Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/77/01. The contractual start date was in December 2011. The draft report began editorial review in May 2016 and was accepted for publication in October 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Catherine E Hewitt declares membership of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) commissioning board, and Sarah E Lamb declares membership of the NIHR HTA prioritisation board. Lorraine Green reports that she works as an independent private practitioner and is an associate of Mr Andrew Horwood, design consultant at Healthystep Ltd. Robin Hull reports that his employers, North Yorkshire and York Primary Care Trust (now Harrogate and District NHS Foundation Trust), received payment for clinical assessment of REducing Falls with ORthoses and a Multifaceted podiatry intervention (REFORM) patients from the REFORM HTA grant.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Cockayne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Burden of falls and falling in the UK
Falls and fall-related fractures are a serious cause of morbidity and cost to individuals and society. 1 This burden is likely to increase owing to an ageing population. Falls are associated with a loss of independence and functional decline, and may result in the need for long-term care. 2 Each year, approximately 30% of people aged ≥ 65 years living in the community will have a fall, and among those aged ≥ 80 years this increases to 50%. 3,4 Older adults who fall once are two to three times more likely to fall again within 1 year. One-fifth of all falls require medical attention, with 5% of falls leading to a fracture. 5 The financial cost of injurious falls has been estimated at £2B per annum, a cost that is mainly attributed to resultant hip fractures. 6 The National Service Framework for Older People highlighted the importance of fall-related injuries and called for health improvement plans to reduce this burden. 7
Risk factors for falling
It is well recognised that falls occur for a variety of reasons. They may result from interactions between environmental hazards, medical conditions and physiological risk factors. 3 Foot problems, which affect one in three community-dwelling people aged ≥ 65 years,8 have been associated with reduced walking speed and difficulty in performing activities of daily living. Results from cohort studies have indicated that there is a relationship between foot and ankle problems and risk of falling. 9,10
In addition to causing foot problems, inappropriate footwear may contribute to poor balance and an increased risk of falling. 11 Footwear characteristics that are considered detrimental to balance include higher heels, soft soles and inadequate slip resistance. 11,12 Prospective studies have shown that walking barefoot, wearing only stockings inside the home and wearing shoes with an increased heel height and smaller contact area all increase the risk of falling. 9,13,14
Podiatry interventions to improve balance
Given the emerging evidence that foot problems and inappropriate footwear increase the risk of falling, it has been suggested that podiatry may have a role to play in falls prevention, with several guidelines recommending that older people have their feet and footwear examined by a podiatrist. 15,16 Previous studies have looked at treatments that may improve balance in older adults, such as lesion debridement,17 foot orthoses,18 foot and ankle exercises19,20 and footwear advice. Lesion debridement can improve function during gait if pain is reduced, exercise programmes focus on internal strengthening and flexibility, and appropriate footwear fitted with orthotic devices can provide external support, improved kinaesthesia and improved function. Combining these therapies could, therefore, improve function and stability.
At the time of designing the current study there were two published Cochrane reviews on falls prevention. One related to falls in community-dwelling older people21 and one focused on falls in hospitals and aged care facilities. 22 Neither identified any randomised controlled trials (RCTs) focusing on podiatry-related interventions. A subsequent update identified one Australian trial of a podiatry-based intervention for the prevention of falls. 23 In this study of 305 community-dwelling older people who had foot pain, participants allocated to receive a multifaceted podiatry intervention (n = 153) experienced 36% fewer falls than participants in the control group [incidence rate ratio (IRR) 0.64, 95% confidence interval (CI) 0.45 to 0.91; p = 0.01]. The intervention comprised foot and ankle exercises, foot orthoses, footwear advice, subsidy for new footwear and a falls prevention booklet combined with routine podiatry care and was compared with those receiving only routine podiatry. This trial did not include an economic evaluation.
Aims and objectives of the podiatry intervention for podiatry patients at increased risk of falling
The REducing Falls with ORthoses and a Multifaceted podiatry intervention (REFORM) study was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme in response to a call to evaluate the clinical effectiveness and cost-effectiveness of foot orthoses. Its aim was to establish the clinical effectiveness and cost-effectiveness of a package of podiatric care within a UK health-care setting.
The main objectives of the REFORM study were to:
-
investigate the clinical effectiveness of a multifaceted podiatry intervention for falls prevention
-
investigate the cost-effectiveness of a multifaceted podiatry intervention for falls prevention
-
assess the participants’ and podiatrists’ views and experiences of the intervention and trial processes.
Chapter 2 Methods
Trial design
The REFORM study was a pragmatic multicentred cohort RCT. 24 We chose this approach to test whether or not the cohort RCT design could address some of the issues that other trial designs encounter with regard to recruitment, attrition and participant preference. We expected that using this design would offer the following advantages. First, trial recruitment rates would be enhanced. Some participants would be immediately eligible for the study and could be randomised straight away and others who subsequently fell over time would become eligible and could also then be randomised. If a traditional trial design had been used, then these additional participants who were not immediately eligible would have been lost. In addition, as we were undertaking an internal pilot, this could be undertaken while recruitment proceeded for the main study. Second, we expected that this design would minimise the possibility of introducing attrition and reporting bias. As participants were receiving routine podiatry care outside the study, the only incentive to take part in the study, apart from altruistic reasons, was the possibility of receiving the intervention. Under this design, all participants were informed upon enrolment into the cohort that they may at some point be offered a package of podiatry care. This was offered to participants subsequently randomised into the intervention group of the RCT; however, the usual-care group were not explicitly notified of their group allocation as they would have been in the classic randomised design. We expected that this would reduce attrition caused by ‘resentful demoralisation’ and minimise the risk of participants in the usual-care group either knowingly or unknowingly biasing the trial by reporting the number of falls they had experienced less conscientiously than those allocated to the intervention group. Third, we also expected that that the inclusion of a ‘run-in’ period of falls data collection before randomisation would reduce post-randomisation attrition rates and, therefore, the risk of selection bias. Participants had to demonstrate engagement with the study by returning at least one falls calendar before they were randomised, which enriched the sample with those participants most likely to keep responding.
The cohort RCT design allowed us to test the feasibility of this design and determine whether or not it would enhance recruitment, minimise attrition and lower participant preference effects. It also enabled us to establish a cohort of older adults who could be followed up, thereby helping to inform the knowledge base around health and well-being in older adults. This approach also allowed the possibility for us to invite participants, who had agreed to be contacted again, to take part in future studies.
Participants in the REFORM trial were randomised to receive one of either:
-
a multifaceted intervention consisting of footwear advice (and footwear provision if required), an orthotic insole or review of an existing insole prescription, a programme of foot and ankle balance exercises and a falls prevention leaflet
-
a falls prevention leaflet and usual care from their podiatrist and general practitioner (GP).
Approvals obtained
The study protocol was approved by the East of England – Cambridge East Research Ethics Committee (REC) (multicentre REC) (and substantial amendments) on 9 November 2011 (REC reference number 11/EE/0379). Galway REC approved the study (and substantial amendments) on 26 April 2013 (REC reference number C.A 886). The University of York, Department of Health Sciences Research Governance Committee approved the study (and substantial amendments) on 2 August 2011. Research management and governance approval was obtained for each trust thereafter (see Appendix 1).
The trial was registered as ISRCTN68240461 on 1 July 2011.
Study sites
Recruitment of all participants into the study took place through 37 NHS podiatry clinics based in either primary or secondary care in nine NHS trusts across the UK, and at one international site in a university school of podiatry in Ireland. Each participating podiatry clinic was associated with the trust under which it operates, and each trust acted as a trial ‘centre’, except the Harrogate and District NHS Foundation Trust, which cares for the population of North Yorkshire. As this is a particularly large and diverse area, the clinics in this trust were split into four groups according to their geographical location: Scarborough, York, Harrogate and Skipton. These four groups were also considered as trial ‘centres’, resulting in a total of 13. The Ireland centre was set up to aid study recruitment. This site was chosen because some of the authors had previously collaborated with this site on another NIHR HTA-funded podiatry study in which recruitment had gone well. The NIHR HTA programme gave permission to include the site.
REFORM observational cohort
The REFORM study was initially designed to include people aged ≥ 70 years; however, following the pilot phase, the age limit was reduced to include adults aged ≥ 65 years (see Chapter 3) to facilitate recruitment and reflect the age range seen within the routine podiatry clinics. Participants were first recruited to the REFORM observational cohort. While this cohort was being assembled, we invited a selection of eligible participants from the pilot sites to take part in the internal REFORM pilot trial. After completion of the pilot phase, the remaining eligible participants were invited to take part in the REFORM trial. Figure 1 reports how participants were recruited to the observational cohort and when they were randomised to the trial.
FIGURE 1.
Recruitment of participants to the REFORM study.

Participant recruitment
Recruitment of all participants into the study took place through NHS podiatry clinics based in either primary or secondary care in the UK and at one international site in a university school of podiatry in Ireland. The reasoning for recruiting only from podiatry clinics and not from general practices was because of the requirement for all participants to be receiving routine podiatry care so that we could disentangle the effects of the novel intervention from those of routine podiatry care. Recruitment directly from general practices would most probably have identified many patients who were not receiving routine podiatry care. For these patients to have been entered into the REFORM study, they would have to have been receiving routine podiatry care. NHS podiatry service managers informed us that the burden of providing routine podiatry care for all trial participants as well as delivering the intervention would have made the study unfeasible.
Potential participants were identified by either the REFORM research podiatrist or a podiatrist within the clinic undertaking a search of either electronic or paper medical records of patients registered with the service. Two search criteria were used: (1) age ≥ 65 years and (2) having attended routine podiatry services within the past 6 months from the date of the search. People living in nursing homes were excluded, as participants had to be community dwelling to be eligible for the study. At the time of undertaking the search, it was not possible to easily identify those patients with neuropathy who would be ineligible for the study. Therefore, to minimise the risk of approaching these patients, those who had attended high-risk clinics, for example diabetes mellitus clinics, were excluded from the search. Potential participants were invited to participate in the REFORM study by their podiatry clinic via a postal recruitment pack. This pack comprised an invitation letter (see Appendix 2) electronically signed by the principal investigator (PI) at the site, a consent form (see Appendix 3), a participant information sheet (see Appendix 4), a background information form (see Appendix 5) and a prepaid return envelope addressed to the York Trials Unit (YTU). During the pilot phase of the study, a decline form (see Appendix 6) was also included so that data could be collected on people’s reasons for declining to participate. No identifiable data were available to the study teams until a participant had returned their consent and background information forms.
To aid recruitment, the opportunistic screening of patients attending routine podiatry clinics was undertaken when clinics had capacity to do so. Potential participants were given the recruitment pack and verbal information about the study.
Potential participants who wished to take part in the REFORM study returned their completed consent and background information forms by post to the YTU. The research team assessed the forms for eligibility.
Consenting participants
Participation in the REFORM study was voluntary. Participants who wished to take part were given written information about the study and contact details for the research team should they have had any queries about the study. The participants were asked to complete a consent form to indicate that they wished to take part in the study. The qualitative researcher obtained consent for the qualitative study either face to face or, for interviews conducted over the telephone, by post.
At the consent stage, participants were informed about the opportunity to participate in other related studies. Participants were informed about the ‘possibility’ of being offered an additional podiatric intervention for the prevention of falls and were asked to tick a box if they were interested in taking part in such an intervention. If participants did not wish to be contacted about these studies, they were asked to indicate this by ticking a box on the consent form.
Baseline assessment
On receipt of written consent, researchers at the YTU assessed the participants’ responses on the background information forms for eligibility. Participants assessed as being ineligible for the study were notified in writing and no further correspondence was sent. Participants who were deemed eligible were then sent a baseline questionnaire (see Appendix 7) and a pack of falls calendars (see Appendix 8).
Participant eligibility
Exclusion criteria for the REFORM cohort
Participants were ineligible for the REFORM cohort if they:
-
were > 65 years of age
-
reported having neuropathy, dementia or another neurological condition such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, Lou Gehrig’s disease/amyotrophic lateral sclerosis or Huntington’s disease
-
were unable to walk household distances (10 metres) without the help of a walking aid, such as a walking frame, a walker or a person to assist
-
had had a lower limb amputation
-
were unwilling to attend their podiatry clinic for a REFORM appointment.
Inclusion criteria for the REFORM cohort
All eligible consenting participants who completed a baseline questionnaire and at least one monthly falls calendar were eligible for inclusion in the REFORM cohort.
Inclusion criteria for the REFORM trial
Participants in the cohort were eligible for inclusion in the REFORM trial if they:
-
had had a fall in the past 12 months, or a fall in the past 24 months requiring hospital attention, or reported worrying about falling at least some of the time in the 4 weeks prior to completing their baseline questionnaire
-
were community dwelling
-
were able to read and speak English.
If participants did not report a recent fall on their screening form but later reported a fall on the baseline questionnaire or monthly falls calendar, they became eligible to be randomised.
REFORM internal pilot
An internal pilot was conducted at the start of the study. The objectives of the pilot trial were to:
-
develop and pilot the multifaceted podiatry intervention
-
develop the podiatry training package
-
pilot the falls calendar and other participant data collection questionnaires
-
pilot, review and refine if necessary the recruitment methodology for the main trial.
In order to progress to the main REFORM trial, the study team were asked by the NIHR HTA monitoring team to fulfil the following progression criteria by the end of November 2013:
-
Recruit 580 participants to the REFORM cohort study.
-
Randomise 70 participants to the REFORM pilot trial.
-
Decide which orthotic insole would be used in the main trial.
Sample size
Pilot sample size
The pilot phase of the study ran from October 2012 until November 2013. No formal sample size calculation was conducted but we aimed to randomise at least 70 participants into the pilot trial (35 in each group), which we believed to be sufficient to test the objectives.
REFORM trial sample size
The primary outcome measure for the trial was the incidence rate of falls reported by the participants over the 12 months post randomisation. This was analysed using a mixed-effects negative binomial regression model. However, because of the inherent difficulties of estimating the parameters required to power a trial for a count outcome, such as the IRR to detect and the measure of overdispersion, the trial was instead powered for the binary outcome of whether or not the participants experienced at least one fall, which was one of our key secondary outcomes. We retained incidence rate of falls as the primary outcome as we believed the extra information contained in this outcome would result in the sample size being conservative for this outcome.
A previous falls prevention trial conducted by some of the authors in community-dwelling older adults with a history of recent falls found a 12% absolute reduction in the proportion of participants who fell among those allocated to receive an environmental falls prevention intervention delivered by qualified occupational therapists, relative to the control group. 25 The REFORM trial was powered at 80% using a two-sided 5% significance level to detect a more conservative absolute difference of 10 percentage points from 50% to 40% in the number of people experiencing at least one fall over the 12 months following randomisation. The total sample size required, allowing for a 10% loss to follow-up, was 890 participants (445 in each group).
Randomisation
Participants who fulfilled the eligibility criteria for the REFORM trial and who had provided written informed consent and indicated that they were interested in receiving the intervention were eligible for randomisation. Randomisation was carried out by the YTU secure remote computer randomisation service. Trial clinics informed the YTU when they had capacity to schedule baseline appointments for participants and how many participants they felt that they could manage to schedule appointments for at that time. A group of participants waiting to be randomised from the centre associated with that clinic were selected and randomised in a single block (mainly) 1 : 1 to either the intervention or usual-care groups; however, when clinics had the capacity to see more or less than half the group size, an appropriate alternative allocation ratio was used. Prediction of allocated group by clinician was not possible because of the dynamic nature of the randomisation and the use of a remote service; thus, allocation concealment was maintained. Once intervention participants had been randomised, they were sent a letter informing them of their group allocation and that the podiatry clinic would be in contact to arrange a trial appointment. Participants who were allocated to the usual-care group were not informed of their group allocation in order to minimise potential attrition and the possibility of resentful demoralisation.
Trial interventions
Intervention group
Participants in the intervention group were allocated to receive a multifaceted intervention comprising footwear advice (and footwear provision if required), an orthotic insole or review of an existing prescription, a programme of foot and ankle balance exercises and a falls prevention leaflet. The trial protocol recommended that participants be invited to attend two appointments: the first as soon as possible after randomisation and the second 2–4 weeks later. Further trial visits could be offered if required, in addition to routine podiatry care appointments in accordance with usual practice.
Footwear advice and provision
Participants were asked to bring their indoor and outdoor footwear to their REFORM appointment. The podiatrist assessed the following characteristics of the participant’s footwear that have been identified in the literature as risk factors for falls in older people:12 correct size, method of fastening, height and width of the heel, thickness of outsole, heel counter stiffness, longitudinal sole rigidity, sole flexion point and tread pattern. Footwear was assessed as inappropriate if it had any of the following characteristics: (1) heel height > 4.5 cm, (2) no adjustable fixation of the upper, (3) no heel counter or a heel counter that could be depressed to > 45°, (4) a fully worn/smooth/thin sole, (5) heel width narrower than the participant’s heel width by ≥ 20% or (6) incorrect shoe size. Participants were counselled about any hazardous footwear features identified during the assessment and advised on safer footwear characteristics to select when purchasing footwear in the future.
If a participant’s footwear was deemed inappropriate, and they did not own a suitable pair of shoes that they could be advised to wear instead, new footwear was provided where possible. The podiatrists ordered footwear directly from one of two companies participating in the Healthy Footwear Guide scheme:26 DB shoes (DB Shoes Ltd, Rushden, UK) or Hotter company (Beaconsfield Footwear Limited, Skelmersdale, UK). Not all of the footwear manufactured by these companies fulfil the characteristics of a ‘safe’ shoe; therefore, participants chose footwear from a catalogue of preselected makes and models that the trial team had previously assessed as being suitable. In order to avoid incentivising participants to take part in the study, participants were told about footwear provision only if they were assessed as requiring new footwear.
Foot orthoses
Participants were considered for fitting with an X-Line standard orthotic insole (Healthystep, Mossley, UK). If required, the insole was modified with prefabricated self-adhesive additions to improve the participant’s foot posture. For those participants already wearing an orthotic insole, the treating podiatrist made a clinical judgement on the suitability of replacing the insole with one used in the trial. If the participant’s current insole was replaced, then any current prescription or modifications were repeated. If, however, the podiatrist deemed it to be detrimental to replace their current insole with that of the trial insole, then the participant continued to wear their own insole and this component of the intervention was considered to be addressed. In cases in which the treating podiatrist felt that the participant required more or a prescription that the trial insole could not provide, then a referral was made in line with routine practice.
Participants were advised to ‘wear-in’ the orthotic insole slowly. It was suggested that it should be worn for 1 hour on the first day and wear time increased by a few hours each day, and that the insole could be transferred from one pair of shoes to another.
Home-based foot and ankle exercise programme
When safe and appropriate, participants were prescribed a 30-minute home-based foot and ankle exercise programme to be undertaken three times a week, indefinitely. The aim of the exercises was to stretch and strengthen the muscles of the foot and ankle and improve balance. The exercises were based on the programme developed by Spink et al.,23 which had been adapted for a UK and Irish setting during the pilot phase of the study. A summary of the individual exercises is listed in Table 1. The podiatrist assessed competence and safety at the baseline appointment through demonstration and participant repetition of the exercises. These were supplemented by an explanatory illustrated booklet and a digital versatile disc (DVD), which the participant took home along with the resistive bands and therapy ball that were required to undertake the exercises. At subsequent appointments the podiatrists reviewed the participant’s exercise techniques and, when required, advised the participant to ensure that the exercises were being conducted safely and as intended.
| Activity | Description | Dosage | Increments |
|---|---|---|---|
| Ankle range of motion/warm-up | Sitting, with the knee at 90°. Lift the foot to clear the ground and then rotate the foot slowly in a clockwise direction and then an anticlockwise direction | 1 × 10 repetitions for each foot in each direction | None |
| Ankle inversion strength | Sitting upright, with the hip, knee and ankle at 90°. Invert foot against resistive exercise band. The band should be fixed at 90° to the foot from an additional chair/table leg | 3 × 10 repetitions for each foot | Increase resistance strength of resistive exercise band |
| Ankle eversion strength | Sitting upright, with hip, knee and ankle at 90°. Evert foot against resistive exercise band. The band should be fixed at 90° to the foot from an additional chair/table leg | 3 × 10 repetitions for each foot | Increase resistance strength of resistive exercise band |
| Ankle dorsiflexion strength | Sitting, with hip, knee and ankle at 90°. Dorsiflex both feet to end range of motion and hold. Keep pulling feet up towards the body during the hold | Hold feet in dorsiflexion for 3 × 10 seconds | Increase repetitions up to a maximum of 10 |
| Intrinsic strengthening, toe plantarflexion strength and toe stretch | Sitting, with hip, knee and ankle at 90°. (1) Use the therapy ball under the toes to stretch the toes. The rest of the foot should be plantigrade. Then curl and point the toes up and over the ball. (2) Use the therapy ball under the toes to stretch the toes. The rest of the foot should be plantigrade. With the heel on/close to the floor, curl the toes over the ball and attempt to pick up the ball with the toes | 3 × 10 repetitions for each exercise for both feet. Have a 30-second break between each repetition | Increase up to a maximum of 50 repetitions |
| Ankle plantarflexion strength | From standing position, rise up onto toes of both feet and then slowly lower back down. Just before the heels contact the floor, rise back up onto the toes | 3 × 10 repetitions | Increase repetitions up to a maximum of 50 |
| Calf stretch | Facing a wall and using hands on the wall for balance, step one foot in front of the other keeping feet hip width apart and hips, knees and feet facing the wall. Bend the knee closest to the wall and keep the back leg straight. Keep both heels in contact with the floor | Hold stretch for 3 × 20 seconds on each leg | Increase the stride length and forward lean to increase the stretch |
| Proprioception/balance training | From a standing position and holding on to a work surface/chair/wall for support, stand on one leg. Repeat on the other side | Hold for 30 seconds, repeat for three repetitions | Increase slowly to hold for 1 minute per repetition. If competent, rise up on to toes on the one supporting leg: 3 × 10 repetitions |
Routine podiatry care
Participants continued to receive routine podiatry care as separate podiatry appointments in accordance with usual practice. The aim of these appointments was to reduce painful conditions such as corns and calluses that have been found to be associated with an increased risk of falls.
Podiatrist training to deliver the intervention
The podiatrists delivering the trial intervention attended a half-day face-to-face training session facilitated by the research podiatrist (author LG). The training included instructions on the delivery of the individual components of the intervention including footwear assessment and provision, prescribing and fitting trial insoles and prescribing foot and ankle exercises. Podiatrists were given the opportunity to practice delivering the intervention during role-play sessions. In addition, information about the day-to-day management of podiatry tasks, for example booking appointments or ordering footwear, adverse event reporting and completion of trial paperwork, was provided. When possible, the research podiatrist attended the first participant appointment delivered by each podiatrist to give advice on the delivery of the intervention when requested.
Falls prevention leaflet and trial newsletter
Participants were sent a falls prevention leaflet in the post along with their baseline questionnaire. Participants living in the UK received the Age UK Staying Steady leaflet27 and those in Ireland received the Irish Osteoporosis Society Fall Prevention leaflet. 28
A postal group-specific trial newsletter was sent to participants at 3 months post randomisation, as well as a generic trial newsletter at 12 months. The aim of the newsletters was to keep participants updated with the progress of the trial in an attempt to minimise attrition and improve response rates to postal questionnaires. 29 The 3-month newsletter to the intervention group also included information about how to undertake the foot and ankle exercises and wear the insoles and it aimed to aid compliance. It included anonymised quotations reporting the benefit some participants had experienced after following the package of care. The content of the newsletter was informed by issues raised by participants with the research team during the course of the trial.
Usual-care group
Participants in the control group continued to receive usual care from their podiatrist and GP, which may have included prescription of an orthosis and footwear advice. They also received the same falls prevention advice leaflet sent to the intervention participants and a group-specific trial newsletter at the same time points.
Participant follow-up
All participants in the REFORM trial were followed up with monthly falls calendars for 12 months post randomisation. If a participant did not return their falls calendar after 10 days, a member of the study team telephoned or wrote to them to collect the primary outcome data. The study team contacted participants who had reported a fall to collect further information relating to the nature, cause and location of the fall (see Appendix 9). Participants were also sent follow-up questionnaires at 6 (see Appendix 10) and 12 months (see Appendix 11) post randomisation. Follow-up questionnaires were posted to participants, along with a pre-addressed envelope, and reminder letters were sent after 2 and 4 weeks if unreturned. Participants also received an unconditional £5 in cash with their 12-month postal questionnaire in recognition of their participation in the study and to offset any incidental expenses that they may have incurred when completing postal questionnaires. Telephone follow-up by one of the study team’s researchers was conducted 2 weeks after the second postal reminder for any participant who had not returned a questionnaire to complete the primary outcome data as a minimum. In addition, intervention participants were sent an exercise and orthosis compliance questionnaire at 3, 6 and 12 months (see Appendix 12). Any change in the participant’s trial status during the course of the study was recorded by the study team (see Appendix 13). Data collection ceased in December 2015.
Trial completion and exit
Participants were deemed to have exited the trial when they:
-
had been in the trial for 12 months post randomisation
-
withdrew from the trial, that is, they wished to exit the trial with no further contact for follow-up or treatment
-
were lost to follow-up
-
died.
Withdrawals
Withdrawals could occur at any point during the study at the request of the participant. The reason for their withdrawal did not have to be declared; however, if a reason was provided, then it was recorded. Participants could inform the trial team of their decision to withdraw from the study by contacting them either by telephone or in writing. When possible, a researcher would clarify to what extent they wished to withdraw: from the intervention only or from all aspects of the study. Treating podiatrists could also withdraw participants from the intervention or from all aspects of the trial when they felt that this was appropriate. When withdrawal was from the intervention only, follow-up data continued to be collected. Data were retained for all participants, unless a participant specifically requested that their details be removed.
Patient and public involvement in research
The REFORM trial was informed by the involvement of older people with a history of falls throughout the research period. A patient reference group was established at the start of the study. The group comprised four older people who provided valuable insights into the relevance and readability of the study documentation and advice regarding recruitment methods. They provided input into the content and layout of the patient information sheet, the exercise booklet, newsletters and recruitment posters. They reviewed the exercise DVD and the package of care, and provided feedback on the selection of footwear offered to participants. The patient reference group contributed to this HTA report by reviewing the plain English summary and they will provide guidance about our dissemination strategies on how best to share the study findings with trial participants.
Clinical effectiveness
Primary outcome
The primary end point for the trial was the incidence rate of falls per participant in the 12 months following randomisation. A fall was defined as ‘an unexpected event in which the participant comes to rest on the ground, floor, or lower level’. 30 Data were collected via participant self-reported monthly falls calendars. These took the form of A5 pieces of card with a calendar grid of individual months printed on one side along with a definition of a fall and a freepost address to the YTU on the other. Participants were asked to record the day of the month on which they fell or to record that they did not fall that month and return the calendar to the YTU. Participants who did not return their monthly falls calendar were either telephoned or written to by the YTU to obtain the missing data. Participants were also given a freephone number to report any falls as soon as possible after they occurred, and these were recorded by research staff on a falls telephone data collection sheet (see Appendix 9). The information collected included the date and location of fall, the reason for the fall, any injuries sustained (e.g. a superficial wound or a broken bone), hospital admissions, the footwear worn at the time of the fall and if the participant was wearing an insole or using a walking aid.
Secondary outcomes
All secondary outcomes were self-reported by the participant and collected by questionnaires at 6 and 12 months post randomisation or by monthly falls calendars. Secondary outcomes include:
-
proportion of fallers (at least one fall and multiple falls)
-
time to first fall from date of randomisation
-
incidence rate of falls in 12 months post randomisation as recorded on the 6- and 12-month participant questionnaires
-
fear of falling as measured by the question ‘During the past 4 weeks have you worried about having a fall?’ at 6 and 12 months
-
fear of falling as measured by the Short Falls Efficacy Scale – International (FES-I) at 6 and 12 months31
-
activities of daily living as measured by the Frenchay Activities Index (FAI) at 6 and 12 months32
-
depression as measured by the short form Geriatric Depression Scale (GDS) at 6 and 12 months33
-
proportion of participants with depression (score of ≥ 6 on GDS) at 12 months
-
adaptability and resilience as measured by the 2-item abbreviated version of the Connor-Davidson Resilience Scale (CD-RISC2) at 6 months34
-
level of pain in the feet as measured on a visual analogue scale from 0 (no pain) to 10 (worst possible pain) at 12 months
-
fracture rate (single and multiple)
-
health-related quality of life (HRQoL) as measured by the EuroQoL-5 Dimensions-3 Levels (EQ-5D-3L)35
-
health service utilisation.
Scoring of instruments
Fear of falling
Fear of falling was measured by the question ‘During the past 4 weeks have you worried about having a fall?’ at screening and baseline and at 6 and 12 months. Response categories were all of the time, most of the time, a good bit of the time, some of the time, a little of the time and none of the time. These were scored from 1 to 6 and were treated as continuous data for analysis.
Short Falls Efficacy Scale – International
The Short FES-I asked participants at baseline and at 6 and 12 months to indicate how concerned they were about falling when performing seven different activities: not at all concerned, somewhat concerned, fairly concerned or very concerned. These were scored from 1 to 4, and a total score for the Short FES-I was obtained by summing the seven item scores. When a participant selected two or more responses to an item, this was treated as missing data. If data were missing on two or more items then the questionnaire was considered invalid. If data were missing on no more than one of the seven items then the total score was calculated for the six completed items, divided by six and multiplied by seven. The new total score was then rounded up to the nearest whole number. 31 A final total score of 7 or 8 indicates no/low concern, 9–13 indicates moderate concern and 14–28 indicates a high degree of concern about falling.
Frenchay Activities Index
This 15-item instrument was administered at baseline and at 6 and 12 months and assessed a broad range of activities of daily living. The frequency with which each item or activity was undertaken over the previous 3 or 6 months (depending on the nature of the activity) was assigned a score of 1–4, where a score of 1 is indicative of the lowest level of activity (e.g. never performed). The scale provides a summed total score from 15 to 60. When a participant selected two or more responses to an item, this was treated as missing data. Only when there were no missing item responses was a total score computed for an individual for this instrument.
Geriatric Depression Scale
The GDS is a 15-item scale used as a screening tool for geriatric depression and was administered at baseline and at 6 and 12 months. Each item requires a ‘yes’ or ‘no’ response. A score of 1 is assigned when the item response indicates a negative state of mind, for example responding ‘no’ to ‘Are you basically satisfied with your life?’. A total score out of 15 can be calculated. When a participant selected both ‘yes’ and ‘no’, the worst-case scenario was assumed and a score of 1 was assigned to the item. More than five missing item responses invalidated the scale; otherwise, a total score when there were missing data was calculated by summing the item scores, dividing by the total number of completed items and multiplying by 15. The new total score was then rounded up to the nearest whole number to give the score for an individual (https://web.stanford.edu/∼yesavage/GDS.html). A score of 0–5 is considered normal, whereas a score of > 5 suggests depression. Any participant reporting a score of ≥ 10 on the GDS,33,36 that is, more severe depression, was referred to their GP.
The two-item abbreviated version of the Connor-Davidson Resilience Scale
The CD-RISC2 is a two-item abbreviated version of the full 25-item Connor-Davidson Resilience Scale. It is based on items 1 (‘I am able to adapt to change’) and 8 (‘I tend to bounce back after illness or hardship’) of the original instrument. Each item is scored from 0 (‘not true at all’) to 4 (‘true nearly all the time’), so the CD-RISC2 can be scored from 0 to 8. Higher scores reflect greater ‘bounce-back’ and adaptability. This instrument was administered at baseline and at 6 months.
Other data collected
Non-consenting participants
Participants who did not wish to take part in the study were not required to return any forms to the YTU; however, some chose to complete the screening form, thus providing us with some demographic information. In addition, all participants in the pilot phase of the study were sent an invitation pack that included a decline form, so that if they were willing they could provide a reason for declining. This provided us with sufficient information to document the reasons why participants did not wish to take part in the study, and allowed us to compare participants who declined with those who participated. The recruitment pack in the main study did not contain a decline form.
Intervention: details and adherence
Treatment details were recorded by the podiatrist, including the number of podiatry visits, an eligibility checklist with details on relevant health conditions and test results, characteristics of current indoor and outdoor shoes, details relating to shoes ordered, details on the type and prescription of any current insole use, the type of insole issued/retained with any modifications made, details of the size of any therapy ball and the strength of any resistive band prescribed and any amendments or advice given on the intervention owing to safety reasons.
Information on adherence to the exercise, footwear advice and orthotic insole components of the intervention was collected from participant self-reported questionnaires at 3, 6 and 12 months from participants in the intervention group only. Participants were asked if, during the past month, they had worn their insole all of the time, most of the time, some of the time, a little of the time or none of the time. Participants were also asked, for the past month, typically how many times a week they had done the exercises: not at all, once, twice, three times or more than three times. In addition, all participants were asked on the 12-month follow-up questionnaire if they had been given footwear advice by the trial podiatrist and whether or not they had followed the advice given.
Adverse events
Details of any adverse events reported to the YTU directly by the participant, a member of their family or by a member of the research team at the recruiting site were recorded. Details of the event were recorded on a REFORM Adverse Event Form (see Appendix 14). Any serious adverse events (SAEs) judged to have been related and unexpected were required to be reported to the REC under the current terms of the standard operating procedures for RECs.
In this study, a SAE was defined as any untoward occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Expected events included aches and pains in the lower limb, new callus/corn formation, blisters or ulcers and skin irritation/injury including pressure sores and soft tissue injury.
The occurrence of adverse events during the trial was monitored by an independent Data Monitoring Ethics Committee and the Trial Steering Committee (TSC). The Data Monitoring and Ethics Committee/TSC would have immediately seen all SAEs that were thought to be related to treatment.
Clinical effectiveness analysis
All analyses were conducted on a modified intention-to-treat (ITT) basis using available cases, using a two-sided statistical significance level of 0.05 unless otherwise stated. The analyses were conducted using Stata® version 13 (StataCorp LP, College Station, TX, USA).
Data collected at screening and on the baseline questionnaire are summarised for (1) consenting individuals and those who assented to provide screening data but not to enter the trial, (2) the cohort and (3) trial participants as randomised and as analysed in the primary outcome model by treatment group. Comparisons between groups were made using chi-squared tests for categorical data, independent t-tests for continuous variables and negative binomial regression for count data.
Primary analysis
The primary analysis model controlled, as fixed effects, for sex (coded 0 = female, 1 = male), age at randomisation in years (integer) and history of falling. All participants had to have fallen at least once in the previous 12 months, have had a fall in the last 24 months requiring hospitalisation or have a fear of falling in order to be eligible for randomisation. Participants were classified into two groups for the history of falling covariate: (1) one or no falls in the 12 months prior to completion of the background information sheet; or (2) two or more falls reported in the 12 months prior to completion of the background information sheet. These were coded as 0 and 1, respectively.
As there was evidence of overdispersion in the data, Poisson regression was not considered to be appropriate, and so the incidence rate of falls was analysed using a mixed-effects negative binomial regression model. Participants recruited from the same centre, and therefore residing in a particular geographical area, are more likely to be similar to one another than to participants from other centres. This can result in a correlation between participant outcomes within centres. Failure to account for this clustering of outcomes in the analysis can lead to an increase in the type 1 error rate. Therefore, to account for the potential correlation of participant outcomes from participants in the same centre, we included trial centre (n = 13) as a random effect in the model. The model also took account of the different observation periods for each individual by including a variable for the number of months for which the participant returned a monthly falls calendar (using the exposure option within the Stata command).
The model equation is:
where
-
E(yij) is the expected number of falls for participant i in centre j in time tij
-
tij is the length of exposure (follow-up) for participant i in centre j
-
β is a vector of fixed-effect regression coefficients and
-
exp(β) is a vector of the IRRs.
Coefficients are presented as IRRs with 95% CIs and p-values.
Sensitivity analyses
A sensitivity analysis of the primary outcome was conducted, adjusting for any pre-randomisation variables found to be imbalanced by chance between the randomised groups.
Non-compliance
A complier average causal effect (CACE) analysis to assess the impact of compliance on the treatment estimate was undertaken for the primary analysis. CACE analysis allows an unbiased treatment estimate of, in this case, the podiatry intervention in the presence of non-compliance. It is less prone to biased estimates than the more commonly used approaches of per protocol or ‘on treatment’ analysis, as it preserves the original randomisation and uses the randomisation status as an instrumental variable to account for the non-compliance. The CACE analysis employed a two-stage regression process: first, compliance with the intervention was predicted using a linear mixed model adjusted for randomised group, sex, age and history of falling, with centre as a random effect; and, second, the primary analysis model was repeated but the variable for group allocation was replaced with the variable for compliance and the predicted residuals from the first regression was added as a covariate.
Compliance was based on whether or not the participant was seen in clinic for a trial appointment; therefore, all participants in the usual-care group and those in the intervention group who did not attend an appointment were assigned a compliance value of 0 and those in the intervention group who attended an appointment were assigned a value of 1. As this was a multifaceted intervention, it did not make sense to try and measure the extent to which participants used the orthotic insole, performed their prescribed exercises or wore their provided footwear. This would have been measured with too much error.
Excluding fear of falling participants
Over the course of the trial, it was observed that having a fear of falling was a strong predictor of having a fall in the near future. A protocol amendment was submitted to, and approved by, the REC to include ‘fear of falling’ as an inclusion criterion. Therefore, a small number of participants in the cohort were randomised into the trial who reported a fear of falling on their baseline form but who had not reported a previous fall. On advice from the TSC and the HTA programme, the trial over-recruited to make up for the number of participants recruited using the fear of falling criterion. A sensitivity analysis was conducted excluding these ‘fear of falling’ participants from the primary analysis to determine their effect on the estimates.
Missing data
We compared data collected prior to randomisation for participants who are included in the primary analysis to ensure that any attrition had not produced imbalance in the groups in important covariates. To account for any possible selection bias, univariate logistic regressions were run to predict missing outcome data. As the number of participants who did not return any falls calendars after randomisation was low, missing outcome data were based on returning fewer than 6 months’ worth of data post randomisation. All variables found to be predictive of missingness were then included in a single stepwise logistic regression model, which used a p-value of 0.1 to refine the covariates. The primary analysis was then repeated including as covariates the variables found to be significantly predictive of non-response to determine if this affected the parameter estimates.
Podiatrist effects
In 6 of the 13 sites, only one podiatrist delivered the intervention; therefore, podiatrist effects are to some extent captured by centre effects that are being accounted for in the primary analysis. However, in other sites, more than one podiatrist delivered the intervention to the participants. We therefore have potential clustering by podiatrist in the intervention group that is not completely captured by centre. The success of the intervention may depend on the skill/experience of the podiatrist and their relationship with the participant. To account for this variation between podiatrists, a sensitivity analysis was conducted in which every participant, whether allocated to the intervention or usual-care group, was associated with a podiatrist. For intervention participants, this podiatrist was the podiatrist who delivered their intervention appointments. For usual care participants or intervention participants who did not attend an appointment, we assigned them a counterfactual podiatrist, that is, one that they could have seen had they received the intervention. All participants at sites with only one trial podiatrist were assigned this podiatrist. In sites with more than one trial podiatrist, the participants were randomly assigned one of the podiatrists who saw participants who were randomised in the same month as them, in the proportion that they saw intervention participants. Each podiatrist then had their own cluster of usual care and intervention participants. The primary analysis was then repeated with podiatrist, rather than centre, as a random effect.
Secondary analyses
The incidence rate of falls over the 12 months following randomisation (as reported for the previous 6 months on the 6- and 12-month participant questionnaires) was analysed in the same way as the primary outcome.
The proportion of fallers versus non-fallers, and of multiple fallers versus single or non-fallers, in each group was compared using a mixed logistic regression model adjusting for sex, age and history of falling, with centre included as a random effect. 37
The time from randomisation to first fall in days was derived. Participants who did not have a fall were censored at their date of death or, if alive, their withdrawal from the trial, the date of the last available assessment or 365 days after randomisation, whichever was latest. Kaplan–Meier survival curves were produced for each group. The time to first fall was analysed by a Cox proportional hazard regression with shared centre frailty effects adjusting for sex, age and history of falling. 38
Fear of falling in the past 4 weeks, and the total scores for the Short Falls Efficacy Scale – International, GDS and FAI were compared between the two groups using a covariance pattern mixed model incorporating all post-randomisation time points (6 and 12 months) adjusting for baseline score, sex, age, history of falling, treatment group, time and a treatment group-by-time interaction term, with centre as a random effect. Such an approach models the correlation of observations within participants over time. Different covariance structures for the repeated measurements, which are available as part of Stata version 13 (unstructured, exchangeable, independent and banded), were explored and the most appropriate pattern used for the final model based on the Akaike’s information criterion (smaller values are preferred). 39 Participants were included in the model if they had full data for the baseline covariates and outcome data for at least one post-randomisation time point (6 or 12 months). An estimate of the difference between treatment groups in the outcome was extracted for each time point with a 95% CI and p-value.
The assumptions of the covariance pattern mixed model were checked visually. The normality of the standardised residuals was assessed via a histogram and Q–Q plot, and the homoscedasticity of the errors was checked by plotting the residuals against the fitted values.
The CD-RISC2 score at 6 months was compared between the two groups using a linear mixed model adjusting for baseline CD-RISC2 score, sex, age and history of falling, with centre as a random effect.
Participants with a score of ≥ 6 on the GDS were categorised as having depression; the proportion of people with depression in each group was compared at 12 months using a mixed logistic regression model adjusting for sex, age and history of falling, with centre as a random effect.
The proportion of participants obtaining at least one fracture over the 12-month follow-up period was compared using a mixed logistic regression adjusting for sex, age and history of falling, with centre as a random effect.
At 12 months, participants were asked to indicate their level of pain or discomfort in their feet on a visual analogue scale from 0 (no pain) to 10 (worst possible pain). This was analysed using a linear mixed model adjusting for sex, age and history of falling, with centre as a random effect in an ITT analysis, and also in a CACE analysis. We based compliance on whether or not the participant was seen in clinic for a trial appointment. The CACE analysis employed a two-stage regression process: first, compliance with the intervention was predicted using a linear mixed model adjusting for randomised group allocation, sex, age and history of falling, with centre as a random effect; and second, foot pain score was predicted using a linear mixed model adjusting for compliance, sex, age, history of falling and the predicted residuals from the first regression, with centre as a random effect.
Economic analysis
The economic analysis was conducted on an ITT basis from the NHS and Personal Social Services perspective. Data on HRQoL, obtained from the EuroQoL-5 Dimensions (EQ-5D) instrument collected from self-reported questionnaires, were converted into quality-adjusted life-years (QALYs) for each participant using the area under the curve method. Costs were expressed in UK pounds sterling (£) at 2015 prices.
Differences in mean costs and QALYs at 12 months post randomisation, estimated by means of regression methods, were used to assess the cost-effectiveness of the intervention compared with usual care. Multiple imputation (MI) was used to impute missing cost and QALY data, and the base-case analysis was conducted on this imputed data set. Sensitivity analyses were conducted to test assumptions regarding the missing data mechanism, level of imputation on HRQoL, resource use and perspective of analysis. Cost-effectiveness acceptability curves (CEACs) were used to express the probability of whether or not the intervention is cost-effective at the willingness-to-pay (WTP) threshold used by the National Institute for Health and Care Excellence (NICE).
In addition, HRQoL was extrapolated to 5 years in order to explore how the differences in HRQoL evolve beyond the study follow-up. For this exploratory projection, we used a decision-modelling approach and assumed that the difference in HRQoL and costs observed at 1 year would remain unchanged.
Qualitative study
A qualitative study was undertaken to explore the views, experiences and acceptability of the REFORM package of care from the perspective of both service users and service providers. In particular, this qualitative study considered the barriers to and facilitators of delivering and receiving the intervention, in the context of podiatry care. An in-depth appreciation of these issues is useful for the future successful implementation of complex podiatry interventions in this population group.
Design
A semistructured interview study was used to gather in-depth information on the trial participants’ experiences of receiving the podiatry intervention, alongside the podiatrists’ experiences of delivering the intervention. The interviews were conducted either face to face or over the telephone with participants and podiatrists in the trial (at the end of the intervention period).
Sampling
A purposive sampling strategy was used to achieve a hetergeneous sample of trial participants from the intervention group to ensure maximum variation40 according to age, sex and history of falls. Previous studies have indicated that a sample of approximately 20–30 trial participants is sufficient to address the aforementioned aims from the point of view of the service users.
As podiatrists delivering the intervention were based in a wide variety of clinics, it was expected that their views and experiences may differ. For example, some podiatrists worked in biomechanics and others worked in routine podiatry clinics. All 28 podiatrists who delivered the REFORM intervention were invited for interviews through the PI at each site.
Recruitment and consent
All REFORM trial participants living in the Yorkshire or Lincolnshire areas who expressed an interest in undertaking other associated REFORM research studies on the consent form and who had received the intervention were eligible for participation in the qualitative study. Following sampling, study participants were approached by letter, which contained an information sheet (see Appendix 15) and two consent forms (see Appendix 16). The letter also informed trial participants that a qualitative researcher (authors AC and SC) would contact them via telephone to find out if they would be willing to take part and, if so, to arrange a time for the interview to take place. In accordance with ethics guidelines, informed consent was gained by the researcher before the commencement of the interview. The aim of the interview was explained to the participant, and this was followed by an opportunity for them to ask questions about the study. The anonymity and confidentiality of participants’ personal information were assured by the researcher.
Podiatrists were also invited to take part in the qualitative interviews. The PI at each site was sent an e-mail asking if he or she and the podiatrists who delivered the intervention would like to be interviewed. The PI was asked to forward the e-mail on to podiatrists at their site who delivered the intervention. Podiatrists were asked to contact the research team directly if they wished to take part. The recruitment e-mail included an invitation, information sheet (see Appendix 17) and consent form (see Appendix 18). This was followed up by a telephone call or an e-mail. Prior to the interviews, podiatrists were assured anonymity and confidentiality and were given the opportunity to ask questions. For podiatrists interviewed face to face, a similar process to that used to obtain consent for trial participants was used. For interviews conducted over the telephone, verbal consent was obtained prior to the start of the interview and a copy of the consent form was sent to the qualitative researcher either in the post or via e-mail.
Data collection
The semistructured interviews with trial participants were carried out in participants’ homes or at the University of York between November 2013 and March 2016 and on average lasted 40 minutes using a topic guide (see Appendix 19). All interviews were audio-recorded, transcribed and anonymised before data analysis.
The semistructured interviews with podiatrists were carried out between July 2015 and January 2016 in a private room on premises where the podiatrist was based or over the telephone. The interviews lasted between 30 and 70 minutes and were conducted using a topic guide (see Appendix 20).
The topic guides provided a framework for the semistructured interviews and ensured that all podiatrists and trial participants were asked the same questions, allowing comparisons to be made during the analysis. However, the wording of questions was not fixed to allow interviews to flow and to allow for probing when more detail was required.
Data analysis
An initial thematic analysis was carried out using the stages as outlined by Braun and Clarke:41 (1) familiarisation, (2) generating initial codes, (3) searching for themes, (4) reviewing themes, (5) defining and naming themes and (6) data reporting. An initial coding framework was developed based on a priori themes relating to issues included in the topic guide while allowing for emergent themes. Descriptive coding was conducted, following familiarisation with the data, by the main qualitative researcher on the project (author SC), informed by regular discussion with the qualitative team (authors JA and AC). Subsequently, initial codes were refined in order to address the aims of the qualitative study outlined above, following the analysis of the main trial. A constant comparison method42 was used to check and compare across the data set and to establish appropriate analytical categories. This also ensured that any additional codes were added to reflect as many of the nuances or outlier views in the data as possible, taking into consideration the participants’ wider contexts. Anonymised participant identifiers are used for the reporting of results.
To promote quality, the following strategies were used: description of the participants to provide context (credibility and transferability), transparency of the research process (transferability), evidence of consistency using multiple examples from data (dependability) and engagement of the wider research team with interim findings (confirmability). In addition, a reflexive approach was taken to data analysis. The main interviewers (authors SC and AC) were academic research fellows with no podiatry training. SC was the main REFORM trial co-ordinator and AC had no prior knowledge or experience of podiatry interventions, orthotic insoles or RCTs. The other member of the qualitative team (author JA) had an academic research background and also did not have previous knowledge or experience of podiatry care. This placed the qualitative research team in a very neutral position relating to any prior expectations relating to the study intervention.
Chapter 3 Protocol changes
Clarification to trial documentation and collection of data
Following review of the trial documentation in March 2012, we decided to simplify the participant self-report question regarding neuropathy status. Participants were asked to report if they had any numbness or tingling in their feet or lower limbs as opposed to being asked if they had neuropathy. This was because it was felt that participants may not have known that they had neuropathy but would be able to report if they had numbness or tingling in their lower limbs. Baseline data regarding referrals to a falls service were also collected.
Following completion of the pilot study, it was felt that sufficient data had been collected about the reasons why potential participants did not wish to take part in the study. To reduce the postage costs for the study and on advice of the patient representative, who expressed concerns about this additional data collection, it was agreed that data on reasons for declining participation in the study would not be collected in the main study.
To minimise participant burden and to improve data collection, it was agreed that qualitative data from participants could be collected over the telephone and that information regarding exercise and orthosis compliance would be collected via postal questionnaire at 3, 6 and 12 months post randomisation rather than by the completion of an exercise diary.
Amendments were made to the participant information sheet and consent form in August 2012 to clarify that not all participants would be offered an additional podiatry visit.
Recruitment
The original protocol stated that we planned to recruit 1700 participants to the REFORM cohort, of whom we would randomise 890 to the REFORM trial over a 24-month period from three NHS trusts (Harrogate, Sheffield and Leeds). However, the study commencement was delayed by approximately 5 months because of contractual issues and delays in obtaining service support costs for the study and research and development approval. As the trial progressed, recruitment fell below the expected level because, in the main, a lower than expected uptake rate to the study by participants and a lower than expected number of eligible participants expressing an interest in taking part. Approval was obtained from the funder to extend the study by 10 months to a total of 52 months (December 2011 to March 2016). This permitted the recruitment of seven additional sites. The details of the recruiting sites and date on which research governance approval was received can be found in Appendix 1.
With the number of eligible participants lower than expected, the number of participants in the REFORM cohort had to be increased from 1700. It was decided to aim to recruit 2600 participants into the cohort. To assist with recruitment, approval was obtained to implement the opportunistic screening of participants by podiatrists in clinics, falls practitioners, physiotherapists and the research podiatrist.
Inclusion criteria
The following changes were made to the eligibility criteria during the study.
-
Responses on the ‘decline forms’ received from participants who did not wish to take part in the study during the pilot phase indicated that participants declined because they assumed that they would not be eligible as they considered themselves either too old or too ill. The participant information sheet was modified to try and address this concern stating that, in effect, no one was ‘too old’ to take part and having a chronic illness did not necessarily exclude people from participation. In addition, the TSC reviewed the age limit and agreed to a change from ≥ 70 years to ≥ 65 years, as it was felt that younger participants may also potentially benefit from the intervention. It was hoped that this change would increase the size of the eligible population and improve recruitment. An amendment to reduce the age limit of participants in the study was approved by the multicentre REC in February 2013.
-
Responses from the screening forms sent out during the pilot phase indicated that participants were being excluded as they were already wearing an insole. In order to aid recruitment the TSC agreed that as the trial was evaluating a multifaceted intervention and not insoles on their own, from February 2013 patients could be included if they were currently wearing a full or three-quarter length insole for the purpose of altering or modifying foot function. For participants allocated to the intervention group who were already wearing an orthotic insole, the treating podiatrist made a clinical judgement on the suitability of replacing the insole with one used in the trial. Usual care participants continued to wear their insole but may have had a new insole prescribed by their podiatrist as part of their routine care.
-
To minimise post-randomisation attrition rates, participants had to demonstrate their commitment to the study by returning three falls calendars before they could be randomised. This was thought to cause considerable delay in participants being randomised at new sites. Therefore, to aid recruitment, in April 2013 the need to return a minimum of three falls calendars was reduced to a minimum of one.
-
We undertook an analysis of the predictors of falling in those participants who had been recruited to the study but were in the 3-month ‘run-in’ phase, so were yet to be randomised. As expected, those who reported having had a previous fall were at a higher risk of falling during the run-in period than those who had not [odds ratio (OR) 2.4]. We also observed that those who reported having a fear of falling on their baseline questionnaire were at an elevated risk (OR 2.1). In a previous trial of fracture prevention, a similar relationship was found: fear of falling is a risk factor nearly as strong as a history of falls. 43 Following advice from our TSC and the funders, it was agreed that from June 2014 an additional inclusion criterion (i.e. fear of falling) could be used at clinics that had the capacity to see participants but did not currently have any participants eligible under the current criteria. We felt that this would improve the generalisability of the study and aid recruitment.
To enhance participant safety, it was agreed that, from February 2015, participants should be excluded from the study if they:
-
Had a lower limb amputation.
-
Were unable to walk household distances without the help of a walking aid such as a walking frame, a walker or a person to assist. Participants who used one walking stick, however, were still eligible for the study.
Orthoses
In the original protocol, we intended to use the same orthotic insole (Formthotics™, Foot Science International, Sockburn, New Zealand) used by one of the authors (HBM) in an Australian study,23 as it was found to be acceptable to participants and had been associated with a reduction in falls. However, during the setup of the pilot phase of the study, podiatrists at the recruiting sites reported difficulties using Formthotics, particularly in relation to fitting and modifying. Feedback from a group of podiatrists at recruiting sites indicated that they frequently used an alternative range of orthotic insoles called the ‘X-Line range’, as they were reportedly easier to fit in participant’s current shoes and easily modifiable. In addition to basic functional foot support and control, cushioning properties were also identified as desirable. Therefore, during the pilot phase of the study, 31 participants were given both a Formthotics and an X-Line insole to take home and wear; they were then questioned about their insole preference. As the majority of participants (84%, 26/31) preferred the X-Line range, it was decided to use it in the main trial instead of the Formthotics insole.
Exercises
The exercises developed by one of the authors (HBM) for his Australian trial were reviewed and adapted to take on board lessons learned from the study and to make them more suitable for a UK population. Owing to cost and safety reasons the use of the Archxerciser™ device (Elgin Archxerciser Foot Exercisers, Elgin Division, IL, USA) and marbles were replaced with a therapy ball, which simplified the toe exercises using one device, reflecting current UK practice. Standing calf stretch exercises were adapted for an older UK population by providing an option to use a firm belt/band to stretch while in a sitting position. A further proprioception/balance training exercise was also added.
Additional criteria to expected adverse events
Following discussion with the Trial Management Group it was decided to include some additional expected adverse events relating to wearing an orthotic insole or undertaking foot- and ankle-strengthening exercises to the protocol. These included aches and pains in the lower limb for longer than 48 hours, new callus/corn formation, blisters or ulcers, skin irritation/injury including pressure sores and soft tissue injury.
Provision of footwear
In the original protocol participants were to be provided with a voucher allowing them to purchase their new footwear from participating designated shoe shops. However, this system became unworkable as the number of sites increased and sites became more geographically dispersed. Participants therefore chose their footwear from a catalogue of footwear reviewed and compiled by the research team for suitability. These were then ordered directly from the company by the podiatrist. Footwear that did not fit the participant could be returned to the supplier and exchanged for a different size.
Chapter 4 Clinical effectiveness results
Participant flow
Participants were enrolled into the REFORM study from nine NHS trusts based in either primary or secondary care in the UK (Harrogate and District NHS Foundation Trust; Sheffield Teaching Hospitals NHS Foundation Trust; Leeds Community Healthcare NHS Trust; Solent NHS Trust; Kent Community Health NHS Foundation Trust; Humber NHS Foundation Trust; Northern Lincolnshire and Goole NHS Foundation Trust; South Tyneside NHS Foundation Trust; and North Tees and Hartlepool Hospitals NHS Foundation Trust) and one international site in a university school of podiatry in Galway, Ireland. Harrogate and District NHS Foundation Trust was split into four geographical locations, which were considered to serve distinct populations (Scarborough, York, Harrogate and Skipton), thus forming 13 trial centres. A total of 42 podiatry clinics consented to screen their practice lists and identify participants who met the initial inclusion criteria: those aged ≥ 65 years who were registered with the service and had attended routine podiatry services within the past 6 months. Patients who had attended high-risk clinics (e.g. a diabetes clinic) or who lived in a nursing home were excluded from the invitation mail-out. In addition, sites were requested to screen out, when possible, patients in the following groups: patients with a life expectancy of < 6 months, patients known to have dementia, a neurodegenerative disorder, neuropathy or a lower limb amputation, and patients who were chair or bed bound.
A total of 37,389 recruitment packs were mailed out to potential participants between October 2012 and August 2014: 4428 background information forms (screening forms) were returned to the YTU, of which 3458 (78.1%) were also sent back with a valid consent form. Consenting participants were screened for eligibility to the cohort and potentially eligible participants were sent a baseline questionnaire and a pack of falls calendars (n = 2536). Of the 2389 participants who returned a baseline questionnaire, 88 did not ever return a falls calendar; the remaining 2301 participants joined the epidemiological cohort. Within the cohort, 990 participants were immediately eligible to be randomised, as they reported that they had had at least one fall in the previous 12 months, or one fall in the previous 24 months requiring hospitalisation; 750 of these went on to be randomised into the main trial (participants could be randomised only as and when there was capacity at the clinic to schedule them a baseline appointment). A further 234 participants were randomised after a subsequent fall, and 26 participants were randomised when the eligibility criteria were widened to include participants who had not had a fall but reported a fear of falling. A median of 47 participants were recruited from each centre (range 20–323).
Patents were mostly randomised 1 : 1, although at some sites the ratio was fixed depending on the number of participants the clinic had capacity to see and the number of participants available to be randomised. Of the 1010 participants randomised, 493 were allocated to the intervention group and 517 to the usual-care group. The randomised number of 1010 participants exceeded that of the planned sample size of 890 participants. The flow of participants is illustrated in a Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 2.
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participants in the REFORM study. Reproduced from Cockayne et al. 44 This is an open access article distributed under the terms of the Creative Commons Attribution Licence (CC BY 4.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/).
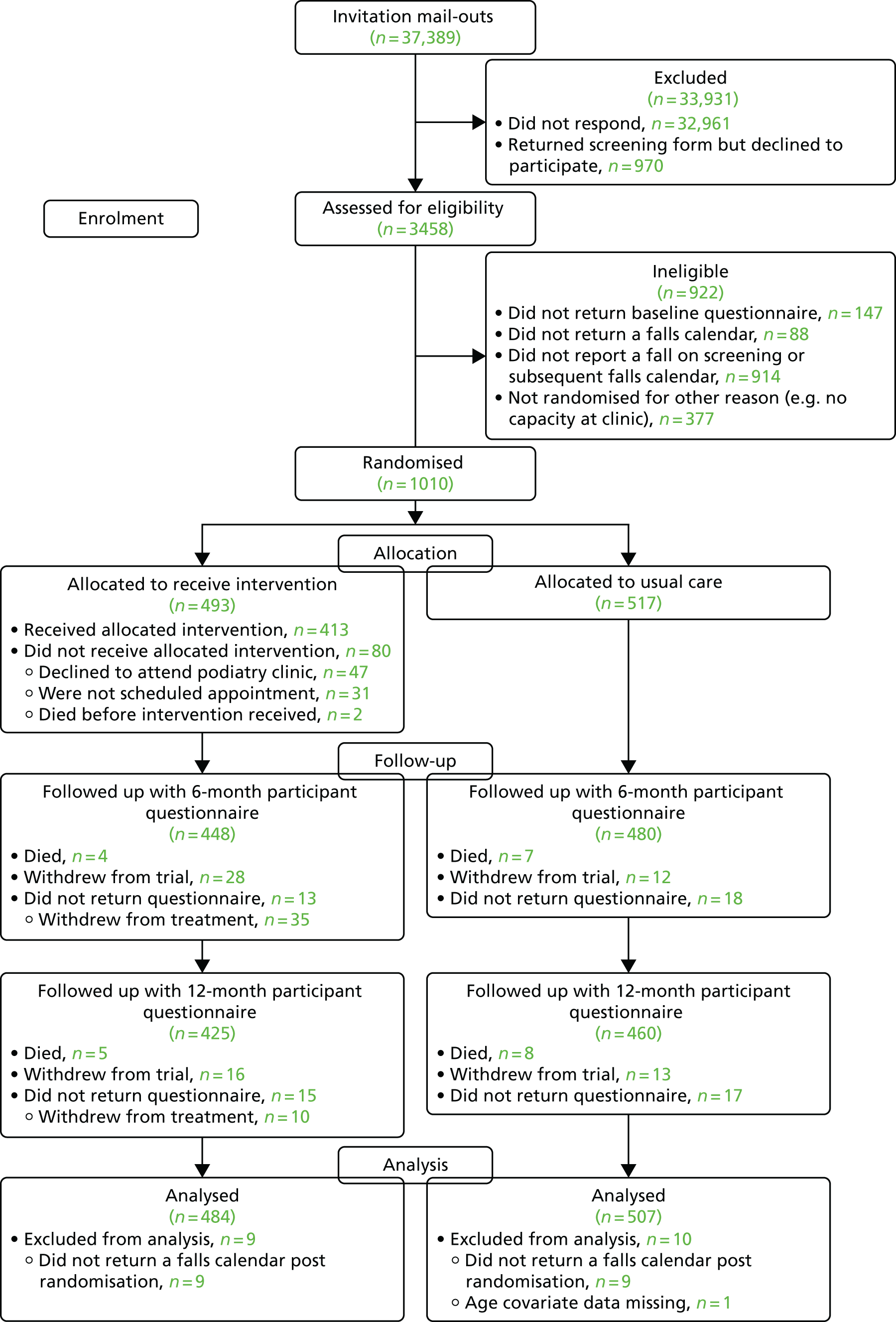
Pilot trial
During the pilot phase of the study, the following quantitative progression criteria were imposed to permit continuation to the main trial: (1) recruit 580 participants to the REFORM cohort study and (2) randomise 70 participants to the REFORM pilot trial. By the end of November 2013, 972 participants had been recruited to the REFORM cohort, and 78 had been randomised (39 participants per group) from York and Scarborough podiatry clinics.
Reasons for non-participation
In total, 567 potential participants provided a reason on a decline form for their decision not to participate in the study (Table 2). The most commonly cited reason was not having an interest in the study (n = 330, 58.2%). Many of the ‘other’ reasons stated by participants could potentially fall into one of the other three broad categories and included the participant feeling too old/unwell to take part, having too many other commitments (e.g. being a carer to a partner) or feeling they would be unsuitable for the study as they do not fall. Collection of this form ceased after the pilot phase of the study.
| Reason for non-participationa | Frequency (%) |
|---|---|
| I am not interested in taking part in this study | 330 (58.2) |
| I feel too unwell to take part in this study | 167 (29.5) |
| I do not have time to take part in this study | 137 (24.2) |
| Other reason | 107 (18.9) |
Trial completion and trial exit
Participants were able to withdraw from the study at any point. They were offered the options of withdrawing from the intervention only or from all aspects of the study. Data were retained for all participants who withdrew, as no participant specifically requested that their details be removed. Of the 493 (9.1%) participants in the intervention group, 45 (9.1%) formally withdrew from treatment, 38 (7.7%) withdrew fully from the trial, 17 (3.5%) were withdrawn by the podiatrist, and there were 9 (1.8%) reported deaths (including one participant who died shortly before they were randomised but whose death was only reported later). These withdrawals were not necessarily mutually exclusive: six participants who withdrew from treatment later went on to fully withdraw from the trial and one of the participants who died had previously withdrawn from treatment. In the usual-care group, 28 out of 517 (5.4%) participants withdrew from the trial and 15 out of 517 (2.9%) participants died (mutually exclusive participants). When reasons for withdrawal were provided, these were grouped into common categories, which are listed in Table 3.
| Participation in the trial | Treatment group, n (%) | Total (N = 1010), n (%) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Full participation | 438 (88.8) | 489 (94.6) | 927 (91.8) |
| Withdrew from trial | 38 (7.7) | 28 (5.4) | 66 (6.5) |
| Ill health | 15 (3.0) | 11 (2.1) | 26 (2.6) |
| Relative is ill | 3 (0.6) | 0 (0.0) | 3 (0.3) |
| Too busy | 1 (0.2) | 2 (0.4) | 3 (0.3) |
| Feels cannot contribute/study is not relevant to them | 2 (0.4) | 3 (0.6) | 5 (0.5) |
| Other | 4 (0.8) | 0 (0.0) | 4 (0.4) |
| No reason given | 13 (2.6) | 12 (2.3) | 25 (2.5) |
| Podiatrist withdrew participant from trial | 17 (3.5) | – | – |
| Neuropathy in feet or poor mobility | 9 (1.8) | – | – |
| Ill health | 3 (0.6) | – | – |
| No reason given | 5 (1.0) | – | – |
| Withdrawal from treatment | 45 (9.1) | – | – |
| Ill health/mobility issues | 12 (2.4) | – | – |
| Problem with interventiona | 7 (1.4) | – | – |
| Declined to attend baseline appointment | 5 (1.0) | – | – |
| Does not attend routine podiatry care | 3 (0.6) | – | – |
| Too busy | 3 (0.6) | – | – |
| Moved out of area | 2 (0.4) | – | – |
| Clinic too far away | 2 (0.4) | – | – |
| No reason given | 11 (2.2) | – | – |
Timing of follow-up and response
The median time between completion of the screening form and baseline questionnaire for participants in the entire cohort was 38 days. Owing to the study design, there was often a delay between completion of the baseline questionnaire and randomisation (median 152 days, range 2–584 days). The timing of the 6- and 12-month participant questionnaires was based on the date of randomisation. Time between randomisation and completion of the 6- and 12-month participant questionnaires and the time between when the questionnaires were sent and returned is presented by randomised group in Table 4.
| Time in days between time points | Treatment group | Total (N = 1010) | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | |||||
| Mean (SD) | Median (minimum, maximum) | Mean (SD) | Median (minimum, maximum) | Mean (SD) | Median (minimum, maximum) | |
| Completion of baseline questionnaire and randomisation | 175.0 (133.3) | 149 (2, 544) | 183.1 (135.5) | 161 (5, 584) | 179.1 (134.4) | 152 (2, 584) |
| Randomisation and completion of 6-month participant questionnaire | 198.0 (13.8) | 194 (180, 294) | 196.5 (14.3) | 193 (174, 279) | 197.2 (14.0) | 193 (174, 294) |
| Sent and returned dates for 6-month participant questionnaire | 16.4 (13.0) | 13 (5, 111) | 14.9 (14.4) | 12 (4, 126) | 15.6 (13.7) | 12 (4, 126) |
| Randomisation and completion of 12-month participant questionnaire | 376.9 (13.0) | 373 (359, 467) | 375.9 (13.0) | 372 (337, 465) | 376.4 (13.0) | 373 (337, 467) |
| Sent and returned dates for 12-month participant questionnaire | 13.6 (10.0) | 11 (4, 101) | 12.1 (10.0) | 10 (1, 147) | 12.8 (10.0) | 11 (1, 147) |
Questionnaire return rates by randomised group
The return rates for participant questionnaires administered at 6 and 12 months post randomisation and exercise and orthosis diaries administered to intervention participants only at 3, 6 and 12 months are presented in Table 5. The ‘expected’ columns take into account participants who withdrew or died before the questionnaire was due to be completed. Response rates were consistently high in this trial; overall, 885 participants (87.6% of randomised participants) returned the 12-month participant questionnaire.
| Questionnaire | Treatment group, n (%) | Total (N = 1010), n (%)a | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 493)a | Usual care (N = 517)a | |||||
| Expected | Received | Expected | Received | Expected | Received | |
| Questionnaire | ||||||
| 6 months | 461 (93.5) | 448 (97.2) | 498 (96.3) | 480 (96.4) | 959 (95.0) | 928 (96.8) |
| 12 months | 440 (89.2) | 425 (96.6) | 477 (92.3) | 460 (96.4) | 917 (91.8) | 885 (96.5) |
| Intervention diary | ||||||
| 3 months | 476 (96.6) | 457 (96.0) | – | – | – | – |
| 6 months | 461 (93.5) | 427 (92.6) | – | – | – | – |
| 12 months | 440 (89.2) | 408 (92.7) | – | – | – | – |
The intervention: package of podiatry care
Of the 493 participants allocated to receive the podiatry intervention, 412 (83.6%) attended at least one podiatry appointment and one further participant had a telephone baseline appointment, as the podiatrist was ill on both occasions on which appointments were booked. The trial protocol stated that participants were to be invited to attend two podiatry visits, one as soon as possible after randomisation and another 2–4 weeks later. Further appointments could be offered if required in addition to the participant’s routine podiatry care. In total, 38 participants attended only one appointment and 375 had more than one contact with the podiatrist (occasionally follow-up appointments were conducted over the telephone rather than in person). Participants received a median of two podiatry appointments each (range 1–7 appointments). The first appointment occurred a median of 22 days after randomisation (range 3–275 days) and the second appointment occurred a median of 20 days after the first (range 6–184 days, with one outlier at 343 days for one participant who failed to attend several follow-up appointments booked for them because of conflicting hospital appointments).
The reasons why the remaining 80 participants did not receive the intervention are detailed in Table 6. The intervention was delivered by 28 podiatrists across the 13 centres. Podiatrists saw a median of 10 participants each (range 2–83 participants).
| Intervention received, or reason participant did not receive intervention | Intervention (N = 493), n (%) |
|---|---|
| Attended at least one podiatry appointment | 413 (83.8) |
| Was offered but declined baseline appointmenta | 47 (9.5) |
| Was not made an appointmentb | 31 (5.3) |
| Died shortly before or after randomisation | 2 (0.4) |
Package of podiatry care
A baseline appointment form completed by the treating podiatrist was received for 380 participants. From this, we can summarise details of the intervention delivered, when this was provided. Twenty of 351 participants (5.7%) were reported as currently wearing custom-made shoes. Of the 364 participants who had their usual outdoor footwear assessed by the podiatrist at this visit, 249 (68.4%) were deemed to be wearing appropriate footwear; therefore, 115 (31.6%) exhibited a feature that made the shoes a risk for falling. The most common reason was inappropriate fixation/fastening (n = 81, 70.4%), followed by an inappropriate heel counter (n = 57, 49.5%), incorrect size (n = 43, 37.4%), unsuitable sole (n = 42, 36.5%), inappropriate heel width (n = 17, 14.8%) and excessive heel height (n = 15, 13.0%). New footwear was provided to 260 (52.7% of 493, 63.0% of 413) intervention participants throughout the course of the trial.
At the baseline appointment, 115 out of 363 (31.7%) participants were currently wearing an insole. The type of current insole being used was not recorded for six participants, and two participants reported wearing two different types of insole in different shoes or for different occasions. The most common type of insole being used was a simple flat-bed insole (n = 37, 32.2%), followed by a contoured prefabricated insole with simple modification (n = 25, 21.7%), a bespoke total contact insole (n = 23, 20.0%), a simple contoured prefabricated insole (n = 21, 18.2%) and a contoured prefabricated insole with complex modifications (n = 5, 4.4%).
An orthotic insole was fitted for 241 of the 413 (58.4%) participants who received the intervention (X-Line blue, n = 209; X-Line red, n = 23; Formthotics, n = 9). A therapy ball and theraband were prescribed for 355 (93.4%) and 358 (94.2%) participants, respectively, of the 380 for whom we have this information.
Screening and baseline characteristics
Data collected on the screening form are summarised for consenting participants and those who declined to participate but completed a screening form in Tables 7–9. Consenting participants appear to be more physically able than those who chose not to participate (e.g. tended to be slightly younger, more likely to be willing to attend their local podiatry clinic if required and more likely to be able to walk 10 metres unaided); however, they also appeared to be at a higher risk of falling (e.g. more likely to require a modification to shoes or wear an insole, to have had a previous fall or have concern about falling).
| Characteristic | Consent (N = 3458) | Did not consent (N = 970) | Total (N = 4428) |
|---|---|---|---|
| Age (years)* | |||
| Mean (SD) | 76.7 (7.1) | 78.8 (7.5) | 77.1 (7.2) |
| Median (minimum, maximum) | 77 (64, 99) | 79 (64, 99) | 77 (64, 99) |
| Sex, n (%)* | |||
| Male | 1621 (47.1) | 381 (40.5) | 2002 (45.7) |
| Female | 1819 (52.9) | 559 (59.5) | 2378 (54.3) |
| Ethnic group, n (%) | |||
| White | 3386 (98.8) | 905 (97.6) | 4291 (98.5) |
| Asian or Asian British | 18 (0.5) | 11 (1.2) | 29 (0.7) |
| Black or Black British | 19 (0.6) | 8 (0.9) | 27 (0.6) |
| Other | 6 (0.2) | 3 (0.3) | 9 (0.2) |
| Willing to attend local podiatry clinic if required, n (%)* | 1800 (52.1) | 298 (30.7) | 2098 (47.4) |
| Characteristic | Consent (N = 3458), n (%) | Did not consent (N = 970), n (%) | Total (N = 4428), n (%) |
|---|---|---|---|
| Able to walk for 10 metres unaided* | 2987 (86.4) | 711 (73.3) | 3698 (83.5) |
| Had lower limb surgery in the previous 3 months | 109 (3.2) | 23 (2.4) | 132 (3.0) |
| Lower limb surgery planned in the next 6 months* | 94 (2.7) | 13 (1.3) | 107 (2.4) |
| Has had any toe or lower limb amputations | 98 (2.8) | 18 (1.9) | 116 (2.6) |
| Requires modifications to shoes* | 556 (16.1) | 122 (12.6) | 678 (15.3) |
| Currently wearing an insole or orthosis* | 1156 (33.4) | 201 (20.7) | 1357 (30.7) |
| Comorbiditiesa | |||
| ALS/Lou Gehrig’s disease | 21 (0.6) | 5 (0.5) | 26 (0.6) |
| Alzheimer’s disease* | 38 (1.1) | 27 (2.8) | 65 (1.5) |
| Arthritis* | 1945 (56.3) | 472 (48.7) | 2417 (54.6) |
| Dementia* | 52 (1.5) | 43 (4.4) | 95 (2.2) |
| Depression* | 345 (10.0) | 76 (7.8) | 421 (9.5) |
| Diabetes | 1339 (38.7) | 374 (38.6) | 1713 (38.7) |
| Dizziness/vertigo* | 642 (18.6) | 152 (15.7) | 794 (17.9) |
| Huntington’s disease | 22 (0.6) | 7 (0.7) | 29 (0.7) |
| Ménière’s disease/conditions affecting balance | 142 (4.1) | 29 (3.0) | 171 (3.9) |
| Multiple sclerosis | 25 (0.7) | 9 (0.9) | 34 (0.8) |
| Numbness or tingling in feet or lower limbs* | 1067 (30.9) | 222 (22.9) | 1289 (29.1) |
| Osteoporosis | 466 (13.5) | 122 (12.6) | 588 (13.3) |
| Parkinson’s disease | 61 (1.8) | 15 (1.6) | 76 (1.7) |
| Characteristic | Consent (N = 3458) | Did not consent (N = 970) | Total (N = 4428) |
|---|---|---|---|
| Experienced at least one fall in previous 12 months, n (%)* | 1342 (38.8) | 283 (29.2) | 1625 (36.7) |
| If yes, number of falls,* median (minimum, maximum) | 2 (1, 90) | 2 (1, 24) | 2 (1, 90) |
| Experienced at least one fall in previous 24 months, n (%)* | 1581 (45.7) | 323 (33.3) | 1904 (43.0) |
| If yes, did any require hospitalisation?, n (%)* | 518/1581 (32.8) | 137/323 (42.4) | 655/1904 (34.4) |
| Worried about falling during previous 4 weeks, n (%)* | |||
| All of the time | 199 (5.8) | 48 (5.3) | 247 (5.7) |
| Most of the time | 193 (5.6) | 51 (5.6) | 244 (5.6) |
| A good bit of the time | 242 (7.1) | 35 (3.9) | 277 (6.4) |
| Some of the time | 619 (18.0) | 144 (15.9) | 763 (17.6) |
| A little of the time | 894 (26.0) | 162 (17.9) | 1056 (24.3) |
| None of the time | 1288 (37.5) | 465 (51.4) | 1753 (40.4) |
| Broken a bone in the previous 12 months, n (%)a | 146 (4.2) | 44 (4.5) | 190 (4.3) |
Characteristics for all participants in the cohort (eligible, consenting participants who returned a baseline questionnaire and at least one falls calendar, n = 2301) at screening and baseline are presented in Tables 10–12. The average age of participants in the cohort was 76 years (range 64–99 years), and 44.3% were male (n = 1015). One-third of participants reported experiencing at least one fall in the 12 months prior to completing the screening questionnaire (n = 784, 34.1%). The median number of falls reported in this time was two; however, participants reported up to 60 falls in this time.
| Characteristic | Cohort (N = 2301) |
|---|---|
| Age (years) | |
| Mean (SD) | 76.7 (7.0) |
| Median (minimum, maximum) | 77 (64,a 99) |
| Sex, n (%) | |
| Male | 1015 (44.3) |
| Female | 1279 (55.8) |
| BMI (kg/m2) | |
| Mean (SD) | 27.6 (5.2) |
| Median (IQR) | 27.0 (24.0–30.5) |
| Ethnic group, n (%) | |
| White | 2267 (99.1) |
| Asian or Asian British | 8 (0.4) |
| Other | 9 (0.4) |
| Missing | 4 (0.2) |
| Living arrangements, n (%)b | |
| Lives alone | 925 (40.2) |
| Lives with a partner or spouse | 1257 (54.6) |
| Lives with a friend or relative | 97 (4.2) |
| Lives in sheltered accommodation | 69 (3.0) |
| Education continued after minimum school leaving age, n (%) | 1227 (53.3) |
| Has degree or equivalent professional qualification, n (%) | 758 (32.9) |
| Comorbidities, n (%)b | |
| ALS/Lou Gehrig’s disease | 4 (0.2) |
| Alzheimer’s disease | 6 (0.3) |
| Arthritis | 1234 (53.6) |
| Dementia | 4 (0.2) |
| Depression | 185 (8.0) |
| Diabetes | 834 (36.3) |
| Dizziness/vertigo | 368 (16.0) |
| Huntington’s disease | 5 (0.2) |
| Ménière’s disease/conditions affecting balance | 67 (2.9) |
| Multiple sclerosis | 4 (0.2) |
| Numbness or tingling in feet or lower limbs | 373 (16.2) |
| Osteoporosis | 289 (12.6) |
| Parkinson’s disease | 5 (0.2) |
| Taking more than four medications prescribed by a doctor, n (%) | 1347 (58.5) |
| Able to walk for 10 metres unaided, n (%)a | 2144 (93.2) |
| Had lower limb surgery in the previous 3 months, n (%) | 56 (2.4) |
| Lower limb surgery planned in the next 6 months, n (%)a | 35 (1.5) |
| Has had any toe or lower limb amputations, n (%) | 8 (0.4) |
| Requires modifications to shoes, n (%) | 301 (13.1) |
| Currently wearing an insole or orthosis, n (%) | 739 (32.1) |
| Characteristic | Cohort (N = 2301) |
|---|---|
| Experienced at least one fall in previous 12 months, n (%) | 784 (34.1) |
| If yes, number of falls, median (minimum, maximum) | 2 (1, 60) |
| Experienced at least one fall in previous 24 months, n (%) | 955 (42.0) |
| If yes, did any require hospitalisation?, n (%) | 292/955 (30.6) |
| Worried about falling during previous 4 weeks, n (%) | |
| All of the time | 72 (3.1) |
| Most of the time | 90 (3.9) |
| A good bit of the time | 125 (5.5) |
| Some of the time | 379 (16.5) |
| A little of the time | 651 (28.4) |
| None of the time | 976 (42.6) |
| Broken a bone in the previous 12 months, n (%)a | 87 (3.8) |
| Characteristic | Cohort (N = 2301) |
|---|---|
| Experienced at least one fall in previous 6 months, n (%) | 647 (28.1) |
| If yes, number of falls, median (minimum, maximum) | 1 (1, 30) |
| Worried about falling during previous 4 weeks, n (%) | |
| All of the time | 72 (3.2) |
| Most of the time | 98 (4.3) |
| A good bit of the time | 130 (5.7) |
| Some of the time | 396 (17.4) |
| A little of the time | 732 (32.1) |
| None of the time | 851 (37.3) |
| Referred to a falls clinic/service, n (%) | 103 (4.5) |
Data collected at screening and baseline are presented for all 1010 randomised participants (intervention, n = 493; usual care, n = 517) in Tables 13–16. The average age of the trial participants was 77 years (range 65–99 years) and 39.6% were male (n = 400). The proportion of participants currently wearing an orthotic insole was slightly higher in the intervention group than in the usual-care group (38.7% vs. 31.5%), as was the proportion who reported at least one fall in the 6 months prior to baseline (51.5% vs. 47.6%); otherwise, the randomised groups appear comparable.
| Characteristic | Treatment group | Total (N = 1010) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Age (years) | |||
| Mean (SD) | 78.1 (7.2) | 77.7 (7.0) | 77.9 (7.1) |
| Median (minimum, maximum) | 78 (65, 96) | 78 (65, 99) | 78 (65, 99) |
| Sex, n (%) | |||
| Male | 190 (38.5) | 210 (40.6) | 400 (39.6) |
| Female | 303 (61.5) | 307 (59.4) | 610 (60.4) |
| BMI (kg/m2) | |||
| Mean (SD) | 27.6 (5.3) | 27.7 (5.4) | 27.6 (5.4) |
| Median (IQR) | 26.9 (23.9–30.6) | 27.1 (24.1–30.6) | 27.0 (24.0–30.6) |
| Ethnic group, n (%) | |||
| White | 492 (99.8) | 510 (98.7) | 1002 (99.2) |
| Asian or Asian British | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Other | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Missing | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| Living arrangements, n (%)a | |||
| Lives alone | 236 (47.9) | 220 (42.6) | 456 (45.2) |
| Lives with a partner or spouse | 230 (46.7) | 266 (51.5) | 496 (49.1) |
| Lives with a friend or relative | 22 (4.5) | 27 (5.2) | 49 (4.9) |
| Lives in sheltered accommodation | 19 (3.9) | 14 (2.7) | 33 (3.3) |
| Education continued after minimum school leaving age, n (%) | 269 (54.6) | 296 (57.3) | 565 (55.9) |
| Has degree or equivalent professional qualification, n (%) | 170 (34.5) | 194 (37.5) | 364 (36.0) |
| Characteristic | Treatment group, n (%) | Total (N = 1010), n (%) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Comorbiditiesa | |||
| Arthritis | 292 (59.2) | 300 (58.0) | 592 (58.6) |
| Depression | 49 (9.9) | 48 (9.3) | 97 (9.6) |
| Diabetes | 158 (32.1) | 175 (33.9) | 333 (33.0) |
| Dizziness/vertigo | 107 (21.7) | 95 (18.4) | 202 (20.0) |
| Ménière’s disease/conditions affecting balance | 21 (4.3) | 15 (2.9) | 36 (3.6) |
| Numbness or tingling in feet or lower limbs | 76 (15.4) | 85 (16.4) | 161 (15.9) |
| Osteoporosis | 86 (17.4) | 65 (12.6) | 151 (15.0) |
| Taking more than four medications prescribed by a doctor | 313 (63.5) | 304 (58.8) | 617 (61.1) |
| Requires modifications to shoes | 67 (13.6) | 69 (13.4) | 136 (13.5) |
| Currently wearing an insole or orthosis | 191 (38.7) | 163 (31.5) | 354 (35.1) |
| Characteristic | Treatment group | Total (N = 1010) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Experienced at least one fall in previous 12 months, n (%) | 325 (65.9) | 332 (64.2) | 657 (65.0) |
| If yes, number of falls, median (minimum, maximum) | 2 (1, 25) | 2 (1, 20) | 2 (1, 25) |
| Experienced at least one fall in previous 24 months, n (%) | 329 (66.7) | 330 (63.8) | 659 (65.2) |
| If yes, did any require hospitalisation?, n (%) | 113/329 (34.4) | 99/330 (30.0) | 212/659 (32.2) |
| Worried about falling during previous 4 weeks, n (%) | |||
| All of the time | 23 (4.7) | 22 (4.3) | 45 (4.5) |
| Most of the time | 30 (6.1) | 25 (4.9) | 55 (5.5) |
| A good bit of the time | 42 (8.5) | 43 (8.4) | 85 (8.4) |
| Some of the time | 100 (20.3) | 129 (25.1) | 229 (22.7) |
| A little of the time | 168 (34.1) | 154 (29.9) | 322 (31.9) |
| None of the time | 130 (26.4) | 142 (27.6) | 272 (27.0) |
| Broken a bone in the previous 12 months, n (%) | 38 (7.7) | 27 (5.2) | 65 (6.4) |
| Bones broken, n (%)a | |||
| Crown or facial bone | 1 (2.6) | 0 (0.0) | 1 (1.5) |
| Breast or collar bone | 1 (2.6) | 0 (0.0) | 1 (1.5) |
| Rib | 4 (10.5) | 2 (7.4) | 1 (1.5) |
| Back or spine | 1 (2.6) | 2 (7.4) | 3 (4.6) |
| Shoulder | 5 (13.2) | 2 (7.4) | 7 (10.8) |
| Arm | 3 (7.9) | 3 (11.1) | 6 (9.2) |
| Wrist | 8 (21.1) | 6 (22.2) | 14 (21.5) |
| Hand or finger | 1 (2.6) | 4 (14.8) | 5 (7.7) |
| Hip or pelvis | 2 (5.3) | 2 (7.4) | 4 (6.2) |
| Leg | 4 (10.5) | 4 (14.8) | 8 (12.3) |
| Ankle | 3 (7.9) | 2 (7.4) | 5 (7.7) |
| Foot or toe | 11 (29.0) | 3 (11.1) | 14 (21.5) |
| Characteristic | Treatment group | Total (N = 1010) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Experienced at least one fall in previous 6 months, n (%) | 254 (51.5) | 246 (47.6) | 500 (49.5) |
| If yes, number of falls, median (minimum, maximum) | 1 (1, 20) | 1 (1, 8) | 1 (1, 20) |
| Worried about falling during previous 4 weeks, n (%) | |||
| All of the time | 24 (4.9) | 18 (3.5) | 42 (4.2) |
| Most of the time | 40 (8.2) | 27 (5.3) | 67 (6.7) |
| A good bit of the time | 47 (9.6) | 42 (8.2) | 89 (8.9) |
| Some of the time | 109 (22.2) | 138 (27.0) | 247 (24.6) |
| A little of the time | 165 (33.6) | 178 (34.8) | 343 (34.2) |
| None of the time | 106 (21.6) | 109 (21.3) | 215 (21.4) |
| Referred to a falls clinic/service, n (%) | 32 (6.5) | 35 (6.8) | 67 (6.6) |
Primary outcome
Raw data
In total, 992 (98.2%) trial participants returned at least one falls calendar following randomisation [intervention, n = 484, 98.2%; usual care, n = 508, 98.3%], with 762 (75.5%) returning a complete 12 months’ worth of calendars post randomisation (intervention, n = 360, 73.0%; usual care, n = 402, 77.8%). In total, 1423 falls were reported: 661 in the intervention group (median 1 fall, range 0–23 falls) over a median of 365 days (range 6–365 days), and 762 in the usual-care group (median 1 fall, range 0–28 falls) over a median of 365 days (range 27–365 days) (Figures 3 and 4).
FIGURE 3.
Histogram displaying the distribution of the number of falls by treatment group. (a) Intervention; and (b) usual care.


FIGURE 4.
Histogram displaying the distribution of the length of follow-up for falls data by treatment group. (a) Intervention; and (b) usual care.

Information, such as the cause and location, was available for 1172 (82.7%) falls (intervention, n = 549, 83.3%; usual care, n = 623, 82.1%; Table 17). Over one-third of the falls were reportedly caused by a trip (n = 457, 39%), and an injury was sustained in over half of the falls (n = 655, 55.9%). These injuries include 31 broken bones (from 17 falls in the intervention group and 14 in usual care). The most common bones broken in a fall were the hip or bones in the hand (n = 5 each).
| Information about fall | Treatment group, n (%) | Total (N = 1172), n (%) | |
|---|---|---|---|
| Intervention (N = 549) | Usual care (N = 623) | ||
| Cause of/reason for fall | |||
| Trip | 205 (37.3) | 252 (40.5) | 457 (39.0) |
| Slip | 55 (10.0) | 76 (12.2) | 131 (11.2) |
| Turning | 51 (9.3) | 42 (6.7) | 93 (7.9) |
| Legs gave way | 50 (9.1) | 67 (10.8) | 117 (10.0) |
| Dizzy | 23 (4.2) | 34 (5.5) | 57 (4.9) |
| Lost balance | 60 (10.9) | 48 (7.7) | 108 (9.2) |
| Unknown/cannot remember | 48 (8.7) | 42 (6.7) | 90 (7.7) |
| Other | 70 (12.8) | 77 (12.4) | 147 (12.5) |
| Location of fall | |||
| Inside own home | 221 (40.3) | 263 (42.2) | 484 (41.3) |
| Inside, but not in own home | 40 (7.3) | 54 (8.7) | 94 (8.0) |
| Outside | 259 (47.2) | 280 (44.9) | 539 (46.0) |
| Missing | 29 (5.3) | 26 (4.2) | 55 (4.7) |
| If fall was inside, was it . . ? | |||
| On one level | 169 (64.5) | 195 (61.5) | 364 (62.9) |
| Accessing shower/bath | 6 (2.3) | 23 (7.3) | 29 (5.0) |
| Getting out of bed | 17 (6.5) | 23 (7.3) | 40 (6.9) |
| Getting out of a chair | 22 (8.4) | 19 (6.0) | 41 (7.1) |
| Walking up or down stairs | 42 (16.0) | 52 (16.4) | 94 (16.2) |
| Accessing the toilet | 6 (2.3) | 5 (1.6) | 11 (1.9) |
| If fall was outside, was it. . ? | |||
| Car park/driveway | 16 (6.2) | 13 (4.6) | 29 (5.4) |
| Crossing a street | 4 (1.5) | 8 (2.9) | 12 (2.2) |
| Garden/grassed area | 73 (28.2) | 76 (27.1) | 149 (27.6) |
| Getting in or out of a vehicle | 5 (1.9) | 5 (1.8) | 10 (1.9) |
| On a bus or train | 3 (1.2) | 1 (0.4) | 4 (0.7) |
| On a footpath | 77 (29.7) | 86 (30.7) | 163 (30.2) |
| On a kerb | 13 (5.0) | 13 (4.6) | 26 (4.8) |
| On a step/escalator | 18 (7.0) | 29 (10.4) | 47 (8.7) |
| On one level | 8 (3.1) | 11 (3.9) | 19 (3.5) |
| Other | 27 (10.4) | 21 (7.5) | 48 (8.9) |
| Missing | 15 (5.8) | 17 (6.1) | 32 (5.9) |
| Footwear worn | |||
| Barefoot | 45 (8.2) | 66 (10.6) | 111 (9.5) |
| Slippers | 126 (23.0) | 121 (19.4) | 247 (21.1) |
| Shoes/boots | 292 (53.2) | 332 (53.3) | 624 (53.4) |
| Wellington boots | 11 (2.0) | 4 (0.6) | 15 (1.3) |
| Flip-flops/sandals | 22 (4.0) | 36 (5.8) | 58 (5.0) |
| Cannot remember | 24 (4.4) | 34 (5.5) | 58 (5.0) |
| Missing | 29 (5.3) | 30 (4.8) | 59 (5.0) |
| Using a walking aid | |||
| Yes | 83 (15.1) | 91 (14.6) | 174 (14.9) |
| No | 414 (75.4) | 483 (77.5) | 897 (76.5) |
| Missing | 52 (9.5) | 49 (7.9) | 101 (8.6) |
| Wearing an insole or orthosis | |||
| Yes | 200 (36.4) | 135 (21.7) | 335 (28.6) |
| No | 289 (52.6) | 410 (65.8) | 699 (59.6) |
| Missing | 60 (10.9) | 78 (12.5) | 138 (11.8) |
| Injuries suffered | |||
| None | 195 (35.5) | 259 (41.6) | 454 (38.7) |
| Superficial | 305 (55.6) | 319 (51.2) | 624 (53.2) |
| Broken bone | 17 (3.1) | 14 (2.3) | 31 (2.7) |
| Missing | 32 (5.8) | 31 (5.0) | 63 (5.4) |
| Bones broken | |||
| Crown or facial bone | 1 (5.9) | 0 (0.0) | 1 (3.2) |
| Breast or collar bone | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rib | 2 (11.8) | 1 (7.1) | 3 (9.7) |
| Back or spine | 1 (5.9) | 0 (0.0) | 1 (3.2) |
| Shoulder | 2 (11.8) | 0 (0.0) | 2 (6.5) |
| Arm | 0 (0.0) | 1 (7.1) | 1 (3.2) |
| Wrist | 1 (5.9) | 14 (14.3) | 3 (9.7) |
| Hand or finger | 2 (11.8) | 3 (21.4) | 5 (16.1) |
| Hip or pelvis | 3 (17.7) | 14 (14.3) | 5 (16.1) |
| Leg | 1 (5.9) | 1 (7.1) | 2 (6.5) |
| Ankle | 1 (5.9) | 0 (0.0) | 1 (3.2) |
| Foot or toe | 2 (11.8) | 14 (14.3) | 4 (12.9) |
| Other/unknown | 1 (5.9) | 14 (14.3) | 3 (9.7) |
| Overnight stay in hospital required | |||
| Yes | 15 (2.7) | 16 (2.6) | 31 (2.7) |
| No | 473 (86.2) | 527 (84.6) | 1000 (85.3) |
| Missing | 61 (11.1) | 80 (12.8) | 141 (12.0) |
| If yes, how many nights? | |||
| Median (minimum, maximum) | 7 (1, 21) | 5 (1, 21) | 6 (1, 21) |
Covariates
The primary analysis model controlled for sex, age at randomisation and history of falling. The age of one participant in the usual-care group was not available so the primary model was based on 991 participants (484 in the intervention group and 507 in the usual-care group).
Screening and baseline data for participants analysed in the primary model
Data collected on the screening form and baseline questionnaire are presented by randomised group for the 991 participants included in the primary analysis (‘as analysed’ population; Tables 18–21). The composition of the analysis groups is virtually identical to that at randomisation, indicating that the loss of the 19 participants from the primary analysis has not introduced any selection bias.
| Characteristic | Treatment group | Total (N = 991) | |
|---|---|---|---|
| Intervention (N = 484) | Usual care (N = 507) | ||
| Age (years) | |||
| Mean (SD) | 78.1 (7.2) | 77.6 (7.0) | 77.8 (7.1) |
| Median (minimum, maximum) | 78 (65, 96) | 78 (65, 99) | 78 (65, 99) |
| Sex, n (%) | |||
| Male | 189 (39.1) | 207 (40.8) | 396 (40.0) |
| Female | 295 (61.0) | 300 (59.2) | 595 (60.0) |
| BMI (kg/m2) | |||
| Mean (SD) | 27.6 (5.3) | 27.7 (5.4) | 27.6 (5.4) |
| Median (IQR) | 27.0 (23.9–30.6) | 27.1 (24.1–30.6) | 27.1 (24.0–30.6) |
| Ethnic group, n (%) | |||
| White | 483 (99.8) | 500 (98.6) | 983 (99.2) |
| Asian or Asian British | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Other | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Missing | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| Living arrangements, n (%)a | |||
| Lives alone | 230 (47.5) | 214 (42.2) | 444 (44.8) |
| Lives with a partner or spouse | 227 (46.9) | 262 (51.7) | 489 (49.3) |
| Lives with a friend or relative | 22 (4.6) | 27 (5.3) | 49 (4.9) |
| Lives in sheltered accommodation | 18 (3.7) | 14 (2.8) | 32 (3.2) |
| Education continued after minimum school leaving age, n (%) | 263 (54.3) | 289 (57.0) | 552 (55.7) |
| Has degree or equivalent professional qualification, n (%) | 167 (34.5) | 191 (37.7) | 358 (36.1) |
| Characteristic | Treatment group, n (%) | Total (N = 991), n (%) | |
|---|---|---|---|
| Intervention (N = 484) | Usual care (N = 507) | ||
| Comorbiditiesa | |||
| Arthritis | 286 (59.1) | 290 (57.2) | 576 (58.1) |
| Depression | 46 (9.5) | 48 (9.5) | 94 (9.5) |
| Diabetes | 155 (32.0) | 168 (33.1) | 323 (32.6) |
| Dizziness/vertigo | 102 (21.1) | 91 (18.0) | 193 (19.5) |
| Ménière’s disease/conditions affecting balance | 20 (4.1) | 15 (3.0) | 35 (3.5) |
| Numbness or tingling in feet or lower limbs | 76 (15.7) | 83 (16.4) | 159 (16.0) |
| Osteoporosis | 83 (17.2) | 65 (12.8) | 148 (14.9) |
| Taking more than four medications prescribed by a doctor | 305 (63.0) | 297 (58.6) | 602 (60.8) |
| Requires modifications to shoes | 67 (13.8) | 68 (13.4) | 135 (13.6) |
| Currently wearing an insole or orthosis | 189 (39.1) | 161 (31.8) | 350 (35.3) |
| Characteristic | Treatment group | Total (N = 991) | |
|---|---|---|---|
| Intervention (N = 484) | Usual care (N = 507) | ||
| Experienced at least one fall in previous 12 months, n (%) | 319 (65.9) | 323 (63.7) | 642 (64.8) |
| If yes, number of falls, median (minimum, maximum) | 2 (1, 25) | 1 (1, 20) | 2 (1, 25) |
| Experienced at least one fall in previous 24 months, n (%) | 323 (66.7) | 321 (63.3) | 644 (65.0) |
| If yes, did any require hospitalisation?, n (%) | 110/323 (34.1) | 96/321 (29.9) | 206/644 (32.0) |
| Worried about falling during previous 4 weeks, n (%) | |||
| All of the time | 22 (4.6) | 21 (4.2) | 43 (4.4) |
| Most of the time | 27 (5.6) | 24 (4.8) | 51 (5.2) |
| A good bit of the time | 42 (8.7) | 41 (8.1) | 83 (8.4) |
| Some of the time | 100 (20.7) | 128 (25.4) | 228 (23.1) |
| A little of the time | 163 (33.7) | 151 (29.9) | 314 (31.8) |
| None of the time | 130 (26.9) | 140 (27.7) | 270 (27.3) |
| Broken a bone in the previous 12 months, n (%)a | 36 (7.4) | 27 (5.3) | 63 (6.4) |
| Characteristic | Treatment group | Total (N = 991) | |
|---|---|---|---|
| Intervention (N = 484) | Usual care (N = 507) | ||
| Experienced at least one fall in previous 6 months, n (%) | 248 (51.2) | 246 (47.6) | 500 (49.5) |
| If yes, number of falls, median (minimum, maximum) | 1 (1, 20) | 1 (1, 8) | 1 (1, 20) |
| Worried about falling during previous 4 weeks, n (%) | |||
| All of the time | 22 (4.6) | 18 (3.6) | 40 (4.1) |
| Most of the time | 40 (8.3) | 26 (5.2) | 66 (6.7) |
| A good bit of the time | 44 (9.1) | 40 (8.0) | 84 (8.5) |
| Some of the time | 106 (22.0) | 137 (27.3) | 243 (24.7) |
| A little of the time | 165 (34.2) | 173 (34.5) | 338 (34.4) |
| None of the time | 105 (21.8) | 108 (21.5) | 213 (21.7) |
| Referred to a falls clinic/service, n (%) | 30 (6.2) | 35 (6.9) | 65 (6.6) |
Number of falls by centre
A summary of the number of falls for participants contributing to the primary analysis is presented by centre in Table 22, in order of largest to smallest contributing centre.
| Centre | Number of participants contributing to primary analysis | Number of falls | |
|---|---|---|---|
| Mean (SD) | Median (minimum, maximum) | ||
| 1 | 320 | 1.4 (2.2) | 1 (0, 15) |
| 2 | 131 | 1.6 (3.0) | 1 (0, 23) |
| 3 | 102 | 1.3 (2.0) | 1 (0, 10) |
| 4 | 91 | 1.5 (2.4) | 0.5 (0, 10) |
| 5 | 63 | 0.8 (1.4) | 0 (0, 6) |
| 6 | 56 | 1.9 (3.2) | 1 (0, 18) |
| 7 | 46 | 1.5 (2.3) | 1 (0, 13) |
| 8 | 41 | 1.6 (2.7) | 1 (0, 15) |
| 9 | 38 | 1.6 (2.9) | 1 (0, 17) |
| 10 | 31 | 2.2 (5.1) | 1 (0, 28) |
| 11 | 27 | 1.1 (1.3) | 1 (0, 5) |
| 12 | 26 | 1.2 (1.6) | 0.5 (0, 6) |
| 13 | 19 | 1.1 (1.7) | 0 (0, 6) |
Primary analysis
The adjusted negative binomial model indicated a non-statistically significant reduction in the fall rate in the intervention group relative to usual care (IRR 0.88, 95% CI 0.73 to 1.05; p = 0.16). History of falling was seen to be a significant predictor in the model (IRR 2.10, 95% CI 1.74 to 2.54; p < 0.001; Table 23). Little difference in the estimate of the treatment effect was observed when centre was included as a fixed, as opposed to a random, effect in the primary analysis model in a sensitivity analysis (IRR 0.88, 95% CI 0.73 to 1.07; p = 0.20).
| Variable | IRR (standard error) | 95% CI (p-value) |
|---|---|---|
| Allocation (0, usual care; 1, intervention) | 0.88 (0.08) | 0.73 to 1.05 (p = 0.16) |
| Sex (0, female; 1, male) | 1.10 (0.10) | 0.91 to 1.32 (p = 0.33) |
| Age at randomisation (years) | 1.01 (0.01) | 0.99 to 1.02 (p = 0.31) |
| History of falling (0, fewer than two falls in 12 months before screening; 1, more than two falls) | 2.10 (0.20) | 1.74 to 2.54 (p < 0.001) |
| Constant | 0.002 (0.001) | < 0.001 to 0.005 (p < 0.001) |
Sensitivity analyses
Non-compliance
When non-compliance with the intervention was accounted for using an instrumental variable CACE analysis approach, the intervention was seen to have a marginally greater benefit than in the ITT analysis but the conclusions were otherwise consistent (IRR 0.86, 95% CI 0.69 to 1.06; p = 0.16).
Podiatrist effects
A single podiatrist appeared to deliver the intervention to all participants in 6 of the 13 centres. In the other seven centres, two, three, four (two centres each) or six (one centre) podiatrists held intervention appointments. Counterfactual podiatrists were assigned to the 80 participants in the intervention group who did not receive the intervention and the 517 usual care participants. Repeating the primary analysis with podiatrist as a random effect in the place of centre had a negligible effect on the treatment estimate (IRR 0.88, 95% CI 0.73 to 1.05; p = 0.16).
Baseline imbalance by chance
A sensitivity analysis of the primary outcome was planned, which adjusted the model for any pre-randomisation variables found to be imbalanced by chance between the randomised groups, namely proportion of participants wearing an insole, number of falls in 6 months prior to completion of baseline questionnaire and total FAI score. However, owing to concerns that this model could be overparameterised by including two variables relating to past falls history (a dichotomous variable indicating two or more falls in the 12 months prior to screening and a continuous variable of the number of falls recalled in the 6 months before baseline), the number of falls variable was not included.
The resultant model was based on 912 participants (intervention, n = 448, 90.9%; usual care, n = 464, 89.7%). Of those included in the primary model, 77 did not have a valid baseline FAI score and three did not provide a response to whether or not they were wearing an orthotic insole at screening; 79 participants were missing at least one of these additional covariates.
When these variables were added to the primary model, the predicted IRR was 0.88 (95% CI 0.72 to 1.06; p = 0.18), which is virtually unchanged from the primary model.
Fear of falling participants
Excluding the 26 fear of falling participants from the primary model had a negligible effect on the treatment estimate (IRR 0.88, 95% CI 0.73 to 1.05; p = 0.16).
Missing data
Not living with a partner or spouse, not having a degree or equivalent professional qualification, reporting dizziness or vertigo at screening and number of falls in the 6 months prior to completion of the baseline questionnaire were observed to predict returning less than 6 months’ worth of falls calendar data post randomisation. When these variables were included in the primary analysis model (excluding the number of falls variable for the same reasons as cited above), the parameter estimate for the treatment effect was IRR 0.86 (95% CI 0.72 to 1.04; p = 0.11). This model was based on 976 participants (intervention, n = 477, 96.8%; usual care, n = 499, 96.5%).
Post hoc analysis
Prior to the analysis of this study, a trial of structured physical activity for the prevention of serious fall injuries in adults aged 70–89 years [Lifestyle Interventions and Independence for Elders (LIFE) study] was published. 45 In subgroup analyses the authors observed that the hazard ratio for time to first serious fall injury did not differ significantly according to sex (interaction p = 0.14); however, a clinically meaningful qualitative difference was observed with a hazard ratio of 0.62 (95% CI 0.34 to 1.12) in men and of 1.05 (95% CI 0.72 to 1.52) in women. In an analysis that was not prespecified, we repeated the primary analysis in the subgroups of males and females and found similar treatment effects in each (men: IRR 0.87, 95% CI 0.64 to 1.17; women: 0.86, 95% CI 0.68 to 1.09). When an interaction between sex and treatment allocation was included in the primary model, the interaction was not observed to be statistically significant (p = 0.93).
Secondary analyses
Number of falls as reported for the previous 6 months on the 6- and 12-month participant questionnaires
This outcome was computed for 450 (91.3%) participants in the intervention group and 484 (93.6%) in the usual-care group. In the intervention group, 423 (85.8%) participants responded to this question at both 6 and 12 months and 27 (5.5%) responded at either 6 or 12 months only; the average number of falls reported in this group was 1.5 (median 1 fall, range 0–20 falls). In the usual-care group, 457 (88.4%) participants responded to this question at both 6 and 12 months and 27 (5.2%) responded at either 6 or 12 months only; the average number of falls reported in this group was 1.7 (median 1 fall, range 0–29 falls). The adjusted IRR obtained from the negative regression model was 0.87 (95% CI 0.72 to 1.06; p = 0.17).
Proportion of fallers and multiple fallers
In total, 245 out of 493 (49.7%) intervention participants and 284 out of 517 (54.9%) usual care participants reported at least one fall on their monthly falls calendars (adjusted OR 0.78, 95% CI 0.60 to 1.00; p = 0.05). An OR of 0.6 (lower confidence limit) approximately relates to a decrease in the percentage of fallers from 55% in the usual-care group to 42% in the intervention group, which exceeds the 10 percentage point difference for which the trial was powered.
To calculate the percentage of the intervention group, let us say that the 2 × 2 table for falls by allocation is as given in Table 24.
| Treatment group | Fall | No fall | Total |
|---|---|---|---|
| Intervention | a | b | a + b |
| Usual care | c | d | c + d |
| Total | a + c | b + d | N |
In this case, we know that a + b = 493, c = 284, d = 233, c + d = 517 and N = 1010. The calculation for the OR is:
We want to know the values of a and b if c and d are as observed in the trial and the OR is 0.6. Rearranging (2), we get:
We know that a + b = 493, so if we rearrange and solve these equations, we conclude that a = 208 and b = 285. Therefore, if there were 208 fallers, this would equate to a percentage of 208/493 × 100 = 42%.
The analysis of fallers assumes that the 18 participants who did not return any falls calendars following randomisation into the trial did not fall. To test the sensitivity of the results to this assumption we repeated the logistic regression (1) dropping these participants and (2) assuming, conversely, that they did fall at least once. Estimates were robust: adjusted OR 0.77 (95% CI 0.59 to 0.99; p = 0.04); and OR 0.77 (95% CI 0.60 to 0.99; p = 0.05), respectively. In addition, if participants did not report a fall, it is implicitly assumed that these participants did not fall in the months for which they did not return a falls calendar. To test this, we assumed that a random 50% of the 185 participants [99/493 (20.1%) participants in the intervention group and 86/517 (16.6%) participants in the usual-care group] who did not report a fall and did not complete a falls calendar for all 12 months post randomisation fell at least once in a month for which data were missing. This increased the effect (adjusted OR 0.72, 95% CI 0.55 to 0.93; p = 0.01).
The proportion of participants who reported two or more falls on their falls calendars following randomisation was also lower in the intervention group than in the usual-care group [27.6% (n = 136/493) vs. 34.6% (n = 179/517); adjusted OR 0.69, 95% CI 0.52 to 0.90; p = 0.01].
Time to first fall
The median time to the first fall and its associated 95% CIs were estimated at 314 days (95% CI 267 days, upper limit not calculable) in the intervention group and 257 days in the usual-care group (95% CI 209 to 319 days). Kaplan–Meier survival curves are presented for each group in Figure 5. The adjusted hazard ratio from the Cox proportional hazards model for the treatment effect was 0.88 (95% CI 0.74 to 1.04; p = 0.13), indicating that the hazard or chance of falling at any particular time is lower in the intervention group than in the usual-care group, but this ratio is not statistically significant. Log-log plots of the categorical covariates indicated slight violation of the proportional hazard assumption for sex and allocation (and indeed we observe that the survival curves cross one another on the Kaplan–Meier curve but this occurs relatively early on); however, the Grambsch and Therneau test did not provide evidence that the assumptions did not hold.
FIGURE 5.
Kaplan–Meier curves by randomised group for time to first fall. Reproduced from Cockayne et al. 44 This is an open access article distributed under the terms of the Creative Commons Attribution Licence (CC BY 4.0), which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited (https://creativecommons.org/licenses/by/4.0/).
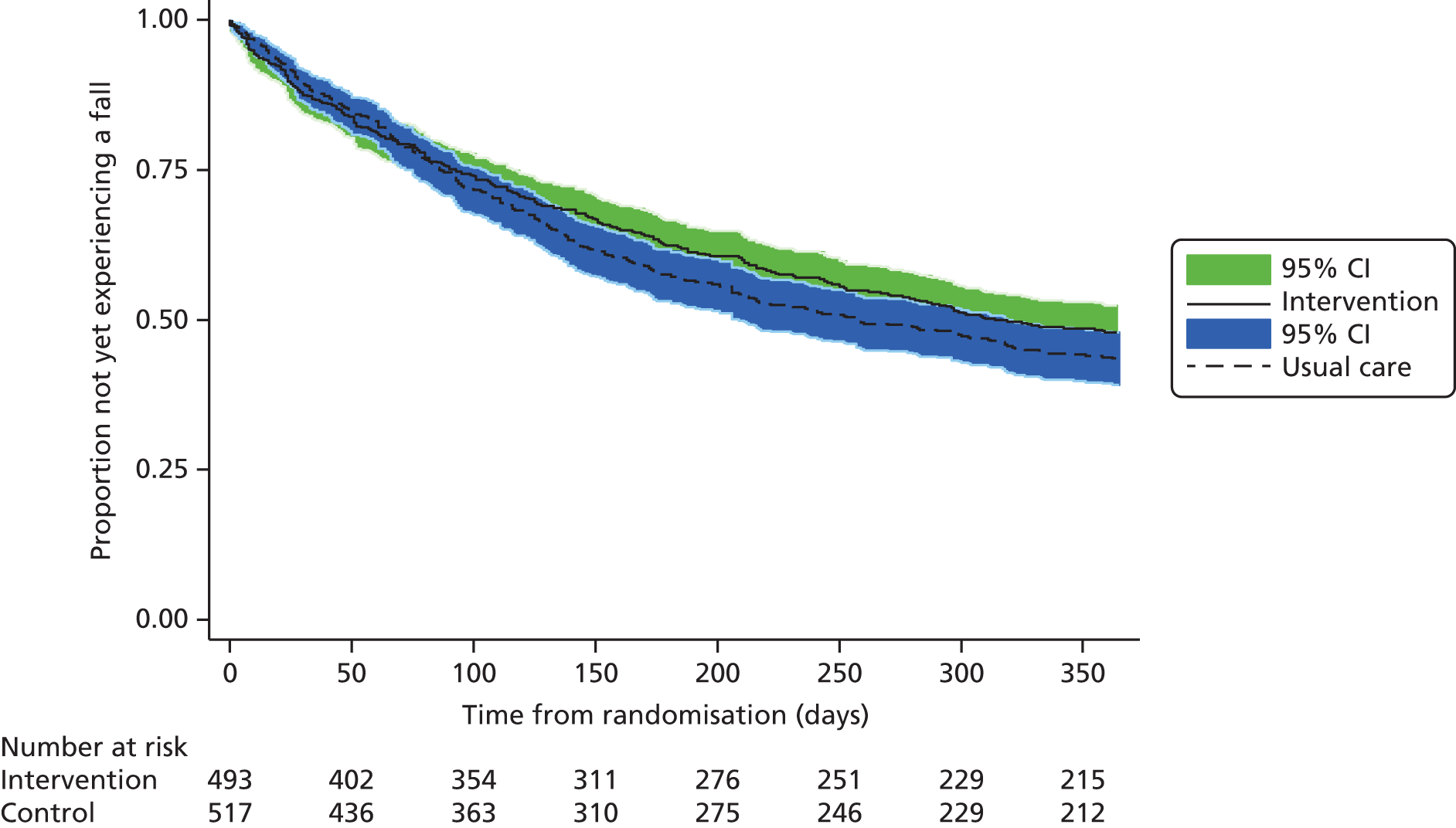
Participant-reported outcome measures
No statistically significant differences between the two groups were observed at 6 or 12 months in the fear of falling question, the Short FES-I, the FAI or the GDS (Tables 25–28). For the Short FES-I and the GDS, the residuals from the model showed slight violation from normality, so the models were repeated using a log-transformed outcome, but this did not change the conclusions. The results presented in the main body of the table are for the untransformed analysis, with the results from the transformed analysis included in the footer. The Short FES-I total score was categorised and is presented in Table 29. At 12 months, a similar proportion of participants in each group reported no, or low, concern about falling (intervention, 21.2%; usual care, 22.2%), but, of those who were concerned, the proportion reporting high concern about falling was slightly higher in the intervention group (32.2% vs. 30.0%). No difference in CD-RISC2 score at 6 months was observed between the groups (adjusted means: intervention 6.4, usual care 6.3; adjusted mean difference: 0.10, 95% CI –0.08 to 0.28; p = 0.26). Higher scores reflect greater resilience and adaptability.
| Time point | Unadjusted | Adjusteda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Total (N = 1010) | Treatment group | Mean difference (95% CI); p-value | |||||||||||
| Intervention (N = 493) | Usual care (N = 517) | Intervention | Usual care | |||||||||||
| n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 491 | 4.4 (1.4) | 5 (1, 6) | 512 | 4.5 (1.3) | 5 (1, 6) | 1003 | 4.4 (1.3) | 5 (1, 6) | – | – | – | – | – |
| Month 6 | 424 | 4.4 (1.4) | 5 (1, 6) | 461 | 4.4 (1.3) | 5 (1, 6) | 885 | 4.4 (1.3) | 5 (1, 6) | 4.4 (0.06) | 4.3 to 4.5 | 4.4 (0.05) | 4.3 to 4.5 | 0.08 (–0.05 to 0.21); p = 0.24 |
| Month 12 | 417 | 4.4 (1.4) | 5 (1, 6) | 453 | 4.3 (1.3) | 4 (1, 6) | 870 | 4.3 (1.4) | 5 (1, 6) | 4.4 (0.06) | 4.3 to 4.5 | 4.3 (0.06) | 4.2 to 4.4 | 0.13 (–0.01 to 0.27); p = 0.07 |
| Time point | Unadjusted | Adjusteda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Total (N = 1010) | Treatment group | Mean difference (95% CI); p-value | |||||||||||
| Intervention (N = 493) | Usual care (N = 517) | Intervention | Usual care | |||||||||||
| n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 481 | 12.4 (4.6) | 11 (7, 28) | 504 | 12.2 (4.2) | 11 (7, 27) | 985 | 12.3 (4.4) | 11 (7, 28) | – | – | – | – | – |
| Month 6 | 425 | 12.4 (4.9) | 11 (7, 28) | 451 | 12.2 (4.3) | 11 (7, 28) | 876 | 12.3 (4.6) | 11 (7, 28) | 12.4 (0.16) | 12.1 to 12.7 | 12.2 (0.15) | 11.9 to 12.5 | 0.13 (–0.30 to 0.56); p = 0.56 |
| Month 12 | 410 | 12.7 (4.9) | 11 (7, 28) | 447 | 12.2 (4.4) | 11 (7, 28) | 857 | 12.4 (4.7) | 11 (7, 28) | 12.6 (0.16) | 12.3 to 12.9 | 12.3 (0.16) | 12.0 to 12.6 | 0.30 (–0.14 to 0.73); p = 0.19 |
| Time point | Unadjusted | Adjusteda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Total (N = 1010) | Treatment group | Mean difference (95% CI); p-value | |||||||||||
| Intervention (N = 493) | Usual care (N = 517) | Intervention | Usual care | |||||||||||
| n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 456 | 45.5 (7.9) | 47 (20, 60) | 473 | 46.8 (7.0) | 48 (19, 60) | 929 | 46.2 (7.5) | 48 (19, 60) | – | – | – | – | – |
| Month 6 | 365 | 45.2 (8.3) | 47 (18, 59) | 405 | 45.9 (7.9) | 48 (16, 59) | 770 | 45.5 (8.1) | 47 (16, 59) | 45.7 (0.26) | 45.2 to 46.2 | 45.9 (0.25) | 45.4 to 46.4 | –0.22 (–0.84 to 0.41); p = 0.50 |
| Month 12 | 372 | 45.3 (8.0) | 46 (18, 59) | 388 | 45.8 (8.0) | 48 (15, 60) | 760 | 45.6 (8.0) | 47 (15, 60) | 45.4 (0.27) | 44.9 to 46.0 | 45.4 (0.26) | 44.9 to 45.9 | 0.01 (–0.65 to 0.67); p = 0.98 |
| Time point | Unadjusted | Adjusteda | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Total (N = 1010) | Treatment group | Mean difference (95% CI); p-value | |||||||||||
| Intervention (N = 493) | Usual care (N = 517) | Intervention | Usual care | |||||||||||
| n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | n | Mean (SD) | Median (minimum, maximum) | Mean (SE) | 95% CI | Mean (SE) | 95% CI | ||
| Baseline | 484 | 3.8 (3.1) | 3 (0, 14) | 510 | 3.6 (3.0) | 3 (0, 15) | 994 | 3.7 (3.0) | 3 (0, 15) | – | – | – | – | – |
| Month 6 | 439 | 3.8 (3.2) | 3 (0, 15) | 467 | 3.6 (3.0) | 3 (0, 14) | 906 | 3.7 (3.1) | 3 (0, 15) | 3.7 (0.10) | 3.5 to 3.9 | 3.7 (0.09) | 3.5 to 3.9 | 0.05 (–0.21 to 0.32); p = 0.70 |
| Month 12 | 418 | 3.7 (3.3) | 3 (0, 14) | 450 | 3.4 (3.0) | 3 (0, 15) | 868 | 3.5 (3.2) | 3 (0, 15) | 3.7 (0.11) | 3.5 to 3.9 | 3.5 (0.10) | 3.3 to 3.7 | 0.22 (–0.07 to 0.51); p = 0.13 |
| Time point | Treatment group | Total (N = 1010) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | |||||||||||
| n | Level of concern | n | Level of concern | n | Level of concern | |||||||
| None/low | Moderate | High | None/low | Moderate | High | None/low | Moderate | High | ||||
| Baseline | 481 | 75 (15.6) | 260 (54.1) | 146 (30.4) | 504 | 73 (14.5) | 293 (58.1) | 138 (27.4) | 985 | 148 (15.0) | 553 (56.1) | 284 (28.8) |
| Month 6 | 425 | 90 (21.2) | 201 (47.3) | 134 (31.5) | 451 | 89 (19.7) | 226 (50.1) | 136 (30.2) | 876 | 179 (20.4) | 427 (48.7) | 270 (30.8) |
| Month 12 | 410 | 87 (21.2) | 183 (44.6) | 140 (34.2) | 447 | 99 (22.2) | 214 (47.9) | 134 (30.0) | 857 | 186 (21.7) | 397 (46.3) | 274 (32.0) |
Proportion of participants with depression
The proportion of participants who were depressed was higher in the intervention group than in the usual-care group (as measured by a score of ≥ 6 on the GDS) at all three assessment time points (12 months: 23.2% vs. 19.1%; Table 30). The adjusted OR at 12 months was 1.26 but this effect was not statistically significant (95% CI 0.91 to 1.75; p = 0.16).
| GDS score of ≥ 6 | Treatment group | Total (N = 1010) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Baseline | |||
| n | 484 | 510 | 994 |
| Depressed, n (%) | 120 (24.8) | 104 (20.4) | 224 (22.5) |
| Not depressed, n (%) | 364 (75.2) | 406 (79.6) | 770 (77.5) |
| Month 6 | |||
| n | 439 | 467 | 906 |
| Depressed, n (%) | 113 (25.7) | 101 (21.6) | 214 (23.6) |
| Not depressed, n (%) | 326 (74.3) | 366 (78.4) | 692 (76.4) |
| Month 12 | |||
| n | 418 | 450 | 868 |
| Depressed, n (%) | 97 (23.2) | 86 (19.1) | 183 (21.1) |
| Not depressed, n (%) | 321 (76.8) | 364 (80.9) | 685 (78.9) |
Proportion of participants obtaining a fracture or multiple fractures
Over the 12-month follow-up, 31 participants (intervention, n = 17; usual care, n = 14) reported breaking or fracturing a bone as a result of a fall (adjusted OR 1.21, 95% CI 0.59 to 2.49; p = 0.60). Two participants, both in the intervention group, reported fractures from two distinct events. The types of fractures reported are presented in Table 31.
| Type of fracture | Treatment group, n (%) | Total (N = 33), n (%) | |
|---|---|---|---|
| Intervention (N = 19) | Usual care (N = 14) | ||
| Hip | 5 (26.3) | 2 (14.3) | 7 (21.2) |
| Hand/finger | 2 (10.5) | 3 (21.4) | 5 (15.2) |
| Toe/foot | 2 (10.5) | 2 (14.3) | 4 (12.1) |
| Wrist | 2 (10.5) | 2 (14.3) | 4 (12.1) |
| Leg | 2 (10.5) | 1 (7.1) | 3 (9.1) |
| Rib | 1 (5.3) | 1 (7.1) | 2 (6.1) |
| Shoulder | 2 (10.5) | 0 (0.0) | 2 (6.1) |
| Ankle | 1 (5.3) | 0 (0.0) | 1 (3.0) |
| Arm | 0 (0.0) | 1 (7.1) | 1 (3.0) |
| Spine/back | 1 (5.3) | 0 (0.0) | 1 (3.0) |
| Unknown | 1 (5.3) | 2 (14.3) | 3 (9.1) |
Foot pain
Participants in the intervention group reported greater foot pain at 12 months (adjusted mean 3.1 vs. 2.6; adjusted mean difference 0.43, 95% CI 0.06 to 0.80; p = 0.02). When non-compliance with the intervention was accounted for through CACE analysis, the predicted mean pain score among compliers in the intervention group was 3.1, and among the counterfactual group of compliers in the usual-care group was 2.6 (adjusted mean difference 0.50, 95% CI 0.08 to 0.92; p = 0.02).
Adverse events
Serious adverse events
A total of 95 SAEs were reported in the period between randomisation and 1 month following the trial end (12 months after randomisation), by 49 (9.9%) participants in the intervention group and 37 (7.2%) participants in the usual-care group (Table 32). The majority of participants (90.7%) reported only one event. During the reporting period, there were 23 reported deaths (eight in the intervention group and 15 in usual care); all deaths were considered expected. For seven deaths, the relationship to research procedures could not be assessed owing to a lack of information, but, for those that could, none was deemed to be related. Nearly two-thirds of all SAEs were hospitalisations (n = 62, 65.3%). The two events considered to be life- or limb-threatening were in the intervention group, and one of these was related to the intervention. Details of the SAEs deemed to be at least possibly related to the research are presented in Table 33. None of these events was attributable to the exercise programme but tended to relate to the trial shoes or orthosis.
| SAEs | Treatment group | Total (N = 1010) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Total number of SAEs | 53 | 42 | 95 |
| Number of participants with one or more SAEs | 49 | 37 | 86 |
| Number of events per participant, n (%) | |||
| 1 | 45 (91.8) | 33 (89.2) | 78 (90.7) |
| 2 | 4 (8.2) | 3 (8.1) | 7 (8.1) |
| 3 | 0 (0.0) | 1 (2.7) | 1 (1.2) |
| Event details, n (%) | |||
| Death | 8 (15.1) | 15 (35.7) | 23 (24.2) |
| Hospital required/prolonged | 36 (67.9) | 26 (61.9) | 62 (65.3) |
| Life-/limb-threatening | 2 (3.8) | 0 (0.0) | 2 (2.1) |
| Disability | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 7 (13.2) | 1 (2.4) | 8 (8.4) |
| Intensity, n (%) | |||
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 5 (9.4) | 2 (4.8) | 7 (7.4) |
| Severe | 47 (88.7) | 40 (95.2) | 87 (91.6) |
| Missinga | 1 (1.9) | 0 (0.0) | 1 (1.1) |
| Outcome, n (%) | |||
| Recovered fully | 22 (41.5) | 12 (28.6) | 34 (35.8) |
| Recovered partially | 6 (11.3) | 2 (4.8) | 8 (8.4) |
| Ongoing | 16 (30.2) | 13 (31.0) | 29 (30.5) |
| Died | 8 (15.1) | 15 (35.7) | 23 (24.2) |
| Missing | 1 (1.9) | 0 (0.0) | 1 (1.1) |
| Relationship to any of the research procedures, n (%) | |||
| Unrelated | 35 (66.0) | 31 (73.8) | 66 (69.5) |
| Unlikely | 8 (15.1) | 6 (14.3) | 14 (14.7) |
| Possibly | 3 (5.7) | 0 (0.0) | 3 (3.2) |
| Probably | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Definitely | 2 (3.8) | 0 (0.0) | 2 (2.1) |
| Not able to assess | 4 (7.6) | 5 (11.9) | 9 (9.5) |
| Missing | 1 (1.9) | 0 (0.0) | 1 (1.1) |
| Expectedness, n (%) | |||
| Expected | 48 (90.6) | 37 (88.1) | 85 (89.5) |
| Unexpected | 4 (7.6) | 5 (11.9) | 9 (9.5) |
| Missing | 1 (1.9) | 0 (0.0) | 1 (1.1) |
| Event type | Description | Intensity | Outcome | Relationship | Expectedness |
|---|---|---|---|---|---|
| Hospitalisation | Participant tripped while wearing trial shoes, fell and was hospitalised (found to have elevated blood pressure and low blood glucose) | Severe | Recovered partially | Possible related | Yes |
| Hospitalisation | Participant fell and fractured wrist and skull; they were not wearing trial shoes | Severe | Recovered partially | Possible related | Yes |
| Hospitalisation | Participant fell while wearing trial shoes with insoles. Laces were correctly fastened. Injured elbow and shoulder, and was hospitalised | Severe | Ongoing | Possible related | Yes |
| Hospitalisation | Participant fell while wearing trial shoes and broke hip | Severe | Recovered partially | Definitely | Yes |
| Life-/limb-threatening | Participant’s shoes with an insole caused pressure ulceration at the toes and subsequent cellulitis, which required antibiotics | Severe | Recovered fully | Definitely | Yes |
Non-serious adverse events
Non-SAEs that occurred within the reporting period and were deemed to be at least possibly related to any of the research procedures are summarised in Table 34. These were all in the intervention group. Participant self-reported occurrences of pain or cramp possibly resulting from the exercises were forwarded to the treating podiatrist for review. If these events lasted for > 48 hours, then an adverse event was recorded. Pain and cramp lasting for < 48 hours was considered an expected occurrence within this population and for this component of the intervention, and so was not recorded.
| Non-SAEs | Intervention (N = 493) |
|---|---|
| Total number of non-SAEs | 58 |
| Number of participants with one or more non-SAEs | 49 |
| Number of events per participant, n (%) | |
| 1 | 42 (85.7) |
| 2 | 5 (10.2) |
| 3 | 2 (4.1) |
| Event details, n (%) | |
| Aches/pains in lower limbs lasting for ≥ 48 hours | 26 (44.8) |
| Injury attributable to exercise equipment | 1 (1.7) |
| Soft tissue injury | 5 (8.6) |
| Skin irritation/injury (e.g. pressure sore, callus/corn) | 9 (15.5) |
| Other | 22 (37.9) |
| Intensity, n (%) | |
| Mild | 36 (62.1) |
| Moderate | 22 (37.9) |
| Severe | 0 (0.0) |
| Outcome, n (%) | |
| Recovered fully | 28 (48.3) |
| Recovered partially | 15 (25.9) |
| Ongoing | 15 (25.9) |
| Relationship to any of the research procedures, n (%) | |
| Possibly | 11 (19.0) |
| Probably | 29 (50.0) |
| Definitely | 18 (31.0) |
| Expectedness, n (%) | |
| Expected | 46 (79.3) |
| Unexpected | 12 (20.7) |
Chapter 5 Economic evaluation
Introduction
As stated in earlier chapters, the aim of the REFORM trial is to provide rigorous trial evidence for the role of a complex podiatry care intervention that combines foot and ankle exercise with footwear advice and orthotic inserts for falls prevention within a UK setting.
Economic evaluation supports decision-making in prioritising the allocation of limited health-care resources. 46 Economic evaluation alongside clinical trials, as in the REFORM trial, can therefore be a valuable tool to help decide what interventions should be implemented, based not only on clinical effectiveness but also on cost-effectiveness. Moreover, RCTs are often the best means for providing unbiased estimates of both health effects and costs. 47
This chapter reports on the economic evaluation that was conducted alongside the REFORM trial. The aim of this economic analysis is to help decision-making in determining whether or not the multifaceted intervention represents a cost-effective alternative within the UK NHS for falls prevention compared with usual care provided by the podiatrist or GP and a falls prevention leaflet.
Methods
Overview
Individual participant data collected in the REFORM trial were used to perform a within-trial economic analysis that comprised (1) a cost–utility analysis, in terms of the cost per QALY, and (2) a cost-effectiveness analysis (CEA), in terms of the cost per fall averted (i.e. using the primary effectiveness outcome of the trial). Costs are presented in UK pounds sterling (£) at 2015 prices, and the analysis has been undertaken in Stata version 13.1. The NICE guidelines were applied to all methods used for this economic analysis. 48
Base-case analysis
The base-case analysis was conducted on an ITT basis using multiple imputed data and from the perspective of the UK NHS and Personal Social Services, which included resource use related to falls only. The ITT aspect compares participants in the two groups (intervention vs. usual care) on the basis of their initial random allocation, irrespective of protocol deviations or withdrawal. A secondary analysis was undertaken from the societal perspective. Costs and outcome data are compared for the two groups over 12 months and, hence, discounting was not required.
Owing to the impact of missing data for the within-trial CEA, our economic analysis plan indicated that the base-case analysis would be conducted as an imputed analysis by means of MI at the utility level. This has been recommended as the appropriate method to reflect the uncertainty in the results of the economic evaluation attributable to missing data. 49
Sensitivity analysis
Additional sensitivity analyses were conducted to explore the extent to which the results change with different assumptions. Sensitivity analyses were conducted in order to test (1) complete case as an alternative method to MI for handling missing data, (2) the impact of imputing HRQoL at aggregated level (e.g. QALY level), (3) the impact of including both fall- and non-fall-related visits and hospitalisations in the calculation of total costs and (4) the societal perspective (e.g. cost of the shoes as a personal expense for the patient). Finally, we used a probabilistic sensitivity analysis to explore the uncertainty associated with the mean difference in costs and health outcomes using both the imputed and the complete data sets.
Economic data collection
Data for outcomes and resource use for the economic analysis were collected prospectively. Health service usage was measured using participant-reported questionnaires at baseline and at 6 and 12 months during the 12 months’ follow-up.
Health-related quality of life
Health-related quality of life is expressed in terms of utilities, which were assessed at baseline and at 6 months and 12 months using the EQ-5D-3L. 50 The EQ-5D is a standardised and validated generic instrument for the measurement of HRQoL that allows the translation of patient utilities into QALYs, which is the primary outcome for the base-case analysis.
The QALY is a measure of health that simultaneously incorporates changes in both morbidity (related to quality of life) and mortality (related to the quantity of years lived). As well as being one of the most widely used generic health status measures, the EQ-5D is the instrument recommended by the NICE appraisal guidance. 48 The EQ-5D considers health (functioning) in terms of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three possible levels: no problems, moderate problems and severe problems. This five-domain and three-level system generates 245 mutually exclusive health states, including unconscious and dead. To estimate HRQoL weights (known as utilities) and to reflect the preferences of the UK population, each of these health states has been validated in a large UK population sample30 using the time trade-off method, ranging from 1 for perfect health (thus, the maximum value possible) to –0.594 for severe problems; 0 corresponds to death. Utility values were generated by valuing health status (using a social tariff) as measured using the EQ-5D system. Mean utility values were reported for each trial group, and differences in utilities between the two treatment groups were estimated using ordinary least squares (OLS) regression.
The EQ-5D has been recommended by The Prevention of Falls Network Europe Consensus as the measure of HRQoL to be used in fall prevention trials. 32 The rationale behind this is that the EQ-5D is simple and responsive to changes in health and, more importantly, it has been used widely in older populations. 51 Similarly, the EQ-5D has been used before in a UK setting to assess HRQoL and costs implications of falls in elderly people. 1 We converted the utilities derived from the EQ-5D into QALYs for each participant using the area under the curve method, following the trapezium rule, which assumes linear interpolation between follow-up points. 52 Despite the randomisation process, which should ensure that, on average, baseline variables are balanced between the groups of the trial, in practice (regardless of sample size) it is not unusual to find imbalance in mean baseline utility. As baseline utility is likely to be correlated with participants’ QALY gains over time, there are robust reasons to control for baseline utility when estimating QALYs;53 therefore, incremental mean QALYs between treatment groups were estimated with and without adjustment for baseline utility, using regression methods according to ITT allocation. In addition, incremental mean QALYs were adjusted for the same set of covariates as in the primary clinical effectiveness analysis model: age at randomisation, sex and history of falling. Centre was treated as a fixed effect in all models.
Health benefits in terms of falls
The primary outcome of the trial was the incidence rate of falls per participant during the 12 months following randomisation. The primary clinical effectiveness analysis used a mixed negative binomial regression model to analyse the number of falls per person per year controlling, as fixed effects, for sex, age at randomisation and history of falling, with centre as a random effect.
In order to interpret the cost-effectiveness results, the health outcome was reported as ‘falls averted’. The number of falls averted was estimated as the difference in mean reduction in the fall rate between the two groups in the trial estimated as per the adjusted negative binomial model used for the primary clinical effectiveness analysis.
Health-care resource use
Health-care resource use data were collected via participant-reported questionnaires. Participants were asked to complete information on their number of visits to primary care facilities (e.g. contacts with a GP and general practice nurse), use of community care (e.g. contacts with occupational therapist) and their number of hospital visits [inpatient, day case, outpatient, and accident and emergency (A&E) department] at baseline and at 6 and 12 months. Patients were also asked about the number of times they made an emergency service call or the used the Patient Transportation Service. All resource use (except inpatient hospital stay) was split into ‘fall-related’ and ‘non-fall-related’. The base-case analysis was based on fall-related resource use, except for inpatient hospital stay, given the format of the questionnaire. A sensitivity analysis explored the impact of including both fall- and non-fall-related resource use in the analysis.
Participants were asked to record the total number of times they stayed in hospital as an inpatient as well as the number of nights for each visit and the reason for attendance. The number of inpatient visits did not differentiate fall- from non-fall-related incidents and, hence, the base-case analysis included all inpatient stays reported by participants during the trial. Following a reported fall, participants were contacted to obtain information about the nature, location and cause of the fall. They were asked whether or not they sustained any injuries from the fall and, if so, whether or not they required an overnight stay in hospital. Only 28 participants (intervention, n = 14; usual care, n = 14) reported that they had to spend a night in hospital as a result of their fall. We assumed that missing answers (boxes left blank) to the second question (i.e. number of nights in hospital) when participants reported no hospital stay indicated no use of services and, thus, no overnight stays. There were participants with missing responses to number of nights but who reported the reason for their stay; for these cases the length of stay (nights in hospital) was assumed to be one night. Similarly, there were participants who reported not being in hospital but who gave information on the reason for their attendance; it was assumed that these participants stayed in hospital for one night. Finally, there were participants who reported that they stayed overnight in hospital but left blank the remaining information regarding number of occasions, number of nights and reason for stay. As a conservative assumption, we assumed that these participants stayed in hospital for one night on one occasion.
The number of visits made by the participant to their podiatry clinic in the previous 12 months was collected on the 12-month participant questionnaire. This information was available for participants in both treatment groups. In the case of participants in the intervention group, the number of visits made to the podiatry clinic as part of the intervention was collected via a trial-specific podiatrist database. Therefore, for participants in the intervention group, the number of podiatry appointments self-reported at 12 months was assumed to consist of trial appointments and unrelated routine care appointments. We knew the number of appointments received as part of the intervention and, therefore, assumed that all other reported visits were unrelated; hence, these were not included as part of the cost of the intervention.
We also asked about the use of Meals on Wheels and paid care; however, few participants reported using either Meals on Wheels (96.14% did not use this service) or paid-for help (92.87% did not pay for care). Therefore, it was decided not to incorporate these into the societal perspective analysis. Resource use was valued in monetary terms and unit costs were reported in UK pounds sterling (£) for the financial year 2014/15. The cost for each participant in the REFORM trial was calculated by multiplying health-care resource use by associated unit costs. Table 35 details the unit costs for the estimation of costs related to patient care that were used in the analysis.
| Resource use | Unit cost (£) | Source |
|---|---|---|
| Cost component: primary care | ||
| Visit to GP | 44.00 | Unit Costs of Health and Social Care 2015 54 |
| Visit to general practice nurse | 25.00 | Unit Costs of Health and Social Care 2015 54 |
| Occupational therapist | 44.00 | Unit Costs of Health and Social Care 2015 54 |
| Cost component: secondary care | ||
| Hospital stay | 3106.00 | a NHS Reference Costs 2014–15 55 |
| Excess hospital stay | 303.00 | b NHS Reference Costs 2014–15 55 |
| Outpatient visit | 114.50 | c NHS Reference Costs 2014–15 55 |
| Day case | 720.00 | d NHS Reference Costs 2014–15 55 |
| A&E | 140.60 | e NHS Reference Costs 2014–15 55 |
| Emergency service call | 154.00 | f Unit Costs of Health and Social Care 2015 54 |
| Patient transportation service | 99.00 | Unit Costs of Health and Social Care 2015 54 |
| Cost component: podiatrist | ||
| Podiatrist first visit (assessment) | 46.00 | g NHS Reference Costs 2014–15 55 |
| Podiatrist second visit | 44.00 | g NHS Reference Costs 2014–15 55 |
| Podiatrist follow-up visit | 39.00 | g NHS Reference Costs 2014–15 55 |
Costing the intervention
The cost of the podiatry intervention was assessed based on the data collected as part of a baseline appointment questionnaire and the podiatrist database, which included information directly related to the podiatrist assessments and the intervention package received by the participant (e.g. orthosis prescription, exercise programme and exercise equipment).
Unit costs, together with their sources, for the podiatry intervention are provided in Table 36. Aside from manufacturer prices, the unit costs used in the analysis were obtained from published national sources: Unit Costs of Health and Social Care (Personal Social Services Research Unit)54 and NHS Reference Costs. 55
| Resource use | Unit cost (£) | Source |
|---|---|---|
| Cost component: podiatry intervention | ||
| Shoes provided, per pair | 64.00 | Manufacturer price 2015 |
| Therapy ball (large) | 0.95 | Manufacturer price 2013a |
| Therapy ball (small) | 0.90 | Manufacturer price 2013a |
| Resistive exercise band (band 1) | 1.11 | Manufacturer price 2013a |
| Resistive exercise band (band 2) | 1.21 | Manufacturer price 2013a |
| Resistive exercise band (band 3) | 1.28 | Manufacturer price 2013a |
| Resistive exercise band (band 4) | 1.45 | Manufacturer price 2013a |
| Resistive exercise band (band 5) | 1.72 | Manufacturer price 2013a |
| Resistive exercise band (band 6) | 1.50 | Manufacturer price 2013a |
| Resistive exercise band (band 7) | 1.74 | Manufacturer price 2013a |
| Resistive exercise band (band 8) | 1.89 | Manufacturer price 2013a |
| Resistive exercise band (band 9) | 1.97 | Manufacturer price 2013a |
| Resistive exercise band (band 10) | 2.09 | Manufacturer price 2013a |
| X-Line Extra insoles | 5.95 | Manufacturer price 2015 |
| X-Line Pressure Perfect insoles | 4.75 | Manufacturer price 2015 |
| Formthotics Dual insoles | 14.99 | Manufacturer price 2015 |
| DVD and booklet | 3.82 | Manufacturer price 2013a |
| Podiatrist first visit (assessment) | 46.00 | NHS Reference Costs 2014–15 55 |
| Podiatrist second visit | 44.00 | NHS Reference Costs 2014–15 55 |
| Podiatrist follow-up visit | 39.00 | NHS Reference Costs 2014–15 55 |
The base-case analysis includes only costs falling within the NHS and, hence, the cost of the shoe was not included in the primary analysis. This issue was discussed at length within the trial team and with the trial podiatrists; all were in strong agreement that, if the intervention were implemented, the NHS would not cover the cost of the shoes. We therefore considered the price of the shoes as a personal expense for the patient (e.g. as part of the societal perspective). A secondary analysis from the societal perspective that included the cost for the shoe was conducted.
We calculated the cost for each participant in the trial by multiplying their use of health-care resources by the associated unit costs. The total costs for the base-case analysis included only fall-related resource use (except for inpatient stay, which included both fall- and non-fall-related resource use). The total cost comprises five main components: (1) podiatrist visits, (2) hospital visits (inpatient, outpatient and day cases), (3) visits to primary and community health-care professionals (GP, practice nurse and occupational therapist), (4) patient transportation and (5) the cost of the podiatrist intervention. Other scenarios were tested as part of the sensitivity analysis, in which we explored the impact of incorporating both types of resource use (fall- and non-fall-related) into the analysis.
Multiple imputation
Missing data occur frequently in RCTs, irrespective of how well designed the data collection is. This is a major concern for within-trial CEA, as costs and QALYs, the main outcomes in CEAs, are cumulative measures collected over the trial follow-up. Therefore, missing data at one follow-up time point (e.g. one dimension response missing to the EQ-5D at one time point) result in missing aggregate data (e.g. total QALYs over the trial) for that participant. This problem is common in economic evaluations, as the analysis has to draw on all aspects of the study, including resource use and health outcomes. Non-response to questionnaires and returned but incomplete questionnaires reduce, often considerably, the number of data on resource use that are available for analysis. The problem is amplified when there are frequent assessments, as in the REFORM trial.
Complete-case assessment and available case analysis are proposed as useful preliminary estimations for economic evaluation but should not constitute the base case for within-trial economic evaluation. 56 An alternative method to address missing data in CEAs alongside clinical trials is MI,57 which has been recommended as the appropriate method to reflect the uncertainty in the results of the economic evaluation attributable to missing data. 49 As already stated, our economic analysis plan indicated that the base case would be conducted as an imputed analysis using MI with chained equations. Given the extent of missing data in the REFORM trial, this initial decision is justified; therefore, the base-case analysis was conducted on the imputed data set. A descriptive analysis of missing data was conducted in order to help inform the base-case assumption regarding the missing data mechanism. We described the number of missing data by treatment group at each follow-up point. We also examined the missing data pattern to find out whether or not participants with missing data were lost to follow-up throughout the duration of the trial.
Multiple imputation comprises three steps. First, the imputed data set is created through the use of regression models to predict plausible values for the missing observations from the observed values. The process includes all the variables that might be associated with the missingness mechanism (here, sex, age, history of falls, centre, baseline costs, primary care and hospital costs) and QALY utilities (at baseline and at 6 and 12 months). Costs and utilities were imputed simultaneously rather than separately in the model. Therefore, the covariates registered in the model were used for both costs and utilities, when a regression model was fitted for each variable with missing values, with the previous variables as covariates. Based on the resulting model, a new regression model is then estimated and used to impute the missing values for each variable. A random component is included to reflect the uncertainty around the predictions. Thus, MI reflects the uncertainty in the prediction of missing values while preserving the distribution and correlations in the data. 58 These values are then used to fill in the gaps in the data set. This process is repeated m times, creating m imputed data sets. It is suggested that, if missing values account for 20% of the total number of data, three imputations are sufficient. 59 Given the extent of missing data in REFORM, five imputations were performed. In the second stage, each data set is analysed independently using complete-case methods. Finally, the estimates obtained from each imputed data set are combined to generate mean estimates of costs and QALYs, variances and CIs using Rubin’s rules. 60
The correct specification of the regression model is vital to ensure that the distribution of imputed values does not differ from the observed values and, thus, that unbiased estimates are obtained. The specification of the regression models depends on the type and distribution of the variable to be imputed. Costs and QALYs are both continuous and non-normally distributed. Two alternative methods are proposed to deal with this difficulty when using MI with chained equations: (1) data transformation and (2) predictive mean matching. 61 Predictive mean matching was used for the imputation of REFORM data. This method ensures that observed data were used to estimate a predictive model (using the specified covariates) but, instead of replacing missing values with the model predicted values, the nearest observed value is used to fill the missing one. This guarantees that the imputed values are sampled from values in the original data set, and, therefore, that no imputed values will lie outside the bounds of the original data distribution. In addition to the description of the missing data mechanism, we used graphical plots to visualise whether or not the distribution of imputed data resembles the distribution of original data.
The main assumption that drives the MI mechanism is that the data are missing at random (MAR). Additional sensitivity analyses were conducted to test the robustness of the results to deviations from this assumption. In that sense we explored (1) the use of the complete data set [e.g. assume that the data are missing completely at random (MCAR)] and (2) imputation at various levels of aggregation (e.g. at utility level rather than QALY level).
Incremental analysis
Total health-care expenditure must be covered from a limited budget and, therefore, the most informative estimate for CEA is based on the difference of arithmetic mean effect from both budgetary and social perspectives. 62 Therefore, the focus of this economic evaluation was to estimate the mean costs and mean health outcomes. The cost-effectiveness of the podiatry intervention was evaluated by comparing the mean costs and outcomes (QALYs and falls) incurred in the intervention group with the mean costs and outcomes (QALYs and falls) in the usual-care group at 12 months’ follow-up, using conventional decision rules and estimating incremental cost-effectiveness ratios (ICERs) when appropriate.
As expected, costs in the REFORM trial were right-skewed, and we found that some participants had costs that far exceeded the mean value. In order to deal with skewness and heteroscedasticity, non-parametric bootstrap procedures62–64 are usually implemented as the primary statistical test for making inferences about arithmetic means for moderately sized samples of skewed cost data (such as the REFORM sample). Bootstrap methods assume that the empirical distribution of the data is an adequate representation of the true distribution of the data; the analysis is based on repeatedly sampling (with replacement) from the observed data. For the REFORM analysis we repeatedly randomly drew a sample of 1000 for each of the imputed five data sets. Each bootstrap repetition is the equivalent of a repetition of the trial. To obtain reliable results in practice it has been recommended to use at least 1000 resamples to estimate a bootstrap CI. 63 For the REFORM analysis we used 5000 resamples (bias corrected and accelerated). The mean difference in costs and QALYs for the base-case analysis was estimated using seemingly unrelated regression (SUR) equations for data on costs and QALYs. The SUR model used the same set of covariates as the mixed-effect regression model used for the clinical effectiveness analysis (sex, age at randomisation, fall history) as well as total number of falls and baseline utility. Incremental costs were also adjusted for baseline costs. SUR is used to address the correlation of standard errors between costs and QALYs. 65 This brings efficiency gains over unrelated OLS regression for three reasons: (1) it allows for explicit modelling of both costs and effects while allowing the inclusion of a set of different covariates in the two equations; (2) it exploits the existence of correlation between costs and effects; and (3) SUR does not require a new regression for every value of the cost-effectiveness threshold. 66 Again, the same set of covariates as used in the clinical effectiveness analysis was used. The baseline EQ-5D utility was also included in the utility regression to adjust for possible baseline imbalance and to reduce the standard errors of post-test EQ-5D. Incremental costs were also adjusted for baseline costs.
The ICER was estimated as the difference in mean total costs divided by the difference in mean total QALYs from baseline to 12 months. The ICER is estimated to inform decision-makers about the optimal use of NHS resources. According to standard cost-effectiveness decision rules, four different eventualities are plausible when comparing incremental costs and QALYs. If the new intervention provides better outcomes (positive incremental QALYs) at lower costs (negative incremental costs) it is considered a dominant intervention and, hence, cost-effective. If the new intervention achieves poorer outcomes (negative incremental QALYs) at higher costs (positive incremental costs) it is considered a dominated option and, hence, not cost-effective. Thus, the ICER is considered only if either intervention does not dominate, that is, both incremental costs and incremental QALYs are positive (or negative). In these last two situations, to determine whether or not the incremental health gain is worth the incremental cost, the ICER needs to be compared against a threshold value. For positive incremental costs and QALYs (the most frequent situation in HTA), an intervention will be considered cost-effective only if the ICER is lower than the threshold. According to NICE, the WTP threshold for an additional QALY ranges from £20,000 to £30,000. 48 This threshold has been used by NICE for more than a decade; however, it has recently been suggested that the threshold should be decreased to £13,000 per QALY gained. 67 According to the current established decision rules, if the result of this cost–utility analysis, namely the estimated cost per QALY, is below the £30,000 threshold, the podiatry intervention would be considered cost-effective in terms of QALYs gained.
The ICER can be rearranged in terms of net benefit, which is a more intuitive way of expressing whether or not the health benefits of the podiatry intervention are worth the additional costs. 68 The net benefit can be estimated on the cost scale as the incremental health gain, expressed in terms of money, minus the incremental cost of the intervention. The health benefits are translated into monetary value using the cost-effectiveness threshold, that is, incremental QALYs are multiplied by the WTP threshold. Therefore, the net monetary benefit (NMB) provides an estimation of the gain (or loss) in resources of investing in a particular intervention when those resources might be used elsewhere. 69 Current NICE guidance recommends presenting the NMB using values of £20,000 and £30,000 per QALY for the WTP threshold. The podiatry intervention would be considered cost-effective only if the NMB were positive.
Analysis of uncertainty
Uncertainty in economic evaluation is related to the expected values of model inputs but not to patient variability. The uncertainty around the cost-effectiveness estimates was explored by means of sensitivity analyses in order to test the robustness of the results under different scenarios. These scenarios captured variability in the estimates of costs and outcomes, which resulted from either different methods (e.g. imputation methods), from the costs included in the analysis or from the perspective for the analysis.
The extent of missing data in the REFORM trial justified the use of the imputed data set as the base-case analysis. Nevertheless, a complete-case analysis was explored as part of a sensitivity analysis. Similarly, we also conducted sensitivity analyses to test different levels of aggregation when imputing costs and QALYs.
The base case was based on the imputed data set and included only fall-related visits and hospitalisations. One-way sensitivity analyses were conducted to test the impact of including all visits and hospitalisations regardless of them having being classified as fall- or non-fall-related.
Finally, we used probabilistic sensitivity analysis to investigate the uncertainty associated with the mean difference in costs and QALYs between the two treatment groups using both the imputed and the complete-case data sets. Non-parametric bootstrapping was used to plot the joint distribution of costs and effects (QALYs) on the cost-effectiveness plane and to derive the CEAC to express the (Bayesian) probability that the podiatry intervention is cost-effective as a function of the WTP threshold. 70
Exploration of the need for a long-term model
An exploratory model was developed to explore how the differences in HRQoL observed during the trial (e.g. at 1 year) evolve beyond the study (up to 5 years). For this exploratory projection, we used a decision-modelling approach and assumed that the difference in HRQoL and costs observed at 1 year would remain unchanged.
Validation of results
In order to validate the results of the analysis, two statistical codes (written in Stata) were independently developed and their results compared. The codes were developed by one analyst and checked independently by another. The distributions of the observed and imputed values were compared graphically.
Results
Patient population and missing data mechanism
Twenty-four participants died during the trial: 9 out of 493 (1.8%) in the intervention group and 15 out of 517 (2.9%) in the usual-care group. When there were missing data before these participants’ deaths, the imputation process was applied in the same way as for the rest of the patients in the trial. The questionnaires that should have been received at any other assessment after their deaths were considered as part of the complete-case analysis with zero resource use and zero utilities. The complete-case analysis comprised those participants for whom data were available for the whole trial duration for utilities and all cost categories.
The proportion of participants with complete data decreased with the duration of follow-up but remained similar in both groups (Table 37): from 72.0% (baseline) to 54.4% (12 months) for the intervention group and from 71.8% (baseline) to 61.3% (12 months) for the usual-care group. In the usual-care group, more individuals are observed at 12 months than at 6 months; therefore, the missing data follow not a monotonic pattern but an intermittent one (i.e. there are participants with missing 6-month data but complete data at 12 months). A complete-case assessment would be, as a minimum, inefficient because it would discard observed data from individuals with some missing outcomes.
| Time point | Intervention (N = 493), n (%) | Usual care (N = 517), n (%) |
|---|---|---|
| Baseline | 355 (72.0) | 371 (71.8) |
| 6 months | 285 (57.8) | 305 (59.0) |
| 12 months | 268 (54.4) | 317 (61.3) |
| Total trial duration | 129 (26.2) | 157 (30.4) |
Table 38 presents the ORs from a logistic regression of indicators of missing QALY data on treatment group allocation and the covariates used for the main statistical model. Lower EQ-5D at baseline is associated with missing QALY data but is not statistically significant at the 5% level. This suggests that the data are unlikely to be MCAR. It was found that older and frailer (lower utility) participants with a history of falling were more likely to have missing QALY data. This information would support the MAR assumption (e.g. MI assumed missing data mechanism).
| Missing data on QALYs | OR (95% CI) |
|---|---|
| Treatment group | 1.13 (0.82 to 1.55) |
| Sex | 1.27 (0.92 to 1.77) |
| Age | 1.04a (1.02 to 1.06) |
| History of fall | 1.26 (0.89 to 1.77) |
| EQ-5D at baseline | 0.68 (0.35 to 1.32) |
Multiple imputation and likelihood-based methods can handle non-monotonic missing data under the MAR assumption while incorporating the uncertainty around the unobserved data and maintaining the correlation structure. 56 Therefore, the base case for the REFORM analysis uses MI. A complete-case analysis, which is not valid under MAR, is presented only for comparison.
The MI model was validated by comparing the distributions of the observed and the imputed data (Figures 6 and 7). The distributions of imputed data are similar to the distribution of the observed data. The MI data sets were analysed with the same SUR model used for the complete-case analysis.
FIGURE 6.
Comparison of the distribution of imputed values (imputations 1–5) with the observed data (imputation 0) for total costs.
FIGURE 7.
Comparison of the distribution of imputed values (imputations 1–5) with the observed data (imputation 0) for total QALYs.

Health-related quality of life
The complete-case analysis for utilities consisted of the participants who returned all questionnaires and completed the EQ-5D questions. The EQ-5D is classified as complete only if its five dimensions contain a response. Table 39 shows the number of questionnaires returned (including those with missing dimensions) and the number of completed EQ-5D questions for each time point. The number of questionnaires returned decreases with time.
| Time point | Treatment group, n (%) | |||
|---|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | |||
| Completed EQ-5D | Missing EQ-5D | Completed EQ-5D | Missing EQ-5D | |
| Baseline | 468 (94.9) | 25 (5.1) | 497 (96.1) | 20 (3.9) |
| 6 months | 422 (85.6) | 71 (14.4) | 448 (86.7) | 69 (13.4) |
| 12 months | 405 (82.2) | 88 (17.9) | 440 (85.1) | 77 (14.9) |
| Total trial duration | 369 (74.9) | 113 (21.9) | 404 (78.1) | 113 (21.9) |
Table 40 describes the number and proportion of participants in the REFORM trial reporting each of the levels on each EQ-5D dimension.
| Description | Dimension | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | ||||||||||||||||
| Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | |||||||||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |
| Level 1, n (%) | 220 (43) | 165 (37) | 194 (40) | 162 (39) | 425 (85) | 374 (84) | 385 (81) | 333 (80) | 267 (53) | 218 (48) | 221 (46) | 182 (44) | 120 (24) | 101 (22) | 104 (22) | 93 (22) | 371 (73) | 319 (70) | 323 (67) | 285 (69) |
| Level 2, n (%) | 290 (57) | 286 (63) | 287 (60) | 256 (61) | 77 (15) | 73 (16) | 90 (19) | 78 (19) | 232 (46) | 223 (49) | 243 (51) | 218 (52) | 343 (68) | 315 (70) | 334 (69) | 285 (69) | 128 (25) | 126 (28) | 155 (32) | 122 (30) |
| Level 3, n (%) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 2 (4) | 3 (7) | 8 (16) | 10 (22) | 14 (29) | 16 (38) | 44 (87) | 34 (8) | 43 (9) | 37 (9) | 8 (2) | 8 (2) | 4 (1) | 7 (2) |
| Total | 510 | 452 | 481 | 418 | 503 | 448 | 477 | 414 | 507 | 451 | 478 | 416 | 507 | 450 | 481 | 415 | 507 | 453 | 482 | 414 |
| Number reporting some problems, n (%) | 290 (58) | 287 (64) | 287 (60) | 256 (61) | 78 (16) | 74 (17) | 92 (19) | 81 (20) | 240 (47) | 233 (52) | 257 (54) | 234 (56) | 387 (57) | 349 (78) | 377 (78) | 322 (78) | 136 (27) | 134 (30) | 159 (33) | 129 (31) |
| Change in numbers reporting problems | –3 | –31 | –4 | –11 | –7 | –23 | 62 | –55 | –2 | –30 | ||||||||||
| % change in numbers reporting problems | –1 | –11 | –5 | –12 | –3 | –9 | –10 | –15 | –1.5 | –19 | ||||||||||
| Rank of dimensions in terms of changes | 4 | 4 | 1 | 3 | 2 | 5 | 5 | 2 | 3 | 1 | ||||||||||
At baseline, participants reported problems in mobility and pain more than in the other dimensions. These domains are worse for the participants in the intervention group: mobility (56.9% usual care vs. 59.7% intervention) and pain (56.6% usual care vs. 78.4% intervention). As expected, the intervention improved mobility as data showed an 11% reduction in the number of participants reporting problems from baseline to 12 months in the intervention group (compared with 1% change in the usual-care group). Similarly, the intervention improved pain (15% reduction in participants reporting problems in the intervention group compared with 10% change in the usual-care group).
The likelihood of remaining in perfect health decreased with time (Tables 41 and 42). However, the reduction in the number of participants in perfect health is lower in the intervention group (7.4%) than in the usual-care group (17.7%). The data also suggested that improvement in anxiety/depression is proportionally even greater than the improvement in other dimensions, especially among participants in the intervention group (19% reduction in numbers reporting anxiety problems).
| Description | Treatment group | |
|---|---|---|
| Usual care (%) | Intervention (%) | |
| Baseline | ||
| Perfect health | 15.3 | 13.6 |
| Problems | 84.7 | 86.4 |
| 6 months | ||
| Perfect health | 12.8 | 11.6 |
| Problems | 87.2 | 88.4 |
| 12 months | ||
| Perfect health | 12.6 | 12.6 |
| Problems | 87.4 | 87.4 |
| Description | Treatment group | |
|---|---|---|
| Usual care (%) | Intervention (%) | |
| Variation in perfect health | –17.7 | –7.4 |
| Variation in problems | 3.2 | 1.2 |
Utility values were generated by valuing health status (using a social tariff) as measured using the EQ-5D system. The analysis of utilities (Table 43) shows that participants in the intervention group start from a lower baseline utility, on average (0.67 for the intervention group vs. 0.69 for usual care). The data also showed that participants in the intervention group had, on average, 0.14 [standard deviation (SD) 0.38] admissions to the hospital during the 6 months before randomisation, whereas participants in the usual-care group had, on average, 0.12 (SD 0.45) admissions. This emphasises the need to adjust the utilities gained for baseline utility level. When considering this baseline imbalance, the data show that by the end of the trial participants allocated to intervention obtained, on average, a marginally higher HRQoL gain than participants allocated to usual care.
| Time point | Treatment group, EQ-5D score | Unadjusted mean difference (intervention – usual care) (95% CI)a | Mean difference adjusted for baseline utility (intervention – usual care) (95% CI)a | |||
|---|---|---|---|---|---|---|
| Intervention | Usual care | |||||
| n | Mean score (SD) | n | Mean score (SD) | |||
| Baseline | 468 | 0.67 (0.24) | 467 | 0.70 (0.23) | –0.023 (–0.053 to 0.008) | –0.023 (–0.053 to 0.008) |
| 6 months | 426 | 0.65 (0.27) | 455 | 0.65 (0.27) | –0.005 (–0.041 to 0.031) | 0.013 (–0.016 to 0.041) |
| 12 months | 414 | 0.66 (0.27) | 455 | 0.66 (0.26) | –0.005 (–0.041 to 0.031) | 0.015 (–0.013 to 0.043) |
The overall distribution of EQ-5D scores (utilities) for the different follow-up time points is illustrated by treatment group in Figure 8. Utilities at baseline ranged from –0.181 to 1 for both groups; at the end of the trial, utilities ranged from –0.239 to 1 for both groups.
FIGURE 8.
Distribution of EQ-5D scores by treatment allocation and time point. (a) Baseline: usual care; (b) baseline: intervention; (c) 6 months: usual care; (d) 6 months: intervention; (e) 12 months: usual care; and (f) 12 months: intervention.
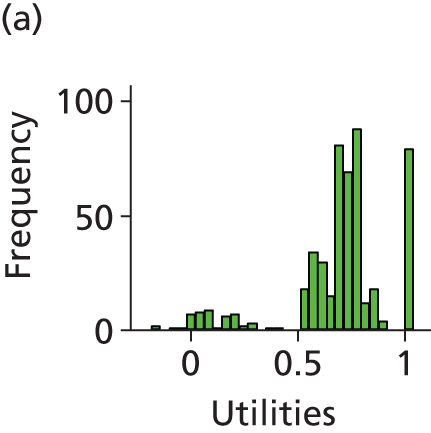



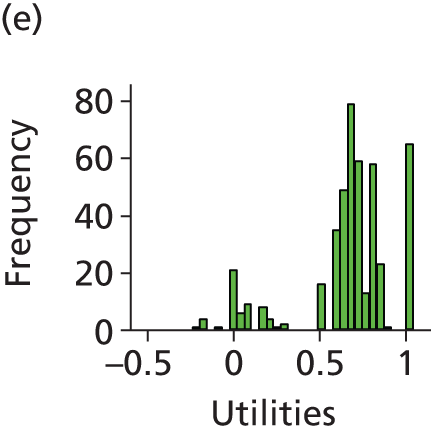

The distribution of mean utilities across the 12-month follow-up for the two groups is shown in Figure 9. The usual care participants reported higher HRQoL at baseline and at 6 and 12 months. All of the differences were small, and the 95% CIs overlap at each time point.
FIGURE 9.
Mean EQ-5D scores at baseline and follow-up time points by treatment group.
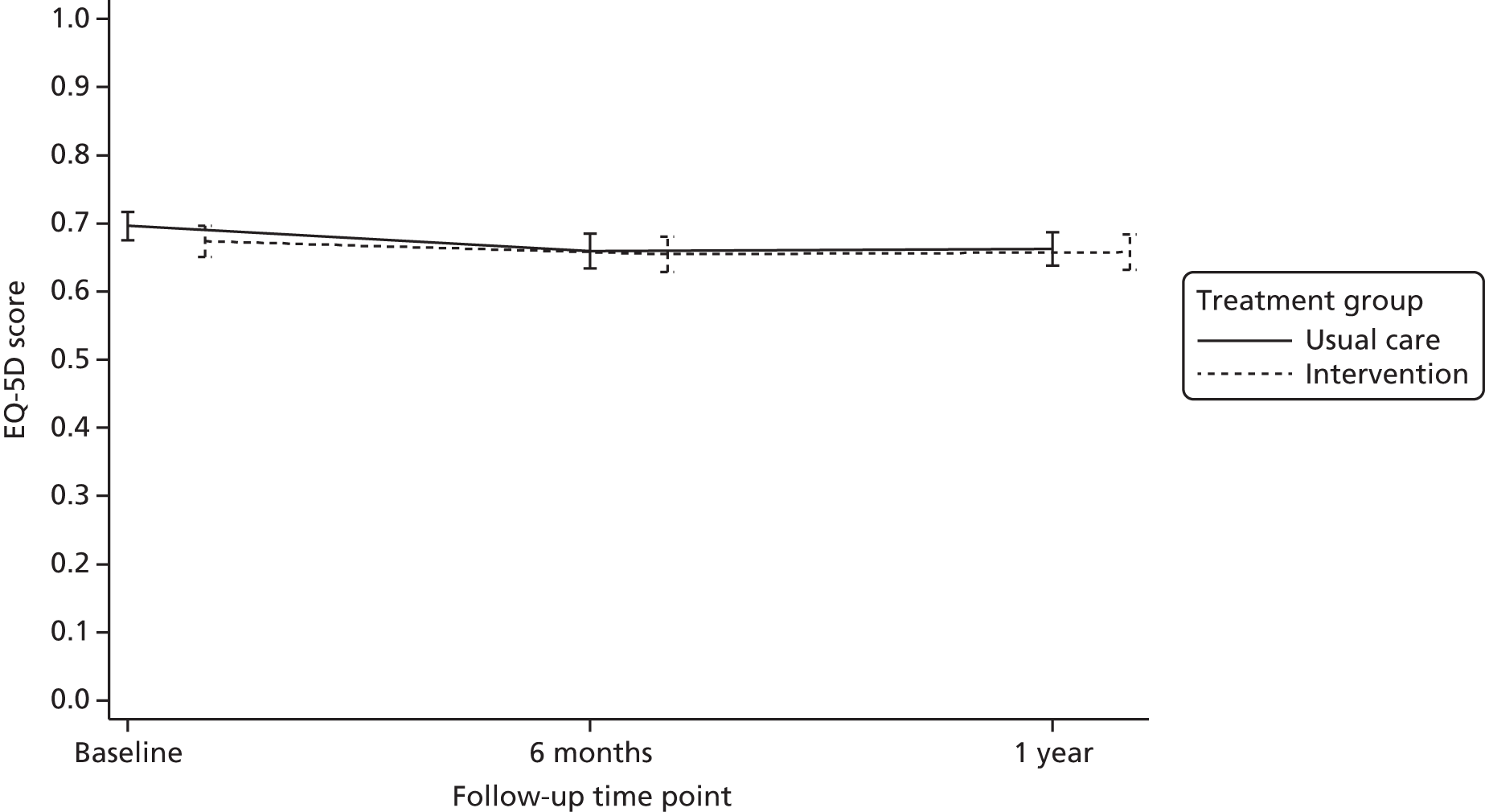
The mean QALYs were estimated based on individual participants’ utilities. Table 44 summarises the mean QALYs and the difference between treatment groups for all available cases. At the end of the trial, participants allocated to the intervention obtained, on average, a marginally higher QALY gain than participants allocated to usual care (Figure 10) when adjusted for baseline utility (0.010 QALY gain). The difference is 0.083 when adjusted for all covariates.
| Treatment group | Total | Mean (SD) QALYs | Difference (intervention – usual care) (95% CI)a | Difference (intervention – usual care) (95% CI)b |
|---|---|---|---|---|
| Intervention | 377 | 0.67 (0.24) | 0.010 (–0.010 to 0.031) | 0.008 (–0.009 to 0.026) |
| Usual care | 415 | 0.68 (0.23) |
FIGURE 10.
Mean total QALYs for the intervention and usual-care groups.
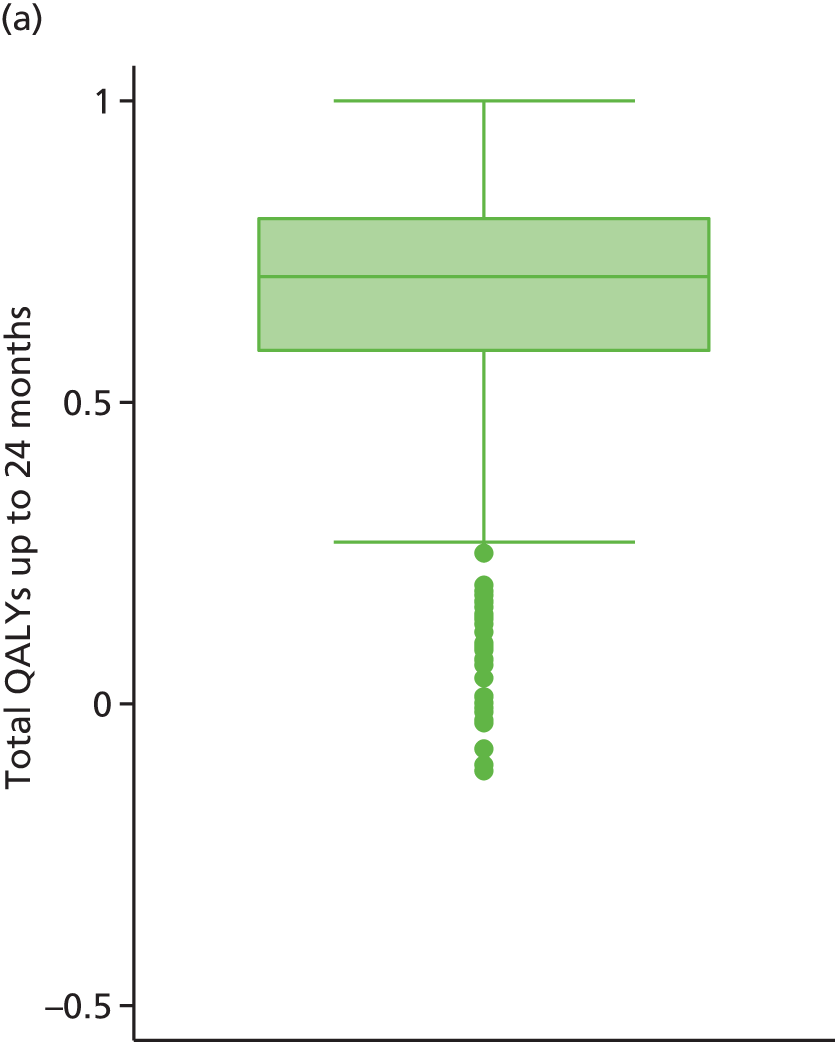
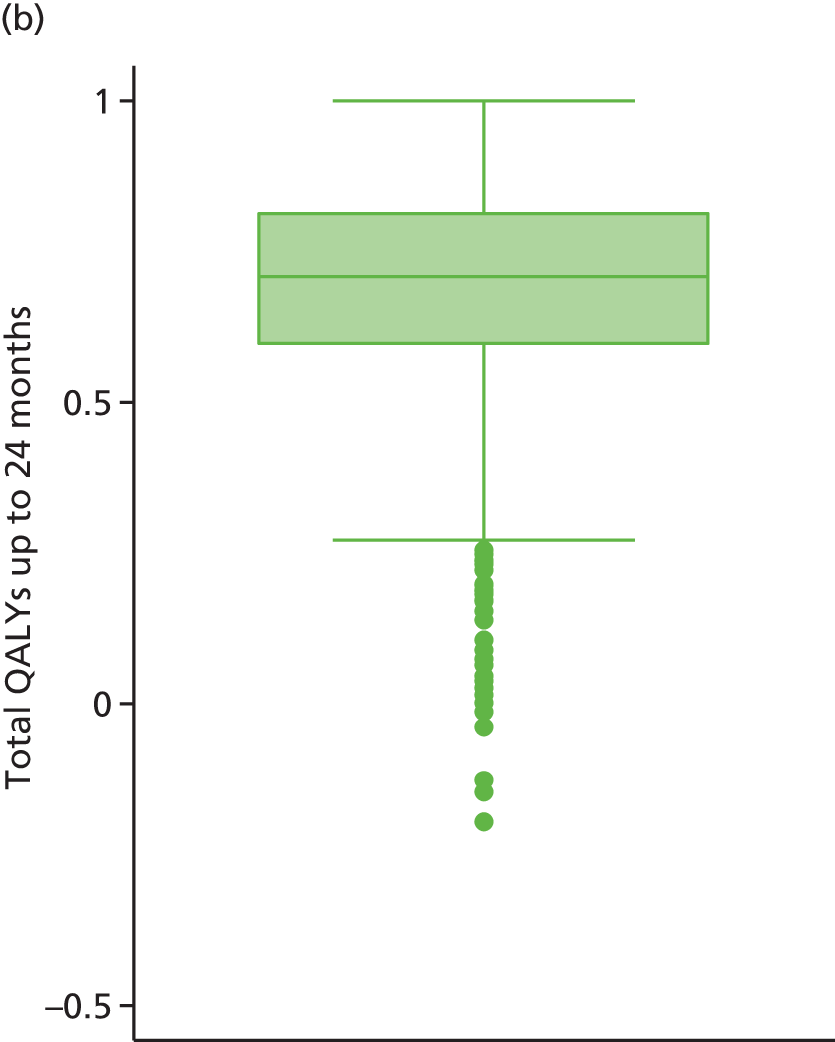
Health-care resource use and costs
The mean levels of resource use over the trial based on all available data are shown for the two treatment groups in Table 45. Although participants in the intervention group had, on average, fewer hospital day cases and used the patient transportation service fewer times, they had, on average, more hospital admissions, more outpatient visits and more A&E attendances than usual care participants over the trial duration.
| Type of resource use | Treatment group | |
|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | |
| Hospital admissions | ||
| At 6 months | ||
| n | 444 | 475 |
| Mean (SD) | 0.20 (0.50) | 0.16 (0.46) |
| Median (minimum, maximum) | 0 (0, 3) | 0 (0, 3) |
| Missing (%) | 49 (9.94) | 42 (8.12) |
| At 12 months | ||
| n | 425 | 467 |
| Mean (SD) | 0.14 (0.45) | 0.13 (0.41) |
| Median (minimum, maximum) | 0 (0, 4) | 0 (0, 3) |
| Missing (%) | 68 (13.79) | 50 (9.67) |
| Over the trial | ||
| n | 418 | 452 |
| Mean (SD) | 0.34 (0.81) | 0.30 (0.71) |
| Median (minimum, maximum) | 0 (0, 7) | 0 (0, 6) |
| Missing (%) | 75 (15.21) | 65 (12.57) |
| Hospital outpatient visits | ||
| At 6 months | ||
| n | 341 | 359 |
| Mean (SD) | 0.17 (0.92) | 0.12 (0.52) |
| Median (minimum, maximum) | 0 (0, 13) | 0 (0, 5) |
| Missing (%) | 152 (30.83) | 158 (30.56) |
| At 12 months | ||
| n | 315 | 362 |
| Mean (SD) | 0.15 (1.11) | 0.12 (0.50) |
| Median (minimum, maximum) | 0 (0, 18) | 0 (0, 4) |
| Missing (%) | 178 (36.11) | 155 (29.88) |
| Over the trial | ||
| n | 259 | 292 |
| Mean (SD) | 0.32 (1.66) | 0.23 (0.73) |
| Median (minimum, maximum) | 0 (0, 20) | 0 (0, 4) |
| Missing (%) | 234 (47.46) | 225 (43.52) |
| Hospital day case | ||
| At 6 months | ||
| n | 331 | 354 |
| Mean (SD) | 0.04 (0.26) | 0.07 (0.43) |
| Median (minimum, maximum) | 0 (0, 3) | 0 (0, 5) |
| Missing (%) | 162 (32.86) | 163 (31.53) |
| At 12 months | ||
| n | 313 | 363 |
| Mean (SD) | 0.07 (0.57) | 0.11 (1.10) |
| Median (minimum, maximum) | 0 (0, 7) | 0 (0, 20) |
| Missing (%) | 180 (36.51) | 154 (29.79) |
| Over the trial | ||
| n | 245 | 287 |
| Mean (SD) | 0.11 (0.80) | 0.12 (1.33) |
| Median (minimum, maximum) | 0 (0, 10) | 0 (0, 22) |
| Missing (%) | 248 (50.30) | 230 (44.49) |
| Hospital A&E | ||
| At 6 months | ||
| n | 346 | 375 |
| Mean (SD) | 0.13 (0.56) | 0.10 (0.38) |
| Median (minimum, maximum) | 0 (0, 6) | 0 (0, 3) |
| Missing (%) | 147 (29.82) | 142 (27.47) |
| At 12 months | ||
| n | 328 | 373 |
| Mean (SD) | 0.07 (0.32) | 0.09 (0.37) |
| Median (minimum, maximum) | 0 (0, 3) | 0 (0, 4) |
| Missing (%) | 165 (33.47) | 144 (27.85) |
| Over the trial | ||
| N | 268 | 309 |
| Mean (SD) | 0.22 (0.73) | 0.19 (0.58) |
| Median (minimum, maximum) | 0 (0, 6) | 0 (0, 4) |
| Missing (%) | 225 (45.64) | 208 (40.23) |
| Emergency service call | ||
| At 6 months | ||
| n | 365 | 384 |
| Mean (SD) | 0.06 (0.30) | 0.05 (0.39) |
| Median (minimum, maximum) | 0 (0, 3) | 0 (0, 6) |
| Missing (%) | 128 (25.96) | 133 (25.73) |
| At 12 months | ||
| n | 338 | 381 |
| Mean (SD) | 0.02 (0.26) | 0.03 (0.19) |
| Median (minimum, maximum) | 0 (0, 4) | 0 (0, 2) |
| Missing (%) | 156 (31.64) | 136 (26.31) |
| Over the trial | ||
| n | 287 | 321 |
| Mean (SD) | 0.08 (0.43) | 0.09 (0.48) |
| Median (minimum, maximum) | 0 (0, 6) | 0 (0, 6) |
| Missing (%) | 206 (41.78) | 196 (37.91) |
| Patient transportation | ||
| At 6 months | ||
| n | 360 | 382 |
| Mean (SD) | 0.013 (0.13) | 0.031 (0.30) |
| Median (minimum, maximum) | 0 (0, 2) | 0 (0, 5) |
| Missing (%) | 133 (26.98%) | 136 (26.31%) |
| At 12 months | ||
| n | 337 | 381 |
| Mean (SD) | 0.053 (0.58) | 0.015 (0.21) |
| Median (minimum, maximum) | 0 (0, 10) | 0 (0, 4) |
| Missing (%) | 156 (31.64) | 136 (26.31) |
| Over the trial | ||
| Patient transportation (N) | 283 | 319 |
| Mean (SD) | 0.021 (0.20) | 0.015 (0.14) |
| Median (minimum, maximum) | 0 (0, 3) | 0 (0, 2) |
| Missing (%) | 210 (42.60) | 198 (38.30) |
Participants in the intervention group had, on average, fewer visits to the GP than the usual-care group (Table 46); however, they had, on average, more visits to the practice nurse and the occupational therapist. Participants in the intervention group undertook more podiatrist visits in total than the usual-care group, as this group received this service as part of the intervention.
| Type of resource use | Treatment group | |
|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | |
| GP visit at GP practice | ||
| At 6 months | ||
| n | 328 | 364 |
| Mean (SD) | 0.17 (1.17) | 0.15 (0.55) |
| Median (minimum, maximum) | 0 (0, 20) | 0 (0, 5) |
| Missing (%) | 165 (33.47%) | 153 (29.59%) |
| At 12 months | ||
| n | 323 | 364 |
| Mean (SD) | 0.11 (0.47) | 0.14 (0.57) |
| Median (minimum, maximum) | 0 (0, 4) | 0 (0, 5) |
| Missing (%) | 170 (34.48) | 153 (29.59) |
| Over the trial | ||
| n | 250 | 295 |
| Mean (SD) | 0.25 (1.53) | 0.30 (0.95) |
| Median (minimum, maximum) | 0 (0, 23) | 0 (0, 10) |
| Missing (%) | 243 (49.29) | 222 (42.94) |
| Nurse visit at GP practice | ||
| At 6 months | ||
| n | 324 | 360 |
| Mean (SD) | 0.12 (0.62) | 0.25 (1.48) |
| Median (minimum, maximum) | 0 (0, 6) | 0 (0, 20) |
| Missing (%) | 169 (34.28) | 157 (30.37%) |
| At 12 months | ||
| n | 309 | 359 |
| Mean (SD) | 0.23 (2.89) | 0.13 (0.86) |
| Median (minimum, maximum) | 0 (0, 50) | 0 (0, 10) |
| Missing (%) | 184 (32.32) | 158 (30.56) |
| Over the trial | ||
| n | 241 | 287 |
| Mean (SD) | 0.37 (3.54) | 0.35 (1.88) |
| Median (minimum, maximum) | 0 (0, 54) | 0 (0, 20) |
| Missing (%) | 252 (51.12) | 230 (44.49) |
| Occupational therapist visit | ||
| At 6 months | ||
| n | 342 | 376 |
| Mean (SD) | 0.10 (0.71) | 0.04 (0.46) |
| Median (minimum, maximum) | 0 (0, 10) | 0 (0, 6) |
| Missing (%) | 151 (30.63) | 141 (27.27) |
| At 12 months | ||
| n | 334 | 370 |
| Mean (SD) | 0.04 (0.31) | 0.07 (0.53) |
| Median (minimum, maximum) | 0 (0, 4) | 0 (0, 7) |
| Missing (%) | 159 (32.25) | 147 (28.43) |
| Over the trial | ||
| n | 268 | 304 |
| Mean (SD) | 0.12 (0.65) | 0.11 (0.67) |
| Median (minimum, maximum) | 0 (0, 7) | 0 (0, 6) |
| Missing (%) | 225 (45.64) | 213 (41.20) |
| Podiatrist visits | ||
| Not intervention related | ||
| n a | 355 | 380 |
| Mean (SD) | 2.09 (3.84) | 4.01 (2.63) |
| Median (minimum, maximum) | 1 (0, 46) | 3 (0, 20) |
| Missing (%) | 138 (27.99) | 137 (26.50) |
| Intervention related | ||
| n | 413 | N/A |
| Mean (SD) | 2.52 (0.88) | N/A |
| Median (minimum, maximum) | 0 (0, 7) | N/A |
| Missing (%) | 0 (0%) | N/A |
Table 47 summarises the mean cost by item of resource use based on all available cases and according to treatment group. Costs associated with hospital inpatient stay and the intervention itself were the major cost drivers for the participants in the intervention group.
| Cost item | Total mean cost, £ (SD) | Mean difference (intervention – usual care) (95% CI) | |
|---|---|---|---|
| Intervention (N = 493) | Usual care (N = 517) | ||
| Hospital inpatient length of staya | 1314.29 (3290.81) | 1089.96 (2791.51) | 224.3 (–181.0 to 629.6) |
| Hospital outpatient visits | 37.57 (190.09) | 26.66 (84.41) | 10.91 (–13.24 to 35.06) |
| Hospital day case | 85.22 (578.58) | 90.31 (962.86) | –5.08 (–143.31 to 133.13) |
| A&E visit | 30.95 (103.97) | 27.75 (82.85) | 3.19 (–12.09 to 18.48) |
| Podiatry visits | 73.35 (134.43) | 140.64 (92.35) | –67.29 (–83.90 to –50.68) |
| GP visit at GP practice | 11.24 (64.47) | 13.42 (42.01) | –2.15 (–11.47 to 7.15) |
| Nurse visit at GP practice | 9.43 (88.51) | 8.88 (47.06) | 0.55 (–11.31 to 12.42) |
| Occupational therapist | 5.58 (28.71) | 4.92 (29.71) | 0.66 (–4.15 to 5.47) |
| Emergency service call | 12.34 (67.62) | 14.87 (74.05) | –2.53 (–13.87 to 8.81) |
| Patient transportation | 21.80 (116.51) | 24.20 (132.79) | –2.39 (–22.47 to 17.68) |
| Cost of interventionb | 155.79 (55.02) | N/A | N/A |
| Podiatry visits | 104.74 (32.14) | N/A | N/A |
| Shoes | 40.29 (30.94) | N/A | N/A |
| Insoles | 3.60 (3.33) | N/A | N/A |
| Exercise therabands | 2.32 (1.17) | N/A | N/A |
| Exercise ball | 1.00 (0.58) | N/A | N/A |
| Exercise DVD | 3.82 (0) | N/A | N/A |
Twenty-eight participants (intervention, n = 14; usual care, n = 14) reported that they had to spend a night in hospital as a result of a fall. The average cost per inpatient stay was £7121.00 (SD £1535.83) in the intervention group and £6666.50 (SD £1156.84) in the usual-care group. Therefore, inpatient stay based on fall calendars was £454.50 more expensive for participants in the intervention group (95% CI –£601.80 to £1510.80). Given the small sample size, it was decided to estimate inpatient stay based on data from the participant questionnaires. The only limitation of this is that the participant questionnaire did not differentiate inpatient stay as fall- and non-fall-related, although the rest of hospital stay (outpatient, day case and A&E) did differentiate between these.
Costing the intervention
The protocol stated that participants allocated to the intervention would receive at least one baseline visit to the podiatrist plus at least one follow-up appointment. In total, 413 out of 493 (83.8%) participants allocated to the intervention had at least one visit to the podiatry clinic and 183 (37.1%) had at least two. The first appointment was assumed to last for 1 hour, the second appointment for 30 minutes and all the rest were assumed to be the same duration as a GP clinic consultation (11.7 minutes). The cost for the visits was estimated according to NHS pay scales on the Agenda for Change (https://healthcareers.nhs.uk/glossary%32Agenda_for_Change) for NHS podiatrist staff in England, Wales, Scotland and Northern Ireland from 1 April 2015. Podiatrists delivering the intervention ranged from band 6 to band 8. The annual and unit costs per podiatry visit were estimated, excluding qualifications but including overheads on a community basis.
A total of 260 participants received a new pair of shoes. The price of the shoes ranged between £39 and £89. There was insufficient information to determine the exact make and model of shoe received by the participant; hence, an average shoe price of £64 was assumed for the analysis. As the NHS will not cover the cost of the provision of new footwear, this was not considered for the base-case analysis. A sensitivity analysis on the societal perspective looked at the impact of the shoe price on the cost-effectiveness results. A total of 241 participants also received a pair of insoles: X-Line red (n = 23), X-Line blue (n = 209) or Formthotics insoles (n = 9). They also received resistive therapy bands and therapy balls for the exercises.
The intervention cost on average was £155.79 (SD £55.02) for the 413 participants who received the intervention when we include the price of the shoes (societal perspective) and £115.5 (SD £33.06) when we exclude the price of the shoes.
Cost–utility analysis and uncertainty
The base-case analysis (Table 48) shows that the participants who were randomised to the intervention experienced (marginally) improved health outcomes. At the end of the trial, the intervention group had experienced 0.0129 (95% CI –0.0050 to 0.0314) more QALYs. However, the intervention is more costly than usual care [on average £252.17 more per participant than usual care (95% CI –£69.48 to £589.38)] when adjusted for all covariates (including baseline utility). ICERs ranged between £19,494 and £20,593 (societal perspective adjusted for all covariates) per additional QALY. For both the base-case and secondary analysis (societal perspective), the probability of the intervention being the more cost-effective option is > 0.60 for the incremental analysis adjusted for baseline EQ-5D, and > 0.65 when incremental QALYs are adjusted for all covariates. The NMB associated with the intervention is positive, indicating that the intervention is cost-effective, as the resources to be displaced would be less than the benefit to be gained if the intervention was implemented in the NHS. However, these results were calculated from the point estimate of the difference in QALYs; the lower-bound confidence limit for the 95% CI was negative, and, therefore, there is the potential for a negative QALY gain.
| Analysis | Difference in costsa (95% CI) | Difference in QALYsa (95% CI) | ICER for the intervention (£ per QALY) | Probability intervention was cost-effective £30,000/QALY (%) |
|---|---|---|---|---|
| Base case (MI), NHS perspective | 252.17 (–69.48 to 589.38) | 0.0129 (–0.00 to 0.03) | 19,494.35 | 65.58 |
| Sensitivity 1 (complete case) | 272.86 (–349.6 to 916.56) | –0.0091 (–0.04 to 0.02) | Intervention dominated | 17 |
| Sensitivity 2 | 222.34 (–156.6 to 605.1) | 0.0109 (–0.007 to 0.029) | 20,385.75 | 61 |
| Sensitivity 3 | 441.88 (–273.1 to 1052.4) | 0.0150 (–0.002 to 0.033) | 29,454.34 | 49 |
| Sensitivity 4 | 327.17 (–65.17 to 451.09) | 0.0140 (–0.003 to 0.032) | 23,341.72 | 60 |
The incremental cost-effectiveness plane (Figure 11) demonstrates the uncertainty associated with the mean difference in costs and QALYs between both intervention groups by plotting the non-parametric bootstrapping results. A total of 5000 bootstrapped replicates of differences in costs and QALYs are shown. The majority of the replicates falls within the north-east quadrant, indicating that the intervention is more effective but more costly.
FIGURE 11.
Cost-effectiveness plane (ITT analysis) for the base-case analysis: base-case NHS perspective (MI adjusted for baseline utility).

The CEAC derived from the joint distribution of costs and effects is represented in Figure 12. The curve was constructed by plotting the proportion of incremental cost–effect pairs that are cost-effective for a range of thresholds. The horizontal interrupted line indicates a 50% probability of the intervention representing value for money for the NHS. The probability of the intervention being cost-effective is > 60% given the current NICE WTP threshold of £30,000 per additional QALY.
FIGURE 12.
Cost-effectiveness acceptability curve (ITT analysis) for the base-case analysis: base-case NHS perspective (MI adjusted for baseline utility).
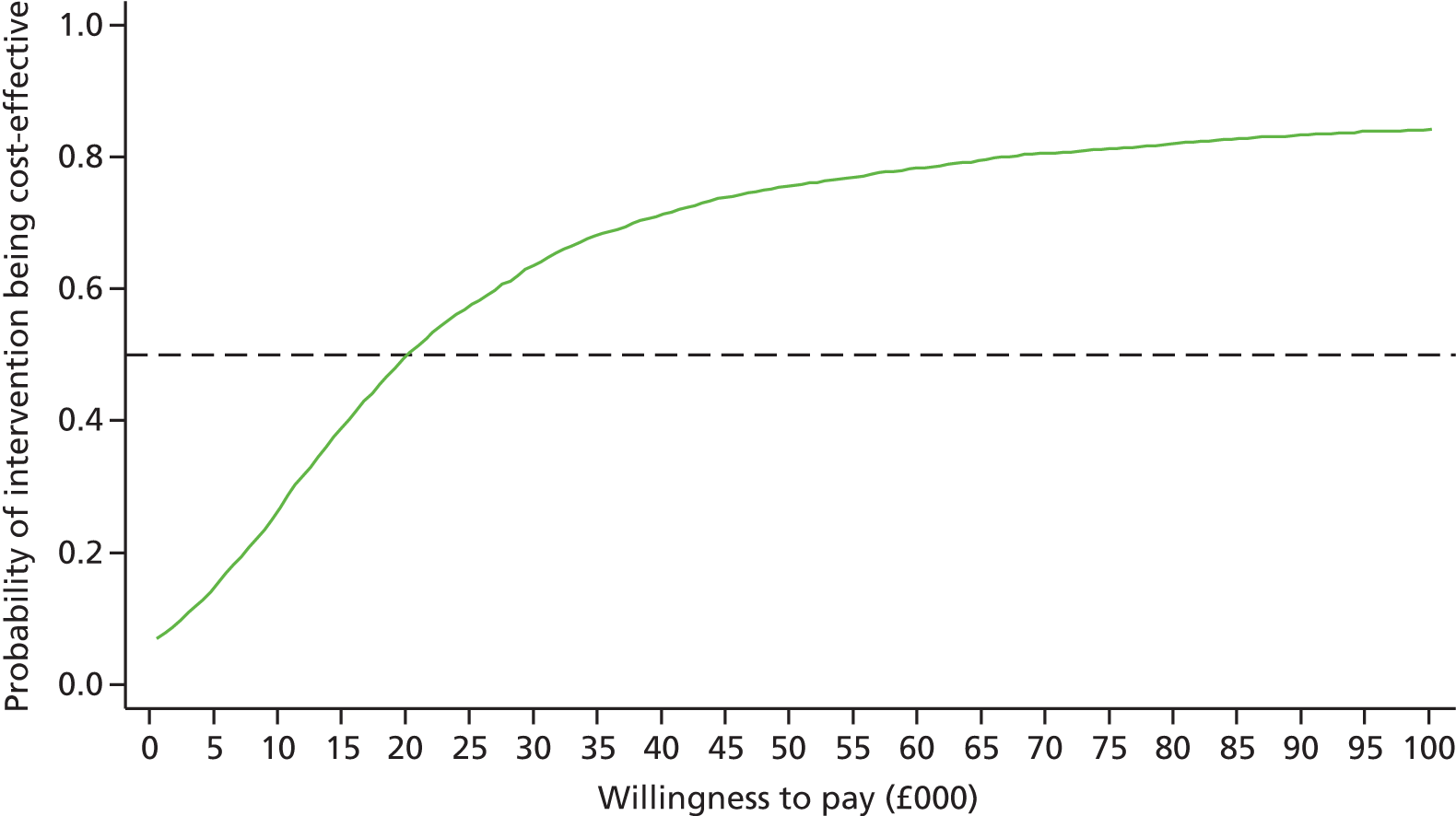
Cost-effectiveness analysis and uncertainty
A cost-effectiveness analysis, in which the outcome is expressed in terms of number of falls averted, may be more intuitive to interpret for health-care professionals. However, there is no established WTP threshold for an additional fall averted. Therefore, the cost per fall averted was assessed for comparison, as allocation decisions can be made based only on cost per QALY estimates. In the base case, the podiatry intervention was both more costly (mean incremental cost £241.64, 95% CI £–98.08 to £581.37) and more effective (mean incremental effect 0.19 falls averted per person year, 95% CI –0.05 to 0.44 falls averted per person year), with an incremental cost per fall averted of £1253.82 (ICER); however, for both of these parameters the lower 95% confidence limit is negative, and so this does not exclude the possibility of a negative result. Figure 13 shows the incremental costs and incremental effects on the form of a cost-effectiveness plane. There is significant uncertainty in the effectiveness estimates, as the estimates fall on both sides of the x-axis. Figure 14 shows the CEAC per fall averted.
FIGURE 13.
Cost-effectiveness plane (ITT analysis) per fall averted.
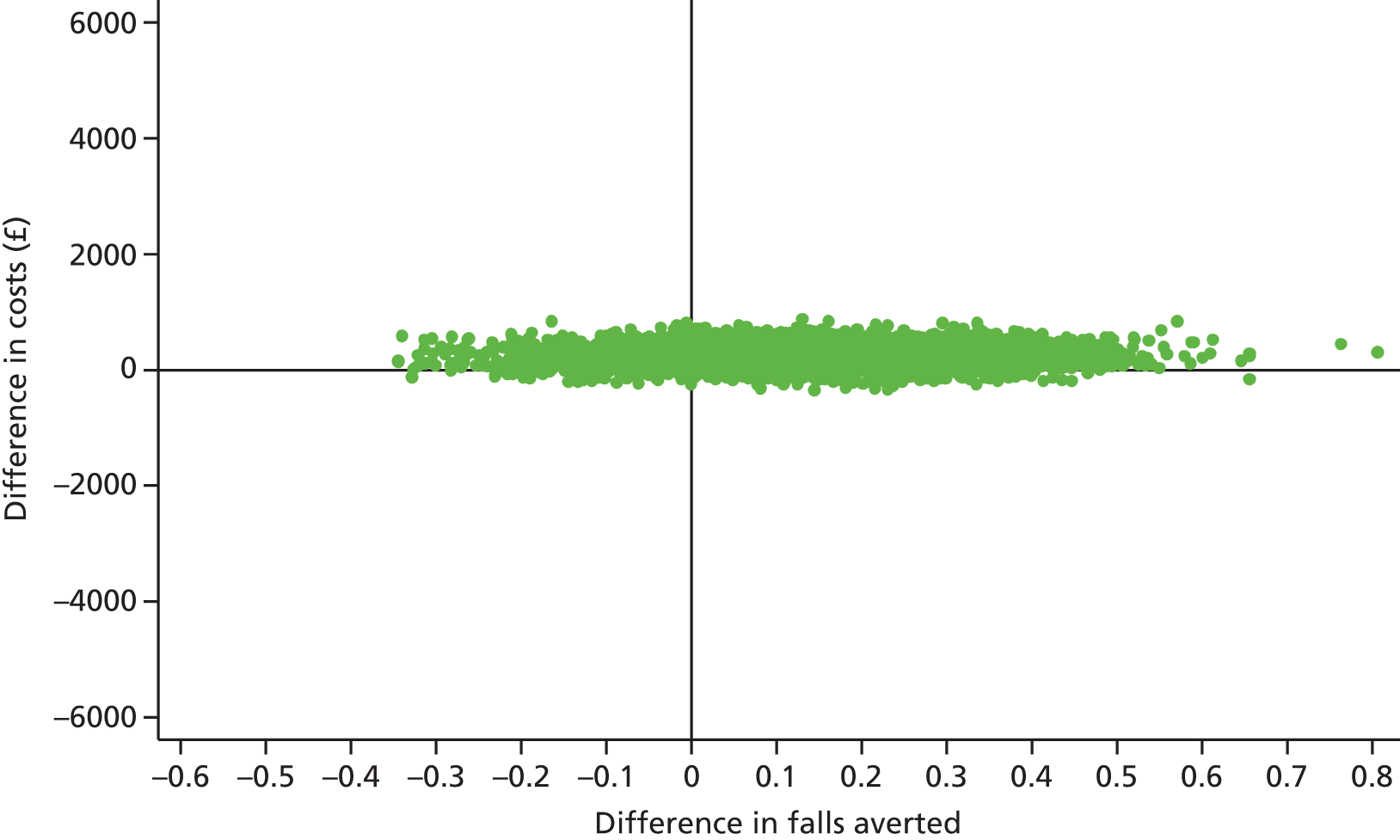
FIGURE 14.
Cost-effectiveness acceptability curve (ITT analysis) per fall averted.
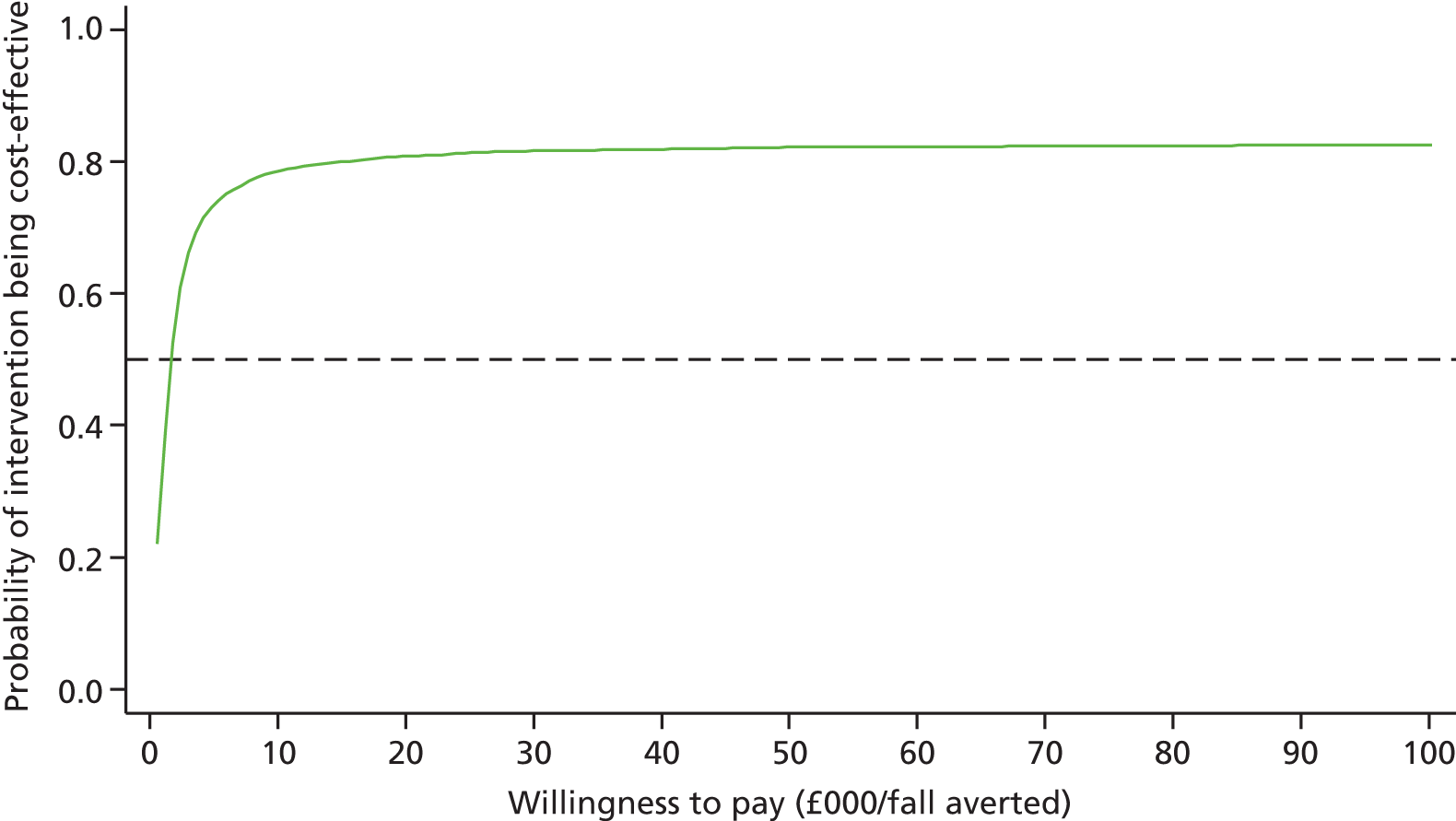
Sensitivity analysis
Handling missing data
The complete-case analysis was tested as an alternative method to MI for handling missing data. The complete-case scenario comprised 286 (28.3%) participants, of whom 129 (26.2%) were in the intervention group and 157 (30.4%) were in the usual-care group. The complete-case analysis shows that the intervention group accumulated greater costs and reported lower HRQoL than participants randomised to usual care. The intervention costs were, on average, £272.86 more per participant than usual care (95% CI –£349.63 to £916.56), although accumulated total QALYs are smaller than those for usual care (mean difference –0.0091, 95% CI –0.0396 to 0.0196). Therefore, complete-case results indicate that the intervention is dominated by usual care (Figure 15). The NMB associated with the intervention indicates that the resources to be displaced would be greater than the benefit to be gained if the intervention were implemented in the NHS. Figure 16 represents the CEACs for the complete case when adjusted for all covariates. The probability of the intervention being cost-effective is < 20% given the WTP for an additional QALY up to £30,000; therefore, the intervention is unlikely to be cost-effective based on a complete-case analysis.
FIGURE 15.
Cost-effectiveness plane (ITT analysis) for the complete-case analysis (NHS perspective, adjusted for covariates).

FIGURE 16.
Cost-effectiveness acceptability curve (ITT analysis) for the complete-case analysis (NHS perspective, adjusted for covariates).

Given the accumulative nature of costs and QALYs, these variables can be dealt with at different levels of aggregation. The base-case analysis estimated QALYs by imputing missing utilities (e.g. disaggregated level). A sensitivity analysis was conducted for the base case on the imputed data set in which we explored the impact of imputing HRQoL at QALY level (e.g. aggregated level). Imputing HRQoL at an aggregated level has no impact on the cost-effectiveness of the intervention).
Resource use
A one-way sensitivity analysis was conducted to test the impact of including both fall- and non-fall-related resource use. There is no major impact in the results when we investigate the impact of considering all resource use in the assessment. The intervention is still cost-effective at the £30,000 per QALY gained threshold.
Exploration of the need for a long-term model
The economic evaluation conducted alongside REFORM found that the podiatry intervention was likely to be cost-effective over a 1-year time horizon. A sufficient condition for surgery to be definitely cost-effective over a longer term is that in each year after 12 months, HRQoL is lower (and costs are the same or increasing faster) in the usual-care group than in the intervention group. This section develops an exploratory model to explore how the differences in QALYs evolve beyond the study. A straightforward way of projecting QALYs beyond the trial is to assume that the difference in HRQoL observed at 1 year remains unchanged. To compare the cost-effectiveness estimates, we defined two health states (alive and dead). The podiatry intervention, when displacing usual care, is expected to bring gains of 0.0129 QALYs per patient (per year). In addition, it was assumed that patients undergoing the podiatry intervention incur costs of £251 more per year when alive. When looking at the first 5 years, the results of the model show that adopting the podiatry intervention over usual care provides a higher HRQoL over a 5-year time horizon. Although the difference in HRQoL between the intervention and usual-care groups decreases over time (e.g. 0.0126 at year 2 vs. 0.0117 at year 5), it remains higher for patients who received the intervention. The expected ICER related to the adoption of the podiatry intervention ranged between £19,950 (year 2) and £21,460 (year 5) per QALY gained. Nonetheless, the value for money of the intervention is decreasing with time. We consider that this exploratory projection is likely to be conservative, as it excludes potential costs savings associated with the intervention. Therefore, from this exploratory analysis we can conclude that this relatively low-cost intervention appears to improve health outcomes within the short term. We are currently conducting a long-term model to validate these preliminary results. The findings of this model will be published in a peer-reviewed journal.
Chapter 6 Qualitative results
Participants
Qualitative semistructured interviews were conducted with 15 podiatrists: 14 who delivered the REFORM intervention and one PI from a site who was not involved in delivering the intervention but who assisted with the day-to-day management of the study at the site. All trial podiatrists were invited to take part in the qualitative interviews. The sample consisted of five men and 10 women, representing seven NHS trusts and a university podiatry school in Ireland. Participating podiatrists had between 6 and 32 years’ experience. Various grades of podiatrist were represented: one at band 5, six at band 6, six at band 7 and two at band 8. All podiatrists worked predominantly with patients from the community and were skilled in providing footwear advice, exercises and insole therapy for the management of foot and ankle pathology and biomechanical imbalance. The sample included podiatrists with postgraduate training at master’s (three podiatrists) and doctoral (two podiatrists) level.
Further details of the REFORM podiatrists are provided in Table 49.
| Podiatrist identifier | Sex | Years’ experience | Qualification |
|---|---|---|---|
| 1P | Male | 27 | BSc and MSc |
| 2P | Male | 10 | BSc and MSc |
| 3P | Female | 11 | BSc |
| 4P | Male | 10 | BSc |
| 5P | Male | 6 | BSc |
| 6P | Female | 8 | BSc |
| 7P | Female | 10 | BSc |
| 8P | Female | 32 | MSc |
| 9P | Female | 13 | BSc |
| 10P | Female | 22 | BSc |
| 11P | Female | 9 | BSc |
| 12P | Male | 28 | DPodM |
| 13P | Female | 18 | BSc |
| 14P | Female | 10 | PhD and BSc |
| 15P | Female | 9 | PhD and BSc |
Twenty-one participants from the REFORM trial were interviewed. The sample comprised 10 men and 11 women aged between 65 and 87 years. Fifteen participants said that they lived with their spouse and/or other family members and the remaining six lived alone. Further details of the REFORM trial participants are provided in Table 50.
| Participant | Age at randomisation (years) | Sex | Reported fear of falling at baseline | Fallen during the 12 months after randomisation |
|---|---|---|---|---|
| 1 | 70 | Male | Some of the time | No |
| 2 | 75 | Male | Some of the time | No |
| 3 | 77 | Male | A little of the time | No |
| 4 | 80 | Female | None of the time | Yes |
| 5 | 81 | Female | Most of the time | Yes |
| 6 | 77 | Female | A little of the time | No |
| 7 | 67 | Female | Some of the time | No |
| 8 | 65 | Male | A little of the time | No |
| 9 | 80 | Female | All of the time | Yes |
| 10 | 84 | Male | A little of the time | No |
| 11 | 80 | Female | A little of the time | No |
| 12 | 67 | Female | Some of the time | Yes |
| 13 | 87 | Female | None of the time | Yes |
| 14 | 79 | Male | All of the time | No |
| 15 | 79 | Female | A little of the time | No |
| 16 | 84 | Male | A little of the time | Yes |
| 17 | 69 | Male | A good bit of the time | Yes |
| 18 | 78 | Male | Some of the time | Yes |
| 19 | 86 | Male | None of the time | Yes |
| 20 | 79 | Female | Some of the time | Yes |
| 21 | 79 | Female | A good bit of the time | No |
The qualitative interviews with trial participants and podiatrists discussed experiences of receiving and delivering the REFORM podiatry intervention, respectively. The findings are reported according to the three components of the REFORM trial intervention in turn: (1) footwear assessment, advice and provision, (2) orthoses and (3) exercises. For each intervention component, three main themes are discussed: (1) current usual practice, (2) acceptability and barriers to implementation among service providers and (3) acceptability and adherence among service users. Within the adherence subsection, quantitative data from the whole of the trial intervention group are included as appropriate.
Footwear assessment, advice and provision
Assessing and ordering footwear during usual practice
Podiatrists reported how patients frequently wore inappropriate footwear and cited common issues seen during practice as narrow shoes, inappropriate heel height or shoe style and a lack of appropriate fastening. Although some issues with men’s footwear were reported (e.g. fastening and inappropriate slippers), it was the perception of the podiatrists that the majority of issues were with women’s footwear:
It tended to be the women that weren’t wearing the sort of shoes that would balance and that were comfortable, they tended to wear, I don’t know, more fashionable shoes forgetting about what sort of age they were and they hadn’t thought about the fact that the shoes were possibly causing their instability.
Podiatrist 7
All podiatrists discussed how general footwear advice was provided as part of their usual practice, with a small number of podiatrists basing this advice on falls prevention. Routinely, footwear advice involved discussions around the types and styles of shoes and placed a particular emphasis on the importance of indoor shoes. Central to these discussions was the need to enhance the patient’s understanding of ‘good footwear’. To facilitate this, podiatrists described how they often directed patients to cheaper footwear alternatives or specific companies, to demonstrate that a good shoe was not necessarily reflected by its price. In addition, podiatrists spoke of using prompts such as shoe catalogues, leaflets and sample shoes that were compared with patients’ current footwear, in order to further patients’ understanding.
Variation in the criteria to assess patients’ footwear was reported within routine practice; for instance, some podiatrists described how they made assessments by glancing at the patients’ footwear or how the assessment was ‘second nature’. The characteristics of footwear commonly assessed during routine practice tended to include fastening, length, width, heel height, sole and fabric. Podiatrists confirmed that there is currently no formal checklist used within routine practice for footwear assessment:
Yes it’s in your head and then you tick a box on SystmOne to say appropriate footwear worn . . . it’s second nature.
Podiatrist 5
Podiatrists reported that footwear was not routinely provided, with only one podiatrist (podiatrist 5) referring to fitting ‘stock hospital shoes’ when there was a clinical need.
Experiences of assessing and ordering footwear during the REFORM trial
For the purposes of the REFORM trial, participants were asked to bring samples of their indoor and outdoor footwear to the clinic for assessment. As described in Chapter 2, podiatrists were asked to assess participants’ footwear against a checklist provided by the research team of characteristics of suitable footwear identified in the literature12 (see Appendix 21). If trial participants failed to bring samples of their footwear, the assessment was based on a description. The majority of the podiatrists found the trial criteria for assessing footwear straightforward to follow and described the checklist as clear and logical:
It [the checklist] was very similar to what I would normally do and it actually reiterated all the things that I was doing before so it was, you know, clarified everything that I was doing and thinking yep that it was [what] I would do anyway, so I mean that was quite useful because it took me back to basics to actually think about it a bit more.
Podiatrist 7
However, a few podiatrists mentioned that the current electronic medical record did not have sufficient data fields for the more detailed footwear checklist; therefore, alternative ways of capturing these data would have to be found:
In our paperwork we have whether it’s a good fitting shoe, whether it was too big or too small, whether it’s a slipper or bespoke orthopaedic aid and then we have an optional heel height and what kind of fastening they have and we do have a comment box as well. So that’s our kind of footwear assessment of what we can document.
Podiatrist 10
The REFORM trial also enabled podiatrists to order footwear for participants, which is something that is not currently provided in usual podiatry care. Prior to ordering shoes, podiatrists were required to measure participants’ feet. The measuring guide was a laminated picture of a foot, annotated with different shoe sizes. Participants stood on the guide and their corresponding shoe size was read. The width of shoe was determined by measuring the circumference of the foot using a tape measure and referring to a chart to find the required width (www.dbshoes.co.uk/measuring_chart.php, accessed 2 October 2012; www.hotter.com/gb/en/info/Hotter-Shoes-Fitting-Guide, accessed 2 October 2012). Although the majority of podiatrists described the measuring process as straightforward, a number of difficulties were reported, especially among those who did not have training in shoe fitting outside the trial (the trial did not provide additional training on this). Some podiatrists commented that the measuring guide on which participants stood during the measuring process would sometimes slip unless it was taped to the floor. Challenges for less mobile patients were also reported, as they found it hard to stand on the measure, which had to be placed against a wall.
Despite some difficulties, the majority of podiatrists felt that footwear assessment and advice was generally straightforward and a central element of their clinical role; however, some expressed concerns that in routine practice there would be insufficient time to undertake a full footwear assessment:
It is time-consuming but we were given enough time to do it, so in an ordinary clinic if you had 20 minutes to do a routine treatment and educate them on footwear and have a discussion around it, it would really eat into the time but for the study we had enough time to do that.
Podiatrist 1
Given these time pressures within usual practice, a number of the podiatrists did suggest that this element of the intervention could potentially be conducted by a podiatry assistant or technician:
. . . probably our technicians or podiatry assistants are probably very, very competent at doing that type of thing.
Podiatrist 7
In contrast, however, one podiatrist (podiatrist 4) felt very strongly that footwear measurement was outside the role of podiatrist and should be conducted by an orthotist, working as part of a multidisciplinary team.
Additional issues with the measuring process included the accuracy of the sizing guide, fitting slippers that were available only in full sizes (not half sizes), the lack of footwear under size two and the time required to fit footwear:
That was out of frustration because of the backwards and forwards process of ordering, fitting and finding out that the shoe wasn’t right.
Podiatrist 3
A minority of podiatrists also found the shoe ordering process very time-consuming, something that was attributed mainly to patients spending large amounts of time selecting footwear from what some podiatrists considered an excessive number of options.
Experiences of and adherence to footwear advice/trial purchased shoes
As would be expected, adherence to footwear advice varied among participants. From the 12-month follow-up questionnaire administered to the whole of the trial intervention group, we observed that nearly two-thirds (n = 137, 63.7%) of the participants who reported that they had their shoes checked said that their podiatrist gave them advice about their footwear or suggested that they should wear a different style of shoe, of whom 104 (77.0%) reported that they followed this advice (80.8% of men and 74.7% of women).
It was the view of both podiatrists and trial participants that it can be difficult to action footwear advice and change shoes/slippers, as high-street shops do not always stock footwear that is a suitable fit, and in some areas there is a lack of stockists:
But you see they say what makes an everyday shoe unsafe . . . what they mean about secure fastenings but they’re not easy to find either . . . That’s what I need, depth for my toes and they have depth but you don’t get a great deal of choice and I believe there’s a shop down [name of street where shop is] that deals in, just a little shop and they deal in shoes, I haven’t been in. I’ve just looked in the window as I’ve passed by.
Trial participant 8
Several podiatrists highlighted that, for many service users, the cost of the appropriate footwear recommended could be prohibitive:
Obviously these brands that you recommended, you know, in the shops they can be 70 to 80 pounds, so you know, unfortunately some people just can’t afford to get more specialised footwear.
Podiatrist 6
The availability of shoes through the trial had provided respondents with access to appropriate footwear by overcoming these barriers. In addition, podiatrists were at pains to discuss with service users that, although many shoes were expensive, ultimately the cost of the shoe did not always reflect whether or not it was appropriate for them:
. . . so I think [the service providing shoes] is a great idea in theory but I think it’s probably quite costly and maybe, you know, you can buy, you know, if you’ve got to buy a pair of shoes there’s a lot of shoes you can buy that aren’t as expensive but still have those good qualities, you know, like a cheaper pair of trainers really.
Podiatrist 12
The response to the footwear advice/footwear provided was mixed. Many participants reported that they wore their ‘appropriate’ outdoor shoes all the time and were really satisfied with the fit and choice of shoe:
I think I’ll always wear Hotter shoes now because they’re so good.
Trial participant 2
Other participants described how they stopped wearing the footwear, largely because the shoes did not fit properly and were uncomfortable; in some cases, even the recommended shoe suppliers did not have shoes that were a good comfortable fit for people who are older (some of whom had various foot problems including arthritis, bunions or corns). In addition, there were also pragmatic reasons why appropriate shoes were more difficult to identify for some service users, such as difficulty in putting shoes on among this age group:
The age group of the patients in the REFORM trial were very elderly, or a lot of them were, and for that reason they had difficulty getting down to their feet, so they tend to go for slip-on shoes for ease of fitting them whereas a lace-up or Velcro-fastening shoe created additional problems.
Podiatrist 3
Despite participants demonstrating a good general understanding of ‘appropriate footwear’, there were still a number of examples of inappropriate footwear being worn, reflecting the pragmatic solutions the participants had found in order to incorporate the advice into their daily routines and practices:
I think if you wear sensible shoes, I think that’s the big thing. I mean I’m past all these stiletto heels now. I used to wear them once but no, I do wear a little heel sometimes when I go out. I like these flip-flops in the summer because they’ve got the heighted heel at the back and I find that very comfortable. I’m not very keen on dead flat but because of my neck I don’t like a very flat shoe. Yeah I think sensible shoes.
Trial participant 6
Although not particularly supported by the quantitative data, podiatrists perceived that women were less likely to action the footwear advice and that this needed to be taken into account when such advice was being provided:
It’s about the complete loss of identity . . . you have to try and see if from the patient’s point of view because it’s very cut and dry for us. This is what you need, this is what’s going to be really good for you and that’s what you’re going to get and sometimes you have to work a little bit more around the patient and I think, you know, going back to the original discussion about footwear, I think women have an idea about how they want to present themselves to the world and if say that foot support or orthotic is not going to fit in that shoe or the shoe is not going to support the function of the device, it can be very difficult.
Podiatrist 4
Orthoses
Using orthoses during routine practice
The majority of podiatrists described how they had prescribed some form of orthosis in their usual practice, predominantly for clinical conditions such as Achilles tendon problems or plantar fasciitis; however, one podiatrist (podiatrist 1) reported that they had prescribed an orthosis for a participant who had fallen. Of the podiatrists who had prescribed orthoses, the majority had prescribed the trial orthosis (X-Line) or a similar orthosis. Issues encountered when routinely prescribing orthoses were reported and included complaints that they were uncomfortable or too bulky to fit in participants’ shoes:
Sometimes they [the participants]find them a bit too bulky, so they can’t get their foot in as well as the insole and just to be able to get them in the shoes themselves.
Podiatrist 2
Experiences of the X-Line orthosis during the REFORM trial
The majority of podiatrists and trial participants reported positive experiences of using the X-Line orthosis and described the X-Line as a good, cost-effective orthosis. Podiatrists also explained how the X-Line was good for participants who had arthritis or deformities, as the orthosis was slim and so could easily fit into footwear. The arch support, control on the heel and met dome support were also cited along with the fact that the X-Line was not overly corrective and so was not expected to cause problems such as lesions or balance problems. Direct comparisons with other routinely prescribed orthoses were also made, with podiatrists often stating a preference for the trial orthosis. Podiatrists’ positive experiences of the X-Line orthosis were also demonstrated when one site reported that it may consider changing its routinely prescribed orthosis to the X-Line, although this would ultimately depend on the cost of the device and whether or not those who purchased equipment in the trust would agree to order this type of device:
These weren’t bad at all because they’re quite thin on the front as well, that’s another problem we usually have of patients especially if they’ve got arthritis in their toes, claw toes that type of thing but even the slight raise sort of lifts the foot up and then the shoes are pressing on the toes. But no I was quite impressed with those.
Podiatrist 8
On a practical level, the majority of podiatrists found the X-Line insoles easy to fit into participants’ footwear. Trimming the insole was generally not found to be an issue, as this could mainly be done at the clinic with scissors. If required, the insole could also be trimmed to three-quarter length, to aid fitting into participants’ footwear. Attaching postings and additions was also not perceived to be a problem, as they were self-adhesive. This meant that they could be attached in the clinic and so did not require special equipment or to be sent to the laboratory, as is sometimes the case:
The additions we just had the peel back, so like the sticky on the back and you just peel it off and away you go whereas the ones we use at the minute, they have to go to the lab and like be glued on. So obviously this is easy because you’ve got them in clinic and you can put them on there and then and off they go.
Podiatrist 2
However, although the majority of podiatrists related positive experiences, theere were some difficulties reported with fitting and trimming the X-Line. For instance, one podiatrist (podiatrist 3) stated that trying to fit the orthosis into a participant’s footwear made the shoes too tight and inappropriate:
The only difficulty came in with trying to fit an orthotic [insole] to what was an appropriate shoe and if the shoe then became too tight then it was no longer an appropriate shoe.
Podiatrist 3
At study set-up, some sites raised concerns about the possibility of the trial identifying patients with an unmet clinical need, who would require a full biomechanics assessment. Sites were apprehensive about the impact that this would have on their clinics. These concerns were not represented within the interviews; the majority of podiatrists were willing to prescribe the insole without a full assessment, as they considered the device unlikely to cause participants any problems. However, three podiatrists reported giving participants an assessment to check that it was clinically appropriate to prescribe the orthosis:
Yeah I mean it was a slight assessment with checking muscle strength and basic things but I wouldn’t say it was a full complex biomechanical assessment.
Podiatrist 8
Experiences of and adherence to the orthosis prescribed during the REFORM trial
For those in the trial as a whole, Table 51 presents responses to the adherence questions for the intervention participants who received an orthotic insole and responded to this question. At 12 months, 66.4% of participants reported wearing their orthosis most or all of the time, and 85.0% reported wearing it at least a little of the time.
| Time point (month) | |||
|---|---|---|---|
| 3 | 6 | 12 | |
| Number of questionnaires received | 457 | 427 | 408 |
| Of which received intervention | 393 | 372 | 357 |
| Of which received an orthosis | 237 | 224 | 215 |
| In the past month, typically how often was foot orthosis (insole) worn in shoes, n (%) | |||
| All of the time | 89 (38.5) | 75 (33.9) | 87 (40.7) |
| Most of the time | 61 (26.4) | 73 (33.0) | 55 (25.7) |
| Some of the time | 34 (14.7) | 29 (13.1) | 30 (14.0) |
| A little of the time | 10 (4.3) | 15 (6.8) | 10 (4.7) |
| None of the time | 37 (16.0) | 29 (13.1) | 32 (15.0) |
It is clear that wearing orthoses was generally acceptable; however, the podiatrists adapted their use to the individual circumstances of the participant, for example by adapting the insole to accommodate foot deformities. As might be expected, how comfortable the participant found the insole was a significant determinant in whether or not it was worn with any regularity:
They’re comfortable and that’s the main thing as well, if they weren’t comfortable they would have been chucked out.
Trial participant 7
However, those who felt that the orthosis was likely to have a benefit, through either previous experiences of using orthoses or anecdotes from family or friends, seemed to be prepared to endure some discomfort, at least initially. Some participants were also willing to persevere with the orthotic, despite some initial discomfort, because it had been recommended by the podiatrist and they felt that it was likely to be of benefit. Several reported that they considered the insoles to be a good idea, especially as they had either fallen several times or because they wanted to find something to help improve their walking, and demonstrated a willingness to try:
Well it took several weeks to get accustomed to the insoles, it was quite painful but I was determined to persevere.
Trial participant 4
Although in some cases the perseverance paid off, others ceased to wear the orthosis completely:
I used the orthotics, I had some from before, and they are not comfortable with my back. I mean there’s nothing much the matter with my back but I ended up with a sore back, with wearing them.
Trial participant 13
A contributing factor in the resultant comfort was whether or not appropriate footwear was available. Although the trial had attempted to accommodate the requirement for footwear when necessary, for some participants, even with this option, the orthosis was still not a comfortable fit. This was especially the case for individuals who had pre-existing problems with their feet, which made it difficult to cope with the inserts, or who had to adapt the use of the inserts to make them tolerable:
Yes but on one foot I could wear them but not on the other because I’ve got very high arch, I broke this foot when I was very, very young and it just made a difference. It made the shoe too tight.
Trial participant 3
However, for many the whole package of obtaining the appropriate footwear and orthosis worked well and participants could feel the benefit from the increased support. Positive experiences were mainly associated with comfort and support, for example improvement in posture or shoes fitting better and being more comfortable to wear. This was in addition to the perceived effect it had on participants’ balance, the number of falls they had and their confidence:
. . . [the podiatrist] decided the shoes, although they were Clarks [C&J Clark Ltd, Somerset, UK] the shoes I was wearing, they weren’t as good so she measured me for some shoe inserts and I got a pair of Hotter shoes and a pair of Hotter slippers and these shoes and the inserts they’ve made a dramatic difference, you know, to me walking and lifting; because I think my arches have probably fallen a bit, so it gives me support in that way.
Trial participant 2
Those who were experiencing a noticeable benefit tended to wear the orthoses all the time:
I’ve got one set for these shoes and then another set I use if I go out anywhere or leisure, I just slip them into whatever shoes I’m using.
Trial participant 2
However, others, who did not feel such a palpable benefit or who did not have the expectation that orthoses were likely to affect their risk of falls, tended to use the inserts in a more pragmatic way, and adapted their use to what was practical and feasible:
I’ve got them in one of the pairs of shoes that I wear most of the time . . . [I wear them] probably four times a week, because I can’t get them in, like I say, I’ve got a problem with my toe, so if I can’t put them into any of my other shoes because there’s not enough room in there for my toes and that in sole . . . I can’t say that I’ve really felt a difference, do you know what I mean – I’ve got used to wearing them so they’re very comfortable.
Trial participant 7
Exercises
Prescribing exercises in routine practice
Some podiatrists reported prescribing exercises in their routine practice, largely for conditions such as plantar fasciitis or Achilles tendon injuries. The podiatrists were not currently prescribing exercises for falls prevention in routine care. They acknowledged issues associated with prescribing exercises, which included the potential for the exercises to cause injury:
Yeah but with older people a lot of the exercises you’ve got to be really careful with that you don’t cause further problems. Some of the exercises I think the patients go a bit too far with them and would actually sort of damage tendons if they overstretch.
Podiatrist 8
Exercises prescribed during routine practice differed from those in the REFORM trial. Although podiatrists reported having prescribed some, if not all, of the exercises provided during the trial, these exercises had not been prescribed as a ‘package’ or in combination with each other. In terms of the individual exercises, resistive bands and foot therapy balls were rarely provided but may have been prescribed in biomechanics clinics. Some podiatrists raised the issue of professional domains, remarking that, in current practice, prescribing exercises is the responsibility of physiotherapists rather than podiatrists:
. . . but we’ve never had therabands within podiatry stock.
I think some of it is expensive, is that the reason?
I think that would be, yeah the main reason I would think and it just seems to be for our trust, it seems to be the role of the physiotherapist. So we maybe refer patients to physiotherapy for that part of an exercise programme but we haven’t done it within the podiatry clinics.
To negate the need for specialist equipment in routine practice, podiatrists would suggest alternative ways of conducting exercises; for example, they might advise patients to use a dressing gown cord instead of a resistive band. In addition, over half of the podiatrists reported that they had prescribed exercises using alternatives to a therapy ball that required patients to roll their feet over a can, or to pick up pencils or golf or tennis balls.
Experiences of the exercises during the REFORM trial
Podiatrists mostly spoke positively of the trial exercises. Indeed, two podiatrists reported having changed their routine practice to prescribe exercises when appropriate. However, some practical issues were reported, including the difficulty with prescribing exercises for elderly and frail patients, especially given the number of exercises that were included and the length of time it took to explain them. Podiatrists took a pragmatic approach and modified the trial exercise package to adapt the regime for people with comorbidities, to reduce the number of exercises or to suggest that particular exercises were not undertaken:
Yes when we were finding exercises for the very elderly patients, a lot of them struggled to actually be able to go on tiptoe, stand on one foot because they were quite frail. Some patients also had other health problems which made exercise a lot more difficult such as osteoarthritis of the feet, so they would often phone up and say, I’ve tried very hard to do these exercises but they’re causing me a lot of pain.
Right and so in those cases what was the advice you gave them?
We asked them to modify their approach and to do as many of the exercises as they could do but not feel too bad if they had to reduce the frequency of the exercise or maybe even miss one exercise out, for instance, if they had osteoarthritis of the first MTP [metatarsophalangeal], big toe joint, we would say to them, don’t worry about getting on tiptoe but do and try and do the other ones.
To facilitate trial participants’ understanding of and adherence to the exercises, a booklet and a DVD were provided. Podiatrists and trial participants spoke positively of the booklet, describing it as well written and easy to follow, with clear instructions and a good combination of pictures and text. The booklet was viewed as a useful resource that helped podiatrists to remind and explain to patients how to do the exercises, something that was considered particularly important given the amount of information patients received at their first appointment. During the trial, podiatrists used the booklet as a guide when delivering the whole intervention and also worked through the booklet with patients, using the pictures to aid their description of the exercises during clinic. Although one podiatrist (podiatrist 3) felt that some participants viewed the information in the booklet as too simplistic, others saw its simplicity as a strength, stating that it could have been issued without additional instructions. The positive feedback provided by podiatrists is exemplified by the fact that a few sites asked if they could give out the booklet in their routine practice prior to the end of the trial, with one podiatrist (podiatrist 10) also presenting the booklet at a staff meeting:
I thought it was very good. It was very well written. It was very easy to follow and because it had the pictures, the people could, you know, go back to it and have a look and it did make sense. It was written well.
Podiatrist 7
In light of podiatrists’ initial concerns regarding the amount of information given to trial participants at the start of the study, both the DVD and the booklet were considered helpful reminders. However, only a small proportion of trial participants reported having watched the DVD. The podiatrists provided some insight into this during their interviews, as they reported that patients had difficulty playing the DVD or, in some cases, did not have a DVD player. In addition, one podiatrist (podiatrist 3) suggested that the DVD may be more suitable for younger people and felt that those in the age group taking part in the trial would prefer written information:
Some of the DVDs did not work and not every patient had access to a DVD player and the patients tended to report back that while they found the booklet very helpful because that generation tends to like to read things rather than play things, if you’re dealing with a younger generation the DVD would have been more useful.
Podiatrist 3
Podiatrists felt that a follow-up appointment was important to ensure that the exercises were being conducted correctly:
. . . showing the patient how to do them correctly and getting them to do them correctly, because occasionally when they came back to the clinic for their review appointment and you asked them to demonstrate the exercises again, some of them hadn’t been doing them correctly, so it just needed a bit of re-education really.
Podiatrist 1
Experiences of and adherence to the exercises prescribed during the REFORM trial
At 12 months, 28.9% of participants in the intervention group reported performing the exercises at least three times per week and 74.5% reported performing them at least once per week (Table 52).
| Time point (month) | |||
|---|---|---|---|
| 3 | 6 | 12 | |
| Number of questionnaires received | 457 | 427 | 408 |
| Of which received intervention | 393 | 372 | 357 |
| In the past month, typically how many times a week were foot and ankle exercises undertaken, n (%) | |||
| More than three times a week | 51 (13.4) | 43 (11.8) | 45 (12.9) |
| Three times a week | 90 (23.7) | 66 (18.1) | 56 (16.1) |
| Twice a week | 89 (23.4) | 86 (23.6) | 71 (20.3) |
| Once a week | 75 (19.7) | 79 (21.7) | 88 (25.2) |
| Not undertaken | 75 (19.7) | 90 (24.7) | 89 (25.5) |
In the interviews, participants reported varying levels of adherence to the exercises, with some reporting undertaking them every day, some reporting undertaking them three times a week and some reporting that they did not do them at all. The length of time that participants complied for also varied, with some reporting trying the exercises for a short time before stopping (e.g. 1 month), although others persevered because of their desire to prevent falls.
In the main, the trial participants spoke positively about the trial exercises, although, in a similar vein to the podiatrist interviews, the number and challenging nature of some exercises was discussed. Trial participants and podiatrists expressed varying opinions regarding the resistive band and therapy ball exercises, which are outlined in Table 53.
| Type of exercise | Trial participant and podiatrist opinions |
|---|---|
| Resistive band exercises | |
| Some podiatrists thought that the exercises were good but expressed concerns over whether or not the participant could site the band properly and undertake the exercises correctly | The place at where they put the theraband over the foot, so sometimes it might have been more along the arches of the foot rather than across the forefoot, so just where to place the theraband. Sometimes they were trying to move their whole leg with the therabandPodiatrist 13 |
| Some trial participants reported difficulties in siting the band and others developed strategies to site the band correctly | Mainly because I don’t think I have furniture that lends itself to it but it just kept sliding off and we tried and triedTrial participant 1I used to put like the big elastic band around the table leg and move my legTrial participant 8 |
| Therapy ball exercises | |
| The majority of podiatrists liked the exercise and found it easy to determine which size of ball to prescribe, although patients with foot deformities encountered difficulties in performing the exercises | It was quite useful idea, get the intrinsic muscles working a little bit, help with the stability a good ideaPodiatrist 1There were a few patients who couldn’t do it but just due to their foot deformity they’ve got like arthritis of the toes or they couldn’t actually bend, you knowPodiatrist 10 |
| Most of the trial participants liked these exercises. Not all could pick up the ball but those who did often reported a sense of achievement | I did find them very good and I felt very pleased with myself when I could grip the ball. I used to say [name] come and see what I can do now, you know, it’s an achievement because again you get to a certain age and you don’t do, you know, generally you don’t do exercises reallyTrial participant 9I find the ones with the ball I find it very good to do that and I can more or less, at first I couldn’t lift it, you know, but I am getting more flexibility in my toes now with thatTrial participant 3 |
Although some participants reported that they had not noticed any differences since undertaking the exercises, for many participants the exercises had led to perceived benefits, such as improvements in their walking, balance, confidence and body awareness:
I didn’t do them every day to start with but I did them at least three times a week and within several weeks I felt the benefit. I did feel the benefit from, you know. In my walking and in my balance and the ability to get to stand up.
Trial participant 2
Whether or not participants perceived the exercises to be beneficial may have influenced their adherence. For example, one participant commented that they had continued to undertake the exercises even when they were tired, as their perception of improvements had given them an incentive to continue.
A number of participants also described strategies that they had adopted to make the exercises easier to fit into their daily lives, for example goal-setting (e.g. wanting to stand up easily), splitting the exercises throughout the day, and doing the exercises ‘first thing in the morning’ or while watching television, which may have improved adherence:
I do them three times a week all at once, one after the other. Monday, Wednesday and Friday and I’ve done them this morning before 7 o’clock. Well I make sure because I do it at a time that suits me that doesn’t interfere with my life, you know, because I’m out of the house by quarter to nine every morning, so you know, that’s the time it’s done.
Trial participant 1
For participants who did not regularly undertake the exercises, a range of reasons for non-adherence were provided, including the length of time required to undertake them. Medical conditions such as heart problems or arthritis also made performing the exercises difficult or painful for a number of participants:
. . . because I’ve got a bad heart, it doesn’t take me long to get out of breath, so I found them hard work, very hard work.
Trial participant 7
Others had to adapt the exercise package to accommodate their own physical limitations:
One I couldn’t do. The one where you had to stand up with your back up against a wall. I couldn’t do that because I couldn’t balance on one leg because this knee is so bad. There is no way I could stand and put all my weight on one leg, so I tried that one once and thought wow.
Trial participant 7
For others, the exercises were not a priority either because they were not motivated to do them or because they had other priorities such as being a full-time carer for a relative.
The REFORM package of care
Of the 211 participants who received an orthosis as part of the intervention and who provided a response to both questions relating to adherence to the insole and to the exercises at 12 months, 68.7% (n = 145) reported having worn their orthotic and preformed the exercises in the previous 4 weeks, 5.2% (n = 11) reported no adherence to either aspect and the remaining 55 reported that they either only wore the orthotic (n = 35) or only undertook the exercises (n = 20).
Given the multifaceted nature of the REFORM intervention, coupled with the often complex health issues of the population group, the majority of participants were able to complete only some aspects of the care package and had to adapt what was available to suit their own circumstances and perceptions of impact. A similar pragmatic approach was taken by the podiatrists, who were able to see how they could incorporate the basis of the intervention into routine practice:
I mean the majority I think I would probably give a few exercises and an insole to.
Podiatrist 7
Although podiatrists found the intervention acceptable and were, in principle, willing to administer all of the elements, some did question how the intervention could be incorporated into routine care, given the way that services were currently configured:
I think just, I suppose it just reminded me more of what I should be doing, what our roles should involve because the way we work in [centre], we’re all quite fragmented I suppose. So I would not normally have a patient in a clinic, in a routine clinic for a 20-minute appointment slot and so, if you’ve got a caseload of patients and they’re coming in to see you every 4 months, if they’ve had a fall, you’re just not going to start taking them through a falls exercise programme and there isn’t any other specialised clinic that you book them into to have that time to do it. So we have falls, so you know if somebody has a fall, our service accesses the falls clinic and the GP would refer onto the falls clinic and that’s nothing to do with podiatry.
Podiatrist 13
Some highlighted that, for the exercise element in particular, there may be more appropriate contexts in which this could be delivered:
Yeah because we have got dedicated biomechanics clinics, so I think it would sort of fit in well within that area of our service.
Podiatrist 9
Given the probable time restraints in routine care, some podiatrists suggested delivering certain elements of the package of care in group sessions to save time and money; however, opinion was split on whether or not this would be the best mode of delivery from the patients’ point of view:
Something that we could use later as maybe a group session and getting patients in to talk them through as a group session and showing them the different exercises and the type of shoe that they should be wearing.
Podiatrist 7
I think on a one-to-one basis it is better. If you have them in a class, you’ll have people who are self-conscious or not really willing to try things just out of fear and I think one-on-one situations they feel comfortable, in a safe environment and it’s a better way to deliver it.
Podiatrist 1
Summary
Footwear
Podiatrists provide footwear advice in routine practice and are well versed in doing so. The trial footwear checklist was detailed, provided a more formal evidence-based tool with which to assess footwear and was acceptable to podiatrists. For the checklist to be used in routine practice, attention would have to be paid to how the information on the checklist was recorded in the current electronic patient record systems.
Although most podiatrists found measuring for shoes straightforward, this could be time-consuming and may be difficult within the constraints of normal clinic appointments. It was suggested that this aspect could be conducted by technicians, podiatry assistant or orthotists rather than podiatrists as part of a multidisciplinary team.
It is questionable whether appropriate footwear would be provided within current NHS budgets. Outside the trial it may be more difficult to achieve adherence to footwear advice, given the financial constraints of many of the service users. In addition, there is a subgroup of service users who will be unable to access shoe retailers, who will not be able to achieve a good fit owing to existing foot problems, or who will be resistant to wearing the footwear options available.
Orthotics
Podiatrists do not routinely prescribe orthotics for falls prevention. However, they were positive about the trial orthotic and found it easy and acceptable to implement without the need for more complex biometrics assessment. Appropriate footwear is required to achieve a good fit for the orthotic; this will be less achievable for a wide range of service users outside the context of the trial if footwear is not being provided. Service users will find a pragmatic solution to incorporating wearing an orthotic, if comfortable to do so, into their everyday lives.
Exercises
Podiatrists do not currently prescribe exercise packages such as those in the REFORM intervention. They did find it acceptable to do so, in particular with the aid of the trial booklet. However, explaining the exercises properly was time-consuming and would be difficult to fit into a routine podiatry appointment. Podiatrists also felt that a follow-up appointment would be necessary to check that the exercises were being conducted appropriately to avoid injury.
The equipment necessary for the REFORM exercises was not always routinely available in podiatry clinics, meaning that additional resources would be required or that alternatives to the formal equipment would have to be suggested. Although podiatrists were happy to implement the exercise component of the intervention, this was more commonly seen as being the domain of physiotherapy or biomechanics, and in routine practice a way of incorporating the intervention into the current configuration of podiatry/falls services would have to be developed. For example, it was suggested the exercises and footwear advice could be explained in a group setting, perhaps in the context of a multidisciplinary falls clinic.
Chapter 7 Discussion
Here we report the results of a large RCT assessing the clinical effectiveness and cost-effectiveness of a multifaceted podiatric intervention for the prevention of falls among podiatry patients within a NHS setting and one international site in Ireland. Previous reviews, including the most recent Cochrane review, have identified only one previous RCT of a similar intervention in an Australian setting. 23 A meta-analysis of eight RCTs using foot and ankle exercises noted improvements in surrogate measures of outcomes, such as balance. 19 In this discussion, we summarise our key findings, compare these with previous studies and discuss the strengths and limitations of our study.
Key findings
The REFORM trial is the largest study of a podiatric programme that includes a foot and ankle exercise programme to reduce the risk of falling. A total of 1010 participants were randomised. Our sample size allowed for a 10% loss to follow-up. The actual overall loss to follow-up observed at 12 months was 12.4% [in total, a 12-month questionnaire was returned for 885/1010 (87.6%) randomised participants]. Although this loss was higher than expected, we still had sufficient numbers relative to the target sample size of 890, as the trial over-recruited to 1010 participants. The primary clinical outcome for the trial was the incidence rate of falls reported on monthly falls calendars in the 12 months following randomisation. In practice, it is difficult to calculate the required sample size for a regression model, such as a Poisson or negative binomial regression model, to analyse count data. This requires an estimate of the measure of overdispersion and a justifiable treatment effect to detect. There were a limited number of data on which to base these parameters and so the decision was made to power the trial to detect a difference in the percentage of participants who reported at least one fall over the 12-month follow-up.
In total, 992 (98.2%) trial participants returned at least one falls calendar following randomisation, with similar proportions across the two groups [484 (98.2%) participants in the intervention group and 508 (98.3%) participants in the usual-care group]. We found a reduction in the rate of falls per person-year (IRR 0.88, 95% CI 0.73 to 1.05) and in the proportion of participants who had one or more falls over the 12 months from randomisation (OR 0.78, 95% CI 0.60 to 1.00). The difference was not statistically significant in our prespecified primary outcome of rate of falls (p = 0.16); however, the difference in the proportion of participants who had at least one fall (54.9% and 49.7% for usual care and intervention groups, respectively), a key secondary outcome, was of borderline statistical significance (p = 0.05). In our sample size calculation, we assumed that 50% of the usual-care group would fall during the 12-month follow-up, and we powered to detect a fall to 40% in the intervention group. In fact, 55% of the usual-care group experienced a fall. With the numbers recruited, we had 80% power to detect a fall to 46%, and approximately 36% power to detect the difference of 5% observed. Although a 5% decrease in the number of participants falling is of borderline statistical significance, it is difficult to say whether or not it is clinically meaningful. The estimated number of participants to whom we would need to offer the intervention to prevent one person from experiencing a fall is 20, which is relatively low.
A small, and similar, proportion of participants reported at baseline that they had been referred to a falls clinic or service in the previous 12 months in the two groups. At the end of the 12-month follow-up, we asked this question again: 30 out of 416 (7.2%) intervention participants and 22 out of 452 (4.9%) usual care participants said that they had been referred to a falls clinic or service in the previous 12 months. It is possible that participants in the intervention group interpreted this question as referring to their trial appointments at the podiatry clinic. If participants in the usual-care group received some form of intervention shortly before or during the trial follow-up, this could potentially have diluted the treatment effect. However, with only small numbers reporting this, we do not believe that this could have significantly influenced the results, and in any case we ran this as a pragmatic trial and so the results will reflect usual practice.
Time to first fall was reduced in the intervention group but this was not statistically significantly (hazard ratio 0.88, 95% CI 0.74 to 1.04; p = 0.14). No statistically significant differences between the two groups were observed at 6 or 12 months in the fear of falling question, the Short Falls Efficacy Scale-International, the FAI, the GDS or the CD-RISC2. The intervention group did, however, report higher levels of foot pain at 12 months on a 10-cm visual analogue scale from 0 (no pain) to 10 (worst pain possible). The mean pain in the intervention group was 3.1, compared with 2.6 in the usual-care group (adjusted mean difference 0.43, 95% CI 0.06, 0.80; p = 0.02); however, although statistically significant, a difference of 4.3 mm may not be clinically meaningful. It is unclear why participants in the intervention group reported higher pain scores. Evidence from the qualitative study suggests that, in some cases, increased foot pain could have been a result of insoles reducing the space in footwear. In other cases it may be that intervention participants were simply more aware and more critical of (problems with) their feet, or they were using their feet more while performing the exercises. Alternatively, this could be a chance finding.
Cost-effectiveness
The results of the economic evaluation conducted alongside the REFORM trial suggest that the multifaceted intervention could be a cost-effective option for falls prevention in terms of QALYs gained calculated using the EQ-5D. The ICER for the ITT approach in the imputed data set ranged between £19,494 and £20,593 per additional QALY. The probability of being cost-effective for the base-case analysis is > 60%. The results are robust to the sensitivity analyses testing the assumptions regarding resource use, perspective of analysis and level of imputation regarding missing data on HRQoL. With the one exception of when the missing data mechanism is tested, the complete-case analysis suggests that the multifaceted podiatry intervention is expected to be more costly and slightly less beneficial than usual care. However, the complete case in REFORM is not without limitations. In addition to the much reduced sample size of the original data (28.3%), missing data patterns showed that incomplete data followed a non-monotonic pattern, which suggests that the complete-case assessment would be inefficient, as it would discard observed data from individuals who have some missing outcomes. A logistic regression analysis showed that advancing age and lower EQ-5D at baseline are associated with missing QALY data. This suggests that the data are unlikely to be MCAR; consequently, the results from the multiple imputed data set are likely to be more accurate and more reliable than complete care results.
The main limitation of this economic evaluation, conducted alongside the REFORM trial, is that it does not account for any differences in costs and QALYs that may be expected over the longer term (> 12 months post randomisation). The HRQoL data showed that the reduction in the number of participants in perfect health in the intervention group is lower than that in the usual-care group (17.7%); the increase in the number of participants having problems is also lower in the intervention group. The effectiveness analysis also indicated a reduction, albeit a non-statistically significant one, in fall rate in the intervention group relative to usual care. Cost-effectiveness did not noticeably differ when we projected HRQoL beyond the trial duration (up to 5 years). However, we consider this exploratory projection likely to be conservative, and it would be important to explore the long-term impact of reducing the number of falls, as this might also lead to a reduction in the number of fractures, which in turn will make it more likely that the intervention yields long-term cost savings in the NHS.
Qualitative findings
The qualitative study explored issues of acceptability and implementation from the perspectives of both patients and podiatrists. It found that most podiatrists could implement some elements of the programme, such as the footwear advice and the provision of the orthotic, as part of their normal clinic practice, with some podiatrists continuing to offer the intervention outside the trial. Some concerns were raised about the ability of podiatrists to effectively deliver the exercise component within the time constraints of a routine clinical appointment. Although the podiatrists generally felt confident in doing so, time (and equipment) would have to be allocated for this purpose, alongside any necessary follow-up appointments. Given the way in which most falls prevention services are set up, some podiatrists felt that the intervention may be well suited to a multidisciplinary falls service, which would include podiatry alongside physiotherapy input for the exercise intervention, particularly in a group setting.
The trial participants were largely content with the intervention, and adherence was generally good. Some trial participants, especially those with comorbidities, found some of the exercises challenging; however, generally, both podiatrists and participants were able to adapt the exercises to suit individual circumstances. Some participants noticed a benefit of the exercise training after several weeks and felt more confident as a result. The trial participants found pragmatic ways to incorporate wearing an orthotic, when it was comfortable to do so. Some participants, however, were not able to adhere to the footwear advice/orthotic, as they were unable to achieve a good fit owing to existing foot problems or they were resistant to wearing the footwear options available.
Comparison with other studies
Our results to some extent support the earlier findings by Spink et al. 23 In this Australian trial, among 305 community-dwelling men and women (mean age 74 years) who were suffering from disabling foot pain and who had an elevated risk of falling, a reduction in the incidence rate of falls was observed (IRR 0.64, 95% CI 0.45 to 0.91). The Australian population was similar to ours in that they were all receiving routine podiatry care and were recruited from podiatry patient lists. However, participants had to be suffering from disabling foot pain, which was not the case for our population; patients may have had foot pathology but they did not necessarily have significant foot pain. Our population had a higher risk of falling; the usual-care group sustained an average of 1.5 falls per year, compared with 1.06 for the Australian patient group. Similarly, 55% of our usual care participants sustained one or more falls, compared with 49% in the Spink et al. 23 study.
The key elements of the interventions were similar, comprising foot and ankle exercises, an orthosis and an assessment for poor footwear. Both studies were carried out among patients who were receiving ‘standard’ podiatry. However, there were some differences. We did not use exactly the same orthosis as that used in the Australian study, and the foot and ankle exercises were modified partly in light of lessons learned from the Australian study. In our study, when possible, new footwear was provided to participants in the intervention group whose own current footwear was inappropriate. In the Spink et al. 23 trial, participants were provided with a subsidy for new footwear in the form of a voucher. Furthermore, the participants in our study did not need to have ‘disabling foot pain’, as was the case in the Australian study. Forest plots to compare the results of the two studies graphically are presented in Figures 17 and 18. An analysis of the REFORM data was repeated including only treatment groups in the models for comparability with the Spink et al. 23 trial. Individual patient data were provided by the authors of the Spink et al. trial, and so, whereas results for the proportion of fallers are presented as a risk ratio in the publication, here we were able to present these as an OR.
FIGURE 17.
Impact of multifaceted podiatry intervention on the incidence rate of falls over 12 months in older adults. Reproduced from Spink et al. 23 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License (CC BY-NC 2.0), which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license (http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode).
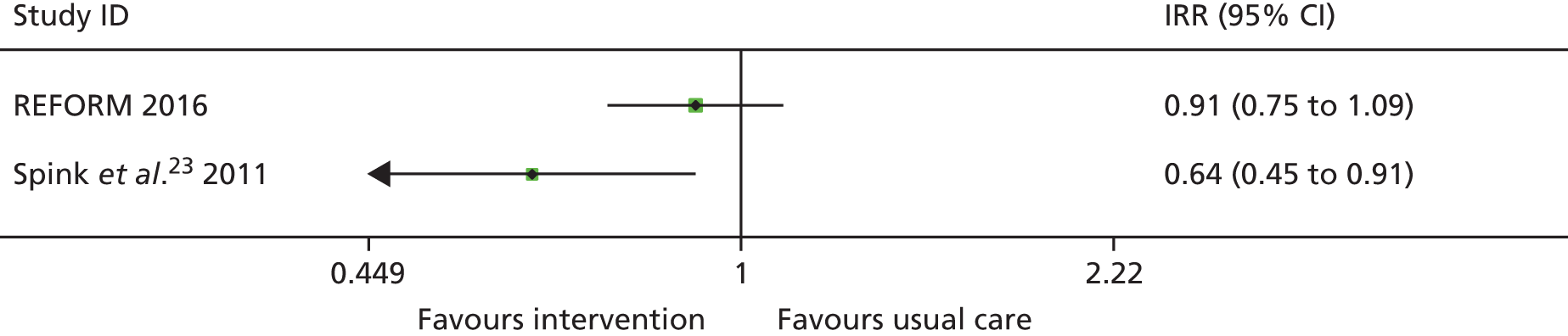
FIGURE 18.
Impact of multifaceted podiatry intervention on proportion of participants who fall at least once over 12 months. Reproduced from Spink et al. 23 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License (CC BY-NC 2.0), which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license (http://creativecommons.org/licenses/by-nc/2.0/).
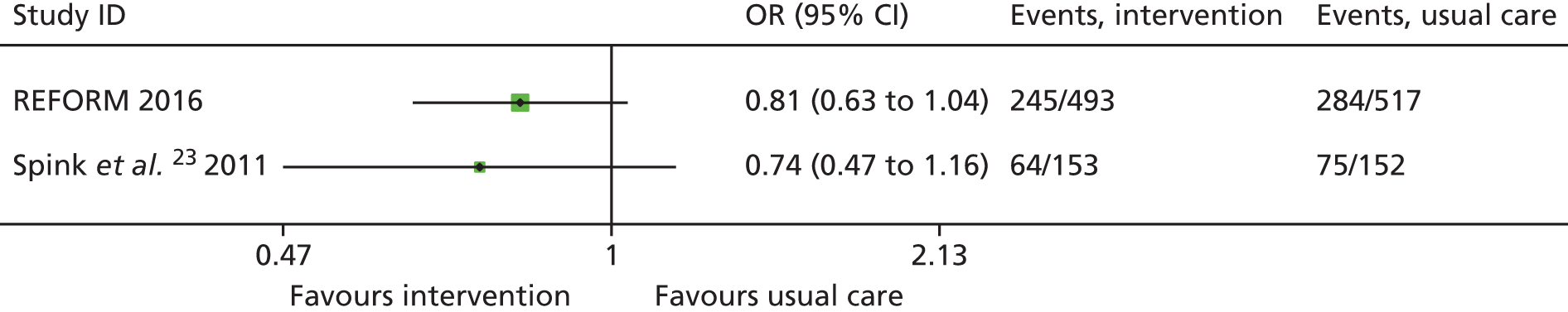
Strengths and limitations of the study
This was a large pragmatic trial, and we used a novel design, namely a cohort randomised trial, to evaluate this podiatric intervention. The design had several strengths: the use of a run-in period with outcome data collection could have reduced the incidence of post-randomisation attrition; those in the usual-care group were unaware of the exact time at which they were randomised, and, in theory, this should have limited resentful demoralisation. The design also allowed us to recruit participants who initially were ineligible because they had not fallen but later became eligible because they had fallen while part of the observational cohort. The initial engagement of participants with the intervention was high; 84% of intervention participants attended a trial appointment. Compliance with the exercise component was reasonable (at 12 months, 29% of intervention participants reported performing the exercises at least three times per week and 75% reported doing them at least once per week). However, in the qualitative interviews, some podiatrists stated that they felt that this could have been higher if they had had additional contact with the participants. Another limitation of the study is that the sample size was based on detecting a difference not in the primary outcome of incidence rate of falls but in the proportion of participants reporting at least one fall in 12 months. This was because of the difficulty in calculating a sample size for a count outcome, as discussed in Key findings. It is not possible, therefore, to confirm that the trial was sufficiently powered for the primary outcome. In addition, participants were recruited from podiatry clinics; therefore, the estimated impact of the intervention among people who do not regularly see a NHS podiatrist or who receive care from a private podiatrist may be different. Using a run-in period may also have biased the sample towards volunteers with a heightened interest and commitment to the intervention. Furthermore, the intervention is a ‘complex’ one, and our design does not allow us to estimate the different contributions of changes in footwear, the addition of an orthotic insole or the undertaking of foot and ankle exercises to the observed effect. It may well be that one or more of the interventions included in the ‘package of care’ is ineffective. There is also the possibility that some participants in the usual-care group had enrolled in another falls prevention programme as part of their NHS care, which could have diluted the treatment effect. This dilution effect is likely to be minimal, however, given that only a small proportion of participants in the usual-care group reported being referred to a falls clinic or service during the trial.
Generalisability of the results
The REFORM intervention was a pragmatic RCT across nine sites in the UK and one site in Ireland. All participants were recruited from podiatry clinic lists. This was to ensure that we could identify an additional effect of the intervention not confounded by routine podiatric care. Consequently, the trial cannot answer the question of whether or not the intervention is effective among patients who do not have routine podiatry care. However, approximately one in six people aged > 65 years receives NHS podiatry care and, therefore, our results are applicable to a significant proportion of the older population.
The trial results may also not be generalisable to patients who would not fulfil the eligibility criteria, that is, those with lower limb amputations, neuropathy, dementia or other neurological conditions; those unable to walk household distances without the help of a walking aid; those living in residential or nursing care homes; and those aged < 65 years. The views of the podiatrists interviewed in the qualitative part of the study were mixed on whether or not people with neuropathy or amputations could have benefited from the intervention, and the majority agreed that a more intensive follow-up would have been required in order to ensure patient safety.
Implications for health care
Our results suggest that there is a role for NHS podiatrists in reducing the risk of falling among their patients. Although cost-effectiveness was demonstrated based on QALYs gained calculated via the EQ-5D and not necessarily on reducing falls, falls could potentially have a negative effect of patients’ quality of life and any intervention to improve this is valid. However, in terms of the current intervention, some of the podiatrists felt that additional podiatry contact was required to maximise compliance with the individual intervention components. There is the potential for the cost of the intervention to be further reduced if a podiatry assistant rather than the podiatrist undertook the assessment of participants’ footwear and the measuring, ordering and fitting of new footwear.
Implications for research
The impact of falls risk among these patients was relatively modest. As falls are a major source of morbidity in an older population, research into combining different interventions to develop a more effective overall strategy might be worth pursuing. Further research could also examine the risk and cost of falls in other populations or settings (e.g. people with neuropathy or residential aged care facilities). Additionally, the intervention could be tested in populations deemed to be at high risk of falling.
There is evidence to suggest that exercise is an effective falls prevention strategy, and it may be the case that it is equally, or possibly more, effective when demonstrated to patients in group sessions, as opposed to one on one. This would have the additional benefit of being cheaper to deliver and, therefore, being more cost-effective. Further research could be undertaken to test the clinical effectiveness and cost-effectiveness of a group exercise programme, which could also investigate whether or not the intervention could be delivered equally effectively across the professional boundaries of podiatry and physiotherapy. Alternatively, further research into the intensity of the exercise could be undertaken to see how much is actually needed.
Acknowledgements
We would like to thank the participants for taking part in the trial, the podiatrists for delivering the intervention and completing the trial documentation, the research nurse and support teams who helped with the sending out of recruitment packs and confirmation of eligibility, the PIs at each site for co-ordinating the study and the reception teams at sites for organising study appointments. We would like to thank Hylton Menz, Martin Spink, Mohammad R Fotoohabadi, Elin Wee, Karl B Landorf, Keith D Hill and Stephen R Lord for agreeing to us using and adapting the package of podiatry care that was originally designed for their original research work, which was funded by The National Health and Medical Research Council of Australia and La Trobe University.
We would like to acknowledge the support of the NIHR Clinical Research network. We would also like to thank the members of the TSC/Data Monitoring Committee for overseeing the study.
We would specifically like to thank Deborah Armstrong, Cathy Bellman, Mark Brooksbank, Graeme Carter, Tony Carter, Lindsay Cherry, Daniel Crow, Nina Davies, Nicola Edmund, Dominic Evans, Lisa Farndon, Tristan Grant, Jordan Green, Carole Greig, Clair Holmes, Christine Hudson, Keith Littlewood, Claire MacGilchrist, Rezwana Malik, Wendy Monaghan, Carmel Moran, Geoffrey Phillips, Abby Platts, Allison Pringle, Sandra Robson, Derek Sant, Christine Scott, Rachel Sedgewick, Hannah Smith, Justine Stirling, Janette Thompson, Maria Trotman, Joanna Veal, Wesley Vernon, Mariann Waller, Amanda Walsh, Wanita Wayman, Hayley Wilkinson, Nadia Winborn and Annette Woods, who were the members of the research team at each site and who either delivered the intervention to participants in the study or provided administrative support.
We would like to thank Ryan Whitaker for the artwork used on the trial advertisements and Judith Watson for designing the REFORM logo.
We would like to thank Dr Sara Brookes, Professor Roger Francis, Dr Ian Mathieson, Dr Margaret May, Professor Chris Nester and Mrs Christine Thomas, who were the independent members of the TSC/Data Monitoring Committee, and Mrs June Dixon, Mr Mawson, Mrs Christine Thomas and Mr Kenneth Watson, the members of our patient and public involvement group.
Contributions of authors
Sarah Cockayne (Research Fellow, Health Sciences) was a coinvestigator and the REFORM study manager. She contributed to the development of the grant application, trial protocol and was the lead for study management. She undertook the qualitative interviews and analysis and was involved in writing the report. She also was responsible for co-ordinating the compilation, formatting, proofreading and final approval of the report.
Sara Rodgers (Research Fellow Health Sciences) was a trial co-ordinator, assisted with the day-to-day management of the study and contributed to writing the report.
Lorraine Green (Research Podiatrist) contributed to the development of the protocol, trained the podiatrists delivering the intervention and assisted with the day-to-day management of the study.
Caroline Fairhurst (Statistician, Health Sciences) wrote the statistical analysis plan, conducted the statistical data analysis and contributed to writing the report.
Joy Adamson (Senior Research Fellow, Health Sciences) was a coinvestigator and contributed to the development of the grant application and trial protocol. She supervised the conduct of qualitative research and was involved in the qualitative analysis and writing the report.
Arabella Scantlebury (Research Fellow, Health Sciences) undertook the qualitative interviews and analysis and wrote sections of the report.
Belen Corbacho (Research Fellow, Health Sciences) wrote the health economics analysis plan, conducted the health economics analysis and wrote the health economics section of the report.
Catherine E Hewitt (Professor in Statistics, Health Sciences) was a coinvestigator and contributed to the overall study design and implementation, supervised the statistical analyses and approved the final version of the report.
Kate Hicks (Research Fellow, Health Sciences) was a trial co-ordinator, assisted with the day-to-day management of the study and proofread the final report.
Robin Hull (General Manager Acute and Cancer Care and Podiatrist) was a coinvestigator and contributed to the overall study design.
Anne-Maree Keenan (Professor, Assistant Director, NIHR Leeds Musculoskeletal Biomedical Research Unit and Musculoskeletal Research Lead for the Leeds Teaching Hospitals Trust) was a coinvestigator and contributed to the overall study design and protocol, provided podiatry advice and supervised the research podiatrist.
Sarah E Lamb (Professor of Rehabilitation) was a coinvestigator and contributed to the overall study design and provided expertise in the field of falls.
Caroline McIntosh (Established Professor and Head of Podiatric Medicine) was a coinvestigator and contributed to the overall study design and provided podiatry expertise.
Hylton B Menz (Professor, National Health and Medical Research Council Senior Research Fellow) was a coinvestigator and contributed to the overall study design and protocol, provided podiatry advice and critically reviewed drafts of the report and approved the final version.
Anthony Redmond (Professor, Head of Section of Clinical Biomechanics and Physical Medicine) was a coinvestigator and contributed to the overall study design and provided podiatry expertise and supervised the research podiatrist.
Zoe Richardson (Trials Support Officer) assisted with the day-to-day management of the trial.
Wesley Vernon (Professor in Podiatric Medicine) was a coinvestigator and contributed to the overall study design and provided podiatry expertise.
Judith Watson (Research Fellow Health Sciences) was a coinvestigator and contributed to the overall study design and protocol, gave advice on study management and critically reviewed drafts of the report and approved the final version.
David J Torgerson (Professor, Director of the YTU, University of York) was the lead applicant and Chief Investigator for the REFORM study. He had overall responsibility for the design and implementation of the study and the writing of the report with final approval of the report submission.
All authors were invited to comment on the final manuscript.
Publications from REFORM podiatry intervention
Cockayne S, Adamson J, Hewitt C, Hull R, Keenan AM, Redmond A, et al. The reform study: a cohort multiple randomised controlled trial. Trials 2013;14(Suppl. 1):44.
Arundel C, Torgerson D, Jefferson L, Cockayne S. A nested randomised controlled trial of a leaflet, containing information on research, to increase the recruitment rate of reform (reducing falls with orthoses and a multifaceted podiatry) trial participants. Trials 2013;14(Suppl. 1):109.
Cockayne S, Adamson J, Corbacho, Martin B, et al. The REFORM study protocol: a cohort randomised controlled trial of a multifaceted podiatry intervention for the prevention of falls in older people. BMJ Open 2014;4:e006977.
Cockayne S, Adamson J, Bower P, Corbacho B, Fairhurst C, Farndon L, et al. The reform patient information sheet sub study – an embedded trial evaluating the enhancement of patient information sheets to improve recruitment. Trials 2015;16(Suppl. 2):87.
Cockayne S, Adamson J, Corbacho B, Fairhurst C, Farndon L, Hicks K, et al. The reform study: a case study of embedded trials. Trials 2015;16(Suppl. 2):174.
Tong JWK, Kong VP, Sze L, Gale S, Veto J, McArdle C, et al. The College of Podiatry Annual Conference 2015: meeting abstracts. J Foot Ankle Res 2016;9:1–14.
Cockayne S, Adamson J, Clarke A, Corbacho B, Fairhurst C, Green L, et al. Cohort randomised controlled trial of a multifaceted podiatry intervention for the prevention of falls in older people (the REFORM trial). PLOS ONE 2017;12:e0168712.
Data sharing statement
Requests to access REFORM data can be made to the corresponding author and will be considered on a case-by-case basis by the Trial Management Group. All data requests will be managed in accordance with YTU, University of York, processes and procedures.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Iglesias CP, Manca A, Torgerson DJ. The health-related quality of life and cost implications of falls in elderly women. Osteoporos Int 2009;20:869-78. http://dx.doi.org/10.1007/s00198-008-0753-5.
- Bischoff-Ferrari HA. The role of falls in fracture prediction. Curr Osteoporos Rep 2011;9:116-21. http://dx.doi.org/10.1007/s11914-011-0059-y.
- Lord SR, Sherrington C, Menz HB, Close JCT. Falls in Older People: Risk Factors and Strategies for Prevention. Cambridge: Cambridge University Press; 2007.
- O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol 1993;137:342-54. https://doi.org/10.1093/oxfordjournals.aje.a116681.
- Vetter NJ, Lewis PA, Ford D. Can health visitors prevent fractures in elderly people?. BMJ 1992;304:888-90. https://doi.org/10.1136/bmj.304.6831.888.
- The Care of Patients with Fragility Fracture. London: British Orthopaedic Association; 2007.
- National Service Framework for Older People. London: DH; 2001.
- Dunn JE, Link CL, Felson DT, Crincoli MG, Keysor JJ, McKinlay JB. Prevalence of foot and ankle conditions in a multiethnic community sample of older adults. Am J Epidemiol 2004;159:491-8. https://doi.org/10.1093/aje/kwh071.
- Menz HB, Morris ME, Lord SR. Foot and ankle risk factors for falls in older people: a prospective study. J Gerontol A Biol Sci Med Sci 2006;61:866-70. https://doi.org/10.1093/gerona/61.8.866.
- Mickle KJ, Munro BJ, Lord SR, Menz HB, Steele JR. Foot pain, plantar pressures, and falls in older people: a prospective study. J Am Geriatr Soc 2010;58:1936-40. http://dx.doi.org/10.1111/j.1532-5415.2010.03061.x.
- Hatton AL, Rome K, Dixon J, Martin DJ, McKeon PO. Footwear interventions: a review of their sensorimotor and mechanical effects on balance performance and gait in older adults. J Am Podiatr Med Assoc 2013;103:516-33. https://doi.org/10.7547/1030516.
- Menant JC, Steele JR, Menz HB, Munro BJ, Lord SR. Optimizing footwear for older people at risk of falls. J Rehabil Res Dev 2008;45:1167-81. https://doi.org/10.1682/JRRD.2007.10.0168.
- Koepsell TD, Wolf ME, Buchner DM, Kukull WA, LaCroix AZ, Tencer AF, et al. Footwear style and risk of falls in older adults. J Am Geriatr Soc 2004;52:1495-501. http://dx.doi.org/10.1111/j.1532-5415.2004.52412.x.
- Kelsey JL, Procter-Gray E, Nguyen US, Li W, Kiel DP, Hannan MT. Footwear and falls in the home among older individuals in the MOBILIZE Boston study. Footwear Sci 2010;2:123-9. http://dx.doi.org/10.1080/19424280.2010.491074.
- American Geriatrics Society, British Geriatrics Society and American Academy of Orthopaedic Surgeons Panel on Falls Prevention . Guideline for the prevention of falls in older persons. J Am Geriatr Soc 2001;49:664-72. https://doi.org/10.1046/j.1532-5415.2001.49115.x.
- Preventing Falls and Harm from Falls in Older People: Best Practice Guidelines for Australian Community Care. Sydney, NSW: Australian Commission on Safety and Quality in Healthcare; 2009.
- Balanowski KR, Flynn LM. Effect of painful keratoses debridement on foot pain, balance and function in older adults. Gait Posture 2005;22:302-7. https://doi.org/10.1016/j.gaitpost.2004.10.006.
- Hijmans JM, Geertzen JHB, Dijkstra PU, Postema K. A systematic review of the effects of shoes and other ankle or foot appliances on balance in older people and people with peripheral nervous system disorders. Gait Posture 2007;25:316-23. https://doi.org/10.1016/j.gaitpost.2006.03.010.
- Schwenk M, Jordan ED, Honarvararaghi B, Mohler J, Armstrong DG, Najafi B. Effectiveness of foot and ankle exercise programs on reducing the risk of falling in older adults: a systematic review and meta-analysis of randomized controlled trials. J Am Podiatr Med Assoc 2013;103:534-47. https://doi.org/10.7547/1030534.
- Kobayashi R, Hosoda M, Minematsu A, Sasaki H, Maejima H, Tanaka S, et al. Effects of toe grasp training for the aged on spontaneous postural sway. J Phys Ther Sci 1999;11:31-4. https://doi.org/10.1589/jpts.11.31.
- Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.cd007146.pub2.
- Cameron ID, Murray GR, Gillespie LD, Robertson MC, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev 2010;1. https://doi.org/10.1002/14651858.cd005465.pub2.
- Spink MJ, Menz HB, Fotoohabadi MR, Wee E, Landorf KB, Hill KD, et al. Effectiveness of a multifaceted podiatry intervention to prevent falls in community dwelling older people with disabling foot pain: randomised controlled trial. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d3411.
- Relton C, Torgerson D, O’Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the ‘cohort multiple randomised controlled trial’ design. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1066.
- Pighills AC, Torgerson DJ, Sheldon TA, Drummond AE, Bland JM. Environmental assessment and modification to prevent falls in older people. J Am Geriatr Soc 2011;59:26-33. http://dx.doi.org/10.1111/j.1532-5415.2010.03221.x.
- Vernon W. The healthy footwear guide. Podiatry Now 2009;5.
- Age UK . Staying Steady. Improving Your Strength and Balance n.d. http://psnc.org.uk/doncaster-lpc/wp-content/uploads/sites/51/2013/07/Appendix-4-staying-sready-leaflet.pdf (accessed 10 August 2011).
- Irish Osteoporosis Society . Fall Prevention n.d. www.irishosteoporosis.ie/downloads/Fall_Prevention.pdf (accessed 11 February 2013).
- Mitchell N, Hewitt CE, Lenaghan E, Platt E, Shepstone L, Torgerson DJ. SCOOP study team . Prior notification of trial participants by newsletter increased response rates: a randomized controlled trial. J Clin Epidemiol 2012;65:1348-52. http://dx.doi.org/10.1016/j.jclinepi.2012.05.008.
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Kempen GI, Yardley L, van Haastregt JC, Zijlstra GA, Beyer N, Hauer K, et al. The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing 2008;37:45-50. https://doi.org/10.1093/ageing/afm157.
- Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke 1993;24:1173-7. https://doi.org/10.1161/01.STR.24.8.1173.
- Brink TL, Yesavage JA, Lum O, Heersema PH, Adey M, Rose TL. Screening tests for geriatric depression. Clin Gerontol 1982;1:37-43. https://doi.org/10.1300/J018v01n01_06.
- Vaishnavi S, Connor K, Davidson JR. An abbreviated version of the Connor-Davidson Resilience Scale (CD-RISC), the CD-RISC2: psychometric properties and applications in psychopharmacological trials. Psychiatry Res 2007;152:293-7. https://doi.org/10.1016/j.psychres.2007.01.006.
- Kind P. The EuroQoL Instrument: An Index of Health-Related Quality of Life. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- Sheikh JI, JA Y. Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986.
- Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome – when, why, and how?. BMC Med Res Methodol 2014;14. http://dx.doi.org/10.1186/1471-2288-14-20.
- Glidden DV, Vittinghoff E. Modelling clustered survival data from multicentre clinical trials. Stat Med 2004;23:369-88. http://dx.doi.org/10.1002/sim.1599.
- Akaike H. A new look at the statistical model identification. IEEE T Automat Contr 1974;19:716-23.
- Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179-83. http://dx.doi.org/10.1002/nur.4770180211.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Lincoln YS, Guba EG. Naturalistic Inquiry. Newbury Park, CA: Sage; 1985.
- Birks YF, Porthouse J, Addie C, Loughney K, Saxon L, Baverstock M, et al. Randomized controlled trial of hip protectors among women living in the community. Osteoporos Int 2004;15:701-6. http://dx.doi.org/10.1007/s00198-004-1599-0.
- Cockayne S, Adamson J, Clarke A, Corbacho B, Fairhurst C, Green L, et al. Cohort randomised controlled trial of a multifaceted podiatry intervention for the prevention of falls in older people (the REFORM trial). PLOS ONE 2017;12. http://dx.doi.org/10.1371/journal.pone.0168712.
- Gill TM, Pahor M, Guralnik JM, McDermott MM, King AC, Buford TW, et al. Effect of structured physical activity on prevention of serious fall injuries in adults aged 70–89: randomized clinical trial (LIFE Study). BMJ 2016;352. http://dx.doi.org/10.1136/bmj.i245.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Torgerson DJ, Torgerson C. Designing Randomised Trials in Health, Education and the Social Sciences: An Introduction. Basingstoke: Palgrave Macmillan; 2008.
- Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Appl Health Econ Health Policy 2005;4:65-7. https://doi.org/10.2165/00148365-200504020-00001.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Brooks R. EuroQoL: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: a structured review of generic self-assessed health instruments. Qual Life Res 2005;14:1651-68. https://doi.org/10.1007/s11136-005-1743-0.
- Billingham LJ, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- NHS Reference Costs 2014–15. London: DH; 2015.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473-89. https://doi.org/10.1080/01621459.1996.10476908.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004.
- Royston P. Multiple imputation of missing values. Stata Tech J 2005;5:527-36.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521-33. https://doi.org/10.1111/j.1524-4733.2005.00045.x.
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: CRC Press; 1994.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Gomes M, Grieve R, Nixon R, Ng ES, Carpenter J, Thompson SG. Methods for covariate adjustment in cost-effectiveness analysis that use cluster randomised trials. Health Econ 2012;21:1101-18. http://dx.doi.org/10.1002/hec.2812.
- Manca A, Lambert PC, Sculpher M, Rice N. Cost-effectiveness analysis using data from multinational trials: the use of bivariate hierarchical modeling. Med Decis Making 2007;27:471-90. https://doi.org/10.1177/0272989X07302132.
- Claxton K. Methods for the Estimation of the NICE Cost Effectiveness Threshold. York: University of York, Centre for Health Economics; 2013.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Claxton K. Exploring uncertainty in cost-effectiveness analysis. PharmacoEconomics 2008;26:781-98. https://doi.org/10.2165/00019053-200826090-00008.
- Briggs AH. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ 1999;8:257-61. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3%3C257::AID-HEC427%3E3.0.CO;2-E.
Appendix 1 Regulatory approvals
| Research site | Date of research and development approval |
|---|---|
| Sheffield Teaching Hospitals NHS Foundation Trust | 19 September 2012 |
| Harrogate and District NHS Foundation Trust | 2 July 2012 |
| Leeds Community Healthcare NHS Trust | 7 March 2013 |
| National University of Ireland, Galway | 26 April 2013 |
| Humber NHS Foundation Trust | 13 May 2013 |
| Solent NHS Trust | 29 October 2013 |
| North Lincolnshire and Goole Hospitals NHS Foundation Trust | 11 November 2013 |
| Kent Community Health NHS Foundation Trust | 11 February 2014 |
| South Tyneside NHS Foundation Trust | 1 April 2014 |
| North Tees and Hartlepool Hospitals NHS Foundation Trust | 9 May 2014 |
Approval was gained at two additional sites; neither was able to start recruitment.
Appendix 2 REFORM invitation letter
Appendix 3 REFORM consent form
Appendix 4 REFORM patient information sheet
Appendix 5 REFORM background information form
Appendix 6 REFORM decline form
Appendix 7 REFORM baseline questionnaire
Appendix 8 REFORM sample falls calendar
Appendix 9 Falls telephone data collection sheet
Appendix 10 REFORM 6-month follow-up questionnaire
Appendix 11 REFORM 12-month follow-up questionnaire
Appendix 12 REFORM participant 6-month exercise and orthosis diary
Appendix 13 REFORM change of circumstance form
Appendix 14 REFORM adverse event form
Appendix 15 REFORM participant information sheet (qualitative)
Appendix 16 REFORM participant interview consent form
Appendix 17 REFORM qualitative podiatrist information sheet
Appendix 18 REFORM podiatrist interview consent form
Appendix 19 REFORM participant qualitative topic guide
Appendix 20 REFORM podiatrist qualitative topic guide
Appendix 21 REFORM footwear assessment checklist
| Outdoor | Yes | No |
|---|---|---|
| Appropriate heel height? | ||
| Appropriate heed width? | ||
| Appropriate fixation/fastening? | ||
| Appropriate heel counter? | ||
| Suitable sole? | ||
| Correct size? | ||
| Indoor | ||
| Appropriate heel height? | ||
| Appropriate heed width? | ||
| Appropriate fixation/fastening? | ||
| Appropriate heel counter? | ||
| Suitable sole? | ||
| Correct size? |
List of abbreviations
- A&E
- accident and emergency
- CACE
- complier average causal effect
- CD-RISC2
- 2-item abbreviated version of the Connor-Davidson Resilience Scale
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DVD
- digital versatile disc
- EQ-5D
- EuroQoL-5 Dimensions
- EQ-5D-3L
- EuroQoL-5 Dimensions, 3 Level
- FAI
- Frenchay Activities Index
- FES-I
- Falls Efficacy Scale – International
- GDS
- Geriatric Depression Scale
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IRR
- incidence rate ratio
- ITT
- intention to treat
- MAR
- missing at random
- MCAR
- missing completely at random
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OLS
- ordinary least squares
- OR
- odds ratio
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- research ethics committee
- REFORM
- REducing Falls with ORthoses and a Multifaceted podiatry intervention
- SAE
- serious adverse event
- SD
- standard deviation
- SUR
- seemingly unrelated regression
- TSC
- Trial Steering Committee
- WTP
- willingness to pay
- YTU
- York Trials Unit