Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/302/129. The contractual start date was in April 2007. The draft report began editorial review in July 2015 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Hisham Mehanna reports that he is a member of the Health Technology Assessment Clinical Trials Board and Claire Hulme reports that she is a member of the Health Technology Assessment Commissioning Board. Andrew GJ Hartley and Max Robinson report grants during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Mehanna et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide, with approximately 500,000 new cases per year. 1 It poses a significant therapeutic problem and has a high mortality and morbidity. Survival rates [apart from those for oropharyngeal cancer (OPC)] have not considerably improved over the past three decades despite newer aggressive surgical and chemoradiotherapy (CRT) regimens. Furthermore, both the disease and its treatments have considerable effects on vital functions (such as speech, eating, swallowing and appearance) and can result in significant functional deficits and quality-of-life effects.
Organ preservation treatment protocols, using radical concomitant CRT, have evolved in the past three decades, resulting in improved control rates (6.5% absolute improvement) at the primary site compared with radiotherapy alone. 2–4 Therefore, in many centres, CRT has become the preferred first line of treatment for several types of HNSCC, especially of the base of the tongue, tonsil, larynx and hypopharynx.
For patients who have advanced nodal metastasis in the neck [tumour, node, metastases (TNM) stages N2 or N3 nodes > 3 cm in size], the evidence for management is controversial. 1 Previously, the standard care in these patients was to perform a neck dissection (ND) (an operation to remove all lymph glands in the neck), either before or after CRT. A ND can result in morbidity, which can be lifelong, and (even a small risk of) mortality. 4 Since 2000, it appears that there has been a shift towards active surveillance guided by imaging, but there remains a significant proportion of patients being treated by planned routine ND. 5 Therefore, there continues to be lack of consensus regarding the best management for advanced nodal disease in patients receiving CRT.
This controversy continues mainly because of the poor quality and contradictory evidence (level 3/4) from prospective and retrospective case series for both management strategies. Furthermore, the advent of newer and more accurate functional modalities for the detection of persistent disease [such as positron emission tomography (PET)–computerised tomography (CT) scanning6,7] has further strengthened this debate. Several studies and systematic reviews have reported a high negative predictive value for PET scanning for the detection of persistent nodal disease. However, studies are small, mainly retrospective, single-centre studies. Many authorities on head and neck (H&N) cancer and literature reviews7,8 have stressed the need for a multicentre randomised trial to obtain an answer to this important question.
Existing research
Evidence in support of planned (routine) neck dissection
Until recently the standard care in the UK was to perform a ND with CRT. In other countries, a significant proportion of patients are still treated with planned ND. 5 Proponents of this management policy maintain that CRT does not eradicate large-volume nodal disease in a large proportion of patients (up to 50%), putting them at risk of recurrence. Some believe that for most of these patients, salvage by surgery will not be possible,9 resulting in devastating consequences.
The only randomised controlled trial available in the literature on the subject compared conservative follow-up with planned ND before radical CRT. 10 It found that there was significantly improved disease-specific survival following ND. However, it was small (50 patients) and had several major limitations, casting strong doubts on the validity of its results and findings. Other single-arm and retrospective studies have reported that planned ND demonstrated excellent locoregional control, although some did not detect improvement in overall or disease-specific survival in their series. 11–14
In addition, it has been found that in up to 40% of patients who show a complete clinical response to CRT but who undergo ND 8–10 weeks later tumour deposits can still be detected histologically in the ND specimen. 11,14,15 It has therefore been suggested that CRT does not completely eradicate the tumour in up to 40% of patients. Furthermore, proponents of ND maintain that selecting patients at a high risk of persistent disease is not possible using CT and/or magnetic resonance imaging (MRI), as studies have found that clinical and imaging evidence of complete response (CR) of nodal disease to CRT does not predict a complete pathological response (i.e. it does not correlate with an absence of pathological evidence of the disease in the ND specimen). 12,14
Evidence supporting a watch-and-wait policy in patients with complete response to chemoradiotherapy
Many clinicians now advocate a conservative surveillance policy, performing ND only if there is clinical evidence of persistent nodal disease after CRT. The rationale is that, among patients who exhibit a clinical CR in the neck following CRT, the recurrence rate in the neck is low (< 10%), and similar to that (8%) in patients with pathologically negative neck following ND. 8,16 Other level 2/3 studies9,17,18 have also found that ND in patients who show a CR to CRT does not confer any benefit in terms of improved survival or reduction in nodal recurrence compared with a watch-and-wait policy. Importantly, a large retrospective cohort study found that 43% of patients showed CR on CT, with a control rate of 92% at 5 years. Among those who experienced less than a CR, the 5-year control rate among those who underwent ND was similar, at 90%, but among those who did not undergo ND it was significantly lower (76%). 19
There is also evidence to suggest that, in many cases, the cells in the residual nodal deposits found on histology in ND specimens following CRT are not viable. 7,18 An experimental study, examining a proliferation marker, Ki-67, appears to confirm this hypothesis. 20
Accuracy of PET–CT scanning in the assessment of response to chemoradiotherapy and detection of residual nodal disease
Crucial to a watch-and-wait policy is the ability to detect residual disease in the neck post CRT, so that these patients can be targeted to have a ND. Detection of residual disease in the neck is not accurately assessed by examination in the clinic or by CT and/or MRI. Evidence suggests that PET is able to accurately identify those patients who do not have residual tumour in their neck following CRT (i.e. PET has a high negative predictive value).
Positron emission tomography has been reported in several small single-institution prospective and retrospective series and two meta-analyses21,22 to have a negative predictive value of 90–100% for the detection of persistent nodal metastasis following radiotherapy or CRT, with a variable positive predictive value of approximately 30–60%. 23–31 It has also been shown in several studies to have higher predictive values than CT and/or MRI,21,27,32,33 and better than combined clinical examination and ultrasound. 31 Finally, studies have found that co-registering PET and CT (PET–CT) is even more accurate than PET alone33,34 (Table 1).
| Modality | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|---|
| PET–CT | 98 | 92 | 94 | 88 | 99 |
| PET | 87 | 91 | 90 | 85 | 92 |
| CT | 74 | 75 | 74 | 63 | 83 |
There is also evidence from same studies that PET–CT is highly sensitive to the detection of residual tumour at the primary site following CRT and radiotherapy, with a high negative predictive value of 90–100%. 35–37 Indeed, it has been suggested that PET–CT may have similar a predictive value in the exclusion of residual disease at the primary site to examination under anaesthesia (EUA), the current gold standard, and may be capable of replacing it as a result of it being less invasive. 38,39 However, there is no existing level 1 evidence to corroborate this theory.
The study had not intended to measure human papillomavirus (HPV) status at the time of inception. However, over the past 5 years, there have been reports on the significant increasing incidence of HPV-associated OPC, with the proportion of OPCs that are HPV associated increasing from 40.5% to 72% over a period of 20 years. 40 HPV association appears to have a remarkable effect on the prognosis and outcomes of treatment, with a 58% reduction in the risk of death [hazard ratio (HR) 0.42, 95% confidence interval (CI) 0.27 to 0.66]. 41 This has necessitated examining outcomes by HPV status. 42
Research objectives
-
To test the hypotheses that a PET–CT-guided watch-and-wait policy (experimental arm) is non-inferior to the current practice of planned ND (control arm) when comparing overall survival (OS) in the management of advanced (N2 or N3) nodal metastasis in patients treated with CRT for primary HNSCC.
-
To assess the cost-effectiveness of the PET–CT-guided watch-and-wait policy.
-
To compare, as secondary outcomes, the efficacy of the PET–CT-guided watch-and-wait policy in terms of disease-specific survival, recurrence and quality of life.
-
To describe the accuracy of PET–CT in the detection of persistent disease at the primary site and the neck following CRT.
Chapter 2 Methods
Trial design
The trial was a two-arm, pragmatic, multicentre, randomised, non-inferiority trial comparing a PET–CT-guided watch-and-wait policy (experimental arm) with the current planned ND policy (control arm) in HNSCC patients with advanced neck metastases and treated with radical CRT.
Target recruitment was 560 patients. Patients were randomised in a 1 : 1 ratio. Primary outcomes were OS and health economics. Secondary outcomes were recurrence in the neck, complication rates and quality of life.
Amendments to the protocol
Substantial amendments to the trial protocol were approved since conception, which were significant to evaluate the economic costs, to help increase recruitment and to clarify eligibility and which included the following:
-
The introduction of health economics evaluation at local sites. A health economic cost analysis was required because the avoidance of a ND with its costs and possible complications and morbidity is expected to be one of the most significant benefits of the watch-and-wait policy. Furthermore, the economic effect of replacing EUA and CT with a PET–CT scan would need to be evaluated to assess its potential for the future. The aim of the economic evaluation was to identify the within-trial (WT) and long-term incremental cost-effectiveness of PET–CT-guided watch-and-wait compared with planned ND in HNSCC patients.
-
To extend the inclusion criteria to allow patients with occult primary tumours to enter the PET-NECK trial. Patients occasionally have pathologically occult tumours but, if all other diagnostic procedures point towards a H&N primary, and all other primary sites are excluded, they are routinely diagnosed and treated as having H&N cancer. There was no reason why these patients should not also have been given the opportunity to enter the PET-NECK trial if they wished and if they met all other criteria.
-
To allow ND to be performed before or after CRT in patients randomised to receive standard ND (control arm). There had been a change in practice in the previous 2–3 years regarding the timing of planned ND. In the USA and Europe, planned ND is usually carried out after CRT, rather than before, and there has been a gradual shift towards this practice in the UK over the previous 2–3 years. Therefore, amending the control arm to allow this was intended to increase recruitment and ‘future-proof’ the study if this trend continues in the UK.
-
To exclude patients with tumours that were not histologically squamous cell carcinoma and patients with primary nasopharyngeal carcinoma. Both non-squamous cell carcinomas and nasopharyngeal carcinomas have different biological behaviours and natural histories from squamous cell carcinoma of the H&N, and they should not be treated in a similar manner. Nasopharyngeal carcinoma is highly sensitive to radiotherapy and should not be treated by a ND. Non-squamous cell carcinoma is much less radiosensitive and, therefore, a ND is indicated in these cases. The literature available pertains to only squamous cell carcinoma in sites of the H&N other than nasopharyngeal.
-
To clarify that patients with equivocal PET–CT scans should receive a ND. The management policy in the UK for patients with equivocal PET–CT scans at the time of conducting the study was that they should receive a ND.
Ethics and research and development approvals
A favourable opinion was given by the Oxfordshire Multi-Research Ethics Committee in May 2007 (reference number 07/Q1604/35). Research and development approval was obtained from University Hospitals Coventry and Warwickshire (UHCW) in June 2007, and permissions to conduct the study at each site were obtained from the NHS trusts covering the Heart of England, Coventry and Warwickshire, Bath, Velindre, London North West, London Central, London South East, Marsden, Poole, Barnet and Chase Farm, Blackpool, Lancashire, Beatson West of Scotland, Abertawe Bro Morgannwg, Sunderland, Luton and Dunstable, Hull and East Yorkshire, Basildon and Thurrock, Southend, Sheffield and Chesterfield, Bradford, Nottingham, East Lancashire, Mid Essex, Aintree, Derby, Royal Devon, Bristol North, Bristol South, East Kent, Portsmouth, Plymouth, Manchester Central, Christie, Pennine, Hertfordshire, Dumfries and Galloway, Lothian, Royal Surrey, Newcastle Upon Tyne, Dudley, Grampian, Wolverhampton, Belfast, South Devon, Gloucestershire, Walsall, Birmingham West Midlands and South Tees. Participating hospitals are listed in the Acknowledgements of this report. All sites were activated between September 2007 and May 2012.
Sponsorship
The PET-NECK trial was co-ordinated by the Warwick Clinical Trials Unit and it has a co-sponsorship agreement with UHCW. The Warwick Clinical Trials Unit stores the data within the University of Warwick central information technology service host computers. All data are securely stored under the Data Protection Act 200443 and adhere to the University of Warwick Clinical Trials Unit data sharing standard operating procedure in which data sharing agreements have to be approved by both the Trial Management Group and the sponsor.
Participants
The study sought to recruit patients diagnosed with HNSCC with advanced nodal metastases who had not received previous treatment for their HNSCC and who had had no other cancer diagnoses within the past 5 years (exceptions given in Exclusion criteria), from H&N cancer-treating centres throughout UK NHS hospital trusts.
Inclusion criteria
Patients who met all of the criteria listed below were eligible to participate in the study:
-
had a histological diagnosis of oropharyngeal, laryngeal, oral, hypopharyngeal or occult HNSCC
-
had a clinical and CT/MRI imaging evidence of nodal metastases, stage N2 (a, b or c) or N3
-
had a multidisciplinary team (MDT) decision to receive curative radical concurrent CRT for primary
-
had an indication to receive one of the CRT regimens approved by the study
-
were fit for ND surgery
-
ND was technically feasible to perform to remove nodal disease (e.g. no carotid encasement, no direct extension between tumour and nodal disease)
-
were aged ≥ 18 years
-
were able to provide written informed consent.
Patients with N2 or N3 histologically and/or cytologically proven squamous cell carcinoma and an occult primary (after EUA and PET–CT scan) were eligible for the trial if they were going to be treated with CRT.
Exclusion criteria
Patients who met any of the criteria listed below were ineligible:
-
had tumours that were not squamous cell carcinomas histologically
-
were undergoing resection for their primary tumour, for example resection of the tonsil or base of tongue with flap reconstruction (diagnostic tonsillectomy was not considered an exclusion criteria)
-
had N1 stage nodal metastasis
-
were receiving neoadjuvant CRT with no concomitant chemotherapy
-
were receiving adjuvant chemotherapy
-
were undergoing chemotherapy with or without radiotherapy for palliative purposes
-
were undergoing radiotherapy alone (this is not an optimal treatment for neck node disease)
-
had distant metastases to the chest, liver, bones or other sites
-
were unfit for surgery or CRT
-
had received previous treatment for HNSCC
-
had primary nasopharyngeal carcinoma
-
were pregnant
-
had had another cancer diagnosis in the past 5 years (with the exception of basal cell carcinoma or carcinoma of the cervix in situ).
Patients undergoing neoadjuvant chemotherapy followed by concomitant CRT were eligible to enter the trial.
The patients in the study were similar to those in the studies that established the efficacy of CRT of the larynx42 and oropharynx,44 and were similar to the patients included in a wide range of studies examining PET–CT in the assessment of persistent nodal disease. 21,22
Settings and locations
In total, 38 centres (57 NHS hospitals) throughout the UK took part in the study. Participating centres were Department of Health-approved MDTs working in NHS clinics and undertaking the management of H&N cancer. MDTs are assessed according to defined criteria set by the Department of Health, including national standards for diagnosis, staging, therapy, radiology, pathology and patient support. 45 All clinicians have to be core members of the approved MDTs meeting minimum qualifications and throughput criteria. All trusts undertaking the management of H&N cancer undergo regular peer review and quality assurance (QA). 46 To participate, centres had to have centrally satisfactorily conducted a peer review report in the last 2 years. A list of participating hospitals is given in the Acknowledgements of this report.
All centres were required to provide confirmation of trust research and development and Administration of Radioactive Substances Advisory Committee (ARSAC) approval to conduct the study at each site. Centres also needed access to a PET–CT scanner either locally or at a distant site.
Administration of Radioactive Substances Advisory Committee approval
Administration of Radioactive Substances Advisory Committee licences were study specific and related only to the PET–CT centre, which was stated on the application.
Therefore, when a licence was held for the PET-NECK trial at a particular PET–CT centre, the site could send their patients to be scanned there as long as the ARSAC licence holder agreed to oversee the safety of the patients. The trials office had a list of ARSAC licence holders and arranged this for the centre. However, when PET–CT centres were not close by or when the site had its own PET–CT centre but did not hold a licence, its radiologist had to make an application under the Ionising Radiation (Medical Exposure) 2000 Regulations47 to the appropriate ARSAC. Approval of applications took around 1 month and patients could not be scanned without the licence being in place. ARSAC licences were issued for 2 years and had to be renewed after that time. The trials office kept a log of all expiry dates for ARSAC certificates and reminded the radiologist to renew them and send a copy of the renewal. A list of ARSAC licence holders is given in the Acknowledgements section of this report.
Quality assurance testing for PET–CT scanners
Before sites could scan patients in the surveillance arm of the trial, the PET–CT scanner to be used had to receive a successful QA review by the study’s central physicist.
All scanners had to be quality assured by the physicist. Each site had to arrange with the PET–CT centre to send a test case to the PET-NECK trial core laboratory for standardisation and quality control for each scanner that would be used in the trial.
The test case had to be an 18F or 68Ge Dicom phantom scan. All Dicom images had to be anonymised with patient trial number and initials and the compact disc or digital versatile disc had to be correctly labelled with ‘PET-NECK Trial’, scan date, site name and coded identifier and be sent along with an anonymised copy of the local report and a completed study Dicom patient scan transfer form.
Providers of PET–CT scanning units used in the study were InHealth (InHealth Group, High Wycombe, UK), Alliance (Alliance Healthcare, Hinckley, UK) and Cobalt Healthcare (Cobalt Healthcare, Cheltenham, UK). Scanners used for the study were either ‘static’, that is, fixed at a specific hospital site, or ‘mobile’, that is, able to travel various hospitals around the country.
Static scanners had to be quality assured every 2 years; however, mobile scanning units had to be tested by the core laboratory half yearly. The trial team kept a log of the pass dates for scanning units and organised with the PET–CT providers to renew the QA review.
A set-up visit to each centre by the trial co-ordinator also took place, during which information about the trial protocol was presented and site staff were given the opportunity to clarify information. Each site was provided with the TNM staging manual and written procedural requirements for PET–CT scanning and reporting. Once all approvals were in place, an activation letter was sent to the principal investigator and trust research and development department for each site to activate the start of recruitment.
Recruitment procedure
Participants were identified following pre-study assessments for diagnosis and tumour staging. Clinical, radiological and pathological staging was performed in accordance with the Union for International Cancer Control’s TNM Classification of Malignant Tumours staging manual. 48
Assessments for diagnosis and tumour staging
Each of the following assessments had to be done within the 4 weeks prior to randomisation, or at least CT/MRI or EUA had to have been carried out within the 4 weeks before randomisation:
-
CT/MRI of the primary site and neck (which had to be carried out before biopsy or not less than 2 weeks after biopsy).
-
Examination of the primary site with biopsy. In most cases, this necessitated examination under general anaesthesia. However, it was not mandatory if adequate clinical examination and biopsy could be performed without general anaesthetic.
-
CT of the chest.
-
Assessment for fitness for anaesthetic by treating clinician or anaesthetist.
Patient treatment was discussed by the MDT and each patient was considered for the PET-NECK trial. Eligible patients were invited to the clinic to discuss treatment and were told about the PET-NECK study. The study was discussed in detail with the patient and carer and all questions were answered. Once a patient decided to enter the study, informed consent was taken.
Informed consent
Adequate time was allowed for patients to consider their participation in the trial. There was no pre-agreed specified time by which to consent. Consent was required to be informed and voluntary, with time for questions and reflection. However, the patient also had the right to make an immediate decision to consent.
Consent to participate in the PET-NECK study was sought by the clinician involved in the patient’s care, with the involvement of the research nurse in the consent discussion. Patients were asked to confirm their consent to participate in the PET-NECK trial by initialling the appropriate boxes on the consent form and signing the form in the presence of the person taking consent. A copy was given to the patient, another copy was kept in the patient notes and the original was kept in the local site file.
The PET-NECK trial intervention
Patient randomisation
In total, 564 patients were recruited from 37 centres (43 NHS hospitals). For all patients recruited to the study, written informed consent was obtained.
Randomisation occurred centrally through the Warwick Clinical Trials Unit, where the study was being managed. Treatment allocation was performed using a minimisation algorithm and was stratified by the following: centre, timing of planned ND (before or after CRT), chemotherapy schedule {concomitant platinum, concomitant cetuximab [Erbitux® (Merck Biopharma, Darmstadt, Germany)], neoadjuvant and concomitant platinum, neoadjuvant docetaxel [Taxotere® (Sanofi-Aventis, Gentilly, France)] and platinum and 5-fluorouracil (TPF) with concomitant platinum} disease site (oropharyngeal, laryngeal, oral, hypopharyngeal or occult), tumour (T) stage (T1–T2 vs. T3–T4) and nodal (N) stage (N2a–N2b vs. N2c–N3) (Figure 1).
FIGURE 1.
Trial schema. a, EUA; b, if the patient’s primary tumour showed no response or an incomplete response to CRT or the patient developed a recurrence in primary site or neck, the patient was immediately referred back to the MDT for consideration for salvage surgery.

At randomisation, the eligibility of the patient was checked and the trial number and treatment arm allocated. Before being informed of the random allocation, each patient was asked to complete a quality-of-life questionnaire. Following randomisation, the patient’s general practitioner (GP) was notified of study participation by letter.
Treatment
Chemoradiotherapy regimens
To be enrolled in the study, patients had to have been recommended for concomitant CRT by the MDT. Patients randomised to the ND arm underwent ND either within 4 weeks before or within 4–8 weeks after CRT. For each patient, the participating centre had to specify at randomisation the schedule to be used. The CRT schedule was required to be one that the centre uses in its normal peer-reviewed practice and on the list of approved trial schedules in Box 1.
The recommended standard schedule for the trial was:
-
radiotherapy doses of 65–70 Gy in 30–35 daily fractions of 2 Gy or more with at least two doses of concomitant 3-weekly i.v. cisplatin (100 mg/m2) or carboplatin (4.5–5 AUC). 2,3
The following radiotherapy schedules are examples of approved schedules and are considered equivalent: 70 Gy in 35 fractions and 65 Gy in 30 fractions.
Approved variations to the recommended trial chemoradiotherapy scheduleThe following variations to the recommended trial standard regimen were also permitted:
-
Radiotherapy schedule variations: doses of 55 Gy in 20 daily fractions or equivalent.
-
Concomitant chemotherapy schedule variations:
-
Weekly cisplatin doses (30–40 mg/m2) to a minimum cumulative cisplatin dose of 200 mg/m2 or more or weekly carboplatin (1.5 AUC). 49,50
-
The use of epidermal growth factor receptor modulation with cetuximab instead of cisplatin-based chemotherapy was permitted: loading dose of 400 mg/m2 body surface area followed by weekly i.v. infusions of 250 mg/m2. 51,52
-
Variations from the approved trial schedules above as a result of emerging evidence or changes in the practice of a centre were at times permitted, after consultation with the PET-NECK trial management team.
-
-
Patients undergoing neoadjuvant chemotherapy followed by concomitant CRT were eligible for the trial. If these patients were randomised to the ND (control) arm, it was recommended that patients have their ND within 2 weeks of randomisation if possible, followed by the neoadjuvant schedule.
-
Neoadjuvant with concomitant chemotherapy schedules could be used at the centre’s discretion, provided that the following schedules were used:
-
Up to three cycles of neoadjuvant platinum-based chemotherapy were permissible, for example cisplatin 75–100 mg/m2 or carboplatin AUC 5–6 and 5-fluorouracil 1000 mg/m2 a day for 4–5 days or equivalent.
-
Docetaxel 75 mg/m2 with platinum (75–100 mg/m2) and 5-fluorouracil (1000 mg/m2) schedules were also permitted. 53
-
-
Variations from the approved trial schedules above, as a result of emerging evidence or changes in the practice of a centre may have been permitted, after consultation with the PET-NECK trial management team.
-
Adjuvant CRT and neoadjuvant-only chemotherapy followed by radiotherapy schedules were not permissible.
AUC, area under the curve; i.v., intravenous.
The recommended CRT schedule and the approved variations to the schedule were selected after consultation with several oncologists nationwide. The approved schedules were selected because they each fulfilled the following criteria: the schedule was supported by a strong evidence base and the schedule was well established and a standard schedule in UK centres. Variations from the approved trial schedules as a result of emerging evidence or changes in the practice of a centre were individually appraised and approved when appropriate by the PET-NECK trial management team. Patients receiving radiotherapy only (even if receiving accelerated radiotherapy schedules) were not eligible to enter the trial.
Planned neck dissections
The randomising centre was required to decide before randomisation whether, in the case of a patient being randomised to the ND (control) arm, the planned ND would be performed before or after CRT.
Timing of neck dissection
If the ND was to be performed before CRT, it had to be performed within 4 weeks of randomisation.
If the ND was to be performed after CRT, it had to be performed 4–8 weeks after completion of CRT. It was recommended that patients undergo assessment by CT when possible to assess the primary site (but this was not mandatory) and clinical examination with or without EUA before ND.
Type of neck dissection
The recommended surgical procedure was a modified radical ND. This involves the removal of lymphatic structures in levels I–V, with preservation of one or more of the following: spinal accessory nerve, internal jugular vein and sternocleidomastoid muscle. 54
Selective NDs (including lymphatic tissues in fewer than the five levels I–V) were also acceptable provided the following conditions were met:
-
All clinically evident involved nodal groups were included in the ND.
-
The lymphatic tissues of levels I–V that had not been removed in the ND were included in the radiotherapy fields to a minimum of 50 Gy.
The type of access incision was left to the surgeon to decide.
Quality assurance of histological assessment of neck dissection specimens
Histological assessment of ND specimens was performed in accordance with the requirements and standards of the minimum pathology data set published by the Royal College of Pathologists. 46
A central pathology review of 10% of pathology specimens was performed for the purposes of ensuring quality control. The cases were selected at random by the PET-NECK trial office and requested from the participating centres. There were no differences in the results of the local centre pathologist and the central pathology reviewer.
Three-month post-chemoradiotherapy assessment
All patients were assessed for response after CRT. In the case of patients randomised to the PET–CT (surveillance) arm, this assessment took place 9–13 weeks after completion of CRT. In patients who were randomised to ND (control) arm and who received ND before CRT, assessment was performed 9–13 weeks after CRT. In patients in the control arm who received ND after CRT, assessment for response to CRT was done 4 weeks prior to the ND.
The timing of the assessment was chosen to reflect literature findings that PET–CT is most accurate when done at least 8 weeks post CRT, and preferably > 12 weeks after CRT. However, this was balanced by the need to perform the ND as soon as possible to avoid regrowth of the residual tumour post CRT, and the increased technical difficulty in performing ND later than 12 weeks post CRT because of ensuing fibrosis and scarring. As this was a pragmatic trial, some flexibility was afforded.
At any time, if a patient was found to have residual primary disease, he or she was immediately referred back to the treating clinician and MDT for consideration for salvage treatment.
Neck dissections for persistent disease identified on PET–CT post chemoradiotherapy
Persistent disease on PET–CT was defined as metastatic nodal disease in the neck identified by PET–CT at the 3-month post-CRT assessment. Patients in whom PET–CT findings 3 months post CRT were equivocal had to be treated as if they had persistent disease and had to undergo ND. The ND had to be performed within 4 weeks of a decision at the multidisciplinary meeting (MDM) to perform surgery following identification of residual disease on PET–CT. As for planned NDs, both modified radical ND and selective NDs were acceptable, provided that the conditions were met. The type of access incision was left to the surgeon to decide.
Recommended clinical guidelines for PET–CT scanning
-
Patient scheduling:
-
PET–CT should have ideally been performed between 10 and 12 weeks after completion of the last dose of CRT. However, it could be performed between 9 and 13 weeks after completion of CRT and before any biopsies.
-
-
Preparation:
-
Patients had to fast for 6 hours prior to the scan.
-
Patients had to be weighed without shoes and coats (ensuring that the weighing device was calibrated).
-
Blood glucose had to be recorded using Boehringer Mannheim’s Glucometer (Boehringer Mannheim, Mannheim, Germany) (ensuring that the device was calibrated).
-
Patients had to drink 2–3 glasses of water prior to the test to ensure hydration.
-
Metal denture fixtures had to be removed whenever possible (to reduce CT artefacts and improve semiquantitative accuracy).
-
-
Injection:
-
Fludeoxyglucose had to be injected via a butterfly cannula under quiet conditions.
-
The patient had to be asked to remain silent.
-
The injected dose had to be 4.5 MBq of fludeoxyglucose per kilogram of body weight up to a maximum of 400 MBq.
-
-
Fludeoxyglucose uptake:
-
The patient had to remain inactive in a comfortable and quiet environment during the uptake.
-
The patient had to empty their bladder just prior to positioning on scanner bed.
-
The emission scan had to start at 90 minutes post injection.
-
-
Positioning:
-
The patient had to be scanned on a regular couch top.
-
Scanning had to begin at the skull vertex and end at the groin.
-
The scan had to be done with arms down if a single whole-body scan was performed. If the body was scanned separately from the H&N, then the body had to be scanned with the arms up.
-
-
Acquisition parameters:
-
Each PET–CT centre was advised to use routine local protocols.
-
-
Reconstruction parameters:
-
The PET–CT centre was advised to use ordered subset expectation maximisation, with CT for attenuation correction using local routine parameters.
-
-
Archive:
-
The reconstructed CT, PET attenuation-corrected and non-attenuation-corrected data had to be archived locally.
-
-
QA and quality control:
-
This was done under an agreed QA and quality control protocol.
-
-
Reporting PET–CT findings:
-
A standardised criterion was used for reporting PET–CT findings.
-
The PET–CT findings had to be reported by the local hospital imaging team.
-
The PET–CT findings had to be reviewed at the central laboratory by an expert in the PET core laboratory, who was independent of the local report.
-
Differences in reporting between the local hospital and the central laboratory were resolved by consensus, otherwise a third expert at the core laboratory reported on the scan and the majority view was taken.
-
-
PET–CT data transfer to the central laboratory:
This was under an agreed protocol transfer following reconstructed and anonymised files:
-
CT
-
PET attenuation corrected
-
PET non-attenuation corrected
-
PET–CT report from local imaging team.
-
-
Adverse events and serious adverse events (SAEs) during PET CT:
-
Adverse events and SAEs were required to be recorded and documented by the local investigational team in accordance with agreed protocols. However, none of these was reported.
-
Quality assurance review of PET–CT scans
For patients in the PET–CT arm of the trial, the PET–CT scan was reviewed by both the local reporter and the trial core laboratory radiologist. When there were significant differences between the reports of the local PET–CT clinician and the central PET–CT reviewer that might have affected patient management, the central PET–CT reviewer (from the core laboratory) and the participating centre radiologist conferred and a final report was agreed. If agreement could not be reached, a second independent reviewer from the core laboratory was asked to review the scans and issue a second opinion report. If there was still a significant difference between the local PET–CT report and the core laboratory reports, the final decision on which report to use rested with the local PET–CT clinician, local treating clinicians and MDT.
Any discordance between the reports of the local PET–CT clinician and central PET–CT reviewer was documented in the patient’s assessment form including which report was used to make the final clinical decision.
Quality assurance review of staging and response scans
Central QA review was performed on 10% of staging scans (and response scans for patients in the ND arm) to check for concordance. The results of the review are not yet finalised and this will be reported in a later paper.
Patient assessments
The schedule of assessments is shown in Table 2. All biobank samples collected at baseline and follow-up will be retained for future work.
| Assessments | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-study entry | 2 weeks post NDa | Last CRT dose | Monthly follow-up visits during year 1 | Post-CRT assessmentb | 6-month follow-upb | 12-month follow-upb | 2-monthly follow-up visits during year 2 | 24-month follow-upb | |
| MDM discussion | ✗ | ||||||||
| Informed consent for trial | ✗ | ||||||||
| Evaluations | |||||||||
| Complete history/physical | ✗ | ✗c | ✗ | ✗c | ✗c | ✗c | ✗c | ||
| Complications reporting | ✗d | ✗e | |||||||
| Examination with or without biopsyf | ✗g | ✗h | ✗ | ||||||
| Imaging | |||||||||
| H&N CT/MRI | ✗i | ✗i | |||||||
| Thoracic CT | ✗g | ||||||||
| PET–CT | (✗j) | ✗k,l | |||||||
| CXR | ✗ | ✗ | |||||||
| Quality-of-life assessments | |||||||||
| EORTC QLQ-C30 and H&N35 | ✗ | ✗m | ✗ | ✗ | ✗ | ||||
| MDADI | ✗ | ✗m | ✗ | ✗ | ✗ | ||||
| EQ-5D | ✗ | ✗m | ✗ | ✗ | ✗ | ||||
| Health economic questionnairesn | ✗ | ✗m | ✗ | ✗ | ✗ | ✗ | |||
| Blood sample collectiono | ✗ | ✗o | ✗o | ||||||
Patient follow-up
Patient follow-up was monthly for the first year up to the 12th month after randomisation, and 2-monthly for the second year up to the 24th month after randomisation. It was recommended, but not mandatory, that patients should have chest radiography annually.
At each follow-up visit, the patient received a full clinical examination with palpation of the neck and visualisation of primary site when possible, with appropriate trial forms and questionnaires completed at the completion of CRT, and at 6, 12 and 24 months after randomisation.
Patients with confirmed recurrence
Recurrent disease was defined as a confirmed tumour in the primary site after a clear 3-month post-CRT assessment or metastatic nodal disease identified in the neck after negative PET–CT post CRT or after a ND, during the follow-up period. A tumour was confirmed as recurrent if disease was confirmed by biopsy or radiological cross-sectional imaging.
Patients with recurrence, confirmed by biopsy or needle aspiration and CT/MRI or by their PET–CT scan were referred back to the treating clinician and MDM urgently for consideration for salvage treatment, including ND for patients with isolated nodal recurrence.
Serious adverse events
Investigators were required to inform the trials unit immediately of any SAEs following CRT, PET–CT or ND.
Each time the patient was seen in clinic he or she was asked if any SAEs had occurred. The occurrence of SAEs was based on information provided by either patients or their carers. The following adverse events were considered serious:
-
death
-
life-threatening disease
-
hospitalisation or prolongation of hospitalisation
-
congenital abnormality
-
persistent disability
-
other medically significant event.
Trial-specific exclusions from reporting SAEs included any of the following:
-
elective hospitalisations for the treatment of the primary cancer and its effects
-
elective hospitalisation for social reasons
-
elective hospitalisation for pre-existing conditions that were not exacerbated by the treatment.
Adverse effects of PET–CT scanning
If, after administration of the radiopharmaceutical required for PET–CT, the biodistribution within the patient was not as would be expected, the ARSAC certificate holder had to be informed and a check made to determine that the correct radiopharmaceutical was administered. If the correct radiopharmaceutical was administered, the supplier of the radiopharmaceutical had to be informed.
In the case of an incorrect radiopharmaceutical being administered to a patient, the ARSAC licence certificate holder and a senior member of staff had to be informed immediately who was then required to follow the guidance in Health and Safety Guidance Number 95. 55
-
The ARSAC certificate holder was required to inform the patient of the error.
-
The referring clinician had to be informed.
-
The GP of the patient had to be informed.
-
A risk assessment was required to be requested from the radiation protection advisor.
-
The staff member was required to inform the chief investigator and the sponsor.
A report of any incident, including action taken in order to prevent a recurrence, was required to be compiled by the radiation protection supervisor and forwarded to the radiation protection advisor. Any such incident was required to be recorded as an unexpected SAE and also reported through the individual trust critical incident policies.
Patient withdrawal
Patients were informed about the right to withdraw from the study at any time without giving a reason. However, site staff were asked to make every effort to identify the reason for withdrawal. Withdrawn patients were asked if any data collected prior to their withdrawal could be used in the analyses.
The reason for withdrawal (when known) was recorded on the patient notes and the trial office was informed of the withdrawal.
Patients who elected to withdraw from the trial interventions remained in the trial for follow-up per protocol unless they withdrew their consent.
Patients who changed their mind about withdrawal and wished to rejoin the study could chose to do so at any time, but they needed to be reconsented and follow-up data were to be collected only from that point onwards.
Patients moving out of the area
When patients moved away from the area to another participating centre, every effort had to be made, with the patients’ consent, to transfer the follow-up of patients so that the new centre could take over the responsibility for follow-up. Close co-ordination with the PET-NECK trial office was essential for this.
Outcomes
Overall survival (primary outcome)
Information on death and survival was obtained from centres via death and follow-up forms.
Cost-effectiveness (primary outcome)
Data for the health economic analysis were collected from the trial EuroQoL-5 Dimensions (EQ-5D) and resource use questionnaires, as well as the case report forms. Full details of the health economic analysis are given in Chapter 4.
Complication rates
Complications following ND surgery were reported.
Disease-specific survival
Causes of death were reported on the death form. Deaths were regarded as caused by H&N cancer if they were reported as such, if there was persistent, recurrent or metastatic disease present at death or if the patient died as a result of complications of treatment for H&N cancer.
Recurrence in neck nodes
Details of recurrences were reported at follow-up visits. Any notification of recurrence within 3 months of radiotherapy was regarded as persistent disease and notifications after that date were regarded as recurrences. Recurrences in the neck nodes were reported as ipsilateral or contralateral in the notification of recurrence.
Quality of life
The questionnaires included the following instruments:
-
EQ-5D: five 3-point scales and one summary 100-point scale. This was used for the health economics evaluation
-
European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire for Cancer (with 30 questions) (QLQ-C30): five functional, three symptom and a global scale and six single items for assessment of general quality of life
-
EORTC Quality of Life Questionnaire for Cancer head and neck module with 35 questions (QLQ-H&N35): seven scales and 11 single items for H&N cancer-related quality of life
-
MD Anderson Dysphagia Inventory (MDADI): an overall function scale and four subscales.
Patients were requested to complete these questionnaires at five time points. The first was completed in clinic prior to randomisation. Later questionnaires were received either in clinic or through the post before the assessment dates were due at these time points: within 2 weeks of CRT completion, 6 months after randomisation, and 12 and 24 months after randomisation.
Accuracy of PET–CT scanning
Positron emission tomography–computerised tomography scans were assessed both by the local radiologist and by the trial core radiologist.
Sample size
The trial was designed to allow demonstration of non-inferiority of the PET–CT surveillance arm compared with the control arm (planned ND), which was assumed to have a 2-year OS of 75%. The margin for non-inferiority was set at 10%, implying that the 2-year OS of the experimental arm should not be below 65%, a difference equivalent to a HR of 1.50.
The trial was expected to recruit for 3 years, with an additional follow-up period of 2 years. The sample size was set at 560 patients in total (280 in each treatment arm), giving a 90% power with a type I error of 5%, allowing for a 3% loss to follow-up. The sample size was calculated by simulation assuming unadjusted analysis by a Cox’s proportional hazards model and assuming follow-up to the end of the trial 5-year period (using data from the Office for National Statistics). If no follow-up existed beyond the 2-year requested data, then the power would be 76%. It was expected that the true power would lie between these figures (i.e. 76% and 90%). The assumptions underlying the sample size calculation were monitored by the Independent Data and Safety Monitoring Committee (IDSMC) throughout the study.
Randomisation
Treatments were centrally allocated through the PET-NECK trial office at the Warwick Clinical Trials Unit. Allocation was performed using a minimisation algorithm balancing by the following: centre, timing of planned ND (before or after CRT), chemotherapy schedule (concomitant platinum, concomitant cetuximab, neoadjuvant and concomitant platinum, neoadjuvant TPF with concomitant platinum, other approved), disease site (oropharyngeal, laryngeal, oral, hypopharyngeal or occult), T stage (T1–T2 vs. T3–T4) and N stage (N2a–N2b vs. N2c–N3). At randomisation, the eligibility of the patient was checked and the trial number and treatment arm were allocated. Each patient was asked to complete a quality-of-life questionnaire (before being informed of the randomisation allocation).
Allocation concealment
When the randomisation service was telephoned, participant details were taken and registration into the trial followed. The allocation was then generated, ensuring concealment.
Blinding
It was not possible to blind either the doctor or the patient to the allocated treatment.
Statistical methods
Participants were analysed according to the treatment group to which they were randomised. Analyses were guided by an analysis plan prepared before data were available.
A preplanned early stopping guideline was applied, assuming that at least 2 years’ follow-up would be available for each patient, and that 140 deaths (25%) would be expected. Three analyses of the primary outcome were prespecified, equally spaced at 47, 94 and 140 deaths, to be viewed only by the IDSMC and trial statistician. At each of these points, it could be concluded that the experimental treatment is equivalent (non-inferior). The 5% one-sided type I error rate for testing non-inferiority was controlled by an O’Brien–Fleming-like alpha spending rule set at p-values of 0.004, 0.007 and 0.047. It could alternatively be concluded that the experimental arm is inferior. A similar 10% type I rate for testing non-equivalence was used, with p-values set at 0.02, 0.032 and 0.084. At the first interim analysis (48 deaths), when 84% of patients had been randomised, the p-value for non-inferiority was 0.0025, which was below the (0.004) threshold for consideration of stopping. The IDSMC considered that data were immature and that the trial should continue. At the second interim analysis (102 deaths) the p-value for non-inferiority was 0.008, that is, above the threshold for the second analysis, and the trial continued to the final analysis.
Demographic and clinical characteristics and baseline measurements are presented to evaluate the comparability of intervention groups and generalisability to clinical settings. A Consolidated Standards of Reporting Trials (CONSORT) flow diagram was produced. 56
The primary end point, OS, was measured for every patient from the date of randomisation to the date of death. Survival times for patients on follow-up and patients lost to follow-up were censored at the latest date at which they were known to be alive. Kaplan–Meier survival curves were plotted. To test non-inferiority of the surveillance arm the HR was estimated using a Cox’s proportional hazards model stratified by intended timing of planned ND (before or after CRT), with the trial treatment arm as the only covariate. Non-inferiority is demonstrated by the 95% quantile of the estimated HR of the surveillance arm being less than the inferiority limit (HR 1.50). Proportionality of hazards was checked using a time-dependent treatment effect. A secondary analysis adjusting for N stage, T stage, tumour site, chemotherapy schedule and timing, sex and centre was performed. Kaplan–Meier OS was also calculated for the planned timing of ND (before or after CRT) subgroups and also for p16-positive and -negative tumours. The treatment effect on OS was also presented for subgroups of site of tumour, T stage, planned chemotherapy schedule and p16 status, using HR plots with interaction statistics using methods described by the Early Breast Cancer Trialists’ Collaborative Group. 57
Causes of death were classified as H&N cancer or other causes. Cumulative incidence rates by treatment arm were plotted for these competing risk groups and the difference between treatments was tested using Gray’s test. 58
Patients were deemed at risk of recurrence when they completed their CRT and time to recurrence was measured from that date. This meant that patients in the planned ND group who underwent surgery before CRT were not at risk until they had finished all their treatment. Counts of neck node recurrences by treatment were reported.
Quality-of-life scales at five time points were available for the EORTC QLQ-C30 version 3, EORTC QLQ-H&N35 H&N-specific quality-of-life questionnaire and the MDADI. The recommended scoring methods were applied59 (see also Fayers60 and Chen et al. 61). The differences between treatments in mean changes from baseline scores were calculated and compared for each of the assessment points separately using the Wilcoxon signed-rank test. The proportion of patients whose scores changed by at least 10% was also reported.
Complications of ND surgery were reported as counts and as proportion of NDs with Agresti–Coull binomial CIs.
Positron emission tomography–computerised tomography accuracy was difficult to assess, as it is difficult to define a false positive. Therefore, it was not possible to measure PET–CT accuracy in a meaningful way.
Concordance of local PET–CT scan assessments with that of the trial review radiologist were reported.
Statistical analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
Database and data processing
The database was held on the Warwick Clinical Trials Unit’s Microsoft Structured Query Language server system (Microsoft Corporation, Redmond, WA, USA) and imposed rules for data entry that included valid range for responses, linked dates and patient identification numbers.
Data were single entered into the database by study personnel. The trial statistician carried out checks of plausibility of values, missing data and form return rates to enable further queries to be resolved prior to freezing data for scheduled IDSMC reports and analysis. A 100% check on the details in death reports was applied.
Chapter 3 Results
Screening and recruitment
Recruitment
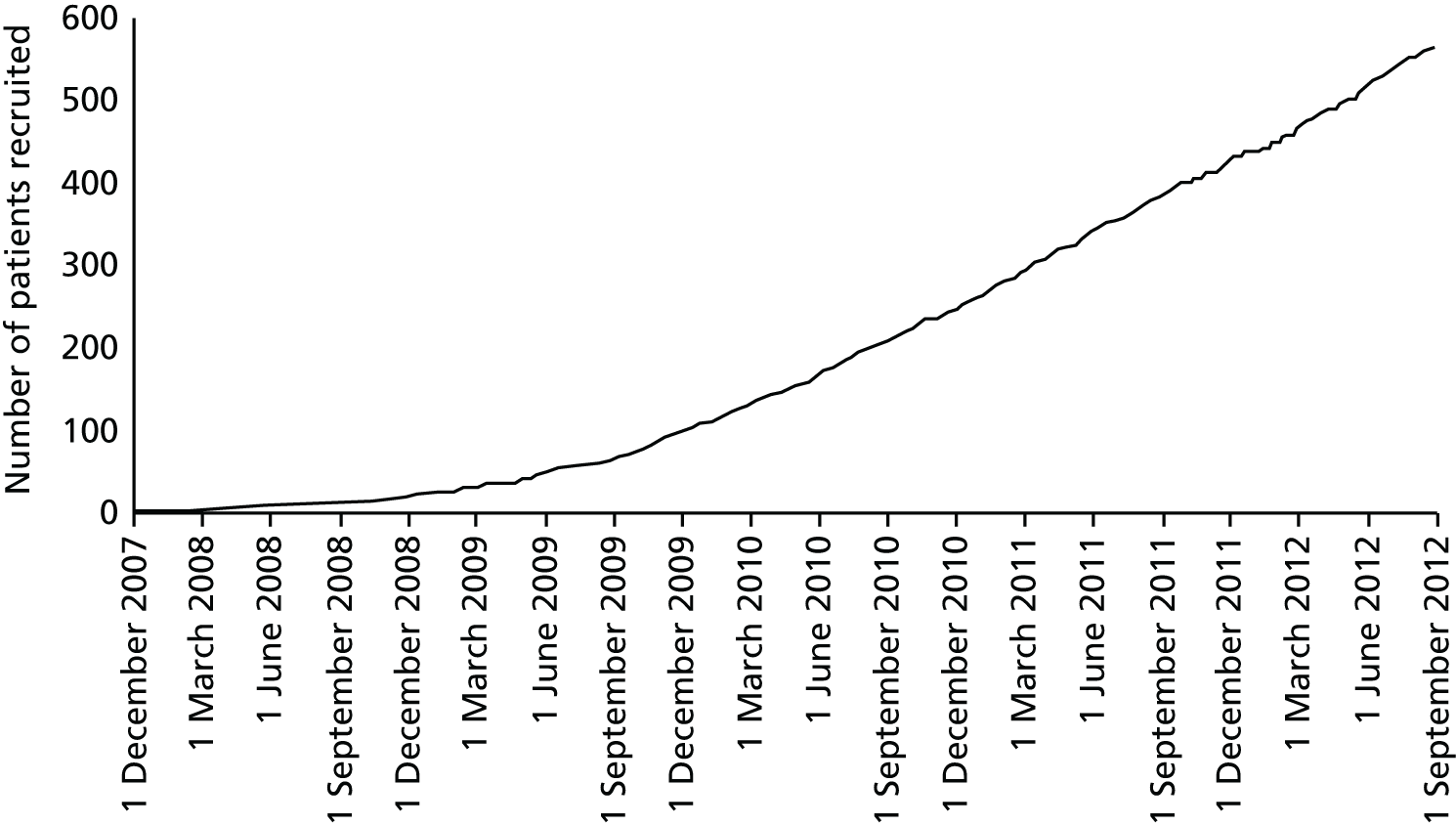
A total of 564 patients were recruited between 2 October 2007 and 23 August 2012: 282 to each trial arm (Figure 2).
FIGURE 2.
Recruitment.
Two patients in the surveillance arm were subsequently found to be ineligible: one patient’s disease was found to be T1N1 and another patient had metastatic disease at the time of randomisation. Follow-up data were collected and the patients are included in analyses.
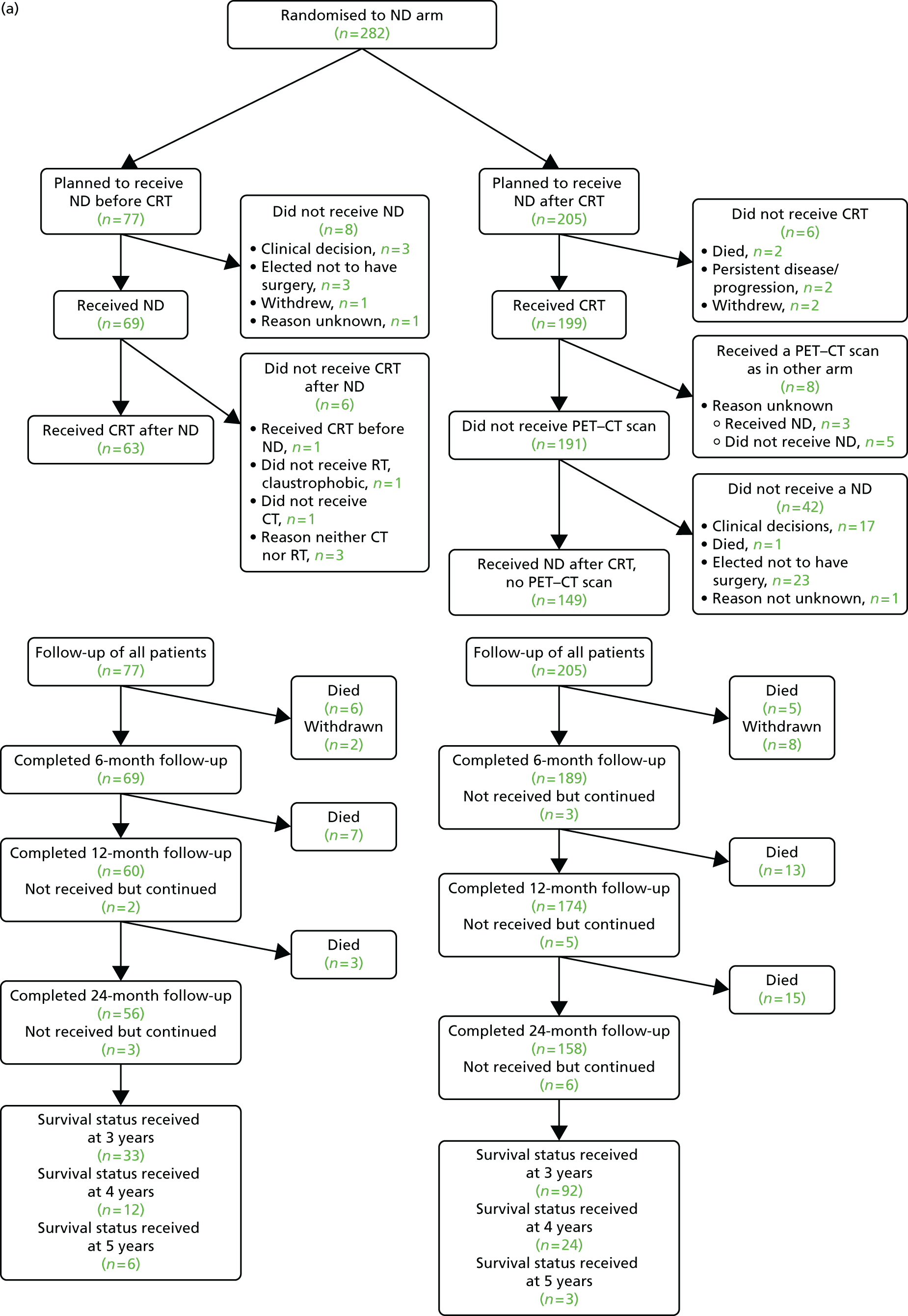
The participant flow diagram outlines the passage of patients through the study; more detail is given in later sections (Figure 3).
FIGURE 3.
Participant flow diagram. (a) ND arm; and (b) surveillance arm. Adapted from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62

Screening
All patients newly diagnosed with HNSCC were considered potentially eligible and had to be screened by the MDT prior to their clinic appointment.
Both screened patients and those approached for study participation had to be recorded anonymously on the screening log. The log was updated monthly and passed to the co-ordinating centre.
If a screened patient was not eligible for the trial, an anonymous record of the case was recorded on the screening log. Recorded details included the name of the centre, the patient’s initials and the reason for non-randomisation, if not randomised. Patients randomised to the trial were also recorded.
In total, 1792 patients were screened. The number of patients screened by centre is given in Table 3.
| Site | Number of patients screened |
|---|---|
| Aberdeen Royal Infirmary | 7 |
| Barnet and Chase Farm Hospitals | 2 |
| Beatson West of Scotland Cancer Centre | 31 |
| Belfast City Hospital | 33 |
| Blackpool Victoria Hospital | 29 |
| Birmingham Heartlands Hospital | 70 |
| Bradford Royal Infirmary | 23 |
| Bristol Haematology and Oncology Centre | 40 |
| Castle Hill Hospital | 22 |
| Cheltenham General Hospital | 5 |
| Christie Hospital | 25 |
| Derriford Hospital | 12 |
| Guy’s and St Thomas’ Hospitals | 41 |
| James Cook University Hospital | 10 |
| Kent and Canterbury Hospital | 77 |
| Mount Vernon Hospital | 17 |
| New Cross Hospital | 11 |
| North Manchester General Hospital | 12 |
| Nottingham University Hospital | 17 |
| Poole Hospital | 10 |
| Queen Alexandra Hospital | 5 |
| Queen Elizabeth Hospital Birmingham | 77 |
| Royal Blackburn Hospital | 126 |
| Royal Derby Hospital | 59 |
| Royal Devon and Exeter Hospital | 35 |
| Royal Marsden Hospital | 5 |
| Royal Preston Hospital | 21 |
| Royal Surrey County Hospital | 42 |
| Royal United Hospital | 44 |
| Russells Hall Hospital | 11 |
| Singleton Hospital | 101 |
| Sir Bobby Robson Cancer Trials Research Centre (Freeman Hospital) | 32 |
| Southend University Hospital | 4 |
| Sunderland Royal Hospital | 153 |
| Torbay Hospital | 11 |
| UHCW | 143 |
| University College London Hospital | 32 |
| University Hospital Aintree | 131 |
| Velindre Cancer Centre | 26 |
| Walsall Manor Hospital | 12 |
| Western General Hospital | 39 |
| Weston Park Hospital | 188 |
| William Harvey Hospital | 1 |
Of the 1792 patients screened, 564 were randomised, 1032 did not fulfil the eligibility criteria, and 196 patients refused consent. Table 4 shows reasons for patients not meeting the standard eligibility criteria and Table 5 shows reasons for unwillingness of eligible patients to enter the study.
| Ineligibility criteria | Number of patients in category |
|---|---|
| Patient did not have the required histological diagnosis | 187 |
| Patient did not have N2 or N3 disease | 223 |
| Patient had distant metastases | 57 |
| Patient had previous treatment for HNSCC | 59 |
| Previous cancer in past 5 years | 4 |
| Patient would not receive protocol-driven CRT regimen | 26 |
| Patient was unfit for surgery and/or CRT | 115 |
| Patient required resection of tumour | 151 |
| Inoperable nodes | 5 |
| Patient required palliative treatment | 69 |
| For radiotherapy only | 3 |
| Clinical decision not to enter patient in the trial | 15 |
| Other treatment planned | 41 |
| Other reasons not specified above | 47 |
| Reason for ineligibility not specified | 30 |
| Total | 1032 |
| Reason | Number of patients in category |
|---|---|
| Patient wanted ND or standard treatment | 47 |
| Patient did not want surgery | 36 |
| Patient wanted more control over the type or place of treatment | 16 |
| Patient had anxieties about the large amount of information and making decisions on randomisation and treatment | 12 |
| Patient did not want to take part in clinical study | 11 |
| Total | 122 |
The majority of patients who were ineligible either had the wrong type or severity of disease or a different type of treatment was indicated. There were 47 patients who were not approached about the study for reasons such as cognitive impairment, psychiatric issues or short-term memory problems that were likely to affect compliance. Other examples include language barriers, too much information to give to a patient as a result of having to tell them about their disease at the same time as giving study information, lack of clinicians available to discuss trial/consent patients, patient travelling elsewhere for treatment, a patient refusing to be treated or a patient being entered into another incompatible study. For 30 patients, the reason for ineligibility was not known.
Of the 196 patients who refused to participate, 74 did not give a reason for non-participation. The reasons for non-participation of the other 122 patients are given below.
One patient was anxious about being randomised in the trial, 11 patients were not interested in participating in a clinical trial, two patients did not want to travel for PET–CT if randomised to the surveillance arm of the trial, one patient felt that more imaging would be too much, four patients were overwhelmed with the amount of information given (trial information as well as diagnosis and treatment information), seven patients were too distressed after receiving diagnosis information to make a decision about the trial, one patient requested treatment at another clinic, one patient wanted to remain private, one patient wanted to retain control over his/her treatment and 93 patients had specific treatment preferences. Of these, 42 patients preferred ND and 36 patients did not want ND. There were two patients whose treatment preference was not specified. One patient wanted radiotherapy only, one wanted photodynamic therapy, four wanted CRT only, one wanted CRT followed by PET and one patient wanted to proceed immediately to adjuvant chemotherapy. Finally, five patients wanted standard CRT with ND, before or after CRT.
Baseline characteristics
Baseline characteristics of participants by trial arm
Treatment allocation by minimisation was balanced by the six characteristics in Table 6. A total of 38 centres took part, randomising mainly oropharyngeal patients (84.4%). Neoadjuvant chemotherapy was intended in 35.8% of patients.
| Baseline characteristic | Trial arm, n (%) | |
|---|---|---|
| Surveillance | ND | |
| Centre | ||
| Arden | 30 (10.6) | 30 (10.6) |
| Sheffield and Chesterfield | 25 (8.9) | 25 (8.9) |
| Birmingham | 23 (8.2) | 24 (8.5) |
| Bristol | 19 (6.7) | 19 (6.7) |
| Edinburgh | 17 (6.0) | 19 (6.7) |
| Lancashire | 14 (5.0) | 14 (5.0) |
| Glasgow | 13 (4.6) | 12 (4.3) |
| Royal Surrey | 12 (4.3) | 13 (4.6) |
| East Peninsula | 11 (3.9) | 11 (3.9) |
| Guy’s and St Thomas’ | 11 (3.9) | 11 (3.9) |
| Newcastle | 11 (3.9) | 11 (3.9) |
| Black Country | 10 (3.5) | 9 (3.2) |
| Manchester | 11 (3.9) | 8 (2.8) |
| Cardiff | 9 (3.2) | 9 (3.2) |
| Swansea | 8 (2.8) | 9 (3.2) |
| East and North Herts | 6 (2.1) | 7 (2.5) |
| Bath | 5 (1.8) | 7 (2.5) |
| West Peninsula | 6 (2.1) | 5 (1.8) |
| Nottingham | 5 (1.8) | 5 (1.8) |
| Sunderland | 4 (1.4) | 6 (2.1) |
| Derby | 5 (1.8) | 3 (1.1) |
| East Kent | 5 (1.8) | 3 (1.1) |
| Liverpool | 3 (1.1) | 4 (1.4) |
| East Lancashire | 3 (1.1) | 2 (0.7) |
| Gloucestershire | 3 (1.1) | 2 (0.7) |
| South Tees | 3 (1.1) | 2 (0.7) |
| Hull & East Yorkshire | 1 (0.4) | 3 (1.1) |
| Portsmouth | 2 (0.7) | 2 (0.7) |
| Dorset | 1 (0.4) | 2 (0.7) |
| Essex | 3 (1.1) | – |
| Barnet | 1 (0.4) | 1 (0.4) |
| Aberdeen | 2 (0.7) | – |
| Bradford | – | 1 (0.4) |
| Belfast | – | 1 (0.4) |
| Royal Marsden | – | 1 (0.4) |
| University College London Hospital | – | 1 (0.4) |
| ND policy before or after CRT | ||
| ND before CRT | 76 (27.0) | 77 (27.3) |
| ND after CRT | 206 (73.1) | 205 (72.7) |
| Approved chemotherapy schedules | ||
| Concomitant platinum | 163 (57.8) | 169 (59.9) |
| Concomitant cetuximab | 14 (5.0) | 14 (5.0) |
| Neoadjuvant and concomitant platinum | 10 (3.6) | 9 (3.2) |
| Neoadjuvant TPF with concomitant platinum | 89 (31.6) | 85 (30.1) |
| Other agreed schedules | 6 (2.1) | 5 (1.8) |
| Tumour site | ||
| Oral | 4 (1.4) | 7 (2.5) |
| Oropharyngeal | 240 (85.1) | 236 (83.7) |
| Laryngeal | 18 (6.4) | 19 (6.7) |
| Hypopharyngeal | 15 (5.3) | 14 (5.0) |
| Occult H&N | 5 (1.8) | 6 (2.1) |
| T stage | ||
| T1/T2 | 162 (57.4) | 160 (56.7) |
| T3/T4 | 116 (41.1) | 116 (41.1) |
| Occult H&N | 4 (1.4) | 6 (2.1) |
| N stage | ||
| N2a/N2b | 221 (78.4) | 222 (78.7) |
| N2c/N3 | 61 (21.6) | 60 (21.3) |
Other baseline characteristics of participants by trial arm
The patient characteristics in Table 7 were collected either at randomisation or via baseline information forms. The mean age was 57.9 years and 83.3% were male. Three-quarters of participants were either current or past smokers and, of those tested for p16 status, 335 out of 446 (75%) were positive.
| Baseline characteristic | Trial arm | |
|---|---|---|
| Surveillance | ND | |
| Age (years) | ||
| n | 282 | 282 |
| Mean (SD) | 57.6 (7.5) | 58.2 (8.1) |
| Median (IQR) | 57 (53–63) | 58 (53–63) |
| Minimum, maximum | 37, 79 | 38, 83 |
| Sex, n (%) | ||
| n | 282 | 282 |
| Male | 223 (79.1) | 237 (84.0) |
| Female | 59 (20.9) | 45 (16.0) |
| Primary site, n (%) | ||
| n | 277 | 277 |
| Tonsil | 138 (49.1) | 134 (48.4) |
| Base of tongue | 82 (29.6) | 83 (30.0) |
| Floor of mouth | 1 (0.4) | – |
| Palate | 4 (1.4) | 3 (1.1) |
| Tongue | 1 (1.1) | 2 (0.4) |
| Supraglottis | 15 (5.4) | 17 (6.1) |
| Glottis/subglottis/transglottis | 2 (0.7) | 1 (0.7) |
| Pyriform fossa | 14 (5.1) | 12 (4.3) |
| Posterior pharyngeal wall | 3 (1.1) | 6 (2.2) |
| More than one site | 17 (6.1) | 19 (6.9) |
| Tonsil, base of tongue | 9 | 9 |
| Tonsil, base of tongue, pyriform fossa | 1 | – |
| Tonsil, base of tongue, palate | 1 | – |
| Tonsil, base of tongue, post-pharyngeal wall | – | 1 |
| Tonsil, palate, tongue | – | 1 |
| Tonsil, palate | 1 | 2 |
| Tonsil, tongue | – | 1 |
| Tonsil, pyriform fossa | – | 1 |
| Tonsil, supraglottis, pyriform fossa | – | 1 |
| Tonsil, posterior pharyngeal wall | 1 | – |
| Tonsil, supraglottis | 1 | – |
| Base of tongue, supraglottis | – | 2 |
| Base of tongue, floor of mouth | 1 | 1 |
| Base of tongue, posterior pharyngeal wall | 1 | – |
| Supraglottis, posterior pharyngeal wall | 1 | – |
| T stage, n (%) | ||
| n | 282 | 282 |
| T1 | 48 (17.0) | 52 (18.4) |
| T2 | 114 (40.4) | 108 (38.3) |
| T3 | 61 (21.6) | 52 (18.4) |
| T4 | 55 (19.5) | 64 (22.7) |
| Occult | 4 (1.4) | 6 (2.1) |
| N stage, n (%) | ||
| n | 282 | 282 |
| N2a | 54 (19.1) | 44 (15.6) |
| N2b | 167 (59.2) | 178 (63.1) |
| N2c | 52 (18.4) | 52 (18.4) |
| N3 | 9 (3.2) | 8 (2.8) |
| Side of primary, n (%) | ||
| n | 280 | 278 |
| Left | 120 (42.9) | 102 (36.7) |
| Right | 129 (46.1) | 142 (51.1) |
| Midline and/or left and right | 31 (11.1) | 34 (12.2) |
| Differentiation, n (%) | ||
| n | 237 | 235 |
| Well differentiated | 15 (6.3) | 10 (4.3) |
| Moderately differentiated | 101 (42.6) | 90 (38.3) |
| Poorly differentiated | 119 (50.2) | 134 (57.0) |
| Undifferentiated | 2 (0.8) | 1 (0.4) |
| Type of staging scan, n (%) | ||
| n | 282 | 281 |
| PET–CT | 27 (9.6) | 27 (9.6) |
| CT | 169 (59.9) | 173 (61.6) |
| MRI | 86 (30.5) | 81 (28.8) |
| ECOG performance status, n (%) | ||
| n | 281 | 281 |
| 0 | 220 (78.3) | 218 (77.6) |
| 1 | 60 (21.4) | 60 (21.4) |
| 2 | 1 (0.4) | 3 (1.1) |
| Smoking, n (%) | ||
| n | 281 | 281 |
| Current | 88 (31.3) | 76 (27.0) |
| Past | 119 (42.3) | 134 (47.7) |
| Never | 74 (26.3) | 71 (25.3) |
| Alcohol, n (%) | ||
| n | 280 | 279 |
| Current | 222 (79.3) | 234 (83.9) |
| Past | 34 (12.1) | 18 (6.5) |
| Never | 24 (8.6) | 27 (9.7) |
| Ethnic group, n (%) | ||
| n | 281 | 280 |
| White | 280 (99.6) | 278 (99.3) |
| Black or black British | 1 (0.4) | 2 (0.7) |
| p16 status, n (%) | ||
| n | 226 | 220 |
| p16 positive | 164 (72.6) | 171 (77.7) |
| p16 negative | 62 (27.4) | 49 (22.3) |
| p16 test not done or result not available | 56 | 62 |
Further details of the tumour and nodal stages of randomised patients are given in Tables 8 and 9.
| Stage | ND intended (n) | |||
|---|---|---|---|---|
| Before CRT | After CRT | |||
| Surveillance | ND | Surveillance | ND | |
| T1N2a | 3 | 4 | 12 | 11 |
| T1N2b | 5 | 12 | 24 | 21 |
| T1N2c | 1 | 2 | 3 | – |
| T1N3 | – | 1 | – | 1 |
| T2N2a | 14 | 4 | 12 | 11 |
| T2N2b | 21 | 24 | 48 | 50 |
| T2N2c | 2 | 4 | 12 | 12 |
| T2N3 | 1 | 1 | 4 | 2 |
| T3N2a | 2 | 1 | 7 | 5 |
| T3N2b | 11 | 5 | 24 | 25 |
| T3N2c | 3 | 5 | 11 | 9 |
| T3N3 | 1 | 1 | 2 | 1 |
| T4N2a | – | 4 | 2 | 4 |
| T4N2b | 8 | 4 | 25 | 32 |
| T4N2c | 3 | 2 | 17 | 17 |
| T4N3 | – | – | – | 1 |
| Occult N2a | – | – | 2 | – |
| Occult N2b | 1 | 3 | – | 2 |
| Occult N2c | – | – | – | 1 |
| Occult N3 | – | – | 1 | – |
| Stage | ND intended, n (%) | |||
|---|---|---|---|---|
| Before CRT | After CRT | |||
| Surveillance | ND | Surveillance | ND | |
| T stage | ||||
| T1 | 9 (11.8) | 19 (24.7) | 39 (18.9) | 33 (16.1) |
| T2 | 38 (50.0) | 33 (42.9) | 76 (36.9) | 75 (36.6) |
| T3 | 17 (22.4) | 12 (15.6) | 44 (21.4) | 40 (19.5) |
| T4 | 11 (14.5) | 10 (13.0) | 44 (21.4) | 54 (26.3) |
| Occult | 1 (1.3) | 3 (3.9) | 3 (1.5) | 3 (1.5) |
| N stage | ||||
| N2a | 19 (25.0) | 13 (16.9) | 35 (17.0) | 31 (15.1) |
| N2b | 46 (60.5) | 48 (62.3) | 121 (58.7) | 130 (63.4) |
| N2c | 9 (11.8) | 13 (16.9) | 43 (20.9) | 39 (19.0) |
| N3 | 2 (2.6) | 3 (3.9) | 7 (3.4) | 5 (2.4) |
Withdrawals
There were 13 withdrawals in the ND group and three in the surveillance group (Table 10).
| Reason | Trial arm (n) | |
|---|---|---|
| Surveillance | ND | |
| Ineligible | – | 1 |
| Patient did not want ND/wanted surveillance arm | – | 4 |
| Wished to consent to another clinical trial | – | 1 |
| Migrated to the USA for IMRT | – | 1 |
| Patient decision | 1 | 2 |
| Patient and clinician decision | 1 | – |
| Clinician decision | 1 | – |
| No reason given | – | 4 |
Protocol deviations
Categories and frequencies of protocol deviations are given in Table 11. A number of patients were found to have been randomised on the basis of incorrect information. The tumour site category (oral, oropharyngeal, laryngeal, hypopharyngeal or occult) was inconsistent with the more detailed subsites. These errors were later corrected. Chemotherapy schedules were incorrectly recorded because of confusion over the three schedules containing cisplatin. Correction of these errors did not adversely affect the balance of the treatment allocation (see Baseline characteristics). The reasons why some patients in the ND arm did not undergo a ND are better described in Neck dissections. Twenty patients were randomised after they had already started CRT because the centre misunderstood the correct trial procedure.
| Description | Trial arm (n) | Total number | |
|---|---|---|---|
| Surveillance | ND | ||
| Related to randomisation | |||
| Randomised on the basis of incorrect ND timing | – | 4 | 4 |
| Randomised on the basis of incorrect T stage | 1 | – | 1 |
| Randomised on the basis of incorrect chemotherapy schedule | 27 | 30 | 57 |
| Randomised on the basis of incorrect tumour site | 39 | 33 | 72 |
| Subtotal | 67 | 67 | 134 |
| Others related to either trial arm | |||
| Started CRT prior to randomisation (mostly one centre) | 11 | 9 | 20 |
| Timeline of response | – | 1 | 1 |
| Chest radiography not CT of chest | 1 | 1 | 2 |
| MRI standardised uptake value details | – | 1 | 1 |
| Different chemotherapy schedule to planned | 2 | – | 2 |
| Ineligible | 2 | 2 | 4 |
| Subtotal | 16 | 14 | 30 |
| Others related to surveillance arm only | |||
| No PET–CT scan done | 1 | – | 1 |
| Watch and wait on partial response | 1 | – | 1 |
| Timeline of PET–CT | 2 | – | 2 |
| Partial response in nodes led to biopsy taken after PET–CT | 2 | – | 2 |
| Subtotal | 6 | – | 6 |
| Others related to ND arm only | |||
| Randomised to ND arm but patient elected not to undergo ND | – | 25 | 25 |
| No surgery, clinician decision | – | 9 | 9 |
| ND surgery later than protocol timeline | – | 2 | 2 |
| Timing of surgery changed (before/after CRT) | – | 1 | 1 |
| Underwent PET–CT | – | 1 | 1 |
| Subtotal | – | 38 | 38 |
Chemotherapy
Chemotherapy schedules planned at randomisation
Planned chemotherapy schedules are counted separately for patients in whom ND was planned to take place before CRT (Table 12) and for those in whom ND was planned to take place after CRT (Tables 13 and 14). Neoadjuvant schedules were more frequent when the planned timing was ND after CRT. A slightly higher proportion of patients in the ND arm whose ND was planned to be before CRT had a planned concomitant platin schedule.
| Schedule | Trial arm, n (%) | |
|---|---|---|
| Surveillance | ND | |
| Concomitant cisplatin or carboplatin | 55 (72.4) | 63 (81.8) |
| Concomitant cetuximab | 5 (6.6) | 5 (6.5) |
| Neoadjuvant and concomitant platinum | 3 (3.9) | – |
| Neoadjuvant docetaxel, cisplatin and 5-fluorouracil and concomitant cisplatin | 12 (15.8) | 9 (11.7) |
| Other agreed (neoadjuvant carboplatin) | 1 (1.3) | – |
| Total | 76 | 77 |
| Schedule | Trial arm, n (%) | |
|---|---|---|
| Surveillance | ND | |
| Concomitant cisplatin or carboplatin | 108 (52.4) | 106 (51.7) |
| Concomitant cetuximab | 9 (4.4) | 9 (4.4) |
| Neoadjuvant and concomitant platinum | 7 (3.4) | 9 (4.4) |
| Neoadjuvant docetaxel, cisplatin and 5-fluorouracil and concomitant cisplatin | 77 (37.4) | 76 (37.1) |
| Other agreed (see Table 14) | 5 (2.4) | 5 (2.4) |
| Total | 206 | 205 |
| Chemotherapy received | Number of patients |
|---|---|
| Surveillance arm | |
| Neoadjuvant cisplatin and 5-fluorouracil, concomitant cisplatin | 2 |
| Cisplatin and 5-fluorouracil | 1 |
| TPF | 2 |
| ND arm | |
| Neoadjuvant docetaxel, cisplatin and 5-fluorouracil, concomitant cetuximab | 1 |
| Neoadjuvant cisplatin and 5-fluorouracil, concomitant cisplatin (2) | 2 |
| Induction cisplatin and 5-fluorouracil followed by concomitant cisplatin neoadjuvant and concomitant platinum | 1 |
| Neoadjuvant docetaxel and cisplatin and 5-fluorouracil, concomitant carboplatin | 1 |
Type of chemotherapy delivered
Chemotherapy details were received for 272 patients in the ND arm and 277 patients in the surveillance arm. Overall, 10 ND patients and five surveillance patients did not have chemotherapy for the reasons given in Table 15.
| Reason for no chemotherapy | Trial arm (n) | |
|---|---|---|
| Surveillance | ND | |
| Refused | 1 | – |
| Patient unwell | – | 2 |
| Death | 1 | 3 |
| Did not want ND | – | 2 |
| Wanted surgery | 1 | – |
| Progression/metastases | 1 | 1 |
| Went to the USA for treatment | – | 1 |
| Withdrew | 1 | – |
| Not known | – | 1 |
One patient in the ND arm, who was planned (at randomisation) to receive neoadjuvant and concomitant chemotherapy, received only concomitant chemotherapy. At the same time, two patients randomised to concomitant platinum received only neoadjuvant and concomitant therapy.
Individual drug details were available for 269 patients in the ND arm and 274 patients in the surveillance arm (Table 16). The type of chemotherapy delivered was very similar in the trial arms.
| Chemotherapy received | Trial arm (n) | |
|---|---|---|
| Surveillance | ND | |
| Concomitant cisplatin or carboplatin schedule planned | ||
| Concomitant (cisplatin or carboplatin) | 154 | 153 |
| Concomitant (cisplatin or carboplatin) and 5-fluorouracil | – | 1 |
| Concomitant (cisplatin or carboplatin) then changed to cetuximab | – | 3 |
| Concomitant cetuximab | 1 | 2 |
| TPF | 2 | – |
| Concomitant cetuximab schedule planned | ||
| Concomitant cetuximab | 12 | 13 |
| Neoadjuvant and concomitant platin schedule planned | ||
| Neoadjuvant (cisplatin or carboplatin) and 5-fluorouracil, concomitant (cisplatin or carboplatin) | 8 | 7 |
| Neoadjuvant (cisplatin or carboplatin) and 5-fluorouracil | 1 | 2 |
| Neoadjuvant (cisplatin or carboplatin) | 1 | – |
| TPF schedule planned | ||
| TPF | 71 | 61 |
| Neoadjuvant (cisplatin or carboplatin), 5-fluorouracil and docetaxel | 6 | 8 |
| Neoadjuvant (cisplatin or carboplatin), 5-fluorouracil and docetaxel, concomitant cetuximab | 8 | 6 |
| Neoadjuvant (cisplatin or carboplatin) | – | 1 |
| Neoadjuvant docetaxel and concomitant cisplatin or carboplatin | – | 1 |
| Neoadjuvant (cisplatin or carboplatin), 5-fluorouracil and cetuximab, concomitant cetuximab | 1 | – |
| Neoadjuvant (cisplatin or carboplatin), cetuximab, 5-fluorouracil, docetaxel | – | 1 |
| Neoadjuvant (cisplatin or carboplatin) and 5-fluorouracil, concomitant (cisplatin or carboplatin) | 3 | 4 |
| Concomitant (cisplatin or carboplatin) | – | 1 |
| Other approved schedules planned | ||
| Neoadjuvant (cisplatin or carboplatin) and 5-fluorouracil, concomitant (cisplatin or carboplatin) | 4 | 3 |
| TPF | – | 2 |
| Neoadjuvant (cisplatin or carboplatin), 5-fluorouracil and docetaxel, concomitant cetuximab | 1 | – |
| Neoadjuvant (cisplatin or carboplatin), 5-fluorouracil and docetaxel | 1 | – |
| Total | 274 | 269 |
Chemotherapy dose information
Table 17 gives median doses received as reported on the CRT chemotherapy forms.
| Schedule and drug | Trial arm | |||
|---|---|---|---|---|
| Surveillance | ND | |||
| n | Dose (mg), median (Q1, Q3) | n | Dose (mg), median (Q1, Q3) | |
| Concomitant platin | ||||
| Cisplatin | 136 | 340 (240, 420) | 140 | 337 (240, 400) |
| Carboplatin (excluding AUC)a | 19 | 780 (550, 1050) | 22 | 645 (440, 960) |
| Schedule 2: concomitant cetuximab | ||||
| Concomitant cetuximab | 11 | 3500 (2580, 4210) | 13 | 3260 (1400, 3870) |
| Neoadjuvant and concomitant platin | ||||
| Concomitant cisplatin | 7 | 300 (282, 320) | 6 | 335 (120, 360) |
| Neoadjuvant cisplatin | 8 | 236 (180, 266) | 8 | 284 (210, 400) |
| Neoadjuvant 5-fluorouracil | 8 | 7625 (4450, 12,450) | 8 | 10500 (7260, 14,820) |
| Schedule 4: neoadjuvant TPF and concomitant platin | ||||
| Concomitant cisplatin | 46 | 332 (183, 400) | 47 | 312 (200, 400) |
| Concomitant carboplatin (when not AUC) | 27 | 920 (740, 1310) | 18 | 972 (650, 1270) |
| Cetuximab | 9 | 2120 (1650, 2300) | 6 | 2250 (2000, 2650) |
| Neoadjuvant cisplatin | 84 | 373 (264, 450) | 74 | 400 (300, 450) |
| Neoadjuvant docetaxel | 81 | 390 (240, 440) | 71 | 420 (300, 450) |
| Neoadjuvant 5-fluorouracil | 86 | 14500 (6000, 18,000) | 75 | 14600 (5700, 18,000) |
Radiotherapy
There were 270 completed case report forms for patients randomised to ND and 278 for patients randomised to PET–CT surveillance. Intensity-modulated radiotherapy (IMRT) was administered in 159 out of 263 (60%) ND patients and 173 out of 269 (64%) surveillance patients. Reasons why patients did not receive radiotherapy are given in Table 18.
| Reason | Trial arm (n) | |
|---|---|---|
| Surveillance | ND | |
| Refused (claustrophobic) | – | 1 |
| Patient unwell | – | 1 |
| Death | 1 | 4 |
| Did not want ND | – | 2 |
| Progression/metastases | 2 | 2 |
| Went to the USA for IMRT | – | 1 |
| Withdrew | 1 | – |
| Not known | – | 1 |
There were few modifications to radiotherapy duration (Table 19). The duration of treatment in six ND patients and three surveillance patients was 5 or more days longer than planned.
| Reason | Trial arm (n) | |
|---|---|---|
| Surveillance | ND | |
| Skin toxicity and mucositis | 1 | – |
| Myelosuppression | – | 1 |
| Pneumonia | – | 1 |
| Perforated duodenal ulcer | 1 | – |
| Did not attend | 1 | – |
| Weight loss: required replan | – | 1 |
| Neutropenia and infection | – | 1 |
| Admitted to hospital | – | 1 |
| Constipation/poor nutritional intake | – | 1 |
The planned doses to primary in the two treatment arms were similar (Table 20).
| Radiotherapy dose fractionation | Trial arm, n (%) | |
|---|---|---|
| Surveillance | ND | |
| 68–70 Gy in 34 or 35 fractions | 95 (34.7) | 86 (32.2) |
| 60–66 Gy in 30 fractions | 144 (52.6) | 144 (53.9) |
| 55 Gy in 20 fractions | 28 (10.2) | 28 (10.5) |
| Other | 7 (2.6) | 9 (3.4) |
| Not known | 4 | 3 |
Median and interquartile range (IQR) planned doses to involved neck nodes were also the same (54.0 Gy, IQR 50.0–54.0 Gy).
Neck dissections
Neck dissection was performed in 221 patients in the ND arm and in 54 patients in the surveillance arm.
Patients in neck dissection arm who did not undergo a neck dissection
Of the 282 patients in the ND arm, 221 underwent a planned ND. In total, 25 refused to have surgery. A summary of reasons for the 61 who did not receive a planned ND is given in Table 21.
| Reason | ND planned (n) | |
|---|---|---|
| Before CRT | After CRT | |
| Clinical decision | – | 3 |
| Inoperable or unsuitable for surgery | 1 | 1 |
| Progression/metastases/residual disease | 1 | 8 |
| Patient unwell | 1 | 6 |
| Early death | - | 4 |
| Patient elected not to have ND | 2 | 23 |
| Went to the USA for IMRT | 1 | |
| Patient elected not to have ND after delay in finding operation list date | 1 | |
| Had PET–CT scan and CR | – | 2 |
| Patient wanted PET–CT arm | – | 3 |
| No information | 1 | 3 |
Summary of surveillance arm patients who had a neck dissection
According to the trial protocol, patients in the surveillance arm should undergo a ND if the PET–CT findings are positive or equivocal. Fifty-four patients underwent ND, with 48 (36 incomplete responders in nodes and 12 incomplete responders in primary and nodes) of them conforming to protocol in that PET–CT was positive or equivocal. Of the remaining six, two patients did not undergo PET–CT, in two patients PET–CT findings were equivocal or node positive in the central reviewer’s PET–CT read and in two cases the reason is unknown.
Summary of surveillance arm patients who did not have a neck dissection
There were 228 patients in the surveillance arm who had no ND (Table 22).
| Reason | n |
|---|---|
| PET–CT, CRT, CR in primary and nodes | 181 |
| PET–CT, CRT, CR in nodes but not in primary | 15 |
| PET–CT, CRT, incomplete response in primary and nodes | 7 |
| Did not receive CRT | 6 |
| CR in primary, not in nodes | 11 |
| Retroperitoneal nodes | 1 |
| Nodes reported as CR, but after PET–CT reviewed reassigned as involved | 1 |
| New lung primary | 1 |
| Distant metastases | 1 |
| Neck node judged by MDT to be reactive | 1 |
| Recurred 5 weeks after post-CRT assessment | 1 |
| Metastases of liver and bone found at assessment | 1 |
| Biopsy confirmed no malignancy | 1 |
| Faint activity in lymph node on PET–CT but deemed to be a CR | 1 |
| MDT deemed nodal response as clear, CR | 1 |
| Biopsy, found only residuum of previously involved node | 1 |
| No information about response in primary | 4 |
| Had imaging other than PET–CT | 2 |
| Missed appointment and died | 1 |
| Had progressive disease | 1 |
| No response information having died early | 2 |
Surgical details
Surgery forms were received for 275 patients who received ND surgery (Table 23).
| ND details | ND, n (%) | Trial arm, n (%) | ||
|---|---|---|---|---|
| Before CRT (N = 69) | After CRT (N = 152) | ND (N = 221) | Surveillance (N = 54) | |
| Laterality | ||||
| Unilateral left | 26 (37.7) | 62 (41.3) | 88 (40.2) | 23 (42.6) |
| Unilateral right | 36 (52.2) | 68 (45.3) | 104 (47.5) | 28 (51.9) |
| Bilateral | 7 (10.1) | 20 (13.3) | 27 (12.3) | 3 (5.6) |
| Not available | – | 2 | 2 | – |
| Type of ND | ||||
| Modified radical | 39 (56.5) | 57 (37.5) | 96 (43.4) | 19 (35.2) |
| Selective | 29 (42.0) | 87 (57.2) | 116 (52.5) | 30 (55.6) |
| Super selective | 1 (1.4) | 8 (5.3) | 9 (4.1) | 5 (9.3) |
| Type of incision | ||||
| J-shaped | 15 (22.7) | 39 (27.1) | 54 (25.7) | 14 (26.4) |
| Y-shaped (triradiate) | 13 (19.7) | 9 (6.3) | 22 (10.5) | 1 (1.9) |
| U-shaped (utility) | 11 (16.7) | 26 (18.1) | 37 (17.6) | 10 (18.9) |
| McFee | 3 (4.5) | 8 (5.6) | 11 (5.2) | 9 (17.0) |
| Other | 24 (36.4) | 62 (43.1) | 86 (41.0) | 19 (35.8) |
| Not available | 3 | 8 | 11 | 1 |
| Neck structures removed | ||||
| Internal jugular vein | 20 (29.0) | 24 (15.8) | 44 (19.9) | 14 (25.9) |
| Spinal accessory nerve | 12 (17.4) | 10 (6.6) | 22 (10.0) | 4 (7.4) |
| Sternocleidomastoid muscle | 20 (29.0) | 30 (19.7) | 50 (22.6) | 11 (20.4) |
| Common and internal carotid artery | 2 (2.9) | 2 (1.3) | 4 (1.8) | – |
| Skin | 3 (4.3) | 1 (0.7) | 4 (1.8) | 1 (1.9) |
| Number of patients with any of the above structures removed | 30 (43.5) | 42 (27.6) | 72 (32.6) | 17 (31.5) |
From the above, it is important to note that, overall, the number of participants who experienced nerve sacrifice (which is morbid) was considerably lower in the surveillance arm (4 out of 220) than in the ND arm (22 out of 220); similar findings were obtained for sacrifice of the internal jugular vein and sternocleidomastoid muscle.
If the numbers of structures removed are summed (counting potentially one for left and one for right), the mean number of structures removed per ND is 0.59 in the ND arm and 0.56 in the surveillance arm. The mean number of structures removed in the ND before group is 0.78, whereas in the ND after group it is 0.49. Combining the five structures given, fewer patients whose planned ND was after CRT had one or more of the structures removed (see Table 23, p = 0.02). On examination of these data, this does not correlate with more advanced disease.
Follow-up
Patients were due to be followed up by examination at the clinic at 6, 12 and 24 months after randomisation. Late requests for survival and recurrence information were made in the last year of the trial to obtain follow-up data for up to 5 years after randomisation.
Median follow-up for survival in the ND arm is 36.1 months (IQR 25.6–43.7 months) and for the surveillance arm is 35.6 months (IQR 29.2–42.5 months). The survival status at 24 months is unknown for 28 patients in the ND arm and 16 in the surveillance arm. Eleven patients in the ND arm and one in the surveillance arm have no follow-up, having, with one exception, withdrawn early. Table 24 gives information on the timing of follow-up visits.
| Trial follow-up time point | Trial arm | |||
|---|---|---|---|---|
| Surveillance | ND | |||
| Number of patients | Time (months) from randomisation to examination median (IQR) | Number of patients | Time (months) from randomisation to examination median (IQR) | |
| 6 months | 270 | 6.3 (5.9–7.1) | 258 | 6.3 (5.8–7.3) |
| 12 months | 257 | 12.2 (11.7–12.9) | 234 | 12.3 (11.8–13.1) |
| 24 months | 227 | 24.3 (23.7–25.3) | 210 | 24.3 (23.8–25.3) |
Serious adverse events
Summary
In the study, 282 SAEs were reported and are summarised in Table 25. There were 169 in the ND arm and 113 in the PET–CT arm; none was deemed unexpected by the chief investigator.
| SAE summary | Trial arm, n | |||
|---|---|---|---|---|
| ND | Surveillance | |||
| Planned | Total (N = 282) | Total (N = 282) | ||
| Before CRT (N = 77) | After CRT (N = 205) | |||
| Number of SAEs | 35 | 134 | 169a | 113a |
| Number of patients with at least one SAE | 25 | 87 | 112 | 89 |
| Percentage of patients experiencing SAEs | 32.5 | 42.4 | 39.7 | 31.6 |
Tables 26 and 27 show that the most common SAE was hospitalisation or prolongation of hospitalisation due to CRT, which was very high for ND post CRT. The rates of other SAEs are similar in the ND before and after CRT trial arms and in the surveillance ND arm.
| Primary reasons for reporting (> 1 allowed per patient) | Trial arm, n | |||
|---|---|---|---|---|
| ND | Surveillance | |||
| Planned | Total (N = 282) | Total (N = 282) | ||
| Before CRT (N = 77) | After CRT (N = 205) | |||
| Death | 2 | 1 | 3 | 3 |
| Life-threatening event | 2 | 13 | 15 | 11 |
| Inpatient hospitalisation or prolongation of existing hospitalisation | 29 | 129 | 158 | 104 |
| Persistent or significant disability/incapacity | 3 | 6 | 9 | 10 |
| Other medically significant reason for reporting | 2 | 4 | 6 | 5 |
| Causality | Trial arm, n | |||
|---|---|---|---|---|
| ND | Surveillance | |||
| Planned | Total | Total | ||
| Before CRT | After CRT | |||
| Surgery | 4 | 6 | 10 | 5 |
| CRT | 22 | 88 | 110 | 75 |
| CRT and surgery | – | 2 | 2 | – |
| CRT and other | – | 4 | 4 | 1 |
| Other | 9 | 34 | 43 | 32 |
Of the SAEs with hospitalisation as a result of to CRT in the ND after CRT group, 67 out of 71 patients (94.4%) had their SAE before surgery (Table 28).
| Outcomes | Trial arm, n | |||
|---|---|---|---|---|
| ND | Surveillance | |||
| Planned | Total | Total | ||
| Before CRT (N = 35 events) | After CRT (N = 134 events) | |||
| Resolved | 27 | 107 | 134 | 94 |
| Not yet resolved | 6 | 23 | 29 | 15 |
| Deaths | 2 | 4 | 6 | 4 |
The highest symptom grades of the events are given comparing trial arms in Table 29 and comparing ND before CRT with ND after CRT in Table 30. No difference is seen between groups in either case (p = 0.99 and p = 0.42 for trend).
| Highest symptom grade of event | Trial arm, number of SAEs (%) | |
|---|---|---|
| Surveillance (N = 89) | ND (N = 112) | |
| 5 | 1 (0.4) | 1 (0.4) |
| 4 | 8 (2.8) | 15 (5.3) |
| 3 | 64 (22.7) | 71 (25.2) |
| 2 | 10 (3.5) | 18 (6.4) |
| 1 | 6 (2.1) | 7 (2.5) |
| Highest symptom grade of event | ND, number of SAEs (%) | |
|---|---|---|
| Before CRT (N = 77) | After CRT (N = 205) | |
| 5 | – (–) | 1 (0.5) |
| 4 | 4 (5.2) | 11 (5.4) |
| 3 | 13 (16.9) | 58 (28.3) |
| 2 | 6 (7.8) | 12 (5.9) |
| 1 | 2 (2.6) | 5 (2.4) |
| 0, that is, no SAE | 52 (67.5) | 118 (57.6) |
Overall survival
Median follow-up for survival was 35.6 months (IQR 29.2–42.5 months) in the surveillance arm and 36.1 months (IQR 25.6–43.7 months) in the ND arm.
There have been 123 deaths reported in the trial: 62 in the ND arm and 61 in the surveillance arm. Of these, 92 occurred within 2 years of randomisation. One death in the surveillance arm occurred > 5 years after randomisation and is not included in the time-to-event analysis.
Overall survival is plotted by treatment arm (Figure 4). The 2-year OS rate for the trial was 83.2% (95% CI 80.1% to 86.4%). This was higher than that expected at the start of the trial (75%). Consequently, the retrospective power of the study with 122 deaths was 72%.
FIGURE 4.
Overall survival by treatment arm. Reproduced from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62
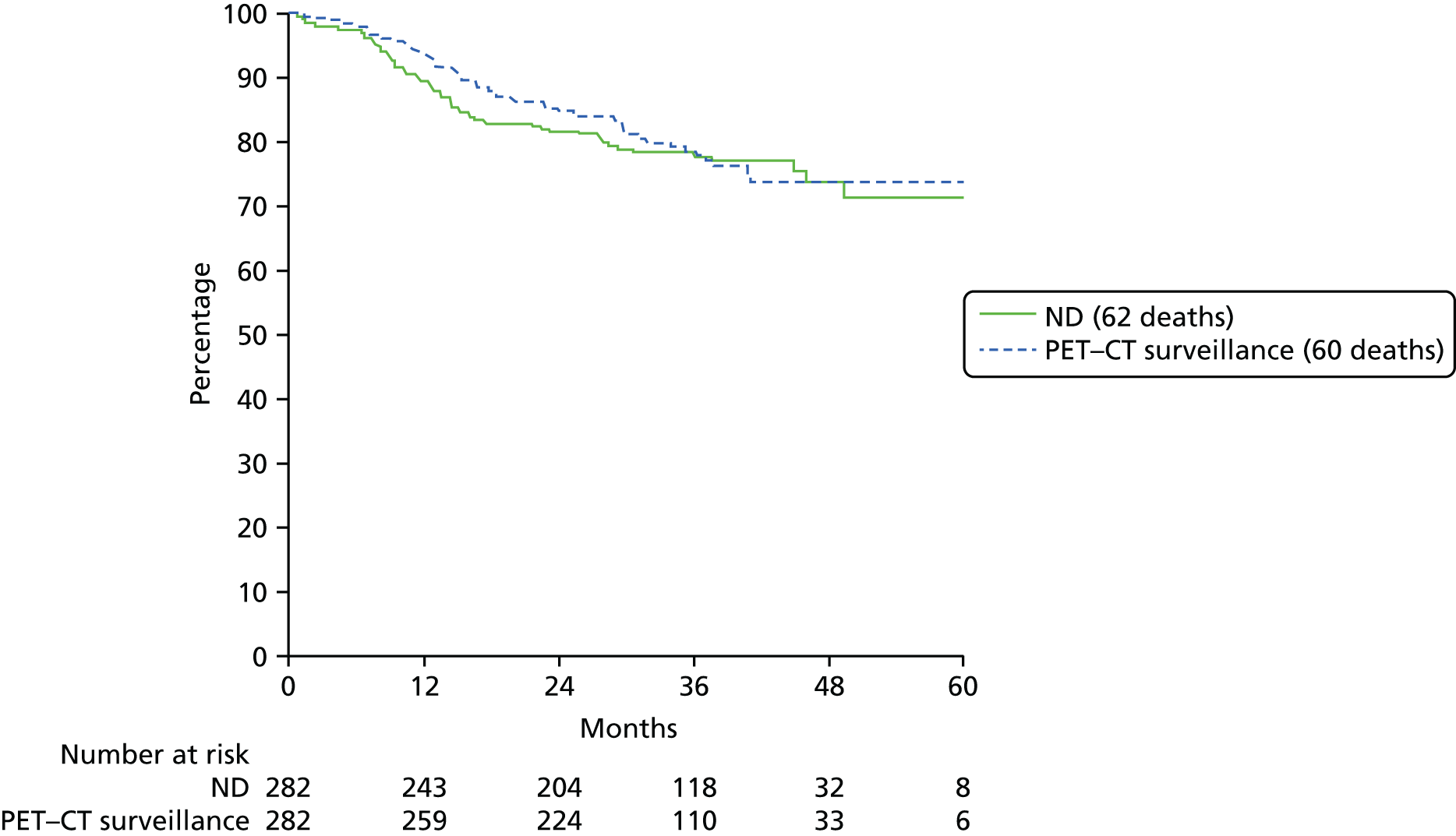
The 2-year OS in the ND arm was 81.5% (95% CI 76.9% to 86.2%) and in the PET–CT surveillance arm was 84.9% (95% CI 80.7% to 89.1%).
Primary outcome overall survival
The prespecified analysis takes account of timing of planned ND by stratification (planned ND before CRT and planned ND after CRT). These are plotted separately in Figures 5 and 6, respectively.
FIGURE 5.
Overall survival by treatment arm for planned ND before CRT. Reproduced from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62

FIGURE 6.
Overall survival by treatment arm for planned ND after CRT. Reproduced from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62

Analysing by proportional hazards model (Table 31) stratified by intended timing of ND before or after CRT, the HR is 0.924 (95% CI 0.648 to 1.318) in favour of the surveillance arm. The limit for non-inferiority in the trial design was set at a HR of 1.50, which lies at the 99.63th percentile of the estimated CI for the HR, which can be interpreted as a one-sided p-value of 0.0037, that is, indicating non-inferiority (HR < 1.5). The conventional two-sided p-value for difference between treatments is 0.6621.
| OS proportional hazards model | Number of events | Treatment HR estimate (95% CI) | HR 1.50 (%) |
|---|---|---|---|
| Stratified by ND pre/post (prespecified analysis of primary outcome) | 122 | 0.924 (0.648 to 1.318) | 99.63 |
Further analyses of overall survival
The early drop in the Kaplan–Meier curve of the group that had a planned ND before CRT suggests some non-proportionality of hazards between treatment groups. This is confirmed by testing an interaction between treatment and log-(time) (p-value for the time interaction is 0.03). No evidence of non-proportionality is seen in the planned ND after CRT stratum (p = 0.38). An alternative analysis to the Cox’s model is stratifying analysis into two risk periods, namely the first year and rest of the period at risk. The result of this additional unplanned analysis gives a very similar HR of 0.922 (instead of 0.924 in Table 31).
Adjusting for centre, T stage, N stage, site of primary, chemotherapy schedule, sex and age gives a very similar result, as does further adjustment for p16 status on a reduced sample (Table 32).
| OS proportional hazards model | Number of events | Treatment HR estimate (95% CI) | HR 1.50 (%) |
|---|---|---|---|
| Stratified by ND pre/post adjusted for centre, T stage, N stage, tumour site, chemotherapy schedule, sex and age | 122 | 0.835 (0.571 to 1.222) | 99.87 |
| Stratified by ND pre/post, adjusted as above plus p16 status (categorised as positive/negative/not known) | 122 | 0.759 (0.513 to 1.124) | 99.97 |
| ND before CRT subgroup | 31 | 0.664 (0.325 to 1.356) | 98.7 |
| ND after CRT subgroup | 91 | 1.033 (0.685 to 1.558) | 96.2 |
| p16-positive subgroup stratified by ND pre/post | 40 | 0.736 (0.393 to 1.378) | 98.7 |
| p16-negative subgroup stratified by ND pre/post | 58 | 0.982 (0.583 to 1.654) | 94.4 |
p16 status was highly prognostic, but there was no difference between p16-positive and p16-negative status in terms of the trial result, as shown in Table 32 and Figures 7 and 8.
FIGURE 7.
Overall survival: p16 positive.

FIGURE 8.
Overall survival: p16 negative.
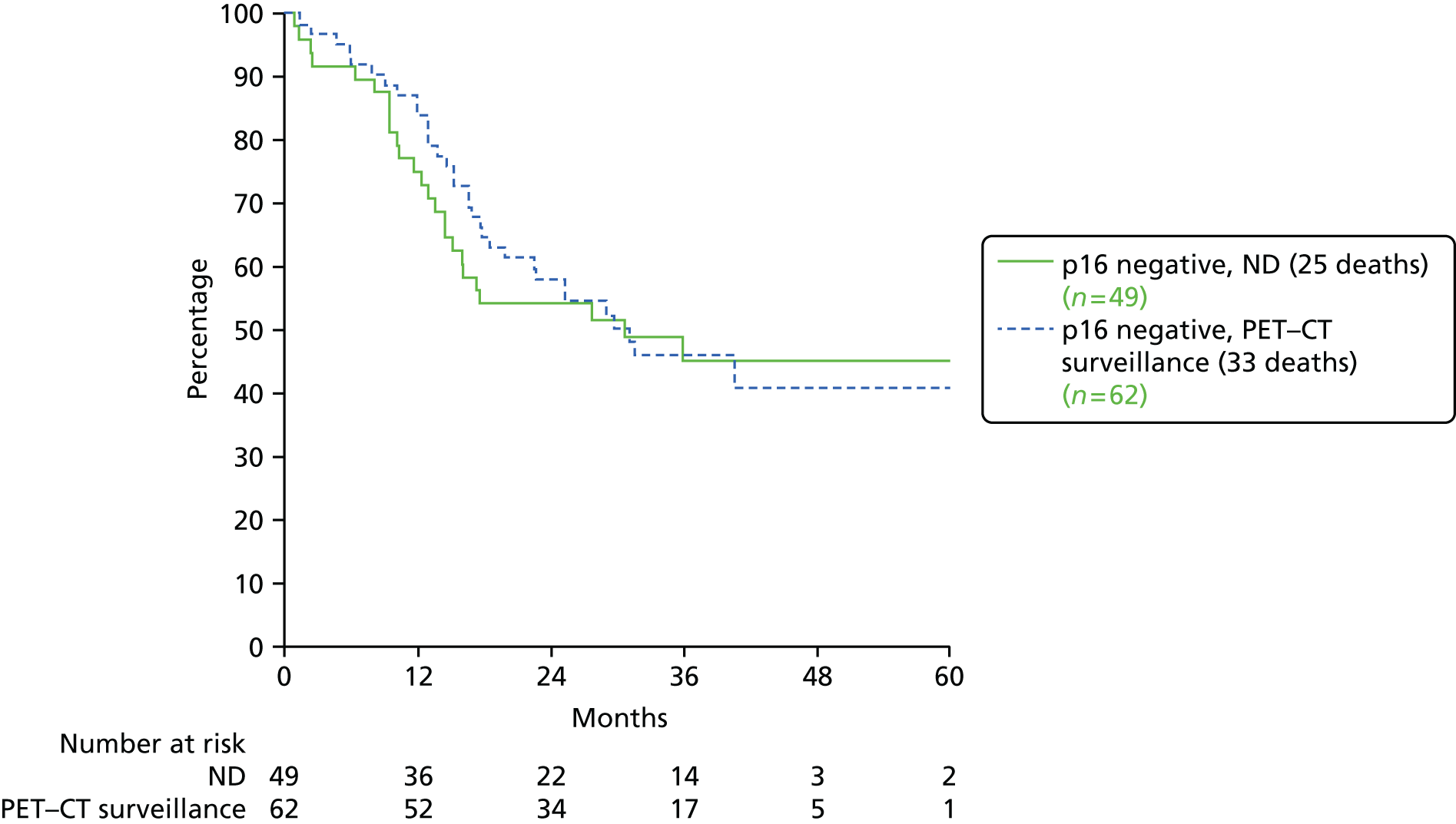
The HRs in Figure 9 are stratified by planned ND timing (before or after CRT). Some of the groups are small and differences between them should be interpreted cautiously. There is some evidence that the ND arm did better in the T stage 1/2 group than in the T3–T4 group. The small group of females in the trial did particularly well in the surveillance arm (treatment interaction p = 0.01), although with a total of only 18 deaths this is not a reliable result. There was no difference in the trial result by p16 status, which was highly prognostic. In unplanned analyses, the ND arm did worse in the first year at risk, and no difference was seen in the trial result by radiotherapy dose, IMRT versus conformal radiotherapy or high- versus low-recruiting centres.
FIGURE 9.
Overall survival of subgroups. Method as described in Early Breast Cancer Trialists’ Collaborative Group. Treatment of Early Breast Cancer. Volume 1. Worldwide Evidence 1985–1990. Oxford: Oxford University Press; 1990. 57 (a) Overall survival of subgroups 1; and (b) overall survival of subgroups 2. Adapted from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62
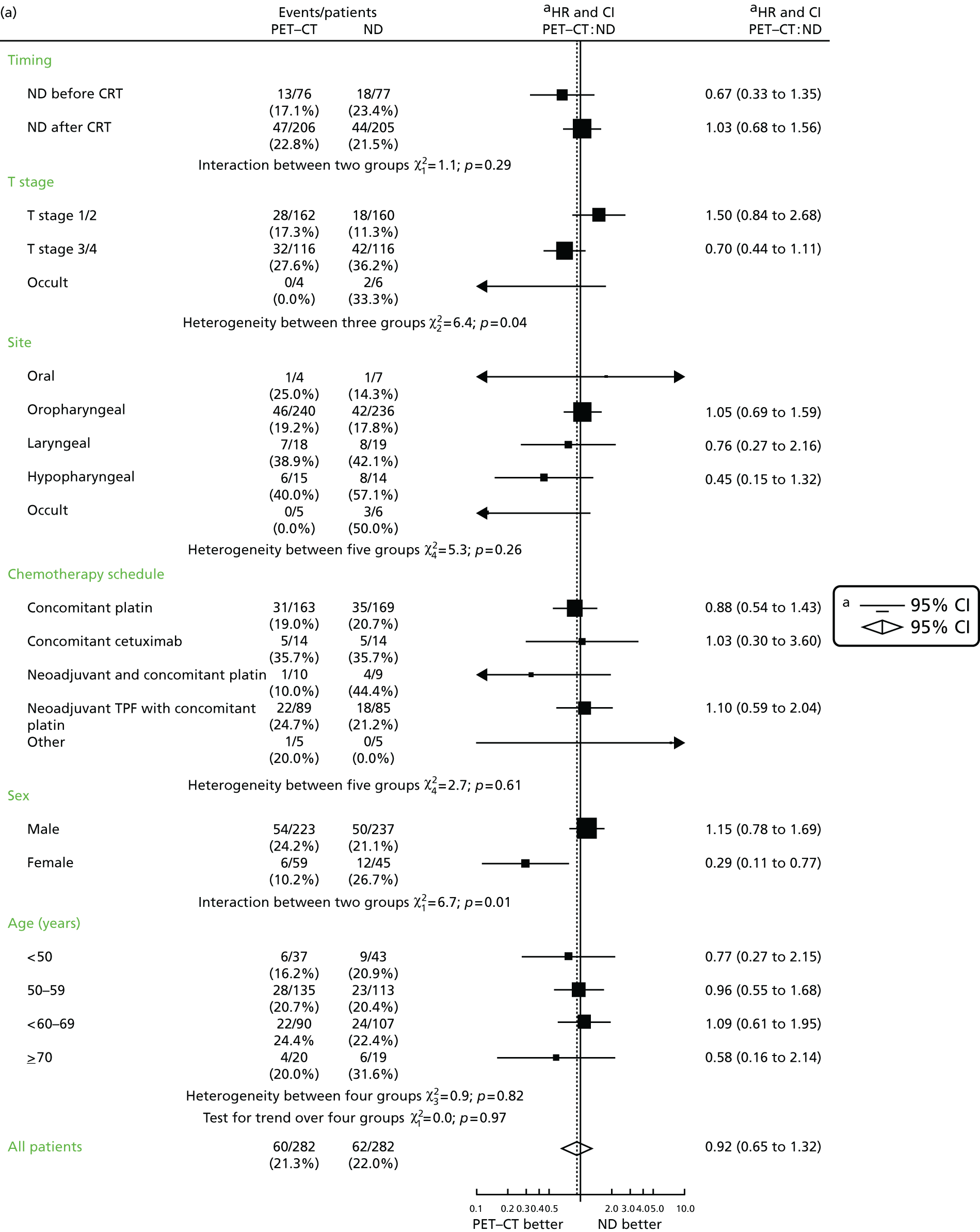
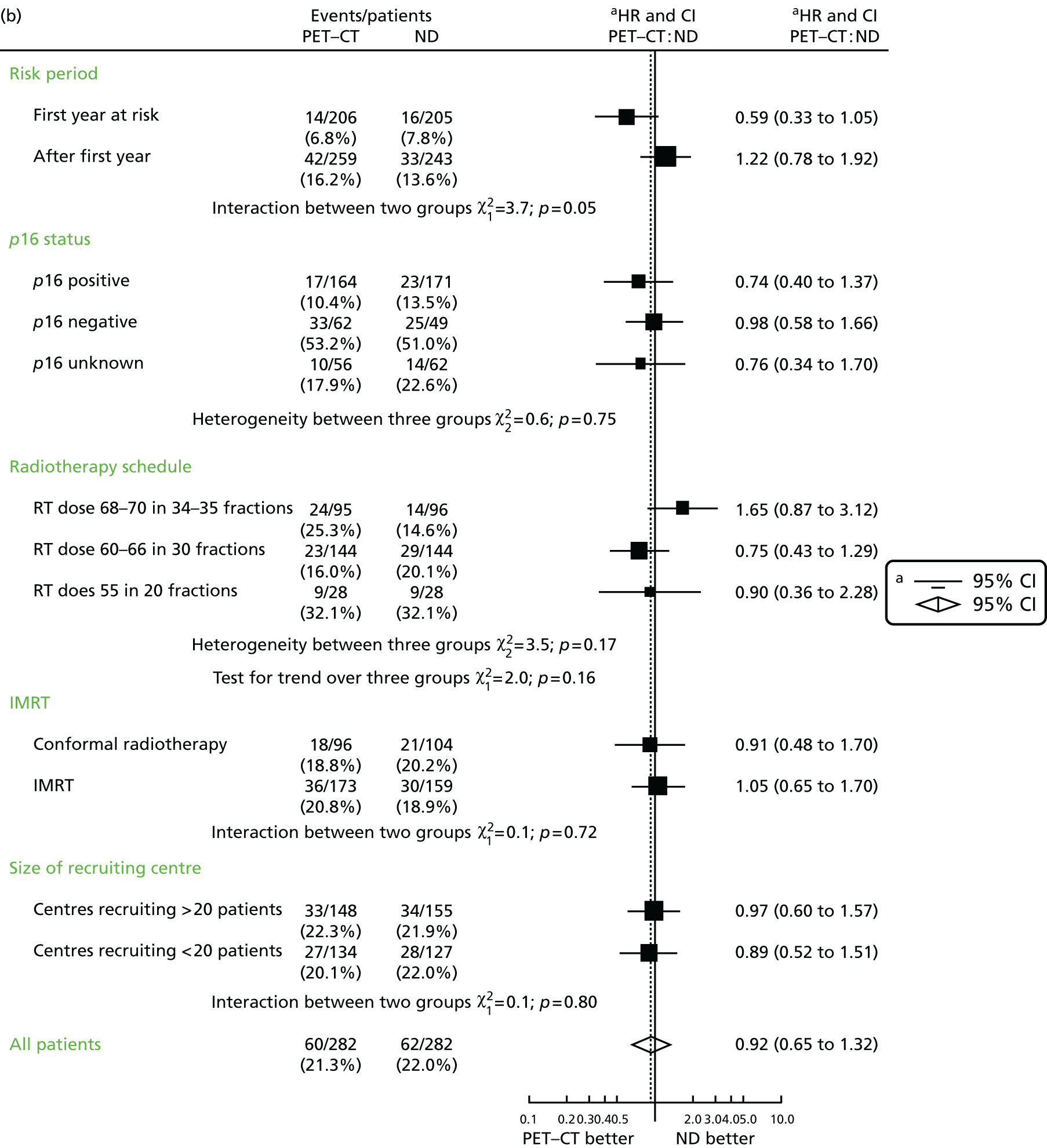
Mortality by cause
Deaths attributable to head and neck cancer
The causes of death are summarised in Table 33. These include complications attributable to surgery or CRT and persistent, recurrent or metastatic H&N cancer at death. There were 92 deaths due to H&N cancer and 29 deaths from other causes (detailed in Table 34).
| Cause of death | Randomised as ND policy (n) | Total (n) | ||||
|---|---|---|---|---|---|---|
| Before CRT | After CRT | |||||
| Surveillance | ND | Surveillance | ND | Surveillance | ND | |
| H&N cancer | 11 | 13 | 37 | 31 | 48 | 44 |
| Other causes | 3 | 5 | 10 | 11 | 13 | 16 |
| Not known | – | – | – | 2 | – | 2 |
| Total | 14 | 18 | 47 | 44 | 61 | 62 |
| Arm | Cause |
|---|---|
| PET–CT | |
| Before CRT stratum | Cardiac failure |
| Malignant plasma cell neoplasm: extramedullary plasmacytoma | |
| Pneumonia | |
| After CRT stratum | Road traffic accident |
| New primary non-small cell lung cancer | |
| Extensive bilateral consolidation secondary to severe infection | |
| Second primary lung | |
| Bronchopneumonia: squamous cell carcinoma oropharynx | |
| Severe depression | |
| Post-operative complications for second primary oesophagus | |
| Chest sepsis | |
| Unknown primary: probably lung, widespread metastases | |
| Renal cancer, cardiovascular and respiratory failure. Low anterior resection plus ileostomy | |
| ND | |
| Before CRT | Pneumonia |
| Aspiration pneumonia | |
| Glioblastoma | |
| Bilateral pneumonia | |
| Infection | |
| After CRT | Unknown |
| Patient on dialysis, died of renal failure | |
| Metastatic non-small cell lung cancer | |
| Left ventricular failure, myocardial infarction | |
| Second primary | |
| Sudden death at home, no recurrence of H&N cancer | |
| Neutropenic sepsis as a result of colitis | |
| Bronchopneumonia | |
| Cerebrovascular accident | |
| Coronary artery atherosclerosis | |
| Not known as patient moved away | |
| Aspiration pneumonia | |
Head and neck cancer mortality: cumulative incidence
There is little difference between the trial arms in the incidence of H&N cancer-related deaths (Figure 10).
FIGURE 10.
Mortality attributable to H&N cancer.

The 2-year cumulative incidence rates for H&N mortality are 13.66% (95% CI 9.88% to 18.04%) in the ND arm and 12.25% (95% CI 8.72% to 16.42%) in the surveillance arm. A Gray’s test for difference between cumulative incidence functions gives a p-value of 0.7992.
Other cause mortality: cumulative incidence
The difference in non-cancer deaths is also small (Figure 11).
FIGURE 11.
Mortality attributable to causes other than H&N cancer.
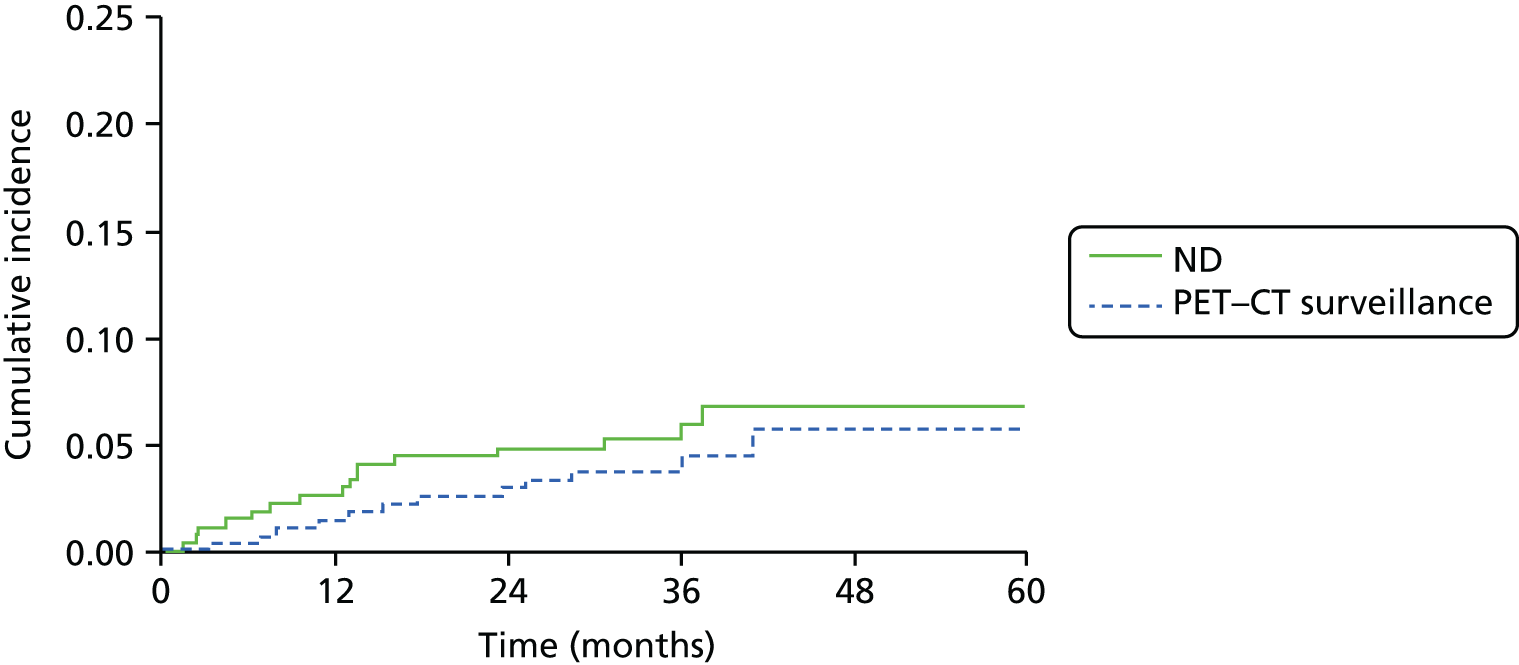
The 2-year cumulative incidence rates for other causes of mortality are 4.80% (95% CI 2.68% to 7.82%) in the ND arm and 2.88% (95% CI 1.36% to 5.37%) in the surveillance arm. A Gray’s test for difference between cumulative incidence functions gives a p-value of 0.4124.
Recurrences
Information on recurrences and persistent disease is obtained mainly from recurrence forms, and also via cause of death information. Recurrence is defined using the date of completion of radiotherapy. Disease that is apparent < 3 months after radiotherapy is defined as persistent disease. Disease that is apparent later than 3 months is defined as a recurrence. Frequencies of persistent disease and reported sites of recurrence are shown in Table 35.
| Recurrence/persistent disease | Trial arm, n | |
|---|---|---|
| Surveillance | ND | |
| Total reports of recurrence/persistent disease | 59 | 52 |
| Reports via recurrence forms | 54 | 46 |
| Reports via cause of death only | 5 | 6 |
| Persistent disease (< 3 months after radiotherapy completion) | 14 | 10 |
| Recurrence (> 3 months after radiotherapy completion) | 45 | 42 |
| Recurrence in primary | 21 | 16 |
| In primary only | 15 | 12 |
| Recurrence in neck nodes | 6 | 2 |
| In neck nodes only | 3 | 1 |
| Recurrence site distant | 21 | 23 |
| Distant only | 17 | 18 |
| Site unknown | 4 | 6 |
For Kaplan–Meier analysis of recurrence (Figure 12), the time of recurrence is taken as the time from completion of radiotherapy to reported recurrence or to cancer death if there is no reported recurrence. Consequently, the time at risk for some patients starts after the ND, while for others it is before ND and others still have no ND. The 2-year recurrence rates are 16.0% (95% CI 11.4% to 20.6%) in the ND arm and 14.4% (95% CI 10.1% to 18.7%) in the surveillance arm.
FIGURE 12.
Time to recurrence by treatment arm.
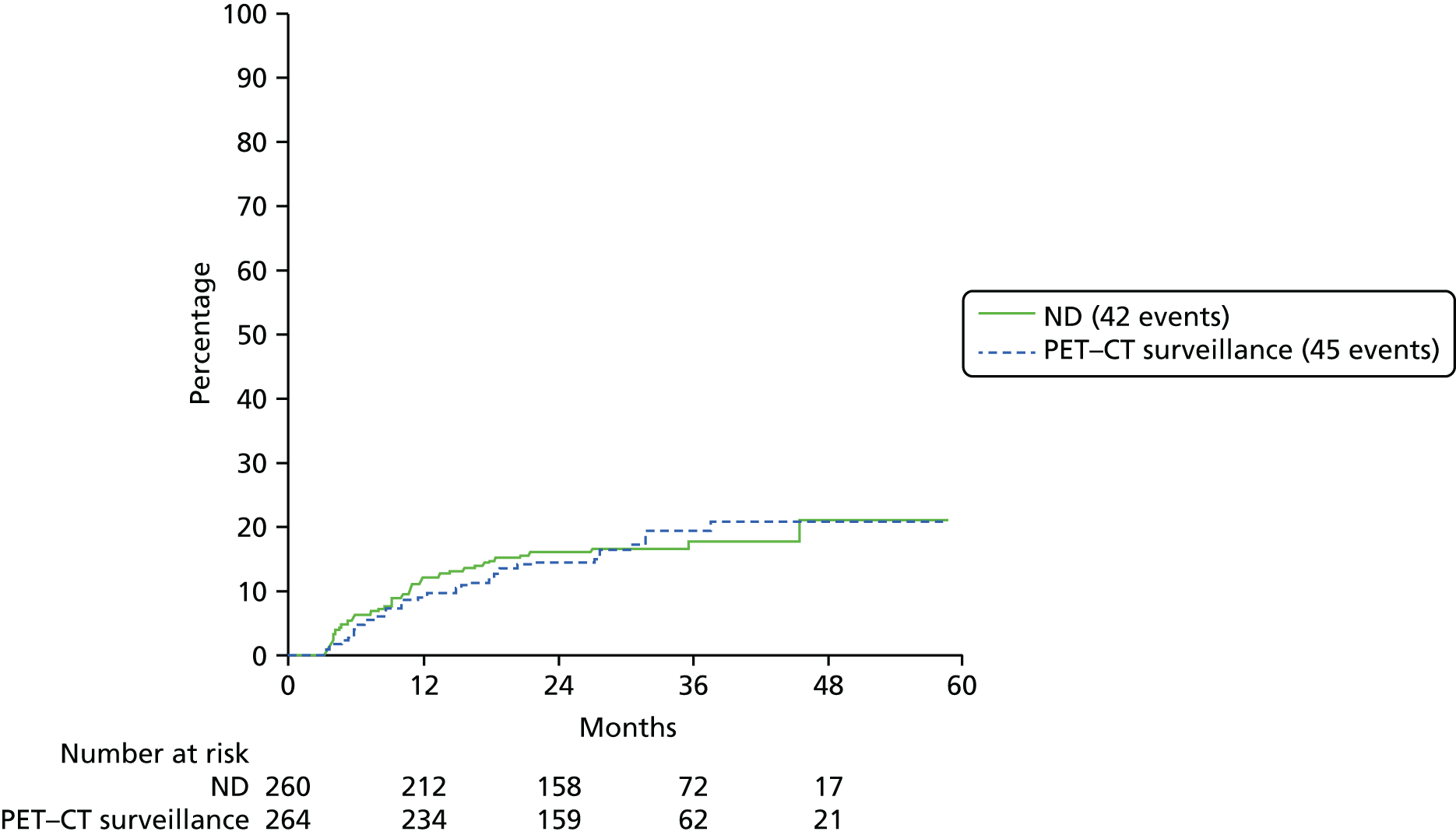
Four of the six surveillance patients with recurrence in neck nodes were complete responders on the post-CRT PET–CT scan.
Two of the three surveillance patients with recurrence in the neck had a CR only on post-CRT PET–CT.
Complications of neck dissection
Information on surgical complications was supplied for 220 of the 221 NDs in the ND arm and 53 of 54 in the surveillance arm. Table 36 separates the results by ND pre/post CRT planned at the time of randomisation. The overall complication rate per ND is 0.38 in the ND arm and 0.42 in the surveillance arm.
| Surgical complication | Trial arm, n (rate) [95% CI] | |||
|---|---|---|---|---|
| ND (N = 220) | Surveillance (N = 53) | |||
| Pre CRT (n = 69) | Post CRT (n = 151) | Pre CRT (n = 16) | Post CRT (n = 37) | |
| No complications | 50 (72.46%) [60.89% to 81.67%] | 118 (78.15%) [70.86% to 84.03%] | 10 (62.50%) [38.53% to 81.63%] | 24 (64.86%) [48.70% to 78.23%] |
| General | ||||
| Chest infection | 1 (1.45%) [0.00% to 8.52%] | 3 (1.99%) [0.42% to 5.94%] | – | – |
| Urinary tract infection | – | – | – | 2 (5.41%) [0.57% to 18.63%] |
| Septicaemia | – | 1 (0.66%) [0.00% to 4.03%] | – | – |
| Other general (see Table 37) | – | 2 (1.32%) [0.06% to 5.01%] | – | 1 (2.70%) [0.00% to 15.05%] |
| Local | ||||
| Breach of tumour | 1 (1.45%) [0.00% to 8.52%] | – | – | – |
| Intraoperative chyle leak | 1 (1.45%) [0.00% to 8.52%] | 1 (0.66%) [0.00% to 4.03%] | – | 2 (5.41%) [0.57% to 18.63%] |
| Wound infection | 3 (4.35%) [0.99% to 12.52%] | 9 (5.96%) [3.02% to 11.09%] | 1 (6.25%) [0.00% to 30.31%] | 3 (8.11%) [2.06% to 22.03%] |
| Postoperative chyle leak | 1 (1.45%) [0.00% to 8.52%] | 3 (1.99%) [0.42% to 5.94%] | 1 (6.25%) [0.00% to 30.31%] | – |
| Vagal palsy | 1 (1.45%) [0.00% to 8.52%] | – | – | – |
| Marginal mandibular nerve palsy | 5 (7.25%) [2.77% to 16.23%] | 3 (1.99%) [0.42% to 5.94%] | 1 (6.25%) [0.00% to 30.31%] | – |
| Postoperative haematoma | 3 (4.35%) [0.99% to 12.52%] | 5 (3.31%) [1.21% to 7.72%] | 1 (6.25%) [0.00% to 30.31%] | – |
| Breakdown of wound | 2 (2.90%) [0.20% to 10.57%] | 6 (3.97%) [1.65% to 8.59%] | – | 1 (2.70%) [0.00% to 15.05%] |
| Shoulder movement disability | 4 (5.80%) [1.85% to 14.40%] | 10 (5.96%) [3.02% to 11.09%] | – | 3 (8.11%) [2.06% to 22.03%] |
| Hypoglossal nerve palsy | – | 2 (1.32%) [0.06% to 5.01%] | 1 (6.25%) [0.00% to 30.31%] | 2 (5.41%) [0.57% to 18.63%] |
| Seroma | 1 (1.45%) [0.00% to 8.52%] | 5 (3.31%) [1.21% to 7.72%] | – | 1 (2.70%) [0.00% to 15.05%] |
| Other local (see Table 37) | 1 (1.45%) [0.00% to 8.52%] | 9 (5.96%) [3.02% to 11.09%] | 1 (6.25%) [0.00% to 30.31%] | 1 (2.70%) [0.00% to 15.05%] |
| Total number of complications | 24 | 59 | 6 | 16 |
The complications given in the table as ‘other local’ and ‘other general’ are as in Table 37.
| Arm | ND pre/post | Other general complication |
|---|---|---|
| ND | Pre | Fistula |
| ND | Post | Throat pain |
| ND | Post | Tracheostomy |
| ND | Post | Other nerve damage |
| ND | Post | Reduced oral intake |
| ND | Post | Oedema |
| ND | Post | Other nerve damage |
| ND | Post | Reduced sensation over right ear |
| ND | Post | Oedema |
| ND | Post | Infection |
| ND | Post | Dysphagia |
| ND | Post | Oedema |
| PET–CT | Pre | Fistula |
| PET–CT | Post | Intraoperative possible air embolism unconfirmed |
| PET–CT | Post | Required continuous pressure airway support for 1 day |
In Table 38, complication counts are given by whether or not the NDs were actually performed before or after CRT.
| Surgical complication | Trial arm, n (rate) [95% CI] | ||
|---|---|---|---|
| ND arm | Surveillance arm | ||
| ND performed pre CRT (N = 64) | ND performed post CRT (N = 152) | ND performed (N = 54) | |
| No complications | 49 (76.56%) [64.76% to 85.35%] | 118 (77.63%) [70.34% to 83.56%] | 34 (62.96%) [49.60% to 74.60%] |
| General | |||
| Chest infection | 1 (1.56%) [0.00% to 9.14%] | 3 (1.97%) [0.41% to 5.90%] | – |
| Urinary tract infection | – | – | 2 (3.70%) [0.30% to 13.26%] |
| Septicaemia | – | 1 (0.66%) [0.00% to 4.00%] | – |
| Other general | – | 2 (1.32%) [0.06% to 4.97%] | 1 (1.85%) [0.00% to 10.69%] |
| Local | |||
| Intraoperative chyle leak | 1 (1.56%) [0.00% to 9.14%] | 1 (0.66%) [0.00% to 4.00%] | 2 (3.70%) [0.30% to 13.26%] |
| Wound infection | 1 (1.56%) [0.00% to 9.14%] | 9 (5.92%) [3.00% to 11.02%] | 4 (7.41%) [2.42% to 18.05%] |
| Postoperative chyle leak | 1 (1.56%) [0.00% to 9.14%] | 3 (1.97%) [0.41% to 5.90%] | 1 (1.85%) [0.00% to 10.69%] |
| Vagal palsy | 1 (1.56%) [0.00% to 9.14%] | – | – |
| Marginal mandibular nerve palsy | 5 (7.81) [3.00% to 17.4%] | 3 (1.97%) [0.41% to 5.90%] | 1 (1.85%) [0.00% to 10.69%] |
| Postoperative haematoma | 2 (3.13%) [0.23% to 11.33%] | 5 (3.29%) [1.21% to 7.68%] | 1 (1.85%) [0.00% to 10.69%] |
| Breakdown of wound | 1 (1.56%) [0.00% to 9.14%] | 6 (3.95%) [1.63% to 8.53%] | 1 (1.85%) [0.00% to 10.69%] |
| Shoulder movement disability | 4 (6.25%) [2.01% to 15.44%] | 10 (6.58%) [3.48% to 11.82%] | 3 (5.56%) [1.32% to 15.70%] |
| Hypoglossal nerve palsy | – | 2 (1.32%) [0.06% to 4.97%] | 3 (5.56%) [1.32% to 15.70%] |
| Seroma | 1 (1.56%) [0.00% to 9.14%] | 5 (3.29%) [1.21% to 7.68%] | 1 (1.85%) [0.00% to 10.69%] |
| Other local | 1 (1.56%) [0.00% to 9.14%] | 9 (5.92%) [3.00% to 11.02%] | 2 (3.70%) [0.30% to 13.26%] |
| Total number of complications | 19 | 59 | 22 |
Quality of life
Completion of quality-of-life questionnaires
The total return rate of quality-of-life questionnaires, based on the number that could have been received if all surviving patients completed all of them, was 76.4% in the ND arm and 80.6% in the surveillance arm. Generally, the return rates (Table 39) were at least 70% at each time point and they were returned at the required times.
| Quality-of-life questionnaire status | Trial arm | |
|---|---|---|
| Surveillance | ND | |
| Total randomised | 282 | 282 |
| Number of quality-of-life questionnaires received | 1 | 5 |
| Baseline questionnaire | ||
| Received (% of total alive) | 262 (92.9) | 261 (92.6) |
| Not received | 19 | 16 |
| 2 weeks after CRT | ||
| Received (% of total alive) | 211 (75.4) | 196 (70.8) |
| Not received | 67 | 71 |
| Died | 2 | 5 |
| Withdrawn from trial | 1 | 5 |
| 6 months after randomisation | ||
| Received (% of total alive) | 208 (76.5) | 183 (68.3) |
| Not received | 61 | 71 |
| Died | 10 | 14 |
| Withdrawn from trial | 2 | 9 |
| 12 months after randomisation | ||
| Received (% of total alive) | 197 (76.1) | 181 (72.7) |
| Not received | 58 | 53 |
| Died | 23 | 33 |
| Withdrawn from trial | 3 | 10 |
| 24 months after randomisation | ||
| Received (% of total alive) | 195 (81.9) | 179 (76.8) |
| Not received yet | 38 | 39 |
| Died | 44 | 49 |
| Withdrawn from trial | 4 | 10 |
The patients who responded to the questionnaire at 24 months were broadly similar in terms of baseline characteristics to those living patients from whom there was no response, but the non-responders had slightly worse survival beyond 24 months.
Questionnaires in both trial arms were completed at the designated times (Table 40). The questions were well answered, allowing most scales to be computable in the majority of cases (Table 41). The questionnaires were well completed, with most scales computable in the majority of cases.
| Time point | Time from randomisation to completion, median (IQR) | |
|---|---|---|
| Surveillance | ND | |
| 2 weeks after CRT | 87 days (69–117 days) | 95 days (72–117 days) |
| 6 months | 6.2 months (5.8–7.1 months) | 6.4 months (5.8–7.3 months) |
| 12 months | 12.2 months (11.9–13.1 months) | 12.4 months (11.9–13.2 months) |
| 24 months | 24.5 months (23.9–25.8 months) | 24.5 months (23.9–25.7 months) |
| Completeness | Trial arm, n (%) | Total, N (%) | |
|---|---|---|---|
| Surveillance | ND | ||
| EORTC QLQ-C30 | |||
| All scales computable | 1000 (93.2) | 928 (92.8) | 1928 (93.0) |
| One scale (out of 15) missing | 54 (5.0) | 52 (5.2) | 106 (5.1) |
| More than one scale missing | 19 (1.8) | 20 (2.0) | 39 (1.9) |
| EORTC QLQ-H&N35 | |||
| All scales computable | 865 (80.5) | 818 (81.9) | 1683 (81.2) |
| One scale (out of 18) missing | 142 (13.2) | 115 (11.5) | 257 (12.4) |
| More than one scale missing | 67 (6.2) | 66 (6.6) | 133 (6.4) |
| MDADI dysphagia questionnaire | |||
| All scales computable | 1023 (95.3) | 949 (94.9) | 1972 (95.1) |
| One scale (out of five) missing | 19 (1.8) | 18 (1.8) | 37 (1.8) |
| More than one scale missing | 31 (2.9) | 33 (3.3) | 64 (3.1) |
| EQ-5D (3L) | |||
| All items present | 1008 (93.9) | 937 (93.7) | 1945 (93.8) |
| At least one item missing | 65 (6.1) | 63 (6.3) | 128 (6.2) |
Baseline quality-of-life questionnaire scores
The quality-of-life, H&N function and dysphagia-related scores were well balanced between the treatment arms (Table 42).
| Quality-of-life scale | Trial arm, mean (SD) | |
|---|---|---|
| Surveillance (n = 281) | ND (n = 277) | |
| QLQ-C30 scores (scale 0–100, high values are good) | ||
| Global health status | 71.3 (20.5) | 71.0 (21.4) |
| Physical functioning | 91.2 (15.6) | 90.6 (16.7) |
| Role functioning | 84.3 (26.3) | 84.0 (25.7) |
| Emotional functioning | 74.5 (22.3) | 74.8 (22.6) |
| Cognitive functioning | 85.1 (20.7) | 84.8 (20.9) |
| Social functioning | 82.5 (25.1) | 80.3 (24.4) |
| QLQ-C30 symptoms and side effects (scale 0–100, low values are good) | ||
| Fatigue | 77.0 (24.1) | 77.4 (23.6) |
| Nausea and vomiting | 94.7 (13.4) | 95.8 (11.6) |
| Pain | 78.4 (26.2) | 77.8 (26.1) |
| Dyspnoea | 90.6 (18.6) | 89.5 (22.3) |
| Insomnia | 72.2 (30.7) | 69.1 (31.3) |
| Appetite loss | 81.5 (28.3) | 83.5 (25.6) |
| Constipation | 87.0 (23.4) | 86.9 (23.2) |
| Diarrhoea | 95.3 (13.7) | 94.6 (14.9) |
| Financial difficulties | 76.8 (33.9) | 79.7 (30.7) |
| H&N35 scores (H&N quality of life) (scale 0–100, high values are good) | ||
| Pain | 75.7 (23.7) | 73.5 (25.2) |
| Swallowing | 87.0 (19.9) | 85.1 (22.5) |
| Senses problems | 91.6 (17.4) | 90.6 (17.8) |
| Speech problems | 87.8 (18.5) | 87.5 (18.2) |
| Trouble with social eating | 86.7 (22.2) | 87.5 (21.8) |
| Trouble with social contact | 93.0 (15.7) | 93.9 (14.0) |
| Less sexuality | 75.9 (32.3) | 78.0 (32.9) |
| Teeth | 87.1 (26.6) | 85.5 (26.6) |
| Opening mouth | 87.5 (25.8) | 85.3 (28.0) |
| Dry mouth | 77.4 (28.6) | 81.7 (24.4) |
| Sticky saliva | 84.0 (25.4) | 84.4 (27.2) |
| Coughing | 74.2 (25.4) | 74.8 (26.9) |
| Felt ill | 84.2 (25.5) | 86.5 (21.0) |
| Used painkillers | 36.4 (48.2) | 33.8 (47.4) |
| Taken nutritional supplements | 84.3 (36.5) | 84.5 (36.3) |
| Used a feeding tube | 97.3 (16.2) | 94.6 (22.7) |
| Weight loss | 72.7 (44.7) | 71.4 (45.3) |
| Weight gain | 85.0 (35.8) | 85.5 (35.3) |
| MDADI (dysphagia scales) (scale 0–100, high values are good) | ||
| Global | 76.5 (29.0) | 75.3 (29.7) |
| Emotional | 77.8 (18.9) | 76.8 (18.0) |
| Functional | 81.2 (19.7) | 80.8 (19.1) |
| Physical | 76.2 (24.5) | 75.4 (23.3) |
| Total | 78.1 (20.3) | 77.2 (19.4) |
The European Organisation for Research and Treatment of Cancer’s Quality of Life Questionnaire for Cancer (with 30 questions) quality-of-life outcomes
In Tables 43–47, ‘mean treatment difference’ is the mean change from baseline in the surveillance arm minus the mean change from baseline in the ND arm. For these preplanned analyses of quality-of-life scores, no p-value was specified for determining a significant difference. In Table 43, global and functioning scales are low = bad, high = good. So, a positive mean treatment difference is good for the PET–CT arm. There are no clear differences as, for example, in global health status depicted in Figure 13, but results are slightly more favourable for the surveillance arm. It suggests that the symptoms and quality-of-life effects of ND are less impactful that those of the CRT.
| Scales/measures | Trial arm, number of patients | Mean treatment difference | p-value (Wilcoxon) | |
|---|---|---|---|---|
| PET–CT | ND | |||
| QLQ-C30 global health status | ||||
| Post CRT | 196 | 186 | 0.12 | 0.97 |
| 6 months | 192 | 169 | 4.94 | 0.03 |
| 12 months | 182 | 171 | 3.03 | 0.09 |
| 24 months | 180 | 166 | –0.81 | 0.85 |
| QLQ-C30 physical functioning | ||||
| Post CRT | 198 | 190 | –0.45 | 0.93 |
| 6 months | 190 | 173 | 3.51 | 0.08 |
| 12 months | 183 | 173 | 4.29 | 0.01 |
| 24 months | 179 | 170 | –0.9 | 0.89 |
| QLQ-C30 role functioning | ||||
| Post CRT | 196 | 187 | 0.81 | 0.93 |
| 6 months | 188 | 172 | 3.71 | 0.14 |
| 12 months | 182 | 169 | 12.09 | 0.001 |
| 24 months | 177 | 169 | –0.71 | 0.74 |
| QLQ-C30 emotional functioning | ||||
| Post CRT | 197 | 185 | 0.97 | 0.82 |
| 6 months | 192 | 168 | 3.83 | 0.26 |
| 12 months | 183 | 170 | 4.05 | 0.34 |
| 24 months | 180 | 166 | 0.86 | 0.48 |
| QLQ-C30 cognitive functioning | ||||
| Post CRT | 194 | 181 | –0.09 | 0.82 |
| 6 months | 190 | 168 | 1.24 | 0.48 |
| 12 months | 178 | 167 | 4.63 | 0.31 |
| 24 months | 175 | 161 | 0.15 | 0.93 |
| QLQ-C30 social functioning | ||||
| Post CRT | 191 | 180 | –2.69 | 0.64 |
| 6 months | 184 | 166 | 2.51 | 0.41 |
| 12 months | 181 | 168 | 5.37 | 0.07 |
| 24 months | 176 | 163 | –5.57 | 0.04 |
| Scales/measures | Trial arm, number of patients | Mean treatment difference | p-value (Wilcoxon) | |
|---|---|---|---|---|
| PET–CT | ND | |||
| QLQ-C30 fatigue | ||||
| Post CRT | 199 | 190 | –2.35 | 0.52 |
| 6 months | 191 | 173 | 5.25 | 0.08 |
| 12 months | 184 | 173 | 4.75 | 0.07 |
| 24 months | 180 | 170 | 4.52 | 0.20 |
| QLQ-C30 nausea and vomiting | ||||
| Post CRT | 195 | 189 | 2.81 | 0.62 |
| 6 months | 189 | 172 | –1.48 | 0.29 |
| 12 months | 184 | 171 | 3.09 | 0.06 |
| 24 months | 180 | 167 | –1.17 | 0.43 |
| QLQ-C30 pain (i.e. general) | ||||
| Post CRT | 191 | 182 | 0.46 | 0.92 |
| 6 months | 185 | 165 | 7.65 | 0.01 |
| 12 months | 179 | 169 | 8.49 | 0.01 |
| 24 months | 176 | 165 | 3.98 | 0.11 |
| QLQ-C30 dyspnoea | ||||
| Post CRT | 197 | 188 | 0.91 | 0.69 |
| 6 months | 188 | 173 | 0.3 | 0.60 |
| 12 months | 183 | 171 | 3.79 | 0.08 |
| 24 months | 179 | 170 | 1.34 | 0.69 |
| QLQ-C30 insomnia | ||||
| Post CRT | 197 | 190 | –2.45 | 0.28 |
| 6 months | 191 | 173 | 5.07 | 0.21 |
| 12 months | 184 | 172 | –1.1 | 0.93 |
| 24 months | 179 | 170 | –4.14 | 0.19 |
| QLQ-C30 appetite loss | ||||
| Post CRT | 195 | 189 | 6.42 | 0.10 |
| 6 months | 189 | 172 | 7.22 | 0.11 |
| 12 months | 184 | 172 | 7.49 | 0.09 |
| 24 months | 179 | 167 | 4.35 | 0.35 |
| QLQ-C30 constipation | ||||
| Post CRT | 198 | 189 | –4.48 | 0.28 |
| 6 months | 191 | 172 | –0.43 | 0.50 |
| 12 months | 183 | 171 | 0.99 | 0.57 |
| 24 months | 180 | 169 | –0.33 | 0.70 |
| QLQ-C30 diarrhoea | ||||
| Post CRT | 198 | 186 | –0.21 | 0.99 |
| 6 months | 192 | 169 | –3.27 | 0.13 |
| 12 months | 182 | 170 | –2.88 | 0.05 |
| 24 months | 180 | 167 | –0.17 | 0.94 |
| QLQ-C30 financial difficulties | ||||
| Post CRT | 195 | 183 | –1.28 | 0.79 |
| 6 months | 189 | 168 | –0.15 | 0.87 |
| 12 months | 181 | 170 | 4.24 | 0.28 |
| 24 months | 178 | 165 | –1.69 | 0.73 |
| Scales/measures | Trial arm, number of patients | Mean treatment difference | p-value (Wilcoxon) | |
|---|---|---|---|---|
| PET–CT | ND | |||
| H&N35 pain (mouth, jaw or throat) | ||||
| Post CRT | 197 | 189 | –1.48 | 0.77 |
| 6 months | 190 | 172 | –1.83 | 0.53 |
| 12 months | 182 | 172 | –1.43 | 0.86 |
| 24 months | 180 | 168 | –3.67 | 0.42 |
| H&N35 swallowing | ||||
| Post CRT | 188 | 185 | –1.41 | 0.68 |
| 6 months | 188 | 169 | –0.68 | 0.69 |
| 12 months | 182 | 173 | –1.07 | 0.75 |
| 24 months | 180 | 168 | –3.08 | 0.43 |
| H&N35 senses problems | ||||
| Post CRT | 193 | 182 | –1.17 | 0.89 |
| 6 months | 186 | 168 | –1.76 | 0.70 |
| 12 months | 178 | 169 | 0.6 | 0.89 |
| 24 months | 176 | 167 | –4.08 | 0.06 |
| H&N35 speech problems | ||||
| Post CRT | 198 | 189 | 0.31 | 0.99 |
| 6 months | 190 | 173 | –0.33 | 0.84 |
| 12 months | 182 | 172 | 1.23 | 0.75 |
| 24 months | 179 | 167 | –1.21 | 0.38 |
| H&N35 trouble with social eating | ||||
| Post CRT | 173 | 178 | –1.43 | 0.84 |
| 6 months | 182 | 166 | 2.55 | 0.54 |
| 12 months | 180 | 170 | 2.77 | 0.30 |
| 24 months | 176 | 166 | 0.23 | 0.99 |
| H&N35 trouble with social contact | ||||
| Post CRT | 198 | 189 | 1.96 | 0.55 |
| 6 months | 190 | 173 | 1.47 | 0.38 |
| 12 months | 182 | 172 | 6.96 | 0.01 |
| 24 months | 179 | 167 | 0.12 | 0.87 |
| H&N35 less sexuality | ||||
| Post CRT | 163 | 161 | 2.79 | 0.62 |
| 6 months | 163 | 156 | 2.03 | 0.54 |
| 12 months | 161 | 156 | 6.42 | 0.21 |
| 24 months | 154 | 148 | 1.08 | 0.77 |
| H&N35 problems with teeth | ||||
| Post CRT | 191 | 178 | 0.09 | 0.86 |
| 6 months | 186 | 168 | 3.12 | 0.21 |
| 12 months | 179 | 169 | 6.92 | 0.10 |
| 24 months | 176 | 164 | –2.9 | 0.44 |
| H&N35 problems opening mouth wide | ||||
| Post CRT | 198 | 185 | –6.13 | 0.23 |
| 6 months | 191 | 172 | 0.92 | 0.59 |
| 12 months | 181 | 172 | 2.93 | 0.29 |
| 24 months | 179 | 167 | 5.75 | 0.08 |
| H&N35 dry mouth | ||||
| Post CRT | 198 | 188 | –0.12 | 0.95 |
| 6 months | 191 | 172 | –0.73 | 0.66 |
| 12 months | 181 | 171 | 7.38 | 0.08 |
| 24 months | 179 | 168 | –2.39 | 0.44 |
| H&N35 sticky saliva | ||||
| Post CRT | 196 | 189 | 0.49 | 0.95 |
| 6 months | 188 | 172 | –4.7 | 0.14 |
| 12 months | 181 | 171 | –4.69 | 0.23 |
| 24 months | 177 | 168 | –3.47 | 0.42 |
| H&N35 coughing | ||||
| Post CRT | 197 | 189 | –4.35 | 0.20 |
| 6 months | 192 | 171 | 3.08 | 0.73 |
| 12 months | 182 | 172 | 2.77 | 0.65 |
| 24 months | 180 | 167 | –0.05 | 0.81 |
| H&N35 felt ill | ||||
| Post CRT | 197 | 189 | 4.04 | 0.26 |
| 6 months | 190 | 172 | 5.21 | 0.07 |
| 12 months | 182 | 173 | 4.91 | 0.19 |
| 24 months | 180 | 167 | 1.63 | 0.55 |
| H&N35 used painkillers (yes/no) | ||||
| Post CRT | 198 | 188 | –1.32 | 0.79 |
| 6 months | 189 | 172 | 3.87 | 0.53 |
| 12 months | 181 | 171 | 16.45 | 0.01 |
| 24 months | 179 | 166 | 4.75 | 0.61 |
| H&N35 taken nutritional supplements (yes/no) | ||||
| Post CRT | 195 | 186 | 2 | 0.53 |
| 6 months | 189 | 171 | 6.18 | 0.26 |
| 12 months | 182 | 167 | 0.97 | 0.89 |
| 24 months | 177 | 165 | 1.79 | 0.74 |
| H&N35 used a feeding tube (yes/no) | ||||
| Post CRT | 197 | 187 | –0.04 | 0.93 |
| 6 months | 189 | 171 | 1.34 | 0.82 |
| 12 months | 180 | 169 | –2.16 | 0.62 |
| 24 months | 178 | 167 | –2.17 | 0.40 |
| H&N35 weight loss (yes/no) | ||||
| Post CRT | 186 | 183 | –3.5 | 0.61 |
| 6 months | 185 | 163 | –5.93 | 0.38 |
| 12 months | 175 | 169 | 1.53 | 0.83 |
| 24 months | 175 | 163 | 1.49 | 0.78 |
| H&N35 weight gain (yes/no) | ||||
| Post CRT | 180 | 173 | 0.56 | 0.91 |
| 6 months | 181 | 156 | 7.27 | 0.21 |
| 12 months | 167 | 166 | 1.32 | 0.88 |
| 24 months | 172 | 159 | 10.48 | 0.10 |
| Scales/measures | Trial arm, number of patients | Mean treatment difference | p-value (Wilcoxon) | |
|---|---|---|---|---|
| PET–CT | ND | |||
| MDADI dysphagia global | ||||
| Post CRT | 187 | 182 | 0.95 | 0.65 |
| 6 months | 180 | 167 | 8.46 | 0.05 |
| 12 months | 173 | 161 | 4.31 | 0.17 |
| 24 months | 168 | 161 | –0.27 | 0.92 |
| MDADI dysphagia emotional | ||||
| Post CRT | 185 | 179 | –1.68 | 0.36 |
| 6 months | 178 | 163 | 2.11 | 0.43 |
| 12 months | 173 | 164 | 2.83 | 0.44 |
| 24 months | 168 | 160 | –1.05 | 0.91 |
| MDADI dysphagia functional | ||||
| Post CRT | 189 | 181 | –1.39 | 0.54 |
| 6 months | 186 | 166 | 1.46 | 0.48 |
| 12 months | 175 | 166 | 1.22 | 0.76 |
| 24 months | 172 | 162 | –0.87 | 0.77 |
| MDADI dysphagia physical | ||||
| Post CRT | 185 | 177 | 0.53 | 0.87 |
| 6 months | 178 | 161 | 0.27 | 0.97 |
| 12 months | 173 | 164 | 1.38 | 0.70 |
| 24 months | 168 | 159 | –0.49 | 0.99 |
| MDADI dysphagia total | ||||
| Post CRT | 189 | 183 | –0.98 | 0.56 |
| 6 months | 183 | 166 | 1.62 | 0.46 |
| 12 months | 175 | 166 | 1.75 | 0.58 |
| 24 months | 171 | 162 | –0.64 | 0.99 |
| Scales/measures | Trial arm, number of patients | Mean treatment difference | p-value (Wilcoxon) | |
|---|---|---|---|---|
| PET–CT | ND | |||
| EQ-5D overall health status | ||||
| Post CRT | 187 | 174 | 0.04 | 0.20 |
| 6 months | 181 | 166 | 0.04 | 0.09 |
| 12 months | 173 | 161 | 0.07 | 0.007 |
| 24 months | 175 | 156 | 0.02 | 0.21 |
FIGURE 13.
The EORTC’s QLQ-C30 global health status. Reproduced from the New England Journal of Medicine, Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, PET-CT Surveillance versus Neck Dissection in Advanced Head and Neck Cancer, 374, 1444–54. Copyright © (2016) Massachusetts Medical Society. Reprinted with permission. 62

The European Organisation for Research and Treatment of Cancer’s Quality of Life Questionnaire for Cancer (with 30 questions) symptom scales
For simplicity of interpretation the symptom scales in Table 44 have been transformed so that, here also, a positive mean treatment difference is good for the PET–CT arm. There are no clear differences, but results are slightly more favourable for the surveillance arm.
The European Organisation for Research and Treatment of Cancer’s Quality of Life Questionnaire for Cancer head and neck module with 35 questions quality-of-life outcomes
All scales in Table 45 have been set to low as bad and high as good, so a positive difference in mean treatment difference is good for the PET–CT arm. There is little to suggest differences between trial treatments. No difference is seen in pain in H&N cancer and in problems with swallowing (Figures 14 and 15). The difference in problems with teeth is small (Figure 16). Reported use of painkillers at 12 months was higher in the ND arm (Figure 17).
FIGURE 14.
The EORTC’s H&N35 pain in H&N cancer: mean scores unadjusted.
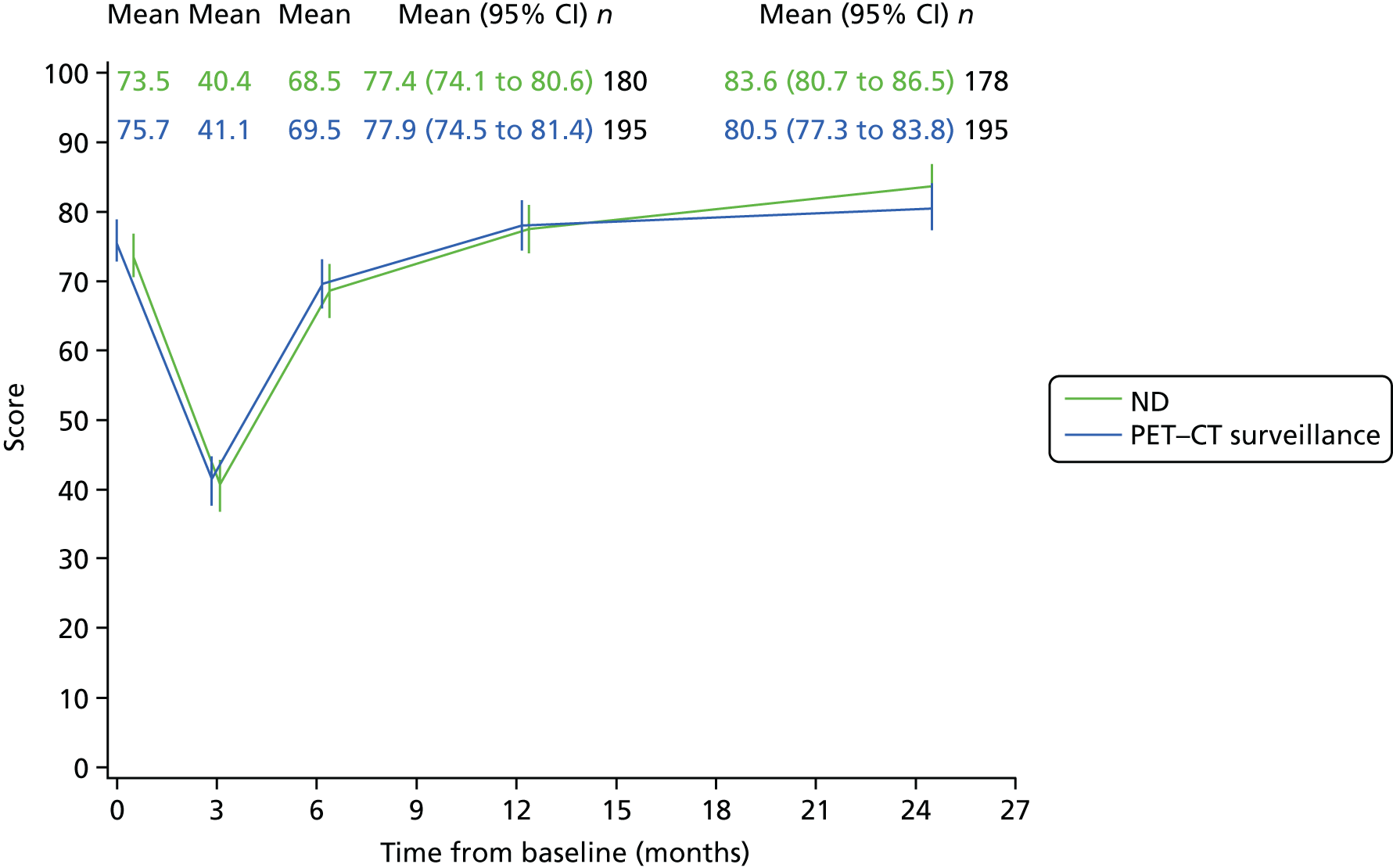
FIGURE 15.
The EORTC’s H&N35 problems with swallowing: mean scores unadjusted.
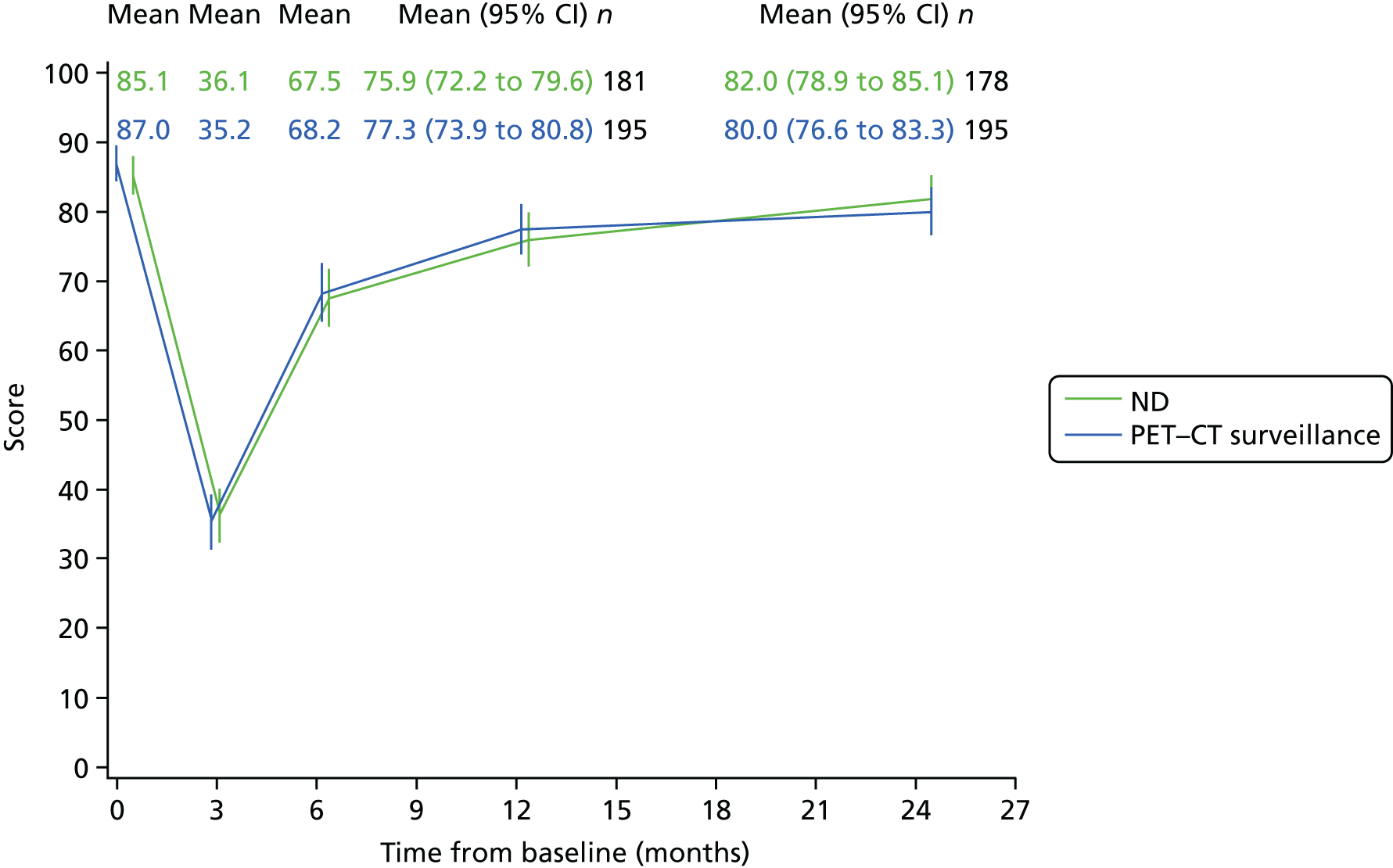
FIGURE 16.
The EORTC’s H&N35 problems with teeth: mean scores unadjusted.

FIGURE 17.
The EORTC’s H&N35 used painkillers: mean scores unadjusted (higher score = less pain).
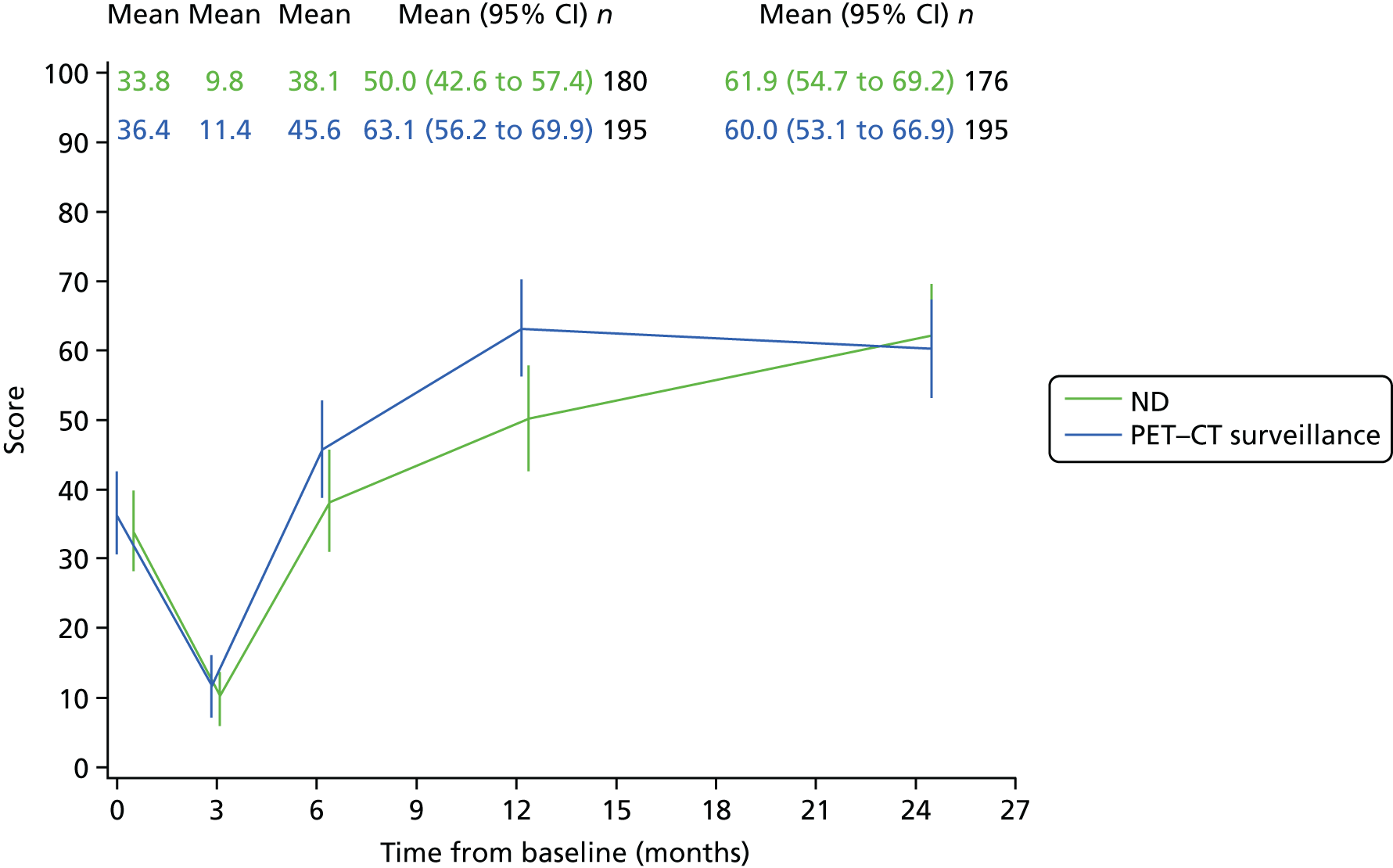
The MD Anderson Dysphagia Inventory dysphagia scales
All scales in Table 46 have been set to low as bad and high as good, so a positive difference in mean treatment difference is good for the PET–CT arm. There is little to suggest differences between trial treatments (see, e.g., Figure 18).
FIGURE 18.
The MDADI dysphagia total: mean scores unadjusted.

European Quality of Life-5 Dimensions
The EQ-5D is used for the health resource study. In Table 47, positive values for mean treatment difference indicate a better response in the PET–CT arm.
Differences of at least 10% in quality-of-life scales
Another way of presenting these data is to display differences from baseline of at least 10%. Figures 19–25 compare the randomised groups on that basis. Tables 48–54 give similar information, comparing patients who underwent ND before CRT with those who did not. The swallowing measures in Figures 21, 24 and 25 show no difference between trial treatments, with both having a low proportion of improvers.
FIGURE 19.
The EORTC’s QLQ-C30 global health status, percentages changed by ≥ 10%.

FIGURE 20.
The EORTC’s QLQ H&N35 pain scale, percentages changed by ≥ 10%.
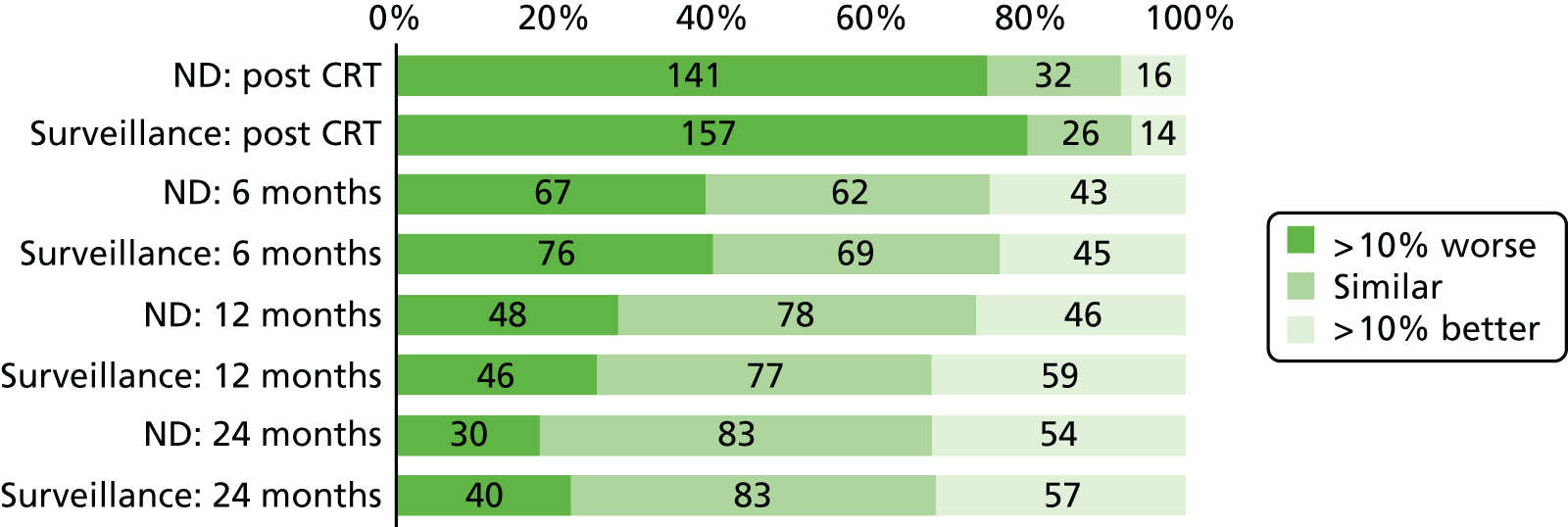
FIGURE 21.
The EORTC’s QLQ H&N35 swallowing scale, percentages changed by ≥ 10%.
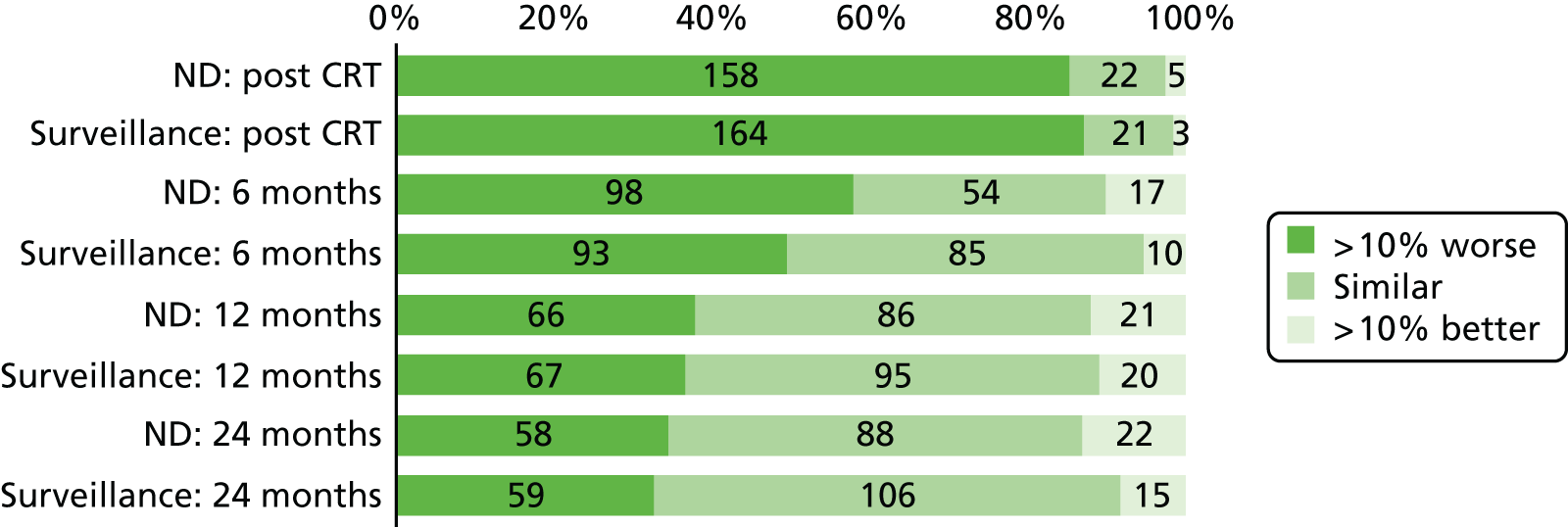
FIGURE 22.
The EORTC’s QLQ H&N35 problems with teeth, percentages changed by ≥ 10%.
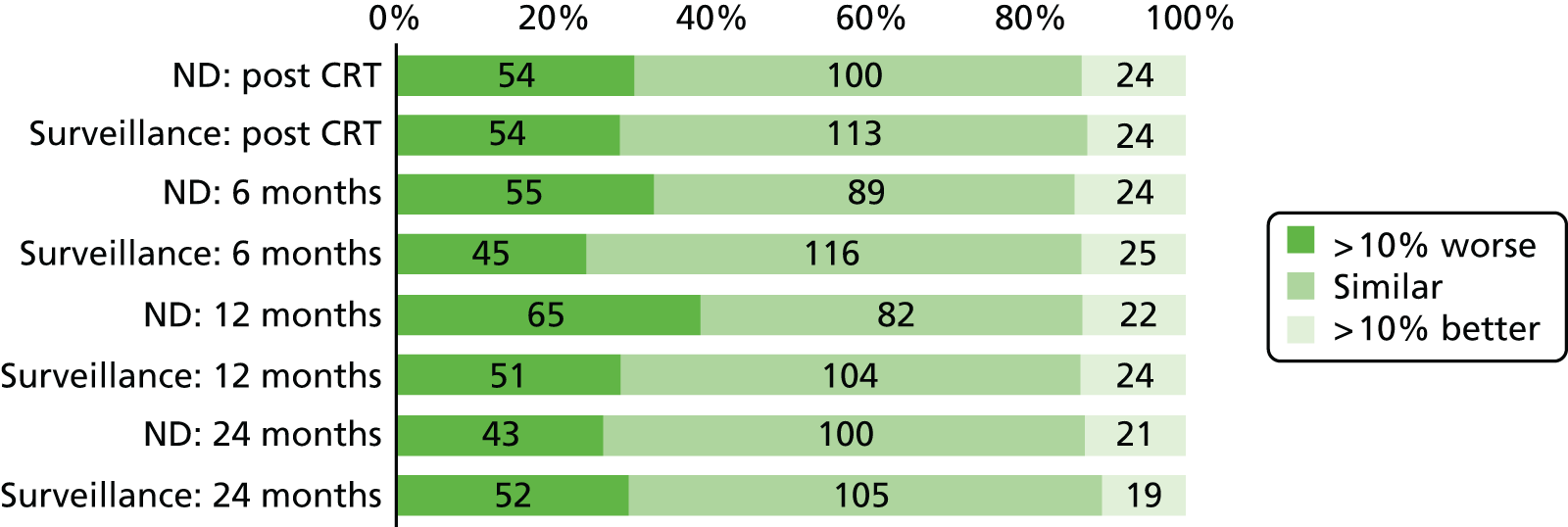
FIGURE 23.
The EORTC’s QLQ H&N35 use of painkillers, percentages changed by ≥ 10%.
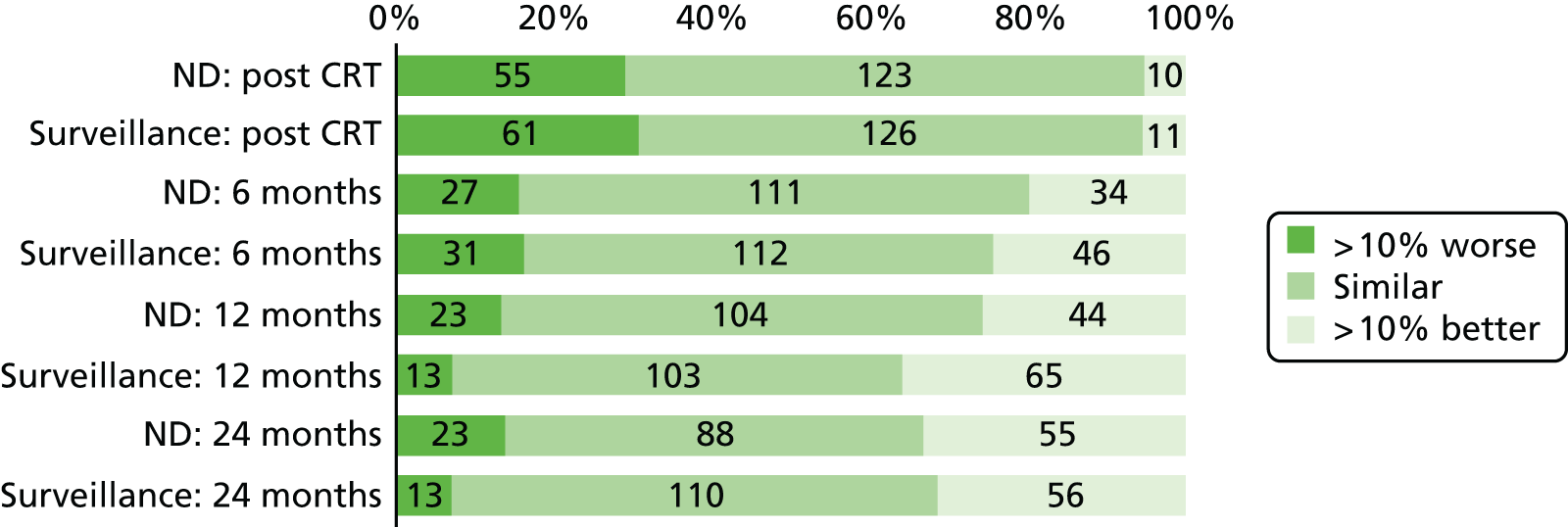
FIGURE 24.
The MDADI dysphagia global scale, percentages changed by ≥ 10%.
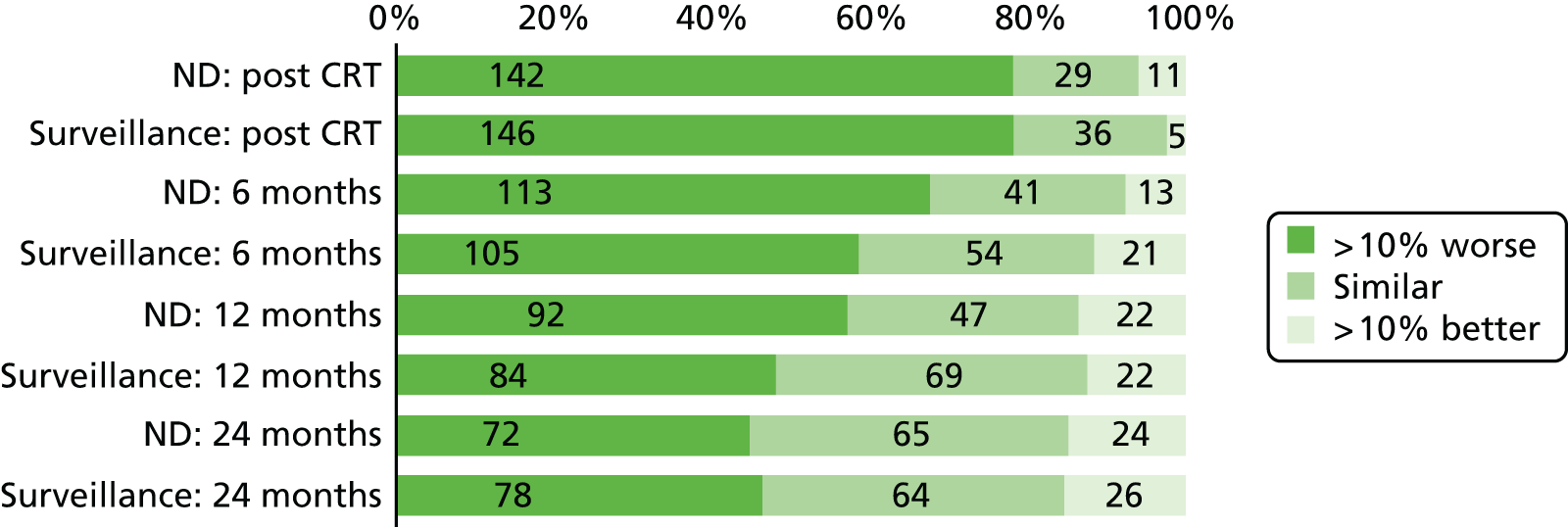
FIGURE 25.
The MDADI dysphagia total scale, percentages changed by ≥ 10%.
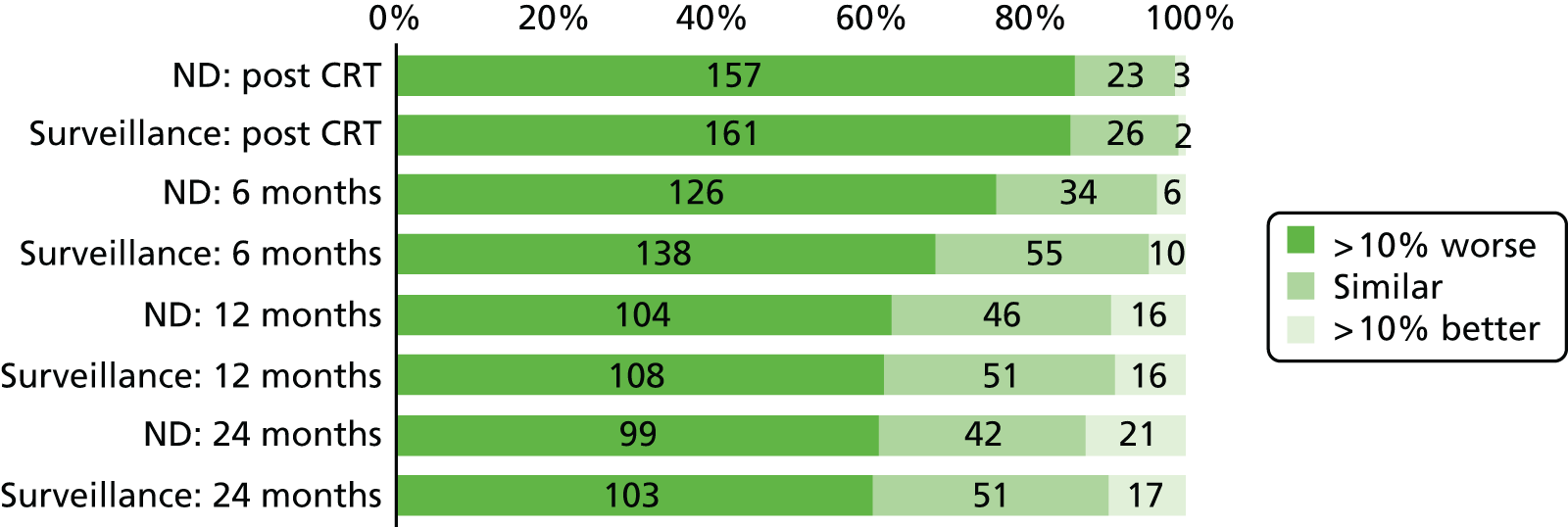
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 42 (84.0) | 6 (12.0) | 2 (4.0) | 50 |
| All others | 254 (76.5) | 63 (19.0) | 15 (4.5) | 332 |
| 6 months, n (%) | ||||
| ND before CRT | 28 (54.9) | 17 (33.3) | 6 (11.8) | 51 |
| All others | 144 (46.5) | 131 (42.3) | 35 (11.3) | 310 |
| 12 months, n (%) | ||||
| ND before CRT | 21 (43.8) | 17 (35.4) | 10 (20.8) | 48 |
| All others | 107 (35.1) | 131 (43.0) | 67 (22.0) | 305 |
| 24 months, n (%) | ||||
| ND before CRT | 14 (29.2) | 25 (52.1) | 9 (18.8) | 48 |
| All others | 75 (25.2) | 152 (51.0) | 71 (23.8) | 298 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 36 (72.0) | 6 (12.0) | 8 (16.0) | 50 |
| All others | 262 (78.0) | 52 (15.5) | 22 (6.6) | 336 |
| 6 months, n (%) | ||||
| ND planned before CRT | 23 (44.2) | 17 (32.7) | 12 (23.1) | 52 |
| All others | 120 (38.7) | 114 (36.8) | 76 (24.5) | 310 |
| 12 months, n (%) | ||||
| ND planned before CRT | 16 (33.3) | 18 (37.5) | 14 (29.2) | 48 |
| All others | 782 (25.5) | 137 (44.8) | 91 (29.7) | 306 |
| 24 months, n (%) | ||||
| ND planned before CRT | 9 (18.8) | 23 (47.9) | 16 (33.3) | 48 |
| All others | 62 (20.7) | 143 (47.7) | 95 (31.7) | 300 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 39 (81.3) | 8 (16.7) | 1 (2.1) | 48 |
| All others | 283 (87.1) | 35 (10.8) | 7 (2.2) | 325 |
| 6 months, n (%) | ||||
| ND planned before CRT | 27 (51.9) | 18 (34.6) | 7 (13.5) | 52 |
| All others | 164 (53.8) | 121 (39.7) | 20 (6.6) | 305 |
| 12 months, n (%) | ||||
| ND planned before CRT | 20 (41.7) | 21 (43.8) | 7 (14.6) | 48 |
| All others | 113 (36.8) | 160 (52.1) | 34 (11.1) | 307 |
| 24 months, n (%) | ||||
| ND planned before CRT | 17 (35.4) | 25 (52.1) | 6 (12.5) | 48 |
| All others | 100 (33.3) | 169 (56.3) | 31 (10.3) | 300 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 17 (37.8) | 26 (57.8) | 2 (4.4) | 45 |
| All others | 91 (28.1) | 187 (57.7) | 46 (14.2) | 324 |
| 6 months, n (%) | ||||
| ND planned before CRT | 16 (32.0) | 29 (58.0) | 5 (10.0) | 50 |
| All others | 84 (27.6) | 176 (57.9) | 44 (14.5) | 304 |
| 12 months, n (%) | ||||
| ND planned before CRT | 15 (33.3) | 24 (53.3) | 6 (13.3) | 45 |
| All others | 101 (33.3) | 162 (53.5) | 40 (13.2) | 303 |
| 24 months, n (%) | ||||
| ND planned before CRT | 14 (30.4) | 29 (63.0) | 3 (6.5) | 46 |
| All others | 81 (27.6) | 176 (59.9) | 37 (12.6) | 294 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 24 (48.0) | 25 (50.0) | 1 (2.0) | 50 |
| All others | 92 (27.4) | 224 (66.7) | 20 (5.6) | 336 |
| 6 months, n (%) | ||||
| ND planned before CRT | 13 (24.5) | 34 (64.2) | 6 (11.3) | 53 |
| All others | 45 (14.6) | 189 (61.4) | 74 (24.0) | 308 |
| 12 months, n (%) | ||||
| ND planned before CRT | 12 (25.0) | 33 (68.8) | 3 (6.3) | 48 |
| All others | 24 (7.9) | 174 (57.2) | 106 (34.9) | 304 |
| 24 months, n (%) | ||||
| ND planned before CRT | 15 (31.3) | 25 (52.1) | 8 (16.7) | 48 |
| All others | 21 (7.1) | 173 (58.3) | 103 (34.7) | 297 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 35 (74.5) | 9 (19.2) | 3 (6.4) | 47 |
| All others | 253 (78.6) | 56 (17.4) | 13 (4.0) | 322 |
| 6 months, n (%) | ||||
| ND planned before CRT | 32 (65.3) | 15 (30.6) | 2 (40.1) | 49 |
| All others | 186 (62.4) | 80 (26.9) | 32 (10.7) | 298 |
| 12 months, n (%) | ||||
| ND planned before CRT | 21 (47.7) | 17 (38.6) | 6 (13.6) | 44 |
| All others | 155 (53.5) | 99 (34.1) | 36 (12.4) | 290 |
| 24 months, n (%) | ||||
| ND planned before CRT | 20 (45.5) | 17 (38.6) | 7 (15.9) | 44 |
| All others | 130 (45.6) | 112 (39.3) | 43 (15.1) | 285 |
| Time point and group | Decrease by ≥ 10% | Similar | Increase by ≥ 10% (improvement) | Total |
|---|---|---|---|---|
| Post CRT, n (%) | ||||
| ND planned before CRT | 43 (89.6) | 5 (10.4) | 0 (0.0) | 48 |
| All others | 275 (84.9) | 44 (13.6) | 5 (1.5) | 324 |
| 6 months, n (%) | ||||
| ND planned before CRT | 35 (70.0) | 14 (28.0) | 1 (2.0) | 50 |
| All others | 229 (76.6) | 55 (18.4) | 15 (5.0) | 299 |
| 12 months, n (%) | ||||
| ND planned before CRT | 31 (67.4) | 12 (26.1) | 3 (6.5) | 46 |
| All others | 181 (61.4) | 85 (28.8) | 29 (9.8) | 295 |
| 24 months, n (%) | ||||
| ND planned before CRT | 31 (68.9) | 10 (22.2) | 4 (8.9) | 45 |
| All others | 171 (59.4) | 83 (28.8) | 34 (11.8) | 288 |
A slightly greater percentage of patients who had an early ND had a decreased global health status. This is not generally the case for the more specific symptom scores in Figures 20–25.
p16 subgroups
The baseline scores are better in the p16-positive group than in the negative group. No difference is seen between treatment groups, but the plots in Figures 26 and 27 suggest that the p16-positive group is affected more by CRT than the p16-negative group.
FIGURE 26.
The EORTC’s QLQ-C30 global health status: p16-positive group.
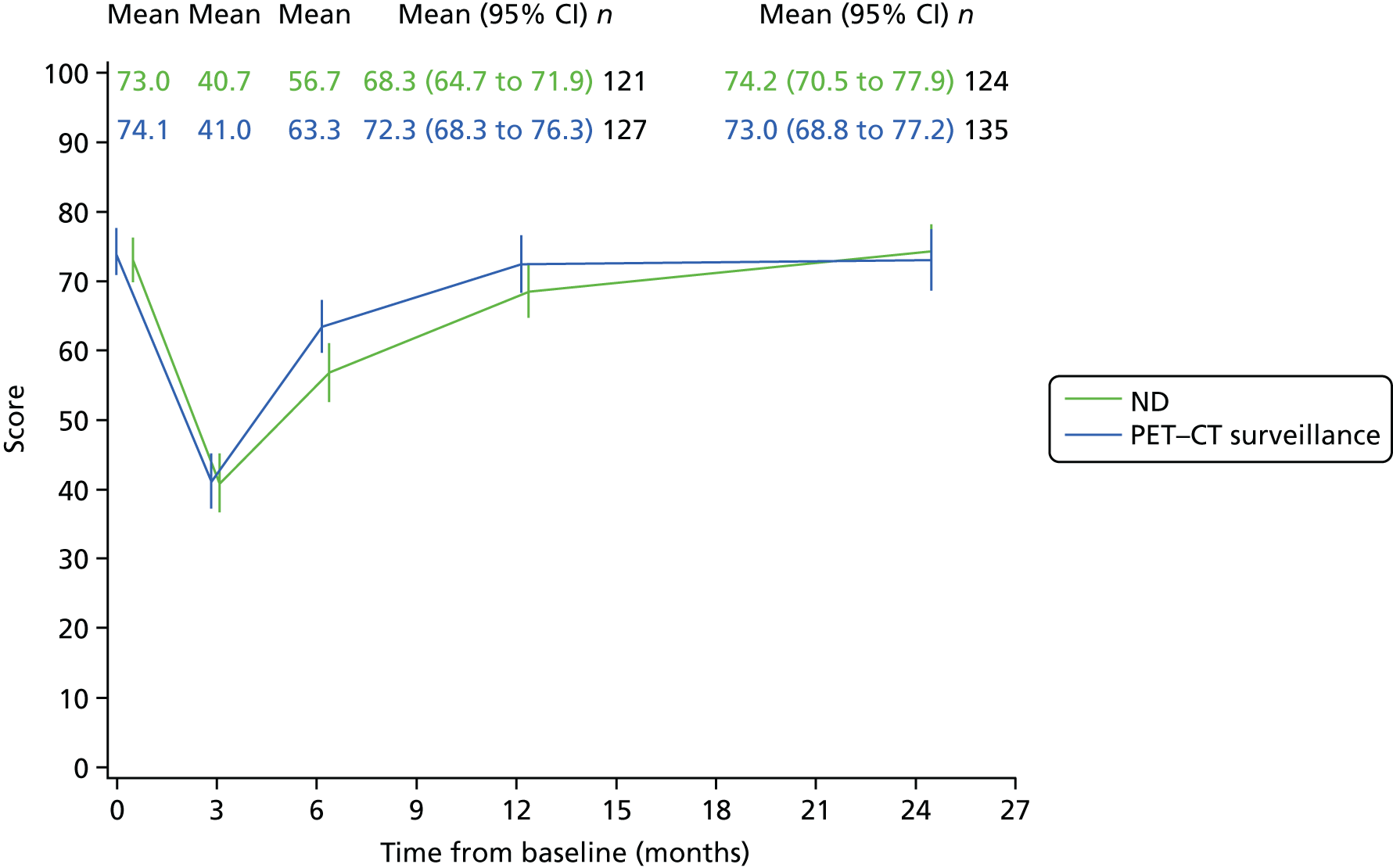
FIGURE 27.
The EORTC’s QLQ-C30 global health status: p16-negative group.
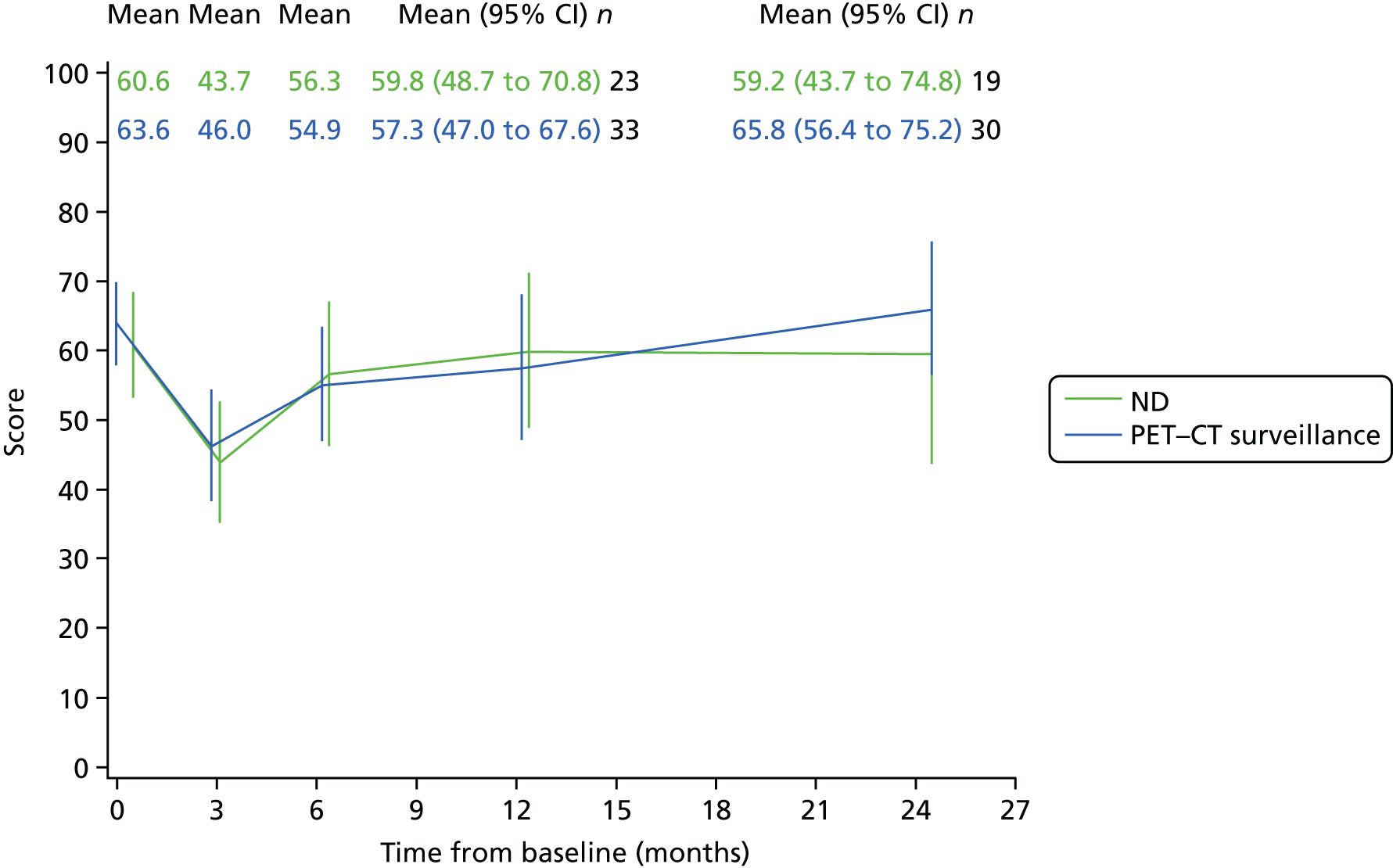
PET–CT scans and concordance
The PET–CT surveillance arm of the trial contained 282 patients, of whom 276 received CRT. Six patients did not receive PET–CT imaging: two died early, one progressed, two proceeded directly to ND and one missed an appointment and died before the rearranged date. PET–CT was performed in 270 patients, and the resulting assessments are summarised in Table 55.
| Assessment | Concordance | n | |||
|---|---|---|---|---|---|
| Local | Central review | ||||
| Response in primary tumour | Response in neck nodes | Response in primary tumour | Response in neck nodes | ||
| Concordant in primary tumour and nodes | |||||
| CR | CR | CR | CR | Yes | 169 |
| CR | Not CR | CR | Not CR | Yes | 36 |
| Not CR | CR | Not CR | CR | Yes | 8 |
| Not CR | Not CR | Not CR | Not CR | Yes | 13 |
| N/A | CR | N/A | CR | Yes | 2 |
| N/A | CR | CR | CR | Yes | 2 |
| Concordant in nodes only | |||||
| CR | Not CR | Not CR | Not CR | Yes | 4 |
| Not CR | Not CR | CR | Not CR | Yes | 5 |
| CR | CR | Not CR | CR | Yes | 3 |
| Not CR | CR | CR | CR | Yes | 5 |
| Discordant | |||||
| CR | CR | Not CR | Not CR | No | 3 |
| CR | CR | CR | Not CR | No | 3 |
| CR | Not CR | CR | CR | No | 1 |
| Discordant summary (local radiologist), concordant summary (central reviewer) | |||||
| CR | CR | Not CR | Not CR | No | 3a |
| CR | CR | CR | Not CR | No | 4b |
| CR | Not CR | CR | CR | No | 6c |
| Not CR | CR | Not CR | Not CR | No | 2d |
| Not CR | Not CR | Not CR | CR | No | 1c |
Concordance rate for nodal disease
The concordance rate, on the basis of the readings reported by the local sites, is 247 out of 270, or 91.5%, but on review and a correct reading of the local report, the last group in the table (3 + 4 + 6 + 2 + 1) should be interpreted as concordant, giving a rate of 263 out of 270, or 97.4%. In total, 243 patients are concordant in primary tumour and neck nodes, 20 are concordant in neck nodes only and seven are discordant in neck nodes. Therefore, the concordance rate for the sites and the central laboratory is 97.4%, with a kappa value of 0.78 (95% CI 0.69 to 0.87). The summary assessments are related to NDs performed in Table 56.
| CR in | ND | Number | |
|---|---|---|---|
| Primary tumour | Neck nodes | ||
| Yes | Yes | No | 181 |
| Yes | Yes | Yes | 4 |
| Yes | No | Yes | 36 |
| Yes | No | No | 11a |
| No | Yes | Yes | – |
| No | Yes | No | 15 |
| No | No | Yes | 12b |
| No | No | No | 7 |
| Missing | Yes | No | 4 |
Concordance in primary tumour
Similarly, for primary tumour the concordance rate for the sites and the central laboratory is 243 out of 266 or 91.4%, with a kappa value of 0.63 (95% CI 0.49 to 0.77).
True-negative rate
The number of patients in whom no neck cancer was seen on PET–CT on review and for whom no ND was carried out was 191. Among this group, 26 experienced recurrence, giving a true-negative rate of 86.4%. Only one recurrence was in the neck alone. Similarly, the true-negative rate for primary tumour was 29 out of 192 (84.9%).
Chapter 4 Economic evaluation
Introduction
An economic evaluation was conducted to assess the cost-effectiveness of the PET–CT-guided watch-and-wait policy compared with planned ND. The evaluation consisted of two components: (1) a WT analysis, in which cost-effectiveness was assessed over the 24-month trial period using individual patient data collected in the trial; and (2) a decision-analytic model analysis, in which cost-effectiveness was assessed over a lifetime horizon, using standard modelling techniques applied to the trial data in order to extrapolate the trial results. The primary analysis was conducted from a NHS secondary care perspective (i.e. including NHS hospital costs). In addition, sensitivity analyses were conducted using (1) a NHS and Personal Social Services (PSS) perspective and (2) a societal perspective.
Methods
Within-trial analysis
Individual patient data collected in the trial were used to determine the costs and quality-adjusted life-years (QALYs) associated with each treatment arm. QALYs were derived using patient EQ-5D questionnaire responses, and costs were calculated using information collected in case report forms and patient- and carer-reported questionnaires. Cost-effectiveness was assessed as the incremental cost-effectiveness ratio (ICER) and incremental net health benefit (INB). Future costs and health outcomes (beyond 1 year) were discounted at an annual rate of 3.5%, as per the National Institute for Health and Care Excellence (NICE)’s Guide to the Methods of Technology Appraisal 2013. 63
Quality-adjusted life-years
In the economic evaluation [both WT and lifetime decision model (DM)] patient health benefit was measured in terms of QALYs. QALYs provide a generic measure of overall patient health and are a composite measure of patient survival weighted by quality of life (utility) over time; for example, 1 year in full health is equivalent to 1 QALY, whereas 1 year at half of full health is equivalent to 0.5 QALYs. Expression of health benefit in terms of QALYs allows decision-makers to make a direct comparison of the cost-effectiveness of interventions across different disease areas and indications, and NICE currently recommends the use of QALYs in cost-effectiveness analyses in its reference case. 63
Information on patient health-related quality of life during the trial was collected using patient responses to the EQ-5D-3L (three levels) questionnaire. 64 The EQ-5D questionnaire asks patients to identify their current health status across five health domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Patients can respond that they currently have no problems, some problems or major problems within each of the given domains; these responses are coded as 1, 2 or 3, respectively. According to their responses to the EQ-5D questionnaire, patients may therefore be classified in one of 243 (35) health states.
Patients in the PET-NECK trial were asked to complete the EQ-5D questionnaire at baseline, during treatment (2 weeks post CRT) and at 3, 6, 12 and 24 months post randomisation. For each time point patients’ identified health state classifications were converted into quality-of-life values by applying the standard European Quality of Life UK tariff values;65 these values represent the UK general public’s preference for each of the possible EQ-5D-defined health states and provide a utility value for each of the health states. For each time point, patient utilities across the two arms were compared using the Wilcoxon signed-rank test. 66
Quality-adjusted life-years were calculated by combining patient utility values with OS data. The Kaplan–Meier method was used to account for loss to follow-up and life-years were calculated by taking the area under the survival curve. The area under the survival curve over each time period was then weighted by the corresponding utility to calculate patient QALYs. 67
Resource use and costs
Data on patient and carer consumption of NHS, PSS and personal resources during the trial were obtained from a combination of case report forms (completed by nurses/clinicians at the enrolled hospital) and patient- and carer-reported questionnaires. Case report forms were completed throughout the trial for all participants to collect information on resource use related to a range of secondary care activities, including node dissection/salvage surgery, radiotherapy, chemotherapy, SAEs, patient follow-up assessments and recurrence events. Patient report forms were used for a subset of patients (n = 42) to collect additional data on secondary care activity outside the patient’s enrolled hospital oncology department, primary and community care activity (such as GP visits, nurse visits, counselling and therapy services, carers and social worker visits) and patient societal costs (i.e. patient travel expenses, equipment costs, one-off expenses and lost earnings resulting from illness). For this subset of patients, carer report forms were also used for those patients with a friend or family member acting as an informal carer, in order to identify carer societal costs (i.e. carer travel expenses, equipment costs, one-off expenses and lost earnings resulting from the associated patient’s illness). In order to ensure quality of reported data and to minimise the burden of reporting on patients and carers, patient and carer report forms were collected for patients enrolled at the two highest-recruiting sites only [University Hospital Birmingham (UHB) and UHCW]. Individuals were asked to recall their use of services over the previous 3-month period (or since completion of the last form when appropriate), at the same time points as for the EQ-5D questionnaire (i.e. at baseline, treatment and 3, 6, 12 and 24 months post randomisation).
Owing to the small sample numbers of patients and carers providing self-reported cost data (42 patients and 35 carers) and to the significant uncertainty involved in imputing these data for the whole trial population, these data were excluded from the base-case analysis of costs. The base-case analysis includes data on secondary care resource use collected in the case report forms only and, therefore, adopts a NHS secondary care perspective. Sensitivity analyses were conducted in order to assess the impact of (1) including the additional patient-reported NHS and PSS data (i.e. using a NHS and PSS perspective) and (2) including all of the additional patient- and carer-reported data (i.e. using a societal perspective).
Costs were estimated by combining the resource use data with unit costs (Table 57) obtained from national sources including NHS national reference costs69 and the Personal Social Services Research Unit (PSSRU)70 costs for NHS and PSS service use costs, and the British National Formulary72 and Drugs and Pharmaceutical Electronic Market Information (eMit)71 for medication costs. The results of the economic evaluation are reported in pounds sterling (price year 2015). Unit costs reported in 2013/14 prices were inflated to 2015 prices in the analysis using the consumer price index inflation value reported by the Office for National Statistics (note that the prices reported in the original source were non-inflated). 76 Costs were compared between the two arms using the Wilcoxon signed-rank test. 66
| Resource item | Unit cost | Source | Details/assumptions |
|---|---|---|---|
| Secondary care costs (relating to case report form items) | |||
| Inpatient ‘hotel cost’ (per night cost) | £200 | East and North Hertfordshire NHS Trust’s Performance Report Month 1168 | |
| Oncology assessment | £181 | Reference Costs 2013–2014 69 | Medical oncology first face-to-face attendance. CC: WF01B. SC: 370 |
| Cardiology assessment | £160 | Reference Costs 2013–2014 69 | Cardiology first face-to-face attendance. CC: WF01B. SC: 320 |
| Respiratory assessment | £186 | Reference Costs 2013–2014 69 | Respiratory medicine first face-to-face attendance. CC: WF01B. SC: 340 |
| Other assessment | £196 | Reference Costs 2013–2014 69 | General medicine first face-to-face attendance. CC: WF01B. SC: 300 |
| Dental assessment | £126 | Reference Costs 2013–2014 69 | Dental medicine first face-to-face attendance. CC: WF01B. SC: 450 |
| Nasopharyngoscopy | £114 | Reference Costs 2013–2014 69 | Diagnostic nasopharyngoscopy, aged ≥ 19 years. CC: CA71A. SC: 370 |
| Fine-needle aspiration | £164 | Reference Costs 2013–2014 69 | Minor maxillofacial procedures. CC: CA95Z. SC: 144 |
| Surgery assessment | £150 | Reference Costs 2013–2014 69 | General surgery first face-to-face attendance. CC: WF01B. SC: 100 |
| CT scan | £147 | Reference Costs 2013–2014 69 | CT scan, more than three areas. CC: RA14Z. SC: DIAGIMOP |
| PET–CT scan | £649 | Reference Costs 2013–2014 69 | Nuclear medicine, category 8 (PET–CT). CC: RA42Z |
| MRI scan | £145 | Reference Costs 2013–2014 69 | MRI scan, one area, post contrast only, aged ≥ 19 years. CC: RA02A. SC: DIAGIMOP |
| Radiography | £40 | Personal communication with LTHT (Dr Peter Hall, University of Leeds, 2015, personal communication) | |
| Ultrasound | £76 | Reference Costs 2013–2014 69 | Ultrasound mobile scan or intraoperative procedures, < 20 minutes. CC: RA25Z. SC: DIAGIMOP |
| Other radiography assessment | £88 | Reference Costs 2013–2014 69 | Clinical oncology (previously radiotherapy) first attendance. CC: WF01D. SC: 800 |
| Nurse assessment | £100 | Unit Costs of Health and Social Care 70 | Assume an assessment is equivalent to 1 hour of contact time |
| Palliative care assessment | £97 | Unit Costs of Health and Social Care 70 | |
| Social other | £37 | Unit Costs of Health and Social Care 70 | Assume equivalent to speech/diet assessment |
| Speech assessment | £37 | Unit Costs of Health and Social Care 70 | Assume an assessment is equivalent to 1 hour of contact time |
| Dietitian assessment | £37 | Unit Costs of Health and Social Care 70 | As above |
| Rehabilitation assessment | £36 | Unit Costs of Health and Social Care 70 | Assume equivalent to 1 hour of occupational therapist contact time |
| Psychology assessment | £138 | Unit Costs of Health and Social Care 70 | Assume hospital assessment cost equivalent to community visit cost |
| Counselling assessment | £50 | Unit Costs of Health and Social Care 70 | As above |
| Peg swab | £7 | Reference Costs 2013–2014 69 | Directly accessed – pathology services. Microbiology. CC: DAPS07 |
| Bloods: haematology | £3 | Reference Costs 2013–2014 69 | Directly accessed – pathology services. Haematology. CC: DAPS05 |
| Bloods: biochemistry | £1 | Reference Costs 2013–2014 69 | Directly accessed – pathology services. Clinical biochemistry. CC: DAPS04 |
| Bloods: microbiology | £7 | Reference Costs 2013–2014 69 | Directly accessed – pathology services. Microbiology. CC: DAPS07 |
| Bloods: other | £8 | Reference Costs 2013–2014 69 | Directly accessed – pathology services. Other. CC: DAPS09 |
| Secondary care costs (relating to patient-reported items) | |||
| Short-stay (≤ 2 days) inpatient cost | £611 | Unit Costs of Health and Social Care 70 | |
| Long-stay (> 2 days) inpatient cost | £2716 | Unit Costs of Health and Social Care 70 | |
| Hospital day centre | £119 | Reference Costs 2013–2014 69 | Inpatient specialist palliative care, same day, aged ≥ 19 years and over. SC: DCRDN. CC: SD02A |
| Outpatient visit | £109 | Unit Costs of Health and Social Care 70 | |
| Accident and emergency visit | £135 | Reference Costs 2013–2014 69 | Total outpatient attendances. SC: 180 |
| Nursing/convalescent home | £82 | Unit Costs of Health and Social Care 70 | Assume cost for 1 day and night equals the reported private-sector nursing home cost per week/7 |
| Primary and community care service costs (relating to patient-reported items) | |||
| GP surgery visit (telephone call) | £46 (28) | Unit Costs of Health and Social Care 70 | |
| GP home visit | £67 | Unit Costs of Health and Social Care 70 | Assume equal to reported cost for 17-minute surgery visit |
| District nurse home visit (telephone call) | £66 (£11) | Unit Costs of Health and Social Care 70 | Assume each visit equal to 1 hour of contact time and a call is equivalent to 10 minutes of contact time |
| Social worker visit (telephone call) | £79 (£13) | Unit Costs of Health and Social Care 70 | As above |
| Physiotherapist visit (telephone call) | £36 (£6) | Unit Costs of Health and Social Care 70 | As above |
| Occupational therapist visit (telephone call) | £36 (£6) | Unit Costs of Health and Social Care 70 | As above |
| Counsellor visit (telephone call) | £50 (£8) | Unit Costs of Health and Social Care 70 | As above |
| Home help service | £24 (£4) | Unit Costs of Health and Social Care 70 | Assume a visit is equal to 1 hour of weekday contact and a call is equivalent to 10 minutes of this time |
| Psychiatrist | £138 | Unit Costs of Health and Social Care 70 | Assume a visit is equal to 1 hour of contact time and a call is equivalent to 10 minutes of contact time |
| Day centre | £24 | Unit Costs of Health and Social Care 70 | Assume equivalent to home help service visit |
| Chemotherapy drug costs | |||
| 5-fluorouracil | £3.47 | Drugs and pharmaceutical electronic market information (eMit), 201571 | 5 g/100-ml vial, 5%, size 1 |
| Cisplatin | £16.69 | Drugs and pharmaceutical electronic market information (eMit), 201571 | 100 mg/100-ml vial |
| Carboplatin | £28.89 | Drugs and pharmaceutical electronic market information (eMit), 201571 | 600 mg/60-ml vial |
| Cetuximab | £890.50 | British National Formulary, 201572 | 5 mg/ml, 100-ml vial |
| Docetaxel | £29.78 | Drugs and pharmaceutical electronic market information (eMit), 201571 | 160 mg/8-ml vial |
| Delivery cost | £328 | Reference Costs 2013–2014 69 | Deliver subsequent elements of a chemotherapy cycle. CC: SB15Z. SC: DCRDN |
| Radiotherapy costs | |||
| Radiotherapy delivery | £149 | Reference Costs 2013–2014 69 | Deliver a fraction of complex treatment on a megavoltage machine. CC: SC23Z. SC: DCRDN |
| Radiotherapy planning visit | £1587 | Reference Costs 2013–2014 69 | Preparation for IMART, with technology support. CC: SC41Z. SC: DCRDN |
| Surgery costs | |||
| Node dissection | £3548 | Reference Costs 2013–2014 69 | Elective inpatient. Intermediate maxillofacial procedures. CC: CA94Z |
| Salvage surgery | £7722 | Reference Costs 2013–2014 69 | Elective inpatient. Major maxillofacial procedures, aged ≥ 19 years, with a complexity and comorbidity score of ≥ 1. CC: CA93A |
| Follow-up visit assessment costs | |||
| Anaesthetic examination | £85 | Reference Costs 2013–2014 69 | Anaesthetics: diagnostic, laryngoscopy or pharyngoscopy, aged ≥ 19 years. CC: CA69A |
| Biopsy | £164 | Reference Costs 2013–2014 69 | Maxillofacial surgery: minor maxillofacial procedures. CC: CA95Z |
| Clinical examination | £109 | Reference Costs 2013–2014 69 | Maxillofacial surgery: Diagnostic, laryngoscopy or pharyngoscopy, aged ≥ 19 years. CC: CA69A |
| Recurrence treatment costs | |||
| Brachytherapy | £2393 | Reference Costs 2013–2014 69 | 1 × preparation for interstitial brachytherapy (£1196). CC: DCRDN. SC: SC55Z |
| 1 × deliver a fraction of intraluminal brachytherapy (£1197). CC: DCRDN. SC: SC30Z | |||
| Chemotherapy course | £4753 | Reference Costs 2013–2014 69 | Six × procure chemotherapy drugs for regimens in band 2 (£323). CC: DCRDN. SC: SB02Z |
| 1 × deliver more complex parenteral chemotherapy at first attendance (£317). CC: DCRDN. SC: SB13Z | |||
| 5 × deliver subsequent elements of a chemotherapy cycle (£328). CC: DCRDN. SC: SB15Z | |||
| 6 × medical oncology follow-up. CC: WF01A. SC: 370 | |||
| Radiotherapy course | £1744 | Assumes 1 radiotherapy planning visit and 1 delivery | |
| Annual supportive care cost post distant recurrence | £1682 | Hall et al., 201473 | |
| Terminal month palliative care cost post distant recurrence | £1051 | Hall et al., 201473 | |
| Resection/free flap reconstruction | £7722 | Reference Costs 2013–2014 69 | Elective inpatient. Major maxillofacial procedures, aged ≥ 19 years , with a complexity and comorbidity score of ≥ 1. CC: CA93A |
| Societal costs (relating to patient- and carer-reported forms) | |||
| Average wage per hour | £15.11 | Office for National Statistics’ Annual Survey of Hours and Earnings, 2014 Provisional Results74 | |
| Cost per mile of car travel | £0.67 | NHS reimbursement data75 | |
Missing data
For the calculation of QALYs, multiple imputation using chained equations was conducted using the ‘mice’ package in R (The R Foundation for Statistical Computing, Vienna, Austria). Data on patient baseline cancer stage, sex and age were used to impute missing EQ-5D data across the data collection time points. A sensitivity analysis was conducted based on a complete-case analysis in order to explore the impact of using imputation (see Within-trial analysis, Uncertainty and sensitivity analyses).
For costs, data on societal, primary and community care resource use and secondary care resource use outside the enrolled hospitals were collected for only a small subset of the overall trial population using patient- and carer-reported questionnaires. As the sample size of this subpopulation was small (patients, n = 42; carers, n = 35), the base-case analysis was conducted using costs derived from the case report forms (collected for the whole trial population and with complete data) only. Sensitivity analyses were conducted, which included the additional resource use items reported in the patient- and carer-reported questionnaires. These forms were relatively complete, with most patients returning their forms at each of the follow-up time points (Table 58). In the case of missing data within each of the forms, values were based on imputation using the mean of reported values. Ideally, in the case of missing data, multiple imputation methods should be employed in order to provide reliable estimates for the missing data values; however, because of the small sample size it is unlikely that complex imputation methods would yield any more reliable results in this instance.
| Follow-up time point | Strategy | |||||
|---|---|---|---|---|---|---|
| ND | PET–CT surveillance | |||||
| Received (n) | Expected (n) | Missing (%) | Received (n) | Expected (n) | Missing (%) | |
| Within 2 weeks of CRT | 39 | 42 | 7 | 38 | 40 | 5 |
| 3 months post-CRT assessment | 37 | 42 | 12 | 38 | 40 | 5 |
| 6 months post randomisation | 36 | 40 | 10 | 36 | 37 | 3 |
| 12 months post randomisation | 36 | 36 | 0 | 37 | 37 | 0 |
| 24 months post randomisation | 34 | 34 | 0 | 35 | 35 | 0 |
Cost-effectiveness analysis
Cost-effectiveness was measured in terms of the ICER and INB. The ICER is calculated by dividing the difference in mean cost between the two arms by the difference in mean QALYs between the two arms:
where Ci and Ei are the expected cost and effectiveness of the intervention (PET–CT surveillance), Cc and Ec are the expected cost and effectiveness of the comparator strategy (planned ND), and ΔC and ΔE are the incremental cost and effect of the intervention compared with the comparator.
When the new intervention dominates the standard care strategy (i.e. is more effective and less costly), or when the new intervention is dominated by the standard care strategy (i.e. is less effective and more costly), the decision of whether or not to adopt the new strategy is straightforward (accept and reject, respectively). In these cases the ICER calculation is meaningless. The ICER becomes important when we are considering adopting an intervention that requires a trade-off between additional effect for additional cost and less effect for less cost. Assuming that a new intervention is more costly and more effective than the current standard care strategy, the ICER represents the additional cost associated with the intervention per additional unit of benefit (QALY) that it provides compared with current treatment; alternatively, if the intervention is less costly and less effective than the standard care intervention, the ICER represents the cost saved per QALY lost compared with current treatment. The cost-effectiveness of an intervention is then determined by whether or not the ICER value falls above or below the decision-maker’s willingness to pay per QALY. In the UK, NICE adopts a willingness-to-pay threshold value of £20,000 per QALY: if a new intervention has an ICER value below £20,000 per additional QALY (or > £20,000 saved per QALY lost), then it is likely to be considered a cost-effective use of NHS resources, whereas an ICER value above £20,000 per additional QALY (or < £20,000 saved per QALY lost) indicates that the intervention is not expected to be a cost-effective use of resources. For the case of positive incremental cost and QALYs (i.e. the intervention is more effective and more costly), this decision rule is expressed in the following formula:
where λ is the adopted willingness-to-pay threshold (£ per QALY).
When the threshold is known, cost-effectiveness can be expressed in terms of net health benefit (NB). The NB for the given intervention (NBi) and comparator strategy (NBc) and the INB of the intervention are calculated as follows:77
The NB equation expresses the overall benefit of a strategy in terms of QALYs by converting the expected cost of the intervention on to the QALY scale using the given threshold value. Unlike the ICER, for which interpretation depends on whether or not the incremental cost and QALYs are positive or negative, the interpretation of NB is straightforward: for any given set of strategies, the strategy with the highest net benefit is the most cost-effective; equivalently, a strategy is cost-effective if its INB is positive. All results of the economic evaluation are reported in terms of both ICER and NB values.
Uncertainty and sensitivity analyses
Non-parametric bootstrapping was used to determine the level of sampling uncertainty around the WT cost-effectiveness result by generating 10,000 estimates of incremental costs and benefits from the trial results. The bootstrap approach considers the original sample as if it is the population and draws multiple random samples with replacements from the original sample in order to simulate possible alternative sample sets. Results are presented using scatterplots on the cost-effectiveness plane (which plots incremental QALYs against incremental costs) to illustrate the uncertainty surrounding the cost-effectiveness estimates.
On the cost-effectiveness plane a result is considered cost-effective if it falls on or below the given cost-effectiveness willingness to pay per QALY threshold. The cost-effectiveness acceptability curve is derived by calculating the proportion of bootstrapped estimates that are cost-effective across a range of willingness-to-pay thresholds, to show the probability that PET–CT-guided treatment is cost-effective across different threshold values.
For the WT analysis, three additional sensitivity analyses were conducted to explore additional uncertainties in the analysis.
Within-trial sensitivity analysis 1: imputation of additional patient-reported cost data (NHS and Personal Social Services perspective)
In the base-case analysis, costs were calculated using data from the trial case report forms that provided information on a range of secondary care resource usage for the total trial population (n = 564). Additional data on primary and community care resource use, as well as additional secondary care resource usage (outside the enrolled oncology department), were collected for a subgroup of the trial population (n = 42) enrolled at the two main recruiting centres (UHB and UHCW). Patients were asked to recall their use of NHS and PSS services over the past 3 months (or since completion of the last form when appropriate) at baseline, during treatment (2 weeks post CRT), and at 3, 6, 12 and 24 months post randomisation. In the base-case analysis this information was excluded because of the small sample size available for these data and the significant uncertainty incorporated in any attempt to impute these data to the whole trial population. A sensitivity analysis was conducted to assess the potential impact of including these additional cost data by imputing the mean reported values for the additional resource use items collected in the patient-reported forms to the total trial population. Mean values were calculated using a complete-case analysis of the patient- and carer-reported data. Owing to the small sample size and significant number of missing data, it was deemed inappropriate to attempt to conduct multiple imputation for missing data.
On the patient-reported questionnaires, patients were asked to report any additional visits to hospital (inpatient, day centre, outpatient, accident and emergency department or nursing home visits), not including visits to their enrolled oncology department; the aim was to capture additional secondary care resource use not already captured in the case report forms routinely completed at the patient’s enrolled oncology department. However, when additional hospital visits were reported, patients were asked to give the name of the hospital they visited, and a significant number of patients identified the hospital as UHB or UHCW. The majority of patients did not specify the department visited, so it is unclear if these hospital visits constituted unique events that have not already been captured in the case report forms. Two analyses were therefore conducted:
-
WT sensitivity analysis 1.1: in this analysis it was assumed that patients correctly reported their use of secondary care resources, that is, if the hospital site was reported as UHB or UHCW, patients reported only those hospital events that occurred outside the oncology department and, therefore, each reported event represented a unique resource use item not already contained within the case report forms
-
WT sensitivity analysis 1.2: in this analysis it was assumed that for any instance in which patients identified the hospital visited as either UHB or UHCW these data would already have been captured in the case report forms and these events are therefore excluded from the analysis.
Within-trial sensitivity analysis 2: imputation of additional patient- and carer-reported societal costs (societal perspective analysis)
For the subgroup of patients enrolled at UHB and UHCW, patients and carers were asked to report on the impact of the patient’s illness on travel expenses, one-off expenses, equipment costs and lost productivity (i.e. lost earnings), at the same time points as for the patient questionnaires discussed in WT sensitivity analysis 1 (baseline, treatment and 3, 6, 12 and 24 months post randomisation). A sensitivity analysis was conducted to assess the potential impact of including these additional cost data, in addition to the costs included in WT sensitivity analysis 1, by imputing the mean reported values for the additional resource use items collected in the patient- and carer-reported forms to the total trial population. Owing to the fact that the additional costs included in WT sensitivity analysis 1 have been split into two analyses (WT sensitivity analysis 1.1 and sensitivity analysis 1.2), two corresponding analyses have been conducted from a societal perspective:
-
WT sensitivity analysis 2.1, based on inclusion of societal costs plus additional patient-reported costs as given in WT sensitivity analysis 1.1 (i.e. using patient-reported costs as reported)
-
WT sensitivity analysis 2.2, based on inclusion of societal costs plus additional patient-reported costs as given in WT sensitivity analysis 1.2 (i.e. excluding patient-reported secondary care visits where the attended hospital was identified as one of the enrolled hospitals).
Within-trial sensitivity analysis 3: complete-case analysis for calculation of quality-adjusted life-years
In the base-case analysis, multiple imputation methods were used in order to account for missing EQ-5D data within the trial. A sensitivity analysis was conducted using complete-case analysis for the calculation of QALYs in each arm (i.e. ignoring missing data).
Lifetime decision model analysis
In order to estimate the lifetime cost-effectiveness of PET–CT-guided management, a de novo decision-analytical model was constructed. In line with the WT analysis, the base-case model adopts a UK secondary care perspective and future costs and QALYs were discounted at an annual rate of 3.5% in line with NICE guidance. 63 A sensitivity analysis was conducted in which a broader NHS and PSS perspective was adopted. The model was built and analysed using R software.
Model structure
The model structure (Figure 28) is split into two phases: an initial 6-month treatment phase, in which patients receive CRT and (potential) ND; and a follow-up phase, in which patients may go on to recover, develop local recurrence (LR) or distant recurrence (DR), or die. Within the treatment period, cost and QALYs for each of the treatment arms are taken directly from the results of the first 6 months of the WT analysis. For the follow-up period, longer-term cost and QALYs for each of the treatment arms are estimated using a modified Markov model. The model begins at this point in order to enable the analysis to capture the full cost and utility decrements associated with LRs and DRs, as these data were not fully captured in the trial.
FIGURE 28.
Lifetime DM structure.
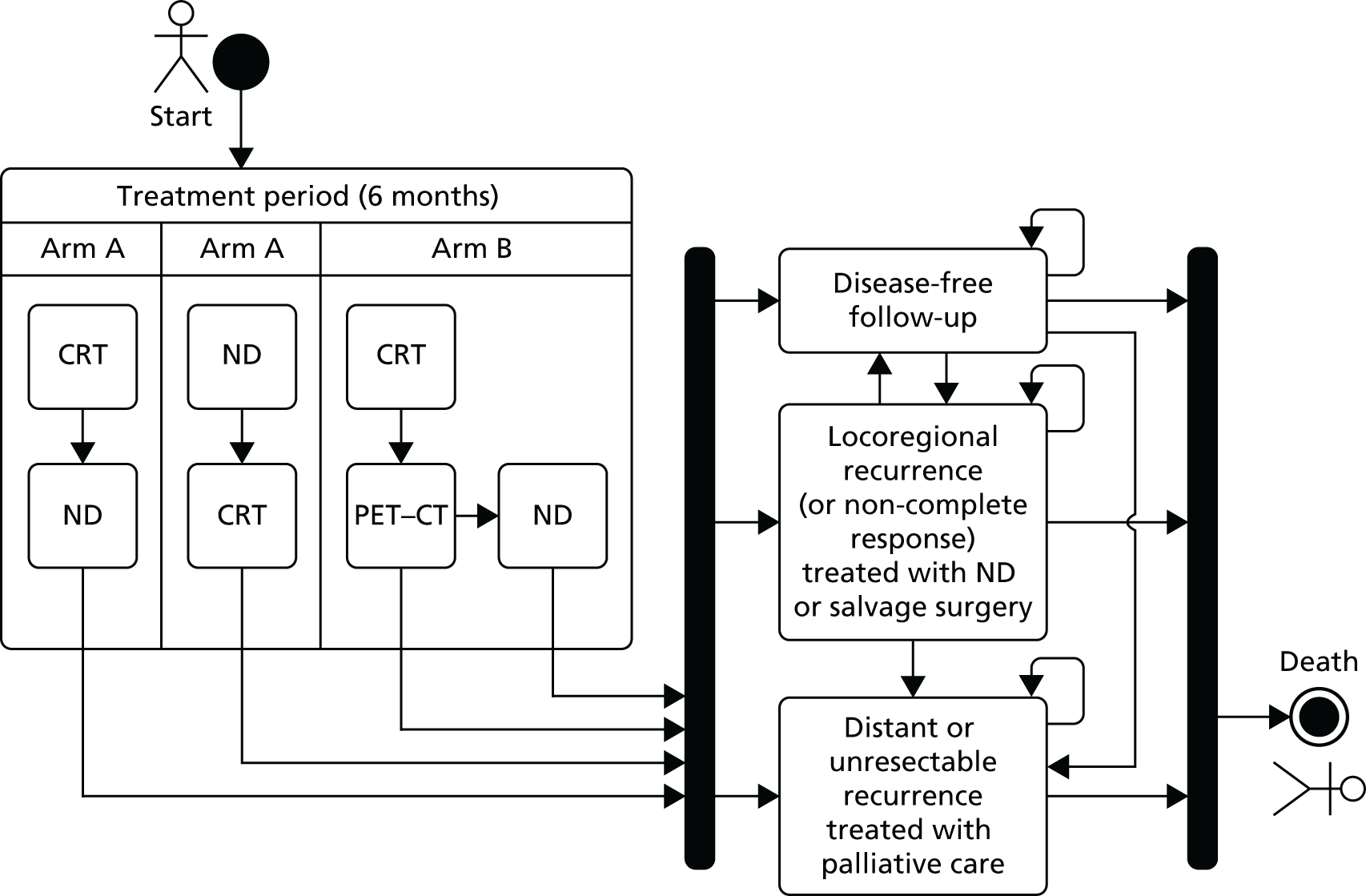
Markov models describe patient progression over time through a pathway of health states, with movement between the health states being triggered by events such as disease progression or death. Resource use and costs are associated with each health state, and patients accumulate costs and health benefits in each state over monthly cycles. The analysis adopts a lifetime horizon, truncated at 100 years.
During the treatment phase of the model, patients in the standard care arm (arm A) receive planned ND either before or after CRT. Patients in the intervention arm (arm B) receive CRT followed by PET–CT at 9–13 weeks post CRT, which dictates whether or not patients go on to receive ND. In the subsequent follow-up phase of the model, patients enter one of four health states: disease free (DF), LR, DR or death. During any cycle in the follow-up period patients in the DF state may remain DF, develop a LR or DR, or die. Similarly, patients in the LR state may recover, develop a DR or die, and patients in the DR state may remain in DR or die. Entry into the LR state is associated with a one-off treatment cost and utility decrement, after which patients either recover (with costs and utility equal to those in the DF state) or advance to the DR state. Patients who enter the DR state similarly incur a one-off treatment cost and further utility decrement and, subsequently, either remain in the DR state (with an ongoing supportive care cost and utility decrement) or die. Once in the DR state, it is assumed that patients cannot recover and either remain in the DR state or die.
Model parameters
The model input parameters were derived using information from a range of sources. When possible, data from the trial were used directly and, when necessary, published literature was used to inform remaining parameters.
For the 6-month initial treatment phase of the model, costs and QALYs for each of the arms were taken directly from the trial-reported data. Resource use items relevant to the treatment phase (i.e. surgery, radiotherapy, chemotherapy, treatment response assessment and adverse events and recurrence) were assigned costs and evaluated, as discussed earlier (see Methods, Within-trial analysis). Bootstrap methods were used to account for sampling uncertainty around the results.
For the follow-up modified Markov phase of the model, the proportion of patients beginning in each of the health states of the model was derived directly from the trial data using data on OS, recurrence-free survival and types of recurrences experienced.
The cost for the DF state was derived from the trial data, based on calculating the average monthly cost in each arm for DF patients over the follow-up period (6–24 months). The cost of LR and DR treatment (applied to patients’ first cycle in each of the recurrence states) was derived from the trial data on recurrence treatments administered on patients’ first recurrence event. No data were available from the trial regarding the ongoing treatment of patients who experienced a recurrence event; the subsequent cost of LR and DR beyond initial treatment is therefore uncertain. For patients who experienced a LR and, subsequently, recovered after treatment, ongoing costs were assumed to be equal to those for patients in the DF state. For patients remaining in the DR state it was assumed that patients would incur an ongoing supportive care cost, which was derived from the literature. 73
For the health state utilities, DF utility was derived directly from the trial data by calculating the average utility for patients in the trial who were DF over the second year trial follow-up period (12–24 months). There were no data available from the trial from which the utility of patients experiencing a LR or DR could be derived. Several studies were identified in the literature that have explored health-related quality-of-life for patients with H&N cancers. Most of these studies were deemed inappropriate for the derivation of the current model recurrence health states because the majority either reported only utilities derived from the EORTC QLC-C30 questionnaire (a cancer-specific health-related quality-of-life questionnaire), which cannot be easily converted in to EQ-5D utilities,78–81 or did not report health state utilities that could be reliably mapped to the health states used in the current model. 82 One UK study83 was identified. However, this study calculated EQ-5D utilities from a sample of 50 oncology nurses; because health-care professionals are known to be poor proxies for patients, this study was similarly excluded from consideration for the base-case analysis. de Almeida et al. 84 conducted an elicitation of utilities from a sample of 50 healthy Canadian subjects using both standard gamble and visual analogue scale (VAS) methods. The authors report utility values for a range of health states, including several remission, LR and DR states. As this study was deemed to be the best-quality study identified in the literature, it was used to derive the recurrence health state values for the current DM. Probabilistic utility decrement values for the local and DR health states were derived by sampling from the remission, LR and DR distributions in the de Almeida et al. 84 study and taking the resulting mean differences and standard error values. The base-case analysis uses the reported utilities in de Almeida et al. 84 based on the standard gamble elicitation. A sensitivity analysis was conducted using the VAS-reported values.
The mortality risk within the DF, LR and DF after LR states was assumed to be equal to the mortality risk within the general population (taken from Office for National Statistics 2013 statistics85) multiplied by an excess mortality factor of 20% derived from the literature. 86 The mortality risk within the DR state was derived by calibrating the model survival curve with the Kaplan–Meier survival data from the trial. The rate of disease progression from the DF state to LR and DR within the first 5 years was derived directly from the trial data, using the trial recurrence-free survival Kaplan–Meier data to derive monthly primary recurrence transition probabilities, accounting for loss to follow-up. Stochastic primary recurrence probabilities were derived by simulating 10,000 bootstrap data samples from the original Kaplan–Meier data (i.e. sample with replacement), which were used to derive separate recurrence probabilities for each of the 10,000 model simulations in the probabilistic analysis (see Lifetime decision model analysis, Uncertainty and sensitivity analyses). In the base-case analysis recurrence events were assumed to occur only within the first 5 years post randomisation, based on observations within the literature, which indicate that the majority of recurrences occur within the first 5 years from initial diagnosis. 87 This assumption was also supported by the trial follow-up data, which indicated that the number of recurrence events had fallen close to zero at 5 years post treatment. A sensitivity analysis was conducted to assess the potential impact of recurrences beyond 5 years (see Lifetime decision model analysis, Uncertainty and sensitivity analyses). The proportion of patients experiencing a LR or DR in each arm was derived from the trial data by calculating the relative proportion of LR versus DR events within the trial. This proportion was assumed to be constant over time in the model.
There were no data available from the trial regarding the rate of progression from LR and DF after LR to subsequent LR or DR (i.e. only primary recurrences were captured in the trial follow-up). The rate of subsequent recurrences were therefore derived from a recent study88 that reported observed LRs and DRs in 176 patients with local recurrent disease after primary curative treatment of HNSCC.
A full list of model parameters and distributions applied in the base-case model is given in Table 59.
| Parameter | Mean | Distribution | SD | Source |
|---|---|---|---|---|
| Global parameters | ||||
| Discount rate | 0.035 | Fixed | – | NICE guidance63 |
| Start age (years) | 57 | Fixed | – | PET-NECK baseline trial data |
| Percentage male | 82% | Fixed | – | |
| Health state starting distribution (i.e. end of 6-month treatment period) | ||||
| ND | ||||
| Recurrence | 0.06 | Beta | 0.015 | PET-NECK trial data |
| Proportion of recurrences local (vs. distant) | 0.35 | Beta | 0.069 | |
| Dead | 0.03 | Beta | 0.010 | |
| PET–CT | ||||
| Recurrence | 0.05 | Beta | 0.013 | PET-NECK trial data |
| Proportion of recurrences local (vs. distant) | 0.41 | Beta | 0.066 | |
| Dead | 0.02 | Beta | 0.008 | |
| Monthly health state costs | ||||
| DF | £70 | Gamma | £95 | PET-NECK trial data on DF patients (n = 439) |
| LR initial treatment | £3964 | Gamma | £4343 | PET-NECK trial data on LR patients (n = 39) |
| DF after LR | £70 | Gamma | £95 | Assumed equivalent to DF cost |
| DR initial treatment | £3635 | Gamma | £3197 | PET-NECK trial data on DR patients (n = 63) |
| DR follow-up (ongoing care) | £140 | Gamma | £32 | Hall et al., 201473 |
| Terminal-month cost | £1051 | Gamma | £115 | Hall et al., 201473 |
| Health state utilities | ||||
| DF | 0.71 | Beta | 0.04 | PET-NECK trial data on DF patients during second year of follow-up |
| LR decrement | –0.11 | Beta | 0.12 | de Almeida et al., 201484 |
| DF after LR | 0.71 | Beta | 0.03 | Assumed equivalent to DF utility |
| DR decrement | –0.47 | Beta | 0.20 | de Almeida et al., 201484 |
| Dead | 0 | Fixed | – | |
| Transition probabilities/effects | ||||
| Primary recurrence over first 5 years | Derived from the trial Kaplan–Meier curves for recurrence-free survival in each arm | PET-NECK trial data | ||
| Proportion of recurrences local (vs. distant) in planned ND arm | 0.35 | Beta | 0.069 | PET-NECK trial |
| Proportion of recurrences local (vs. distant) in PET–CT surveillance arm | 0.41 | Beta | 0.066 | |
| Probability of LR from DF after LR state | 0.02 | Beta | 0.002 | Matoscevic et al., 201488 |
| Probability of DR from LR/DF after LR states | 0.02 | Beta | 0.003 | Matoscevic et al., 201488 |
| Baseline mortality in DF and LR states | Life table | Fixed | – | Office for National Statistics,85 2013 age- and sex-standardised rates |
| Excess mortality factor for DF and LR states | 1.20 | Fixed | – | van der Schroeff et al., 201086 |
| Mortality in DR state | 0.30 | Beta | 0.30 | Calibration of model survival curve against the trial survival data |
Cost-effectiveness analysis
As for the WT analysis, cost-effectiveness was measured in terms of the ICER and INB (see Lifetime decision model analysis, Uncertainty and sensitivity analyses for more details on the calculation of ICERs and INB).
Uncertainty and sensitivity analyses
Probabilistic sensitivity analyses were conducted to assess the impact of joint parameter uncertainty on the results. Probabilistic analysis accounts for joint parameter uncertainty in non-linear models by assigning probability distributions to each of the input parameters and randomly drawing from these probabilities over 10,000 Monte Carlo model simulations to produce different cost and QALY estimates in each simulation of the model. As for the bootstrap WT analysis, the results are presented on the cost-effectiveness plane as a scatterplot, using cost-effectiveness acceptability curves to show the probability that the two strategies are cost-effective across different willingness-to-pay per QALY thresholds.
One-way sensitivity analyses were conducted to test the impact of changes in individual parameter estimates on the results. Individual parameters were altered by ± 25% from their baseline value, with the impact on the expected INB reported in a tornado diagram.
For the lifetime DM analysis, three additional sensitivity analyses were conducted to assess the impact of key assumptions used in the model.
Decision model sensitivity analysis 1: imputation of additional patient-reported cost data (NHS and personal social services perspective)
As for the WT cost-effectiveness analysis, the base-case DM analysis was based on utilisation of data from the trial relating to patients’ consumption of NHS secondary care resources, using the trial case report forms, which were collected for the total trial population. In line with the sensitivity analyses conducted in the WT sensitivity analysis 1.1 and sensitivity analysis 1.2 (see Within-trial analysis, Uncertainty and sensitivity analyses), two corresponding sensitivity analyses were conducted in the model analysis to assess the impact of including additional patient-reported cost data:
-
DM sensitivity analysis 1.1: in this analysis the treatment-phase costs for the model were taken from the treatment-phase costs generated from the WT sensitivity analysis that included the additional patient-reported cost data, assuming that patients correctly reported their use of additional secondary care resources (i.e. corresponding to analysis WT sensitivity analysis 1.1). Similarly, the monthly cost of the DF state was recalculated using the results of WT sensitivity analysis 1.1. In addition, the average monthly patient-reported cost associated with the use of primary, community and additional secondary care (i.e. outside the enrolled oncology department) was applied to the local and DR health states. As this is likely to underestimate the primary and community care costs for these states (because the added cost data are derived from primarily DF patients), no other data were available to determine the exact cost of primary and community care for recurrent patients.
-
DM sensitivity analysis 1.2: this analysis involved the same steps as outlined in DM sensitivity analysis 1.1 above, but instead used data from the WT sensitivity analysis, including additional patient-reported data and excluding secondary care resource use where patients identified the visited hospital as one of the trial centres (i.e. WT sensitivity analysis 1.2; see Within-trial analysis, Uncertainty and sensitivity analyses for further details).
Decision model sensitivity analysis 2: visual analogue scale utility values for the local recurrence and distant recurrence states
In the base-case analysis, utility in the recurrence health states was derived using data reported in de Almeida et al. ,84 based on an elicitation of utility values using the standard gamble method. The authors also reported utilities based on using the VAS tool. A sensitivity analysis was conducted using these alternative values in order to assess the impact of the method of utility derivation on the results.
Decision model sensitivity analysis 3: recurrences beyond 5 years post diagnosis estimated using a parametric survival curve
In the base-case analysis, no recurrence events were assumed to occur beyond 5 years in the model. A sensitivity analysis was conducted assuming that recurrences could occur beyond 5 years: long-term recurrence probabilities for the planned ND arm were estimated using a Gompertz parametric survival curve fitted to the trial baseline Kaplan–Meier data on recurrence-free survival; a HR was then applied to this curve in order to derive DF survival within the PET–CT surveillance arm (using the HR observed across the trial follow-up period), as outlined in Briggs et al. 89 The Gompertz distribution was identified as the best-fitting curve to estimate long-term recurrence events (compared with the exponential, Weibull, gamma and log-normal distributions), based on an analysis of the Akaike information criterion (AIC) and Bayesian information criterion (BIC) (Table 60; better-fitting curves are indicated by lower AIC and BIC values) and a visual inspection of the curve fits (Figure 29).
| Trial arm | Model specification | |
|---|---|---|
| AIC | BIC | |
| Planned ND | ||
| Exponential | 679.50 | 683.14 |
| Gamma | 674.96 | 682.96 |
| Weibull | 673.83 | 681.11 |
| Log-normal | 665.86 | 673.14 |
| Gompertz | 659.03 | 666.31 |
| PET–CT surveillance | ||
| Exponential | 734.08 | 737.72 |
| Gamma | 735.58 | 742.86 |
| Weibull | 735.16 | 742.44 |
| Gompertz | 727.89 | 735.17 |
| Log-normal | 726.19 | 733.48 |
FIGURE 29.
Plot of parametric survival curves (using Gompertz and log-normal model specifications) against trial data on patient recurrence-free survival. (a) Planned ND arm; and (b) PET–CT surveillance arm. Log-normal and Gompertz specifications are shown here, as these were the two models identified as having the best fit via an analysis of AIC and BIC. The Gompertz curve was used in the sensitivity analysis, as this had the best overall fit when taking into account AIC, BIC and a visual inspection of the goodness of fit.
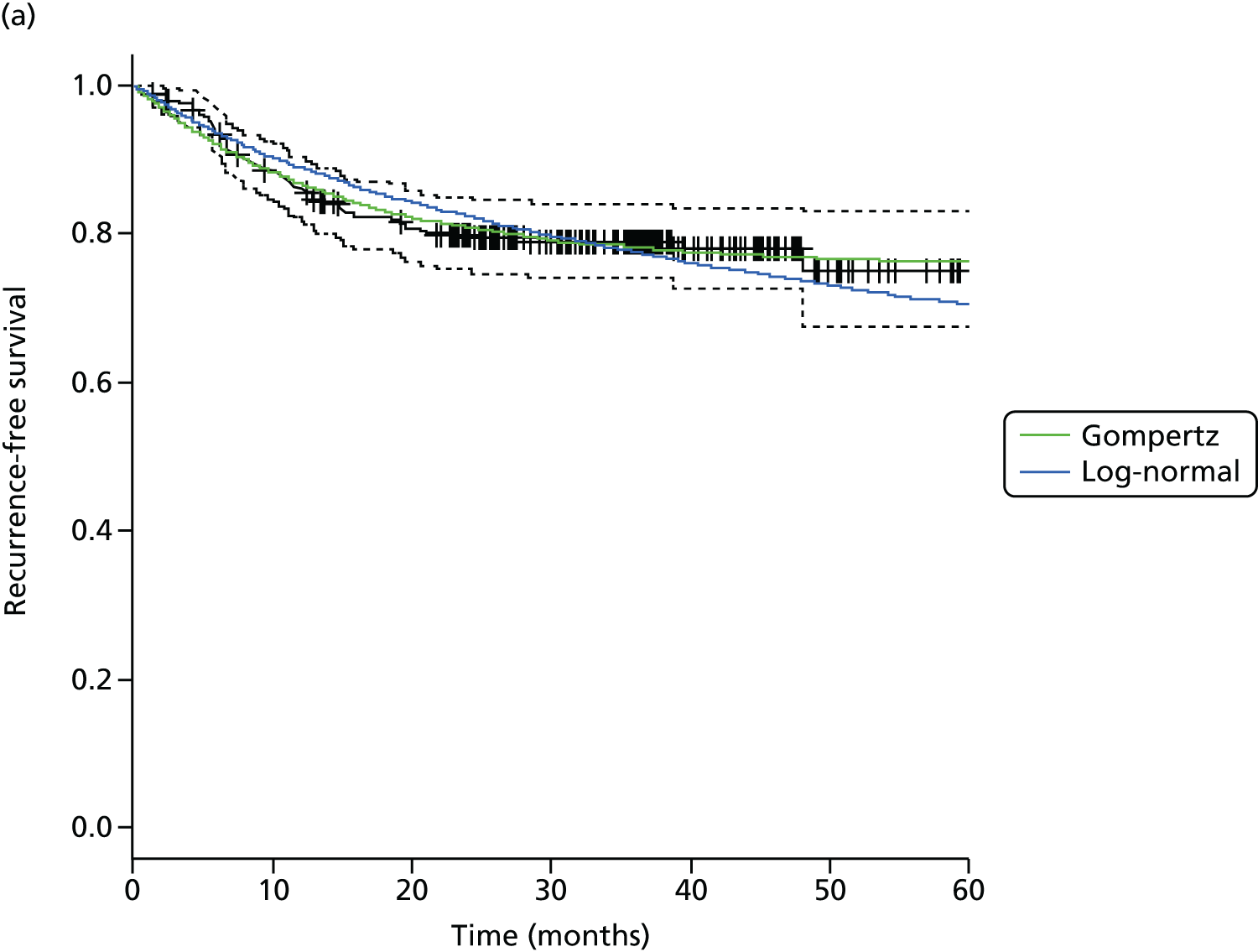
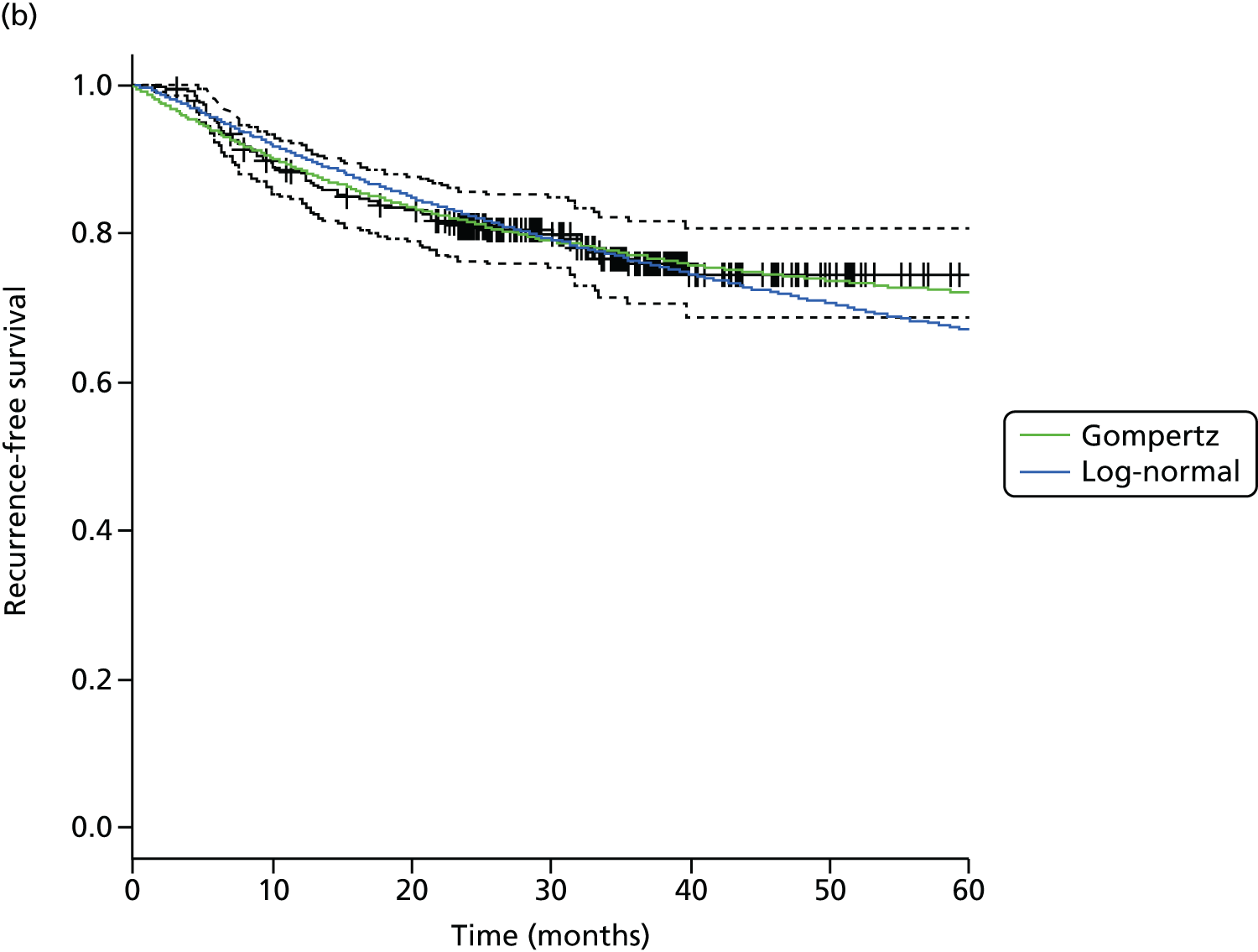
Results
Within-trial analysis
Within-trial costs and utilities
Costs: base case (NHS secondary care perspective)
A summary of the 2-year WT costs included in the base-case analysis (i.e. NHS secondary care costs) is provided in Table 61.
| Cost category | Strategy (£) | Mean difference (£) | p-value (Wilcoxon signed-rank test) | |||||
|---|---|---|---|---|---|---|---|---|
| Planned ND (n = 282) | PET–CT (n = 282) | |||||||
| Mean | Median | SD | Mean | Median | SD | |||
| Treatment period (0–6 months) | ||||||||
| Surgery | 2542 | 3566 | 1616 | 303 | 0 | 997 | –2238 | 0.000 |
| Radiotherapy | 5768 | 6087 | 1677 | 5963 | 6087 | 1334 | 194 | 0.451 |
| Chemotherapy: concomitant | 1444 | 724 | 1811 | 1527 | 764 | 1837 | 82 | 0.361 |
| Chemotherapy: neoadjuvant | 774 | 0 | 1252 | 922 | 0 | 1313 | 148 | 0.224 |
| Treatment assessment | 437 | 508 | 183 | 354 | 360 | 123 | –83 | 0.000 |
| PET–CT | 69 | 0 | 230 | 833 | 652 | 358 | 763 | 0.000 |
| SAE | 1167 | 0 | 2081 | 655 | 0 | 1185 | –512 | 0.010 |
| Recurrence | 88 | 0 | 633 | 21 | 0 | 151 | –67 | 0.166 |
| Total treatment period cost | 12,289 | 12,052 | 3969 | 10,578 | 10,081 | 3155 | –1711 | 0.000 |
| Follow-up period (6–24 months) | ||||||||
| Surgery | 266 | 0 | 938 | 467 | 0 | 1203 | 201 | 0.029 |
| Radiotherapy | 24 | 0 | 407 | 0 | 0 | 0 | –24 | 0.319 |
| Follow-up assessments | 416 | 484 | 223 | 449 | 484 | 192 | 33 | 0.271 |
| PET–CT | 70 | 0 | 237 | 103 | 0 | 274 | 34 | 0.065 |
| SAE | 429 | 0 | 1316 | 208 | 0 | 706 | –221 | 0.146 |
| Recurrence | 496 | 0 | 1846 | 671 | 0 | 2181 | 175 | 0.248 |
| Total follow-up costs | 1700 | 542 | 5041 | 1898 | 589 | 2970 | 198 | 0.243 |
| Total costs (0–24 months) | ||||||||
| Surgery | 2807 | 3566 | 1462 | 770 | 0 | 1468 | –2037 | 0.000 |
| Radiotherapy | 5793 | 6087 | 1643 | 5963 | 6087 | 1334 | 170 | 0.533 |
| Chemotherapy: concomitant | 1444 | 724 | 1811 | 1527 | 764 | 1837 | 82 | 0.361 |
| Chemotherapy: neoadjuvant | 774 | 0 | 1252 | 922 | 0 | 1313 | 148 | 0.224 |
| Treatment assessment | 437 | 508 | 183 | 354 | 360 | 123 | –83 | 0.000 |
| Follow-up assessments | 416 | 484 | 223 | 629 | 652 | 121 | 214 | 0.271 |
| PET–CT | 139 | 338 | 0 | 936 | 652 | 464 | 797 | 0.000 |
| SAE | 1596 | 0 | 3057 | 863 | 0 | 1690 | –733 | 0.609 |
| Recurrence | 584 | 0 | 1932 | 693 | 0 | 2181 | 109 | 0.609 |
| Total overall cost | 13,989 | 13,278 | 5041 | 12,476 | 11,940 | 4613 | –1513 | 0.000 |
The total per-patient costs over the 2-year trial follow-up period were significantly higher in the standard care (planned ND) arm than in the PET–CT surveillance arm (mean £13,989 vs. £12,476; p = 0.000). This is primarily because of the higher up-front cost of surgery (£2542 vs. £303) as well as higher adverse event costs (£1167 vs. £655), which result in higher treatment-phase costs for planned ND than for PET–CT surveillance (£12,289 vs. £10,578). In contrast, subsequent costs in the follow-up period (6–24 months post randomisation) were non-significantly higher in the PET–CT arm (mean £1898 vs. £1700, p = 0.24). This is mainly because of higher costs associated with surgery (£467 vs. £266) and recurrence (£671 vs. £496) in the PET–CT arm during the follow-up phase.
Costs: sensitivity analysis 1 (NHS and Personal Social Services perspective)
A summary of the patient-reported costs used in sensitivity analysis 1 is presented in Table 62. These costs include patient-reported primary, (additional) secondary and community care costs collected for a subset (n = 42) of patients enrolled at the two main recruiting centres (UHB and UHCW). WT sensitivity analysis 1.1 includes patient-reported costs as they were reported; WT sensitivity analysis 1.2 excludes hospital visits for patients who identified the visited hospital as either UHB or UHCW (because of the likelihood that these events will be duplicated in the trial case report forms).
| Questionnaire time point | Strategy (£) | Mean difference (£) | p-value (Wilcoxon signed-rank test) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Planned ND | PET–CT surveillance | |||||||||
| n | Mean | Median | SD | n | Mean | Median | SD | |||
| Sensitivity analysis 1.1: analysis using patient data as reported | ||||||||||
| Treatment period | 17 | 3170 | 3261 | 2059 | 25 | 2244 | 1191 | 1994 | –926 | 0.118 |
| 3 months post randomisation | 15 | 3225 | 3183 | 1898 | 20 | 1098 | 1007 | 501 | –2127 | 0.000 |
| 6 months post randomisation | 4 | 2009 | 2166 | 1281 | 10 | 1076 | 928 | 814 | –933 | 0.240 |
| 12 months post randomisation | 16 | 1224 | 825 | 1406 | 21 | 1125 | 872 | 1137 | –99 | 0.963 |
| 24 months post randomisation | 15 | 1183 | 797 | 1406 | 14 | 1087 | 843 | 1099 | –96 | 0.963 |
| Totala | – | 18,245 | 18,245 | 1469 | – | 13,869 | 13,869 | 1494 | –4377 | 0.000 |
| Sensitivity analysis 1.2: analysis excluding patient-reported secondary care resource usage in which the visited hospital was indicated as UHB or UHCW | ||||||||||
| Treatment period | 17 | 962 | 753 | 667 | 25 | 1032 | 846 | 720 | 70 | 0.547 |
| 3 months post randomisation | 15 | 1216 | 787 | 914 | 20 | 883 | 787 | 304 | –333 | 0.802 |
| 6 months post randomisation | 4 | 855 | 843 | 502 | 10 | 748 | 751 | 358 | –107 | 0.733 |
| 12 months post randomisation | 16 | 1121 | 721 | 1430 | 21 | 935 | 707 | 684 | –186 | 0.914 |
| 24 months post randomisation | 15 | 1083 | 696 | 667 | 14 | 904 | 683 | 661 | –179 | 0.929 |
| Totala | – | 11,291 | 11,219 | 1355 | – | 10,193 | 10,193 | 810 | –1098 | 0.000 |
For both analyses the total mean patient-reported costs were higher in the planned ND arm; however, the cost difference was greater in WT sensitivity analysis 1.1 (£18,245 vs. £13,869) than in WT sensitivity analysis 1.2 (£11,291 vs. £10,193). Note that because of the small numbers of completed questionnaires at each time point, particularly within the planned ND arm, these results are subject to significant sampling uncertainty (as is reflected in the large standard deviation values) and should therefore be interpreted with caution.
Costs: sensitivity analysis 2 (societal perspective)
A summary of the patient- and carer-reported societal costs used in sensitivity analysis 2 is presented in Table 63. These costs include reported equipment costs, one-off expenses, transport costs and lost earnings. As for the patient-reported costs reported in Table 62, the reported societal costs were higher in the planned ND arm than in the PET–CT surveillance arm, and this difference was most substantial for the patient-reported costs (total patient costs £17,136 vs. £11,567; total carer costs £6762 vs. £5388). However, as for the previous sensitivity analysis, these costs should be interpreted with caution given the substantial uncertainty because of the small sample sizes contributing to each of the cost estimates.
| Questionnaire time point | Strategy (£) | Mean difference | p-value (Wilcoxon signed-rank test) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Planned ND | PET–CT surveillance | |||||||||
| n | Mean | Median | SD | n | Mean | Median | SD | |||
| Sensitivity analysis 2: patient costs | ||||||||||
| Treatment period | 17 | 1951 | 1211 | 3026 | 25 | 1547 | 34 | 2530 | –404 | 0.421 |
| 3 months post randomisation | 15 | 3462 | 58 | 6756 | 20 | 2804 | 20 | 5193 | –657 | 0.891 |
| 6 months post randomisation | 3 | 3475 | 1200 | 5468 | 10 | 940 | 103 | 1394 | –2535 | 0.942 |
| 12 months post randomisation | 16 | 1932 | 482 | 2979 | 21 | 1831 | 1 | 3170 | –101 | 0.747 |
| 24 months post randomisation | 15 | 286 | 0 | 871 | 14 | 125 | 0 | 458 | –161 | 0.649 |
| Totala | – | 17,136 | 17,136 | 4018 | – | 11,567 | 11,567 | 4013 | –5569 | 0.000 |
| Sensitivity analysis 2: carer costs | ||||||||||
| Treatment period | 13 | 1644 | 1688 | 859 | 22 | 1732 | 1365 | 1159 | 88 | 0.733 |
| 3 months post randomisation | 10 | 445 | 414 | 135 | 16 | 849 | 528 | 760 | 405 | 0.861 |
| 6 months post randomisation | 3 | 1538 | 1723 | 395 | 8 | 416 | 209 | 527 | –1122 | 0.085 |
| 12 months post randomisation | 10 | 381 | 302 | 469 | 14 | 451 | 253 | 516 | 70 | 0.861 |
| 24 months post randomisation | 6 | 294 | 212 | 284 | 11 | 276 | 119 | 347 | –18 | 0.733 |
| Totala | – | 6762 | 6762 | 429 | – | 5388 | 5388 | 660 | –1374 | 0.000 |
Utilities
A summary of the mean utilities in each arm over time based on both the base-case analysis (multiple imputation for missing data) and sensitivity analysis 3 (complete-case analysis) is given in Table 64. Patient-reported utilities were largely similar between the two arms, with the largest difference occurring at 3 months post randomisation.
| Time point | Statistic | Strategy (sensitivity analysis 3) | p-value (base case; Wilcoxon signed-rank test) | |
|---|---|---|---|---|
| Planned ND | PET–CT | |||
| Baseline | Mean | 0.76 (0.76) | 0.76 (0.77) | 0.884 |
| SD | 0.24 (0.23) | 0.24 (0.23) | ||
| Median | 0.80 (0.80) | 0.80 (0.80) | ||
| Treatment | Mean | 0.55 (0.50) | 0.49 (0.52) | 0.764 |
| SD | 0.27 (0.30) | 0.31 (0.30) | ||
| Median | 0.62 (0.62) | 0.62 (0.62) | ||
| 3 months post randomisation | Mean | 0.35 (0.41) | 0.67 (0.64) | 0.000 |
| SD | 0.39 (0.38) | 0.31 (0.32) | ||
| Median | 0.19 (0.62) | 0.73 (0.73) | ||
| 6 months post randomisation | Mean | 0.65 (0.66) | 0.68 (0.64) | 0.015 |
| SD | 0.28 (0.28) | 0.30 (0.32) | ||
| Median | 0.73 (0.73) | 0.76 (0.73) | ||
| 12 months post randomisation | Mean | 0.72 (0.70) | 0.70 (0.73) | 0.556 |
| SD | 0.30 (0.31) | 0.34 (0.31) | ||
| Median | 0.76 (0.75) | 0.80 (0.80) | ||
| 24 months post randomisation | Mean | 0.71 (0.78) | 0.74 (0.77) | 0.007 |
| SD | 0.29 (0.25) | 0.34 (0.31) | ||
| Median | 0.76 (0.80) | 0.85 (0.85) | ||
Base-case cost-effectiveness results
The results of the WT cost-effectiveness analysis are presented in Table 65 (deterministic results) and Table 66 (probabilistic results). Over the 2-year trial period, PET–CT-guided management was associated with an expected incremental cost saving of £1492 and an expected incremental effect of +0.08 QALYs compared with planned ND. PET–CT surveillance was therefore found to be cost-effective and dominates the standard care strategy, being less costly and more effective with an INB of +0.16 QALYs (95% CI 0.03 to 0.28 QALYs).
| Strategy | Total cost (£) | Total QALY | Incremental cost (£) | Incremental QALY | ICER | NB (QALYs) | Incremental NB (QALYs) |
|---|---|---|---|---|---|---|---|
| Planned ND | 13,989 | 1.20 | – | – | – | 0.50 | – |
| PET–CT | 12,476 | 1.27 | –1513 | 0.07 | Dominant | 0.66 | 0.15 |
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (95% CI) | Incremental NB (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 13,944 (13,269 to 14,509) | 1.19 (1.10 to 1.27) | – | – | – | 0.49 (0.41 to 0.58) | – |
| PET–CT | 12,457 (11,964 to 12,961) | 1.27 (1.18 to 1.34) | –1492 (–698 to –2239) | 0.08 (–0.03 to 0.19) | Dominant | 0.65 (0.56 to 0.72) | 0.16 (0.03 to 0.28) |
Figure 30 shows the results of the WT probabilistic cost-effectiveness analysis on the incremental cost-effectiveness plane. Each of the points represents one of the 10,000 simulated bootstrap results, and provides an indication of the expected uncertainty around the mean cost-effectiveness result. From this graph we can see that in almost every simulation PET–CT surveillance is expected to be cost-saving compared with planned ND (i.e. the majority of points lie below the zero line for the incremental cost results). The expected incremental benefit of PET–CT-guided treatment is more uncertain, with points lying across both the positive and negative incremental benefit domains. At a £20,000 per QALY threshold, PET–CT surveillance is cost saving 99% of the time, and more effective than planned ND 91% of the time.
FIGURE 30.
Within-trial analysis base-case probabilistic results: incremental cost and QALYs of PET–CT-guided care vs. planned ND (bootstrap analysis and 10,000 simulations).

Figure 31 shows the cost-effectiveness acceptability curve for the WT base-case analysis. This graph shows the probability that each strategy is the most cost-effective alternative (i.e. has the highest expected net benefit) across a range of willingness to pay per QALY thresholds. At a £20,000 per QALY threshold, PET–CT surveillance is associated with a 99% probability of being cost-effective compared with planned ND. This probability remains above 93% up to a £150,000 per QALY threshold.
FIGURE 31.
Cost-effectiveness acceptability curve for the base-case WT cost-effectiveness analysis.

Sensitivity analysis
Sensitivity analysis 1: imputation of additional patient-reported cost data (NHS and personal social services perspective)
The results of the sensitivity analysis, including the additional patient-reported cost data collected for a subgroup of patients in the trial [including data on resource usage of primary, (additional) secondary and community care services], are presented in Table 67. Inclusion of the patient-reported additional costs leads to substantial increases in the expected per patient lifetime cost in both arms, with an increase from £13,944 in the base case to £32,469 in the planned ND arm, and from £12,457 to £26,350 per patient in the PET–CT surveillance arm. As the increase in costs is greater in the planned ND arm than in the PET–CT surveillance arm, the relative cost-effectiveness of PET–CT surveillance is increased, with a rise in the expected INB from 0.16 QALYs to 0.39 QALYs. The probability that PET–CT is cost-effective compared with planned ND is 100% at a £20,000 per QALY thresholds, and remains above 99% up to a threshold value of £150,000/QALY (results not shown).
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 32,469 (31,948 to 32,966) | 1.18 (1.09 to 1.27) | – | – | – | –0.446 (–0.47 to –0.40) | – |
| PET–CT | 26,350 (25,452 to 26,586) | 1.26 (1.19 to 1.33) | –6119 (–6915 to –5270) | 0.09 (–0.02 to 0.17) | Dominant | –0.054 (–0.14 to 0.02) | 0.39 (0.29 to 0.48) |
Table 68 shows the results of the same analysis but with the additional cost data amended to account for potential error in patient-reported values. Again, the expected lifetime cost associated with both arms is increased from the base-case analysis; however, the increase is smaller in this instance and the relative difference in cost increase between arms is reduced. The result is that the INB associated with PET–CT surveillance is slightly reduced to 0.21 QALYs, compared with 0.39 QALYs in the previous analysis.
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 25,274 (24,646 to 25,879) | 1.19 (1.09 to 1.27) | – | – | – | –0.08 (–0.17 to 0.01) | – |
| PET–CT | 22,630 (22,134 to 23,186) | 1.26 (1.18 to 1.35) | –2645 (–3467 to –1805) | 0.08 (–0.05 to 0.21) | Dominant | 0.13 (0.03 to 0.24) | 0.21 (0.08 to 0.35) |
Within-trial sensitivity analysis 2: inclusion of societal costs (societal perspective)
The results of the WT sensitivity analysis including both the additional costs included in WT sensitivity analysis 1 and patient and carer-reported societal costs (such as travel, equipment and lost earning expenses) are shown in Tables 69 and 70.
As in WT sensitivity analysis 1, including societal costs in the analysis leads to substantial increases in the expected cost of each strategy. Again, this cost increase appears to be more pronounced in the planned ND arm, leading to PET–CT surveillance being more cost-effective with increased INB values (to +0.70 QALYs in WT sensitivity analysis 2.1 and to +0.53 QALYs in WT sensitivity analysis 2.2).
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 48,753 (48,107 to 49,485) | 1.19 (1.09 to 1.27) | – | – | – | –1.25 (–1.35 to –1.16) | – |
| PET–CT | 36,464 (35,780 to 37,126) | 1.27 (1.19 to 1.34) | –12,289 (–13,218 to –11,394) | 0.08 (–0.03 to 0.19) | Dominant | –0.56 (–0.64 to –0.47) | 0.70 (0.57 to 0.82) |
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 41,786 (41,107 to 42,489) | 1.19 (1.10 to 1.27) | – | – | – | –0.90 (–0.99 to –0.81) | – |
| PET–CT | 32,799 (32,175 to 33,472) | 1.27 (1.19 to 1.34) | –8987 (–9928 to –8047) | 0.08 (–0.04 to 0.19) | Dominant | –0.38 (0.46 to –0.28) | 0.53 (0.39 to 0.65) |
Within-trial sensitivity analysis 3: complete-case analysis for quality-adjusted life-years calculation
The results of the sensitivity analysis using complete-case analysis for the WT QALY calculation are reported in Table 71. The PET–CT-guided watch-and-wait policy remains the dominant strategy, being more effective and less costly than planned ND. The expected incremental cost and QALY outcomes are very similar to the base case and result in the same expected INB (0.16 QALYs).
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 13,985 (13,395 to 14,584) | 1.22 (1.15 to 1.29) | – | – | – | 0.52 (0.44 to 0.60) | – |
| PET–CT | 12,481 (11,946 to 13,031) | 1.31 (1.24 to 1.37) | –1504 (–2312 to –709) | 0.09 (–0.01 to 0.19) | Dominant | 0.68 (0.61 to 0.76) | 0.16 (0.06 to 0.27) |
The results of this analysis indicate that the use of multiple imputation versus complete-case analysis for the calculation of QALYs in the WT analysis has minimal impact on the overall results. As a result of this finding, no corresponding sensitivity analysis using complete-case QALYs in the DM was conducted.
Lifetime decision model analysis
Base-case results
Results of the lifetime DM base-case analysis are presented in Table 72. Compared with planned ND, PET–CT surveillance is expected to lead to a lifetime cost saving of –£1485 and a health gain of +0.13 QALYs per patient. The intervention therefore dominates standard care, being more effective and less costly than planned ND and resulting in an expected INB of 0.21 QALYs. There is, however, uncertainty around this result, with a wide CI around the mean value (INB 95% CI –0.41 to +0.85 QALYs).
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 24,074 (12,947 to 63,200) | 9.01 (7.87 to 10.46) | – | – | – | 7.80 (5.65 to 9.32) | – |
| PET–CT | 22,589 (11,319 to 62,155) | 9.14 (8.05 to 10.55) | –1485 (–2815 to 159) | 0.13 (–0.49 to 0.79) | Dominant | 8.01 (5.85 to 9.51) | 0.21 (–0.41 to 0.85) |
Figure 32 shows the results of the model base-case analysis on the incremental cost-effectiveness plane. Each of the points represents one of the 10,000 simulated model results and provides an indication of the expected uncertainty around the mean cost-effectiveness result. The diagonal line represents NICE’s willingness to pay per QALY threshold of £20,000 per QALY: points lying under the line indicate simulations where the intervention is expected to be cost-effective, whereas points lying above the line are not cost-effective. This graph illustrates the increased uncertainty around the lifetime expected incremental cost and QALY results compared with the WT analysis, with points being widely distributed across both cost-effective and non-cost-effective areas of the graph. The majority of the uncertainty lies around the expected effectiveness of the PET–CT surveillance strategy: at a willingness to pay per QALY threshold of £20,000 per QALY, the probability that PET–CT is cost saving compared with planned ND is 96% (compared with 99% in the WT analysis), whereas the probability that PET–CT is more effective than planned ND is 66% (compared with 91% in the WT analysis).
FIGURE 32.
Lifetime DM base-case cost-effectiveness results: scatterplot of incremental costs vs. incremental QALYs.

The uncertainty around the lifetime modelled results is reflected in the cost-effectiveness acceptability curve shown in Figure 33.
FIGURE 33.
Lifetime DM: cost-effectiveness acceptability curve.

This graph shows the probability that each strategy is the most cost-effective alternative (i.e. has the highest expected net benefit) across a range of willingness to pay per QALY thresholds. At a £20,000 per QALY threshold, PET–CT surveillance is associated with a 75% probability of being cost-effective compared with planned ND (compared with 99% in the WT analysis), dropping to 68% at a £100,000/QALY threshold, and remaining above 67% at a £150,000/QALY threshold.
Sensitivity analysis
One-way sensitivity analysis: tornado plot
The results of the one-way sensitivity analysis, altering individual model parameters by 25%, are presented in Figure 34. The results are most sensitive to changes in the treatment period costs and QALYs, and parameters concerned with the rate of recurrences in each arm. In particular, increasing the rate of primary recurrences in the PET–CT surveillance arm (or decreasing the rate of primary recurrences in the planned ND arm) results in the PET–CT watch-and-wait strategy no longer being cost-effective, with the INB falling below zero. Changes to other cost and utility parameters had little impact on the results.
FIGURE 34.
The results of DM one-way sensitivity analysis (tornado plot). DFaLR, disease free after local recurrence.

Decision model sensitivity analysis 1: inclusion of additional patient-reported cost data (NHS and personal social services perspective)
The results of DM sensitivity analysis 1 are presented in Table 73 (DM sensitivity analysis 1.1) and Table 74 (DM sensitivity analysis 1.2). As for the base-case analysis, PET–CT surveillance dominates standard care (i.e. is cost saving and more effective); however, the expected costs of each strategy are substantially higher than in the base-case analysis. In DM sensitivity analysis 1.1, the per patient lifetime expected costs of planned ND and PET–CT surveillance increase from £24,074 and £22,589 to £125,147 and £120,872, respectively, and in DM sensitivity analysis 1.2 the costs increase to £99,898 and £99,198, respectively. At a £20,000 per QALY threshold the probability that PET–CT surveillance is the most cost-effective strategy is 98% in DM sensitivity analysis 1.1 and 81% in DM sensitivity analysis 1.2 (compared with 75% in the base case). In DM sensitivity analysis 1.1, this value remains above 70% up to a threshold of £150,000/QALY, whereas in DM sensitivity analysis 1.2, this value remains above 67%.
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 125,147 (91,267 to 166,177) | 9.01 (7.88 to 10.47) | – | – | – | 2.74 (0.50 to 4.65) | – |
| PET–CT | 120,872 (86,905 to 161,955) | 9.13 (8.05 to 10.54) | –4276 (–11,277 to 3154) | 0.13 (–0.49 to 0.78) | Dominant | 3.09 (0.88 to 5.04) | 0.34 (0.02 to 0.70) |
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 99,898 (68,360 to 139,654) | 9.01 (7.87 to 10.46) | – | – | – | 4.01 (1.90 to 5.87) | – |
| PET–CT | 99,198 (67,304 to 139,049) | 9.13 (8.05 to 10.54) | –700 (–6190 to 5362) | 0.13 (–0.49 to 0.79) | Dominant | 4.18 (2.04 to 6.04) | 0.17 (–0.22 to 0.57) |
Although these results suggest that PET–CT surveillance remains cost-effective over a broader NHS and PSS perspective, the results should be considered as exploratory only because of the substantial uncertainty around the additional costs used to inform this analysis (which were based on data from a small subpopulation of the trial cohort).
Decision model sensitivity analysis 2: visual analogue scale utility values for the local recurrence and distant recurrence states
The results of the DM sensitivity analysis using health state utilities derived from reported VAS utilities from de Almeida et al. 84 are reported in Table 75. Using the alternative utility values (for the recurrence states) has little impact on the results. The expected QALY values in both arms are slightly reduced compared with the base case, but the incremental effect is unchanged and PET–CT surveillance remains cost-effective.
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 24,136 (12,977 to 63,956) | 8.94 (7.85 to 10.09) | – | – | – | 7.73 (5.60 to 9.10) | – |
| PET–CT | 22,651 (11,323 to 62,958) | 9.07 (8.02 to 10.09) | –1485 (–2805 to 223) | 0.13 (–0.50 to 0.79) | Dominant | 7.94 (5.78 to 9.28) | 0.21 (–0.41 to 0.86) |
Decision model sensitivity analysis 3: additional recurrences allowed beyond 5 years post diagnosis in local and distant recurrence health states
The results of DM sensitivity analysis 3 are presented in Table 76. Allowing primary and subsequent recurrences to occur beyond 5 years has little impact on the results. The QALY increment associated with PET–CT surveillance is slightly reduced (from 0.13 QALYs in the base case to 0.10 QALYs), which results in a small decrease in the expected INB (from 0.21 QALYs in the base case to 0.18 QALYs); however, PET–CT surveillance remains cost-effective. As in the base-case analysis, there is uncertainty around this result, with wide CIs around the expected health gain (0.18 QALYs, 95% CI –0.43 to 0.87 QALYs).
| Strategy | Total cost (£) (95% CI) | Total QALY (95% CI) | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER | NB (QALYs) (95% CI) | Incremental NB (QALYs) (95% CI) |
|---|---|---|---|---|---|---|---|
| Planned ND | 24,313 (13,149 to 63,642) | 8.76 (7.60 – 10.32) | – | – | – | 7.55 (5.41 to 9.12) | – |
| PET–CT | 22,841 (11,492 to 62,359) | 8.86 (7.73 – 10.40) | –1472 (–2934 to 381) | 0.10 (–0.56 to 0.80) | Dominant | 7.72 (5.55 to 9.29) | 0.18 (–0.43 to 0.87) |
Summary
An economic evaluation was conducted to assess the cost-effectiveness of PET–CT-guided watch-and-wait policy compared with planned ND. The evaluation consisted of two components: (1) a WT analysis, in which cost-effectiveness is assessed over the 24-month trial period using individual patient data collected in the trial; and (2) a decision-analytic model analysis, in which cost-effectiveness is assessed over a lifetime horizon, using standard modelling techniques applied to the trial data in order to extrapolate the trial results. Owing to the availability of data within the trial, the base-case analysis was conducted from a NHS secondary care perspective. Additional sensitivity analyses were conducted using a NHS and PSS perspective and a societal perspective (including patient and carer one-off expenses, travel costs and lost earnings).
Results of the WT base-case analysis indicate that the PET–CT watch-and-wait strategy is cost-effective over a 2-year time horizon. Using a NHS secondary care perspective, PET–CT surveillance yielded an average per-patient cost saving of £1492 (95% CI –£698 to –£2239) and an expected health gain of 0.08 QALYs (95% CI –0.03 to 0.19 QALYs) compared with planned ND. The PET–CT watch-and-wait policy therefore dominates standard care, being more effective and less costly, producing a 2-year incremental net health gain of 0.16 QALYs (95% CI 0.03 to 0.28 QALYs) per patient, with a 99% probability of being cost-effective at a £20,000 per QALY threshold.
The results of the base-case lifetime DM analysis indicate that PET–CT surveillance is expected to remain cost-effective over a lifetime horizon; however, there is increased uncertainty around this result compared with the WT analysis. In the base-case analysis the PET–CT watch-and-wait policy is associated with an expected lifetime cost saving of –£1485 (95% CI –£2815 to £159) compared with planned ND, and an expected health gain of 0.13 QALYs (95% CI –0.41 to 0.85 QALYs), resulting in an incremental net health gain of 0.21 QALYs (95% CI –0.41 to 0.85 QALYs). At a £20,000 per QALY threshold there is a 75% probability that PET–CT surveillance is the most cost-effective strategy, dropping to 68% at a £100,000 per QALY threshold. These results were sensitive to parameters relating to the rate of recurrence in each arm, although the incremental health benefit associated with PET–CT surveillance dropped below zero (i.e. indicating non-cost-effectiveness) in only two scenarios: (1) increasing the expected rate of primary recurrence in the PET–CT arm by 25% or (2) decreasing the rate of primary recurrence in the planned ND arm by 25%.
The results of both the WT and DM sensitivity analyses indicate that the inclusion of additional primary, secondary, community and societal care costs is likely to result in substantial increases in the expected cost of both treatment strategies, and may lead to an increase in the expected cost-effectiveness of PET–CT surveillance. However, these results should be considered with caution given the significant uncertainty involved in including the additional costs that were derived from small sample sizes from the trial.
Discussion
In the WT base-case analysis PET–CT surveillance was found to be cost-effective, producing an incremental cost saving of –£1415 and an incremental health benefit of 0.07 QALYs compared with planned ND over a 2-year time horizon. This result is relatively certain, with an expected INB of 0.15 QALYs (95% CI 0.02 to 0.27 QALYs) and a probability of cost-effectiveness of 99% at a £20,000 per QALY threshold, and with results being robust to sensitivity analyses. In the lifetime DM base-case analysis PET–CT surveillance remained cost-effective, producing a lifetime cost saving of –£1485 and an incremental health benefit of 0.13 QALYs. However, this result was more uncertain, with a wide CI around the expected incremental net benefit (0.21 QALYs, 95% CI –0.41 to 0.85 QALYs), and a probability of cost-effectiveness of 75% at a £20,000 per QALY threshold, dropping to 68% at a £100,000 per QALY threshold. This uncertainty is largely attributable to uncertainty around the QALY benefit associated with PET–CT surveillance (mean 0.13 QALYs, 95% CI –0.49 to 0.79 QALYs), which is likely to be a result of uncertainty around the relative rate of recurrences between the two arms. Although there is uncertainty around the lifetime results, PET–CT surveillance remained cost-effective across a range of sensitivity analyses, and was found to be non-cost-effective only when assuming a significant increase in the rate of primary recurrences in the PET–CT arm compared with the planned ND arm, which is expected to represent a highly unlikely scenario. Thus, although uncertainty exists, the mean result was relatively robust to sensitivity analyses.
The results of this analysis are in line with previous economic evaluations of PET–CT surveillance strategies to guide the use of surgery in H&N cancer patients. 90–94 In a recent study, Pryor et al. 94 found that a similar PET–CT-guided strategy was a safe and significantly less costly alternative strategy than planned surgery from an Australian health service perspective. 94 Similarly, Rabalais et al.,92 Sher et al. 90 and Hollenbeak et al. 93 all found PET–CT surveillance to be cost-effective from an American health-care perspective. As far as these authors are aware, no full economic evaluation of PET–CT surveillance for H&N cancer has previously been conducted from a UK NHS perspective; therefore, these results represent the first indication that PET–CT is likely to provide a cost-effective alternative to planned ND within the UK health-care system, and adds support to the current body of studies in favour of adopting PET–CT into routine clinical practice.
Ideally, cost-effectiveness analyses should be conducted from a NHS and PSS or societal perspective, in order to fully account for resources that will be consumed as a result of implementing the new intervention. 63 In this evaluation, however, we have adopted a NHS secondary care perspective in the base case, which accounts only for the use of secondary care (i.e. hospital) resources. This is a clear limitation of the analysis, and is a result of a lack of reliable and sufficient data within the trial on which to derive full NHS and PSS or societal costs; such data were available for only a small subsample of 42 patients and 35 carers. Sensitivity analyses were conducted to assess the potential impact of including these additional costs, the results of which indicate that inclusion of broader costs within the analysis is likely to result in substantial increases in the expected cost of both treatment strategies, and may in fact lead to PET–CT surveillance becoming more cost-effective than in the base case. This suggests that the use of PET–CT may lead to resource savings across primary and societal care, in addition to the savings evident in the hospital secondary care setting. As previously stated, these results should be interpreted with caution because of the uncertainty around the additional cost data used in these analyses; nevertheless, it is encouraging that these exploratory results indicate that PET–CT surveillance remains cost-effective when adopting a broader perspective. Future work should focus on obtaining more accurate estimates of NHS, PSS and societal care costs, in order to enable future UK economic evaluations to adopt a broader perspective.
Chapter 5 Discussion
Interpretation
The results of this study show that PET–CT-guided surveillance followed by ND appears to be equivalent to a planned ND regimen for the management of advanced (N2/N3) nodal disease in patients with H&N cancer being treated with CRT, and demonstrated non-inferiority for differences of > 4% in OS. This strongly supports the efficacy of the PET–CT-guided surveillance policy. The policy is also cost-effective in the short term, and potentially in the long term.
Surveillance policy resulted in far fewer patients requiring ND and, as a result, considerably fewer complications of surgery. The effect of ND on quality of life, however, was not high in that both groups appear to have similar quality of life in the longer term. It is notable, however, that patients randomised to the planned ND experienced more SAEs. One would expect these to be related to surgery, but these were mainly related to CRT, especially in the arm of patients who were randomised to ND after CRT. It is not clear why this should be, or whether or not this is a significant finding. The locoregional control rate and death from other causes were the same in both arms. Importantly, we demonstrated the feasibility and success of a PET–CT-guided policy in a multicentre randomised prospective setting, something that had not been demonstrated before.
The fact that concordance between the central laboratory and the local radiology readings was very high demonstrates the applicability of the surveillance regimen across PET–CT sites in the UK. It is also a testament to the quality of the training of PET–CT radiologists in the UK.
It is important to note that most of the patients had OPC and a large proportion of these were caused by the human papillomavirus (HPV-positive OPC). The subgroup analysis suggests that the surveillance policy is particularly effective in the HPV-positive subgroup. This may be because HPV-positive OPC responds well to CRT and, therefore, there is a smaller risk of persistent disease in HPV-negative patients. However, the subgroup analysis also shows the surveillance policy to be non-inferior for HPV-negative patients.
Generalisability
It is important to note that most of the patients had N2a or N2b disease and that very few patients had N3 disease. This may be partly because N3 disease is not common. However, there may also be a degree of selection bias by investigators, who may have been less keen to submit patients with N3 disease to randomisation. On the one hand, this may have been because they were concerned that the surveillance policy may result in residual disease in the N3 population, which would be difficult to salvage. On the other hand, clinicians may have been concerned that subjecting a patient to a planned ND, especially before CRT, may not be in the patient’s best interest if there was a high chance that the primary site would not respond to CRT.
Overall evidence
The study supports claims that ND does not confer any survival benefits or an increase in nodal recurrence in patients who show a CR compared with patients who undergo a surveillance policy. 17,18
The rate of complications following ND is only slightly higher following PET–CT than planned NDs done before or immediately after CRT. The differences in the rate of complications were not significant.
As was expected, outcomes were significantly better among patients with HPV-positive OPC than among those with HPV-negative disease. However, there is no difference between the planned ND and surveillance arms by HPV status. Indeed, HPV-positive patients may benefit from undergoing surveillance.
Of interest is the fact that there were no quality-of-life differences between the ND and surveillance arms. This suggests that the main determinant of quality of life in these patients is the CRT.
Also of interest is the fact that HPV-positive patients had significantly worse quality of life during treatment, but that they recovered from that and had an overall better quality of life than HPV-negative patients in the longer term. The reasons for this may be that the HPV-positive patients are a younger group who are usually employed and have young families; therefore, the effect of treatment may have more of an impact on their daily lives during that period. However, because they are younger and fitter, in the longer term they recover more quickly and have a higher baseline quality of life when considering the effect of age on quality of life.
Overall conclusions
In the authors’ opinion, this study strongly supports the efficacy of the PET–CT-guided surveillance policy. The policy is also cost-effective in the short term, and potentially in the long term. Importantly, surveillance resulted in 80% of patients avoiding ND and, therefore, fewer complications of surgery. Patients in the surveillance arm had similar quality-of-life scores as patients in the ND arm and, when accounting for the cost-savings associated with PET–CT surveillance, the intervention was found to be more cost-effective in the short term and, potentially, in the long term also.
This is the largest report in the literature on the accuracy of PET–CT scanning in assessing residual disease at the primary site that provides sufficient evidence to allow the replacement of EUA (the current gold standard) by PET–CT scanning, which is a less invasive non-operative technique.
Most of the patients in this study had N2a or N2b disease and few patients had N3 disease; therefore, we cannot confirm the efficacy of the surveillance policy for very large N3 nodal disease.
Further research
The aim of the economic evaluation was to identify the WT and long-term incremental cost-effectiveness of a PET–CT-guided watch-and-wait policy compared with planned ND in HNSCC patients. As yet, comparisons of CT-driven response systems and PET–CT-driven systems in multicentre randomised settings have not been published. Unfortunately, because of cost constraints, we did not undertake contrast CT at the same time as PET–CT, so we cannot compare the efficacies of the two modalities. Therefore, the relative efficacy and cost-effectiveness of PET–CT compared with CT-based approaches is still unclear.
The majority of patients recruited into the study had N2 disease. This means that the study results and the PET–CT surveillance policy may not be applicable to very large N3 nodal disease. This requires further assessment.
Further research is also required for the equivocal cases. This is especially the case with PET-negative disease with persistent nodes on anatomical CT scanning. Research to assess prolonged surveillance with CT or assessment of the persistent node using ultrasound-guided fine-needle aspiration is warranted.
Acknowledgements
Trial Management Group
The Trial Management Group comprised Professor Hisham Mehanna (as chief investigator), Ms Joy Rahman (as trial co-ordinator), Mr Chris McConkey (as statistician), Professor Janet A Dunn (as senior statistician), Mr Dharmesh Patel (as trial administrator) and Ms Jennifer Cooper (as data input clerk).
Research nurses
June Jones and Lynda Wagstaff, research nurses, were funded by the trial to recruit patients at UHCW and Queen Elizabeth Hospital, Birmingham, respectively.
The Administration of Radioactive Substances Advisory Committee licence holders
The ARSAC licence holders for the study covered various PET–CT centres and included Dr Margaret Brookes for Aberdeen, Dr Anil Kumar for Basildon, Dr Julian Kabala for Bristol, Dr Peter Guest for Birmingham, Dr Jonathan Hill for Preston, Dr Richard Smith for Bradford, Dr Iain Lyburn for Linton House Clinic Cheltenham, Dr Paul Hulse for Christie, Dr Peter Guest for University Hospital Coventry and Warwickshire, Dr Rakesh Fanatra for Nottingham, Dr Dilip Patel for Edinburgh, Dr Thomas Gruning for Derriford, Dr Eddie Kalkman for West of Scotland, Dr Lucy Richardson for Hull, Dr Andrew Scarsbrook for Leeds, Dr Nuribe Wieshmann for Clatterbridge, Dr Michael O’Doherty and Dr Sally Barrington for St Thomas’, Dr Gary Cook for Royal Marsden, Dr Irfan Kayani for University College London, Dr Wai-Lup Wong for Alliance Imaging London, Dr John Wilsdon for Newcastle, Dr Nicola Robson for Poole and Dr John Hall for Guildford Diagnostic Imaging Surrey.
Patient and public involvement
Mrs Ethel Culling and Ms Vivienne Reed of the Laryngectomy Society were members of the Trial Steering Committee and commented on the drafting of the protocol and the patient information sheets for the study.
The local head and neck teams
The PET-NECK team would like to thank the NHS trusts and participating principal investigators and their colleagues for recruiting and monitoring trial participants. These include (in alphabetical order of participating hospitals followed by the local principal investigators and their colleagues): Dr David Hurman of Aberdeen Royal Infirmary; Professor Terry Jones, Dr Hulya Weishmann, Mr Jeffrey Lancaster, Dr Aditya Shenoy, Ms Shirley Pringle and Mr Paul Banks of Aintree University Hospital; Mr Imtiaz Ahmed, Dr Sree Palvai, Dr Krinaswami Madhavan and Mrs Anne Hill of Basildon Hospital and Southend University Hospital; Dr Anna Thompson of Barnet and Chase Farm Hospitals; Mr Mohammed Rizwanullah of the Beatson West of Scotland Cancer Centre; Mr William Primrose of Belfast City Hospital and Royal Victoria Hospital; Dr Muthiah Sivaramalingham, Dr Ajay Mehta, Dr Ajay Nigam, Mr N Kazmi, Leanne Davies, Sue Hesketh, Alison Mackle, Karen Gratrix and Christopher Pemberton of Blackpool Victoria Hospital; Dr James McCaul, Andrew Pick, Shelley Berry, Raghav Kulkarni and Kayleigh Gilbert of Bradford Royal Infirmary; Dr Hoda Booz and Dr Matthew Beasley of the Bristol Haematology and Oncology Centre; Dr Vivienne Loo of Broomfield Hospital; Dr Ashoke Biswas of Burnley General Hospital; Mr Jemy Jose of Castle Hill Hospital; Dr Audrey Cook of Cheltenham General Hospital and Gloucester Royal Hospital; Dr Om Purohit of Chesterfield Royal Hospital; Professor Jarrod Homer, Professor Nicholas Slevin, Dr Lip Wai Lee and Mrs Kim Denton of Christie Hospital; Dr Amy Roy of Derriford Hospital; Dr Elizabeth Junor and Mrs Kirsty Peebles of Dumfries and Galloway and Western General Hospital; Mr Jean-Pierre Jeannon and Dr Teresa Guerrerourbano of Guy’s and St Thomas’ Hospitals; Mr Richard Wight, Ms Rita Mohan, Mr Shane Lester, Dr Peter Dunlop, Mr Colin Edge, Mr Douglas Byant and Dr Eleanor Aynsley of James Cook University Hospital; Mr Alistair Balfour of Queen Elizabeth Queen Mother Hospital, Kent and Canterbury Hospital, and William Harvey Hospital; Dr Kate Goodchild and Ann Downs of Luton and Dunstable Hospitals, Mount Vernon Hospital and Northwick Park; Dr Caroline Brammer of New Cross Hospital; Dr Lip Lee, Joanne Allsop and Tracey Hodgkiss of North Manchester General Hospital; Dr Anna Thompson of North Middlesex Hospital; Dr Judy Christian and Sarah Fleet of Nottingham University Hospital; Dr Joseph Davies of Poole Hospital; Mr Kim Dae, Debi Barnes, Mr Costa Repanos, Dr Katy Bradley, Mr Tim Mellor, Mr Jean Daniel Dubois and Professor Peter Brennan of Queen Alexandra Hospital; Mr Keith Webster, Dr Andrew Hartley, Mrs Lynda Wagstaff, Dr Paul Sanghera, Dr John Glaholm, Mr Huw Griffiths, Mr Ijaz Ahmed, Mr Paul Pracey, Mr Christopher Jennings, Mr Sat Parmar, Mr Timothy Martin, Mrs Janet O’Connell, Dr Adrian Warfield, Dr Peter Colloby, Dr John Henderson, Dr Steve Colley, Dr Alison Page, Dr Julie Olliff, Dr Tunde Ogunremi and Dr Peter Guest of the Queen Elizabeth Hospital Birmingham; Dr Ashoke Biswas and Mr Pardeep Morar of Royal Blackburn Hospital; Mr Jerry Sharp of the Royal Derby Hospital; Dr David Hwang of the Royal Devon and Exeter Hospital; Professor Christopher Nutting of the Royal Marsden Hospital; Dr Muthiah Sivaramalingham, Dr Ajay Mehta, Dr Ashoke Biswas and Mr A Cardozo of the Royal Preston Hospital; Dr Stephen Whittaker, Dr Katie Wood and Mrs Ceri Jamieson of the Royal Surrey County Hospital; Dr Emma De Winton and Mr Tomas Tylee of the Royal United Hospital; Dr Prakash Ramachandra of Russells Hall Hospital; Dr Martin Rolles, Tracey Ford and Karen Phillips of the Singleton Hospital; Mr Vinidh Paleri and Dr Charles Kelly of the Sir Bobby Robson Cancer Trials Centre (Freeman Hospital); Mr Paul Tierney of Southmead Hospital; Mr James Moor, Eleanor Dungca, Ms Helen Cocks and Mr James O’Hara of Sunderland Royal Hospital; Dr Geoffrey Cogill of Torbay Hospital; Dr Martin Forster and Dr Dawn Carnell of University College Hospital; Professor Hisham Mehanna, Ms June Jones, Ms Rachel O’Beney, Mr Mark Wake, Mr Gary Walton, Mr Raj Sandhu, Dr Lydia Fresco, Dr Andrew Chan and Dr David Jones of UHCW; Dr Mererid Evans, Ms Amanda Jackson, Ms Caroline Vitolo, Mr Nachi Palaniappan, Ms Laura Moss, Mr Carl Badger Watts and Ms Catherine John of the Velindre Cancer Centre; Dr Andrew Hartley and Lynda Wagstaff of Walsall Manor Hospital; and Professor Martin Robinson, Dr Bernadette Foran, Gail Jessop and John Martindale of Weston Park Hospital.
The PET-NECK trial team would like to thank Jennifer Cooper (research team) for her contributions to the administration of the trial; Dr Bal Sanghera (Paul Strickland Scanner Centre) for performing quality checks on all the PET–CT scanners used in the trial; Dr Hulya Wieshmann for conducting a review of 10% of CT and MRI scans for QA; Dr Sandra Ventorin von Zeildler for undertaking the histopathology review for 10% of patients; the PET-NECK Collect team (Sean James, Andrew White and Adrian Fisk) for managing the translational aspects of the study; and Dr Max Robinson for carrying out p16 and HPV testing for trial participants. The team would also like to thank the Independent Data Monitoring Committee members (Dr Roger A’Hern, Professor Fons Balm and Professor Vincent Gregoire) and the Trial Steering Committee members (Professor Jonathan Ledermann, Professor Michael Langman, Dr Andrew Hartley, Professor Christopher Nutting, Professor Patrick Bradley, Ethel Culling and Vivien Reed) for their invaluable input and advice. Finally, we would like to thank the UK PET–CT centres for scanning patients and co-operating with the QA review, the providers of PET–CT scanning units (Inhealth, Alliance and Cobalt Healthcare) and the ARSAC licence holders for overseeing the safety aspects of patient PET–CT scanning.
Other acknowledgements
Alison F Smith, Peter Hall and Claire Hulme are supported by the National Institute for Health Research Diagnostic Evidence Co-operative Leeds.
This project benefited from facilities funded through the Department of Health: PET–CT scans performed for half the patients in the trial were paid for by the NHS. The project also received support from the National Institute for Health Research Clinical Research Networks. The PET-NECK team thank both sources for their valuable contributions.
Contributions of authors
Hisham Mehanna (Chief Investigator) and Janet A Dunn (Co-Applicant and Senior Statistician) were responsible for identifying the research question and writing the study protocol.
Chris C McConkey (Trial Statistician) and Janet A Dunn (Senior Statistician) carried out the analysis and interpreted the results.
Joy K Rahman (Trial Co-ordinator) managed the administration of the trial.
Wai-Lup Wong (Co-Applicant and Principal Radiologist) reviewed all trial PET–CT scans and advised hospital clinicians on treatment response.
Alison F Smith, Peter Hall and Claire Hulme (Health Economics Team) conducted the analysis of economic models for the trial.
Chris Nutting (Professor of Clinical Oncology) provided expertise on CR treatment.
Andrew GJ Hartley (Consultant Oncologist) provided advice on eligibility and treatment regimens.
Dharmesh K Patel (Trial Administrator) assisted with the administration of the trial.
Sandra Ventorin von Zeidler (Professor of Pathology) performed the histopathology review for 10% of patients and contributed to the writing of the manuscript.
Max Robinson (Senior Lecturer in Oral Pathology) undertook the p16 staining.
Bal Sanghera (Clinical Scientist) performed quality checks on all the PET–CT scanners used in the trial and contributed to the writing of the manuscript.
Lydia Fresco (Clinical Oncologist) recruited patients and is the clinical lead at the sponsor site.
Hisham Mehanna, Chris C McConkey, Joy K Rahman, Alison F Smith and Janet A Dunn were responsible for drafting this report, although all authors provided comments on drafts and approved the final version.
Publications
Rossignol M. Indications de la tomographie par émission de positrons (TEP): mise à jour sommaire. ETMIS 2011;7:1–44.
Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C. Barriers to recruitment in surgical trials in head and neck oncology: a survey of trial investigators. BMJ 2013;3:e002625.
Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, et al. PET-NECK. A multi-centre, randomised, phase III, controlled trial (RCT) comparing PETCT guided active surveillance with planned neck dissection (ND) for locally advanced (N2/N3) nodal metastases (LANM) in patients with head and neck squamous cell cancer (HNSCC) treated with primary radical chemoradiotherapy (CRT). J Clin Oncol 2015;33. 2015 ASCO Annual Meeting, 29 May–2 June, Chicago, IL, abstract no. 6009.
Smith AF, Hall P, Hulme C, Dunn J, Rahman JK, McConkey CC, Mehanna H. Is PET-CT-guided management for patients with locally advanced head and neck squamous cell cancer (HNSCC) cost-effective? Results from a UK non-inferiority phase III randomized trial. J Clin Oncol 2015;33. 2015 ASCO Annual Meeting, 29 May–2 June, Chicago, IL, abstract no. 6010.
Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016;374:1444–54.
Data sharing statement
All requests for data sharing will adhere to the Warwick Clinical Trials Unit data sharing agreement policy. Warwick Clinical Trials Unit is supportive of data sharing and will endeavour to assist in requests for data sharing. All requests are dealt with on a case-by-case basis. Any request should be submitted on the Warwick Clinical Trials Unit data request form, which is then reviewed by the Trial Management Group and the sponsor.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Effective Head and Neck Cancer Management. London: The Royal College of Surgeons; 2002.
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937-44. http://dx.doi.org/10.1056/NEJMoa032646.
- Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945-52. http://dx.doi.org/10.1056/NEJMoa032641.
- Pignon JP, le Maître A, Maillard E, Bourhis J. MACH-NC Collaborative Group . Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4-14. http://dx.doi.org/10.1016/j.radonc.2009.04.014.
- Thariat J, Hamoir M, Garrel R, Cosmidis A, Dassonville O, Janot, et al. Management of the neck in the setting of definitive chemoradiation: is there a consensus? A GETTEC study. Ann Surg Oncol 2012;19:2311-19. http://dx.doi.org/10.1245/s10434-012-2275-9.
- Gupta T, Agarwal JP. Planned neck dissection following chemo-radiotherapy in advanced HNSCC. Int Semin Surg Oncol 2004;1. http://dx.doi.org/10.1186/1477-7800-1-6.
- Kutler DI, Patel SG, Shah JP. The role of neck dissection following definitive chemoradiation. Oncology 2004;18:993-8.
- Garg M, Beitler JJ. Controversies in management of the neck in head and neck cancer. Curr Treat Options Oncol 2004;5:35-40. https://doi.org/10.1007/s11864-004-0004-8.
- Mendenhall WM, Villaret DB, Amdur RJ, Hinerman RW, Mancuso AA. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck 2002;24:1012-18. http://dx.doi.org/10.1002/hed.10187.
- Carinci F, Cassano L, Farina A, Pelucchi S, Calearo C, Modugno V, et al. Unresectable primary tumor of head and neck: does neck dissection combined with chemoradiotherapy improve survival?. J Craniofac Surg 2001;12:438-43. https://doi.org/10.1097/00001665-200109000-00007.
- Lavertu P, Adelstein DJ, Saxton JP, Secic M, Wanamaker JR, Eliachar I, et al. Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck 1997;109:559-66. https://doi.org/10.1002/(SICI)1097-0347(199710)19:7 <559::AID-HED1>3.0.CO;2-6.
- McHam SA, Adelstein DJ, Rybicki LA, Lavertu P, Esclamado RM, Wood BG, et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer?. Head Neck 2003;25:791-8. http://dx.doi.org/10.1002/hed.10293.
- Brizel DM, Prosnitz RG, Hunter S, Fisher SR, Clough RL, Downey MA, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;58:1418-23. http://dx.doi.org/10.1016/j.ijrobp.2003.09.004.
- Frank DK, Hu KS, Culliney BE, Persky MS, Nussbaum M, Schantz SP, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope 2005;115:1015-20. https://doi.org/10.1097/01.MLG.0000162648.37638.76.
- Stenson KM, Haraf DJ, Pelzer H, Recant W, Kies MS, Weichselbaum RR, et al. The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: the feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg 2000;126:950-6. https://doi.org/10.1001/archotol.126.8.950.
- Clayman GL, Johnson CJ, Morrison W, Ginsberg L, Lippman SM. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck Surg 2001;127:135-9. https://doi.org/10.1001/archotol.127.2.135.
- Grabenbauer GG, Rödel C, Ernst-Stecken A, Brunner T, Hornung J, Kittel K, et al. Neck dissection following radiochemotherapy of advanced head and neck cancer – for selected cases only?. Radiother Oncol 2003;66:57-63. https://doi.org/10.1016/S0167-8140(02)00193-7.
- Argiris A, Stenson KM, Brockstein BE, Mittal BB, Pelzer H, Kies MS, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck 2004;26:447-55. http://dx.doi.org/10.1002/hed.10394.
- Thariat J, Ramus L, Maingon P, Odin G, Gregoire V, Darcourt V, et al. Dental maps: automatic dental delineation for radiotherapy planning in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012;82:1858-65. http://dx.doi.org/10.1016/j.ijrobp.2011.03.035.
- Strasser MD, Gleich LL, Miller MA, Saavedra HI, Gluckman JL. Management implications of evaluating the N2 and N3 neck after organ preservation therapy. Laryngoscope 1999;109:1776-80. http://dx.doi.org/10.1097/00005537-199911000-00010.
- Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 2008;33:210-22. https://doi.org/10.1111/j.1749-4486.2008.01688.x.
- Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2011;38:2083-95. http://dx.doi.org/10.1007/s00259-011-1893-y.
- Wong WL, Chevretton EB, McGurk M, Hussain K, Davis J, Beaney R, et al. A prospective study of PET-FDG imaging for the assessment of head and neck squamous cell carcinoma. Clin Otolaryngol Allied Sci 1997;22:209-14. https://doi.org/10.1046/j.1365-2273.1997.00852.x.
- Wong RJ, Lin DT, Schöder H, Patel SG, Gonen M, Wolden S, et al. Diagnostic and prognostic value of [(18)F]fluorodeoxyglucose positron emission tomography for recurrent head and neck squamous cell carcinoma. J Clin Oncol 2002;20:4199-208. https://doi.org/10.1200/JCO.2002.02.590.
- Fischbein NJ, AAssar OS, Caputo GR, Kaplan MJ, Singer MI, Price DC, et al. Clinical utility of positron emission tomography with 18F-fluorodeoxyglucose in detecting residual/recurrent squamous cell carcinoma of the head and neck. AJNR Am J Neuroradiol 1998;19:1189-96.
- Greven KM. Positron-emission tomography for head and neck cancer. Semin Radiat Oncol 2004;14:121-9. https://doi.org/10.1053/j.semradonc.2003.12.005.
- Kubota K, Yokoyama J, Yamaguchi K, Ono S, Qureshy A, Itoh M, et al. FDG-PET delayed imaging for the detection of head and neck cancer recurrence after radio-chemotherapy: comparison with MRI/CT. Eur J Nucl Med Mol Imaging 2004;3:590-5. https://doi.org/10.1007/s00259-003-1408-6.
- Schmidt M, Schmalenbach M, Jungehülsing M, Theissen P, Dietlein M, Schröder U, et al. 18F-FDG PET for detecting recurrent head and neck cancer, local lymph node involvement and distant metastases. Comparison of qualitative visual and semiquantitative analysis. Nuklearmedizin 2004;43:91-101. http://dx.doi.org/10.1267/NUKL04030091.
- Yao M, Graham MM, Hoffman HT, Smith RB, Funk GF, Graham SM, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2004;59:1001-10. http://dx.doi.org/10.1016/j.ijrobp.2004.01.040.
- Ryan WR, Fee WE, Le QT, Pinto HA. Positron-emission tomography for surveillance of head and neck cancer. Laryngoscope 2005;115:645-50. https://doi.org/10.1097/01.mlg.0000161345.23128.d4.
- Goerres GW, Schmid DT, Bandhauer F, Huguenin PU, von Schulthess GK, Schmid S, et al. Positron emission tomography in the early follow-up of advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2004;130:105-9. http://dx.doi.org/10.1001/archotol.130.1.105.
- Hanasono MM, Kunda LD, Segall GM, Ku GH, Terris DJ. Uses and limitations of FDG positron emission tomography in patients with head and neck cancer. Laryngoscope 1999;109:880-5. https://doi.org/10.1097/00005537-199906000-00007.
- Branstetter BF, Blodgett TM, Zimmer LA, Snyderman CH, Johnson JT, Raman S, et al. Head and neck malignancy: is PET/CT more accurate than PET or CT alone?. Radiology 2005;235:580-6. http://dx.doi.org/10.1148/radiol.2352040134.
- Rusthoven KE, Koshy M, Paulino AC. The role of PET-CT fusion in head and neck cancer. Oncology 2005;19:241-6.
- Terhaard CH, Bongers V, van Rijk PP, Hordijk GJ. F-18-fluoro-deoxy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/ pharyngeal cancer. Head Neck 2001;23:933-41. https://doi.org/10.1002/hed.1135.
- Lowe VJ, Boyd JH, Dunphy FR, Kim H, Dunleavy T, Collins BT, et al. Surveillance for recurrent head and neck cancer using positron emission tomography. J Clin Oncol 2000;18:651-8.
- Bongers V, Hobbelink MG, van Rijk PP, Hordijk GJ. Cost-effectiveness of dual-head 18F-fluorodeoxyglucose PET for the detection of recurrent laryngeal cancer. Cancer Biother Radiopharm 2002;17:303-6. http://dx.doi.org/10.1089/10849780260179260.
- Brouwer J, Bodar EJ, De Bree R, Langendijk JA, Castelijns JA, Hoekstra OS, et al. Detecting recurrent laryngeal carcinoma after radiotherapy: room for improvement. Eur Arch Otorhinolaryngol 2004;261:417-22. http://dx.doi.org/10.1007/s00405-003-0708-6.
- Stokkel MP, Terhaard CH, Hordijk GJ, van Rijk PP. The detection of local recurrent head and neck cancer with fluorine-18 fluorodeoxyglucose dual-head positron emission tomography. Eur J Nucl Med 1999;26:767-73. https://doi.org/10.1007/s002590050448.
- Mehanna H, Olaleye O, Licitra L. Oropharyngeal cancer – is it time to change management according to human papilloma virus status?. Curr Opin Otolaryngol Head Neck Surg 2012;20:120-4. http://dx.doi.org/10.1097/MOO.0b013e3283509735.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. http://dx.doi.org/10.1056/NEJMoa0912217.
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003;349:2091-8. http://dx.doi.org/10.1056/NEJMoa031317.
- Data Protection Act 2004. London: The Stationery Office; 2004.
- Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 1999;91:2081-6. https://doi.org/10.1093/jnci/91.24.2081.
- Cancer Action Team . Head and Neck Measures for the Manual for Cancer Services 2006. www.cquins.nhs.uk (accessed 26 January 2017).
- Helliwell T, Woolgar J. Minimum Dataset for Histopathology Reports on Head and Neck Carcinomas and Salivary Neoplasms. London: The Royal College of Pathologists; 2005.
- Ionising Radiation (Medical Exposure) Regulations 2000. London: The Stationery Office; 2000.
- Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. New York, NY: John Wiley & Sons; 2002.
- Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730-8. http://dx.doi.org/10.1200/JCO.2005.16.790.
- Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys 1996;36:999-1004. https://doi.org/10.1016/S0360-3016(96)00430-0.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. http://dx.doi.org/10.1056/NEJMoa053422.
- Bernier J, Schneider D. Cetuximab combined with radiotherapy: an alternative to chemoradiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck?. Eur J Cancer 2007;43:35-4. http://dx.doi.org/10.1016/j.ejca.2006.08.035.
- Remenar E, Van Herpen C, Germa Lluch J, Stewart S, Gorlia T, Degardin M, et al. A randomized phase III multicenter trial of neoadjuvant docetaxel plus cisplatin and 5-fluorouracil (TPF) versus neoadjuvant PF in patients with locally advanced unresectable squamous cell carcinoma of the head and neck (SCCHN). Final analysis of EORTC 24971. J Clin Oncol 2006;24.
- Diagnosis and Management of Head and Neck Cancer: Guideline No. 90. Edinburgh: Scottish Intercollegiate Guidelines Network; 2006.
- Health and Safety Guidance HSG95. Radiation Safety of Lasers used for Display Purposes. Merseyside: Health and Safety Executive; 1996.
- Moher D, Schulz K, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Treatment of Early Breast Cancer. Volume 1. Worldwide Evidence 1985–1990. Oxford: Oxford University Press; 1990.
- Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141-54. https://doi.org/10.1214/aos/1176350951.
- Bjordal K, de Graeff A, Fayers PM, Hammerlid E, van Pottelsberghe C, Curran D, et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 2000;36:1796-807. https://doi.org/10.1016/S0959-8049(00)00186-6.
- Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer 2001;37:1331-4. https://doi.org/10.1016/S0959-8049(01)00127-7.
- Chen AY, Frankowski R, Bishop-Leone J, Hebert T, Leyk S, Lewin J, et al. The Development and Validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2001;127:870-6.
- Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016;374:1444-54. http://dx.doi.org/10.1056/NEJMoa1514493.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- EuroQol. Rotterdam: EuroQol Research Foundation; 2015.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin 1945;1:80-3. https://doi.org/10.2307/3001968.
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48. https://doi.org/10.1191/0962280202sm269ra.
- Associate Director of Operations . Performance Report Month 11 2014.
- Reference Costs 2013–2014. London: Department of Health; 2014.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Drugs and Pharmaceutical Electronic Market Information (eMit). London: Department of Health; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Hall P, Walkington L, Newsham A, Hall G, Glaser A. Costs of hospital care over ten years from diagnosis of early breast cancer in England. Eur J Cancer 2014;50:S79-80.
- Annual Survey of Hours and Earnings, 2014 Provisional Results. London: Office for National Statistics; 2014.
- NHS Employers . Agenda for Change Pay 2014. www.nhsemployers.org/your-workforce/pay-and-reward/pay/agenda-for-change-pay (accessed April 2015).
- Rate of Inflation Joint Lowest Since Records Began. London: Office for National Statistics; 2015.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Schliephake H, Jamil MU. Prospective evaluation of quality of life after oncologic surgery for oral cancer. Int J Oral Maxillofac Surg 2002;31:427-33. https://doi.org/10.1054/ijom.2001.0194.
- Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer – a longitudinal study. Head Neck 2001;23:113-25. https://doi.org/10.1002/1097-0347(200102)23:2 <113::AID-HED1006>3.0.CO;2-W.
- Chandu A, Sun KC, DeSilva RN, Smith AC. The assessment of quality of life in patients who have undergone surgery for oral cancer: a preliminary report. J Oral Maxillofac Surg 2005;63:1606-12. http://dx.doi.org/10.1016/j.joms.2005.07.012.
- De Graeff A, De Leeuw J, Ros W, Hordijk G, Blijham G, Winnubst J. A prospective study on quality of life of patients with cancer of the oral cavity or oropharynx treated with surgery with or without radiotherapy. Oral Oncol 1999;35:27-32. https://doi.org/10.1016/S1368-8375(98)00049-9.
- Parthan A, Posner MR, Brammer C, Beltran P, Jansen JP. Cost utility of docetaxel as induction chemotherapy followed by chemoradiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2009;31:1255-62. http://dx.doi.org/10.1002/hed.21096.
- Brown B, Diamantopoulos A, Bernier J, Schöffski P, Hieke K, Mantovani L, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health 2008;11:791-9.
- de Almeida JR, Villanueva NL, Moskowitz AJ, Miles BA, Teng MS, Sikora A, et al. Preferences and utilities for health states after treatment for oropharyngeal cancer: transoral robotic surgery versus definitive (chemo)radiotherapy. Head Neck 2014;36:923-33. http://dx.doi.org/10.1002/hed.23340.
- How Have Mortality Rates by Age Changed over the Last 50 Years?. London: Office for National Statistics; 2014.
- van der Schroeff MP, van de Schans SA, Piccirillo JF, Langeveld TP, Baatenburg de Jong RJ, Janssen-Heijnen ML. Conditional relative survival in head and neck squamous cell carcinoma: Permanent excess mortality risk for long-term survivors. Head Neck 2010;32:1613-18. http://dx.doi.org/10.1002/hed.21369.
- Wong LY, Wei WI, Lam LK, Yuen AP. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck 2003;25:953-9. http://dx.doi.org/10.1002/hed.10310.
- Matoscevic K, Graf N, Pezier TF, Huber GF. Success of salvage treatment: a critical appraisal of salvage rates for different subsites of HNSCC. Otolaryngol Head Neck Surg 2014;151:454-61. http://dx.doi.org/10.1177/0194599814535183.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Sher DJ, Tishler RB, Annino D, Punglia RS. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol 2010;21:1072-7. http://dx.doi.org/10.1093/annonc/mdp405.
- van Hooren AC, Brouwer J, de Bree R, Hoekstra OS, Leemans CR, Uyl-de Groot CA. The cost-effectiveness of 18FDG-PET in selecting patients with suspicion of recurrent laryngeal carcinoma after radiotherapy for direct laryngoscopy. Eur Arch Otorhinolaryngol 2009;266:1441-8. http://dx.doi.org/10.1007/s00405-008-0878-3.
- Rabalais A, Walvekar RR, Johnson JT, Smith KJ. A cost-effectiveness analysis of positron emission tomography-computed tomography surveillance versus up-front neck dissection for management of the neck for N2 disease after chemoradiotherapy. Laryngoscope 2012;122:311-14. http://dx.doi.org/10.1002/lary.22464.
- Hollenbeak CS, Lowe VJ, Stack BC. The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer 2001;92:2341-8. https://doi.org/10.1002/1097-0142(20011101)92:9 <2341::AID-CNCR1581>3.0.CO;2-8.
- Pryor DI, Porceddu SV, Scuffham PA, Whitty JA, Thomas PA, Burmeister BH. Economic analysis of FDG-PET-guided management of the neck after primary chemoradiotherapy for node-positive head and neck squamous cell carcinoma. Head Neck 2013;35:1287-94. http://dx.doi.org/10.1002/hed.23108.
List of abbreviations
- AIC
- Akaike information criterion
- ARSAC
- Administration of Radioactive Substances Advisory Committee
- BIC
- Bayesian information criterion
- CI
- confidence interval
- CR
- complete response
- CRT
- chemoradiotherapy
- CT
- computerised tomography
- DF
- disease free
- DM
- decision model
- DR
- distant recurrence
- eMIT
- Drugs and Pharmaceutical Electronic Market Information
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- EuroQoL-5 Dimensions
- EUA
- examination under anaesthesia
- GP
- general practitioner
- H&N
- head and neck
- HNSCC
- head and neck squamous cell carcinoma
- HPV
- human papillomavirus
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- IMRT
- intensity-modulated radiotherapy
- INB
- incremental net health benefit
- IQR
- interquartile range
- LR
- local recurrence
- MDADI
- MD Anderson Dysphagia Inventory
- MDM
- multidisciplinary meeting
- MDT
- multidisciplinary team
- MRI
- magnetic resonance imaging
- NB
- net health benefit
- ND
- neck dissection
- NICE
- National Institute for Health and Care Excellence
- N stage
- nodal stage
- OPC
- oropharyngeal cancer
- OS
- overall survival
- PET
- positron emission tomography
- PSS
- personal social services
- PSSRU
- Personal Social Services Research Unit
- QA
- quality assurance
- QALY
- quality-adjusted life-year
- QLQ-C30
- Quality of Life Questionnaire for Cancer (with 30 questions)
- QLQ-H&N35
- Quality of Life Questionnaire for Cancer head and neck module with 35 questions
- SAE
- serious adverse event
- TPF
- docetaxel, platinum and 5-fluorouracil
- TNM
- tumour, node, metastases
- T stage
- tumour stage
- UHB
- University Hospital Birmingham
- UHCW
- University Hospitals Coventry and Warwickshire
- VAS
- visual analogue scale
- WT
- within trial