Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/31/01. The contractual start date was in January 2014. The draft report began editorial review in July 2016 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors report grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study. Martin Underwood also reports personal fees from the NIHR HTA programme and personal fees from the National Institute for Health and Care Excellence (NICE). Martin Underwood was also chairperson of the NICE back pain development group that produced the 2009 guidelines and is a member of the NIHR Journals Library Editorial Group. James HL Antrobus was a pain physician with a NHS practice and an independent practice. He offered a service for and performed facet joint injections in both the NHS and independent sector while this study was in progress. James HL Antrobus was a member of the British Pain Society and a member of the British Pain Society Interventional Medicine Special Interest Group. David A Walsh reports consultancy on over-the-counter analgesic preparations that is not directly relevant to the submitted work (Novartis Consumer Health S.A).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Ellard et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Low back pain
A global problem
Low back pain (LBP) is ranked as highest in the Global Burden of Disease in terms of years lived with disability. 1 The most recent UK ‘cost-of-illness’ study was in 1998. 2 At that time, the direct health-care costs of LBP were £1067M for the UK NHS and £565M for private health care, resulting in a cost of £28 per head of population. A 1998 US study3 estimated direct health-care costs as US$90,601M and a cost of US$335 per person. Much has changed since then. In the USA, there has been a 2.4-fold increase in spinal fusions and a massive increase in facet joint interventions, both of which are still increasing. 4,5 In response to this problem, the National Institute for Health and Care Excellence (NICE) commissioned guidelines for the management of non-specific LBP lasting between 6 weeks and 1 year. 6 The excluded treatment approaches included the injection of therapeutic substances into the back. Deep divisions in the scientific and clinical communities have become clear since the publication of the 2009 NICE guidelines and the 2009 American Pain Society guidelines for LBP,7 which also indicated that there was insufficient evidence to support the injection of therapeutic substances into the back. 8 Although the methodological approach used by the American Pain Society has been challenged, the American Society of Interventional Pain Physicians also concluded that the evidence for therapeutic intra-articular facet joint injections (FJIs) was limited. 9,10 A new NICE guideline for LBP and sciatica,11 published in 2016, did not support the use of intra-articular FJIs (www.nice.org.uk/guidance/conditions-and-diseases/musculoskeletal-conditions/low-back-pain). It does, however, in contrast to the 2009 guidance, support the use of radiofrequency denervation in people who have had a positive diagnostic medial nerve branch block.
There is a variety of different interpretations of the available evidence from randomised controlled trials (RCTs) and observational studies on the effectiveness of intra-articular FJIs. 12–18 Controversy surrounding this issue remains in the USA, and in the UK there has been new research evaluating lumbar facet joint interventions. 9,19,20 However, the outcome of all this work was that, at the outset of this study, the data were not a robust evidence base on which to inform decisions about the use of therapeutic intra-articular FJIs. Notwithstanding current NICE guidance and insufficient evidence to justify the use of FJIs, Hospital Episode Statistics21 record that, in 2014/15, 81,963 FJI procedures were performed in England for the NHS, an increase from 62,671 in 2012/13. Although there may be some inaccuracies in the coding of different facet joint-related procedures, this still represents a 30% increase in activity over 2 years.
Thus, there was a clear need for a trial to test the effectiveness of adding intra-articular FJIs to usual care for the treatment of persistent LBP when usual care as recommended by NICE or the American Pain Society has been ineffective. It is important that the proposed trial provides data that all parties can agree on. If the trial has positive results, then reinvestment in this treatment will be justified. If the trial has negative results, its conclusions need to be sufficiently robust that all parties to the debate on current guidance are satisfied that the evidence does not support the use of therapeutic intra-articular FJIs.
The UK National Institute for Health Research, via a specific call, funded two feasibility studies in preparation for trials of therapeutic intra-articular FJIs. We were funded to carry out one of these studies. Our proposed main study will test the addition of a therapeutic intra-articular FJI to best usual care (BUC) [Health Technology Assessment (HTA) programme reference number 11/31/01]. 22 A different team is funded to test the feasibility of a more explanatory trial comparing active intra-articular injection with a sham control in people with a positive diagnostic medial branch nerve block (HTA 11/31/02). 23 These two studies will produce complementary data that will inform decisions on the merit of offering therapeutic intra-articular FJIs to selected people with LBP.
Diagnosis of low back pain
Low back pain is pain or discomfort felt in the back between the bottom of the rib cage and the buttock creases. Once medically serious causes of LBP have been excluded (infection, fracture, malignancy, inflammatory disorders such as ankylosing spondylitis), LBP is labelled as being non-specific. None of the early treatment options recommended in the 2009 NICE guidance requires any further diagnostic work-up.
Unless there is a concern that there is a serious medical cause for the pain, imaging of the lumbar spine is not indicated. This approach recognises the difficulty of reliably identifying subgroups of people who may respond better to any particular treatment approach. 24,25 Notwithstanding this overall approach to the diagnosis of LBP, it is possible that there are subgroups of individuals who would benefit from one or more specific approaches to the treatment of their LBP. One subgroup who may benefit from a more specific back treatment is patients whose pain wholly, or partially, arises from the facet joints. This notion is supported by the 2016 NICE back pain guideline, which recommends the use of diagnostic medial branch nerve blocks before considering radiofrequency denervation in people with suspected facet joint pain. The clinical assessment of suspected facet joint pain suggested in NICE 2016 guidance is based on work described later in this report. 26
Facet joint pain
The facet joints are paired structures between the superior and inferior articular processes of adjacent vertebrae that, in the lumbar spine, allow flexion and a degree of rotation of the spine. They are synovial joints whose capsule is richly innervated. With increasing age, progressively more people develop radiological osteoarthritis of the facet joints. A systematic review estimated the prevalence of facet joint degeneration increased from 4% for those in their twenties through 32% for those in their fifties to 83% for those in their eighties. 27 There is not, however, an association observed between radiological change in facet joints and the presence of back pain. 28 If pain arising from the facet joint is suspected clinically, it may be abolished temporarily by the injection of local anaesthetic, which may be used as a diagnostic test. A depot preparation of steroid added to the injection may prolong the analgesic benefit. The relief or reduction of pain may facilitate compliance with a programme of exercise designed to improve lumbar range of movement and muscular stability.
Clinical features of facet joint pain
There are few good epidemiological data on the prevalence of facet joint pain in different populations or on how to diagnose suspected facet joint pain clinically. In some studies, 5–15% of people with chronic LBP are believed to have disease of one or more facet joints that is contributing to their pain. 29 The gold-standard test for the presence of facet joint pain is pain relief after the injection of affected joints with a local anaesthetic. There is considerable diagnostic uncertainty about how to identify people with pain of facet joint origin among the wider chronic LBP population.
Revel et al. 30 in 1998 found that people with pain of facet joint origin were characterised by:
-
being aged > 65 years
-
pain that is well relieved by recumbency
-
absence of pain exacerbation
-
by coughing
-
by forward flexion
-
when rising from flexion
-
by hyperextension
-
by extension rotation.
-
All predicted a benefit from injection of anaesthetic into facet joints. The presence of five of these characteristics, including pain on recumbency, correctly identified 92% of responders and 80% of non-responders. 30 Others, however, were unable to replicate their findings. 31 Subsequent reviews suggest that 62% of those with these clinical features of facet joint pain obtained immediate relief from FJIs; one-third of these were false positives. 32,33
Laslett et al. 34 in 2006 found that seven factors were predictive of facet joint pain:
-
age ≥ 50 years
-
pain is best when walking
-
pain is best when sitting
-
onset of pain is paraspinal
-
modified somatic perceptions questionnaire score exceeding 13 (suggesting a somatisation disorder)
-
positive extension/rotation test
-
absence of centralisation during repeated movement testing.
They found that presence of three or more factors of age ≥ 50 years, pain is best when walking, pain is best when sitting, onset of pain is paraspinal and positive extension/rotation test was 85% sensitive and 91% specific for facet joint pain. 34 In a 2007 systematic review, Hancock et al. 35 did not find evidence for a robust diagnostic test for facet joint pain. In an initial scoping review in preparation for this work, we found one additional relevant article. In a retrospective chart review (n = 170), DePalma et al. 36 found that the presence of isolated paramidline LBP increased the probability of facet or sacroiliac joint dysfunction and slightly reduced the likelihood of lumbar disc degeneration. The sensitivity of reporting paramidline pain if the patient has facet joint pain was 96% [95% confidence interval (CI) 83% to 99.4%] for sacroiliac pain and 67% for internal disc disruption. This supports other work indicating that paraspinal or paramidline pain is a clinical indicator of possible facet joint involvement. Similarly, although not empirically proven, lumbar segmental motion may also be useful in identifying the level of facet joint involvement. 37 A 2007 consensus study38 identified clinical features thought to be associated with facet joint pain, such as:
-
localised unilateral LBP
-
lack of radicular features
-
pain eased in flexion
-
pain, if referred, is above the knee
-
palpation: local unilateral passive movement shows reduced range of motion or increased stiffness on the side of pain
-
unilateral muscle spasm over the affected facet joint
-
pain in extension
-
pain in extension, lateral flexion or rotation to the ipsilateral side.
In a prospective cohort study of medial branch blocks for suspected lumbar or cervical facet pain, Wasan et al. 39 used selection criteria including a history of axial pain with radiation in an established facet joint referral pattern and tests for facet joint loading signs (extension, side bending and rotation). Although they acknowledged that their study was not designed to confirm diagnosis, Wasan et al. 39 concluded that the selection criteria reduced the likelihood of radicular pain due to nerve root involvement or non-specific LBP.
In the 2011 protocol for a trial of specific physiotherapy compared with advice, Hahne et al. 40 argued that if three or more of the following factors are present then there is facet joint dysfunction:
-
unilateral LBP
-
pain reproduced with lumbar extension and ipsilateral lateral-flexion movements
-
pain on ipsilateral passive postero-anterior accessory movement applied through the transverse process or zygapophyseal joint at one or two segments
-
improvement in pain or range of movement following a ‘mini-treatment’ of manual therapy directed at the zygapophyseal joint.
The choices of Hahne et al. are grounded in the Maitland’s clinical reasoning approach to identifying a group who would respond to manual therapy. 41 The use of such phenotypically defined subgroups, grounded on clinical reasoning, may be the most appropriate approach to subgroup identification in LBP. 42 This approach has not been tested empirically and may not be directly relevant to identifying people likely to respond to FJIs. It does, however, represent current best practice to identify people likely to respond to the physical component of our proposed control intervention. Interestingly, this trial is restricted to people aged < 65 years, even though others have found increasing age to be a feature predicting the presence of facet joint pain. Nevertheless, 20% of recruits were identified clinically as having facet joint dysfunction. Diagnoses were not confirmed using diagnostic injections.
During the early stage of this study we identified a further study, published as a conference abstract,43 that identified combined movements as a predictor of pain relief from a diagnostic lumbar medial branch block.
Why might injecting facet joints be helpful?
The use of corticosteroid injection has been shown to be effective in producing, at least in the short term (1–4 weeks), benefits for a range of musculoskeletal disorders including frozen shoulder and osteoarthritis of the knee and hip. 44–46 Analgesic benefits of intra-articular injection of corticosteroids in rheumatoid arthritis may be more sustained (up to 3 months). 47 Drawing on these data from other parts of the musculoskeletal system, it is a reasonable hypothesis that intra-articular injection of corticosteroids could produce at least short-term pain relief in a different synovial joint that is causing pain. The focus of this study is on intra-articular FJIs.
Evidence on the safety of facet joint injections
Robust evidence on the complications from FJIs is very sparse. Manchikanti et al. ,15 however, in an observational study of facet joint nerve blocks (3162 LBP episodes with 15,654 lumbar nerve blocks) found no major complications. However, 73% of encounters had local bleeding, 10% had oozing, 4% had intravascular injection and 0.4% had profuse bleeding. These figures may be indicative of minor adverse event rates from FJIs. The number of people with a short-term increase in pain was not reported.
Evidence for treatment of facet joints
That pain can arise from facet joints has been proven. There are several good double-blind studies that show, in selected patients, that immediate pain relief can be obtained from injecting local anaesthetic into facet joints that is not obtained from injecting saline. 48 Indeed, demonstrating such pain relief is the gold-standard test for diagnosing facet joint pain.
Observational studies
There is a body of observational data suggesting that FJIs are an effective treatment for lumbar facet joint pain. Boswell et al. 49 identified six prospective studies (n = 253) of FJIs. All but one of these studies found positive short- and long-term effects. There are, of course, substantial limitations to using such uncontrolled observational data to inform practice because of the natural history of LBP. There is substantial improvement even in the usual-care arms in nearly all trials of chronic LBP. 50
Placebo-controlled trials
At the time this research started, there were five recent reviews of the RCT evidence for FJIs compared with a placebo or sham procedure. NICE7 and Boswell et al. 51 each identified one trial (Carette et al. 52); Chou et al. 53 and Henschke et al. 54 each identified two trials (Carette et al. ,52 n = 97; Lilius et al. ,55 n = 86). 54 Datta et al. 56 identified both of these studies but excluded them: Carette et al. ,52 unusually, because they had not excluded placebo responders, and Lilius et al. 55 because theirs was considered to be an observational study. Neither of these trials was reported by the original authors as showing a positive result.
The National Institute for Health and Care Excellence, in both 20096 and 2016,11 and Chou et al. 7 concluded that the evidence did not support the use of FJIs. Henschke et al. 54 concluded, based on very low-quality evidence, that there was no difference between FJIs with placebo and corticosteroids. Datta et al. 56 concluded that, because of the lack of evidence, there could be only a very weak positive recommendation or a recommendation not to provide FJIs. Boswell et al. ,49 on the other hand, felt that there was evidence for a positive effect from FJIs. This was based on categorising one study (Carette et al. 52) as a positive trial. This was because, although there was not a positive effect at 3 months, there was a strong positive effect in the 6-month analysis. Both Carette et al. 52 and Chou et al. 53 discounted this observation because the patients who gained a benefit at 6 months were not the same as those who had gained a benefit at 1 month and because of the large number of cointerventions in the steroid arm of the trial. Neither group felt it to be biologically plausible that a steroid injection would be effective at 6 months if it had not been effective at 1 month. Notwithstanding differences in interpretation, and the absence of statistical significance at 3 months, the point estimates for benefit from FJIs in the Carette et al. 52 study are competitive with currently recommended treatments. 57 The proportion of patients who reported substantial improvement was 42% versus 33% (95% CI –11% to 28%) and 46% versus 15% (95% CI 14% to 48%) at 3 and 6 months, respectively. These equate to numbers needed to treat (NNTs) of 11 and 5, respectively. If such NNTs were reproduced in a definitive trial, then FJIs could be an attractive addition to recommended treatments for selected people with LBP. These results, however, were obtained in participants who had already had a diagnostic FJI, suggesting that this study was carried out in patients who were most likely to benefit. Not excluding those with a placebo response to the diagnostic injections might also have reduced the apparent effect size. Carette et al. 52 also did not include those who found the diagnostic injections too painful; 7 out of 110 participants (6%) with a positive result from diagnostic injections did not want therapeutic injections because they found the process too painful.
Facet joint injections compared with other treatments
At the start of this study there were two reviews of FJIs compared with other treatments. Henschke identified five studies (n = 420) of FJIs with corticosteroids compared with other interventions. 13,16–18,58 Marks et al. 18 found that FJIs gave superior pain relief to facet nerve blocks (both used corticosteroid and lignocaine) at 1 month but not at immediate follow-up or at 3 months (n = 86). Mayer et al. 58 found no benefit from adding a FJI with local anaesthetic and corticosteroid to a home stretching exercise programme (n = 70). Fuchs et al. 13 found no significant differences between FJIs with steroid and sodium hyaluronate (n = 60). Manchikanti et al. 16 found no difference when comparing multiple medial facet nerve blocks with local anaesthetic with or without steroids (n = 84). Manchikanti et al. 17 found no differences between medial branch blocks with and without corticosteroid (n = 120). Datta et al. 56 identified two of the same trials of facet joint blocks. 16,17 and concluded that there was strong evidence for facet joint nerve blocks because of the good outcome in both groups. Celik et al. 12 reported a positive effect from FJIs in a subsequent trial (n = 80) comparing FJIs with a combination of bed rest, a non-steroidal anti-inflammatory and a muscle relaxant. In a subsequent trial (n = 100) comparing FJIs with facet joint radiofrequency denervation, Civelek et al. 59 found better immediate results from injection and better long-term results from denervation.
Cost-effectiveness
Our scoping reviews did not identify any studies of the cost-effectiveness of FJIs. FJIs require specialist facilities and experienced operators but the potential benefit observed in observational studies may well outweigh these costs.
Conclusion of initial scoping literature review
In our own 2016 review,60 we identified six relevant randomised placebo/sham-controlled trials of intra-articular FJIs. 12,52,55,58,61,62 Two studies (Lilius et al. ,55 n = 109; Carette et al. ,52 n = 97) used placebo injections into the facet joints as the control treatment, one study (Ribeiro et al. ,62 n = 60) used intramuscular corticosteroid as the control treatment, two studies (Mayer et al. ,58 n = 70; Kawu et al. ,61 n = 18) used exercise as the control treatment and one (Celik et al. ,12 n = 80) used bed rest plus analgesia and non-steroidal anti-inflammatory drugs as the control treatment. Four studies made a clinical diagnosis of facet joint pain,12,55,61 one study62 used clinical and radiographic features to make the diagnosis and two studies52,58 used diagnostic blocks. We considered the study populations, and the comparisons made, to be too heterogeneous for any robust conclusions to be drawn. 60
The quality of reporting of these trials of FJIs is generally poor and it is not a robust evidence base to inform decisions about the use of FJIs.
Measurement of outcome
The measurement of outcome in LBP trials is problematic. 63–65 Although there are well-established standard packages of outcome measures, endorsed by expert groups, the theoretical underpinning of these is poor and they may not capture those outcomes that are important to individuals. 63,66 It is of note that neither of these consensus exercises included input from patients and the decision about which outcomes should be measured in LBP trials was from a consensus of clinicians and researchers. 67 More recent recommendations from the expert advisory group, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), do include patient group consultations to explore the relevance and acceptability of outcomes used to determine recovery. 68
Studies of patients suggest that other domains, not included in current recommendations, may be of similar or even greater importance. For example, domains such as enjoyment of life and fatigue were rated most highly in a patient survey. 69 A review of outcomes measured in RCTs of LBP found that 5 out of 19 outcomes rated as important by respondents to the patient survey were never reported and a further eight measured only rarely. 70 There is a need to broaden the pool of outcome measures used beyond the established package to include measures that more effectively capture the patient perspective.
Where might facet joint injections fit in the care pathway?
For people with acute or subacute LBP or early persistent LBP, the prognosis is generally very good and there is little need for invasive treatments. Low-intensity, low-risk and therapist-delivered interventions, as recommended by NICE, are sufficient. Although there are some differences between the 2009 NICE guidance6 and the 2016 guidance,11 both support the use of therapist-delivered interventions as the first treatment approach after advice and analgesics. Those with substantial problems persisting after such interventions (typically ≥ 6 months from onset) require more intensive treatment. Persistent LBP is a complex biopsychosocial phenomenon. In those people with pain persisting beyond 6 months, in spite of good conservative care, a syndrome of chronic pain and disability will already be present. For this reason, one would not expect FJIs, on their own, to resolve the problem. Rather, pain relief obtained from a FJI may give the person with back pain the confidence and a window of opportunity to engage more fully with a rehabilitation programme.
There is a clear need for a trial to test the effectiveness of adding FJIs to usual care as recommended by NICE for the treatment of persistent LBP. It is important for this trial that it provides conclusive results. If the trial has positive results then this will be a justification for investment in this area. On the other hand, if the trial is negative then its conclusions need to be sufficiently robust that all parties to the debate on the role of therapeutic intra-articular FJIs are satisfied that the evidence does not support their use. There are methodological challenges to setting up and running such a trial; principally, these centre on the identification of people with LBP that is, at least in part, from facet joints. Our feasibility study addressed these methodological issues, tested trial processes and recruitment in an external pilot, conducted an interim analysis and identified sites to conduct the main study.
For the NHS to consider reinstating FJIs for people with otherwise non-specific LBP, a package of care including FJIs, for selected patients, needs to be an effective and cost-effective addition to BUC. This is true regardless of the result of any placebo-controlled trial of the efficacy of FJIs. The components of any overall effect (positive or negative) of FJIs will be the non-specific effects of attending for the injection (including any advice from the treating clinician), any local effects from injecting fluid into the facet joint and the specific effects of the drug/s injected. A positive efficacy study in a tightly controlled population will not necessarily transfer to a treatment that is effective in real life. Conversely, failure to show a positive effect in an efficacy study using a placebo or sham injection would not necessarily exclude the possibility that the overall package of care is effective. Furthermore, such an efficacy study will not be able to answer a question on the cost-effectiveness of adding the intervention to usual care.
The situation here is perhaps analogous to interpreting the evidence on the use of acupuncture, a treatment with a much weaker theoretical base than FJIs. There is a substantial body of evidence that acupuncture is superior to usual care for a range of common disorders, with meaningful effect sizes. The evidence that verum acupuncture is superior to a sham control is, however, much weaker, with very small apparent effect sizes. Nevertheless, acupuncture was recommended by NICE for LBP in 2009,6 although not in 2016 guidance,11 and for headaches in 2012. 71 For these reasons we propose a two-arm study testing the effect of adding FJIs to a BUC package.
Aims and objectives
The aim of the study was to carry out a feasibility study for a trial to assess the clinical effective and cost-effectiveness of intra-articular FJIs for selected patients with chronic LBP.
In this feasibility study we explored the feasibility of running a RCT to test the hypothesis that, for people with suspected facet joint pain contributing to persistent LBP, the addition of the option of FJIs, with local anaesthetic and corticosteroids, to best usual non-invasive care available from the NHS is clinically effective and cost-effective.
The specific objectives for this feasibility study were to:
-
develop, and evaluate, agreed criteria for identifying people with suspected facet joint pain
-
develop an agreed protocol for the injection of facet joints in a consistent manner
-
develop, and evaluate, a standardised control treatment deliverable in the NHS and congruent with NICE guidance (BUC)
-
develop and test systems for collecting short-term and long-term pain outcomes, including measures required for economic evaluation
-
demonstrate that recruitment to the main trial is feasible
-
collect the recruitment and outcome data required to inform sample size and number of sites needed for the main study
-
conduct a between-group trial to inform the decision on the need for a full trial
-
do a process evaluation of patient experience within the trial.
Overview of the Facet Injection Study
This report is split into a number of key sections. The Facet Injection Study (FIS) includes a considerable body of development work to inform the study processes, the diagnoses and the feasibility study’s control, intervention and injection technique as well as the pilot trial. The feasibility trial was terminated by the funder because of poor recruitment.
First, we present this development work, which was mostly informed by a consensus conference. We summarise the methodologies used and the outcomes that informed the development of the trial protocol, a diagnostic manual, a ‘BUC manual’ and an injection manual. Reports of the full consensus methods, results and conclusions are available elsewhere as online resources. 72–76 Second, we report the experience of running the feasibility RCT research methods, including the process evaluation, and the outcome/results of the study. The final section, the discussion and conclusion, is a synthesis of all of the results and outcomes.
Patient and public involvement
We are not able to report on patient and public involvement (PPI) in the framing of the original commissioning brief from the HTA programme. Throughout the development of this proposal, in response to the HTA brief, and subsequent conduct of the study there has been input from PPI representatives and groups. All of the project’s development work and interpretation of the results have had input from PPI representatives.
-
Two very active co-applicant PPI representatives on the studies trial management group played an important part in the development of study material and intervention development at the consensus conference, and one provided a reflection of their experience working on the trial (see Appendix 1).
-
One PPI representative on the trial steering committee who gave clear guidance throughout.
-
Six lay representatives participated in our consensus meeting. 74,76
We also acknowledge the help and support provided by University/User Teaching and Research Action Partnership (UNTRAP) at the University of Warwick, which provides training and support to ensure effective PPI.
Chapter 2 Consensus: developing the study protocols
A four-stage process was adopted to ensure that the FIS protocol was robust and informed by current evidence and expert opinion, was acceptable to the academic community and practising clinicians and reflected NHS practice. First, the FIS team identified key design considerations that are of vital importance for the production of robust and acceptable evidence on an implementable FJI programme. Second, an evidence review of each design consideration was conducted using systematic methodology. Third, an evidence document was prepared that contextualised the pragmatic FIS, outlined the methodological challenges of designing a credible pragmatic trial and presented the outputs from the evidence reviews. Fourth, using the evidence document as a delegate pack, the FIS design considerations were considered by a consensus conference of clinicians, experts, academics and lay representatives.
Methods
Before the conference
The three stages of the study are presented in Figure 1: (1) scoping review and identification of key design considerations, (2) evidence reviews and (3) consensus conference.
FIGURE 1.
Facet Injection Study protocol development process. Adapted with permission from Mars et al. 26
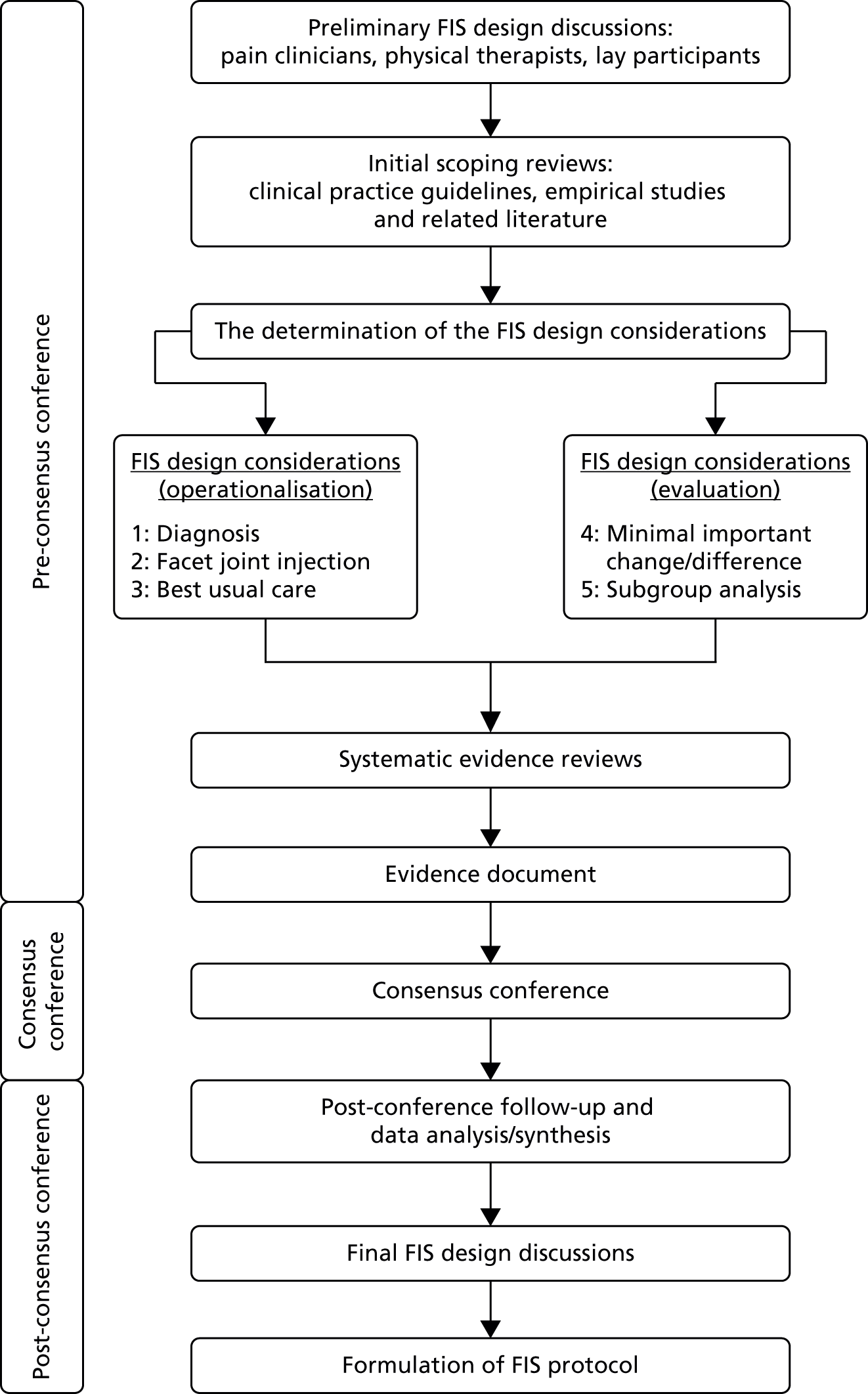
Scoping reviews and formulation of key design considerations
Our study team includes pain clinicians, physical therapists, radiologist and lay representatives, as well as research methodologists. Based on scoping reviews of clinical practice guidelines, empirical studies and related literature and team discussion, five design considerations for the proposed trial were identified and questions posed, as follows.
-
Diagnosis: what is the best choice of clinical assessment to identify patients with facet joint pain?
-
Injection technique: what is the agreed technique for the therapeutic intra-articular injection of facet joints?
-
BUC: what is the optimal conservative management/rehabilitation for patients with LBP for whom facet joints have been identified as a contributing source of symptoms?
-
Between-group differences: what is the difference in magnitude of response between treatment and control groups that should be considered large enough to establish the scientific or therapeutic importance of the results?
-
A priori subgroup analyses: which variable(s) should be used for a priori subgroup analyses in the main trial?
The consensus conference
Potential conference participants were invited through relevant professional and lay organisations (Box 1). We sought participation from experts from across the UK. By expert, we mean that participants were professionals or lay people with an interest in, or experience of, back pain, its treatment and, in particular, its treatment with therapeutic intra-articular FJIs. The invitation was to a 1-day conference with no attendance charge and travel expenses were reimbursed. This was held at the University of Warwick on 27 June 2014.
-
Professors/consultants in pain management via Binley mailing services (www.binleys.com) (accessed 22 February 2014).
-
British Association of Spinal Surgeons (www.spinesurgeons.ac.uk) (accessed 3 March 2014).
-
Association of British Neurologists (www.theabn.org) (accessed 3 March 2014).
-
British Society of Skeletal Radiologists (www.bssr.org.uk) (accessed 3 March 2014).
-
British Society of Interventional Radiologists (www.bsir.org) (accessed 5 March 2014).
-
Primary Care Rheumatology Society (www.pcrsociety.org) (accessed 19 March 2014).
-
Council for Allied Health Professions Research (www.csp.org.uk/professional-union/research/networking-support/council-allied-health-professions-research) (accessed 28 March 2014).
-
Midlands Health Psychology Network (www.mhpn.co.uk) (accessed 19 March 2014).
-
Back Care – a lay advocacy and support organisation (www.backcare.org.uk) (accessed 14 March 2014).
-
UNTRAP is a partnership between users of health and social care services and carers, the University of Warwick and the NHS (www2.warwick.ac.uk/fac/cross_fac/healthatwarwick/untrap) (accessed 10 March 2014).
Adapted with permission from Mars et al. 26
Approximately 1 week before the consensus conference, a document consisting of the design considerations and related evidence was sent to all those registered to attend.
We used nominal group technique to gain consensus. This allows for discussion, while avoiding individuals or groups dominating the consensus process, and allows participants to draw on available evidence and expertise. 79 We started the conference with a brief reminder of the key design considerations and evidence. We then held 15 small group consensus sessions, each lasting 1 hour, with five groups meeting in parallel at any one time (Figure 2). Small group results were fed back to a plenary session, in which final consensus was reached. With participant consent, all sessions were audio-recorded for reference during analysis. Participants were randomly assigned to small group sessions stratified by profession (approximately 10–12 per group) with each participant discussing three different design considerations. Each small group had a trained facilitator, a scribe and a subject expert from our team. The subject expert did not participate in the discussions but answered questions about technical issues when invited to by the facilitator. Discussions centred on the particular design consideration, with the suggested ‘protocol’ as a starting point when appropriate. Nominal group technique was adapted to the design consideration under discussion as described in Figure 2. Each participant confidentially ranked the acceptable approaches identified by the group. Results were collated by the scribes.
FIGURE 2.
A diagrammatic representation of the consensus process. Adapted with permission from Mars et al. 26 NGT, nominal group technique.

In the sections following, we outline the five design considerations and how the discussions on these were conducted during the consensus conference day. More detailed information is provided in three documents:
Diagnosis: assessment for facet joint pain
Participants were presented with summaries of the information about diagnosis from the evidence reviews and asked to suggest components of clinical assessment (Box 2). These were then discussed to identify any that were similar and then grouped as sets forming complete clinical assessments. Participants then ranked the clinical assessments.
-
Current empirical evidence on the clinical diagnosis of facet joint pain is limited.
-
Some signs/symptoms or aggravating factors have been suggested to be indicative of facet joint pain but their use is not supported by the research evidence.
-
Small-scale and provisional research suggests that a regular compression pattern when testing combined movements may have some validity for identifying facet joint pain.
-
The ability to identify patients where the facet joints are a suspected source of pain is important as it is one of the entry criteria for enrolment in the study.
-
Being able to accurately identify a relatively homogenous group of back pain patients with facet joint pain will allow a true evaluation of the potential benefits of FJIs.
Injection technique
Participants were presented with summaries of the information about injection technique from the evidence reviews (Box 3).
-
Key educational/instructional texts for FJI describe details of each author’s technique. A broad methodology emerges that varies in detail within a narrow range of options.
-
For this study we need to achieve a single detailed process for therapeutic injection of lumbar facet joints that is acceptable to the professional community and can be applied consistently across all participating study centres.
A potential protocol was presented to them to aid with discussions (Box 4). This included 14 different aspects of the process of injection. For each aspect a proposal was made for the technique to be used. Group members first identified which of these they considered acceptable. After collation of these results, the facilitator invited discussion in turn on each of the aspects for which there was not agreement on acceptability. For each of these, alternative processes were identified and then ranked.
When they attend for injection the operator will make a brief clinical assessment to satisfy themselves that FJIs are appropriate. Consent for the procedure will be obtained and the current pre-injection risk management procedures of the participating study centres will be adhered to. The operator will then inject the facet joint(s). We expect to inject up to six facet joints in each individual (L3/L4, L4/L5 and L5/S1) bilaterally. However, when, on clinical assessment, there is unilateral pain or involvement of only some levels the operator may choose to do unilateral injection or be selective on levels injected. We expect that everyone should receive at least two injections. This pragmatic approach reflects what actually happens in NHS practice. This approach is consistent with that used in trials of other complex interventions for LBP, for example manual therapy or a cognitive–behavioural approach, where practitioners choose from a limited range of options based on their clinical assessment of the patient.
Procedure to position the needle-
We do not expect to use intravenous sedation.
-
Prone position with pillow under abdomen to flatten lumbar lordosis.
-
Intravenous access, resuscitation equipment available.
-
Skin cleansing with 0.5% or 2% chlorhexidine in alcohol, sterile drapes. (Some clinicians think that 2% chlorhexidine is neurotoxic and like to use 0.5% as skin cleansing before nerve blocks. On the other hand, 2% chlorhexidine is recommended by the control of infection experts as optimum skin cleansing before intravenous cannulation and may be preferred in some trusts.)
-
Radiography (C-arm fluoroscopy) oblique view to visualise joint.
-
The dose of radiation used will be adequate to visualise the joint while minimising X-ray exposure.
-
Skin weal at needle entry point: 1% lidocaine via a 25-gauge hypodermic needle.
-
22-gauge × 3.5-in (0.7 × 90 mm) needle with Quincke type point: guide needle to joint cleft.
-
Entry to the joint cleft may be indicated by radiograph appearance: observation of the needle tip on the joint line with medial/lateral movement of the X-ray beam to cause parallax shift.
-
If entry to the joint has not been achieved after repositioning the needle twice, the needle will be positioned on the joint line without further attempts at capsular puncture.
-
Aspiration should be negative for blood or cerebrospinal fluid.
-
We do not expect to use contrast medium because of the restriction of available joint volume and the risk of serious allergic reactions.
-
The immediate post-injection advice will be in accordance with the current procedures of the participating study centre.
-
Pre-filled syringes containing 7.5 mg of bupivacaine and 20 mg of methyl prednisolone in total volume; 2 ml will be used for each joint.
-
The full volume, 2 ml, will be injected through the spinal needle placed into each joint. Some facet joints may not be sufficiently large to take this volume of injectate meaning in practice that the injections will be intra- and periarticular. This reflects what we believe to be current practice in the UK.
-
Resistance to injection may occur because of abutment of the needle bevel to a surface or because of filling of the intra-articular space:
-
Force should not be used.
-
The needle should first be rotated 90° and a further attempt at injection made.
-
If, after two further 90° rotations resistance to injection persists or if, after successful injection of a part-volume resistance develops, gentle pressure should be maintained on the plunger and the needle withdrawn gradually until resistance to injection falls.
-
-
After completion of the injection, the needle is removed and a sterile dressing applied.
Adapted with permission from Mars et al. 26
Best usual care
Group participants were asked to suggest what treatment approaches should be included in the package from which a therapist could pick and tailor treatment for each patient. This could include manual therapy, home exercises and cognitive approaches. The content of the initial assessment and the number and duration of individual treatment sessions was also discussed. A basic outline and protocol was presented to the groups to aid in the discussions (Box 5). Group participants identified which aspects of the proposed BUC they considered acceptable. After collation of these results, the facilitator invited discussion in turn on each of the treatment approaches for which there was no agreement on acceptability. The group voted on inclusion/exclusion of treatment approaches from the ‘toolbox’ and assessment session content. They ranked alternatives for the number/duration of individual treatment sessions.
Initial assessment of 60 minutes. Assessment includes discussion of expectations, fear avoidance and self-efficacy to assess any perceived challenges and barriers that patients feel may be preventing them from engaging in self-management of chronic pain and to allow subsequent treatment sessions to be tailored to individual need. For the intervention group, the FJIs are given in the period between this first assessment and the first follow-up appointment.
Individual sessionsFive further sessions each of 30 minutes incorporating elements of manual therapy, pacing, motor control retraining, therapeutic exercise, soft tissue stretches/release, postural and general advice, goal-setting and challenging negative thoughts associated with physical activity and chronic LBP, as appropriate.
Manual therapy intervention may include-
Passive accessory intervertebral movements: either central or unilateral applied to either the symptomatic level or the level adjacent depending on the severity and irritability.
-
Soft tissue release/trigger point release/muscle energy techniques: as indicated in order to facilitate motor control retraining and effectiveness of manual therapy.
-
Manipulation treatment: as indicated.
-
Active exercise: to increase mobility, improved motor control and core stability, improve overall strength and stretch any tight muscle groups.
-
Mobility techniques: such as flexion in lying, pelvic tilt, side glides in standing and gym ball exercises.
-
Motor control retraining exercises (depending on individual assessments): this may include all muscles involved in core stabilising of the spine and also reducing activity in more superficial muscles that have been shown to become overactive in the presence of LBP. Treatment focuses on retraining the ‘coactivation’ pattern of stabilising muscles such as transversus abdominus and lumbar multifidus. This includes retraining of lumbar multifidus, as it is innervated by the medial branch and becomes inhibited ipsilateral to the pain in chronic back pain conditions. There is also evidence that specific retraining of ‘core muscles’ can improve pain and disability in some back pain patients.
-
Passive stretches: muscle groups identified during assessment as tight or overactive may be stretched within the therapy sessions in order to allow for improved spinal mobility and facilitate motor control retraining. Stretches are taught as part of the home exercise regime.
-
Bespoke exercise programme to complement face-to-face sessions: prescription to include frequency, dose, repetitions and progressions.
-
Advice on positions of ease, strategies to use in event of a ‘flare-up’ and strategies to reduce increasing pain: for example use of pelvic tilt prior to standing after prolonged sitting.
-
Pacing: including discussion of what is meant by pacing, relevance of pacing and methods to incorporate pacing into daily activities such as pacing by time, pacing by numbers or pacing by grading activities.
-
Goal-setting: including discussion of setting mutually agreed goals related to functional activities as well as general daily goals and long-term goals. Goals agreed between the physiotherapist and patient participant. In line with a cognitive–behavioural approach, goals may be based on Specific, Measurable, Achievable, Realistic and have a Time frame (a date for competition) (SMART) principles.
-
Challenging negative automatic thoughts (cognitive restructuring): including working with patients to identify particular negative thoughts they may have in relation to physical activity, fear avoidance and helping patients challenge their thoughts and adapt positive coping strategies.
Homework tasks between each session tailored to each individual and what is discussed during the session. For example, using pacing on a particular activity identified by the patient, keeping a diary of negative automatic thoughts that may trigger anxieties about movement or exercise and pain.
Adapted with permission from Mars et al. 26
Between-group difference
Participants were asked to consider each of the questions based on the evidence with which they were provided. These were quite technical questions, and the ‘expert’ in the room provided much needed support and clarification. Participants provided suggestions that were then voted on and/or rank-ordered. This particular topic is discussed in more detail in Chapter 3.
Subgroups for analysis
Participants were presented with a list of potential moderators from the evidence reviews. These were discussed and edited by either the addition of new items or the removal of an item (through agreement within the group). Items were ranked in order of preference/importance.
The results from all the small group sessions were collated and presented to the plenary session. When small group results were consistent, no further discussion took place. When there were inconsistencies between small group results, these were discussed and further ranking undertaken, collated and reported to the plenary session. We discussed and reranked issues until one option was clearly the preferred option and there was no objection to its adoption from conference delegates. We considered consensus to have been reached when there was 75% agreement.
Post conference
All results were checked and verified from all small group sessions and the plenary session. A small number of errors were found in the collation of rankings. The team therefore contacted participants with relevant expertise via e-mail to clarify and reach a consensus on these items.
Results
Fifty-seven people confirmed their attendance, of whom 52 attended on the day. Table 1 summarises their professional or lay roles. Of the 52 attendees, three asked not to be associated with the final consensus document: one was not happy with the way the day was organised and with the involvement of laypersons, one did not agree with having a physiotherapist-led BUC package and one noted no conflict but stated that they felt unable to contribute as they were not statistically minded. All other attendees agreed to being identified as part of the consensus group.
| Specialty/role | Number of attendees |
|---|---|
| Pain consultants and physicians | 19 |
| Anaesthetists | 6 |
| Physiotherapist or physical specialists | 12 |
| Academics | 4 |
| Psychologists | 3 |
| Radiographers | 2 |
| Lay representatives | 6 |
Evidence reviews
A full evidence document was produced for each delegate, which was distributed electronically before the day and provided in hard copy on the day. 76 This included tabulated results of the searches, brief summaries and, in several cases, suggested ‘protocols’.
Consensus conference
We present a brief summary of the results from the consensus conference for each of the five design considerations. Full data are available. 74 Results related to the first three of these design considerations (diagnosis, injection technique and BUC) have been published elsewhere and data and a number of tables have been reproduced here with the full permission of the editors.
Diagnosis
The four ‘diagnosis’ group sessions all approached the problem in different ways. In three of the groups lists were generated and items were then ranked, with the top-ranked items going forward to the plenary discussions. However, in one group there was considerable discussion and the group agreed/proposed a diagnostic pathway. This was taken forward to the plenary session. Key components of diagnostic assessment that were discussed in all groups included increased pain on extension/rotation and extension/lateral flexion and no pain on rising from flexion. In addition, the following were considered: no radicular symptoms, no sacroiliac joint pain on pain provocation testing and flexion less painful than extension. Consensus was not reached on the day.
Injection technique: the process of therapeutic intra-articular facet joint injection
There were 14 aspects of the injection process for the groups to consider. In each group a number of these were considered acceptable without discussion, although these varied between the groups. All 14 aspects were brought to the plenary session, but 10 were discussed very briefly before consensus was reached. The following items prompted considerable discussion and were ranked: administration of local anaesthetic and its composition, confirmation of needle position, injectate volume and injectate composition. Owing to errors in ranking identified, we undertook post-conference ranking of injectate volume and composition among participants with experience of injecting.
Best usual care
All four of the BUC group discussions followed a similar format. First, the group discussed and voted on agreement/disagreement with the suggested protocol items. The groups then proposed and voted on new items for inclusion. Comprehensive packages were proposed in all groups and these were taken forward to the afternoon plenary session. Although a consensus was reached regarding the key components to be included, some clarification was sought after the conference with participants who had experience of treatment delivery.
Size of signal
Table 2 summarises the results of the consensus meeting discussions on the size of the signal. There was considerable discussion in these groups and some questions were not covered because of time constraints.
| Question | Group 1 (total votes) | Group 2 (total votes) | |
|---|---|---|---|
| 1.1: at 3 months, should we be seeking a mean between-group difference in change scores that is smaller/the same/or larger than that observed for the trials of manual therapy? | |||
| A | Smaller | 1 | 0 |
| B | Larger | 6 | 9 |
| C | Same | 2 | 2 |
| 1.1a: additional question asked in group 1 – should we be asking for the number who got better/the difference in benefit? | |||
| A | Smaller | 2 | N/A |
| B | Larger | 4 | N/A |
| C | Same | 3 | N/A |
| 1.2: informed by the MID units calculated for the trials of manual therapy (supporting evidence), at 3 months should we be seeking a small (< 0.5), medium (0.5–1.0) or large (> 1.0) MID unit as proof of important difference? | |||
| A | Small (< 0.5) | 8 | 2 |
| B | Medium (0.5–1.0) | 1 | 5 |
| C | Larger (> 1.0) | 0 | 1 |
| 1.3: what magnitude of reduction in pain after the injection constitutes immediate pain relief? | Group 1 (ranking)a | ||
| A | 80% | 2 | N/A |
| B | > 50% | 4 | N/A |
| C | 0% | 3 | N/A |
| D | 60% | 1 | N/A |
The results from the morning sessions were taken forward to the afternoon plenary session, in which there was a considerable amount of discussion about this topic. As there was a difference of opinion from the morning session for question 1.2, the whole group were asked to vote on the two items, (A) small (< 0.5) and (B) medium (0.5–1.0). There were 48 out of 49 valid votes, with the outcome of eight votes for small (< 0.5) and 40 votes for medium.
An additional question was posed: ‘What difference in those who achieve minimally important change (MIC) is good?’. The group was asked to vote on three options: larger, the same and smaller. In total, 44 out of 49 ballots were valid, with the result of 22 votes for larger, nine votes for the same and 13 votes for smaller.
During discussion, the question of measuring pain relief at 1 hour in the study was raised. A vote was therefore held that asked participants ‘should we assess pain at 1 hour?’. The result was inconclusive, with a total of 46 out of 49 valid votes: 22 said yes and 24 said no.
Finally, the group revisited question 1.3: ‘What magnitude of reduction in pain after the injection constitutes immediate pain relief?’. Four options were suggested (some extracted from the morning session) and 46 out of 48 valid votes were included. There were four votes for 30%, 22 votes for 50%, 12 votes for 60% and eight votes for 80%.
Subgroup analysis
There was only one group discussion on this topic. The participants were presented with current evidence and asked to consider the variables that they felt were important. Lists were generated and items collapsed into categories. This resulted in a list of 10 variables, which were then ranked in order of importance.
Table 3 summarises the result from this group (concerning subgroup analysis).
| Final rank | Identifier | Variables |
|---|---|---|
| 1 | A | Severity |
| 2 | D | Anxiety/depression |
| 3 | M | Do you think you need an injection to get better |
| 4 | E | Treatment expectations |
| 5 | H | Back beliefs |
| 6 | G | Quality of life |
| 7 | B | Age |
| 8 | F | Self-efficacy |
| = 9 | L | Forward flexion pain (yes/no) |
| = 9 | N | Does the therapist think the treatment is effective |
The top five ranked items were presented to the plenary session as the adopted items (see Table 3).
Post conference
Clarifications were needed for three of the design considerations (diagnosis, the process of FJI and the BUC package).
Diagnosis
In order to confirm the diagnostic criteria for the study, 45 of the professional delegates were e-mailed to ask the following question:
We would like you to review the following text and confirm if the suggested clinical diagnostic criteria proposed for the study is ‘acceptable’? Stating ‘YES’ or ’NO’.
Increased pain unilaterally or bilaterally, on lumbar paraspinal palpation. AND. Increased LBP on one or more of the following; Extension (more than flexion), Rotation, extension/side flexion, extension/rotation. AND. No radicular symptoms (defined as pain radiating below the knee). AND No sacroiliac joint pain elicited using a pain provocation tests.
Responses received: acceptable, n = 23; yes, n = 22, no, n = 1.
Box 6 outlines the diagnostic criteria that emerged from the consensus and which went on to be used in the study.
There is considerable diagnostic uncertainty about how to identify people with pain of facet joint origin among the wider chronic LBP population. Therefore, the diagnostic criteria used in this trial have been drawn from the available evidence base and following consensus gained from a range of experts and clinicians.
Diagnostic criteria for trialA summary of the diagnostic criteria is shown below. Criteria 1 and 2 cover the issue of presence of pain on palpation or symptom reproduction on movement testing. The second two criteria relate to the absence of symptoms, namely radicular symptoms and sacroiliac pain.
-
Increased pain unilaterally or bilaterally, on lumbar paraspinal palpation.
AND
-
Increased LBP on one or more of the following;
AND
-
No radicular symptoms (defined as pain radiating below the knee or objective neurological signs above the kneeb).
AND
-
No sacroiliac joint pain elicited using a pain provocation test.
The process of facet joint injection
Following the consensus conference there was uncertainty about the injectate to be used in the study. Six options were sent, via e-mail, to 27 pain consultants, anaesthetists or professionals (delegates) who indicated that they were responsible for injection. We received 11 responses; the results can be seen in Table 4. Box 7 provides a summary of the final agreed injection protocol.
| Injectate options | Preferred option | Acceptable | |
|---|---|---|---|
| Yes | No | ||
| Triamcinolone (10 mg/ml)/Levobupivacaine (2.5 mg/ml) | 4 | 5 | 2 |
| Triamcinolone (10 mg/ml)/levobupivacaine (5.0 mg/ml) | 4 | 5 | 0 |
| Triamcinolone (10 mg/ml)/levobupivacaine (7.5 mg/ml) | 1 | 6 | 3 |
| Triamcinolone (20 mg/ml)/levobupivacaine (3.75 mg/ml) | 1 | 3 | 5 |
| Triamcinolone (20 mg/ml)/levobupivacaine (7.5 mg/ml) | 0 | 4 | 4 |
| Triamcinolone (20-mg/ml)/levobupivacaine (11.25-mg/ml) | 0 | 2 | 6 |
Prior to the study injection procedure, following normal local trust clinical practice, the investigator will obtain informed consent for the injection from the participant prior to injecting the facet joints . . .
Skin cleansing with chlorhexidine 0.5% or 2% in alcohol sterile drapes are recommended to be used.
No intravenous sedation is required.
Prone position with measures to reduce the lumbar lordosis, for example a pillow under the abdomen.
Intravenous accessRadiography (C-arm fluoroscopy or other suitable equipment) for visualisation of the joint.
The dose of radiation will be adequate to visualise the joint while minimising X-ray exposure.
Entry to the joint cleft may be indicated by radiograph appearance. Medial/lateral movement of the X-ray beam with intermittent screening to cause parallax shift may be used . . .
For the FIS, contrast will not be administered.
InjectionLocal anaesthesia at needle entry point: 1% lidocaine via 25-gauge hypodermic needle . . .
. . . The investigator responsible for the injection will prepare the injection syringe to contain 1 ml of Levobupivacaine (5 mg/ml) and 1 ml of Triamcinolone (10 mg/ml) in total volume; 2 ml will be used for each joint . . .
. . . Up to six facet joints (L3/L4, L4/L5 and L5/S1) bilaterally in each participant will be injected. However, when on clinical assessment there is unilateral pain or involvement of only some levels, the investigator may choose to do unilateral injection or be selective on levels injected.
The full volume, 2 ml, will be injected through the spinal needle placed into each joint. Some facet joints may not be sufficiently large to take this volume of injectate, meaning, in practice, that the injections will be intra- and periarticular. This reflects what we believe to be current practice in the UK.
If there is resistance to injection may occur because of abutment of the needle bevel to a surface or because of filling of the intra-articular space. Force should not be used.
The needle should first be rotated 90° and a further attempt at injection made.
If, after two further 90° rotations, resistance to injection persists or if, after successful injection of a part-volume resistance develops, gentle pressure should be maintained on the plunger and the needle withdrawn gradually until resistance to injection falls.
After completion of the injection the needle is removed and a sterile dressing applied.
Best usual-care package
Confirmation of the number and duration of sessions was sought post conference. We e-mailed 15 delegates who were physiotherapists, extended scope practitioners or clinical/health psychologists. Two alternatives, (1) and (2), were sent and delegates were asked to state a preferred option and to also say if they felt that it was acceptable or not.
There were 12 responses:
-
one session of 60 minutes plus five sessions of 30 minutes (nine preferred, seven yes, zero no)
-
up to six sessions of 45 minutes each (three preferred, six yes, one no).
Among the 12 responses reported, two responders answered that both options were acceptable, one responder provided only a preference and did not state whether the options were acceptable and two responders preferred option (1) and thought that this was the acceptable option. Box 8 summarises the agreed BUC package agreed.
Patients initially undergo a thorough physical assessment based on the principles of Maitland manual therapy assessment and clinical reasoning.
Sessions 2–6 (30 minutes each)The aim of BUC for this trial is to provide a fully integrated psychological and physical rehabilitation. It is important therefore to integrate the two elements of care as far as possible so that participants do not see them as ‘stand alone’.
Treatment should be directed at pain arising from the facet joint. Physiotherapists should use their full range of skills and knowledge in constructing a personalised rehabilitation programme using the comprehensive ‘tool kit’ provided.
The section below outlines component parts of the ‘tool kit’. The BUC manual provides full instructions and examples for the physiotherapists to use.
Acceptance (session 1), goal-setting (session 1 or 2), pacing (session 1 or 2) and challenging negative thoughts and mindfulness Manual therapy| Kaltenborn. |
| McKenzie. |
| Maitland. |
| Cyriax. |
| Osteopathic techniques. |
| Mulligans. |
| (Natural apophyseal glides/ sustained natural apophyseal glides/movement valued manual therapies.) |
| Other. |
| Myofascial. |
| Trigger point. |
| Soft tissue massage. |
| Manipulation. |
| Soft tissue release. |
| Other. |
| Specific. |
| Motor control retraining/core stability. |
| Cardiovascular. |
| Strength. |
| Stretches. |
| Other. |
| Pain terminology, mechanisms and pathways. |
| Activities of daily living. |
| Work and ergonomics. |
| Lifestyle changes. |
| Management of flare ups and changing. |
| Symptoms. |
| Paced home exercises. |
| Other. |
Summary/conclusions
We have established consensus from health professionals concerned with the treatment of facet joint pain in the UK on the assessment of facet joint pain, injection of facet joints, BUC, minimal important difference and subgroup analysis for use in a feasibility study for a proposed clinical trial of FJIs. The process was evidence based and open to all those with a professional interest in this topic. It included lay participants and was undertaken in a transparent way. The use or not of FJI is controversial internationally and, therefore, consensus and transparency is essential for the design of the proposed trial of FJIs to ensure that the results are acceptable to the whole pain treatment community. The results of the consensus process have provided much-needed clarity into key components of the study and have shaped the protocol for the subsequent feasibility RCT.
Chapter 3 Interpreting treatment effects: ‘What is the minimal between-group difference in change scores necessary for facet joint injection to be considered worthwhile?’
Background
The important aspects of LBP and how it impacts individuals’ ability to live their life can be assessed using well-developed patient-reported outcome measures (PROMs). 63,82 Increasingly, such measures, for example the Roland–Morris Disability Questionnaire (RMDQ)83 and the Pain Numerical Rating Scale (Pain-NRS),84 are used as primary or secondary outcomes in clinical trials of LBP management. 57,85,86 However, the effect size even in positive effectiveness trials comparing physiotherapist-delivered interventions with ‘usual care’ are typically modest. These may be expressed either as natural units of measures such as the RMDQ (typically 1–2 points on a 24-point scale) or as a standardised mean difference [between-groups difference/baseline standard deviation (SD), typically 0.2–0.4 points in trials with positive results]. By way of benchmarking, there is consensus that a 5-point change, or a 30% improvement from baseline, in the RMDQ represents a worthwhile benefit to an individual patient. 87 Although determination of the clinical relevance or meaningfulness of these scores is crucial to determining if treatment is worthwhile,88 interpretation guidance is largely unavailable. 89 Moreover, the lack of interpretation guidance is often a barrier to appropriate utilisation of trial data. 90
There are two aspects of score interpretation relevant to clinical trials: (1) between-groups difference or the ‘minimally important difference’ (MID) and (2) the within-individual change (‘MIC’) or ‘responder definition’. 91,92 International consensus for the reporting of continuous patient-reported outcomes in LBP trials supports the reporting of:
-
between-group differences, with guidance for MID when available
-
a responder analysis that adopts an empirically derived MIC within patients, reporting both proportion improved and deteriorated according to a predefined responder definition
-
a calculation of the NNTs. 89 However, the authors acknowledge that guidance for MID is often lacking and is difficult to estimate empirically.
The MID compares the average change from baseline across all patients in treatment and control groups92,93 and has been defined as the difference in magnitude of response between treatment and control groups that should be considered large enough to establish scientific or therapeutic importance of the results. It is usually reported through the comparison of summary measures (e.g. mean between-group differences for continuous measures). 91 Although it is common to declare that there is a single MID for an outcome measure, in reality one might expect that the MID for an invasive procedure would be larger than that for a low-risk educational intervention. Analytical approaches that report statistical significance of score change fail to convey the clinical value or the patient perspective on the value of the difference. 88,94,95 An alternative approach that takes into consideration meaningful individual-level change is afforded by the calculation of the MID unit. 96,97 The MID unit divides the between-group difference found in a trial by the established MIC for the outcome of interest: estimates of < 0.5 MID units suggest that it is increasingly less likely that an appreciable number of patients will achieve important benefits from treatment, whereas values between 0.5 and 1.0 suggest that treatment may benefit an appreciable number of patients. This approach, increasingly used within meta-analyses of trial evidence,96,97 grounds the calculation in clinical reality (a within-person individual change) while tailoring the MID to the nature of the intervention. Interpretation provides an evaluation of whether or not an appreciable number of patients achieve clinically important benefits, with MID units of < 1 reflecting increasingly lower likelihood of benefit.
Minimally important difference units have been applied in meta-analyses supporting new treatment guidelines for knee osteoarthritis. 98 The authors concluded that for MID units < 0.5 there was a low likelihood that an appreciable number of patients achieved clinically important benefits. Although MIC guidance for several legacy measures in LBP exists,87 the application of MID units that incorporate individual MIC values has not been described. Informed by calculation of the MID unit, we sought to provide guidance for the minimal between-group difference in change scores (MID) for the RMDQ and/or the Pain-NRS necessary for FJI to be considered worthwhile.
Methods
There were three stages of this work package:
-
meta-analysis of published data from large trials of physiotherapist-delivered interventions for chronic LBP
-
calculation of the between-group differences of change scores and the MID unit from a large UK trial [the UK Back pain Exercise And Manipulation (BEAM) trial]86
-
consensus meeting – score interpretation.
Meta-analysis of data from large trials of physiotherapist-delivered interventions for chronic low back pain
To gain an indication of the likely magnitude of MID unit differences that may be expected in a positive study of an intervention to treat LBP, we conducted a meta-analysis using published data from large trials with which we were already familiar. We selected studies that we had previously included in a database of individual patient data from RCTs of physiotherapist-delivered interventions for back pain. 25 Studies were included if they included data on ≥ 300 participants and had used the RMDQ. 25 Our original intention was to also include a meta-analysis of Pain-NRSs; however, none of the studies in our sampling frame included both a RMDQ and useable Pain-NRS data. The purpose of this analysis was to obtain illustrative data for the consensus meeting rather than to systematically report all studies meeting these criteria. For this reason, we made the pragmatic decision to include only those studies that we had assessed as part of this previous project.
Full-text versions of the included studies were retrieved. Two reviewers extracted study-level information from the included articles: a standardised data extraction list included study-specific information (authors and trial population) and outcome-specific information [primary and secondary outcomes, mean (SD) between-group differences in scores at baseline and follow-up and MIC if calculated].
The mean between-group differences in change scores for the RMDQ and across treatment groups and at different time points were reported for each trial. Each value was compared with the known MIC for the RMDQ. 87
For all included studies, MID units were calculated using published MIC guidance. MID units were calculated per trial and as an overall value (all trials combined) at 3 and 12 months. As two MIC values are recommended for the RMDQ, two forms of MID unit were calculated, reflecting the MIC score change and MIC 30% improvement from baseline. 87
Calculation of the between-group differences of change scores and the minimally important difference unit from a large UK trial (UK Back pain Exercise And Manipulation trial)
We carried out a further analysis of data from a large UK trial of therapist-delivered interventions (n = 1169) (the UK BEAM trial86). Using individual patient data, we were able to obtain Pain-NRS data for pain today as a single item extracted from the Modified Von Korff pain grade scale. 86,99 For this analysis, all three active treatments in UK BEAM trial were pooled for comparison with the control intervention. Scores for the RMDQ and Pain-NRS were adjusted for sex, age and scores at baseline. Mean between-group differences of change in RMDQ and Pain-NRS scores and MID units (30% and score change) at 4 weeks, 3 months and 12 months were calculated.
Consensus meeting: score interpretation
Finally, a consensus meeting was held. 76 The results from the two previous stages were used to inform a 1-day consensus meeting of clinical and academic experts and lay representatives who sought to determine and make recommendations for between-group score interpretation. All participants received an evidence synthesis in advance of the meeting. A nominal group technique was adopted to gain consensus. Following a brief reminder of key evaluation considerations and evidence, delegates were randomly assigned to small group sessions that were stratified by profession (approximately 10–12 per group). Discussions lasted up to 1 hour. Each group had a trained facilitator, a scribe and a subject expert. Participants were invited to consider the following overall question: what is the difference in magnitude of response between treatment and control groups that should be considered large enough to establish the scientific or therapeutic importance of the results?
Specific subquestions, pertaining to the RMDQ and/or Pain-NRS, included:
-
At 3 months should we be seeking a mean between-group difference in change scores that is smaller than/the same/or larger than that observed for the trials of physiotherapy?
-
Informed by the MID units calculated for the trials of physiotherapy, at 3 months should we be seeking a small (< 0.5), medium (0.5–1.0) or large (> 1.0) MID unit as proof of important difference?
The results from all small group sessions were collated and presented during the final plenary session, in which there was considerable discussion on this topic. When there were inconsistencies between small groups, these were discussed and further ranking undertaken. Final consensus was sought for all questions.
Results
Meta-analysis of data from large trials of physiotherapist-delivered interventions for chronic low back pain
Following application of our inclusion criteria, three out of five shortlisted large trials were included in the analysis (Table 5).
| Author (year) | Treatment | Number at randomisation | Age (years), mean (SD) | Female, n (%) | RMDQ | Pain (Pain-NRS) |
|---|---|---|---|---|---|---|
| Lamb et al. (2010)100 | Control | 233 | 54 (14.9) | 142 (61) | Yes | No (Von Korff)99 |
| Advice plus cognitive–behavioural therapy | 468 | 53 (14.6) | 278 (59) | |||
| UK BEAM trial (2004)86 | Control | 338 | 43 (10.6) | 178 (53) | Yes | No (Von Korff)99 |
| BUC and exercise | 310 | 44 (11.0) | 170 (55) | |||
| BUC and manipulation | 353 | 43 (11.4) | 212 (60) | |||
| BUC, manipulation and exercise | 333 | 43 (11.9) | 189 (57) | |||
| Hay et al. (2005)101 | Manual physiotherapy | 201 | 41 (11.6) | 110 (55) | Yes | No (VAS) |
| Brief pain management programme | 201 | 40 (12.0) | 100 (50) |
Mean between-group differences for the Roland–Morris Disability Questionnaire
The trial data suggest that differences in mean functional ability between control or ‘best care’ groups and a range of physical modalities, such as cognitive–behavioural therapy (CBT) with advice, exercise, manipulation, manipulation followed by exercise and physical therapy, following 3 months of treatment/follow-up were small and ranged between 0.8 and 1.87 points in the RMDQ (Table 6). 89,101
| Author (year) | Control/intervention | Number of participants in experimental arm | Number of participants in control arm | Mean baseline RMDQ (SD) | Mean score for the intervention group at follow-up (SE) | Mean change for the intervention group (95% CI) | Mean score for the control group at follow-up | Mean change for the control group (95% CI) | Mean between-group difference in change score (compared with control) (95% CI) | Outcome assessment time points (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lamb et al. (2010)85 | Control: Best practice Intervention: Advice plus CBT |
355 | 190 | 9.0 (SD 4.7–5.0) | Advice plus CBT: 2.0 (1.58 to 2.43) | Best practice: 1.1 (0.35 to 1.54) | Advice plus CBT:1.1 (0.38 to 1.71) | 3 | ||
| 393 | 189 | Advice plus CBT: 2.5 (1.96 to 3.03) | Best practice: 1.0 (0.40 to 1.67) | Advice plus CBT: 1.5 (0.70 to 2.22) | 6 | |||||
| 399 | 199 | Advice plus CBT: 2.4 (1.89 to 2.84) | Best practice: 1.1 (0.39 to 1.72) | Advice plus CBT: 1.3 (0.56 to 2.06) | 12 | |||||
| UK BEAM trial (2004)86 | Control: BUC in general practice Interventions: BUC and exercise OR BUC and manipulation (private or NHS) OR BUC and manipulation (private or NHS) plus exercise |
BUC and exercise: 225 | 256 (‘best care’) | Total: 9.0 (4.0) | BUC and exercise: 5.47 (0.29) | BUC and exercise: 3.5 | BUC and exercise: 6.83 (0.28) | BUC and exercise: 2.1 | BUC and exercise: 1.36 (0.63 to 2.10) | 3 |
| BUC and manipulation:287 | BUC and manipulation: 5.09 (0.28) | BUC and manipulation: 3.9 | BUC and manipulation: 6.66 (0.30) | BUC and manipulation: 2.3 | BUC and manipulation: 1.57 (0.82 to 2.32) | |||||
| BUC, manipulation and exercise: 258 | BUC, manipulation and exercise: 4.84 (0.28) | BUC, manipulation and exercise: 4.1 | BUC, manipulation and exercise: 6.71 (0.28) | BUC, manipulation and exercise: 2.3 | BUC, manipulation and exercise: 1.87 (1.15 to 2.60) | |||||
| BUC and exercise: 216 | 248 (‘best care’) | BUC and exercise: 5.74 (0.31) | BUC and exercise: 3.2 | BUC and exercise: 6.13 (0.30) | BUC and exercise: 2.8 | BUC and exercise: 0.39 (–0.41 to 1.19) | 12 | |||
| BUC and manipulation:273 | BUC and manipulation: 5.15 (0.29) | BUC and manipulation: 3.8 | BUC and manipulation: 6.16 (0.31) | BUC and manipulation: 2.8 | BUC and manipulation: 1.01 (0.22 to 1.81) | |||||
| BUC, manipulation and exercise: 257 | BUC, manipulation and exercise: 4.72 (0.29) | BUC, manipulation and exercise: 4.2 | BUC, manipulation and exercise: 6.02 (0.30) | BUC, manipulation and exercise: 2.9 | BUC and manipulation and exercise: 1.30 (0.54 to 2.07) | |||||
| Hay et al. (2005)101 | Control: Brief pain management programme Intervention: PT |
162 | 157 | PT 13.3 (4.9); Control 13.8 (4.8) | PT: 5.1 (5.8) | PT: 8.1 (6.0) | Pain management: 6.0 (5.9) | Pain management: 7.8 (6.6) | PT: 0.8 (–0.5 to 2.1) | 3 |
| 165 | 164 | PT: 4.4 (5.5) | PT: 8.8 (6.1) | Pain management: 5.2 (5.7) | Pain management: 8.8 (6.4) | 0.8 (–0.5 to 2.0) | 12 |
Following 12 months of follow-up the mean between-group difference in RMDQ scores ranged from 0.8101 to 1.30 points (see Table 6). 85,89,100
It is noteworthy that the between-group score differences are considerably lower than the suggested within-individual change or MIC for the RMDQ (5 points or 30% improvement from baseline).
Meta-analysis and calculation of the minimally important difference units for the Roland–Morris Disability Questionnaire
We calculated MID units per trial and as an overall score for the three trials (trials combined) at 3 and 12 months (Table 7). Larger MID units were calculated when the 30% MIC was used than for the score change of 5 points, ranging between 0.20 and 0.69 points at 3 months (combined-trials MID unit 0.49 points, 95% CI 0.37 to 0.61 points). At 12 months, MID units were smaller, ranging from 0.14 to 0.49 points (combined-trials MID unit 0.34 points, 95% CI 0.21 to 0.48 points).
| Author (year) | Mean between-group difference in change score (95% CI) | MID units (95% CI) (mean change/MIC of 5 points) | MID units (95% CI) (mean change/MIC 30% change from baseline) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 3 months | 12 months | 4 weeks | 3 months | 12 months | 4 weeks | 3 months | 12 months | |
| RMDQ83 | |||||||||
| Lamb et al. (2010)85,100 | – | 1.1 (0.38 to 1.71) | 1.3 (0.56 to 2.06) | – | 0.22 (0.09 to 0.35) | 0.26 (0.11 to 0.41) | – | 0.42 (0.16 to 0.67) | 0.49 (0.21 to 0.78) |
| UK BEAM trial (2004)86 | – | BUC and exercise: 1.36 (0.63 to 2.10) | BUC and exercise: 0.39 (–0.41 to 1.19) | – | BUC and exercise: 0.27 (0.13 to 0.42) | BUC and exercise: 0.08 (–0.08 to 0.24) | – | BUC and exercise: 0.50 (0.23 to 0.77) | BUC and exercise: 0.14 (–0.15 to 0.44) |
| BUC and manipulation: 1.57 (0.82 to 2.32) | BUC and manipulation: 1.01 (0.22 to 1.81) | BUC and manipulation: 0.31 (0.16 to 0.46) | BUC and manipulation: 0.20 (0.04 to 0.36) | BUC and manipulation: 0.58 (0.31 to 0.86) | BUC and manipulation: 0.38 (0.08 to 0.67) | ||||
| BUC, manipulation and exercise: 1.87 (1.15 to 2.60) | BUC, manipulation and exercise: 1.30 (0.54 to 2.07) | BUC, manipulation and exercise: 0.37 (0.23 to 0.52) | BUC, manipulation and exercise: 0.26 (0.11 to 0.41) | BUC, manipulation and exercise: 0.69 (0.42 to 0.96) | BUC, manipulation and exercise: 0.48 (0.20 to 0.76) | ||||
| Hay et al. (2005)101 | – | 0.8 (–0.5 to 2.1) | 0.8 (–0.5 to 2.0) | – | 0.16 (–0.10 to 0.42) | 0.16 (–0.09 to 0.41) | – | 0.20 (–0.12 to 0.52) | 0.20 (–0.11 to 0.50) |
| Meta-analysis (combined) | – | – | – | – | 0.28 (0.21 to 0.35) | 0.20 (0.13 to 0.27) | – | 0.49 (0.37 to 0.61) | 0.34 (0.21 to 0.48) |
| Raw data (UK BEAM trial) | |||||||||
| RMDQ (n = 1169) | 1.0 (0.45 to 1.50) | 1.5 (0.94 to 2.15) | 1.0 (0.31 to 1.62) | 0.20 (0.09 to 0.30) | 0.31 (0.12 to 0.43) | 0.25 (0.08 to 0.41) | 0.42 (0.20 to 0.64) | 0.68 (0.43 to 0.93) | 0.48 (0.20 to 0.75) |
| Pain-NRS | |||||||||
| Raw data (n = 1126) | 0.43 (0.12 to 0.74) | 0.81 (0.45 to 1.17) | 0.50 (0.12 to 0.87) | 0.22 (0.06 to 0.37) | 0.41 (0.22 to 0.59) | 0.25 (0.06 to 0.43) | 0.13 (–0.15 to 0.42) | 0.54 (0.22 to 0.86) | 0.41 (0.06 to 0.75) |
The use of the MIC 30% change from baseline score as a denominator produced consistently larger MID units than the use of the MIC raw score change.
Calculation of the between-group differences of change scores and the minimally important difference unit from a large UK trial (UK Back pain Exercise And Manipulation trial): Roland–Morris Disability Questionnaire and Pain Numerical Rating Scale
The two suggested MIC values for the RMDQ and Pain-NRS were used to inform calculation of the MID unit: (1) a score change of 5 points (RMDQ) or 2 points (Pain-NRS) and (2) 30% score improvement.
Minimally important difference units (mean change/minimal important change score change)
Small MID units were calculated for the RMDQ at 4 weeks, 3 months and 12 months ranging from 0.20 points (0.09–0.30 points) at 4 weeks to 0.31 points (0.12–0.43 points) at 3 months. Small to moderate MID units for the Pain-NRS were calculated at 4 weeks (0.22 points, 0.06–0.37 points), 3 months (0.41 points, 0.22–0.59 points) and 12 months (0.25 points, 0.06–0.43 points) (see Table 7).
Minimally important difference units (mean change/minimal important change 30% improvement)
The use of the 30% MIC produced larger MID units for the RMDQ at all time points: the MID units were moderate at 4 weeks (0.42 points, 0.20–0.64 points) and at 1 year (0.48 points, 0.20–0.75 points) and large at 3 months (0.68 points, 0.43–0.93 points). Use of the MIC 30% produced small to moderate MID units for the Pain-NRS, ranging from 0.13 points (–0.15 to 0.42 points) at 4 weeks to 0.54 points (0.22–0.86 points) at 3 months and 0.41 points (0.06–0.75 points) at 12 months.
Interpretation of minimally important difference units
In general, larger MID units were calculated for both the RMDQ and Pain-NRS when applying the 30% MIC in comparison to the MIC score change (see Table 7). The larger MID units for the RMDQ suggest that treatment may result in the improvement in function in an appreciable number of patients at all time points (all MID units greater than 0.4 points) but particularly at 3 months (MID unit 0.68 points).
Interpretation suggests that few people will achieve important benefits from treatment at 4 weeks and 12 months (MID units > 0.5 points) with regard to an improvement in their pain experience. However, an appreciable number of patients may experience a reduction in pain at 3 months (MID unit 0.54 points).
Consensus meeting: score interpretation
These data were presented at the consensus meeting. The outcome of the meeting was that any trial of FJIs should show a larger effect size than that typically seen in trials of physical interventions and that a medium MID unit difference of 0.5–1.0 points should be sought. The largest effect size seen on the RMDQ at 3 months in the example trials was 1.87 points (UK BEAM trial combined treatment). This is a MID unit difference of 0.37 using an absolute difference of 5 points, or a MID unit difference of 0.69 based on a 30% reduction from baseline. Delegates were also keen that results should be presented as responder criteria and that they would expect any trial of FJIs to have a greater number of responders compared with usual care than a trial of physiotherapy.
Discussion
The findings from the meta-analysis and secondary analysis demonstrated that the largest MID units (range 0.2–0.69 points) were consistently reported at the 3-month follow-up: this suggests that physiotherapist-delivered interventions might result in an improvement in functional ability (RMDQ) and reduction in pain (Pain-NRS) in an appreciable number of patients at 3 months and to a lesser extent at 12 months. However, fewer people will achieve important benefits from treatment at 4 weeks.
Participants in the consensus meeting agreed that if FJIs resulted in an additional improvement similar to that achieved in trials of therapist-delivered interventions, it would be a worthwhile intervention. A MID unit score of 0.5–1.0 points was therefore recommended. By way of illustration, if the baseline value of the RMDQ is 10.0 points, this would equate to a MIC (30% improvement) of 3 points; accepting a MID unit of 0.5–1.0 points would result in a between-group difference of 1.5–3.0 points. The alternative approach based on absolute RMDQ scores (score change of 5), a worthwhile between-group difference of interest would be 2.5 points regardless of baseline values. Similarly, if the mean baseline Pain-NRS is 7.5 points this would equate to a MIC (30% improvement) of 2.25 points; accepting a MID unit of 0.5–1.0 points would imply, therefore, that a worthwhile benefit would be a between-group difference of 1.125–2.25 points.
The purpose of this work was to inform the decision regarding progression to a main trial, for example based on the estimated difference and CI calculated for these two measures, a main trial would be considered to be appropriate if the upper limit of the 95% CI, for either assessment, exceeded the minimal worthwhile effect. Conservatively, for the RMDQ we suggest use of the smaller of the two values estimated for the RMDQ. If the limit of the 95% CI does not exceed this value, this indicates that it would not be worthwhile proceeding to a main trial. As the pilot study closed prematurely and no between-group comparisons were carried out, we cannot use this finding to inform a decision to progress to a main study; it will, however, inform the sample size estimate for any main study.
The suggestion that responder criteria were used has many attractions. Previous work has shown that the NNT within the UK BEAM trial data set for an improvement on the RMDQ at 3 months is 5.2 patients (95% CI 3.7 to 8.8 patients) for a 5-point improvement and 5.4 patients (95% CI 3.8 to 9.9 patients) for a 30% improvement. At 12 months the NNTs are 8.4 patients (95% CI 5.0 to 28.6 patients) for a 5-point improvement and 8.0 patients (95% CI 4.9 to 24.3 patients) for a 30% improvement. 57 These previous analyses could also serve to inform sample size calculations, however, they do not take into account any effects on deteriorations prevented by treatment or indeed deteriorations as a consequence of treatment. The previous work did consider these but was limited in its analysis by the absence of consensus on what represents a deterioration. This may be particularly pertinent in the context of this study, in which any deterioration as a consequence of the intervention is likely to be in the short term and may not be measured at the same time point used for any responder analyses. Thus, although we think that this approach is important for presentation, we do not think that it should be used to estimate sample size. 89
Chapter 4 Feasibility randomised controlled trial
In Chapter 3 we outlined the FIS consensus conference. Data from this event have played a considerable role in shaping key methodological components of our protocol, including the diagnosis of patients with suspected facet joint pain, the technique/protocol for injecting the facet joints and a package of BUC combining both physical and psychological components for the treatment of patients with suspected facet joint pain. In this chapter we bring these together into the methods used to carry out the feasibility RCT.
Trial summary
The FIS was a mixed-methods randomised multicentre feasibility trial to test the addition of FJIs to a bespoke BUC package. The trial was conducted in the UK only. We planned to randomise up to 150 participants into two equal groups stratified by trust, participant age and severity. Recruitment was expected to take around 6 months.
The randomised pilot trial was planned to be conducted in up to six NHS acute trusts. Patients referred for treatment of LBP present for at least 6 months, after failure of conservative treatment, were considered as potential participants. Potential participants were sent a trial screening information sheet and screening questionnaire for completion and return with their contact details on an expression of interest form. The screening questionnaire assessed preliminary eligibility for enrolment into the trial. If the potential participant appeared eligible and interested, an appointment with a research physiotherapist was made during which a diagnostic assessment was undertaken to determine if facet joint pain was probable. If the potential participant was deemed to be unsuitable for the trial, his or her standard treatment of care continued as normal. In the event that the assessing physiotherapist had concerns that a potential participant having a specific cause of back pain (malignancy, fracture, infection or possible ankylosing spondylitis), cauda equina compression or radicular pain suitable for surgery, the assessing physiotherapist, as appropriate, expedited a specialist assessment or referred the potential participant back to their general practitioner (GP). If the potential participant was confirmed to have suspected facet joint pain, he or she was considered for enrolment. If the potential participant was able and willing to attend on specified dates for the scheduled intervention, written informed consent was obtained and the participant was considered enrolled into the trial.
For this study we wished to test a pragmatic approach in which those with suspected facet joint pain receive a therapeutic intra-articular injection. This is congruent with how any such service would be delivered in the NHS. Outside a research environment, performing an initial diagnostic intra-injection or medial branch block to confirm the diagnosis would be unnecessarily resource intensive. The resources necessary to carry out a therapeutic injection are almost identical to those needed to carry out a diagnostic procedure.
Randomisation was performed centrally by Warwick Clinical Trials Unit (WCTU) using randomised permuted blocks, stratified by trust, participant age and troublesomeness of LBP. Participants were randomised to receive either FJI with BUC or BUC only. Pain outcomes were collected, immediately before and after injection (intervention only), daily for up to 7 days before first physiotherapy treatment session until 28 days after randomisation (including 7 days after the notional injection date; all injections should have taken place within 21 days of randomisation) and weekly for 3 months after the intervention. Health utility data [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)] were collected daily for 8 days from around the notional injection date and then weekly until 3 months after randomisation.
Aims and objectives
Primary objective
The primary objective of this trial was to explore the feasibility of running a RCT to test the hypothesis that for people with suspected facet joint pain contributing to persistent LBP, adding the option of FJIs to best usual non-invasive care available from the NHS is clinically effective and cost-effective.
Secondary objectives
-
To evaluate a standardised control treatment deliverable in the NHS and congruent with NICE guidance (BUC).
-
To develop and test systems for collecting short-term and long-term pain outcomes, including measures required for economic evaluation.
-
To demonstrate that recruitment to the main trial is feasible.
-
To collect the recruitment and outcome data required to inform sample size and number of sites needed for the main trial.
-
To conduct a between-group analysis to inform the decision on the need for a full trial.
-
To undertake a process evaluation of patient experience within the trial data from exploratory work, data from the pilot trial and data from both exploratory work and the pilot trial.
Research methods
A protocol paper has been published for this part of the study. 102
Study design
The FIS was a mixed-methods randomised multicentre feasibility trial to compare FJIs with BUC.
Setting
The feasibility RCT was conducted in five NHS acute trusts in the centres based in England. These were:
-
University Hospitals Coventry and Warwickshire NHS Trust
-
Kidderminster Hospital and Treatment Centre, Worcestershire Acute Hospital NHS Trust
-
King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust
-
The James Cook University Hospital, South Tees Hospitals NHS Foundation Trust
-
Warwick Hospital, South Warwickshire NHS Foundation Trust.
In each hospital we were working with the clinicians providing pain services for patients. One further trust, Hull and East Yorkshire Hospitals NHS Trust, was preparing to join the study at the time recruitment stopped.
Participants
Patients referred to the trust for treatment of LBP present for at least 6 months, after failure of conservative treatment, were considered as potential participants. We aimed to recruit 150 patients. We expected up to 40 participants to be recruited at each participating centre, with up to 20 participants randomised to each group at each site. Recruitment was planned to be primarily from pain clinic services, which was an approach that was congruent with the commissioning brief.
Inclusion criteria
-
Was able and willing to comply with the trial procedures and signed and dated informed consent was obtained.
-
Was aged ≥ 18 years with at least moderately troublesome LBP present for at least 6 months. 103
-
Had LBP as their predominant musculoskeletal pain.
-
Had undergone registered health professional delivered treatment for LBP in the 2 years prior to study entry.
-
Met clinical criteria for possible facet joint pain when there is no radicular symptoms (defined as pain radiating below the knee) and no sacroiliac joint pain elicited using a pain provocation test and increased pain unilaterally, bilaterally on lumbar paraspinal palpation and increased LBP on one or more of the following: extension (more than flexion), rotation, extension/side flexion or extension/rotation.
-
Was able to manage text messaging, or an alternative means of daily data collection (paper-based diary).
-
Was fluent in written and spoken English.
Exclusion criteria
-
Was unable to attend for randomised treatment, other circumstances that would significantly decrease the chance of obtaining reliable data, achieving trial objectives or completing the trial and follow-up assessments or was considered unsuitable to participate in the trial by an investigator.
-
Was unable/unwilling to undergo injections.
-
Had used oral corticosteroids or had a corticosteroid injection in the preceding 3 months.
-
Had an underlying serious psychiatric or psychological disorder that precludes participation in either intervention.
-
Had previously undergone spinal injections.
-
Had previously undergone spinal surgery.
-
Had a contraindication to FJIs, for example a serious comorbidity (e.g. severe chronic obstructive pulmonary disease, poorly controlled diabetes), such as malignancy, infection, inflammatory disorder or fracture, or was taking anticoagulant medications.
-
Had a known allergy to the constituents of the planned injections.
-
Pregnancy or suspected pregnancy.
-
Was previously randomised in this trial.
-
Was currently participating in another clinical trial (with an unregistered medicinal product) or < 90 days had passed since completing participation in such a trial.
Study treatments
Facet joint injection with ‘best usual care’
Participants received detailed information (verbal and written) about the procedure prior to and during the diagnostic physiotherapy assessment. When the participant attended for injection, the clinician (consultant) responsible for injection made a clinical assessment to ensure FJI remained appropriate. Briefly, the clinical assessment, as per normal clinical practice, included a review of the medical history and an assessment of the participant’s suitability for FJIs. There was a particular emphasis on ensuring that the participant had no important comorbidities such as malignancy, inflammatory disease, severe chronic obstructive pulmonary disease, poorly controlled diabetes, fracture or infection (trial exclusions). The clinician could also ‘postpone’ a treatment session if the patient presented with a short-term ailment (e.g. influenza). Following normal local trust clinical practice, the clinician obtained informed consent for the injection procedure from participants prior to injecting the facet joints. If during the assessment the clinician identified significant pathologies that had not been identified previously that excluded a potential participant, they ‘flagged’ these in the patient notes or to a local investigator site clinician or a GP, whichever was the most appropriate. Participants were fully informed that they may not receive the injection at this consultation. If it was determined that the injection was not appropriate, the participant would continue to receive the ‘BUC’ physiotherapy package.
Up to six facet joints (L3/L4, L4/L5 and L5/S1) bilaterally in each participant were injected. However, when, on clinical assessment, there was unilateral pain or involvement of only some levels, the operator may have chosen to carry out a unilateral injection or to be selective on levels injected. This pragmatic approach reflects current clinical practice within the NHS and was informed by the earlier consensus meeting.
This approach is consistent with that used in trials of other complex interventions for LBP, for example a manual therapy or a cognitive–behavioural approach, in which practitioners choose from a limited range of options based on their clinical assessment of the patient. 13,14
Control treatment: ‘best usual-care’ package (standardised treatment deliverable in the NHS and congruent with 2009 National Institute for Health and Care Excellence guidance)
A study ‘physiotherapist and cognitive–behavioural treatment manual’ was prepared to ensure that all sites adhere to the same procedures when delivering the ‘BUC’ package. Prior to commencement of the trial, physiotherapists within the investigator site team were trained in the delivery of the package and its processes. Below we outline the principles and theoretical underpinnings of our ‘BUC’ package. This builds on previous work showing that physiotherapists can deliver an effective cognitive intervention but has an additional emphasis on a physical approach to treating facet joint dysfunction. 104 Informed by the Medical Research Council framework for complex interventions, we developed a best practice intervention that is congruent with NICE. 105 Consensus agreement ensured that the ‘BUC’ package was deliverable within the NHS at a reasonable cost, that is, for much less than 100 hours of contact time recommended by NICE at the time this study took place. 106
Fundamentally, treatment followed guidance from NICE but tailored to individual patients. Participants underwent a thorough initial physical assessment (60 minutes) based on the principles of Maitland manual therapy. 107 The assessment included discussion of patient expectations, fear avoidance and perceived self-efficacy.
Participants randomised to the injection arm had their FJIs between their first and second physiotherapy treatment sessions and within no more than 3 weeks. They then had four further sessions, of 30 minutes each, which incorporated manual therapy, active and motor control exercises, soft tissue stretches and a cognitive–behavioural approach. This included cognitive restructuring, challenging unhelpful thoughts associated with physical activity and chronic LBP, pacing and goal-setting. The sessions were delivered in a participant-centred manner, with each session tailored to the participant’s needs.
The ‘BUC’ intervention was piloted with five participants recruited from participating trusts before the trial went into the randomised phase.
Procedures
Procedures for screening
A member of the local principal investigator (PI)’s site trial team actively identified referrals to secondary care for patients with LBP. We expected that most of these patients, having been referred after failure of conservative treatment, would already have a diagnosis of non-specific LBP. The referring GP would have referred the patient based on their knowledge and the patient’s history of chronic back pain and previous treatments. Those patients in whom the GP suspected a specific cause for their back pain would typically have been referred in through a different pathway, such as the 2-week wait pathway for suspected cancer. Any patients for whom the referral from the GP queried a specific cause of back pain were not approached for the study. As the entry criteria for this study required participants to have chronic pain, to have been assessed by their GP and to have already had a course of conservative treatment, it is extremely unlikely that any patients with malignancy, infection or inflammatory disorders were approached. We were, however, aware that the 2009 NICE guidance6 for the management of non-specific LBP recommends keeping the diagnosis under review. As originally planned, we recruited from secondary care pain services. It was here that we perceived the question on the effectiveness of intra-articular FJIs to be of greatest importance. Recruitment in this environment was challenging. At the time the study closed because of poor recruitment, we were working towards being able to recruit from neurosurgery, rheumatology and orthopaedic clinics. We were also setting up a GP and community physiotherapy referral system into a secondary care physiotherapist-led research clinic. Posters were placed in GP and community clinics. Interested patients could contact local research nurses in their participating trusts.
A member of the investigator’s trial team based at the local site (e.g. a research nurse or physiotherapist) sent a brief screening information sheet, a screening questionnaire and an expression of interest form to potential participants, for whom there were no concerns about a specific cause of LBP, to assess preliminarily their eligibility for enrolment into the trial. At this time, the brief screening information sheet was sent to potential participants to provide them with sufficient information to make an informed judgement about whether or not they might be interested in finding out more about the study. At some sites, patients with LBP who were attending clinics were approached by their treating clinician (e.g. the local PI or team) to assess their possible participation in the trial. In these cases, the treating clinician gave the patient a trial information pack containing a brief screening information sheet, a screening questionnaire and an expression of interest form. The potential participant was invited to return the completed screening questionnaire and expression of interest form to the PI’s trial team at the local site using an envelope provided. From review of the questionnaire, if the potential participant appeared eligible and interested, a member of the local site trial team sent out the full participant information sheet regarding the trial. Specifically, the full participant information sheet informed the patient about the eligibility assessment they were being invited to attend, which helped to determine their possible inclusion in the trial, and clearly explained that it was only at this assessment that their eligibility would be confirmed. They were invited to attend an appointment for their clinical/physical (diagnostic) assessment at a nominated research clinic. Each site was required to maintain an anonymised trial screening log (see Appendix 2) monitoring the number of screening packs sent out, returned and recruited, noting reasons for ineligibility (Figure 3).
FIGURE 3.
Participant pathway and procedures. EQ-5D, EuroQol-5 Dimensions.

Clinical diagnosis of suspected facet joint pain (visit A)
A study-trained physiotherapist met with potential participants who had agreed to attend the assessment appointment. On arrival the physiotherapist carried out the FIS diagnostic assessment.
Based on the agreement from the consensus conference, suspected facet joint pain was considered to be present when there was:
-
no radicular symptoms (defined as pain radiating below the knee)
-
no sacroiliac joint pain elicited using a pain provocation test
-
increased pain, unilaterally or bilaterally, on lumbar paraspinal palpation
-
increased LBP on one or more of the following:
-
extension (more than flexion)
-
rotation
-
extension/side flexion
-
extension/rotation.
-
Note that both extension/side flexion and extension/rotation were representative of regular compression patterns. 49
A manual was developed for the diagnostic assessment to aid the physiotherapist. 72
After it had been confirmed that the patient met all of the inclusion criteria and none of the exclusion criteria, and was deemed ‘eligible’ following the diagnostic assessment, their written informed consent to participate in the trial was obtained by suitably trained physiotherapists or research nurses, or by the PI as a delegated responsibility.
If potential participants were considered not to have facet joint pain, the physiotherapist informed them that they would not be included in the trial and that their standard treatment/management care plan would continue as referred.
The outcome of the eligibility assessment for suspected facet joint pain was documented for all potential participants. Those potential participants who were considered not eligible based on eligibility assessment were asked to consent to the use of the data generated from their assessment. This was documented in each person’s clinical record.
After giving written informed consent, the participant was scheduled to attend a 1-hour preliminary ‘BUC’ physiotherapy session delivered by a specially trained physiotherapist.
First ‘best usual-care’ session (visit B1)
Participants initially underwent a thorough physical assessment based on the principles of Maitland manual therapy assessment and clinical reasoning,107 in which symptomatic levels are identified, and the severity and nature of the symptoms recorded and used to direct treatment.
Assessment included discussions of acceptance, goal-setting and pacing as well as general discussion of patient expectations, fear avoidance and self-efficacy to assess any perceived challenges and barriers that patients felt may be preventing them from engaging in self-management of chronic pain and to allow subsequent treatment sessions to be tailored to individual need. A manual was developed for the ‘BUC’ intervention to aid the physiotherapist. 73
Once the first trial physiotherapy session was completed, the participant was randomised to receive FJI with BUC package or BUC package only. The treating physiotherapist, within 1 working day, informed the investigator trial team that the first treatment session has been completed. The investigator trial team then completed the randomisation process and informed the participant whether or not they needed to attend for an injection.
This approached ensured that all participants had received the introductory physiotherapy session and that we were testing the addition of intra-articular FJIs to BUC physiotherapy package rather than comparing FJIs with physiotherapy. Our concern here was that those allocated to injections might have elected not to attend for physiotherapy assessment if they expected a substantial benefit from injections.
At this visit we also collected an immediate pre-treatment pain score that would be important for any analysis of short-term benefits, or harms, from treatment. This is pertinent because there would inevitably be a delay between consent and arranging treatment sessions.
If a patient was randomised to receive FJI, the injection was scheduled between the preliminary and secondary physiotherapy treatment session. This took place within 3 weeks of randomisation.
Facet joint injections (visit C)
For those participants who were randomised to undergo FJI, the injection took place between the first and second BUC sessions. The treating clinician at this time made their own assessment of the participant’s suitability for FJI and obtained consent for the procedure following normal practice in each participating trust. The treating clinician could postpone injection in the presence of short-term illness (e.g. influenza). If significant comorbidity was identified at this time that contraindicated the injection, this was ‘flagged’ in the patient notes or to the local investigator, site clinician or GP, whoever was most appropriate. The participant would continue to receive the control intervention. A manual was developed for the clinician, which gave clear instructions on how the injection(s) should be carried out. 75
‘Best usual-care package’ (visits B2–B6)
It was expected that all 150 participants would have up to a total of six ‘BUC’ physiotherapy sessions (including the first treatment session immediately prior to randomisation). The five remaining sessions were to last approximately 30 minutes. All participants (those in the intervention and those in the control arms) were encouraged to attend all of these sessions. The package was a series of one-to-one sessions with a study physiotherapist who would use the BUC manual informed by consensus to help them tailor the treatment to the participant’s needs. 26 Sessions were a bespoke package of physical and behavioural rehabilitation. All treatment sessions were to be completed within 12 weeks of randomisation.
Follow-up
Follow-up was conducted at 3 months after randomisation using self-administered questionnaires. The majority of questionnaires were completed in postal format. Response was tracked carefully by the trial office and a reminder questionnaire was sent after 2 weeks if a follow-up questionnaire had not been returned. If the questionnaire had still not been returned after a further 2 weeks, participants were telephoned to check that they were receiving the questionnaires and to arrange for another to be sent if needed. A core data set was requested via the telephone, at a time convenient to the participant, if a participant had still not responded.
The case report form (CRF) was stamped with the date and initialled on receipt at the WCTU office. It was checked for correctness and completeness and coded for data entry. Any queries were checked with the statistician. Missing data were clarified with participants when possible.
The baseline and follow-up data were entered into the study database and a random sample check of 10% was undertaken to ensure accuracy.
Outcome assessment
Table 8 outlines the outcome measures and delivery time points.
| Type of data | Outcome measures | Time points | ||||
|---|---|---|---|---|---|---|
| 1a | 2b | 3c | 4d | 5e | ||
| Demographic | Age, gender, ethnic group, age at leaving full-time education, occupation, current work status | Yes | ||||
| History | Time since completely free of back pain | Yes | ||||
| History | Previous back pain treatments | Yes | ||||
| Medications | Current medications | Yes | Yes | |||
| History | Satisfaction with health state | Yes | Yes | |||
| History | Troublesomeness question | Yes | Yes | |||
| Back pain related disability | RMDQ83 | Yes | Yes | |||
| Back pain related disability | The MVK questionnaire disability score84,99 | Yes | Yes | |||
| Back Pain Severity | The MVK questionnaire pain scale99 | Yes | Yes | |||
| Modified form of PGI109,110 | Yes | Yes | ||||
| Psychological distress | DAPOS111 | Yes | Yes | |||
| Pain self-efficacy | Pain self-efficacy questionnaire112 | Yes | Yes | |||
| Health-related quality of life | SF-12v2, reported as physical and mental component scores113 | Yes | Yes | |||
| Health utilities | EQ-5D-5L114,115 | Yes | Yes | Yes | Yes | |
| Well-being | WEMWEBS116 | Yes | Yes | |||
| Pain distribution | Troublesomeness grid103 | Yes | ||||
| Back Pain Severity today | 11-point pain rating scale84 | Yes | Yes | |||
| Current work status | If appropriate date of return to work | Yes | ||||
| Health and social service resource use | Including hospital and community resource, as well as costs to individuals and carers | Yes | ||||
Demography and baseline assessment
Appendix 3 includes an example of the CRF listing the demographic and clinical data that were collected at pre-enrolment and pre-randomisation stages. This includes the baseline assessments that are carried out during visit ‘A’ at the time of enrolment.
Clinical outcomes
For the randomised pilot trial we used a package of outcome measures consistent with consensus recommendations for outcome assessment in back pain trials. These included pain measurement, physical function, emotional function, back-related function, generic well-being, disability (social role), satisfaction with care, patient rating of improvement and satisfaction with treatment, symptoms (pain), adverse events, participant disposition and a modified form of the Patient Generated Index (PGI). 67,108 In this feasibility trial we assessed performance of these measures and with a view to reducing the questionnaire burden in the main trial.
Table 8 summarises the outcome measures and their time of completion by participants. The main questionnaire packages are completed at baseline (at trial entry assessment) and follow-up (3 and 6 months post randomisation). A pain severity (today) score was recorded daily for 35 days from 7 days before first treatment session and following this weekly until 3-month follow-up. Health utility (EQ-5D-5L) was recorded weekly from 1 week prior to first treatment session until the night before injection appointment when we asked participants to record it daily for 8 days and then weekly until the 3-month follow-up. In addition, intervention participants record a pain severity 45–60 minutes before and after receiving an injection. Clinical data were collected and recorded by physiotherapists and clinicians, covering patients’ assessments, injections and involvement in the physiotherapy intervention. Copies of questionnaires are provided in Appendices 4 and 5.
Primary outcome
The primary outcome for this feasibility trial was a numerical rating scale for pain collected via text messaging over 3 months following randomisation. 84
A method for frequent (daily) collection of pain-related outcome data using a text messaging system was developed and piloted within the feasibility trial. To test the feasibility of using an electronic diary, a review of studies using electronic diary-related pain or pain-related disability measurement was conducted, as well as the identification or development of an application or text system with candidate diary prompts and choice of prompts and frequency. 117 Research participants find electronic symptom diaries acceptable and can generate valid symptom data. 118 During the exploratory phase, initial feasibility testing, we refined the diary recording for the needs of the trial.
For those participants unable or unwilling to use a text messaging system, we used a paper-based system. We collected data on pain severity using an 11-point numerical rating scale (Pain-NRS). 84 Data on pain were collected daily for 35 days starting 1 week prior to randomisation and then weekly for until 3 months after randomisation. It was felt that any benefit or harm in the immediate post-injection period was likely to change on a daily basis, and, therefore, less frequent data collection should identify any between-group differences.
Our second primary outcome focused on back pain related disability. We used the RMDQ at baseline and 3 months (follow-up collected using a postal questionnaire).
It was planned that the between-group analysis would be looking for a ‘signal’ that the intervention package might be effective. If we failed to find an indication that there may be a worthwhile benefit, there would be no need to proceed to a full trial. We were not, here, interested in the point estimate for any between-group differences. Rather, we were interested in whether or not it was plausible that FJIs would achieve a benefit of sufficient magnitude to make them a worthwhile intervention. Thus, a decision not to proceed to a main trial would have been based on it being implausible that the intervention would achieve a worthwhile clinical effect. The size of effect below which the clinical and academic community accept that the use of FJIs was not worth considering was decided at the consensus development conference. However, because of the early closure of recruitment, no between-group analyses were undertaken (see Analyses).
Sample size
There were several drivers for the sample size estimate: gaining sufficient experience to be confident of recruitment rates for a main trial, estimating the proportion who gain immediate pain relief and obtaining sufficient outcome data to inform a decision to proceed while keeping absolute numbers manageable in a short time frame. We aimed to randomise 150 participants over 6 months into two equal groups stratified by trust. This is as large as any existing RCT of FJIs. 60
We considered how the effect size found in a between-group comparison within this pilot may influence a decision to run a main trial. Essentially, if the limit for the 95% CI includes a value that would be indicative of a clinically important difference then we should proceed and if it does not then we should not proceed. At the design stage we calculated the probability of deciding to proceed to main study when the true treatment effect is zero for a range of desired standardised mean differences that would be within the 95% CI from the analysis of pilot data. This calculation suggested that if the desired standardised mean difference indicative of a minimally clinically important difference is in the range 0.3–0.4, if we recruit around 150 participants, after allowing for 20% loss to follow-up, then the probability of proceeding to a full trial if true effect is zero is around 50% (see Appendix 6). This effect size expressed as a standardised mean difference is consistent with the effect size agreed at the consensus conference expressed as MID units for the RMDQ, meaning that no change was required for this calculation.
The resulting 75 patients in the active injection group would allow us to estimate the proportion with ‘true’ facet joint pain, based on achieving immediate pain relief, with a precision of 11% if the true proportion was 62%. 119,120
Our original plan for our primary analysis here, for pain, was to be the difference in the area under the curve (AUC) values from our pain measurements over 3 months. For the second primary outcome, we were going to use the difference in RMDQ at 3 months. If there was not a positive signal suggesting an early reduction in pain, or in the RMDQ score, then we would not have wanted to proceed to a full trial.
Randomisation
Written informed consent for entry into the trial and the immediate pre-treatment pain score were obtained prior to randomisation. Participants were randomised sequentially, using randomised permuted blocks, stratified by trust, participant age and troublesomeness of LBP.
Randomisation was performed centrally by the WCTU using a remote telephone randomisation system to ensure concealment and avoidance of bias. The randomisation system was managed by the WCTU randomisation service.
Randomisation was sequential in a 1 : 1 ratio to FJI plus ‘BUC’ or ‘BUC’ only. A unique trial number was assigned to the participant in order to maintain anonymity and this was recorded in the CRF.
Any telephone contact for data collection after randomisation was undertaken by a member of the investigator trial team blind to randomisation.
When possible, an entry was recorded in the clinical record of each participant noting the date of enrolment, name of the investigator’s trial team member that authorised enrolment and the unique trial number of the patient.
All forms related to randomisation are included in Appendix 7.
Monitoring and quality assurance of trial procedures
Monitoring and quality assurance was carried out in line with WCTU standard operating procedures.
Formal approvals
Sponsor and governance arrangements
The University Hospitals Coventry and Warwickshire NHS Trust and University of Warwick acted as cosponsors for this trial.
Ethics
Originally we submitted an ethics application for approval with a full protocol and all of the relevant paperwork (e.g. patient information sheets, consent forms) on 7 April 2014. In this application we noted that we were seeking approval for the general methodology of the study but that it was possible that some things, such as the drugs used for the intervention, would change after our developmental conference. This application was rejected and we were instructed to reapply after the consensus conference. After resubmission we received approval on 11 August 2014 (Research Ethics Committee reference 14/YH/0161). This initial refusal prevented us from being able to set up sites for the trial when originally planned. There were a number of amendments, which are outlined below along with their dates of approval:
-
approval for new documents (24 October 2014):
-
a cover letter to supplement the follow-up questionnaires
-
a reminder letter for non-return of follow-up questionnaires
-
a daily/weekly pain diary
-
a weekly health diary
-
a daily health diary
-
-
approval to add a new site/PI (20 November 2014):
-
added Castle Hill Hospital, Cottingham, Mr Marek Karpinski
-
-
approval to add a new site/PI and to remove a site/PI (as pain service discontinued at this time) (13 February 2015):
-
added The James Cook University Hospital, Middlesbrough, Dr Sam Eldabe
-
removed South Warwickshire NHS Foundation Trust, Warwick Hospital, Warwick, Dr James (Hugh) Antrobus
-
-
approval to add a new site/PI (29 June 2015) (reinstating site after pain management service restored):
-
added South Warwickshire NHS Foundation Trust, Warwick Hospital, Warwick, Dr James (Hugh) Antrobus
-
-
approval to make changes to the protocol and information sheets and gain approval for a new clinic poster (14 September 2015)
-
approval for the new scheduled diagnostic assessment and screening questionnaire response letters (16 December 2015).
Regulatory authorities
The FIS comes under the definition of a Clinical Trial of an Investigational Medicinal Product under the European Union Clinical Trials Directive 2001/20/EC121 and, therefore, required submission to the Medicines and Healthcare products Regulatory Agency (MHRA). The trial did not start before the necessary approvals from Research Ethics Committee and the MHRA were obtained in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use E6-Good Clinical Practice. 122 Before commencing recruitment, each PI was required to obtain local NHS permission from within their trust.
Approval was granted on 18 November 2014. A copy of all regulatory/ethics/NHS permission approval documentation was provided to WCTU prior to commencing recruitment of participants into the trial.
Adverse event management
As this study was classified as an Investigational Medicinal Product study we carefully prepared an adverse event management system that covered all areas of the trial. We did not expect any adverse events, however, it was important to consider this aspect of the trial and have policies in place to manage any that occurred in line with Good Clinical Practice122 and MHRA guidance. 123
Adverse events
The monitoring and reporting of potential adverse events was carried out in line with WCTU standard operating procedures.
The following were expected adverse events and, if found, were recorded in the CRF:
-
pain, bleeding, discomfort and minor bruising at the injection site (transient)
-
numbness in the buttocks and legs from local anaesthetic (transient)
-
infection of injection site; inadvertent intravenous injection (uncommon)
-
musculoskeletal injuries requiring medical attention including serious sprains, joint dislocation, falls or other injuries occurring as a direct consequence of the intervention (i.e. while participating in the ‘BUC’ physiotherapy treatment intervention in real time).
Procedures in case of pregnancy
Pregnancy was an exclusion criterion for entry into the study; however, we planned to follow up and document the outcome of all pregnancies (spontaneous miscarriage, elective termination, normal birth or congenital abnormality) even if the patient had been discontinued from the trial.
All female participants were advised to notify the investigator immediately if they became pregnant during the trial. The investigator would then report any pregnancy to the WCTU. As noted, we planned to follow up any pregnancy, and any complications were recorded as an adverse event. All reports of congenital abnormalities/birth defects would be reported and followed up as serious adverse events.
Complete study procedures for adverse reactions, adverse events, serious adverse events and suspected unexpected serious adverse events were included in the study protocol.
Data management
Submitted data were reviewed for completeness and entered onto a secure, backed-up, bespoke database. Due care was taken to ensure data safety and integrity and compliance with the Data Protection Act 1998. 124 Participants were identified using a unique trial number, which was allocated at the time of enrolment, and their initials in order to maintain anonymity. The unique trial number was recorded in the participant’s CRF. We outline our data management in the following section (see Data collection and management), but more detail on data storage, access, quality assurance, archiving and data sharing are included in the protocol.
Data collection and management
All data for an individual participant were collected by a member of the investigator’s research team and recorded in the CRF. Participant identification in the CRF was through their initials and unique trial number allocated at the time of enrolment. Data were collected from the time the patient was considered for entry into the trial through to the completion of the intervention.
All data were entered into the CRF; a copy of each form was returned to the WCTU and a duplicate was retained at the participating site. The unique trial number and other contact details of all participants were supplied to the WCTU to allow follow-up telephone or postal questionnaires to be administered to the participant at 3 months and 6 months after randomisation.
Data collection was restricted to variables required to define patient characteristics at enrolment, to monitor the treatment received, to monitor adverse effects and to determine quality of life and the use of health-care resources.
Database
The database was developed and managed by the programming team at the WCTU, and all specifications (i.e. database variables, validation checks and screens) were agreed between members of the project team, including the programmer and statistician.
Pre-pilot study
A pre-pilot study was planned ahead of the main feasibility randomised trial, with the aim of testing all of the trial processes. Our objectives were to:
-
test our recruitment process (by recruiting 10–12 participants)
-
allow our assessing physiotherapists to gain experience of the diagnostic assessment
-
evaluate the acceptability of our baseline questionnaire
-
test, when possible, our procedures for injections
-
allow our treating physiotherapists to gain experience of delivering ‘BUC’ package
-
explore patient experiences of trial processes.
Staff training
As noted in Chapter 2, Consensus conference (Diagnosis; Injection technique the process of therapeutic intra-articular facet joint injection; and Best usual care), the research team developed manuals/protocols for diagnosis, BUC and injection technique, informed by best current evidence and consensus. Training days were scheduled for the appropriate staff in these trial procedures (e.g. physiotherapists for diagnosis and BUC, and clinicians for injections). We piloted this training in the pilot study. The manuals/training were updated before the main feasibility trial based on the experiences and observations of their use in the pilot study.
Pilot procedure
We planned to recruit around 10–12 participants to the pilot study from one or more of our participating trusts. The recruitment and assessment processes would be the same as those designed for the main study. At the end of the diagnostic assessment, participants with suspected facet joint pain who gave informed consent were asked to complete the baseline questionnaire. The participants in the pilot study were not randomised.
All of those participants who met the eligibility criteria were offered the whole treatment package (including the injection and ‘BUC’ package). Once treatment was complete, a member of the research team contacted participants by telephone to get their feedback on their experience of the assessment and treatment process. This included asking for comments on the face validity and acceptability of the baseline questionnaire. The research team obtained feedback from the treating physiotherapists about the study processes. The data from participants and therapists were used to refine the assessment process and treatment package prior to the randomised feasibility trial being launched.
Analyses
Statistical analysis
At the outset it was planned that the main quantitative outputs from this feasibility trial would, first, be process outcomes, including:
-
proportion of eligible participants who are randomised and complete follow-up
-
proportion who obtain immediate (diagnostic) pain relief
-
recruitment rates, that is, the number of referrals per site and proportion of referrals converted to participants. These data are needed to estimate the number of sites needed for, and the duration of, the main trial
-
completeness of data from short-term electronic data collection.
Our primary effectiveness analyses was planned to be the overall difference in average pain and back pain related disability between intervention and control groups with a 95% CI. We planned to adjust our models for baseline stratification factors and other baseline covariates and missing values imputed. However, early closure precluded us from taking this approach, and the following plan was developed.
Overview
The proposed statistical analyses were conducted on data collected from participants randomised prior to study closure. The objectives outlined in this plan are based on previously stated objectives from the FIS protocol and an earlier version of a full statistical analysis plan developed before recruitment began.
None of our prespecified effectiveness analyses outlined in the protocol and statistical analysis plan was carried out. Given the number of participants recruited at study closure, there is insufficient statistical power to detect any ‘true’ treatment difference between interventions, if one did exist. If any underpowered comparative analyses were presented, inappropriate inferences and conclusions may be made. However, overall descriptive data summaries (e.g. means, SDs) are presented.
Key objectives
The main objectives of the revised analyses are to:
-
present descriptive summaries and graphical representations of baseline and 3-month outcomes, with no between-group analyses
-
describe participant flow through the study, including a Consolidated Standards of Reporting Trials diagram, to summarise participation from screening through to follow-up
-
evaluate recruitment rates, number of referrals per site and conversion percentages in order to better inform decisions regarding estimates of parameters for a main trial
-
assess the completeness of data collected via text message (the text messaging service used to collect daily and weekly pain scores from participants) and the quality of these data in comparison with postal paper pain diary data, when appropriate
-
evaluate the performance of CRFs and patient questionnaires for collecting data at baseline and at follow-up time points by presenting summaries of data completeness at baseline and 3-month follow-up
-
estimate the proportion of participants obtaining immediate pain relief in the FJI group as a measure of diagnostic accuracy
-
assess performance of secondary outcome measures to inform decisions regarding questionnaire burden for a main trial.
Scoring of measures
Roland–Morris Disability Questionnaire scores were calculated using the recommended scoring approach, suggested by the creators, whereby all positive responses are summed: the instrument does not have any ‘no’ boxes to indicate activities that are not affected. Modified Von Korff scores were calculated only for those participants with a full set of responses to ensure domain scores can be calculated. Short Form questionnaire-12 items version 2 (SF-12v2) scoring software was used to calculate SF-12v2113 mental and physical component scores. 2009 US norms were used to calculate scores in line with developer advice. 125 EQ-5D-5L scores were calculated using the most recent available UK value set published in January 2016. 126 Warwick–Edinburgh Mental Well-Being Scale (WEMWEBS)116 and all three Depression, Anxiety, and Positive Outlook Scale (DAPOS)111 domain scores were calculated for those participants with a full set of responses. The Pain Self-Efficacy Questionnaire (PSEQ)112 was scored and presented as the creators recommended. The modified PGI109,110 scores were calculated by multiplying the score given to each domain by the points allocated to the domain. This sum was then divided by the number of points awarded, which was 10 for all participants, and transformed into a 0–100 scale for ease of interpretation.
Text messaging scores
For the interpretation of the text messaging results, we developed a set of three simple assumptions and imputations to manage duplicate text responses, a mixture of text and diary responses and missing data:
-
If two scores were submitted on the same day then the earliest score that was received after 6 p.m. was used for analysis. The other score was ignored.
-
If a text response was received instead of an integer, it was then converted into a score (e.g. nine converted to 9).
-
If a weekly score was received after the specified day, then the score was used. For example, if a score was requested on day 49 and received on day 50 during the weekly follow-ups then this observation counted as the day 50 score.
Area under the curve calculation: scoring algorithm details and details of assumptions
To calculate AUC scores, a full set of 42 observations (35 daily and seven weekly scores) must be specified. There is missingness in both the text message and paper diaries so a scoring algorithm was created in order to analyse a complete set of scores for each participant. The development of the algorithm used the following rules.
-
A blank score for each day is created.
-
If there is a valid text message response for this day, then the blank value is replaced with the text message score.
-
If the participant did not register with the text messaging service and there is a valid paper diary score, then the blank value is replaced with this paper diary score.
-
If the participant has completed only one data source (either text message or paper diaries) and day X is missing in the middle of the data set (not day 1 or day 84), then the score for day X is calculated as:
-
If two or more adjacent scores are missing, then a monotonic assumption is made for the missing values between the most recent valid score and the next available valid daily score. For example if two consecutive scores are missing, day X and day X + 1, then the scores for day X – 1 and day X + 2 are used to calculate the imputed values for day X and day X + 1 as follows:
-
If the participant has agreed to complete both data sources and has completed both, then the text message score is used.
-
If the participant has agreed to complete both data sources and the text message score is missing, then the paper-based score is used.
-
If the participant has agreed to complete both data sources and both scores are missing, then the method described above to calculate day X scores is used.
-
If day 1 is missing, then the day 1 score will be calculated using the Modified Von Korff pain scale from the baseline questionnaire, if available. If this is missing then the first valid observation for this participant is backfilled.
-
If day 84 is missing, then the pain score reported on the 3-month follow-up questionnaire will be used, if available. If this score is missing then the last observation carried forward is used.
Area under the curve analysis
The AUC was calculated for each participant with a complete set of pain scores from day 0 to day 84 (week 12). Stata/SE 14.1 was used for all data analysis. The pkexamine command was used to calculate an AUC value for each participant. The pkexamine command is part of a family of commands available in Stata, namely the pk family of commands, which allows users to perform analyses of pharmacokinetic data including AUC analyses. 127 The pkexamine command allows the user to specify which rule should be used for calculating AUC results. For these, data cubic splines were used to calculate scores. AUC was then calculated based on all data points from 0 (day 1) to the final measurement (day 84/week 12).
Health economic analyses
Objectives
An economic analysis was conducted as part of the early termination. The objectives of the economic analysis were to:
-
estimate the health-care and broader resource use and costs of FJIs and BUC, the two interventions being evaluated in the study
-
evaluate the performance of trial CRFs (and our client service receipt inventory forms) for collecting health-care and broader resource utilisation data over a 3-month follow-up period that will inform any future trial-based economic evaluation
-
estimate the health-care and broader resource utilisation and costs over the 3-month follow-up period of the feasibility study and provide a description of key cost drivers
-
describe the health-related quality of life profile of study participants over the 3-month follow-up period.
Cost of facet joint injections and best usual care for low back pain
Prior to receiving the decision to terminate the feasibility study, a micro-costing exercise was conducted to estimate the resource use and costs associated with provision of FJIs and BUC for LBP. BUC consists of a package of physiotherapy sessions and exercises, details of which are outlined in the study protocol. Relevant resource inputs associated with the comparator interventions were collected from each participating centre and were combined with the unit cost of relevant resource inputs to estimate the likely costs of delivering the FJIs and BUC to NHS patients with LBP as well as variability in costs across centres.
Analyses of resource utilisation, economic costs and health-related quality of life
As part of the feasibility study, health-care and broader utilisation data were collected prospectively from participants recruited and randomised into the study using CRFs designed specifically for this study. The performance of the CRFs for collecting resource utilisation and health-related quality-of-life data for patients with LBP was evaluated. Appropriate sources of unit costs of resource use variables reported in the CRFs were obtained. Between-group comparisons of clinical effectiveness and cost-effectiveness outcomes were considered inappropriate given the small number of patients recruited before termination of the study. Hence, only descriptive summaries and graphs have been generated using all of the available data to give an indication of the probable health-related quality-of-life profile of patients with LBP as well as the health-care and broader resource utilisation and costs associated with the condition. To gauge appropriate time intervals for collecting health-related quality-of-life data (expressed in terms of health utilities) to inform a cost-effectiveness analysis of the interventions being evaluated, comparisons were made of quality-adjusted life-year (QALY) estimates based on daily and weekly reports of health-related quality-of-life (EQ-5D-5L) data over the 3-month follow-up period and the QALYs generated using only baseline and 3-month data.
Process evaluation
A process evaluation was undertaken using mixed methodologies. Data included quantitative data collected as part of recording trial activity (e.g. attendance rates, compliance) and qualitative data from interviews and small group discussions with patients, research therapists and staff.
Table 9 outlines the key items of the evaluation and the data and how these were analysed. Our items are based on established frameworks128,129 and our experience of implementing these frameworks. 130–133 In addition, we include outcome data in this process evaluation, as these are not being analysed elsewhere.
| Item | Data | Analysis |
|---|---|---|
| Context: description of the clinical site where recruitment was undertaken |
|
|
| Reach: have we reached the right population? |
|
Proportion of expected patient population recruited to trial at each site and overall |
| Recruitment: of field sites and trial participants |
|
|
| Dose delivered |
|
|
| Dose received | Patient interviews | Patient perception of intervention received |
| Outcome data |
|
|
| Impact | Patient interviews | Thematic analysis of patient interviews for patient perception of impact of intervention |
Patient Generated Index
The majority of PROMs, such as the RMDQ83 and Von Korff pain grade,99 contain a set number of standardised questions that may or may not include the outcomes that are considered important to patients. Individualised measures, such as the PGI, provide an alternative assessment format that enables respondents to specify those areas of life affected by the nominated condition that they judge to be most important, thus increasing content validity and relevance of assessment to the individual. 134 An international expert panel has recommended use of the PGI, alongside more standardised PROMs specific to LBP assessment, as a method of assessing the individualised nature of LBP and the meaning of recovery. 63
The PGI was originally developed in the UK in the 1990s for application across a range of conditions,135 including LBP. 135,136 Although PGI was widely utilised in prospective cohort studies to complement more traditional PROM-based assessment (e.g. Haywood et al. 137), the complicated formats of earlier versions meant that its application was limited in clinical trial settings. 134,138 However, recent revisions of the PGI have resulted in improved acceptability and completion rates. 137,139 The version of the PGI evaluated in this study retains the three completion stages of the original, but scoring (stage 2) and point spending (stage 3) have been revised. Stage 1 requires the respondents to list up to five important areas of life affected by their LBP. This is facilitated by the provision of a ‘trigger list’ generated for the target condition:134 a 17-item trigger list was created, informed by qualitative evidence of the impact of LBP,63,64,140,141 items included in widely used LBP-specific measures63,65 and recent application of the PGI in a Norwegian LBP population113 (Box 9).
Pain, sleep, fatigue.
Work, social life, hobbies, completing tasks, housework.
Driving, travelling, walking.
Relationships – with family or friends, sexual relationships.
Feeling depressed, loss of self-esteem, self-image.
Slow to do things.
A sixth predefined area relates to all other areas of life affected by their LBP and not listed separately. Stage 2 utilises a shorter, 7-point response scale (each nominated area is scored between 0 and 6, where 0 is as ‘bad as could possibly be’ and 6 is ‘as good as could possibly be’). Finally, in stage 3, a reduced number of points are available; respondents spend a total of 10 points to indicate the areas in which they would most value an improvement. A closed format version of the PGI was adopted for the clinical trial. At follow-up, respondents were presented with the first and the last columns completed using data brought forward from their baseline forms. They were then asked to score each item (stage 2) related to how they felt about it now.
The PGI score is generated by multiplying the area scores (stage 2) by the proportion of points awarded (stage 3) and summing and transforming to a 0–100 score, in which higher scores are representative of a better quality of life. 134 Here, we describe the first application of a revised format of the PGI in a UK LBP patient cohort and the first application of the revised format in a clinical trial setting.
Chapter 5 Results
Recruitment started on 26 June 2015 and was terminated by the funder on 11 December 2015 because of poor recruitment, at which point 26 participants had been randomised. The reasons for slow recruitment are described in detail in the process evaluation. In total, 320 people were approached about the study and 164 (51%) completed a screening questionnaire. Of these, 56 (34%) were interested in and appeared to be eligible for the study. At the time that recruitment closed 33 people had been assessed for the study, 27 of whom (81%) had suspected facet joint pain. One was not randomised because of the termination of recruitment, which meant that the final randomised total was 26 people. Assuming that a similar conversion rate in potential participants had not yet been assessed, we would have randomised around 42 participants from the 320 approached, a conversion rate of around 13% (Figure 4 and Table 10). In the original grant application we expected to approach 1500 people, with the aim of recruiting 150 participants: a conversion rate of 10%.
FIGURE 4.
The Consolidated Standards of Reporting Trials (CONSORT) chart.

| Variables | N | Mean | SD | Median | Minimum | Maximum | Missing | N/A |
|---|---|---|---|---|---|---|---|---|
| Continuous variables | ||||||||
| Age (years) | 25 | 53 | 14.4 | 56 | 30 | 80 | 0 | |
| Age left full-time education (years) | 25 | 17 | 3.0 | 16 | 15 | 27 | 0 | |
| If working, number of hours’ work per week | 15 | 33 | 9.4 | 37 | 16 | 48 | 0 | 10 |
| Categorical variables | ||||||||
| Gender, n (%) | ||||||||
| Male | 6 (24) | |||||||
| Female | 19 (76) | |||||||
| Missing | 0 (0) | |||||||
| Current work status, n (%) | ||||||||
| Full-time | 11 (44) | |||||||
| Part-time | 4 (16) | |||||||
| Not at the moment | 10 (40) | |||||||
| Missing | 0 (0) | |||||||
| If not working, which applies, n (%) | ||||||||
| Retired | 8 (80) | |||||||
| At home | 0 | |||||||
| Unable to work because of LBP | 0 | |||||||
| Unable to work because of illness | 1 (10) | |||||||
| Unemployed and looking | 0 | |||||||
| In full-time education | 0 | |||||||
| Other | 1 (10) | |||||||
| N/A | 15 | |||||||
Feasibility randomised controlled trial results
One baseline questionnaire (4%) was not received by the co-ordinating centre. The mean age of participants was 53 years (range 30–80 years). Three-quarters (19/25, 76%) of participants were female and 10 out of 25 (40%) were not working at the time. No participants were prevented from working by back pain; 8 out of 25 (32%) were retired from work.
Baseline patient-reported outcomes (Table 11) indicate a population with substantial pain and disability and an overall poor quality of life. Many participants had at least moderately troublesome pain in other parts of the body (Table 12). Five (20%) participants had ‘at least moderately troublesome’ pain for only LBP. Approximately half of our participants (12/25, 48%) also had at least moderately troublesome headaches.
| Outcome measures | N | Mean | SD | Median | Minimum | Maximum | Missing |
|---|---|---|---|---|---|---|---|
| Continuous variables | |||||||
| RMDQ83 (0–24)a | 25 | 11.6 | 4.5 | 12 | 2 | 20 | 0 |
| MVK questionnaire disability score84,99 (0–100)a | 24 | 65 | 20.5 | 68 | 30 | 100 | 1 |
| MVK questionnaire pain scale84 (0–100)a | 25 | 70 | 18.9 | 70 | 34 | 96 | 0 |
| SF-12v2 physical component score113 (0–100)b | 24 | 31.6 | 7.7 | 30.0 | 19.0 | 50.0 | 1 |
| SF-12v2 mental component score113 (0–100)b | 24 | 42.7 | 9.6 | 42.1 | 26.0 | 60.3 | 1 |
| EQ-5D-5L score114,115 (–0.11 to 1)b | 24 | 0.547 | 0.25 | 0.543 | 0.117 | 0.942 | 1 |
| EQ-5D VAS114,115 (0–100)b | 25 | 59 | 20.6 | 60 | 15 | 95 | 0 |
| WEMWEBS116 (14–70)b | 24 | 45.7 | 9.6 | 44.5 | 26 | 62 | 1 |
| DAPOS – Depression Scale111 (5–25)a | 25 | 9.8 | 3.9 | 9 | 5 | 20 | 0 |
| DAPOS – Anxiety Scale111 (3–15)a | 25 | 6.1 | 3.2 | 5 | 3 | 15 | 0 |
| DAPOS – Positive Outlook Scale111 (3–15)a | 25 | 10.7 | 2.5 | 11 | 5 | 15 | 0 |
| PSEQ112 (0–60)b | 24 | 32.7 | 12.8 | 34 | 3 | 52 | 1 |
| PGI109,110 (0–100)b | 25 | 38.6 | 18.3 | 36 | 0 | 72 | 0 |
| Categorical variables | |||||||
| Current health satisfaction, n (%) | |||||||
| Very dissatisfied | 12 (48) | ||||||
| Somewhat dissatisfied | 10 (40) | ||||||
| Neither satisfied nor dissatisfied | 2 (8) | ||||||
| Somewhat satisfied | 0 (0) | ||||||
| Very satisfied | 1 (4) | ||||||
| Missing | 0 | ||||||
| Troublesomeness grid symptom | Less than moderately troublesome, n (%) | At least moderately troublesome, n (%) | Missing, n (%) |
|---|---|---|---|
| Headache | 13 (52) | 12 (48) | 0 (0) |
| Neck pain | 18 (72) | 7 (28) | 0 (0) |
| Shoulder pain | 21 (84) | 4 (16) | 0 (0) |
| Elbow pain | 23 (92) | 2 (8) | 0 (0) |
| Wrist/hand pain | 21 (84) | 4 (16) | 0 (0) |
| Chest pain | 25 (100) | 0 (0) | 0 (0) |
| Abdominal pain | 25 (100) | 0 (0) | 0 (0) |
| Upper back pain | 20 (80) | 4 (16) | 1 (4) |
| LBP | 0 (0) | 25 (100) | 0 (0) |
| Hip/thigh pain | 17 68) | 8 (32) | 0 (0) |
| Knee pain | 21 (84) | 4 (16) | 0 (0) |
| Ankle/foot pain | 24 (96) | 1 (4) | 0 (0) |
| Other pains | 23 (92) | 2 (8) | 0 (0) |
| Pain in at least one other area | 5 (20) | 20 (80) | 0 (0) |
Diagnostic assessment
We obtained diagnostic assessment data on 27 participants (Table 13). These show that our participants typically had very longstanding LBP, had tried multiple previous treatments and had continued with very or extremely troublesome LBP.
| Condition | Item | Total, n (%) |
|---|---|---|
| How long has the participant had back pain? | 6–12 months | 3 (11) |
| 1–2 years | 3 (11) | |
| 2–5 years | 8 (30) | |
| > 5 years | 13 (48) | |
| Previous back pain treatmentsa | Physiotherapy | 25 (93) |
| Osteopathy | 3 (11) | |
| Chiropractic | 8 (30) | |
| Acupuncture | 7 (26) | |
| Other | 3 (11) | |
| Troublesomeness of back pain during assessment visit | Moderately troublesome | 10 (37) |
| Very troublesome | 8 (30) | |
| Extremely troublesome | 9 (33) | |
| When undertaking active movements did the patient indicatea | Increased pain on rising from flexion | 13 (48) |
| Symptoms best on walking | 16 (63) | |
| Symptoms best when sitting | 11 (41) | |
| Onset of pain paraspinal | 20 (74) |
Best usual care
Adherence to study treatments was good. All participants, inevitably, attended the first BUC session. Three people (23%) randomised to BUC only subsequently withdrew from BUC, two because their LBP did not improve and one because they had problems travelling to the intervention. One of these participants also withdrew from follow-up. Attendance at BUC was good, with 21 out of 26 (81%) attending at least four treatment sessions (Table 14). Overall, the process data indicate that participants received the essential components of the package. This included acceptance, goal-setting and pacing. Participants were also given specific homework tasks to complete between sessions. In comparison with other studies, adherence for CBT for chronic back pain has shown to be associated with treatment gains in a variety of outcome measures including accomplishment of daily goals. 142 Attendance was good, with 23 participants (88%) attending at least half of the sessions.
| Category | Subcategory | Session | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Number of participants attending session, n (%) | 26a (100) | 24 (92) | 23 (88) | 21 (81) | 17 (65) | 15 (58) | |
| Components used during sessions | |||||||
| Acceptance | 26 | 19 | 15 | 12 | 9 | 8 | |
| Goal-setting | 24 | 19 | 18 | 12 | 9 | 8 | |
| Pacing | 25 | 20 | 16 | 14 | 12 | 8 | |
| Exercises | Specific | 9 | 10 | 11 | 10 | 8 | 5 |
| Motor control retraining | 7 | 10 | 11 | 7 | 8 | 6 | |
| Cardiovascular | 1 | 2 | 3 | 3 | 2 | 3 | |
| Strength | 6 | 9 | 11 | 7 | 10 | 5 | |
| Stretches | 19 | 10 | 14 | 10 | 6 | 5 | |
| Other exercises | 4 | 0 | 0 | 0 | 0 | 0 | |
| Advice | Pain terminology, mechanisms and pathways | 12 | 8 | 7 | 3 | 2 | 1 |
| Activities of daily living | 11 | 12 | 8 | 6 | 4 | 6 | |
| Work and ergonomics | 6 | 3 | 5 | 3 | 4 | 1 | |
| Lifestyle changes | 5 | 6 | 5 | 4 | 4 | 2 | |
| Management of flare ups and changing symptoms | 2 | 8 | 5 | 6 | 5 | 8 | |
| Paced home exercises | 12 | 9 | 12 | 4 | 6 | 8 | |
| Other advice | 2 | 1 | 0 | 0 | 1 | 1 | |
| Manual therapy | Kaltenborn | 0 | 0 | 0 | 0 | 0 | 0 |
| McKenzie | 4 | 3 | 3 | 2 | 2 | 1 | |
| Maitland | 5 | 5 | 5 | 3 | 0 | 0 | |
| Cyriax | 0 | 0 | 0 | 0 | 0 | 0 | |
| Osteopathic techniques | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mulligans | 1 | 0 | 1 | 1 | 0 | 0 | |
| NAGs/SNAGs/MVMs | 1 | 1 | 1 | 1 | 0 | 0 | |
| Other manual therapy | 0 | 0 | 0 | 0 | 0 | 0 | |
| Soft tissue | Myofascial | 4 | 4 | 2 | 1 | 3 | 2 |
| Trigger point | 3 | 4 | 1 | 2 | 2 | 2 | |
| Soft tissue massage | 0 | 0 | 0 | 0 | 1 | 1 | |
| Manipulation | 0 | 0 | 0 | 0 | 0 | 1 | |
| Soft tissue release | 1 | 1 | 2 | 2 | 1 | 1 | |
| Other soft tissue | 0 | 0 | 0 | 0 | 0 | 0 | |
| Challenging negative thoughts | 5 | 5 | 5 | 3 | 3 | 1 | |
| Mindfulness | 3 | 1 | 3 | 3 | 4 | 3 | |
Facet joint injections
One person randomised to injection decided against this after their first BUC session. We obtained injection records for 12 out of 12 (100%) of those injected. These showed that most people had had at least one injection at each spinal level and over one-third had had six facet joints injected. There is evidence that clinicians were making a judgement as to which joints were appropriate to inject. There was a wide variation in the reported time of exposure to radiation, ranging from 35 to 600 seconds. We have not been able to resolve queries on the total radiation exposure because of the incompatibility of different approaches to radiation exposure and different machinery. We also have some concerns about the quality of recorded data on the duration of radiation exposure because of the wide difference in times recorded.
We had immediate pre-injection and 60-minute post-injection data on pain for 12 participants (Figure 5). There was an overall reduction in pain scores, falling from 5.9 to 2.6 following injection. Seven participants reported a > 50% reduction in pain, five of whom reported that they were pain free following the injection. One participant reported that their pain was worse, and their pain score increased from 3 to 6 (Table 15). This study was not designed to assess the validity of our clinical assessment of possible facet joint pain against a gold-standard test. 119 Nevertheless, these data suggest that we have identified a population that has some potential to benefit from intra-articular FJIs, with one-third of participants achieving the target level of immediate pain relief (a 50% reduction) suggested by the consensus conference.
FIGURE 5.
Box plot of pre- and post-injection pain scores.

| Variable | Description | FJI and BUC only (N = 12) |
|---|---|---|
| Category | ||
| Pain score immediately before injection (within 60 minutes) | Mean | 5.9 |
| n | 12 | |
| SD | 2.1 | |
| Median | 6.5 | |
| Minimum | 3 | |
| Maximum | 9 | |
| Missing | 0 | |
| Total number of facet joints injected, n (%) | 1 | 1 (8) |
| 2 | 2 (17) | |
| 3 | 2 (17) | |
| 4 | 2 (17) | |
| 5 | 0 (0) | |
| 6 | 5 (42) | |
| Missing | 0 (0) | |
| Which joints injected, n (%) | L3/L4, L4/L5 and L5/S1 | 7 (59) |
| L4/L5 and L5/S1 | 4 (33) | |
| L3/L4 only | 1 (8) | |
| Missing | 0 (0) | |
| Resistance to injection?, n (%) | Yes | 5 (42) |
| No | 7 (58) | |
| Missing | 0 (0) | |
| If yes to resistance, reason for resistance (more than one reason allowed), n (%) | Abutment of needle bevel to surface | 3 (60) |
| Filing of the intra-articular space | 4 (80) | |
| Other | 0 (0) | |
| Pain score immediately AFTER injection (within 60 minutes) | Mean | 2.6 |
| n | 12 | |
| SD | 3.0 | |
| Median | 2 | |
| Minimum | 0 | |
| Maximum | 9 | |
| Missing | 0 | |
| Cumulative exposure time (seconds) | Mean | 199 |
| n | 12 | |
| SD | 242 | |
| Median | 60.5 | |
| Minimum | 35 | |
| Maximum | 600 | |
| Missing | 0 | |
| Injection site monitoring pre injection, if done, n (%) | Normal | 12 (100) |
| Bleeding | 0 (0) | |
| Haematoma | 0 (0) | |
| Redness | 0 (0) | |
| Infection | 0 (0) | |
| Other | 0 (0) | |
| Injection site monitoring post injection, if done, n (%) | Normal | 12 (100) |
| Bleeding | 0 (0) | |
| Haematoma | 0 (0) | |
| Redness | 0 (0) | |
| Infection | 0 (0) | |
| Other | 0 (0) | |
Adverse events
No adverse events resulting from either intervention were recorded.
Area under the curve for pain
Although there were missing data in the text messaging responses for pain, these were largely replaced with the results of the written pain diary (Table 16). A priori we set three assumptions for this, which were as follows:
-
If two text scores were submitted on the same day, then the earliest score that was received after 6 p.m. was used for the analysis. The other score would be ignored (n = 20 occasions).
-
If a written response was received instead of a number, then this was converted into a score (n = 1 occasion on which a participant replied ‘nine’ = pain and the score was calculated as 9 for this day).
-
If a weekly score was received after the specified day, then the score would be used. For example, if a score was requested on day 49 and received on day 50 during the weekly follow-ups, then this observation would count as the day 50 score (n = 8 occasions).
| Day | Text messaging (N = 16 registered) | Paper diaries (N = 14 registered) | ||
|---|---|---|---|---|
| n replies | % replied | n replies | % replied | |
| 1 | 12 | 75 | 14 | 100 |
| 2 | 12 | 75 | 14 | 100 |
| 3 | 12 | 75 | 14 | 100 |
| 4 | 11 | 69 | 14 | 100 |
| 5 | 12 | 75 | 14 | 100 |
| 6 | 14 | 88 | 14 | 100 |
| 7 | 15 | 94 | 14 | 100 |
| 8 | 14 | 88 | 14 | 100 |
| 9 | 16 | 100 | 14 | 100 |
| 10 | 16 | 100 | 14 | 100 |
| 11 | 16 | 100 | 14 | 100 |
| 12 | 15 | 94 | 14 | 100 |
| 13 | 16 | 100 | 14 | 100 |
| 14 | 15 | 94 | 13 | 93 |
| 15 | 16 | 100 | 14 | 100 |
| 16 | 14 | 88 | 14 | 100 |
| 17 | 14 | 88 | 14 | 100 |
| 18 | 13 | 81 | 14 | 100 |
| 19 | 14 | 88 | 14 | 100 |
| 20 | 14 | 88 | 13 | 93 |
| 21 | 13 | 81 | 13 | 93 |
| 22 | 11 | 69 | 13 | 93 |
| 23 | 13 | 81 | 13 | 93 |
| 24 | 11 | 69 | 12 | 86 |
| 25 | 15 | 94 | 13 | 93 |
| 26 | 14 | 88 | 13 | 93 |
| 27 | 13 | 81 | 13 | 93 |
| 28 | 15 | 94 | 13 | 93 |
| 29 | 13 | 81 | 13 | 93 |
| 30 | 14 | 88 | 13 | 93 |
| 31 | 15 | 94 | 13 | 93 |
| 32 | 14 | 88 | 13 | 93 |
| 33 | 14 | 88 | 13 | 93 |
| 34 | 11 | 69 | 12 | 86 |
| 35 | 14 | 88 | 12 | 86 |
| Week | ||||
| 6 | 13 | 81 | 12 | 86 |
| 7 | 13 | 81 | 13 | 93 |
| 8 | 13 | 81 | 12 | 86 |
| 9 | 11 | 69 | 12 | 86 |
| 10 | 12 | 75 | 12 | 86 |
| 11 | 11 | 69 | 12 | 86 |
| 12 | 12 | 75 | 11 | 79 |
A simple imputation rule allowed us to estimate the AUC when no values were available for any individual day. This has provided good-quality data, allowing us to see the pattern of pain over the initial post-randomisation period. We achieved usable outcome data on 23 out of 26 (88%) of our participants for this, our primary outcome (Table 17). Visual inspection of the graph of the pooled outcome over time shows that there is a modest improvement over the first 35 days of daily measurement, with little further improvement after this time (Figure 6). This is also reflected in the responses given to questions on improvement since baseline in the 3-month questionnaire.
| Measure | Mean | 95% CI | n | SD | Median | Minimum | Maximum | Missing |
|---|---|---|---|---|---|---|---|---|
| AUC | 454 | 392.8 to 515.2 | 23 | 141.5 | 464.6 | 178.6 | 696.8 | 0 |
FIGURE 6.
Participants’ average pain score over lifetime of the project (n = 23).
The plot (see Figure 6) represents the mean pain score for all participants on each given day of data collection and shows the overall trajectory of pain scores. The dotted vertical line indicates the proposed date of the intervention at day 7 and the solid line at day 35 represents the transition point from daily scores to weekly scores.
Three-month questionnaire
Seven 3-month questionnaires were not received, resulting in a follow-up rate of 73% for the 3-month questionnaire outcomes. One of these participants withdrew from questionnaire follow-up prior to 3-month follow-up and the remainder did not respond.
The small numbers mean that extreme caution is needed in interpreting the follow-up findings. Nevertheless, all pain-related outcomes show the expected improvement between baseline and follow-up. For example, for the RMDQ the difference is 2.8 points on a 0–24 scale and for the Modified Von Korff pain scale the difference is 10 points on a 0–100 scale. However, for psychological and attitudinal outcomes (SF-12v2 mental component score, WEMWEBS, DAPOS and PSEQ) there does not appear to have been any meaningful change between baseline and follow-up. With the exception of the PGI, all of the measures used are established outcome measures and we will not assess their performance further within this study (Table 18).
| Outcome measures | Mean | N | SD | Median | Minimum | Maximum | Missing |
|---|---|---|---|---|---|---|---|
| Continuous variables | |||||||
| Back pain scale143 (0–10)a | 5.8 | 18 | 1.9 | 6 | 3 | 8 | 1 |
| RMDQ83 (0–24)a | 8.8 | 18 | 4.9 | 9 | 1 | 19 | 1 |
| MVK questionnaire disability score84,99 (0–100)a | 48.7 | 19 | 27.7 | 54 | 4 | 100 | 0 |
| MVK questionnaire pain scale84 (0–100)a | 60 | 19 | 21.2 | 64 | 30 | 86 | 0 |
| SF-12v2 physical component score113 (0–100)b | 46.6 | 17 | 8.1 | 46 | 32.5 | 61.4 | 2 |
| SF-12v2 mental component score113 (0–100)b | 37.6 | 17 | 9 | 37.9 | 22.3 | 52.9 | 12 |
| EQ-5D-5L Crosswalk Index Score114,115 (–0.11 to 1)b | 0.644 | 19 | 0.249 | 0.71 | 0.084 | 0.942 | 0 |
| EQ-5D VAS114,115 (0–100)b | 65.3 | 19 | 17.4 | 65 | 20 | 90 | 0 |
| WEMWEBS116 (14–70)b | 42.7 | 15 | 12.2 | 47 | 24 | 58 | 4 |
| DAPOS – Depression Scale111 (5–25)a | 10.4 | 17 | 5.0 | 11 | 5 | 20 | 2 |
| DAPOS – Anxiety Scale111 (3–15)a | 6.7 | 17 | 3.4 | 7 | 3 | 13 | 2 |
| DAPOS – Positive Outlook Scale111 (3–15)a | 9.7 | 17 | 2.8 | 9 | 5 | 15 | 2 |
| PSEQ112 (0–60)b | 34.2 | 17 | 15.4 | 38 | 8 | 56 | 2 |
| PGI109,110 (0–100)b | 50 | 16 | 23.2 | 49 | 12 | 100 | 3 |
| Categorical variables | |||||||
| Change in LBP, n (%) | |||||||
| Vastly worse | 0 (0) | ||||||
| Much worse | 1 (5) | ||||||
| Slightly worse | 0 (0) | ||||||
| No change | 7 (37) | ||||||
| Slightly better | 6 (32) | ||||||
| Much better | 4 (21) | ||||||
| Completely better | 0 (0) | ||||||
| Missing | 1 (5) | ||||||
| Change in ability to perform daily tasks, n (%) | |||||||
| Vastly worse | 0 (0) | ||||||
| Much worse | 0 (0) | ||||||
| Slightly worse | 1 (5) | ||||||
| No change | 8 (42) | ||||||
| Slightly better | 6 (32) | ||||||
| Much better | 3 (16) | ||||||
| Completely better | 0 (0) | ||||||
| Missing | 1 (5) | ||||||
| Troublesomeness of lower back symptoms in past 4 weeks, n (%) | |||||||
| No pain experienced | 0 (0) | ||||||
| Not at all troublesome | 0 (0) | ||||||
| Slightly troublesome | 2 (11) | ||||||
| Moderately troublesome | 10 (53) | ||||||
| Very troublesome | 6 (31) | ||||||
| Extremely troublesome | 0 (0) | ||||||
| Missing | 1 (5) | ||||||
| Benefit gained from treatment or advice received since joining the study, n (%) | |||||||
| Substantial harm | 0 (0) | ||||||
| Moderate harm | 1 (5) | ||||||
| No benefit | 5 (26) | ||||||
| Moderate benefit | 10 (53) | ||||||
| Substantial benefit | 2 (11) | ||||||
| Missing | 1 (5) | ||||||
| Satisfaction with treatment received for back pain, n (%) | |||||||
| Very dissatisfied | 2 (11) | ||||||
| Somewhat dissatisfied | 2 (11) | ||||||
| Neither satisfied nor dissatisfied | 3 (16) | ||||||
| Somewhat satisfied | 6 (31) | ||||||
| Very satisfied | 5 (26) | ||||||
| Missing | 1 (5) | ||||||
| Satisfaction with current health, in relation to LBP, n (%) | |||||||
| Very dissatisfied | 3 (16) | ||||||
| Somewhat dissatisfied | 3 (16) | ||||||
| Neither satisfied nor dissatisfied | 5 (26) | ||||||
| Somewhat satisfied | 7 (37) | ||||||
| Very satisfied | 0 (0) | ||||||
| Missing | 1 (5) | ||||||
Results of Patient Generated Index completion
The PGI was correctly completed by all patients who returned a baseline questionnaire (n = 25); a score was calculable for all participants. All participants correctly completed stage 1. The small numbers limited a meaningful evaluation of the psychometric properties of the PGI in this population [COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN)]. 144 However, the content validity of PGI completion was explored for stage 1.
The baseline PGI scores were a mean of 38.6 (SD 18.3, median 36, range 0–72), on a scale of 0–100, where 100 is the best quality of life.
At the 3-month follow-up, 16 out of 19 (84%) participants completed the PGI. The mean score had increased to 50 (SD 23.2, median 49, range 12–100) (see Table 18).
Content validity
All patients completing the PGI entered at least three areas in stage 1 (n = 23, range 3–5). Five areas were entered by 18 participants (69.5), four by two participants (8.6%) and three by five participants (21.7%). Most respondents (24/25) completed the sixth item: ‘all other areas affected by your LBP and not listed above’.
A total of 33 areas, including 14 out of 17 of the trigger list items, were listed in stage 1 (Table 19). The most frequently listed areas affected by LBP were walking (n = 13), sleeping (n = 12), driving (n = 10) and work (n = 10) (see Table 19).
| Trigger list item? | Rank | Area of importance | Frequency (%) |
|---|---|---|---|
| Yes | 1 | Walking | 13 (52) |
| Yes | 2 | Sleeping | 12 (48) |
| Yes | 3 | Driving | 10 (40) |
| Yes | 4 | Work | 10 (40) |
| Yes/no | = 5 | Hobbies (4) plus recreation (2) plus dancing (1) (combined) | 7 (28) |
| Yes | = 5 | Housework | 7 (28) |
| Yes | 7 | Pain | 6 (24) |
| Yes | 8 | Fatigue | 5 (20) |
| No | = 9 | Sitting | 4 (16) |
| Yes | = 9 | Sexual relations | 3 (12) |
| Yes | = 9 | Travelling | 3 (12) |
| Yes | = 9 | Self-esteem | 3 (12) |
| Yes | = 9 | Relationships (1) plus relationships family/friends (1) plus family (1) | 3 (12) |
| Yes | = 9 | Social life | 3 (12) |
| No | = 14 | Sport: cycling/football/rugby | 2 (8) |
| No | = 14 | Decorating (1)/do-it-yourself (1) | 2 (8) |
| Yes | = 14 | Slow to do things | 2 (8) |
| No | = 14 | Shower (1)/washing (1) | 2 (8) |
| No | Lifting granddaughter/lifting | 2 (8) | |
| No | Family activities | 1 (4) | |
| No | Spectating: sport | 1 (4) | |
| No | Standing | 1 (4) | |
| No | Toilet | 1 (4) | |
| No | Putting underwear on | 1 (4) | |
| No | Motorcycle riding | 1 (4) | |
| No | Gardening | 1 (4) | |
| No | Looking after my kids | 1 (4) | |
| No | Playing with my child | 1 (4) | |
| No | Weight gain because of lack of exercise | 1 (4) | |
| No | Quality of life | 1 (4) | |
| No | Not being able to do what other people my age do and what I used to do | 1 (4) | |
| No | Shopping | 1 (4) | |
| No | Moaning | 1 (4) |
The 33 areas were categorised into seven broad themes (Table 20): symptoms, activities/function, basic activities of daily living, emotional well-being, participation (social function and social relationships) and other. A miscellaneous category was also created. The frequency with which items were listed reflects the importance of the impact of LBP on functional activities and the symptomology associated with LBP, followed by the impact on social function and relationships.
| Area of importance | Count (total 113) | Frequency % |
|---|---|---|
| Symptoms | ||
| Sleeping | 12 | 48 |
| Pain | 6 | 24 |
| Fatigue | 5 | 22 |
| Activities/function | ||
| Walking | 13 | 52 |
| Housework | 7 | 28 |
| Lifting granddaughter/lifting | 2 | 4 |
| Decorating/do-it-yourself | 2 | 9 |
| Gardening | 1 | 4 |
| Shopping | 1 | 4 |
| Sitting | 4 | 16 |
| Standing | 1 | 4 |
| Driving | 10 | 43 |
| Travelling | 3 | 12 |
| Motorcycle riding | 1 | 4 |
| Basic activities of daily living | ||
| Shower/washing | 2 | 9 |
| Toilet | 1 | 4 |
| Putting underwear on | 1 | 4 |
| Emotional well-being | ||
| Self-esteem | 3 | 12 |
| Moaning | 1 | 4 |
| Participation | ||
| Social function | ||
| Work | 10 | 39 |
| Recreation/hobbies (combined) | 7 | 26 |
| Sport: cycling/football/rugby | 2 | 8 |
| Spectating: sport | 1 | 4 |
| Family activities | 1 | 4 |
| Social relationships | ||
| Relationships family/friends | 3 | 12 |
| Social life | 3 | 12 |
| Looking after my kids | 1 | 4 |
| Playing with my child | 1 | 4 |
| Sexual relations | 3 | 12 |
| Other | ||
| Slow to do things | 2 | 8 |
| Weight gain because of lack of exercise | 1 | 4 |
| Quality of life | 1 | 4 |
| Not being able to do what other people my age do and what I used to do | 1 | 4 |
Health economic results
Resource use and cost estimates of facet joint injection treatment for low back pain relief
The average resource use and costs estimates associated with the delivery of the FJI procedure for LBP relief are presented in Table 21. Cost categories included use of the treatment room/injection facility (equipped with a radiograph or equipped with C-arm fluoroscopy machine, resuscitation trolley and/or monitoring back-up equipment), staff time, anaesthetic drugs and disposable medical consumables. The mean duration of the FJI procedure was estimated to be 36 minutes (range 15–60 minutes) based on data from four of the trial participating centres. The unit costs for resource use variables were obtained primarily from the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2015 compendium146 and the NHS Prescription Cost Analysis 2015 database. 147 The unit costs of syringes and needles and other medical consumables were obtained from online sources (www.medisave.co.uk, www.farlamedical.co.uk; both accessed 21 November 2015) when more direct NHS sources were unavailable. The cost of the treatment room facility was estimated to be £183.34. This was based on treatment lasting 36 minutes on average and on a treatment room cost of £175 per hour as reported in a report for NICE Technology Appraisal 279 (Stevenson et al. 145) and updated to 2015 prices using the NHS Hospital & Community Health Services inflation index. 146 It was assumed that the FJIs are performed in a similar treatment facility as percutaneous vertebroplasty or percutaneous balloon kyphoplasty for treatment of osteoporotic vertebral fractures, the interventions being appraised in NICE Technology Appraisal 279. The mean total cost of 36 minutes of clinical staff time was £191.60 and the mean total cost of drugs and disposable medical equipment/consumables was £44.28 per patient, respectively. Thus, the mean total cost of performing a FJI was estimated at £419.22 per patient.
| Resource use category | Unit of analysis | Quantity (range) | Unit costa (£) | Source of unit cost | Cost per patient (£) |
|---|---|---|---|---|---|
| Mean treatment duration | Minutes | 36 (15–60) | – | – | – |
| Treatment room | Minutes | 36 | 305.57b | Stevenson et al. (2012)145 | 183.34 |
| Staffc | |||||
| Consultant physician | 1 | 140.00 | PSSRU 2015146 | 84.00 | |
| Nurse, band 6 | 2 | 51.00 | PSSRU 2015146 | 61.20 | |
| Radiographer, band 6 | 1 | 40.00 | PSSRU 2015146 | 24.00 | |
| Health-care assistant | 1 | 24.00 | PSSRU 2015146 | 14.40 | |
| Administration support | Minutes | 20 | 20.00 | PSSRU 2015146 | 8.00 |
| Total staffing cost | 191.60 | ||||
| Injection drugs/and medical disposables | |||||
| Intravenous access | 1 | 0.60 | Prescription Cost Analysis: England 2015 147 | 0.60 | |
| ChloraPrep® with Tint (CareFusion, Basingstoke, UK), 3-ml applicator | 1 | 18.55 | Prescription Cost Analysis: England 2015 147 | 18.55 | |
| Sterile pack/dressing | 1 | 0.43 | www.medisave.co.uk d | 0.43 | |
| Levobupivacaine (5.0 mg/ml) | 1 | 3.15 | Prescription Cost Analysis: England 2015 147 | 3.15 | |
| Triamcinolone (10 mg/ml), 1-mg ampoule | 3 (1–6) | 1.59 | Prescription Cost Analysis: England 2015 147 | 4.77 | |
| Local anaesthesia (Lidocaine) | 1 | 2.65 | Prescription Cost Analysis: England 2015 147 | 2.65 | |
| BD™ spinal needle (Becton Dickinson, Franklin Lakes, NJ, USA) (22 gauge) | 3 (1–6) | 2.06 | www.farlamedical.co.uk d | 6.18 | |
| Filter needle | 1 | 7.22 | www.medisave.co.uk d | 7.22 | |
| Green needle (21 gauge) | 1 | 0.03 | www.medisave.co.uk d | 0.03 | |
| Orange needle (25 gauge) | 1 | 0.03 | www.medisave.co.uk d | 0.03 | |
| Syringes (10 cc) | 1 | 0.15 | www.medisave.co.uk d | 0.15 | |
| Syringes (2 cc) | 3 (1–6) | 0.16 | www.medisave.co.uk d | 0.48 | |
| Disposable gloves | 1 (1–2) | 0.04 | www.medisave.co.uk d | 0.04 | |
| Total cost of drugs and disposables | 44.28 | ||||
Resource use and cost estimates for programme of physiotherapy treatment for low back pain
Table 22 presents the resource use and cost estimates associated with the delivery of the programme of physiotherapy treatment to relieve LBP as specified in the FIS feasibility protocol. The unit costs were obtained from the PSSRU Unit Cost 2015 compendium146 and the published Department of Health National Reference Cost 2014–15 schedules. 148 The mean total cost of the full course of physiotherapy treatment, consisting of one initial consultation (diagnostic assessment) followed by four follow-up treatment consultations, was estimated to be £264 per participant.
| Resource use variable | Number | Unit cost (£)a | Source | Total cost (£) |
|---|---|---|---|---|
| Physiotherapy, band 6 | ||||
| Diagnostic assessment (initial consultation) | 1 | 54.00 | WF01B, non-consultant lead services (National Reference Costs 2014/15)148 | 54.00 |
| Follow-up consultation | 5 | 42.00 | WF01A, non-consultant lead services (National Reference Costs 2014/15)148 | 210.00 |
| Total cost of full treatment programme | 264.00 | |||
The mean total cost of the overall treatment package (comprising one injection and six physiotherapy sessions) was estimated at £683.22 per participant. This is similar to a NHS tariff cost for a course of FJIs of £686.84 (National Reference Costs 2014/15148 – main schedule, currency code AB16Z). What is not included here is cost of the initial consultant appointment (£144.79, National Reference Costs 2014/15148 – main schedule) at which a decision to offer FJIs is made. The minimum overall cost of the overall FJI package is thus £831.63. This does not account for the care costs of patients who have a consultant appointment and do not proceed to FJIs.
Health-care utilisation and costs during trial follow-up
Tables 23–27 present summaries of patient self-reports of health-care resource use and costs for the 3-month period prior to randomisation (medication use only) and the 3-month post-randomisation follow-up period for all resource use categories including pain relief medication. In total, 25 (96%) and 19 (73%) of the 26 participants provided complete data on medication use at baseline and the 3-month follow-up time point, respectively. The total cost of prescribed pain relief tablets, patches and gels was £86.27 (standard error £37.33) per patient and £33.34 (standard error £18.79) per patient in the 3 months prior to randomisation and the 3 months following randomisation, respectively (see Table 23).
| Type of medication | Cost per patient (£), mean (standard error) | |
|---|---|---|
| 3 months pre randomisation (n = 25) | 3 months post randomisation (n = 19) | |
| Pain relief medication, gels and patches | ||
| Tabletsa | 85.64 (37.36) | 31.81 (18.84) |
| Gels | 0.00 (0.00) | 1.53 (1.53) |
| Patches/padsb | 0.63 (0.44) | 0.00 |
| Total medication use | 86.27 (37.33) | 33.34 (18.79) |
| Resource use variable | Resource utilisation, mean (SE) | Unit cost per contact (£) | Unit cost sourcea | Cost per patient (£), mean (SE) |
|---|---|---|---|---|
| Inpatient care | ||||
| Admitted care | 0.000 | 0.00 | 0.00 | |
| Day case | 0.059 (0.059) | 686.84 | AB16Z148 | 40.4 (40.40) |
| Outpatient care | ||||
| Consultant (pain clinic) | 0.412 (0.173) | 144.79 | Service code 191148 | 59.62 (25.01) |
| Physiotherapy | 1.000 (0.411) | 46 | Service code 650148 | 46 (18.92) |
| Radiology: MRI | 0.235 (0.106) | 137 | RD01A148 | 32.24 (14.53) |
| Radiology: CT | 0.059 (0.059) | 93 | RD20A148 | 5.47 (5.47) |
| Radiology: ultrasound | 0 | 55.00 | RD40Z148 | 0.00 |
| Blood testsb | 0 | 0.00 | 0.00 | |
| Hospital nurse (band 6)c | 0.059 (0.059) | 62.5 | PSSRU 2015146 | 3.68 (3.68) |
| Occupational therapyd | 0.059 (0.059) | 65.99 | Service code 651148 | 3.88 (3.88) |
| General surgery | 0.059 (0.059) | 138.65 | Service code 100148 | 8.16 (8.16) |
| Primary care | ||||
| GP surgery visit | 0.824 (0.395) | 44 | PSSRU 2015146 | 36.24 (17.39) |
| GP home visit | 0 | 59.05e | PSSRU 2013149 | 0.00 |
| Practice nurse | 0.118 (0.081) | 7.88 | PSSRU 2015146 | 0.93 (0.63) |
| District nurse: surgery | 0 | 12.73 | PSSRU 2015146 | 0.00 |
| District nurse: home visit | 0 | 12.73 | PSSRU 2015146 | 0.00 |
| Rehabilitation specialist | 0 | PSSRU 2015146 | 0.00 | |
| Physiotherapist: surgery visit | 0 | 38.00 | PSSRU 2015146 | 0.00 |
| Physiotherapist: home visit | 0 | 38.00 | PSSRU 2015146 | 0.00 |
| Occupational therapist | 0 | 38.00 | PSSRU 2015146 | 0.00 |
| Counsellor | 0 | 58.00 | PSSRU 2015146 | 0.00 |
| Psychologist | 0 | 225.37 | PSSRU 2015146 | 0.00 |
| Social worker | 0 | 95.00 | PSSRU 2015146 | 0.00 |
| Resource use variable | Mean (SE) resource use | Costs (£), mean (SE) | |
|---|---|---|---|
| Medical insurance contribution | Personal contribution | ||
| Physiotherapy | 0.059 (0.059) | 0.00 | 17.65 (17.65) |
| Bowen practitioner | 0.059 (0.059) | 0.00 | 20.59 (20.59) |
| Occupational therapy | 0 | 0.00 | 0.00 |
| Counsellor | 0 | 0.00 | 0.00 |
| Psychologist | 0 | 0.00 | 0.00 |
| Radiology: MRI | 0 | 0.00 | 0.00 |
| Radiology: CT | 0 | 0.00 | 0.00 |
| Radiology: radiograph | 0 | 0.00 | 0.00 |
| Radiology: ultrasound | 0 | 0.00 | 0.00 |
| Consultant service | 0 | 0.00 | 0.00 |
| Osteopath | 0 | 0.00 | 0.00 |
| Chiropractor | 0 | 0.00 | 0.00 |
| Acupuncturist | 0 | 0.00 | 0.00 |
| Homeopath | 0 | 0.00 | 0.00 |
| Special equipment use | 0 | 0.00 | 0.00 |
| Total private medical cost | 0.00 | 38.24 (38.24) | |
| Resource use variable | 3 months pre randomisation | 3 months post randomisation | ||
|---|---|---|---|---|
| n (%)a | Cost (£), mean (SE) | n (%)a | Cost (£), mean (SE) | |
| Pain relief medication, gels and patches | ||||
| Tablets | 25 (96) | 2.48 (1.26) | 19 (73) | 9.09 (3.46) |
| Gels | 25 (96) | 0.44 (0.44) | 19 (73) | 6.83 (2.63) |
| Patchesb | 25 (96) | 0.00 | 19 (73) | 2.37 (1.64) |
| Total over-the-counter medication | 25 (96) | 2.92 (1.30) | 19 (73) | 18.28 (5.05) |
| Travel costs (e.g. bus fares) incurred by patient | 14 (54) | 1.08 (1.08) | ||
| Travel costs incurred by partner of patient | 14 (54) | 1.86 (1.86) | ||
| Child care costs | 14 (54) | 0.00 | ||
| Cost of help with housework | 14 (54) | 0.00 | ||
| Cost of laundry services | 14 (54) | 0.00 | ||
| Total out-of-pocket costs | 14 (54) | 24.42 (6.89)c | ||
| Productivity variable | n (%) | Mean (SE) |
|---|---|---|
| Reported current employment status | 17 (65) | – |
| Employed | 9 (35) | 0.53 (0.121) |
| Not in employment because of ill health | 2 (8) | 0.25 (0.11) |
| Not in employment because of retirement | 6 (23) | 0.75 (0.11) |
| Lost income because of ill health (yes/no) | 11 (42) | 0.08 (0.08) |
| Work days lost because of ill health | 8 (31) | 2.78 (2.22) |
| Lost income (monetary terms) | 11 (42) | £34.08 (£34.08) |
Complete primary and secondary care utilisation data were available from 17 (65%) of the 26 study participants for the 3-month post-randomisation period (see Table 24). None of the study participants was admitted for an overnight stay in hospital and one patient was treated as a day case, generating a mean cost of £40.40 per patient. There was no reported use of some outpatient services such as blood tests and ultrasound investigations, as well as the majority of community care services. The mean (standard error) cost per patient for outpatient services ranged from £3.68 (£3.68) for hospital nursing services to £59.62 (£25.01) for consultant pain clinic services. The mean (standard error) cost of primary care services was £36.24 (£17.39) for GP surgery services and £0.93 (£0.63) for practice nursing services, with both lasting 11.7 minutes on average. The mean (standard error) total cost to the health services over the first 3 months post randomisation was £236.60 (£79.65). Private health-care utilisation was entirely self-funded by one patient reporting private health-care use and cost £38.24 per patient on average (see Table 25).
The health-care resource use and out-of-pocket expenses incurred by patients and their relatives are presented in Table 26. The mean cost per patient of over-the-counter pain relief medication, gels and patches increased from £2.92 (standard error £1.30) in the 3-month pre-randomisation period to £18.28 (standard error £5.05) in the 3-month post-randomisation period. The mean cost per patient of health-care-related travel (e.g. bus fares and taxis) was £1.15 (standard error £1.15) for study participants and £2.00 (standard error £2.00) for their partners. Overall, the mean total health-related out-of-pocket expenditure was £26.23 (standard error £7.19) per patient in the 3 months post randomisation. There were no reported costs associated with child care, laundry- and household-related services that could have been associated with the delivery of the study interventions.
The employment- and income-related status of trial participants at the end of the 3-month follow-up period is summarised in Table 27. Nine of the 17 patients with complete data on employment status were in work, six were retired and two were unemployed because of ill health. Twelve patients reported information related to loss of income: one patient reported lost income of £409 because of ill health, translating into an income loss of £34.08 (£34.08) per patient on average. The mean number of work days lost was 2.78 (standard error 2.22).
Health-related quality of life at baseline and 3 months’ post randomisation
Self-reports of the health-related quality of life of patients with LBP were made using the EQ-5D-5L instrument at baseline and at 3-month follow-up; the results are summarised in Tables 28 and 29, respectively. The proportion of missing data among responders was negligible and there was evidence of floor effects in the EQ-5D-5L dimensions related to self-care and anxiety and depression and, to some extent, the mobility, dimension at both baseline and end of follow-up. The mean health utility score on a scale (range –0.28 to 1), generated using recently derived UK tariffs for the EQ-5D-5L,126 changed from 0.50 (standard error 0.10) at baseline to 0.60 (standard error 0.20) by the end of the 3-month follow-up period. Similarly, patients’ self-rating of their health-related quality of life on the EQ-5D visual analogue scale (VAS; 0–100) indicated an improvement from 59.3 (standard error 4.4) at baseline to 65.6 (standard error 17.88) at the end of follow-up.
| EQ-5D-5L dimensions | Range | Baseline scores (n = 25) | 3 months (n = 19) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % missing | Median | % floora | % ceilingb | % missing | Median | % floora | % ceilingb | ||
| Mobility | 1–5 | 0 | 3 | 15.4 | 0 | 0 | 2 | 11.5 | 0 |
| Self-care | 1–5 | 3.8 | 2 | 38.5 | 0 | 0 | 2 | 34.6 | 0 |
| Usual activities | 1–5 | 0 | 3 | 7.7 | 0 | 0 | 2 | 7.7 | 0 |
| Pain and discomfort | 1–5 | 0 | 4 | 0 | 3.8 | 0 | 3 | 0 | 0 |
| Anxiety and depression | 1–5 | 0 | 2 | 26.9 | 11.5 | 0 | 2 | 34.6 | 0 |
| Follow-up time point | Quality of life weight | Range | % missing | % floora | % ceilingb | Lower quartile | Mean | Median | Upper quartile | Standard error |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 25) | EQ-5D index | –0.28 to 1 | 3.8 | 0 | 0 | 0.4 | 0.5 | 0.5 | 0.7 | 0.1 |
| EQ-5D VAS | 0 to 100 | 0 | 0 | 0 | 45 | 59 | 60 | 75 | 4.1 | |
| 3 months (n = 19) | EQ-5D index | 0 | 0 | 0 | 0 | 0.5 | 0.6 | 0.7 | 0.8 | 0.1 |
| EQ-5D VAS | 0 | 0 | 0 | 0 | 55 | 65.3 | 65 | 75 | 4 |
The plots in Figure 7 compare 3-month health-related quality-of-life profiles of patients generated using (1) self-completed questionnaires at baseline and at the end of follow-up (3-month time point) and (2) daily (during the first week) and then weekly (for the remainder of the 3-month follow-up period) self-assessment of health-related quality of life recorded in daily/weekly health diaries. Not all patients completed both versions of the questionnaire at each time point. Figure 7a shows the mean health-related quality-of-life profiles of all participants who completed the baseline/end of follow-up version of the EQ-5D-5L questionnaire (baseline, n = 25; follow-up, n = 19) and the daily/weekly diary records (n = 15). Figure 7b shows the profiles for a subset of participants (n = 11) completing both approaches to data collection. The diary scores indicate marked fluctuations in health-related quality of life (both EQ-5D-5L index and VAS scores) in the first few weeks post randomisation. In particular, there is a substantial short-lived improvement in both EQ-5D-5L index and VAS scores in the first few days after the initial treatment. The fluctuation in health-related quality of life is less pronounced during the remainder of the follow-up period, almost returning to the baseline rating at the end of the 3-month follow-up period.
FIGURE 7.
Three months’ QALY profile of patients with LBP generated via daily and weekly self-reports of EQ-5D-5L scores and self-completed questionnaire at baseline and at end of follow-up. (a) Scores of all participants who completed the postal questionnaire or diary records; and (b) scores from a subset of participants who provided postal questionnaire and diary EQ-5D data.

The QALY estimates for the 3-month follow-up period were generated based on the EQ-5D index-derived utility values for the 11 patients who reported both baseline/follow-up questionnaire and daily/weekly diary data. The mean QALY estimate was 0.141 (standard error 0.018) based on health-related quality-of-life outcomes measured at baseline and at the end of follow-up, and 0.170 (standard error 0.017) based on the more regular diary records. Over the 3-month follow-up period, the mean QALY difference generated by daily/weekly assessment of health-related quality of life, compared with the normal practice of assessing health-related quality of life at less regular time points (typically at baseline and end of follow-up), was 0.029 (standard error 0.01).
Figure 7a is based on the scores of all participants who completed the postal questionnaire (baseline, n = 24; follow-up, n = 19) or on diary records (n = 15). Figure 7b is based on scores from a subset of participants (n = 11) who provided both postal questionnaire and diary EQ-5D data.
Process evaluation results
This evaluation draws on national published data, audit data from one study site, study process data, observation notes made by the research team during site initiation visits and visits during recruitment, and group interviews with all study site teams.
On recruitment, 21 patients consented to be approached for interview. Interviews were planned to take place shortly after the participant completed 3-month follow-up. Of those 21 patients, 13 were sent information and invited to consent to an interview, six responded/consented to be interviewed and five were interviewed, with one withdrawing on the day of the interview. The five interviewees, all female, were from three sites (James Cook, n = 3; Kidderminster, n = 1; Kingsmill, n = 1). Of these five, four received the FJI and one received the control intervention. As there were so few interviews with study participants, we cannot be confident about the representativeness of these data. We have limited ourselves to interrogating them for the study participant voice on issues raised by the study site teams.
Project timeline of study approvals
Figure 8 gives a brief overview of the study approvals and includes planned and revised timelines. Full ethics approval was obtained in September 2014 in line with our planned timeline, although we had originally planned to have an outline approval in early 2014 with an updated/amended approval post consensus in July/August 2014. The ethics committee rejected our early submission and advised that we should resubmit after we had held our consensus conference, which we did.
FIGURE 8.
Original revised and actual timelines, approvals and site set-up.

Full ethics approval is the driver to start the formal processes of obtaining research and development (R&D) and site approvals. This proved to be a long process. This study sought approvals at the time that an older system of approvals was being changed over to a newer system, operated by the Health Research Authority, and this may explain some of the severe delays we experienced in setting up sites. Our request for global R&D approval was submitted in August 2014 but was not approved until February 2015, which was a far longer period of time than normal and meant that approval was gained far later than planned. Repeated requests for updates from the team, who had provided all of the relevant materials, did not help to resolve this. Once global R&D was approved, each of the sites required site approval that had to be in place before any recruitment took place. This again proved to be a slow process. One site was approved within 1 month, three sites were approved in 4 months, and for the final two sites approval took over 5 months.
These delays, which were largely outside the study team’s control, impacted on the timeline of the study. The planned 6-month recruitment process that was meant to start in November 2014 was delayed for 7 months until June 2015. The study team provided the staff training needed at three of our sites in preparation for the start of recruitment in late 2014.
Study research sites
Sites
Table 30 provides an overview of the study sites outlining the type and level of pain management services offered in the pain clinics. Services vary across all six trusts; some trusts offer a fully integrated service that is well provisioned and others have more fragmented services.
| Research site | Service | Approximate population serveda | Consultants | Total number of WTE consultants at the facility | Other clinical staff | Multidisciplinary team meetings | Research active | Change in service commissioning during studyb | Style of recruitment | Number of participants recruitedb |
|---|---|---|---|---|---|---|---|---|---|---|
| University Hospitals Coventry and Warwickshire NHS Trust | Pain management | 6,203,700 | Three anaesthetists | 1.5 | Four nurse specialists (4 WTE) | No | No | Yesc | Screening letter | 4 |
| Warwick Hospital, South Warwickshire Foundation Trust | Pain management | 270,000 | Two anaesthetists | 0.7 | One nurse specialist/acupuncturist (1 WTE) | No | No | Yesd | Screening letter | 1 |
| Kingsmill Hospital, Sherwood Forest Hospitals NHS Foundation Trust | Back pain management | 400,000 | Four anaesthetists and one rheumatologist | 5 | Three physiotherapists (1.8 WTE); two specialist clinical psychologists (1 WTE); three occupational therapists (1.4 WTE); two acupuncturists (2 WTE) | Yes | Yes | No | Face to face | 8 |
| Kidderminster treatment centre, Worcestershire Acute Hospitals NHS Trust | Pain management | Missing data | One anaesthetistb | Nursesb Physiotherapistsb |
No | No | No | Face to face | 4 | |
| James Cook Hospital, South Tees NHS Foundation Trust | Pain management (clinics at four locations; two included in study) | 794,000 | Five anaesthetists and one neurosurgeon | 3 | Two trainee doctors (2 WTE); three physiotherapists, (3 WTE); three specialist clinical psychologist (1.5 WTE); one occupational therapist (0.1 WTE); three nurse specialists (3 WTE); all shared across four clinics | No | Yes | No | Face to face and screening letter | 9 |
| Castle Hill Hospital, Hull and East Yorkshire Hospitals NHS Trust | Pain management | 475,118 | Three anaesthetists | 3 | One trainee doctor (1 WTE); two nurse specialists (2 WTE) | Yes | Yes | No | N/A | N/A |
Site initiation
As noted, recruitment was delayed because of severe delays in gaining research governance approvals that were beyond the control of the study team. In addition, recruitment from site 2 was delayed because the pain service was suspended by commissioners after a consultant retired, and recruitment from site 5 did not occur before the study closed. Figure 8 details site set-up timelines. Site initiation took place within 1 month of approvals being granted. In two sites, staff training for the study ran in the month prior to recruitment initiation. However, in three sites staff training took place up to 9 months prior to the initiation of recruitment because of the delay in gaining approvals. Staff at these sites reported frustration at being unable to initiate recruitment.
Reach of the study across the eligible population
It was necessary to know the size and characteristics of the population that had the potential to benefit from the intervention to identify if the recruited population was comparable. Although Table 30 gives an indication of the overall population that our study pain clinics serve, we have little information on the local prevalence of back pain.
Study site 2, which provided a pain management service, collected data on referrals received and treatments provided during a period of 8 months that overlapped with the study period. During this time they received 335 referrals, of which 116 (35%) had back pain. Of the patients with back pain, 34% received physiotherapy, 22% received advice only and 13% were referred to a pain management group. Four patients received FJI, of whom one was recruited to our study and the remainder were seen by the service outside our recruitment phase. It is not possible to draw a conclusion about the reach of our study from this audit but it suggests that pain management services may see only small numbers of patients who may benefit from FJIs.
Screening and recruitment participants
The study plan was to recruit 150 participants in a 6-month recruitment period. It was calculated that each site would recruit around 30 patients. Recruitment was planned to be primarily from pain clinic services, which was an approach that was congruent with the commissioning brief. As noted at the beginning of this chapter, recruitment started on 26 June 2015 and was terminated by the funder on 11 December 2015 when only 320 patients had been approached, which had resulted in the 26 patients who were randomised. In the following sections we look at the recruitment process in more detail.
Screening for eligible patients
The research team had planned for the site teams to identify patients who were referred with back pain from referral letters and to send a screening letter to these patients. This proved to be more complex than originally thought. Numbers of referrals at sites were not at the level originally expected and site teams were finding that it was impractical to mass-mail all referrals, as this was a very labour-intensive task. Most site teams adopted a pre-screening process to try to ensure that letters went out only to potentially eligible participants, but even this process proved difficult and time-consuming, requiring time that the site staff did not have. Hence not as many letters were sent out from sites as we would have expected. Three sites adopted the pre-screening approach, one site chose to approach patients when they attended the clinic and one site used both approaches (see Table 30). Although the numbers are small, the data suggest that the face-to-face approach resulted in a higher proportion of the people who were approached being recruited. The delays in gaining approvals also had an impact on the screening and selection process. Similar to the study team, sites were expecting study activities to take place on or near a particular time. Sites planned and provisioned for this, with staff in place. Delays proved costly, as recruiting and screening staff were either no longer available or on sick or holiday leave by the time we were ready to begin recruitment.
During group interviews, staff said that they found it straightforward to identify patients in the clinic and that patients appreciated this type of approach. Staff who identified potential participants through referral letters found that this was a difficult task. Referral letters (e.g. GP referral letters) were often either very specific, such as requesting a particular treatment, or non-specific when the reason for referral was unclear. Thus, staff found it hard to identify patients who had been referred for treatment of back pain.
Patient expectation of receiving facet joint injection
The expectation of receiving a FJI was identified by the site teams as a barrier to recruitment. Our study protocol excluded patients who had previously received FJIs (or any injection in the back). Site staff reported that some patients excluded themselves because they specifically wanted a FJI as they knew of others who had received this treatment. Others had been referred to the service by their GP because the service was known to provide FJIs. Of the five sites that recruited to the study, three were commissioned to provide injections, although NICE guidance,6 which was current at the time of the study, did not support this approach for those patients with LBP present for < 1 year. Study site staff reported that the name of the study and its logo showing an injection going into the spine raised expectations with patients that they were going to get an injection. The patient who withdrew from interview and from the study cited the fact that they wanted the injection treatment but had been randomised to the control. None of the patients who were interviewed said that they expected an injection but talked more generally about wanting to be rid of their back pain:
. . . I thought to myself at the time was whatever you offered me, if it took away some of the pain, I would have done anything.
#1
Well I just thought if I had this injection I might not have to take tramadol.
#2
I don’t know, I was hoping it would get rid of my back [pain], that’s all.
#3
I was hoping for that I would get a vast improvement, or a lot better than what I was . . .
#4
Anything! Anything at all, I knew it wouldn’t make it worse and I’d got nothing to lose.
#5
It is unclear the extent to which the expectation of injection impacted on patient decisions to engage or not engage with the study, as we have no data from non-recruited patients.
Additional recruitment approaches
Positive discussions were held in three sites with orthopaedic teams about recruiting to the study, but this was not initiated prior to study closure.
Study site staff suggested recruiting from primary care, as many of those attending a pain service were patients who had undergone procedures before and were likely to be ineligible for the trial. We sought approval to put up posters in community physiotherapy departments and GP surgeries in the locality of our local study site. This commenced approximately 3 weeks prior to study closure. The local site staff noted that the posters were generating enquires, but there was no indication of numbers.
Eligibility assessment and consent
Physiotherapists reported that our manual and training had prepared them well for undertaking the eligibility assessment, which was a set of specific tasks that all patients were to attempt. One physiotherapist reported a reluctance to put patients through this number of tests, particularly if they were elderly and frail. Another described the tests as necessary and appropriate and stated that they were not too onerous on the patient; this physiotherapist had specialised in back care for many years. One physiotherapist commented that our manual and training had led them to change their clinical practice.
Initially, study site staff had expressed fears about the amount of paperwork required for the study, but in practice they found completing the paperwork to be straightforward. No site team reported problems with the study processes or paperwork. The laminated guides were useful when undertaking tasks such as explaining the text messaging and diaries to patients. All of the teams found the consent and trial registration process straightforward. Initially, there had been concerns about the number of tasks to be done with patients after registration: explaining the collection of pain scores by text, providing paper diaries as an alternative, asking patients to complete the EQ-5D diaries and completion of the baseline questionnaire. Most staff felt that they found these tasks straightforward once they had become accustomed to them. All sites noted that time to complete these tasks varied from < 30 minutes to nearly 1 hour, with some patients requiring more time and support than others.
Four out of the five interviewees used the text messaging service to report their pain score and reported no problems. The fifth interviewee used the paper version to report their daily pain score. No interviewee reported problems with completing the pain diaries. The key themes that emerged from the participant interviews about how they made their decision were mainly in terms of their own appraisal of what they could physically do and, to a lesser extent, how they perceived that they were feeling:
What I couldn’t do . . . what it stopped me doing, like what I used to be able to do?
#1
But some days I really think about what I’m doing and how I feel down sometimes with it and that would sort of affect how I was scoring things.
#2
It’s very difficult to put into words really sometimes, never mind on a bit of paper on numbers! I looked at that paper many a time and I was thinking ‘well they’re numbers, how do you gauge, at this minute what number you’re at?’ It’s alright when it’s at the most, you can ‘oh right, yeah, it’s nine, it’s ten or whatever’ but then when it comes down but it never goes away, it’s difficult to rate it.
#3
I think I worried that I was thinking sometimes it was quite . . . it was quite good, you know, if things had gone well that day for me and then other days they hadn’t.
#4
How I’d felt on the previous day and what I’d put on the previous day from that and that and it was the things that I could do . . .
#5
When asked about the frequency of completing these scores, interviewees had no concerns. One found score completion to be a tedious task, but another said that they missed sending the scores once the trial ended:
If you weren’t in a lot of pain, you think ‘well it is there, it does ache, it hasn’t gone away’ . . . yeah, it was a little bit tedious at times near the end when I wasn’t in drastic pain.
#1
Oh no, no, I felt quite lost when I finished actually! I was looking for it thinking, ‘where’s it gone, where’s my bits of paper?’.
#2
Interviewees expressed no concerns about completing the EQ-5D. One participant said it helped them to reflect on how they had improved:
You know, it made you think about yourself. Yeah, that was okay and then I could see as I was coming out it, literally as I was feeling better, I could sort of look back and think ‘yeah, I do feel better than what I did, I do feel the improvements’.
#1
Patients generally found the baseline questionnaires a bit repetitive:
I did feel that some of the questions were repeating themselves but I know that the questions would have been set for a reason so I just had to try and read each question on its own, without thinking ‘oh I’ve just answered something like this’.
#5
First best usual-care session: assessment and randomisation
The physiotherapists who delivered these sessions appreciated the longer than normal time that they had with the patient. Most said that they were able to carry out a full assessment of the patient and develop a personalised treatment plan as outlined in our manual. All said that the training manual and support were excellent. Staff at sites that received training many months before recruitment found that they needed to revisit this material before starting. Randomisation took place after this first assessment session. All staff spoken to reported that the assessment and randomisation processes were straightforward. Four of the patient interviewees reported no problems with the assessment processes:
Now I found that gradually, beginning to talk to the lady and her giving me the courses of exercises to do and I actually said to her – because we had nice conversations which I found in general quite helpful because the one question she said to me, ‘what do you for relaxation?’ and I laughed, I said ‘work’. She said ‘don’t you have any hobbies?’, I said, ‘no, I work’, and she said, really kindly she said, ‘I think it’s time you took time for you, like to do something that you like to do’. And that’s when we discussed about the exercises, my hobby used to be going to the gym, riding my bike, going swimming, you know?
#1
I mean she was very, very good, she sat down and asked me what sorts of things I was looking to get on with and how far ahead did I want to go and what sort of things I was looking forward to. But I mean we went through quite a bit together, yes, it was very good.
#4
One felt that the BUC was ‘basic’:
I found the physiotherapy appointment strange in all honesty. After I’d had a full assessment [from the lead physiotherapist], who’d gone into quite detail about where my problems are with my back and what it could be [during eligibility assessment], but then physiotherapy was very much ‘how have you been today’, ‘where’s the pain’ . . . it was very basic.
#5
Delivery of the interventions to recruited patients
Facet injection procedure
Four out of the five clinicians who were ‘injectors’ in this study were interviewed, and the research nurse who attended the injections at the fifth site commented on the process during group interviews. Staff from all sites stated that there were no problems with the procedure and that the study instructions and material were easy to follow. One clinician noted that because of their site’s late start in the study they had not given anyone an injection, but they felt that, if patients at the site had been entered into the study, good systems were in place.
Best usual-care package (five sessions per patient)
Physiotherapists at all of the sites reported that these sessions were straightforward to deliver and that the materials provided by the study team were useful. The physiotherapists were positive about the package but several reported that they were less confident with the psychological components than with the physical components. They felt that they needed a little more training in these areas.
A number of the sites reported that they had patients who did not attend organised sessions. The first session (pre randomisation) was attended by all 26 patients. All patients were offered five follow-up sessions and over half attended all five sessions, three attended no follow-up and one of these had withdrawn from the study (Table 31). Study site staff suggested that some patients expected to have an injection and when they were not randomised to receive this they chose not to attend. At one site the availability of car parking was cited as a possible barrier to attendance.
| BUC sessions | n |
|---|---|
| First BUC session | 26 |
| Plus ≥ 1 follow-up session | 23 |
| Plus ≥ 2 follow-up sessions | 22 |
| Plus ≥ 3 follow-up sessions | 18 |
| Plus ≥ 4 follow-up sessions | 16 |
| Plus 5 follow-up sessions | 15 |
| No follow-up sessions attended | 3 |
The physiotherapists, when asked, were of the opinion that if participants completed all of the therapy sessions then they benefited from the treatment package.
All interviewees attended all of their sessions. Most interviewees liked the physiotherapy sessions and reported benefits. One, however, felt that it was strange that the session was not more ‘hands on’:
From start to finish and I think to myself ‘well if I’ve got no pain today, there’s no reason why if I carry on doing this, that I shouldn’t have less pain, regularly, as long as I keep doing what I was, you know, shown what to do . . .‘ So therefore it’s taught me to give myself time to unwind, you know, to let myself relax, you know, to make myself more comfortable rather than . . . I’m more conscious of my body, let me put it that way. If I think ‘oh that aches’, well why does it ache and you do a couple of breathing sessions and you think ‘well that’s alright, it’s gone now’. You’ve just got too uptight if you’ve been too stressed over something.
#1
Yes, yes, I’m not doing all of them but I am doing the ones that I felt were more beneficial. When I get up on the mornings sometimes I think ‘my back is really sore’ and then I do some of these exercises and I can move around alright, I feel better for it. So I’ve found what works for me and what doesn’t.
#2
Well I went before all this [outside of the study], she was hands on but this one wasn’t, she was just exercising, she didn’t touch me at all on my back . . . It just was exercising, I could do most of them but they gave me these bands and they’re so strong I just didn’t do them so I just did the bottles – I still exercise, I do the bottles every day and all the bending and everything else, you know?
#3
Yes, I still use them, I’ve even got myself an exercise bike which I have a go on now and again.
#4
I found the physiotherapy appointment strange in all honesty. After I’d had a full assessment from the nurse, Steve [Surname], who’d gone into quite detail about where my problems are with my back and what it could be, but then physiotherapy was very much ‘how have you been today’, ‘where’s the pain’ . . . it was very basic.
#5
Intervention delivery: paperwork
In general, it was felt that the paperwork was well organised and fairly simple to complete. Two physiotherapists noted that the CRF was at times difficult to follow when completing the assessments, as statements seemed contradictory or were not in the order outlined in the manual. Support provided over the telephone was helpful. One of the lead physiotherapists who had no significant research nurse support found the paperwork burdensome.
Process evaluation strengths and weaknesses
The process evaluation provided us with insights about implementing this complex study within the NHS. Engagement with the clinical teams throughout the study allowed us to collect qualitative data about the study while they were fresh to those involved with the study. Study teams were willing to share positives and negatives about the study.
Our recruitment of participants to interview was disappointing and reflects the low and slow recruitment rate to the study. We have used the interview data from study participants cautiously to avoid overclaiming from this very small data set.
Chapter 6 Discussion
This was a feasibility study. Our overall aim was to explore the feasibility of running a RCT to test the hypothesis that, for people with suspected facet joint pain contributing to persistent LBP, adding the option of FJIs, with local anaesthetic and corticosteroids, to best usual non-invasive care available from the NHS is clinically effective and cost-effective.
The results of this feasibility study are both encouraging and disappointing. At the start of the project we set ourselves eight specific objectives and it is very pleasing to report that we were successful in achieving most of these. However, the recruitment of participants to the study through pain clinics was not at the level that had been expected and we failed to recruit to an acceptable level within the planned timeline. This in itself, although disappointing, is an important outcome from a feasibility study.
To develop, and evaluate, agreed criteria for identifying people with suspected facet joint pain
A major success of this project was to achieve consensus from a multidisciplinary group on a clinical assessment to identify people with suspected facet joint pain (Box 10). The absence of such criteria has been a major barrier to performing research in this area. We have some preliminary validation from within this study. Although the numbers providing immediate data in the injection arm of the study were small, most reported a substantial level of immediate pain relief. Seven (58%) report a > 50% reduction on pain, which is congruent with the benchmark of 62% used in our original proposal. Although far from conclusive, these data suggest that facet joint problems may be making a contribution to the pain affecting our participants, that is, we have the correct population group that might be considered for intra-articular FJIs within the NHS. However, it is not possible in this study to isolate the effect of local anaesthetic from the placebo effect of having spinal injections. Future work could validate these criteria for suspected facet joint pain against the results of double-blind diagnostic medial branch blocks or intra-articular injections with local anaesthetic. 119 Nevertheless, our criteria have been cited in the 2016 NICE guidance on LBP and sciatica to identify people who should be considered for a diagnostic medial branch block prior to radiofrequency denervation. 11 This approach is a stand-alone output from this project.
-
Increased pain unilaterally or bilaterally on lumbar paraspinal palpation.
-
Increased back pain on one or more of the following:
-
extension (more than flexion)
-
rotation
-
extension/side flexion
-
extension/rotation.
-
AND
-
No radicular symptoms.
AND
-
No sacroiliac joint pain elicited using provocation tests.
Based on consensus. 26
To develop an agreed protocol for the injection of facet joints in a consistent manner
Failure to adequately define the intervention is a recurring criticism of interventional studies for LBP. 152 There was some variation in the detail of what we originally proposed and the final protocol. This protocol was acceptable to our clinical leads and has been satisfactorily implemented across several sites, although with a limited number of participants. There is evidence from our process data that clinicians are using their clinical assessment to decide on the numbers and levels of joints to inject. Pragmatically, this reflects what is likely to happen in clinical practice and represents an intervention that we can test in a main study. One concern for the conduct of the main study is that sites were unable to record radiation exposure in a consistent manner. This will need addressing for any main study to ensure that the potential risk from radiation exposure during the injections can be quantified. Radiation exposure during screening for FJIs is, however, part of normal clinical practice. We have no concerns that this poor recording indicates that any patients may have been put at unnecessary risk. Nevertheless, for any main trial these data will be needed to allow us to model any long-term consequences from low-level radiation exposure during FJIs.
We now have an agreed injection procedure to use in the main trial.
To develop, and evaluate, a standardised control treatment deliverable in the NHS and congruent with 2009 National Institute for Health and Care Excellence guidance (best usual care)
We have successfully developed a package of care using a multimodal approach for people with suspected facet joint pain. This approach has been endorsed by the multidisciplinary group at our consensus meeting. We have successfully implemented this. The overall approach was acceptable to participants, with 81% attending four sessions and 58% attending all six sessions. Our process data indicate that the components of the care package were delivered, and that the physiotherapists found them to be straightforward to deliver and were generally positive. Unsurprisingly, they were less confident about the psychological components than about the physical components. There may need to be more focus on this aspect in training for any future use of the programme. Interview data with study participants were largely supportive of the approach used in the BUC package.
We have developed a manualised package of BUC for people with suspected facet joint pain that is deliverable in the NHS. It has not been formally tested against alternative conservative approaches. Nevertheless, the rigour of its development means that clinicians may wish to adopt this approach in preference to other less structured approaches. There is the potential for this package to be assessed in a RCT against an alternative treatment package, or no treatment, in this population of people with LBP. The package is ready to deliver as the control arm in a future main trial. This package is a stand-alone outcome from this project, although it should be noted that we have developed a one-to-one package, whereas 2009 NICE back pain guidelines favour group interventions.
To develop and test systems for collecting short-term and long-term pain outcomes, including measures required for economic evaluation
Here, we focus on the developmental aspects of outcome assessment.
Early pain outcomes
Short-term benefits and harms from interventional procedures are important to capture. The conventional approach of using postal questionnaires for which outcomes are often not collected until 1 or 3 months after randomisation will not capture these. It may be that, even if there is little difference in long-term outcomes, the short-term benefits mean that FJIs are worthwhile. Our original intention had been to collect these using text messaging only. It became clear as the project developed that we also needed to offer a paper alternative. Indeed, the age range of our participants, up to 80 years, suggests that using text messaging alone might have meant that we missed useful information. Although there were missing data in both text and paper versions of daily/weekly pain scores, we obtained sufficient data to have useable outcome data on the pattern of pain over the first 3 months following randomisation of 23 out of 26 (88%) participants. This is an important development.
Patient Generated Index
The revised PGI was correctly completed by all baseline questionnaire respondents, although these constituted a small cohort. This compares favourably with completion rates in another study that applied the revised format in a LBP population (89% completion)113 and with the original format that was completed in a LBP population (68% completion). 136
A Norwegian translation of the revised PGI has recently been completed by LBP participants in a longitudinal postal survey. 113 Participants listed more than 380 areas in stage 1; the most frequently reported were pain (65%), sleep (47%), stiffness (34%), socialising (34%) and housework (33%).
We found that the impact of LBP on an individual’s ability to walk and sleep were the most frequently reported areas of concern, at 52% and 48%, respectively. In the Norwegian study,113 sleep disturbance was identified as the second most frequent area of concern at 48% after pain, at 65%; however, only 24% of our participants identified pain as an important symptom. Fatigue is increasingly recognised as an important symptom across many chronic, long-term and rheumatological conditions. 153,154 We found that 20% identified fatigue as a concern. Neither sleep nor fatigue is part of the RMDQ. 83 The Oswestry Disability Index155 includes sleep but not fatigue. Comparisons between the areas of concern identified within these two aforementioned studies of the PGI and the domains within leading back pain in specific outcome measures indicate discrepancies between what participants consider to be important and the items we are measuring in our trials. A systematic review of qualitative studies has also identified that current outcome measures may not be adequately capturing the social component of the impact of LBP. 156
Although the numbers in our study are limited, people appear to have been able to complete the adapted follow-up PGI score satisfactorily. The results show the expected improvement over time. This was also the experience in the Norwegian study. 113 Overall, our preliminary data indicate that it would be worthwhile exploring further the performance of the PGI as an outcome measure in back pain trials.
Health economic outcomes
There are two components ensuring that we have satisfactory health economic outcomes: costs and outcomes. We have produced detailed micro-costing of both the BUC and the injection treatments that reflect the true cost of delivery.
Our overall micro-costing of £419.22 per injection compares with a NHS tariff cost for FJIs of £686.84 (National Reference Costs 2014/15148 – main schedule, currency code AB16Z). What is not included here is cost of the initial consultant appointment (£144.79, National Reference Costs 2014/15148) at which a decision to offer FJIs is made.
Although the number of completed follow-up questionnaires is limited, we have been able to satisfactorily estimate NHS and personal health-care costs from our participants.
An important concern at the start of the study was how to capture short-term benefits and harms. We described earlier the process for collecting and analysing pain data. We also wanted overall health utility data to assess how these early changes might impact on cost-effectiveness. For these, we sought to collect data weekly throughout follow-up rather than the early data we used for pain. This was to reduce questionnaire burden. We had originally planned to collect these electronically, but the only licensed versions of the EQ-5D were not suitable for use when linked to smartphones, which would have allowed a seamless collection of data on pain and the EQ-5D. For this reason, the pain score was completed as weekly diaries. Participants found these difficult to complete fully and we received only 13 fully completed sets of data. These limited data show a very interesting pattern, with a marked short-term increase in health utility soon after joining the study (see Figure 7). At the end of 3 months, the diary data are similar to the data collected in the final postal questionnaire. There is a clinically important difference in QALYs accruing over this time according to the two collection methods of 0.029 (standard error 0.01). This provides us with a challenge for the main study, as there are potentially important short-term effects that should be captured in the main trial for which we do not have an approach to data collection that yields data on enough participants.
In developing our main trial proposal, we will develop a decision-analytic model to simulate the clinical pathway of patients with LBP within the UK NHS and over the lifetime of the individual. The model parameters for prevalence of LBP in the target population, health outcomes (in QALYs) and health-care utilisation and associated costs will be informed by results of the FIS feasibility study and systematic review of the relevant literature. Bayesian evidence synthesis methods will be used to derive distributions for model parameters based on all available information. The model will be used to estimate the expected value of sample information of a trial of the protocolised intervention and management plan, using algorithms developed by Madan et al. 157 and Strong et al. 158 The expected value of sample information calculations will be performed for alternative study designs to determine the optimal design in terms of factors such as sample size, length of follow-up and study population.
To demonstrate that recruitment to the main trial is feasible
That we did not achieve our recruitment target within the lifetime of the study was disappointing. The randomised pilot faced some substantial challenges, not least that, congruent with the commissioning brief, recruitment was planned to be primarily from pain clinic or orthopaedic services. Although initially this seemed to be a good idea, it soon became apparent that patients fitting our criteria were sparse in these areas. Study site staff were comfortable with face-to-face recruitment. Screening referral letters proved to be more problematic than expected. Identifying those referred for treatment of non-specific LBP from referral letters proved to be very problematic. A further unexpected problem was that many of those who were identified as being referred because of non-specific LBP had had previous spinal injections, meaning that they were not eligible to be invited to take part in the study. This may reflect our decision to mainly use sites with an active programme of FJIs. We made this decision in the expectation that this would make delivery of the study interventions easier. In fact, perversely, it may have meant that many potential participants were excluded. The research and site teams discussed this problem at length, trying to identify at which point in the patient journey it would be appropriate to capture the patients who could benefit the most from our treatments. A number of solutions were put forward, including recruiting through physiotherapy services, primary care and/or through orthopaedic clinics, as suggested in the brief but not operationalised in this study.
We also note that delays in getting approvals made it difficult to maintain momentum at sites, and staff who were prepared to start on or around the planned start date were often not available because of the delay.
There was no culture of applied research within most of the clinics in which we were working, there was little history of the pain clinics working collaboratively with the physiotherapy services and there were some anxieties on the part of some staff as to how the study would run. At one site, staff illness essentially prevented any recruitment.
A further unexpected problem was our approach to the naming and marketing of the study. The working title of ‘Facet Injection Study’ and the use of a trial logo with an image of an injection appears to have led to people interpreting this to be a study of FJIs rather than a study of adding FJI to best conservative treatment and to not have understood the nature of the randomisation and our overall aim.
Notwithstanding all of these issues, at the time the study was closed a number of barriers to recruitment had been identified. Our initial sites were all actively recruiting: one site had just started recruiting and a final site had just obtained all the relevant approvals. We had made changes to recruitment processes so that we could recruit from physiotherapy departments, from orthopaedic clinics and take referrals to the study directly from primary care. At the time the study closed, we had established a very good flow of referrals into the study. There were 28 patients who were awaiting initial assessments for study entry. Thus, having identified barriers to recruitment, we had addressed these and we have some evidence that we could recruit satisfactorily to the main study.
To collect the recruitment and outcome data required to inform sample size and number of sites needed for the main study
We have obtained sufficient baseline data to inform development of a sample size estimate for the main trial. Our data on recruitment indicate that we need a different approach to participant identification. Notwithstanding the 47 potential participants who were pending, at different stages of the recruitment process, at the time of closure we randomised 8% of those approached. Allowing for just five of the 47 potential participants to eventually have been randomised, our conversion rate from initial approach to randomisation would have been 10%. This is a good conversion rate for a study of this nature, which gives us confidence that we can recruit to a main study. In calculating the number of sites for the main study, we will use throughput of physiotherapy back pain services in interested localities to estimate number of sites needed.
Drawing on our work on defining the MID for this study and our baseline data, we can estimate the sample size needed (Table 32). Here we have estimated sample size needed for 90% power and 5% or 2.5% significance. If any future study has two primary outcomes, for example AUC for pain over 3 months and back pain disability at 3 months, we may need to make a formal correction for multiple comparisons. In each case, we have looked for sample size needed for either a 0.5 or 1.0 MID unit difference for which this is expressed in natural units and for a 30% difference in baseline score.
| MIC | Parameters | Effect size (MID unit) | Significance (%) | Sample sizea (MID unit) | ||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 0.5 | 1.0 | |||
| RMDQ | ||||||
| 30% | Baseline 11.5, 3/12 SD 5.1 | 1.725 | 3.45 | 5 | 370 | 94 |
| 2.5 | 438 | 112 | ||||
| 5 | 2.5 | 5 | 5 | 176 | 46 | |
| 2.5 | 210 | 56 | ||||
| Pain-NRS | ||||||
| 30% | Baseline 7.1, 3/12 SD 2.0 | 1.07 | 2.13 | 5 | 152 | 40 |
| 2.5 | 178 | 46 | ||||
| 2 | 1.0 | 2.0 | 5 | 172 | 46 | |
| 2.5 | 202 | 52 | ||||
| AUC | ||||||
| 30% | AUC 454, SD 142 | 68 | 136 | 5 | 186 | 46 |
| 2.5 | 218 | 58 | ||||
| 168 | 84 | 168 | 5 | 124 | 34 | |
| 2.5 | 146 | 38 | ||||
For the AUC analysis we have set our MID to be 84% of the values we arrived at for a Pain-NRS/visual analogue score MIC, that is, 2 × 0.84. This reflects that the data are collected over 84 days, meaning that the maximum AUC (a pain score of 10 on every occasion) cannot exceed 0.84 and that the AUC, similar to a visual analogue score, allows for a full range of values between 0 and 10. Thus, we consider that the smaller MIC suggested for a visual analogue score is the appropriate benchmark. 87 MIDs and changes for the AUC in this context have not, however, been formally established.
We suggest that a definitive study should aim to recruit 275 participants. Allowing for a maximum of 20% loss to follow-up, data on 220 participants will have 90% power at the 2.5% significance level to show a 15% difference in the AUC and a 2.5-point difference on the RMDQ at 3 months. If there are no positive effects at 3 months, then long-term benefits are unlikely, making clinical outcomes up to 3 months appropriate for the primary analysis. One-year follow-up will still be needed for health economic analyses and to identify any long-term benefits if there is a positive result at 3 months. We are aware that for the RMDQ we are suggesting the use of absolute difference and for the AUC the use of a percentage difference to define the sample size. Using a percentage difference approach to setting the MID difference for the RMDQ would require a doubling of sample size. We do not think this can be justified when we expect that the primary mode of action is through the reduction of pain. However, as the AUC approach to assessment of short-term pain has not been established as an outcome measure in back pain trials, we are not suggesting that only pain measurements should be used as a primary outcome. For this reason, we consider it important to include an established measure of back pain disability as a second primary outcome.
Based on an assumption that 500 people annually will be seen in a physiotherapy service with continuing pain after treatment and that 10% of these will choose to join the study, six sites should be sufficient for recruitment. At each site, physiotherapy referral can be supplemented by referral from other secondary care services and from direct GP referral to the trial.
To conduct a between-group analysis to inform the decision on the need for a full trial
We were unable to achieve this objective because of the closure of the trial.
To carry out a process evaluation of patient experience within the trial
We have satisfactorily completed a formative process evaluation. Data were limited by small numbers in the study. Nevertheless, we have gained some important insights into how the trial ran in our sites and identified some key areas to address in order to ensure any main trial recruits in a timely manner.
Strengths and weaknesses
Many of the key uncertainties that needed to be addressed before running the main trial have been considered. We have also demonstrated in the randomised pilot that eligible patients who are invited to join the study are interested in the study and can comply with the study procedures. We have also successfully collected a frequent short-term outcome allowing us to identify any relevant short-term harms and benefits.
That we have achieved consensus on describing our population of interest and our control and active interventions is a real strength of this project. Furthermore, we have demonstrated that participants are able to comply with the recruitment process for the study and provide initial data for us to assess our non-standard approaches to clinical data collection: the PGI. Interestingly, the SD of the AUC as an outcome is quite small compared with the plausible reduction in pain over 3 months that might be considered clinically worthwhile. This means that by using this as a primary outcome for a future study a smaller overall study size may be possible and that any short-term benefits or harms for intervention will be captured.
Study procedures and paperwork require only minor improvements but the title of the study needs to be reconsidered to avoid raising patient expectations of receiving an injection. In a future trial, additional training may be needed for physiotherapists in the psychological treatment approaches.
In undertaking this process evaluation we aimed to identify aspects of the study design that have potential to threaten the success of a full trial. Although our data were limited in their scope, particularly from patients, we would argue that it was sufficient to identify major threats.
The key weakness of this study was failure to achieve our expected recruitment targets and the consequent early closure by the funder. There had been very substantial organisational barriers to the set-up of the trials. Long delays, outside our control, in receiving governance approvals giving us the green light to start recruitment at our first site meant that adequate recruitment would be a challenge. Although the funding brief specified that recruitment should be sought from pain clinics, it became apparent in the course of this study that this was insufficient. The people attending these clinics were, on the whole, less likely to be suitable for the study than those who had not yet been referred. There were also substantial operational issues with the clinics, which were unfamiliar with recruiting to RCTs, meaning that the start of recruitment, even after approvals had been obtained, was delayed. Although not drawn out in the process evaluation, our impression was that clinical staff were reluctant to start recruitment because of uncertainty as to how patients might feel about the study. Once sites had started recruitment, there seemed to be less concern. At our best recruiting site, all recruitment stopped for several months because staff were either no longer available or on sick leave and there was, as a result, lack of capacity on the part of the comprehensive research network. At the time the study was terminated, we had established a good recruitment flow that was starting to feed into an increased randomisation rate.
Is a main study still needed?
It has been argued that the question of the effectiveness of intra-articular FJIs may not be important, as this approach has been superseded by radiofrequency denervation of the medial branch of relevant lumbar nerves. The 2016 NICE guidance recommends radiofrequency denervation in selected patients who have had a positive diagnostic block. Their conclusions do not agree with the most recent Cochrane review of radiofrequency denervation. 159 Our view is that neither the NICE nor the Cochrane meta-analysis on radiofrequency denervation are robust because of the poor quality of the underpinning data. There is an economic model in 2016 NICE back pain and sciatica guidance suggesting that radiofrequency denervation is cost-effective with a cost per QALY of £11,178. The cost of a diagnostic block and radiofrequency used by NICE were £521 and £640, respectively, based on Healthcare Resource Group codes AB05Z and AB08Z. This compares with a cost of £420 that we have calculated for delivery of FJIs. Thus, intra-articular FJIs are substantially cheaper than radiofrequency denervation.
A Dutch RCT of radiofrequency denervation has now been carried out but, at the time of writing, the findings are available only as a conference abstract. 160 These data were not available to the 2016 NICE guideline development group. This study, which is substantially larger than previous studies, did not show a benefit from radiofrequency denervation, and there was a very low probability that it would be a cost-effective intervention at any willingness to pay. A smaller Egyptian trial, the results of which were published immediately prior to the publication of NICE guidance, found that radiofrequency denervation in people with proven facet joint pain was more beneficial than a sham procedure. 161
Intra-articular FJIs remain a treatment option for suspected facet joint pain that has not been adequately assessed in RCTs. The NICE guideline development group did not find sufficient evidence to support their use and our 2016 systematic review did not find data that could support the use of FJIs. 60 Their acquisition costs are, however, substantially lower than those of radiofrequency denervation, and FJIs may represent good value even if they are less effective than radiofrequency denervation.
There remains a need for robust studies of both the efficacy and the effectiveness of invasive procedures such as intra-articular FJIs and radiofrequency denervation for people with suspected facet joint pain.
Conclusions
We are cautious here in our overall interpretation of the results of this feasibility study. A feasibility study can be defined as ‘an assessment of the practicality of a proposed plan or method’ and, in this respect, this study has been very successful. Study procedures and paperwork require only minor improvements and the title of the study needs to be reconsidered to avoid raising patient expectation of receiving an injection. In a future trial, additional training may be needed for physiotherapists in the psychological treatment approaches. However, we cannot ignore the poor recruitment into the study. The feasibility has shown us that recruitment from pain clinics alone (undertaken in accordance with the funding brief) is not sufficient and this is an important finding. In response to the poor recruitment rates, we put considerable effort into trying to identify the most appropriate places to access our required patient sample. A number of these were being tested when recruitment was stopped. We have insufficient data to determine if these recruitment strategies would have been successful, but they were encouraging.
The evidence still shows the need for a RCT of intra-articular FJIs for patients with suspected facet joint pain when added to a package of best usual conservative care. Here, we provide a solid methodology for screening, assessment and monitoring as well as an agreed package of conservative care. We also provide valuable information about recruitment to such a trial. We therefore suggest that a full trial with an in-built pilot study to test recruitment is feasible.
Recommendations for research
A definitive trial of adding intra-articular FJIs to BUC for people with suspected facet joint pain that is not resolving after conservative treatment.
Acknowledgements
We would like to acknowledge and thank all of the people involved in all aspects of the FIS.
Trial Management Group
Martin Underwood (Chief Investigator).
Hugh Antrobus (Co-applicant and PI).
Frances Griffiths (Professor of Medicine in Society).
Nigel Stallard (Co-applicant and Statistician).
Stavros Petrou (Co-applicant and Health Economist).
Charles Hutchinson (Co-applicant and Professor of Clinical Imaging).
Melinda Cairns (Co-applicant and Clinical Research Fellow).
Kirstie Haywood (Co-applicant and Senior Research Fellow).
Harbinder Sandhu (Associate Professor in Health Psychology).
Shyam Balasubramanian (Co-applicant and PI).
David Walsh (Co-applicant and PI).
Alan Bennett (PI).
Sallie Brown (Co-applicant and Lay Member).
Colin Tysall (Co-applicant and Lay Member).
Ranjit Lall (Statistician).
James Griffin (Statistician).
Felix Achana (Health Economist).
David R Ellard (Principal Research Fellow).
Becky Chadwick (Sponsor Representative, Co-applicant and Delivery Manager – Research, Development and Innovation).
Katie Bruce (Sponsor Representative and Trial Manager).
Trial Steering Committee
Nefyn Williams (Chairperson).
Obi Ukoumunne.
Sujaan Singh.
Nadine Foster.
Bronek Boszcyzk.
Deb Smith.
Owen Bodycombe.
Data management and administrative support
Sarah Lowe (Trial Co-ordinator).
Sukhdeep Dosanjh (Senior Project Manager).
Suzanne Keohane (Senior Project Manager).
Constance Jacobs (Data Clerk).
Penny Cox (Data Clerk).
Craig Turner (Trial Administrator).
Kate Packard (Trial Administrator).
Hayley Johnson (Trial Administrator).
Cheryl Ritchie (Research Facilitator).
Paul Beeby (Management Accountant).
Janice Denham (Senior Finance Assistant).
Danny Wesley (Finance Assistant).
Paul Lindford (Senior Research Finance Assistant).
Claire Daffern (Quality Assurance Manager).
Natalie Strickland (Clinical Trials Unit Manager).
Sarah Duggan (Clinical Trials Unit Manager).
Sonia Davies (Trial Co-ordinator).
Kerry Barkan (Administration Support).
Morna Wood (Transcriber).
Adrian Willis (Senior Programmer).
Henry Adjei (Programmer).
Emmanuel Oluwaseun (Programmer).
Rebecca Silver (Assistant Research Contracts/Funding Officer).
Phil Ryan (Contracts Officer).
Erika Wright (Research Funding Officer).
Debbie Ranger (Head of Governance and Quality).
Graham Hewitt (Programme Quality Manager).
Emily Dight (Research and Ethics Governance Administrator).
Jane Prewitt (Lead Sponsor and Deputy Director).
Ceri Jones (Cosponsor and Head of Research, Development and Innovation).
Navdeep Bains (Head of Research Support Funding and Contracts).
Sonia Kandola (Research Governance Specialist).
Randomisation Service (Warwick CTU Randomisation Team).
Jonathan Dove (Community Physiotherapist, Coventry and Warwickshire NHS Trust).
Tom Mars (Research Fellow, University of Warwick).
Melina Dritsaki (Research Fellow, Health Economist, University of Warwick).
Research team
University Hospitals Coventry and Warwickshire NHS Trust
Shyam Balasubramanian (PI and Injector).
Davina Hewitt (Research Nurse).
Nicolas Aldridge (Research Nurse).
Sarah O’Toole (Research Nurse).
Stefanie Gwynne (Administrator).
Janet King (Administrator).
Jo Freelove (Diagnostic Assessor).
Tim Rhodes Jones (Treating Physiotherapist).
Lizzie Fort (Treating Physiotherapist).
Lois Burton (Treating Physiotherapist).
Allan Jackson (Treating Physiotherapist).
Atique Rehman (Treating Physiotherapist).
Mojid Khan (Lead Pharmacist).
Worcestershire Acute Hospitals NHS Trust
Alan Bennett (PI and Injector).
Julie Wollaston (Research Nurse).
Terry Martin (Research Nurse).
Sharon Williams (Research Sister).
Janice Gastinger (Diagnostic Assessor).
Vicky Chapman (Diagnostic Assessor).
Dianne Spalding (Treating Physiotherapist).
Judith Jehring (Treating Physiotherapist).
Ann White (Lead Pharmacist).
Sherwood Forest Hospitals NHS Foundation Trust
David Walsh (PI and Diagnostic Assessor).
Bala Veemaraon (Injector).
Yvette Girvan (Research Nurse).
Deborah Wilson (Research Nurse).
Debbie Bottomley (Research Nurse).
Stephen Bliss (Diagnostic Assessor).
Asha Gudibandi (Treating Physiotherapist).
Amanda Buckley (Treating Physiotherapist).
Linda Otter (Lead Pharmacist).
South Warwickshire NHS Foundation Trust
Hugh Antrobus (PI and Injector).
Dev Talik (Injector).
Cheryl Ritchie (Research Facilitator).
Suzanne Jones (Diagnostic Assessor).
Roger Wedden (Diagnostic Assessor).
Glynn Alderman (Treating Physiotherapist).
Elizabeth Rourke (Treating Physiotherapist).
Julia Jones (Lead Pharmacist).
South Tees Hospitals NHS Foundation Trust
Sam Eldabe (PI and Injector).
Vicky Phelps (Research Nurse).
Morag Brookes (Research Nurse).
Grace Madzinga (Research Nurse).
Karoline Middleton (Administrator).
Nicolas Harland (Diagnostic Assessor).
Amada Hughes (Diagnostic Assessor).
Victoria Robinson (Diagnostic Assessor).
Crystal Rosser (Treating Physiotherapist).
Richard King (Treating Physiotherapist).
Samantha Cook (Treating Physiotherapist).
Jason Wong (Lead Pharmacist).
The team wish to acknowledge all of those who contributed to the FIS consensus conference (see tables below).
The team acknowledge the support of the Revalidation and Continuing Professional Development Team at The Royal College of Anaesthetists for providing continuing professional development points for this event. We also extend thanks to Warwick conferences for the excellent facilities and refreshments.
The Facet Injection Study team
Professor Martin Underwood. a
Dr Hugh Antrobus. a
Dr Shyam Balasubramanian. a
Ms Kerry Barkan.
Dr Alan Bennett.
Mrs Sally Brown. a
Dr Melinda Cairns. a
Ms Sonia Davies.
Dr David Ellard.
Professor Frances Griffiths. a
Dr Kirstie Haywood. a
Professor Charles Hutchinson. a
Ms Amy Ismay.
Ms Suzanne Keohane.
Dr Ranjit Lall.
Ms Claudia Lega.
Mr Tom Mars.
Mr Luke Parsons.
Professor Stavros Petrou. a
Dr Rachel Potter.
Ms Sophie Radford.
Ms Marie Reynolds.
Azam Saied.
Dr Harbinder Sandhu. a
Professor Nigel Stallard. a
Mr Colin Tysall. a
Professor David Walsh. a
Ms Christelle Kennedy.
Mr James Griffin.
The facet joint consensus delegatesOlayinka Agbejule.
Kanar Al-Quragooli.
Hugh Antrobus.
Shyam Balasubramanian.
Keith Bell.
Alan Bennett.
Stephen Bliss.
Sally Brown.
Julian Campbell.
Helen Challinor.
Robin Correa.
Rob Froud.
Janice Gastinger.
David George.
Aditi Ghei.
Dorothy Goodwin.
Jane Griffiths.
Andrew Gunatilleke.
Julie Hall.
Jing Lee.
Richard Makin.
John O’Hanlon.
Shilpa Patel.
Sarah Paterson.
Helen Richmond.
Cheryl Ritchie.
Nicholas Roberts.
David Rogers.
Audrey Rose.
Yadi Jayran-Nejad.
Louise C Jeynes.
Janet King.
Zbigniew M Kirkor.
Yvonne Krantz.
Karen Le Marchand.
Geoff Rose.
Sujaan Singh.
Eilish Simpson.
Di Spalding.
Bhaskar Tandon.
Madan Thirugnanam.
Justin Turner.
Colin Tysall.
Bala Veemarajan.
Thirunavukkarasu Vishwanathan.
David Walsh.
Rebecca Warren.
Helen Welch.
Lars Williams.
Denotes co-applicants on the funding proposal.
All reported delegates consented to inclusion.
The team also acknowledges the American Society of Interventional Pain Physicians (Holly Long) for permission to reproduce material from our consensus conference results paper within our consensus conference results section.
Finally, the team would like to thank Mosio (www.mosio.com) for providing the text messaging service for the collection of daily/weekly pain scores from participants, in particular Charlotte Justice, who was our account manager at Mosio.
This project benefited from facilities funded through Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands.
Contributions of authors
All of the named authors contributed substantially to the development of the research question and study design, implementation, analysis and/or interpretation of data and submission of the final report.
Particular contributions are as follows.
David R Ellard (Principal Research Fellow, University of Warwick) contributed to the design of the process evaluation, collected the process evaluation data, undertook the qualitative and quantitative analyses of the process evaluation and managed and contributed to the writing of the background, methods, results and discussion sections of this report.
Martin Underwood (Professor of Primary Care Research, University of Warwick) was the lead applicant and chief investigator for the trial. He made substantial contributions to the overall design of the study, the statistical analysis plan and the writing of the background, methods, results and discussion sections of the report.
Felix Achana (Research Fellow, University of Warwick) contributed to the design, conduct, analysis and writing of the health economic evaluation.
James HL Antrobus (Consultant, Anaesthesia & Pain Medicine, South Warwickshire NHS Foundation Trust) was a co-applicant and contributed to the design and conduct of the study. In particular, he was involved with the development of the injection procedure used in this trial. As PI at his trust, within the pain management service, he provided the injection procedure to participants randomised to this arm.
Shyam Balasubramanian (Consultant, Anaesthesia & Pain Medicine, University Hospitals Coventry and Warwickshire NHS Trust) was a co-applicant and contributed to the design and conduct of the study. As PI at his trust, within the pain management service, he provided the injection procedure to participants randomised to this arm.
Sally Brown (Lay Representative, University of Warwick) was a co-applicant and provided a much-needed lay perspective, overseeing and commenting on all aspects of the trial and its documentation.
Melinda Cairns (Postdoctoral Research Fellow, University of Hertfordshire) was a co-applicant and physiotherapy lead. She contributed to the design and conduct of the study. In particular, she was involved in bringing together the consensus data and building both the studies diagnostic criteria and ‘BUC’ package.
James Griffin (Research Fellow, University of Warwick) contributed to the statistical analysis plan and undertook the statistical analyses and their reporting.
Frances Griffiths (Professor of Medicine in Society, University of Warwick) was a co-applicant and made substantial contributions to the overall design of the study and the writing of the background, methods, results and discussion sections of the report.
Kirstie Haywood (Senior Research Fellow, University of Warwick) was a co-applicant and contributed to the overall design of the study, in particular the PROMs, and the writing of the report.
Charles Hutchinson (Professor of Clinical Imaging, University of Warwick) was a co-applicant and contributed to the overall design of the study and the writing of the report.
Ranjit Lall (Principal Research Fellow, University of Warwick) contributed to the statistical analysis plan, the design of the study and the writing of the report.
Stavros Petrou (Professor in Health Economics, University of Warwick) was a co-applicant and health economics lead. He contributed to the health economic analysis plan and commented on the final draft of the report.
Nigel Stallard (Professor of Medical Statistics, University of Warwick) was a co-applicant, lead trial statistician and contributed to the statistical analysis plan and writing of the results section of the report.
Colin Tysall (Lay Representative, University of Warwick) was a co-applicant and provided a much-needed lay perspective overseeing and commenting on all aspects of the trial and its documentation.
David A Walsh (Professor of Rheumatology, University of Nottingham) was a co-applicant and contributed to the overall design of the study and the writing of the report.
Harbinder Sandhu (Associate Professor, University of Warwick) was a co-applicant and health psychology lead on the study. She contributed to the design and conduct of the study. In particular, she was involved in bringing together the consensus data and building the ‘BUC’ package.
Publications
Cairns M. Diagnostic Assessment Manual. University of Warwick, Warwick Medical School, Clinical Trials Unit; 2014. URL: http://wrap.warwick.ac.uk/78982 (accessed 23 May 2016).
Cairns M, Sandhu H. Best Usual Care Physical Rehabilitation Combined with Psychology Physiotherapy Manual. University of Warwick, Warwick Medical School, Clinical Trials Unit; 2014. URL: http://wrap.warwick.ac.uk/78986 (accessed 23 May 2016).
Underwood M, Antrobus H. Injection Manual. University of Warwick, Warwick Medical School, Clinical Trials Unit; 2014. URL: http://wrap.warwick.ac.uk/78985 (accessed 23 May 2016).
Underwood M, Antrobus H, Barkan K, Cairns M, Ellard DR, Griffiths F, et al. Facet-Joint Injections for People with Persistent Non-Specific Low Back Pain (FIS). University of Warwick, Warwick Medical School, Clinical Trials Unit; 2014. URL: http://wrap.warwick.ac.uk/78978 (accessed 23 May 2016).
Ellard DR, Griffiths F. Facet-joint Injections for People with Persistent Nonspecific Low Back Pain (FIS). University of Warwick, Warwick Medical School, Clinical Trials Unit; 2015. URL: http://wrap.warwick.ac.uk/78987 (accessed 23 May 2016).
Mars T, Ellard DR, Antrobus JH, Cairns M, Haywood K, Keohane S, et al. Intraarticular facet injections for low back pain: design considerations, consensus methodology to develop the protocol for a randomized controlled trial. Pain Physician 2015;18:473–93.
Sandhu H, Ellard DR, Achana F, Antrobus JH, Balasubramanian S, Brown S, et al. Facet-joint injections for people with persistent non-specific low back pain (FIS): study protocol for a randomised controlled feasibility trial. Trials 2015;16:588.
Vekaria R, Bhatt R, Ellard DR, Henschke N, Underwood M, Sandhu H. Intra-articular facet joint injections for low back pain: a systematic review. Eur Spine J 2016;25:1266–81.
Data sharing statement
We shall make data available to the scientific community with as few restrictions as feasible. Requests for the data should be made to the corresponding author (Professor Martin Underwood).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968-74. http://dx.doi.org/10.1136/annrheumdis-2013-204428.
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95-103. https://doi.org/10.1016/S0304-3959(99)00187-6.
- Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 2004;29:79-86. http://dx.doi.org/10.1097/01.BRS.0000105527.13866.0F.
- Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine 2005;30:1441-5. https://doi.org/10.1097/01.brs.0000166503.37969.8a.
- Manchikanti L, Pampati V, Singh V, Falco FJ. Assessment of the escalating growth of facet joint interventions in the medicare population in the United States from 2000 to 2011. Pain Physician 2013;16:E365-78.
- Low Back Pain: Early Management of Persistent Non-specific Low Back Pain. London: NICE; 2009.
- Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine 2009;34:1066-77.
- The Pain Society . The NICE Back Pain Issue. The Controversy Discussed 2009. http://britishpainsociety.org/bps_nl_autumn_2009.pdf (accessed 23 May 2014).
- Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician 2013;16:49-283.
- Manchikanti L, Datta S, Gupta S, Munglani R, Bryce DA, Ward SP, et al. A critical review of the American Pain Society clinical practice guidelines for interventional techniques: part 2. Therapeutic interventions. Pain Physician 2010;13:E215-64.
- Low Back Pain and Sciatica in Over 16s: Assessment and Management 2016. London: NICE; 2016.
- Celik B, Er U, Simsek S, Altug T, Bavbek M. Effectiveness of lumbar zygapophysial joint blockage for low back pain. Turk Neurosurg 2011;21:467-70. http://dx.doi.org/10.5137/1019-5149.JTN.4057-10.1.
- Fuchs S, Erbe T, Fischer HL, Tibesku CO. Intraarticular hyaluronic acid versus glucocorticoid injections for nonradicular pain in the lumbar spine. J Vasc Interv Radiol 2005;16:1493-8. https://doi.org/10.1097/01.RVI.0000175334.60638.3F.
- Manchikanti L, Hirsch JA, Falco FJ, Boswell MV. Management of lumbar zygapophysial (facet) joint pain. World J Orthop 2016;7:315-37. http://dx.doi.org/10.5312/wjo.v7.i5.315.
- Manchikanti L, Malla Y, Wargo BW, Cash KA, Pampati V, Fellows B. Complications of fluoroscopically directed facet joint nerve blocks: a prospective evaluation of 7,500 episodes with 43,000 nerve blocks. Pain Physician 2012;15:E143-50.
- Manchikanti L, Pampati V, Bakhit CE, Rivera JJ, Beyer CD, Damron KS, et al. Effectiveness of lumbar facet joint nerve blocks in chronic low back pain: a randomized clinical trial. Pain Physician 2001;4:101-17.
- Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, double-blind controlled trial: Clinical Trial NCT00355914. Pain Physician 2008;11:121-32.
- Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain 1992;49:325-8. https://doi.org/10.1016/0304-3959(92)90239-8.
- Falco FJ, Manchikanti L, Datta S, Sehgal N, Geffert S, Onyewu O, et al. An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician 2012;15:E869-907.
- Manchikanti L, Singh V, Falco FJ, Cash KA, Fellows B. Comparative outcomes of a 2-year follow-up of cervical medial branch blocks in management of chronic neck pain: a randomized, double-blind controlled trial. Pain Physician 2010;13:437-50.
- Hospital Episode Statistics, Admitted Patient Care – England 2016. Leeds: Health and Social Care Information Centre; 2016.
- HTA – 11/31/01: Facet Feasibility (FF). Southampton: National Institute for Health Research; 2015.
- NIHR Evaluation, Trials and Studies Coordinating Centre . HTA – 11 31 02: A Multicentre Double-Blind Randomised Controlled Trial to Assess the Clinical- and Cost-Effectiveness of Facet-Joint Injections in Selected Patients With Non-Specific Low Back Pain: A Feasibility Study 2015. www.nets.nihr.ac.uk/projects/hta/113102 (accessed 28 July 2016).
- Hancock M, Herbert RD, Maher CG. A guide to interpretation of studies investigating subgroups of responders to physical therapy interventions. Phys Ther 2009;89:698-704. http://dx.doi.org/10.2522/ptj.20080351.
- Patel S, Hee S, Mistry D, Jordan J, Brown S, Dritsaki M, et al. Identifying back pain subgroups: developing and applying approaches using individual patient data collected within clinical trials. Programme Grants Appl Res 2016;4.
- Mars T, Ellard DR, Antrobus JH, Cairns M, Underwood M, Haywood K, et al. Intraarticular Facet injections for low back pain: design considerations, consensus methodology to develop the protocol for a randomized controlled trial. Pain Physician 2015;18:473-93.
- Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36:811-16. http://dx.doi.org/10.3174/ajnr.A4173.
- Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O’Donnell CJ, et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine 2008;33:2560-5. http://dx.doi.org/10.1097/BRS.0b013e318184ef95.
- van Kleef M, Vanelderen P, Cohen SP, Lataster A, Van Zundert J, Mekhail N. 12. Pain originating from the lumbar facet joints. Pain Pract 2010;10:459-69. http://dx.doi.org/10.1111/j.1533-2500.2010.00393.x.
- Revel M, Poiraudeau S, Auleley GR, Payan C, Denke A, Nguyen M, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine 1998;23:1972-6. https://doi.org/10.1097/00007632-199809150-00011.
- Laslett M, Oberg B, Aprill CN, McDonald B. Zygapophysial joint blocks in chronic low back pain: a test of Revel’s model as a screening test. BMC Musculoskelet Disord 2004;5. https://doi.org/10.1186/1471-2474-5-43.
- Hooten WM, Martin DP, Huntoon MA. Radiofrequency neurotomy for low back pain: evidence-based procedural guidelines. Pain Med 2005;6:129-38. https://doi.org/10.1111/j.1526-4637.2005.05022.x.
- Sharma H, Duggan S, Nazir J, Andrews J, Fender D, Sanderson P, et al. Setting ‘diagnostic reference levels’ for fluoroscopy-guided spinal procedures. J Bone Joint Surg Br 2012;94–B.
- Laslett M, McDonald B, Aprill CN, Tropp H, Oberg B. Clinical predictors of screening lumbar zygapophyseal joint blocks: development of clinical prediction rules. Spine J 2006;6:370-9. https://doi.org/10.1016/j.spinee.2006.01.004.
- Hancock MJ, Maher CG, Latimer J, Spindler MF, McAuley JH, Laslett M, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J 2007;16:1539-50. http://dx.doi.org/10.1007/s00586-007-0391-1.
- DePalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW. Does the location of low back pain predict its source?. PM R 2011;3:33-9. http://dx.doi.org/10.1016/j.pmrj.2010.09.006.
- Mayer TG, Robinson R, Pegues P, Kohles S, Gatchel RJ. Lumbar segmental rigidity: can its identification with facet injections and stretching exercises be useful?. Arch Phys Med Rehabil 2000;81:1143-50. https://doi.org/10.1053/apmr.2000.9170.
- Wilde VE, Ford JJ, McMeeken JM. Indicators of lumbar zygapophyseal joint pain: survey of an expert panel with the Delphi technique. Phys Ther 2007;87:1348-61. https://doi.org/10.2522/ptj.20060329.
- Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-22.
- Hahne AJ, Ford JJ, Surkitt LD, Richards MC, Chan AY, Thompson SL, et al. Specific treatment of problems of the spine (STOPS): design of a randomised controlled trial comparing specific physiotherapy versus advice for people with subacute low back disorders. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-104.
- Ford J, Thompson S, Hahne A. A classification and treatment protocol for low back disorders: part 1 – specific manual therapy. Phys Ther Rev 2011;16:168-77.
- Underwood M, Mistry D, Lall R, Lamb S. Predicting response to a cognitive-behavioral approach to treating low back pain: secondary analysis of the BeST data set. Arthritis Care Res 2011;63:1271-9. http://dx.doi.org/10.1002/acr.20518.
- Challinor H, Hourigan P, Powell R, Conn D. Movement Test Diagnose Lumbar Facet Joint Pain?. London: Society of Back Pain Research; 2013.
- Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2. http://dx.doi.org/10.1002/14651858.CD005328.pub2.
- Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev 2003;1. http://dx.doi.org/10.1002/14651858.CD004016.
- Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669-81. https://doi.org/10.1136/ard.2004.028886.
- van Vliet-Daskalopoulou E, Jentjens T, Scheffer RT. Intra-articular rimexolone in the rheumatoid knee: a placebo-controlled, double-blind, multicentre trial of three doses. Br J Rheumatol 1987;26:450-3. https://doi.org/10.1093/rheumatology/26.6.450.
- Sehgal N, Dunbar EE, Shah RV, Colson J. Systematic review of diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: an update. Pain Physician 2007;10:213-28.
- Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician 2007;10:229-53.
- Artus M, van der Windt DA, Jordan KP, Hay EM. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology 2010;49:2346-56. http://dx.doi.org/10.1093/rheumatology/keq245.
- Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 2007;10:7-111.
- Carette S, Marcoux S, Truchon R, Grondin C, Gagnon J, Allard Y, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med 1991;325:1002-7. http://dx.doi.org/10.1056/NEJM199110033251405.
- Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine 2009;34:1078-93.
- Henschke N, Kuijpers T, Rubinstein SM, van Middelkoop M, Ostelo R, Verhagen A, et al. Injection therapy and denervation procedures for chronic low-back pain: a systematic review. Eur Spine J 2010;19:1425-49. http://dx.doi.org/10.1007/s00586-010-1411-0.
- Lilius G, Laasonen EM, Myllynen P, Harilainen A, Grönlund G. Lumbar facet joint syndrome. A randomised clinical trial. J Bone Joint Surg Br 1989;71:681-4.
- Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician 2009;12:437-60.
- Froud R, Eldridge S, Lall R, Underwood M. Estimating the number needed to treat from continuous outcomes in randomised controlled trials: methodological challenges and worked example using data from the UK Back Pain Exercise and Manipulation (BEAM) trial. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-35.
- Mayer TG, Gatchel RJ, Keeley J, McGeary D, Dersh J, Anagnostis C. A randomized clinical trial of treatment for lumbar segmental rigidity. Spine 2004;29:2199-205. https://doi.org/10.1097/01.brs.0000142009.73869.8d.
- Civelek E, Cansever T, Kabatas S, Kircelli A, Yilmaz C, Musluman M, et al. Comparison of effectiveness of facet joint injection and radiofrequency denervation in chronic low back pain. Turk Neurosurg 2012;22:200-6. http://dx.doi.org/10.5137/1019-5149.JTN.5207-11.1.
- Vekaria R, Bhatt R, Ellard DR, Henschke N, Underwood M, Sandhu H. Intra-articular facet joint injections for low back pain: a systematic review. Eur Spine J 2016;25:1266-81. http://dx.doi.org/10.1007/s00586-016-4455-y.
- Kawu AA, Olawepo A, Salami AO. Facet joints infiltration: a viable alternative treatment to physiotherapy in patients with low back pain due to facet joint arthropathy. Niger J Clin Pract 2011;14:219-22. http://dx.doi.org/10.4103/1119-3077.84021.
- Ribeiro LH, Furtado RN, Konai MS, Andreo AB, Rosenfeld A, Natour J. Effect of facet joint injection versus systemic steroids in low back pain: a randomized controlled trial. Spine 2013;38:1995-2002. http://dx.doi.org/10.1097/BRS.0b013e3182a76df1.
- Hush JM, Kamper SJ, Stanton TR, Ostelo R, Refshauge KM. Standardized measurement of recovery from nonspecific back pain. Arch Phys Med Rehabil 2012;93:849-55. http://dx.doi.org/10.1016/j.apmr.2011.11.035.
- Hush JM, Refshauge K, Sullivan G, De Souza L, Maher CG, McAuley JH. Recovery: what does this mean to patients with low back pain?. Arthritis Rheum 2009;61:124-31. http://dx.doi.org/10.1002/art.24162.
- Kamper SJ, Stanton TR, Williams CM, Maher CG, Hush JM. How is recovery from low back pain measured? A systematic review of the literature. Eur Spine J 2011;20:9-18. http://dx.doi.org/10.1007/s00586-010-1477-8.
- Staniszewska S, Haywood KL, Brett J, Tutton L. Patient and public involvement in patient-reported outcome measures: evolution not revolution. Patient 2012;5:79-87. http://dx.doi.org/10.2165/11597150-000000000-00000.
- Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine 1998;23:2003-13. https://doi.org/10.1097/00007632-199809150-00018.
- Turk DC, Dworkin RH, Burke LB, Gershon R, Rothman M, Scott J, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125:208-15. https://doi.org/10.1016/j.pain.2006.09.028.
- Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137:276-85. https://doi.org/10.1016/j.pain.2007.09.002.
- Beale M, Cella M, Williams AC. Comparing patients’ and clinician-researchers’ outcome choice for psychological treatment of chronic pain. Pain 2011;152:2283-6. http://dx.doi.org/10.1016/j.pain.2011.06.007.
- Diagnosis and Management of Headache in Young People and Adults. London: NICE; 2012.
- Cairns M. Diagnostic Assessment Manual 2014. http://wrap.warwick.ac.uk/78982 (accessed 23 May 2016).
- Cairns M, Sandhu H. Facet Injection Study: Best Usual Care Physical Rehabilitation Combined With Psychology Physiotherapy Manual 2014. http://wrap.warwick.ac.uk/78986 (accessed 23 May 2016).
- Ellard DR, Griffiths F. Facet-Joint Injections for People With Persistent Nonspecific Low Back Pain (FIS) 2015. http://wrap.warwick.ac.uk/78987 (accessed 23 May 2016).
- Underwood M, Antrobus H. Facet Injection Study: Injection Manual 2014. http://wrap.warwick.ac.uk/78985 (accessed 23 May 2016).
- Underwood M, Antrobus H, Barkan K, Cairns M, Ellard DR, Griffiths F, et al. Facet-Joint Injections for People With Persistent Non-Specific Low Back Pain (FIS): Facet Injection Study Consensus Conference (FISCC) Evidence Document 2014. http://wrap.warwick.ac.uk/78978 (accessed 23 May 2016).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009. www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf.
- Nair R, Aggarwal R, Khanna D. Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 2011;41:95-105. http://dx.doi.org/10.1016/j.semarthrit.2010.12.001.
- Edwards B. Manual of Combined Movements. Oxford: Butterworth-Heinemann; 1999.
- McCarthy C, McCarthy C. Combined Movement Theory. Rotational Mobilization of the Vertebral Column. London: Churchill Livingstone; 2010.
- Chapman JR, Norvell DC, Hermsmeyer JT, Bransford RJ, DeVine J, McGirt MJ, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine 2011;36:54-68. http://dx.doi.org/10.1097/BRS.0b013e31822ef74d.
- Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141-4. https://doi.org/10.1097/00007632-198303000-00004.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133-49. https://doi.org/10.1016/0304-3959(92)90154-4.
- Lamb SE, Hansen Z, Lall R, Castelnuovo E, Withers EJ, Nichols V, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. Lancet 2010;375:916-23. http://dx.doi.org/10.1016/S0140-6736(09)62164-4.
- UK BEAM Trial Team . United Kingdom back pain exercise and manipulation (UK BEAM) randomised trial: effectiveness of physical treatments for back pain in primary care. BMJ 2004;329. https://doi.org/10.1136/bmj.38282.669225.AE.
- Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine 2008;33:90-4. http://dx.doi.org/10.1097/BRS.0b013e31815e3a10.
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146:238-44. http://dx.doi.org/10.1016/j.pain.2009.08.019.
- Froud R, Eldridge S, Kovacs F, Breen A, Bolton J, Dunn K, et al. Reporting outcomes of back pain trials: a modified Delphi study. Eur J Pain 2011;15:1068-74. http://dx.doi.org/10.1016/j.ejpain.2011.04.015.
- Froud R, Underwood M, Carnes D, Eldridge S. Clinicians’ perceptions of reporting methods for back pain trials: a qualitative study. Br J Gen Pract 2012;62:e151-9. http://dx.doi.org/10.3399/bjgp12X630034.
- Henschke N, van Enst A, Froud R, Ostelo RW. Responder analyses in randomised controlled trials for chronic low back pain: an overview of currently used methods. Eur Spine J 2014;23:772-8. http://dx.doi.org/10.1007/s00586-013-3155-0.
- Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007;10:125-37. https://doi.org/10.1111/j.1524-4733.2007.00275.x.
- Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7:541-6. https://doi.org/10.1016/j.spinee.2007.01.008.
- Guyatt G. Understanding the fundamentals of quality of life measurement. Evid Based Cardiovasc Med 1998;2:35-6. https://doi.org/10.1016/S1361-2611(98)80079-1.
- Wyrwich KW, Norquist JM, Lenderking WR, Acaster S. Industry Advisory Committee of International Society for Quality of Life Research (ISOQOL) . Methods for interpreting change over time in patient-reported outcome measures. Qual Life Res 2013;22:475-83. http://dx.doi.org/10.1007/s11136-012-0175-x.
- Johnston BC, Patrick DL, Thorlund K, Busse JW, da Costa BR, Schünemann HJ, et al. Patient-reported outcomes in meta-analyses-part 2: methods for improving interpretability for decision-makers. Health Qual Life Outcomes 2013;11. http://dx.doi.org/10.1186/1477-7525-11-211.
- Johnston BC, Thorlund K, Schünemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-116.
- Marx RG, Jones EC, Angel M, Wickiewicz TL, Warren RF. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy 2003;19:762-70. https://doi.org/10.1016/S0749-8063(03)00398-0.
- Underwood MR, Barnett AG, Vickers MR. Evaluation of two time-specific back pain outcome measures. Spine 1999;24:1104-12. https://doi.org/10.1097/00007632-199906010-00010.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14410.
- Hay EM, Mullis R, Lewis M, Vohora K, Main CJ, Watson P, et al. Comparison of physical treatments versus a brief pain-management programme for back pain in primary care: a randomised clinical trial in physiotherapy practice. Lancet 2005;365:2024-30. https://doi.org/10.1016/S0140-6736(05)66696-2.
- Sandhu H, Ellard DR, Achana F, Antrobus JH, Balasubramanian S, Brown S, et al. Facet-joint injections for people with persistent non-specific low back pain (FIS): study protocol for a randomised controlled feasibility trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-1117-z.
- Parsons S, Carnes D, Pincus T, Foster N, Breen A, Vogel S, et al. Measuring troublesomeness of chronic pain by location. BMC Musculoskelet Disord 2006;7. https://doi.org/10.1186/1471-2474-7-34.
- Lamb SE, Mistry D, Lall R, Hansen Z, Evans D, Withers EJ, et al. Back Skills Training Trial Group . Group cognitive behavioural interventions for low back pain in primary care: extended follow-up of the Back Skills Training Trial (ISRCTN54717854). Pain 2012;153:494-501. http://dx.doi.org/10.1016/j.pain.2011.11.016.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. https://doi.org/10.1136/bmj.39108.379965.BE.
- Savigny P, Watson P, Underwood M. Guideline Development Group . Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1805.
- Maitland G, Hengeveld E, Banks K, English L. Maitland’s Vertebral Manipulation. Oxford: Elsevier; 2005.
- Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003;106:337-45. https://doi.org/10.1016/j.pain.2003.08.001.
- Klokkerud M, Grotle M, Lochting I, Kjeken I, Hagen KB, Garratt AM. Psychometric properties of the Norwegian version of the patient generated index in patients with rheumatic diseases participating in rehabilitation or self-management programmes. Rheumatology 2013;52:924-32.
- Løchting I, Grotle M, Storheim K, Werner E, Garratt A. Individualised quality of life in patients with low back pain: reliability and validity of the Patient Generated Index (PGI). J Rehab Med 2014;46:781-7. https://doi.org/10.2340/16501977-1826.
- Pincus T, Rusu A, Santos R. Responsiveness and construct validity of the depression, anxiety, and positive outlook scale (DAPOS). Clin J Pain 2008;24:431-7. http://dx.doi.org/10.1097/AJP.0b013e318164341c.
- Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007;11:153-63. https://doi.org/10.1016/j.ejpain.2005.12.008.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-Being Scale (WEMWBS): development and UK validation. Health Quality Life Outcomes 2007;5. https://doi.org/10.1186/1477-7525-5-63.
- Morren M, van Dulmen S, Ouwerkerk J, Bensing J. Compliance with momentary pain measurement using electronic diaries: a systematic review. Eur J Pain 2009;13:354-65. http://dx.doi.org/10.1016/j.ejpain.2008.05.010.
- Burton C, Weller D, Sharpe M. Are electronic diaries useful for symptoms research? A systematic review. J Psychosom Res 2007;62:553-61. https://doi.org/10.1016/j.jpsychores.2006.12.022.
- Bogduk N. Diagnosing lumbar zygapophysial joint pain. Pain Med 2005;6:139-42. https://doi.org/10.1111/j.1526-4637.2005.05023.x.
- Bogduk N. A narrative review of intra-articular corticosteroid injections for low back pain. Pain Med 2005;6:287-96. https://doi.org/10.1111/j.1526-4637.2005.00048.x.
- European Commission . European Union Clinical Trials Directive 2001 20 EC 2001.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . Integrated Addendum to ICH E6 (R1): Guideline for Good Clinical Practice E6(R2). ICH Harmonised Guideline n.d. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4.pdf (accessed 21 February 2017).
- Gov.UK . Good Clinical Practice for Clinical Trials n.d. www.gov.uk/guidance/good-clinical-practice-for-clinical-trials (accessed 21 February 2017).
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Medical Care 2007;45. https://doi.org/10.1097/MLR.0b013e31814848f1.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England 2016. www.ohe.org/publications/valuing-health-related-quality-life-eq-5d-5l-value-set-england (accessed 10 June 2016).
- Stata 14 Base Reference Manual. College Station, TX: Stata Press; 2015.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process Evaluation of Complex Interventions: UK Medical Research Council (MRC) Guidance 2014. www.ioe.ac.uk/MRC_PHSRN_Process_evaluation_guidance_final(2).pdf (accessed January 2015).
- Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Ellard D, Parsons S, Thorogood M, Coombes Y. Evaluating Health Promotion Practice and Methods. Oxford: Oxford University Press; 2010.
- Ellard DR, Chimwaza W, Davies D, O’Hare JP, Kamwendo F, Quenby S, et al. ETATMBA Study Group . Can training in advanced clinical skills in obstetrics, neonatal care and leadership, of non-physician clinicians in Malawi impact on clinical services improvements (the ETATMBA project): a process evaluation. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005751.
- Ellard DR, Taylor SJ, Parsons S, Thorogood M. The OPERA trial: a protocol for the process evaluation of a randomised trial of an exercise intervention for older people in residential and nursing accommodation. Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-28.
- Ellard DR, Thorogood M, Underwood M, Seale C, Taylor SJ. Whole home exercise intervention for depression in older care home residents (the OPERA study): a process evaluation. BMC Med 2014;12. http://dx.doi.org/10.1186/1741-7015-12-1.
- Garratt AM. Evaluation of the stages of completion and scoring of the Patient Generated Index (PGI) in patients with rheumatic diseases. Qual Life Res 2015;24:2625-35. http://dx.doi.org/10.1007/s11136-015-1014-7.
- Ruta DA, Garratt AM, Leng M, Russell IT, MacDonald LM. A new approach to the measurement of quality of life. The Patient-Generated Index. Med Care 1994;32:1109-26. https://doi.org/10.1097/00005650-199411000-00004.
- Ruta DA, Garratt AM, Russell IT. Patient centred assessment of quality of life for patients with four common conditions. Qual Health Care 1999;8:22-9. https://doi.org/10.1136/qshc.8.1.22.
- Haywood KL, Garratt AM, Dziedzic K, Dawes PT. Patient centered assessment of ankylosing spondylitis-specific health related quality of life: evaluation of the Patient Generated Index. J Rheumatol 2003;30:764-73.
- Patel KK, Veenstra DL, Patrick DL. A review of selected patient-generated outcome measures and their application in clinical trials. Value Health 2003;6:595-603. https://doi.org/10.1046/j.1524-4733.2003.65236.x.
- Martin F, Camfield L, Rodham K, Kliempt P, Ruta D. Twelve years’ experience with the Patient Generated Index (PGI) of quality of life: a graded structured review. Qual Life Res 2007;16:705-15. http://dx.doi.org/10.1007/s11136-006-9152-6.
- Hush JM, Refshauge KM, Sullivan G, De Souza L, McAuley JH. Do numerical rating scales and the Roland-Morris Disability Questionnaire capture changes that are meaningful to patients with persistent back pain?. Clin Rehabil 2010;24:648-57. http://dx.doi.org/10.1177/0269215510367975.
- Ong BN, Konstantinou K, Corbett M, Hay E. Patients’ own accounts of sciatica: a qualitative study. Spine 2011;36:1251-6. http://dx.doi.org/10.1097/BRS.0b013e318204f7a2.
- Kerns RD, Burns JW, Shulman M, Jensen MP, Nielson WR, Czlapinski R, et al. Can we improve cognitive-behavioral therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol 2014;33:938-47. http://dx.doi.org/10.1037/a0034406.
- Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95-103. https://doi.org/10.1097/BRS.0b013e31815e7f94.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010;63:737-45. http://dx.doi.org/10.1016/j.jclinepi.2010.02.006.
- Stevenson M, Gomersall T, Lloyd Jones M, Rawdin A, Hernández M, Dias S, et al. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18170.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- NHS Digital . Prescription Cost Analysis: England 2015 2016. http://content.digital.nhs.uk/catalogue/PUB20200 (accessed 28 February 2017).
- NHS Reference Costs 2014 to 2015. London: DH; 2015.
- Curtis L. Unit Costs of Health and Social Care 2013 2013. www.pssru.ac.uk/project-pages/unit-costs/2013/ (accessed 28 February 2017).
- National Pain Audit – Find a Clinic. London: Dr Foster Research Ltd; 2015.
- Care Quality Commission . Hospitals n.d. www.cqc.org.uk/content/hospitals (accessed 26 April 2017).
- Slade SC, Keating JL. Exercise prescription: a case for standardised reporting. Br J Sports Med 2012;46:1110-13. http://dx.doi.org/10.1136/bjsports-2011-090290.
- Haywood KL, Packham JC, Jordan KP. Assessing fatigue in ankylosing spondylitis: the importance of frequency and severity. Rheumatology 2014;53:552-6. https://doi.org/10.1093/rheumatology/ket397.
- Hewlett S, Ambler N, Almeida C, Cliss A, Hammond A, Kitchen K, et al. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis 2011;70:1060-7.
- Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271-3.
- Froud R, Patterson S, Eldridge S, Seale C, Pincus T, Rajendran D, et al. A systematic review and meta-synthesis of the impact of low back pain on people’s lives. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-50.
- Madan J, Ades AE, Price M, Maitland K, Jemutai J, Revill P, et al. Strategies for efficient computation of the expected value of partial perfect information. Med Decis Making 2014;34:327-42. http://dx.doi.org/10.1177/0272989X13514774.
- Strong M, Oakley JE, Brennan A, Breeze P. Estimating the expected value of sample information using the probabilistic sensitivity analysis sample: a fast, nonparametric regression-based method. Med Decis Making 2015;35:570-83. http://dx.doi.org/10.1177/0272989X15575286.
- Maas ET, Ostelo RW, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, et al. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev 2015;10. http://dx.doi.org/10.1002/14651858.CD008572.pub2.
- Maas E, Juch J, Ostelo R, Groeneweg G, Kallewaard JW, Koes B, et al. Effectiveness and cost-effectiveness of radiofrequency denervation for chronic low back pain originating from the facet joints. Pain Practice 2016;16.
- Salman OH, Gad GS, Mohamed AA, Rafae HH, Abdelfatah AM. Randomized, controlled blind study comparing sacroiliac intra-articular steroid injection to radiofrequency denervation for sacroiliac joint pain. Egy J Anaesthesia 2016;32:219-25.
- Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Physics 1980;6:371-4. https://doi.org/10.1016/0360-3016(80)90153-4.
- Stallard N. Optimal sample sizes for phase II clinical trials and pilot studies. Stat Med 2012;31:1031-42. https://doi.org/10.1002/sim.4357.
Appendix 1 Patient and public involvement perspective
One of our lay representatives reflected on his experience of taking part in this study from early meetings to its conclusion:
My associate lay coapplicant and I were invited to the first planning meeting when the idea of a randomised controlled feasibility trial to test the theory that FJIs might be therapeutically beneficial for people with low back pain.
We had experience this sort of intervention with varying degrees of benefit. We were also known to the researchers by our involvement in other lower back pain projects. At the first meeting the makeup of the team was discussed. Then, at subsequent meeting, the necessary expertise was brought together and the structure of the trial was formulated.
We were fully involved in all discussions and our ‘expertise by experience’ was called on when discussing the protocol and PPI element of the proposed trial. We tested the use of mobile phones to relay pain scores to the trial team. We checked all documentation that was used with patients and asked for justification for use of quality measures and data analysis to achieve the trials goals.
We took part in the consensus meeting when it was discovered that there were no standard guidelines for the methods of both giving FJIs or what to inject.
We were fully involved in determining the exclusion criteria for the trial and the data collection time scale. The BUC had to be standardised so that we had a baseline for the treatment. Also it was necessary to find out what the professionals meant by facet joint pain. If all this was possible and we were able to bring together many and varied sites around the country so that recruitment and procedures could be co-ordinated. We could then proceed to a full trial to prove or otherwise that FJIs were both therapeutically beneficial and cost-effective.
Appendix 2 Example screening log
Appendix 3 Case report form
Appendix 4 Baseline questionnaire
Appendix 5 Three-month follow-up questionnaire
Appendix 6 Sample size calculation
Facet feasibility study sample size calculations
At the end of the feasibility trial, we will obtain an estimate, θ^ of the unknown treatment difference along with a 95% CI given by:
We will consider proceeding to the main study if the upper limit exceeds some prescribed target δ.
For a true treatment difference of θ, we have, θ^ ∼ N(θ, 2σ2/(n/2)), so that
Calculation of this expression gives the probability of recommending progression to the main study with total sample size n when the true treatment effect is θ.
If the true treatment effect is equal to the specified target value, that is, θ = δ, this probability is 1 – Φ (1.96) = 0.975, so that the probability is 0.975 irrespective of the sample size or choice of δ (this follows from the definition of a 95% CI). For θ = 0, we get the probability of erroneously proceeding to the main study when the true effect is zero. Values for a range of values of n and δ are given in Table 33.
| δ | n = 80 | n = 90 | n = 100 | n = 110 | n = 120 | n = 130 | n = 140 |
|---|---|---|---|---|---|---|---|
| 0.20 | 0.857 | 0.844 | 0.831 | 0.819 | 0.806 | 0.794 | 0.781 |
| 0.25 | 0.800 | 0.781 | 0.761 | 0.742 | 0.723 | 0.704 | 0.685 |
| 0.30 | 0.732 | 0.704 | 0.677 | 0.651 | 0.624 | 0.599 | 0.573 |
| 0.35 | 0.653 | 0.618 | 0.583 | 0.550 | 0.517 | 0.486 | 0.456 |
| 0.40 | 0.568 | 0.525 | 0.484 | 0.445 | 0.409 | 0.374 | 0.342 |
These probabilities can be interpreted in a number of ways. The test can be viewed as a test of the null hypothesis θ = δ conducted at the one-sided 2.5% level. The probabilities in Table 33 then correspond to the power of this test to detect the alternative θ = 0.
Alternatively, considering a conventional test of the null hypothesis that θ = 0, this approach fixes the power to detect a difference of size δ to be 97.5% (and does so irrespective of the unknown variance). The type I error rate is then determined by the sample size (and σ2) and is given by the values in Table 33.
The approach of using a high power and a high type I error rate in pilot studies has been advocated by a number of authors (e.g. see Schoenfeld162 and Stallard163).
It is proposed to take n to be approximately 130 (150 less about 20% dropout). For a δ of 0.35, this gives a probability of proceeding to the main study when the true treatment effect is zero of approximately 50%. Equivalently, this decision will be taken provided the treatment effect estimate at the end of this study is at least positive.
Appendix 7 Patient enrolment, contact details and randomisation case report form
Glossary
- Best usual care
- There are a number of definitions that can be attributed to this term, but in this study it refers to the package of physical and psychological support that is provided for every participant (six sessions) by our study physiotherapists.
- Feasibility study
- An assessment of the practicality of a proposed project.
- Intra-articular facet joint injections
- An injection of a steroid and a local anaesthetic into the facet joints in the lower back with the aim to relieve pain.
List of abbreviations
- AUC
- area under the curve
- BEAM
- Back pain Exercise And Manipulation
- BUC
- best usual care
- CBT
- cognitive–behavioural therapy
- CI
- confidence interval
- CRF
- case report form
- DAPOS
- Depression, Anxiety, and Positive Outlook Scale
- EQ-5D
- EuroQoL-5 Dimensions
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- FIS
- Facet Injection Study
- FJI
- facet joint injection
- GP
- general practitioner
- HTA
- Health Technology Assessment
- LBP
- low back pain
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MIC
- minimally important change
- MID
- minimally important difference
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- Pain-NRS
- Pain Numerical Rating Scale
- PGI
- Patient Generated Index
- PI
- principal investigator
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSEQ
- Pain Self-Efficacy Questionnaire
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- RMDQ
- Roland–Morris Disability Questionnaire
- SD
- standard deviation
- SF-12v2
- Short Form questionnaire-12 items version 2
- UNTRAP
- University/User Teaching and Research Action Partnership
- VAS
- visual analogue scale
- WCTU
- Warwick Clinical Trials Unit
- WEMWEBS
- Warwick–Edinburgh Mental Well-Being Scale