down yourself and just think what I, you know, what you’re doin’ and, and just try and rest a bit’, or ‘do something for yourself’, and, and, erm, little things. When I read through it all I think, you know, think about it, and just writing down helps ya to, to you know, do things. It’s like I said to me sister-in-law, if you feel, you know, sometimes if you just write it down you’re getting it off your chest.
Q10075F
A safe sounding board
Many respondents referred to the intervention having allowed them to talk openly about very personal issues in a way that they were not able to with friends or family members and that those delivering the intervention had managed to achieve the necessary trust:
I think I, I think I’ve said in the past that I’ve found both of you very easy to converse with. And, and, you know, if you’re at ease with someone it’s easier to bring out your inner thoughts, you know. There are things that, err, . . . I won’t say are secrets ‘cos that would be too strong, but you know, . . . it isn’t always easy to reveal exactly what you’re thinking about things, erm, with someone that you don’t know or you’ve never met.
That’s right. You have to feel comfortable don’t you.
Yes, yes I think so and I don’t know whether you’ve had that feeling, but ever since we met and, and [case manager] and I as well, I’ve felt I’ve been able to discuss things with you without fear of embarrassment.
Providing tools for self-help
For some participants, the intervention had provided them with the necessary tools to work out the best solution for themselves:
Well yeah, some people have, have kind of from the discussions, you know, I think . . . the people who’ve engaged with it, it’s not like I’ve had to suggest things. They, you know, have just, from having, it’s just triggered stuff, like made them think and they come up with their own solutions and their own, a lot, I mean I try and contribute to that as well, but . . . I haven’t sort of felt like I’ve had to . . ., you know, erm, yeah I’ve felt like a lot of it has come from them.
CMQ7
Although for most interviewees their low mood had been brought on by specific events in the recent past, one participant had been suffering from depression for many years and had received medication for it intermittently. He was surprised to have benefited from this psychological intervention, as he had been very sceptical initially. The intervention had given him the tools to be able to cope with the situation differently:
Yes, so do you feel that . . . this [intervention] has given you the tools to, to keep it going?
What I think it’s done is, I mean what I find is that I probably had the tools I just wasn’t aware, and I, and what this has done it’s, well it’s done something that has made me able to think and I hope that thinking process won’t be too . . . badly influenced if I come off the paroxetine. I don’t think it will, because I think it’s saying to me, ‘It doesn’t matter how you feel, this is what you have got to do’. And I think that’s an important thing. As I said, we’ll just have to wait and see whether it does . . . Well I’m, I am genuinely surprised. That’s why, that’s why I’ve been so cheerful about it. I just didn’t expect it! I didn’t. So it shows, you’re never too old to learn!
Beyond an increasing awareness of their present and future well-being, several participants translated into action the learning from their reflections and suggestions made by the case managers. This was often the result of a particular engagement with the idea of functional equivalence. In this trial the manual was adapted to emphasise this stage of the intervention because of its preventative purpose. These participants obviously found it useful to consider their future and how they might cope with the ageing process:
But I think it was very good later on in the thing [booklet] where it said, ‘What could you do to replace, erm, you know, various activities if you had to stop doing them’. And I think that was good, to help you think round things. Although in many cases there aren’t really any great solutions are there?
Ah ha, that’s it.
You know I mean, yes, if you could, if you can’t go out, well you can invite people to you and things like that, but, erm, . . . and if I didn’t have the car, for example, obviously if I couldn’t drive the car I wouldn’t find it easy to get on the bus either, so you’d have to have a taxi, and then you get back to the finances of things again don’t you. Erm, . . . but I found, the whole thing I did find quite . . . interesting, and it was a very, you know, good process of going, it was well structured wasn’t it, starting from the beginning.
Regaining control
Several participants reported having benefited from the intervention through successfully addressing their low mood or depression. Participants reported feeling empowered to take control of their lives and make positive changes, especially those who had lost their confidence after a bereavement:
So do you want to just tell me what you think you got out of this?
Oh I got, I got a, a, I got . . . oh I got a sort of almost a life saving sort of . . ., err, support out of it in a way. I mean it REALLY did keep me going, erm, this feeling of, err, . . . ’Alright, you’re not . . . in a little boat with a storm round you, you actually are rowing that boat and you can’, [you’re] in control, yeah. Err, I haven’t got the feeling of . . . confidence back . . . haven’t got a feeling of sort of light-hearted happiness back, but yeah, but then I mean . . . G and I were together for 60 years. We sort of grew up together. When he came out of the Navy he was 21 and I was just 21 and that’s a long time, to be you know, totally together and completely happy with each other. So you’ve got to grieve a long time haven’t you really?
Yes. When did your husband die?
Err, 3 years ago exactly. So you, you’ve got to give yourself time over it.
Intervention delivery
In this section we present the findings relating to aspects of intervention delivery and highlight any issues that would facilitate the implementation of the intervention on a larger scale.
Acceptability of the telephone intervention
The intervention was designed to begin with a case manager visit and assessment in the participant’s home. As a rule, all subsequent contacts between the case manager and the participant took place over the telephone at a prearranged date and time. However, there were some exceptions, with these contacts continuing face to face. This could be because the participant had expressed an explicit preference for face-to-face interactions or because of sensory impairments (particularly hearing impairment) that might impede communication over the telephone.
Participants and case managers felt that keeping the first meeting as face to face was essential to develop a rapport and to glean contextual information:
Yeah, yeah. I, I would say so. I mean I think it is really, you know, it is really important. Erm, erm, I would, I would always prefer to see somebody face to face first, just so they, you know, they know what you look like and, and you’ve been able to, erm, err, you know, to be able to build up that, erm, . . . that therapeutic relationship face to face first. Erm, . . . and then I think it is, it is easy, easier to, to, you know, maintain that and build on that, erm, through, through subsequent, erm, sessions. Erm, because I think with, with a lot of the people that, that I’ve seen, as I said, they, they’ve not got a, an issue or a problem with, with how they’re, they’re feeling. Erm, so a lot of them do, do see it as, erm, . . . not, not necessarily just a chat but it’s, it’s kind of, erm, it’s difficult to describe it really. I mean it’s not therapy. Yeah, it’s not therapy, that’s, that’s not what, what we’re doing.
CMQ3
Although telephone delivery was considered perfectly adequate, some participants voiced an assumption that face-to-face delivery would have a greater impact. However, many considered this as an indulgence in practical terms:
I was a bit apprehensive . . ., but it was fine, it was OK.
What were you apprehensive about?
Erm, . . . [coughs] Because I don’t like talking on the telephone all that much. Erm, because of sort of interviews where you try and sort something out like your telephone or your inter, internet or something, and people talk too fast and too quickly and wait, and you feel terribly slow, but she wasn’t like that at all. She was very clear and patient . . . and it was fine, and relaxed and yeah. I thought it would be odd talking over a telephone about yourself, but it was alright.
Ah ha. So would you have preferred having it face to face, all the sessions face to face?
Well I think, yes I mean one would say that, but it wouldn’t matter. It’s, erm, . . . it’s slightly self-indulgent having face-to-face [interactions] I think, perhaps . . .
Mmm, mmm. Because we’re try, that’s another thing we’re trying out here is whether, you know, delivering the intervention over the telephone, (a) is acceptable to people and (b) has it got, you know, the same benefits as, as doing it face to face?
No I don’t think it could have. I think face to face has got to be different because it’s, erm, a person. You’re a person looking at me, talking. Erm, and it’s got to have more impact hasn’t it? BUT if it’s not possible then a telephone [is alright].
Case managers too thought that telephone delivery was acceptable; however, they did mention a few practical difficulties with this, for example not being able to see the workbook, a lack of non-verbal cues and having to accommodate a variety of potential impairments experienced by the person in receipt of the intervention. Although the intervention was designed to be delivered over the telephone, this required case managers to be sensitive to the needs of individual participants and to recognise when telephone delivery was unsuitable. Older people may not necessarily request a face-to-face meeting as they may consider it an imposition on a busy professional:
Are there any examples where you had to continue seeing people face to face?
Erm, . . . I’ve got somebody at the moment I’m going to have to go and see face to face, a new participant, and I’ll have to see her face to face because, erm, she can’t manage the book, the participant pack and the phone. Erm, . . . and it causes her neck pain, so that’s fine. So it’s not always about hearing, it’s about, and also this person has got a physical disability . . ., erm, . . . so . . . that, you know, doesn’t help with the fact of having to sort of have the phone like this, and holding her arm up to have a phone. So for them, so there can be hearing problems but there can be physical problems, and you’ve got to remember that we’re asking them to look through a participant pack whilst being on the phone, which we all know is difficult to try and read something and be on the phone, and it’s that cognitive ability as well, the quickness of having somebody ask you a question and having to think without having the other non-verbal signs of somebody’s face, being able to read facial expressions and things.
Appropriate closure
Over the course of the intervention the developing client–practitioner relationship became, for some participants, an important support for coping with life. Several participants therefore spoke of how they missed the interaction with the case manager. One participant, for example, articulated this as a feeling of abandonment at the end of the intervention and she suggested a follow-up conversation:
You know you develop a bit of a relationship with the person that you’ve been seeing, and it’s all been very helpful and useful and then it suddenly stops . . . I think you might feel a bit abandoned at the end.
Did you [feel abandoned]?
You do feel a bit abandoned.
The quote illustrates the importance of case managers managing the final session of the intervention in such a way that participants are prepared for the end of this supportive relationship. Possibly not all case managers were aware of the extent to which participants may come to rely on the relationship with the case manager for their mental well-being:
‘Cos one of mine [participants] wanted to see me at the end as well, and I was like [thinking]: ‘Well we can, but [laughs] I’ve only met you once’, [laughs] you know.
CMQ10
Understanding of the study/intervention
Some of the narratives from the participants revealed that they did not always have a clear understanding of the purpose of the study/intervention:
Not really. Well, as I say, I, I used to feel sorry for [case manager] because she were asking all these questions and to me they didn’t seem to make sense of what, what you were trying to do. I couldn’t understand what you were trying to do.
Q11217
Some of the confusion came from being asked to participate in a RCT, which for some participants was difficult to disentangle from also being provided with an intervention that was supposed to assist their mental well-being. Of course, this issue is specific to the study and would not be problematic in normal clinical practice:
But it was only when I realised that this is going to actually affect me personally. I didn’t realise it was, it was actually a treatment plan. I thought it was just a, an information gatherer plan. And once you, once I realised it was a treatment plan you’re starting to be affected by what is asked of you.
Q10675
However, the confusion was not all related to the trial. Some case managers expressed difficulties in explaining the rationale behind the intervention and building a convincing case for a participant’s engagement; this was influential for those who expressed a concern that their participation was a waste of time:
In fact I used to think sometimes she’d [case manager] go away thinking: ‘Oh God, oh me head’ [laughs]. Is she [case manager] at university as well?
No, no, she’s a professional, she’s got training to do this kind of thing.
You need training to do that?
Yes.
Oh [laughs]. I can’t see why, why she, what she would have accomplished in long run then. I say, I can’t see what she would have accomplished in long run.
Case managers were aware of the difficulties in trying to explain quite a complex idea and felt that they were getting better with time:
So it’s difficult to know whether actually you probably could be maybe interacting with people on this level, it’s just the way that we’re discussing it doesn’t quite fit with how they would see it, you know . . . That, that’s probably rather than the intervention being irrelevant, it’s the selling of it. It’s the sort of getting people kind of on board with it really . . . And I think that is very much to do with, sort of, you know, it’s quite subtle the way that you word things and the way that you . . . and I’ve found throughout this time that that’s what I found myself doing automatically, is just changing the way I’m describing things a little bit.
CMXX
Discussion
Collaborative care was shown to be effective at an aggregate level within the clinical trial. The qualitative evaluation brings a greater level of context by showing that the level of engagement that an individual has with the collaborative-care intervention is central to his or her capacity to benefit from it. Engagement with the intervention appeared to be related to several factors. Primarily, the participants had to see the relevance of the intervention for themselves, in particular to be able to relate to the concept of subclinical depression. Those who did not see themselves as having a problem with their mental well-being were less likely to fully engage with the intervention and often withdrew or ‘went through the motions’.
In some cases the participants were not able to understand the nature or purpose of what was being asked of them – this was a complex study in which participants had to understand the concept of a RCT in addition to potentially being invited to undergo an intervention for symptoms that they had not identified themselves as being a problem, nor had they sought help for. Case managers concurred that the reasoning behind the intervention could be difficult to explain, as well as the purpose of each of the activities, sometimes feeling that they had to ‘sell’ the concepts to participants.
Study participants were sometimes limited in their ability to carry out the intervention tasks because of sensory or physical problems causing difficulties with a writing-dominated task. In addition, these sorts of activities seemed to appeal more to those people who were more used to reading and expressing themselves through writing – those with higher levels of education. Some of the tasks were quite challenging and required participants to open up to the case managers and reflect on their lives; although this was therapeutic for some, for others it was detrimental.
Although a proportion (28%) of individuals withdrew from the intervention programme before completion, others found enormous benefit. Those who engaged with the intervention experienced a greater awareness of themselves and their situation through being given the space to devote time to think about their own well-being. The case managers provided a safe sounding board for participants to talk about issues that were often difficult to raise with family members and friends, despite some people finding this hard to do. The intervention provided participants with various tools for self-help, with many specifically mentioning that the concept of the functional equivalent was especially useful. In general, the intervention gave those participants a way to regain some control of their lives within the context of the ageing process.
Although several participants would have preferred the whole intervention to be delivered face to face, most were happy to accept telephone contact. Case managers did not feel that this particularly impacted on the success of the intervention; however, they did recognise that for some clients this made the delivery more difficult. Intervention delivery does require some flexibility, especially for those for whom telephone use is limited because of physical or cognitive problems. For those who had come to rely on the interactions with their case manager, having appropriate closure from the intervention would help to negate any possible feelings of abandonment.
Strengths and weaknesses
The qualitative sampling approach succeeded in achieving a broad range of views and experiences of the collaborative-care intervention. We achieved the aims of our purposive sampling by incorporating men and women of different ages, from different social backgrounds and with a range of depression scores. It was particularly important to include the views of those who withdrew from the intervention to provide insight into the possible reasons for this. By including all case managers, based across two different trial centres, we were able to obtain a range of views on the delivery of the intervention. However, the qualitative sample was obtained from the pilot phase of the CASPER trial only and therefore the views and experiences expressed obviously reflected the start of the process; these views may have changed during the main trial, particularly for case managers with greater experience in the role.
Conclusions
The embedded qualitative study generated insights to inform the overall trial results, by adding depth and context to the acceptability and practicality of the intervention. Collaborative care represents an innovative treatment in the UK NHS as it involves the delivery of a psychological intervention by a novel mode of delivery (over the telephone). The intervention is used for a group of older people who might not necessarily recognise themselves as being depressed. As older adults they are likely to have concurrent physical health problems and may also have communication difficulties.
The qualitative study suggests that the intervention was acceptable to a large proportion of participants but that others did not engage. The main reasons for non-engagement were explored and these related to participants having misgivings about the potential benefits of behaviourally based programmes or the fact that they did not view themselves to be sufficiently unwell to justify treatment. The importance of the adaptation of treatment to those with long-term conditions or limitations was underlined. The positive aspects of treatment included the fact that people saw the benefits of behavioural activation and engaged well with their case manager, even if there were initial misgivings. Case managers and older people with subthreshold depression were generally happy to deliver/receive collaborative care over the telephone. However, the importance of an initial face-to-face meeting was highlighted, as well as the importance of appropriate closure of the treatment intervention. The preventative aspects of collaborative care were highlighted, such as the importance of modifying unhelpful behavioural patterns and spotting future symptoms. This might, in part, explain the longer-term and preventative benefits seen in the clinical trial.
Chapter 8 Discussion
The CASPER trial is, to our knowledge, the first large-scale evaluation of the effectiveness of collaborative care in older adults in the UK. Moreover, it appears to be the largest evaluation of a brief low-intensity psychosocial intervention for older adults with subthreshold depression. The area of research was one that was prioritised by the National Institute for Health Research HTA programme. We designed an intervention that was potentially suitable for older people with subthreshold depression; this represents a non-pharmacological intervention that could feasibly be delivered by expansion of psychological care by the IAPT programme. In the CASPER trial, outcomes were measured across a broad range of domains including psychological well-being, quality of life, resilience and health state utility. Important aspects of health service resource use were also recorded. The CASPER trial included concurrent qualitative and economic evaluations.
The main findings of the CASPER study in relation to (1) trial-based estimates of the clinical effectiveness of collaborative care, (2) trial-based estimates of cost-effectiveness and (3) a qualitative examination of the acceptability and use of collaborative care will now be discussed in turn.
Trial-based estimates of the clinical effectiveness of collaborative care for subthreshold depression
A group of older adults with mild levels of depression were recruited to the CASPER study (mean PHQ-9 score 7.5, where the cut-off point for case-level depression is held to be 10). The mean age of the population was 77 years. There was a high prevalence of co existing long-term health problems, with an average of 2.2 self-reported problems per participant (selected from a list of 10 prespecified conditions and excluding patient-defined ‘other’ conditions). Commonly reported long-term conditions included high blood pressure (46% of collaborative-care participants), eye disease (38%), arthritis (29%), heart disease (26%) and diabetes (16%).
When offered collaborative care, the greater proportion (85%) engaged with this telephone-based intervention. Just under half (46%) of the collaborative-care group completed the treatment programme and the median number of sessions was seven.
At 4 months’ follow-up there was improvement over time in both groups in terms of depression severity as measured by a commonly used measure of depression severity (PHQ-9), but a greater level of improvement was recorded in the collaborative-care group. There was a statistically significant benefit of collaborative care in terms of the primary outcome of depression severity at 4 months. The magnitude of difference in favour of collaborative care at 4 months was 1.31 PHQ-9 score points (95% CI 0.67 to 1.95 score points; p < 0.001). This benefit for collaborative care was sustained at 12 months (difference 1.33 score points, 95% CI 0.55 to 2.10 score points; p = 0.001). The score difference at 4 months equates to a standard effect size of 0.30, the exact value for which the trial was powered. This finding was robust to a range of sensitivity analyses. For example, adjustments for clustering within therapists reduced the between-group effect size estimate by 0.1 score points.
An effect in terms of preventing case-level depression at 12 months was also observed. At 4 months’ follow-up, 17% in the collaborative-care arm were found to be moderately to severely depressed compared with 28% in the usual-care group (OR 1.35, 95% CI 0.85 to 2.16; p = 0.205). By 12 months this had reached a level of significance, with 16% in the collaborative-care group and 28% in the usual-care group found to be moderately to severely depressed (OR 1.98, 95% CI 1.21 to 3.25; p = 0.007).
When a number of secondary outcomes were analysed there was also a benefit for collaborative care. There was a significant and sustained 12-month improvement in anxiety (as measured by the GAD7) and somatic complaints (as measured by the PHQ-15). Of note was the fact that common somatic complaints among older people (such as pain, constipation and sleep) were found to be specifically improved in the collaborative-care group compared with the usual-care group.
The population of older adults had important limitations of function consistent with the high levels of physical comorbidity and this was reflected in scores on the PCS scale of the SF-12. Physical functioning was below the average adult physical health status (scores of < 50) for participants throughout the trial period, as would be expected in an older population. Patients in the usual-care arm maintained physical functioning scores at approximately 35–36 score points, whereas patients in the collaborative-care arm improved on average to up to 40 points over follow-up. The differences in physical functioning were statistically significant at 4 months (p < 0.001) and 12 months (p = 0.020). Improvements and between-group differences were observed for the MCS scale of the SF-12 in favour of collaborative care and in line with changes on other psychological function scales. Improvements were also noted for resilience as measured by the CD-RISC 2.
In summary, statistically significant improvements in depression severity were observed in favour of collaborative care in both the short term (4 months) and the medium term (12 months). In addition, benefits were observed across the range of psychological, quality of life and resilience outcomes in the short and medium term.
Summary of trial-based estimates of the cost-effectiveness of collaborative care
There was a concurrent cost-effectiveness analysis within the CASPER trial and we were able to derive utility-based estimates of quality of life alongside resource use derived from scrutiny of GP records and self-report. Collaborative care was a relatively brief intervention delivered by a low-intensity IAPT therapist. When all costs associated with a fully completed episode of collaborative-care were accounted for, the costs to the NHS were £495 per patient. Only around half of the collaborative care participants completed six or more of the eight planned sessions and, when the costs of collaborative care as might be delivered within a typical IAPT service were accounted for, these were £224 per patient. There was a statistically significant improvement in health state utility associated with collaborative care compared with usual care (adjusted QALY gain at 12 months 0.044; p = 0.003). Resource use was not substantially offset in the collaborative-care group, with the total costs reduced by around £51 in the collaborative-care group. In the base-case incremental cost-effectiveness analysis, collaborative care achieved gains at a cost of £9633 per QALY. The probability that the incremental cost-effectiveness of collaborative care was < £20,000 per QALY was 93% and the probability that it fell below the £30,000 per QALY willingness-to-pay threshold was 97%. When the actual costs of collaborative care were accounted for, the cost per QALY estimate fell to £3328.
Summary of the main findings from the qualitative examination of acceptability and uptake of collaborative care
The qualitative evaluation brought insights to the overall trial results by adding depth and context to the acceptability and practicality of the intervention. Collaborative care represents an innovative treatment in the UK NHS as it involves the delivery of a psychological intervention by a novel mode of delivery (over the telephone). The intervention is used for a group of older people who might not necessarily recognise themselves as being depressed or for whom depression might not be the main reason for consultation. As older adults they were likely to have concurrent physical health problems and may also have communication or sensorial difficulties.
The qualitative evaluation showed that the intervention was acceptable to a large proportion of participants, but that some did not engage. The main reasons for non-engagement or failure to complete the median course of seven sessions were explored and these related to participants having misgivings about the potential benefits of behaviourally based programmes or the fact that they did not view themselves to be sufficiently unwell to justify treatment with a collaborative-care intervention. Some participants disliked certain aspects of behavioural activation such as the need to reflect and self-monitor. Others found the activity diaries and ‘homework’ difficult, requiring too much time and effort. However, case managers learned to adapt treatment and tailor collaborative care to the individual and this improved as case managers gained experience.
The importance of the adaptation of treatment to those with long-term conditions or limitations was underlined and it was clear that the use of functional equivalence had been helpful in developing a repertoire of new self-reinforcing activities in the face of disabilities and impairments. The positive aspects of treatment included the fact that people saw the benefits of behavioural activation and engaged well with their case managers, even if there had been initial misgivings.
The qualitative evaluation highlighted that case managers and older adults with subthreshold depression were generally happy to deliver/receive collaborative care over the telephone. However, the importance of an initial face-to-face meeting was highlighted in ensuring engagement. Participants were also appreciative of certain aspects of the treatment and the relationship with the case manager. Participants mentioned that the case manager became a ‘sounding board’ for emotional and mood-related matters.
The preventative aspects of collaborative care were highlighted such as the importance of modifying unhelpful behavioural patterns and spotting future symptoms. This might, in part, explain the longer-term and preventative benefits seen in the clinical trial. It is important to recognise this aspect of treatment in the light of the longer-term and preventative findings of the trial.
Discussion of the main findings
The observed standard effect of 0.30 for the primary outcome represents a moderate effect size according to criteria used to classify the magnitude of effect for psychological interventions. 48 The effect size is consistent with findings from systematic reviews of collaborative care as summarised in a recent Cochrane review20 and is also of the same order of magnitude as that seen in UK trials of collaborative care for working-age adults, such as those observed in the recently published CADET trial. 65 The CASPER trial also showed benefits across a range of secondary outcomes and it was notable that there were improvements in anxiety symptoms, somatoform symptoms and quality of life (in both physical and mental domains as measured by the SF-12). These benefits were seen in the short term (4 months) and were also sustained at 12 months.
An additional finding was that the proportion of participants with case-level depression at 12 months was reduced among those who received collaborative care. This provides evidence that collaborative care may have a preventative role in relation to depression. To our knowledge this is the first time that this has been observed in a large-scale trial of a psychosocial intervention for older people with low-severity depression. This is a finding that deserves further research and it would be of interest to know whether or not this benefit persists beyond the 12-month period of follow-up in the CASPER trial. We note that other studies have found longer-term benefits of collaborative care,66 including studies of collaborative care for older populations. 22 People with subthreshold depression are at a particular risk of developing more severe disorders and might be a target high-risk population in any preventative strategy. The results of the CASPER trial are, to our knowledge, the first demonstration of preventative effects in subclinically depressed older people.
We noted from the rates of uptake of the intervention that a greater proportion of participants (85%) engaged well and completed a large number of the planned sessions (median seven out of eight planned sessions). The qualitative evaluation of collaborative care pointed to aspects of the intervention that participants found helpful. The initial appointment was face to face to establish a relationship between the case manager and the participant before continuing the sessions as telephone appointments. What was notable was that participants were generally happy to receive collaborative care over the telephone, but that the initial face-to-face meeting was felt to be important. There was some uncertainty whether or not a telephone intervention would be acceptable to older people with subthreshold depression. It was encouraging to find from the qualitative study and from comments made to case managers that this was seen by most people to be an acceptable method of delivery. This is important for those who plan services or for therapists who might have misgivings about the telephone-based mode of delivery of a psychosocial intervention.
The evidence-supported psychological intervention at the centre of collaborative care in the CASPER trial was behavioural activation. 67 The psychological intervention was adapted for use in an older age group at the developmental pilot phase of the study. 47 A reduction in social isolation is an important aspect of the intervention and much of the collaborative care for some participants focused around this. Although face-to-face contact with the case manager may have provided initial social contact it would be in the short term only. The case managers sought to reduce social isolation in the long term by ascertaining a participant’s needs and preferences regarding social contact. Putting them in touch with organisations, groups and individuals who could help them to increase their social network and opportunities for interaction afforded them long-term benefits.
Case managers worked in a patient-centred way with each participant. If participants reported that reflecting on any particular aspects of the intervention made them feel uncomfortable or upset then this could be worked around. For example, some participants did not wish to think about not being able to carry on doing certain activities in the future and how they might replace these activities with ones that were functionally equivalent. In this case, this aspect of the intervention could be omitted or discussed in a non-threatening way by using the premise of a temporary situation, such as bad weather. Alternatively, if the participant had identified an activity that they had been forced to stop doing in the past, the way that they had managed this could be used to illustrate the principle of functional equivalence.
We also found that a small but significant minority of participants did not engage with the psychologically based intervention. This may be explained by the fact that some people with subthreshold depression did not see themselves as psychologically unwell and did not endorse treatment. Nevertheless, it is notable that the uptake of collaborative care in the context of the CASPER trial was broadly in line with the uptake of a range of primary care-based low-intensity interventions such as those offered by IAPT services. 68 The results of the CASPER trial therefore add to an emerging evidence base that behavioural activation is effective for older adults. 69
The results of the economic evaluation provide robust evidence relating to the cost-effectiveness of collaborative care for people with lower-severity depression. The CASPER trial provides estimates of the overall costs of the intervention, which will be useful for those who might plan services. Within a range of scenarios collaborative care was found to provide QALY gains within a range of willingness-to-pay thresholds. There are relatively few cost-effectiveness analyses of collaborative care from the perspective of the UK health-care system. The worldwide body of randomised economic research generally shows that collaborative care is cost-effective. 21 The results of the CASPER trial add to emerging evidence of the cost-effectiveness of collaborative care in the UK. The economic results of the CASPER trial are broadly in line with the results of the only other UK cost-effectiveness analysis of collaborative care (cost per QALY £14,248 in working-age adults65) and also replicate findings from large-scale US studies of collaborative care in older people. 70
Finally, we note that there has hitherto been a paucity of non-pharmacological interventions that have been evaluated among older people with subthreshold depression. 51 The most recent NICE guidance6 in relation to the management of depression was unable to recommend collaborative care in this population and the CASPER trial represents a significant advance in the development of randomised knowledge in this area. This research knowledge will be helpful to those who formulate guidelines for the management of depression, including the next iteration of NICE guidelines for the care of depression and the care of psychological problems in the context of long-term physical ill health. 7
Limitations
The results of the CASPER trial need to be considered in the light of limitations that emerged during the study. First, regarding trial design, blinding was not feasible, which means that there was potential for contamination at the GP level as well as at an individual level. Each participating GP practice treated an average of 22 participants (minimum 2, maximum 83) and it is possible that GPs, who needed to be aware if a patient was currently receiving collaborative care, managed cases differently based on their knowledge. Furthermore, many participants would be living geographically close to one another in the same catchment area. Within a population of that age, it is reasonable to assume that some would know each other and share their trial experiences. In either case, we anticipate that contamination would result in additional benefits to control-arm participants, thereby reducing any group differences during follow-up and rendering our result a conservative estimate of the treatment effect. Also relating to study design, participants were recruited by means of postal screening from age-based GP practice lists and so participants identified with subthreshold depression had not necessarily presented with this problem. Therefore, the results of the CASPER trial might not automatically apply to older people who screen positive for depression in the context of primary care attendance or physical health checks for older people.
Retention and differential attrition between the trial arms was a further limitation. Although follow-up rates were high overall (83% at 4 months) and exceeded the anticipated trial retention on which the trial was powered, there was a much higher rate of attrition in the collaborative-care arm than in the usual-care arm (24% in the collaborative-care arm and 10% in the usual-care arm). This was in part accounted for by a number of participants who disengaged from the collaborative-care intervention and fully withdrew from the trial at the same time. It remains possible, however, that the patients who withdrew from the trial and who did not provide outcome data may have presented a very different outcome profile from those who continued, which may have biased the treatment effect. Based on the very similar baseline characteristics between randomised patients and those available for the primary analysis as well as our exploration of the impact of missingness, such bias appears less likely, however. We did adapt our withdrawal procedure when it came to light that participants disengaging from the intervention were actually tending to withdraw in full. In the early stages of the study, participants wishing to withdraw were able to do so through their case manager. Once we had identified the problem we changed the procedure so that case managers could no longer withdraw participants. The case managers would inform the research team if a participant wished to withdraw and the research team would make contact with those participants to discuss which level of withdrawal they required and to encourage them to remain in the study for follow-up. This proved successful and rates of retention to follow-up improved following this procedure change.
There are a couple of limitations relating to the cost-effectiveness analysis. First, it was not possible to include antidepressant use in the cost calculations. This was because of the high levels of polypharmacy in older adults, which impacted on the level of medication data obtained from GP practices. Some participants were prescribed large quantities of products during the 12-month follow-up period and any who exceeded 20 products were subjectively censored at the practice level, meaning that the level of detail to cost pharmaceutical use (e.g. product name, dose, duration of treatment, schedule of use) was not available. It was therefore not possible to obtain a reliable overall cost related to the use of prescribed medicines, or specifically antidepressants, from the available data. Additional learning from the process of collecting objective data relates to the mechanism of obtaining the data. Our experience was time-consuming and labour intensive, requiring data on the number of practice contacts for each patient to be manually copied and pasted into a document. In the future it would be helpful if the process could be made more efficient and effective by working with practices to develop ways of exporting anonymous objective data on a patient’s resource use directly from GP practice database systems (such as SystmOne, EMIS Web and Vision). This would make the task less onerous for time-deprived GP practices and provide a richer set of objective data, allowing for more accurate future cost-effectiveness analysis. It should also be recognised that the results presented are of the within-trial cost-effective analysis with an explicitly stated 12-month time horizon and without introducing external assumptions required to extend our results to a lifetime horizon. However, we have aimed to provide sufficient detail to inform a future state transition decision model. A further limitation of the cost-effectiveness analysis is that the reported economic evaluation considers only primary care NHS costs. Future cost-effectiveness estimates would benefit from having a wider perspective, factoring in secondary and tertiary care costs, along with unpaid costs such as family carers and volunteering.
The lowering of the age limit from the pilot phase to the main trial, from ≥ 75 years to ≥ 65 years, is likely to have affected the results as the proportion of ‘older’ older adults will have been reduced. It is also recognised that the qualitative findings are not representative of the entire trial. The data were collected during the pilot phase when the intervention was in its early stages of development. Had interviews been conducted with participants in the main trial, the findings may have been different. It is likely that, as case managers’ confidence grew through becoming more accomplished at delivering the intervention, participants would have experienced a more established intervention, which could have impacted on them more positively.
A final limitation is the truncation of follow-up at 12 months. Although we now know collaborative care to be effective in the medium term, the longer-term benefits beyond 12 months remain unknown.
Conclusions
There is currently little provision for older adults with subthreshold depression. Lower-severity depression is relatively common among older people and is often associated with long-term health conditions. The CASPER trial represents the largest trial-based evaluation of a psychosocial intervention for this group. It was found to be effective across a range of depression, psychological and quality-of-life outcomes in the short term. The effects remained at 12 months’ follow-up and the intervention was also shown to reduce the proportion of older people with case-level depression at this point. The longer-term benefits beyond this are not known. The intervention was delivered over the telephone by low-intensity psychological therapists such as those who work in NHS IAPT services. Qualitative research showed this to be an acceptable and valued treatment by the greater proportion of people who were offered collaborative care. A concurrent economic evaluation found that the intervention resulted in gains in QALYs at a cost threshold that is acceptable to the UK health system.
Implications for health care
Collaborative care was acceptable for many of the older adults with low subthreshold depression and could readily be delivered over the telephone following a first face-to-face meeting. However, the experience of using IAPT workers to deliver the intervention demonstrated that older adults were not the priority of IAPT services. As a result, it may be worth exploring other methods of delivering the intervention such as through nurses who conduct comorbidity checks or healthy living workers. Certainly, the provision of care for older people with subthreshold depression would require substantial expansion in the scope of IAPT services if this intervention were to be delivered through that mechanism.
Collaborative care proved clinically effective at improving depression scores and preventing the onset of case-level depression for older people with subthreshold depression. The small to moderate effect size of 0.3 may represent limited change at the individual level but it has a substantial impact at the population level. 48 Moreover, the robust cost-effectiveness estimates for the use of collaborative care to treat subthreshold depression were cost-effective under conventional willingness-to-pay thresholds. This study has shown that collaborative care represents a new way of treating subthreshold depression in primary care. Depression is a relatively common condition, affecting about 5% of older adults. Given that subthreshold depression affects a larger proportion of the population, around 15–20%, this trial’s findings demonstrate the scope for collaborative care at the population/epidemiological level if it were to be rolled out into primary care. The CASPER trial evidence could be used by policy-makers and primary care to improve services and reduce the disease burden of our ageing population.
A final implication for health care relates to the relatively high dropout rate from the collaborative-care arm and what this would mean for take-up of the intervention in the real world. Some participants found the intervention intrusive and felt that talking and thinking about their symptoms made them feel uncomfortable. This may signal a potential problem if collaborative care were to be offered as part of NHS services. As with all psychological services, this type of intervention will not necessarily suit everyone and care should be taken to ascertain the likelihood of this being the case before any referral to such a service.
Recommendations for research
-
Investigate the longer-term effect of collaborative care on relapse and the prevention of future case-level depression. Extended follow-up of CASPER participants, potentially at 60 months, represents a possible method to examine the longer-term effect of collaborative care, to see if the clinical benefits identified at 12 months were maintained over a longer duration.
-
Investigate the longer-term effect of collaborative care on multimorbidities. A large proportion of participants in the CASPER trial had at least one long-term physical health condition. Although there were some improvements in function and quality of life among participants, there is little evidence on the effectiveness of collaborative care at treating comorbidities. Evidence from a US trial71 that tested collaborative care for the treatment of comorbid depression or subthreshold depression and diabetes mellitus showed that it helped improve depression care and outcomes but did not result in improved glycaemic control. Given that the population is ageing and the rate of long-term conditions is rising, there is demand for future trials to investigate the effectiveness of collaborative care at improving physical and mental health outcomes in older adults with multimorbidities.
-
Many patients in the collaborative-care arm discontinued treatment or dropped out of the trial. Further qualitative and quantitative work should explore the reasons for this and identify the most appropriate target population for the intervention.
-
Translating the research findings into clinical practice will be challenging and would benefit from further research. This relates to both enabling capacity to deliver the intervention to patients, and being able to target it at those most likely to complete the process and make use of the resource.
-
There are no trials of collaborative care for people of working age with subthreshold depression. It would be useful to decision-makers to know whether or not the results of the CASPER trial can be replicated in this population.
Acknowledgements
We would especially like to thank all of the participants who took part in this trial. Thanks also to the GPs and other professionals from participating general practices who enabled patients to be recruited and data to be collected, ensuring the success of the trial. We are grateful to York Trials Unit for managing and storing the data securely and to the PC-MIS team for providing support in using their patient case management information system. We also wish to thank the TSC and the DMEC members for overseeing the study.
The CASPER trial also benefited from working with Yorkshire and Humber NHS IAPT and the North East NHS IAPT services. In addition, it was supported by the North and East Yorkshire and Northern Lincolnshire Primary Care Research Network; Yorkshire and Humber Clinical Research Network (previously known as the Comprehensive Local Research Network); North East and North Cumbria Clinical Research Network; Tees, Esk and Wear Valleys NHS Foundation Trust; and Northumberland, Tyne and Wear NHS Foundation Trust.
CASPER trial team (past and present)
-
Chief investigator: Simon Gilbody.
-
Trial managers: Helen Lewis and Natasha Mitchell.
-
Principal investigators: Dave Ekers, Simon Gilbody, John Holmes and Esther Cohen-Tovee.
-
Trial co-ordinators: Katharine Bosanquet, Emily Clare, Lesley Haley, Jahnese Maya, Shaista Meer and Gemma Traviss-Turner.
-
Statisticians: Rhian Gabe, Catherine Hewitt and Ada Keding.
-
Health economists: Steve Parrot and Dominic Trépel.
-
Qualitative researchers: Joy Adamson, Karen Spilsbury and Friederike Ziegler.
-
Trial administrators: Catherine Arundel, Pauline Holloway, Sarah Mercer and Denise Womersley.
-
Data management Team: Matthew Bailey, Ben Cross, Lynne Hornby, Tanya Pawson, Julie Ross, Kevin Sherratt, Jo Waddington, Val Wadsworth and Maureen Wakefiled.
-
Research team: Katie Atherton, Della Bailey, Catherine Baxter, Jules Beresford-Dent, Jacqueline Birtwistle, Daniel Brett, Simon Carver, Deborah Foster, Samantha Gascoyne, Rebecca Hargate, Gillian Johnson, Amanda Lilly, Jodi Meredith, Rachel Mann, Sarah Nutbrown, Karen Overend, Madeline Pasterfield, Norah Phipps, Katherine Richardson, Helen Riding and Rebecca Woodhouse.
-
TMG: Joy Adamson, Katie Atherton, Della Bailey, Jacqueline Birtwistle, Katharine Bosanquet, Emily Clare, Esther Cohen-Tovee, Jaime Delgadillo, Dave Ekers, Deborah Foster, Samantha Gascoyne, Simon Gilbody, Lesley Haley, Catherine Hewitt, John Holmes, Helen Lewis, Ada Keding, Peter Knapp, Rachel Mann, Jahnese Maya, Dean McMillan, Shaista Meer, Jodi Meredith, Natasha Mitchell, Sarah Nutbrown, Karen Overend, Steve Parrot, Madeline Pasterfield, Karen Spilsbury, Gemma Traviss-Turner, Dominic Trépel, Rebecca Woodhouse and Friederike Ziegler.
Trial Steering Committee members
-
Mr Mike Beckett, Director of York MIND, York (now Director of Development at the Retreat, York).
-
Dr David Geddes, Medical Director of NHS North Yorkshire & York and GP at Clifton Medical Practice, York (now National Head of Primary Care Commissioning, NHS Commissioning Board, Leeds).
-
Dr Alison Layton (Chair), Co-director of North and East Yorkshire and Northern Lincolnshire Comprehensive Local Research Network and Harrogate and District NHS Foundation Trust lead for Research and Development, Harrogate District Hospital (now Clinical Director of Yorkshire and Humber Clinical Research Network).
-
Dr Waquas Waheed, Academic Consultant Psychiatrist, Lancashire Care NHS Foundation Trust, Preston (now National Primary Care Research and Development Centre, University of Manchester, Manchester).
-
Plus members of the CASPER trial’s TMG.
Data Monitoring and Ethics Committee
-
Dr David Kessler (Chair), National Institute for Health Research Clinical Lecturer, Primary Health Care, University of Bristol, Bristol.
-
Dr Judy Leibowitz, Primary Care Mental Health Development Coordinator, Camden Primary Care Trust, London (now Head of IAPT, Camden and Islington NHS Foundation Trust).
-
Professor Stephen Walters, Medical Statistics and Clinical Trials, Health Services Research, School of Health and Related Research, University of Sheffield, Sheffield.
Patient and public involvement in research
The CASPER trial owes great thanks to the users of mental health services and carers who were part of the advisory group, established at the end of the pilot phase; their insights and understanding helped improve the relevance and readability of the study documentation.
Contributions of authors
Helen Lewis (Research Fellow, Health Sciences) was the CASPER study manager overseeing all sites and was involved in writing the report.
Joy Adamson (Senior Research Fellow, Health Sciences) contributed to the development of the grant application and trial protocol, supervised the conduct of the qualitative research and was involved in the qualitative analysis and writing the report.
Katie Atherton (Clinical Studies Officer, Health Sciences) was a case manager and contributed to the day-to-day running of the trial.
Della Bailey (Research Fellow, Health Sciences) developed the manual/intervention post pilot phase, was a case manager who also trained and supervised case managers and was involved in writing the report.
Jacqueline Birtwistle (Research Assistant, Health Sciences) contributed to the day-to-day running of the trial.
Katharine Bosanquet (Research Fellow, Health Sciences) co-ordinated recruitment and the running of trial at the core site (York), managed the collection of objective data from all GP practices across all sites and was responsible for drafting the report.
Emily Clare (Clinical Studies Officer, Clinical Research Network) co-ordinated recruitment and the running of the trial at the Newcastle upon Tyne site.
Jaime Delgadillo (Researcher and Cognitive Behavioural Psychotherapist, Leeds Community Healthcare NHS Trust) supervised the case managers and provided clinical input and advice during the trial.
David Ekers (Senior Clinical Lecturer, Health Sciences) contributed to the development of the grant application and trial protocol, provided expertise and training in behavioural activation, provided clinical input and advice during the trial and was a local Principal Investigator.
Deborah Foster (Research Fellow, Health Sciences) developed the manual/intervention post pilot phase and was a case manager who also trained and supervised case managers.
Rhian Gabe (Senior Statistician, Health Sciences) provided statistical support during the study.
Samantha Gascoyne (Trial Support Officer, Health Sciences) contributed to the day-to-day running of the trial and was involved in writing the report.
Lesley Haley (Clinical Studies Officer, Clinical Research Network) co-ordinated recruitment and the running of the trial at the Durham site.
Rebecca Hargate (Clinical Studies Officer, Health Sciences) contributed to the day-to-day running of the trial.
Catherine Hewitt (Senior Statistician, Health Sciences) contributed to the development of the grant application and trial protocol, provided statistical support throughout the study, supervised the conduct of the statistical analysis and undertook the second checking of the final analysis for the report.
John Holmes (Senior Clinical Lecturer, Health Sciences) contributed to the development of the grant application and trial protocol, provided expertise in the design and evaluation of psychosocial interventions for older adults with comorbidities, provided clinical input and advice during the trial and was a local Principal Investigator.
Ada Keding (Statistician, Health Sciences) wrote the statistical analysis plan and performed the statistical analysis and was involved in writing the report.
Amanda Lilley-Kelly (Clinical Studies Officer, Health Sciences) contributed to the day-to-day running of the trial.
Jahnese Maya (Clinical Studies Officer, Clinical Research Network) co-ordinated recruitment and the running of the trial at the Newcastle upon Tyne site.
Dean McMillan (Senior Clinical Lecturer, Health Sciences and Hull York Medical School) contributed to the development of the grant application and trial protocol, provided clinical input and advice during the trial and conducted supervision with the case manager supervisors.
Shaista Meer (Research Officer, Health Sciences) co-ordinated recruitment and the running of the trial at the Leeds site.
Jodi Meredith (Trial Support Officer, Health Sciences) was a case manager and contributed to the day-to-day running of the trial.
Natasha Mitchell (Research Fellow, Health Sciences) contributed to the trial protocol and setting up of the CASPER study, and was the initial CASPER study manager.
Sarah Nutbrown (Research Fellow) contributed to the day-to-day running of the trial and developed site-specific procedures.
Karen Overend (Trial Support Officer, Health Sciences) contributed to the day-to-day running of the trial.
Madeline Pasterfield (Research Assistant, Health Sciences) was a case manager and tailored the training manual to be appropriate for older adults.
David Richards (Professor, Mental Health Services Research) contributed to the development of the grant application and trial protocol, and provided content expertise in the design of low-intensity collaborative care.
Karen Spilsbury (Professor, Nursing) contributed to the development of the grant application and trial protocol, supervised the conduct of the qualitative research, and was involved in the qualitative analysis and writing of the report.
David Torgerson (Professor, Trial Methodology) provided advice on efficient and effective trial conduct, and contributed to the development of the grant application and trial protocol.
Gemma Traviss-Turner (Senior Research Fellow, Health Sciences) co-ordinated recruitment and the running of the trial at the Leeds site.
Dominic Trépel (Health Economist, Health Sciences) conducted all of the cost-effectiveness analysis and was involved in writing the report.
Rebecca Woodhouse (Trial Support Officer, Health Sciences) contributed to the day-to-day running of the trial and was involved in writing the report.
Friederike Ziegler (Research Fellow, Health Sciences) conducted the qualitative research and wrote a final report. She also established a public and patient involvement group that advised on the development of study materials.
Simon Gilbody (Professor, Psychological Medicine and Health Services Research) contributed to the development of the grant application and trial protocol, provided clinical input and advice during the trial, was involved in writing the report and was the Chief Investigator who oversaw the study.
All authors approved and/or commented on the final manuscript.
Publications
Papers
Mitchell N, Hewitt C, Adamson J, Parrott S, Torgerson D, Ekers D, et al. A randomised evaluation of CollAborative care and active surveillance for Screen-Positive EldeRs with sub-threshold depression (CASPER): study protocol for a randomised controlled trial. Trials 2011;12:225.
Lewis H, Hems D, Bosanquet K, Overend K. Editorial: is enough being done to treat depression in the elderly? Age Health 2013;9:243–5.
Pasterfield M, Bailey D, Hems D, McMillan D, Richards D, Gilbody S. Adapting manualized behavioural activation treatment for older adults with depression. Cogn Behav Ther 2014;7:e5.
Bosanquet K, Mitchell N, Gabe R, Lewis H, McMillan D, Ekers D, et al. Diagnostic accuracy of the Whooley depression tool in older adults based in primary care. J Affect Disord 2015;182:39–43.
Gilbody S, Lewis H, Adamson J, Atherton K, Bailey D, et al. Effect of collaborative care vs usual care on depression symptoms in older adults with subthreshold depression. The CASPER randomized clinical trial. JAMA 2017;317:728–37.
Posters
Lewis H, Mitchell N, McMillan D, Bosanquet K, Hall R, Lilley A, et al. Collaborative Care in Screen Positive Elders – the CASPER trial: a randomised controlled trial pilot study. Presented at the UK Primary Care Mental Health Research Conference, Bristol, 2012.
Bosanquet K, Mitchell N, Lewis H, Bailey D, Gabe R, McMillan D, Gilbody S. Diagnostic accuracy of Whooley depression tool in older adults based in primary care in the UK. Presented at the UK Primary Care Mental Health Research Conference, Manchester, 2013. Won best academic poster award.
Traviss G, Holmes J, Lewis H, Mitchell N, McMillan D, Hems D, et al. CASPER – CollAborative care for Screen Positive EldeRs. Presented at the British Psychological Society Annual Conference, Harrogate, 2013.
Radio broadcasts
BBC1 ‘Inside out’ North East focused on care for older adults and interviewed Simon Gilbody, Deborah Jane Foster and Della Bailey regarding the CASPER and CASPER Plus trials; BBC Radio York interviewed Simon Gilbody regarding mental health and older adults in connection with the CASPER study; BBC1 ‘Look North’ focused on depression in older adults, interviewing a GP whose practice participated in the CASPER study, 15 October 2012.
Data sharing statement
Reasonable requests for data sharing and collaborative analysis can be made to the corresponding author, who will then consider the request in consultation with the TMG and coinvestigators of the CASPER trial.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries and Risk Factors in 1990. Boston, MA: Harvard School of Public Health on behalf of the World Bank; 1996.
- Chew-Graham C, Baldwin R, Burns A. Treating depression in later life. BMJ 2004;329:181-2. http://dx.doi.org/10.1136/bmj.329.7459.181.
- Rapp S, Parisi S, Walsh D. Psychological dysfunction and physical health among elderly medical inpatients. J Consult Clin Psychol 1988;56:851-5. http://dx.doi.org/10.1037/0022-006X.56.6.851.
- Cahachamovich E, Fleck M, Laidlaw K, Power M. Impact of major depression and subsyndromal symptoms on quality of life and attitudes toward aging in an international sample of older adults. Gerontologist 2008;48:593-602. http://dx.doi.org/10.1093/geront/48.5.593.
- Walker Z, Leek A, D’ath P, Katona C. Psychiatric morbidity in elderly attenders at an accident and emergency department. Int J Geriatr Psychiatry 1995;10:951-7. http://dx.doi.org/10.1002/gps.930101107.
- The Treatment and Management of Depression in Adults. London: NICE; 2009.
- Depression in Adults with a Chronic Physical Health Problem. London: NICE; 2009.
- Revisions to the GMS Contract 2006/07. London: NHS Employers; 2006.
- Whooley M, Avins A, Miranda J, Browner W. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. http://dx.doi.org/10.1046/j.1525-1497.1997.00076.x.
- Baldwin R, Anderson D, Black S, Evans S, Jones R, Wilson K, et al. Guideline for the management of late-life depression in primary care. Int J Geriatr Psychiatry 2003;18:829-38. http://dx.doi.org/10.1002/gps.940.
- Quality and Outcomes Framework Guidance for GMS Contract 2013/14. London: NHS Employers; 2013.
- Diagnostic and Statistical Manual. Washington, DC: American Psychiatric Association; 1994.
- International Statistical Classification of Disease and Related Health Problems – 10th Revision. Geneva: World Health Organization; 2007.
- Sheehan D, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Judd L, Akiskal H, Maser J, Zeller P, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry 1998;55:694-700. http://dx.doi.org/10.1001/archpsyc.55.8.694.
- Davies S. Annual Report of the Chief Medical Officer (CMO) 2013. Public Mental Health Priorities: Investing in the Evidence. London: Department of Health; 2014.
- Iliffe S, Haines A, Gallivan S, Booroff A, Goldenberg E, Morgan P. Assessment of elderly people in general practice. 1. Social circumstances and mental state. Br J Gen Pract 1991;41:9-12.
- Lovell K, Richards D. Multiple Access Points and Levels of Entry (MAPLE): ensuring choice, accessibility and equity for CBT services. Behav Cogn Psychother 2000;28:379-91.
- Simon G. Collaborative care for depression. BMJ 2006;332:249-50. http://dx.doi.org/10.1136/bmj.332.7536.249.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.cd006525.pub2.
- Gilbody S, Bower P, Whitty P. Costs and consequences of enhanced primary care for depression: systematic review of randomised economic evaluations. Br J Psychiatry 2006;189:297-308. http://dx.doi.org/10.1192/bjp.bp.105.016006.
- Unützer J, Katon W, Callahan C, Williams J, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836-45. http://dx.doi.org/10.1001/jama.288.22.2836.
- Chew-Graham C, Lovell K, Roberts C, Baldwin R, Morley M, Burns A, et al. A randomised controlled trial to test the feasibility of a collaborative care model for the management of depression in older people. Br J Gen Pract 2007;57:364-70.
- Ciechanowski P, Wagner E, Schmaling K, Schwartz S, Williams B, Diehr P, et al. Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA 2004;291:1569-77. http://dx.doi.org/10.1001/jama.291.13.1569.
- Richards D, Hill J, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Jacobson N, Dobson K, Truax P, Addis M, Koerner K, Gollan J, et al. A component analysis of cognitive–behavioural treatment for depression. J Consult Clin Psychol 1996;64:295-304. http://dx.doi.org/10.1037/0022-006X.64.2.295.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23. http://dx.doi.org/10.1017/S0033291707001614.
- Richards D, Lovell K, Gilbody S, Torgerson D, Barkham M, Bland M, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med 2008;38:279-87. http://dx.doi.org/10.1017/S0033291707001365.
- Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Quality and Outcomes Framework Guidance for GMS Contract 2009/10. Delivering Investment in General Practice. London: NHS Employers; 2009.
- Arroll B, Goodyear-Smith F, Kerse N, Fishman T, Gunn J. Effect of the addition of a ‘help’ question to two screening questions on specificity for diagnosis of depression in general practice: diagnostic validity study. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38607.464537.7C.
- World Health Organization . Definition of an Older or Elderly Person 2015. www.who.int/healthinfo/survey/ageingdefnolder/en/ (accessed 30 June 2015).
- Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the ‘cohort multiple randomised controlled trial’ design. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1066.
- Kronke K, Spitzer R. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:1-7. http://dx.doi.org/10.3928/0048-5713-20020901-06.
- Ware J, Kosinski M, Keller S. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Spitzer R, Kroenke K, Williams J, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer R, Williams J. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66. http://dx.doi.org/10.1097/00006842-200203000-00008.
- Vaishnavi S, Connor K, Davidson J. An abbreviated version of the Connor–Davidson Resilience Scale (CD-RISC), the CD-RISC2: psychometric properties and applications in psychopharmacological trials. Psychiatry Res 2007;152:293-7. http://dx.doi.org/10.1016/j.psychres.2007.01.006.
- Gruenberg A, Goldstein R, Pincus H. Classification of Depression: Research and Diagnostic Criteria: DSM-IV and ICD-10. Weinheim: Wiley; 2005.
- Adams K, Moon H. Subthreshold depression: characteristics and risk factors among vulnerable elders. Aging Ment Health 2009;13:682-92. http://dx.doi.org/10.1080/13607860902774501.
- Bosanquet K, Mitchell N, Gabe R, Lewis H, McMillan D, Ekers D, et al. Diagnostic accuracy of the Whooley depression tool in older adults in UK primary care. J Affect Disord 2015;182:39-43. http://dx.doi.org/10.1016/j.jad.2015.04.020.
- Zeiss AM, Lewinsohn PM, Rohde P, Kato P, Mann T. Handbook of Diversity Issues in Health Psychology. New York, NY: Plenum Press; 1996.
- Blazer DG. Depression in late life: review and commentary. Focus 2009;7:118-36. http://dx.doi.org/10.1176/foc.7.1.foc118.
- Joiner TE, Conwell Y, Fitzpatrick KK, Witte TK, Schmidt NB, Berlim MT, et al. Four studies on how past and current suicidality relate even when ‘everything but the kitchen sink’ is covaried. J Abnorm Psychol 2005;114:291-303. http://dx.doi.org/10.1037/0021-843X.114.2.291.
- Vinkers DJ, Gussekloo J, Stek ML, Westendorp RGJ, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ 2004;329:881-5. http://dx.doi.org/10.1136/bmj.38216.604664.DE.
- Pasterfield M, Bailey D, Hems D, McMillan D, Richards D, Gilbody S. Adapting manualized behavioural activation treatment for older adults with depression. Cogn Behav Ther 2014;7. http://dx.doi.org/10.1017/s1754470x14000038.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Lawrence Erlbaum Associates; 1988.
- National Research Ethics Service . Standard Operating Procedures for Research Ethics Committees in the United Kingdom 2009. http://webarchive.nationalarchives.gov.uk/20110729124925/nres.npsa.nhs.uk/ (accessed 1 October 2010).
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Netten A, Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- O’Brien B, Briggs A. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. http://dx.doi.org/10.1191/0962280202sm304ra.
- Glick H, Doshi J, Sonnad S. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Manning W, Mullahy J. Estimating log models: to transform or not to transform?. J Health Econ 2001;20:461-94. http://dx.doi.org/10.1016/S0167-6296(01)00086-8.
- Vuong Q. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989;57:307-33. http://dx.doi.org/10.2307/1912557.
- Patton M. Qualitative Research and Evaluation Methods. Thousands Oaks, CA: Sage; 2001.
- O’Cathain A, Thomas K, Drabble S, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Cresswell J. Qualitative Inquiry and Research Design. London: Sage; 1998.
- Friese S. Qualitative Data Analysis with ATLAS.ti. London: Sage; 2012.
- Strauss A, Corbin J. Basics of Qualitative Research. Techniques and Procedures for Developing Grounded Theory. London: Sage; 2008.
- Lincoln Y, Guba G. Naturalistic Inquiry. Newbury Park, CA: Sage; 1985.
- Green C, Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, et al. Cost-effectiveness of collaborative care for depression in UK primary care: economic evaluation of a randomised controlled trial (CADET). PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0104225.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton A. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. http://dx.doi.org/10.1001/archinte.166.21.2314.
- Kanter J, Manos R, Bowe W, Baruch D, Busch A, Rusch L. What is behavioural activation? A review of the empirical literature. Clin Psychol Rev 2010;30:608-20. http://dx.doi.org/10.1016/j.cpr.2010.04.001.
- IAPT Three-Year Report: The First Million Patients. London: The Stationery Office; 2012.
- Samad Z, Brealey S, Gilbody S. The effectiveness of behavioural therapy for the treatment of depression in older adults: a meta-analysis. Int J Geriatr Psychiatry 2011;26:1211-20. http://dx.doi.org/10.1002/gps.2680.
- Katon W, Schoenbaum M, Fan M-Y, Callahan C, Williams J, Hunkeler E, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry 2005;62:1313-20. http://dx.doi.org/10.1001/archpsyc.62.12.1313.
- Katon W, Von Korff M, Lin E, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042-9. http://dx.doi.org/10.1001/archpsyc.61.10.1042.
Appendix 1 Regulatory approvals
| Trust | R&D approval granted |
|---|---|
| NHS East Riding of Yorkshire | 18 November 2010 |
| NHS Hull | 6 January 2011 |
| NHS North Yorkshire and York | 18 November 2010 |
| NHS Leeds | 29 September 2011 |
| NHS County Durham | 21 October 2011 |
| NHS Darlington | 21 October 2011 |
| NHS Middlesbrough | 21 October 2011 |
| NHS Stockton-on-Tees | 21 October 2011 |
| NHS Hartlepool | 21 October 2011 |
| NHS Redcar and Cleveland | 21 October 2011 |
| Northumberland, Tyne and Wear NHS Foundation Trust | 15 February 2013 |
| NHS North of Tyne | 5 March 2013 |
Appendix 2 The CASPER trial documents
Appendix 2.1 CASPER trial participant invite letter
Appendix 2.2 CASPER trial consent form and background information sheet
Appendix 2.3 CASPER trial decline form
Appendix 2.4 CASPER trial patient information sheet
Appendix 3 Baseline questionnaire
Appendix 4 Exploring risk assessment tool
Appendix 5 The CASPER trial 4-month follow-up questionnaire
Appendix 6 The CASPER trial 12-month follow-up questionnaire
Appendix 7 Suicide protocols
Appendix 7.1 CASPER trial case managers’ suicide protocol

This Reporting Risk Study Specific Procedure (SSP) has been devised to provide guidance for case managers in instances where a participant’s mental well-being causes them concern, specifically when they present with risk of self-harm or suicide. Case managers are part of a system of collaborative care. They do not work alone, but receive weekly supervision from their clinical supervisor and communicate regularly with their participants’ GPs by sending them progress reports. Communication with participants’ GPs is central to the collaborative care process so if case managers are in any way concerned about a participant’s well-being they are obliged to share this information with their GP. As stated in the manual, no case manager should be managing participants at significant risk of suicide or self-harm. If participants express any suicide ideation, case managers must enact this procedure.
Classifying level of risk
Case managers determine a participant’s level of suicide ideation by asking the risk questions provided in the CASPER case manager manual. This will identify whether the suicide ideation was in the past or is in the present (current) and whether it is active or passive. As such, there are four types of risk: past passive, past active, current passive and current active. The procedure for reporting these risks differs – past passive, past active and current passive risks follow a similar procedure; current active risk needs reporting urgently and follows a different procedure.
Reporting risk: non-urgent (past active, past passive and current passive suicide ideation)
In cases where suicide ideation was in the past tense, whether or not it was passive or active, case managers need to document it in the participant’s notes on PC-MIS. There is no need to communicate past risk immediately to either the participant’s GP or their clinical supervisor. Rather, case managers should inform the GP in writing in the next letter they have to send as part of collaborative care progress updates. Similarly, case managers can wait until their next supervision session to raise it with their supervisor; there is no need to contact the supervisor directly. This procedure also applies to current passive risk where no level of current intent exists. However, if in any doubt, always ‘err on the side of caution’ and classify risk as greater than it may actually be. Remember to explain to the participant that you will inform their GP next time you send a progress update letter to them. If the participant objects to this, discuss with your clinical supervisor at the next supervision session who will advise you; in some cases it may not be necessary to inform the GP of past suicide ideation. However, any current passive suicide ideation needs to be communicated to the GP in the next letter to the GP.
Reporting risk: urgent (current active suicide ideation)
If a participant presents current active suicide ideation, the case manager is to explain that they are concerned about the participant’s well-being and will have to inform their (participant’s) GP. First, they must immediately report risk to their clinical supervisor. If no answer to telephone call, text the clinical supervisor’s mobile, alerting them to suicide risk. If unable to contact clinical supervisor, contact GP directly without clinical advice. If participant’s named GP cannot be reached, speak to another GP from the practice. Inform the GP of the participant’s suicide ideation, explaining that it is now their decision as how to proceed with the participant’s care. Remember that even if the participant has objected to you informing their GP, in cases where there is current active suicide ideation the participant’s wish must be overridden. Document the procedure in the participant’s notes on PC-MIS. Fill in the suicide ideation form (overleaf) to be signed off by the clinical supervisor. E-mail the clinical supervisor, chief investigator and trial manager to confirm that participant ‘x’ has presented suicidal ideation, which has been dealt with following the reporting risk procedure. In addition, case manager to send a letter to the GP as confirmation of reporting risk telephone call.
| Site | Role | Name |
|---|---|---|
| York | 1. Clinical Supervisor | |
| 2. Case Manager Supervisor | ||
| 3. Case Manager Supervisor | ||
| 4. Principal Investigator (Chief Investigator) | ||
| Leeds | 1. Clinical Supervisor | |
| 2. Case Manager Supervisor | ||
| 3. Case Manager Supervisor | ||
| 4. Principal Investigator | ||
| Durham | 1. Clinical Supervisor/Principal Investigator | |
| 2. Case Manager Supervisor | ||
| 3. Case Manager Supervisor | ||
| Newcastle upon Tyne | 1. Clinical Supervisor | |
| 2. Risk contact | ||
| 3. Case Manager Supervisor | ||
| 4. Principal Investigator |
Exploring other risk areas
-
Risk from others includes abuse of elders and can take many forms including physical, psychological, emotional, sexual or financial abuse. If risk from others is detected follow Procedure 4, Safeguarding Older Adults.
-
Self-neglect does occur sometimes in older adults. Examples include not eating or caring for one’s physical needs. If concerned about self-neglect contact local site clinical supervisor/principal investigator.
-
Cognitive impairment does occur in older adults and typically presents as loss of memory. If concerned about cognitive impairment contact local site clinical supervisor.
Appendix 7.2 CASPER trial researchers’ suicide protocol
Appendix 8 Zero-inflated negative binomial
| GP appointments | IRR | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 0.9168 | 0.0646 | –1.23 | 0.217 | 0.7985378 to 1.052469 |
| Constant | 6.1156 | 0.2881 | 38.43 | 0 | 5.576135 to 6.707199 |
| GP home visits | IRR | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 0.6808 | 0.1625 | –1.61 | 0.107 | 0.4264942 to 1.086881 |
| Constant | 0.8190 | 0.1774 | –0.92 | 0.356 | 0.5356569 to 1.252091 |
| GP telephone consultations | IRR | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 1.0661 | 0.1302 | 0.52 | 0.6 | 0.8391497 to 1.354371 |
| Constant | 1.8136 | 0.1472 | 7.34 | 0 | 1.546874 to 2.126225 |
| Nurse appointments | IRR | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 0.8501 | 0.0768 | –1.8 | 0.072 | 0.7121701 to 1.014706 |
| Constant | 4.2063 | 0.2488 | 24.29 | 0 | 3.745931 to 4.723286 |
| Nurse telephone consultations | IRR | SE | z | p > z | 95% CI |
|---|---|---|---|---|---|
| Collaborative care | 0.5319 | 0.1797 | –1.87 | 0.062 | 0.2743071 to 1.031285 |
| Constant | 0.3961 | 0.2942 | –1.25 | 0.212 | 0.0923805 to 1.698212 |
Appendix 9 The CASPER trial participant topic guide

Interview topic guide for ‘collaborative care’ participants (one interview only)
This topic guide summarises the main areas to be explored in each interview about mental well-being, collaborative care, adherence to the intervention and issues related to participating in a trial. As with any qualitative interviews, these headings are intended as a starting point to ensure that the primary issues are covered, whilst allowing flexibility for new issues to emerge. Within each group of participants, preliminary analysis of data from earlier interviews will shape the topics covered in later interviews.
Approximately 40 participants randomised to ‘collaborative care plus usual GP care’ will be purposively sampled. Participants will be interviewed once during the study.
Introduction and background
-
Background information on participant (e.g. age, general health, health problems that they are currently experiencing and how these affect their everyday life).
Understanding mental well-being
-
Study participant’s understanding of mental well-being, where they have gained this information from (e.g. family, friends, professionals, media), how much of a problem they perceive this to be.
-
Their thoughts on the relationship between mental well-being and any current health problems they are currently experiencing.
-
Extent to which mental well-being is an important issue to them.
-
Views about how they manage their mental well-being (i.e. keeping their spirits up, feeling fed-up) including medication management.
Views of their personal mental well-being
-
Understanding of ‘risk factors’: what kind of things do they think might cause older adults, friends or themselves to have poor mental well-being (e.g. whether they mention poor social support, loss of friends/family, death of partner, poor health).
-
Experience of being identified as experiencing subthreshold or above-threshold symptoms (e.g. how did this compare with what they were expecting – whether they expected not to be experiencing symptoms, thought their feelings were part of normal ageing).
-
Thoughts on collaborative care:
-
advantages of collaborative care (ease of delivery, method of delivery, meeting with case manager, adherence to collaborative care, maintenance over time, usefulness of collaborative care for mental well-being/future behaviour, practical issues)
-
disadvantages of collaborative care (ease of delivery, method of delivery, meeting with case manager, adherence to collaborative care, maintenance over time, usefulness of collaborative care for mental well-being/future behaviour, practical issues)
-
thoughts on mental well-being and health-care professionals having had collaborative care [any change in beliefs about mental well-being (positive/negative/no change) having been involved in collaborative care].
-
-
Views and feelings about future mental well-being.
-
Views about how well they feel that they cope with difficulties in their life and in comparison to their friends.
Participating in the study
-
Views of their experience of being involved in a study like CASPER.
-
Views about being randomised to the intervention group (e.g. experiences of randomisation process and understanding of this, feelings about completing questionnaires for the study).
-
Views about value of the trial.
Any other issues
-
Any other issue the participant would like to raise.
-
Experiences of taking part in the interview.
-
Clarify what next in terms of CASPER study.
-
Thank them for their time.
Appendix 10 The CASPER trial case manager topic guide

Interview topic guide for case managers (up to three interviews)
This topic guide summarises the main areas to be explored in each interview about mental well-being, collaborative care, adherence to the intervention and issues related to participating in a trial. As with any qualitative interviews, these headings are intended as a starting point to ensure that the primary issues are covered, whilst allowing flexibility for new issues to emerge. Within each group of participants, preliminary analysis of data from earlier interviews will shape the topics covered in later interviews. (This topic guide will be used for each of the interviews to follow up any changes that may have occurred during participation in the study; subsequent interviews will be tailored to explore topics that were highlighted during the initial interview.)
Introduction and background
-
Background information on participant.
Understanding mental well-being
-
Study participant’s understanding of mental well-being.
-
Their thoughts on the relationship between mental well-being and health problems.
-
Extent to which mental well-being is an important issue to them.
-
Views about how they manage mental well-being, including medication management.
-
Understanding of ‘risk factors’: what kind of things do they think might cause older adults, friends or themselves to have poor mental well-being (e.g. whether they mention poor social support, loss of friends/family, death of partner, poor health).
-
Thoughts on collaborative care:
-
advantages of collaborative care (ease of delivery, method of delivery, adherence to collaborative care, maintenance over time, usefulness of collaborative care for mental well-being/future behaviour, practical issues)
-
disadvantages of collaborative care (ease of delivery, method of delivery, adherence to collaborative care, maintenance over time, usefulness of collaborative care for mental well-being/future behaviour, practical issues).
-
-
Views and feelings about future mental well-being.
Participating in the study
-
Views of their experience of being involved in a study like CASPER.
-
Views about value of the trial.
Any other issues
-
Any other issue the participant would like to raise.
-
Experiences of taking part in the interview.
-
Clarify what next in terms of CASPER study.
-
Thank them for their time.
List of abbreviations
- CADET
- CollAborative DEpression Trial
- CASPER
- CollAborative care and active surveillance for Screen-Positive EldeRs with subthreshold depression
- CD-RISC 2
- Connor–Davidson Resilience Scale two-item version
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CM
- case manager
- CONSORT
- Consolidated Standards of Reporting Trials
- DMEC
- Data Monitoring and Ethics Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D
- European Quality of Life-5 Dimensions
- GAD-7
- Generalised Anxiety Disorder seven-item scale
- GP
- general practitioner
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- MCS
- mental component summary
- MINI
- Mini International Neuropsychiatric Interview
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PC-MIS
- Patient Case Management Information System
- PCS
- physical component summary
- PHQ-9
- Patient Health Questionnaire-9 items
- PHQ-15
- Patient Health Questionnaire-15 items
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee