Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/163. The contractual start date was in November 2011. The draft report began editorial review in June 2015 and was accepted for publication in October 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jon Deeks is a member of the Health Technology Assessment Commissioning Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Thangaratinam et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Burden of pre-eclampsia
Pre-eclampsia is a multisystem disorder in pregnancy associated with hypertension and proteinuria. 1–3 Hypertension is defined as systolic blood pressure (BP) of ≥ 140 mmHg and diastolic BP of ≥ 90 mmHg on two occasions between 4 and 6 hours apart. 1–3 Proteinuria is defined as ≥ 300 mg of protein in a 24-hour urine collection period or urine dipstick of 1+ or more in two samples collected 6 hours apart or a spot urine protein-to-creatinine ratio (PCR) of at least 30 mg/mmol. 2–4 Hypertensive diseases in pregnancy remain one of the leading causes of direct maternal deaths in the UK and account for 20% of all stillbirths. 5 In 1% of pregnant women, pre-eclampsia develops before 34 weeks’ gestation and thus is called early-onset pre-eclampsia. 6,7
Early-onset pre-eclampsia is considered to be a pathophysiologically different disease from late-onset pre-eclampsia, with considerably increased risk of maternal complications, including a 20-fold higher maternal mortality. 8–10 The only known cure for this condition is delivery of the baby and placenta. In women with early-onset pre-eclampsia, decisions on the timing of delivery can be difficult, as fetal and neonatal benefits from prolongation of pregnancy beyond preterm gestation need to be balanced against the risk of multisystem dysfunction in the mother. Preterm delivery accounts for 65% of neonatal deaths and 50% of neurological disability in childhood. 11 Many of the current practice guidelines do not consider gestational age at presentation as a criterion for diagnosis, severity or subclassification to stratify risk in women with pre-eclampsia. 2,12
The complexity of the treatment in early-onset pre-eclampsia gives rise to high health-care costs. 6,7 Women are often admitted to a tertiary care facility, and one-third experience complications, which may necessitate admission to an intensive care facility. 13 Infants usually need prolonged intensive care treatment for the management of complications, including lifelong handicaps arising as a result of prematurity. The additional cost to the NHS of caring for a preterm baby born before 33 weeks’ gestation is £61,509 and and of a baby born before 28 weeks’ gestation is £94,190. 14 Each year, the care of preterm babies costs the NHS £939M, largely accounted for by neonatal care such as incubation and hospital readmissions. 14 Delaying premature births by a week could potentially save £260M a year. 14
One of the key recommendations in the last Confidential Enquiries into Maternal and Child Health (CEMACH) report for policy-makers, service commissioners and providers, and health-care professionals (now known as the Centre for Maternal and Child Enquiries, CMACE) is the need to adopt an early-warning system to help in the timely recognition, referral and treatment of women who have or are developing critical conditions. 5 This applies to women with early-onset pre-eclampsia, as early recognition of women at risk of adverse outcomes will allow timely transfer from a secondary to a tertiary unit to enable care in a high-dependency unit or neonatal intensive care unit if needed.
Timely prediction of complications in women with early-onset pre-eclampsia involves the use of a combination of patients’ characteristics, symptoms, physical signs and investigations;15 these ‘tests’ are performed routinely in all obstetric units, but, in the absence of a structured approach, somewhat haphazardly. Gestational age is the most important determinant of perinatal outcome with more than half the chance of intact fetal survival when the gestational age is > 27 weeks and the birthweight is > 600 g. 16 Clinicians are hesitant to advocate expectant management because of uncertainties about the scale of maternal risk. Development of a prediction model for adverse maternal and fetal outcomes will help clinicians make appropriate decisions, after discussion with the parents.
Existing evidence
Evidence on assessment of risk of complications in early-onset pre-eclampsia
At present, it is difficult to identify those mothers with early-onset pre-eclampsia at increased risk of developing complications, and individual risk estimates for complications at various time points cannot be provided. 9 Current classification systems of pre-eclampsia [Royal College of Obstetricians and Gynaecologists (RCOG); Australia and New Zealand School of Government (ANZOG); International Society for the Study of Hypertension in Pregnancy (ISSHP); Community Health Partnerships (CHP); and the Society of Obstetricians and Gynaecologists in Canada (SOGC)] are based on the severity of the disease. 9,12,17–19 All of them include BP and proteinuria to dichotomise the severity but do not take into account gestational age to assess severity of pre-eclampsia, with the exception of the SOGC, which classifies all early-onset pre-eclampsia as severe. 19 However, in this subgroup the predictors that influence maternal and fetal outcomes are not well established.
Our systematic reviews on the accuracy of tests in predicting complications in women with pre-eclampsia were based on very few, poor-quality primary studies. 20–23 They did not take into account the predictive role of more than one test result on the outcome. Furthermore, there was no separate quantification of risks, especially in women with early-onset pre-eclampsia.
Prediction models, such as Pre-eclampsia Integrated Estimate of RiSk (PIERS), were developed in women with any onset pre-eclampsia and not particularly in those with early onset. 9 Furthermore, the PIERS model did not fully account for the treatment paradox, whereby a strong predictor of a common complication triggers an effective treatment, thereby preventing the occurrence of a certain proportion of adverse outcomes. In this situation, the predictor that triggered the treatment in the first place will look poorer in its predictive performance in a simple model. 24 Hence, tests such as BP and proteinuria were not identified to be significant in the PIERS model. This had a negative impact on the face validity of the model, as traditionally clinicians prioritise these tests and have a very low threshold for intervention when they are abnormal.
Management of early-onset pre-eclampsia
Currently, the only definitive treatment in pre-eclampsia is delivery. Antenatal corticosteroids are administered to improve fetal lung maturation whenever preterm delivery is anticipated. As steroids achieve their optimal effect after 48 hours,25,26 clinicians tend to postpone delivery until this time unless complications have occurred or are anticipated. Neonatal morbidity from early preterm delivery could be reduced by stabilising the woman’s condition and, if possible, by delaying delivery. Expectant management of early-onset pre-eclampsia has been shown to improve perinatal outcomes in randomised trials. 27,28 A Cochrane review13 that compared early intervention with expectant management in women with early-onset severe pre-eclampsia27,28 showed that babies born to mothers in the early intervention group had more hyaline membrane disease [relative risk (RR) 2.3, 95% confidence interval (CI) 1.4 to 3.8] and more necrotising enterocolitis (RR 5.50, 95% CI 1.04 to 29.60) and were more likely to be admitted to the neonatal intensive care unit (RR 1.3, 95% CI 1.1 to 1.6) than those allocated an expectant policy. Infants in the expectant group were delivered approximately 2 weeks later and were 300 g heavier at birth than infants in the early intervention group. A recent systematic review of observational studies suggested that expectant management in carefully selected cases of pre-eclampsia before 34 weeks’ gestation was associated with few serious maternal complications (median < 5%), similar to interventionist care. 9,29 There is consensus that fetal outcome is poor before 24 weeks’ gestation in women with early-onset pre-eclampsia. 27,30,31 However, many centres do not practise expectant management because of the poorly quantified maternal risk. Our study will establish a predictive rule to allow clinicians to confidently provide expectant care when risk of complications in early-onset pre-eclampsia is low.
Objectives
-
To develop, and internally validate, a prediction model in women admitted with early-onset pre-eclampsia from 20+0 weeks to 33+6 weeks’ gestation, for assessment of the risk of adverse maternal outcome by discharge and at various time points after diagnosis.
-
To externally validate and update the model through two external data sets of patients with a diagnosis of early-onset pre-eclampsia.
-
To assess the risk of adverse fetal and neonatal outcomes at birth and at any time until discharge, and to summarise the unadjusted and adjusted prognostic ability of a set of candidate predictor variables.
Chapter 2 Development and internal validation of the prediction model: PREP prospective observational study
Study methods
The study protocol was developed according to existing recommendations on prognostic research, model development and validation, and prediction rule development,32–34 and reported in line with the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement. 35 The study received ethics approval from the National Research Ethics Service Committee West Midlands (approval number 11/WM/0248).
Study design and conduct
We undertook a prospective, observational cohort study to develop the prediction model(s). All consecutive women with suspected or confirmed diagnosis of early-onset pre-eclampsia were approached to take part in the study. Women were recruited from December 2011 to April 2014 based on a set of prespecified eligibility criteria and the follow-up of the last participant was completed in July 2014. Potential mothers were identified and recruited by research midwives and clinicians from the antenatal clinics, wards, day assessment units and delivery suites. We obtained information routinely collected as part of the antenatal booking process in the UK such as maternal age, ethnicity, smoking, alcohol intake and substance misuse. The ethnicity classification was applied using the NHS criteria. 36,37
Setting
The multicentre study was conducted in 53 obstetric units within secondary and tertiary care hospitals in England and Wales.
Participants
Women with suspected or confirmed diagnosis of early-onset pre-eclampsia (before 34 weeks’ gestation) were recruited to the study. Only women with confirmed early-onset pre-eclampsia were included in the final models.
Patient and public involvement
The Action on Pre-Eclampsia Charity (APEC) was vital in providing important input. The charity was involved from the very start with the development and design of the study protocol and will help with the dissemination of findings from the study. A member of the organisation sat as an independent member of the study steering committee and contributed to the overall supervision and management of the research project. They were also involved with the development of study materials, including the informed consent forms and patient information sheets. The APEC was involved in promoting the study to midwives and clinicians, attending the study days and meetings.
Inclusion criteria
The inclusion criteria were gestational age between 20+0 and 33+6 weeks; maternal age of ≥ 16 years; and a diagnosis of new-onset or superimposed pre-eclampsia. We also included women with a diagnosis of haemolysis, elevated liver enzymes, low platelets (HELLP) syndrome with no proteinuria or hypertension and those with one episode of eclamptic seizures but no hypertension or proteinuria. 9,38 All women provided written informed consent and were capable of understanding the information provided. We used an interpreter if required.
The definitions for diagnosis of pre-eclampsia are provided in Table 1.
| Condition | Definition |
|---|---|
| New-onset pre-eclampsia | New-onset hypertension (systolic BP of ≥ 140 mmHg or diastolic BP of ≥ 90 mmHg on two occasions between 4 and 6 hours apart in women) after 20 weeks of pregnancy and new-onset proteinuria (≥ 2+ on urine dipstick or PCR of > 30 mg/mmol or 300 mg of protein excretion in 24 hours)39 |
| Suspected pre-eclampsia | New-onset hypertension (systolic BP of ≥ 140 mmHg or diastolic BP of ≥ 90 mmHg on two occasions between 4 and 6 hours apart in women) after 20 weeks of pregnancy, and 1+ proteinuria on urine dipstick |
| Superimposed pre-eclampsia | |
| In women with chronic hypertension and no proteinuria before 20 weeks’ gestation | New-onset proteinuria (as defined previously) |
| In women with significant proteinuria before 20 weeks’ gestation | Elevated serum alanine aminotransferase concentration (> 70 units per litre) or worsening hypertension (either two diastolic BP measurements of at least 110 mmHg 4 hours apart or one diastolic BP measurement of at least 110 mmHg if the woman had been treated with an antihypertensive drug), plus one of the following: increasing proteinuria, persistent severe headaches or epigastric pain |
| HELLP syndrome | HELLP syndrome: presence of haemolysis based on examination of the peripheral smear, elevated indirect bilirubin levels, or low serum haptoglobin levels in association with significant elevation in liver enzymes and a platelet count below 100,000/mm3 after ruling out other causes of haemolysis and thrombocytopenia |
| One episode of eclamptic seizures without hypertension or proteinuria9,38 | Other neurological conditions of seizures have been excluded |
Exclusion criteria
Women were excluded if the outcome (including recurrent eclamptic seizures) occurred prior to the tests or if there was insufficient time for gaining informed consent or if the mother did not comprehend spoken and written English adequately. A flow chart of study conduct is shown in Figure 1.
FIGURE 1.
Flow chart of the Prediction of Risks in Early-onset Pre-eclampsia (PREP) study conduct. a, Only reduced PREP logistic model validated.

Predictors
Identification of predictors
Our previous Delphi survey of international experts on pre-eclampsia prioritised the tests that were considered to be clinically important in women with pre-eclampsia. 15,41 Additional predictors were identified from our systematic reviews on the accuracy of tests for complications in pre-eclampsia and other relevant studies. 9 This provided face validity to the choice of tests evaluated in the development of the Prediction of Risks in Early-onset Pre-eclampsia (PREP) model.
The list of preselected candidate predictor variables evaluated in the PREP study is provided in Box 1 and the list of predictor variables for fetal complications in the study is shown in Box 2. In addition, we included management strategies that had the potential to reduce risk of complications to minimise bias from treatment paradox. 24 This included administration of antihypertensive drugs (oral and/or parenteral) and/or magnesium sulphate if women were on them at the time of diagnosis of early-onset pre-eclampsia, or if they were commenced within a day of diagnosis. We forced maternal age and gestational age at diagnosis into the model.
Maternal age at diagnosis (years).
Gestational age at diagnosis (weeks).
Number of fetuses in pregnancy at time of consent (1, 2 or 3).
HistorySummary score for medical history – 1 point for each of the following: pre-existing hypertension, renal disease, diabetes mellitus, autoimmune disease, previous history of pre-eclampsia (0, 1, 2 or more).
SymptomsHeadache and/or visual disturbance (yes/no).
Epigastric pain, nausea and/or vomiting (yes/no).
Chest pain and dyspnoea (yes/no).
Bedside examination and testsSystolic BP (mmHg, highest measurement over 6 hours).
Diastolic BP (mmHg, highest measurement over 6 hours).
Clonus (yes/no).
Exaggerated tendon reflexes (yes/no).
Abnormal oxygen saturation (< 95% on air) (yes/no).
Urine dipstick (0, 1+, 2+, 3+, 4+ or more).
Laboratory testsHaemoglobin (g/l).
Platelet count (× 109/l).
ALT concentration (IU/l).
Serum uric acid concentration (µmol/l).
Serum urea concentration (mmol/l).
Serum creatinine concentration (µmol/l).
Urine PCR (mg/mmol).
Management at baselineAdministration of oral and/or parenteral antihypertensives (ongoing or commenced within 1 day of diagnosis) (yes/no).
Administration of magnesium sulphate (commenced before or within 1 day of diagnosis) (yes/no).
ALT, alanine aminotransaminase; IU, international unit.
Maternal age at diagnosis (years).
Gestational age at diagnosis (weeks).
Number of fetuses in pregnancy at time of consent (1, 2 or 3).
HistorySummary score for history, for example 1 point for each of pre-existing hypertension, renal disease, diabetes mellitus, autoimmune disease, previous history of pre-eclampsia (0, 1, 2 or more).
SymptomsHeadache and/or visual disturbance (yes/no).
Epigastric pain, nausea and/or vomiting (yes/no).
Chest pain and dyspnoea (yes/no).
Bedside examination and testsSystolic BP (mmHg, highest measurement over 6 hours).
Diastolic BP (mmHg, highest measurement over 6 hours).
Clonus (yes/no).
Exaggerated tendon reflexes (yes/no).
Abnormal oxygen saturation (< 94% on air) (yes/no).
Urine dipstick (0, 1+, 2+, 3+, 4+ or more).
Laboratory testsHaemoglobin (g/l).
Platelet count (× 109/l).
ALT concentration (IU/l).
Serum uric acid concentration (µmol/l).
Serum urea concentration (mmol/l).
Serum creatinine concentration (µmol/l).
Urine PCR in 24 hours (mg/mmol).
Ultrasound and cardiotocographyUterine artery Doppler at 20–24 weeks’ gestation (normal/abnormal).
CTG findings (normal/abnormal).
Estimated fetal weight by ultrasound (< 10th centile).
Liquor volume (normal/abnormal).
Management at baselineAdministration of oral and/or parenteral antihypertensives (ongoing or commenced within 1 day of diagnosis) (yes/no).
Administration of magnesium sulphate (commenced within 1 day of diagnosis) (yes/no).
Administration of corticosteroids (commenced within 1 day of diagnosis) (yes/no).
ALT, alanine aminotransaminase; CTG, cardiotocography; IU, international unit.
Outcome
The primary outcome was composite adverse maternal outcome that included at least one of the components in Table 2. In addition to maternal complications, prior to the analysis, we added delivery before 34 weeks’ gestation as an additional component to the composite maternal adverse outcome to minimise bias caused by treatment paradox. The components of the composite outcome were developed through Delphic consensus and had previously undergone piloting and validation in the Canadian cohort of patients in the PIERS (Pre-eclampsia Integrated Estimate of RiSk) study. 9 A composite measure for fetal outcome was also developed by the Delphi consensus9 (Table 3).
| Outcome | Definition |
|---|---|
| Mortality | Maternal death attributable to complications of pre-eclampsia |
| Hepatic dysfunction | INR > 1.2 indicative of disseminated intravascular coagulation (DIC) in the absence of treatment with warfarin. DIC is defined as having both abnormal bleeding and consumptive coagulopathy (i.e. low platelets, abnormal peripheral blood film or one or more of the following: increased INR, increased PTT, low fibrinogen, of increased fibrin degradation products that are outside normal non-pregnancy ranges) |
| Hepatic haematoma or rupture | Blood collection under the hepatic capsule as confirmed by ultrasound or laparotomy |
| Glasgow Coma Scale score of < 13 | Based on the Glasgow Coma Scale score system42 |
| Stroke | Acute neurological event, with deficits lasting > 48 hours |
| Cortical blindness | Loss of visual acuity in the presence of intact papillary response to light |
| Reversible ischaemic neurological deficit | Cerebral ischaemia lasting > 24 hours but < 48 hours revealed through clinical examination |
| Retinal detachment | Separation of the inner layers of the retina from the underlying retinal pigment epithelium (choroid) and is diagnosed by ophthalmological examination |
| Acute renal insufficiency | For women with an underlying history of renal disease defined as a creatinine concentration of > 200 µM; for patients with no underlying renal disease defined as a creatinine concentration of > 150 µM |
| Dialysis | Including haemodialysis and peritoneal dialysis |
| Transfusion of blood products | Includes transfusion of any units of blood products: fresh-frozen plasma, platelets, red blood cells, cryoprecipitate or whole blood |
| Positive ionotropic support | The use of vasopressors to maintain a systolic BP of > 90 mmHg or mean arterial pressure of > 70 mmHg |
| Myocardial ischaemia/infarction | ECG changes (ST segment elevation or depression) without enzyme changes and/or any one of the following:
|
| Require > 50% oxygen for > 1 hour | Oxygen given at greater than 50% concentration based on local criteria for > 1 hour |
| Intubation other than for caesarean section | Intubation may be by ventilation, electrical impedance tomography or continuous positive airway pressure |
| Pulmonary oedema | Clinical diagnosis with radiographic confirmation or requirement of diuretic treatment and SaO2 < 94% |
| Postpartum haemorrhage | Blood loss of > 1 l after delivery |
| Early preterm delivery | Delivery at a gestational age of < 34 weeks |
| Outcome | Definition |
|---|---|
| Perinatal or infant mortality | Death of a fetus or neonate. Infant mortality is the death of a child < 1 year of age |
| Bronchopulmonary dysplasia | Oxygen requirement at 36 weeks corrected gestational age unrelated to an acute respiratory episode |
| Necrotising enterocolitis including only Bell’s stage 2 or 3 | Evidence of pneumatosis intestinalis on an abdominal radiography and/or surgical intervention |
| Grade III/IV intraventricular haemorrhage | Bleeding into the brain’s ventricular system, where ventricles are enlarged by the accumulated blood or bleeding extends into the brain tissue around the ventricles |
| Cystic periventricular leukomalacia | Softening and necrosis in the hemispheric white matter in newborns that may result from impaired perfusion at the interface between ventriculopetal and ventriculofugal arteries |
| Stages 3–5 retinopathy of prematurity | Abnormal blood vessel development in the retina of the eye, where blood vessel growth is severely abnormal, where there is a partially or totally detached retina |
| Hypoxic–ischaemic encephalopathy | Apgar score of ≤ 5 at 10 minutes and/or pH 7.00 in first 60 minutes of life and/or base deficit ≥ –16 in first 60 minutes of life associated with abnormal consciousness level (lethargy, stupor or coma) and seizures and/or poor/weak suck and/or hypotonia and/or abnormal reflexes |
When more than one outcome occurred in the same woman, we chose the adverse outcome that occurred first for the purpose of the survival model. A panel of clinicians with expertise in pre-eclampsia and prognosis research ranked the maternal outcomes for their importance to clinical care (see Appendix 1).
Sample size
We aimed to examine about 10 candidate predictor variables for inclusion in the model(s). Simulation studies examining predictor variables for inclusion in logistic regression models suggest that approximately 10 events are necessary for each candidate predictor to avoid overfitting. 43–45 Therefore, to examine 10 candidate predictors, we required at least 100 women with adverse maternal outcomes in our cohort. From our systematic reviews,20–23 20% of women (100 of 500) with early-onset pre-eclampsia were expected to have adverse maternal outcomes at any time before discharge. Thus, the original target sample size was 500 women with confirmed pre-eclampsia. As the event rate was lower than predicted, we revised the sample size to continue recruitment until 100 women had experienced adverse events. Appendix 2 lists the changes compared to the original protocol submitted to the National Institute for Health Research Health Technology Assessment programme.
Prior to analysis, after discussion with the steering committee, the study group additionally classified delivery before 34 weeks’ gestational age as an adverse maternal outcome to avoid treatment paradox from delivery. The sample size criteria remained the same. With the increased number of outcomes, we were able to consider about 20 candidate predictors but we maintained at least 10 events per predictor in the modelling process.
Data sets for external validation: PIERS and PETRA studies
The PREP model was externally validated in two external independent data sets from the PIERS9 and the Pre-Eclampsia TRial Amsterdam (PETRA)46 studies.
PIERS study
The aim of this prospective observational study was to develop a prediction model for adverse maternal outcomes in women with pre-eclampsia of any onset (both early and late). 9 Two thousand and twenty-three women were recruited from tertiary perinatal units in Canada, New Zealand, the UK and Australia between 1 September 2003 and 31 January 2010. Women were included if they were admitted with pre-eclampsia or had developed pre-eclampsia after admission. Women were excluded if they were admitted in spontaneous labour or had achieved any component of the maternal outcome before either fulfilling eligibility criteria or collection of predictor data. The primary outcome was a composite maternal outcome. The combined adverse maternal outcome included one or more of the following: maternal mortality or a serious central nervous system, cardiorespiratory, hepatic, renal, or haematological morbidity. We used the anonymised data set of women with early-onset pre-eclampsia in the PIERS study to validate the PREP model.
PETRA study
The PETRA study was a randomised controlled trial evaluating the effectiveness of plasma expansion in expectant management of early-onset hypertensive disease in pregnancy, including pre-eclampsia. 46 Women were recruited from two university hospitals, the Department of Obstetrics at the Academic Medical Centre and the Vrije University Medical Centre Amsterdam, between April 2000 and May 2003. Patients across the spectrum of severe hypertensive disorders of pregnancy were included in the trial. Patients were excluded if severe fetal distress or lethal fetal congenital abnormalities were diagnosed, if language difficulties prevented informed consent-taking, or if plasma volume expansion had already been given. A total of 216 women were randomised, 111 to plasma volume expansion and 105 to no plasma volume expansion (the control group). The primary outcomes were neonatal neurological development at term age (Prechtl score),47 perinatal death, neonatal morbidity and maternal morbidity. The intervention showed no significant difference in outcomes between the two groups. Data from the entire study cohort were used in the external validation of the PREP model.
Analysis plan development
We convened a panel of 24 experts in the field of pre-eclampsia and prognostic research, to explore the challenges and potential solutions in the development of a prediction model. The panel focused its discussion on methods to reduce the risk of bias in the PREP models as a result of treatment paradox. 24 After consideration of various methods, it was decided to include effective treatments such as antihypertensive drugs and magnesium sulphate as predictors to avoid bias. The appropriateness of the population, predictors and outcomes was discussed. The methodological issues pertinent to the analysis, such as the choice of model (logistic or survival), were considered and the panel suggested the development of both models.
Statistical analysis
We used a transparent process with appropriate prognostic research methodology for our analysis, and reported using TRIPOD recommendations. 35 We developed and externally validated two models: a logistic model (PREP-L) for overall risk of any adverse outcome by discharge, and a survival model (PREP-S) to assess the risk of adverse outcomes at various time points from diagnosis of pre-eclampsia until 34 weeks’ gestation.
Data preparation
We produced descriptive tables of baseline characteristics, candidate predictors and outcomes. Candidate predictors were checked for normality and log-transformed if applicable. We avoided dichotomisation of continuous variables to avoid loss of information. Only pulse oximetry findings were dichotomised because of the very small variations in values.
Methods for handling missing values
During model development, to deal with missing predictor values in some patients, multiple imputation was performed (under a missing at random assumption) using the user-written ICE package in Stata version 12 (StataCorp LP, College Station, TX, USA) with five imputations. We combined the estimates across imputed data sets using Rubin’s rules to produce final parameter estimates for the model. 48 All missing values of candidate predictor variables were multiply imputed except for pulse oximetry and previous occurrence of pre-eclampsia. Previous occurrence of pre-eclampsia was always classed as a ‘no’ in nulliparous women. Pulse oximetry was assumed normal if missing. The imputation of missing data was performed on the complete data set of all participants with suspected or confirmed pre-eclampsia. This was to allow as much information as possible into the imputation procedure. Apart from the eight women lost to follow-up after baseline, no outcome data were missing; therefore, no outcomes were imputed.
Selection of predictor variables
For both primary models, all 22 variables listed in Box 1 were considered to be candidate predictor variables for inclusion in our maternal model. A backwards selection procedure was used to decide which of the candidate predictor variables should be included in the final prediction model (with a p-value of < 0.15 conservatively taken to warrant inclusion and prevent omission of important predictors). Gestational age and maternal age at diagnosis were forced into the model, to ensure clinical acceptability of the final model. For categorical variables, such as medical history and urine dipstick, we used the lowest p-value of any category (relative to the reference category) to indicate inclusion or exclusion.
Continuous variables were initially selected based on an assumed linear trend. After inclusion, non-linear trends were also evaluated using fractional polynomials (FPs), with a p-value of < 0.01 (for the change in model fit) used to justify the inclusion of non-linear trends. Any continuous variables that were originally dropped were double-checked for whether or not non-linear trends would alternatively suggest their inclusion.
Model development for adverse maternal outcomes
We applied the modelling process to women with confirmed diagnosis of early-onset pre-eclampsia and had complete outcome data (Figure 2).
FIGURE 2.
Flow of women recruited in the PREP study for development of the prediction model(s) for adverse maternal and fetal outcomes.

Definition of survival time
For survival analysis, the end of follow-up was defined as the time of occurrence of the first adverse outcome or end of 34 weeks’ gestation, whichever occurred first. A woman was considered to be at risk from the time of diagnosis of early-onset pre-eclampsia and the failure event was defined as maternal adverse outcome occurring before 34 weeks’ gestation. The survival data information was described in the original data set and copied into all five imputed data sets. The survival information was independent of the multiple imputation, as no outcome was imputed and the time of diagnosis data were available for all women.
Survival model: flexible parametric model
A flexible parametric survival model was used via the Royston–Parmar approach,49–51 with the cumulative baseline hazard scale modelled using restricted cubic splines (implemented as the stpm2 package in Stata12). We chose this approach over a Cox regression as it allowed us to explicitly model the baseline hazard rate allowing non-linear functions via cubic splines, which are very flexible and relatively simple to work with. Simpler parametric models may not be flexible enough to adequately represent the hazard function.
Splines are flexible mathematical functions defined by piecewise polynomials, with some constraints to ensure that the overall curve is smooth. The points at which the polynomials join are called knots. Royston and Lambert50 explain that the stpm2 uses restricted cubic splines which force the function to be linear before the first knot and after the final knot. Let s(x) be the restricted cubic spline function. Defining m interior knots, k1, . . ., km, and also two boundary knots, kmin and kmaxs(x), can be written as a function of parameters γ and some newly created variables z1, . . ., zm + 1 giving:
The derived variables zj (also known as the basis functions) can be calculated as follows:
where j = 2, . . ., m + 1,
When choosing the location of the knots for the restricted cubic splines, it is useful to have some sensible default locations. In stpm2, the default knot locations are at the centiles of the distribution of uncensored log-event times.
Survival null model
We identified the number of knots to go into the model by fitting the null model with an increasing number of knots. The number of knots was chosen based on the lowest Akaike information criterion/Bayesian information criterion (AIC/BIC) and visual inspection of the change in fitted shape, with preference for simplicity (i.e. fewer knots) to avoid overfitting. AIC and BIC are measurements of model fit.
Univariable model, full model and variable selection process
Univariable analyses were performed for both models on all 22 candidate predictors in their linear (log-transformed, if applicable) form. These were fitted in each imputed data set and combined using Rubin’s rules. 48 Univariable analyses were performed only to summarise the unadjusted associations in the data, and were not used to inform the selection of predictors in the final multivariable models. Where applicable and computationally possible, all analyses were performed with the imputed data sets and the results were combined appropriately. A backwards selection procedure was applied to both full models as previously described. Maternal age and gestational age at diagnosis were forced into the model. The model was refitted after dropping each individual predictor.
Non-linear terms
We identified the non-linear terms using the multivariable FP (MFP) procedure in Stata, which selects the MFP model that best predicts the outcome variable. The MFP procedure allows the selection of non-linear terms for continuous variables and the procedure was applied to each of the five multiply imputed data sets separately, and the pattern that was identified by the majority of multiply imputed data sets was used (on consensus between the lead statisticians). In order to avoid overfitting, only non-linear terms that improved the model fit at a minimum significance level of 1% (test of deviance) were considered. The final models were refitted by including the FP terms and checked for dropping further predictors at a p-value of < 0.150. Such variables were dropped only if their exclusion did not change the FP terms already identified in the previous step. This step was performed only once and was not repeated if additional predictors had p-values of ≥ 0.150.
Sensitivity analyses
Logistic and survival model
We included treatment with any antihypertensive drug (oral and/or parenteral) within 1 day of diagnosis in the final models. As parenteral antihypertensive drugs are usually commenced in severe pre-eclampsia to prevent complications, the predictive values could be different for oral and parenteral antihypertensive drugs. A sensitivity analysis was conducted by including oral and parenteral antihypertensive drugs separately in the final models to check if model fitting is improved.
Survival model only
The full survival model needed to be fitted in each of the imputed data sets and the results combined using Rubin’s rules. This procedure is not officially supported for use with the stpm2 command in Stata, although it does perform the estimation if forced. In order to confirm accuracy of the results, we fitted a Cox regression for the same model. The stcox command is supported for the combination of estimations using Rubin’s rules. We also checked the final survival model for time-dependent effects.
Apparent performance
The apparent performance of the fitted models was examined by calculating discrimination performance using the c-statistic for the logistic model and Harrell’s c-statistic for the survival model,52 with a 95% confidence interval (CI), in the same data used to generate the model. A c-statistic close to 1 indicates excellent discrimination and 0.5 indicates no discrimination beyond chance. The calibration performance (fit of observed to expected risk across all individuals) was examined by checking that the calibration slope was 1. As the model was developed using the same data, we expected the calibration to show perfect agreement on average across the individuals.
Internal validation
To evaluate the potential for overfitting of our developed models, we used non-parametric bootstrapping. The variable selection procedure was repeated in 100 bootstrap data sets from each of the five multiple imputation data sets (thereby giving a total of 500 data sets). This led to a new final model being produced in each of the bootstrap samples. The performance of the models (in terms of c-statistic and calibration slope) in the bootstrap sample itself represents an estimation of the apparent performance, and their performance in the original sample represents test performance. The difference between these performances is an estimate of the optimism in the apparent performance. This difference was averaged to obtain a single estimate of optimism for the c-statistic and the calibration slope. This optimism was then subtracted from the original apparent performance statistics to produce optimism-adjusted performance statistics.
Production of the final models
The coefficients in the final models were adjusted for optimism. The optimism-adjusted calibration slope was taken as the uniform shrinkage factor, and the original predictor effects (beta coefficients) were multiplied by this value. Following this, the intercept (for the logistic model) or baseline hazard (for the survival model) were re-estimated to ensure that the overall calibration of the final model predictions to the observed data were maintained, that is, to ensure that calibration-in-the-large was zero. For the survival model, only the intercept term was modified in the baseline hazard function (i.e. the shape of the original baseline hazard was maintained). For sensitivity analysis, we applied these optimism-adjusted models to women with an unconfirmed diagnosis of pre-eclampsia.
After developing and validating the prediction model based on the final set of (close to) 20 predictors, we additionally investigated whether or not any of the candidate predictors we excluded would actually significantly improve the accuracy of the model; however, this was clearly noted as secondary analyses.
The models were available as Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) files (see Appendices 3 and 4) to allow clinicians to input the findings of their patients, and obtain estimates of overall risk of adverse outcomes (PREP-L) and risks at daily intervals after diagnosis (PREP-S).
External validation
The final models were externally validated using the PIERS and PETRA data. We compared the availability of predictors, missing values and the outcome components in the external data sets with the PREP data. If there were any missing predictors in the external cohorts, we planned to re-estimate a reduced version form of our model (using exactly the same process as above) using only those predictors that were available in the external data sets.
If predictors were centred in the PREP model, then predictors in the external data were centred by the same value. The reduced PREP models were used to estimate predicted risks (or risk scores) for women in the PIERS or PETRA population. To produce calibration plots, the risks were grouped into tenths (defined by centiles) of predicted risk in the PREP-L model and into four risk groups for the PREP-S model. As the predictions were not compatible with the Stata 12.1 facilities for combining results using Rubin’s rules, we calculated the fitted values or predictions of risk within each imputed data set and then averaged them.
Secondary analysis of fetal outcomes
The analysis of fetal outcomes by discharge was done in the same way as for the logistic model described above, although non-linear terms were not considered. Analysis was performed on the level of the mother/pregnancy rather than on the fetal level. In multiple pregnancies with multiple sets of predictors and outcomes, we used the worst predictor and considered any outcome regardless of whether an outcome occurred in one of the babies or in both. A variable selection process on the full list of maternal and fetal candidate predictors provided the final fetal model. No adjustment for optimism, external validation or sensitivity analyses was performed for the fetal model.
All the above analyses were carried out using Stata version 12.0. Definitions of key terms are provided in Table 4.
| Terms | Definitions |
|---|---|
| Calibration | Calibration indicates the ability of the model to correctly estimate the absolute risks and was examined using calibration plots |
| Reproducibility (internal validation) | The process of determining internal validity. Internal validation assesses validity for the setting from which the development data originated |
| Generalisability/transportability (external validation) | The process of determining external validity of the prediction model to populations that are plausibly related |
| Discrimination | Discrimination describes the ability of the model to correctly distinguish those who will have an adverse outcome from those who will not |
| Calibration plot | In a calibration plot the predictive risk plotted against the observed incidence of the outcome. Ideally the predicted risk equals the observed incidence throughout the entire risk spectrum and the calibration plot follows the 45° line |
Chapter 3 Maternal characteristics, predictors and outcomes in women with early-onset pre-eclampsia
Flow of participants in the study
Between December 2011 and April 2014, we screened 3302 pregnant women from 53 maternity units for inclusion in the PREP study. Of these, 2099 did not meet the inclusion criteria: 882 did not have raised proteinuria, 650 did not have raised BP readings, 457 were classed as other, 53 had underlying comorbidities, 36 were participating in a clinical trial of an investigational medicinal product and 21 did not understand English and an interpreter could not be used at the time of recruitment. Of the 1203 eligible women, 1101 were recruited to the study with a suspected or confirmed diagnosis of early-onset pre-eclampsia. Of those recruited, 954 women had confirmed pre-eclampsia, 142 women had a suspected diagnosis of pre-eclampsia that was not subsequently confirmed, baseline information data were not available in five participants and nine were lost to follow-up. The final maternal prediction models included data from 946 women and the fetal prediction model included data from 945 pregnancies (see Figure 2).
Baseline characteristics of women included in the PREP study
Table 5 shows the women recruited into the study according to the various inclusion criteria. Over 90% (866/954) of all participants had a diagnosis of new-onset pre-eclampsia, 75 women (75/954, 7.9%) had superimposed pre-eclampsia, 10 (10/954, 1.0%) had HELLP syndrome and three women (3/954, 0.3%) had a single episode of eclamptic seizure in the absence of raised BP or proteinuria.
| Inclusion criteria | Women, n (%) |
|---|---|
| New-onset pre-eclampsia | 866 (91.0) |
| Superimposed pre-eclampsia | 75 (7.9) |
| HELLP syndrome | 10 (1.0) |
| Eclamptic seizures | 3 (0.3) |
| Total | 954 |
The mean age of participants was 30.2 years [standard deviation (SD) 6.1 years] (Table 6). Two-thirds of women identified themselves as European (631/950, 66%), one-fifth as South or South-East Asian (18%, 172/950) and about one-tenth (81/950, 9%) as African. Around 3% of all women were from the Caribbean (31/950), 1% from the Far East (8/950) and 1% from the Middle East (6/950). Ninety-one per cent (866/954) of all pregnancies were singletons, while twins and triplets accounted for 9% (83/954) and 1% (5/954) of pregnancies, respectively. More than half of all women were nulliparous (551/954, 58%). Recurrent miscarriage (three or more) had occurred in 42 women (4.4%). About one-tenth (87/943, 9%) of women reported smoking in pregnancy at booking appointment and alcohol intake in pregnancy was reported by 5% (47/937) of all participants.
| Maternal characteristics | Women with early-onset pre-eclampsia (n = 954) |
|---|---|
| Mean (SD) or n (%) | |
| Maternal age (years), mean (SD) | 30.2 (6.1) |
| Alcohol intake | 47 (5%) |
| Currently smoking | 87 (9%) |
| Drug use | 4 (0.4%) |
| Mother’s ethnic group | |
| Europe | 631 (66%) |
| Africa | 81 (9%) |
| South and South East Asia | 172 (18%) |
| Far East | 8 (1%) |
| Middle East | 6 (1%) |
| Caribbean | 31 (3%) |
| Other | 21 (2%) |
| Parity | |
| 0 | 551 (58%) |
| 1 | 207 (22%) |
| 2 | 109 (11%) |
| 3 | 55 (6%) |
| 4 | 20 (2%) |
| 5–9 | 12 (1%) |
| Total number of miscarriages | |
| 0 | 607 (64%) |
| 1 | 225 (24%) |
| 2 | 72 (8%) |
| > 3 | 42 (4%) |
Predictor characteristics in women with early-onset pre-eclampsia
The values of the various candidate predictors of women in the PREP study are shown in Table 7. The mean gestational age at which the diagnosis of early-onset pre-eclampsia was made was 30.5 weeks (SD 2.9 weeks) and there were no missing values for gestational age at diagnosis.
| Candidate predictor | Women with early-onset pre-eclampsia (N = 954) | Women with missing data, n (%) |
|---|---|---|
| Mean (SD) or n (%) | ||
| Maternal characteristics | ||
| Maternal age (years), mean (SD) | 30.2 (6.1) | 2 (0.2) |
| Gestational age at diagnosis (weeks), mean (SD) | 30.5 (2.9) | – |
| Number of fetuses in pregnancya | ||
| Singleton | 866 (91%) | – |
| Twins | 83 (9%) | – |
| Triplets | 5 (1%) | – |
| History | ||
| Summary score for medical historyb | 1 (0.1) | |
| 0 | 601 (63%) | – |
| 1 | 251 (26%) | – |
| ≥ 2 | 101 (11%) | – |
| Chronic hypertension | 139 (15%) | 10 (1.0) |
| Renal disease | 30 (3%) | 10 (1.0) |
| Previous history of pre-eclampsia | 169 (43%) | 558c |
| Autoimmune disease | 18 (2%) | 32 (3.4) |
| Pre-existing DM | 109 (11%) | 6 (0.6) |
| Type I DM | 56 (51%) | – |
| Type II DM | 16 (15%) | – |
| Gestational DM | 37 (34%) | – |
| Symptoms | ||
| Headache and/or visual disturbance | 382 (40%) | 28 (2.9) |
| Headache generalised | 293 (31%) | 36 (3.8) |
| Headache localised | 121 (13%) | 92 (9.6) |
| Visual disturbance | 139 (15%) | 56 (5.9) |
| Epigastric pain, nausea and/or vomiting | 202 (22%) | 47 (4.9) |
| Epigastric pain | 131 (14%) | 68 (7.1) |
| Nausea | 111 (12%) | 130 (13.6) |
| Vomiting | 54 (6%) | 120 (12.6) |
| Chest pain and/or breathlessness | 60 (6%) | 126 (13.2) |
| Chest pain | 30 (3%) | 164 (17.2) |
| Breathlessness | 38 (4%) | 162 (17.0) |
| Bedside examination and tests | ||
| Systolic BP (mmHg), mean (SD) | 159 (19) | 5 (0.5) |
| Diastolic BP (mmHg), mean (SD) | 99 (12) | 5 (0.5) |
| Clonus | 95 (10%) | 403 (42.2) |
| Exaggerated tendon reflexes | 147 (15%) | 353 (37.0) |
| Oxygen saturation by pulse oximetry (%), mean (SD) | 98 (2) | 521 (54.6) |
| Oxygen saturation abnormal (< 94%) | 4 (≥ 1%) | 521 (54.6) |
| Urine dipstick | ||
| None/trace | 39 (4%) | – |
| 1+ | 170 (18%) | – |
| 2+ | 314 (33%) | 19 (2.0) |
| 3+ | 306 (32%) | – |
| ≥ 4 | 106 (11%) | – |
| Laboratory tests | ||
| Haemoglobin (g/l), mean (SD) | 11.9 (1.3) | 37 (3.9) |
| Platelet count (× 109/l), mean (SD) | 226 (78) | 41 (4.3) |
| ALT concentration (U/l), mean (SD) | 31.0 (71.0) | 75 (7.9) |
| Serum uric acid concentration (µmol/l), mean (SD) | 0.6 (2.7) | 165 (17.3) |
| Serum urea concentration (mmol/l), mean (SD) | 4.6 (4.4) | 70 (7.3) |
| Serum creatinine concentration (µmol/l), mean (SD) | 61.0 (17.8) | 38 (4.0) |
| Urine PCR 24 hour (mg/mmol), mean (SD) | 273 (492) | 109 (11.4) |
| Treatment provided | ||
| Any antihypertensive therapyd | 753 (79%) | 6 (0.6) |
| Oral antihypertensive therapy | 734 (77%) | 6 (0.6) |
| Parenteral antihypertensive therapy | 111 (12%) | 6 (0.6) |
| Intravenous magnesium sulphatee | 144 (15%) | 6 (0.6) |
Clinical history
One-quarter (251/953, 26%) of all women for whom data were available had at least one of the following risk factors: previous history of pre-eclampsia (169/396, 43%), chronic hypertension (139/944, 15%), diabetes mellitus (109/948, 11%), renal disease (30/944, 3%) and autoimmune disease (18/922, 2%). One-tenth (101/953, 11%) had two or more risk factors.
Symptoms
Symptoms such as headache and/or visual disturbances were experienced by 41% (382/926) of women, epigastric pain and/or vomiting by 22% (202/907) and chest pain and/or dyspnoea by 7% (60/828).
Bedside examination and tests
The mean systolic and diastolic BP at the time of diagnosis of early-onset pre-eclampsia was 159 mmHg (SD 19 mmHg) and 99 mmHg (SD 12 mmHg), respectively. Around two-thirds of women had demonstrable clonus (551/954, 58%) and exaggerated tendon reflexes (601/954, 63%). The oxygen saturation levels were ≤ 94% in only 1% (4/433) of women, and more than half of the women did not have documented results (521/954, 55%). There were no missing values for BP or proteinuria.
Laboratory tests
Serum alanine aminotransaminase (ALT) was measured more often than aspartate transaminase (AST) (in 93% of women vs. 30%); consequently, ALT was used in the analysis. Less than one-tenth of values were missing for haemoglobin level, platelet count and concentrations of serum urea and serum creatinine concentration. Furthermore, < 20% of values were missing for serum uric acid concentration.
Treatment provided
More than three-quarters (753/948, 79%) of women were previously on antihypertensive drugs or were started on them within the first 24 hours of diagnosis of pre-eclampsia. Three-quarters of women (734/948, 77%) were on oral antihypertensive therapy and 12% (111/948) were receiving parenteral antihypertensive therapy. Fifteen per cent (144/948) of women started magnesium sulphate treatment to prevent or treat eclamptic seizures in the first 24 hours after diagnosis.
Additional fetal predictors
For the analysis of fetal outcomes, five additional predictors were included, as shown in Table 8. Around one-quarter (91/342, 27%) of women had an abnormal uterine artery Doppler at 20–24 weeks’ gestation. Only 6% of women had abnormal liquor volume (57/898) and 5% had abnormal cardiotocography (CTG) findings (46/713). Over 40% (291/717) of pregnancies had an estimated fetal weight < 10th centile. More than half (430/783, 55%) of the women received treatment with corticosteroids at baseline.
| Additional fetal predictors | Women with early-onset pre-eclampsia (n = 954), mean (SD) or n (%) | Women with missing data, n (%) |
|---|---|---|
| Abnormal uterine artery Doppler | 91 (10%) | 612 (64) |
| Abnormal liquor volume | 57 (6%) | 56 (6) |
| Abnormal CTG findings | 46 (5%) | 241 (25) |
| Estimated fetal weight < 10th centile | 291 (31%) | 237 (25) |
| Baseline treatment: steroids | 430 (45%) | 171 (18) |
Maternal and fetal adverse outcomes in women with early-onset pre-eclampsia
Outcome data were available for 99% (946/954) of all participants in the PREP study. The rates of individual components of the composite adverse maternal and fetal outcomes are provided in Tables 9 and 10, respectively.
| Adverse maternal outcome | Women with complications (N = 946), n (%) |
|---|---|
| Maternal death | – |
| Neurological | |
| Eclamptic seizures | 12 (1.3) |
| Glasgow Coma Scale score of < 13 | 3 (0.3) |
| Stroke or RIND | – |
| Cortical blindness | – |
| Retinal detachment | – |
| Posterior reversible encephalopathy | 2 (0.2) |
| Bell’s palsy | – |
| Hepatic | |
| Hepatic dysfunction | 12 (1.3) |
| Subcapsular haematoma | – |
| Hepatic capsule rupture | – |
| Cardiorespiratory | |
| Need for positive inotrope support | 1 (0.1) |
| Myocardial ischaemia or infarction | – |
| At least 50% FiO2 for > 1 hour | 7 (0.7) |
| Intubation | 9 (1.0) |
| Pulmonary oedema | 6 (0.6) |
| Renal | |
| Acute renal insufficiency | 5 (0.5) |
| Dialysis | 5 (0.5) |
| Haematological | |
| Transfusion | 51 (5.4) |
| Abruptions | 25 (2.6) |
| Postpartum haemorrhage | 74 (7.8) |
| Preterm delivery | |
| Delivery at < 34 weeks’ gestational age | 580 (61.3) |
| At least one of the above occurred by discharge | 633 (66.9) |
| At least one occurred before 34 weeks’ gestational age | 584 (61.7) |
| Adverse fetal outcome | Pregnancies with complications (N = 945a), n (%) |
|---|---|
| Neonatal death | 23 (2.4) |
| Bronchopulmonary dysplasia | 41 (4.3) |
| Necrotising enterocolitis | 34 (3.6) |
| Grade III/IV intraventricular haemorrhage | 11 (1.2) |
| Cystic periventricular leukomalacia | 5 (0.5) |
| Stage 3–5 retinopathy | 7 (0.7) |
| Hypoxic ischaemic encephalopathy | 2 (0.2) |
| Stillbirth | 16 (1.7) |
| Admission to NICU at any time | 681 (72.1) |
| At least one of the above occurred by discharge | 702 (74.3) |
Overall, 66.9% (633/946) of all women with early-onset pre-eclampsia experienced at least one adverse maternal outcome and 74.3% (702/945) had at least one adverse fetal outcome. The most frequently reported outcome was preterm delivery before 34 weeks’ gestation, occurring in 61.3% (580/946) of women. The second most common outcome was postpartum haemorrhage (7.8%, 74/946), followed by transfusion of any blood products (5.4%, 51/946) and abruptio placentae (2.6%, 25/946). The least reported maternal complications were need for positive inotrope support (0.1%, 1/946), posterior reversible encephalopathy (0.2%, 2/946) and a Glasgow Coma Scale score of < 13 (0.3%, 3/946). When preterm delivery was excluded as a component of the composite outcome, 15.5% of all women (147/946) had at least one adverse maternal outcome.
Chapter 4 Prediction of overall risk of adverse maternal outcome by discharge in women with early-onset pre-eclampsia: PREP-L model
Of the 946 women for whom outcome data were available, 633 (67%) experienced at least one adverse maternal outcome at any time from diagnosis to discharge. The number of women who had experienced an adverse outcome was 228 (24%) at 48 hours, 410 (43%) at 1 week and 624 (66%) at 30 days after diagnosis.
Modelling continuous predictors
Maternal age, systolic and diastolic BPs, haemoglobin level and platelet count were normally distributed, and hence we did not apply any transformation. Concentrations of ALT, AST, serum uric acid, serum urea and serum creatinine and the PCR were strongly right skewed, and we log-transformed these values. As gestational age at diagnosis was an inclusion criterion and limited to 34 weeks, a log transformation was applied to decrease the range. We were not able to fully evaluate serum uric acid concentration as a predictor because of data coding issues in this variable at the time of model development. Subsequent to model development being completed, and after the data coding issues were resolved for this variable, we calculated how the c-statistic changed after adding log-transformed serum uric acid concentration to the final models to assess whether or not this variable improved model performance (see Apparent performance and internal validation of the PREP-L model).
Development of PREP-L model: predictor selection
Table 11 shows the univariable and multivariable analysis for association of predictors and adverse maternal outcomes. The models were fitted in each of the imputed data sets and the results combined using Rubin’s rules. In the univariable analysis, lower gestational age at diagnosis, symptoms of epigastric pain and/or nausea and vomiting, clonus, exaggerated tendon reflexes, raised systolic and diastolic BPs, urine dipstick-detectable proteinuria, high levels of haemoglobin, low platelet counts, raised concentrations of ALT, serum urea, serum uric acid and creatinine, increased urine PCR, management with antihypertensives and use of magnesium sulphate were significantly associated with adverse maternal outcomes (p < 0.05). Relevant medical history of one or more conditions such as chronic hypertension, diabetes mellitus, renal disease, autoimmune disease and a history of pre-eclampsia in previous pregnancy were associated with a reduced risk of complications.
| Candidate predictors | Women,a n | No adverse maternal outcome (n = 313), mean (SD) or n (%) | Adverse maternal outcome (n = 633), mean (SD) or n (%) | Univariable models after multiple imputation (n = 946) | Multivariable full model after multiple imputation (n = 946) | ||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | ||||
| Maternal characteristics | |||||||
| Maternal age (years) | 944 | 30.7 (6.3) | 30.0 (6.0) | 0.981 (0.959 to 1.003) | 0.088 | 0.978 (0.951 to 1.006) | 0.123 |
| Log-transformed gestational age at diagnosis | 946 | 3.4 (0.1) | 3.4 (0.1) | 0.005 (0.001 to 0.028) | < 0.001 | 0.001 (0.000 to 0.009) | < 0.001 |
| Multiple pregnancy | |||||||
| Singleton (reference) | 946 | 284 (91%) | 579 (91%) | ||||
| Twins | 28 (9%) | 51 (8%) | 0.893 (0.552 to 1.447) | 0.647 | 1.381 (0.790 to 2.416) | 0.257 | |
| Triplets | 1 (< 1%) | 3 (< 1%) | 1.472 (0.152 to 14.209) | 0.738 | 2.826 (0.257 to 31.104) | 0.396 | |
| Global test | 0.849 | 0.556 | |||||
| Medical history score | |||||||
| 0 (reference) | 945 | 170 (54%) | 425 (67%) | ||||
| 1 | 98 (31%) | 152 (24%) | 0.622 (0.456 to 0.848) | 0.003 | 0.708 (0.487 to 1.030) | 0.071 | |
| ≥ 2 | 45 (14%) | 55 (9%) | 0.488 (0.317 to 0.753) | 0.001 | 0.515 (0.296 to 0.897) | 0.019 | |
| Global test | < 0.001 | 0.032 | |||||
| Symptoms | |||||||
| Headache and/or visual disturbance | 920 | 128 (42%) | 252 (41%) | 0.967 (0.734 to 1.275) | 0.813 | 0.839 (0.582 to 1.210) | 0.349 |
| Epigastric pain, nausea and/or vomiting | 901 | 52 (18%) | 148 (25%) | 1.491 (1.035 to 2.147) | 0.033 | 1.001 (0.610 to 1.643) | 0.997 |
| Chest pain and/or dyspnoea | 822 | 17 (6%) | 43 (8%) | 1.254 (0.644 to 2.441) | 0.510 | 1.074 (0.441 to 2.618) | 0.876 |
| Bedside examination and tests | |||||||
| Clonus | 545 | 19 (12%) | 75 (19%) | 1.976 (1.127 to 3.464) | 0.029 | 1.157 (0.537 to 2.492) | 0.716 |
| Exaggerated tendon reflexes | 594 | 25 (15%) | 121 (29%) | 2.244 (1.534 to 3.284) | < 0.001 | 1.037 (0.643 to 1.673) | 0.881 |
| Systolic BP (mmHg) | 942 | 151 (15) | 162 (20) | 1.038 (1.029 to 1.047) | < 0.001 | 1.025 (1.013 to 1.038) | < 0.001 |
| Diastolic BP (mmHg) | 942 | 96 (10) | 101 (12) | 1.047 (1.032 to 1.061) | < 0.001 | 1.009 (0.989 to 1.029) | 0.384 |
| Oxygen saturation: abnormal (< 94%) | 429 | 0 (0%) | 4 (< 1%) | No abnormal values in women without outcome | No abnormal values in women without outcome | ||
| Urine dipstick: none/trace (reference) | 928 | 16 (5%) | 23 (4%) | ||||
| 1+ | 80 (26%) | 89 (14%) | 0.780 (0.384 to 1.584) | 0.491 | 0.884 (0.404 to 1.933) | 0.757 | |
| 2+ | 120 (39%) | 193 (31%) | 1.133 (0.575 to 2.233) | 0.719 | 0.894 (0.420 to 1.905) | 0.772 | |
| 3+ | 71 (23%) | 232 (37%) | 2.243 (1.117 to 4.501) | 0.023 | 1.171 (0.520 to 2.637) | 0.703 | |
| ≥ 4 | 19 (6%) | 85 (14%) | 3.035 (1.355 to 6.794) | 0.007 | 1.233 (0.484 to 3.145) | 0.661 | |
| Global test | < 0.001 | 0.712 | |||||
| Laboratory tests | |||||||
| Haemoglobin (g/l) | 910 | 11.8 (1.2) | 12.0 (1.4) | 1.114 (1.004 to 1.237) | 0.042 | 1.054 (0.917 to 1.212) | 0.461 |
| Platelet count (× 109/l) | 906 | 245 (77) | 217 (77) | 0.995 (0.993 to 0.997) | < 0.001 | 0.996 (0.994 to 0.999) | 0.001 |
| Log-transformed ALT concentration | 871 | 2.8 (0.6) | 3.0 (0.8) | 1.561 (1.257 to 1.937) | < 0.001 | 1.189 (0.914 to 1.548) | 0.197 |
| Log-transformed serum uric acid concentration | 782 | –1.3 (1.0) | –1.0 (0.7) | 1.566 (1.210 to 2.028) | 0.001 | ||
| Log-transformed serum urea concentration | 877 | 1.2 (0.4) | 1.5 (0.5) | 3.812 (2.598 to 5.594) | < 0.001 | 2.634 (1.588 to 4.369) | < 0.001 |
| Log-transformed serum creatinine concentration | 909 | 4.0 (0.3) | 4.1 (0.3) | 3.120 (1.863 to 5.226) | < 0.001 | 1.157 (0.598 to 2.240) | 0.665 |
| Log-transformed PCR | 838 | 4.2 (1.4) | 4.9 (1.5) | 1.369 (1.230 to 1.524) | < 0.001 | 1.111 (0.955 to 1.293) | 0.173 |
| Treatment provided | |||||||
| Antihypertensive therapy | 945 | 225 (72%) | 526 (83%) | 1.931 (1.398 to 2.667) | < 0.001 | 1.555 (1.055 to 2.292) | 0.026 |
| Administration of magnesium sulphate | 945 | 8 (3%) | 136 (22%) | 10.433 (5.042 to 21.587) | < 0.001 | 3.886 (1.746 to 8.653) | 0.001 |
Predictor variables were dropped stepwise based on the largest p-value. The final list of predictors for the logistic model were maternal age, log-transformed gestational age at diagnosis, summary score for medical history, systolic BP, platelet count, log-transformed serum urea concentration, log-transformed PCR, baseline treatment with any antihypertensive and baseline treatment with magnesium sulphate.
Transformation of predictors for the final PREP-L model
We considered the following continuous variables for non-linear terms: maternal age, log-transformed gestational age at diagnosis, systolic BP, platelet count, log-transformed serum urea concentration and log-transformed PCR.
Appendix 5 shows the FP terms identified within each multiply imputed data set and the p-value for the test of deviance comparing the FP model with the model including linear terms only. Non-linear terms were identified as significant at the 1% level for log-transformed gestational age at diagnosis and serum urea concentration.
Final PREP-L model before adjusting for optimism
Table 12 shows the final logistic model after multiple imputation, including FP terms, and prior to adjustment for optimism.
| Candidate predictors | Odds ratio (95% CI) | p-value |
|---|---|---|
| Maternal age (years) | 0.977 (0.950 to 1.004) | 0.099 |
| FP (log-gestational age at diagnosis)3 | 1,188,051.840 (29,739.511 to 47,461,008.565) | < 0.001 |
| FP (log-gestational age at diagnosis)3 × ln(log-gestational age at diagnosis) | 0.000 (0.000 to 0.001) | < 0.001 |
| Effect of one pre-existing condition | 0.681 (0.467 to 0.994) | 0.046 |
| Effect of more than two pre-existing conditions | 0.510 (0.295 to 0.884) | 0.016 |
| Systolic BP (mmHg) | 1.028 (1.017 to 1.039) | < 0.001 |
| Platelet count (× 109/l) | 0.995 (0.993 to 0.997) | < 0.001 |
| FP (log-serum urea concentration)–1 | 0.332 (0.191 to 0.575) | < 0.001 |
| Log-transformed PCR | 1.185 (1.030 to 1.362) | 0.019 |
| Baseline treatment: any antihypertensive drug | 1.607 (1.085 to 2.380) | 0.018 |
| Baseline treatment: magnesium sulphate | 4.279 (1.963 to 9.325) | < 0.001 |
| Constant | 0.000 (0.000 to 0.000) | < 0.001 |
The final PREP-L model identified that maternal age, early gestational age at diagnosis of pre-eclampsia, raised systolic BP, high urine PCR, high serum urea concentration, low platelet counts, need for treatment with antihypertensive drugs and administration of magnesium sulphate were associated with increased risk of adverse maternal outcomes. A positive medical history for pre-existing medical conditions or a previous history of pre-eclampsia was associated with a reduced risk of complications.
Apparent performance and internal validation of the PREP-L model
The apparent c-statistic for the PREP-L model (averaged across all multiply imputed data sets) was 0.84 (95% CI 0.82 to 0.87) and after adjustment for optimism it was 0.82 (95% CI 0.80 to 0.84). A sensitivity analysis showed that when all predictors were added to the final model, the c-statistic increased by < 0.01. Another sensitivity analysis of the model using oral and parenteral antihypertensive drugs separately showed no change in the c-statistic; therefore, the combined antihypertensive variable was retained. The addition of log-transformed serum uric acid concentration increased the c-statistic by < 0.004.
The predicted risk was grouped into tenths defined by centiles of predicted risk. Table 13 shows the proportions of outcomes observed within each centile of risk. Figure 3 shows predicted versus observed risk in the model development data set PREP.
| Centile of risk | Women, n | Outcomes observed, n (%) |
|---|---|---|
| < 10th centile | 11 | 3 (27) |
| 10–20th centile | 35 | 11 (31) |
| 20–30th centile | 58 | 15 (26) |
| 30–40th centile | 78 | 20 (26) |
| 40–50th centile | 80 | 34 (43) |
| 50–60th centile | 92 | 47 (51) |
| 60–70th centile | 87 | 54 (62) |
| 70–80th centile | 122 | 86 (70) |
| 80–90th centile | 159 | 144 (91) |
| > 90th centile | 224 | 219 (98) |
FIGURE 3.
Predicted versus observed risk for maternal complications in the PREP-L model. Reproduced from Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided that appropriate credit to the original authors and the source is given. 40

Final adjusted PREP-L model for adverse maternal outcomes in women with early-onset pre-eclampsia
Based on the optimism in calibration, the predictor effect estimates of the developed model coefficient were reduced by the uniform shrinkage factor of 0.862. Appendix 6 shows the coefficient for the final logistic model adjusted for optimism.
Based on the women’s characteristics the probability of adverse outcome by discharge is:
where:
β1–βn are the coefficients for predictors in Appendix 6. Written formally, the equation used to derive individual risk predictions by discharge is as shown in Box 3.
GA, gestational age; MgSO4, magnesium sulphate; SBP systolic BP.
The predicted probability of an outcome is exp(X)/(1 + exp(X)), where X is the predicted logit-p.
Application of the PREP-L model
We have shown examples of application of the PREP-L model below for two women recruited in the PREP study.
Scenario 1
BVH007, a 24-year-old woman with no relevant medical history, was admitted with a diagnosis of pre-eclampsia at 33 + 6 weeks’ gestation. Her highest systolic BP was 200 mmHg and her urine PCR was 4907.6 mg/mmol. Her blood profile showed a platelet count of 75 × 109/l and a serum urea concentration of 9.5 mmol/l. She required parenteral antihypertensive therapy to manage her BP and was started on magnesium sulphate by her clinicians.
Applying the equation exp(3.649)/[1 + exp(3.649)], her predicted risk of adverse maternal outcome by discharge was 97%. The mother was observed to need a blood transfusion following an emergency caesarean section as a result of worsening pre-eclampsia at 9 hours after diagnosis (Table 14).
| Predictor variables | Example 1: BVH007 | Example 2: BWH012 | ||||
|---|---|---|---|---|---|---|
| Predictor values | Calculation | Predictor values | Calculation | |||
| Maternal age (years) | 24 | –0.020 × 24 | –0.480 | 28 | –0.020 × 28 | –0.560 |
| Summary score of medical history | 0 | +0 | +0 | 1 | +1 | +1 |
| Gestational age (weeks) at diagnosis | 33.857 | +12.052 × {[log(33.857)]3 – 39.90241} – 7.930 × [log(33.857)]3 × log{[log(33.857)] – 49.08188} | –1.344 | 32.857 | + 12.052 × {[log(32.857)]3 – 39.90241} – 7.930 × [log(32.857)]3 × log{[log(32.857)] – 49.08188} | –0.744 |
| PCR (mg/mmol) | 4907.6 | +0.146 × log(4907.6) | +1.241 | 0.32 | +0.146 × log(0.32) | –0.166 |
| Serum urea concentration (mmol/l) | 9.5 | –0.951 × [log(9.5) – 1] | –0.422 | 3.5 | –0.951 × [log(3.5) – 1] | –0.759 |
| Platelet count (× 109/l) | 75 | –0.004 × 75 | –0.300 | 283 | –0.004 × 283 | –1.132 |
| Systolic BP (mmHg) | 200 | +0.024 × 200 | +4.800 | 136 | +0.024 × 136 | +3.264 |
| Baseline treatment | ||||||
| Any antihypertensive drug | 1 (‘yes’) | +0.409 | +0.409 | 1 (‘yes’) | +0.409 | +0.409 |
| Magnesium sulphate | 1 (‘yes’) | +1.252 | +1.252 | 0 (‘no’) | +0 | +0 |
| – 1.507 | –1.507 | |||||
| = 3.649 | = –1.525 | |||||
| Predicted risk by discharge | 0.976 | 0.179 | ||||
| Adverse maternal outcomes | Blood transfusion within 9 hours of diagnosis | None | ||||
Scenario 2
BWH012, a 28-year-old woman, was admitted with a diagnosis of pre-eclampsia at 32 + 6 weeks’ gestation. She had a summary score of 1 for relevant medical history and her highest systolic BP was 136 mmHg. Her PCR was 0.32 mg/mmol and her blood profile showed a platelet count of 283 × 109/l and a serum urea concentration of 3.5 mmol/l. She was started on parenteral antihypertensives by her clinician to manage her BP.
Applying the equation, exp (–1.525)/[1 + exp(–1.525)], her predicted risk of adverse maternal outcome by discharge was 18%. The mother was discharged without having any adverse maternal outcome (see Table 14).
Sensitivity analysis of the PREP-L model in participants with unconfirmed diagnosis of pre-eclampsia
Of the 142 participants recruited with a suspected diagnosis of pre-eclampsia, 138 had a 1+ urine dipstick. There were two perfect predictions by baseline treatment with magnesium sulphate and these two observations were dropped. The optimism-adjusted logistic model, as described in Appendix 6, was applied to 136 women with an unconfirmed diagnosis of pre-eclampsia. The apparent c-statistic was 0.68 (95% CI 0.58 to 0.79) and the calibration slope was 0.64 (95% CI 0.25 to 1.04).
Chapter 5 Prediction of adverse maternal outcome in women with early-onset pre-eclampsia: PREP-S model
Overall, 946 women contributed towards 584 failures. Five of these failures occurred on the same day as diagnosis and were forced into the survival model by adding 10 minutes to the time of their occurrence. The total analysis time at risk was 10,923 days, the median analysis time per participant was 6 days (interquartile range 2–14 days) and the longest follow-up period is 98 days. The mean gestational age at delivery was 33.0 weeks (SD 3.2 weeks). For the survival model, the first adverse events are defined as the failure event and are shown in Table 15. Delivery before 34 weeks’ gestation contributed the most (85%, 497/584) to failures.
| Failure defining adverse event | Number of women (N = 584), n (%) |
|---|---|
| Eclamptic seizures after diagnosis | 11 (1.9) |
| Glasgow Coma Scale score of < 13 | 1 (0.2) |
| Hepatic dysfunction | 4 (0.7) |
| At least 50% FiO2 for > 1 hour | 1 (0.2) |
| Intubation | 1 (0.2) |
| Pulmonary oedema | 4 (0.7) |
| Transfusion of any blood product | 12 (2.1) |
| Abruption | 16 (2.7) |
| PPH | 31 (5.3) |
| Delivery at < 34 weeks’ gestational age | 497 (85.1) |
| Combined events | |
| Acute renal insufficiency and preterm delivery (< 34 weeks’ gestation) | 1 (0.2) |
| Abruption and preterm delivery (34 weeks’ gestation) | 3 (0.5) |
| Intubation, PPH and transfusion of any blood product | 1 (0.2) |
| Abruption and PPH | 1 (0.2) |
Modelling continuous predictors
We modelled the continuous predictors as shown in Chapter 4, Modelling continuous predictors.
Development of the PREP-S model: predictor selection
Table 16 shows the hazard ratios for adverse pregnancy outcomes for various candidate predictors by univariable and by multivariable analysis summarised across using the multiply imputed data sets. After dropping the candidate predictor variables stepwise based on the largest p-value, the following were included as the final list of predictors in the survival model: maternal age, log-transformed gestational age at diagnosis, summary score for medical history, systolic BP, clonus, exaggerated tendon reflexes, oxygen saturation, platelet count, log-transformed ALT concentration, log-transformed serum urea concentration, log-transformed serum creatinine concentration, log-transformed PCR, baseline treatment with any antihypertensive drug and baseline treatment with magnesium sulphate.
| Candidate predictors | Women,a n | No adverse maternal outcome (n = 374), mean (SD) or n (%) | Adverse maternal outcome (n = 572), mean (SD) or n (%) | Univariable analysis (N = 946) | Multivariable analysis (N = 946) | ||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | ||||
| Maternal characteristics | |||||||
| Maternal age (years) | 944 | 30.9 (6.4) | 29.8 (6.0) | 0.972 (0.959 to 0.984) | < 0.001 | 0.968 (0.954 to 0.982) | < 0.001 |
| Log-transformed gestational age (weeks) at diagnosis | 946 | 3.45 (0.1) | 3.40 (0.1) | 10.198 (4.162 to 24.987) | < 0.001 | 22.425 (8.528 to 58.970) | < 0.001 |
| Multiple pregnancy | |||||||
| Singleton (reference) | 946 | 319 (88%) | 544 (93%) | ||||
| Twins | 41 (11%) | 38 (7%) | 0.790 (0.568 to 1.098) | 0.160 | 0.895 (0.631 to 1.270) | 0.535 | |
| Triplets | 2 (1%) | 2 (< 1%) | 0.816 (0.203 to 3.273) | 0.774 | 1.194 (0.291 to 4.904) | 0.806 | |
| Global test | 0.360 | 0.794 | |||||
| Medical history score | |||||||
| 0 (reference) | 945 | 195 (54%) | 400 (69%) | ||||
| 1 | 116 (32%) | 134 (23%) | 0.604 (0.496 to 0.736) | < 0.001 | 0.828 (0.671 to 1.022) | 0.078 | |
| ≥ 2 | 51 (14%) | 49 (8%) | 0.468 (0.347 to 0.631) | < 0.001 | 0.658 (0.479 to 0.905) | 0.010 | |
| Global test | < 0.001 | 0.017 | |||||
| Symptoms | |||||||
| Headache and/or visual disturbance | 920 | 142 (40%) | 238 (42%) | 1.136 (0.962 to 1.341) | 0.133 | 1.007 (0.835 to 1.215) | 0.940 |
| Epigastric pain, nausea and/or vomiting | 901 | 61 (18%) | 139 (25%) | 1.495 (1.223 to 1.828) | < 0.001 | 0.943 (0.745 to 1.194) | 0.627 |
| Chest pain and/or dyspnoea | 822 | 19 (6%) | 41 (8%) | 1.227 (0.844 to 1.785) | 0.293 | 1.172 (0.766 to 1.793) | 0.472 |
| Bedside examination and tests | |||||||
| Clonus | 545 | 24 (13%) | 70 (19%) | 1.622 (1.303 to 2.020) | < 0.001 | 0.763 (0.547 to 1.064) | 0.129 |
| Exaggerated tendon reflexes | 594 | 28 (14%) | 118 (30%) | 1.966 (1.602 to 2.413) | < 0.001 | 1.249 (0.996 to 1.566) | 0.055 |
| Systolic BP (mmHg) | 942 | 151 (14) | 163 (20) | 1.028 (1.023 to 1.032) | < 0.001 | 1.018 (1.012 to 1.024) | < 0.001 |
| Diastolic BP (mmHg) | 942 | 96 (10) | 102 (12) | 1.033 (1.026 to 1.040) | < 0.001 | 1.002 (0.993 to 1.011) | 0.695 |
| Oxygen saturation: abnormal (< 94%) | 429 | 0 (0%) | 4 (1%) | 5.769 (2.154 to 15.449) | < 0.001 | 4.342 (1.496 to 12.607 | 0.007 |
| Urine dipstick: none/trace (reference) | 928 | 18 (5%) | 21 (4%) | ||||
| 1+ | 95 (27%) | 74 (13%) | 0.731 (0.449 to 1.190) | 0.208 | 0.864 (0.522 to 1.433) | 0.572 | |
| 2+ | 136 (38%) | 177 (31%) | 1.065 (0.676 to 1.679) | 0.786 | 0.994 (0.619 to 1.597) | 0.981 | |
| 3+ | 84 (24%) | 219 (38%) | 1.853 (1.178 to 2.914) | 0.008 | 1.293 (0.795 to 2.103) | 0.300 | |
| ≥ 4 | 21 (6%) | 83 (14%) | 2.487 (1.536 to 4.026) | < 0.001 | 1.216 (0.717 to 2.062) | 0.469 | |
| Global test | < 0.001 | 0.076 | |||||
| Laboratory tests | |||||||
| Haemoglobin (g/l) | 910 | 11.8 (1.2) | 12.0 (1.4) | 1.102 (1.032 to 1.176) | 0.004 | 1.051 (0.984 to 1.121) | 0.137 |
| Platelet count (× 109/l) | 906 | 242 (76) | 217 (77) | 0.995 (0.994 to 0.997) | < 0.001 | 0.997 (0.996 to 0.998) | < 0.001 |
| Log-transformed ALT concentration | 871 | 2.8 (0.6) | 3.0 (0.8) | 1.503 (1.338 to 1.689) | < 0.001 | 1.181 (1.040 to 1.341) | 0.011 |
| Log-transformed serum uric acid concentration | 782 | –1.3 (1.0) | –1.0 (0.7) | 1.431 (1.280 to 1.600) | < 0.001 | ||
| Log-transformed serum urea concentration | 877 | 1.2 (0.4) | 1.5 (0.5) | 1.985 (1.734 to 2.273) | < 0.001 | 1.555 (1.296 to 1.865) | < 0.001 |
| Log-transformed serum creatinine concentration | 909 | 4.0 (0.3) | 4.1 (0.3) | 3.381 (2.474 to 4.620) | < 0.001 | 1.549 (1.081 to 2.219) | 0.017 |
| Log-transformed PCR | 838 | 4.2 (1.4) | 5.0 (1.5) | 1.405 (1.303 to 1.516) | < 0.001 | 1.082 (1.001 to 1.169) | 0.049 |
| Treatment provided | |||||||
| Antihypertensive therapy | 945 | 264 (73%) | 487 (84%) | 1.477 (1.187 to 1.838) | < 0.001 | 1.239 (0.983 to 1.562) | 0.070 |
| Magnesium sulphate administered | 945 | 9 (2%) | 135 (23%) | 6.654 (5.442 to 8.137) | < 0.001 | 3.540 (2.708 to 4.627) | < 0.001 |
We identified non-linear terms for log-transformed gestational age at diagnosis and serum urea concentration using FPs. We chose terms with a p-value of > 0.001 to avoid overfitting. After including non-linear terms for gestational age at diagnosis and serum urea concentration, clonus then filled the criterion for exclusion at a p-value of > 0.150 and was therefore removed. Running the multivariable FP procedure without clonus confirmed that the FP terms identified originally were still valid (results not shown).
In the full multivariable model, the risk of adverse maternal outcomes were significantly increased at the 5% level with lower maternal age, greater gestational age at diagnosis, lower number of components of medical history, raised systolic BP, lower platelet count, raised ALT concentration, raised serum urea concentration, increased urine PCR and administration of magnesium sulphate.
We applied the variable selection process and included the non-linear terms for gestational age at diagnosis and serum urea concentration. The PREP-S survival model prior to adjustment for optimism is shown in Table 17.
| Flexible parametric model after multiple imputation | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| Maternal age (years) | 0.964 | 0.951 to 0.978 | < 0.001 |
| FP [log(GA at diagnosis/10)]–2 centred at 0.8345136 | 5.794 | 0.299 to 112.276 | 0.245 |
| aFP [log(GA at diagnosis/10)]–2 ln[log(GA at diagnosis/10)] centred at 0.0652155 | 750.276 | 2.380 to 236561.167 | 0.024 |
| Exaggerated tendon reflexes | 1.152 | 0.935 to 1.420 | 0.185 |
| Number of pre-existing conditions | |||
| 0 | |||
| Effect of a medical history score of 1 | 0.822 | 0.668 to 1.010 | 0.062 |
| Effect of a medical history score of ≥ 2 | 0.640 | 0.464 to 0.883 | 0.007 |
| Systolic BP (mmHg) | 1.018 | 1.013 to 1.023 | < 0.001 |
| Oxygen saturation < 94% | 2.520 | 0.870 to 7.298 | 0.089 |
| Platelet count (× 109/l) | 0.997 | 0.996 to 0.998 | < 0.001 |
| Log-transformed ALT concentration | 1.157 | 1.031 to 1.299 | 0.013 |
| FP (log-serum urea concentration)2 | 2.017 | 1.611 to 2.526 | < 0.001 |
| FP (log-serum urea concentration)3 | 0.846 | 0.795 to 0.900 | < 0.001 |
| Log-transformed serum creatinine concentration centred at 4.067578 | 1.361 | 0.952 to 1.944 | 0.091 |
| Log-transformed PCR | 1.097 | 1.025 to 1.173 | 0.007 |
| Baseline treatment | |||
| Any antihypertensive drug | 1.227 | 0.976 to 1.543 | 0.080 |
| Magnesium sulphate | 3.445 | 2.675 to 4.437 | < 0.001 |
Apparent performance of the PREP-S model
The apparent c-statistic of the developed survival model was 0.78 (95% CI 0.76 to 0.80). Estimation of risks in the five groups defined by the 10th, 25th, 75th and 90th centiles of predicted risk showed that the probability of adverse outcome was around 52% in the highest risk group at 48 hours after diagnosis, 95% by 1 week, and 100% by 1 month. Ninety-seven per cent (91/94) of women in the > 90th centile group experienced an adverse maternal outcome, with a mean follow-up time of 1.2 days (SD 1.3 days). In the lowest risk group (≤ 10th centile), the probability of outcome-free survival was around 97% at 48 hours after diagnosis, 87% by 1 week, and 56% by 1 month. In this group, 24% (23/95) had an adverse outcome, with a mean follow-up time of 28.9 days (SD 23.1 days) (Table 18).
| Centile of predicted risk | Number of women, n | Adverse maternal outcome, n (%) | Follow-up time (days), mean (SD) | |
|---|---|---|---|---|
| Adverse outcome | No adverse outcome | |||
| ≤ 10th | 95 | 22 (23) | 28.2 (23.3) | 40.2 (29.8) |
| 10–25th | 143 | 64 (45) | 16.1 (12.7) | 15.9 (13.7) |
| 25–75th | 471 | 289 (61) | 8.6 (8.4) | 10.1 (9.4) |
| 75–90th | 143 | 118 (83) | 3.9 (4.4) | 6.3 (5.9) |
| > 90th | 94 | 91 (97) | 1.2 (1.3) | 3.7 (4.6) |
In women in the groups defined by the highest centiles of predicted risk, there was good agreement between those with observed and predicted adverse outcomes. About 97% (91/94) of women with risks above the 90th centile had an adverse outcome; 83% (118/143) of women with predicted risks between the 75th and 90th centiles had complications; and 61% (289/471) with risks between the 25th and 75th centiles experience an adverse outcome.
Figure 4 shows the model-based mean survival curves for the five prognostic groups compared with their observed Kaplan–Meier survival curves, for 1 month after diagnosis-days. Agreement is generally excellent, perhaps with the exception of the lowest risk group, although this has fewest events and so more uncertainty about the mean predicted curve. CIs are not shown on the figure for aesthetic reasons.
FIGURE 4.
Mean survival curves for groups of prognostic index compared with their observed Kaplan–Meier survival curves up to 30 days from diagnosis. Reproduced from Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided that appropriate credit to the original authors and the source is given. 40

Sensitivity analysis
Inclusion of all candidate predictors in the model only improved the Harrell’s c-statistic by < 0.001. Comparison of the Cox regression model with our flexible parametric model yielded similar hazard ratios, as expected. The full models are presented in Appendix 7. Time-dependent effects on the model were not significant for all covariates except baseline medication. Inclusion of this effect for baseline medication into the model improved Harrell’s c-statistic by 0.005 (results not shown). In order to avoid overfitting and achieve simplicity, we considered this improvement too small to include a time-dependent effect into our final model. Another sensitivity analysis of the model using oral and parenteral antihypertensive therapy separately showed no change in the c-statistic; therefore, the combined antihypertensive variable was retained. Addition of log-transformed uric acid concentration changed the Harrell’s c-statistic by < 0.001.
Internal validation and shrinkage of estimates for the final PREP-S model
The bootstrap approach showed an optimism of 0.019 (SD 0.010) in the c-statistic and 0.138 (SD 0.002) in the calibration slope. Based on the optimism in calibration, the predictor effect estimates of the developed model coefficient were reduced by the uniform shrinkage factor: 1 – 0.138 = 0.862. The intercept of the baseline spline term was re-estimated to ensure perfect calibration-in-the-large. The optimism-adjusted Harrell’s c-statistic of the survival model was 0.75 (95% CI 0.73 to 0.78).
Appendix 8 shows the coefficients of the final PREP-S model and the baseline hazard after adjusting for optimism. Table 19 gives the baseline survival at various time points to calculate the predicted survival probability for a woman diagnosed with early-onset pre-eclampsia.
| Time point (days) | Baseline survival, S0(t) |
|---|---|
| 2 | 0.99142 |
| 3 | 0.98542 |
| 4 | 0.97973 |
| 5 | 0.97452 |
| 6 | 0.96962 |
| 7 | 0.96492 |
| 14 | 0.93404 |
| 21 | 0.90373 |
| 28 | 0.87377 |
| 35 | 0.84432 |
| 42 | 0.81549 |
Based on the woman’s characteristics, the survival probability at time point t is:
where β1–βn are the coefficients for predictors shown in Appendix 8, and X1–Xn are the predictor values for the patient. Written formally, the equation used to derive individual risk predictions over time is shown in Box 4.
where S0(t) is the value of the baseline survival function at time t (see Table 19).
Positive regression coefficients suggest an increase in the risk of adverse maternal outcome with increasing values of continuous predictors or the presence of dichotomous predictor variables, and vice versa for negative coefficients.
Application of the PREP-S model
We have provided examples of calculating the individual risk of adverse maternal outcomes at 48 hours using the PREP-S model. The predictor values of two women are provided in Table 20. The calculation of risk can easily be amended to a different time point by replacing the value for baseline survival with the baseline survival for the desired time point from Table 18.
| Candidate predictors | Example 1: BVH007 | Example 2: BWH012 | ||||
|---|---|---|---|---|---|---|
| Predictor values | Calculation | Predictor values | Calculation | |||
| Time point | 48 hours | 48 hours | ||||
| Baseline survival | = 0.99142exp(. . . | = 0.99142exp(. . . | ||||
| Maternal age (years) | 24 | – 0.031 × 24 | –0.744 | 28 | –0.031 × 28 | –0.868 |
| Gestational age (weeks) at diagnosis | 33.857 | + 1.514 × (log(33.857/10)–2 –0.8345136)+ 5.707 × (log(33.857/10)–2 × log(log(33.857/10)) – 0.0652155) | +0.144 | 32.857 | + 1.514 × (log(32.857/10)–2 –0.8345136)+ 5.707 × (log(32.857/10)–2× log(log(32.857/10)) – 0.0652155) | +0.134 |
| Exaggerated tendon reflexes | 0 (‘no’) | +0 | +0 | 0 (‘no’) | +0 | +0 |
| Summary score for medical history | 0 | +0 | +0 | 1 | – 0.169 | –0.169 |
| Systolic BP (mmHg) | 200 | +0.016 × 200 | +3.200 | 136 | +0.016 × 136 | +2.176 |
| ALT concentration (U/l) | 72 | +0.126 × log(72) | +0.539 | 11 | +0.126 × log(11) | +0.302 |
| PCR (mg/mmol) | 4907.6 | +0.080 × log(4907.6) | +0.680 | 0.32 | +0.080 × log(0.32) | –0.091 |
| Serum urea concentration (mmol/l) | 9.5 | +0.605 × log(9.5)2 – 0.144 × log(9.5)3 | +1.423 | 3.5 | +0.605 × log(3.5)2 – 0.144 × log(3.5)3 | +0.666 |
| Serum creatinine concentration (µmol/l) | 74 | +0.265 × log(74) | +1.141 | 33 | +0.265 × log(33) | +0.927 |
| Platelet count (× 109/l) | 75 | –0.002 × 75 | –0.150 | 283 | –0.002 × 283 | –0.566 |
| Oxygen saturation < 94% | 99 | +0 | +0 | Assumed normal | +0 | +0 |
| Baseline treatment | ||||||
| Any antihypertensive drug | 1 (‘yes’) | +0.176 | +0.176 | 1 (‘yes’) | +0.176 | +0.176 |
| Magnesium sulphate | 1 (‘yes’) | +1.066 | +1.066 | 0 (‘no’) | +0 | +0 |
| = 7.475 | = 2.687 | |||||
| Predicted survival by 48 hours | = 0.00000025 | = 0.881 | ||||
| Adverse maternal outcomes | Blood transfusion within 9 hours of diagnosis | None | ||||
Sensitivity analysis of survival model in participants with unconfirmed diagnosis of pre-eclampsia
Of the 142 participants recruited with a suspected diagnosis of pre-eclampsia, 138 had a 1+ urine dipstick. For one woman the time of outcome was missing. The optimism-adjusted survival model, as described in Appendix 8, was applied to this population. The apparent c-statistic was 0.64 (95% CI 0.53 to 0.76) and the calibration scope was 0.88 (95% CI 0.17 to 1.58).
Chapter 6 External validation of the prediction models for complications in women with early-onset pre-eclampsia
Inclusion criteria and availability of data in external data sets
The PIERS study
The PIERS study evaluated the effects of 48 predictors in 2023 women with pre-eclampsia of any onset. Of these, 636 (31%) were diagnosed with early-onset pre-eclampsia and 634 had available data for external validation of the PREP models. The majority of women with early-onset pre-eclampsia were classified as having new-onset disease (519/636, 82%), followed by those with superimposed pre-eclampsia (95/636, 15%) and HELLP syndrome (22/636, 3%) (Table 21).
| Inclusion criteria | Study | ||
|---|---|---|---|
| Women in the PREP cohort (N = 954), n (%) | Women in the PIERS cohort (N = 636), n (%) | Women in the PETRA cohort (N = 216), n (%) | |
| New-onset pre-eclampsia | 866 (91.0) | 519 (82) | 96 (44)a |
| Chronic hypertension | 75 (7.9) | ||
| Superimposed pre-eclampsia | 10 (1.0) | 95 (15) | |
| HELLP syndrome | 3 (0.3) | 22 (3) | 54 (25)a |
| Eclampsia | 5 (2.3)a | ||
| Fetal growth restriction or pregnancy-induced hypertension | 125 (58)a | ||
Of the 13 predictors in the PREP models, 10 were also evaluated in the PIERS study. Exaggerated tendon reflexes, serum urea concentration and autoimmune diseases (one element of the medical history) were assessed in the PREP study, but were not available in the PIERS data set.
The PETRA study
The PETRA study evaluated the effect of plasma volume expansion in 111 patients with severe hypertensive disorders of pregnancy compared with a control group of 105 patients with severe hypertensive disorders of pregnancy. All patients (n = 216) had a diagnosis of early-onset pre-eclampsia and had available data for external validation of the PREP-L model only. The majority of women with early-onset pre-eclampsia were those classified as having fetal growth restriction or pregnancy-induced hypertension (125/216, 58%), followed by those with new-onset pre-eclampsia (96/216, 44%), HELLP syndrome (54/216, 25%) and eclampsia (5/216, 2.3%) (see Table 21).
Characteristics of women with early-onset pre-eclampsia in the PIERS and PETRA studies
There were no significant differences in the mean gestational age at diagnosis of pre-eclampsia, which was around 30 weeks. The PETRA study included only singleton pregnancies, while around 91% (870/954) of pregnancies in the PREP study and 85% (542/634) in the PIERS study were singletons. Two-thirds (601/953, 63%) of women in the PREP study did not have any significant medical history, such as pre-existing medical conditions or previous history of pre-eclampsia, compared with 45% (284/634) and 84% (182/216) in the PIERS and PETRA studies, respectively.
The PETRA study did not have any data on symptoms or examination findings such as deep-tendon reflexes or clonus. In addition, the study did not report any tests for proteinuria, oxygen saturation and serum creatinine concentration that were reported in the other two cohorts. Table 22 compares patient characteristics and candidate predictor variables in the PREP development data set and the PIERS and PETRA validation data sets.
| Characteristics of women | Study | |||||
|---|---|---|---|---|---|---|
| PREP | PIERS | PETRA | ||||
| Women for whom data were available (n) | Mean (SD) or n (%) | Women for whom data were available (n) | Mean (SD) or n (%) | Women for whom data were available (n) | Mean (SD) or n (%) | |
| Gestational age at diagnosis (weeks), mean (SD) | 954 | 30.5 (2.9) | 634 | 30.2 (3.0) | 216 | 29.4 (2.6) |
| Maternal characteristics | ||||||
| Maternal age (years), mean SD | 952 | 30.2 (6.1) | 634 | 31.2 (6.3) | 216 | 30.0 (5.0) |
| Number of fetuses in pregnancy | 954 | 634 | 216 | |||
| Singleton | 866 (91%) | 542 (85%) | 216 (100%) | |||
| Twins | 83 (9%) | 88 (14%) | – | |||
| Triplets | 5 (1%) | 4 (1%) | – | |||
| History | ||||||
| Summary score for medical history | 953 | 634 | 216 | |||
| 0 | 601 (63%) | 284 (45%) | 182 (84%) | |||
| 1 | 251 (26%) | 251 (40%) | 30 (14%) | |||
| ≥ 2 | 101 (11%) | 99 (15%) | 4 (2%) | |||
| Symptoms | ||||||
| Headache and/or visual disturbance, present | 926 | 382 (41%) | 634 | 319 (50%) | – | – |
| Epigastric pain, nausea and/or vomiting, present | 907 | 202 (22%) | 634 | 220 (35%) | – | – |
| Chest pain and/or dyspnoea, present | 828 | 60 (7%) | 634 | 42 (7%) | – | – |
| Examination | ||||||
| Clonusa | 551 | 95 (17%) | – | – | – | – |
| Exaggerated tendon reflexes,a mean (SD) | 601 | 139 (15%) | – | – | – | – |
| Systolic BP, mean (SD) | 949 | 159 (19) | 634 | 168 (20) | 216 | 157 (18) |
| Diastolic BP, mean (SD) | 949 | 99 (12) | 634 | 105 (11) | 216 | 104 (11) |
| Oxygen saturation by pulse oximetry (%), mean (SD) | 433 | 98.1 (1.6) | 474 | 96 (2) | – | – |
| Oxygen saturation abnormal (< 94%), present | 433 | 4 (1%) | 474 | 72 (15%) | – | – |
| Laboratory tests | ||||||
| Haemoglobin (g/l), mean (SD) | 917 | 11.9 (1.3) | – | – | – | – |
| Platelet count (× 109/l), mean (SD) | 913 | 226 (78) | 630 | 204 (77) | 215 | 172 (87) |
| ALT concentration (U/l), mean (SD) | 879 | 31.0 (71.0) | 630 | 65.5 (157.6) | 207 | 79.9 (139.3) |
| AST concentration (U/l), mean (SD) | 275 | 36.9 (61.1) | 600 | 74.3 (196.5) | 212 | 91.9 (160.7) |
| Serum uric acid concentration (µmol/l), mean (SD) | 789 | 0.6 (2.7) | – | – | – | – |
| Serum urea concentration (mmol/l), mean (SD) | 884 | 4.6 (4.4) | – | – | – | – |
| Serum creatinine concentration (µmol/l), mean (SD) | 916 | 61.9 (17.8) | 626 | 69.3 (20.5) | 214 | 67.8 (16.8) |
| Urine dipstick | ||||||
| None/trace | 935 | 39 (4%) | 613 | 129 (21%) | – | – |
| 1+ | 170 (18%) | 69 (11%) | – | – | ||
| 2+ | 314 (34%) | 111 (18%) | – | – | ||
| 3+ | 306 (33%) | 141 (23%) | – | – | ||
| ≥ 4 | 106 (11%) | 163 (27%) | – | – | ||
| Urine PCR 24 hour (mg/mmol), mean (SD) | 433 | 98.1 (1.6) | 437 | 276 (437) | – | – |
| Baseline treatment | ||||||
| Antihypertensive therapy, present | 948 | 753 (79%) | 634 | 551 (87%) | 216 | 123 (57%) |
| Magnesium sulphate administration, present | 948 | 144 (15%) | 634 | 325 (51%) | 216 | 34 (16%) |
Risk of adverse outcomes in the PIERS and PETRA cohorts
Overall, 67% (633/946) of women with early-onset pre-eclampsia in the PREP study had adverse maternal outcomes by discharge, compared with 77% (489/634) and 86% (185/216) in the PIERS and PETRA cohorts, respectively. The date and time of occurrence of adverse maternal outcomes was consistently reported in the PIERS data set and not in the PETRA study. Maternal and fetal composite outcomes not reported in the PIERS and PETRA data sets are provided in Tables 23 and 24.
| Components of adverse maternal outcome evaluated in the PREP study | PIERS | PETRA |
|---|---|---|
| Maternal death | ✓ | ✓ |
| Eclamptic seizures | ✓ | ✓ |
| Glasgow Coma Scale score of < 13 | ✓ | ✓ |
| Stroke or RIND | ✓ | ✓ |
| Cortical blindness | ✓ | ✓ |
| Retinal detachment | – | – |
| Posterior reversible encephalopathy | – | ✓ |
| Bell’s palsy | ✓ | – |
| Hepatic dysfunction | ✓ | ✓ |
| Subcapsular haematoma | – | – |
| Hepatic rupture | ✓ | ✓ |
| Need for positive inotrope support | ✓ | – |
| Myocardial ischaemia or infarction | ✓ | – |
| At least 50% FiO2 for > 1 hour | ✓ | ✓ |
| Intubation | ✓ | ✓ |
| Pulmonary oedema | ✓ | ✓ |
| Acute renal insufficiency (creatinine concentration of > 200 uM) | ✓ | ✓ |
| Dialysis | ✓ | ✓ |
| Transfusion of any blood product | ✓ | ✓ |
| Abruptions | – | ✓ |
| Postpartum haemorrhage | – | ✓ |
| Delivery at < 34 weeks’ gestational age | ✓ | ✓ |
| Components of adverse fetal outcome evaluated in the PREP study | PIERS | PETRA |
|---|---|---|
| Neonatal death | ✓ | ✓ |
| Bronchopulmonary dysplasia | ✓ | ✓ |
| Necrotising enterocolitis | ✓ | ✓ |
| Grade III/IV intraventricular haemorrhage | ✓ | ✓ |
| Cystic periventricular leukomalacia | ✓ | ✓ |
| Stage 3–5 retinopathy | ✓ | – |
| Hypoxic–ischaemic encephalopathy | – | ✓ |
| Stillbirth | ✓ | ✓ |
| Admission to NICU at any time | ✓ | ✓ |
External validation of the models
As not all predictors in the PREP models were available in the PIERS and PETRA data sets, we externally validated a slightly reduced version of our final models, with the model parameters re-estimated with a reduced set of predictors. We re-estimated the coefficients and intercept terms of the model, and then adjusted for optimism as before. We validated the survival model in only the PIERS data set, because of the non-availability of time of outcome occurrence in the PETRA cohort.
External validation of the PREP-L model in the PIERS data set
Complete records on the predictors considered were available for 437 of 654 women in whom pre-eclampsia was diagnosed at < 34 weeks’ gestation.
Obtaining the reduced PREP-L model
Serum urea concentration was identified as a predictor in the PREP data, but it was not recorded in the PIERS data set. We obtained the reduced PREP-L (rPREP-L) model and adjusted for optimism after excluding serum urea concentration (see Appendix 9). The calibration slope for this optimism-adjusted rPREP-L model was 1.01 (95% CI 0.86 to 1.15) when averaged across all imputed data sets. The apparent c-statistic was 0.82 (95% CI 0.80 to 0.85).
Application of the reduced PREP-L model in the PIERS data set
The optimism-adjusted c-statistic of the rPREP-L model was 0.81 (95% CI 0.77 to 0.85), indicating a good discrimination in the external validation data set. The calibration slope was 0.93 (95% CI 0.72 to 1.13), indicating very good calibration and model fit in the PIERS data on average across all individuals (Figure 5).
FIGURE 5.
Validation plot of the predicted vs. observed risk for adverse maternal outcome using the rPREP-L model in the PIERS cohort. Reproduced from Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided that appropriate credit to the original authors and the source is given. 40

The predicted risk was grouped into centiles of predicted risk. Table 25 shows the risk of outcome for each centile of predicted risk. When the intercept term was recalibrated to the PIERS data, the calibration slope and c-statistic remained the same.
| Groups of predicted risk | Women with predicted outcomes, n | Women with observed outcomes, n (%) |
|---|---|---|
| < 10th centile | 0 | – |
| 10–20th centile | 3 | 0 (0) |
| 20–30th centile | 20 | 6 (30) |
| 30–40th centile | 24 | 8 (33) |
| 40–50th centile | 33 | 16 (48) |
| 50–60th centile | 34 | 21 (62) |
| 60–70th centile | 38 | 19 (50) |
| 70–80th centile | 58 | 42 (72) |
| 80–90th centile | 72 | 59 (82) |
| > 90th centile | 155 | 147 (95) |
External validation of the reduced PREP-L model in the PETRA cohort
Complete records on the predictors considered were available for 211 of 216 women in whom pre-eclampsia was diagnosed at < 34 weeks’ gestation .
Harrell’s c-statistic was 0.75 (95% CI 0.64 to 0.86), indicating a moderate discrimination in the external validation data set. The calibration slope was 0.90 (95% CI 0.48 to 1.3), indicating some slight miscalibration, with observed risk generally higher than predicted. However, predictions showed reasonably close agreement at predicted risks above 0.7 (Figure 6). Table 26 shows the risk of outcome for groups defined by tenths of predicted risk.
FIGURE 6.
Validation plot of the predicted vs. observed risk for adverse maternal outcome using the rPREP-L model in the PETRA cohort. Reproduced from Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided that appropriate credit to the original authors and the source is given. 40
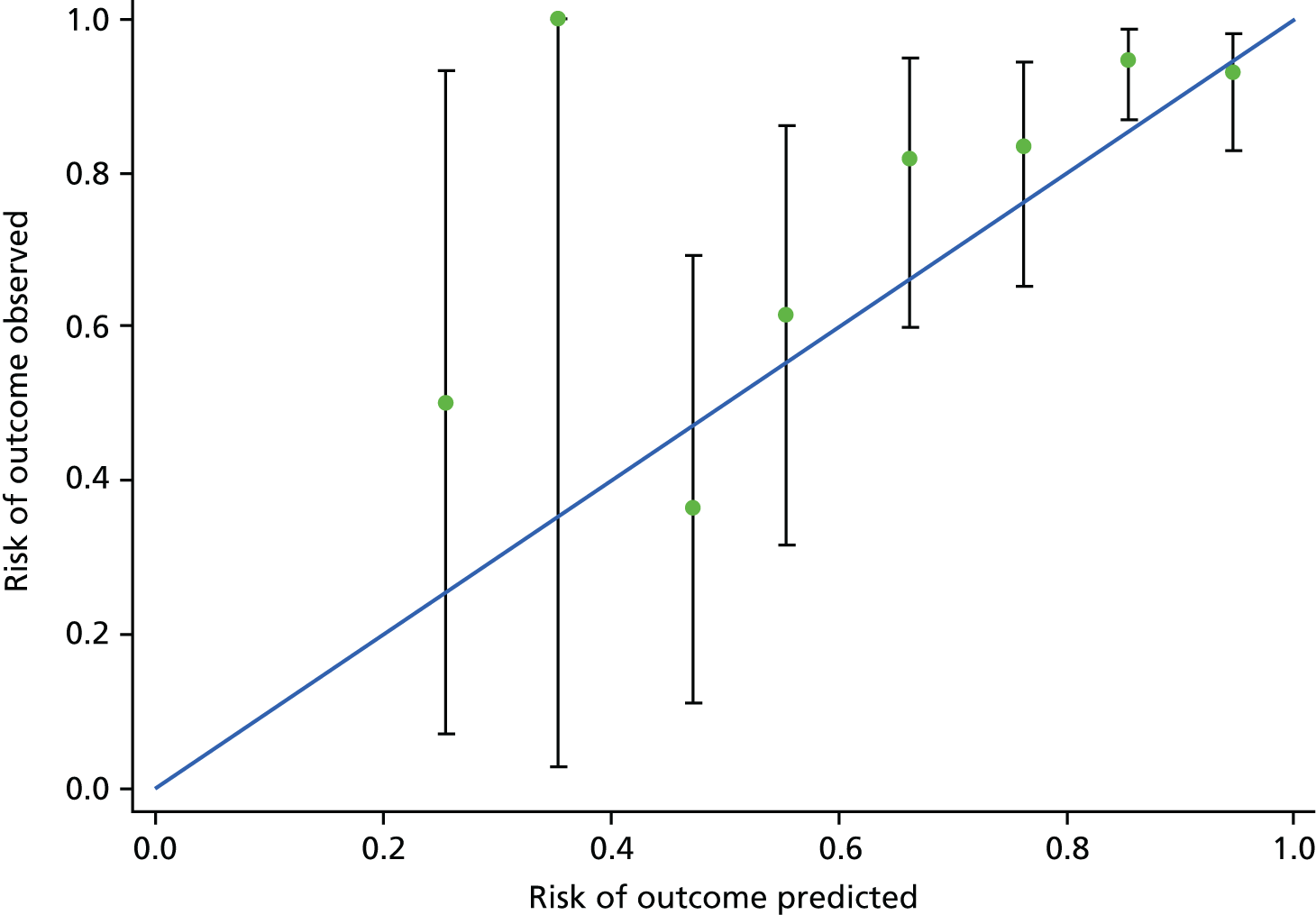
| Groups of predicted risk | Women with predicted outcomes, n | Women with observed outcomes, n (%) |
|---|---|---|
| < 10th centile | 0 | – |
| 10–20th centile | 0 | – |
| 20–30th centile | 4 | 2 (50) |
| 30–40th centile | 1 | 1 (100) |
| 40–50th centile | 11 | 4 (36) |
| 50–60th centile | 13 | 8 (62) |
| 60–70th centile | 22 | 18 (82) |
| 70–80th centile | 30 | 25 (83) |
| 80–90th centile | 74 | 70 (95) |
| > 90th centile | 56 | 52 (93) |
Recalibration of the intercept to the PETRA data did not improve the calibration slope. Table 27 shows the performance of the reduced PREP models in the derivation cohorts and external validation data sets.
| Model performance | PREP | rPREP (for PIERS) | PIERS | rPREP (for PETRA) | PETRA |
|---|---|---|---|---|---|
| PREP-L model | |||||
| Number analysed | 946 | 946 | 437 | 946 | 211 |
| Number of outcomes | 633 | 633 | 318 | 633 | 180 |
| Apparent c-statistic (95% CI) | 0.84 (0.82 to 0.87) | 0.82 (0.80 to 0.85) | 0.81 (0.77 to 0.85) | 0.81 (0.79 to 0.84) | 0.75 (0.64 to 0.86) |
| Optimism-adjusted c-statistic (95% CI) | 0.82 (0.80 to 0.84) | – | – | – | – |
| Calibration slope (95% CI) | 1 | 1 | 0.93 (0.72 to 1.13) | 1 | 0.90 (0.48 to 1.32) |
| PREP-S model | |||||
| Number analysed | 946 | 946 | 339 | – | – |
| Number of events | 584 | 584 | 239 | – | – |
| Apparent c-statistic (95% CI) | 0.77 (0.75 to 0.79) | 0.76 (0.74 to 0.78) | 0.71 (0.67 to 0.75) | – | – |
| Optimism-adjusted c-statistic (95% CI) | 0.75 (0.73 to 0.78) | – | – | – | – |
| Calibration slope (95% CI) | 1 | 1 | 0.67 (0.56 to 0.79) | – | – |
External validation of the PREP-S model in the PIERS data set
In the PIERS data set, 634 women were diagnosed with pre-eclampsia before 34 weeks’ gestation. Four hundred and sixty-one failures occurred during follow-up, six of which occurred on the same day as diagnosis and were included by adding a fraction of a day. One hundred and thirty-two had failures by 48 hours, 332 by 1 week and 458 by 30 days after diagnosis. We evaluated the reduced PREP-S (rPREP-S) model in 339 women with complete predictor value data. The total analysis time was 5425 days, with the last observed exit at 89 days of follow-up.
Obtaining the reduced PREP-S model
As serum urea concentration and exaggerated tendon reflexes were identified to be a predictor in the PREP-S model but were not recorded in the PIERS data set, we refitted the PREP-S model. Appendix 10 shows the coefficients of the rPREP-S prediction model after excluding serum urea concentration and exaggerated tendon reflexes, adjusted for optimism. Harrell’s c-statistic of the optimism-adjusted rPREP-S model was 0.76 (95% CI 0.74 to 0.78).
Applying the reduced PREP-S model in the PIERS data set
The rPREP-S model with coefficients as described in Appendix 10 was fitted to the PIERS data set. Figures 7 and 8 compare the predictions made by the PREP model in four prognostic groups of the PIERS data until 34 weeks’ gestation up to 30 days after diagnosis, respectively.
FIGURE 7.
Validation of the PREP-S model in the PIERS data set up to 30 days from diagnosis.

FIGURE 8.
Validation of the PREP-S model in the PIERS data set up to 7 days from diagnosis.

The c-statistic in the PIERS cohort was 0.71 (95% CI 0.67 to 0.75), which was slightly lower than that of the rPREP-S model. However, the four risk groups are still noticeably distinct and ordered appropriately for the majority of the 30-day period, with the exception of the two intermediate-risk groups before 3 days. The calibration slope of the rPREP-S model in the PIERS data set was 0.67 (95% CI 0.56 to 0.79), suggesting large overprediction of the reduced PREP model, and this was observed predominantly in the third of four groups (which had the largest patient numbers), especially after 5 days. Importantly, those identified as ‘high risk’ by the PREP model were still in the high-risk category in the PIERS cohort, but the observed absolute risk values were lower than expected from the reduced PREP model.
Calibration slope for each of the four risk groups are:
-
≤ 15th centile (n = 59): 0.21 (95% CI –0.51 to 0.92)
-
15–50th centile (n = 70): 0.65 (95% CI –0.80 to 2.10)
-
50–85th centile (n = 123): 0.25 (95% CI –0.34 to 0.84)
-
> 85th centile (n = 87) 0.73 (95% CI 0.42 to 1.00).
Recalibration of the intercept of the baseline hazard function in the rPREP-S model to the PIERS data set did not improve calibration. Figure 9 shows that the agreement between observed and predicted survival was much improved in the high-risk group. However, it was noticeably worse in the other risk groups.
FIGURE 9.
Validation of the rPREP-S model in the PIERS data set after recalibration up to 30 days from diagnosis. Reproduced from Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided that appropriate credit to the original authors and the source is given. 40
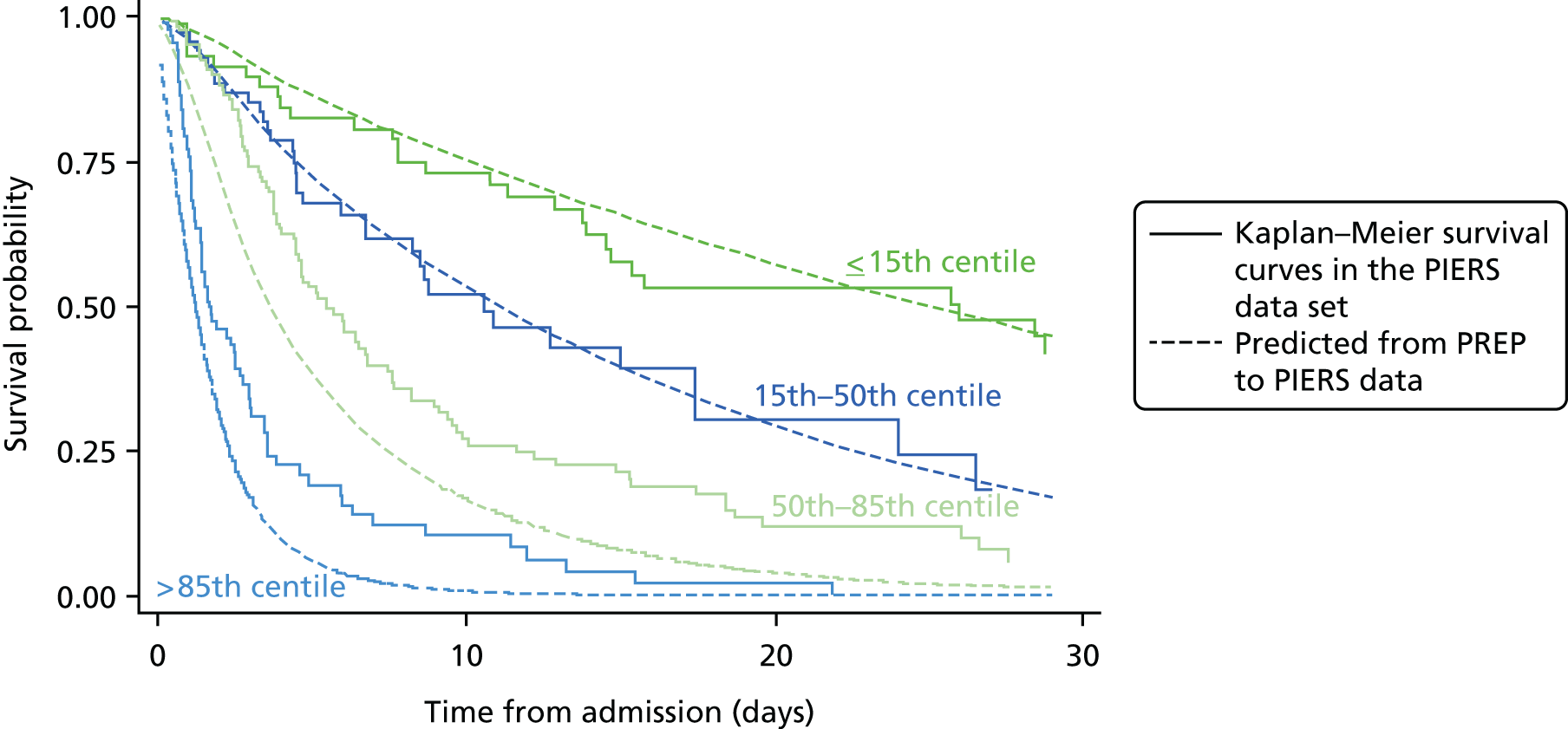
Chapter 7 Prediction of fetal complications in women with early-onset pre-eclampsia
We assessed the predictive value of individual tests on fetal outcomes in the 945 pregnancies for which outcome data were available. The rates of individual fetal and neonatal complications observed in women with early-onset pre-eclampsia can be seen in Table 10.
Performance of the PREP-L model for adverse fetal outcomes
We assessed the performance of the PREP-L model (see Table 12) for the fetal composite outcome, using the same predictors. Table 28 and Figure 10 show the proportions of outcomes observed within each centile of risk. The c-statistic was 0.76 (95% CI 0.73 to 0.79) and the calibration slope was 0.77 (95% CI 0.63 to 0.91), indicating overprediction of risk, especially for prediction of 0.6 or below. In women predicted to be at high risk (> 80th centile), around 90% had adverse fetal outcomes.
| Decile of risk | Women with predicted outcomes, n | Women with observed outcomes, n (%) |
|---|---|---|
| < 10th centile | 7 | 2 (29) |
| 10–20th centile | 23 | 8 (35) |
| 20–30th centile | 53 | 26 (49) |
| 30–40th centile | 73 | 35 (48) |
| 40–50th centile | 91 | 51 (56) |
| 50–60th centile | 101 | 69 (68) |
| 60–70th centile | 103 | 76 (74) |
| 70–80th centile | 140 | 109 (78) |
| 80–90th centile | 171 | 149 (87) |
| > 90th centile | 183 | 177 (97) |
FIGURE 10.
Calibration plot of the predicted vs. observed risk for adverse fetal outcome using the PREP-L model.
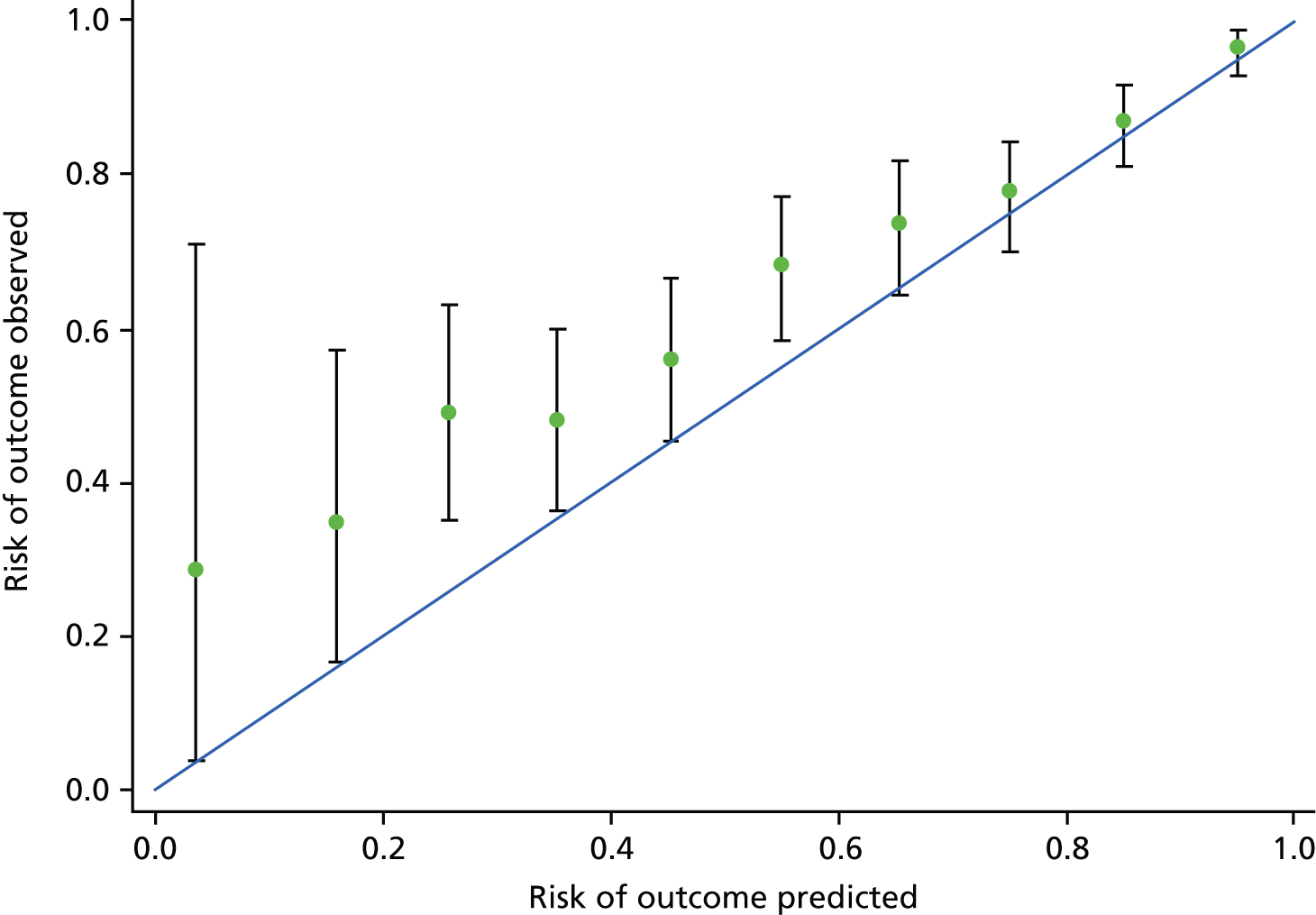
Predictive value of tests for adverse fetal and neonatal outcomes
We evaluated the prognostic value of all candidate predictors associated with maternal outcomes and the following five additional predictors: ultrasound (uterine artery Doppler in second trimester, expected fetal weight and liquor volume), CTG findings and use of steroids within or before 24 hours of diagnosis of pre-eclampsia. Table 29 shows the descriptive values of the candidate predictors and their crude and multivariate association with adverse fetal outcomes.
| Candidate predictors | Women, na | No adverse fetal outcomes (n = 243), mean (SD) or n (%) | Adverse fetal outcomes (n = 702), mean (SD) or n (%) | Univariable analysis (N = 945) | Multivariable analysis (N = 945) | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||||
| Maternal characteristics | |||||||
| Maternal age (years) | 943 | 31.0 (6.1) | 30.0 (6.1) | 0.972 (0.949 to 0.995) | 0.018 | 0.984 (0.954 to 1.015) | 0.301 |
| Log-transformed gestational age (weeks) at diagnosis | 945 | 3.4 (0.1) | 3.4 (0.1) | 0.111 (0.022 to 0.554) | 0.007 | 0.089 (0.013 to 0.607) | 0.014 |
| Multiple pregnancy | |||||||
| Singleton (reference) | 945 | 225 (93%) | 637 (91%) | ||||
| Twins | 17 (7%) | 62 (9%) | 1.288 (0.738 to 2.250) | 0.373 | 1.676 (0.862 to 3.260) | 0.128 | |
| Triplets | 1 (0%) | 3 (0%) | 1.060 (0.110 to 10.239) | 0.960 | 1.245 (0.112 to 13.774) | 0.858 | |
| Global test | 0.673 | 0.313 | |||||
| Medical history score | |||||||
| 0 (reference) | 944 | 116 (48%) | 478 (68%) | ||||
| 1 | 83 (34%) | 167 (24%) | 0.490 (0.351 to 0.683) | < 0.001 | 0.654 (0.435 to 0.984) | 0.041 | |
| ≥ 2 | 44 (18%) | 56 (8%) | 0.309 (0.198 to 0.481) | < 0.001 | 0.434 (0.246 to 0.767) | 0.004 | |
| Global test | < 0.001 | 0.009 | |||||
| Symptoms | |||||||
| Headache and/or visual disturbance | 919 | 102 (43%) | 278 (41%) | 0.891 (0.658 to 1.206) | 0.456 | 0.812 (0.552 to 1.193) | 0.289 |
| Epigastric pain, nausea and/or vomiting | 900 | 43 (19%) | 157 (23%) | 1.331 (0.906 to 1.955) | 0.145 | 0.958 (0.578 to 1.590) | 0.869 |
| Chest pain and/or dyspnoea | 821 | 12 (6%) | 48 (8%) | 1.340 (0.668 to 2.687) | 0.410 | 1.190 (0.502 to 2.822) | 0.693 |
| Bedside examination and tests | |||||||
| Clonus | 545 | 10 (8%) | 84 (20%) | 2.399 (1.262 to 4.562) | 0.008 | 1.487 (0.609 to 3.633) | 0.384 |
| Exaggerated tendon reflexes | 594 | 20 (16%) | 126 (27%) | 2.063 (1.318 to 3.231) | 0.002 | 0.852 (0.461 to 1.575) | 0.610 |
| Systolic BP (mmHg) | 941 | 153 (16) | 161 (20) | 1.025 (1.016 to 1.034) | < 0.001 | 1.005 (0.992 to 1.019) | 0.414 |
| Diastolic BP (mmHg) | 941 | 96 (11) | 101 (11) | 1.044 (1.029 to 1.059) | < 0.001 | 1.018 (0.996 to 1.039) | 0.103 |
| Oxygen saturation abnormal (< 94%) | 428 | 1 (1%) | 3 (1%) | 1.039 (0.108 to 10.032) | 0.974 | 0.107 (0.009 to 1.214) | 0.071 |
| Urine dipstick: none/trace (reference) | 927 | 11 (5%) | 28 (4%) | ||||
| 1+ | 71 (30%) | 98 (14%) | 0.549 (0.256 to 1.179) | 0.124 | 0.609 (0.259 to 1.435) | 0.257 | |
| 2+ | 100 (42%) | 212 (31%) | 0.840 (0.401 to 1.760) | 0.644 | 0.781 (0.341 to 1.791) | 0.560 | |
| 3+ | 42 (18%) | 261 (38%) | 2.429 (1.117 to 5.284) | 0.025 | 1.445 (0.587 to 3.555) | 0.424 | |
| ≥ 4 | 13 (5%) | 91 (13%) | 2.739 (1.111 to 6.751) | 0.029 | 0.974 (0.334 to 2.840) | 0.961 | |
| Global test | < 0.001 | 0.045 | |||||
| Laboratory tests | |||||||
| Haemoglobin (g/l) | 909 | 11.8 (1.1) | 12.0 (1.4) | 1.096 (0.983 to 1.223) | 0.100 | 1.019 (0.885 to 1.173) | 0.792 |
| Platelet count (× 109/l) | 905 | 244 (77) | 220 (77) | 0.996 (0.994 to 0.998) | < 0.001 | 0.998 (0.996 to 1.001) | 0.128 |
| Log-transformed ALT concentration | 870 | 2.7 (0.6) | 3.0 (0.8) | 1.641 (1.244 to 2.165) | < 0.001 | 1.330 (0.958 to 1.848) | 0.089 |
| Log-transformed serum uric acid concentration | 781 | –1.3 (1.2) | –1.0 (0.7) | 1.409 (1.135 to 1.750) | 0.002 | ||
| Log-transformed serum urea concentration | 876 | 1.2 (0.4) | 1.4 (0.5) | 3.679 (2.482 to 5.452) | < 0.001 | 1.718 (1.068 to 2.764) | 0.026 |
| Log-transformed serum creatinine concentration | 908 | 4.0 (0.3) | 4.1 (0.3) | 2.823 (1.662 to 4.795) | < 0.001 | 1.039 (0.506 to 2.135) | 0.916 |
| Log-transformed PCR | 837 | 3.9 (1.4) | 4.9 (1.4) | 1.569 (1.397 to 1.762) | < 0.001 | 1.290 (1.111 to 1.497) | 0.001 |
| Treatment provided | |||||||
| Antihypertensive therapy | 944 | 177 (73%) | 573 (82%) | 1.663 (1.182 to 2.339) | 0.004 | 1.558 (1.026 to 2.368) | 0.038 |
| Magnesium sulphate administered | 944 | 9 (4%) | 135 (19%) | 6.190 (3.100 to 12.363) | < 0.001 | 2.402 (1.036 to 5.573) | 0.041 |
| Steroids administered | 783 | 66 (41%) | 364 (59%) | 2.186 (1.549 to 3.085) | < 0.001 | 1.208 (0.795 to 1.835) | 0.376 |
| Ultrasound and CTG | |||||||
| Uterine artery Doppler abnormal | 339 | 12 (14%) | 79 (31%) | 2.365 (1.536 to 3.639) | < 0.001 | 1.944 (1.077 to 3.510) | 0.027 |
| CTG findings abnormal | 710 | 10 (6%) | 36 (7%) | 1.395 (0.680 to 2.865) | 0.364 | 0.625 (0.254 to 1.538) | 0.306 |
| Estimated fetal weight < 10th centile | 712 | 27 (15%) | 261 (49%) | 3.835 (2.453 to 5.995) | < 0.001 | 2.538 (1.462 to 4.405) | 0.001 |
| Liquor volume abnormal | 890 | 10 (4%) | 46 (7%) | 1.548 (0.776 to 3.087) | 0.215 | 1.279 (0.519 to 3.152) | 0.593 |
Association of maternal and fetal characteristics with adverse fetal outcomes
In the multivariable analysis of predictors, increased gestational age at diagnosis of pre-eclampsia reduced the odds of fetal complications (OR 0.09, 95% CI 0.01 to 0.61). A medical history of pre-existing chronic hypertension, diabetes mellitus, autoimmune disease, renal disease or a history of pre-eclampsia in previous pregnancies reduced the odds of composite adverse fetal outcomes for one pre-existing medical complication (OR 0.65 95% CI 0.44 to 0.98) and for two or more pre-existing medical complications (OR 0.43 95% CI 0.25 to 0.77).
The odds of fetal complications were significantly increased in women with raised urine PCR (OR 1.29, 95% CI 1.11 to 1.50), serum urea concentration (OR 1.72, 95% CI 1.07 to 2.76), treatment with antihypertensives (OR 1.56, 95% CI 1.04 to 2.37), treatment with magnesium sulphate (OR 2.40, 95% CI 1.04 to 5.57), abnormal uterine artery Doppler (OR 1.94, 95% CI 1.08 to 3.51) and when expected fetal weight was less than the 10th centile by ultrasound (OR 2.54, 95% CI 1.46 to 4.40).
Chapter 8 Discussion
In women with early-onset pre-eclampsia, the PREP prediction models provide robust estimates of the overall risk of adverse maternal outcomes by discharge, and the risks at various time points following diagnosis. The PREP-L model showed good discrimination and calibration and appeared to be useful in predicting risk of complications as a result of early-onset pre-eclampsia in pregnancy and until discharge for UK populations. Given that the rPREP-L model was easily transportable, with good performance in the non-UK populations, we expect the original PREP-L model to have similar performance externally. The PREP-S model showed good discrimination in external data sets, with reasonable calibration. The use of this model will be useful to health-care professionals in deciding on the appropriate setting for management and for commencement of interventions, such as steroids, if preterm delivery is anticipated.
Strengths and limitations
A well-performing prediction model is one that is relevant, accurate, validated in populations and data sets external to those used to develop the model and applicable to clinical practice. With these properties, it has the potential to improve clinical outcomes by helping clinicians and patients make more informed decisions.
The PREP models were developed in a sample of women with early-onset pre-eclampsia, a condition that is considered to be pathophysiologically different from late onset disease,8–10 and with a high proportion of adverse outcomes. We used prospective cohorts with high-quality data for both model development and validation, and with standardised definitions of variables and outcomes. The model was developed with data from 53 units in the UK, making the results as generalisable as possible within the NHS.
We ensured that all routinely performed tests in clinical practice were evaluated. The choice of predictors and components of the composite outcome were made by using Delphi surveys of experts in the field. 9,15,41 We chose delivery before 34 weeks as an outcome to further minimise treatment paradox-related bias, as delivery is planned at this gestation only if there are concerns regarding the health of the mother.
Prediction models often evaluate a large number of predictors in a population with few events, making the findings less robust. We ensured that we had adequate sample size for the number of candidate predictors to avoid overfitting. 43–45 The rates of follow-up were very high in our PREP cohort and very few individuals had missing values for most predictors.
One of the main reasons why clinicians lack confidence in applying risk scores in practice is the lack of sufficient evidence to demonstrate the reproducibility and transportability of the model in an external data set. 32 Furthermore, they are less likely to accept the model if it does not include important predictors such as BP. Guided by an a priori expert workshop (see Chapter 2, Analysis plan development), we minimised bias due to treatment by the inclusion of management decisions such as use of antihypertensives and magnesium sulphate as predictors. We transparently reported the development of the model and have provided the regression coefficients to enable clinical use and future validation of the model.
Our prediction study used rigorous statistical methods to develop the model, to assess its accuracy and to formally validate its performance in external data sets. 32–35,39,48–51 We developed two prediction models for the dual purpose of obtaining overall complication risks arising from pre-eclampsia and risk estimates for complications at various time points after diagnosis. A logistic model alone would not have sufficient sample size to provide estimates of adverse outcomes at time points close to diagnosis, such as 48 hours after delivery, given the low rates of serious complications. However, the PREP-S model allowed us to overcome this problem, and is the first to provide individualised risks of adverse maternal outcomes at various time points after the diagnosis of early-onset pre-eclampsia.
We performed geographic, temporal and domain validation of the model. The external data sets of the PIERS and PETRA cohorts were geographically different (Canada and the Netherlands) and were conducted earlier than the PREP study. We validated reduced models in external data sets as fewer predictors were evaluated therein. However, the rPREP-L model validated with good discrimination and calibration for predicting overall risk, and we expect the original PREP-L model to have similar, if not better, performance if fully externally validated.
The aim of the model was to provide reliable, accurate and precise information of risks to the mother and baby based on tests done at the time of diagnosis of early-onset pre-eclampsia. We only evaluated the tests and variables measured routinely in clinical practice. The added value of biomarkers and ultrasound to the accuracy of the model is not known. We refrained from using predictors such as fetal weight estimated by ultrasound, which may have been a significant predictor, as access to ultrasound may not always be available close to the diagnosis of pre-eclampsia in most units. We arbitrarily chose the components of relevant medical history and scored them. Inclusion of a different set of medical conditions may have altered the results. Women with a medical history score appeared to have a reduction in the risk of complications. It is likely that specialists in joint obstetric specialist clinics closely monitor these mothers resulting in early diagnosis of pre-eclampsia. Targeted and intense follow-up of these women may have led to prolongation of pregnancy beyond 34 weeks and with low complications. When developing our PREP models, we were unable to properly examine serum uric acid concentration as a predictor because at the time of the model development there was a coding error in the data for this variable. However, subsequent to the PREP models being developed and this coding error being corrected, we examined if the inclusion of serum uric acid concentration was important and found that the c-statistic barely changed for either the PREP-L or PREP-S model.
Women with earlier diagnosis of pre-eclampsia appeared to be at lower risk of maternal complications in the PREP-S model. This is likely, as the primary outcome is largely driven by delivery before 34 weeks. In women who were close to 34 weeks’ gestation, clinicians may have a lower threshold for delivery in the next few days or weeks. However, if the diagnosis was made much earlier in the pregnancy, clinicians would aim to prolong gestation as long as possible, leading to a longer survival time.
Our primary outcome was a composite of maternal complications. A different choice of outcomes may have identified a different set of predictors. However, given the rarity of individual complications in women with early-onset pre-eclampsia, we felt that our approach to include delivery before 34 weeks is a close representative measure for the severity of the disease. However, we did not separately report iatrogenic preterm deliveries from spontaneous preterm deliveries. As it is difficult to accurately identify the cause of spontaneous preterm delivery, which could still be related to pre-eclampsia such as small abruption, we grouped them together as one outcome.
The external data sets were limited in the number of variables evaluated, and hence we were unable to validate our full PREP model in either of them. The reduced PREP models, especially rPREP-L, showed good performance in the development and validation data sets, although the rPREP-S model showed reduced performance. Although the overall values of predictors may be similar to the PREP cohort, the management of women with early-onset pre-eclampsia may be different in the various health-care systems of the external cohorts; for example, magnesium sulphate treatment was provided in 51% of all women with early-onset pre-eclampsia in the PIERS cohort, compared with only 15% in the PREP cohort. Furthermore, the variation in the proportion of women giving birth before 34 weeks’ gestation, which was the major component of the composite outcome, may have contributed to the reduced performance of the model in the external data sets. The narrow spectrum of diseases in individuals in the PETRA cohort may have contributed to the reduced performance of the PREP model.
Comparison with existing evidence
So far, systematic reviews have not been able to produce robust estimates of accuracy of the individual tests. 20,22,23,39,53,54 Tests widely used in clinical practice, such as measurement of BP and proteinuria, suffered from treatment paradox and those such as clonus and deep-tendon reflexes were not studied in sufficient detail. This study is the first to address the above deficiencies.
The PREP study is the first to develop and validate the models for predicting adverse maternal outcomes specifically in women with early-onset disease. Previously, the PIERS and mini-PIERS models have provided estimates for overall risk of adverse outcomes in women with pre-eclampsia of any onset. Their sample sizes were too small to predict complications in women with early-onset pre-eclampsia. This is also the first study to provide individualised risk estimates for adverse outcomes at various time points after diagnosis of early-onset pre-eclampsia. Although the PIERS model included predictors such as gestational age at diagnosis, and concentrations of liver enzymes (AST) and serum creatinine to predict a composite maternal outcome, other important variables, such as BP and proteinuria, were not included.
The performance of any prediction model is influenced by effective treatment measures, such as antihypertensive drugs, magnesium sulphate and delivery, which reduces the probability of adverse outcomes. To avoid such bias, we included management strategies such as need for antihypertensive drugs and magnesium sulphate as predictors. Furthermore, as delivery is considered to be the cure for the condition, we incorporated preterm delivery before 34 weeks’ gestation as a component of the maternal composite adverse outcome. We considered early preterm delivery to be indicative of the severity of the condition, as clinicians usually aim to prolong pregnancy beyond 34 weeks’ gestation to reduce prematurity-related complications in the neonate unless there are overwhelming concerns about the health of the mother. This approach has led to the inclusion of important tests such as measurement of BP, proteinuria and the need for antihypertensives and magnesium sulphate as significant predictors in the final PREP models.
To ensure that the prediction model could be applied in clinical practice, the findings at the time of baseline (i.e. at diagnosis) should be used to assess the risk. Many models include the worst value of the predictor in the same time period used for outcome ascertainment. This is likely to overestimate the predictive performance of the model. Rule of thumb indicates that at least 10 events should be available per candidate predictor for model development. Compared with the PIERS model, that evaluated 34 predictors for 106 outcomes at 48 hours, the survival analysis approach taken with the PREP-S model allowed us to have sufficient sample size to predict complications accurately at various time points, including 48 hours after diagnosis. The PIERS model did not provide estimates of overall risk of complications in pregnancy and reported a discrimination index of just > 0.7 for prediction of adverse outcomes by 1 week. The PREP-L model showed good discrimination of > 0.8 to predict overall risk until discharge and the PREP-S model had an estimate of > 0.75. The performance validated well in the external data set with good discrimination and calibration. Given the potentially rapid changes in predictor values over time in pregnancy, these measures are impressive for their predictive power at the time of diagnosis of the condition.
Implications for clinical practice
The PREP models were developed with the explicit purpose of providing relevant information to mothers and clinicians on individualised risks at the time of diagnosis of pre-eclampsia. The Microsoft Excel file is user-friendly and easily accessible. It should be emphasised that the PREP models should not be used to choose between administration or non-administration of antihypertensives and magnesium sulphate, which are predictor variables. The risk estimates provided by the model are relevant to women who are managed as per the current clinical guidelines. 55 For example, when managing women admitted with very high BP, the clinicians are expected to manage the mother as per current guidelines with antihypertensives and, if appropriate, magnesium sulphate, and not to base treatment on the probability of risk provided by the model. However, the PREP-S model will be useful in providing the mother’s individualised risk of adverse outcomes at various time points, such as 48 hours after diagnosis, given the clinical characteristics and the choice of treatment.
The PREP-L model provides mothers with the overall risk of experiencing an adverse outcome by the time of discharge. The PREP-L model had high discrimination and calibration estimates in both development and validation data sets, and thus appears accurate and transportable to the non-UK populations examined. Clinicians and mothers should be informed that the model provides overall risks by discharge and should not be used to plan immediate management.
The calibration of the PREP-S model was good in the development data set, as expected, and reasonable in the validation cohort, especially for those with high risk, which suggests that women deemed to be at high risk of outcomes are also more likely to experience the outcome. The PREP-S model can be used as a tool for triaging mothers with a diagnosis of pre-eclampsia before 34 weeks’ gestation to decide on the optimal place of delivery. In women identified as high risk by the model, efforts should be made for early transfer of the mother to a tertiary unit for neonatal care, in addition to care for the mother. As the tool provides risk estimates at various time points, resources can be mobilised appropriately when required for transfer of the mother. The risk estimates will identify mothers who require prophylactic corticosteroids when preterm delivery is anticipated. In addition, this tool will allow neonatologists to provide individualised prognostic estimates for the baby after delivery, depending on the predicted risk of complications and for that gestational age.
Research recommendations
The PREP models assessed the risk of composite adverse outcomes. Individual patient data meta-analysis of the studies evaluating the prediction of the accuracy of tests for complications would provide an increased sample size to assess the risk of individual outcomes or outcomes grouped by the organ system involvement. We have undertaken the first two stages (model development and external validation) in prognostic research aimed at improving patient care. A final evaluation of the impact of PREP models on clinical practice is required, especially in the improvement of health outcomes for the mother and baby, but is beyond the remit of this project. In other words, further research may examine the impact of implementing the PREP-S and PREP-L models into clinical practice, in terms of their uptake by clinicians and their impact on patient outcomes. This might be in the form of a cluster randomised trial, for example, with practices randomised to either using or not using the PREP-S and PREP-L models.
Acknowledgements
We thank all the research midwives and nurses, community and hospital midwives, midwifery assistants, nursing staff and trainee doctors from each of the recruiting hospitals for promoting the PREP study. We are grateful for their hard work and support in facilitating and managing the study within their hospitals.
We thank the members of the Joint Steering and Data Monitoring Committee, which included Professor Arri Coomarasamy (Chairperson, University of Birmingham, UK), Dr Aris Papageorghiou (St George’s University Hospital, UK), Mrs Nicola Bandy (Action on Pre-eclampsia, UK), Professor Javier Zamora (Hospital Ramon y Cajal (IRYCIS) and CIBER Epidemiologia y Salud Publica, Madrid, Spain), Dr Gerben ter Riet (Academisch Medisch Centrum, Universiteit van Amsterdam, the Netherlands), Dr Teresa Pérez Pérez (Complutense University of Madrid, Spain), Professor Andrew Ewer (University of Birmingham, UK) and Professor Harry Gee (Ammalife, UK) for their guidance and support throughout the project.
The study was co-ordinated at the Women’s Health Research Unit of Queen Mary University of London and we acknowledge the hard work of all the staff at the unit involved in the study. We thank John Allotey (Study Co-ordinator), Julie Dodds (Senior Trials Manager), Sian Newton (Research Assistant), Aikaterini Nikolaou (Research Assistant), Glenn Poon (Data Assistant), Maria D’Amico (Data Assistant) and Malika Barakat (Data Assistant). We also thank the Pragmatic Clinical Trials Unit of Queen Mary University of London for its help in the development and management of the study database.
We also thank members of the PREP prognostic meeting expert panel for their contribution in prioritising the composite outcomes and for their help in addressing the challenges encountered with developing the PREP prediction models: Joost Akkermans (Leiden University Medical Centre, the Netherlands), Gary Collins (University of Oxford, UK), Thomas Debray (University Medical Centre, Utrecht, the Netherlands), Bill Grobman (Northwestern University Feinberg School of Medicine, USA), Henk Groen (University Medical Centre, Groningen, the Netherlands), Richard Hooper (Queen Mary University of London, UK), Miland Joshi (Queen Mary University of London, UK), Brenda Kazemier (Academisch Medisch Centrum, Universiteit van Amsterdam, the Netherlands) Emily Kleinrouweler (Academisch Medisch Centrum, Universiteit van Amsterdam, the Netherlands) Ewelina Rogozinska (Queen Mary University of London, UK), Ewoud Schuit (University Medical Centre, Utrecht, the Netherlands) and Jonathan Sterne (University of Bristol, UK).
We thank the members of the Action on Pre-eclampsia Charity for their support in promoting the PREP study at their midwifery meetings and study days, as well as for their guidance in developing the study materials.
We would finally like to thank all the women who consented to participate in the study. This work would not have been completed without their participation and the PREP study would not have been possible without them.
Additional thanks go to the following people:
Mr Raajkumar Sundararajah, Southend Hospital, Southend; Miss Avideah Nejad, Basingstoke and North Hampshire Hospital, Basingstoke; Mr Rehan Khan, Dr Fiona Cheong-See and Dr Madhavi Kalidindi, Barts Health NHS Trust, London; Dr Celia Burrell, Queen’s Hospital, Romford; Mr Manish Gupta, Barts Health NHS Trust, London; Mr Vincent Oon, Barts Health NHS Trust, London; Dr Rezan Kadir, Royal Free Hospital, London; Dr Zeudi Ramsey-Marcelle, North Middlesex Hospital, London; Dr Louise Page, West Middlesex University Hospital, Isleworth; Professor Baskaran Thilaganathan, St George’s Hospital, London; Mr Bill Martin, Birmingham Women’s Hospital, Birmingham; Ms Shagaf Haj Bakour, City Hospital, Birmingham; Mr Hassan Morsi, Russells Hall Hospital, Dudley; Dr David Churchill, New Cross Hospital, Wolverhampton; Miss Fidelma O’Mahony, City General Hospital, Stoke-on-Trent; Miss Karen Powell, Staffordshire General Hospital, Stafford; Dr Jayasree Srinivasan, Queen’s Hospital, Burton-on-Trent; Dr Michele Mohajer, Royal Shrewsbury Hospital, Shrewsbury; Dr Siobhan Quenby, University Hospitals Coventry & Warwickshire, Coventry; Dr Lakshmi Thirumalaikumar, Worcestershire Royal Hospital, Worcester; Professor Justin Konje, Leicester Royal Infirmary, Leicester; Professor Jim Thornton, Nottingham City Hospital, Nottingham; Mr George Bugg, Queen’s Medical Centre, Nottingham; Dr Shonag Mackenzie, Wansbeck General Hospital, Ashington; Dr Aarti Ullal, Sunderland Royal Hospital, Sunderland; Dr Marie Smith, Royal Victoria Infirmary, Newcastle; Dr Rita Arya, Warrington Hospital, Warrington; Dr Simon Cunnigham, Leighton Hospital, Crewe; Professor James Walker and Dr Nigel Simpson, Leeds General Infirmary, Leeds; Dr Joanne Page, Derriford Hospital, Plymouth; Miss Claire Oxby, York Hospital, York; Dr Karen Watkins, Royal Cornwall Hospital (Treliske), Truro; Professor Derek Tuffnell, Bradford Royal Infirmary, Bradford; Mr S Bober, West Cumberland Hospital, Whitehaven; Mr A Wijesiriwardana, Cumberland Infirmary, Carlisle; Dr Helene Brandon, Queen Elizabeth Hospital, Gateshead; Mr Saif El-Badawy, North Devon District Hospital, Barnstaple; Dr Sara Brigham, Countess of Chester hospital, Cheshire; Mr Lanre Shorinola, Warwick Hospital, Warwick; Dr Aethele Khunda, James Cook University Hospital, Middlesbrough; Dr Shaku Kalla and Dr Mohammed M Abdullah Agha, Wexham Park Hospital, Slough; Mr Stephen Poku and Mr Ayo Olawo, Rotherham Hospital, Rotherham; Mr Johnson Amu, Blackpool Victoria Hospital, Blackpool; Mr Philip Banfield, Glan Clwyd Hospital, Rhyl; Mr Franz Majoko, Singleton Hospital, Swansea; Dr Julia Alcide, Furness General Hospital, Cumbria; Dr Jyothi Rajeswary, King’s Mill Hospital, Sutton-In-Ashfield; Dr Marwan Salloum, Queen Alexandra Hospital, Portsmouth; Dr Alexandra Rees, University Hospital of Wales, Cardiff; Dr Odiri Oteri, Lincoln County Hospital, Lincoln; Dr Sunday Ikhena, Pilgrim Hospital, Boston; Dr Janet Cresswell, Chesterfield and North Derbyshire Royal Hospital, Chesterfield; Dr Feroza Dawood and Dr Umber Agarwal, Liverpool Women’s Hospital, Liverpool.
Contributions of authors
Shakila Thangaratinam (Professor of Maternal and Perinatal Health) designed and led the project, provided clinical direction and prepared and edited the final report.
John Allotey (Senior Trials Co-ordinator) oversaw the day-to-day management of the study, ensured the study protocol was followed at participating hospitals and assisted in preparing the draft and final version of the manuscript.
Nadine Marlin (Statistician) performed the statistical analysis of the study and contributed to Chapter 2 of the final report.
Ben W Mol (Professor of Obstetrics and Gynaecology) provided clinical direction and edited the final version of the report.
Peter Von Dadelszen (Professor of Maternal Fetal Medicine) provided the PIERS data set used for validation of the PREP model.
Wessel Ganzevoort (Gynaecologist, Fellow of Perinatology) provided the PETRA data set used for validation of the PREP model.
Joost Akkermans (Research Physician, Resident Obstetrician and Gynaecologist) provided the PETRA data set used for validation of the PREP model.
Asif Ahmed (Pro-Vice-Chancellor for Health) contributed to the protocol and study development.
Jane Daniels (Deputy Director/Senior Research Fellow) contributed to the write-up of the final report.
Jon Deeks (Professor of Biostatistics, Joint School Research Lead) contributed to the protocol and study development.
Khaled Ismail (Professor of Obstetrics and Gynaecology) contributed to the protocol and study development.
Ann Marie Barnard (CEO Action on Pre-eclampsia Charity) helped draft and review the lay summary, contributing to the writing of the final report.
Julie Dodds (Senior Clinical Trials Manager) provided management direction by supervising the study co-ordinator (John Allotey), ensuring the implementation of the protocol, preparing the data for analysis and editing the final report.
Sally Kerry (Reader in Medical Statistics) provided guidance and management to the statistician (Nadine Marlin), oversaw the statistical analysis and edited the final report.
Carl Moons (Professor of Clinical Epidemiology) provided statistical guidance on analysis of data and presentation of the results and contributed to the write-up of the final report.
Richard D Riley (Professor of Biostatistics) provided statistical guidance, contributed to the analysis plan for the study and edited the final report.
Khalid S Khan (Professor of Women’s Health and Clinical Epidemiology) designed the project, provided clinical and overall direction, contributed to and edited the final report.
Publication
Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15:68. https://doi.org/10.1186/s12916-017-0827-3
Data sharing statement
Study data can be obtained from the corresponding author on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust N Z J Obstet Gynaecol 2000;40:133-8. http://dx.doi.org/10.1111/j.1479-828X.2000.tb01136.x.
- Anon . Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:s1-s22. http://dx.doi.org/10.1067/mob.2000.107928.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 2003;102:181-92. http://dx.doi.org/10.1097/00006250-200307000-00033.
- Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol 1988;158:892-8. http://dx.doi.org/10.1016/0002-9378(88)90090-7.
- Lewis G. Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer – 2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London: CEMACH; 2007.
- Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy 2000;19:221-31. http://dx.doi.org/10.1081/PRG-100100138.
- Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol 1993;169:1000-6. http://dx.doi.org/10.1016/0002-9378(93)90043-I.
- MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol 2001;97:533-8. http://dx.doi.org/10.1097/00006250-200104000-00011.
- von Dadelszen P, Menzies JM, Payne B, Magee LA. PIERS Study Group . Predicting adverse outcomes in women with severe pre-eclampsia. Semin Perinatol 2009;33:152-7. http://dx.doi.org/10.1053/j.semperi.2009.02.009.
- von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy 2003;22:143-8. http://dx.doi.org/10.1081/PRG-120021060.
- Shennan AH. Recent developments in obstetrics. BMJ 2003;327:604-8. http://dx.doi.org/10.1136/bmj.327.7415.604.
- The Management of Severe Pre-Eclampsia/Eclampsia. Guidelines and Audit Committee of the Royal College of Obstetricians and Gynaecologists; 2006.
- Churchill D, Duley L. Interventionist versus expectant care for severe pre-eclampsia before term. Cochrane Database Syst Rev 2002. http://dx.doi.org/10.1002/14651858.cd003106.
- Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123:e312-27. http://dx.doi.org/10.1542/peds.2008-1827.
- Thangaratinam S, Ismail K, Sharp S, Coomarasamy A, O’Mahony F, Khan KS, et al. Prioritisation of tests for the prediction of preeclampsia complications: a Delphi survey. Hypertens Pregnancy 2007;26:131-8. http://dx.doi.org/10.1080/10641950601148000.
- von Dadelszen P, Magee LA, Lee SK, Stewart SD, Simone C, Koren G, et al. Activated protein C in normal human pregnancy and pregnancies complicated by severe preeclampsia: a therapeutic opportunity?. Crit Care Med 2002;30:1883-92. http://dx.doi.org/10.1097/00003246-200208000-00035.
- Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol 2000;40:139-55. http://dx.doi.org/10.1111/j.1479-828X.2000.tb01137.x.
- Helewa ME, Burrows RF, Smith J, Williams K, Brain P, Rabkin SW. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ 1997;157:715-25.
- Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Strategic Training Initiative in Research in the Reproductive Health Sciences Scholars . Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can 2008;30:1-48. http://dx.doi.org/10.1016/S1701-2163(16)32776-1.
- Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS. Tests in Prediction of Pre-eclampsia Severity review group . Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG 2006;113:369-78. http://dx.doi.org/10.1111/j.1471-0528.2006.00908.x.
- Thangaratinam S, Coomarasamy A, Sharp S, O’Mahony F, O’Brien S, Ismail KM, et al. Tests for predicting complications of pre-eclampsia: a protocol for systematic reviews. BMC Pregnancy Childbirth 2008;8. http://dx.doi.org/10.1186/1471-2393-8-38.
- Thangaratinam S, Koopmans CM, Iyengar S, Zamora J, Ismail KM, Mol BW, et al. Accuracy of liver function tests for predicting adverse maternal and fetal outcomes in women with preeclampsia: a systematic review. Acta Obstet Gynecol Scand 2011;90:574-85. http://dx.doi.org/10.1111/j.1600-0412.2011.01112.x.
- Thangaratinam S, Coomarasamy A, O’Mahony F, Sharp S, Zamora J, Khan KS, et al. Estimation of proteinuria as a predictor of complications of pre-eclampsia: a systematic review. BMC Med 2009;7. http://dx.doi.org/10.1186/1741-7015-7-10.
- Cheong-See F, Allotey J, Marlin N, Mol BW, Schuit E, ter Riet G, et al. Prediction models in obstetrics: understanding the treatment paradox and potential solutions to the threat it poses. BJOG 2016;123:1060-4. http://dx.doi.org/10.1111/1471-0528.13859.
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev 2000.
- Shear RM, Rinfret D, Leduc L. Should we offer expectant management in cases of severe preterm preeclampsia with fetal growth restriction?. Am J Obstet Gynecol 2005;192:1119-25. http://dx.doi.org/10.1016/j.ajog.2004.10.621.
- Odendaal HJ, Pattison RC, Dutoit R. Fetal and neonatal outcome in patients with severe pre-eclampsia before 34 weeks. S Afr Med J 1987;71:555-8.
- Sibai BM, Mercer BM, Schiff E, Friedman SA. Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomised controlled trial. Am J Obstet Gynecol 1994;171:818-22. http://dx.doi.org/10.1016/0002-9378(94)90104-X.
- Magee LA, Yong PJ, Espinosa V, Cote AM, Chen I, von Dadelszen P. Expectant management of severe preeclampsia remote from term: a structured systematic review. Hypertens Pregnancy 2009;28:312-47. http://dx.doi.org/10.1080/10641950802601252.
- Paruk F, Moodley J. Maternal and neonatal outcome in early- and late-onset pre-eclampsia. Semin Fetal Neonatal Med 2000;5:197-20. http://dx.doi.org/10.1053/siny.2000.0023.
- Sibai BM, Taslima M, Abdella TN, Brooks TF, Spinnato JA, Anderson GD, et al. Maternal and perinatal outcome of conservative management of severe preeclampsia in the mid-trimester. Am J Obstet Gynecol 1985;152. http://dx.doi.org/10.1016/S0002-9378(85)80171-X.
- Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b605.
- Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how?. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b375.
- Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b604.
- Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:55-63. http://dx.doi.org/10.7326/M14-0698.
- Department of Health Data Standards: Ethnic Category. London: ONS; 2009.
- Standardisation Committee for Care Information (SCCI), Ethnic Category Coding – DSCN11/2008 . Statement of Need for Standard Review 2014. https://groups.ic.nhs.uk/SCCIDsupport/dashboard/SCCISecretariat/2014-06-25/SCCI2023%20SoN%202011%20Ethnic%20Category%20Coding.pdf.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol 2005;105:402-10. http://dx.doi.org/10.1097/01.AOG.0000152351.13671.99.
- Brunelli VB, Prefumo F. Quality of first trimester risk prediction models for pre-eclampsia: a systematic review. BJOG 2015;122:904-14. http://dx.doi.org/10.1111/1471-0528.13334.
- Thangaratinam S, Allotey J, Marlin N, Dodds J, Cheong-See F, von Dadelszen P, et al. Prediction of complications in early-onset pre-eclampsia (PREP): development and external multinational validation of prognostic models. BMC Med 2017;15. https://doi.org/10.1186/s12916-017-0827-3.
- Thangaratinam S, Redman CWE. The Delphi technique. Obstet Gynaecol 2005;7:120-5. http://dx.doi.org/10.1576/toag.7.2.120.27071.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet 1974;304:81-4. http://dx.doi.org/10.1016/S0140-6736(74)91639-0.
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. http://dx.doi.org/10.1016/0895-4356(95)00048-8.
- Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol 2005;58:475-83. http://dx.doi.org/10.1016/j.jclinepi.2004.06.017.
- Westerhuis ME, Schuit E, Kwee A, Zuithoff NP, Groenwold RH, Van Den, et al. Prediction of neonatal metabolic acidosis in women with a singleton term pregnancy in cephalic presentation. Am J Perinatol 2012;29:167-74. http://dx.doi.org/10.1055/s-0031-1284226.
- Ganzevoort W, Rep A, Bonsel GJ, Fetter WP, van Sonderen L, De Vries JI, et al. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early-onset pre-eclampsia. BJOG 2005;112:1358-68. http://dx.doi.org/10.1111/j.1471-0528.2005.00687.x.
- Prechtl HFR, Beintema D. The Neurological Examination of the Full-Term Newborn Infant. London: William Heinemann Medical Books; 1964.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987.
- Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J 2009;9:265-90.
- Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press; 2011.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. http://dx.doi.org/10.1002/sim.1203.
- Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543-6. http://dx.doi.org/10.1001/jama.1982.03320430047030.
- Thangaratinam S, Datta A, Ismail KMK, Khan KS. What is the accuracy of blood pressure in predicting complications in pre-eclampsia?. Arch Dis Child Fetal Neonatal Ed 2011;96. http://dx.doi.org/10.1136/adc.2011.300163.15.
- Thangaratinam S, Gallos ID, Meah N, Usman S, Ismail KM, Khan KS, et al. How accurate are maternal symptoms in predicting impending complications in women with preeclampsia? A systematic review and meta-analysis. Acta Obstet Gynecol Scand 2011;90:564-73. http://dx.doi.org/10.1111/j.1600-0412.2011.01111.x.
- Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. NICE Clinical Guidelines, No. 107. London: RCOG Press; 2010.
Appendix 1 Prioritisation of outcomes for inclusion in the composite adverse maternal outcome based on clinical importance by expert panel
| Outcome | Score | Median | Range | Ranking |
|---|---|---|---|---|
| Transfusion of blood products | 11 | 1 | 1–2 | Mild |
| Bell’s palsy | 12 | 1 | 1–2 | Mild |
| Postpartum haemorrhage > 1 l | 15 | 2 | 1–3 | Moderate |
| Hepatic dysfunction | 15.5 | 2 | 1–3 | Moderate |
| Acute renal insufficiency | 17 | 2 | 1–3 | Moderate |
| Positive inotrope support | 18 | 2 | 1–3 | Moderate |
| Requirement for > 50% oxygen for > 1 hour | 18 | 2 | 1–3 | Moderate |
| Posterior reversible encephalopathy | 20.5 | 2 | 2–3 | Moderate |
| Reversible ischaemic neurological deficit | 21 | 2 | 2–3 | Moderate |
| Hepatic haematoma | 22 | 2 | 2–3 | Moderate |
| Intubation | 22 | 3 | 2–3 | Severe |
| Glasgow coma scale score of < 13 | 23 | 3 | 2–3 | Severe |
| Pulmonary oedema | 23 | 3 | 2–3 | Severe |
| Abruptio placentae | 23 | 3 | 1–3 | Severe |
| Retinal detachment | 24 | 3 | 2–3 | Severe |
| Eclamptic seizures | 25 | 3 | 2–3 | Severe |
| Cortical blindness | 26 | 3 | 3–3 | Severe |
| Stroke | 26.5 | 3 | 3–3 | Severe |
| Maternal mortality | 27 | 3 | 3–3 | Severe |
| Hepatic rupture | 27 | 3 | 3–3 | Severe |
| Dialysis | 27 | 3 | 3–3 | Severe |
| Myocardial ischaemia/infarction | 27 | 3 | 3–3 | Severe |
Appendix 2 Changes since original application
| What was proposed in original grant application | What was done in the PREP study |
|---|---|
| The original target sample size was 500 women with confirmed diagnosis of pre-eclampsia | The sample size was revised so we continued recruitment until 100 women had experienced an adverse event. The population did not change |
| Update on maternal predictor variables | Chest pain and dyspnoea were added as candidate predictors. Gestational age, maternal age and platelet count were also added to the maternal prognostic factors |
| One general list of candidate prognostic factors | Candidate prognostic factors were split into maternal and fetal predictor variables and only the fetal predictor variables included ultrasound |
| Symptoms of headache, epigastric pain, nausea, chest pain, dyspnoea or visual disturbance were one variable | These were split and regrouped into:
|
| BP was one variable | This was split into systolic BP and diastolic BP |
| Outcome assessment by 48 hours and by discharge | For the logistic model we had insufficient sample size to assess model performance at 48 hours. Therefore, we developed a second model, the survival model, to provide risks at various time points including 48 hours. However, we censored at 34 weeks, as one of the components of the outcome is delivery by 34 weeks |
| Develop the PREP model in the ASTRONAUT cohort of women | The ASTRONAUT study (gtr.rcuk.ac.uk/projects?ref=G0601295) did not commence and we were therefore unable to work on its data |
| Validate the PREP model in the PIERS and PETRA cohort | We validated the rPREP-L model in both external data sets. We were unable to validate the rPREP-S model in the PETRA data set; dates and times of outcome occurrence were not reported |
| Assess the added predictive contribution of biomarkers (sFlt1, sEng, PIGF) in maternal blood or urine | The ASTRONAUT study, planned to provide data on biomarkers, did not commence and we were therefore unable to work on its data |
| Update of maternal outcomes | Platelet count and infusion of any third parenteral antihypertensive removed as maternal outcomes. Preterm delivery < 34 weeks’ gestation added as a maternal outcome |
Appendix 3 PREP-L model
Appendix 4 PREP-S model
Appendix 5 Multivariable fractional polynomial terms that best predict outcome in the logistic model
| Multiple imputation data set | Variables | Best powers identified | p-value |
|---|---|---|---|
| 1 | Maternal age (years) | 1 | 0.782 |
| Log-transformed gestational age at diagnosis | 3 3 | < 0.001 | |
| Systolic BP (mmHg) | 1 | 0.788 | |
| Platelet count (× 109/l) | 1 | 0.250 | |
| Log-transformed serum urea concentration | –1 | 0.002 | |
| Log-transformed PCR | 1 | 0.261 | |
| 2 | Maternal age (years) | 1 | 0.835 |
| Log-transformed gestational age at diagnosis | 3 3 | < 0.001 | |
| Systolic BP (mmHg) | 1 | 0.761 | |
| Platelet count (× 109/l) | 1 | 0.176 | |
| Log-transformed serum urea concentration | –1 | 0.003 | |
| Log-transformed PCR | 1 | 0.202 | |
| 3 | Maternal age (years) | 1 | 0.821 |
| Log-transformed gestational age at diagnosis | 3 3 | < 0.001 | |
| Systolic BP (mmHg) | 1 | 0.682 | |
| Platelet count (× 109/l) | 1 | 0.177 | |
| Log-transformed serum urea concentration | –1 | 0.002 | |
| Log-transformed PCR | 1 | 0.364 | |
| 4 | Maternal age (years) | 1 | 0.799 |
| Log-transformed gestational age at diagnosis | 3 3 | < 0.001 | |
| Systolic BP (mmHg) | 1 | 0.653 | |
| Platelet count (× 109/l) | 1 | 0.219 | |
| Log-transformed serum urea concentration | –1 | 0.001 | |
| Log-transformed PCR | 1 | 0.462 | |
| 5 | Maternal age (years) | 1 | 0.958 |
| Log-transformed gestational age at diagnosis | 3 3 | < 0.001 | |
| Systolic BP (mmHg) | 1 | 0.684 | |
| Platelet count (× 109/l) | 1 | 0.288 | |
| Log-transformed serum urea concentration | –1 | 0.007 | |
| Log-transformed PCR | 1 | 0.342 |
Appendix 6 Coefficients of the final multivariable logistic model after adjustment for optimism
| Predictor | Coefficient |
|---|---|
| Maternal age (years) | –0.020 |
| FP (log-GA at diagnosis)3 centred at 39.90241 | 12.047 |
| FP (log-GA at diagnosis)3 × ln(log-GA at diagnosis) centred at 49.08188 | –7.926 |
| Effect of a medical history score of 1 | –0.330 |
| Effect of a medical history score of ≥ 2 | –0.579 |
| Systolic BP (mmHg) | 0.024 |
| Platelet count (× 109/l) | –0.004 |
| FP (log-serum urea concentration)–1 | –0.950 |
| Log-transformed PCR | 0.146 |
| Baseline treatment: any antihypertensive drug | 0.409 |
| Baseline treatment: magnesium sulphate | 1.252 |
| Constant | –1.507 |
Appendix 7 Comparison of the flexible parametric approach with the Cox model for the full survival model
| Candidate predictors | Flexible parametric model after multiple imputation | Cox regression after multiple imputation | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Maternal age (years) | 0.968 (0.954 to 0.982) | < 0.001 | 0.967 (0.954 to 0.982) | < 0.001 |
| Log-transformed gestational age (weeks) at diagnosis | 22.425 (8.528 to 58.970) | < 0.001 | 21.552 (8.156 to 56.952) | < 0.001 |
| Symptoms of headache and/or visual disturbance | 1.007 (0.835 to 1.215) | 0.940 | 1.004 (0.832 to 1.211) | 0.968 |
| Symptoms of epigastric pain, nausea and/or vomiting | 0.943 (0.745 to 1.194) | 0.627 | 0.942 (0.744 to 1.192) | 0.618 |
| Symptoms of chest pain and/or dyspnoea | 1.172 (0.766 to 1.793) | 0.465 | 1.183 (0.771 to 1.813) | 0.442 |
| Clonus | 0.763 (0.547 to 1.064) | 0.111 | 0.763 (0.545 to 1.068) | 0.115 |
| Exaggerated tendon reflexes | 1.249 (0.996 to 1.566) | 0.054 | 1.261(1.004 to 1.584) | 0.046 |
| Medical history score (reference 0) | ||||
| Effect of a medical history score of 1 | 0.828 (0.671 to 1.022) | 0.078 | 0.831 (0.673 to 1.025) | 0.084 |
| Effect of a medical history score of 2 | 0.658 (0.479 to 0.905) | 0.010 | 0.660 (0.480 to 0.908) | 0.011 |
| Twins vs. singleton | 0.895 (0.631 to 1.270) | 0.535 | 0.904 (0.637 to 1.283) | 0.571 |
| Triplets vs. singleton | 1.194 (0.291 to 4.904) | 0.806 | 1.177 (0.287 to 4.836) | 0.821 |
| Systolic BP (mmHg) | 1.018 (1.012 to 1.024) | < 0.001 | 1.017 (1.012 to 1.023) | 0.000 |
| Diastolic BP (mmHg) | 1.002 (0.993 to 1.011) | 0.695 | 1.002 (0.993 to 1.011) | 0.677 |
| Oxygen saturation < 94% | 4.342 (1.496 to 12.607) | 0.007 | 4.514 (1.557 to 13.088) | 0.006 |
| Haemoglobin level (g/l) | 1.051 (0.984 to 1.121) | 0.137 | 1.053 (0.986 to 1.124) | 0.123 |
| Platelet count (× 109/l) | 0.997 (0.996 to 0.998) | < 0.001 | 0.997 (0.996 to 0.998) | < 0.001 |
| Log-transformed ALT concentration | 1.181 (1.040 to 1.341) | 0.010 | 1.179 (1.038 to 1.340) | 0.011 |
| Log-transformed serum uric acid concentration | 1.052 (0.957 to 1.157) | 0.289 | 1.051 (0.956 to 1.155) | 0.302 |
| Log-transformed serum urea concentration | 1.555 (1.296 to 1.865) | < 0.001 | 1.558 (1.300 to 1.868) | < 0.001 |
| Log-transformed serum creatinine concentration | 1.549 (1.081 to 2.219) | 0.017 | 1.549 (1.080 to 2.220) | 0.017 |
| Urine dipstick (reference: none/trace) | ||||
| 1+ | 0.864 (0.522 to 1.433) | 0.572 | 0.855 (0.516 to 1.417) | 0.544 |
| 2+ | 0.994 (0.619 to 1.597) | 0.981 | 0.987 (0.614 to 1.585) | 0.956 |
| 3+ | 1.293 (0.795 to 2.103) | 0.300 | 1.282 (0.789 to 2.085) | 0.316 |
| ≥ 4 | 1.216 (0.717 to 2.062) | 0.469 | 1.200 (0.707 to 2.036) | 0.499 |
| Log-transformed PCR | 1.082 (1.001 to 1.169) | 0.047 | 1.084 (1.004 to 1.170) | 0.040 |
| Baseline treatment | ||||
| Any antihypertensive drug | 1.239 (0.983 to 1.562) | 0.070 | 1.231 (0.976 to 1.552) | 0.079 |
| Magnesium sulphate | 3.540 (2.708 to 4.627) | < 0.001 | 3.523 (2.693 to 4.609) | < 0.001 |
Appendix 8 Coefficients of the survival model after adjusting for optimism
| Predictor | Coefficient |
|---|---|
| Maternal age (years) | –0.031 |
| FP (log(GA at diagnosis/10))–2 centred at 0.8345136 | 1.514 |
| FP (log(GA at diagnosis/10))–2 × ln(log(GA at diagnosis/10)) centred at 0.0652155 | 5.707 |
| Exaggerated tendon reflexes | 0.122 |
| Summary score of medical history | |
| Effect of a medical history score of 1 | –0.169 |
| Effect of a medical history score of ≥ 2 | –0.385 |
| Systolic BP (mmHg) | 0.016 |
| Pulse oximetry < 94% | 0.797 |
| Platelet count (× 109/l) | –0.002 |
| Log-transformed ALT concentration | 0.126 |
| FP (log-serum urea concentration)2 | 0.605 |
| FP (log-serum urea concentration)3 | –0.144 |
| Log-transformed serum creatinine concentration centred at 0.4067578 | 0.265 |
| Log-transformed PCR | 0.080 |
| Baseline treatment | |
| Any antihypertensive | 0.176 |
| Magnesium sulphate | 1.066 |
| Baseline H(t) terms | |
| Spline basis function 1 | 1.500 |
| Spline basis function 2 | –0.116 |
| Spline basis function 3 | 0.141 |
| Spline basis function 4 | –0.054 |
| Spline basis function 5 | –0.011 |
| Constant | –3.724 |
Appendix 9 Coefficients of the final adapted PREP-L model adjusted for optimism excluding serum urea concentration
| Predictor variables | Coefficient |
|---|---|
| Maternal age (years) | –0.020 |
| FP (log-GA at diagnosis)3 centred at 39.90241 | 11.386 |
| FP (log-GA at diagnosis)3 × ln(log-GA at diagnosis) centred at 49.08188 | –7.492 |
| Summary score of medical history | |
| Effect of a medical history score of 1 | –0.340 |
| Effect of a medical history score of ≥ 2 | –0.518 |
| Systolic BP (mmHg) | 0.023 |
| Platelet count (× 109/l) | –0.005 |
| Log-transformed PCR | 0.203 |
| Baseline treatment | |
| Any antihypertensive | 0.453 |
| Magnesium sulphate | 1.287 |
| Constant | –3.577 |
Appendix 10 Coefficients of the final adapted PREP-S model adjusted for optimism and excluding serum urea concentration, clonus and exaggerated tendon reflexes
| Predictor variables | Coefficient |
|---|---|
| Maternal age (years) | –0.029 |
| FP (log(GA at diagnosis/10))–2 centred at 0.8345136 | 1.076 |
| FP (log(GA at diagnosis/10))–2 × ln(log(GA at diagnosis/10)) centred at 0.0652155 | 4.635 |
| Effect of a medical history score of 1 | –0.188 |
| Effect of a medical history score of ≥ 2 | –0.339 |
| Systolic BP (mmHg) | 0.016 |
| Oxygen saturation < 94% | 1.587 |
| Platelet count (× 109/l) | –0.003 |
| Log-transformed ALT concentration | 0.141 |
| Log-transformed serum creatinine concentration centred at 4.067578 | 0.669 |
| Log-transformed PCR | 0.138 |
| Baseline treatment: any antihypertensive | 0.178 |
| Baseline treatment: magnesium sulphate | 1.083 |
| Spline basis function 1 | 1.455 |
| Spline basis function 2 | –0.090 |
| Spline basis function 3 | 0.148 |
| Spline basis function 4 | –0.052 |
| Spline basis function 5 | –0.009 |
| Constant | –6.009 |
Appendix 11 PREP study data collection forms
List of abbreviations
- AIC
- Akaike information criterion
- ALT
- alanine aminotransaminase
- APEC
- Action on Pre-Eclampsia Charity
- AST
- aspartate transaminase
- BIC
- Bayesian information criterion
- BP
- blood pressure
- CI
- confidence interval
- CTG
- cardiotocography
- FP
- fractional polynomial
- HELLP
- haemolysis, elevated liver enzymes, low platelets
- MFP
- multivariable fractional polynomial
- PCR
- protein-to-creatinine ratio
- PETRA
- Pre-Eclampsia TRial Amsterdam
- PIERS
- Pre-eclampsia Integrated Estimate of RiSk
- PREP
- Prediction of Risks in Early-onset Pre-eclampsia
- PREP-L
- Prediction of Risks in Early-onset Pre-eclampsia – logistic model
- PREP-S
- Prediction of Risks in Early-onset Pre-eclampsia – survival model
- rPREP-L
- Prediction of Risks in Early-onset Pre-eclampsia – reduced logistic model
- rPREP-S
- Prediction of Risks in Early-onset Pre-eclampsia – reduced survival model
- RR
- relative risk
- SD
- standard deviation
- SOGC
- Society of Obstetricians and Gynaecologists in Canada
- TRIPOD
- Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis