Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/01/38. The contractual start date was in January 2009. The draft report began editorial review in January 2015 and was accepted for publication in June 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dr Rawstron reports personal fees from Roche, personal fees from Biogen Idec, personal fees from Gilead, personal fees from Abbvie, personal fees and non-financial support from BD Biosciences, personal fees from Celgene and personal fees from GlaxoSmithKline, outside the submitted work. Professor Gregory reports personal fees from Janssen and personal fees from Celgene, outside the submitted work. Professor Hillmen received research funding and speakers’ fees from Roche Pharmaceuticals and grants and personal fees from GlaxoSmithKline, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Howard et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Sections of this chapter have been reproduced from Howard et al. 1 with permission.
Scientific background
Chronic lymphocytic leukaemia (CLL) is the most common adult leukaemia, affecting approximately 6.9 per 100,000 of the population. The incidence of CLL increases with age and twice as many men are affected as women. CLL results from the clonal proliferation of B-cells and is diagnosed by the pattern of expression of various cell surface antigens on the CLL cells. Patients most commonly present with lymphocytosis, lymphadenopathy, splenomegaly and systemic symptoms, such as fatigue, weight loss and malaise. The clinical course of CLL is highly variable, with a median survival from diagnosis in the region of 7 years. Patients with more advanced disease (Binet stages B, C and stage A progressive) have a significantly worse survival.
Standard therapy for chronic lymphocytic leukaemia
Fludarabine combined with cyclophosphamide is one of the more frequently used combinations of drugs for treating CLL in second and subsequent line use. The MD Anderson Cancer Center reported the use of fludarabine and cyclophosphamide combined with rituximab (Mabthera®, Roche Products Ltd) (FCR) in both previously untreated and refractory CLL. 2,3 The response rates for FCR are very impressive and compare extremely positively with historical controls treated with fludarabine, either alone or in combination with cyclophosphamide. In previously untreated patients, complete remission was demonstrated in 217/300 (72%) patients, nodular partial remission in 31 (10%), partial remission in 37 (12%), no response in 13 (4%) and early death in 2 (< 1%) patients. 2 The same group also reported their experience with FCR in 284 patients with previously treated CLL. 3 The estimated median progression-free survival (PFS) was 21 months, with a median overall survival (OS) of 47 months. The median number of prior treatments was two: 67 patients were alkylating agent refractory, 52 were fludarabine refractory and 98 patients had prior rituximab. Using National Cancer Institute (NCI) criteria, 30% of patients achieved a complete remission rate, 14% achieved nodular partial remission and 30% had partial response (PR), giving an overall response rate (ORR) of 74%.
The German CLL Study Group (GCLLSG) completed the German CLL8 trial, which compared FCR with fludarabine and cyclophosphamide (FC) in patients with CLL who had previously been untreated and required therapy according to conventional criteria. 4 It was reported that 811 patients were entered into the GCLLSG CLL8 trial and randomly assigned to receive either FC or FCR. The ORR was significantly higher in the FCR arm (95%; 370/390 patients) than the FC arm (88%; 328/371) (p = 0.001). The complete response (CR) rate of the FCR arm was 52% compared with 27.0% in the FC arm (p < 0.0001). PFS was 65% at 3 years in the FCR arm and 45% in the FC arm (p < 0.0001). Updated data showed that at a median follow-up of 5.9 years, the PFS was 38% in the FCR group compared with 27.4% in the FC group (p < 0.0001). A total of 69.4% of the patients were alive in the FCR group versus 62.3% in the FC group. The median OS was 86 months in the FC group but the median OS was not reached in the FCR arm (p < 0.001). 5 In 2009, the European Medicines Evaluation Agency granted a product licence for rituximab combined with FC in previously untreated CLL.
Rituximab dose
The dose of rituximab has not been established systematically in CLL. It has been extrapolated from the earlier trials with use of rituximab in B-cell malignancies. However, rituximab monotherapy at a dose of 375 mg/m2 induced an ORR of 13% in previously treated CLL/small lymphocytic lymphoma (SLL). 6,7 This poor response was thought to be attributable to low CD20 expression on CLL cells and binding of rituximab to CD20 positive cellular debris. Subsequent studies investigating thrice weekly doses of rituximab (375 mg/m2) and higher weekly doses of rituximab (500–2250 mg/m2) in previously untreated patients induced modest ORRs of 43% and 40%, respectively. 8–10 The combination of intravenous FC along with rituximab at variable dose (375 mg/m2 in cycle 1 and 500 mg/m2 in cycles 2–6) was used in the Phase III CLL8 trial, showing an excellent ORR, PFS and OS. 5 However, the rationale of using higher doses of rituximab has not been formally assessed.
Rituximab binds specifically to the transmembrane antigen CD20, a non-glycosylated phosphoprotein located on pre-B and mature B lymphocytes. The antigen is expressed on > 95% of all B-cell non-Hodgkin’s lymphomas. CD20 is found on both normal and malignant B-cells, but not on haematopoietic stem cells, pro-B-cells, normal plasma cells or other normal tissue. The phenomenon of CD20 shaving on CLL cells with rituximab has been established in CLL. Most of the CLL cells were cleared after 30 mg of rituximab followed by recrudescence of CLL cells which have lost > 90% of CD20 expression. These data suggested that low-dose rituximab thrice weekly at much lower doses of 20–60 mg/m2 may promote enhanced clearance of CLL cells by preserving CD20 expression. 11 Subcutaneous rituximab at a dose of 20 mg three times a week resulted in the reduction of CD20 expression on CLL cells, but sufficient expression was maintained during the course of 6–12 weeks in another study. 12 A combination of low-dose rituximab (20 mg/m2 three times a week), alemtuzumab (Lemtrada®, Genzyme Therapeutics) and pentostatin (Nipent®, Hospira UK Ltd) in high-risk CLL showed that this low dose of rituximab is able to opsonise and clear the majority of circulating cells, but the loss of CD20 is less pronounced. There was also evidence of complement activation owing to C3d deposition on CLL cells and natural killer cell activation owing to down-modulation of CD16, up-regulation of CD54 and a decrease in the number of natural killer cells. 13 Hence, there is considerable evidence that rituximab at doses as low as 20 mg/m2 can be effective and can reduce the phenomenon of CD20 shaving, as seen with the higher dosing of rituximab used in CLL.
Rituximab has also been used in lower doses in a variety of autoimmune conditions, such as refractory systemic lupus erythematosus and rheumatoid arthritis, where it is standard to use two intravenous doses of 1000 mg 2 weeks apart. 14–17 Rituximab at a dose of 100 mg once a week for 4 weeks has been used in autoimmune haemolytic anaemia and immune thrombocytopenic purpura with relative similar efficacy to the standard dose of 375 mg/m2, although there are no randomised controlled trials to compare the two doses. 18,19 Furthermore, two infusions of 250 mg/m2 of rituximab in mixed cryoglobulinaemia are as effective as four infusions of standard-dose rituximab. 20
In summary, the dose of rituximab in the treatment of CLL has not been systematically established and there is good evidence to suggest that low-dose rituximab would be effective in combination with chemotherapy.
Addition of mitoxantrone
Mitoxantrone is a synthetic anthracenedione that is structurally similar to doxorubicin and daunorubicin. It was synthesised with the aim of reducing side effects, especially cardiotoxicity. It is indicated, either in combination therapy or as a single agent, in the treatment of acute non-lymphocytic leukaemia, metastatic breast cancer, hepatoma, lymphoma and paediatric sarcoma.
The addition of mitoxantrone to the fludarabine-based therapy has been found to result in high response rates in a variety of indolent lymphoproliferative disorders, including follicular lymphoma21 and mantle cell lymphoma. 22 The combination of fludarabine, cyclophosphamide and mitoxantrone (FCM) has been reported in 60 patients who have relapsed or resistant CLL. 23 The ORR in this series was of 78% with 30 patients (50%) achieving a complete remission. It was of considerable importance that 10 of the patients in CR had an eradication of detectable minimal residual disease (MRD) by a sensitive four-colour flow cytometric test, and that these patients had a significantly prolonged survival compared with the other patients in this series. In addition, FCM plus rituximab (FCM-R) appears to be a very promising combination in Phase II trials for CLL. The Barcelona group have reported the use of FCM-R in a non-randomised Phase II trial reporting a complete remission rate of 82% and an ORR of 93% in previously untreated CLL. 24 In this study, 46% of the CR patients had undetectable MRD. The National Cancer Research Institute (NCRI) CLL subgroup has recently completed a randomised Phase II study including FCM and FCM-R in previously treated patients with CLL. This study recruited 52 patients, with 26 in each arm, and reported a 65% CR rate for FCM-R compared with a 58% CR rate for FCM, with five and three patients, respectively, achieving eradication of MRD following FCM-R and FCM. 25
Rationale for design
As we previously demonstrated that the combination of fludarabine, cyclophosphamide, mitoxantrone and rituximab can be delivered safely25 and that there is evidence of synergistic effect in this combination, the aim of this trial was to test the hypothesis that the low dose of rituximab (100 mg per cycle) in combination with FCM would be as effective as the current standard care, which is the combination of FCR. The data from the use of low-dose rituximab suggest that it can result in effective B-cell depletion with relative preservation of CD20 expression on CLL cells, which would be important in terms of maintaining the efficacy of rituximab. The higher dose of rituximab used in CLL is based primarily on the efficacy of the drug as a single agent where higher doses resulted in better ORRs. However, it can be postulated that higher doses are required as a single agent owing to the tumour burden. The combination of chemotherapy with rituximab might not require the higher dose of rituximab as there is effective clearance of tumour load, and preservation of CD20 expression on CLL cells may be important to maintain the efficacy of rituximab.
Based on scientific rationale, another important aspect in the design of the trial was to assess the cost-effectiveness of delivering the combination of FCM and rituximab at a low dose. The total cost of six cycles of rituximab at the current recommended dose in the UK is estimated to be £10,128 for an average body surface area (BSA) of 1.93m2 (average BSA in CLL8 trial). 17 This does not include the hospital cost for delivery of the infusion. The cost of six cycles of rituximab at a standard dose of 100 mg would be £1048. The infusion time to deliver this dose will be considerably lower than the standard dose. It can be suggested that the chances of developing infusion-related reactions requiring hospital admission would be lower at the lower dose of rituximab. The cost of six cycles of mitoxantrone at a dose of 6 mg/m2 intravenously with this combination is estimated to be £600. The cost-effectiveness analysis of comparing the two arms of the trial would be crucial in establishing whether or not the use of a lower dose of rituximab is a reasonable alternative to the standard-arm FCR. Also, the non-inferiority design of the trial helps to ascertain whether lowering the dose of rituximab, and hence reducing the cost of treatment, does not affect the efficacy in terms of CR rates, as well as the longer-term outcomes of PFS and OS.
In summary, the trial answers a critical scientific question of whether or not reducing the dose of rituximab and using a combination of mitoxantrone with oral FC would be as effective as standard care, and whether or not this would, in turn, have an effect on the toxicity and cost-effectiveness of the regimes.
Chapter 2 Methods
Sections of this chapter have been reproduced from Howard et al. 1 with permission.
Aims and objectives
The aim of the ARCTIC trial was to establish whether the addition of mitoxantrone, with a low dose of rituximab, to fludarabine and cyclophosphamide (i.e. FCM-miniR), is as effective as FCR in terms of response in patients with previously untreated CLL.
The primary objective of the statistical analysis was to compare the CR rates as defined by IWCLL criteria26 in each treatment group, in order to determine whether FCM-miniR was non-inferior to FCR.
The secondary objectives were:
-
to assess the rate of eradication of detectable MRD following treatment with FCR or FCM-miniR
-
to assess the ORR (complete or partial remission defined by IWCLL criteria) between the treatment groups
-
to assess the safety and toxicity of low-dose rituximab and mitoxantrone in combination with FC
-
to evaluate PFS
-
to evaluate OS
-
to evaluate time to MRD relapse.
The primary objective of the economic evaluation was to evaluate the incremental cost-effectiveness of treating patients with CLL with FCM-miniR compared with the standard treatment of FCR. Two economic evaluations were undertaken in this phase:
-
a within-trial analysis comparing the outcomes and costs up to 24 months’ follow-up
-
a long-term cost-effectiveness analysis modelling outcomes and costs over a lifetime horizon.
The evaluation followed the reference case guidance for technology appraisals set out by the National Institute for Health and Care Excellence (NICE). 27
Trial design
The ARCTIC trial is a multicentre, randomised, controlled, open, Phase IIB non-inferiority trial in patients who are newly diagnosed with B-cell chronic lymphocytic leukaemia (B-CLL). Patients were randomised on a 1 : 1 basis to receive one of two trial interventions, FCR or FCM-miniR.
The trial was reviewed and approved by the National Research Ethics Service Leeds (East) Research Ethics Committee (REC) (reference 09/H1306/54) and was registered as an International Standard Randomised Controlled Trial, number ISRCTN16544962. The trial was registered on the European Clinical Trials Database (EudraCT), number 2009–010998–20.
Patient and public involvement
The trial was overseen by the NCRI CLL Subgroup Committee which includes two patient and public involvement (PPI) representatives. Trial updates were presented to this committee three times per year and the PPI representatives would provide feedback on the trial during these meetings. There was involvement from a PPI representative on the Trial Steering Committee (TSC) who provided input into the initial production of, and any amendments to, the Participant Information Sheet and other trial documentation intended for use by participants. Through membership of the TSC the PPI representatives also provided input into the design and conduct of the trial through annual meetings. The Plain English summary has been reviewed by a PPI representative who is part of the Trial Management Group (TMG).
Participants
The trial sought to recruit 206 participants with previously untreated CLL from ethically approved hospitals around the UK. Participants had to meet the following eligibility criteria in order to participate in the trial:
Inclusion criteria
-
At least 18 years of age.
-
B-CLL with a characteristic immunophenotype, including SLL.
-
Binet’s stage A progressive or B, or stage C.
-
Requiring therapy by the IWCLL criteria in that they must have at least one of the following:
-
evidence of progressive marrow failure as manifested by the development of, or worsening of, anaemia and/or thrombocytopenia
-
massive (i.e. 6 cm below the left costal margin) or progressive or symptomatic splenomegaly
-
massive nodes (i.e. 10 cm in longest diameter) or progressive or symptomatic lymphadenopathy
-
progressive lymphocytosis with an increase of more than 50% over a 2-month period or lymphocyte doubling time of < 6 months as long as the lymphocyte count is over 30 × 109/l
-
a minimum of any one of the following disease-related symptoms must be present:
-
unintentional weight loss more than or equal to 10% within the previous 6 months
-
significant fatigue (i.e. Eastern Cooperative Oncology Group performance status 2 or worse; cannot work or unable to perform usual activities)
-
fevers of greater than 38.0 °C for 2 or more weeks without other evidence of infection
-
night sweats for more than 1 month without evidence of infection.
-
-
-
No prior therapy for CLL.
-
World Health Organization (WHO) performance status of 0, 1 or 2.
-
Able to provide written informed consent.
Exclusion criteria
-
Prior therapy for CLL.
-
Active infection.
-
Past history of anaphylaxis following exposure to rat- or mouse-derived complementarity determining region-grafted humanised monoclonal antibodies.
-
Pregnancy, lactation or women of child-bearing potential unwilling to use medically approved contraception while receiving treatment and for 12 months after treatment has finished.
-
Men whose partners are capable of having children but who are not willing to use appropriate medically approved contraception while receiving treatment and for 12 months after treatment has finished, unless they are surgically sterile.
-
Central nervous system involvement with CLL.
-
Mantle cell lymphoma.
-
Symptomatic cardiac failure not controlled by therapy or unstable angina not adequately controlled by current therapy (in patients with a significant cardiac history the left ventricular function should be assessed and patients with severe impairment should be excluded).
-
Other severe, concurrent diseases or mental disorders.
-
Known to be human immunodeficiency virus (HIV)-positive.
-
Patient has active or prior hepatitis B or C.
-
Active secondary malignancy excluding basal cell carcinoma.
-
Persisting severe pancytopenia (neutrophils < 0.5 × 109/l or platelets < 50 × 109/l) or transfusion-dependent anaemia unless attributable to direct marrow infiltration by CLL.
-
Active haemolysis (patients with haemolysis controlled with prednisolone at a dose of 10 mg or less per day can be entered into the trial).
-
Patients with a creatinine clearance of < 30 ml/minute (either measured by or derived from the Cockcroft–Gault formula).
Recruitment procedure
Participants were recruited from multiple research centres around the UK. Research centres were identified via a feasibility assessment to determine the most appropriate centres to participate in the trial. Research centres were required to have obtained ethical and management approvals and undertaken a site initiation meeting with the Clinical Trials Research Unit (CTRU) based at the University of Leeds prior to the start of recruitment into the trial. Potential participants were identified by the clinical team at participating centres and were approached to participate in the trial during standard clinic visits. Each participating centre was required to maintain a log of all patients screened for eligibility and to record reasons for non-randomisation.
Randomisation
Participants who fulfilled the eligibility criteria were randomised on a 1 : 1 basis to receive either FCR or FCM-miniR. A computer-generated minimisation program that incorporated a random element was used to ensure that treatment groups were well-balanced for the following characteristics:
-
centre
-
Binet staging (A progressive or B, C)
-
age (≤ 65 years, > 65 years)
-
sex (male, female).
Informed consent
A verbal explanation of the trial was provided by the attending medical staff and a Participant Information Sheet and Informed Consent Document was provided for the patient to consider. This included detailed information about the rationale, design and personal implications of the trial. Following information provision, participants had as long as they needed to consider participation (normally a minimum of 24 hours) and were given the opportunity to discuss the study with their family and other health-care professionals before they decided whether they would be willing to take part in the study.
Assenting patients were then invited to provide informed, written consent and to be formally assessed for eligibility. A record of the consent process including the date of consent and all those present was to be kept in the participants’ medical notes. The original consent form was kept at the research centre, filed in the Investigator Site File, and copies of the consent form were given to the participant and the CTRU at the University of Leeds.
Participants were free to withdraw from the trial at any time. The specific wishes of any participant wanting to withdraw consent for further involvement in the trial, be that from further treatment and/or follow-up data collection, was documented to ensure appropriate processes were followed after withdrawal.
Interventions
Participants were randomised to receive six cycles of either FCR or FCM-miniR according to the regimens outlined below (Tables 1 and 2).
| Drug name | Entry route | Dosage | Number of days |
|---|---|---|---|
| Fludarabine | Oral | 24 mg/m2/day | Days 1–5 |
| Cyclophosphamide | Oral | 150 mg/m2/day | Days 1–5 |
| Rituximab | Intravenous | 375 mg/m2 | Day 1 (Cycle 1) |
| Rituximab | Intravenous | 500 mg/m2 | Day 1 (Cycles 2–6) |
| Drug name | Entry route | Dosage | Number of days |
|---|---|---|---|
| Fludarabine | Oral | 24 mg/m2/day | Days 1–5 |
| Cyclophosphamide | Oral | 150 mg/m2/day | Days 1–5 |
| Mitoxantrone | Intravenous | 6 mg/m2/day | Day 1 |
| Low-dose rituximab | Intravenous | 100 mg | Day 1 |
Cycles of FCR and FCM-miniR were repeated every 28 days for a total of six cycles.
Participants who experienced nausea and vomiting or diarrhoea were given FC via the intravenous route owing to concerns over drug absorption. Intravenous fludarabine was given at a dose of 25 mg/m2/day for 3 days (bioequivalent to 24 mg/m2/day for 5 days given orally) and cyclophosphamide was given at a dose of 250 mg/m2/day for 3 days.
Routine concomitant medications
Participants received prophylaxis against Pneumocystis carinii pneumonia (PCP) with 960 mg of co-trimoxazole bi-daily on Monday/Wednesday/Friday or 480 mg on a daily basis. Participants who were allergic to co-trimoxazole received an alternative, such as dapsone (Dapsone, Actavis UK Ltd) [100 mg once daily (OD)] or nebulised pentamidine (Pentacavinat, Sanofi) (monthly). PCP prophylaxis continued throughout treatment and for at least 2 months after the last course of treatment. Aciclovir (400 mg bi-daily) was recommended as prophylaxis against herpes virus reactivation for all participants. Allopurinol at a dose of 300 mg/day was recommended for all participants for at least the first 28 days of therapy.
Dose delays and reductions
Treatment was delayed or reduced in the following circumstances:
Rituximab-related infusion reactions
The infusion was temporarily stopped until the reaction was resolved and then restarted at half the speed of infusion.
Impaired renal function
Fludarabine was not to be given to participants with a creatinine clearance of < 30 ml/minute. Participants with a creatinine clearance of < 30 ml/minute could have a delay of treatment for up to 4 weeks but were withdrawn from the trial treatment if their creatinine clearance did not improve. Participants with a creatinine clearance of between 30 and 60 ml/minute were permitted to have a 50% dose of fludarabine at the discretion of the treating clinician.
Neutropenia
If neutrophils were < 1.0 × 109/l owing to trial chemotherapy rather than bone marrow involvement, treatment was delayed for up to 2 weeks, with a 25% dose reduction of FC in subsequent treatment cycles. Participants who had a neutrophil count of < 1.0 × 109/l at day 28 of any cycle of therapy received granulocyte colony-stimulating factor {GCSF [lenograstim (Granuocyte, Chungai Pharma UK Ltd)]} at a recommended dose of 263 µg/day from days 7–13 for the next and all subsequent cycles of chemotherapy. Further dose reductions were permitted if neutropenia recurred after the 25% dose reduction. If the neutrophil count recovered to > 1.0 × 109/l the doses of chemotherapy were re-escalated with continuing GCSF support.
Other haematological toxicities
If platelets were < 75 × 109/l as a result of trial chemotherapy rather than bone marrow involvement, treatment was delayed for up to 2 weeks, with a 25% dose reduction of FC in subsequent treatment cycles. If on subsequent cycles of therapy platelets had recovered to over 100 × 109/l, the chemotherapy doses were re-escalated. If further haematological toxicity occurred after the 25% dose reduction further dose reductions were permitted.
Data collection and management
Data collection took place via paper case report forms (CRFs), which centres returned to the CTRU for entry onto a central database. Initial validation checks of the forms were carried out and the trial database also validated most dates and data in line with pre-programmed validation rules.
Safety monitoring
All AEs, both related and unrelated to the treatment of CLL, were collected for all patients and evaluated for duration and intensity according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. AEs were collected from randomisation until 30 days after the last dose of treatment with FCR or FCM-miniR.
Serious adverse events (SAEs) were defined as any untoward medical occurrence or effect that:
-
resulted in death
-
was life-threatening
-
required inpatient hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect
-
may have jeopardised the patient and required medical or surgical intervention to prevent one of the outcomes listed above.
Where a SAE was deemed to have been related to an Investigational Medicinal Product (IMP) used within the trial (fludarabine, cyclophosphamide, rituximab or mitoxantrone) the event was termed as a serious adverse reaction (SAR).
A Suspected Unexpected Serious Adverse Reaction (SUSAR) was defined as a SAR that also demonstrated the characteristics of being unexpected, the nature and severity of which was not consistent with the information about the medicinal product in question set out in the summary of product characteristics for that product.
Serious adverse events were collected from the time of randomisation until 30 days post treatment. SARs and SUSARs were collected from the time of randomisation and for the duration of the trial.
All SAEs, SARs and SUSARs were reported by the CTRU to the Chief Investigator of the trial as they occurred. A summary of how many SAEs and SUSARs had been received was reported at each TMG meeting. A summary of all SAEs and SUSARs was presented by treatment arm to the DMEC at all annual meetings and in an interim report every 6 months, with any safety concerns being fed back to the TSC.
Outcome measures
Primary outcome measure
-
Proportion of participants achieving a CR at 3 months post therapy, as assessed by IWCLL criteria. 26
Secondary outcome measures
-
Proportion of participants with eradicated MRD at 3 months post therapy: MRD is defined as negative or undetectable owing to the presence of < 0.01% CLL cells in the blood or bone marrow by IWCLL criteria. 26
-
Overall response rate at 3 months post therapy: defined as complete or partial remission by IWCLL criteria. 26
-
Safety and toxicity: reported based on AEs (as graded by CTCAE V3.0), SAEs, SUSARs and treatment-related mortalities within 3 months of discontinuing protocol treatment. Determined by routine clinical assessments at each centre.
-
Economic evaluation: quality-adjusted life-years (QALYs) were used to measure health benefit. Health-related quality of life was estimated using responses to health economics participant questionnaires, which included the European Quality of Life-5 Dimensions (EQ-5D™) and Short Form questionnaire-12 items (SF-12) [converted to Short Form questionnaire-6 Dimensions (SF-6D)]. 28
-
Progression-free survival at 2 years: time from randomisation to first documented evidence of disease progression or death. Participants without evidence of disease progression at the time of analysis are censored at the last date on which they were known to be alive and progression free. The initial analysis was planned once all participants had been followed for 2 years post randomisation.
-
Overall survival: time from randomisation to date of death. Participants still alive at the time of analysis are censored at the date on which they were last followed up. The initial analysis was planned once all participants had been followed for 2 years post randomisation.
-
Time to MRD relapse in participants who are MRD negative 3 months post treatment: time from the 3-month post-treatment visit, for those participants who became MRD negative, to when the participant became MRD positive. Participants who were alive and MRD negative at the time of analysis, or participants dying from causes unrelated to CLL, were censored. If participants were lost to follow-up, their MRD relapse-free survival time was censored at the time at which they were last known to be alive and MRD negative.
Independent primary end point review
The primary end point data, response at 3 months post treatment by IWCLL criteria, was centrally reviewed by an independent panel in order to enhance the consistency and accuracy in the reporting of the primary end point, and to eliminate potential local assessment bias. The independent review panel consisted of CLL clinicians who were identified via the NCRI CLL Subgroup Committee. The independent reviews were performed using anonymised data with no information regarding which treatment was provided for each participant. Each response assessment was made by two independent clinicians and where the outcome of the assessments differed the data were sent to a third clinician, an independent arbiter, to make a final decision on response.
Sample size
A total of 206 participants were required. From previous studies it was anticipated that FCR would produce response rates of at least 50%. In particular, the results from the GCLLSG, presented at the American Society of Haematology conference 2008, showed a 52% CR rate with FCR. 4,29 It was anticipated that FCM-miniR would actually have a superior response rate to FCR. This was based on, firstly, the assumption that miniR was as good, or nearly as good, as full-dose rituximab,30 and, secondly, the hypothesis that mitoxantrone increases the response rate when added to FCR. It was, therefore, hypothesised that FCM-miniR may increase the response rate by approximately 10%. If this was the case, and FCM-miniR really had a 10% better response rate when compared with FCR, a non-inferiority Phase IIB trial was considered practicable. Under this assumed 10% difference in favour of FCM-miniR, to have 80% power to show non-inferiority, where this is defined as FCM-miniR being not more than 10% worse in terms of response rate than FCR, the trial would require the randomisation of 98 participants per arm, 196 in total. 31
Note that if the FCR response rate deviates in either direction from 50%, the sample size required to show that the CR rate in the FCM-miniR group is not inferior by more than 10% would decrease. Therefore, this calculation was conservative, in that deviations from this assumption increase the power to assess FCM-miniR being not more than 10% worse in terms of response rate than FCR.
To account for a 5% dropout, 206 participants (103 per arm) were sought to be randomised. This approach used a one-sided 97.5% confidence interval (CI), that is, an α (type I) error rate of 2.5%, equivalent to a conventional α of 5% for the superiority setting. 31
Statistical analysis
A full statistical analysis plan was written and signed off in accordance with current CTRU standard operating procedures.
Analysis populations
All analyses were conducted on the intention-to-treat (ITT) population, in which participants were included according to the treatment they were randomised to.
For the outcome measures assessing response, participants without an available response assessment, who had not withdrawn for toxicity or died, were excluded from the denominators. This was felt to be appropriate as it was strongly assumed that response end point data would be missing completely at random, given that participants were unlikely to refuse to have assessments performed owing to their level of response or treatment allocation. Assessments were more likely to be unavailable as a result of samples being un-assessable or missed in error. Reasons for missing response data were monitored and have been summarised (see Table 24).
A per-protocol (PP) analysis was planned for the primary end point assessment in addition to the ITT, where only participants who received at least one cycle of treatment in line with the protocol, who were not major eligibility violators and for whom primary end point data were available are included. For the primary analysis, equal weighting is given to both the ITT analysis and the PP analyses, as the ITT analysis is likely to be the least conservative approach when assessing non-inferiority.
Safety end points were assessed based on the safety population, which included participants according to the treatment they actually received and who had been exposed to at least one dose of the study treatment.
Missing data handling
In the evaluation of response for the primary end point, the analysis was based on the centrally reviewed data at 3 months post treatment. Participants without an assessment of response were treated as non-responders in the ITT and PP analyses if they either:
-
died from CLL or protocol treatment prior to the 3-month post-treatment assessment or
-
discontinued treatment early owing to non-response or toxicity.
Trephine data are required to confirm a CR in participants who are known to be at least a PR. For participants with at least a PR but with missing trephine data, the following rules, defined in advance within the Statistical Analysis Plan, were applied:
-
For participants assessed as at least a PR with no evidence that were not CR/CRi:
-
MRD-negative participants were reported as ‘CR/CRi’
-
MRD-positive participants were reported as ‘PR’
-
Participants with missing MRD status were excluded from the analysis.
-
-
Participants assessed as PR with evidence that they were not a CR or CRi by the IWCLL criteria, or participants with stable/progressive disease (PD), were treated as non-responders in the analysis.
Evaluation of MRD was based on an assessment of the bone marrow, performed centrally at the Haematological Malignancy Diagnostic Service (HMDS), St James’s University Hospital, Leeds, at 3 months post treatment. For participants with a missing MRD assessment, the next available observation based on the peripheral blood was carried backwards and imputed in place of the missing 3-month observation. This was considered to be a conservative approach as participants are not expected to improve over time without treatment.
Frequency of analyses
Interim reports presenting recruitment, demographic, safety and toxicity data along with treatment and protocol compliance were presented by treatment arm to the DMEC in strict confidence at approximately yearly intervals. In addition to the full annual reports, safety data were presented to the DMEC on a 6-monthly basis. The DMEC reported its recommendations regarding the continuation of the trial to the TSC.
A single formal interim analysis was planned on the short-term efficacy data when half the number of participants (103) had reached their primary end point; this was reported to the DMEC in September 2012. A separate formal analysis plan was written for the interim analysis and signed off before the final data download. The results and outcome of the meeting are reported below (see Interim analysis).
Final analyses were carried out on all but the survival end points when the response data became available for all participants, approximately 9 months after the close of recruitment. The survival end points were analysed 2 years after the close of recruitment and will be updated as appropriate.
Interim analysis
The interim analysis was carried out at a stringent alpha level in order to retain an overall 5% level (two-sided) for the final analysis. The O’Brien and Fleming32 alpha spending function was used to adjust for multiple testing, requiring an alpha level of 0.005 (two-sided) for the interim analysis, and an alpha level of 0.048 (two-sided) for the final analysis.
Primary end point analysis
An overall one-sided 2.4% significance level was used for the final primary response analysis.
The proportion of participants who achieved at least a CR are summarised by treatment group, and the lower limit of the 95.2% CI (one-sided type I error rate of 2.4%) for the difference in the proportions of participants achieving a CR between the treatment groups reported. This was obtained using one-sided binary logistic regression to adjust for the minimisation factors: Binet stage, sex and age, but excluding centre. The treatment estimate is presented along with the odds ratio (OR) and 95.2% Wald CI around the OR estimate. In order to determine whether FCM-miniR was non-inferior to FCR, defined as FCM-miniR being no more than 10% worse in terms of response rates, the lower limit of the CI for the treatment effect is compared with the non-inferiority margin of 10%, expressed as an OR using the following formula:
where π1 = the proportion of responders in the FCR arm and π2 = proportion of responders in the FCR arm minus 10%.
For the primary end point analysis, the ITT and PP populations are of joint primacy. In the event that the analyses do not concur (i.e. one demonstrates non-inferiority and the other does not), non-inferiority cannot be concluded.
Sensitivity analyses assess the robustness of results of the primary analysis, and the assumptions regarding missing data:
-
treating all participants will miss primary end point data as non-responders
-
treating all participants will miss primary end point data as responders
-
excluding all participants will miss trephine data from the analysis.
Secondary end point analyses
A two-sided 5% significance level was used for all secondary superiority efficacy end point comparisons.
The proportion of participants with undetectable MRD following treatment, and the proportion of participants who achieved an OR of at least a PR by IWCLL criteria following treatment, are each summarised by treatment group. The differences in the proportions and exact 95% CIs are reported. Binary logistic regression is used to provide treatment estimates with corresponding standard errors (SEs) and p-values, along with ORs and 95% CIs around the OR estimates, after adjusting for the minimisation factors, excluding centre.
Cox regression analysis is used to analyse time to MRD relapse, progression and death, both overall and between treatment arms, accounting for the minimisation factors, excluding centre. Treatment and covariate estimates, SEs, hazard ratios (HRs) and corresponding 95% CIs and p-values are presented for all variables incorporated in the models. Median PFS, OS and time to MRD relapse and corresponding 95% CIs are also presented per treatment arm and overall. The proportional hazards assumption is assessed by plotting the hazards over time (i.e. the log-cumulative hazard plot) for each treatment arm, after adjusting for the minimisation factors. In addition, MRD relapse, PFS and OS curves are calculated using the Kaplan–Meier method.
Safety analyses summarise the number of AEs, SAEs and SUSARs occurring after randomisation. Safety data are presented by treatment group using the safety population. Summaries of the total numbers of SAEs/SUSARs reported and numbers of participants experiencing each event are presented, along with details of the suspected relationship with trial medication or other causality, duration of recovered SAEs/SUSARs, seriousness criteria, event outcome and Medical Dictionary for Regulatory Activities (MedDRA) body system coding. The number and causes of deaths occurring from randomisation until 3 months post treatment are summarised, and the proportion of participants with each cause of death is calculated. No statistical testing is performed between the two groups.
Subgroup analyses
Exploratory subgroup summaries are presented to assess the heterogeneity of the treatment effect among the following subgroups of interest for the primary end point and, where relevant, PFS and OS:
-
sex (male, female)
-
age group (≤ 65 years, > 65 years)
-
Binet stage (A progressive or B, C)
-
creatinine clearance levels (30–60 ml/minute, > 60 ml/minute)
-
β2-microglobulin (β2M) concentration (< 4 mg/l, ≥ 4 mg/l)
-
number of cycles of treatment received (three or fewer, more than three)
-
GCSF received (yes, no)
-
17p deletion (yes, no)
-
11q deletion (yes, no)
-
heavy-chain variable-region (VH) mutation risk (poor risk, standard risk).
In addition, PFS and OS are analysed by IWCLL response and MRD response to treatment, both alone and by treatment arm.
The analyses carried out use the same populations as for the main analyses on the primary and secondary end points. Analyses on the PP population were considered unnecessary owing to the similarity with the ITT population. Subgroup analyses may, by chance, generate false-negative or false-positive results and must be interpreted with caution and treated as hypothesis generating.
Economic evaluation
An economic evaluation was conducted to assess the cost-effectiveness of FCM-miniR compared with FCR from a UK NHS and Personal Social Services (PSS) perspective. The evaluation consists of two components: a within-trial analysis, in which cost-effectiveness is assessed within the 24-month trial period using individual participant data collected in the trial, and a decision analytic model analysis, in which cost-effectiveness is assessed over a lifetime horizon using standard modelling techniques applied to the trial data in order to extrapolate the trial results.
Measurement of outcomes
The economic analysis used QALYs to measure health benefit. Health-related quality of life was estimated using responses to health economics participant questionnaires, which included the EQ-5D. 28 QALYs represent a quality-weighted survival value in which one QALY is equivalent to 1 year of full health. All participants were asked to complete these questionnaires at the following time points:
-
baseline
-
after three cycles of therapy
-
at the end of therapy
-
3 months after the end of therapy
-
every 3 months after the end of therapy until 24 months post randomisation (i.e. at 6, 9, 12, 18 and 24 months post randomisation).
Standard UK tariff values were applied to these responses at each time point to obtain participant utility values for the within-trial period. QALYs were calculated using the ‘area under the curve’ method and formed the primary outcome measure of the cost-effectiveness study. As NICE currently recommends the use of EQ-5D derived utilities in its reference case, EQ-5D utilities were used in all base-case analyses; sensitivity analysis using SF-12 (converted to SF6D) utilities was also conducted. 27
Measurement of costs
Participant-reported data on resource usage were collected in the trial using the health economics participant questionnaires at similar time points as the health outcomes, except at baseline. For the 3-month follow-up data, the recall period was 3 months (i.e. participants were asked to provide information on their use of health-care resources over the previous 3-month period). For all other cases, the recall period was either since entering the study or since the last questionnaire was completed. The questionnaires included the number and length of hospital inpatient stays, the number of outpatient visits, and the number of primary/community care visits. Participants were also asked to report on the use of PSS related to their treatment (such as the number of visits of carers and social workers).
Costs were estimated by combining participant-reported resource usage with unit cost data obtained from national databases such as the Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care and British National Formulary (BNF). 33,34
The analyses took the perspective of the NHS including the costs of health and social care. All costs are reported in 2013 GBP (£) and future costs were discounted at an annual rate of 3.5%, as per the NICE Methods Guide. 27
Missing data
Missing data for participant-reported health-related quality of life were dealt with by using the multiple imputation method. 35,36 This method assumes that data are missing at random; missing data values are replaced with plausible substitutes based on the distribution of observed data, with uncertainty around the observed data values incorporated using iterative multivariable regression techniques. A set of baseline variables and cost data from that time point were used to impute missing health outcomes data. This approach is recommended for economic analyses alongside clinical trials as it reflects the uncertainty inherent in missing data. 37
Within-trial analysis
Main characteristics of the analysis
The within-trial analysis aimed to determine the cost-effectiveness of FCM-miniR compared with FCR over the 24-month trial period. Individual participant data collected in the trial were used to determine the cost and QALYs associated with each treatment arm. QALYs were derived using participant EQ-5D questionnaire responses, and cost-effectiveness is assessed as the incremental cost per incremental QALY.
Following the trial interim analysis, carried out on the first half of participants randomised to the trial (n = 103), 21 participants randomised to FCM-miniR transferred over to treatment with FCR. The difference in the CR rates at the interim analysis between the treatment arms, although not statistically significant, was deemed by the DMEC to be clinically relevant in favour of the control group. In light of this, and evidence of additional toxicity in the FCM-miniR arm, the trial was closed early at the recommendation of the DMEC and all participants still receiving FCM-miniR were recommended to transfer to treatment with FCR for the remainder of their treatment cycles. The economic evaluation base-case analysis was therefore conducted using the trial sample with these 21 participants removed, as the treatment transfer occurred as a result of the planned interim analysis rather than as a result of an independent participant or clinician decision, which does not meet the definition of ITT. Sensitivity analyses were conducted to assess the impact of removing these participants.
As the analysis spans more than 1 year, future costs and health outcomes (beyond one year) were discounted at an annual rate of 3.5% as per the NICE Methods Guide. 27 Cost-effectiveness is measured in terms of the incremental cost-effectiveness ratio (ICER), which is calculated by dividing the mean difference in cost between the two arms by the mean difference in QALYs between the two arms, as follows:
where ΔC is the incremental cost of FCM-miniR and ΔE is the incremental health benefit of FCM-miniR and λ is the societal willingness to pay (WTP) for one QALY. The ICER represents the additional cost per one unit of outcome gained. This indicates the trade-off between total cost and effectiveness when choosing between FCM-miniR and FCR therapies. When compared against the marginal trade-off for the NHS as a whole – the cost-effectiveness threshold – this gives an indication of whether spending money on FCM-miniR is an efficient use of resources. As a guideline rule, we used the NICE implicit WTP threshold of £20,000–30,000 per QALY to determine cost-effectiveness. In general, a new intervention is considered cost-effective so long as its ICER is within or below the £20,000–30,000 per QALY range.
Uncertainty
Non-parametric bootstrapping was used to determine the level of sampling uncertainty around the ICER by generating 10,000 estimates of incremental costs and benefits from the trial results. The bootstrap approach is a non-parametric method that considers the original sample as though it were the population and draws multiple random samples from the original sample. Results are presented using cost-effectiveness scatterplots to illustrate the uncertainty surrounding the cost-effectiveness estimates. On the cost-effectiveness plane (which plots incremental QALYs against incremental costs), a result is considered cost-effective if it falls on or below the given cost-effectiveness threshold. The cost-effectiveness acceptability curve (CEAC) is derived by calculating the proportion of bootstrapped estimates which are cost-effective across a range of WTP thresholds, to show the probability that FCM-miniR is cost-effective across different threshold values. 37 The CEACs were constructed using the net benefit approach. The net monetary benefit (NB) is a simple rearrangement of the ICER decision formula as shown:
where ΔC is the incremental cost of the treatment strategy, ΔE is the incremental benefit of the treatment strategy, and λ is the societal WTP per QALY threshold. Across any number of alternative interventions, the intervention with the highest NB is considered cost-effective [i.e. an intervention is cost-effective if the incremental net monetary benefit (INB) is positive]. 27 Using the NB statistic, the cost-effectiveness of each of the bootstrap estimates can be determined in order to derive the overall probability of cost-effectiveness for the CEAC.
Mean INBs between the two arms were reported with 95% bootstrap CIs calculated using the bias-corrected method. 38
Sensitivity analysis
To investigate the appropriateness of the EQ-5D as the principal outcome measure, a sensitivity analysis using SF-6D utility values derived from the SF-12 trial data was conducted.
In addition, to assess the potential impact of participant crossover in the trial, two additional sensitivity analyses were conducted:
-
An ITT analysis was conducted, in which any transfer of participants between arms was ignored and participants who crossed over from FCM-miniR to FCR were retained in the analysis of the FCM-miniR arm.
-
Participants who were transferred are deemed to have been in the FCR treatment arm from randomisation.
Decision economic model analysis
Decision analytical modelling was used to compare FCR and FCM-miniR therapies over a lifetime horizon. A discrete-time state-transition (modified-Markov) model was developed to estimate the long-term cost-effectiveness of treating participants with CLL with FCM-miniR compared with the standard treatment of FCR. In line with the within-trial economic evaluation, the model analysis adopts a UK NHS and PSS perspective and future costs and QALYs were discounted at an annual rate of 3.5% in line with NICE guidance. 27 The model was built in Microsoft Excel version 2010 (Microsoft Corporation, Redmond, WA, USA).
Model structure and parameters
The model structure is presented in Figure 1. The model included three possible health states: PFS, PD and death.
FIGURE 1.
Three-state Markov model.

Markov models describe patient progression over time through a pathway of health states, with movement between the health states being triggered by events such as disease progression or death. Resource use and costs are associated with each health state and patients accumulate costs and health benefits in each state over 3-monthly cycles. As 2-year individual patient cost and utility data were available from the trial data, the model runs from the end of the trial in order to estimate long-term costs and health benefits. The lifetime cost and QALY results are calculated as the sum of the trial 2-year results and the model lifetime estimates (truncated at age 100 years).
The model inputs were derived using information from a range of sources. Where possible, data from the trial were used directly, and published literature was used to inform remaining parameters. Where published literature was required, focused non-systematic reviews were conducted to identify potential sources of information. Appropriate distributions were applied using observed or published variance data; where no such data were available, the standard deviation was assumed to be equal to the mean. As for the within-trial analysis, the model base-case analysis excludes participants who crossed over from the FCM-miniR arm to the FCR arm; a sensitivity analysis was conducted to assess the impact of this. The proportion of participants beginning in each health state in the model was derived directly from the proportion of participants in the trial who remained progression-free and mortality-free at the last follow-up (24 months). Derivation of the post-24-month rate of transition between the progression-free state and progressed disease state required significant extrapolation beyond the relatively short follow-up period of the trial, which contained low numbers of progression events upon which to base the extrapolation. Owing to the short follow-up of the trial, the usual practice of fitting survival curves to the trial data in order to extrapolate results over time is likely to produce highly uncertain, and most likely implausible, results. An alternative approach was therefore adopted that involved fitting a parametric survival model using the Remission Duration Model (RDM) model. The RDM model is based on a plausible biological rationale for the mechanism of disease progression. 39 The model was fitted, using maximum likelihood estimation, to the control arm of the CLL8 trial conducted in Germany which represents a comparable patient population treated with a comparable FCR regimen. 4,40 The fitted model parameters were then calibrated to the PFS curve from the FCR arm of ARCTIC (Figure 2).
FIGURE 2.
Progression-free survival in the FCR arm.

As chemotherapy is expected to exert its effect on PFS only during the treatment period, which was captured within the trial follow-up period, the progression-free event rate beyond 2 years (i.e. in the model) was assumed to be the same between arms. In a sensitivity analysis, the transition rate for FCM-miniR was derived by applying the HR observed in the trial period to the FCR survival curve over various durations to allow for the possibility of a carry-over effect.
Adverse events (AEs) relating to each of the treatment strategies were assumed to occur only within the trial follow-up period (2 years post randomisation to treatment). The cost of AEs has therefore been captured in the within-trial analysis period (in which AEs were costed separately for each arm based on the trial individual patient data) and has not been included within the model follow-up period.
The probability of dying from the PF state was assumed to be equivalent to the general population age- and sex-specific mortality. The probability of dying from the PD state was derived from the literature and assumed to be identical between arms.
Death was assumed to be associated with zero utility and zero cost. For the PF health state, the associated 3-month cost and utility values were derived directly from the trial second-year data, using available data on participants prior to disease progression. Cost and utility values were calculated using second-year data only in order to avoid using data from participants’ primary treatment period, which would bias the results. For the PD state, a combination of a lack of progression events and lack of completed questionnaires at the time of progression in the trial meant that cost and utility estimates for this health state had to be derived from the literature. For the cost of the PD, the 3-month health state cost was taken from a previously conducted cost–utility analysis which looked at the cost-effectiveness of bendamustine (Levact, Napp Pharmaceuticals) versus chlorambucil (Leukeven, Aspen) for the first line treatment of CLL in England and Wales. Woods et al. derived resource use for CLL health states (including PD) from an advisory board conducted in January 2010, which consisted of five haematologists who worked in the UK NHS and were experienced in treating CLL. The reported 3-month progressed disease state cost was £1924, based on 2009 NHS reference costs; this cost was inflated to a 2013 price for use in the current model. 41 For the PD state utility, a decrement was applied to the PF state, using data from Beusterien et al. 42 This was a cross-sectional study in which 89 members of the general population in the UK (England and Scotland) were asked to value health states describing CLL response status using standard gamble methodology. 42
A full list of model parameters and distributions applied in the model is given in Table 3.
| Model parameter | Mean | Distribution | SE | Source | |
|---|---|---|---|---|---|
| Global parameters | Annual discount rate | 0.035 | Fixed | – | NICE27 |
| Start age, years | 62.38 | Fixed | – | ARCTIC trial data | |
| Proportion male, % | 0.66 | Fixed | – | ||
| Starting distribution | PF (FCR) | 0.894 | Dirichlet | 0.03 | ARCTIC trial distribution at 24-month follow-up |
| PD (FCR) | 0.064 | Dirichlet | 0.04 | ||
| Dead (FCR) | 0.042 | Dirichlet | 0.02 | ||
| PF (FCM-miniR) | 0.791 | Dirichlet | 0.05 | ||
| PD (FCM-miniR) | 0.094 | Dirichlet | 0.06 | ||
| Dead (FCM-miniR) | 0.115 | Dirichlet | 0.04 | ||
| Health state costs (3 months), £ | PF | 268 | Gamma | 43 | ARCTIC trial data |
| PD | 2146 | Gamma | 2146 | Woods et al., 201241 | |
| Dead | 0.00 | Fixed | – | – | |
| Health state utilities (3 months) | PF (EQ-5D) | 0.82 | Beta | 0.01 | ARCTIC trial data |
| PF (SF-6D) | 0.70 | Beta | 0.03 | ||
| PD utility decrement applied to PF state value | –0.16 | Log-normal | 0.02a | Beusterien et al., 201042 | |
| Dead | 0.00 | Fixed | – | – | |
| Transition probabilities/effects (3 months) | Risk of progression (PF to PD) in FCR arm | Varies (drawn from survival curve figure) | Fixed | – | Survival analysis of ARCTIC within-trial data (see Figure 2) |
| Risk of progression (PF to PD) in FCM-miniR arm | Varies (drawn from survival curve figure) | Fixed | – | ||
| Mortality in PF state | Varies (age- and sex-dependent) | Fixed | – | Office for National Statistics, 2013 age- and sex-standardised rates43 | |
| Mortality in PD state | 0.14 | Beta | 0.03 | Wierda et al., 201044 | |
| Log of HR for risk of progression in FCM-miniR arm vs. FCR arm (used in SA only) | 0.33 | Normal | 0.30 | ARCTIC trial data | |
Sensitivity analyses
Probabilistic sensitivity analyses were conducted to assess the impact of joint parameter uncertainty on the results. Probabilistic analysis accounts for joint parameter uncertainty in non-linear models by assigning probability distributions to each of the input parameters and randomly drawing from these probabilities over 10,000 Monte Carlo model simulations to produce different cost and QALY estimates in each simulation of the model. As for the bootstrap within-trial analysis, the results are presented on the cost-effectiveness plane as a scatterplot, and using CEACs to show the probability that the two arms are cost-effective across different WTP per additional QALY thresholds.
Deterministic sensitivity analysis was used to assess the impact of individual parameter uncertainty on the results. Parameters were independently varied between upper and lower bands of plausible values, based on increasing and decreasing each parameter by 25% of its initial value.
Three additional sensitivity analyses were conducted to assess the impact of key model assumptions:
-
SF-6D utilities. The base-case analysis was conducted using utilities derived from participant responses to the EQ-5D questionnaire (which NICE recommends). A sensitivity analysis was conducted using utilities derived from participant responses to the SF-12 questionnaire. For a discussion on the relative merits of SF-12 versus EQ-5D please see Chapter 5, Economic evaluation discussion.
-
Intention to treat analysis. In the base-case analysis participants who crossed over from FCM-miniR to FCR as a result of the trial interim analysis were excluded from the cost-effectiveness analysis. A sensitivity analysis was conducted in which these participants were instead retained in the analysis of the FCM-miniR arm.
-
Treatment effect. As chemotherapy is expected to exert its effect on PFS only during the treatment period, which was captured within the trial follow-up period, the progression-free event rate beyond 2 years was assumed to be the same between arms in the base case. In a sensitivity analysis, the transition rate for FCM-miniR was derived by applying the HR observed in the trial to the FCR survival curve over various durations to allow for the possibility of a carry-over effect; in addition to the base-case analysis, in which a differential rate of progression occurs in the initial 2-year trial period only, analyses were conducted extending the observed HR over 3, 5, 10 years and a lifetime horizon in the FCM-miniR arm.
Value of information analysis
Any model is subject to uncertainty around model parameters and assumptions, which may result in a wrong decision being made based on the model results. If an incorrect decision is made, there will be consequences in terms of health benefit and resources lost. The maximum amount the NHS should be willing to invest to reduce uncertainty in the decision can be informed by the expected value of perfect information (EVPI). 45 The EVPI evaluates the expected opportunity cost of current uncertainty by assessing the probability that a decision based on current information is wrong, multiplied by the costs of making a wrong decision. It is calculated by applying non-parametric methods to the output from the Monte Carlo simulation of the model. 46 It is the difference between the expected value (E) of the decision made with perfect information across j interventions and θ parameters, Eθ maxj NB(j,θ), and the expected value of the decision made on the basis of existing evidence, maxj EθNB(j,θ):
Additional research should be considered only if the EVPI exceeds the expected cost of research. The EVPI therefore provides an upper limit on the amount that the NHS should be willing to spend on further research in order to resolve current uncertainties.
The value of future research can be further explored using the expected value of perfect parameter information (EVPPI). The EVPPI is a calculation of the maximum value attributable to specific components (parameters) of the evidence base. The EVPPI is calculated as the difference between the expected value of the decision made with perfect information for a particular parameter or set of parameters, ϕ, across the remaining uncertain parameters, ψ, and the expected value of the decision made on the basis of existing evidence:
The EVPPI represents the maximum cost the NHS should be willing to spend on further research to resolve uncertainty for the given parameter or set of parameters evaluated. It is useful for isolating which specific parameters of the decision model are fuelling the overall EVPI, and thereby indicates in what direction future research should be focused.
The formulae above give the per-person EVPI and EVPPI values. To generate population value of information statistics, the per-person estimates must be multiplied by the population expected to be affected by the new treatment. This value is derived from the annual incidence of disease multiplied by the number of years the treatment decision is expected to be relevant. Based on cancer research statistics, the annual incidence of CLL across England and Wales is expected to be 2943. 47 In the absence of quantitative data it was assumed that the period over which the current decision problem will be relevant (i.e. the effective lifetime over which the new treatment will be used) was 10 years. An annual discount factor of 3.5% was applied to the population estimate.
Summary of changes to the protocol
From the time of initial ethical approval (25 June 2009), four substantial amendments to the protocol were submitted to and subsequently approved by the REC.
Amendment 1, dated 5 November 2009 and approved 26 November 2009, included the following key changes:
-
introduction of a participant diary card to collect compliance with oral treatments
-
addition of a health economics participant questionnaire
-
clarification regarding data collection and storage in the participant information sheet.
Amendment 2, dated 21 February 2011 and approved 17 March 2011, included the following key changes:
-
amending inclusion criteria to allow participants with SLL to enter the trial
-
allowing two schedules for splitting the dose of rituximab
-
stipulating the re-escalation of chemotherapy after dose reductions
-
making the 6-month post-treatment blood sample compulsory for all participants
-
adding information to include participant name on all samples sent to HMDS.
Amendment 3, dated 15 July 2011 and approved 29 July 2011, included the following key changes:
-
correcting an error in one of the rituximab dose-splitting schedules.
Amendment 4, dated 1 October 2012 and approved 13 November 2012, included the following key changes:
-
noting the early trial closure
-
updating the end of trial definition
-
providing clarifications to the pharmacovigilance reporting and review requirements.
Chapter 3 Statistical trial results
Sections of this chapter have been reproduced from Howard et al. 1 with permission.
Recruitment
Between December 2009 and September 2012, 200 of a planned 206 patients were recruited into the ARCTIC trial (Figure 3), with 100 participants randomly allocated to the FCR control arm and 100 to the FCM-miniR intervention arm. Written informed consent was received from all participants.
FIGURE 3.
Cumulative and monthly recruitment.
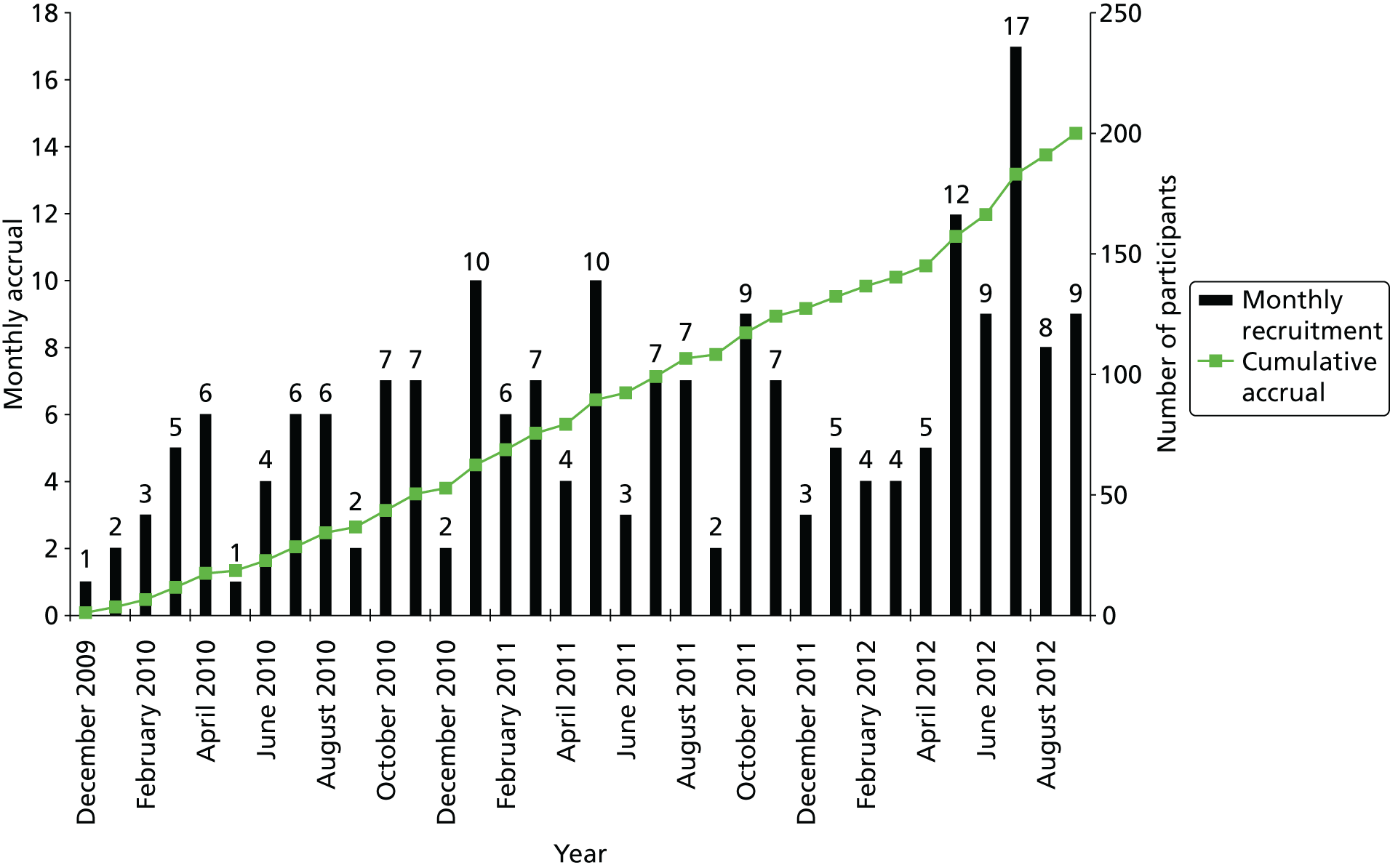
Thirty-eight centres across the UK received local ethical and management approval for the trial and had permission to randomise patients, of which 34 centres were recruited into the trial. Table 4 summarises recruitment per centre and by allocated treatment arm. The top five recruiting centres were the Oxford Cancer and Haematology Centre (n = 25), Birmingham Heartlands Hospital (n = 16), St James’s University Hospital (Leeds) (n = 15), Southampton General Hospital (n = 13) and Castle Hill Hospital (Hull) (n = 12).
| Randomising centre | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Arrowe Park Hospital, Upton | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Birmingham Heartlands Hospital, Birmingham | 8 (8.0) | 8 (8.0) | 16 (8.0) |
| Borders General Hospital, Melrose | 3 (3.0) | 3 (3.0) | 6 (3.0) |
| Buckinghamshire Hospitals NHS Trust, Wycombe and Stoke Mandeville | 6 (6.0) | 5 (5.0) | 11 (5.5) |
| Castle Hill Hospital, Hull | 6 (6.0) | 6 (6.0) | 12 (6.0) |
| Christie Hospital, Manchester | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Colchester General Hospital, Colchester | 2 (2.0) | 1 (1.0) | 3 (1.5) |
| Epsom St Helier University Hospital NHS Trust, Epsom and Carshalton | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Glan Clwyd Hospital, Rhyl | 4 (4.0) | 4 (4.0) | 8 (4.0) |
| Good Hope Hospital, Birmingham | 6 (6.0) | 5 (5.0) | 11 (5.5) |
| Great Western Hospital, Swindon | 2 (2.0) | 2 (2.0) | 4 (2.0) |
| Harrogate District Hospital, Harrogate | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| King’s College Hospital, London | 0 (0.0) | 2 (2.0) | 2 (1.0) |
| Manchester Royal Infirmary, Manchester | 2 (2.0) | 3 (3.0) | 5 (2.5) |
| Northwick Park Hospital, London | 0 (0.0) | 2 (2.0) | 2 (1.0) |
| Oxford Cancer and Haematology Centre, Oxford | 12 (12.0) | 13 (13.0) | 25 (12.5) |
| Pinderfields Hospital, Wakefield | 3 (3.0) | 5 (5.0) | 8 (4.0) |
| Princess Royal University Hospital, London | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| Queen Elizabeth Hospital, Birmingham | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Queen Elizabeth Hospital, Gateshead | 2 (2.0) | 3 (3.0) | 5 (2.5) |
| Queen Elizabeth Hospital, Woolwich | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| Royal Blackburn Hospital, Blackburn | 2 (2.0) | 4 (4.0) | 6 (3.0) |
| Royal Liverpool University Hospital, Liverpool | 2 (2.0) | 2 (2.0) | 4 (2.0) |
| Royal Marsden Hospital, London | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Royal United Hospital, Bath | 2 (2.0) | 3 (3.0) | 5 (2.5) |
| Russells Hall Hospital, Dudley | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| St Batholomew’s Hospital, London | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| St James’s University Hospital, Leeds | 8 (8.0) | 7 (7.0) | 15 (7.5) |
| Southampton General Hospital, Southampton | 6 (6.0) | 7 (7.0) | 13 (6.5) |
| Southmead Hospital, Bristol | 3 (3.0) | 0 (0.0) | 3 (1.5) |
| Sunderland Royal Hospital, Sunderland | 3 (3.0) | 3 (3.0) | 6 (3.0) |
| University Hospital of North Tees, Stockton-on-Tees | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Western General Hospital, Edinburgh | 3 (3.0) | 3 (3.0) | 6 (3.0) |
| Ysbyty Gwynedd Hospital, Bangor | 3 (3.0) | 4 (4.0) | 7 (3.5) |
Early closure to recruitment
The ARCTIC trial closed early to recruitment in September 2012 owing to an urgent safety measure, on advice from the DMEC and the TSC. This decision was made following the planned interim analysis of the short-term efficacy data for the first half of participants randomised to the trial (n = 103).
The unblinded CR and ORRs (based on an independent assessment of response following the IWCLL criteria26) were presented along with baseline demographic, treatment compliance, MRD eradication and safety and toxicity data. The data presented showed higher CR rates in the control arm (FCR) than were expected, and the difference in the CR rates between the treatment arms, although not statistically significant, was deemed by the DMEC to be clinically significant in favour of FCR over FCM-miniR. In addition, there appeared to be an increased toxicity rate and increased number of dose omissions and reductions in the FCM-miniR arm. A detailed summary of the interim analysis results is presented in Statistical trial results.
Interim analysis recommendations
The primary aim of this Phase IIB trial was to determine whether FCM-miniR should continue to be investigated in a Phase III trial. Given the strength and robustness of the interim data, the DMEC felt that continuing this trial was futile, as it was highly unlikely that the data would warrant continued investigation of the experimental treatment. As such, they recommended that recruitment into the trial should stop with immediate effect, and these recommendations were ratified by the TSC. It was agreed that the local site investigators should be informed of the findings of the interim analysis as soon as possible. All participants receiving FCM-miniR were recommended to transfer to treatment with FCR for the remainder of their treatment cycles, although this was not mandated. The decision was to be made by the participant following detailed discussions with their clinician. It was also agreed that participants should continue to be followed up as per the protocol.
At the time of trial closure, 200 participants had been recruited. Twenty-one out of the 23 participants still receiving FCM-miniR at the time of trial closure chose to transfer over to receive treatment with FCR on discussion with their treating clinician. Where appropriate, the results will categorise participants into three treatment categories: FCR, FCM-miniR and FCM-miniR/FCR for the patients randomised to FCM-miniR who were recommended to transfer. It is not appropriate to include the participants who transferred to FCR in either of the other categories according to the ITT analysis, because the decision to stop FCM-miniR was on recommendation from the DMEC, rather than a decision from the treating clinician or participant, which violates the ITT assumption.
Participant flow
Figure 4 presents the Consolidated Standards of Reporting Trials (CONSORT) diagram of participant flow through the trial. In total, 548 patients were reported as having been assessed for eligibility and, of these, 200 (36.5%) provided written informed consent and were randomised into the trial. Of the 348 patients who were reported as having been assessed for the trial but who were not randomised, 177 of these were from a single centre which used a very broad screening process which was inconsistent with other centres. The majority of patients were excluded owing to being clinically ineligible (n = 228, 65.5%). Again, this is biased towards the single centre which assessed 172 of these patients as being ineligible as they did not meet key eligibility criteria such as having had no prior therapy for CLL. If this centre is excluded from the screening data, a total of 371 patients were reported as having been assessed for eligibility with 56 (15.1%) being excluded owing to clinical ineligibility. A total of 100 participants were randomised to the FCR control arm and 100 to the FCM-miniR intervention arm.
FIGURE 4.
Consolidated Standards of Reporting Trials diagram. a, One participant did not receive any FCR and also had missing primary end point data and is therefore recorded twice; and b, one participant received FCR and had missing primary end point data and is therefore recorded twice. Reproduced from Howard et al. 1 with permission.

In the FCR arm, all but two participants received their allocated treatment (n = 98, 98.0%). One participant was ineligible (owing to prior therapy for CLL) and went on to receive FCR off trial. The other participant withdrew from the trial on the advice of the treating clinician as they had a 17p deletion and were treated with a more ‘appropriate regime’ off trial. In the FCM-miniR arm, 79 participants (79.0%) received their allocated intervention throughout the trial, with 21 participants transferring over to receive treatment with FCR as a result of the interim analysis, two from their first cycle of treatment.
A total of nine participants (4.5%) withdrew their consent for the trial (five in the FCR arm and four in the FCM-miniR arm). Table 5 summarises the number of participants who withdrew consent from the trial, the type of withdrawal and reason for withdrawal. Two participants withdrew their consent for further trial treatment only (one each in the FCR and FCM-miniR arms). Six participants withdrew their consent from further trial treatment and follow-up data collection (four in the FCR arm and two in the FCM-miniR), of which one ineligible participant in the FCR arm did not receive any trial treatment. Primary end point data are available for the two FCM-miniR participants, but all participants in the FCR arm who withdrew from follow-up did so prior to the assessment of the primary end point. One participant in the FCM-miniR arm withdrew their consent for further follow-up data collection only after the assessment of the primary end point, having received all six cycles of treatment.
| Participant withdrawal details | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| Participant withdrawn, n (%) | 5 (5.0) | 4 (4.0) | 9 (4.5) |
| Type of withdrawal, n (%) | |||
| Withdrawn from trial treatment only | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Withdrawn from trial treatment and follow-up data collection | 4 (4.0) | 2 (2.0) | 6 (3.0) |
| Withdrawn from follow-up data collection only | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Reason for withdrawal | |||
| Unwilling to continue with treatment, owing to toxicity | 0 (0.0) | 2 (2.0) | 2 (1.0) |
| Unwilling to continue with treatment, owing to being too unwell | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Unwilling to continue with treatment | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Unwilling to continue with visits | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Non-response to treatment | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Clinician decision | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Other reason | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Trial duration (months) from randomisation to withdrawal | |||
| Mean (SD) | 2.2 (2.0) | 9.6 (8.8) | 5.4 (6.8) |
| Median (range) | 1.8 (0.4–5.7) | 8.1 (1.4–20.7) | 2.0 (0.4–20.7) |
| N | 5 | 4 | 9 |
Three participants in the FCM-miniR arm withdrew because they were unwilling to continue with trial treatment as a result of either toxicity or being too unwell. In the FCR arm, one participant withdrew owing to non-response to treatment, another participant felt that ‘Not enough information was given regarding neutropenic sepsis’ and that their ‘GCSF injections were delayed due to the trial’ (categorised as ‘Other reason’ in Table 5). The median time from randomisation to withdrawal was 2.0 months (range: 0.4–20.7 months) with a shorter median time to withdrawal in the FCR arm (1.8 months) compared with the FCM-miniR arm (8.1 months).
Two participants in the FCR arm were found to be ineligible for the trial post randomisation. One participant had received prior therapy for CLL; they received FCR off-trial and continued to be followed up as per the protocol and therefore had available primary end point data within the definition of the ITT population, although they were excluded from the PP population. The second participant had been previously infected with hepatitis B; they continued on the trial under the approval of the Chief Investigator, with the justification that the participant had ‘antibodies but no infection’ and, therefore, the eligibility deviation was felt to be minor. They received all six cycles of treatment and were followed up for their primary end point.
A total of 13 participants (6.5%) were lost to follow-up and were classed as participants with missing primary end point data, with a similar proportion in each treatment arm (n = 8, 8.0% FCR; n = 5, 5.0% FCM-miniR). Ten participants (six in the FCR arm and four in the FCM-miniR arm) had a missing trephine sample at 3 months post treatment, which is required to confirm a CR. Four participants (one in the FCR arm and three in the FCM-miniR arm) missed their 3-month post-treatment visit, and one FCM-miniR participant failed to have sufficient clinical evaluations performed. Four participants in the FCR arm withdrew from further follow-up prior to their assessment of response; however, two did so owing to toxicity and non-response to treatment and were therefore included in the ITT analysis as non-responders. For further information on the reasons for missing trephine samples, missed clinic visits, withdrawals and how participants have been handled in the analysis, see Final analysis: primary end point.
Overall, 167 participants (83.5%) were included in the ITT analysis, with a higher proportion coming from the FCR arm (n = 92, 92.0%) than the FCM-miniR arm (n = 75, 75.0%). Participants were excluded from both trial arms owing to missing primary end point data (n = 13) and a further 21 participants were excluded from the FCM-miniR arm as a result of receiving treatment with FCR following the closure of the FCM-miniR arm. The PP population was similar to that of the ITT, with an additional participant excluded from the FCR arm owing to breaching the eligibility criteria and not receiving any of their randomised treatment. The participant who had previously been infected with hepatitis B was not excluded from the PP population as this was not classed as a major protocol violation.
Baseline characteristics
Tables 6–10 summarise the baseline characteristics, including the minimisation factors, participant characteristics, assessment of disease, clinical details and genetic markers for all randomised participants.
| Minimisation factors | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| Age group (years), n (%) | |||
| ≤ 65 | 63 (63.0) | 62 (62.0) | 125 (62.5) |
| > 65 | 37 (37.0) | 38 (38.0) | 75 (37.5) |
| Sex, n (%) | |||
| Male | 68 (68.0) | 67 (67.0) | 135 (67.5) |
| Female | 32 (32.0) | 33 (33.0) | 65 (32.5) |
| Binet stage, n (%) | |||
| A progressive or B | 67 (67.0) | 69 (69.0) | 136 (68.0) |
| C | 33 (33.0) | 31 (31.0) | 64 (32.0) |
| Participant characteristics | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| Age summaries, years | |||
| Mean (SD) | 61.8 (8.3) | 62.6 (8.3) | 62.2 (8.3) |
| Median (range) | 63 (41–77) | 63 (36–80) | 63 (36–80) |
| Age categories (years), n (%) | |||
| 30–39 | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| 40–49 | 9 (9.0) | 5 (5.0) | 14 (7.0) |
| 50–59 | 24 (24.0) | 25 (25.0) | 49 (24.5) |
| 60–69 | 48 (48.0) | 48 (48.0) | 96 (48.0) |
| ≥ 70 | 19 (19.0) | 21 (21.0) | 40 (20.0) |
| Ethnicity, n (%) | |||
| White | 98 (98.0) | 96 (96.0) | 194 (97.0) |
| Mixed: white and black Caribbean | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Asian: Indian | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Black: Caribbean | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Black: African | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Other ethnic group | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Baseline assessment | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| Duration of CLL (years) | |||
| Mean (SD) | 3.37 (3.60) | 2.81 (3.32) | 3.09 (3.46) |
| Median (range) | 2.47 (0.01–22.74) | 1.64 (0.03–17.88) | 2.17 (0.01–22.74) |
| Binet criteria, n (%) | |||
| A progressive | 20 (20.0) | 14 (14.0) | 34 (17.0) |
| B | 41 (41.0) | 54 (54.0) | 95 (47.5) |
| C | 39 (39.0) | 32 (32.0) | 71 (35.5) |
| B symptoms, n (%) | |||
| Yes | 46 (46.0) | 57 (57.0) | 103 (51.5) |
| No | 54 (54.0) | 43 (43.0) | 97 (48.5) |
| Signs of extranodal/extramedullary CLL, n (%) | |||
| Yes | 11 (11.0) | 14 (14.0) | 25 (12.5) |
| No | 88 (88.0) | 85 (85.0) | 173 (86.5) |
| Missing | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| WHO performance status, n (%) | |||
| 0 | 55 (55.0) | 61 (61.0) | 116 (58.0) |
| 1 | 40 (40.0) | 37 (37.0) | 77 (38.5) |
| 2 | 5 (5.0) | 2 (2.0) | 7 (3.5) |
| Baseline assessment | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| β2M concentration (mg/l), n (%) | |||
| < 4 | 37 (37.0) | 35 (35.0) | 72 (36.0) |
| ≥ 4 | 53 (53.0) | 62 (62.0) | 115 (57.5) |
| Missing | 10 (10.0) | 3 (3.0) | 13 (6.5) |
| Creatinine clearance (ml/minute), n (%) | |||
| 30–60 | 17 (17.0) | 14 (14.0) | 31 (15.5) |
| > 60 | 83 (83.0) | 86 (86.0) | 169 (84.5) |
| DCT, n (%) | |||
| Positive | 19 (19.0) | 15 (15.0) | 34 (17.0) |
| Negative | 71 (71.0) | 73 (73.0) | 144 (72.0) |
| Missing | 10 (10.0) | 12 (12.0) | 22 (11.0) |
| Paraprotein type, n (%) | |||
| None | 71 (71.0) | 68 (68.0) | 139 (69.5) |
| IgA | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| IgG | 17 (17.0) | 23 (23.0) | 40 (20.0) |
| IgM | 2 (2.0) | 4 (4.0) | 6 (3.0) |
| Missing | 10 (10.0) | 4 (4.0) | 14 (7.0) |
| Hepatitis B, n (%) | |||
| Previously infected | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Vaccinated | 4 (4.0) | 1 (1.0) | 5 (2.5) |
| Negative | 94 (94.0) | 98 (98.0) | 192 (96.0) |
| Missing | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Hepatitis C, n (%) | |||
| Negative | 100 (100) | 100 (100) | 200 (100) |
| Genetic markers | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| 17p deletion, n (%) | |||
| Yes (poorer risk) | 4 (4.0) | 3 (3.0) | 7 (3.5) |
| No (standard risk) | 88 (88.0) | 88 (88.0) | 176 (88.0) |
| Missing | 8 (8.0) | 9 (9.0) | 17 (8.5) |
| 11q deletion, n (%) | |||
| Yes (poorer risk) | 10 (10.0) | 20 (20.0) | 30 (15.0) |
| No (standard risk) | 83 (83.0) | 75 (75.0) | 158 (79.0) |
| Missing | 7 (7.0%) | 5 (5.0) | 12 (6.0) |
| VH mutation risk group, n (%) | |||
| Poor riska | 52 (52.0) | 52 (52.0) | 104 (52.0) |
| Standard riskb | 30 (30.0) | 31 (31.0) | 61 (30.5) |
| Missing | 18 (18.0) | 17 (17.0) | 35 (17.5) |
| CD38 status, n (%) | |||
| Positive (poorer risk) | 41 (41.0) | 46 (46.0) | 87 (43.5) |
| Negative (standard risk) | 55 (55.0) | 52 (52.0) | 107 (53.5) |
| Missing | 4 (4.0) | 2 (2.0) | 6 (3.0) |
Overall, 62.5% (n = 125) of the trial population were aged 65 years or less, 67.5% (n = 135) were male and 68.0% (n = 136) had Binet stage A progressive or B (Table 6). The median age of a trial participant was 63 years (range 36–80 years) and 20.0% (n = 40) were aged 70 years or more. Of the trial population, 97.0% (n = 194) were of white ethnicity (Table 7). The two treatment arms were well balanced for the minimisation factors and participant characteristics.
Table 8 summarises the baseline assessments of disease. The median duration of disease was approximately 2 years, with a range of less than 1 month to approximately 23 years. A total of 35.5% of participants (n = 71) were Binet stage C, with a higher proportion in the FCR arm (n = 39, 39.0%) than in the FCM-miniR arm (n = 32, 32.0%). These proportions are slightly different from those presented in Table 4 because, for a minor number of cases, the incorrect Binet staging was provided at randomisation. Overall, 51.5% (n = 103) of the trial population had B symptoms, with a higher proportion in the FCM-miniR arm (n = 57, 57.0%) than in the FCR arm (n = 46, 46.0%). Twenty-five (12.5%) participants had signs of extranodal or extramedullary CLL and the majority of participants (n = 116, 58.0%) were WHO performance status 0, with 38.5% and 3.5% at performance status 1 and 2, respectively.
Table 9 summarises the baseline clinical assessments, which are reasonably well balanced between the treatment arms. Seventy-two participants (36.0%) had a β2M concentration of < 4 mg/l and 31 (15.5%) participants had a creatinine clearance between 30 and 60 ml/minute. Overall, 34 participants (17.0%) had a positive direct Coombs test (DCT) and immunoglobulin G was the predominant paraprotein type after ‘None’ in 20% of participants. Results of the clinical assessments are in line with what would be expected for this population of patients. One participant in the FCR arm was assessed as being previously infected with hepatitis B, as described in Participant flow.
Table 10 summarises the genetic markers which were assessed at baseline and considered to be prognostic for response. Seven (3.5%) participants had a 17p deletion and 30 (15.0%) participants had an 11q deletion, a higher proportion in the FCM-miniR arm (n = 20, 20.0%) than in the FCR arm (n = 10, 10.0%). This chance difference might be important, as 11q-deleted CLL may be sensitive to rituximab and so this imbalance could affect the results.
Overall, 52.0% (n = 104) of participants were assessed to be within the VH mutation poor risk group and 43.5% (n = 87) had a positive CD38 status, a slightly higher proportion in the FCM-miniR arm (n = 46, 46.0%) than in the FCR arm (n = 41, 41.0%).
Treatment received
Tables 11–14 summarise the treatment details including treatment received, treatment discontinuations and modifications and GCSF usage by randomisation allocation (FCR, FCM-miniR). After the interim analysis, 21 participants still receiving FCM-miniR transferred over to receive treatment with FCR. Of these, two participants received a full six cycles of FCR; four received FCR from cycle 2; four from cycle 3; six from cycle 4; four from cycle 5; and one participant received FCR for their final cycle of treatment, cycle 6. These participants are summarised in addition to those who received FCR and FCM-miniR throughout the trial.
| Treatment details | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Number of treatment cycles received, n (%) | ||||
| 0 | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| 1 | 1 (1.0) | 7 (8.9) | 0 (0.0) | 8 (4.0) |
| 2 | 9 (9.0) | 3 (3.8) | 0 (0.0) | 12 (6.0) |
| 3 | 3 (3.0) | 6 (7.6) | 0 (0.0) | 9 (4.5) |
| 4 | 6 (6.0) | 5 (6.3) | 0 (0.0) | 11 (5.5) |
| 5 | 9 (9.0) | 7 (8.9) | 1 (4.8) | 17 (8.5) |
| 6 | 70 (70.0) | 51 (64.6) | 20 (95.2) | 141 (70.5) |
| Treatment cycles received, n (%) | ||||
| ≤ 3 | 15 (15.0) | 16 (20.3) | 0 (0.0) | 31 (15.5) |
| > 3 | 85 (85.0) | 63 (79.7) | 21 (100.0) | 169 (84.5) |
| Summary statistics | ||||
| Mean (SD) | 5.2 (1.5) | 5.0 (1.7) | 6.0 (0.2) | 5.2 (1.5) |
| Median (range) | 6 (0–6) | 6 (1–6) | 6 (5–6) | 6 (0–6) |
| Early discontinuation details | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Number of participants discontinuing treatment early, n (%) | 30 (30.0) | 28 (35.4) | 1 (4.8) | 59 (29.5) |
| Reason for early discontinuation of treatment, n (%) | ||||
| Disease progression, requiring further treatment | 0 (0.0) | 3 (10.7) | 0 (0.0) | 3 (5.1) |
| Toxicity | 22 (73.3) | 21 (75.0) | 1 (100) | 44 (74.6) |
| A prior eligibility criteria has been violated (discussed with CTRU/CI) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Stable disease with no or minimal response | 1 (3.3) | 2 (7.1) | 0 (0.0) | 3 (5.1) |
| Participant decision | 2 (6.7) | 1 (3.6) | 0 (0.0) | 3 (5.1) |
| Clinician decision | 3 (10.0) | 1 (3.6) | 0 (0.0) | 4 (6.8) |
| Other reason discontinued | 1 (3.3) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Treatment modifications | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Number of participants experiencing at least one dose modification, n (%) | 67 (67.0) | 52 (65.8) | 16 (76.2) | 135 (67.5) |
| Dose modification, n (%) | ||||
| Dose omission | 6 (6.0) | 9 (11.4) | 2 (9.5) | 17 (8.5) |
| Dose reduction | 31 (31.0) | 28 (35.4) | 11 (52.4) | 70 (35.0) |
| Dose delay | 53 (53.0) | 47 (59.5) | 14 (66.7) | 114 (57.0) |
| Dose stopped early | 11 (11.0) | 7 (8.9) | 1 (4.8) | 19 (9.5) |
| Alternative route of administration | 7 (7.0) | 5 (6.3) | 1 (4.8) | 13 (6.5) |
| GCSF usage | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Received GCSF during the first three cycles, n (%) | ||||
| Yes | 31 (31.0) | 28 (35.4) | 9 (42.9) | 68 (34.0) |
| No | 57 (57.0) | 41 (51.9) | 12 (57.1) | 110 (55.0) |
| Unknown | 12 (12.0) | 10 (12.7) | 0 (0.0) | 22 (11.0) |
| Received GCSF during treatment, n (%) | ||||
| Yes | 42 (42.0) | 40 (50.6) | 12 (57.1) | 94 (47.0) |
| No | 53 (53.0) | 34 (43.0) | 9 (42.9) | 96 (48.0) |
| Unknown | 5 (5.0) | 5 (6.3) | 0 (0.0) | 10 (5.0) |
Overall, 31 participants (15.5%) received three or fewer cycles of treatment (n = 15, 15.0% FCR; n = 16, 20.3% FCM-miniR; n = 0, 0.0% FCM-miniR/FCR), with 70.5% of participants receiving all six cycles (n = 70, 70.0% FCR; n = 51, 64.6% FCM-miniR; n = 20, 95.2% FCM-miniR/FCR) (Table 11). Two participants randomised to FCR did not receive any trial treatment. One was found to be ineligible post randomisation as they had received prior therapy for CLL; they went on to receive FCR off trial and continued to be followed up as per the protocol. The other participant withdrew from the trial (treatment and follow-up) on the advice of the treating clinician as they had a 17p deletion and were treated with a more ‘appropriate regime’ off trial.
Fifty-nine participants (29.5%) discontinued treatment early: 30 of those participants who were receiving FCR (30.0%), 28 of those participants receiving FCM-miniR (35.4%) and one of those participants in the FCM-miniR/FCR group. Toxicity was the majority reason for early discontinuation of treatment (74.6% of cases), with a similar proportion in the FCR and FCM-miniR treatment arms (FCR: n = 22, 73.3%; FCM-miniR: n = 21, 75.0%) (Table 12).
Table 13 summarises the number and proportion of participants experiencing at least one modification to their protocol-defined dose of treatment (i.e. dose omission, reduction, delay, stopped early or alternative route of administration). A total of 135 participants (67.5%) experienced at least one modification to their protocol treatment with a similar proportion in the FCR (n = 67, 67.0%) and FCM-miniR (n = 52, 65.8%) treatment arms. Overall, 57.0% (n = 114) of participants experienced a delay to at least one of their doses of treatment and 35.0% (n = 70) experienced a dose reduction with a higher proportion in the FCM-miniR arm.
Overall, 68 participants (34.0%) received treatment with GCSF during the cycles of therapy with a higher proportion in the FCM-miniR arm (35.4%, n = 28) than the FCR arm (31.0%, n = 31) and the FCM-miniR/FCR arm (42.9%, n = 9). Throughout the whole of the treatment period (cycles 1–6), 94 participants (47.0%) received GCSF at some stage (FCR: 42%, n = 42; FCM-miniR: 50.6%, n = 40; and FCM-miniR/FCR: 57.1%, n = 12) (Table 14). Rates are in line with what would be expected for these treatments.
Of the 68 participants known to have received treatment with GCSF at some stage during the first three cycles, 88.2% (n = 60) received four or more cycles of treatment; in contrast, 98.2% (n = 108) of those who did not receive GCSF received four or more cycles of treatment (Table 15).
| Number of treatment cycles received | Received GCSF within first three cycles (n = 68), n (%) | Did not receive GCSF within first three cycles (n = 110), n (%) | Unknown (n = 20), n (%) | Total (n = 198), n (%) |
|---|---|---|---|---|
| 1 | 0 (0.0) | 0 (0.0) | 8 (40.0) | 8 (4.0) |
| 2 | 2 (2.9) | 0 (0.0) | 10 (50.0) | 12 (6.1) |
| 3 | 6 (8.8) | 2 (1.8) | 1 (5.0) | 9 (4.5) |
| 4 | 5 (7.4) | 6 (5.5) | 0 (0.0) | 11 (5.6) |
| 5 | 5 (7.4) | 12 (10.9) | 0 (0.0) | 17 (8.6) |
| 6 | 50 (73.5) | 90 (81.8) | 1 (5.0) | 141 (71.2) |
Interim analysis
An interim analysis of the short-term efficacy data was performed on the first 103 participants randomised into the study: 51 allocated to the FCR control arm and 52 allocated to the FCM-miniR intervention arm. The results of the interim analysis were presented to the DMEC in September 2012.
The interim analysis was based on all data received and entered into the trial database up to 30 August 2012. Owing to the ongoing nature of the trial, the DMEC was aware that the data on which the interim analysis was based may not have been fully validated and could be subject to change by the time of the final analysis. This report summarises the data as they were reported to the DMEC, even if there were changes to these results prior to the final, clean, analysis data set.
By the time the final participant to be included in the interim analysis had reached their primary end point (i.e. 3 months post treatment), 191 participants out of a target of 206 had been randomised from 32 UK centres. The primary aim of the formal interim analysis was to be able to release information on any potential large differences in efficacy between the treatment arms earlier than would have been the case with the final analysis.
Treatment received
Of the first 103 participants randomised, 72 (69.9%) received all six cycles of treatment, with a higher proportion of participants coming from the FCR arm (n = 38, 74.5%) than the FCM-miniR arm (n = 34, 65.4%) (Table 16). Thirty-one participants (30.1%) had discontinued treatment early (FCR: 25.5%, n = 13; FCM-miniR: 34.6%, n = 18).
| Treatment details | FCR (n = 51) | FCM-miniR (n = 52) | Total (n = 103) |
|---|---|---|---|
| Number of treatment cycles received, n (%) | |||
| 1 | 0 (0.0) | 3 (5.8) | 3 (2.9) |
| 2 | 5 (9.8) | 1 (1.9) | 6 (5.8) |
| 3 | 1 (2.0) | 2 (3.8) | 3 (2.9) |
| 4 | 3 (5.9) | 5 (9.6) | 8 (7.8) |
| 5 | 4 (7.8) | 7 (13.5) | 11 (10.7) |
| 6 | 38 (74.5) | 34 (65.4) | 72 (69.9) |
Table 17 summarises the number and proportion of participants experiencing at least one modification to their protocol-defined dose of treatment. A higher proportion of participants in the FCM-miniR arm experienced at least one dose omission, dose reduction and dose delay than in the FCR arm.
| Treatment modifications | FCR (n = 51) | FCM-miniR (n = 52) | Total (n = 103) |
|---|---|---|---|
| Dose modification, n (%) | |||
| Dose omission | 1 (2.0) | 7 (13.5) | 8 (7.8) |
| Dose reduction | 15 (29.4) | 20 (38.5) | 35 (34.0) |
| Dose delay | 23 (45.1) | 28 (53.8) | 51 (49.5) |
Efficacy
An independent central assessment of response was carried out in order to assess formally the primary end point data on response to treatment as defined by IWCLL criteria. 26 The interim analysis of the primary end point data was based on the ITT population, which included participants for whom written informed consent had been received and for which primary end point data were available. A PP analysis was not performed as, out of the first 103 participants, all received at least one cycle of their randomised treatment. The FCR participant who had been previously infected with hepatitis B was not excluded as this was not classed as a major protocol violation.
Of the 103 participants assessed in the interim analysis, 18 (17.5%) were excluded from the formal analysis of the primary end point, with a slightly higher proportion coming from the FCR arm (FCR: n = 10, 19.6%; FCM-miniR: n = 8, 15.4%) (Table 18). Fifteen participants had a missing trephine sample (7 FCR; 8 FCM-miniR), which is required by the IWCLL criteria to confirm a CR, with no further evidence that they were definitely not a CR/CRi. Of these, two trephine samples were taken but were inadequate for analysis. Thirteen samples were not taken owing to a sample being missed in error (n = 4); a clinician’s decision (n = 2); a participant’s decision (n = 2); a participant being unwell (n = 1); it being too painful/difficult to sample a participant (n = 4).
| Exclusion | FCR (n = 51) | FCM-miniR (n = 52) | Total (n = 103) |
|---|---|---|---|
| Participants excluded from the primary end point analysis, n (%) | 10 (19.6) | 8 (15.4) | 18 (17.5) |
| Reasons for exclusion, n (%) | |||
| Missing 3-month post-treatment trephine sample | 7 (13.7) | 8 (15.4) | 15 (14.6) |
| Withdrew from further trial treatment and follow-up data collection | 2 (3.9) | 0 (0.0) | 2 (1.9) |
| Lost to follow-up | 1 (2.0) | 0 (0.0) | 1 (1.0) |
In the FCR arm, two participants withdrew their consent for further trial treatment and follow-up data collection prior to the assessment of the primary end point, and one participant discontinued treatment after their second cycle and was subsequently lost to follow-up.
Table 19 presents the number of participants achieving a CR and the difference in response rates between the treatment arms. Overall, 71.8% (n = 61) of participants achieved a CR, with a higher proportion in the FCR arm (n = 34, 82.9%) than in the FCM-miniR arm (n = 27. 61.4%). The difference in the CR rates was 21.6% in favour of the FCR arm, with a 99.5% CI (–48.0% to 4.8%).
| Achievement of the primary end point | FCR (n = 41) | FCM-miniR (n = 44) | Total (n = 85) | Difference in response rates (FCM – miniR-FCR) | 99.5% CI for difference in response rate |
|---|---|---|---|---|---|
| Achieved a CR/CRi, n (%) | 34 (82.9) | 27 (61.4) | 61 (71.8) | –21.6 | –48.0% to 4.8% |
| Did not achieve a CR/CRi, n (%) | 7 (17.1) | 17 (38.6) | 24 (28.2) |
A binary multivariate logistic regression model was used to assess formally the effect of treatment on the proportion of participants achieving a CR at 3 months post treatment, adjusting for the minimisation factors, excluding centre (Table 20). The O’Brien and Fleming32 alpha spending function was used to adjust for multiple testing, requiring an alpha level of < 0.005 (two-sided) to indicate significance, in order to preserve the alpha for the final analysis.
| Parameter | df | Parameter estimate | SE | Wald χ2 | Pr > χ2 | OR (99.5% CI) |
|---|---|---|---|---|---|---|
| Intercept | 1 | 0.81 | 0.31 | 6.86 | 0.009 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.56 | 0.27 | 4.34 | 0.037 | 0.32 (0.07 to 1.48) |
| Sex: female vs. male | 1 | 0.31 | 0.31 | 1.02 | 0.312 | 1.87 (0.33 to 10.55) |
| Age group: > 65 years vs. ≤ 65 years | 1 | –0.56 | 0.28 | 3.91 | 0.048 | 0.32 (0.07 to 1.60) |
| Binet stage: C vs. A progressive or B | 1 | –0.42 | 0.28 | 2.35 | 0.125 | 0.43 (0.09 to 2.02) |
The OR for achieving a CR in the FCM-miniR arm compared with the FCR arm was 0.32 (99.5% CI: 0.07 to 1.48). With a p-value of 0.037, the difference between the treatment arms in terms of CR rates was not significant at the reduced 0.5% significance level, although it was noted that the experimental treatment was somewhat worse in terms of response.
Of the 103 participants included in the interim analysis, 51.5% (n = 53) had undetectable MRD at 3 months post treatment, with a higher proportion of participants in the FCR arm (n = 29, 56.9%) than in the FCM-miniR arm (n = 24, 46.2%) (Table 21).
| MRD assessment | FCR (n = 51) | FCM-miniR (n = 52) | Total (n = 103) |
|---|---|---|---|
| Assessment of MRD, n (%) | |||
| MRD negative | 29 (56.9) | 24 (46.2) | 53 (51.5) |
| MRD positive | 19 (37.3) | 22 (42.3) | 41 (39.8) |
| Unknown, samples not taken | 0 (0.0) | 5 (9.6) | 5 (4.9) |
| Early death | 0 (0.0) | 1 (1.9) | 1 (1.0) |
| Withdrew from further trial treatment and follow-up data collection | 2 (3.9) | 0 (0.0) | 2 (1.9) |
| Lost to follow-up | 1 (2.0) | 0 (0.0) | 1 (1.0) |
Safety and toxicity
A total of 103 SAEs were reported from 60 participants out of the first 103 randomised into the trial. Of the 103 reported SAEs, 44 events (42.7%) were from 26 participants receiving FCR and 59 events (57.3%) were from 34 participants receiving FCM-miniR. The mean number of SAEs reported per participant (1.7) was the same in both treatment arms (Table 22).
| SAE summary | FCR (n = 51) | FCM-miniR (n = 52) | Total (n = 103) |
|---|---|---|---|
| Has the participant experienced an SAE?, n (%) | |||
| Yes | 26 (51.0) | 34 (65.4) | 60 (58.3) |
| No | 25 (49.0) | 18 (34.6) | 43 (41.7) |
| Number of participants with one or more SAE | 26 | 34 | 60 |
| Number of SAEs reported | 44 | 59 | 103 |
| Number of SAEs per participant | |||
| Mean (SD) | 1.7 (1.2) | 1.7 (1.1) | 1.7 (1.1) |
| Median (range) | 1 (1–5) | 1 (1–5) | 1 (1–5) |
Of the 103 SAEs reported, 85 (82.5%) were suspected to be related to trial treatment (51 reported from participants receiving FCM-miniR and 34 reported from participants receiving FCR). One SUSAR was reported from a participant in the FCR arm. The participant received all six cycles of treatment and was diagnosed with a ‘squamous cell carcinoma’ approximately 4 months after their last cycle of treatment. The event was felt by the Principal Investigator at the site to be related to trial treatment (F, C and R) and unexpected. The majority of SAEs required hospitalisation (n = 89, 86.4%); three participants died as a result of their SAE (one in the FCR arm and two in the FCM-miniR arm) (Table 23).
| SAE relationship and seriousness criteria | FCR, N (%) | FCM-miniR, N (%) | Total, N (%) |
|---|---|---|---|
| Relationship to experimental treatment | |||
| Suspected, unexpected | 1 (2.3) | 0 (0.0) | 1 (1.0) |
| Suspected, expected | 33 (75.0) | 51 (86.4) | 84 (81.6) |
| Not suspected | 10 (22.7) | 8 (13.6) | 18 (17.5) |
| Total | 44 (100) | 59 (100.0) | 103 (100) |
| Seriousness criteria (not mutually exclusive) | |||
| Participant died as a result of the SAE | 1 (2.3) | 2 (3.4) | 3 (2.9) |
| Life-threatening | 1 (2.3) | 3 (5.1) | 4 (3.9) |
| Congenital anomaly/birth defect | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Required/prolonged hospitalisation | 39 (88.6) | 50 (84.7) | 89 (86.4) |
| Persistent or significant disability/incapacity | 1 (2.3) | 0 (0.0) | 1 (1.0) |
| Jeopardised patient/required intervention to prevent one of the above criteria | 6 (13.6) | 10 (16.9) | 16 (15.5) |
| Missing | 1 (2.3) | 0 (0.0) | 1 (1.0) |
A total of 1469 AEs were reported from 101 participants. A total of 669 events were from 49 participants receiving FCR and 770 events from 52 participants receiving FCM-miniR.
Conclusions from the Data Monitoring and Ethics Committee
The difference in the CR rates between the treatment arms (82.9% FCR; 61.4% FCM-miniR), although not statistically significant, was deemed by the DMEC to be clinically relevant. In light of this, and the indication of an increased toxicity rate and increased number of dose omissions and reductions in the FCM-miniR arm, the DMEC recommended that the trial should close the recruitment with immediate effect, and all participants receiving FCM-miniR were recommended to transfer to treatment with FCR for the remainder of their treatment cycles. At the time of trial closure, 200 participants had been recruited into the trial.
Final analysis: primary end point
The final participant’s clinic visit to assess their primary end point (3 months post treatment) was carried out on 31 July 2013; the data lock for the analysis of the primary end point was performed on 30 October 2013. Owing to the non-inferiority nature of the primary end point, the primary analysis was carried out on both the ITT and PP populations.
Participants randomised to FCM-miniR who transferred over to receive treatment with FCR after the interim analysis (see Interim analysis for further details) were summarised in a separate group and excluded from any formal comparisons of the treatment arms. This was felt to be appropriate, as the decision to stop FCM-miniR was on the recommendation of the DMEC, rather than a decision from the treating clinician or participant, which violates the ITT assumption.
Central assessment of response by International Workshop on chronic lymphocytic leukaemia
Table 24 summarises the proportion of participants with a centrally reviewed IWCLL assessment of response. Of the 200 participants, a response assessment was available for 166 participants (83.0%), with the same proportion in each treatment arm. Twenty-four (12.0%) participants assessed as being at least a PR had a missing trephine sample, which is required by the IWCLL criteria to confirm a CR. Four participants in the FCR arm withdrew from follow-up and one FCM-miniR participant died prior to the assessment of response. In addition, in the FCM-miniR arm, one participant’s response to treatment was unable to be assessed and four participants (one in the FCR and three in the FCM-miniR arm) missed their 3-month post-treatment visit.
| IWCLL assessment of response available | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| IWCLL assessment of response, n (%) | |||
| Yes | 83 (83.0) | 63 (63.0) | 146 (73.0) |
| Yes (received FCM-miniR/FCR) | 0 (0.0) | 20 (20.0) | 20 (10.0) |
| No IWCLL assessment of response, n (%) | |||
| Missing trephine sample | 12 (12.0) | 11 (11.0) | 23 (11.5) |
| Missing trephine sample (received FCM-miniR/FCR) | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Withdrew from follow-up prior to assessment of response | 4 (4.0) | 0 (0.0) | 4 (2.0) |
| Early death | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Unable to assess | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Missed 3-month post-treatment visit | 1 (1.0) | 3 (3.0) | 4 (2.0) |
Numbers analysed and reasons for non-inclusion in the intention-to-treat population
Of the 200 participants randomised, 167 (83.5%) participants were included in the ITT analysis, with a higher proportion in the FCR arm (n = 92, 92.0% FCR; n = 75, 75.0% FCM-miniR) (Table 25). Participants who were randomised to FCM-miniR but transferred over to receive treatment with FCR after the interim analysis were excluded from the ITT population (n = 21). In addition to the 146 participants in the FCR and FCM-miniR arms with an assessment of response, a further 14 participants who were at least a PR but had missing trephine samples were included in the ITT analysis owing to further clinical evidence that confirmed that they were either a CR/CRi or PR, as per the pre-specified decision rules, specified in Chapter 2, Missing data handling. Two participants in the FCR arm withdrew consent for the trial owing to non-response and toxicity, and a further FCM-miniR participant died as a result of their CLL, all prior to the assessment of their primary end point (3-month post-treatment visit). Four participants (one in the FCR and three from the FCM-miniR arms) discontinued treatment early owing to non-response or toxicity and subsequently missed their 3-month post-treatment visit. All seven participants (three FCR, four FCM-miniR) were included in the ITT analysis as non-responders, as per the missing data decision rules.
| Reason for exclusion, n (%) | FCR (n = 100) | FCM-miniR (n = 100) | Total (n = 200) |
|---|---|---|---|
| Participants included in the ITT population | 92 (92.0) | 75 (75.0) | 167 (83.5) |
| IWCLL response assessment available | 84 (84.0) | 66 (66.0) | 150 (75.0) |
| Missing trephine sample but clinical evidence that participant was either a CR/CRi or PR | 6 (6.0) | 8 (8.0) | 14 (7.0) |
| Withdrew from follow-up prior to assessment of response owing to toxicity or non-response | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| Early death as a result of CLL | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Participants excluded from the ITT population | 8 (8.0) | 25 (25.0) | 33 (16.5) |
| Received FCM-miniR/FCR | 0 (0.0) | 21 (21.0) | 21 (10.5) |
| Missing trephine sample with at least a PR and no evidence that participant was not a CR/CRi | 6 (6.0) | 3 (3.0) | 9 (4.5) |
| Withdrew from follow-up prior to assessment of response | 2 (2.0) | 0 (0.0) | 2 (1.0) |
| Unable to assess | 0 (0.0) | 1 (1.0) | 1 (0.5) |
A total of 33 participants were not able to be included in the ITT analysis, including the 21 treatment arm transfers:
-
There were nine participants with a missing trephine sample (six in the FCR and three in the FCM-miniR arm), with no further clinical evidence they were definitely not a CR/CRi. Of these, three were missed in error; two were at the discretion of the treating clinician; two were the participants’ decisions; two were a result of it being too painful/difficult to sample participants.
-
Two participants withdrew their consent for further trial treatment and follow-up data collection prior to the assessment of the primary end point, both randomised to FCR. Of these, one participant had a 17p deletion and on the advice of the treating clinician was treated with a ‘more appropriate regime’ off trial, prior to receiving any trial treatment. The second participant withdrew consent after receiving one cycle of treatment, for non-treatment-related reasons: ‘Due to other tests/treatment, the participant no longer wishes to remain on trial and feels they do not have time to continue with trial follow-up’.
-
One participant, who received two cycles of treatment with FCM-miniR, was unable to be assessed for response owing to insufficient clinical evaluations performed at the 3-month post-treatment visit (i.e. missing haematology tests, liver and spleen examinations and blood/trephine samples), with no reason provided.
These 33 participants were excluded from the ITT population in line with the guidance within the Statistical Analysis Plan, which was signed off prior to any analysis being conducted.
In the PP population, an additional participant with an assessment of response in the FCR arm was excluded as a result of breaching the eligibility criteria and not receiving any of their randomised treatment. The participant in the FCR arm who had been previously infected with hepatitis B was not excluded as this was not classed as a major protocol violation.
Table 26 presents the baseline characteristics for the ITT population compared with the 33 participants who were not able to be included in the analysis. In the group of participants who were not included in the ITT analysis, participants tended to be older, with a higher proportion of females and Binet stage A progressive or B disease, although these were relatively small numbers for comparison. The excluded population contained a slightly higher proportion of poorer risk participants in terms of 17p and 11q deletions.
| Baseline characteristics | ITT population (n = 167) | Excluded from the ITT population (n = 33) | Total (n = 200) |
|---|---|---|---|
| Minimisation factors, n (%) | |||
| Age group | |||
| ≤ 65 years | 106 (63.5) | 19 (57.6) | 125 (62.5) |
| > 65 years | 61 (36.5) | 14 (42.4) | 75 (37.5) |
| Sex | |||
| Male | 117 (70.1) | 18 (54.5) | 135 (67.5) |
| Female | 50 (29.9) | 15 (45.5) | 65 (32.5) |
| Binet stage | |||
| A progressive or B | 111 (66.5) | 25 (75.8) | 136 (68.0) |
| C | 56 (33.5) | 8 (24.2) | 64 (32.0) |
| Genetic markers | |||
| 17p deletion | |||
| Yes (poorer risk) | 6 (3.6) | 1 (3.0) | 7 (3.5) |
| No (standard risk) | 147 (88.0) | 29 (87.9) | 176 (88.0) |
| Missing | 14 (8.4) | 3 (9.1) | 17 (8.5) |
| 11q deletion | |||
| Yes (poorer risk) | 24 (14.4) | 6 (18.2) | 30 (15.0) |
| No (standard risk) | 133 (79.6) | 25 (75.8) | 158 (79.0) |
| Missing | 10 (6.0) | 2 (6.1) | 12 (6.0) |
| VH risk group | |||
| Poor risk | 87 (52.1) | 17 (51.5) | 104 (52.0) |
| Standard risk | 52 (31.1) | 9 (27.3) | 61 (30.5) |
| Unknown | 28 (16.8) | 7 (21.2) | 35 (17.5) |
Formal analysis of the primary end point
Overall, 66.5% (n = 111) of participants in the ITT population achieved a CR, a higher proportion in the FCR arm (n = 70, 76.1%) than the FCM-miniR arm (n = 41, 54.7%) (Table 27). The difference between the arms was 21.4%, in favour of the FCR arm (95% CI –35.8% to –7.0%), indicating that FCM-miniR appears to be performing worse in terms of response.
| Achievement of the primary end point, n (%) | FCR (n = 92) | FCM-miniR (n = 75) | Total (n = 167) | Difference in CR rates and 95% CIs (FCM-miniR – FCR) |
|---|---|---|---|---|
| Achieved a CR/CRi | 70 (76.1) | 41 (54.7) | 111 (66.5) | –21.4% (–35.8% to –7.0%) |
| Did not achieve a CR/CRi | 22 (23.9) | 34 (45.3) | 56 (33.5) |
A binary multivariate logistic regression model was used to assess formally the primary end point: the effect of treatment on the proportion of participants achieving a CR at 3 months post treatment, after adjusting for the minimisation factors, excluding centre (Table 28). The OR for achieving a CR in the FCM-miniR arm compared with the FCR arm was 0.37 (95% CI: 0.19 to 0.73), indicating that participants in the FCM-miniR are less likely to achieve a CR. The trial was powered on demonstrating that FCM-miniR was no more than 10% worse in terms of CR rates than FCR. With a CR rate of 76.1% in the FCR arm, a 10% reduction gives an OR of 0.61 (see Primary end point analysis for further details of the derivation). As the lower limit of the 95% CI for the treatment effect is < 0.61, and even the mean OR is below this level, there is very strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates. As the upper limit of the 95% CI is also below 1, there is evidence that FCM-miniR is significantly inferior to FCR.
| Parameter | df | Parameter estimate | SE | OR (95% CI estimate) |
|---|---|---|---|---|
| Intercept | 1 | 1.38 | 0.33 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.98 | 0.34 | 0.37 (0.19 to 0.73) |
| Sex: female vs. male | 1 | 0.22 | 0.38 | 1.25 (0.59 to 2.63) |
| Age group: > 65 years vs. ≤ 65 years | 1 | –0.55 | 0.35 | 0.58 (0.29 to 1.15) |
| Binet stage: C vs. A progressive or B | 1 | –0.21 | 0.36 | 0.81 (0.40 to 1.63) |
The 95% CIs around the ORs for the minimisation factors all contain 1, indicating that there is no evidence that any of the minimisation factors are significantly associated with response, although there is a suggested trend towards participants who are aged over 65 years and with Binet stage C performing worse.
The analysis of the primary end point using the PP population concurred with the outcome of the ITT analysis and demonstrated inferiority of FCM-miniR to FCR with an OR of 0.38 (95% CI 0.19 to 0.75) (Table 29).
| Parameter | df | Parameter estimate | SE | OR (95% CI estimate) |
|---|---|---|---|---|
| Intercept | 1 | 1.37 | 0.33 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.97 | 0.34 | 0.38 (0.19 to 0.75) |
| Sex: female vs. male | 1 | 0.23 | 0.38 | 1.26 (0.60 to 2.66) |
| Age group: > 65 years vs. ≤ 65 years | 1 | –0.57 | 0.35 | 0.57 (0.28 to 1.13) |
| Binet stage: C vs. A progressive or B | 1 | –0.20 | 0.36 | 0.82 (0.41 to 1.66) |
The analysis of the primary end point on both the ITT and PP populations strongly demonstrated that FCM-miniR is not non-inferior to FCR in terms of CR rates and, in fact, there is evidence to suggest that FCM-miniR is significantly inferior to FCR.
Sensitivity analyses
Sensitivity analyses were performed in order to assess the reliability of the analysis of primacy and the assumptions regarding the missing primary end point data. The responses of participants that were not included in the ITT population were imputed, with the exception of those participants who received FCM-miniR/FCR after the FCM-miniR arm was closed (see Table 25).
The first sensitivity analysis assumed that participants who were not included in the ITT population were all non-responders, and thus these participants were treated as not having achieved a CR in the analysis of the primary end point (eight in the FCR arm and four in the FCM-miniR). A total of 179 participants were included in this analysis, with 62.0% (111/179) achieving a CR compared with 66.5% (111/167) in the analysis of primacy. A total of 70.0% (70/100) of FCR participants achieved a CR compared with 51.9% (41/79) in the FCM-miniR arm. The OR for achieving a CR in the FCM-miniR arm compared with the FCR arm was 0.46 (95% CI 0.24 to 0.86) (Table 30), indicating that FCM-miniR participants are less likely to achieve a CR. With a response rate of 70.0% in the control arm, a 10% reduction (i.e. the non-inferiority margin) gives an OR of 0.64. As the lower limit of the 95% CI for the treatment effect and the mean OR are both < 0.64, there is very strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates, even after treating participants with missing data as non-responders.
| Parameter | df | Parameter estimate | SE | OR (95% CI estimate) |
|---|---|---|---|---|
| Intercept | 1 | 1.14 | 0.30 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.78 | 0.32 | 0.46 (0.24 to 0.86) |
| Sex: female vs. male | 1 | 0.03 | 0.34 | 1.03 (0.52 to 2.02) |
| Age group: > 65 years vs. ≤ 65 years | 1 | –0.52 | 0.32 | 0.60 (0.31 to 1.13) |
| Binet stage: C vs. A progressive or B | 1 | –0.28 | 0.33 | 0.75 (0.39 to 1.45) |
The second sensitivity analysis assumed that the participants who were not included in the ITT population were all responders, and thus these participants were treated as having achieved a CR in the analysis of the primary end point (8 FCR, 4 FCM-miniR). A total of 179 participants were included in this analysis, with 68.7% (123/179) achieving a CR compared with 66.5% (111/167) in the analysis of primacy. A total of 78.0% (78/100) of FCR participants achieved a CR compared with 57.0% (45/79) in the FCM-miniR arm. The OR for achieving a CR in the FCM-miniR arm compared with the FCR arm was 0.37 (95% CI: 0.19 to 0.72) (Table 31) indicating that participants in the FCM-miniR are less likely to achieve a CR. With a response rate of 78.0% in the control arm, a 10% reduction (i.e. the non-inferiority margin) gives an OR of 0.60. As the lower limit of the 95% CI for the treatment effect and the mean OR are < 0.60, there is very strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates, even after treating participants with missing data as responders.
| Parameter | df | Parameter estimate | SE | OR (95% CI estimate) |
|---|---|---|---|---|
| Intercept | 1 | 1.45 | 0.33 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –1.00 | 0.34 | 0.37 (0.19 to 0.72) |
| Sex: female vs. male | 1 | 0.32 | 0.37 | 1.38 (0.66 to 2.85) |
| Age group: > 65 vs. ≤ 65 | 1 | –0.51 | 0.34 | 0.60 (0.31 to 1.18) |
| Binet stage: C vs. A progressive or B | 1 | –0.22 | 0.35 | 0.81 (0.40 to 1.60) |
The third sensitivity analysis excluded all participants with missing trephine data from the ITT population and thus the analysis of the primary end point (6 FCR, 8 FCM-miniR). A total of 153 participants were included in this analysis, with 69.9% (107/153) achieving a CR compared with 66.5% (111/167) in the analysis of primacy. A total of 79.1% (68/86) of FCR participants achieved a CR compared with 58.2% (39/67) of participants in the FCM-miniR arm. The OR for achieving a CR in the FCM-miniR arm compared with the FCR arm was 0.37 (95% CI: 0.18, 0.77) (Table 32), indicating that participants in the FCM-miniR are less likely to achieve a CR. With a response rate of 79.1% in the control arm, a 10% reduction (i.e. the non-inferiority margin) gives an OR of 0.59. As the lower limit of the 95% CI for the treatment effect and the mean OR are < 0.59, there is very strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates, even after excluding participants with missing trephine data.
| Parameter | df | Parameter estimate | SE | OR (95% CI estimate) |
|---|---|---|---|---|
| Intercept | 1 | 1.54 | 0.36 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.99 | 0.37 | 0.37 (0.18 to 0.77) |
| Sex: female vs. male | 1 | 0.47 | 0.43 | 1.61 (0.69 to 3.75) |
| Age group: > 65 vs. ≤ 65 | 1 | –0.62 | 0.37 | 0.54 (0.26 to 1.13) |
| Binet stage: C vs. A progressive or B | 1 | –0.25 | 0.39 | 0.78 (0.36 to 1.67) |
The results of the sensitivity analyses are consistent with the analysis of primacy and confirm that there is strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates at 3 months post treatment. The results do not change considerably even after imputing the missing data, demonstrating the robustness of the conclusions. After excluding participants with missing trephine data, the results remained consistent with the analysis of primacy, supporting the approach to impute the response for participants with missing trephine data but who were known to be at least a PR (as described in Missing data handling).
Subgroup analyses
Pre-specified subgroup analyses were carried out to assess the heterogeneity of the treatment effect among subgroups of interest for the primary end point, using the ITT population. As the PP population was very similar to that of the ITT, subgroup analyses on this population were considered unnecessary. As the trial was not powered to look for differences in subgroups of participants, the results are to be treated as exploratory and should be used for hypothesis-generating purposes only.
Tables 33–36 present the subgroups of interest by whether or not a CR was achieved, the difference in response rates and 95% CIs.
| Minimisation factors | Difference in rates (95% CI) | ||
|---|---|---|---|
| Sex | Male (n = 117) | Female (n = 50) | |
| Achieved a CR/CRi, n (%) | 76 (65.0) | 35 (70.0) | –5.0% (–20.4% to 10.3%) |
| Did not achieve a CR/CRi, n (%) | 41 (35.0) | 15 (30.0) | |
| Age group | ≤ 65 years (n = 105) | > 65 years (n = 58) | |
| Achieved a CR/CRi, n (%) | 75 (70.8) | 36 (59.0) | 11.7% (–3.3% to 26.8%) |
| Did not achieve a CR/CRi, n (%) | 31 (29.2) | 25 (41.0) | |
| Binet stage | Stage A progressive or B (n = 106) | Stage C (n = 56) | |
| Achieved a CR/CRi, n (%) | 76 (68.5) | 35 (62.5) | 6.0% (–9.4% to 21.3%) |
| Did not achieve a CR/CRi, n (%) | 35 (31.5) | 21 (37.5) | |
| Baseline clinical details | Difference in rates (95% CI) | ||
|---|---|---|---|
| Creatinine clearance levels | 30–60 ml/minute (n = 25) | > 60 ml/minute (n = 142) | |
| Achieved a CR/CRi, n (%) | 15 (60.0) | 96 (67.6) | –7.6% (–28.3% to 13.1%) |
| Did not achieve a CR/CRi, n (%) | 10 (40.0) | 46 (32.4) | |
| β2M concentration | < 4 mg/l (n = 60) | ≥ 4 mg/l (n = 97) | |
| Achieved a CR/CRi, n (%) | 43 (71.7) | 60 (61.9) | 9.8% (–5.1% to 24.8%) |
| Did not achieve a CR/CRi, n (%) | 17 (28.3) | 37 (38.1) | |
| Treatment cycles received | Three cycles or fewer (n = 25) | More than three cycles (n = 142) | Difference in rates (95% CI) |
|---|---|---|---|
| Achieved a CR/CRi, n (%) | 7 (28.0) | 104 (73.2) | –45.2% (–64.3% to –26.2%) |
| Did not achieve a CR/CRi, n (%) | 18 (72.0) | 38 (26.8) | |
| Received GCSF during first three cycles | Yes (n = 56) | No (n = 95) | |
| Achieved a CR/CRi, n (%) | 38 (67.9) | 69 (72.6) | –4.8% (–19.9% to 10.4%) |
| Did not achieve a CR/CRi, n (%) | 18 (32.1) | 26 (27.4) | |
| Received GCSF during treatment | Yes (n = 77) | No (n = 83) | |
| Achieved a CR/CRi, n (%) | 50 (64.9) | 59 (71.1) | –6.1% (–20.6% to 8.3%) |
| Did not achieve a CR/CRi, n (%) | 27 (35.1) | 24 (28.9) |
| 17p deletion | Yes (poorer risk) (n = 6) | No (standard risk) (n = 147) | Difference in rates (95% CI) |
|---|---|---|---|
| Achieved a CR/CRi, n (%) | 0 (0.0) | 102 (69.4) | –69.4% (–76.8% to –61.9%) |
| Did not achieve a CR/CRi, n (%) | 6 (100) | 45 (30.6) | |
| 11q deletion | Yes (poorer risk) (n = 24) | No (standard risk) (n = 133) | |
| Achieved a CR/CRi, n (%) | 14 (58.3) | 90 (67.7) | –9.3% (–30.6% to 11.9%) |
| Did not achieve a CR/CRi, n (%) | 10 (41.7) | 43 (32.3) | |
| VH mutation risk | Poor risk (n = 87) | Standard risk (n = 52) | |
| Achieved a CR/CRi, n (%) | 54 (62.1) | 36 (69.2) | –7.2% (–23.3% to 9.0%) |
| Did not achieve a CR/CRi, n (%) | 33 (37.9) | 16 (30.8) |
Of the 163 participants included in the ITT analysis of the primary end point, a similar proportion of males and females achieved a CR (65.0% vs. 70.0%; Table 33). The difference in response rates between the two age groups was 11.7% (95% CI –3.3% to 26.8%), suggesting a trend towards younger participants (aged ≤ 65 years) being more likely to achieve a CR; as the CI contains zero this difference is not statistically significant. A higher proportion of participants with Binet stage A progressive or B achieved a CR than those with stage C (68.5% vs. 62.5%). The 95% CI contains zero, indicating that this difference is not statistically significant but suggesting a trend in favour of Binet stage A progressive and B participants.
The difference in response rates between the two creatinine clearance groups was –7.6% (95% CI –28.3% to 13.1%), suggesting a trend towards participants with a creatinine clearance level between 30 and 60 ml/minute being less likely to achieve a CR than those with a creatinine clearance level of > 60 ml/minute. As the CI contains zero, this difference was not statistically significant. A higher proportion of participants achieved a CR with a β2 M concentration < 4 mg/l compared with a concentration ≥ 4 mg/l (71.7% vs. 61.9%). The 95% CI contains zero, indicating that this difference is not statistically significant but suggesting a trend in favour of the lower β2M concentration group (Table 34).
A much higher proportion of participants who received more than three cycles of treatment achieved a CR than those who received three cycles or fewer (73.2% vs. 28.0%) (Table 35). The difference in response rates was –45.2% (95% CI –64.3% to –26.2%), indicating a significant trend in favour of participants who received more than three cycles of treatment. Of the participants who received treatment with GCSF at some stage during the first three treatment cycles, 67.9% achieved a CR, compared with 72.6% of those who did not. This difference (–4.8%) was non-significant, with 95% CIs that contain zero (–19.9% to 10.4%). Of the participants who received treatment with GCSF during any treatment cycle, 64.9% achieved a CR compared with 71.1% of those who did not receive treatment with GCSF. This difference (–6.1%) was non-significant, with 95% CIs that contain zero (–20.6% to 8.3%). This suggests that the use of GCSF was successful in allowing the delivery of more cycles of therapy and that this enabled participants to achieve better responses which were similar to those seen in participants not requiring GCSF.
All participants who were 17p deleted and who had an available assessment of response (n = 6) failed to achieve a CR. The 95% CI (–76.8% to –61.9%) is highly statistically significant. A higher proportion of participants who did not have an 11q deletion achieved a CR compared with those who did (67.7% vs. 58.3). The difference in response rates (–9.3%) was non-significant with 95% CIs which contain zero (Table 36). Of the 10 participants with an 11q deletion receiving FCR who had an available response assessment, a greater number achieved a CR compared with those participants receiving FCM-miniR [FCR = 8/10 (80.0%), FCM-miniR = 6/14 (42.9%)]. This might be expected as patients with 11q-deleted CLL classically have a relatively high expression of CD20 and are very sensitive to rituximab when combined with FC. Of the participants who were in the ‘poor’ VH mutation risk group, 62.1% achieved a CR compared with 69.2% of those who were in the ‘standard’ risk group. This difference (–7.2%) was non-significant with 95% CIs that contain zero (–23.3% to 9.0%).
Secondary end points
Overall response rate
At 3 months post treatment, of the 197 participants randomised and with a response assessment, a total of 184 (93.4%) achieved at least a PR, including 10 participants with missing trephine data for whom it was known that they were at least a PR (Table 37) but for whom it could not be confirmed if they were a CR for the primary analysis. A slightly higher proportion of participants in the FCR arm achieved at least a PR compared with the FCM-miniR arm (n = 94, 95.9% FCR; n = 69, 88.5% FCM-miniR) with all participants receiving FCM-miniR/FCR achieving at least a PR. Thirteen participants (6.6%) did not achieve an overall response, and there were three participants with missing data (Table 37).
| ORR | FCR (n = 98) | FCM-miniR (n = 78) | FCM-miniR/FCR (n = 21) | Total (n = 197) |
|---|---|---|---|---|
| Achieved at least a PR, n (%) | 94 (95.9) | 69 (88.5) | 21 (100) | 184 (93.4) |
| Did not achieve at least a PR, n (%) | 4 (4.1) | 9 (11.5) | 0 (0.0) | 13 (6.6) |
Table 38 presents the proportion of participants achieving an OR (at least a PR) at 3 months post treatment and the difference in the response rates between the arms, after excluding those FCM-miniR participants (n = 21) who received treatment with FCR post-interim analysis. The ORR is high at 92.6% (n = 163), with a slightly higher proportion of participants in the FCR arm achieving at least a PR compared with participants in the FCM-miniR arm (95.9% FCR; 88.5% FCM-miniR). Approximately 7.5% fewer participants achieved at least a PR in the FCM-miniR arm (95% CI –15.6% to 0.6%). As the CI contains zero, the difference between the arms is not statistically significant, although it is bordering on significance.
| ORR | FCR (n = 98) | FCM-miniR (n = 78) | Total (n = 176) | Difference in OR rates (95% CI) (FCM-miniR – FCR) |
|---|---|---|---|---|
| Achieved at least a PR, n (%) | 94 (95.9) | 69 (88.5) | 163 (92.6) | –7.5% (–15.6% to 0.6%) |
| Did not achieve at least a PR, n (%) | 4 (4.1) | 9 (11.5) | 13 (7.4) |
When the responses to treatment are broken down into categories, as per IWCLL, 128/200 (64.0%) participants achieved a CR, with 33.5% achieving complete remission and 30.5% achieving complete remission with incomplete marrow recovery at 3 months’ treatment. Forty-six participants (23.0%) were assessed as achieving partial remission. Three participants were recorded as having stable disease (one FCR, two FCM-miniR) and three as having PD (three FCM-miniR). A total of 15 participants had missing response data owing to being lost to follow-up prior to the 3-month post-treatment visit (n = 4), insufficient clinical evaluations to be able to assess response (n = 1) and missing trephine data (n = 10). The participants with missing trephine data were known to have achieved at least a PR and so are included in the ORR analysis (Table 39). The participant who died as a result of their CLL, two of the participants who withdrew owing to toxicity and non-response and the four participants who discontinued treatment early owing to toxicity/non-response and were lost to follow-up are included in the CR and OR analyses as non-responders.
| IWCLL response assessment | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Complete remission, n (%) | 40 (40.0) | 18 (22.8) | 9 (42.9) | 67 (33.5) |
| Complete remission with incomplete marrow recovery (CRi), n (%) | 30 (30.0) | 23 (29.1) | 8 (38.1) | 61 (30.5) |
| Partial remission, n (%) | 18 (18.0) | 25 (31.6) | 3 (14.3) | 46 (23.0) |
| Stable disease, n (%) | 1 (1.0) | 2 (2.5) | 0 (0.0) | 3 (1.5) |
| PD, n (%) | 0 (0.0) | 3 (3.8) | 0 (0.0) | 3 (1.5) |
| Early death, n (%) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (0.5) |
| Withdrew from further follow-up data collection prior to assessment of response, n (%) | 4 (4.0) | 0 (0.0) | 0 (0.0) | 4 (2.0) |
| Missing, n (%) | 7 (7.0) | 7 (8.9) | 1 (4.8) | 15 (7.5) |
Minimal residual disease
Minimal residual disease was assessed in the bone marrow at 3 months post treatment. At this time, 85/200 participants (42.5%) were MRD negative and 81/200 (40.5%) were MRD positive (Table 40). A total of 29 participants (14.5%) had missing MRD data with proportions reasonably balanced across the treatment arms (n = 15, 15.0% FCR; n = 13, 16.5% FCM-miniR). Reasons for missing MRD data included: procedure attempted but unable to get sample (n = 1); participant refused to have sample taken (n = 3); sample not taken as participant unwell (n = 2); sample not taken owing to investigator’s discretion (n = 2); sample not taken owing to an administrative error or clinical omission (n = 10); missed visit (n = 3); reasons unknown (n = 8).
| Assessment of MRD | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| MRD negative, n (%) | 45 (45.0) | 29 (36.7) | 11 (52.4) | 85 (42.5) |
| MRD positive, n (%) | 36 (36.0) | 36 (45.6) | 9 (42.9) | 81 (40.5) |
| Withdrew from further follow-up data collection prior to assessment of MRD, n (%) | 4 (4.0) | 0 (0.0) | 0 (0.0) | 4 (2.0) |
| Early death, n (%) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (0.5) |
| Missing, n (%) | 15 (15.0) | 13 (16.5) | 1 (4.8) | 29 (14.5) |
For participants with missing MRD data at 3 months post treatment, the missing value was imputed using the next available observation, if available, as described in Chapter 2, Missing data handling. This was the case for 14 participants (10 FCR; three FCM-miniR; one FCM-miniR/FCR). Twelve MRD-negative results were imputed (eight FCR; three FCM-miniR; one FCM-miniR/FCR) and two MRD-positive results (two FCR). Of the four participants in the FCR arm who withdrew from follow-up data collection prior to the assessment of response, two did so owing to toxicity and non-response. These participants and the participant in the FCM-miniR arm who died early as a result of CLL (prior to 3 months post treatment) have been classed as ‘MRD positive’.
Of the 183 participants with an assessment of MRD after imputation, over half (53.0%) were MRD negative at 3 months post treatment (Table 41). A total of 57.0% (n = 53) of FCR participants were MRD negative compared with 46.4% (n = 32) of FCM-miniR participants and 57.1% (n = 12) of those participants randomised to FCM-miniR who swapped over to receive treatment with FCR.
| Assessment of MRD | FCR (n = 93) | FCM-miniR (n = 69) | FCM-miniR/FCR (n = 21) | Total (n = 183) |
|---|---|---|---|---|
| MRD negative, n (%) | 53 (57.0) | 32 (46.4) | 12 (57.1) | 97 (53.0) |
| MRD positive, n (%) | 40 (43.0) | 37 (53.6) | 9 (42.9) | 86 (47.0) |
Table 42 presents the proportion of participants with undetectable MRD (MRD negative) at 3 months post treatment and the difference in rates between the arms, excluding those FCM-miniR participants (n = 21) who received treatment with FCR following the interim analysis. A higher proportion of participants in the FCR arm were MRD negative than participants in the FCM-miniR arm (n = 53, 57.0% FCR; n = 32, 46.4% FCM-miniR). Approximately 11% fewer participants were MRD negative in the FCM-miniR arm (95% CI –26.1% to 4.9%). As the CI contains zero, there is no evidence of a significant difference between the treatment arms, although the overall trend is in favour of the FCR arm.
| MRD status | FCR (n = 93) | FCM-miniR (n = 69) | Total (n = 162) | Difference in MRD-negative rates (95% CI) (FCM-miniR – FCR) |
|---|---|---|---|---|
| MRD negative, n (%) | 53 (57.0) | 32 (46.4) | 85 (52.5) | –10.6% (–26.1% to 4.9%) |
| MRD positive, n (%) | 40 (43.0) | 37 (53.6) | 77 (47.5) |
A binary multivariate logistic regression model was used to assess formally the effect of treatment on the proportion of participants achieving MRD negativity at 3 months post treatment, after adjusting for the minimisation factors, excluding centre (Table 43).
| Parameter | df | Parameter estimate | SE | Wald χ2 | Pr > χ2 | OR (95% CI) |
|---|---|---|---|---|---|---|
| Intercept | 1 | 0.54 | 0.29 | 3.47 | 0.062 | |
| Treatment group: FCM-miniR vs. FCR | 1 | –0.46 | 0.32 | 1.97 | 0.160 | 0.63 (0.34 to 1.20) |
| Sex: female vs. male | 1 | 0.10 | 0.35 | 0.08 | 0.775 | 1.10 (0.56 to 2.18) |
| Age group: > 65 years vs. ≤ 65 years | 1 | –0.67 | 0.34 | 3.93 | 0.048 | 0.51 (0.27 to 0.99) |
| Binet stage: C vs. A progressive or B | 1 | –0.12 | 0.34 | 0.13 | 0.721 | 0.89 (0.46 to 1.72) |
The OR for achieving MRD negativity in the FCM-miniR arm compared with the FCR arm was 0.63 (95% CI 0.34 to 1.20), indicating that there is a non-significant trend towards FCM-miniR participants being less likely to achieve MRD negativity at 3 months post treatment than participants in the FCR arm.
Progression-free survival
The final participant’s 24-month clinic visit (after which they are followed up annually) was carried out on 13 October 2014; the data lock for the analysis of the long-term follow-up data was performed on 15 October 2014. At the time of analysis, the median follow-up time of participants who were event-free and still in follow-up was 37.3 months (range: 24.7–58.1 months). PFS was defined as the time from randomisation to the first documented evidence of disease progression or death. Overall, 38 participants (19.0%) had progressed, 17 in the FCR arm (17.0%), 19 in the FCM-miniR arm (24.1%) and two participants who received both FCM-miniR and FCR. A total of 49 (24.5%) participants had either progressed or died: 24 in the FCM-miniR arm (30.4%) compared with 22 in the FCR arm (22.0%) (Table 44).
| Status | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| Number of participants with PD, n (%) | 17 (17.0) | 19 (24.1) | 2 (9.5) | 38 (19.0) |
| Number of participants with PD and/or who have died, n (%) | 22 (22.0) | 24 (30.4) | 3 (14.3) | 49 (24.5) |
Figure 5 presents the Kaplan–Meier curves for PFS in months by randomisation allocation. Participants still alive and progression-free at the time of analysis were censored at the last date on which they were known to be alive and progression-free, indicated by a cross. Participants who were randomised to FCM-miniR but transferred over to receive treatment with FCR were excluded from the analysis (n = 21).
FIGURE 5.
Kaplan–Meier plot of time to progression by randomisation allocation.

The PFS curves indicate an overall trend towards an improvement in PFS for participants in the FCR arm. More early progressions are seen with FCM-miniR than with FCR within the first 12 months, but after that the curves remain roughly proportional. At 24 months post randomisation, the PFS probability in the FCR arm is 89.4% compared with 79.1% in the FCM-miniR arm. There is a high number of censored observations around and beyond the 24-month point, the time to which the majority of participants have been followed up, so care must be taken at this stage not to over-interpret the results until longer-term follow-up data are acquired.
A log-rank test was used to compare the differences between the PFS curves. There was no evidence of a significant difference between the treatment arms at the 5% significance level with respect to time to progression (p = 0.279). The Wilcoxon rank-sum test, which gives greater weight to earlier differences, was also non-significant (p = 0.1081) (Table 45).
| Randomisation allocation | Median time to progression (months) (95% CI) | Total number of participants | Total number of events | Total number of censored observations | Test | χ2 | df | p-value |
|---|---|---|---|---|---|---|---|---|
| FCM-miniR | 48.5 (40.4 to NE) | 79 | 24 | 55 | Log-rank | 1.1719 | 1 | 0.2790 |
| FCR | 52.7 (42.9 to NE) | 100 | 22 | 78 | Wilcoxon rank-sum | 2.5811 | 1 | 0.1081 |
| Total | 52.7 (44.5 to NE) | 179 | 46 | 133 |
In a formal Cox regression analysis, after adjusting for the minimisation factors, excluding centre, there was no evidence of a significant difference in PFS between the treatment arms at the 5% significance level with a HR of 1.39 (95% CI 0.77 to 2.49; p = 0.2771) (Table 46), although it should be noted that the CIs are wide owing to the relatively short follow-up period. Although non-significant, a HR of 1.39 indicates that there is a trend in favour of the FCR arm, and that participants in the FCM-miniR arm are around 40% more likely to progress at each time point. The trial was not powered to detect a difference in PFS and CIs around the HR are wide, indicating a lack of precision of the HR estimate owing to the small number of events, and indicating that longer-term follow-up data are required.
| Parameter | df | Parameter estimate | SE | χ2 | p-value | HR (95% CI) |
|---|---|---|---|---|---|---|
| Randomisation allocation: FCM-miniR vs. FCR | 1 | 0.33 | 0.30 | 1.18 | 0.2771 | 1.39 (0.77 to 2.49) |
| Age group: > 65 years vs. ≤ 65 years | 1 | 0.05 | 0.32 | 0.03 | 0.8702 | 1.05 (0.56 to 1.97) |
| Sex: female vs. male | 1 | –0.04 | 0.32 | 0.02 | 0.8952 | 0.96 (0.51 to 1.81) |
| Binet stage: C vs. A progressive or B | 1 | –0.16 | 0.32 | 0.23 | 0.6298 | 0.86 (0.45 to 1.61) |
The 95% CIs around the HRs for the minimisation factors all contain 1, indicating that there is no significant evidence that any of the minimisation factors are significantly associated with PFS, although it should be noted that the CIs are wide owing to the relatively short follow-up period.
Overall survival
At the time of analysis (October 2014), the median follow-up time for survivors was 37.7 months (range 24.7–58.1 months). OS was defined as the time from randomisation to death from any cause. In total, 24 participants (12.0%) had died: 10 in the FCR arm (10.0%), 13 in the FCM-miniR arm (16.5%) and one participant who received FCM-miniR followed by one cycle of FCR (Table 47). The most common primary cause of death was ‘overwhelming tumour load’ in 33.3% of cases (n = 8) and there was only one treatment-related death in the trial, occurring in the FCR arm.
| Number of participants who have died, n (%) | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 21) | Total (n = 200) |
|---|---|---|---|---|
| 10 (10.0) | 13 (16.5) | 1 (4.8) | 24 (12.0) | |
| Primary cause of death | ||||
| Treatment-related death | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Overwhelming tumour load | 2 (20.0) | 6 (46.2) | 0 (0.0) | 8 (33.3) |
| Infection owing to CLL | 3 (30.0) | 4 (30.8) | 0 (0.0) | 7 (29.2) |
| Infection owing to treatment | 2 (20.0) | 0 (0.0) | 1 (100) | 3 (12.5) |
| High-grade transformation on the background of CLL | 0 (0.0) | 1 (7.7) | 0 (0.0) | 1 (4.2) |
| Other malignancies (non-haematopoietic) | 0 (0.0) | 1 (7.7) | 0 (0.0) | 1 (4.2) |
| Other | 2 (20.0)a | 1 (7.7)b | 0 (0.0) | 3 (12.5) |
Figure 6 presents the Kaplan–Meier curves for OS in months by randomisation allocation. Participants still alive at the time of analysis were censored at the last date on which they were known to be alive, indicated by a cross. Participants who were randomised to FCM-miniR but transferred over to receive treatment with FCR were excluded from the analysis (n = 21).
FIGURE 6.
Kaplan–Meier plot of OS by randomisation allocation.
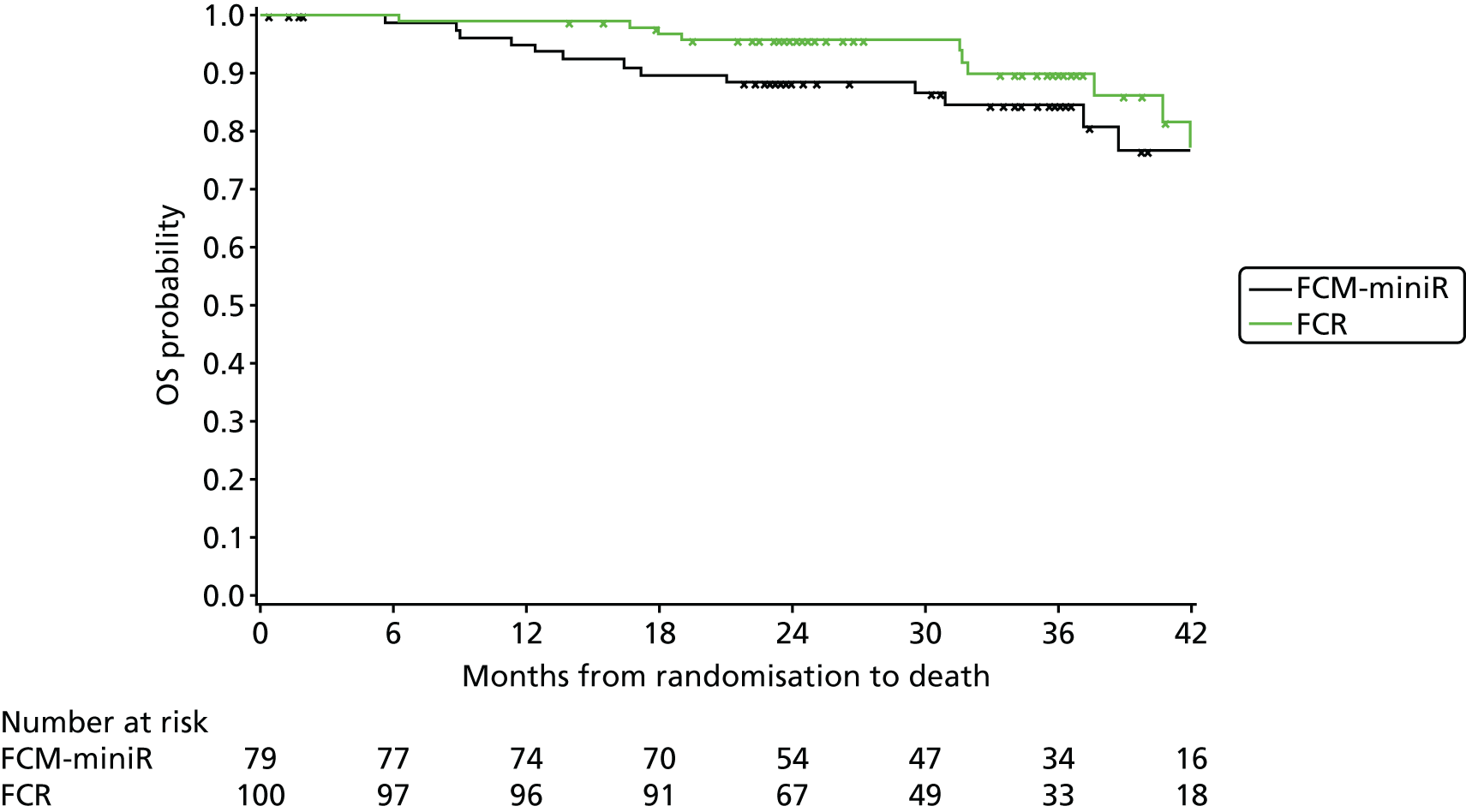
The survival curves indicate an overall trend towards an improvement in OS for participants in the FCR arm. As with PFS, there are a greater number of earlier events in participants on FCM-miniR, with the curves becoming closer to parallel after 18 months. At 24 months post randomisation, the survival probability is 95.8% in the FCR arm compared with 88.5% in the FCM-miniR arm. There are a high number of censored observations around and beyond the 24-month point, the time to which the majority of participants have been followed up, so care must be taken at this stage not to overinterpret the results until longer-term follow-up data are acquired.
The log-rank test was used to compare the difference between the survival curves. There was no evidence of a significant difference between the treatment arms at the 5% significance level with respect to OS (p = 0.2779). The Wilcoxon rank-sum test, which gives greater weight to earlier differences, was also non-significant (p = 0.1013) (Table 48). Note, however, that there have been few events in either arm owing to the relatively short follow-up period.
| Randomisation allocation | Median time to death (months) (95% CI) | Total number of participants | Total number of events | Total number of censored observations | Test | χ2 | df | p-value |
|---|---|---|---|---|---|---|---|---|
| FCM-miniR | NE | 79 | 13 | 66 | Log-rank | 1.1771 | 1 | 0.2779 |
| FCR | NE | 100 | 10 | 90 | Wilcoxon rank-sum | 2.6854 | 1 | 0.1013 |
| Total | NE | 179 | 23 | 156 |
In the formal Cox regression analysis, after adjusting for the minimisation factors, excluding centre, there was no evidence of a significant difference in OS between the treatment arms at the 5% significance level with a HR of 1.57 (95% CI 0.68 to 3.58; p = 0.2876) (Table 49). Although non-significant, a HR of 1.57 indicates that there is a trend in favour of the FCR arm, and that participants in the FCM-miniR arm are around 57% more likely to not survive at any time point. The trial was not powered to detect a difference in OS and the CIs around the HR are wide, indicating a lack of precision of the HR estimate owing to the small number of events, so longer-term follow-up data would be beneficial.
| Parameter | df | Parameter estimate | SE | χ2 | p-value | HR (95% CI) |
|---|---|---|---|---|---|---|
| Randomisation allocation: FCM-miniR vs. FCR | 1 | 0.45 | 0.42 | 1.13 | 0.2876 | 1.57 (0.68 to 3.58) |
| Age group: > 65 vs. ≤ 65 | 1 | 0.46 | 0.42 | 1.17 | 0.2797 | 1.58 (0.69 to 3.61) |
| Sex: female vs. male | 1 | –0.29 | 0.48 | 0.36 | 0.5485 | 0.75 (0.29 to 1.92) |
| Binet stage: C vs. A progressive or B | 1 | –0.05 | 0.46 | 0.01 | 0.9053 | 0.95 (0.39 to 2.33) |
The 95% CIs around the HRs for the minimisation factors all contain 1, indicating that there is no evidence that any of the minimisation factors are significantly associated with OS, although there is only a limited period of follow-up at the time of reporting. There is a suggested trend in favour of participants aged 65 years or younger to have an improved survival than those aged over 65.
Time to minimal residual disease relapse
Participants who were MRD negative at 3 months post treatment were followed up 6-monthly until MRD relapse (i.e. until they became MRD positive) or until 24 months post randomisation. Longer follow-up was not possible owing to funding constraints. In the population of participants who were assessable for MRD at 3 months post treatment, 85/162 (52.5%) participants had achieved MRD negativity (see Table 42). At the time of analysis, it was reported that only seven participants had relapsed at the MRD level (four FCR, three FCM-miniR); however, there was a high proportion of missing MRD data at each of the follow-up visits. Owing to the small number of events, high proportion of missing data and lack of longer-term follow-up data, the time to MRD relapse analysis was unable to be performed.
Subgroup analyses
Subgroup analyses were carried out to assess PFS and OS based on IWCLL and MRD response groups, the minimisation factors, number of cycles of treatment received, GCSF usage and genetic risk groups. As the trial was not powered for survival end points or to look for differences in subgroups of participants, the results are to be treated as exploratory and used for hypothesis generating purposes only.
Progression-free survival by minimal residual disease response status
Figure 7 presents the Kaplan–Meier curves for time to progression by MRD status at 3 months post treatment. Of the 85 participants who were MRD negative at this time point, eight (9.4%) have reported an event (i.e. progression or death) compared with 30/77 (39.0%) who were MRD positive.
FIGURE 7.
Kaplan–Meier plot of time to progression by MRD status at 3 months post treatment.
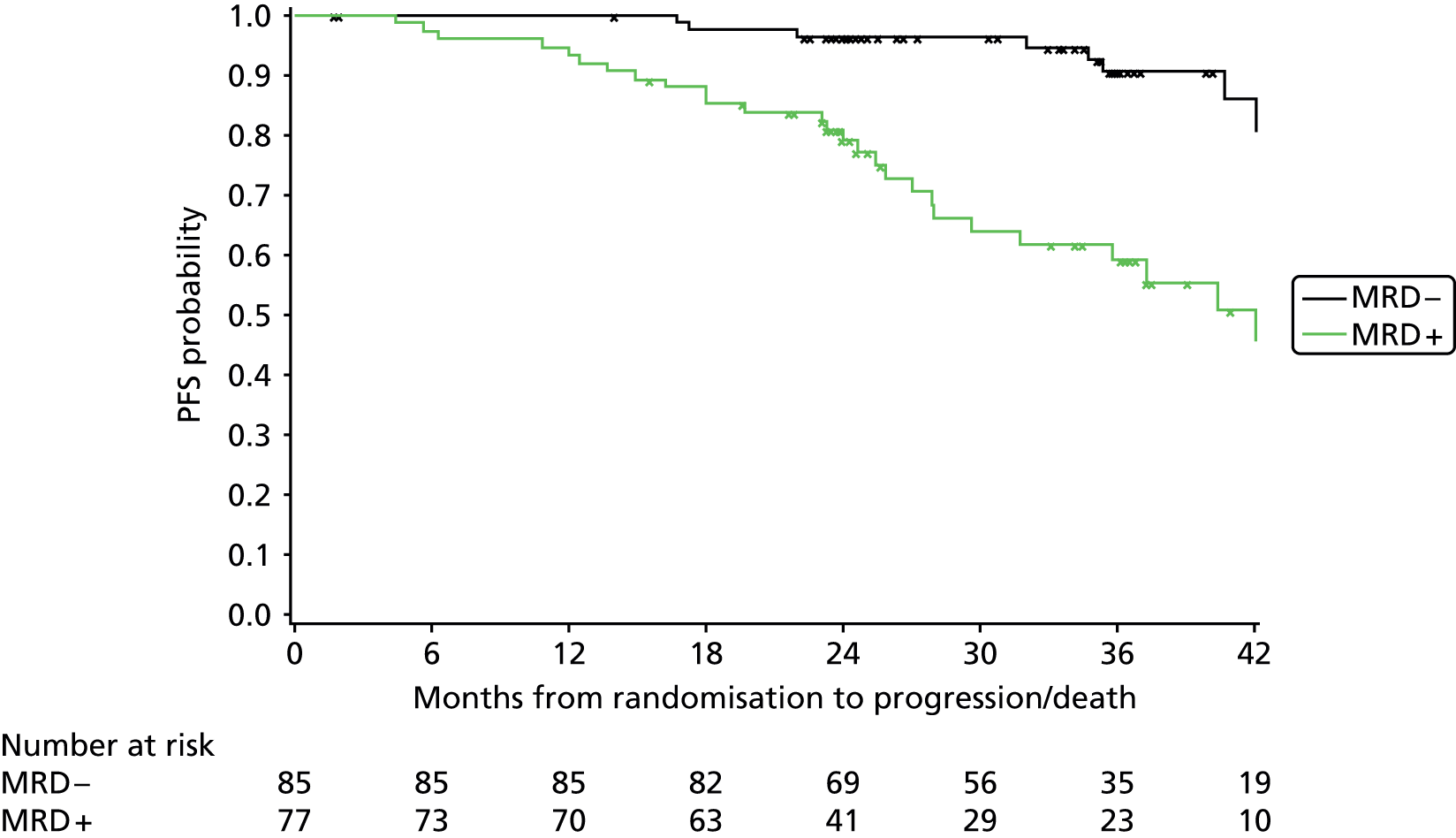
The curves show clear divergence from 6 months post randomisation, the end of treatment visit, with an overall trend towards an improvement in PFS for participants who were MRD negative at 3 months post treatment. This difference was significant at the 5% level in favour of the MRD-negative participants (log-rank: χ2 = 23.20; p < 0.0001). At 24 months post randomisation, the PFS probability is 96.4% for MRD-negative participants compared with 79.2% for the MRD-positive group.
Progression-free survival by minimal residual disease response status and randomisation allocation
Figure 8 presents the Kaplan–Meier curves for time to progression by randomisation allocation and MRD status at 3 months post treatment.
FIGURE 8.
Kaplan–Meier plot of time to progression by randomisation allocation and MRD status at 3 months post treatment.
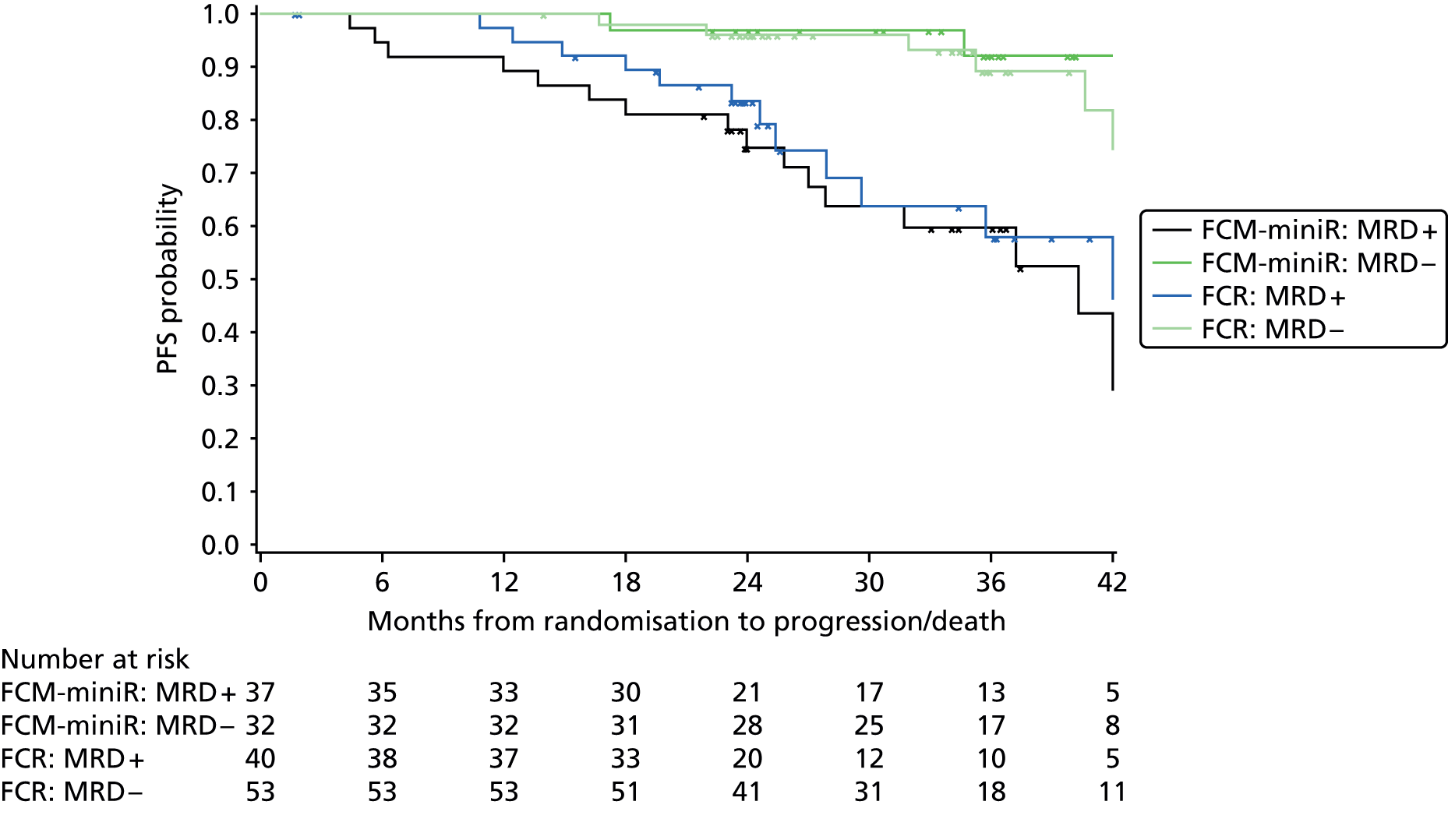
For the participants who became MRD negative, the PFS curves are similar for the two treatment arms. For the participants who were MRD positive, participants in the FCR arm show a trend towards a slightly improved time to progression compared with participants in the FCM-miniR arm until around 24–30 months post randomisation, at which point there are a number of censored observations, indicating that longer term follow-up would be beneficial to interpretation. At 24 months post randomisation, the PFS probabilities for the MRD-negative participants were similar between the treatment groups (96.2% FCR, 96.9% FCM-miniR). For MRD-positive participants, the PFS probability was 83.7% in the FCR arm compared with 74.9% in the FCR-miniR arm.
Progression-free survival by complete response status
Figure 9 presents the Kaplan–Meier curves for time to progression by CR status at 3 months post treatment. Of the participants who had achieved a complete remission or CRi at this time point, 16/111 (14.4%) had an event (i.e. progression or death) compared with 27/56 (48.2%) who had not achieved at least a CR.
FIGURE 9.
Kaplan–Meier plot of time to progression by CR status at 3 months post treatment.

The curves show clear divergence from 6 months post randomisation, the end of treatment visit, with an overall trend towards an improvement in PFS for participants who achieved a CR at 3 months post treatment. This difference was significant at the 5% level in favour of participants who achieved at least a CR (log-rank: χ2 = 29.41; p < 0.0001). At 24 months post randomisation, the PFS probability is 93.4% for participants who achieved a CR compared with 65.4% for those who did not.
Progression-free survival by complete response status and randomisation allocation
Figure 10 presents the Kaplan–Meier curves for time to progression by randomisation allocation and CR status at 3 months post treatment.
FIGURE 10.
Kaplan–Meier plot of time to progression by randomisation allocation and CR status at 3 months post treatment.
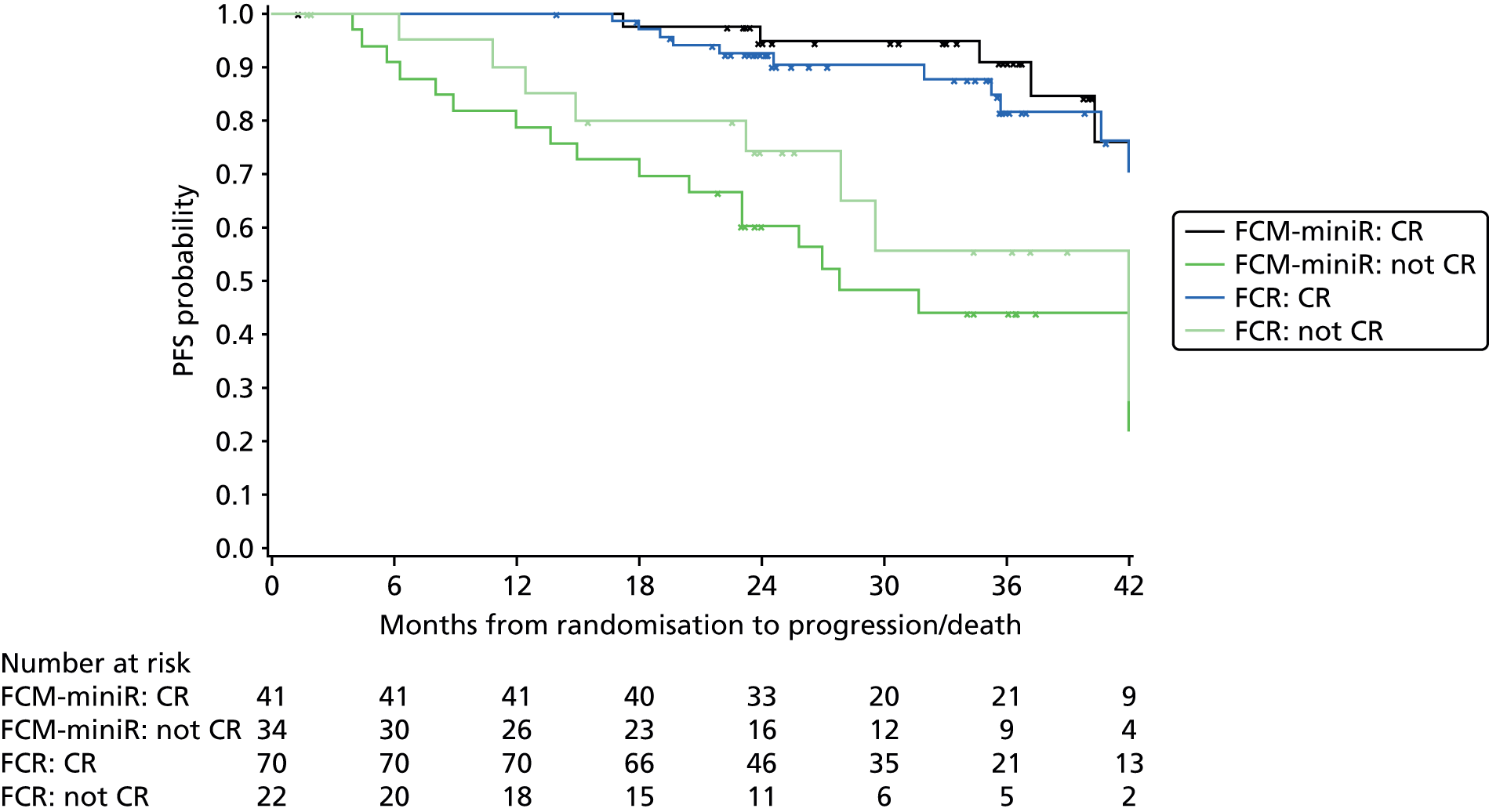
For the participants who achieved a CR, the curves are similar for the two treatment arms, with a slight trend towards the FCM-miniR participants doing better. For the participants who did not achieve a CR, participants in the FCR arm have an improved time to progression compared with FCM-miniR participants. At 24 months post randomisation, the PFS probabilities for the participants who achieved a CR were similar between the treatment groups (92.6% FCR, 94.8% FCM-miniR). For participants who did not achieve a CR, the PFS survival probability was 74.3% in the FCR arm compared with 60.3% in the FCR-miniR arm.
Overall survival by minimal residual disease response status
Figure 11 presents the Kaplan–Meier curves for OS by MRD status at 3 months post treatment. Of the participants who were MRD negative at this time point, 4/85 (4.7%) had died compared with 14/77 (18.2%) who were MRD positive.
FIGURE 11.
Kaplan–Meier plot of OS by MRD status at 3 months post treatment.

The curves show clear divergence from the end of treatment visit, with an overall trend towards an improvement in OS for participants who were MRD negative at 3 months post treatment. This difference was significant at the 5% level in favour of the MRD-negative participants (log-rank: χ2 = 9.25; p = 0.003). At 24 months post randomisation, the OS probability for MRD-negative participants is 97.6% compared with 91.9% for the MRD-positive group.
Overall survival by minimal residual disease response status and randomisation allocation
Figure 12 presents the Kaplan–Meier curves for OS by randomisation allocation and MRD status at 3 months post treatment.
FIGURE 12.
Kaplan–Meier plot of OS by randomisation allocation and MRD status at 3 months post treatment.
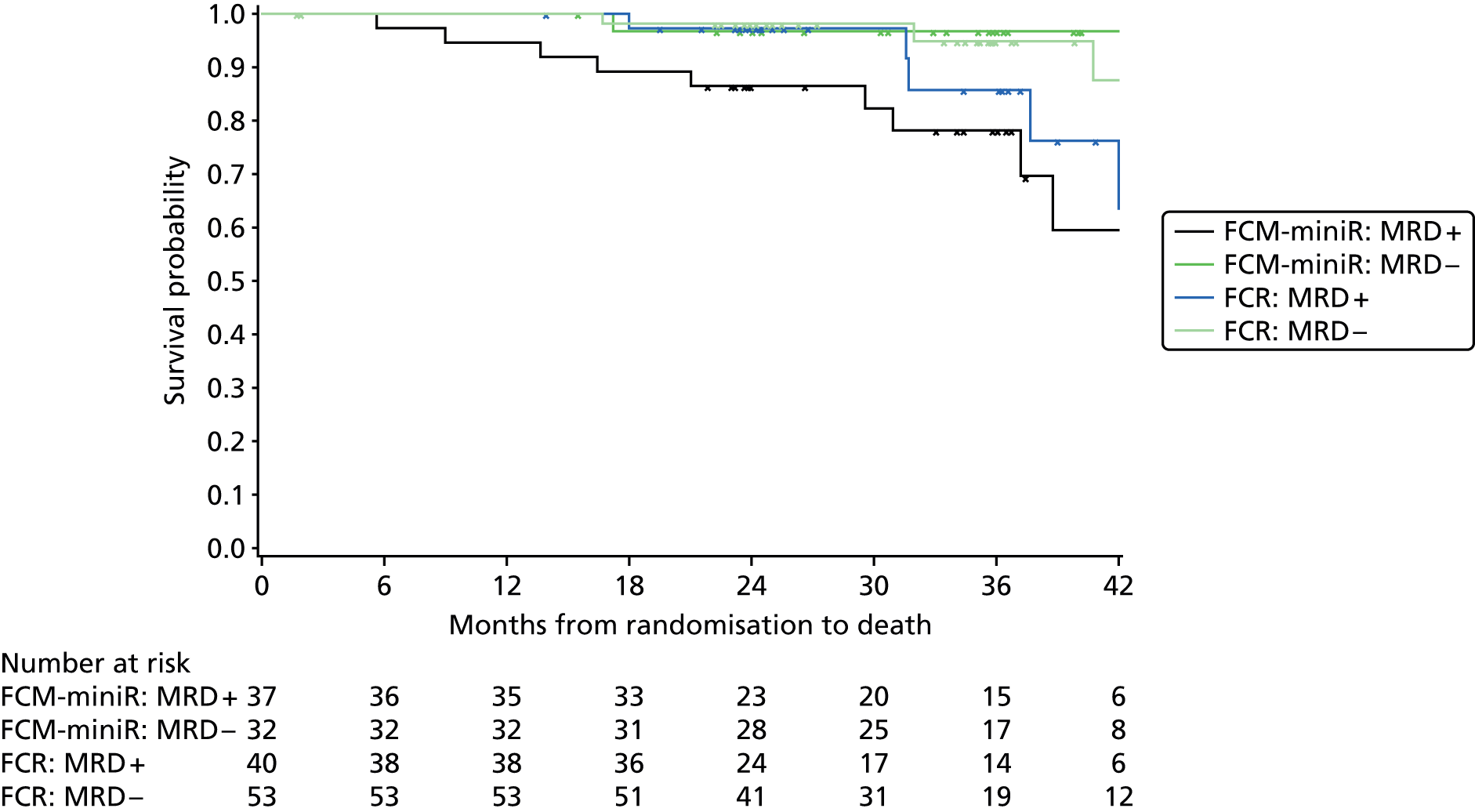
For the participants who became MRD negative, the OS curves are similar for the two treatment arms. A similar survival pattern was observed for MRD-positive participants receiving FCR until around 30 months post randomisation, at which point survival worsened. OS was poorest for MRD-positive participants who received FCM-miniR. At 24 months post randomisation, the OS probabilities for the MRD-negative participants were similar between the treatment groups (98.1% FCR, 96.9% FCM-miniR), and for MRD-positive participants receiving FCR (97.3%). The FCM-miniR, MRD-positive group showed the poorest OS probability at 24 months (86.5%).
Overall survival by complete response status
Figure 13 presents the Kaplan–Meier curves for OS by CR status at 3 months post treatment. Of the participants who had achieved a CR at this time point, 8/111 (7.2%) had died compared with 15/56 (26.8%) who had not achieved a CR.
FIGURE 13.
Kaplan–Meier plot of OS by CR status at 3 months post treatment.
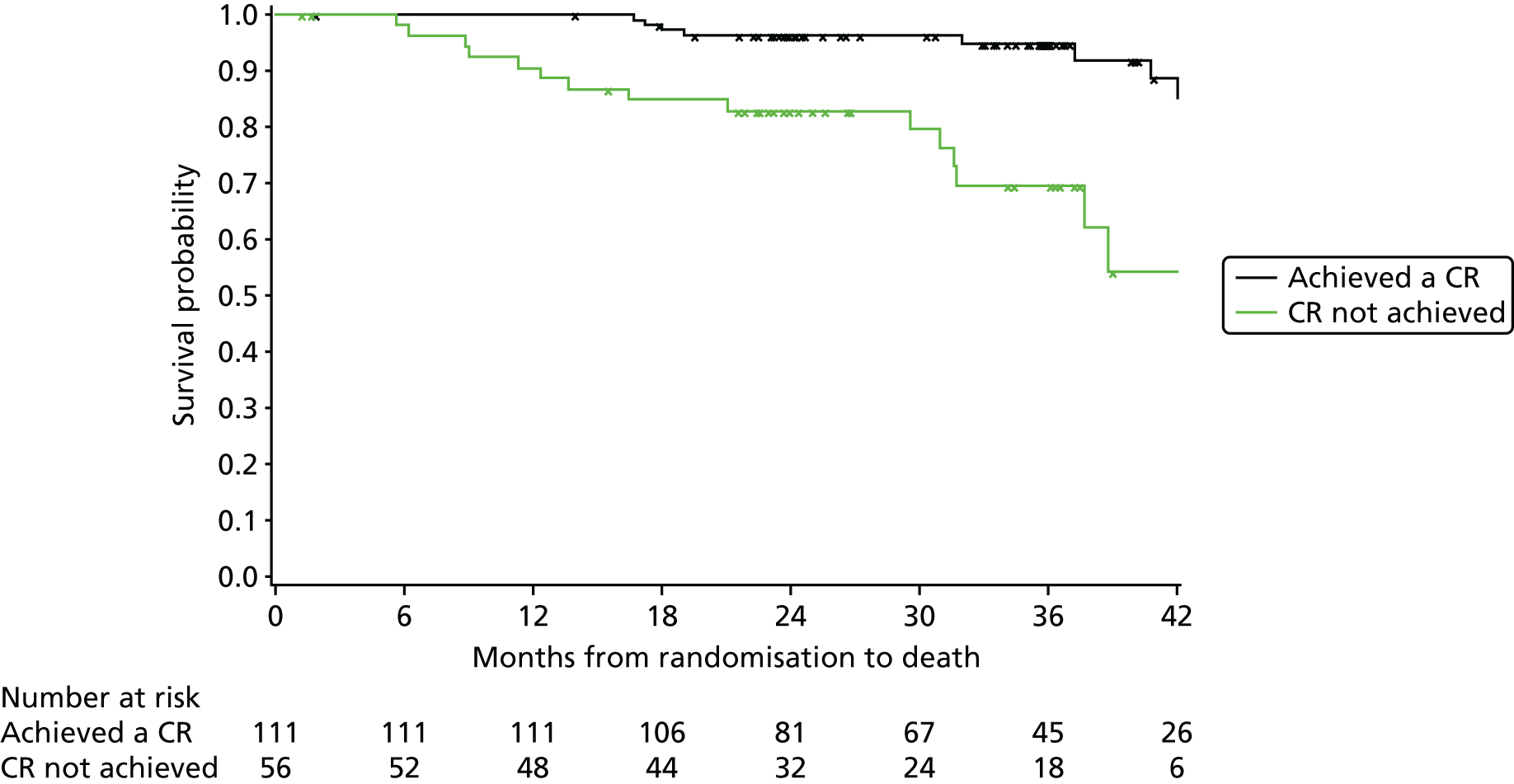
The curves show clear divergence from 6 months post randomisation, the end of treatment visit, with an overall trend towards an improvement in OS for participants who achieved a CR at 3 months post treatment. This difference was significant at the 5% level in favour of participants who achieved a CR (log-rank: χ2 = 16.92; p < 0.001). At 24 months post randomisation, the OS probability for participants who achieved a CR is 96.4% compared with 82.9% for those who did not.
Overall survival by complete response status and randomisation allocation
Figure 14 presents the Kaplan–Meier curves for OS by randomisation allocation and CR status at 3 months post treatment.
FIGURE 14.
Kaplan–Meier plot of OS by randomisation allocation and response status at 3 months post treatment.

For the participants who achieved a CR, the curves are similar for the two treatment arms. A similar survival pattern was observed for participants who did not achieve a CR who were receiving FCR until around 30 months post randomisation, at which point survival worsened. OS was poorest for participants who did not achieve a CR and who received FCM-miniR. At 24 months post randomisation, the OS probabilities for the participants who achieved a CR were similar between the treatment groups (95.6% FCR, 97.6% FCM-miniR), and for participants who did not achieve a CR receiving FCR (95.0%). The FCM-miniR participants who did not achieve a CR showed the poorest OS probability at 24 months (75.8%).
Progression-free survival and overall survival by minimisation factors at baseline: sex
Figure 15 presents the Kaplan–Meier curves for time to progression by participant sex. At the time of analysis, of the 123 male participants, 32 (26.0%) had reported an event (i.e. progression or death) compared with 14/56 (25.0%) female participants.
FIGURE 15.
Kaplan–Meier plot of time to progression by sex.

At 24 months post randomisation, the PFS probability for male participants was 84.4% compared with 85.5% for females. The difference between the PFS curves for sex was non-significant (log-rank: χ2 = 0.045; p = 0.833).
Figure 16 presents the Kaplan–Meier curves for OS by participant sex. At the time of analysis, of the 123 male participants, 17 (13.8%) had died compared with 6/56 (10.7%) female participants.
FIGURE 16.
Kaplan–Meier plot of OS by sex.

At 24 months post randomisation, the OS probability for male participants was 92.4% compared with 92.8% for females. The difference between the OS curves for sex was non-significant (log-rank: χ2 = 0.411; p = 0.522).
Progression-free survival and overall survival by minimisation factors at baseline: age group
Figure 17 presents the Kaplan–Meier curves for time to progression by age group (≤ 65 years, > 65 years). At the time of analysis, of the 113 participants aged ≤ 65 years, 31 (27.4%) had reported an event (i.e. progression or death) compared with 15/66 (22.7%) participants aged over 65 years.
FIGURE 17.
Kaplan–Meier plot of time to progression by age group.
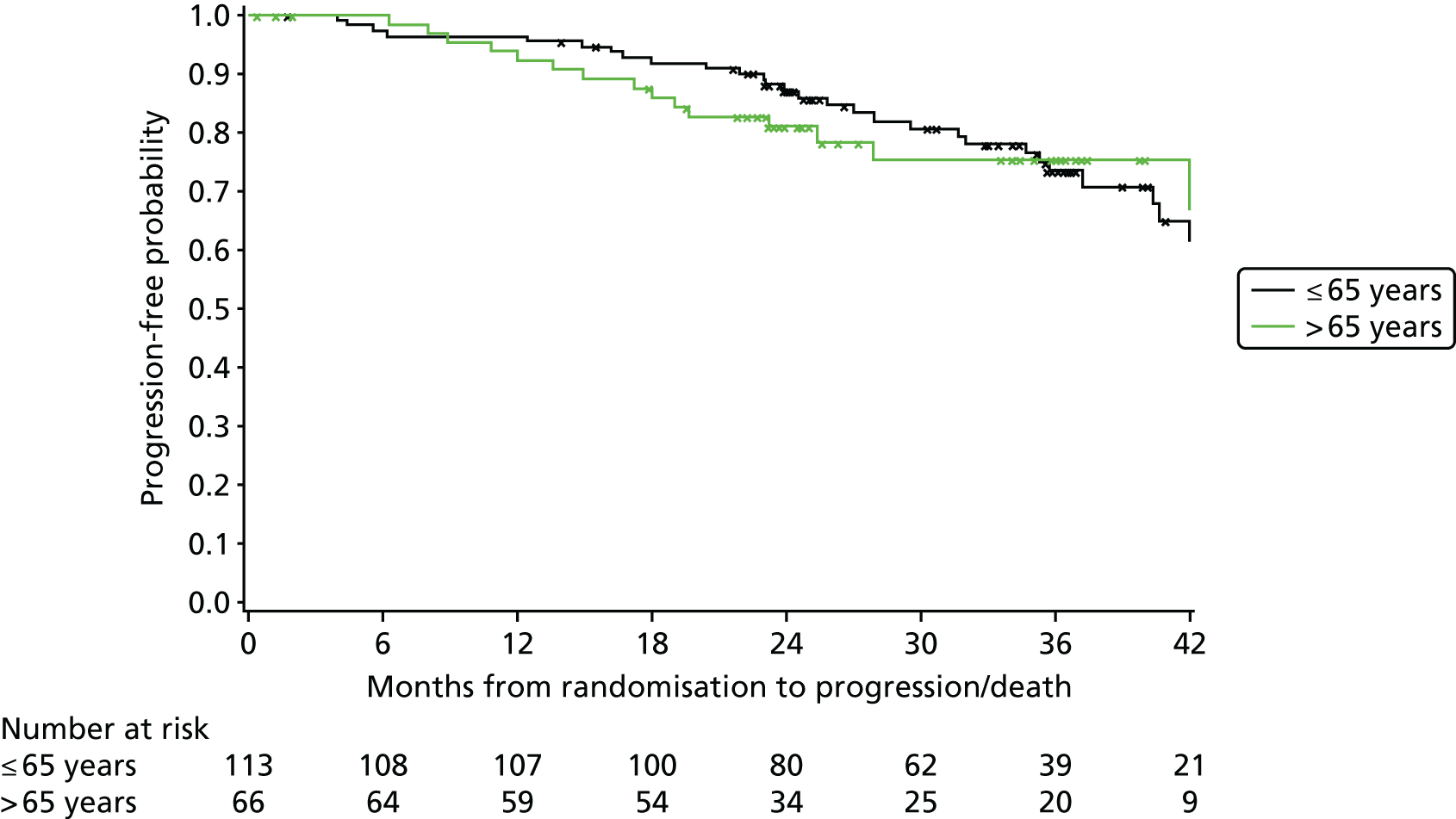
At 24 months post randomisation, the PFS probability for participants aged ≤ 65 years was 87.0% compared with 80.9% for those older than 65 years. Although there appears to be a trend in favour of younger participants performing better in terms of PFS, the overall difference between the PFS curves for age group was non-significant (log-rank: χ2 = 0.015; p = 0.902).
Figure 18 presents the Kaplan–Meier curves for OS by age group (≤ 65 years, > 65 years). At the time of analysis, of the 113 participants aged ≤ 65 years, 13 (11.5%) had died compared with 10/66 (15.2%) participants aged over 65 years.
FIGURE 18.
Kaplan–Meier plot of OS by age group.

At 24 months post randomisation, the OS probability for participants aged ≤ 65 years was 94.5% compared with 89.0% for those over 65 years of age. The difference between the OS curves for age group was non-significant (log-rank: χ2 = 1.106; p = 0.293), although there is a suggested trend for younger participants performing better in terms of OS.
Progression-free survival and overall survival by minimisation factors at baseline: Binet stage
Figure 19 presents the Kaplan–Meier curves for time to progression by Binet stage (A progressive or B, C). At the time of analysis, of the 118 participants who were Binet stage A progressive or B, 32 (27.0%) had reported an event (i.e. progression or death) compared with 14/61 (23.0%) participants who were Binet stage C.
FIGURE 19.
Kaplan–Meier plot of time to progression by Binet stage.
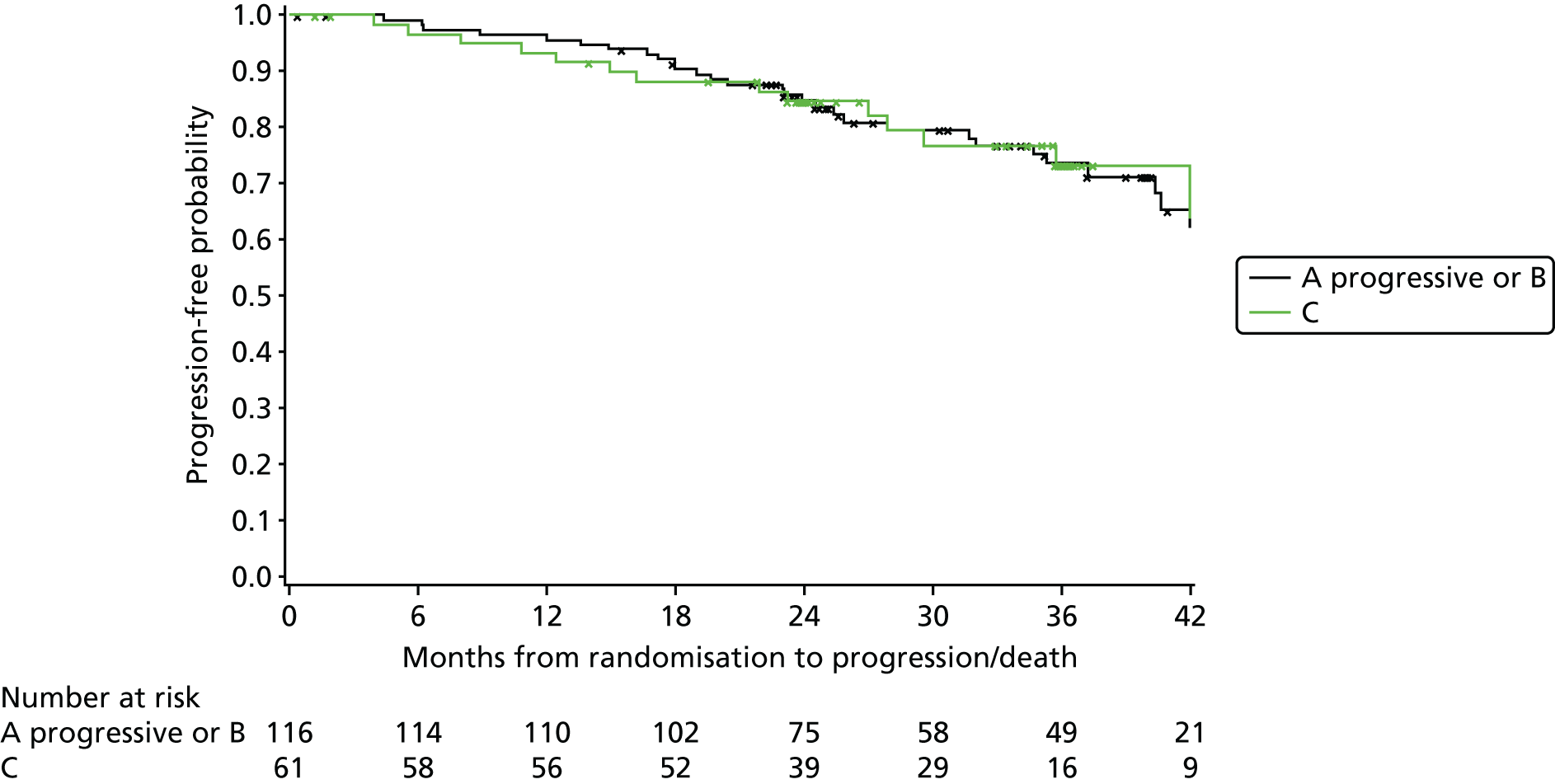
At 24 months post randomisation, the PFS probability for participants with Binet stage A progressive or B was 84.8% compared with 84.7% for Binet stage C participants. The difference between the PFS curves for Binet stage was non-significant (log-rank: χ2 = 0.217; p = 0.641).
Figure 20 presents the Kaplan–Meier curves for OS by Binet stage (A progressive or B, C). At the time of analysis, of the 118 participants who were Binet stage A progressive or B, 16 (13.6%) had died compared with 7/61 (11.5%) participants who were Binet stage C.
FIGURE 20.
Kaplan–Meier plot of OS by Binet stage.

At 24 months post randomisation, the OS probability for participants with Binet stage A progressive or B was 91.2% compared with 95.0% for Binet stage C participants. The difference between the OS curves for Binet stage was non-significant (log-rank: χ2 = 0.030; p = 0.863).
Progression-free survival and overall survival by treatment received
Figure 21 presents the Kaplan–Meier curves for time to progression by number of treatment cycles received (three or fewer, more than three). At the time of analysis, of the 31 participants who received three cycles or fewer, 16 (51.6%) had reported an event (i.e. progression or death) compared with 30/148 (20.3%) participants who received more than three cycles of treatment.
FIGURE 21.
Kaplan–Meier plot of PFS by number of treatment cycles received.
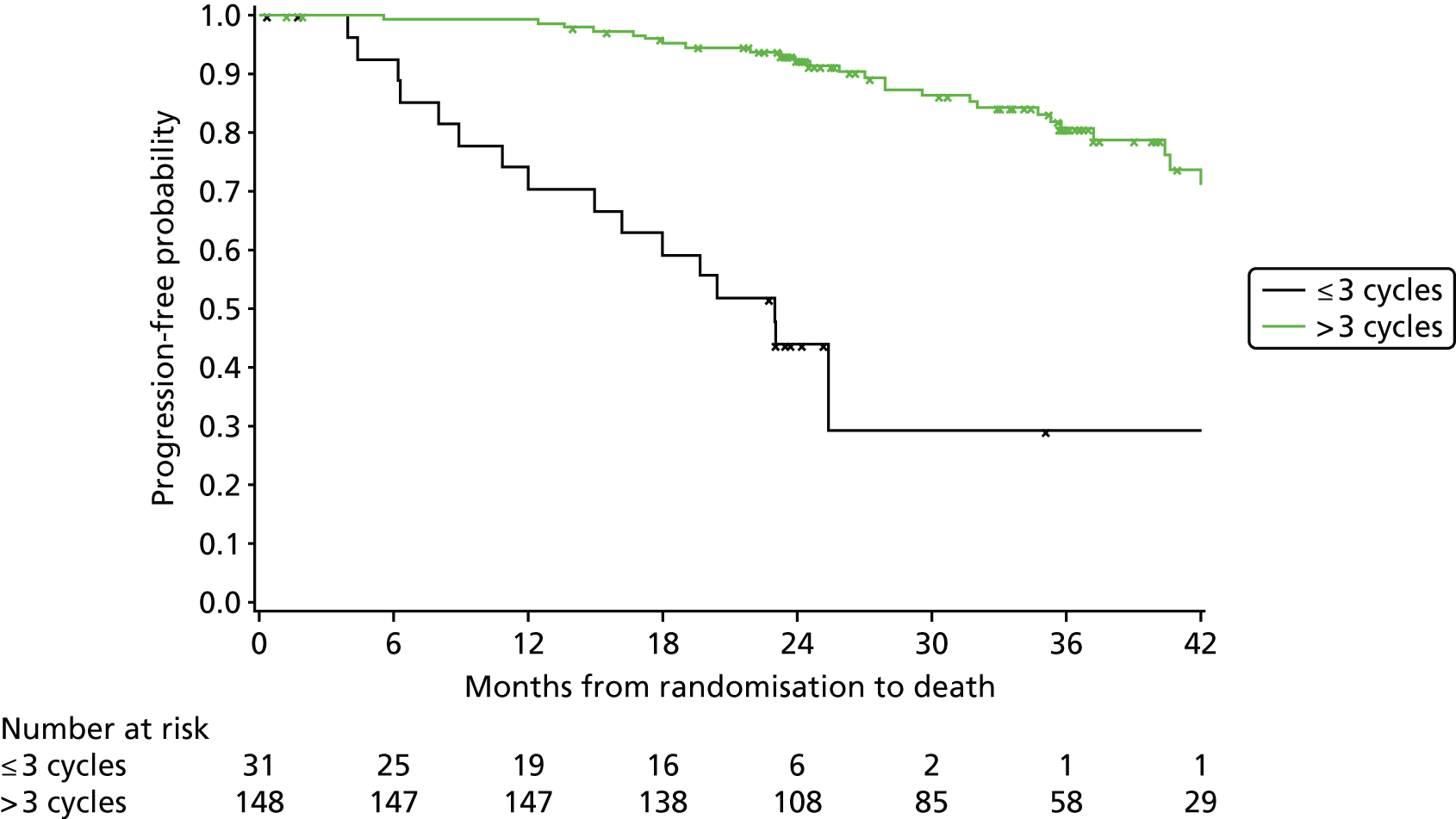
The curves show clear divergence from the end of treatment with an overall trend towards an improvement in PFS for participants who received more than three cycles of treatment. At 24 months post randomisation, the PFS probability for participants receiving more than three cycles of treatment was 92.3% compared with 43.9% for those receiving three or less. The overall difference between the PFS curves was significant at the 5% level (log-rank: χ2 = 51.629; p < 0.0001).
Figure 22 presents the Kaplan–Meier curves for OS by number of treatment cycles received (three or fewer, more than three). At the time of analysis, of the 31 participants who received three cycles or less, 8 (25.8%) had died compared with 15/148 (10.1%) participants who received more than three cycles of treatment.
FIGURE 22.
Kaplan–Meier plot of OS by number of treatment cycles received.

The curves show clear divergence from the end of treatment with an overall trend towards a worse OS for participants who received three or fewer cycles of treatment. At 24 months post randomisation, the OS probability for participants receiving more than three cycles of treatment was 95.9% compared with 74.1% for those receiving three or fewer. The overall difference between the OS curves was significant at the 5% level (log-rank: χ2 = 14.167; p = 0.0002).
Progression-free survival and overall survival by granulocyte-colony stimulating factor usage
Figure 23 presents the Kaplan–Meier curves for time to progression by GSCF usage during treatment. At the time of analysis, of the 82 participants who had received GCSF at some stage during their treatment, 21 (25.6%) had reported an event (i.e. progression or death) compared with 23/87 (26.4%) participants who had not received any GSCF.
FIGURE 23.
Kaplan–Meier plot of PFS by GCSF usage.
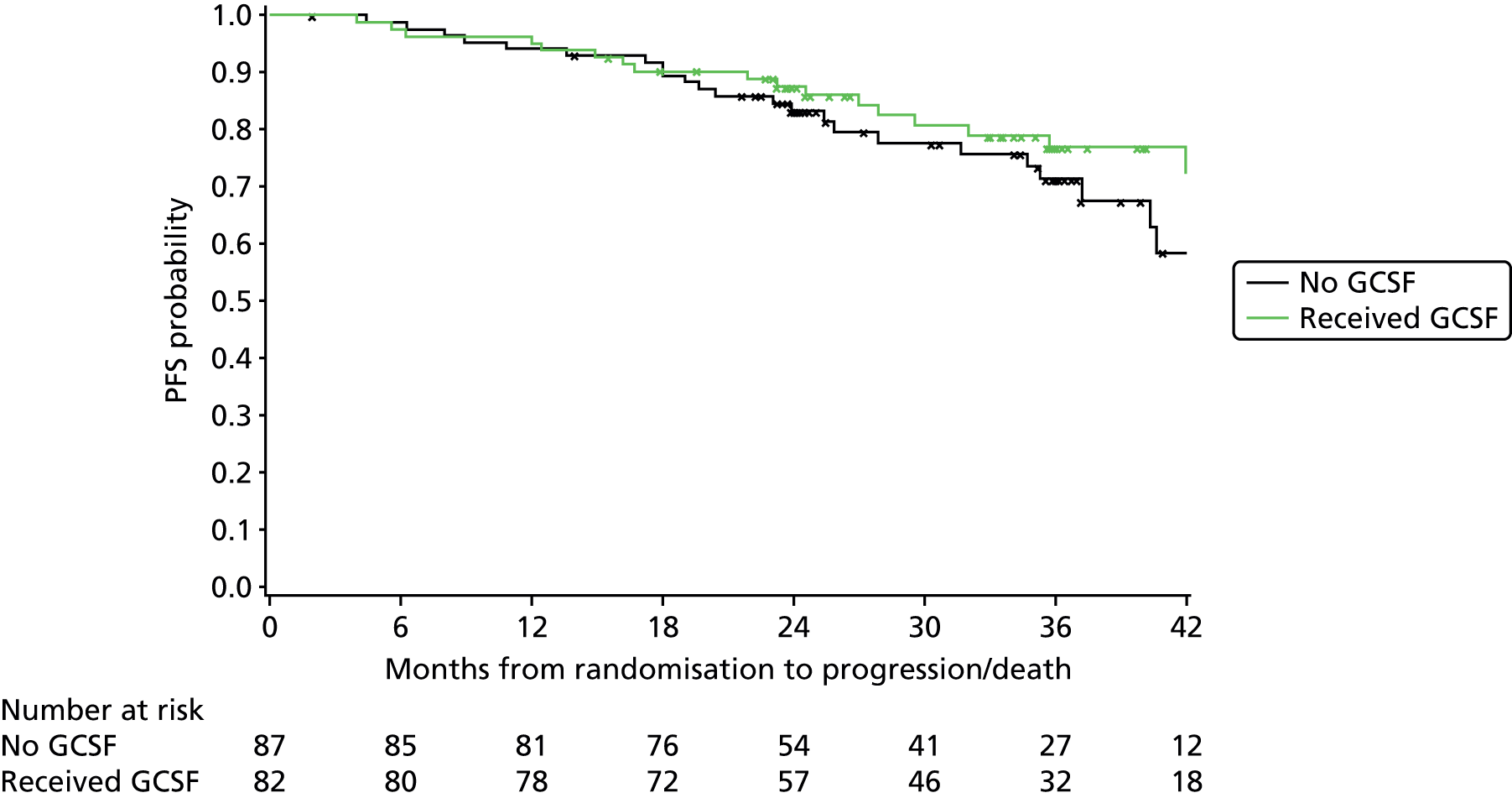
At 24 months post randomisation, the PFS probability for participants who had received GCSF at some stage during their treatment was 87.6% compared with 83.3% for participants who had not received any GCSF. The difference between the PFS curves for GCSF usage was non-significant (log-rank: χ2 = 0.249; p = 0.618).
Figure 24 presents the Kaplan–Meier curves for OS by GCSF usage during treatment. At the time of analysis, of the 82 participants who had received GCSF at some stage during their treatment, 11 (13.4%) had died compared with 12/87 (13.8%) participants who had not received any GSCF.
FIGURE 24.
Kaplan–Meier plot of OS by GCSF usage.
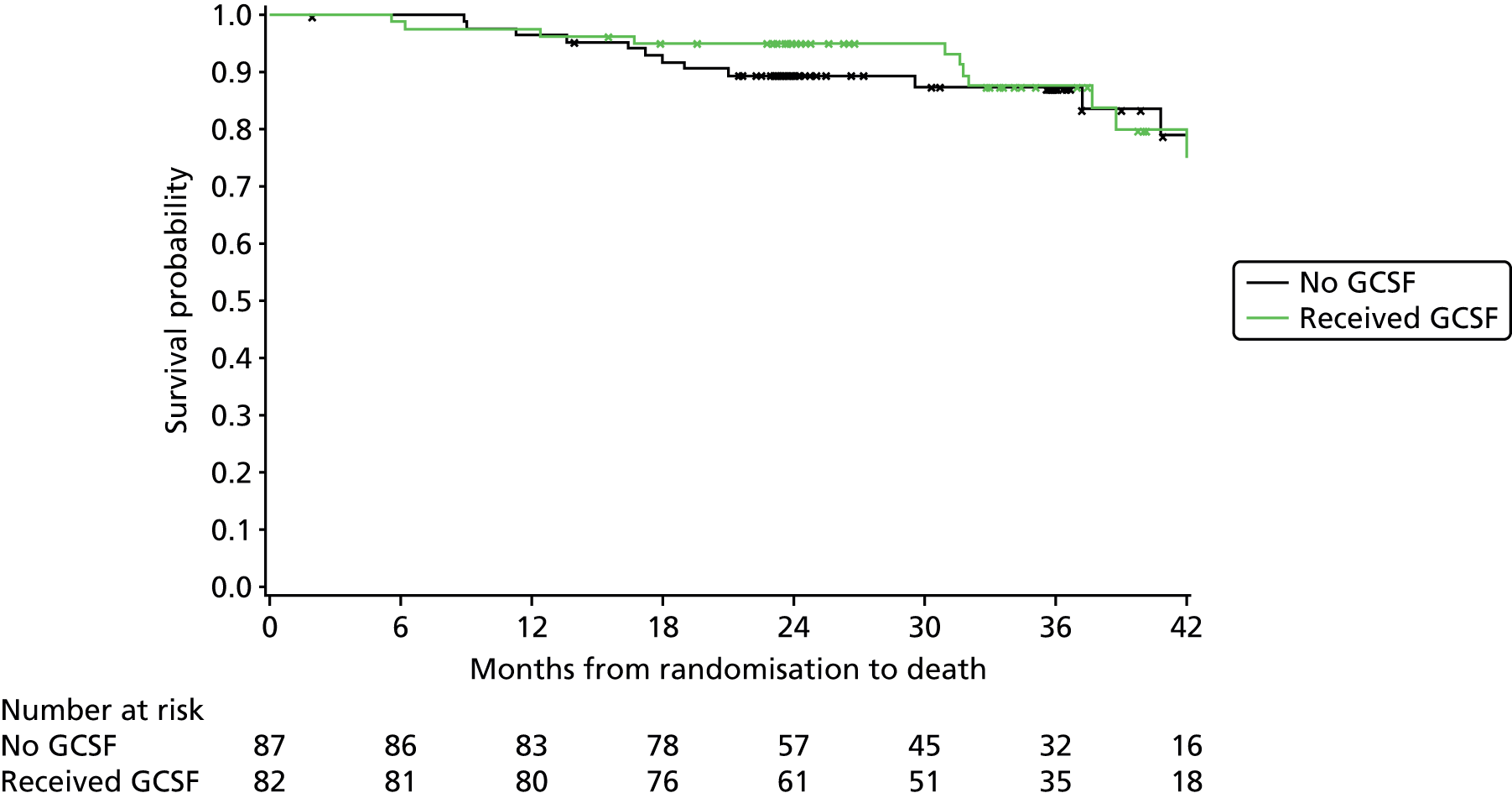
At 24 months post randomisation, the OS probability for participants who had received GCSF at some stage during their treatment was 95.1% compared with 89.5% for participants who had not. The difference between the OS curves for GCSF usage was non-significant (log-rank: χ2 = 0.067; p = 0.796).
Progression-free survival and overall survival by genetic risk factors: 17p and 11q
Given that there were only five participants with 17p deletion this analysis was not felt to be appropriate.
Figure 25 presents the Kaplan–Meier curves for time to progression by whether or not participants were 11q deleted. At the time of analysis, of the 26 participants who had an 11q deletion, six (23.1%) had reported an event (i.e. progression or death) compared with 37/142 (26.1%) participants who reported no event.
FIGURE 25.
Kaplan–Meier plot of PFS by 11q deletion.
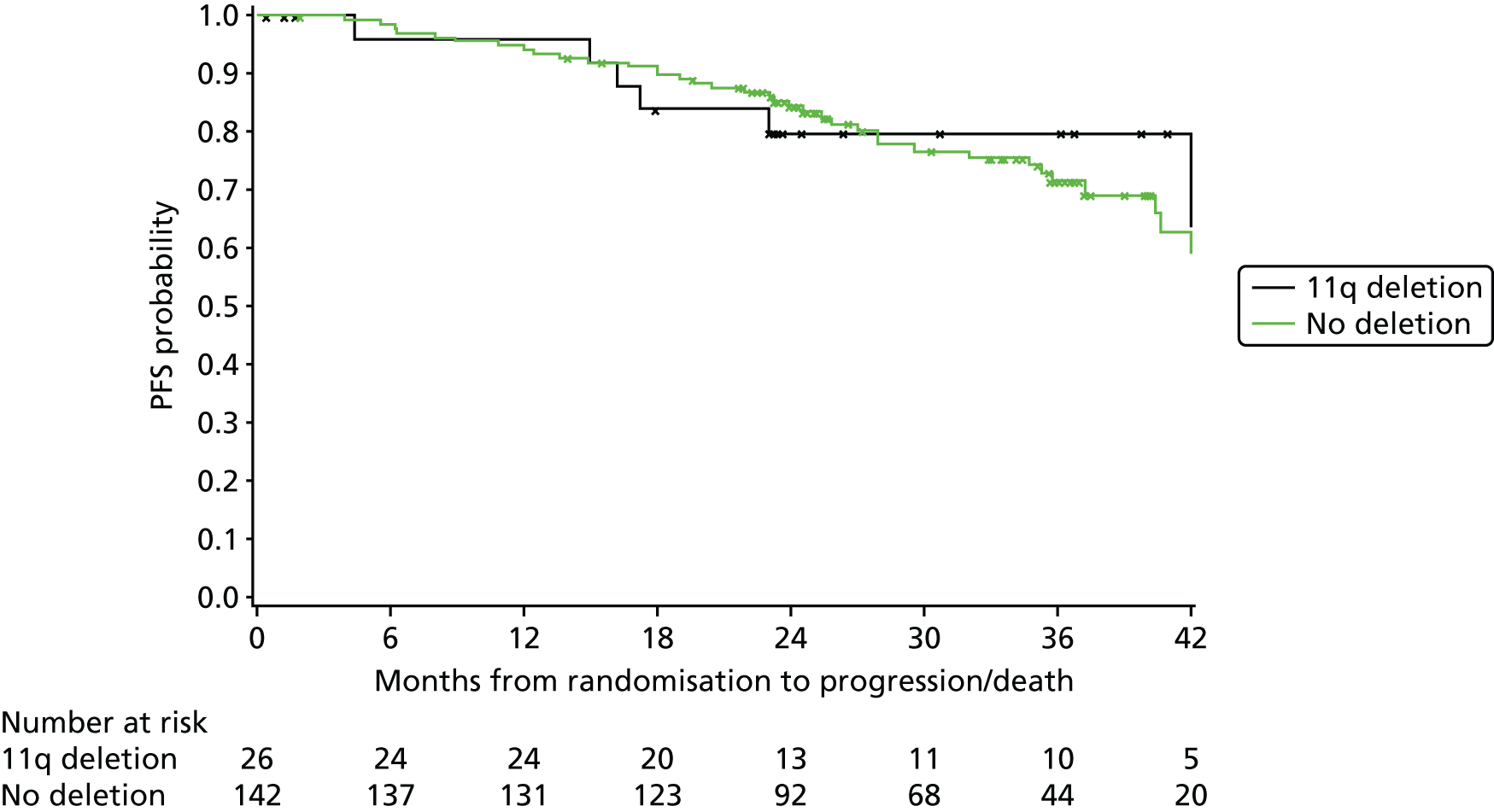
At 24 months post randomisation, the PFS probability for participants who were 11q deleted was 79.8% compared with 84.5% for participants who were not. The difference between the PFS curves was non-significant (log-rank: χ2 = 0.032; p = 0.858).
Figure 26 presents the Kaplan–Meier curves for PFS by whether or not participants were 11q deleted and by treatment group. The numbers are small, with only 10 and 16 participants in the FCR and FCM-miniR groups, respectively; however, it can be seen that only one of the FCR patients with 11q deletion progressed after over 3 years, compared with 5/16 (31.3%) of 11q-deleted patients on FCM-miniR. Excluding these 11q-deleted patients from the PFS curves brings the FCR and FCM-miniR curves closer together than those in Figure 5, although it can be seen that there is still a trend towards a PFS advantage with FCR over FCM-miniR.
FIGURE 26.
Kaplan–Meier plot of PFS by 11q deletion and treatment group.

Figure 27 presents the Kaplan–Meier curves for OS by whether or not participants were 11q deleted and by treatment group. The numbers are small, with only 10 and 16 participants in the FCR and FCM-miniR groups, respectively. No FCR patients with 11q deletion died, compared with 2/16 (12.5%) of 11q-deleted patients on FCM-miniR. After excluding these 11q-deleted patients from the OS curves, there is still a trend towards a survival advantage in the FCR group.
FIGURE 27.
Kaplan–Meier plot of OS by 11q deletion and treatment group.
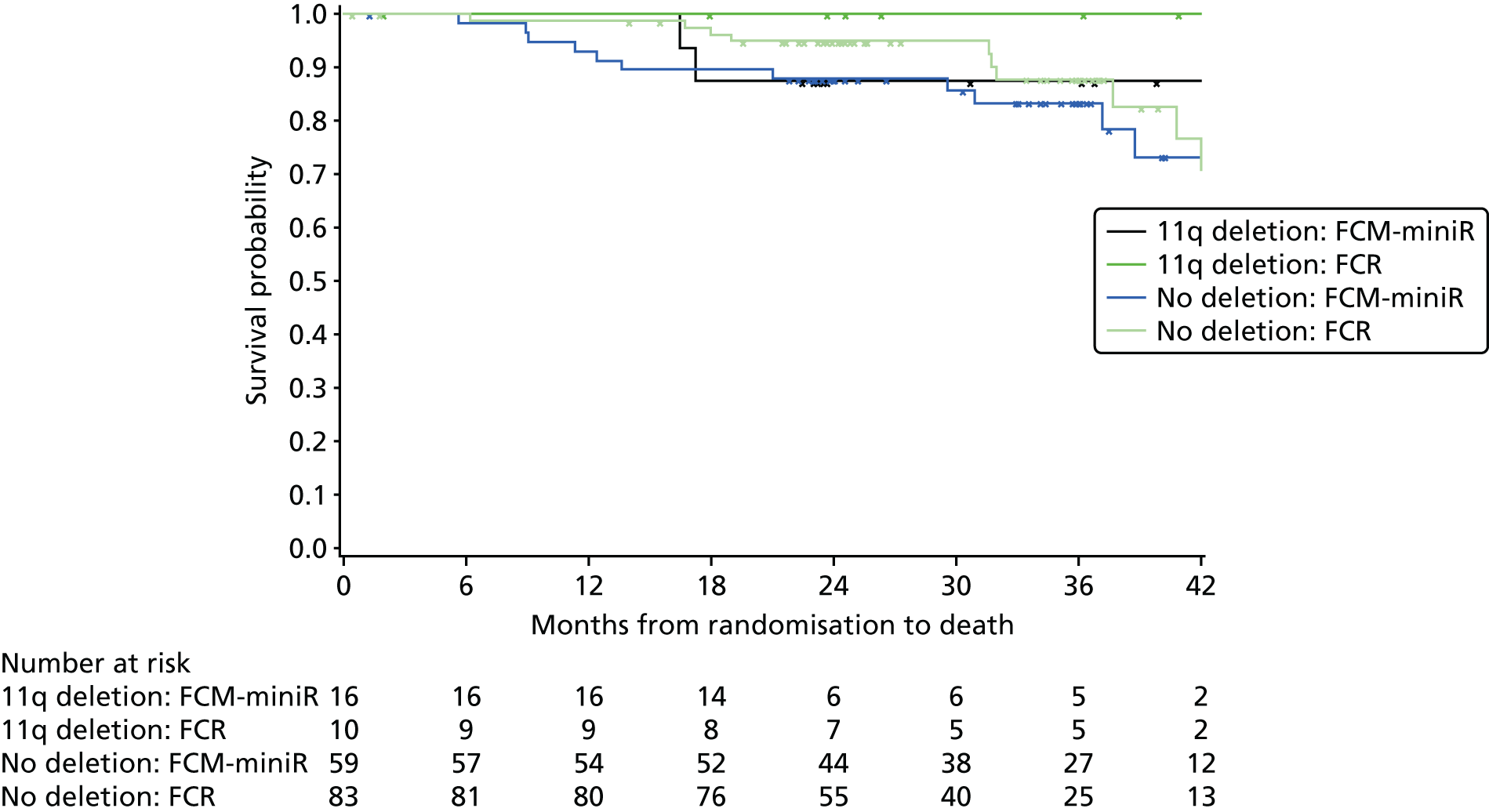
Figure 28 presents the Kaplan–Meier curves for OS by whether or not participants were 11q deleted. At the time of analysis, of the 26 participants who had an 11q deletion, 2 (7.7%) had died compared with 21/142 (14.8%) participants who had not.
FIGURE 28.
Kaplan–Meier plot of OS by 11q deletion.

At 24 months post randomisation, the OS probability was the same for participants who were 11q deleted and those who were not (92.0%). The difference between the OS curves was non-significant (log-rank: χ2 = 0.648; p = 0.421).
Progression-free survival and overall survival by genetic risk factors: VH mutation risk
Figure 29 presents the Kaplan–Meier curves for time to progression by VH mutation risk, where standard risk indicates that the participant had a VH mutation not involving the VH3–21 gene and poor risk indicates that the participant did not have a VH mutation, or that the VH3–21 gene was involved. At the time of analysis, of the 91 participants with a poor VH mutation risk, 27 (29.7%) had reported an event (i.e. progression or death) whereas 11/58 (18.0%) participants with a standard VH mutation risk had reported an event.
FIGURE 29.
Kaplan–Meier plot of PFS by VH mutation risk.

At 24 months post randomisation, the PFS probability for the poor risk group was 82.6% compared with 87.4% for the standard-risk group. Although there appears to be a slight trend in favour of standard-risk participants performing better in terms of PFS, the difference between the PFS curves was non-significant (log-rank: χ2 = 1.672; p = 0.1960).
Figure 30 presents the Kaplan–Meier curves for OS by VH mutation risk. At the time of analysis, of the 91 participants with a poor VH mutation risk, 16 (17.6%) had died whereas 5/58 (8.6%) participants with a standard VH mutation risk had died.
FIGURE 30.
Kaplan–Meier plot of OS by VH mutation risk.
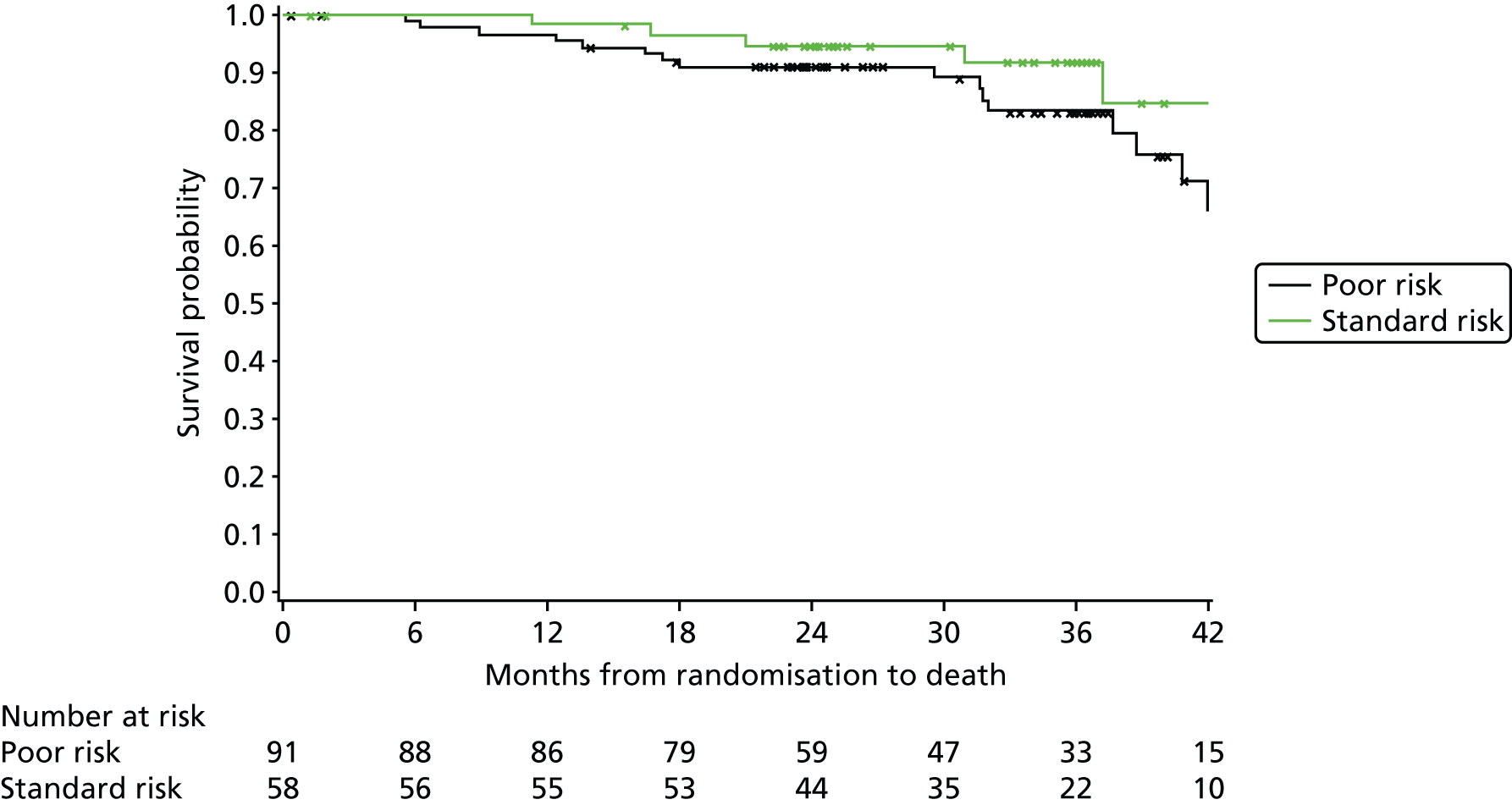
At 24 months post randomisation, the OS probability was 91.0% for the poor-risk group compared with 94.6% for the standard-risk group. The difference between the OS curves was non-significant (log-rank: χ2 = 2.172; p = 0.141), although there is a suggested trend for standard-risk participants performing better in terms of OS.
Safety and toxicity
Safety summaries are based on the safety population, which includes 198 participants, 100 who received FCR, 79 who received FCM-miniR and 19 who received FCM-miniR followed by FCR. The FCR arm excludes two participants randomised to FCR who did not receive any protocol treatment and includes two FCM-miniR participants who received FCR from their first treatment cycle, as planned and documented in Chapter 2, Analysis populations.
Serious adverse events
A total of 183 SAEs [80 (43.7%) FCR; 81 (44.3%) FCM-miniR; 22 (12.0%) FCM-miniR/FCR] have been reported from 104 (52.5%) participants. In the FCR arm, 49/100 (49.0%) participants reported at least one SAE compared with 46/79 (58.2%) receiving FCM-miniR and 9/22 (47.4%) receiving FCM-miniR followed by FCR (Table 50). Of the participants experiencing at least one SAE, the mean number of SAEs reported was 1.8, with a similar number for the FCR (mean = 1.6) and FCM-miniR (mean = 1.8) arms, as for rates for all participants.
| SAEs | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 19) | Total (n = 198) |
|---|---|---|---|---|
| Number of participants experiencing at least one SAE, n (%) | 49 (49.0) | 46 (58.2) | 9 (47.4) | 104 (52.5) |
| Total number of SAEs reported | 80 | 81 | 22 | 183 |
| Number of SAEs per participant who had at least one event | ||||
| Mean (SD) | 1.6 (1.0) | 1.8 (1.2) | 2.4 (1.3) | 1.8 (1.1) |
| Median (range) | 1 (1–5) | 1 (1–5) | 2 (1–4) | 1 (1–5) |
| N | 49 | 46 | 9 | 104 |
| Number of SAEs per participant (all participants) | ||||
| Mean (SD) | 0.8 (1.1) | 1.0 (1.2) | 1.2 (1.5) | 0.9 (1.2) |
| Median (range) | 0 (0–5) | 1 (0–5) | 0 (0–4) | 1 (0–5) |
| N | 100 | 79 | 19 | 198 |
Of the 183 SAEs reported, 145 (79.2%) were suspected to be related to protocol treatment (SARs), with a slightly higher proportion in the FCM-miniR arm (n = 67, 82.7%) than in the FCR arm (n = 62, 77.5%) (Table 51). SARs were reported from 89 participants (44.9%), with a higher proportion of SARs reported from participants receiving FCM-miniR (n = 39, 49.4%), compared with FCR (n = 41, 41.0%), and from nine participants (47.4%) receiving FCM-miniR followed by FCR. There was one SUSAR reported in the trial, from a participant in the FCR arm. This SUSAR was reported to the regulatory authorities (Medicines and Healthcare products Regulatory Agency), sponsor and the main REC within the required timelines for expedited reporting. The participant received all six cycles of treatment and was diagnosed with a ‘squamous cell carcinoma’ approximately 4 months after their last cycle of treatment. The event was felt by the Principal Investigator at the site to be related to trial treatment (FCR) and unexpected. Further details on this event, and all SAEs, are provided in Appendix 1.
| Relationship to experimental treatment | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Suspected, unexpected | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Suspected, expected | 61 (76.3) | 67 (82.7) | 16 (72.7) | 144 (78.7) |
| Not suspected | 18 (22.5) | 14 (17.3) | 6 (27.3) | 38 (20.8) |
| Total | 80 (100) | 81 (100) | 22 (100) | 183 (100) |
| Suspected to be related to | ||||
| Fludarabine | 2 (3.2) | 6 (9.0) | 1 (6.3) | 9 (6.2) |
| Cyclophosphamide | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| Rituximab | 7 (11.3) | 0 (0.0) | 0 (0.0) | 7 (4.8) |
| Low-dose rituximab | 0 (0.0) | 5 (7.5) | 2 (12.5) | 7 (4.8) |
| Fludarabine and cyclophosphamide | 21 (33.9) | 0 (0.0) | 4 (25.0) | 25 (17.2) |
| Fludarabine and mitoxantrone | 0 (0.0) | 2 (3.0) | 0 (0.0) | 2 (1.4) |
| Fludarabine and rituximab | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (0.7) |
| Mitoxantrone and low-dose rituximab | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| FCM | 0 (0.0) | 21 (31.3) | 1 (6.3) | 22 (15.2) |
| FCR | 32 (51.6) | 0 (0.0) | 2 (12.5) | 34 (23.4) |
| Fludarabine, cyclophosphamide and low-dose rituximab | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| FCM-miniR | 0 (0.0) | 30 (44.8) | 5 (31.3) | 35 (24.1) |
| Total | 62 (100) | 67 (100) | 16 (100) | 145 (100) |
Of the 145 SAEs suspected to be related to protocol treatment, in the FCR arm the majority (51.6%) were suspected to be related to all three IMPs, F, C and R, with 33.9% suspected to be related to just F and C, and 11.3% suspected to be related to rituximab only. In the FCM-miniR arm, SAEs were most commonly (44.8%) suspected to be related to all four IMPs, F, C, M and reduced-dose rituximab (miniR) with 31.3% suspected to be related to F, C and M.
The majority of SAEs required (prolonged) hospitalisation (89.1%), with 92.5% coming from the FCR arm and 84.0% from the FCM-miniR arm (Table 52).
| Seriousness criteria and outcome | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Seriousness criteria | ||||
| Participant died | 1 (1.3) | 2 (2.5) | 0 (0.0) | 3 (1.6) |
| Life-threatening | 2 (2.5) | 4 (4.9) | 0 (0.0) | 6 (3.3) |
| Required/prolonged hospitalisation | 74 (92.5) | 68 (84.0) | 21 (95.5) | 163 (89.1) |
| Persistent or significant disability/incapacity | 2 (2.5) | 0 (0.0) | 0 (0.0) | 2 (1.1) |
| Jeopardised participant/required intervention to prevent one of the above | 10 (12.5) | 14 (17.3) | 1 (4.5%) | 25 (13.7%) |
| Outcome | ||||
| Recovered | 72 (90.0) | 71 (87.7) | 19 (86.4) | 162 (88.5) |
| Recovered with sequelae | 5 (6.3) | 7 (8.6) | 3 (13.6) | 15 (8.2) |
| Condition improving | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Condition still present and unchanged | 1 (1.3) | 1 (1.2) | 0 (0.0) | 2 (1.1) |
| Death | 1 (1.3) | 2 (2.5) | 0 (0.0) | 3 (1.6) |
| Total | 80 (100) | 81 (100) | 22 (100) | 183 (100) |
Six SAEs in the FCM-miniR arm were deemed to be life-threatening or resulted in death, compared with three in the FCR arm. In the FCR arm, one participant died as a result of an ‘infection’ which was suspected to be related to trial treatment within 5 months of their last course of treatment. This participant received two cycles of treatment. In the FCM-miniR arm, one participant died as a result of a ‘Bilateral pneumonia’ which was suspected to be related to trial treatment within 9 months of their last course of treatment. The participant received all six cycles of treatment. A further FCM-miniR participant died as a result of ‘Neutropenic sepsis and infected shoulder’, which was not suspected to be related to trial treatment, within 3 months of their last course of treatment. This participant received four cycles of treatment. Further information on the SAEs that resulted in death are provided in Appendix 1.
All but three SAEs had recovered at the time of reporting.
The majority of SARs required (or prolonged) hospitalisation (90.3%) (93.5% in the FCR arm compared with 86.6% in the FCM-miniR arm). All but three SARs had recovered at the time of reporting (Table 53).
| Seriousness criteria and outcome | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Seriousness criteria | ||||
| Participant died | 1 (1.6) | 1 (1.5) | 0 (0.0) | 2 (1.3) |
| Life-threatening | 2 (3.2) | 4 (6.0) | 0 (0.0) | 6 (4.1) |
| Required/prolonged hospitalisation | 58 (93.5) | 58 (86.6) | 15 (93.8) | 131 (90.3) |
| Persistent or significant disability/incapacity | 2 (3.2) | 0 (0.0) | 0 (0.0) | 2 (1.3) |
| Jeopardised participant/required intervention to prevent one of the above | 8 (12.9) | 11 (16.4) | 1 (6.3) | 20 (13.8) |
| Outcome | ||||
| Recovered | 58 (93.5) | 60 (89.6) | 15 (93.8) | 133 (91.7) |
| Recovered with sequelae | 1 (1.6) | 5 (7.5) | 1 (6.3) | 7 (4.8) |
| Condition improving | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Condition still present and unchanged | 1 (1.6) | 1 (1.5) | 0 (0.0) | 2 (1.4) |
| Death | 1 (1.6) | 1 (1.5) | 0 (0.0) | 2 (1.4) |
| Total | 62 (100) | 67 (100) | 16 (100) | 145 (100) |
Of the 198 participants in the safety population, 96 (48.5%) required hospitalisation during the trial as a result of an SAE, with a higher proportion in the FCM-miniR arm (n = 41, 51.9%) than the FCR arm (n = 46, 46.0%) (Table 54).
| Required hospitalisation for an SAE | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 19) | Total (n = 198) |
|---|---|---|---|---|
| Yes, n (%) | 46 (46.0) | 41 (51.9) | 9 (47.4) | 96 (48.5) |
| No, n (%) | 54 (54.0) | 38 (48.1) | 10 (52.6) | 102 (51.5) |
Of the 180 SAEs that were not ongoing, the median duration of an event was 5 days (range: < 1–303 days) with a similar duration in each of the treatment groups (Table 55). The median duration of SARs that were suspected to be related to protocol treatment was the same, although there was variability in the mean durations between the treatment arms.
| Duration | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Duration of SAE (days from when SAE became serious to recovery/death) | ||||
| Mean (SD) | 15.9 (40.9) | 15.3 (41.2) | 15.9 (53.3) | 15.6 (42.5) |
| Median (range) | 4.0 (< 1–280.0) | 5.0 (< 1–303.0) | 4.5 (< 1–254.0) | 5.0 (< 1–303.0) |
| N | 78 | 80 | 22 | 180 |
| Missing | 2 | 1 | 0 | 3 |
| Duration of SAR (days from when SAR became serious to recovery/death) | ||||
| Mean (SD) | 11.7 (22.8) | 15.2 (44.5) | 19.9 (62.5) | 14.2 (39.4) |
| Median (range) | 5.0 (< 1–144.0) | 5.0 (< 1–303.0) | 4.5 (< 1–254.0) | 5.0 (< 1–303.0) |
| N | 60 | 66 | 16 | 142 |
| Missing | 2 | 1 | 0 | 3 |
Of the 145 SAEs suspected to be related to protocol treatment (SARs), the majority (n = 90, 62.1%) were classed as ‘infections and infestations’ according to the MedDRA System Organ Class system (Table 56). A higher proportion of events reported in the FCR arm were classed as ‘general disorders and administration site conditions’ than those reported in the FCM-miniR arm [10 (16.1%) FCR; 6 (9.0%) FCM-miniR].
| MedDRA system organ class | FCR, n (%) | FCM-miniR, n (%) | FCM-miniR/FCR, n (%) | Total, n (%) |
|---|---|---|---|---|
| Blood and lymphatic system disorders | 8 (12.9) | 8 (11.9) | 0 (0.0) | 16 (11.0) |
| Gastrointestinal disorders | 4 (6.5) | 4 (6.0) | 2 (12.5) | 10 (6.9) |
| General disorders and administration site conditions | 10 (16.1) | 6 (9.0) | 3 (18.8) | 19 (13.1) |
| Immune system disorders | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| Infections and infestations | 36 (58.1) | 43 (64.2) | 11 (68.8) | 90 (62.1) |
| Musculoskeletal and connective tissue disorders | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (1.6) | 1 (1.5) | 0 (0.0) | 2 (1.4) |
| Psychiatric disorders | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Renal and urinary disorders | 0 (0.0) | 2 (3.0) | 0 (0.0) | 2 (1.4) |
| Skin and subcutaneous tissue disorders | 2 (3.2) | 1 (1.5) | 0 (0.0) | 3 (2.1) |
| Total | 62 (100) | 67 (100) | 16 (100) | 145 (100) |
Adverse events
A total of 2163 AEs have been reported from 192 (97.0%) participants. A total of 1117 events have been reported from 96 participants receiving FCR, 863 events from 77 participants receiving FCM-miniR and 183 from 19 participants who received FCM-miniR followed by FCR. The mean number of AEs reported was similar for the FCR and FCM-miniR treatment groups for the population of participants experiencing at least one event (Table 57). Line listings of all AEs by treatment received are presented in Appendix 2, Adverse event listings.
| AEs | FCR (n = 100) | FCM-miniR (n = 79) | FCM-miniR/FCR (n = 19) | Total (n = 198) |
|---|---|---|---|---|
| Number of participants experiencing at least one AE, n (%) | 96 (96.0) | 77 (97.5%) | 19 (100%) | 192 (97.0%) |
| Number of AEs reported | 1117 | 863 | 183 | 2163 |
| Number of AEs per participant who had at least one event | ||||
| Mean (SD) | 11.6 (7.1) | 11.2 (6.9) | 9.6 (5.7) | 11.3 (6.9) |
| Median (range) | 11 (1–38) | 10 (1–36) | 8 (1–19) | 10 (1–38) |
| N | 96 | 77 | 19 | 192 |
| Number of AEs per participant (overall) | ||||
| Mean (SD) | 11.2 (7.3) | 10.9 (7.0) | 9.6 (5.7) | 10.9 (7.1) |
| Median (range) | 11 (0–38) | 10 (0–36) | 8 (1–19) | 10 (0–38) |
| N | 100 | 79 | 19 | 198 |
New AEs most commonly occurred during the first treatment cycle, with the number of new AEs occurring gradually declining with the more treatment cycles received, although this trend will be influenced by the declining number of participants receiving each treatment cycle (Table 58). The proportion of new AEs occurring at each treatment cycle appears to be reasonably well balanced between FCR and FCM-miniR.
| Treatment cycle AE first occurred | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| 1 | 280 (25.1) | 237 (27.5) | 51 (27.9) | 568 (26.3) |
| 2 | 217 (19.4) | 156 (18.1) | 45 (24.6) | 418 (19.3) |
| 3 | 185 (16.6) | 144 (16.7) | 22 (12.0) | 351 (16.2) |
| 4 | 164 (14.7) | 114 (13.2) | 26 (14.2) | 304 (14.1) |
| 5 | 142 (12.7) | 99 (11.5) | 24 (13.1) | 265 (12.3) |
| 6 | 129 (11.5) | 113 (13.1) | 15 (8.2) | 257 (11.9) |
| Total | 1117 (100) | 863 (100) | 183 (100) | 2163 (100) |
Table 59 presents the maximum CTCAE grade experienced for each AE by treatment received. The majority of AEs were reported as a maximum CTC grade 1 (50.5%). A higher percentage of CTC grade 3/4 AEs were experienced in the FCM-miniR arm (n = 193, 22.4%) than the FCR (n = 168, 15.0%) and FCM-miniR/FCR arms (n = 27, 14.6%).
| Maximum CTCAE grade | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| 1 | 589 (52.7) | 405 (46.9) | 99 (54.1) | 1093 (50.5) |
| 2 | 354 (31.7) | 262 (30.4) | 57 (31.1) | 673 (31.1) |
| 3 | 107 (9.6) | 118 (13.7) | 17 (9.3) | 242 (11.2) |
| 4 | 61 (5.5) | 75 (8.7) | 10 (5.5) | 146 (6.7) |
| Missing | 6 (0.5) | 3 (0.3) | 0 (0.0) | 9 (0.4) |
| Total | 1117 (100) | 863 (100) | 183 (100) | 2163 (100) |
Treatment-related mortalities within 3 months of ending protocol treatment
There were no treatment-related mortalities within 3 months of the protocol treatment ending.
One trial participant, receiving FCM-miniR, died within 3 months of discontinuing the protocol treatment but the cause was not suspected to be related to trial treatment. This participant discontinued treatment after their fourth cycle owing to ‘Disease progression, requiring further treatment’. After discontinuing treatment, and prior to death, they reported a SAE of ‘Neutropenic sepsis and infected shoulder’, which was not suspected to be related to the trial treatment. The participant gradually deteriorated and died within 3 months of ending protocol treatment. The primary cause of death was given as ‘Infection due to CLL’ and the participant’s disease status at the time of death was recorded as ‘stable disease’.
Secondary cancers
The following tables summarise the secondary cancers that were reported at follow-up.
Table 60 shows that the incidence of secondary cancers was similar across the trial arms, with 11.1% of patients reporting a secondary cancer. The most common types of secondary cancer were skin- and haematological-related cancers.
| Incidence and type of secondary cancer | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Has the participant reported a secondary cancer? | ||||
| Yes | 11 (11.0) | 10 (12.7) | 1 (5.3) | 22 (11.1) |
| No | 89 (89.0) | 69 (87.3) | 18 (94.7) | 176 (88.9) |
| Total | 100 (100) | 79 (100) | 19 (100) | 198 (100) |
| Secondary cancer type | ||||
| Haematological (lymphoma) | 2 (16.7) | 2 (18.2) | 0 (0.0) | 4 (16.7) |
| Haematological (AML/MDS) | 3 (25.0) | 2 (18.2) | 0 (0.0) | 5 (20.8) |
| Skin (non-melanoma) | 2 (16.7) | 7 (63.6) | 1 (100) | 10 (41.7) |
| Skin (melanoma) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Non-haematological (solid tumours) | 4 (33.3) | 0 (0.0) | 0 (0.0) | 4 (16.7) |
| Total | 12 (100) | 11 (100) | 1 (100) | 24 (100) |
Table 61 further summarises the type of secondary cancer reported. Note that the results are not mutually exclusive, as one patient was diagnosed with basal cell carcinoma twice, and another with both squamous cell carcinoma and melanoma.
| Secondary cancer type | FCR, N (%) | FCM-miniR, N (%) | FCM-miniR/FCR, N (%) | Total, N (%) |
|---|---|---|---|---|
| Lymphoma (other) | 2 (16.7) | 2 (18.2) | 0 (0.0) | 4 (16.7) |
| Myelodysplastic syndrome | 2 (16.7) | 2 (18.2) | 0 (0.0) | 4 (16.7) |
| Acute myeloid leukaemia | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Basal cell carcinoma | 0 (0.0) | 3 (27.3) | 0 (0.0) | 3 (12.5) |
| Squamous cell carcinoma | 2 (16.7) | 4 (36.4) | 1 (100) | 7 (29.2) |
| Melanoma | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Lung | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Hepatobiliary | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (4.2) |
| Urological (prostate) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (8.3) |
| Total | 12 (100) | 11 (100) | 1 (100) | 24 (100) |
Table 62 shows the mean, median and ranges of when the secondary cancers were diagnosed from randomisation and from end of treatment.
| Timing of secondary cancer | FCR | FCM-miniR | FCM-miniR/FCR | Total |
|---|---|---|---|---|
| Months from randomisation to diagnosis | ||||
| Mean (SD) | 23.9 (14.9) | 18.7 (9.4) | 24.3 | 21.5 (12.3) |
| Median (range) | 25.4 (1.6–46.5) | 20.0 (6.4–31.6) | 24.3 (24.3–24.3) | 22.3 (1.6–46.5) |
| n | 12 | 11 | 1 | 24 |
| Months from end of treatment to diagnosis | ||||
| Mean (SD) | 19.9 (14.3) | 13.2 (8.7) | 19.2 | 16.8 (11.9) |
| Median (range) | 21.7 (0.3–41.5) | 14.6 (1.7–25.7) | 19.2 (19.2–19.2) | 16.1 (0.3–41.5) |
| n | 12 | 11 | 1 | 24 |
Summary of statistical results
There is strong evidence to suggest that FCM-miniR is not non-inferior to FCR in terms of CR at 3 months post treatment.
From December 2009 to September 2012, 200 of a planned 206 patients were recruited from 34 centres across the UK. A good proportion of participants (n = 141, 70.5%) completed the recommended six cycles of treatment, with a slightly higher proportion in the FCR arm than in the FCM-miniR arm (70% vs. 64.6%), and the majority of participants who discontinued treatment early doing so for reasons of toxicity. A similar proportion of participants in each of the treatment arms experienced at least one dose modification to their protocol-defined dose of treatment, and just under half (47.0%) received treatment with GCSF at some stage during their treatment period.
At the interim analysis, carried out on the first half of patients randomised to the trial (n = 103), of those with available data, 82.9% of participants achieved a CR in the FCR arm compared with 61.4% in the FCM-miniR arm. The difference in proportions (FCM-miniR – FCR) was –21.6% (99.5% CI –48.0% to 4.8%), which was not statistically significant at the 0.5% (p = 0.005) level, although the experimental treatment had the worst performance. This was confirmed by the adjusted analysis (OR 0.32, 99.5% CI 0.07 to 1.48; p = 0.037). The primary aim of the interim analysis was to be able to release information of any potential large differences in efficacy between the treatment arms and inform the continued treatment of trial participants earlier than would have been the case with the final analysis. As the results were approaching significance in favour of the control group, and there was evidence of additional toxicity in the FCM-miniR arm, the trial was closed early at the recommendation of the DMEC and participants still receiving FCM-miniR were recommended to transfer to treatment with FCR for the remainder of their treatment cycles.
At the final analysis of the primary end point (at 3 months post treatment), 76.1% of participants in the FCR arm achieved a CR compared with 54.7% in the FCM-miniR arm. The difference in proportions (FCM-miniR – FCR) was –21.4% (95% CI –35.8% to –7.0%) and the adjusted analysis gave an OR of 0.37 for the treatment effect (95% CI 0.19 to 0.73), indicating that participants in the FCM-miniR were significantly less likely to achieve a CR. As the lower limit of the 95% CI and the mean OR were < 0.61 (equivalent to a difference in proportions of 10% based on the observed control rate) and the upper limit of the 95% CI was also below 1, there was very strong evidence that FCM-miniR is not non-inferior to FCR in terms of CR rates at 3 months post treatment, and that it is in fact inferior. The analysis of the PP population and the sensitivity analyses agreed with this conclusion. The exploratory subgroup analyses indicated that there was a significant trend towards participants who received more than three cycles of treatment, and those who did not have a 17p deletion, performing better in terms of response.
The ORR was high at 92.6% with 7.5% fewer participants achieving at least a PR in the FCM-miniR arm compared with the FCR arm (95% CI –15.6% to 0.6%). The difference in the ORR proportions was not statistically significant, although it is approaching significance.
At 3 months post treatment, 53.0% of participants were MRD negative, a higher percentage of participants in the FCR arm (57.0%) than in the FCM-miniR arm (46.4%). The difference in proportions (FCM-miniR – FCR) was –10.6% (95% CI –26.1% to 4.9%) and the adjusted analysis gave an OR of 0.63 for the treatment effect (95% CI 0.34 to 1.20) which was not statistically significant (χ2 = 1.97; p = 0.160), although it was approaching significance.
There was no conclusion of a significant difference between the treatment arms with respect to time to progression (log-rank test, p = 0.2790; Wilcoxon rank-sum test, p = 0.1081), confirmed by the adjusted Cox regression analysis (HR 1.39, 95% CI 0.77 to 2.49; p = 0.2771). There was also no conclusion of a significant difference between the treatment arms with respect to OS (log-rank test, p = 0.2779; Wilcoxon rank-sum test, p = 0.1013), confirmed by the adjusted Cox regression analysis (HR 1.57, 95% CI 0.68 to 3.58; p = 0.2876). However, there was a non-significant trend towards the FCM-miniR participants performing worse. At 24 months from randomisation, 89.4% of the FCR participants remained progression-free compared with 79.1% of the FCM-miniR participants. In terms of OS at 24 months, 95.8% of the FCR participants remained alive compared with 88.5% of the FCM-miniR participants. In the exploratory subgroup analyses, PFS and OS were significantly improved for participants who were MRD negative or had achieved a CR at 3 months post treatment, or who received more than three cycles of treatment. In addition, of those participants who were MRD positive, OS was worse in participants who received FCM-miniR than in those who received FCR, suggesting that after progression the participants initially treated with FCM-miniR responded worse to salvage therapies or died before further treatment was possible. Longer follow-up data are required to be able to assess reliably the time-to-event outcomes, and these will be updated in future.
More participants experienced an SAE in the FCM-miniR arm than the FCR arm (58.2% vs. 49.0%), as well as an SAR (49.4% vs. 41.0%). One SUSAR (‘squamous cell carcinoma’) was reported during the trial in the FCR arm. More participants in the FCM-miniR arm were hospitalised for an SAE during the trial (51.9% vs. 46.0%) and six SAEs were deemed to be life-threatening or resulted in death compared with three in the FCR arm. A similar proportion of participants experienced an AE in each treatment arm, but a higher proportion of CTCAE grade 3 and 4 AEs were reported in the FCM-miniR arm (22.4% vs. 15.0%). There were no treatment-related mortalities within 3 months of completing protocol treatment.
Throughout the duration of the trial there were nine withdrawals (4.5%), with a similar number of participants coming from each treatment arm.
Chapter 4 Economic evaluation
Sections of this chapter have been reproduced from Howard et al. 1 with permission.
The health economics analysis was designed to provide an economic evaluation of previously untreated patients with CLL to compare FCR and FCM-miniR. The aim was to assess the cost-effectiveness of FCR compared with FCM-miniR from a UK NHS and PSS perspective.
Unit cost data
Unit cost of resource use
Individual-level resource use was combined with unit costs to calculate the total health-care use cost for each participant in the trial. In order to convert resource usage figures into costs, unit cost figures were assigned from national sources such as the PSSRU Unit Costs of Health and Social Care 2013. 33 Table 63 presents the summary of unit costs.
| Resource item | Face-to face-visits, £ | Phone call, £ | Source |
|---|---|---|---|
| GP surgery visit | 45.00 | 27.00 | PSSRU (2013) p. 191: including direct care staff costs with qualification; per participant contact lasting 11.7 minutes |
| GP out of office hours visit | 114.00 | 27.00 | PSSRU (2013) p. 191: including direct care staff costs with qualification (we consider it as out of surgery visit lasting 23.4 minutes) |
| District nurse | 70.00 | N/A | PSSRU (2013) p. 183: per hour of home visit including qualification |
| Health visitor | 71.00 | N/A | PSSRU (2013) p. 185: per hour of home visit including qualification |
| Occupational therapist | 44.00 | N/A | PSSRU (2013) p. 201: per hour participant contact (costs including training) |
| Physiotherapist | 34.00 | N/A | PSSRU (2013) p. 175: per hour participant contact (costs including qualification) |
| Counsellor | 58.00 | N/A | PSSRU (2013) p. 54: cost per consultation |
| Home help or care worker | 24.00 | N/A | PSSRU (2013) p. 202: cost per hour weekday. Each home visit lasting 30 minutes |
| Psychiatrist | 362.00 | N/A | PSSRU (2013) p. 247: per face-to-face contact |
| Psychologist | 134.00 | N/A | PSSRU (2013) p. 179: per hour participant contact |
| Hospital inpatient stay | 598.00 | PSSRU (2013) p. 107: national average non-elective short stay | |
| 2581.00 | PSSRU (2013) p. 107: national average non-elective long stay | ||
| Hospital day centre | 697.00 | N/A | PSSRU (2013) p. 107: day cases Healthcare Resource Group data |
| Hospital outpatient clinic | 135.00 | N/A | PSSRU (2013) p. 107: weighted average of all outpatient procedures |
| Hospital A&E department | 131.00 | N/A | PSSRU (2010) p. 119: national average A&E treatments leading to admitted (not admitted) [not updated in the latest publication] |
| Nursing home | 750.00 | N/A | PSSRU (2013) p. 37: establishment cost per permanent resident week |
Unit cost of medications
Participants were randomised to receive six cycles of either FCR or FCM-miniR. Each cycle was 28 days. Participants were evaluated after three cycles of chemotherapy and if no response or disease progression was observed they were stopped from receiving further therapy. These participants would still attend follow-up assessments until 24 months after randomisation. Unit costs for medications were obtained from the BNF 67th edition of September 2013. 34 Details for medication dosages given within the six treatment cycles were collected. The total medication costs using the dosage and the frequency provided by the participant were calculated. If the dose of the drug was not recorded, it was assumed that the participant received the same dosage as other participants who were given the same treatment. If the quantity was not recorded, the average quantity for that drug as reported in the data was applied. Table 64 shows all the unit costs for the drugs that were reported in the trial.
| Drug name | Description | Price (BNF) August 2014 |
|---|---|---|
| Cyclophosphamide | ||
| Tablets | Cyclophosphamide monohydrate BP 53.0 mg equivalent to 50 mg anhydrous cyclophosphamide | Tablets, s/c, cyclophosphamide (anhydrous) 50 mg, net price 100 = £70.70. Label: 25, 27 |
| Solution | Cyclophosphamide monohydrate powder for solution injection or infusion | Injection, powder for reconstitution, cyclophosphamide, net price 500-mg vial = £9.20; 1-g vial = £17.06 |
| Fludarabine | ||
| Tablets | Fludarabine phosphate 50 mg | Tablets, f/c, pink, fludarabine phosphate 10 mg, net price 15-tab pack = £302.48, 20-tab pack = £403.31 |
| Solution | Fludarabine phosphate 50 mg | Injection, powder for reconstitution, fludarabine phosphate, net price 50-mg vial = £147.07 |
| Mitoxantrone | ||
| Solution | Each millilitre of concentrate contains 2 mg mitoxantrone (as hydrochloride) | Concentrate for intravenous infusion, mitoxantrone (as hydrochloride) 2 mg/ml, net price 10-ml vial = £121.85 |
| Each 10-ml vial contains 20 mg mitoxantrone as hydrochloride | ||
| Rituximab (MabThera) | ||
| Solution | MabThera solution for infusion | Concentrate for intravenous infusion, rituximab 10 mg/ml, net price 10-ml vial = £174.63, 50-ml vial = £873.15 |
| Composition rituximab 100 mg/10 ml or 500 mg/50 ml | ||
Treatment costs
In addition to the cost of medications used during chemotherapy, an additional cost was identified which was the cost of administering the drug. The costs are shown in Table 65.
| Drug namea | Cost per cycle, £ | Source |
|---|---|---|
| Rituximab (i.v. administration) | 430 | NICE, Rituximab for the first-line treatment of chronic lymphocytic leukaemia, 200948 |
| FC | 230 | NICE, Rituximab for the first-line treatment of chronic lymphocytic leukaemia, 200948 |
Utility and quality-adjusted life-years
Participant health-related quality of life was assessed using EQ-5D,28 which was included along with the participant resource-use questionnaires. Changes in EQ-5D scores at baseline, 3 months after treatment ends, and at 12, 18 and 24 months post randomisation were evaluated using two-sample t-tests to explore any important differences in these end points within the time frame of the trial.
In line with the NICE reference case27 the primary health outcome for the economic evaluation was QALYs measured using the EQ-5D questionnaire. Participant responses to the EQ-5D questionnaire at each time point were converted to utilities using the standard UK tariff values28 and the ‘area under the curve’ approach. QALYs were calculated by multiplying these values with the time spent in each state, with quality of life linearly interpolated for the periods between the four observations provided in the trial data. Average QALYs between adjacent time points were calculated to generate smoothed estimates between the time points. A sensitivity analysis was conducted using the SF-12 trial questionnaire responses (instead of the EQ-5D) to calculate QALYs.
Missing data
The mean total cost per participant was calculated for NHS and PSS perspectives by adding the costs of inpatient stay, outpatient visit, consultations, medication, treatment and applicable interventions for all participants where response data were available. Missing QALY data were predicted in terms of baseline EQ-5D, visual analogue scale of health-related quality of life, treatment received, age, sex and resource use for that time period. From the overall sample, missing data represented 33.98%. Importantly, the health economics criteria for inclusion were slightly more restrictive than those for the statistical analysis; for those cases in which either resource usage or quality of life data were unavailable, these figures cannot be calculated. The complete data analysis was based on 136 participants. We addressed missingness using multiple imputations via chained equations35,36 to complete missing data assuming missing at random and using Stata 13 (StataCorp LP, College Station, TX, USA). A total of 10 imputations were created to stabilise the result. Following multiple imputations, data for 162 participants were accounted for. The reported cost-effectiveness results were synthesised based on all imputed data sets.
Within-trial cost-effectiveness analysis
Cost-effectiveness results: base case
Resource use and QALY data were available for 162 participants, with 92 participants being treated with FCR and 70 participants with FCM-miniR.
Health-care resource use
The total costs associated with resource use during the trial are shown in Tables 66 and 67. The mean total NHS and PSS resource use costs during therapy are £15,492 for FCR and £9049 for FCM-miniR. Costs were significantly higher for FCR (+£6444; p = 0.00). In the 18 months following the therapy period, the difference in resource use costs between the two arms diminished, with a mean total cost of £1756 for FCR and £1570 for FCM-miniR (p = 0.71). There were no significant differences in NHS and PSS resource use costs between the treatment arms following the end of therapy according to the t-tests.
| Cost type | FCR (n = 92), £ | FCM-miniR (n = 70), £ | Difference: p-value of t-test | |
|---|---|---|---|---|
| Cycles 1–3 (medication and treatment) | Mean (SD) | 6152 (1811) | 2763 (901) | 0.0000 |
| Cycles 4–6 (medication and treatment) | Mean (SD) | 5701 (2769) | 2916 (1411) | 0.0000 |
| Health and social services usea | Mean (SD) | 218 (448) | 254 (417) | 0.5779 |
| Hospital-based care services | Mean (SD) | 3421 (3989) | 3116 (4261) | 0.6198 |
| Total | Mean (SD) | 15,492 (6478) | 9049 (5516) | 0.0000 |
| Cost type | FCR (n = 92), £ | FCM-miniR (n = 70), £ | Difference: p-value of t-test | |
|---|---|---|---|---|
| Health and social services usea | Mean (SD) | 269 (381) | 265 (306) | 0.5780 |
| Hospital-based care services | Mean (SD) | 1487 (2285) | 1472 (4075) | 0.9757 |
| Total resource use | Mean (SD) | 1756 (2402) | 1570 (3720) | 0.7133 |
Health outcomes
Table 68 presents the EQ-5D scores at baseline, 3 months after therapy ended, and 12, 18 and 24 months post randomisation with missing values imputed. Both treatment groups showed increasing EQ-5D scores from baseline up to 3 months after the end of therapy, with a slight dip at 12 months and 18 months post randomisation in both arms before increasing again.
| Parameter | FCR (n = 92) | FCM-miniR (n = 70) | Difference: p-value of t-test | |
|---|---|---|---|---|
| Baseline | Mean (SD) | 0.829 (0.200) | 0.774 (0.275) | 0.169 |
| Median (min.–max.) | 0.814 (–0.016–1.00) | 0.812 (–0.0.16–1.00) | ||
| 3 months after end of therapy | Mean (SD) | 0.852 (0.141) | 0.868 (0.194) | 0.546 |
| Median (min.–max.) | 0.858 (0.378–1.00) | 0.870 (–0.239–1.00) | ||
| 12 months post randomisation | Mean (SD) | 0.838 (0.177) | 0.863 (0.218) | 0.434 |
| Median (min.–max.) | 0.883 (0.189–1.00) | 0.934 (–0.74–1.00) | ||
| 18 months post randomisation | Mean (SD) | 0.833 (0.180) | 0.851 (0.184) | 0.517 |
| Median (min.–max.) | 0.927 (0.145–1.00) | 0.937 (–0.003–0.965) | ||
| 24 months post randomisation | Mean (SD) | 0.852 (0.161) | 0.871 (0.097) | 0.383 |
| Median (min.–max.) | 0.895 (–0.071–0.965) | 0.876 (0.498–0.965) | ||
| Total QALYs | Mean (SD) | 1.610 (0.329) | 1.552 (0.414) | 0.316 |
| Median (min.–max.) | 1.714 (0.418–2.00) | 1.722 (0.049–1.974) | ||
On average, the difference between arms was marginal. Independent sample t-tests indicated that the changes in EQ-5D score over time were not statistically significant. The average total QALYs gained over the 24 months was marginally higher in the FCR arm (1.61) than in the FCM-miniR arm (1.55) (p = 0.40).
Cost-effectiveness results within the NHS and Personal Social Services perspectives
Table 69 shows the total costs and EQ-5D-generated QALYs for each of the treatment arms. Differences in QALYs between groups were minimal and suggested marginal health decrements in the FCM-miniR arm compared with the FCR arm. The FCR group had the highest EQ-5D-generated QALYs over the trial period. The mean total cost was significantly higher for the FCR group. The high standard deviation (SD) for the deterministic cost estimates indicates the presence of a few outlying individuals who incurred significant health service costs.
| Total costs and QALYs | FCR | FCM-miniR |
|---|---|---|
| n | 92 | 70 |
| Total QALYs (SD) | 1.610 (0.329) | 1.552 (0.412) |
| Total cost, £ (SD) | 17,248 (7156) | 10,619 (7312) |
Table 70 below provides the probabilistic cost-effectiveness results, showing the incremental costs and benefits as well as the ICER. The results suggest that FCM-miniR is associated with an incremental cost saving of £6619 and an incremental QALY loss of 0.059. Note that, owing to the fact that FCM-miniR is associated with both negative cost and QALY increments, the ICER result cannot be interpreted in the usual way as the cost per additional QALY associated with FCM-miniR; the ICER instead represents the cost saved per QALY lost. That is, if the NHS adopts FCM-miniR, it could expect to save £112,193 per QALY lost. As this cost saving outweighs the value of a lost QALY based on a WTP of £20,000 per QALY, the overall net benefit associated with FCM-miniR is positive (£5349) and FCM-miniR is therefore expected to be cost-effective over a 24-month time horizon.
| Strategy | Total cost, £ (SD) | Total QALY (based on EQ-5D) (SD) | Incremental cost, £ (SD) | Incremental QALY (SD) | ICER, £ | Incremental NB, £ (SD) |
|---|---|---|---|---|---|---|
| FCR | 17,241 (745) | 1.610 (0.04) | ||||
| FCM-miniR | 10,622 (758) | 1.551 (0.05) | –6619 (1061) | –0.059(0.06) | 112,193a | 5439 (1546) |
The uncertainty around the cost-effectiveness estimate is represented graphically on the cost-effectiveness plane (Figure 31) using bootstrapping with 10,000 iterations. This method samples at random with replacement from each of the 10 imputed data sets, producing 10,000 incremental cost and incremental QALY estimates.
FIGURE 31.
Cost-effectiveness plane showing the incremental costs and QALYs for FCM-miniR compared with FCR from the bootstrap analysis of the within-trial results over 24 months (EQ-5D, NHS and PSS perspectives).
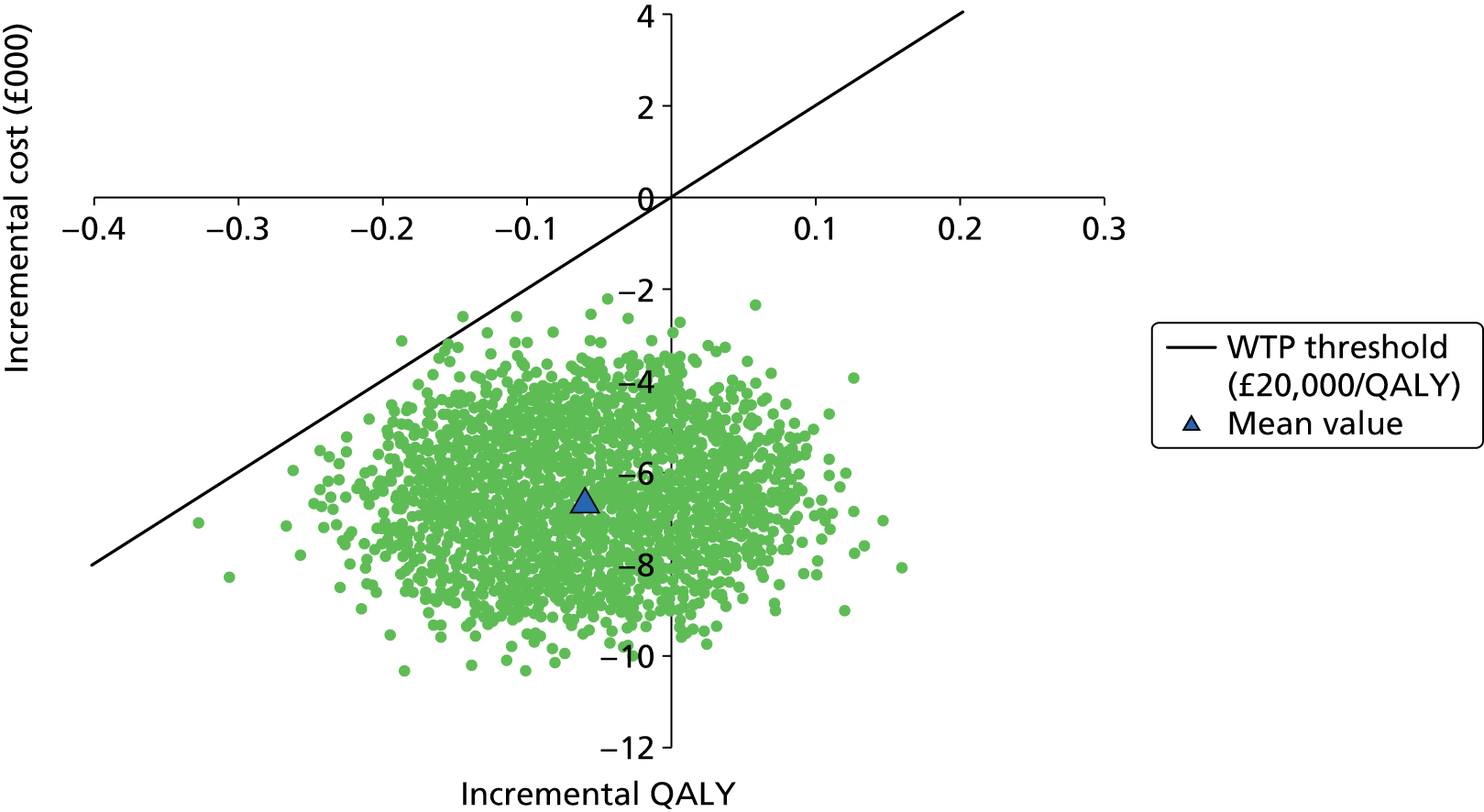
Figure 31 shows that all the points were below the x-axis, indicating that FCM-miniR is cost-saving compared with FCR, and most points were to the left of the y-axis, indicating that FCM-miniR produces fewer QALYs than FCR. The majority of points lie below the £20,000/QALY threshold line, indicating that FCM-miniR is cost-effective.
The CEAC showing the probability that FCR is cost-effective is presented below in Figure 32 with a range of cost-effectiveness WTP thresholds values. The probability that FCM-miniR is cost-effective is high and remains above 60% up to a threshold value of £100,000 per QALY.
FIGURE 32.
Cost-effectiveness acceptability curves at 12 months (NHS and PSS perspectives, using EQ-5D generated QALYs).

Sensitivity analyses within trial in the NHS and Personal Social Services perspective
In order to test the robustness of the within-trial analysis a number of different cost-effectiveness analyses were completed.
Sensitivity analysis of health utility measurement
A similar cost-effectiveness analysis was completed using SF-6D-generated QALYs. When the equivalent SF-6D figures were analysed, a different pattern was observed. There was a decrease in mean SF-6D values between baseline and 3 months post randomisation, with fluctuations in either direction at the follow-up time points. The average total QALYs gained were slightly higher in the FCM-miniR arm (1.211) than the FCR arm (1.195) (p = 0.81), in contrast to the EQ-5D analysis in which FCR produced a higher overall QALY value. A statistically significant difference in the SF-6D utility values was observed at the 24 months post randomisation time point only (Table 71).
| Parameter | FCR (n = 92) | FCM-miniR (n = 89) | Difference: p-value of t-test | |
|---|---|---|---|---|
| Baseline | Mean (SD) | 0.651 (0.290) | 0.670 (0.269) | 0.713 |
| Median (min.–max.) | 0.723 (–0.01–1.00) | 0.723 (–0.128–1.00) | ||
| 3 months after end of therapy | Mean (SD) | 0.606 (0.261) | 0.593 (0.242) | 0.645 |
| Median (min.–max.) | 0.6592 (–0.024–1.00) | 0.634 (–0.074–1.00) | ||
| 12 months post randomisation | Mean (SD) | 0.715 (0.238) | 0.706 (0.237) | 0.814 |
| Median (min.–max.) | 0.770 (–0.127–1.00) | 0.770 (–0.060–1.00) | ||
| 18 months post randomisation | Mean (SD) | 0.670 (0.239) | 0.722 (0.219) | 0.157 |
| Median (min.–max.) | 0.711 (–0.075–0.965) | 0.777 (–0.071–0.965) | ||
| 24 months post randomisation | Mean (SD) | 0.612 (0.289) | 0.763 (0.170) | 0.000a |
| Median (min.–max.) | 0.704 (–0.059–1.00) | 0.808 (–0.014–0.965) | ||
| Total QALYs | Mean (SD) | 1.195 (0.414) | 1.211 (0.412) | 0.812 |
| Median (min.–max.) | 1.242 (0.038–1.850) | 1.305 (0.126–1.820) | ||
Table 72 below shows the total costs and SF-6D-generated QALYs for each of the treatment arms. FCM-miniR is associated with an incremental cost saving of £6492 and an incremental QALY gain of 0.016 compared with FCR; FCM-miniR therefore dominates FCR, being more effective and less costly, with a positive INB of £6805.
| Strategy | Total cost, £ | Total QALY (based on SF-6D) | Incremental cost, £ | Incremental QALY | ICER | INB, £ |
|---|---|---|---|---|---|---|
| FCR | 17,248 | 1.1949 | ||||
| FCM-miniR | 10,756 | 1.2105 | –£6492 | 0.016 | FCM-miniR dominates | 6805 |
Using the NICE WTP threshold of £20,000, FCM-miniR is expected to be 100% cost-effective over a 24-month time horizon. This is illustrated in Figure 33 by the fact that all of the simulated points in the probabilistic sensitivity analysis lie under the WTP threshold.
FIGURE 33.
Cost-effectiveness plane generated from bootstrapped mean cost and SF-6D-generated QALY differences over 24 months (NHS and PSS perspectives).

Impact of crossover participants
To assess the impact of removing the 21 participants who crossed over from the FCM-miniR arm to FCR in the base-case analysis, two further sensitivity analyses were conducted:
-
ITT, whereby any transfer of participants from one arm to another is ignored and participants who crossed over from FCM-miniR to FCR are retained in the analysis of the FCM-miniR arm
-
participants who were transferred are deemed to have been in the FCR treatment arm from randomisation.
The results of these analyses can be seen in Table 73. The results show consistency in the trend of costs and QALYs in both types of analyses (i.e. using EQ-5D- or SF-6D-generated QALYs).
| EQ-5D | Incremental cost, £ | Incremental QALY gain | ICER, £ | INB, £ |
|---|---|---|---|---|
| Base case | ||||
| FCM-miniR vs. FCR | –6619 | –0.0590 | 112,193a | 5439 |
| ITT | ||||
| FCM-miniR vs. FCR | –5761 | –0.0467 | 123,298a | 4826 |
| Transferred from baseline | ||||
| FCM-miniR vs. FCR | –6000 | –0.0523 | 114,657a | 4954 |
| SF-6D | ||||
| Base case | ||||
| FCM-miniR vs. FCR | –6456 | 0.0176 | FCM-miniR dominates | 6807 |
| ITT | ||||
| FCM-miniR vs. FCR | –5674 | 0.0305 | FCM-miniR dominates | 6284 |
| Transferred from baseline | ||||
| FCM-miniR vs. FCR | –6031 | 0.0138 | FCM-miniR dominates | 6308 |
Summary of within-trial cost-effectiveness analysis
Using the EQ-5D to generate QALYs, the results showed that participants with CLL, treated in the FCM-miniR arm, did not show a higher QALY gain when compared with participants in the FCR arm (1.552 vs. 1.610). However, FCM-miniR presented negative incremental costs (£6443), indicating that treatment with FCM-miniR would lead to cost savings. This difference was driven by the higher cost of delivery of FCR, with cost differences stemming from treatment costs during the six cycles of chemotherapy.
Based on imputed data, the analysis found the incremental QALY gain to favour FCR, despite the difference being minimal at 0.059 (roughly equivalent to 21.5 days of full health). This result is robust to sensitivity analysis and stochastic bootstrapping.
However, using SF-6D to generate QALYs, the results showed that participants with CLL treated with FCM-miniR showed a slightly higher QALY gain than participants in the FCR arm (1.211 vs. 1.195). This gain, together with the incremental cost saving of £6805, suggests that FCM-miniR dominates FCR and is cost-effective at a £20,000 WTP threshold. Despite this difference in QALY findings between the two quality-of-life measures, FCM-miniR was found to be cost-effective in both analyses.
The within-trial analysis is conducted on data collected over a 24-month time period. The majority of CLL participants go on to live much longer than this; therefore, the within-trial analysis is unlikely to capture all of the relevant differences in long-term costs and health outcomes between the two participant groups. Hence, there is a need for decision model analysis to extrapolate these results to lifetime horizons.
Lifetime cost-effectiveness analysis
A de novo decision analytic model was developed to estimate the lifetime cost-effectiveness of FCM-miniR compared with FCR. The base-case analysis was conducted using QALYs derived from participant EQ-5D questionnaire responses in the trial, and it was assumed that any difference between the two treatments in terms of rate of disease progression was contained within the first 2 years from treatment initiation (i.e. within the trial period). The analysis was conducted from an NHS and PSS perspective and adhered to current NICE reference case standards. 27 For the base-case model, probabilistic analysis (using 10,000 Monte-Carlo simulations) was conducted in order to account for uncertainty around the model parameter input values. In addition, deterministic one-way sensitivity analyses were conducted to assess the influence of changes to individual model parameters and key model assumptions on the results. A value of information analysis was conducted to determine the potential value to the NHS of conducting additional research on the cost-effectiveness of FCM-miniR compared with FCR.
Base-case model results
Results of the base-case analyses are presented in Table 74 (deterministic results) and Table 75 (probabilistic results). Taking into account uncertainty around the model parameters (i.e. using the probabilistic results), FCM-miniR is associated with an expected lifetime cost saving of £7723 and a lifetime QALY loss of 0.73 compared with FCR; an incremental loss of over two-thirds of a healthy life-year. As the incremental cost and QALYs are both negative, the ICER does not represent the incremental cost per additional QALY, but rather represents the cost saved per QALY lost associated with adoption of FCM-miniR. The ICER therefore indicates that one QALY will be lost for every £10,624 saved by adopting FCM-miniR. As the amount saved is less than the societal WTP for a QALY (£20,000), the overall expected INB of FCM-miniR is negative. This indicates that adoption of FCM-miniR would lead to a lifetime loss of benefit for the NHS and PSS, and FCM-miniR is therefore not expected to be cost-effective. The expected lost net benefit associated with FCM-miniR is –£6780, which is equivalent to a net health loss of 0.34 QALYs (assuming that QALYs are valued at £20,000 per QALY). There is significant uncertainty around this result, as can be seen by the large SD value for the INB (SD = 7907).
| Strategy | Total cost, £ | Total QALY | Incremental cost, £ | Incremental QALY | ICER, £ | Net benefit, £ | Incremental net benefit, £ |
|---|---|---|---|---|---|---|---|
| FCR | 31,176 | 6.12 | 91,324 | ||||
| FCM- miniR | 23,468 | 5.46 | –7708 | –0.67 | 11,576a | 85,715 | –5609 |
| Strategy | Total cost, £ (SD) | Total QALY (SD) | Incremental cost, £ (SD) | Incremental QALY (SD) | ICER, £ | Net benefit, £ (SD) | Incremental net benefit, £ (SD) |
|---|---|---|---|---|---|---|---|
| FCR | 31,314 (7237) | 7.76 (0.26) | 123,917 (8447) | ||||
| FCM-miniR | 23,590 (6997) | 7.04 (0.36) | –7723 (3281) | –0.73 (0.42) | 10,651a | 117,137 (9449) | –6780 (7907) |
The results of the probabilistic analysis are presented in Figure 34. Each point on the graph represents the result of one probabilistic simulation of the model and indicates a potential incremental cost and QALY for FCM-miniR compared with FCR. The diagonal line represents the currently accepted WTP per QALY threshold of £20,000 per QALY. Points that lie below the threshold line are considered cost-effective; points above the threshold line are not considered to be cost-effective. In this analysis, the points are widely distributed, with many points lying in both cost-effective and non-cost-effective regions. This indicates that there is significant uncertainty around the cost-effectiveness of FCM-miniR compared with FCR over a lifetime horizon. The mean of the simulated values lies above the threshold line, indicating that, on average, FCM-miniR is not expected to be cost-effective.
FIGURE 34.
Scatterplot of model lifetime cost-effectiveness results for FCM-miniR vs. FCR.

The CEAC shows the proportion of model simulation points that lie under the cost-effectiveness threshold plane across different threshold values, which indicates the probability that each treatment is cost-effective at given WTP values. The CEAC for FCM-miniR versus FCR is presented in Figure 35. At low cost per additional QALY thresholds, FCM-miniR is associated with a high probability of being cost-effective. However, as the threshold value increases the probability of FCM-miniR being cost-effective rapidly diminishes, and FCR is associated with an increasing probability of cost-effectiveness. At a threshold value of £20,000 per QALY, FCM-miniR is associated with 19% probability of being cost-effective; increasing the threshold value to £30,000 per QALY results in a drop to a 12% probability of being cost-effective.
FIGURE 35.
Cost-effectiveness acceptability curve for model lifetime analysis.

Sensitivity analyses
One-way deterministic sensitivity analysis
Results of the one-way deterministic sensitivity analysis are presented in Figure 36. The vertical line indicates the base-case deterministic INB value for FCM-miniR versus FCR (–£5609). The horizontal bars indicate the extent to which this base-case value is altered when individual model parameter values are increased or decreased by 25% of their base-case value. From the diagram it appears that altering the discount rate and utility of the progression-free state results in the greatest change to the cost-effectiveness result. However, FCM-miniR is associated with a negative INB in all of the analyses considered, indicating that FCM-miniR is not expected to be cost-effective in any of the analyses. Changes in the input parameters therefore have no effect on the overall result of the analysis.
FIGURE 36.
Tornado plot of one-way deterministic sensitivity analysis results.

Intention-to-treat analysis (including participants who crossed over from fludarabine, cyclophosphamide, mitoxantrone and low-dose rituximab to fludarabine, cyclophosphamide and mitoxantrone)
Results of the sensitivity analysis using the ITT population are shown in Table 76. Compared with the base-case analysis in which participants who crossed over from FCM-miniR to FCR were excluded from the analysis, the incremental cost saving associated with FCM-miniR compared with FCR is slightly reduced (–£7708 vs. –£6593), and the incremental QALY loss is reduced (–0.67 vs. –0.56). Nevertheless, FCM-miniR remains non-cost-effective, with a negative INB value (–£4591 compared with the base-case deterministic value of –£5609). This indicates that the exclusion of the participants who crossed over from FCM-miniR to FCM has no effect on the overall cost-effectiveness result.
| Strategy | Total cost, £ | Total QALY | Incremental cost, £ | Incremental QALY | ICER, £ | NB, £ | Incremental NB, £ |
|---|---|---|---|---|---|---|---|
| FCR | 30,741 | 7.63 | 121,924 | ||||
| FCM-miniR | 24,148 | 7.07 | –6593 | –0.56 | 11,790a | 117,333 | –4591 |
Short Form questionnaire-6 Dimensions
Results of the sensitivity analysis using QALYs derived from participant-reported SF-12 forms (converted to SF-6D utilities) are shown in Table 77. As for the base-case analysis using EQ-5D-derived utilities, FCM-miniR is associated with a negative incremental cost and utility value, indicating that FCM-miniR is expected to be cost saving but less effective than FCR. Compared with the EQ-5D analysis, using SF-6D utilities leads to a smaller QALY loss associated with FCM-miniR (–0.55 compared with –0.67); however, this QALY decrement still outweighs the expected cost saving, resulting in a negative INB value. FCM-miniR is therefore still expected to be non-cost-effective over a lifetime horizon, as in the base-case analysis.
| Strategy | Total cost, £ | Total QALY | Incremental cost, £ | Incremental QALY | ICER, £ | NB, £ | Incremental NB, £ |
|---|---|---|---|---|---|---|---|
| FCR | 31,437 | 6.41 | 96,678 | ||||
| FCM-miniR | 23,842 | 5.85 | –7595 | –0.55 | 13,721a | 93,203 | –3475 |
Treatment effect
Results of the sensitivity analysis extending the differential rates of progression observed between FCR and FCM-miniR in the trial beyond the trial period are shown in Table 78. Owing to the fact that in the trial period participants in the FCR arm progressed at a slower rate than participants in the FCM-miniR arm, extending this effect beyond the trial period by applying the observed HR in the trial benefits the FCR arm. The expected net benefit associated with FCM-miniR therefore decreases as the HR is applied over longer time periods, with FCM-miniR becoming increasingly non-cost-effective as the treatment effect is extended. The expected INB falls from –£5609 in the base case to –£9750 when the differential rates of progression are applied over a lifetime horizon. FCM-miniR therefore remains non-cost-effective across all the analyses.
| Outcome | Base case (differential effect in 2-year trial period) | 3-year time horizon (HR applied up to year 3) | 5-year time horizon | 10-year time horizon | Lifetime time horizon |
|---|---|---|---|---|---|
| Incremental cost, £ | –7708 | –7717 | –7720 | –7716 | –7705 |
| Incremental QALY | –0.67 | –0.76 | –0.84 | –0.86 | –0.87 |
| FCR-miniR incremental NB, £ | –5609 | –7549 | –9198 | –9520 | –9750 |
Value of information analysis
Results of the population EVPI analysis are shown in Figure 37. The EVPI value represents the absolute maximum that the NHS should be willing to spend on further research on the cost-effectiveness of FCM-miniR versus FCR. At a £20,000 per QALY threshold, and assuming a 10-year effective time horizon for the new treatment, the expected value of information is £21M. The maximum EVPI value (£43M) is reached at a £10,600/QALY threshold value (equivalent to the ICER value for FCM-miniR), as this is the point at which uncertainty around parameter estimates in the model has the greatest influence on the decision of whether or not to adopt the new treatment.
FIGURE 37.
Population EVPI.
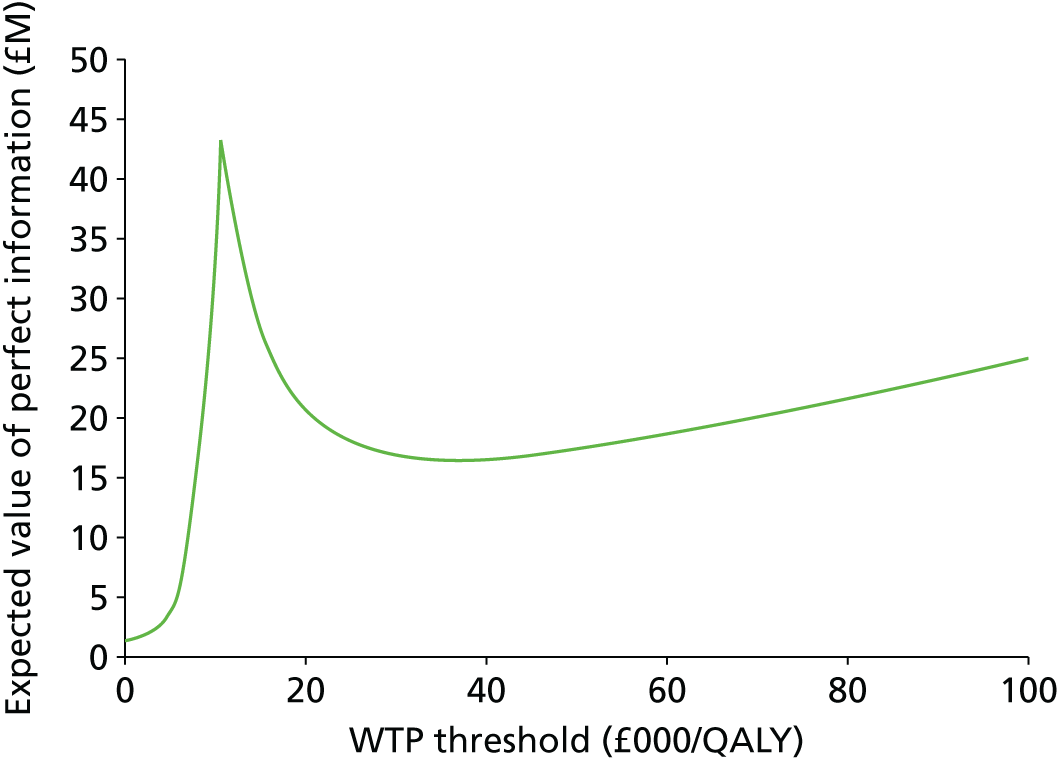
Results of the EVPPI analysis are shown in Figure 38. Uncertainty around the starting distributions of the model (i.e. the proportion of participants who begin the model with progressed disease as opposed to being disease free) is found to be the most influential factor, with the associated EVPPI values for these parameters being substantial. These parameters are directly related to the efficacy of each of the treatments in terms of delaying disease progression, so it is unsurprising that these have a large impact on the model results.
FIGURE 38.
Population EVPPI for base-case model (£20,000/QALY threshold, 10-year effective lifetime and annual incidence of 2943).
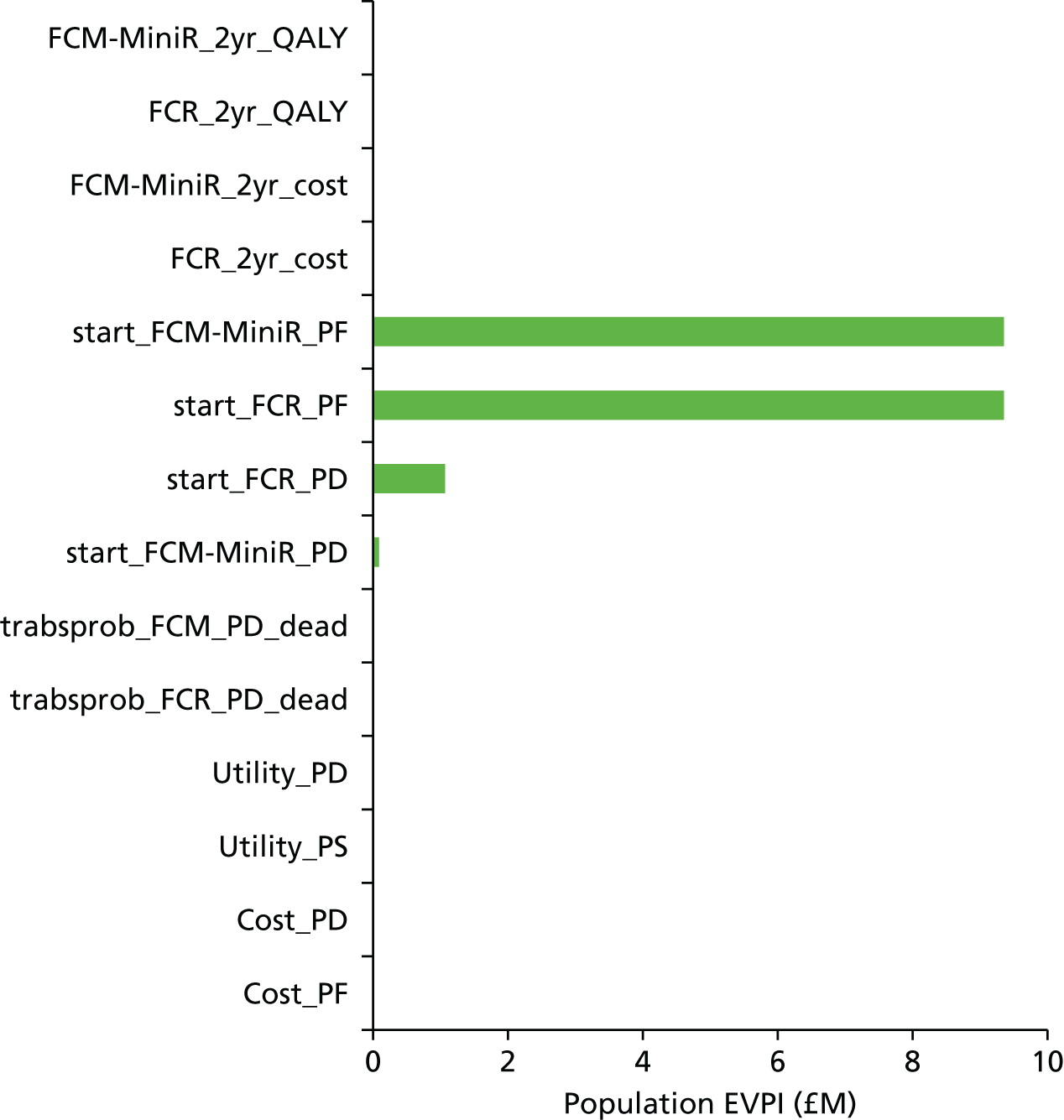
The value of information results suggest that there is the potential for further research into the cost-effectiveness of FCM-miniR compared with FCR to be of value to the NHS. However, the EVPI and EVPPI values provide only a necessary (but not sufficient) condition for the decision to fund further research (i.e. a positive EVPI/EVPPI value indicates that there may be value in conducting further research). The decision of whether or not to invest in further research and what design that research should take requires further analysis of the cost of research and the value of particular trial designs.
Predicted overall survival
The predicted OS curves simulated by the base-case model are presented in Figure 39. The difference in OS between the two arms is greatest at the beginning of the model simulation; this is attributable to the fact that the model begins from the end of the trial 24-month period, and the proportion of participants surviving in each arm was taken directly from the trial follow-up data which showed the given discrepancy between the two arms. Subsequently in the model, however, it is assumed that the risk of mortality in both the progression-free and progressed disease states is the same in both arms. In the future, validation of the model could be achieved by comparing the predicted OS with real-world data; currently these data are unavailable and we therefore show the OS curves for illustrative purposes only.
FIGURE 39.
Overall survival curves simulated from the lifetime decision analytic model.

Summary of decision model lifetime cost-effectiveness analysis
The results of the decision analytic model indicate that FCM-miniR is not expected to be cost-effective over a lifetime horizon. Compared with FCR, FCM-miniR is associated with an average lifetime cost saving of £7723 per patient, and a lifetime QALY loss of 0.73. The resulting expected INB associated with FCM-miniR is –£6780 (equivalent to a net health loss of –0.34 QALYs), indicating that if FCM-miniR were to be adopted instead of FCR the NHS would be worse off as a whole.
Results of the deterministic sensitivity analyses found that the results were robust to changes in individual model parameters, with FCM-miniR remaining non-cost-effective in all of the analyses conducted. However, when joint parameter uncertainty is considered (using probabilistic sensitivity analysis), there is some uncertainty around the results: at a £20,000 per QALY threshold, there is a 19% chance that FCM-miniR is cost-effective. The expected value of information analysis indicates that there is potential benefit to conducting further research into the cost-effectiveness of FCM-miniR; however, additional considerations (such as the cost of research and specific research design) would need to be considered before a definitive recommendation for further research could be made.
Summary of the economic evaluation
An economic evaluation was conducted to assess the cost-effectiveness of FCM-miniR compared with FCR from a UK NHS and PSS perspective. The evaluation consisted of two components: a within-trial analysis, in which cost-effectiveness was assessed within the 24-month trial period using individual participant data collected in the trial; and a decision analytic model analysis, in which cost-effectiveness was assessed over a lifetime horizon using standard modelling techniques to extrapolate the trial analysis to a lifetime horizon.
The results of the within-trial analysis indicate that FCM-miniR is cost-effective compared with FCR over a 24-month time horizon, although it should be noted that cost-effectiveness in this instance is achieved only by virtue of the fact that the cost savings associated with FCM-miniR in the short term outweigh the associated QALY losses. In the base-case analysis, FCM-miniR was associated with a mean cost saving of £6619 and a mean QALY loss of –0.059 compared with FCR. The corresponding ICER was £112,193, indicating that for every £112,193 saved by adopting FCM-miniR, one QALY would be lost. Assuming that one QALY is valued at £20,000 (the threshold currently adopted by NICE), FCM-miniR is therefore associated with a positive INB of £5439, indicating that it is cost-effective compared with FCR. In a sensitivity analysis using SF-6D QALYs derived from the SF-12 questionnaire (instead of EQ-5D as in the base case), FCM-miniR was estimated to result in an overall QALY increase (+0.016) compared with FCR, resulting in FCM-miniR dominating FCR (being more effective and less costly). Altering the analysis to include participants who crossed over from FCM-miniR to FCR had minimal effect on the results. The base-case probabilistic analysis found that at a £20,000 per QALY threshold, there is a 100% probability that FCM-miniR is cost-effective compared with FCR over a 24-month time horizon.
However, the cost-effectiveness of FCM-miniR was not sustained in the long-term analysis. Results of the economic decision model indicate that FCM-miniR is not expected to be cost-effective over a lifetime horizon. The base-case analysis indicates that over a lifetime horizon FCM-miniR is expected to result in a mean cost saving of £7723 and a mean QALY loss of 0.73 QALYs. The associated ICER is £10,651, indicating that, for every £10,651 saved by adopting FCM-miniR, one QALY would be lost. In contrast to the within-trial analysis, the cost saving associated with FCM-miniR is no longer sufficient to outweigh the QALY loss, and FCM-miniR is no longer cost-effective, with an incremental net loss of –£6780. However, lack of available data regarding long-term outcomes means that there is uncertainty around this result; at a £20,000 per QALY threshold there is a 19% chance that FCM-miniR is cost-effective. Owing to this uncertainty, the value of information analysis found that there is potential value in conducting further research on the cost-effectiveness of FCM-miniR. Nevertheless, the cost of further research and the likely value of specific research designs would need to be considered before making a definitive recommendation regarding the value of further research.
Chapter 5 Discussion
Sections of this chapter have been reproduced from Howard et al. 1 with permission.
Interpretation
The ARCTIC trial demonstrated that the combination of FCM-miniR was not non-inferior to FCR for the primary end point of CR. However, the CR rates observed in the trial were very high compared with previous studies involving FCR in both Europe4,5 and the USA. 2,3 It appears that the addition of mitoxantrone to the regime created more toxicity, limiting the dose and/or duration of therapy compared with the FCR arm. There is no sign that low-dose rituximab is as effective as the full dose when given with chemoimmunotherapy. The trial also confirmed that patients who respond well, achieving either a CR and/or the eradication of detectable MRD, have a better outcome than those with worse responses.
The ARCTIC trial protocolised the use of haematopoietic growth factors, namely GCSF, to support the blood counts if they were low and delaying further courses of therapy. It appears that this planned use of GCSF as secondary prophylaxis (given only to patients whose neutropenia was delaying subsequent cycles of treatment) allows more patients to complete their planned therapy and that this leads to a similar outcome when compared with those patients who do not require GCSF.
The reason for the very high response rates and the favourable PFS and OS in ARCTIC is unclear. The reasons for this, compared with international trials with similar entry criteria, are probably multifactorial, and there are at least five possible factors that could account for this: (1) FC were given by the oral route in ARCTIC rather than intravenously as in previous studies; (2) the exposure for each cycle of treatment was 5 days rather than the 3 days over which FCR is given intravenously – this might be important as when alkylating agents are used alone they appear to be more effective when given over a few days; (3) patients in ARCTIC received primary prophylaxis against PCP with co-trimoxazole or equivalent and aciclovir prophylaxis to prevent herpes zoster virus reactivation; (4) the use of GCSF as secondary prophylaxis allowed the optimisation of therapy in patients who were struggling to complete the full six cycles of treatment; (5) the centres in the UK that recruited patients are generally large cancer centres and the patients are cared for by haematologists rather than by small community practices.
The ARCTIC trial confirmed some important factors regarding the outcome of therapy in CLL. In particular, patients who achieved a CR had a better outcome, with 93.4% of CR patients being progression-free at 24 months, compared with 65.4% of patients who did not achieve a CR. In addition, patients who achieved the eradication of detectable MRD from their bone marrow 3 months after completing therapy had a 96.4% probability of being progression-free after 24 months, compared with 79.2% for patients not achieving a MRD-negative response. A somewhat surprising finding was that the PFS was similar for patients aged over 65 years and those under 65 years of age, suggesting that the selection of patients for therapy by fitness rather than age is effective. This justifies the selection of patients for FCR and the inclusion of certain groups of patients such as those with renal dysfunction, who also fared reasonably well in ARCTIC and who in most previous series have been excluded from FCR-like therapies. In comparison with historical series of FC given prior to the advent of rituximab, it does appear that the addition of low-dose rituximab to FC with mitoxantrone (FCM-miniR) is better than FC in terms of response and PFS, although it is inferior to FCR. This indicates that low-dose rituximab is clinically active but probably less so than the full conventional dose.
Economic evaluation discussion
In both the within-trial and the decision model analyses, FCM-miniR was found to be associated with negative incremental costs and benefits compared with FCR. Care needs to be taken when interpreting the results of both analyses, given that ICERs have a different meaning when both cost and benefit increments are negative. Typically, a new intervention is found to be more expensive and more effective than the standard-care treatment (with results of probabilistic analyses lying in the north-east quadrant of the cost-effectiveness plane). In such cases, the ICER represents the additional cost required to be spent on the new treatment in order to gain an additional QALY compared with the standard-care treatment. However, in the case of new treatment that is less costly and less effective than the comparator treatment (with results of probabilistic analyses lying in the south-west quadrant of the cost-effectiveness plane), the ICER instead represents the incremental cost saved per QALY lost compared with the standard-care treatment. For example, in the economic analysis FCM-miniR is associated with an ICER of £10,651, indicating that for every £10,651 saved by adopting FCM-miniR, one QALY will be lost compared with FCR. Assuming a WTP of £20,000 per QALY, this means that FCM-miniR is not cost-effective, which may not be immediately clear from the ICER result. For this reason, it is preferable to look at the INB results rather than the ICERs, as interpretation of net benefit results does not depend on the direction of the incremental cost and QALYs. If the INB associated with a new treatment is positive, then that treatment is cost-effective, whereas if it is negative the treatment is not cost-effective. Thus, for the case of the decision model analysis, FCM-miniR is associated with an INB of –£6780, indicating that FCM-miniR is not cost-effective over a lifetime horizon.
Treatments that are both cost- and QALY-decreasing can still be cost-effective because the cost saved can be spent on treatments or interventions elsewhere in the NHS in order to gain additional QALYs, which may outweigh the QALY loss associated with the given intervention. That is, the overall INB of the new treatment compared with the standard-care treatment may be positive (indicating cost-effectiveness) if the cost saving associated with the new treatment is sufficient to outweigh the QALY loss. In the case of the within-trial analysis, FCM-miniR was associated with a mean cost saving of £6619 and a mean QALY loss of –0.056. In this case, the cost saved outweighs the QALYs lost, resulting in a positive INB. However, for the decision model analysis, the cost saving associated with FCM-miniR (£7723) was unable to outweigh the QALY loss (–0.73), resulting in a negative INB (i.e. –0.735 × 20,000 + 7723 = –£6780; equivalent to a net health loss of –0.735 + 7723/20,000 = –0.34 QALYs).
The discrepancy in the short- and long-term cost-effectiveness analyses results (i.e. the fact that FCM-miniR is found to be cost-effective in the within-trial analysis but not in the model lifetime analysis) is attributable to the fact that all cost-savings associated with FCM-miniR occur in the short term (i.e. when therapy costs are incurred). In the model it is assumed that costs in the progression-free and progressed disease states are the same for both treatments, so the benefit of FCM-miniR in terms of reducing treatment costs is contained in the initial trial 24-month period of the model. Over the lifetime analysis, the cost savings associated with FCM-miniR are, therefore, diluted and are no longer sufficient to outweigh the QALY losses associated with the new treatment.
For the within-trial analysis, the results of QALY calculations were not consistent across health utility measures. The base-case EQ-5D analysis found FCM-miniR to be inferior to FCR in terms of QALY production (resulting in a –0.059 QALY decrement), whereas the SF-6D analysis found FCM-miniR to be superior to FCR (resulting in a +0.016 QALY increment). The reason for this discrepancy is unclear. The SF-12 questionnaire used to derive the SF-6D QALYs asks more detailed questions regarding patients’ emotional states, and t-test results showed significant differences using SF-6D at 24 months post randomisation, whereas no significant differences between arms were shown using EQ-5D. This could suggest that the SF-12 form is more sensitive to changes in health states for this population. Nevertheless, despite the difference in expected QALY values between the EQ-5D and SF-6D analyses, both analyses indicate that FCM-miniR is associated with a positive INB value and therefore represents a cost-effective investment for the NHS. Overall, there is some uncertainty around the model results (see Generalisability) which will not have been captured in the deterministic or probabilistic sensitivity analyses. It is unclear in which direction this uncertainty is likely to impact on the results; therefore, some caution should be maintained when drawing conclusions from the model results.
Summary
In summary, we have shown that FCM-miniR does not have a non-inferior CR rate compared with FCR for the frontline therapy of CLL and that, over the lifetime of the patients, FCM-miniR was not found to be cost-effective compared with FCR. Therefore, there is no evidence against FCR remaining the gold-standard treatment for patients with CLL who require therapy and are considered fit for fludarabine-based combinations.
Generalisability
The ARCTIC trial was a randomised Phase IIB rather than a Phase III trial, but it was large and well-powered to show non-inferiority in terms of CR rates. The primary end point CR is associated with outcome in many other studies and was rigorously assessed by three independent assessors who were blinded to the treatment that patients received. In addition, the secondary end points, such as the eradication of MRD, are supportive, which is strongly associated with outcome; and the follow-up of the study, with a median of 35.6 months since randomisation, is mature enough to allow some interpretation of both PFS and OS. The efficacy outcome was consistent across the primary and secondary end points, which validates the conclusions of the trial.
In terms of the health economic assessment, there are some limitations to the analysis. The decision analytic model has several important limitations that should be considered when interpreting the results. These are highlighted below:
-
The model structure is unlikely to be sufficiently detailed to capture all the relevant differences between the two treatments in terms of costs and benefits. In particular, the progression-free state aggregates information on several distinct disease stages that each treatment may impact differently on and, once in the progressed disease state, patients were not permitted to return to the progression-free state, as does occur temporarily in practice.
-
The rate of progression in each arm for a given time point was set to the value given by the calibrated RDM model; each value in time was therefore fixed and this variable was not included in the probabilistic analysis or in the value of information analysis. The results are, therefore, likely to underestimate the uncertainty around the cost-effectiveness results and the associated value of additional information to resolve that uncertainty.
-
The cost and utility values for the progressed disease state are unlikely to be accurate estimates. Owing to a lack of available data, the cost and utility values for the progressed disease state were sourced from the literature; although care was taken to identify the most relevant estimates available, these values are still unlikely to reflect accurately the true cost of progressed disease for CLL patients. In addition, it may be that the cost of progressed disease is not the same across the two arms; however, without any evidence to the contrary the cost of progressed disease was assumed equivalent.
In addition for the within-trial analysis, previous 3-month costs at baseline would have allowed for adjustment in case of higher (or lower) initial costs for patients at the beginning of the study; however, these costs were not collected and were instead assumed to be equivalent between arms.
The question of the value of adding mitoxantrone to FCR has been answered clearly. It also appears that the question of whether lower doses of rituximab are as effective has been answered, although one of the limitations of the trial is that the toxicity of adding mitoxantrone could have confounded the interpretation of the low-dose rituximab question.
A real strength of ARCTIC is that it is the first randomised trial in which FC were given orally in the FCR combination. This has demonstrated that the outcomes for oral FCR appear to be superior to the historical control series where all the drugs were given intravenously.
The patients recruited into ARCTIC were entered at 34 centres throughout the UK. Given the large number of patients recruited and the geographical distribution of the patients, the trial population is very similar to fit patients in the UK generally, and thus the outcome of the trial is generalisable to the whole UK CLL patient population.
We have managed to address the key questions without the need for a larger Phase III trial. A weakness of the trial is that over the past few years, since the design of the trial, there has been an extremely rapid increase in the number of therapies for CLL and this has meant that the interest in chemoimmunotherapy, such as FCR, has weakened in favour of the novel targeted treatments.
Overall evidence
In summary, the ARCTIC trial showed that the combination of FCM-miniR was not non-inferior to FCR and, in fact, that FCM-miniR was significantly inferior in terms of CR. Even though FCM-miniR was less expensive, it was not cost-effective in the long term to use FCM-miniR rather than FCR. Therefore, there is no evidence against FCR remaining the gold-standard therapy for CLL in patients considered fit for fludarabine-based therapy.
Chapter 6 Conclusions
Implications for health care
-
There is no evidence against FCR remaining the gold-standard therapy for CLL.
-
Oral FC and intense supportive care (primary antibiotic prophylaxis, secondary GCSF support, etc.) optimise the delivery of FCR-like therapies.
-
Improved responses (both CR and MRD negativity) are associated with better outcomes, justifying the planned use of MRD negativity by the European Medicines Agency as a surrogate end point for drug approval in CLL.
-
In countries in which the cost of ‘conventional’ dose rituximab is prohibitive, it appears that low-dose rituximab (100 mg per cycle) is active and may be worth testing further.
Recommendations for research
Fludarabine, cyclophosphamide and rituximab remains the gold-standard therapy for frontline CLL and should be used as the ‘standard’ arm in subsequent trials. It is acceptable, maybe even advisable, to allow the use of oral FC in future trials. The surrogate end point of the eradication of MRD is a good prognostic marker for PFS and therefore acceptable in future Phase II CLL trials.
Acknowledgements
Clinical Trials Research Unit staff, University of Leeds
Ms Gillian Booth (co-applicant).
Professor Julia Brown.
Ms Corinne Collett.
Ms Vicky Napp (co-applicant).
Mr David Phillips.
Academic Unit of Health Economics staff, University of Leeds
Professor Claire Hulme.
Professor Chris McCabe (co-applicant).
Trial Steering Committee
Ms Jane Barnard (independent member and patient representative).
Professor Daniel Catovsky (independent member), retired.
Professor Terry Hamblin (independent chairperson until 2011), deceased.
Dr Steve Johnson (independent member and independent chairperson from 2011 onwards), retired.
Data Monitoring and Ethics Committee
Dr Derek Norfolk (independent member), Leeds Teaching Hospitals NHS Trust.
Professor Chris Twelves (independent chairperson), Leeds Teaching Hospitals NHS Trust.
Dr Christina Yap (independent member), University of Birmingham.
Trial Management Group
Mr Arthur Graley (patient representative).
National Cancer Research Institute Chronic Lymphocytic Leukaemia Subgroup Committee
Dr David Allsup, Hull and East Yorkshire Hospitals NHS Trust.
Mr Garry Bisshopp (patient representative).
Dr Adrian Bloor, Christie Hospital NHS Foundation Trust.
Professor Daniel Catovsky, retired.
Dr Claire Dearden, The Royal Marsden NHS Foundation Trust.
Professor Steve Devereux, King’s College Hospital NHS Foundation Trust.
Dr Caroline Duncan, NHS Grampian.
Dr Andrew Duncombe, University Hospital Southampton NHS Foundation Trust.
Professor Martin Dyer, University Hospitals of Leicester NHS Trust.
Dr Chris Fegan, Cardiff and Vale University Health Board.
Dr George Follows, Cambridge University Hospitals NHS Foundation Trust.
Dr Francesco Forconi, University Hospital Southampton NHS Foundation Trust.
Dr Chris Fox, Nottingham University Hospitals NHS Trust.
Professor John Gribben, Barts Health NHS Trust.
Dr Ben Kennedy, University Hospitals of Leicester NHS Trust.
Dr Helen McCarthy, The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust.
Dr Alison McCaig, NHS Greater Glasgow and Clyde.
Dr Scott Marshall, Gateshead Health NHS Foundation Trust.
Dr Melanie Oates, University of Liverpool.
Dr Shankara Paneesha, Heart of England NHS Foundation Trust.
Dr Piers Patten, Guy’s and St Thomas’ NHS Foundation Trust.
Dr Chris Pepper, Cardiff and Vale University Health Board.
Professor Andrew Pettitt, Royal Liverpool and Broadgreen University Hospitals NHS Trust.
Dr Chris Pocock, East Kent Hospitals University NHS Foundation Trust.
Dr Guy Pratt, Heart of England NHS Foundation Trust.
Mr John Reeve (patient representative).
Dr Anna Schuh, Oxford University Hospitals NHS Trust.
Dr John Strefford, University of Southampton.
Dr Elisabeth Vandenberghe, St James’s Hospital, Dublin.
Dr Renata Walewska, The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust.
Participating centres
We would like to thank all research staff at participating centres who provided and cared for trial participants and collected trial data. Especial thanks go to the Principal Investigators:
Dr David Allsup, Castle Hill Hospital, Hull.
Dr Norbert Blessing, Great Western Hospital, Swindon.
Dr Adrian Bloor, Christie Hospital, Manchester.
Dr Ranjit Dasgupta, Arrowe Park Hospital, Upton.
Dr Claire Dearden, Royal Marsden Hospital, London.
Dr Corinne DeLord, Princess Royal University Hospital and Queen Elizabeth Hospital, Woolwich.
Professor Steve Devereux, King’s College Hospital, London.
Dr Andrew Duncombe, Southampton General Hospital, Southampton.
Professor John Gribben, St Bartholomew’s Hospital, London.
Dr Claire Hall, Harrogate District Hospital, Harrogate.
Dr Michael Hamblin, Colchester General Hospital, Colchester.
Dr Earnest Heartin, Glan Clwyd Hospital, Rhyl.
Dr Christopher Knechli, Royal United Hospital, Bath.
Dr Scott Marshall, Queen Elizabeth Hospital, Gateshead and Sunderland Royal Hospital, Sunderland.
Dr Jane Mercieca, Epsom St Helier University Hospital NHS Trust, Epsom and Carshalton.
Professor Donald Milligan, Birmingham Heartlands Hospital and Good Hope Hospital, Birmingham.
Dr Paul Moreton, Pinderfields Hospital, Wakefield.
Dr Philip Mounter, University Hospital of North Tees, Stockton-on-Tees.
Dr Jim Murray, Queen Elizabeth Hospital, Birmingham.
Dr Jeff Nielson, Russells Hall Hospital, Dudley.
Dr Nicki Panoskaltsis, Northwick Park Hospital, London.
Dr Jonathan Pattinson, Buckinghamshire Hospitals NHS Trust, Wycombe and Stoke Mandeville.
Professor Andrew Pettitt, Royal Liverpool University Hospital, Liverpool.
Dr Malgorzata Rokicka, Royal Blackburn Hospital, Blackburn.
Dr Kate Ryan, Manchester Royal Infirmary, Manchester.
Dr Anna Schuh, Oxford Cancer and Haematology Centre, Oxford.
Dr Fiona Scott, Western General Hospital, Edinburgh.
Dr Jim Seale, Ysbyty Gwynedd Hospital, Bangor.
Dr John Tucker, Borders General Hospital, Melrose.
Dr Alastair Whiteway, Southmead Hospital, Bristol.
Participants
Thank you to all trial participants for their essential contribution to the trial.
Contribution of authors
Dena R Howard (Principal Statistician, Leeds Institute for Clinical Trials Research at the University of Leeds) had overall responsibility for the statistics and research methodology.
Talha Munir (Consultant Haematologist, St James’s University Hospital) and Abraham Varghese (Consultant Haematologist, St James’s University Hospital) contributed to the conduct and monitoring of the trial, clinical queries and interpretation of data, and Talha Munir updated the review of the literature.
Lucy McParland (Senior Statistician at the Leeds Institute for Clinical Trials Research, University of Leeds) and Dena Howard conducted the statistical analysis and interpreted the results.
Andy C Rawstron (Consultant Clinical Scientist, St James’s University Hospital) analysed and interpreted the laboratory data.
Anna Chalmers (Head of Trial Management, Leeds Institute for Clinical Trials Research at the University of Leeds) oversaw and co-ordinated the running of the trial, acquisition of data, trial monitoring, safety reporting and GCP requirements.
Dena Howard and Peter Hillmen (Consultant Haematologist, Leeds Institute of Cancer and Pathology at the University of Leeds), along with Walter M Gregory (Professor of Statistical Methodology in Clinical Trials and Cancer Division Director, Leeds Institute for Clinical Trials Research at the University of Leeds), designed the randomised controlled trial and were involved in obtaining funding.
For the health-economics analysis, John L O’Dwyer (Research Officer, Leeds Institute of Health Sciences at the University of Leeds) was responsible for the within-trial economic analysis, Alison Smith (Research Fellow, Leeds Institute of Health Sciences at the University of Leeds) was responsible for the decision analytical model analysis and Roberta Longo (Lecturer, Leeds Institute of Health Sciences at the University of Leeds) was responsible for the development of the decision analytical model.
Alexandra Smith (Head of Trial Management, Leeds Institute for Clinical Trials Research at the University of Leeds) contributed to the trial design, protocol development and implementation, and co-ordination of data acquisition. All authors contributed to the writing of the report and had the opportunity to critically revise it.
The ARCTIC trial was conceived by Peter Hillmen, who had overall responsibility for the trial.
Publications
Hillmen P, Milligan D, Schuh A, McParland L, Chalmers A, Munir T, et al. Results of the randomised phase II NCRI ARCTIC (Attenuated dose Rituximab with ChemoTherapy In CLL) trial of low dose rituximab in previously untreated CLL). Presented at the 55th ASH Annual Meeting and Exposition, New Orleans, LA, December 2013.
Munir T, Cohen D, Pocock C, Rawstron A, McParland L, Chalmers A, et al. Oral FCR induces higher complete remission rates and MRD negativity in untreated CLL than previous reports of intravenous therapy: Combined results of the NCRI ADMIRE and ARCTIC trials. Paper presented at the 19th Congress of the European Haematology Association, Milan, June 2014.
Munir T, Cohen D, Milligan D, Schuh A, McParland L, Chalmers A, et al. Results of the randomised phase II NCRI ARCTIC (Attenuated dose Rituximab with ChemoTherapy In CLL) trial of low dose rituximab in previously untreated CLL). Paper presented at the British Society for Haematology (BSH) 54th Annual Scientific Meeting, Birmingham, April 2014.
Howard DR, Munir T, McParland L, Rawstron AC, Milligan D, Schuh A, et al. Results of the randomized phase IIB ARCTIC trial of low-dose rituximab in previously untreated CLL [published online ahead of print May 2 2017]. Leukaemia 2017.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Howard DR, Munir T, McParland L, Rawstron AC, Milligan D, Schuh A, et al. Results of the randomized phase IIB ARCTIC trial of low-dose rituximab in previously untreated CLL. Leukaemia 2017. http://dx.doi.org/10.1038/leu.2017.96.
- Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do K-A, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975-80. http://dx.doi.org/10.1182/blood-2008-02-140582.
- Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 2011;117:3016-24. http://dx.doi.org/10.1182/blood-2010-08-304683.
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164-74. http://dx.doi.org/10.1016/S0140-6736(10)61381-5.
- Fischer K, Bahlo J, Fink AM, Bucsch R, Bottcher S, Mayer J, et al. Extended Follow up of the CLL8 Protocol, a Randomized Phase-III Trial of the German CLL Study Group (GCLLSG) Comparing Fludarabine and Cyclophosphamide (FC) to FC Plus Rituximab (FCR) for Previously Untreated Patients With Chronic Lymphocytic Leukemia (CLL): Results On Survival, Progression-Free Survival, Delayed Neutropenias and Secondary Malignancies Confirm Superiority of the FCR Regimen n.d.
- Nguyen DT, Amess JA, Doughty H, Hendry L, Diamond LW. IDEC-C2B8 anti-CD20 (Rituximab) immunotherapy in patients with low-grade non-Hodgkin’s lymphoma and lymphoproliferative disorders: evaluation of response on 48 patients. Eur J Haematol 1999;62:76-82. http://dx.doi.org/10.1111/j.1600-0609.1999.tb01725.x.
- Almasri NM, Duque RE, Iturraspe J, Everett E, Braylan RC. Reduced expression of CD20 antigen as a characteristic marker for chronic lymphocytic leukemia. Am J Hematol 1992;40:259-63. http://dx.doi.org/10.1002/ajh.2830400404.
- Keating M, O’Brien S. High-dose rituximab therapy in chronic lymphocytic leukemia. Seminars Oncol 2000;27:86-90.
- O’Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 2001;19:2165-70.
- Byrd JC, Murphy T, Howard RS, Lucas MS, Goodrich A, Park K, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 2001;19:2153-64.
- Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol 2006;177:7435-43. http://dx.doi.org/10.4049/jimmunol.177.10.7435.
- Aue G, Lindorfer MA, Beum PV, Pawluczkowycz AW, Vire B, Hughes T, et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica 2010;95:329-32. http://dx.doi.org/10.3324/haematol.2009.012484.
- Zent CS, Taylor RP, Lindorfer MA, Beum PV, LaPlant B, Wu W, et al. Chemoimmunotherapy for relapsed/refractory and progressive 17p13-deleted chronic lymphocytic leukemia (CLL) combining pentostatin, alemtuzumab, and low-dose rituximab is effective and tolerable and limits loss of CD20 expression by circulating CLL cells. Am J Hematol 2014;89:757-65. http://dx.doi.org/10.1002/ajh.23737.
- Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: Results from 136 patients from the French autoimmunity and rituximab registry. Arthritis Rheum 2010;62:2458-66. http://dx.doi.org/10.1002/art.27541.
- Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: The randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222-33. http://dx.doi.org/10.1002/art.27233.
- Smolen JS, Keystone EC, Emery P, Breedveld FC, Betteridge N, Burmester GR, et al. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 2007;66:143-50. http://dx.doi.org/10.1136/ard.2006.061002.
- Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor. London: NICE; 2010.
- Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica 2007;92:1695-8.
- Barcellini W, Zaja F, Zaninoni A, Imperiali FG, Battista ML, Di Bona E, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood 2012;119:3691-7. http://dx.doi.org/10.1182/blood-2011-06-363556.
- Visentini M, Ludovisi S, Petrarca A, Pulvirenti F, Zaramella M, Monti M, et al. A phase II, single-arm multicenter study of low-dose rituximab for refractory mixed cryoglobulinemia secondary to hepatitis C virus infection. Autoimmunity Rev 2011;10:714-19. http://dx.doi.org/10.1016/j.autrev.2011.04.033.
- Wilder DD, Ogden JL, Jain VK. Efficacy of fludarabine/mitoxantrone/dexamethasone alternating with CHOP in bulky follicular non-Hodgkin’s lymphoma. Clin Lymphoma 2002;2:229-37. http://dx.doi.org/10.3816/CLM.2002.n.004.
- Zinzani P, Magagnoli M, Moretti L, Battista R, Ronconi F, De Renzo A, et al. Fludarabine-based chemotherapy in untreated mantle cell lymphomas: an encouraging experience in 29 patients. Haematologica 1999;84:1002-6.
- Bosch F, Ferrer A, Villamor N, González M, Briones J, González-Barca E, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res 2008;14:155-61. http://dx.doi.org/10.1158/1078-0432.CCR-07-1371.
- Bosch F, Abrisqueta P, Villamor N, Terol MJ, González-Barca E, Ferra C, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol 2009;27:4578-84. http://dx.doi.org/10.1200/JCO.2009.22.0442.
- Hillmen P, Cohen DR, Cocks K, Pettitt A, Sayala HA, Rawstron AC, et al. A randomized phase II trial of fludarabine, cyclophosphamide and mitoxantrone (FCM) with or without rituximab in previously treated chronic lymphocytic leukaemia. Br J Haematol 2011;152:570-8. http://dx.doi.org/10.1111/j.1365-2141.2010.08317.x.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute – Working Group 1996 Guidelines 2008;111:5446-56. http://dx.doi.org/10.1182/blood-2007-06-093906.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Hallek M, Fingerle-Rowson G, Fink A-M, Busch R, Mayer J, Hensel M, et al. Immunochemotherapy With Fludarabine (F), Cyclophosphamide (C), and Rituximab (R) (FCR) Versus Fludarabine and Cyclophosphamide (FC) Improves Response Rates and Progression-Free Survival (PFS) of Previously Untreated Patients (pts) With Advanced Chronic Lymphocytic Leukemia (CLL) 2008;112.
- Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 2004;172:3280-8. http://dx.doi.org/10.4049/jimmunol.172.5.3280.
- Kay R. Issues in Non-Inferiority Trials. 2009.
- O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-56. http://dx.doi.org/10.2307/2530245.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- British National Formulary . Drug costs from the BNF. 67th edition [Internet]. British Medical Association and The Royal Pharmaceutical Society of Great Britain 2013. www.bnf.org.uk (accessed 16 December 2014).
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons; 1987.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: John Wiley and Sons; 2002.
- Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess 2001;5:1-79. http://dx.doi.org/10.3310/hta5120.
- Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987;82:171-85. http://dx.doi.org/10.1080/01621459.1987.10478410.
- Gregory WM, Richards MA, Slevin ML, Souhami RL. A mathematical model relating response durations to amount of subclinical resistant disease. Cancer Res 1991;51:1210-16.
- Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 2012;30:980-8. http://dx.doi.org/10.1200/JCO.2011.36.9348.
- Woods B, Hawkins N, Dunlop W, O’Toole A, Bramham-Jones S. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in england and wales: a cost–utility analysis. Value Health 2012;15:759-70. http://dx.doi.org/10.1016/j.jval.2012.03.1389.
- Beusterien K, Davies J, Leach M, Meiklejohn D, Grinspan J, O’Toole A, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-50.
- How Have Mortality Rates by Age Changed Over the Last 50 Years?. 2013.
- Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010;28:1749-55. http://dx.doi.org/10.1200/JCO.2009.25.3187.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8:1-103. http://dx.doi.org/10.3310/hta8310.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation (Handbooks for Health Economic Evaluation). New York, NY: Oxford University Press; 2006.
- Cancer Research UK . Data Table: Cancer Cases and Rates by Country in the UK 2014 n.d. http://publications.cancerresearchuk.org/publicationformat/formatstats/dtinccountries.html (accessed 16 December 2014).
- Rituximab for the First-line Treatment of Chronic Lymphocytic Leukaemia. NICE; 2009.
Appendix 1 Serious adverse event listings
| Treatment received | MedDRA system organ class | SAE ID number | SAE medical description | Country | Age at randomisation, years | Sex | SAE case description | Seriousness criteria |
|---|---|---|---|---|---|---|---|---|
| FCR | Blood and lymphatic system disorders | N00014/00028/002 | Neutropenia | UK | 67 | Male | Neutropenia | Required/prolonged hospitalisation |
| FCR | Blood and lymphatic system disorders | N00076/00187/001 | Platelet count decreased | UK | 65 | Male | Platelets already low prior to starting treatment on trial (Professor Hillmen aware) but further decrease has prompted a halt to chemotherapy. Also halved owing to Coombs-negative haemolytic anaemia. F-Up 30 September 14 ?ITP ?chemotherapy-induced hypoplasia continues to be observed | Jeopardised patient/required intervention to prevent one of the above |
| FCR | Blood and lymphatic system disorders | N00080/00032/001 | Infection | UK | 55 | Male | Anaemic and very tired and nauseated, poor oral intake. Admitted for 3-unit blood transfusion and i.v. antiemetics and fluids. Hb post 3 units of blood = 8; 1 day later Hb was 7.9. Follow-up report: prolonged hospital admission to ITU. Recurrent sepsis requiring ionotropes and invasive ventilation. Followed by fungal liver abscess; died despite appropriate fungal treatment | Required/prolonged hospitalisation/patient died/life-threatening/persistent or significant disability/incapacity |
| FCR | Blood and lymphatic system disorders | N00098/00017/001 | Extremely low Hb | UK | 50 | Female | Came for end-of-therapy visit at day 23 of cycle 6. Looked extremely pale, slightly yellow, felt faint with effort of walking. Breathless, BP 98/P134. Hb found to be 3.9 g/dl. Results awaited | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCR | Blood and lymphatic system disorders | N00098/00017/002 | Low Hb: 5.9 g/dl on Pentra | UK | 50 | Female | Pale, breathless on exertion, Hb of 7.5 g/dl on main lab. Plan: to transpose 3 units of blood | Required/prolonged hospitalisation |
| FCR | Blood and lymphatic system disorders | N00114/00088/001 | Persistent neutropenia related to chemotherapy | UK | 72 | Male | Patient has had five cycles. He has had persistent neutropenia. Upon discussion with Chief Investigator, final course to be omitted. D/W Professor Hillmen 1 November 2011 decision to stop treatment | Jeopardised patient/required intervention to prevent one of the above |
| FCR | Blood and lymphatic system disorders | N00175/00155/001 | Anaemia | UK | 61 | Female | Admitted for a planned hospital visit for a 4-unit blood transfusion. Additional information: not reported at time of event as only admitted to ward; no day case space available | Required/prolonged hospitalisation |
| FCR | Blood and lymphatic system disorders | N00230/00033/002 | Myelodysplasia: RAES | UK | 61 | Female | Anaemia and thrombocytopenia | Persistent or significant disability/incapacity/jeopardised patient/required intervention to prevent one of the above |
| FCR | Gastrointestinal disorders | N00050/00120/001 | Diarrhoea and vomiting | UK | 65 | Male | Admitted with diarrhoea and vomiting. Plan for patient to stay overnight. Outcome not known yet | Required/prolonged hospitalisation |
| FCR | Gastrointestinal disorders | N00098/00060/002 | Nausea and vomiting | UK | 52 | Male | Admitted with nausea and vomiting post chemotherapy | Required/prolonged hospitalisation |
| FCR | Gastrointestinal disorders | N00114/00088/003 | Gastroenteritis | UK | 72 | Male | Diarrhoea and vomiting, abdominal pain. Stool isolated C. diff (non-toxin variety). Discharged 2 April 2012 | Required/prolonged hospitalisation |
| FCR | Gastrointestinal disorders | N00349/00035/001 | Nausea | UK | 66 | Female | Presented with uncontrolled nausea. Reduced intake of oral fluids and difficulty taking oral medication. Follow-up report: discharged from hospital 17 March 2011 | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00014/00028/004 | Infection/anaemia | UK | 67 | Male | General weakness, lethargy, headache, sweating, sore throat, cough (expectorating). Follow-up report: confirmed swine flu on 7 January 2011 | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00014/00028/005 | Anaemia grade 4 | UK | 67 | Male | General weakness/fatigue, lethargy, anaemia, cough (expectoration). Follow-up report – Hb of 4.6. Discharged 10 February 2011 with Hb of 10.8 g/dl (grade 1) | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00014/00029/001 | Rigors | UK | 53 | Female | High temperature and shaking | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00098/00019/001 | Pyrexial 38.2 post first dose of rituximab | UK | 67 | Male | Reacted to rituximab with vomiting and cold sweat. Given hydrocortisone, piriton and ondansetron. Recovered. One infusion finished, vomited again and had rigor. Settled again but then spiked temperature of 38.2 °C and was admitted for observation, i.v. fluids, i.v. antibiotics. Note: this was day 1, cycle 1 of split rituximab. This was day 1, cycle 1 for the patient, who is having first dose of rituximab split over 2 days (has commenced second rituximab day today). Therefore received on 100 mg rituximab | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00114/00055/001 | Fever and rigors | UK | 65 | Male | Fever, increased CRP, probable delayed rituximab reaction, no evidence of sepsis. Chest radiograph normal, urine microbiology negative. Follow-up report: admitted to hospital 13 January 2011, treated with prophylactic i.v. antibiotics and i.v. hydrocortisone. Discharged: 18 January 2011 | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00173/00011/001 | Hypoxia | UK | 72 | Female | Developed breathlessness 15 April 2010 during rituximab infusion. Symptoms improved with oxygen therapy. Oxygen saturations are 84% on room air. Awaiting V/Q scan and chest radiography ?Chest infection ?Pulmonary embolism. CTPA 21 April 2010 showed no evidence of pulmonary | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00173/00137/001 | Vomiting | UK | 59 | Female | Post chemotherapy nausea and vomiting requiring admission for antiemetics and i.v. fluids | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00218/00168/001 | Fever and non-specifically unwell | UK | 63 | Male | See above: admitted for antibiotics and assessment, no evidence of TLS. Dramatic reduction in WBC? All chemotherapy-related – probably | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00319/00125/001 | Allergic reaction | UK | 61 | Female | Pyrexia. Rigor. Hypotension | Required/prolonged hospitalisation |
| FCR | General disorders and administration site conditions | N00391/00194/002 | Probable rituximab reaction | UK | 74 | Male | Emergency admission with fever, cough and vomiting one day after chemotherapy on 9 January 2013. (See attached discharge summary.) Required emergency medical admission with fever, cough and vomiting. Had similar episode following fourth cycle of chemotherapy on 13 December 2012 and treatment was terminated at that time. On this occasion, received pre-medication with hydrocortisone, piriton and paracetamol prior to receiving rituximab and commencing oral FC. On admission looked reasonably well with a good colour and well perfused. Temperature 39.4 °C. Pulse 106 per minute. BP 119/60. Respiratory rate 20 per minute. Saturations 93% breathing room air. Had no lymphadenopathy. Heart sounds were normal and there were a few crackles at both lung bases. The abdomen was soft and non-tender and no organs or masses felt. Hb 12.7 g/dl, WBC 4.9 × 109/l, platelets 163 × 109/l. CRP < 5 mg/l, lactate 2.6 mmol/l, routine biochemistry otherwise normal. Chest radiograph from 9 January 2013 was thought to be clear but the clinical report has suggested minor patchy change around the upper pole of the right hilum possibly representing early infection. Blood culture was negative. Was treated as according to sepsis six protocol with i.v. fluids and antibiotics consisting of Co-Amoxiclav and Clarithromycin. Developed hypotension and SIRS score increased from 1 to 2. With i.v. fluids blood pressure recovered and SIRS score fell to 0. Was well enough to be discharged home on the following medication: Aciclovir 400 mgs twice daily, paracetamol 1 g as required. Chemotherapy was discontinued. We have decided to avoid any further rituximab in the future. We plan one further final course of chemotherapy with oral FC plus oral Mesna to protect against possible chemotherapy-induced cystitis. Remains on Pentamidine inhalations on a monthly basis as prophylaxis against PCP and this will continue for up to 6 months after last dose of fludarabine | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00014/00028/003 | Febrile neutropenia | UK | 67 | Male | Neutropenia grade 4, pyrexia 38 °C | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00014/00112/001 | Shingles | UK | 62 | Male | Itchy painful rash | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00040/00056/001 | Neutropenic sepsis | UK | 59 | Male | Admitted 22 February 2011. Neutrophils 0.09 10/l on 24 February 2011. Discharged 28 February 2011; neutrophils 1.10 10/l | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00046/00185/001 | Infection with grade 4 neutrophils | UK | 63 | Male | Pyrexial with cough and signs of infection, last few days at home. Attended 74 day ward for routine i.v. immunoglobulins today. Neutropenic and septic therefore admitted for tazocin and gentamicin. Blood cultures, viral screen and chest radiograph taken – results pending. Additional information: neutrophils 0.0 × 109/l, Hb 11.1 g/dl, phs 174. 31 December 2012. Follow-up SAE viral screen, chest radiography, blood cultures – negative. Given i.v. tazocin + gentamicin for 5 days. Neutropenic so given × 4 days G-GP, GP; CSF after this plus 7 days of oral antibiotics. Discharged 4 January 2013 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00050/00041/001 | Febrile neutropenia | UK | 69 | Female | Pyrexia, feeling unwell. Follow-up report: neutrophil counts recovered on 14 December 2010. However, patient remained hospitalised for respiratory infection | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00098/00001/001 | Neutropenic sepsis | UK | 41 | Male | Patient felt shivery. Temperature checked at home – 38 °C. Rang ward and was admitted. Neutrophils 0.28 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00098/00060/001 | Fever – no focus identified | UK | 52 | Male | Patient admitted feeling hot and cold, decreased eating and drinking. Generally unwell for 4/7 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00098/00060/003 | Probable late PCP/PJP | UK | 52 | Male | Admitted with shortness of breath on exertion and cough. Also vomiting and nausea. Patient noted to be lymphopenic with lymphocyte count of 0.74. CT scan was suggestive of PCP or atypical infection. Information on cause and outcome to follow. Additional information CT scan on 2 June 2012 stated that appearances were felt to be pneumocystis. Clinically appeared to be PCP and all other microbiology performed showed no other organism. Patient commenced on co-trimoxazole prophylactically | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00099/00084/001 | Pyrexia ?neutropenic sepsis | UK | 59 | Female | Pyrexia, patient feeling unwell | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00099/00105/001 | Neutropenic sepsis | UK | 68 | Male | Admitted 2 days after the chemotherapy with sudden drop of WBCs and neutrophils. He is on broad spectrum antibiotics | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00106/00130/001 | Neutropenic sepsis | UK | 70 | Male | Fever/rigors. Now apyrexial, on tazocin + gentamicin: gentamicin now stopped. Completed 5 days tazocin, now on oral ciprofloxacin and GCSF – discharged home | Life-threatening/required/prolonged hospitalisation |
| FCR | Infections and infestations | N00106/00130/002 | Neutropenic sepsis | UK | 70 | Male | Presented with fever + neutropenia – responded to i.v. antibiotics. Neutrophils 0.3 on 27 April 2012 – recovered to 1.5 1 May 2012 – Discharged home | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00106/00179/001 | Pyrexial, neutropenic | UK | 68 | Female | Nausea and vomiting. ?chest infection. Additional information received by fax 6 August 2012: rituximab 600 mg given i.v. statutory dose on 26 July 2012. NB: patient had rituximab given over 2 days = 700 mg in total. Follow-up report: Neutrophils recovered to 1.1 on 7 August 2012. Chest radiograph showed Lt base atelectasis – no growth in urine/stool or blood | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00106/00179/003 | Neutropenic sepsis | UK | 68 | Female | Neutropenic sepsis. Drug reaction, facial swelling and itchy rash. Fever 40 °C. Follow-up report. Neutropenic sepsis. 0.0 on 23 October 2012 – recovered to 0.9 on 27 October 2012. No sepsis found. Drug reaction – itchy rash and pyrexia. Resolved by 27 October 2012. Patient discharged 27 October 2012 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00114/00067/001 | Neutropenic sepsis | UK | 61 | Female | Increasingly unwell for 4 days prior to admission with high temperature. Temperature on admission: 38 °C. Neutrophils 1.2 CRP 13. Follow-up report: increasingly unwell. Temperature 38 °C, neutrophils 0.2 CRP 13 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00114/00067/002 | Neutropenic sepsis? cause | UK | 61 | Female | Febrile 38.1 on 17 July 2011. Nausea/vomited × 2 in 24 hours. Decreased appetite | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00114/00067/003 | ?Neutropenic sepsis | UK | 61 | Female | Temperature 38.1 °C, nausea and vomiting, decreased appetite | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00114/00067/004 | Neutropenic sepsis | UK | 61 | Female | Neutropenic sepsis. Recently noticed infection under arm. GP prescribed Flucloxacillin. Not neutropenic + pyrexial. Admitted to hospital 23 January 2012 for i.v. antibiotics. Follow-up report received: admitted 23 January 2012 with febrile neutropenia (having been on Flucloxacillin for 3 days for axillary gland infection).Treated with i.v. tazocin + GCSF. Subsequent rise in neutrophils. Discharged home 27 January 2012 on oral antibiotics | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCR | Infections and infestations | N00114/00088/002 | Neutropenic sepsis | UK | 72 | Male | 4-day history of dysuria and left loin pain. Follow-up report: admitted following 4-day history of dysuria, loin pain and haematuria. Found to be neutropenic. Discharged home 3 January 2011 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00153/00026/001 | Infection | UK | 53 | Male | Patient admitted with fever 37.8 °C, feeling generally unwell. Not neutropenic. Treated for 24 hours with i.v. antibiotics, then discharged home | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00153/00050/001 | Chest infection | UK | 53 | Male | Seen in triage unit on 24 February 2011 with SOB, commenced on oral antibiotics. Symptoms worsened. Admitted on 27 February 2011 with pyrexia, productive cough, commenced i.v. antibiotics. Follow-up report: patient admitted via triage unit with shortness of breath, productive cough, treated on i.v. antibiotics | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00173/00011/003 | Lower respiratory tract inf. | UK | 72 | Female | Cough and pyrexia, not neutropenic | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00218/00168/002 | Neutropenic sepsis | UK | 63 | Male | Admitted neutropenic sepsis. Temperature: 38.4 °C. Neutropenic. Painful area around anus. Awaiting surgical review. Started imipenem | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00230/00033/001 | Neutropenic sepsis | UK | 61 | Female | Temperature: 38.8 °C. Loose stools, decreased appetite. Follow-up report: allergic reaction to meropenum/Tazocin (penicillin) | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00231/00174/001 | Febrile, rigors, ?septic. Episodes of diarrhoea for 2 days | UK | 67 | Male | 22 August 2012, 19.50, arrived in A&E complaining of feeling unwell and high temperature 38.1 °C. Triage assessment BP 134/80, Temperature 36.6 °C, O2 sats 97%. Admitted to acute medical unit. i.v. antibiotics started. No obvious focus of sepsis identified. Discharged on 26 August 2012 with oral Ciprofloxacin | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00231/00174/002 | Febrile illness (pyrexia, rigors) | UK | 67 | Male | Admitted via A&E 27/8/12. Complained of feeling unwell, malaise, T 37.9 °C. Treated with i.v. teicoplanin (Targocid, Sanofi). Follow-up report: CRP remains high, unwell and malaise, no site of infection. i.v. antibiotics changed to meropenam. CT – NAD. Diabetes unstable blood sugars. Now discharged on insulin, 80 mg Glucazide, Novami × 30 14/g units. See attached CRP report | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00231/00174/003 | Neutropenic sepsis | UK | 67 | Male | Pyrexia, neutropenic (0.9). Presumed neutropenic sepsis, site of infection unknown. CRP 19.7. Pyrexia at home 38.9 °C on arrival to A&E 37.8 °C. Treated with Tazocin and Gentamicin and Meropenem i.v. Discharged from hospital to home 18 October 2012 | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00255/00138/001 | Neutropenic sepsis | UK | 61 | Female | Patient was admitted to hospital with chief complaint of headache and temperature of 39.1 °C. Developed neutropenic sepsis and low haemoglobin. Commenced on i.v. antibiotics and received 4 units of blood. Day 20 post chemotherapy | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00255/00166/001 | Febrile neutropenia | UK | 66 | Female | Admitted with left earache, fevers, rigors and feeling unwell | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00255/00166/002 | Neutropenic sepsis | UK | 66 | Female | Temperature 39.6 °C, rigors, neutrophils 0.1, haemoptysis | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00280/00008/001 | Neutropenic sepsis | UK | 60 | Male | Persistent pyrexia, feeling generally unwell – admitted 31 May 2010 – i.v. antibiotics given. Blood culture – negative after 5 days’ incubation | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00319/00125/002 | Febrile neutropenia | UK | 61 | Female | Temperature at home 38.8° C, admitted to hospital, on admission neutrophils 0.04. Noted rash at home improved on admission | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00361/00123/002 | Neutropenic sepsis | UK | 67 | Female | Pyrexial (39.5 at home)? Source. Full infection screen completed – all specimens negative. Commenced i.v. Meropenem 1 g TDS. Pyrexia settled 7 July 2012 at 0.2 onwards. Patient remains apyrexial at present. Still no cause identified | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00391/00111/001 | Neutropenic sepsis | UK | 75 | Male | GP referral. 2-day history of feeling unwell, dizzy, breathless, rash over back and arms. Temperature 38.5 °C on admission. Neutrophils 0.8. Commenced i.v. antibiotics and GCSF. Follow-up report received: please see attached discharge summary to up-date SAE information. Treated with Gentamicin and Tazocin, and Co-trimoxazole was discontinued as the most likely culprit for rash. Also received Filgrastim to stimulate neutrophil recovery. Fever and rash settled then rash appeared to worsen on legs. Were set to send home on 9 November 2011 but developed unexplained fever which settled the following day. By this time was switched to oral Ciprofloxacin. Neutrophils on discharge had improved to 2.0 × 10/l. Plan to keep off Co-trimoxazole and considering rechallenging when rash is fully settled. Will give prophylactic Pegfilgrastim to try to prevent further admissions with neutropenic sepsis | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCR | Infections and infestations | N00391/00194/001 | ?Neutropenic sepsis. ?Viral infection. ?Drug-induced gastritis | UK | 74 | Male | Admitted via A&E with 1-day history of nausea/vomiting and sore throat. Neutrophils on 12 December 2012 1.1. Temperature 37.3 °C at home but increased to 40.0 °C on admission. Shaking limbs and sore head. Follow-up report: admitted 13 December 2012. Fever of unknown origin. He has already shown clinical remission to three cycles of treatment. Discharged 15/12/12. On admission he was febrile (38.3 °C) with pulse 95 per minute, BP 135/70, saturations 94% breathing air. SIRS score 0. There was no evidence of any oral thrush and no lymphadenopathy. Heart sounds normal, chest clear, abdomen soft and non-tender with no organs or masses felt. Haemoglobin on admission 12.4 g/dl, WBC 3.3 × 109/l, neutrophils 2.8 × 109/l, platelets 139 × 109/l. Routine biochemistry was normal other than a minor rise in his creatinine to 129 µmol/l. Cultures of blood/urine negative. Treated with i.v. fluids and received single dose of paracetamol. Temperature settled nicely and did not require antibiotics. Cause for fever uncertain but possibly reaction to 4th cycle of chemotherapy which was discontinued after just 24 hours. Discharged home taking maintenance acyclovir 400 mg twice daily. Will be reviewed at Macmillan Centre on 24/12/12 with an up-to-date blood test. Hope to resume the chemotherapy as planned providing this is tolerated | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00698/00198/001 | Febrile neutropenia | UK | 71 | Male | Neutrophils – 0.1 on admission to local hospital, commenced on i.v. antibiotics. Pyrexial on admission. Follow-up: SAE G4 febrile neutropenia – admitted to local hospital on 1 January 2013. Treated successfully i.v. antibiotics. No source of infection found but urinalysis positive for nitrates | Jeopardised patient/required intervention to prevent one of the above/required/prolonged hospitalisation |
| FCR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N00098/00002/001 | Squamous cell carcinoma – two lesions: lower back and central chest | UK | 63 | Male | Central chest lesion seen 20 August 2010 and initially thought to be Giant Molluscum. Increased in size over next few weeks. Patient went to GP – referred to dermatologist. Biopsy done and also lesion on back noted and excised. Biopsy revealed both to be SCC | Jeopardised patient/required intervention to prevent one of the above |
| FCR | Psychiatric disorders | N00349/00104/002 | Psychotic episode | UK | 69 | Male | Catatonic. Follow up received 24 February 2012 – Discharged from hospital 10 January 2012 on diazepam. 24 February 2012 no longer on medication for his anxiety | Required/prolonged hospitalisation |
| FCR | Skin and subcutaneous tissue disorders | N00349/00070/001 | Rash | UK | 63 | Male | Reaction to Rituximab infusion | Required/prolonged hospitalisation |
| FCR | Skin and subcutaneous tissue disorders | N00353/00122/001 | Erythemous rash grade 3 | UK | 72 | Male | Rash covering all of body and face. Skin red, inflamed and hot to touch. | Required/prolonged hospitalisation |
| FCM-miniR | Blood and lymphatic system disorders | N00009/00127/001 | Pleural effusion and pulmonary embolism | UK | 57 | Male | Increased shortness of breath since day 7 of cycle 1 of FCM treatment. No improvement despite increasing asthma inhalers. Became very breathless and admitted on 31 December 2011. CTPA on 3 January 2012 confirmed huge bilateral pleural effusion and pulmonary embolisms in right upper lobe artery | Required/prolonged hospitalisation |
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/001 | Neutropenic sepsis | UK | 65 | Female | Patient admitted with a low-grade pyrexia and neutropenic at 0.2. Discharged from hospital but remains severely neutropenic (sequelae) | Required/prolonged hospitalisation |
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/002 | Neutropenic sepsis | UK | 65 | Female | Patient admitted with feeling unwell for a few days. Feeling hot and cold and shaky. Diarrhoea for past few days. Neutrophil count 0.1 × 10/l. Was previously discharged with neutropenia (sequelae) – readmitted on 17 October 2010 | Required/prolonged hospitalisation |
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/003 | DCT negative autoimmune haemolysis | UK | 46 | Male | Probably owing to fludarabine. Discussed with Chief Investigator – to omit cycle 6 and treat ATHA as needed. Professor Hillmen 1 November 11 agreed action | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/004 | Pulmonary embolism | UK | 46 | Male | Following post-treatment CT scan. Patient noted to have pulmonary embolism. Commenced on Clexane/Warfarin. Possibly attributable to haemolytic anaemia. Follow-up report: following post-treatment CT scan, patient noted to have pulmonary embolism. Commenced on Clexane and Warfarin | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Blood and lymphatic system disorders | N00153/00010/001 | Neutropenia | UK | 58 | Male | Patient neutrophils grade 4 – 0.37 10/l. Follow-up report: neutrophils grade 4. Dose reduction of 25% and ratiograstim with each cycle | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Blood and lymphatic system disorders | N00391/00144/001 | Marked pancytopenia post chemotherapy. Cough and short of breath | UK | 57 | Female | Reason for admission: (1) marked pancytopenia following chemotherapy with low HB, leucopenia and thrombocytopenia; (2) shortness of breath and slight cough. This 57-year-old lady was admitted to the medical ward under the care of the physician on 6 November 2012. She had a temperature of 37.5 °C but she was feeling very tired and also had a cough. At the time of her admission her full blood count was as follows: HB 5.9 g/dl, white cell count 0.9 × 109/l, neutrophils 0.2 × 109/l, platelets 72 × 109/l. She was comfortable but looked very pale. Heart sounds were normal and there were no audible murmurs. There was no oedema. Chest was clear. Abdomen was soft, there was no hepatosplenomegaly. Central nervous system was grossly normal. She was transfused with 4 units of red cells. She was also given Pentamidine 300 mg by nebuliser route on 8 November 2012. NB: Research Nurse only aware of this on 14 December 2012? If needs reported as 43 days past end of chemotherapy | Required/prolonged hospitalisation |
| FCM-miniR | Blood and lymphatic system disorders | N01527/00030/001 | Anaemia | UK | 49 | Male | Headaches, dizziness, shortness of breath since Sunday. GI bleeding does not seem to be cause. DAT-positive haemolysts | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00050/00083/001 | Vomiting | UK | 54 | Male | Nausea and vomiting – medication taken with no effect. Admitted to ward 96 | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00153/00034/001 | Vomiting | UK | 62 | Female | Patient admitted with vomiting, unresolved with oral antiemetics. Last day of oral chemotherapy. Given i.v. fluids and further antiemetic. Normal renal function and FBC | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00153/00034/002 | Nausea and vomiting | UK | 62 | Female | Patient admitted with nausea and vomiting during cycle 2 FMC-miniR. Given i.v. fluids and i.v. antiemetics | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N01527/00184/001 | Vomiting | UK | 63 | Female | Patient admitted to MAU 4 October 2012 at Royal Blackburn Hospital with vomiting post chemotherapy. Last cycle 27 September 2012. Observations: BP 138/80, HR 79, temperature 36.6 °C, RR12, sats 99% RA. Blood reports attached. Chest radiograph – clear | Required/prolonged hospitalisation |
| FCM-miniR | General disorders and administration site conditions | N00040/00180/001 | Serious Rituximab reaction | UK | 65 | Male | Patient became unresponsive, BP unrecordable, HR 34, sats 94% on air. Patient lost output – IM adrenaline given – swift recovery post adrenaline. Hypotension. CTCAE grade 4 hypotension | Life-threatening/required/prolonged hospitalisation |
| FCM-miniR | General disorders and administration site conditions | N00098/00004/001 | High-grade fever | UK | 60 | Male | Developed high-grade fever during first cycle of FCM-miniR. Temperature maximum 39.1 °C at 16.20 and not reducing with paracetamol. Decision by medical team to admit to ward. i.v. fluids | Required/prolonged hospitalisation |
| FCM-miniR | General disorders and administration site conditions | N00098/00005/003 | Anaphylaxis | UK | 56 | Male | Acute anaphylaxis, leading to collapse, loss of consciousness, generalised seizure, associated bronchospasm | Life-threatening/jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | General disorders and administration site conditions | N00098/00099/001 | Vasovagal episode with first dose of rituximab | UK | 61 | Male | Patient became diapheretic, hypotensive and lost consciousness for approx. 1 minute. Bradycardic on recovery. BP and pulse recovered back to baseline within 3–4 minutes. Second episode of collapse after mitoxantrone, recovered very quickly | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | General disorders and administration site conditions | N00099/00036/001 | Reaction to Rituximab | UK | 61 | Male | During rituximab infusion suddenly felt unwell, light headed and sweaty. Required oxygen. Later in the day felt sick, had slight rigors and lost consciousness for approx. 10–20 seconds. Oxygen reapplied. Rituximab stopped | Life-threatening |
| FCM-miniR | General disorders and administration site conditions | N00153/00156/001 | Infusion reaction | UK | 58 | Male | Patient had a reaction to the rituximab infusion, BP dropped to 70/40, HR 41, momentary loss of consciousness, < 1 minute. i.v. hydration given and additional i.v. hydrocortisone. Rituximab infusion was omitted – patient recovered. WCC = 434 | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Immune system disorders | N00361/00109/001 | Haemolytic anaemia | UK | 66 | Female | Patient was due second cycle of FCM-miniR on 17 November 2011. Hb reduced to 79, bilirubin increased to 22. Reviewed by doctor – Heamolysing. Doctor completed haemolysis screen, given 2 units red cell concentrate (blood) and commenced Prednisolone 50 mgs. Reviewed 18 November 2011: Hb 95, bilirubin 16 | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Infections and infestations | N00014/00054/001 | Sepsis | UK | 52 | Male | Possible viral infection?? Swine flu with grade 3 neutropenia | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00040/00108/001 | Neutropenic sepsis | UK | 56 | Male | Fever 39.4 °C. Lethargy | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00040/00108/002 | Pyrexia | UK | 56 | Male | Previous admission for neutropenic sepsis – discharged home. Re-admitted with pyrexia, antibiotics given i.v., no cause for pyrexia found as yet. Follow-up report: 1 November 2011, discharged 18 October 2011 and recommenced treatment on 20 October 2010 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00040/00108/004 | Neutropenic sepsis | UK | 56 | Male | Pyrexia. Feeling generally unwell. Developed rash following dose of co-amoxiclav. Treatment changed. No new drugs on discharge | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00050/00095/001 | Neutropenic sepsis | UK | 60 | Male | Admitted with neutropenic sepsis (with haematura). Generally cytopenic. Results on 01 January 12, Hb 7.7, WBC 0.50, neutrophils 0.13, Platelets 59 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00080/00058/001 | Infection with grade 4 neutrophils | UK | 51 | Female | Cough with yellow sputum, chest tightness, pyrexial | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00080/00058/002 | Chest infection/febrile neutropenia | UK | 51 | Female | Started feeling unwell on the 14 October 2011 came to clinic. No temperature and chest clear. On the 27 October 2011 increased temperature and feeling unwell with productive cough | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00005/001 | Pyrexia and rigors | UK | 56 | Male | Possible neutropenic sepsis, spiking temperatures. i.v. antibiotics given, no source identified. Neutrophils > 1.0 × 10/l | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00005/002 | Pyrexia and diarrhoea | UK | 56 | Male | Recurrent fever 38.8 °C, episodes of diarrhoea, flushing, readmitted. No source identified – i.v. antibiotics. Neutrophils > 1.0 × 10/l | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00005/004 | Neutropenic sepsis | UK | 56 | Male | Felt hot and cold. Temperature 38.6 °C. Admitted. Commenced i.v. antibiotics (finished chemotherapy tablets for this cycle 4 days previously) | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00005/005 | Fever, unknown origin, nausea | UK | 56 | Male | Spike temperature of 38 °C evening of 18 May 2010. Very nauseated and vomiting. Temperature resolved, but up again this morning. Reviewed by haem doctor. Decision to admit | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00115/001 | Urinary tract infection | UK | 69 | Male | Admitted with chills + rigors + lower abdominal pain + dysuria and dark urine. Apyrexial. Not neutropenic CRP 236. Given i.v. antibiotics + discharged 7 May 2012 on 7 days’ oral antibiotics. For USS urinary tract. Treatment already stopped as end of 6 cycles | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00099/00027/003 | Neutropenic sepsis | UK | 65 | Female | High temperature, neutrophils 0.3, platelets 130. Follow-up report: patient also presented with diarrhoea which settled prior to discharge | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00099/00036/002 | Neutropenic sepsis | UK | 61 | Male | Patient admitted to hospital with pyrexia and feeling unwell. Symptoms included increased fatigue and dysuria. Temperature 37.8 °C. Neutrophil count 0.6 × 10/l | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00099/00045/001 | Neutropenic sepsis | UK | 73 | Male | Patient admitted with sore throat and fever. Clinically septic with tachycardia, fever (temperature 39 °C), neutrophils 0.10 × 10/l. Treatment – i.v. fluids, i.v. antibiotics | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00106/00075/001 | URTI (not neutropenic) and anaemia | UK | 63 | Male | Admitted with fever, non-productive cough, shortness of breath at rest, sore throat. Oral ciprofloxacin given 4/7 days prior to admission. Treated as per hospital protocol with i.v. antibiotics. Transfused 4 units of RBC. Condition improved. Likely to be viral | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00106/00075/003 | Neutropenic sepsis | UK | 63 | Male | Admitted on 30 April 2011 with febrile neutropenia. Treated with i.v. Gentamicin and Tazocin and discharged home on 2 May 2011. He was then readmitted on the same day with rigor, pyrexia, oedema of the face, hands and feet with rash. Likely to be a drug reaction, not yet determined. Treated with Clarithromycin and Ciprofloxacin. E-mail text from Hazel Wynn, Research Nurse – ‘In response to our telephone call I can confirm that pt 75, MD has had an adverse event form sent on 26 April 11 and 4 May 2011 and that after discussing with Dr Eagleton, Dr Pushkarran and Dr Pattison these two SAEs are to be counted as 1 ongoing adverse event. Note therefore that this SAE is linked with SAE 00106/00075/02, additional information received recorded here in order to be able to capture on database’ | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00111/00132/001 | Pyrexia neutropenia | UK | 73 | Male | Increasingly unwell, urinary symptoms, confusion, pyrexia, admitted to hospital – neutropenic | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00111/00132/003 | Opportunistic infection associated with > grade 2 lymphopenia | UK | 73 | Male | Began feeling not right in head on 15 February 2013, frequency of urine. Started on i.v. antibiotics. PCP diagnosed (likely), diagnosed 22 February 2013. Septrim commenced | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00114/00006/002 | Infection | UK | 70 | Male | Productive cough for 5 days. Headache. Pyrexia (38.1 °C). Associated rigors. Admitted to hospital 22 February 2012, diagnosed influenza A. Treated with oseltamivir and GCSF. Discharged home 27 February 2012 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00114/00023/001 | Bilateral pneumonia | UK | 69 | Female | Admitted to hospital 8 July 2011 with shortness of breath. Diagnosed with bilateral pneumonia. Admitted to intensive care unit on 25 July 2011 with worsening pneumonia. Intubated 28 July 2011. Patient deteriorated and died 26 August 2011. Cause of death: (1) pneumonia; (2) CLL. Event likely to have been caused by immunosuppression from disease and treatment. Queried with trials office at time of admission whether this needed reporting as SAE. Informed that as it was more than 30 days post treatment SAE not required. However, have since been informed that SAE was required | Patient died/required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00114/00086/001 | Neutropenic sepsis | UK | 46 | Male | Rigors. Temperature 38.4 °C (19 August 2011). Spiked temperature 17 August 2011, which settled with paracetamol. Coeyzal symptoms for 2 weeks. Commenced i.v. Tazocin (19 August 2011). Follow-up report: admitted 19 August 2011. i.v. antibiotics | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00114/00094/001 | Dehydration and diarrhoea, secondary to low haemoglobin | UK | 75 | Female | Patient collapsed at home prior to pre-booked blood transfusion. Following transfusion patient had episode of loose stool. Follow-up report: admitted 5 September 2011. Discharged 7 September 2011. Campylobacter found in stool | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00132/00069/001 | Neutropenic sepsis | UK | 65 | Male | Patient admitted with neutropenic fever of 37.7 °C post chemotherapy. Patient received i.v. antibiotics and a sepsis screen and was discharged on oral antibiotics | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00132/00069/002 | Grade 2 fevers after chemotherapy | UK | 65 | Male | Admitted with grade 2 fevers, day 5 of cycle 4 chemotherapy with neutrophils of 1.30 × 10/l. No focal localising signs of infection. Placed on broad spectrum antibiotics. Discharged 3 August 2011 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00153/00010/002 | Febrile neutropenia | UK | 58 | Male | Patient admitted to hospital febrile and neutropenic, feeling dizzy and light headed. Treated as per neutropenic protocol. Follow-up report: Patient admitted febrile and neutropenic. Treated with i.v. antibiotics and PEG Filgrastim. Temperature settled, patient discharged. Awaiting decision on future chemotherapy treatment. 4 August 2010: bone marrow exam outstanding. 16 August 2010 – ratiograstim stopped, PEG filgrastim given, neutropenia resolved | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00153/00016/001 | Neutropenic sepsis/Neutropenic fever | UK | 54 | Female | Upper respiratory tract infection. Neutropenic fever. Follow-up report: patient admitted to hospital on 31 August 2010 with fever and 5-day history of productive cough. Treated with i.v. antibiotics; blood cultures negative. Discharged on oral antibiotics for 5 days | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Infections and infestations | N00153/00020/001 | Neutropenic sepsis | UK | 61 | Female | Patient admitted after 2-day history of temperature, FBC shows neutropenia. More detail to follow. Follow-up report: presented to A&E with fever and flu-like symptoms. Treated with i.v. antibiotics as per neutropenic sepsis protocol. GCSF FCVR 4 days. 5 days of oral antibiotics on discharge | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00153/00020/002 | Neutropenic sepsis | UK | 61 | Female | Patient admitted with fever and neutropenic count of 0.63 × 109/l. Treated with i.v. antibiotic as per local neutropenic sepsis protocol. To delay next cycle of treatment – due 20 September 2010. Follow-up report: discharged on oral antibiotics with neutrophil count > 2.0 10/l. Cycle 4 FCM-miniR delayed | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00153/00156/002 | Lower respiratory tract infection | UK | 58 | Male | Admitted owing to cough/fever – patient’s diagnosis at discharge = neutropenic sepsis/lower respiratory tract infection. 5 days of i.v. tazocin + 2 days of i.v. gentamicin was given. Discharged on 10 days of oral antibiotics | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00161/00047/001 | Neutropenic sepsis | UK | 60 | Male | Low-grade pyrexia, productive cough, neutrophils 0.06 10/l. Treatment of Tazocin and Gentamycin – i.v. Follow-up report: discharged on 3 December 2010 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00161/00047/002 | Suspected neutropenic sepsis | UK | 60 | Male | Temperature of 38.7 °C on admission – increased to 39.5 °C. Also had cough. WBC = 1.8 10/l, neutrophils = 1.3 10/l | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00161/00047/003 | Chest infection | UK | 60 | Male | Cough, temperature and shaking. Neutrophils = 1.3 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00280/00039/001 | Septic/sepsis | UK | 72 | Female | Temperature 39.1 °C. Diarrhoea, abdominal pain, headache and vomiting. Patient to be admitted. Follow-up: neutrophils 1.0 on admission. Dropped to nadir of 0.5 during admission. Now recovered | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00319/00080/001 | Neutropenia | UK | 63 | Male | Temperature 37.6 °C, admitted neutrophils 0.53 | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00319/00080/002 | Temperature 37.8°C | UK | 63 | Male | Temperature of 37.8 °C, no other signs or symptoms | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00319/00080/003 | Temperature 37.5°C | UK | 63 | Male | Temperature 37.7 °C at home, fatigue. (Hospital policy to admit patients with 37.5 °C or above) | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00319/00080/004 | Fever. Grade 1 | UK | 63 | Male | Non-neutropenic fever. Temperature 38.6 °C. Treated with intravenous antibiotics 2 g Ceftazidime and 280 mg Gentamicin. Chest radiography performed, no findings | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00353/00065/001 | Fever | UK | 57 | Male | Constant temperature 38.3 °C | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00391/00071/001 | Grade 3 anaemia and grade 4 febrile neutropenia | UK | 60 | Female | Felt cold and shivery for 3 days, chronic cough. Admitted 1 May 2011. Neutrophils = 0.2. Responded to i.v. antibiotics, two doses Filgrastim. 2-unit transfusion | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Infections and infestations | N00391/00142/001 | Neutropenia and fever and sweats | UK | 73 | Male | Ongoing neutropenia post cycle 1 chemotherapy on 27 April 2012. Fevers at night up to 38.5 °C. Day 46 post chemotherapy with intermittent GCSF cover. Apyrexial on admission. Neutrophils 0.6 and blood cultures taken. Follow-up report: please see attached discharge summary for full details of event. On admission looked well and was afebrile. Pulse 85 per minute, BP 110/60, sats 99% breathing air. Had significant cervical and predominantly left axillary lymphadenopathy. Heart sounds normal, chest clear, abdomen soft with no organs or masses felt. Commenced i.v. Gentamicin and Tazocin but despite this developed temperature of 39 °C. Received a 2-unit blood transfusion. Temperature fluctuated and SIRS score reached 2 and received i.v. fluids for hypotension. We were not altogether happy with his ongoing fevers of up to 38 °C but in view of neutrophils recovering to 1.1 and RB anxiety to go home we agreed to this early review. Was seen again on 21 June 2012. Had been quite well at home with no fevers during the day but admitted to sweats at night. Neutrophils had recovered to 10 × 109/l so we therefore discontinued figrastim and continues with fluconazole 100 mg daily, co-trimoxazole 960 mg M,W,F and acyclovir 400 mg twice daily | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N01527/00081/002 | Febrile neutropenia | UK | 74 | Female | Fever, rigors, generally unwell. ?Neutropenic sepsis | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N01527/00081/003 | Febrile neutropenia | UK | 74 | Female | Pyrexia, rigors, neutropenic sepsis. This event is 31 days after last treatment dose but have reported as second episode since last treatment. Follow-up report: a1 onset of first sign has been updated following review of medical notes | Required/prolonged hospitalisation |
| FCM-miniR | Musculoskeletal and connective tissue disorders | N01527/00081/001 | Infection with normal ANC ?septic arthritis | UK | 74 | Female | Right knee/left lower leg swelling, pain, pyrexia, anaemia. No action taken as yet, see section B. However tx may be delayed, next cycle due 9 June 2011. Follow-up report: medical opinion is condition probably gout | Required/prolonged hospitalisation |
| FCM-miniR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N01527/00081/004 | EBV – Associated lymphoproliferativedisorder | UK | 74 | Female | Patient presented with cough and was referred for chest radiography by GP. CT scan urgently arranged as well as bronchial biopsy. Verbal notification via PI confirming diagnosis. Additional information: see attached reports. Supplementary report with diagnosis to follow when available. Supplementary report now updated. This is an EBV-driven lymphoproliferative disorder. This is unlikely to be clonally related to the underlying CLL but may have occurred as a consequence of the disease and treatment related immunosuppression. Update: patient received 6 cycles RCHOP (January–May 2013) with a CR | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Renal and urinary disorders | N00071/00021/001 | Acute renal failure – tumour lysis syndrome | UK | 56 | Female | Nausea and vomiting since chemotherapy 24/6/10. Refused admission 1/7/10 (to GP). Admitted 2/7/10. Na 129, K+ 8.3, Ur –52.5, Cr 853, Ca 1.64, PO4 –10.46, urate 2.36, WBC count 178.6 on 28/6/10, 6.5 on 2/7/10. Tumour lysis syndrome | Life-threatening |
| FCM-miniR | Renal and urinary disorders | N00353/00165/001 | Renal failure CTC grade 3 | UK | 66 | Male | Shortness of breath, hypotension, acute tubular necrosis. CRP 9.7 mg/l, urea 25.6 mmol/l, creatinine 258 µmol/l. Bloods on 16 July 2012: potassium 6.3 mmol/l, urea 6.2 mmol/l, creatinine 530 mmol/l. Follow-up report: patient reviewed on 23 July 2013 and clinician happy for treatment to continue | Required/prolonged hospitalisation |
| FCM-miniR | Skin and subcutaneous tissue disorders | N00106/00075/002 | Pyrexia/rash – macular/papular | UK | 63 | Male | Flu-like symptoms. Rash covering entire body. Temperature. E-mail text from Hazel Wynn, Research Nurse – ‘In response to our telephone call I can confirm that pt 75, MD has had an adverse event form sent on 26 April 11 and 4 May 11 and that after discussing with Dr Eagleton, Dr Pushkarran and Dr Pattison these two SAEs are to be counted as 1 ongoing adverse event. Note therefore that this SAE is linked with SAE 00106/00075/03, additional information received recorded there in order to be able to capture on database’ | Required/prolonged hospitalisation/jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR/FCR | Gastrointestinal disorders | N00173/00145/001 | Nausea and vomiting | UK | 72 | Male | Nausea + vomiting + unable to eat or drink from day after chemotherapy | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Gastrointestinal disorders | N00319/00189/001 | Vomiting (grade 3) | UK | 66 | Female | Patient called triage line with vomiting on Day 4 chemotherapy. GP went out. Patient attended clinic Day 5 still experiencing uncontrolled nausea/vomiting. Not able to eat/drink, feeling weak. Admitted from clinic for i.v. fluids/antiemetics | Required/prolonged hospitalisation |
| FCM-miniR/FCR | General disorders and administration site conditions | N00137/00196/002 | Nausea, vomiting, and dehydration | UK | 50 | Female | Informed today (25 September 2012) that R-T was admitted with nausea, vomiting and dehydration. Given antiemetics and i.v. fluids | Required/prolonged hospitalisation |
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/003 | Fever (absence neutropenia) | UK | 46 | Male | Rituximab and mitoxantrone given in clinic. 2 hours post infusion patient started to feel cold and shivery at home. Temperature 38.8 °C and vomited. Admitted to hospital. Infection? Drug reaction? | Required/prolonged hospitalisation |
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/004 | Cytokine release grade 3 | UK | 46 | Male | Pyrexia, moderate chills, rigor, commencing 12 hours post infusion of rituximab. Severe delayed reaction post #2 course – inpatient admission with fever + hypotension. Intervention given promptly post course 3, therefore reaction not as severe | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR/FCR | Infections and infestations | N00076/00169/001 | Febrile neutropenia | UK | 67 | Female | Pyrexia unknown origin | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00076/00169/002 | Febrile | UK | 67 | Female | Patient reported pyrexia. Follow-up information: 11 December 2012 – Possible viral infection – unknown – nil found on blood cultures | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00137/00196/003 | Neutropenic sepsis | UK | 50 | Female | Admitted post chemotherapy with nausea and vomiting. Low neutrophils noted. Commenced neutropenic sepsis protocol. Given i.v. fluids then, once settled, oral fluids. Discharged to finish course of oral antibiotics. Mildly raised bilirubin – abdominal ultrasound showed nil liver or gall bladder issues | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00137/00196/004 | Neutropenic sepsis | UK | 50 | Female | Admitted with sweating and generally unwell. Follow-up report: admitted post chemotherapy with nausea and vomiting. Low neutrophils noted. Commenced neutropenic sepsis protocol. Given i.v. fluids, then once settled oral fluids. Discharged to finish course oral antibiotics. Mildly raised bilirubin. Abdominal ultrasound showed nil liver or gall bladder issues | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00153/00148/001 | Febrile neutropenia | UK | 54 | Female | Patient was admitted into hospital owing to febrile neutropenia, i.v. antibiotics commenced | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00153/00177/001 | Febrile neutropenia | UK | 59 | Female | Patient felt unwell/shivery at home. Temperature = 37.9 °C. Neutrophils 0.6. i.v. antibiotics given. Patient was discharged on oral antibiotics | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00153/00186/001 | Febrile neutropenia | UK | 52 | Female | Patient was admitted into hospital with neutropenic sepsis, neutrophils = 0.27 | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00218/00162/001 | Neutropenic fever | UK | 64 | Male | Hospital admission with pyrexia and neutropenia. ?VZV. Fever and intermittent rash at present but suspicious for VZV (was on prophylactic aciclovir). Follow-up report: 4 September 2012 blood culture negative. VZV not diagnosed | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/002 | Neutropenic sepsis (febrile neutropenia) | UK | 66 | Female | Developed a fever at home, 37.5 °C, and felt unwell. Neutrophils 0.30 on admission. Commenced on i.v. antibiotics and i.v. fluids. Additional information: patient had FCM-miniR for first cycle and FCR thereafter | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/003 | Febrile neutropenia | UK | 66 | Female | Developed a fever during hospital visit (clinic appointment). 38.5°C. Neutrophils 0.94 on admission. Commenced i.v. ABs. Additional information: patient had FCM-miniR for cycle 1 then switched to FCR | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/004 | Neutropenia with fever 37.8°C | UK | 66 | Female | Generally feeling unwell; pyrexial, temperature 37.8 °C. Neutrophils 0.54 | Required/prolonged hospitalisation |
| Treatment received | MedDRA system organ class | SAE ID number | SAE medical description | Suspected relationship to trial treatment | Suspected product(s) | Other causality (in addition to trial medications) | Randomisation date | Date SAE became serious | SAE recovery date | SAE duration (days) | Outcome of SAE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCR | Blood and lymphatic system disorders | N00014/00028/002 | Neutropenia | Suspected, expected | FCR | 21 July 2010 | 27 October 2010 | 1 November 2010 | 5 | Recovered | |
| FCR | Blood and lymphatic system disorders | N00076/00187/001 | Platelet count decreased | Suspected, expected | FC | CLL | 21 August 2012 | 31 December 2012 | Condition still present and unchanged | ||
| FCR | Blood and lymphatic system disorders | N00080/00032/001 | Infection | Suspected, expected | FC | Other illness: general frailty/possible GBS | 23 August 2010 | 6 October 2010 | 144 | Death | |
| FCR | Blood and lymphatic system disorders | N00098/00017/001 | Extremely low Hb | Suspected, expected | FCR | CLL | 30 April 2010 | 3 November 2010 | 6 November 2010 | 3 | Recovered with sequelae |
| FCR | Blood and lymphatic system disorders | N00098/00017/002 | Low Hb 5.9 g/dl on Pentra | Suspected, expected | FCR | 30 April 2010 | 12 November 2010 | 13 November 2010 | 1 | Recovered | |
| FCR | Blood and lymphatic system disorders | N00114/00088/001 | Persistent neutropenia related to chemotherapy | Suspected, expected | FCR | Concomitant medications | 24 May 2011 | 2 November 2011 | 2 January 2012 | 61 | Recovered |
| FCR | Blood and lymphatic system disorders | N00175/00155/001 | Anaemia | Suspected, expected | FCR | 30 May 2012 | 16 October 2012 | 16 October 2012 | 0 | Recovered | |
| FCR | Blood and lymphatic system disorders | N00230/00033/002 | Myelodysplasia: RAES | Suspected, expected | FC | 24 August 2010 | 6 May 2014 | . | Condition improving | ||
| FCR | Gastrointestinal disorders | N00050/00120/001 | Diarrhoea and vomiting | Suspected, expected | FC | 7 November 2011 | 20 December 2011 | 21 December 2011 | 1 | Recovered | |
| FCR | Gastrointestinal disorders | N00098/00060/002 | Nausea and vomiting | Suspected, expected | FC | 24 January 2011 | 28 April 2011 | 28 April 2011 | 0 | Recovered | |
| FCR | Gastrointestinal disorders | N00114/00088/003 | Gastroenteritis | Suspected, expected | FCR | Other: suspect take-away meal | 24 May 2011 | 1 April 2012 | 2 April 2012 | 1 | Recovered |
| FCR | Gastrointestinal disorders | N00349/00035/001 | Nausea | Suspected, expected | FC | 10 September 2010 | 16 March 2011 | 15 April 2011 | 30 | Recovered | |
| FCR | General disorders and administration site conditions | N00014/00028/004 | Infection/anaemia | Suspected, expected | FCR | 21 July 2010 | 4 January 2011 | 11 February 2011 | 38 | Recovered | |
| FCR | General disorders and administration site conditions | N00014/00028/005 | Anaemia grade 4 | Suspected, expected | FCR | 21 July 2010 | 19 January 2011 | 10 February 2011 | 22 | Recovered | |
| FCR | General disorders and administration site conditions | N00014/00029/001 | Rigors | Suspected, expected | Rituximab | 4 August 2010 | 24 August 2010 | 26 August 2010 | 2 | Recovered | |
| FCR | General disorders and administration site conditions | N00098/00019/001 | Pyrexial 38.2 °C post first dose of Rituximab | Suspected, expected | Rituximab | 8 June 2010 | 17 June 2010 | 18 June 2010 | 1 | Recovered | |
| FCR | General disorders and administration site conditions | N00114/00055/001 | Fever and rigors | Suspected, expected | Rituximab | CLL | 5 January 2011 | 12 January 2011 | 18 January 2011 | 6 | Recovered |
| FCR | General disorders and administration site conditions | N00173/00011/001 | Hypoxia | Suspected, expected | Rituximab | CLL; asthma | 29 March 2010 | 16 April 2010 | 21 April 2010 | 5 | Recovered |
| FCR | General disorders and administration site conditions | N00173/00137/001 | Vomiting | Suspected, expected | FC | Other: constipation | 5 March 2012 | 8 April 2012 | 9 April 2012 | 1 | Recovered |
| FCR | General disorders and administration site conditions | N00218/00168/001 | Fever and non-specifically unwell | Suspected, expected | FCR | Other: ? infection underlying – none proven | 4 July 2012 | 11 July 2012 | 13 July 2012 | 2 | Recovered |
| FCR | General disorders and administration site conditions | N00319/00125/001 | Allergic reaction | Suspected, expected | Rituximab | CLL | 12 December 2011 | 2 February 2012 | 2 February 2012 | 0 | Recovered |
| FCR | General disorders and administration site conditions | N00391/00194/002 | Probable rituximab reaction | Suspected, expected | Rituximab | 12 September 2012 | 10 January 2013 | 14 January 2013 | 4 | Recovered | |
| FCR | Infections and infestations | N00014/00028/003 | Febrile neutropenia | Suspected, expected | FCR | 21 July 2010 | 6 December 2010 | 13 December 2010 | 7 | Recovered | |
| FCR | Infections and infestations | N00014/00112/001 | Shingles | Suspected, expected | FCR | 19 October 2011 | 8 February 2012 | 11 February 2012 | 3 | Recovered | |
| FCR | Infections and infestations | N00040/00056/001 | Neutropenic sepsis | Suspected, expected | FC | 7 January 2011 | 22 February 2011 | 28 February 2011 | 6 | Recovered | |
| FCR | Infections and infestations | N00046/00185/001 | Infection with grade 4 neutrophils | Suspected, expected | FCR | CLL | 13 August 2012 | 31 December 2012 | 4 January 2013 | 4 | Recovered |
| FCR | Infections and infestations | N00050/00041/001 | Febrile neutropenia | Suspected, expected | FCR | 19 October 2010 | 11 December 2010 | 14 December 2010 | 3 | Recovered | |
| FCR | Infections and infestations | N00098/00001/001 | Neutropenic sepsis | Suspected, expected | FC | 14 December 2009 | 2 January 2010 | 5 January 2010 | 3 | Recovered | |
| FCR | Infections and infestations | N00098/00060/001 | Fever: no focus identified | Suspected, expected | FC | CLL | 24 January 2011 | 4 March 2011 | 7 March 2011 | 3 | Recovered |
| FCR | Infections and infestations | N00098/00060/003 | Probable late PCP/PJP | Suspected, expected | FCR | 24 January 2011 | 30 May 2012 | 6 June 2012 | 7 | Recovered | |
| FCR | Infections and infestations | N00099/00084/001 | Pyrexia ?neutropenic sepsis | Suspected, expected | FC | 11 May 2011 | 24 October 2011 | 27 October 2011 | 3 | Recovered | |
| FCR | Infections and infestations | N00099/00105/001 | Neutropenic sepsis | Suspected, expected | FC | 26 August 2011 | 7 September 2011 | 17 September 2011 | 10 | Recovered | |
| FCR | Infections and infestations | N00106/00130/001 | Neutropenic sepsis | Suspected, expected | FCR | 16 January 2012 | 24 March 2012 | 28 March 2012 | 4 | Recovered | |
| FCR | Infections and infestations | N00106/00130/002 | Neutropenic sepsis | Suspected, expected | FCR | 16 January 2012 | 27 April 2012 | 1 May 2012 | 4 | Recovered | |
| FCR | Infections and infestations | N00106/00179/001 | Pyrexial, neutropenic | Suspected, expected | FCR | 17 July 2012 | 2 August 2012 | 7 August 2012 | 5 | Recovered | |
| FCR | Infections and infestations | N00106/00179/003 | Neutropenic sepsis | Suspected, expected | FCR | Concomitant medications | 17 July 2012 | 24 October 2012 | 27 October 2012 | 3 | Recovered |
| FCR | Infections and infestations | N00114/00067/001 | Neutropenic sepsis | Suspected, expected | FCR | 9 February 2011 | 23 May 2011 | 28 May 2011 | 5 | Recovered | |
| FCR | Infections and infestations | N00114/00067/002 | Neutropenic sepsis ?cause | Suspected, expected | FC | 9 September 2011 | 18 July 2011 | 25 July 2011 | 7 | Recovered | |
| FCR | Infections and infestations | N00114/00067/003 | ?Neutropenic sepsis | Suspected, expected | FCR | CLL | 9 February 2011 | 7 October 2011 | 14 October 2011 | 7 | Recovered |
| FCR | Infections and infestations | N00114/00067/004 | Neutropenic sepsis | Suspected, expected | FCR | 9 February 2011 | 23 January 2012 | 27 January 2012 | 4 | Recovered | |
| FCR | Infections and infestations | N00114/00088/002 | Neutropenic sepsis | Suspected, expected | FCR | CLL | 24 May 2011 | 24 December 2011 | 3 January 2012 | 10 | Recovered |
| FCR | Infections and infestations | N00153/00026/001 | Infection | Suspected, expected | FCR | 20 July 2010 | 10 August 2010 | 11 August 2010 | 1 | Recovered | |
| FCR | Infections and infestations | N00153/00050/001 | Chest infection | Suspected, expected | FCR | 26 November 2010 | 27 February 2011 | 1 March 2011 | 2 | Recovered | |
| FCR | Infections and infestations | N00173/00011/003 | Lower respiratory tract infection | Suspected, expected | FCR | CLL | 29 March 2010 | 6 July 2010 | 12 July 2010 | 6 | Recovered |
| FCR | Infections and infestations | N00218/00168/002 | Neutropenic sepsis | Suspected, expected | FC | 4 July 2012 | 25 September 2012 | 4 October 2012 | 9 | Recovered | |
| FCR | Infections and infestations | N00230/00033/001 | Neutropenic sepsis | Suspected, expected | FC | 24 August 2010 | 26 September 2010 | 5 October 2010 | 9 | Recovered | |
| FCR | Infections and infestations | N00231/00174/001 | Febrile, rigors, ?septic. Episodes of diarrhoea for 2 days | Suspected, expected | FC | Other: infection | 9 July 2012 | 22 August 2012 | 26 August 2012 | 4 | Recovered |
| FCR | Infections and infestations | N00231/00174/002 | Febrile illness (pyrexia, rigors) | Suspected, expected | FC | Other: infection | 9 July 2012 | 27 August 2012 | 10 September 2012 | 14 | Recovered |
| FCR | Infections and infestations | N00231/00174/003 | Neutropenic sepsis | Suspected, expected | FC | 9 July 2012 | 1 October 2012 | 18 October 2012 | 17 | Recovered | |
| FCR | Infections and infestations | N00255/00138/001 | Neutropenic sepsis | Suspected, expected | FCR | CLL | 13 March 2012 | 7 April 2012 | 16 April 2012 | 9 | Recovered |
| FCR | Infections and infestations | N00255/00166/001 | Febrile neutropenia | Suspected, expected | FCR | 29 June 2012 | 13 September 2012 | 18 September 2012 | 5 | Recovered | |
| FCR | Infections and infestations | N00255/00166/002 | Neutropenic sepsis | Suspected, expected | FCR | 29 June 2012 | 17 March 2013 | 30 March 2013 | 13 | Recovered | |
| FCR | Infections and infestations | N00280/00008/001 | Neutropenic sepsis | Suspected, expected | FC | 22 March 2010 | 31 May 2010 | 07 June 2010 | 7 | Recovered | |
| FCR | Infections and infestations | N00319/00125/002 | Febrile neutropenia | Suspected, expected | FCR | CLL | 12 December 2011 | 20 February 2012 | 24 February 2012 | 4 | Recovered |
| FCR | Infections and infestations | N00361/00123/002 | Neutropenic sepsis | Suspected, expected | FCR | CLL | 21 November 2011 | 5 July 2012 | 9 July 2012 | 4 | Recovered |
| FCR | Infections and infestations | N00391/00111/001 | Neutropenic sepsis | Suspected, expected | FC | 6 October 2011 | 4 November 2011 | 10 November 2011 | 6 | Recovered | |
| FCR | Infections and infestations | N00391/00194/001 | ?Neutropenic sepsis. ?Viral infection. ?Drug-induced gastritis | Suspected, expected | FC | 12 September 2012 | 13 December 2012 | 15 December 2012 | 2 | Recovered | |
| FCR | Infections and infestations | N00698/00198/001 | Febrile neutropenia | Suspected, expected | FCR | 19 September 2012 | 31 December 2012 | 5 January 2013 | 5 | Recovered | |
| FCR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N00098/00002/001 | SCC: two lesions (lower back and central chest) | Suspected, unexpected | FCR | 13 January 2010 | 6 October 2010 | 14 December 2010 | 69 | Recovered | |
| FCR | Psychiatric disorders | N00349/00104/002 | Psychotic episode | Suspected, expected | Fludarabine | Other illness: long history anxiety | 25 August 2011 | 20 December 2011 | 24 February 2012 | 66 | Recovered |
| FCR | Skin and subcutaneous tissue disorders | N00349/00070/001 | Rash | Suspected, expected | Rituximab | 09 March 2011 | 12 April 2011 | 13 April 2011 | 1 | Recovered | |
| FCR | Skin and subcutaneous tissue disorders | N00353/00122/001 | Erythemous rash grade 3 | Suspected, expected | Fludarabine | Concomitant medications | 11 November 2011 | 19 December 2011 | 9 January 2012 | 21 | Recovered |
| FCM-miniR | Blood and lymphatic system disorders | N00009/00127/001 | Pleural effusion and pulmonary embolism | Suspected, expected | Cyclophosphamide | 15 December 2011 | 31 December 2011 | 11 January 2012 | 11 | Recovered | |
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/001 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 20 July 2010 | 1 October 2010 | 7 October 2010 | 6 | Recovered with sequelae | |
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/002 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 20 July 2010 | 17 October 2010 | 25 October 2010 | 8 | Recovered with sequelae | |
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/003 | DCT-negative autoimmune haemolysis | Suspected, expected | Fludarabine | CLL | 18 May 2011 | 02 November 2011 | Condition still present and unchanged | ||
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/004 | Pulmonary embolism | Suspected, expected | Fludarabine | CLL; auto-immune haemolytic anaemia | 18 May 2011 | 16 January 2012 | 14 November 2012 | 303 | Recovered with sequelae |
| FCM-miniR | Blood and lymphatic system disorders | N00153/00010/001 | Neutropenia | Suspected, expected | FCM-mini-R | Concomitant medications | 25 March 2010 | 7 April 2010 | 26 April 2010 | 19 | Recovered |
| FCM-miniR | Blood and lymphatic system disorders | N00391/00144/001 | Marked pancytopenia post chemotherapy. Cough and SOB | Suspected, expected | FCM | 17 April 2012 | 6 November 2012 | 9 November 2012 | 3 | Recovered | |
| FCM-miniR | Blood and lymphatic system disorders | N01527/00030/001 | Anaemia | Suspected, expected | Fludarabine | 9 August 2010 | 27 August 2010 | 31 August 2010 | 4 | Recovered | |
| FCM-miniR | Gastrointestinal disorders | N00050/00083/001 | Vomiting | Suspected, expected | FCM | 6 May 2011 | 10 September 2011 | 11 September 2011 | 1 | Recovered | |
| FCM-miniR | Gastrointestinal disorders | N00153/00034/001 | Vomiting | Suspected, expected | FCM | 31 August 2010 | 21 September 2010 | 23 September 2010 | 2 | Recovered | |
| FCM-miniR | Gastrointestinal disorders | N00153/00034/002 | Nausea and vomiting | Suspected, expected | FCM | 31 August 2010 | 16 October 2010 | 19 October 2010 | 3 | Recovered | |
| FCM-miniR | Gastrointestinal disorders | N01527/00184/001 | Vomiting | Suspected, expected | Fludarabine and mitoxantrone | 06 August 2012 | 04 October 2012 | 05 October 2012 | 1 | Recovered | |
| FCM-miniR | General disorders and administration site conditions | N00040/00180/001 | Serious Rituximab reaction | Suspected, expected | Low-dose Rituximab | 20 July 2012 | 30 July 2012 | 31 July 2012 | 1 | Recovered | |
| FCM-miniR | General disorders and administration site conditions | N00098/00004/001 | High-grade fever | Suspected, expected | Mitoxantrone and low-dose Rituximab | 2 February 2010 | 24 February 2010 | 25 February 2010 | 1 | Recovered | |
| FCM-miniR | General disorders and administration site conditions | N00098/00005/003 | Anaphylaxis | Suspected, expected | Low-dose Rituximab | 15 February 2010 | 17 February 2010 | 17 February 2010 | 0 | Recovered | |
| FCM-miniR | General disorders and administration site conditions | N00098/00099/001 | Vasovagal episode with first dose of rituximab | Suspected, expected | Low-dose Rituximab | Other: high vagal tone | 27 July 2011 | 10 August 2011 | 11 August 2011 | 1 | Recovered |
| FCM-miniR | General disorders and administration site conditions | N00099/00036/001 | Reaction to Rituximab | Suspected, expected | Low-dose Rituximab | 16 September 2010 | 27 September 2010 | 27 September 2010 | 0 | Recovered | |
| FCM-miniR | General disorders and administration site conditions | N00153/00156/001 | Infusion reaction | Suspected, expected | Low-dose Rituximab | 31 May 2012 | 7 June 2012 | 7 June 2012 | 0 | Recovered | |
| FCM-miniR | Immune system disorders | N00361/00109/001 | Haemolytic anaemia | Suspected, expected | Fludarabine | 5 October 2011 | 17 November 2011 | 15 February 2012 | 90 | Recovered | |
| FCM-miniR | Infections and infestations | N00014/00054/001 | Sepsis | Suspected, expected | FCM-mini-R | 5 January 2011 | 4 February 2011 | 22 February 2011 | 18 | Recovered | |
| FCM-miniR | Infections and infestations | N00040/00108/001 | Neutropenic sepsis | Suspected, expected | FCM | 8 September 2011 | 24 September 2011 | 2 October 2011 | 8 | Recovered | |
| FCM-miniR | Infections and infestations | N00040/00108/002 | Pyrexia | Suspected, expected | FCM-mini-R | CLL | 8 September 2011 | 7 October 2011 | 18 October 2011 | 11 | Recovered |
| FCM-miniR | Infections and infestations | N00040/00108/004 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | CLL | 8 September 2011 | 7 January 2012 | 13 January 2012 | 6 | Recovered |
| FCM-miniR | Infections and infestations | N00050/00095/001 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 21 July 2011 | 31 December 2011 | 4 January 2012 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00080/00058/001 | Infection with grade 4 neutrophils | Suspected, expected | FCM | 17 January 2011 | 28 April 2011 | 3 May 2011 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00080/00058/002 | Chest infection/febrile neutropenia | Suspected, expected | FCM | 17 January 2011 | 28 October 2011 | 3 November 2011 | 6 | Recovered | |
| FCM-miniR | Infections and infestations | N00098/00005/001 | Pyrexia and rigors | Suspected, expected | FCM | 15 February 2010 | 5 March 2010 | 8 March 2010 | 3 | Recovered | |
| FCM-miniR | Infections and infestations | N00098/00005/002 | Pyrexia and diarrhoea | Suspected, expected | FCM | 15 February 2010 | 8 March 2010 | 12 March 2010 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00098/00005/004 | Neutropenic sepsis | Suspected, expected | FCM | 15 February 2010 | 22 April 2010 | 28 April 2010 | 6 | Recovered | |
| FCM-miniR | Infections and infestations | N00098/00005/005 | Fever, unknown origin, nausea | Suspected, expected | FCM | Concomitant medications infection | 15 February 2010 | 19 May 2010 | 21 May 2010 | 2 | Recovered |
| FCM-miniR | Infections and infestations | N00098/00115/001 | Urinary tract infection | Suspected, expected | FCM-mini-R | CLL | 27 October 2011 | 4 May 2012 | 14 May 2012 | 10 | Recovered |
| FCM-miniR | Infections and infestations | N00099/00027/003 | Neutropenic sepsis | Suspected, expected | FCM | 20 July 2010 | 22 February 2011 | 25 February 2011 | 3 | Recovered with sequelae | |
| FCM-miniR | Infections and infestations | N00099/00036/002 | Neutropenic sepsis | Suspected, expected | Fludarabine and mitoxantrone | 16 September 2010 | 03 November 2010 | 10 November 2010 | 7 | Recovered with sequelae | |
| FCM-miniR | Infections and infestations | N00099/00045/001 | Neutropenic sepsis | Suspected, expected | FCM | 9 November 2010 | 29 March 2011 | 3 April 2011 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00106/00075/001 | URTI (not neutropenic) and anaemia | Suspected, expected | FCM-mini-R | CLL | 21 March 2011 | 6 April 2011 | 8 April 2011 | 2 | Recovered |
| FCM-miniR | Infections and infestations | N00106/00075/003 | Neutropenic sepsis | Suspected, expected | FCM | 21 March 2011 | 2 May 2011 | 4 May 2011 | 2 | Recovered | |
| FCM-miniR | Infections and infestations | N00111/00132/001 | Pyrexia neutropenia | Suspected, expected | FCM | CLL | 30 January 2012 | 8 May 2012 | 14 May 2012 | 6 | Recovered |
| FCM-miniR | Infections and infestations | N00111/00132/003 | Opportunistic infection associated with > grade 2 lymphopenia | Suspected, expected | FCM-mini-R | CLL | 30 January 2012 | 16 February 2013 | 8 March 2013 | 20 | Recovered |
| FCM-miniR | Infections and infestations | N00114/00006/002 | Infection | Suspected, expected | FCM-mini-R | 22 February 2010 | 22 February 2012 | 27 February 2012 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00114/00023/001 | Bilateral pneumonia | Suspected, expected | FCM-mini-R | CLL | 8 July 2010 | 8 July 2011 | 50 | Death | |
| FCM-miniR | Infections and infestations | N00114/00086/001 | Neutropenic sepsis | Suspected, expected | FCM | Other: in-dwelling Hickman line | 18 May 2011 | 19 August 2011 | 26 August 2011 | 7 | Recovered |
| FCM-miniR | Infections and infestations | N00114/00094/001 | Dehydration and diarrhoea, secondary to low Hb | Suspected, expected | FCM | CLL | 8 July 2011 | 5 September 2011 | 7 September 2011 | 2 | Recovered |
| FCM-miniR | Infections and infestations | N00132/00069/001 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 7 March 2011 | 10 May 2011 | 12 May 2011 | 2 | Recovered | |
| FCM-miniR | Infections and infestations | N00132/00069/002 | Grade 2 fevers after chemotherapy | Suspected, expected | FCM-mini-R | 7 March 2011 | 30 July 2011 | 3 August 2011 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00153/00010/002 | Febrile neutropenia | Suspected, expected | FCM-mini-R | 25 March 2010 | 3 August 2010 | 7 August 2010 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00153/00016/001 | Neutropenic sepsis/neutropenic fever | Suspected, expected | FCM-mini-R | 30 April 2010 | 31 August 2010 | 8 September 2010 | 8 | Recovered | |
| FCM-miniR | Infections and infestations | N00153/00020/001 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 16 June 2010 | 2 September 2010 | 12 September 2010 | 10 | Recovered | |
| FCM-miniR | Infections and infestations | N00153/00020/002 | Neutropenic sepsis | Suspected, expected | FCM-mini-R | 16 June 2010 | 14 September 2010 | 19 September 2010 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00153/00156/002 | Lower respiratory tract infection | Suspected, expected | FC and low-dose R | 31 May 2012 | 6 September 2012 | 10 September 2012 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00161/00047/001 | Neutropenic sepsis | Suspected, expected | FCM | 12 November 2010 | 1 December 2010 | 3 December 2010 | 2 | Recovered | |
| FCM-miniR | Infections and infestations | N00161/00047/002 | Suspected neutropenic sepsis | Suspected, expected | Fludarabine | Other illness: lung scarring from previous infection | 12 November 2010 | 12 August 2011 | 15 August 2011 | 3 | Recovered |
| FCM-miniR | Infections and infestations | N00161/00047/003 | Chest infection | Suspected, expected | FCM-mini-R | CLL | 12 November 2010 | 26 August 2011 | 30 August 2011 | 4 | Recovered |
| FCM-miniR | Infections and infestations | N00280/00039/001 | Septic/sepsis | Suspected, expected | FCM | 13 October 2010 | 11 February 2011 | 16 February 2011 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00319/00080/001 | Neutropenia | Suspected, expected | FCM-mini-R | 3 May 2011 | 5 July 2011 | 18 July 2011 | 13 | Recovered | |
| FCM-miniR | Infections and infestations | N00319/00080/002 | Temperature 37.8 °C | Suspected, expected | FCM-mini-R | CLL | 3 May 2011 | 18 July 2011 | 18 July 2011 | 0 | Recovered |
| FCM-miniR | Infections and infestations | N00319/00080/003 | Temperature 37.5 °C | Suspected, expected | FCM-mini-R | 3 May 2011 | 31 August 2011 | 1 September 2011 | 1 | Recovered | |
| FCM-miniR | Infections and infestations | N00319/00080/004 | Fever: grade 1 | Suspected, expected | FCM-mini-R | 3 May 2011 | 1 October 2011 | 6 October 2011 | 5 | Recovered | |
| FCM-miniR | Infections and infestations | N00353/00065/001 | Fever | Suspected, expected | FCM | 9 February 2011 | 22 April 2011 | 23 April 2011 | 1 | Recovered | |
| FCM-miniR | Infections and infestations | N00391/00071/001 | Grade 3 anaemia and grade 4 febrile neutropenia | Suspected, expected | FCM | 11 March 2011 | 1 May 2011 | 5 May 2011 | 4 | Recovered | |
| FCM-miniR | Infections and infestations | N00391/00142/001 | Neutropenia and fever and sweats | Suspected, expected | FCM-mini-R | CLL | 10 April 2012 | 12 June 2012 | 18 June 2012 | 6 | Recovered |
| FCM-miniR | Infections and infestations | N01527/00081/002 | Febrile neutropenia | Suspected, expected | FCM-mini-R | CLL | 5 May 2011 | 17 August 2011 | 25 August 2011 | 8 | Recovered |
| FCM-miniR | Infections and infestations | N01527/00081/003 | Febrile neutropenia | Suspected, expected | FCM-mini-R | CLL | 5 May 2011 | 30 September 2011 | 7 October 2011 | 7 | Recovered |
| FCM-miniR | Musculoskeletal and connective tissue disorders | N01527/00081/001 | Infection with normal ANC ?septic arthritis | Suspected, expected | FCM-mini-R | Other illness: previous septic arthritis | 5 May 2011 | 6 June 2011 | 9 June 2011 | 3 | Recovered |
| FCM-miniR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N01527/00081/004 | EBV – associated lymphoproliferative disorder | Suspected, expected | Fludarabine | 5 May 2011 | 10 December 2012 | 20 June 2013 | 192 | Recovered | |
| FCM-miniR | Renal and urinary disorders | N00071/00021/001 | Acute renal failure: tumour lysis syndrome | Suspected, expected | FCM-mini-R | Other illness: vomiting/dehydration ?did not take allopurinol (patient stopped taking aciclovir/septrin/allopurinol when nauseous/vomiting) | 17 June 2010 | 2 July 2010 | 20 July 2010 | 18 | Recovered |
| FCM-miniR | Renal and urinary disorders | N00353/00165/001 | Renal failure CTCAE grade 3 | Suspected, expected | FCM-mini-R | Other illness: acute tubular necrosis; Hypotension | 28 June 2012 | 13 July 2012 | 23 July 2012 | 10 | Recovered |
| FCM-miniR | Skin and subcutaneous tissue disorders | N00106/00075/002 | Pyrexia/rash: macular/papular | Suspected, expected | FCM-mini-R | Other illness: ?Viral/bacterial infection | 21 March 2011 | 22 April 2011 | 01 June 2011 | 40 | Recovered |
| FCM-miniR/FCR | Gastrointestinal disorders | N00173/00145/001 | Nausea and vomiting | Suspected, expected | FCM-mini-R | 30 April 2012 | 20 June 2012 | 21 June 2012 | 1 | Recovered | |
| FCM-miniR/FCR | Gastrointestinal disorders | N00319/00189/001 | Vomiting (grade 3) | Suspected, expected | Fludarabine | 30 August 2012 | 11 September 2012 | 17 September 2012 | 6 | Recovered | |
| FCM-miniR/FCR | General disorders and administration site conditions | N00137/00196/002 | Nausea, vomiting and dehydration | Suspected, expected | FCM-mini-R | 17 September 2012 | 24 September 2012 | 27 September 2012 | 3 | Recovered | |
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/003 | Fever (absence neutropenia) | Suspected, expected | Low-dose Rituximab | 28 June 2012 | 14 August 2012 | 18 August 2012 | 4 | Recovered | |
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/004 | Cytokine release grade 3 | Suspected, expected | Low-dose Rituximab | 28 June 2012 | 19 September 2012 | 19 September 2012 | 0 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00076/00169/001 | Febrile neutropenia | Suspected, expected | FC | 4 July 2012 | 6 August 2012 | 13 August 2012 | 7 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00076/00169/002 | Febrile | Suspected, expected | Fludarabine and rituximab | 4 July 2012 | 26 November 2012 | 3 December 2012 | 7 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00137/00196/003 | Neutropenic sepsis | Suspected, expected | FCR | 17 September 2012 | 16 January 2013 | 22 January 2013 | 6 | Recovered with sequelae | |
| FCM-miniR/FCR | Infections and infestations | N00137/00196/004 | Neutropenic sepsis | Suspected, expected | FCR | 17 September 2012 | 13 February 2013 | 25 October 2013 | 254 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00153/00148/001 | Febrile neutropenia | Suspected, expected | FCM-mini-R | 4 May 2012 | 27 May 2012 | 31 May 2012 | 4 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00153/00177/001 | Febrile neutropenia | Suspected, expected | FCM | 13 July 2012 | 4 August 2012 | 5 August 2012 | 1 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00153/00186/001 | Febrile neutropenia | Suspected, expected | FCM-mini-R | CLL | 13 August 2012 | 30 August 2012 | 2 September 2012 | 3 | Recovered |
| FCM-miniR/FCR | Infections and infestations | N00218/00162/001 | Neutropenic fever | Suspected, expected | FCM-mini-R | 20 June 2012 | 28 August 2012 | 2 September 2012 | 5 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/002 | Neutropenic sepsis (febrile neutropenia) | Suspected, expected | FC | 30 August 2012 | 14 November 2012 | 17 November 2012 | 3 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/003 | Febrile neutropenia | Suspected, expected | FC | 30 August 2012 | 8 January 2013 | 13 January 2013 | 5 | Recovered | |
| FCM-miniR/FCR | Infections and infestations | N00319/00189/004 | Neutropenia with fever 37.8 °C | Suspected, expected | FC | 30 August 2012 | 31 January 2013 | 10 February 2013 | 10 | Recovered |
| Treatment received | MedDRA system organ class | SAE ID number | SAE medical description | Trial medication | Product form | First trial dose | Date most recent dose | Most recent dose | Units | Route | Frequency |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FCR | Blood and lymphatic system disorders | N00014/00028/002 | Neutropenia | Fludarabine | Oral | 2 August 2010 | 3 October 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 August 2010 | 3 October 2010 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 August 2010 | 29 September 2010 | 1000 | mg | i.v. | Monthly | ||||
| FCR | Blood and lymphatic system disorders | N00076/00187/001 | Platelet count decreased | Fludarabine | Oral | 29 August 2012 | 22 November 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 29 August 2012 | 22 November 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 29 August 2012 | 22 November 2012 | 900 | mg | i.v. | Once | ||||
| FCR | Blood and lymphatic system disorders | N00080/00032/001 | Infection | Fludarabine | Oral | 2 September 2010 | 3 October 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 September 2010 | 3 October 2010 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 September 2010 | 3 October 2010 | 850 | mg | i.v. | STAT | ||||
| FCR | Blood and lymphatic system disorders | N00098/00017/001 | Extremely low Hb | Fludarabine | Oral | 10 May 2010 | 11 October 2010 | 30 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 10 May 2010 | 11 October 2010 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 10 May 2010 | 11 October 2010 | 700 | mg | i.v. | Once | ||||
| FCR | Blood and lymphatic system disorders | N00098/00017/002 | Low Hb 5.9 g/dl on Pentra | Fludarabine | Oral | 10 May 2010 | 11 October 2010 | 30 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 10 May 2010 | 11 October 2010 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 10 May 2010 | 11 October 2010 | 700 | mg | i.v. | Once | ||||
| FCR | Blood and lymphatic system disorders | N00114/00088/001 | Persistent neutropenia related to chemotherapy | Fludarabine | Oral | 31 May 2011 | 24 September 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 24 September 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 31 May 2011 | 21 September 2011 | 1000 | mg | i.v. | Daily | ||||
| FCR | Blood and lymphatic system disorders | N00175/00155/001 | Anaemia | Fludarabine | Oral | 7 June 2012 | 28 September 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 7 June 2012 | 28 September 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 7 June 2012 | 28 September 2012 | 950 | mg | i.v. | Once a day | ||||
| FCR | Blood and lymphatic system disorders | N00230/00033/002 | Myelodysplasia: RAES | Fludarabine | Oral | 16 September 2010 | 7 February 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 16 September 2010 | 7 February 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 16 September 2010 | 7 February 2011 | 900 | mg | i.v. | Once | ||||
| FCR | Gastrointestinal disorders | N00050/00120/001 | Diarrhoea and vomiting | Fludarabine | Oral | 9 November 2011 | 11 December 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 9 November 2011 | 11 December 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 9 November 2011 | 11 December 2011 | 670 | mg | i.v. | Once | ||||
| FCR | Gastrointestinal disorders | N00098/00060/002 | Nausea and vomiting | Fludarabine | Oral | 27 January 2011 | 25 April 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 January 2011 | 25 April 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 27 January 2011 | 21 April 2011 | 900 | mg | i.v. | Day 1 | ||||
| FCR | Gastrointestinal disorders | N00114/00088/003 | Gastroenteritis | Fludarabine | Oral | 31 May 2011 | 24 September 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 24 September 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 31 May 2011 | 21 September 2011 | 1000 | mg | i.v. | Over 2 days | ||||
| FCR | Gastrointestinal disorders | N00349/00035/001 | Nausea | Fludarabine | i.v. | 5 October 2010 | 16 March 2011 | 27.5 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 5 October 2010 | 16 March 2011 | 280 | mg | i.v. | Daily | ||||
| Rituximab | i.v. | 5 October 2010 | 15 March 2011 | 700 | mg | i.v. | Daily | ||||
| FCR | General disorders and administration site conditions | N00014/00028/004 | Infection/anaemia | Fludarabine | Oral | 2 August 2010 | 28 November 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 August 2010 | 28 November 2010 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 August 2010 | 24 November 2010 | 1000 | mg | i.v. | Daily | ||||
| FCR | General disorders and administration site conditions | N00014/00028/005 | Anaemia grade 4 | Fludarabine | Oral | 2 August 2010 | 28 November 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 August 2010 | 28 November 2010 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 August 2010 | 24 November 2010 | 1000 | mg | i.v. | Monthly | ||||
| FCR | General disorders and administration site conditions | N00014/00029/001 | Rigors | Fludarabine | Oral | 24 August 2010 | 28 August 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 24 August 2010 | 28 August 2010 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 24 August 2010 | 25 August 2010 | 500 | mg | i.v. | Monthly | ||||
| FCR | General disorders and administration site conditions | N00098/00019/001 | Pyrexial 38.2 °C post first dose of Rituximab | Rituximab | i.v. | 17 June 2010 | 17 June 2010 | 100 | mg | i.v. | Once |
| FCR | General disorders and administration site conditions | N00114/00055/001 | Fever and rigors | Fludarabine | Oral | 11 January 2011 | 15 January 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 11 January 2011 | 15 January 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 11 January 2011 | 12 January 2011 | 650 | mg | i.v. | Daily | ||||
| FCR | General disorders and administration site conditions | N00173/00011/001 | Hypoxia | Fludarabine | Oral | 15 April 2010 | 17 April 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 15 April 2010 | 16 April 2010 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 15 April 2010 | 15 April 2010 | 85 | mg | i.v. | Once | ||||
| FCR | General disorders and administration site conditions | N00173/00137/001 | Vomiting | Fludarabine | Oral | 7 March 2012 | 8 April 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 7 March 2012 | 8 April 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 7 March 2012 | 8 April 2012 | 900 | mg | i.v. | Once only | ||||
| FCR | General disorders and administration site conditions | N00218/00168/001 | Fever and non-specifically unwell | Fludarabine | Oral | 9 July 2012 | 11 July 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 9 July 2012 | 11 July 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 9 July 2012 | 10 July 2012 | 750 | mg | i.v. | Days 1–2 | ||||
| FCR | General disorders and administration site conditions | N00319/00125/001 | Allergic reaction | Fludarabine | Oral | 3 February 2012 | 3 February 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 3 February 2012 | 3 February 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 February 2012 | 2 February 2012 | 100 | mg | i.v. | Once | ||||
| FCR | General disorders and administration site conditions | N00391/00194/002 | Probable rituximab reaction | Fludarabine | Oral | 19 September 2012 | 9 January 2013 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 19 September 2012 | 9 January 2013 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 19 September 2012 | 9 January 2013 | 950 | mg | i.v. | Day 1 | ||||
| FCR | Infections and infestations | N00014/00028/003 | Febrile neutropenia | Fludarabine | Oral | 2 August 2010 | 28 November 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 August 2010 | 28 November 2010 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 2 August 2010 | 24 November 2010 | 1000 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00014/00112/001 | Shingles | Fludarabine | Oral | 31 October 2011 | 4 February 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 October 2011 | 4 February 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 31 October 2011 | 30 January 2012 | 1000 | mg | i.v. | Monthly | ||||
| FCR | Infections and infestations | N00040/00056/001 | Neutropenic sepsis | Fludarabine | Oral | 12 January 2011 | 20 February 2011 | 54 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 12 January 2011 | 20 February 2011 | 340 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 12 January 2011 | 20 February 2011 | 1100 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00046/00185/001 | Infection with grade-4 neutrophils | Fludarabine | i.v. | 23 August 2012 | 14 December 2012 | 50 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 23 August 2012 | 14 December 2012 | 500 | mg | i.v. | Daily | ||||
| Rituximab | i.v. | 23 August 2012 | 12 December 2012 | 1000 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00050/00041/001 | Febrile neutropenia | Fludarabine | i.v. | 3 November 2010 | 3 December 2010 | 42 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 3 November 2010 | 3 December 2010 | 420 | mg | i.v. | Daily | ||||
| Rituximab | i.v. | 3 November 2010 | 1 December 2010 | 840 | mg | i.v. | OD 1/7 | ||||
| FCR | Infections and infestations | N00098/00001/001 | Neutropenic sepsis | Fludarabine | Oral | 15 December 2009 | 20 December 2009 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 15 December 2009 | 20 December 2009 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 15 December 2009 | 16 December 2009 | 750 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00098/00060/001 | Fever: no focus identified | Fludarabine | Oral | 27 January 2011 | 28 February 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 January 2011 | 28 February 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 27 January 2011 | 24 February 2011 | 900 | mg | i.v. | 1 day | ||||
| FCR | Infections and infestations | N00098/00060/003 | Probable late PCP/PJP | Fludarabine | Oral | 27 January 2011 | 19 June 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 January 2011 | 19 June 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 27 January 2011 | 15 June 2011 | 900 | mg | i.v. | 1 day | ||||
| FCR | Infections and infestations | N00099/00084/001 | Pyrexia ?neutropenic sepsis | Fludarabine | Oral | 18 May 2011 | 9 October 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 18 May 2011 | 9 October 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 18 May 2011 | 5 October 2011 | 1000 | mg | i.v. | Day 1 | ||||
| FCR | Infections and infestations | N00099/00105/001 | Neutropenic sepsis | Fludarabine | Oral | 5 September 2011 | 7 September 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 5 September 2011 | 7 September 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 5 September 2011 | 6 September 2011 | 600 | mg | i.v. | 1/2 dose daily | ||||
| FCR | Infections and infestations | N00106/00130/001 | Neutropenic sepsis | Fludarabine | Oral | 23 January 2012 | 23 March 2012 | 50 | mg | Oral | OD × 4 days |
| Cyclophosphamide | Oral | 23 January 2012 | 23 March 2012 | 300 | mg | Oral | OD × 4 days | ||||
| Rituximab | i.v. | 23 January 2012 | 19 March 2012 | 1000 | mg | i.v. | 1 Day | ||||
| FCR | Infections and infestations | N00106/00130/002 | Neutropenic sepsis | Fludarabine | Oral | 23 January 2012 | 17 April 2012 | 50 | mg | Oral | OD × 5 days |
| Cyclophosphamide | Oral | 23 January 2012 | 17 April 2012 | 300 | mg | Oral | OD × 5 days | ||||
| Rituximab | i.v. | 23 January 2012 | 17 April 2012 | 1000 | mg | i.v. | OD × 1 day | ||||
| FCR | Infections and infestations | N00106/00179/001 | Pyrexial, neutropenic | Fludarabine | Oral | 25 July 2012 | 30 July 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 25 July 2012 | 30 July 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 25 July 2012 | 25 July 2012 | 700 | mg | i.v. | OD over 2 days | ||||
| FCR | Infections and infestations | N00106/00179/003 | Neutropenic sepsis | Fludarabine | Oral | 25 July 2012 | 2 September 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 25 July 2012 | 2 September 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 25 July 2012 | 31 August 2012 | 900 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00114/00067/001 | Neutropenic sepsis | Fludarabine | Oral | 22 February 2011 | 21 May 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 February 2011 | 21 May 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 22 February 2011 | 21 May 2011 | 950 | mg | i.v. | Day 1 | ||||
| FCR | Infections and infestations | N00114/00067/002 | Neutropenic sepsis ?cause | Fludarabine | Oral | 22 February 2011 | 16 July 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 February 2011 | 16 July 2011 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 22 February 2011 | 13 July 2011 | 950 | mg | i.v. | For second day of cycle | ||||
| FCR | Infections and infestations | N00114/00067/003 | ?Neutropenic sepsis | Fludarabine | Oral | 22 February 2011 | 16 July 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 February 2011 | 16 July 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 22 February 2011 | 16 July 2011 | 950 | mg | i.v. | Once | ||||
| FCR | Infections and infestations | N00114/00067/004 | Neutropenic sepsis | Fludarabine | Oral | 22 February 2011 | 16 July 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 February 2011 | 16 July 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 22 February 2011 | 16 July 2011 | 950 | mg | i.v. | Over 2 days | ||||
| FCR | Infections and infestations | N00114/00088/002 | Neutropenic sepsis | Fludarabine | Oral | 31 May 2011 | 24 September 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 24 September 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 31 May 2011 | 21 September 2011 | 1000 | mg | i.v. | Over 2 days | ||||
| FCR | Infections and infestations | N00153/00026/001 | Infection | Fludarabine | Oral | 28 July 2010 | 1 August 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 28 July 2010 | 1 August 2010 | 350 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 28 July 2010 | 29 July 2010 | 800 | mg | i.v. | Once (split 2 days) | ||||
| FCR | Infections and infestations | N00153/00050/001 | Chest infection | Fludarabine | Oral | 30 November 2010 | 26 February 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 30 November 2010 | 26 February 2011 | 350 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 30 November 2010 | 22 February 2011 | 1100 | mg | i.v. | Once | ||||
| FCR | Infections and infestations | N00173/00011/003 | Lower respiratory tract infection | Fludarabine | Oral | 15 April 2010 | 23 June 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 15 April 2010 | 23 June 2010 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 15 April 2010 | 23 June 2010 | 850 | mg | i.v. | Once only on day 1 of cycle | ||||
| FCR | Infections and infestations | N00218/00168/002 | Neutropenic sepsis | Fludarabine | Oral | 9 July 2012 | 7 September 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 9 July 2012 | 7 September 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 9 July 2012 | 3 September 2012 | 1000 | mg | i.v. | Day 1 | ||||
| FCR | Infections and infestations | N00230/00033/001 | Neutropenic sepsis | Fludarabine | Oral | 16 September 2010 | 14 October 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 16 September 2010 | 14 October 2010 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 16 September 2010 | 14 October 2010 | 700 | mg | i.v. | Once | ||||
| FCR | Infections and infestations | N00231/00174/001 | Febrile, rigors, ?septic. Episodes of diarrhoea for 2 days | Fludarabine | Oral | 24 July 2012 | 22 August 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 24 July 2012 | 22 August 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 24 July 2012 | 21 August 2012 | 900 | mg | i.v. | Per cycle | ||||
| FCR | Infections and infestations | N00231/00174/002 | Febrile illness (pyrexia, rigors) | Fludarabine | Oral | 24 July 2012 | 22 August 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 24 July 2012 | 22 August 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 24 July 2012 | 21 August 2012 | 900 | mg | i.v. | Per cycle | ||||
| FCR | Infections and infestations | N00231/00174/003 | Neutropenic sepsis | Fludarabine | Oral | 24 July 2012 | 30 September 2012 | 44 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 24 July 2012 | 30 September 2012 | 270 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 24 July 2012 | 25 September 2012 | 900 | mg | i.v. | Once per cycle | ||||
| FCR | Infections and infestations | N00255/00138/001 | Neutropenic sepsis | Fludarabine | Oral | 18 March 2012 | 22 March 2012 | 40 | mg | Oral | OD × 5 days |
| Cyclophosphamide | Oral | 18 March 2012 | 22 March 2012 | 250 | mg | Oral | OD × 5 days | ||||
| Rituximab | i.v. | 16 March 2012 | 19 March 2012 | 600 | mg | i.v. | Every 28 days | ||||
| FCR | Infections and infestations | N00255/00166/001 | Febrile neutropenia | Fludarabine | Oral | 4 July 2012 | 12 September 2012 | 30 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 4 July 2012 | 12 September 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 4 July 2012 | 7 September 2012 | 950 | mg | i.v. | Start dose | ||||
| FCR | Infections and infestations | N00255/00166/002 | Neutropenic sepsis | Fludarabine | Oral | 4 July 2012 | 2 January 2013 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 4 July 2012 | 2 January 2013 | 200 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 4 July 2012 | 2 January 2013 | 950 | mg | i.v. | Start dose | ||||
| FCR | Infections and infestations | N00280/00008/001 | Neutropenic sepsis | Fludarabine | Oral | 25 March 2010 | 31 May 2010 | 40 | mg | Oral | 40 mg daily for 5 days |
| Cyclophosphamide | Oral | 25 March 2010 | 31 May 2010 | 300 | mg | Oral | 300 mg daily for 5 days | ||||
| Rituximab | i.v. | 25 March 2010 | 27 May 2010 | 900 | mg | i.v. | Single dose | ||||
| FCR | Infections and infestations | N00319/00125/002 | Febrile neutropenia | Fludarabine | Oral | 2 February 2012 | 6 February 2012 | 40 | mg | Oral | Once a day for 5 days |
| Cyclophosphamide | Oral | 2 February 2012 | 6 February 2012 | 250 | mg | Oral | Once a day for 5 days | ||||
| Rituximab | i.v. | 2 February 2012 | 6 February 2012 | 500 | mg | i.v. | Once a day | ||||
| FCR | Infections and infestations | N00361/00123/002 | Neutropenic sepsis | Fludarabine | Oral | 5 December 2011 | 19 June 2012 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 5 December 2011 | 19 June 2012 | 120 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 5 December 2011 | 19 June 2012 | 800 | mg | i.v. | Daily | ||||
| FCR | Infections and infestations | N00391/00111/001 | Neutropenic sepsis | Fludarabine | Oral | 19 October 2011 | 23 October 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 19 October 2011 | 23 October 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 19 October 2011 | 19 October 2011 | 700 | mg | i.v. | Days 1 and 2 of cycle 1 | ||||
| FCR | Infections and infestations | N00391/00194/001 | ?Neutropenic sepsis ?Viral infection ?Drug-induced gastritis | Fludarabine | Oral | 19 September 2012 | 13 December 2012 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 19 September 2012 | 13 December 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 19 September 2012 | 12 December 2012 | 900 | mg | i.v. | Day 1 | ||||
| FCR | Infections and infestations | N00698/00198/001 | Febrile neutropenia | Fludarabine | Oral | 20 September 2012 | 24 December 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 20 September 2012 | 24 December 2012 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 20 September 2012 | 20 December 2012 | 900 | mg | Oral | Daily | ||||
| FCR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N00098/00002/001 | SCC: two lesions (lower back and central chest) | Fludarabine | i.v. | 14 January 2010 | 3 June 2010 | 55 | mg | i.v. | Once |
| Cyclophosphamide | i.v. | 14 January 2010 | 3 June 2010 | 550 | mg | i.v. | Once | ||||
| Rituximab | i.v. | 14 January 2010 | 2 June 2010 | 1100 | mg | i.v. | Once | ||||
| FCR | Psychiatric disorders | N00349/00104/002 | Psychotic episode | Fludarabine | Oral | 13 September 2011 | 13 December 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 13 September 2011 | 13 December 2011 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 13 September 2011 | 9 December 2011 | 800 | mg | i.v. | Daily | ||||
| FCR | Skin and subcutaneous tissue disorders | N00349/00070/001 | Rash | Fludarabine | Oral | 11 March 2011 | 12 April 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 11 March 2011 | 12 April 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 11 March 2011 | 12 April 2011 | 1000 | mg | i.v. | OD | ||||
| FCR | Skin and subcutaneous tissue disorders | N00353/00122/001 | Erythemous rash grade 3 | Fludarabine | Oral | 17 November 2011 | 16 December 2011 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 November 2011 | 16 December 2011 | 300 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 17 November 2011 | 15 December 2011 | 930 | mg | i.v. | Once | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00009/00127/001 | Pleural effusion and pulmonary embolism | Fludarabine | Oral | 20 December 2011 | 24 December 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 20 December 2011 | 24 December 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 20 December 2011 | 20 December 2011 | 13 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 20 December 2011 | 20 December 2011 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/001 | Neutropenic sepsis | Fludarabine | Oral | 22 July 2010 | 20 September 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 July 2010 | 20 September 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 July 2010 | 16 September 2010 | 10 | mg | i.v. | 15-minute infusion | ||||
| Mini Rituximab | i.v. | 22 July 2010 | 16 September 2010 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00099/00027/002 | Neutropenic sepsis | Fludarabine | Oral | 22 July 2010 | 20 September 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 July 2010 | 20 September 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 July 2010 | 16 September 2010 | 10 | mg | i.v. | 15-minute infusion | ||||
| Mini Rituximab | i.v. | 22 July 2010 | 16 September 2010 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/003 | DCT-negative autoimmune haemolysis | Fludarabine | Oral | 31 May 2011 | 8 October 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 8 October 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 31 May 2011 | 4 October 2011 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 31 May 2011 | 4 October 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00114/00086/004 | Pulmonary embolism | Fludarabine | Oral | 31 May 2011 | 8 October 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 8 October 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 31 May 2011 | 4 October 2011 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 31 May 2011 | 4 October 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00153/00010/001 | Neutropenia | Fludarabine | Oral | 29 March 2010 | 10 July 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 29 March 2010 | 10 July 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 29 March 2010 | 5 July 2010 | 9 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 29 March 2010 | 5 July 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Blood and lymphatic system disorders | N00391/00144/001 | Marked pancytopenia post chemotherapy; cough and SOB | Fludarabine | Oral | 3 May 2012 | 24 September 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 3 May 2012 | 24 September 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 3 May 2012 | 20 September 2012 | 10 | mg | i.v. | Day 1 only | ||||
| Mini Rituximab | i.v. | 3 May 2012 | 20 September 2012 | 100 | mg | i.v. | Day 1 only | ||||
| FCM-miniR | Blood and lymphatic system disorders | N01527/00030/001 | Anaemia | Fludarabine | Oral | 12 August 2010 | 17 August 2010 | 60 | mg | Oral | 5 days |
| Cyclophosphamide | Oral | 12 August 2010 | 17 August 2010 | 350 | mg | Oral | 5 days | ||||
| Mitoxantrone | i.v. | 12 August 2010 | 12 August 2010 | 14.2 | mg | i.v. | Every 28 days | ||||
| Mini Rituximab | i.v. | 12 August 2010 | 12 August 2010 | 100 | mg | i.v. | Every 28 days | ||||
| FCM-miniR | Gastrointestinal disorders | N00050/00083/001 | Vomiting | Fludarabine | i.v. | 11 May 2011 | 7 October 2011 | 52.5 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 11 May 2011 | 7 October 2011 | 520 | mg | i.v. | Daily | ||||
| Mitoxantrone | i.v. | 11 May 2011 | 5 October 2011 | 13 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 11 May 2011 | 5 October 2011 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Gastrointestinal disorders | N00153/00034/001 | Vomiting | Fludarabine | Oral | 17 September 2010 | 21 September 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 September 2010 | 21 September 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 17 September 2010 | 17 September 2010 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 17 September 2010 | 17 September 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Gastrointestinal disorders | N00153/00034/002 | Nausea and vomiting | Fludarabine | Oral | 17 September 2010 | 18 October 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 September 2010 | 18 October 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 17 September 2010 | 14 October 2010 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 17 September 2010 | 14 October 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Gastrointestinal disorders | N01527/00184/001 | Vomiting | Fludarabine | Oral | 23 August 2012 | 27 September 2012 | 30 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 23 August 2012 | 27 September 2012 | 200 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 23 August 2012 | 27 September 2012 | 7.6 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 23 August 2012 | 27 September 2012 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | General disorders and administration site conditions | N00040/00180/001 | Serious rituximab reaction | Cyclophosphamide | Oral | 30 July 2012 | 30 July 2012 | 300 | mg | Oral | Daily |
| Mini Rituximab | i.v. | 30 July 2012 | 30 July 2012 | ||||||||
| FCM-miniR | General disorders and administration site conditions | N00098/00004/001 | High-grade fever | Fludarabine | Oral | 25 February 2010 | 1 March 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 25 February 2010 | 1 March 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 24 February 2010 | 24 February 2010 | 11 | mg | i.v. | Once (given on day 1 only) | ||||
| Mini Rituximab | i.v. | 24 February 2010 | 24 February 2010 | 100 | mg | i.v. | Once (given on day 1 only) | ||||
| FCM-miniR | General disorders and administration site conditions | N00098/00005/003 | Anaphylaxis | Mini Rituximab | i.v. | 17 February 2010 | 17 February 2010 | 35 | Other | i.v. | Once |
| FCM-miniR | General disorders and administration site conditions | N00098/00099/001 | Vasovagal episode with first dose of rituximab | Fludarabine | |||||||
| Cyclophosphamide | |||||||||||
| Mitoxantrone | i.v. | 10 August 2011 | 10 August 2011 | 12 | mg | i.v. | Once | ||||
| Rituximab | i.v. | 10 August 2011 | 10 August 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | General disorders and administration site conditions | N00099/00036/001 | Reaction to rituximab | Fludarabine | Oral | 27 September 2010 | 1 October 2010 | 50 | mg | Oral | For 5 days |
| Cyclophosphamide | Oral | 27 September 2010 | 1 October 2010 | 300 | mg | Oral | For 5 days | ||||
| Mitoxantrone | i.v. | 27 September 2010 | 27 September 2010 | 12 | mg | i.v. | 1-day infusion | ||||
| Mini Rituximab | i.v. | 27 September 2010 | 27 September 2010 | 15 | mg | i.v. | 1-day infusion | ||||
| FCM-miniR | General disorders and administration site conditions | N00153/00156/001 | Infusion reaction | Fludarabine | Oral | 7 June 2012 | 11 June 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 7 June 2012 | 11 June 2012 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 7 June 2012 | 7 June 2012 | 11 | mg | i.v. | OD | ||||
| Mini Rituximab | i.v. | 7 June 2012 | 7 June 2012 | 100 | mg | i.v. | OD | ||||
| FCM-miniR | Immune system disorders | N00361/00109/001 | Haemolytic anaemia | Fludarabine | Oral | 20 October 2011 | 16 November 2011 | 36 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 20 October 2011 | 16 November 2011 | 230 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 20 October 2011 | 16 November 2011 | 9 | mg | i.v. | D1 daily | ||||
| Mini Rituximab | i.v. | 20 October 2011 | 16 November 2011 | 100 | mg | i.v. | D1 daily | ||||
| FCM-miniR | Infections and infestations | N00014/00054/001 | Sepsis | Fludarabine | Oral | 19 January 2011 | 23 January 2011 | 50 | mg | Oral | 5 × days/28 days |
| Cyclophosphamide | Oral | 19 January 2011 | 23 January 2011 | 300 | mg | Oral | 5 × days/28 days | ||||
| Mitoxantrone | i.v. | 19 January 2011 | 19 January 2011 | 13 | mg | i.v. | 1 × 28 days | ||||
| Mini Rituximab | i.v. | 19 January 2011 | 19 January 2011 | 100 | mg | i.v. | 28 days | ||||
| FCM-miniR | Infections and infestations | N00040/00108/001 | Neutropenic sepsis | Fludarabine | Oral | 13 September 2011 | 17 September 2011 | 48 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 13 September 2011 | 17 September 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 13 September 2011 | 17 September 2011 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 13 September 2011 | 13 September 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00040/00108/002 | Pyrexia | Fludarabine | Oral | 13 September 2011 | 17 September 2011 | 48 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 13 September 2011 | 17 September 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 13 September 2011 | 17 September 2011 | 12 | mg | i.v. | 1 day | ||||
| Mini Rituximab | i.v. | 13 September 2011 | 17 September 2011 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Infections and infestations | N00040/00108/004 | Neutropenic sepsis | Fludarabine | Oral | 13 September 2011 | 21 December 2011 | 48 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 13 September 2011 | 21 December 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 13 September 2011 | 21 December 2011 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 13 September 2011 | 21 December 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00050/00095/001 | Neutropenic sepsis | Fludarabine | Oral | 27 July 2011 | 25 December 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 July 2011 | 25 December 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 27 July 2011 | 21 December 2011 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 27 July 2011 | 21 December 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00080/00058/001 | Infection with grade 4 neutrophils | Fludarabine | Oral | 26 January 2011 | 24 April 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 26 January 2011 | 24 April 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 26 January 2011 | 20 April 2011 | 12 | mg | i.v. | STAT | ||||
| Mini Rituximab | i.v. | 26 January 2011 | 20 April 2011 | 100 | mg | i.v. | STAT | ||||
| FCM-miniR | Infections and infestations | N00080/00058/002 | Chest infection/febrile neutropenia | Fludarabine | Oral | 26 January 2011 | 3 July 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 26 January 2011 | 3 July 2011 | 200 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 26 January 2011 | 29 June 2011 | 9 | mg | i.v. | Stat | ||||
| Mini Rituximab | i.v. | 26 January 2011 | 29 June 2011 | 100 | mg | i.v. | Stat | ||||
| FCM-miniR | Infections and infestations | N00098/00005/001 | Pyrexia and rigors | Fludarabine | Oral | 17 February 2010 | 16 March 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 February 2010 | 16 March 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 17 February 2010 | 16 March 2010 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 17 February 2010 | 16 March 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00098/00005/002 | Pyrexia and diarrhoea | Fludarabine | Oral | 17 February 2010 | 16 March 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 February 2010 | 16 March 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 17 February 2010 | 16 March 2010 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 17 February 2010 | 16 March 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00098/00005/004 | Neutropenic sepsis | Fludarabine | Oral | 17 February 2010 | 18 April 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 February 2010 | 18 April 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 17 February 2010 | 14 April 2010 | 12 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00098/00005/005 | Fever, unknown origin, nausea | Fludarabine | Oral | 17 February 2010 | 12 May 2010 | 50 | mg | Oral | 5 days |
| Cyclophosphamide | Oral | 17 February 2010 | 12 May 2010 | 300 | mg | Oral | 5 days | ||||
| Mitoxantrone | i.v. | 17 February 2010 | 12 May 2010 | 12 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00098/00115/001 | Urinary tract infection | Fludarabine | Oral | 2 November 2011 | 26 April 2012 | 50 | mg | Oral | OD Days 1–5 |
| Cyclophosphamide | Oral | 2 November 2011 | 26 April 2012 | 300 | mg | Oral | OD Days 1–5 | ||||
| Mitoxantrone | i.v. | 2 November 2011 | 26 April 2012 | 13 | mg | i.v. | Once only | ||||
| Mini Rituximab | i.v. | 2 November 2011 | 26 April 2012 | 100 | mg | i.v. | Once only | ||||
| FCM-miniR | Infections and infestations | N00099/00027/003 | Neutropenic sepsis | Fludarabine | Oral | 22 July 2010 | 9 January 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 July 2010 | 9 January 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 July 2010 | 5 January 2011 | 10 | mg | i.v. | 15-minute infusion | ||||
| Mini Rituximab | i.v. | 22 July 2010 | 5 January 2011 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Infections and infestations | N00099/00036/002 | Neutropenic sepsis | Fludarabine | Oral | 27 September 2010 | 29 September 2010 | 50 | mg | Oral | For 5 days |
| Cyclophosphamide | Oral | 27 September 2010 | 29 October 2010 | 300 | mg | Oral | For 5 days | ||||
| Mitoxantrone | i.v. | 27 September 2010 | 25 October 2010 | 12 | mg | i.v. | 1-day infusion | ||||
| Mini Rituximab | i.v. | 27 September 2010 | 27 September 2010 | 15 | mg | i.v. | 1-day infusion | ||||
| FCM-miniR | Infections and infestations | N00099/00045/001 | Neutropenic sepsis | Fludarabine | Oral | 17 November 2010 | 16 January 2011 | 50 | mg | Oral | 5 days |
| Cyclophosphamide | Oral | 17 November 2010 | 16 January 2011 | 300 | mg | Oral | 5 days | ||||
| Mitoxantrone | i.v. | 17 November 2010 | 12 January 2011 | 12 | mg | i.v. | Day 1 only | ||||
| Mini Rituximab | i.v. | 17 November 2010 | 12 January 2011 | 100 | mg | i.v. | Day 1 only | ||||
| FCM-miniR | Infections and infestations | N00106/00075/001 | URTI (not neutropenic) and anaemia | Fludarabine | Oral | 23 March 2011 | 28 March 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 23 March 2011 | 28 March 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 23 March 2011 | 23 March 2011 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 23 March 2011 | 23 March 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00106/00075/003 | Neutropenic sepsis | Fludarabine | Oral | 23 March 2011 | 1 May 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 23 March 2011 | 2 May 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 23 March 2011 | 23 March 2011 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 23 March 2011 | 23 March 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00111/00132/001 | Pyrexia neutropenia | Fludarabine | Oral | 1 February 2012 | 2 May 2012 | 45 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 1 February 2012 | 2 May 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 1 February 2012 | 27 April 2012 | 11.5 | mg | i.v. | 28 days | ||||
| Mini Rituximab | i.v. | 1 February 2012 | 27 April 2012 | 100 | mg | i.v. | 28 days | ||||
| FCM-miniR | Infections and infestations | N00111/00132/003 | Opportunistic infection associated with > grade 2 lymphopenia | Fludarabine | Oral | 1 February 2012 | 28 July 2012 | 30 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 1 February 2012 | 28 July 2012 | 200 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 1 February 2012 | 28 July 2012 | 8 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 1 February 2012 | 28 July 2012 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00114/00006/002 | Infection | Fludarabine | Oral | 2 March 2010 | 24 July 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 2 March 2010 | 24 July 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 2 March 2010 | 20 July 2010 | 12 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 2 March 2010 | 20 July 2010 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Infections and infestations | N00114/00023/001 | Bilateral pneumonia | Fludarabine | Oral | 13 July 2010 | 4 November 2010 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 13 July 2010 | 4 November 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 13 July 2010 | 30 November 2010 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 13 July 2010 | 30 November 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00114/00086/001 | Neutropenic sepsis | Fludarabine | Oral | 31 May 2011 | 4 August 2011 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 31 May 2011 | 4 August 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 31 May 2011 | 4 August 2011 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 31 May 2011 | 4 August 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00114/00094/001 | Dehydration and diarrhoea, secondary to low Hb | Fludarabine | Oral | 9 August 2011 | 5 September 2011 | 20 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 9 August 2011 | 5 September 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 9 August 2011 | 5 September 2011 | 9 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 9 August 2011 | 5 September 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00132/00069/001 | Neutropenic sepsis | Fludarabine | Oral | 27 April 2011 | 27 April 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 April 2011 | 27 April 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 27 April 2011 | 27 April 2011 | 10 | mg | i.v. | Day 1 | ||||
| Mini Rituximab | i.v. | 27 April 2011 | 27 April 2011 | 100 | mg | i.v. | Day 1 | ||||
| FCM-miniR | Infections and infestations | N00132/00069/002 | Grade 2 fevers after chemotherapy | Fludarabine | Oral | 27 April 2011 | 25 July 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 27 April 2011 | 25 July 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 27 April 2011 | 25 July 2011 | 10 | mg | i.v. | 1 day | ||||
| Mini Rituximab | i.v. | 27 April 2011 | 25 July 2011 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Infections and infestations | N00153/00010/002 | Febrile neutropenia | Fludarabine | Oral | 29 March 2010 | 10 July 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 29 March 2010 | 10 July 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 29 March 2010 | 5 July 2010 | 9 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 29 March 2010 | 5 July 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00153/00016/001 | Neutropenic sepsis/neutropenic fever | Fludarabine | Oral | 5 May 2010 | 30 August 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 5 May 2010 | 30 August 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 5 May 2010 | 26 August 2010 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 5 May 2010 | 26 August 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00153/00020/001 | Neutropenic sepsis | Fludarabine | Oral | 28 June 2010 | 23 August 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 28 June 2010 | 23 August 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 28 June 2010 | 23 August 2010 | 10 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 28 June 2010 | 23 August 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00153/00020/002 | Neutropenic sepsis | Fludarabine | Oral | 28 June 2010 | 23 August 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 28 June 2010 | 23 August 2010 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 28 June 2010 | 23 August 2010 | 10 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 28 June 2010 | 23 August 2010 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00153/00156/002 | Lower respiratory tract infection | Fludarabine | Oral | 7 June 2012 | 11 June 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 7 June 2012 | 11 June 2012 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 7 June 2012 | 7 June 2012 | 11 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 7 June 2012 | 7 June 2012 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Infections and infestations | N00161/00047/001 | Neutropenic sepsis | Fludarabine | Oral | 16 November 2010 | 20 November 2010 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 16 November 2010 | 20 November 2010 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 16 November 2010 | 16 November 2010 | 11 | mg | i.v. | D1 | ||||
| Mini Rituximab | i.v. | 16 November 2010 | 16 November 2010 | 100 | mg | i.v. | D1 | ||||
| FCM-miniR | Infections and infestations | N00161/00047/002 | Suspected neutropenic sepsis | Fludarabine | i.v. | 16 November 2010 | 12 June 2011 | 30 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 16 November 2010 | 12 June 2011 | 200 | mg | i.v. | Daily | ||||
| Mitoxantrone | i.v. | 16 November 2010 | 12 June 2011 | 8 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 16 November 2010 | 12 June 2011 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Infections and infestations | N00161/00047/003 | Chest infection | Fludarabine | i.v. | 16 November 2010 | 12 June 2011 | 30 | mg | i.v. | Daily |
| Cyclophosphamide | i.v. | 16 November 2010 | 12 June 2011 | 200 | mg | i.v. | Daily | ||||
| Mitoxantrone | i.v. | 16 November 2010 | 12 June 2011 | 8 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 16 November 2010 | 12 June 2011 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Infections and infestations | N00280/00039/001 | Septic/sepsis | Fludarabine | Oral | 4 November 2010 | 1 February 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 4 November 2010 | 1 February 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 4 November 2010 | 27 January 2011 | 9 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 4 November 2010 | 27 January 2011 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR | Infections and infestations | N00319/00080/001 | Neutropenia | Fludarabine | Oral | 22 June 2011 | 26 June 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 June 2011 | 26 June 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 June 2011 | 26 June 2011 | 11 | mg | i.v. | Once only | ||||
| Mini Rituximab | i.v. | 22 June 2011 | 26 June 2011 | 100 | mg | i.v. | Once only | ||||
| FCM-miniR | Infections and infestations | N00319/00080/002 | Temperature 37.8 °C | Fludarabine | Oral | 22 June 2011 | 26 June 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 June 2011 | 26 June 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 June 2011 | 22 June 2011 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 22 June 2011 | 22 June 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Infections and infestations | N00319/00080/003 | Temperature 37.5 °C | Fludarabine | Oral | 22 June 2011 | 17 August 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 June 2011 | 17 August 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 June 2011 | 17 August 2011 | 11 | mg | i.v. | Stat | ||||
| Mini Rituximab | i.v. | 22 June 2011 | 17 August 2011 | 100 | mg | i.v. | Stat | ||||
| FCM-miniR | Infections and infestations | N00319/00080/004 | Fever grade 1 | Fludarabine | Oral | 22 June 2011 | 14 September 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 22 June 2011 | 14 September 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 22 June 2011 | 14 September 2011 | 11 | mg | i.v. | 1 day | ||||
| Mini Rituximab | i.v. | 22 June 2011 | 14 September 2011 | 100 | mg | i.v. | 1 day | ||||
| FCM-miniR | Infections and infestations | N00353/00065/001 | Fever | Fludarabine | Oral | 18 February 2011 | 20 April 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 18 February 2011 | 20 April 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 18 February 2011 | 15 April 2011 | 11 | mg | i.v. | 4 weekly | ||||
| Mini Rituximab | i.v. | 18 February 2011 | 15 April 2011 | 100 | mg | i.v. | 4 weekly | ||||
| FCM-miniR | Infections and infestations | N00391/00071/001 | Grade 3 anaemia and grade 4 febrile neutropenia | Fludarabine | Oral | 24 March 2011 | 21 April 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 24 March 2011 | 21 April 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 24 March 2011 | 21 April 2011 | 10 | mg | i.v. | Day 1 | ||||
| Mini Rituximab | i.v. | 24 March 2011 | 21 April 2011 | 100 | mg | i.v. | Day 1 | ||||
| FCM-miniR | Infections and infestations | N00391/00142/001 | Neutropenia and fever and sweats | Fludarabine | Oral | 27 April 2012 | 1 May 2012 | 40 | mg | Oral | OD Days 1–5 |
| Cyclophosphamide | Oral | 27 April 2012 | 1 May 2012 | 250 | mg | Oral | OD Days 1–5 | ||||
| Mitoxantrone | i.v. | 27 April 2012 | 1 May 2012 | 10 | mg | i.v. | Day 1 | ||||
| Mini Rituximab | i.v. | 27 April 2012 | 1 May 2012 | 100 | mg | i.v. | Day 1 | ||||
| FCM-miniR | Infections and infestations | N01527/00081/002 | Febrile neutropenia | Fludarabine | Oral | 12 May 2011 | 31 August 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 12 May 2011 | 31 August 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 12 May 2011 | 31 August 2011 | 10 | mg | i.v. | OD | ||||
| Mini Rituximab | i.v. | 12 May 2011 | 31 August 2011 | 100 | mg | i.v. | OD | ||||
| FCM-miniR | Infections and infestations | N01527/00081/003 | Febrile neutropenia | Fludarabine | Oral | 12 May 2011 | 31 August 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 12 May 2011 | 31 August 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 12 May 2011 | 31 August 2011 | 10 | mg | i.v. | OD | ||||
| Mini Rituximab | i.v. | 12 May 2011 | 31 August 2011 | 100 | mg | i.v. | OD | ||||
| FCM-miniR | Musculoskeletal and connective tissue disorders | N01527/00081/001 | Infection with normal ANC; ?septic arthritis | Fludarabine | Oral | 12 May 2011 | 9 June 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 12 May 2011 | 9 June 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 12 May 2011 | 9 June 2011 | 10 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 12 May 2011 | 9 June 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N01527/00081/004 | EBV – associated lymphoproliferative disorder | Fludarabine | Oral | 12 May 2011 | 8 August 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 12 May 2011 | 8 August 2011 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 12 May 2011 | 8 August 2011 | 10 | mg | i.v. | Day 1 of cycle | ||||
| Mini Rituximab | i.v. | 12 May 2011 | 8 August 2011 | 100 | mg | i.v. | Day 1 of cycle | ||||
| FCM-miniR | Renal and urinary disorders | N00071/00021/001 | Acute renal failure – tumour lysis syndrome | Fludarabine | Oral | 24 June 2010 | 28 June 2010 | 40 | mg | Oral | 4 weekly |
| Cyclophosphamide | Oral | 24 June 2010 | 28 June 2010 | 200 | mg | Oral | 4 weekly | ||||
| Mitoxantrone | i.v. | 24 June 2010 | 24 June 2010 | 9 | mg | i.v. | 4 weekly | ||||
| Mini Rituximab | i.v. | 24 June 2010 | 24 June 2010 | 100 | mg | i.v. | 4 weekly | ||||
| FCM-miniR | Renal and urinary disorders | N00353/00165/001 | Renal failure CTC grade 3 | Fludarabine | Oral | 6 July 2012 | 10 July 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 6 July 2012 | 10 July 2012 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 6 July 2012 | 6 July 2012 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 6 July 2012 | 6 July 2012 | 100 | mg | i.v. | Once | ||||
| FCM-miniR | Skin and subcutaneous tissue disorders | N00106/00075/002 | Pyrexia/rash – macular/papular | Fludarabine | Oral | 23 March 2011 | 23 April 2011 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 23 March 2011 | 23 April 2011 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 23 March 2011 | 20 April 2011 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 23 March 2011 | 20 April 2011 | 100 | mg | i.v. | Once | ||||
| FCM-miniR/FCR | Gastrointestinal disorders | N00173/00145/001 | Nausea plus vomiting | Fludarabine | Oral | 17 May 2012 | 18 June 2012 | 40 | mg | Oral | Daily for 5 days |
| Cyclophosphamide | Oral | 17 May 2012 | 18 June 2012 | 300 | mg | Oral | Daily for 5 days | ||||
| Mitoxantrone | i.v. | 17 May 2012 | 18 June 2012 | 11 | mg | i.v. | Once on day 1 | ||||
| Mini Rituximab | i.v. | 17 May 2012 | 18 June 2012 | 100 | mg | i.v. | Once on day 1 | ||||
| FCM-miniR/FCR | Gastrointestinal disorders | N00319/00189/001 | Vomiting (grade 3) | Fludarabine | Oral | 7 September 2012 | 12 September 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 7 September 2012 | 12 September 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 7 September 2012 | 7 September 2012 | 9 | mg | i.v. | Once (Day 1) | ||||
| Mini Rituximab | i.v. | 7 September 2012 | 7 September 2012 | 100 | mg | i.v. | Once (Day 1) | ||||
| FCM-miniR/FCR | General disorders and administration site conditions | N00137/00196/002 | Nausea, vomiting and dehydration | Fludarabine | Oral | 18 September 2012 | 22 September 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 18 September 2012 | 22 September 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 18 September 2012 | 18 September 2012 | 10 | mg | i.v. | Once (18 September 2012) | ||||
| Mini Rituximab | i.v. | 18 September 2012 | 18 September 2012 | 100 | mg | i.v. | Once (18 September 2012) | ||||
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/003 | Fever (absence neutropenia) | Fludarabine | Oral | 10 July 2012 | 14 July 2012 | 50 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 10 July 2012 | 14 July 2012 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 10 July 2012 | 14 August 2012 | 12 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 10 July 2012 | 14 August 2012 | 100 | mg | i.v. | Once | ||||
| FCM-miniR/FCR | General disorders and administration site conditions | N00349/00164/004 | Cytokine release grade 3 | Fludarabine | Oral | 10 July 2012 | 18 August 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 10 July 2012 | 18 August 2012 | 300 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 10 July 2012 | 14 August 2012 | 12 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 10 July 2012 | 19 September 2012 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR/FCR | Infections and infestations | N00076/00169/001 | Febrile neutropenia | Fludarabine | Oral | 20 July 2012 | 25 July 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 20 July 2012 | 25 July 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 20 July 2012 | 20 July 2012 | 11 | mg | i.v. | OD | ||||
| Mini Rituximab | i.v. | 20 July 2012 | 20 July 2012 | 100 | mg | i.v. | OD | ||||
| FCM-miniR/FCR | Infections and infestations | N00076/00169/002 | Febrile | Fludarabine | Oral | 20 July 2012 | 15 November 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 20 July 2012 | 15 November 2012 | 250 | mg | Oral | Daily | ||||
| Rituximab | i.v. | 20 July 2012 | 15 November 2012 | 900 | mg | i.v. | Once | ||||
| FCM-miniR/FCR | Infections and infestations | N00137/00196/003 | Neutropenic sepsis | Fludarabine | Oral | 18 September 2012 | 7 February 2013 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 18 September 2012 | 7 February 2013 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 18 September 2012 | 18 September 2012 | ||||||||
| Rituximab | i.v. | 16 October 2012 | 7 February 2013 | 840 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 18 September 2012 | 18 September 2012 | ||||||||
| FCM-miniR/FCR | Infections and infestations | N00137/00196/004 | Neutropenic sepsis | Fludarabine | Oral | 18 September 2012 | 11 February 2013 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 18 September 2012 | 11 February 2013 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 18 September 2012 | 18 September 2012 | ||||||||
| Rituximab | i.v. | 16 October 2012 | 11 February 2013 | 840 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 18 September 2012 | 18 September 2012 | ||||||||
| FCM-miniR/FCR | Infections and infestations | N00153/00148/001 | Febrile neutropenia | Fludarabine | Oral | 21 May 2012 | 21 May 2012 | 40 | mg | Oral | OD for 5 days |
| Cyclophosphamide | Oral | 21 May 2012 | 21 May 2012 | 250 | mg | Oral | OD for 5 days | ||||
| Mitoxantrone | i.v. | 21 May 2012 | 21 May 2012 | 10 | mg | i.v. | OD | ||||
| Mini Rituximab | i.v. | 21 May 2012 | 21 May 2012 | 100 | mg | i.v. | OD | ||||
| FCM-miniR/FCR | Infections and infestations | N00153/00177/001 | Febrile neutropenia | Fludarabine | Oral | 17 July 2012 | 21 July 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 17 July 2012 | 21 July 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 18 July 2012 | 18 July 2012 | 10 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 17 July 2012 | 17 July 2012 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR/FCR | Infections and infestations | N00153/00186/001 | Febrile neutropenia | Fludarabine | Oral | 15 August 2012 | 15 August 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 15 August 2012 | 15 August 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 15 August 2012 | 15 August 2012 | 11 | mg | i.v. | Daily | ||||
| Mini Rituximab | i.v. | 15 August 2012 | 15 August 2012 | 100 | mg | i.v. | Daily | ||||
| FCM-miniR/FCR | Infections and infestations | N00218/00162/001 | Neutropenic fever | Fludarabine | Oral | 25 June 2012 | 24 August 2012 | 40 | mg | Oral | Daily |
| Cyclophosphamide | Oral | 25 June 2012 | 24 August 2012 | 250 | mg | Oral | Daily | ||||
| Mitoxantrone | i.v. | 25 June 2012 | 20 August 2012 | 11 | mg | i.v. | Once | ||||
| Mini Rituximab | i.v. | 25 June 2012 | 20 August 2012 | 100 | mg | i.v. | Once | ||||
| FCM-miniR/FCR | Infections and infestations | N00319/00189/002 | Neutropenic sepsis (febrile neutropenia) | Fludarabine | i.v. | 7 September 2012 | 2 November 2012 | 20 | mg | i.v. | Every 28 days for 3 days |
| Cyclophosphamide | i.v. | 7 September 2012 | 2 November 2012 | 370 | mg | i.v. | Every 28 days for 3 days | ||||
| Rituximab | i.v. | 7 September 2012 | 31 October 2012 | 700 | mg | i.v. | Every 28 days | ||||
| FCM-miniR/FCR | Infections and infestations | N00319/00189/003 | Febrile neutropenia | Fludarabine | i.v. | 7 September 2012 | 29 December 2012 | 20 | mg | i.v. | Every 28 days for 3 days |
| Cyclophosphamide | i.v. | 7 September 2012 | 29 December 2012 | 370 | mg | i.v. | Every 28 days for 3 days | ||||
| Rituximab | i.v. | 7 September 2012 | 27 December 2012 | 700 | mg | i.v. | Every 28 days | ||||
| FCM-miniR/FCR | Infections and infestations | N00319/00189/004 | Neutropenia with fever 37.8 °C | Fludarabine | i.v. | 7 September 2012 | 25 January 2013 | 20 | mg | i.v. | Every 28 days for 3 days |
| Cyclophosphamide | i.v. | 7 September 2012 | 25 January 2013 | 370 | mg | i.v. | Every 28 days for 3 days | ||||
| Rituximab | i.v. | 7 September 2012 | 23 January 2013 | 700 | mg | i.v. | Every 28 days |
| Treatment received | MedDRA system organ class | SAE ID number | SAE medical description | Country | Age at randomisation, years | Sex | SAE case description | Seriousness criteria |
|---|---|---|---|---|---|---|---|---|
| FCR | Blood and lymphatic system disorders | N00153/00091/001 | Anaemia | UK | 59 | Female | Patient phoned feeling short of breath, palpitations. Hb found to be 7.6 g/dl. Admitted for blood transfusions. Follow-up report: discharged 28 June 2011 | Required/prolonged hospitalisation |
| FCR | Cardiac disorders | N00050/00178/001 | Atrial fibrillation | UK | 61 | Male | Admitted with gradual onset chest pain, associated SOB, palpitations and dizziness. Chest radiography and ECG done. Discharged apyrexial and asymptomatic. No action required. Neutropenic but well | Required/prolonged hospitalisation |
| FCR | Cardiac disorders | N00173/00011/002 | Palpitations | UK | 72 | Female | Tachycardia, palpitations and heaviness in central chest area Awaiting 24 hour tape result | Required/prolonged hospitalisation |
| FCR | Cardiac disorders | N00173/00011/004 | Pericardial effusion | UK | 72 | Female | Incidental finding of pericardial effusion at mid-point assessment on CT. An echo is requested, patient is well | Jeopardised patient/required intervention to prevent one of the above |
| FCR | Eye disorders | N00014/00112/002 | Left vitrectomy | UK | 62 | Male | Planned admission for eye surgery for removal of left vitreous haemorrhage | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00014/00028/001 | ?Varicella zoster infection | UK | 67 | Male | Rash and pruritus grade 3. Results from virology screen show inconclusive to Varicella zoster infection | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00098/00001/002 | Viral infection, previously febrile neutropenia | UK | 41 | Male | Elevated temperature and mildly neutropenic. Fever with mild neutropenia. Temperature resolved. Blood cultures and chest radiograph = NAD. Probable viral infection | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00098/00019/002 | Pyrexia of unknown origin | UK | 67 | Male | Recurrent spikes of temperature above 38 °C for several days. No associated symptoms. Admitted 29 April 2011. 5 months since any chemotherapy. CRP high | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00349/00070/002 | Pyrexia | UK | 63 | Male | History of rigors and pyrexia for 2 days – unknown cause of pyrexia. white blood cells 3.5, platelets 127, neutrophils 3.22, Hb 12.3, CRP 26 on 14/4/11. MSU result outstanding. Follow-up report: MSU negative, chest radiograph clear | Required/prolonged hospitalisation |
| FCR | Infections and infestations | N00361/00123/001 | Infection? source | UK | 67 | Female | Admitted secondary to pyrexia 39.5, patient not neutropenic. × 6 episodes of loose stool 13 April 2012. Lower abdominal discomfort. Commenced i.v. Meropenem 1 g (× 3 daily) as allergic to Penicillin. Blood cultures + stool specimen taken. No other obvious source of infection | Required/prolonged hospitalisation |
| FCR | Injury, poisoning and procedural complications | N00014/00025/001 | Corneal abrasion | UK | 76 | Female | Patient had fall and hit head/eye resulting in headache and loss of sight in left eye | Required/prolonged hospitalisation |
| FCR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N01527/00076/001 | Dermatology/skin/other. Metastatic SCC | UK | 72 | Male | Right neck large level II and III nodes and forehead lesions. ?early SCC recurrence from fully excised SCC December 2010. (1-cm margins and no CT evidence of active malignancy). Follow-up report: patient had radical right neck dissection | Jeopardised patient/required intervention to prevent one of the above |
| FCR | Psychiatric disorders | N00349/00104/001 | Steroid-induced agitation | UK | 69 | Male | Steroid-induced psychosis. Intermittent mumbling, confusion, agitation | Required/prolonged hospitalisation |
| FCR | Renal and urinary disorders | N00230/00158/001 | Kidney stone/Renal colic | UK | 53 | Male | Admitted 11 August 2012 with renal colic. Ureteric stent inserted 11 August 2012. Temperature 40 °C. Tonsillitis ± LRTI treated with i.v. antibiotics. Temperature now stable, changed to oral antibiotics. Infection clearing. A/W lithotripsy and uteroscopy to treat kidney stone. Discharged 15 August 2012 | Required/prolonged hospitalisation |
| FCR | Respiratory, thoracic and mediastinal disorders | N00218/00159/001 | SOB?PE. | UK | 65 | Female | SOB, tachycardic, admitted to hospital. Additional information: CTPA result awaited – performed today. Chest radiograph – normal | Required/prolonged hospitalisation |
| FCR | Respiratory, thoracic and mediastinal disorders | N00391/00147/001 | Pleural effusion | UK | 69 | Female | Increased SOB owing to right-sided pleural effusion. Admitted for insertion of right pleural drain, bloods, ECG and chest radiography | Required/prolonged hospitalisation |
| FCR | Skin and subcutaneous tissue disorders | N00050/00093/001 | Rash | UK | 44 | Male | Rash developed within half an hour. On inside of arms, abdomen and thighs. Itchiness started after fourth cycle but rash not present then | Required/prolonged hospitalisation |
| FCR | Skin and subcutaneous tissue disorders | N00106/00179/002 | Severe rash | UK | 68 | Female | Patient noticed knees very red on 22 August 2012, by 25 August 2012 spreading rash everywhere. 2 September 2012: eyes swollen and face swollen. 2 September 2012: seen by consultant – given prednisolone and piriton. 3 September 2012: antibiotics stopped, nausea and temperature 38 °C. Admitted 4 September 2012 owing to rash and associated symptoms. Cycle and fludarabine stopped early. Follow-up report: patient discharged 10 September 2012 – rash much improved | Required/prolonged hospitalisation |
| FCM-miniR | Cardiac disorders | N00098/00003/001 | Tachycardia | UK | 73 | Male | Patient c/o high heart rate, asymptomatic | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00050/00046/001 | Diarrhoea | UK | 66 | Male | Started with severe diarrhoea, more than seven stools a day. Weight loss of over 10 lb since Monday. Struggling to drink. Awaiting investigations | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00111/00132/002 | Diarrhoea | UK | 73 | Male | 4 days of diarrhoea | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N00114/00006/001 | Constipation | UK | 70 | Male | Day 1 course1 FCM-miniR on 2 March 2010. Following treatment, unable to open bowels. Admitted 7 March 2010 with nausea, abdominal discomfort and constipation. Ondansetron stopped, laxatives administered, symptoms settled. Discharged 9 March 2010 | Required/prolonged hospitalisation |
| FCM-miniR | Gastrointestinal disorders | N01527/00184/002 | Bowel obstruction + femoral hernia (right side) | UK | 63 | Female | Patient was admitted to MAU at Royal Blackburn Hospital on 14 October 2012, as patient experienced 5–6 episodes of vomiting (coffee ground vomit), temperature 35.7 °C. Last cycle chemotherapy 27 September 2012. Initially diagnosis was ?GI bleed. However, OGD on 16 October 12 showed ‘nothing abnormal detected’. Ultrasound scan performed on 19 October 12 suggested possible obstruction. CT scan performed on 23 October 12 suggested small bowel obstruction, possibly caused by a right inguinal hernia. Surgery Salpingo-Oophorectomy right side plus repair of right femoral hernia performed on 25 October 2012. Patient will remain in-patient until recovered | Required/prolonged hospitalisation |
| FCM-miniR | General disorders and administration site conditions | N00153/00121/001 | Allergic reaction | UK | 64 | Male | Patient’s wife phoned stating that patient’s mouth was swollen, during phone call symptoms worsened, tongue swelling, throat pain, difficult swallowing. Advised to dial 999 – attended A&E. Had taken second dose Fludarabine 30 minutes prior. Co-trimoxazole started this morning. Further symptoms experienced in A&E when con meds administered again (no chemo taken) | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | General disorders and administration site conditions | N01527/00184/003 | Abdominal pain and vomiting | UK | 63 | Female | Patient admitted to Royal Blackburn Hospital 25 November 2012 with abdominal pain and bile-coloured vomiting. Observations Temperature 36.4 °C, RR14 sats 100%, RA blood pressure 138/102, heart rate 68, AXR – No obstructions. Additional information: Blood results attached. MSU report attached. Will update with more information when available. Additional information in updated SAE (7 December 2012). CT on 30 November 12 showed acute cholecystitis (see attached report). Antibiotic therapy given. Symptoms improved and patient discharged home on 5 December 12. Patient’s case was discussed at Haematology MDT and, owing to abdominal complications recently and having achieved a satisfactory response to 2 cycles FCM-miniR, decision made to stop chemotherapy at this time. Any further treatment given will be off trial | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00098/00040/001 | Neutropenic sepsis and infected shoulder | UK | 63 | Male | Patient admitted with neutropenic sepsis on 11 March 2011 – neutrophils 0.02 × 10/l. Patient gradually deteriorated and died on 3 April 2011 | Patient died |
| FCM-miniR | Infections and infestations | N00099/00096/001 | Low magnesium, neutropenic sepsis | UK | 62 | Female | Admitted from day unit, ?neutropenic sepsis on 29 December 2011, abnormal blood results. Borderline CMV antigen PCR positive | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00114/00086/002 | Ongoing line sepsis | UK | 46 | Male | Tachycardic. Spiking temperatures. 20 September 2011 Temperature 40 °C. Patient took paracetamol. Follow-up report: admitted with tachycardia, temperature spikes. 21 September 2011 sepsis of Hickman line. Discharge 25 September 11 after line removal | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N00349/00135/001 | Pyrexia | UK | 65 | Male | Admitted with temperature 31.7 °C and feeling generally unwell. Neutrophils 1.13. Blood cultures on 1 June 12 Staphylococcus aureus | Required/prolonged hospitalisation |
| FCM-miniR | Infections and infestations | N01527/00081/005 | Anaemia grade 3 | UK | 74 | Female | Patient was already admitted to hospital on 17 August 2011 with neutropenic sepsis. Bloods taken on 18 August 2011 showed grade 3 anaemia and patient was treated with 2 units of blood | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR | Renal and urinary disorders | N00040/00108/003 | Renal colic | UK | 56 | Male | Patient presented with loin pain – renal colic. No surgical input needed. Awaiting CT scan. Discharged home 2 December 2011. Follow-up report: CT scan 4 January 2012 – kidney stone visualised in left ureter at distal end. For radiography again on 17 January 2012 – may need future stone removal if pain persists. Follow-up report: 22 February 2012 No stones visible on radiograph. Discharged from Urology team | Required/prolonged hospitalisation |
| FCM-miniR | Skin and subcutaneous tissue disorders | N00153/00014/001 | Rash | UK | 51 | Male | All over body rash, swollen eyes, rash, red and inflamed not itchy. Subject went to A&E on 16.5.10. Grade 3 SAE. Temperature = 38.7 °C. Follow up: all over body rash – peeling skin, swollen eyes. Temperature 38.7 °C. Patient stopped co-trimoxazole, started pentamidine | Jeopardised patient/required intervention to prevent one of the above |
| FCM-miniR/FCR | Infections and infestations | N00349/00164/001 | Viral URTI | UK | 46 | Male | Profound cough, mild intermittent pyrexia, general malaise | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Infections and infestations | N00349/00164/002 | Pyrexia grade 1 | UK | 46 | Male | Persisting cough since 16 July 12, none productive. Admitted on 4 August 2012 with cough and pyrexia | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Musculoskeletal and connective tissue disorders | N00137/00196/001 | Back pain | UK | 50 | Female | Started chemotherapy 19 September 2012 and BM done 18 September 2012 pre-chemotherapy. c/o lethargic, nausea, sweaty and presented with lower back pain. Reviewed by Doctor at local hospital, localised pain owing to BM, infection ruled out. Admitted for observation and pain control. Discharged 20 September 2012 in stable condition. Cycle 1, Day 1 started 18 September 2012 – cycle ongoing at present. No disruptions to treatment | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00153/00148/002 | All over body rash | UK | 54 | Female | Patient has an all over body rash, admitted into hospital for i.v. fluids, piriton and hydrocortisone | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00218/00162/002 | Allergic rash | UK | 64 | Male | Admitted to hospital c/o allergic rash following antibiotic therapy for neutropenic fever | Required/prolonged hospitalisation |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00218/00162/003 | Rash + swelling of face | UK | 64 | Male | Patient admitted with rash and swelling over face after restarting septrin and aciclovir last week. History of fever at home. Treated with i.v. tazocin and gentamicin – afebrile. Treated with prednisolone. Discharged 17 September 2012. Additional comments Difficult case! Not sure which antibiotic/viral over last 2–3 weeks is responsible | Required/prolonged hospitalisation |
| Treatment received | MedDRA system organ class | SAE ID number | SAE medical description | Suspected relationship to trial treatment | Randomisation date | Date SAE became serious | SAE recovery date | SAE duration (days) | Causality (in addition to trial medications) | Outcome of SAE |
|---|---|---|---|---|---|---|---|---|---|---|
| FCR | Blood and lymphatic system disorders | N00153/00091/001 | Anaemia | Not suspected | 21 June 2011 | 27 June 2011 | 28 June 2011 | 1 | CLL | Recovered |
| FCR | Cardiac disorders | N00050/00178/001 | Atrial fibrillation | Not suspected | 16 July 2012 | 27 September 2012 | 28 September 2012 | 1 | Presumed primary cardiac disorder | Recovered |
| FCR | Cardiac disorders | N00173/00011/002 | Palpitations | Not suspected | 29 March 2010 | 6 June 2010 | 7 June 2010 | 1 | Has a history of palpitations | Recovered |
| FCR | Cardiac disorders | N00173/00011/004 | Pericardial effusion | Not suspected | 29 March 2010 | 28 July 2010 | 18 January 2011 | 174 | CLL | Recovered |
| FCR | Eye disorders | N00014/00112/002 | Left vitrectomy | Not suspected | 19 October 2011 | 9 May 2012 | 9 May 2012 | 0 | Vitreous haemorrhage caused by diabetic retinopathy | Recovered |
| FCR | Infections and infestations | N00014/00028/001 | ?Varicella zoster virus infection | Not suspected | 21 July 2010 | 27 October 2010 | 3 August 2011 | 280 | Varicella zoster virus infection | Recovered |
| FCR | Infections and infestations | N00098/00001/002 | Viral infection, previously febrile neutropenia | Not suspected | 14 December 2009 | 6 December 2009 | 10 December 2009 | 4 | CLL | Recovered |
| FCR | Infections and infestations | N00098/00019/002 | Pyrexia of unknown origin | Not suspected | 8 June 2010 | 29 April 2011 | 4 May2011 | 5 | Recovered | |
| FCR | Infections and infestations | N00349/00070/002 | Pyrexia | Not suspected | 9 March 2011 | 14 June 2011 | 20 June 2011 | 6 | Recovered | |
| FCR | Infections and infestations | N00361/00123/001 | Infection? source | Not suspected | 21 November 2011 | 11 April 2012 | 15 April 2012 | 4 | Acquired viral infection | Recovered |
| FCR | Injury, poisoning and procedural complications | N00014/00025/001 | Corneal abrasion | Not suspected | 16 July 2010 | 21 December 2010 | 23 December 2010 | 2 | Patient tripped over shoe and fell | Recovered with sequelae |
| FCR | Neoplasms benign, malignant and unspecified (including cysts and polyps) | N01527/00076/001 | Dermatology/skin/other. Metastatic SCC | Not suspected | 5 April 2011 | 26 May 2011 | 20 June 2011 | 25 | Previous SCC | Recovered |
| FCR | Psychiatric disorders | N00349/00104/001 | Steroid-induced agitation | Not suspected | 25 August 2011 | 9 September 2011 | 12 September 2011 | 3 | Concomitant medications | Recovered |
| FCR | Renal and urinary disorders | N00230/00158/001 | Kidney stone/renal colic | Not suspected | 12 June 2012 | 11 August 2012 | 15 August 2012 | 4 | Pre-existing condition | Recovered with sequelae |
| FCR | Respiratory, thoracic and mediastinal disorders | N00218/00159/001 | SOB ?PE | Not suspected | 14 June 2012 | 17 November 2012 | 3 December 2012 | 16 | Immobility | Recovered with sequelae |
| FCR | Respiratory, thoracic and mediastinal disorders | N00391/00147/001 | Pleural effusion | Not suspected | 3 May 2012 | 8 May 2012 | 10 May 2012 | 2 | CLL | Recovered |
| FCR | Skin and subcutaneous tissue disorders | N00050/00093/001 | Rash | Not suspected | 6 July 2011 | 14 November 2011 | 14 November 2011 | 0 | Viral fever | Recovered |
| FCR | Skin and subcutaneous tissue disorders | N00106/00179/002 | Severe rash | Not suspected | 17 July 2012 | 31 August 2012 | 10 September 2012 | 10 | Concomitant medications | Recovered with sequelae |
| FCM-miniR | Cardiac disorders | N00098/00003/001 | Tachycardia | Not suspected | 15 January 2010 | 29 January 2010 | 30 January 2010 | 1 | Recovered | |
| FCM-miniR | Gastrointestinal disorders | N00050/00046/001 | Diarrhoea | Not suspected | 10 November 2010 | 24 March 2011 | 4 April 2011 | 11 | Rotavirus infection | Recovered |
| FCM-miniR | Gastrointestinal disorders | N00111/00132/002 | Diarrhoea | Not suspected | 30 January 2012 | 4 June 2012 | 7 June 2012 | 3 | Gastroenteritis | Recovered |
| FCM-miniR | Gastrointestinal disorders | N00114/00006/001 | Constipation | Not suspected | 22 February 2010 | 7 March 2010 | 9 March 2010 | 2 | Concomitant medications; past history of constipation and abdominal surgery | Recovered |
| FCM-miniR | Gastrointestinal disorders | N01527/00184/002 | Bowel obstruction + femoral hernia (right side) | Not suspected | 6 August 2012 | 15 October 2012 | 14 November 2012 | 30 | Now known to have been a bowel obstruction + right sided femoral hernia | Recovered |
| FCM-miniR | General disorders and administration site conditions | N00153/00121/001 | Allergic reaction | Not suspected | 7 November 2011 | 11 November 2011 | 11 November 2011 | 0 | Concomitant medications: co-trimoxazole and aciclovir | Recovered with sequelae |
| FCM-miniR | General disorders and administration site conditions | N01527/00184/003 | Abdominal pain and vomiting | Not suspected | 6 August 2012 | 25 November 2012 | 5 December 2012 | 10 | Cholecystitis | Recovered |
| FCM-miniR | Infections and infestations | N00098/00040/001 | Neutropenic sepsis and infected shoulder | Not suspected | 15 October 2010 | 11 March 2011 | 23 | CLL | Died | |
| FCM-miniR | Infections and infestations | N00099/00096/001 | Low magnesium, neutropenic sepsis | Not suspected | 25 July 2011 | 29 December 2011 | 4 January 2012 | 6 | Diarrhoea of unknown cause | Recovered with sequelae |
| FCM-miniR | Infections and infestations | N00114/00086/002 | Ongoing line sepsis | Not suspected | 18 May 2011 | 21 September 2011 | 27 September 2011 | 6 | CLL | Recovered |
| FCM-miniR | Infections and infestations | N00349/00135/001 | Pyrexia | Not suspected | 22 February 2012 | 31 May 2012 | 4 June 2012 | 4 | CLL | Recovered |
| FCM-miniR | Infections and infestations | N01527/00081/005 | Anaemia grade 3 | Not suspected | 5 May 2011 | 18 August 2011 | 8 September 2011 | 21 | Sepsis | Recovered |
| FCM-miniR | Renal and urinary disorders | N00040/00108/003 | Renal colic | Not suspected | 8 September 2011 | 1 December 2011 | 20 February 2012 | 81 | Kidney stone | Recovered |
| FCM-miniR | Skin and subcutaneous tissue disorders | N00153/00014/001 | Rash | Not suspected | 26 April 2010 | 16 May 2010 | 7 June 2010 | 22 | Concomitant medications | Recovered |
| FCM-miniR/FCR | Infections and infestations | N00349/00164/001 | Viral URTI | Not suspected | 28 June 2012 | 13 July 2012 | 23 July 2012 | 10 | Viral URTI | Recovered |
| FCM-miniR/FCR | Infections and infestations | N00349/00164/002 | Pyrexia grade 1 | Not suspected | 28 June 2012 | 4 August 2012 | 10 August 2012 | 6 | Viral illness | Recovered |
| FCM-miniR/FCR | Musculoskeletal and connective tissue disorders | N00137/00196/001 | Back pain | Not suspected | 17 September 2012 | 20 September 2012 | 20 September 2012 | 0 | Recovered with sequelae | |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00153/00148/002 | All over body rash | Not suspected | 4 May 2012 | 15 June 2012 | 19 June 2012 | 4 | Concomitant medications | Recovered |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00218/00162/002 | Allergic rash | Not suspected | 20 June 2012 | 5 September 2012 | 12 September 2012 | 7 | Concomitant medications | Recovered with sequelae |
| FCM-miniR/FCR | Skin and subcutaneous tissue disorders | N00218/00162/003 | Rash + swelling of face | Not suspected | 20 June 2012 | 14 September 2012 | 17 September 2012 | 3 | Concomitant medications | Recovered |
Appendix 2 Adverse event listings
| Patient number | Treatment received | AE description | Other AE description | Maximum CTCAE grade | Treatment cycle AE started | Related to fludarabine? | Related to cyclophosphamide? | Related to rituximab? | Date of onset | Recovered? | Date of recovery | Duration of AE (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 15 December 2009 | Yes | 15 December 2009 | 0 | |
| FCR | Anaemia | 3 | 1 | Probably | Probably | Possibly | 21 December 2009 | No | ||||
| FCR | Anaemia | 4 | 1 | Probably | Probably | Possibly | 2 January 2010 | Yes | 12 January 2010 | 10 | ||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Possibly | 20 January 2010 | No | ||||
| FCR | Nausea | 2 | 3 | Probably | Probably | Probably | 17 February 2010 | Yes | 22 February 2010 | 5 | ||
| FCR | Neutropenia | 3 | 4 | Probably | Probably | Possibly | 12 April 2010 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 1 | 4 | Possibly | Possibly | Possibly | No | |||||
| 2 | FCR | Hypotension | 2 | 1 | Unrelated | Unrelated | Almost certainly | 14 January 2010 | Yes | 14 January 2010 | 0 | |
| FCR | Nausea | 2 | 1 | Probably | Probably | Possibly | 15 January 2010 | Yes | 25 January 2010 | 10 | ||
| FCR | Infusional reaction | 2 | 2 | Unlikely | Unlikely | Almost certainly | 11 February 2010 | Yes | 11 February 2010 | 0 | ||
| FCR | Nausea | 2 | 2 | Probably | Probably | Possibly | 11 February 2010 | Yes | 22 February 2010 | 11 | ||
| FCR | Vomiting | 2 | 2 | Probably | Probably | Possibly | 11 February 2010 | Yes | 22 February 2010 | 11 | ||
| FCR | Nausea | 1 | 3 | Probably | Probably | Possibly | 11 March 2010 | Yes | 13 March 2010 | 2 | ||
| FCR | Vomiting | 1 | 3 | Probably | Probably | Possibly | 11 March 2010 | Yes | 11 March 2010 | 0 | ||
| FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Possibly | 9 April 2010 | Yes | 15 April 2010 | 6 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Possibly | Possibly | Possibly | 19 April 2010 | Yes | 29 April 2010 | 10 | ||
| FCR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Possibly | 6 May 2010 | Yes | 12 May 2010 | 6 | ||
| FCR | Vomiting | 2 | 5 | Almost certainly | Almost certainly | Possibly | 6 May 2010 | Yes | 7 May 2010 | 1 | ||
| FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Possibly | 3 June 2010 | Yes | 8 June 2010 | 5 | ||
| FCR | Vomiting | 2 | 6 | Almost certainly | Almost certainly | Possibly | 3 June 2010 | Yes | 4 June 2010 | 1 | ||
| FCR | Rash/flushing | 1 | 6 | Possibly | Possibly | Possibly | No | |||||
| 7 | FCR | Constipation | 1 | 1 | Unrelated | Unrelated | Unrelated | 24 March 2010 | Yes | 29 March 2010 | 5 | |
| FCR | Mucositis/thrush | 2 | 5 | Unrelated | Unrelated | Unrelated | 14 July 2010 | No | ||||
| 8 | FCR | Fatigue | 1 | 1 | Probably | Unrelated | Unrelated | 26 March 2010 | Yes | 1 April 2010 | 6 | |
| FCR | Hypotension | 1 | 1 | Unrelated | Unrelated | Almost certainly | 25 March 2010 | Yes | 25 March 2010 | 0 | ||
| FCR | Diarrhoea | 1 | 3 | Unrelated | Unrelated | Possibly | 26 March 2010 | Yes | 8 June 2010 | 74 | ||
| FCR | Fatigue | 2 | 6 | Probably | Unrelated | Unrelated | 3 August 2010 | Yes | 8 August 2010 | 5 | ||
| 9 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 31 March 2010 | Yes | 3 April 2010 | 3 | |
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 31 March 2010 | Yes | 1 April 2010 | 1 | ||
| FCR | Diarrhoea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 2 April 2010 | Yes | 4 April 2010 | 2 | ||
| FCR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 31 March 2010 | Yes | 4 April 2010 | 4 | ||
| FCR | Fatigue | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 27 April 2010 | Yes | 30 April 2010 | 3 | ||
| FCR | Non-specific pain | 1 | 2 | Unlikely | Unlikely | Unlikely | Yes | |||||
| FCR | Fatigue | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 May 2010 | Yes | 28 May 2010 | 2 | ||
| FCR | Alopecia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCR | Other AE description | Varicocele | 2 | 3 | Unlikely | Unlikely | Unlikely | No | ||||
| FCR | Fatigue | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 22 July 2010 | Yes | 25 July 2010 | 3 | ||
| FCR | Dry skin/erythema | 1 | 4 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Almost certainly | 17 August 2010 | No | ||||
| FCR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 17 August 2010 | No | ||||
| 11 | FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 May 2010 | Yes | 26 May 2010 | 22 | |
| FCR | Anaemia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 19 May 2010 | Yes | 26 May 2010 | 7 | ||
| FCR | Nausea | 1 | 1 | Probably | Probably | Unlikely | 15 April 2010 | Yes | 26 April 2010 | 11 | ||
| FCR | Constipation | 1 | 1 | Unrelated | Unrelated | Unrelated | 14 April 2010 | Yes | 26 April 2010 | 12 | ||
| FCR | Thrombocytopenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 24 April 2010 | Yes | 4 May 2010 | 10 | ||
| FCR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 11 June 2010 | No | ||||
| FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 18 June 2010 | No | ||||
| FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Almost certainly | 12 July 2010 | No | ||||
| FCR | Anaemia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 8 July 2010 | Yes | 27 July 2010 | 19 | ||
| FCR | Renal impairment | 1 | 3 | Unlikely | Unlikely | Unlikely | 6 July 2010 | Yes | 7 July 2010 | 1 | ||
| 13 | FCR | Infusional reaction | 2 | 2 | Unrelated | Unrelated | Unrelated | 9 June 2010 | Yes | 9 June 2010 | 0 | |
| FCR | Vomiting | 1 | 3 | Probably | Probably | Unrelated | 15 June 2010 | Yes | 16 June 2010 | 1 | ||
| FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | 1 September 2010 | Yes | 6 September 2010 | 5 | ||
| 17 | FCR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Possibly | 12 May 2010 | Yes | 17 May 2010 | 5 | |
| FCR | Vomiting | 2 | 1 | Almost certainly | Almost certainly | Possibly | 12 May 2010 | Yes | 12 May 2010 | 0 | ||
| FCR | Rash/flushing | 2 | 1 | Possibly | Possibly | Possibly | 21 May 2010 | Yes | 28 May 2010 | 7 | ||
| FCR | Cough | 1 | 1 | Possibly | Possibly | Possibly | 15 May 2010 | Yes | 25 May 2010 | 10 | ||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Possibly | No | |||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Possibly | 7 June 2010 | Yes | ||||
| FCR | Rash/flushing | 2 | 2 | Possibly | Possibly | Possibly | 12 June 2010 | Yes | 16 June 2010 | 4 | ||
| FCR | Headache | 2 | 2 | Possibly | Possibly | Possibly | 28 June 2010 | Yes | 28 June 2010 | 0 | ||
| FCR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Possibly | 7 July 2010 | Yes | 10 July 2010 | 3 | ||
| FCR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Possibly | 8 July 2010 | Yes | 10 July 2010 | 2 | ||
| FCR | Fatigue | 2 | 3 | Probably | Probably | Probably | 7 July 2010 | Yes | 23 July 2010 | 16 | ||
| FCR | Cough | 1 | 3 | Possibly | Possibly | Possibly | 12 July 2010 | Yes | ||||
| FCR | Anorexia/cachexia | 1 | 3 | Possibly | Possibly | Possibly | No | |||||
| FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Possibly | 4 August 2010 | Yes | 6 August 2010 | 2 | ||
| FCR | Vomiting | 2 | 4 | Almost certainly | Almost certainly | Possibly | 4 August 2010 | Yes | 4 August 2010 | 0 | ||
| FCR | Fatigue | 1 | 4 | Almost certainly | Almost certainly | Possibly | 2 August 2010 | Yes | ||||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Possibly | No | |||||
| FCR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Possibly | 14 September 2010 | Yes | 21 September 2010 | 7 | ||
| FCR | Vomiting | 1 | 5 | Almost certainly | Almost certainly | Possibly | 20 September 2010 | Yes | 21 September 2010 | 1 | ||
| FCR | Fatigue | 1 | 5 | Probably | Probably | Probably | No | |||||
| FCR | Nausea | 3 | 6 | Almost certainly | Almost certainly | Possibly | 13 October 2010 | Yes | 20 October 2010 | 7 | ||
| FCR | Vomiting | 2 | 6 | Almost certainly | Almost certainly | Possibly | 13 October 2010 | Yes | 15 October 2010 | 2 | ||
| FCR | Fatigue | 2 | 6 | Almost certainly | Almost certainly | Possibly | 13 October 2010 | No | ||||
| FCR | Anaemia | 4 | 6 | Almost certainly | Almost certainly | Possibly | 3 November 2010 | Yes | 4 November 2010 | 1 | ||
| FCR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Possibly | 5 November 2010 | Yes | 6 November 2010 | 1 | ||
| FCR | Thrombocytopenia | 3 | 6 | Almost certainly | Almost certainly | Possibly | 4 November 2010 | No | ||||
| FCR | Fatigue | 4 | 6 | Probably | Probably | Possibly | 1 November 2010 | Yes | 4 November 2010 | 3 | ||
| FCR | Anorexia/cachexia | 3 | 6 | Probably | Probably | Unlikely | 13 October 2010 | Yes | 4 November 2010 | 22 | ||
| 18 | FCR | Nausea | 1 | 1 | Probably | Probably | Unlikely | 2 June 2010 | Yes | |||
| FCR | Neutropenia | 3 | 1 | Probably | Probably | Probably | 7 June 2010 | Yes | 10 June 2010 | 3 | ||
| FCR | Nausea | 1 | 2 | Probably | Probably | Unlikely | Yes | |||||
| FCR | Neutropenia | 4 | 2 | Probably | Probably | Probably | 6 July 2010 | Yes | 26 July 2010 | 20 | ||
| FCR | Diarrhoea | 1 | 3 | Possibly | Possibly | Unlikely | Yes | |||||
| FCR | Neutropenia | 4 | 3 | Probably | Probably | Probably | 23 August 2010 | Yes | 31 August 2010 | 8 | ||
| FCR | Headache | 1 | 3 | Unlikely | Unlikely | Unlikely | Yes | |||||
| FCR | Neutropenia | 4 | 4 | Probably | Probably | Probably | 13 September 2010 | Yes | 27 September 2010 | 14 | ||
| FCR | Neutropenia | 4 | 5 | Probably | Probably | Probably | 25 October 2010 | Yes | 01 November 2010 | 7 | ||
| FCR | Cough | 1 | 5 | Unlikely | Unlikely | Unlikely | Yes | |||||
| 19 | FCR | Vomiting | 2 | 1 | Unrelated | Unrelated | Almost certainly | 17 June 2010 | Yes | 17 June 2010 | 0 | |
| FCR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Possibly | 21 June 2010 | Yes | 25 June 2010 | 4 | ||
| FCR | Diarrhoea | 1 | 1 | Probably | Probably | Possibly | 19 June 2010 | Yes | 20 June 2010 | 1 | ||
| FCR | Constipation | 1 | 1 | Possibly | Possibly | Possibly | Yes | |||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Possibly | 19 July 2010 | Yes | 25 July 2010 | 6 | ||
| FCR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Possibly | 21 July 2010 | Yes | 22 July 2010 | 1 | ||
| FCR | Constipation | 1 | 2 | Possibly | Possibly | Possibly | 22 July 2010 | Yes | 23 July 2010 | 1 | ||
| FCR | Fatigue | 1 | 2 | Probably | Probably | Probably | 23 July 2010 | Yes | 25 July 2010 | 2 | ||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 11 August 2010 | Yes | 8 September 2010 | 28 | ||
| FCR | Anaemia | 1 | 2 | Probably | Probably | Probably | 11 August 2010 | Yes | 8 September 2010 | 28 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Possibly | 15 August 2010 | Yes | 22 August 2010 | 7 | ||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Possibly | 11 September 2010 | Yes | 19 September 2010 | 8 | ||
| FCR | Fatigue | 1 | 4 | Probably | Probably | Possibly | Yes | 19 September 2010 | ||||
| FCR | Other AE description | Gum recession resulting in loose tooth | 1 | 4 | Possibly | Possibly | Possibly | No | ||||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Possibly | 10 October 2010 | No | ||||
| FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Possibly | 8 November 2010 | Yes | 13 November 2010 | 5 | ||
| FCR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Possibly | 1 December 2010 | No | ||||
| 24 | FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Unlikely | 29 July 2010 | Yes | 16 August 2010 | 18 | |
| FCR | Abdominal pain/bloating | 1 | 1 | Possibly | Possibly | Possibly | 22 July 2010 | Yes | 29 July 2010 | 7 | ||
| FCR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Unlikely | 8 September 2010 | Yes | 13 September 2010 | 5 | ||
| FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 6 October 2010 | Yes | 11 October 2010 | 5 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Unlikely | 3 November 2010 | No | ||||
| FCR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 3 November 2010 | Yes | 27 June 2011 | 236 | ||
| FCR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 3 November 2010 | Yes | 6 December 2011 | 398 | ||
| 25 | FCR | Vomiting | 2 | 2 | Probably | Probably | Unlikely | 27 August 2010 | Yes | 29 August 2010 | 2 | |
| FCR | Thrombocytopenia | 2 | 2 | Probably | Probably | Probably | 20 September 2010 | No | ||||
| FCR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 29 September 2010 | Yes | 4 October 2010 | 5 | ||
| FCR | Diarrhoea | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 29 September 2010 | Yes | 4 October 2010 | 5 | ||
| FCR | Infections (not neutropenic sepsis) | 1 | 3 | Unlikely | Unlikely | Unlikely | 17 September 2010 | Yes | 29 September 2010 | 12 | ||
| FCR | Rash/flushing | 2 | 3 | Possibly | Possibly | Possibly | 28 August 2010 | Yes | 29 September 2010 | 32 | ||
| FCR | Thrombocytopenia | 2 | 4 | Probably | Probably | Unlikely | 27 September 2010 | Yes | 29 November 2010 | 63 | ||
| FCR | Diarrhoea | 1 | 5 | Unrelated | Unrelated | Unrelated | 30 December 2010 | Yes | 10 January 2011 | 11 | ||
| 26 | FCR | Rash/flushing | 2 | 1 | Unrelated | Unrelated | Unrelated | 12 August 2010 | Yes | 14 August 2010 | 2 | |
| FCR | Sore throat | 1 | 6 | Possibly | Possibly | Possibly | ||||||
| FCR | Dyspnoea | 1 | 6 | Unlikely | Unlikely | Unlikely | ||||||
| 28 | FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 3 August 2010 | Yes | 7 August 2010 | 4 | |
| FCR | Pruritus | 1 | 3 | Unlikely | Unlikely | Unlikely | 15 September 2010 | No | ||||
| FCR | Rash/flushing | 3 | 4 | Possibly | Unlikely | Possibly | 10 November 2010 | Yes | 22 December 2010 | 42 | ||
| FCR | Neutropenic sepsis | 4 | 4 | Almost certainly | Almost certainly | Possibly | 4 December 2010 | Yes | 13 December 2010 | 9 | ||
| FCR | Fatigue | 2 | 4 | Probably | Probably | Possibly | 4 December 2010 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | 28 December 2010 | No | ||||
| FCR | Anaemia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 4 January 2011 | No | ||||
| FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | 4 January 2011 | No | ||||
| 29 | FCR | Cough | 2 | 3 | Unlikely | Unlikely | Unlikely | 4 October 2010 | Yes | 17 October 2010 | 13 | |
| FCR | Vomiting | 1 | 3 | Unlikely | Unlikely | Unlikely | 22 October 2010 | Yes | 26 October 2010 | 4 | ||
| FCR | Infections (not neutropenic sepsis) | 1 | 4 | Probably | Probably | Possibly | 23 November 2010 | Yes | 30 December 2010 | 37 | ||
| FCR | Nausea | 1 | 6 | Probably | Probably | Unlikely | 10 January 2011 | Yes | 18 January 2011 | 8 | ||
| 32 | FCR | Anorexia/cachexia | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 8 September 2010 | Yes | 10 September 2010 | 2 | |
| FCR | Fatigue | 3 | 2 | Almost certainly | Almost certainly | Unlikely | 6 October 2010 | Yes | 7 October 2010 | 1 | ||
| FCR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 6 October 2010 | Yes | 8 October 2010 | 2 | ||
| FCR | Anorexia/cachexia | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 6 October 2010 | Yes | 9 October 2010 | 3 | ||
| 33 | FCR | Neutropenic sepsis | 4 | 1 | Probably | Probably | Probably | 26 September 2010 | Yes | 5 October 2010 | 9 | |
| FCR | Allergic reaction | 3 | 1 | Unrelated | Unrelated | Unrelated | 28 September 2010 | Yes | 8 October 2010 | 10 | ||
| FCR | Nausea | 1 | 2 | Probably | Probably | Probably | 14 October 2010 | Yes | 18 October 2010 | 4 | ||
| FCR | Neuropathy (sensory) | 1 | 2 | Probably | Probably | Probably | 20 October 2010 | No | ||||
| FCR | Bone pain | 1 | 2 | Unlikely | Unlikely | Unlikely | 22 October 2010 | Yes | 7 December 2010 | 46 | ||
| FCR | Anxiety/depression | 1 | 2 | Unrelated | Unrelated | Unrelated | 12 October 2010 | Yes | 7 December 2010 | 56 | ||
| FCR | Fatigue | 1 | 3 | Probably | Probably | Probably | 11 November 2010 | No | ||||
| FCR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 16 November 2010 | Yes | 19 November 2010 | 3 | ||
| FCR | Insomnia | 1 | 3 | Unrelated | Unrelated | Unrelated | 11 November 2010 | No | ||||
| FCR | Alopecia | 1 | 5 | Possibly | Possibly | Possibly | 12 January 2011 | No | ||||
| FCR | Vomiting | 1 | 5 | Missing | Missing | Missing | 6 January 2011 | Yes | 12 January 2011 | 6 | ||
| FCR | Anxiety/depression | 1 | 5 | Unlikely | Unlikely | Unlikely | 10 January 2011 | Yes | 3 February 2011 | 24 | ||
| FCR | Night sweats | 1 | 5 | Unrelated | Unrelated | Unrelated | 15 November 2010 | Yes | 3 February 2011 | 80 | ||
| FCR | Insomnia | 1 | 6 | Unrelated | Unrelated | Unrelated | 11 December 2010 | No | ||||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 10 December 2010 | Yes | 1 March 2011 | 81 | ||
| FCR | Taste alteration | 1 | 6 | Possibly | Possibly | Possibly | 26 February 2011 | Yes | 01 March 2011 | 3 | ||
| FCR | Rash/flushing | 1 | 6 | Possibly | Possibly | Possibly | 1 February 2011 | Yes | 1 March 2011 | 28 | ||
| 35 | FCR | Dizziness | 2 | 1 | Possibly | Possibly | Unlikely | 8 October 2010 | Yes | 10 October 2010 | 2 | |
| FCR | Nausea | 2 | 1 | Probably | Probably | Unlikely | 8 October 2010 | Yes | 10 October 2010 | 2 | ||
| FCR | Fatigue | 2 | 1 | Probably | Probably | Unlikely | 8 October 2010 | Yes | 10 October 2010 | 2 | ||
| FCR | Anorexia/cachexia | 2 | 1 | Probably | Probably | Unlikely | 8 October 2010 | Yes | 10 October 2010 | 2 | ||
| FCR | Diarrhoea | 1 | 1 | Probably | Probably | Unlikely | 10 October 2010 | Yes | 10 October 2010 | 0 | ||
| FCR | Anaemia | 1 | 1 | Probably | Probably | Unlikely | 5 October 2010 | No | ||||
| FCR | Neutropenia | 2 | 1 | Probably | Probably | Unlikely | 19 October 2010 | Yes | 5 November 2010 | 17 | ||
| FCR | Thrombocytopenia | 1 | 1 | Probably | Probably | Unlikely | 7 October 2010 | Yes | 12 October 2010 | 5 | ||
| FCR | Nausea | 2 | 2 | Probably | Probably | Unlikely | 7 November 2010 | Yes | 26 November 2010 | 19 | ||
| FCR | Vomiting | 2 | 2 | Probably | Probably | Unlikely | 7 November 2010 | Yes | 9 November 2010 | 2 | ||
| FCR | Fatigue | 2 | 2 | Probably | Probably | Unlikely | 7 November 2010 | Yes | 12 November 2010 | 5 | ||
| FCR | Diarrhoea | 2 | 2 | Probably | Probably | Unlikely | 10 November 2010 | Yes | 10 November 2010 | 0 | ||
| FCR | Anorexia/cachexia | 2 | 2 | Probably | Probably | Unlikely | 7 November 2010 | Yes | 12 November 2010 | 5 | ||
| FCR | Anaemia | 2 | 2 | Probably | Probably | Unlikely | 18 November 2010 | No | ||||
| FCR | Nausea | 2 | 3 | Probably | Probably | Unlikely | 7 December 2010 | No | ||||
| FCR | Vomiting | 3 | 3 | Probably | Probably | Unlikely | 8 December 2010 | Yes | 9 December 2010 | 1 | ||
| FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Unlikely | 18 November 2010 | Yes | 9 December 2010 | 21 | ||
| FCR | Fatigue | 2 | 3 | Probably | Probably | Unlikely | 7 December 2010 | Yes | 24 December 2010 | 17 | ||
| FCR | Anorexia/cachexia | 2 | 3 | Probably | Unlikely | Unlikely | 7 December 2010 | Yes | 10 December 2010 | 3 | ||
| FCR | Anaemia | 1 | 3 | Probably | Probably | Unlikely | 18 December 2010 | No | ||||
| FCR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 17 December 2010 | Yes | 23 December 2010 | 6 | ||
| FCR | Anorexia/cachexia | 2 | 4 | Probably | Probably | Unlikely | 4 January 2011 | Yes | 21 January 2011 | 17 | ||
| FCR | Fatigue | 2 | 4 | Probably | Probably | Unlikely | 4 January 2011 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Unlikely | Unlikely | Unlikely | 19 January 2011 | No | ||||
| FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Unlikely | 11 January 2011 | Yes | 1 February 2011 | 21 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Unlikely | 4 January 2011 | No | ||||
| FCR | Nausea | 1 | 5 | Probably | Probably | Unlikely | 6 February 2011 | Yes | 4 March 2011 | 26 | ||
| FCR | Fatigue | 2 | 5 | Probably | Probably | Unlikely | 1 February 2011 | Yes | 4 March 2011 | 31 | ||
| FCR | Non-specific pain | 2 | 5 | Unlikely | Unlikely | Unlikely | 7 January 2011 | Yes | 4 March 2011 | 56 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 5 | Unlikely | Unlikely | Unlikely | 11 February 2011 | Yes | 21 February 2011 | 10 | ||
| FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | 11 February 2011 | No | ||||
| FCR | Anaemia | 2 | 5 | Probably | Probably | Unlikely | 11 February 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Unlikely | 11 February 2011 | No | ||||
| FCR | Nausea | 2 | 6 | Probably | Probably | Unlikely | 15 March 2011 | Yes | 15 April 2011 | 31 | ||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Unlikely | 7 April 2011 | No | ||||
| FCR | Fatigue | 2 | 6 | Probably | Probably | Unlikely | 15 March 2011 | No | ||||
| FCR | Anaemia | 2 | 6 | Probably | Probably | Unlikely | 31 March 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 15 March 2011 | No | ||||
| 38 | FCR | Dry skin/erythema | 1 | 1 | Unrelated | Unrelated | Probably | 12 October 2010 | Yes | 12 October 2010 | 0 | |
| FCR | Chest pain | 1 | 1 | Unrelated | Unrelated | Probably | 12 October 2010 | Yes | 12 October 2010 | 0 | ||
| FCR | Fever | 1 | 1 | Unrelated | Unrelated | Probably | 12 October 2010 | Yes | 12 October 2010 | 0 | ||
| FCR | Rigors | 2 | 1 | Unrelated | Unrelated | Probably | 13 October 2010 | Yes | 13 October 2010 | 0 | ||
| FCR | Nausea | 1 | 1 | Possibly | Possibly | Unlikely | Yes | |||||
| FCR | Thrombocytopenia | 2 | 1 | Unlikely | Unlikely | Unlikely | 11 October 2010 | No | ||||
| FCR | Thrombocytopenia | 2 | 6 | Unlikely | Unlikely | Unlikely | 11 October 2011 | No | ||||
| 41 | FCR | Rigors | 1 | 1 | Unrelated | Unrelated | Almost certainly | 3 November 2010 | Yes | 3 November 2010 | 0 | |
| FCR | Back pain | 1 | 1 | Unrelated | Unrelated | Almost certainly | 3 November 2010 | Yes | 3 November 2010 | 0 | ||
| FCR | Headache | 1 | 1 | Unrelated | Unrelated | Almost certainly | 3 November 2010 | Yes | 3 November 2010 | 0 | ||
| FCR | Nausea | 1 | 1 | Unrelated | Unrelated | Almost certainly | 3 November 2010 | Yes | 4 November 2010 | 1 | ||
| FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Unlikely | Yes | |||||
| FCR | Neutropenia | 4 | 1 | Probably | Probably | Probably | 15 November 2010 | Yes | 29 November 2010 | 14 | ||
| FCR | Nasal symptoms | 2 | 1 | Unrelated | Unrelated | Unrelated | 26 November 2010 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 3 | 2 | Probably | Probably | Probably | 11 December 2010 | Yes | 11 January 2011 | 31 | ||
| FCR | Infections (not neutropenic sepsis) | 3 | 2 | Probably | Probably | Probably | 14 January 2011 | Yes | 21 January 2011 | 7 | ||
| FCR | Neutropenia | 4 | 2 | Probably | Probably | Probably | 11 December 2010 | Yes | 14 December 2010 | 3 | ||
| 42 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Probably | Yes | ||||
| FCR | Neutropenia | 1 | 1 | Possibly | Possibly | Possibly | 23 November 2010 | Yes | 25 November 2010 | 2 | ||
| FCR | Anaemia | 1 | 1 | Possibly | Possibly | Possibly | 19 July 2010 | Yes | 23 November 2010 | 127 | ||
| FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 28 October 2010 | Yes | 28 October 2010 | 0 | ||
| FCR | Vomiting | 2 | 2 | Possibly | Possibly | Possibly | 27 November 2010 | Yes | 27 November 2010 | 0 | ||
| FCR | Diarrhoea | 1 | 2 | Possibly | Possibly | Possibly | Yes | 20 January 2011 | ||||
| FCR | Thrombocytopenia | 1 | 3 | Possibly | Possibly | Possibly | 21 September 2010 | Yes | 18 January 2011 | 119 | ||
| FCR | Lymphopenia | 2 | 3 | Possibly | Possibly | Possibly | 21 December 2010 | Yes | 18 January 2011 | 28 | ||
| FCR | Lymphopenia | 3 | 3 | Possibly | Possibly | Possibly | 18 January 2011 | No | ||||
| FCR | Anaemia | 1 | 3 | Possibly | Possibly | Possibly | 18 January 2011 | No | ||||
| FCR | Myalgias | 1 | 4 | Possibly | Possibly | Possibly | Yes | 17 February 2011 | ||||
| FCR | Cutaneous herpes/shingles | 2 | 5 | Possibly | Possibly | Possibly | 18 February 2011 | Yes | 5 March 2011 | 15 | ||
| FCR | Vomiting | 1 | 5 | Probably | Probably | Possibly | 2 March 2011 | Yes | 3 March 2011 | 1 | ||
| FCR | Fatigue | 1 | 5 | Probably | Probably | Possibly | Yes | 19 March 2011 | ||||
| FCR | Neutropenia | 1 | 5 | Possibly | Possibly | Possibly | 15 February 2011 | Yes | 15 March 2011 | 28 | ||
| FCR | Nausea | 2 | 6 | Probably | Probably | Probably | 2 March 2011 | Yes | 14 April 2011 | 43 | ||
| FCR | Neutropenia | 2 | 6 | Possibly | Possibly | Possibly | 18 January 2011 | Yes | 14 April 2011 | 86 | ||
| 43 | FCR | Anaemia | 1 | 1 | Probably | Probably | Possibly | No | ||||
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Possibly | 26 October 2010 | Yes | ||||
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Possibly | Yes | |||||
| FCR | Fatigue | 1 | 1 | Probably | Probably | Probably | Yes | |||||
| FCR | Rigors | 1 | 1 | Possibly | Possibly | Probably | 26 October 2010 | Yes | 26 October 2010 | 0 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Possibly | 25 November 2010 | Yes | 28 November 2010 | 3 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 3 | Possibly | Possibly | Possibly | Yes | |||||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Possibly | 20 January 2011 | Yes | 29 January 2011 | 9 | ||
| FCR | Constipation | 1 | 4 | Possibly | Possibly | Possibly | Yes | 16 February 2011 | ||||
| FCR | Pruritus | 1 | 4 | Probably | Unlikely | Unlikely | Yes | 16 February 2011 | ||||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Possibly | 17 February 2011 | Yes | ||||
| 44 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 November 2010 | Yes | 10 November 2010 | 6 | |
| FCR | Constipation | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 November 2010 | No | ||||
| FCR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 November 2010 | No | ||||
| FCR | Alopecia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 November 2010 | No | ||||
| FCR | Mucositis/thrush | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 4 November 2010 | Yes | ||||
| FCR | Thrombocytopenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 30 November 2010 | Yes | 14 December 2010 | 14 | ||
| FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 30 November 2010 | Yes | 14 December 2010 | 14 | ||
| FCR | Neuropathy (sensory) | 1 | 1 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | 11 January 2011 | Yes | 13 January 2011 | 2 | ||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 11 January 2011 | No | ||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 16 December 2010 | Yes | ||||
| FCR | Neutropenic sepsis | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 24 December 2010 | Yes | 27 December 2010 | 3 | ||
| FCR | Diarrhoea | 2 | 3 | Unrelated | Unrelated | Unrelated | Yes | |||||
| FCR | Myalgias | 1 | 3 | Possibly | Possibly | Possibly | No | |||||
| FCR | Constipation | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Yes | |||||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Yes | |||||
| FCR | Infections (not neutropenic sepsis) | 1 | 4 | Unrelated | Unrelated | Unrelated | 1 March 2011 | Yes | 8 March 2011 | 7 | ||
| FCR | Fatigue | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 10 February 2011 | Yes | 14 February 2011 | 4 | ||
| FCR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 15 March 2011 | Yes | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 5 | Unrelated | Unrelated | Unrelated | 21 March 2011 | Yes | ||||
| FCR | Vomiting | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 15 March 2011 | Yes | 15 March 2011 | 0 | ||
| 48 | FCR | Headache | 2 | 3 | Unlikely | Unlikely | Unlikely | 26 December 2010 | No | |||
| FCR | Sore throat | 2 | 4 | Possibly | Possibly | Possibly | 14 February 2011 | Yes | 14 March 2011 | 28 | ||
| FCR | Urinary symptoms | 2 | 4 | Possibly | Possibly | Possibly | 14 February 2011 | Yes | 14 March 2011 | 28 | ||
| FCR | Dizziness | 1 | 5 | Unrelated | Unrelated | Unrelated | 14 March 2011 | Yes | 11 April 2011 | 28 | ||
| FCR | Abdominal pain/bloating | 1 | 5 | Possibly | Possibly | Possibly | 14 March 2011 | Yes | 11 April 2011 | 28 | ||
| FCR | Fatigue | 1 | 6 | Almost certainly | Unlikely | Unlikely | 11 April 2011 | Yes | 9 May 2011 | 28 | ||
| FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 11 April 2011 | Yes | 9 May 2011 | 28 | ||
| 50 | FCR | Infections (not neutropenic sepsis) | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | 27 February 2011 | Yes | 1 March 2011 | 2 | |
| 51 | FCR | Neutropenia | 4 | 1 | Probably | Probably | Probably | 30 December 2010 | Yes | 31 January 2011 | 32 | |
| FCR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Missing | 12 February 2011 | Yes | 14 March 2011 | 30 | ||
| FCR | Neutropenia | 3 | 3 | Missing | Missing | Missing | 11 April 2011 | Yes | 15 April 2011 | 4 | ||
| FCR | Thrombocytopenia | 1 | 3 | Missing | Missing | Missing | 14 March 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 4 | Probably | Probably | Probably | 6 May 2011 | No | ||||
| FCR | Neutropenia | 3 | 4 | Probably | Probably | Probably | 12 May 2011 | No | ||||
| FCR | Anaemia | 1 | 4 | Probably | Probably | Probably | 12 May 2011 | No | ||||
| 55 | FCR | Thrombocytopenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | 14 January 2011 | Yes | 7 February 2011 | 24 | |
| FCR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | 14 January 2011 | Yes | 15 February 2011 | 32 | ||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 7 March 2011 | Yes | 4 April 2011 | 28 | ||
| FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 15 February 2011 | Yes | 4 April 2011 | 48 | ||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 22 March 2011 | Yes | 4 April 2011 | 13 | ||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 22 March 2011 | Yes | 4 April 2011 | 13 | ||
| FCR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 7 February 2011 | No | ||||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | 15 June 2011 | Yes | 29 June 2011 | 14 | ||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 7 February 2011 | Yes | 20 September 2011 | 225 | ||
| FCR | Rash/flushing | 2 | 6 | Unrelated | Unrelated | Unrelated | 12 September 2011 | No | ||||
| 59 | FCR | Nausea | 1 | 3 | Probably | Probably | Unlikely | 22 March 2011 | Yes | 26 March 2011 | 4 | |
| FCR | Anaemia | 1 | 3 | Possibly | Possibly | Unlikely | 19 April 2011 | No | ||||
| FCR | Nausea | 1 | 4 | Probably | Probably | Unlikely | 19 April 2011 | Yes | 23 April 2011 | 4 | ||
| FCR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 17 May 2011 | Yes | 8 September 2011 | 114 | ||
| 60 | FCR | Vomiting | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 24 January 2011 | Yes | 27 January 2011 | 3 | |
| FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 27 January 2011 | Yes | 27 January 2011 | 0 | ||
| FCR | Fever | 3 | 2 | Probably | Probably | Probably | 4 March 2011 | Yes | 7 March 2011 | 3 | ||
| FCR | Nausea | 1 | 2 | Unlikely | Unlikely | Unlikely | 7 March 2011 | Yes | 11 March 2011 | 4 | ||
| FCR | Dyspnoea | 1 | 2 | Unlikely | Unlikely | Unlikely | 7 March 2011 | Yes | 11 March 2011 | 4 | ||
| FCR | Neutropenia | 1 | 2 | Probably | Probably | Probably | 4 March 2011 | Yes | 23 March 2011 | 19 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Possibly | 26 March 2011 | Yes | 31 March 2011 | 5 | ||
| FCR | Vomiting | 3 | 4 | Almost certainly | Almost certainly | Unlikely | 23 April 2011 | Yes | 04 May 2011 | 11 | ||
| FCR | Thrombocytopenia | 1 | 4 | Probably | Probably | Possibly | 4 March 2011 | Yes | 18 May 2011 | 75 | ||
| FCR | Neutropenia | 1 | 4 | Probably | Probably | Probably | 20 April 2011 | Yes | 18 May 2011 | 28 | ||
| FCR | Nausea | 3 | 5 | Almost certainly | Almost certainly | Possibly | 21 May 2011 | Yes | 8 June 2011 | 18 | ||
| FCR | Vomiting | 3 | 5 | Almost certainly | Almost certainly | Possibly | 21 May 2011 | Yes | 8 June 2011 | 18 | ||
| FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Possibly | No | |||||
| FCR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Possibly | 15 June 2011 | No | ||||
| FCR | Fatigue | 2 | 5 | Possibly | Possibly | Probably | 19 May 2011 | No | ||||
| FCR | Constipation | 1 | 5 | Unlikely | Unlikely | Unlikely | 18 May 2011 | Yes | 1 June 2011 | 14 | ||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Possibly | 24 February 2011 | Yes | 2 July 2011 | 128 | ||
| FCR | Vomiting | 1 | 6 | Almost certainly | Almost certainly | Possibly | 27 June 2011 | Yes | 2 July 2011 | 5 | ||
| FCR | Sore throat | 1 | 6 | Almost certainly | Almost certainly | Unlikely | Yes | |||||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Possibly | 13 July 2011 | Yes | 27 July 2011 | 14 | ||
| 61 | FCR | Infections (not neutropenic sepsis) | 2 | 1 | Missing | Missing | Missing | 15 February 2011 | Yes | 24 February 2011 | 9 | |
| FCR | Thrombocytopenia | 2 | 1 | Missing | Missing | Missing | 8 February 2011 | Yes | 18 February 2011 | 10 | ||
| FCR | Neutropenia | 4 | 1 | Missing | Missing | Missing | 18 February 2011 | Yes | 24 February 2011 | 6 | ||
| FCR | Constipation | 1 | 1 | Unlikely | Unlikely | Unlikely | 18 February 2011 | Yes | 24 February 2011 | 6 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 7 March 2011 | Yes | 13 March 2011 | 6 | ||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | 24 March 2011 | Yes | 24 March 2011 | 0 | ||
| FCR | Other AE description | Pancytopenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 7 March 2011 | Yes | 24 March 2011 | 17 | |
| FCR | Thrombocytopenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 28 April 2011 | Yes | 5 May 2011 | 7 | ||
| FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | 14 April 2011 | Yes | 28 April 2011 | 14 | ||
| FCR | Fatigue | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 14 April 2011 | Yes | 28 April 2011 | 14 | ||
| FCR | Fatigue | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 5 May 2011 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 16 May 2011 | Yes | 24 May 2011 | 8 | ||
| FCR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | 19 May 2011 | Yes | 15 June 2011 | 27 | ||
| FCR | Thrombocytopenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | 15 June 2011 | No | ||||
| FCR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | 6 July 2011 | Yes | 20 July 2011 | 14 | ||
| FCR | Fatigue | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 6 July 2011 | Yes | 14 July 2011 | 8 | ||
| FCR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Almost certainly | 4 August 2011 | Yes | 22 September 2011 | 49 | ||
| FCR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 17 August 2011 | Yes | 26 August 2011 | 9 | ||
| 62 | FCR | Hypotension | 1 | 1 | Unlikely | Unlikely | Unlikely | 2 February 2011 | Yes | 2 February 2011 | 0 | |
| FCR | Fever | 1 | 1 | Unlikely | Unlikely | Unlikely | 2 February 2011 | Yes | 2 February 2011 | 0 | ||
| FCR | Vomiting | 1 | 3 | Probably | Probably | Unlikely | Yes | |||||
| FCR | Bone pain | 1 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| 66 | FCR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 15 March 2011 | Yes | 22 March 2011 | 7 | |
| FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 22 March 2011 | Yes | 29 March 2011 | 7 | ||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 20 April 2011 | Yes | 26 April 2011 | 6 | ||
| FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 12 April 2011 | Yes | 20 April 2011 | 8 | ||
| FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Almost certainly | 10 May 2011 | Yes | 23 May 2011 | 13 | ||
| FCR | Diarrhoea | 2 | 3 | Possibly | Possibly | Possibly | 3 May 2011 | Yes | 5 May 2011 | 2 | ||
| FCR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | 7 June 2011 | Yes | 20 June 2011 | 13 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 31 May 2011 | Yes | 7 June 2011 | 7 | ||
| FCR | Diarrhoea | 1 | 4 | Probably | Probably | Probably | 29 May 2011 | Yes | 30 May 2011 | 1 | ||
| FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 5 July 2011 | Yes | 26 July 2011 | 21 | ||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 26 July 2011 | Yes | 2 August 2011 | 7 | ||
| FCR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 2 August 2011 | No | ||||
| 67 | FCR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 8 March 2011 | Yes | 21 March 2011 | 13 | |
| FCR | Neutropenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 8 March 2011 | Yes | 21 March 2011 | 13 | ||
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 9 March 2011 | Yes | 9 March 2011 | 0 | ||
| FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Almost certainly | 16 May 2011 | Yes | 1 June 2011 | 16 | ||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 3 May 2011 | Yes | 16 May 2011 | 13 | ||
| FCR | Anaemia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 23 May 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 23 May 2011 | Yes | 28 May 2011 | 5 | ||
| FCR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 14 June 2011 | No | ||||
| FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | 24 June 2011 | No | ||||
| FCR | Anaemia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 16 May 2011 | No | ||||
| FCR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 18 July 2011 | Yes | 29 July 2011 | 11 | ||
| FCR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Almost certainly | 21 June 2011 | Yes | 29 July 2011 | 38 | ||
| FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 17 July 2011 | Yes | 19 July 2011 | 2 | ||
| FCR | Vomiting | 2 | 6 | Almost certainly | Almost certainly | Unrelated | 17 July 2011 | Yes | 19 July 2011 | 2 | ||
| 68 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Possibly | 6 March 2011 | Yes | 13 March 2011 | 7 | |
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Possibly | 8 March 2011 | Yes | 9 March 2011 | 1 | ||
| FCR | Infusional reaction | 3 | 1 | Unrelated | Unrelated | Almost certainly | 3 March 2011 | Yes | 3 March 2011 | 0 | ||
| FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Possibly | No | |||||
| FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 27 April 2011 | No | ||||
| FCR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 5 April 2011 | Yes | 14 April 2011 | 9 | ||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 5 April 2011 | Yes | 11 April 2011 | 6 | ||
| FCR | Allergic reaction | 2 | 2 | Unrelated | Unrelated | Almost certainly | 4 April 2011 | Yes | 4 April 2011 | 0 | ||
| FCR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 4 May 2011 | Yes | 10 May 2011 | 6 | ||
| FCR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 4 May 2011 | Yes | 13 May 2011 | 9 | ||
| FCR | Constipation | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 5 May 2011 | Yes | 7 May 2011 | 2 | ||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Possibly | 3 June 2011 | Yes | 5 June 2011 | 2 | ||
| FCR | Headache | 2 | 4 | Probably | Unlikely | Possibly | 2 June 2011 | Yes | 2 June 2011 | 0 | ||
| FCR | Dizziness | 2 | 4 | Probably | Unlikely | Possibly | 3 June 2011 | Yes | 3 June 2011 | 0 | ||
| FCR | Constipation | 1 | 4 | Unlikely | Unlikely | Unlikely | 4 June 2011 | Yes | 7 June 2011 | 3 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Possibly | 2 July 2011 | Yes | 6 July 2011 | 4 | ||
| FCR | Fatigue | 1 | 5 | Almost certainly | Possibly | Probably | 2 July 2011 | No | ||||
| FCR | Rash/flushing | 2 | 6 | Unrelated | Unrelated | Unrelated | 31 July 2011 | Yes | 4 August 2011 | 4 | ||
| FCR | Nausea | 2 | 6 | Possibly | Possibly | Unrelated | 30 July 2011 | Yes | 1 August 2011 | 2 | ||
| FCR | Anxiety/depression | 2 | 6 | Unrelated | Unrelated | Unrelated | Yes | 26 October 2011 | ||||
| FCR | Headache | 1 | 6 | Unrelated | Unrelated | Unrelated | 27 July 2011 | Yes | 29 July 2011 | 2 | ||
| FCR | Abdominal pain/bloating | 2 | 6 | Unrelated | Unrelated | Unrelated | 7 August 2011 | Yes | 8 August 2011 | 1 | ||
| 70 | FCR | Anaemia | 3 | 1 | Almost certainly | Almost certainly | Possibly | 11 March 2011 | No | |||
| FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Possibly | 11 March 2011 | No | ||||
| FCR | Thrombocytopenia | 3 | 1 | Almost certainly | Almost certainly | Possibly | 11 March 2011 | No | ||||
| FCR | Allergic reaction | 1 | 1 | Unlikely | Unlikely | Almost certainly | 11 March 2011 | Yes | 12 March 2011 | 1 | ||
| FCR | Infections (not neutropenic sepsis) | 3 | 1 | Probably | Probably | Possibly | 12 March 2011 | Yes | 25 March 2011 | 13 | ||
| FCR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Possibly | 12 April 2011 | No | ||||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Possibly | 12 April 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Possibly | 12 April 2011 | Yes | 27 April 2011 | 15 | ||
| FCR | Allergic reaction | 1 | 2 | Unlikely | Unlikely | Almost certainly | 12 April 2011 | Yes | 12 April 2011 | 0 | ||
| FCR | Urinary symptoms | 1 | 2 | Unlikely | Possibly | Unlikely | 1 May 2011 | Yes | 12 May 2011 | 11 | ||
| FCR | Myalgias | 1 | 2 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Possibly | 12 May 2011 | No | ||||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Possibly | 12 May 2011 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 3 | 4 | Probably | Probably | Possibly | 14 June 2011 | Yes | 20 June 2011 | 6 | ||
| FCR | Constipation | 1 | 4 | Unlikely | Unlikely | Unlikely | 11 June 2011 | Yes | 16 June 2011 | 5 | ||
| FCR | Anaemia | 1 | 4 | Almost certainly | Almost certainly | Possibly | 10 June 2011 | Yes | 14 July 2011 | 34 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Possibly | 14 June 2011 | Yes | 23 June 2011 | 9 | ||
| FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Possibly | 17 June 2011 | No | ||||
| FCR | Abnormal electrolytes | 1 | 4 | Unlikely | Unlikely | Unlikely | 16 June 2011 | Yes | 17 June 2011 | 1 | ||
| FCR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Possibly | 4 August 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Possibly | 15 July 2011 | No | ||||
| FCR | Non-specific pain | 1 | 5 | Unlikely | Unlikely | Unlikely | 22 July 2011 | No | ||||
| FCR | Constipation | 1 | 5 | Unlikely | Unlikely | Unlikely | 9 July 2011 | Yes | 13 July 2011 | 4 | ||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Possibly | 19 August 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Possibly | 12 August 2011 | No | ||||
| 72 | FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | 6 April 2011 | Yes | 3 May 2011 | 27 | |
| FCR | Thrombocytopenia | 2 | 1 | Unrelated | Unrelated | Unrelated | 23 February 2011 | No | ||||
| FCR | Anaemia | 1 | 1 | Unrelated | Unrelated | Unrelated | 10 March 2011 | No | ||||
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 20 March 2011 | Yes | 22 March 2011 | 2 | ||
| FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 3 May 2011 | Yes | 17 May 2011 | 14 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 24 April 2011 | Yes | 26 April 2011 | 2 | ||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 22 April 2011 | Yes | 24 April 2011 | 2 | ||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 1 June 2011 | Yes | 5 July 2011 | 34 | ||
| FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 14 June 2011 | Yes | 5 July 2011 | 21 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 22 May 2011 | Yes | 23 May 2011 | 1 | ||
| FCR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 22 May 2011 | Yes | 23 May 2011 | 1 | ||
| FCR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 12 July 2011 | Yes | 2 August 2011 | 21 | ||
| FCR | Diarrhoea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 21 July 2011 | Yes | 27 July 2011 | 6 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 13 July 2011 | Yes | 17 July 2011 | 4 | ||
| FCR | Thrombocytopenia | 3 | 5 | Unrelated | Unrelated | Unrelated | 23 February 2001 | No | ||||
| FCR | Neutropenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 9 August 2011 | Yes | 6 September 2011 | 28 | ||
| FCR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Unrelated | 6 September 2011 | No | ||||
| FCR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Unrelated | 16 August 2011 | Yes | 6 September 2011 | 21 | ||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Unrelated | 17 August 2011 | Yes | 25 August 2011 | 8 | ||
| 74 | FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 22 March 2011 | Yes | 22 March 2011 | 0 | |
| FCR | Myalgias | 1 | 1 | Possibly | Possibly | Possibly | 27 March 2011 | Yes | 27 March 2011 | 0 | ||
| FCR | Nausea | 1 | 1 | Probably | Probably | Possibly | 28 March 2011 | Yes | 28 March 2011 | 0 | ||
| FCR | Vomiting | 1 | 1 | Probably | Probably | Possibly | 28 March 2011 | Yes | 28 March 2011 | 0 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 1 | Unlikely | Unlikely | Unlikely | 4 April 2011 | Yes | 10 April 2011 | 6 | ||
| FCR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | 19 April 2011 | No | ||||
| FCR | Constipation | 1 | 1 | Possibly | Possibly | Possibly | 28 March 2011 | Yes | 31 March 2011 | 3 | ||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Possibly | 17 May 2011 | No | ||||
| FCR | Fatigue | 1 | 2 | Almost certainly | Possibly | Probably | 20 April 2011 | No | ||||
| FCR | Nausea | 3 | 3 | Possibly | Possibly | Possibly | 21 May 2011 | Yes | 22 May 2011 | 1 | ||
| FCR | Vomiting | 3 | 3 | Possibly | Possibly | Possibly | 21 May 2011 | Yes | 22 May 2011 | 1 | ||
| FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Possibly | 14 June 2011 | No | ||||
| FCR | Nausea | 3 | 4 | Almost certainly | Almost certainly | Possibly | 18 June 2011 | Yes | 20 June 2011 | 2 | ||
| FCR | Vomiting | 2 | 4 | Almost certainly | Almost certainly | Possibly | 19 June 2011 | Yes | 20 June 2011 | 1 | ||
| FCR | Thrombocytopenia | 1 | 4 | Possibly | Possibly | Possibly | 29 April 2009 | Yes | 13 July 2011 | 805 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Possibly | 16 July 2011 | Yes | 19 July 2011 | 3 | ||
| FCR | Vomiting | 1 | 5 | Almost certainly | Almost certainly | Possibly | 17 July 2011 | Yes | 17 July 2011 | 0 | ||
| FCR | Constipation | 1 | 5 | Possibly | Possibly | Unlikely | No | |||||
| FCR | Vomiting | 2 | 6 | Probably | Probably | Unlikely | 15 September 2011 | Yes | 16 September 2011 | 1 | ||
| FCR | Nausea | 1 | 6 | Probably | Probably | Unlikely | 11 September 2011 | Yes | 17 September 2011 | 6 | ||
| 78 | FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Possibly | 8 May 2011 | Yes | 11 May 2011 | 3 | |
| FCR | Anorexia/cachexia | 1 | 1 | Unlikely | Unlikely | Unlikely | 9 May 2011 | Yes | 31 May 2011 | 22 | ||
| FCR | Fever | 1 | 1 | Possibly | Possibly | Possibly | 6 May 2011 | Yes | 3 June 2011 | 28 | ||
| FCR | Anaemia | 2 | 1 | Possibly | Possibly | Possibly | 9 May 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 1 | Possibly | Possibly | Possibly | 9 May 2011 | Yes | 28 June 2011 | 50 | ||
| FCR | Anaemia | 1 | 3 | Possibly | Possibly | Possibly | 26 July 2011 | No | ||||
| FCR | Urinary symptoms | 1 | 3 | Unlikely | Unlikely | Unlikely | 3 June 2011 | Yes | 26 July 2011 | 53 | ||
| FCR | Fatigue | 1 | 4 | Possibly | Possibly | Possibly | 9 May 2011 | Yes | 26 August 2011 | 109 | ||
| FCR | Diarrhoea | 1 | 4 | Possibly | Possibly | Possibly | 1 July 2011 | Yes | 26 August 2011 | 56 | ||
| FCR | Vomiting | 1 | 4 | Possibly | Possibly | Possibly | 2 August 2011 | Yes | 26 August 2011 | 24 | ||
| FCR | Rash/flushing | 1 | 5 | Possibly | Possibly | Possibly | 26 August 2011 | Yes | 20 September 2011 | 25 | ||
| FCR | Fatigue | 2 | 6 | Possibly | Possibly | Possibly | 23 September 2011 | Yes | 30 September 2011 | 7 | ||
| FCR | Anorexia/cachexia | 1 | 6 | Possibly | Possibly | Possibly | 23 September 2011 | Yes | 30 September 2011 | 7 | ||
| 82 | FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | 29 June 2011 | Yes | 4 July 2011 | 5 | |
| FCR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 20 July 2011 | No | ||||
| FCR | Thrombocytopenia | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 20 July 2011 | No | ||||
| FCR | Neutropenia | 3 | 2 | Probably | Almost certainly | Unlikely | 3 August 2011 | No | ||||
| FCR | Neutropenia | 4 | 3 | Probably | Probably | Probably | 10 August 2011 | No | ||||
| FCR | Thrombocytopenia | 3 | 3 | Probably | Probably | Probably | 5 August 2011 | No | ||||
| FCR | Anaemia | 2 | 3 | Probably | Probably | Probably | 12 August 2011 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 3 | 6 | Unlikely | Unlikely | Unlikely | 9 December 2011 | No | ||||
| FCR | Thrombocytopenia | 2 | 6 | Possibly | Possibly | Possibly | 30 September 2011 | No | ||||
| 84 | FCR | Neutropenia | 1 | 1 | Possibly | Almost certainly | Unrelated | 9 June 2011 | No | |||
| FCR | Myalgias | 1 | 1 | Unlikely | Unlikely | Unlikely | 25 May 2011 | No | ||||
| FCR | Neuropathy (sensory) | 1 | 1 | Possibly | Unlikely | Possibly | 25 May 2011 | No | ||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 20 June 2011 | Yes | 22 June 2011 | 2 | ||
| FCR | Rash/flushing | 1 | 3 | Unlikely | Unlikely | Unlikely | 17 July 2011 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 3 | Unlikely | Possibly | Unlikely | 17 July 2011 | No | ||||
| FCR | Ophthalmic infections | 1 | 4 | Unlikely | Unlikely | Unlikely | 17 July 2011 | No | ||||
| FCR | Neuropathy (sensory) | 1 | 4 | Possibly | Unlikely | Unlikely | 26 August 2011 | No | ||||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Unlikely | 7 October 2011 | Yes | 24 October 2011 | 17 | ||
| FCR | Fever | 2 | 5 | Almost certainly | Possibly | Unlikely | 22 October 2011 | Yes | 28 October 2011 | 6 | ||
| FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Unlikely | 24 October 2011 | Yes | 1 November 2011 | 8 | ||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 9 September 2011 | Yes | 21 September 2011 | 12 | ||
| 87 | FCR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | 15 June 2011 | Yes | 1 August 2011 | 47 | |
| FCR | Thrombocytopenia | 1 | 5 | Probably | Probably | Probably | 31 October 2011 | No | ||||
| FCR | Anaemia | 1 | 6 | Probably | Probably | Unlikely | 30 November 2011 | No | ||||
| 88 | FCR | Thrombocytopenia | 1 | 1 | Unrelated | Unrelated | Unrelated | 24 May 2011 | No | |||
| FCR | Neutropenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 15 June 2011 | Yes | 28 June 2011 | 13 | ||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 13 July 2011 | No | ||||
| FCR | Diarrhoea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 28 August 2011 | Yes | 29 August 2011 | 1 | ||
| FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | 17 July 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Possibly | Possibly | Possibly | 3 July 2009 | No | ||||
| 89 | FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Possibly | 5 July 2011 | No | |||
| FCR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Unlikely | 12 June 2011 | Yes | 16 June 2011 | 4 | ||
| FCR | Vomiting | 2 | 1 | Almost certainly | Almost certainly | Unlikely | 12 June 2011 | Yes | 16 June 2011 | 4 | ||
| FCR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 8 July 2011 | Yes | 17 July 2011 | 9 | ||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 8 July 2011 | Yes | 17 July 2011 | 9 | ||
| FCR | Fatigue | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 8 July 2011 | Yes | 22 July 2011 | 14 | ||
| FCR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 5 August 2011 | Yes | 15 August 2011 | 10 | ||
| FCR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 5 August 2011 | Yes | 15 August 2011 | 10 | ||
| FCR | Fatigue | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 5 August 2011 | Yes | 19 August 2011 | 14 | ||
| FCR | Abdominal pain/bloating | 2 | 3 | Possibly | Possibly | Unlikely | 4 August 2011 | No | ||||
| FCR | Vomiting | 2 | 4 | Almost certainly | Almost certainly | Unlikely | 8 September 2011 | Yes | 20 September 2011 | 12 | ||
| FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Unlikely | 8 September 2011 | No | ||||
| FCR | Abdominal pain/bloating | 2 | 4 | Possibly | Possibly | Unlikely | 8 September 2011 | No | ||||
| FCR | Vomiting | 1 | 5 | Almost certainly | Almost certainly | Unlikely | 6 October 2011 | Yes | 9 October 2011 | 3 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Unlikely | 5 October 2011 | Yes | 12 October 2011 | 7 | ||
| FCR | Fatigue | 1 | 5 | Almost certainly | Almost certainly | Unlikely | 5 October 2011 | No | ||||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 2 November 2011 | Yes | 5 November 2011 | 3 | ||
| FCR | Vomiting | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 2 November 2011 | Yes | 2 November 2011 | 0 | ||
| 91 | FCR | Anxiety/depression | 2 | 1 | Unrelated | Unrelated | Unrelated | 1 July 2011 | Yes | 21 July 2011 | 20 | |
| FCR | Mucositis/thrush | 1 | 2 | Unrelated | Unrelated | Unrelated | 21 July 2011 | No | ||||
| FCR | Anxiety/depression | 2 | 3 | Unrelated | Unrelated | Unrelated | 18 August 2011 | No | ||||
| FCR | Mucositis/thrush | 1 | 3 | Unrelated | Unrelated | Unrelated | 18 August 2011 | Yes | 19 September 2011 | 32 | ||
| FCR | Anxiety/depression | 2 | 4 | Unrelated | Unrelated | Unrelated | 19 September 2011 | No | ||||
| FCR | Anxiety/depression | 2 | 5 | Unrelated | Unrelated | Unrelated | 17 October 2011 | No | ||||
| FCR | Anaemia | 2 | 6 | Possibly | Possibly | Possibly | 14 November 2011 | Yes | 28 November 2011 | 14 | ||
| 92 | FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Possibly | 28 June 2011 | Yes | 4 July 2011 | 6 | |
| FCR | Non-specific pain | 1 | 3 | Unrelated | Unrelated | Unrelated | 31 August 2011 | Yes | 26 September 2011 | 26 | ||
| FCR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Possibly | 23 November 2011 | Yes | 30 November 2011 | 7 | ||
| 93 | FCR | Headache | 1 | 1 | Unlikely | Unlikely | Unlikely | Yes | ||||
| FCR | Allergic reaction | 1 | 2 | Unrelated | Unrelated | Probably | 12 July 2011 | Yes | 12 July 2011 | 0 | ||
| FCR | Lymphopenia | 1 | 4 | Probably | Probably | Probably | 5 September 2011 | No | ||||
| FCR | Pruritus | 1 | 4 | Unlikely | Unlikely | Unlikely | 5 October 2011 | No | ||||
| FCR | Rash/flushing | 3 | 5 | Unlikely | Unlikely | Unlikely | 13 November 2011 | Yes | ||||
| FCR | Neutropenia | 4 | 5 | Probably | Probably | Probably | 14 November 2011 | Yes | 28 November 2011 | 14 | ||
| FCR | Fever | 2 | 5 | Probably | Probably | Probably | Yes | |||||
| FCR | Lymphopenia | 3 | 5 | Probably | Probably | Probably | 14 November 2011 | No | ||||
| FCR | Lymphopenia | 1 | 6 | Probably | Probably | Probably | 28 November 2011 | No | ||||
| 98 | FCR | Back pain | 1 | 1 | Possibly | Possibly | Possibly | 30 August 2011 | No | |||
| FCR | Non-specific pain | 1 | 1 | Possibly | Possibly | Possibly | 30 August 2011 | No | ||||
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 15 August 2011 | Yes | 19 August 2011 | 4 | ||
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 15 August 2011 | Yes | 29 August 2011 | 14 | ||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 15 September 2011 | Yes | 16 September 2011 | 1 | ||
| FCR | Abdominal pain/bloating | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 15 September 2011 | Yes | 6 October 2011 | 21 | ||
| FCR | Dizziness | 2 | 2 | Possibly | Possibly | Possibly | 16 September 2011 | Yes | 20 September 2011 | 4 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 10 October 2011 | Yes | 12 October 2011 | 2 | ||
| FCR | Abdominal pain/bloating | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 7 October 2011 | Yes | 7 October 2011 | 0 | ||
| FCR | Headache | 1 | 3 | Possibly | Possibly | Possibly | 8 October 2011 | Yes | 8 October 2011 | 0 | ||
| FCR | Constipation | 1 | 3 | Probably | Probably | Unlikely | 15 September 2011 | Yes | 20 September 2011 | 5 | ||
| FCR | Fatigue | 1 | 4 | Almost certainly | Probably | Unlikely | 6 November 2011 | No | ||||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Unlikely | 6 November 2011 | Yes | 10 November 2011 | 4 | ||
| FCR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Unlikely | 4 December 2011 | Yes | 8 December 2011 | 4 | ||
| FCR | Vomiting | 2 | 5 | Almost certainly | Almost certainly | Unlikely | 4 December 2011 | Yes | 8 December 2011 | 4 | ||
| FCR | Vomiting | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 7 January 2012 | Yes | 10 January 2012 | 3 | ||
| FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Unlikely | 7 January 2012 | Yes | 10 January 2012 | 3 | ||
| 101 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Unrelated | 25 August 2011 | Yes | 25 August 2011 | 0 | |
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Unrelated | 26 August 2011 | Yes | 28 August 2011 | 2 | ||
| FCR | Otalgia | 2 | 1 | Possibly | Possibly | Unrelated | 28 September 2011 | No | ||||
| FCR | Infusional reaction | 1 | 1 | Possibly | Possibly | Unrelated | 25 August 2011 | Yes | 26 August 2011 | 1 | ||
| FCR | Neutropenia | 3 | 1 | Probably | Probably | Unrelated | 14 September 2011 | Yes | 23 September 2011 | 9 | ||
| FCR | Allergic reaction | 1 | 2 | Unrelated | Unrelated | Almost certainly | 26 September 2011 | Yes | 26 September 2011 | 0 | ||
| FCR | Neutropenia | 3 | 2 | Probably | Probably | Unrelated | 21 October 2011 | Yes | 4 November 2011 | 14 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Unlikely | Unlikely | Unlikely | 4 January 2012 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 5 | Unrelated | Unrelated | Unrelated | 23 January 2012 | Yes | 27 January 2012 | 4 | ||
| FCR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Unrelated | 9 February 2012 | Yes | 13 February 2012 | 4 | ||
| FCR | Fatigue | 1 | 6 | Almost certainly | Almost certainly | Unrelated | 8 February 2012 | Yes | 15 February 2012 | 7 | ||
| 102 | FCR | Anaemia | 2 | 1 | Probably | Probably | Probably | 6 September 2011 | Yes | 12 September 2011 | 6 | |
| FCR | Thrombocytopenia | 2 | 1 | Probably | Probably | Probably | 6 September 2011 | Yes | 12 September 2011 | 6 | ||
| 104 | FCR | Fatigue | 2 | 1 | Probably | Possibly | Unlikely | 13 September 2011 | Yes | 23 September 2011 | 10 | |
| FCR | Nausea | 2 | 1 | Probably | Probably | Unlikely | 16 September 2011 | Yes | 18 September 2011 | 2 | ||
| FCR | Vomiting | 2 | 1 | Probably | Probably | Unlikely | 17 September 2011 | Yes | 18 September 2011 | 1 | ||
| FCR | Anaemia | 2 | 1 | Possibly | Possibly | Unlikely | 13 September 2011 | No | ||||
| FCR | Neutropenia | 4 | 1 | Possibly | Possibly | Unlikely | 13 September 2011 | Yes | 29 September 2011 | 16 | ||
| FCR | Anxiety/depression | 2 | 1 | Unlikely | Unlikely | Unlikely | 9 September 2011 | Yes | 19 September 2011 | 10 | ||
| FCR | Taste alteration | 1 | 1 | Possibly | Possibly | Unlikely | 23 September 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 1 | Possibly | Possibly | Unlikely | 13 September 2011 | Yes | 29 September 2011 | 16 | ||
| FCR | Fatigue | 1 | 2 | Probably | Possibly | Unlikely | 14 October 2011 | Yes | 4 November 2011 | 21 | ||
| FCR | Nausea | 2 | 2 | Probably | Probably | Unlikely | 15 October 2011 | Yes | 21 October 2011 | 6 | ||
| FCR | Anaemia | 2 | 2 | Possibly | Possibly | Unlikely | 14 October 2011 | No | ||||
| FCR | Neutropenia | 2 | 2 | Probably | Probably | Unlikely | 1 November 2011 | No | ||||
| FCR | Anorexia/cachexia | 1 | 2 | Probably | Probably | Unlikely | 21 October 2011 | Yes | 11 November 2011 | 21 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 2 | Possibly | Possibly | Unlikely | 28 October 2011 | No | ||||
| FCR | Rash/flushing | 2 | 2 | Probably | Probably | Unlikely | 18 October 2011 | Yes | 19 October 2011 | 1 | ||
| FCR | Rash/flushing | 2 | 3 | Unlikely | Unlikely | Unlikely | 28 October 2011 | Yes | 2 December 2011 | 35 | ||
| FCR | Nausea | 1 | 3 | Probably | Probably | Unlikely | 14 November 2011 | Yes | 15 November 2011 | 1 | ||
| FCR | Fatigue | 1 | 3 | Probably | Possibly | Unlikely | 18 November 2011 | Yes | 25 November 2011 | 7 | ||
| FCR | Neutropenia | 4 | 3 | Probably | Probably | Unlikely | 18 November 2011 | Yes | 25 November 2011 | 7 | ||
| FCR | Anaemia | 2 | 3 | Probably | Probably | Unlikely | 25 November 2011 | No | ||||
| FCR | Anxiety/depression | 2 | 3 | Unlikely | Unlikely | Unlikely | 29 November 2011 | No | ||||
| FCR | Pruritus | 1 | 3 | Unrelated | Unrelated | Unrelated | 9 December 2011 | No | ||||
| FCR | Rash/flushing | 2 | 4 | Possibly | Possibly | Unlikely | 16 December 2011 | Yes | 18 December 2011 | 2 | ||
| FCR | Anxiety/depression | 3 | 4 | Unlikely | Unlikely | Unlikely | 16 December 2011 | No | ||||
| FCR | Other AE description | Confusion | 3 | 4 | Unlikely | Unlikely | Unlikely | 20 December 2011 | Yes | 6 January 2012 | 17 | |
| FCR | Anaemia | 2 | 4 | Probably | Probably | Unlikely | 24 December 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 4 | Probably | Probably | Unlikely | 16 December 2011 | No | ||||
| FCR | Neutropenia | 4 | 4 | Probably | Probably | Possibly | 21 December 2011 | Yes | 6 January 2012 | 16 | ||
| FCR | Nausea | 1 | 5 | Probably | Probably | Unlikely | 6 January 2012 | Yes | 10 January 2012 | 4 | ||
| FCR | Anaemia | 1 | 5 | Probably | Probably | Unlikely | 6 January 2012 | No | ||||
| FCR | Neutropenia | 2 | 5 | Probably | Probably | Possibly | 13 January 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 5 | Probably | Probably | Unlikely | 6 January 2012 | No | ||||
| FCR | Nausea | 1 | 6 | Probably | Probably | Unlikely | 6 February 2012 | Yes | 8 February 2012 | 2 | ||
| FCR | Anxiety/depression | 2 | 6 | Unlikely | Unlikely | Unlikely | 20 December 2011 | Yes | 24 February 2012 | 66 | ||
| FCR | Anaemia | 1 | 6 | Probably | Probably | Unlikely | 3 February 2012 | No | ||||
| FCR | Neutropenia | 2 | 6 | Probably | Probably | Possibly | 03 February 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Probably | Probably | Unlikely | 3 February 2012 | No | ||||
| 105 | FCR | Infusional reaction | 2 | 1 | Unlikely | Unlikely | Almost certainly | 5 September 2011 | Yes | 5 September 2011 | 0 | |
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Possibly | 6 September 2011 | Yes | 7 September 2011 | 1 | ||
| FCR | Oedema | 1 | 1 | Unlikely | Unlikely | Unlikely | 22 October 2011 | Yes | 24 October 2011 | 2 | ||
| FCR | Constipation | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 7 September 2011 | Yes | 14 September 2011 | 7 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Possibly | 4 October 2011 | Yes | 5 October 2011 | 1 | ||
| FCR | Infusional reaction | 2 | 2 | Unlikely | Unlikely | Almost certainly | 3 October 2011 | Yes | 3 October 2011 | 0 | ||
| FCR | Constipation | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 5 October 2011 | Yes | 12 October 2011 | 7 | ||
| FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Possibly | 27 October 2011 | Yes | 11 November 2011 | 15 | ||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Possibly | 27 October 2011 | Yes | 11 November 2011 | 15 | ||
| FCR | Lymphopenia | 3 | 3 | Almost certainly | Almost certainly | Possibly | 27 October 2011 | No | ||||
| FCR | Non-specific pain | 2 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Urinary symptoms | 2 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| 107 | FCR | Anaemia | 1 | 1 | Possibly | Possibly | Unlikely | 5 September 2011 | No | |||
| FCR | Vomiting | 2 | 1 | Possibly | Possibly | Unlikely | 19 September 2011 | Yes | 22 September 2011 | 3 | ||
| FCR | Neutropenia | 2 | 2 | Possibly | Possibly | Unlikely | 13 October 2011 | Yes | 10 November 2011 | 28 | ||
| FCR | Vomiting | 2 | 2 | Possibly | Possibly | Unlikely | 14 October 2011 | Yes | 19 October 2011 | 5 | ||
| FCR | Constipation | 1 | 2 | Unlikely | Unlikely | Unlikely | 20 October 2011 | Yes | 22 October 2011 | 2 | ||
| FCR | Thrombocytopenia | 2 | 3 | Possibly | Possibly | Unlikely | 22 September 2011 | Yes | 10 November 2011 | 49 | ||
| FCR | Nausea | 1 | 3 | Probably | Probably | Unlikely | 10 November 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 4 | Possibly | Possibly | Unlikely | 5 December 2011 | No | ||||
| FCR | Fatigue | 1 | 4 | Possibly | Possibly | Unlikely | 8 December 2011 | Yes | 15 December 2011 | 7 | ||
| FCR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Unlikely | 5 January 2012 | Yes | 2 February 2012 | 28 | ||
| FCR | Diarrhoea | 2 | 5 | Possibly | Possibly | Unlikely | 28 January 2012 | Yes | 16 February 2012 | 19 | ||
| 110 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Unlikely | Yes | ||||
| FCR | Rash/flushing | 1 | 3 | Unrelated | Unrelated | Unrelated | 16 January 2012 | Yes | 12 March 2012 | 56 | ||
| FCR | Diarrhoea | 1 | 5 | Possibly | Possibly | Unlikely | 14 February 2012 | Yes | 18 February 2012 | 4 | ||
| 111 | FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Possibly | Yes | 16 November 2011 | |||
| FCR | Rash/flushing | 2 | 1 | Unrelated | Unrelated | Unrelated | 2 November 2011 | Yes | 10 November 2011 | 8 | ||
| FCR | Neutropenic sepsis | 3 | 1 | Almost certainly | Almost certainly | Unlikely | 4 November 2011 | Yes | 10 November 2011 | 6 | ||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Possibly | 19 October 2011 | No | ||||
| FCR | Fatigue | 1 | 4 | Unlikely | Unlikely | Unlikely | Yes | 11 January 2012 | ||||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Possibly | 19 December 2011 | No | ||||
| FCR | Rash/flushing | 1 | 5 | Unlikely | Unlikely | Unlikely | Yes | 6 February 2012 | ||||
| FCR | Pruritus | 1 | 5 | Unlikely | Unlikely | Unlikely | Yes | 6 February 2012 | ||||
| FCR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Possibly | 5 March 2012 | No | ||||
| FCR | Anorexia/cachexia | 1 | 6 | Unrelated | Unrelated | Unrelated | Yes | 8 February 2012 | ||||
| 112 | FCR | Pruritus | 2 | 2 | Unrelated | Unrelated | Unrelated | 13 December 2011 | No | |||
| FCR | Rash/flushing | 2 | 3 | Unrelated | Unrelated | Unrelated | Yes | 27 February 2012 | ||||
| 116 | FCR | Thrombocytopenia | 3 | 1 | Unrelated | Unrelated | Unrelated | 31 October 2011 | No | |||
| FCR | Anaemia | 2 | 1 | Unrelated | Unrelated | Unrelated | 31 October 2011 | No | ||||
| FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 23 November 2011 | No | ||||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Possibly | 28 November 2011 | Yes | 1 December 2011 | 3 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Possibly | 28 December 2011 | Yes | 6 January 2012 | 9 | ||
| FCR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Possibly | 28 December 2011 | Yes | 2 January 2012 | 5 | ||
| FCR | Fatigue | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 25 January 2012 | Yes | 8 February 2012 | 14 | ||
| FCR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | 8 February 2012 | Yes | 21 February 2012 | 13 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 8 February 2012 | No | ||||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 24 February 2012 | Yes | ||||
| FCR | Fatigue | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 24 February 2012 | Yes | ||||
| FCR | Thrombocytopenia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 20 March 2012 | No | ||||
| FCR | Fatigue | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 30 March 2012 | Yes | ||||
| 120 | FCR | Nausea | 1 | 1 | Probably | Probably | Unlikely | Yes | ||||
| FCR | Rash/flushing | 1 | 2 | Unlikely | Unlikely | Unlikely | Yes | |||||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 30 January 2012 | Yes | 27 February 2012 | 28 | ||
| FCR | Fatigue | 1 | 3 | Possibly | Possibly | Possibly | 11 January 2012 | Yes | ||||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Possibly | 1 February 2012 | Yes | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Unlikely | Unlikely | Unlikely | 14 February 2012 | Yes | ||||
| FCR | Fatigue | 1 | 4 | Possibly | Possibly | Possibly | 8 February 2012 | Yes | ||||
| FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 26 March 2012 | Yes | 23 April 2012 | 28 | ||
| FCR | Mucositis/thrush | 1 | 5 | Almost certainly | Almost certainly | Possibly | 29 February 2012 | Yes | 13 March 2012 | 13 | ||
| FCR | Cough | 1 | 5 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Fatigue | 1 | 5 | Possibly | Possibly | Possibly | 7 March 2012 | Yes | ||||
| FCR | Fatigue | 1 | 6 | Possibly | Possibly | Possibly | 4 April 2012 | Yes | ||||
| 122 | FCR | Nausea | 1 | 1 | Probably | Probably | Unlikely | 18 November 2011 | Yes | 23 November 2011 | 5 | |
| FCR | Vomiting | 2 | 1 | Probably | Probably | Unlikely | 18 November 2011 | Yes | 23 November 2011 | 5 | ||
| FCR | Rash/flushing | 2 | 1 | Unlikely | Unlikely | Unlikely | 27 November 2011 | No | ||||
| FCR | Anaemia | 1 | 1 | Possibly | Possibly | Unlikely | 18 November 2011 | No | ||||
| FCR | Nausea | 1 | 2 | Probably | Probably | Unlikely | 16 December 2011 | Yes | 20 December 2011 | 4 | ||
| FCR | Rash/flushing | 3 | 2 | Probably | Unlikely | Unlikely | 9 December 2011 | Yes | 9 January 2012 | 31 | ||
| FCR | Allergic reaction | 1 | 2 | Probably | Unlikely | Unlikely | 18 November 2011 | Yes | 9 January 2012 | 52 | ||
| FCR | Anaemia | 2 | 2 | Possibly | Possibly | Unlikely | 21 December 2011 | Yes | 9 January 2012 | 19 | ||
| 123 | FCR | Fatigue | 1 | 1 | Unlikely | Unlikely | Unlikely | 5 December 2011 | No | |||
| FCR | Common cold | 1 | 1 | Possibly | Possibly | Unlikely | 5 December 2011 | No | ||||
| FCR | Anaemia | 2 | 1 | Unlikely | Unlikely | Unlikely | Yes | 10 January 2012 | ||||
| FCR | Thrombocytopenia | 2 | 1 | Possibly | Possibly | Possibly | 5 December 2011 | No | ||||
| FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Possibly | 22 December 2011 | Yes | 22 March 2012 | 91 | ||
| FCR | Abdominal pain/bloating | 1 | 4 | Unlikely | Unlikely | Unlikely | 11 April 2012 | Yes | ||||
| FCR | Headache | 2 | 5 | Unlikely | Unlikely | Unlikely | 15 March 2012 | Yes | 26 May 2012 | 72 | ||
| FCR | Back pain | 1 | 6 | Unlikely | Unlikely | Unlikely | 28 May 2012 | Yes | 5 June 2012 | 8 | ||
| FCR | Dizziness | 2 | 6 | Unlikely | Unlikely | Unlikely | 29 May 2012 | Yes | 3 June 2012 | 5 | ||
| FCR | Neutropenia | 4 | 6 | Probably | Probably | Probably | 14 April 2012 | Yes | 30 July 2012 | 107 | ||
| FCR | Anaemia | 2 | 6 | Possibly | Possibly | Possibly | 6 July 2012 | Yes | 2 August 2012 | 27 | ||
| 125 | FCR | Anorexia/cachexia | 3 | 1 | Possibly | Possibly | Possibly | No | ||||
| FCR | Alopecia | 1 | 1 | Possibly | Possibly | Unlikely | No | |||||
| FCR | Diarrhoea | 1 | 1 | Unlikely | Unlikely | Unlikely | 22 February 2012 | Yes | 24 February 2012 | 2 | ||
| FCR | Infections (not neutropenic sepsis) | 3 | 1 | Probably | Probably | Probably | 20 February 2012 | Yes | 24 February 2012 | 4 | ||
| FCR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | 20 February 2012 | Yes | 24 February 2012 | 4 | ||
| FCR | Fatigue | 1 | 2 | Probably | Probably | Possibly | Yes | |||||
| FCR | Rash/flushing | 1 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Anaemia | 3 | 4 | Almost certainly | Possibly | Possibly | 22 May 2012 | Yes | 5 June 2012 | 14 | ||
| FCR | Neutropenia | 3 | 5 | Probably | Probably | Possibly | 25 May 2012 | Yes | 20 June 2012 | 26 | ||
| FCR | Nausea | 1 | 5 | Possibly | Possibly | Unlikely | 8 June 2012 | Yes | 10 June 2012 | 2 | ||
| FCR | Bone pain | 2 | 5 | Unlikely | Unlikely | Unlikely | 13 June 2012 | No | ||||
| FCR | Fatigue | 2 | 6 | Unrelated | Unrelated | Unrelated | 12 July 2012 | No | ||||
| FCR | Constipation | 1 | 6 | Unrelated | Unrelated | Unrelated | Yes | |||||
| FCR | Non-specific pain | 2 | 6 | Unrelated | Unrelated | Unrelated | 12 July 2012 | No | ||||
| 129 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 2 February 2012 | Yes | 3 February 2012 | 1 | |
| FCR | Neutropenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 14 February 2012 | No | ||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 1 March 2012 | Yes | 2 March 2012 | 1 | ||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 12 March 2012 | Yes | 17 April 2012 | 36 | ||
| FCR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | 12 March 2012 | Yes | 2 April 2012 | 21 | ||
| FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 12 March 2012 | Yes | 26 March 2012 | 14 | ||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 March 2012 | Yes | 17 April 2012 | 22 | ||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 17 April 2012 | Yes | 30 April 2012 | 13 | ||
| FCR | Urinary symptoms | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 18 April 2012 | No | ||||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 1 May 2012 | Yes | 5 May 2012 | 4 | ||
| FCR | Anaemia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 14 May 2012 | Yes | 28 May 2012 | 14 | ||
| FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 14 May 2012 | Yes | 28 May 2012 | 14 | ||
| FCR | Fatigue | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 29 May 2012 | No | ||||
| FCR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 11 June 2012 | Yes | 25 June 2012 | 14 | ||
| FCR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 11 June 2012 | No | ||||
| FCR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 9 July 2012 | Yes | 26 September 2012 | 79 | ||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 25 June 2012 | No | ||||
| 130 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 26 January 2012 | Yes | 29 January 2012 | 3 | |
| FCR | Vomiting | 1 | 2 | Possibly | Probably | Possibly | 24 February 2012 | Yes | 27 February 2012 | 3 | ||
| FCR | Anaemia | 2 | 2 | Possibly | Probably | Unlikely | 13 March 2012 | No | ||||
| FCR | Neutropenia | 4 | 2 | Possibly | Possibly | Unlikely | 13 March 2012 | Yes | 19 March 2012 | 6 | ||
| FCR | Neutropenia | 4 | 3 | Possibly | Possibly | Unlikely | 25 March 2012 | Yes | 10 April 2012 | 16 | ||
| FCR | Thrombocytopenia | 2 | 3 | Possibly | Possibly | Possibly | 25 March 2012 | Yes | 27 March 2012 | 2 | ||
| FCR | Anaemia | 2 | 3 | Possibly | Probably | Unlikely | 22 March 2012 | Yes | 28 March 2012 | 6 | ||
| FCR | Anaemia | 2 | 3 | Possibly | Probably | Unlikely | 10 April 2012 | No | ||||
| FCR | Neutropenia | 4 | 4 | Possibly | Possibly | Unlikely | 24 April 2012 | Yes | 1 May 2012 | 7 | ||
| FCR | Anaemia | 2 | 5 | Possibly | Probably | Unlikely | 15 May 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 5 | Possibly | Possibly | Possibly | 15 May 2012 | No | ||||
| FCR | Anaemia | 2 | 6 | Possibly | Probably | Unlikely | 12 June 2012 | Yes | 10 July 2012 | 28 | ||
| 134 | FCR | Thrombocytopenia | 1 | 1 | Unlikely | Unlikely | Unlikely | 8 November 2011 | No | |||
| FCR | Rash/flushing | 2 | 2 | Unrelated | Unrelated | Unrelated | 29 March 2012 | Yes | 1 April 2012 | 3 | ||
| FCR | Nausea | 1 | 2 | Possibly | Possibly | Possibly | 21 March 2012 | Yes | 22 March 2012 | 1 | ||
| FCR | Nausea | 2 | 2 | Probably | Probably | Possibly | 14 April 2012 | Yes | 18 April 2012 | 4 | ||
| FCR | Nausea | 2 | 3 | Probably | Probably | Possibly | 12 May 2012 | Yes | 16 May 2012 | 4 | ||
| FCR | Neutropenia | 3 | 5 | Possibly | Possibly | Possibly | 3 July 2012 | No | ||||
| FCR | Nausea | 2 | 5 | Probably | Probably | Possibly | 10 July 2012 | Yes | 14 July 2012 | 4 | ||
| FCR | Fatigue | 1 | 5 | Probably | Probably | Probably | 10 July 2012 | Yes | 24 July 2012 | 14 | ||
| 136 | FCR | Fatigue | 2 | 1 | Unrelated | Unrelated | Unrelated | No | ||||
| FCR | Nausea | 2 | 1 | Probably | Probably | Unlikely | 16 April 2012 | Yes | 18 April 2012 | 2 | ||
| FCR | Pruritus | 1 | 1 | Possibly | Possibly | Possibly | 5 May 2012 | No | ||||
| FCR | Constipation | 1 | 1 | Unlikely | Unlikely | Unlikely | 21 April 2012 | Yes | 25 April 2012 | 4 | ||
| FCR | Pruritus | 2 | 3 | Probably | Probably | Unlikely | 16 April 2012 | No | ||||
| FCR | Nausea | 1 | 3 | Probably | Probably | Unlikely | 13 May 2012 | Yes | 16 May 2012 | 3 | ||
| FCR | Rigors | 1 | 3 | Unlikely | Unlikely | Probably | 9 May 2012 | Yes | 9 May 2012 | 0 | ||
| FCR | Nausea | 2 | 4 | Probably | Probably | Unlikely | 10 June 2012 | Yes | 16 June 2012 | 6 | ||
| FCR | Mucositis/thrush | 1 | 5 | Probably | Probably | Unlikely | Yes | 27 August 2012 | ||||
| FCR | Nausea | 2 | 5 | Probably | Probably | Unlikely | 5 July 2012 | Yes | 8 July 2012 | 3 | ||
| FCR | Constipation | 1 | 5 | Probably | Probably | Possibly | 4 July 2012 | Yes | 11 July 2012 | 7 | ||
| FCR | Other AE description | Sore gums | 1 | 5 | Probably | Probably | Possibly | Yes | 01 August 2012 | |||
| FCR | Otalgia | 1 | 5 | Unlikely | Unlikely | Unlikely | 14 August 2012 | No | ||||
| FCR | Common cold | 1 | 5 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Nausea | 1 | 5 | Probably | Probably | Unlikely | 3 August 2012 | Yes | 10 August 2012 | 7 | ||
| FCR | Constipation | 1 | 5 | Probably | Probably | Possibly | 5 August 2012 | Yes | 11 August 2012 | 6 | ||
| 137 | FCR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | 23 March 2012 | Yes | 2 April 2012 | 10 | |
| FCR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 10 March 2012 | Yes | 10 March 2012 | 0 | ||
| FCR | Visual symptoms | 1 | 1 | Unlikely | Unlikely | Unlikely | 2 April 2012 | No | ||||
| FCR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 16 March 2012 | Yes | 2 April 2012 | 17 | ||
| FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Possibly | 14 March 2012 | Yes | 14 March 2012 | 0 | ||
| FCR | Anorexia/cachexia | 1 | 1 | Probably | Probably | Probably | 16 March 2012 | Yes | 2 April 2012 | 17 | ||
| FCR | Rash/flushing | 3 | 2 | Unlikely | Unlikely | Unlikely | 4 April 2012 | Yes | 9 April 2012 | 5 | ||
| FCR | Fatigue | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 19 April 2012 | Yes | 30 April 2012 | 11 | ||
| FCR | Pruritus | 1 | 2 | Unlikely | Unlikely | Unlikely | 2 April 2012 | Yes | 3 April 2012 | 1 | ||
| FCR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | 19 April 2012 | Yes | 30 April 2012 | 11 | ||
| FCR | Nausea | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | 7 April 2012 | Yes | 9 April 2012 | 2 | ||
| FCR | Diarrhoea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 8 April 2012 | Yes | 9 April 2012 | 1 | ||
| FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | 16 May 2012 | Yes | 28 May 2012 | 12 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 2 May 2012 | Yes | 7 May 2012 | 5 | ||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 30 May 2012 | Yes | 3 June 2012 | 4 | ||
| FCR | Vomiting | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 30 May 2012 | Yes | 3 June 2012 | 4 | ||
| 138 | FCR | Rash/flushing | 2 | 1 | Probably | Probably | Probably | 7 April 2012 | No | |||
| FCR | Headache | 1 | Possibly | Possibly | Possibly | 7 April 2012 | Yes | 11 April 2012 | 4 | |||
| FCR | Fever | 3 | 1 | Possibly | Possibly | Possibly | 7 April 2012 | Yes | 10 April 2012 | 3 | ||
| FCR | Neutropenic sepsis | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | 7 April 2012 | Yes | 16 April 2012 | 9 | ||
| FCR | Other AE description | Weight change | 1 | 2 | Probably | Probably | Probably | Yes | ||||
| FCR | Diarrhoea | 2 | 4 | Possibly | Possibly | Possibly | 15 July 2012 | Yes | 15 July 2012 | 0 | ||
| FCR | Allergic reaction | 2 | 4 | Unlikely | Unlikely | Unlikely | 13 April 2012 | Yes | 13 April 2012 | 0 | ||
| 140 | FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 6 April 2012 | Yes | 7 April 2012 | 1 | |
| FCR | Constipation | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 7 April 2012 | No | ||||
| FCR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Unlikely | 7 April 2012 | No | ||||
| FCR | Non-specific pain | 1 | 1 | Possibly | Possibly | Possibly | 7 April 2012 | Yes | 3 May 2012 | 26 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 11 April 2012 | No | ||||
| FCR | Constipation | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 3 April 2012 | No | ||||
| FCR | Fatigue | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 3 April 2012 | No | ||||
| FCR | Back pain | 1 | 2 | Possibly | Possibly | Possibly | 3 April 2012 | Yes | 3 May 2012 | 30 | ||
| FCR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 1 May 2012 | Yes | 27 May 2012 | 26 | ||
| FCR | Taste alteration | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 3 April 2012 | No | ||||
| FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 3 July 2012 | Yes | 9 July 2012 | 6 | ||
| FCR | Vomiting | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 3 July 2012 | No | ||||
| 141 | FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 2 May 2012 | Yes | 30 May 2012 | 28 | |
| FCR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 2 May 2012 | Yes | 30 May 2012 | 28 | ||
| FCR | Back pain | 1 | 4 | Unrelated | Unrelated | Unrelated | 15 May 2012 | Yes | 27 June 2012 | 43 | ||
| FCR | Vomiting | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 23 June 2012 | Yes | 25 July 2012 | 32 | ||
| FCR | Other AE description | Dry mouth | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 27 June 2012 | Yes | 25 July 2012 | 28 | |
| FCR | Mucositis/thrush | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 27 June 2012 | Yes | 25 July 2012 | 28 | ||
| FCR | Other AE description | Lump on leg | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 27 June 2012 | Yes | 25 July 2012 | 28 | |
| FCR | Infections (not neutropenic sepsis) | 4 | 5 | Probably | Probably | Probably | 25 July 2012 | Yes | 15 August 2012 | 21 | ||
| FCR | Night sweats | 2 | 5 | Unrelated | Unrelated | Unrelated | 25 July 2012 | Yes | 15 August 2012 | 21 | ||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | 12 September 2012 | Yes | 17 September 2012 | 5 | ||
| 143 | FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 30 April 2012 | Yes | 30 April 2012 | 0 | |
| FCR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Unrelated | 5 May 2012 | Yes | 7 May 2012 | 2 | ||
| FCR | Diarrhoea | 1 | 2 | Unlikely | Unlikely | Unlikely | 2 June 2012 | Yes | 3 June 2012 | 1 | ||
| FCR | Fatigue | 3 | 2 | Unlikely | Unlikely | Unlikely | 19 June 2012 | Yes | 20 June 2012 | 1 | ||
| FCR | Other AE description | Worsening of diabetes | 2 | 2 | Unlikely | Unlikely | Unlikely | 20 June 2012 | No | |||
| FCR | Urinary symptoms | 2 | 2 | Unlikely | Unlikely | Unlikely | 20 June 2012 | No | ||||
| FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Unlikely | 20 July 2012 | Yes | 31 August 2012 | 42 | ||
| FCR | Thrombocytopenia | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 20 July 2012 | No | ||||
| FCR | Gout/hyperuricemia | 2 | 4 | Probably | Probably | Unrelated | 16 August 2012 | Yes | 31 August 2013 | 380 | ||
| FCR | Neutropenia | 3 | 5 | Probably | Probably | Unlikely | 3 October 2012 | No | ||||
| FCR | Thrombocytopenia | 2 | 5 | Probably | Probably | Unlikely | 26 September 2012 | No | ||||
| 146 | FCR | Neutropenia | 3 | 1 | Almost certainly | Probably | Probably | 26 June 2012 | Yes | 1 August 2012 | 36 | |
| FCR | Anaemia | 1 | 1 | Almost certainly | Probably | Probably | 26 June 2012 | Yes | 28 June 2012 | 2 | ||
| FCR | Rash/flushing | 3 | 1 | Unrelated | Unrelated | Unrelated | 3 July 2012 | Yes | 1 August 2012 | 29 | ||
| FCR | Urinary symptoms | 2 | 3 | Unrelated | Probably | Unrelated | 4 September 2012 | Yes | ||||
| FCR | Mucositis/thrush | 1 | 5 | Probably | Probably | Probably | Yes | |||||
| FCR | Constipation | 2 | 5 | Probably | Probably | Probably | Yes | 21 November 2012 | ||||
| FCR | Dyspnoea | 1 | 5 | Possibly | Unlikely | Possibly | Yes | |||||
| FCR | Rash/flushing | 1 | 6 | Unrelated | Unrelated | Unrelated | 27 November 2012 | Yes | 3 December 2012 | 6 | ||
| FCR | Abdominal pain/bloating | 1 | 6 | Unrelated | Unrelated | Unrelated | 25 December 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 30 January 2013 | No | ||||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | 30 January 2013 | No | ||||
| 147 | FCR | Fever | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 16 May 2012 | Yes | 20 May 2012 | 4 | |
| FCR | Mucositis/thrush | 1 | 1 | Almost certainly | Almost certainly | Unlikely | Yes | 13 June 2012 | ||||
| FCR | Anaemia | 2 | 1 | Almost certainly | Almost certainly | Possibly | 18 May 2012 | Yes | 22 May 2012 | 4 | ||
| FCR | Constipation | 1 | 2 | Possibly | Possibly | Possibly | Yes | 11 July 2012 | ||||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 11 July 2012 | Yes | 15 July 2012 | 4 | ||
| FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Unlikely | 22 May 2012 | Yes | 7 August 2012 | 77 | ||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 7 August 2012 | No | ||||
| FCR | Fatigue | 1 | 4 | Unlikely | Unlikely | Unlikely | Yes | 5 September 2012 | ||||
| FCR | Rash/flushing | 2 | 5 | Unlikely | Unlikely | Unlikely | No | |||||
| 151 | FCR | Fatigue | 1 | 1 | Possibly | Possibly | Possibly | Yes | ||||
| FCR | Anaemia | 3 | 2 | Possibly | Possibly | Possibly | 9 July 2012 | Yes | 23 July 2012 | 14 | ||
| FCR | Neutropenia | 1 | 3 | Probably | Probably | Unrelated | 20 August 2012 | Yes | 17 September 2012 | 28 | ||
| FCR | Thrombocytopenia | 2 | 3 | Probably | Probably | Unrelated | 20 August 2012 | Yes | 28 August 2012 | 8 | ||
| FCR | Lymphopenia | 3 | 3 | Probably | Unrelated | Probably | 20 August 2012 | No | ||||
| FCR | Neutropenia | 3 | 4 | Probably | Probably | Unrelated | 17 September 2012 | Yes | 24 September 2012 | 7 | ||
| FCR | Thrombocytopenia | 1 | 6 | Probably | Probably | Unrelated | 22 October 2012 | No | ||||
| 155 | FCR | Vomiting | 2 | 1 | Probably | Probably | Unlikely | 8 June 2012 | Yes | 10 June 2012 | 2 | |
| FCR | Vomiting | 2 | 2 | Probably | Probably | Unlikely | 7 July 2012 | Yes | 9 July 2012 | 2 | ||
| FCR | Vomiting | 3 | 3 | Probably | Probably | Unrelated | 4 August 2012 | Yes | 6 August 2012 | 2 | ||
| FCR | Neutropenia | 3 | 4 | Probably | Probably | Unrelated | 27 September 2012 | Yes | 15 October 2012 | 18 | ||
| FCR | Anaemia | 4 | 5 | Probably | Probably | Unrelated | 15 October 2012 | No | ||||
| 157 | FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 6 June 2012 | No | |||
| FCR | Fatigue | 1 | 2 | Unrelated | Unrelated | Unrelated | 6 June 2012 | No | ||||
| FCR | Diarrhoea | 1 | 3 | Unrelated | Unrelated | Unrelated | 1 August 2012 | No | ||||
| FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 23 August 2012 | Yes | 27 August 2012 | 4 | ||
| FCR | Diarrhoea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 23 August 2012 | Yes | 27 August 2012 | 4 | ||
| FCR | Cough | 1 | 5 | Unrelated | Unrelated | Unrelated | 26 September 2012 | No | ||||
| FCR | Diarrhoea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 28 September 2012 | Yes | 7 October 2012 | 9 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 26 September 2012 | Yes | 2 October 2012 | 6 | ||
| FCR | Diarrhoea | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 17 October 2012 | Yes | 23 October 2012 | 6 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | 2 December 2012 | Yes | 2 January 2013 | 31 | ||
| 158 | FCR | Pruritus | 2 | 1 | Unlikely | Unlikely | Unlikely | 1 July 2012 | Yes | 6 July 2012 | 5 | |
| FCR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | 1 July 2012 | Yes | 6 July 2012 | 5 | ||
| FCR | Diarrhoea | 1 | 1 | Unlikely | Probably | Unlikely | 20 June 2012 | Yes | 25 June 2012 | 5 | ||
| FCR | Nausea | 2 | 1 | Unlikely | Probably | Unlikely | 20 June 2012 | Yes | 25 June 2012 | 5 | ||
| FCR | Headache | 1 | 1 | Unlikely | Unlikely | Probably | 18 June 2012 | Yes | 19 June 2012 | 1 | ||
| FCR | Common cold | 2 | 2 | Unlikely | Unlikely | Unrelated | 31 July 2012 | Yes | 10 August 2012 | 10 | ||
| FCR | Mucositis/thrush | 1 | 2 | Unlikely | Probably | Unrelated | 9 August 2012 | No | ||||
| FCR | Back pain | 2 | 2 | Unrelated | Unrelated | Unrelated | 9 August 2012 | No | ||||
| FCR | Nausea | 2 | 2 | Unlikely | Probably | Unrelated | 20 July 2012 | Yes | 22 July 2012 | 2 | ||
| FCR | Vomiting | 2 | 2 | Unlikely | Probably | Unlikely | 20 July 2012 | Yes | 22 July 2012 | 2 | ||
| FCR | Other AE description | Kidney stone – had lithotripsy and surgery | 3 | 3 | Unrelated | Unrelated | Unrelated | 9 August 2012 | Yes | 26 September 2012 | 48 | |
| FCR | Nausea | 2 | 3 | Unlikely | Probably | Unlikely | 3 October 2012 | Yes | 9 October 2012 | 6 | ||
| FCR | Vomiting | 2 | 3 | Unlikely | Probably | Unlikely | 4 October 2012 | Yes | 5 October 2012 | 1 | ||
| FCR | Insomnia | 1 | 3 | Unrelated | Unrelated | Unrelated | 4 October 2012 | Yes | 5 October 2012 | 1 | ||
| FCR | Common cold | 2 | 3 | Unlikely | Unlikely | Unlikely | 2 October 2012 | No | ||||
| FCR | Fatigue | 1 | 3 | Unlikely | Unlikely | Unlikely | 18 June 2012 | No | ||||
| FCR | Nausea | 1 | 4 | Unlikely | Probably | Unlikely | 1 November 2012 | Yes | 6 November 2012 | 5 | ||
| FCR | Nausea | 1 | 5 | Unlikely | Probably | Unlikely | 27 November 2012 | Yes | 4 December 2012 | 7 | ||
| FCR | Vomiting | 1 | 5 | Unlikely | Probably | Unlikely | 29 November 2012 | Yes | 29 November 2012 | 0 | ||
| FCR | Diarrhoea | 1 | 5 | Unlikely | Probably | Unlikely | 30 November 2012 | Yes | 5 December 2012 | 5 | ||
| FCR | Non-specific pain | 1 | 5 | Unrelated | Unrelated | Unrelated | 30 November 2012 | No | ||||
| FCR | Nausea | 1 | 6 | Unlikely | Probably | Unlikely | 26 December 2012 | Yes | 1 January 2013 | 6 | ||
| FCR | Diarrhoea | 1 | 6 | Unlikely | Probably | Unlikely | 29 December 2012 | Yes | 3 January 2013 | 5 | ||
| FCR | Rash/flushing | 2 | 6 | Unlikely | Unlikely | Unlikely | 9 January 2013 | No | ||||
| FCR | Arthralgias | 1 | 6 | Unlikely | Unlikely | Unlikely | 15 January 2013 | Yes | 18 January 2013 | 3 | ||
| 159 | FCR | Nausea | 2 | 1 | Probably | Probably | Possibly | 19 July 2012 | Yes | 25 July 2012 | 6 | |
| FCR | Fatigue | 2 | 1 | Possibly | Possibly | Possibly | 25 July 2012 | No | ||||
| FCR | Taste alteration | 2 | 1 | Possibly | Possibly | Unlikely | 19 July 2012 | No | ||||
| FCR | Anorexia/cachexia | 2 | 1 | Probably | Probably | Unlikely | 19 July 2012 | No | ||||
| FCR | Nausea | 1 | 2 | Probably | Probably | Possibly | 18 August 2012 | Yes | 21 August 2012 | 3 | ||
| FCR | Anorexia/cachexia | 1 | 2 | Probably | Probably | Unlikely | 20 August 2012 | No | ||||
| FCR | Alopecia | 1 | 2 | Possibly | Possibly | Unlikely | 20 August 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Possibly | 30 August 2012 | Yes | 6 September 2012 | 7 | ||
| FCR | Taste alteration | 1 | 3 | Possibly | Possibly | Unlikely | 11 September 2012 | No | ||||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Possibly | 21 September 2012 | No | ||||
| FCR | Nausea | 1 | 4 | Probably | Probably | Possibly | 11 September 2012 | Yes | 5 November 2012 | 55 | ||
| FCR | Thrombocytopenia | 2 | 4 | Almost certainly | Almost certainly | Possibly | 16 October 2012 | No | ||||
| 166 | FCR | Infusional reaction | 1 | Unlikely | Unlikely | Probably | 4 July 2012 | Yes | 5 July 2012 | 1 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 1 | Possibly | Possibly | Unlikely | 23 July 2012 | Yes | 6 August 2012 | 14 | ||
| FCR | Fatigue | 2 | 1 | Possibly | Possibly | Possibly | 10 July 2012 | Yes | 6 August 2012 | 27 | ||
| FCR | Constipation | 1 | 1 | Possibly | Possibly | Possibly | 11 July 2012 | Yes | 26 July 2012 | 15 | ||
| FCR | Anaemia | 3 | 1 | Possibly | Possibly | Possibly | 30 July 2012 | Yes | 6 August 2012 | 7 | ||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | 20 August 2012 | Yes | 4 September 2012 | 15 | ||
| FCR | Neutropenia | 4 | 4 | Possibly | Possibly | Possibly | 30 October 2012 | Yes | 20 November 2012 | 21 | ||
| FCR | Neutropenia | 4 | 5 | Almost certainly | Probably | Probably | 14 December 2012 | Yes | 21 December 2012 | 7 | ||
| FCR | Urinary symptoms | 1 | 5 | Unlikely | Unlikely | Unlikely | 28 December 2012 | |||||
| FCR | Anaemia | 1 | 5 | Almost certainly | Possibly | Possibly | 21 December 2012 | No | ||||
| FCR | Neutropenia | 4 | 6 | Almost certainly | Probably | Probably | 28 December 2012 | Yes | 22 January 2013 | 25 | ||
| FCR | Raised GGT/bilirubin | 1 | 6 | Possibly | Possibly | Possibly | 14 December 2012 | No | ||||
| 167 | FCR | Nausea | 1 | 1 | Probably | Probably | Possibly | 25 July 2012 | Yes | 27 July 2012 | 2 | |
| FCR | Vomiting | 1 | 1 | Probably | Probably | Possibly | 25 July 2012 | Yes | 27 July 2012 | 2 | ||
| FCR | Nausea | 1 | 2 | Probably | Probably | Possibly | 25 August 2012 | Yes | 26 August 2012 | 1 | ||
| FCR | Vomiting | 1 | 2 | Probably | Probably | Possibly | 25 August 2012 | Yes | 26 August 2012 | 1 | ||
| FCR | Nausea | 1 | 3 | Probably | Probably | Possibly | 19 September 2012 | Yes | 23 September 2012 | 4 | ||
| FCR | Vomiting | 1 | 3 | Probably | Probably | Possibly | 19 September 2012 | Yes | 23 September 2012 | 4 | ||
| FCR | Neuropathy (sensory) | 1 | 3 | Possibly | Unlikely | Unlikely | 17 September 2012 | No | ||||
| FCR | Fatigue | 1 | 3 | Probably | Unlikely | Probably | 15 October 2012 | No | ||||
| FCR | Nausea | 1 | 4 | Probably | Probably | Possibly | 17 October 2012 | Yes | 19 October 2012 | 2 | ||
| FCR | Vomiting | 1 | 4 | Probably | Probably | Possibly | 19 October 2012 | Yes | 19 October 2012 | 0 | ||
| FCR | Nausea | 1 | 5 | Probably | Probably | Possibly | 15 November 2012 | Yes | 16 November 2012 | 1 | ||
| FCR | Nausea | 1 | 6 | Probably | Probably | Unlikely | 13 December 2012 | Yes | 14 December 2012 | 1 | ||
| 168 | FCR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Possibly | 1 August 2012 | Yes | 6 August 2012 | 5 | |
| FCR | Nausea | 1 | 1 | Probably | Probably | Possibly | 10 July 2012 | Yes | 13 July 2012 | 3 | ||
| FCR | Rash/flushing | 1 | 1 | Probably | Possibly | Unlikely | 9 July 2012 | Yes | 9 July 2012 | 0 | ||
| FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Possibly | 1 August 2012 | Yes | 27 September 2012 | 57 | ||
| FCR | Infections (not neutropenic sepsis) | 3 | 3 | Probably | Probably | Possibly | 21 September 2012 | Yes | 1 October 2012 | 10 | ||
| FCR | Anaemia | 1 | 4 | Almost certainly | Almost certainly | Possibly | 15 August 2012 | Yes | 1 October 2012 | 47 | ||
| FCR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Possibly | 4 October 2012 | No | ||||
| FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Possibly | 7 November 2012 | Yes | 21 November 2012 | 14 | ||
| FCR | Neutropenia | 1 | 5 | Almost certainly | Almost certainly | Possibly | 5 December 2012 | No | ||||
| FCR | Taste alteration | 1 | 5 | Possibly | Possibly | Possibly | 12 November 2012 | Yes | 20 November 2012 | 8 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 6 | Almost certainly | Almost certainly | Possibly | 25 December 2012 | No | ||||
| 171 | FCR | Abnormal electrolytes | 3 | 1 | Unrelated | Unrelated | Unrelated | 9 July 2012 | Yes | 11 July 2012 | 2 | |
| FCR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Unlikely | 30 July 2012 | Yes | 6 August 2012 | 7 | ||
| FCR | Constipation | 2 | 1 | Unlikely | Unlikely | Unlikely | 9 July 2012 | Yes | 12 July 2012 | 3 | ||
| FCR | Nausea | 1 | 1 | Probably | Probably | Unlikely | 9 July 2012 | Yes | 12 July 2012 | 3 | ||
| FCR | Nausea | 1 | 2 | Probably | Probably | Unlikely | 6 August 2012 | Yes | 14 August 2012 | 8 | ||
| FCR | Constipation | 2 | 2 | Possibly | Possibly | Unlikely | 6 August 2012 | Yes | 14 August 2012 | 8 | ||
| FCR | Back pain | 1 | 2 | Unrelated | Unrelated | Unrelated | 4 July 2012 | Yes | 3 September 2012 | 61 | ||
| FCR | Nausea | 3 | Probably | Probably | Unlikely | 3 September 2012 | Yes | 11 September 2012 | 8 | |||
| FCR | Constipation | 3 | Possibly | Possibly | Unlikely | 3 September 2012 | Yes | 11 September 2012 | 8 | |||
| FCR | Neutropenia | 1 | 3 | Probably | Probably | Unlikely | 27 September 2012 | Yes | 8 October 2012 | 11 | ||
| FCR | Renal impairment | 1 | 3 | Possibly | Possibly | Possibly | 6 August 2012 | No | ||||
| FCR | Nausea | 4 | Probably | Probably | Unlikely | 1 October 2012 | Yes | |||||
| FCR | Renal impairment | 1 | 4 | Possibly | Possibly | Possibly | 3 September 2012 | No | ||||
| FCR | Rash/flushing | 2 | 5 | Unlikely | Unlikely | Unlikely | ||||||
| FCR | Renal impairment | 1 | 5 | Possibly | Possibly | Possibly | 1 October 2012 | No | ||||
| FCR | Rash/flushing | 2 | 6 | Unlikely | Unlikely | Unlikely | 29 October 2012 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 3 | 6 | Unlikely | Unlikely | Unlikely | 1 January 2013 | Yes | 8 January 2013 | 7 | ||
| 174 | FCR | Infusional reaction | 1 | 1 | Unrelated | Unrelated | Almost certainly | 24 July 2012 | Yes | 24 July 2012 | 0 | |
| FCR | Thrombocytopenia | 2 | 1 | Unlikely | Unlikely | Unlikely | 24 July 2012 | No | ||||
| FCR | Anaemia | 1 | 1 | Unlikely | Unlikely | Unlikely | 24 July 2012 | No | ||||
| FCR | Mucositis/thrush | 1 | 1 | Possibly | Probably | Unlikely | 7 August 2012 | Yes | 21 August 2012 | 14 | ||
| FCR | Other AE description | Unstable diabetes control hyperglycaemia – insulin | 3 | 2 | Unlikely | Unlikely | Unlikely | 7 September 2012 | No | |||
| FCR | Neutropenia | 4 | 3 | Probably | Probably | Unlikely | 28 August 2012 | Yes | 7 October 2012 | 40 | ||
| FCR | Fever | 1 | 3 | Possibly | Possibly | Unrelated | 17 September 2012 | Yes | 18 October 2012 | 31 | ||
| FCR | Sore throat | 1 | 3 | Unrelated | Unrelated | Unrelated | 29 August 2012 | Yes | 23 October 2012 | 55 | ||
| FCR | Anxiety/depression | 1 | 3 | Unlikely | Unlikely | Unlikely | 21 August 2012 | Yes | 23 October 2012 | 63 | ||
| FCR | Other AE description | Unstable diabetes control (hyperglycaemia) | 3 | 3 | Unlikely | Unlikely | Unlikely | 7 September 2012 | No | |||
| FCR | Taste alteration | 1 | 4 | Probably | Probably | Unlikely | 21 August 2012 | Yes | 27 November 2012 | 98 | ||
| FCR | Fatigue | 1 | 4 | Probably | Probably | Possibly | 24 July 2012 | Yes | 7 November 2012 | 106 | ||
| FCR | Neutropenia | 4 | 5 | Probably | Probably | Unlikely | 7 November 2012 | Yes | 27 December 2012 | 50 | ||
| FCR | Neutropenia | 2 | 6 | Probably | Probably | Unlikely | 4 January 2013 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 1 | 6 | Possibly | Possibly | Unrelated | 31 December 2012 | Yes | 22 January 2013 | 22 | ||
| FCR | Arthralgias | 1 | 6 | Unrelated | Unrelated | Unrelated | Yes | 12 December 2012 | ||||
| FCR | Other AE description | Unstable diabetic control | 1 | 6 | Unlikely | Unlikely | Unlikely | 7 September 2012 | Yes | 26 February 2013 | 172 | |
| 175 | FCR | Infections (not neutropenic sepsis) | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 25 September 2012 | No | |||
| FCR | Dyspnoea | 2 | 2 | Unrelated | Unrelated | Unrelated | 23 August 2012 | Yes | 24 August 2012 | 1 | ||
| FCR | Chest pain | 2 | 2 | Unrelated | Unrelated | Unrelated | 23 August 2012 | Yes | 24 August 2012 | 1 | ||
| 178 | FCR | Diarrhoea | 1 | 1 | Possibly | Possibly | Unlikely | Yes | ||||
| FCR | Nausea | 1 | 1 | Possibly | Possibly | Unlikely | Yes | |||||
| FCR | Thrombocytopenia | 1 | 3 | Probably | Probably | Probably | 10 September 2012 | No | ||||
| FCR | Lymphopenia | 3 | 3 | Probably | Probably | Probably | 10 September 2012 | Yes | 8 October 2012 | 28 | ||
| FCR | Pruritus | 2 | 4 | Unlikely | Unlikely | Unlikely | 15 October 2012 | Yes | ||||
| FCR | Other AE description | Squamous cell carcinoma | 3 | 4 | Unrelated | Unrelated | Unrelated | 22 October 2012 | Yes | |||
| FCR | Lymphopenia | 3 | 5 | Probably | Probably | Probably | 5 November 2012 | No | ||||
| FCR | Infections (not neutropenic sepsis) | 2 | 6 | Possibly | Possibly | Possibly | Yes | |||||
| FCR | Other AE description | Squamous cell carcinoma in situ of wrist | 3 | 6 | Unrelated | Unrelated | Unrelated | Yes | ||||
| FCR | Neutropenia | 1 | 6 | Probably | Probably | Probably | 21 January 2013 | Yes | 18 February 2013 | 28 | ||
| FCR | Back pain | 1 | 6 | Unrelated | Unrelated | Unrelated | No | |||||
| FCR | Fatigue | 1 | 6 | Unlikely | Unlikely | Unlikely | No | |||||
| 179 | FCR | Neutropenia | 4 | 1 | Probably | Probably | Unlikely | 25 July 2012 | Yes | 7 August 2012 | 13 | |
| FCR | Anaemia | 3 | 1 | Probably | Probably | Possibly | 3 August 2012 | Yes | 14 August 2012 | 11 | ||
| FCR | Thrombocytopenia | 2 | 1 | Possibly | Possibly | Possibly | 25 July 2012 | No | ||||
| FCR | Nausea | 2 | 1 | Unlikely | Unlikely | Possibly | 31 July 2012 | Yes | 24 August 2012 | 24 | ||
| FCR | Vomiting | 2 | 1 | Unlikely | Unlikely | Possibly | 31 July 2012 | Yes | 24 August 2012 | 24 | ||
| FCR | 1 | Missing | Missing | Missing | Yes | |||||||
| FCR | Diarrhoea | 1 | 2 | Unlikely | Unlikely | Possibly | 8 September 2012 | Yes | 12 September 2012 | 4 | ||
| FCR | Thrombocytopenia | 1 | 2 | Possibly | Possibly | Possibly | 7 September 2012 | Yes | 20 September 2012 | 13 | ||
| FCR | Anaemia | 1 | 2 | Probably | Probably | Possibly | 9 September 2012 | Yes | 18 September 2012 | 9 | ||
| FCR | Rash/flushing | 3 | 2 | Unrelated | Unrelated | Unrelated | 22 August 2012 | No | ||||
| 181 | FCR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | 22 August 2012 | Yes | 30 August 2012 | 8 | |
| FCR | Rash/flushing | 2 | 2 | Unrelated | Unrelated | Unrelated | 22 August 2012 | Yes | 9 October 2012 | 48 | ||
| FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 September 2012 | Yes | 9 October 2012 | 13 | ||
| FCR | Fatigue | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 September 2012 | Yes | 27 September 2012 | 1 | ||
| FCR | Anorexia/cachexia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 September 2012 | Yes | 27 September 2012 | 1 | ||
| FCR | Oedema | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 26 September 2012 | Yes | 27 September 2012 | 1 | ||
| FCR | Anxiety/depression | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 6 November 2012 | No | ||||
| FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | 4 December 2012 | Yes | 17 December 2012 | 13 | ||
| FCR | Anxiety/depression | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | 17 December 2012 | No | ||||
| FCR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | 14 January 2013 | Yes | 22 January 2013 | 8 | ||
| 182 | FCR | Rash/flushing | 2 | 3 | Unlikely | Unlikely | Unlikely | 12 September 2012 | No | |||
| FCR | Other AE description | Keratoacanthoma (removed) | 2 | 4 | Unrelated | Unrelated | Unrelated | Yes | 4 December 2012 | |||
| FCR | Nausea | 1 | 6 | Probably | Probably | Possibly | 11 December 2012 | Yes | 17 December 2012 | 6 | ||
| FCR | Headache | 1 | 6 | Unlikely | Unlikely | Possibly | 11 December 2012 | Yes | 17 December 2012 | 6 | ||
| 185 | FCR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Probably | 25 August 2012 | Yes | 30 August 2012 | 5 | |
| FCR | Fatigue | 3 | 1 | Almost certainly | Possibly | Probably | 27 August 2012 | Yes | 31 August 2012 | 4 | ||
| FCR | Fatigue | 1 | 1 | Almost certainly | Possibly | Probably | 16 September 2012 | No | ||||
| FCR | Constipation | 1 | 1 | Unlikely | Unlikely | Possibly | 25 August 2012 | No | ||||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Probably | 20 September 2012 | Yes | 21 September 2012 | 1 | ||
| FCR | Mucositis/thrush | 1 | 2 | Possibly | Unlikely | Possibly | 20 September 2012 | Yes | 27 September 2012 | 7 | ||
| FCR | Rash/flushing | 2 | 2 | Unlikely | Unlikely | Unlikely | 11 October 2012 | No | ||||
| FCR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Probably | 19 October 2012 | Yes | 19 October 2012 | 0 | ||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Probably | 18 October 2012 | Yes | 20 October 2012 | 2 | ||
| FCR | Infections (not neutropenic sepsis) | 2 | 4 | Possibly | Possibly | Possibly | 17 November 2012 | Yes | 19 November 2012 | 2 | ||
| FCR | Neutropenic sepsis | 4 | 5 | Almost certainly | Almost certainly | Probably | 28 December 2012 | Yes | 4 January 2013 | 7 | ||
| FCR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Probably | 31 December 2012 | Yes | 8 January 2013 | 8 | ||
| 187 | FCR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 11 December 2012 | Yes | 20 December 2012 | 9 | |
| FCR | Anaemia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 11 December 2012 | No | ||||
| FCR | Thrombocytopenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | 11 December 2012 | No | ||||
| 191 | FCR | Fatigue | 1 | 1 | Possibly | Possibly | Possibly | No | ||||
| FCR | Cystitis | 1 | 1 | Unrelated | Unrelated | Unrelated | 5 November 2012 | No | ||||
| FCR | Anaemia | 1 | 1 | Unrelated | Unrelated | Unrelated | 19 October 2011 | No | ||||
| FCR | Thrombocytopenia | 1 | 1 | Unrelated | Unrelated | Unrelated | 30 March 2011 | No | ||||
| FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Unlikely | 17 December 2012 | No | ||||
| FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Unlikely | No | |||||
| FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Unlikely | No | |||||
| 192 | FCR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Almost certainly | 10 September 2012 | Yes | 10 September 2012 | 0 | |
| FCR | Nausea | 1 | 1 | Probably | Probably | Probably | 13 September 2012 | Yes | 17 September 2012 | 4 | ||
| FCR | Fatigue | 2 | 1 | Possibly | Possibly | Possibly | 12 September 2012 | Yes | 17 September 2012 | 5 | ||
| FCR | Anaemia | 1 | 1 | Unrelated | Unrelated | Unrelated | 28 July 2012 | Yes | 9 October 2012 | 73 | ||
| FCR | Neutropenia | 2 | 1 | Unrelated | Unrelated | Unrelated | 7 September 2012 | No | ||||
| FCR | Infusional reaction | 1 | 2 | Unrelated | Unrelated | Almost certainly | 10 October 2012 | Yes | 10 October 2012 | 0 | ||
| FCR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 15 October 2012 | Yes | 15 October 2012 | 0 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Unlikely | 10 October 2012 | Yes | 17 October 2012 | 7 | ||
| FCR | Neuropathy (sensory) | 1 | 2 | Possibly | Unlikely | Unlikely | 5 November 2012 | Yes | 05 November 2012 | 0 | ||
| FCR | Fatigue | 2 | 2 | Probably | Probably | Possibly | 10 October 2012 | Yes | 17 October 2012 | 7 | ||
| FCR | Thrombocytopenia | 1 | 2 | Possibly | Possibly | Possibly | 28 July 2012 | Yes | 6 November 2012 | 101 | ||
| FCR | Lymphopenia | 3 | 2 | Almost certainly | Almost certainly | Possibly | 6 November 2012 | No | ||||
| FCR | Bone pain | 2 | 3 | Possibly | Possibly | Possibly | 28 November 2012 | No | ||||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 8 November 2012 | Yes | 15 November 2012 | 7 | ||
| FCR | Fatigue | 2 | 3 | Almost certainly | Almost certainly | Possibly | 7 November 2012 | Yes | 14 November 2012 | 7 | ||
| FCR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Possibly | 4 December 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 3 | Probably | Probably | Possibly | 4 December 2012 | No | ||||
| FCR | Vomiting | 1 | 4 | Possibly | Possibly | Possibly | 8 December 2012 | Yes | 8 December 2012 | 0 | ||
| FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | 9 December 2012 | Yes | 16 December 2012 | 7 | ||
| FCR | Fatigue | 2 | 4 | Almost certainly | Almost certainly | Possibly | 7 December 2012 | Yes | 14 December 2012 | 7 | ||
| FCR | Cystitis | 1 | 4 | Unlikely | Unlikely | Unlikely | 5 November 2012 | Yes | 26 November 2012 | 21 | ||
| FCR | Rash/flushing | 1 | 4 | Possibly | Possibly | Possibly | 27 November 2012 | Yes | 4 December 2012 | 7 | ||
| FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 4 January 2013 | Yes | 12 January 2013 | 8 | ||
| FCR | Constipation | 1 | 5 | Unlikely | Unlikely | Unlikely | 7 January 2013 | Yes | 9 February 2013 | 33 | ||
| FCR | Fatigue | 2 | 5 | Almost certainly | Almost certainly | Possibly | 5 January 2013 | Yes | 12 January 2013 | 7 | ||
| FCR | Vomiting | 2 | 6 | Almost certainly | Almost certainly | Possibly | 31 January 2013 | Yes | 6 February 2013 | 6 | ||
| 193 | FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | 26 October 2012 | Yes | 5 November 2012 | 10 | |
| FCR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 26 October 2012 | Yes | 5 November 2012 | 10 | ||
| FCR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 31 December 2012 | No | ||||
| FCR | Other AE description | Ruptured Achilles tendon | 2 | 4 | Unrelated | Unrelated | Unrelated | 21 January 2013 | No | |||
| FCR | Thrombocytopenia | 1 | 4 | Probably | Probably | Probably | 28 January 2013 | No | ||||
| FCR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | 14 February 2013 | Yes | 26 February 2013 | 12 | ||
| FCR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 14 February 2013 | Yes | 26 February 2013 | 12 | ||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 14 March 2013 | No | ||||
| FCR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | 14 March 2013 | Yes | 25 March 2013 | 11 | ||
| 194 | FCR | Abdominal pain/bloating | 1 | 1 | Possibly | Possibly | Possibly | No | ||||
| FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | 3 October 2012 | Yes | 16 October 2012 | 13 | ||
| FCR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Yes | |||||
| FCR | Anaemia | 1 | 1 | Unlikely | Unlikely | Unlikely | 27 June 2012 | Yes | 16 October 2012 | 111 | ||
| FCR | Cystitis | 2 | 2 | Possibly | Almost certainly | Unlikely | 24 September 2012 | Yes | 17 October 2012 | 23 | ||
| FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | 17 October 2012 | Yes | 22 October 2012 | 5 | ||
| FCR | Anaemia | 1 | 3 | Possibly | Possibly | Possibly | 29 October 2012 | Yes | 12 December 2012 | 44 | ||
| FCR | Fatigue | 2 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Cystitis | 1 | 3 | Almost certainly | Almost certainly | Unlikely | Yes | |||||
| FCR | Pruritus | 1 | 3 | Unlikely | Unlikely | Unlikely | No | |||||
| FCR | Alopecia | 2 | 3 | Almost certainly | Almost certainly | Unlikely | No | |||||
| FCR | Cough | 1 | 4 | Unlikely | Unlikely | Unlikely | Yes | 30 January 2013 | ||||
| FCR | Fever | 2 | 4 | Unlikely | Unlikely | Possibly | 13 December 2012 | Yes | 15 December 2012 | 2 | ||
| FCR | Dyspnoea | 1 | 4 | Unlikely | Unlikely | Possibly | Yes | 9 January 2013 | ||||
| FCR | Vomiting | 2 | 4 | Possibly | Possibly | Possibly | 12 December 2012 | Yes | 15 December 2012 | 3 | ||
| FCR | Abdominal pain/bloating | 2 | 4 | Possibly | Possibly | Possibly | 12 December 2012 | Yes | 15 December 2012 | 3 | ||
| FCR | Fever | 2 | 4 | Unlikely | Unlikely | Possibly | 10 January 2013 | Yes | 14 January 2013 | 4 | ||
| FCR | Vomiting | 2 | 4 | Possibly | Possibly | Possibly | 10 January 2013 | Yes | 14 January 2013 | 4 | ||
| 195 | FCR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 17 October 2012 | Yes | 16 January 2013 | 91 | |
| FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 11 October 2012 | Yes | 17 October 2012 | 6 | ||
| FCR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Unlikely | 17 October 2012 | Yes | 16 January 2013 | 91 | ||
| 198 | FCR | Thrombocytopenia | 3 | 1 | Probably | Probably | Possibly | 4 October 2012 | Yes | 24 October 2012 | 20 | |
| FCR | Urinary symptoms | 2 | 1 | Possibly | Possibly | Unlikely | 22 September 2012 | Yes | 26 September 2012 | 4 | ||
| FCR | Fatigue | 1 | 1 | Probably | Probably | Unlikely | 21 September 2012 | No | ||||
| FCR | Bone pain | 2 | 1 | Unrelated | Unrelated | Unrelated | No | |||||
| FCR | Dizziness | 1 | 1 | Unlikely | Unlikely | Unlikely | 11 October 2012 | Yes | 18 October 2012 | 7 | ||
| FCR | Fatigue | 1 | 2 | Probably | Probably | Unlikely | No | |||||
| FCR | Constipation | 1 | 2 | Unlikely | Unlikely | Possibly | 27 October 2012 | Yes | 1 November 2012 | 5 | ||
| FCR | Nasal symptoms | 1 | 2 | Unlikely | Unlikely | Possibly | 2 November 2012 | No | ||||
| FCR | Arthralgias | 1 | 2 | Unlikely | Unlikely | Possibly | 26 October 2012 | No | ||||
| FCR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Unlikely | 22 November 2012 | Yes | 26 November 2012 | 4 | ||
| FCR | Constipation | 1 | 3 | Unlikely | Unlikely | Possibly | 22 November 2012 | Yes | 26 November 2012 | 4 | ||
| FCR | Taste alteration | 1 | 3 | Almost certainly | Almost certainly | Unlikely | No | |||||
| FCR | Urinary symptoms | 1 | 3 | Unlikely | Probably | Unrelated | 29 November 2012 | No | ||||
| FCR | Nasal symptoms | 1 | 4 | Unlikely | Unlikely | Possibly | No | |||||
| FCR | Fatigue | 2 | 4 | Probably | Probably | Unlikely | 22 November 2012 | No | ||||
| FCR | Urinary symptoms | 1 | 4 | Unlikely | Probably | Unrelated | No | |||||
| FCR | Nasal symptoms | 2 | 4 | Unlikely | Unlikely | Unlikely | 10 January 2013 | No | ||||
| FCR | Anorexia/cachexia | 1 | 4 | Almost certainly | Almost certainly | Unlikely | No | |||||
| FCR | Fatigue | 2 | 5 | Almost certainly | Almost certainly | Unlikely | 17 January 2013 | Yes | 21 January 2013 | 4 | ||
| FCR | Constipation | 1 | 5 | Unlikely | Unlikely | Possibly | 14 February 2013 | No | ||||
| FCR | Fatigue | 2 | 6 | Almost certainly | Almost certainly | Unlikely | 14 February 2013 | Yes | 28 February 2013 | 14 | ||
| FCR | Fatigue | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 1 March 2013 | Yes | 14 March 2013 | 13 | ||
| FCR | Other AE description | Bruising to L orbital region | 1 | 6 | Probably | Probably | Unlikely | Yes | ||||
| 199 | FCR | Vomiting | 2 | 2 | Probably | Almost certainly | Unlikely | 14 October 2012 | Yes | 14 October 2012 | 0 | |
| FCR | Allergic reaction | 2 | 2 | Unlikely | Unlikely | Almost certainly | 10 October 2012 | Yes | 10 October 2012 | 0 | ||
| FCR | Myalgias | 1 | 2 | Unlikely | Unlikely | Unlikely | 2 November 2012 | Yes | 2 November 2012 | 0 | ||
| FCR | Other AE description | Right upper jaw lump | 1 | 2 | Unlikely | Unlikely | Unlikely | No | ||||
| FCR | Fatigue | 1 | 3 | Probably | Probably | Unrelated | 10 October 2012 | Yes | 10 December 2012 | 61 | ||
| FCR | Nausea | 2 | 3 | Probably | Almost certainly | Unlikely | 14 October 2012 | Yes | 10 December 2012 | 57 | ||
| FCR | Other AE description | Right upper jaw lump | 1 | 3 | Unlikely | Unlikely | Unlikely | Yes | ||||
| FCR | Vomiting | 2 | 3 | Probably | Almost certainly | Unlikely | 7 December 2012 | Yes | 9 December 2012 | 2 | ||
| FCR | Nausea | 2 | 4 | Probably | Almost certainly | Unlikely | 7 January 2013 | Yes | 10 January 2013 | 3 | ||
| FCR | Vomiting | 2 | 4 | Probably | Almost certainly | Unlikely | 7 January 2013 | Yes | 10 January 2013 | 3 | ||
| FCR | Fatigue | 1 | 4 | Probably | Probably | Unrelated | 10 December 2012 | No | ||||
| FCR | Nausea | 2 | 6 | Probably | Almost certainly | Unlikely | 6 February 2013 | Yes | 8 February 2013 | 2 | ||
| FCR | Common cold | 2 | 6 | Unrelated | Unrelated | Unrelated | 18 February 2013 | No | ||||
| FCR | Abdominal pain/bloating | 1 | 6 | Unrelated | Unrelated | Unrelated | 5 February 2013 | Yes | 9 February 2013 | 4 | ||
| FCR | Night sweats | 1 | 6 | Possibly | Possibly | Unrelated | 21 February 2013 | Yes | 24 February 2013 | 3 | ||
| 200 | FCR | Constipation | 2 | 1 | Unrelated | Unrelated | Unrelated | 10 October 2012 | Yes | 12 October 2012 | 2 | |
| FCR | Vomiting | 2 | 1 | Unrelated | Unrelated | Possibly | 8 October 2012 | Yes | 8 October 2012 | 0 | ||
| FCR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | 31 October 2012 | No | ||||
| FCR | Anaemia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | 22 October 2012 | No | ||||
| FCR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | 10 September 2012 | No | ||||
| FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | 24 September 2012 | No | ||||
| FCR | Renal impairment | 1 | 2 | Unrelated | Unrelated | Unrelated | 31 October 2012 | Yes | 5 November 2012 | 5 | ||
| FCR | Dry skin/erythema | 1 | 3 | Possibly | Possibly | Possibly | No | |||||
| FCR | Abdominal pain/bloating | 1 | 3 | Possibly | Possibly | Possibly | Yes | |||||
| FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | 3 October 2012 | Yes | ||||
| FCR | Lymphopenia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | 22 October 2012 | No | ||||
| FCR | Neutropenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | 27 February 2013 | No | ||||
| FCR | Lymphopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 10 April 2013 | No | ||||
| FCR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Unlikely | 10 April 2013 | No |
| Patient number | Treatment received | AE description | Other AE description | Maximum CTCAE grade | Treatment cycle AE started | Related to fludarabine? | Related to cyclophosphamide? | Related to mitoxantrone? | Related to low-dose rituximab? | Date of onset | Recovered? | Date of recovery | Duration of AE (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | FCM-miniR | Infusional reaction | 1 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 18 January 2010 | Yes | 18 January 2010 | 0 | |
| FCM-miniR | Arrhythmias/palpitation | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 29 January 2010 | Yes | 30 January 2010 | 1 | ||
| FCM-miniR | Anaemia | 4 | 1 | Almost certainly | Possibly | Possibly | Unlikely | 11 February 2010 | Yes | 12 February 2010 | 1 | ||
| FCM-miniR | Abdominal pain/bloating | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 24 February 2010 | Yes | 11 March 2010 | 15 | ||
| FCM-miniR | Anaemia | 2 | 3 | Almost certainly | Possibly | Possibly | Unlikely | 8 April 2010 | No | ||||
| FCM-miniR | Fatigue | 1 | 4 | Almost certainly | Unlikely | Almost certainly | Unlikely | 7 May 2010 | No | ||||
| FCM-miniR | Sore throat | 1 | 4 | Unlikely | Possibly | Almost certainly | Unlikely | Yes | 7 May 2010 | ||||
| FCM-miniR | Anaemia | 2 | 4 | Almost certainly | Possibly | Possibly | Unlikely | 6 May 2010 | No | ||||
| FCM-miniR | Pruritus | 2 | 5 | Possibly | Possibly | Possibly | Possibly | 17 May 2010 | Yes | 3 June 2010 | 17 | ||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Probably | Probably | Probably | Possibly | 1 July 2010 | Yes | 13 January 2011 | 196 | ||
| 4 | FCM-miniR | Hypotension | 2 | 1 | Unrelated | Unrelated | Unrelated | Probably | 24 February 2010 | Yes | 24 February 2010 | 0 | |
| FCM-miniR | Allergic reaction | 2 | 1 | Unrelated | Unrelated | Possibly | Probably | 24 February 2010 | Yes | 25 February 2010 | 1 | ||
| FCM-miniR | Dizziness | 2 | 1 | Unrelated | Unrelated | Probably | Probably | 24 February 2010 | Yes | 24 February 2010 | 0 | ||
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Possibly | 24 February 2010 | Yes | 3 March 2010 | 7 | ||
| FCM-miniR | Hypotension | 2 | 2 | Unlikely | Unlikely | Unlikely | Possibly | 24 March 2010 | Yes | 24 March 2010 | 0 | ||
| FCM-miniR | Hypotension | 1 | 3 | Unlikely | Unlikely | Unlikely | Probably | 21 April 2010 | Yes | 21 April 2010 | 0 | ||
| FCM-miniR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 19 May 2010 | Yes | 5 June 2010 | 17 | ||
| FCM-miniR | Nausea | 1 | 6 | Probably | Probably | Probably | Possibly | 14 July 2010 | Yes | 19 July 2010 | 5 | ||
| FCM-miniR | Fatigue | 1 | 6 | Probably | Probably | Probably | Possibly | 14 July 2010 | Yes | 4 August 2010 | 21 | ||
| 5 | FCM-miniR | Neutropenia | 1 | 1 | Probably | Probably | Probably | Unlikely | 5 March 2010 | Yes | 7 March 2010 | 2 | |
| FCM-miniR | Anaemia | 1 | 1 | Probably | Probably | Probably | Unlikely | 5 March 2010 | No | ||||
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unrelated | 18 February 2010 | Yes | 26 February 2010 | 8 | ||
| FCM-miniR | Abdominal pain/bloating | 2 | 2 | Possibly | Possibly | Possibly | Missing | No | |||||
| FCM-miniR | Diarrhoea | 1 | 2 | Possibly | Possibly | Possibly | Missing | No | |||||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Missing | 18 March 2010 | Yes | 23 March 2010 | 5 | ||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Probably | Missing | 16 April 2010 | Yes | 20 April 2010 | 4 | ||
| FCM-miniR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Probably | Missing | 17 April 2010 | Yes | 17 April 2010 | 0 | ||
| FCM-miniR | Anaemia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Missing | 22 April 2010 | Yes | 26 April 2010 | 4 | ||
| FCM-miniR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Missing | 15 May 2010 | Yes | 19 May 2010 | 4 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 4 | Possibly | Possibly | Possibly | Missing | 17 May 2010 | Yes | 21 May 2010 | 4 | ||
| FCM-miniR | Fever | 3 | 4 | Possibly | Possibly | Possibly | Missing | 19 May 2010 | Yes | 21 May 2010 | 2 | ||
| FCM-miniR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Missing | 9 June 2010 | Yes | 12 June 2010 | 3 | ||
| FCM-miniR | Fatigue | 2 | 5 | Almost certainly | Possibly | Almost certainly | Missing | 10 June 2010 | Yes | 18 June 2010 | 8 | ||
| FCM-miniR | Vomiting | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Missing | 9 June 2010 | Yes | 10 June 2010 | 1 | ||
| FCM-miniR | Hypotension | 3 | 6 | Unrelated | Unrelated | Unrelated | Missing | 7 July 2010 | Yes | 7 July 2010 | 0 | ||
| FCM-miniR | Fatigue | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Missing | 12 July 2010 | Yes | 18 July 2010 | 6 | ||
| FCM-miniR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Missing | 7 July 2010 | Yes | 11 July 2010 | 4 | ||
| FCM-miniR | Vomiting | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Missing | 8 July 2010 | Yes | 8 July 2010 | 0 | ||
| FCM-miniR | Anaemia | 2 | 6 | Almost certainly | Possibly | Possibly | Missing | 4 August 2010 | No | ||||
| FCM-miniR | Neutropenia | 3 | 6 | Probably | Almost certainly | Probably | Missing | 4 August 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Probably | Probably | Almost certainly | Missing | 4 August 2010 | No | ||||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Missing | 11 August 2010 | No | ||||
| 6 | FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Probably | 16 March 2010 | Yes | 30 March 2010 | 14 | |
| FCM-miniR | Constipation | 4 | 2 | Possibly | Possibly | Possibly | Possibly | 2 March 2010 | Yes | 9 March 2010 | 7 | ||
| FCM-miniR | Vomiting | 1 | 3 | Probably | Probably | Probably | Probably | 21 April 2010 | Yes | 27 April 2010 | 6 | ||
| 10 | FCM-miniR | Anaemia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 22 March 2010 | No | |||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 8 April 2010 | No | ||||
| FCM-miniR | Anaemia | 3 | 2 | Probably | Probably | Probably | Probably | 8 April 2010 | Yes | 7 May 2010 | 29 | ||
| FCM-miniR | Rash/flushing | 1 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 10 May 2010 | Yes | 17 June 2010 | 38 | ||
| FCM-miniR | Abnormal electrolytes | 3 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 1 July 2010 | Yes | 5 July 2010 | 4 | ||
| 12 | FCM-miniR | Rigors | 1 | 1 | Unrelated | Unrelated | Unrelated | Probably | 27 April 2010 | Yes | 27 April 2010 | 0 | |
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unlikely | 28 April 2010 | Yes | 9 May 2010 | 11 | ||
| FCM-miniR | Thrombocytopenia | 2 | 1 | Probably | Probably | Probably | Probably | 10 May 2010 | No | ||||
| FCM-miniR | Neutropenia | 2 | 1 | Probably | Probably | Probably | Probably | 26 April 2010 | No | ||||
| 14 | FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 August 2010 | Yes | 12 September 2010 | 13 | |
| FCM-miniR | Diarrhoea | 1 | 6 | Possibly | Possibly | Possibly | Possibly | Yes | |||||
| FCM-miniR | Thrombocytopenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 October 2010 | No | ||||
| 15 | FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 1 May 2010 | Yes | 7 May 2010 | 6 | |
| FCM-miniR | Vomiting | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 1 May 2010 | Yes | 7 May 2010 | 6 | ||
| FCM-miniR | Diarrhoea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 1 May 2010 | Yes | 7 May 2010 | 6 | ||
| FCM-miniR | Constipation | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCM-miniR | Abdominal pain/bloating | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 April 2010 | Yes | 7 May 2010 | 7 | ||
| FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 April 2010 | No | ||||
| FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 May 2010 | Yes | 21 June 2010 | 42 | ||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 April 2010 | Yes | 19 July 2010 | 81 | ||
| FCM-miniR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 May 2010 | Yes | 5 June 2010 | 7 | ||
| FCM-miniR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 May 2010 | Yes | 31 May 2010 | 2 | ||
| FCM-miniR | Fatigue | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 27 May 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 21 June 2010 | Yes | 19 July 2010 | 28 | ||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 26 June 2010 | Yes | 30 June 2010 | 4 | ||
| FCM-miniR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 26 June 2010 | Yes | 30 June 2010 | 4 | ||
| FCM-miniR | Fatigue | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 June 2010 | No | ||||
| FCM-miniR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 22 July 2010 | Yes | 29 July 2010 | 7 | ||
| FCM-miniR | Vomiting | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 July 2010 | Yes | 29 July 2010 | 5 | ||
| FCM-miniR | Fatigue | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 22 July 2010 | No | ||||
| FCM-miniR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 21 August 2010 | Yes | 28 August 2010 | 7 | ||
| FCM-miniR | Fatigue | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 19 August 2010 | No | ||||
| FCM-miniR | Alopecia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 19 August 2010 | No | ||||
| FCM-miniR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 3 October 2010 | Yes | 13 October 2010 | 10 | ||
| FCM-miniR | Fatigue | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 September 2010 | No | ||||
| 16 | FCM-miniR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 5 May 2010 | Yes | 5 May 2010 | 0 | |
| FCM-miniR | Headache | 1 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | Yes | 3 June 2010 | ||||
| FCM-miniR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 3 June 2010 | Yes | 3 June 2010 | 0 | ||
| FCM-miniR | Myalgias | 2 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 17 June 2010 | Yes | 1 July 2010 | 14 | ||
| FCM-miniR | Fever | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 27 August 2010 | Yes | 8 September 2010 | 12 | ||
| FCM-miniR | Anaemia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 September 2010 | Yes | 6 September 2010 | 4 | ||
| FCM-miniR | Neutropenia | 4 | 6 | Unlikely | Almost certainly | Almost certainly | Almost certainly | 31 August 2010 | Yes | 6 September 2010 | 6 | ||
| 20 | FCM-miniR | Nausea | 2 | 1 | Almost certainly | Possibly | Possibly | Unrelated | 1 July 2010 | Yes | 5 July 2010 | 4 | |
| FCM-miniR | Vomiting | 2 | 1 | Almost certainly | Possibly | Possibly | Unrelated | 1 July 2010 | Yes | 5 July 2010 | 4 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 25 September 2010 | Yes | 4 October 2010 | 9 | ||
| FCM-miniR | Rash/flushing | 1 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 11 October 2010 | Yes | 8 November 2010 | 28 | ||
| FCM-miniR | Nausea | 1 | 6 | Almost certainly | Possibly | Possibly | Almost certainly | 11 December 2010 | Yes | 13 December 2010 | 2 | ||
| FCM-miniR | Cough | 1 | 6 | Almost certainly | Unlikely | Unlikely | Unlikely | 30 December 2010 | Yes | 4 January 2011 | 5 | ||
| FCM-miniR | Fatigue | 1 | 6 | Almost certainly | Unlikely | Unlikely | Unlikely | 11 December 2010 | No | ||||
| FCM-miniR | Rigors | 1 | 6 | Almost certainly | Unlikely | Unlikely | Almost certainly | 8 December 2010 | Yes | 4 January 2011 | 27 | ||
| 21 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 June 2010 | Yes | 4 July 2010 | 4 | |
| FCM-miniR | Vomiting | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 June 2010 | Yes | 4 July 2010 | 4 | |||
| FCM-miniR | Renal impairment | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 July 2010 | Yes | 20 July 2010 | 18 | ||
| FCM-miniR | Other AE description | Acidosis | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 July 2010 | Yes | 10 July 2010 | 8 | |
| FCM-miniR | Abnormal electrolytes | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 July 2010 | Yes | 5 July 2010 | 3 | ||
| FCM-miniR | Abnormal electrolytes | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 3 July 2010 | Yes | 30 July 2010 | 27 | |||
| FCM-miniR | Diarrhoea | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 June 2010 | Yes | 5 July 2010 | 5 | |||
| 22 | FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 20 July 2010 | Yes | 3 August 2010 | 14 | |
| FCM-miniR | Fatigue | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 July 2010 | Yes | 21 July 2010 | 4 | ||
| FCM-miniR | Fatigue | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 3 August 2010 | Yes | 7 August 2010 | 4 | ||
| FCM-miniR | Neutropenia | 1 | 3 | Probably | Probably | Probably | Probably | 17 August 2010 | Yes | 31 August 2010 | 14 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Unrelated | Unrelated | Unrelated | Unrelated | 31 August 2010 | No | ||||
| FCM-miniR | Fatigue | 1 | 3 | Probably | Probably | Probably | Probably | 31 August 2010 | Yes | 4 September 2010 | 4 | ||
| FCM-miniR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 12 October 2010 | Yes | 25 October 2010 | 13 | ||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 25 October 2010 | No | ||||
| FCM-miniR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 9 November 2010 | Yes | 22 November 2010 | 13 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 22 November 2010 | No | ||||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 7 December 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 7 December 2010 | No | ||||
| 23 | FCM-miniR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 28 July 2010 | Yes | 9 August 2010 | 12 | |
| FCM-miniR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 28 July 2010 | Yes | 18 August 2010 | 21 | ||
| FCM-miniR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 August 2010 | Yes | 6 September 2010 | 20 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 7 September 2010 | No | ||||
| FCM-miniR | Anaemia | 1 | 4 | Probably | Probably | Probably | Probably | 20 September 2010 | Yes | 28 September 2010 | 8 | ||
| FCM-miniR | Neutropenia | 1 | 4 | Probably | Probably | Probably | Probably | 20 September 2010 | Yes | 28 September 2010 | 8 | ||
| FCM-miniR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 16 November 2010 | Yes | 29 November 2010 | 13 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 16 November 2010 | No | ||||
| FCM-miniR | Leucocytosis/lymphocytosis | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 16 November 2010 | Yes | 29 November 2010 | 13 | ||
| FCM-miniR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 October 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 December 2010 | No | ||||
| FCM-miniR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 December 2010 | No | ||||
| 27 | FCM-miniR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 12 August 2010 | No | |||
| FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 27 July 2010 | Yes | 30 July 2010 | 3 | ||
| FCM-miniR | Vomiting | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 28 July 2010 | Yes | 29 July 2010 | 1 | ||
| FCM-miniR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | Yes | 16 September 2010 | ||||
| FCM-miniR | Abdominal pain/bloating | 1 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | 16 September 2010 | ||||
| FCM-miniR | Fatigue | 1 | 2 | Probably | Probably | Probably | Probably | Yes | 16 September 2010 | ||||
| FCM-miniR | Neuropathy (sensory) | 1 | 2 | Possibly | Possibly | Probably | Possibly | Yes | 16 September 2010 | ||||
| FCM-miniR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | 14 September 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Unlikely | Probably | 14 September 2010 | No | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 1 October 2010 | Yes | 18 October 2010 | 17 | ||
| FCM-miniR | Diarrhoea | 2 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 29 September 2010 | Yes | 17 October 2010 | 18 | ||
| FCM-miniR | Neuropathy (sensory) | 1 | 3 | Possibly | Possibly | Probably | Possibly | 3 October 2010 | Yes | 3 October 2010 | 0 | ||
| FCM-miniR | Abdominal pain/bloating | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 4 October 2010 | Yes | 4 October 2010 | 0 | ||
| FCM-miniR | Anaemia | 2 | 3 | Probably | Probably | Probably | Probably | 19 October 2010 | No | ||||
| FCM-miniR | Neutropenia | 4 | 3 | Almost certainly | Probably | Probably | Probably | 29 September 2010 | Yes | 19 October 2010 | 20 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Almost certainly | Probably | Probably | Probably | 19 October 2010 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | No | |||||
| FCM-miniR | Dry skin/erythema | 1 | 4 | Possibly | Possibly | Possibly | Possibly | No | |||||
| FCM-miniR | Neutropenia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 October 2010 | Yes | 25 November 2010 | 28 | ||
| FCM-miniR | Mucositis/thrush | 2 | 4 | Probably | Possibly | Probably | Possibly | 1 October 2010 | Yes | 1 November 2010 | 31 | ||
| FCM-miniR | Anaemia | 1 | 4 | Probably | Probably | Probably | Probably | 25 October 2010 | Yes | 25 November 2010 | 31 | ||
| FCM-miniR | Cystitis | 1 | 5 | Possibly | Possibly | Possibly | Possibly | Yes | 29 November 2010 | ||||
| FCM-miniR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 29 December 2010 | Yes | 4 January 2011 | 6 | ||
| FCM-miniR | Neutropenia | 2 | 5 | Almost certainly | Probably | Probably | Probably | 25 October 2010 | Yes | 4 January 2011 | 71 | ||
| FCM-miniR | Common cold | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 23 December 2010 | Yes | 5 January 2011 | 13 | ||
| FCM-miniR | Neutropenic sepsis | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 21 February 2011 | Yes | 25 February 2011 | 4 | ||
| FCM-miniR | Diarrhoea | 1 | 6 | Possibly | Possibly | Possibly | Possibly | 21 February 2011 | Yes | 24 February 2011 | 3 | ||
| FCM-miniR | Neutropenia | 4 | 6 | Almost certainly | Probably | Probably | Probably | 22 February 2011 | Yes | 24 February 2011 | 2 | ||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Possibly | Possibly | Possibly | Possibly | 22 February 2011 | No | ||||
| 30 | FCM-miniR | Anaemia | 4 | 1 | Almost certainly | Possibly | Possibly | Possibly | 22 August 2010 | Yes | 31 August 2010 | 9 | |
| 31 | FCM-miniR | Rigors | 1 | 1 | Unlikely | Unlikely | Unlikely | Probably | 18 August 2010 | Yes | 18 August 2010 | 0 | |
| FCM-miniR | Other AE description | Hypertension | 2 | 1 | Unlikely | Unlikely | Unlikely | Probably | 18 August 2010 | Yes | 18 August 2010 | 0 | |
| FCM-miniR | Fever | 1 | 1 | Unlikely | Unlikely | Unlikely | Probably | 18 August 2010 | Yes | 18 August 2010 | 0 | ||
| FCM-miniR | Back pain | 3 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 8 October 2010 | No | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Probably | Probably | Probably | Probably | 1 September 2010 | Yes | 11 October 2010 | 40 | ||
| FCM-miniR | Diarrhoea | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 13 October 2010 | Yes | 15 October 2010 | 2 | ||
| FCM-miniR | Thrombocytopenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 4 January 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 4 January 2011 | No | ||||
| FCM-miniR | Anaemia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 4 January 2011 | No | ||||
| 34 | FCM-miniR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 17 September 2010 | Yes | 17 September 2010 | 0 | |
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 13 November 2010 | Yes | 17 November 2010 | 4 | ||
| 36 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 27 September 2010 | No | |||
| FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Probably | 25 October 2010 | No | ||||
| FCM-miniR | Neutropenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 21 October 2010 | No | ||||
| FCM-miniR | Cystitis | 1 | 2 | Possibly | Possibly | Possibly | Missing | 30 October 2010 | Yes | 18 November 2010 | 19 | ||
| FCM-miniR | Neutropenia | 3 | 2 | Almost certainly | Probably | Probably | Missing | 3 November 2010 | Yes | 18 November 2010 | 15 | ||
| FCM-miniR | Lymphopenia | 3 | 2 | Possibly | Possibly | Possibly | Missing | 3 November 2010 | Yes | 25 November 2010 | 22 | ||
| FCM-miniR | Anaemia | 1 | 2 | Probably | Probably | Probably | Missing | 3 November 2010 | No | ||||
| FCM-miniR | Constipation | 2 | 3 | Possibly | Possibly | Possibly | Missing | 10 November 2010 | Yes | 23 December 2010 | 43 | ||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Missing | 29 November 2010 | Yes | 23 December 2010 | 24 | ||
| FCM-miniR | Cough | 1 | 3 | Possibly | Possibly | Possibly | Missing | 27 October 2010 | Yes | 23 December 2010 | 57 | ||
| FCM-miniR | Anorexia/cachexia | 1 | 4 | Probably | Probably | Probably | Missing | Yes | 10 February 2011 | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 4 | Probably | Probably | Probably | Missing | No | |||||
| FCM-miniR | Anorexia/cachexia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Missing | 5 January 2011 | Yes | 10 February 2011 | 36 | ||
| FCM-miniR | Oedema | 1 | 4 | Possibly | Possibly | Possibly | Missing | 27 January 2011 | Yes | 10 February 2011 | 14 | ||
| FCM-miniR | Neutropenia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Missing | 23 December 2010 | Yes | 10 February 2011 | 49 | ||
| FCM-miniR | Lymphopenia | 3 | 4 | Possibly | Possibly | Possibly | Missing | 23 December 2010 | Yes | 10 February 2011 | 49 | ||
| 37 | FCM-miniR | Mucositis/thrush | 1 | 5 | Probably | Probably | Probably | Unlikely | Yes | ||||
| FCM-miniR | Anorexia/cachexia | 1 | 5 | Probably | Probably | Probably | Unlikely | Yes | |||||
| FCM-miniR | Fatigue | 1 | 5 | Probably | Probably | Probably | Unlikely | Yes | |||||
| FCM-miniR | Nausea | 1 | 5 | Probably | Probably | Probably | Unlikely | 2 February 2011 | Yes | 4 February 2011 | 2 | ||
| FCM-miniR | Nausea | 1 | 6 | Probably | Probably | Probably | Unlikely | 2 March 2011 | Yes | 4 March 2011 | 2 | ||
| 39 | FCM-miniR | Vomiting | 1 | 1 | Probably | Probably | Probably | Unlikely | 8 November 2010 | Yes | 9 November 2010 | 1 | |
| FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Unrelated | Unrelated | Unrelated | 6 November 2010 | Yes | 12 November 2010 | 6 | ||
| FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Possibly | 15 November 2010 | Yes | 30 December 2010 | 45 | ||
| FCM-miniR | Thrombocytopenia | 2 | 1 | Possibly | Possibly | Possibly | Possibly | 4 November 2010 | Yes | 16 December 2010 | 42 | ||
| FCM-miniR | Anaemia | 1 | 1 | Probably | Probably | Probably | Possibly | 15 November 2010 | Yes | 30 December 2010 | 45 | ||
| FCM-miniR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 3 December 2010 | Yes | 7 December 2010 | 4 | ||
| FCM-miniR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 3 December 2010 | Yes | 4 December 2010 | 1 | ||
| FCM-miniR | Neuropathy (sensory) | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 3 December 2010 | No | ||||
| FCM-miniR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 4 January 2011 | Yes | 6 January 2011 | 2 | ||
| FCM-miniR | Fatigue | 2 | 3 | Almost certainly | Unrelated | Unrelated | Unrelated | 31 December 2010 | No | ||||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 4 January 2011 | Yes | 6 January 2011 | 2 | ||
| FCM-miniR | Neutropenia | 1 | 4 | Missing | Missing | Missing | Missing | 11 February 2011 | Yes | 15 February 2011 | 4 | ||
| FCM-miniR | Vomiting | 2 | 4 | Missing | Missing | Missing | Missing | 11 February 2011 | Yes | 13 February 2011 | 2 | ||
| FCM-miniR | Diarrhoea | 1 | 4 | Missing | Missing | Missing | Missing | 11 February 2011 | Yes | 12 February 2011 | 1 | ||
| FCM-miniR | Neutropenia | 1 | 5 | Missing | Missing | Missing | Missing | 9 March 2011 | Yes | 24 March 2011 | 15 | ||
| FCM-miniR | Anaemia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 5 April 2011 | No | ||||
| FCM-miniR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 5 April 2011 | Yes | 19 April 2011 | 14 | ||
| 40 | FCM-miniR | Anaemia | 3 | 1 | Possibly | Possibly | Possibly | Unlikely | 16 November 2010 | No | |||
| FCM-miniR | Infusional reaction | 1 | 2 | Unrelated | Unrelated | Unrelated | Almost certainly | 17 November 2010 | Yes | 17 November 2010 | 0 | ||
| FCM-miniR | Neutropenia | 3 | 2 | Probably | Probably | Probably | Unlikely | No | |||||
| FCM-miniR | Renal impairment | 1 | 2 | Possibly | Possibly | Possibly | Possibly | 16 November 2010 | Yes | 14 December 2010 | 28 | ||
| FCM-miniR | Allergic reaction | 2 | 3 | Unrelated | Unrelated | Unrelated | Almost certainly | 22 December 2010 | Yes | 22 December 2010 | 0 | ||
| FCM-miniR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | 17 January 2011 | Yes | 23 January 2011 | 6 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | 22 November 2010 | Yes | 23 January 2011 | 62 | ||
| FCM-miniR | Anaemia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | 17 January 2011 | Yes | 23 January 2011 | 6 | ||
| FCM-miniR | Bone pain | 4 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 7 March 2011 | No | ||||
| FCM-miniR | Pruritus | 3 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 19 January 2011 | Yes | 26 January 2011 | 7 | ||
| FCM-miniR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 February 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 7 March 2011 | No | ||||
| FCM-miniR | Renal impairment | 1 | 4 | Possibly | Possibly | Possibly | Possibly | 17 January 2011 | Yes | 15 February 2011 | 29 | ||
| FCM-miniR | Anaemia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 15 February 2011 | No | ||||
| 45 | FCM-miniR | Vomiting | 3 | 1 | Probably | Probably | Probably | Possibly | Yes | ||||
| FCM-miniR | Anaemia | 1 | 1 | Possibly | Possibly | Possibly | Possibly | 13 December 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 1 | Possibly | Possibly | Possibly | Possibly | 1 February 2010 | No | ||||
| FCM-miniR | Neutropenia | 1 | 1 | Probably | Probably | Probably | Possibly | 13 December 2010 | No | ||||
| FCM-miniR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 17 November 2010 | Yes | 17 November 2010 | 0 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | 9 January 2011 | ||||
| FCM-miniR | Constipation | 2 | 2 | Possibly | Possibly | Possibly | Possibly | 18 November 2010 | Yes | 12 January 2011 | 55 | ||
| FCM-miniR | Dizziness | 1 | 2 | Possibly | Possibly | Possibly | Possibly | 21 December 2010 | Yes | 21 December 2010 | 0 | ||
| FCM-miniR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 7 February 2011 | Yes | 21 February 2011 | 14 | ||
| FCM-miniR | Thrombocytopenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 7 February 2011 | No | ||||
| FCM-miniR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 15 January 2011 | Yes | 16 January 2011 | 1 | ||
| 46 | FCM-miniR | Rigors | 1 | 1 | Unlikely | Unlikely | Unlikely | Probably | 11 November 2010 | Yes | 11 November 2010 | 0 | |
| FCM-miniR | Hypotension | 2 | 1 | Unlikely | Unlikely | Unlikely | Probably | 11 November 2010 | Yes | 11 November 2010 | 0 | ||
| FCM-miniR | Arrhythmias/palpitation | 2 | 1 | Unlikely | Unlikely | Unlikely | Probably | 11 November 2010 | Yes | 11 November 2010 | 0 | ||
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unlikely | Yes | |||||
| FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Probably | 22 November 2010 | Yes | 6 December 2010 | 14 | ||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Unlikely | 9 December 2010 | Yes | 10 December 2010 | 1 | ||
| FCM-miniR | Sore throat | 2 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 20 December 2010 | Yes | ||||
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Probably | Unlikely | 31 December 2010 | Yes | 5 January 2011 | 5 | ||
| FCM-miniR | Diarrhoea | 2 | 2 | Probably | Probably | Probably | Unlikely | 31 December 2010 | Yes | 5 January 2011 | 5 | ||
| FCM-miniR | Nausea | 1 | 3 | Probably | Probably | Probably | Unlikely | 6 January 2011 | Yes | ||||
| FCM-miniR | Diarrhoea | 3 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 6 January 2011 | Yes | 4 April 2011 | 88 | ||
| FCM-miniR | Abdominal pain/bloating | 2 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Diarrhoea | 3 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | Yes | 28 April 2011 | ||||
| 47 | FCM-miniR | Cough | 2 | 1 | Possibly | Possibly | Possibly | Possibly | 6 December 2010 | Yes | 14 January 2010 | –326 | |
| FCM-miniR | Hypotension | 1 | 1 | Unrelated | Unrelated | Unrelated | Possibly | 26 November 2010 | Yes | 26 November 2010 | 0 | ||
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unrelated | 26 November 2010 | Yes | 26 November 2010 | 0 | ||
| FCM-miniR | Neutropenia | 2 | 2 | Probably | Probably | Probably | Unrelated | 14 January 2011 | Yes | 26 January 2011 | 12 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 2 | Probably | Probably | Probably | Probably | 7 January 2011 | Yes | 2 February 2011 | 26 | ||
| FCM-miniR | Fatigue | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 23 February 2011 | No | ||||
| FCM-miniR | Diarrhoea | 1 | 3 | Probably | Probably | Probably | Unrelated | 26 February 2011 | Yes | 4 March 2011 | 6 | ||
| FCM-miniR | Vomiting | 1 | 3 | Probably | Probably | Probably | Unrelated | 1 March 2011 | Yes | 3 March 2011 | 2 | ||
| FCM-miniR | Neutropenia | 4 | 3 | Probably | Probably | Probably | Unrelated | 7 March 2011 | Yes | 14 March 2011 | 7 | ||
| FCM-miniR | Neutropenia | 4 | 5 | Probably | Probably | Probably | Unrelated | 21 March 2011 | Yes | 13 April 2011 | 23 | ||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Possibly | 18 April 2011 | Yes | 16 May 2011 | 28 | ||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Possibly | 26 May 2011 | No | ||||
| FCM-miniR | Cough | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 6 May 2011 | Yes | 10 June 2011 | 35 | ||
| 49 | FCM-miniR | Gout/hyperuricemia | 2 | 1 | Probably | Probably | Probably | Probably | 13 April 2011 | Yes | 24 April 2011 | 11 | |
| FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 April 2011 | Yes | 21 April 2011 | 7 | ||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 April 2011 | Yes | 27 April 2011 | 13 | ||
| FCM-miniR | Anaemia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 April 2011 | No | ||||
| FCM-miniR | Renal impairment | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | Yes | 11 July 2011 | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 May 2011 | Yes | 17 May 2011 | 7 | ||
| FCM-miniR | Thrombocytopenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 May 2011 | Yes | 13 July 2011 | 64 | ||
| FCM-miniR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 May 2011 | No | ||||
| FCM-miniR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 June 2011 | Yes | 21 June 2011 | 19 | ||
| FCM-miniR | Thrombocytopenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 May 2011 | Yes | 13 July 2011 | 50 | ||
| FCM-miniR | Anaemia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 June 2011 | No | ||||
| 52 | FCM-miniR | Nausea | 1 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 21 December 2010 | Yes | 27 December 2010 | 6 | |
| FCM-miniR | Vomiting | 2 | 1 | Probably | Probably | Probably | Probably | 26 December 2010 | Yes | 27 December 2010 | 1 | ||
| FCM-miniR | Rash/flushing | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 20 December 2010 | Yes | 20 December 2010 | 0 | ||
| FCM-miniR | Rash/flushing | 2 | 2 | Unrelated | Unrelated | Unrelated | Almost certainly | 17 January 2011 | Yes | 17 January 2011 | 0 | ||
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Probably | Probably | 20 January 2011 | Yes | 24 January 2011 | 4 | ||
| FCM-miniR | Allergic reaction | 1 | 2 | Unrelated | Unrelated | Unrelated | Almost certainly | 17 January 2011 | Yes | 17 January 2011 | 0 | ||
| FCM-miniR | Vomiting | 2 | 3 | Probably | Probably | Probably | Probably | 15 February 2011 | Yes | 17 February 2011 | 2 | ||
| FCM-miniR | Nausea | 1 | 4 | Probably | Probably | Probably | Probably | 14 March 2011 | Yes | 20 March 2011 | 6 | ||
| FCM-miniR | Cough | 2 | 5 | Probably | Probably | Probably | Unlikely | 7 May 2011 | No | ||||
| FCM-miniR | Neutropenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 9 May 2011 | No | ||||
| 53 | FCM-miniR | Dyspnoea | 2 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 23 January 2011 | Yes | |||
| FCM-miniR | Vomiting | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Unrelated | Yes | 8 February 2011 | ||||
| FCM-miniR | Urinary symptoms | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | Yes | 22 March 2011 | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | 22 March 2011 | ||||
| FCM-miniR | Abnormal electrolytes | 3 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 11 January 2011 | No | ||||
| FCM-miniR | Rash/flushing | 2 | 3 | Unrelated | Unrelated | Unrelated | Unrelated | 8 March 2011 | Yes | 19 April 2011 | 42 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 7 June 2011 | Yes | 27 July 2011 | 50 | ||
| 54 | FCM-miniR | Vomiting | 2 | 1 | Missing | Missing | Missing | Missing | 23 January 2011 | Yes | 24 January 2011 | 1 | |
| FCM-miniR | Nausea | 2 | 1 | Missing | Missing | Missing | Missing | 22 January 2011 | Yes | 28 January 2011 | 6 | ||
| FCM-miniR | Neutropenic sepsis | 4 | 1 | Missing | Missing | Missing | Missing | 16 February 2011 | Yes | ||||
| FCM-miniR | Vomiting | 2 | 2 | Unrelated | Probably | Probably | Unrelated | 18 February 2011 | Yes | 23 February 2011 | 5 | ||
| FCM-miniR | Vomiting | 2 | 3 | Probably | Probably | Probably | Unlikely | 17 March 2011 | Yes | 27 March 2011 | 10 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Probably | Probably | Probably | Possibly | 13 April 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Probably | Probably | Probably | Unlikely | 27 April 2011 | Yes | 7 May 2011 | 10 | ||
| FCM-miniR | Fatigue | 1 | 5 | Probably | Probably | Probably | Unrelated | 25 May 2011 | No | ||||
| FCM-miniR | Vomiting | 1 | 5 | Probably | Probably | Probably | Unrelated | 26 May 2011 | Yes | 4 June 2011 | 9 | ||
| FCM-miniR | Thrombocytopenia | 2 | 5 | Probably | Probably | Probably | Unrelated | 22 June 2011 | No | ||||
| FCM-miniR | Anaemia | 1 | 5 | Probably | Probably | Probably | Unrelated | 22 June 2011 | Yes | 6 July 2011 | 14 | ||
| 57 | FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unlikely | 13 January 2011 | Yes | 25 January 2011 | 12 | |
| FCM-miniR | Insomnia | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 13 January 2011 | No | ||||
| FCM-miniR | Neutropenia | 3 | 1 | Probably | Probably | Probably | Unlikely | 25 January 2011 | Yes | 8 February 2011 | 14 | ||
| FCM-miniR | Mucositis/thrush | 1 | 1 | Probably | Probably | Probably | Unlikely | 25 January 2011 | No | ||||
| FCM-miniR | Headache | 1 | 1 | Possibly | Possibly | Possibly | Missing | 5 February 2011 | Yes | 8 February 2011 | 3 | ||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Unlikely | 8 February 2011 | Yes | 12 February 2011 | 4 | ||
| FCM-miniR | Insomnia | 1 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 8 February 2011 | No | ||||
| FCM-miniR | Neutropenia | 3 | 2 | Probably | Probably | Probably | Unlikely | 22 February 2011 | Yes | 8 March 2011 | 14 | ||
| FCM-miniR | Fatigue | 1 | 2 | Probably | Probably | Probably | Unlikely | 22 February 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 3 | Probably | Probably | Probably | Unlikely | 8 March 2011 | Yes | 14 March 2011 | 6 | ||
| FCM-miniR | Insomnia | 1 | 3 | Unrelated | Unrelated | Unrelated | Unrelated | 8 March 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 3 | Probably | Probably | Probably | Probably | 14 March 2011 | No | ||||
| FCM-miniR | Fatigue | 1 | 3 | Probably | Probably | Probably | Unlikely | 8 March 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Probably | Probably | Probably | Unlikely | 5 April 2011 | Yes | 11 April 2011 | 6 | ||
| FCM-miniR | Insomnia | 1 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 5 April 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 4 | Probably | Probably | Probably | Probably | 18 April 2011 | No | ||||
| FCM-miniR | Fatigue | 1 | 4 | Probably | Probably | Probably | Unlikely | 5 April 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 5 | Probably | Probably | Probably | Unlikely | 3 May 2011 | Yes | 9 May 2011 | 6 | ||
| FCM-miniR | Insomnia | 1 | 5 | Unrelated | Unrelated | Unrelated | Unrelated | 3 May 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 5 | Probably | Probably | Probably | Unlikely | 16 May 2011 | No | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 5 | Probably | Probably | Probably | Unlikely | Yes | 31 May 2011 | ||||
| FCM-miniR | Fatigue | 1 | 5 | Probably | Probably | Probably | Unlikely | 3 May 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 6 | Probably | Probably | Probably | Unlikely | 31 May 2011 | Yes | 6 June 2011 | 6 | ||
| FCM-miniR | Insomnia | 1 | 6 | Unrelated | Unrelated | Unrelated | Probably | 31 May 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Unlikely | 31 May 2011 | Yes | 27 June 2011 | 27 | ||
| FCM-miniR | Fatigue | 1 | 6 | Probably | Probably | Probably | Unlikely | 31 May 2011 | No | ||||
| 58 | FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Possibly | 26 January 2011 | Yes | 5 February 2011 | 10 | |
| FCM-miniR | Headache | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 29 January 2011 | Yes | 5 February 2011 | 7 | ||
| FCM-miniR | Vomiting | 1 | 1 | Probably | Probably | Probably | Unrelated | 29 January 2011 | Yes | 30 January 2011 | 1 | ||
| FCM-miniR | Dry skin/erythema | 1 | 1 | Possibly | Possibly | Possibly | Unrelated | 27 January 2011 | No | ||||
| FCM-miniR | Back pain | 2 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 31 January 2011 | Yes | 5 February 2011 | 5 | ||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Possibly | 25 February 2011 | Yes | 3 March 2011 | 6 | ||
| FCM-miniR | Vomiting | 1 | 2 | Probably | Probably | Probably | Unrelated | 28 February 2011 | Yes | 1 March 2011 | 1 | ||
| FCM-miniR | Headache | 1 | 2 | Possibly | Possibly | Possibly | Unlikely | 25 February 2011 | Yes | 28 February 2011 | 3 | ||
| FCM-miniR | Cough | 1 | 2 | Possibly | Possibly | Possibly | Unlikely | 8 March 2011 | Yes | 14 March 2011 | 6 | ||
| FCM-miniR | Fatigue | 2 | 2 | Possibly | Possibly | Possibly | Probably | 27 January 2011 | Yes | 28 February 2011 | 32 | ||
| FCM-miniR | Fatigue | 1 | 2 | Possibly | Possibly | Possibly | Probably | 1 March 2011 | Yes | 26 March 2011 | 25 | ||
| FCM-miniR | Nausea | 1 | 3 | Probably | Probably | Probably | Possibly | 25 March 2011 | Yes | 29 March 2011 | 4 | ||
| FCM-miniR | Fatigue | 2 | 3 | Probably | Probably | Probably | Possibly | 27 March 2011 | No | ||||
| FCM-miniR | Headache | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 2 April 2011 | No | ||||
| FCM-miniR | Cough | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 5 April 2011 | No | ||||
| FCM-miniR | Neutropenia | 2 | 3 | Possibly | Possibly | Possibly | Probably | 15 April 2011 | No | ||||
| FCM-miniR | Neuropathy (sensory) | 1 | 3 | Possibly | Unlikely | Probably | Unrelated | 24 March 2011 | Yes | 27 March 2011 | 3 | ||
| FCM-miniR | Nausea | 1 | 4 | Probably | Probably | Probably | Possibly | 22 April 2011 | Yes | 24 May 2011 | 32 | ||
| FCM-miniR | Cough | 1 | 4 | Possibly | Possibly | Possibly | Possibly | 21 April 2011 | Yes | 13 May 2011 | 22 | ||
| FCM-miniR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 27 April 2011 | Yes | 16 May 2011 | 19 | ||
| FCM-miniR | Headache | 2 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 13 May 2011 | Yes | 27 May 2011 | 14 | ||
| FCM-miniR | Constipation | 2 | 4 | Possibly | Possibly | Possibly | Unlikely | 27 January 2011 | Yes | 20 April 2011 | 83 | ||
| FCM-miniR | Vomiting | 1 | 4 | Probably | Probably | Probably | Unlikely | 22 May 2011 | Yes | 24 May 2011 | 2 | ||
| FCM-miniR | Nausea | 1 | 5 | Probably | Probably | Probably | Possibly | 3 June 2011 | Yes | 25 June 2011 | 22 | ||
| FCM-miniR | Headache | 2 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 11 June 2011 | Yes | 25 June 2011 | 14 | ||
| FCM-miniR | Vomiting | 1 | 5 | Probably | Probably | Probably | Unlikely | 7 June 2011 | Yes | 21 June 2011 | 14 | ||
| FCM-miniR | Neutropenia | 3 | 5 | Probably | Probably | Probably | Possibly | 8 June 2011 | Yes | 28 June 2011 | 20 | ||
| FCM-miniR | Nausea | 1 | 6 | Probably | Probably | Probably | Possibly | 29 June 2011 | Yes | 10 July 2011 | 11 | ||
| FCM-miniR | Headache | 1 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | 30 June 2011 | Yes | 14 July 2011 | 14 | ||
| FCM-miniR | Vomiting | 1 | 6 | Probably | Probably | Probably | Unlikely | 1 July 2011 | Yes | 1 July 2011 | 0 | ||
| FCM-miniR | Dizziness | 2 | 6 | Possibly | Possibly | Probably | Unlikely | 2 July 2011 | Yes | 19 July 2011 | 17 | ||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Possibly | 6 July 2011 | Yes | 13 July 2011 | 7 | ||
| FCM-miniR | Thrombocytopenia | 4 | 6 | Probably | Probably | Probably | Possibly | 6 July 2011 | No | ||||
| FCM-miniR | Mucositis/thrush | 2 | 6 | Probably | Probably | Probably | Possibly | 9 July 2011 | No | ||||
| FCM-miniR | Other AE description | Loss of sense of smell | 1 | 6 | Possibly | Possibly | Possibly | Possibly | 7 July 2011 | No | |||
| FCM-miniR | Dry skin/erythema | 1 | 6 | Possibly | Possibly | Possibly | Probably | 17 July 2011 | No | ||||
| 63 | FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 September 2011 | No | |||
| FCM-miniR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 September 2011 | No | ||||
| FCM-miniR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 September 2011 | Yes | 7 October 2011 | 7 | ||
| FCM-miniR | Anaemia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 9 January 2012 | No | ||||
| FCM-miniR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 11 January 2012 | No | ||||
| FCM-miniR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 9 December 2011 | Yes | 12 March 2012 | 94 | ||
| 64 | FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 12 February 2011 | Yes | 18 February 2011 | 6 | |
| FCM-miniR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 9 March 2011 | Yes | 15 March 2011 | 6 | ||
| FCM-miniR | Other AE description | Restlessness | 2 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 9 March 2011 | Yes | 15 March 2011 | 6 | |
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 8 April 2011 | Yes | ||||
| FCM-miniR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 1 June 2011 | Yes | 28 July 2011 | 57 | ||
| 65 | FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 23 February 2011 | Yes | 3 March 2011 | 8 | |
| FCM-miniR | Anaemia | 3 | 1 | Possibly | Possibly | Possibly | Unlikely | 28 February 2011 | Yes | 18 March 2011 | 18 | ||
| FCM-miniR | Dry skin/erythema | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 22 April 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Possibly | Possibly | Possibly | Unlikely | 15 April 2011 | Yes | 17 May 2011 | 32 | ||
| FCM-miniR | Neutropenia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 12 May 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 5 | Possibly | Possibly | Possibly | Unlikely | 10 June 2011 | Yes | 15 June 2011 | 5 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 10 June 2011 | Yes | 11 July 2011 | 31 | ||
| FCM-miniR | Anaemia | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 8 July 2011 | Yes | 11 July 2011 | 3 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 20 June 2011 | Yes | 15 July 2011 | 25 | ||
| FCM-miniR | Anaemia | 4 | 6 | Probably | Probably | Probably | Unlikely | 22 August 2011 | No | ||||
| 69 | FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 May 2011 | Yes | 16 May 2011 | 6 | |
| FCM-miniR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 11 May 2011 | Yes | 16 May 2011 | 5 | ||
| FCM-miniR | Diarrhoea | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 7 May 2011 | Yes | 16 May 2011 | 9 | ||
| FCM-miniR | Diarrhoea | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 2 July 2011 | Yes | 7 July 2011 | 5 | ||
| FCM-miniR | Neutropenia | 2 | 4 | Probably | Probably | Probably | Probably | 19 July 2011 | Yes | 12 September 2011 | 55 | ||
| FCM-miniR | Fatigue | 1 | 4 | Probably | Probably | Probably | Probably | 7 June 2011 | Yes | 20 July 2011 | 43 | ||
| FCM-miniR | Neutropenia | 3 | 5 | Probably | Probably | Probably | Probably | 16 August 2011 | Yes | 9 September 2011 | 24 | ||
| 71 | FCM-miniR | Fatigue | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | No | ||||
| FCM-miniR | Cough | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Anaemia | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 11 August 2010 | No | ||||
| FCM-miniR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | Yes | 5 May 2011 | ||||
| FCM-miniR | Diarrhoea | 2 | 3 | Possibly | Possibly | Possibly | Possibly | 1 May 2011 | Yes | 5 May 2011 | 4 | ||
| FCM-miniR | Nausea | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 22 May 2011 | Yes | 16 June 2011 | 25 | ||
| FCM-miniR | Vomiting | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 22 May 2011 | Yes | 16 June 2011 | 25 | ||
| FCM-miniR | Alopecia | 1 | 5 | Possibly | Possibly | Possibly | Unlikely | No | |||||
| FCM-miniR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | Yes | 11 August 2011 | ||||
| FCM-miniR | Diarrhoea | 1 | 6 | Possibly | Possibly | Possibly | Possibly | Yes | 11 August 2011 | ||||
| 73 | FCM-miniR | Neutropenia | 3 | 1 | Possibly | Possibly | Almost certainly | Possibly | 4 April 2011 | Yes | 13 April 2011 | 9 | |
| FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Almost certainly | Unlikely | 21 March 2011 | Yes | 25 March 2011 | 4 | ||
| FCM-miniR | Vomiting | 1 | 1 | Probably | Probably | Almost certainly | Unlikely | 21 March 2011 | Yes | 22 March 2011 | 1 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Possibly | Almost certainly | Possibly | Possibly | 26 May 2011 | Yes | 3 June 2011 | 8 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Possibly | Possibly | Possibly | 19 May 2011 | Yes | 22 May 2011 | 3 | ||
| FCM-miniR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Probably | Probably | 11 July 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Probably | Probably | 19 July 2011 | No | ||||
| FCM-miniR | Nausea | 2 | 5 | Probably | Probably | Probably | Almost certainly | 19 July 2011 | Yes | 22 July 2011 | 3 | ||
| FCM-miniR | Rigors | 1 | 5 | Probably | Probably | Probably | Probably | 19 July 2011 | Yes | 22 July 2011 | 3 | ||
| FCM-miniR | Anorexia/cachexia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 11 July 2011 | No | ||||
| 75 | FCM-miniR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 8 April 2011 | Yes | 11 April 2011 | 3 | |
| FCM-miniR | Pruritus | 2 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 11 April 2011 | Yes | 12 April 2011 | 1 | ||
| FCM-miniR | Neutropenia | 4 | 2 | Probably | Probably | Probably | Possibly | 26 April 2011 | Yes | 2 May 2011 | 6 | ||
| FCM-miniR | Anaemia | 2 | 2 | Probably | Probably | Probably | Possibly | 20 April 2011 | Yes | 3 May 2011 | 13 | ||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Possibly | Possibly | Possibly | Possibly | 1 May 2011 | Yes | 5 May 2011 | 4 | ||
| FCM-miniR | Anaemia | 3 | 3 | Probably | Missing | Probably | Missing | 4 May 2011 | No | ||||
| FCM-miniR | Mucositis/thrush | 1 | 4 | Probably | Probably | Unlikely | Unlikely | 13 June 2011 | Yes | 28 June 2011 | 15 | ||
| FCM-miniR | Pruritus | 1 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 24 May 2011 | Yes | 17 June 2011 | 24 | ||
| FCM-miniR | Anaemia | 2 | 4 | Probably | Probably | Probably | Possibly | 27 June 2011 | Yes | 11 July 2011 | 14 | ||
| FCM-miniR | Neutropenia | 4 | 4 | Probably | Probably | Probably | Possibly | 20 June 2011 | Yes | 7 July 2011 | 17 | ||
| FCM-miniR | Anaemia | 2 | 5 | Probably | Probably | Probably | Unrelated | 27 July 2011 | Yes | 3 August 2011 | 7 | ||
| FCM-miniR | Fever | 1 | 6 | Unrelated | Unrelated | Unrelated | Unrelated | 16 August 2011 | Yes | 24 August 2011 | 8 | ||
| FCM-miniR | Anaemia | 2 | 6 | Probably | Probably | Probably | Possibly | 17 August 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Possibly | Possibly | Possibly | Probably | 10 August 2011 | No | ||||
| FCM-miniR | Neutropenia | 2 | 6 | Probably | Probably | Probably | Possibly | 31 August 2011 | No | ||||
| 77 | FCM-miniR | Vomiting | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 20 April 2011 | Yes | 23 April 2011 | 3 | |
| FCM-miniR | Abdominal pain/bloating | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 24 April 2011 | Yes | 25 April 2011 | 1 | ||
| FCM-miniR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 21 May 2011 | Yes | 23 May 2011 | 2 | ||
| FCM-miniR | Abdominal pain/bloating | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 22 May 2011 | Yes | 13 June 2011 | 22 | ||
| FCM-miniR | Nausea | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 18 June 2011 | Yes | 21 June 2011 | 3 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 22 June 2011 | No | ||||
| FCM-miniR | Dehydration | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 18 June 2011 | Yes | 22 June 2011 | 4 | ||
| FCM-miniR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 19 June 2011 | Yes | 22 June 2011 | 3 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 13 June 2011 | No | ||||
| FCM-miniR | Neutropenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 1 September 2011 | Yes | 7 September 2011 | 6 | ||
| FCM-miniR | Thrombocytopenia | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 13 July 2011 | No | ||||
| FCM-miniR | Fatigue | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 26 August 2011 | Yes | 7 September 2011 | 12 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 10 August 2011 | No | ||||
| 79 | FCM-miniR | Pruritus | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 2 July 2011 | Yes | 9 July 2011 | 7 | |
| FCM-miniR | Pruritus | 1 | 3 | Unlikely | Unlikely | Unrelated | Unlikely | 13 July 2011 | Yes | 20 July 2011 | 7 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Unlikely | Unlikely | Unrelated | Unlikely | 23 June 2011 | Yes | 6 July 2011 | 13 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Unlikely | Unlikely | Unrelated | Unlikely | 6 July 2011 | Yes | 20 July 2011 | 14 | ||
| FCM-miniR | Neutropenia | 3 | 4 | Probably | Probably | Probably | Probably | 17 August 2011 | Yes | 31 August 2011 | 14 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 4 | Probably | Probably | Probably | Probably | 17 August 2011 | Yes | 31 August 2011 | 14 | ||
| FCM-miniR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 28 September 2011 | Yes | 5 October 2011 | 7 | ||
| 80 | FCM-miniR | Infections (not neutropenic sepsis) | 3 | 1 | Probably | Probably | Probably | Probably | 5 July 2011 | Yes | 18 July 2011 | 13 | |
| FCM-miniR | Fatigue | 1 | 1 | Probably | Probably | Probably | Probably | 17 July 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Probably | Probably | Probably | Probably | 2 August 2011 | Yes | 16 August 2011 | 14 | ||
| FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Probably | Probably | 21 July 2011 | Yes | 24 July 2011 | 3 | ||
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Probably | Probably | 21 July 2011 | Yes | 24 July 2011 | 3 | ||
| FCM-miniR | Alopecia | 1 | 2 | Probably | Probably | Probably | Probably | 24 July 2011 | No | ||||
| FCM-miniR | Anorexia/cachexia | 1 | 2 | Probably | Probably | Probably | Probably | 21 July 2011 | Yes | 24 July 2011 | 3 | ||
| FCM-miniR | Nausea | 2 | 3 | Probably | Probably | Probably | Probably | 21 August 2011 | Yes | 28 August 2011 | 7 | ||
| FCM-miniR | Fatigue | 2 | 3 | Probably | Probably | Probably | Probably | 22 August 2011 | Yes | 2 September 2011 | 11 | ||
| FCM-miniR | Anxiety/depression | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 28 August 2011 | Yes | 2 September 2011 | 5 | ||
| FCM-miniR | Pruritus | 1 | 3 | Possibly | Unrelated | Unrelated | Unrelated | 31 August 2011 | Yes | 14 September 2011 | 14 | ||
| FCM-miniR | Rash/flushing | 2 | 3 | Possibly | Unrelated | Unrelated | Unrelated | 31 August 2011 | Yes | 1 September 2011 | 1 | ||
| FCM-miniR | Fever | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 31 August 2011 | Yes | 1 September 2011 | 1 | ||
| FCM-miniR | Neutropenia | 3 | 4 | Probably | Probably | Probably | Probably | 4 October 2011 | Yes | 5 October 2011 | 1 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 3 | 4 | Probably | Probably | Probably | Probably | 1 October 2011 | Yes | 6 October 2011 | 5 | ||
| FCM-miniR | Nausea | 2 | 4 | Probably | Probably | Unrelated | Unrelated | 20 September 2011 | Yes | 22 September 2011 | 2 | ||
| FCM-miniR | Vomiting | 1 | 4 | Probably | Probably | Unrelated | Unrelated | 20 September 2011 | Yes | 20 September 2011 | 0 | ||
| FCM-miniR | Fatigue | 1 | 4 | Probably | Probably | Probably | Probably | 20 September 2011 | Yes | 6 October 2011 | 16 | ||
| FCM-miniR | Anxiety/depression | 1 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 1 October 2011 | No | ||||
| FCM-miniR | Rash/flushing | 2 | 4 | Probably | Unlikely | Unlikely | Unlikely | 1 October 2011 | Yes | 2 October 2011 | 1 | ||
| FCM-miniR | Neutropenia | 1 | 6 | Probably | Probably | Probably | Probably | 7 December 2011 | No | ||||
| FCM-miniR | Anxiety/depression | 2 | 6 | Unrelated | Unrelated | Unrelated | Unrelated | 7 December 2011 | No | ||||
| 81 | FCM-miniR | Neutropenia | 3 | 1 | Possibly | Probably | Possibly | Possibly | 24 May 2011 | Yes | 6 June 2011 | 13 | |
| FCM-miniR | Anaemia | 3 | 1 | Possibly | Probably | Possibly | Possibly | 3 June 2011 | Yes | 7 June 2011 | 4 | ||
| FCM-miniR | Vomiting | 2 | 1 | Almost certainly | Possibly | Possibly | Possibly | 13 May 2011 | Yes | 16 May 2011 | 3 | ||
| FCM-miniR | Nausea | 2 | 1 | Almost certainly | Possibly | Possibly | Possibly | 13 May 2011 | Yes | 16 May 2011 | 3 | ||
| FCM-miniR | Thrombocytopenia | 1 | 1 | Almost certainly | Probably | Almost certainly | Unlikely | 24 May 2011 | Yes | 9 June 2011 | 16 | ||
| FCM-miniR | Anaemia | 2 | 1 | Possibly | Probably | Possibly | Possibly | 7 June 2011 | Yes | 9 June 2011 | 2 | ||
| FCM-miniR | Anaemia | 3 | 2 | Possibly | Probably | Possibly | Possibly | 29 June 2011 | Yes | 7 July 2011 | 8 | ||
| FCM-miniR | Neutropenia | 4 | 2 | Possibly | Probably | Possibly | Possibly | 29 June 2011 | Yes | 7 July 2011 | 8 | ||
| FCM-miniR | Neutropenia | 4 | 3 | Probably | Almost certainly | Probably | Probably | 14 July 2011 | Yes | 21 July 2011 | 7 | ||
| FCM-miniR | Nausea | 2 | 3 | Possibly | Possibly | Possibly | Almost certainly | 7 July 2011 | Yes | 21 July 2011 | 14 | ||
| FCM-miniR | Anaemia | 3 | 3 | Possibly | Probably | Possibly | Possibly | 21 July 2011 | Yes | 2 August 2011 | 12 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Almost certainly | Probably | Almost certainly | Unlikely | 29 June 2011 | Yes | 14 July 2011 | 15 | ||
| FCM-miniR | Anaemia | 2 | 3 | Possibly | Probably | Possibly | Possibly | 7 July 2011 | Yes | 21 July 2011 | 14 | ||
| FCM-miniR | Neutropenia | 4 | 4 | Probably | Almost certainly | Probably | Probably | 15 August 2011 | Yes | 24 August 2011 | 9 | ||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Probably | Almost certainly | Unlikely | 15 August 2011 | Yes | 20 August 2011 | 5 | ||
| FCM-miniR | Thrombocytopenia | 2 | 4 | Almost certainly | Probably | Almost certainly | Unlikely | 20 August 2011 | Yes | 21 August 2011 | 1 | ||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Probably | Almost certainly | Unlikely | 21 August 2011 | Yes | 8 September 2011 | 18 | ||
| FCM-miniR | Anaemia | 2 | 4 | Possibly | Probably | Possibly | Possibly | 2 August 2011 | Yes | 18 August 2011 | 16 | ||
| FCM-miniR | Anaemia | 2 | 4 | Possibly | Probably | Possibly | Possibly | 19 August 2011 | Yes | 8 September 2011 | 20 | ||
| 83 | FCM-miniR | Vomiting | 1 | 1 | Probably | Probably | Probably | Unlikely | 15 May 2011 | Yes | 15 May 2011 | 0 | |
| FCM-miniR | Headache | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 15 May 2011 | Yes | 15 May 2011 | 0 | ||
| FCM-miniR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Probably | 20 June 2011 | Yes | 4 July 2011 | 14 | ||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 6 July 2011 | Yes | 9 July 2011 | 3 | ||
| FCM-miniR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 6 July 2011 | Yes | 9 July 2011 | 3 | ||
| FCM-miniR | Infusional reaction | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 1 August 2011 | No | ||||
| FCM-miniR | Vomiting | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 August 2011 | Yes | ||||
| FCM-miniR | Rash/flushing | 2 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 1 August 2011 | No | ||||
| FCM-miniR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 2 August 2011 | Yes | ||||
| FCM-miniR | Vomiting | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 9 September 2011 | Yes | 11 September 2011 | 2 | ||
| FCM-miniR | Fatigue | 2 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Dyspnoea | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Nausea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 9 September 2011 | Yes | ||||
| FCM-miniR | Constipation | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 11 September 2011 | Yes | ||||
| FCM-miniR | Diarrhoea | 1 | 6 | Probably | Probably | Probably | Unlikely | No | |||||
| 85 | FCM-miniR | Thrombocytopenia | 2 | 1 | Possibly | Possibly | Possibly | Unlikely | 23 June 2011 | No | |||
| FCM-miniR | Anaemia | 2 | 2 | Possibly | Possibly | Possibly | Unlikely | 23 June 2011 | Yes | 18 August 2011 | 56 | ||
| FCM-miniR | Neutropenia | 3 | 2 | Possibly | Possibly | Possibly | Unlikely | 23 June 2011 | Yes | 18 July 2011 | 25 | ||
| FCM-miniR | Anaemia | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 18 July 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 18 July 2011 | No | ||||
| FCM-miniR | Neutropenia | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 18 July 2011 | No | ||||
| FCM-miniR | Fatigue | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 18 August 2011 | Yes | 29 September 2011 | 42 | ||
| FCM-miniR | Abnormal electrolytes | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 15 September 2011 | Yes | 13 October 2011 | 28 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 13 October 2011 | Yes | 20 October 2011 | 7 | ||
| FCM-miniR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 20 October 2011 | Yes | 10 November 2011 | 21 | ||
| FCM-miniR | Fatigue | 1 | 6 | Possibly | Possibly | Possibly | Unlikely | 20 October 2011 | Yes | 10 November 2011 | 21 | ||
| 86 | FCM-miniR | Thrombocytopenia | 2 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | No | ||||
| FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 31 May 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 15 June 2011 | No | ||||
| FCM-miniR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 13 July 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 13 July 2011 | Yes | 25 July 2011 | 12 | ||
| FCM-miniR | Vomiting | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 June 2011 | Yes | 30 June 2011 | 0 | ||
| FCM-miniR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 August 2011 | Yes | 19 August 2011 | 2 | ||
| FCM-miniR | Anaemia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 August 2011 | No | ||||
| FCM-miniR | Fatigue | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 19 August 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 4 | Possibly | Possibly | Possibly | Possibly | 22 August 2011 | Yes | 23 September 2011 | 32 | ||
| FCM-miniR | Arrhythmias/palpitation | 1 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 21 September 2011 | Yes | 22 September 2011 | 1 | ||
| FCM-miniR | Fatigue | 2 | 5 | Probably | Probably | Probably | Probably | 4 October 2011 | No | ||||
| FCM-miniR | Anaemia | 2 | 5 | Probably | Probably | Probably | Probably | 24 May 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 5 | Possibly | Possibly | Possibly | Possibly | 21 September 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 19 October 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 5 October 2011 | Yes | 19 October 2011 | 14 | ||
| FCM-miniR | Anaemia | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 19 October 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 1 September 2011 | No | ||||
| FCM-miniR | Fatigue | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 1 November 2011 | No | ||||
| 90 | FCM-miniR | Neutropenia | 3 | 5 | Probably | Probably | Probably | Probably | 6 October 2011 | No | |||
| FCM-miniR | Neutropenia | 2 | 6 | Probably | Probably | Probably | Probably | 8 November 2011 | No | ||||
| 94 | FCM-miniR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 22 August 2011 | No | |||
| FCM-miniR | Anaemia | 3 | 1 | Possibly | Possibly | Possibly | Possibly | No | |||||
| FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 13 August 2011 | Yes | 13 August 2011 | 0 | ||
| FCM-miniR | Vomiting | 1 | 1 | Possibly | Possibly | Possibly | Possibly | 5 September 2011 | Yes | 5 September 2011 | 0 | ||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 26 September 2011 | Yes | 10 October 2011 | 14 | ||
| FCM-miniR | Rash/flushing | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 28 September 2011 | Yes | 28 September 2011 | 0 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 12 October 2011 | Yes | 12 October 2011 | 0 | ||
| FCM-miniR | Vomiting | 1 | 3 | Probably | Probably | Probably | Unlikely | 12 October 2011 | Yes | 12 October 2011 | 0 | ||
| FCM-miniR | Anaemia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 28 September 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 October 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 4 | Probably | Probably | Probably | Probably | 31 October 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 12 November 2011 | Yes | 13 November 2011 | 1 | ||
| FCM-miniR | Vomiting | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 12 November 2011 | Yes | 13 November 2011 | 1 | ||
| FCM-miniR | Rash/flushing | 1 | 4 | Possibly | Possibly | Possibly | Possibly | 11 November 2011 | Yes | 20 November 2011 | 9 | ||
| FCM-miniR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Yes | 5 December 2011 | ||||
| 95 | FCM-miniR | Nausea | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 27 July 2011 | Yes | 29 July 2011 | 2 | |
| FCM-miniR | Fatigue | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 27 July 2011 | Yes | 27 July 2011 | 0 | ||
| FCM-miniR | Fatigue | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 24 August 2011 | No | ||||
| FCM-miniR | Other AE description | Weight gain | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | Yes | ||||
| FCM-miniR | Infusional reaction | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 8 August 2011 | No | ||||
| FCM-miniR | Fatigue | 2 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Dry skin/erythema | 1 | 5 | Possibly | Possibly | Possibly | Unlikely | No | |||||
| FCM-miniR | Thrombocytopenia | 3 | 6 | Probably | Probably | Probably | Probably | 16 January 2012 | No | ||||
| FCM-miniR | Lymphopenia | 4 | 6 | Probably | Probably | Probably | Probably | 8 August 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Probably | 1 January 2012 | Yes | 16 January 2012 | 15 | ||
| FCM-miniR | Anaemia | 3 | 6 | Probably | Probably | Probably | Probably | 1 January 2012 | Yes | 4 January 2012 | 3 | ||
| FCM-miniR | Haematuria | 1 | 6 | Probably | Probably | Probably | Probably | Yes | |||||
| FCM-miniR | Fever | 1 | 6 | Probably | Probably | Probably | Probably | 31 December 2011 | Yes | 1 January 2012 | 1 | ||
| 96 | FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Possibly | 3 August 2011 | Yes | 12 August 2011 | 9 | |
| FCM-miniR | Fatigue | 1 | 1 | Probably | Possibly | Possibly | Possibly | 18 August 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Possibly | Almost certainly | Possibly | 25 August 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Almost certainly | Possibly | Possibly | Possibly | 25 August 2011 | No | ||||
| FCM-miniR | Anaemia | 2 | 1 | Almost certainly | Almost certainly | Possibly | Possibly | 25 August 2011 | No | ||||
| FCM-miniR | Diarrhoea | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Probably | 15 October 2011 | No | ||||
| 97 | FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 18 August 2011 | Yes | 26 August 2011 | 8 | |
| FCM-miniR | Vomiting | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 18 August 2011 | Yes | 24 August 2011 | 6 | ||
| FCM-miniR | Diarrhoea | 1 | 1 | Almost certainly | Possibly | Possibly | Unlikely | 18 August 2011 | Yes | 25 August 2011 | 7 | ||
| FCM-miniR | Fatigue | 2 | 1 | Possibly | Possibly | Possibly | Unlikely | 18 August 2011 | Yes | 31 August 2011 | 13 | ||
| FCM-miniR | Cystitis | 2 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 8 October 2011 | Yes | 13 October 2011 | 5 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 8 October 2011 | Yes | 13 October 2011 | 5 | ||
| FCM-miniR | Vomiting | 1 | 2 | Almost certainly | Unlikely | Probably | Unlikely | 22 September 2011 | Yes | 26 September 2011 | 4 | ||
| FCM-miniR | Nausea | 2 | 2 | Almost certainly | Unlikely | Probably | Unlikely | 22 September 2011 | Yes | 26 September 2011 | 4 | ||
| FCM-miniR | Diarrhoea | 1 | 2 | Almost certainly | Unlikely | Unlikely | Unlikely | 25 September 2011 | Yes | 25 September 2011 | 0 | ||
| FCM-miniR | Infusional reaction | 2 | 2 | Unrelated | Unrelated | Unrelated | Almost certainly | 20 September 2011 | Yes | 20 September 2011 | 0 | ||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 3 March 2010 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 3 | Possibly | Possibly | Possibly | Possibly | 17 October 2011 | No | ||||
| FCM-miniR | Vomiting | 1 | 3 | Probably | Probably | Probably | Probably | 28 October 2011 | Yes | 30 October 2011 | 2 | ||
| FCM-miniR | Nausea | 1 | 3 | Probably | Probably | Probably | Probably | 27 October 2011 | Yes | 1 November 2011 | 5 | ||
| FCM-miniR | Constipation | 1 | 3 | Probably | Probably | Probably | Probably | 29 October 2011 | Yes | 31 October 2011 | 2 | ||
| FCM-miniR | Constipation | 1 | 3 | Probably | Probably | Probably | Probably | 15 November 2011 | Yes | 17 November 2011 | 2 | ||
| FCM-miniR | Ophthalmic infections | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 10 November 2011 | Yes | 15 November 2011 | 5 | ||
| FCM-miniR | Fatigue | 2 | 4 | Possibly | Possibly | Possibly | Unlikely | 10 December 2011 | Yes | 12 December 2011 | 2 | ||
| FCM-miniR | Constipation | 1 | 4 | Probably | Probably | Probably | Probably | 1 December 2011 | Yes | 2 December 2011 | 1 | ||
| FCM-miniR | Constipation | 1 | 5 | Probably | Probably | Probably | Probably | 29 January 2012 | Yes | 31 January 2012 | 2 | ||
| FCM-miniR | Vomiting | 1 | 5 | Probably | Probably | Probably | Probably | 26 January 2012 | Yes | 27 January 2012 | 1 | ||
| FCM-miniR | Ophthalmic infections | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 9 February 2012 | Yes | 12 February 2012 | 3 | ||
| FCM-miniR | Constipation | 1 | 6 | Probably | Probably | Probably | Probably | 23 February 2012 | Yes | 24 February 2012 | 1 | ||
| 99 | FCM-miniR | Allergic reaction | 3 | 1 | Unrelated | Unrelated | Possibly | Probably | 10 August 2011 | Yes | 10 August 2011 | 0 | |
| FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 10 June 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 5 September 2011 | No | ||||
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Unlikely | Unlikely | 16 September 2011 | Yes | 19 September 2011 | 3 | ||
| FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Unlikely | Unlikely | 16 September 2011 | Yes | 19 September 2011 | 3 | ||
| FCM-miniR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | 14 September 2011 | Yes | 12 October 2011 | 28 | ||
| FCM-miniR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 5 September 2011 | Yes | 12 October 2011 | 37 | ||
| FCM-miniR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 14 October 2011 | Yes | 17 October 2011 | 3 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 14 October 2011 | Yes | 18 October 2011 | 4 | ||
| FCM-miniR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 7 November 2011 | No | ||||
| FCM-miniR | Nausea | 1 | 4 | Probably | Probably | Probably | Unrelated | 10 November 2011 | Yes | 15 November 2011 | 5 | ||
| FCM-miniR | Fatigue | 1 | 4 | Probably | Probably | Probably | Unrelated | 7 December 2011 | No | ||||
| FCM-miniR | Mucositis/thrush | 1 | 4 | Probably | Probably | Probably | Unrelated | 7 December 2011 | No | ||||
| FCM-miniR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 7 December 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 10 September 2011 | No | ||||
| FCM-miniR | Vomiting | 1 | 4 | Probably | Probably | Probably | Unrelated | 9 November 2011 | Yes | 13 November 2011 | 4 | ||
| FCM-miniR | Nausea | 2 | 5 | Probably | Probably | Probably | Unrelated | 7 December 2011 | Yes | 13 December 2011 | 6 | ||
| FCM-miniR | Vomiting | 2 | 5 | Probably | Probably | Probably | Unrelated | 7 December 2011 | Yes | 13 December 2011 | 6 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | No | |||||
| FCM-miniR | Dyspnoea | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | No | |||||
| FCM-miniR | Thrombocytopenia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 3 January 2012 | No | ||||
| FCM-miniR | Anaemia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 3 January 2012 | No | ||||
| 100 | FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Unrelated | Probably | 15 September 2011 | Yes | 19 September 2011 | 4 | |
| FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Unrelated | Probably | 15 September 2011 | Yes | 19 September 2011 | 4 | ||
| FCM-miniR | Nausea | 2 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 21 October 2011 | Yes | 10 November 2011 | 20 | ||
| FCM-miniR | Back pain | 2 | 3 | Unrelated | Unrelated | Unrelated | Unrelated | 5 November 2011 | Yes | 12 November 2011 | 7 | ||
| FCM-miniR | Fever | 1 | 4 | Possibly | Possibly | Possibly | Possibly | 2 December 2011 | Yes | 18 December 2011 | 16 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 4 | Possibly | Possibly | Possibly | Possibly | 2 December 2011 | Yes | 18 December 2011 | 16 | ||
| 103 | FCM-miniR | Anorexia/cachexia | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 19 September 2011 | No | |||
| FCM-miniR | Fatigue | 1 | 1 | Probably | Probably | Possibly | Unlikely | 26 August 2011 | Yes | 27 August 2011 | 1 | ||
| FCM-miniR | Anorexia/cachexia | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | No | |||||
| FCM-miniR | Anaemia | 1 | 2 | Probably | Probably | Probably | Probably | 19 September 2011 | Yes | 17 October 2011 | 28 | ||
| FCM-miniR | Rash/flushing | 2 | 4 | Unrelated | Unrelated | Possibly | Unrelated | 4 December 2011 | No | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 18 January 2012 | No | ||||
| 106 | FCM-miniR | Neutropenia | 3 | 1 | Possibly | Possibly | Possibly | Possibly | 9 September 2011 | Yes | 27 September 2011 | 18 | |
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 28 January 2012 | Yes | 7 February 2012 | 10 | ||
| FCM-miniR | Sore throat | 1 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | 12 March 2012 | Yes | 19 March 2012 | 7 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Possibly | Possibly | Possibly | Possibly | 5 April 2012 | Yes | 8 May 2012 | 33 | ||
| FCM-miniR | Cough | 1 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | 12 March 2012 | No | ||||
| 108 | FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Probably | Possibly | Unlikely | 24 September 2011 | Yes | 2 October 2011 | 8 | |
| FCM-miniR | Thrombocytopenia | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 15 July 2011 | Yes | 2 October 2011 | 79 | ||
| FCM-miniR | Anaemia | 1 | 1 | Probably | Probably | Possibly | Unrelated | 9 September 2011 | Yes | 16 November 2011 | 68 | ||
| FCM-miniR | Nausea | 1 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | 16 November 2011 | ||||
| FCM-miniR | Vomiting | 1 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | 16 November 2011 | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Possibly | Possibly | Possibly | Possibly | No | |||||
| FCM-miniR | Neutropenia | 2 | 3 | Possibly | Possibly | Possibly | Possibly | 21 December 2011 | No | ||||
| FCM-miniR | Neutropenia | 4 | 4 | Possibly | Possibly | Possibly | Possibly | 8 January 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Possibly | Possibly | Possibly | Possibly | 3 January 2012 | Yes | 12 January 2012 | 9 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Possibly | Possibly | Possibly | Possibly | 15 February 2012 | No | ||||
| FCM-miniR | Mucositis/thrush | 1 | 6 | Probably | Probably | Probably | Probably | 3 April 2012 | Yes | 7 May 2012 | 34 | ||
| FCM-miniR | Dyspnoea | 2 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | 10 April 2012 | Yes | 17 April 2012 | 7 | ||
| 109 | FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Probably | Unlikely | 21 October 2011 | Yes | 1 December 2011 | 41 | |
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Probably | Unlikely | 21 October 2011 | Yes | 1 December 2011 | 41 | ||
| FCM-miniR | Headache | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 21 October 2011 | Yes | 26 November 2011 | 36 | ||
| FCM-miniR | Rash/flushing | 1 | 2 | Possibly | Possibly | Possibly | Possibly | 11 November 2011 | Yes | 12 November 2011 | 1 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | 17 November 2011 | Yes | 21 December 2011 | 34 | ||
| FCM-miniR | Nausea | 2 | 4 | Missing | Probably | Probably | Unlikely | 22 December 2011 | Yes | 31 December 2011 | 9 | ||
| FCM-miniR | Vomiting | 2 | 4 | Missing | Probably | Probably | Unlikely | 22 December 2011 | Yes | 31 December 2011 | 9 | ||
| FCM-miniR | Fatigue | 1 | 5 | Missing | Probably | Probably | Unlikely | Yes | 19 January 2012 | ||||
| FCM-miniR | Nausea | 1 | 6 | Missing | Probably | Probably | Unlikely | 18 February 2012 | Yes | 22 February 2012 | 4 | ||
| FCM-miniR | Fatigue | 1 | 6 | Missing | Probably | Probably | Unlikely | 18 February 2012 | Yes | 23 February 2012 | 5 | ||
| 113 | FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 30 November 2011 | Yes | 5 December 2011 | 5 | |
| FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Possibly | 30 November 2011 | Yes | 5 December 2011 | 5 | ||
| FCM-miniR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 December 2011 | No | ||||
| FCM-miniR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 December 2011 | Yes | 1 February 2012 | 35 | ||
| FCM-miniR | Mucositis/thrush | 2 | 4 | Possibly | Possibly | Possibly | Unlikely | 18 February 2012 | Yes | 22 February 2012 | 4 | ||
| FCM-miniR | Gout/hyperuricemia | 3 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 22 February 2012 | Yes | 27 February 2012 | 5 | ||
| FCM-miniR | Anaemia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 29 February 2012 | No | ||||
| FCM-miniR | Neutropenia | 4 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 20 February 2012 | Yes | 29 February 2012 | 9 | ||
| FCM-miniR | Gout/Hyperuricemia | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 22 March 2012 | Yes | 28 March 2012 | 6 | ||
| FCM-miniR | Gout/Hyperuricemia | 2 | 6 | Unlikely | Unlikely | Unlikely | Unlikely | 21 April 2012 | Yes | 24 April 2012 | 3 | ||
| 114 | FCM-miniR | Anaemia | 3 | 1 | Possibly | Possibly | Possibly | Unrelated | 5 December 2011 | Yes | 4 January 2012 | 30 | |
| FCM-miniR | Thrombocytopenia | 1 | 1 | Possibly | Possibly | Possibly | Unrelated | 5 December 2011 | Yes | 2 April 2012 | 119 | ||
| 115 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 5 November 2011 | Yes | 19 November 2011 | 14 | |
| FCM-miniR | Constipation | 1 | 1 | Probably | Probably | Probably | Unlikely | Yes | |||||
| FCM-miniR | Fatigue | 1 | 1 | Probably | Probably | Probably | Unlikely | Yes | |||||
| FCM-miniR | Abdominal pain/bloating | 2 | 2 | Probably | Probably | Probably | Unlikely | 3 November 2011 | Yes | 11 November 2011 | 8 | ||
| FCM-miniR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 November 2011 | Yes | 25 January 2012 | 58 | ||
| FCM-miniR | Abdominal pain/bloating | 3 | 3 | Probably | Probably | Probably | Unlikely | 30 December 2011 | Yes | 7 January 2012 | 8 | ||
| FCM-miniR | Constipation | 1 | 3 | Probably | Probably | Probably | Unlikely | 7 January 2012 | Yes | 10 January 2012 | 3 | ||
| FCM-miniR | Diarrhoea | 2 | 3 | Possibly | Possibly | Probably | Unlikely | 11 January 2012 | Yes | 12 January 2012 | 1 | ||
| FCM-miniR | Abdominal pain/bloating | 1 | 3 | Probably | Probably | Possibly | Unlikely | 18 January 2012 | No | ||||
| FCM-miniR | Cystitis | 1 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | 18 January 2012 | Yes | 25 January 2012 | 7 | ||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 December 2011 | Yes | 22 February 2012 | 56 | ||
| FCM-miniR | Abdominal pain/bloating | 2 | 4 | Possibly | Possibly | Possibly | Unlikely | 26 January 2012 | Yes | 30 January 2012 | 4 | ||
| FCM-miniR | Abnormal electrolytes | 2 | 5 | Unlikely | Unlikely | Unlikely | Unlikely | 23 February 2012 | Yes | 23 February 2012 | 0 | ||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 28 March 2012 | No | ||||
| FCM-miniR | Abdominal pain/bloating | 2 | 6 | Possibly | Possibly | Possibly | Unlikely | 3 April 2012 | Yes | 8 April 2012 | 5 | ||
| FCM-miniR | Constipation | 1 | 6 | Possibly | Possibly | Possibly | Unlikely | 6 April 2012 | Yes | 10 April 2012 | 4 | ||
| FCM-miniR | Anorexia/cachexia | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 3 April 2012 | Yes | 8 April 2012 | 5 | ||
| FCM-miniR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 27 April 2012 | No | ||||
| 117 | FCM-miniR | Anaemia | 4 | 1 | Unrelated | Unrelated | Probably | Unlikely | 16 November 2011 | No | |||
| FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Unlikely | 1 November 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 2 | Probably | Probably | Probably | Unlikely | 1 November 2011 | Yes | 28 December 2011 | 57 | ||
| FCM-miniR | Nausea | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 8 March 2012 | Yes | 12 March 2012 | 4 | ||
| FCM-miniR | Vomiting | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 8 March 2012 | Yes | 12 March 2012 | 4 | ||
| FCM-miniR | Neutropenia | 4 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 15 March 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 15 March 2012 | Yes | 28 March 2012 | 13 | ||
| FCM-miniR | Anaemia | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Possibly | 15 March 2012 | No | ||||
| FCM-miniR | Nausea | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 8 April 2012 | Yes | 12 April 2012 | 4 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 11 April 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 11 April 2012 | No | ||||
| FCM-miniR | Anaemia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 11 April 2012 | No | ||||
| 118 | FCM-miniR | Fatigue | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 3 August 2011 | No | |||
| FCM-miniR | Non-specific pain | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Yes | 20 November 2011 | ||||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Possibly | 4 December 2011 | Yes | 7 December 2011 | 3 | ||
| FCM-miniR | Diarrhoea | 1 | 4 | Possibly | Possibly | Possibly | Unlikely | 16 January 2012 | Yes | 16 January 2012 | 0 | ||
| FCM-miniR | Nausea | 1 | 4 | Possibly | Possibly | Possibly | Unlikely | 31 December 2011 | Yes | 3 January 2012 | 3 | ||
| FCM-miniR | Fatigue | 1 | 5 | Possibly | Possibly | Possibly | Possibly | 26 January 2012 | Yes | 31 January 2012 | 5 | ||
| FCM-miniR | Nausea | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 29 January 2012 | Yes | 2 February 2012 | 4 | ||
| FCM-miniR | Vomiting | 2 | 5 | Possibly | Possibly | Possibly | Unlikely | 29 January 2012 | Yes | 2 February 2012 | 4 | ||
| FCM-miniR | Nausea | 2 | 6 | Possibly | Possibly | Possibly | Unlikely | 26 February 2012 | Yes | 2 March 2012 | 5 | ||
| FCM-miniR | Fatigue | 1 | 6 | Possibly | Possibly | Possibly | Possibly | 26 February 2012 | Yes | 28 February 2012 | 2 | ||
| 119 | FCM-miniR | Fatigue | 2 | 1 | Unlikely | Almost certainly | Unlikely | Unlikely | 11 November 2011 | No | |||
| FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 2 December 2011 | Yes | 7 December 2011 | 5 | ||
| FCM-miniR | Constipation | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 12 November 2011 | Yes | 20 November 2011 | 8 | ||
| FCM-miniR | Anaemia | 1 | 1 | Unlikely | Almost certainly | Unlikely | Unlikely | 21 November 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 1 | Unlikely | Probably | Unlikely | Unlikely | 21 November 2011 | No | ||||
| FCM-miniR | Anaemia | 1 | 2 | Probably | Almost certainly | Unlikely | Unlikely | 9 December 2011 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 2 | Unlikely | Probably | Unlikely | Unlikely | 9 December 2011 | No | ||||
| FCM-miniR | Non-specific pain | 2 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 1 December 2011 | No | ||||
| 124 | FCM-miniR | Nausea | 2 | 1 | Almost certainly | Almost certainly | Unlikely | Unlikely | 6 December 2011 | Yes | 12 December 2011 | 6 | |
| FCM-miniR | Constipation | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 6 December 2011 | Yes | 15 December 2011 | 9 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Probably | Probably | Probably | Unlikely | 2 May 2012 | Yes | 15 May 2012 | 13 | ||
| 126 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 January 2012 | Yes | 11 January 2012 | 1 | |
| FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 10 January 2012 | Yes | 23 January 2012 | 13 | ||
| FCM-miniR | Thrombocytopenia | 4 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 16 January 2012 | Yes | 6 February 2012 | 21 | ||
| FCM-miniR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 21 February 2012 | Yes | 29 February 2012 | 8 | ||
| FCM-miniR | Neutropenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 February 2012 | No | ||||
| FCM-miniR | Anaemia | 1 | 4 | Possibly | Possibly | Possibly | Possibly | Yes | 8 August 2012 | ||||
| FCM-miniR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 1 May 2012 | No | ||||
| FCM-miniR | Neutropenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 May 2012 | No | ||||
| 127 | FCM-miniR | Infusional reaction | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 20 December 2011 | Yes | 20 December 2011 | 0 | |
| FCM-miniR | Fatigue | 2 | 1 | Possibly | Possibly | Possibly | Unlikely | 22 December 2011 | Yes | 27 December 2011 | 5 | ||
| FCM-miniR | Dyspnoea | 2 | 1 | Possibly | Possibly | Possibly | Unlikely | 26 December 2011 | Yes | 23 February 2012 | 59 | ||
| FCM-miniR | Other AE description | Pulmonary embolism | 3 | 1 | Unlikely | Possibly | Unlikely | Unlikely | 3 January 2012 | Yes | 11 January 2012 | 8 | |
| FCM-miniR | Other AE description | Pleural effusion | 3 | 1 | Unlikely | Possibly | Unlikely | Unlikely | 3 January 2012 | Yes | 23 February 2012 | 51 | |
| FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 31 December 2011 | Yes | 5 January 2012 | 5 | ||
| FCM-miniR | Constipation | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 29 January 2012 | Yes | 31 January 2012 | 2 | ||
| FCM-miniR | Fatigue | 2 | 2 | Possibly | Possibly | Possibly | Unlikely | 21 January 2012 | Yes | 2 February 2012 | 12 | ||
| FCM-miniR | Rash/flushing | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 30 January 2012 | Yes | 9 February 2012 | 10 | ||
| FCM-miniR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 25 January 2012 | Yes | 23 February 2012 | 29 | ||
| FCM-miniR | Lymphopenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 25 January 2012 | Yes | 13 November 2012 | 293 | ||
| FCM-miniR | Nausea | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 24 February 2012 | Yes | 1 March 2012 | 6 | ||
| FCM-miniR | Vomiting | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 24 February 2012 | Yes | 1 March 2012 | 6 | ||
| FCM-miniR | Constipation | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 24 February 2012 | Yes | 8 March 2012 | 13 | ||
| FCM-miniR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 22 March 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 26 April 2012 | Yes | 28 February 2013 | 308 | ||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Possibly | 24 May 2012 | Yes | 23 August 2012 | 91 | ||
| FCM-miniR | Fatigue | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 1 June 2012 | Yes | 21 June 2012 | 20 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 6 | Probably | Probably | Probably | Possibly | 21 June 2012 | Yes | 28 February 2013 | 252 | ||
| 128 | FCM-miniR | Neutropenia | 3 | 1 | Possibly | Possibly | Possibly | Possibly | 20 January 2012 | Yes | 30 January 2012 | 10 | |
| FCM-miniR | Thrombocytopenia | 1 | 1 | Possibly | Possibly | Possibly | Possibly | 11 January 2012 | Yes | 30 January 2012 | 19 | ||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 6 February 2012 | Yes | 10 April 2012 | 64 | ||
| 132 | FCM-miniR | Rigors | 1 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | 1 February 2012 | Yes | 1 February 2012 | 0 | |
| FCM-miniR | Constipation | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 8 February 2012 | Yes | 10 February 2012 | 2 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 3 | Unrelated | Unrelated | Unrelated | Unrelated | 11 April 2012 | Yes | 13 April 2012 | 2 | ||
| FCM-miniR | Neutropenic sepsis | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Unrelated | 7 May 2012 | Yes | 16 May 2012 | 9 | ||
| FCM-miniR | Neutropenia | 4 | 4 | Probably | Probably | Probably | Unlikely | 8 May 2012 | Yes | 12 May 2012 | 4 | ||
| FCM-miniR | Diarrhoea | 2 | 4 | Unrelated | Unrelated | Unrelated | Unrelated | 1 June 2012 | Yes | 7 June 2012 | 6 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 3 | 4 | Probably | Probably | Probably | Unlikely | 8 May 2012 | Yes | 14 May 2012 | 6 | ||
| FCM-miniR | Other AE description | Confusion | 3 | 4 | Unlikely | Unlikely | Unlikely | Unlikely | 8 May 2012 | Yes | 13 May 2012 | 5 | |
| FCM-miniR | Neutropenia | 4 | 4 | Almost certainly | Unlikely | Unlikely | Unlikely | 3 June 2012 | Yes | 20 June 2012 | 17 | ||
| FCM-miniR | Anaemia | 2 | 5 | Probably | Probably | Probably | Unrelated | 7 June 2012 | Yes | 18 July 2012 | 41 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 6 | Possibly | Possibly | Possibly | Possibly | 29 July 2012 | Yes | 5 August 2012 | 7 | ||
| FCM-miniR | Haematuria | 1 | 6 | Possibly | Possibly | Possibly | Possibly | 29 July 2012 | No | ||||
| FCM-miniR | Neutropenia | 4 | 6 | Probably | Probably | Probably | Unlikely | 22 August 2012 | No | ||||
| FCM-miniR | Anaemia | 2 | 6 | Probably | Probably | Probably | Unlikely | 22 August 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 6 | Probably | Probably | Probably | Unlikely | 22 August 2012 | No | ||||
| 133 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | Yes | ||||
| FCM-miniR | Diarrhoea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | Yes | |||||
| FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 27 February 2012 | Yes | 12 March 2012 | 14 | ||
| FCM-miniR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 12 March 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 11 April 2012 | No | ||||
| FCM-miniR | Fatigue | 1 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | |||||
| FCM-miniR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 14 May 2012 | No | ||||
| FCM-miniR | Constipation | 1 | 5 | Unrelated | Unrelated | Unrelated | Unrelated | Yes | |||||
| FCM-miniR | Vomiting | 1 | 5 | Probably | Probably | Probably | Unrelated | 15 June 2012 | Yes | 15 June 2012 | 0 | ||
| FCM-miniR | Neutropenia | 1 | 6 | Probably | Probably | Probably | Unrelated | 6 August 2012 | No | ||||
| FCM-miniR | Anaemia | 1 | 6 | Probably | Probably | Probably | Unrelated | 9 July 2012 | No | ||||
| 135 | FCM-miniR | Nausea | 1 | 1 | Probably | Probably | Probably | Unlikely | 16 March 2012 | Yes | 23 March 2012 | 7 | |
| FCM-miniR | Vomiting | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | 23 March 2012 | Yes | 23 March 2012 | 0 | ||
| FCM-miniR | Rash/flushing | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | 5 April 2012 | No | ||||
| FCM-miniR | Anaemia | 1 | 1 | Possibly | Possibly | Possibly | Unlikely | 14 March 2012 | No | ||||
| FCM-miniR | Neutropenia | 4 | 1 | Possibly | Possibly | Possibly | Unlikely | 30 March 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Possibly | Possibly | Possibly | Unlikely | 23 March 2012 | No | ||||
| FCM-miniR | Rash/flushing | 2 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 13 April 2012 | No | ||||
| FCM-miniR | Nausea | 1 | 2 | Probably | Probably | Probably | Unlikely | 15 April 2012 | Yes | 18 April 2012 | 3 | ||
| FCM-miniR | Fatigue | 1 | 2 | Possibly | Possibly | Possibly | Unlikely | 14 April 2012 | Yes | 26 April 2012 | 12 | ||
| FCM-miniR | Anaemia | 1 | 2 | Possibly | Possibly | Possibly | Unlikely | 13 April 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 2 | Possibly | Possibly | Possibly | Unlikely | 26 April 2012 | No | ||||
| FCM-miniR | Neutropenia | 4 | 2 | Possibly | Possibly | Possibly | Unlikely | 26 April 2012 | No | ||||
| FCM-miniR | Infections (not neutropenic sepsis) | 2 | 3 | Possibly | Possibly | Possibly | Unlikely | 31 May 2012 | Yes | 4 June 2012 | 4 | ||
| FCM-miniR | Anaemia | 1 | 3 | Possibly | Possibly | Possibly | Unlikely | 1 June 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 3 | 3 | Possibly | Possibly | Possibly | Unlikely | 17 May 2012 | No | ||||
| FCM-miniR | Neutropenia | 4 | 3 | Possibly | Possibly | Possibly | Unlikely | 23 May 2012 | No | ||||
| 139 | FCM-miniR | Anaemia | 3 | 1 | Probably | Probably | Probably | Probably | 9 May 2012 | No | |||
| FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Probably | 9 May 2012 | Yes | 11 July 2012 | 63 | ||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Probably | Probably | Probably | Probably | 9 May 2012 | No | ||||
| 142 | FCM-miniR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 30 May 2012 | Yes | 4 June 2012 | 5 | |
| FCM-miniR | Anaemia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 May 2012 | Yes | 30 May 2012 | 6 | ||
| FCM-miniR | Thrombocytopenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 24 May 2012 | Yes | 4 June 2012 | 11 | ||
| 144 | FCM-miniR | Nausea | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 3 May 2012 | Yes | 7 May 2012 | 4 | |
| FCM-miniR | Fatigue | 1 | 1 | Almost certainly | Unlikely | Unlikely | Unlikely | 3 May 2012 | Yes | 10 May 2012 | 7 | ||
| FCM-miniR | Neuropathy (sensory) | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCM-miniR | Back pain | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Yes | |||||
| FCM-miniR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 31 May 2012 | No | ||||
| FCM-miniR | Fatigue | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | No | |||||
| FCM-miniR | Constipation | 1 | 2 | Possibly | Possibly | Possibly | Possibly | Yes | |||||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | Yes | |||||
| FCM-miniR | Neutropenia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 26 June 2012 | Yes | 17 July 2012 | 21 | ||
| FCM-miniR | Nausea | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCM-miniR | Vomiting | 2 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Yes | 26 July 2012 | ||||
| FCM-miniR | Mucositis/thrush | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | No | |||||
| FCM-miniR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 July 2012 | Yes | 21 August 2012 | 35 | ||
| FCM-miniR | Anaemia | 1 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 17 July 2012 | Yes | 24 July 2012 | 7 | ||
| FCM-miniR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 21 August 2012 | Yes | 23 October 2012 | 63 | ||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 29 May 2012 | Yes | 23 October 2012 | 147 | ||
| FCM-miniR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 18 September 2012 | Yes | 23 October 2012 | 35 | ||
| FCM-miniR | Anaemia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 23 October 2012 | No | ||||
| FCM-miniR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 23 October 2012 | No | ||||
| FCM-miniR | Neutropenia | 3 | 6 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | 23 October 2012 | No | ||||
| 149 | FCM-miniR | Nausea | 2 | 1 | Probably | Probably | Probably | Unrelated | 21 May 2012 | Yes | 28 May 2012 | 7 | |
| FCM-miniR | Alopecia | 2 | 1 | Probably | Probably | Unlikely | Unlikely | 11 June 2012 | No | ||||
| FCM-miniR | Diarrhoea | 1 | 1 | Probably | Unlikely | Possibly | Unlikely | 28 May 2012 | Yes | 12 June 2012 | 15 | ||
| FCM-miniR | Mucositis/thrush | 1 | 1 | Possibly | Possibly | Probably | Unrelated | 28 May 2012 | Yes | 19 June 2012 | 22 | ||
| FCM-miniR | Taste alteration | 4 | 1 | Probably | Probably | Probably | Unlikely | 28 May 2012 | Yes | 28 May 2012 | 0 | ||
| FCM-miniR | Fatigue | 2 | 1 | Probably | Probably | Probably | Unlikely | 15 May 2012 | No | ||||
| FCM-miniR | Fatigue | 2 | 2 | Probably | Probably | Probably | Unlikely | 18 June 2012 | No | ||||
| FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Probably | Unrelated | 19 July 2012 | No | ||||
| FCM-miniR | Constipation | 2 | 2 | Probably | Unlikely | Possibly | Unlikely | 19 July 2012 | No | ||||
| FCM-miniR | Diarrhoea | 2 | 2 | Probably | Unlikely | Possibly | Unlikely | 19 July 2012 | Yes | 24 July 2012 | 5 | ||
| FCM-miniR | Neutropenia | 3 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 18 June 2012 | Yes | 16 July 2012 | 28 | ||
| FCM-miniR | Non-specific pain | 1 | 2 | Unlikely | Unlikely | Unlikely | Unlikely | 18 June 2012 | Yes | 16 July 2012 | 28 | ||
| FCM-miniR | Neutropenia | 3 | 3 | Probably | Probably | Probably | Unlikely | 24 August 2012 | Yes | 3 September 2012 | 10 | ||
| FCM-miniR | Nausea | 2 | 3 | Probably | Probably | Probably | Unrelated | 24 August 2012 | Yes | 3 September 2012 | 10 | ||
| 150 | FCM-miniR | Anaemia | 3 | 2 | Possibly | Possibly | Possibly | Possibly | 1 June 2012 | Yes | 3 June 2012 | 2 | |
| FCM-miniR | Neutropenia | 4 | 3 | Probably | Probably | Probably | Probably | 25 June 2012 | Yes | 15 August 2012 | 51 | ||
| FCM-miniR | Anaemia | 2 | 3 | Probably | Probably | Probably | Probably | 3 June 2012 | Yes | 9 July 2012 | 36 | ||
| FCM-miniR | Neutropenia | 1 | 4 | Probably | Probably | Probably | Probably | 10 October 2012 | No | ||||
| FCM-miniR | Neutropenia | 1 | 5 | Probably | Probably | Probably | Probably | 10 September 2012 | No | ||||
| FCM-miniR | Lymphopenia | 2 | 5 | Probably | Probably | Probably | Probably | 10 September 2012 | No | ||||
| FCM-miniR | Neutropenia | 2 | 6 | Probably | Probably | Probably | Probably | 8 October 2012 | Yes | 14 January 2013 | 98 | ||
| FCM-miniR | Lymphopenia | 3 | 6 | Probably | Probably | Probably | Probably | 5 November 2012 | Yes | 3 December 2012 | 28 | ||
| 154 | FCM-miniR | Neutropenia | 4 | 1 | Probably | Probably | Probably | Probably | 11 June 2012 | No | |||
| FCM-miniR | Thrombocytopenia | 3 | 1 | Probably | Probably | Probably | Probably | 11 June 2012 | No | ||||
| FCM-miniR | Anaemia | 2 | 1 | Probably | Probably | Probably | Probably | 11 June 2012 | No | ||||
| 165 | FCM-miniR | Neutropenia | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Unlikely | 13 July 2012 | Yes | 2 August 2012 | 20 | |
| FCM-miniR | Thrombocytopenia | 3 | 1 | Possibly | Possibly | Possibly | Unlikely | 13 July 2012 | Yes | 23 July 2012 | 10 | ||
| FCM-miniR | Anaemia | 2 | 2 | Unlikely | Unlikely | Unlikely | Unrelated | 13 July 2012 | Yes | 31 August 2012 | 49 | ||
| FCM-miniR | Raised GGT/bilirubin | 1 | 3 | Possibly | Possibly | Possibly | Possibly | 30 August 2012 | No | ||||
| 180 | FCM-miniR | Constipation | 2 | 1 | Almost certainly | Almost certainly | Missing | Missing | 2 August 2012 | Yes | 7 August 2012 | 5 | |
| FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Missing | Missing | 14 August 2012 | Yes | 19 October 2012 | 66 | ||
| FCM-miniR | Vomiting | 2 | 2 | Almost certainly | Almost certainly | Missing | Missing | Yes | |||||
| FCM-miniR | Lymphopenia | 3 | 2 | Almost certainly | Almost certainly | Missing | Missing | 5 September 2012 | No | ||||
| FCM-miniR | Urinary symptoms | 2 | 3 | Unlikely | Unlikely | Missing | Missing | Yes | 15 October 2012 | ||||
| FCM-miniR | Nausea | 2 | 4 | Unlikely | Probably | Missing | Missing | Yes | 23 November 2012 | ||||
| FCM-miniR | Constipation | 2 | 4 | Unlikely | Unlikely | Missing | Missing | Yes | 14 November 2012 | ||||
| FCM-miniR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Missing | Missing | 19 October 2012 | Yes | 14 November 2012 | 26 | ||
| FCM-miniR | Infections (not neutropenic sepsis) | 1 | 5 | Probably | Probably | Missing | Missing | Yes | 14 December 2012 | ||||
| FCM-miniR | Sore throat | 1 | 6 | Possibly | Possibly | Missing | Missing | Yes | |||||
| FCM-miniR | Pruritus | 1 | 6 | Unrelated | Unrelated | Missing | Missing | Yes | |||||
| FCM-miniR | Dry skin/erythema | 1 | 6 | Unrelated | Unrelated | Missing | Missing | 11 January 2013 | Yes | ||||
| FCM-miniR | Anaemia | 1 | 6 | Almost certainly | Almost certainly | Missing | Missing | 22 December 2012 | Yes | 1 March 2013 | 69 | ||
| FCM-miniR | Thrombocytopenia | 1 | 6 | Almost certainly | Almost certainly | Missing | Missing | 14 December 2012 | Yes | 1 March 2013 | 77 | ||
| 184 | FCM-miniR | Thrombocytopenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Probably | 13 September 2012 | No | |||
| FCM-miniR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Probably | 13 September 2012 | No | ||||
| FCM-miniR | Nausea | 2 | 1 | Probably | Probably | Probably | Probably | 23 August 2012 | Yes | 20 September 2012 | 28 | ||
| FCM-miniR | Vomiting | 1 | 1 | Probably | Probably | Probably | Probably | 28 August 2012 | Yes | 20 September 2012 | 23 | ||
| FCM-miniR | Fatigue | 2 | 1 | Probably | Possibly | Probably | Possibly | 23 August 2012 | No | ||||
| FCM-miniR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Probably | 19 October 2012 | No | ||||
| FCM-miniR | Nausea | 2 | 2 | Probably | Probably | Probably | Probably | 30 September 2012 | Yes | 6 October 2012 | 6 | ||
| FCM-miniR | Vomiting | 2 | 2 | Probably | Probably | Probably | Probably | 30 September 2012 | Yes | 6 October 2012 | 6 | ||
| FCM-miniR | Nausea | 2 | 2 | Possibly | Possibly | Possibly | Possibly | 13 October 2012 | No | ||||
| FCM-miniR | Vomiting | 2 | 2 | Possibly | Possibly | Possibly | Possibly | 13 October 2012 | No | ||||
| FCM-miniR | Other AE description | Small bowel obstruction | 3 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 14 October 2012 | No | |||
| FCM-miniR | Other AE description | Femoral hernia | 3 | 2 | Unrelated | Unrelated | Unrelated | Unrelated | 14 October 2012 | No |
| Patient number | Treatment received | AE description | Other AE description | Maximum CTCAE grade | Treatment cycle AE started | Related to Fludarabine? | Related to Cyclophosphamide? | Related to Mitoxantrone? | Related to low-dose Rituximab? | Related to Rituximab? | Date of onset | Recovered? | Date of recovery | Duration of AE (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 145 | FCM-miniR/FCR | Constipation | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | Missing | 31 May 2012 | Yes | |||
| FCM-miniR/FCR | Fatigue | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 31 May 2012 | Yes | 3 June 2012 | 3 | ||
| FCM-miniR/FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 31 May 2012 | Yes | 12 June 2012 | 12 | ||
| FCM-miniR/FCR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 31 May 2012 | Yes | 3 June 2012 | 3 | ||
| FCM-miniR/FCR | Allergic reaction | 3 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 17 May 2012 | Yes | 17 May 2012 | 0 | ||
| FCM-miniR/FCR | Nausea | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 16 June 2012 | Yes | 21 June 2012 | 5 | ||
| FCM-miniR/FCR | Anorexia/cachexia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 17 May 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 20 June 2012 | Yes | 28 June 2012 | 8 | ||
| FCM-miniR/FCR | Anaemia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 28 June 2012 | Yes | 29 June 2012 | 1 | ||
| FCM-miniR/FCR | Raised aminotransferases | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 9 July 2012 | Yes | 11 July 2012 | 2 | ||
| FCM-miniR/FCR | Cutaneous herpes/shingles | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 25 June 2012 | Yes | 30 June 2012 | 5 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 22 August 2012 | Yes | 3 September 2012 | 12 | ||
| FCM-miniR/FCR | Diarrhoea | 2 | 5 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 11 September 2012 | Yes | 4 October 2012 | 23 | ||
| FCM-miniR/FCR | Diarrhoea | 1 | 6 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 12 October 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 2 | 6 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 11 October 2012 | Yes | 15 October 2012 | 4 | ||
| 148 | FCM-miniR/FCR | Neutropenic sepsis | 4 | 2 | Almost certainly | Almost certainly | Almost certainly | Possibly | Missing | 27 May 2012 | Yes | 31 May 2012 | 4 | |
| FCM-miniR/FCR | Anaemia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Unrelated | Missing | 28 May 2012 | Yes | 28 May 2012 | 0 | ||
| FCM-miniR/FCR | Anaemia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Unrelated | Missing | 16 June 2012 | Yes | 16 June 2012 | 0 | ||
| 152 | FCM-miniR/FCR | Diarrhoea | 1 | 2 | Unrelated | Unrelated | Probably | Possibly | Missing | 25 July 2012 | Yes | 7 August 2012 | 13 | |
| FCM-miniR/FCR | Neutropenia | 4 | 3 | Probably | Probably | Probably | Unlikely | Missing | 3 August 2012 | Yes | 28 August 2012 | 25 | ||
| FCM-miniR/FCR | Diarrhoea | 1 | 3 | Unrelated | Unrelated | Probably | Possibly | Missing | 4 August 2012 | Yes | 6 August 2012 | 2 | ||
| 153 | FCM-miniR/FCR | Abnormal electrolytes | 1 | 1 | Probably | Probably | Possibly | Probably | Missing | 1 June 2012 | Yes | 3 June 2012 | 2 | |
| FCM-miniR/FCR | Anorexia/cachexia | 2 | 2 | Almost certainly | Almost certainly | Unrelated | Almost certainly | Missing | 2 July 2012 | No | ||||
| FCM-miniR/FCR | Thrombocytopenia | 2 | 2 | Almost certainly | Almost certainly | Unrelated | Almost certainly | Missing | 25 June 2012 | Yes | 16 July 2012 | 21 | ||
| FCM-miniR/FCR | Nausea | 2 | 4 | Unlikely | Unlikely | Possibly | Unlikely | Missing | 17 September 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 15 October 2012 | No | ||||
| FCM-miniR/FCR | Thrombocytopenia | 2 | 6 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 12 November 2012 | No | ||||
| 160 | FCM-miniR/FCR | Hypotension | 2 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | Missing | 19 June 2012 | Yes | 19 June 2012 | 0 | |
| FCM-miniR/FCR | Dyspnoea | 3 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | Missing | 19 June 2012 | Yes | 19 June 2012 | 0 | ||
| FCM-miniR/FCR | Infections (not neutropenic sepsis) | 2 | 1 | Possibly | Possibly | Unrelated | Unrelated | Missing | 12 July 2012 | Yes | 17 July 2012 | 5 | ||
| FCM-miniR/FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Probably | Probably | Missing | 13 July 2012 | No | ||||
| FCM-miniR/FCR | Abdominal pain/bloating | 2 | 2 | Possibly | Possibly | Possibly | Possibly | Missing | 20 August 2012 | Yes | 24 August 2012 | 4 | ||
| FCM-miniR/FCR | Thrombocytopenia | 1 | 2 | Almost certainly | Probably | Probably | Probably | Missing | 20 August 2012 | Yes | 30 August 2012 | 10 | ||
| FCM-miniR/FCR | Nausea | 1 | 3 | Probably | Probably | Probably | Probably | Missing | 30 July 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 2 | 4 | Possibly | Possibly | Missing | Missing | Unrelated | 8 October 2012 | Yes | ||||
| FCM-miniR/FCR | Neutropenia | 3 | 4 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 31 October 2012 | No | ||||
| FCM-miniR/FCR | Dyspnoea | 1 | 5 | Unrelated | Unrelated | Missing | Missing | Possibly | 8 November 2012 | No | ||||
| FCM-miniR/FCR | Anaemia | 2 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 26 November 2012 | No | ||||
| FCM-miniR/FCR | Thrombocytopenia | 1 | 5 | Almost certainly | Probably | Missing | Missing | Unlikely | 5 November 2012 | Yes | 26 November 2012 | 21 | ||
| FCM-miniR/FCR | Neutropenia | 2 | 6 | Almost certainly | Almost certainly | Missing | Missing | Unlikely | Yes | 22 March 2013 | ||||
| 162 | FCM-miniR/FCR | Nausea | 1 | 1 | Probably | Probably | Probably | Possibly | Missing | 27 June 2012 | Yes | 30 June 2012 | 3 | |
| FCM-miniR/FCR | Neutropenia | 4 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | Missing | 4 July 2012 | Yes | 15 September 2012 | 73 | ||
| FCM-miniR/FCR | Thrombocytopenia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | Missing | 14 August 2012 | Yes | 5 September 2012 | 22 | ||
| FCM-miniR/FCR | Mucositis/thrush | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Possibly | Missing | 2 August 2012 | Yes | 20 August 2012 | 18 | ||
| FCM-miniR/FCR | Rash/flushing | 2 | 3 | Unlikely | Unlikely | Unlikely | Unlikely | Missing | 5 September 2012 | Yes | 12 September 2012 | 7 | ||
| FCM-miniR/FCR | Thrombocytopenia | 1 | 4 | Almost certainly | Almost certainly | Almost certainly | Possibly | Missing | 24 September 2012 | Yes | 24 October 2012 | 30 | ||
| FCM-miniR/FCR | Neutropenia | 2 | 5 | Almost certainly | Almost certainly | Missing | Missing | Possibly | 4 October 2012 | Yes | 1 November 2012 | 28 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Missing | Missing | Possibly | 15 November 2012 | No | ||||
| 164 | FCM-miniR/FCR | Fever | 1 | 1 | Unlikely | Unlikely | Probably | Probably | Missing | 13 July 2012 | Yes | 16 July 2012 | 3 | |
| FCM-miniR/FCR | Neutropenia | 4 | 1 | Possibly | Possibly | Possibly | Unlikely | Missing | 23 July 2012 | Yes | 14 August 2012 | 22 | ||
| FCM-miniR/FCR | Vomiting | 1 | 1 | Unlikely | Unlikely | Unlikely | Possibly | Missing | 13 July 2012 | Yes | 16 July 2012 | 3 | ||
| FCM-miniR/FCR | Fever | 1 | 1 | Unlikely | Unlikely | Probably | Probably | Missing | 4 August 2012 | Yes | 8 August 2012 | 4 | ||
| FCM-miniR/FCR | Rash/flushing | 1 | 1 | Unlikely | Unlikely | Unlikely | Possibly | Missing | 6 August 2012 | Yes | 14 August 2012 | 8 | ||
| FCM-miniR/FCR | Cough | 1 | 2 | Possibly | Possibly | Possibly | Unlikely | Missing | 10 July 2012 | Yes | 14 August 2012 | 35 | ||
| FCM-miniR/FCR | Vomiting | 1 | 2 | Unlikely | Unlikely | Unlikely | Possibly | Missing | 14 August 2012 | Yes | 17 August 2012 | 3 | ||
| FCM-miniR/FCR | Rash/flushing | 1 | 2 | Unlikely | Unlikely | Unlikely | Possibly | Missing | 14 August 2012 | Yes | 21 August 2012 | 7 | ||
| FCM-miniR/FCR | Fever | 1 | 3 | Unlikely | Unlikely | Unrelated | Probably | Missing | 19 September 2012 | Yes | 19 September 2012 | 0 | ||
| FCM-miniR/FCR | Nausea | 1 | 3 | Possibly | Possibly | Possibly | Possibly | Missing | 18 September 2012 | Yes | 23 September 2012 | 5 | ||
| FCM-miniR/FCR | Fatigue | 1 | 3 | Possibly | Possibly | Unlikely | Unlikely | Missing | 18 September 2012 | Yes | 23 September 2012 | 5 | ||
| FCM-miniR/FCR | Nausea | 2 | 4 | Possibly | Possibly | Missing | Missing | Missing | 16 October 2012 | Yes | 20 October 2012 | 4 | ||
| FCM-miniR/FCR | Anorexia/cachexia | 1 | 4 | Possibly | Possibly | Missing | Missing | Missing | 16 October 2012 | Yes | 20 October 2012 | 4 | ||
| FCM-miniR/FCR | Fatigue | 1 | 4 | Possibly | Possibly | Missing | Missing | Missing | 16 October 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 1 | 5 | Possibly | Possibly | Missing | Missing | Missing | 13 November 2012 | Yes | 20 November 2012 | 7 | ||
| FCM-miniR/FCR | Fatigue | 1 | 5 | Possibly | Possibly | Missing | Missing | Missing | 13 November 2012 | Yes | 4 December 2012 | 21 | ||
| FCM-miniR/FCR | Nausea | 1 | 6 | Possibly | Possibly | Missing | Missing | Missing | 11 December 2012 | Yes | 18 December 2012 | 7 | ||
| FCM-miniR/FCR | Fatigue | 1 | 6 | Possibly | Possibly | Missing | Missing | Missing | 11 December 2012 | No | ||||
| 169 | FCM-miniR/FCR | Neutropenia | 4 | 5 | Probably | Probably | Missing | Missing | Probably | 28 November 2012 | Yes | 10 December 2012 | 12 | |
| 170 | FCM-miniR/FCR | Anaemia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 15 August 2012 | Yes | 28 August 2012 | 13 | |
| FCM-miniR/FCR | Thrombocytopenia | 1 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 13 June 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 2 | 1 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 15 August 2012 | Yes | 28 August 2012 | 13 | ||
| FCM-miniR/FCR | Allergic reaction | 1 | 1 | Unrelated | Unrelated | Unrelated | Almost certainly | Missing | 31 July 2012 | Yes | 31 July 2012 | 0 | ||
| FCM-miniR/FCR | Neutropenia | 2 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 12 September 2012 | Yes | 24 September 2012 | 12 | ||
| FCM-miniR/FCR | Anaemia | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 12 September 2012 | Yes | 24 September 2012 | 12 | ||
| FCM-miniR/FCR | Anaemia | 1 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 10 October 2012 | Yes | 19 October 2012 | 9 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 3 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 10 October 2012 | Yes | 19 October 2012 | 9 | ||
| FCM-miniR/FCR | Nausea | 1 | 4 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 23 October 2012 | Yes | 27 October 2012 | 4 | ||
| FCM-miniR/FCR | Fatigue | 1 | 4 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 23 October 2012 | Yes | 27 October 2012 | 4 | ||
| FCM-miniR/FCR | Anorexia/cachexia | 1 | 4 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 23 October 2012 | Yes | 23 October 2012 | 0 | ||
| FCM-miniR/FCR | Nausea | 1 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 24 November 2012 | Yes | 1 December 2012 | 7 | ||
| FCM-miniR/FCR | Nasal symptoms | 1 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 24 November 2012 | Yes | 1 December 2012 | 7 | ||
| FCM-miniR/FCR | Fatigue | 1 | 6 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 18 December 2012 | No | ||||
| 173 | FCM-miniR/FCR | Constipation | 2 | 1 | Possibly | Unlikely | Unlikely | Unlikely | Missing | 14 July 2012 | Yes | 17 July 2012 | 3 | |
| FCM-miniR/FCR | Renal impairment | 1 | 1 | Probably | Probably | Probably | Probably | Missing | 7 August 2012 | Yes | 5 September 2012 | 29 | ||
| FCM-miniR/FCR | Anaemia | 2 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Missing | 7 August 2012 | Yes | 5 September 2012 | 29 | ||
| FCM-miniR/FCR | Constipation | 2 | 2 | Possibly | Unlikely | Unlikely | Unlikely | Missing | 11 August 2012 | Yes | 13 August 2012 | 2 | ||
| 176 | FCM-miniR/FCR | Neutropenia | 4 | 1 | Almost certainly | Almost certainly | Almost certainly | Probably | Missing | 15 August 2012 | Yes | 3 September 2012 | 19 | |
| FCM-miniR/FCR | Thrombocytopenia | 2 | 1 | Almost certainly | Probably | Probably | Probably | Missing | 15 August 2012 | Yes | 3 September 2012 | 19 | ||
| FCM-miniR/FCR | Rash/flushing | 2 | 1 | Possibly | Possibly | Possibly | Possibly | Missing | 21 August 2012 | Yes | 29 August 2012 | 8 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 2 | Almost certainly | Almost certainly | Almost certainly | Probably | Missing | 3 October 2012 | Yes | 16 October 2012 | 13 | ||
| FCM-miniR/FCR | Thrombocytopenia | 2 | 2 | Almost certainly | Probably | Probably | Probably | Missing | 9 October 2012 | Yes | 16 October 2012 | 7 | ||
| FCM-miniR/FCR | Infections (not neutropenic sepsis) | 2 | 6 | Possibly | Possibly | Missing | Missing | Possibly | 1 January 2013 | Yes | 20 January 2013 | 19 | ||
| 177 | FCM-miniR/FCR | Rash/flushing | 2 | 3 | Unrelated | Unrelated | Possibly | Unrelated | Missing | 20 August 2012 | Yes | 31 August 2012 | 11 | |
| FCM-miniR/FCR | Fever | 2 | 3 | Probably | Probably | Possibly | Probably | Missing | 20 August 2012 | Yes | 31 August 2012 | 11 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 3 | Probably | Probably | Possibly | Probably | Missing | 5 September 2012 | Yes | 19 September 2012 | 14 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 16 October 2012 | Yes | 23 October 2012 | 7 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 5 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 20 November 2012 | Yes | 27 November 2012 | 7 | ||
| FCM-miniR/FCR | Bone pain | 1 | 5 | Unrelated | Unrelated | Missing | Missing | Unrelated | 27 November 2012 | Yes | 27 December 2012 | 30 | ||
| FCM-miniR/FCR | Neutropenia | 4 | 6 | Almost certainly | Almost certainly | Missing | Missing | Unlikely | 27 December 2012 | Yes | 3 January 2013 | 7 | ||
| 183 | FCM-miniR/FCR | Nausea | 1 | 1 | Probably | Probably | Probably | Probably | Missing | 8 August 2012 | No | |||
| FCM-miniR/FCR | Vomiting | 1 | 1 | Probably | Probably | Probably | Probably | Missing | 9 August 2012 | Yes | 9 August 2012 | 0 | ||
| FCM-miniR/FCR | Constipation | 1 | 1 | Probably | Probably | Probably | Probably | Missing | 8 August 2012 | Yes | 5 September 2012 | 28 | ||
| FCM-miniR/FCR | Fatigue | 1 | 1 | Possibly | Probably | Probably | Probably | Missing | 8 August 2012 | No | ||||
| FCM-miniR/FCR | Taste alteration | 1 | 1 | Possibly | Possibly | Possibly | Probably | Missing | 8 August 2012 | No | ||||
| FCM-miniR/FCR | Vomiting | 1 | 2 | Probably | Probably | Probably | Probably | Missing | 5 September 2012 | Yes | 7 September 2012 | 2 | ||
| FCM-miniR/FCR | Diarrhoea | 1 | 2 | Probably | Probably | Probably | Probably | Missing | 5 September 2012 | Yes | 10 October 2012 | 35 | ||
| FCM-miniR/FCR | Vomiting | 1 | 3 | Probably | Probably | Missing | Missing | Probably | 3 October 2012 | Yes | 3 October 2012 | 0 | ||
| 186 | FCM-miniR/FCR | Infections (not neutropenic sepsis) | 2 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Missing | 6 August 2012 | Yes | 12 September 2012 | 37 | |
| FCM-miniR/FCR | Otalgia | 3 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Missing | 15 August 2012 | Yes | 12 September 2012 | 28 | ||
| FCM-miniR/FCR | Nausea | 1 | 2 | Almost certainly | Almost certainly | Almost certainly | Almost certainly | Missing | 22 August 2012 | Yes | 10 October 2012 | 49 | ||
| FCM-miniR/FCR | Constipation | 1 | 2 | Unlikely | Unlikely | Unrelated | Unlikely | Missing | 22 August 2012 | No | ||||
| FCM-miniR/FCR | Pruritus | 1 | 3 | Unrelated | Unrelated | Missing | Missing | Almost certainly | 10 October 2012 | Yes | 6 November 2012 | 27 | ||
| FCM-miniR/FCR | Neutropenia | 2 | 3 | Almost certainly | Almost certainly | Missing | Missing | Almost certainly | 10 October 2012 | Yes | 6 November 2012 | 27 | ||
| 188 | FCM-miniR/FCR | Nausea | 1 | 1 | Probably | Probably | Probably | Possibly | Missing | 1 September 2012 | Yes | 4 September 2012 | 3 | |
| FCM-miniR/FCR | Vomiting | 2 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | Missing | 3 September 2012 | Yes | 5 September 2012 | 2 | ||
| FCM-miniR/FCR | Headache | 1 | 1 | Unlikely | Unlikely | Unlikely | Almost certainly | Missing | 30 August 2012 | Yes | 31 August 2012 | 1 | ||
| FCM-miniR/FCR | Fatigue | 2 | 1 | Probably | Unlikely | Probably | Possibly | Missing | 4 September 2012 | Yes | 22 September 2012 | 18 | ||
| FCM-miniR/FCR | Alopecia | 1 | 1 | Unlikely | Almost certainly | Unlikely | Probably | Missing | 21 September 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 1 | 2 | Probably | Probably | Probably | Possibly | Missing | 27 September 2012 | Yes | 30 September 2012 | 3 | ||
| FCM-miniR/FCR | Abdominal pain/bloating | 2 | 2 | Unlikely | Unlikely | Possibly | Possibly | Missing | 30 September 2012 | Yes | 6 October 2012 | 6 | ||
| FCM-miniR/FCR | Fatigue | 2 | 2 | Probably | Unlikely | Probably | Possibly | Missing | 3 October 2012 | Yes | 11 October 2012 | 8 | ||
| FCM-miniR/FCR | Non-specific pain | 1 | 2 | Unlikely | Unlikely | Unlikely | Possibly | Missing | 13 October 2012 | Yes | 18 October 2012 | 5 | ||
| FCM-miniR/FCR | Nausea | 1 | 3 | Probably | Probably | Missing | Missing | Possibly | 26 October 2012 | Yes | 2 November 2012 | 7 | ||
| FCM-miniR/FCR | Fatigue | 2 | 3 | Probably | Unlikely | Missing | Missing | Possibly | 27 October 2012 | No | ||||
| FCM-miniR/FCR | Non-specific pain | 1 | 3 | Unlikely | Unlikely | Missing | Missing | Possibly | 15 October 2012 | Yes | 19 October 2012 | 4 | ||
| FCM-miniR/FCR | Abdominal pain/bloating | 2 | 3 | Unlikely | Unlikely | Missing | Missing | Possibly | 15 October 2012 | Yes | 19 October 2012 | 4 | ||
| FCM-miniR/FCR | Nausea | 2 | 4 | Probably | Probably | Missing | Missing | Possibly | 23 November 2012 | Yes | 16 December 2012 | 23 | ||
| FCM-miniR/FCR | Non-specific pain | 1 | 4 | Unlikely | Unlikely | Missing | Missing | Possibly | 29 November 2012 | Yes | 30 November 2012 | 1 | ||
| FCM-miniR/FCR | Abdominal pain/bloating | 1 | 4 | Unlikely | Unlikely | Missing | Missing | Possibly | 29 November 2012 | Yes | 30 November 2012 | 1 | ||
| FCM-miniR/FCR | Nausea | 2 | 5 | Probably | Probably | Missing | Missing | Possibly | 24 December 2012 | Yes | 7 January 2013 | 14 | ||
| FCM-miniR/FCR | Mucositis/thrush | 2 | 5 | Probably | Unlikely | Missing | Missing | Probably | 7 January 2013 | Yes | 11 January 2013 | 4 | ||
| 189 | FCM-miniR/FCR | Vomiting | 3 | 1 | Probably | Possibly | Possibly | Unlikely | Missing | 10 September 2012 | Yes | 17 September 2012 | 7 | |
| FCM-miniR/FCR | Fatigue | 1 | 1 | Possibly | Possibly | Possibly | Possibly | Missing | 2 October 2012 | No | ||||
| FCM-miniR/FCR | Myalgias | 1 | 1 | Possibly | Possibly | Possibly | Possibly | Missing | 2 October 2012 | No | ||||
| FCM-miniR/FCR | Anaemia | 2 | 1 | Possibly | Possibly | Possibly | Unlikely | Missing | 12 September 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 4 | 1 | Probably | Possibly | Probably | Possibly | Missing | 17 September 2012 | Yes | 2 October 2012 | 15 | ||
| FCM-miniR/FCR | Abdominal pain/bloating | 1 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 3 October 2012 | Yes | 23 October 2012 | 20 | ||
| FCM-miniR/FCR | Fatigue | 2 | 4 | Probably | Probably | Missing | Missing | Unlikely | 28 November 2012 | Yes | 7 December 2012 | 9 | ||
| FCM-miniR/FCR | Arthralgias | 1 | 4 | Unlikely | Unlikely | Missing | Missing | Unlikely | 5 December 2012 | Yes | 7 December 2012 | 2 | ||
| FCM-miniR/FCR | Neutropenia | 1 | 4 | Probably | Probably | Missing | Missing | Unlikely | 24 December 2012 | No | ||||
| FCM-miniR/FCR | Fatigue | 2 | 5 | Probably | Probably | Missing | Missing | Unlikely | 27 January 2013 | No | ||||
| FCM-miniR/FCR | Arthralgias | 1 | 5 | Unlikely | Unlikely | Missing | Missing | Possibly | 27 January 2013 | No | ||||
| FCM-miniR/FCR | Neutropenia | 3 | 5 | Probably | Probably | Missing | Missing | Possibly | 8 January 2013 | Yes | 11 January 2013 | 3 | ||
| FCM-miniR/FCR | Anaemia | 1 | 5 | Probably | Probably | Missing | Missing | Unlikely | 13 January 2013 | No | ||||
| FCM-miniR/FCR | Neutropenia | 2 | 5 | Probably | Probably | Missing | Missing | Possibly | 13 January 2013 | Yes | 22 January 2013 | 9 | ||
| FCM-miniR/FCR | Fatigue | 2 | 6 | Probably | Probably | Missing | Missing | Unlikely | 24 January 2013 | Yes | 12 February 2013 | 19 | ||
| FCM-miniR/FCR | Arthralgias | 1 | 6 | Unlikely | Unlikely | Missing | Missing | Unlikely | Yes | 12 February 2013 | ||||
| FCM-miniR/FCR | Headache | 1 | 6 | Unlikely | Unlikely | Missing | Missing | Unlikely | Yes | 12 February 2013 | ||||
| FCM-miniR/FCR | Thrombocytopenia | 1 | 6 | Probably | Probably | Missing | Missing | Unlikely | 31 January 2013 | Yes | 12 February 2013 | 12 | ||
| FCM-miniR/FCR | Neutropenia | 3 | 6 | Probably | Probably | Missing | Missing | Unlikely | 31 January 2013 | Yes | 5 March 2013 | 33 | ||
| 190 | FCM-miniR/FCR | Nausea | 1 | 1 | Probably | Probably | Probably | Unlikely | Missing | 8 September 2012 | Yes | 9 September 2012 | 1 | |
| FCM-miniR/FCR | Vomiting | 1 | 1 | Probably | Probably | Probably | Unlikely | Missing | 8 September 2012 | Yes | 9 September 2012 | 1 | ||
| FCM-miniR/FCR | Neuropathy (sensory) | 1 | 1 | Unlikely | Unlikely | Unlikely | Unlikely | Missing | 9 September 2012 | Yes | 10 September 2012 | 1 | ||
| FCM-miniR/FCR | Abdominal pain/bloating | 1 | 1 | Probably | Probably | Probably | Unlikely | Missing | 9 September 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 1 | 2 | Probably | Probably | Probably | Unlikely | Missing | 5 October 2012 | Yes | 8 October 2012 | 3 | ||
| FCM-miniR/FCR | Nausea | 1 | 4 | Possibly | Almost certainly | Missing | Missing | Missing | 28 November 2012 | Yes | 3 December 2012 | 5 | ||
| FCM-miniR/FCR | Vomiting | 1 | 6 | Possibly | Possibly | Missing | Missing | Missing | 5 February 2013 | Yes | 7 February 2013 | 2 | ||
| 196 | FCM-miniR/FCR | Fatigue | 1 | 2 | Probably | Probably | Missing | Missing | Unlikely | 20 September 2012 | No | |||
| FCM-miniR/FCR | Back pain | 2 | 2 | Unlikely | Unlikely | Missing | Missing | Unlikely | 17 September 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 2 | 2 | Almost certainly | Almost certainly | Missing | Missing | Unlikely | 20 September 2012 | Yes | ||||
| FCM-miniR/FCR | Oedema | 1 | 2 | Unlikely | Unlikely | Missing | Missing | Unlikely | 2 October 2012 | Yes | 5 October 2012 | 3 | ||
| FCM-miniR/FCR | Bone pain | 2 | 3 | Unlikely | Unlikely | Missing | Missing | Unrelated | 29 October 2012 | No | ||||
| FCM-miniR/FCR | Anorexia/cachexia | 1 | 4 | Probably | Probably | Missing | Missing | Unrelated | 21 November 2012 | No | ||||
| FCM-miniR/FCR | Nausea | 2 | 4 | Almost certainly | Almost certainly | Missing | Missing | Unrelated | 21 November 2012 | No | ||||
| FCM-miniR/FCR | Constipation | 1 | 4 | Unrelated | Unrelated | Missing | Missing | Unrelated | 12 December 2012 | Yes | 13 December 2012 | 1 | ||
| FCM-miniR/FCR | Back pain | 2 | 4 | Unlikely | Unlikely | Missing | Missing | Unrelated | 29 October 2012 | No | ||||
| FCM-miniR/FCR | Constipation | 1 | 6 | Unrelated | Unrelated | Missing | Missing | Unrelated | 7 February 2013 | No | ||||
| 197 | FCM-miniR/FCR | Fatigue | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Missing | 11 September 2012 | No | |||
| FCM-miniR/FCR | Myalgias | 1 | 1 | Unrelated | Unrelated | Unrelated | Unrelated | Missing | Yes | 23 October 2012 | ||||
| FCM-miniR/FCR | Oedema | 1 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 16 October 2012 | No | ||||
| FCM-miniR/FCR | Insomnia | 1 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 2 October 2012 | No | ||||
| FCM-miniR/FCR | Anaemia | 2 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 22 October 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 2 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 22 October 2012 | No | ||||
| FCM-miniR/FCR | Abdominal pain/bloating | 1 | 2 | Possibly | Possibly | Missing | Missing | Possibly | 24 October 2012 | No | ||||
| FCM-miniR/FCR | Neutropenia | 2 | 3 | Probably | Probably | Missing | Missing | Unlikely | 19 November 2012 | No | ||||
| FCM-miniR/FCR | Lymphopenia | 2 | 3 | Probably | Probably | Missing | Missing | Unlikely | 19 November 2012 | No | ||||
| FCM-miniR/FCR | Dry skin/erythema | 1 | 4 | Unlikely | Unlikely | Missing | Missing | Possibly | 27 November 2012 | No | ||||
| FCM-miniR/FCR | Non-specific pain | 1 | 4 | Unlikely | Unlikely | Missing | Missing | Unlikely | 13 December 2012 | Yes | ||||
| FCM-miniR/FCR | Lymphopenia | 2 | 4 | Probably | Probably | Missing | Missing | Unlikely | 17 December 2012 | No | ||||
| FCM-miniR/FCR | Other AE description | Cold hands | 1 | 5 | Possibly | Possibly | Missing | Missing | Possibly | 17 December 2012 | No | |||
| FCM-miniR/FCR | Dyspnoea | 1 | 5 | Possibly | Possibly | Missing | Missing | Possibly | 19 October 2012 | Yes | 24 January 2013 | 97 | ||
| FCM-miniR/FCR | Infections (not neutropenic sepsis) | 1 | 5 | Unlikely | Unlikely | Missing | Missing | Unlikely | 26 December 2012 | Yes | 24 January 2013 | 29 | ||
| FCM-miniR/FCR | Other AE description | R pulmonary effusion | 1 | 5 | Unlikely | Unlikely | Missing | Missing | Unlikely | 26 December 2012 | No | |||
| FCM-miniR/FCR | Rash/flushing | 1 | 6 | Possibly | Possibly | Missing | Missing | Possibly | 6 February 2013 | No |
Appendix 3 Baseline patient health economics questionnaire booklet
Appendix 4 Follow-up patient health economics questionnaire booklet
Appendix 5 Participant Information Sheet
List of abbreviations
- β2M
- β2-microglobulin
- AE
- adverse event
- ARCTIC
- Attenuated dose Rituximab with ChemoTherapy In CLL trial
- B-CLL
- B-cell chronic lymphocytic leukaemia
- BNF
- British National Formulary
- BSA
- body surface area
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CLL
- chronic lymphocytic leukaemia
- CR
- complete remission
- CRF
- case report form
- CRi
- complete remission with incomplete marrow recovery
- CTCAE
- Common Terminology Criteria for Adverse Events
- CTRU
- Clinical Trials Research Unit
- DCT
- direct Coombs test
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D™
- European Quality of Life-5 Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- FC
- fludarabine and cyclophosphamide
- FCM
- fludarabine, cyclophosphamide and mitoxantrone
- FCM-miniR
- fludarabine, cyclophosphamide, mitoxantrone and low-dose rituximab
- FCM-R
- fludarabine, cyclophosphamide, mitoxantrone and rituximab
- FCR
- fludarabine, cyclophosphamide and rituximab
- GCLLSG
- German CLL study group
- GCSF
- granulocyte colony-stimulating factor
- HMDS
- Haematological Malignancy Diagnostic Service
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IMP
- Investigational Medicinal Product
- INB
- incremental net monetary benefit
- ITT
- intention to treat
- IWCLL
- International Workshop on Chronic Lymphocytic Leukaemia
- MedDRA
- Medical Dictionary for Regulatory Activities
- MRD
- minimal residual disease
- NB
- net monetary benefit
- NCI
- National Cancer Institute
- NCRI
- National Cancer Research Institute
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institutes of Health Research
- OD
- once daily
- OR
- odds ratio
- ORR
- overall response rate
- OS
- overall survival
- PCP
- Pneumocystis carinii pneumonia
- PD
- progressive disease
- PFS
- progression-free survival
- PP
- per-protocol
- PPI
- patient and public involvement
- PR
- partial remission
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RDM
- Remission Duration Model
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SLL
- small lymphocytic lymphoma
- SUSAR
- suspected unexpected serious adverse reaction
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VH
- heavy-chain variable-region
- WHO
- World Health Organization
- WTP
- willingness to pay