Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/107/01. The contractual start date was in June 2011. The draft report began editorial review in March 2016 and was accepted for publication in July 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The insulin pumps were provided free of charge and unconditionally by Medtronic, which had no involvement in the design of the protocol; the collection, analysis and interpretation of the data; the writing of this report; or the decision to submit the report for publication. Simon Heller is a Health Technology Assessment Clinical Evaluation and Trials Board Member, who reports personal fees from Sanofi-Aventis, and personal fees and other from Novo Nordisk and Eli Lilly, outside the submitted work. Katharine Barnard reports personal fees from Roche Diabetes Care, outside the submitted work. Michael Campbell was a National Institute for Health Research Health Services and Delivery Research Board Member from 2010 to 2014. Jackie Elliott reports personal fees from AstraZeneca, Merck Sharpe & Dohme and Takeda, and personal fees and non-financial support from Eli Lilly, Novo Nordisk and Sanofi-Aventis, outside the submitted work. Mark Evans reports personal fees and other from Abbott Diabetes Care, Medtronic, Roche, Eli Lilly, Novo Nordisk and Cellnovo, and grants from Senseonics and Oxford Medical Diagnostics, outside the submitted work. Peter Hammond reports personal fees from Medtronic, Johnson & Johnson, Roche, Novo Nordisk and Eli Lilly, outside the submitted work. Alan Jaap reports personal fees and non-financial support from Novo Nordisk, and personal fees from Eli Lilly, Takeda, Merck Sharpe & Dohme and AstraZeneca, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Heller et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Type 1 diabetes mellitus and its treatment
People with type 1 diabetes mellitus (T1DM), around 250,000 individuals in the UK, have lost the ability to make insulin because of autoimmune destruction of the insulin-secreting β cells within the islets of the pancreas. Insulin is essential in the short term to prevent the onset of ketoacidosis, a potentially fatal condition. In the long term, the aim of insulin therapy is to keep blood glucose close to normal and so prevent the development of microvascular complications, such as retinopathy, neuropathy and diabetic kidney disease. Insulin is generally administered by intermittent subcutaneous injection, with the dose adjusted according to eating and other activities, such as exercise. Traditionally, insulin was given twice a day, often as premixed insulin, but such an approach imposes a rigid lifestyle and makes it difficult to maintain a glucose level close to normal. The need for intensification of therapy and its integration into flexible lifestyles is promoted in DAFNE (Dose Adjustment for Normal Eating) and other structured education courses. It involves giving quick-acting insulin just before eating and administering longer-acting background insulin, preferably twice daily, to maintain blood glucose levels in between meals. 1,2 This multiple daily injection (MDI) regimen involves a total of five or six injections a day. Blood glucose levels are monitored from finger-prick samples using a portable meter, and insulin dose calculations are based on self-assessed carbohydrate estimations on a meal-by-meal basis.
Insulin given subcutaneously cannot reproduce the physiological insulin profiles of non-diabetic individuals because of the limitations of insulin formulations and the site of delivery. The relatively slow rate of insulin absorption leads initially to postprandial hyperglycaemia, followed, 1 or 2 hours later, by an increased risk of postabsorptive hypoglycaemia, particularly during the night. Thus, keeping blood glucose close to normal can delay or prevent complications, but brings with it frequent periods of hypoglycaemia. These are categorised as mild, moderate or severe episodes, ranging from mild symptoms, self-managed by ingesting rapid-acting carbohydrate, through to greater disruption in daily routine due to cerebral dysfunction, through to major episodes of coma and seizure requiring third-party assistance. The inability of intermittent injection therapy to control blood glucose tightly without an attendant risk of hypoglycaemia results in many individuals keeping their blood glucose at higher than desirable levels. This leads to an increased risk of serious diabetic complications, which can affect the eyes, feet and kidneys. These complications, plus the associated high risk of cardiovascular disease, reduce both the length and quality of the individuals’ lives.
Insulin analogues
Short- and long-acting insulin analogues have slightly more physiological profiles than insulins of human or animal structure, but cannot reproduce those observed in people without diabetes. 2 Systematic reviews of clinical trials of insulin analogues involving people with T1DM have reported only minor advantages compared with human insulin, with a reduced risk of symptomatic hypoglycaemia, particularly at night. 3,4 This may be, in part, because those people who are at the greatest risk of hypoglycaemia are frequently excluded from clinical trials. Interestingly, in a recent crossover trial comparing MDI of human insulin with analogue insulin, the investigators specifically recruited individuals who had experienced problems with hypoglycaemia, and found that those using analogue insulin had significantly lower risks of severe hypoglycaemia, particularly at night. 5
Insulin pumps
There is clearly an urgent need for better methods of insulin delivery. Insulin pumps were first used clinically in the early 1980s, but randomised controlled trials (RCTs) conducted in the UK failed to show any clinical benefit. At the time, the technology was poorly developed, but has advanced considerably, particularly in the last few years. Insulin pumps are now the size of a small mobile phone and deliver insulin continuously under the skin via a small plastic tube and cannula [continuous subcutaneous insulin infusion (CSII)]. 6,7 These devices are filled with reservoirs of quick-acting insulin only (usually an insulin analogue), which provides insulin replacement by delivering both the mealtime and background insulin. When infused continuously at low rates they ‘mimic’ basal insulin secretion, and this is generally delivered more consistently and accurately than is achievable by the longer-acting insulins, particularly at night. The insulin boluses used to cover meals and correct high blood glucose levels are delivered much more rapidly. All of the insulin doses can be controlled by the patient, based on calculations similar to those required for insulin dosing with a MDI regimen.
The purchase and use of pumps is more expensive than MDI, with pumps at current prices costing around £2500 each, plus £1500 per year extra for running costs. 8 The marginal cost per annum over MDI is about £1800. 9 The potential advantages are more stable blood glucose levels, a reduced risk of hypoglycaemia and a more flexible lifestyle. Pump treatment may deliver insulin more effectively than MDI but does not provide a technological ‘cure’. The same competencies needed for successful insulin self-management, previously described for MDI, are required for pumps, but with additional skills required to operate the pump device itself. Thus, pumps are probably more useful to those individuals who are actively and effectively self-managing their diabetes rather than those who expect the pump to ‘manage’ their diabetes for them.
Pumps are currently used by around 40% of people with T1DM in the USA and > 15% in Europe. 10 In contrast, the proportion in the UK was around 6% in adults in 2012. 11,12 Proponents of pump treatment have proposed that far more patients should be offered treatment in the UK and that current policies are depriving many of the opportunity to improve glycaemic control, reduce hypoglycaemia and improve quality of life (QoL). 12 The UK’s National Institute for Health and Care Excellence (NICE) has recently extended recommendations for the use of pumps for adults with T1DM. The guidance suggests that pump treatment be considered for individuals who are experiencing problems with hypoglycaemia, particularly when this limits the ability to improve glycaemic control. NICE has noted the paucity of evidence for efficacy from RCTs. 13
Problems with evidence in National Institute for Health and Care Excellence appraisals
There have been two appraisals9,14 of pumps by NICE, both supported by technology assessment reports undertaken by some of the present authors, which reviewed the evidence on clinical effectiveness and cost-effectiveness. The first report14 noted that there were no trials of pumps against ‘best MDI’ with long- and short-acting analogue insulins; some trials had unequal amounts of education in the arms (with more in the pump arms); and the trials had focused on easily measurable outcomes such as glycated haemoglobin (HbA1c), rather than on benefits in terms of flexibility of lifestyle and QoL. The report recommended trials of pumps against analogue-based MDI.
The second report9 found that few such trials had been done: one in children, not relevant to this work, and three in adults. Furthermore, the three adult studies15–17 presented data for a small number of participants who were followed over a short period only. The first of these studies was a 24-week pilot study15 in adults with altered hypoglycaemia awareness and debilitating hypoglycaemia. The three study arms consisted of seven patients each and compared (1) analogue MDI, (2) pump and (3) education and relaxation of glycaemic targets. All of the subjects were naive to analogue insulin use and some had never tried MDI, and so were not representative of the type of patients for whom NICE recommends pumps.
The second trial16 recruited 39 adults with T1DM, who had already been on pump therapy for at least 6 months, and who were randomised to stay on pump or switch to glargine (Lantus, Sanofi-Aventis, Guildford, UK)-based MDI for 4 months. The primary end point was glucose variability, which was 5–12% less with the pump. Despite this, there was no significant difference in the frequency of hypoglycaemic episodes or HbA1c.
The third study17 studied 50 patients with T1DM from Italy, UK (Newcastle, Bournemouth) and France, who were naive to pumps and glargine, to which they were switched for the trial, having been previously managed on neutral protamine Hagedorn (NPH)-based regimens. Follow-up was for 24 weeks. Patients were randomised to pump or analogue MDI in an equivalence study. The difference in HbA1c at the study end was only 0.1% (approximately 1 mmol/mol) and the costs with the pump were three times higher.
Thus, the evidence base from trials for comparing pumps and ‘best MDI’ remains weak in terms of numbers, with a total of only 103 patients and short-term follow-up. Furthermore, the patients in the trials were dissimilar to those considered suitable for a pump by NICE, which expects patients to have tried analogue-based MDI before using the pump.
Given the paucity of RCTs, the assessment group also looked at observational studies of adults in which a pump was clinically indicated, mostly because of the limitations of intermittent injections. This comparison has the advantage of measuring change in glycaemic control and hypoglycaemia in those who have most to gain, and these studies showed improved HbA1c of the order of around 0.5% (5.5 mmol/mol). Interpretation of data from observational studies face limitations from bias, and, furthermore, of the 48 observational studies, only nine reported QoL. Study numbers were small and duration was usually short. The longest study noted that initial benefits from pumps might not be sustained.
Therefore, again, NICE was faced with an evidence base with considerable shortcomings, too few trials, durations too short, numbers too small and a need to use observational studies. A recent meta-analysis by Monami et al. 18 concluded that ‘available data justify the use of CSII for basal-bolus insulin therapy in type 1 diabetic patients unsatisfactorily controlled with MDI’. However, most of the RCTs in their analysis were NPH-based and the Bolli et al. 17 trial, with its negative result, was missed.
A systematic review of the cost-effectiveness of insulin pump therapy in adults with T1DM was conducted by Roze et al. 19 They identified four cost-effectiveness studies in the UK setting, three of which presented an incremental cost-effectiveness ratio (ICER). 9,14,20,21 The ICERs in these studies were £11,461 per quality-adjusted life-year (QALY) gained, £25,648 per QALY gained and £37,712 per QALY gained. Two out of the three studies had ICERs that lie within, or below, the £20,000 to £30,000-per-QALY-gained range that NICE usually uses to determine if a health technology is cost-effective. 22 These two studies did receive commercial sponsorship, whereas the study with an ICER of £37,712 was commissioned on behalf of NICE.
Rationale for the trial
We hypothesised that much of the benefit of pumps may come from the retraining and education in intensive insulin management, which allows patients to use pumps safely. 23 In many DAFNE centres, reimbursement for pump use is conditional on patients having attended a DAFNE education course and so some patients undertake DAFNE training with the intention of moving to pump treatment thereafter. It has been our clinical experience that many individuals decide not to switch to the pump after attending a DAFNE course, as they then realise that what they required was training in insulin self-adjustment rather than a different technical way of delivering insulin. Ray et al. 24 found that 69% of those being considered for insulin pump therapy stay on MDI after completing DAFNE. Importantly, trials and observational studies of high-quality training alone (with standard insulin injections) show benefits in blood glucose control, hypoglycaemia and QoL, which are as good, if not better, than those reported after pump therapy. 2,25,26
To our knowledge, no trials in adults, comparing pump treatment with modern MDI, used the same structured training in insulin adjustment, resulting in the added benefit of the pump technology remaining unclear. 23 There was an urgent need to establish this, and identify patients who benefit the most. A RCT was needed to establish these outcomes without bias.
The DAFNE course is a 1-week structured education course, teaching adults with T1DM the skills in insulin self-adjustment and carbohydrate counting. 2 DAFNE courses are currently delivered in more than 70 centres across the UK, with over 37,000 individuals (DAFNE graduates) now trained. We therefore set out to conduct a novel study in which adults waiting for a DAFNE course were randomly allocated to undertake either the standard MDI course or DAFNE incorporating use of pump therapy.
The investigators involved in this work have been undertaking research into other aspects of DAFNE for many years. During recent work funded by a National Institute for Health Research (NIHR) programme grant [Programme Grants for Applied Research (PGfAR)] we measured cost-effectiveness and identified which components of the course are crucial, as well as identifying the factors determining which DAFNE patients managed their diabetes more effectively. 27 This work included funding to pilot a combined DAFNE and pump course, which enabled us to develop a pump curriculum and associated pump-specific resources, ensure that the outcome measures that we wanted to use were feasible and estimate the likely recruitment and retention rates.
We then assembled a study group with expertise in structured T1DM education, pump therapy (having trained in total over 700 pump patients) and health economic assessment of diabetes interventions.
Decision problem: aim of the REPOSE Trial
The aim of our trial was to establish for patients, professionals and those funding the service, the added benefit of using a pump during intensive insulin therapy. We conducted a RCT comparing optimised MDI therapy (using rapid and twice-daily, long-acting insulin analogues) with pump therapy in adults with T1DM, for which both were provided with high-quality structured education (DAFNE).
Research objectives
The project had the following specific objectives:
-
To measure, over 2 years, (1) biomedical, (2) psychosocial (quantitative and qualitative) and (3) adverse event (AE) outcomes. The primary outcome was HbA1c at 2 years, with a minimum clinically significant difference defined as 0.5% (5.5 mmol/mol).
-
To undertake a cost-effectiveness analysis to determine whether or not the marginal benefits of pump therapy over optimised MDI (if demonstrated) are commensurate with the marginal costs, as reflected in an ICER, expressed in terms of a cost per QALY gained that is acceptable to NICE.
-
To conduct a mixed-methods psychosocial evaluation of pump therapy in order to identify factors that predict and/or help explain outcomes on the pump.
Members of the research team have been involved in the NICE appraisal of insulin pumps, have been members of NICE appraisal committees and have a good understanding of what evidence NICE needs. Thus, a further objective was to inform the next NICE reviews of insulin pumps and structured education.
Chapter 2 Overview of evidence base for pump therapy
As noted in Chapter 1, the evidence on the clinical effectiveness and cost-effectiveness up to June 2007 was reviewed in the two assessment reports for NICE,9,14 both published in this monograph series. This chapter is concerned mainly with studies that have emerged since 2007, but we also provide a complete overview of all of the trials.
Methods
Searches were performed for RCTs that compared the clinical effectiveness of pump and MDI in adults, from June 2007 to the present in MEDLINE and EMBASE (see Appendix 1 for search methods). We checked inclusion lists of seven past systematic reviews. 9,14,18,28–31
Reasons for exclusion included:
-
control group not on MDI
-
pump therapy from diagnosis of diabetes
-
studies in pregnancy
-
paediatric age group
-
studies in type 2 diabetes mellitus
-
pump plus continuous glucose monitoring (CGM) versus MDI plus self-monitoring of blood glucose (SMBG) levels
-
closed-loop trials
-
low-glucose suspend (LGS) pumps
-
all on pump therapy, with different pumps
-
trials of catheter duration in pump therapy
-
not a trial
-
peritoneal infusion
-
protocols
-
pumps infusing substances other than insulin
-
reviews
-
trials of exercise on pump therapy.
During the course of the REPOSE Trial, weekly auto-alerts were run in MEDLINE and EMBASE to identify any emerging research that might affect the trial. The search strategy used was:
-
(insulin and pump*).tw.
-
(CSII or continuous subcutaneous insulin infusion).tw.
-
(continuous adj3 insulin adj3 infusion).tw.
-
(subcutaneous adj3 insulin adj3 infusion).tw.
-
1 or 2 or 3 or 4.
-
DAFNE.tw.
-
(dose adjust* adj2 normal eating).tw.
-
6 or 7.
-
5 or 8.
In addition, final searches were performed for RCTs that compared the clinical effectiveness of pump and MDI in adults, from 2007 to 7 January 2016 in MEDLINE, EMBASE, Web of Science and the Cochrane Central Register of Controlled Trials.
Trials may be done in selected groups of patients and, as noted in Chapter 1, they are often of short duration. We therefore carried out a search for longer-term observational studies in large groups of patients as a guide to the results of pump therapy in routine care. We selected studies with at least 3 years of follow-up, and ≥ 100 patients, published since January 2008. Older observational studies were reviewed in a previous monograph,9 and the findings summarised as follows:
-
There were much greater improvements in HbA1c in observational studies than reported in the RCTs.
-
There were considerable reductions in severe hypoglycaemia. This may reflect selection for pump therapy of people having particular problems with hypoglycaemia, but that would make the results more applicable to the patients who would get a pump in routine care.
-
The majority of studies showed no increase in diabetic ketoacidosis (DKA).
-
Weight gain was reported but usually minor.
-
There was a reduction in daily insulin dose, which will provide some savings to offset the cost of pump therapy.
-
There were gains in QoL, with comments on items such as flexibility of meal choices and timings and other aspects of lifestyle, and diabetes being easier to manage.
During the course of the REPOSE Trial, we looked for any important developments in:
-
pump therapy
-
structured education
-
new insulins used in MDI or pump
-
the evidence base on QoL on pump and MDI.
Findings
Pump therapy
Table 1 shows the trials of pump therapy against MDI in adults with T1DM, excluding those in pregnancy. There have been only four trials of pump versus MDI with analogue insulin use in both arms, and the longest follow-up period was 24 weeks, which, as we will show in Chapter 3, is insufficient to achieve the full potential of pump therapy.
| Trial | Year of publication | n of participants | Design | Pump | MDI | Duration on pump |
|---|---|---|---|---|---|---|
| Bak et al., 198732 | 1987 | 20 | Crossover | Actrapida | Actrapid and NPH | 6 months |
| Bode et al., 199633 | 1996 | 55 | Crossover | Soluble | 12 months | |
| Bolli et al., 200917 | 2009 | 43 | Parallel | Lisprob | Lispro t.i.d., glargine once | 24 weeks |
| Bruttomesso et al., 200816 – incorporates Maran et al., 200534 | 2008, 2005 | 42 | Crossover | Lispro | Lispro, glargine | 4 months on each |
| Chiasson et al., 198435 | 1984, 1985 | 12 | Crossover | Regular | Regular and Ultralente | 3 months on each |
| DeVries et al., 200236 | 2002 | 55 completed of 79 starters | Started as crossover but reduced to parallel | Aspart | Aspart and NPH | 16 weeks |
| Düsseldorf Study group (Ziegler et al., 199037) | 1990 | 96 | Parallel | Not specified | Mixture of b.i.d. and MDI with regular and NPH | 2 years |
| Haakens et al., 199038 | 1990 | 52 started, 35 completed | Crossover | Soluble | Soluble, Ultralente, isophane | 6 months |
| Hanaire-Broutin et al., 200039 | 2000 | 40 | Crossover | Lispro | Lispro and NPH | 4 months on each |
| Hirsch et al., 200540 | 2005 | 100 | Crossover | Aspart | Aspart and glargine | 4 weeks |
| Home et al., 198241 | 1982 | 10 | Crossover | Actrapid | Actrapid, Ultralente | 10 weeks |
| Hoogma et al., 200642,43 | 2006 | 256 | Crossover | Lispro | Lispro and NPH | 6 months on each |
| Lepore et al., 200344 | 2003 | 32 | Parallel | Lispro | Lispro and NPH | 12 months |
| Nathan et al., 198245 | 1982 | 5 | Crossover | Soluble | NPH and regular | 8–12 weeks |
| Nosadini et al., 198846 | 1988 | 44 | Parallel | Soluble | Soluble t.i.d. and NPH | 1 year |
| Oslo, 1988,47 198648 | 1985–92 | 30 | Parallel | Velosulina | Regular porcine and NPH | 4 years |
| Saurbrey et al., 198849 | 1988 | 21 | Crossover | Actrapid | Actrapid (NovoPena) and NPH | 10 weeks |
| Schiffrin and Belmonte, 198250 | 1982 | 16 | Crossover | Soluble | Three soluble, one NPH | 6 months |
| Schmitz et al., 198951 | 1989 | 10 | Crossover | Velosulin, porcine regular | Velosulin and Insulatarda NPH | 6 months on each |
| Schottenfeld-Naor et al., 198552 | 1985 | 9 | Crossover | Velosulin | Velosulin and Insulatard | 4 months on each |
| Thomas et al., 200715 | 2007 | 14 | Parallel | Lispro | Lispro and glargine | 24 weeks |
| Tsui et al., 200153 | 2001 | 27 | Parallel | Lispro | Lispro and NPH | 9 months |
Table 1 shows that only five trials (assuming that Lepore et al. 44 is a trial – the paper does not mention randomisation but Misso et al. 31 in the Cochrane review say it was a RCT and that it had access to unpublished data) had a duration of ≥ 12 months, and none used analogue MDI. Lepore et al. 44 report HbA1c only at baseline and 12 months. 44 Dahl-Jørgensen et al. 54 reported a steep drop in HbA1c with a plateau after 3 months, but this reduction started in the 2-month run-in period before pump therapy was started.
Four trials15–17,40 used analogue insulin in both arms. The Hirsch et al. 40 trial had patients on pump and MDI for only 4 weeks. The Thomas et al. 15 trial was a pilot, with only seven patients per arm.
One new trial has been published since the last appraisal by NICE: Bruttomesso et al. 16 This trial recruited 42 patients already well controlled on the pump (mean HbA1c 7.4% at randomisation) and randomised them to continuing pump therapy, or to MDI with lispro and glargine. The aim was to see if the need for pump therapy was reduced by the arrival of the analogue insulins. Patients had only 4 months on MDI. Three patients withdrew shortly after starting MDI because of poorer glycaemic control. After 4 months the patients switched to the other treatment arm. The primary outcome was glucose variability, as assessed by SMBG. HbA1c during the study was 7.3% in both arms. There was no difference in the frequency of severe hypoglycaemia, but moderate hypoglycaemia was about 23% less frequent on pump therapy, although the definition of this is not stated in the published study. Glucose variability was 5–12% less on pump therapy, depending on time of day and method used. At the study end, patients could choose between pump therapy and glargine-based MDI. Thirty patients chose pump, five chose MDI and four opted for summer MDI and winter pump. The study was supported by Disetronic and one author worked for the company. 16
The Bolli et al. 17 trial (see Table 1) was published in 2009 but had been available in abstract form for the last assessment report.
Overall, therefore, there was still a poor evidence base with only one new trial, and that being of short duration (4 months on each arm) and limited sample size (only 39 patients).
Observational studies
Bacon et al. 55 reported 10-year follow-up data on 197 patients on pump therapy. The main indications for the pump were recurrent hypoglycaemia and poor control. HbA1c improved by about 0.7% and the number of severe hypoglycaemic episodes by about 80%. Only about 5% discontinued pump therapy.
Beato-Vibora et al. ,56 from King’s College Hospital, looked back over 12 years of pump therapy in 327 patients, with a mean duration of 4.3 years on the pump. An initial reduction in HbA1c of 8 mmol/mol or 0.7% was partially maintained with reduction at year 5 of 0.4%. The proportion of people having frequent mild-to-moderate hypoglycaemia fell from 29% to 12% and the frequency of severe hypoglycaemia was halved.
Bruttomesso et al. ,57 from the Veneto region of Italy, provide a retrospective study of all patients in their region who started pump therapy. Of 138 patients, 20 stopped pump therapy, although mostly in the earlier years. Strict eligibility criteria had to be met, including ‘the technical, physical and intellectual abilities’, plus motivation, stable personality and realistic expectations of pump therapy. All were familiar with MDI and received extra education. HbA1c was 9.3% when starting pump therapy, fell to 7.9 by end of year 1 and was largely sustained there for 7 years.
Carlsson et al.,58 from Sweden, reported results of 272 patients with at least 5.5 years of follow-up. They compared their results with a much larger group on MDI. HbA1c was reduced by 0.42% at 1 year and 0.43% at 2 years, but some of the effect was lost by 5 years when the reduction compared with the MDI group was only 0.2%. 58 A later paper59 reported that the reduction in HbA1c varied by baseline levels, with a small reduction of 0.29% (85% CI 0.11% to 0.47%) in those with baseline HbA1c of 7%, a reduction of 0.39% (85% CI 0.27% to 0.52%) in those with baseline HbA1c of 8% and a larger reduction of 0.50% (85% CI 0.36% to 0.67%) in those with baseline HbA1c of 9%, which would take them nowhere near target.
Cohen et al. 60 compared two cohorts from Melbourne in a non-randomised comparison. One group received pump therapy and the other received intensified MDI. Both were previously on analogue MDI. Among 126 patients on the pump, HbA1c fell by 0.64% at 6 months, but then rose again, with a reduction of about 0.4% at 2 years and about 0.2% at 5 years. The reduction in HbA1c on intensified MDI was smaller: 0.15% at 6 months. This was despite a similar programme of education, based on DAFNE but shorter, in both pump and MDI groups.
Lepore et al.,61 from three Italian centres, compared results in two matched groups of 110 patients on pump therapy and 110 on MDI, followed for 3 years. HbA1c fell by 0.7% in the pump group and this reduction persisted for the 3 years. HbA1c fell by 0.3% in the MDI group at 3 years.
Nixon et al. 62 reported a study of 35 patients on pump therapy. There was an initial fall of 1.7% in HbA1c but by 5 years the reduction was only 0.9%. However, this reflected a mix of results, with one-third of patients reducing HbA1c by 2.2% and maintaining it there, whereas others had no change on the pump or had an initial reduction not sustained.
Orr et al. ,63 from Ontario, report results among 235 patients on pump therapy. The overall baseline HbA1c was 8.7%, which was reduced to 7.5% after 6 months on the pump, after which it drifted up again to 8.2% in years 3–10. In 39 patients who were followed for 10–15 years, the mean HbA1c was 8.03%. However, two groups of patients who started with high baselines (8.5–10% and > 10%) reduced their HbA1c to about 8% by 8–10 years.
Quiros et al. ,64 from Barcelona, followed 151 patients on pump therapy for 5 years. Overall, HbA1c was reduced from a mean of 8.0% at baseline to 7.8% at 5 years. However, in the 61% of patients who started pump therapy because of poor glycaemic control, HbA1c fell from 8.4% at baseline to 8.0% at 5 years. There was a marked reduction in severe hypoglycaemia. 64
Rosenlund et al. 65 from Denmark looked at the effects of 4 years of pump therapy on albuminuria compared with an unmatched group on MDI. On pump therapy, HbA1c fell from 8.4% to 7.8%, maintained to 4 years. 65
Steineck et al. 66 from Sweden reported mortality data from a cohort of 18,168 people with T1DM in Sweden, of whom 13% were on pump therapy. Total mortality at 7 years was 6% in the pump group and 8% in the MDI group. There were many small differences that would increase the risk in the MDI group – more hypertension, more on lipid-lowering drugs, more with low physical activity, more smokers and more with low education levels. Steineck et al. 66 used propensity matching to adjust for the differences, and concluded that those on pump therapy had a 0.73 hazard ratio for total mortality. However, there could have been confounding factors for which they could not allow.
Most long-term studies show a disappointing waning of the initial HbA1c improvement. Perhaps there is a case for educational updates. In a small trial with only 23 patients, Carlone et al. 67 randomised patients on long-standing pump therapy to standard care or to six educational weekly group meetings on advanced features of the pump, carbohydrate counting and other aspects of diet. After 6 months, the intervention group had reduced their HbA1c by 1%. The control group did not change.
New developments
The main developments in pump therapy have been the use in combination with CGM systems, of which there are two forms that are now relevant to the pump. The first is when the CGM device is integrated with the pump, which means that it sends glucose results to the pump every 5 minutes or so, from a sensor just under the skin. Strictly speaking this means that the glucose result is for the level in interstitial tissue, not in the bloodstream, but the two are closely related. With the integrated CGM system, the pump can send alarms to the user, following which they can take action. This helps users to avoid hypoglycaemic episodes, but some find the alarms to be a nuisance and may disable the alarms. False alarms are not uncommon.
Four trials of CGM compared the pump with CGM against MDI with SMBG, which confounds things [Hermanides et al. (Eurythmic trial),68 Lee et al. ,69 Peyrot and Rubin,70 Bergenstal et al. (STAR-3)71]. The durations of these trials were only 6, 3.5, 3.7 and 12 months, respectively.
Continuous glucose monitoring would have implications for the use of pump therapy rather than MDI if the effectiveness of CGM differed between the two forms of treatment. Garg et al. 72 found that the effects of real-time CGM in reducing HbA1c and hypoglycaemia were similar in two matched groups on MDI and pump.
More recently, the Medtronic Veo (Medtronic, Watford, UK) has been introduced, which has a facility to link with a CGM system and suspend insulin infusion (the LGS facility) if the glucose level goes too low, for up to 2 hours. This means that the pump can take action. In practice, most suspensions are for much less than 2 hours because the wearer takes action. However, at night when the wearer is asleep, this may not happen. 73
There have been two trials of the Veo suspend pump. In the ASPIRE (Automation to Simulate Pancreatic Insulin Response) trial74 in the USA and Canada, the recruits were familiar with pump therapy. They had a 2-week run-in period and were selected for the trial if they had nocturnal hypoglycaemia (defined as plasma glucose < 3.7 mmol/l) at least twice in that period. They also had to be prepared to wear the sensors at least 80% of the time. They were randomised to the Veo with its LGS facility, or to the Medtronic Paradigm Revel, which has integrated CGM but no LGS action. The trial was sponsored by Medtronic, and Medtronic staff were involved in data analysis and editorial assistance. 74 The trial showed no difference in HbA1c after 3 months, perhaps not surprisingly because the baseline HbA1c was very good at 7.2% or 55 mmol/mol. There was reduced hypoglycaemia, especially nocturnal. In the Veo group, 111 of 121 patients had at least one nocturnal suspension on the pump that lasted 2 hours. A 2-hour suspension does not lead to significant ketosis. QoL measures, EuroQol-5 Dimensions (EQ-5D) and the Hypoglycaemia Fear Survey (HFS) score, showed no difference between the arms of the trial. There were only four severe hypoglycaemic episodes, none in the Veo arm.
So the main benefit of the Veo LGS over the integrated CGM pump system is reduction of nocturnal hypoglycaemia. There is quite a large extra capital cost for the device (£2692) with an annual cost, including consumables, of £4862. This will make it difficult to prove cost-effectiveness. The group in which the Veo is most likely to be cost-effective will be patients with recurrent severe hypoglycaemia, but that group is covered by existing NICE guidance on the pump and is not recruited to the REPOSE Trial.
The other trial of the Veo was by Ly et al. 75 in Australia. This trial recruited mainly children and adolescents, with only 31% aged > 18 years. Patients were selected on the basis of impaired awareness of hypoglycaemia. They had been on pump therapy for an average of 4 years. They were randomised to the Veo suspend pump or to stay on their previous pump and use SMBG – not CGM. This immediately raises a problem because the Veo arm has both the LGS facility and CGM. It would have been better to have CGM in both arms. A more serious problem with the study is that, despite reasonable numbers (49 to pump plus SMBG, 45 to the Veo) and randomisation, there was a very marked baseline mismatch in previous severe (defined as seizure or coma) and moderate (defined as requiring assistance) hypoglycaemia, with a rate of 130 per 100 patient-months [95% confidence interval (CI) 111 to 150 patient-months] in the Veo group, but only 21 per 100 patient-months in the control arm (95% CI 14 to 30 patient-months). At study end after 6 months, the rate of moderate and severe hypoglycaemic episodes was 28.4 in the Veo group and 11.9 in the control arm. However, these figures were reversed when the authors adjusted for baseline rates, from 28.4 to 9.5, and from 11.9 to 34.2, all per 100 patient-months. There were no significant changes in HbA1c, but both groups started with quite reasonable levels of 7.6% and 7.4%.
The analysis by Ly et al. 75 has been strongly criticised by the German Institute for Quality and Efficiency in Healthcare [Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG)], as reported by Heinemann and Hermanns. 76
The Veo has been appraised by the NICE Diagnostics Assessment Programme. Their guidance is shown in Box 1.
1.1 The MiniMed Paradigm Veo system is recommended as an option for managing blood glucose levels in people with type 1 diabetes only if:
-
they have episodes of disabling hypoglycaemia despite optimal management with continuous subcutaneous insulin infusion and
-
the company arranges to collect, analyse and publish data on the use of the MiniMed Paradigm Veo system (see section 7.1).
1.2 The MiniMed Paradigm Veo system should be used under the supervision of a trained multidisciplinary team who are experienced in continuous subcutaneous insulin infusion and continuous glucose monitoring for managing type 1 diabetes only if the person or their carer:
-
agrees to use the sensors for at least 70% of the time
-
understands how to use it and is physically able to use the system and
-
agrees to use the system while having a structured education programme on diet and lifestyle, and counselling.
1.3 People who start to use the MiniMed Paradigm Veo system should only continue to use it if they have a decrease in the number of hypoglycaemic episodes that is sustained. Appropriate targets for such improvements should be set.
1.4 The Vibe and G4 PLATINUM CGM system shows promise but there is currently insufficient evidence to support its routine adoption in the NHS for managing blood glucose levels in people with type 1 diabetes. Robust evidence is needed to show the clinical effectiveness of using the technology in practice.
1.5 People with type 1 diabetes who are currently provided with the MiniMed Paradigm Veo system or the Vibe and G4 PLATINUM CGM system by the NHS for clinical indications that are not recommended in this NICE guidance should be able to continue using them until they and their NHS clinician consider it appropriate to stop.
Reproduced with permission from NICE. © National Institute for Health and Care Excellence 2016. Integrated sensor-augmented pump therapy systems for managing blood glucose levels in type 1 diabetes (the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system). Available from: www.nice.org.uk/guidance/dg21. NICE guidance is prepared for the NHS in England, and is subject to regular review and may be updated or withdrawn. NICE has not checked the use of its content in this article to confirm that it accurately reflects the NICE publication from which it is taken.
The patient group in which it has been recommended is different from that in the REPOSE Trial, and so the arrival of the Veo and its LGS facility has no implications for the implementations of the results of the REPOSE Trial.
Findings: structured education
The DAFNE course has changed little since the original trial published in 2002. 2 A programme of work has included a trial comparing the 5-day course in 1 week with 1 day a week for 5 weeks, which found little difference in outcomes. 78
One finding from the DAFNE research programme has been that many patients doing the DAFNE course, in preparation for going on to pump therapy, no longer need to progress to a pump after completing the course. Ray et al. 24 reported that after DAFNE education, 69% of patients previously being considered for pump therapy could remain on MDI.
However, another study from the programme (Mansell et al. 79) found that some patients who had been through DAFNE education still benefited from pump therapy in terms of a reduction in stress [measured by the PAID (Problem Areas in Diabetes Questionnaire) score] and improved glycaemic control at 12 months of follow-up. This may have been because individuals who progress to the pump after doing a DAFNE course have higher pre-course stress levels. 80
Conversely, attendance at DAFNE courses sometimes identifies individuals for whom pump therapy is indicated because of a troublesome dawn phenomenon.
Research into DAFNE education has also been undertaken by the Irish DAFNE group. 81 They carried out a large randomised trial of group follow-up compared with individual clinic visits for patients who had completed the DAFNE course. The intervention group received group education at 6 and 12 months after DAFNE, following a semistructured curriculum, whereas the control group had individual clinic appointments with doctor, nurse or dietitian. The additional education conferred no benefit over individual clinic visits.
The Irish DAFNE group also carried out a qualitative study to identify factors that influenced how well DAFNE graduates incorporated what they had learned into long-term daily living. 82 They identified four themes:
-
Being empowered, and feeling able to manage their diabetes, which some people did not manage to do.
-
Embedded knowledge, which increased over time, for example as patients got better at carbohydrate counting.
-
Maintaining motivation, including coping with uncertainty. The researchers commented that this was most marked at 6 months but improved later. Reducing the risk of complications was a strong motivation factor.
-
Continued support from health-care professionals.
The Australian OzDAFNE group83 also looked at psychological changes after the DAFNE course, and found increases in what they called ‘mastery/control’ and a reduction in diabetes-related distress. One of their key points was that the mean duration of diabetes in their participants was 18 years, but they had low self-assessment of their ability to manage their diabetes, so they recommended that referral to DAFNE courses should not be restricted to recently diagnosed patients.
Findings: new insulins
Some new basal insulins have been introduced, including degludec (Tresiba, Novo Nordisk, Gatwick, UK) and glargine 300 (Toujeo, Sanofi-Aventis, Guildford, UK). However, these are very long-acting basal insulins, and may not have the flexibility in dosing that is needed in MDI for T1DM, and with no data to date about how these might be used effectively in patients with T1DM who are undergoing structured education.
Newer short-acting insulins include ‘fast aspart’, which, in pump therapy, is reported to have a faster glucose-lowering effect but with the same effect overall. The implications for glycaemic control and hypoglycaemia were not reported by Zijlstra et al. 84
Findings: quality of life
Past reviews found a disappointingly low amount of evidence on QoL. This has implications for cost-effectiveness analysis. The Thomas et al. 15 pilot trial of pump therapy versus analogue MDI reported QoL as measured by the Diabetes Quality of Life questionnaire (DQOL) but found no difference. With only seven patients in each arm this may not be surprising. The 2008 health technology assessment (HTA)9 for NICE identified 48 observational studies of pump therapy, but only one reported QoL in adults, and it was a before-and-after study in which patients switched to the pump from conventional insulin therapy, not analogue MDI.
One observational study85 published since then has compared QoL. This study85 by the EQuality1 Study Group from Italy, has both strengths and weaknesses. It was a very large case–control study, with 1341 people with T1DM from 62 clinics, with 481 on the pump and 860 on MDI. The MDI patients came from centres both with and without pump services. Reliable instruments were used: the diabetes-specific quality of life scale (DSQOL) for QoL, Diabetes Treatment Satisfaction Questionnaire (DTSQ) for treatment satisfaction and Short Form questionnaire-36 items (SF-36) for health status. Eighty-four per cent of patients on the pump had been on it for > 1 year; 90% of the MDI group were on glargine-based MDI with the rest using NPH. All of the MDI patients had been on at least four insulin injections a day for > 6 months. The pump and MDI groups were well matched on some variables, but there were striking differences in carbohydrate counting (56% of pump group vs. 40% on MDI) and self-adjustment of insulin doses (80.5% vs. 66.5%), suggesting a marked educational imbalance.
Some DSQOL results were slightly better among the pump group, but this was statistically significant only for diet restrictions (65.5 vs. 60.8; p = 0.0003). DTSQ scores were better on pump (30.2 vs. 26.2; p < 0.0001). SF-36 scores were better on MDI, but in most domains, not statistically significantly so. However, the authors report that multiple regression analysis (details not provided, but adjusted for clinical factors including complications, which were more common in the pump group) showed that the pump group had significantly better scores in DSQOL diet, daily hassles and fear of hypoglycaemia. No differences were found between NPH and glargine-based MDI. The study was supported by Medtronic.
The lack of difference between the QoL effects of NPH and glargine-based MDI may not apply to detemir (Levemir, Novo Nordisk, Gatwick, UK)-based MDI, because detemir given twice daily may provide a more flexible lifestyle than once-daily glargine.
The Five Nations Study43 was a good-quality trial completed before long-acting analogues became available. It compared pump therapy with lispro and NPH-based MDI, but, unusually, the NPH insulin could be given up to four times a day and only 41% of patients had it once daily, with 32% getting it twice a day and 23% thrice daily. The study reported QoL using DQOL and Short Form questionnaire-12 items (SF-12). With SF-12 there was no difference in physical state but the pump group did better on mental health. The end of trial DQOL was 75 on pump and 71 on MDI, a small but statistically significant difference (p < 0.001). The difference reflected gains in treatment satisfaction, flexibility of eating and lifestyle, and reduced worry.
Conclusions
The evidence base for pump therapy compared with modern MDI is still quite sparse, and REPOSE has more participants than in all of the previous trials put together, even if we include the Hirsch et al. trial40 with its 100 patients on very short duration of 4 weeks on each therapy. If we exclude the Hirsch et al. trial,40 REPOSE has more than double the number in the other three trials, which had a total of 99 patients. 15–17 It also recruited a different group of patients from most previous trials, as it excluded those who met the NICE criteria for pump therapy. So it recruited patients in a band of need below those for whom the pump has been approved by NICE.
Chapter 3 Methods
Methods for the randomised controlled trial
The trial protocol was published in a separate paper. 86
Study design
The REPOSE Trial was a pragmatic, multicentre, parallel-group, open-label, confirmatory cluster RCT. Participants were allocated a place on a week-long DAFNE course, depending on their availability to attend the course. The course (cluster element) groups were then randomly allocated in pairs to either pump or MDI treatment, with allocation concealed. A cluster design was chosen because of the impracticality of randomising individuals and then finding suitable times for that participant to attend a course of the correct allocation. 23 Such an approach was more likely to have resulted in significantly higher attrition rates pre course. Following the course, participants received the trial treatment for 2 years and outcome measures were collected at 6, 12 and 24 months post course. Outcome measurement was not blinded (see Data collection).
Approvals obtained
The protocol was approved by the Research Ethics Committee (REC) North West, Liverpool East, on 26 April 2011 (REC reference number 11/H1002/10). Each participating centre gave UK NHS Research and Development (R&D) approval (see Appendix 2). The protocol received Medicines and Healthcare products Regulatory Agency (MHRA) clinical trials authorisation on 26 May 2011 [European Union Drug Regulating Authorities Clinical Trials (EudraCT) reference no: 2010-023198-21].
Setting
The trial was conducted in eight secondary care diabetes centres in Sheffield, Cambridge, Dumfries and Galloway, Edinburgh, Glasgow, Harrogate, London and Nottingham (see Table 11). Participating centres all had experience in delivering high-quality structured education using DAFNE and had variable levels of experience delivering pump therapy; most were established pump centres but some were relatively new to pump therapy. Nottingham was a reserve centre, activated midway through the trial. The seven centres involved from the outset were asked to recruit 40 participants to three pump and three MDI courses (5–8 patients on each course) over 11 months. Owing to a higher than anticipated dropout rate prior to the DAFNE courses we then recruited to an additional pair of courses at Harrogate, and a pair of courses at the reserve centre, Nottingham.
Participants
Participants were eligible for the trial if they met the following inclusion criteria:
-
were aged ≥ 18 years
-
had T1DM for at least 12 months at the time of the DAFNE course
-
were fluent in speaking, reading and understanding English
-
were willing to undertake intensive insulin therapy with SMBG, carbohydrate counting and insulin self-adjustment
-
had no preference for either pump or MDI, and were happy to be randomised
-
were currently using, or willing to switch to, insulin detemir
-
had a need for structured education to optimise diabetes control.
Furthermore, participants were excluded if they met any of the following criteria:
-
had already completed a diabetes education course
-
used a pump in the previous 3 years (defined as > 2 weeks’ use in the last 3 years) or had strong clinical indications for pump therapy in the view of the investigator
-
had renal impairment with a chance of needing renal replacement therapy within the next 2 years (enrolment staff to check that creatinine levels not > 200 µmol/l).
-
had uncontrolled hypertension (diastolic blood pressure of > 100 mmHg and/or sustained systolic level of > 160 mmHg)
-
had a history of heart disease within the past 3 months
-
had severe needle phobia (severity of phobia assessed, considering if the phobia might preclude full participation in either treatment arm or influence the participant’s preference for pump therapy)
-
had a current history of alcohol or drug abuse
-
had serious or unstable medical or psychological conditions that are active enough to preclude the participant safely taking part in the trial (based on investigatory judgement)
-
had recurrent episodes of skin infections
-
were pregnant or planning to become pregnant within the next 2 years
-
had taken part in any other investigational clinical trial during the 4 months prior to screening
-
had any other issue that might have precluded them from satisfactory participation in the study based on investigatory judgement
-
were unable to give informed consent.
Interventions
Dose Adjustment For Normal Eating with multiple daily injection
Participants on the MDI arm attended a standard DAFNE structured education course, described in detail elsewhere. 2 Courses are conducted over 5 consecutive days, providing an average of 38 hours of structured education, delivered to groups of 5–8 adults, aged ≥ 18 years, in an outpatient setting. Courses are delivered by diabetes specialist nurses and dietitians who attend an educator training course, the DAFNE education programme, a seven-part programme consisting of 105 hours of structured training.
The DAFNE curriculum uses a progressive modular-based structure to improve self-management in a variety of medical and social situations. Content is designed to deliver key learning topics at the appropriate time during the week. In this way, knowledge and skills are built up throughout the course with active participant involvement and problem-solving as key methods of learning. The key modules are: ‘What is diabetes?’, ‘Food and diabetes’, ‘Insulin management’, ‘Management of hypoglycaemia’ and ‘Sick day rules’. Lesson plans give guidance on timing and a student activity section serves to give an idea of expected responses. Each meal and snack during the course is used as an opportunity to practise carbohydrate estimation and insulin dose adjustment.
Dose Adjustment For Normal Eating with pump
Participants on the pump arm attended a modified DAFNE course, which had been tested in a pilot study, previously published. 27 The 5-day structure of the standard adult DAFNE course was maintained while incorporating the additional skills and learning outcomes that were considered necessary to use pumps successfully. The principles of insulin dose adjustment taught on the standard adult course were maintained. 23 The need to introduce ‘pump skills’ required the addition of a pre-course group session, delivered 1–3 weeks before the DAFNE course. This session gave participants the opportunity to learn about the basics of insulin pump therapy, including how to set up the pump, so that they could practise using it with saline before starting on insulin at the beginning of the course. The session included the theory of pump therapy, understanding cannulas and infusion sets, skin care, pump maintenance and the advantages and disadvantages of the insulin pump. Participants switched to insulin on the evening before the DAFNE course or on the first day of the course.
Ongoing treatment
After attending the DAFNE course, participants received the trial treatment for 2 years from the secondary care service. All of the participants in both groups were invited to an additional DAFNE follow-up group session at 6 weeks post course, which is standard for DAFNE course attendees.
Multiple daily injection participants used a combination of quick-acting insulin analogues and twice-daily injections of insulin detemir. Pump participants used a Medtronic Paradigm® VeoTM insulin pump (Model X54) with short-acting analogue insulin, as in a meta-analysis87 this was shown to lower HbA1c to a greater extent than traditional soluble insulin. As insulin is already marketed and licensed for use, and as the participants were already accessing insulin through prescription on a regular basis, there was no need to change how the insulin was accessed for the trial – participants collected insulin from their pharmacist as normal.
The insulin pumps include, as standard, a Medtronic Bolus Wizard (Medtronic, Watford UK) to aid calculation of insulin doses. In order to reduce any potential bias, MDI participants were also given access to a bolus calculator (Accu-Chek Aviva Expert Bolus Advisor System, Roche Diagnostics Ltd, Burgess Hill, UK).
Fidelity testing (FT) of pump courses was undertaken in order to assess whether or not courses were delivered in accordance with DAFNE philosophy and principles, and that the educators had the necessary skills to deliver these principles. The results of the FT are reported in Chapter 5. Standard DAFNE courses were not tested, as there is a rigorous quality assurance programme of MDI courses in standard care.
Treatment was changed (pump to MDI or MDI to pump) at the discretion of the local principal investigator (PI) if self-management of diabetes had become ineffective and was considered a risk to the individual. If the participant failed to attend the pump course then they were withdrawn from pump treatment.
Primary outcomes
The main primary end point was the change in HbA1c at 24 months, in those participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol). The key secondary end point was the proportion of participants reaching the NICE target of a HbA1c level of ≤ 7.5% (58 mmol/mol) at 24 months (of all participants).
Glycated haemoglobin is the accepted gold standard measure of glycaemic control and provides a measure of efficacy. Most health economic models of T1DM estimate the cost-effectiveness by primarily modifying HbA1c levels, which subsequently affect the risk of diabetic complications. 88 However, it is important to note that HbA1c may not have fallen in patients who entered the trial with low baseline levels of HbA1c, but who might have been experiencing frequent hypoglycaemia or wished to increase dietary freedom. Success for such individuals would be a HbA1c level that is maintained, or even rises slightly, with a reduction in the frequency of hypoglycaemia. 23 We included such patients as they could provide important information about QoL and the potential of pump therapy to reduce rates of hypoglycaemia. However, as their glycaemic control may not alter, including their HbA1c data would have reduced our statistical power to establish improvement in our primary end point. We therefore powered the trial on the number of participants with a baseline HbA1c of ≥ 7.5% (58 mmol/mol) and in whom a fall would reflect a worthwhile improvement in glycaemic control. We ensured standardisation by testing HbA1c in a central laboratory.
Exploratory outcomes on the primary end points
The primary outcome and key secondary outcome were also evaluated at 6 and 12 months in order to explore the short- and medium-term effects of the intervention.
Secondary outcomes
Secondary outcomes were evaluated in all participants and were measured at 6, 12 and 24 months. Blood and urine samples for secondary outcomes were tested in local laboratories.
Hypoglycaemia
We recorded episodes of both moderate and severe hypoglycaemia and specifically recorded episodes at night (those occurring between 23.00 and 07.00). We used a standard definition of severe hypoglycaemia,89,90 being ‘an episode leading to cognitive impairment sufficient to cause either coma or requiring the assistance of another person to recover’. The number of severe episodes are reliably recorded by patients for up to 1 year. 91
During the last NICE appraisal of pump therapy, the question of the impact of moderate hypoglycaemia was raised. 13 The modelling had included only severe hypoglycaemia, and the point was made that moderate hypoglycaemia, sufficient to interrupt activities of daily living, might, because of greater frequency, have a more cumulative effect on QoL than severe hypoglycaemia. We therefore also recorded rates of moderate hypoglycaemia in an attempt to increase power and identify the ability of pumps to reduce rates of hypoglycaemia. With no standard definition of moderate hypoglycaemia, the Trial Management Group (TMG) agreed to define these as ‘any episodes which could be treated by that individual, but where hypoglycaemia caused significant interruption of current activity, such as having caused impaired performance or embarrassment or having been woken during nocturnal sleep’. As these episodes are more frequent, reliable recall of such events is unlikely to be sustained for more than a few weeks. We therefore asked participants to record the number and timing of moderate episodes over the 4 weeks prior to each follow-up visit. We used this approach successfully to record the frequency of mild episodes in a recent epidemiological study of hypoglycaemic burden in diabetes. 89
Insulin dose and body weight
Pump treatment may result in the use of less insulin, leading to a favourable effect on body weight. We recorded total insulin dose at each time point and calculated units per kilogram of body weight.
Lipids and proteinuria
A recent study61 reported little difference in HbA1c on pump therapy compared with MDI but found less progression to microalbuminuria in the pump group, and also lower cholesterol levels. We measured high-density lipoprotein (HDL) cholesterol and total cholesterol (TC). Proteinuria was measured using the albumin-to-creatinine ratio (ACR).
Diabetic ketoacidosis
Diabetic ketoacidosis was measured throughout the trial through the assessment of serious adverse events (SAEs). 23 As all significant episodes of ketosis require hospital admission, we were confident in capturing all of the relevant episodes.
Quantitative psychosocial outcomes
The quantitative psychosocial outcomes are described later (see Outcomes).
Sample size
It is generally accepted that a difference of 0.5% (5.5 mmol/mol) in HbA1c is clinically worthwhile. To detect this difference with a standard deviation (SD) of 1% at 80% power and 5% two-sided significance using a t-test requires 64 patients per group, for subjects > 7.5% HbA1c. To allow for a clustering effect of the educators, with an average of seven patients per DAFNE group and a within-course intraclass correlation coefficient (ICC) of 0.05, common in diabetes care, the sample size increases to 84. Allowing for a 10% dropout over 24 months, the sample size per group becomes 93. Audit of the DAFNE database showed us that 75% of subjects had a HbA1c of ≥ 7.5%, therefore requiring 124 subjects per group and 248 in total. We planned to recruit 280 subjects, which increased the power to 85% but allowed for some variation in dropout rates and the proportion of patients with HbA1c ≥ 7.5%. However, monitoring of baseline data showed that the actual proportion of participants with HbA1c ≥ 7.5% was around 90% rather than 75%. A modelling exercise undertaken during recruitment, with conservative estimates of 85% (HbA1c ≥ 7.5%) and dropout rate of 15%, suggested that the trial would require at least 240 participants with primary outcome data at 2 years in order to preserve power of at least 85%. 23
Recruitment
A number of methods were used to approach potential participants:
-
PIs or educators identified people from DAFNE waiting lists. They then telephoned or wrote to potentially eligible individuals.
-
Individuals attending a clinic appointment with a trial PI or educator were offered the option of a future or immediate consultation regarding the trial.
-
Clinicians [general practitioner (GP), dietitian, nurse] provided information to patients and referred them to PIs to be screened and enrolled.
-
Details of the trial were advertised through the use of posters and leaflets in clinics (diabetes outpatient, dietetic, GP surgery).
-
Reception staff in diabetes clinics were informed about the trial and provided with leaflets to give to patients who expressed an interest.
-
Participant identification centres were used at some research centres to assist in the identification of suitable participants.
Interested individuals were given the opportunity to discuss the trial with the PI or educator. Those who were still interested in taking part were screened for eligibility. Those who were eligible were either invited to attend a local information meeting, at which the trial was discussed in detail and questions answered, or were provided with a patient information sheet and consent form and given the opportunity to ask further questions. Individuals who were still wanting to take part consented to the trial by one of three methods: (1) by returning a completed consent form (see Appendix 3) in the post, (2) by completing the form with the PI or educator or (3) by completing the form at a local information meeting. The participants’ contact details, GP details and ethnicity were also collected.
Allocation to Dose Adjustment For Normal Eating courses and randomisation
Following consent, participants were allocated to a REPOSE DAFNE course, depending on the participants’ availability. 23 Up to eight participants were allocated to each course, with a minimum of five preferable. Courses were randomised, in pairs, to either DAFNE with pump or DAFNE with MDI. 23 Participant allocation to courses was finalised for each course pair before randomisation took place, no less than 6 weeks prior to the date of the first DAFNE course in that pair. For the first seven centres, a simple randomisation procedure in block size of ‘2’, stratified by centre, was used for courses 1–4. Courses 5 onwards were allocated in pairs using minimisation of the overall and number of participants, with most recent baseline HbA1c value of ≥ 7.5% or < 7.5% between the treatment groups. Any additional courses were allocated using minimisation. Known dropouts prior to the DAFNE course were excluded from the minimisation algorithm for future course allocation. A validated user-written Stata® 13 (StataCorp LP, College Station, TX, USA) code was produced to generate the allocation by a statistician within Sheffield Clinical Trials Research Unit (CTRU), who implemented the randomisation. The trial co-ordinator revealed the allocation to study centres. 23
Blinding of the course allocation was not possible because of the nature of the treatment. Course allocations were revealed to centres 4–6 weeks prior to the date of the first course to allow sufficient preparation time. Participants were informed of the allocation of their DAFNE course no earlier than 4 weeks prior to that course. At this point they were asked to keep a record of any new episodes of moderate hypoglycaemia, which would be collected at the baseline assessment. If the course was a pump course, the participant was booked into a pre-course pump session, up to 3 weeks prior to the course date, in addition to the baseline assessment, which had to take place before the pump session.
If, for any reason, participants were unable to take part in the course at short notice, they could be allocated to a later course date, but only in the same trial arm as in the course to which they were originally allocated. Centres could also keep a list of reserve participants for courses, agreed prior to the time when the course allocation had been revealed to the educators. In the case of participants dropping out, the next person on the reserve list would be invited to participate in that course.
Data collection
Study visits took place at the participants’ diabetes centre. A data collection form (DCF) (see Appendix 4) was completed by the educator with the participant. Blood and urine samples were taken and analysed at local laboratories. Two blood samples were taken for measurement of the primary outcome (HbA1c). One of these was analysed at a central laboratory as the primary measure and the second was tested at the local laboratory as a back-up. DCF data were entered at local centres on to the in-house Prospect web-based electronic data capture system, managed by the CTRU.
Baseline assessments took place up to 3 weeks prior to the DAFNE course. The educator completed the DCF with the participant and handed him/her the self-complete psychosocial questionnaire, asking for return of the completed questionnaire at the forthcoming DAFNE course. Additional demographic data collected at baseline were date of birth, sex, qualifications (highest qualification obtained) and current occupation. Participants were also handed a SAE contact card to aid in contacting their diabetes centre in the event of an AE.
At the DAFNE course, an attendance form was completed, detailing any missed sessions. The completed baseline psychosocial questionnaire was collected and the baseline DCF moderate hypoglycaemic episodes section was updated so that a full 4 weeks of hypoglycaemic episodes were recorded. At all time points, psychosocial questionnaires were posted from centres to Sheffield CTRU and entered on to Prospect by Sheffield CTRU clerical staff.
Participants were followed up at 6, 12 and 24 months after the DAFNE course. Participants were sent the blood glucose diary (see Appendix 5) and instructions for recording moderate hypoglycaemic episodes 4 weeks prior to each visit. Additionally, participants were posted the self-complete psychosocial questionnaire pack prior to the visit and asked to bring their completed questionnaire to the appointment, along with the blood glucose diary and record of moderate hypoglycaemic episodes.
Severe hypoglycaemic episodes or SAEs were collected from participants if reported over the telephone or in clinic. Any additional diabetes-related contacts (DRCs) were also recorded (see Appendix 6 for ongoing data collection booklet).
Blinding of outcome measures was considered impractical because of the intervention-specific nature of outcome measures and the necessity of a local diabetes nurse to collect the data. However, use of an objective outcome (HbA1c) measured in a central laboratory will have minimised bias on the primary end point.
Trial completion
Participants were deemed to have completed the study if they had trial data recorded at baseline and 24 months. Participants were withdrawn from the study if:
-
The participant asked to fully withdraw from the trial. On requesting withdrawal from the trial, participants were able to consent to continue to have their routine HbA1c results recorded.
-
The participant died.
Participants who were changing treatment continued in the trial unless formally withdrawn. Participants were deemed lost to follow-up if they failed to attend the baseline visit, DAFNE course or 24-month follow-up.
Research governance
The trial sponsor was Sheffield Teaching Hospitals NHS Foundation Trust. The trial was conducted in accordance with Good Clinical Practice (GCP) and the Medicines for Human Use (Clinical Trials) Regulations 2004. 92 All staff recruiting participants to the trial had undertaken GCP training. In line with the three-level categorisation of clinical trial risk in the Medical Research Council/Department of Health (DH)/MHRA report on risk-adapted approaches to the management of clinical trials of investigational medicinal products93 (based on the classification by Brosteanu et al. 94), the REPOSE Trial was classified as a Type A study: no higher than the risk of standard medical care. The trial treatment in REPOSE was licensed and administered according to its market authorisation. Trial-specific labelling was not used. Given the lack of criticality of the investigational medicinal product (IMP) with the data analysis and trial results, and the design of the trial being equivalent to standard care, there was no IMP tracking and accountability undertaken.
Three committees were established to govern the conduct of the study: an independent Trial Steering Committee (TSC), an independent Data Monitoring and Ethics Committee (DMEC) and a TMG. Full membership of the TSC and DMEC are listed at the end of this report. The committees functioned in accordance with Sheffield CTRU standard operating procedures (SOPs). The TSC was responsible for overall supervision and monitoring of the trial; it considered any recommendations from the DMEC and provided advice on any actions to be taken. The DMEC operated within a charter agreed by all members and was responsible for monitoring efficacy and safety data. Any concerns were reported to the TSC with recommendations. The TMG was responsible for supporting the implementation of the trial.
Reporting of adverse events
Adverse events were defined as any untoward medical occurrence in a participant to whom a medicinal product has been administered, including occurrences that are not necessarily caused by or related to that product. SAEs were defined as any AE that results in death; is life-threatening (subject at immediate risk of death); requires inpatient hospitalisation or prolonging existing hospitalisation; results in persistent or significant disability or incapacity, or consists of congenital anomaly or birth defect; or is another important medical event that may jeopardise the participant. Pregnancy was also recorded as a SAE, so that any AEs could be identified if and when the child was born.
Included as AEs were an increase in frequency of hypoglycaemia, a blood glucose reading > 30 mmol/l, unexplained constantly raised blood glucose readings, suspicion of pump malfunction and pump site infection. Excluded as AEs were non-serious episodes of hypoglycaemia and ketonuria.
Details of AEs were collected during follow-up appointments. Participants were also provided with a contact card and encouraged to get in touch with their diabetes team if they had experienced any adverse health events. SAEs were reported in accordance with the Sheffield CTRU and REPOSE SAE SOPs. SAEs were assessed by the local PI and reported to Sheffield CTRU within 24 hours of becoming aware of the event, with the exception of events that had been stated as exempt from immediate reporting, for which 28 days was allowed. These exemptions were episodes of severe hypoglycaemia requiring hospitalisation, episodes of DKA and pregnancy. SAEs were assessed for seriousness, frequency, intensity, relationship to study product and, when applicable, relationship to pump. The Summary of Product Characteristics for NovoRapid and Levemir (Novo Nordisk, Gatwick, UK) were kept on file as the reference safety information for the assessment of events. AEs were reviewed at regular intervals by the three study oversight committees. The chief investigator and DMEC chairperson were notified of all SAEs on the event being reported.
Reporting of protocol non-compliances
Protocol non-compliances were reported and assessed in accordance with the Sheffield CTRU and REPOSE non-compliances SOPs. A non-compliance was defined as ‘a departure from the protocol or GCP that has been identified retrospectively’. Non-compliances were addressed with staff training or, when appropriate, an amendment to the protocol. In line with MHRA guidance, deliberate prospective protocol non-compliances or ‘waivers’ were deemed to be unacceptable. A prospective list of exemptions from reporting and of pre-specified major and minor non-compliances was drawn up by the CTRU, the chief investigator and the sponsor.
Trial monitoring
Responsibility for monitoring was delegated to the CTRU and conducted in accordance with CTRU SOPs. Both on-site and central monitoring methods were adopted. Onsite monitoring visits took place at all centres at study set-up, prior to delivery of the first DAFNE course, post delivery of DAFNE course 2 and at study closeout. A further monitoring visit took place during follow-up at seven centres. At each visit, the study site file and key essential logs were reviewed for completeness. Source data verification was conducted for 100% of consent and SAE forms. Patient hospital records were reviewed to substantiate participant existence and eligibility (for which criteria were verifiable from hospital records). Monitoring reports were issued after each visit detailing any remedial actions required. Central monitoring tasks included point of entry validation, verification of data and post-entry validation checks. One participant per DAFNE course per centre was randomly selected for verification. Case report forms at all data collection time points were reviewed for completeness and quality, and verified to monitor data entry. Source data verification also took place for 100% of central laboratory HbA1c results. Feedback on verification was provided and additional verification was undertaken when concerns were identified.
Statistical methods
All statistical analyses were performed in Stata 13 onwards. The MDI is the reference group for all treatment comparisons.
Analysis populations
The intention-to-treat (ITT) data set includes all participants who were randomised according to randomised treatment assignments (ignoring any occurrences post randomisation, such as protocol or treatment non-compliance and withdrawals) with at least one HbA1c assessment measure after baseline. Sensitivity analysis of the ITT primary outcome set was performed using six additional analysis sets, as described later in this section.
The per-protocol group is a subset of the ITT group who complied with the protocol. Protocol compliance was defined as adhering to both the DAFNE course and to pump/MDI. Compliance was reviewed and assessed on a case-by-case basis with the following general considerations applied:
-
adherence to DAFNE course – in general, a participant was adherent to the course if they attended at least 4 of the 5 days, including the first 2 days (as adjudicated by the course leader)
-
adherence to the pump or MDI – a participant was classed as adherent to treatment if he/she adhered to the pump/MDI for the full 2 years (excluding any reasonable temporary interruptions of around 2 weeks).
A review group (SH and JE), ‘blinded’ to patient outcome data, convened to decide any contentious cases for treatment interruptions with the help of the trial statistician (EL).
The complete-case group is a subset of the ITT group who had outcome measurements at a specific follow-up time.
An additional four analysis sets were performed to examine the sensitivity of primary results to multiple imputation and exclusions, as described later in this section.
Data completeness
A CONSORT (Consolidated Standards Of Reporting Trials) flow diagram was used to display data completeness and patient throughput from first contact to final follow-up.
Baseline characteristics
The baseline participant characteristics, diabetes history and laboratory tests were summarised and assessed for comparability between the intervention and control group. No statistical significance testing was carried out to test baseline imbalances between the arms, but any noted differences are reported descriptively.
Primary effectiveness analysis
The primary end point for this study is the change in HbA1c after 2 years in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol). The mean change in HbA1c at 24 months post DAFNE course was compared between participants allocated to pump and participants allocated to MDI using a mixed-effects model. The model was adjusted for clustering by DAFNE course (random effects), centre and baseline HbA1c as a continuous covariate (fixed effects).
The mean (SD) HbA1c change from baseline for the pump and MDI groups, and the number in each group, are displayed. The efficacy of the intervention is reported as mean difference (MD) in HbA1c change at 2 years, with its associated 95% CI and p-value, adjusted for the factors stated above.
Multiple imputation of missing data
Multiple imputation was used to impute missing data on the primary outcome in order to fulfil the ITT principle and for sensitivity analysis. Multiple imputation was used to impute 24-month HbA1c data for patients with at least one assessment after randomisation (i.e. at 6 or 12 months), but without 24-month primary outcome data. Participants’ baseline characteristics were summarised and compared between completers and non-completers. Data were imputed using chained equations (regression) with 50 imputations using baseline, 6- and 12-month HbA1c measurements, DAFNE course, centre, age, sex and HFS behaviour as covariates in the imputation equation. Initially, 10 imputation replicates were planned; however, this was increased to 50 in order to produce a stable and reliable estimate of variability.
The following sensitivity analyses were undertaken on the primary outcome and displayed alongside the ITT results:
-
per-protocol cases (subset of ITT who did not deviate from the protocol)
-
complete cases (subset of ITT including only participants with complete HbA1c data at 24 months)
-
multiple imputation of all missing cases (including those without any follow-up data who are excluded from the ITT analysis)
-
horizontal mean value imputation of all missing cases
-
excluding participants who withdrew from the study because of pregnancy
-
excluding participants with measurements outside a time window of 6 weeks before and after the 24-month follow-up.
A sensitivity analysis on the primary outcome – adjusted for duration of diabetes, number of moderate hypoglycaemic episodes and number of severe hypoglycaemic episodes – was to be performed if notable baseline imbalances were observed; however, none was observed.
An exploratory analysis (on available data) to assess whether or not there were differences in primary outcome between DAFNE lead course educators was conducted using a multilevel model with three levels – patients nested in DAFNE course, which, in turn, are nested within the course lead. Baseline HbA1c, treatment group and centre were treated as fixed effects in the model. The ICCs from this model are presented.
The effect of centre was explored using a mixed-effects regression model. The primary outcome was regressed against treatment, centre (fixed effects) and an interaction term between treatment and centre, and it was also adjusted for course (random effects). The p-value for the interaction between treatment and centre is presented. The MDs between treatment groups with associated 95% CIs, estimated from the mixed-effects model, are presented by centre with the aid of forest plots.
Key secondary effectiveness analysis
The key secondary end point is the proportion of patients reaching the NICE target of a HbA1c level of ≤ 7.5% (58 mmol/mol) at 2 years (including all participants regardless of baseline HbA1c value). The treatment effect was investigated using a mixed-effects logistic regression model adjusted for baseline HbA1c, centre (fixed effect), and a random effect around DAFNE course. The proportion of patients with HbA1c of ≤ 7.5% is presented by treatment group alongside the odds ratio (OR) of HbA1c ≤ 7.5% on pump compared with HbA1c ≤ 7.5% on MDI and its associated 95% CI and p-value.
Secondary effectiveness analysis
Glycated haemoglobin at 6 and 12 months
Secondary analyses on the primary outcome and key secondary outcome were repeated for HbA1c at 6 and 12 months to explore the short- and medium-term effects of the intervention:
-
the change in HbA1c at 6 and 12 months in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol)
-
the proportion of participants reaching the NICE target of HbA1c level of ≤ 7.5% (58 mmol/mol) at 6 and 12 months.
These outcomes were analysed using statistical models as for the primary and key secondary outcome.
Episodes of severe and moderate hypoglycaemia
The number of episodes of moderate hypoglycaemia reported in the 4-week period prior to the 6-, 12- and 24-month visits were compared between treatment groups using a mixed-effects negative binomial linear regression model, with centre and baseline continuous HbA1c included as fixed effects and course as a random effect. The occurrence of at least one moderate hypoglycaemic episode in the 4 weeks prior to starting the DAFNE course was also included as a covariate.
Each episode of moderate hypoglycaemia was classed as ‘confirmed’ or ‘unconfirmed’ by an educator and the blood glucose level was recorded by the participant. The following outcomes were analysed:
-
all recorded episodes
-
confirmed episodes, defined as episodes that were confirmed and for which the blood glucose level (if recorded) was < 3.5 mmol/l
-
confirmed episodes (US definition), defined as episodes that were both confirmed and for which the blood glucose level (if recorded) was < 4 mmol/l.
Severe hypoglycaemic episodes were collected on an ongoing basis. The number of episodes recorded post baseline was analysed in a similar manner to moderate hypoglycaemic episodes, but with the addition of study follow-up time as the exposure. A sensitivity analysis was conducted in the same manner by excluding the first 6 months of data in order to explore any effect of a ‘settling in’ period on the pump.
The incidence rates of moderate hypoglycaemic episodes in the 4 weeks before each time point are displayed by treatment group, and the treatment effect is reported as an adjusted incidence rate ratio (IRR) with its associated 95% CI and p-value. The incidence rates of severe hypoglycaemic episodes over the study duration are displayed as episodes per patient-year and are reported alongside the IRR, its associated 95% CI and p-value.
The overall change in the rate of episodes of severe hypoglycaemia was estimated for the treatment groups combined using a mixed-effects negative binomial linear regression model. The numbers of episodes were compared pre and post baseline, using participant as the random effect, adjusted for treatment, time by treatment interaction, baseline HbA1c and centre. Length of follow-up was included as the exposure variable. Length of follow-up before baseline was set at 365 days, as participants recorded a 12-month history of severe hypoglycaemic episodes at baseline.
The proportions of participants who experienced at least one moderate hypoglycaemic episode at 6, 12 and 24 months were compared between treatment groups using a mixed-effects logistic regression model adjusted for DAFNE course (random effect), centre, presence of at least one episode before baseline and baseline HbA1c (fixed effects). The proportion of patients who experienced at least one episode of severe hypoglycaemia during the study period was compared between groups using a mixed-effects logistic regression adjusted for DAFNE course, centre and baseline HbA1c. Presence of at least one severe episode before baseline was not used as a covariate in the logistic regression model as all participants with at least one episode before baseline experienced at least one episode post baseline. The proportion of patients reporting hypoglycaemic episodes is presented by treatment group alongside the adjusted OR and its associated 95% CI and p-value.
Insulin dose, body weight and lipids
Insulin dose was calculated as:
In the calculation of insulin dose, weight was taken as the value on the same visit the dose was recorded. If weight was not recorded, it was estimated from other study visits as follows:
-
If 24-month weight was missing, 12-month weight was used.
-
If 12-month weight was missing, it was imputed as the time-weighted average of 6- and 24-month weight or as 6- or 24-month weight if only one observation was available.
-
If 6-month weight was missing, it was imputed as the average of baseline and 12-month data, or imputed as baseline or 12-month data if only one observation was available.
-
In all other situations the missing data were left blank.
The analysis of weight was based on available data only.
The mean change from baseline in insulin dose, weight, TC and HDL cholesterol was compared between treatment groups using a mixed-effects linear regression model with independent correlation adjusted for clustering by DAFNE course (random effect), centre and baseline HbA1c (fixed effects). The MD between the groups in change from baseline is displayed with its associated 95% CI and p-value.
Proteinuria
Proteinuria was defined from the ACR at each visit. At each visit a patient was defined as:
-
macroalbuminuria – if ACR ≥ 30
-
microalbuminuria – if 3 ≤ ACR < 30
-
normal – if ACR < 3.
If ACR was missing at a time point, proteinuria status was imputed, based on data from recorded conditions at the same time point.
Proteinuria was analysed using mixed-effects ordered logistic regression adjusted for clustering by DAFNE course (random effect), centre and baseline HbA1c (fixed effects). The OR of being in a higher category (for which macroalbuminuria is the highest category) compared with a lower category is displayed with its associated 95% CI and p-value.
Blood glucose testing
The self-reported number of blood glucose tests performed in the 2 weeks prior to 24-month follow-up was compared between treatment groups, in a post hoc analysis, using a mixed-effects model adjusted for clustering by DAFNE course (random effect), centre and baseline number of blood glucose tests (fixed effects). For both treatment groups combined, the change in the number of tests performed at 24 months compared with baseline was analysed using a paired t-test. Blood glucose testing is presented as number of tests per day, taken as an average over the 2 weeks reported.
Psychosocial questionnaires
Methods for the analysis of questionnaire data are described later (see Methods for the psychosocial evaluation).
Subgroup analysis
Pre-planned subgroup analyses were undertaken and regarded as exploratory; significant results from the analysis were interpreted with caution, as recommended for subgroup analyses. 95 The following subgroups were investigated:
-
baseline HbA1c (< 7.5% or 58 mmol/mol, ≥ 7.5% to < 8.5% or 69 mmol/mol, ≥ 8.5%)
-
duration of diabetes (< 15 years, ≥ 15 years)
-
symptoms of hypoglycaemia (do not feel symptoms or < 3 mmol/l, ≥ 3 mmol/l)
-
self-reported use of the bolus advisor over the study duration (never or rarely, sometimes, often or always)
-
age (< 35, 35–49, ≥ 50 years)
-
sex
-
body mass index (BMI) (normal, < 25 kg/m2; overweight, 25–29.9 kg/m2; obese, ≥ 30 kg/m2)
-
level of education [up to Advanced level (A-level) equivalent, vocational/beyond A-level]
-
occupational status (Office for National Statistics levels 1–4)
-
socioeconomic status as defined by the Office for National Statistics Index of Multiple Deprivation (above/below median in England, and above/below median in Scotland)
-
insulin dose at start of therapy (< 0.7 or ≥ 0.7 IU/weight)
-
frequency of moderate hypoglycaemic episodes within the 4 weeks prior to baseline (none, 1, 2 or 3, 4–9, 10+)
-
experience of lead DAFNE course educator {‘less experienced’ [six courses or fewer within previous 3 years or completed the DAFNE Educator Programme (DEP) within previous year] vs. ‘higher-level experience [seven or more courses within previous 3 years or had continuous ‘educator’ status for > 6 years]}.
The subgroup analysis used mixed-effects linear regression modelling with the primary outcome, change in HbA1c (%), as the response. The model included main effects of the treatment group and subgroup, an interaction term between treatment and subgroup, and covariates of centre (fixed effect) and DAFNE course (random effect). Treatment effect estimates and 95% CIs are presented within each subgroup category. We used a statistical test for interaction between the randomised intervention group and the subgroup to examine the evidence for treatment effect varying between subgroup; the p-value for this interaction is reported unadjusted for multiple testing. Subgroup analyses were also summarised visually using forest plots.
Safety and harms analysis
Serious adverse events and AEs were summarised and assessed for similarity between the treatment groups. Both SAEs and AEs are reported on an ITT basis (i.e. according to the group to which the participants was randomised), but the number occurring following a treatment switch are highlighted.
Patient and public involvement
As part of our recent work funded by a NIHR programme grant (PGfAR),27 15 DAFNE graduates were recruited to act as a ‘user group’ and contribute to different aspects of the work. We invited two members to join both the steering group and other investigator meetings. In addition, one of the project team is a pump user. They provided input to the trial design, implementation and dissemination, including all participant materials. 23 The work supported by the programme grant included qualitative studies in which the barriers to self-management in T1DM were explored. This work led to the development of a pilot study within the PGfAR work, in which a modified DAFNE course incorporating a pump curriculum was developed and piloted in three centres.
Methods of the fidelity assessment
Aim
To ensure that there was consistency in:
-
the delivery of the 5-day DAFNE pump curriculum
-
the timing and content of pump pre-assessment/setting up on pump session.
Multiple daily injection courses were not included in the FT, as there exists a rigorous quality assurance programme of MDI courses in standard care, and trial centres are routinely audited.
Methods
An experienced DAFNE educator and peer reviewer from Sheffield Teaching Hospitals NHS Foundation Trust was employed as the fidelity assessor (FA); this educator was not directly involved in the delivery of REPOSE courses. The FA assessed whether or not the pump courses delivered the correct DAFNE content and philosophy. The FA visited each centre to observe the ‘Wednesday’ of the pump DAFNE course. Wednesday was chosen as pump curriculum sessions that incorporated key differences to MDI would be delivered by DAFNE educators from both dietetic and nursing specialties. In addition, patients on the course should have settled into the course, be more relaxed and be starting to establish patterns and adjustments to their regimen by the third day. It was planned that the FT take place on the first or second pump course at each centre.
Experienced educators who devised the pump curriculum and the national director of the DAFNE programme discussed which sessions differed most between the pump and MDI DAFNE curricula and, thus, warranted observation. These sessions were decided as follows:
-
daily goals, blood glucose results and insulin doses
-
insulin dose adjustment theory, basal rate testing
-
dose adjustment practice – reducing and increasing insulin
-
setting up Bolus Wizard
-
sick day rules
-
alcohol
-
exercise.
All but one session was scheduled for observation on the FT visit, as it was not possible to timetable all sessions that differed between the MDI and pump courses on 1 day. In lieu of observation, the FA reviewed the lesson plan for the sick day rules session.
The following data and documents were requested to be made available for the FT visit:
-
pre-course pump session details including patient attendance, session timings and lesson plan
-
pump course timetable
-
list of course participants and details
-
lesson plan for all observed sessions and the sick day rules lesson plan.
A DEP peer support learning outcomes form was completed for each session observed. This form listed the essential learning outcomes for each session and the FA evaluated whether or not these were met (or partially met). For each learning outcome, the FA provided evidence for its achievement. A template report was devised and used to collate the data collected from the FT visit.
Once the FA had completed the assessment, feedback was given immediately so that educators could resolve any problems. The report was completed within 3 days of the visit and sent to the trial management office.
Methods for the economic evaluation
Setting and perspective
The health economic analyses are designed to inform UK decision-makers within the UK NHS on the potential resource implications of choosing to use pump therapy with DAFNE structured education (pump + DAFNE) or MDI with DAFNE structured education (MDI + DAFNE) for the group of adults with T1DM in the REPOSE Trial, comprising adults with T1DM who are naive to pump therapy.
To ensure that all economic analyses were applicable to the UK decision-making setting, all economic analyses took the NHS and Personal Social Services perspective in line with (NICE) guidance. 22
Two approaches: economic evaluation alongside the clinical trial and long-term cost-effectiveness modelling
The cost-effectiveness of ‘pump + DAFNE’ compared with ‘MDI + DAFNE’ was assessed using an Economic Evaluation Alongside Clinical Trials (EEACT) and long-term modelling exercise. The EEACT took a 2-year time horizon and the long-term modelling took a lifetime horizon. As the long-term modelling takes a lifetime time horizon, and includes all clinically important complications of diabetes, this should be considered as the primary analysis.
Price year and discounting
All costs are reported in 2013–14 prices; if costs were obtained from a previous financial year they were inflated to 2013–14 prices using the Hospital and Community Health Services Pay and Prices index. 96 All costs and QALYs were discounted at a rate of 3.5% in line with NICE guidance. 22 All costs and QALYs were assumed to fall at the end of the year, apart from the cost of the structured education courses, which were assumed to occur at the start of the first year.
Population and subgroups for analysis
The individuals in the REPOSE Trial were adults with T1DM who were eligible to receive a structured education course. Furthermore, all individuals were naive to insulin pump therapy and did not have a preference to receive the pump. The average age of participants was 40.4 years and their mean duration of their diabetes was 18.0 years. Data were collected from individuals at baseline and at 6 months, 1 and 2 years post randomisation. In the MDI + DAFNE arm, 6, 3 and 5 individuals out of 135 were lost to follow-up at 6 months, 1 and 2 years, respectively. A further individual in the MDI + DAFNE arm withdrew from the trial at 6 months. In the pump + DAFNE arm, 0, 1 and 2 individuals out of 132 were lost to follow-up at 6 months, 1 and 2 years, respectively. A further individual in the pump + DAFNE arm withdrew from the trial at 1 year.
The data collected in the REPOSE Trial were considered to be the only relevant evidence on the relative effectiveness of pump + DAFNE compared with MDI + DAFNE. This is because REPOSE is the only large study in a UK setting in which adults with T1DM in both trial arms have received equivalent diabetes education in both the pump and MDI trial arms.
There are two populations in the REPOSE Trial: (1) the ITT population and (2) the per-protocol population. The ITT population includes all individuals who graduated their DAFNE course and had follow-up data for at least one data collection period. In the ITT population, individuals were assigned to their randomised treatment irrespective of whether or not they switched to the other insulin delivery mechanism. The per-protocol population includes all of the individuals who were in the ITT population and adhered to their insulin delivery mechanism (either pump or MDI). Unless otherwise stated, all analyses of the REPOSE Trial data to inform the health economic analyses were conducted in the ITT population.
The population analysed in the primary health economic analyses is all individuals in the REPOSE Trial, regardless of whether or not the individual’s baseline HbA1c was ≥ 58 mmol/mol (7.5%). The analysis population differs from the population used in the primary clinical end point statistical analysis, as the base-case health economic analysis focuses on the whole trial population rather than those individuals with a HbA1c of < 58 mmol/mol (7.5%). For the health economic analyses, it is important to assess the cost-effectiveness of pump + DAFNE compared with MDI + DAFNE for all adults with T1DM who would be potentially eligible to receive either treatment if they were adopted as standard practice.
Subgroup analyses 1–6 were conducted in the long-term modelling only, because of concerns about the reduction in sample size potentially producing spurious results in the EEACT. However, subgroup analysis 7 was conducted in the EEACT, as this was an important subgroup analysis for the estimation of treatment effect of HbA1c (see Statistical methods). The subgroup analyses were conducted in following subgroups of the REPOSE Trial participants:
-
baseline HbA1c ≥ 58 mmol/mol (7.5%)
-
baseline HbA1c ≥ 58 mmol/mol (7.5%) and < 69 mmol/mol (8.5%)
-
baseline HbA1c ≥ 69 mmol/mol (8.5%) and < 80 mmol/mol (9.5%)
-
baseline HbA1c ≥ 80 mmol/mol (9.5%)
-
baseline HbA1c < 69 mmol/mol (8.5%)
-
baseline HbA1c ≥ 69 mmol/mol (8.5%)
-
all individuals in the per-protocol population.
Cost of the Dose Adjustment For Normal Eating course
A detailed within-trial costing of the DAFNE courses was not undertaken because DAFNE is an already established intervention within the NHS. The cost of DAFNE training for adults with T1DM using MDI has been calculated by DAFNE UK as being £359.10 per course attendee in 2012–13 prices (£363.10 in 2013–14 prices). 97
Based on discussion with experts involved in the REPOSE Trial, including a Professor of Clinical Diabetes and Honorary Consultant Physician, and a Professor in Public Health and Health Technology Assessment, it was assumed that the cost of a DAFNE course in the pump + DAFNE arm is identical to the cost of a DAFNE course in the MDI + DAFNE arm, except for the cost of staff time spent conducting an additional pre-course pump-fitting session.
Data were collected on the time spent delivering a pre-course fitting session for pump + DAFNE participants in the FT process. The FT process was conducted for one pump + DAFNE course at each trial centre to ensure that the pump + DAFNE course taught the principles of insulin adjustment in a similar fashion to the MDI + DAFNE course. These data were utilised to estimate the additional cost of the pre-course pump fitting session in the pump + DAFNE arm. Expert advice was sought from two centres in which it was unclear whether reported time use as part of the FT referred to the educator time spent or the total time individuals spent at the venue (which could include non-contact waiting time). To ensure consistency between the estimated costs of a MDI + DAFNE course and a pump + DAFNE course, the cost of staff time was obtained from the estimated cost of staff time for the MDI + DAFNE course. The cost of the pre-course fitting session was estimated to be £28.82 per adult with T1DM.
Economic analysis alongside the clinical trial of pump + Dose Adjustment For Normal Eating versus multiple daily injection + Dose Adjustment For Normal Eating
Resource use by individuals in the REPOSE Trial over the 2-year follow-up period
Resource use was collected either on an ongoing basis or was self-reported by the individuals in the trial at baseline or at a follow-up period (6 months, 1 and 2 years post randomisation). All unit costs used to value the reported resource use in the EEACT, apart from the costs associated with insulin use, are presented later (see Table 3). The unit costs associated with insulin use are reported separately later (see Table 4).
Diabetes-related contacts were collected using two methods in the REPOSE Trial. Patient’s self-reported number and type (either face to face or not face to face) of diabetes-related contacts since the last REPOSE visit (or in the year prior to baseline) were collected in each of the REPOSE DCFs (baseline, 6, 12 and 24 months). Ongoing information was collected from the sites on the number of visits, the type of visit and the time spent at each visit. The self-reported contacts were used in the health economic analysis for two reasons: (1) this method was consistent with the method used to collect information on the baseline number of contacts; and (2) national-level commissioning information provides only a cost per outpatient appointment (rather than for a specified time for a specific health-care professional to conduct an appointment), so from a costing perspective it is the number of contacts that is important rather than the time spent at each contact.
Table 2 shows that numbers of diabetes-related contacts were higher in the CSII + DAFNE arm of the REPOSE Trial than the MDI + DAFNE arm in the first year of the trial. However, most of these differences disappear in the second year of the trial. It should also be noted that the average time spent delivering diabetes-related contacts is higher for pump + DAFNE individuals than MDI + DAFNE individuals, except for telephone contacts delivered between 12 and 24 months post randomisation. This indicates that there are important differences in the number and time spent at diabetes-related contacts for MDI and pump users in the NHS.
| Diabetes-related contacts | Year prior to baseline | Months post randomisation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–6 | 6–12 | 12–24 | |||||||
| Face to face | Not face to face | Face to face | Not face to face | Face to face | Not face to face | Face to face | Not face to face | ||
| MDI + DAFNE | |||||||||
| Ongoing data collection (n = 95) | n, mean (SD) | – | – | 0.432 (1.048) | 0.474 (1.590) | 0.621 (0.947) | 0.516 (2.178) | 1.295 (2.539) | 1.263 (5.260) |
| Time (minutes), mean (SD) | – | – | 16.47 (52.89) | 5.47 (23.90) | 26.17 (44.23) | 7.68 (48.05) | 46.58 (90.73) | 13.32 (53.99) | |
| Self-reported (n = 128) | n, mean (SD) | 4.125 (7.374) | 1.242 (3.089) | 1.531 (2.159) | 0.477 (2.230) | 1.156 (1.492) | 0.336 (1.642) | 2.875 (4.719) | 1.094 (3.852) |
| Pump + DAFNE | |||||||||
| Ongoing data collection (n = 118) | n, mean (SD) | – | – | 0.814 (1.402) | 1.220 (1.913) | 0.924 (1.334) | 0.788 (2.095) | 1.576 (2.878) | 0.703 (1.458) |
| Time (minutes), mean (SD) | – | – | 39.03 (76.63) | 15.89 (28.24) | 37.37 (68.36) | 10.26 (28.66) | 60.13 (155.82) | 9.32 (21.22) | |
| Self-reported (n = 132) | n, mean (SD) | 4.197 (6.211) | 2.167 (4.489) | 1.795 (2.436) | 1.076 (2.092) | 1.242 (1.564) | 0.962 (2.813) | 2.787 (4.199) | 0.576 (1.393) |
The unit costs used to estimate the total cost diabetes-related contacts are presented in Table 3.
| Costs used in the within-trial analyses | Cost (2013–14, £) | Notes |
|---|---|---|
| DAFNE courses | ||
| Cost of a DAFNE course | 363.10 | DAFNE fact sheet 697 |
| Cost of a pre-course pump fitting session | 28.82 | REPOSE Trial data and DAFNE fact sheet 697 |
| Hypoglycaemia | ||
| Cost of hypoglycaemia admission | 446.73 | NHS Reference Costs 2013–14.98 Non-elective inpatient short stay. FCE weighted average of the currency codes: KB01C, KB01D, KB01E, KB01F, KB02G, KB02H, KB02J, KB02K |
| Paramedic cost per case | 233.58 | Elliot et al. 2014,99 table 5 |
| Cost of inpatient admissions | ||
| DKA | ||
| Cost of the first day | 527.78 | NHS Reference Costs 2012–13.100 Non-elective inpatient short stay. Currency code PA67Z |
| Cost of subsequent days | 284.42 | NHS Reference Costs 2012–13.100 Non-elective inpatients excess bed-days. Currency code PA67Z |
| Renal hospitalisation | ||
| Cost of the first day | 471.70 | NHS Reference Costs 2013–14.98 Non-elective inpatients short stay. Weighted average of the currency codes: LA09J, LA09K, LA09L, LA09M, LA09N, LA09P, LA09Q |
| Cost of subsequent days | 257.87 | NHS Reference Costs 2013–14.98 Non-elective inpatients excess bed-days. Currency codes: LA09J, LA09K, LA09L, LA09M, LA09N, LA09P, LA09Q |
| MI | ||
| Cost of the first day | 560.60 | NHS Reference Costs 2013–14.98 Non-elective inpatients short stay. Weighted average of the currency codes: EB10A, EB10B, EB10C, EB10D, EB10E |
| Cost of subsequent days | 248.89 | NHS Reference Costs 2013–14.98 Non-elective inpatients excess bed-days. Currency codes: EB10A, EB10B, EB10C, EB10D, EB10E |
| Foot ulcer | ||
| Cost of the first day | 509.39 | NHS Reference Costs 2012–13.100 Non-elective inpatient short stay. Currency codes: KB03C, KB03D, KB03E |
| Cost of subsequent days | 156.34 | NHS Reference Costs 2012–13.100 Non-elective inpatient short stay day. Currency codes: KB03C, KB03D, KB03E |
| Other inpatient stays | ||
| Cost of the first day | 755.44 | NHS Reference Costs 2012–13.100 Non-elective inpatient short stay. Currency code PA68Z |
| Cost of subsequent days | 335.81 | NHS Reference Costs 2012–13.100 Non-elective inpatient excess bed-day. Currency code PA68Z |
| Medication costs (per quarter) | ||
| Cost of lipid medication | 9.27 | Prescription Cost Analysis: England 2011 (BNF,101 chapter 2, section 12) |
| Cost of antiplatelet medication | 1.87 | Prescription Cost Analysis: England 2011 (BNF,101 chapter 2, section 9) |
| Cost of depression medication | 6.08 | Prescription Cost Analysis: England 2011 (BNF,101 chapter 4, section 3) |
| Cost of diabetes-related contacts | ||
| Cost of a face-to-face clinic | 105.49 | NHS Reference Costs 2013–14.98 Non-consultant-led outpatient attendance. Non-admitted face-to-face follow-up. Service description: Diabetic Medicine |
| Cost of a telephone contact | 75.80 | NHS Reference Costs 2013–14.98 Non-consultant-led outpatient attendance. Non-admitted non-face-to-face follow-up. Service description: Diabetic Medicine |
The individual’s self-reported resource use was collected on whether they were using lipid-lowering, antiplatelet or depression medication at the time of each REPOSE visit. No information was collected on the type of drug or the quantity used. It was assumed that, if an individual reported use of medication received medication, they had been receiving that specific medication since the last REPOSE visit. The average quarterly cost of each type of medication is reported in Table 3.
Data were collected on an ongoing basis for all inpatient hospitalisations that were not scheduled to treat a pre-existing condition. Therefore, the only missing data were for individuals who were lost to follow-up or withdrew from the trial. At each admission, information was collected on the cause. The possible causes for each admission were DKA, myocardial infarction (MI), severe hypoglycaemia, ischaemic heart disease, unstable angina, heart failure (HF), foot ulcer and renal disease. If the admission was not due to one of these causes, the reason was recorded. This occurred for only one inpatient admission in the REPOSE Trial. The NHS Reference Costs 2013–1498 (and all previous years used to inform the unit costs) present the cost of non-elective inpatient stays as short stays, excess bed-days and long stays. The cost of inpatient stays were estimated as the cost of a short stay if the length of stay was ≤ 1 day. If the length of stay was ≥ 2 days then the cost of the visit was estimated using the following formula:
At baseline, self-reported data were collected on the number of diabetes-related admissions in the past year, days spent in hospital and the reason for admission. The possible causes included DKA, hypoglycaemia, MI, ischaemic heart disease, unstable angina, HF, foot ulcer and renal disease. It was possible that individuals had missing information on the number of days that they were in hospital or the reason for the admission. Mean value imputation was used to impute the number of missing days. All of the admissions for which the reason was missing were treated as an ‘other cause inpatient stay’.
Data were also collected on an ongoing basis for individual’s severe hypoglycaemic events. Severe hypoglycaemia was defined in the REPOSE Trial as been any hypoglycaemic episode that an individual was unable to treat themselves. Information was collected on whether each severe hypoglycaemic event required either a paramedic call-out and/or an inpatient admission. If it was reported that an individual did not have an inpatient admission or a paramedic call-out then it was assumed that a friend or family member provided aid to the individual, which meant that no admission or paramedic call-out was required. This had no implications for NHS resource use, so these episodes of severe hypoglycaemia were assumed to have no monetary cost to the NHS in the EEACT. The unit costs for a paramedic call-out or an inpatient admission for severe hypoglycaemia are presented in Table 3.
Information was collected for all individuals in the REPOSE Trial (at baseline, 6, 12 and 24 months post randomisation) on their current insulin regimen (including type of insulin used), the typical daily insulin dose in the week preceding data collection, the number of injections per day, the type of insulin used by the individual and the method of insulin delivery. As information on insulin type was available, the cost of insulin and insulin pens was estimated separately for each insulin type.
The unit costs associated with insulin use are presented separately from the rest of the unit costs in Table 4. The daily cost of insulin was multiplied by the number of days between each data collection period (6 months, 1 and 2 years) to calculate the cost of insulin in the first and second year. If an individual was receiving insulin pump therapy then the cost of needles, insulin pens and syringes were not applied, as these were already included in the estimates of the cost of insulin pump consumables. From this information a cost of insulin for each individual in the REPOSE Trial was calculated.
| Item | Average unit cost (£) | Number of units | Cost per unit (£) | Associated yearly cost of an insulin pen (£) | Source |
|---|---|---|---|---|---|
| Consumables related to MDI | |||||
| Cost of an insulin needle | 0.11 | N/A | N/A | N/A | HSCIC102 |
| Cost of an insulin syringe | 0.13 | N/A | N/A | N/A | HSCIC102 |
| Quick-acting insulin | |||||
| Human insulin | |||||
| Vial | 9.87 | 1000 | 0.01 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 18.97 | 1500 | 0.01 | 8.78 | |
| Animal insulin | |||||
| Vial | 26.15 | 1000 | 0.03 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 38.29 | 1500 | 0.03 | 5.97 | |
| Insulin aspart (NovoRapid) | |||||
| Vial | 14.08 | 1000 | 0.01 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 28.31 | 1500 | 0.02 | 9.59 | |
| Disposable pen | 30.63 | 1500 | 0.02 | N/A | |
| Insulin lispro (Humalog) | |||||
| Vial | 16.61 | 1000 | 0.02 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 28.31 | 1500 | 0.02 | 8.86 | |
| Disposable pen | 28.31 | 1500 | 0.02 | N/A | |
| Insulin glulisine (Apidra) | |||||
| Vial | 16.00 | 1000 | 0.02 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 28.30 | 1500 | 0.02 | 7.86 | |
| Disposable pen | 28.30 | 1500 | 0.02 | N/A | |
| Background insulin | |||||
| Human insulin | |||||
| Vial | 10.41 | 988 | 0.01 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 21.52 | 1500 | 0.01 | 9.30 | |
| Disposable pen | 21.05 | 1500 | 0.01 | N/A | |
| Animal insulin | |||||
| Vial | 26.17 | 1000 | 0.03 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 38.32 | 1500 | 0.03 | 9.57 | |
| Insulin detemir (Levemir) | |||||
| Cartridges for a reusable pen | 42.00 | 1500 | 0.03 | 9.59 | BNF,101 HSCIC102 |
| Disposable pen | 42.10 | 1500 | 0.03 | N/A | |
| Insulin glargine (Lantus) | |||||
| Vial | 30.68 | 1000 | 0.03 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 41.50 | 1500 | 0.03 | 7.86 | |
| Disposable pen | 41.50 | 1500 | 0.03 | N/A | |
| Mixed insulin | |||||
| Biphasic isophane insulin | |||||
| Animal insulin | |||||
| Vial | 25.20 | 1000 | 0.03 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 37.80 | 1500 | 0.03 | 5.97 | |
| Human insulin | |||||
| Vial | 15.43 | 987 | 0.02 | N/A | BNF,101 HSCIC102 |
| Cartridges for a reusable pen | 18.94 | 1500 | 0.01 | 7.74 | |
| Disposable pen | 21.43 | 1500 | 0.01 | N/A | |
| Biphasic insulin aspart | |||||
| Cartridges for a reusable pen | 28.79 | 28.79 | 0.02 | 9.59 | BNF,101 HSCIC102 |
| Disposable pen | 29.89 | 29.89 | 0.02 | ||
| Biphasic insulin lispro | |||||
| Vial | 16.61 | 1000 | 0.02 | BNF,101 HSCIC102 | |
| Cartridge for reusable pen | 29.03 | 1500 | 0.02 | 8.93 | |
| Disposable pen | 30.13 | 1500 | 0.02 | ||
Cost of the insulin pumps and consumables
The annual cost of an insulin pump and insulin pump consumables was estimated using a survey, which was conducted in all of the trial centres. This survey obtained information on the unit costs and the quantities of insulin pumps and insulin pump consumables purchased by centres in routine clinical practice. Information was also collected on the insulin pump consumables used by participants in the REPOSE Trial. Data were collected over a 6-month period for the insulin pump consumables and a 12-month period for the insulin pumps. The Scottish centres purchased insulin pumps and insulin pump consumables through the Scottish Government. Instead of completing the survey, information was obtained on the average price that the Scottish Government paid for insulin pumps and consumables.
One centre did not report any price information and two centres did not report the quantities of insulin pump consumables used by REPOSE participants. This missingness was addressed by using the mean price and mean resource use at the other trial centres to calculate the cost of insulin pumps and consumables.
For some centres, data collection on individuals’ use of consumables was for a period that was somewhat shorter than 6 months and we estimated their consumables use for 12 months assuming a pro rata uplift. The cost of insulin pumps and insulin pump consumables during the trial duration was estimated by multiplying the annual cost of insulin pumps and consumables by the fraction of each year that each individual spent on insulin pump therapy.
The annual cost of an insulin pump was calculated assuming a pump lifetime of 4.5 years, based on the clinical expert opinion of a diabetes specialist nurse. The annualised cost was multiplied by the number of days that an individual spent on an insulin pump to give the total cost of insulin pump therapy in the trial period.
The effect of a price reduction of insulin pumps and insulin pump consumables of 25% and 50% from the pump costing survey prices was tested in scenario analyses. A further scenario analysis was conducted by using a cost of £2002 per annum for a Medtronic pump and consumables reported in Riemsma et al. 8 Riemsma et al. 8 conducted an appraisal of integrated sensor-augmented pump therapy systems for managing blood glucose levels compared with stand-alone insulin pumps with a separate CGM system in the UK for NICE’s diagnostics advisory committee. One of the comparators in this appraisal was stand-alone insulin pumps with an additional continuous blood glucose monitoring system. As such, this cost for a Medtronic insulin pump was obtained from the estimated cost of a stand-alone insulin pump estimated in this study. The costs were obtained from the stated prices of an insulin pump from the London New Drugs Group in November 2014. 103
Treatment switching
During the REPOSE Trial, it was possible for individuals to switch from insulin delivery mechanism to the other, that is to switch from insulin pump therapy to MDI and vice versa. It is important to include treatment switching in a health economic analysis, as it is unreasonable to assume that (1) people who switch treatment will use the same resources over a lifetime as someone who does not use a pump and (2) someone still receiving an insulin pump has the same benefit from treatment as someone who has switched to using MDI. As a consequence of including treatment switching in the long-term model, the mean cost and QALY gain per patient in the pump + DAFNE arm is more likely to represent the true lifetime costs and QALYs than an analysis that ignored treatment switching.
It was possible to switch treatment twice, and two individuals did so in the REPOSE Trial. The data in the REPOSE Trial were analysed to assess the number of people with diabetes who switched treatment. The estimated cost of insulin and insulin pumps was adjusted to reflect the fact that individuals switch treatments. As the EEACT uses a microcosting approach to estimate costs and obtains QALY data from the self-reported EQ-5D data to calculate QALYs, all other cost and QALY effects due to switching are included in the analysis.
The cost of insulin was adjusted for treatment switching by using the data on an individual’s insulin use. If an individual switched treatment once, insulin use between the last follow-up period and the treatment switching date was estimated using the reported insulin use at his/her last follow-up period (individuals were followed up at 6 months, 1 and 2 years post randomisation). Similarly, insulin use between his/her treatment switching date and the next follow-up period was estimated using the data observed in the next follow-up period. For example, if an individual switched treatment 11 months post randomisation, his/her insulin use reported at 6-month follow-up would be used to estimate the cost of insulin between 6 and 11 months, and insulin use at 1-year follow-up would be used to estimate insulin use between 11 months and 1 year. If an individual switched between the baseline and the 6-month follow-up period then his/her data were treated as missing, as no information was available on insulin use when using his/her randomised treatment allocation, after receiving DAFNE education. Furthermore, if an individual switched treatment twice (n = 2) then the individual was excluded from the EEACT analysis population. This was because individuals both switched and switched back to their original treatment within the time period between two consecutive follow-up periods. Therefore, no information was available on their resource use when they received the other treatment.
The cost of an individual’s use of insulin pumps and consumables in each year was calculated by multiplying the fraction of the year for which they used insulin pumps by the associated yearly cost of insulin pumps and insulin pump consumables.
Estimating the within-trial cost effects
The total cost for each individual consisted of the cost of the following components: inpatient admissions; paramedic call-outs for severe hypoglycaemia; the cost of a pump fitting session for individuals who received pump + DAFNE; the cost of pump-fitting session for individuals who switched from MDI to insulin pump therapy and insulin; annual cost of an insulin pump; annual cost of insulin pump consumables; and the cost of DAFNE course.
In the base-case analysis, complete cost information was used in the EEACT. Complete total cost information was available for 98%, 90% and 92% of individuals in the ITT population at baseline, 1 and 2 years, respectively.
In a scenario analysis, missing cost data were imputed for those individuals who attended at least one REPOSE Trial follow-up visit. Total discounted cost data were imputed using chained equations (predictive mean matching), utilising baseline HbA1c, treatment allocation, age at baseline and baseline cost values as covariates in the imputation equations. Ten different imputed values were calculated for each individual with missing data.
Estimating within-trial quality-adjusted life-year effects using the EuroQol-5 Dimensions and the Short Form questionnaire-12 items
To generate QALY measures over the 2-year trial follow-up, information was collected on an individual’s utility using two different instruments: the EQ-5D and the SF-12. The EQ-5D and SF-12 questionnaires were completed by individuals at baseline and all follow-up visits (6, 12 and 24 months).
In the base-case within-trial analysis, the utility values measured by the EuroQol-5 Dimensions, 3-level version (EQ-5D-3L) were used to calculate QALYs using an area-under-the-curve analysis. EQ-5D utility scores were used in the base case because they are NICE’s preferred utility measure. 1 In a scenario analysis, utility values measured using the Short Form questionnaire-6 Dimensions (SF-6D) (a measure derived from the SF-12) were used to calculate QALY values. 104
In the base case, only individuals with complete QALY data were included in the analysis. Utilities, as measured by the EQ-5D-3L, were completed by 99%, 93%, 88% and 90% of individuals at baseline, 6, 12 and 24 months, respectively. If an individual had a missing 6-month utility value, then it was assumed that the 6-month utility value would be the average of the baseline and 1-year utility values. If an individual had a missing utility score at 12 or 24 months, then they were excluded from the base-case analysis. The 6-month utility values of individuals with missing utility data at 12 or 24 months were similar in both model arms. The individuals in the pump + DAFNE arm, who did not have 1- or 2-year EQ-5D-3L data, had a mean EQ-5D-3L utility score of 0.8177 [standard error (SE) 0.0602] at 6-month follow-up. The individuals in the pump + DAFNE arm, who did not have 1- or 2-year EQ-5D-3L data, had a mean EQ-5D-3L utility score of 0.904 (SE 0.0256) at 6-month follow-up. The hypothesis that the difference between these two distributions was equal to zero could not be rejected using a two-sided t-test with equal variances at the 10% significance level. Therefore, there is no indication that excluding these individuals from the base case would bias the results.
In a scenario analysis, multiple imputation was used to impute missing QALY values for individuals with assessment data for least one follow-up point. Data were imputed using chained equations (predictive mean matching), utilising baseline HbA1c, treatment allocation, age at baseline and baseline cost or QALY values as covariates in the imputation equations. Ten imputed values were calculated for each individual, with missing data in the analyses using imputed data.
Statistical model used for the within-trial analysis
A seemingly unrelated regression model was used to estimate the costs and QALYs in the EEACT. A seemingly unrelated regression is a type of statistical model that allows for multiple outcome variables to be modelled simultaneously. 105 This approach is advantageous, as any covariances between covariates across the different outcome variables are estimated. One seemingly unrelated regression was fitted to four outcome variables: (1) total discounted costs in year 1, (2) total discounted costs in year 2, (3) total discounted QALYs in year 1 and (4) total discounted QALYs in year 2, using the ‘mysureg’ command in the ‘ml_ado’ package in Stata version 13.1. For the QALY outcome variables, baseline HbA1c, treatment allocation and baseline utility were included as covariates, and clustering was controlled for in each DAFNE course. Baseline utility was included as a covariate to estimate QALYs so that any baseline differences in health between the two treatment arms was controlled for. 106 For the cost outcome variables, baseline HbA1c, centre, treatment allocation and baseline resource use were included as covariates, and clustering was controlled for in each DAFNE course.
A scenario analysis was conducted in which both missing cost and QALY data were imputed for individuals with at least one assessment during the REPOSE Trial follow-up period. A regression was conducted in each imputed data set and combined using Rubin’s rules. 107 Details of the imputation procedures used in this scenario analysis are given in Estimating the within-trial cost effects and Estimating within-trial quality-adjusted life-year effects using the EuroQol-5 Dimensions and the Short Form questionnaire-12 items.
The impact of treatment allocation on total costs was calculated by adding the treatment allocation parameters relating to the cost outcomes in years 1 and 2. Likewise, the impact of treatment allocation on total QALYs was calculated by adding the treatment allocation parameters relating to the QALY outcomes in years 1 and 2. CIs around the effect of treatment allocation on total costs and total QALYs were calculated using the formula for calculating the variance of a variable that is a sum of correlated variables. The variances and covariance used in this calculation were obtained from the variance–covariance matrix of the seemingly unrelated regression.
Analysis
The key measure of cost-effectiveness in the EEACT was the ICER base on the mean incremental effect of pump + DAFNE compared with MDI + DAFNE on total costs and total QALYs. The CIs around these effects were estimated from the variance–covariance matrix of the regression model. The results were presented on a cost-effectiveness plane and the uncertainty around the mean effect was presented using a confidence ellipse.
Long-term cost-effectiveness
The Sheffield Type 1 Diabetes Policy Model Overview
The Sheffield Type 1 Diabetes Policy Model (henceforth, the Model) was used to estimate the lifetime costs and QALYs for individuals receiving MDI + DAFNE and pump + DAFNE. The Model has been developed and used over several years, and a detailed description is provided in a journal article108 and a detailed report to the NIHR on the DAFNE programme grant research. 27 In this analysis, we have updated some aspects of the evidence used within the Model. We term the version used here as ‘The Sheffield Type 1 Diabetes Policy Model version 1.3’.
The Model is an individual-level simulation model, which consists of a series of submodels that simulate the progression of diabetic complications (microvascular and macrovascular), SAEs (severe hypoglycaemia and DKA) and mortality in a given population with T1DM. Each of the modelled microvascular (nephropathy, neuropathy, retinopathy and macular oedema) and macrovascular complications (MI, stroke, HF and angina) are included in the model as separate Markov submodels with an annual time cycle. Short-term AEs (severe hypoglycaemia and DKA) are modelled as the annual incidence of these complications, dependent on each patient’s characteristics. The Model structure is also presented in Figure 1. The Model attaches utilities and ongoing costs to health states and one-off costs to events (the move to another health state in a submodel). These costs and utilities are combined with the length of time that a patient spends in a health state to estimate lifetime costs and QALYs. The Model estimates patient’s disease progression over their lifetime.
FIGURE 1.
The structure of the Sheffield Type 1 Diabetes Policy Model (from Thokala et al. 108 with permission from John Wiley & Sons, Inc.). CVD, cardiovascular disease; ESRD, end-stage renal disease; hypos, hypoglycaemic episodes; PAD, peripheral arterial disease.

The disease progression parameters in the Model were not updated in these analyses. However, the costs and utilities associated with health states and events were updated. A full probabilistic sensitivity analysis (PSA) was conducted with 500 probabilistic runs, each with 5000 individuals in each Model arm. All Model runs were conducted using the SIMUL8 2010 professional (Simul8 Corporation, Boston, MA, USA) programme.
Microvascular events and disease progression
For each microvascular complication (retinopathy, neuropathy and nephropathy), individuals progress to the more severe health states within each annual time cycle according to the probabilities reported in table 29 of Heller et al. 27 The health states included for retinopathy include no retinopathy, background retinopathy, proliferative retinopathy and blindness. The health states included for neuropathy include no neuropathy, clinical neuropathy, clinically confirmed neuropathy, diabetic foot syndrome and peripheral arterial disease (PAD) with amputation. The health states included for nephropathy include no nephropathy, microalbuminuria, macroalbuminuria, end-stage renal disease (ESRD) and death from ESRD.
Macrovascular events and disease progression
The risks of fatal and non-fatal macrovascular complications (MI, stroke, HF and angina) are modelled in three stages. First, the annual probability of experiencing any cardiovascular event is estimated based on individual characteristics, as per the 5-year cardiovascular risk model of Cederholm et al. 109 Second, if the individual is deemed to experience a cardiovascular event, the type of event (MI, stroke, HF or angina) is determined using methods outlined in Palmer,110 based on data from the Diabetes Control and Complications Trial (DCCT) Epidemiology of Diabetes Interventions and Complications study. 111 Third, is the issue of fatality. If the event experienced is a MI, stroke or HF, it is determined whether or not the event is fatal using methods outlined in Palmer110 and as shown in table 31 of Heller et al. 27
Individuals can also die from other causes; this other-cause mortality is modelled based on UK life tables from 2012–14, adjusted to exclude the causes either attributed to diabetes mellitus (either type 1, type 2 or unspecified) or modelled directly in the microvascular and macrovascular disease components (deaths due to ESRD, MI, stroke and HF).
Utilities: health-related quality of life for health states in the long-term model
Heller et al. 27 (pp. 108–9) detail the utility analyses undertaken to inform version 1.2 of the model. Since that report, further analysis has taken place in the course of peer-reviewed journal publication, and the utilities presented in this analysis are based primarily on those revised analyses, which are now published in Peasgood et al. 112 The main change in this analysis is that the preferred statistical model to estimate utility values in the publication is a random-effects model rather than a Tobit model. Riemsma et al. 8 conducted the independent economic analysis for NICE on the cost-effectiveness of integrate CGM and insulin pump therapy. For the independent analysis, a systematic review of utilities in type 2 diabetes mellitus published in 2014 by Beaudet et al. 113 was used for many of the health states of their economic model. The utilities presented in version 1.2 of the model were considered for updating by the new information presented in Peasgood et al. 112 and Beaudet et al. 113
The following criteria were applied to decide if a utility value should be updated. Utility values estimated in a population with T1DM were preferred to values estimated in a population with type 2 diabetes mellitus. If multiple values were available in a T1DM population, utility values that were estimated using the EQ-5D were preferred to other utility values. If a paper presented more than one parameter value, the parameter from the best-fitting model was the preferred source. If two papers analysed the same data source then the most recent paper was the preferred source. The utility parameters used in the Model version 1.3 base-case analyses, and the distributions used in the PSA, are given in Table 5.
| Health state for event | Utility | SE | Beta distribution | Source | |
|---|---|---|---|---|---|
| Alpha | Beta | ||||
| Baseline utility value | |||||
| Male with T1DM and no complications | 0.866 | 0.010 | 947.789 | 146.898 | Peasgood et al. 2016112 |
| Disutility | Gamma distribution | ||||
| Alpha | Beta | ||||
| Complications or covariates | |||||
| Female with T1DM and no complications | 0.0236 | 0.008 | 8.703 | 0.003 | aPeasgood et al. 2016112 |
| Nephropathy | |||||
| Microalbuminuria | 0 | Assumption | |||
| Microalbuminuria | –0.017 | 0.01 | 2.89 | 0.006 | Coffey et al. 2002114 |
| ESRD | –0.078 | 0.026 | 9 | 0.009 | Coffey et al. 2002114 |
| Neuropathy | |||||
| Clinical neuropathy | –0.055 | 0.01 | 30.25 | 0.002 | Coffey et al. 2002114 |
| Clinically confirmed neuropathy | –0.055 | 0.01 | 30.25 | 0.002 | Coffey et al. 2002114 |
| Diabetic foot syndrome | –0.1042 | –0.119 | 0.767 | 0.136 | Peasgood et al. 2016112 |
| PAD with amputation | –0.1172 | –0.055 | 4.541 | 0.026 | aPeasgood et al. 2016112 |
| Retinopathy | |||||
| Background retinopathy | –0.0544 | –0.023 | 5.594 | 0.010 | Peasgood et al. 2016112 |
| Proliferative retinopathy | –0.0288 | –0.026 | 1.227 | 0.023 | Peasgood et al. 2016112 |
| Blindness | –0.208 | 0.013 | 256 | 0.001 | Coffey et al. 2002114 |
| Cardiovascular | |||||
| MI (first year) | –0.065 | 0.03 | 4.694 | 0.014 | Alva et al. 2014115 |
| MI (subsequent years) | –0.057 | 0.03 | 3.61 | 0.016 | Alva et al. 2014115 |
| HF | –0.101 | 0.032 | 9.962 | 0.010 | Alva et al. 2014115 |
| Stroke | –0.165 | 0.035 | 22.224 | 0.007 | Alva et al. 2014115 |
| Angina | –0.09 | 0.018 | 25 | 0.004 | bClarke et al. 2002116 |
| AEs | |||||
| Hypoglycaemia episode unable to treat yourself | –0.002 | –0.002 | 1 | 0.002 | Peasgood et al. 2016112 |
| DKA | –0.0091 | –0.01 | 0.828 | 0.011 | aPeasgood et al. 2016112 |
Unit costs for health states in the long-term model
The base case unit costs, which are presented in Heller et al. ,27 were inflated to 2013–14 prices using the Hospital and Community Health Services Index. 2 The base-case health-state costs used, and the distributions used in the PSA, are given in Table 6.
| Health state | Mean cost (£) | SE | Gamma distribution | Source | |
|---|---|---|---|---|---|
| Alpha | Beta | ||||
| Microalbuminuria (ongoing) | 36 | 3.56 | 100 | 0.36 | BNF 2012,117 McEwan et al. 2007118 |
| Microalbuminuria (ongoing) | 36 | 3.56 | 100 | 0.36 | |
| ESRD (ongoing) | 24,436 | 2444 | 100 | 244.36 | NHS Reference Costs 2011119 (activity-weighted average of LD01A, LD02A, LD03A, LD04A, LD05A, LD06A, LD07A, LD08A, LD09A, LD010A and LD011A and LD012A) |
| Death due to ESRD | 0 | 0 | 0 | 0.00 | Assumption |
| Clinically confirmed neuropathy | 271 | 27.14 | 100 | 2.71 | Currie et al. 2007120 |
| Clinical neuropathy | 271 | 27.14 | 100 | 2.71 | Assumed equal to clinically confirmed neuropathy |
| Diabetic foot syndrome | 2848 | 285 | 100 | 28.48 | NHS Reference Costs 2011119 [activity-weighted average of ‘Non-Elective Inpatient (Long Stay)’, ‘Non-Elective Inpatient (Long Stay) Excess Bed-day’ and ‘Non-Elective Inpatient (Short Stay)’ for currency code QZ17B] |
| PAD with amputation (year 1) | 7221 | 722 | 100 | 72.21 | NHS Reference Costs 2011119 [activity-weighted average of ‘Non-Elective Inpatient (Long Stay)’, ‘Non-Elective Inpatient (Long Stay) Excess Bed-day’ and ‘Non-Elective Inpatient (Short Stay)’ for currency codes QZ12Z and QZ11B] |
| PAD with amputation (ongoing) | 439 | 43.93 | 100 | 4.39 | McEwan et al. 2007118 |
| Background retinopathy | 145 | 14.47 | 100 | 1.45 | |
| Proliferative retinopathy | 661 | 66.11 | 100 | 6.61 | |
| Macular oedema | 661 | 66.11 | 100 | 6.61 | Assumed equal to proliferative retinopathy |
| Blindness (year 1) | 1584 | 158 | 100 | 15.84 | Clarke et al. 2003121 |
| Blindness (ongoing) | 519 | 51.88 | 100 | 5.19 | |
| First MI (year 1) | 6788 | 679 | 100 | 67.88 | Clarke et al. 2003121 |
| Second MI | 6788 | 679 | 100 | 67.88 | |
| Final MI | 6788 | 679 | 100 | 67.88 | |
| MI (ongoing) | 904 | 90.43 | 100 | 9.04 | |
| Fatal MI | 2101 | 210 | 100 | 21.01 | |
| First stroke (year 1) | 4361 | 436 | 100 | 43.61 | |
| Second stroke | 4361 | 436 | 100 | 43.61 | |
| First stroke (ongoing) | 559 | 55.90 | 100 | 5.59 | |
| Fatal stroke | 5684 | 568.45 | 100 | 56.84 | |
| HF (year 1) | 3818 | 382 | 100 | 38.18 | |
| HF (ongoing) | 1173 | 117 | 100 | 11.73 | |
| Fatal HF | 3818 | 382 | 100 | 38.18 | |
| Angina (year 1) | 3397 | 340 | 100 | 33.97 | |
| Angina (ongoing) | 951 | 95.09 | 100 | 9.51 | |
| Hypoglycaemia | 187 | 18.69 | 100 | 1.87 | Previous calculation (weighted average of the following HRG codes, with activities obtained from the hypoglycaemia rates observed before and after DAFNE: KB02D, KB02E, KB02F. KB02D, KB02E, KB02F, KB01B, KB01B, KB01A, KB01A, PS13A, PS13B, PS13C, VB09Z, VB09Z) |
| DKA with hospitalisation | 1399 | 140 | 100 | 13.99 | NHS Reference Costs 2011119 [activity-weighted average of ‘Non-Elective Inpatient (Long Stay)’, ‘Non-Elective Inpatient (Long Stay) Excess Bed-day’ and ’Non-Elective Inpatient (Short Stay)’ for currency codes KB01B and PA67Z, respectively] |
Pre-specified subgroup analyses
A series of subgroup analyses were conducted in the long-term modelling. The same subgroup analyses were not conducted in the within-trial analysis, as conducting analyses in these subgroups would lead to a reduced sample size and increase the chance that a spurious result would be found. The cost-effectiveness of pump + DAFNE against MDI + DAFNE was compared for subgroups:
-
baseline HbA1c ≥ 7.5% (58 mmol/mol)
-
baseline HbA1c ≥ 7.5% (58 mmol/mol) and < 8.5% (69 mmol/mol)
-
baseline HbA1c ≥ 8.5% (69 mmol/mol) and < 9.5% (80 mmol/mol)
-
baseline HbA1c ≥ 9.5% (80 mmol/mol)
-
baseline HbA1c < 8.5% (69 mmol/mol)
-
baseline HbA1c ≥ 8.5% (69 mmol/mol)
-
all individuals in the per-protocol population.
For people with a baseline HbA1c of < 7.5% (58 mmol/mol), there were insufficient numbers (n = 12 people in the MDI + DAFNE arm, n = 13 in the pump + DAFNE arm) to conduct a subgroup analysis.
The subgroup analyses were conducted by changing only the individual characteristics that were inputted into the model. All of the other parameters and assumptions in the model were identical to those in the base case.
Modelled cohort of 5000 simulated individuals
Individual characteristics were drawn from the baseline characteristics of all individuals, irrespective of treatment arm, in the ITT population. The variables included in the baseline individual characteristics are HbA1c, age, diabetes duration, triglycerides, TC, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, systolic blood pressure, baseline cost of insulin, baseline cost of diabetes-related contacts, sex, physical activity (measured as being either low, medium or high, based on the time spent walking, fast walking or running per week), smoking status, ethnicity, history of nephropathy, history of neuropathy, history of retinopathy, history of MI, history of stroke, history of HF and history of angina.
The observed characteristics (including missing values) of the REPOSE Trial individuals were sampled with replacement to generate a cohort of 5000 individuals to be used in the economic model. After the cohort of 5000 individuals was obtained, missing data values were observed for TC, HDL cholesterol, LDL cholesterol, systolic blood pressure and sex. To obtain the missing data, these values were imputed. The data were imputed in the cohort of 5000 individuals, rather than for the individuals in the trial data set, as this allowed the missing data to vary across different replications of an individual with missing data. If the data were imputed before the sampling, then the missing data would take on a fixed value in the cohort of 5000 individuals rather than being uncertain.
The imputation procedure depended on whether the missing value was a continuous or a categorical variable. TC, HDL cholesterol, LDL cholesterol and systolic blood pressure were imputed using chained equations, utilising the truncated regression procedure. Sex was imputed separately from TC, HDL cholesterol and systolic blood pressure, using the Poisson procedure. In both sets of imputation models, all of the complete individual characteristics were included as predictive covariates. LDL cholesterol was calculated using the imputed data for TC and HDL cholesterol, using the following formula: (3)LDL cholesterol=total plasma cholesterol (cholesterol) – HDL cholesterol – (triglycerides/2.19). 122
All of the imputations were performed using single imputation. The reason for using one imputed value was that as more imputations were performed, the average value of these imputations would converge for the different replications of an individual from the trial population. Therefore, the uncertainty in the values of the missing data would not be fully reflected in the model cohort.
Summaries of the baseline characteristics of the 5000 simulated individuals for the base-case cohort and the 260 individuals sampled from the REPOSE Trial are given in Table 7. The summary of baseline characteristics for the 5000 simulated individuals for each of the pre-specified subgroup analyses is provided in Appendix 7.
| Characteristic | REPOSE ITT population (N = 260) | Simulated cohort (N = 5000) |
|---|---|---|
| Continuous variables, mean (SD) | ||
| Baseline HbA1c (mmol/mol) | 76.0 (18.6) | 76.1 (18.8) |
| Age (years) | 40.4 (13.4) | 40.3 (13.3) |
| Diabetes duration (years) | 18.0 (12.5) | 18.0 (12.5) |
| Triglycerides (mmol/mol) | 1.4 (1.0) | 1.3 (1.0) |
| TC (mmol/mol) | 4.9 (0.9) | 4.9 (0.9) |
| HDL cholesterol (mmol/mol) | 1.6 (0.4) | 1.6 (0.4) |
| LDL cholesterol (mmol/mol) | 2.8 (0.9) | 2.8 (0.9) |
| Systolic blood pressure | 131.4 (16.4) | 131.3 (16.3) |
| Baseline cost of insulin (£) | 357.24 (147.65) | 360.39 (157.92) |
| Baseline cost of diabetes-related contacts (£) | 561.61 (885.92) | 571.63 (928.92) |
| Categorical variables n/N (%) | ||
| Sex | ||
| Female | 104/260 (40.0) | 2050/5000 (41.0) |
| Male | 152/260 (58.5) | 2950/5000 (59.0) |
| Missing | 4/260 (1.5) | 0/5000 (0.0) |
| Physical activity | ||
| Low | 67/260 (25.8) | 1245/5000 (24.9) |
| Medium | 128/260 (49.2) | 2440/5000 (48.8) |
| High | 65/260 (25.0) | 1320/5000 (26.4) |
| Smoking status | ||
| Current | 50/260 (19.2) | 960/5000 (19.2) |
| Former | 67/260 (25.8) | 1315/5000 (26.3) |
| Never | 143/260 (55.0) | 2725/5000 (54.5) |
| Ethnicity | ||
| White | 258/260 (99.2) | 4955/5000 (99.1) |
| Black | 2/260 (0.8) | 45/5000 (0.9) |
| Nephropathy | ||
| No complications | 239/260 (91.9) | 4645/5000 (92.2) |
| Microalbuminuria | 13/260 (5.0) | 235/5000 (4.7) |
| Macroalbuminuria | 7/260 (2.7) | 135/5000 (2.7) |
| Dialysis or transplant | 1/260 (0.4) | 20/5000 (0.4) |
| Neuropathy | ||
| No complications | 238/260 (91.5) | 4535/5000 (90.7) |
| Neuropathy or ulcers | 22/260 (8.5) | 465/5000 (9.3) |
| Retinopathy | ||
| No complications | 145/260 (55.8) | 2800/5000 (56.0) |
| Background diabetic retinopathy | 91/260 (35.0) | 1740/5000 (34.8) |
| Proliferative diabetic retinopathy | 24/260 (9.2) | 465/5000 (9.3) |
| MI | ||
| No complications | 255/260 (98.1) | 4890/5000 (97.8) |
| MI | 5/260 (1.9) | 110/5000 (2.2) |
| Stroke | ||
| No complications | 259/260 (99.6) | 4985/5000 (99.7) |
| Stroke | 1/260 (0.4) | 15/5000 (0.3) |
| HF | ||
| No complications | 259/260 (99.6) | 4970/5000 (99.4) |
| HF | 1/260 (0.4) | 30/5000 (0.6) |
| Angina | ||
| No complications | 257/260 (98.9) | 4940/5000 (98.8) |
| Angina | 3/260 (1.2) | 60/5000 (1.2) |
Incorporating estimated clinical effectiveness from the REPOSE Trial: glycated haemoglobin
The probability of switching treatment, changes in HbA1c, the probability of a severe hypoglycaemic event and the probability of the DKA were based on data from the REPOSE Trial. These four clinical effects have been included in the health economic model, as they all would impact on the costs of treatment and QALYs gained by people if either option were to be adopted in routine clinical practice. HbA1c has been included as it is the key driver of all modelled diabetic complications in the Model. Changes in HbA1c were estimated using a beta regression. The probability of severe hypoglycaemia and DKA have been included, so that any benefits of either arm in reducing the incidence of these events is included in the economic model. The probability of severe hypoglycaemia and DKA were estimated using negative binomial models. Treatment switching has been included, as it is expected that when an individual switched treatment from pump to MDI or from MDI to pump that the cost of managing their diabetes and their clinical outcomes would change. The probability of switching treatment was estimated using parametric survival curves, using treatment switching as the event of interest.
Incorporating treatment switching
During REPOSE, individuals in both trial arms could switch their insulin delivery mechanism; because of effects on both costs and clinical outcomes, it was important to incorporate treatment switching into the model. A total of 17 of 132 (12.88%) individuals, initially randomised to the pump, switched once to MDI to deliver their insulin. A further two individuals, initially randomised to the pump, switched from pump to MDI and then switched again from MDI back to pump. A total of 8 of 128 (6.25%) individuals, initially randomised to MDI, switched to pump.
Kaplan–Meier curves were fitted to individual-level data using treatment switching as the event. Parametric survival curves were fitted to the data with HbA1c prior to switching, number of DKAs and number of severe hypoglycaemic events in the year prior to switching (or 2 years’ follow-up if no switching occurred) included as covariates. The SEs of the parametric models were adjusted for clustering within each course. Separate models were fitted to individuals initially randomised to insulin pump therapy and MDI, so no assumption of proportion hazards or accelerated failure time was made. Exponential, Weibull, Gompertz, log-logistic, log-normal and generalised gamma distributions for the parametric curves were considered. The goodness of fit of the different curves was assessed using visual assessment of the Kaplan–Meier plots and the Akaike information criterion (AIC) and the Bayesian information criterion (BIC).
Based on expert clinical opinion of a Professor of Clinical Diabetes and Honorary Consultant Physician, and a Professor in Public Health and Health Technology Assessment, it was assumed, in the base case, that if an individual was on an insulin pump after 2 years then they would remain on the pump; this assumption was made, as, in their experience, once an adult with T1DM was successfully using an insulin pump then they were unlikely to change the method of insulin delivery.
In the model, treatment switching impacted on HbA1c and the cost of pumps, diabetes-related contacts and insulin. Details on how the HbA1c of patients who switched are given later (see Estimation of each individual’s glycated haemoglobin); details on how the costs of treatment were updated for people who switched are also given later (see Cost of insulin, diabetes-related contacts and insulin pumps). No explicit inclusion of treatment switching on the risk of DKA and severe hypoglycaemia was included in the model because of the relatively small numbers of these events in each trial arm (see Table 7). However, the risk of DKA and severe hypoglycaemia does depend on the HbA1c of the individual in the model; therefore, there are differences in the risk of DKA and severe hypoglycaemia between those who switched treatment and those who did not.
Estimation of each individual’s glycated haemoglobin
To develop the method to incorporate HbA1c treatment effect evidence into the model, several factors were considered. Data were collected on each individual’s HbA1c at each follow-up visit. As HbA1c is the key predictor of clinical events in the model, it is important that the distribution of HbA1c is reflective of what was observed in the REPOSE Trial. Because 5000 replicated individuals are included in the model from the n = 260 sample, we are able to incorporate heterogeneity of individual outcomes into the cost-effectiveness analysis using statistical modelling of the REPOSE data set. A clinical expert (Senior Clinical Lecturer/Honorary Consultant) commented that few adults with T1DM were able to sustain a HbA1c level of < 31 mmol/mol (5%) for a full year, and that, in the expert’s experience, no adult with T1DM had a HbA1c of > 200 mmol/mol (20.5%). A final consideration was that the lowest HbA1c observed in the DAFNE research database was 30 mmol/mol (4.9%). 27
The effect of pump + DAFNE treatment compared with MDI + DAFNE treatment on HbA1c was estimated using a beta regression. 123 Beta regressions estimate outcome parameters, which are bound by, but do include, a range of 0–1. HbA1c from the trial data was transformed so that a HbA1c level of 29 mmol/mol (4.8%) was equal to zero. The upper limit of HbA1c was taken to be 201 mmol/mol (20.5%). A beta regression estimates two parameters of interest for simulating each individual’s HbA1c response to pump + DAFNE in the model, the mean effect (µ) and a dispersion parameter (φ). The expectation and the variance of each individual’s outcome, yi, are estimated using the following formulae:
To estimate the mean effect on 1-year HbA1c, treatment allocation, baseline HbA1c and centre were included as covariates. To estimate the dispersion parameter in the 1-year HbA1c regression, only baseline HbA1c was included as a covariate. All of the parameters that were included in the mean effect regression were tested as covariates, but were not statistically significant at the 5% level.
To estimate the mean effect on 2-year HbA1c, all of the covariates used to estimate the mean effect on 1-year HbA1c were used, and 1-year HbA1c was included as an additional covariate. To estimate the dispersion parameter, HbA1c at 1 year was used. All of the parameters that were included in the mean effect regression were tested as covariates, but were not statistically significant at the 5% level.
The uncertainty in each individual’s outcome was parameterised using a beta distribution, which was individualised, based on their covariates. Independent beta distributions were fitted to 1- and 2-year HbA1c outcomes, as they had different expectations of the mean effect and the variance in the mean effect in the first and second year. For each individual, two independent random draws were taken: one from their individualised beta distribution for 1-year HbA1c and the other from their individualised beta distribution for 2-year HbA1c, to determine their HbA1c at 1 and 2 years, respectively.
In the base case, it was assumed that if an individual switched treatments then they had a change in HbA1c equal to the difference between the predicted mean effect on HbA1c in their randomised treatment arm and the predicted mean effect on HbA1c in their non-randomised treatment arm. The mean effects were obtained from their individualised outcomes from the beta regressions. In the base case, the estimates of changes in HbA1c were obtained from the per-protocol population, as individuals who switched insulin delivery mechanism were not included in this population. Therefore, treatment effect parameters in this population reflect the relative effectiveness of pump + DAFNE versus MDI + DAFNE for those individuals who did not switch insulin delivery mechanism.
The Model is designed to use a mean HbA1c value, using the DCCT% scale, for each individual in each yearly time cycle. In the base-case analysis, an individual’s HbA1c for the first model cycle (0–1 years) is given by their baseline HbA1c, an individual’s HbA1c for the second model cycle (1–2 years) is given by their 1-year HbA1c sampled from their individualised beta distribution for 1-year HbA1c and an individual’s HbA1c for the third model cycle (2–3 years) is given by their 2-year HbA1c sampled from their individualised beta distribution for 2-year HbA1c. These sampled values of HbA1c on the beta scale were then transformed on to the DCCT% scale for use in the long-term modelling.
The trial population – used to estimate HbA1c effect, changes to HbA1c on treatment switching and the timing of changes in HbA1c – was tested in three deterministic scenario analyses. In the first scenario analysis, the treatment effect was estimated in the ITT population, and when an individual switched insulin delivery mechanism his/her HbA1c still changed so that it was reflective of the other trial arm of REPOSE. In the second, the treatment effect was estimated in the ITT population, but there was no variation in HbA1c changes for those individuals who switched. This scenario was conducted as in the ITT analysis population: individuals who switched insulin delivery mechanism remained in the arm to which they were originally randomised. In the third scenario analysis, HbA1c effects were modelled as occurring one model cycle earlier than they did in the base case. For example, an individual’s 2-year HbA1c was used as their modelled HbA1c value in the second model cycle in the scenario analysis rather than the third model cycle in the base case.
Estimating severe hypoglycaemic events and diabetic ketoacidosis events
To develop the method to incorporate severe hypoglycaemic events and DKA treatment effect evidence into the model, several factors were considered. Data on severe hypoglycaemic events and DKA were collected on an ongoing basis throughout the trial. A summary of the numbers of DKAs and severe hypoglycaemic events is given in Table 8. It can be seen that the number of DKAs and severe hypoglycaemic events declines in the second year on every measure except self-reported DKAs in the MDI + DAFNE arm, where the number of events was the same in both years. As such, the statistical models used in the economic data estimated the incidence of severe hypoglycaemia and DKA in the first and second years separately.
| AE | Year 1 | Year 2 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pump + DAFNE (n = 132) | MDI + DAFNE (n = 128) | Total (n = 260) | Pump + DAFNE (n = 132) | MDI + DAFNE (n = 128) | Total (n = 260) | Pump + DAFNE (n = 132) | MDI + DAFNE (n = 128) | Total (n = 260) | |
| DKAs: SAEs | |||||||||
| Number of (%) participants with ≥ 1 DKA | 15 (11.4) | 1 (0.8) | 16 (6.2) | 4 (3.0) | 2 (1.5) | 6 (2.2) | 17 (12.9) | 3 (2.3) | 20 (7.7) |
| Number of hospital admissions | 16 | 5 | 21 | 5 | 4 | 9 | 21 | 9 | 30 |
| DKAs: self-reported admissions | |||||||||
| Number of (%) participants with ≥ 1 DKA | 17 (12.9) | 6 (4.7) | 23 (8.8) | 6 (4.5) | 5 (3.7) | 11 (4.1) | 18 (13.6) | 8 (6.3) | 26 (10.0) |
| Number of self-reported DKAs | 24 | 11 | 35 | 7 | 11 | 18 | 26a | 13a | 39a |
| Severe hypoglycaemia | |||||||||
| Number of (%) participants with ≥ 1 severe hypoglycaemic event | 10 (7.6) | 9 (7.0) | 19 (7.3) | 4 (3.0) | 7 (5.5) | 11 (4.2) | 14 (10.6) | 11 (8.6) | 25 (9.6) |
| Number of severe hypoglycaemic events | 21 | 12 | 33 | 4 | 12 | 16 | 25 | 24 | 49 |
Negative binomial regressions were used to predict the number of DKAs and severe hypoglycaemic events in years 1 and 2 for each outcome separately. When the outcome variable was the number of hypoglycaemic events in year 1, year-1 HbA1c and treatment group were included as covariates. When the outcome variable was the number of hypoglycaemic events in year 2, year-2 HbA1c and treatment group were included as covariates. When the outcome variable was the number of DKAs in year 1, year-1 HbA1c and treatment group were included as covariates. When the outcome variable was the number of DKAs in year 2, year-2 HbA1c and treatment group were included as covariates. The possibility of using the number of events in the previous year, baseline events for the 1-year outcomes and year-1 events for the 2-year outcomes, as a covariate was explored. However, because of the low number of events, the negative binomial models often did not converge when this was included as a covariate.
The statistical models did not converge for DKAs reported as SAEs in the first year. This was not the case for self-reported DKAs and there were more self-reported cases of DKA than were picked up through the reporting of SAEs. Therefore, the rates of DKA were estimated using self-reported DKAs as the outcome measure.
The statistical models were fitted using the Zellig package in R version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria) and using specifications described above; it was used to simulate the predicted number of severe hypoglycaemia and DKA events in each trial arm 10,000 times. The simulations were separately in each trial arm and for HbA1c values every 0.1% between 4% and 20.5%. The number of events observed in the simulations was truncated at 20 events per year to reduce the effect of extreme values in the simulation on the cost-effectiveness results. These simulations were then used to determine the probability that an individual would suffer a given number of severe hypoglycaemic events and DKA events in 1 year, dependent on their HbA1c that year and the trial arm to which they were allocated. The probability that an individual would suffer a given number of events was a fixed parameter in the PSA; therefore, any differences in the rates of DKA or severe hypoglycaemia for an individual between any two model runs will solely be due to differences in their HbA1c.
In the base case, the statistical models fitted to the incidence of severe hypoglycaemia and DKA in years 1 and 2 were used in the first and second model cycles, respectively, to predict the incidence of severe hypoglycaemia and DKA. The statistical models for year 2 were also used in all subsequent model years because we assumed that year 1 models might not be representative of ongoing event rates because of ‘teething problems’ with treatments given, which are in a sense ‘ironed out’ by year 2. This assumption was based on the clinical opinion of the clinical members of the REPOSE TMG, including honorary consultants in diabetes and diabetes nurse specialists.
In a scenario analysis, individuals in both model arms returned to their baseline rate of severe hypoglycaemia and DKA after the second year. Self-reported information was collected at baseline on the number of severe hypoglycaemic events and DKAs experienced by the individuals in the 12 months prior to baseline data collection. The baseline incidence of these events was estimated using the same methods used to estimate the probability of experiencing these events in year 1 or year 2; however, treatment allocation was not included as covariate. This is because all of the events in the baseline rate models occurred prior to an individual’s randomisation in the REPOSE Trial.
Cost of insulin, diabetes-related contacts and insulin pumps
The cost of insulin, diabetes-related contacts and insulin pumps (including consumables) in the long-term model was estimated based on resource use data from the REPOSE Trial data and the unit costs used in the EEACT (see Resource use by individuals in the REPOSE Trial over the 2-year follow-up period). Statistical models were fitted to these subcomponents of total cost in the EEACT, as it is expected that the covariates that predict the cost of insulin in year 1 may be correlated with the covariates that predict the cost of insulin in year 2. It is also expected that this may be true for the cost of diabetes-related contacts and the cost of insulin pumps (including consumables). Therefore, instead of fitting six independent regression models, three seemingly unrelated regressions were fitted [one seemingly unrelated regression for the cost of insulin, another for the cost of diabetes-related contacts and, finally, one for the cost of insulin pumps (including consumables)].
In the ‘cost insulin seemingly unrelated regression model’, the cost of insulin in year 1 and the cost of insulin in year 2 were used as the outcome variables for the seemingly unrelated regression model. Baseline cost of insulin, baseline HbA1c, treatment allocation, whether or not the individual switched from MDI to insulin pump infusion in year 1 and whether or not the individual switched from insulin pump infusion to MDI in year 1 were included as covariates to predict the cost of insulin in year 1. Baseline cost of insulin, baseline HbA1c, the actual method of insulin delivery that an individual was using at the end of the first year, whether or not the individual switched from MDI to insulin pump infusion in year 2 and whether or not the individual switched from insulin pump infusion to MDI in year 2 were included as covariates to predict the cost of insulin in year 2. The SEs were adjusted for clustering in each DAFNE course. For each individual in the model, their baseline cost of using insulin, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin.
In the ‘cost of diabetes-related contacts seemingly unrelated regression model’, the cost of diabetes-related contacts in year 1 and year 2 were used as the outcome variables for the seemingly unrelated regression model. Baseline cost of diabetes-related contacts, baseline HbA1c, and treatment allocation – whether or not the individual switched from MDI to insulin pump infusion in year 1 and whether or not the individual switched from insulin pump infusion to MDI in year 1 – were included as covariates to predict the cost of insulin in year 1. Baseline cost of diabetes-related contacts, baseline HbA1c, the actual method of insulin delivery that an individual was using at the end of the first year, whether or not the individual switched from MDI to insulin pump infusion in year 2 and whether or not the individual switched from insulin pump infusion to MDI in year 2 were included as covariates to predict the cost of insulin in year 2. The SEs were adjusted for clustering in each DAFNE course. For each individual in the model, their baseline cost of diabetes-related contact resource use, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin pump therapy.
In the ‘cost of insulin pump seemingly unrelated regression model’, the cost of insulin pumps and consumables in year 1 and the cost of insulin pumps and consumables in year 2 were the two outcome variables used in the model. No control was made for baseline resource use or baseline HbA1c for either outcome variable, as no individual in the REPOSE Trial had a previous history of using an insulin pump. The individual’s randomised treatment arm, whether or not they switched from pump to MDI in the first year and whether or not they switched from MDI to pump in the first year were included as covariates to predict the cost of insulin pumps and consumables in year 1. An individual’s actual treatment at the end of the first year, whether or not they switched from pump to MDI in year 2 and whether or not they switched from MDI to pump in year 2 were included as covariates to predict the cost of insulin pumps and consumables in year 2. For each individual in the model, their HbA1c, their treatment at the start of the year and whether or not they switched treatment were used with the parameter values from the regression to predict their cost of insulin pump therapy.
Duration of treatment effectiveness beyond the trial period
A key parameter for the long-term cost-effectiveness modelling is the duration of effectiveness of the two interventions and, in particular, the length of time that HbA1c improvements last. The REPOSE Trial provides data only up to 2 years after randomisation. Therefore, the available literature on the long-term duration of treatment effectiveness for MDI individuals taking a DAFNE course and pump + DAFNE individuals needs to be assessed to determine the assumptions to be used for HbA1c progression beyond the 2-year trial period.
A literature search was conducted for studies on the duration of HbA1c improvements for MDI + DAFNE individuals and insulin pump therapy individuals. Seven potentially relevant studies were identified. Two studies124,125 were identified as being potentially relevant for MDI + DAFNE individuals. Five studies56–59,63 were identified as being potentially relevant for insulin pump therapy individuals. Two studies56,59 were excluded: Beato-Vibora et al. 56 was not included because fewer than one-quarter of the individuals in the initial sample had follow-up data for any given year; Clements et al. 59 was excluded because it was a subgroup analysis of the data presented by Carlsson et al. 58 As such, if Carlsson et al. 58 was included to estimate the long-term duration of treatment effect of pump therapy, the effect estimated from Clements et al. 59 would be given double the weight of the other studies because of a published subgroup analysis being available.
For the five included studies56–59,63 (two studies for adults receiving MDI + DAFNE and three studies for adults receiving pump + DAFNE), the average yearly increase in HbA1c was estimated, pragmatically, using data from the point of largest reduction in HbA1c and the last observation in which the sample size was greater than one-quarter of the initial sample size. A weighted average of these studies’ evidence (using the initial sample size) calculated the mean yearly HbA1c increase for both trial arms (Table 9). The weighted average yearly HbA1c increase for insulin pump therapy individuals was 0.052% per annum. The weighted average yearly HbA1c increase for MDI + DAFNE individuals was 0.054% per annum.
| Study | Treatment group | ||||
|---|---|---|---|---|---|
| Pump | MDI | ||||
| aOrr et al., 201563 | Carlsson et al., 201358 | Bruttomesso et al., 200257 | Gunn and Mansell, 2012125 | Speight et al., 2010124 | |
| Initial sample size | 200 | 272 | 138 | 111 | 104 |
| Baseline HbA1c, % | 8.68 | 8.39 | 9.30 | 8.6 | 9.3 |
| Peak HbA1c improvement, % | –1.18 | –0.43 | –1.34 | –0.5 | –0.6 |
| Time of peak HbA1c improvement, years | 0.5 | 2 | 1 | 1 | 1 |
| Last observed HbA1c improvement with n ≥ 25% the initial sample size, % | –0.49 | –0.20 | –1.31 | –0.37 | –0.4 |
| Time of last HbA1c improvement | 9 years | 5 years | 10 years | 7 years | 44 months |
| Average yearly HbA1c increase (from peak to last observed value) | 0.08 | 0.06 | 0.00 | 0.03 | 0.08 |
| Implied time to baseline (years from baseline) | 9.9 | 8.5 | 408.6 (lifetime effect) | 19.0 | 8.2 |
| Funding | None stated | Region of Gotland in Sweden | None stated | NIHR | Diabetes UK and DAFNE collaborative |
The uncertainty in these long-term changes was parameterised using a normal distribution in the PSA. There was no SD for the mean HbA1c increases across the studies in each model arm; data were obtained from the REPOSE Trial on the SD of the mean change in HbA1c between years 1 and 2. The mean observed change in HbA1c between years 1 and 2 for individuals receiving MDI + DAFNE was –0.08%, with a SD of 0.84%. The mean observed change in HbA1c between years 1 and 2 for individuals receiving pump + DAFNE was –0.09%, with a SD of 0.98%. To estimate the SE for each trial arm, the SD associated with each trial arm was divided by the combined sample size of the studies used to estimate the long-term changes in HbA1c. The estimated mean effect and the estimated SE for each model arm were used to parameterise a normal distribution for the PSA.
In the base-case analysis, data from the studies on the HbA1c increases for MDI + DAFNE individuals and pump individuals were used for each individual’s lifetime. To ensure that each individual could not have implausibly high or low HbA1c values, their HbA1c was constrained so that it could not fall below 4.8% or go above 20.5%.
In addition to these five studies, the cost-effectiveness model used by Riemsma et al. 8 used an annual progression of 0.045% per annum derived from the DCCT trial. This was assumed to apply equally to all comparators analysed in the study. In a further sensitivity analysis it was assumed that individuals would return to their baseline HbA1c at the end of the third year in the model with no further progression of their HbA1c.
Threshold analysis
A two-way price and effectiveness threshold analysis was conducted to assess the HbA1c reduction and/or annual cost reduction necessary to potentially make CSII cost-effective in the UK for the whole UK population of adults with T1DM who are eligible to receive a structured education course, are naive to pump therapy and do not have a preference to receive the pump. A conservative assumption was made, in that all HbA1c changes did not apply to 1-year HbA1c, but did apply to all future years. The treatment effect associated with pump + DAFNE compared with MDI + DAFNE was varied between HbA1c changes of –0.3% and –1.2%, in –0.1% increments.
There were two methods used for estimating the change in HbA1c due to receiving pump therapy. In the first method, the following steps were taken: (1) all individuals’ HbA1c values were estimated as if they were a MDI + DAFNE recipient and then (2) a treatment effect (HbA1c change) of pump + DAFNE versus MDI + DAFNE was inputted into the model. In the second method (1) the reduction in HbA1c was applied to the individual’s mean effect in the beta regression; (2) this reduction in HbA1c resulted in a different variance to an equivalent MDI patient as their mean effect was lower; and (3) HbA1c was sampled from the individualised beta distribution, which reflected the mean effect and variance parameters. The second method of conducting the threshold analysis will help future investigators to understand the effect of including heterogeneity in an individual’s response to CSII on the HbA1c reductions that are required to make CSII cost-effective. However, it should be noted that this method assumes that the heterogeneity is defined by the equations estimated from the REPOSE Trial and, as such, may not be valid if CSII were to be clinically more effective.
In both scenario analyses, the cost of insulin pumps and insulin pump consumables was changed from 100% of the mean cost obtained from the pump costing survey to 50% of the mean price observed at REPOSE sites in 5% price reduction increments. Deterministic model runs were used to produce all of the results in the threshold analysis.
It should be noted that other than the method used to estimate HbA1c, all of the other parameters were the same, and assumptions were the same as were used in the base case. As all assumptions were the same as those presented for the base-case analysis, all individuals who switched from MDI + DAFNE to pump + DAFNE received the HbA1c associated with pump + DAFNE, and the individuals who switched from pump + DAFNE to MDI + DAFNE received their HbA1c associated with MDI + DAFNE. This means that the modelled HbA1c reductions are equivalent to per-protocol analysis (treatment switchers removed) rather than an ITT analysis (treatment switchers included in their originally randomised groups) of any future study of pump + DAFNE versus MDI + DAFNE.
As no study other than REPOSE has been conducted to assess the cost-effectiveness of pump + DAFNE versus MDI + DAFNE for adults in the UK with T1DM, the results should are indicative of the HbA1c reductions that pump + DAFNE would need to achieve if it were to be deemed cost-effective compared with MDI + DAFNE.
Methods for the psychosocial evaluation
Aims and objectives
As noted in Chapter 2, evidence on QoL effects of the pump has been inconsistent, with some studies reporting no difference between the pump and MDI groups, and others reporting improved QoL on the pump. A previous HTA report identified some gains in QoL that could be described as ‘social related’ rather than ‘health related’. 14 These included flexibility of lifestyle and fewer problems dealing with variations in daily life, such as timing of meals. For this reason, we included a range of psychosocial measures alongside embedded qualitative research in the REPOSE Trial.
The psychosocial study employed a mixed-methods quantitative (questionnaires) and qualitative (interviews) approach to:
-
establish whether or not, and why, there were any differences in QoL and other psychological or psychosocial outcomes between participants using pump and MDI regimens
-
examine whether or not, and why, QoL and other outcomes changed over time
-
understand and explore the added benefit (if any) of pump technology over MDI from participants’ and educators’ perspectives
-
explore why some patients may do better than others using the pump
-
examine acceptability of, and reasons for, discontinuing (pump) treatment
-
enhance understanding and assist in the interpretation of trial outcomes.
Quantitative methods
Validated and reliable questionnaires were used to assess generic and health-specific QoL, treatment satisfaction, fear of hypoglycaemia, hypoglycaemia unawareness, self-efficacy, social support, adherence to treatment, emotional well-being and acceptability of technology. A repeated-measures longitudinal questionnaire study explored both differences in outcomes between the two trial arms and the short- and long-term predictors and mediators of outcomes. Outcomes were measured at baseline and at 6, 12 and 24 months after the DAFNE course. These time points were selected to capture both short- and long-term post-treatment changes in psychosocial outcomes. Questionnaires were posted to participants and self-completed within 6 weeks of the specified time point.
Outcomes
Quantitative psychosocial end points were measured via participant self-completed questionnaires, which included items assessing QoL (generic and diabetes specific), fear of hypoglycaemia, treatment satisfaction and emotional well-being. There has been limited examination of the impact of pump therapy on these areas, on how and why these may change over time, and why individuals are able or unable to use pump therapy to improve glycaemic control. Rubin and Peyrot126 reviewed the evidence on ‘patient-reported outcomes’ and concluded that, at present, there is little evidence that pump therapy improves them.
Diabetes-specific QoL was assessed using the DSQOL, a reliable and valid measure. 127 Specifically designed for the German study on which UK DAFNE is based, it was included to facilitate important comparisons between the UK and German studies. In addition, generic measures of QoL, the World Health Organization Quality of Life Abbreviated Questionnaire (WHOQOL-BREF)128 and functional health status using the SF-12129 and EQ-5D130 were used. The SF-12 was used to facilitate comparison with ‘healthy controls’ and other long-term conditions.
The HFS131 is a well-validated psychometric tool assessing participants’ fear of hypoglycaemia, both overall and separately, for behaviour and worry. A specific benefit to the survey is that it may be able to identify participants who are likely to maintain high blood glucose levels, thus aiding understanding of potential reasons for poor glycaemic control. A study by Nixon and Pickup,132 in people who had been using a pump for an average of 5 years, found that fear of hypoglycaemic episodes remained a problem.
The DTSQ133 has proven to be highly sensitive in clinical trials. 134 Treatment satisfaction refers to an individual’s subjective appraisal of their experience of treatment, including ease of use, side effects and efficacy. Improvements in satisfaction are not necessarily accompanied by improvements in QoL; treatment satisfaction can be high despite diabetes having a negative impact on QoL, which is why it is important to measure both separately.
The Hospital Anxiety and Depression Scale (HADS)135 measures anxiety on one subscale and depression on another through the use of seven questions for each characteristic. It was important to measure emotional well-being in the trial, as participants may find it easier to manage their condition after DAFNE education or with one of the treatments. This could have a substantial effect on their emotional well-being, which the QoL measures are not sensitive enough to pick up.
The DAFNE Principles Questionnaire was completed at 24 months only. This questionnaire (12 items) assesses the impact of the DAFNE course on self-management behaviours, such as bolus and basal rate changes, correction dose practices, timing of injections/bolus doses and review of blood glucose data. It was included partly in order to establish if there were differences in self-management practices between participants in the pump and MDI arms, to aid interpretation of the final trial findings. The DAFNE Principles Questionnaire was administered to all of the participants irrespective of treatment group. This measure was previously used in DAFNE. 78
Statistical power was calculated for the primary outcome of HbA1c, thus the psychosocial outcomes are either over- or underpowered, depending on the underlying effect size. Statistically significant results are considered in combination with qualitative data in order to answer the key psychosocial research aims.
Statistical analysis of questionnaire data
Short Form questionnaire-12 items
The Physical Component Summary was calculated using physical functioning, body pain, role physical and general health domain scores. The Mental Component Summary was calculated using vitality, social functioning, role emotional and mental health domain scores. When the questionnaires were only partially completed, missing items were imputed using a single imputation procedure based on the mean calculated from complete items on that domain. 136 The scores were standardised and scaled to range from 0 to 100, with higher scores representing better outcomes. 129,137
Diabetes-specific quality of life
The DSQOL domain scores (social relations, leisure time restrictions and flexibility, physical complaints, worries about the future, daily hassle or functions, diet restrictions) and DSQOL total score were calculated if at least 80% of the items from the domain were complete, using the following formula:
Preference-weighted treatment satisfaction was calculated by multiplying the various treatment goals with the corresponding degree of satisfaction (scores of –2.5 = totally dissatisfied to 2.5 = extremely satisfied) and summing the results.
Finally, all DSQOL scores were converted to a 0–100 scale, in which a higher value means worse outcome (more burden) on all scores.
World Health Organization Quality of Life Abbreviated Questionnaire
Four subdomains of WHOQOL-BREF were calculated (physical health, psychological, social relationships and environment) if at least 80% of the questions in that domain were present. The domains were scored by calculating the mean of the items within each domain, and scaling to range from 0 to 100,138 with higher scores representing better outcomes.
Hypoglycaemia Fear Survey
The HFS behaviour and worry scores were calculated if at least 80% of items within that domain were complete, using Equation 5 (see Diabetes-specific quality of life). 131
The HFS behaviour score ranges from 10 to 50 and the HFS worry score ranges from 17 to 85; in both cases, higher scores represent more fear.
Diabetes Treatment Satisfaction Questionnaire
The DTSQ, which measured satisfaction with diabetes treatment, was administered at baseline and 6- and 24-month follow-up. The DTSQc [Diabetes Treatment Satisfaction Questionnaire (change)], which measures change in satisfaction from pre-trial treatment, was administered at 12 months’ follow-up.
Treatment satisfaction (DTSQ) and treatment satisfaction change (DTSQc) were calculated if at least five of the six items were complete using the following formula:
For the treatment satisfaction domain, higher scores represent higher satisfaction (range 0 to 36 on DTSQ and –18 to 18 on DTSQc). Two further domains, perceived frequency (change) in hyperglycaemia and perceived frequency (change) in hypoglycaemia, were calculated based on single items. Only complete data were used for these scores and low scores represent good perceived blood glucose control (scoring ranges of 0 to 6 in DTSQ, and –3 to 3 in DTSQc).
Hospital Anxiety and Depression Scale
Anxiety and depression domain scores were calculated by summing the items in the respective domains. Mean value imputation based on the other six items of a domain was used to impute missing data if a single item was missing. If more than one item was missing then the domain score was not calculated. The HADS scores range from 0 to 21, with higher scores indicating more anxiety/depression (scoring: normal is 0–7; borderline abnormal 8–10; 11–21 abnormal). 135
EuroQol-5 Dimensions
The EQ-5D-3L tariff was derived from five three-level questions using UK norms. The tariff was calculated only if all five questions were answered. It is measured on a scale from –0.56 to 1.00 (good health).
The availability of questionnaire outcome data was summarised for each time point.
The DTSQ domains at 6, 12 and 24 months post course were compared between the treatment groups using a non-parametric Wilcoxon–Mann–Whitney U-test. The median and interquartile range (IQR) of change from baseline for the DTSQ domains at 6 and 24 months, and the median and IQR score for the DTSQc domains at 12 months, are displayed by treatment group. The differences between groups post course are displayed as the median difference (in change from baseline for DTSQ scores) with its associated 95% CI, which was calculated as described in the study by Newson. 139
Other QoL outcomes (DSQOL, SF-12, WHOQOL-BREF, HFS, EQ-5D, HADS) at 6 months post course are compared between the treatment groups using a mixed-effects linear regression model of change from baseline adjusted for DAFNE course (random effect), baseline HbA1c, baseline score and centre. The means and SDs for the treatment and control groups with adjusted MDs and associated CIs and p-values (unadjusted for multiple testing) are reported. This analysis is repeated for the 12- and 24-month outcomes. A complementary sensitivity analysis, in which the analysis described above was repeated only including patients with complete data, was performed.
Qualitative methods
Study design
An inductive, thematic approach was used, informed by the principles of Grounded Theory research. 140 This entailed concurrent data collection and analysis, allowing findings and themes arising from early phases of data collection to inform the areas explored in later phases, as well as sampling. In-depth interviews, informed by topic guides, were used as the main method of data collection, as these helped to ensure that the discussion remained relevant to areas under investigation, while affording the flexibility needed for participants to raise and discuss issues that they perceived as salient, including those unforeseen at the study’s outset. 141,142
Patient participants, recruited from both trial arms, were interviewed at two time points: within 2 weeks of completing their DAFNE courses (round 1) and 6 months later (round 2). This longitudinal design permitted patients’ initial understandings and experiences of using the pump and MDI regimens to be explored, and any continuities and changes in their diabetes self-management practices to be tracked and compared over time. Six months was selected as the time point for follow-up to coincide with collection of 6-month clinical and psychological data, and because previous experience of undertaking longitudinal qualitative research with DAFNE graduates had demonstrated that this allowed sufficient time to establish whether, and for what reasons, patients are able/unable to put their skills training into practice. 143–146 In addition, cost considerations (including a request by the funder to reduce the costings for the qualitative component prior to the protocol being finalised) meant that it was not possible to do follow-up interviews with patients at later time points.
Educators were interviewed once, following completion of their centre’s sixth REPOSE DAFNE course. This time point was chosen to avoid any risk of inadvertent contamination of the trial intervention by the qualitative questioning, and also because, at this point, it was anticipated that staff would have had considerable experience of trial recruitment and delivery on which they could reflect.
Recruitment and sampling
As per the original protocol, participants (patients and educators) were recruited from seven of the eight trial centres (with roughly equal numbers recruited from each centre); recruitment to the qualitative research was not undertaken in the eighth centre (Nottingham), as this centre came on board only in the later phases of the trial and recruited patients to only one set of courses.
When they were consented to take part in the trial, patient participants were asked whether or not they would be willing to be approached to take part in the qualitative research (see Appendix 8). Of the 317 patients who were randomised, 315 (99.37%) agreed to be approached. Participants who gave this agreement were purposively sampled so that both those randomised to pump and MDI arms of the trial were recruited and there was broad, and roughly equal, representation of ages, sex, diabetes duration and occupational/socioeconomic groups in the final sample.
It was initially planned that one nurse and one dietitian would be recruited and interviewed from each of the seven main trial centres. However, after initial interviews had been conducted and analysed, a decision was made to increase the number of nurse educators interviewed. This is because the initial interviews had made apparent that these staff members tended to have the greatest involvement in recruitment and notifying patients of the outcome of randomisation, and, as reported elsewhere,147 these aspects of trial work proved to be particularly challenging for staff. Educators were sent recruitment packs and invited to ‘opt in’ to the study; all of those approached agreed to take part.
Recruitment of patients and educators continued until data saturation occurred, that is until no new findings or themes were identified in new data collected. All participants provided written consent prior to their interviews.
Data collection
Baseline interviews with patients were undertaken face to face to establish rapport and were conducted at a time and location convenient to them (mostly their own homes). Follow-up interviews were done by telephone (again at a time most convenient to the interviewee). There was no apparent difference in the quality and disclosure of information between interviews undertaken face to face and those done on the telephone. All staff opted to be interviewed by telephone.
Topic guides for the patient interviews were developed in light of literature reviews, course observations, inputs from the trial team and patient representatives, and revised in light of emerging findings. Full details of the topics explored in patients’ round 1 and round 2 interviews are provided in Appendix 9. In brief, round 1 interviews explored patients’ understandings of the trial and the pump, and their reasons for agreeing to take part; their views about the outcome of randomisation; and their early experiences of using a MDI or pump regimen to undertake diabetes self-management practices following course attendance and training in DAFNE principles. Round 2 interviews were used to explore whether or not, how and why patients’ experiences of managing their diabetes had changed since their last interview (including reasons for adhering or not adhering to treatment recommendations, discontinuing treatment, etc.); how the use of their regimen (pump or MDI) had impacted on their perceptions of their diabetes, their confidence and perceived ability to undertake diabetes self-management practices; and their everyday (work and family) lives. Although broadly the same areas were explored in the follow-up interviews, each participant’s round 1 interview account was reviewed before their round 2 interview was undertaken to enable follow-up of specific issues raised by particular individuals.
Staff interviews explored their experiences of recruiting into the REPOSE Trial, delivering the 5-day courses and undertaking patient follow-up as part of the trial; perceptions of patients’ engagement with pump therapy compared with MDI during the trial; previous experiences (if any) of using insulin pumps in routine clinical practice; and views about the potential benefits of the pump compared with MDI regimens. In light of emerging findings, staff were also invited to reflect on whether or not their views about the potential benefits, and beneficiaries, of insulin pumps had changed in light of their experiences of delivering, and observing, patients during the REPOSE Trial. Full details of the areas explored in the staff interviews are also contained within Appendix 9.
Interviews with patients were conducted between June 2012 and June 2013, and those with staff between December 2012 and April 2013. All interviews averaged 1 hour, were digitally recorded and transcribed in full for in-depth analysis.
Data analysis
Data were analysed thematically using the method of constant comparison. 148 This entailed members of the qualitative research team reading patient and educator transcripts (which were treated as ‘stand-alone’ data sets) repeatedly before cross-comparing them to identify issues and experiences that cut across different patient and educator accounts. To address the study aims and objectives, a longitudinal analysis of the patient data was also undertaken. Each individual’s round 1 and round 2 accounts were cross-compared and attention paid to continuities and changes in their experiences, views and diabetes self-management practices (using pump or MDI) over time, and the reasons for these. A key aspect of this analysis also focused on comparison of the (longitudinal) accounts of patients using pump and MDI regimens. This was done to better understand the impact of using pump (compared with MDI) regimens on patients’ diabetes self-management practices, disease perceptions and everyday life.
Members of the qualitative team undertook their own independent analyses and wrote separate reports before meeting (both during and after data collection) to compare their interpretations, discuss discrepant cases, and reach agreement on recurrent themes and findings. For both patient and educator interviews, a final coding frame, which reflected the original study aims/questions and emergent themes, was developed once all of the data had been reviewed and consensus reached on key themes and findings. NVivo9 (Doncaster, VIC, Australia), a qualitative software package, was used to facilitate data coding and retrieval. Coded data sets were subjected to further analyses to allow for the identification of subthemes and illustrative quotations.
Confidentiality
To protect participants’ identities, each individual was allocated a unique identifier and these identifiers are used in the reporting of interview data. In the case of staff, ‘N’ is used to refer to a nurse and ‘D’ to a dietitian. In the case of patients, data are tagged with the participant’s treatment arm (‘M’ for MDI, ‘P’ for pump), identifying number and interview round (e.g. ‘M7.2’ refers to the second interview with MDI participant 7).
Chapter 4 Changes to the protocol
All study amendments are listed in Appendix 10. The most significant changes are explained below.
Inclusion/exclusion criteria
As NICE guidance advises that all patients who have poor diabetic control are considered for pump therapy, early concern was raised at the TMG regarding the potential for inclusion of individuals who had a definite need for pump therapy. Such participants were not the intended trial population for REPOSE. Prior to the start of recruitment, the inclusion and exclusion criteria were therefore changed in order to clarify that suitable participants were those who, in the opinion of the investigator, have a need for structured education to optimise their diabetes control, but do not have a clear indication for pump therapy. In the early stages of recruitment (January 2012), the criteria were clarified to exclude those who have a strong need for pump therapy. Further clarifications of the exclusion criteria for patients who have used pump therapy within the last 3 years were made in April 2012, defined as > 2 weeks’ use within the last 3 years. Following early site monitoring visits in July 2012 some further minor clarifications were made to the exclusion criteria to confirm that a severe needle phobia must preclude full participation in either treatment arm or influence the participants’ preference for pump therapy, and an unstable psychological condition must be active enough to preclude the participant safely taking part in the trial.
Recruitment target
The ITT population was defined as participants who consent to take part in the trial and who attend their DAFNE course at least in part. Although the trial was on course to meet the set recruitment target, it was noted that larger than anticipated numbers were withdrawing consent prior to the DAFNE course, resulting in lower than anticipated numbers eligible for the ITT analysis. The trial statistician undertook a review in August 2012 to determine the need for additional DAFNE courses and participants to maintain study power. Scenarios were modelled based on the current and predicted HbA1c population prevalence of ≥ 7.5%, dropout rate (10% or 15%) and size of DAFNE course (four, five, six or seven participants). Assuming that these variables remained similar to those observed (as at August 2012), it was estimated that the trial would need to run an additional two to seven courses in order to maintain power for the primary outcome. Therefore, a reserve centre (Nottingham University Hospitals NHS Trust) was initiated. The target recruitment was increased to ‘no more than 340 participants’, with 280 expected to attend the DAFNE course (as originally planned).
Data collection procedures
The study power was calculated on a 90% retention rate at 24 months. Although in May 2013 the 6-month participant retention was high (95%), the trial team anticipated challenges in maintaining this rate at 24 months. To ensure that follow-up rates remained adequate, we added the option for site staff to collect appropriate data from participants over the telephone when participants had been unable to attend for follow-up. Furthermore, we included the possibility of obtaining outcome data from participants’ medical records, for which participants had given consent. We also added an option for data to be collected at participants’ homes or appropriate NHS location, if they had been unable to attend at their centre.
Diabetic ketoacidosis/illness letter to participants
Episodes of DKA were expected to occur in some REPOSE participants – as this is a known complication for individuals with T1DM – and were reported as SAEs. The TMG, TSC and DMEC regularly reviewed all SAEs and it was noted early in the trial that some centres were reporting an unexpectedly high number of DKA events. The DMEC reviewed the events and, although the numbers were not considered a major concern, recommended that a troubleshooting document be issued to participants as a precautionary measure, which was then agreed by the TSC. This provided written guidelines on how to manage illness. These were sent to all participants, following approval by the REC in August 2013. The pump troubleshooting document was based on a hand-out already issued to participants during the DAFNE education course and an equivalent version was provided to MDI participants (see Appendix 11). The letter also served as a reminder for participants to contact their diabetes team regarding any problems that they may be experiencing and to report any adverse health events that may have occurred.
The 24-month letter incorporating information about severe hypoglycaemia reporting
In October 2011 the UK Driver and Vehicle Licensing Agency released new medical standards for people with diabetes, containing stricter rules advising that people experiencing more than one severe episode of hypoglycaemia in 1 year should not drive. It was noted that reporting of severe hypoglycaemic events during the trial had been low and the TMG had concerns that patients may have been under-reporting events. The letter issued to all participants at 24 months – reminding them of their appointment and enclosing a copy of the psychosocial questionnaire for completion – was updated to reassure them that all of the information provided as part of the trial ‘is kept completely anonymous and not sent to any organisation where participants could be identified’ (see Appendix 12).
Bolus calculators letter
Participants on both arms of the trial were provided with bolus calculators. These devices help patients to calculate the correct pre-meal insulin dose to inject. Patients on MDI therapy would not always be provided with these devices; however, this was deemed necessary to reduce any potential bias, as pump participants had access to a bolus calculator via the pump. The qualitative research undertaken post course, and at 6 months, had indicated that some patients had become de-skilled and dependent on the devices, whereas others misunderstood the need to change the parameters, believing they had been pre-programmed during the DAFNE course. In some cases this could have been leading to ineffective management of their diabetes, potentially affecting their health. The issue was discussed with the trial DMEC, who suggested that a brief, light-touch intervention was administered to all of the participants in the trial, highlighting effective use of the devices. The TSC chairperson agreed with this action. Therefore, as a precautionary safety measure, in January 2014 we sent a document to all of the participants in the trial detailing appropriate use of the devices (see Appendix 13).
Chapter 5 Results of the randomised controlled trial
Trial recruitment
Participant recruitment initially took place at seven centres between November 2011 and December 2012. A review of recruitment and retention in August 2012 revealed higher than expected dropout rates prior to the DAFNE courses. The recruitment target was therefore increased to a maximum of 340, but with no more than 280 in the ITT population. In order to achieve this we facilitated an additional pair of DAFNE courses at an existing centre (Harrogate) and introduced a reserve centre (Nottingham) to facilitate a further two courses. Recruitment continued until April 2013. Figure 2 illustrates recruitment and course attendance rates against targets. Table 10 summarises course attendance by treatment group, with similar mean participant numbers per course. Table 11 shows recruitment details by centre.
FIGURE 2.
Participant recruitment and attendance targets and rates.
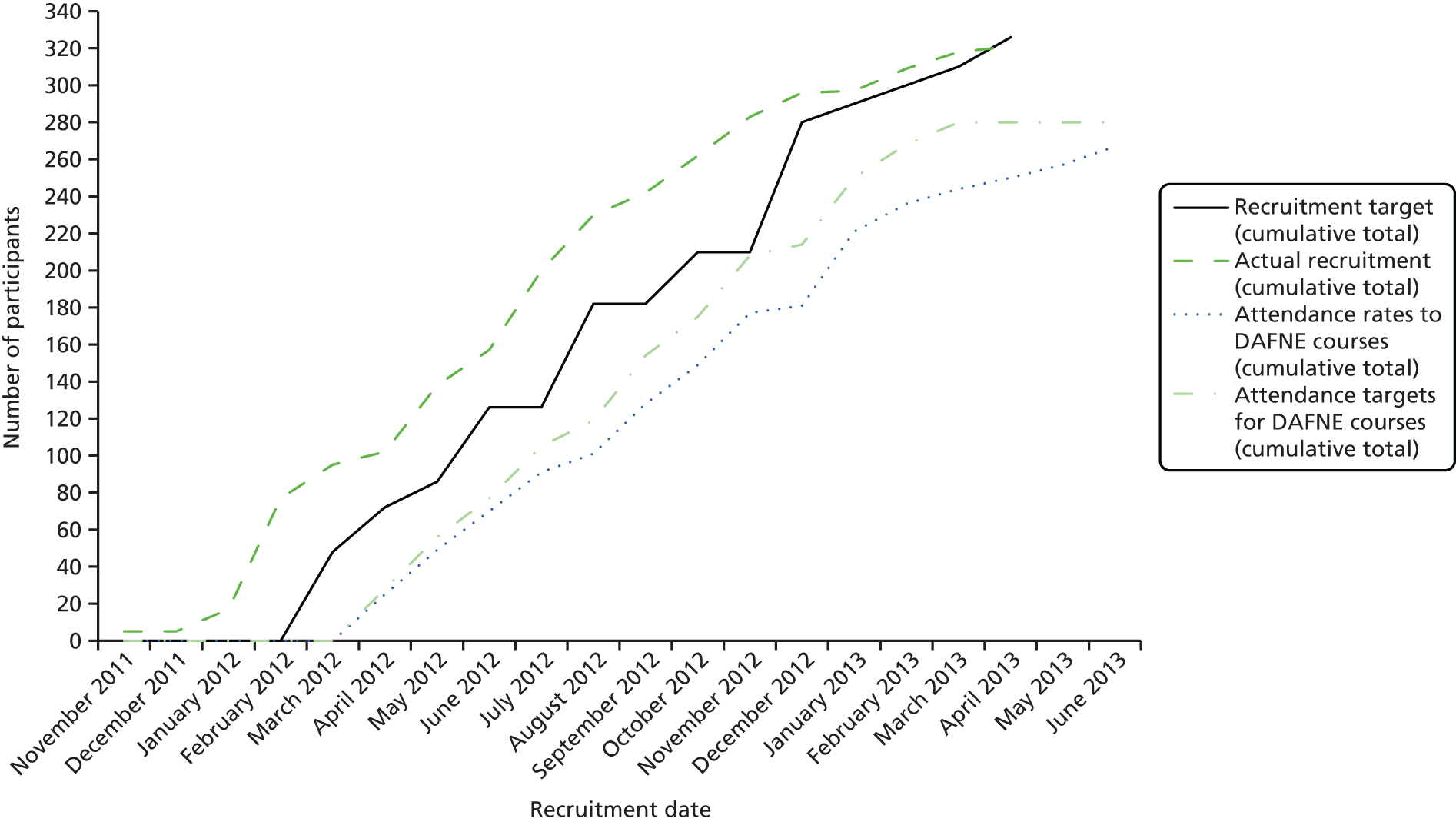
| Summary of course attendance | Treatment group | ||
|---|---|---|---|
| MDI | Pump | Total | |
| Mean number of participants (SD) | 5.87 (1.39) | 5.74 (1.39) | 5.80 (1.38) |
| Median number of participants (IQR) | 6 (5–7) | 6 (5–7) | 6 (5–7) |
| Minimum to maximum | 3–8 | 3–8 | 3–8 |
| Centres | Number of courses per centre | Number of participants attended |
|---|---|---|
| Sheffield Teaching Hospitals NHS Foundation Trust | ||
| Course 1: MDI | 7 | |
| Total number of participants recruited: 41 | Course 2: Pump | 7 |
| Recruited: | Course 3: MDI | 5 |
| First participant 10 January 2012 | Course 4: Pump | 4 |
| Last participant 19 October 2012 | Course 5: MDI | 8 |
| Course 6: Pump | 7 | |
| NHS Greater Glasgow and Clyde | ||
| Course 1: Pump | 8 | |
| Total number of participants recruited: 45 | Course 2: MDI | 6 |
| Recruited: | Course 3: Pump | 7 |
| First participant 1 February 2012 | Course 4: MDI | 8 |
| Last participant 27 November 2012 | Course 5: MDI | 5 |
| Course 6: Pump | 6 | |
| King’s College Hospital NHS Trust | ||
| Course 1: Pump | 5 | |
| Total number of participants recruited: 41 | Course 2: MDI | 6 |
| Recruited: | Course 3: MDI | 5 |
| First participant 12 February 2012 | Course 4: Pump | 3 |
| Last participant 5 December 2012 | Course 5: Pump | 6 |
| Course 6: MDI | 4 | |
| Cambridge University Hospitals NHS Foundation Trust | ||
| Course 1: Pump | 6 | |
| Total number of participants recruited: 43 | Course 2: MDI | 4 |
| Recruited: | Course 3: MDI | 7 |
| First participant 23 November 2011 | Course 4: Pump | 5 |
| Last participant 20 December 2012 | Course 5: Pump | 8 |
| Course 6: MDI | 4 | |
| Harrogate and District NHS Foundation Trust | ||
| Course 1: Pump | 6 | |
| Total number of participants recruited: 55 | Course 2: MDI | 7 |
| Recruited: | Course 3: Pump | 7 |
| First participant 28 February 2012 | Course 4: MDI | 6 |
| Last participant 10 April 2013 | Course 5: MDI | 7 |
| Course 6: Pump | 5 | |
| Course 7: Pump | 7 | |
| Course 8: MDI | 6 | |
| NHS Dumfries and Galloway | ||
| Course 1: Pump | 5 | |
| Total number of participants recruited: 41 | Course 2: MDI | 7 |
| Recruited: | Course 3: Pump | 7 |
| First participant 3 February 2012 | Course 4: MDI | 4 |
| Last participant 2 October 2012 | Course 5: MDI | 3 |
| Course 6: Pump | 4 | |
| NHS Lothian | ||
| Course 1: MDI | 7 | |
| Total number of participants recruited: 43 | Course 2: Pump | 5 |
| Recruited: | Course 3: Pump | 6 |
| First participant 8 May 2012 | Course 4: MDI | 6 |
| Last participant 20 November 2012 | Course 5: MDI | 7 |
| Course 6: Pump | 4 | |
| Nottingham University Hospitals NHS Trust | ||
| Course 1: MDI | 6 | |
| Total number of participants recruited: 12 | Course 2: Pump | 4 |
| Recruited: | ||
| First participant 21 February 2013 | ||
| Last participant 15 March 2013 | ||
| Total recruited: 321 | Total attendance | 267 |
Participant flow
Figure 3 shows the CONSORT flow of participants through the trial. In total 1278 people were invited to take part, of which 885 responded. Of these responders, 362 were interested in taking part. Reasons given for non-participation are listed in Table 12. Of those interested, 334 were assessed as eligible and 321 of these consented to take part. Four of these dropped out prior to randomisation. Forty-six courses (23 course pairs) were randomised, comprising 317 participants (156 pump and 161 MDI). Fifty patients were excluded from the analysis: 40 patients withdrew before baseline data were collected and 10 withdrew before they attended a DAFNE course. All randomised courses were delivered. 23 One participant was deemed protocol non-compliant, as he/she had not adhered to the DAFNE course (as adjudicated by the course leader). Of the 267 participants (132 pump and 135 MDI) who were randomised, attended baseline visit and attended a DAFNE course, 260 (132 pump and 128 MDI) had HbA1c data for at least one post-baseline follow-up visit and these make the ITT set. A total of 248 participants (128 pump and 120 MDI) had complete primary outcome data at 24 months’ follow-up. 23
FIGURE 3.
The CONSORT flow diagram. Note: for footnotes, see Table 12. Reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
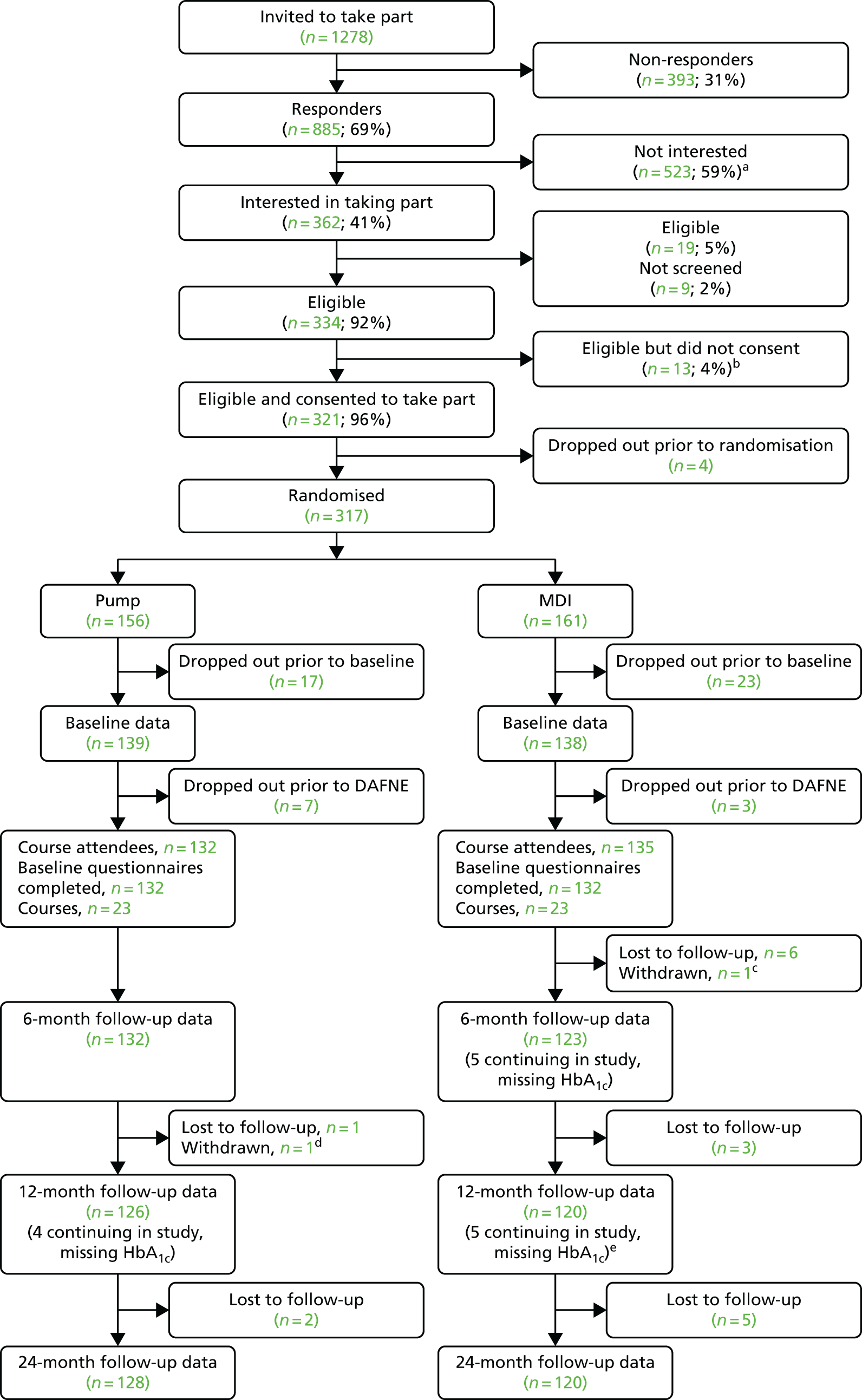
| Reason | n (%) |
|---|---|
| (a) Most common reasons for non-participation based on 521 completed forms (multiple reasons per individual) | |
| Not interested in having a pump | 189 (36) |
| Could not take week off work | 123 (24) |
| Satisfied with my current treatment and management of diabetes | 93 (18) |
| Lack of time | 76 (15) |
| No reason documented/provided | 29 (6) |
| Other | 27 (5) |
| Does not meet eligibility criteria | 26 (5) |
| Difficulty travelling to the course | 21 (4) |
| Dependants at home | 19 (4) |
| Moving/moved away from area/transferred care | 16 (3) |
| Not willing to take part if not receiving a pump | 13 (2) |
| Not interested in REPOSE | 13 (2) |
| (b) Reasons for non-participation, based on seven completed forms (multiple reasons per individual) | |
| Not interested in having a pump | 2 (29) |
| Lack of time | 2 (29) |
| Could not take week off work | 2 (29) |
| Other | 1 (14) |
| Satisfied with my current treatment and management of diabetes | 1 (14) |
| Not willing to take part if not receiving a pump | 1 (14) |
| Medical reasons: | 1 (14) |
| (c) Participant does not wish to continue because of personal/family issues | |
| (d) Participant does not wish to continue (switched from pump to MDI, did not like the practicalities of pump therapy) | |
| (e) Of the five participants continuing in study at 12 months – but without 12-month data – three had available 6-month data, two had no 6-month data | |
Baseline data
Table 13 shows the baseline demographics and characteristics of the trial population. Overall, eight centres recruited to the study contributing between 10 and 51 participants per centre. Patients were more likely to be male (60%) and were generally white British (91%). The average age of participants was 41 years.
| Variable | Scoring | Treatment group | ||
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | Total (N = 267) | ||
| Recruitment centre, n (%) | London (King’s College Hospital) | 14 (10.6) | 15 (11.1) | 29 (10.9) |
| Sheffield | 18 (13.6) | 20 (14.8) | 38 (14.2) | |
| Glasgow | 21 (15.9) | 19 (14.1) | 40 (15.0) | |
| Dumfries | 16 (12.1) | 14 (10.4) | 30 (11.2) | |
| Cambridge | 19 (14.4) | 15 (11.1) | 34 (12.7) | |
| Harrogate | 25 (18.9) | 26 (19.3) | 51 (19.1) | |
| Edinburgh | 15 (11.4) | 20 (14.8) | 35 (13.1) | |
| Nottingham | 4 (3.0) | 6 (4.4) | 10 (3.7) | |
| Sex, n (%) | Male | 78 (59.1) | 82 (60.7) | 160 (59.9) |
| Female | 54 (40.9) | 53 (39.3) | 107 (40.1) | |
| Smoking status, n (%) | Smoker | 23 (17.4) | 30 (22.2) | 53 (19.9) |
| Ex-smoker | 42 (31.8) | 27 (20.0) | 69 (25.8) | |
| Never smoker | 67 (50.8) | 78 (57.8) | 145 (54.3) | |
| Ethnicity, n (%) | White Britisha | 125 (94.7) | 119 (88.1) | 244 (91.4) |
| Irish | 1 (0.8) | 0 (0.0) | 1 (0.4) | |
| Any other white background | 1 (0.8) | 3 (2.2) | 4 (1.5) | |
| Indian | 1 (0.8) | 1 (0.7) | 2 (0.7) | |
| Chinese | 0 (0.0) | 1 (0.7) | 1 (0.4) | |
| African | 0 (0.0) | 1 (0.7) | 1 (0.4) | |
| Caribbean | 0 (0.0) | 1 (0.7) | 1 (0.4) | |
| Arab | 0 (0.0) | 1 (0.7) | 1 (0.4) | |
| Any other ethnic group | 0 (0.0) | 4 (3.0) | 4 (1.5) | |
| Prefer not to say | 1 (0.8) | 3 (2.2) | 4 (1.5) | |
| Missing | 3 (2.3) | 1 (0.7) | 4 (1.5) | |
| ONS occupational status,b n (%) | Level 1 | 32 (24.2) | 24 (17.8) | 56 (21.0) |
| Level 2 | 37 (28.0) | 43 (31.9) | 80 (30.0) | |
| Level 3 | 39 (29.5) | 46 (34.1) | 85 (31.8) | |
| Level 4 | 12 (9.1) | 14 (10.4) | 26 (9.7) | |
| Not classifiable | 4 (3.0) | 2 (1.5) | 6 (2.2) | |
| Missing | 8 (6.1) | 6 (4.4) | 14 (5.2) | |
| Highest qualification obtained, n (%) | No formal qualifications | 7 (5.3) | 8 (5.9) | 15 (5.6) |
| GCSE level | 24 (18.2) | 26 (19.3) | 50 (18.7) | |
| A-level | 10 (7.6) | 8 (5.9) | 18 (6.7) | |
| Vocational qualification | 40 (30.3) | 32 (23.7) | 72 (27.0) | |
| Undergraduate degree | 25 (18.9) | 32 (23.7) | 57 (21.3) | |
| Postgraduate degree | 15 (11.4) | 17 (12.6) | 32 (12.0) | |
| Other | 9 (6.8) | 9 (6.7) | 18 (6.7) | |
| Missing | 2 (1.5) | 3 (2.2) | 5 (1.9) | |
| Age (years) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 41.5 (14.2) | 39.9 (12.5) | 40.7 (13.4) | |
| Median (IQR) | 40.7 (27.9–52.3) | 41.0 (28.0–48.8) | 40.8 (28.0–49.4) | |
| Minimum to maximum | 18.5–77.6 | 18.5–73.1 | 18.5–77.6 | |
| Body weight (kg) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 82.4 (18.2) | 80.0 (17.4) | 81.2 (17.8) | |
| Median (IQR) | 81.2 (69.1–91.6) | 78.1 (67.0–91.0) | 79.6 (68.4–91.2) | |
| Minimum to maximum | 50.4–144.8 | 46.5–148.4 | 46.5–148.4 | |
| BMI (kg/m2) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 27.4 (5.0) | 27.0 (5.0) | 27.2 (5.0) | |
| Median (IQR) | 27.1 (23.8–29.7) | 26.6 (23.5–29.2) | 26.9 (23.7–29.5) | |
| Minimum to maximum | 17.4–47.9 | 17.2–45.9 | 17.2–47.9 | |
| HFS behaviour score | n (%) | 130 (98.5) | 132 (97.8) | 262 (98.1) |
| Mean (SD) | 30.3 (5.8) | 29.1 (5.5) | 29.7 (5.7) | |
| Median (IQR) | 29.0 (27.0–34.0) | 29.5 (25.0–33.0) | 29.0 (26.0–33.0) | |
| Minimum to maximum | 17.0–50.0 | 16.0–42.0 | 16.0–50.0 | |
| HFS worry score | n (%) | 131 (99.2) | 132 (97.8) | 263 (98.5) |
| Mean (SD) | 40.7 (14.6) | 37.9 (13.3) | 39.3 (14.0) | |
| Median (IQR) | 37.0 (30.0–47.0) | 36.0 (28.0–45.0) | 37.0 (29.0–46.0) | |
| Minimum to maximum | 17.0–82.0 | 17.0–85.0 | 17.0–85.0 | |
| IMDc | n (%) | 78 (59.1) | 79 (58.5) | 157 (58.8) |
| Mean (SD) | 15.9 (13.7) | 17.2 (11.3) | 16.5 (12.5) | |
| Median (IQR) | 11.0 (7.7–18.9) | 13.3 (9.5–22.3) | 13.0 (8.6–19.6) | |
| Minimum to maximum | 2.3–73.2 | 3.1–54.0 | 2.3–73.2 | |
| SIMDd | n (%) | 51 (38.6) | 53 (39.3) | 104 (39.0) |
| Mean (SD) | 22.0 (18.3) | 22.5 (17.0) | 22.2 (17.6) | |
| Median (IQR) | 16.9 (8.0–26.4) | 18.6 (10.5–29.9) | 17.5 (8.8–28.5) | |
| Minimum to maximum | 2.4–73.8 | 1.9–74.7 | 1.9–74.7 | |
Table 14 shows the history of diabetes among study participants at baseline. The median (IQR) duration of diabetes was 16 (8–26) years, 12% of the participants had an episode of severe hypoglycaemia in the 12 months prior to baseline and around half of the participants had a prior history of complications (55%).
| Variable | Scoring | Treatment group | ||
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | Total (N = 267) | ||
| Duration of diabetes (years) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 18.5 (12.9) | 17.5 (12.1) | 18.0 (12.5) | |
| Median (IQR) | 16.5 (7.8–27.7) | 14.9 (7.7–25.4) | 15.8 (7.7–26.4) | |
| Minimum to maximum | 1.1–56.9 | 1.1–51.9 | 1.1–56.9 | |
| Prior history of complications | No | 63 (47.7) | 56 (41.5) | 119 (44.6) |
| Yes | 69 (52.3) | 79 (58.5) | 148 (55.4) | |
| Retinopathy as a complication | Yes | 51 (38.6) | 65 (48.1) | 116 (43.4) |
| No | 81 (61.4) | 70 (51.9) | 151 (56.6) | |
| Neuropathy as a complication | Yes | 13 (9.8) | 6 (4.4) | 19 (7.1) |
| No | 119 (90.2) | 129 (95.6) | 248 (92.9) | |
| Number of all forms of complications | ≥ 1 (%) | 68 (51.5) | 79 (58.5) | 147 (55.1) |
| n (%) | 132 (100) | 135 (100) | 267 (100) | |
| Mean (SD) | 1.0 (1.3) | 1.1 (1.2) | 1.1 (1.3) | |
| Median (IQR) | 1.0 (0–2) | 1.0 (0–2) | 1.0 (0–2) | |
| Minimum to maximum | 0–6 | 0–5 | 0–6 | |
| Number of confirmed moderate hypoglycaemic episodesa | ≥ 1 (%) | 89 (67.4) | 90 (66.7) | 179 (67.0) |
| n (%) | 132 (100) | 135 (100) | 267 (100) | |
| Mean (SD) | 2.6 (3.9) | 2.0 (2.7) | 2.3 (3.4) | |
| Median (IQR) | 1.0 (0–3) | 1.0 (0–3) | 1.0 (0–3) | |
| Minimum to maximum | 0–27 | 0–16 | 0–27 | |
| Number of moderate nocturnal hypoglycaemic episodesa | ≥ 1 (%) | 41 (31.1) | 46 (34.1) | 87 (32.6) |
| n (%) | 132 (100) | 135 (100) | 267 (100) | |
| Mean (SD) | 0.5 (1.0) | 0.7 (1.3) | 0.6 (1.2) | |
| Median (IQR) | 0.0 (0–1) | 0.0 (0–1) | 0.0 (0–1) | |
| Minimum to maximum | 0–5 | 0–10 | 0–10 | |
| Number of severe hypoglycaemiab | ≥ 1 (%) | 16 (12.1) | 15 (11.1) | 31 (11.6) |
| n (%) | 132 (100) | 135 (100) | 267 (100) | |
| Mean (SD) | 0.17 (0.52) | 0.16 (0.50) | 0.16 (0.51) | |
| Median (IQR) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) | |
| Minimum to maximum | 0–3 | 0–3 | 0–3 | |
Table 15 shows the history of severe hypoglycaemic episodes by baseline HbA1c category: 5 (20%) of the 25 participants with HbA1c of < 7.5% had an episode in the 12 months prior to baseline; 26 (11%) of the 242 participants with baseline HbA1c of ≥ 7.5% had an episode in the 12 months prior to baseline.
| Scoring | Number of severe hypoglycaemic episodesa | ||
|---|---|---|---|
| HbA1c < 7.5% (N = 25) | HbA1c ≥ 7.5% (N = 242) | Total (N = 267) | |
| ≥ 1, n (%) | 5 (20.0) | 26 (10.7) | 31 (11.6) |
| n (%) | 25 (100) | 242 (100) | 267 (100) |
| Mean (SD) | 0.36 (0.81) | 0.14 (0.46) | 0.16 (0.51) |
| Median (IQR) | 0.0 (0–0) | 0.0 (0–0) | 0.0 (0–0) |
| Minimum to maximum | 0–3 | 0–3 | 0–3 |
Table 16 shows laboratory results of participants at baseline. The mean HbA1c was 9.3% or 77.9 mmol/mol in the pump group and 9.0% or 74.8 mmol/mol in the MDI group. Other than this difference in baseline HbA1c, the data appear to be well balanced across treatment groups.
| Variable | Scoring | Treatment group | ||
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | Total (N = 267) | ||
| HbA1c, n (%) | < 7.5 | 13 (9.8) | 12 (8.9) | 25 (9.4) |
| ≥ 7.5 | 119 (90.2) | 123 (91.1) | 242 (90.6) | |
| Proteinuria (unconfirmed), n (%) | Normal | 90 (68.2) | 86 (63.7) | 176 (65.9) |
| Microalbuminuria | 18 (13.6) | 14 (10.4) | 32 (12.0) | |
| Macroalbuminuria | 3 (2.3) | 9 (6.7) | 12 (4.5) | |
| Missing | 21 (15.9) | 26 (19.3) | 47 (17.6) | |
| Classification of chronic kidney disease, n (%) | None | 82 (62.1) | 85 (63.0) | 167 (62.5) |
| Mild | 19 (14.4) | 15 (11.1) | 34 (12.7) | |
| Moderate | 5 (3.8) | 2 (1.5) | 7 (2.6) | |
| Severe | 2 (1.5) | 7 (5.2) | 9 (3.4) | |
| Missing | 24 (18.2) | 26 (19.3) | 50 (18.7) | |
| HbA1c | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 9.3 (1.9) | 9.0 (1.4) | 9.1 (1.7) | |
| Median (IQR) | 8.9 (8.1–10.2) | 8.6 (8.0–9.9) | 8.7 (8.1–9.9) | |
| Minimum to maximum | 5.7–16.7 | 6.1–14.1 | 5.7–16.7 | |
| HbA1c (mmol/mol) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 77.9 (21.0) | 74.8 (15.6) | 76.3 (18.5) | |
| Median (IQR) | 74.0 (65.0–88.0) | 71.0 (64.0–85.0) | 72.0 (65.0–85.0) | |
| Minimum to maximum | 39.0–159.0 | 43.0–131.0 | 39.0–159.0 | |
| Creatinine (µmol/l) | n (%) | 129 (97.7) | 134 (99.3) | 263 (98.5) |
| Mean (SD) | 76.8 (17.4) | 78.4 (20.5) | 77.6 (19.0) | |
| Median (IQR) | 73.0 (64.0–85.0) | 73.0 (64.0–89.0) | 73.0 (64.0–86.0) | |
| Minimum to maximum | 49.0–163.0 | 42.0–158.0 | 42.0–163.0 | |
| ACR (mg/mol) | n (%) | 130 (98.5) | 129 (95.6) | 259 (97.0) |
| Unable to calculate | 20 (15.4) | 20 (15.5) | 40 (15.4) | |
| < 3 | 90 (69.2) | 86 (66.7) | 176 (68.0) | |
| 3–10 | 10 (7.7) | 12 (9.3) | 22 (8.5) | |
| 10–30 | 8 (6.2) | 2 (1.6) | 10 (3.9) | |
| 30+ | 2 (1.5) | 9 (7.0) | 11 (4.2) | |
| eGFR (mmol/l) | n (%) | 129 (97.7) | 135 (100.0) | 264 (98.9) |
| 30–44 | 3 (2.3) | 2 (1.5) | 5 (1.9) | |
| 45–59 | 5 (3.9) | 6 (4.4) | 11 (4.2) | |
| ≤ 60–90 | 90 (69.8) | 89 (65.9) | 179 (67.8) | |
| ≥ 90 | 31 (24.0) | 38 (28.1) | 69 (26.1) | |
| Cholesterol (mmol/l) | n (%) | 132 (100.0) | 134 (99.3) | 266 (99.6) |
| Mean (SD) | 5.0 (1.0) | 4.9 (0.9) | 5.0 (0.9) | |
| Median (IQR) | 5.0 (4.4–5.6) | 4.8 (4.2–5.4) | 4.9 (4.3–5.6) | |
| Minimum to maximum | 2.8–8.6 | 2.7–8.0 | 2.7–8.6 | |
| Triglycerides (mmol/l) | n (%) | 132 (100.0) | 135 (100.0) | 267 (100.0) |
| Mean (SD) | 1.4 (1.2) | 1.4 (0.8) | 1.4 (1.0) | |
| Median (IQR) | 1.1 (0.8–1.6) | 1.2 (0.8–1.7) | 1.1 (0.8–1.6) | |
| Minimum to maximum | 0.3–11.2 | 0.3–5.9 | 0.3–11.2 | |
| HDL cholesterol (mmol/l) | n (%) | 125 (94.7) | 133 (98.5) | 258 (96.6) |
| Mean (SD) | 1.6 (0.4) | 1.5 (0.4) | 1.6 (0.4) | |
| Median (IQR) | 1.6 (1.3–1.9) | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | |
| Minimum to maximum | 0.6–3.2 | 0.5–2.7 | 0.5–3.2 | |
| QAID, units/body weight (kg) | n (%) | 128 (97.0) | 133 (98.5) | 261 (97.8) |
| Mean (SD) | 0.37 (0.17) | 0.37 (0.16) | 0.37 (0.16) | |
| Median (IQR) | 0.33 (0.25–0.49) | 0.35 (0.24–0.47) | 0.33 (0.24–0.47) | |
| Minimum to maximum | 0.10–0.99 | 0.12–1.17 | 0.10–1.17 | |
| BID, units/body weight (kg) | n (%) | 128 (97.0) | 134 (99.3) | 262 (98.1) |
| Mean (SD) | 0.35 (0.17) | 0.38 (0.21) | 0.37 (0.19) | |
| Median (IQR) | 0.32 (0.23–0.45) | 0.34 (0.26–0.45) | 0.33 (0.25–0.45) | |
| Minimum to maximum | 0.08–1.04 | 0.10–1.48 | 0.08–1.48 | |
| PMID, units/body weight (kg) | n (%) | 4 (3.0) | 1 (0.7) | 5 (1.9) |
| Mean (SD) | 0.78 (0.32) | 1.43a | 0.91 (0.40) | |
| Median (IQR) | 0.84 (0.53–1.03) | 1.43 (1.43–1.43) | 0.99 (0.69–1.07) | |
| Minimum to maximum | 0.36–1.07 | 1.43–1.43 | 0.36–1.43 | |
Table 17 summarises the proportion of participants with above and below 7.5% HbA1c at baseline in each centre, stratified by treatment group.
| Recruitment centre | HbA1c (%) | Treatment group | ||
|---|---|---|---|---|
| Pump (N = 132), n (%) | MDI (N = 135), n (%) | Total (N = 267), n (%) | ||
| All | ≥ 7.5 | 119 (90.2) | 123 (91.1) | 242 (90.6) |
| London (King’s College Hospital) | < 7.5 | 1 (7.1) | 5 (33.3) | 6 (20.7) |
| ≥ 7.5 | 13 (92.9) | 10 (66.7) | 23 (79.3) | |
| Sheffield | < 7.5 | 2 (11.1) | 1 (5.0) | 3 (7.9) |
| ≥ 7.5 | 16 (88.9) | 19 (95.0) | 35 (92.1) | |
| Glasgow | < 7.5 | 0 (0.0) | 1 (5.3) | 1 (2.5) |
| ≥ 7.5 | 21 (100.0) | 18 (94.7) | 39 (97.5) | |
| Dumfries | < 7.5 | 2 (12.5) | 0 (0.0) | 2 (6.7) |
| ≥ 7.5 | 14 (87.5) | 14 (100.0) | 28 (93.3) | |
| Cambridge | < 7.5 | 3 (15.8) | 1 (6.7) | 4 (11.8) |
| ≥ 7.5 | 16 (84.2) | 14 (93.3) | 30 (88.2) | |
| Harrogate | < 7.5 | 4 (16.0) | 1 (3.8) | 5 (9.8) |
| ≥ 7.5 | 21 (84.0) | 25 (96.2) | 46 (90.2) | |
| Edinburgh | < 7.5 | 1 (6.7) | 3 (15.0) | 4 (11.4) |
| ≥ 7.5 | 14 (93.3) | 17 (85.0) | 31 (88.6) | |
| Nottingham | < 7.5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥ 7.5 | 4 (100.0) | 6 (100.0) | 10 (100.0) | |
Protocol deviations
One participant was excluded from the per-protocol analysis set, as they did not adhere to the DAFNE course (this is not including the dropouts prior to the DAFNE course).
Twenty-five patients had a single treatment change form that recorded change across study treatments; 17 patients switched from pump to MDI and eight patients switched from MDI to pump. Two patients on the pump arm changed to MDI and back again (recorded on treatment change forms), and other participants recorded temporary treatment breaks at the follow-up appointments. After review, excluding any reasonable temporary treatment interruptions, 236 out of the 260 ITT participants were considered as compliant with the protocol. Of the 235 ITT participants with baseline HbA1c of ≥ 7.5%, 18 were considered protocol deviations, leaving 217 in the per-protocol analysis set. Participants who deviated from the protocol started with higher baseline HbA1c across both the treatment groups (Table 18); however, greater improvement was seen for the protocol deviants in the MDI group. The reasons for protocol deviation/treatment change are shown in Table 19.
| Timing | HbA1c unit | Statistics | Protocol deviation | Per protocol | ||
|---|---|---|---|---|---|---|
| Pump (n = 11) | MDI (n = 7) | Pump (n = 108) | MDI (n = 109) | |||
| Baseline | n | 11 | 7 | 108 | 109 | |
| % | Mean (SD) | 10.4 (2.4) | 10.3 (1.4) | 9.5 (1.7) | 9.1 (1.3) | |
| mmol/mol | Mean (SD) | 90.5 (26.1) | 89.4 (15.3) | 80.1 (18.7) | 75.6 (14.0) | |
| 6 months | n | 11 | 7 | 108 | 104 | |
| % | Mean (SD) | 9.9 (2.5) | 10.0 (2.6) | 8.7 (1.4) | 8.6 (1.4) | |
| mmol/mol | Mean (SD) | 84.2 (26.9) | 85.7 (28.8) | 71.5 (15.8) | 71.0 (15.1) | |
| 12 months | n | 7 | 7 | 106 | 101 | |
| % | Mean (SD) | 10.6 (2.3) | 9.5 (2.0) | 8.8 (1.5) | 8.6 (1.4) | |
| mmol/mol | Mean (SD) | 92.7 (25.4) | 79.9 (21.4) | 72.4 (16.6) | 70.5 (15.0) | |
| 24 months | n | 9 | 7 | 106 | 102 | |
| % | Mean (SD) | 9.7 (2.1) | 8.3 (1.6) | 8.6 (1.4) | 8.7 (1.4) | |
| mmol/mol | Mean (SD) | 82.4 (23.1) | 67.1 (17.8) | 70.5 (15.7) | 71.5 (15.5) | |
| Treatment change | ID | Month of treatment changea | Reason for treatment change |
|---|---|---|---|
| Switched from pump to MDI | 1 | 0 | Participant withdrawal from the DAFNE course |
| 2 | 0 | Participant did not tolerate trial treatment: risk of DKA as a result of not following safety protocols | |
| 3 | 3 | Participant did not tolerate trial treatment: problems with cannula sites | |
| 4 | 4 | Participant did not tolerate trial treatment: problems with cannulas | |
| 5 | 7 | Participant did not tolerate trial treatment: headaches, erratic blood glucose and stress | |
| 6 | 12 | Participant did not tolerate trial treatment: inconvenience of delivery method | |
| 7 | 13 | Participant did not tolerate trial treatment: found pump difficult to manage | |
| 8 | 13 | Other: did not like the practicalities of being on a pump | |
| 9 | 14 | Participant did not tolerate trial treatment: pump did not suit him | |
| 10 | 23 | Other: patient decision without input from team | |
| 11 | 23 | Participant did not tolerate trial treatment: painful cannula sites reported | |
| Switched from MDI to pump | 12 | 9 | Other: fear of disabling hypoglycaemia |
| 13 | 14 | Other: persistently elevated morning glycaemia | |
| 14 | 15 | Other: pump therapy clinically appropriate | |
| 15 | 15 | Other: clinical need for pump therapy | |
| 16 | 18 | Investigator decision: deterioration in HbA1c; meets criteria for trial of pump therapy | |
| 17 | 19 | Other: continuing problems with hypoglycaemia | |
| 18 | 20 | Other: dawn phenomenon |
Primary outcome
Table 20 shows the primary outcome, change in HbA1c at 24 months in participants whose baseline HbA1c was ≥ 7.5%. The mean change in the pump group was a decrease of 0.85% or 9.3 mmol/mol, whereas the mean decrease in the MDI group was 0.42% or 4.5 mmol/mol. After adjusting for centre, course and baseline HbA1c, the MD in HbA1c change from baseline was –0.24% (95% CI –0.53% to 0.05%) or –2.7 mmol/mol (95% CI –5.8 to 0.5 mmol/mol; p = 0.098). 23 Figure 4 shows the distribution of HbA1c change at 2 years, by treatment group.
| Primary outcome | Treatment group | Difference in mean changea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Pump | MDI | |||||
| n | Mean (SD) | n | Mean (SD) | |||
| Change in HbA1c (%) | 119 | –0.85 (1.25) | 116 | –0.42 (1.21) | –0.24 (–0.53 to 0.05) | 0.098 |
| Change in HbA1c (mmol/mol) | 119 | –9.3 (13.66) | 116 | –4.5 (13.19) | –2.7 (–5.8 to 0.5) | |
FIGURE 4.
Changes in HbA1c (%) at 24 months in participants whose baseline HbA1c was ≥ 7.5%. (a) Pump; and (b) MDI.
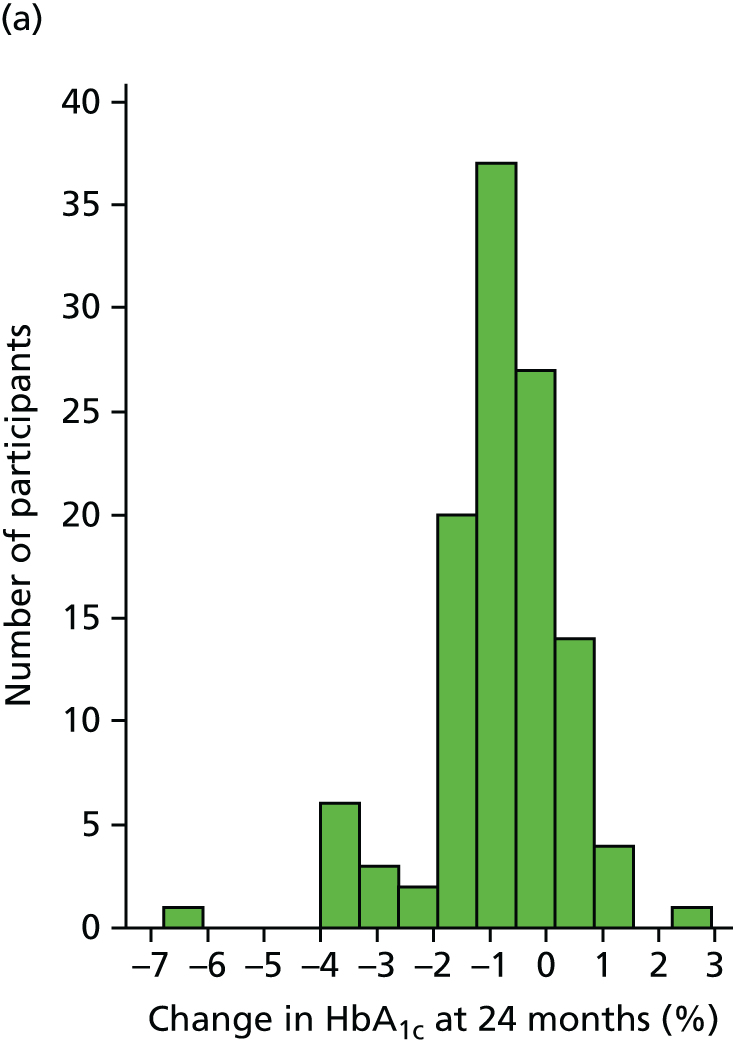

The treatment difference was larger for the per-protocol analysis: MD –0.36% (95% CI –0.64% to –0.07%) or –3.9 mmol/mol (95% CI –7.0 to –0.8 mmol/mol) in favour of the pump; p = 0.015. However, the observed point estimate was still smaller than the minimum clinically important difference of 0.5% or 5.5 mmol/mol,23 although the 95% CI includes this clinically important effect.
Table 21 shows sensitivity analysis on the primary outcome; the analysis was repeated for complete case, imputing data for all participants, excluding mistimed measurements and excluding pregnant women. The results from Tables 20 and 21 are presented graphically in Figure 5. All sensitivity analyses show similar results to the primary analysis shown in Table 20.
| Sensitivity analysis set | HbA1c unit of measurement | Treatment group | Differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean change (SD) | n | Mean change (SD) | ||||
| Per protocol | % | 108 | –0.85 (1.28) | 109 | –0.31 (1.12) | –0.36 (–0.64 to –0.07) | 0.015 |
| mmol/mol | 108 | –9.3 (13.96) | 109 | –3.4 (12.23) | –3.9 (–7.0 to –0.8) | ||
| Multiple imputationb | % | 119 | –0.85 (1.25) | 123 | –0.44 (1.21) | –0.24 (–0.53 to 0.05) | 0.104 |
| mmol/mol | 119 | –9.3 (13.66) | 123 | –4.8 (13.19) | –2.7 (–5.8 to 0.5) | ||
| Mean value imputation | % | 119 | –0.83 (1.23) | 123 | –0.45 (1.14) | –0.22 (–0.49 to 0.05) | 0.105 |
| mmol/mol | 119 | –9.1 (13.43) | 123 | –4.9 (12.44) | –2.4 (–5.3 to 0.5) | ||
| Complete case | % | 115 | –0.84 (1.25) | 109 | –0.43 (1.21) | –0.22 (–0.50 to 0.06) | 0.127 |
| mmol/mol | 115 | –9.2 (13.66) | 109 | –4.7 (13.19) | –2.4 (–5.4 to 0.7) | ||
| Excluding mistimed measurements | % | 114 | –0.85 (1.25) | 104 | –0.44 (1.16) | –0.19 (–0.47 to 0.09) | 0.186 |
| mmol/mol | 114 | –9.3 (13.69) | 104 | –4.8 (12.72) | –2.1 (–5.2 to 1.0) | ||
| Excluding pregnant women | % | 115 | –0.84 (1.25) | 107 | –0.41 (1.21) | –0.23 (–0.52 to 0.05) | 0.104 |
| mmol/mol | 115 | –9.2 (13.66) | 107 | –4.5 (13.22) | –2.6 (–5.7 to 0.5) | ||
FIGURE 5.
Forest plot of MD in change from baseline in HbA1c (%) at 24 months between groups for the sensitivity analysis samples. MCID, minimum clinically important difference, adjusted for baseline HbA1c, centre and DAFNE course.

Notes: (1) Thirteen local laboratory HbA1c values were used in the final analysis (two at 6 months, one at 12 months, 10 at 24 months), seven HbA1c values were taken from patient notes (one at 6 months, four at 12 months, two at 24 months). (2) ICC from complete case model at 24 months is 0.005. If centre is excluded from the model (as a fixed effect), the ICC is 0.08.
The change in HbA1c for participants with data at all four time points is displayed, by treatment group, in Figure 6. The majority of improvement in HbA1c occurred in the first 6 months; HbA1c stayed roughly constant between 6 and 24 months. The change in HbA1c over time is also displayed in Figure 7, but here all of the participants with post-baseline data are included. Each coloured line represents a participant, and the thick black line is the mean for each treatment group.
FIGURE 6.
Mean HbA1c (%) over time in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol) (including only participants with data at all four visits, n = 208). Numbers in parentheses are mmol/mol equivalent. Reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.

FIGURE 7.
Glycated haemoglobin (mmol/mol) over time in participants whose baseline HbA1c was ≥ 7.5% (58 mmol/mol) (including participants with any post-baseline HbA1c data, n = 235). (a) Pump; and (b) MDI.


Table 22 shows the mean change at 24 months for the treatment groups combined; the change in all participants with complete 24-month HbA1c data was a decrease of 0.54% (95% CI 0.38% to 0.69%) or 5.9 mmol/mol (95% CI 4.2 to 7.6 mmol/mol). For participants with baseline HbA1c ≥ 7.5%, the reduction was slightly bigger, of 0.64% (95% CI 0.48% to 0.80%) or 7 mmol/mol (95% CI 5.2 to 8.8 mmol/mol). 23
| HbA1c (mmol/mol) | HbA1c units | n | Mean change (95% CI) |
|---|---|---|---|
| All participants with complete 24-month data | % | 248 | –0.54 (–0.69 to –0.38) |
| mmol/mol | 248 | –5.9 (–7.6 to –4.2) | |
| Participants with baseline HbA1c ≥ 7.5% and complete 24-month data | % | 224 | –0.64 (–0.80 to –0.48) |
| mmol/mol | 224 | –7.0 (–8.8 to –5.2) |
Sensitivity analysis: effect of centre and lead Dose Adjustment For Normal Eating course educator
We undertook a further analysis that used a nested model of patients within courses, which, in turn, are nested within course lead educators, to investigate differences in outcomes between lead educators. For this nested model, the ICC of the lower-level clustering variable, DAFNE course, is 0.5%; for the upper-level clusters, lead educator, ICC < 0.1%. We found no evidence of notable differences in outcomes between lead course educators. This analysis was performed for available data only.
We explored the centre effect through an interaction test between centre and treatment group. Results of estimated MD in HbA1c change (% or mmol/mol) at 24 months are presented by centre with the aid of forest plots (Figure 8). The overall p-value for the interaction between treatment and centre was 0.565, suggesting that there is no centre effect. The centre with the largest difference between treatments was Nottingham, although the CI for this centre is large because of the small number of participants with outcome data at that centre.
FIGURE 8.
Forest plot of MD in 24-month HbA1c change (%) at 24 months between groups for participants with baseline HbA1c ≥ 7.5% by centre (complete cases with HbA1c ≥ 7.5% at baseline, n = 224). MDs are calculated from mixed-effects regression analysis adjusted for DAFNE course. KCH, King’s College Hospital; MCID, minimum clinically important difference.

Secondary outcomes
Glycated haemoglobin
The proportion of participants reaching the NICE target of HbA1c of ≤ 7.5% (58 mmol/mol) after 2 years is displayed in Table 23 (including all participants regardless of baseline HbA1c value). The proportion of patients with HbA1c ≤ 7.5% was similar across the groups. The results are very similar at 6 and 12 months (Table 24).
| Outcome | Treatment group, n/N (%) | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Pump | MDI | |||
| HbA1c ≤ 7.5% | 32/128 (25.0) | 28/120 (23.3) | 1.22 (0.62 to 2.39) | 0.566 |
| Outcome | Follow-up (months) | Treatment group, n/N (%) | ORa (95% CI) | p-value | |
|---|---|---|---|---|---|
| Pump | MDI | ||||
| HbA1c ≤ 7.5% (58 mmol/mol) | 6 | 26/132 (20.5) | 26/123 (21.1) | 1.03 (0.51 to 2.10) | 0.930 |
| 12 | 29/126 (23.0) | 27/120 (22.5) | 1.32 (0.62 to 2.80) | 0.478 | |
Table 25 shows the distribution of HbA1c categories at baseline and 24 months. Of the participants who ended with HbA1c of ≤ 7.5% at 24 months, 12 began the study with baseline HbA1c of ≥ 8.5%.
| Outcome | Category | Treatment group, n (%) | Total (N = 267), n (%) | |
|---|---|---|---|---|
| Pump (n = 132) | MDI (n = 135) | |||
| HbA1c (%) at baseline | < 7 | 10 (7.6) | 7 (5.2) | 17 (6.4) |
| ≥ 7 to < 7.5 | 3 (2.3) | 5 (3.7) | 8 (3.0) | |
| ≥ 7.5 to < 8 | 18 (13.6) | 15 (11.1) | 33 (12.4) | |
| ≥ 8 to < 8.5 | 21 (15.9) | 31 (23.0) | 52 (19.5) | |
| ≥ 8.5 to < 9 | 15 (11.4) | 21 (15.6) | 36 (13.5) | |
| ≥ 9 to < 10 | 28 (21.2) | 29 (21.5) | 57 (21.3) | |
| ≥ 10 | 37 (28.0) | 27 (20.0) | 64 (24.0) | |
| HbA1c (%) at 24 months | < 7 | 15 (11.4) | 12 (8.9) | 27 (10.1) |
| ≥ 7 to < 7.5 | 14 (10.6) | 13 (9.6) | 27 (10.1) | |
| ≥ 7.5 to < 8 | 26 (19.7) | 18 (13.3) | 44 (16.5) | |
| ≥ 8 to < 8.5 | 25 (18.9) | 29 (21.5) | 54 (20.2) | |
| ≥ 8.5 to < 9 | 9 (6.8) | 20 (14.8) | 29 (10.9) | |
| ≥ 9 to < 10 | 17 (12.9) | 12 (8.9) | 29 (10.9) | |
| ≥ 10 | 22 (16.7) | 16 (11.9) | 38 (14.2) | |
| No data | 4 (3.0) | 15 (11.1) | 19 (7.1) | |
The primary analysis at 24 months displayed above (see Primary outcome) is repeated for 6- and 12-month follow-up visits among participants with complete data. The results for these interim follow-ups are consistent with the primary outcome analysis and are displayed in Table 26. The largest MD in HbA1c change from baseline was observed at 6 months, –0.25% (95% CI –0.52% to 0.02%) or –2.7 mmol/mol (95% CI –5.6 to 0.2 mmol/mol), but is not clinically relevant or statistically significant at the 5% nominal level.
| Follow-up (months) | HbA1c units | Treatment group | MD in changea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean change (SD) | n | Mean change (SD) | ||||
| 6 | % | 118 | –0.76 (1.19) | 111 | –0.36 (1.06) | –0.25 (–0.52 to 0.02) | 0.069 |
| mmol/mol | 118 | –8.3 (12.05) | 111 | –3.9 (11.56) | –2.7 (–5.6 to 0.2) | ||
| 12 | % | 111 | –0.70 (1.10) | 107 | –0.40 (1.02) | –0.13 (–0.40 to 0.14) | 0.349 |
| mmol/mol | 111 | –7.6 (12.04) | 107 | –4.4 (11.10) | –1.4 (–4.3 to 1.5) | ||
Episodes of moderate and severe hypoglycaemia
Few severe hypoglycaemic episodes were observed post baseline; 49 episodes recorded from 25 participants23 (Table 27). All severe hypoglycaemic episodes occurred while participants were on their allocated treatment. Across both treatment groups the number of severe hypoglycaemic episodes reduced: the average number of episodes per patient-year in the study reduced from 0.17 before baseline to 0.10 during follow-up. The IRR for the number of severe hypoglycaemic episodes in the 24-month follow-up, compared with the year before baseline, is 0.46 (95% CI 0.24 to 0.89; p = 0.021). 23 Therefore, compared with the year before baseline, the number of severe hypoglycaemic episodes per year were roughly halved in the 2 years of follow-up post baseline. There was no statistically significant difference in the rate of severe hypoglycaemia during follow-up between the treatment groups having adjusted for centre, DAFNE course, baseline HbA1c and presence of at least one severe hypoglycaemic episode in the 12 months before baseline (IRR 1.13; 95% CI 0.51 to 2.51; p = 0.766). The comparison of severe hypoglycaemic episodes between groups was repeated excluding the first 6 months of follow-up, which is the ‘settling in’ period on the pump. This time the estimated IRR was almost equivocal, but the large CI around this reflects the amount of uncertainty as a result of these analyses being based on so few episodes from few participants (IRR 1.05; 95% CI 0.44 to 2.53; p = 0.912).
| Time period | Number of events: event per patient-year, incidence rate | ||
|---|---|---|---|
| Pump (n = 132) | MDI (n = 135) | Total (N = 267) | |
| Before baseline | 24, 0.18 | 21, 0.16 | 45, 0.17 |
| Between baseline and 6-month follow-up | 13, 0.18 | 7, 0.10 | 20, 0.14 |
| Between 6 and 12 months’ follow-up | 8, 0.13 | 5, 0.09 | 13, 0.11 |
| Between 12 and 24 months’ follow-up | 4, 0.03 | 12, 0.11 | 16, 0.07 |
| Overall (post baseline) | 25, 0.10 | 24, 0.10 | 49, 0.10 |
| (Excluding first 6 months) | 12, 0.06 | 17, 0.10 | 29, 0.08 |
| IRR (95% CI):a 1.13 (0.51 to 2.51); p = 0.766 | |||
| (Excluding first 6 months) IRR (95% CI):b 1.05 (0.44 to 2.53); p = 0.912 | |||
| Test of overall change over time: IRRc (95% CI) (study follow-up compared with the year before baseline, treatment groups combined) 0.46 (0.24 to 0.89); p = 0.021 | |||
Across both treatment arms, on average, three moderate hypoglycaemic episodes were recorded per patient over a 4-week history at 6 months (Table 28). By 24 months, the average number of recorded moderate hypoglycaemic episodes during a 4-week history was slightly lower (2.6 for pump, 2.3 for MDI). There was no statistically significant difference between the groups in the rate of moderate hypoglycaemic episodes at any time point. 23
| Outcome | Follow-up (months) | Classification | Treatment group, n, IRa | IRRb (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Pump | MD | |||||
| Episodes of moderate hypoglycaemia in 4 weeks before follow-up visit | 6 | All recorded episodes | 131, 2.95 | 125, 3.04 | 1.21 (0.87 to 1.66) | 0.258 |
| Confirmed episodes | 131, 2.29 | 125, 2.14 | 1.24 (0.91 to 1.68) | 0.168 | ||
| Confirmed episodes (US definition) | 131, 2.92 | 125, 2.66 | 1.17 (0.87 to 1.57) | 0.299 | ||
| 12 | All recorded episodes | 124, 2.73 | 119, 2.90 | 0.89 (0.66 to 1.19) | 0.416 | |
| Confirmed episodes | 124, 2.03 | 119, 2.22 | 0.88 (0.65 to 1.20) | 0.433 | ||
| Confirmed episodes (US definition) | 124, 2.71 | 119, 2.82 | 0.88 (0.66 to 1.18) | 0.402 | ||
| 24 | All recorded episodes | 127, 2.56 | 119, 2.26 | 1.00 (0.71 to 1.41) | 0.992 | |
| Confirmed episodes | 127, 1.81 | 119, 1.76 | 1.02 (0.72 to 1.46) | 0.894 | ||
| Confirmed episodes (US definition) | 127, 2.51 | 119, 2.16 | 1.04 (0.73 to 1.48) | 0.832 | ||
Few participants reported one or more severe hypoglycaemic episode during study follow-up: 14 (10.6%) in the pump group and 11 (8.6%) in the MDI group (Table 29). There was no evidence that the number of patients reporting at least on severe hypoglycaemic episode was different in the two groups: OR 1.22 (95% CI 0.49 to 3.03). More than half of the participants reported at least one moderate hypoglycaemic episode in the 4 weeks prior to follow-up at each time point and across both treatment groups. Slightly more participants reported at least one episode at 6 months in the pump group than in the MDI group (p = 0.088). However, a smaller proportion of participants reported episodes in the pump group at 12 and 14 months, although not statistically significant.
| Outcome | Follow-up | Treatment group, n/N (%) | ORa (95% CI) | p-value | |
|---|---|---|---|---|---|
| Pump | MDI | ||||
| Severe hypoglycaemic episode | Entire duration | 14/132 (10.6) | 11/128 (8.6) | 1.22 (0.49 to 3.03) | 0.666 |
| Moderate hypoglycaemic episode | 6 months | 89/131 (67.9) | 72/125 (57.6) | 1.64 (0.93 to 2.91) | 0.088 |
| 12 months | 68/124 (54.8) | 76/119 (63.9) | 0.66 (0.37 to 1.19) | 0.171 | |
| 24 months | 70/127 (55.1) | 67/119 (56.3) | 0.95 (0.49 to 1.85) | 0.890 | |
Figure 9 shows the distribution of the number of severe hypoglycaemic episodes for those who had one or more episodes post baseline. The majority of participants had only one severe hypoglycaemic episode, 10 participants recorded more than one episode during the follow-up period and the maximum recorded by a participant was seven. The number of patients who had an episode makes up a small proportion of the study population (10%).
FIGURE 9.
Distribution of number of severe hypoglycaemic episodes in participants with at least one episode post baseline, by treatment group. (a) Pump, n = 14; and (b) MDI, n = 11.
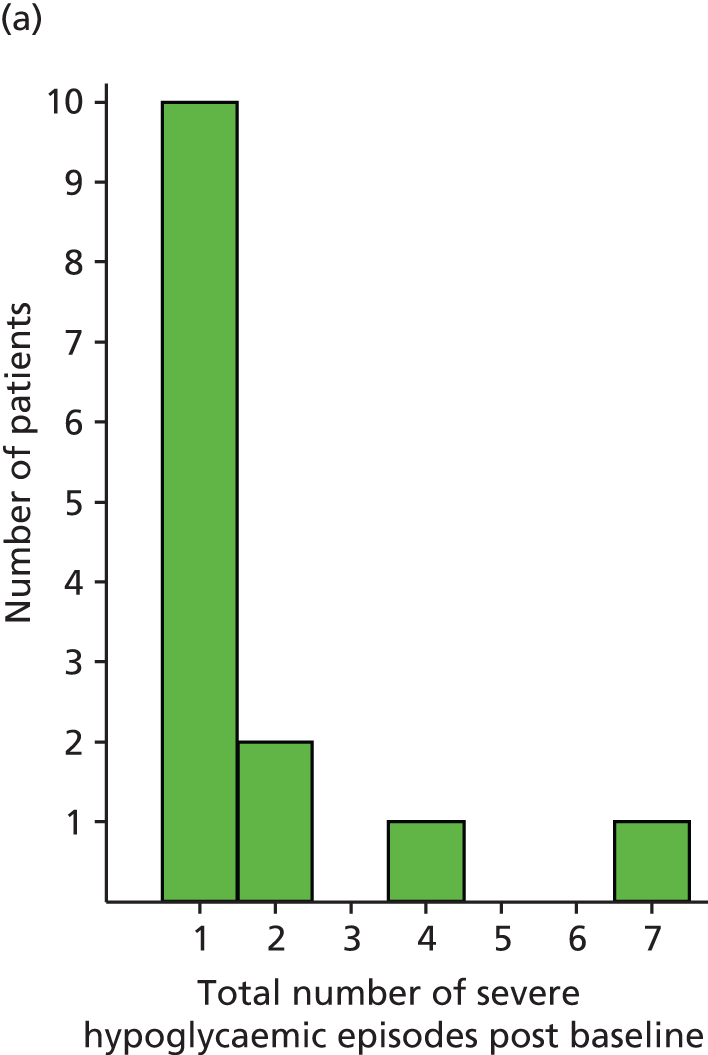

Figure 10 shows the timing of severe hypoglycaemic episodes. Each dot represents a severe hypoglycaemic episode. Dots connected by a line represent severe hypoglycaemic episodes experienced by the same person.
FIGURE 10.
Severe hypoglycaemic episodes over time per participant with at least one episode post baseline, by treatment group. (a) Pump, n = 14; and (b) MDI, n = 11.
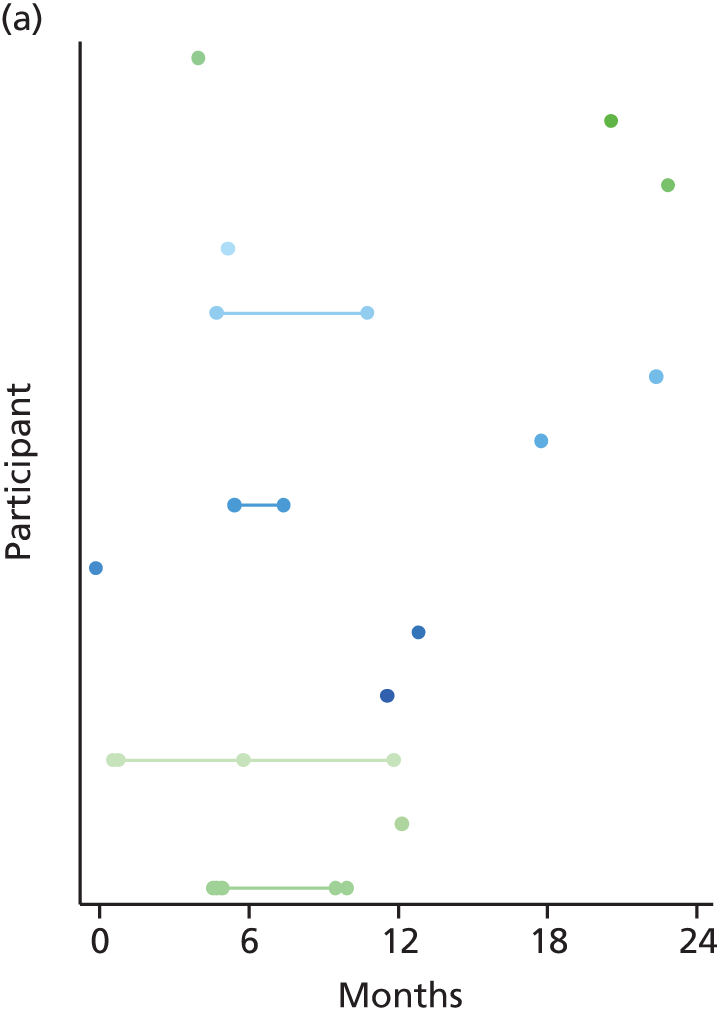

There is no statistically significant difference in the odds of proteinuria between the treatment groups (Table 30). At 6 months, the odds of being in a higher proteinuria category (where macroalbuminuria is the highest category) are estimated to be 21% lower in the pump group than the MDI group (OR 0.79), but 14% higher at 12 months, and almost identical at 24 months.
| Follow-up (months) | Secondary outcome | Treatment group, n (%) | ORa (95% CI) | p-value | |
|---|---|---|---|---|---|
| Pump | MDI | ||||
| 6 | Normal | 76 (80.0) | 81 (81.0) | ||
| Microalbuminuria | 17 (17.9) | 14 (14.0) | |||
| Macroalbuminuria | 2 (2.1) | 5 (5.0) | 0.79 (0.36 to 1.73) | 0.558 | |
| 12 | Normal | 65 (75.6) | 67 (80.7) | ||
| Microalbuminuria | 16 (18.6) | 10 (12.0) | |||
| Macroalbuminuria | 5 (5.8) | 6 (7.2) | 1.14 (0.53 to 2.48) | 0.736 | |
| 24 | Normal | 77 (81.1) | 70 (83.3) | ||
| Microalbuminuria | 16 (16.8) | 9 (10.7) | |||
| Macroalbuminuria | 2 (2.1) | 5 (6.0) | 1.04 (0.46 to 2.32) | 0.932 | |
Table 31 shows exploratory descriptive analyses of self-reported physical activity for the two groups at each study visit; no formal statistical tests have been performed on these data. The amount of physical activity appears similar across the groups.
| Follow-up | Physical activity | Treatment group, n (%) | All, N (%) | |
|---|---|---|---|---|
| Pump | MDI | |||
| Baseline | High | 33 (25.0) | 33 (24.4) | 66 (24.7) |
| Medium | 63 (47.7) | 70 (51.9) | 133 (49.8) | |
| Low | 36 (27.3) | 32 (23.7) | 68 (25.5) | |
| 6 months | High | 36 (27.7) | 36 (28.8) | 72 (28.2) |
| Medium | 60 (46.2) | 63 (50.4) | 123 (48.2) | |
| Low | 34 (26.2) | 26 (20.8) | 60 (23.5) | |
| 12 months | High | 35 (28.5) | 39 (32.8) | 74 (30.6) |
| Medium | 60 (48.8) | 51 (42.9) | 111 (45.9) | |
| Low | 28 (22.8) | 29 (24.4) | 57 (23.6) | |
| 24 months | High | 41 (32.3) | 37 (31.4) | 78 (31.8) |
| Medium | 61 (48.0) | 53 (44.9) | 114 (46.5) | |
| Low | 25 (19.7) | 28 (23.7) | 53 (21.6) | |
Table 32 shows the results of comparing secondary continuous outcomes across treatment groups. Weight remained roughly constant throughout the study duration, and was not statistically significantly different between the treatment groups at any follow-up. 23 A slight increase in HDL cholesterol and a slight decrease in TC was observed across both treatment groups. 23 There was no evidence of a difference between treatment groups in cholesterol change from baseline, with p-values ranging from 0.219 to 0.856. Insulin dose decreased across both pump and MDI arms. There was evidence of a difference in the mean change in insulin dose at 12 months between treatment groups; on average, participants in the pump group had a 0.07-IU/weight larger decrease (95% CI 0.01 to 0.013 IU/weight; p = 0.017) in insulin dose than those in the MDI group. However, the difference between treatments in insulin dose was slightly smaller at 6 and 24 months, but not statistically significant.
| Outcome | Follow-up (months) | Treatment group | Adjusted differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean change (SD) | n | Mean change (SD) | ||||
| Body weight (kg) | 6 | 131 | –0.05 (4.35) | 124 | –0.61 (4.32) | 0.45 (–0.66 to 1.55) | 0.430 |
| 12 | 123 | 0.78 (4.95) | 116 | –0.05 (4.65) | 0.67 (–0.64 to 1.98) | 0.316 | |
| 24 | 127 | 0.71 (5.45) | 117 | 0.20 (6.37) | 0.42 (–1.17 to 2.01) | 0.607 | |
| HDL cholesterol (mmol/l) | 6 | 123 | 0.01 (0.28) | 116 | 0.04 (0.36) | –0.05 (–0.13 to 0.03) | 0.264 |
| 12 | 109 | 0.04 (0.29) | 113 | 0.04 (0.38) | –0.01 (–0.10 to 0.08) | 0.801 | |
| 24 | 117 | 0.03 (0.30) | 112 | 0.06 (0.39) | –0.04 (–0.12 to 0.05) | 0.428 | |
| Cholesterol (mmol/l) | 6 | 130 | –0.17 (0.84) | 122 | –0.01 (0.84) | –0.14 (–0.35 to 0.08) | 0.219 |
| 12 | 121 | –0.14 (1.02) | 116 | –0.08 (0.83) | –0.02 (–0.26 to 0.22) | 0.856 | |
| 24 | 127 | –0.21 (0.95) | 116 | –0.19 (1.03) | 0.03 (–0.25 to 0.30) | 0.848 | |
| Total insulin dose (IU/weight) | 6 | 130 | –0.07 (0.27) | 124 | –0.03 (0.21) | –0.04 (–0.10 to 0.02) | 0.199 |
| 12 | 123 | –0.09 (0.26) | 117 | –0.02 (0.22) | –0.07 (–0.13 to –0.01) | 0.017 | |
| 24 | 125 | –0.06 (0.27) | 116 | –0.01 (0.23) | –0.05 (–0.11 to 0.02) | 0.152 | |
Table 33 summarises blood glucose testing per day averaged over a 2-week recorded period, stratified by the baseline HbA1c category. A post hoc analysis indicated that there was no difference in the mean blood glucose testing frequency between treatment groups at 24 months, having adjusted for baseline number of blood glucose tests, centre and DAFNE course. 23 The adjusted MD in blood glucose tests (95% CI) was 0.22 (–0.24 to 0.68) per day or 3.1 (–3.4 to 9.6) over 2 weeks; p = 0.352. Overall, the number of blood glucose tests increased from 3.6 at baseline to 4.1 per day at 24 months (95% CI 0.33 to 0.82; p < 0.001). 23
| Outcome | Baseline HbA1c category (%) | Treatment group | Adjusted MDa (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Baseline number of blood glucose tests performed per day (averaged over 2 weeks) | < 7.5 | 13 | 3.8 (1.7) | 11 | 4.9 (2.1) | ||
| ≥ 7.5% and < 8.5 | 38 | 3.9 (1.8) | 43 | 3.8 (1.9) | |||
| ≥ 8.5 | 76 | 3.3 (1.7) | 65 | 3.2 (1.8) | |||
| All | 127 | 3.6 (1.8) | 119 | 3.6 (1.9) | |||
| 24 months: number of blood glucose tests performed per day (averaged over 2 weeks) | < 7.5 | 13 | 4.8 (1.0) | 11 | 4.3 (1.9) | ||
| ≥ 7.5 and < 8.5 | 38 | 5.2 (2.4) | 43 | 3.8 (1.9) | |||
| ≥ 8.5 | 76 | 3.7 (2.1) | 64 | 4.1 (1.7) | |||
| All | 127 | 4.3 (2.2) | 118 | 4.0 (1.8) | 0.22 (–0.24 to 0.68) | 0.352 | |
Subgroup analysis
The potential moderating effects of subgroups were explored using mixed-effects linear regression, with an interaction between treatment and subgroup. Results of the subgroup analyses are presented in Tables 34–37, and the results are summarised graphically using forest plots in Figures 11 and 12.
| Variable | Subgroup | Treatment group | MD in change (95% CI)a | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Sex | Male | 75 | –5.97 (11.61) | 73 | –3.55 (11.72) | –0.19 (–0.59 to 0.21) | 0.441 |
| Female | 53 | –9.85 (16.73) | 47 | –4.98 (14.57) | –0.43 (–0.91 to 0.05) | ||
| Level of education | Up to A level/equivalent | 43 | –10.58 (16.46) | 40 | –2.92 (15.75) | –0.67 (–1.21 to –0.14) | 0.07 |
| Vocational/beyond A level | 82 | –5.61 (12.46) | 75 | –4.76 (11.57) | –0.07 (–0.47 to 0.33) | ||
| IMD | IMD below median | 22 | –2.64 (14.69) | 23 | 0.57 (13.40) | –0.25 (–0.95 to 0.46) | 0.929 |
| IMD above median | 54 | –6.20 (13.62) | 49 | –3.94 (12.34) | –0.21 (–0.68 to 0.26) | ||
| SIMD | SIMD below median | 24 | –13.21 (14.27) | 24 | –5.38 (14.48) | –0.54 (–1.29 to 0.21) | 0.351 |
| SIMD above median | 25 | –8.36 (13.10) | 22 | –8.05 (11.43) | –0.07 (–0.80 to 0.66) | ||
| Age (years) | < 35 | 43 | –0.89 (1.59) | 42 | –0.36 (1.35) | –0.53 (–1.05 to –0.01) | 0.538 |
| 35–49 | 49 | –0.60 (1.19) | 55 | –0.43 (1.16) | –0.15 (–0.62 to 0.32) | ||
| ≥ 50 | 36 | –0.59 (0.98) | 23 | –0.28 (0.89) | –0.22 (–0.85 to 0.41) | ||
| BMI (kg/m2) | Normal < 25 | 45 | –0.65 (1.43) | 47 | –0.52 (1.22) | –0.10 (–0.60 to 0.40) | 0.626 |
| Overweight 25–29.9 | 53 | –0.69 (1.16) | 48 | –0.27 (0.94) | –0.42 (–0.90 to 0.05) | ||
| Obese ≥ 30 | 30 | –0.77 (1.31) | 25 | –0.32 (1.49) | –0.35 (–0.99 to 0.29) | ||
| ONS occupational status | Level 1 | 31 | –7.03 (13.45) | 22 | –1.91 (18.35) | –0.38 (–1.05 to 0.30) | 0.915 |
| Level 2 | 35 | –6.40 (12.83) | 36 | –5.86 (11.60) | –0.17 (–0.74 to 0.40) | ||
| Level 3 | 38 | –8.24 (17.13) | 41 | –3.32 (13.27) | –0.36 (–0.90 to 0.17) | ||
| Level 4 | 12 | –4.67 (7.64) | 13 | –4.54 (5.98) | –0.06 (–1.02 to 0.89) | ||
| Variable | Subgroup | Treatment group | MD in changea (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Sex | Male | 75 | –6.0 (11.6) | 73 | –3.5 (11.7) | –2.1 (–6.4 to 2.3) | 0.441 |
| Female | 53 | –9.8 (16.7) | 47 | –5.0 (14.6) | –4.7 (–9.9 to 0.6) | ||
| Level of education | Up to A level/equivalent | 43 | –10.6 (16.5) | 40 | –2.9 (15.8) | –7.4 (–13.2 to –1.5) | 0.07 |
| Vocational/beyond A level | 82 | –5.6 (12.5) | 75 | –4.8 (11.6) | –0.8 (–5.1 to 3.6) | ||
| English IMDc | Below median | 22 | –2.6 (14.7) | 23 | 0.6 (13.4) | –2.7 (–10.4 to 5.0) | 0.929 |
| Above median | 54 | –6.2 (13.6) | 49 | –3.9 (12.3) | –2.3 (–7.4 to 2.8) | ||
| SIMDd | Below median | 24 | –13.2 (14.3) | 24 | –5.4 (14.5) | –5.9 (–14.1 to 2.3) | 0.351 |
| Above median | 25 | –8.4 (13.1) | 22 | –8.0 (11.4) | –0.7 (–8.7 to 7.2) | ||
| Age (years) | < 35 | 43 | –9.7 (17.4) | 42 | –3.9 (14.8) | –5.8 (–11.5 to –0.1) | 0.538 |
| 35 to 49 | 49 | –6.5 (13.0) | 55 | –4.7 (12.6) | –1.6 (–6.8 to 3.5) | ||
| ≥ 50 | 36 | –6.5 (10.7) | 23 | –3.0 (9.7) | –2.4 (–9.3 to 4.5) | ||
| BMI (kg/m2) | Normal < 25 | 45 | –7.1 (15.6) | 47 | –5.6 (13.4) | –1.1 (–6.6 to 4.4) | 0.626 |
| Overweight/25–29.9 | 53 | –7.5 (12.7) | 48 | –2.9 (10.2) | –4.6 (–9.8 to 0.6) | ||
| Obese ≥ 30 | 30 | –8.4 (14.3) | 25 | –3.5 (16.3) | –3.8 (–10.9 to 3.2) | ||
| ONS occupational statuse | Level 1 | 31 | –7.0 (13.5) | 22 | –1.9 (18.3) | –4.1 (–11.5 to 3.2) | 0.915 |
| Level 2 | 35 | –6.4 (12.8) | 36 | –5.9 (11.6) | –1.9 (–8.1 to 4.3) | ||
| Level 3 | 38 | –8.2 (17.1) | 41 | –3.3 (13.3) | –4.0 (–9.9 to 1.9) | ||
| Level 4 | 12 | –4.7 (7.6) | 13 | –4.5 (6.0) | –0.7 (–11.1 to 9.7) | ||
| Variable | Subgroup | Treatment Group | MD in change (95% CI)a | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Diabetes duration (years) | < 15 | 59 | –7.25 (16.51) | 59 | –2.69 (12.66) | –0.43 (–0.87 to 0.02) | 0.364 |
| ≥ 15 | 69 | –7.86 (11.62) | 61 | –5.48 (13.03) | –0.15 (–0.58 to 0.27) | ||
| Experience of lead DAFNE course educatorc | Less experienced | – | – | 10 | 0.90 (13.37) | – | – |
| More experienced | 128 | –7.58 (14.03) | 110 | –4.56 (12.79) | –0.26 (–0.59 to 0.06) | ||
| Insulin dose (IU/weight) | < 0.7 | 68 | –5.56 (10.48) | 57 | –3.32 (9.03) | –0.22 (–0.65 to 0.20) | 0.607 |
| ≥ 0.7 | 60 | –9.87 (17.00) | 63 | –4.83 (15.60) | –0.38 (–0.81 to 0.05) | ||
| HbA1c (%) | < 7.5 | 13 | 0.59 (0.78) | 11 | 0.14 (0.66) | 0.42 (–0.47 to 1.31) | 0.183 |
| ≥ 7.5 to < 8.5 | 34 | –0.10 (0.82) | 39 | 0.05 (0.65) | –0.12 (–0.63 to 0.38) | ||
| ≥ 8.5 | 81 | –1.15 (1.27) | 70 | –0.70 (1.36) | –0.42 (–0.77 to –0.07) | ||
| Symptoms of hypoglycaemia usually occur at blood glucose level (mmol/l) | ≥ 3 | 92 | –7.37 (13.94) | 85 | –3.41 (13.31) | –0.33 (–0.70 to 0.04) | 0.660 |
| < 3 or do not feel symptoms | 36 | –8.11 (14.46) | 35 | –5.80 (11.75) | –0.18 (–0.75 to 0.38) | ||
| Use of bolus advisor | Never or rarely | 18 | –0.44 (0.84) | 55 | –0.32 (1.23) | –0.18 (–0.81 to 0.46) | 0.736 |
| Sometimes | 10 | –0.39 (1.49) | 5 | 0.27 (1.94) | –0.72 (–2.00 to 0.55) | ||
| Often or always | 100 | –0.77 (1.33) | 60 | –0.48 (1.05) | –0.21 (–0.60 to 0.18) | ||
| Moderate hypoglycaemic episodesd | 0 | 41 | –9.73 (15.92) | 36 | –4.14 (13.57) | –0.45 (–0.98 to 0.09) | 0.795 |
| 1 | 29 | –7.17 (11.44) | 33 | –4.09 (12.80) | –0.26 (–0.85 to 0.33) | ||
| 2 or 3 | 30 | –9.07 (15.01) | 25 | –3.52 (9.67) | –0.35 (–0.98 to 0.29) | ||
| 4–9 | 16 | –8.13 (11.59) | 19 | –5.79 (15.62) | –0.41 (–1.20 to 0.37) | ||
| ≥ 10 | 12 | 3.25 (9.42) | 7 | –1.57 (14.58) | 0.32 (–0.77 to 1.42) | ||
| Variable | Subgroup | Treatment group | MD in changea (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Diabetes duration (years) | < 15 | 59 | –7.3 (16.5) | 59 | –2.7 (12.7) | –4.7 (–9.5 to 0.2) | |
| ≥ 15 | 69 | –7.9 (11.6) | 61 | –5.5 (13.0) | –1.7 (–6.3 to 3.0) | 0.364 | |
| Experience of lead DAFNE course educator | Less experienced | – | – | 10 | 0.9 (13.4) | – | |
| More experienced | 128 | –7.6 (14.0) | 110 | –4.6 (12.8) | –2.9 (–6.4 to 0.7) | – | |
| Insulin dose (IU/weight) | < 0.7 | 68 | –5.6 (10.5) | 57 | –3.3 (9.0) | –2.4 (–7.1 to 2.2) | |
| ≥ 0.7 | 60 | –9.9 (17.0) | 63 | –4.8 (15.6) | –4.1 (–8.8 to 0.6) | 0.607 | |
| HbA1c (%) | < 7.5 | 13 | 6.5 (8.6) | 11 | 1.5 (7.2) | 4.6 (–5.2 to 14.4) | |
| ≥ 7.5 to < 8.5 | 34 | –1.1 (8.9) | 39 | 0.6 (7.1) | –1.4 (–6.9 to 4.2) | ||
| ≥ 8.5 | 81 | –12.5 (13.9) | 70 | –7.6 (14.9) | –4.6 (–8.5 to –0.8) | 0.183 | |
| Symptoms of hypoglycaemia usually occur at blood glucose level | ≥ 3 mmol/l | 92 | –7.4 (13.9) | 85 | –3.4 (13.3) | –3.6 (–7.7 to 0.4) | |
| < 3 mmol/l or do not feel symptoms | 36 | –8.1 (14.5) | 35 | –5.8 (11.8) | –2 (–8.2 to 4.2) | 0.660 | |
| Use of bolus advisor | Never or rarely | 18 | –4.8 (9.2) | 55 | –3.5 (13.4) | –1.9 (–8.9 to 5.0) | |
| Sometimes | 10 | –4.3 (16.3) | 5 | 3.0 (21.2) | –7.9 (–21.8 to 6.0) | ||
| Often or always | 100 | –8.4 (14.5) | 60 | –5.3 (11.5) | –2.3 (–6.6 to 2.0) | 0.736 | |
| Moderate hypoglycaemic episodesc | 0 | 41 | –9.7 (15.9) | 36 | –4.1 (13.6) | –4.9 (–10.7 to 1.0) | |
| 1 | 29 | –7.2 (11.4) | 33 | –4.1 (12.8) | –2.9 (–9.3 to 3.6) | ||
| 2 or 3 | 30 | –9.1 (15.0) | 25 | –3.5 (9.7) | –3.8 (–10.7 to 3.1) | ||
| 4–9 | 16 | –8.1 (11.6) | 19 | –5.8 (15.6) | –4.5 (–13.1 to 4.1) | ||
| ≥ 10 | 12 | 3.3 (9.4) | 7 | –1.6 (14.6) | 3.5 (–8.5 to 15.6) | 0.795 | |
FIGURE 11.
Subgroup evaluation (demographic characteristics) MD in HbA1c change (%) at 24 months by demographic subgroup. MCID, minimum clinically important difference; ONS, Office for National Statistics; SIMD, Scottish Index of Multiple Deprivation. Reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
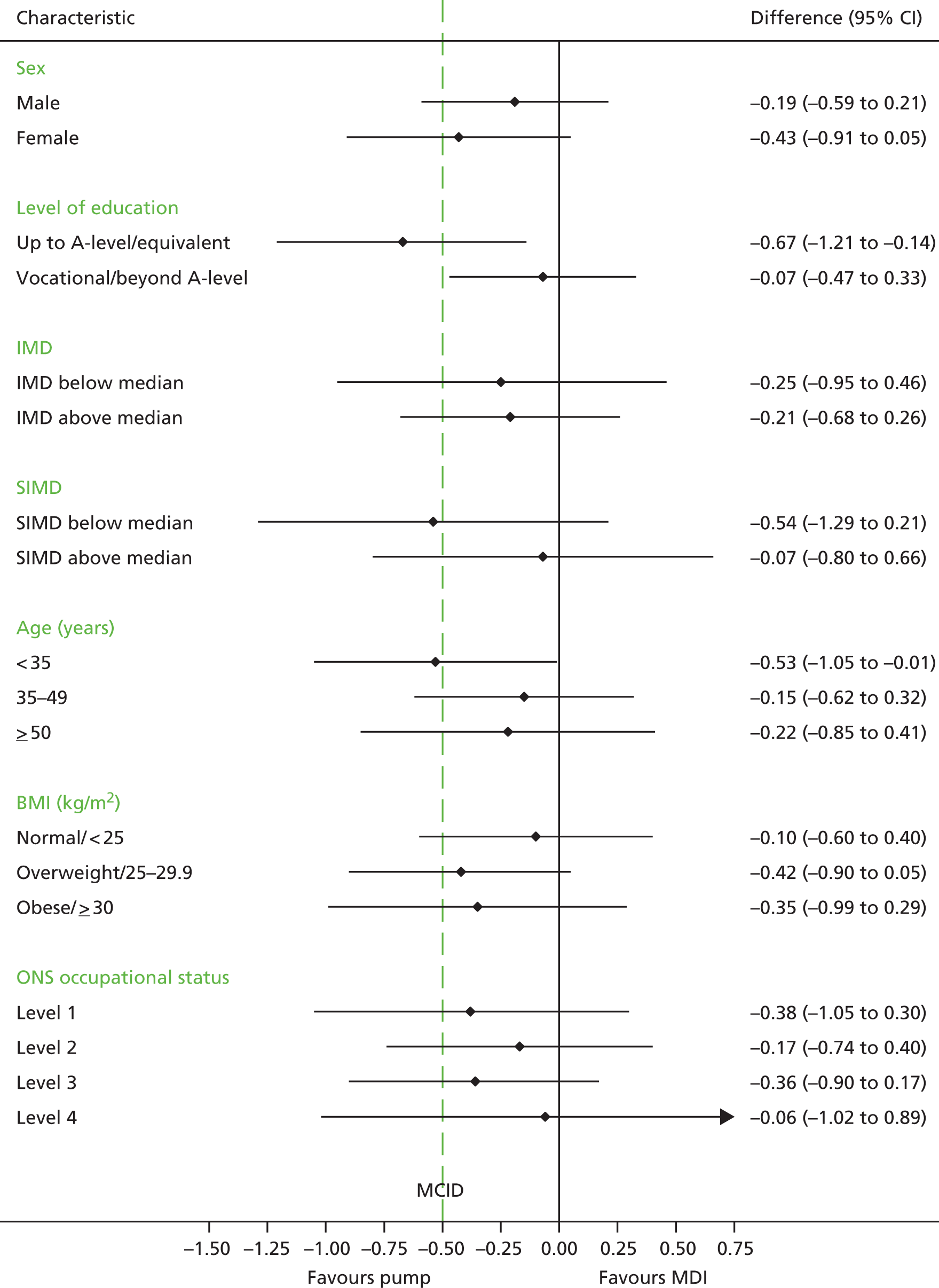
FIGURE 12.
Subgroup evaluation (diabetes characteristics): MD in HbA1c change (%) at 24 months by subgroup. MCID, minimum clinically important difference. Reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.

We found no reliable statistical evidence of any subgroup effects or interactions between the pump and MDI groups. However, there was some indication that participants with qualifications up to A-level/equivalent did better in the pump arm than in the MDI arm – MD in HbA1c change (95% CI) at 24 months of –0.67% (–1.21% to –0.14%) vs. –0.07% (–0.47% to 0.33%) or –7.4 mmol (–13.2 to –1.5 mmol) vs. –0.8 (–5.1 to 3.6 mmol) – although the interaction test was not statistically significant (p = 0.07). 23
Ancillary analyses
Adverse events
Table 38 shows the AEs recorded throughout study follow-up. More participants in the pump arm (66%) reported AEs than in the MDI arm (37%). However, part of this difference can be attributed to the 23 cases of suspicion of pump malfunction, which, by definition, could occur only for participants using pump therapy. Table 39 shows the AEs that were recorded over different time periods during study follow-up. During each time period, more participants in the pump arm experienced AEs than in the MDI arm. A total of 142 AEs were recorded for the pump group during the first 6 months of follow-up in comparison with 84 in the following 6 months, and 94 in the final 12 months of follow-up, suggesting that more AEs occurred during the early ‘settling in’ period on pump therapy.
| Outcome | Classification | Treatment group | Total (N = 267) | |
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | |||
| Participants with ≥ 1 AE, n (%) | Any AE | 87 (65.9) | 50 (37.0) | 137 (51.3) |
| Increase in hypoglycaemic episode frequency | 11 (8.3) | 8 (5.9) | 19 (7.1) | |
| Blood glucose reading > 30 mmol/l | 55 (41.7) | 16 (11.9) | 71 (26.6) | |
| Raised blood glucosea | 16 (12.1) | 6 (4.4) | 22 (8.2) | |
| Suspicion of pump malfunction | 23 (17.4) | – | 23 (8.6) | |
| Pregnancy | 6 (4.5) | 4 (3.0) | 10 (3.7) | |
| Infection at pump cannula site | 2 (1.5) | – | 2 (0.7) | |
| DKA | 7 (5.3) | 2 (1.5) | 9 (3.4) | |
| Other | 28 (21.2) | 28 (20.7) | 56 (21.0) | |
| Participants with ≥ 1 AE related to study drug, n (%) | Any AE | 40 (30.3) | 14 (10.4) | 54 (20.2) |
| Increase in hypoglycaemic episode frequency | 4 (3.0) | 5 (3.7) | 9 (3.4) | |
| Blood glucose reading > 30 mmol/l | 27 (20.5) | 5 (3.7) | 32 (12.0) | |
| Raised blood glucoseb | 5 (3.8) | 1 (0.7) | 6 (2.2) | |
| Suspicion of pump malfunction | 13 (9.8) | – | 13 (4.9) | |
| Pregnancy | – | – | – | |
| Infection at pump cannula site | 1 (0.8) | – | 1 (0.4) | |
| DKA | 1 (0.8) | – | 1 (0.4) | |
| Other | 3 (2.3) | 3 (2.2) | 6 (2.2) | |
| Number of AEsb | 321 | 102 | 423 | |
| Participants with ≥ 1 AE | Follow-up period (months) | Treatment group | Total (N = 267) | |
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | |||
| Any AE, n (%) | 0–6 | 57 (28.9) | 22 (15.0) | 79 (23.0) |
| 6–12 | 48 (24.4) | 16 (10.9) | 64 (18.6) | |
| 12–24 | 46 (23.4) | 23 (15.6) | 69 (20.1) | |
| Number of AEsa | 0–6 | 142 | 37 | 179 |
| 6–12 | 84 | 22 | 106 | |
| 12–24 | 94 | 42 | 136 | |
Table 40 shows the AEs that were classified as being SAEs. The distribution of SAEs was similar across the treatment groups, with the exception that more participants experienced DKA in the pump group. Table 41 shows SAEs by study time period. Again, for the pump group, more SAEs were recorded in the first 6 months (n = 17) than in the following 6 months (n = 11) or when compared with the last 12 months (n = 17).
| Outcome | Classification | Treatment group | Total (N = 267) | |
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | |||
| Participants with ≥ 1 SAE, n (%) | Any SAE | 31 (23.5) | 26 (19.3) | 57 (21.3) |
| DKA | 17 (12.9) | 5 (3.7) | 22 (8.2) | |
| MI | 2 (1.5) | – | 2 (0.7) | |
| Severe hypoglycaemia | – | 1 (0.7) | 1 (0.4) | |
| Foot ulcer | 1 (0.8) | – | 1 (0.4) | |
| Renal disease | 1 (0.8) | – | 1 (0.4) | |
| Abdominal pain | 1 (0.8) | 2 (1.5) | 3 (1.1) | |
| Pregnancya | 4 (3.0) | 3 (2.2) | 7 (2.6) | |
| Hyperglycaemia | 3 (2.3) | 4 (3.0) | 7 (2.6) | |
| Migraine | – | 1 (0.7) | 1 (0.4) | |
| Overdose/suicide attempt | 1 (0.8) | 1 (0.7) | 2 (0.7) | |
| Chest pain | 2 (1.5) | 1 (0.7) | 3 (1.1) | |
| Infection | 2 (1.5) | 2 (1.5) | 4 (1.5) | |
| Other | 5 (3.8) | 12 (8.9) | 17 (6.4) | |
| Participants with ≥ 1 SAE related to a treatment, n (%) | All | 5 (3.8) | 2 (1.5) | 7 (2.6) |
| Participants with ≥ 1 SAE by intensity, n (%) | Mild | 6 (4.5) | 6 (4.4) | 12 (4.5) |
| Moderate | 20 (15.2) | 18 (13.3) | 38 (14.2) | |
| Severe | 9 (6.8) | 5 (3.7) | 14 (5.2) | |
| Participants with ≥ 1 treatment-related SAE by intensity, n (%) | Mild | – | – | – |
| Moderate | 4 (3.0) | 2 (1.5) | 6 (2.2) | |
| Severe | 1 (0.8) | – | 1 (0.4) | |
| Number of SAEs | All | 45b | 44b | 89b |
| Number of SAEs related to treatment | Definite | 4 | 1 | 5 |
| Probable | 1 | 1 | 2 | |
| Possible | 5 | 2 | 7 | |
| Unlikely | 14 | 13 | 27 | |
| Unrelated | 21 | 27 | 48 | |
| Number of SAEs by intensity | Mild | 6 | 8 | 14 |
| Moderate | 29 | 26 | 55 | |
| Severe | 10 | 10 | 20 | |
| Outcome | Classification | Follow-up period (months) | Treatment group | Total (N = 267) | |
|---|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | ||||
| Participants with ≥ 1 SAE, n (%) | Any SAE | 0–6 | 14 (10.1) | 7 (5.1) | 21 (7.6) |
| 6–12 | 10 (7.2) | 5 (3.6) | 15 (5.4) | ||
| 12–24 | 13 (9.4) | 17 (12.3) | 30 (10.9) | ||
| DKA | 0–6 | 8 (5.8) | 1 (0.7) | 9 (3.3) | |
| 6–12 | 7 (5.1) | – | 7 (2.5) | ||
| 12–24 | 4 (2.9) | 4 (2.9) | 8 (2.9) | ||
| Number of SAEsa | 0–6 | 17 | 10 | 27 | |
| 6–12 | 11 | 5 | 16 | ||
| 12–24 | 17 | 29 | 46 | ||
Note: All of the DKAs that occurred were reported as SAEs and resulted in hospitalisation. All of the SAEs have a corresponding AE; however, in some cases, a DKA SAE had a corresponding AE that was not labelled as DKA, which is why there are more DKA SAEs recorded than DKA AEs.
Characteristics of participants by missing data status
Tables 42 and 43 show baseline characteristics of patients with missing data.
| Variable | Statistic | Non-completers, n (%) | Completers, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Pump (N = 4) | MDI (N = 15) | All (N = 19) | Pump (N = 128) | MDI (N = 120) | All (N = 248) | ||
| Age (years) | Mean (SD) | 33.4 (15.4) | 32.6 (10.9) | 32.8 (11.5) | 41.7 (14.2) | 40.8 (12.4) | 41.3 (13.3) |
| Median (IQR) | 27.7 (22.9–44.0) | 29.9 (23.6–39.8) | 29.9 (23.0–39.8) | 40.9 (29.0–52.3) | 42.8 (30.9–49.1) | 41.9 (30.2–49.5) | |
| Diabetes duration (years) | Mean (SD) | 21.6 (18.2) | 13.8 (9.6) | 15.4 (11.7) | 18.5 (12.8) | 18.0 (12.3) | 18.2 (12.5) |
| Median (IQR) | 17.3 (9.7–33.5) | 12.4 (5.4–20.2) | 14.2 (5.4–20.2) | 16.5 (7.8–27.7) | 15.4 (8.2–25.9) | 15.9 (7.9–26.7) | |
| HbA1c (mmol/mol) | Mean (SD) | 94.0 (24.3) | 83.4 (17.5) | 85.6 (18.9) | 77.4 (20.8) | 73.7 (15.1) | 75.6 (18.3) |
| Median (IQR) | 97 (73.5–114.5) | 85 (68.0–97.0) | 85 (68.0–103.0) | 73 (65.0–87.0) | 71 (64.0–82.0) | 71.5 (64.0–85.0) | |
| HbA1c (%) | Mean (SD) | 10.8 (2.2) | 9.8 (1.6) | 10.0 (1.7) | 9.2 (1.9) | 8.9 (1.4) | 9.1 (1.7) |
| Median (IQR) | 11.0 (8.9–12.6) | 9.9 (8.4–11.0) | 9.9 (8.4–11.6) | 8.8 (8.1–10.1) | 8.6 (8.0–9.7) | 8.7 (8.0–9.9) | |
| BMI (kg/m2) | Mean (SD) | 29.8 (12.4) | 27.0 (5.1) | 27.6 (6.9) | 27.3 (4.7) | 27.0 (5.0) | 27.1 (4.8) |
| Median (IQR) | 25.8 (22.5–37.2) | 26.9 (22.3–29.8) | 26.4 (22.3–29.8) | 27.2 (23.8–29.7) | 26.5 (23.7–29.2) | 27 (23.8–29.5) | |
| Variable | Scoring | Non-completers, n (%) | Completers, n (%) | ||||
|---|---|---|---|---|---|---|---|
| Pump (N = 4) | MDI (N = 15) | All (N = 19) | Pump (N = 128) | MDI (N = 120) | All (N = 248) | ||
| Sex | Male | 3 (75.0) | 9 (60.0) | 12 (63.2) | 75 (58.6) | 73 (60.8) | 148 (59.7) |
| Female | 1 (25.0) | 6 (40.0) | 7 (36.8) | 53 (41.4) | 47 (39.2) | 100 (40.3) | |
| Smoking status | Smoker | 2 (50.0) | 5 (33.3) | 7 (36.8) | 21 (16.4) | 25 (20.8) | 46 (18.5) |
| Ex-smoker | 1 (25.0) | 6 (40.0) | 7 (36.8) | 41 (32.0) | 21 (17.5) | 62 (25.0) | |
| Never smoker | 1 (25.0) | 4 (26.7) | 5 (26.3) | 66 (51.6) | 74 (61.7) | 140 (56.5) | |
| Ethnicity | White British | 4 (100.0) | 14 (93.3) | 18 (94.7) | 121 (94.5) | 105 (87.5) | 226 (91.1) |
| Other | – | 1 (6.7) | 1 (5.3) | 3 (2.3) | 11 (9.2) | 14 (5.6) | |
| Prefer not to say | – | – | – | 1 (0.8) | 3 (2.5) | 4 (1.6) | |
Findings of the fidelity assessment
Course characteristics
All eight REPOSE centres were fidelity tested. Four centres were fidelity tested on their second pump course and two centres were fidelity tested on their first pump course. One centre was fidelity tested on their third and final course as a result of personal circumstances of the FA precluding the assessment being undertaken on the second pump course. Nottingham ran one pair of courses and, thus, FT took place on its only pump course.
The number of REPOSE participants on the fidelity-tested pump courses ranged from 3 to 7 (for course sizes, see Table 44). The range of participants on the remaining pump courses was 3–8, with a mean of 5.7.
| Centre | Number of participants on fidelity-tested course |
|---|---|
| Cambridge | 7 |
| Dumfries | 7 |
| Edinburgh | 4 |
| Glasgow | 7 |
| Harrogate | 6 |
| London (King’s College Hospital) | 3 |
| Nottingham | 4 |
| Sheffield | 7 |
One pump course (Cambridge) included a non-REPOSE participant who had been on a pump for 10 years and was very keen to do DAFNE.
Pump pre-course session
All participants attended the pump pre-course session to learn the mechanics of pump therapy and to programme and load the pump with saline to enable practice and familiarisation prior to undertaking the course. This session was scheduled to run for 2 hours and 30 minutes (± 15 minutes). Seven out of the eight centres ran sessions within this duration window. The centre that did not (Nottingham) ran a pump pre-course session of 2 hours and so was 15 minutes short of the specified duration window.
The majority of centres delivered the pump pre-course session solely by REPOSE educators (diabetes specialist nurses and dietitians). Two centres (Glasgow and Edinburgh) had a Medtronic representative present to provide technical support and help with elements of pump set-up. One centre (Glasgow) also had the PI present.
All pump pre-course session lesson plans were evaluated by the FA as relating to the objectives set for this session.
Insulin switchover
Participants were asked to switch over their pump from saline to insulin the evening before their pump DAFNE course if they felt happy to do so.
All course participants switched the evening before their course at three centres (Nottingham, Edinburgh and Harrogate). At Glasgow, all participants switched to insulin on the morning of course. This was a decision taken by the personnel at that centre who, after already having run one pump course, felt that this approach worked best, and course participants had not expressed any preference for the Sunday evening. At the remaining centres, the majority of participants switched to insulin the night before their course. Those who did not cited the following reasons:
-
unsure of how to fit reservoir
-
started in previous week but stopped, as wanted support from health professionals
-
anxiety regarding change
-
timing issues and technical problems with pump
-
pump failure/motor alarming problem
-
ran out of consumables and had cannula problems.
Pump courses
The pump course timetable was reviewed by the FA. All centres provided timetables that were evaluated as incorporating all elements of the pump DAFNE curriculum in a logical order. Based on the times allocated for sessions on the pump course timetable, all centres planned to deliver the curriculum in the specified duration window of ≥ 1870 minutes but ≤ 2280 minutes. The mean course duration was 2006 minutes, that is 33 hours and 26 minutes.
All sessions planned to be observed were reviewed during the fidelity visit and their lesson plans were reviewed.
The sick day rules lesson plan was reviewed for each centre. Seven of the eight centres were evaluated as having no issues with this session lesson plan, with only minor problems noted, for example no aims or objectives listed, timings not written on. One centre (Glasgow) was evaluated as having an issue with the sick day rule lesson plan. The lesson plan was lifted directly from the pump DAFNE curriculum without personalisation. The Glasgow educator explained that there was no time to personalise the lesson plan but agreed to remedy for future courses.
Essential learning outcomes
The FA recorded (with evidence) if all essential learning outcomes were met in the sessions observed. Sessions were recorded as having met all learning outcomes: ‘yes’ or ‘no’ or partially achieving essential learning outcomes. Table 45 provides a summary.
| Centre | Daily goals, blood glucose results and insulin dosesb | Insulin dose adjustment theory, basal rate testing | DA escalation | DA reduction | Setting up bolus wizard | Exercise | Alcohol | Lunchtime CP | Corrections |
|---|---|---|---|---|---|---|---|---|---|
| Sheffield | Y | Y | Y | P (95%) | Y | P (95%) | Y | Y | Not observed |
| Cambridge | P (70%) | Y | Y | Y | Y | Y | Y | Not observed | Y |
| London (King’s College Hospital) | P (60%) | Y | Y | P (80%) | Y | Y | Y | Y | P (95%) |
| Harrogate | P (90%) | Y | P (95%) | P (95%) | Y | Y | Y | P (98%) | Not observed |
| Nottingham | P (80%) | Y | P (90%) | P (90%) | Y | Not observedc | Not observedc | P (70%) | Not observed |
| Glasgow | P (85%) | Y | P (98%) | P (95%) | Y | Y | Y | Y | Y |
| Edinburgh | P (85%) | Y | Y | Y | Y | Y | Y | P (60%) | Not observed |
| Dumfries | P (55%) | Y | Y | Y | Y | Y | Y | Not observed | Y |
For three sessions (‘Insulin dose adjustment theory and basal rate testing’, ‘Setting up the bolus wizard’ and ‘Alcohol’) all of the centres met all of the essential learning outcomes. For the ‘Exercise’ session, seven centres met the essential learning outcomes and the remaining centre (Sheffield) met 95% of learning outcomes.
For the dose escalation and reduction sessions, all of the centres either met or partly met all of the essential learning outcomes. For the centres that partly met the learning outcomes for these sessions, 80–98% of learning outcomes were met.
The essential learning outcomes for the session ‘Daily goals, blood glucose results and insulin doses’ were partly met at seven of the eight centres and fully met at one centre (100%). It is important to note for this session, which is delivered at the beginning and end of each day, it is expected that some essential learning outcomes will not be covered in one session, as it is guided by situations that the patients have recorded in their diaries. During the DAFNE course, as new situations are observed, further essential learning outcomes are generally covered.
Although not essential for the FT, the FA observed the ‘Lunchtime CP (carbohydrate portion)’ and the ‘Corrections’ sessions at some centres.
Overall fidelity assessment concerns and action plans
The FA was asked to make an overall assessment of whether or not there were any major concerns about the delivery of the pump course and, if there were, any recommended actions to be taken. These are summarised by centre in Table 46.
| Centre | Concerns | Action |
|---|---|---|
| Sheffield | Main issue was timing. Bolus wizard set-up also took longer than timetabled | Team to discuss and consider allocating more time for these |
| London (King’s College Hospital) | Pump set-up lesson plan not seen, as not available on the day. Some learning outcomes not observed on the day but not a cause for concern | Pump set-up plan to be e-mailed |
| Cambridge | No concerns. Discussion regarding CP estimation and corrections that were not observed, as covered in detail on other days at beginning of the week. No deviation from curriculum | None |
| Harrogate | Discussed timings around pump set-up session. Some learning outcomes were covered earlier in the week or will be covered in other sessions, especially around dose adjustment | Educators to reflect on the week and consider group evaluation and timings, etc. |
| Nottingham | Some lesson plans were not very detailed, for example dose escalation and reduction, and so some essential learning outcomes were left out. Educator agreed and noted that no internal QA had been done for a while because of the inconsistency of staffing levels | This is a priority to rewrite lesson plans and to think about QA once their new DAFNE educator has run some courses and had the training |
| Glasgow | Term ‘rebound hyperglycaemia’ used, that is, when blood glucose level is normal at bedtime but high in the morning. Educator said it is due to hypoglycaemic episodes in the night and the liver releasing glucose. DAFNE does not say this and educator referred to p. 134 of curriculum escalation. Educator said that they did not know that this was the case and will make it clear to educators that the explanation will be overtreatment of a hypoglycaemia/dawn phenomenon/basal rate not correct Lesson plan for sick day rules not personalised Some DA practice escalation and reduction essential learning outcomes not covered |
Personalisation of sick days rules lesson plan Educator to ensure that all of the team knows not to use ‘rebound hyperglycaemia’ term |
| Edinburgh | No major concerns regarding delivery. Lots of questions from participants on one session, so it went over time. Rebound hyperglycaemia (after night-time hypoglycaemia and increased blood glucose in the morning) was used | Explained that the term ‘rebound hyperglycaemia’ was not used in the DAFNE curriculum. The group understood, however, that they should not correct a raise of blood glucose following an episode of hypoglycaemia |
| Dumfries | Rebound hyperglycaemia term being used (i.e. raised blood glucose in the morning after a normal bedtime reading owing to rebound after night-time hypoglycaemia); discussed that not used in DAFNE and educator confirmed that they had not wished it to come over like this but patients obviously interpreting as such | Discussed the need to word things differently and educators agreed to make sure that the group understood this over the rest of the course |
Conclusion
Overall, the pump courses appear to have been delivered according to the pump course curriculum. The pump courses observed seem representative of pump courses on REPOSE in terms of course characteristics. The pre-course session was delivered consistently and met the objectives set. All pump courses were planned to run in a logical order within the time frame specified. There were problems with the term ‘rebound hyperglycaemia’ being used (three centres) and non-personalisation of lesson plan (one centre).
Generally, essential learning outcomes were consistently delivered during the sessions. The session ‘Daily goals, blood glucose results and insulin doses’ had the lowest percentage of essential outcomes met. This is not unusual for this session, when learning outcomes are met during the week of the course. In standard care, learning outcomes may also be omitted in other sessions during the week of the DAFNE course, but are subsequently covered in other sessions. This can be for various reasons, for example more pressing issues and questions raised by the participants. With appropriate timetabling and timings allocated to sessions, there should be sufficient time for experienced educators to deliver all of the essential learning outcomes for all sessions. The key thing is that educators have awareness of any learning outcomes that have been missed and can produce a strategy for how they will incorporate the missed content at another relevant stage of the week, or indeed at the 6-week follow-up session if necessary.
The quality assurance programme for MDI DAFNE courses in standard care audits the entire DAFNE course week, whereas the REPOSE FT was restricted to 1 day of the course. Therefore, although the quality assurance programme of MDI courses can examine if missed learning outcomes are covered in later sessions, this was not possible for the REPOSE FT of the pump courses, and is a limitation. Nevertheless, the number of missed learning outcomes was still low.
Chapter 6 Results of the economic evaluation
Cost of insulin pumps and consumables
The weighted average cost of an insulin pump from the pump costing survey was £2571. The cost of insulin pumps was converted into a yearly cost using annuitisation. The lifetime of the insulin pumps was taken to be 4.5 years and the discount rate was that used by NICE (3.5%). 22 This gave a weighted average yearly cost of insulin pumps to be £627. The weighted average yearly cost of insulin pump consumables was £1433.
Within-trial cost-effectiveness analysis
Base-case analysis
The results of the within-trial cost-effectiveness analysis are presented using a confidence ellipse in Figure 13. In the base case, pump + DAFNE was dominated by MDI + DAFNE, as pump + DAFNE produced fewer mean QALYs at a higher mean cost. The confidence ellipse shows that pump + DAFNE was associated with statistically significantly higher costs than MDI + DAFNE at the 5% significance level, as the confidence ellipse does not cross the x-axis at £0. The confidence ellipse also shows that pump + DAFNE was not associated with statistically significantly lower QALYs than MDI + DAFNE at the 5% significance level. This is because the confidence ellipse crosses the y-axis of the graph at 0. Another point to note is that the confidence ellipses do not cross a threshold ICER of £20,000 per QALY gained; therefore, the ICER of pump + DAFNE compared with MDI + DAFNE is greater than £20,000 per QALY gained at the 95% confidence level.
FIGURE 13.
Confidence ellipse for the base-case within-trial analysis: controlling for baseline utility.
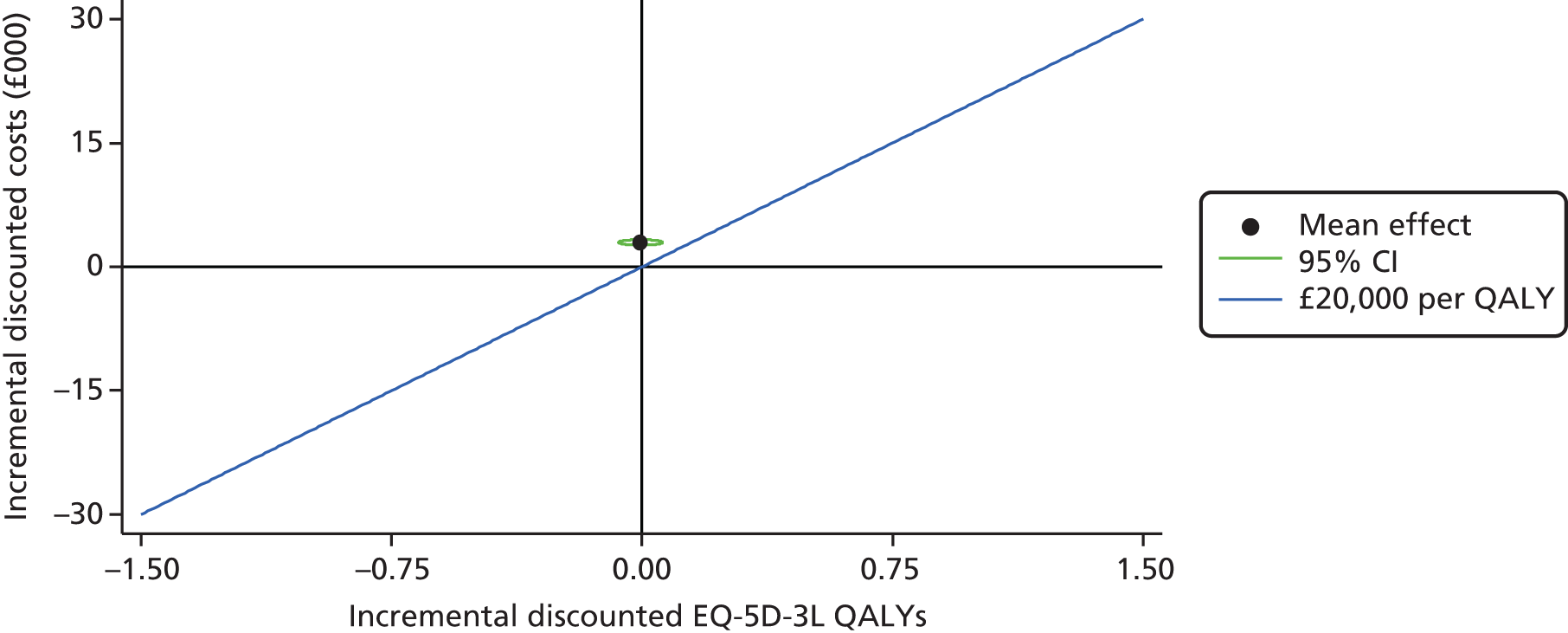
The cost-effectiveness acceptability curve is presented in Figure 14. It shows that pump + DAFNE has a 0.0% chance of being considered cost-effective at threshold ICERs of £20,000 per QALY gained and £30,000 per QALY gained. This is important as, based on the data in the REPOSE Trial, pump + DAFNE has a 0% probability of being cost-effective at the thresholds used by NICE in the UK for decision-making. 22
FIGURE 14.
Cost-effectiveness acceptability curve for the base-case within-trial analysis: controlling for baseline utility.

Table 47 presents the incremental cost and QALY outcomes of pump + DAFNE compared with MDI + DAFNE in each year of the trial and for both years combined. In the base case, the incremental cost in year 2 is lower than the cost in year 1. This is probably due to (1) treatment switching and (2) the rate of DKAs and severe hypoglycaemic events being noticeably lower in the pump + DAFNE arm in the second year than in the first year. The incremental QALYs are negative in the first year and positive in the second year. However, in neither year is this result statistically significant and, in both years, the central estimates are less than one-hundredth of a QALY. This is not unusual in diabetes trials, in which the crucial QALY gains due to an intervention come much later in the patient experience because of a reduced risk of long-term complications.
| n | Incremental costs (£), mean (95% CI) | Incremental QALYs, mean (95% CI) | ICER, £/QALY gained | |||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Total | Year 1 | Year 2 | Total | |||
| Base case | ||||||||
| ITT population with complete costs and QALYs | 205 | 1732 (1511 to 1952) | 1228 (1063 to 1392) | 2959 (2692 to 3227) | –0.007 (–0.036 to 0.022) | 0.003 (–0.029 to 0.035) | –0.004 (–0.057 to 0.048) | Dominated |
| Scenario analyses | ||||||||
| Per-protocol population | 188 | 1780 (1520 to 2041) | 1434 (1328 to 1539) | 3214 (2916 to 3513) | –0.003 (–0.034 to 0.027) | 0.006 (–0.026 to 0.037) | 0.002 (–0.051 to 0.056) | 1,369,287 |
| Imputed data | 260 | 1697 (1492 to 1901) | 1175 (1006 to 1345) | 2872 (2602 to 3142) | –0.013 (–0.039 to 0.014) | 0.004 (–0.029 to 0.037) | –0.009 (–0.058 to 0.04) | Dominated |
| SF-6D QALYs | 196 | 1746 (1514 to 1978) | 1254 (1096 to 1412) | 3000 (2729 to 3271) | –0.001 (–0.021 to 0.019) | –0.002 (–0.027 to 0.023) | –0.003 (–0.045 to 0.039) | Dominated |
| Imputed data and SF-6D QALYs | 256 | 1701 (1494 to 1908) | 1186 (1016 to 1357) | 2888 (2616 to 3159) | –0.003 (–0.021 to 0.015) | –0.001 (–0.024 to 0.022) | –0.004 (–0.041 to 0.034) | Dominated |
| Riemsma et al.,8 pump costs | 205 | 1679 (1450 to 1908) | 1184 (1024 to 1343) | 2863 (2586 to 3140) | –0.007 (–0.036 to 0.022) | 0.003 (–0.029 to 0.035) | –0.004 (–0.057 to 0.048) | Dominated |
| Imputed data Riemsma et al.,8 pump costs | 260 | 1648 (1434 to 1861) | 1125 (964 to 1286) | 2772 (2498 to 3047) | –0.013 (–0.039 to 0.014) | 0.004 (–0.029 to 0.037) | –0.009 (–0.058 to 0.04) | Dominated |
| The cost of pumps and consumables are 25% lower | 205 | 1285 (1022 to 1547) | 955 (850 to 1059) | 2239 (1786 to 2314) | –0.007 (–0.036 to 0.022) | 0.003 (–0.104 to 0.11) | –0.004 (–0.057 to 0.048) | Dominated |
| The cost of pumps and consumables are 25% lower in a per-protocol analysis | 188 | 1223 (1010 to 1436) | 768 (634 to 902) | 1991 (1939 to 2540) | –0.003 (–0.034 to 0.027) | 0.006 (–0.026 to 0.037) | 0.002 (–0.051 to 0.056) | 966,218 |
| The cost of pumps and consumables are 50% lower | 205 | 767 (532 to 1001) | 375 (255 to 494) | 1141 (873 to 1409) | –0.007 (–0.036 to 0.022) | 0.003 (–0.029 to 0.035) | –0.004 (–0.057 to 0.048) | Dominated |
| The cost of pumps and consumables are 50% lower in a per-protocol analysis | 188 | 789 (524 to 1053) | 475 (372 to 579) | 1264 (961 to 1567) | –0.004 (–0.034 to 0.027) | 0.006 (–0.026 to 0.037) | 0.002 (–0.051 to 0.056) | 552,866 |
Summary of the scenario analyses
The following scenario analyses were undertaken to explore structural uncertainty in the base-case analysis:
-
per-protocol population
-
missing cost and QALY data were imputed
-
QALYs measured by the SF-6D were used instead of QALYs measured using the EQ-5D
-
imputed data and QALYs measured by SF-6D QALYs
-
pump costs measured by Riemsma et al. 8 were used
-
Riemsma et al. 8 pump costs were used and missing data were imputed
-
the cost of pumps and consumables are 25% lower
-
the cost of pumps and consumables are 25% lower in a per-protocol population
-
the cost of pumps and consumables are 50% lower
-
the cost of pumps and consumables are 50% lower in a per-protocol population.
In the first scenario, EEACT was conducted in the per-protocol population as this was a pre-specified subgroup analysis (see Chapter 2, Population and subgroups for analysis). In the second scenario, missing cost and QALY data were imputed to explore the uncertainty that may result from having incomplete data, as in the base-case analysis only 78.85% of people had complete cost and QALY data. Details of the imputation procedure used are provided in Chapter 3 (see Estimating the within-trial cost effects and Estimating within-trial quality-adjusted life-year effects using EuroQol-5 Dimensions and Short Form questionnaire-12 items). A further sensitivity analysis was conducted, for which the SF-6D measure, instead of the EQ-5D, was used to calculate QALYs. This scenario analysis was conducted to explore if changing the preference-based measure of health changed the estimated QALY values significantly enough to potentially change the conclusions on cost-effectiveness (see Chapter 3, Estimating within-trial quality-adjusted life-year effects using EuroQol-5 Dimensions and Short Form questionnaire-12 items). Uncertainty in insulin pump costs was also explored, to see if significant discounts from the prices observed at the REPOSE Trial sites would lead to pump + DAFNE being considered to be cost-effective compared with MDI + DAFNE. Several of these uncertainties were also combined in other scenarios to determine if the joint effect of the uncertainties had any meaningful effect on the conclusions.
Results of the scenario analyses
The results of the scenario analyses are also presented in Table 47. It is clear that pump + DAFNE compared with MDI + DAFNE generated fewer QALYS at a higher cost in all analyses, apart from those scenarios conducted in the per-protocol population. The lowest ICER is observed in the scenario for which the per-protocol population is used, and there is a cost reduction in insulin pumps and consumables of 50%. The ICER in this scenario is £552,866, which is above the £20,000–30,000 per QALY gained threshold considered by NICE. 22 Therefore, based on the data observed directly in the REPOSE Trial, pump + DAFNE would be unlikely to be considered cost-effective if it were to be assessed by NICE.
Clinical evidence used to inform the cost-effectiveness of pump + Dose Adjustment For Normal Eating compared with multiple daily injection + Dose Adjustment For Normal Eating
This section details the results of the statistical models fitted to estimate the incidence of treatment switching, HbA1c, the risk of severe hypoglycaemia, the risk of DKA, the cost of insulin, the cost of diabetes-related contacts and the cost of insulin pumps. The parameters presented in these statistical models were directly included in the long-term health economic model, except for the risk of severe hypoglycaemia and the risk of DKA, for which simulations of the expected number of events were inputted into the long-term health economic model. In the PSA, the uncertainty in the parameters of these statistical models was assumed to follow a multivariate normal distribution. Variance–covariance matrices are available from the authors on request.
Treatment switching
The results of the exponential and Weibull parametric survival models for individuals randomised to MDI + DAFNE and pump + DAFNE are given in Table 48. The results for the Gompertz, log-logistic and log-normal parametric models are given in Appendix 14. It was not possible to estimate a survival curve using a generalised gamma distribution, as the model did not converge in either trial arm.
| Parameter | Coefficient | Robust SE | 95% CI |
|---|---|---|---|
| Pump + DAFNE | |||
| Exponential model | |||
| HbA1c | 0.222 | 0.241 | –0.251 to 0.695 |
| Number of DKAs | –0.972 | 0.474 | –1.901 to –0.042 |
| Number of severe hypoglycaemic events | 0.427 | 0.087 | 0.257 to 0.598 |
| Constant | –4.616 | 2.125 | –8.781 to –0.451 |
| Weibull model | |||
| HbA1c | 0.221 | 0.234 | 0.016 to 0.694 |
| Number of DKAs | –0.981 | 0.471 | –7.113 to –4.910 |
| Number of severe hypoglycaemic events | 0.404 | 0.085 | 0.337 to 0.684 |
| Constant | –4.460 | 2.100 | –10.607 to –4.696 |
| ln-scale parameter | –0.258 | 0.220 | 0.111 to 1.377 |
| MDI + DAFNE | |||
| Exponential model | |||
| HbA1c | 0.336 | 0.164 | 0.014 to 0.657 |
| Number of DKAs | –5.555 | 0.561 | –6.655 to –4.455 |
| Number of severe hypoglycaemic events | 0.460 | 0.074 | 0.315 to 0.605 |
| Constant | –6.725 | 1.450 | –9.567 to –3.884 |
| Weibull model | |||
| HbA1c | 0.355 | 0.173 | 0.016 to 0.694 |
| Number of DKAs | –6.012 | 0.562 | –7.113 to –4.910 |
| Number of severe hypoglycaemic events | 0.510 | 0.089 | 0.337 to 0.684 |
| Constant | –7.652 | 1.508 | –10.607 to –4.696 |
| ln-scale parameter | 0.744 | 0.323 | 0.111 to 1.377 |
In the pump + DAFNE arm, it was predicted that an individual was more likely to switch treatment if they had a severe hypoglycaemic event or if they had a higher HbA1c. It was also observed that an individual was less likely to switch from CSII to MDI if they had experienced a DKA event in the previous year. All of these results are statistically significant at the 5% level in the Weibull and exponential models, except for the effect of HbA1c on the probability of switching in the exponential model.
In the MDI + DAFNE arm, the relationships between HbA1c, number of severe hypoglycaemic episodes in the year prior to switching and the number of DKAs in the year prior to switching worked in a similar way to the pump + DAFNE arm. It should be noted that the effect sizes are different in the two arms for different covariates. In the MDI + DAFNE arm, all of the coefficients were statistically significant at the 5% level.
Table 49 shows the AIC and BIC for the different survival models fitted to pump + DAFNE individuals and MDI + DAFNE individuals. In the pump + DAFNE arm, the curve with lowest AIC and BIC was the exponential model. For the MDI + DAFNE individuals, the curve with the lowest AIC was the Weibull model and the curve with the lowest BIC was the exponential model.
| Distribution | AIC | BIC |
|---|---|---|
| Pump + DAFNE | ||
| Exponential | 145.77 | 157.24 |
| Weibull | 146.46 | 160.80 |
| Gompertz | 147.25 | 161.59 |
| Log-logistic | 147.49 | 161.83 |
| Log-normal | 148.48 | 162.82 |
| MDI + DAFNE | ||
| Exponential | 64.36 | 75.77 |
| Weibull | 62.55 | 76.81 |
| Gompertz | 63.76 | 78.02 |
| Log-logistic | 63.78 | 78.04 |
| Log-normal | 64.97 | 79.23 |
The visual plot of the survival curves for remaining on the initially allocated treatment for the pump + DAFNE and MDI + DAFNE individuals are presented in Figures 15 and 16, respectively. The curves fitted to the treatment switching data show a reasonable fit to the Kaplan–Meier curves for individuals who were randomised to pump + DAFNE and MDI + DAFNE. A visual inspection of curves showed that the exponential curve for pump + DAFNE showed the best fit to the Kaplan–Meier curve at the 1- and 2-year time points, although a visual check does not indicate that it has the best fit for all of the time points. A visual inspection of the curves in the MDI + DAFNE arm shows that all curves had a reasonable fit to the Kaplan–Meier curve, except the exponential curve, which had a poor fit to the Kaplan–Meier curve, especially in the first year.
FIGURE 15.
A visual plot of the Kaplan–Meier and parametric survival curves for those individuals who were randomised to pump with DAFNE. (a) Exponential curve; (b) Weibull curve; (c) Gompertz curve; (d) log-logistic curve; and (e) log-normal curve.



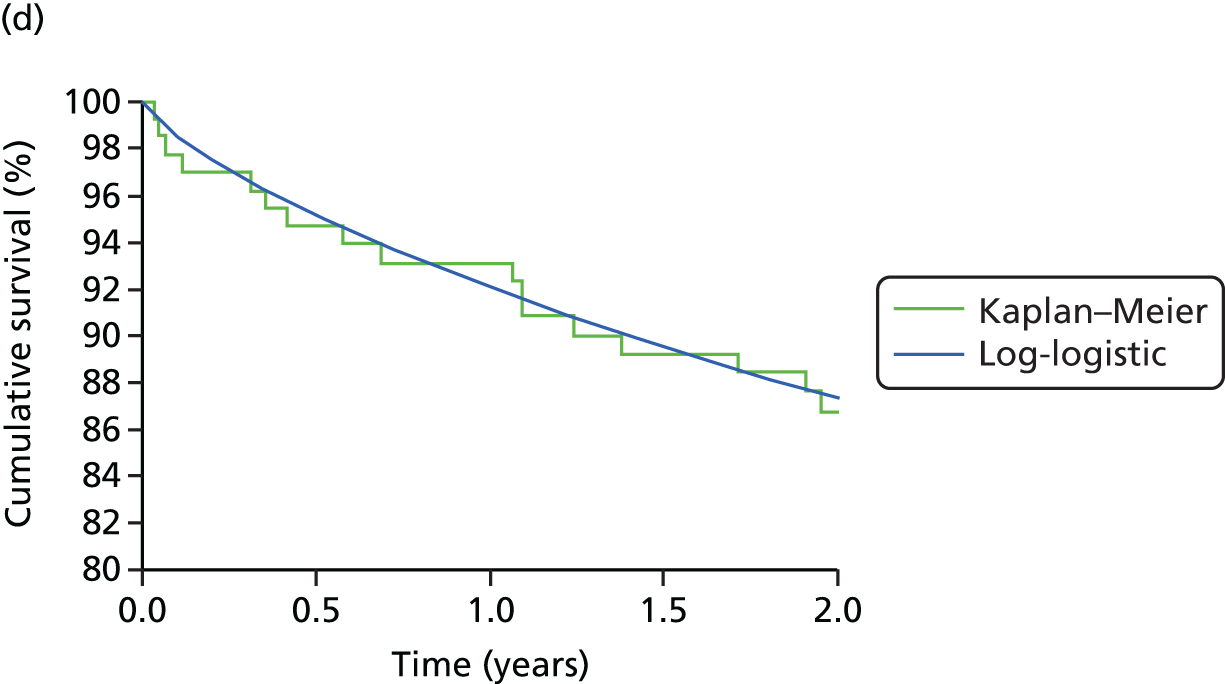
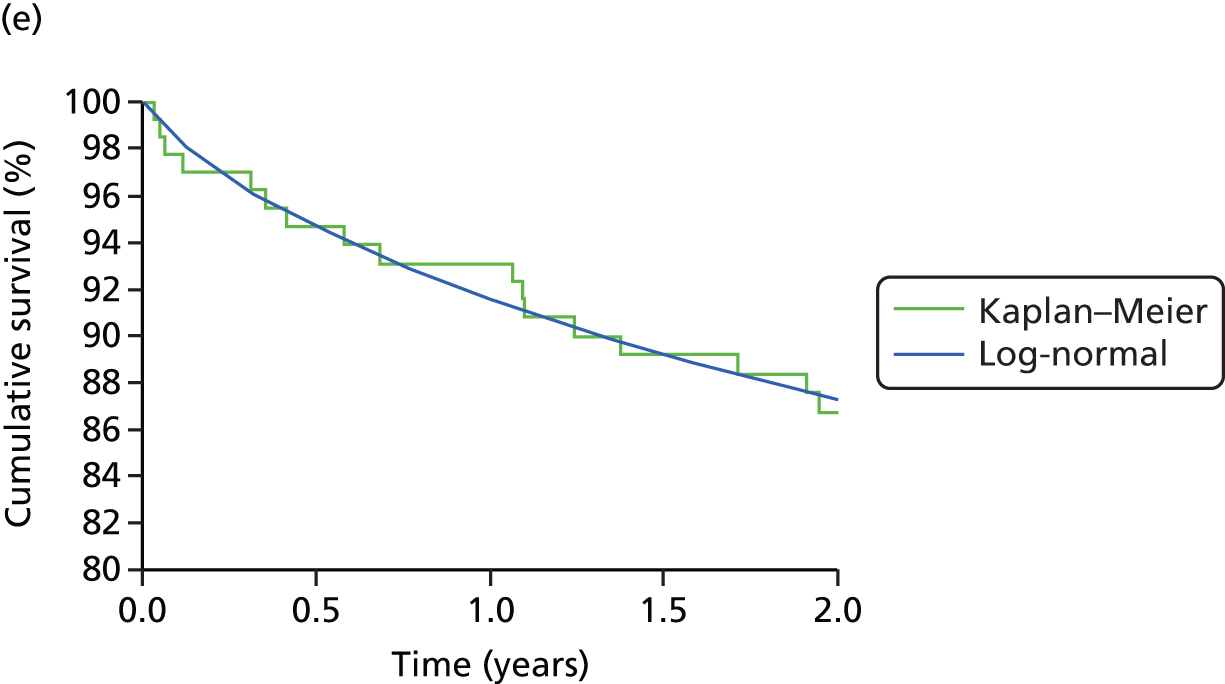
FIGURE 16.
A visual plot of the Kaplan–Meier and parametric survival curves for those individuals who were randomised to MDI with DAFNE. (a) Exponential curve; (b) Weibull curve; (c) Gompertz curve; (d) log-logistic curve; and (e) log-normal curve.
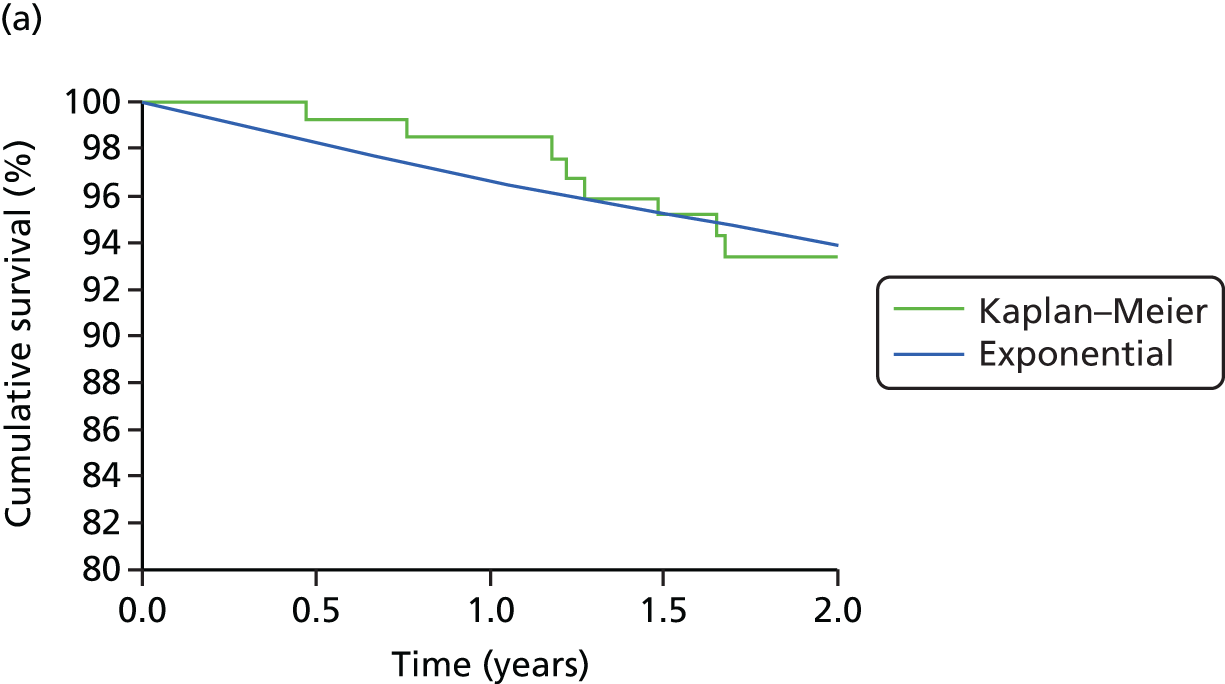




In the base case, the exponential model will be used to model the treatment switching of individuals in the pump + DAFNE arm, and the Weibull model will be used to model the treatment switching of individuals in the MDI + DAFNE arm. Uncertainties in the coefficients of these models were included in the PSA using a multivariate normal distribution. Scenario analyses were conducted when the risk of switching treatment was estimated directly from the Kaplan–Meier curves at years 1 and 2. The risk of switching treatment for a pump individual, given that he/she was receiving pump therapy at the start of the year, was 6.94% at year 1 and 6.89% at year 2. The risk of switching for a MDI individual, given that they were receiving MDI at the start of the year, was 1.58% in year 1 and 5.13% in year 2.
Glycated haemoglobin
The results of the beta regressions used to model the effectiveness of pump + DAFNE versus MDI + DAFNE in the ITT population is given in Table 50. Pump + DAFNE has a coefficient on HbA1c reduction of –0.056 at year 1 and –0.018 at year 2; neither result was statistically significant at the 5% significance level. These coefficients are not easily interpretable, as changes in HbA1c, as the mean effects are estimated using a logit link function.
| HbA1c | Coefficient | SE | t | p > t | 95% CI |
|---|---|---|---|---|---|
| At 1 year (beta scale) | |||||
| Mean effect (Mu) | |||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –0.056 | 0.038 | –1.49 | 0.137 | –0.131 to 0.018 |
| Baseline HbA1c (beta scale) | 3.978 | 0.248 | 16.01 | 0 | 3.491 to 4.465 |
| Constant | –2.223 | 0.088 | –25.28 | 0 | –2.395 to –2.050 |
| Centre effects (Cambridge is the reference centre): | |||||
| Dumfries and Galloway | –0.025 | 0.074 | –0.33 | 0.738 | –0.171 to 0.121 |
| Edinburgh | –0.019 | 0.065 | –0.3 | 0.768 | –0.147 to 0.108 |
| Glasgow | –0.154 | 0.099 | –1.55 | 0.12 | –0.348 to 0.040 |
| Harrogate | 0.022 | 0.041 | 0.52 | 0.602 | –0.060 to 0.103 |
| London (King’s College Hospital) | 0.013 | 0.065 | 0.21 | 0.837 | –0.114 to 0.140 |
| Nottingham | 0.214 | 0.060 | 3.58 | 0 | 0.097 to 0.331 |
| Sheffield | 0.066 | 0.057 | 1.17 | 0.241 | –0.045 to 0.178 |
| Natural logarithm of the dispersion parameter [ln(phi)] | |||||
| Baseline HbA1c (beta scale) | –2.996862 | 0.9980645 | –3 | 0.003 | –4.954 to –1.040 |
| Constant | 4.912 | 0.332 | 14.79 | 0 | 4.261 to 5.563 |
| At 2 years (beta scale) | |||||
| Mean effect (Mu) | |||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –0.018 | 0.035 | –0.52 | 0.603 | –0.086 to 0.050 |
| 1-year HbA1c (beta scale) | 0.797 | 0.318 | 2.51 | 0.012 | 0.175 to 1.419 |
| Baseline HbA1c (beta scale) | 3.599 | 0.342 | 10.51 | 0 | 2.927 to 4.271 |
| Constant | –2.380 | 0.091 | –26.14 | 0 | –2.558 to –2.201 |
| Centre effects (Cambridge is the reference centre): | |||||
| Dumfries and Galloway | 0.047 | 0.093 | 0.5 | 0.617 | –0.137 to 0.230 |
| Edinburgh | 0.067 | 0.085 | 0.8 | 0.426 | –0.098 to 0.233 |
| Glasgow | 0.137 | 0.097 | 1.42 | 0.155 | –0.052 to 0.327 |
| Harrogate | 0.123 | 0.087 | 1.41 | 0.158 | –0.048 to 0.294 |
| London (King’s College Hospital) | 0.079 | 0.087 | 0.9 | 0.366 | –0.092 to 0.249 |
| Nottingham | 0.120 | 0.110 | 1.09 | 0.279 | –0.098 to 0.337 |
| Sheffield | 0.156 | 0.080 | 1.96 | 0.05 | 0.000 to 0.312 |
| Natural logarithm of the dispersion parameter [ln(phi)] | |||||
| 1-year HbA1c (beta scale) | –4.667 | 1.129 | –4.13 | 0 | –6.881 to –2.453 |
| Constant | 5.422 | 0.277 | 19.56 | 0 | 4.879 to 5.966 |
In the per-protocol population as in this group, the statistical analysis showed a significant improvement in HbA1c for pump + DAFNE. The results of the beta regression fitted to the per-protocol population is given in Table 51. Pump + DAFNE was associated with a coefficient of –0.056 in year 1 and –0.047 in year 2. Neither of these coefficients was statistically significant at the 5% level. The uncertainty in the coefficients in these statistical models was included in the PSA of the Sheffield Type 1 Diabetes Model by sampling the coefficients from a multivariate normal distribution using the known variance covariance matrices.
| HbA1c | Coefficient | SE | t | p > t | 95% CI |
|---|---|---|---|---|---|
| At 1 year (beta scale) | |||||
| Mean effect (Mu) | |||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –0.056 | 0.044 | –1.37 | 0.171 | –0.148 to 0.026 |
| Baseline HbA1c (beta scale) | 3.938 | 0.255 | 13.62 | 0 | 2.978 to 3.980 |
| Constant | –2.219 | 0.093 | –23.94 | 0 | –2.401 to –2.038 |
| Centre effects (Cambridge is the reference centre): | |||||
| Dumfries and Galloway | –0.019 | 0.078 | –0.25 | 0.805 | –0.172 to 0.134 |
| Edinburgh | 0.020 | 0.056 | 0.37 | 0.714 | –0.089 to 0.130 |
| Glasgow | –0.129 | 0.095 | –1.36 | 0.175 | –0.315 to 0.057 |
| Harrogate | 0.025 | 0.040 | 0.62 | 0.534 | –0.054 to 0.104 |
| London (King’s College Hospital) | 0.018 | 0.064 | 0.28 | 0.779 | –0.107 to 0.143 |
| Nottingham | 0.172 | 0.039 | 4.46 | 0 | 0.096 to 0.247 |
| Sheffield | 0.084 | 0.064 | 1.31 | 0.191 | –0.042 to 0.209 |
| Natural logarithm of the dispersion parameter [ln(phi)] | |||||
| Baseline HbA1c (beta scale) | –3.504 | 1.050 | –3.34 | 0.001 | –5.563 to –1.446 |
| Constant | 5.062 | 0.351 | 14.41 | 0 | 4.373 to 5.751 |
| At 2 years (beta scale) | |||||
| Mean effect (Mu) | |||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –0.047 | 0.035 | –1.35 | 0.177 | –0.116 to 0.021 |
| 1-year HbA1c (beta scale) | 3.475 | 0.340 | 10.23 | 0 | 2.809 to 4.141 |
| Baseline HbA1c (beta scale) | 1.053 | 0.351 | 3 | 0.003 | 0.365 to 1.740 |
| Constant | –2.382 | 0.092 | –26.01 | 0 | –2.562 to –2.203 |
| Centre effects (Cambridge is the reference centre): | |||||
| Dumfries and Galloway | 0.022 | 0.088 | 0.26 | 0.799 | –0.150 to 0.194 |
| Edinburgh | 0.076 | 0.085 | 0.89 | 0.374 | –0.091 to 0.243 |
| Glasgow | 0.105 | 0.096 | 1.1 | 0.271 | –0.082 to 0.293 |
| Harrogate | 0.092 | 0.085 | 1.08 | 0.28 | –0.075 to 0.258 |
| London (King’s College Hospital) | 0.053 | 0.085 | 0.62 | 0.538 | –0.115 to 0.220 |
| Nottingham | 0.109 | 0.100 | 1.1 | 0.276 | –0.089 to 0.308 |
| Sheffield | 0.157 | 0.078 | 2.02 | 0.043 | 0.005 to 0.310 |
| Natural logarithm of the dispersion parameter [ln(phi)] | |||||
| 1-year HbA1c (beta scale) | –4.809 | 1.231 | –3.9 | 0 | –7.223 to –2.394 |
| Constant | 5.474 | 0.302 | 18.13 | 0 | 4.882 to 6.066 |
Severe hypoglycaemia and diabetic ketoacidosis
The results of the negative binomial regressions for the incidence of severe hypoglycaemia are given in Table 52. The regression predicts that the number of severe hypoglycaemic events increases as a patient’s HbA1c decreases; however, this result is not statistically significant at the 5% level in the second year. Pump + DAFNE compared with MDI + DAFNE was associated with a higher incidence of severe hypoglycaemia in year 1 and a lower incidence of severe hypoglycaemia in year 2. Neither result was statistically significant at the 5% level.
| Severe hypoglycaemia | Coefficient | SE | z-value | p > z |
|---|---|---|---|---|
| Year 1 | ||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | 0.2861 | 0.5149 | 0.556 | 0.578 |
| 1-year HbA1c (DCCT% scale) | –0.5010 | 0.2323 | 2.157 | 0.03 |
| Number of severe hypoglycaemic events experienced in the year prior to baseline | 2.0708 | 0.5638 | 3.673 | > 0.000 |
| Constant | 1.2689 | 1.8676 | 0.679 | 0.49687 |
| Year 2 | ||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –1.1141 | 0.7202 | –1.547 | 0.122 |
| 2-year HbA1c (DCCT% scale) | –0.2019 | 0.2668 | –0.757 | 0.449 |
| Constant | –0.6367 | 2.2625 | –0.281 | 0.778 |
The results of the negative binomial regressions for the incidence of DKA are given in Table 53. The predicted number of DKAs increase with a patient’s HbA1c. This result is statistically significant in the first year, but not in the second year. Pump + DAFNE when compared with MDI + DAFNE was associated with a higher incidence of DKA in year 1 and a lower incidence of DKA in year 2. Neither result was statistically significant at the 5% level.
| DKA | Coefficient | SE | z-value | p > z |
|---|---|---|---|---|
| Year 1 | ||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | 0.3369 | 0.4786 | 0.704 | 0.481 |
| 1-year HbA1c (DCCT% scale) | 0.4089 | 0.1246 | 3.283 | 0.001 |
| Constant | –5.9443 | 1.1879 | –5.004 | > 0.00 |
| Year 2 | ||||
| Treatment allocation (1 = pump + DAFNE, 0 = MDI + DAFNE) | –0.07564 | 0.70426 | –0.107 | 0.914 |
| 2-year HbA1c (DCCT% scale) | 0.32667 | 0.19447 | 1.680 | 0.093 |
| Number of DKAs in year 1 | 0.86618 | 0.51682 | 1.676 | 0.094 |
| Constant | –5.98206 | 1.82156 | –3.284 | 0.01 |
Cost of insulin, diabetes-related contacts and insulin pumps
The results of the analyses on the cost of insulin used in the long-term modelling are given in Table 54. Pump treatment was associated with a reduction in insulin costs of around £500 per annum in years 1 and 2 compared with MDI treatment. This result was statistically significant in both years. Switching from pump to MDI treatment was associated with an increase in insulin costs of around £550 in year 1 and £150 in year 2. No coefficient could be estimated on whether or not a MDI individual switched to pump, as this parameter was collinear with model parameters. Switching from MDI to pump was associated with a decrease in insulin costs of around £350 in year 2. All of these results were statistically significant at the 5% level.
| Parameter | Coefficient | Robust SE | 95% CI |
|---|---|---|---|
| Year 1 | |||
| Baseline insulin cost | 0.97 | 0.14 | 0.69 to 1.25 |
| Baseline HbA1c (DCCT% scale) | 5.08 | 6.65 | –7.95 to 18.10 |
| Randomised treatment group (1 = pump + DAFNE, 0 = MDI + DAFNE) | –517.91 | 25.57 | –568.02 to –467.80 |
| Did the individual switch from pump to MDI in year 1? (1 = switched, 0 = did not switch) | 554.47 | 114.26 | 330.53 to 778.41 |
| Constant | 381.77 | 70.20 | 244.19 to 519.36 |
| Year 2 | |||
| Baseline insulin cost | 1.04 | 0.11 | 0.82 to 1.26 |
| Baseline HbA1c (DCCT% scale) | 12.81 | 8.72 | –4.27 to 29.90 |
| Patient’s treatment at 1 year follow-up (1 = pump, 0 = MDI) | –527.64 | 30.22 | –586.87 to –468.42 |
| Did the individual switch from pump to MDI in year 2? (1 = switched, 0 = did not switch) | 153.35 | 55.96 | 43.67 to 263.02 |
| Did the individual switch from MDI to pump in year 2? (1 = switched, 0 = did not switch) | –353.27 | 80.06 | –510.18 to –196.36 |
| Constant | 324.53 | 79.15 | 169.40 to 479.66 |
The model uses the parameters in the regression model presented in Table 53 to estimate their cost of insulin. For example, the formula used to estimate the cost of insulin beyond the second year in a deterministic analysis is as follows:
The results of the analyses on the cost of diabetes-related contacts are given in Table 55. Pump + DAFNE was associated with an increase in the cost of diabetes-related contacts of £130 per annum in year 1 and £90 per annum in year 2 compared with MDI. These results were not statistically significant in year 1 or 2. Switching from insulin pump therapy to MDI was associated with an increase in diabetes-related contact costs of £280 per annum in year 1 and a decrease of £50 per annum in year 2. Switching from MDI to insulin pump therapy was associated with an increase in diabetes-related contact costs of £730 per annum in year 1 and £300 per annum in year 2. None of the treatment switching coefficients was statistically significant at the 5% significance level.
| Parameter | Coefficient | Robust SE | 95% CI | |
|---|---|---|---|---|
| Year 1 | ||||
| Baseline diabetes-related contacts cost | 0.11 | 0.04 | 0.04 | 0.18 |
| Baseline HbA1c (DCCT% scale) | –21.66 | 20.72 | –62.27 | 18.94 |
| Randomised treatment group (1 = pump + DAFNE, 0 = MDI + DAFNE) | 129.08 | 68.35 | –4.88 | 263.05 |
| Did the individual switch from pump to MDI in year 1? (1 = switched, 0 = did not switch) | 280.16 | 368.38 | –441.86 | 1002.17 |
| Did the individual switch from MDI to pump in year 1? (1 = switched, 0 = did not switch) | 733.95 | 633.94 | –508.55 | 1976.45 |
| Constant | 415.46 | 132.54 | 155.69 | 675.24 |
| Year 2 | ||||
| Baseline diabetes-related contacts cost | 0.03 | 0.02 | –0.02 | 0.07 |
| Baseline HbA1c (DCCT% scale) | 12.15 | 25.18 | –37.20 | 61.50 |
| Patient’s treatment at 1 year follow-up (1 = pump, 0 = MDI) | 88.99 | 69.17 | –46.58 | 224.56 |
| Did the individual switch from pump to MDI in year 2? (1 = switched, 0 = did not switch) | –47.10 | 66.92 | –178.25 | 84.05 |
| Did the individual switch from MDI to pump in year 2? (1 = switched, 0 = did not switch) | 299.80 | 153.43 | –0.92 | 600.52 |
| Constant | 201.93 | 171.22 | –133.64 | 537.51 |
The cost of DRCs for each individual was predicted using the values in Table 55. For example, in a deterministic model run, a patient’s cost of DRCs in the first year was given by the following formula:
The results of the analyses on the cost in insulin pump therapy (includes the yearly cost of the pump and the associated consumables) is given in Table 56. Insulin pump therapy was associated with a cost per annum of £2056 in year 1 and £2051 in year 2. Switching from insulin pump therapy to MDI was associated with a decrease in insulin pump therapy costs of £1140 in year 1 and a reduction of £910 in year 2. Switching from MDI to insulin pump therapy was associated with an increase in costs of £840 in year 1 and £130 in year 2. All of these results were statistically significant at 5% level.
| Parameter | Coefficient | Robust SE | 95% CI |
|---|---|---|---|
| Year 1 | |||
| Randomised treatment group (1 = pump + DAFNE, 0 = MDI + DAFNE) | 2056.11 | 15.54 | 2025.65 to 2086.56 |
| Did the individual switch from pump to MDI in year 1? (1 = switched, 0 = did not switch) | –1143.68 | 287.44 | –1707.04 to –580.31 |
| Did the individual switch from MDI to pump in year 1? (1 = switched, 0 = did not switch) | 804.57 | 208.95 | 395.03 to 1214.11 |
| Constant | 0.00 | 0.00 | 0.00 to 0.00 |
| Year 2 | |||
| Patient’s treatment at 1 year follow-up (1 = pump, 0 = MDI) | 2050.99 | 13.79 | 2023.97 to 2078.01 |
| Did the individual switch from pump to MDI in year 2? (1 = switched, 0 = did not switch) | –905.03 | 226.55 | –1349.07 to –461.00 |
| Did the individual switch from MDI to pump in year 2? (1 = switched, 0 = did not switch) | 1134.27 | 152.67 | 835.04 to 1433.49 |
| Constant | 0.00 | 0.00 | 0.00 to 0.00 |
The coefficients in these statistical models were included in the model to predict the cost of insulin, diabetes-related contact and insulin pump therapy. The uncertainty in these parameters was included in the PSA by using a multivariate normal distribution for each regression equation.
Long-term cost-effectiveness
Base-case analysis
The results of the long-term cost-effectiveness analysis base case results using the PSA is shown in Table 57. For the pump arm, the mean costs of the intervention are £42,143 discounted over the lifetime horizon, which compares with £20,398 for the MDI arm. The difference between the intervention costs for the two arms is £21,745. AE costs are slightly lower in the pump arm, £1040 versus £1509, a mean lifetime saving of £470 per person. Complication costs are also lower £57,435 versus £59,877, a mean lifetime saving of £2443 per person, which is mostly due to reductions in the occurrence of end-stage renal failure in the nephropathy complications. The net incremental lifetime cost of pump versus MDI is therefore estimated as £18,832 (95% CI £535 to £34,978) per person.
| MDI + DAFNE | Pump + DAFNE | Incremental (95% CI) | |
|---|---|---|---|
| Mean lifetime discounted costs per person (£) | |||
| Intervention costs | |||
| Insulin | 12,542 | 5634 | –6908 (–8329 to –5344) |
| Diabetes-related contacts | 5166 | 6451 | 1285 (–426 to 3108) |
| Insulin pumps and consumables | 2327 | 29,667 | 27,339 (22,771 to 31,368) |
| DAFNE course | 363 | 392 | 29 (29 to 29) |
| Subtotal intervention costs | 20,398 | 42,143 | 21,745 (17,321 to 25,569) |
| AE costs | |||
| Severe hypoglycaemia | 136 | 42 | –94 (–221 to –54) |
| DKA | 1373 | 998 | –375 (–1811 to 285) |
| Subtotal cost of AEs | 1509 | 1040 | –470 (–1880 to 160) |
| Long-term complication costs | |||
| Nephropathy | 51,515 | 49,139 | –2376 (–19,397 to 11,957) |
| Neuropathy | 1975 | 1915 | –60 (–419 to 255) |
| Retinopathy + macular oedema | 2212 | 2203 | –8 (–85 to 58) |
| MI | 1996 | 1994 | –2 (–258 to 206) |
| HF | 663 | 666 | 2 (–76 to 89) |
| Stroke | 278 | 278 | 0 (–43 to 41) |
| Angina | 1238 | 1239 | 1 (–143 to 123) |
| Total cost of long-term complications | 59,877 | 57,435 | –2443 (–20,177 to 12,381) |
| Total costs | 81,785 | 100,617 | 18,832 (535 to 34,978) |
| Mean discounted QALYs per person | |||
| QALYs lived (excluding decrements due to complications) | 14.2894 | 14.3898 | 0.1005 (–0.6522 to 0.8383) |
| QALYs lost because of AEs | |||
| Severe hypoglycaemia | –0.0014 | –0.0004 | 0.0010 (0 to 0.0042) |
| DKA | –0.0088 | –0.0064 | 0.0024 (–0.0018 to 0.0171) |
| Subtotal QALYs due to AEs | –0.0102 | –0.0068 | 0.0034 (–0.0009 to 0.0174) |
| QALYs lost because of complications | |||
| Nephropathy | –0.2179 | –0.2105 | 0.0074 (–0.0527 to 0.0714) |
| Neuropathy | –0.3301 | –0.3210 | 0.0091 (–0.0387 to 0.0629) |
| Retinopathy and macular oedema | –0.5202 | –0.5139 | 0.0064 (–0.0292 to 0.0488) |
| MI | –0.0647 | –0.0649 | –0.0002 (–0.0072 to 0.0067) |
| HF | –0.0420 | –0.0422 | –0.0002 (–0.0062 to 0.0055) |
| Stroke | –0.0376 | –0.0378 | –0.0002 (–0.0065 to 0.0061) |
| Angina | –0.0821 | –0.0822 | –0.0001 (–0.0081 to 0.0084) |
| Subtotal QALYs lost because of complications | –1.2947 | –1.2725 | 0.0222 (–0.112 to 0.1773) |
| Total QALYs | 12.9845 | 13.1105 | 0.1260 (–0.7533 to 0.9705) |
| Summary | |||
| Total mean discounted costs per person (£) | 81,785 | 100,617 | 18,832 |
| Total mean discounted QALYs per person | 12.9845 | 13.1105 | 0.1260 |
| ICER (£/QALY gained) | – | – | 149,483 |
| Probability (%) that pump + DAFNE is cost-effective at a threshold of £20,000 per QALY gained | – | – | 15.4 |
The ‘QALYs lived without diabetic complications’ captures all of the QALYs gains from the increased life expectancy of patients who receive pump + DAFNE prior to adjusting their utility downwards for the incidence of diabetic complications. The ‘QALYs lived without complications’ in the pump + DAFNE arm is 14.3898 QALYs compared with 14.2894 QALYs in the MDI + DAFNE arm, a mean increase of 0.1005 QALYs. The QALYs lost because of AEs are slightly lower in the pump + DAFNE arm than in the MDI + DAFNE arm, –0.0068 versus –0.0102 QALYs, leading to a mean increase of 0.0034 QALYs in favour of pump + DAFNE. The overall QALYs lost because of the incidence of diabetic complications was again slightly lower in the pump + DAFNE arm than in the MDI + DAFNE arm, –1.2725 versus –1.2947 QALYs, a mean increase in lifetime QALYs of 0.0222. However, pump + DAFNE is not associated with a mean increase in QALYs for each of the individual long-term diabetic complications. This is because although the incidence of the complications is expected to be lower in the pump + DAFNE arm, as they have a lower HbA1c, people are also expected to live longer in the pump + DAFNE arm, so they may be at a greater overall risk of suffering a diabetic complication within their lifetime. The net incremental QALY gain per person is 0.1260 QALYs (95% CI –0.7381 to 0.9705 QALYs) per person.
Pump + DAFNE generated more QALYs – 0.1260 QALYs (95% CI –0.7381 to 0.9705 QALYs) – at a higher incremental cost of £18,832 (95% CI £535 to £34,978) than MDI + DAFNE. The ICER associated with pump + DAFNE was £149,483 per QALY gained. This is outside the range of £20,000–30,000 per QALY gained at which NICE would usually consider to be cost-effective. Figure 17 shows the base-case cost-effectiveness plane for the PSA. It is clear that, when using the £20,000 per QALY gained threshold, most PSA runs lie in the region where pump + DAFNE would not be considered to be cost-effective, as they are above the £20,000 per QALY gained line. The cost-effectiveness acceptability curve presented in Figure 18 shows the probability that pump + DAFNE and MDI + DAFNE are cost-effective across a range of cost-effectiveness thresholds. 152 It is clear that MDI + DAFNE has a higher probability of being cost-effective than pump + DAFNE at all cost-effectiveness thresholds in the range of £0–50,000 per QALY gained.
FIGURE 17.
Cost-effectiveness plane of the base-case analysis using the Sheffield Type 1 Diabetes Policy Model.

FIGURE 18.
Cost-effectiveness acceptability curve of the base-case analysis using the Sheffield Type 1 Diabetes Policy Model.

The modelled lifetime incidence of diabetic complications in the PSA is given in Table 58. Pump + DAFNE was associated with fewer diabetic complications than MDI + DAFNE. However, this is to be expected, as the treatment effect coefficient was negative in the beta regression that was used to estimate HbA1c in the model (see Tables 50 and 51). It should also be noted that the incidence of proliferative retinopathy, macular oedema and blindness were higher in the pump + DAFNE arm than the MDI + DAFNE arm. This seems to be counterintuitive; however, there are two effects. The first is that, in a given year, patients in the pump + DAFNE arm are at a lower risk of these complications. The second effect is that as the HbA1c of patients in the pump + DAFNE arm is, on average, lower than the MDI + DAFNE arm then they are expected to live longer, increasing their absolute risk of experiencing a complication. For the proliferative retinopathy, macular oedema and blindness complications, the increased risk as a result of living longer outweighs the decreased annual risk of a complication as a result of these patients having a lower HbA1c value.
| Diabetic complication | MDI + DAFNE | Pump + DAFNE | Incremental |
|---|---|---|---|
| Microalbuminuria | 2.1610 | 2.0818 | –0.0792 |
| Macroalbuminuria | 1.9461 | 1.8419 | –0.1042 |
| ESRD | 1.7084 | 1.6162 | –0.0922 |
| Death due to ESRD | 1.0070 | 0.9474 | –0.0597 |
| Clinical neuropathy | 1.6179 | 1.5292 | –0.0888 |
| PAD with amputation | 0.4526 | 0.4320 | –0.0205 |
| Background retinopathy | 1.1388 | 1.0645 | –0.0743 |
| Proliferative retinopathy | 0.0441 | 0.0457 | 0.0015 |
| Macular oedema | 0.0385 | 0.0398 | 0.0013 |
| Blindness | 0.0252 | 0.0254 | 0.0002 |
| First cardiovascular disease | 1.9191 | 1.9106 | –0.0085 |
| Cardiovascular disease | 4.7381 | 4.7198 | –0.0183 |
| MI | 2.4818 | 2.4709 | –0.0110 |
| First MI | 1.6111 | 1.6048 | –0.0062 |
| Fatal MI | 1.2331 | 1.2306 | –0.0025 |
| Stroke | 0.3251 | 0.3241 | –0.0010 |
| First stroke | 0.2924 | 0.2915 | –0.0008 |
| Fatal stroke | 0.0713 | 0.0713 | 0.0000 |
| HF | 0.5720 | 0.5695 | –0.0025 |
| First HF | 0.4601 | 0.4581 | –0.0020 |
| Fatal HF | 0.0334 | 0.0330 | –0.0004 |
| Angina | 1.3592 | 1.3553 | –0.0039 |
| Severe hypoglycaemia | 4.3911 | 1.2783 | –3.1128 |
| DKA | 7.0059 | 4.6286 | –2.3773 |
| Life expectancy (years) | 29.7615 | 30.0851 | 0.3236 |
Summary of the scenario analyses in the long-term model
The following scenario analyses were conducted in the long-term modelling:
-
pump costs estimated using data in Riemsma et al. 8 on the yearly cost of insulin pump therapy
-
a 25% price reduction in insulin pumps and consumables
-
a 50% price reduction in insulin pumps and consumables
-
the ITT estimate of treatment effect was used
-
the ITT estimate of treatment effect was used and there was no change in HbA1c if an individual switches treatment
-
post-trial HbA1c progression in both arms is estimated from the DCCT at +0.045% per annum
-
individuals return to their baseline HbA1c after 3 years and experience no progression in their HbA1c thereafter
-
HbA1c effects occur one model cycle earlier
-
individuals return to baseline risk of hypoglycaemic episodes and DKA at 3 years
-
treatment switching probabilities were estimated directly from the Kaplan–Meier curves
-
subgroup – individuals with a baseline HbA1c of < 8.5% (69 mmol/mol)
-
subgroup – individuals with a baseline HbA1c of ≥ 8.5% (69 mmol/mol)
-
subgroup – individuals with a baseline HbA1c of ≥ 7.5% (58 mmol/mol)
-
subgroup – individuals with a baseline HbA1c of ≥ 7.5% (58 mmol/mol) and < 8.5% (69 mmol/mol)
-
subgroup – individuals with a baseline HbA1c of ≥ 8.5% (69 mmol/mol) and < 9.5% (80 mmol/mol)
-
subgroup – individuals with a baseline HbA1c of ≥ 9.5% (80 mmol/mol)
-
subgroup – individuals in the per-protocol population
-
subgroup – individuals in the per-protocol population and no treatment switching is included in the model.
Structural uncertainty and potential subgroup effects were explored in the scenario analyses with the long-term model.
Much like the EEACT, uncertainty due to potential decreases in price of insulin pumps was explored in these scenario analyses.
Four further scenario analyses were conducted around the different methods that could be used to estimate each patient’s HbA1c in the model. A scenario analysis was conducted in which HbA1c was estimated using beta regression in the ITT population rather than the per-protocol population. As the ITT population includes switchers in their originally assigned treatment groups, a further scenario analysis was conducted using the regression estimated in the ITT population where the individuals in the model did not experience a change in HbA1c when they switched treatment, as these effects were already included in the estimate of the relative treatment effect of pump + DAFNE versus MDI + DAFNE. Uncertainty in the long-term changes in HbA1c was explored by using data observed in the DDCT trial for both of the model arms. As there was no information in the DCCT trial on different HbA1c trajectories for pump or MDI users, the same trajectory was used in both model arms, which effectively assumes that the treatment effect for pump users in the REPOSE Trial is maintained for a lifetime. Uncertainty in the HbA1c of individuals after the REPOSE Trial was also explored by assuming that all individuals returned to their baseline HbA1c after the third model year. This is a very conservative assumption, but gives some idea of the least favourable scenario to pump + DAFNE. The effect of assuming that changes in HbA1c occurred one model cycle (1 year) earlier than the base case on the model outcomes was explored. Full details on the reason for and rationale behind scenario analyses 4–8 are given earlier (see Chapter 3, Estimation of each individual’s glycated haemoglobin and Duration of treatment effectiveness beyond the trial period).
The effect of assuming that the second-year risk functions for severe hypoglycaemia and DKA were applied for the rest of an individual’s lifetime was tested by instead assuming that individuals in both arms returned to their risk of severe hypoglycaemia and DKA at baseline. Full details on this scenario analysis is given earlier (see Chapter 3, Estimating severe hypoglycaemic events and diabetic ketoacidosis events).
Finally, the validity of the treatment switching models was testing by assuming directly using the risks of switching observed in the Kaplan–Meier curves. In this scenario, treatment switching was a random event that did not depend on HbA1c, number of severe hypoglycaemic episodes in the last year and number of DKAs last year, as was used in the base case. Full details on this scenario analysis are given earlier (see Chapter 3, Incorporating treatment switching).
Further to the one-way scenario analyses, a threshold analysis was conducted to determine the HbA1c fall that future pumps would need to have to be considered cost-effective. Full details on this threshold analysis are given earlier (see Chapter 3, Threshold analysis).
Results of the one-way scenario analyses
The one-way scenario analyses are presented in Table 59. The ICER did not fall below £30,000 per QALY gained in any of the scenario analyses. Furthermore, the subgroup analyses did not indicate that the ICER for pump + DAFNE compared with MDI + DAFNE will fall below £30,000 per QALY gained for any identified pre-specified subgroup in the REPOSE Trial patient population. The most favourable ICER to pump + DAFNE was observed when a 50% reduction in the price of insulin and insulin pump consumables was modelled; however, the ICER in this scenario was £46,578, which is above the maximum acceptable ICER range of £20,000–30,000 that is usually used by UK decision-makers when deciding whether or not a health technology is cost-effective. Although the ICERs are more favourable to pump + DAFNE in the long-term modelling than in the EEACT, the long-term modelling does not indicate that pump + DAFNE is likely to be considered a cost-effective treatment pathway if it were to be appraised by NICE.
| Analysis | MDI + DAFNE | Pump + DAFNE | Incremental | ||||
|---|---|---|---|---|---|---|---|
| Total discounted costs (£) | Total discounted QALYs | Total discounted costs (£) | Total discounted QALYs | Total discounted costs (£) | Total discounted QALYs | ICER (£ per QALY gained) | |
| Base case | |||||||
| PSA | 81,785 | 12.9845 | 100,617 | 13.1105 | 18,832 | 0.1260 | 149,483 |
| Deterministic | 70,132 | 12.6719 | 90,581 | 12.8166 | 20,448 | 0.1447 | 141,312 |
| Scenario | |||||||
| Pump prices were estimated from Riemsma et al.8 | 70,083 | 12.6719 | 89,759 | 12.8166 | 19,677 | 0.1447 | 135,977 |
| 25% price reduction in insulin pumps and consumables | 69,690 | 12.6719 | 83,285 | 12.8166 | 13,594 | 0.1447 | 93,945 |
| 50% price reduction in insulin pumps and consumables | 69,248 | 12.6719 | 75,989 | 12.8166 | 6740 | 0.1447 | 46,578 |
| ITT estimate of treatment effect | 71,238 | 12.7130 | 91,307 | 12.7935 | 20,069 | 0.0805 | 249,338 |
| ITT estimate of treatment effect and no change in HbA1c if an individual switches treatment | 70,994 | 12.8239 | 70,994 | 12.6475 | 19,390 | 0.1764 | 109,897 |
| Post-trial HbA1c progression in both arms is estimated from the DCCT | 69,382 | 12.7211 | 89,523 | 12.8412 | 20,141 | 0.1202 | 167,613 |
| Individuals return to their baseline HbA1c after 3 years and no progression thereafter | 67,471 | 12.9472 | 88,462 | 12.9162 | 20,991 | –0.0310 | Dominated |
| HbA1c effects occur one model cycle earlier | 71,220 | 12.6514 | 90,589 | 12.7528 | 19,369 | 0.1014 | 190,974 |
| Individuals return to their baseline risk of hypoglycaemic episodes and DKA at 3 years | 70,102 | 12.6725 | 90,719 | 12.8565 | 20,616 | 0.1841 | 111,998 |
| Switching probabilities were estimated directly from the Kaplan–Meier curves | 69,318 | 12.6740 | 90,904 | 12.7735 | 21,586 | 0.0995 | 216,871 |
| Subgroup | |||||||
| Individuals with a baseline HbA1c < 8.5% | 54,473 | 13.2733 | 76,758 | 13.3434 | 22,284 | 0.0701 | 317,893 |
| Individuals with a baseline HbA1c ≥ 8.5% | 82,769 | 12.1320 | 100,508 | 12.2979 | 17,739 | 0.1659 | 106,909 |
| Individuals with a baseline HbA1c ≥ 7.5% | 73,944 | 12.4866 | 92,481 | 12.6614 | 18,536 | 0.1747 | 106,090 |
| Individuals with a baseline HbA1c ≥ 7.5% and < 8.5% | 58,654 | 12.9513 | 79,560 | 13.0973 | 20,906 | 0.1460 | 143,214 |
| Individuals with a baseline HbA1c ≥ 8.5% and < 9.5% | 62,515 | 13.3038 | 83,006 | 13.4234 | 20,491 | 0.1195 | 171,447 |
| Individuals with a baseline HbA1c ≥ 9.5% | 97,111 | 11.5164 | 111,862 | 11.6564 | 14,751 | 0.1400 | 105,351 |
| Individuals in the per-protocol population | 69,739 | 12.5982 | 89,363 | 12.7142 | 19,623 | 0.1160 | 169,143 |
| Individuals in the per-protocol population and no treatment switching | 69,874 | 12.6018 | 92,601 | 12.7380 | 22,727 | 0.1362 | 166,831 |
An important scenario to note is the one in which the HbA1c effects occur one model cycle earlier.
Results of the threshold analysis
The results of the two-way price and effectiveness threshold analysis for a certain reduction in HbA1c are given in Table 60. When the annual pump cost is assumed to be £2060 then the analysis shows that the reduction in HbA1c (for CSII compared with MDI) would need to be ≥ 11 mmol/mol (1.0%) for pumps to be considered cost-effective (ICER £22,757). When the annual cost is 25% lower (£1545) then a HbA1c reduction of > 7.7 mmol/mol (0.7%) would be needed to have an ICER of < £20,000 per QALY gained. When the annual cost is halved (£1030) then a HbA1c reduction of 4.4 mmol/mol (0.4%) would be sufficient to have an ICER of < £20,000 per QALY gained.
| The annual cost (£) of insulin pumps and insulin pump consumables | HbA1c reduction associated with an insulin pump, mmol/mol (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.3 (0.3) | 4.4 (0.4) | 5.5 (0.5) | 6.6 (0.6) | 7.7 (0.7) | 8.7 (0.8) | 9.8 (0.9) | 10.9 (1.0) | 12.0 (1.1) | 13.1 (1.2) | |
| £2060 | £102,654 | £63,887 | £51,752 | £48,912 | £41,272 | £32,840 | £30,560 | £22,757 | £20,852 | £18,409 |
| £1957 | £95,154 | £59,254 | £47,299 | £44,867 | £37,634 | £29,712 | £27,863 | £20,365 | £18,727 | £16,252 |
| £1854 | £87,653 | £54,621 | £42,846 | £40,822 | £33,997 | £26,584 | £25,167 | £17,973 | £16,602 | £14,094 |
| £1751 | £80,153 | £49,988 | £38,392 | £36,777 | £30,359 | £23,456 | £22,471 | £15,582 | £14,477 | £11,937 |
| £1648 | £72,652 | £45,354 | £33,939 | £32,732 | £26,722 | £20,328 | £19,775 | £13,190 | £12,352 | £9780 |
| £1545 | £65,151 | £40,721 | £29,486 | £28,687 | £23,084 | £17,200 | £17,079 | £10,799 | £10,227 | £7623 |
| £1442 | £57,651 | £36,088 | £25,032 | £24,642 | £19,446 | £14,072 | £14,382 | £8407 | £8102 | £5465 |
| £1339 | £50,150 | £31,455 | £20,579 | £20,597 | £15,809 | £10,944 | £11,686 | £6015 | £5977 | £3308 |
| £1236 | £42,650 | £26,822 | £16,126 | £16,552 | £12,171 | £7816 | £8990 | £3624 | £3852 | £1151 |
| £1133 | £35,149 | £22,189 | £11,672 | £12,506 | £8534 | £4688 | £6294 | £1232 | £1727 | Dominates |
| £1030 | £27,648 | £17,555 | £7219 | £8461 | £4896 | £1560 | £3598 | Dominates | Dominates | Dominates |
The results of the two-way price and effectiveness threshold analysis for when the uncertainty in the treatment effect is estimated using the dispersion parameter formula used in the REPOSE Trial is given in Table 61. When the annual cost is assumed to be £2060 then the analysis shows that the reduction in HbA1c (for pumps vs. MDI) would need to be > 9.8 mmol/mol (0.9%) for pumps to have an ICER of < £30,000 per QALY gained. When the annual cost of insulin pumps and consumables is 25% lower (£1545), then a HbA1c reduction of 7 mmol/mol (0.6%) would be needed to have an ICER of < £30,000 per QALY gained. When the annual cost is halved (£1030) then a HbA1c reduction of 4.4 mmol/mol (0.4%) would be sufficient to have an ICER of < £20,000 per QALY gained.
| The annual cost (£) of insulin pumps and insulin pump consumables | HbA1c reduction associated with an insulin pump, mmol/mol (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3.3 (0.3) | 4.4 (0.4) | 5.5 (0.5) | 6.6 (0.6) | 7.7 (0.7) | 8.7 (0.8) | 9.8 (0.9) | 10.9 (1.0) | 12.0 (1.1) | 13.1 (1.2) | |
| £2060 | £90,343 | £80,074 | £53,002 | £47,577 | £37,626 | £33,373 | £26,946 | £23,903 | £18,344 | £20,968 |
| £1957 | £83,668 | £74,071 | £48,550 | £43,798 | £34,341 | £30,216 | £24,424 | £21,325 | £16,340 | £18,670 |
| £1854 | £76,994 | £68,069 | £44,099 | £40,018 | £31,056 | £27,058 | £21,901 | £18,746 | £14,336 | £16,371 |
| £1751 | £70,319 | £62,066 | £39,647 | £36,239 | £27,771 | £23,901 | £19,379 | £16,167 | £12,332 | £14,073 |
| £1648 | £63,644 | £56,064 | £35,195 | £32,459 | £24,486 | £20,744 | £16,857 | £13,588 | £10,328 | £11,775 |
| £1545 | £56,970 | £50,061 | £30,743 | £28,680 | £21,201 | £17,587 | £14,334 | £11,010 | £8324 | £9477 |
| £1442 | £50,295 | £44,059 | £26,292 | £24,900 | £17,916 | £14,430 | £11,812 | £8431 | £6320 | £7178 |
| £1339 | £43,621 | £38,056 | £21,840 | £21,120 | £14,631 | £11,272 | £9290 | £5852 | £4316 | £4880 |
| £1236 | £36,946 | £32,054 | £17,388 | £17,341 | £11,346 | £8115 | £6767 | £3273 | £2312 | £2582 |
| £1133 | £30,271 | £26,052 | £12,936 | £13,561 | £8061 | £4958 | £4245 | £695 | £308 | £284 |
| £1030 | £23,597 | £20,049 | £8485 | £9782 | £4776 | £1801 | £1723 | Dominates | Dominates | Dominates |
The threshold analysis indicates if a future study of pumps + DAFNE versus MDI + DAFNE were to be conducted then the cost of insulin pumps and their associated consumables should be taken into account when determining the appropriate effect size to power the study on. At current prices, per-protocol effect sizes of > 5.5 mmol/mol would be required in the whole population who would be eligible for pump therapy for insulin pumps to have an ICER in the £20,000–30,000 per QALY gained range at which NICE is likely to consider them to be a cost-effective use of NHS resources.
Summary of the economic analysis results
None of the analyses conducted in the EEACT or the long-term modelling had an ICER of < £30,000 per QALY gained. Furthermore, no subgroup was identified for which the ICER was < £30,000 per QALY gained. This indicates that pump + DAFNE is unlikely to be considered to be a cost-effective use of NHS resources by NICE in the UK compared with the current practice of MDI + DAFNE, as the ICERs are all above the ICER range of £20,000–30,000 per QALY gained, which is usually used by NICE to determine the cost-effectiveness of health technologies. The findings of this analysis are consistent with the current recommended care pathway for adults with T1DM in the UK, who should be offered structured education with MDI, ideally around 12 months after diagnosis (but failing that at any later stage).
Chapter 7 Results of the psychosocial evaluation
Completion rates and final sample
Quantitative data
Questionnaires were administered to all of the participants at all of the time points. Table 62 shows completion rates at each time point. High levels of questionnaire completeness were observed across all questionnaires and follow-up (around 90% completed at each follow-up). A total of 264 participants of the 267 participants attending the DAFNE course completed at least one of the psychosocial questionnaires (n = 128 pump, n = 117 MDI), with a minimum of 236 participants completing questionnaires at all time points. The lowest completion rate on any individual measure was 86% of participants. The completion rate was slightly higher for participants who were allocated to pump than participants allocated to MDI, which reflects the slightly higher dropout rate in the MDI group.
| Questionnaire | Follow-up | Treatment group, n (%) | Total (N = 267), n (%) | |
|---|---|---|---|---|
| Pump (N = 132) | MDI (N = 135) | |||
| SF-12 | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 128 (97.0) | 120 (88.9) | 248 (92.9) | |
| 12 months | 121 (91.7) | 119 (88.1) | 240 (89.9) | |
| 24 months | 124 (93.9) | 117 (86.7) | 241 (90.3) | |
| DSQOL | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 129 (97.7) | 120 (88.9) | 249 (93.3) | |
| 12 months | 121 (91.7) | 119 (88.1) | 240 (89.9) | |
| 24 months | 124 (93.9) | 116 (85.9) | 240 (89.9) | |
| WHOQOL-BREF | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 129 (97.7) | 120 (88.9) | 249 (93.3) | |
| 12 months | 121 (91.7) | 119 (88.1) | 240 (89.9) | |
| 24 months | 124 (93.9) | 117 (86.7) | 241 (90.3) | |
| HFS | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 129 (97.7) | 120 (88.9) | 249 (93.3) | |
| 12 months | 121 (91.7) | 119 (88.1) | 240 (89.9) | |
| 24 months | 124 (93.9) | 117 (86.7) | 241 (90.3) | |
| HADS | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 129 (97.7) | 120 (88.9) | 249 (93.3) | |
| 12 months | 121 (91.7) | 119 (88.1) | 240 (89.9) | |
| 24 months | 124 (93.9) | 117 (86.7) | 241 (90.3) | |
| EQ-5D | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 128 (97.0) | 120 (88.9) | 248 (92.9) | |
| 12 months | 120 (90.9) | 116 (85.9) | 236 (88.4) | |
| 24 months | 124 (93.9) | 116 (85.9) | 240 (89.9) | |
| DTSQ | Baseline | 131 (99.2) | 132 (97.8) | 264 (98.9) |
| 6 months | 128 (97.0) | 119 (88.1) | 247 (92.5) | |
| 12 months | 121 (91.7) | 118 (87.4) | 239 (89.5) | |
| 24 months | 124 (93.9) | 116 (85.9) | 240 (89.9) | |
Qualitative interviews
A total of 45 patients were recruited, of whom 25 were randomised to the pump and 20 to the MDI arm of the trial. Full details of the sample are provided in Table 63. Three participants (two ‘pump’, one ‘MDI’) could not be contacted for round 2 interviews.
| Variable | Scoring | Total (N = 45) |
|---|---|---|
| Age (years) | Mean (SD) | 40 (12.8) |
| Range | 19–66 | |
| Sex (%) | Female | 48.9 |
| Diabetes duration (years) | Mean (SD) | 17.4 (12.4) |
| Range | 1 to 41 | |
| Occupation, n (%) | Professional | 14 (31) |
| Semiskilled | 16 (35.5) | |
| Student | 4 (9) | |
| Unemployed | 4 (9) | |
| Unskilled | 7 (15.5) | |
| HbA1c | ||
| mmol/mol | Mean (SD) | 71 (14) |
| Range | 46–109 | |
| % | Mean (SD) | 8.6 (1.3) |
| Range | 6.4–11.7 | |
The final educator sample comprised 12 nurses and six dietitians; owing to staff leave it was not possible to interview the dietitian in one of the centres. See Table 64 for full details of the educator sample. As can be seen from this table, there was diversity among the educators in terms of diabetes, DAFNE and pump experience. All of the educators were women.
| Variable | Scoring | Total (N = 18) |
|---|---|---|
| Occupation, n (%) | Nurse | 12 (67) |
| Dietitian | 6 (33) | |
| Experience of working in T1DM (years) | Mean (SD) | 14 (7.7) |
| Range | 5–29 | |
| Experience of DAFNE (years) | Mean (SD) | 7.9 (4.3) |
| Range | 1–15 | |
| Experience of pump therapy (years) | Mean (SD) | 4 (4.3) |
| Range | 0–15 |
Findings
The findings of the mixed-methods study are structured and reported under the six original study aims, with qualitative and/or quantitative data drawn on, as appropriate, to answer and address particular questions.
Study aims 1 and 2
-
To establish whether or not, and why, there are differences in QoL and other psychological outcomes between patients using pump and MDI regimens.
-
To examine whether or not, and why, QoL and other outcomes change over time.
Overview
Material that is relevant to addressing aims 1 and 2 has been combined in this final report because of the strong overlaps in the content. In this section we begin by presenting quantitative data on ≥ 236 participants before going on to draw on patients’ interview accounts to aid interpretation of quantitative findings.
Quantitative data
Tables 65–67 show QoL outcomes at 6, 12 and 24 months, respectively. Table 68 shows DTSQ data at the same time points.
| QoL outcome | Domain | Treatment group | Adjusted differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| SF-12 | PCS | 127 | 1.2 (6.1) | 116 | 0.5 (8.6) | 0.3 (–1.4 to 2.0) | 0.721 |
| MCS | 127 | 0.2 (8.8) | 117 | 0.9 (9.9) | –0.8 (–2.9 to 1.3) | 0.452 | |
| DSQOL | Total score | 128 | –5.2 (12.2) | 117 | –4.4 (11.2) | –0.1 (–2.8 to 2.6) | 0.935 |
| Social relations | 128 | –2.2 (11.8) | 117 | –3.0 (13.2) | 1.5 (–1.2 to 4.2) | 0.276 | |
| Leisure time restrictions and flexibility | 128 | –5.1 (16.6) | 117 | –4.4 (18.5) | –0.1 (–3.8 to 3.7) | 0.968 | |
| Physical complaints | 128 | –6.0 (17.0) | 117 | –4.8 (13.8) | –0.1 (–3.5 to 3.3) | 0.953 | |
| Worries about the future | 128 | –7.9 (20.4) | 117 | –7.5 (19.4) | –0.7 (–5.5 to 4.1) | 0.779 | |
| Daily hassle of functions | 128 | –6.3 (18.9) | 117 | –5.0 (18.7) | –0.8 (–5.0 to 3.4) | 0.700 | |
| Diet restrictions | 128 | –11.3 (18.3) | 117 | –6.4 (16.0) | –3.3 (–6.9 to 0.2) | 0.061 | |
| Treatment satisfaction (PWTSS) | 118 | 2.1 (4.4) | 109 | 2.1 (4.8) | 0.1 (–0.7 to 1.0) | 0.791 | |
| WHOQOL-BREF | Physical health | 127 | 0.4 (2.3) | 117 | 0.2 (2.3) | 0.1 (–0.4 to 0.6) | 0.740 |
| Psychological | 128 | 0.1 (1.9) | 117 | 0.4 (2.2) | –0.3 (–0.7 to 0.2) | 0.225 | |
| Social relationships | 127 | –0.3 (2.7) | 117 | 0.3 (3.0) | –0.7 (–1.3 to –0.1) | 0.026 | |
| Environment | 128 | 0.1 (1.7) | 117 | 0.4 (1.6) | –0.3 (–0.7 to 0.1) | 0.170 | |
| HFS | Behaviour score | 127 | –1.7 (4.9) | 117 | –0.2 (4.8) | –0.9 (–2.0 to 0.1) | 0.074 |
| Worry score | 128 | –4.0 (10.9) | 117 | –2.8 (9.5) | –0.1 (–2.4 to 2.1) | 0.906 | |
| HADS | Anxiety score | 128 | –0.2 (3.0) | 117 | –0.6 (3.3) | 0.4 (–0.3 to 1.1) | 0.260 |
| Depression score | 128 | –0.3 (2.9) | 117 | –0.2 (2.5) | 0.1 (–0.5 to 0.7) | 0.735 | |
| EQ-5D | Utility index | 127 | –0.02 (0.17) | 117 | –0.01 (0.18) | –0.02 (–0.06 to 0.02) | 0.382 |
| QoL outcome | Domain | Treatment group | Adjusted differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| SF-12 | PCS | 119 | 0.7 (7.7) | 115 | 1.1 (6.9) | –0.4 (–2.1 to 1.3) | 0.669 |
| MCS | 121 | –1.1 (10.8) | 116 | –1.0 (10.9) | –0.1 (–2.6 to 2.3) | 0.912 | |
| DSQOL | Total score | 121 | –5.8 (11.4) | 116 | –3.6 (10.1) | –1.5 (–4.0 to 1.1) | 0.254 |
| Social relations | 121 | –2.9 (12.4) | 116 | –1.5 (11.2) | –0.7 (–3.6 to 2.1) | 0.620 | |
| Leisure time restrictions and flexibility | 121 | –5.2 (17.7) | 115 | –4.5 (15.9) | 0.0 (–3.8 to 3.7) | 0.981 | |
| Physical complaints | 121 | –5.6 (15.2) | 115 | –4.4 (13.0) | –0.4 (–3.5 to 2.8) | 0.824 | |
| Worries about the future | 121 | –8.1 (21.7) | 116 | –6.4 (20.9) | –2.0 (–7.0 to 2.9) | 0.421 | |
| Daily hassle or functions | 121 | –9.1 (19.4) | 116 | –3.5 (18.7) | –5.0 (–9.2 to –0.8) | 0.019 | |
| Diet restrictions | 121 | –12.8 (17.1) | 115 | –7.0 (16.7) | –4.1 (–7.2 to –1.0) | 0.010 | |
| Treatment satisfaction (PWTSS) | 109 | 1.5 (4.6) | 112 | 1.4 (4.4) | 0.1 (–0.8 to 1.0) | 0.839 | |
| WHOQOL-BREF | Physical health | 121 | 0.0 (2.0) | 116 | 0.1 (2.2) | –0.1 (–0.6 to 0.4) | 0.596 |
| Psychological | 121 | –0.1 (1.9) | 116 | 0.1 (2.0) | –0.2 (–0.7 to 0.2) | 0.341 | |
| Social relationships | 121 | –0.2 (3.0) | 116 | –0.1 (2.5) | –0.3 (–0.9 to 0.4) | 0.375 | |
| Environment | 121 | 0.2 (1.7) | 116 | 0.3 (1.7) | –0.1 (–0.5 to 0.3) | 0.727 | |
| HFS | Behaviour score | 120 | –1.2 (5.2) | 116 | –0.1 (5.1) | –1.0 (–2.1 to 0.2) | 0.091 |
| Worry score | 121 | –4.3 (12.5) | 116 | –3.3 (10.7) | –0.6 (–3.1 to 1.8) | 0.602 | |
| HADS | Anxiety score | 121 | –0.1 (3.2) | 116 | –0.3 (3.1) | 0.2 (–0.6 to 0.9) | 0.664 |
| Depression score | 121 | –0.3 (3.3) | 116 | 0.4 (2.9) | –0.5 (–1.2 to 0.2) | 0.180 | |
| EQ-5D | Utility Index | 120 | –0.03 (0.15) | 113 | –0.02 (0.17) | 0.00 (–0.04 to 0.04) | 0.876 |
| QoL outcome | Domain | Treatment group | Adjusted differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| SF-12 | PCS | 122 | 0.3 (7.9) | 112 | 1.0 (8.3) | –0.4 (–2.1 to 1.3) | 0.657 |
| MCS | 123 | 2.1 (11.2) | 114 | 0.5 (10.3) | 1.6 (–0.7 to 4.0) | 0.175 | |
| DSQOL | Total score | 123 | –8.2 (13.1) | 114 | –4.2 (13.2) | –3.8 (–6.5 to –1.1) | 0.006 |
| Social relations | 123 | –5.7 (12.9) | 113 | –2.7 (14.8) | –2.5 (–5.4 to 0.4) | 0.092 | |
| Leisure time restrictions and flexibility | 123 | –8.1 (17.0) | 113 | –3.6 (19.7) | –4.6 (–8.4 to –0.9) | 0.016 | |
| Physical complaints | 123 | –8.7 (17.2) | 113 | –4.8 (16.6) | –3.6 (–7.3 to 0.0) | 0.049 | |
| Worries about the future | 123 | –11.9 (23.3) | 113 | –7.8 (21.2) | –4.8 (–9.7 to 0.2) | 0.058 | |
| Daily hassle or functions | 123 | –9.6 (21.2) | 113 | –3.6 (21.5) | –6.3 (–10.9 to –1.8) | 0.006 | |
| Diet restrictions | 123 | –12.8 (19.5) | 113 | –6.9 (19.3) | –5.1 (–8.6 to –1.6) | 0.004 | |
| Treatment satisfaction (PWTSS) | 113 | 1.9 (4.5) | 108 | 1.5 (5.4) | 0.5 (–0.5 to 1.4) | 0.317 | |
| WHOQOL-BREF | Physical health | 123 | 0.5 (2.4) | 114 | –0.1 (2.2) | 0.5 (0.0 to 1.0) | 0.067 |
| Psychological | 123 | 0.5 (2.5) | 114 | 0.3 (2.4) | 0.2 (–0.4 to 0.7) | 0.567 | |
| Social relationships | 123 | 0.0 (3.3) | 114 | 0.1 (2.9) | –0.2 (–0.9 to 0.5) | 0.627 | |
| Environment | 122 | 0.4 (2.2) | 114 | 0.3 (2.0) | 0.3 (–0.2 to 0.8) | 0.211 | |
| HFS | Behaviour score | 122 | –1.4 (5.6) | 114 | –0.6 (5.1) | –0.4 (–1.5 to 0.7) | 0.442 |
| Worry score | 123 | –6.7 (13.0) | 114 | –2.9 (12.5) | –3.4 (–6.0 to –0.8) | 0.010 | |
| HADS | Anxiety score | 123 | –1.0 (4.0) | 114 | –0.5 (3.5) | –0.5 (–1.3 to 0.4) | 0.255 |
| Depression score | 123 | –1.0 (3.8) | 114 | –0.2 (3.3) | –0.7 (–1.5 to 0.1) | 0.105 | |
| EQ-5D | Utility Index | 123 | 0.00 (0.18) | 113 | –0.02 (0.18) | 0.02 (–0.03 to 0.06) | 0.464 |
| Follow-up (months) | QoL outcome | Treatment group | Differencea (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Pump | MDI | ||||||
| n | Median (IQR) | n | Median (IQR) | ||||
| 6 | Perceived frequency of hyperglycaemia | 126 | –1 (–2 to 0) | 116 | –1 (–2 to 1) | 0.0 (–1.0 to 0.0) | 0.182 |
| Perceived frequency of hypoglycaemia | 127 | 0 (–1 to 1) | 116 | –1 (–2 to 0) | 0.0 (0 to 1.0) | 0.296 | |
| Treatment satisfaction | 126 | 8 (3 to 12) | 116 | 5 (1 to 10) | 2.0 (1.0 to 4.0) | 0.067 | |
| 12 | Perceived change in frequency of hyperglycaemia | 121 | 0 (–2 to 1) | 118 | 1 (–1 to 2) | 0.0 (0.0 to 1.0) | 0.131 |
| Perceived change in frequency of hypoglycaemia | 121 | –1 (–2 to 0) | 118 | –1 (–2 to 0) | 0.0 (0.0 to 1.0) | 0.345 | |
| Treatment satisfaction (change) | 121 | 16 (13 to 18) | 118 | 12 (7 to 16) | –3.0 (–4.0 to –1.0) | < 0.001 | |
| 24 | Perceived frequency of hyperglycaemia | 122 | –1 (–2 to 0) | 113 | –1 (–2 to 0) | 0.0 (–1.0 to 0.0) | 0.071 |
| Perceived frequency of hypoglycaemia | 123 | 0 (–1 to 1) | 113 | 0 (–2 to 0) | 0.0 (0.0 to 1.0) | 0.504 | |
| Treatment satisfaction | 122 | 8 (3 to 12) | 113 | 5 (0 to 9) | 4.0 (2.0 to 5.0) | < 0.001 | |
Improvement was seen across most psychosocial outcomes and time points for both treatment groups. There were no statistically significant differences at 6 months between the pump and MDI cohorts on any psychosocial measure. Participants in the pump group had better improvement in treatment satisfaction at all time points using DTSQ, but not using DSQOL; however, the difference was statistically significant only at 12 and 24 months (p = 0.067 at 6 months; p < 0.001 at both 12 and 24 months). Furthermore, participants in the pump group reported statistically improved diabetes-specific QoL at 24 months compared with the MDI group (p = 0.006); however, this was not the case at 6 or 12 months and could be due to chance rather than the treatment effect. We note that, if 6-month treatment satisfaction is reanalysed using a mixed-effects linear regression adjusted for baseline score, HbA1c, centre and course, as was done for the other Qol measures (rather than a non-parametric test), the treatment difference is similar and is statistically significant (p = 0.004), in part due to the increased precision from covariate adjustment.
There were some statistically significant differences on some subdomains, using p < 5% as the level for statistical significance. A caveat is required concerning the number of variables examined and tests performed, as multiple testing was not adjusted for. A statistically significant difference was observed on the social relations domain of the WHOQoL-BREF generic QoL measure in favour of the pump participants (p = 0.026), but this was seen only at 6 months, was one of 12 tests of significance and is likely to be a chance finding.
The DSQOL results (see Table 69) at 24 months showed statistically significant improvements (reductions) in both the pump (mean reduction of 8.2, 95% CI 5.84 to 10.50; p < 0.00001) and MDI (mean reduction 4.2, 95% CI 1.71 to 6.61; p = 0.001) groups, but with greater improvements in the pump group in the overall score (difference 3.8; p = 0.006) and some subdomains. The improvement in DSQOL diet restrictions was larger for the pump group than the MDI group at both 12 and 24 months (12-month adjusted MD in change from baseline –4.1, 95% CI –7.2 to –1.0; p = 0.010; 24-month adjusted MD in change from baseline –5.1, 95% CI –8.6 to –1.6; p = 0.004: lower scores represent better outcomes). A slightly smaller difference was observed at 6 months, which was not statistically significant (adjusted MD –3.3, 95% CI –6.9 to 0.2; p = 0.061). The pump group also had better improvement in DSQOL daily hassle or functions at both 12 and 24 months: at 24 months the score had decreased by 9.6 points in the pump group compared with 3.6 points in the MDI group (adjusted MD –6.3, 95% CI –10.9 to –1.8; p = 0.006).
However, there was a wide spread of changes in DSQOL, with some patients in both groups reporting deterioration at 24 months compared with baseline, as shown in Figure 19.
FIGURE 19.
Changes in overall DSQOL at 24 months. (a) Pump; and (b) MDI.

The HFS showed no difference in behaviour score but less worry about hypoglycaemia in the pump arm at 24 months only (p = 0.01). Higher treatment satisfaction by DTSQ was reported by pump users at all time points, and although this was not statistically significant at 6 months, statistical significance was reached at 12- and 24-month follow-up periods, although the absolute difference at 24 months was small at 4.0. EQ5D, SF-12, WHOQOL-BREF and HADS scores showed no differences between groups at any time.
Per-protocol results
Some patients switched from pump to MDI and vice versa, and they may be atypical. We therefore carried out an exploratory per-protocol analyses of psychosocial outcomes after excluding those who switched, and obtained, the following results.
Both groups showed statistically significant improvements in DSQOL as shown in Table 69.
| Treatment group | n | Mean change (SD) | 95% CI | p-valuea |
|---|---|---|---|---|
| MDI | 114 | –4.16 (13.20) | –6.61 to –1.71 | 0.0010 |
| Pump | 123 | –8.17 (13.07) | –10.50 to –5.84 | < 0.00001 |
The findings were similar with HADS-anxiety – both groups showed small improvements but this reached statistical significance only in the pump arm (Table 70). Large improvements would not be expected because baselines scores were quite low (pump 6.8, MDI 6.1)
| Treatment group | n | Mean change (SD) | 95% CI | p-value |
|---|---|---|---|---|
| MDI | 114 | –0.51 (3.49) | –1.16 to 0.14 | 0.1221 |
| Pump | 123 | –0.95 (3.95) | –1.66 to –0.25 | 0.0087 |
Hospital Anxiety and Depression Scale-depression scores improved in both groups but the change only reached statistical significance in the pump group (Table 71). Again, baseline scores were low (pump 4.4, MDI 3.7).
| Treatment group | n | Mean change (SD) | 95% CI | p-value |
|---|---|---|---|---|
| MDI | 114 | –0.15 (3.35) | –0.77 to 0.47 | 0.6355 |
| Pump | 123 | –0.99 (3.79) | –1.67 to –0.31 | 0.0044 |
Qualitative interpretation: cross-cutting improvements in quality of life
We turn now to qualitative data to (1) help explain the general improvements found across most psychosocial outcomes for both treatment groups and (2) aid the interpretation of those findings that reached statistical significance at more than one time point (i.e. findings relating to treatment satisfaction, dietary restrictions and daily hassles of function).
The overarching improvements in QoL observed in this study mirror those experienced by other cohorts of patients who have attended the DAFNE programme2,78,124,153 and, arguably, are largely attributable to conversion to a DAFNE approach. Indeed, when they were interviewed after their courses and 6 months later, patients in both arms reported very similar benefits and improvements to their lives. For example, patients in both arms – like other DAFNE graduates who have taken part in longitudinal qualitative research154 – reported a renewed enthusiasm for managing their diabetes after attending their courses and being more open to discussing aspects of their condition and self-management practices with family and friends. As a consequence, patients also discussed being more open to seeking and accepting support from these family members and friends.
Patients in both arms – like other DAFNE graduates143 – also reported feeling more in control of their diabetes/blood glucose levels and more committed to adhering to their treatment regimens (e.g. undertaking SMBG, administering insulin to cover the carbohydrate content of meals/snacks). Notably, however, although participants in the MDI arm tended to attribute these kinds of benefits and improvements to the education and instruction in DAFNE principles received during their courses, those in the pump arm – such as the participant quoted below – tended to accredit them to use of the insulin pump:
Because the pumps given me more awareness, like well if I do eat this and I give myself some insulin for it I’ll need to know what my blood sugar is then, so I will test, so I’ve been doing more tests as a result of doing more insulin with the pump.
P43.2
In addition, patients in both arms reported similar improvements in QoL arising from use of their automated bolus advisors. As described in detail elsewhere,143 patients who lacked confidence in their mathematical skills, or whose concentration could be compromised by high/low blood glucose, described the benefits and ‘peace of mind’ that arose from having the advisor to calculate their insulin doses for them. Those who were more confident about their mathematical ability also described liking and benefiting from using their advisor as these devices saved time and effort when calculating doses. Others still reported liking the data storage facility, as this reduced the burden of maintaining a paper diary. 155
After attending their course, patients in both arms reported high levels of satisfaction with their new regimens. Specifically, patients using pump and MDI – like other cohorts of DAFNE graduates143 – described feeling more confident and in control of their condition by virtue of having been given what they saw as a more logical approach and a better toolkit for managing their diabetes:
And I think the DAFNE course gave, gave me the confidence to, to manipulate my dosing . . . be more consistent with corrections. And once, and better carb-counting so once the corrections . . . once you’re right then it’s, you don’t need the corrections. I’ve found it much easier to maintain now.
M01.1
I’m testing me blood sugars a lot more, I’m counting, I’ve learnt how to count me carbohydrate properly. And I’ve learnt how to manage, if I ever get really sick, really bad sick days, I’ve learnt how to control them and deal with them a lot better, a lot better.
P13.1
However, patients using pump therapy also reported treatment benefits that were specific to using the pump, which helps explain the higher treatment satisfaction levels reported by those in the pump arm of the trial. For example, patients described how the pump delivered a drip-feed of insulin, which, as P04 suggested, enabled them to enjoy a more flexible lifestyle than had been possible using an injection regimen because they no longer had to adhere to routines to maintain their supply of background insulin by injecting at similar times each day:
Having the basal has just been amazing, just having that constant [supply] and being able to see your sugars just so constant. And not having to get up at . . . like I used to try and take mine at ten in the morning and ten at night . . . Whereas now I can just, if I want to sleep in till midday and not eat anything and I can still wake up with blood sugars at 6 and 7 and be totally fine.
P04.1
Patients using insulin pumps also described liking and valuing having access to a method of insulin delivery that enabled them to avoiding the pain and discomfort of injecting five or six times a day, as well as being able to administer insulin doses effortlessly and discreetly, and without the inconvenience of having to find somewhere private to inject:
. . . if I get taken out to lunch with a client or a supplier, then I don’t need to excuse myself or I don’t need to say sorry . . . I can do it from where I stand and, and taking something off your belt and so easy to do in so little time is, is, is great.
P17.1
I can just take my pump out of my pocket and key it, key it in and stick it back in my pocket. I don’t have to, I don’t have to get my needles out at dinner time and that’s quite nice. And it is nice for it not to be such a big issue and not to have to get half undressed every time you, you want to have some insulin.
P01.2
Some patients who engaged in sporting activities described how the device provided them with a more effective self-management tool to undertake such activities than was possible with MDI. Specifically, such individuals described liking being able to use a regimen that allowed them to suspend or adjust the rate of insulin infusion, depending on blood glucose readings, both to take into account the effects of long-duration physical activity, or, in P09’s case, to permit spontaneous visits to the gym:
Going skiing and having the pump . . . to have that and to be able to just tweak it constantly throughout the day if just great.
P04.2
Before if you were wanting to go to the gym you’d have to know hours and hours before it, before your last [background] insulin so that you could either reduce that . . . whereas now you can just say right I’m going to the gym I’ll just reduce it now or . . . take it off even.
P09.2
The above accounts stood in contrast to those of some individuals in the MDI arm, who identified exercise and physical activity as areas in which they continued to struggle to manage their blood glucose effectively, despite making the changes recommended during their DAFNE course:
I wasn’t given that much confidence with regard to doing physical activity and adjusting the dosage. Um, because my workout varies day in day out, so one time I go to the gym I might be there for an hour, um, but then one time I go to the gym on the weekend I might be there for an hour plus an hour in the pool or something like that. And it was just . . . the near enough generic way they give you of, um, adjusting your dosage, it’s like drop it by 10% or something like that, I didn’t find that that was effective [ . . . ] that side of things [exercise], it hasn’t really had much of, any impact on.
M09.2
The greater treatment satisfaction found in the pump arm of the trial can also be explained by patients’ perceptions of the added benefits of pump technology over MDI. These data are considered further under Study aim 3.
Mirroring the accounts of other cohorts of DAFNE graduates,146 patients in both arms described how their newly acquired knowledge and skills had allowed them to be more flexible and spontaneous in their food choices. For instance, patients in both arms described feeling more confident about eating less carbohydrate (which, for some, eliminated a perceived need to eat a snack before going to bed) and, in certain circumstances, skipping consumption of carbohydrates entirely. Relatedly, patients also described being more able to alter the timing of meals, as they no longer feared hypoglycaemia if they did not eat at specified times:
I was so happy the first night I was thinking ‘Oh I don’t need to eat a snack, that’s brilliant, I can just go to my bed if I want to go to my bed’. Whereas before I’d to wait till like 9, half past 9, to have my last insulin and have my snack before I went to bed and I was like ‘This is fantastic! I don’t even need to eat anything before I go to my bed!’.
P18.1
[I] was always very strict, ‘this is what I need to eat, it’s eight o’clock, I need to eat, otherwise there’s going to be trouble’ . . . I’ve definitely found some freedom in that I don’t have to eat when I don’t want to eat.
M07.2
However, patients in the pump arm highlighted additional benefits that appeared to be more specific to using an insulin pump, and which can be used to help explain the greater improvement in DSQOL diet restrictions in this arm of the trial. For instance, patients using the pump described how they could now eat a carbohydrate-based snack and administer a bolus accordingly, whereas, when using a MDI regimen, some reported having skipped a snack because they did not want to have a further injection:
I would rather have a pump than keep on injections and stuff, and it does mean I can have a snack. Um, you know, I don’t, I don’t really want to, let’s say, have a bag, have a bag of crisps and then inject myself, it wasn’t very appealing.
P31.2
Some such patients also discussed how, since moving on to pump therapy, they no longer had to restrict consumption of snacks containing carbohydrates to near to a mealtime in order to avoid having to inject more than once:
Before, if you were having something to eat, if you wanted something sweet, you’d have it with a meal, whereas it’s a lot more flexible now. If you want to go out in the afternoon and have a cake or something, you could . . . you could have a cake and just have a bit of insulin for it.
P25.2
Patients also described feeling more confident and able to dine out because the pump afforded an easy means of administering a separate bolus for each course. As P27 observed, this made it easier to make an impromptu decision to have a dessert without the burden of also having to administer a further injection. Others, such as P33.2, described how the ease with which they could stagger their insulin doses during a meal meant that they no longer had to worry about hypoglycaemia, particularly if a course arrived later than expected:
The pump is good because you can make fine adjustments, fine-tuning. You go out for a meal in the evening and decide to have a dessert at the last minute, so you just take, you know, a few more units in the bolus. Far nicer than getting out the pen and all of that.
P27.2
Some people would take it [a single dose] before their meal and then if their meal doesn’t come for so long, they sit and go, ‘right, where’s the cans of Coke’ but I just feel as if you’ve got more freedom. You can actually stagger your insulin over a meal, which is good. I find, maybe if you’re sitting for a long meal, a couple of hours, you can stagger your insulin so it’s, you’re not getting too much at once.
P33.2
Although there were no statistically significant differences at 6 months, patients did highlight factors and experiences in their 6-month interview accounts that might help to explain why the pump group also had better improvement in the DSQOL daily hassle of functions at both 12 and 24 months. Notably, patients using pump therapy reflected on how using a pump to administer insulin required less time and effort, and was ‘less of a chore’ (P25.2) than using pens. This was partly because pressing a button to administer insulin was a more convenient and expedient option than ‘having the hassle and worry of getting the needle out’ (P04) and ‘having to crank it up on the pen and then inject’ (P33.2). In addition, patients, including P30.2, highlighted the advantages of no longer having to take time of out of their everyday activities to find private locations in which to inject (see also aim 4):
‘Cos when I went to work, with pens, I’d often go into the locker room to inject myself. And now with the pump, I’ll just take it out of my shirt pocket, type in what I am having, put it back in my shirt pocket and it’s done.
P30.2
Some patients also described how pump therapy was a less burdensome and time-consuming option because of the ability to use, set and alter basal rates:
If I was on the pen, you know, I’d be having to take an extra insulin mid-morning, you know, if my blood sugar was rising . . . So for me it’s just so much easier to be able to set things on a temporary basis.
P39.2
Cos I’m going to bed and I reach to take my insulin before I go to bed, and it’s like, ‘no, no that doesn’t have to happen anymore’, so it’s good.
P40.1
Patients also highlighted the advantages of having ‘less paraphernalia to lug around’ (P06.2) by virtue of using the pump, whether this be when travelling to and from work (P03) or, in P04’s case, when undertaking recreational activities, such as skiing on a recent holiday:
You’ve for that freedom with the pump, you can do anything whereas [with] the injections you’ve got to take your pen, you’ve got to take your needles, you’ve got to take your sharps bin, you’ve got to make sure you’ve got a spare pen in case that one don’t work. Whereas with your pump, I always carry a spare quick inserter [cannula], a spare tube insert, just in case you’ve any problems or get a blockage or whatever . . . but they’re nothing, they’ll slip in a rucksack or in your pocket.
P03.2
There is no stress, it’s there, it’s attached. Going skiing and having the pump on was on me was just so much better than having pens, having to take pens and needles and stuff up the mountain . . . you’ve just got this thing attached to you and that’s it done with now.
P04.2
Study aim 3
-
To understand and explore the added benefit (if any) of pump technology over MDI from patients’ and educators’ perspectives.
Qualitative data are drawn on to address this study aim; here we begin with patients’ perspectives before moving on to those of staff.
Patients’ perspectives
Preconceptions about insulin pumps
Many patients described having had misconceptions prior to the trial about how the pump worked and how it could be used to manage their diabetes. Specifically, some described how they had thought that the pump would be a small device implanted under the skin. Others had perceived the pump as being more akin to a closed-loop system, which would alleviate much of the burden of diabetes management by monitoring blood glucose and calculating and administering insulin doses:
I actually thought the pump was some kind of implant . . . and I thought it was something you connected . . . some kind of pipe or cannula and you filled up this implant and then once it was full you disconnected it and then you just had like a remote [control].
P09.1
I think my preconceived ideas were slightly wrong . . . I thought it would be a continuous monitoring system and adjust accordingly . . . And I didn’t realise that you had to keep on testing yourself.
P14.1
Despite some such patients’ initial hopes and expectations not being met, most of those who used pump therapy during the trial described the pump as offering benefits over a MDI regimen. Although some of the benefits described by these patients were also highlighted by those in the MDI arm of the trial (and, hence, arguably were due to the use of the DAFNE approach rather than pump therapy per se), some did appear to arise specifically from use of an insulin pump and these are considered below.
Drip-feeding basal insulin and altering basal rates
Most patients using pump therapy described feeling that they had better control over their blood glucose levels because the device supplied a constant drip-feed of basal insulin, which, as they suggested, more accurately mimicked the natural release of insulin by the pancreas.
Now, because it’s such a little trickle, it’s really, I think, that’s made a huge difference, because it’s made me operate, my body operates more like somebody that’s got a, you know, a pancreas that works.
P24.2
Some patients also highlighted the benefits of being able to set different rates of basal insulin infusion during the day and night. This included P09, who described using a lower basal rate for a specific period of time to counter recurring nocturnal hypoglycaemia, and P19, who reported using higher basal rates to counter rises in blood glucose during periods of inactivity and lower rates when more active (e.g. at weekends):
. . . it’s a lot easier, like, at the moment, my blood sugar tends to dip between midnight and four in the morning, so the pump slightly reduces the insulin . . . whereas on the pen [MDI] then I’d have to reduce the whole of the insulin from before I go to bed until I get up in the morning.
P09.2
[During the week] I’ll sit at my desk until lunchtime, whereas obviously at the weekend I’ll get up, have breakfast, and then I’ll probably go out and about and do something active, so that was . . . weekends were my problem for blood sugar. But that, you know, now I’ve changed that, I’ve put on temporary, er, temporary basals for then, during the morning, and er, it’s been fine.
P19.2
Others highlighted the clinical and personal benefits gained from being able to use a temporary basal rate to accommodate sporting and other physical activities (see Study aims 1 and 2) or, in P18’s case, to minimise the risk of hypoglycaemia after drinking alcohol:
I’d set a temporary basal on it because I was having a drink and so I lowered the basal so as that I could, to stop me hypo-ing through the night sort of thing.
P18.1
Fine-tuning and administering small doses of bolus insulin
As well as being able to alter basal rates, some patients reported additional benefits arising from being able to administer very small and/or precise bolus doses of insulin. Reflecting back on their experiences using an injection regimen, such patients described how this feature had enabled them to more precisely match insulin doses to carbohydrate intake in order to fine-tune their blood glucose control:
I love that you can, you can just give 0.1 of a unit now and before I was on, like, you know, 1 unit, so the accuracy’s much better . . . I’m excited that you can just fine-tune it so much . . . the control that it’s given me already is just fab.
P04.1
. . . it’s more clinical isn’t it, so, you know, it’s easier to be, to be able to drill down into it and to fine-tune it, which is, which is what really I need to do, it doesn’t need to be massive changes, it just needs to be slight, you know, slight changes to make it that much better.
P19.2
As a consequence of being able to administer very precise and small doses, some patients who were sensitive to insulin also described how using the pump had lessened their perceived risk of hypoglycaemia:
If you’re on the edge of going hypo[glycaemic] and you’re having something to eat . . . so you take your insulin, that half a, extra half a unit can send you down again. Whereas on this [pump] you can, like I say, you can fine-tune it to half a unit, so you know exactly what you’re taking. If you need one and a half units for a sausage roll, you’re not trying to think, ‘well, do I take 1 or do I take 2?’. You can take one and a half.
P13.1
Advanced settings: dual- and square-wave boluses
A small number of patients also suggested that they benefited from using advanced pump settings, such as the dual- or square-wave function, to offset the delayed effect of carbohydrate-dense foods, such as pasta, or when eating a meal over an extended period of time:
Then there was the dual wave, you know like when we’ve, if we’ve had pasta and you know your carb[ohydrate]s are going to be long acting and things like that, I think that’s brilliant, whereas before when I were having injections, you just had your injection and then 3, 3 hours later your blood sugar would still be really high.
P05.2
At Christmas time, parties, right? Buffets and things like that, this is a lot easier because you can put it on a dual wave or a square bolus or something and you can forget, you know, right, I’ve dealt with the insulin, and then you can just eat little bits over however many hours, um, and I did that and it worked . . . you couldn’t do that with injections.
P07.1
Wearing the pump prompts patients to perform self-management practices
Although some patients described disliking being connected to the device (see Study aim 5) an additional benefit identified by some individuals was that the pump’s presence prompted them to undertake DAFNE-specific self-management practices, such as SMBG:
. . . it’s a very useful kind of physical manifestation of the fact that, ah, you have this, you have this condition and you’re eating right now, and so do something about your blood test, do something about what you’re eating . . . It’s a very, kind of very useful as a, as a way of, er, reminding you to, you to employ the, er, the techniques . . . that we’re that we’re taught on the DAFNE course.
P23.2
No need to inject
Aside from perceived clinical benefits, many patients reported personal benefits arising from no longer having to inject. Despite having to insert a cannula every 2–3 days, most suggested that this procedure was much less onerous than having to inject five or six times a day:
I know you have to mess about with putting the cannulas in every 3 days, but that’s the biggest hardship. It’s still, you know, going from that . . . er, to like four injections a day, morning, lunchtime, teatime and night-time, when you’re out you’ve got to pull the injection out, stick it in you and stuff like that, it’s, it’s totally different.
P10.1
As reported earlier, in Diabetes Treatment Satisfaction Questionnaire: treatment satisfaction (better for patients using pump therapy) and Diabetes-specific quality of life: daily hassles of functions (better for patients using pump therapy), patients using pumps also described benefits and satisfaction arising from being able to administer insulin without having to inject in front of others and/or to find somewhere private to administer an injection when in a public place. As such, and like the adolescent pump users studied by Lowes et al. ,156 patients also described feeling less noticeable, stigmatised and, hence, detached from others as a consequence:
It’s actually more discreet . . . one person thought it was an iPod [Apple Inc., Cupertino, CA, USA] . . . I find it more discreet because you can take a bolus before a meal without having to expose your skin, which, not everybody likes you injecting in public, it’s easier to take your dose in that way, and it means it’s much easier to fit in.
P12.2
Educators’ expectations and perspectives
Educators’ perspectives have already been reported in detail elsewhere;147 hence, readers may wish to reference this work for more detail about particular findings or to access additional quoted material.
Added benefit of pump therapy
All staff were keen to emphasise that a MDI regimen, taught in conjunction with a DAFNE or similar educational approach, presented a very good and effective toolkit for undertaking diabetes self-management. Hence, educators also suggested that, if they were taught to use a MDI regimen effectively, most patients would neither need nor gain added clinical benefit from using pump therapy:
I think we can maximise most people on DAFNE and it’s wonderful, we really are DAFNE advocates and we’ve had a lot of improvements and reductions in hypos.
D4
However, all educators also noted that, because of the constant drip-feed of insulin, the ability to alter basal rates, and also the ability to titrate and deliver very small insulin doses, pump therapy could potentially help certain groups of patients to improve and/or fine-tune their glycaemic control. As educators described, these individuals were principally those who met current NICE criteria for pump referral,13 such as those who suffered from the dawn phenomenon, were very insulin sensitive and/or who undertook a lot of sporting activities that exposed them to risk of hypoglycaemia:
People whose insulin requirements are really small, really low, where sort of injected longer-acting insulin, background insulin, you just can’t adjust them finely enough . . . a pump is great for them because you’ve got the really, you know, minute basal adjustments.
N02
Those that are maybe quite intense when it comes to exercise, you know, there’s definitely a potential for them. Equally, those that are maybe finding that they are on really small doses of insulin because it [the pump] does give them that opportunity to fine-tune.
D05
However, all educators pointed out that, to gain added clinical benefit from using a pump, patients had to be willing and able to their use the pump’s features otherwise, as N3 suggested:
They will just sit on the pump and use it as another method of delivering insulin and they’ll be no better off than on injections.
N3
As is described further later (see Study aim 4), educators also highlighted the difficulties of predicting which patients, or groups of patients, would have this willingness and ability to use the pump to optimal effect.
Study aim 4
-
To look at why some patients may do better than others using pump therapy.
To address this aim, we begin by presenting quantitative data before drawing on educator accounts to reinforce and support the quantitative findings.
In addition to the pre-specified subgroup analyses presented in Chapter 5, Subgroup analysis, we undertook exploratory analyses investigating the relationship between continuous baseline variables and outcome, using scatter plots with superimposed regression splines (Figures 20–22).
FIGURE 20.
Relationship between baseline HbA1c and change in HbA1c at 2 years.
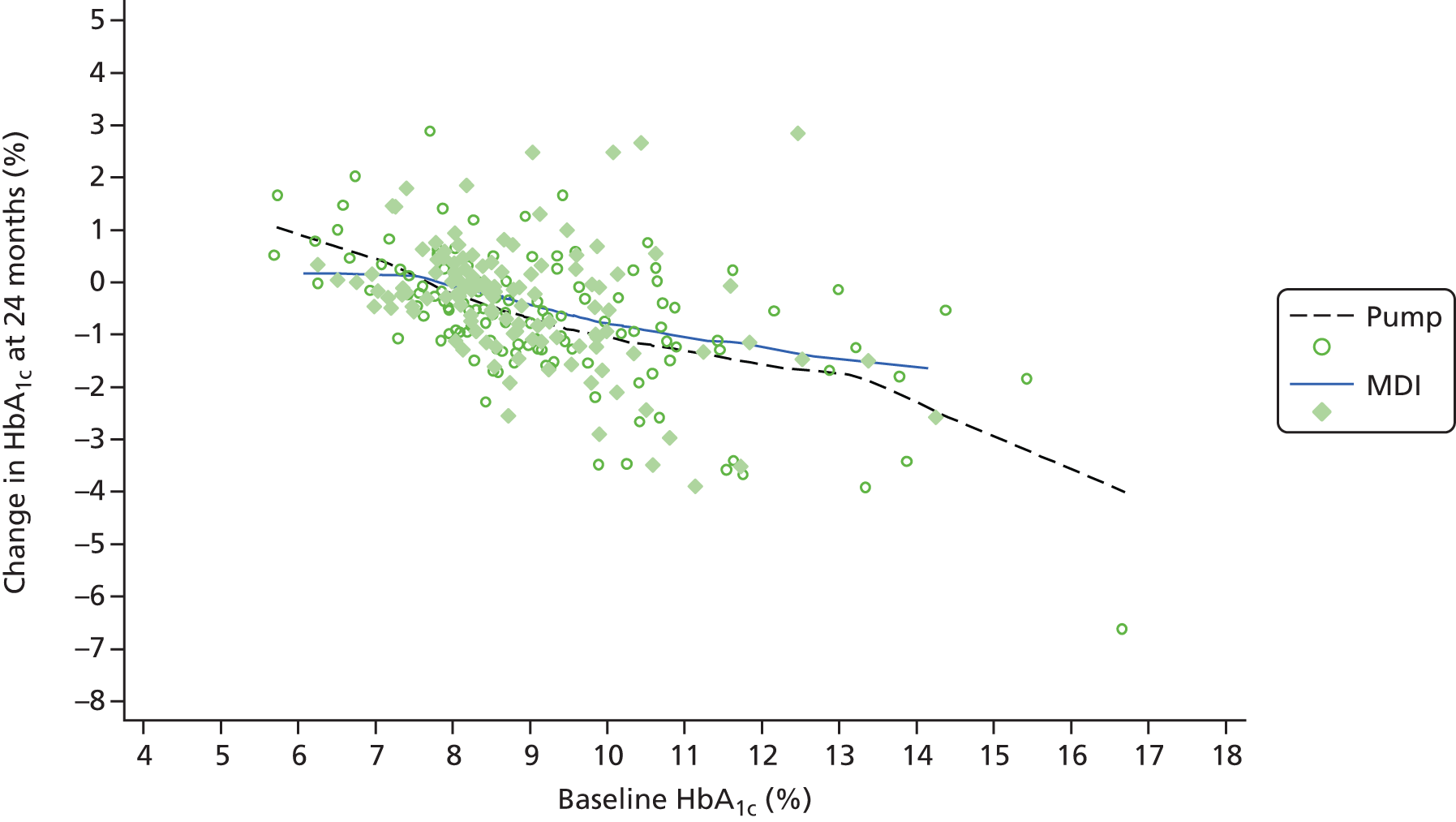
FIGURE 21.
Relationship between duration of diabetes and change in HbA1c at 2 years.
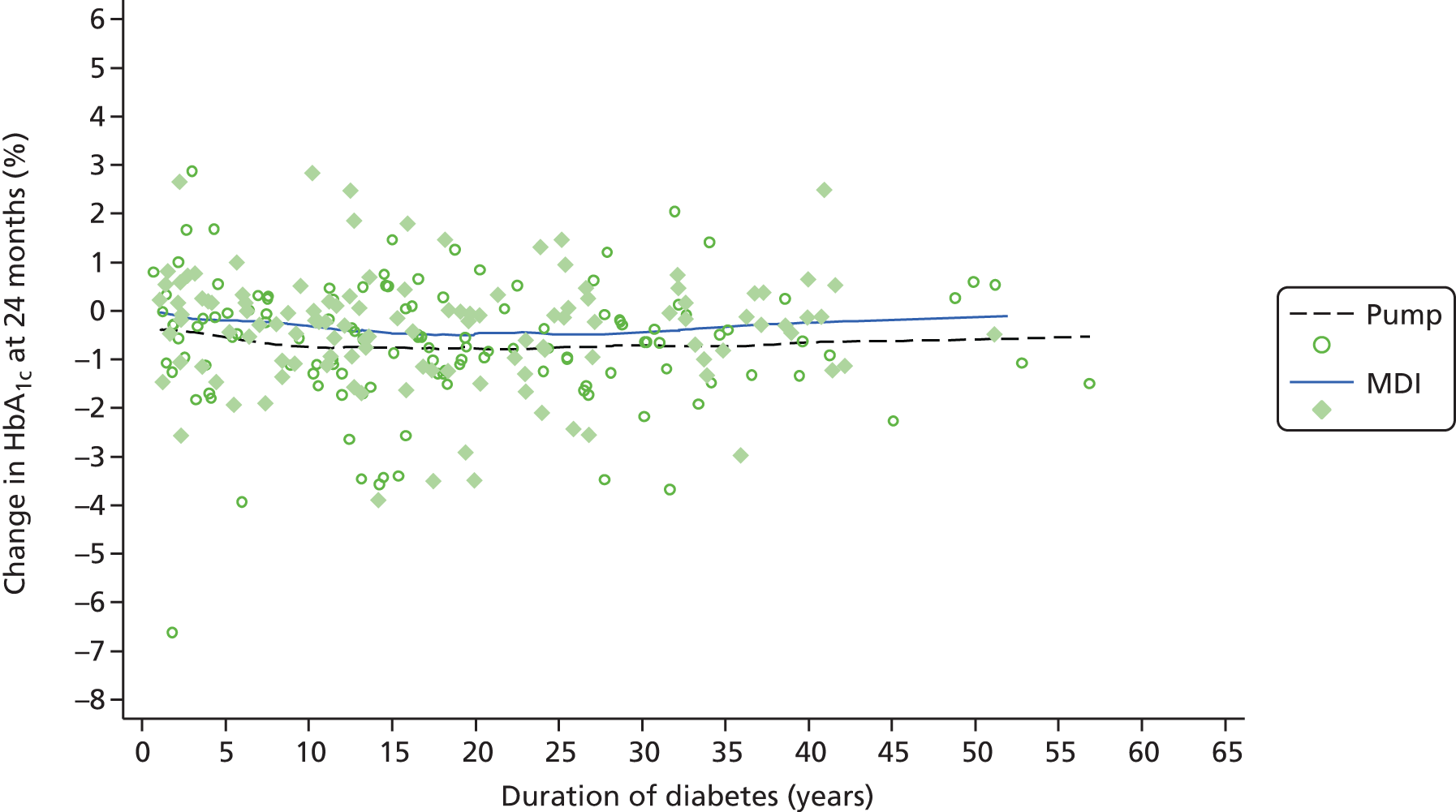
FIGURE 22.
Relationship between baseline age of onset/diagnosis and change in HbA1c at 2 years.
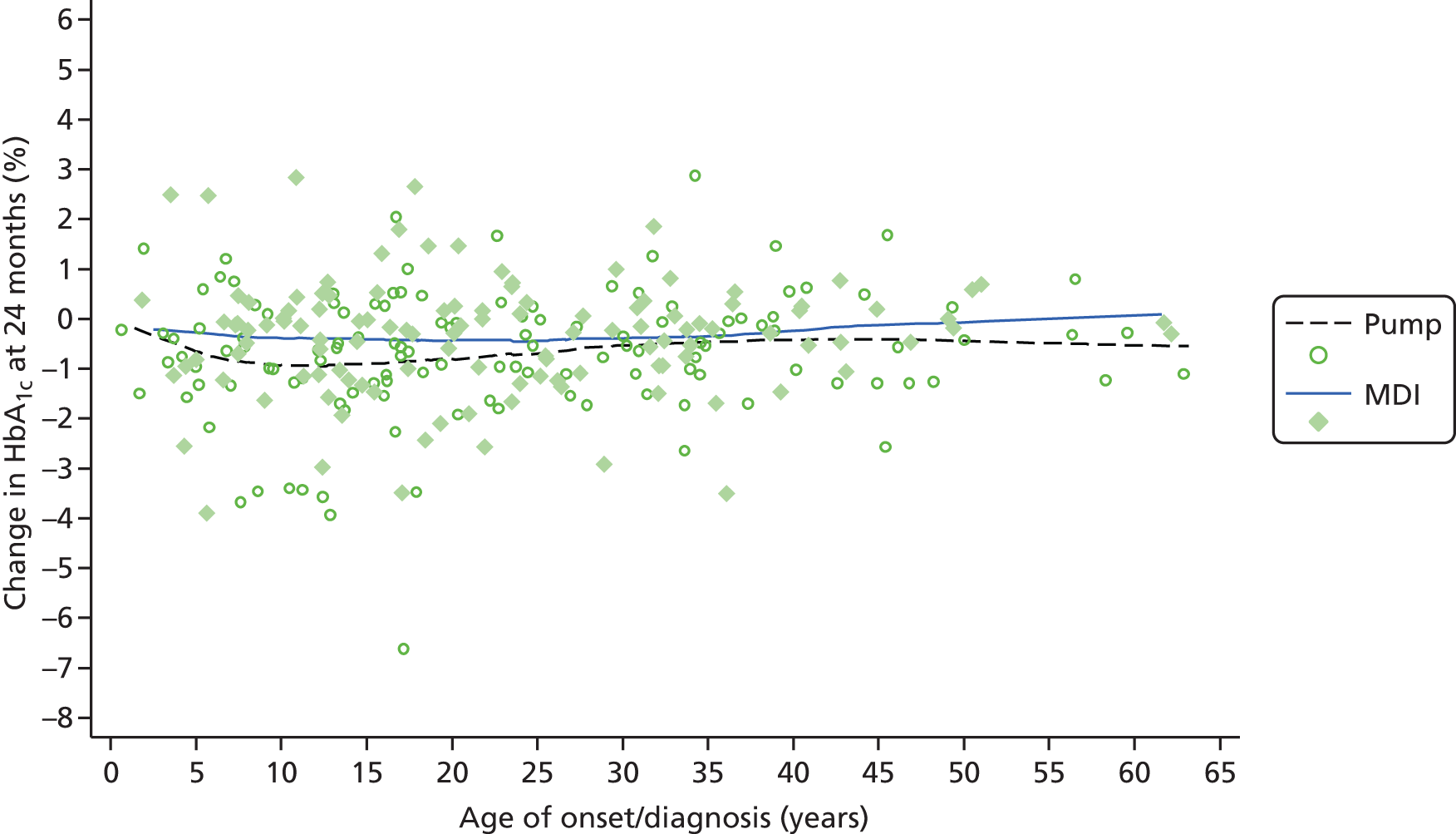
Unsurprisingly, those with the highest HbA1c at baseline tended to have the largest reductions in HbA1c at 24 months in both groups. There were no clear associations seen between HbA1c reduction and age at entry, duration of diabetes, BMI or age at onset in either group. As with HbA1c, no clear patterns were seen between DSQOL at 24 months and mean age at baseline, duration of diabetes, BMI or age of onset. The biggest reduction was seen in those with highest DSQOL at baseline, who had more scope to gain.
The lack of association between duration of diabetes and benefit after DAFNE is an important finding, which supports the recommendation in the updated NICE guideline that structured education should be provided to all patients, not just those recently diagnosed.
We hypothesised that greater use of the facilities in the pump might be an indicator of engagement with self-management. However, we found no association between the number of basal rates used and change in HbA1c (Figure 23).
FIGURE 23.
Change in HbA1c (%) vs. number of different basal settings (pump group only).

We found considerable variability of changes in both HbA1c (see Figure 4) and DSQOL (see Figure 19), with some individuals making very considerable improvements and others deteriorating over time. However, exploratory analysis of factors that might be influencing the changes did not find anything of significance.
Qualitative findings: educator accounts
In advance of the trial, educators described holding certain preconceptions about who would do well on a pump and make full and effective use of its features to optimise glycaemic control. These preconceptions, as will be described, were subsequently challenged and revised in light of educators’ trial delivery experiences.
Pre-trial views about pump candidacy
As indicated earlier (see Study aim 3) educators described having had preconceptions in advance of the trial about the kinds of individuals who would do well on a pump. Specifically, educators discussed how, in their routine clinical practice, in addition to using NICE and other clinical criteria, they had tended to recommend individuals for pump therapy based on tacit and informal assumptions about whether or not they had the right aptitude and technical ability to use the pump to optimal effect. These individuals, as educators also noted, had tended to be those who were younger, technologically savvy and academically able:157
. . . people who [are] more numerate and the more, the more intelligent, the more, you know, sort of educationally able to take on board all the information.
D03
For similar reasons, educators also described how, despite meeting clinical criteria for pump referral, they had not generally recommended individuals for pump therapy in routine clinical practice if they had a poor history of diabetes self-management, were older or were less academically able. 157 This was a result of their concerns that such individuals would be unwilling or unable to ‘put in the extra work required to use a pump properly’ (N11) and, hence, would not gain any added clinical benefit from the pump as compared with MDI.
Revising preconceptions as a result of trial participation
Educators also described how, as a result of their participation in the REPOSE Trial (for which a randomisation process rather than their own judgement was used to determine who was moved onto the pump), they had been exposed to individuals using pumps who they would not have put forward for this regimen in routine clinical practice. As educators further noted, this kind of exposure had led them to reconsider which kinds of people might gain clinical benefit from using a pump. Specifically, and as detailed elsewhere,157 educators recounted experiences during which they had observed individuals during the trial ‘doing really, really well on pump therapy who we would have predicted would have really struggled’ (D2), as well as those ‘such as the likes of the young lad who was desperate for a pump and he’s just not using it’ (N9). As a consequence, some educators described how they ‘had stopped having preconceptions about who it will suit and who it won’t’ (N3), whereas others suggested that, in light of their trial experiences, they now thought that motivation – rather than age, technological aptitude or academic ability – should be used as the main criterion (alongside clinical criteria) for determining future pump referrals:
I’ve found that when you actually sit down, show them it, work way through it, actually they become more efficient. So in a way I don’t think there’s anybody that shouldn’t do well on a pump as long as they are keen and motivated.
D4
Others still noted from their experiences of observing patients during the REPOSE Trial that use of a pump could itself act as a tipping point for increased disease self-management among some erstwhile seemingly demotivated patients. As a consequence, such individuals described having reached the conclusion that pumps ‘should potentially be made available to everyone [meeting clinical criteria] because you simply can’t predict, so maybe you need to give everyone a chance?’ (D1). 147
Summary
Educator accounts thus highlight the difficulties of identifying and using patient characteristics to predict potential clinical success using an insulin pump, thereby reinforcing the findings of the quantitative analysis, which showed that it is not possible to determine which patients, compared with others, are likely to do better on the pump.
Study aim 5
-
To explore acceptability of, and reasons for, discontinuing (pump) treatment.
To address this study aim, we draw on the interview accounts of patients in the pump arm of the trial.
Acceptability
As described in Study aims 1 and 2, very high levels of treatment satisfaction were reported by patients using pump therapy. However, at baseline, and over time, a small number of individuals did describe having struggled to adapt to the presence of the pump and discussed how they had disliked being attached to the device, as it acted as a constant reminder of their disease state:
. . . it just makes me feel like I’ve got, I know I have a disease, but like a diseased person with this thing, a machine attached to me.
P12.2
Although most patients found the pump to be a discreet form of treatment, a few also reported feeling self-conscious when using the device in public settings:
. . . before, obviously, you’ve got nothing . . . there’s nothing on you to say, ‘I’m a diabetic’ and now you’ve got this pump, people are a little bit more . . . inquisitive.
P09.2
In some cases, and mirroring findings reported by Hayes et al. ,158 patients described how they had found the pump inconvenient to carry on their person and awkward to stow in their clothes, both during the day and when in bed. Others spoke about having experienced pain if they had accidentally bumped the site where the cannula had been inserted and/or if they had caught the cannula needle/tubing when performing everyday activities. This included occasions when patients had been in bed asleep, driving, playing with children, wearing tight-fitting clothing, having sex or undertaking sporting activities:
. . . sometimes when I’ve lifted the kids they’ve caught themselves on the tubing . . . and having to say to them ‘you need to watch mummy’s pump’ so they don’t kick it or something when we’re carrying on.
P18.2
It’s not nearly as convenient . . . it’s in the way. And it’s also awkward at night . . . . So I’m still getting to grips with that, and as I, when I played tennis this week I took it off, when I play golf I tend to put it in the pocket and the same with gardening.
P11.1
Despite many patients reporting having experienced practical difficulties, most also indicated that they had quickly adapted to wearing the pump. To do this, patients described having altered where they had stowed the pump or having adapted clothing to ensure the device was more secure or tubing less likely to snag:
I’m mostly wearing it tucked into a belt. And one of the things I have changed recently is I now tend to wear it at the side or even slightly behind the side.
P11.2
I think at first it’s obtrusive because it’s there, isn’t it and it’s in bed and ‘where do the, where the hell do I put it . . . and it’s been under my pillow. But now I’ve got used to it. And as I say, I’ve got some elastic to get it tucked away at night-time.
P05.1
Furthermore, although many patients described how the pump could be a ‘bit of a nuisance sometimes’ (P14.2), most also suggested that the practical inconvenience of having it attached to their body was outweighed by their perception that the device had enabled them to achieve better glycaemic control, and a more flexible lifestyle than was possible using a MDI regimen (see Chapter 9, Research question 3):
I thought ‘oh I’m not sure I’m going to like having something attached to my body the whole time’. But I think, after doing the week [DAFNE course], you can see the benefits that it had in terms of being able to manage your diabetes and make subtle changes in the amount of insulin you have that you can’t really do with pen injections, you know, that kind of outweighed for me the fact that I’m going to have . . . and you just get used to it, like I don’t really feel it on me now so you just kind of get used to it.
P21.1
Similarly, patients who described difficulties siting and inserting a cannula contrasted this level of inconvenience with a MDI regimen, which they considered to be much more cumbersome:
When it comes time for me to change the pump [cannula], I’m like, I can’t be bothered doing this! But then I think to myself, ‘well, it’s either do this or else do six injections a day’ and then I just have a wee argument with myself and tell myself to shut up [laughs]!
P18.2
Limiting the use of the pump
Although none of the patients who participated in the qualitative study reported having discontinued using the pump entirely, there were two individuals, both young women, who reported struggling with disruption to their body image: ‘it’s like having a colostomy bag attached to you’ (P01.1), ‘I think I was like, “oh, this thing’s attached to me and I’m getting fed up with it, I need a break from it otherwise it’ll drive me insane”’ (P04.2).
As a result, both of these individuals described temporarily reverting to MDI on some occasions during the 6-month period of study. They also identified specific trigger points, similar to those reported by Hayes et al. ,158 which had resulted in them disconnecting the pump, including when there was little time available to change a cannula or when a tight-fitting dress had had to be worn and ‘every lump and bump’ was visible. However, despite the unease they had experienced when wearing the pump, both women reported removing the device for only relatively brief periods of time before subsequently reattaching it because, as P04 explained, ‘all the positives outweigh the negatives’.
Study aim 6
-
To enhance understanding and assist in the interpretation of trial outcomes (e.g. differences in HbA1c between the two arms).
As there were no significant differences in HbA1c between the two arms, we are unsurprisingly cautious in drawing any major contributions from the psychosocial work in relation to these outcomes, although it should be noted that, because of our restricted funding, we were limited by our inability to interview patients beyond 6 months. The perceived benefits of the pump user group, both in terms of the qualitative work and the limited benefits in terms of treatment satisfaction and some DSQOL domains, are described in detail within study aims 1–5.
Summary
We used a mixed-methods approach with questionnaires and interviews, and had a good response to questionnaires, with approximately 94% completion in the pump group and 86% in the MDI group. There was also a very good response to invitations to take part in interviews, and attrition in this part of the study was low with only three of 45 recruits not completing the round 2 interviews.
We found little difference in quantitative psychosocial outcomes between the pump and MDI arms, largely because improvements were observed in both following DAFNE. There were some statistically significant differences in the subdomains of the DSQOL in favour of pump therapy, those being leisure time restrictions and flexibility, daily hassle and dietary restrictions.
Treatment satisfaction also improved in both arms, but statistically significantly more in the pump arm. These observations were supported by findings from the qualitative interviews. There was also a greater reduction in the ‘hypoglycaemia worry’ score in the pump arm. The qualitative findings were that patients in both arms felt more in control of their diabetes.
Patients in both arms reported benefiting from automated bolus advisors, although, as reported elsewhere, there may be unintended consequences to giving people access to this technology. 155
A recurrent theme was that after doing the DAFNE course, patients in both arms felt more in control and more confident in self-management. However, those on the pump reported some additional benefits from the pump, mentioning increased flexibility of lifestyles, avoidance of the frequent injections with MDI, more effective self-management around sporting activities and dietary variations, and the ability to administer very small doses of insulin, with different basal rates, at different times of day and night.
Chapter 8 Discussion
Statement of principal findings
We carried out a randomised trial of pump versus MDI in a group of adults with T1DM referred for structured training in flexible insulin therapy because of suboptimal diabetes control. Both groups received training ensuring that education was balanced across the arms. The main results were:
-
The pump group had a slightly greater mean reduction in HbA1c of 0.85% (9.3 mmol/mol) than 0.42% (4.5 mmol/mol) on MDI. After adjusting for baseline difference and accounting for missing data, the MD at 2 years did not reach statistical significance –0.24% (95% CI –0.53% to 0.05%) or –2.7 mmol/mol (95% CI –5.8 to 0.5 mmol/mol).
-
Overall, participants in the trial achieved a clinically worthwhile fall in HbA1c of 0.6% (7 mmol/mol) at 2 years.
-
Some patients switched treatments during the trial and the per-protocol analysis showed a statistically significant MD of –0.36% (95% CI –0.64% to –0.07%) or –3.9 mmol/mol (95% CI –7.0 to –0.8 mmol/mol) in favour of pump therapy (p = 0.015). The 95% CI includes the 5% clinically important effect and so we cannot claim equivalence of pump and MDI in this population.
-
The proportions achieving HbA1c of ≤ 7.5% at 24 months were relatively low in both groups at 25% on pump and 23% on MDI.
-
The frequency of severe hypoglycaemia fell in both groups, although more so in the pump group during months 12–24.
-
At 24 months, there were no significant differences in BMI, insulin dose or lipid levels.
-
Both groups demonstrated improved psychological outcomes over a range of different scales, which included treatment satisfaction and DSQOL. Treatment satisfaction and two subdomains of the DSQOL (daily hassle, diet restrictions) improved to a greater extent in those allocated to pump therapy both at 12 months and 2 years.
-
The qualitative work found that patients in both arms felt more in control of their diabetes and benefited from automated bolus advisors. A recurrent theme was that after undertaking the DAFNE course, participants in both arms felt more in control and more confident in self-management. Those on pump therapy reported some additional benefits from the pump, including increased flexibility of lifestyles, more effective self-management around sporting activities and dietary variations, and the ability to administer very small doses of insulin. These findings are reflected in the differences in the quantitative outcomes, but did not result in significant differences in glycaemic control.
Thus, in terms of the primary outcome, there were no significant differences in change from baseline to 24 months between those randomised to pump therapy or those using MDI, indicating that pump treatment provided no significant additional biomedical benefit over DAFNE skills training. 23
Rates of severe hypoglycaemia were halved in both groups, a benefit maintained to 24 months with no difference between the groups in this or in rates of moderate hypoglycaemia. 23 However, we noted that between months 12 and 24, rates of severe hypoglycaemia were lower in the pump group, although this comparison had not been pre-specified. There were no other differences in biomedical outcomes apart from slightly greater reductions in insulin doses in those randomised to pump treatment. 23 Contrary to most previous studies, insulin dose fell in the MDI arm.
Summary of trial- and model-based estimates of cost-effectiveness
Both the trial- and model-based estimates of cost-effectiveness showed that the addition of pump therapy to a structured training course was not cost-effective compared with the £20,000–30,000 per QALY gained threshold used by NICE. 22 These results were robust to all scenario analyses.
In the base-case EEACT, the addition of insulin pump therapy to structured education for adults with suboptimally controlled diabetes was dominated by current practice as, on average, it produced fewer discounted QALYs over the 2 years (–0.004) at a higher discounted cost (£2959). The lowest ICER was observed in the scenario analyses, in which a 50% reduction in the cost of insulin pumps and insulin pump consumables in the per-protocol population was conducted. The ICER of this strategy was £552,866, indicating that, even with substantial discounts in price, insulin pump therapy was not a cost-effective addition to structured education for adults with suboptimally controlled T1DM.
Any differences in the rates of diabetic complications in the long term are not included in the estimates of cost and QALYs in a within-trial analyses, as they will occur after the last follow-up period. To address this issue, the lifetime costs and QALYs were estimated using the Sheffield Type 1 Diabetes Policy Model.
In the long-term modelling, the addition of insulin pump therapy to structured education for adults with suboptimally controlled diabetes generated more incremental discounted QALYs (0.1447) at a higher incremental cost (£20,448), producing an ICER of £141,312 per QALY gained. The lowest ICER was observed in the scenario in which the prices of insulin pumps and insulin pump consumables were reduced by 50%. The ICER of this strategy was £46,578, again indicating that even with substantial discounts in price, insulin pump therapy was not a cost-effective addition to structured education for adults with suboptimally controlled T1DM.
Strengths and weaknesses of the research
Our study had a robust, multisite design, involved larger participant numbers and had a 2-year follow-up period, which was longer than previous trials of pump therapy and therefore more clinically meaningful. Participants in both arms used analogue insulins and bolus calculators. 23 The study was conducted in secondary care centres, reflecting a range of experience delivering pump therapy and involving attendance at a structured training intervention that has consistently shown improved biomedical and psychological outcomes and is well established across the UK. It included a main outcome measured in a central laboratory and a comprehensive psychological evaluation with high levels of data completeness. The pragmatic study design thus provides good external validity, particularly as participating in the educational course led to sustained improvements in glycaemic control, reduced rates of severe hypoglycaemia and improved psychological outcomes across a range of scales. 23
The follow-up period, although longer than other studies, could have been lengthened to 3 years, as evidence from some previous studies indicates a waning of the effect of pumps over time. 63
It is not possible to blind a trial in which insulin delivery systems are fundamentally different, and this could lead to a bias in any RCT involving pumps. A trial studying individuals who have expressed a desire for pump treatment is likely to struggle to recruit participants if one arm continues on MDI. Those randomised to MDI may also either drop out or exhibit poor outcomes due to ‘disappointment’ and lack of motivation. We studied individuals who had not specifically requested pump therapy, but who were awaiting a course in diabetes self-management to help them improve their glycaemic control. Thus, our aim was to determine any added benefit of pumps above MDI while controlling for the training itself. 23
An important additional limitation is that those randomised to pump treatment might have been insufficiently motivated to make the most of any technological benefit, as they had not expressed a particular wish to use a pump. Anecdotally, one common reason given by patients for not wanting to participate was reluctance to use an insulin pump. However, educators encouraged participants to use pump features and provide additional input if this was requested. Overall, we reasoned that as participants had signed up for a course to improve their glucose control, any additional benefits of pump treatment would emerge. 23
Comparison with other research
Two appraisals of pumps by NICE have reviewed the evidence on clinical effectiveness and cost-effectiveness. The first14 noted that there were no trials of pumps against ‘best MDI’ with long- and short-acting analogue insulins; some trials had unequal amounts of education in the arms (with more in the pump arms); and the trials had focused on easily measurable outcomes, such as HbA1c, rather than on benefits in terms of flexibility of lifestyle and QoL. The report recommended trials of pumps against analogue-based MDI. A more recent report9 found only three trials in adults: one a pilot and the second involving 39 adults with T1DM, already on pump therapy, who were randomised to stay on pump therapy or to switch to glargine-based MDI; patients had 4 months on each form of treatment. A third trial recruited 57 adults who were randomised to pump or analogue MDI in an equivalence study. None showed any difference in HbA1c. Thus, the evidence base from trials for comparing pumps and ‘best MDI’ was weak in terms of numbers, with a total of only 103 patients and short follow-up. (This paragraph is reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.)
The literature on the cost-effectiveness of insulin pump therapy has solely been based on comparisons of insulin pump therapy to MDI rather than ‘optimised MDI’. A recent systematic review on the cost-effectiveness of pump therapy in different countries identified four studies that were conducted in a UK setting, three of which presented an ICER. 19 The base-case ICERs in these studies ranged from £37,712 to £11,461 per QALY gained. These ICERs are much lower than those estimated in the analyses based on the REPOSE Trial data. However, the ability of these studies to determine the cost-effectiveness of insulin pump therapy as a treatment for all adults with suboptimally controlled T1DM is limited, as the effectiveness used in these analyses does not compare insulin pump therapy to ‘best MDI’. Hence, the REPOSE health economic study is the first known evaluation of insulin pumps in the type of individuals enrolled in the REPOSE Trial.
There is limited evidence on the increases in HbA1c that adults with T1DM may experience beyond the trial period. Much of the existing evidence is based on observational studies of adults with T1DM, who received either best-practice MDI or insulin pump therapy. None of these long-term observational studies made a comparison between those adults with T1DM who received MDI and those who received insulin pump therapy. The applicability of this evidence to the individuals in the REPOSE Trial may be limited, especially as, by design, we excluded patients with a clinical indication for a pump as recommended by NICE. 13 Thus, information presented in the observational studies is probably the best available evidence to inform long-term trends in HbA1c for the economic modelling.
The advantage of the observational studies of adults switching to pumps for clinical indications lies in measuring change in glycaemic control and hypoglycaemia in those who have most to gain. These studies showed improved HbA1c of the order of around 0.5%. Bias in observational studies is more of a problem and results must be treated with caution. 23 Furthermore, of 48 observational studies, only nine reported QoL. Study numbers were small, with, at most, 35 patients, and duration was usually short, often ≤ 6 months. The longest study noted that initial benefits from pump therapy might not be sustained. The REPOSE Trial has thus addressed a number of these concerns, with large numbers in an adequately powered trial and a virtually complete data set for both biomedical and psychological outcomes. 23
Discussion of results
Our study suggests that extending the availability of pumps to adults with T1DM with suboptimal glycaemic control and no firm desire to use this form of insulin delivery is unlikely to result either in lower levels of glycaemia as measured by HbA1c or lower rates of hypoglycaemia, or be cost-effective. The results would appear to support the current clinical pathway as proposed by NICE,18 in which people desiring improved diabetes control should undertake structured education in flexible insulin therapy with MDI alone. 23
Clearly some patients improved more than others in terms of glucose control or hypoglycaemia and we explored whether or not there were any demographic differences in those who did particularly well. There was no reliable evidence of any plausible subgroup effects or interactions between the pump and MDI group, and the baseline characteristics of those whose glycaemic control improved to < 7.5% during the trial were no different from the pump population as a whole. (This text, from ‘Clearly’ to ‘whole’, is reproduced from The REPOSE Study Group 2017. 23 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.) Those using insulin pumps did show some QoL benefits, reporting fewer restrictions in diet and daily hassles in the DSQOL, and greater treatment satisfaction. Nevertheless, the differences were modest and observed in comparison to a group given no novel technology. As they were not associated with other positive treatment outcomes, they are probably insufficient to justify a major alteration in guidelines for the use of pumps. 23
One of the more striking results of this trial was the generally high level of HbA1c among adults in the UK enrolling for self-management training in flexible insulin therapy. Participation in the courses produced significant and sustained improvement, but still fell well short of the target recommended by NICE, recently reduced from 7.5% to 6.5%. 159 There is an urgent need to explore the barriers to successful self-management in adults with T1DM in the UK and understand why referral for appropriate training is often left so long. This was also the conclusion of our recently completed research programme, funded by NIHR. 27 The results of the REPOSE Trial show that these problems cannot be overcome merely by providing additional technology in the form of pumps. 23
The possible lack of engagement among some individuals assigned to pump therapy may also explain the increased numbers of episodes of DKA in those randomised to insulin pumps. In the earliest trials of insulin pumps in the 1980s, rates of DKA were also raised among those who had agreed to try a pump when offered. A psychological analysis, undertaken at the time, suggested that those who experienced DKA expressed less personal responsibility for their care. Importantly, in the REPOSE Trial, both MDI and pump courses included instruction in ‘sick day rules’, designed to prevent the development of DKA in the case of illness. Pump courses also included specific guidance in dealing with an interruption of the insulin infusion, although there was no guarantee that participants would follow these.
A detailed review of DKA cases indicated that:
-
more patients on pumps had multiple episodes (five vs. two)
-
differences were confined to the first year; there were comparable numbers of episodes (four in each group) during year 2
-
three episodes occurred in two patients switching to pump and one in a single person switching to MDI
-
most DKA episodes were due to infections; in pump patients, 18% were due to ‘set failure’
-
only five episodes occurred when all sick day rules were implemented.
Implications for health care
The NICE Type 1 diabetes guideline159 states:
1.3.1 Offer all adults with type 1 diabetes a structured education programme of proven benefit, for example the DAFNE (dose-adjustment for normal eating) programme. Offer this programme 6–12 months after diagnosis.
1.3.2 If a structured education programme has not been undertaken by an adult with type 1 diabetes by 12 months after diagnosis, offer it at any time that is clinically appropriate and suitable for the person, regardless of duration of type 1 diabetes.
The REPOSE Trial provided DAFNE to both arms, and we observed significant improvements in both arms, which persisted for the 24 months of the study. The improvements were both in glycaemic control, as reflected by HbA1c, falls in severe hypoglycaemia and measures of QoL, providing support for NICE recommendation 1.3.1.
We found no relationship between duration of diabetes and benefit from DAFNE, which supports NICE recommendation 1.3.2 – that all patients should be offered structured education.
The recent update of the T1DM guideline recommends that people be supported to aim for a tight target of glycaemic control in recommendation 1.6.6159 [6.5% (48 mmol/mol)], which is lower than the treatment target of 7.5% set when the REPOSE Trial was being run. Despite recent evidence dissociating lower HbA1c in T1DM from increasing severe hypoglycaemia rates, fear of hypoglycaemia remains a barrier. The combination of recommendations 1.6.6 and 1.6.8 will be challenging, and is likely to require an increase in the use of insulin pumps as approved under the current NICE technology appraisal guidance. 13 However, we would point out that DAFNE structured training also reduces rates of severe hypoglycaemia.
The REPOSE Trial excluded patients who met the NICE criteria for a pump. We also excluded patients who had a strong desire to use an insulin pump. Therefore the results of REPOSE are not relevant to the recommendations of TA151,13 they apply to a group of patients with a lower level of need.
The results of the REPOSE Trial showed improvements in both arms. The pump group showed slightly greater improvements than the MDI arm, but most of the differences were not statistically significant, and the difference in HbA1c did not reach a clinically meaningful level. Our cost-effectiveness analysis shows that in the type of patients in REPOSE, pumps will not be cost-effective.
It is important to note that the REPOSE Trial may be reported as a ‘negative trial’ of pumps, but the failure to show a significant benefit of pump over MDI was because both groups improved following DAFNE training. The results indicate that, in adults with high levels of HbA1c, training them to self-manage their diabetes with structured training programmes is more useful than providing them with insulin pumps.
Implications for the National Institute for Health and Care Excellence
The implications for NICE guidelines and guidance are:
-
The guideline on the importance of providing structured training programmes is reinforced. Considerable evidence has been found for the effectiveness (and cost-effectiveness) of offering evidence-based structured education to individuals with T1DM.
-
There are no implications for TA151 on insulin pumps. The REPOSE Trial results do not apply to the patient groups to which TA151 refers.
Future research needs
There remains a clinical and economic need to improve the glycaemic control of adults with suboptimally controlled T1DM. The results in the UK, for example in terms of proportions of people reaching HbA1c targets, are poorer than in some other European countries. We need to explore the differences in clinical practice and patient behaviour that underlie these differences. The DAFNEplus programme of research is aiming to develop and evaluate the current DAFNE course (based on previous research, behaviour change theory and technological support). This programme is not expected to report until September 2021.
In both arms of the REPOSE Trial there were marked variations in HbA1c, with some people showing marked improvement and others showing deterioration. Further research is needed to explain why some people do so well, whereas others do not.
We found no relationship between duration and benefit from structured education. This raises a question as to why patients who have been attending diabetic clinics for many years – even decades – have not been offered structured training in diabetes self-management.
More extensive qualitative research should be considered to:
-
explore the issues that influence patients’ use and rejection of technologies, such as insulin pumps and CGM
-
examine patients’ perspectives on both the impact of withdrawal of technology (e.g. pumps and CGM), both at the end of trials and in clinical practice when they are deemed not to meet NICE criteria
-
compare the views of both professionals and patients in other European countries that appear to achieve far better glucose control.
The Cochrane review of pumps by Misso et al. 31 is now well out of date and should be replaced by an up-to-date, but much more focused, review of pump versus analogue MDI, which would include the REPOSE Trial.
Conclusions
In conclusion, people with T1DM might be better served by ensuring far greater availability of high-quality, structured self-management training, which is currently accessed by < 10% of adults with T1DM in the UK. 160 Participants may recognise the limitations of insulin delivery by MDI only once they are attempting to maintain flexible intensive insulin therapy following training. Those individuals could then be offered pump therapy to help them reach the stringent glucose targets that are necessary to achieve an optimal HbA1c or overcome problematic hypoglycaemia. 23
Chapter 9 The challenges of closing out a clinical trial after which treatments may be withdrawn: qualitative study of staff involved in closeout of the REPOSE Trial
Background
Clinical trials are considered the ‘gold standard’ method for assessing the efficacy and safety of pharmaceutical treatments and other health-care interventions. It is common practice for qualitative research to be undertaken with patients and staff who are involved in clinical trials. 161,162 This research usually takes place during a trial’s pilot or early phases to improve recruitment, patients’ understanding of trial processes and the solicitation of informed consent. 163–168 Qualitative research has also been undertaken during trial delivery to explore adherence to trial protocols and treatments, and aid interpretation of trial findings. 169,170 Although the closeout of a trial potentially presents challenges for both patients and health professionals, especially when patients may be required to stop using the treatment(s) under investigation, this aspect of trial participation and delivery remains surprisingly under-researched. The limited work undertaken to date suggests that patients may experience a form of trial bereavement on closeout,171 and some may wish to continue using trial treatment(s) despite the trial failing to show clinical benefit. 172 How staff address closeout issues with patients, and what their own information and support needs are, remain unknown.
Closing out REPOSE
Recruitment to the REPOSE study commenced in November 2011 and the first trial centre began to close out patients [i.e. commenced 2-year (final) follow-up appointments] in April 2014, with the final centre closeout appointment in June 2015. The insulin pumps used during the REPOSE study were provided free of charge by Medtronic, with a warranty that covered only the trial’s 2-year duration. After extensive negotiations, pump consumables were funded at a local or national level (e.g. by the DH, Chief Scientist Office or a primary care trust) for the duration of the trial and on the understanding that pump therapy would be withdrawn post trial unless a clinical benefit could be demonstrated for individual patients and local funding provided. This was communicated to potential trial participants in the patient information sheet for the trial (see Appendix 8). On closeout, each REPOSE centre was advised (as per the trial’s SOP for closeout; see Appendix 15) to make their own clinical decisions about which patients should remain on a pump and who should revert to a MDI regimen, with centres having to find local funding for patients who remained on pump therapy. 86 It was also agreed (as formalised in the SOP) that the patients would not be told whether or not they would keep their pump until after their data had been collected at the final 2-year appointment because of concerns that this knowledge might influence how they completed their questionnaires.
Early reports from trial staff and ongoing review of trial data indicated a large variation in closeout practices between the REPOSE centres (listed in Table 11). Although, in some centres, most or all patients remained on pump therapy, in others, the majority of patients had pump therapy withdrawn and were reverted to a MDI regimen. Early anecdotal reports from staff also indicated that patients’ emotional reactions to withdrawal of pump therapy were variable, with some presenting major challenges to staff. Specifically, some staff expressed concerns about the lack of guidelines, procedures and support structures for themselves to manage and support patients effectively at closeout when withdrawing pump therapy. In light of these reports, it was decided to systematically evaluate staff experiences of closeout to generate insights and recommendations to support the conduct and closeout of future trials, especially those where an expensive health technology is being tested that may be withdrawn at the end of the trial period. A case was made to the funder to undertake this additional piece of qualitative work using some underspend within the grant. Approval from the funder for this substudy was given on 13 April 2015.
Aims
This qualitative study drew on the experiences, understandings and views of health professionals who were involved in closeout of the REPOSE Trial in order to:
-
better understand variations in practices between trial centres on closeout and establish whether or not, and to what extent, these arise from local clinical guidelines and practices, individual physician/health professional beliefs and/or other factors and considerations
-
inform guidance and support for staff involved in the closeout of future clinical trials, particularly those in which investigated treatment(s)/device(s) may be withdrawn.
Research questions
-
What are health professionals’ experiences of closing out the REPOSE Trial? What (if any) practical/ethical/other issues arose for staff, and how did they attempt to address these?
-
What factors and considerations informed health professionals’ decisions to continue or discontinue pump treatment in individual patients?
-
What processes and procedures do staff think should be put in place to support patients and staff involved in the closeout of future clinical trials, especially those where expensive health technologies are being investigated and may be withdrawn?
Overview
The qualitative work was completed to plan and on schedule, enabling a comprehensive investigation of staff members’ experiences of closeout in the seven main REPOSE centres. Although it had originally also been our intention to include patients’ views, we were unable to involve this group because of the limited time available to gain NHS research ethics and R&D approvals at the REPOSE centres, and undertake the data collection and analysis. One journal article has been accepted for publication, which reports key findings from the following analysis (Lawton et al. 173).
Study design and methods
In-depth interviews were used to collect data about staff experiences of study closeout, as these afforded the flexibility needed for participants to raise and discuss issues that they perceived as being salient, including those unforeseen at the study’s outset. 142,174 The use of one-to-one interviews also afforded privacy, allowing participants to share their views about the processes and procedures for closeout at their study centre. The study was informed by the principles of Grounded Theory140 and entailed simultaneous data collection; this allowed the areas explored in the later interviews to be revised in light of emerging findings.
Recruitment and sample
Working closely with the CTRU to identify relevant individuals, we targeted all staff members (physicians, diabetes specialist nurses and dietitians) in the REPOSE centres, who were thought to have been actively involved in closeout appointments. Staff were recruited from seven of the eight participating centres. The eighth centre was not included because it was a reserve centre that was added at the end of the trial to deliver two courses only, and this centre had only one patient using a pump at the end of the trial. Staff were recruited via written (e-mail) invitations accompanied by information sheets and opt-in forms. When staff had opted in, NH contacted them to arrange an interview.
Data collection and analysis
The University of Edinburgh’s Centre for Population Health Sciences, Ethics Review Group granted ethics approval for this study in June 2015. The interviews took place between June and August 2015. Participants were offered the choice of a telephone or face-to-face interview at a time/place most convenient to them; only six (29%) staff members requested a face-to-face interview.
Interviews were informed by a topic guide that was developed in light of literature reviews and findings from qualitative research conducted earlier in the trial,147,155 and were revised in light of emergent findings from the early interviews. The final version of the topic guide is appended to this final report (see Appendix 16). Interviews lasted for ≈60–90 minutes. The key areas in the topic guide were covered and explored in depth in all interviews. Interviews were digitally recorded (with consent) and transcribed in full for in-depth analysis. By the time recruitment and interviewing had stopped, data saturation had been achieved, that is, no new findings or themes were identified in new data collected.
The interviews were analysed thematically by NH and JL using the method of constant comparison. 148 Individual interviews were read through repeatedly to look at differences and similarities in individuals’ perspectives and experiences before being cross-compared to identify common issues and experiences across and within study centres. NH and JL wrote separate reports before meeting (both during and after data collection) to discuss and reach agreement on key themes, identify emerging findings requiring more detailed exploration and develop a coding frame. The qualitative analysis software package NVivo10 (QSR International, Warrington, UK) was used to facilitate data coding and retrieval. Coded data sets were subjected to further, in-depth analysis to identify subthemes and illustrative quotations.
The findings presented below are structured under our original research questions. To safeguard participants’ confidentiality, pseudonyms for individuals, Dr X (diabetes specialist) or EDX (DAFNE educator – diabetes specialist nurse/dietitian), and centres (A–G) are used throughout this report, and all identifying information has been removed or deliberately altered.
Findings
Participants
Twenty-four staff members were invited to participate. In one case a staff member said that they had no direct experience of closeout/end-of-trial consultations. Two others opted in, but an interview could not be arranged at a convenient time, hence 21 (87.5%) staff members were interviewed. Full details of the final sample are provided in Appendix 17.
As can be seen from Appendix 17, we achieved good representation of different types of staff: clinical diabetes specialists, diabetes specialist nurses and dietitians. Between two and five (mode three) members of staff were interviewed at each centre. With the exception of centre A, at least one DAFNE educator and one diabetes specialist was interviewed from each centre.
Staff experience of delivering DAFNE varied from 5 to 17 years (mean 10 years). There was also variability with regard to individuals’ experience of pump therapy, ranging from 2 to 37 years (mean 10 years). It should be noted that the majority of staff interviewed at centres D and E had relatively little experience of pump therapy prior to delivering REPOSE. Although many staff had previous experiences of working on clinical trials, few had been involved with studies that had required new technologies to be withdrawn at the end of the trial. The main exceptions were those staff members (n = 5) who belonged to the three study centres that had been involved in the delivery of a DAFNE pump pilot study. This 12-month study27 – 6 months’ recruitment and 6 months’ follow-up – had taken place between 2009 and 2010.
In order to understand staff members’ experiences of closing out REPOSE, it is necessary to provide an account of what happened at the end of the trial in the various centres. Thus, prior to answering the research questions, we will describe the variations in closeout practices that staff described in the different centres.
Background: closeout practices in REPOSE centres
As noted above, the CTRU issued a SOP for closeout (see Appendix 15), which outlined what was to happen up to the point at which all trial procedures were completed (i.e. final blood samples were taken, QoL measures collected and data from the pump downloaded). What happened to trial participants afterwards – whether or not they remained on MDI/pump, whether or not they had pump therapy withdrawn or initiated – was a clinical decision, taken by staff at the individual centres. In other words, the decision to leave patients on, start or terminate pump therapy was not a trial decision. However, many of the staff involved in delivering REPOSE experienced these post-trial treatment decisions and, more specifically, patients’ reactions to them, as part of their trial experience. Thus, for the purpose of this chapter, we will talk about post-trial treatment decisions as part of the closeout process because this is how the staff perceived and interpreted them.
To ensure that resources (i.e. pumps) were allocated appropriately and fairly at the end of the trial, most centres put site-specific procedures in place for closeout (i.e. what would occur after the final downloads had been logged). Some centres adopted very formalised operational procedures for decision-making about post-trial treatment. In these centres, decisions about individual participants were made at a multidisciplinary team (MDT) meeting, involving all of the research team and other staff members, and which took place a couple of weeks before the closeout of each of the groups. The MDTs’ decisions were governed by strict NICE/Scottish Intercollegiate Guidelines Network (SIGN) criteria (see Research question 2) and (normally) documented. Each patient then attended a post-trial consultation with a clinician/educator after the final data collection session to discuss his/her treatment plan.
Other centres took a less formal approach to closing out their patients. Dr H described what happened in one such centre (centre B):
I guess it wasn’t a formal MDT. But yeah it was just a chat with the educators and myself about each individual patient as they were coming up for the end of the study, about who/what the best way forward was for them.
In some of these centres, the whole research team met in advance of the post-trial appointments to discuss what might happen in individual cases; in others, the educators briefly spoke to the clinician after the patient had provided their final download and before they went in for their post-trial consultation. In these centres, although some, or all, trial team members had some input into post-trial treatment decisions, it was individual clinicians who made the final decision during the post-trial consultation, often taking the patient’s views into account:
We started a fairly neutral conversation about how it been and what would they want to do if the option were that they could keep it. And then if they said well you know, they’d really like to stay on pump therapy then I said ‘OK, well you know, let’s have a look at how you’ve got on with it’ and obviously I’d got a feel for that already. So before they came in [educator] and I sat down and looked through and looked at how they’d got on, and what had happened to hypoglycaemia frequency, what had happened to HbA1c, and then obviously they came through and told us how they felt in terms of, the impact on quality of life and things.
Dr G
In all of the centres, the clinical appointment to discuss post-trial treatment occurred after the final appointment to collect trial data. In some cases, patients were seen on the same day on which they came in for their final trial appointment; in others, this clinical appointment occurred a couple of weeks later.
Research question 1
-
What are health professionals’ experiences of closing out the REPOSE Trial? What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?
What are health professionals’ experiences of closing out the REPOSE Trial?
Staff who had been involved in follow-up appointments during the trial, primarily the educators, said that they had become increasingly aware that withdrawal of pump therapy at closeout/the end of the trial would be difficult. This was a result of their observations (see ED7 below) that patients were becoming increasingly emotionally attached to their pumps as the trial progressed, an issue which became particularly apparent from the 12-month follow-up appointment onwards.
. . . at the kind of the routine REPOSE follow-ups when we asked them how they were feeling about the pump, they all reported that they loved the pump, that they felt it was making their life so much easier, and that they couldn’t imagine going back to having to inject multiple times a day . . . So they were all very vocal that they really wanted to stay on their pump. And that they would be prepared to fight for it, if needs be.
ED7
For this reason, some staff reported worries and concerns about how patients might react to the withdrawal of the pump at the end of the trial: ‘I knew it was going to be difficult and I wasn’t looking forward to it’ (ED11).
Dealing with stressful situations
The ways staff experienced these post-trial consultations varied, and was related to whether or not patients were able to remain on their preferred therapy and, as will be described later [see What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?], whether or not staff had put pre-emptive measures in place to manage and prevent problems arising from the withdrawal of pump therapy. In some cases, when patients who wanted to remain on a pump were told they would have to revert to MDI, staff members, including Dr C, described situations that had been stressful and difficult to manage because patients had become upset and/or angry:
I had trouble in the course, because one lady when she came to the end of her trial, her HbA1c was appalling. I mean it was appalling. There was no way you could justify leaving her on pump, because she was getting no benefit from it biomedically. What she needed was a complete change in how she managed her life. And she was very upset to have the pump removed. But what was fascinating was she did not say that at the closeout interview . . . . Next thing I know she’s written streams of letters of complaints to all and everybody, because we removed the pump from her, and refused to give her any supplies after 3 months.
Dr C
Later in the interview, Dr C reflected on how this and other similar experiences had ‘. . . kind of tainted the whole study for me, because it was really quite difficult for a little while. It was very uncomfortable’. Dr B reported a similarly stressful encounter with a patient:
The one locally that really didn’t go well, was a gentleman whose control had got worse on the pump. And I was explaining that in fact on balance it was actually more dangerous for him to remain on pump. And he was the one that walked out. He didn’t shout or give me any indication. He just stood up and said ‘OK’ and walked out.
Dr B
Like Dr C, Dr B had been taken aback by this experience: they had been ill prepared for it, primarily because, like the other clinicians in the study, they had been less involved in the trial follow-up visits, which had been mainly carried out by educators.
Smooth transitions
However, the withdrawal of pump therapy was not always experienced as generating such negative reactions; indeed, a small group of patients, across the centres, were described as having been ‘happy’ to revert to MDI, with some requesting this transition at the end of the trial. Moreover, in another centre (centre A) at which pump therapy had been withdrawn from the majority of patients, staff said that closeout had been relatively straightforward and non-confrontational. As will be described further later [see What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?], this appeared to be due to staff having put procedures in place to pre-empt, prevent and manage disappointment among those patients.
In approximately half of the centres, patients received the treatment that they wanted at the end of the trial, and closeout, as a result, was experienced as raising few issues for staff. This was particularly the case in Scottish centres where, in 2012, the Scottish Government had made funding available for pump therapy, with a target of ≈5% of patients with T1DM to be using pump therapy between 2013 and 2015. As Dr E reflected, because of the Scottish Government’s largesse, closeout was very straightforward in that centre because the majority of patients were able to remain on pump therapy if they wished to do so:
Our closeout has probably been less complex than most places. And that’s because of this impetus to increase the number of people with pumps . . . Happily for us, because the timing was just perfect, so that the end of the trial was within this expansion up, we were actually able to fairly straightforwardly continue with pumps on a routine NHS way for all of the patients who wished to.
Dr E
ED3, from another resource-rich centre, similarly said ‘I think it [closeout] went well. There was nothing certainly from our side in [site D]. I don’t think there were any issues for us’.
Staff at the Scottish centres did comment that, had government funding not been put in place during the trial, closeout would have been more challenging and problematic:
Well I guess we would have been in the same situation as other centres where there was no funding stream to continue patients. And we would have had to say: ‘sorry. We don’t have any money for you to continue on this’. And I think it would be very difficult. I mean obviously I would imagine in other places it’s caused a bit of damage to the doctor or health-care professional relationship . . . I guess people having invested a lot of time in it over the course of the study you’d feel a bit let down if somebody’s told that there’s no money. Sorry, give it back.
Dr H
Although staff at such centres did not generally have to manage patients’ reactions to the withdrawal of the pump, they did have other issues with which to contend. First, as Dr E noted, they had problems providing timely training for all MDI patients who were offered, and accepted, pump therapy at the end of the trial: ‘Most of our control patients were really quite keen to go on pumps, afterwards. And the, you know, there’s a degree of work just dealing with that’. Second, even though patients usually received the therapy they wanted after closeout, the staff said they still had to reassure and ‘calm down’ patients when they came in for their final downloads because they were anxious about losing their pumps:
On the day of their final visit I think they were all extremely heightened, they were very worried I think most of the patients who came in. We kind of had to almost calm folk down a little bit. We had quite a few who were walking in the door at that final visit very, very scared because they knew it was the end of the trial and they didn’t know what was going to happen now.
ED6
As ED6 commented, dealing with patients’ anxiety throughout the trial was particularly difficult in their centre, as although staff realised that most people would have their pump therapy funded after the trial, they still had to follow the trial SOP, which required staff to be more circumspect when patients asked about post-trial treatment during follow-up visits.
Differences of opinion within multidisciplinary teams
Finally, with regard to their closeout experiences, some staff indicated a lack of consensus within some research teams regarding the decision to keep particular individuals on pump therapy at the end of the trial:
And I think there was a difference in how some of the team viewed it as well, in that some seemed to say: well, it’s a trial for 2 years. And then they come off the pump and we see how they do. And then we may put them back on the pump. Whereas others are saying: well no there’s been significant improvement, So we’ll keep them on the pump. So I had kind of extremes.
ED12
I think others (in our site) were much more, I . . . I think that they thought that they were going to stick to the letter of the law and they’d take pumps away and that would be tricky . . . but then that’s their individual practice it’s not for me to tell colleagues particularly consultants how they should practice, and the practice is very different it’s such a personal thing.
Dr A
This particularly applied to those centres that had adopted less formalised closeout procedures, specifically where final decisions were taken by clinicians alone. In such centres, not only were disparities in decision-making between different team members noted, but also some team members described having not always agreed with their colleagues’ decision to keep individual patients on pump therapy. Several staff commented that they were not always convinced that patients were benefiting over and above what could be achieved using a MDI regimen and DAFNE education. Indeed, some such staff indicated that they would rather have used stricter guidelines for pump allocation at the end of the trial to ensure that NHS resources were distributed in a fair and transparent way in their centre (see Research question 3).
In summary, the interviews confirmed differences in staff experiences of closeout across the study centres. First, in centres at which pump therapy was routinely withdrawn from all but a few patients, staff had needed to manage some of the patients’ negative emotional reactions and some had felt ill prepared for this experience. Second, there was evidence that some centres had managed patient expectations about post-trial treatment more successfully than others, thereby pre-empting patients’ disappointment at having pump therapy withdrawn (see Research question 2). Finally, in centres where ample funding for pump therapy was available, the issues arising at trial closeout focused on calming anxious patients before final data collection and providing timely training for MDI patients commencing pump therapy.
What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?
Staff identified a couple of issues that may have affected their own and patients’ experience of the trial and closeout; these included the length of the trial and the ethical challenges arising from withdrawal of the pump. Although some of these issues had been identified and addressed prior to, or during, the main trial, others emerged only during the interviews, as staff reflected upon their trial experiences.
Length of trial
Some staff, as already indicated, reported that they did not really start picking up on patients’ anxiety about the removal of pump therapy until they attended their 12-month follow-up. Thus, the length of the trial, or, specifically the length of time spent on pump therapy, was identified as an issue by a number of staff who questioned that this may have affected patients’ emotional reactions at closeout:
I have been involved with trials where the treatment has been withdrawn, but it’s been a shorter period of time. I think 2 years is quite a long time and people get very used to things, don’t they. And then they do start to think that the pump’s theirs. So I think that’s more difficult. When I’ve been involved with other trials of equipment it’s been more like a few weeks, 6 weeks or something like that. And so patients are very aware that it’s just for that trial period.
ED11
Although ED11, like others, saw the length of time spent on the pump as affecting patients’ reactions at closeout, ED1 regarded the trial’s relatively long duration as indirectly influencing some of clinicians’ decisions to continue pump therapy for certain individuals in their centre:
The REPOSE SOP for the end of the study was based on what we did for the pilot. And the only thing I could say, I hadn’t really thought about that until just this morning. And whether there was something to do with the length of the trial, the duration, which made it more difficult to make that decision [to remove pump therapy] at the end.
ED1
Learning from experience gained during the pilot study
Three of the centres in the main trial had been involved in the pilot,27 and staff who had taken part in the pilot talked about how these earlier experiences had influenced the ways that they approached the main trial. These staff described how they had entered the trial with some, but perhaps not enough, awareness that terminating pump therapy at closeout might be problematic, and how this had led them to putting some pre-emptive measures in place to prepare patients for removal of their pumps:
When we did the pilot . . . some people were really devastated that they couldn’t keep the pump, even though we told them. So we were much clearer we think, this time round with: you’re not, you know – although everything was signed – with the fact that they needed to give the pump back. And I think we were before. But I think we just reiterated it throughout the process more.
ED10
Indeed, in one such centre (centre A) staff designed a clear formal protocol for ending the trial, which not only set out criteria for who was to stay on pump therapy (see Research question 2), but also helped them to manage patients’ expectations throughout the trial and their emotions at closeout. This centre had reverted the majority of patients to MDI at closeout and they had followed strict procedures for this including explaining why pumps were being removed, what removal meant and how reversion to MDI might be a temporary state of affairs, which could be revisited in the future. This centre also provided patients with spare consumables so that they could continue to use their pumps in the immediate short term before reverting to MDI at a convenient time, thereby giving them a chance to adjust psychologically and practically to the transition. These strategies appeared to work, for although this centre had withdrawn pump therapy from most patients, the staff reported that this had gone reasonably smoothly:
So that we didn’t switch them there and then on that 24-month visit. We reminded them, we had a few people were quite upset and grumpy about it. And we said: look, how can we? We have some kit we can give you that can tide you over for another month or 6 weeks, while we sort out your pens and getting you back – to switch you back onto MDI and doing it in a supportive as way as possible. We didn’t rip the pump off them at that appointment and say: there’s your pens back, off you go. And so having that discussion at the meetings [MDT] before for all of them just helped us come up with a kind of individual plan to sort of, damage limitation really.
ED14
Leaving the door open to revisit patients’ eligibility for a pump
Although centre A was the only centre to consistently allow patients a lead-in period to revert back to MDI, staff in other centres described how they had tried to manage anxiety and disappointment by making patients aware that they could make a case for them to have the pump reinstated in the future if they struggled to manage their diabetes using DAFNE + MDI:
And we did say to him, as we said to others. This is does not mean that pump therapy is completely closed to you. You know, what you need to do now is go back on injections, really apply DAFNE. You know, monitor, keep records, make adjustments, and you know, down the line, if you’re still not managing to achieve an HbA1c or you’re getting hypos, then we can consider a pump again. But you need to put the work in.
ED1
Staff in all of the centres, but especially those in pilot centres, also talked about how they had tried to manage patients’ expectations about closeout throughout the trial, particularly the likelihood that they may not continue on the pump:
Cause we did – had done the pilot as well we’d sort of expected – we knew what to expect, cause you know, we’d done the pilot before REPOSE. So we’d been involved and the same sort of thing had happened: people you know, of course if they liked the pump, they like the pump and want to keep it. So it was really about just reminding people of the rules and we tried to do that each time we met them as well, just to remind them that this was about the trial, this was about seeing if the pump was effective and if they didn’t meet NICE criteria the pump would go back. So we tried to talk about that at each meeting time as well, not just leave it to the end.
ED11
In a couple of centres, in addition to raising the issue of withdrawal of the pump during trial visits, patients in the pump arm were given encouragement several weeks before closeout to use the remaining period of the trial to demonstrate that they could use their pumps more effectively by the time of their final download or, as Dr H described, to prepare patients for closeout and ease their disappointment if a clinical benefit could not be evidenced:
I think we just felt better that we’d given them the opportunity. You know if you’re pre-warned that there’s going to be an exam[ination] result in another 3 months kind of thing, then if you don’t do so well in it, you think: oh well, at least they told me kind of thing. I think we were just thinking that a warning shot is quite a good idea. And might make the that’s all, no you can’t have a pump any more discussions easier.
Dr H
The ethical challenges of withdrawing pump therapy
For some staff closing out the REPOSE Trial was seen as throwing up distinctive ethical challenges not only because the patients had time to get used to pump treatment, but also because of the nature of the treatments involved. As Dr H noted, unlike drug trials through which treatments might be replaced by seemingly similar forms of therapy, REPOSE required the withdrawn treatment to be replaced by a very different therapeutic option, which, as they noted, may be seen by some patients as not really an option at all:
I mean it doesn’t really have parallels to other studies. I mean I guess if you’re on a new tablet for x, y or z at the end of the study you might not be able to continue it, but there’s usually an alternative. And it’s you know tablet versus tablet instead of you know, pump versus another way of giving insulin which is very different . . . as I say it’s not like this is trying one pump versus another pump, and you at the end of the study you go back to the old pump. But you take away the new fancy one. This is like something, getting something versus getting nothing.
Dr H
Dr A raised further ethical issues regarding the withdrawal of pump therapy. This clinician, like others, argued that if individuals were benefiting, or even perceived themselves as benefiting, in ways that went beyond the clinical criteria outlined in the NICE/SIGN guidelines then it would not be right to remove pump therapy at the end of the trial period, not least because these individuals had given their time to take part in a research project. In other words, as long as patients were using their pump safely then they had the right to keep it after the trial had finished:
Just because we’re doing a research project doesn’t mean you don’t continue to have a therapeutic relationship with people and I mean you can call me a softy, but I think we owe it to our patients who participate in research to do the best by them, and as I said at the beginning you can get a pump for anybody if you want, and I just think making a judgement that they don’t benefit therefore they should stop. If they think they’re benefiting, then I’m not comfortable saying I know better than you.
Dr A
Dr A, like others, also stressed that removing pump therapy at the end of the trial could potentially undermine an ongoing therapeutic relationship with a patient – especially as was the case for this doctor, when health professionals delivering the trial were also responsible for providing patients’ routine diabetes care.
Although Dr A was based in a centre at which access to funding for pump therapy was limited and some patients had pump therapy removed at the end of REPOSE, ED5’s centre, in contrast, had plenty of funding available and the majority of patients had stayed on the pump following closeout. ED5, however, was acutely aware of the different funding situations across the trial centres and commented that the removal of pump therapy at the end of the trial in some centres and not others was just further evidence of the existence of what they regarded as an unethical ‘postcode lottery’:
That’s the state of the NHS that really at the end of the day if the patient’s benefiting then I feel it’s quite sad that someone can remove something from someone that they’re benefiting from. And I think that it just highlights in the NHS a bit of a postcode lottery really regarding pumps, and that hopefully in the future that’s going to be more standardised. Because your care really wherever you are should be equitable.
ED5
In summary, staff in all of the centres anticipated that closeout and the withdrawal of pump therapy might be an issue for patients and, hence, had developed a range of potential solutions to address these, including developing strict protocols for managing expectations and emotions, and reminding patients that pump therapy was a research intervention whenever they attended trial visits. In addition, staff identified a couple of ethical issues, such as the problem of withdrawing treatment from patients who perceived themselves as benefiting from it, potentially compromising an ongoing therapeutic relationship, and the inequity of the postcode lottery for funding treatments in the UK.
Research question 2
-
What factors and considerations informed health professionals’ decisions to continue or discontinue pump treatment in individual patients?
Variability between centres
The number of patients staying on pumps after closeout varied markedly between centres and, as noted in Research question 1, it was clear that the staff in the different centres, including ED10, were aware of this:
And there are always going to be clinical judgement and exceptions. But it feels a bit like people [sites] have done things slightly differently at the end.
What did you do at the end?
We said to everybody, you have to give it back.
Ultimately, it was the availability of resources, specifically the availability of funding to keep/move patients on to pumps in routine clinical practice, which determined what happened to individual patients at the end of the trial. In centres E, B, D and F, for which generous funding was available, the majority of REPOSE patients stayed on/commenced pump therapy if they wanted to:
So we were very fortunate in that sort of financially there wasn’t going to be any problem here about asking patients for the pump back at the end of the study. It was agreed that it would be daft to do that and then restart them again on a pump 3 months later or something. So although the patients didn’t know and obviously we wouldn’t say to them, because that wasn’t, that wouldn’t have been good. You know within the group it was realised that there was a sort of secure funding stream to continue those that were benefiting from the pumps at the end of the study.
Dr H
In centres A, C and G, for which funding was scarce, staff were acutely aware that pump therapy needed to be rationed and restricted to those patients who demonstrated a clinical need or benefit, independent of the patient’s wishes:
[Dr] was quite cut and dried about it. Unless there was a medical reason or unless they met NICE [criteria] already from a hypo[glycaemia] point of view they had to come off. And you know if there was any, you know, trouble, they would come down and talk to the patient themselves if necessary.
ED14
Different interpretations of National Institute for Health and Care Excellence/Scottish Intercollegiate Guidelines Network criteria
The interviews suggested that staff in resource-rich and resource-limited centres tended to use different criteria when making decisions about individual patient’s post-trial therapy. Two resource-limited centres adopted very strict criteria for allocating pump therapy at the end of the trial so that, in general, only those patients with a clinical need who met NICE/SIGN criteria,13,175 as tightly defined (namely, HbA1c > 8.5%, attempts to reach target with MDI resulting in disabling hypoglycaemia), continued using pump therapy following closeout. The remaining patients in these centres were informed that they would revert to MDI:
My view was that if they had shown significant benefit in terms of HbA1c and, and/or reduction in hypoglycaemia frequency, then we would continue them on pump therapy. And that’s effectively what we did . . . . they had to effectively fulfil what NICE would expect. So the NICE guidance is based on an expectation of a 0.9% reduction in HbA1c, and I felt that was what they should be achieving for us to say that they should continue pump therapy.
Dr G
There were some that we knew had done really well on the pump. We knew that they’d really enjoyed being on the pump, that we knew that because they had never had, kind of from a NICE guidance point of view, a period of time having had what we would consider a, you know having done DAFNE and seeing if DAFNE works first, before putting them on a pump, and had never seen that, we couldn’t justify it from a hypo[glycaemia] point of view. They had done really well no doubt. But we couldn’t justify it from NICE to keep them on the pump.
ED14
This approach contrasts with that adopted by resource-rich centres that applied a much looser or subjective interpretation of the NICE/SIGN criteria when determining who remained on pump therapy. In one of these centres, nearly all of the patients in the pump group were allowed to remain on the pump when the trial finished, with some individuals, such as Dr F, justifying their decision by referring to the ambiguity inherent in the NICE/SIGN criteria:
Yeah. I think it was difficult to remove something that somebody’s doing well with, and wants to continue. If you know you have that funding available. And as I say SIGN and NICE are very vague. So you know, you, I felt I could justify it.
Dr F
Using quality-of-life criteria to inform decisions
These centres frequently took QoL issues, as well as biomedical criteria, into account when deciding who should remain on pump therapy. Dr F, for example, commented that they took into consideration how ‘well’ people were doing on pump therapy when making treatment allocation decisions in their centre:
What do you mean by doing well?
It’s interesting isn’t it. So doing well might be having good blood glucose values. But doing well might just be engaging with their diabetes better than they did before. So we had a couple of quite chaotic people who don’t have perfect glycaemic control, but they’re testing, they’re entering information and they’re keeping in touch with us in a way that before they weren’t. So I guess you know, doing well can be something over and above what their blood sugar’s telling us. And certainly their control, it’s not perfect, it’s better and safer than it was before. So I think that’s what I would sort of class as doing well.
Likewise, Dr H, from another resource-rich centre, described how decisions about post-trial therapy at their centre were governed by the team’s ‘global impressions’ about how individuals had coped on pump therapy:
We didn’t have any sort of hard criteria. It was going to be more just a sort of global impression taking into account of all the team’s views. You know, for instance this guy . . . early on in the study I think everybody would have said if he ever makes it to the end of the study, when he gets there he shouldn’t be on a pump. But he eventually got there with using it. So the people kind of relaxed a bit more about it. But I think he was the only person potentially that we would have taken off.
Dr H
Ensuring patient safety
Finally, independently of the availability of resources to fund pump therapy, decisions around individuals’ continuation on pump therapy following the trial were primarily affected by consideration of safety issues. As ED3, who was based in a well-resourced centre, indicated:
So as a team we reviewed all the people on pumps and made the decision about whether we felt, based on the information that we had and their downloads etc. they were using the pump first of all safely, cause that’s the key priority really is, the safeness and then whether they were getting any benefit from it.
ED3
Indeed, in resource-rich centres, safety appears to have been the only reason for removing people from pump therapy at the end of the trial, unless patients requested to come off the pump:
Oh it was definitely individual, definitely. I mean if we had funding but thought that person wasn’t safe. It wouldn’t have mattered if the funding was in place.
ED4
In summary, post-trial treatment decisions in all centres were influenced by assessments of patient safety and efficacy plus the availability of funding for pump therapy. Access to resources ultimately dictated the decision-making strategy that was adopted by the different centres; in resource-limited centres individual treatment decisions were NICE/SIGN-guideline driven and based on strict, objective efficacy criteria, whereas in resource-rich centres, decisions about individuals were based on looser, subjective views of efficacy or patient benefit and a desire to safeguard an ongoing therapeutic relationship.
Research question 3
-
What processes and procedures do staff think should be put in place to support patients and staff involved in the closeout of future clinical trials, especially those where expensive health technologies are being investigated and may be withdrawn?
Strategies used in REPOSE
As already outlined above (see Research question 1), staff had developed some strategies either proactively or during REPOSE to manage and prepare patients for potential withdrawal of the pump. In the main, staff saw these strategies as having been helpful, effective and appropriate, and said they would use them (and recommend them to others for use) in future trials of a similar nature to REPOSE. Such strategies included preparing patients for closeout by reminding them, at the outset, that pump therapy was only funded for the duration of the trial:
. . . it’s about the expectations those people had from the start. And I do think that if you’re very clear from the outset, if people’s expectations are at a certain level, then those conversions (post trial) are much easier. But it’s about being very clear from the outset.
ED14
In addition, staff recommended that patients be given reassurance that they will be monitored to determine whether or not they needed a pump in the future so that a case could be made for them to access one; separating (ideally in space and time) the clinical appointment to discuss post-trial treatment from the final trial appointment and, if possible [see What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?] giving patients a window of time after closeout to adapt before therapy was removed:
You know maybe there should have been a wash-out period or something afterwards, you know like this is the end of the trial maybe you’ll have 2 or 3 months or something to discuss with your team the way forward or whatever rather than people thinking right on the day it finishes and that’s it, it’s very difficult to just whip something off somebody and say here you are go back to your pen so I think that might have been the only thing, and that’s just feedback from the patients really.
ED6
Staff also identified two general areas in which they felt that their practice could have been improved and which could help support patients and staff involved in closing out future trials involving potential withdrawal of treatment. These were formalising post-trial procedures and improving communication between/within teams and with patients.
Formal post-trial procedures
Staff at a number of centres commented that the post-trial period is relatively neglected in trial planning compared with trial set-up and delivery. In light of their experiences of working on REPOSE, these staff members highlighted a need to acknowledge and prepare for the ending of a trial from the outset:
Maybe if I’d been in a trial where something had been taken away, we would have formalised this a bit more . . . But it’s difficult to envisage that when you’re writing a protocol so far in advance isn’t it? At that point the major thing is: can we get enough people into the study. That’s always the major hurdle. And in hindsight we probably ought to have sorted out the closeout in more detail once we were up and running. And set aside time to actually do that. With amendments or whatever it needed.
Dr B
There was widespread acknowledgement that thinking about closeout in advance and adopting a more detailed or formalised set of procedures for decision-making about ending/continuing trial therapy would have been helpful for staff managing this process. Some staff commented that appropriate costings/resources would also be required for this and to ensure staff had dedicated time to manage the closeout effectively rather than trying to fit it into already busy work schedules:
And that we had enough time for it – I think one of the issues as well is, because we’re such a busy clinical team and this was kind of fitted in as part of our clinical work as well. Although there was some backfill and things it was still a very busy time for us. So making sure that we did have the time and it was given to that
ED13
Staff in resource-limited centres, in particular, highlighted a need to develop a more formalised process with regard to decision-making about post-trial therapy, suggesting that this would enable staff to support each other when making difficult treatment allocation decisions and communicating them to patients:
But the final decision [post-trial treatment] was made by different people. In hindsight potentially I think all of the educators and the PI should have been probably together for all of them. And discussed them . . . I would have definitely met and gone through the SOP and gone through everything and checked that everyone was clear with what we were doing. And probably together supported each other and probably have continual meetings with those people particularly involved in the trial.
ED10
Local guidelines
As indicated earlier, staff also thought that having local guidelines in place was important to avoid inconsistent practices within centres and to help promote parity in decision-making, fair allocation of scarce resources (pumps) and also to help prevent potential disagreements and tensions within the team.
Staff in resource-rich centres also suggested that having more formalised procedures at the end of the trial could be useful and result in more transparent and accountable post-trial treatment decisions:
Would I have put something in at the end to kind of reassess, to kind of see whether or not there was, was it right to allow a participant who’d been given a pump to remain on a pump . . . So perhaps something that perhaps brought a bit more structure into that . . . But that perhaps would have been one thing to kind of do a fuller or a more structured assessment about whether or not it was the right thing to keep them on a pump.
ED8
The staff speculated that adopting more formalised procedures for closing out patients would ensure that staff with the requisite skills were available following closeout to train patients to use different technologies, if required, as well as provide emotional support:
When I saw the patients my team weren’t there. So, and that was a technical problem, because I had to teach people how to use MDI and how to use the bolus calculators . . . I struggled a bit, ’cause I’m not a trained educator . . . And it just wasn’t done properly. And that’s entirely my fault, because we didn’t set it up to do it. We didn’t think it through I don’t think. And they were the last patients. So we didn’t get the chance to improve it.
Dr C
Finally, some staff also indicated an explicit need for training/role play to deal with patients’ emotional reactions at closeout and suggested that this training could be usefully incorporated into the costings and design of future trials:
And I guess maybe yeah, just kind of sort of, kind of how to deal maybe with – if people are being – if something’s being withdrawn from the person as well, in terms of a sort of a therapy, how to kind of sort of handle that as well, and what kind of the, so maybe a little bit of training about kind of the best way to kind of present that to people.
ED13
Improving communication
Finally, nearly all of the interviewees talked about the need for better communication about closeout at the end of the trial. As Dr C said, ‘Most of our problems came from breakdowns in communication, I think’. First, many staff noted that better communication across trial centres would have been helpful in the REPOSE Trial, as this would have enabled staff in the different centres to prepare for, and alert others to, patients’ reactions and develop more consistent protocols and/or guidelines for good practice:
I think the only thing I might have done better is we might have had more of a discussion about the scenarios and shared the experiences so people got a more consistent message.
Dr A
Consensus and communication within centre teams was also seen as important for managing closeout effectively. Some staff noted that there had been a communication breakdown in their centre, with the result that some team members were not aware when pump therapy was scheduled to be withdrawn, and that this had caused problems for the staff and patients involved.
I think that was, that was, that was all not – we didn’t manage that very well, if I’m honest, because Dr did it on a day when I was on leave. And Dr didn’t – I didn’t know Dr was going to take the pumps off them there and then. So I would have, I would have liked to have seen them to have gone through their regimes on pens with them. And to have given them a bolus calculator meter, which would be like the bolus calculator on their pumps that they were used to. So we didn’t – I didn’t know they were going to walk into the consultation with a pump and leave without one.
ED12
Other staff members at this centre commented that better communication within the team would have enabled them to better support each other through the closeout process:
I think probably there should have been more of a team effort at the close. Because there was a lot of people involved in the team, but it was more or less left to you know, the educators and the dietitian. And then the consultant saw them later. But I think you know, if the whole team were involved there wouldn’t have been so much awkwardness at the close.
ED10
Finally, staff argued that closeout of these sorts of trials would be potentially easier if there was better communication with research participants. As indicated earlier, some advocated continually reminding participants that pump treatment was funded only for the trial’s duration [see What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?]. Others, who supported adopting more formalised end-of-trial processes, suggested that these could be explained to patients so they are made aware in advance of how decisions about their post-trial therapy would be made:
You need to let the patients know this, you know, you could have some fixed set criteria for whether they keep the pump or not. Or we say, at the end of the trial, you come off the pump for 3 months. And after the 3 months your diabetes control will be reviewed again to see if the pump therapy is suitable for you. So that they actually – and that might be the better way to do it – so that everybody knows they’re going to come off the pump for 3 months. And then they’ll get a review, rather than this.
ED12
As noted earlier [see What (if any) practical/ethical/other issues arose for staff and how did they attempt to address these?], staff made a related point when they argued that it might be useful to make patients aware during the trial when they were currently not reaching the criteria for post-trial treatment (pump) so that they were prepared for the possibility of their treatment being withdrawn:
If we’d had the same conversations all along: your HbA1c‘s no better. You only bolus twice a day. And you have that conversation three or four times, then the patient is going to come to the conclusion: yeah I’m not going to keep the pump. I can’t do this. They would have got to that point themselves.
Dr B
Dedicated trial clinics
Although all staff regarded communication between staff and patients as a crucial factor in facilitating trial closeout, some acknowledged that developing relationships with trial participants is difficult, particularly in the larger trial centres. To overcome this, one member of staff suggested that, in the future, trials should set up clinics for trial participants, which, they reflected, would have been helpful in the REPOSE Trial when communicating with patients, particularly when terminating pump therapy:
Whoever finished the trial with the patient and communicated to them the decision should have known the patient. Just so that it was a – it was much more of a more – the way you would clinically, . . . the person who did know the patient should have been there at the time [the end] to support the patient through the transition. I don’t think we really realised how the patient would perceive the difficulty of the transition . . . unlike other studies that I’ve done I personally didn’t feel that I was engaged with the study’s subjects, which probably is correct from the point of view of the outcomes, but it did make the ending of the study a bit more difficult.
Dr C
As Dr C further suggested, having dedicated trial clinics would result in continuity of care across the trial and thus make it easier at closeout because staff involved would be known to the patients and vice versa: ‘I think the person who is terminating the study should have been involved throughout it – that would have made all of the difference’.
Dr B similarly commented that involving educators who were known to the patients in the post-trial consultations was helpful when it came to communicating with, and managing, patients’ emotional reactions:
I think, she [educator] knew some of the patients better than I did because she’d done the course with some of them. And so she was warning which ones might be tricky. And you know, she is a good judge of character. So that was really helpful I guess. I think one of the other times we ran into a problem where it, the doctor that didn’t know the background to the patient. You know, so if you, I suppose not had the pre-warning, if we’ve not had that discussion beforehand, it would come across, or could be, come across really quite cold and so I think that was helpful.
Dr B
Role of the Clinical Trials Research Unit
Finally, one member of staff, ED11, suggested that CTRUs have a major role to play in communicating and co-ordinating information about trial closeout by offering/co-ordinating training, hosting meetings/teleconferences to allow staff to share experiences of closeout, offering examples of good closeout practices, making sure that there is adequate resourcing to do the closeout/post-trial appointments properly and reminding centres that closeout is approaching so that they can make preparations, particularly for potential negative reactions to the withdrawal of treatment:
I think everyone should have been advised to have those difficult conversation – you know had the conversation about not being able to keep the pump at the end, being advised to do that at every visit and every opportunity, so that people had their expectations managed. [And] . . . maybe a reminder that we needed to meet and discuss who was going to stay on the pumps and who wasn’t. You know, so just a reminder to say: have you had that conversation with your team? Has the patient been primed? Something like that would have been helpful.
ED11
During the trial there were opportunities for staff to share experiences during regular TMG meetings involving local PIs and some lead educators from all of the centres, as well as during regular educator teleconferences. However, these teleconferences primarily focused on issues relating to trial delivery. Although closeout was discussed in advance in both types of teleconference, the educator teleconferences were stopped just before the start of the closeout period, as it was thought that, by this point, further meetings would not be necessary (a decision that may have been partly due to a lack of awareness of the problems which would arise for some staff at closeout). Hence, there were limited opportunities for some staff members at the different centres to share and discuss the difficulties they went on to encounter when withdrawing treatment.
In summary, although most staff did not regard themselves as having needed support for the closeout of the REPOSE Trial, they outlined a number of ideas that they felt would facilitate closeout in trials when treatments are withdrawn. In addition to the strategies they had already developed during REPOSE, staff suggested that more formalised procedures for ending trials should be adopted; specifically, procedures for post-trial treatment decision-making and for transitioning research participants back into clinical care. Second, they advocated for improved communication among trial staff both between and within centres and with patients.
Key findings
This study has highlighted and explored differences in staff members’ experiences of closeout, both within and across the REPOSE centres. In most of the centres with limited funding for pump therapy, all but a few patients were reverted to MDI at the end of the trial. In some such cases, staff had had to manage patients’ negative emotional reactions to the withdrawal of pump therapy. In other centres at which funding for pumps was more readily available, all patients who were safely using the pump, who wished to continue using it and who were benefiting, as broadly defined from pump therapy, were allowed to continue this treatment following trial closeout. As patients in these centres were able to remain on the pump if they wanted to, closeout in these centres was perceived as less challenging.
Most staff, but particularly those involved in the pilot phase, anticipated that the withdrawal of pump therapy might be an issue for patients, and hence had developed a range of potential solutions to address this. These included developing strict protocols for managing patients’ expectations and pre-empting potential disappointment/anger by reminding patients that pump therapy was a research intervention that may terminate at the end of trial whenever they attended trial appointments.
All centres developed site-specific procedures for decision-making about post-trial treatment, although some were more formalised than others. These decisions were influenced by assessments of patient safety and efficacy plus the availability of funding for pump therapy. Access to resources ultimately dictated the decision-making strategy adopted by the different centres (and, in some cases, by different individuals within those centres); in resource-limited centres individual treatment decisions were NICE/SIGN-guideline driven and based on objective efficacy criteria, whereas in resource-rich centres the treatment decisions were based on more subjective views of efficacy or patient benefit and a desire to safeguard an ongoing therapeutic relationship.
Staff described a number of ethical questions and issues concerning the withdrawal of treatment, which they felt had emerged in closing out the REPOSE Trial. These included whether or not it was right to remove a therapy if patients were deriving some benefit, or perceived themselves as benefiting, from it; the fact that removal of therapy might undermine the trust and confidence in an ongoing therapeutic relationship; and the existence of inequity in funding for post-trial treatment.
Staff identified a number of things that they felt could facilitate closeout of future trials when treatments may be withdrawn. In addition to the particular strategies they had developed during the REPOSE Trial, staff suggested that more formalised procedures for ending trials should be adopted; specifically, procedures for post-trial treatment decision-making and for transitioning research participants back into clinical care. Second, they advocated for improved communication among trial staff, both between and within centres, and with patients. In addition, staff highlighted the potential value of having several team members involved in post-trial consultations, including staff who had contact with patients during the trial.
Key recommendations
-
Planning for closeout should begin at a trial’s inception. Closeout should be addressed in the risk assessment for the trial, and consideration given to whether or not there may be ethical and practical issues related to removing a trial treatment.
-
Ensuring that the necessary resources, training and protocols are in place will require that realistic costings for closeout (e.g. training for staff, making sure they have dedicated time for post-trial clinic appointments and MDTs) are included in the grant application or are negotiated with trusts during the planning stage.
-
Having formal closeout procedures for decision-making about post-trial treatment and transitioning patients back into clinical care/other therapies may increase accountability and transparency, and aid the communication of treatment decisions to patients.
-
Consensus and communication within centre teams is important for managing closeout effectively.
-
If closeout is staggered across/within centres then regular meetings/debriefs during the closeout period would allow staff to share and learn from each others’ experiences.
-
If a treatment may be withdrawn at the end of the trial, trial staff should communicate this to patients at every opportunity during the trial to prevent/pre-empt disappointment.
-
Information about the potential withdrawal of treatment should be included in formal trial materials (e.g. the patient information sheet, see Appendix 8), as occurred in REPOSE, as well as informal trial communications (e.g. trial newsletters). Participants could also receive a separate (local) closeout information sheet before the end of the trial, which explains the timescales involved, the training/support provided and arrangements for future monitoring of (new) treatment. Consideration could also be given to whether or not a statement about the potential withdrawal of treatment should be included on the consent form.
-
Continuity of care across the trial could be encouraged; this could take the form of running dedicated clinics for trial participants or, at the very least, ensuring that staff closing out the trial are known to the patients.
-
Research appointments to collect trial data and clinical appointments to discuss post-trial therapy should be distinct; if possible, these should occur at different times and in different places.
-
Allowing patients a period of time after the trial is ended to continue on trial therapy and adjust to the idea of the withdrawal of treatment may be valuable. Funding for this period of adjustment may need to be included in the grant application.
-
Examples of good and bad practice at closeout could be documented and used to create scenarios for role play/training staff involved in the closeout of future trials involving potential withdrawal of treatments.
Strengths and limitations
This study had very high opt-in levels from staff, providing us with a sample size sufficient to achieve data saturation and allowing good representation of a diverse range of views. Recruitment from the seven main trial centres enabled us to identify a number of variations in practice in closing out the trial and the impact of contextual (e.g. availability of funding for pumps), as well as individual, factors (previous experience of delivering pilot, exposure/lack of exposure to patients during follow-ups, etc.) on closeout experiences and practices. This wide-ranging approach also enabled us to identify broader cross-cutting ethical issues and challenges, experienced in most/all of the centres.
There are, however, a couple of limitations that must be considered. First, in some cases there was a time lag between closeout and the interviews; hence, some of the accounts may have been subject to a recall bias. Second, the interviews required staff to reflect on what proved, for some, to be sensitive experiences. This may have impacted on staff willingness to discuss these issues in too much depth, although this was not evident in the interviews. Third, the fact that all of the staff interviewed come from a relatively small research community has affected the material that we are able to report because of our ethical mandate to safeguard confidentiality. Finally, one major limitation of this study is that we were unable to interview REPOSE patients about their closeout experiences because, as noted above, there was insufficient time available to secure ethical and other approvals and to collect the data.
In summary, the REPOSE Trial, presented an opportunity to undertake research on the experiences, views and information/support needs of staff members involved in the closeout of a trial, which potentially involved the withdrawal of trial treatments, thereby allowing us to provide data on a much neglected topic. All of the objectives of this qualitative study were achieved, and all of the original research questions answered. A peer-reviewed journal article is in press. 173
Acknowledgements
We are particularly grateful to those with diabetes who participated in the trial. We thank the members of the TSC and DMEC for their contribution.
Medtronic Ltd, UK, provided the insulin pumps for the trial.
The REPOSE Study Group
Simon Heller was the chief investigator.
Norman Waugh was the deputy chief investigator.
Stephanie Amiel, Mark Evans, Fiona Green, Peter Hammond, Alan Jaap, Brian Kennon, Robert Lindsay and Peter Mansell were site PIs and contributed to the study design and data interpretation.
Jane Baillie, Anita Beckwith, Helen Brown, Karen Callaby, Katy Davenport, Sarah Donald, Jackie Elliott, Leila Faghahati, Sara Hartnell, Allison Housden, Kalbir Kaur Pabla, Nicola Croxon, Sheena Macdonald, Muna Mohammed, Vicky Steel, Katy Valentine, Pamela Young, Ann Boal, Patsy Clerkin, Lynn Doran, Joanne Flynn, Emma Gibb, Hilary Peddie, Bernie Quinn, Helen Rogers, Janice Shephard, Janet Carling, Ann Collins, Laura Dinning, Christine Hare, Joyce Lodge, Sutapa Ray, Debora Brown, Jenny Farmer, Alison Cox, Chris Cheyette, Pratik Choudhary, Linda East, June Ellul, Katherine Hunt, Kimberley Shaw, Ben Stothard, Lucy Diamond, Lindsay Aniello, Debbie Anderson, Kathy Cockerell, Vida Heaney, Alyson Hutchison, Nicola Zammitt, Gayna Babington, Gail Bird, Janet Evans, Tasso Gazis, Nicola Maude, Karen Nunnick, Dawn Spick, Laura Fenn, Carla Gianfrancesco, Valerie Gordon, Linda Greaves, Susan Hudson, Valerie Naylor, Chloe Nisbet, Carolin Taylor, Karen Towse and Candice Ward contributed at sites to participant recruitment, intervention delivery and data collection.
Cindy Cooper, Gemma Hackney, Diana Papaioannou, Emma Whatley and David White provided central trial management, oversight and monitoring.
Mike Bradburn, Michael Campbell, Munya Dimairo and Ellen Lee contributed to the statistics.
Hasan Basarir, Alan Brennan, Simon Dixon and Daniel Pollard contributed to the health economics.
Nina Hallowell, Jackie Kirkham, Julia Lawton and David Rankin designed and undertook the qualitative work.
Katharine Barnard provided expert input to the quantitative psychosocial work.
Timothy Chater and Kirsty Pemberton provided data management.
Fiona Allsop and Lucy Carr provided central administration.
Pamela Royle conducted literature searches and exploratory analyses.
Gill Thompson and Sharon Walker provided central DAFNE support.
Pauline Cowling conducted the fidelity assessment.
W Henry Smithson provided user input to the design and implementation of the trial.
Trial Steering Committee
Professor Richard Holt, Independent Chair (Professor in Diabetes & Endocrinology, Human Development and Health Academic Unit, Faculty of Medicine, University of Southampton).
Dr Sean Dinneen (Consultant Endocrinologist, National University of Ireland Galway).
Dawn Kitchener (Diabetes Specialist Nurse, Leicester Royal Infirmary).
Jane Morgan (Diabetes Specialist Nurse, Northumbria Healthcare NHS Foundation Trust).
Mark Mullee (Statistician, Director NIHR Research Design Service South Central, Southampton General Hospital).
Professor John Pickup (Professor of Diabetes & Metabolism, Guy’s Hospital, London).
Brian Trench (Patient Representative, Chairperson DAFNE User Action Group).
Data Monitoring and Ethics Committee
Professor Robert Tattersall, independent chairperson (retired professor of clinical diabetes at the University of Nottingham).
Dr Paul Ewings (Statistician, Peninsula Research & Development Support Unit, Director NIHR Research Design Service South West).
Ms Florence Findlay White (National Care Advisor for Diabetes UK Northern Ireland).
Contributions of authors
Simon Heller (Professor of Clinical Diabetes), David White (Trial Manager), Ellen Lee (Research Associate, statistics), Julia Lawton (Professor of Health and Social Science), Daniel Pollard (Research Associate, health economics), Norman Waugh (Professor in Public Health) and Nina Hallowell (Research Fellow, Population Health Science) produced the first draft of the report. All of the authors contributed to the content of the report.
Simon Heller, Julia Lawton, Norman Waugh, Stephanie Amiel (Professor of Diabetic Medicine), Katharine Barnard (Associate Professor of Health Psychology), Alan Brennan (Professor of Health Economics and Decision Modelling), Michael Campbell (Professor of Medical Statistics), Cindy Cooper (Director, Sheffield CTRU), Simon Dixon (Professor of Health Economics), Mark Evans (University Lecturer/Honorary Consultant Physician, Institute of Metabolic Science), Fiona Green (Consultant Physician, Diabetes and Endocrinology), Peter Hammond (Consultant Physician, Diabetes and Endocrinology), W Henry Smithson (Professor, General Practice) and Carolin Taylor (Diabetes Specialist Nurse) were applicants on the Health Technology Assessment grant and contributed to the study design.
Simon Heller, David White, Ellen Lee, Julia Lawton, Daniel Pollard, Norman Waugh, Stephanie Amiel, Katharine Barnard, Anita Beckwith (Clinical Lead Dietitian, diabetes), Alan Brennan, Michael Campbell, Cindy Cooper, Munyaradzi Dimairo (Research Associate, statistics), Simon Dixon, Jackie Elliott (Senior Clinical Lecturer in Diabetes), Mark Evans, Fiona Green, Gemma Hackney (Research Assistant), Peter Hammond, Alan Jaap (Consultant Physician, Diabetes and Endocrinology), Brian Kennon (Consultant Physician, Diabetes and Endocrinology), Jackie Kirkham (Research Fellow, Population Health Science), Robert Lindsay (Reader in Diabetes & Endocrinology), Peter Mansell (Consultant Physician, Diabetes and Endocrinology), Diana Papaioannou (Research Fellow), David Rankin (Research Fellow, Population Health Science), Pamela Royle (Senior Research Fellow), W Henry Smithson and Carolin Taylor were members of the TMG and contributed to the delivery of the trial and revisions of the protocol.
Nina Hallowell conducted the qualitative substudy on the challenges of closing out a clinical trial.
Publications
Lawton J, Kirkham J, Rankin D, Barnard K, Cooper CL, Taylor C, et al. Perceptions and experiences of using automated bolus advisors amongst people with type 1 diabetes: a longitudinal qualitative investigation. Diabetes Res Clin Pract 2014;106:443–50. http://dx.doi.org/10.1016/j.diabres.2014.09.011
White D, Waugh N, Elliott J, Lawton J, Barnard K, Campbell MJ, et al. The Relative Effectiveness of Pumps Over MDI and Structured Education (REPOSE): study protocol for a cluster randomised controlled trial. BMJ Open 2014;4:e006204. http://dx.doi.org/10.1136/bmjopen-2014-006204
Lawton J, Kirkham J, White D, Rankin D, Cooper C, Heller S. Uncovering the emotional aspects of working on a clinical trial: a qualitative study of the experiences and views of staff involved in a type 1 diabetes trial. Trials 2015;16:3.
Lawton J, Kirkham J, Rankin D, White DA, Elliott J, Jaap A, et al. Who gains clinical benefit from using insulin pump therapy? A qualitative study of the perceptions and views of health professionals involved in the Relative Effectiveness of Pumps over MDI and Structured Education (REPOSE) trial. Diabet Med 2016;33:243–51. http://dx.doi.org/10.1111/dme.12879
The REPOSE Study Group. A cluster randomised trial comparing insulin pump therapy to multiple injections during flexible intensive insulin therapy for type 1 diabetes - The Relative Effectiveness of Pumps Over MDI and Structured Education (REPOSE) Trial. BMJ 2017; in press.
Lawton J, White D, Rankin D, Elliott J, Taylor C, Cooper C, et al. Staff experiences of closing out a clinical trial involving withdrawal of treatment: qualitative study. Trials 2017;18:61.
The REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ 2017;356:j1285.
Data sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author and will be considered by the REPOSE TMG, which, despite the fact that specific consent for data sharing was not obtained, will release data on a case-by-case basis following the principles for sharing patient-level data as described by Smith et al. 176 The presented data do not contain any direct identifiers; we will minimise indirect identifiers and remove free-text data to minimise the risk of identification.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Mühlhauser I, Bruckner I, Berger M, Cheţa D, Jörgens V, Ionescu-Tîrgovişte C, et al. Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf Study. Diabetologia 1987;30:681-90. https://dx.doi.org/10.1007/BF00296989.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746-9. https://dx.doi.org/10.1136/bmj.325.7367.746.
- Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005;165:1337-44. http://dx.doi.org/10.1001/archinte.165.12.1337.
- Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;2. https://dx.doi.org/10.1002/14651858.cd005613.pub3.
- Pedersen-Bjergaard U, Kristensen PL, Beck-Nielsen H, Nørgaard K, Perrild H, Christiansen JS, et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol 2014;2:553-61. http://dx.doi.org/10.1016/S2213-8587 (14)70073-7.
- Tamborlane WV, Bonfig W, Boland E. Recent advances in treatment of youth with type 1 diabetes: better care through technology. Diabet Med 2001;18:864-70. https://dx.doi.org/10.1046/j.1464-5491.2001.00626.x.
- Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care 2002;25:593-8. https://dx.doi.org/10.2337/diacare.25.3.593.
- Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Type 1 diabetes: integrated sensor-augmented pump therapy systems for managing blood glucose levels (The MiniMed Paradigm Veo System and the Vibe and G4 PLATINUM CGM system), a systematic review and economic evaluation. Health Technol Assess 2016;20. https://dx.doi.org/10.3310/hta20170.
- Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation. Health Technol Assess 2010;14. https://dx.doi.org/10.3310/hta14110.
- Pickup J. Insulin pumps. Int J Clin Pract Suppl 2011;170:16-9. https://dx.doi.org/10.1111/j.1742-1241.2010.02574.x.
- White HD, Goenka N, Furlong NJ, Saunders S, Morrison G, Langridge P, et al. The U.K. service level audit of insulin pump therapy in adults. Diabet Med 2014;31:412-18. http://dx.doi.org/10.1111/dme.12325.
- Pickup JC. Are insulin pumps underutilized in type 1 diabetes? Yes. Diabetes Care 2006;29:1449-52. http://dx.doi.org/10.2337/dc06-0011.
- Continuous Subcutaneous Insulin Infusion for the Treatment of Diabetes Mellitus. London: NICE; 2008.
- Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N. Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes. Health Technol Assess 2004;8. https://dx.doi.org/10.3310/hta8430.
- Thomas RM, Aldibbiat A, Griffin W, Cox MAA, Leech NJ, Shaw JAM. A randomized pilot study in type 1 diabetes complicated by severe hypoglycaemia, comparing rigorous hypoglycaemia avoidance with insulin analogue therapy, CSII or education alone. Diabet Med 2007;24:778-83. https://dx.doi.org/10.1111/j.1464-5491.2007.02196.x.
- Bruttomesso D, Crazzolara D, Maran A, Costa S, Dal Pos M, Girelli A, et al. In type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med 2008;25:326-32. http://dx.doi.org/10.1111/j.1464-5491.2007.02365.x.
- Bolli GB, Kerr D, Thomas R, Torlone E, Sola-Gazagnes A, Vitacolonna E, et al. Comparison of a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care 2009;32:1170-6. http://dx.doi.org/10.2337/dc08-1874.
- Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol 2010;47:S77-81. https://dx.doi.org/10.1007/s00592-009-0132-5.
- Roze S, Smith-Palmer J, Valentine W, de Portu S, Nørgaard K, Pickup JC. Cost-effectiveness of continuous subcutaneous insulin infusion versus multiple daily injections of insulin in type 1 diabetes: a systematic review. Diabet Med 2015;32:1415-24. http://dx.doi.org/10.1111/dme.12792.
- Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of type 1 diabetes in the UK. Diabet Med 2005;22:1239-45. https://dx.doi.org/10.1111/j.1464-5491.2005.01576.x.
- Scuffham P, Carr L. The cost-effectiveness of continuous subcutaneous insulin infusion compared with multiple daily injections for the management of diabetes. Diabet Med 2003;20:586-93. https://dx.doi.org/10.1046/j.1464-5491.2003.00991.x.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- The REPOSE Study Group . Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ 2017;356. https://doi.org/10.1136/bmj.j1285.
- Ray T, Choudhary P, Mansell P, Heller S, Amiel SA, Hopkins D. Dose adjustment for normal eating (DAFNE) structured education reduces progression to continuous subcutaneous insulin infusion (CSII) among patients being considered for insulin pump therapy at enrolment. Diabet Med 2013;30:7-8.
- Mühlhauser I, Jörgens V, Berger M, Graninger W, Gürtler W, Hornke L, et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia 1983;25:470-6. https://dx.doi.org/10.1007/BF00284453.
- Sämann A, Mühlhauser I, Bender R, Kloos Ch, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 2005;48:1965-70. https://dx.doi.org/10.1007/s00125-005-1905-1.
- Heller S, Lawton J, Amiel S, Cooke D, Mansell P, Brennan A, et al. Improving management of type 1 diabetes in the UK: the Dose Adjustment For Normal Eating (DAFNE) programme as a research test-bed. A mixed-method analysis of the barriers to and facilitators of successful diabetes self-management, a health economic analysis, a cluster randomised controlled trial of different models of delivery of an educational intervention and the potential of insulin pumps and additional educator input to improve outcomes. Programme Grants Appl Res 2014;2. https://dx.doi.org/10.3310/pgfar02050.
- Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002;324. https://dx.doi.org/10.1136/bmj.324.7339.705.
- Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab 2009;94:729-40. http://dx.doi.org/10.1210/jc.2008-1415.
- Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941-51. https://dx.doi.org/10.1007/s00125-008-0974-3.
- Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD005103.pub2.
- Bak JF, Nielsen OH, Pedersen O, Beck-Nielsen H. Multiple insulin injections using a pen injector versus insulin pump treatment in young diabetic patients. Diabetes Res 1987;6:155-8.
- Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care 1996;19:324-7. https://dx.doi.org/10.2337/diacare.19.4.324.
- Maran A, Crazzolara D, Nicoletti M, Costa S, dal Pos M, Tiengo A, et al. A randomized crossover study to compare continuous subcutaneous insulin infusion (CSII) with multiple daily injection (MDI) in type 1 diabetic patients previously treated with CSII. Diabetologia 2005;48.
- Chiasson JL, Ducros F, Poliquin-Hamet M, Lopez D, Lecavalier L, Hamet P. Continuous subcutaneous insulin infusion (Mill-Hill Infuser) versus multiple injections (Medi-Jector) in the treatment of insulin-dependent diabetes mellitus and the effect of metabolic control on microangiopathy. Diabetes Care 1984;7:331-7. https://dx.doi.org/10.2337/diacare.7.4.331.
- DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ. Dutch Insulin Pump Study Group . A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care 2002;25:2074-80. https://dx.doi.org/10.2337/diacare.25.11.2074.
- Ziegler D, Dannehl K, Koschinsky T, Toeller M, Gries FA. Comparison of continuous subcutaneous insulin infusion and intensified conventional therapy in the treatment of type I diabetes: a two-year randomized study. Diabetes Nutr Metab Clin Exp 1990;3:203-13.
- Haakens K, Hanssen KF, Dahl-Jørgensen K, Vaaler S, Aagenaes O, Mosand R. Continuous subcutaneous insulin infusion (CSII), multiple injections (MI) and conventional insulin therapy (CT) in self-selecting insulin-dependent diabetic patients. A comparison of metabolic control, acute complications and patient preferences. J Intern Med 1990;228:457-64. https://dx.doi.org/10.1111/j.1365-2796.1990.tb00263.x.
- Hanaire-Broutin H, Melki V, Bessières-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care 2000;23:1232-5. https://dx.doi.org/10.2337/diacare.23.9.1232.
- Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, et al. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care 2005;28:533-8. https://dx.doi.org/10.2337/diacare.28.3.533.
- Home PD, Capaldo B, Burrin JM, Worth R, Alberti KG. A crossover comparison of continuous subcutaneous insulin infusion (CSII) against multiple insulin injections in insulin-dependent diabetic subjects: improved control with CSII. Diabetes Care 1982;5:466-71. https://dx.doi.org/10.2337/diacare.5.5.466.
- Hoogma R, Hoekstra JB, Michels BP, Levi M. Comparison between multiple daily insulin injection therapy (MDI) and continuous subcutaneous insulin infusion therapy (CSII), results of the five nations study. Diabetes Res Clin Pract 2006;74:S144-7. https://dx.doi.org/10.1016/S0168-8227(06)70019-5.
- Hoogma RP, Hammond PJ, Gomis R, Kerr D, Bruttomesso D, Bouter KP, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med 2006;23:141-7. http://dx.doi.org/10.1111/j.1464-5491.2005.01738.x.
- Lepore G, Dodesini AR, Nosari I, Trevisan R. Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care 2003;26:1321-2. https://dx.doi.org/10.2337/diacare.26.4.1321.
- Nathan DM, Lou P, Avruch J. Intensive conventional and insulin pump therapies in adult type I diabetes. A crossover study. Ann Intern Med 1982;97:31-6. https://dx.doi.org/10.7326/0003-4819-97-1-31.
- Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic-ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes Nutr Metab Clin Exp 1988;1:289-96.
- Brinchmann-Hansen O, Dahl-Jørgensen K, Hanssen KF, Sandvik L. The response of diabetic retinopathy to 41 months of multiple insulin injections, insulin pumps, and conventional insulin therapy. Arch Ophthalmol 1988;106:1242-6. https://dx.doi.org/10.1001/archopht.1988.01060140402041.
- Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J 1986;293:1195-9. https://dx.doi.org/10.1136/bmj.293.6556.1195.
- Saurbrey N, Arnold-Larsen S, Møller-Jensen B, Kühl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabet Med 1988;5:150-3. https://dx.doi.org/10.1111/j.1464-5491.1988.tb00962.x.
- Schiffrin A, Belmonte MM. Comparison between continuous subcutaneous insulin infusion and multiple injections of insulin. A one-year prospective study. Diabetes 1982;31:255-64. https://dx.doi.org/10.2337/diab.31.3.255.
- Schmitz A, Christiansen JS, Christensen CK, Hermansen K, Mogensen CE. Effect of pump versus pen treatment on glycaemic control and kidney function in long-term uncomplicated insulin-dependent diabetes mellitus (IDDM). Dan Med Bull 1989;36:176-8.
- Schottenfeld-Naor Y, Galatzer A, Karp M, Josefsberg Z, Laron Z. Comparison of metabolic and psychological parameters during continuous subcutaneous insulin infusion and intensified conventional insulin treatment in type I diabetic patients. Isr J Med Sci 1985;21:822-8.
- Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care 2001;24:1722-7. https://dx.doi.org/10.2337/diacare.24.10.1722.
- Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J 1985;290:811-15. https://dx.doi.org/10.1136/bmj.290.6471.811.
- Bacon S, McCarthy A, Costa-Pozza A, Condron A, Vizzard N, O’Shea H, et al. The effectiveness and safety of continuous subcutaneous insulin infusion in a large adult cohort over a 10 year period. Ir J Med Sci 2014;1:S450-1.
- Beato-Víbora P, Yeoh E, Rogers H, Hopkins D, Amiel SA, Choudhary P. Sustained benefit of continuous subcutaneous insulin infusion on glycaemic control and hypoglycaemia in adults with type 1 diabetes. Diabet Med 2015;32:1453-9. http://dx.doi.org/10.1111/dme.12869.
- Bruttomesso D, Pianta A, Crazzolara D, Scaldaferri E, Lora L, Guarneri G, et al. Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med 2002;19:628-34. https://dx.doi.org/10.1046/j.1464-5491.2002.00750.x.
- Carlsson BM, Attvall S, Clements M, Gumpeny SR, Pivodic A, Sternemalm L, et al. Insulin pump-long-term effects on glycemic control: an observational study at 10 diabetes clinics in Sweden. Diabetes Technol Ther 2013;15:302-7. http://dx.doi.org/10.1089/dia.2012.0286.
- Clements M, Matuleviciene V, Attvall S, Ekelund M, Pivodic A, Dahlqvist S, et al. Predicting the effectiveness of insulin pump therapy on glycemic control in clinical practice: a retrospective study of patients with type 1 diabetes from 10 outpatient diabetes clinics in Sweden over 5 years. Diabetes Technol Ther 2015;17:21-8. http://dx.doi.org/10.1089/dia.2014.0139.
- Cohen ND, Hong ES, Van Drie C, Balkau B, Shaw J. Long-term metabolic effects of continuous subcutaneous insulin infusion therapy in type 1 diabetes. Diabetes Technol Ther 2013;15:544-9. http://dx.doi.org/10.1089/dia.2012.0331.
- Lepore G, Bruttomesso D, Bonomo M, Dodesini AR, Costa S, Meneghini E, et al. Continuous subcutaneous insulin infusion is more effective than multiple daily insulin injections in preventing albumin excretion rate increase in type 1 diabetic patients. Diabet Med 2009;26:602-8. http://dx.doi.org/10.1111/j.1464-5491.2009.02736.x.
- Nixon R, Folwell R, Pickup JC. Variations in the quality and sustainability of long-term glycaemic control with continuous subcutaneous insulin infusion. Diabet Med 2014;31:1174-7. http://dx.doi.org/10.1111/dme.12486.
- Orr CJ, Hopman W, Yen JL, Houlden RL. Long-term efficacy of insulin pump therapy on glycemic control in adults with type 1 diabetes mellitus. Diabetes Technol Ther 2015;17:49-54. http://dx.doi.org/10.1089/dia.2014.0131.
- Quiros C, Gimenez M, Rios P, Careaga M, Roca D, Vidal M, et al. Long-term outcome of insulin pump therapy: reduction of hypoglycaemia and impact on glycaemic control. Diabet Med 2016;33:1422-6. https://dx.doi.org/10.1111/dme.13094.
- Rosenlund S, Hansen TW, Andersen S, Rossing P. Effect of 4 years subcutaneous insulin infusion treatment on albuminuria, kidney function and HbA1c compared with multiple daily injections: a longitudinal follow-up study. Diabet Med 2015;32:1445-52. http://dx.doi.org/10.1111/dme.12950.
- Steineck I, Cederholm J, Eliasson B, Rawshani A, Eeg-Olofsson K, Svensson AM, et al. Insulin pump therapy, multiple daily injections, and cardiovascular mortality in 18,168 people with type 1 diabetes: observational study. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h3234.
- Carlone A, Cipolloni L, Gillanti G, Gnessi C, Leto G, Buzzetti R. Effectiveness of therapeutic-educational re-training in patients affected by type 1 diabetes treated with CSII (continuous subcutaneous insulin infusion). Diabetologia 2011;54.
- Hermanides J, Nørgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes; a randomized controlled trial. Diabet Med 2011;28:1158-67. http://dx.doi.org/10.1111/j.1464-5491.2011.03256.x.
- Lee SW, Sweeney T, Clausen D, Kolbach C, Hassen A, Firek A, et al. Combined insulin pump therapy with real-time continuous glucose monitoring significantly improves glycemic control compared to multiple daily injection therapy in pump naïve patients with type 1 diabetes; single center pilot study experience. J Diabetes Sci Technol 2007;1:400-4. https://dx.doi.org/10.1177/193229680700100313.
- Peyrot M, Rubin RR. Patient-reported outcomes for an integrated real-time continuous glucose monitoring/insulin pump system. Diabetes Technol Ther 2009;11:57-62. http://dx.doi.org/10.1089/dia.2008.0002.
- Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311-20. http://dx.doi.org/10.1056/NEJMoa1002853.
- Garg SK, Voelmle MK, Beatson CR, Miller HA, Crew LB, Freson BJ, et al. Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study. Diabetes Care 2011;34:574-9. http://dx.doi.org/10.2337/dc10-1852.
- Choudhary P, Shin J, Wang Y, Evans ML, Hammond PJ, Kerr D, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023-5. http://dx.doi.org/10.2337/dc10-2411.
- Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224-32. http://dx.doi.org/10.1056/NEJMoa1303576.
- Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs. standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240-7. http://dx.doi.org/10.1001/jama.2013.277818.
- Heinemann L, Hermanns N. IQWiG Reanalyzes and raises questions about an article by Ly et al. which concluded low glucose suspend is very beneficial. J Diabetes Sci Technol 2016;10:185-90. http://dx.doi.org/10.1177/1932296815597918.
- Integrated sensor-dugmented pump therapy systems for managing blood glucose levels in type 1 diabetes (the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system). 2016.
- Elliott J, Rankin D, Jacques RM, Lawton J, Emery CJ, Campbell MJ, et al. A cluster randomized controlled non-inferiority trial of 5-day Dose Adjustment for Normal Eating (DAFNE) training delivered over 1 week versus 5-day DAFNE training delivered over 5 weeks: the DAFNE 5 × 1-day trial. Diabet Med 2015;32:391-8. http://dx.doi.org/10.1111/dme.12621.
- Mansell P, Cullum R, Cole F, Taylor C, Cooke D, Murphy H. Outcomes in people with type 1 diabetes transferring to insulin pump therapy after structured education. Diabetologia 2013;56.
- Cole F, Cullum R, Cooke D, Taylor C, Murphy H, Mansell P. Higher levels of psychological distress in people with type 1 diabetes who transfer to insulin pump therapy after Dose Adjustment for Normal Eating (DAFNE) structured education. Diabet Med 2013;30.
- Dinneen SF, O’Hara MC, Byrne M, Smith D, Courtney CH, McGurk C, et al. Group follow-up compared to individual clinic visits after structured education for type 1 diabetes: a cluster randomised controlled trial. Diabetes Res Clin Pract 2013;100:29-38. http://dx.doi.org/10.1016/j.diabres.2013.01.017.
- Casey D, Murphy K, Lawton J, White FF, Dineen S. A longitudinal qualitative study examining the factors impacting on the ability of persons with T1DM to assimilate the Dose Adjustment for Normal Eating (DAFNE) principles into daily living and how these factors change over time. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-672.
- Engel L, Cummins R. Impact of dose adjustment for normal eating in Australia (OzDAFNE) on subjective wellbeing, coping resources and negative affects in adults with type 1 diabetes: a prospective comparison study. Diabetes Res Clin Pract 2011;91:271-9. http://dx.doi.org/10.1016/j.diabres.2010.11.023.
- Zijlstra E, Heise T, Rikte T, Thorsson L, Nosek L, Haahr H. Faster-acting insulin aspart by continuous subcutaneous insulin infusion: earlier exposure and greater early pharmacokinetic/pharmacodynamic effects vs. insulin aspart. Diabetologia 2015;1.
- Nicolucci A, Maione A, Franciosi M, Amoretti R, Busetto E, . EQuality1 Study Group . Quality of life and treatment satisfaction in adults with type 1 diabetes: a comparison between continuous subcutaneous insulin infusion and multiple daily injections. Diabet Med 2008;25:213-20. https://dx.doi.org/10.1111/j.1464-5491.2007.02346.x.
- White D, Waugh N, Elliott J, Lawton J, Barnard K, Campbell MJ, et al. The Relative Effectiveness of Pumps Over MDI and Structured Education (REPOSE): study protocol for a cluster randomised controlled trial. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-006204.
- Colquitt J, Royle P, Waugh N. Are analogue insulins better than soluble in continuous subcutaneous insulin infusion? Results of a meta-analysis. Diabet Med 2003;20:863-6. https://dx.doi.org/10.1046/j.1464-5491.2003.01018.x.
- Henriksson M, Jindal R, Sternhufvud C, Bergenheim K, Sörstadius E, Willis M. A systematic review of cost-effectiveness models in type 1 diabetes mellitus. PharmacoEconomics 2016;34:569-85. http://dx.doi.org/10.1007/s40273-015-0374-8.
- UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140-7. https://dx.doi.org/10.1007/s00125-007-0599-y.
- Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384-95. http://dx.doi.org/10.2337/dc12-2480.
- Pedersen-Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycaemia and self-estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev 2003;19:232-40. http://dx.doi.org/10.1002/dmrr.377.
- The Medicines for Human Use (Clinical Trials) Regulations 2004. 2004.
- Medical Research Council/DH/MHRA Joint Project . Risk-Adapted Approaches to the Management of Clinical Trials of Investigational Medicinal Products 2011. www.gov.uk/government/uploads/system/uploads/attachment_data/file/343677/Risk-adapted_approaches_to_the_management_of_clinical_trials_of_investigational_medicinal_products.pdf (accessed February 2002).
- Brosteanu O, Houben P, Ihrig K, Ohmann C, Paulus U, Pfistner B, et al. Risk analysis and risk adapted on-site monitoring in noncommercial clinical trials. Clin Trials 2009;6:585-96. http://dx.doi.org/10.1177/1740774509347398.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5. https://dx.doi.org/10.3310/hta5330.
- Curtis L. Unit Costs of Health and Social Care 2014 2014. www.pssru.ac.uk/project-pages/unit-costs/2014/index.php (accessed August 2015).
- DAFNE . DAFNE Fact Sheet Six 2012. www.dafne.uk.com/uploads/135/documents/06_factsheetsix_12pt_18_06_12.pdf (accessed August 2015).
- Department of Health . NHS Reference Costs 2013 to 2014 2014. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed August 2015).
- Elliott J, Jacques RM, Kruger J, Campbell MJ, Amiel SA, Mansell P, et al. Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with type 1 diabetes. Diabet Med 2014;31:847-53. http://dx.doi.org/10.1111/dme.12441.
- Department of Health . NHS Reference Costs 2012 to 2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 1 August 2015).
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; n.d.
- Health and Social Care Information Centre . Prescription Cost Analysis England 2014 2015. www.hscic.gov.uk/catalogue/PUB17274 (accessed August 2015).
- London New Drugs Group . Comparative Table of Insulin Pumps 2013. www.medicinesresources.nhs.uk/upload/documents/Evidence/Comparative table of insulin pumps.pdf (accessed August 2015).
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. https://dx.doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc 1962;57:348-68. https://dx.doi.org/10.1080/01621459.1962.10480664.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://dx.doi.org/10.1002/hec.944.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987.
- Thokala P, Kruger J, Brennan A, Basarir H, Duenas A, Pandor A, et al. Assessing the cost-effectiveness of type 1 diabetes interventions: the Sheffield type 1 diabetes policy model. Diabet Med 2014;31:477-86. http://dx.doi.org/10.1111/dme.12371.
- Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Gudbjörnsdottir S. A new model for 5-year risk of cardiovascular disease in type1 diabetes; from the Swedish National Diabetes Register (NDR). Diabet Med 2011;28:1213-20. https://dx.doi.org/10.1111/j.1464-5491.2011.03342.x.
- Palmer JL. Cost-Effectiveness of Angiotensin-Converting Enzyme Inhibitors for the Prevention of Cardiovascular and End-Stage Renal Disease in Patients With Type 1 Diabetes 2012.
- Nathan DM, Clearly PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53. https://dx.doi.org/10.1056/NEJMoa052187.
- Peasgood T, Brennan A, Mansell P, Elliot J, Basarir H, Kruger J. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Mak 2016;36:1020-33. https://dx.doi.org/10.1177/0272989X16658660.
- Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462-70. http://dx.doi.org/10.1016/j.jval.2014.03.003.
- Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238-43. https://dx.doi.org/10.2337/diacare.25.12.2238.
- Alva M, Gray A, Mihaylova B, Clarke P. The effect of diabetes complications on health-related quality of life: the importance of longitudinal data to address patient heterogeneity. Health Econ 2014;23:487-500. http://dx.doi.org/10.1002/hec.2930.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9. https://dx.doi.org/10.1177/027298902400448902.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- McEwan P, Poole CD, Tetlow T, Holmes P, Currie CJ. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 1 diabetes in the UK. Curr Med Res Opin 2007;23:S7-19. https://dx.doi.org/10.1185/030079907X167561.
- Department of Health . NHS Reference Costs 2010–11 2011. www.gov.uk/government/publications/2010-11-reference-costs-publication (accessed August 2015).
- Currie CJ, Poole CD, Woehl A, Morgan CL, Cawley S, Rousculp MD, et al. The financial costs of healthcare treatment for people with type 1 or type 2 diabetes in the UK with particular reference to differing severity of peripheral neuropathy. Diabet Med 2007;24:187-94. http://dx.doi.org/10.1111/j.1464-5491.2006.02057.x.
- Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50. https://dx.doi.org/10.1046/j.1464-5491.2003.00972.x.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
- Cribari-Neto F, Zeileis A. Beta regression in R. J Stat Softw 2010;34:1-24. https://dx.doi.org/10.18637/jss.v034.i02.
- Speight J, Amiel SA, Bradley C, Heller S, Oliver L, Roberts S, et al. Long-term biomedical and psychosocial outcomes following DAFNE (Dose Adjustment For Normal Eating) structured education to promote intensive insulin therapy in adults with sub-optimally controlled type 1 diabetes. Diabetes Res Clin Pract 2010;89:22-9. http://dx.doi.org/10.1016/j.diabres.2010.03.017.
- Gunn D, Mansell P. Glycaemic control and weight 7 years after Dose Adjustment For Normal Eating (DAFNE) structured education in type 1 diabetes. Diabet Med 2012;29:807-12. http://dx.doi.org/10.1111/j.1464-5491.2011.03525.x.
- Rubin RR, Peyrot M. Patient-reported outcomes and diabetes technology: a systematic review of the literature. Pediatr Endocrinol Rev 2010;7:405-12.
- Bott U, Mühlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care 1998;21:757-69. https://dx.doi.org/10.2337/diacare.21.5.757.
- The WHOQOL Group . Development of the World Health Organisation WHOQOL-BREF quality of life assessment. Psychol Med 1998;28:551-8. https://dx.doi.org/10.1017/S0033291798006667.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://dx.doi.org/10.1097/00005650-199603000-00003.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16:199-208.
- Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617-21. https://dx.doi.org/10.2337/diacare.10.5.617.
- Nixon R, Pickup JC. Fear of hypoglycemia in type 1 diabetes managed by continuous subcutaneous insulin infusion: is it associated with poor glycemic control?. Diabetes Technol Ther 2011;13:93-8. http://dx.doi.org/10.1089/dia.2010.0192.
- Lewis KS, Bradley C, Knight G, Boulton AJ, Ward JD. A measure of treatment satisfaction designed specifically for people with insulin-dependent diabetes. Diabet Med 1988;5:235-42. https://dx.doi.org/10.1111/j.1464-5491.1988.tb00976.x.
- Secnik Boye K, Matza LS, Oglesby A, Malley K, Kim S, Hayes RP, et al. Patient-reported outcomes in a trial of exenatide and insulin glargine for the treatment of type 2 diabetes. Health Qual Life Outcomes 2006;4. http://dx.doi.org/10.1186/1477-7525-4-80.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Perneger TV, Burnand B. A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol 2005;58:142-9. https://dx.doi.org/10.1016/j.jclinepi.2004.06.005.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. https://dx.doi.org/10.1016/S0895-4356(98)00109-7.
- WHOQOL-Bref: Introduction, Administration, Scoring and Generic Version of the Assessment. Geneva: World Health Organization; 1996.
- Newson R. Confidence intervals for rank statistics: percentile slopes, differences, and ratios. Stata J 2006;6:497-520.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3. https://dx.doi.org/10.1136/bmj.311.6999.251.
- Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ 1995;311:42-5. https://dx.doi.org/10.1136/bmj.311.6996.42.
- Lawton J, Rankin D, Cooke D, Elliott J, Amiel S, Heller S. UK NIHR DAFNE Study Group . Patients’ experiences of adjusting insulin doses when implementing flexible intensive insulin therapy: a longitudinal, qualitative investigation. Diabetes Res Clin Pract 2012;98:236-42. http://dx.doi.org/10.1016/j.diabres.2012.09.024.
- Rankin D, Cooke DD, Elliott J, Heller SR, Lawton J. UK NIHR DAFNE Study Group . Supporting self-management after attending a structured education programme: a qualitative longitudinal investigation of type 1 diabetes patients’ experiences and views. BMC Public Health 2012;12. http://dx.doi.org/10.1186/1471-2458-12-652.
- Lawton J, Rankin D, Cooke DD, Elliott J, Amiel S, Heller S. UK NIHR DAFNE Study Group . Self-treating hypoglycaemia: a longitudinal qualitative investigation of the experiences and views of people with type 1 diabetes. Diabet Med 2013;30:209-15. http://dx.doi.org/10.1111/dme.12007.
- Lawton J, Rankin D, Cooke DD, Clark M, Elliot J, Heller S. UK NIHR DAFNE Study Group . Dose Adjustment for Normal Eating: a qualitative longitudinal exploration of the food and eating practices of type 1 diabetes patients converted to flexible intensive insulin therapy in the UK. Diabetes Res Clin Pract 2011;91:87-93. http://dx.doi.org/10.1016/j.diabres.2010.11.007.
- Lawton J, Kirkham J, White D, Rankin D, Cooper C, Heller S. Uncovering the emotional aspects of working on a clinical trial: a qualitative study of the experiences and views of staff involved in a type 1 diabetes trial. Trials 2015;16. http://dx.doi.org/10.1186/1745-6215-16-3.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Beverly Hills, CA: Sage Publications; 1990.
- Standard occupational classification 2010. 2010.
- English indices of deprivation 2010. 2011.
- Scottish Index of Multiple Deprivation 2012. 2012.
- Van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://dx.doi.org/10.1002/hec.4730030505.
- Cooke D, Bond R, Lawton J, Rankin D, Heller S, Clark M, et al. U.K. NIHR DAFNE Study Group . Structured type 1 diabetes education delivered within routine care: impact on glycemic control and diabetes-specific quality of life. Diabetes Care 2013;36:270-2. http://dx.doi.org/10.2337/dc12-0080.
- Rankin D, Barnard K, Elliott J, Cooke D, Heller S, Gianfrancesco C, et al. Type 1 diabetes patients’ experiences of, and need for, social support after attending a structured education programme: a qualitative longitudinal investigation. J Clin Nurs 2014;23:2919-27. http://dx.doi.org/10.1111/jocn.12539.
- Lawton J, Kirkham J, Rankin D, Barnard K, Cooper CL, Taylor C, et al. Perceptions and experiences of using automated bolus advisors amongst people with type 1 diabetes: a longitudinal qualitative investigation. Diabetes Res Clin Pract 2014;106:443-50. http://dx.doi.org/10.1016/j.diabres.2014.09.011.
- Lowes L, Eddy D, Channon S, McNamara R, Robling M, Gregory JW. DEPICTED study team . The experience of living with type 1 diabetes and attending clinic from the perception of children, adolescents and carers: analysis of qualitative data from the DEPICTED study. J Pediatr Nurs 2015;30:54-62. http://dx.doi.org/10.1016/j.pedn.2014.09.006.
- Lawton J, Kirkham J, Rankin D, White DA, Elliott J, Jaap A, et al. Who gains clinical benefit from using insulin pump therapy? A qualitative study of the perceptions and views of health professionals involved in the Relative Effectiveness of Pumps over MDI and Structured Education (REPOSE) trial. Diabet Med 2016;33:243-51. http://dx.doi.org/10.1111/dme.12879.
- Hayes M, Frearson S, Keller C, Cartmale A, Lewis-Hayes S. A hermeneutic phenomenological study of why adults with type 1 diabetes choose to discontinue CSII. Eur Diabetes Nurs 2011;8:12-6. https://dx.doi.org/10.1002/edn.167.
- NICE . Type 1 Diabetes in Adults: Diagnosis and Management n.d. www.nice.org.uk/guidance/ng17 (accessed 29 February 2016).
- Health and Social Care Information Centre . National Diabetes Audit 2012–13. Report 1: Care Processes and Treatment Targets 2014. www.hscic.gov.uk/catalogue/PUB14970 (accessed 18 February 2016).
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Cooper C, O’Cathain A, Hind D, Adamson J, Lawton J, Baird W. Conducting qualitative research within clinical trials units: avoiding potential pitfalls. Contemp Clin Trials 2014;38:338-43. http://dx.doi.org/10.1016/j.cct.2014.06.002.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-5.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. http://dx.doi.org/10.1016/j.jclinepi.2014.03.010.
- Eborall HC, Dallosso HM, Daly H, Martin-Stacey L, Heller SR. The face of equipoise: delivering a structured education programme within a randomized controlled trial: qualitative study. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-15.
- Featherstone K, Donovan JL. ‘Why don’t they just tell me straight, why allocate it?’ The struggle to make sense of participating in a randomised controlled trial. Soc Sci Med 2002;55:709-19. https://dx.doi.org/10.1016/S0277-9536(01)00197-6.
- Wardle K, Murphy D, Ireland L, Holbeck C, Davidson C, Rissel C, et al. What we learnt: recruiting prenatal mothers to an RCT addressing the prevention of overweight in early childhood?. Aust J Adv Nurs 2010;28:41-5.
- Lawton J, Jenkins N, Darbyshire J, Farmer A, Holman R, Hallowell N. Understanding the outcomes of multi-centre clinical trials: a qualitative study of health professional experiences and views. Soc Sci Med 2012;74:574-81. http://dx.doi.org/10.1016/j.socscimed.2011.11.012.
- Lawton J, Jenkins N, Darbyshire JL, Holman RR, Farmer AJ, Hallowell N. Challenges of maintaining research protocol fidelity in a clinical care setting: a qualitative study of the experiences and views of patients and staff participating in a randomized controlled trial. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-108.
- Lawton J, Fox A, Fox C, Kinmonth AL. Participating in the United Kingdom Prospective Diabetes Study (UKPDS): a qualitative study of patients’ experiences. Br J Gen Pract 2003;53:394-8.
- Wynne A. Is it any good? The evaluation of therapy by participants in a clinical trial. Soc Sci Med 1989;29:1289-97. https://dx.doi.org/10.1016/0277-9536(89)90069-5.
- Lawton J, White D, Rankin D, Elliott J, Taylor C, Cooper C, et al. Staff experiences of closing out a clinical trial involving withdrawal of treatment: qualitative study. Trials 2017;18.
- Britten N, Jones R, Murphy E, Stacy R. Qualitative research methods in general practice and primary care. Fam Pract 1995;12:104-14. https://dx.doi.org/10.1093/fampra/12.1.104.
- SIGN . Management of Diabetes: A National Clinical Guideline 2013. www.sign.ac.uk/pdf/sign116.pdf (accessed August 2015).
- Smith C, Hopkins C, Sydes M, Woolfall K, Clarke M, Murray G, et al. Good practice principles for sharing individual participant data from publicly funded clinical trials. Trials 2015;16. https://dx.doi.org/10.1186/1745-6215-16-S2-O1.
Appendix 1 Search methods
The Ovid MEDLINE search strategy was adapted, as appropriate, to the other databases.
-
((continuous or subcutaneous) adj3 insulin adj3 infusion).mp.
-
(csii or insulin pump*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
(insulin and pump*).m_titl.
-
Insulin Infusion Systems/
-
1 or 2 or 3 or 4
-
Diabetes Mellitus, Type 1/ or type 1.mp.
-
random*.tw.
-
randomized controlled trial.pt.
-
7 or 8
-
5 and 6 and 9
-
limit 10 to yr=“2007-Current”
The searches yielded 1341 records, and 749 remained after duplicates were removed. After screening the titles and abstracts to exclude studies not in adults, 180 records remained, and the titles and abstracts of these were screened by two authors. Only 128 were RCTs. We excluded trials for the reasons reported in Chapter 2 (see Methods). Twenty-three papers were included in the table of previous trials. Some trials were reported in more than one paper.
We also checked inclusion lists of six past systematic reviews (Colquitt et al. ,14 Cummins et al. ,9 Pickup et al. ,28 Monami et al. ,18 Fatourechi et al. 29 and the Cochrane review by Misso et al. 31).
Searches were run in Ovid MEDLINE for observational and audit studies of insulin pumps from 2012 to 7 January 2016.
The search strategy was
-
((continuous or subcutaneous) adj3 insulin adj3 infusion).mp.
-
(csii or insulin pump*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
(insulin and pump*).m_titl.
-
Insulin Infusion Systems/
-
1 or 2 or 3 or 4
-
*Diabetes Mellitus, Type 1/
-
type 1 diabet*.tw.
-
6 or 7
-
5 and 8
-
limit 9 to yr=“2012-Current”
-
(editorial or letter or randomized controlled trial).pt.
-
(10 not (editorial or letter or randomized controlled trial)).pt.
This retrieved 603 records and, after screening, 33 were retained for screening by a second reviewer. Of these 22 were included.
Appendix 2 Regulatory approvals
Research Ethics Committee approval was obtained for the study from the Liverpool East REC on 26 April 2011. MHRA approval was received on the 26 May 2011.
The relevant R&D departments were approached and approval was given for the relevant primary care trusts/trusts on the following dates:
| R&D department | Date of approval |
|---|---|
| Sheffield Teaching Hospitals NHS Foundation Trust | 27 October 2011 |
| NHS Greater Glasgow and Clyde | 10 January 2012 |
| King’s College Hospital NHS Trust | 13 January 2012 |
| Cambridge University Hospitals NHS Foundation Trust | 26 October 2011 |
| Harrogate and District NHS Foundation Trust | 20 January 2012 |
| NHS Dumfries & Galloway | 24 October 2011 |
| NHS Lothian | 5 January 2012 |
| Nottingham University Hospitals NHS Trust | 31 October 2012 |
Appendix 3 Consent forms
Appendix 4 Sample of data collection booklet: 24-month follow-up
Appendix 5 Blood glucose diary: pump arm
Appendix 6 Ongoing data collection booklet
Appendix 7 Individual characteristics for the simulated cohort in each of the pre-specified subgroup analyses
| Characteristic | Full cohort | Baseline HbA1c ≥ 58 mmol/mol | 69 mmol/mol > baseline HbA1c ≥ 58 mmol/mol | 80 mmol/mol > baseline HbA1c ≥ 69 mmol/mol | Baseline HbA1c ≥ 80 mmol/mol | Baseline HbA1c < 69 mmol/mol | Baseline HbA1c ≥ 69 mmol/mol | Per-protocol population |
|---|---|---|---|---|---|---|---|---|
| Baseline HbA1c, mmol/mol (SD) | 76.1 (18.8) | 78.5 (17.0) | 63.3 (3.0) | 73.3 (3.1) | 96.6 (16.3) | 60.3 (6.8) | 85.9 (17.0) | 75.5 (17.5) |
| Age, years (SD) | 40.3 (13.3) | 40.4 (13.1) | 43.0 (12.3) | 40.6 (13.2) | 37.7 (13.0) | 42.6 (13.2) | 39.5 (13.2) | 40.6 (13.0) |
| Diabetes duration, years (SD) | 18.0 (12.5) | 17.9 (11.8) | 19.3 (12.2) | 19.0 (11.7) | 16.1 (11.4) | 19.3 (13.9) | 17.4 (11.7) | 18.3 (12.5) |
| Triglycerides, mmol/mol (SD) | 1.3 (1.0) | 1.4 (1.0) | 1.3 (0.9) | 1.2 (0.6) | 1.6 (1.3) | 1.3 (0.9) | 1.4 (1.1) | 1.3 (0.8) |
| TC, mmol/mol (SD) | 4.9 (0.9) | 5.0 (0.9) | 4.8 (0.9) | 4.8 (0.8) | 5.3 (1.0) | 4.8 (0.9) | 5.1 (1.0) | 4.9 (0.9) |
| HDL cholesterol, mmol/mol (SD) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.6 (0.5) | 1.5 (0.4) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) |
| LDL cholesterol, mmol/mol (SD) | 2.8 (0.9) | 2.8 (0.9) | 2.7 (0.9) | 2.7 (0.8) | 3.0 (0.9) | 2.6 (0.8) | 2.9 (0.9) | 2.8 (0.9) |
| Systolic blood pressure, mmHg (SD) | 131.3 (16.3) | 131.6 (15.9) | 133.7 (18.1) | 130.6 (13.6) | 130.3 (16.1) | 132 (17.7) | 130.7 (15.0) | 131.4 (15.9) |
| Characteristic | Full cohort, % | Baseline HbA1c ≥ 58 mmol/mol, % | 69 mmol/mol > baseline HbA1c ≥ 58 mmol/mol, % | 80 mmol/mol > baseline HbA1c ≥ 69 mmol/mol, % | Baseline HbA1c ≥ 80 mmol/mol, % | Baseline HbA1c < 69 mmol/mol, % | Baseline HbA1c ≥ 69 mmol/mol, % | Per-protocol population, % |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 41.0 | 41.0 | 34.8 | 38.1 | 47.0 | 36.2 | 43.0 | 40.5 |
| Male | 59.0 | 59.0 | 65.2 | 61.9 | 53.0 | 63.8 | 57.0 | 59.5 |
| Physical activity | ||||||||
| Low | 24.9 | 28.0 | 17.0 | 28.7 | 34.9 | 17.1 | 31.6 | 25.6 |
| Medium | 48.8 | 48.9 | 53.7 | 54.8 | 39.7 | 28.9 | 47.3 | 50.4 |
| High | 26.4 | 23.1 | 29.3 | 16.5 | 25.4 | 56.4 | 21.1 | 24.1 |
| Smoking status | ||||||||
| Current | 19.2 | 20.6 | 16.0 | 17.3 | 27.2 | 14.7 | 23.7 | 20.0 |
| Former | 26.3 | 26.4 | 28.9 | 22.9 | 24.6 | 28.9 | 24.9 | 26.4 |
| Never | 54.5 | 53.1 | 55.0 | 59.8 | 48.2 | 56.4 | 51.4 | 53.5 |
| Ethnicity | ||||||||
| White | 99.1 | 99.3 | 98.5 | 100 | 99.2 | 98.9 | 43.0 | 99.3 |
| Black | 0.9 | 0.7 | 1.5 | 0 | 0.8 | 1.1 | 57.0 | 0.7 |
| Nephropathy | ||||||||
| No complications | 92.2 | 92.0 | 88.7 | 94.7 | 92.4 | 88.7 | 94.2 | 91.5 |
| Microalbuminuria | 4.7 | 4.7 | 6.6 | 3.8 | 3.6 | 7.1 | 3.5 | 5.0 |
| Macroalbuminuria | 2.7 | 2.9 | 3.3 | 1.5 | 4.0 | 3.0 | 2.3 | 2.9 |
| Dialysis or transplant | 0.4 | 0.4 | 1.3 | 0 | 0 | 1.2 | 0 | 0.6 |
| Neuropathy | ||||||||
| No complications | 90.7 | 91.9 | 94.6 | 97.2 | 85.3 | 92.2 | 90.5 | 91.8 |
| Neuropathy or foot ulcers | 9.3 | 8.1 | 5.4 | 2.8 | 14.7 | 7.8 | 9.5 | 8.2 |
| Retinopathy | ||||||||
| No complications | 56.0 | 54.6 | 50.3 | 52.7 | 59.6 | 54.0 | 56.7 | 54.8 |
| Background diabetic retinopathy | 34.8 | 35.6 | 40.5 | 36.7 | 28.8 | 38.0 | 32.9 | 35.6 |
| Proliferative diabetic retinopathy | 9.3 | 9.8 | 9.2 | 8.6 | 11.5 | 8.0 | 10.4 | 9.6 |
| MI | ||||||||
| No complications | 97.8 | 97.8 | 98.6 | 95.8 | 98.6 | 98.9 | 97.0 | 98.0 |
| MI | 2.2 | 2.2 | 1.4 | 4.2 | 1.4 | 1.1 | 3.0 | 2.0 |
| Stroke | ||||||||
| No complications | 99.7 | 99.6 | 100 | 100 | 99.0 | 100 | 99.3 | 99.5 |
| Stroke | 0.3 | 0.4 | 0 | 0 | 1.0 | 0 | 0.7 | 0.5 |
| HF | ||||||||
| No complications | 99.4 | 99.6 | 99.0 | 100 | 100 | 99.2 | 100 | 99.5 |
| HF | 0.6 | 0.4 | 1.0 | 0 | 0 | 0.8 | 0 | 0.5 |
| Angina | ||||||||
| No complications | 98.8 | 99.0 | 98.6 | 98.5 | 100 | 97.8 | 99.2 | 98.8 |
| Revascularised | 1.2 | 1.0 | 1.4 | 1.5 | 0 | 2.2 | 0.8 | 1.2 |
Appendix 8 Participant Information Sheets
Appendix 9 Qualitative substudy topic guides
Qualitative substudy – topic guide for patient interviews at baseline.
Appendix 10 Summary of amendments
| Amendment number and type | Date submitted | Summary of amendment | Documents changed | Date approved |
|---|---|---|---|---|
| Substantial amendment 1 | 13 June 2011 | To allow ethical review of the consent forms, patient information sheets and interview topic guides for the qualitative substudy/component of the REPOSE Trial | None | 20 June 2011 |
| Substantial amendment 2 | 6 July 2011 | Protocol:
|
Protocol to v3, 28 June 2011 | 20 July 2011 |
Patient information sheet:
|
Patient information sheet to v3, 28 June 2011 | |||
REPOSE leaflet:
|
REPOSE leaflet, to v3, 28 June 2011; participant consent form, to v3, 28 June 2011; baseline hypoglycaemic recall forms: 1 × severe, 2 × moderate, to v2, 28 June 2011; follow-up hypoglycaemic recall forms: 1 × severe, 2 × moderate, to v2, 28 June 2011 | |||
CTA:
|
CTA | |||
No changes made to the following documents, but omitted from original application to ethics:
|
||||
| Substantial amendment 3 | 8 August 2011 | To allow ethical review of a consent form, patient information sheet and interview topic guide for interviews undertaken with two to three participants who were involved in a pump pilot study (a smaller-scale version of the REPOSE Trial) | No documents changed but the following documents were reviewed:
|
22 August 11 |
| The aim was to create a short video clip to show to potential participants for the REPOSE Trial at local information meetings for the trial | ||||
| The aim of the video clips is to give potential participants an understanding of what it is like to take part in a clinical trial and be on pump therapy to control their diabetes, from the perspective of someone who has taken part in a similar trial (i.e. the pump pilot) | ||||
| In addition, the video clip is introduced and ended by short foreword and ending by the chief investigator | ||||
| Substantial amendment 4 | 6 September 2011 | 1. To add and remove centres:
|
N/A | 7 September 2011 |
| 2. Notification that Harrogate and District NHS Foundation Trust (PI: Dr Peter Hammond) are delivering part of the trial intervention using a venue that is not owned by Harrogate and District NHS Foundation Trust: Henshaws Society for Blind People, Bogs Lane, Harrogate, North Yorkshire, HG1 4ED | ||||
| Substantial amendment 5 | 12 September 2011 | Protocol:
|
|
NRES, 19 September 2011 |
| Participant consent form |
|
|||
| REPOSE participant information sheet |
|
|||
| SAE contact card |
|
|||
| Psychosocial questionnaire |
|
|||
| Follow-up instructions for filling in your diary |
|
|||
Minor amendments included for notification:
|
|
|||
New documents:
|
|
|||
| CTA |
|
|||
| Minor amendment 1 | 24 October 2011 | 1. Reformatting or minor changes to the following documents: | NRES approval: N/A; notified in substantial amendment 6 | |
| REPOSE leaflet, v3, 7 October 2011 (previously v2, 28 June 2011) | REPOSE leaflet, v3, 7 October 2011 | |||
| REPOSE poster, v2, 7 October 2011 (previously v1, 7 January 2011) | REPOSE poster, v2, 7 October 2011 | |||
| SAE contact card, v3, 7 October 2011 | SAE contact card, v3, 7 October 2011 | |||
| 2. Name changes of centres: The names of some centres on the CTA are listed slightly incorrectly (e.g. Cambridge centre is listed as Cambridge University rather than Cambridge University Hospitals NHS Foundation Trust) |
||||
| Minor amendment 2 | 3 November 2011 | Consent, v6, 3 November 2011: revised so the participant ID is now the participant’s DAFNE number | Consent, v6, 3 November 2011 | NRES approval: N/A; notified in substantial amendment 6 |
| Minor amendment 3 | 9 January 2012 | REPOSE invite letter, v3, 6 January 2012: addition of an optional sentence to inform potential participants the date of local recruitment evenings/afternoons. | REPOSE invitation letter, v3, 6 January 2012 | NRES approval: N/A; notified in substantial amendment 6 |
| Minor amendment 4 | 12 January 2012 | REPOSE GP letters – MDI and pump: minor amendments so the trial name listed is REPOSE not the pump pilot study | REPOSE GP letter: MDI, v2, 9 January 2012 | NRES approval: N/A; notified in substantial amendment 6 |
| REPOSE GP letter: Pump, v2, 9 January 2012 | ||||
| Minor amendment 5 | 18 January 2012 | Agreed in risk assessment meeting with Sponsor on 9 January 2012 – amendments to the REPOSE protocol:
|
NRES approval: N/A; notified in substantial amendment 6 | |
| Substantial amendment 6 | 17 January 2012 |
|
Psychosocial questionnaire, v3, 16 January 2012; protocol, v5, 4 January 2012 | 6 February 2012 |
| Notification of minor amendments 1–5 | ||||
| Minor amendment 6 | 18 January 2012 | The lost to follow-up definition (p. 33) has been amended to clarify that lost to follow-up participants are those who fail to attend more than two follow-up visits, including the 24-month follow-up appointment (previous definition was participants who failed to attend more than two follow-up visits) | REPOSE protocol, v6, 16 January 2012 | NRES approval: N/A; notified in substantial amendment 7 |
| Substantial amendment 7 | 17 January 2012 | 1. Blinded review of HbA1c (measure of the level of blood glucose control): To allow the trial statistician to conduct a blinded review after course 2, 4 and 5 to examine the proportions of recruited participants who are in each HbA1c category (i.e. ≥ 7.5% or < 7.5%). The trial statistician will look at the proportions in each HbA1c category, and numbers of participants with a HbA1c of ≥ 7.5% threatens the ability of the trial to detect a difference in primary outcome (i.e. there are substantially more subjects recruited with a HbA1c of < 7.5% than anticipated), then an additional inclusion criteria will be added to limit recruitment only to participants with a HbA1c of ≥ 7.5% in order to ensure that the trial can detect a difference in the primary outcome |
REPOSE protocol, v6, 16 January 2012 | 6 February 2012 |
| 2. Withdrawal from the pump criteria: Removal of ‘Participant becomes pregnant’ as a reason for withdrawal from the pump. Amended so that the decision whether or not a participant who becomes pregnant during the trial stays on the pump is purely a clinical decision based on the participant’s blood glucose control on the pump (i.e. if the participant was managing their diabetes well on the pump, they remain on the pump) |
||||
| 3. Consent process: Amended to allow the witnessing of the consent by the educator can take place when the consent form (signed by the participant) is received in the post (instead of at the baseline appointment) |
||||
| Notification of minor amendment 6 | ||||
| Substantial amendment 8 | 23 January 2012 | Amendment to the psychosocial questionnaire so that the HFS and DSQOL are exact copies of the validated versions | Psychosocial questionnaire, v4, 20 January 2012 | 6 February 2012 |
| Minor amendment 7 | 7 February 2012 |
|
Qualitative substudy consent form, v3, 7 February 2012 | Submitted with substantial amendment 9 to REC |
| (Please note that no other versions of the pump diary have been used. The v2 pump diary reflects internal editing at DAFNE, who modified the MDI diary) | ||||
| Minor amendment 8 | 13 April 2012 | Clarification of the exclusion criteria of having used pump therapy in the last 3 years | REPOSE protocol, v7, 3 April 2012 | Submitted with substantial amendment 9 to REC |
| Clarified that this must be ‘significant use’, which is defined as no more than 2 weeks use of the pump in the last 3 years | ||||
| Discussed and agreed this definition with the TMG today | ||||
| Substantial amendment 9 | 16 April 2012 | Creation of a new participant letter to be sent with the follow-up psychosocial questionnaire | Psychosocial questionnaire letter, v1, 11 April 2012 | 1 May 2012 |
| Addition of Nottingham as a research centre | ||||
| Notification to REC of minor amendments 7 and 8 | ||||
| Minor amendment 9 | 24 May 2012 | Change of sponsor/lead NHS R&D details from Jim Lithgow to Erica Wallis | None | Ethics approved on 24 May 2012 |
| Substantial amendment 10 | 29 May 2012 | Change of PI at KCH centre from Professor Stephanie Amiel to Dr Pratik Choudary | None | 27 June 2012 |
| Minor amendment 10 | 12 June 2012 | Increased number of centres where qualitative research will take place from 3–4 to 7 | REPOSE protocol, v7.1, 12 June 2012 | Submitted with substantial amendment 10 to REC |
| Altered time the educators find out about treatment allocation from 6 weeks to 4–6 weeks | ||||
| Clarified inclusion criteria regarding having a 12-month history of diabetes: participants must have had a 12-month history of diabetes by the time of baseline/DAFNE course | ||||
| Minor amendment 11 | 6 July 2012 | Clarification that the review of baseline HbA1c is not blinded, as it does not need to be | REPOSE protocol, v7.2, 9 July 2012 | Submitted with substantial amendment 11 to REC |
| Clarification of severe needle phobia exclusion criteria: clarification that the severity of phobia assessed considering if the phobia might preclude full participation in either treatment arm or influence the participant’s preference for pump therapy | ||||
| Clarification of unstable psychological problems: clarification that such conditions are active enough to preclude the participant safely taking part in the trial (based on investigatory judgement) | ||||
| KCH course 3 and 4: change of venue for DAFNE course, Springfield Medical Centre | ||||
| Substantial amendment 11 | 7 August 2012 | Creation of a participant newsletter to be issued just before each of the scheduled follow-up appointments, (i.e. 6, 12 and 24 months post baseline) | 6-month follow-up participant newsletter, v1, 24 July 2012 | Ethics approval: 21 August 2012 |
| Substantial amendment 12 | 24 August 2012 | To increase the number recruited to the study. Dropouts are occurring prior to DAFNE course attendance and thus these participants do not count towards the ITT. This change does not increase the number of participants who will receive the intervention or comparator treatment. | REPOSE protocol, v8, 20 August 2012 | Ethics approval: 12 September 2012 |
| Minor amendment 12 | 24 August 2012 | Psychosocial questionnaire, v4.1, 28 June 2012:
|
Psychosocial questionnaire, v4.1, 28 June 2012 | Submitted with substantial amendment number 12 to REC |
REPOSE protocol v8:
|
REPOSE protocol, v8, 20 August 2012 | |||
| Minor amendment 13 | 12 November 2012 | To amend the patient information sheet to include the new research centre (Nottingham); the consent form references the patient information sheet and it is therefore necessary to amend this | Patient information sheet, v5.1, 4 September 2012 | Submitted with substantial amendment 13 to REC |
| Informed consent form, v6.1, 4 September 2012 | ||||
| Substantial amendment 13 | 9 November 2012 | Change of PI at KCH centre, back to Professor Stephanie Amiel from Dr Pratik Choudhary | None | Ethics approval: 12 November 2012 |
| Substantial amendment 14 | 12 January 2013 | Creation of a participant newsletter to be issued just before the 12-month follow-up appointment | 12-month follow-up participant newsletter, v1, 7 January 2013 | Ethics approval: 13 February 2013 |
| Substantial amendment 15 | 20 May 2013 | REPOSE protocol v9:
|
REPOSE protocol, v9, 9 May 2013 | Ethics approval: 10 June 2013 |
| Substantial amendment 16 | 26 July 2013 | Creation of a letter and supporting documentation to send to all REPOSE participants reminding them of how to deal with illness and other problems that may occur | REPOSE Ketone Management Reminder 2013 – CSII – v1, 28 June 2013 | Ethics approval: 5 August 2013 |
| REPOSE Ketone Management Reminder 2013 – MDI – v1, 28 June 2013 | ||||
| REPOSE Ketone Management Reminder Letter – CSII v1, 28 June 2013 | ||||
| REPOSE Ketone Management Reminder Letter – MDI v1, 28 June 2013 | ||||
| Substantial amendment 17 | 23 September 2013 | Creation of a participant newsletter to be issued 18 months post course | 18-month follow-up participant newsletter, v1, 15 August 2013 | Ethics approval: 3 October 2013 |
| Substantial amendment 18 | 24 December 2013 | Three additional questionnaires to be added to the psychosocial questionnaire pack at the 24-month time point only:
|
DAFNE principles questionnaire, v1, 12 December 2013 | Ethics approval: 3 February 2014 |
| Use of bolus calculators questionnaire, v1, 12 December 2013 | ||||
| Pump use questionnaire, v1, 12 December 2013 | ||||
Creation of a new participant letter to be sent with the follow-up psychosocial questionnaire prior to the 24-month follow-up appointment incorporating information regarding:
|
24-month psychosocial questionnaire letter, v2, 22 January 2014 | |||
REPOSE protocol v10:
|
REPOSE protocol, v10, 11 December 2013 | |||
| Substantial amendment 19 | 24 December 2013 | Creation of a participant newsletter to be issued 24 months post course | 24-month follow-up participant newsletter, v1, 3 December 2013 | Ethics approval: 13 January 2014 |
| Creation of a letter to send to all REPOSE participants reminding them of how to use their bolus calculator | Bolus Calculator Intervention Letter, v1, 11 December 2013 | |||
| Substantial amendment 20 | 5 March 2014 | REPOSE Protocol v11.1:
|
REPOSE protocol, v11.1, 31 March 2014 | Ethics approval: 4 April 2014 |
| Minor amendment 14 | 23 March 2015 | REPOSE protocol v11.2:
|
REPOSE protocol, v11.2, 24 March 2015 | Acknowledgement received from REC: 8 April 2015 |
Appendix 11 Diabetic ketoacidosis/illness letter and troubleshooting documents issued to participants
Appendix 12 Twenty-four month letter incorporating information about severe hypoglycaemia reporting
Appendix 13 Bolus calculator letter issued to participants
Appendix 14 Results of the Gompertz, log-logistic and log-normal parametric survival models used to predict treatment switching
| Parameter | Coefficient | Robust SE | 95% CI |
|---|---|---|---|
| Gompertz model | |||
| HbA1c | 0.220 | 0.236 | –0.243 to 0.684 |
| Number of DKAs | –0.983 | 0.468 | –1.901 to –0.065 |
| Number of severe hypoglycaemic events | 0.407 | 0.090 | 0.230 to 0.584 |
| Constant | –4.307 | 2.232 | –8.682 to 0.068 |
| Gamma parameter | –0.316 | 0.479 | –1.256 to 0.624 |
| Log-logistic model | |||
| HbA1c | –0.294 | 0.286 | –0.855 to 0.267 |
| Number of DKAs | 1.406 | 0.676 | 0.081 to 2.730 |
| Number of severe hypoglycaemic events | –0.554 | 0.170 | –0.887 to –0.220 |
| Constant | 5.637 | 2.510 | 0.718 to 10.557 |
| ln gamma parameter | 0.215 | 0.230 | –0.235 to 0.665 |
| Log-normal model | |||
| HbA1c | –0.307 | 0.292 | –0.879 to 0.264 |
| Number of DKAs | 1.867 | 0.755 | 0.387 to 3.347 |
| Number of severe hypoglycaemic events | –0.656 | 0.180 | –1.009 to –0.304 |
| Constant | 6.406 | 2.520 | 1.466 to 11.346 |
| ln sigma parameter | 1.002 | 0.206 | 0.598 to 1.405 |
| Parameter | Coefficient | Robust SE | 95% CI |
|---|---|---|---|
| Gompertz model | |||
| HbA1c | 0.350 | 0.170 | 0.016 to 0.683 |
| Number of DKAs | –6.009 | 0.562 | –7.110 to –4.908 |
| Number of severe hypoglycaemic events | 0.512 | 0.094 | 0.329 to 0.696 |
| Constant | –8.080 | 1.471 | –10.963 to –5.197 |
| Gamma parameter | 1.055 | 0.669 | –0.256 to 2.366 |
| Log-logistic model | |||
| HbA1c | –0.181 | 0.121 | –0.418 to 0.055 |
| Number of DKAs | 2.609 | 0.799 | 1.044 to 4.175 |
| Number of severe hypoglycaemic events | –0.232 | 0.070 | –0.368 to –0.095 |
| Constant | 3.676 | 1.317 | 1.094 to 6.258 |
| ln gamma parameter | –0.780 | 0.317 | –1.401 to –0.160 |
| Log-normal model | |||
| HbA1c | –0.190 | 0.107 | –0.400 to 0.021 |
| Number of DKAs | 1.617 | 0.517 | 0.603 to 2.630 |
| Number of severe hypoglycaemic events | –0.283 | 0.101 | –0.481 to –0.086 |
| Constant | 4.117 | 1.291 | 1.587 to 6.647 |
| ln sigma parameter | 0.066 | 0.338 | –0.596 to 0.728 |
Appendix 15 End of trial for pump participants: standard operating procedure
Appendix 16 Closeout qualitative substudy: staff topic guide
Demographic
-
Role/occupation.
-
Years of diabetes/DAFNE experience.
-
Years of experience of working with insulin pumps prior to the trial.
Clinical use of pumps in site
-
How are pumps usually funded in your centre?
-
What clinical and other criteria are used to determine who is referred for a pump?
-
Prompt NICE/SIGN: how are NICE guidelines on pumps interpreted at your centre/by you? Why interpreted in this way?
-
REPOSE
-
Tell me about your work on REPOSE – how were you involved (recruitment, training, delivery, contact with patients during the trial, closeout)?
-
Was recruitment difficult in your centre? How did it end up regarding pumps and MDI?
-
-
Why do you think the patients recruited from your centre agreed to take part in the trial? (Probe patients’ preferences: what did you think about this and how did you manage this?)
-
Do you think more patients really wanted pumps even if they did not’ fess up at the time?
-
-
What do you think about the inclusion/exclusion criteria used in REPOSE and the fact that the trial’s criteria were different to NICE criteria?
-
What do you think about these differences? How did these make you feel about dealing with these patients?
-
Any problems and difficulties encountered during the trial (e.g. patient complaints, withdrawals)? How did you address these?
-
Impact of trial on clinical practice? CLOSEOUT
-
What do you think about the information given at recruitment about closeout (probe around information about potential withdrawal of pump at end of trial)?
-
How did patients recruited react to this at the time?
-
What expectations did you have about closeout? WHY?
-
What did you think closeout would be like? WHY?
-
What problems or challenges did you think might arise at closeout? WHY? When did these change?
-
What expectations did patients in your site have about closeout do you think?
-
So what happened in the end? Can you talk me through your experiences of closeout?
-
What happened in your site?
-
Did you have a SOP/protocol for closeout? How did this differ from trial protocol? Why?
-
What decisions were made about whether to continue or discontinue pump treatment in your site? To what extent were NICE guidelines used/followed?
-
How were they made? Who made them? Why?
-
-
Were these decisions about closeout made in general or on a case to case basis – WHY?
-
How did patients react to closeout decisions?
-
How did you manage their reactions?
-
-
In hindsight, what would you have done differently at closeout? Why?
General topics
-
What did you think of the trial? (Design, rationale.)
-
Do you think the trial will work? Why?
-
How did people do in the MDI arm?
-
Do you think there will be a difference between pump and MDI arms?
-
If you think there are benefits in clinical outcomes, why do you think they have occurred?
Generic experiences and needs
-
What information and support did you receive when closing out REPOSE? Where/from whom did it come? (CTU, local PI.)
-
What unmet needs for support did you have and how could these be addressed in future trials? (Prompt debriefing – pre-empting? Who should provide?)
-
Experiences of working on and closing out other trials before or since to RESPOSE? Issues or problems that have arisen on the closeout of earlier trials. How did REPOSE differ and why?
-
Anything missed out?
-
Anything to add?
Appendix 17 Closeout qualitative substudy: participants
| Variable | n | % |
|---|---|---|
| REPOSE centres | ||
| Number of centres | 7 | |
| Interviewees per centre range | 1–5 | |
| Interviewees per centre mode | 3 | |
| Role | ||
| Diabetes consultants | 7 | 33 |
| DAFNE educators | 14 | 66 |
| Diabetes specialist nurses-to-dietitians | 8 : 6 | 38 : 29 |
| DAFNE experience, years | ||
| 5–10 | 14 | 66 |
| 10–15 | 4 | 21 |
| > 15 | 3 | 14 |
| Pump therapy experience, years | ||
| < 5 | 5 | 24 |
| 5–10 | 9 | 42 |
| 10–15 | 5 | 24 |
| > 15 | 2 | 10 |
List of abbreviations
- ACR
- albumin-to-creatinine ratio
- AE
- adverse event
- AIC
- Akaike information criterion
- A-level
- Advanced level
- BIC
- Bayesian information criterion
- BMI
- body mass index
- CGM
- continuous glucose monitoring
- CI
- confidence interval
- CONSORT
- Consolidated Standards Of Reporting Trials
- CP
- carbohydrate portion
- CSII
- continuous subcutaneous insulin infusion
- CTRU
- Clinical Trials Research Unit
- DAFNE
- Dose Adjustment For Normal Eating
- DCCT
- Diabetes Control and Complications Trial
- DCF
- data collection form
- DEP
- DAFNE Educator Programme
- DH
- Department of Health
- DKA
- diabetic ketoacidosis
- DMEC
- Data Monitoring and Ethics Committee
- DQOL
- Diabetes Quality of Life (questionnaire)
- DRC
- diabetes-related contact
- DSQOL
- diabetes-specific quality of life (scale)
- DTSQ
- Diabetes Treatment Satisfaction Questionnaire
- DTSQc
- Diabetes Treatment Satisfaction Questionnaire (change)
- EEACT
- Economic Evaluation alongside Clinical Trials
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, 3-level version
- ESRD
- end-stage renal disease
- FA
- fidelity assessor
- FT
- fidelity testing
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HF
- heart failure
- HFS
- Hypoglycaemia Fear Survey
- HTA
- Health Technology Assessment
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LDL
- low-density lipoprotein
- LGS
- low glucose suspend
- MD
- mean difference
- MDI
- multiple daily injection
- MDT
- multidisciplinary team
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MI
- myocardial infarction
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPH
- neutral protamine Hagedorn
- OR
- odds ratio
- PAD
- peripheral arterial disease
- PGfAR
- Programme Grants for Applied Research
- PI
- principal investigator
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMBG
- self-monitoring of blood glucose
- SOP
- standard operating procedure
- T1DM
- type 1 diabetes mellitus
- TC
- total cholesterol
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WHOQOL-BREF
- World Health Organization Quality of Life Abbreviated Questionnaire