Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/144/04. The contractual start date was in May 2014. The draft report began editorial review in July 2016 and was accepted for publication in November 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lisa Hampson reports grants from the Medical Research Council (MRC) and the pharmaceutical industry outside the submitted work. Francesco Muntoni reports grants from the European Union 7th Framework Programme, MRC, Ionis Pharmaceuticals/Biogen, Inc., PTC Therapeutics, Summit Pharmaceutical International, Roche, L’Association Française contre les Myopathies and Muscular Dystrophy UK and personal fees from Pfizer, Biogen, Inc. and Summit outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Hind et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Duchenne muscular dystrophy
Epidemiology
Duchenne muscular dystrophy (DMD) is a genetic disease mainly affecting boys. It affects between 1 in 3600 and 1 in 6000 live male births;1–3 the prevalence is 5 in 100,000. 4
Aetiology
Duchenne muscular dystrophy is caused by deletions, duplication or point mutations in a gene on the X chromosome. 5 The gene codes for a protein, dystrophin, that links the cell’s cytoskeleton to a transmembrane complex. Absence of this protein causes disease in muscle cells and brain neurons. The precise mechanism is still uncertain.
Pathology
In muscle, pathological studies show a ‘dystrophic’ picture in which there are dying muscle cells and regenerating muscle cells, together with inflammatory cells and excess amounts of fat and connective tissue. 6,7 Clinically, this manifests as weakness, first of the larger skeletal muscles around the shoulders and hips, and later of all skeletal muscles in the limbs and trunk. With increasing age, this causes a progressive loss of functional abilities that affect mobility (going up stairs, walking, standing, sitting and transferring between objects such as a chair or bed), activities of daily living (dressing, bathing and eating) and eventually breathing. As the disease progresses, muscles and tendons become shorter. These ‘contractures’ then prevent the joints they operate from moving through their full range, because the muscle can no longer stretch as completely as it should. The calf and long finger muscles are often involved by the age of 5 years, causing an inability to bend the foot fully up towards the shin (dorsiflexion) or straighten the fingers back. If untreated, contractures become more severe with time. Later, weakness of the trunk muscles causes scoliosis or curvature of the spine. The heart muscle is increasingly affected with age, eventually leading to impaired function and cardiac failure. The smooth muscle of the bowel deteriorates, affecting bowel function. The lack of dystrophin subtypes in the brain increases the risk of non-progressive cognitive dysfunction, which is associated with communication difficulties. 8
Prognosis
Untreated, half of boys will lose independent ambulation by the age of 9 years, and all will do so by the age of 12 years. Scoliosis and impaired heart function start in early adolescence, whereas breathing difficulty develops later in adolescence. In the 1960s, survival beyond mid-adolescence was unusual. With better therapies, survival increased to late adolescence in the 1970s to the 1990s. In the past two decades, the introduction of more effective respiratory support and heart treatment has increased survival into the early 30s, or longer. The more widespread use of corticosteroids from a young age has slowed disease progression; in those taking daily steroids, the average age at loss of ambulation has increased to 14 years. 9
Significance in terms of ill health
Health-related quality of life for boys with DMD and their carers is lower than for the general population. 10,11 Although physical and psychosocial domains of health-related quality of life are comparatively low in boys with DMD, psychosocial quality of life is sometimes higher in adolescents than in school-age children, indicating the development of coping strategies. 12–14 The psychosocial well-being of parents is greatly impacted, particularly around the time of a boy’s transition to wheelchair use. 15,16 Parents, especially mothers, report high levels of anxiety, depression and guilt. 17,18 Early diagnosis and the resilience of the family unit, expressed as their commitment and control, were associated with improved psychological adjustment by the parent, resilience on the part of the boy and response from siblings. 18–22 Parents, and mothers in particular, report care activities, such as including help for bathing and toileting, as time-intensive and contributing to social isolation. 23
Health-care costs are associated with access to specialist paediatricians/physicians in neurology, respiratory, cardiac and endocrine fields, orthopaedic surgeons, psychologists, physiotherapists and occupational therapists. Boys will sometimes have care co-ordinators or advisors, dietitians or nutritionists, or speech/language/swallowing therapists. Young men older than most of those in our study are likely to make increased use of emergency and respite care. Outside the health services, older boys may access home help, personal assistants and transportation services. From around 8 years of age, the median age of boys entering our study, families will typically make investments in and reconstructions of the home, for instance making adaptations for wheelchair accessibility. 24 Adaptations may also be required to educational facilities. A systematic review of the cost of illness4 noted only three studies that reported cost-impact data, all of which were > 20 years old. 25–27 However, a questionnaire study24 involving people with DMD in Germany, Italy, the UK and the USA provided per-patient annual costs of DMD in 2012 in international dollars. The questionnaire elicited information about hospital admissions, visits to health-care professionals, tests, assessments, medications, non-medical community services, aids, devices, alterations to the home and informal care. Mean per-patient annual direct cost was $23,920–54,270, which is between 7 and 16 times higher than the mean per-capita health expenditure. The total societal costs were $80,120–120,910 per patient per annum, increasing sharply with disease progression. Mean household costs were estimated at $58,440–71,900. 24
Current service provision
Pharmacological management and multidisciplinary care excluding physiotherapy
At diagnosis, a boy and his family will typically be seen by a paediatrician at their local hospital, often a district general hospital. They will then be referred to a specialist muscle clinic, usually involving a paediatric neurologist, in their regional teaching hospital. 28 Regular review and ongoing medical care is provided by these services. The boy and his family will also be seen by the regional genetics service. As part of this service provision they will also be referred to the local paediatric physiotherapy team for assessment and management (see Physiotherapy). If needed, they may be referred for occupational therapy advice as well. Educational services will also be involved. Some children and their families may also benefit from psychological support.
From the age of 3–4 years, most boys will start treatment with corticosteroids, which requires 6-monthly monitoring visits. If complications develop, extra visits and referrals may be needed. One frequent early complication is excess weight gain, which would lead to referral to dietetic services. Approximately one-third of the current population of ambulant boys is eligible for trials of newer treatments designed to increase the amount of the missing dystrophin protein in muscle cells, leading to a milder disease course. If successful, these treatments are likely to become generally available, and additional products are likely to be designed to expand the number of boys who could benefit.
Physiotherapy
Although physiotherapy has been a mainstay of treatment since the 1960s, there is relatively little evidence for its efficacy. There have been no generally accepted guidelines on what type or dose of physiotherapy intervention should be provided. 29,30 Many recommendations are based on animal studies in which contraction-induced muscle injury was observed in dystrophinopathy. 31 A Muscular Dystrophy Campaign workshop29 agreed that the main aims of physiotherapy in neuromuscular disease should be to:
-
maintain or improve muscle strength by exercise
-
maximise functional ability through the use of exercise and the use of orthoses
-
minimise the development of contractures by stretching and splinting.
The prevention of joint contractures is a multidisciplinary effort, involving specialist hubs and community spokes. 32 Regional consultant neurologists and specialist neuromuscular physiotherapists review disease progress and treatment regimens at clinic visits, typically twice a year, and offer management suggestions to community physiotherapists. 28 While a boy is still able to walk, a community paediatric physiotherapist will monitor him for hip and ankle contractures until he becomes wheelchair dependent. 33 The community physiotherapist is responsible for tailoring a programme of stretches and exercises to the boy’s individual needs and tolerance levels,28 and for training parents, carers, school staff or, occasionally, other health and social care staff to deliver the stretches. 33
Management should consist of a variety of treatment options aimed at maintaining the length and extensibility of affected muscle groups. 34 Although evidence to support interventions aimed at improving the range of movement is lacking, there are generally recognised principles that should be carried out to delay or, where possible, prevent the development of contractures. 35 These include the prescription of a regular targeted stretching regime and the use of specific orthotics (e.g. resting or night splints are generally recommended). Stretches are typically prescribed to be performed at home, in school or occasionally in community clinics, on a minimum of 4–6 days per week. 28 They are intended to maintain dorsiflexion and hip flexion range, among other targets, with a view to postponing the onset of contractures and prolonging the length of time the child can walk independently. 33 There are no clear guidelines to specific exercise prescription, but regular submaximal exercise is recommended to maintain existing muscle strength and avoid secondary disuse atrophy,36,37 along with general advice on regular activity such as walking, cycling and swimming. Although there is still the need for further research, there is general agreement that exercise that contains a substantial eccentric component (such as trampolining, stair descending) should be avoided because of the risk of exacerbating muscle damage. 38
Physiotherapy plays its part in the holistic, multidisciplinary management of children with DMD, providing specialist assessment, physiotherapy prescription and ongoing monitoring and evaluation of a complex and progressive condition. 28 Liaison with other specialist services, such as orthotics, wheelchair services, social and housing services, and schools to ensure the provision of appropriate equipment and support in a timely manner to maximise function and independence wherever possible is key. Hyde et al. 39 recommend the use of night splints. Resting or night splints, generally ankle–foot orthoses, are provided for use in combination with a regular stretching regime to optimise the length of the tendo-Achilles complex and maintain ankle dorsiflexion at night. 28,33,39 Children who are losing the ability to walk may be provided with knee–ankle–foot orthoses and may have surgical intervention to release tendons with a view to maintaining joint motion and independent ambulation. 28,33 Postponing wheelchair use may also defer the more or less inevitable associated onset of spinal scoliosis. 33
Aquatic therapy
Introduction
Exercise that is safe and controlled but still sufficiently intense to maintain physical function is a challenge for children with altered muscle tone, balance or motor control problems and severe contractures. 40 Warm water allows children with DMD to perform targeted stretches, exercises and function-based and play activities that are progressively lost to them on dry land. 33 An aquatic therapy (AT) pool may be the only setting in which these children can learn new postures or skills and maintain fitness without damaging their joints.
Although definitions sometimes overlap, common usage distinguishes hydrotherapy and ‘aquatic therapy’ and, especially, ‘aquatic exercise’, from ‘balneotherapy’, which denotes seated immersion or spa therapy without exercise. 41–43 The Aquatic Therapy Association of Chartered Physiotherapists (ATACP) defined aquatic physiotherapy as:
A physiotherapy programme utilising the properties of water, designed by a suitably qualified Physiotherapist. The programme should be specific for an individual to maximise function which can be physical, physiological, or psychosocial. Treatments should be carried out by appropriately trained personnel, ideally in a purpose built, and suitably heated pool.
ATACP43
They permit the use of the term ‘aquatic therapy’ for water-based programmes, designed by a suitably qualified physiotherapist, but carried out by non-specialist physiotherapists, or by carers and teaching assistants without specialist knowledge of anatomy and physiology.
Theoretical basis
In addition to the theories underpinning land-based therapy (LBT) (see Physiotherapy), the theoretical bases for AT are our understanding of the physical properties of water and a learning theory that accounts for developmental aquatic readiness, a model of systematic desensitisation44 and the biomechanical principles of the floating human body,45 as encapsulated in the Halliwick Concept. 46
The physical properties of water that are relevant to physiotherapy are density and specific gravity, hydrostatic pressure, buoyancy, viscosity and thermodynamics. 47 For DMD, a musculoskeletal condition, immersion brings the following benefits. First, warm water may increase cardiac output away from the splanchnic beds to the skin and musculature. 48–50 Blood flow to the muscles may be enhanced at rest51 and during exercise. 52 Second, body weight is offloaded with immersion, with the desired amount of loading variable by depth. 53 In musculoskeletal conditions, then, it is hypothesised that the hydrostatic effects and the warmth of the water, compared with cooler water in community swimming pools, make muscles more supple. The buoyancy and antigravity effects relieve pressure on joints, leading to a reduction in pain and an increase in joint mobility compared with strengthening and stretching exercises performed on dry land. 47 The metacentric or rotational effects, caused by altering the amount of the body that is immersed or the shape of the body in water so that the body is displaced requiring postural adjustments, are used to develop balance, core/proximal stability (stomach, shoulder and hip muscles) and to simulate function (transitions between positions – sit to stand, rolling over, going up steps, lying to sitting).
Systems such as Halliwick, which teach people with disabilities to return to a safe breathing position in water,46 thereby producing confidence and safety, are essential for an AT/physiotherapy programme. 54 They combine two elements: first, the ‘Ten Point Program’, which covers aspects of mental adjustment (including water confidence and breath control), balance control and movement; and, second, a protocol for ‘Water Specific Therapy’, involving assessment and objective setting, based on which the therapist chooses appropriate exercise patterns and treatment techniques. 46
Intervention methods and materials
Aquatic therapy pools typically operate at temperatures of 32–36 °C, higher than the 28 °C of conventional swimming pools, with a greater level of disinfectant and more frequent microbial sampling. 55 Pool size varies, with a recommended minimum space of 2.5 m × 2.25 m per patient and a typical depth of 1–1.2 m, often on a gradient. The provision of changing rooms with adequate space and wide entrances is optimal, along with hoists, both in the changing rooms and to get in and out of the pool. An ATACP foundation programme for chartered physiotherapists is necessary for safe and effective treatment. 56 Subjective, Objective, Assessment and Plan (SOAP) record keeping is recommended. 33
Evidence for effectiveness
In an overview,42 three systematic reviews evaluating aquatic exercise against controls in adults with musculoskeletal conditions showed small post-intervention improvements in function [n = 648, standardised mean difference 0.26, 95% confidence interval (CI) 0.11 to 0.42], quality of life (n = 599, standardised mean difference 0.32, 95% CI 0.03 to 0.61) and mental health (n = 642, standardised mean difference 0.16, 95% CI 0.01 to 0.32). 57–59 They also found a 3% absolute reduction and a 6.6% relative reduction in pain, measured on a visual analogue scale (n = 638, standardised mean difference 0.19, 95% CI 0.04 to 0.35). The meta-analyses identified no differences for walking ability or stiffness.
Although annotated bibliographies on the use of AT in disabled children exist,60,61 we are aware of only one relevant systematic review of aquatic interventions for children with neuromotor or neuromuscular impairments. 62 It included one randomised controlled trial (RCT) and 10 observational studies. Only three studies, all with low levels of evidence, investigated neuromuscular disorders. Seven articles indicated improvements in physical function and activity level, and two out of four articles investigated levels of participation-indicated improvements. The review concluded that there was a lack of quality evidence on the effects of AT in this population.
Current aquatic therapy provision in the UK
Although many health professionals believe that AT can improve mobility, strength, flexibility and cardiopulmonary fitness,63 and although AT is a routine part of care in other countries, access is uneven and restricted in the UK. 64 At the end of 2015, the charity Muscular Dystrophy UK (MDUK) identified 179 AT pools in the UK that people with muscle-wasting conditions could potentially use, but highlight that:
many hydrotherapy pools are based in schools and are only open during school hours and terms
privately-owned hydrotherapy pools can be expensive to access – often more than £75 for a half-hour session
people have to travel long distances to get to a pool, and this is not sustainable
hydrotherapy pools do not always have hoists, or accessible changing facilities.
Reproduced with permission from MDUK, Hydrotherapy in the UK: The Urgent Need for Increased Access64
Muscular Dystrophy UK asserts that NHS-funded AT ‘is often restricted to patients whose improvements can be demonstrably measured’;64 as such, functional improvements are difficult to demonstrate in degenerative conditions and funding is often absent or limited. In MDUK’s report, a young person with DMD is quoted as saying:
We have an ongoing need for hydrotherapy, which is not fulfilled by just being given a block of sessions for six weeks. This does not allow us to continue to maintain our condition because once our block of six sessions is over we struggle to be able to access hydrotherapy treatment anywhere else.
Reproduced with permission from MDUK, Hydrotherapy in the UK: The Urgent Need for Increased Access64
The aspiration to access AT throughout the year, rather than in 4- to 6-week blocks, is confirmed by our patient and public involvement (PPI) author, James Parkin, who notes ‘If you have AT regularly, it improves self-esteem; self-esteem improves how you deal with self-management’. A long hiatus between sessions can decrease water confidence and result in a loss of skills. Our qualitative research (see Chapter 5, Environmental factors and Operational work) confirms that, where publicly funded AT is available, it often comes in blocks of 4 or 6 weeks, ≥ 6 months apart, and is not necessarily routine but may be contingent on a successful application. MDUK end their report by arguing that access to AT helps people with muscle-wasting conditions ‘to manage their condition and improve their quality of life by reducing pain and increasing mobility’. 64 They make an appeal to equity, proposing that access should not depend on where a person lives or their disposable income. 64
Rationale and objectives
Rationale
The National Institute for Health Research (NIHR) published a commissioning brief (commissioning brief HTA 12/144) requesting a ‘feasibility study’ to evaluate the addition of ‘manualised hydrotherapy’ to ‘optimised land-based exercise’ for children and young people with DMD who still have some mobility. There are several ways in which we might have interpreted and responded to this brief so, at this point, it is worth taking the reader through the choices we made and why we made them. First, confusion about the definition of feasibility studies, which has since been widely acknowledged,65,66 meant that a decision had to made on the primary objective of this study. The request for a control group, and the focus on the ability to recruit and randomise, led us to interpret the brief as requiring an external pilot RCT, sometimes defined as ‘a version of the main study run in miniature to determine whether the components of the main study can all work together’. 66 In other words, we interpreted the brief primarily as a study to understand the feasibility of a research protocol for a full-scale RCT, rather than of the feasibility of delivering manualised AT per se. As the reader will see, an understanding of both issues is likely to be important for future clinical decision-making and the commissioning of further research.
Primary objective
A future full-scale trial would test the hypothesis that AT in addition to LBT is more effective than LBT alone for the maintenance of functional, participation or quality-of-life outcomes. The primary objective of this external pilot RCT was to determine the feasibility of a full-scale trial, defined in terms of participant recruitment.
Secondary objectives
Secondary objectives were the identification of:
-
the best primary outcome for a full-scale trial
-
the consent rate among eligible boys with DMD who were approached about the study
-
why boys with DMD refuse consent
-
the proportion of boys who provide valid outcome data 6 months after entering the trial
-
reasons for attrition from the trial
-
the views of participants and their families on the acceptability of the research procedures and the AT intervention
-
the robustness of the intended data collection tools
-
the willingness of participating centres to provide an AT service and to recruit participants
-
the ability of participating physiotherapists to deliver the AT intervention faithfully in accordance with a manualised protocol
-
therapist views on the feasibility of the AT intervention and the acceptability of the research protocol.
Chapter 2 Methods
The final protocol for this study can be found on the NIHR website. 67
Developing the intervention and associated theory
Overview
None of the team’s specialist physiotherapists knew of intervention manuals tailored to the needs of boys with DMD, as per the NIHR brief. As they would be interventions ‘with several interacting components’, we followed the steps recommended by the Medical Research Council Framework in developing them in parallel with the grant application process. 68 The first step, identification of the evidence base, has been discussed: there is no standard approach to LBT or AT for boys with DMD based on evidence for improvement in function, participation and emotional well-being. Either may help children with DMD maintain physical function but certain types of exercise are thought to be dangerous (see Chapter 1, Physiotherapy and Evidence for effectiveness). The second step, identifying/developing intervention theory, is addressed in The development of treatment manuals and theory. The third and final step is modelling the process and outcomes of implementation, culminating in the development of a programme theory (see Modelling process: developing programme theory). As researchers do not always have the same vision of what theory is supposed to be or do,69 to clarify, then:
-
The function of a pilot RCT is not to test physiological treatment theory, but to determine whether a future full-scale RCT would be capable of doing so. 70,71
-
Initial testing of the programme theory, using linear logic modelling, is one subject of this report.
-
We also access theory to consider why patterns of observations may have occurred. We use general theories and conceptual frameworks, concerning disability and participation, the burden and implementation of services and the character of AT service delivery traditions, to better understand how the social context mediates programme delivery.
The development of treatment manuals and theory
Key stakeholders attended an all-day meeting on 27 March 2013, 4 hours of which were set aside to inform the design of the study intervention (Table 1).
| Name by category of expertise | Affiliation |
|---|---|
| DMD family | |
| Victoria Whitworth | Parent of service user |
| AT | |
| Heather Epps | Epps Consultancy, Dorking |
| Physiotherapists specialising in DMD | |
| Michelle Eagle | Newcastle University |
| Marion Main | Great Ormond Street Hospital, London |
| Lindsey Pallant | Leeds Teaching Hospitals |
| Elaine Scott | University of Sheffield |
| Allison Shillington | Alder Hey Children’s Hospital, Liverpool |
| Neurology | |
| Peter Baxter | Sheffield Children’s Hospital |
| Valeria Ricotta | University College London (also the North Star Project)a |
| Trials Unit | |
| Daniel Hind | University of Sheffield |
Specialist physiotherapists proposed a set of exercises appropriate to manage the development of DMD in the target population group (see Figure 1 and Interventions). They also agreed that there should be free play at the end of the session, that disease progression should be monitored throughout the study with exercise prescription adapted accordingly and that, in the context of this study, it was worth experimenting with a twice-weekly programme that continued for as long as possible. Members of the team felt that, with no reliable ‘dose–response’ data available for the use of AT in people with DMD, maximising the dose would improve the chances of detecting a treatment effect, if one existed, in any future, full-scale trial. After the meeting, Heather Epps modified an AT manual created for the management of junior idiopathic arthritis,73 Marion Main adapted an existing LBT manual for the management of DMD,34 and the physiotherapists agreed that the manual was suitable for testing. The intervention protocols are described in Interventions.
At successive meetings we developed a treatment theory that hypothesises links between ingredients (observable measurable actions, chemicals, devices or forms of energy), mechanisms of action (processes through which the ingredients bring about change) and targets (measurable aspects of the individual’s functioning that is predicted to be directly changed). 74,75 Figure 1 shows the treatment theory, with ingredients 1 and 2 applying to LBT and all three ingredients applying to the AT intervention. Stretches (ingredient) provide mechanical traction for,76 and relax,37 muscles (mechanisms), which tend to tighten with the progression of DMD, thereby maintaining muscle length and soft tissue structure, while deferring the development of contractures (target). 37 Muscle training and general aerobic, submaximal exercises (ingredients) create biochemical adaptations that increase muscle mass by means of muscle fibre hypertrophy37 and maintained stroke volume77 (mechanisms) to prevent disuse atrophy and maintain or improve skills such as sit to stand, getting up from the floor and stair climbing (targets). Warm water (ingredient) creates haemodynamic changes78 and offers properties such as buoyancy and turbulence (mechanisms)47 that are not available in land-based physiotherapy, thereby reducing mechanical stress during muscle strengthening and allowing activation of muscles in ways not possible on land (target). The mental adjustment and disengagement stages of the Halliwick Concept46 (ingredients) may use mechanisms (e.g. reciprocal inhibition,79 systematic desensitisation80 and extinction81) that psychologists associate with exposure with graded support to achieve water confidence and participation (targets).
FIGURE 1.
Treatment theory for AT programme. VO2, oxygen consumption.

Modelling process: developing programme theory
A treatment theory is essentially physiological and, in this project, limited in scope to the prescribed exercises performed in the pool. Around these exercises is a ‘programme’, that is, a ‘set of planned activities directed toward bringing about specified change(s)’82 in boys with DMD through prescribed exercises. A programme theory recognises that, around the AT exercises, there is a complex series of contracts, actions, interactions and emergent relationships between people and organisational units. For many researchers, the purpose of evaluating a programme is to develop or test a theory about how it works, rather than merely evaluating whether it works. 83 If the programme is successful, then the evaluation can identify essential elements for replication; if the programme is unsuccessful, then it can identify whether or not this was through failures of implementation, translation to unsuitable contexts or treatment theory. 84 A programme theory, then, is broader than a treatment theory; it focuses on assumptions about background behavioural, social and economic mechanisms that operate if the treatment is to be delivered as scheduled. 85 Programme theory was developed iteratively: (1) deductively, through a review of the literature, (2) by articulating mental models, through discussions at stakeholder meetings, and (3) inductively through interviews with participants and professionals. 86
We searched the literature to inform an initial thematic framework about how children with neurological or musculoskeletal problems, their parents and physiotherapists respond to physical therapy programmes. We used Medical Subject Headings, such as ‘musculoskeletal diseases’, ‘nervous system diseases’, ‘children’, ‘adolescents’ and ‘physical therapy modalities’, as well as free-text terms such as ‘qualitative’ and ‘themes’ in a MEDLINE search to find papers. We found four relevant qualitative research studies on the acceptability of physiotherapy in children with neuromuscular conditions, consisting mainly of inductive thematic analyses. 87–89 One study90 drew on two social science theories. The themes of social model theory, distinguishing impairment from disability and eliminating discrimination and removing barriers to access91,92 have been incorporated into the International Classification of Functioning, Disability and Health – Children and Youth version (ICF-CY)93 (see Modelling process: developing programme theory), which is recommended for understanding context in evaluative studies. 94 The theory of psychosocial development95 was used to understand how changes brought on by adolescence affect adherence to therapy and adaptation to illness. Our population was younger and more compliant than that in that study. As neither theory seemed suited to this population, we constructed an initial framework by which to understand responses to the interventions from themes observed in the four studies. Table 2 shows the key themes of the a priori framework.
| Theme | Christy et al., 201089 | Capjon and Bjørk, 201087 | Wiart et al., 201088 | Redmond and Parrish, 200890 |
|---|---|---|---|---|
| (a) Improvement in physical function | ✓ | ✓ | ✓ | ✗ |
| (b) Improvement in confidence and independence | ✓ | ✗ | ✓ | ✗ |
| (c) Increased participation | ✓ | ✓ | ✗ | ✗ |
| (d) Achievement of goals | ✓ | ✗ | ✓ | ✗ |
| (e) Fatigue during the programme | ✓ | ✓ | ✗ | ✗ |
| (f) Pain during the programme | ✗ | ✓ | ✗ | ✗ |
| (g) The duration and spacing of therapy sessions | ✗ | ✗ | ✓ | ✗ |
| (h) The quality of the relationship with the therapist and communication between therapist and families | ✗ | ✓ | ✗ | ✓ |
| (i) Stress associated with the programme and balancing therapy with the demands of everyday life | ✓ | ✗ | ✓ | ✗ |
| (j) Responsiveness of schools to children’s therapy schedule | ✗ | ✓ | ✗ | ✗ |
We later employed a number of conceptual frameworks and models to ‘make sense of or shed light on’69 the empirical data we gathered, to better understand the implementation and acceptability of the AT programme and to consider how processes could be improved.
The ICF-CY is a conceptual framework used to define disability, which has widespread appeal and acceptance among physiotherapists, one target audience of this work. 93 It describes functioning in terms of body structures and function, desired activity or activity limitations, and participation restrictions (the inclusion of the young person in social situations). The ICF-CY Environmental factors component views disability as the outcome of interactions of these factors with other contextual factors, both environmental and personal.
The way in which participation is understood has developed rapidly since this work was commissioned. 96–99 The conceptual framework developed through an integrative review by Kanagasabai et al. 100 suggests contextual conditions under which participation is reduced or maintained (Figure 2). Another review, by Kang et al. ,101 proposes that participation is optimised with physical, social and self-engagement. Social engagement refers to interpersonal interactions during activities, feelings of being included or belonging. Self-engagement refers to feelings of enjoyment, self-determination and how a person thinks about themselves, especially in the setting and achievement of goals.
FIGURE 2.
Conceptual framework of association between motor functioning and leisure participation. Reproduced with permission from Kanagasabai et al. , Association between motor functioning and leisure participation of children with physical disability: an integrative review, Developmental Medicine & Child Neurology,100 John Wiley and Sons © 2014 Mac Keith Press.
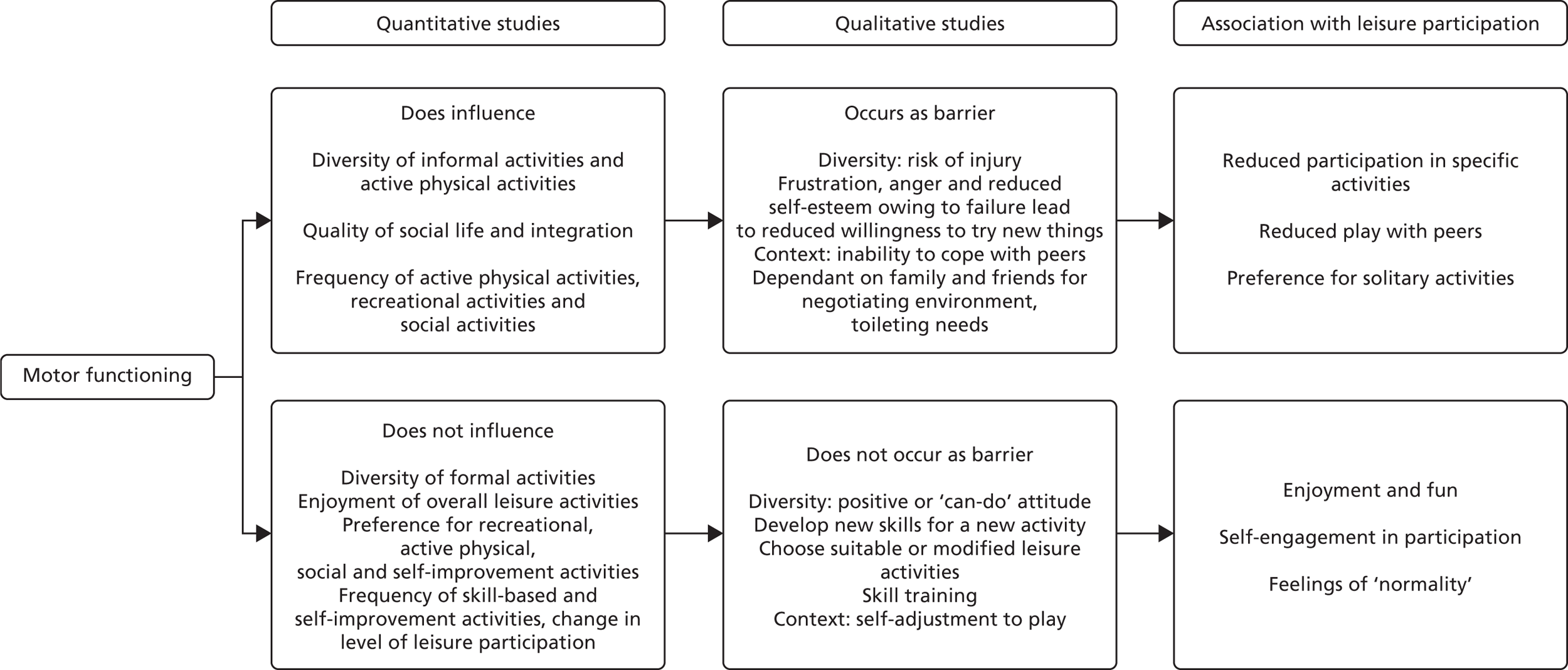
A range of child-, family- and environment-related attributes affect participation. Personal factors covered later in Figure 23 relate to how children adapt to keep engaging with peers or to cope with rejection. 102,103 Age, gender and preferences for particular activities or experiences also play a part in the extent of participation. 104–106 Family socioeconomic status,104 functioning,105 mental well-being,107 orientation towards physical activity105,106 and other factors108 also contribute. A physical environment, societal attitudes and supportive services can provide physical assistance, guidance and broader opportunities for activity. 109–112
Often, the strategies that we develop to manage chronic disease created additional burdens of work for patients, leading to poor adherence and clinical outcomes, as well as wasted resources. 113,114 Minimally disruptive medicine should identify this burden, encourage improved co-ordination of care and design, or prioritise care from the patient perspective. 115 Burden of treatment theory provides a way of understanding the relationship between health-care users, their social networks and the health services they use, with a view to redesigning those services to make them minimally disruptive. 113,116 As burden of treatment theory has been developed around the adult service user, it is useful to consider it in tandem with a conceptual model of parental work of care for children with special health-care needs (Figure 3 and Table 3). 117
FIGURE 3.
The burden of health care in chronic conditions: two conceptual models. a, See Table 3. Adapted from Tran et al. ,114 Hexem et al. 117 and Eton et al. 118 All articles are distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. CSHCN, children with special health-care needs.
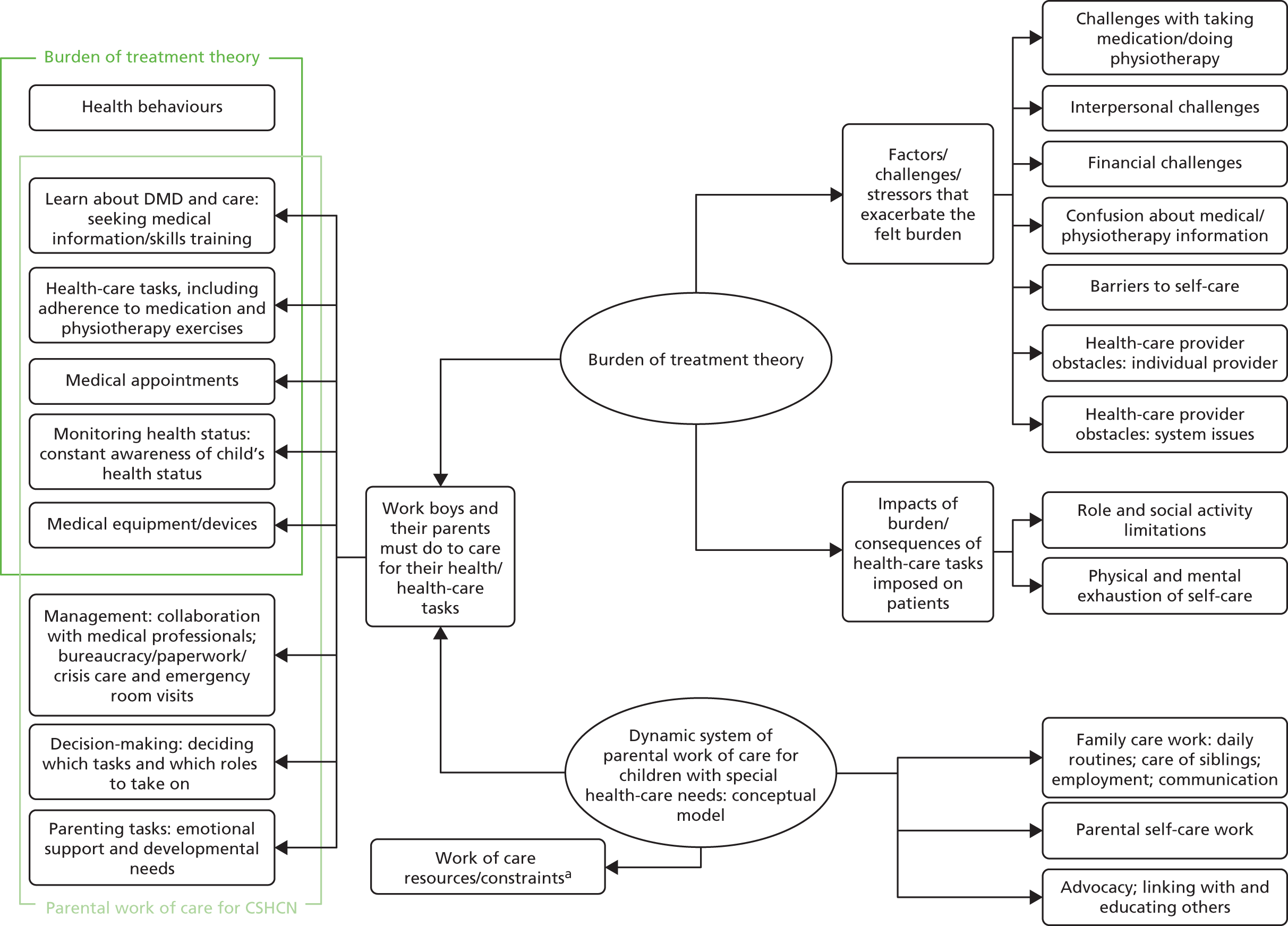
| Category | Selected codes relevant to study population |
|---|---|
| Child | |
| Disease | Severity, symptoms and child’s quality of life |
| Episodic quality of illness and uncertainty | |
| Medical care | Type of technology or equipment |
| Frequency of treatments | |
| Behaviour | Cognitive and emotional function/expression |
| Functional ability/activity limitations | |
| Location | Home, hospital or elsewhere |
| Parent | |
| Gender roles | How the roles of mothers and fathers differ |
| Mental health | Emotions, quality of life and stress |
| Personality | Hardiness, self-esteem and coping style |
| Knowledge | Medical and parenting skills and experience |
| Education level | |
| Social support | Availability of friends and family |
| Family | |
| Family structure | Family cohesion, including marital dynamics |
| Single parents | |
| Siblings | |
| Finances | Employment, income and expenses |
| Society | |
| Geographic locale | Different regions, countries. Immobility |
| Attitudes and norms | Disability and disease in childhood |
| Parental responsibility and gender norms | |
| Health services | Availability of care facilities and providers |
| Awareness of available services | |
| Political system | Government policies and funding |
Burden of treatment theory helps us to understand the response of patients and carers to complex interventions. To understand the perspectives of physiotherapists delivering AT, we draw on normalisation process theory (NPT), a general theory of how complex interventions become routinely embedded in health services. 119,120 NPT categorises the different kinds of work that people do when they implement a new practice as sense-making, relational, operational and appraisal. Problems in any of the categories can result in implementation failure.
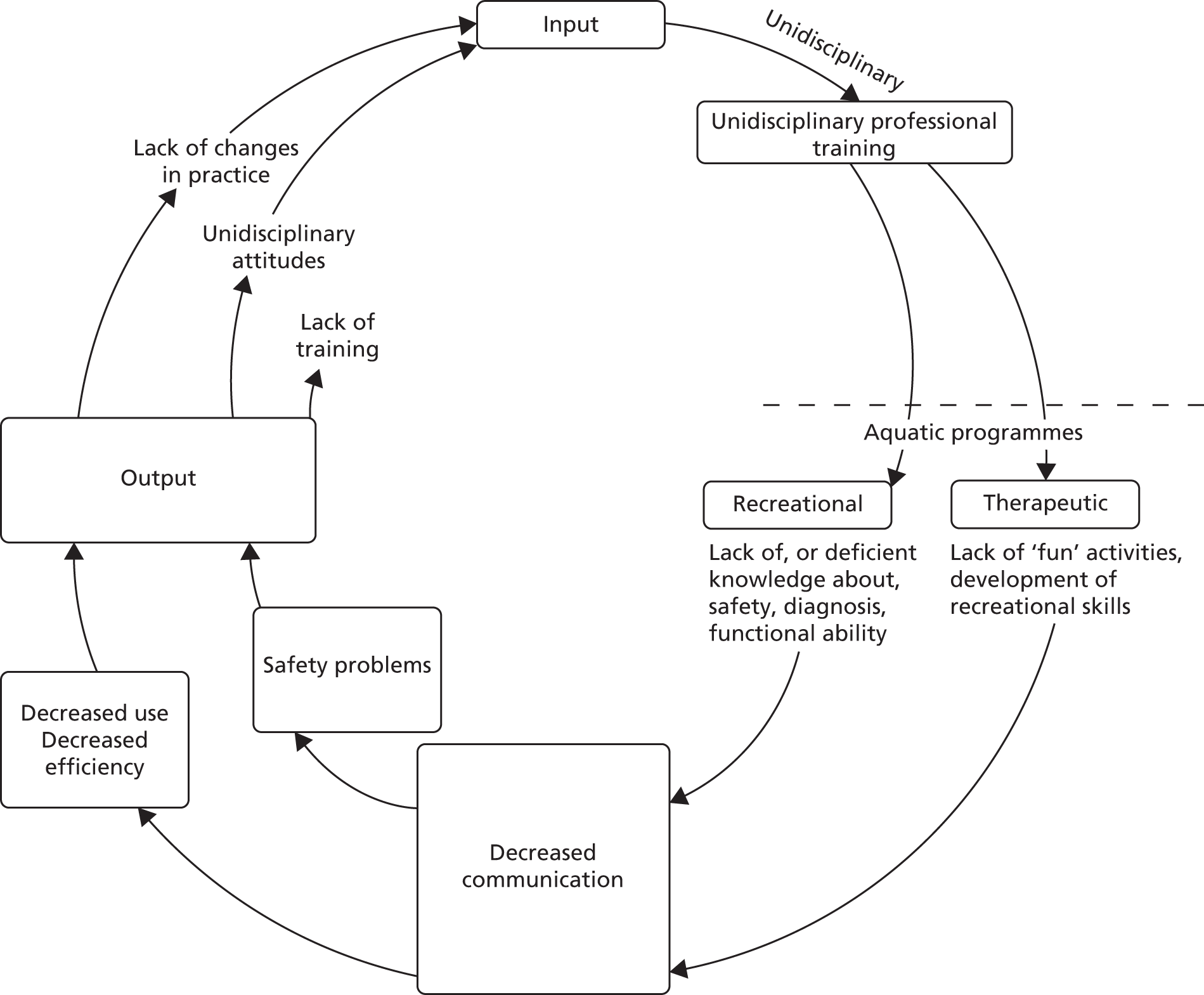
Finally, we draw on themes identified by a systems analysis of problems encountered in aquatic programmes for disabled children (Figure 4). 121 The author describes a split between recreational and therapeutic approaches that existed in both practice and the literature of the early 1980s. 121,122 She identified problems in this division of philosophy, principles, staff composition, roles and training (Figure 5), and described an integrated approach to aquatic intervention for disabled children. Retrospectively, we find this theory useful to interpret the responses of some participating physiotherapists to manualised AT.
FIGURE 4.
Dulcy’s systems analysis of problems in aquatic programmes for disabled children. Copyright 1983 from Aquatic programs for disabled children by Faye H. Dulcy. 121 Reproduced by permission of Taylor & Francis, LLC (www.tandfonline.com). Tx, treatment.
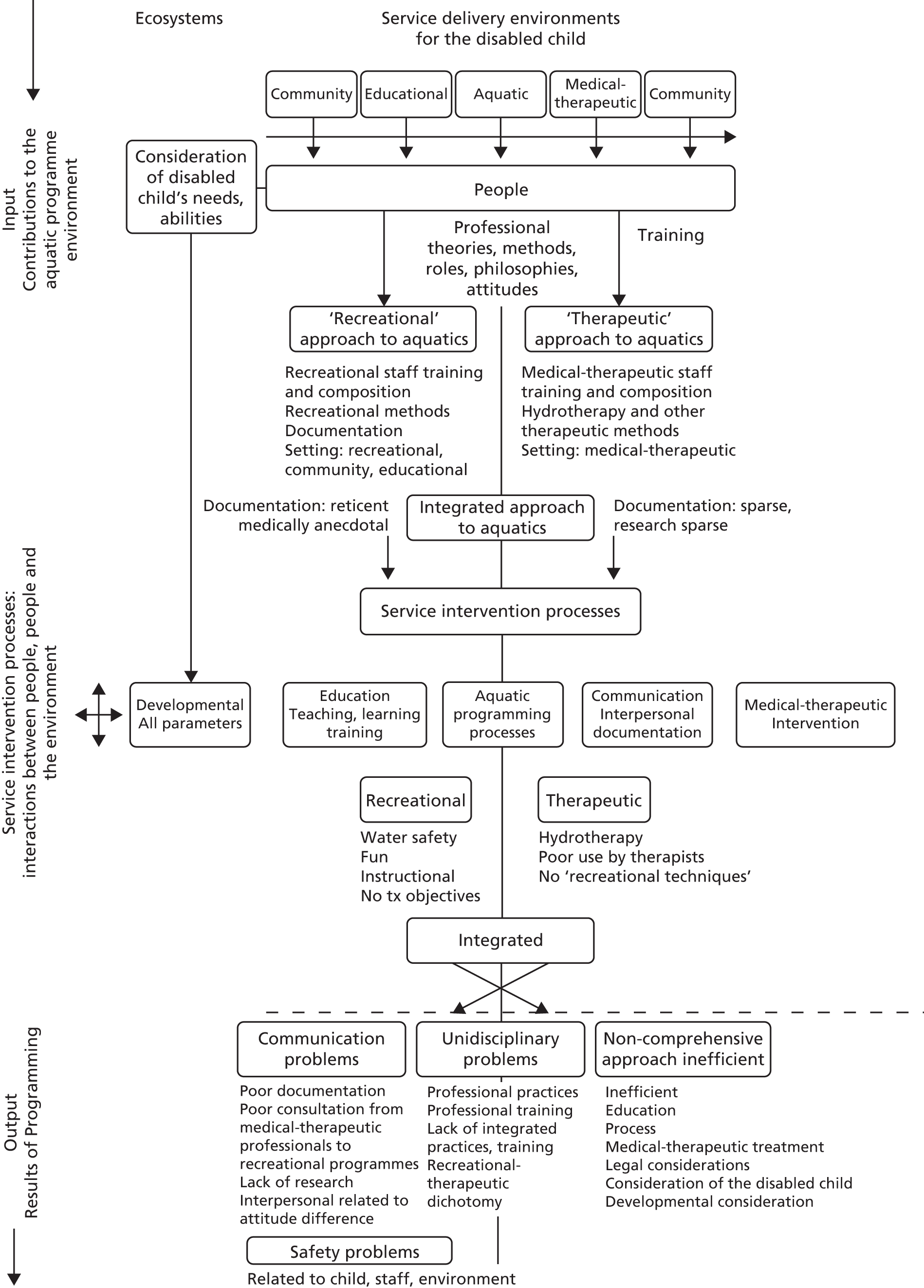
FIGURE 5.
Dulcy’s vicious cycle of problems in aquatic programmes. Copyright 1983 from Aquatic programs for disabled children by Faye H. Dulcy. 121 Reproduced by permission of Taylor & Francis, LLC (www.tandfonline.com).
Based on the above literature and through team discussions, we developed the programme theory, which could be briefly described as follows:
Boys, parents, specialist physiotherapists will be willing and able to conduct twice-weekly AT and tertiary centres will allocate resources for this (inputs and activities). This will be manageable within the existing roles, interactions and relationships that characterise the management of DMD (context). Delivery of the programme per protocol (immediate outcomes) will bring about physiological benefits described in the treatment theory (intermediate outcomes).
We developed a logic model to illustrate how chains of events over time were to bring about the desired outcomes, in accordance with the programme theory (Figure 6). 123,124 Contextual factors that can influence and be influenced by implementation are included.
FIGURE 6.
Logic model (initial).
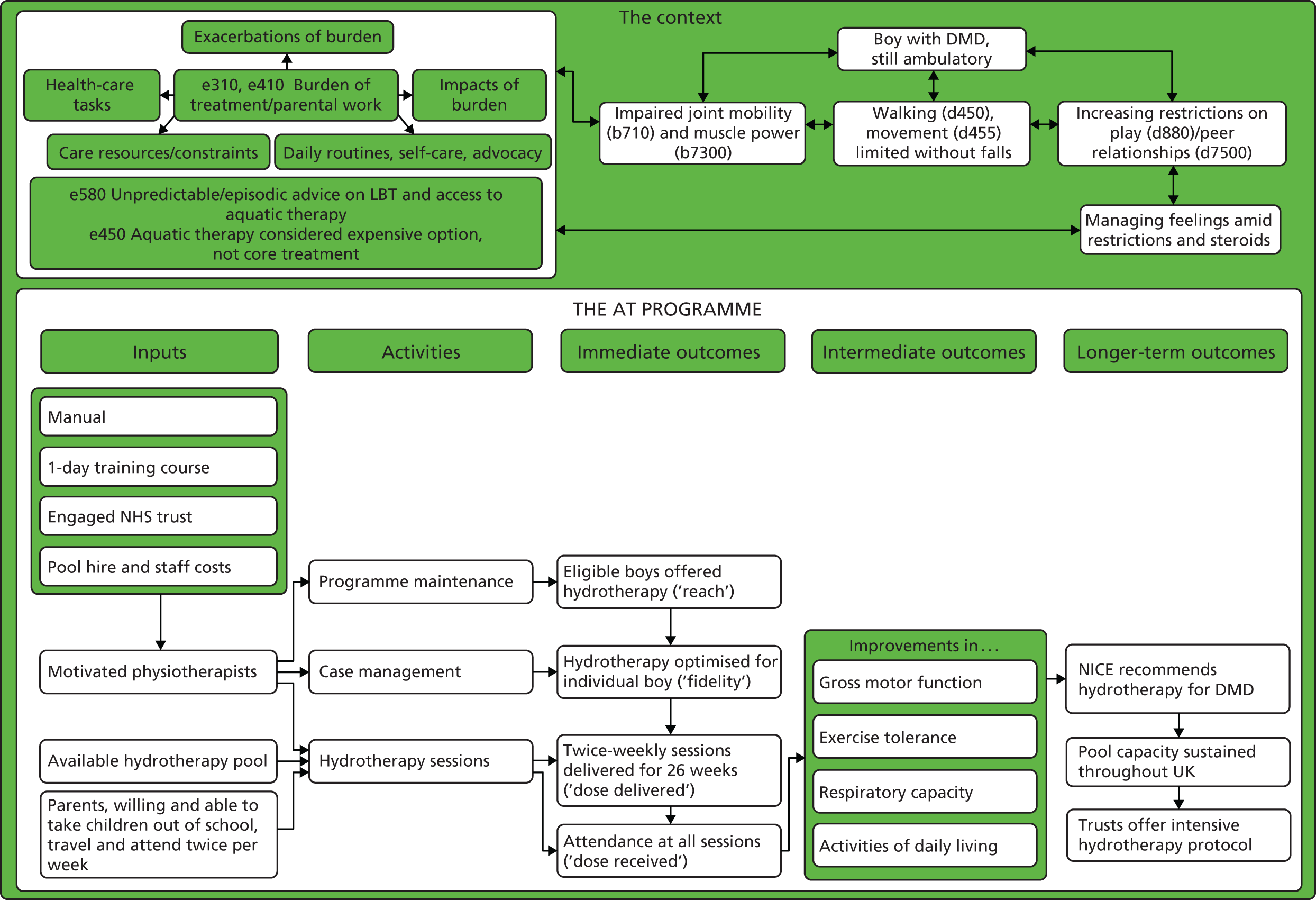
Most elements of the logic model are self-explanatory, but some are worth unpacking. First, there are a number of definitions of ‘case management’ containing functions that also appear in definitions of ‘programme management’. 125,126 Although we acknowledge this overlap, we use the term to mean a micro-level episode of health-care management in a health-care setting in which one is accountable for service user outcomes. To simplify, by ‘case management’, we mean everything that a physiotherapist does with a boy with DMD and their notes, from identifying a boy as suitable for AT to discharging them from the service. By ‘programme maintenance’, we mean everything that the physiotherapist, his or her colleagues and organisation do to sustain (continue and institutionalise) an AT service beyond case management. 127–130
The programme theory and the logic model form the starting point for a process evaluation, which investigates what is delivered and how, mechanisms of impact (broadly defined) and how context affects implementation and outcomes. 131 In the standard format of a process evaluation, there is an imperative to evaluate implementation fidelity,131–134 which is typically interpreted as the consistency with which intervention components are delivered. 135 However, insofar as front-line physiotherapy often involves making sense of rapidly changing and ambiguous phenomena to revise treatment plans, it is not the kind of work that benefits from standardisation. 136 A body of theoretical literature suggests that the standardisation of function, that is, being faithful to the intervention theory, is more important than standardising the form of an intervention. 137–139 In this study, standardisation would not meet the physical and psychological needs or capabilities of individual children. For these reasons, we evaluate treatment optimisation to the child’s needs and capabilities, adjudicated by an independent physiotherapist, rather than fidelity to intervention components. We define optimisation as good adherence to a good-quality prescription (see Optimisation of prescription).
The pilot trial
Trial design
This was a parallel-group, open-label, randomised external pilot trial with a 1 : 1 allocation ratio (Figure 7). Consolidated Standards of Reporting Trials guidelines are observed. 140 Important changes to the methods after trial commencement are reported in Appendix 1.
FIGURE 7.
Pilot RCT summary. 6MWD, Six-Minute Walk Distance; ACTIVLIM, Activity Limitations Measure; CarerQol, Care-related Quality of Life; CHU-9D, Child Health Utility 9D Index; FVC, forced vital capacity; NSAA, North Star Ambulance Assessment.

Participants
Inclusion criteria
-
Genetically confirmed DMD: a muscle biopsy report from a registered NHS pathology laboratory showing dystrophin deficiency compatible with DMD and/or a report from a registered NHS molecular genetics laboratory showing the DMD gene to have a pathogenic deletion, duplication or point mutation.
-
Aged 7–16 years: children aged < 7 years were excluded because natural history studies have shown that there is a continuing improvement in the 6-minute walk distance (6MWD) up until that age and, therefore, any improvement might be spontaneous rather than a result of the intervention. 141
-
Established on glucocorticoid corticosteroids [patient has been treated with prednisolone or deflazacort (Calcort®, Sanofi-Aventis) for at least 6 months with no major change in drug, dosage or frequency for at least 3 months before the initial assessment]. Such changes were defined as follows:
-
Frequency covered a change from daily dose to alternate day or another non-daily regimen (or vice versa).
-
A dose increase in line with weight was acceptable. Any other change was an exclusion criterion.
-
A change in prescription from prednisolone to deflazacort (or vice versa) was an exclusion criterion.
-
-
A North Star Ambulatory Assessment (NSAA) score of ≥ 8 to 34. Those with more than a 20% variation between baseline screens 4 weeks apart (at pre-screen and initial assessment) were excluded.
-
Able to complete a 10-m walk test with no walking aids or assistance.
Exclusion criteria
-
Involvement in another RCT.
-
More than a 20% variation between screening and baseline NSAA scores.
-
Unable to commit to the programme of twice-weekly AT for 6 months.
-
Any absolute contraindications or precautions to AT43 listed in Table 4 at the point of determining eligibility.
| Absolute contraindications | Precautions |
|---|---|
| Severe cardiac failure | Fear of water |
| Resting angina | Behaviour problems that prevent participation in physiotherapy or AT |
| Shortness of breath at rest | Arterial hypotension/hypertension |
| Renal failure | Chemical sensitivity |
| Proven allergy to chlorine or bromine | Indwelling catheter/PEG tube |
| Uncontrolled epilepsy | Tracheostomy |
| Uncontrolled faecal incontinence | Poor skin integrity/open wounds |
| Febrile conditions: acute systemic illness or pyrexia (until resolved) | Unstable angina, cardiac arrhythmias or additional cardiac considerations |
| Acute vomiting/diarrhoea (until resolved) | Dizziness/vertigo |
| Wound infection (until resolved) | Diabetes mellitus |
| Weight in excess of available pool evacuation equipment | Thyroid problems |
| Widespread MRSA (until resolved) | |
| HIV/AIDS | |
| Haemophilia |
Settings and locations where the data were collected
Staff at six specialist neuromuscular clinics identified a sufficient pool of eligible patients. Each expected to be able to recruit 10 patients in the 6-month accrual window, during which all patients would have one routine clinic visit. Site staff received training in protocol procedures by the trials unit; many of the research procedures, especially matters of documentation, were already routine in clinical practice.
Screening and consent
Recruitment was undertaken by good clinical practice-trained site staff who screened health records to identify study candidates. Candidates with absolute contraindications were excluded at this stage. A cover letter and information sheet were sent to the carers of study candidates. Carers were invited to discuss the trial by telephone. For those interested, an appointment was booked at the site at which candidates and carers were invited to ask further questions. We acquired full written consent and assent from carers and participants, respectively.
Assessment of eligibility
Confirmation of eligibility (including repeat NSAA) took place 4 weeks later.
Interventions
Research arm: aquatic therapy programme
Materials
A manual providing a ‘menu’ of aquatic exercises is available as a web-only appendix. 142
Procedures
Exercises, using the properties of water (buoyancy, turbulence, etc.), included:
-
Stretches – active assisted and/or passive regime that targets key muscle groups in ambulatory boys (e.g. hip flexors, hip abductors; iliotibial band; hamstrings; knee extensors, ankle plantar flexors; supination, pronation; wrist and finger flexors; neck flexors). Some movements used the properties of ‘drag’, for example for a passive trunk side flexion stretch.
-
Muscle training/strengthening – hip extension, abduction; knee extension; ankle dorsiflexion; shoulder abduction, horizontal extension, flexion; elbow extension; wrist extension.
-
General aerobic – walking backwards and sideways, bobbing, swimming, punching, ball activities. Submaximal exercise in the water.
-
Simulated or real functional activities (e.g. sit to standing, running, jumping, hopping, using the unencumbered, three-dimensional properties of water). For example, going from a seated to a standing position in water, when there is no actual seat, uses the metacentric effect and the properties of water to develop core strength and balance, and to learn or maintain the task, which is becoming more difficult out of water. A previous protocol was adapted for this aspect, with the exception that children would not be asked to lie fully prone, but only three-quarters prone (face not quite submerged). 34
-
Breath control exercises and games.
-
Swimming and other ‘fun’ activities.
Provider
The intervention was delivered by suitably qualified physiotherapists who worked in tertiary paediatric neuromuscular centres and had experience in the management of DMD. Most, but not all, physiotherapists had previously attended foundation training courses and had experience of delivering aquatic exercise, but not necessarily using the properties of water. Those delivering the intervention from four trusts attended a specialist day course, delivered by Heather Epps, an accredited trainer, in which they were trained in the principles and practice of AT; a training video was also provided. Physiotherapists at a further trust did not attend face-to-face training but did receive the video.
Location and mode of delivery
Aquatic therapy was delivered face to face in a NHS AT pool, heated to a temperature of 34–36 °C. Although group sessions were anticipated, participating trusts could schedule only individual sessions with one exception, in which brothers were randomised to AT.
Schedule
Aquatic therapy was to be delivered twice a week for a maximum of 30 minutes per session in the water (to avoid fatigue) for a period of 6 months. The research physiotherapists at the site were responsible for booking participants into sessions for those randomised to the AT arm and for supervising their treatment regime throughout the study. The research protocol specified that study interventions should commence within 4 weeks of randomisation.
Tailoring
It was intended that the treating physiotherapist should choose options appropriate to each child’s level of ability and particular presenting clinical problems. The prescription should be focused and achievable; it was not expected that most/all exercise categories in the manual would be represented in a prescription or that prescribed LBT exercises would be replicated in the water (training session minutes, Birmingham, 19 November 2014). To avoid excessive fatigue, participants were asked not to undertake their LBT on days that they received AT.
Modifications
No modifications were made to the manual during the project.
Optimisation
We define optimisation as good adherence to a good-quality prescription (see Optimisation of prescription). Details of the AT prescribed for and delivered to the participant were entered on to the patient’s care plan/AT log. An independent physiotherapist who was not a co-applicant was commissioned to assess if AT and LBT had been optimised to the need and capacity of the participant (see Optimisation of prescription). We asked physiotherapists to complete a form detailing why sessions did not take place, with the following options: did not attend, unable to attend (cancelled), pool closed, no pool time available, staff unavailable or other.
Control arm: land-based therapy
Materials
A manual was developed, based on best existing practice, again providing a ‘menu’ of exercises from which the treating physiotherapist could choose options appropriate to the child’s level of ability and particular presenting clinical problems. Physiotherapy intervention/prescription depended on the clinical need and capability of the individual patient (see Chapter 1, Physiotherapy).
Procedures
Depending on the needs and capability of the individual participant, a regular stretching regime (4–6 days/week) would target key muscle groups in ambulatory boys (triceps surae complex, hamstrings, hip flexors, iliotibial tract, long finger flexors). To avoid disuse atrophy, while being aware of the potentially detrimental effects of overexercising, particularly with regard to activities that promote eccentric activity, a directed programme of exercises dependent on the individual’s need was prescribed. General advice on regular activity was also recommended (e.g. walking, cycling and swimming).
Provider and locations
Although it is normal for community physiotherapists to prescribe therapy for children with DMD,28,33 for the purposes of this trial (see Chapter 3, Problems with the delivery of land-based therapy), LBT exercises were prescribed by the research physiotherapist at participating tertiary centres. They informed the community physiotherapist of the exercises prescribed, and asked them to feed back if the prescription changed. Exercises were delivered, in varying proportions, by parents at home, by support workers in school and, less commonly, by local community physiotherapists. Parents and support workers were trained in the delivery of exercises by the prescribing physiotherapist, as is routine practice.
Schedule
Typically 4–6 days per week.
Tailoring
The number of sessions, schedule, duration and intensity were based on the child’s level of ability and particular presenting clinical problems.
Modifications
As in usual practice, community therapists could modify prescriptions depending on the rate of functional change. They were asked to notify the study team when they did so.
Optimisation
Parents were asked to complete the LBT log every week, indicating the number of days on which they had completed each exercise; they were provided with self-addressed envelopes and asked to return completed forms. Research physiotherapists sent out a maximum of three letters reminding participants to return overdue LBT logs over the course of the period of trial involvement. An external assessor evaluated if therapy had been optimised for each participant.
Outcomes
Outcomes are summarised in Table 5.
| Assessment | Where | Completed by | Paper | Electronic | Consent and screen (visit 1) | Eligibility and baseline (visit 2) | Intervention | 26 weeks (visit 3) |
|---|---|---|---|---|---|---|---|---|
| Eligibility criteria form including | ✗ | ✗ | ✗ | |||||
| NSAA | Clinic | Physiotherapist | ✗ | ✗ | ✗ | ✗ | ✗ | |
| 6MWD | Clinic | Physiotherapist | ✗ | ✗ | ||||
| FVC | Clinic | Physiotherapist | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Patient-reported outcomes | ||||||||
| CHU-9D | Clinic | Participant | ✗ | ✗ | ✗ | |||
| ACTIVLIM | Clinic | Participant | ✗ | ✗ | ✗ | |||
| CarerQoL | Clinic | Carer | ✗ | ✗ | ✗ | |||
| Health and social care resource use | Clinic | Participant/carer | ✗ | ✗ | ✗ | |||
| Pain (VAS) | Participant/carer | ✗ | ✗ | |||||
| OMNI scale of perceived exertion | Clinic | Participant/carer | ✗ | ✗ | ||||
| Concomitant medications | Clinic | Physiotherapist | ✗ | ✗ | ✗ | ✗ | ||
| Adverse and serious adverse events | Clinic, home, other | Participant/carer/ physiotherapist | ✗ | ✗ | ✗ | |||
| Process evaluation | ||||||||
| AT log | Pool, clinic | Physiotherapist | ✗ | ✗ | ✗ | |||
| LBT log | Clinic, home | Participant/carer/ physiotherapist | ✗ | ✗ | ✗ | |||
| Participants’ views on intervention and research procedures | Home, CTRU | Participant/researcher | ✗ | ✗ | ||||
| Therapists’ views on intervention/research protocol | Clinic, CTRU | Physiotherapist/researcher | ✗ | ✗ | ||||
| Care plan prescribed for intervention and control | Clinic, pool | Physiotherapist | ✗ | ✗ | ||||
| Feasibility outcomes | ||||||||
| Number and characteristics of eligible patients approached: screening form | Clinic | Physiotherapist | ✗ | ✗ | ✗ | |||
| Reasons for refused consent: screening form | Clinic | Physiotherapist | ✗ | ✗ | ✗ | |||
| Reasons for attrition: withdrawal form | Clinic, home | Physiotherapist | ✗ | ✗ | ||||
| Participant attrition rate | CTRU | Researcher | ✗ | ✗ | ✗ | |||
| Number of missing values/incomplete cases | CTRU | Researcher | ✗ | ✗ | ✗ | |||
| Participants’ and therapists’ views on acceptability | Research site, home | Participant/researcher | ✗ |
Primary outcome
Recruitment rate.
Other feasibility outcomes
-
Decision on the primary end point for the main trial.
-
Number and characteristics of patients who were identified as potentially eligible, approached for the study, at each study visit, randomised, withdrawn and lost to follow-up, discontinued from the AT intervention (with reasons), and included and excluded from analysis (with reasons). We also report the recruitment rate (defined as the proportion of patients approached that consent into the study).
-
Reasons for refused consent.
-
Participant attrition rate (the proportion of the consented and randomised participants who withdrew or were lost to follow up).
-
Reasons for attrition.
-
Number of missing values/incomplete cases. For questionnaires we report the item response rate at each time point.
-
Feasibility of recruiting participating centres and estimation of costs, given as a narrative assessment.
-
Therapist views on intervention/research protocol acceptability/perceived contamination of control arm.
Clinical outcomes
The following outcomes, assumed to be those of any future full-scale trial, were assessed during routine clinical visits at baseline and 6 months (* indicates routine assessment):
-
6MWD disease-related limitations on ambulation143
-
forced vital capacity (FVC)146*
-
Child Health Utility 9D Index (CHU-9D) health-state utility147
-
Activity Limitations Measure (ACTIVLIM) measure of independence and activity148
-
Care-related Quality of Life (CarerQoL) measure of carer burden149
-
health and social care resource use questionnaire for economic evaluation.
The following safety outcomes were assessed at the AT session for those in the intervention arm only after each AT session:
-
pain (visual analogue scale)
-
Children’s OMNI Scale of Perceived Exertion. 150
Changes to trial outcomes after the trial commenced
Changes to outcome assessments, made during the study, are detailed in Appendix 1.
Sample size
The sample size was based on a recommended minimum of 30 participants (15 per group) for feasibility objectives involving parameter estimation. 151 Assuming a dropout rate at 6 months of 20%, we set a target of randomising at least 40 participants (20 per group).
Feasibility criterion
This pilot aimed to recruit 40 children in 6 months and to deliver AT to 20 of them. If this objective success criterion was met, then a full-scale study would be deemed feasible.
Generation of the random allocation sequence
The randomisation schedule was computer generated prior to the study by the clinical trials research unit (CTRU) in accordance with standard operating procedures. It was stratified by centre with randomly permuted blinded block sizes to ensure that enough patients were allocated evenly.
Allocation concealment
The allocation schedule was concealed through the use of the centralised web-based randomisation service.
Implementation
After eligibility and written consent were confirmed, patient details were entered into the randomisation system by good clinical practice- and protocol-trained site staff (physiotherapists), and the treatment allocation was returned.
Blinding
Although the physiotherapists, physicians and participants were not blinded, the data analysts remained blind to treatment allocation until after the statistical analysis plan was finalised, the database was locked and the data review was completed. Initially, blinded data were delivered to the statistician by the data manager to define analysis sets and to test statistical programs. Any queries were communicated to the study and data manager prior to database lock. The database was locked after agreement between the statistician, data manager and study manager. No changes were made once the data had been locked. Database freeze and lock were conducted in accordance with CTRU standard operating procedures.
Statistical methods
Analysis population
The intention-to-treat population included all patients for whom consent was obtained and who were randomised to treatment. This was the primary analysis set, and end points are summarised for the intention-to-treat population unless stated otherwise.
Baseline characteristics
For continuous variables (e.g. age), either mean and standard deviation (SD) or median and interquartile range (IQR) have been presented, along with minimum and maximum variables. The number of observations used has been presented alongside the summaries. For categorical variables (e.g. ethnicity), the number and percentage of participants in each of the categories and the total number of observations are presented.
Feasibility outcomes
Subject-specific AT adherence is reported as the number of AT sessions attended within 6 months and the per cent compliance (out of the 52 anticipated sessions) with mean (SD), median (IQR) and minimum–maximum values for the number of sessions attended. We also report the number and percentage of participants who attended all AT sessions and tabulate reasons for missed sessions. LBT adherence is reported as the number of days for which a participant was prescribed exercise and compliance is reported as the percentage performance (over the total number of days for which exercise adherence was recoded).
Clinical outcomes
Descriptive statistics are presented for the clinical outcomes; significance testing has not been undertaken. NSAA measures (final measure and change from baseline) are presented as mean differences between groups and their associated 95% CIs. Clinical outcomes have been presented for the intention-to-treat set with available 6-month outcome data, by group and overall. Spaghetti plots of participant trajectories have been provided for various outcomes, stratified by treatment group, to provide a visual display of the change over time.
Missing spurious and unused data
The extent of missing data has been reported as it was one of the fidelity outcomes of the study. No sensitivity analyses involving imputation for missing data was performed. Any spurious data were queried and checked for consistency with data management before data lock. Patient and carer questionnaires were scored only if all relevant items that make up a domain were complete.
Ethical aspects
The study received a favourable opinion from the National Research Ethics Committee, East of England – Cambridge South, on 4 July 2014 (reference 14/EE/0204).
Patient and public involvement
James Parkin, a young man with DMD, and Victoria Whitworth, his mother and former carer, were involved in the design of the intervention, the study, the qualitative research analysis and the drafting of the report. They reviewed, made changes to and approved the final lay summary.
The intervention optimisation substudy
Introduction
Physiotherapists are legally obliged to record treatments given in a patient’s record at each intervention session. Participating physiotherapists collected standardised information on the type of exercises, number of repetitions and the time spent on each exercise, in a log based on the AT manual. Parents did the same for LBT. These data were entered onto a web-based data capture system and validated through on-site source data verification. An independent physiotherapist (JS), who was not a co-applicant, acted as an independent reviewer. She assessed whether or not prescribed treatment was ‘optimal’ given the treatment need and capacity of the boy concerned. We tabulated and charted summary data completeness. Missed/cancelled sessions were attributed to provider, patient or unknown factors. In attendance calculations we assumed that when the reasons for a missed session were unknown, then they were due to provider factors; the sum of sessions attended and sessions missed for family reasons were used as the denominator for attendance statistics.
Optimisation of prescription
The assessor made an assessment of whether AT or LBT prescriptions were optimised based on treatment logs and baseline data (NSAA, ACTIVLIM, medical/social history and schooling). The NSAA score was assessed as above or below average depending on the participant’s age and steroid regime. 9 The AT attendance was assessed as good if > 70% of available sessions were attended. The quality of the AT/LBT prescription was classified as ‘good’ if it was individualised to patient’s needs and focused on priorities, as ‘varied’ if the prescription was inconsistent, less focused or too extensive, or as ‘poor’ if it was unfocused and had too much content. In addition, prescriptions were classified as achievable if the exercises were appropriate and could be completed in a half-hour session. The LBT was also classified as realistic if it was clinically appropriate and achievable for the child’s level of ability, contractures and functional score (NSAA). This accepts that prescriptions may need to change with time, loss of function and loss of range of movement.
Adherence to the prescription
As a result of the shortfall in recruitment, sampling was unnecessary and all completed records were evaluated. Full LBT adherence would be demonstrated by completed logs, demonstrating that all prescribed exercises were performed, for each of the seven 4-week periods throughout the 26-week study. Full AT adherence would also be demonstrated by completed logs.
The number of exercises prescribed for AT and LBT is sometimes provided as a range when prescriptions changed during the trial period. Weekly (LBT) or by session (AT) percentage compliance with the prescription was described using mean, median and range values. Prescription compliance of > 50% was assessed as good for both AT and LBT. For both the AT and LBT data, Jennie Sheehan assessed whether or not priority stretches were prescribed according to baseline joint range, NSAA score and knowledge of natural history of DMD. In addition, Jennie Sheehan assessed if any changes in the LBT prescription appeared to affect compliance.
Assessment of overall treatment optimisation
To summarise, the independent reviewer was asked to assess the following:
-
Was the quality of the prescription ‘good’, ‘varied’ or ‘poor’?
-
Was adherence with the prescription ‘good’ or ‘poor’?
If the answer to both (a) and (b) was good, then we considered treatment to be optimised. We considered attendance as a separate variable, but note that it is possible for treatment to be optimised but for there still to be poor receipt of the treatment (see Figure 6). Physiotherapists were given the chance to respond to detailed feedback from the independent rater. Only two took up the opportunity.
The qualitative research
The Consolidated criteria for Reporting Qualitative studies are observed. The topic guides used in the qualitative research are available in Appendices 2 and 3.
Interviewer characteristics
All interviews were conducted by Daniel Hind, a male graduate anthropologist, with 10 years’ experience of qualitative research, employed as a senior research fellow at the University of Sheffield.
Relationship with participants
No relationship was established with interviewees prior to commencement of the qualitative substudy. Participants were informed of the purpose of the research and the professional identity of the interviewer via the information sheet, and reminded, immediately before the interview, that he was an employee of the university, not of their care provider.The interviewer is a health services researcher with no motivational interest in either the population or the success of either intervention.
Theoretical and thematic framework
Rationale
Our rationale for using qualitative research alongside the pilot RCT is that it can tell stakeholders how to optimise interventions and research protocols, or why trials are likely to be infeasible. 152–154 Qualitative methods also enable research teams to capture how an intervention is implemented and experienced, thereby enabling better understanding of causal pathways. 131
Worldview155/epistemology156
Our rationale is pragmatic;157 it is concerned less with building, testing or advancing social science theory131,158 than with the ‘conceivable practical consequences’159 of different lines of action and with establishing a basis for ‘organising future observations and experiences’. 160 In other words, we hope to guide those who might want to develop, evaluate, commission or use AT services in the future by describing the experiences of those involved in our study. To do so, we do not rely on a single ‘favourite theory’,161 but aim to be ‘informed theoretical agnostics’,162 exploring how different theories of change might work at different levels and in different contexts.
Research design155/methodology156/approach163
Holistic single-case design with the unit of analysis at the intervention programme and research protocol level. 164
Theory
We used the four papers to inform our participant interview schedule. 87–90 To understand the conditions necessary to support the introduction and embedding of protocolised AT as a routine element of care, and to support a future evaluation, the health professional interview schedule was based around NPT. 120,165–167 Prompts related to the Theoretical Domains Framework168 were later added, but insufficient time was available for this additional coding work.
Participant selection
Convenience samples of children/parents and interventionists were taken. The consent of children and their parents was sought by the site principal investigators at the same time as RCT consent, but was not a precondition of the trial entry. Interventionists were informed at site initiation and approached for consent by a member of the research team directly. We interviewed all of the seven families whose boys (n = 8) received AT. In one case, the child was unavailable on the day and only the parent was interviewed. We interviewed seven physiotherapists who had delivered the intervention at five NHS trusts and a consultant paediatric neurologist at a sixth trust where the only participant had been randomised to the control arm (total health professional interviews, n = 8). None of the families approached declined an interview; one physiotherapist declined an interview, without giving a reason. Once recruited, no-one dropped out. All interviewed physiotherapists (n = 7) had experience of delivering AT prior to this project. Using the NHS Agenda for Change paygrade system,169 two physiotherapists we interviewed were band 6 (the most junior), three were band 7 and two were band 8. To put this in context, all those interviewed would have specialised in a particular condition (whereas it is typical that grade 5 physiotherapists rotate around specialties). Band 8 is usually an indicator that the individual is a physiotherapy service manager.
Setting
Semistructured interviews took place between 22 September 2015 and 14 January 2016 for participants and between 17 September 2015 and 29 January 2016 for physiotherapists. Parents chose the setting for data collection: most parent and child dyads were interviewed in their own home, in person; one dyad was interviewed by SkypeTM (Microsoft Corporation, Redmond, WA, USA); and one parent was interviewed by telephone after the failure of a Skype call. In general, interviews were conducted in quiet and private settings to reduce distractions; one interview was somewhat disrupted by the unavoidable presence of a younger child, otherwise, parents aside, no non-participants were present at interviews. All health professionals were interviewed by telephone around the time of site closure.
Data collection
In addition to the a priori themes identified in Table 2, semistructured interview guides for participants contained questions about the acceptability of intervention and research protocols. Interview guides were piloted with interventionist and patient/carer members of the study management group. The interview guide for health professionals adapted questions suggested by the NPT developers166 and was not piloted. No repeat interviews were undertaken. All interviews were recorded on an encrypted digital recorder and fully transcribed, with transcriptions anonymised. Field notes were taken after interviews as required. Participant/parent interviews lasted a median of 32 minutes (range 20–42 minutes), with durations typically related to the responsiveness of the child; the researcher’s sensitivity and judgement were used to determine the length of the interview. 170 Physiotherapist interviews took a median of 52 minutes (range 44–81 minutes). Formal assessment of whether or not saturation has occurred or of stopping criteria for qualitative data collection was not employed. 171 Although fewer than planned (because of the trial’s recruitment shortfall), some researchers would consider 16 interviews with families and professionals adequate for thematic172 (if not other sorts173,174 of) saturation. There was, for the most part, great consistency in messages from both groups. Transcripts were not returned to participants for correction.
Data analysis
We used the National Centre for Social Research ‘Framework’ approach to analysis, with its five ‘key stages’: (1) familiarisation, (2) identifying a thematic framework, (3) indexing, (4) charting and (5) mapping/interpretation. 175 ‘Framework’ analysis allows sufficient flexibility for analysts to pre-specify themes of anticipated importance as coding categories and to combine them with others that are identified during inductive analysis, allowing the reformulation of ideas during the progress of the analytical process. 176 Transcripts were imported into NVivo version 11 (QSR International, Warrington, UK). Daniel Hind, James Parkin and Victoria Whitworth read and re-read transcripts (familiarisation), considering them in light of the initial thematic framework (see Table 2), NPT and the logic model (Figure 8; see also Figure 6), with notes being taken on new categories inductively derived from the data. We did not develop subthemes because of the number of different theoretical approaches that we were interested in accommodating. Daniel Hind, James Parkin and Victoria Whitworth independently coded a sample of the transcripts (indexing), before conferring with each other with regard to the coded transcripts for items relating to the thematic framework (see Table 2). Daniel Hind also coded within the NPT166 and against items in the logic model. Further literature reviews were conducted to understand emergent themes (see Modelling process: developing programme theory). New frameworks were added to NVivo, where necessary transcripts were recoded and categories refined or merged. We summarised coded data using NVivo matrices, linked to the relevant quotations (charting). Completed charts (available on request) were compared within and between participants.
FIGURE 8.
Coding framework.
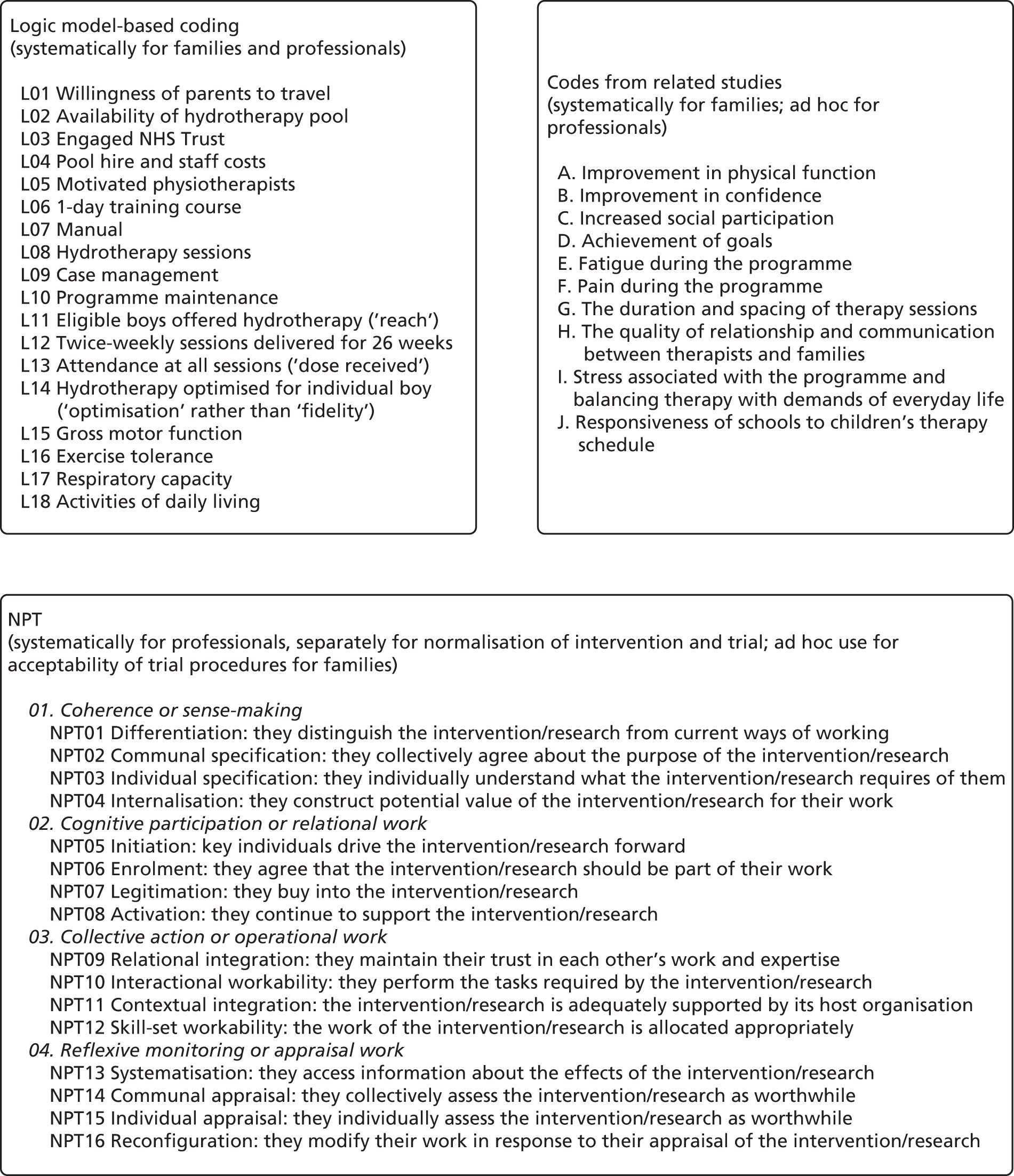
We noted if a participant held strong views on a subject and if there was considerable agreement or disagreement between participants. Emerging ideas were mapped out on paper to aid interpretation (mapping). In addition to the involvement of patient representatives, protection against the researcher’s own views and prejudices were minimised by involving a specialist physiotherapist (ES) in three discursive debriefing sessions in which we reviewed coding and discussed interpretations. We actively sought discrepant and divergent views to combat confirmatory bias and to avoid overly simplistic interpretations of phenomena. 177 Participants were not asked to provide feedback on the findings.
Reporting
Before the main results, we provide an exploration of context, which critical interpretivists define as more than the physical environment,178,179 factors, variables and causal relationships,180 but as something sociorelational, which incorporates settings, ‘roles, interactions and relationships’;178 ‘people’s social connectedness, their social locations, or their affinity with the intervention itself’;179 and conflicts, ‘perspectives, relationships, and trust’. 180 As proposed elsewhere,181 to add greater insight,182 participant quotations are sometimes supplemented with accounts from PPI representatives. We provide quotations for major and some minor themes.
The cost analysis substudy
As a result of the shortfall in participant recruitment, we had insufficient numbers to meet our original objectives of identifying key resource use items for a larger study and conducting a value-of-information analysis. We took the decision not to examine individual resource use items, as it would be difficult to establish whether a potential key resource item was just that or whether it was observed by chance in a small sample. In order to estimate the cost of AT intervention, we have supplemented information from the quantitative study with information from the qualitative interviews to estimate the costs of AT to the NHS as well as the costs borne by participants and their carers in participating in AT.
Triangulation protocol
Rationale
The rationale for using different methods and informants in this study, and a formal framework to compare their findings, was to address different aspects of the overall research question, making the study more comprehensive, increasing confidence in findings and providing a platform for feedback by professionals and patient representatives. 183
Design
Quantitative and qualitative methods described above were used concurrently, with no priority granted to either, to assess the feasibility of a research protocol and an intervention. We used a modified version of the protocol proposed by Farmer et al. 184 to compare quantitative and qualitative findings (methodological triangulation of data sets), with the following stages: sorting, convergence coding, convergence assessment, completeness assessment and feedback (researcher comparison was not undertaken because of resource constraints).
We reviewed the various data sets to identify key components of the intervention logic model (see Figure 6) to compare for presence and examples (‘sorting’). A convergence coding matrix summarised similarities and differences between data sets for each of the 17 logic model components in the Resources (n = 6), Activities (n = 3), Immediate outcomes (n = 4) and Intermediate outcomes (n = 4) categories. We compared the prominence of logic model components in the data sets, selecting examples to support or explain how each component had contributed to the success or failure of the intervention (‘convergence coding’). We applied the following convergence coding scheme: ‘agreement’ – full agreement between data sets in terms of the interpretation, partial agreement (some disagreement within or between either data sets), silence (only one set of results covers a logic mode component) and dissonance (disagreement between data sets). We quantified the level of agreement between the data sets (‘convergence assessment’) and highlighted differences in the contribution to the research question (‘completeness comparison’). We shared the triangulated results with team members and other selected stakeholders at a face-to-face meeting on 13 April 2016 for ‘feedback’, allowing points of disagreement to be discussed and changes in interpretation to be incorporated if supported by the data.
Chapter 3 Results of the pilot trial
Implementation of the intervention and trial
Implementation summary
Overall, 17 sites were approached to participate in the study: six sites opened and 11 were unable to do so. Of the six sites involved in the grant application, four opened between October 2014 and December 2014, one was unable to proceed because of difficulties securing treatment costs and another because of pool access. Ten sites approached between December 2014 and May 2015 either declined or were unable to gain the relevant approvals in time. Reasons for non-involvement included treatment costs, a lack of eligible participants within travelling range, lack of AT pool availability, organisational change (e.g. moving premises) and therapists being on maternity leave. In April 2015, two additional sites were initiated. The duration between site initiation and the first participant consent was 30–40 days for two sites, 50–60 days for another two sites, 90 days for one site and 169 days for another site.
NHS treatment costs as a cause of centre attrition
Considerable problems were encountered in accessing treatment costs for the research. Where the cost of an experimental treatment is greater than the cost of usual care, in the UK this cost falls on health-care commissioners rather than grant-awarding bodies. 185 Although at the time at which the trial was run most NHS commissioning was devolved, local commissioners recognised that the commissioning of services for rare disease groups was the responsibility of NHS England, an executive non-departmental public body of the Department of Health, established in 2013. However, NHS England responded that they did not have a process in place to support the alignment of commissioning priorities and research needs and refused to meet the treatment costs. After negotiating for several months with local commissioners, one of the original sites withdrew from participation in the study because they could not meet the treatment costs for the trial locally [minutes, Trial Management Group (TMG), 19 January 2015]. Neither local commissioners nor the participating trusts effectively met the treatment costs at other trusts, with physiotherapy teams absorbing the costs within their units or, more usually, participating physiotherapists delivering the intervention and trial procedures in their own time (see Chapter 5, Therapist views of the service, Operational work and Chapter 5, Comments on the trial procedures, Operational work). This was possible only because of the low levels of recruitment and the goodwill of research enthusiasts in research-active trusts.
Specialist centres and distance from the target population
The use of highly specialist physiotherapists from tertiary centres to deliver the AT intervention proved problematic. The eligible patients who were approached for participation in a RCT, which could see them come into the centre twice per week, would have to travel for long distances. Eligible participants at Leeds lived as far afield as Hull and York; Great Ormond Street Hospital, London, drew its patients from as far as Watford and Guildford. At other centres, the majority of study candidates lived > 20 miles away. Unlike in a previous paediatric AT trial, in which usual care involved a 2-week inpatient stay in a specialist centre, at the beginning of which children could be randomised, the contemporary DMD treatment pathway was wholly based on outpatient visits. The team at Great Ormond Street Hospital planned to deliver the intervention not on their own premises, but at AT suites in east and north-west London, which were closer to, and more accessible for, the target population (minutes, TMG, 7 July 2014). However, on investigation, the costs proved prohibitive (minutes, TMG, 3 November 2014). The inability to reimburse travels costs for travel to intervention sessions caused some consternation (minutes, TMG, 7 July 2014).
The idea of subcontracting delivery of AT to therapists at satellite centres, nearer to participants’ homes was discussed (minutes, TMG, 7 July 2014, 19 January 2015). However, given that the population is small and geographically dispersed, we have had to contract with almost as many community trusts as participants would, not to mention agree treatment costs (see previous paragraph) for interventionist training and the delivery of the intervention. The team’s perception was that less research-active community trusts were less likely to tolerate implementation of the study and intervention without access to treatment costs. All trusts had intended to run AT as a group intervention with two or more children in the pool, something that was prevented by under-recruitment. All the trusts were limited to providing AT sessions in office hours because of staffing requirements for safety and evacuation procedures and lone-working policies (minutes, TMG, 3 November 2014).
Participant recruitment and the prohibition on co-enrolment
The general prohibition on co-enrolment of patients,186 especially those deemed to be vulnerable, considerably reduced the pool of candidates available to the study. For instance, at Great Ormond Street Hospital, the children with the best cardiac function tended to be already enrolled in the DMD Heart Protection Study (ISRCTN50395346), meaning that the potential sample could have poor external validity. In addition, there were concerns that many parents were anticipating the opening of well-advertised drug studies (NCT02383511, NCT02369731, NCT01957059, NCT01826474) and would withhold their children from the AT trial, or enrol and then drop out, in the hope of access to a disease-modifying drug therapy (minutes, TMG, 7 July 2014). Although the NIHR urged us to co-enrol patients who had already consented to drug trials (Emma Catlin, NIHR, 2013, personal communication), the research ethics committee refused our request to do so (Leslie Gelling, NRES Committee East of England-Cambridge South 2014, personal communication).
Problems with the delivery of land-based therapy
The commissioning brief required that LBT be ‘optimised’. Normally, community physiotherapists see families between once every 6 weeks and once every 6 months to give an exercise prescription (the minority of patients that are at special schools have therapists who come into school to deliver exercises two or three times per week). Specialist physiotherapists on the team felt that the delivery of LBT would be variable between participants in between seeing community physiotherapists. In routine practice, specialist physiotherapists can recommend problems to work on and specific techniques to community physiotherapists, but varying degrees of co-operation between specialist and community physiotherapists were reported. The reorganisation of services in 2013, when this research was commissioned, following the Health and Social Care Act 2012,187 resulted in complexity and a lack of uniformity of services previously hosted by primary care trusts, including community physiotherapy. 188 As we anticipated difficulties finding out with whom to engage and approach, in order to gain the appropriate NHS Research and Development permissions to implement the study, we asked physiotherapists at participating specialist centres to prescribe LBT for the duration of the trial (minutes, TMG, 15 September 2015). We then relied on parents to capture the weekly delivery of LBT on a log.
Problems with data collection
The ability to deliver the 6MWD, which requires a 30-m corridor,189 was a source of great anxiety in the set-up period. Some hospital sites, where the NSAA would normally have been conducted, did not have passageways of this length (minutes, TMG, 7 July 2014, 15 September 2014, 3 November 2014). It was also posited that, in trusts that were not research active, training would have to be given regarding the administration of the 6MWD (minutes, TMG, 15 September 2014). Although the costs of data capture and entry were adequately funded, this money did not translate into extra resource for the units in terms of a data entry clerk at any of the sites. Instead, relatively expensive physiotherapists ended up entering data, with the CTRU personnel team making on-site visits to help out as the trial advanced (minutes, TMG, 3 November 2014).
Recruitment and participant flow
The first site was initiated on 24 October 2014 (Figure 9). All sites were instructed to cease consenting new patients when recruitment closed on 30 June 2015, so that those consenting could be randomised by 31 July 2015 because of the need to take two NSAA scores 1 month apart (see Chapter 2, Participants). The first patient was consented on 23 December 2014 and randomised on 10 February 2015. The last patient was consented on 24 June 2015 and randomised on 17 July 2015. The trial ended as planned when the window for 6-month follow-up closed on 28 January 2016. In 40 centre-months we consented and randomised 12 participants (0.3 per centre-month), of whom 10 have 6-month follow-up data for the NSAA and between five and nine have 6-month follow-up data for other outcomes. The six sites screened 348 boys for eligibility, of whom only 17 were interested and eligible (Figure 10). Thirteen were formally screened and consented (32.5% of the recruitment target, n = 40), and 12 were randomised (30% of the recruitment target, n = 40). Eight participants were randomised to AT plus LBT and four to LBT alone.
FIGURE 9.
Number of participants randomised by month.
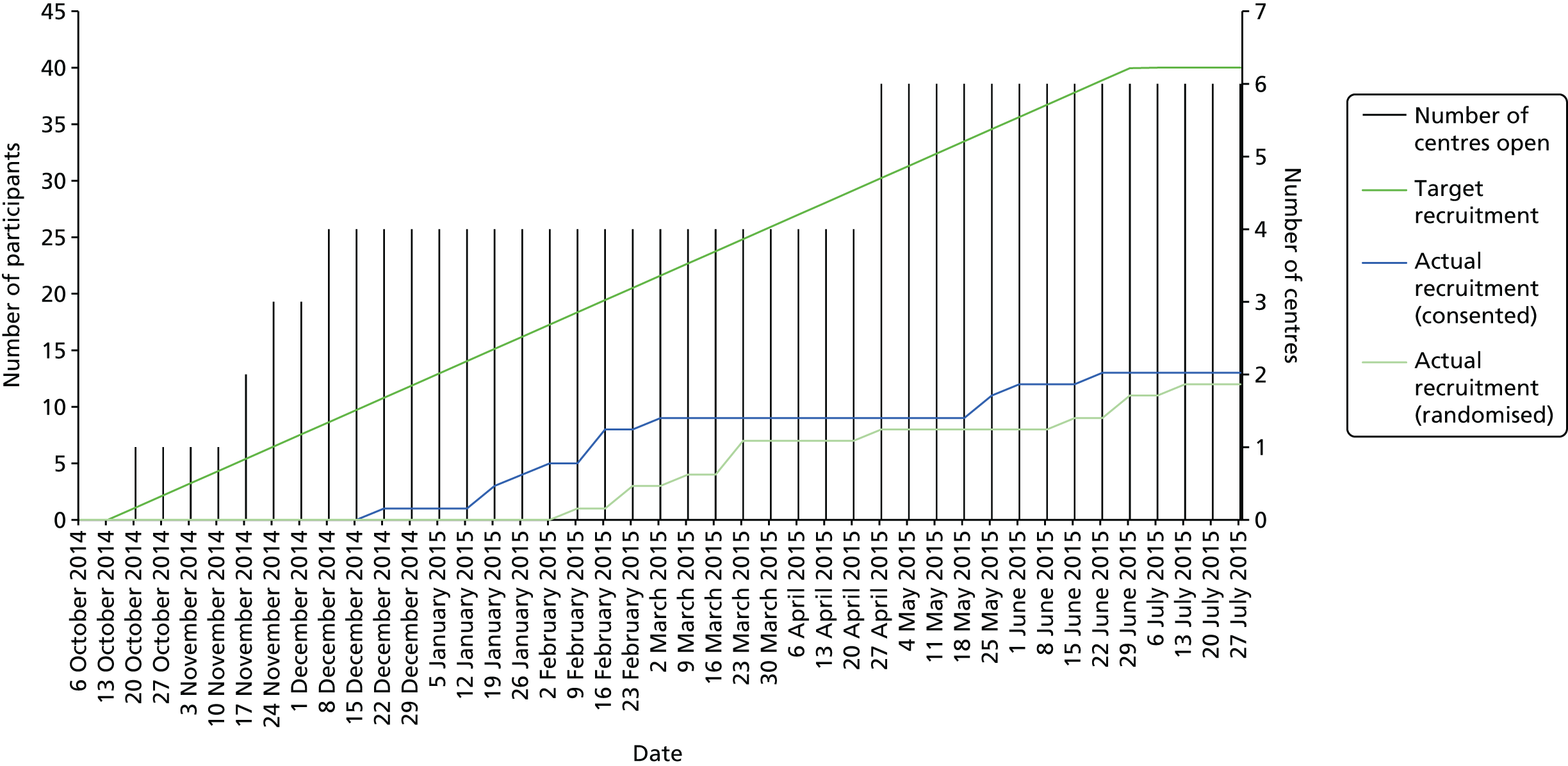
FIGURE 10.
Participant flow diagram.

Protocol non-compliances
Table 6 shows protocol non-compliances. In general, these were to do with the timing of assessments not being within acceptable ‘windows’. Issues with consent included getting verbal but not written assent at the same time as consent and using superseded versions of consent/assent forms.
| Site | Event date | Category | Class | Date reported to sponsor | Corrective action | Preventative action |
|---|---|---|---|---|---|---|
| R02 | 16 March 2015 | Consent | Minor | 01 July 2015 | NA | NA |
| R07 | 24 July 2015 | MW | Minor | 28 July 2015 | Other | NA |
| R05 | 28 January 2015 | MW | Minor | Not reported | Other | NA |
| R05 | 24 February 2015 | MW | Minor | Not reported | Other | NA |
| R01 | 22 January 2015 | Other | Major | 02 April 2015 | Other | Staff training |
| R01 | 28 April 2015 | MW | Minor | 28 October 2015 | NA | NA |
| R06 | 7 September 2015 | MW | Minor | 28 October 2015 | NA | NA |
| R01 | 23 July 2015 | Consent | Major | 28 July 2015 | Other | NA |
| R04 | 10 August 2015 | Other | Major | 18 August 2015 | Other | NA |
| R05 | 11 September 2015 | MW | Minor | 11 September 2015 | NA | NA |
| R01 | 9 October 2015 | MW | Minor | 13 October 2015 | NA | NA |
| R02 | 9 October 015 | MW | Minor | 13 October 2015 | NA | NA |
| R01 | 28 April 2015 | MW | Minor | 28 October 2015 | NA | NA |
| R05 | 14 October 2015 | Other | Major | 28 October 2015 | Other | Other |
| R02 | 12 October 2015 | Other | Major | 28 October 2015 | Other | Other |
| R05 | 3 November 2015 | MW | Minor | 17 November 2015 | NA | NA |
| R04 | 6 July 2015 | MW | Minor | 30 November 2015 | NA | NA |
| R01 | 13 November 2015 | MW | Minor | 11 December 2015 | NA | NA |
| R04 | 28 January 2016 | MW | Minor | 29 January 2016 | NA | NA |
Losses and exclusions after randomisation
Table 7 shows the pilot trial completion rate by site. One of the 13 boys for whom the study team received parental consent withdrew from the study before randomisation; his family could not be contacted to confirm the reason, which was, according to a physiotherapist, to enter a drug industry trial. Two participants formally withdrew from the study before completing, both from the control arm: one gave the reason as ‘burden of attending the trial procedure for child’ (R06/001), the other was ‘accepted onto another trial’ (R01/001). One participant was lost to follow-up in the control arm (R07/002). No patients were excluded by the study team.
| Site | Date initiated | Consented | Randomised | 6-month visit (completed) | Withdrew consent | Lost to follow-up | Other withdrawn |
|---|---|---|---|---|---|---|---|
| R01 | 24 October 2014 | 5 | 5 | 4 | 1 | 0 | 0 |
| R02 | 27 November 2014 | 2 | 2 | 2 | 0 | 0 | 0 |
| R04 | 11 December 2014 | 1 | 1 | 1 | 0 | 0 | 0 |
| R05 | 19 November 2014 | 2 | 1 | 1 | 0 | 0 | 1 |
| R06 | 28 April 2015 | 1 | 1 | 0 | 1 | 0 | 0 |
| R07 | 29 April 2015 | 2 | 2 | 1 | 0 | 1 | 0 |
Baseline data
Demographic and social and schooling information for randomised participants is displayed in Tables 8 and 9, respectively.
| Characteristic | Control | Intervention | Total |
|---|---|---|---|
| Age | |||
| n | 4 | 8 | 12 |
| Mean (SD) | 9.8 (2.5) | 8.0 (0.9) | 8.6 (1.7) |
| Median (IQR) | 9.5 (8.0–11.5) | 8.0 (7.5–8.0) | 8.0 (7.5–9.5) |
| Min., max. | 7, 13 | 7, 10 | 7, 13 |
| Ethnicity, n (%) | |||
| English/Welsh/Scottish/Northern Irish/British | 3 (75.0) | 2 (25.0) | 5 (41.7) |
| Any other white background | 1 (25.0) | 2 (25.0) | 3 (25.0) |
| Indian | 0 | 1 (12.5) | 1 (8.3) |
| Any other Asian background | 0 | 2 (25.0) | 2 (16.7) |
| Any other mixed/multiple ethnic background | 0 | 1 (12.5) | 1 (8.3) |
| Other (specify) | |||
| Korean | 0 | 1 | 1 |
| Filipino | 0 | 1 | 1 |
| Polish | 1 | 2 | 3 |
| Weight (kg) | |||
| n | 2 | 5 | 7 |
| Mean (SD) | 25.550 (2.616) | 26.480 (4.572) | 26.214 (3.910) |
| Median (IQR) | 25.550 (23.700–27.400) | 26.500 (23.800–26.600) | 26.500 (23.700–27.400) |
| Min., max. | 23.70, 27.40 | 21.70, 33.80 | 21.70, 33.80 |
| Height (cm) | |||
| n | 2 | 5 | 7 |
| Mean (SD) | 117.000 (0.849) | 119.960 (6.280) | 119.114 (5.339) |
| Median (IQR) | 117.000 (116.400–117.600) | 121.000 (114.600–121.200) | 117.600 (114.600–121.200) |
| Min., max. | 116.40, 117.60 | 113.70, 129.30 | 113.70, 129.30 |
| Characteristic | Control, n (%) | Intervention, n (%) | Total, n (%) |
|---|---|---|---|
| Housing adaptions | |||
| n/a at present | 1 (25.0) | 0 | 1 (8.3) |
| Pending | 1 (25.0) | 3 (37.5) | 4 (33.3) |
| In process | 0 | 2 (25.0) | 2 (16.7) |
| Completed | 2 (50.0) | 2 (25.0) | 4 (33.3) |
| Educational statement of needs | |||
| No | 0 | 4 (50.0) | 4 (33.3) |
| Yes | 4 (100.0) | 4 (50.0) | 8 (66.7) |
| Substantial learning difficulties | |||
| No | 4 (100.0) | 7 (87.5) | 11 (91.7) |
| Do not know | 0 | 1 (12.5) | 1 (8.3) |
| Level of educational support needed | |||
| Mainstream: no dedicated | 0 | 5 (62.5) | 5 (41.7) |
| Mainstream: > 50% support | 2 (50.0) | 1 (12.5) | 3 (25.0) |
| Mainstream: < 50% support | 2 (50.0) | 1 (12.5) | 3 (25.0) |
| Special school | 0 | 1 (12.5) | 1 (8.3) |
Feasibility outcomes
Owing to reporting guidelines, several protocol-specified feasibility outcomes sit more comfortably elsewhere in the report. For information on eligible patients approached for the study, see Recruitment and participant flow. For reasons for refused consent and participant attrition rate, see Losses and exclusions after randomisation. Reasons for attrition from the research protocol are detailed in Losses and exclusions after randomisation. Participant and parent views on the feasibility and acceptability of the intervention can be found in Chapter 5, Context understood through the International Classification of Functioning, Disability and Health – Child and Youth version and burden of treatment theory and Patient and parent views of the aquatic therapy intervention; those of the therapists can be found in Chapter 5, Therapist views of the service analysed within normalisation process theory. Participant, parent and therapist views on the acceptability and feasibility of the research protocol can be found in Chapter 5, Comments on the trial procedures. We did not consult, as originally stated in the protocol, therapists on the risk of control arm contamination after understanding how technical, and therefore impossible to replicate without training, the intervention was. The feasibility of recruiting participating centres is addressed in Implementation of the intervention and trial and the estimation of costs is addressed in Chapter 6. Finally, intervention optimisation (in place of intervention fidelity) is addressed in Chapter 4.
Decision on the primary end point and sample size for a full-scale trial
Although the 6MWD is the most popular primary outcome in drug trials for ambulant children with DMD, the study raised concerns about its feasibility (see Problems with data collection). Such concerns would be magnified if, as seems likely, any future study involved community trusts, which are more likely to lack the necessary 30-m corridors for the shuttle walk or staff already trained in assessment. For this reason, the NSAA, which is routinely collected for all boys with DMD in the UK, seems like the most feasible outcome for any future full-scale trial. Not only could data collection costs be minimised through its use, but, given the small number of DMD patients available, it is essential that we minimise any loss of information – especially in the control arm – as a result of patient attrition.
Table 10 lists the maximum sample sizes and expected sample sizes on termination required by three-stage ρ-family error spending tests of H0. We fix the power to detect a minimum important difference of 9 points at 0.8, and take the response SD to be 15 points, which looks sensible given the results of Mayhew et al. 190 We consider designs for a range of values for the type I error rate; given the small sample sizes available, we may be willing to conduct a trial at higher than conventional significance levels, acknowledging the increased risk of a false-positive conclusion when interpreting the trial results.
| Significance level | Fixed sample sizea | Maximum sample sizea | Expected sample size on termination | |||
|---|---|---|---|---|---|---|
| θ = 0 | θ = δ/2 | θ = δ | θ = 3δ/2 | |||
| ρ = 1 | ||||||
| α = 0.025 | 87.2 | 104.7 | 58.5 | 74.1 | 73.2 | 57.9 |
| α = 0.05 | 68.7 | 82.9 | 50.5 | 60.2 | 58.3 | 47.8 |
| α = 0.1 | 50.1 | 60.7 | 41.0 | 45.6 | 43.7 | 37.5 |
| ρ = 2 | ||||||
| α = 0.025 | 87.2 | 93.3 | 62.1 | 75.9 | 74.4 | 60.3 |
| α = 0.05 | 68.7 | 73.5 | 53.0 | 61.2 | 59.3 | 49.8 |
| α = 0.1 | 50.1 | 53.5 | 42.2 | 46.0 | 44.3 | 38.9 |
To interpret the numbers listed in Table 10, please note that once a patient enters the trial, there will be a 6-month delay before their primary response to treatment can be measured. As a result of this delay, when the trial is conducted, at each interim analysis there will be patients in the pipeline who have not yet been followed up for their 6-month response. Conservatively, this external pilot indicates that a future trial might recruit up to 16 boys per year, which implies that approximately eight boys would be in the pipeline at an interim analysis. The standard group sequential designs summarised in Table 10 make stopping decisions using only those data available at an interim analysis. However, the expected sample sizes listed are the expected numbers of patients recruited on termination, incorporating those who are in the pipeline when a stopping decision is made.
From Table 10 we see that the maximum sample sizes needed to conduct a definitive trial with a conventional type I error rate of α = 0.025 are likely to be prohibitive in the context of a national UK trial, which could recruit up to 16 boys with DMD per year. At this rate of recruitment, it would take > 6 years to reach the maximum sample size in the absence of early stopping. If we relax the type I error constraint to test at the 10% significance level, we would expect to take around 2.5 years to come to a conclusion and, in the absence of early stopping, just under 4 years to recruit the maximum sample size.
Table 11 summarises the findings of a simulation study, listing the percentage of trials satisfying the proposed success criterion for various sample sizes when prior distributions are as previously defined. The frequentist type I error rate at θ = 0 is approximately 10%, which is much higher than the conventional 2.5% significance level permitted for one-sided tests of superiority. Under the assumptions of the simulation study, we estimate that a future Bayesian trial would have frequentist power of ≥ 0.7 to detect a clinically relevant treatment effect if ≥ 40 patients could be recruited and followed up for their primary response at 6 months.
| θ | Percentage of trials declaring E as superior to | |||
|---|---|---|---|---|
| n = 20 | n = 30 | n = 40 | n = 60 | |
| θ = 0 | 9.99 | 10.41 | 10.51 | 9.99 |
| θ = δ/2 | 26.79 | 33.04 | 37.63 | 39.32 |
| θ = δ | 51.61 | 64.47 | 73.15 | 74.87 |
| θ = 3δ/2 | 75.23 | 88.08 | 93.98 | 93.46 |
Results are based on 10,000 simulations. N is the total sample size divided equally between interventions. Data are simulated according to the model θ^ ∼ N(θ, 4σ2/N) and s2 ∼ (σ2/N) χN − 22 setting σ = 15 and δ = 9.
Delivery and receipt of the aquatic therapy and land-based therapy interventions
If eight participants allocated to AT attended all 52 AT sessions, there would be 416 session reports. In fact, several participants did not have their first AT session until some time after randomisation (Table 12). The median time between randomisation and commencement of AT was 47 days (range 7–211 days); the mean was 63 days. As a result, not all 416 sessions were possible, especially for those randomised late in the project because the 6-month assessment is anchored to the randomisation date. Of the 349 scheduled sessions for which we have data, 203 (58.2%) expected sessions took place and 146 (41.8%) did not. Reasons for aggregate non-attendance are reported in Table 13 (for individual non-attendance, see Chapter 4, Attendance). Where reasons for session cancellation were discernible (10% of sessions were unaccounted for), there was an even split between participant/family factors (43%) and health-care provider factors (47%).
| Participant | Days between randomisation and intervention | Number of sessions done (by AT log entered) (n/N) | Number of sessions done (by attendance log) (n/N) |
|---|---|---|---|
| 1 | 80 | 29/52 | 29/52 |
| 2 | 80 | 29/52 | 29/52 |
| 3 | 48 | 29/52 | 30/52 |
| 4 | 21 | 23/52 | 23/52 |
| 5 | 7 | 18/52 | 18/52 |
| 6 | 46 | 28/52 | 28/52 |
| 7 | 211 | 16/52 | 16/52 |
| 8 | 11 | 30/52 | 30/52 |
| Process outcome | Centre number | Total | ||||
|---|---|---|---|---|---|---|
| R001 | R002 | R004 | R005 | R007 | ||
| Hydrosession counts | ||||||
| n (number of participants) | 3 | 2 | 1 | 1 | 1 | 8 |
| Mean (SD) | 29.3 (0.6) | 20.5 (3.5) | 28.0 | 16.0 | 30.0 | 25.4 (5.7) |
| Median (IQR) | 29.0 (29.0–30.0) | 20.5 (18.0–23.0) | 28.0 (28.0–28.0) | 16.0 (16.0–16.0) | 30.0 (30.0–30.0) | 28.5 (20.5–29.5) |
| Min., max. | 29, 30 | 18, 23 | 28, 28 | 16, 16 | 30, 30 | 16, 30 |
| Did the session occur?, n (%) | ||||||
| No | 47 (30.1) | 63 (60.6) | 10 (19.2) | 12 (23.1) | 14 (26.9) | 146 (35.1) |
| Yes | 88 (56.4) | 41 (39.4) | 28 (53.8) | 16 (30.8) | 30 (57.7) | 203 (48.8) |
| Missing | 21 (13.5) | 0 | 14 (26.9) | 24 (46.2) | 8 (15.4) | 67 (16.1) |
| Reason for non-attendance, n (%) | ||||||
| DNA | 2 (1.3) | 6 (5.8) | 0 | 1 (1.9) | 4 (7.7) | 13 (3.1) |
| UTA | 12 (7.7) | 27 (26.0) | 5 (9.6) | 3 (5.8) | 3 (5.8) | 50 (12.0) |
| Pool closed | 3 (1.9) | 27 (26.0) | 1 (1.9) | 0 | 5 (9.6) | 36 (8.7) |
| Staff unavailable | 24 (15.4) | 0 | 4 (7.7) | 6 (11.5) | 1 (1.9) | 35 (8.4) |
| Other | 3 (1.9) | 2 (1.9) | 0 | 2 (3.8) | 1 (1.9) | 8 (1.9) |
| Bank holiday | 3 | 1 | 0 | 0 | 0 | 4 |
| Dental work | 0 | 1 | 0 | 0 | 0 | 1 |
| Forgot swimming trunks | 0 | 0 | 0 | 2 | 0 | 2 |
| Patient unwell | 0 | 0 | 0 | 0 | 1 | 1 |
Of the 12 participants who were randomised, only five returned any LBT data, and for one of those there was only 1 week’s worth of data (Table 14). The other four participants returned more or less full sets of data. The median duration between randomisation and the first date on a LBT parent-completed data collection form was 25 days (range 11–52 days); the mean was 28 days. The LBT adherence data completion summary is shown in Table 15.
| Participant | Group | Days between randomisation and LBT | Number of weeks for which data returned |
|---|---|---|---|
| 1 | AT | 41 | 27/26a |
| 2 | AT | 25 | 24/26 |
| 3 | AT | 12 | 24/26 |
| 4 | LBT | 52 | 1/26 |
| 5 | AT | 11 | 20/26 |
| Process outcome | Control | Intervention | Total |
|---|---|---|---|
| Land-based session count | |||
| n (number of participants) | 1 | 4 | 5 |
| Number of weekly forms submitted | |||
| Mean (SD) | 1.0 | 23.8 (2.9) | 19.2 (10.5) |
| Median (IQR) | 1.0 (1.0–1.0) | 24.0 (22.0–25.5) | 24.0 (20.0–24.0) |
| Min., max. | 1, 1 | 20, 27 | 1, 27 |
Number of missing values/incomplete cases
Data completeness is described in Tables 16 and 17.
| Instrument | Follow-up time point | Control (N = 4), n (%) | Intervention (N = 8), n (%) | Overall (N = 12), n (%) |
|---|---|---|---|---|
| NSAA score | Consent | 4 (100) | 8 (100) | 12 (100) |
| Baseline | 4 (100) | 8 (100) | 12 (100) | |
| 6 months | 2 (100) | 8 (100) | 10 (100) | |
| FVC absolute | Consent | 4 (100) | 7 (88) | 11 (92) |
| Baseline | 2 (50) | 5 (63) | 7 (58) | |
| 6 months | 0 (0) | 5 (63) | 5 (56) | |
| FVC % predicted for height | Consent | 2 (50) | 4 (50) | 6 (50) |
| Baseline | 2 (50) | 5 (63) | 7 (58) | |
| 6 months | 0 (0) | 5 (63) | 5 (56) | |
| 6MWD | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 4 (100) | 8 (100) | 12 (100) | |
| 6 months | 1 (100) | 8 (100) | 9 (100) | |
| CHU-9D utility value | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 3 (75) | 8 (100) | 11 (92) | |
| 6 months | 1 (100) | 8 (100) | 9 (100) | |
| CarerQoL score | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 3 (75) | 7 (88) | 10 (83) | |
| 6 months | 1 (100) | 7 (88) | 8 (89) | |
| CarerQoL happy VAS | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 3 (75) | 8 (100) | 11 (92) | |
| 6 months | 1 (100) | 8 (100) | 9 (100) | |
| ACTIVLIM patient score | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 3 (75) | 8 (100) | 11 (92) | |
| 6 months | 1 (100) | 8 (100) | 9 (100) | |
| ACTIVLIM patient measure | Consent | 0 (0) | 0 (0) | 0 (0) |
| Baseline | 3 (75) | 8 (100) | 11 (92) | |
| 6 months | 1 (100) | 8 (100) | 9 (100) |
| Scoring | Follow-up time point | Control | Intervention | Overall | |||
|---|---|---|---|---|---|---|---|
| Min.–max. | Median | Min.–max. | Median | Min.–max. | Median | ||
| NSAA score | Consent | 100–100 | 100 | 100–100 | 100 | 100–100 | 100 |
| Baseline | 100–100 | 100 | 100–100 | 100 | 100–100 | 100 | |
| 6 months | 100–100 | 100 | 100–100 | 100 | 100–100 | 100 | |
| CHU-9D | Baseline | 100–100 | 100 | 100–100 | 100 | 100–100 | 100 |
| 6 months | 100–100 | 100 | 100–100 | 100 | 100–100 | 100 | |
| ACTIVLIM | Baseline | 81.82–100 | 100 | 63.64–100 | 95.45 | 63.64–100 | 100 |
| 6 months | 81.82–81.82 | 81.82 | 72.73–100 | 81.82 | 72.73–100 | 81.82 | |
| CarerQoL | Baseline | 100–100 | 100 | 85.71–100 | 100 | 85.71–100 | 100 |
| 6 months | 100–100 | 100 | 71.43–100 | 100 | 71.43–100 | 100 | |
Clinical outcomes and estimation
In the statistics that follow, a difference in means starting with a zero reflects a direction of effect that would favour AT, were the trial adequately powered; those starting with a ‘1’ would favour the control arm. Owing to control arm attrition, comparative statistics are presented for the NSAA only. NSAA measures of functional exercise capacity are shown in Figure 11, in which we include the observed average annual decline in function on the NSAA scale for UK boys diagnosed with DMD aged > 7 years, calculated as 3.7 units per year. 72 Therefore, over the study period of 6 months, the expected decline for the boys in our sample is estimated to be 1.85 units. On average, the 12 study participants who were randomised had a NSAA value of 24.75 and would be expected to have an estimated value at 6 months of 22.9 units. The mean score at 6 months was 21.0 (SD 15.6) in the control arm (n = 2) and 21.4 (SD 8.5) in the AT arm (n = 8), a difference of –0.38 (95% CI –17.95 to 17.2). The mean change score was –5.5 (SD 7.8) in the control arm and –2.8 (SD 4.1) in the AT arm, a difference of –2.8 (95% CI –11.3 to 5.8). The clinical outcomes are displayed in Table 18 and the change in 6MWD over 6 months is shown in Figure 12.
| Outcome measure | Follow-up | Control | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | Min.–max. | n | Mean (SD) | Median (IQR) | Min.–max. | ||
| 6MWD total distance | Baseline | 4 | 360 (84.98) | 362 (294.5–425.5) | 259–457 | 8 | 369.63 (78.39) | 376.5 (313.5–393) | 266–525 |
| 6 months | 1 | 255 | 255 (255–255) | 255–255 | 8 | 347.63 (81.88) | 369.5 (288–388.5) | 226–463 | |
| NSAA score | Consent | 4 | 25.75 (3.4) | 26.5 (23.5–28) | 21–29 | 8 | 23.38 (5.93) | 22.5 (18.5–29) | 16–31 |
| Baseline | 4 | 26 (4.55) | 25.5 (23–29) | 21–32 | 8 | 24.13 (5.49) | 23.5 (19.5–28.5) | 18–32 | |
| 6 months | 2 | 21 (15.56) | 21 (10–32) | 10–32 | 8 | 21.38 (8.47) | 21 (16–28) | 8–33 | |
| FVC absolute | Consent | 4 | 1.42 (0.21) | 1.43 (1.27–1.57) | 1.16–1.66 | 7 | 1.52 (0.2) | 1.5 (1.27–1.74) | 1.27–1.78 |
| Baseline | 2 | 1.29 (0.21) | 1.29 (1.14–1.43) | 1.14–1.43 | 5 | 1.34 (0.19) | 1.39 (1.3–1.48) | 1.03–1.5 | |
| 6 months | 0 | 5 | 1.33 (0.42) | 1.52 (1–1.6) | 0.77–1.76 | ||||
| FVC % predicted for height | Consent | 2 | 90.5 (9.19) | 90.5 (84–97) | 84–97 | 4 | 97.25 (11.62) | 97.5 (89–105.5) | 83–111 |
| Baseline | 2 | 88.5 (7.78) | 88.5 (83–94) | 83–94 | 5 | 90.8 (17.09) | 91 (79–101) | 70–113 | |
| 6 months | 0 | 5 | 83.8 (22.57) | 88 (68–93) | 56–114 | ||||
| CHU-9D utility value | Baseline | 3 | 0.92 (0.07) | 0.89 (0.87–1) | 0.87–1 | 8 | 0.77 (0.23) | 0.88 (0.59–0.94) | 0.39–0.96 |
| 6 months | 1 | 0.95 | 0.95 (0.95–0.95) | 0.95–0.95 | 8 | 0.87 (0.09) | 0.87 (0.82–0.95) | 0.71–1 | |
| CarerQol score | Baseline | 3 | 31.27 (10.37) | 26.2 (24.4–43.2) | 24.4–43.2 | 7 | 40.6 (22.9) | 29.3 (24.4–59.4) | 19.7–81.6 |
| 6 months | 1 | 50.1 | 50.1 (50.1–50.1) | 50.1–50.1 | 7 | 51.27 (6.78) | 50.1 (48.8–50.4) | 44–65.8 | |
| ACTIVLIM patient score | Baseline | 3 | 32.67 (9.71) | 35 (22–41) | 22–41 | 8 | 30.38 (7.58) | 32 (26.5–34) | 17–41 |
| 6 months | 1 | 21 | 21 (21–21) | 21–21 | 8 | 26.88 (6.36) | 26 (22.5–31.5) | 19–36 | |
| ACTIVLIM patient measure | Baseline | 3 | 2.98 (2.62) | 4.15 (–0.02–4.82) | –0.02–4.82 | 8 | 2.1 (1.37) | 2.22 (1.61–2.78) | –0.6 to 4.15 |
| 6 months | 1 | 0.18 | 0.18 (0.18–0.18) | 0.18–0.18 | 8 | 1.29 (1.13) | 1.48 (0.4–2.1) | –0.35 to 2.73 | |
FIGURE 12.
Change in 6MWD over 6 months.
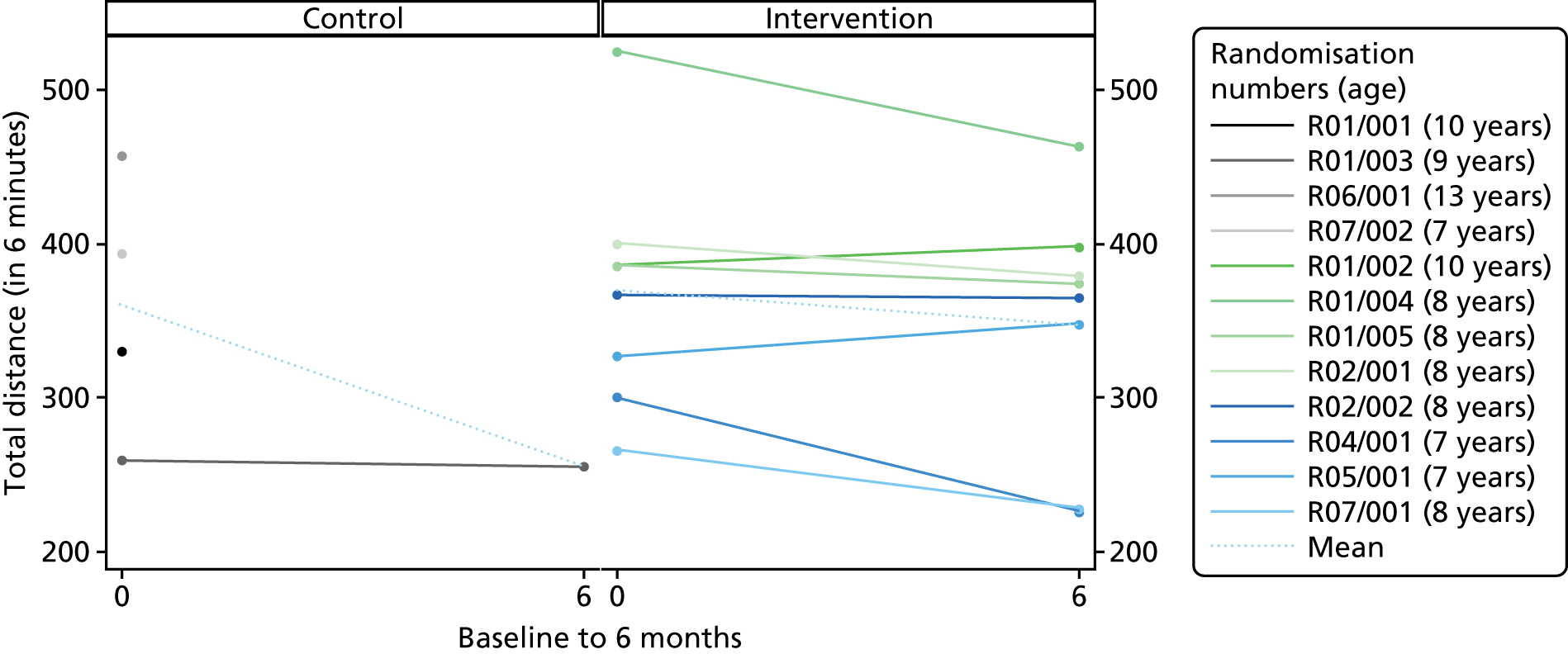
Figures 13 and 14 show the FVC absolute and FVC percentage predicted for height, respectively.
FIGURE 13.
Forced vital capacity absolute.

FIGURE 14.
Forced vital capacity percentage predicted for height.
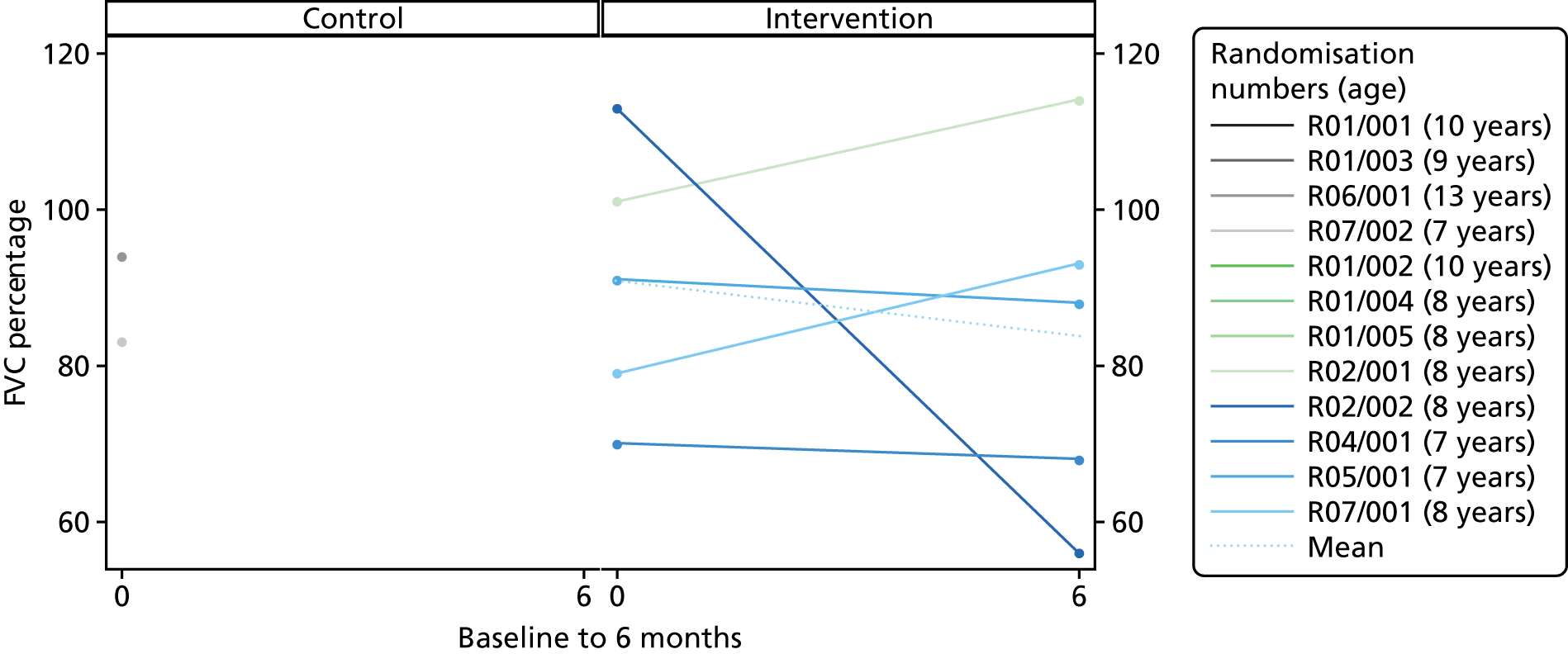
Figures 15 and 16 display the CHU-9D and CarerQol scores over 6 months, respectively.
FIGURE 15.
Child Health Utility 9D Index.

FIGURE 16.
Care-related quality of life.

Adverse events
A total of 15 adverse events were reported to the trial team (Table 19). The only event related to the intervention was delayed muscle soreness, which was expected. Of the rest, 10 were falls related, two were related to influenza immunisation and the remainder were related to chest infection and sleep hypoventilation. There were no serious adverse events. In addition, two parents reported back pain, which they attributed to home delivery of LBT exercise (see Chapter 5, Fatigue and pain). Post-AT pain, as measured on the Wong–Baker pain inventory, and fatigue, as measured on the Children’s OMNI Scale of perceived exertion, are addressed in Table 20.
| Randomisation group | Category | Details | Ongoing | Serious? | Intervention discontinued |
|---|---|---|---|---|---|
| Intervention | Other | Participant falls 2–3 times a week | Yes | No | No |
| Control | Other | Fell and sprained ankle resulting in visit to A&E. Had radiography, no fracture | No | No | No |
| Control | Other | Participant falls four times a week | Yes | No | No |
| Intervention | Other | Participant falls 15–20 times a week | Yes | No | No |
| Intervention | Other | Participant falls once a week | Yes | No | No |
| Intervention | Pain | Fell off slide, reported back pain, spinal radiography clear, self-resolved | No | No | No |
| Intervention | Other | Participant falls occasionally: two falls in past 6 months | Yes | No | No |
| Intervention | Acute infection | Chest infection | No | No | No |
| Intervention | Other | Symptoms of sleep hypoventilation – headaches and tiredness | Yes | No | No |
| Intervention | Other | Falls | Yes | No | No |
| Intervention | Other | Influenza immunisation | No | No | No |
| Intervention | Other | Falls daily | Yes | No | No |
| Intervention | Other | Influenza immunisation nasal spray given. Information shared as part of expected adverse events questions at visit 3 | No | No | No |
| Intervention | Other | Regular falls 2–3 times a week | Yes | No | No |
| Intervention | Other | Delayed-onset muscle soreness – as part of expected adverse events questions for visit 3 | Yes | No | No |
| ID | Scoring | Attendance status | Session counts | Before | After | Change | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Median (IQR) | Min.–max. | n | Mean (SD) | Median (IQR) | Min.–max. | n | Mean (SD) | Median (IQR) | Min.–max. | ||||
| R01/002 | Wong–Baker | Full | 28 | 21 | 0.43 (1.03) | 0 (0–0) | 0–4 | 21 | 0.38 (0.8) | 0 (0–0) | 0–2 | 21 | –0.05 (0.5) | 0 (0–0) | –2 to 1 |
| OMNI | Full | 28 | 10 | 2.5 (0.53) | 2.5 (2–3) | 2–3 | 27 | 2.44 (0.8) | 2 (2–3) | 1–4 | 10 | 0.5 (0.71) | 0 (0–1) | 0–2 | |
| R01/004 | Wong–Baker | Full | 29 | 21 | 0.1 (0.44) | 0 (0–0) | 0–2 | 21 | 0.1 (0.44) | 0 (0–0) | 0–2 | 21 | 0 (0) | 0 (0–0) | 0–0 |
| OMNI | Full | 29 | 10 | 2.7 (0.48) | 3 (2–3) | 2–3 | 28 | 2.43 (0.79) | 3 (2–3) | 1–4 | 10 | 0.3 (0.48) | 0 (0–1) | 0–1 | |
| R01/005 | Wong–Baker | Full | 29 | 28 | 0.04 (0.19) | 0 (0–0) | 0–1 | 28 | 0.04 (0.19) | 0 (0–0) | 0–1 | 28 | 0 (0) | 0 (0–0) | 0–0 |
| OMNI | Full | 29 | 10 | 0.9 (1.6) | 0 (0–1) | 0–5 | 28 | 0.75 (1.14) | 0 (0–1) | 0–5 | 10 | 0.1 (0.32) | 0 (0–0) | 0–1 | |
| R02/001 | Wong–Baker | Full | 17 | 16 | 0.06 (0.25) | 0 (0–0) | 0–1 | 16 | 0.19 (0.54) | 0 (0–0) | 0–2 | 16 | 0.13 (0.5) | 0 (0–0) | 0–2 |
| OMNI | Full | 17 | 15 | 0.2 (0.41) | 0 (0–0) | 0–1 | 16 | 3.13 (0.96) | 3 (3–4) | 1–5 | 15 | 3.07 (0.7) | 3 (3–4) | 2–4 | |
| R02/002 | Wong–Baker | Full | 16 | 16 | 0.25 (0.68) | 0 (0–0) | 0–2 | 16 | 1.06 (1.44) | 0 (0–2) | 0–4 | 16 | 0.81 (1.22) | 0 (0–2) | 0–4 |
| OMNI | Full | 16 | 14 | 0.5 (0.85) | 0 (0–1) | 0–2 | 16 | 1.88 (1.75) | 2 (0–2.5) | 0–6 | 14 | 1.07 (1.82) | 1.5 (0–2) | –2 to 4 | |
| R04/001 | Wong–Baker | Full | 28 | 28 | 0 (0) | 0 (0–0) | 0–0 | 26 | 0 (0) | 0 (0–0) | 0–0 | 26 | 0 (0) | 0 (0–0) | 0–0 |
| OMNI | Full | 28 | 0 | – | – | – | 28 | 0 (0) | 0 (0–0) | 0–0 | 0 | – | – | – | |
| R05/001 | Wong–Baker | Full | 15 | 15 | 0 (0) | 0 (0–0) | 0–0 | 15 | 0 (0) | 0 (0–0) | 0–0 | 15 | 0 (0) | 0 (0–0) | 0–0 |
| OMNI | Full | 15 | 2 | 0.5 (0.71) | 0.5 (0–1) | 0–1 | 15 | 0.33 (0.82) | 0 (0–0) | 0–3 | 2 | 1 (1.41) | 1 (0–2) | 0–2 | |
| R07/001 | Wong–Baker | Full | 26 | 26 | 1.65 (1.65) | 2 (0–2) | 0–5 | 26 | 2.65 (2) | 2 (2–4) | 0–7 | 26 | 1 (1.67) | 0.5 (0–2) | –2 to 4 |
| OMNI | Full | 26 | 8 | 4 (0.76) | 4 (3.5–4.5) | 3–5 | 26 | 3.85 (2.54) | 4 (2–5) | 0–8 | 8 | 2.38 (1.6) | 2 (1–3.5) | 1–5 | |
Sample size calculations for candidate future trials
We calculated a sample size calculation for a full-scale trial comparing optimised LBT versus LBT plus AT for boys with DMD. Based on feasibility data, we assumed that the primary end point would be the NSAA score at 6 months from randomisation (see Problems with data collection and Decision on the primary end point and sample size for a full-scale trial). The linearised version of this score was used with transformed scores lying between 0 and 100. 190 This is preferred to ensure that a unit change in score implies the same change in function across the breadth of the scale. We restricted our attention to frequentist and Bayesian designs for randomised designs. It may be difficult to learn about the effectiveness of AT from observational studies, as patients will receive varying background therapies of glucocorticoid steroids, which may influence disease progression. The designs were proposed under the simplifying assumption that linearised NSAA scores are approximately normally distributed. When performing sample size calculations, we take a minimum important treatment effect to be a 9-point change on the transformed NSAA scale.
Frequentist group sequential trial
In the following, we refer to optimised LBT and LBT plus AT as interventions C and E, respectively. We assume that transformed NSAA scores at 6 months would be analysed by fitting a general linear model adjusting for baseline NSAA score and other relevant baseline covariates. Therefore, the 6-month response of the ith patient would be modelled as:
where XEi is an indicator variable that takes the value 1 if patient i is randomised to the intervention, and 0 otherwise; XBi is the NSAA score at baseline; and εi is an independent random-error term with εi ∼ N(0, σ2). We interpret θ as the adjusted difference between expected transformed NSAA scores at 6 months on E versus C; positive values indicate that E is superior to C.
Our first proposal is to conduct a future trial according to a group sequential test of H0: θ ≤ 0 against H1: θ > 0 with type I error rate α at θ = 0 and type II error rate β at θ = δ. We take δ = 9 as the minimum important difference we wish to detect. We shall consider group sequential designs, which permit early stopping either for futility (i.e. to abandon a lost cause) or for success (i.e. to declare E superior to C). By testing the H0 group sequentially, we reduce the expected number of patients needed to conduct the trial, which is particularly desirable in this context when sample sizes are small. We consider one-sided, rather than two-sided, tests of null hypotheses as AT would be adopted in practice only if it can be shown to be superior to standard care.
We propose group sequential tests of H0 following error spending designs, so called because stopping rules are derived such that certain probabilities of making a type I or type II error are ‘spent’ at each interim analysis. The advantage of this type of design is that it can accommodate unpredictable group sizes, which are likely to occur if recruitment rates are unpredictable and Data Monitoring and Ethics Committee meetings are scheduled at fixed calendar times. We consider designs spending error probabilities according to the ρ-family of functions:
where t, 0 ≤ t ≤ 1, represents the fraction of the test’s maximum information level that has been accrued. Here, f and g stipulate the cumulative type I and type II error probabilities to be spent by the time information fraction t has been accrued. The error-spending parameter, ρ, governs how rapidly error probabilities are spent as a function of the statistical information available for θ: smaller values of ρ imply more aggressive stopping rules with greater opportunity for very early stopping.
Bayesian design
We could shift the aim of a future trial from reaching definitive conclusions on the relative merits of interventions E and C to increasing our understanding of these merits. A future trial would then proceed by recruiting as many patients as possible over a reasonable time frame; based on the HydroDMD pilot study, we believe that 32 patients could be recruited over 2 years. The accumulated data would then be analysed using Bayesian methods to quantify our current thinking about treatment benefits.
Before the future trial begins, the Bayesian approach would start with a thorough evaluation of what is already known about probable patient responses on interventions E and C. For simplicity, we shall take µE and µC to represent the average change from baseline to 6 months in the linearised NSAA score on interventions E and C, respectively. Furthermore, let σ2 denote the common variance of the change from baseline scores. We propose that our prior understanding of the relative merits of interventions E and C could be summarised by placing the following independent prior distributions on θ = µE – µC and σ:
The prior for θ summarises the findings of the HydroDMD pilot study: the mean is equal to the observed sample mean difference while we set the prior SD equal to twice the estimated standard error of the sample mean. This inflation is made in order to downweight the contribution of the pilot information to a future efficacy trial and reflects our uncertainty about the estimated standard error. An independent and vague prior is used for σ to reflect our uncertainty about the response variance. We could seek to incorporate into the stated prior distributions data from other relevant historical controlled trials; however, the natural history of DMD is known to have changed markedly in recent years with the introduction of glucocorticoid therapy and the standardisation of usual care (Ricotti et al. 72). Therefore, it is unlikely that the average responses seen in historical trials would be commensurate with those seen in contemporary studies.
The next step would be to conduct the Bayesian trial, recruiting as many patients as possible across a network of UK centres. Patients would be randomised in a 1 : 1 ratio between interventions E and C. On termination of the trial, once all recruited patients have been followed up for their 6-month outcome, the new trial data would be summarised by the pair of sufficient statistics for θ and σ, that is, the maximum likelihood estimate of θ and the sample variance. A Bayesian analysis would then be performed, using Bayes’ theorem to update the priors to derive posterior distributions incorporating the trial data. A provisional decision to introduce intervention E would be made if the posterior probability that θ > 0 exceeds 0.9.
Figures 17 and 18 show the OMNI after the session and change for each intervention participant, respectively.
FIGURE 17.
OMNI score after the session.

FIGURE 18.
OMNI score change.
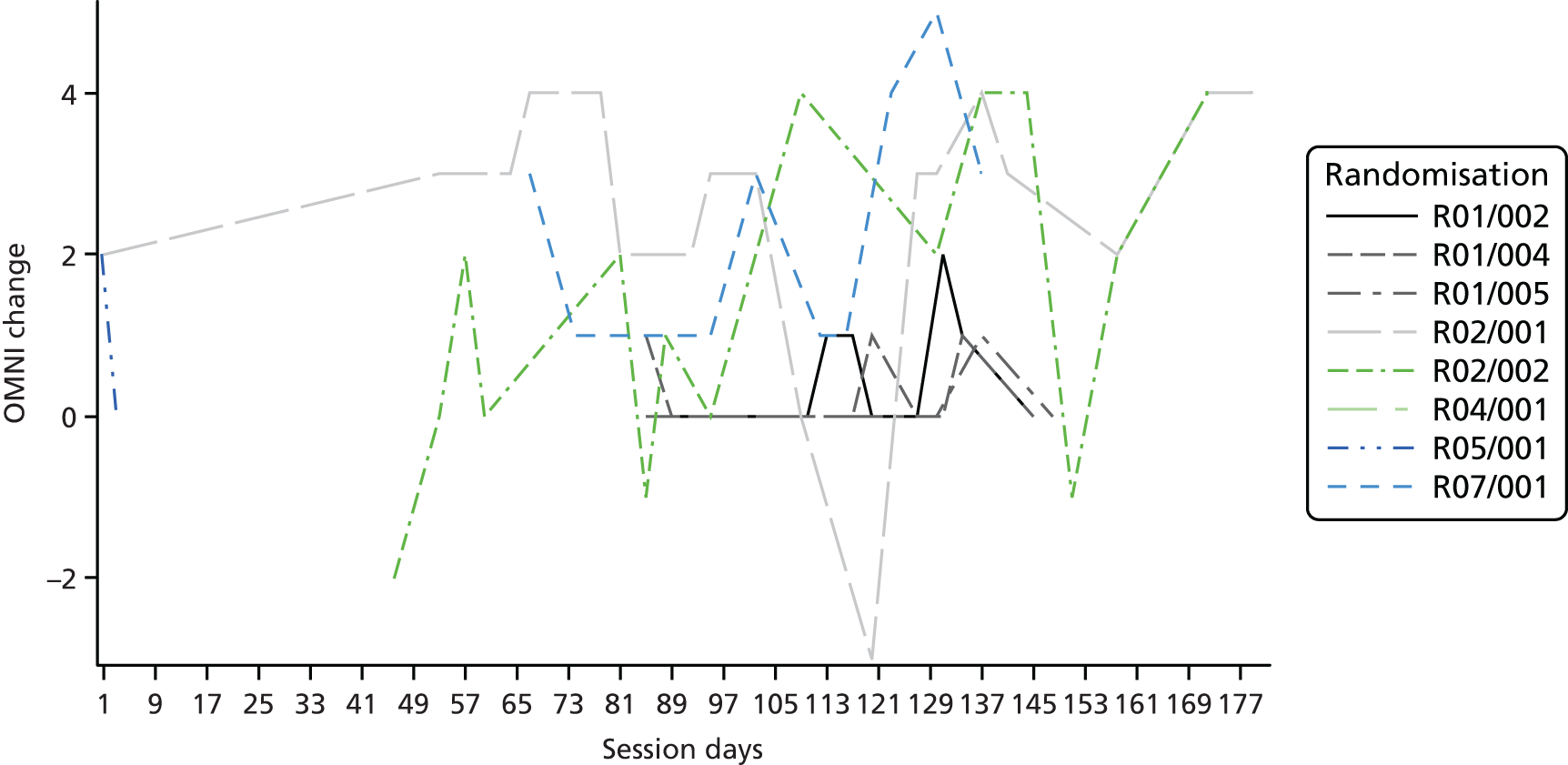
Figures 19 and 20 show the Wong–Baker visual analogue scale after the session and change for each intervention participant, respectively.
FIGURE 19.
Wong–Baker visual analogue scale after the session.
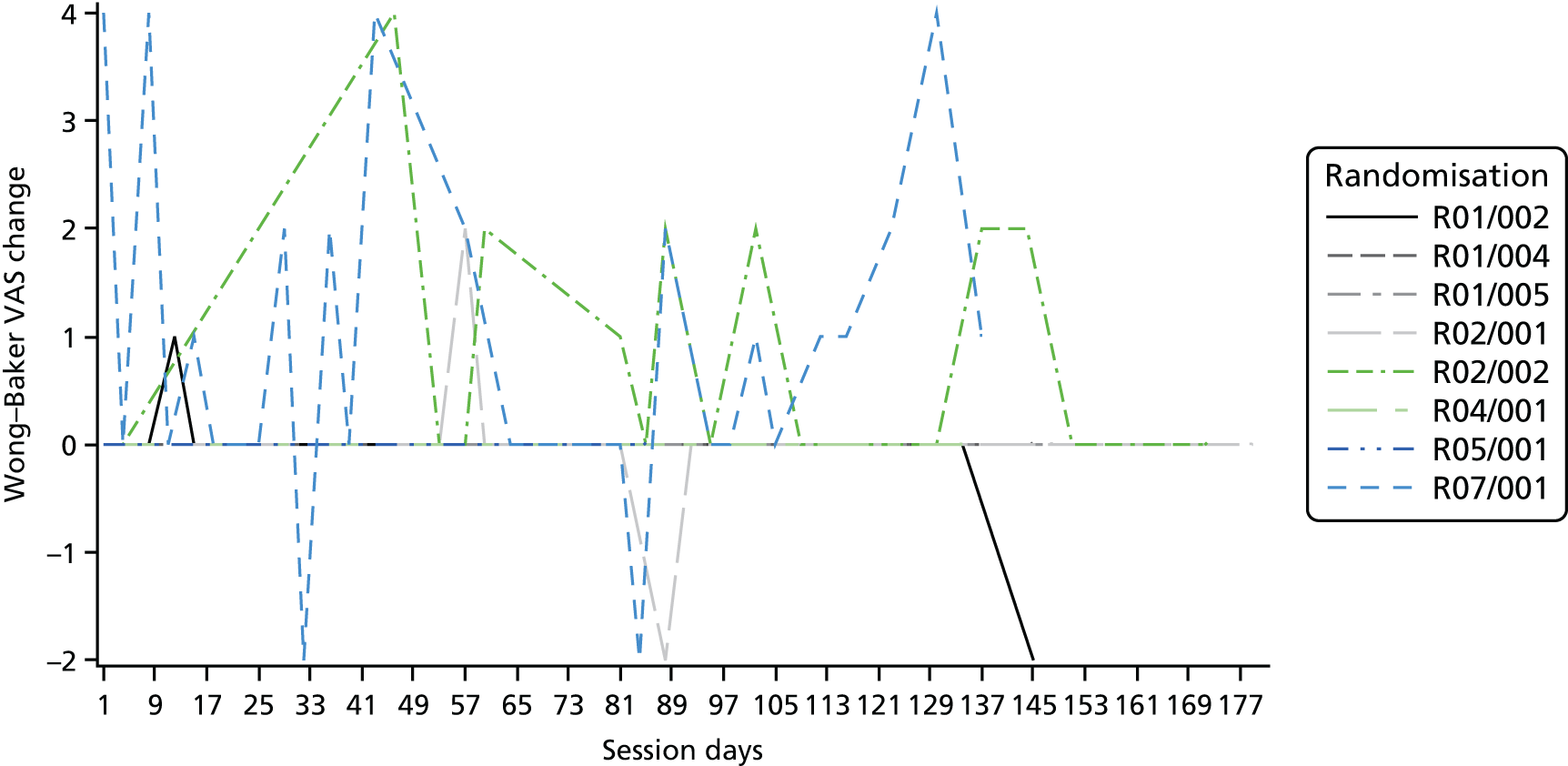
FIGURE 20.
Wong–Baker change.
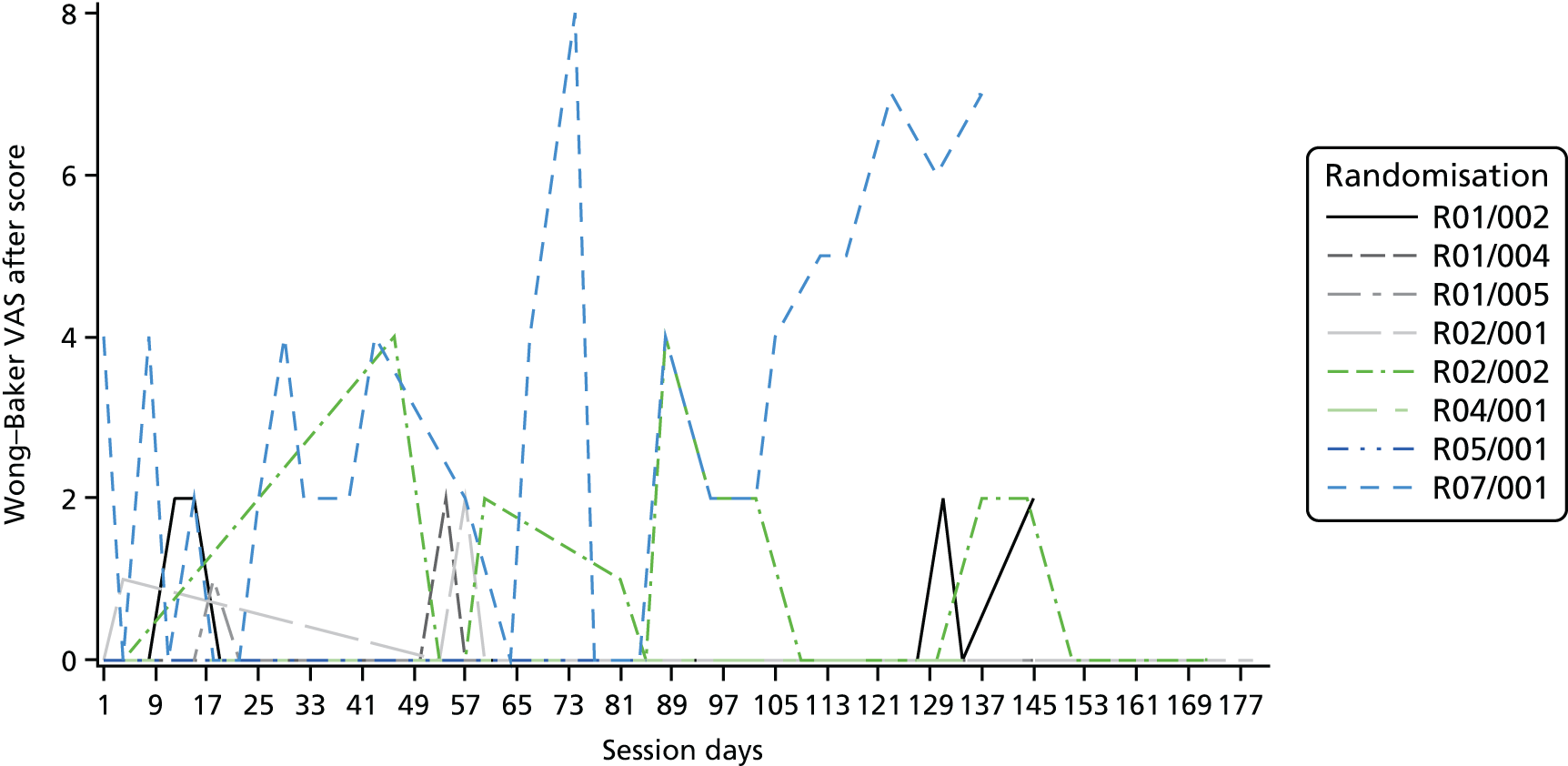
Chapter 4 Intervention optimisation study results
Introduction: characterisation of participant functional ability
Good-quality data on the optimisation of AT and LBT were available for only eight and four participants, respectively (Table 21); all were from the research arm, and control arm participants contributed no useful data. The mother of AT arm participants R01/002 and R01/004 and control arm participant R01/003 indicated that no LBT was delivered at home, but that some was delivered at school (see also Chapter 5, Environmental factors). The total number of AT sessions with data available ranged from 16 to 30 sessions per participant. The total number of LBT sessions with data available ranged from 20 to 27 sessions. The median total number of stretches, with data as a percentage of the total number of stretches prescribed, varied from 66.67% to 100% for participants (minimum 30.77%, maximum 100%). The mean total number of stretches with data as a percentage of the total number of stretches prescribed varied from 72.16% to 97.92% for participants.
| Participant | Total number of AT sessions with data | Total number of weeks with LBT data |
|---|---|---|
| R01/002 | 29 | 0 |
| R01/004 | 29 | 0 |
| R01/005 | 29 | 27 |
| R02/001 | 23 | 24 |
| R02/002 | 18 | 24 |
| R04/001 | 28 | 0 |
| R05/001 | 16 | 0 |
| R07/001 | 30 | 20 |
Aquatic therapy
Attendance
Aquatic therapy attendance (Table 22 and Figures 21 and 22) overall was assessed as good for six of the eight participants. The actual number of sessions attended by participants varied between 16 and 30 sessions overall, and the actual number of sessions not attended ranged between 10 and 34 sessions overall. Patient and pool factors for non-attendance were equal for three participants, pool factors were higher for three participants and patient factors were higher for two participants. Two participants had overall attendance of < 40%, one had overall attendance of between 40% and 50% and five participants had attendance levels of between 50% and 60%.
| Randomisation number | Actual sessions attended | Actual sessions not attended | Patient factors | Pool factors | Unknown | Available sessionsa | Per cent attendance based on available pool |
|---|---|---|---|---|---|---|---|
| R01/002 | 29 | 16 | 5 | 10 | 1 | 34 | 85 |
| R01/004 | 29 | 16 | 4 | 10 | 2 | 33 | 88 |
| R01/005 | 30 | 15 | 5 | 10 | 0 | 35 | 86 |
| R02/001 | 23 | 29 | 14 | 14 | 1 | 37 | 62 |
| R02/002 | 18 | 34 | 20 | 14 | 0 | 38 | 47 |
| R04/001 | 28 | 10 | 5 | 5 | 0 | 33 | 85 |
| R05/001 | 16 | 12 | 6 | 6 | 0 | 22 | 73 |
| R07/001 | 30 | 14 | 8 | 6 | 0 | 38 | 79 |
FIGURE 21.
Number of AT sessions completed.
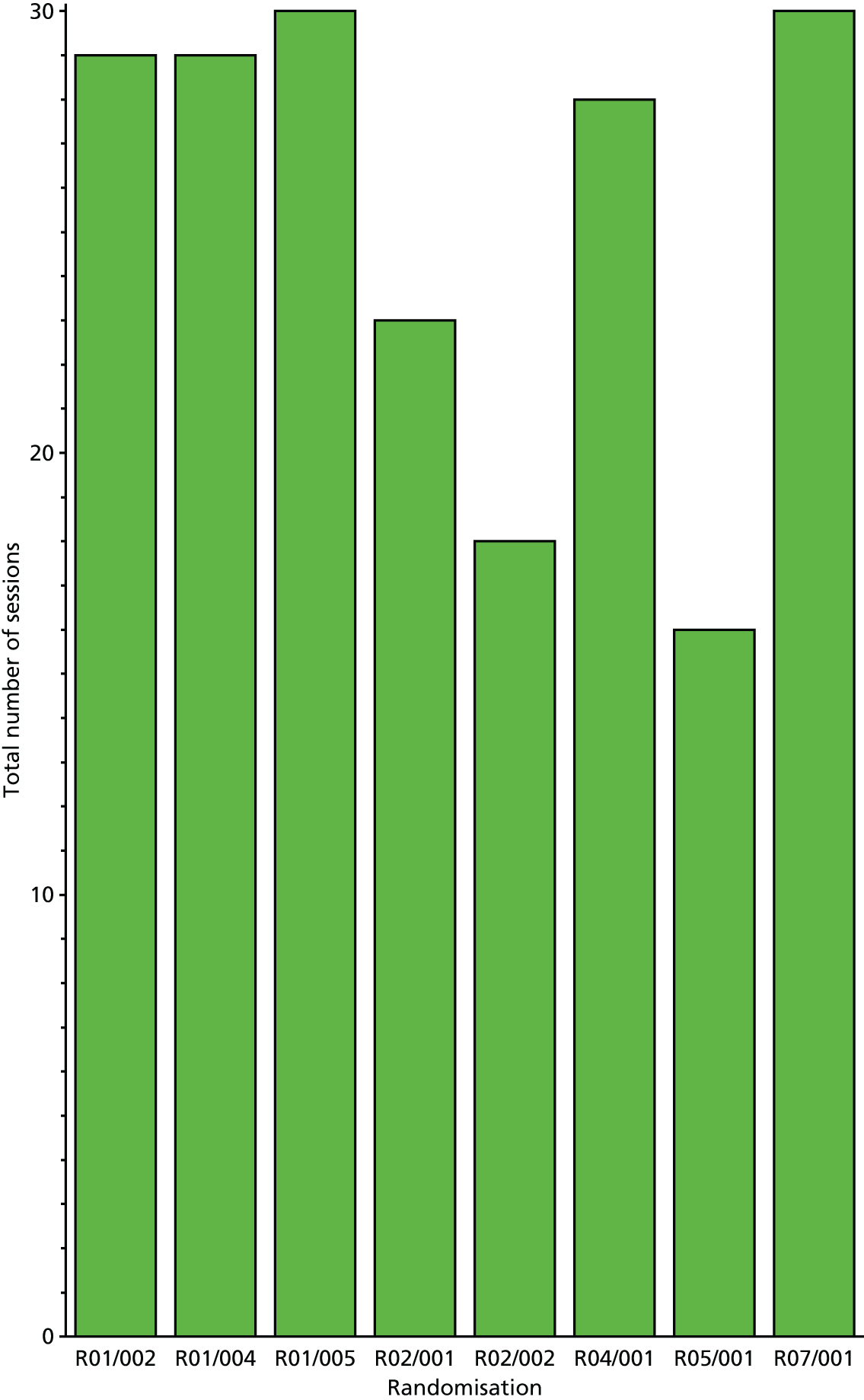
FIGURE 22.
Timing of AT sessions.

The aquatic therapy prescription
The NSAA score was assessed by the independent rater as above expected for age for three participants and below expected for five participants. Between the consent and baseline assessments (a gap of 1 month), five participants improved, one maintained function and two declined on the NSAA. Seven of the participants had contractures and all participants had a reduced range of movement (Table 23). Note the rapid decline in NSAA score for participants R04/001 and, in particular, R07/001, during the 6-month trial period.
| Randomisation number | NSAA score by age: above or below average9 | NSAA consent | NSAA baseline | NSAA 6 months | Baseline 6MWD (metres) | Contractures Y/N | Attendance | Quality of prescription (focused) | Achievable prescription? (Can exercises be done in a half-hour session) | Prescription (number of exercises prescribed per session) | Mean; median (range) compliance with prescription; independent rater’s subjective opinion on compliance | Non-prescribed exercises with no explanation (number of sessions) | Priority stretches prescribed? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R01/002 | Above | 25 | 27 | 26 | 386 | Y | Good | Poor | No | 27 | 30.99; 33.33 (14.81–48.15); poor | 0 | Partially – ankles but not hips |
| R01/004 | Above | 31 | 30 | 30 | 525 | Y | Good | Poor | No | 23 | 28.91; 29.17 (12.5–52.17); poor | 0 | Partially – hip extension (hip and trunk missing, and high emphasis on wrist unexplained) |
| R01/005 | Below | 16 | 18 | 17 | 386 | Y | Good | Poor | No | 22–24 | 38.63; 40.91 (8.7–68.18); poor | 6 | Yes – ankles |
| R02/001 | Above | 31 | 32 | 33 | 400 | Y | Poor | Good | Yes | 14–15 | 86.77; 92.86 (42.86–100); good | 10 | Yes – ankles |
| R02/002 | Below | 18 | 20 | 17 | 367 | Y | Poor | Good | Yes | 15 | 90; 93.33 (46.67–100); good | 2 | Yes – ankles |
| R04/001 | Below | 20 | 19 | 15 | 300 | Y | Good | Varied | No | 11–23 | 51.55; 41.21 (21.21–100); good | 10 | Yes – ankles |
| R05/001 | Above | 27 | 27 | 25 | 327 | N | Good | Varied | Yes | 4–10 | 49.76; 51.67 (26.67–63.64); good | 7 | Yes – ankles |
| R07/001 | Below | 19 | 20 | 8 | 266 | Y | Good | Good | Yes | 11 | 91.52; 100 (36.36–100); good | 0 | Partial – ankles but not ITBs |
The prescription overall was assessed as good for three participants, variable by session for two and poor for three participants. The independent assessor assessed the AT prescriptions as realistic and achievable for four of the eight participants. The prescription was assessed as unachievable for four participants at two centres. The independent assessor stated that the number of exercises prescribed per session ranged from 4 to 27 exercises across all participants who contributed data. In relation to overall prescription compliance, three participants were assessed as poor and five as having good levels of compliance. Overall prescription compliance was between 20% and 30% for one participant, 30% and 40% for two participants, 70% and 80% for two participants, and 80% and 90% for three participants. The prescription compliance per session ranged from 9% to 100% across participants. One physiotherapist, who was deemed to have optimised treatment based on the data given to the independent rater, commented that her participant (participant R07/001) encountered significant fatigue and dissatisfaction (see Chapter 5, Fatigue and pain and Appraisal work). She explained that, although the prescription remained the same, the actual number of exercise repetitions was adjusted depending on the participant’s fatigue, which the independent assessor agreed was appropriate.
Five of the eight participants had additional non-prescribed exercises completed during sessions with no explanation provided. The independent rater considered exercises not to be appropriately prioritised in three cases: with R01/002, she would have prioritised hips in addition to ankles; with R01/004 she would have prioritised more hip and trunk exercises, and fewer wrist exercises; with R07/001 she would have prioritised iliotibial band exercises in addition to ankles. The independent rater felt that too much emphasis was placed on wrist exercise in a number of prescriptions (R01/002, R01/005, R01/004 and R05/001). Although wrist stretching was appropriate, it did not require the properties of water and, with limited time, other exercises that do benefit from the properties of water (particularly lower limb exercises) could have been prioritised. The independent rater remarked that wrist exercises were placed first on the manual menu of exercises and that this may have led to overprescription.
Land-based therapy
The LBT prescription was assessed as appropriately focused and achievable at baseline for all four of the AT participants who returned the LBT pro forma, and compliance was initially good (Table 24). However, after intervention by the community physiotherapist, midway through the course, the number of exercises increased and compliance decreased (R01/005, R02/001 and R02/002). Across the course, the number of exercises prescribed ranged from 5 to 13 per day. Participant prescription compliance was generally good and compliance with priority stretches did not fall below 85% for this participant (R02/001).
| Randomisation number | NSAA score by age: above or below average | NSAA consent | NSAA baseline | NSAA 6 months | Baseline 6MWD (metres) | Contractures Y/N | Prescription (good varied, poor) | Realistic prescription at baseline | Prescription (number of exercises prescribed per session) | Mean; median (range) compliance with prescription;a rater’s subjective opinion on compliance | Priority stretches prescribed? | Did the change in prescription affect compliance? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R01/002 | Above | 25 | 27 | 26 | 386 | Y | n/a | n/a | n/a | n/a | n/a | n/a |
| R01/004 | Above | 31 | 30 | 30 | 525 | Y | n/a | n/a | n/a | n/a | n/a | n/a |
| R01/005 | Below | 16 | 18 | 17 | 386 | Y | Good | Achievable | 5–12 | 74.69; 66.67 (58.33–100); good | Yes | No |
| R02/001 | Above | 31 | 32 | 33 | 400 | Y | Good | Achievable | 5–13 | 72.16; 81.87 (30.77–100); good | Yes | Yes |
| R02/002 | Below | 18 | 20 | 17 | 367 | Y | Good | Achievable | 8–12 | 97.92; 100 (87.5–100); good | Yes | Yes |
| R04/001 | Below | 20 | 19 | 15 | 300 | Y | n/a | n/a | n/a | n/a | n/a | n/a |
| R05/001 | Below | 27 | 27 | 25 | 327 | N | n/a | n/a | n/a | n/a | n/a | n/a |
| R07/001 | Below | 19 | 20 | 8 | 266 | Y | Good | Achievable | 5–6 | 93.67; 100 (80–100); good | Yes | No |
Attendance at AT was variable, with provider and patient factors playing an equal part in non-attendance (see Chapter 3, Delivery and receipt of the aquatic therapy and land-based therapy interventions and Chapter 5, Balancing the demands of the programme with work, school and family life). AT prescriptions were well optimised (focused and realistic) for three boys. For others, there was insufficient focus on the prescription, with too many/less important exercises for those that did not require the properties of water included. This resulted in insufficient clarity about the need to develop, at the outset, a prescription that was achievable and focused on the needs of the boy. There had been a difference in understanding whether or not it was intended that exercises from most categories of the AT manual should be implemented or if the prescription should be more focused (see Chapter 5, Sense-making). More or less complete data were available for LBT in only four boys. In general, compliance was good and the prescriptions were appropriately optimised.
Chapter 5 Qualitative research results
Context understood through the International Classification of Functioning, Disability and Health – Children and Youth version and burden of treatment theory
Figure 23 shows the ICF-CY as it applies to DMD; ICF-CY codes are provided in the text for reference.
FIGURE 23.
Disability of ambulatory boys with DMD understood through the ICF-CY. Adapted with permission from International Classification of Functioning Disability and Health, Children and Youth Version. Geneva: World Health Organization; 2007,93 http://apps.who.int/iris/bitstream/10665/43737/1/9789241547321_eng.pdf.
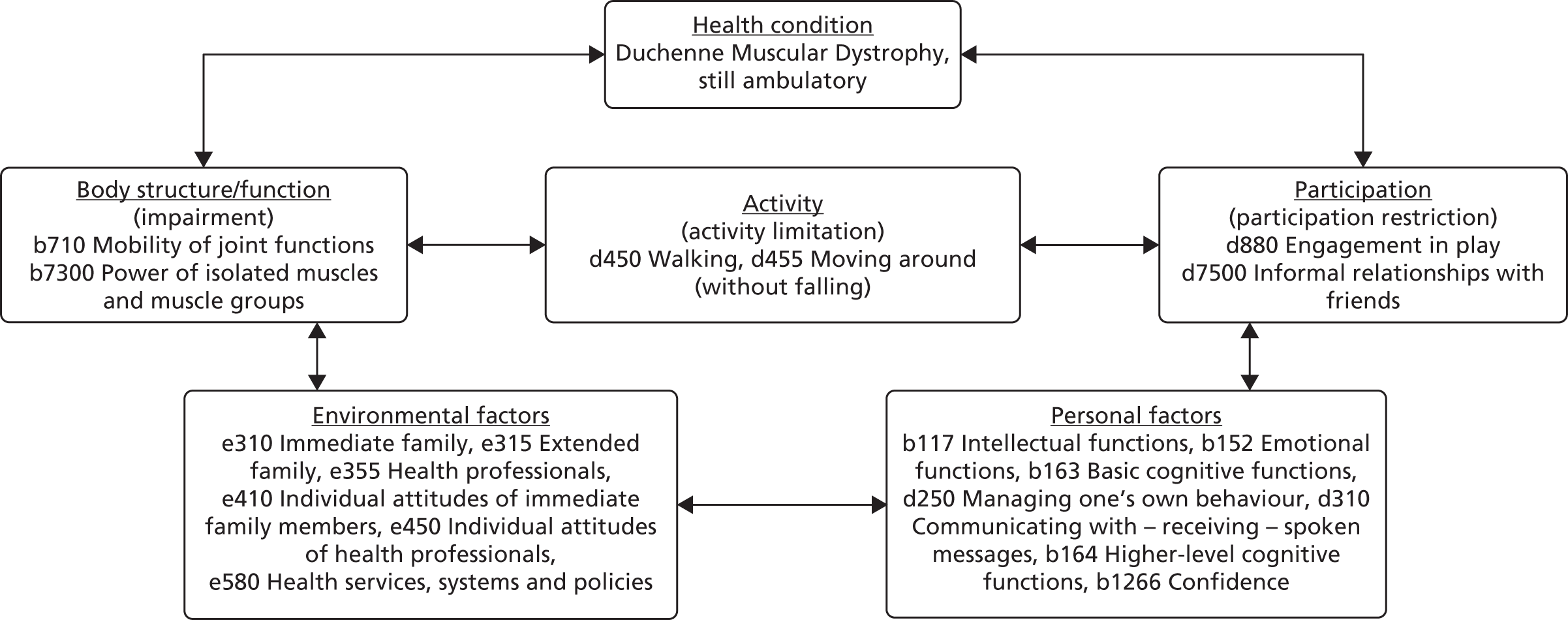
Impairment and activity limitation
The children in our study were already starting to have problems walking (d450) and moving around as a result of increasing muscle weakness and secondary joint contractures (b710). Many had already transitioned into a phase involving adaptive equipment to improve mobility and quality of life. Some were using canes, walkers or power wheelchairs for parts of the day to maintain independence of mobility. One interview took place as an occupational therapist was inspecting the participant’s house for modifications. To assist with care, one child’s bedroom had recently been moved to the ground floor. Modified vans and ramps were often in evidence as aids to assist with care. Several children were falling frequently (d455), and some were using motorised wheelchairs for portions of the day.
Yeah, playing in the street, he walk, and when he walking, he fell down very easily . . . now he can’t walk maybe more than hundred metre, something like that.
Parent R05/001
Participation restriction
Predictably,100,191 some parents reported that their boys were increasingly frustrated at the loss of function (see Perceived improvement in confidence, independence or participation) and consequent withdrawal from sport, play (d880) and their peer group (d7500).
Personal factors
Typically for the age group, most participants were on steroid therapy with the anticipation of prolonging ambulation,9,192 sometimes with attendant weight gain and behavioural issues (b152, d310). 193 We are not aware that any of our participants had substantial learning difficulties (b117, b164), which are sometimes associated with DMD. 193 With life expectancy now much longer as a result of improved disease management,194 the prospect of further education and profession was likely to be an important goal for many families. However, at an average age of 9 years, a child’s awareness of possible futures is somewhat limited.
We are now having a population that lives to go on to university and, in that case, academia is very important . . . the ones that are cognitively good, you know, that’s really important.
Physiotherapist S07/001
I have a 14-year-old son and obviously [his brother and participant] is 8 . . . now. [Participant] knows he’s got poorly muscles, he knows he’s got something called DMD; he knows that he’ll need a wheelchair more and more. But, for him, it’s the day by day. I think, as a parent, you keep it as normal as possible for as long as possible . . . we’ve also got to think about the 14-year-old who can go on Google [Google Inc., Mountain View, CA, USA] and see all the worst case and the future . . . we’re pumping [participant] full of steroids every day so, emotionally, that changes him, gives him high and lows . . . When he’s tired he becomes quite emotional he can laugh uncontrollably at the slightest thing or cry uncontrollably at the slightest thing.
Parent R02/001
Environmental factors
Immediate and extended family (e310, e315) and their attitudes (e450)93
Burden of treatment theory: workload113
Although we did interview one father who was the principal carer for his children, it was relatively clear that, in most families, mothers shouldered the burden of care, regardless of the fact that many of them did work, or had until recently worked, full-time (see Balancing the demands of the programme with work, school and family life). In at least two families, working patterns involved both parents working long days to guarantee mutually exclusive non-working time where one of them could be available for care. In five interviews (six participating boys), there were multiple DMD boys in the household, with one family having four DMD boys and an unaffected girl (participants R01/002, R01/003 and R01/004).
The difficulty faced by the parents of disabled children in accessing appropriate services within striking distance of their home and resorting to private provision is well charted. 64,195–197 It is also well established that services are often fragmented, that intra-agency service planning is poor and that professionals, as well as families, are confused about provision. 195,197,198 The obstacles to accessing services (see Figure 3) were in evidence, as in other studies, and parents had to ‘fight for everything’,199 and relationships with providers are described as ‘repeatedly asymmetrical, non-collaborative, and negative’:199–202
I’ve had to fight . . . to put everything in place . . . they used to have statements; they got rid of statements and replaced it so, but to get someone to assist [participant] to come in especially for school for him has been a nightmare. It’s been an absolute nightmare and you know it’s been quite upsetting that they’re failing him . . . the government not providing the right level of care, but I’m on it.
Parent R07/001
Burden of treatment theory: capacity113
The ability of parents to do this work, which ensures care for their boys, is constrained and enabled by a variety of factors (see Table 3). 117,203 Their own health, morale, beliefs, parenting skills, personality and potential to absorb adversity (resilience) all have a role in how parents cope. 113,196,203 By way of illustration, two parents, each with two DMD boys, responded differently to the expectations that they would deliver LBT exercises, which are causing each of them back pain:
I’m always concerned I’m not quite doing it right . . . but yeah it’s part of the norm. We sit down, he watches Simpsons, I do his physio[therapy] . . . roughly about 15 to 20 minutes per night . . . perhaps, you know, he’ll have a Friday or Saturday night off . . . I’ve pulled my back many times with [participant] . . . to be honest with you it’s part of the reason I think he does so well . . . if anything I’m probably lighter on him than the actual physiotherapists.
Parent R02/001
It’s me who’s got the aches and pains yeah, because when I do his stretches I also have to exert some effort you see . . . this one time that I hardly get up because of the pain . . . I even went to see my GP [general practitioner] . . . that’s why I can’t really do [the LBT exercises] every day, it’s . . . stressful for me.
Parent R02/002
The often endemic stress experienced by mothers of disabled children (see Chapter 1, Significance in terms of ill health) is frequently related to inadequate, unco-ordinated and hard-to-access services. 195,197,200 As social networks have often shrunk,196 close family becomes the main support. 204–206 Grandparents, who often enable parents to undertake paid employment by providing informal daytime care,207–209 were mentioned in only one interview:
My little un, he goes to me [sic] mum’s [while the participant goes to hydrotherapy] . . . we all have tea at me mum’s so we don’t get home till about 7.
Parent R01/005
Other parents described the struggle to manage without such informal support. For at least two families, for whom English was a second language, the extended family was living outside the UK. Economic capital can buffer stress, but having a disabled child is typically linked with a loss of earnings and a range of additional household costs (see Chapter 1, Significance in terms of ill health and Chapter 6); employment can be a stressor as well as a coping resource. 196
Twenty-first century DMD parents are, by reputation, proactive and knowledgeable about their sons’ condition and associated treatment. 192 Cultural capital (education) often results in improved access to information and problem-solving skills. 196 As in other studies,200 there was no sense that parents who were more highly educated could more easily access professional support for disabled children, but some demonstrated skill in exploiting alternative opportunities:
I have had contact with hydro[therapy] since [participant] was a little boy. That’s purely my just nosing around . . . and finding out so, there is one [a pool] not far from the general hospital and it’s a special needs school, so weekends and some weekdays they have availability so we go. It’s £16 – it’s expensive . . .
Parent R07/001
There was little or evidence in the way of ‘bridging social capital’210 the creation of ‘weak ties’211 between people with a common purpose. An example of such social capital is provided by PPI coauthor, Victoria Whitworth. Victoria Whitworth’s family used to block-book an AT pool for successive Sunday afternoons, sharing the cost and use with other families, whom she had met via MDUK and whose boys also had DMD. The boys would develop friendships and spend time together in an environment in which they were not restricted, having fun and doing AT exercises. Some parents who we interviewed had contemplated this kind of arrangement, but did not yet see how it could be done:
I’m probably being very utopian but I’m sure other parents would volunteer to enable their son to have the treatment . . . do a 2-hour session and four children get treatment.
Parent R02/001
Health services, systems and policies (e580)
In theory, community physiotherapists are seeing boys with DMD and prescribing exercises for them at regular intervals of at least twice per year, but it was not clear that this was happening in all cases:
So do you see a local physiotherapist?
Err yes, yeah we do err, we only see them when we need, when we need erm, when he’s, when he’s got a problem.
So you wouldn’t see that physiotherapist regularly?
Oh no, oh no, no.
But that physiotherapist has given [participant’s name] some exercises and . . . ?
Err no, no exercise at all.
So you’re not doing any exercise . . . ?
No we seldom see the physio[therapist].
Parent R02/002
As related previously (see Chapter 1, Current aquatic therapy provision in the UK), access to AT was unpredictable and, at best, involved the episodic provision of blocks of sessions:
Community physiotherapists hire a pool [at a local hospital] . . . they do like eight sessions and then you have to wait for maybe 6 months and then another eight sessions.
Parent R04/001
Health professionals and their attitudes (e450)93
Aquatic therapy is often considered to be a very expensive form of treatment; even those who dispute this advocate a careful, problem-based selection of clients, so that it is seen as ‘extra’ rather than core to a treatment programme:33
The issue with hydrotherapy with muscular dystrophy is that patients all want it but for no particular reason other than they think it will be nice to exercise in warm water . . . I think there is a real place for hydrotherapy in certain periods within them and I think the period is when they’re actually going off their feet to supplement them maybe going into KAFOs [knee–ankle–foot orthoses] to support a particular objective . . . As long as they’re swimming . . . and there’s appropriate access in and out of a pool, I personally don’t see that there is more benefit of doing a specific hydro[therapy] programme than swimming and doing some things at home . . . So I think you need to have a clear goal . . . You’ve gotta think, what is the point of hydrotherapy over and above land. And at the end of the day, [LBT] can be done by teaching assistants, can be done by parents; hydrotherapy you have to go somewhere or pay extra money [yeah]. So I think you have to really work out what you’re getting over and above swimming, which we encourage . . . (and as I say there are certain pools that you can also go to that are warmer pools) . . . You just have to rationalise what, what an extra intervention is giving you.
Physiotherapist S07/001
Patient and parent views of the aquatic therapy intervention
Perceived improvement or decline in physical function
In five cases, parents or boys perceived some improvement (R01/005, R01/004, R01/002, R02/001, R07/001), although in one case the child and physiotherapist disagreed. In two cases (R02/001 and R01/005) this was based on specific observations:
I actually think his range on his heels improved.
Parent R02/001
The next morning [after the AT], his legs were like really, really good. They weren’t stiff and they didn’t feel heavy . . . it keeps him flexible and it stops the cramps. On the assessment that [the physiotherapist] did at the hospital [at the 6-month assessment] he actually had to step up . . . without holding onto anything. He didn’t do it the first time . . . then he did. We were all amazed. He did absolutely fantastic.
Have you been able to do that before?
Not as high as what it were, no.
So it’s actually improved over the last 6 months you think?
Oh definitely . . .
And I couldn’t jump either. I couldn’t jump and now I can.
Parent and child R01/005
In some cases, the perceived benefits were less specific, but still valued:
They’re seeing a difference to what it was before and we think it’s helping . . . They’re more active, more active in the water that’s for sure.
Parent R01/002 and R01/004
It makes his muscles relax and then when I bring him home to massage him after the hydro[therapy] session you know he’s much more looser and I can tell the difference . . . I think he benefited from it . . . I mean the calf muscle is definitely something that was tight always before anyway and it’s much looser now. And I find that I can stretch him a bit further . . . I can see the difference.
Parent R07/001
In another case, a parent felt that function had remained the same, but also commented on the beneficial morning-after effect, also observed in child R01/005 previously mentioned:
His mobility, his strength, everything stayed where it was, even if it has not improved much, but it’s not going down . . . he was more relaxed, had a nice sleep at night when he does the [AT] exercises and the next day the body is more refreshed actually . . . it was nice way to start the day.
Parent R04/001
One parent perceived that his son’s condition had deteriorated despite the AT:
Unfortunately, I couldn’t find any, any kind of good positive result from hydrotherapy . . . I expected you know hydrotherapy would have more, er . . . stopped progressing in his symptom . . . but recently I found that [participant] quite often fell quite easily when he was playing or when he’s walking.
Parent R05/001
Perceived improvement in confidence, independence or participation
Some parents discussed fewer physiological benefits when asked about benefits, volunteering that they perceived improvements in confidence and participation. Sometimes, as with child R01/005, this related to a sense of achievement linked to a goal of maintaining or improving function:
The step up, me and [the physiotherapist], we were gobsmacked . . . You were happy weren’t you? You were buzzing.
Parent and child R01/005
For others, the outcomes might be less tangible, but the psychosocial response was nonetheless appreciable.
He really did come out of himself and be excitable in the pool and actually chat to the physiotherapists . . . rather than being the reserved [child’s name] he often is in public . . . he’s incredibly competitive, and [the physiotherapists] were able to fetch that out of him . . . a lot of the time when [child’s name] does physical activity he’s, he’s the slow one, he’s behind the standard, he’s the one being specially accommodated for. Whereas, this being one on one, it was just at his own ability to be able to push that. He goes swimming at school now, no matter how hard he tries, he’s not going to keep up with the other children so it becomes a negative. So to be able to do this physical, fun thing without any kind of peer pressure or benchmark it may be why [he] enjoyed it so much.
Parent R02/001
Patient representative and coauthor, James Parkin, confirms the importance of swimming and, therefore, water confidence as DMD progresses in terms of cardiovascular exercise, self-esteem and enjoyment: ‘When every other sport or exercise is taken away from you with Duchenne, that’s what you’re left with’. As something you can do as a family, it can be incredibly important. In that sense, AT can be about more than just stretching muscles for the assessment of function in 6 months’ time, it is about longer-term outcomes (see Chapter 2, Methods Modelling process: developing programme theory and Figure 4 on the views of Faye Dulcy,121,122 and physiotherapist S01/001 on water confidence in Appraisal work below). Here, another parent describes the importance of a perceived improvement in water confidence at a time when the child is beginning to be marginalised in participation terms:
In school he’s not involved in any sports because, if he was hit by other kids then he’ll just fall . . . I could see what [the physiotherapists] are doing and that, from day one until the last session, he knows how to swim. So yeah it’s really amazing what it [the AT] did.
Parent R02/002
Fatigue and pain
Fatigue was not generally a concern for either parents or children. In general, descriptors for how children felt after the session ranged from ‘not tired at all’ (R02/002) to ‘a bit tired’ (R05/001), with some indicating that it depended on the pressures of the school day when AT sessions immediately followed school (R001/002 and R001/004). One parent maintained that the child’s energy levels had decreased since the AT programme stopped:
After he stopped [the 6-month AT programme] he feel more tired.
Parent R04/001
In one case, the child, mother and physiotherapist expressed concerns about pain and, particularly, fatigue arising from sessions delivered before the school day, with school missed as a result of fatigue:
Well I get really exhausted I feel like I wanna go to bed, but obviously I can’t go to bed because I have to go to school . . . when I get in class my legs are in a little bit of pain but then, when it’s the afternoon my legs feel better.
Between the trip from the hospital to school it’s about half hour in the car so [participant] then sits in the car and has a good rest . . . some of those days [the school] would call and say ‘he is extra tired, could you come and pick him up?’.
Child and parent R07/001
We had huge issues with fatigue . . . it was too much with the burden for the patients . . . and I also think the community teams had a bit of a panic about it, and were wanting to do different exercises and we ended up cutting them back because they were too tired. I also think that we didn’t get involved in what they were doing at school, we kept them doing the same things at school but obviously with each new school term the physio[therapist] goes in and changes things and then I think by the time they did [LBT exercises] at school, then parents felt they need to do [LBT exercises] as well . . . the fatigue was way too much, our patient that did do the hydro[therapy] was constantly fatigued: we ended up stopping all the land exercises except the stretches; we’ve ended up cancelling a lot of the hydro[therapy]; the fatigue was a real issue.
Physiotherapist S07/001
No other child receiving AT complained of pain. Two parents volunteered information about back injuries brought on by the delivery of LBT exercises to their children in the home (see Environmental factors).
Quality of the therapist–family relationship and communication
Parents and children were almost universally effusive about their physiotherapists. Only in two interviews did parents distinguish between physiotherapists who had delivered AT to their sons, and then only to highlight the difference in their skill at making physiotherapy fun, which they perceived as crucial to maintain their children’s engagement (see Chapter 2, Modelling process: developing programme theory and Figure 4):
It’s a testament to the team there for understanding his learning style . . . and adapting to it. They build rapport . . . they all got to know him as an individual and were able to kinda trigger conversations about the things he likes doing, like splashing me at the side of the pool or whatever that might be . . . you’d hope to get a good physio[therapist] to be concerned about his journey . . . rather than just being about physical [function].
Parent R02/001
We thought they were amazing, because they had the skills to deal with children. So they treated [participant] as how he should be treated at his age and made it fun in the pool.
Parent R07/001
This conscious effort to engage children in what could be fairly unpalatable exercises was summed up by one physiotherapist as follows:
[Children] don’t really see it as exercising; they see it as a fun thing to do . . . It’s the therapist’s skill, isn’t it, in making the exercises into a game so they don’t even really realise what they’re doing.
Physiotherapist S02/001
Balancing the demands of the programme with work, school and family life
There was general agreement on the burden of a twice-weekly programme. Universally, this related to travel time to the AT sessions, often through rush hour traffic to make slots at the beginning and end of the day. Even those with strong relational networks, for example, extended family who could help with sibling child care, said that ‘it takes a lot of organisation’ and felt that ‘it would be better if it was a bit closer to home’ (parent R01/005). Two mothers had given up full-time work during the last year for reasons related to the burden of care for their sons; one stated unequivocally that this had happened before involvement with the trial, in the other case, the mother had moved to casual work as a response to being allocated twice-weekly AT. Some parents had employers who were fairly inflexible about working arrangements or whose self-employment made for an uncomfortable choice:
New business, not having a wage and having to turn down work due to attend is, is something I’m more than willing to do and I’ll do every single time but obviously a challenge . . . It was probably only 15 miles each way, but via the school and then rush hour traffic. Bear in mind my session was at 4 o’clock so sometimes it was an hour and a half on the way back, not a challenge unless you’ve got a 5-month-old screaming baby in the back.
Parent R02/001
Mother (via translator): There was no other option, no other choice but to attend. It was really important for the boys . . . we’ve got five children . . .
Father (via translator): Sometimes there were child [care] problems and I finish my work at 8 p.m.
Family R01/002 and R01/004
No parent highlighted that the programme had created any problems with school. For some children, this was because the session was after the working day. Even those who had attended 4 p.m. sessions remarked that it had not been a problem to miss the last 30 minutes to 1 hour of the day. Only one parent mentioned concern that the programme might compromise his son’s education, a concern that had been allayed by the school:
[School] were happy, because they said like most of the work is done by 1 o’clock, with respect to the main studies. After 2 o’clock is more like activities, fun things, so it’s OK. And they normally give him homework before 1.
Parent R04/001
The two boys whose therapy took place before school found this particularly difficult and, although the parents reported that the schools were co-operative, the therapists were concerned (see Fatigue and pain and Appraisal work):
He did miss a fair few sessions . . . he’s now said that he can’t do the Wednesday [morning] because it’s just too early for him because that’s all we could offer him was an early appointment . . . Basically it’s not as convenient a time that we were offering him.
Physiotherapist S05/001
Summary
Notwithstanding the burden of treatment and the fact that some boys experienced functional decline while receiving AT, there was a widespread enthusiasm for continuing to access AT, more locally, once per week, for reasons of participation and the hope of maintenance of function:
A lot of them did sort of say, ‘Oh, what’s next? Is there any way we can continue?’ or ‘Will the service be continuing?’. The feedback that I’ve had is that they’re dying [for] it to be continued or to, to access more if there’s the opportunity.
Physiotherapist S01/001
Therapist views of the service analysed within normalisation process theory
Sense-making
Unsurprisingly, physiotherapists distinguished the AT intervention from standard LBT (differentiation). Many specified that the properties of water, particularly buoyancy, which were unavailable on dry land, were highly relevant and helpful to people with muscle conditions who wished to exercise. There was a shared sense of the aims of physical therapy (communal specification), but differing understandings about the desired objectives (deliverables) (individual specification), for which the research team must take some responsibility:
We were confused . . . we didn’t realise we were supposed to go through the exercises and decide at the beginning which ones we were going to do and then each week tick them off. So we . . . didn’t quite get it right at the beginning. So I don’t know whether that was explained quite clearly enough.
Physiotherapist S05/002
The collective understanding within some centres was as specified in the training, that the manual formed a ‘menu’ of exercises that could be selected to address specific, emerging problems. However, some centres, which had sent physiotherapists on face-to-face training, characterised the manual as prescriptive and prescribed exercises from most categories in the manual, regardless of relevance. This was not the intended message of the training, which asked for focus and realism (see Chapter 2, Interventions). At other centres, physiotherapists who did optimise treatment delivery (see Chapter 4, The aquatic therapy prescription) nonetheless perceived the manual as prescriptive and questioned whether certain types of exercises or intensive AT in general were necessary:
I know the purpose of physio[therapy] for this type of condition, but . . . I’m not convinced that stretching in the pool is a better way of stretching than stretching out of the pool.
Physiotherapist S05/002
I think just putting somebody in hydrotherapy all the time just as an extra form of therapy I don’t think is a purpose . . . I think you can make hydrotherapy have a purpose in specific.
Physiotherapist S07/001
All but one participating physiotherapist constructed some sort of potential value of the AT intervention for their work (internalisation). Part of this value included ensuring that stretches were actually being conducted:
People used to go for physiotherapy, but that doesn’t happen now: stretches are done by parents or carers . . . the benefit of hydro[therapy] is that . . . you can tell it’s happening because you’re in the pool.
Physiotherapist S02/001
Relational work
Several centres were unable to set up the service because of problems accessing treatment costs (see Chapter 3, NHS treatment costs as a cause of centre attrition). At all centres that eventually participated in the study, key individuals drove the intervention forward (initiation). In each case, the site principal investigator was charged with setting up systems and procedures, as well as engaging with others to make the intervention happen. Once a senior physiotherapist got agreement from a service manager, then there was no problem getting others’ implementation as part of their work (enrolment), at least at the scale required by and for the duration of the study:
I have to go to a manager to say ‘Right, this is what we want to do, this is how long it’s going to take, this is how many staff I’m going to need’ . . . and the staff then have to agree to take it on.
Physiotherapist S02/001
Although the majority of physiotherapists thought that increased access to AT was beneficial for boys with DMD, none believed that it was right for them, as specialist physiotherapists in tertiary centres, to be involved in its delivery (a problem of ‘legitimation’). They also questioned if it was a sound use of scarce resources, with some explicitly addressing the issue of opportunity cost, that is, the benefits other populations could have received by diverting resources to them:
My role is mainly assessment and communication with the community services, who communicate with the physiotherapist who provides the treatment, so [delivery of AT] is in addition to what I would normally do.
Physiotherapist S05/001.
You’re having to look at it and say, ‘where am I going to pull staff from?’. If you’ve got the resources, then it’s quite straightforward to set it up. What’s difficult is if everybody’s saying, ‘well hang on a minute, where are we going to find people to do it?’, because they’d be pulling them from other work.
Physiotherapist S02/001
Thinking of costs, you’ve got to think, ‘what is the point of hydrotherapy over and above land[-based therapy]?’.
Physiotherapist S07/001
The physiotherapists continued to support the intervention (activation), but stressed that this could be done only for the duration of the study and because of the scale required by the low participant study consent rate:
So we are willing to invest the time but the problem is . . . we said originally we said we could probably take five hydro[therapy] people [participants who were allocated to do AT] and we only ended up having one, which was jolly lucky because actually in reality I don’t think we could have fitted five in . . . we haven’t got the staffing numbers, we haven’t got the pool availability, you know, it just doesn’t work.
Physiotherapist S07/001
It’s not really very sustainable to be able to offer two sessions a week for 6 months to a family, that’s certainly over and above what would be sustainable to be able to offer on a regular basis.
Physiotherapist S01/001
Operational work
Most physiotherapists described a process of translating or adapting instructions from the AT manual in order to get participants to perform a particular procedure in the pool (interactional workability):
Yeah it seemed like the right thing to be doing but we also applied our own assessment and clinical reasoning. We spent quite a bit of time looking at our patients and what their needs were, and modifying it a little bit to address their problems.
Physiotherapist S02/002
As more than one physiotherapist was involved at each trust, this knowledge work was a communal process and helped to maintain confidence in the intervention and in each other (relational integration):
At the beginning when we first started doing it we had to really think, sit down and write down what our treatment plans for our two children in the pool, looking at the manual and then saying, ‘Right so we want to do that exercise, how are we going to get it done? How are we going to do it? How are we going to deliver it?’. I’ve got our treatment plan that we came up with . . . we used the manual to do it, but we needed to streamline it to make it more user friendly for the sessions.
Physiotherapist S02/001
Other staff were involved in the delivery of AT, and their availability was among the factors that affected delivery:
The staffing’s got to be there to run it safely . . . you need some experienced physio[therapist]s but you also need some of the junior staff . . . who are very capable of delivering the service, but there’s training and supervision issues. And then you need your pool-side assistant who is usually a physio assistant or a technical instructor . . . then we also had to have three other people in the department in case of pool rescue . . . Then you need all your maintenance people who keep the pool up and running . . . I mean we had our pool closed for a lot of sessions because we had some building work that had to be done.
Physiotherapist S02/001
Although the work of delivering AT was not always considered appropriate to the role, physiotherapists were consistently confident that they had the expertise to enact it (skill set workability). This was not just based on their technical knowledge of physiotherapy technique (intervention) but also because, as physiotherapists who were highly specialised in musculoskeletal conditions, they understand how to apply it given the complicated changing needs of the condition (which would not apply to community physiotherapists):
The complexity of deciding on the treatment plan, knowing the boys well enough to know how to do their treatment plan and then how to deliver it just wasn’t something that could just be handed out.
Physiotherapist S02/001
Critically, there was no participating NHS trust in which the delivery of the intervention was adequately supported by a host organisation (absence of ‘contextual integration’). Although all physiotherapists reported that their NHS trusts were enthusiastic about participating in the research study, this did not extend to backfilling their posts so that they could deliver the study intervention after NHS England declined to pay excess treatment costs (see Chapter 3, NHS treatment costs as a cause of centre attrition):
We’ve done this over and above what we normally do . . . we’ve ended up having to do this on our non-working days . . . By the time we’re doing it all on extra hours and fitting it around our child care and home life and everything else . . . it’s a non-viable service.
Physiotherapist S07/001
No participating physiotherapist believed that a twice-weekly, ongoing AT service delivered from a tertiary centre was viable (programme maintenance), with some indicating that their organisation’s commitment to AT more generally was wavering in a period of fiscal constraint:
It’s not really very sustainable to be able to offer two sessions a week for 6 months to a family . . . But you know, more blocks of hydro[therapy] to try and show them activities so they can go away and do [them], is a good idea.
Physiotherapist S01/001
I know that they would love to shut the pool . . . because of the financial drain.
Physiotherapist S05/001
Even those physiotherapists who believed that AT was a useful intervention for boys with DMD thought that it should be delivered elsewhere in the health-care system:
I just see the patients when they come to clinic, do the assessments and provide advice. So I don’t provide any treatment, I don’t go to the schools, I don’t go to the homes, whereas the community physio[therapist]s do that and set up their treatment programmes . . . where there is a pool, they will have a designated physio or physio team, that provide the hydrotherapy.
Physiotherapist S05/001
Appraisal work
All highly specialised physiotherapists who see boys with DMD enter their physical function data on to the North Star database every 6 months and so are keenly aware of changing rates of disease progression. However, with the disease progression being so unpredictable and treatment effects being confounded by concomitant therapies, especially steroids, physiotherapists were sceptical that they could attribute objective clinical outcomes to AT:
Most of the boys that had the therapy maintained their function which, for those boys, is a goal . . . maintenance is part of the battle. But, then again, it’s hard to know whether they would have maintained [that level of physical function] over that 6-month period without the hydro[therapy] . . . so it is quite difficult to say explicitly that they’ve been able to maintain [physical function] because of that input.
Physiotherapist S01/001
However, there are other effects of AT that physiotherapists were able to access (systematisation), both positive, such as perceived small physical gains, water confidence and ‘carryover’ (‘the extent to which treatment gains are maintained and used functionally between treatment sessions’212), and negative, in particular fatigue:
[The family] perhaps don’t always see a lot of the small gains that you might be able to get in the water in terms of a bit of extra range or a bit of activation in muscles that they might not necessarily have used in that way on land . . . the one boy in particular he had never really been in water before, he’d never done activity in water, he was quite water cautious and was quite fearful of coming in the pool, and by the end of the intervention he was very water confident and was able to independently float and swim in the water . . . I think it’s definitely helped to encourage families to carry that over into what they’re doing at home in terms of like recreation err and sort of sport and extracurricular activities.
Physiotherapist S01/001
All he’s done is get progressively worse and had more and more issues at school with fatigue, and I think we’ve affected his quality of life very negatively.
Physiotherapist S07/001
The majority of participating physiotherapists contributed to the triangulation exercise detailed in Chapter 7, Convergence assessment (communal appraisal). Although most of them assessed the AT intervention to be worthwhile in principle (with some dissent), they were united in their agreement that it was too costly as currently implemented and that AT interventions should be delivered in community settings. Those who believed that the intervention was worthwhile thought that the manual represented a good starting point for treatment but that it should be used as a menu of options from which exercises could be selected and adapted to the individual patient and available materials (reconfiguration; see Sense-making). This flexible implementation of complex interventions to local circumstances is often held to be inevitable, desirable and an indicator of sustainability. 137,213 It is also, to some extent, a normal, if arduous, part of physiotherapy case management, manifested through SOAP note keeping (see Chapter 1, Intervention methods and materials):33
You do need to think about planning the treatment programme and then you need to reassess it and modify it and try different things . . . and you need time to do that and then time obviously to reflect and evaluate on it afterwards. So it’s not just about being in the pool, it’s the time before and after the sessions and time to document it all. Because it all has to be documented, even though we were filling in the forms for the [RCT]; we had to write patient notes and a patient treatment plan each time as well . . . It is, again, quite resource heavy.
Physiotherapist S01/001
Some participating physiotherapists did individually assess the AT programme’s effects on themselves and their work contexts in terms other than those already discussed (individual appraisal). These reflections were generally positive:
I definitely benefited from additional training to help skill me up more, into delivering a more pure sort of hydrotherapy-based activity [where you use the properties of the water] as opposed to what you would describe as exercises or activity in water.
Physiotherapist S01/001
To actually be able to see one child over that length of time is extremely unusual in tertiary referral and I think it’s been a positive experience for me in terms of, not only [because] I enjoyed it, but also that I really feel that this is something that I would advocate even more than I was already doing. I mean I’ve often said ‘this child needs hydro[therapy]’ but I think I would be even more dogmatic now and say ‘look it really is a good intervention’.
Physiotherapist S04/001
Summary of therapist views within normalisation process theory
Normalisation process theory is a framework for understanding how new complex interventions are implemented and embedded in health service contexts. 119,120 When we used NPT to analyse the views of seven specialist physiotherapists who delivered the AT programme in their tertiary centres, we found that the roll-out of the intervention had not been successful. Although the intervention made sense to all, not everybody thought it to be of value and there were different perceptions about how flexible their use of the AT manual should be, with those who took the most flexible approach being most satisfied with the intervention. Although participating physiotherapists implemented the intervention energetically, they believed that community settings, not tertiary settings, were the right place for the intervention, and their organisations permitted its delivery, with sometimes indifferent support, only for the duration of the trial (programme maintenance). Case management was arduous and is unlikely to be feasible at scale. Nevertheless, most of the physiotherapists were pleased with the new skills that they had acquired and felt that they would advocate the use of frequent and intensive AT in other, more patient-convenient, settings where resources permitted. Figure 24 shows a summary of physiotherapist views within NPT.
FIGURE 24.
Summary of physiotherapist views within NPT.

Comments on the trial procedures
Sense-making
Stakeholders (physiotherapists and patients) distinguished the evaluation from other, mainly pharmaceutical, research (differentiation). Physiotherapists understood correctly and collectively agreed on the feasibility purpose of the research (communal specification); some thought that many patients saw the study as a way of getting AT for 6 months. This view was borne out by interviewed parents who professed a lack of equipoise in favour of AT and worried that participation would exclude their son from a drug trial, a declared reason for non-participation by parents who declined involvement and by some in the trial:
That was one of the worries that by partaking in this would it stop him being eligible for another trial because, as great as this is, as a parent, you’re always online seeing what NICE [National Institute for Health and Care Excellence] are going to approve.
Parent R02/001
Physiotherapists understood what the research required of them (individual specification) and constructed potential value for the research (internalisation). Specific value was expressed in terms of the study being a necessary precursor to a definitive evaluation and the general value of being involved in research. Physiotherapists believed that parental guilt (see Chapter 1, Significance in terms of ill health) or a sense that they had to do everything they could for their sons drove participation, and this was confirmed by interviews with parents.
Relational work
Site investigators drove the research forward (initiation) and physiotherapists agreed that the research should be part of their work (enrolment). They confirmed that participant enrolment was difficult because of competition with more attractive industry trials, and challenged the reasoning behind the Research Ethics Committee’s prohibition on co-enrolment (see Chapter 3, Participant recruitment and the prohibition on co-enrolment). Participant enrolment was particularly challenging at one site where access to AT was good. Two physiotherapists questioned the restrictiveness of the eligibility criteria, proposing that younger and more disabled children should be eligible. Physiotherapists bought into the research (legitimisation), but some questioned whether or not long follow-up (several years) might be needed to disaggregate the treatment effects of physical therapy from those of other interventions. They continued to support the research (activation) despite finding research procedures burdensome. They valued support from one individual in the trials unit, felt that they worked well as site teams and continued to support the trial (relational integration). However, they felt that participants’ continued engagement was contingent on their allocation to AT, something borne out in quantitative findings and parent interviews. Allocation to the control had been difficult for those with friends or brothers in the research arm.
Operational work
Physiotherapists performed the tasks required by the research (interactional workability), although, as elsewhere,214 clinicians selected out eligible patients thought to be capricious. They reported that families were struggling to complete LBT documentation or were dropping out because of it. Physiotherapists found documentation version control, data capture and data query resolution overly burdensome, but appreciated the rationale for them (relational integration). In particular, one interviewee found the AT logs confusing and restrictive compared with SOAP note taking, and this may account for certain data quality issues. Although some complained of insufficient administrative support, physiotherapists said that the research was adequately supported by host organisations (contextual integration). However, they said that backfilling hours was difficult with the resources available, and, other than at two organisations where dedicated research professionals were used for data collection and entry, physiotherapists conducted research procedures on top of their clinical workload (see Operational work):
Just the fact that we only got the one patient [randomised to AT in the pilot RCT] made it feasible for us. But if we’d have got a bigger uptake then we probably would have struggled.
Physiotherapist S05/002
The physiotherapists felt that, although research tasks were not always part of their normal job role, they were nonetheless appropriate for them (skillset workability). Most were resistant to involving generic research nurses, believing that they would not understand the clinical environment.
Appraisal work
Physiotherapists have access information about the trial and intervention feasibility (systematisation) and dissemination to participant families is planned. Communal and individual appraisal was positive. Physiotherapists made proposals on how the study design and procedures could be modified in response to their appraisal (reconfiguration): streamlining of documentation, smartphone/tablet access to the study database for easier data collection and extending routine clinical sessions by 1 hour to allow for completion of research-specific patient assessments. One physiotherapist wanted more mechanistic outcome assessments about fatigue, joint range and muscle power. More than one requested a longer, observational, design. The importance of measuring fatigue and pain pre and post session was stressed.
Although signed assent forms were available for all participants, interviewed children often could not remember being approached about entry into the study. Those who could remember the approach described feeling ‘good’, ‘excited’ or ‘nervous’. Parents were pleased to be approached about entry to the study, although expressed anxiety about forgoing the opportunity to be in future drug trials. Only one child recalled the randomisation process and reported not liking it. Only two parents (both in the AT arm) described a positive response to the randomisation process. Three families reported the number of items on the questionnaire battery as being too many. Only two families made more specific remarks, in each case in relation to well-documented problems with responding to vague questions on health-related quality-of-life instruments. 215
Chapter 6 Cost analysis
Cost of aquatic therapy
The cost of AT to the NHS was estimated by taking the NHS 2013 tariff cost of AT of £110 for a first session and £74 for subsequent sessions inflated to current prices using the Hospital and Community Health Services Index. 216 We also examined some of the individual components of AT including staff time and maintenance costs. Qualitative interviews and discussions at our TMG showed that a minimum of five staff members were needed on site to run a 30-minute AT session: one physiotherapist at NHS band 5, 6 or 7; one physiotherapist assistant (NHS band 3); and three other staff (band 2 or 3). We took the midsalary point on NHS Agenda for Change169 and assumed that staff needed to be available for 1 hour to deliver a half-hour session. We were unable to obtain maintenance costs directly from the centres participating in our study; however, a NHS report from trusts showed annual costs of £12,000 per year. 217
Land-based therapy was prescribed by the specialist physiotherapist during this study. Outside this study, in usual care, it would be prescribed by a community-based physiotherapist. The frequency between visits to a community physiotherapist varied between once every 6 weeks and once every 6 months. The unit cost of a physiotherapist was taken from the Personal Social Services Research Unit. 216 The exercises prescribed were then carried out by carers and teaching assistants.
Costs borne by patients
Although not a NHS cost, there is an opportunity cost to participants and their carers and family to attend an AT session; this includes time off work or usual activities for the carer, time out of school for the participant, child care costs for other children, journey time getting to the session and parking.
The intervention also included LBT two to four times a week, which may be delivered by a community-based physiotherapist, parent or teaching assistant. For NHS costs it was assumed that the community physiotherapist saw the participant once per month and that sessions were delivered by parents or teaching assistants at all other times. It was assumed that the amount of contact time the community physiotherapist had with participants was the same in both groups. However, we allowed for the fact that parents in the usual care group would have to spend more time delivering LBT, as they would have to provide, on average, three more LBT sessions than required for the AT group.
Using information from the qualitative interviews and from discussions with our PPI representatives on the TMG, we made the following assumptions about time:
-
time off work or usual activities to take participant to an appointment – 3 hours
-
time to look after other siblings during the session, assuming that each carer had at least one other child who would need child care arrangements – 3 hours
-
time out of school for participant – 30 minutes
-
travel to appointment by car – 30-mile round trip
-
car parking costs.
Productivity loss was taken to assume any time taken away from usual activities by the carer regardless of whether this was time away from paid or unpaid activities. In the main analysis we excluded the time the child was taken out of school but this was then included in an alternative scenario. Costs per hour were taken from the Office for National Statistics Annual Survey for Hours and Earnings for 2015218 and were £528 per week or £14.08 per hour. Based on the qualitative interviews it was assumed that all participants travelled to the AT sessions by car; average running costs were obtained from the Automobile Association (Basingstoke, UK) website and assumed to be, for a petrol car costing £18,000–25,000 when new and covering 20,000 miles per year (median cost and travel distance), 42.52p per mile. 219 Car parking costs were assumed to cover 1–2 hours of parking at a NHS car parking fee of £2.50.
Land-based therapy was delivered 4–6 times per week in the usual care group and 2–4 times a week in the intervention group. This tended to be shared between teachers and parents. We assumed that parents would help with LBT two or three times a week in the usual care arm and once or twice a week in the intervention arm, for approximately 30 minutes per session. Costs per hour were taken from the Annual Survey for Hours and Earnings for 2015. 218
For both NHS cost and costs borne by patients, perspectives costs are estimated per patient per session and for a 6-month block of treatment. Discounting was not applied as costs are presented over the 6-month time period of the study. All costs are presented in 2015 prices.
Attendance
Alternative costs for a course of AT were estimated after allowing for non-attendance. If the AT pool had been available for all sessions over the 6-month period, then participants would have had the opportunity to attend 52 sessions. In reality, participants were offered anywhere between 28 and 52 sessions (349 over eight participants). This resulted in an attendance rate of 58% over the 349 sessions that were offered, or 49% if all 416 sessions had been available. In 50 of the 349 sessions, participants were unable to attend and were assumed to cancel the session in advance; assuming that the pool was put to other use over this time would give attendances of 68% over 299 sessions or 55% over 366 sessions.
Results
Using NHS tariff costs, AT cost £113.88 for a first session and £76.61 for subsequent sessions. Over a 6-month course of AT this would result in a cost of £4021 over 52 sessions. Allowing for alternative attendance rates, costs of AT would range between £1970 (49% attendance rate) and £2734 (68% attendance rate) (Table 25).
| Staff | Midpoint salary (£) | Cost per hour (£) |
|---|---|---|
| One band 5/6/7 physiotherapist | 29,043 | 19.85 |
| One band 3 physiotherapy assistant | 17,972 | 12.29 |
| Three band 2/3 staff | 17,179 (×3) | 35.25 |
| Total NHS staff costs | 67.39 |
Over a 6-month course of AT, staff costs would be £3504.28 and range between £1717 (49% attendance rate) and £2383 (68% attendance rate). Assuming annual maintenance costs of £12,424 for an AT pool used by a range of services and offered for 39 weeks per year during core hours would work out at £8.49 per hour.
Land-based therapy provided in its usual setting would cost the NHS between £80 and £320 over a 6-month period, assuming that physiotherapists saw boys once every 6 weeks to once every 6 months. The remainder of the costs of LBT would be borne by the carers and the education system (teaching assistants).
Costs borne by participants and their carers for aquatic therapy
Table 26 shows costs borne by participants and their carers per AT session to be £99.74 or £106.78 if including time lost by a child from being taking out of school early. These costs would rise if child care for other children was undertaken by professionals (e.g. after-school clubs or childminders rather than volunteers).
| Activity | Effort | Cost (£) |
|---|---|---|
| Carer time off work or usual activities | 3 hours | 42.24 |
| Child care costs assuming time borne voluntarily by other carers | 3 hours | 42.24 |
| Travel costs by car | 30-mile round trip | 12.76 |
| Parking | 1–2 hours | 2.50 |
| Total per session | 99.74 | |
| Including time out of school | 0.5 hours | 7.04 |
| Total per session | 106.78 |
If participants attended all 52 sessions, the costs borne by them and their carers would be £5186 (£5552 if including time out of school). Costs would range between £2541 (49% attendance rate) and £3526 (68% attendance rate) if participants’ time out of school is unaccounted for or £2720 (49% attendance rate) and £3775 (68% attendance rate) if participants’ time out of school is accounted for.
Land-based therapy costs to parents in the usual care group ranged from £732.16 to £1098.24 for two or three sessions over the course of 6 months and from £366.08 to £732.16 for one or two sessions over 6 months in the intervention group; a difference in costs of £366.08. Overall, the costs borne by participants and their families for AT plus LBT could be as much as £6000 assuming attendance of all sessions, in comparison with £1098 for LBT alone.
Chapter 7 Triangulation exercise
Convergence assessment
The convergence between quantitative and qualitative findings is presented in Table 27. There was agreement on six components, silence on eight (all expected areas amenable only to qualitative assessment) and dissonance on two. The areas of dissonance concerned attendance and optimisation. In each case, simple reading of the quantitative data might lead to an overly simplistic attribution of cause. In the case of session attendance, most of the quantitative data pointed to illness or simple non-appearance of the family; the qualitative data revealed that the convenience of available time slots had a strong role in non-attendance for some families (see Chapter 5, Balancing the demands of the programme with work, school and family life). Similarly, the quantitative study identified an apparent failure to optimise the intervention on the part of several physiotherapists (see Table 23); the qualitative data revealed this to be part of a misunderstanding, with therapists wrongly assuming that the study required them to apply the manual prescriptively or extensively (see Chapter 5, Sense-making), rather than in the focused and more achievable way proposed at training (see Chapter 2, Interventions). The same therapists were aware and very concerned that therapy was not optimised.
| Theme | Quantitative findings | Qualitative findings | Convergence code |
|---|---|---|---|
| Resources | |||
| Willingness and ability of parents to take children out of school, travel and attend twice per week | Large numbers of eligible families (self-)excluded from study because of geographical distance from pool (see Chapter 3, Recruitment and participant flow) | Families unified in view that twice-weekly travel to the acute trust for AT is too burdensome (see Chapter 5, Balancing the demands of the programme with work, school and family life) | Agreement (on access problems/burden of treatment) |
| Available AT pool | Percentage of missed sessions were because of staff or the pool not being available (see Chapter 3, Delivery and receipt of the aquatic therapy and land-based therapy interventions) | Pool closures and staffing problems were discussed (see Chapter 5, Operational work) | Agreement (on pool availability as a frequent reason for missed sessions) |
| Engaged NHS trust | – | Participating trusts not interested in providing a more intensive, open-ended AT service for DMD (see Chapter 5, Operational work) | Silence (issue appropriate only for qualitative investigation) |
| Pool hire and staff costs | Estimated direct NHS costs range from £1970 to £2734 over 6 months, based on attendance; societal costs range from £2541 to £3775 | Physiotherapists noted that financial pressures and opportunity costs meant that service was hard to justify (see Chapter 5, Operational work) | Agreement: it could be hard for AT to compete with other paediatric treatments of similar costs in QALY terms. NHS costs are less than for haemodialysis in children aged over 6 months (£10,296–46,352 assuming three sessions per week) or the cost of specialist services for children with cystic fibrosis, which range from £2554 to £24,809 over 6 months depending on complexity of care.220 However, the costs of delivering the same intervention in the community could be substantially lower |
| Motivated physiotherapists | – | Motivated, but believed that the acute trust was an inappropriate setting, with one finding it unnecessary (see Chapter 5, Relational work) | Silence (issue appropriate only for qualitative investigation) |
| 1-day training course | Attended by six physiotherapists from four trusts (see Chapter 2, The development of treatment manuals and theory) | Attendees found it useful (see Chapter 5, Appraisal work), although not all optimised treatment subsequently | Agreement (training implemented and successful) |
| Manual | – | Found useful as a ‘starting point’ or ‘menu’ of options. Concern about prescriptive use (see Chapter 5, Appraisal work) | Silence (issue appropriate only for qualitative investigation) |
| Activities | |||
| AT sessions | Implemented in 6 out of 17 NHS trusts approached for involvement (see Chapter 3, Implementation of the intervention and trial) | Treatment cost barrier to the other 11 trusts, confirmed in interviews (see Chapter 5, Operational work) | Agreement (on difficulty of funding AT in context of a RCT) |
| Case management | – | Documentation resource intensive (see Chapter 5, Appraisal work) | Silence (issue appropriate only for qualitative investigation) |
| Programme maintenance | – | Unsustainable in the long term or at scale because of opportunity cost (see Chapter 5, Relational work) | Silence (issue appropriate only for qualitative investigation) |
| Immediate outcomes | |||
| Eligible boys offered AT (‘reach’) | 348 boys identified at six centres. Families of 17/66 eligible boys contacted about RCT interested; 8/12 randomised received AT (see Figure 10) | Distance from centre and (in the context of RCT) involvement in drug trials confirmed as major reasons for non-participation (see Chapter 5, Comments on the trial procedures) | Agreement (on extent and reasons for failure of service to reach families) |
| Twice-weekly sessions delivered for 26 weeks (‘dose delivered’) | 203/349 scheduled sessions took place for eight participants. 69/146 missed sessions were because of NHS factors (unavailable pool/staff) (see Chapter 3, Delivery and receipt of the aquatic therapy and land-based therapy interventions) | Interviews confirmed that closures for repairs were an issue (see Chapter 5, Operational work) | Agreement (on causes of non-delivery) |
| Attendance at all sessions (‘dose received’) | 63/146 missed sessions were because of family factors (e.g. illness) (see Chapter 3, Delivery and receipt of the aquatic therapy and land-based therapy interventions) | Reasons for non-attendance also included inconvenient scheduling (see Chapter 5, Balancing the demands of the programme with work, school and family life) | Dissonance: qualitative findings problematise quantitative findings |
| AT optimised for individual boy (in place of ‘fidelity’) | Independent rater considered AT optimised for 3/8 boys, with other programmes too extensive/insufficiently focused (see Chapter 4) Only one boy had both optimised treatment and good attendance, but he also had high pain and fatigue |
Some physiotherapists misunderstood the manual’s purpose as prescriptively demanding extensive rather than focused programmes (see Chapter 5, Sense-making) One boy was dissatisfied with the AT |
Dissonance: qualitative findings problematise quantitative findings. Lack of clarity from research team/training on need for focus may be responsible for poor optimisation. Good optimisation did not necessarily lead to good outcomes/satisfaction |
| Intermediate outcomes | |||
| Gross motor function | The study is not powered to detect important differences in clinical/patient-important outcomes | Some interviewees perceived small gains (see Chapter 5, Perceived improvement in confidence, independence or participation and Summary of therapist views within normalisation process theory) | Silence |
| Exercise tolerance | The study is not powered to detect important differences in clinical/patient-important outcomes | – | Silence |
| Respiratory capacity | The study is not powered to detect important differences in clinical/patient-important outcomes | – | Silence |
| Activities of daily living | The study is not powered to detect important differences in clinical/patient-important outcomes | Physical and social participation and self-engagement (enjoyment, accomplishment, satisfaction)101 were noted and valued by parents, most boys (see Chapter 5, Perceived improvement in confidence, independence or participation) and some physiotherapists (see Chapter 5, Summary of therapist views within normalisation process theory) | Silence |
Completeness assessment
Qualitative research contributed information to 15 out of 17 logic model components, whereas the quantitative components contributed to nine. This was expected; some components were not amenable to investigation in quantitative terms.
Summary
Figure 25 provides a summary of key points relating to the intervention implementation. Although families are motivated to access AT, the distance from tertiary centres precludes many and adds to the existing burden of disease management for those who engage. Pool availability is at a premium, not constant and often at inconvenient times. Tertiary centres were not committed to delivery at scale or beyond the life of the RCT, owing to the costs and opportunity costs being too great. Estimated direct NHS costs, based on a service at an acute trust, range from £1970 to £2734 over 6 months; this would compete poorly with other specialist paediatric services in terms of value for money, but the costs could be reduced considerably by delivery in the community. Specialist physiotherapists are motivated to help patients and most see a wider role for AT, but not with them or their NHS trusts as providers. The 1-day training course was well received. The AT manual provides a good basis for a flexible but focused and realistic prescription. Only 6 out of 17 NHS trusts were able to deliver the trial, sometimes because of intervention costs. Documentation for case management was burdensome and programme maintenance was unsustainable. About 40% of scheduled sessions were missed because of a mixture of NHS and family factors, but the time of day affects the ability of families to attend. Optimal therapy was delivered in three out of seven participants, but optimal therapy did not necessarily lead to satisfaction or good outcomes.
FIGURE 25.
Three columns from initial logic model showing threats to implementation. Italicised bullet points illustrate the implementation problem.
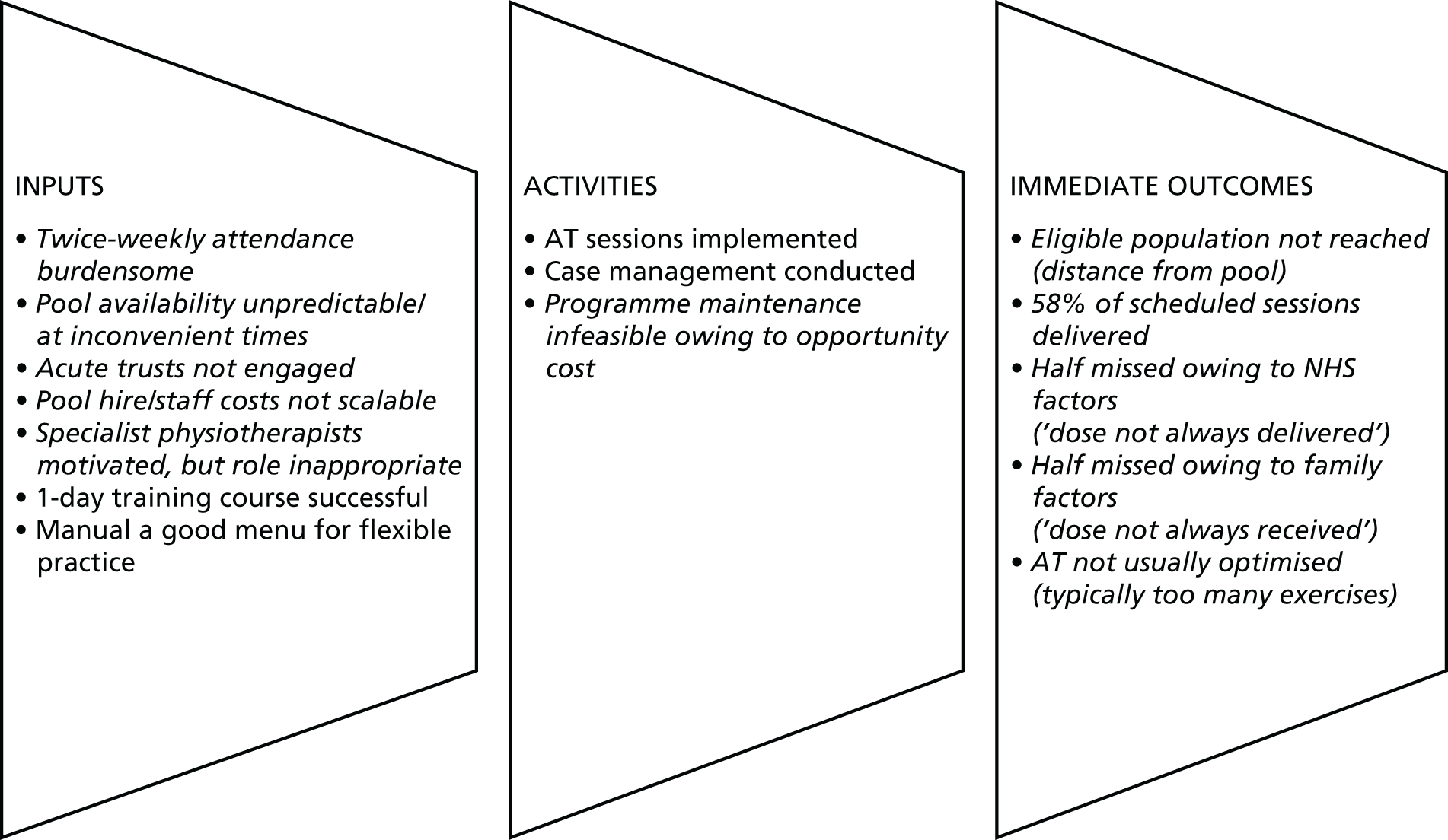
Chapter 8 Discussion
Summary of findings
Over 6 months, 348 boys were screened, most of whom could not be enrolled in the external pilot RCT as they were already in trials; 13 consented and 12 were randomised (30% of target) to AT (n = 8) or control (n = 4). The intention-to-treat analysis involved nine (AT, n = 8; control, n = 1) participants for all outcomes except the routinely collected NSAA score, which was available for 10 participants. Of the 349 scheduled sessions for which we have data, 203 (58.2%) expected sessions took place and 146 (41.8%) did not. Where the reasons for session cancellation were reported (10% of sessions were unaccounted for), there was a fairly even split between participant/family factors (43%) – sometimes related to inconvenient session timing – and health-care provider factors (47%). An independent physiotherapist who assessed optimisation of the interventions found that, based on capability and need, AT had been optimised for only three out of eight boys, with the remainder receiving treatment that was too extensive and insufficiently focused (revealed by qualitative research to be because of a misconception about the rigour with which the study team intended the manual to be implemented).
Interviews with families revealed sometimes isolated parents attempting to ‘keep it as normal as possible’ while having ‘to fight . . . to put everything in place’ for their children. Many had multiple DMD boys. Parents often shared the delivery of LBT with teaching assistants or, less frequently, community physiotherapists who came into school. Some reported back pain and lack of confidence in delivery. Outside the study, some parents had accessed at least one block of 4–6 weekly half-hour AT sessions since their sons had developed mobility problems. Some would be able to apply for another block in 6 months’ time. They valued exercise in warm water, reporting that their sons were unable to function for long in regular pools and that they felt that the break between ‘blocks’ was unhelpful. Most parents perceived gains in function or social/water confidence from the study AT, and valued closer working with the therapy team. Fatigue was a problem for two boys attending morning sessions. One of them, who was the oldest in the study and the boy experiencing the most rapid decline in function, also missed school as a result of his fatigue. This child was the only one who had both optimised therapy and good attendance, but he reported dissatisfaction with the AT and pain, although this resolved the same day. Attending AT at specialist centres involved long journeys, sometimes 3-hour round trips through rush hour traffic, for parents who mostly lived 15 miles away. All would have liked to have accessed AT at more local community pools.
Physiotherapists at two trusts questioned the value of the AT because of the opportunity cost to the trust. The message delivered in AT training was that AT prescriptions should be focused on the needs and capability of the individual, but many therapists thought that they had been asked to deliver stretches that could be delivered on dry land, or a range of exercises regardless of relevance. As a result, some were dissatisfied with the intervention. Participating physiotherapists believed that, in future, AT should be delivered in community rather than tertiary settings. Host organisations did not normalise the ongoing delivery of the intervention. Most of the physiotherapists were pleased with the new skills that they had acquired and felt that they would advocate the use of more frequent and intensive AT in other, more patient-convenient settings, where resources permitted.
Strengths and limitations
Development and implementation of the intervention
We have fully described not only what the intervention is and how it was developed, often poorly reported,221 but also how implementation and context affected its feasibility and acceptability. 131,222 There is no standard way to develop complex interventions. 223 We did not use formal consensus methods that, in any case, may not be important until finalising an intervention’s specifications. 223 Our development group contained too few parents of boys with DMD to reflect a broad range of concerns or to exercise an effective voice, and no community physiotherapists. Future development of AT programmes should fully and effectively involve these and other stakeholder groups, such as charities. Time prohibited iterative testing and adjustment before the pilot224,225 may have improved the intervention. The study team made it insufficiently clear to physiotherapists that they should generate a focused and achievable prescription to guide intervention sessions, leading to suboptimal prescriptions in five boys. Although all participating physiotherapists had been on a Chartered Society of Physiotherapy AT foundation course and received a training video, we are not clear how many watched it and not all took up face-to-face training.
The pilot trial
Pilot trials are not designed or powered to provide estimates of clinical effect that are adequate for decision-making. 70,71 We measured key process variables,70 describing fidelity, dose and reach;132 the study was not powered to test quantitatively for mechanisms of impact or the presence of contextual moderators. 131 As a result of the shortfall in recruitment, data are also inadequate for sample size estimation. 226 Although the consent/assent process was procedurally correct, participant recall of it was poor (see Chapter 5, Appraisal work), indicating that improvements are necessary in any further related research (see Chapter 9).
The decision to deliver AT outside its usual context of delivery by community physiotherapists in community pools to delivery by specialists in specialist centres was taken for the best of reasons. The national reorganisation of services in 2013 (see Chapter 3, Problems with the delivery of land-based therapy) left us lacking in confidence that we would be able to approach and engage the right people to set up the study. Although we have fully described the context of access and delivery, the evaluation we have delivered is of a decontextualised intervention. Many of the barriers that we encountered in the delivery of AT may not be encountered to the same extent if the intervention were to be delivered more locally to the service user and in community settings.
The clinical assessments focused on physiological function and paid insufficient attention to participation outcomes, the importance of which emerged during qualitative research and in consultation with PPI representatives. Agreed approaches to the conceptualisation and measurement of participation have been lacking,97,98,100 but two reviews and a conceptual framework100,227 now provide sound guidance for future research.
The qualitative research
The use of qualitative methods successfully captured breakdowns in implementation and views on the intervention131 and enabled us to recommend changes to the intervention. The use of a logic model, published empirical evaluations and social science theory ensured that key uncertainties and important questions were addressed. 131 Iterative data collection enabled the exploration of emergent themes. We did not use serial interviews to capture changes in the intervention or related experiences over time,131,228 or to adequately explore the relationship between families, community physiotherapists and schools.
Optimisation
As physiotherapy is a process that is poorly understood by analytical reasoning and requires extensive knowledge-based processing, it is not generally helpful to standardise it. 136 The decision to evaluate optimisation rather than fidelity of delivery was appropriate and ensures congruence with the intervention theory. 139,229,230 The use of an experienced independent physiotherapist with content expertise was appropriate, but might have been improved by a second rater working blind to the assessment of the first. Only two of the therapists took up the opportunity to respond to the independent assessment of their work; protocolisation and better scheduling of this opportunity might help to bring out important nuances in future optimisation exercises. We are unclear how adherent most participants were to their LBT exercises; it is plausible that we have good-quality data only on those who were able to comply both with their LBT prescription and with study procedures.
Health economics
This study has shown that the potential investment costs for families could be greater than those to the NHS. However, patients and carers were happy to commit to AT. Owing to the small numbers in this study, these findings have focused on the cost of delivery of the intervention and the impact on families; we have not been able to take into account variability in any assumptions, although we have reported ranges when applicable. In addition, we have not been able to use information on the CHU-9D and additional resource use owing to the small numbers and the lack of any meaningful interpretation. Although we have not used the information the study showed, it was feasible to collect it in this population.
Generalisability
The sample size is inadequate for sample size estimation226 or theoretical development,174 but some findings are generalisable for decision-making in the UK. Attempts to run RCTs on this topic will recruit poorly and generate unrepresentative samples because of the prohibition of co-enrolment and competition with industry studies. 186 Equipoise is poor among parents, with most believing AT to be beneficial. Where these conditions pertain, resentful demoralisation on the part of those randomised to the control arm could have serious psychosocial effects (see Chapter 5, Sense-making). However, future commissioning and evaluations are likely to involve local rather than central provision of services, as preferred by diffuse populations,115 and less intensive AT services amenable to austerity conditions and patient preference. Modifications in design, which are necessary to make a full-scale study feasible, are proposed below. To avoid some of the problems with the intervention and trial delivery reported here, such a study would have to be delivered in a community setting, meaning that, inevitably many of our findings would not be transferable.
Evidence of feasibility
On the basis of our success criterion (see Chapter 2, Feasibility criterion), a full-scale study along traditional frequentist lines is unlikely to be feasible. The main barrier to feasibility, namely the need for large numbers, could be removed by the employment of a Bayesian design, which could recruit 40 participants in a 2-year accrual window (see Chapter 3, Decision on the primary end point and sample size for a full-scale trial). However, the prohibition on co-enrolment raises concerns that the resultant trial population would be unrepresentative of the whole (see Chapter 3, Participant recruitment and the prohibition on co-enrolment). The alternative is to address the co-enrolment prohibition (which applies only to RCTs) and the absence of equipoise among parents (see Chapter 5, Comments on the trial procedures) by sacrificing randomisation and evaluating AT using an observational design (see Chapter 9). The necessary step of delivering AT closer to participants’ homes will increase the number of research and development approvals and so, all things being equal, the costs of the study, thereby replacing one barrier (refusal of consent because of participant travel time) with another (funder willingness to pay). The imperative to understand exactly what was delivered in complex intervention research, in terms of dose and fidelity,68,131 can drive up research costs and presents a barrier to feasibility. Research-literate specialist physiotherapists from tertiary centres may have the capability, opportunity and motivation to invest in trial procedures necessary to assess the optimisation of AT (see Chapter 5, Comments on the trial procedures); there is no guarantee that community physiotherapists will do so. Meanwhile, reliably assessing the delivery of LBT data may require the co-operation of a community physiotherapist, a parent and a teaching assistant for every boy (see Chapter 1, Introduction). Although the use of a tablet or smartphone application with short-messaging service reminders for these stakeholders may overcome the problems with paper-based data collection observed in this study, the prospect of high-quality data still seems remote.
Implications for health professionals and families
The need for new models of aquatic therapy access
Patient and public involvement coauthors, James Parkin and Victoria Whitworth, provide the following challenge for future research.
The ideal is open-ended, weekly, therapist-led AT, but the current service is sporadic 4- to 6-week blocks. James Parkin found that he had to rebuild all his confidence when coming back to therapist-led AT after breaks in service. Sustaining water confidence and self-esteem is very important to self-management. If you have a big break, each time you go back, the likelihood is that you have physically deteriorated – so the break just highlights the physical deterioration. It is easier, psychologically, to experience gradually increasing difficulties over time, rather than perceive step changes in the decline of physical function. The problem then is, how do we do approach the ideal?
Open-ended weekly AT, a priority for families, will be problematic to commission from NHS providers, even in the context of future research studies. More feasible models of care that extend access to AT are needed. The PPI coauthors suggest the following elements that are intended to augment the ‘blocks of sessions’ model (see Chapter 1, Current aquatic therapy provision in the UK). Suggestions a–c are feasible, based on their personal experience.
-
The number of sessions in a NHS AT block might be extended if the numbers of children with neuromuscular conditions in the pool could be increased to capacity, and the incremental cost to the NHS could be minimised by parents being in the pool. The same amount of face-to-face physiotherapy contact could be spread over a greater number of sessions, by leaving parents to practise exercises and allowing more NHS pool time, while a physiotherapist rotates between clients. This model was practised by James Parkin’s provider for many years, allowing increased access (6-week blocks between 12-week intervals) and enabling NHS AT access three times, rather than twice per year.
-
During routine NHS AT session blocks, parents are enabled to safely and effectively deliver AT, through in-pool training and diagram sheets. It is then their responsibility to access warm water pools between NHS session blocks. This merely extends the existing parental role of LBT delivery between physiotherapy consultations.
-
If parents of children with neuromuscular conditions were able to link with each other, then they could share the high costs of warm-water pool hire documented by MDUK (see Chapter 1, Current aquatic therapy provision in the UK), in order to do aquatic physiotherapy and other exercises reguarly. 64 James Parkin’s family was able to link with others in this way through a national charity, to access AT at the most convenient time, Sunday afternoons, with their families.
-
If parent-led weekend AT ‘clubs’ were feasible, then it is possible that community physiotherapists could be attracted to that setting to monitor that treatment is being delivered optimally. This model could reduce the administrative burden on the physiotherapist as the (non-NHS) pool management would be responsible for safety, and the requirement for burdensome SOAP note taking during such sessions (see Chapter 5, Appraisal work) could be reduced.
An obvious limitation of this model is that greater parental time in the pool is predicated on an adult-to-child ratio that can assure safety; some of the parents we interviewed are on their own with more than one DMD boy during pool visits while their partner, if they have one, is working. Such barriers need not be insurmountable; in Leeds, children engage in 20-minute one-to-one tailored AT sessions with qualified physiotherapists supported by volunteer student physiotherapists from Leeds Beckett University. 232 The costs to the family are £7 per session with the remainder of the £40 direct costs made up through charitable donations. The Leeds service is based at the Penny Field School; coauthor and aquatic therapy specialist Heather Epps believes that, for some geographical pockets, training staff at special schools using the manual and course that we have developed may be more efficient and sustainable than delivery through the NHS. In Heather Epps’s catchment area, there are high numbers of boys with DMD at specialist schools. At state-funded Valence School in Westerham, Kent, staff deliver an AT programme, with oversight by NHS physiotherapists. The independent Treloar College in Holybourne, Hampshire, employs its own physiotherapists to deliver AT. Special schools that employ technical instructors and physical therapy assistants can deliver AT more cheaply than the NHS, but still work at a high level. Economies of scale could be achieved if they were to serve neighbouring special schools with no pool of their own.
In the rest of this section, we provide general recommendations for the development of collaborative services, based on the six principles for the future of health and social care services recently outlined by NHS England. 233
Person centred: personalised, co-ordinated and empowering
In theory, community physiotherapists are already delivering personalised care in the sense of working with boys and their parents to develop and implement action plans, and to monitor progress,33 although in practice this is often not the continuous process it should be (see Chapter 5, Environmental factors). Fully personalised care involves shared decision-making to agree goals and to identify support needs. 233,234 Physiotherapists may require training to deliver services that are flexible and responsive to help young people achieve individually defined goals. 235,236 Personalised care should also be minimally disruptive,16 given that it is unreasonable for schools to expect poor outcomes in students with a disability,237 efforts should be made to schedule AT so as to minimise impact on school participation. The integrated model of AT (see Figure 4)121 and models of developmentally appropriate health care238 highlight the rewards gained when delivery focuses not only on narrow physiological outcomes but also on social and psychological gains. Proper co-ordination is required (see Carers are identified, supported and involved), with 6-monthly identification of key problems, safety issues, ideas for exercises and how to make them fun by specialists in consultation with community physiotherapists, parents and teaching assistants.
Services that are created in partnership with citizens and communities
NHS England proposes that communities and local services can work together at all stages of the planning cycle, from the identification of needs through to implementation and evaluation. 233 Critically, if service managers cannot deliver the ongoing physiotherapy service that parents desire, then they may be able to support something else to happen. 239,240 Use of national involvement standards for public involvement241 and sound evaluation principles242 will increase the chances that new service models will meet the needs of service users and their families. At a minimum, stakeholders for the codesign or coproduction of future services should include DMD family members, specialist and community physiotherapists, representatives of schools and/or teaching assistants, community AT pool providers and third-sector organisations.
Focus is on equality and narrowing health inequalities
Participation is rarely equitable and more normally determined by location and income. 64,96 Clear principles are required to guide the coproduction of new services241 and these might be provided by the United Nations Convention on the Rights of Persons with Disabilities, which urges state providers to promote ‘full inclusion and participation in the community’ (Article 19), provide ‘services designed to minimize and prevent further disabilities, including among children . . . as close as possible to people’s own communities’ (Article 25), and to promote recreation, leisure and sport (Article 30). 243 At a national level, since the Children and Families Act 2014244 and associated guidance,245 families can sometimes access AT via Sections F (special educational provision required) and G (health provision reasonably required by learning difficulties or disabilities) of an education, health and care plan.
Carers are identified, supported and involved
Models of coproduction239,240 and developmentally appropriate health care246,247 would suggest that service managers now need to recognise parents and teaching assistants as assets and allow specialist and community physiotherapists to build their capability for safe and effective delivery of LBT and AT outside the NHS. If the distribution of this work is to remain ‘joined up’ in how it works across roles (physiotherapists, parents and teaching assistants) and organisations (tertiary centres, community providers of physiotherapy, schools), then co-ordination is necessary.
New models of participation-based physiotherapy for disabled children see the therapist as a consultant, who shares information and educates to build capacity for the work of rehabilitation in the child, family, and community. 248 To be family centred, the family should identify needs, but also share responsibility, and health professionals should empower the family. 88,249,250 Support for shared management support might include effective training in, and printed information on, exercises that parents can perform safely and effectively with their children, as well as improved signposting to services. Key worker, partnership and skills training models of this kind reduce the stress experienced by parents of disabled children by helping them to understand the service environment, and to understand services to understand their needs. 198 Family-centred and shared-management approaches to physiotherapy for disabled children are emerging, with associated models for implementation and evaluation. 248,250,251 They stress that investment may be needed to train physiotherapists in practical coaching and facilitation skills. 251
This study identified that parents delivering physiotherapy to their children suffered injury as a result. Work-related musculoskeletal disorders, especially back pain, are common among physical therapists and parents of children with musculoskeletal disorders. 252,253 It is particularly important that parents and teaching assistants delivering LBT receive structured education and feedback on the safe delivery of exercises to their sons. We also identified that not all parents habitually prioritise the delivery of LBT. Although this can be seen as a function of burden of treatment, parents who are interested in habituating the evening delivery of LBT to their sons may benefit from interventions such as written exercise instructions or behavioural interventions with booster sessions and goal setting. 254
Careful work is required to ensure that involvement and mutual responsibility works for parents and is not received by them as part of the ‘growing demands’ for them ‘to organize and co-ordinate their own care’. 113 Carer engagement should not be used as a staging post for rationing by denial, selection, deterrence or dilution,255 not least because safety concerns (see Chapter 1, Physiotherapy) mean that carers need some level of education in pathology and oversight and treatment.
Voluntary sector as a key partner and enabler
In the past, the voluntary sector has contributed to the costs of group AT sessions and they are currently active in charting the location of warm water pools in the NHS, specialist schools, gyms, hospices and other areas. 64 They may also be persuaded to fund the training of community physiotherapists to deliver AT, and to act as more effective co-ordinators of the distributed work of care, at least in the context of further research. Perhaps the most important role for the voluntary sector is to build social capital for DMD families. Social capital is defined as a ‘public good consisting of ties, trust and norms’, which is based on social activities rather than private property. 210 The social capital of families is often weakened on receipt of a chronic disease diagnosis for their child. 256 In theory, building social capital increases participation and social inclusion for people with disabilities and their families257 through the removal of social and economic barriers to participation in services and leisure activities. 258 Charities can build social capital in two ways: (1) through changing norms, expectations and attitudes about access to services, and (2) by enabling DMD families to link into social networks of mutual support.
Social action as a key enabler
Social action is about ‘people coming together to help improve their lives and solve the problems that are important in their communities’. 259 If DMD families are able to link together, then other benefits should follow, other than sharing the cost of hiring private warm-water pools (see The need for new models of aquatic therapy access). Peer support groups are of practical importance for parents of disabled children in terms of information, advice and improving relationships with professionals; they also offer an opportunity to forge a community of shared experience. 260
A revised programme theory
Given our findings, it is necessary to revise our programme theory (see Chapter 1, Secondary objectives) to inform future efforts to implement and evaluate AT. Changes and additions are in bold and a revised logic model is presented (Figure 26).
FIGURE 26.
Logic model (revised).

Families will be motivated to access open-ended, once-weekly AT.
Clinical Commissioning Groups currently allocate resources for community physiotherapists to deliver 4- to 8-week blocks of AT, every 6 months at most (inputs and activities) within the existing roles, interactions and relationships that characterise the management of DMD (context). Alteration of this usual provision to teach parents key stretches and exercises, and action by the third sector to link parents, so that they can combine resources and hire warm water pools (immediate outcomes), will enable the desired ongoing access and bring about physiological benefits described in the treatment theory. It will also result in important gains in dimensions of participation – physical, social and self-engagement (intermediate outcomes).
Chapter 9 Further research
The research priorities should be as follows.
-
Families require more regular access to an enhanced AT service that enables them to learn and safely deliver techniques between standard NHS session blocks. The present monograph reviews much of the evidence and theory. A study combining intervention development and feasibility study phases of the Medical Research Council Framework is needed to model process and outcomes and to test procedures in community settings. 68 Participatory action research to understand how the third sector, NHS specialist and community physiotherapists, parents and teaching assistants in more than one location can coproduce a scalable personalised, co-ordinated AT service. Families should be encouraged to link with each other to build capacity for AT beyond limited NHS provision of intermittent session blocks, and contribute to or lead the evaluation. Outcomes should be a new service protocol for evaluation, measurement of participation and function, and participant views. An appropriate plan should be provided, not only to identify successful elements of practice, but for knowledge brokering and scaling up of successful intervention models,261–266 and theoretically informed methods of implementation should be reported in line with appropriate guidance. 267–269
-
The work of LBT is currently distributed but unco-ordinated, with parents and teaching assistants sometimes injuring themselves or failing to deliver treatment; as a result, treatment is not routinely optimised (see Chapter 5, Participation restriction). Taking into account what is known about barriers to interagency working,270,271 stakeholders should produce materials and systems to ensure that prescriptions are clear and regularly updated with specialist input, and that responsibility for training and delivery is transparent. Motivational and scheduling barriers should be addressed through problem-focused coaching. To facilitate evaluation, and for the convenience of parents, a means of sharing prescriptions and document delivery should be developed. The resulting intervention should be subject to evaluation through a series of iterative mini pilots. 223–225
-
Recruitment to large-scale evaluations of AT is hindered by a small, diffuse population of boys with DMD, an absence of parental equipoise and a prohibition on co-enrolment to randomised studies. There are two potential solutions.
-
A small-scale Bayesian RCT recruiting around 40 boys with DMD over 2 years to a randomised comparison (see Chapter 3, Decision on the primary end point and sample size for a full-scale trial) of community-based enhanced AT versus no AT (or usual service provision), with NSAA score at 1 year as the primary outcome. Eligible participants would have to be identified well ahead of time to facilitate implementation of an enhanced AT service in the area if necessary. Health professionals who recruit should communicate the balance of benefits and risks associated with trial treatment, stressing negative physiological outcomes such as pain and fatigue. 272 Professionals should use questions and feedback to ensure that the child understands that they are participating in research, and that participation is voluntary; assent should be continuously renegotiated throughout the trial. 273
-
If a RCT is deemed infeasible in the future then, understood as a quality improvement issue,274 well-developed interventions might be assessed using an interrupted time series design, a useful approach for studying complex and subjective phenomena such as children’s participation. 275 Alternative or supplementary patient-centred outcome assessments for such a study might include the measure yourself medical outcome profile. 276 The complexity of participation as a concept98 and limitations with the ACTIVLIM148 as a measure of participation have recently been pointed out;277 researchers should agree dimensions of participation and corresponding validated instruments101,227 in consultation with service users.
-
Chapter 10 Conclusions
A full-scale RCT based on frequentist methods is not feasible based on the trial protocol piloted in this study. Before a full-scale RCT can be conducted, further formative work is required to develop an intervention that can be delivered safely by community physiotherapists in settings that are more convenient for families of boys with DMD. If such an intervention is delivered, specialist physiotherapists must help focus clinical problems, propose related exercises and collaborate in the monitoring of intervention safety and the disease condition’s development. Although absence of parent equipoise, access to pools and the prohibition on study co-enrolment are likely to preclude a full-scale randomised trial run in the UK alone, a RCT employing one of a number of novel Bayesian designs, designed for use in rare populations, may be feasible in the future.
Acknowledgements
We gratefully acknowledge the hard work, support and advice from the following: Noeleen Goulbourne (Sheffield Children’s NHS Foundation Trust); Alec Musson (Leeds Teaching Hospitals NHS Trust); Jordan Butler (Great Ormond Street Hospital for Children NHS Foundation Trust); Anna Davies (Heart of England NHS Foundation Trust); Victoria Darke, Nicholas Emery, Megan Hyne, Richa Kulshrestha, Tessa Rowlands, Hilary Shepley and Sarah Turner (The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust); and Catherine Howard, Emma Martin and Jenni Palmer (University Hospital Southampton NHS Foundation Trust) for participant screening and data collection; Amanda Loban, Lucy Carr and Katie Walker (University of Sheffield) for data management, administrative and clerical support; and Paul Dimitri (Research and Development Director) and Gillian Gatenby (Research Directorate Manager) at Sheffield Children’s NHS Foundation Trust for support on ethics and governance.
We offer special thanks to the members of our trial steering committee: Cathy White (Independent Chairperson, Morriston Hospital), Alan Rigby (Independent Statistician, The Hull York Medical School), Gita Ramdharry (Lecturer/Physiotherapist, Kingston University) and Laura Merry (Independent Parent Representative).
Contributions of authors
Daniel Hind (Assistant Director), James Parkin (PPI Representative), Victoria Whitworth (PPI Representative), Saleema Rex (Data Specialist), Tracey Young (Health Economist), Lisa Hampson (Statistician), Jennie Sheehan (Clinical Specialist Neuromuscular Physiotherapist), Chin Maguire (Trial Manager), Hannah Cantrill (Research Assistant), Elaine Scott (Study Manager), Marion Main (Senior Physiotherapist), Michelle Geary (Specialist Paediatric Neurology Physiotherapist), Heather McMurchie (Paediatric Neuromuscular Physiotherapist), Lindsey Pallant (Paediatric Neuromuscular Physiotherapist), Daniel Woods (Specialist Children’s Physiotherapist), Jennifer Freeman (Associate Professor in Health Informatics/eHealth), Tracey Willis (Consultant Paediatric Neurologist) and Peter Baxter (Consultant Paediatric Neurologist) together produced the first draft of the report.
The following conceived or designed the work: Daniel Hind, Tracey Young, Chin Maguire, Elaine Scott, Heather Epps (Aquatic Therapist), Marion Main, Michelle Geary, Heather McMurchie, Lindsey Pallant, Daniel Woods, Jennifer Freeman, Ellen Lee (Statistician), Michelle Eagle (Consultant Physiotherapist), Tracey Willis, Francesco Muntoni (Professor Paediatric Neurologist) and Peter Baxter.
The following were involved in the acquisition of data for the work: Daniel Hind, Saleema Rex, Tracey Young, Elaine Scott, Heather Epps, Marion Main, Michelle Geary, Heather McMurchie, Lindsey Pallant, Daniel Woods, Jennifer Freeman, Ellen Lee, Tracey Willis, Francesco Muntoni and Peter Baxter.
The following were involved in the analysis of data: Daniel Hind, James Parkin, Victoria Whitworth, Saleema Rex, Tracey Young, Lisa Hampson, Jennie Sheehan, Elaine Scott, Jennifer Freeman, Ellen Lee and Peter Baxter.
Daniel Hind, James Parkin, Victoria Whitworth, Saleema Rex, Tracey Young, Lisa Hampson, Jennie Sheehan, Elaine Scott, Jennifer Freeman, Ellen Lee and Peter Baxter were involved in the interpretation of data for the work.
Daniel Hind, Saleema Rex, Tracey Young, Lisa Hampson, Jennie Sheehan, Jennifer Freeman and Peter Baxter drafted the monograph.
Daniel Hind, Saleema Rex, Tracey Young, Lisa Hampson, Jennie Sheehan, Elaine Scott, Marion Main, Michelle Geary, Heather McMurchie, Lindsey Pallant, Daniel Woods, Jennifer Freeman, Ellen Lee, Tracey Willis and Peter Baxter critically revised the work for important intellectual content.
Daniel Hind, James Parkin, Victoria Whitworth, Saleema Rex, Tracey Young, Lisa Hampson, Jennie Sheehan, Chin Maguire, Hannah Cantrill, Elaine Scott, Heather Epps, Marion Main, Michelle Geary, Heather McMurchie, Lindsey Pallant, Daniel Woods, Jennifer Freeman, Ellen Lee, Michelle Eagle, Tracey Willis, Francesco Muntoni and Peter Baxter were involved in the final approval of the version to be published.
All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publications
Hind D, Parkin J, Whitworth V, Rex S, Young T, Hampson L, et al. Aquatic therapy for boys with Duchenne muscular dystrophy (DMD): an external pilot randomised controlled trial. Pilot Feasibil Stud 2017;3:16.
Data sharing statement
Requests for patient-level data and statistical code should be made to the corresponding author. Although specific consent for data sharing was not obtained, the management group will consider the release of data on a case-by-case basis following published guidelines. 278 The data presented do not contain any direct identifiers; we shall minimise indirect identifiers and remove free-text data to minimise the risk of identification.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Drousiotou A, Ioannou P, Georgiou T, Mavrikiou E, Christopoulos G, Kyriakides T, et al. Neonatal screening for Duchenne muscular dystrophy: a novel semiquantitative application of the bioluminescence test for creatine kinase in a pilot national program in Cyprus. Genet Test 1998;2:55-60. http://dx.doi.org/10.1089/gte.1998.2.55.
- Bradley D, Parsons E. Newborn screening for Duchenne muscular dystrophy. Semin Neonatol 1998;3. https://doi.org/10.1016/S1084-2756(98)80146-2.
- Emery AE. Population frequencies of inherited neuromuscular diseases – a world survey. Neuromuscul Disord 1991;1:19-2. https://doi.org/10.1016/0960-8966(91)90039-U.
- Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy 2015;119:964-79. http://dx.doi.org/10.1016/j.healthpol.2014.12.016.
- McKusick VA, Hartz PA. 300377.DYSTROPHIN; DMD. OMIM® – Online Mendelian Inheritance in Man® 2016. www.omim.org/entry/300377 (accessed 30 March 2016).
- Anderson L, Karpati G. Structural and Molecular Basis of Skeletal Muscle Diseases. Basel: ISN Neuropath Press; 2002.
- Emery AEH, Muntoni F, Quinlivan RCM, Emery AEH, Muntoni F, Quinlivan RCM. Duchenne Muscular Dystrophy. Oxford: Oxford University Press; 2015.
- Poysky J. Behavior in DMD Study Group . Behavior patterns in Duchenne muscular dystrophy: report on the Parent Project Muscular Dystrophy behavior workshop 8–9 December 2006, Philadelphia, USA. Neuromuscul Disord 2007;17:986-94. https://doi.org/10.1016/j.nmd.2007.06.465.
- Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al. NorthStar Clinical Network. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatr 2013;84:698-705. http://dx.doi.org/10.1136/jnnp-2012-303902.
- Baiardini I, Minetti C, Bonifacino S, Porcu A, Klersy C, Petralia P, et al. Quality of life in Duchenne muscular dystrophy: the subjective impact on children and parents. J Child Neurol 2011;26:707-13. http://dx.doi.org/10.1177/0883073810389043.
- Landfeldt E, Lindgren P, Bell CF, Guglieri M, Straub V, Lochmüller H, et al. Quantifying the burden of caregiving in Duchenne muscular dystrophy. J Neurol 2016;263:906-15. http://dx.doi.org/10.1007/s00415-016-8080-9.
- Kohler M, Clarenbach CF, Böni L, Brack T, Russi EW, Bloch KE. Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med 2005;172:1032-6. https://doi.org/10.1164/rccm.200503-322OC.
- Simon VA, Resende MB, Simon MA, Zanoteli E, Reed UC. Duchenne muscular dystrophy: quality of life among 95 patients evaluated using the Life Satisfaction Index for Adolescents. Arq Neuropsiquiatr 2011;69:19-22. https://doi.org/10.1590/S0004-282X2011000100005.
- Uzark K, King E, Cripe L, Spicer R, Sage J, Kinnett K, et al. Health-related quality of life in children and adolescents with Duchenne muscular dystrophy. Pediatrics 2012;130:e1559-66. http://dx.doi.org/10.1542/peds.2012-0858.
- Bray P, Bundy AC, Ryan MM, North KN, Burns J. Health status of boys with Duchenne muscular dystrophy: a parent’s perspective. J Paediatr Child Health 2011;47:557-62. http://dx.doi.org/10.1111/j.1440-1754.2011.02022.x.
- Bray P, Bundy AC, Ryan MM, North KN, Everett A. Health-related quality of life in boys with Duchenne muscular dystrophy: agreement between parents and their sons. J Child Neurol 2010;25:1188-94. http://dx.doi.org/10.1177/0883073809357624.
- Chen JY, Chen SS, Jong YJ, Yang YH, Chang YY. A comparison of the stress and coping strategies between the parents of children with Duchenne muscular dystrophy and children with a fever. J Pediatr Nurs 2002;17:369-79. https://doi.org/10.1053/jpdn.2002.123525.
- Chen JY, Clark MJ. Family function in families of children with Duchenne muscular dystrophy. Fam Community Health 2007;30:296-304. http://dx.doi.org/10.1097/01.FCH.0000290542.10458.f8.
- Bendixen RM, Senesac C, Lott DJ, Vandenborne K. Participation and quality of life in children with Duchenne muscular dystrophy using the international classification of functioning, disability, and health. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-43.
- Chen JY. Mediators affecting family function in families of children with Duchenne muscular dystrophy. Kaohsiung J Med Sci 2008;24:514-22. http://dx.doi.org/10.1016/S1607-551X(09)70010-5.
- Read J, Kinali M, Muntoni F, Weaver T, Garralda ME. Siblings of young people with Duchenne muscular dystrophy – a qualitative study of impact and coping. Eur J Paediatr Neurol 2011;15:21-8. http://dx.doi.org/10.1016/j.ejpn.2010.07.006.
- van Wijk E, Messelink BJ, Heijnen L, de Groot IJ. Prevalence and psychosocial impact of lower urinary tract symptoms in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2009;19:754-8. http://dx.doi.org/10.1016/j.nmd.2009.07.009.
- Pangalila RF, van den Bos GA, Stam HJ, van Exel NJ, Brouwer WB, Roebroeck ME. Subjective caregiver burden of parents of adults with Duchenne muscular dystrophy. Disabil Rehabil 2012;34:988-96. http://dx.doi.org/10.3109/09638288.2011.628738.
- Landfeldt E, Lindgren P, Bell CF, Schmitt C, Guglieri M, Straub V, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology 2014;83:529-36. http://dx.doi.org/10.1212/WNL.0000000000000669.
- Rosenberg T, Jacobs HK, Thompson R, Horne JM. Cost-effectiveness of neonatal screening for Duchenne muscular dystrophy – how does this compare to existing neonatal screening for metabolic disorders?. Soc Sci Med 1993;37:541-7. https://doi.org/10.1016/0277-9536(93)90289-G.
- Koch SJ, Arego DE, Bowser B. Outpatient rehabilitation for chronic neuromuscular diseases. Am J Phys Med 1986;65:245-57.
- van der Riet AA, van Hout BA, Rutten FF. Cost effectiveness of DNA diagnosis for four monogenic diseases. J Med Genet 1997;34:741-5. https://doi.org/10.1136/jmg.34.9.741.
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010;9:177-89. http://dx.doi.org/10.1016/S1474-4422(09)70272-8.
- Eagle M. Report on the muscular dystrophy campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscul Disord 2002;12:975-83. https://doi.org/10.1016/S0960-8966(02)00136-0.
- Markert CD, Case LE, Carter GT, Furlong PA, Grange RW. Exercise and Duchenne muscular dystrophy: where we have been and where we need to go. Muscle Nerve 2012;45:746-51. http://dx.doi.org/10.1002/mus.23244.
- Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand 2001;171:311-19. https://doi.org/10.1046/j.1365-201x.2001.00833.x.
- Vignos PJ, Wagner MB, Karlinchak B, Katirji B. Evaluation of a program for long-term treatment of Duchenne muscular dystrophy. Experience at the University Hospitals of Cleveland. J Bone Joint Surg Am 1996;78:1844-52. https://doi.org/10.2106/00004623-199612000-00007.
- Ainslie T. The Concise Guide to Physiotherapy – Volume 2: Treatment. London: Elsevier; 2012.
- Jenkins L, Hyde S, Posselt H. A Home Exercise Book: Physiotherapy Management for Duchenne Muscular Dystrophy. London: MDUK; 2009.
- Skalsky AJ, McDonald CM. Prevention and management of limb contractures in neuromuscular diseases. Phys Med Rehabil Clin N Am 2012;23:675-87. http://dx.doi.org/10.1016/j.pmr.2012.06.009.
- Jansen M, van Alfen N, Geurts ACH, de Groot IJM. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial ‘no use is disuse’. Neurorehabil Neural Repair 2013;27:816-27. https://doi.org/10.1177/1545968313496326.
- Vignos PJ. Physical models of rehabilitation in neuromuscular disease. Muscle Nerve 1983;6:323-38. http://dx.doi.org/10.1002/mus.880060502.
- Lovering RM, Brooks SV. Eccentric exercise in aging and diseased skeletal muscle: good or bad?. J Appl Physiol 2014;116:1439-45. http://dx.doi.org/10.1152/japplphysiol.00174.2013.
- Hyde SA, Fløytrup I, Glent S, Kroksmark A-K, Salling B, Steffensen BF, et al. A randomized comparative study of two methods for controlling Tendo Achilles contracture in Duchenne muscular dystrophy. Neuromuscul Disord 2000;10:257-63. https://doi.org/10.1016/S0960-8966(99)00135-2.
- Kelly M, Darrah J. Aquatic exercise for children with cerebral palsy. Dev Med Child Neurol 2005;47:838-42. https://doi.org/10.1017/S0012162205001775.
- Verhagen AP, Cardoso JR, Bierma-Zeinstra SM. Aquatic exercise and balneotherapy in musculoskeletal conditions. Best Pract Res Clin Rheumatol 2012;26:335-43. http://dx.doi.org/10.1016/j.berh.2012.05.008.
- Kamioka H, Tsutani K, Okuizumi H, Mutoh Y, Ohta M, Handa S, et al. Effectiveness of aquatic exercise and balneotherapy: a summary of systematic reviews based on randomized controlled trials of water immersion therapies. J Epidemiol 2010;20:2-12. https://doi.org/10.2188/jea.JE20090030.
- Guidance on Good Practice in Aquatic Physiotherapy. London: ATACP; 2015.
- Stillwell B. The subjective experiences of those afraid in water. Int J Aquat Res Educ 2011;5:51-60.
- Nicol K, Schmidt-Hansberg M, McMillan J, Teraud J, Bedingfield E. International Series on Sport Sciences Volume 8, Swimming III. Baltimore, MD: University Park Press; 1979.
- Johan Lambeck B, Stanat F, Kinnaird D, Cole A, Becker B. Comprehensive Aquatic Therapy. Philadelphia, PA: Elsevier; 2004.
- Becker BE. Aquatic therapy: scientific foundations and clinical rehabilitation applications. PMR 2009;1:859-72. https://doi.org/10.1016/j.pmrj.2009.05.017.
- Epstein M. Renal effects of head-out water immersion in humans: a 15-year update. Physiol Rev 1992;72:563-621.
- Bishop PA, Frazier S, Smith J, Jacobs D. Physiologic responses to treadmill and water running. Phys Sportsmed 1989;17:87-94. http://dx.doi.org/10.1080/00913847.1989.11709707.
- Wilder RP, Brennan DK. Physiological responses to deep water running in athletes. Sports Med 1993;16:374-80. https://doi.org/10.2165/00007256-199316060-00003.
- Balldin UI, Lundgren CE, Lundvall J, Mellander S. Changes in the elimination of 133 xenon from the anterior tibial muscle in man induced by immersion in water and by shifts in body position. Aerosp Med 1971;42:489-93.
- Martin WH, Montgomery J, Snell PG, Corbett JR, Sokolov JJ, Buckey JC, et al. Cardiovascular adaptations to intense swim training in sedentary middle-aged men and women. Circulation 1987;75:323-30. https://doi.org/10.1161/01.CIR.75.2.323.
- Harrison R, Hillman M, Bulstrode S. Loading of the lower limb when walking partially immersed: implications for clinical practice. Physiotherapy 1992;78:164-6. https://doi.org/10.1016/S0031-9406(10)61377-6.
- Guidance on Good Practice in Hydrotherapy. London: Chartered Society of Physiotherapy; 2006.
- ATACP . About the Aquatic Therapy Association of Chartered Physiotherapists (ATACP) 2014. http://atacp.csp.org.uk/about-atacp (accessed 16 March 2016).
- Foundation Course in Aquatic Physiotherapy. London: Chartered Society of Physiotherapy; 2010.
- Bartels EM, Lund H, Hagen KB, Dagfinrud H, Christensen R, Danneskiold-Samsøe B. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.cd005523.pub2.
- Hall J, Swinkels A, Briddon J, McCabe CS. Does aquatic exercise relieve pain in adults with neurologic or musculoskeletal disease? A systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 2008;89:873-83. http://dx.doi.org/10.1016/j.apmr.2007.09.054.
- Pittler MH, Karagülle MZ, Karagülle M, Ernst E. Spa therapy and balneotherapy for treating low back pain: meta-analysis of randomized trials. Rheumatology 2006;45:880-4. https://doi.org/10.1093/rheumatology/kel018.
- Dumas H, Francesconi S. Aquatic therapy in pediatrics: annotated bibliography. Phys Occup Ther Pediatr 2001;20:63-78. https://doi.org/10.1080/j006v20n04_05.
- Attermeier S, Dulcy FH, Harris SR, Martin K. Aquatics for disabled persons. Phys Occup Ther Pediatr 1983;3:83-92. https://doi.org/10.1080/J006v03n01_07.
- Getz M, Hutzler Y, Vermeer A. Effects of aquatic interventions in children with neuromotor impairments: a systematic review of the literature. Clin Rehabil 2006;20:927-36. https://doi.org/10.1177/0269215506070693.
- Ciafaloni E, Moxley RT. Treatment options for Duchenne muscular dystrophy. Curr Treat Options Neurol 2008;10:86-93. https://doi.org/10.1007/s11940-008-0010-4.
- Hydrotherapy in the UK: The Urgent Need for Increased Access. London: MDUK; 2015.
- Eldridge S, Bond C, Campbell M, Lancaster G, Thabane L, Hopwell S. Definition and reporting of pilot and feasibility studies. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-S1-O18.
- Whitehead AL, Sully BG, Campbell MJ. Pilot and feasibility studies: is there a difference from each other and from a randomised controlled trial?. Contemp Clin Trials 2014;38:130-3. http://dx.doi.org/10.1016/j.cct.2014.04.001.
- Baxter P. HTA – 12 144 04: What Is the Clinical Effectiveness of Hydrotherapy in Maintaining Physical Function in People With Duchenne Muscular Dystrophy? 2014. www.nets.nihr.ac.uk/projects/hta/1214404 (accessed 18 March 2016).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Abend G. The meaning of ‘theory.’. Sociol Theory 2008;26:173-99. https://doi.org/10.1111/j.1467-9558.2008.00324.x.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-1.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j.2002.384.doc.x.
- Ricotti V, Ridout DA, Pane M, Main M, Mayhew A, Mercuri E, et al. The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatr 2016;87:149-55. http://dx.doi.org/10.1136/jnnp-2014-309405.
- Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al. Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9390.
- Dijkers MP. Reporting on interventions: issues and guidelines for rehabilitation researchers. Arch Phys Med Rehabil 2015;96:1170-80. https://doi.org/10.1016/j.apmr.2015.01.017.
- Dijkers M, Hart T, Whyte J, Zanca JM, Packel A, Tsaousides T. Rehabilitation treatment taxonomy: implications and continuations. Arch Phys Med Rehabil 2014;95:45-54.e2. http://dx.doi.org/10.1016/j.apmr.2013.05.033.
- Klingler W, Jurkat-Rott K, Lehmann-Horn F, Schleip R. The role of fibrosis in Duchenne muscular dystrophy. Acta Myol 2012;31:184-95.
- Neufer PD. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med 1989;8:302-20. https://doi.org/10.2165/00007256-198908050-00004.
- Weston CFM, O’Hare JP, Evans JM, Corrall RJM. Haemodynamic changes in man during immersion in water at different temperatures. Clin Sci 1987;73:613-16. https://doi.org/10.1042/cs0730613.
- Wolpe J. Psychotherapy by Reciprocal Inhibition. Stanford, CA: Stanford University Press; 1958.
- Watts FN. Habituation model of systematic desensitization. Psychol Bull 1979;86:627-37. https://doi.org/10.1037/0033-2909.86.3.627.
- Waters WF, McDonald DG, Koresko RL. Psychophysiological responses during analogue systematic desensitization and non-relaxation control procedures. Behav Res Ther 1972;10:381-93. https://doi.org/10.1016/0005-7967(72)90061-7.
- Smith M. Evaluability Assessment: A Practical Approach. Waltham, MA: MA Kluwer; 1989.
- Donaldson S, Lipsey M, Shaw I, Greene J, Mark M. The Handbook of Evaluation: Policies, Programs, and Practices. London: Sage; 2006.
- Coryn CLS, Noakes LA, Westine CD, Schroter DC. A systematic review of theory-driven evaluation practice from 1990 to 2009. Am J Eval 2010;32:199-226. https://doi.org/10.1177/1098214010389321.
- Leeuw FL, Donaldson SI. Theory in evaluation: reducing confusion and encouraging debate. Evaluation 2015;21:467-80. https://doi.org/10.1177/1356389015607712.
- Funnell SC, Rogers PJ. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models. San Francisco, CA: Jossey-Bass; 2011.
- Capjon H, Bjørk IT. Rehabilitation after multilevel surgery in ambulant spastic children with cerebral palsy: children and parent experiences. Dev Neurorehabil 2010;13:182-91. http://dx.doi.org/10.3109/17518421003606151.
- Wiart L, Ray L, Darrah J, Magill-Evans J. Parents’ perspectives on occupational therapy and physical therapy goals for children with cerebral palsy. Disabil Rehabil 2010;32:248-58.
- Christy JB, Saleem N, Turner PH, Wilson J. Parent and therapist perceptions of an intense model of physical therapy. Pediatr Phys Ther 2010;22:207-13. http://dx.doi.org/10.1097/PEP.0b013e3181db8151.
- Redmond R, Parrish M. Variables influencing physiotherapy adherence among young adults with cerebral palsy. Qual Health Res 2008;18:1501-10. http://dx.doi.org/10.1177/1049732308325538.
- Tregaskis C. Social Model Theory: the story so far . Disabil Soc 2002;17:457-70. https://doi.org/10.1080/09687590220140377.
- Morris J. Impairment and disability: constructing an ethics of care that promotes human rights. Hypatia 2001;16:1-16. https://doi.org/10.1111/j.1527-2001.2001.tb00750.x.
- International Classification of Functioning, Disability and Health Children & Youth Version. Geneva: World Health Organization; 2007.
- Koutsogeorgou E, Leonardi M, Bickenbach JE, Cerniauskaite M, Quintas R, Raggi A. Social capital, disability, and usefulness of the International Classification of Functioning, Disability and Health for the development and monitoring of policy interventions. Disabil Soc 2014;29:1104-16. https://doi.org/10.1080/09687599.2014.910106.
- Erikson E. Identity: Youth and Crisis. New York, NY: W.W. Norton & Company; 1968.
- Novak I. Evidence to practice commentary: advancing the evidence and the right to participation. Phys Occup Ther Pediatr 2013;33:421-5. https://doi.org/10.3109/01942638.2013.834179.
- Granlund M. Participation – challenges in conceptualization, measurement and intervention. Child Care Health Dev 2013;39:470-3. https://doi.org/10.1111/cch.12080.
- Raghavendra P. Participation of children with disabilities: measuring subjective and objective outcomes. Child Care Health Dev 2013;39:461-5. http://dx.doi.org/10.1111/cch.12084.
- Shikako-Thomas K, Kolehmainen N, Ketelaar M, Bult M, Law M. Promoting leisure participation as part of health and well-being in children and youth with cerebral palsy. J Child Neurol 2014;29:1125-33. http://dx.doi.org/10.1177/0883073814533422.
- Kanagasabai PS, Mulligan H, Mirfin-Veitch B, Hale LA. Association between motor functioning and leisure participation of children with physical disability: an integrative review. Dev Med Child Neurol 2014;56:1147-62. http://dx.doi.org/10.1111/dmcn.12570.
- Kang L-J, Palisano RJ, King GA, Chiarello LA. A multidimensional model of optimal participation of children with physical disabilities. Disabil Rehabil 2014;36:1735-41. https://doi.org/10.3109/09638288.2013.863392.
- King G, McDougall J, DeWit D, Hong S, Miller L, Offord D, et al. Pathways to children’s academic performance and prosocial behaviour: roles of physical health status, environmental, family, and child factors. Int J Disabil Dev Educ 2005;52:313-44. https://doi.org/10.1080/10349120500348680.
- Reijntjes A, Stegge H, Meerum Terwogt M. Children’s coping with peer rejection: the role of depressive symptoms, social competence, and gender. Infant Child Dev 2006;15:89-107. https://doi.org/10.1002/icd.435.
- Law M, King G, King S, Kertoy M, Hurley P, Rosenbaum P, et al. Patterns of participation in recreational and leisure activities among children with complex physical disabilities. Dev Med Child Neurol 2006;48:337-42. https://doi.org/10.1017/S0012162206000740.
- Bennett KS, Hay DA. The role of family in the development of social skills in children with physical disabilities. Int J Disabil Dev Educ 2007;54:381-97. https://doi.org/10.1080/10349120701654555.
- King G, Law M, Hanna S, King S, Hurley P, Rosenbaum P, et al. Predictors of the leisure and recreation participation of children with physical disabilities: a structural equation modeling analysis. Child Heal Care 2006;35:209-34. https://doi.org/10.1207/s15326888chc3503_2.
- Majnemer A, Shevell M, Law M, Birnbaum R, Chilingaryan G, Rosenbaum P, et al. Participation and enjoyment of leisure activities in school-aged children with cerebral palsy. Dev Med Child Neurol 2008;50:751-8. http://dx.doi.org/10.1111/j.1469-8749.2008.03068.x.
- LaForme Fiss A, Chiarello LA, Bartlett D, Palisano RJ, Jeffries L, Almasri N, et al. Family ecology of young children with cerebral palsy. Child Care Health Dev 2014;40:562-71. http://dx.doi.org/10.1111/cch.12062.
- Lawlor K, Mihaylov S, Welsh B, Jarvis S, Colver A. A qualitative study of the physical, social and attitudinal environments influencing the participation of children with cerebral palsy in northeast England. Pediatr Rehabil 2006;9:219-28. https://doi.org/10.1080/13638490500235649.
- Law M, Haight M, Milroy B, Willms D, Stewart D, Rosenbaum P. Environmental factors affecting the occupations of children with physical disabilities. J Occup Sci 1999;6:102-10. https://doi.org/10.1080/14427591.1999.9686455.
- Shikako-Thomas K, Majnemer A, Law M, Lach L. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pediatr 2008;28:155-69. https://doi.org/10.1080/01942630802031834.
- Anaby D, Hand C, Bradley L, DiRezze B, Forhan M, DiGiacomo A, et al. The effect of the environment on participation of children and youth with disabilities: a scoping review. Disabil Rehabil 2013;35:1589-98. http://dx.doi.org/10.3109/09638288.2012.748840.
- May CR, Eton DT, Boehmer K, Gallacher K, Hunt K, MacDonald S, et al. Rethinking the patient: using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Serv Res 2014;14. http://dx.doi.org/10.1186/1472-6963-14-281.
- Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med 2015;13. http://dx.doi.org/10.1186/s12916-015-0356-x.
- May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2803.
- Mair FS, May CR. Thinking about the burden of treatment. BMJ 2014;349. https://doi.org/10.1136/bmj.g6680.
- Hexem KR, Bosk AM, Feudtner C. The dynamic system of parental work of care for children with special health care needs: a conceptual model to guide quality improvement efforts. BMC Pediatr 2011;11. http://dx.doi.org/10.1186/1471-2431-11-95.
- Eton DT, Ridgeway JL, Egginton JS, Tiedje K, Linzer M, Boehm DH, et al. Finalizing a measurement framework for the burden of treatment in complex patients with chronic conditions. Patient Relat Outcome Meas 2015;6:117-26. http://dx.doi.org/10.2147/PROM.S78955.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: Normalization Process Theory. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-29.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of Normalization Process Theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Dulcy FH. Aquatic programs for disabled children: an overview and an analysis of the problems. Phys Occup Ther Pediatr 1983;3:1-20. https://doi.org/10.1080/j006v03n01_01.
- Dulcy FH. A theoretical aquatic service intervention model for disabled children. Phys Occup Ther Pediatr 1983;3:21-38. https://doi.org/10.1080/J006v03n01_02.
- McLaughlin JA, Jordan GB. Logic models: a tool for telling your program’s performance story. Eval Program Plann 1999;22:65-72. https://doi.org/10.1016/S0149-7189(98)00042-1.
- W.K. Kellogg Foundation . Logic Model Development Guide 2004. www.wkkf.org/knowledge-center/resources/2006/02/WK-Kellogg-Foundation-Logic-Model-Development-Guide.aspx (accessed 9 February 2017).
- Tahan H. Essential activities and knowledge domains of case management: new insights from the CCMC role and functions study. Case Manager 2006;17:45-8. https://doi.org/10.1016/j.casemgr.2006.03.005.
- Brault GL, Kissinger LD. Case management: ambiguous at best. J Pediatr Health Care 1991;5:179-83. https://doi.org/10.1016/0891-5245(91)90058-X.
- Scheirer MA, Dearing JW. An agenda for research on the sustainability of public health programs. Am J Public Health 2011;101:2059-67. http://dx.doi.org/10.2105/AJPH.2011.300193.
- Fleiszer AR, Semenic SE, Ritchie JA, Richer MC, Denis JL. The sustainability of healthcare innovations: a concept analysis. J Adv Nurs 2015;71:1484-98. http://dx.doi.org/10.1111/jan.12633.
- Rogers E, Rogers E. Diffusion of Innovations. New York, NY: Free Press; 2003.
- Gruen RL, Elliott JH, Nolan ML, Lawton PD, Parkhill A, McLaren CJ, et al. Sustainability science: an integrated approach for health-programme planning. Lancet 2008;372:1579-89. http://dx.doi.org/10.1016/S0140-6736(08)61659-1.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h1258.
- Linnan L, Steckler A. Process Evaluation for Public Health Interventions and Research: An Overview. San Francisco, CA: Jossey-Bass; 2002.
- Baranowski T, Stables G. Process evaluations of the 5-a-day projects. Health Educ Behav 2000;27:157-66. https://doi.org/10.1177/109019810002700202.
- McGraw SA, Stone EJ, Osganian SK, Elder JP, Perry CL, Johnson CC, et al. Design of process evaluation within the Child and Adolescent Trial for Cardiovascular Health (CATCH). Health Educ Q 1994:5-26. https://doi.org/10.1177/10901981940210S103.
- Dusenbury L, Brannigan R, Falco M, Hansen WB. A review of research on fidelity of implementation: implications for drug abuse prevention in school settings. Health Educ Res 2003;18:237-56. https://doi.org/10.1093/her/18.2.237.
- Wears RL. Standardisation and its discontents. Cogn Technol Work 2015;17:89-94. http://dx.doi.org/10.1007/s10111-014-0299-6.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. http://dx.doi.org/10.1136/bmj.328.7455.1561.
- Hawe P. Minimal, negligible and negligent interventions. Soc Sci Med 2015;138:265-8. https://doi.org/10.1016/j.socscimed.2015.05.025.
- Haynes A, Brennan S, Redman S, Williamson A, Gallego G, Butow P. CIPHER team . Figuring out fidelity: a worked example of the methods used to identify, critique and revise the essential elements of a contextualised intervention in health policy agencies. Implement Sci 2016;11. http://dx.doi.org/10.1186/s13012-016-0378-6.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;11.
- Pane M, Mazzone ES, Sivo S, Sormani MP, Messina S, D’Amico A, et al. Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes. PLOS ONE 2014;9:6-11. https://doi.org/10.1371/journal.pone.0108205.
- Hind D, Parkin J, Whitworth V, Rex S, Young T, Hampson L, et al. Aquatic therapy for boys with Duchenne muscular dystrophy (DMD): an external pilot randomised controlled trial. Pilot Feasibil Stud 2017;3. http://dx.doi.org/10.1186/s40814-017-0132-0.
- McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2010;41:500-10. http://dx.doi.org/10.1002/mus.21544.
- Scott E, Eagle M, Mayhew A, Freeman J, Main M, Sheehan J, et al. Development of a functional assessment scale for ambulatory boys with Duchenne muscular dystrophy. Physiother Res Int 2012;17:101-9. http://dx.doi.org/10.1002/pri.520.
- Mayhew A, Cano S, Scott E, Eagle M, Bushby K, Muntoni F, et al. Moving towards meaningful measurement: Rasch analysis of the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev Med Child Neurol 2011;53:535-42. https://doi.org/10.1111/j.1469-8749.2011.03939.x.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. https://doi.org/10.1183/09031936.05.00034805.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. http://dx.doi.org/10.2165/11587350-000000000-00000.
- Vandervelde L, Van den Bergh PY, Goemans N, Thonnard JL. ACTIVLIM: a Rasch-built measure of activity limitations in children and adults with neuromuscular disorders. Neuromuscul Disord 2007;17:459-69. https://doi.org/10.1016/j.nmd.2007.02.013.
- Brouwer WB, van Exel NJ, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res 2006;15:1005-21. http://dx.doi.org/10.1007/s11136-005-5994-6.
- Robertson RJ, Goss FL, Boer NF, Peoples JA, Foreman AJ, Dabayebeh IM, et al. Children’s OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sports Exerc 2000;32:452-8. https://doi.org/10.1097/00005768-200002000-00029.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40. https://doi.org/10.1002/sim.4780141709.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J. Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study – a mixed methods study. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18380.
- O’Cathain A, Goode J, Drabble SJ, Thomas KJ, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-215.
- O’Cathain A, Hoddinott P, Lewin S, Thomas KJ, Young B, Adamson J, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0026-y.
- Creswell JW. Research Design. London: Sage; 2014.
- Carter SM, Little M. Taking action: epistemologies, methodologies, and methods in qualitative research. Qual Health Res 2007;17:1316-28. https://doi.org/10.1177/1049732307306927.
- Cherryholmes CH. Notes on pragmatism and scientific realism. Educ Res 1992;21:13-7. https://doi.org/10.3102/0013189X021006013.
- Eakin JM. Educating critical qualitative health researchers in the land of the randomized controlled trial. Qual Inq 2016;22:107-18. https://doi.org/10.1177/1077800415617207.
- Peirce CS, Thayer HS. Meaning and Action: A Critical History of Pragmatism. Indianapolis, IN: Hackett; 1984.
- Dewey J, Thayer H. Pragmatism: The Classic Writings. Indianapolis, IN: Hackett; 1989.
- Burawoy M. The extended case method. Sociol Theory 1998;16:4-33. https://doi.org/10.1111/0735-2751.00040.
- Timmermans S, Tavory I. Theory construction in qualitative research: from grounded theory to abductive analysis. Sociol Theory 2012;30:167-86. https://doi.org/10.1177/0735275112457914.
- O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med 2014;89:1245-51. http://dx.doi.org/10.1097/ACM.0000000000000388.
- Yin RK, Yin RK. Case Study Research: Design and Methods. London: Sage; 2014.
- Finch TL, Rapley T, Girling M, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8. http://dx.doi.org/10.1186/1748-5908-8-43.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-63.
- May CR, Finch T, Ballini L, MacFarlane A, Mair F, Murray E, et al. Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-245.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-37.
- NHS Employers . Agenda for Change Pay Bands and Spine Points from April 2016 2016. www.nhsemployers.org/~/media/Employers/Documents/Pay and reward/AfC pay bands from 1 April 2016_FINAL.pdf (accessed 16 May 2016).
- Mahon A, Glendinning C, Clarke K, Craig G. Researching children: methods and ethics. Child Soc 1996;10:145-54. https://doi.org/10.1002/(SICI)1099-0860(199606)10:2<145::AID-CHI19>3.0.CO;2-H.
- Francis JJ, Johnston M, Robertson C, Glidewell L, Entwistle V, Eccles MP, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229-45. http://dx.doi.org/10.1080/08870440903194015.
- Guest G. How many interviews are enough?: an experiment with data saturation and variability. Field Methods 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- O’Reilly M, Parker N. ‘Unsatisfactory saturation’: a critical exploration of the notion of saturated sample sizes in qualitative research. Qual Res 2012;13:190-7. https://doi.org/10.1177/1468794112446106.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2015.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. Abingdon: Routledge; 1994.
- Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med 2011;9. http://dx.doi.org/10.1186/1741-7015-9-39.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol 2005;52:250-60. https://doi.org/10.1037/0022-0167.52.2.250.
- Pfadenhauer LM, Mozygemba K, Gerhardus A, Hofmann B, Booth A, Lysdahl KB, et al. Context and implementation: a concept analysis towards conceptual maturity. Z Evid Fortbild Qual Gesundhwes 2015;109:103-14. http://dx.doi.org/10.1016/j.zefq.2015.01.004.
- Shoveller J, Viehbeck S, Di Ruggiero E, Greyson D, Thomson K, Knight R. A critical examination of representations of context within research on population health interventions. Crit Public Health 2015;1596:1-14.
- Greenhalgh T, Russell J. Why do evaluations of eHealth programs fail? An alternative set of guiding principles. PLOS Med 2010;7. http://dx.doi.org/10.1371/journal.pmed.1000360.
- Morgan H, Thomson G, Crossland N, Dykes F, Hoddinott P. Combining PPI with qualitative research to engage ‘harder-to-reach’ populations: service user groups as co-applicants on a platform study for a trial. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0023-1.
- Staley K, Doherty C. It’s not evidence, it’s insight: bringing patients’ perspectives into health technology appraisal at NICE. Res Involv Engagem 2016;2. https://doi.org/10.1186/s40900-016-0018-y.
- O’Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in health services research in England: a mixed methods study. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-85.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. https://doi.org/10.1177/1049732305285708.
- Simmons T. Attributing the Costs of Health and Social Care Research & Development (AcoRD). London: Department of Health; 2012.
- Myles PS, Williamson E, Oakley J, Forbes A. Ethical and scientific considerations for patient enrollment into concurrent clinical trials. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-470.
- Health and Social Care Act 2012. London: The Stationery Office; 2012.
- Checkland K, Mcdermott I, Coleman A, Perkins N, Checkland KH. Complexity in the new NHS: longitudinal case studies of CCGs in England. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010199.
- McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Atkinson L, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle Nerve 2010;42:966-74. http://dx.doi.org/10.1002/mus.21808.
- Mayhew AG, Cano SJ, Scott E, Eagle M, Bushby K, Manzur A, et al. North Star Clinical Network for Neuromuscular Disease. Detecting meaningful change using the North Star Ambulatory Assessment in Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1046-52. http://dx.doi.org/10.1111/dmcn.12220.
- Lauruschkus K, Nordmark E, Hallström I. ‘It’s fun, but?…’ Children with cerebral palsy and their experiences of participation in physical activities. Disabil Rehabil 2014;8288:1-7.
- Webb CL. Parents’ perspectives on coping with Duchenne muscular dystrophy. Child Care Health Dev 2005;31:385-96. https://doi.org/10.1111/j.1365-2214.2005.00518.x.
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010;9:77-93. https://doi.org/10.1016/S1474-4422(09)70271-6.
- Kieny P, Chollet S, Delalande P, Le Fort M, Magot A, Pereon Y, et al. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann Phys Rehabil Med 2013;56:443-54. http://dx.doi.org/10.1016/j.rehab.2013.06.002.
- Redmond B, Richardson V. Just getting on with it: exploring the service needs of mothers who care for young children with severe/profound and life-threatening intellectual disability. J Appl Res Intellect Disabil 2003;16:205-18. https://doi.org/10.1046/j.1468-3148.2003.00165.x.
- Beresford BA. Resources and strategies: how parents cope with the care of a disabled child. J Child Psychol Psychiatry 1994;35:171-209. https://doi.org/10.1111/j.1469-7610.1994.tb01136.x.
- Read J. There was never really any choice: the experience of mothers of disabled children in the United Kingdom. Women’s Stud Int Forum 1991;14:561-71. https://doi.org/10.1016/0277-5395(91)90026-E.
- Sloper P. Models of service support for parents of disabled children. What do we know? What do we need to know?. Child Care Health Dev 1999;25:85-99. https://doi.org/10.1046/j.1365-2214.1999.25220120.x.
- Beresford B. The needs of disabled children and their families. Soc Care Res 1995;76:1-4.
- McKeever P, Miller KL. Mothering children who have disabilities: a Bourdieusian interpretation of maternal practices. Soc Sci Med 2004;59:1177-91. http://dx.doi.org/10.1016/j.socscimed.2003.12.023.
- Leiter V. The consequences of caring: effects of mothering a child with special needs. J Fam Issues 2004;25:379-403. https://doi.org/10.1177/0192513X03257415.
- Brett J. The experience of disability from the perspective of parents of children with profound impairment: is it time for an alternative model of disability?. Disabil Soc 2002;17:825-43. https://doi.org/10.1080/0968759022000039109.
- Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol 2012;65:1041-51. http://dx.doi.org/10.1016/j.jclinepi.2012.05.005.
- Trute B. Grandparents of children with developmental disabilities: intergenerational support and family well-being. Fam Soc J Contemp Soc Serv 2003;84:119-26. https://doi.org/10.1606/1044-3894.87.
- Knussen C, Sloper P. Stress in families of children with disability: a review of risk and resistance factors. J Ment Heal 1992;1:241-56. https://doi.org/10.3109/09638239209005457.
- Beresford B. Positively Parents – Caring for a Severely Disabled Child. London: Social Policy Research Unit, University of York; 1994.
- Clarke L, Cairns H, Broad B. Kinship Care: The Placement Choice for Children and Young People. Lyme Regis: Russell House Publishing; 2001.
- Wheelock J, Jones K. ‘Grandparents are the next best thing’: informal childcare for working parents in urban Britain. J Soc Policy 2002;31:441-63. https://doi.org/10.1017/S0047279402006657.
- Mitchell W. Research review: the role of grandparents in intergenerational support for families with disabled children: a review of the literature. Child Fam Soc Work 2007;12:94-101. https://doi.org/10.1111/j.1365-2206.2006.00421.x.
- Putnam RD. The prosperous community: social capital and public life. Am Prospect 1993;13:35-42.
- Granovetter MS. The strength of weak ties. Am J Sociol 1973;78:1360-80. https://doi.org/10.1086/225469.
- Kersten P, Stokes M. Physical Management in Neurological Rehabilitation. London: Elsevier; 2004.
- Green LW. From research to ‘best practices’ in other settings and populations. Am J Health Behav 2001;25:165-78. https://doi.org/10.5993/AJHB.25.3.2.
- Weintraub M, Buncher C, Tsay J. Statistics in the Pharmaceutical Industry. Boca Raton, FL: Chapman & Hall/CRC; 1993.
- Mallinson S. Listening to respondents: a qualitative assessment of the Short-Form 36 Health Status Questionnaire. Soc Sci Med 2002;54:11-2. https://doi.org/10.1016/S0277-9536(01)00003-X.
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit; 2015.
- Aneurin Bevan Health Board . Closure of the Hydrotherapy Pool at Llanfrechfa Grange Hospital: Minutes 22 May 2013 2013. www.wales.nhs.uk/sitesplus/documents/866/3.11%20Hydrotherapy%20Pool.pdf (accessed 16 May 2016).
- Annual Survey of Hours and Earnings. London: Office for National Statistics; 2015.
- Automobile Association . Guide to Car Running Costs 2015. www.theaa.com/motoring_advice/running_costs/advice_rcosts_guide.html (accessed 24 May 2016).
- Reference Costs Guidance 2015–16. London: Department of Health; 2016.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Parry G, Power M. To RCT or not to RCT? The ongoing saga of randomised trials in quality improvement. BMJ Qual Saf 2016;25:221-3. http://dx.doi.org/10.1136/bmjqs-2015-004862.
- Hoddinott P. A new era for intervention development studies. Pilot Feasibility Stud 2015;1.
- Asch DA, Rosin R. Innovation as discipline, not fad. N Engl J Med 2015;373:592-4. http://dx.doi.org/10.1056/NEJMp1506311.
- Volpp KG, Terwiesch C, Troxel AB, Mehta S, Asch DA. Making the RCT more useful for innovation with evidence-based evolutionary testing. Healthcare 2013;1:4-7. http://dx.doi.org/10.1016/j.hjdsi.2013.04.007.
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301-8. http://dx.doi.org/10.1016/j.jclinepi.2011.07.011.
- Imms C, Adair B, Keen D, Ullenhag A, Rosenbaum P, Granlund M. ‘Participation’: a systematic review of language, definitions, and constructs used in intervention research with children with disabilities. Dev Med Child Neurol 2016;58:29-38. http://dx.doi.org/10.1111/dmcn.12932.
- Murray SA, Kendall M, Carduff E, Worth A, Harris FM, Lloyd A, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3702.
- Weiss CH. Theory-based evaluation: past, present, and future. New Dir Eval 1997;1997:41-55. https://doi.org/10.1002/ev.1086.
- Saunders RP, Evans MH, Joshi P. Developing a process-evaluation plan for assessing health promotion program implementation: a how-to guide. Health Promot Pract 2005;6:134-47. https://doi.org/10.1177/1524839904273387.
- Caracelli VJ, Riggin L. Mixed-method evaluation: developing quality criteria through concept mapping. Am J Eval 1994;15:139-52. https://doi.org/10.1177/109821409401500204.
- Special Needs and Parent Support . Our Costs 2016. https://snapsyorkshire.org/pricing-page/ (accessed 4 July 2016).
- Jones P. New Care Models: Empowering Patients and Communities – A Call to Action for a Directory of Support. London: NHS England; 2015.
- Coulter A, Roberts S, Dixon A. Delivering Better Services for People with Long-Term Conditions: Building the House of Care. London: The King’s Fund; 2013.
- McDonagh JE, Minnaar G, Kelly K, O’Connor D, Shaw KL. Unmet education and training needs in adolescent health of health professionals in a UK children’s hospital. Acta Paediatr 2006;95:715-19. https://doi.org/10.1080/08035250500449858.
- Sawyer SM. Developmentally appropriate healthcare for young people with chronic illness: questions of philosophy, policy, and practice. Pediatr Pulmonol 2003;36:363-5. https://doi.org/10.1002/ppul.10369.
- Vaz S, Cordier R, Falkmer M, Ciccarelli M, Parsons R, McAuliffe T, et al. Should schools expect poor physical and mental health, social adjustment, and participation outcomes in students with disability?. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0126630.
- Farre A, Wood V, McDonagh JE, Parr JR, Reape D, Rapley T. Transition Collaborative Group. Health professionals’ and managers’ definitions of developmentally appropriate healthcare for young people: conceptual dimensions and embedded controversies. Arch Dis Child 2016;101:628-33. http://dx.doi.org/10.1136/archdischild-2015-309473.
- Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, et al. Coproduction of healthcare service. BMJ Qual Saf 2016;25:509-17. https://doi.org/10.1136/bmjqs-2015-004315.
- Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual Saf 2016;25:626-32. http://dx.doi.org/10.1136/bmjqs-2015-004839.
- Faulkner A, Yiannoullou S, Kalathil J, Crepaz-Keay D, Singer F, James N, et al. Involvement for Influence. 4PI National Involvement Standards. London: National Survivor User Network; 2015.
- Evaluation: What to Consider. London: Health Foundation; 2015.
- United Nations . Convention on the Rights of Persons With Disability 2006. www.un.org/development/desa/disabilities/convention-on-the-rights-of-persons-with-disabilities/convention-on-the-rights-of-persons-with-disabilities-2.html (accessed 20 March 2016).
- Children and Families Act 2014. London: Her Majesty’s Stationery Office; n.d.
- Special Educational Needs and Disability Code of Practice: 0 to 25 years: Statutory Guidance for Organisations which Work with and Support Children and Young People who have Special Educational Needs or Disabilities. London: Department for Education; 2015.
- Department of Health . You’re Welcome: Quality Criteria for Young People Friendly Services 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/ (accessed 8 March 2016).
- Suris JC, Michaud PA, Viner R. The adolescent with a chronic condition. Part I: developmental issues. Arch Dis Child 2004;89:938-42. http://dx.doi.org/10.1136/adc.2003.045369.
- Palisano RJ, Chiarello LA, King GA, Novak I, Stoner T, Fiss A. Participation-based therapy for children with physical disabilities. Disabil Rehabil 2012;34:1041-52. http://dx.doi.org/10.3109/09638288.2011.628740.
- Øien I, Fallang B, Østensjø S. Goal-setting in paediatric rehabilitation: perceptions of parents and professional. Child Care Health Dev 2010;36:558-65. http://dx.doi.org/10.1111/j.1365-2214.2009.01038.x.
- An M, Palisano RJ. Family-professional collaboration in pediatric rehabilitation: a practice model. Disabil Rehabil 2014;36:434-40. https://doi.org/10.3109/09638288.2013.797510.
- Baldwin P, King G, Evans J, McDougall S, Tucker MA, Servais M. Solution-focused coaching in pediatric rehabilitation: an integrated model for practice. Phys Occup Ther Pediatr 2013;33:467-83. http://dx.doi.org/10.3109/01942638.2013.784718.
- Bork BE, Cook TM, Rosecrance JC, Engelhardt KA, Thomason ME, Wauford IJ, et al. Work-related musculoskeletal disorders among physical therapists. Phys Ther 1996;76:827-35. https://doi.org/10.1093/ptj/76.8.827.
- Kavlak E, Altuğ F, Büker N, Şenol H. Musculoskeletal system problems and quality of life of mothers of children with cerebral palsy with different levels of disability. J Back Musculoskelet Rehabil 2015;28:803-10. http://dx.doi.org/10.3233/BMR-150588.
- Peek K, Sanson-Fisher R, Mackenzie L, Carey M. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: a systematic review. Physiotherapy 2015;102:127-35. https://doi.org/10.1016/j.physio.2015.10.003.
- Klein R, Maybin J. Thinking About Rationing. London: The King’s Fund; 2012.
- Buchner T, Smyth F, Biewer G, Shevlin M, Ferreira MAV, Toboso Martín M, et al. Paving the way through mainstream education: the interplay of families, schools and disabled students. Res Pap Educ 2015;30:411-26. https://doi.org/10.1080/02671522.2014.989175.
- Simeonsson RJ, Carlson D, Huntington GS, McMillen JS, Brent JL. Students with disabilities: a national survey of participation in school activities. Disabil Rehabil 2001;23:49-63. https://doi.org/10.1080/096382801750058134.
- Bolin K, Lindgren B, Lindström M, Nystedt P. Investments in social capital – implications of social interactions for the production of health. Soc Sci Med 2003;56:2379-90. https://doi.org/10.1016/S0277-9536(02)00242-3.
- Social Action: Harnessing the Potential: A Discussion Paper. London: Cabinet Office; 2015.
- Solomon M, Pistrang N, Barker C. The benefits of mutual support groups for parents of children with disabilities. Am J Community Psychol 2001;29:113-32. https://doi.org/10.1023/A:1005253514140.
- Rimmer JH, Vanderbom KA, Graham ID. A new framework and practice center for adapting, translating, and scaling evidence-based health/wellness programs for people with disabilities. J Neurol Phys Ther 2016;40:107-14. http://dx.doi.org/10.1097/NPT.0000000000000124.
- Milat AJ, Bauman A, Redman S. Narrative review of models and success factors for scaling up public health interventions. Implement Sci 2015;10. https://doi.org/10.1186/s13012-015-0301-6.
- Wensing M, Bosch M, Grol R. Developing and selecting interventions for translating knowledge to action. CMAJ 2010;182:E85-8. https://doi.org/10.1503/cmaj.081233.
- Gagnon ML. Moving knowledge to action through dissemination and exchange. J Clin Epidemiol 2011;64:25-31. http://dx.doi.org/10.1016/j.jclinepi.2009.08.013.
- Lomas J. The in-between world of knowledge brokering. BMJ 2007;334:129-32. https://doi.org/10.1136/bmj.39038.593380.AE.
- Barker PM, Reid A, Schall MW. A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci 2016;11. http://dx.doi.org/10.1186/s13012-016-0374-x.
- Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-139.
- Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10:1-13. https://doi.org/10.1186/s13012-015-0242-0.
- Pinnock H, Epiphaniou E, Sheikh A, Griffiths C, Eldridge S, Craig P, et al. Developing standards for reporting implementation studies of complex interventions (StaRI): a systematic review and e-Delphi. Implement Sci 2015;10. http://dx.doi.org/10.1186/s13012-015-0235-z.
- Sloper P. Facilitators and barriers for co-ordinated multi-agency services. Child Care Health Dev 2004;30:571-80. https://doi.org/10.1111/j.1365-2214.2004.00468.x.
- Cameron A, Lart R. Factors promoting and obstacles hindering joint working: a systematic review of the research evidence. J Integr Care 2003;11:9-17. https://doi.org/10.1108/14769018200300013.
- Peay HL, Scharff H, Tibben A, Wilfond B, Bowie J, Johnson J, et al. ‘Watching time tick by…’: Decision making for Duchenne muscular dystrophy trials. Contemp Clin Trials 2016;46:1-6. http://dx.doi.org/10.1016/j.cct.2015.11.006.
- Lambert V, Glacken M. Engaging with children in research: theoretical and practical implications of negotiating informed consent/assent. Nurs Ethics 2011;18:781-80. https://doi.org/10.1177/0969733011401122.
- Portela MC, Pronovost PJ, Woodcock T, Carter P, Dixon-Woods M. How to study improvement interventions: a brief overview of possible study types. BMJ Qual Saf 2015;24:325-36. https://doi.org/10.1136/bmjqs-2014-003620.
- Anaby D, Lal S, Huszczynski J, Maich J, Rogers J, Law M. Interrupted time series design: a useful approach for studying interventions targeting participation. Phys Occup Ther Pediatr 2014;34:457-70. http://dx.doi.org/10.3109/01942638.2013.866612.
- Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. BMJ 1996;312:1016-20. https://doi.org/10.1136/bmj.312.7037.1016.
- Raggi A, Leonardi M. Assessing activity limitations in patients with neuromuscular diseases: is the ACTIVLIM questionnaire linked to ICF and ICF-CY?. Int J Rehabil Res 2009;32:148-53. https://doi.org/10.1097/MRR.0b013e32831e4573.
- Tudur Smith C, Hopkins C, Sydes MR, Woolfall K, Clarke M, Murray G, et al. How should individual participant data (IPD) from publicly funded clinical trials be shared?. BMC Med 2015;13. http://dx.doi.org/10.1186/s12916-015-0532-z.
Appendix 1 Changes to protocol
| Changes to protocol | Progress report | Date | Approved by |
|---|---|---|---|
| Protocol Version 2 (11 June 2014) | |||
| This protocol amendment was in relation to the following: the exclusion criterion ‘involvement in another randomised controlled trial’ was added and it was stated that their GP will be informed of the participant’s involvement via post. Both were added as a result of the original ethical review process | 1 (31 October 2014) | 4 July 2014 | NRES Committee East of England – Cambridge South |
| Protocol Version 3 (15 August 2014) | |||
| This protocol amendment was in relation to the following: it was clarified that the 6MWD should be completed only once at baseline and 26 weeks. The NSAA score and FVC for visit 1 are both routine measurements and are completed each time a participant attended clinic; therefore, the protocol was updated to state a NSAA or FVC completed up to 4 weeks before consent could be used as the data for visit 1. The pain VAS and urine dipstick would now be completed before and after each AT session. The chief investigator was removed as being blind from treatment allocation. Inclusion criterion 3 was amended to clarify what classed as ‘no major changes to drug, dosage or frequency’. Renal failure was listed as a contraindication and precaution in error, and was therefore removed as a precaution. Transient conditions in Table 2 were amended to clarify that participants should be excluded until the condition resolved. The window for trial interventions to begin was extended to within 2–4 weeks of the date of randomisation. It was clarified that ‘receipt of intervention’ and ‘the number of sessions attended’ would be feasibility outcomes for the study and not protocol non-compliance. The trial interventions section relating to the control group was amended to clarify that the research physiotherapists would prescribe the LBT and inform the community physiotherapist about the combination of exercises prescribed; in addition, parents would post the completed LBT forms to the university team or hand them to the research physiotherapist. Participants were also asked to take their LBT prescription to the community physiotherapist so that the community physiotherapist can note any changes. The process of sending up to three reminder letters to parents was outlined. Data handling and record keeping were clarified, for example regarding the qualitative element of the trial in which researchers would contact the participants. The words ‘and SARs’ were removed from the description about SUSARs. Finally the sentence ‘The Medicines for Human Use (Clinical Trials) Regulations 2004 SI: 1031 plus subsequent amendments’ was replaced with ‘This clinical trial will be conducted in accordance with Good Clinical Practice Guidelines and CTRU standard operating procedures’ as the trial was not a CTIMP | 2 (1 May 2015) | 26 November 2014 | NRES Committee East of England – Cambridge South |
| Protocol Version 4 (9 January 2015) | |||
| This protocol amendment was in relation to the following: one of the clinical outcomes that the trial intended to measure was whether or not myoglobin was present in the urine of participants who underwent AT. This outcome was removed because of the following reasons: clinically, it was felt that pain and exertion were the most important key outcomes before/after AT sessions, these were already being measured for the trial; research physiotherapists who routinely administer physiotherapy and AT advised that taking urine samples for dipstick testing is not a standard measure taken for children who undergo AT routinely; biochemistry colleagues advised that such testing was unlikely to detect myoglobinuria immediately after exercise such as AT; both the research ethics committee and NIHR expressed concerns about the burden of this testing for participants and their families | 2 (1 May 2015) | 30 January 2015 | NRES Committee East of England – Cambridge South |
| Protocol Version 5 (30 October 2015) | |||
| The references were updated in section 6.4 of the protocol | Approved after final progress report submitted | n/a | NRES Committee East of England – Cambridge South |
| Protocol Version 6 (18 November 2015) | |||
| This protocol amendment was in relation to the following: Version 5 of the protocol allowed the collection of a post-AT session OMNI score to measure the level of exertion in boys after their AT session. The physiotherapists delivering the intervention felt that it was clinically important to measure their pre-AT levels of exertion and, as such, an amendment was submitted to allow the analysis of these data. We gained approval to include the pre-AT OMNI data collected | Approved after final progress report submitted | n/a | NRES Committee East of England – Cambridge South |
Appendix 2 Topic guide for participants and parents/guardians
Appendix 3 Topic guide for health professionals
Glossary
- Aetiology
- The causes of a disease or condition.
- Ambulation
- Walking.
- Bayesian
- Tradition of statistical methods distinct from mainstream ‘frequentist’ approaches.
- Blinded/blinding
- Concealment of group allocation from individuals involved in a randomised controlled trial.
- Contractures
- A condition involving the shortening and hardening of muscles, tendons or other tissue, often leading to deformity and rigidity of joints.
- Contraindications
- Situation in which a procedure should not be used because it may be harmful.
- Convergence coding/assessment
- Summary of similarities and differences between two sets of data.
- Cytoskeleton
- Microscopic network of protein filaments and tubules that give living cells shape and coherence.
- Disuse atrophy
- Withering of muscles as a result of lack of muscle use.
- Dorsiflexion
- Flexion between the foot and the front of the leg.
- Dystrophin
- A protein found in skeletal muscle, which is absent in patients with Duchenne muscular dystrophy.
- Dystrophinopathy
- Duchenne or Becker muscular dystrophy.
- Epidemiology
- The study of incidence, distribution and possible control of diseases.
- Framework analysis
- Qualitative method, suited for applied policy research.
- Frequentist
- Mainstream tradition of statistical methods.
- Glucocorticoid corticosteroids
- A class of anti-inflammatory drugs.
- Haemodialysis
- Kidney dialysis.
- Hydrodynamic
- Motion of fluids and the forces acting on solid bodies immersed in fluids and in motion relative to them.
- Hydrostatic
- Equilibrium of liquids and the pressure exerted by liquid at rest.
- Hydrotherapy
- Use of exercises in a pool as part of treatment for conditions. Increasingly referred to as ‘aquatic therapy’.
- Hypertonia
- An abnormal increase in muscle tension and the reduced ability of a muscle to stretch, caused by injury to motor pathways in the central nervous system.
- Hypertrophy
- Enlargement of an organ or tissue from the increase in size of its cells.
- Hypotonia
- Low muscle tone.
- Iliotibial tract/band
- Long fibrous connective tissue that helps the thigh muscle to extend or rotate the hip.
- Learning support assistant
- A person, often referred to as a teaching assistant, who supports teachers and pupils in mainstream schools with special educational needs units and special schools.
- Logic model
- A tool used to evaluate the implementation of a programme of care.
- MEDLINE
- Bibliographic database.
- Musculature
- The arrangement of muscles in a body.
- Myoglobin/myoglobinuria
- The presence of the protein myoglobin in the urine, usually associated with muscle damage.
- Neurodevelopment
- The growth and development of the brain or central nervous system.
- NVivo (QSR International, Warrington, UK)
- Computer software package for qualitative data analysis.
- Orthosis
- An external device that may support the limbs/spine or prevent/assist movement.
- Orthotics
- Devices such as splints and braces.
- Pathology
- Effects of diseases.
- Prognosis
- The probable future course of a medical condition.
- Scoliosis
- Twisting leading to the lateral curvature of the spine.
- Sequential design
- Sometimes referred to as cross-sequential design, defined as a combination of longitudinal and cross-sectional design by following several differently aged cohorts over time.
- Success criteria
- The basis on which the feasibility of a research study is to be demonstrated.
- Teaching assistant
- See Learning support assistant.
- Tertiary centre
- A large hospital that provides specialist health care and receives referrals from secondary and primary care.
- Thermodynamics
- Relationships between heat and mechanical energy.
- Transmembrane
- Existing or occurring across a cell membrane.
- Triangulation
- Method used by qualitative researchers to establish trustworthiness by comparing the findings of different methods or perspectives.
- Triceps surae
- A muscle in the calf area of the leg.
- Viscoelastic deformation
- The ability of some tissues to stretch and remain stretched for some time before slowly returning to their original length.
- Viscosity
- The body’s inhibition of quick muscle reaction as it slows contraction, preventing tearing during loading.
List of abbreviations
- 6MWD
- 6-minute walk distance
- ACTIVLIM
- Activity Limitations Measure
- AT
- aquatic therapy
- ATACP
- Aquatic Therapy Association of Chartered Physiotherapists
- CarerQoL
- Care-related Quality of Life
- CHU-9D
- Child Health Utility 9D Index
- CI
- confidence interval
- CTRU
- clinical trials research unit
- DMD
- Duchenne muscular dystrophy
- FVC
- forced vital capacity
- ICF-CY
- International Classification of Functioning, Disability and Health – Children and Youth version
- IQR
- interquartile range
- LBT
- land-based therapy
- MDUK
- Muscular Dystrophy UK
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- NSAA
- North Star Ambulatory Assessment
- PPI
- patient and public involvement
- RCT
- randomised controlled trial
- SD
- standard deviation
- SOAP
- Subjective, Objective, Assessment and Plan
- TMG
- Trial Management Group
