Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/403/51. The contractual start date was in October 2008. The draft report began editorial review in June 2015 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Hywel C Williams is Programmes Director for the Health Technology Assessment programme.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Chalmers et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
What is pemphigoid?
Bullous pemphigoid (BP) is the most common form of a group of autoimmune pemphigoid diseases characterised by autoantibodies directed at skin adhesion proteins of the epidermal–dermal junction. 1 BP often starts with intense itching followed by an urticated and/or eczematous-looking rash and tense blisters on erythematous or normal-looking skin (Figure 1). The blisters may develop several months after the appearance of the initial symptoms and may be quite large and filled with blood. They often break down to form raw skin erosions, which can become infected.
FIGURE 1.
Large tense blisters and erosions in a man with BP (by kind permission of Professor Enno Schmidt with written consent from the patient).
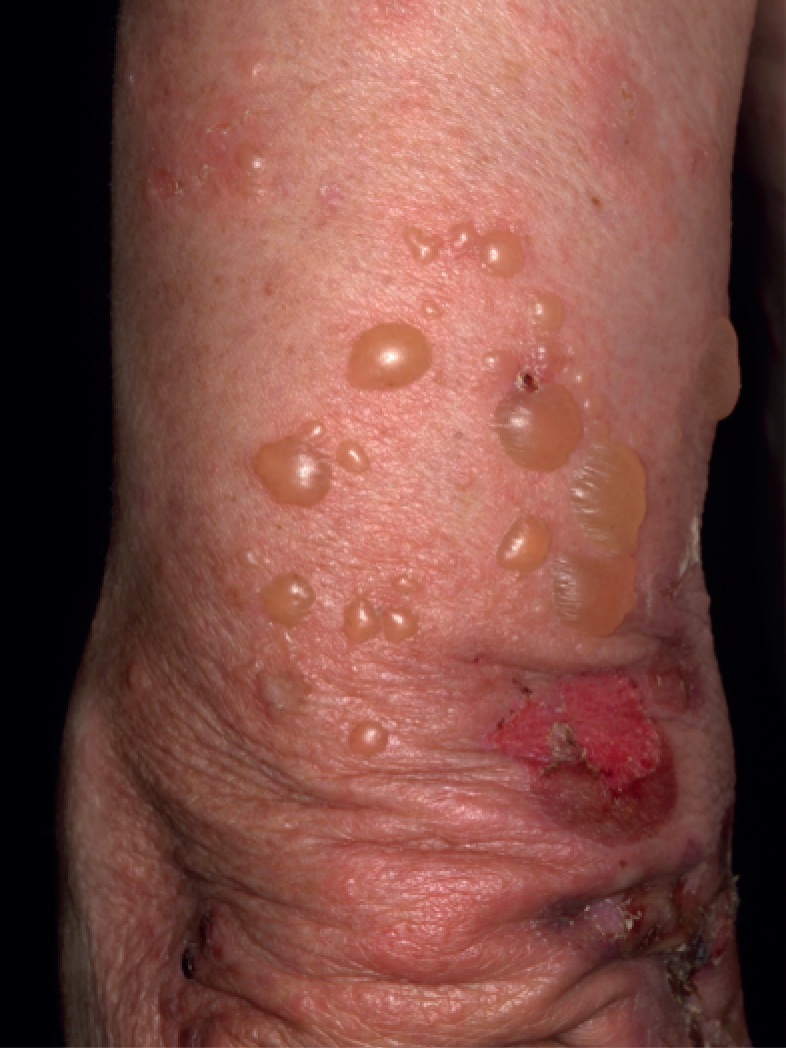
The pain and severe itching associated with BP can greatly impair quality of life. BP typically affects people aged around 80 years and the incidence, which appears to be on the increase, is estimated to be between 6 and 43 cases per million per year. 2,3 The cause of BP is unknown but it is associated with neurological conditions such as cerebrovascular disease, dementia, Parkinson’s disease, epilepsy and multiple sclerosis4,5 and the chronic use of several drugs including spironolactone (Aldactone®, Pharmacia Ltd), neuroleptics, certain diuretics and phenothiazines in some patients. 4,6–9 Neurological disease has been suggested as a predisposing and prognostic factor. 10
Increased mortality
Mortality rates are increased for people with BP. In one study the risk of death was more than six times that of the general population. 11 Mortality rates for people with BP of between 11% and 41% have been suggested to be related to advanced age and comorbid medical conditions rather than the disease itself. 12,13 It is likely that the increase in mortality rate is owing, at least in part, to side effects of oral prednisolone, which is commonly used to treat BP. 14–17
Topical treatment
Some widely reported studies have shown that superpotent topical corticosteroids applied to the whole skin surface are effective and safer than oral corticosteroids. 18,19 Practical guidance on how this can be carried out is provided elsewhere. 15 However, the application of topical steroids to the whole body for weeks or possibly months is not a practical option in many elderly outpatients with limited support. Therefore, there still remains a need for a convenient oral treatment that is both effective and safe.
Rationale for testing tetracyclines further
Treatment of BP with antibiotics possessing anti-inflammatory actions, such as those from the tetracycline group, is widely used in clinical practice. In a national survey of 326 UK dermatologists conducted in 2013, around 80% stated that they had used doxycycline (Efracea®, Galderma), minocycline (Aknemin®, Almirall Hermal GmbH) or lymecycline (Tetralysal®, Galderma). 20 However, a Cochrane systematic review21 found only one small poorly reported clinical trial22 and concluded that further evidence was needed before the effectiveness of tetracyclines could be established. No further trials of tetracyclines were identified in a subsequent systematic review23 nor in a search of the Cochrane Central Register of Controlled Trials using ‘pemphigoid’ as a search term (28 May 2015). The limited data available and practical experience to date suggest that it is very unlikely that tetracyclines would be more effective than long-term use of oral corticosteroids, yet they are likely to be safer in this elderly population given the known adverse effects of oral corticosteroids, including diabetes mellitus, serious infections and osteoporosis, leading to fractures and death. Because some patients with BP might not respond to tetracyclines at all, it was clearly important to test how they might be used in clinical practice by permitting additional application of potent topical corticosteroids to affected areas or a switch to oral corticosteroids if symptoms and blister control were inadequate. Similarly, in the UK, those initiated on oral steroids are typically given topical corticosteroids for localised application to blisters to help with initial control and doses of oral corticosteroids are typically adjusted over several months, according to blister control or side effects. We therefore sought to investigate whether or not a strategy of initial treatment of BP with anti-inflammatory tetracyclines (200 mg/day of doxycycline) is effective enough to produce an acceptable degree of blister control compared with initial treatment with oral corticosteroids (0.5 mg/kg/day of prednisolone) in a non-inferiority comparison and whether or not tetracyclines confer a long-term advantage in terms of safety over oral corticosteroids in a superiority comparison.
Chapter 2 Methods
Parts of this report are based on Williams et al. 24 This article is published open access under the terms of the Creative Commons Attribution 4.0 licence (CC-BY) (https://creativecommons.org/licenses/by/4.0).
Trial design
The Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) trial was a two-arm, parallel-group, multicentre, multinational randomised controlled trial of 52 weeks’ duration. The design was towards the pragmatic end of the explanatory–pragmatic spectrum. 25 We recruited patients with BP from the UK and Germany who had not started systemic treatment. Participants were randomised to receive either doxycycline or prednisolone as initial treatment and were followed up at weeks 3, 6, 13, 26, 39 and 52, with unscheduled visits as required to reflect normal clinical care.
The two primary outcomes in this trial reflect the need for patients and clinicians to balance the likely differences in effectiveness and safety for oral prednisolone and doxycycline when making a shared decision on treatment. Although topical corticosteroids have been shown to be effective for BP, it is not always practical or cost-effective to admit elderly people into hospital for whole body application15 and therefore oral treatment alternatives are needed. Although oral prednisolone is thought to be effective at reducing the blisters in BP, it has many side effects as indicated in previous trials comparing topical corticosteroids with oral corticosteroids. 18,19,26 Doxycycline, on the other hand, is perceived to be less effective by clinicians but probably has fewer side effects. Therefore, a non-inferiority comparison was used to assess effectiveness, the results of which could be considered alongside the superiority comparison of safety in clinical decision-making.
The trial protocol was published prior to the analysis. 27
Choice of intervention
At baseline, patients were randomised to receive either 0.5 mg/kg/day of prednisolone or 200 mg/day of doxycycline, both taken as a single, daily dose (brand not specified). We chose a starting dose of 0.5 mg/kg/day for prednisolone based on safety concerns over higher doses such as 0.75–1 mg/kg/day highlighted in the Cochrane systematic review. 22 The study protocol encouraged investigators to stick to the allocated treatment and dose for the first 6 weeks unless it was medically necessary to change, but after the 6-week effectiveness assessment investigators were free to modify the dose as needed, switch to the other treatment arm or administer an alternative treatment if appropriate, to reflect normal clinical practice. Participants were followed up for the full 52 weeks when possible, regardless of any changes to treatment. Participants recorded study medication use in their diary.
Rescue medication
Up to 30 g/week of topical corticosteroids in the potent class [preferably mometasone furoate (Elocon®, Merck Sharp & Dohme Ltd)] was permitted throughout the study, except between weeks 3 and 6. The cessation of topical corticosteroids from week 3 to week 6 provided a washout period to minimise the potential effect of systemic absorption of topical corticosteroids on the primary effectiveness outcome at 6 weeks. To minimise potential systemic effects, topical corticosteroids were applied only to blisters and erosions. Participants were permitted to apply a moisturiser to blisters and erosions at any time.
Choice of tetracycline
Doxycycline was chosen for this trial because (1) it is associated with a lower incidence of gastrointestinal side effects than other tetracyclines and (2) the alternative option of oxytetracycline would have required participants to swallow approximately eight large tablets a day.
Although the only published randomised controlled trial investigating tetracycline antibiotics for the treatment of BP used a combination therapy of tetracycline plus nicotinamide,21 a single therapy of doxycycline was chosen for this trial to allow the effects of the tetracycline to be clearly defined.
Trial outcomes
We did not identify any core outcome sets for BP when this study was designed, although some consensus criteria have subsequently been suggested by an international group. 28
Primary outcomes
The primary outcomes were the absolute difference between the two treatment arms in the:
-
Non-inferiority comparison. The proportion of participants classed as a treatment success (three or fewer significant blisters present on examination) at 6 weeks. A significant blister was defined as an intact fluid-filled blister at least 5 mm in diameter or a ruptured blister with a flexible (not dry) roof over a moist base. Mucosal blisters were excluded from the count.
-
Superiority comparison. The proportion of participants with grade 3 (severe), 4 (life-threatening) and 5 (death) adverse events that were possibly, probably or definitely related to the treatment in the 52 weeks following randomisation. A modified version of the Common Terminology Criteria for Adverse Events v3.0 was used [see http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 12 November 2015)] and the relatedness of grade 5 (fatal) adverse events was judged by an independent adjudicator. Although only grade 3 and above related adverse events were captured in the primary outcome, less medically important related adverse events (such as weight gain and skin fragility) can bother patients when taking corticosteroids and so were included in a secondary outcome.
For the primary outcome, treatment success was defined as three or fewer significant blisters, regardless of whether or not treatment had been modified because of a poor response (either by changing the dose or by changing the treatment) during the first 6 weeks. However, for all secondary and tertiary end points, participants were classed as a treatment success at each visit only if (1) they had three or fewer significant blisters present on examination and (2) their treatment had not been altered because of a poor response prior to that visit.
Secondary outcomes
The secondary outcomes were the absolute difference between the two treatment arms in the following:
-
non-inferiority comparisons:
-
proportion of participants classed as a treatment success (three or fewer significant blisters present on examination and no treatment modification) at 6 weeks
-
proportion of participants classed as a treatment success at 13 and 52 weeks
-
proportion of participants who had a further episode of BP during the study
-
-
superiority comparisons:
-
proportion of participants reporting adverse events of any grade that were possibly, probably or definitely related to BP medication in the 52 weeks following randomisation
-
quality of life [European Quality of Life-5 Dimension (EQ-5D) and Dermatology Life Quality Index (DLQI) questionnaires at 6, 13, 26, 39 and 52 weeks]
-
cost-effectiveness over 12 months from a NHS perspective
-
-
combined comparison: proportion of participants classed as a treatment success at 6 weeks and who were alive at 52 weeks.
Tertiary outcomes
The tertiary outcomes were the absolute difference between the two treatment arms in the following:
-
non-inferiority comparisons:
-
proportion of participants completely blister free at 6 weeks
-
proportion of participants classed as a treatment success at 3 weeks (to compare the speed of onset of action)
-
-
superiority comparisons:
-
mortality over the 52-week follow-up period
-
amount of potent and superpotent topical corticosteroids used during the 52 weeks following randomisation.
-
Participants
Adults (aged ≥ 18 years) who were capable of giving written informed consent and who had a clinical diagnosis of BP were eligible. To ensure that active disease was present, at least three significant blisters (defined as intact, fluid-filled blisters measuring ≥ 5 mm) must have appeared within the week prior to screening and must have been present across at least two body sites. Recent erosions could be included provided that they had a flexible (not dry) roof over a moist base. Positive direct (skin biopsy) or indirect (serum) immunofluorescence [immunoglobulin G (IgG) and/or complement component 3 (C3) at the epidermal basement membrane zone) was required to confirm diagnosis. Anonymised samples were tested at the Immunofluorescence Laboratory at the Department of Dermatology, John Radcliffe Hospital, Oxford, and the Institute of Dermatology Immunodermatology Laboratory at St John’s Institute of Dermatology, St Thomas’s Hospital, London. Samples with appropriately obtained consent were sent to the Clinical Immunological Laboratory, University of Lübeck, Germany, for additional immunology substudies, which will be published separately.
Patients must have been free of blisters and have not received treatment for previous episodes of BP in the preceding year. Patients were excluded if they had predominantly mucosal pemphigoid, had received any systemic medication for the current episode of BP or had received oral prednisolone or doxycycline for any other conditions in the preceding 12 weeks. Women of childbearing potential who were not taking adequate contraception, as well as those who were pregnant or who planned to become pregnant during the study or who were currently lactating, were excluded. Additional exclusions for safety reasons were live virus vaccine administration within the previous 3 months, allergy to any member of the tetracycline family or a pre-existing condition or use of a medication that precluded the use of either study drug or that made the patient unsuitable for this trial, as assessed by the investigator.
Retention of participants
To help retain participants in the study, in addition to being able to speak to the investigator, participants were able to telephone the trial manager if they wished to discuss any aspect of the study. For medical queries, participants were directed to a medical member of staff. In addition, the trial administrator made telephone calls to participants to support them throughout the duration of the study. Participants were sent birthday and Christmas cards while they were participating in the study.
Withdrawal of participants
Patients whose immunofluorescence test results were not available until after randomisation and which were subsequently both negative, indicating that they did not have BP, were withdrawn from the trial, replaced and not included in the analysis. This approach reflects normal practice: if there is a clinical picture of BP, treatment is commenced and this is changed later if the laboratory tests are subsequently negative.
Participants who withdrew from study treatment were followed up for the remainder of the year unless they had withdrawn their consent.
Informed consent
Patients presenting with suspected BP were assessed by the recruiting investigator as per normal clinical practice. If a diagnosis of BP was suspected, the patient was given details of the study verbally. If the patient was interested in taking part he or she was given time to read the full participant information leaflet and the investigator answered any questions. A consent form was signed before any study procedures were carried out.
If the disease severity was such that immediate oral treatment was required or the patient did not wish to delay the start of treatment, he or she was able to give consent and be randomised to the study during the first visit to the dermatologist. Otherwise, the patient was given a second appointment for consent and randomisation.
Recruitment
To meet the target for this rare disease, recruitment took place at a large number of hospitals, mainly in dermatology clinics (54 in the UK and seven in Germany).
Randomisation
Randomisation was based on a computer-generated pseudorandom code using random permuted blocks of randomly varying size, created by the Nottingham Clinical Trials Unit (NCTU) in accordance with its standard operating procedure and held on a secure server. Access to the sequence during the trial was confined to the NCTU data manager.
Participants were allocated in a 1 : 1 ratio to the doxycycline and prednisolone treatment arms. Randomisation was stratified by disease severity, which was defined as the number of blisters present at baseline (mild: 1–9; moderate: 10–30; severe: > 30).
The investigator or research nurse randomised participants using the web-based NCTU randomisation system. The treatment allocation was sent directly to the pharmacist who dispensed the appropriate medication directly, which allowed the investigator to remain blinded.
Blinding
Investigators (outcome assessors) were unaware of treatment allocation and remained blinded to treatment allocation for the first 6 weeks of the trial. At the week 6 visit, the investigators carried out the blister count (primary effectiveness outcome) while blinded to treatment allocation. The investigators were unblinded for the remaining assessments: that is, for the primary safety outcome (adverse events over the full 52 weeks) and the long-term effectiveness outcomes. Unblinding before the 6-week point was permitted if required for treatment decisions that affected patient safety. Investigators were asked at week 6 if they were aware of the treatment allocation prior to carrying out the blister count for the primary effectiveness outcome to capture the rate of unblinding. A subgroup analysis to assess any potential bias of unblinding on the blister count at 6 weeks was performed. The patients and pharmacists were not blinded to treatment allocation.
Sample size
In total, 256 participants were needed to detect a clinically important absolute difference of 20% in grade 3, 4 and 5 (mortality) side effects within 1 year of randomisation (primary safety outcome). This was based on an expected 60% incidence with prednisolone compared with 40% with doxycycline29 with 80% power at the 5% significance level allowing for a 20% loss to follow-up by 1 year using a 1 : 1 allocation ratio. A survey of UK dermatologists showed that a 20% absolute reduction in side effects was considered to be an acceptable and worthwhile clinical difference. More detailed results can be found in Appendix 1.
The effectiveness outcome at 6 weeks was expressed as a two-sided 90% confidence interval (CI) for the absolute difference in success rates (based on blister count) between the prednisolone (control) arm and the doxycycline (intervention) arm. It was assumed that the point estimate for this difference would be 25%, based on an expected response rate of 95% in the control (prednisolone) arm and 70% in the intervention (doxycycline) arm. The acceptable non-inferiority margin was set at 37% based on the upper bound of the 90% CI for an expected 25% difference. Because the non-inferiority margin is inversely proportional to the sample size, the number of patients who we could realistically expect to recruit in a rare disease of the elderly was factored in when setting the non-inferiority margin. The closer to the expected difference of 25% we set the non-inferiority margin, the larger the sample size that would be required. With 80% power, a total of 111 evaluable participants per group was required. The attrition rate in the initial 6 weeks was expected to be low (5%) and so a total of 234 participants was required, that is, within the 256 required for the primary safety outcome.
Analysis
All superiority analyses were conducted on a modified intention-to-treat (mITT) basis and all non-inferiority analyses were performed on both the mITT and the per-protocol (PP) population according to recommended practice. 30
The mITT population consisted of those participants who fulfilled the eligibility criteria, who were randomised to receive either study drug and who had data on the outcome of interest.
For each non-inferiority outcome, the doxycycline arm was considered to be non-inferior to the prednisolone arm if the upper bound of the CI for the difference in proportions was less than the agreed non-inferiority margin of an absolute difference of 37% in both the mITT and the PP analyses.
All analyses were adjusted for baseline disease severity to optimise power and reduce possible imbalances in possible response predictors. Age and Karnofsky score31 were also adjusted for in analyses as continuous variables when possible using a binomial regression with identity links. Methods for dealing with missing data can be found in the statistical analysis plan [see www.nottingham.ac.uk/research/groups/cebd/projects/5rareandother/index.aspx (accessed 19 May 2015)]. Multiple imputation was used to handle missing data because of missed visits for the primary safety analysis.
Patients were excluded from the PP analysis of the primary outcome if, before their 6-week visit, for reasons other than treatment success or failure, they had:
-
increased the dose of their allocated treatment
-
changed treatment or added a new treatment to their allocated treatment (for a reason other than for treatment failure or success)
-
used topical steroids between visit weeks 3 and 6
-
missed more than 3 consecutive days of treatment.
For non-inferiority outcomes after week 6, the PP populations consisted of those participants who were included in the PP analysis of the 6-week primary effectiveness outcome and who had:
-
not missed more than 3 consecutive weeks of allocated treatment between 6 and 52 weeks (regardless of whether the dose had been increased or decreased) unless they had stopped for good clinical response
-
used no more than 30 g of topical steroids per week after week 6
-
not added systemic steroids to doxycycline (if allocated) or doxycycline or an immunosuppressant to prednisolone (if allocated) unless for poor clinical response.
For the non-inferiority outcomes, 90% CIs are presented. For the study treatment doxycycline to be considered non-inferior to the control treatment, the upper bound of the 90% CI should fall below 37%. For the superiority outcomes, 95% CIs are presented. A difference between treatment arms was considered statistically significant at the 5% level, that is, the 95% CIs do not contain zero (no difference between arms).
The primary analysis had a dual outcome, one primary outcome for effectiveness and one for safety. For the study treatment doxycycline to be considered acceptable as an alternative to prednisolone, non-inferiority had to be demonstrated (as defined above) with regard to effectiveness, as well as the superiority of doxycycline over prednisolone for safety.
Subgroup analyses
For some patients the blister count at 6 weeks may have been performed by an investigator who knew what treatment the patient was on. To determine whether or not this introduced bias into the results of the 6-week effectiveness outcome, an interaction test was performed to compare the treatment effects in patients who were and patients who were not assessed by an investigator who knew the treatment allocation. This interaction analysis was performed on both the primary and the secondary definition of treatment success at 6 weeks.
Subgroup analyses of treatment success (using both definitions) at 6 weeks by the three categories of baseline disease severity (mild, moderate and severe) were also performed. A global test for a treatment interaction was used to determine whether or not treatment effects were different in the three categories of disease severity.
Cost-effectiveness
The EuroQoL tool
The EuroQol tool is a two-page questionnaire consisting of the EQ-5D descriptive system and the EuroQol visual analogue scale (EQ VAS). 32 The tool is a standardised measure of current health status developed by the EuroQol group for clinical and economic studies. The EQ-5D three-level version (EQ-5D-3L) was used, consisting of five questions addressing the health dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension is assessed at three levels: no problems, some problems and extreme problems. EQ-5D and EQ VAS data were collected using patient-completed questionnaires at baseline and 6, 13, 26, 39 and 52 weeks. Scores were converted to a single health-related index ranging from 0 (death) to 1 (perfect health), with negative scores possible for some health states. Patients who died during the study were subsequently scored 0 at later scheduled follow-up visits.
Resource use
Resource use assessments were carried out at 3, 6, 13, 26, 39 and 52 weeks during mandatory clinical visits and were augmented by telephone calls. Recall was assisted by the use of patient diaries. Patients’ use of study and non-study drugs was recorded and costed using weighted average prices determined from Prescription Cost Analysis (PCA) data. 33 Health service contacts were recorded by asking patients to recall general practitioner (GP) clinic and home visits, practice and district nurse visits, outpatient visits and inpatient stays. Health-care resource use was costed using published national reference costs. 34–36 Patient-level resource costs were estimated as the sum of resources used weighted by their national reference costs.
Economic analysis
The economic analysis followed intention-to-treat (ITT) principles and a prospectively agreed analysis plan (see Appendix 2). No discounting was applied to economic data reflecting the follow-up period of 1 year. Costs were estimated in UK pounds sterling using patient resource use and 2013 reference costs. 35 The analysis took an English NHS perspective, reporting generic (EQ-5D, EQ VAS)32 and disease-specific (DLQI)37 health outcomes. Repeated scores over time were used to construct area under the curve (AUC) estimates for each patient, using the trapezoidal method. Missing values at individual follow-up points were managed using two scenarios: multiple imputation (the base-case analysis38) and analysis of complete cases (in which patients with any missing data were excluded).
Patient estimates of costs and quality-adjusted life-years (QALYs) at 1 year were used to derive an estimate of the cost-effectiveness of doxycycline-initiated therapy compared with prednisolone-initiated therapy for patients with BP. Estimates using imputed missing data provided the base-case analysis and estimates using complete data provided supportive sensitivity analysis. Analysis and modelling were undertaken in Stata 13 (StataCorp, College Station, TX, USA). The base-case analysis included the imputed within-trial incremental cost/QALYs gained, adjusted for trial covariates (age, sex, baseline blister severity and baseline Karnofsky score). QALY estimates were also adjusted for baseline EQ-5D score.
Chapter 3 Results
Study population
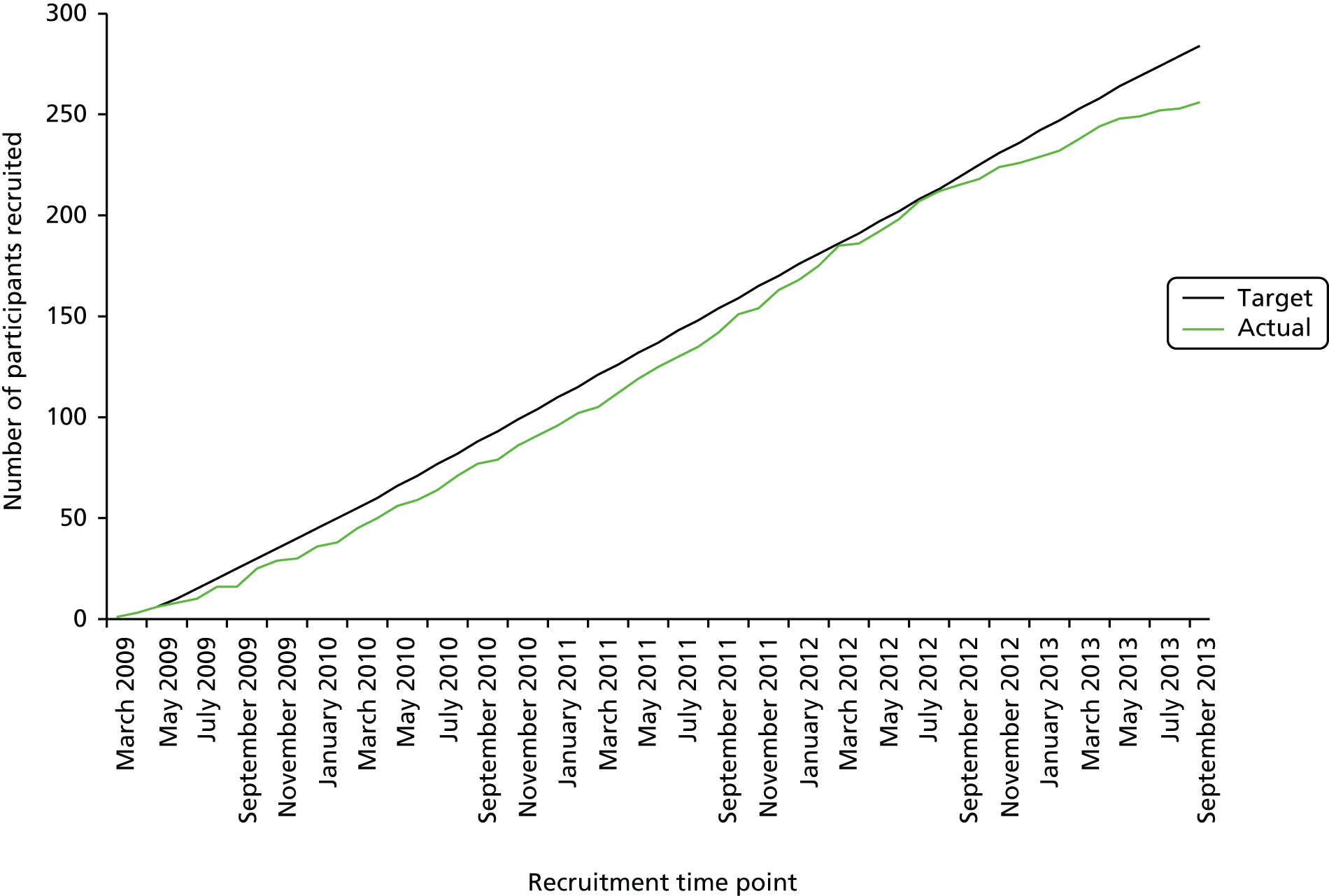
Recruitment commenced in March 2009 in the UK and in February 2010 in Germany and was completed in October 2013. In total, 278 patients were randomised from 54 centres in the UK and seven centres in Germany (Figure 2).
FIGURE 2.
Recruitment graph.
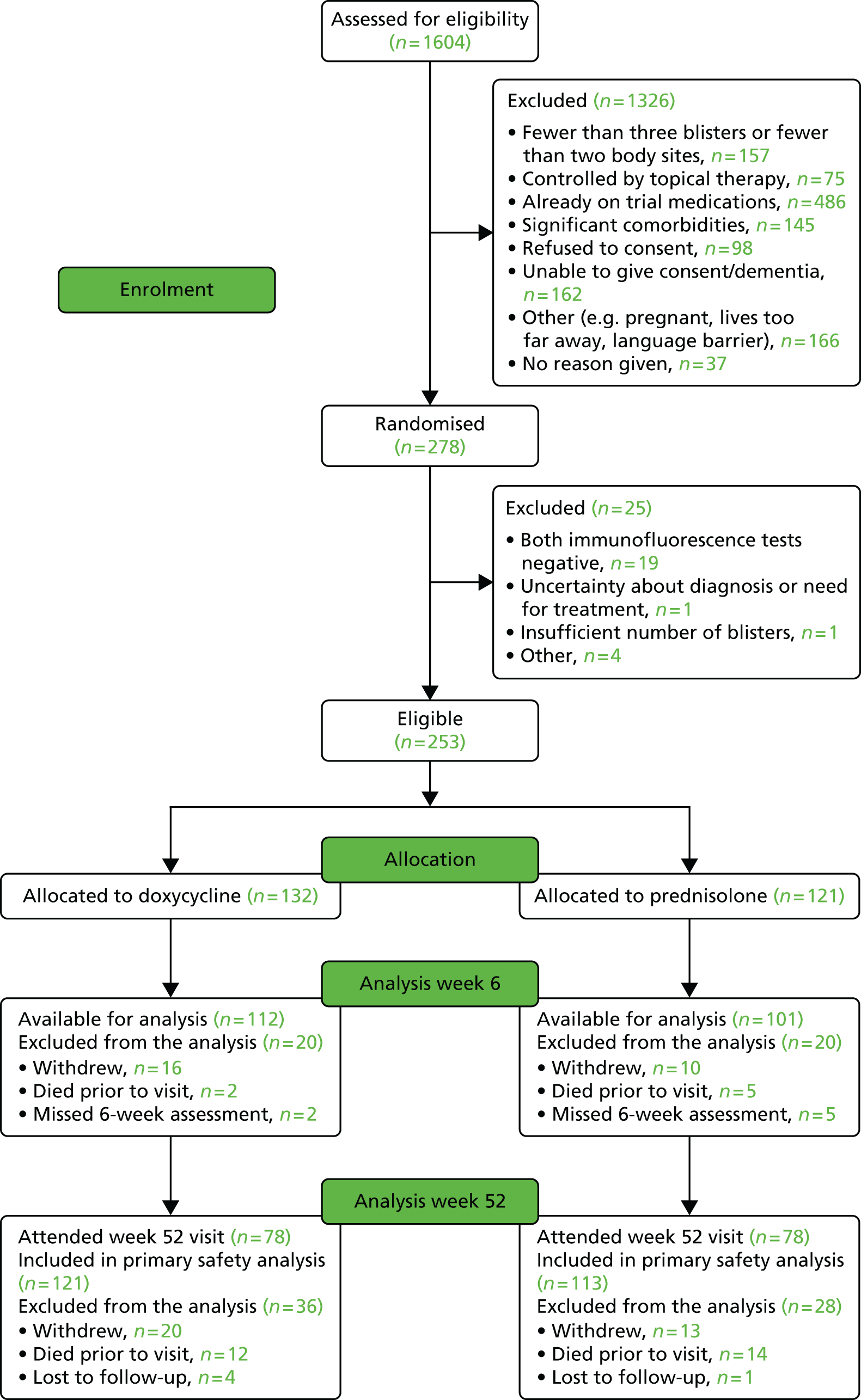
Of the 1604 patients screened for eligibility, 1326 were excluded, mainly because of an inability to provide informed consent, frailty or their disease being too mild or because they had already been started on prednisolone by their GP (Figure 3). Of the 278 patients randomised, 140 were allocated to the doxycycline arm and 138 to the prednisolone arm (Table 1). However, 19 patients were excluded because both the direct and the indirect immunofluorescence tests were negative and a further six were excluded for other eligibility reasons. Therefore, 253 patients were included in the analyses, 132 in the doxycycline arm and 121 in the prednisolone arm (see Figure 3).
FIGURE 3.
Consolidated Standards of Reporting Trials flow chart. Those patients excluded from analysis at week 6 because they missed the week 6 assessment are included in the denominator at week 52 as they have the possibility of attending a visit after week 6 and therefore are not considered lost to follow-up at week 6. Patients who did not attend their week 52 visit are designated as lost to follow-up at week 52. Reproduced from Williams et al. 24 under the terms of the Creative Commons Attribution 4.0 licence (CC-BY) (https://creativecommons.org/licenses/by/4.0).
| Patient status | Doxycycline | Prednisolone | Total |
|---|---|---|---|
| Number randomised | 140 | 138 | 278 |
| Number withdrawn because of ineligibility | 8 | 17 | 25 |
| Reasons for ineligibility | |||
| Both direct and indirect immunofluorescence tests were negative | 4 | 15 | 19 |
| Investigator felt on reflection that patient did not have sufficient blisters to meet the inclusion criteria and therefore should be withdrawn from the trial | 0 | 1 | 1 |
| Uncertainty initially about diagnosis, negative indirect immunofluorescence, need for treatment | 1 | 0 | 1 |
| Patient ineligible for other reasons | 3 | 1 | 4 |
| Number to be analysed | 132 | 121 | 253 |
Baseline characteristics
Of the 253 eligible patients randomised, 52.6% were men and 47.4% were women. The average age was 77.7 years, with 25.3% aged > 85 years, 37.9% aged from 75 to < 85 years, 28.1% aged from 65 to < 75 years and 8.7% aged < 65 years. There was a good distribution of baseline severity of disease: 29.3% of patients had severe BP (> 30 blisters), 39.1% had moderate disease (10–30 blisters) and 31.6% had mild disease (three to nine blisters). The two groups were also balanced for Karnofsky score of functional impairment and ethnicity (Table 2).
| Characteristic | Doxycycline, n (%) | Prednisolone, n (%) | Total, N (%) |
|---|---|---|---|
| Female | 63 (47.7) | 57 (47.1) | 120 (47.4) |
| Male | 69 (52.3) | 64 (52.9) | 133 (52.6) |
| Age (years)a | 78.1 (9.5) | 77.2 (10.0) | 77.7 (9.7) |
| < 65 | 8 (6.1) | 14 (11.6) | 22 (8.7) |
| 65 to < 75 | 38 (28.8) | 33 (27.3) | 71 (28.1) |
| 75 to < 85 | 51 (38.6) | 45 (37.2) | 96 (37.9) |
| ≥ 85 | 35 (26.5) | 29 (24.0) | 64 (25.3) |
| Karnofsky scorea | 69.0 (18.3) | 70.5 (17.6) | 69.7 (18.0) |
| < 40 | 3 (2.3) | 1 (0.8) | 4 (1.6) |
| 40 to < 55 | 32 (24.2) | 26 (21.5) | 58 (22.9) |
| 55 to < 70 | 21 (15.9) | 24 (19.8) | 45 (17.8) |
| 70 to < 85 | 45 (34.1) | 38 (31.4) | 83 (32.8) |
| ≥ 85 | 31 (23.5) | 32 (26.4) | 63 (24.9) |
| Unable to care for self | 16 (12.1) | 11 (9.1) | 27 (10.7) |
| Unable to work | 55 (41.7) | 51 (42.1) | 106 (41.9) |
| Ableb | 61 (46.2) | 59 (48.8) | 120 (47.4) |
| Ethnicity | |||
| White | 112 (84.8) | 100 (82.6) | 212 (83.8) |
| Black – African | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Black – other | 0 | 1 (0.8) | 1 (0.4) |
| Asian – Indian | 2 (1.5) | 1 (0.8) | 3 (1.2) |
| Asian – Chinese | 1 (0.8) | 0 | 1 (0.4) |
| Asian – other | 2 (1.5) | 1 (0.8) | 3 (1.2) |
| Other | 0 | 1 (0.8) | 1 (0.4) |
| Not known/given | 14 (10.6) | 16 (13.2) | 30 (11.9) |
| Severity of BP | |||
| Mild (3–9 blisters) | 42 (31.8) | 38 (31.4) | 80 (31.6) |
| Moderate (10–30 blisters) | 53 (40.2) | 46 (38.0) | 99 (39.1) |
| Severe (> 30 blisters) | 37 (28.0) | 37 (30.6) | 74 (29.2) |
| Total n | 132 | 121 | 253 |
Withdrawals
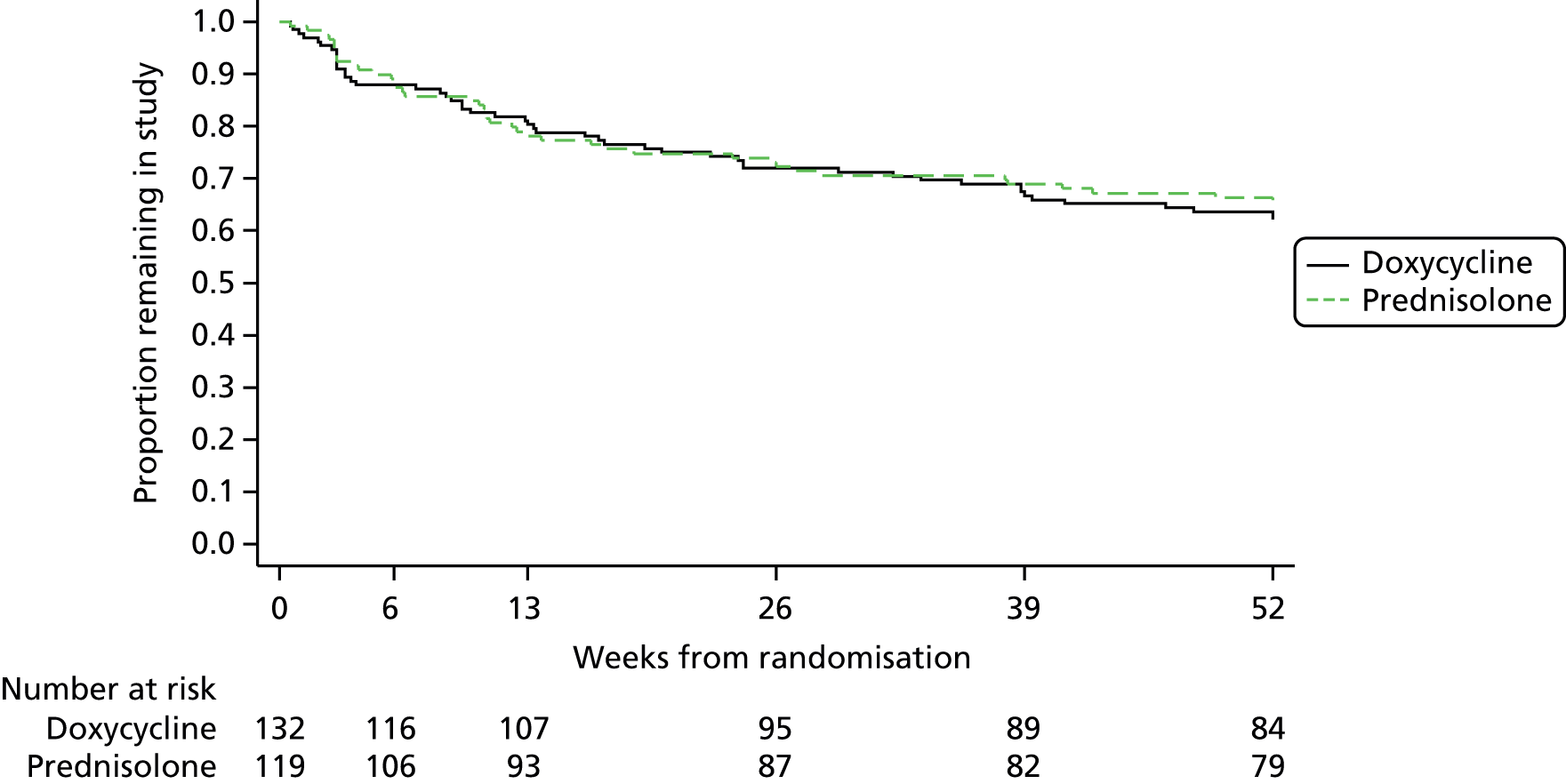
A total of 92 patients (36.8%) withdrew from the trial with a similar rate between the two arms (Tables 3 and 4). The most common reasons were withdrawal of consent and patient died. The withdrawal rate remained similar throughout the 52-week follow-up period (Table 5 and Figure 4).
| Primary reason for withdrawal | Doxycycline (n = 132), n | Prednisolone (n = 121), n | Total, N |
|---|---|---|---|
| Death | 14 | 19 | 33a |
| Adverse event | 2 | 1 | 3 |
| Lost to follow-up | 5 | 4 | 9 |
| Treatment failure | 4 | 1 | 5 |
| Withdrew consent | 23 | 16 | 39 |
| Unable to tolerate trial medications | 1 | 0 | 1 |
| Other | 1 | 1 | 2 |
| Total | 50 | 42 | 92 |
| Week | Patient status | Doxycycline (n = 132), n (%) | Prednisolone (n = 121), n (%) | Total (N = 253), N (%) |
|---|---|---|---|---|
| 3 | Seen | 120 (90.9) | 110 (90.9) | 230 (90.9) |
| Died before visit | 1 (0.8) | 4 (3.3) | 5 (2.0) | |
| Withdrew before visit | 9 (6.8) | 5 (4.1) | 14 (5.5) | |
| Visit not conducted/status not known | 2 (1.5) | 2 (1.7) | 4 (1.6) | |
| Total expected | 132 | 121 | 253 | |
| 6 | Seen | 112 (91.8) | 101 (90.2) | 213 (91.0) |
| Died before visit, but after last scheduled visit | 1 (0.8) | 1 (0.9) | 2 (0.9) | |
| Withdrew before visit, but after last scheduled visit | 7 (5.7) | 5 (4.5) | 12 (5.1) | |
| Visit not conducted/status not known | 2 (1.6) | 5 (4.5) | 7 (3.0) | |
| Total expected | 122 | 112 | 234 | |
| 13 | Seen | 99 (86.8) | 94 (88.7) | 193 (87.7) |
| Died before visit, but after last scheduled visit | 5 (4.4) | 5 (4.7) | 10 (4.5) | |
| Withdrew before visit, but after last scheduled visit | 3 (2.6) | 6 (5.7) | 9 (4.1) | |
| Visit not conducted/status not known | 7 (6.1) | 1 (0.9) | 8 (3.6) | |
| Total expected | 114 | 106 | 220 | |
| 26 | Seen | 88 (83.0) | 81 (85.3) | 169 (84.1) |
| Died before visit, but after last scheduled visit | 2 (1.9) | 6 (6.3) | 8 (4.0) | |
| Withdrew before visit, but after last scheduled visit | 9 (8.5) | 4 (4.2) | 13 (6.5) | |
| Visit not conducted/status not known | 7 (6.6) | 4 (4.2) | 11 (5.5) | |
| Total expected | 106 | 95 | 201 | |
| 39 | Seen | 81 (85.3) | 79 (92.9) | 160 (88.9) |
| Died before visit, but after last scheduled visit | 3 (3.2) | 2 (2.4) | 5 (2.8) | |
| Withdrew before visit, but after last scheduled visit | 5 (5.3) | 3 (3.5) | 8 (4.4) | |
| Visit not conducted/status not known | 6 (6.3) | 1 (1.2) | 7 (4.4) | |
| Total expected | 95 | 85 | 180 | |
| 52 | Seen | 78 (89.7) | 78 (97.5) | 156 (93.4) |
| Died before visit, but after last scheduled visit | 2 (2.3) | 1 (1.3) | 3 (1.8) | |
| Withdrew before visit, but after last scheduled visit | 3 (3.4) | 0 | 3 (1.8) | |
| Visit not conducted/status not known | 4 (4.6) | 1 (1.3) | 5 (3.0) | |
| Total expected | 87 | 80 | 167 |
| Week | Patient status | Doxycycline (n = 132), n (%) | Prednisolone (n = 121), n (%) | Total (N = 253), N (%) |
|---|---|---|---|---|
| 3 | Died before visit | 1 (0.8) | 4 (3.3) | 5 (2.0) |
| Withdrew before visit | 9 (6.8) | 5 (4.1) | 14 (5.5) | |
| 6 | Died before visit | 2 (1.5) | 5 (4.1) | 7 (2.8) |
| Withdrew before visit | 16 (12.1) | 10 (8.3) | 26 (10.3) | |
| 13 | Died before visit | 7 (5.3) | 10 (8.3) | 17 (6.7) |
| Withdrew before visit | 19 (14.4) | 16 (13.2) | 35 (13.8) | |
| 26 | Died before visit | 9 (6.8) | 16 (13.2) | 25 (9.9) |
| Withdrew before visit | 28 (21.2) | 20 (16.5) | 48 (19.0) | |
| 39 | Died before visit | 12 (9.1) | 18 (14.9) | 30 (11.9) |
| Withdrew before visit | 33 (25.0) | 23 (19.0) | 56 (22.1) | |
| 52 | Died before visit | 14 (10.6) | 19 (15.7) | 33 (13.0) |
| Withdrew before visit | 36 (27.3) | 23 (19.0) | 59 (23.3) |
FIGURE 4.
Kaplan–Meier survival plot showing time to withdrawal (including death).
Adherence to interventions for primary effectiveness at 6 weeks
Of the participants who had data available for the week 6 primary effectiveness analysis (mITT population), 18.8% in the doxycycline group missed > 3 days of treatment during the first 6 weeks, compared with 5.0% in the prednisolone group (Table 6). Nausea was cited as the most frequent reason for reduced adherence to doxycycline in the first 6 weeks (mentioned in 10/21 cases).
| Missed > 3 consecutive days of treatment before week 6 | Doxycycline, n (%) | Prednisolone, n (%) | Total, N (%) |
|---|---|---|---|
| Yes | 21 (18.8) | 5 (5.0) | 26 (12.2) |
| No | 91 (81.3) | 96 (95.0) | 187 (87.8) |
| Total | 112 | 101 | 213 |
Primary outcomes
Primary effectiveness outcome
The primary effectiveness outcome was a non-inferiority comparison of the proportions of patients achieving treatment success at 6 weeks, defined as three or fewer significant blisters. It was anticipated that doxycycline would be less effective than prednisolone but that this would be accompanied by an improvement in the safety profile. As is best practice for non-inferiority comparisons, both mITT and PP analyses were performed.
Modified intention-to-treat analysis
The mITT population included all participants regardless of any changes to treatment, provided that they had survived to week 6 and had received a blister count. This analysis reflects the comparison of the two strategies of starting on doxycycline or starting on prednisolone. In total, 91.1% of patients who were randomised to the prednisolone group were considered to be a treatment success at 6 weeks compared with 74.1% of patients who were randomised to the doxycycline group (Table 7). Although this is an 18.6% adjusted difference in effectiveness in favour of prednisolone (90% CI 11.1% to 26.1%), the upper CI falls well within the prespecified bounds of non-inferiority (37%) and doxycycline can therefore be considered non-inferior.
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 83 (74.1) | 92 (91.1) |
| Failure | 29 (25.9) | 9 (8.9) |
| Total | 112 | 101 |
| Difference in proportions (prednisolone – doxycycline) | Adjusted:a 18.6% (90% CI 11.1% to 26.1%) | |
| Unadjusted: 17.0% (90% CI 8.7% to 25.2%) | ||
There was no evidence of an interaction between disease severity and treatment effect in either the mITT or the PP population.
Per-protocol analysis
Patients were excluded from the PP analysis for deviations from the protocol that could significantly affect the outcome. Patients may appear more than once under the various reasons for exclusion if they met more than one of the criteria. The results of the PP analysis were very similar to the results of the ITT analysis, with 92.3% of patients in the prednisolone group achieving success compared with 74.4% in the doxycycline group, an adjusted difference of 18.7% (90% CI 9.8% to 27.6%) (Table 8), which falls well within the 37% upper bound for the 90% CI.
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone, n (%) | |
| Success | 58 (74.4) | 84 (92.3) |
| Failure | 20 (25.6) | 7 (7.7) |
| Total | 78 | 91 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 18.7 (9.8 to 27.6) | |
| Unadjusted: 17.9 (8.6 to 27.3) | ||
Both the mITT and PP analyses are represented graphically in Figure 5.
FIGURE 5.
Proportions of participants who achieved treatment success at 6 weeks: mITT and PP analyses.

Subgroup analyses
Two subgroup analyses were performed to test for treatment interactions. There were no significant interactions in either the mITT or the PP populations between effectiveness at 6 weeks and baseline disease severity (mild, moderate and severe) (Tables 9 and 10 respectively) or between effectiveness at 6 weeks and the blinding status of the investigator who carried out the week 6 blister count (Tables 11 and 12, respectively).
| Outcome | Subgroupa | Number (%) of patients | Proportion a treatment success (90% CI) (%)b | Difference in proportionsb (prednisolone – doxycycline) (90% CI) (%) | Interaction test p-valuec | ||
|---|---|---|---|---|---|---|---|
| Doxycycline (N = 112) | Prednisolone (N = 101) | Doxycycline | Prednisolone | ||||
| Difference in proportions (prednisolone – doxycycline)b | Mild baseline severity | 37 (33.0) | 31 (30.7) | 75.7 (64.1 to 87.3) | 96.8 (91.6 to 102.0) | 21.1 (8.4 to 33.8) | – |
| Moderate baseline severity | 46 (41.1) | 42 (42.6) | 78.3 (68.3 to 88.3) | 97.6 (93.7 to 101.5) | 19.4 (8.6 to 30.1) | 0.863 | |
| Severe baseline severity | 29 (25.9) | 28 (27.7) | 65.5 (51.0 to 80.0) | 75.0 (61.5 to 88.5) | 9.5 (–10.3 to 29.3) | 0.417 | |
| Outcome | Subgroupa | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline)b (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 78) | Prednisolone (N = 91) | ||||
| Difference in proportions (prednisolone – doxycycline)b | Mild baseline severity | 22 (28.2) | 26 (28.6) | 23.4 (6.6 to 40.2) | – |
| Moderate baseline severity | 34 (43.6) | 40 (44.0) | 24.8 (9.8 to 43.0) | 0.672 | |
| Severe baseline severity | 22 (28.2) | 25 (27.5) | 7.3 (–13.1 to 27.7) | 0.477 | |
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline)a (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 112) | Prednisolone (N = 101) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Medication was not known | 70 (62.5) | 64 (63.4) | 20.6 (9.8 to 31.4) | 0.333 |
| Medication was known | 42 (37.5) | 37 (36.6) | 21.9 (10.2 to 33.5) | – | |
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline)a (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 78) | Prednisolone (N = 91) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Medication was not known | 53 (68.0) | 59 (64.8) | 21.5 (10.0 to 33.0) | 0.356 |
| Medication was known | 25 (32.1) | 32 (35.2) | 10.6 (5.0 to 26.3) | – | |
Primary safety outcome
The primary safety outcome was the proportion of patients who, during the 52 weeks following randomisation, experienced at least one adverse event that was judged to be either grade 3 (severe), grade 4 (life-threatening) or grade 5 (death) and possibly, probably or definitely related to study treatment. The mITT population for this primary safety outcome included all those with data from at least one scheduled visit, regardless of any changes to treatment. An adjusted regression model in which preceding visits were set to zero was used to impute missing data to determine the sensitivity of the observed results to the missing data.
The risk of experiencing a treatment-related severe, life-threatening or fatal adverse event for patients started on doxycycline was 18.2%; this compared with 36.3% for those starting on prednisolone (Table 13). This represents a difference of 19.0% (95% CI 7.9% to 30.1%) after adjusting for baseline severity of BP. Similar results were obtained from a regression model in which missing data had been imputed: 22.5% of patients in the doxycycline group experienced a treatment-related severe, life-threatening or fatal adverse event compared with 40.0% in the prednisolone group, a difference of 18.4% (95% CI 6.0% to 30.8%) after adjusting for baseline severity of BP (Table 14).
| Doxycycline | Prednisolone | |
|---|---|---|
| Proportion of patients with an adverse event of grade 3 or above (%)a | 18.2 | 36.3 |
| Difference in proportions (prednisolone – doxycycline) (95% CI) (%) | Adjusted:b 19.0 (7.9 to 30.1); p = 0.001 | |
| Unadjusted: 18.1 (6.9 to 29.3); p = 0.002 | ||
| Doxycycline | Prednisolone | |
|---|---|---|
| Proportion of patients with an adverse event of grade 3 or above (%)a | 22.5 | 40.0 |
| Difference in proportions (prednisolone – doxycycline) (95% CI) (%) | Adjusted:b 18.4 (6.0 to 30.8); p = 0.004 | |
| Unadjusted: 17.5 (4.8 to 30.1); p = 0.007 | ||
A higher number of participants in the prednisolone group than in the doxycycline group had a maximum grade of adverse event of grade 3 (severe) and grade 5 (death) (Table 15). The pattern was the same for the total number of adverse events of each grade (Table 16).
| Adverse event | Number (%) of patients | |
|---|---|---|
| Doxycycline (N = 121) | Prednisolone (N = 113) | |
| No adverse events or maximum grade of 1 (mild) or 2 (moderate) | 99 (81.8) | 72 (63.7) |
| Maximum grade of 3 (severe) | 14 (11.6) | 25 (22.1) |
| Maximum grade of 4 (life-threatening) | 5 (4.1) | 5 (4.4) |
| Maximum grade of 5 (death) | 3 (2.5) | 11 (9.7) |
| Maximum grade of 3, 4 or 5 | 22 (18.2) | 41 (36.3) |
| Adverse event | Number (%) of adverse events (mean per participant) | |
|---|---|---|
| Doxycycline (N = 121) | Prednisolone (N = 113) | |
| Grade 3 (severe) | 33 (0.3)a | 59 (0.5) |
| Grade 4 (life-threatening) | 9 (0.1) | 9 (0.1) |
| Grade 5 (death) | 3 (< 0.1) | 11 (0.1) |
| Grades 3–5 | 45 (0.4) | 79 (0.7) |
Secondary outcomes
Secondary effectiveness outcome: weeks 6, 13 and 52
For secondary effectiveness outcomes, the definition of treatment success was different from that used for the primary effectiveness outcome. A participant was required to have three or fewer significant blisters present on examination to be considered a treatment success but, unlike the primary effectiveness analysis, patients who had had their treatment modified because of a poor response (change of medication or dose of randomised medication increased) prior to the visit did not qualify as a success in the secondary analyses. This difference is because the primary effectiveness outcome assessed the strategy of starting on doxycycline or starting on prednisolone, whereas the secondary outcomes assessed the effectiveness of the treatments used as longer-term monotherapy. All secondary effectiveness outcomes are non-inferiority analyses.
The proportion of patients classed as a treatment success at 6 weeks in the mITT analysis, according to the definition of success described above, was 85.4% in the prednisolone group and 53.6% in the doxycycline group (Table 17). This represents a difference of 31.8% (90% CI 22.5% to 41.2%) in favour of prednisolone after adjusting for baseline severity of BP and age. The PP analysis showed similar results, with a difference of 34.4% (90% CI 23.7% to 45.1%) in favour of prednisolone after adjusting for baseline severity of BP and age (Table 18). The upper CIs for both analyses fall just outside the prespecified upper bound of 37%. Subgroup analyses of the effectiveness outcome data at week 6 using the secondary outcome definition failed to show any evidence of interaction between disease severity and treatment effect (Tables 19 and 20) in either the mITT or the PP population. There was no interaction between blinding status and treatment effect at 6 weeks using the secondary outcome definition in the mITT analysis (Table 21) but there was some evidence of an interaction effect when a PP analysis was done (Table 22).
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Successa | 60 (53.6) | 88 (85.4) |
| Failure | ||
| High blister countb | 29 (25.9) | 9 (8.7) |
| Changed treatment | 23 (20.5) | 4 (3.9) |
| Died before week 6 | 0 | 2 (1.9) |
| Total | 112 | 103 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:c 31.8 (22.5 to 41.2) | |
| Unadjusted: 31.9 (22.2 to 41.5) | ||
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 41 (52.6) | 81 (87.1) |
| Failure | ||
| High blister count | 20 (25.6) | 7 (7.5) |
| Changed treatment | 17 (21.8) | 3 (3.2) |
| Died before week 6 | 0 | 2 (2.2) |
| Total | 78 | 93 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 34.4 (23.7 to 45.1) | |
| Unadjusted: 34.5 (23.6 to 45.4) | ||
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline) (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 112) | Prednisolone (N = 103) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Mild baseline blister severity (< 10) | 37 (33.0) | 32 (31.1) | 28.5 (12.9 to 44.2) | – |
| Moderate baseline blister severity (10–30) | 46 (41.1) | 42 (40.8) | 31.7 (17.5 to 45.8) | 0.806 | |
| Severe baseline blister severity (> 30) | 29 (25.9) | 29 (28.2) | 37.9 (18.0 to 58.8) | 0.543 | |
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline) (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 78) | Prednisolone (N = 93) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Mild baseline blister severity (< 10) | 22 (28.2) | 27 (29.0) | 33.4 (14.3 to 52.5) | – |
| Moderate baseline blister severity (10–30) | 34 (43.6) | 40 (43.0) | 34.2 (18.2 to 50.2) | 0.956 | |
| Severe baseline blister severity (> 30) | 22 (28.2) | 26 (28.0) | 36.1 (14.1 to 58.1) | 0.878 | |
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline) (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 112) | Prednisolone (N = 101) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Medication was not known | 70 (62.5) | 64 (63.4) | 25.1 (13.7 to 36.5) | – |
| Medication was known | 42 (37.5) | 37 (36.6) | 46.2 (31.6 to 60.9) | 0.059 | |
| Outcome | Subgroup | Number (%) of patients | Difference in proportions achieving treatment success (prednisolone – doxycycline) (90% CI) (%) | Interaction test p-value | |
|---|---|---|---|---|---|
| Doxycycline (N = 78) | Prednisolone (N = 91) | ||||
| Difference in proportions (prednisolone – doxycycline)a | Medication was not known | 53 (67.9) | 59 (64.8) | 25.0 (12.2 to 37.8) | – |
| Medication was known | 25 (32.1) | 32 (35.2) | 57.7 (40.3 to 75.1) | 0.013 | |
At 13 weeks, in the mITT analysis, 75.3% of patients in the prednisolone group were considered a treatment success compared with 58.6% in the doxycycline group, a difference of 17.5% (90% CI 6.8% to 28.2%) in favour of prednisolone after adjusting for baseline severity of BP and age (Table 23). Again, similar results were noted for the PP analysis (a difference of 17.3% in favour of prednisolone, 90% CI 4.9% to 29.7%) (Table 24).
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 58 (58.6) | 76 (75.2) |
| Failure | ||
| High blister count | 12 (12.1) | 6 (5.9) |
| Changed treatment | 29 (29.3) | 12 (11.9) |
| Died before week 13 | 0 | 7 (6.9) |
| Total | 99 | 101 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 17.5 (6.8 to 28.2) | |
| Unadjusted: 16.7 (5.9 to 27.4) | ||
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 38 (60.3) | 71 (78.0) |
| Failure | ||
| High blister count | 5 (7.9) | 5 (5.5) |
| Changed treatment | 20 (31.8) | 9 (9.9) |
| Died before week 13 | 0 | 6 (6.6) |
| Total | 63 | 91 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 17.3 (4.9 to 29.7) | |
| Unadjusted: 17.7 (5.3 to 30.1) | ||
The longer-term assessment of effectiveness (the proportion classed as a success at 52 weeks) shows that, in the adjusted mITT analysis, 41.0% of patients started on doxycycline compared with 51.1% of those started on prednisolone achieved treatment success, a difference of 10.0% (90% CI –2.3% to 22.2%) in favour of prednisolone (Table 25). Similar results were seen for the PP analysis, with a difference of 7.1% (90% CI –7.1% to 21.3%) in favour of prednisolone (Table 26).
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 34 (41.0) | 45 (51.1) |
| Failure | ||
| High blister count | 3 (3.6) | 3 (3.4) |
| Changed treatment | 43 (51.8) | 29 (33.0) |
| Died before week 52 | 3 (3.6) | 11 (12.5) |
| Total | 83 | 88 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 10.0 (–2.3 to 22.2) | |
| Unadjusted: 10.2 (–2.3 to 22.6) | ||
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 24 (45.3) | 41 (53.3) |
| Failure | ||
| High blister count | 2 (3.8) | 3 (3.9) |
| Changed treatment | 25 (47.2) | 23 (29.9) |
| Died before week 52 | 2 (3.8) | 10 (13.0) |
| Total | 53 | 77 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 7.1 (–7.1 to 21.3) | |
| Unadjusted: 8.0 (–6.7 to 22.6) | ||
Secondary outcome: relapse rates
Another measure of the long-term effectiveness of the two treatments was the proportion of participants who had a further episode of BP during their participation in the study after previously being classed as a treatment success. A participant was classed as having a relapse if he or she had a further episode of BP, defined as more than three significant blisters, or a change or escalation of treatment because of worsening of disease during participation in the study after previously being classed as a treatment success (either three or fewer significant blisters present on prior examination or previously classed as a treatment success on the treatment log). After adjusting for baseline severity, age and Karnofsky score, a similar number of relapses occurred in the doxycycline group and the prednisolone group in the mITT population (2.1% more in the prednisolone group, 90% CI –8.3% to 12.5%) (Table 27). A larger difference was noted in the PP analysis (11.0% more relapses in the prednisolone group, 90% CI –1.2% to 23.2%) (Table 28).
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Relapse | 37 (32.5) | 39 (35.8) |
| No relapse | 77 (67.5) | 70 (64.2) |
| Total | 114 | 109 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 2.1 (–8.3 to 12.5) | |
| Unadjusted: 3.3 (–7.1 to 13.8) | ||
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Relapse | 20 (27.0) | 34 (38.6) |
| No relapse | 54 (73.0) | 54 (61.4) |
| Total | 74 | 88 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 11.0 (–1.2 to 23.2) | |
| Unadjusted: 11.6 (–0.4 to 23.7) | ||
Secondary outcome: combined outcome of effectiveness at 6 weeks and safety over 52 weeks
To provide an overall measure of success, a combined analysis of effectiveness and safety is presented in a superiority analysis. In the prednisolone group, 74.8% of patients were classed as a treatment success at 6 weeks and were alive at 52 weeks whereas in the doxycycline group 50.0% of patients were classed as a treatment success at 6 weeks and were alive at 52 weeks (Table 29). This represents a difference of 25.0% (95% CI 13.1% to 37.0%) in favour of prednisolone after adjusting for baseline severity and age.
| Treatment success | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 56 (50.0) | 77 (74.8) |
| Failure | ||
| Success at week 6 but not alive at week 52 | 4 (3.6) | 11 (10.7) |
| Not successful at week 6 because of high blister count | 29 (25.9) | 9 (8.7) |
| Not successful at week 6 because of treatment change before week 6 | 23 (20.5) | 4 (3.9) |
| Not successful at week 6 because of death before week 6 | 0 | 2 (1.9) |
| Total | 112 | 103 |
| Difference in proportions (prednisolone – doxycycline) (95% CI) (%) | Adjusted:a 25.0 (13.1 to 37.0); p < 0.001 | |
| Unadjusted: 24.8 (14.3 to 35.2); p < 0.001 | ||
Secondary safety outcome: all adverse events
This secondary outcome includes all adverse events that are possibly, probably or definitely related to the trial medication, regardless of severity. All analyses were carried out on a superiority basis. The maximum grade of related adverse events experienced by participants during the trial is shown in Table 30. Patients in the prednisolone group were significantly more likely to experience an adverse event that was related to the study medication than those in the doxycycline group (95.7% vs. 86.2%, 95% CI 1.8% to 17.2%; p = 0.016, unadjusted because of non-convergence in the model) (Table 31). The total numbers of related adverse events by grade (raw data) are shown in Table 32.
| Adverse event | Number (%) of patients | |
|---|---|---|
| Doxycycline (N = 121) | Prednisolone (N = 113) | |
| No adverse events | 23 (19.0) | 13 (11.5) |
| Maximum grade of adverse event grade 1 (mild) | 20 (16.5) | 16 (14.2) |
| Maximum grade of adverse event grade 2 (moderate) | 56 (46.3) | 43 (38.1) |
| Maximum grade of adverse event grade 3 (severe) | 14 (11.6) | 25 (22.1) |
| Maximum grade of adverse event grade 4 (life-threatening) | 5 (4.1) | 5 (4.4) |
| Maximum grade of adverse event grade 5 (death) | 3 (2.5) | 11 (9.7) |
| Adverse event of any grade | 98 (81.0) | 100 (88.5) |
| Doxycycline | Prednisolone | |
|---|---|---|
| Proportion of patients with an adverse event (%)a | 86.2 | 95.7 |
| Difference in proportion of patients with an adverse event (prednisolone – doxycycline) (95% CI) (%) | Unadjusted:b 9.5 (1.8 to 17.2); p = 0.016 | |
| Adverse event | Number of adverse events (mean per participant) | |
|---|---|---|
| Doxycycline (n = 121) | Prednisolone (n = 113) | |
| Grade 1 (mild) | 210 (1.7) | 234 (2.1) |
| Grade 2 (moderate) | 158 (1.3) | 129 (1.1) |
| Grade 3 (severe) | 33 (0.3) | 59 (0.5) |
| Grade 4 (life-threatening) | 9 (0.1) | 9 (0.1) |
| Grade 5 (death) | 3 (< 0.1) | 11 (0.1) |
| Grades 1–5 | 413 (3.4) | 442 (3.9) |
Secondary outcome: quality of life
Quality of life was assessed using the generic EQ-5D and the skin-specific DLQI. For a specific visit, the EQ-5D tabulations and analyses were conducted on all patients in the ITT population who had all five scores for the five questions for that visit (mobility, self-care, usual activity, pain/discomfort and anxiety/depression). Only patients who had at least one visit (not including baseline) for which data were available were included in the analysis. The EQ-5D score (social preference score) was obtained, with a value assigned to each combination of scores from the individual five questions (see Chapter 4, Data sources). For the DLQI, the tabulations and analyses were conducted for a specific visit on all patients in the ITT population who had all 10 scores for the 10 questions for that visit. Only patients who had at least one visit (not including baseline) for which data were available were included in the analysis. The DLQI score was calculated as the sum of each of the scores for the 10 questions asked at each visit.
There was a median change in EQ-5D score from baseline to week 52 of +0.090 in the doxycycline group and +0.071 in the prednisolone group (Table 33). When this was adjusted for baseline EQ-5D score, baseline severity, age and Karnofsky score, the difference was not significant (0.045, 95% CI –0.015 to 0.106; p = 0.143) (Table 34). Patients in the two groups had a similar improvement in DLQI score, with a median improvement from baseline to week 52 of 9 points in the doxycycline group and 10 points in the prednisolone group (Table 35). When adjusted for baseline DLQI score, baseline severity, age and Karnofsky score, there was a significant difference of –1.8 (95% CI –2.58 to –1.01) in favour of doxycycline (Table 36).
| Time point | Doxycycline (n = 110) | Prednisolone (n = 101) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | Median change from baseline | Total patients with data | Median (IQR) | Median change from baseline | Total patients with data | |
| Baseline | 0.656 (0.273–0.796) | 0 | 110 | 0.656 (0.273–0.760) | 0 | 101 |
| Week 6 | 0.620 (0.353–0.805) | –0.036 | 108 | 0.746 (0.587–1.000) | +0.090 | 96 |
| Week 13 | 0.710 (0.450–1.000) | +0.054 | 96 | 0.779 (0.639–0.925) | +0.123 | 92 |
| Week 26 | 0.746 (0.587–1.000) | +0.090 | 85 | 0.796 (0.638–0.850) | +0.140 | 80 |
| Week 39 | 0.727 (0.587–1.000) | +0.071 | 79 | 0.710 (0.587–1.000) | +0.054 | 77 |
| Week 52 | 0.746 (0.587–1.000) | +0.090 | 78 | 0.727 (0.587–1.000) | +0.071 | 74 |
| Difference in EQ-5D scores (prednisolone – doxycycline) (95% CI) | Adjusted:a 0.045 (–0.015 to 0.106); p = 0.143 |
| Unadjusted:b 0.062 (–0.006 to 0.130); p = 0.076 |
| Time point | Doxycycline (n = 108) | Prednisolone (n = 101) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | Median change from baseline | Total patients with data | Median (IQR) | Median change from baseline | Total patients with data | |
| Baseline | 10 (6–15) | 0 | 108 | 11 (6–14) | 0 | 101 |
| Week 6 | 5 (2–9) | –5 | 106 | 1 (0–3) | –10 | 96 |
| Week 13 | 2 (1–6) | –8 | 98 | 1 (0–4) | –10 | 90 |
| Week 26 | 2 (0–4) | –8 | 86 | 1 (0–3) | –10 | 80 |
| Week 39 | 1 (0–4) | –9 | 80 | 1 (1–3) | –10 | 77 |
| Week 52 | 1 (0–3) | –9 | 79 | 1 (0–3) | –10 | 75 |
| Difference in DLQI scores (prednisolone – doxycycline) (95% CI) (%) | Adjusted:a,b –1.80 (–2.58 to –1.01); p < 0.001 |
Tertiary outcomes
The mITT analysis showed that there was a higher proportion of participants who were completely blister free at 6 weeks (rather than three or fewer significant blisters) in the prednisolone group (73.3% vs. 45.9%) (Table 37). This is a difference of 28.6% in favour of prednisolone after adjusting for baseline severity, age and Karnofsky score (90% CI 18.1 to 39.1%). Table 38 lists the number of patients in the mITT population excluded from the PP analysis. The PP analysis showed very similar results (Table 39).
| Outcome | Number (%) of patients) | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Blister free | 51 (45.9) | 74 (73.3) |
| Not blister free | 60 (54.1) | 27 (26.7) |
| Total | 111 | 101 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 28.6 (18.1 to 39.1) | |
| Unadjusted: 27.3 (16.7 to 38.0) | ||
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 17 | 3 |
| Used topical steroids between weeks 3 and 6 | 7 | 3 |
| Missed more than 3 consecutive days of treatment before week 6 | 21 | 5 |
| Total number of non-PP patients | 34 | 10 |
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Blister free | 35 (45.5) | 68 (74.7) |
| Not blister free | 42 (54.5) | 23 (25.3) |
| Total | 77 | 91 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 30.2 (18.4 to 42.0) | |
| Unadjusted: 29.3 (17.3 to 41.2) | ||
Tertiary effectiveness outcome: proportion of participants who achieved treatment success at 3 weeks (non-inferiority)
As a measure of the speed of onset of action, the proportions of participants classed as a treatment success (three or fewer significant blisters) were assessed after only 3 weeks of treatment. In the mITT population, a higher proportion of those started on prednisolone were classed as a treatment success at 3 weeks than those started on doxycycline, with a difference of 23.4% (90% CI 14.4% to 32.5%) after adjusting for baseline severity, age and Karnofsky score (Table 40), although this remains within the prespecified bounds of non-inferiority. A similar difference was seen in the PP analysis (Table 41).
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 67 (56.3) | 90 (81.1) |
| Failure | ||
| High blister count | 42 (35.3) | 17 (15.3) |
| Changed treatment | 10 (8.4) | 3 (2.7) |
| Died before week 3 | 0 | 1 (0.9) |
| Total | 119 | 111 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 23.4 (14.4 to 32.5) | |
| Unadjusted: 24.8 (15.1 to 34.4) | ||
| Treatment outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 56 (57.1) | 85 (81.7) |
| Failure | ||
| Severe baseline disease | 33 (33.7) | 16 (15.4) |
| Changed treatment | 9 (9.2) | 3 (2.9) |
| Died before week 3 | 0 | 0 |
| Total | 98 | 104 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 23.7 (13.3 to 34.0) | |
| Unadjusted: 24.6 (14.3 to 34.9) | ||
Tertiary safety outcome: mortality over the 52-week follow-up period (superiority)
An assessment of all deaths, regardless of any relatedness to the study medication, was performed. Those who started on prednisolone were more likely to die during the year of follow-up than those started on doxycycline (83.5% alive at 1 year compared with 89.4%, respectively) (Table 42). The hazard ratio for reduction in death in favour of doxycycline, adjusted for baseline severity, age and Karnofsky score, was 0.61 (95% CI 0.30 to 1.24; p = 0.173) (Table 43). Time to death is shown in the Kaplan–Meier plot in Figure 6.
| Mortality outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline (N = 132) | Prednisolone (N = 121) | |
| Died during trial follow-up | 14 (10.6) | 20a (16.5) |
| Relateda | 3 (2.3) | 11 (9.1) |
| Unrelatedb | 11 (8.3) | 9 (7.4) |
| Alive at the end of follow-up (1 year) | 118 (89.4) | 101 (83.5) |
FIGURE 6.
Kaplan–Meier survival plot showing time to death.

| All-cause mortality (hazard ratio) (95% CI) | Adjusted:a 0.61 (0.30 to 1.24); p = 0.173 |
| Unadjusted: 0.59 (0.29 to 1.19); p = 0.139 |
Tertiary effectiveness outcome: use of potent and superpotent topical corticosteroids during the 52-week follow-up period (superiority)
Use of topical steroids (potent or superpotent) was permitted during the first 3 weeks of treatment for symptomatic relief (no more than 30 g per week to localised lesions only) and also after 6 weeks, as might occur in normal practice in the UK. Although use of topical steroids was discouraged from the end of week 3 to the week 6 effectiveness assessment, some participants did use topical corticosteroids for local relief of the affected area. Data on quantities of topical corticosteroids used were not collected accurately over the 1-year study period and so Table 44 presents topical corticosteroid use during different study periods. As anticipated, topical corticosteroid use for symptomatic relief was greater at all time points for those initiated on doxycycline treatment.
| Time period | Doxycycline (N = 112), n (%) | Prednisolone (N = 101), n (%) | ||||
|---|---|---|---|---|---|---|
| Potent | Superpotent | Patients using topical corticosteroids | Potent | Superpotent | Patients using topical corticosteroids | |
| After randomisation up to 3 weeks | 6 (5.4) | 1 (0.9) | 7 (6.3) | 3 (3.0) | 1 (1.0) | 4 (4.0) |
| After 3 weeks up to 6 weeks | 12 (10.7) | 11 (9.8) | 23 (20.5) | 6 (5.9) | 0 | 6 (5.9) |
| After 6 weeks | 5 (4.5) | 7 (6.3) | 12 (10.7) | 2 (2.0) | 0 | 2 (2.0) |
| At any time during the trial | 13 (11.6) | 11 (9.8) | 24 (21.4) | 6 (5.9) | 1 (1.0) | 7 (6.9) |
Additional post-hoc analysis
Post-hoc analysis: primary effectiveness outcome repeated at week 52
The primary effectiveness outcome at 6 weeks assessed the strategy of starting on doxycycline or prednisolone by including all participants regardless of any treatment modification. It was decided at the Trial Steering Committee meeting to conduct an additional post-hoc effectiveness analysis using the same definition of the population included to assess the strategy of starting on doxycycline or prednisolone over the whole 52-week period. There was no significant difference between the two groups when assessed over the full 52 weeks in either the mITT analysis (Table 45) or the PP analysis (Table 46).
| Outcomea | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 77 (96.3) | 74 (96.1) |
| Failure | 3 (3.8) | 3 (3.9) |
| Total | 80 | 77 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%)b | –0.1 (–5.2 to 4.9) | |
| Outcomea | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 49 (96.1) | 64 (95.5) |
| Failure | 2 (3.9) | 3 (4.5) |
| Total | 51 | 67 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%)b | –0.6 (–6.7 to 5.5) | |
Post-hoc analysis: action (treatment changes) taken in response to relapse
In total, 76 patients were identified as having relapsed after previously being classified as a treatment success, either because they had three or fewer significant blisters present or because they had ceased trial medication because of treatment success (Table 47). For those started on prednisolone, if they experienced a relapse, patients usually either received an increased dose of prednisolone (30/39, 76.9%) or recommenced prednisolone if treatment had ceased completely (8/39, 20.5%). For those who started on doxycycline, the most common course of action on relapse after having previously achieved disease control was to change to prednisolone (13/37, 35.1%) or increase the dose of prednisolone (16/37, 43.2%).
| Action taken in response to disease relapse | Number (%) of patients | |
|---|---|---|
| Doxycycline (n = 37) | Prednisolone (n = 39) | |
| Treatment changed to prednisolone | 13 (35.1) | 0 |
| Treatment changed to doxycycline | 0 | 0 |
| Dose of prednisolone increased | 16 (43.2) | 30 (76.9) |
| Dose of doxycycline increased | 3 (8.1) | 0 |
| Prednisolone restarted | 3 (8.1) | 8 (20.5) |
| Doxycycline restarted | 2 (5.4) | 0 |
| Other treatment change | 0 | 1 (2.6) |
Post-hoc analysis: time to switching treatment
For those patients who switched treatments, the time between starting the study and switching treatment was assessed in a post-hoc analysis. This included all of the patients in the mITT population who contributed to the secondary efficacy analysis at week 6. The rate of switching from doxycycline was greatest during the first 6 weeks; after 13 weeks there was very little switching (Figure 7). There was a lower rate of switching for those starting on prednisolone and the rate was more constant over the whole 52 weeks.
FIGURE 7.
Kaplan–Meier survival plot showing time to change of treatment. Note: A change in treatment refers to a change from the allocated treatment (either doxycycline or prednisolone) to the other treatment. This graph includes the 213 patients who formed the mITT population for the primary efficacy outcome.

Post-hoc analysis: sensitivity of the primary effectiveness outcome at 6 weeks to topical corticosteroid use
A slightly different definition of a PP violator was used from that defined in the statistical analysis plan so that any use of a prohibited potent or superpotent topical corticosteroid between weeks 3 and 6 resulted in exclusion from the PP analysis of blister control at 6 weeks. The results of this post-hoc analysis are shown in Table 48. This shows that the non-inferiority status of doxycycline was robust to excluding those who used additional topical corticosteroids in the 3 weeks prior to the 6-week blister count assessment, at which the presence of three or fewer significant blisters was considered a treatment success.
| Outcome | Number (%) of patients | |
|---|---|---|
| Doxycycline | Prednisolone | |
| Success | 49 (74.2) | 82 (93.2) |
| Failure | 17 (25.8) | 6 (6.8) |
| Total | 66 | 88 |
| Difference in proportions (prednisolone – doxycycline) (90% CI) (%) | Adjusted:a 19.3 (9.6 to 29.0) | |
| Unadjusted: 18.9 (9.0 to 28.8) | ||
Post-hoc analysis: safety data at 52 weeks according to baseline disease severity
To assist with interpreting the health economic analysis, an additional post-hoc analysis was performed for the primary safety data according to baseline disease severity (Table 49).
| Subgroupa | Number (%) of patients | Number (%) experiencing a related adverse event (from raw data) | Difference in percentage (prednisolone – doxycycline) (95% CI)b | ||
|---|---|---|---|---|---|
| Doxycycline | Prednisolone | Doxycycline | Prednisolone | ||
| Mild baseline severity | 40 (33.1) | 35 (31.0) | 6 (15.0) | 14 (40.0) | 25.0 (5.4 to 44.6) |
| Moderate baseline severity | 47 (38.8) | 43 (38.1) | 5 (10.6) | 17 (39.5) | 28.9 (11.8 to 46.0) |
| Severe baseline severity | 34 (28.1) | 35 (31.0) | 11 (32.4) | 10 (28.6) | –3.8 (–25.5% to 17.9) |
| Total | 121 | 113 | 22 (18.2) | 41 (36.3) | 18.1% |
Similar post-hoc subgroup analyses for imputed data showed similar results: the differences in the percentages experiencing a related adverse event (prednisolone – doxycycline) were 25.2% (95% CI 4.0% to 46.3%) for mild baseline severity, 28.6% (95% CI 9.9% to 47.3%) for moderate baseline severity and –6.1% (95% CI –32.0% to 19.7%) for severe baseline severity.
Chapter 4 Cost-effectiveness
Objective
The objective of the economic analysis was to estimate the cost-effectiveness of doxycycline-initiated therapy compared with that of prednisolone-initiated therapy for patients with BP from the perspective of the English NHS.
Overview
Economic analysis is intended to inform decision-makers about the value for money of treatment alternatives, in a context in which health-care resources are limited and prioritisation is informed, at least in part, by the efficient use of resources. 39 The economic analysis within the BLISTER trial followed a prospectively agreed analysis plan and provides robust and unbiased evidence of cost-effectiveness, augmenting the trial estimates of clinical effectiveness. The BLISTER trial featured a pragmatic multicentre design reflecting real-world clinical practice and thus cost and outcome profiles are likely to reflect routine care in NHS settings. Individual patient data collected within the BLISTER trial included NHS treatment costs and health status, estimated as QALYs. Cost-effectiveness analysis captures the effect of treatment as changes in costs and QALYs. Given that follow-up was limited to 1 year, no discounting of future costs and benefits was applied.
The analysis followed ITT principles, with patients included in the analysis according to the treatment allocated by randomisation, irrespective of subsequent care.
Because of some missing data during trial follow-up, a base-case analysis was constructed in which missing data were imputed using multiple imputation. Supportive sensitivity analyses included only patients with complete data, thus exploring the impact of imputation.
Methods
Data sources
Resource use and quality-of-life measurements were collected from patients by health-care professionals at mandatory clinics at baseline and 6, 13, 26, 39 and 52 weeks. Generic health-related quality of life was assessed using the EuroQol questionnaire (containing the EQ-5D-3L and EQ VAS). The DLQI was included as a disease-specific quality-of-life measure.
The EQ-5D scores were converted to health status scores using the value set (time trade-off recommended by the EuroQol group). 40,41 Using the trapezoidal rule, the AUC of health status scores was calculated, providing patient-level QALY estimates for the cost-effectiveness analysis. 42 Similarly, EQ VAS and DLQI scores were integrated discretely over time. As AUC estimates were predicted to correlate with baseline scores (and thus potential baseline imbalances), AUC estimates were adjusted for baseline scores. 43 Sensitivity analyses explored adjusted and unadjusted estimation including a range of patient covariates: age, sex, baseline blister severity and baseline Karnofsky score as well as baseline outcome measure score.
Resource capture included use of study and non-study drugs as well as health service contacts: GP clinic and home visits, practice and district nurse visits and outpatient visits and inpatient stays. Patients were asked to recall use of resources since their last clinic visit and were given a diary at the beginning of the study to aid recall. Drugs recorded related to the management of BP or its sequelae. For health service contacts patients were asked to identify all patient contacts as well as those related directly to the management of BP or its sequelae. Consequently, ‘attributable costs’ are used in the base-case analysis and a sensitivity analysis included ‘all costs’.
Unit costs of resources were estimated or drawn from national reference sources for 2013 and are shown in Table 50. Study and non-study drugs were prescribed at varying doses. Using national PCA data,33 average costs per unit weight of therapeutic were determined and applied to patient drug use records. The list of drugs used included a number of occasionally prescribed drugs; pragmatically, drugs that were prescribed on more than five occasions during the course of the trial were costed. Costs for inpatient stays (in days) and outpatient visits were estimated using Hospital Episodes Statistics (HES)36 and NHS reference costs. 35 National HES data were explored for inpatient episodes with a primary diagnosis of International Classification of Diseases L12.0 Bullous Pemphigoid. The 10 most common Healthcare Resource Group (HRG) HRG4+ codes associated with that diagnosis were included, accounting for 96.2% of admissions. Per diem costs for each HRG4+ code were estimated from NHS reference costs and a volume-weighted average cost per admission for BP was estimated allowing for mean stay and cost per day. GP clinic and home visits, and practice and district nurse visits were costed using unit costs provided by the Personal Social Services Research Unit (PSSRU). 34 Patient costs were estimated as the sum of resources used weighted by their reference costs. The base-case analysis included only BP-related resource use.
| Resource | Cost (£) | Unit | Source |
|---|---|---|---|
| Drug | |||
| Doxycycline | 0.0015 | Per mg | PCA33 |
| Prednisolone | 0.0221 | Per mg | PCA33 |
| Azathioprine (Imuran®, Aspen) | 0.0034 | Per mg | PCA33 |
| Betamethasone valerate (Betnovate®, GlaxoSmithKline UK) | 0.0582 | Per g | PCA33 |
| Clobetasol propionate (Dermovate®, GlaxoSmithKline UK) | 0.0828 | Per g | PCA33 |
| Mometasone furoate | 0.1368 | Per g | PCA33 |
| Clobetasone butyrate (Eumovate®, GlaxoSmithKline UK) | 0.0588 | Per g | PCA33 |
| Contact | |||
| Inpatient | 334.22 | Per day | HES,36 NHS reference costs35 |
| Outpatient | 98.00 | Per visit | HES,36 NHS reference costs35 |
| GP clinic | 46.00 | Per visit | PSSRU34 |
| GP home | 92.00 | Per visit | PSSRU34 |
| Practice nurse | 13.34 | Per visit | PSSRU34 |
| District nurse | 39.00 | Per visit | PSSRU34 |
Multiple imputation
In any form of analysis, missing data can cause serious problems. The problem is greater than a simple loss of statistical power as missing data may be not a random event but related to treatment and outcome. Examples include getting better (and no longer needing care) or dying, although QALY estimates allow for and include patients who die during follow-up. Quality-of-life measures typically feature repeated assessments over time, which are used to construct AUC estimates. This approach is both a strength and a weakness. With a complete-case analysis the weakness is evident: more observations may mean more incomplete assessments and loss of one follow-up assessment means the non-inclusion of that patient. However, repeated observations may provide (partial) assessments on more patients, allowing their experience to be reliably imputed. The base-case analysis used multiple imputation, conducted according to good practice guidance. 44,45 A range of alternative model specifications provided sensitivity analyses.
Multiple imputation provides a method of replacing each missing value with a predicted value, potentially permitting analysis of the entire trial sample. The process begins with reporting levels of missingness and the missing at random (MAR) assumption is then explored in the data. The MAR assumption requires that the probability of data being unobserved is dependent on the observed values but independent of unobserved values. Multiple imputation provides unbiased estimates of treatment effect if data are MAR. Additionally, complete-case analysis provides unbiased estimates only if data are missing completely at random, that is, the probability of data being unobserved is independent of both observed and unobserved values. The imputation model included all variables used within analysis models to preserve correlation structures.
A regression model was used to generate multiple imputed data sets (or ‘draws’), in which missing values were predicted drawing on predictive covariates. These included age, sex, baseline blister severity and baseline Karnofsky score as predictors only and outcome measures (at each time point) and costs as predictors and imputed variables. Each draw provided a complete data set that reflected the distributions and correlations between variables. Predictive mean matching was used to enhance the plausibility and robustness of imputed values, as normality could not be assumed. The model used fully conditional Markov chain Monte Carlo methods (multiple imputation by chained equations), which are appropriate when missing and correlated data occur in more than one variable. Each draw was analysed independently and the estimates obtained were pooled to generate mean and variance estimates of costs and QALYs using Rubin’s rule, a method that captures within and between variances for imputed samples. 46 To minimise the information loss of finite imputation sampling, 50 draws were taken. 46 This resulted in a loss of efficiency relative to infinite sampling of < 0.5% in all imputed values. The distribution of imputed and observed values was compared to establish that imputation did not introduce bias into subsequent estimation.
Incremental analysis
The goal of economic analysis is to inform decision-makers about the incremental costs and benefits of change; when expressed together in the cost/QALY metric this facilitates comparison with other technology choices. The analysis reports costs, QALYs and cost per QALY comparing doxycycline-initiated therapy with prednisolone-initiated therapy for patients with BP.
Bivariate regression using seemingly unrelated regression equations was used to model incremental changes in costs and QALYs. This method respects the correlation of costs and outcomes within the data and allows adjustment for a set of covariates that can be explored. 47 Baseline quality-of-life scores were included within all QALY estimation models to allow for potential baseline imbalances. 38 The incremental cost-effectiveness ratio (ICER) was estimated as the difference in mean total costs between treatments divided by the difference in mean total QALYs. Value for money is determined by comparing the ICER with a threshold value, typically the National Institute for Health and Care Excellence threshold of £20,000–30,000 per QALY for UK studies. This represents the willingness to pay for an additional QALY and lower values than the threshold could be considered cost-effective for use in the NHS. The net monetary benefit (NMB) of changing treatment was also reported as a recalculation of the ICER at a range of thresholds of willingness to pay for an additional QALY. The NMB succinctly describes the resource gain (or loss) when investing in a new treatment when resources can be used elsewhere at the same threshold. 48
Uncertainty
A range of decisions are made in the construction of cost-effectiveness models, for example which costs to include, whether or not to adjust for covariates and whether or not to impute missing values. These options are presented and the most plausible assumptions form the base-case analysis; assumptions are explored using a range of supportive sensitivity analyses. The analyses carried out in this study are described in Table 51.
| Analysis | Costs | Outcome | Covariate adjustmenta |
|---|---|---|---|
| Base case | Attributed costs, imputed | EQ-5D, imputed, baseline adjusted | Yes |
| Sensitivity analyses | |||
| 1 | Attributed costs, imputed | EQ-5D, imputed, baseline adjusted | No |
| 2 | Attributed costs, complete case | EQ-5D, complete case, baseline adjusted | Yes |
| 3 | Attributed costs, complete case | EQ-5D, complete case, baseline adjusted | No |
| 4 | All costs, imputed | EQ-5D, imputed, baseline adjusted | Yes |
| 5 | All costs, imputed | EQ-5D, imputed, baseline adjusted | No |
The imputation data set provides the most plausible base-case analyses, but a complete-case analysis and unadjusted analysis provide useful sensitivity analyses. No method of analysis can provide protection from bias if the assumption that data are MAR does not hold (adequately). Joint distributions of costs and outcomes were generated using the (non-parametric) bootstrap method, with replicates used to populate the cost-effectiveness plane and generate cost-effectiveness acceptability curves. The cost-effectiveness acceptability curve compares the likelihood that treatments are cost-effective as the willingness-to-pay threshold varies. 49 Bootstrapping jointly resamples costs and outcomes from the original data with replacement (maintaining the sample correlation structure) to create a new bootstrap sample from which a change in costs and QALYs is estimated. Using bias-corrected non-parametric bootstrapping, 5000 bootstraps were taken per model evaluated. Means estimates are reported with 95% CIs.
Results
Completeness of data
Of the 253 patients included in the primary analysis of effectiveness, 220 patients (87%) had at least one EQ-5D record during follow-up and were included in the economic analysis (Table 52). Subsequent discussion of the completeness of the data focuses on the 220 included patients as most relevant when considering the extent of imputation. In total, 164 patients (75%) had complete EQ-5D assessments for all time periods. Patients who died were subsequently scored zero on visits that followed for both cost and EQ-5D score and are included as observed data. There was a pattern of decreasing completeness as follow-up proceeded. Cost data were complete for 191 patients (87%). It was not possible to explore completeness of health-care costs by follow-up visit as patients could use diaries to complete missing data at a later follow-up visit and the study drug report covered the entire follow-up period. When considering both utilities and resource use, complete information was available for 143 patients (65%). Completeness of data was similar when comparing treatment arms (see Table 52).
| Prednisolone (n = 108), n (%) | Doxycycline (n = 112), n (%) | Total: analysis (n = 220), n (%) | Total: trial (n = 253), % | |
|---|---|---|---|---|
| Health status | ||||
| EQ-5D baseline | 107 (99.1) | 112 (100.0) | 219 (99.5) | 86.6 |
| EQ-5D 6 weeks | 102 (94.4) | 108 (96.4) | 210 (95.5) | 83.0 |
| EQ-5D 13 weeks | 101 (93.5) | 101 (90.2) | 202 (91.8) | 79.8 |
| EQ-5D 26 weeks | 96 (88.9) | 93 (83.0) | 189 (85.9) | 74.7 |
| EQ-5D 39 weeks | 94 (87.0) | 90 (80.4) | 184 (83.6) | 72.7 |
| EQ-5D 52 weeks | 92 (85.2) | 90 (80.4) | 182 (82.7) | 71.9 |
| EQ-5D all visits | 83 (76.9) | 81 (72.3) | 164 (74.5) | 64.8 |
| Resource use | ||||
| Drug use | 95 (88.0) | 102 (91.1) | 197 (89.5) | 77.9 |
| Health service contacts | 103 (95.4) | 107 (95.5) | 210 (95.5) | 83.0 |
| Costs | 92 (85.2) | 99 (88.4) | 191 (86.8) | 75.5 |
| Health status and resource use | ||||
| Costs and EQ-5D | 72 (66.7) | 71 (63.4) | 143 (65.0) | (56.5) |
Thirty-four patients died during the trial period, 14 (10.6%) in the doxycycline arm and 20 (16.5%) in the prednisolone arm. Missing values were imputed to provide a base-case analysis including all 220 patients.
Complete-case estimates
To describe the observed data, findings in this section are reported unadjusted for patient-level covariates. Models in this section (Tables 53–55) are adjusted as shown in Table 51.
Mean EQ-5D scores by treatment group are reported in Table 53. There was a small non-significant difference in health status at baseline (lower in doxycycline-initiated patients). Over the 1-year follow-up period there were no significant differences in QALYs when comparing treatments.
| Prednisolone | Doxycycline | Doxycycline – prednisolonea | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | 95% CI | |
| EQ-5D baseline | 0.42 | 0.31 | 0.39 | 0.31 | –0.034 | –0.117 to 0.046 |
| EQ-5D 6 weeks | 0.52 | 0.33 | 0.43 | 0.34 | –0.094 | –0.188 to –0.001 |
| EQ-5D 13 weeks | 0.51 | 0.33 | 0.42 | 0.37 | –0.084 | –0.179 to 0.013 |
| EQ-5D 26 weeks | 0.46 | 0.34 | 0.45 | 0.35 | –0.010 | –0.107 to 0.087 |
| EQ-5D 39 weeks | 0.41 | 0.34 | 0.40 | 0.34 | –0.009 | –0.106 to 0.092 |
| EQ-5D 52 weeks | 0.38 | 0.33 | 0.39 | 0.32 | 0.007 | –0.091 to 0.106 |
| EQ-5D AUC | 0.46 | 0.30 | 0.42 | 0.30 | –0.034 | –0.126 to 0.057 |
Resource use (in natural units) by treatment group is reported in Table 54. Predictably, study drug use for prednisolone and doxycycline was significantly higher in patients allocated to each treatment. However, there was significantly subsequent crossover to the alternative study drug in patients with poor outcomes. Of patients starting on prednisolone, 12.6% subsequently received at least one prescription of doxycycline; of patients starting on doxycycline, 57.8% subsequently received at least one prescription of prednisolone. Patients commonly used four topical corticosteroids; the overall use of any topical steroid during the trial was very similar for prednisolone (76.8%) and doxycycline (72.5%) patients. However, the level of use (and steroid potency) was notably lower in the prednisolone group (average prescribed amount of all topical steroids among users: prednisolone 121 g vs. doxycycline 277 g). Similar proportions of patients received the immunosuppressant azathioprine (prednisolone 4.2% vs. doxycycline 8.8%) although again the quantity of prescribed use was far lower in the prednisolone group (average prescribed amount among users: prednisolone 2.9 g vs. doxycycline 12.4 g).
| Resource use | Prednisolone | Doxycycline | Doxycycline – prednisolonea | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | 95% CI | |
| Drug use (g)b | ||||||
| Prednisolonec | 4.18 | 2.47 | 2.07 | 2.48 | –2.11 | –2.74 to –1.43 |
| Doxycyclinec | 1.30 | 5.92 | 16.50 | 18.52 | 15.20 | 11.50 to 18.97 |
| Azathioprine | 0.11 | 0.64 | 1.00 | 4.92 | 0.89 | 0.09 to 1.86 |
| Betamethasone valerate | 18.83 | 81.15 | 11.99 | 61.15 | –6.84 | –27.48 to 12.97 |
| Clobetasol propionate | 16.26 | 74.33 | 73.18 | 210.20 | 56.92 | 19.27 to 103.59 |
| Mometasone furoate | 46.63 | 58.21 | 83.37 | 150.4 | 36.74 | 7.72 to 66.49 |
| Clobetasone butyrate | 0.65 | 6.77 | 14.2 | 128.7 | 13.5 | –1.0 to 44.7 |
| NHS contacts (all)b | ||||||
| Inpatient days | 7.77 | 22.44 | 8.09 | 22.27 | 0.32 | –5.59 to 6.1 |
| Outpatient visits | 2.58 | 3.43 | 2.57 | 4.07 | –0.01 | –1.02 to 1.04 |
| GP clinic visits | 2.70 | 3.32 | 1.96 | 3.20 | –0.74 | –1.63 to 0.12 |
| GP home visits | 1.11 | 2.47 | 0.81 | 1.51 | 0.30 | –0.89 to 0.24 |
| Practice nurse visits | 2.38 | 7.61 | 8.52 | 65.39 | 6.14 | –1.48 to 19.7 |
| District nurse visits | 6.59 | 13.14 | 15.21 | 43.24 | 8.62 | 1.62 to 16.87 |
| NHS contacts (attributed to BP)b | ||||||
| Inpatient days | 3.30 | 8.75 | 5.14 | 12.53 | 1.84 | –0.9 to 4.7 |
| Outpatient visits | 0.87 | 1.61 | 1.64 | 3.12 | 0.77 | 0.14 to 1.44 |
| GP clinic visits | 0.81 | 1.61 | 0.64 | 1.79 | –0.17 | –0.61 to 0.29 |
| GP home visits | 0.38 | 1.24 | 0.30 | 0.95 | –0.08 | –0.39 to 0.22 |
| Practice nurse visits | 1.05 | 6.27 | 1.77 | 7.61 | 0.72 | –1.19 to 2.75 |
| District nurse visits | 4.60 | 10.26 | 10.57 | 39.73 | 5.97 | –0.02 to 13.75 |
Although resource-use comparisons (attributed to BP) were generally not statistically significant, there was a pattern of greater care received by doxycycline patients, consistent with a lower level of clinical effectiveness reflected (by design) in the primary outcome.
Patterns of resource use were costed using national reference values (see Table 50) and are reported in Table 55. Although costs for patients starting on doxycycline treatment appeared greater over 1 year, the increase was not statistically significant.
| Cost item | Prednisolone (£) | Doxycycline (£) | Doxycycline – prednisolone (£)a | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | 95% CI | |
| Study drugs | 106 | 49 | 77 | 53 | –29 | –42 to –15 |
| Non-study drugs | 10 | 12 | 25 | 41 | 15 | 7 to 23 |
| Study and non-study drugs | 116 | 53 | 102 | 73 | –14 | –31 to 3 |
| NHS contacts, all | 3364 | 7574 | 3827 | 7863 | 463 | –1591 to 2501 |
| NHS contacts, attributed | 1454 | 3044 | 2371 | 4822 | 917 | –109 to 1990 |
| Total, allb | 3657 | 7953 | 3987 | 8120 | 330 | –1904 to 2546 |
| Total, attributedb | 1687 | 3197 | 2455 | 4945 | 768 | –318 to 1911 |
Cost-effectiveness analysis
The joint distribution of incremental cost and outcome for the base-case analysis is shown graphically in Figure 8. Patients allocated initially to doxycycline experienced a slightly lower average quality of life (–0.024 QALYs, 95% CI –0.088 to 0.041) while tending to incur higher average health costs (£959, 95% CI –£24 to £1941), although neither finding was statistically significant (Table 56). These findings are consistent with the results of the clinical trial, which by design demonstrated a compromise between reduced effectiveness and increased safety for doxycycline. However, there remains the suggestion that doxycycline-initiated care may result in greater medium-term care costs, as shown by the 95% CI in Figure 8.
FIGURE 8.
Cost-effectiveness plane: base-case analysis [cost per QALY (£), 2013].

| Doxycycline-initiated vs. prednisolone-initiated therapy | Incremental cost (95% CI) (£) | Incremental QALYs (95% CI) | ICER (95% CI) (£) | p-valuea | p-valueb | NMB (95% CI) (£)a | NMB (95% CI) (£)b |
|---|---|---|---|---|---|---|---|
| Base case | |||||||
| Imputed attributable costs and QALYs, covariate adjusted | 959 (–24 to 1941) | –0.024 (–0.088 to 0.041) | Dominatedc | 0.046 | 0.070 | –1432 (–3094 to 230) | –1669 (–3886 to 549) |
| Sensitivity analyses | |||||||
| 1. Imputed attributable costs and QALYs, baseline EQ-5D score adjusted | 925 (–126 to 1977) | –0.026 (–0.094 to 0.042) | Dominated | 0.060 | 0.083 | –1443 (–3263 to 377) | –1702 (–4112 to 708) |
| 2. Complete-case attributable costs and QALYs, covariate adjusted | 1214 (33 to 2495) | –0.021 (–0.107 to 0.063) | Dominated | 0.063 | 0.101 | –1628 (–3738 to 437) | –1834 (–4726 to 961) |
| 3. Complete-case attributable costs and QALYs, baseline EQ-5D score adjusted | 1343 (50 to 2754) | –0.019 (–0.108 to 0.065) | Dominated | 0.083 | 0.110 | –1720 (–4132 to 538) | –1909 (–5060 to 1077) |
| 4. Imputed total costs and QALYs, covariate adjusted | 435 (–1481 to 2351) | –0.024 (–0.088 to 0.041) | Dominated | 0.228 | 0.213 | –909 (–3295 to 1477) | –1146 (–3967 to 1676) |
| 5. Imputed total costs and QALYs, baseline EQ-5D score adjusted | 461 (–1564 to 2487) | –0.026 (–0.094 to 0.042) | Dominated | 0.230 | 0.215 | –981 (–3580 to 1617) | –1241 (–4319 to 1836) |
| Base case: subgroup analysis | |||||||
| Blister: mild or moderate | 269 (–662 to 1199) | 0.001 (–0.074 to 0.076) | 298,586 (undefined) | 0.388 | 0.422 | –251 (–1987 to 1485) | –243 (–2647 to 2161) |
| Blister: severe | 2558 (–82 to 5198) | –0.090 (–0.222 to 0.042) | Dominated | 0.015 | 0.019 | –4361 (–8283 to –439) | –5263 (–10237 to –288) |
The likelihood of being cost-effective at different thresholds is shown in Figure 9.
FIGURE 9.
Cost-effectiveness acceptability curve: base-case analysis – doxycycline-initiated therapy.

Using a willingness-to-pay criterion of < £20,000 per QALY gained, there is a 4.6% probability that doxycycline-initiated treatment is cost-effective; conversely, there is a 95.4% chance that prednisolone-initiated therapy is cost-effective. [Note that these (one-sided) model probabilities should not be compared with inferential findings from (two-sided) statistical tests reported in Chapter 3.]
The NMB associated with doxycycline-initiated treatment was found to be negative and diminished with willingness to pay (Figure 10). Using a willingness-to-pay criterion of < £20,000 per QALY gained, the NMB associated with doxycycline-initiated therapy was negative but failed to reach statistical significance (–£1432, 95% CI –£3094 to £230). Although statistically imprecise, analysis of the NMB suggests that resources displaced in the NHS by doxycycline-initiated therapy would be greater than the value of benefit gained.
FIGURE 10.
Relationship between net monetary benefit and willingness to pay for doxycycline-initiated treatment.

Sensitivity and subgroup analyses
Comparing mean costs and QALY estimates using different modelling assumptions supports the base-case finding (see Table 56). The qualitative similarity of NMB estimates comparing imputed and complete-case analysis, covariate adjustment and range of costs included supports the validity of the imputation process and assumptions.
There was no interaction between treatment effect and trial stratifying variables, except in the case of blister severity. Patients recruited with severe blisters demonstrated a different cost and outcome pattern from patients with mild or moderate blisters, as shown in Table 56 and Figures 9 and 11. For patients presenting with mild or moderate blisters, differences in costs and QALYs are very small and thus the costs and outcomes can be thought to be similar. For patients presenting with severe blisters, doxycycline-initiated treatment presents greater costs (£2558, 95% CI –£82 to £5198) and poorer quality of life (–0.090 QALYs, 95% CI –0.222 to 0.042), which together make this strategy appear a poor investment (NMB –£4361, 95% CI –£8283 to –£439) using a willingness-to-pay criterion of < £20,000 per QALY gained.
FIGURE 11.
Cost-effectiveness plane: subgroup analysis [cost per QALY (£), 2013].
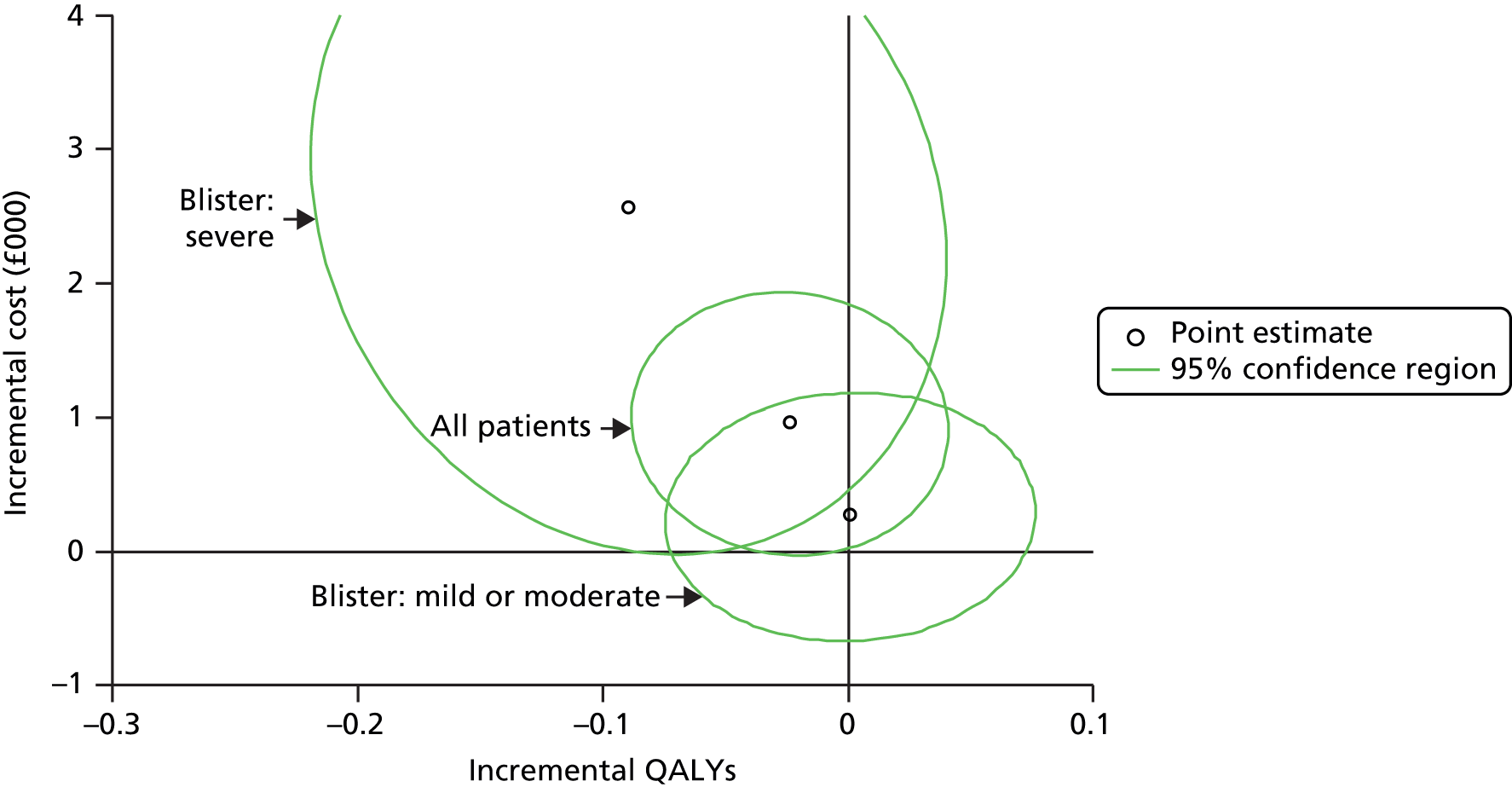
Other quality-of-life measures
Additionally, EQ VAS and DLQI scores were used to derive AUC scores over the course of the 1-year follow-up period (Table 57).
| Analysis | Incremental cost (95% CI) (£) | Incremental outcome (95% CI) |
|---|---|---|
| EQ VAS, imputed, covariate adjusted | 804 (–177 to 1785) | 0.005 (–5.138 to 5.147) |
| EQ VAS, imputed, baseline adjusted | 808 (–248 to 1864) | –0.555 (–5.874 to 4.764) |
| DLQI, imputed, covariate adjusted | 841 (–144 to 1826) | 1.162 (0.376 to 1.948) |
| DLQI, imputed, baseline adjusted | 832 (–228 to 1892) | 1.162 (0.376 to 1.948) |
The EQ VAS is scored from 1 to 100; equivalent QALY scores are obtained by dividing by 100, although the EQ VAS is not recommended for QALY estimation within trials as values are self-rated rather than societal. As with the EQ-5D estimates, there was no significant difference between treatments. DLQI estimates were significantly higher (denoting a poor outcome for doxycycline-initiated therapy). Being a disease-specific quality-of-life measure, the DLQI is potentially more sensitive to change than a generic measure; nonetheless, the changes correspond to an average of 1 point on a 30-point scale and are of uncertain clinical importance.
Conclusion
Patient-level data from the BLISTER trial provide the most robust evidence to date on whether or not doxycycline-initiated therapy is cost-effective as a treatment for patients with BP. The trial is concerned with the comparative short-term healing and long-term safety of doxycycline and prednisolone. Summatively, the profile of EQ-5D scores draws together the short-term and long-term patterns (see Table 52). A small quality-of-life benefit for prednisolone may occur in the first few months but would disappear by 6 months. Hence, although an extrapolation exercise was originally planned as part of the economic analysis, with modelling beyond 12 months, there was no rationale to pursue this.
In the base-case analysis (using multiple imputation) similar costs and outcomes were found regardless of whether patients received doxycycline-initiated therapy or prednisolone-initiated therapy. The joint distribution of costs and QALYs nonetheless suggests that doxycycline-initiated therapy may not be cost-effective. However, this finding seems to have been driven by the performance of the subgroup of patients with severe blisters. For patients presenting with mild or moderate blisters the economic and clinical findings align; given similar costs and outcomes, treatment decisions should be patient led and informed by the different profile of the two drugs. The clinical and economic findings for patients with severe blisters are different from the findings for those with moderate and mild disease. The economic analysis provides a clear preference for prednisolone-initiated therapy for patients presenting with severe blisters. This finding is returned to in the discussion (see Chapter 5).
The findings were robust to a range of sensitivity analyses using the complete-case data set; all rather than attributed costs; and different levels of model adjustment.
Chapter 5 Discussion
Main findings
This pragmatic study suggests that a strategy of starting people with BP on 200 mg/day of oral doxycycline is significantly safer over the course of a year than a strategy of starting with oral prednisolone (0.5 mg/kg/day). These long-term gains in safety for doxycycline are at the expense of short-term compromises in effectiveness in terms of blister control at 6 weeks, as we had anticipated. Both of our primary end points in this study supported our a priori hypothesis of non-inferiority of short-term blister control and superiority of safety for doxycycline compared with prednisolone, and these findings were robust to ITT and PP analysis and a range of sensitivity analyses.
Although the time point for assessing our primary outcome of short-term control of blisters was at 6 weeks, the non-inferiority of doxycycline compared with prednisolone was maintained within our prespecified margin when assessed at 13 and 52 weeks.
Treatment success was defined as having three or fewer significant blisters remaining, rather than complete clearance. There was a greater difference between the groups when the proportions of patients who were completely blister free were compared, but, in clinical practice, treatment is usually considered successful when only a few blisters remain because achieving complete clearance risks potentially ‘overtreating’ the patient.
Given that untreated BP tends to get worse, the study has also produced compelling new evidence that doxycycline does produce a genuine clinical effect in controlling blisters in BP. How tetracyclines, such as doxycycline, work in BP is still unclear, but it is suggested that they may exert an anti-inflammatory effect by inhibiting chemotaxis of neutrophils and eosinophils and blocking certain metalloproteinases. 26
The study also shows that oral prednisolone at a dose of 0.5 mg/kg/day (rather than a higher dose of 1 mg/kg/day) is very effective in controlling blisters for mild and moderately severe BP, with response rates of 97% and 98%, respectively, and provides a clinically useful response rate of 75% for those with severe disease, an evidence gap that has been highlighted in previous guidelines. 15 Corresponding response rates for doxycycline for mild, moderate and severe disease were 76%, 78% and 66%, respectively – still clinically useful given the potential long-terms reduction in adverse effects compared with prednisolone. It should be noted, however, that, although there was a suggestion of less difference between the two treatment strategies in those with severe baseline blisters, we did not find any statistical evidence that the differences in response rates between prednisolone and doxycycline varied according to baseline disease severity according to our planned interaction tests (see Tables 9, 19 and 20).
All safety outcomes pointed in the same direction, showing that is a safer to embark on a strategy of starting patients with BP on doxycycline rather than prednisolone. There were fewer severe, life-threatening or fatal treatment-related adverse events in those started on doxycycline and analysis of all-cause mortality showed that there were fewer deaths, both total and treatment related, in the doxycycline group. The overall differences between the groups are likely to be larger if higher doses of oral prednisolone are used, such as 0.75 mg/kg/day, as recommended in recent guidelines. 15 The safety advantage of doxycycline-initiated treatment appeared to be lost in those with severe disease at baseline in a post-hoc analysis (see Table 49). Overall adverse events (including mild and moderate) were similar in both groups, however, and quite high overall (> 80%) and mainly in keeping with the known adverse event profile for each drug. Nuisance adverse events such as nausea were higher in the first 6 weeks of treatment with doxycycline, which might have accounted for a greater lack of adherence to this treatment policy (see Table 6) compared with prednisolone.
Patients were able to switch treatments in this pragmatic study, which meant that some of the short-term effectiveness at 6 weeks of a strategy of starting with doxycycline could be attributed to oral prednisolone and, conversely, some of the severe, life-threatening and fatal events at 1 year in the doxycycline group could be attributed to those patients who switched to oral prednisolone. It is important therefore to be aware of the pragmatic perspective of this study, that is, to test the strategy of starting a patient with BP on doxycycline rather than a policy of starting with oral prednisolone. Although there was a considerable degree of switching, differences between the groups were clear throughout the study. At 6 weeks there was no switching in the prednisolone group but approximately one-quarter of those started on doxycycline had switched treatment. By 52 weeks approximately half of those who started on doxycycline had switched treatment compared with only approximately 10% of those who started on prednisolone. If all patients started on doxycycline switched to oral prednisolone it is likely that there would be no difference in effectiveness and safety.
The quality of life of patients in both groups improved over time and there were only small differences between the groups by 52 weeks. Although both groups showed a similar improvement in quality of life, as measured by the skin-specific DLQI, the prednisolone group improved more rapidly than the doxycycline group, probably reflecting the more rapid onset of blister control for those taking oral prednisolone.
Cost-effectiveness
Patient-level data from the BLISTER trial provide the most robust evidence to date on whether or not doxycycline-initiated therapy is cost-effective as a treatment for patients with BP. The base-case analysis (using multiple imputation) found similar costs and outcomes regardless of whether patients received doxycycline-initiated therapy or prednisolone-initiated therapy. The joint distribution of costs and QALYs nonetheless suggests that doxycycline-initiated therapy may not be cost-effective, with only a 4.6% probability of doxycycline being more cost-effective than prednisolone if societal willingness to pay is capped at £20,000 per QALY. However, post-hoc subgroup analysis of patients with and without severe blisters produced discrepant findings. For patients presenting with mild or moderate blisters the economic and clinical findings align. Prednisolone- and doxycycline-initiated treatments result in similar costs and outcomes and thus treatment decisions should be patient led and informed by the different profiles of the two drugs. It should be noted that both drugs are inexpensive and widely available worldwide. From a health policy point of view, the clinical and economic analyses both show some variation in findings for patients with severe blisters at baseline; on the grounds of cost-effectiveness there is a clear preference for prednisolone-initiated therapy for those with severe disease.
How our evidence fits with existing evidence
The 2010 Cochrane review on interventions for BP21 found that combination treatment with tetracycline and nicotinamide may be effective, but this was based on only one small and inadequately reported trial. Because tetracyclines are widely used in practice, it was concluded that this treatment option needs further investigation. No further trials of tetracyclines were identified in a subsequent systematic review23 nor in a search of the Cochrane Central Register of Controlled Trials database using ‘pemphigoid’ as a search term (28 May 2015).
Guidelines for the management of BP produced by the British Association of Dermatologists in 201214 discuss the use of potent topical corticosteroids over the entire body (except the face), as these have been shown to be effective and much safer than oral steroids. 18 Potent topical steroids are associated with some adverse effects, such as thinning of the skin and easy bruising. The risk of experiencing adverse effects from use of topical steroids depends on the strength of the steroid, how long it is used for, which area of the body it is applied to and the kind of skin problem; if a high-strength, potent steroid is used, enough may be absorbed through the skin to cause systemic adverse effects such as adrenal insufficiency or Cushing syndrome. For those patients for whom applying topical corticosteroids is not practical, oral steroids are the main recommended systemic medications. Oral steroids are effective in the treatment of BP; however, common adverse effects of oral steroids include weight gain, diabetes, infections, fractures and high blood pressure. The British guidelines recommend oral prednisolone at doses varying from 0.3 mg/kg/day to 1 mg/kg/day, depending on disease severity. 14 Spanish guidelines from 2014 recommended oral prednisolone at doses of 0.5–0.75 mg/kg/day26 and the same doses were recommended in guidelines produced by the European Dermatology Forum in 2015. 15 All three guidelines mention tetracyclines as a possible treatment for BP and point to the need for further high-quality evidence for tetracyclines.
The BLISTER study has now addressed the recommendation in the Cochrane 2010 review and a range of guidelines to further investigate the effects of tetracyclines in patients with BP, confirming its modest effectiveness and superior safety compared with oral prednisolone at a dose of 0.5 mg/kg/day.
Strengths and limitations
Strengths
This was a large, multicentre, multinational trial of 253 evaluable participants that had the power to detect clinically important differences. The trial had good external validity as patients were recruited from a large number of hospitals all over the UK and Germany, covering those with all types of disease severity, and from a range of hospital settings and socioeconomic areas. Additionally, patients were not excluded on the grounds of comorbidities and any patient with active BP was eligible. With the exception of incapacity to consent, patients were excluded only on safety grounds. Typically, BP patients are elderly and present with several comorbidities and so inclusion of such a population as in this study was an important issue.
This trial was designed to reflect clinical practice as far as possible, making the results useful in the real-life setting of a dermatology clinic. We assessed the degree to which the study was pragmatic using the pragmatic–explanatory continuum indicator summary tool25 and concluded that the trial was more pragmatic than explanatory.
There is no evidence of selection bias as the two groups were well matched for all baseline characteristics. Allocation of treatment was concealed by blinding the investigator until the 6-week blister count had been carried out (primary effectiveness outcome). Treatment allocation was revealed only once the blister count was entered into the trial database, thereby minimising the potential for performance and information bias. The investigator was unblinded for the remaining assessments for that participant, for example the primary safety outcome (adverse events over the full 52 weeks), as the investigator was often also the treating physician for this trial and it was not feasible to have a second (blinded) outcome assessor to collect adverse event data at every recruiting site. A relatively objective measure (adverse events graded according to the Common Terminology Criteria for Adverse Events) was chosen to minimise bias for the primary safety outcome measure.
As the investigator was blinded for the first 6 weeks of treatment (until the primary effectiveness outcome had been assessed), it was difficult for local investigators to comment on the relatedness of adverse events. Therefore, an independent adjudicator judged the relatedness of any adverse events to trial medication so that the blinding of the investigator for the primary effectiveness outcome could be maintained. In addition, all severe, life-threatening and fatal adverse events were independently assessed for relatedness to the trial medication to ensure that relatedness was being properly attributed.
The primary effectiveness results were similar in both the ITT population and the PP population. This is particularly important in this trial because of the non-inferiority comparisons, in which dependence on the ITT population alone risks falsely concluding a lack of difference between groups when there are significant amounts of missing data. 50 Similarly, findings from the cost-effectiveness analysis were robust to a range of sensitivity analyses, providing confidence in the modelling assumptions.
To minimise selective reporting bias, the trial was registered before recruitment started, the protocol published and the statistical analysis plan made freely available on the trial website [see https://ctsu.nottingham.ac.uk/ts0614/summary.asp (accessed 12 November 2015)]. All planned outcomes and analyses are reported here and any post-hoc analyses have been clearly indicated as such.
Limitations
This trial was a comparison between initiating treatment with doxycycline and initiating treatment with prednisolone rather than an explanatory trial evaluating the pharmacological effects of these treatments. It is very likely therefore that some of the treatment response in patients who switched from initial doxycycline treatment to prednisolone can be attributed to prednisolone. Despite treatment switches, there were still fewer medically serious side effects in those starting on doxycycline than in those starting on prednisolone over the course of 1 year. The strategy of initiating treatment with doxycycline and thus reducing the overall dose of corticosteroids is a safer treatment approach than starting on prednisolone over the course of 1 year.
To reflect clinical practice in which topical corticosteroids are often used early on in addition to systemic treatments,14 the use of potent topical corticosteroids applied only to blisters was permitted for the first 3 weeks. A washout period from week 3 to week 6 allowed a clearer assessment of the effectiveness of doxycycline at 6 weeks. It is unlikely therefore that topical corticosteroid use only in the first 3 weeks of treatment was responsible for the blister control observed at 6 weeks, given such a substantial 3-week washout period that followed. 51 More participants in the doxycycline group had to use potent topical corticosteroids during the washout period, although the non-inferiority findings for the primary effectiveness outcomes were robust to excluding such participants in a sensitivity analysis. Localised use of topical corticosteroids was permitted after week 6 when needed to reflect clinical practice and so assessments at all other time points could be affected to some degree by such usage.
The prespecified non-inferiority margin for effectiveness was relatively wide (with an upper CI of 37%), resulting in an effectiveness estimate for doxycycline that was both non-inferior and inferior compared with the prednisolone strategy, a paradox that was anticipated beforehand. 52 When surveyed prior to the trial starting (see Appendix 1), there was clearly a willingness among UK dermatologists to accept a considerable reduction in short-term effectiveness of doxycycline for a treatment that did not result in such severe long-term side effects.
There was a relatively high dropout rate in this trial, reflecting the multiple morbidities and frailty of a mainly elderly study population, with the potential for differential reporting bias. A low dropout rate of no more than 5% was predicted for the week 6 visit given the morbidity of the disease, but the dropout rate was higher than anticipated (although it should be noted that 2.8% dropped out as a result of death). There was a gradual loss over time resulting in a higher dropout rate than the 20% at 52 weeks allowed for in the sample size calculation.
It is unlikely that the success of both treatments at 6 weeks is a result of regression to the mean, reflecting the natural progression of the disease, because BP is a progressive disease. 53,54 Additionally, there is evidence that very low doses of prednisolone (0.3 mg/kg/day) are not effective,55 suggesting that there is little evidence to support a placebo effect. Even if one postulates a placebo/natural resolution response of around 20% at 6 weeks, the study response rates for effectiveness at 6 weeks (74.4% and 92.3% for doxycycline and prednisolone, respectively) are much higher and are likely to represent a true therapeutic response. Including a third placebo arm would have been unethical and would have probably failed to recruit.
As we collected data only on adverse events that were deemed to be possibly, probably or definitely related to the study treatment, the side effects of which are well known, it is possible that some degree of attribution bias could have occurred, for example sepsis occurring in a patient taking oral prednisolone might have been more likely to have been attributed to the drug rather than being recorded as a natural event. We think that this is unlikely because it was expected that the rate of severe adverse effects would be higher in the prednisolone group, based on knowledge from other trials,18 and because the number of deaths (regardless of attribution) was higher in the prednisolone group. Mild and moderate treatment-related adverse events for both drugs were fairly similar between groups, with grade 3 and above adverse events more common in the prednisolone group (see Table 32), arguing against a differential bias towards attributing more events to prednisolone. Determining the true attribution of adverse events to drugs is difficult in clinical practice and all unclear grade 3, 4 and 5 events in this trial were reviewed by a senior independent dermatologist who had access to the medical backgrounds of study participants and the chronology of events. In the absence of data on all adverse events, which was deemed unnecessary by the regulatory authorities for two such study drugs with well-established adverse effect profiles, some degree of attribution bias could have occurred in this study, but in a way that reflects how such attribution would be judged in everyday clinical practice, as per the pragmatic design of the study.
The generalisability of these results may be reduced by not including patients with dementia. Such patients were not included, as ethics committees insist that people with dementia, the majority of whom are unable to give informed consent, should not be recruited into trials if a trial can be delivered without involving such patients. However, in a disease that affects mainly the elderly, a large part of the patient population will be affected by dementia. It is unclear, though, whether or not having dementia would be likely to affect the treatment comparisons described in this study, as issues such as adherence are likely to have been similar in both groups. It is also worth noting that large numbers of potentially eligible new cases of BP for our study ended up not being eligible because their GP had already started them on oral prednisolone.
Although relapse rates were assessed, the results should be interpreted with some caution. To make it practical for clinicians in busy clinics to be able to include patients in this trial, they had to decide only whether or not the patient had < 4 significant blisters at each blister count (other than baseline), rather than undertake the time-consuming process of carrying out a full blister count. This means that it is possible that a patient who was considered a treatment success because of the presence of only two significant blisters could then ‘relapse’ by having just four at the next visit. Other patients may go from being almost blister free to having ≥ 30 blisters, which might be considered a more ‘true’ relapse. However, our relapse definition does give some indication of relapse rates and the direction is reflective of the other effectiveness outcomes in the trial.
All economic analyses in modelling the bivariate distribution of costs and QALYs involve assumptions. For example, judgements are made about the base-case model, the estimation method, adjustment for covariates, attribution of resource use and unit costs applied, as well as the quality-of-life measure used and societal weighting applied. As levels of missing data increase, complete-case analyses become progressively less satisfactory, whereas multiple imputation inevitably requires strong assumptions about data being MAR, which are only partially testable. Careful consideration of modelling issues and use of sensitivity analyses, exploring assumptions, provide some indication of the robustness of findings.
Patient and public involvement was not as strong as it could have been in this study. We failed to identify patients with BP or their carers who were willing to join our study team, despite repeated requests across study sites. Most patients were elderly and infirm and not able to travel to team meetings and no patient support group exists for BP that we could tap into. Perhaps we did not try hard enough. We did manage to find a patient and a carer who kindly joined the independent Trial Steering Group, one of whom (Penny Standen) was able to advise the team on the interpretation of the study. She also helped to write the plain English summary once the study was completed. Further qualitative work with patients to explore their views on the trade-off between short-term blister control and long-term safety gains would be worthwhile.
Implications for practice
This was a pragmatic trial, designed to be relevant to the sorts of people with BP typically seen in secondary care. All suitable patients who gave consent were included and the use of localised topical corticosteroids was allowed in the initial few weeks of the trial, along with the ability to change the treatment and the dose as required during the trial.
Some clinicians expressed the view at the study outset that doxycycline, if effective at all, would be useful only in those with mild disease, an assertion not borne out by this study. We found no evidence that the difference in short-term effectiveness between the two treatment strategies was dependent on the baseline disease severity. Higher absolute response rates for mild to moderate disease were seen for both treatment policies, but even those with severe disease had a clinically useful response at 6 weeks (75% and 66% treatment success at 6 weeks for prednisolone and doxycycline respectively). In terms of short-term effectiveness, the policy of starting treatment with doxycycline appears to be a potentially useful approach for patients with all severities of BP. With regard to serious related adverse effects, most of the advantage of doxycycline-initiated therapy resided in the mild and moderate severity groups, and cost-effectiveness analysis suggests that doxycycline-initiated therapy is not cost-effective in those with severe disease at baseline. BP is a disease associated with significant morbidity and mortality and the possible harms of treatment need to be given more attention.
The work by Joly et al. 18 shows that whole-body application of topical corticosteroids is a good treatment strategy for BP. However, such an approach is not always practical: very elderly or immobile patients would need considerable additional support to be able to apply a cream all over their body every day, and in many clinical settings this is not available. This study has suggested that initiating treatment with oral doxycycline is an effective and safe treatment and so, when application of topical corticosteroids is not a practical option, doxycycline may be offered as a safer alternative to oral corticosteroids whenever possible. It is important that patients understand the trade-off between doxycycline and prednisolone. Starting on doxycycline may mean that symptoms last longer than if starting on oral prednisolone and they may experience more mild side effects such as gastrointestinal problems, but they are less likely to experience severe and life-threatening side effects, even if they have to eventually switch to oral prednisolone.
In circumstances in which treatment with oral prednisolone is still indicated, these results have demonstrated that, although not as safe as doxycycline, a dose of 0.5 mg/kg/day probably produces a clinically worthwhile treatment response that is comparable to that seen with higher doses such as 1.0 mg/kg/day mentioned in guidelines,15 even for those with severe disease.
Implications for research
Qualitative work exploring patients’ views on the trade-off between short-term blister control and long-term safety might also be worthwhile, perhaps accompanied by a patient decision aid. Follow-up surveys of the clinicians from the UK Dermatology Clinical Trials Network who took part in determining the initial non-inferiority margin might also be useful to see whether or not the results have changed their practice.
It is clear from the results presented here that reducing the total amount of prednisolone, even if it is not avoided altogether, was beneficial to patients. Therefore, it would be useful to evaluate in a clinical trial whether a strategy of initiating treatment with prednisolone to achieve early disease control followed by a switch to maintenance therapy with doxycycline is safer than maintenance therapy with a reducing dose of prednisolone.
In the absence of a placebo-controlled study of doxycycline in BP, some might argue that such a study should be carried out, perhaps for mild disease and for a short duration of 6 weeks. We think that such a study would be unethical, given that the high response rates for doxycycline seen in this study (74% at 6 weeks) are likely to be much higher than any placebo or natural regression effects for what is thought to be a persistent and progressive disease in most people.
It might also be useful to investigate whether or not other antibiotics such as lymecycline, which are reported to have fewer side effects than doxycycline, are also beneficial in BP. Although doxycycline was far safer than prednisolone with respect to severe and life-threatening side effects, there was a high number of mild and moderately severe side effects with doxycycline, which are a nuisance to people taking long-term medicines. Inclusion of patients with dementia may also be considered in future studies, as they may respond to treatments differently.
A range of other systemic treatments such as azathioprine, methotrexate, dapsone, mycophenolate, high-dose intravenous immunoglobulin, plasmapheresis or rituximab have been used for BP that is more severe or unresponsive to first-line treatments such as topical or oral corticosteroids, but, apart from some combination therapies,56 most have been poorly evaluated. As this study has indicated how conclusions may differ for those with severe disease, future trials should present results according to baseline severity and consider planned subgroup analyses for those with severe disease. Consideration should be given to adopting clearer definitions of remission and early, intermediate and late observation points as recommended in 2012 by an international group. 57 There is also scope for establishing a core outcome set for future clinical trials of pemphigoid which includes outcomes that are important to patients so that the results of new trials can be compared in meta-analysis.
Chapter 6 Conclusions
This study has shown that a strategy of starting people with BP on doxycycline at a dose of 200 mg/day is safer than standard oral treatment with prednisolone at a dose of 0.5 mg/kg/day over the course of a year. Blister control with doxycycline in the first 6 weeks of treatment is inferior to that of oral prednisolone but it is still reasonably effective and was well within our prespecified non-inferiority margin. Overall, there is no significant difference in cost-effectiveness between the two treatment strategies although doxycycline-initiated therapy may not be cost-effective for those with severe baseline disease. The combined analysis of short-term effectiveness and long-term safety in this study provides critical information for clinicians to share with patients in a shared decision-making model, as well as providing reliable data to inform national and international guidelines, especially for those patients in whom extensive daily application of topical corticosteroids is not feasible. Further research may consider the evaluation of oral doxycycline to maintain remission in those who have initially been brought under rapid control with topical or oral corticosteroids.
Acknowledgements
We would like to thank Ms Sunita Rehal and Dr Daniel Bratton for their input into the trial and Dr Natasha Rogers for help with writing, editing and formatting the report. We would also like to thank all of the patients who gave up their time to participate in the trial. We thank the members of our trial oversight committees (see Appendix 4), all those from the Nottingham and Medical Research Council (MRC) Clinical Trials Units (see Appendix 5) who contributed to trial management at various stages of the project and all those who contributed to recruitment and follow-up from the recruiting centres (see Appendix 6).
This trial would not have been possible without the support of the UK Dermatology Clinical Trials Network [see www.ukdctn.org (accessed 6 October 2015)], which helped with various surveys prior to the main study and who were key in identifying recruitment centres. We would also like to acknowledge the support of the UK Clinical Research Network, particularly in providing research nurse support at the many centres around the UK.
This report presents independent research commissioned by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, NIHR, MRC, Central Commissioning Facility (CCF), NIHR Evaluation, Trials and Studies Co-ordinating Centre, Health Technology Assessment programme or Department of Health.
Contributions of authors
Dr Joanne R Chalmers was a lead applicant on the funding application, contributed to the design of the study and was part of the writing team.
Professor Fenella Wojnarowska conceived the study, was a coapplicant on the funding application, contributed to the design of the study and was part of the writing team.
Dr Gudula Kirtschig conceived the study, was a coapplicant on the funding application, contributed to the design of the study, liaised with the study sites in Germany and was part of the writing team.
Professor James Mason was a coapplicant on the funding application, contributed to the design of the study and conducted the cost-effectiveness analysis and was part of the writing team.
Ms Margaret Childs managed the trial and was part of the writing team.
Ms Diane Whitham provided senior clinical trials unit support and contributed to and reviewed the report.
Dr Karen Harman was a principal investigator who contributed to and reviewed the report.
Dr Anna Chapman was a principal investigator who contributed to and reviewed the report.
Dr Shernaz Walton was a principal investigator who contributed to and reviewed the report.
Professor Enno Schmidt was the lead investigator for Germany and was part of the writing team.
Mr Thomas R Godec was the trial statistician and conducted the effectiveness and safety analyses and was part of the writing team.
Professor Andrew J Nunn was senior statistician, a coapplicant on the funding application, contributed to the design of the study and conducted the cost-effectiveness analysis and was part of the writing team.
Professor Hywel C Williams was chief investigator, a coapplicant on the funding application and contributed to the design of the study and was part of the writing team.
Publications
Bratton DJ, Nunn AJ, Wojnarowska F, Kirtschig G, Sandell A, Williams HC. The value of the pragmatic–explanatory continuum indicator summary wheel in an ongoing study: the Bullous Pemphigoid Steroids and Tetracyclines study. Trials 2012;13:50.
Bratton DJ, Williams HC, Kahan BC, Phillips PP, Nunn AJ. When inferiority meets non-inferiority: implications for interim analyses. Clin Trials 2012;9:605–9.
Chalmers JR, Wojnarowska F, Kirtschig G, Nunn AJ, Brattan DJ, Mason J, et al. A randomised controlled trial to compare the safety and effectiveness of doxycycline (200 mg/day) with oral prednisolone (0.5 mg/kg/day) for initial treatment of bullous pemphigoid: a protocol for the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) trial. Br J Dermatol 2015;173:227–34.
Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial [published online ahead of print 6 March 2017]. Lancet 2017.
Data sharing statement
Data included in this manuscript are not able to be included in a public repository because of ethical restrictions. Requests for access to the data should be made to Professor Hywel Williams (hywel.williams@nottingham.ac.uk).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet 2013;381:320-32. http://dx.doi.org/10.1016/S0140-6736(12)61140-4.
- Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris – incidence and mortality in the UK: population based cohort study. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a180.
- Joly P. Incidence of bullous pemphigoid and pemphigus vulgaris. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a209.
- Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case–control study. Arch Dermatol 2010;146:1251-4. http://dx.doi.org/10.1001/archdermatol.2010.322.
- Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case–control study. J Invest Dermatol 2011;131:631-6. http://dx.doi.org/10.1038/jid.2010.357.
- Cordel N, Chosidow O, Hellot MF, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology 2007;215:187-91. http://dx.doi.org/10.1159/000106574.
- Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case–control study. J Invest Dermatol 2011;131:637-43. http://dx.doi.org/10.1038/jid.2010.301.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case–control study. JAMA Dermatol 2013;149:58-62. http://dx.doi.org/10.1001/2013.jamadermatol.376.
- Yang YW, Chen YH, Xirasagar S, Lin HC. Increased risk of stroke in patients with bullous pemphigoid: a population-based follow-up study. Stroke 2011;42:319-23. http://dx.doi.org/10.1161/STROKEAHA.110.596361.
- Fichel F, Barbe C, Joly P, Bedane C, Vabres P, Truchetet F, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol 2014;150:25-33. http://dx.doi.org/10.1001/jamadermatol.2013.5757.
- Joly P, Baricault S, Sparsa A, Bernard P, Bedane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol 2012;132:1998-2004. http://dx.doi.org/10.1038/jid.2012.35.
- Parker SR, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, et al. Mortality of bullous pemphigoid: an evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol 2008;59:582-8. http://dx.doi.org/10.1016/j.jaad.2008.07.022.
- Hogan DJ. Mortality of bullous pemphigoid. J Am Acad Dermatol 2009;60. http://dx.doi.org/10.1016/j.jaad.2008.10.003.
- Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G. British Association of Dermatologists’ guidelines for the management of bullous pemphigoid 2012. Br J Dermatol 2012;167:1200-14. http://dx.doi.org/10.1111/bjd.12072.
- Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol 2015;172:867-77. http://dx.doi.org/10.1111/bjd.13717.
- Rzany B, Partscht K, Jung M, Kippes W, Mecking D, Baima B, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol 2002;138:903-8. http://dx.doi.org/10.1001/archderm.138.7.903.
- Schmidt E, Goebeler M, Hertl M, Sárdy M, Sitaru C, Eming R, et al. S2k guidelines for the diagnosis of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges 2015;13:713-27. http://dx.doi.org/10.1111/ddg.12612.
- Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 2002;346:321-7. http://dx.doi.org/10.1056/NEJMoa011592.
- Joly P, Roujeau JC, Benichou J, Delaporte E, D’Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol 2009;129:1681-7. http://dx.doi.org/10.1038/jid.2008.412.
- Taghipour K, Mohd Mustapa MF, Highet AS, Venning VA, Kirtschig G. The approach of dermatologists in the UK to the treatment of bullous pemphigoid: results of a national survey. Clin Exp Dermatol 2013;38:311-13. http://dx.doi.org/10.1111/ced.12042.
- Kirtschig G, Middleton P, Bennett C, Murrell DF, Wojnarowska F, Khumalo NP. Interventions for bullous pemphigoid. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.cd002292.pub3.
- Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol 1994;130:753-8. http://dx.doi.org/10.1001/archderm.1994.01690060083010.
- Singh S. Evidence-based treatments for pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid: a systematic review. Indian J Dermatol Venereol Leprol 2011;77:456-69. http://dx.doi.org/10.4103/0378-6323.82400.
- Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial [published online ahead of print 6 March 2017]. Lancet 2017.
- Bratton DJ, Nunn AJ, Wojnarowska F, Kirtschig G, Sandell A, Williams HC. The value of the pragmatic–explanatory continuum indicator summary wheel in an ongoing study: the bullous pemphigoid steroids and tetracyclines study. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-50.
- Fuertes de Vega I, Iranzo-Fernandez P, Mascaro-Galy JM. Bullous pemphigoid: clinical practice guidelines. Actas Dermosifiliogr 2014;105:328-46. http://dx.doi.org/10.1016/j.ad.2012.10.022.
- Chalmers JR, Wojnarowska F, Kirtschig G, Nunn AJ, Brattan DJ, Mason J, et al. A randomised controlled trial to compare the safety and effectiveness of doxycycline (200 mg/day) with oral prednisolone (0.5 mg/kg/day) for initial treatment of bullous pemphigoid: a protocol for the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) trial. Br J Dermatol 2015;173:227-34. http://dx.doi.org/10.1111/bjd.13729.
- Murrell DF, Marinovic B, Caux F, Prost C, Ahmed R, Wozniak K, et al. Definitions and outcome measures for mucous membrane pemphigoid: recommendations of an international panel of experts. J Am Acad Dermatol 2015;72:168-74. http://dx.doi.org/10.1016/j.jaad.2014.08.024.
- Goon AT, Tan SH, Khoo LS, Tan T. Tetracycline and nicotinamide for the treatment of bullous pemphigoid: our experience in Singapore. Singapore Med J 2000;41:327-30.
- D’Agostino RB, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues – the encounters of academic consultants in statistics. Stat Med 2003;22:169-86. http://dx.doi.org/10.1002/sim.1425.
- Joly P, Benichou J, Lok C, Hellot MF, Saiag P, Tancrede-Bohin E, et al. Prediction of survival for patients with bullous pemphigoid: a prospective study. Arch Dermatol 2005;141:691-8. http://dx.doi.org/10.1001/archderm.141.6.691.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- NHS Precription Services . Prescription Cost Analysis (PCA) Data, England 2013 2013. www.nhsbsa.nhs.uk/PrescriptionServices/3494.aspx (accessed 19 March 2015).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Department of Health . NHS Reference Costs 2013 to 2014 n.d. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 19 March 2015).
- Health and Social Care Information Centre (HSCIC) . Hospital Episode Statistics, Admitted Patient Care, England – 2013–14 n.d. www.hscic.gov.uk/searchcatalogue?productid = 17192 (accessed 19 March 2015).
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-16. http://dx.doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. http://dx.doi.org/10.2165/00148365-200504020-00001.
- Drummond MF, Sculpher MJ, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Dordrecht: Springer Netherlands; 2007.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2393.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- Schiller P, Burchardi N, Niestroj M, Kieser M. Quality of reporting of clinical non-inferiority and equivalence randomised trials – update and extension. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-214.
- Castela E, Archier E, Devaux S, Gallini A, Aractingi S, Cribier B, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol 2012;26:47-51. http://dx.doi.org/10.1111/j.1468-3083.2012.04523.x.
- Bratton DJ, Williams HC, Kahan BC, Phillips PP, Nunn AJ. When inferiority meets non-inferiority: implications for interim analyses. Clin Trials 2012;9:605-9. http://dx.doi.org/10.1177/1740774512453220.
- Lever WF. Pemphigus. Medicine (Baltimore) 1953;32:1-123. http://dx.doi.org/10.1097/00005792-195302000-00001.
- Savin JA. The events leading to the death of patients with pemphigus and pemphigoid. Br J Dermatol 1979;101:521-34. http://dx.doi.org/10.1111/j.1365-2133.1979.tb11881.x.
- Roujeau JC, Guillaume JC, Morel P, Crickx B, Dalle E, Doutre MS, et al. Plasma exchange in bullous pemphigoid. Lancet 1984;2:486-8. http://dx.doi.org/10.1016/S0140-6736(84)92565-0.
- Beissert S, Werfel T, Frieling U, Bohm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol 2007;143:1536-42. http://dx.doi.org/10.1001/archderm.143.12.1536.
- Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol 2012;66:479-85. http://dx.doi.org/10.1016/j.jaad.2011.06.032.
Appendix 1 Survey results
Q1: What reduction in effectiveness would be acceptable?
-
Assuming the mortality rate with oxytetracycline is 1% less than with prednisolone and prednisolone is 95% effective:
-
a minimum median effectiveness rate of 50% would be required for oxytetracycline to have any place in the management of BP
-
a minimum median effectiveness rate of 80% would be required oxytetracycline to have potential as primary treatment for BP.
-
-
Assuming the mortality rate with oxytetracycline is 10% less than with prednisolone and prednisolone is 95% effective:
-
a minimum median effectiveness rate of 40% would be required for oxytetracycline to have any place in the management of BP
-
a minimum median effectiveness rate of 70% would be required oxytetracycline to have potential as primary treatment for BP.
-
Q2: What reduction in mortality would be useful?
-
Assuming oxytetracycline is 5% less effective than prednisolone and the mortality rate of prednisolone is 40%:
-
a maximum median mortality rate of 35% would be acceptable for oxytetracycline to have any place in the management of BP
-
a maximum median mortality rate of 23% would be acceptable for oxytetracycline to have potential as primary treatment for BP.
-
-
Assuming oxytetracycline is 10% less effective than prednisolone and the mortality rate of prednisolone is 40%:
-
a maximum median mortality rate of 30% would be acceptable for oxytetracycline to have any place in the management of BP
-
a maximum median mortality rate of 20% would be acceptable for oxytetracycline to have potential as primary treatment for BP.
-
-
Assuming oxytetracycline is 20% less effective than prednisolone and the mortality rate of prednisolone is 40%:
-
a maximum median mortality rate of 30% would be acceptable for oxytetracycline to have any place in the management of BP
-
a maximum median mortality rate of 15% would be acceptable for oxytetracycline to have potential as primary treatment for BP.
-
Recruitment feasibility
| Question | Averages |
|---|---|
| Approximately how many new cases of BP do you personally see per year? | 5 |
| Approximately how many new cases of BP are seen in your whole department per year? | 14 |
| Are you interested in helping recruit patients for this study? (approximately two patients per year, followed up for 1 year each) | Most yes |
| Do you think that it would be feasible for your colleagues to refer patients to you for this study? | Most yes |
| If yes, what proportion of the total number of BP patients presenting to your department would you be able to get referred to you for the study? | Approximately half |
Appendix 2 Health economics analysis plan
Appendix 3 Reasons for exclusion from the per-protocol analyses
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment during weeks 0–6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment | 17 | 3 |
| Used topical steroids between weeks 3 and 6 | 7 | 3 |
| Missed more than 3 consecutive days of treatment | 21 | 5 |
| Total number of non-PP patients | 34 | 10 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 17 | 3 |
| Used topical steroids between weeks 3 and 6 | 7 | 3 |
| Missed more than 3 consecutive days of treatment before week 6 | 21 | 5 |
| Total number of non-PP patients | 34 | 10 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 16 | 2 |
| Used topical steroids between weeks 3 and 6 | 6 | 3 |
| Missed more than 3 consecutive days of treatment before week 6 | 19 | 4 |
| Missed more than 3 consecutive weeks of treatment between week 6 and week 13 | 2 | 0 |
| Received > 30 g of topical steroids per week between week 6 and week 13 | 5 | 0 |
| Added systemic steroids to doxycycline (if allocated) or doxycycline or another immunosuppressant to prednisolone (if allocated) between week 6 and week 13 | 5 | 1 |
| Total number of non-PP patients | 36 | 10 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 13 | 2 |
| Used topical steroids between weeks 3 and 6 | 5 | 1 |
| Missed more than 3 consecutive days of treatment before week 6 | 15 | 1 |
| Missed more than 3 consecutive weeks of treatment between week 6 and week 52 | 2 | 0 |
| Received > 30 g of topical steroids per week between week 6 and week 52 | 3 | 2 |
| Added systemic steroids to doxycycline (if allocated) or doxycycline or another immunosuppressant to prednisolone (if allocated) between week 6 and week 52 | 7 | 8 |
| Total number of non-PP patients | 30 | 11 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 16 | 3 |
| Used topical steroids between weeks 3 and 6 | 7 | 3 |
| Missed more than 3 consecutive days of treatment before week 6 | 21 | 8 |
| Missed more than 3 consecutive weeks of treatment between week 6 and week 52 | 3 | 0 |
| Received > 30 g of topical steroids per week between week 6 and week 52 | 5 | 2 |
| Added systemic steroids to doxycycline (if allocated) or doxycycline or another immunosuppressant to prednisolone (if allocated) between week 6 and week 52 | 7 | 9 |
| Total number of non-PP patients | 40 | 21 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 3 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 3 | 14 | 2 |
| Missed more than 3 consecutive days of treatment before week 3 | 10 | 6 |
| Total number of non-PP patients | 21 | 7 |
| Reason for exclusion | Doxycycline, n | Prednisolone, n |
|---|---|---|
| Increased the dose of the allocated treatment before week 6 | 1 | 0 |
| Changed treatment or added a new treatment to the allocated treatment before week 6 | 12 | 1 |
| Used topical steroids between weeks 3 and 6 | 5 | 1 |
| Missed more than 3 consecutive days of treatment before week 6 | 14 | 1 |
| Missed more than 3 consecutive weeks of treatment between week 6 and week 52 | 2 | 0 |
| Received > 30 g of topical steroids per week between week 6 and week 52 | 3 | 2 |
| Added systemic steroids to doxycycline (if allocated) or doxycycline or another immunosuppressant to prednisolone (if allocated) between week 6 and week 52 | 7 | 8 |
| Total number of non-PP patients | 29 | 10 |
Appendix 4 Trial oversight committees
Trial Steering Committee
Independent members
-
Professor Jonathan Barker (chairperson), St John’s Institute of Dermatology, Guy’s Hospital, London, UK.
-
Professor Pascal Joly (clinical expert), Hôpital Charles Nicolle, Rouen, France.
-
Dr Jonathan Leonard (clinical expert), St Mary’s Hospital, London, UK.
-
Ms Helena Haywood (dermatology nurse), Amersham Hospital, Amersham, UK.
Patient representatives
-
Penny Standen.
-
Brian Lockwood.
Non-independent members
-
Professor Hywel Williams (chief investigator), Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK.
-
Professor Fenella Wojnarowska (lead clinician), Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK.
-
Dr Gudula Kirtschig (clinical expert and co-ordinator for German sites), Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK.
-
Professor Andrew Nunn (senior trial statistician), MRC Clinical Trials Unit at University College London, London, UK.
-
Daniel Bratton, Sunita Rehal, Tom Godec (trial statisticians), MRC Clinical Trials Unit at University College London, London, UK.
-
Dr Karen Harman (principal investigator representative), University Hospitals Leicester, Dermatology Department, Leicester Royal Infirmary, Leicester, UK.
-
Dr Phillip Hampton (principal investigator representative), Royal Victoria Infirmary, Newcastle, UK.
-
Dr Joanne Chalmers (research fellow), Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK.
The current trial manager was also a non-independent member of the Trial Steering Committee.
Trial Management Group
-
Professor Hywel Williams (chief investigator), Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK.
-
Professor Fenella Wojnarowska (lead clinician), Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK.
-
Dr Gudula Kirtschig (clinical expert and co-ordinator for European sites), Centre of Evidence Based Dermatology, University of Nottingham, Nottingham, UK.
-
Professor Andrew Nunn (senior trial statistician), MRC Clinical Trials Unit at University College London, London, UK.
-
Daniel Bratton, Sunita Rehal, Thomas R Godec (trial statisticians), MRC Clinical Trials Unit at University College London, London, UK.
-
Professor James Mason (health economist), Durham University, School of Medicine, Pharmacy and Health, Stockton-on-Tees, UK.
The current trial manager was also a member of the Trial Management Group.
Data Monitoring Committee
-
Professor S Lamb (chairperson), Warwick Clinical Trials Unit, University of Warwick, Coventry, UK
-
Dr R Graham-Brown (independent member), Department of Dermatology, Leicester Royal Infirmary, Leicester, UK
-
Dr Tracey Young (independent member), Health Economics and Decision Science, School of Health and Related Research, University of Sheffield, Sheffield, UK.
Appendix 5 Trial management team
All trial management was conducted by the NCTU, Nottingham Health Science Partners, Queen’s Medical Centre, Nottingham, UK.
Senior trial managers
-
Margaret Childs.
-
Diane Whitham.
Trial managers
-
Caroline Onions.
-
Dr Katharine Foster.
-
Dr Anna Sandell.
Data managers, trial co-ordinators and administrators
-
Daniel Simpkins.
-
Aisha Shafayat.
-
Robert Allen.
-
Aimee Tooley.
-
Sally Kucyj.
Appendix 6 Recruiting centres
Recruiting group (UK) in centre number order
-
Walton Hospital, Liverpool (principal investigator: Dr A Alkali; coinvestigator: Dr G Wong; research nurses: Ms M Harrison and Ms P Taylor).
-
Blackpool Victoria Hospital, Blackpool (principal investigator: Dr W Bottomley).
-
St Luke’s Hospital, Bradford (principal investigator: Dr A Wright; coinvestigator: Dr M Whittmann; research nurses: Ms J Ott and Mr A Liu).
-
Brighton General Hospital, Brighton (principal investigator: Dr C DeGiovanni; coinvestigators: Dr S Gossain, Dr S George and Dr F Imran; research nurses: Ms H Santander and Ms M Flowerdew).
-
Addenbrooke’s Hospital, Cambridge (principal investigator: Dr J Sterling; coinvestigators: Dr J Batchelor, Dr M Chattopadhyay, Dr M Wallace, Dr G Ben-Zvi, Dr S Haque-Hussain and Dr A Ranasinghe).
-
Sunderland Royal Infirmary, Sunderland (principal investigator: Dr S Wahie; coinvestigators: Dr K Freeman, Dr S Nataranjan, Dr N Rajan and Dr R Ellis; research nurse: Ms A Thomson).
-
University Hospital of North Durham, Durham (principal investigator: Dr S Wahie; coinvestigators: Dr T Sripathy, Dr V Bajaj, Dr M Vatve and Dr K Freeman; research nurse: Ms A Thomson).
-
London Road Community Hospital, Derby (principal investigator: Dr A Ferguson; research nurse: Ms K Riches).
-
Corbett Hospital, Stourbridge, West Midlands (principal investigators: Dr I Verpetinske, Dr S Cheung; dermatology clinical nurse specialist: Miss M Taylor).
-
Kent and Canterbury Hospital, Canterbury (principal investigator: Dr E Duarte-Williamson; coinvestigators: Dr C Cowley, Dr E Kulakov and Dr J Mann; research nurse: Ms A Potter).
-
Frimley Park Hospital, Frimley, Surrey (principal investigator: Dr F Antony; coinvestigator: Dr J Williams; research nurse: Ms L Moore; dermatology nurse specialist: Mrs J Herzke; clinical researcher: Ms S Atkinson).
-
Guy’s and St Thomas’ Hospital, London (principal investigator: Dr R Groves; coinvestigator: Dr E Benton; research nurses: Ms H Sreeneebus and Ms S Jones).
-
Harrogate District Hospital, Harrogate (principal investigator: Dr A Layton; coinvestigators: Dr A Whitton, Dr B Walker, Dr R Strauss, Dr S Das, Dr E Marshall, Dr N Goddard, Dr L Savage, Dr J Kwok and Dr M Walker; research nurses: Ms M Broome, Ms G Law and Mrs A Wray; clinical trials assistant: Mrs J Hussey; research and development administrator: Mrs J Pearson).
-
Hull Royal Infirmary, Hull (principal investigator: Dr S Walton; coinvestigators: Dr R Zaman, Dr A Kapdia and Dr V Smith; research nurses: Mr P Jones and Ms K Ashton).
-
Ipswich Hospital, Ipswich (principal investigators: Dr O Aziz, Dr S Gibbs, Dr D Rallan; research nurse: Ms S Hood).
-
James Paget Hospital, Great Yarmouth (principal investigators: Dr I Salvary, Dr R Graham; coinvestigator: Dr V Gajawada; research nurses: Ms S Simmons and Ms J Woods).
-
Cannock Chase Hospital, Cannock, Staffordshire (principal investigators: Dr A Azam, Dr R Rotarescu; coinvestigator: Dr S Cheung; research nurses: Miss K Amor, Ms M Harry, Ms N Smith, Ms S Hendy, Miss D Sirdefield and Mrs S Johnson).
-
Norfolk and Norwich University Hospital, Norwich (principal investigator: Dr N Levell; coinvestigators: Dr N Cassie-Chetty, Dr R Coelho, Dr G Millington, Dr M McDermott, Dr A Yong and Dr M Chriba; research nurses: Ms K Banks-Dunnell and Ms D Butcher).
-
Cumberland Infirmary, Carlisle (principal investigators: Dr N Cox and Dr M Nik; research nurse: Ms K Gilbanks).
-
North Devon District Hospital, Barnstaple, Devon (principal investigator: Dr K Davies; research nurses: Mr N Lawton and Ms L Wells).
-
Queen’s Medical Centre, Nottingham (principal investigator: Dr J English; coinvestigators: Dr M Malik, Dr C Wooton, Dr R Murphy, Dr J Batchlor, Dr R Simpson, Dr E Burden-Teh, Dr A Yaakub and Dr M Lam; research nurses: Ms S Davies-Jones and Ms J Llewellyn).
-
Churchill Hospital, Oxford (principal investigator: Dr V Venning; coinvestigators: Dr T McPherson and Dr S Cooper; research nurses: Ms L Matter, Ms M Westmoreland and T McPherson).
-
Queen Elizabeth Hospital, Greenwich (principal investigator: Dr A Chapman; coinvestigators: Dr Y Estfan and Dr N Miller; research nurse: Ms G Reeves).
-
Royal Berkshire Hospital, Reading (principal investigators: Dr G Kaushal, Dr D Seukeran, Dr I Nasr andDr H Malhomme; coinvestigators: Dr J Dua, Dr C Higgins, Dr A Lloyd Lavery, Dr S Ong, Dr C Allen and Dr R Clayton; research nurses: Ms K Wilmott, Ms J Foxton, Ms J King and Ms G Grimwood).
-
Royal Devon and Exeter Hospital, Exeter (principal investigator: Dr C Bower; coinvestigators: Dr C Charman and Dr J Varghese; research nurses: Mr R James and Ms T Hill; clinical trials administrator: Miss M Hayward).
-
Broadgreen Hospital, Liverpool (principal investigators: Dr H Bell and Dr R Azurdia; coinvestigators: Dr M Walsh, Dr K Ngan, Dr P Jayasekera and Dr C Angit; research nurses: Ms A Turner, Ms P Taylor, Ms D Marsh, Ms A Young and Ms T O’Rourke).
-
Royal United Hospital, Bath (principal investigator: Dr C Lovell).
-
Sandwell General Hospital, Birmingham (principal investigator: Dr S Velangi; coinvestigators: Dr W Szczecinska, Dr N Talsamia, Dr G Jutley, Dr M Ogboli and Dr J Halpern; research nurse: Ms T Shumba).
-
Royal Hallamshire Hospital, Sheffield (principal investigator: Professor D Gawkrodger; coinvestigators: Dr P Cousen, Dr N Aldoori, Dr C Morgan and Dr A Diaz).
-
King’s Mill Hospital, Sutton-in-Ashfield, Nottinghamshire (principal investigator: Dr J Ravenscroft; coinvestigators: Dr M Panchal, Dr E Bayliss, Dr J English, Dr A Yaakub and Dr H Trinh; research nurses: Ms C Heeley and Mr A Novak).
-
Torbay Hospital, Torquay (principal investigators: Dr J Adams and Dr T Frost; research nurses: Ms S Burns, Dr A Clepa and Dr D Benham).
-
James Cook University Hospital, Middlesbrough (principal investigator: Dr A Carmichael; coinvestigators: Dr R Ellis, Dr A Kapadia, Dr H Reddy, Dr S Fatah and Dr J Dalrimple).
-
Warwick Hospital, Warwick (principal investigator: Dr R Charles-Holmes; coinvestigators: Dr J Carter and Dr A Bedlow; research nurses: Ms C Jones, Ms W Seaton and Ms K Hotchkiss).
-
St George’s Hospital, London (principal investigator: Dr V Akhras; coinvestigator: Dr J Wee).
-
St Helens Hospital, St Helens (principal investigator: Dr S Winhoven; research nurse: Ms K Rutter).
-
Great Western Hospital, Swindon (principal investigators: Dr D Buckley, Dr S Gibbs; coinvestigators: Dr L Whittam, Dr H Hempel and Dr J Gingell; research nurses: Ms S Toft and Ms J Arnold).
-
Musgrove Park Hospital, Taunton (principal investigator: Dr V Lewis; coinvestigators: Dr J Adams and Dr R Wachsmuth).
-
Royal Victoria Infirmary, Newcastle (principal investigator: Dr P Hampton).
-
Whittington Hospital, London (principal investigator: Dr K Taghipour; coinvestigators: Dr N Kapur, Dr R Wakeel, Dr A Friedman; research nurse: Ms L Reeves and Ms B Akworth).
-
Lincoln County Hospital, Lincoln (principal investigator: Dr K Hussain; research nurses: Ms K Horton and Ms K Warner).
-
University Hospital, Coventry (principal investigator: Dr A Ilchyshyn; coinvestigator: Dr B Dharma; research nurse: Ms K Hotchkiss).
-
Bristol Royal Infirmary, Bristol (principal investigator: Dr G Dunnill; coinvestigator: Dr A Bray).
-
Leicester Royal Infirmary, Leicester (principal investigators: Dr K Harmen, Dr A Alexandrov; coinvestigators: Dr K Narayana, Dr G Johnston and Dr I Helbling; research nurses: Ms C Shelley and Ms A Hill).
-
Weston General Hospital, Weston-super-Mare (principal investigators: Dr M Kirkup, Dr D Simmons and Dr H Lloyd-Jones; research nurse: Mr G Saunders).
-
Whipps Cross University Hospital, London (principal investigator: Dr K Gibbon; coinvestigator: Dr A Bewley).
-
Yeovil District Hospital, Yeovil (principal investigator: R Wachsmuth; coinvestigators: Ms F Edwards and Dr J Boyle; research nurse: Ms M Davey).
-
York Hospital, York (principal investigator: Dr C Lyon; research nurse: Ms J Green).
-
Aberdeen Royal Infirmary, Aberdeen (principal investigator: Dr A Ormerod; coinvestigators: Dr F Craig and Dr F Hussain; research nurse: Ms L Lawson).
-
Royal Gwent Hospital, Newport (principal investigator: Professor A Anstey; coinvestigator: Dr J Ingram; research nurses: Ms S Mitchell and Ms C Watkins).
-
Raigmore Hospital, Inverness (principal investigator: Dr J Vestey; coinvestigator: Ms S Halliday; research nurses: Ms P Martin and Ms S Ross).
-
Glangwili General Hospital, Carmarthen (principal investigators: Dr D Shipley and Dr E Veysey; research nurse: Ms A Johnson).
-
Singleton Hospital, Swansea (principal investigators: Dr E Veysey and Dr S Blackford; coinvestigator: Dr S Sidhu; research nurse: Ms C Thomas).
-
University Hospital of Wales, Cardiff (principal investigators: Dr G Patel and Dr J Ingram; coinvestigators: Dr R Motley, Dr A Morris, Dr C Long, Dr R Abbott, Dr M Chowdhury and Dr S Scourfield; research nurse: Ms A Thomas).
-
Ninewells Hospital, Dundee (principal investigator: Professor J Ferguson; coinvestigators: Dr A Waters, Dr R Dawe and Dr P Rakvit; research nurse: Ms S Yule).
Recruiting group (Germany) in centre number order
-
Universitätsklinikum Carl Gustav Carus, Dresden (principal investigators: Dr C Günther and Professor Wozel; research nurse: Fr Blümlein).
-
Universitätsklinikum Erlangen (principal investigator: Professor M Sticherling; coinvestigator: Dr R Renner; research nurses: Fr P Alt and Fr S Friedel).
-
Universitätsklinikum Schleswig-Holstein, Lübeck [principal investigator: Professor E Schmidt (and chief investigator for Germany); coinvestigators: Dr D Meyersburg and Dr N Van Beek; research nurse: Fr D Knuth-Rehr].
-
Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz (principal investigator: Dr K Steinbrink; research nurse: Fr G Hagedorn).
-
Universitätsklinikum Münster, Münster (principal investigator: Professor Luger; coinvestigators: Dr A Tsianakas and Dr AM Perusquia Ortiz).
-
Klinik und Poliklinik für Dermatologie, Venerologie und Allergologie, Universitätsklinikum Würzburg, Würzburg (principal investigators: Professor E Broecker and Dr Benoit; coinvestigators: Dr C Hosp, Dr J Stoevesandt, Dr D Anders and Dr H Poppe; research nurse: Fr S König).
-
Universitätsklinikum Schleswig-Holstein, Kiel (principal investigator: Professor R Gläser; coinvestigators: Dr Rainer Hügel and Dr F Lipowsky; research nurse: Fr L Wedler).
Appendix 7 Data collection tools
Glossary
- Blinding/masking
- Procedures designed to ensure that those assessing outcomes in a study are not aware of the treatment allocations.
- Bullous pemphigoid
- An autoimmune blistering skin disease.
- Intention to treat
- The principle of including all those originally randomised in the final analysis.
- Non-inferiority comparison
- An approach to hypothesis testing that sets out to show that a treatment is acceptably inferior to another treatment within a predefined acceptability margin.
- Per protocol
- A form of analysis that evaluates only those participants who adhered strictly to the study protocol.
- Skin immunofluorescence
- A technique for detecting whether or not circulating autoantibodies are present in the skin. In pemphigoid, these are found at the junction between the epidermis and the dermis.
- Superiority comparison
- An approach to hypothesis testing that sets out to show that one treatment is superior to another by a defined margin.
List of abbreviations
- AUC
- area under the curve
- BLISTER
- Bullous Pemphigoid Steroids and Tetracyclines
- BP
- bullous pemphigoid
- CI
- confidence interval
- DLQI
- Dermatology Life Quality Index
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- EQ VAS
- EuroQol visual analogue scale
- GP
- general practitioner
- HES
- Hospital Episodes Statistics
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- MAR
- missing at random
- mITT
- modified intention to treat
- MRC
- Medical Research Council
- NCTU
- Nottingham Clinical Trials Unit
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PCA
- Prescription Cost Analysis
- PP
- per protocol
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year