Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 99/10/99. The contractual start date was in April 2004. The draft report began editorial review in January 2016 and was accepted for publication in January 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Mant et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Colorectal cancer is a major health problem. In the UK, around 32,000 cases are diagnosed annually and 16,000 deaths are attributed to the disease. 1 Surgery is the mainstay of treatment and most cases cured are cured by surgery alone. Adjuvant chemotherapy reduces the risk of relapse in high-risk patients and radiotherapy reduces the local recurrence rate in patients with high-risk rectal cancer. For selected patients who develop either local recurrence or distant metastases following surgery for primary disease, resection of recurrent disease, most commonly in the liver, may still result in cure. In a meta-analysis of > 20,000 patients, 40% of patients having liver resection were alive after 5 years and 30% were alive at 10 years. 2 Similar, if less well-documented, results are seen in surgery for local recurrence or lung metastases. 3,4 In those not considered to have upfront resectable recurrent disease, the early initiation of chemotherapy may prolong survival5 or, importantly, may downsize metastases to operability. 6
Traditionally, patients who have curative surgery for colorectal cancer are subject to long-term follow-up, the detection of treatable recurrence being one of the objectives. Curatively treated cancer in this setting is commonly held to refer to a patient without metastatic disease other than in local lymph nodes in whom all disease has been removed. Clinicians use various protocols, but few, if any, are evidence based. The costs to the NHS of follow-up are substantial and they need to be justified by evidence of cost-effectiveness. Although a number of studies have assessed the value of follow-up of patients with curatively resected colorectal cancer, none provides a definitive answer, for a variety of reasons.
At the time this study was developed, a number of randomised trials of colorectal cancer follow-up had been published. 7–10 The largest of these7 showed no benefit of follow-up, but the protocol did not include intensive imaging. Two small and underpowered studies appeared to show benefit of follow-up, but these did not achieve statistical significance. 8,9 Only one of these trials8 included intensive liver imaging. A study10 of 325 patients compared intensive follow-up with computerised tomography (CT) and colonoscopy, with a structured clinical review and simple tests. More patients with asymptomatic metastases were detected in the investigational arm, but the number of potentially curative hepatectomies was not different. However, this study may not have been powerful enough to detect a difference between the two active follow-up schedules.
Preliminary results of a further trial to evaluate the effect of undertaking ‘second-look’ surgery in response to a rise in carcinoembryonic antigen (CEA) during follow-up were reported in 1984. 11 At the time that the Follow-up After Colorectal Surgery (FACS) trial was designed, this trial had not demonstrated any benefit from the intervention, and a subsequent publication confirmed this. 12 However, the trial suffers from the criticism that patients who developed recurrence were not commonly offered treatments that could impact on survival, such as liver/lung resection. Of the non-randomised trials, a meta-analysis suggested benefit if the follow-up strategy included CEA testing. 13 A Cochrane review concluded that there may be a benefit of imaging follow-up (around 8%), but that further adequately powered studies were necessary14 as publication bias was likely.
It is recognised that most of the trials performed in this area preceded the common use of liver resection and modern effective chemotherapy in patients found to have metastatic disease. However, there is sufficient clinical and scientific uncertainty about a potentially important clinical treatment to justify a trial on ethical grounds. A pre-trial economic model was devised to assist in the development of the trial protocol and was used to assess the probable costs, effects and cost-effectiveness of each of the trial options. The results of this modelling (shown in Appendix 1 along with the parameter values) indicated that relatively small gains in survival could well be cost-effective, that it was important to include a primary care-based follow-up option with CEA monitoring in the study, that accurate information was required on palliative care alongside other aspects of resource use and that the cost-effectiveness results would be strongly influenced by the duration of follow-up and analytic horizon.
Four objectives of follow-up were considered in developing the trial.
-
Early detection of metastatic disease. There is unambiguous evidence that the resection of colorectal liver metastases may result in cure. Approximately 40% of patients who have a R0 (≥ 1-mm clearance at lateral margins) resection performed (no residual disease, microscopically clear margins) will survive for 5 years and 30% will survive to 10 years. 2,15 Recurrence after 10 years is not reported. The data for lung metastases are similar, although somewhat more contentious. 3,16 Although it is not absolutely certain how many patients with liver metastases are suitable for resectional surgery, the only prospective analysis suggests that the figure may be as high as 20%. 17 There is also some evidence of a survival benefit (beyond lead time) from early diagnosis and pre-symptomatic treatment with cytotoxic chemotherapy in patients not suitable for resection. 5 Furthermore, cytotoxic chemotherapy increases in a number of patients suitable for resectional liver surgery18 and also improves the outlook of those with resectable disease. 19
-
Removal of further adenomatous polyps and metachronous cancer. Patients who have developed colorectal cancer are at increased risk of further adenomatous polyps and metachronous cancer. The detection and removal of metachronous polyps or early cancer may benefit the patient in survival terms. However, it is held that if a ‘clean colon’ (no polyps) is achieved during initial treatment, then follow-up colonoscopy after several years is adequate for patients without specific genetic susceptibility. 20
-
Early detection of surgically treatable luminal recurrence. Endoscopy may detect luminal recurrence. However, luminal recurrence is uncommon21 and can be a manifestation of more extensive local recurrence. At the time of trial design, there was uncertainty regarding whether or not this is commonly curable. 22
-
Improvement in quality of life. One randomised study suggested that follow-up has a small quality-of-life advantage over no follow-up. 23 However, a randomised trial of hospital- and community-based follow-up of patients with breast cancer in the UK, comparing quality of life and diagnostic delay, showed no advantage of hospital follow-up and found that patient satisfaction with care was higher in the general practice arm. 24
Audit evidence suggests that follow-up, as normally practised in the early 2000s, when the trial was designed, seldom included surveillance with tumour markers or regular imaging. 25 Such practice was unlikely to achieve any of the four ends detailed above. Clinical examination may provide reassurance, but clinical examination is not clear that this reassurance is best provided in a hospital setting and it is unlikely to detect many cases of treatable recurrence. The use of colonoscopy in the detection of metachronous polyps is also established,26 and will ultimately impact on the incidence of metachronous cancer,20 but this need not be frequent. Anastomotic and missed synchronous cancer may also be detected (in spite of apparently complete colonic imaging), but the detection rate is low. 21 The follow-up modalities most likely to influence the primary end point were intensive imaging and serial tumour marker measurements. There was evidence that these could detect recurrence at an early and treatable stage. 14
At the time of developing the trial, spiral CT imaging was probably the best non-invasive, widely available method of detecting colorectal metastases. Even so, the sensitivity reported varied between approximately 75% and 94%,27,28 depending on the gold standard used. Spiral CT imaging is expensive and involves a considerable ionising radiation dose. Magnetic resonance imaging (MRI) does not involve ionising radiation and is more sensitive for detecting liver metastases (around 80%),27 but it is much less effective for the detection of lung and other metastases, is more expensive and is less widely available. Transabdominal ultrasound is widely available and inexpensive, but it is less sensitive for the detection of hepatic metastases (77%)28 and generally unhelpful in the detection of most extrahepatic metastases. Six-monthly CT is affordable in service terms and is currently used, with variable frequency, in most colorectal cancer units.
In order to satisfy Ionising Radiation (Medical Exposure) Regulations 2000 regulations,29 and to reflect the findings published in Radiation Protection Dosimetry,30 the patient information sheet indicated probable radiation doses and the cancer risk associated with CT imaging during the study. The patients randomised to the CT and CEA testing plus CT arms who completed all seven CT scans were exposed to a total effective radiation dose of about 140 mSv, associated with a lifetime risk of induction of a fatal cancer of about 1 in 2000. For the small group of younger (aged < 70 years) participants, the risk is higher, approximately 1 in 300. Risk from a particular radiation dose increases with younger age because there is more time for any genetic damage caused by the radiation to progress to a clinical problem.
The International Commission on Radiological Protection report on ‘Radiological Protection in Biomedical Research’ categorises the level of radiation involved in this study as ‘moderate’, with a ‘substantial’ societal benefit expected. 31 If a participating centre was unable to comply with these dose constraints, the co-ordinating centre (Southampton) would be informed. A local amendment of the patient information sheet would then be produced and submitted for approval by the main Research Ethics Committee (South West Multi-centre Research Ethics Committee).
Although a number of tumour markers are expressed in patients with colorectal cancer, CEA levels are most frequently detected. 32 It has been shown to be a useful adjunct to the detection of recurrent disease in colorectal cancer32 and can be used to monitor the progress of patients undergoing chemotherapy for colorectal cancer. 33 CEA monitoring is risk free and has the advantage of being able to be used in primary care. At the time the protocol was developed, an elevated CEA level was thought to have a 77% sensitivity for recurrence (100% for liver metastases) combined with a 98% specificity,27 although a recently published Cochrane review suggests poorer diagnostic performance. 34 The elevation in CEA level usually occurs prior to progression appearing on imaging. 33
In the context of the radically different follow-up modalities that were being proposed, we considered that assessment of quality of life was essential. The EuroQol-5 Dimensions (EQ-5D) is a generic measure that assesses five broad dimensions of health status: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 35 The EQ-5D also provides utility scores for use in cost–utility analyses. The European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 (quality of life) is a disease-specific instrument widely used in European oncology trials that provides assessments of physical function, role function, cognitive function, emotional function, social function, symptoms and a global judgement of quality of life. 36 The Hospital Anxiety and Depression Scale (HADS) is also widely used in oncology trials, providing assessments of two dimensions of psychological well-being, anxiety and depression, with items that avoid physical symptoms that may arise from physical, rather than psychological, health. 37 Acceptability of follow-up regimes would then be assessed by a modified form of a College of Health Questionnaire that has proved sensitive to differences in follow-up regimes in patients with breast cancer. 38 The instrument addresses patients’ perceptions of three aspects of health care: service delivery, consultations and continuity. A small number of items were also selected and piloted from the seven-item questionnaire used by Kjeldsen et al. 23 in their study of follow-up after radical colorectal surgery. These items more directly address issues of the perceived value and distress of follow-up visits for patients after colorectal surgery.
Health-related quality-of-life (HRQoL) instruments (EQ-5D, EORTC QLQ-C30, HADS) were selected because they have been validated for use in oncology trials; their content is appropriate to assess the impact of follow-up regimes and the number of items (49) should impose a relatively modest burden on respondents. Two more condition-specific instruments were published at the time of writing the FACS trial protocol assessing HRQoL for colorectal cancer, but they were not considered to address any important issues ignored by the selected battery of instruments and did not appear to be developed to address problems experienced by patients during the period following surgery. 39,40
Objective
The study objective was to assess the effect on the number of recurrences treated surgically with curative intent of augmenting symptomatic follow-up in primary care with two intensive methods of follow-up: monitoring of a tumour marker in primary care and intensive imaging in hospital.
Chapter 2 Methods
Trial design
This was a factorial 2 × 2 pragmatic randomised controlled trial to assess the outcome of scheduled follow-up following primary curative treatment of colorectal cancer with blood CEA testing and/or CT imaging (plus additional colonoscopy) for 5 years, compared with minimal symptomatic follow-up. Participants were randomised independently (on a 1 : 1 allocation ratio) to 6- to 12-monthly CT imaging or minimum follow-up and to 3- to 6-monthly CEA testing or minimum follow-up. The minimum and CEA testing arms were permitted a single CT scan at 12–18 months.
Participants
The trial was conducted in 39 NHS hospitals in England that had access to high-volume regional services geared towards offering surgical treatment for metastatic recurrence. Participants were recruited between January 2003 and August 2009. All participants had undergone curative treatment for primary colorectal cancer with no residual disease, microscopically clear margins and Dukes’ A to C stage [tumour, node, metastasis (TNM) stage I–III]. They were disease free on colonic imaging with no evidence of metastatic disease (confirmed by CT or MRI liver scan and chest CT scan) and with a post-operative blood CEA level of ≤ 10 µg/l. For patients having adjuvant therapy, CEA level was measured after the completion of chemotherapy and a CT scan was performed.
For a patient to enter the trial, their primary curative treatment had to be complete. Patients were excluded if they had concurrent serious illness, dominantly inherited colon cancer or an inability to give written informed consent or if they were involved in a primary treatment trial with conflicting follow-up requirements. Potential participants who were aged < 50 years or were > 6 months from completion of primary or adjuvant treatment were included only if a case for their inclusion was agreed by the chief surgical investigator.
Interventions
Follow-up was scheduled for 5 years from trial entry. The factorial design, with independent allocation to the CEA testing and CT interventions, meant that patients received one of four types of follow-up:
-
CEA testing follow-up – 3-monthly measurement of blood CEA level for 2 years, and then 6-monthly for 3 years, with a single chest, abdominal and pelvic CT scan at 12–18 months
-
CT follow-up – 6-monthly chest, abdominal and pelvic CT scan for 2 years, and then annually for 3 years
-
CEA testing and CT follow-up – both blood CEA measurement and CT imaging as above
-
minimum follow-up – no scheduled follow-up except a single chest, abdominal and pelvic CT scan at 12–18 months if requested at study entry by the hospital clinician.
The discretional CT scan in the minimum arm was adopted as a protocol amendment in May 2005 to reflect changing clinical opinion among many participating clinicians on the position of equipoise following publication of new national guidance on follow-up. 41
All patients had been investigated by colonoscopy at trial entry (to ensure that there was no residual or metachronous intraluminal disease) and were offered an end-of-trial colonoscopy at 5 years; in the two CT arms, a colonoscopy to check for luminal recurrence was also undertaken at 2 years.
For those undergoing CEA testing follow-up, blood collection kits were sent directly to the patient, who then attended their own general practice for phlebotomy. The blood was sent by post to the biochemistry laboratory at John Radcliffe Hospital; the CEA testing analysis was carried out using a ADVIA Centaur XP analyser (Siemens Healthcare Limited, Camberley, UK). If the blood CEA level was ≥ 7 µg/l above the patient’s baseline level at trial entry, the test was repeated as soon as possible; if the second test was also above this threshold, the general practitioner (GP) was asked to refer the patient urgently to the local hospital.
Outcomes
The original protocol specified overall survival as the primary clinical outcome. However, by 2006 (after 3 years of recruitment) it was clear that we would fall far short of our original recruitment target. The Data Monitoring and Ethics Committee (DMEC) also conducted an analysis of outcomes in the first 500 patients, which showed that the recurrence and survival rates were substantially better than predicted from routine data. They therefore advised the Health Technology Assessment (HTA) programme in 2007 that the trial would have insufficient power to assess mortality within the projected time scale (i.e. when all participants had completed the 5-year follow-up) and that the primary outcome should be changed to surgical treatment of recurrence with curative intent.
Surgical treatment of recurrence with curative intent is, therefore, reported here as the primary clinical outcome. Overall mortality is reported here as the main secondary clinical outcome. Other pre-specified secondary clinical outcomes reported are deaths from colorectal cancer, time to detection of recurrence and post-recurrence survival.
Data on treatment of recurrence and treatment intent were recorded on case report forms (CRFs) by local National Cancer Research Network (NCRN) staff (who had access to the full clinical records).
Information on deaths was collected by flagging each participant at the Office for National Statistics (ONS) central registry; cause of death was abstracted from death certificates.
Sample size
The trial originally set out to recruit 4800 participants in order to detect a 4% improvement in survival from 49% to 53% in any of the more intensive follow-up arms (CEA testing, CT or CEA testing and CT), compared with the minimum follow-up arm. In advising the change in main outcome from mortality to recurrence treated surgically with curative intent, the DMEC estimated that to detect a 6% absolute difference between minimum follow-up and any of the three other more intensive follow-up arms with 80% power (two-sided alpha 0.05) would require 205 patients in each of the four follow-up arms; 590 subjects allocated to each factorial group (CEA measurement and CT imaging) would provide 80% power to detect a 3% absolute difference in the factorial comparison. We therefore planned to stop recruitment when we reached a minimum of 1180 participants.
Randomisation and blinding
Patients were independently allocated at random in a 1 : 1 ratio to receive or not receive each of the factorial interventions (CEA measurement and CT imaging) using the telephone randomisation service provided by the Oxford Clinical Trials Unit. Research staff at the local centres telephoned the unit, giving patient details and answering a checklist of questions to confirm eligibility. A computerised algorithm was applied using the method of minimisation to balance the patient characteristics within each centre. The balancing variables were adjuvant chemotherapy, gender and age group (three strata).
As this was a pragmatic open trial, it was not possible to conceal allocation arm either to the participants or to the clinical team involved in patient management. However, the research staff who abstracted the outcome data from the clinical notes were employed by the local NCRN teams, independent of the investigators.
The initial protocol had specified that the primary analysis would be undertaken when all patients had completed 5 years’ follow-up. As a result of the extended period of recruitment and the change in the main outcome measure, the DMEC subsequently advised that an interim analysis should be undertaken (and randomisation code broken) in September 2012, when all participants had completed a minimum of 3 years’ scheduled follow-up. This interim analysis programme was undertaken first using dummy variables for the allocation arms and the code was not broken until the syntax was agreed. The analysis reported here (based on outcome data collected up to November 2014) applies the same syntax as for the interim analysis but necessarily was not blinded to allocation arm.
Statistical methods for clinical analyses
The primary analysis was undertaken using IBM Statistical Product and Service Solutions (SPSS) Statistics version 20 (IBM Corporation, Armonk, NY, USA). When feasible, crude data are presented with statistical comparison made between randomisation arms based on chi-squared tests for binary or categorical data, the t-test or analysis of variance as appropriate for comparing group means and the Kruskal–Wallis test for comparing medians. Time to recurrence was analysed by the Kaplan–Meier method, to take account of both time-censoring and the difference in the number of recurrences detected in each arm. The plots of time to recurrence are compared by the log-rank Mantel–Cox statistic. Adjusted odds ratios (ORs) for the main outcome were calculated by binary logistic regression, entering all the baseline characteristics, reported in Table 1, into the model. Cox HRs are also reported for comparison of overall mortality. As there was significant interaction between the CEA testing and CT factorial groups (p = 0.013), the main comparison made in presenting the results is between the three intensive arms and the minimum follow-up arm.
Protocol adherence and withdrawal
Adherence to protocol was ascertained through NHS hospital and laboratory records. A secondary per-protocol analysis is reported that excludes the 308 patients who received any unscheduled investigation or had missed more than one scheduled examination. Reasons for withdrawal were sought. All patients who withdrew gave consent for continued follow-up through ONS mortality records. The analysis of other clinical outcomes (including recurrence and recurrence treated surgically with curative intent) is censored on the date of withdrawal.
Protocol amendments
The two significant amendments to the original 2003 protocol during the trial have been described above. The initial protocol did not specify the single CT scan at 12–18 months in the minimum follow-up arm; 66 patients were randomised before this change took effect in May 2005. Surgical treatment with curative intent rather than overall survival was specified as the main outcome in 2007, when it became clear that we could not recruit the number of participants necessary to estimate an effect on overall survival with adequate statistical power.
Chapter 3 Results
Characteristics of participants
The allocation of the 1202 participants recruited between January 2003 and August 2009 to each randomisation arm and subsequent drop-out is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1). The characteristics of participants at trial entry are shown in Table 1. The mean age of participants was 69 years, 61.2% were male, 29.1% had significant comorbidity, 40.5% had received adjuvant chemotherapy and 11.6% had received pre-operative radiotherapy (for rectal cancer) before randomisation. The randomisation method was successful in achieving a good balance between randomisation arms and factorial comparison groups, with no statistically significant or important difference in age, gender, ethnicity, tumour site and stage, adjuvant treatment or smoking habit.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram. FU, follow-up.

| Characteristics | Individual randomisation arm | Factorial comparison group | ||||||
|---|---|---|---|---|---|---|---|---|
| CEA testing only (N = 300) | CT only (N = 299) | CEA testing and CT (N = 302) | Minimum (N = 301) | CEA testing (N = 602) | No CEA testing (N = 600) | CT (N = 601) | No CT (N = 601) | |
| Age (years) | ||||||||
| Mean (SD) | 68.8 (8.3) | 69.0 (8.9) | 69.5 (8.1) | 69.3 (8.5) | 69.2 (8.2) | 69.1 (8.7) | 69.2 (8.5) | 69.1 (8.4) |
| Median (IQR) | 69 (63–75) | 69 (62–76) | 70 (64–76) | 70 (63–75) | 69 (63–75) | 70 (63–76) | 70 (63–76) | 69 (63–75) |
| Male gender, n (%) | 184 (61.3) | 183 (61.2) | 185 (61.3) | 184 (61.1) | 369 (61.3) | 367 (61.2) | 368 (61.2) | 368 (61.2) |
| Concurrent treatment for other illness, n (%) | 90 (30.0) | 81 (27.1) | 86 (28.5) | 93 (30.9) | 176 (29.2) | 174 (29.0) | 167 (27.8) | 183 (30.4) |
| Pre-treated with chemotherapy, n (%) | 121 (40.3) | 118 (39.5) | 125 (41.4) | 123 (40.9) | 246 (40.9) | 241 (40.2) | 243 (40.4) | 244 (40.6) |
| Pre-treated with radiotherapy, n (%) | 32 (10.7) | 34 (11.4) | 38 (12.6) | 35 (11.7) | 70 (11.6) | 69 (11.6) | 72 (12.1) | 67 (11.2) |
| Site of cancer,a n (%) | (N = 293) | (N = 290) | (N = 292) | (N = 295) | (N = 585) | (N = 585) | (N = 582) | (N = 588) |
| Right colon | 93 (31.7) | 96 (33.1) | 90 (30.8) | 103 (34.9) | 183 (31.3) | 199 (34.0) | 186 (32.0) | 196 (33.3) |
| Left colon | 118 (40.3) | 96 (33.1) | 110 (37.7) | 105 (35.6) | 228 (39.0) | 201 (34.4) | 206 (35.4) | 223 (37.9) |
| Rectum | 82 (28.0) | 98 (33.8) | 92 (31.5) | 87 (29.5) | 174 (29.7) | 185 (31.6) | 190 (32.6) | 169 (28.7) |
| Dukes’ stage,b n (%) | (N = 289) | (N = 293) | (N = 287) | (N = 292) | (N = 576) | (N = 585) | (N = 580) | (N = 581) |
| A | 54 (18.7) | 71 (24.2) | 60 (20.9) | 69 (23.6) | 114 (19.8) | 140 (23.9) | 131 (22.6) | 123 (21.2) |
| B | 144 (49.8) | 132 (45.1) | 146 (50.9) | 131 (44.9) | 290 (50.3) | 263 (45.0) | 278 (47.9) | 275 (47.3) |
| C | 91 (31.5) | 90 (30.7) | 81 (28.2) | 92 (31.5) | 172 (29.9) | 182 (31.1) | 171 (29.5) | 183 (31.5) |
| Smoking status,c n (%) | (N = 290) | (N = 288) | (N = 294) | (N = 290) | (N = 584) | (N = 578) | (N = 582) | (N = 580) |
| Current smoker | 20 (6.9) | 16 (5.6) | 18 (6.1) | 14 (4.8) | 38 (6.5) | 30 (5.2) | 34 (5.8) | 34 (5.9) |
| Ex-smoker | 145 (50.0) | 154 (53.5) | 162 (55.1) | 155 (53.4) | 307 (52.6) | 309 (53.5) | 316 (54.3) | 300 (51.7) |
| Never smoked | 125 (43.1) | 118 (41.0) | 114 (38.8) | 121 (41.7) | 239 (40.9) | 239 (41.3) | 232 (39.9) | 246 (42.4) |
Length and completeness of follow-up
The duration of scheduled follow-up to detect recurrence was 5 years and the median time elapsed since trial entry during which we collected mortality data was 8.7 years at the data lock in the autumn of 2014. As mortality data were collected through ONS flagging of death certificates, follow-up is complete except for three participants who have emigrated from the UK. As the CONSORT diagram (see Figure 1) shows, clinical follow-up continued until a trial end point (death, recurrence or end of scheduled follow-up), except in the 145 patients who withdrew during the study. The main reasons for withdrawal were deteriorating health (40.0%), dislike of the allocated mode of follow-up (41.4%) and moving away (13.8%). Although Table 2 shows that there was no significant difference between arms in the number of withdrawals, there were more withdrawals from dislike of the allocated mode of follow-up in the minimum arm (27/301, 9.0%) than in the more intensive arms (32/901, 3.5%), and the median length of follow-up was shorter.
| Description | Individual randomisation arms | Factorial comparison groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA testing only | CT only | CEA testing and CT | Minimum | p-value | CEA testing | No CEA testing | p-value | CT | No CT | p-value | |
| Withdrawn without diagnosis of recurrence, n (%) | 29 (9.7) | 32 (10.7) | 38 (12.6) | 46 (15.3) | 0.16 | 67 (11.1) | 78 (13.0) | 0.32 | 70 (11.6) | 75 (12.5) | 0.66 |
| Median duration of observation (months) | 26.9 | 20.1 | 24.7 | 7.9 | 19.5 | 20.7 | 20.0 | 20.3 | |||
| Died without diagnosis of recurrence, n (%) | 12 (4.0) | 11 (3.7) | 10 (3.3) | 17 (5.6) | 0.96 | 22 (3.7) | 28 (4.7) | 0.80 | 21 (3.5) | 29 (4.8) | 0.93 |
| Diagnosed with recurrence | |||||||||||
| All sites, n (%) | 56 (18.7) | 61 (20.4) | 48 (15.9) | 38 (12.6) | 0.06 | 104 (17.3) | 99 (16.5) | 0.72 | 109 (18.1) | 94 (15.6) | 0.25 |
| Liver, n | 23 | 30 | 19 | 15 | 42 | 45 | 49 | 38 | |||
| Lung, n | 23 | 18 | 17 | 11 | 40 | 29 | 35 | 34 | |||
| Locoregional, n | 18 | 19 | 15 | 14 | 33 | 33 | 34 | 32 | |||
| Other, n | 12 | 8 | 9 | 14 | 21 | 22 | 17 | 26 | |||
| Recurrences detected by a scheduled follow-up examination, n (%) | 35 (11.7) | 52 (16.1) | 40 (13.2) | 9 (3.0) | < 0.001 | 75 (12.5) | 61 (10.2) | 0.21 | 92 (15.3) | 44 (7.3) | < 0.001 |
| Blood CEA level, n | 30 | 0 | 13 | 0 | 43 | 0 | 13 | 30 | |||
| CT imaging, n | 3 | 49 | 26 | 9 | 29 | 58 | 75 | 12 | |||
| Colonoscopy, n | 0 | 3 | 1 | 0 | 1 | 3 | 4 | 0 | |||
Detection of recurrence
During the 5 years of scheduled follow-up, cancer recurrence was detected in 203 (16.9%) participants (see Table 2). Two-thirds of recurrences (134, 66.0%) were detected by a scheduled follow-up investigation: 87 (64.9%) by CT, 43 (32.1%) by CEA measurement and four at colonoscopy (either by colonoscopy or other investigation initiated at the consultation) (2.98%). Fewer recurrences were detected in the minimum arm than in the intensive follow-up arms (12.6% vs. 18.3%; p = 0.02).
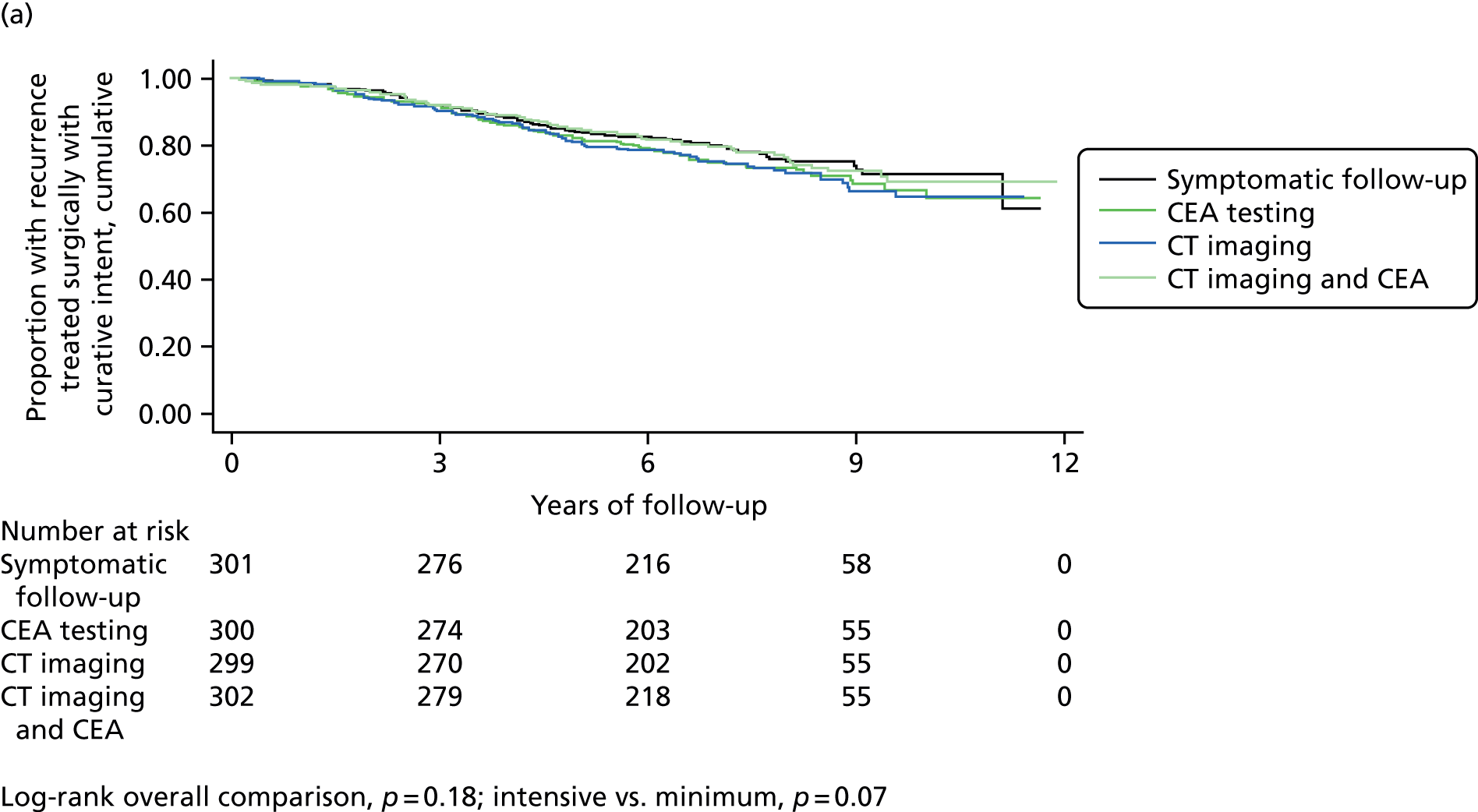
The Kaplan–Meier plots in Figure 2 show the detection of recurrence over time. The difference between the three more intensive arms and the minimum arm in detection of recurrence does not reach statistical significance (log-rank p = 0.07), but the difference in detection of recurrence treatable with curative intent is significant (log-rank p = 0.005), with none detected in the minimum follow-up arm after the end of year 2.
FIGURE 2.
Kaplan–Meier plots of time to diagnosis of (a) any recurrence and (b) any recurrence surgically treatable with curative intent by follow-up group.


Curative treatment and survival
The proportion of participants with recurrence surgically treated with curative intent was 6.3% (76/1202) overall, with little difference according to Dukes’ stage (stage A, 5.1%; stage B, 7.4%; stage C, 5.6%; p = 0.56). Table 3 shows that surgical treatment of recurrence with curative intent was two to three times higher in each of the three more intensive follow-up arms (7.5% overall) than in the minimum follow-up arm (2.7%) (absolute difference 4.8%; p = 0.003). The adjusted OR was 2.5 for CEA measurement only (p = 0.04) and 3.7 for CT only (0.002).
| Description | Individual randomisation arms | Factorial comparison groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA testing only (N = 300) | CT only (N = 299) | CEA testing and CT (N = 302) | Minimum (N = 301) | p-value | CEA testing (N = 602) | No CEA testing (N = 600) | p-value | CT (N = 601) | No CT (N = 601) | p-value | |
| Surgical treatment with curative intent, n (%) | 19 (6.3) | 28 (9.4) | 21 (7.0) | 8 (2.7) | 0.008 | 40 (6.6) | 36 (6.0) | 0.65 | 49 (8.2) | 27 (4.5) | 0.009 |
| Adjusted ORa | 2.40 | 3.69 | 2.78 | 1 | 1.13 | – | 1.87 | – | |||
| 95% CI | 1.02 to 5.65 | 1.63 to 8.38 | 1.19 to 6.49 | 0.70 to 1.82 | 1.14 to 3.07 | ||||||
| Wald’s p-value | 0.041 | 0.002 | 0.020 | 0.63 | 0.014 | ||||||
| Mortality | |||||||||||
| Total deaths, n (%) | 81 (27.0) | 83 (27.8) | 72 (23.8) | 70 (23.3) | 0.49 | 153 (25.4) | 153 (25.5) | 0.97 | 155 (25.8) | 151 (25.1) | 0.79 |
| Deaths attributed to colorectal cancer, n (%) | 48 (16.0) | 45 (15.1) | 38 (12.6) | 38 (12.6) | 0.73 | 86 (14.3) | 83 (13.8) | 0.84 | 83 (13.8) | 86 (14.3) | 0.92 |
| Patients with recurrence still surviving, n (%) | 14 (4.7) | 14 (4.7) | 15 (5.0) | 7 (2.3) | 0.33 | 29 (4.8) | 21 (3.5) | 0.25 | 29 (4.8) | 21 (3.5) | 0.25 |
| Patients with recurrence treated with curative intent still surviving, n (%) | 11 (3.7) | 11 (3.7) | 13 (4.3) | 5 (1.7) | 0.29 | 24 (4.0) | 16 (2.7) | 0.20 | 24 (4.0) | 16 (2.7) | 0.20 |
| Median survival post recurrence (months) | |||||||||||
| All patients with recurrence | 23.7 (n = 56) | 25.5 (n = 61) | 38.0 (n = 48) | 14.6 (n = 38) | 0.16 | 27.7 (n = 104) | 23.1 (n = 99) | 0.44 | 29.2 (n = 109) | 20.7 (n = 94) | 0.08 |
| Treated surgically with curative intent | 51.2 (n = 19) | 43.6 (n = 28) | 58.7 (n = 21) | 76.9 (n = 8) | 0.18 | 56.0 (n = 40) | 51.3 (n = 36) | 0.82 | 52.0 (n = 49) | 59.1 (n = 27) | 1.00 |
| Not treated surgically with curative intent | 19.0 (n = 37) | 13.0 (n = 33) | 22.2 (n = 27) | 10.6 (n = 30) | 0.11 | 19.1 (n = 64) | 12.6 (n = 63) | 0.04 | 15.6 (n = 60) | 12.6 (n = 67) | 0.54 |
Both CT and CEA testing were significantly more effective than minimum follow-up in detecting recurrence treatable with curative intent, but there was no evidence of any additive effect (the adjusted OR for the combined CT + CEA testing arm was not significantly different from that for CT or CEA testing alone). More recurrences were detected in the CT arm than in the CEA testing arm (9.4% vs 6.3%; p = 0.16). Although the difference between CEA testing and CT was not statistically significant, the factorial comparison showed a significant absolute benefit only for CT (absolute difference 3.7%; p = 0.01).
The number of patients with recurrence detected during scheduled follow-up who were still alive at the time of analysis was also higher in the intensive follow-up arms (4.8% vs. 2.3%; p = 0.07), as was the median post-recurrence survival (27.3 vs. 14.6 months; p = 0.11), but neither difference is statistically significant. Of the 76 patients treated surgically with curative intent, around half (40, 52.6%) were still surviving at a median of 4 years and 4 months from time of surgery. Median survival after surgical treatment of recurrence with curative intent was 52.3 months (compared with 14.2 months in those treated without curative intent). The absolute difference in the proportion of patients surviving after curative intent treatment in the intensive arms compared with the minimum arm was 2.2% (p = 0.29); the absolute difference in the factorial comparison was 1.3% for both CEA testing and CT (p = 0.20).
There were no significant differences in the total number of deaths, or in the number of deaths attributed to colorectal cancer (see Appendix 2), between the four randomisation arms or two factorial groups. The Kaplan–Meier overall survival curve by randomisation arm (Figure 3) confirms that there was no significant difference in survival over time between arms (log-rank p = 0.45). The Cox hazard ratio (HR) comparing the minimum and intensive arms and adjusting for the characteristics in Table 1 showed a non-significant advantage to minimum follow-up of 0.87 [95% confidence interval (CI) 0.67 to 1.15]. These CIs imply an upper limit to the absolute mortality benefit from intensive follow-up of 3.8%; the lower limit means that it is impossible to exclude the possibility of harm. However, the outcome of patients treated for recurrence with curative intent is favourable.
FIGURE 3.
Kaplan–Meier plots of overall survival of the cohort by follow-up group for (a) the intention-to-treat population and (b) the per-protocol population.

Adherence to protocol
The extent of adherence to the follow-up protocol is shown in Table 4. Patient adherence to follow-up was good. For imaging, 85% attended for every CT scan and only 3% missed more than one scheduled CT. For CEA testing, 70% of patients attended for every scheduled blood test and only 9% missed more than one test. Although clinician adherence appears less impressive (with 10.5% of participants receiving unscheduled blood CEA level tests, 12.0% receiving unscheduled CT scans and 10.1% receiving unscheduled colonoscopies without documented evidence that these investigations had been triggered by symptomatic presentation), the protocol required investigation of any symptoms presenting between scheduled follow-up tests. Substantially more unscheduled tests were carried out in patients not receiving regular CT scans, with 16.3% compared with 4.7% receiving one or more unscheduled CEA tests (p < 0.001), 18.6% compared with 3.7% receiving one or more unscheduled CT scans (p < 0.001) and 16.0% compared with 4.3% receiving one or more unscheduled colonoscopies (p < 0.001).
| Description | Individual randomisation arms | Factorial comparison groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA testing only (N = 300) | CT only (N = 299) | CEA testing and CT (N = 302) | Minimum (N = 301) | p-value | CEA testing (N = 602) | No CEA testing (N = 600) | p-value | CT (N = 601) | No CT (N = 601) | p-value | |
| Patients receiving less than specified follow-up | |||||||||||
| Missed ≥ 1 scheduled CEA, n (%) | 78 (26.0) | n/a | 99 (32.8) | n/a | n/a | 177 (29.4) | n/a | n/a | 99 (16.5) | 78 (13.0) | 0.09 |
| Missed ≥ 1 scheduled CT scan, n (%) | 3 (1.0)a | 55 (18.4) | 56 (18.5) | 3 (1.0)a | n/a | 59 (9.8) | 58 (9.7) | 0.94 | 111 (18.5) | 6 (1.0)a | n/a |
| Patients receiving more than specified follow-up | |||||||||||
| Received ≥ 1 unscheduled CEA, n (%) | 40 (13.3) | 17 (5.7) | 11 (3.6) | 58 (19.3) | < 0.001 | 51 (8.5) | 75 (12.5) | 0.023 | 28 (4.7) | 98 (16.3) | < 0.001 |
| Received ≥ 1 unscheduled CT scan, n (%) | 56 (18.7) | 14 (4.7) | 8 (2.6) | 56 (18.6) | < 0.001 | 64 (10.6) | 70 (11.7) | 0.57 | 22 (3.7) | 122 (20.3) | < 0.001 |
| Received ≥ 1 unscheduled colonoscopy, n (%) | 43 (14.3) | 13 (4.3) | 13 (4.3) | 53 (17.6) | < 0.001 | 56 (9.3) | 66 (11.0) | 0.33 | 26 (4.3) | 96 (16.0) | < 0.001 |
| Total excluded from per-protocol analysis as had missed > 1 scheduled test or had received any unscheduled test, n (%) | 111 (37.0) | 47 (15.7) | 69 (22.8) | 113 (37.5) | < 0.001 | 180 (29.9) | 160 (26.7) | 0.21 | 116 (19.3) | 224 (37.3) | < 0.001 |
Per-protocol analysis
The results of a per-protocol analysis are shown in Table 5, excluding the 340 (28.3%) patients who missed more than one scheduled investigation or received any unscheduled investigation. The results are consistent with the intention-to-treat (ITT) analysis but effect estimates are higher: the rate of detection of treatable recurrence in the more intensive arms compared with the minimum follow-up was 9.3% versus 2.1% (p = 0.001). As with the ITT analysis, Table 4 and Figure 3 show that there is no difference in overall survival between arms (log-rank p = 0.36). The Cox HR comparing the minimum and intensive arms and adjusting for the characteristics in Table 1 again showed no significant difference in survival (HR 0.97, 95% CI 0.72 to 1.32).
| Description | Individual randomisation arms | Factorial comparison groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEA testing only (N = 189) | CT only (N = 252) | CEA testing and CT (N = 233) | Minimum (N = 188) | p-value | CEA testing (N = 422) | No CEA testing (N = 440) | p-value | CT (N = 485) | No CT (N = 377) | p-value | |
| Surgical treatment with curative intent, n (%) | 16 (8.5) | 27 (10.7) | 20 (8.6) | 4 (2.1) | 0.008 | 36 (8.5) | 31 (7.0) | 0.42 | 47 (9.7) | 20 (5.3) | 0.017 |
| Adjusted ORa | 3.29 | 4.11 | 3.77 | 1 | 1.22 | – | 1.87 | – | |||
| 95% CI | 1.09 to 9.96 | 1.43 to 11.8 | 1.43 to 11.1 | 0.73 to 2.06 | 1.07 to 3.26 | ||||||
| Wald’s p-value | 0.016 | 0.003 | 0.020 | 0.45 | 0.027 | ||||||
| Mortality at median 8.7 years post randomisation | |||||||||||
| Total deaths, n (%) | 63 (33.3) | 80 (31.7) | 63 (27.0) | 52 (27.7) | 0.42 | 126 (29.9) | 132 (30.0) | 0.96 | 143 (29.5) | 115 (30.5) | 0.75 |
| Deaths attributed to colorectal cancer, n (%) | 40 (21.2) | 43 (17.1) | 37 (15.9) | 30 (16.0) | 0.47 | 77 (18.2) | 73 (16.6) | 0.48 | 80 (16.5) | 70 (18.6) | 0.73 |
| Patients with recurrence still surviving, n (%) | 9 (4.8) | 14 (5.6) | 14 (6.0) | 5 (2.7) | 0.41 | 23 (5.5) | 19 (4.3) | 0.44 | 28 (5.8) | 14 (3.7) | 0.16 |
| Median length of survival after recurrence (months) | |||||||||||
| All patients with recurrence | 23.2 (n = 45) | 27.1 (n = 60) | 38.0 (n = 46) | 10.6 (n = 28) | 0.051 | 27.3 (n = 91) | 23.0 (n = 88) | 0.44 | 29.5 (n = 106) | 19.8 (n = 973) | 0.039 |
| Treated surgically with curative intent | 48.7 (n = 16) | 43.8 (n = 27) | 57.8 (n = 20) | 95.3 (n = 4) | 0.082 | 51.7 (n = 36) | 52.0 (n = 31) | 0.91 | 52.0 (n = 47) | 51.7 (n = 20) | 0.85 |
| Not treated surgically with curative intent | 19.0 (n = 29) | 13.0 (n = 33) | 22.2 (n = 26) | 9.4 (n = 24) | 0.035 | 19.1 (n = 55) | 11.3 (n = 57) | 0.02 | 15.6 (n = 59) | 11.0 (n = 53) | 0.326 |
Summary of main findings
The proportion of participants with recurrence treated with curative intent was lower than predicted from earlier trials (6.0% overall) but it was three times higher in the more intensive arms than in the minimum follow-up arm. The proportion of recurrences treatable with curative intent was not related to stage at diagnosis of the primary cancer. Both CEA testing (with a single CT scan) and regular CT are effective modes of follow-up, but conducting CEA testing and CT imaging in parallel provided no additional benefit. There was no statistical difference in overall deaths or colorectal cancer deaths in the minimum compared with the intensive follow-up arms after a median of 8.7 years of observation. If there is a survival benefit from intensive follow-up in the first 10 years, it is very unlikely to exceed 4% in absolute terms and harm cannot be excluded, although this seems unlikely as the survival rate of patients treated for recurrence is high.
Chapter 4 Economic evaluation
Introduction
The FACS trial included a prospective economic analysis, which was designed to be integral to the trial, collecting information on resource use, survival and HRQoL to investigate the cost-effectiveness of each policy. This chapter reports an analysis of the FACS health economic data collected up to 5 years post randomisation for each patient. The details of the study design and interventions have been reported previously in this report.
Methods
Resource use
The planned economic evaluation was a cost–utility analysis conducted from the perspective of the UK NHS. Given the trial’s modified primary end point, cost-effectiveness results were additionally expressed using recurrence treated surgically with curative intent (henceforth referred to as treatable recurrence) as the outcome measure.
The analyses used trial data collected for the duration of the 5-year clinical follow-up period; survival data were also censored at 5 years for all patients. CEA testing was organised centrally by the research team and the number of tests performed was calculated from laboratory test reports received. Numbers of CT scans, colonoscopies, radiography and MRI scans performed were extracted from hospital records by NCRN nurses in each centre and reported on CRFs. Information on confirmed recurrences and treatments (curative or palliative surgery, radiotherapy and chemotherapy) was also obtained from hospital records. Using questionnaires sent at 12, 24, 36, 48 and 60 months post randomisation, patients reported health-care services that they had used over the previous 12 months, including visits to GPs, practice nurses, stoma care nurses, hospital outpatient clinics and visits from district nurses. Patients also reported if they had been admitted to hospital, how many times they had been admitted and their total number of hospital inpatient nights.
Costs
Patient-level resource-use data were costed using national average unit costs (expressed in 2012–13 UK pounds sterling) obtained from a variety of sources. 42–44 The unit costs used are shown in Table 15, Appendix 3. Full details of the costing methods used can also be found in Appendix 3, with abridged information given below. As the time horizon for the analysis was 5 years, costs (and outcomes) were discounted to present values at an annual rate of 3.5%. 45
For each patient, and for each follow-up year, each CEA test, CT scan, colonoscopy, radiography and MRI scan was costed by applying the appropriate unit cost. The associated visits to practice nurses and hospital outpatient clinics for each of these screens/scans were costed by adding the relevant visit cost. Additional practice nurse and outpatient clinic visits, together with visits to GPs and stoma nurses, and home visits from district nurses, were also costed using the appropriate per-visit costs. When costing inpatient hospital admissions for reasons other than surgery for recurrence, an average oncology bed-day cost was used to multiply the number of hospital inpatient days reported. 44
For each patient with recurrence who underwent surgery, the type of surgical procedure performed was recorded and mapped to its corresponding Healthcare Resource Group (HRG) code(s). This code was then located in the NHS Reference Cost database and a weighted average was taken of the cost (and length of stay) across all specialties and subcategories within that code. 44 When a patient’s recorded hospital stay exceeded the average length of stay for their particular procedure, additional days were costed using an excess bed-day cost for an oncology ward. 44
For radiotherapy and/or chemotherapy administered following recurrence, expert clinical opinion provided information on standard regimens (drug doses, frequency and duration) for curative and palliative management; these were then costed (see Table 15, Appendix 3) and assigned to patients accordingly.
Recurrence treated surgically with curative intent
Local NCRN staff extracted data on recurrences and treatment intent (curative vs. non-curative) from hospital clinical records.
Survival
Each patient entered into the study was flagged with the ONS central registry; monitoring is ongoing, but for this analysis it is censored at 5 years post randomisation. Cause of death was abstracted from death certificates.
Health-related quality of life
At baseline and at 12, 24, 36, 48 and 60 months, patients completed the EQ-5D, three-level version (EQ-5D-3L), a questionnaire assessing levels of generic HRQoL and containing questions on five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. 35 Responses to the EQ-5D-3L were converted into a single HRQoL index score on a scale where 0 is representative of dead and 1 of full health with an algorithm developed using data from a sample of the UK general population. 46,47
Quality-adjusted life-years
For each patient in the study, quality-adjusted life-years (QALYs) were generated by adjusting observed survival time in each year for the average level of EQ-5D HRQoL reported during the same year (e.g. 1 year survived with an average level of HRQoL of 0.6 gives 0.6 QALYs). These calculations were repeated for each of the 5 years, discounted and then summed to give the total number of QALYs experienced.
Statistical analysis
Data were missing on 11% of resource-use and EQ-5D data items at 5 years. Data were assumed to be missing at random and multiple imputation (MI) using chained regression equations was used to impute missing values (for additional details see Appendix 3). In line with recommendations, imputation was performed separately for each trial arm and five values were predicted for each missing data point, essentially creating five different data sets. 48 Rubin’s rule, which acknowledges and accounts for variability within as well as between imputed data sets, was used when summarising data across the five data sets. 49,50
Continuous resource use data, costs and QALYs were summarised as means and standard errors (SEs), and categorical data were summarised as numbers and percentages. The three imaging follow-up policies were each compared with minimum follow-up and with each other using mean costs, cases of recurrence treated surgically with curative intent and QALYs. Parametric 95% CIs were estimated around mean differences, and incremental cost-effectiveness ratios (ICERs) were calculated. 51 The ICER is estimated by dividing the difference in cost by the difference in effect between policies and provides an estimate of the additional cost of producing one additional unit of effect (here, an additional treatable recurrence or a QALY) when moving from one policy to another. To interpret an ICER reporting the incremental cost of detecting an additional treatable recurrence, knowledge is required about whether or not such an ‘intermediate’ end point translates into actual patient benefit, such as improved life expectancy, quality of life, or both. ICERs already encompassing ‘final’ end points such as the QALY are more readily interpretable and, with much research already conducted to suggest that society’s maximum willingness to pay for a QALY in England and Wales lies between £20,000 and £30,000, can be compared against this threshold. 45 Policies with ICERs below this threshold are generally considered cost-effective.
Non-parametric bootstrapping was used to explore the uncertainty surrounding the QALY-based cost-effectiveness results and to construct cost-effectiveness acceptability curves, which describe a range of possible cost-effectiveness thresholds. 52
The analyses were performed using Stata® version 12 (StataCorp, College Station, TX, USA).
Sensitivity analysis
To assess the influence of the MI procedure, an alternative complete-case analysis was performed. Uncertainty around the mode of delivery and processing costs of the CEA test was also examined; the baseline test cost of £7.50 was increased (decreased) to £10 (£5).
Results
Table 6 reports the demographic and clinical characteristics for patients in each arm of the trial; the groups were well balanced.
| Characteristics | Minimum follow-up (N = 301) | CEA testing follow-up (N = 300) | CT follow-up (N = 299) | CEA testing and CT follow-up (N = 302) |
|---|---|---|---|---|
| Age (years), mean (SD) | 69.3 (8.5) | 68.8 (8.3) | 69.0 (8.9) | 69.5 (8.1) |
| Gender (male), n (%) | 184 (61.1) | 184 (61.3) | 183 (61.2) | 185 (61.3) |
| Treated comorbidity, n (%) | 93 (30.9) | 90 (30.0) | 81 (27.1) | 86 (28.5) |
| Pre-treated with chemotherapy, n (%) | 123 (40.9) | 121 (40.3) | 118 (39.5) | 125 (41.4) |
| Pre-treated with radiotherapy, n (%) | 35 (11.7) | 32 (10.7) | 34 (11.4) | 38 (12.6) |
| Site of cancer, n (%) | (N = 295) | (N = 293) | (N = 290) | (N = 292) |
| Right colon | 103 (34.9) | 93 (31.7) | 96 (33.1) | 90 (30.8) |
| Left colon | 105 (35.6) | 118 (40.3) | 96 (33.1) | 110 (37.7) |
| Rectum | 87 (29.5) | 82 (28.0) | 98 (33.8) | 92 (31.5) |
| Dukes’ stage, n (%) | (N = 292) | (N = 289) | (N = 293) | (N = 287) |
| A | 69 (23.6) | 54 (18.7) | 71 (24.2) | 60 (20.9) |
| B | 131 (44.9) | 144 (49.8) | 132 (45.1) | 146 (50.9) |
| C | 92 (31.5) | 91 (31.5) | 90 (30.7) | 81 (28.2) |
| Recurrence diagnosed at 5 years, n (%) | 38 (12.6) | 56 (18.7) | 61 (20.8) | 48 (15.9) |
| Treated surgically, n (%) | 14 (36.8) | 25 (44.6) | 30 (49.2) | 22 (45.8) |
| Surgery was with curative intent, n (%) | 8 (21.1) | 19 (33.9) | 28 (45.9) | 21 (43.8) |
| Deaths at 5 years, n (%)a | 48 (16.0) | 53 (17.7) | 57 (19.1) | 46 (15.2) |
| Attributable to colorectal cancer, n (%)a | 28 (58.3) | 35 (66.0) | 36 (63.2) | 28 (60.9) |
Recurrence treated surgically with curative intent
Table 6 also shows the number of patients diagnosed with recurrence in each trial arm after 5 years of clinical follow-up. This figure was 38 (12.6%) in the minimum follow-up arm, 56 (18.7%) in the CEA testing arm, 61 (20.4%) in the CT arm and 48 (15.9%) in the CEA testing and CT arm. The numbers of treatable recurrences were 8 (21.1%), 19 (33.9%), 28 (45.9%) and 21 (43.8%), respectively. When averaged across all patients in each trial arm, the mean numbers of treatable recurrences per patient were 0.027, 0.063, 0.094 and 0.070, respectively.
Resource use and costs
Table 7 summarises the mean resource use by trial arm over the 5-year follow-up period and Table 8 reports the corresponding mean total costs by trial arm. A more detailed version of these tables by year of follow-up is shown in Table 16 in Appendix 3.
| Description | Minimum follow-up (N = 301), mean (SE) | CEA testing follow-up (N = 300), mean (SE) | CT follow-up (N = 299), mean (SE) | CEA testing and CT follow-up (N = 302), mean (SE) |
|---|---|---|---|---|
| CEA tests at 5 years | 0.70 (0.12) | 11.55 (0.26) | 0.12 (0.05) | 11.03 (0.26) |
| CT scans at 5 years | 1.09 (0.06) | 1.01 (0.06) | 5.44 (0.13) | 5.47 (0.13) |
| Colonoscopies at 5 years | 0.57 (0.04) | 0.50 (0.04) | 1.10 (0.04) | 1.15 (0.05) |
| Radiography at 5 years | 0.04 (0.01) | 0.03 (0.01) | 0.35 (0.08) | 0.33 (0.07) |
| MRI scans at 5 years | 0.01 (0.01) | 0.01 (0.01) | 0.03 (0.02) | 0.01 (0.01) |
| ≥ 1 surgeries for recurrence at 5 years, n (%) | 14.00a (4.7) | 25.00 (8.3) | 30.00 (10.0) | 22.00 (7.3) |
| Radiotherapy for recurrence at 5 years, n (%) | 7.00 (2.3) | 4.00 (1.3) | 9.00 (3.0) | 9.00 (3.0) |
| Chemotherapy for recurrence at 5 years, n (%) | 14.00 (4.7) | 32.00 (10.7) | 32.00 (10.7) | 32.00 (10.6) |
| GP visits at 5 years | 2.95 (0.33) | 3.15 (0.31) | 3.39 (0.72) | 3.74 (0.55) |
| Practice nurse visits at 5 years | 2.19 (0.74) | 13.48 (0.67) | 1.95 (0.65) | 12.01 (0.27) |
| District nurse visits at 5 years | 0.75 (0.22) | 3.89 (0.97) | 1.86 (0.39) | 2.71 (0.85) |
| Stoma nurse visits at 5 years | 0.65 (0.15) | 0.39 (0.10) | 0.68 (0.11) | 0.85 (0.21) |
| Outpatient clinic attendances at 5 years | 6.49 (0.62) | 6.17 (0.63) | 11.65 (0.48) | 12.07 (0.58) |
| Other inpatient bed-days at 5 years | 1.98 (0.40) | 2.68 (0.51) | 2.14 (0.46) | 4.52 (1.41) |
| Description | Minimum follow-up (N = 301), mean (SE) | CEA testing follow-up (N = 300), mean (SE) | CT follow-up (N = 299), mean (SE) | CEA testing and CT follow-up (N = 302), mean (SE) |
|---|---|---|---|---|
| CEA tests at 5 years | 5.28 (0.88) | 86.65 (1.94) | 0.88 (0.35) | 82.72 (1.95) |
| CT scans at 5 years | 142.88 (8.06) | 131.59 (7.20) | 711.31 (17.06) | 714.63 (17.31) |
| Colonoscopies at 5 years | 295.42 (21.33) | 258.71 (19.34) | 567.29 (22.96) | 590.59 (24.13) |
| Radiography at 5 years | 1.46 (0.43) | 1.07 (0.37) | 13.91 (3.03) | 13.25 (2.92) |
| MRI scans at 5 years | 1.90 (1.10) | 1.91 (1.10) | 6.39 (4.60) | 2.53 (1.26) |
| Subtotal: tests/scans | 446.95 (25.48) | 479.93 (23.13) | 1299.78 (37.90) | 1403.72 (40.24) |
| ≥ 1 surgeries for recurrence at 5 years, n (%) | 423.96 (114.07) | 760.80 (152.64) | 1042.49 (191.40) | 613.61 (128.45) |
| Radiotherapy for recurrence at 5 years, n (%) | 37.21 (18.39) | 29.97 (18.00) | 37.46 (18.52) | 49.53 (22.10) |
| Chemotherapy for recurrence at 5 years, n (%) | 492.27 (134.59) | 1199.36 (203.61) | 1224.99 (208.08) | 1206.70 (205.39) |
| Subtotal: treatment for recurrence | 953.44 (206.43) | 1990.13 (296.99) | 2304.93 (323.66) | 1869.85 (291.44) |
| GP visits at 5 years | 132.67 (14.84) | 141.72 (14.11) | 152.55 (32.54) | 168.17 (24.97) |
| Practice nurse visits at 5 years | 29.42 (9.89) | 181.09 (8.98) | 26.14 (8.71) | 161.28 (3.66) |
| District nurse visits at 5 years | 29.28 (8.41) | 151.89 (37.91) | 72.60 (15.33) | 105.84 (33.15) |
| Stoma nurse visits at 5 years | 27.91 (6.49) | 16.86 (4.37) | 29.25 (4.78) | 36.74 (9.06) |
| Outpatient clinic attendances at 5 years | 935.19 (88.75) | 888.86 (91.26) | 1677.34 (68.43) | 1737.63 (83.33) |
| Other inpatient bed-days at 5 years | 730.73 (148.76) | 989.31 (190.43) | 793.01 (170.91) | 1670.18 (520.73) |
| Subtotal: health-care contacts | 1885.21 (208.89) | 2369.73 (259.95) | 2750.88 (202.60) | 3879.83 (576.04) |
| Mean total costs at 5 years | 3285.60 (344.99) | 4839.80 (458.05) | 6355.60 (411.54) | 7153.40 (652.70) |
| Of which: year 1 | 1062.80 (189.74) | 1558.17 (227.07) | 1925.07 (226.04) | 2018.09 (252.35) |
| Year 2 | 1038.66 (183.29) | 1363.56 (227.26) | 2024.73 (207.47) | 1876.54 (184.15) |
| Year 3 | 477.66 (81.83) | 758.13 (159.95) | 764.63 (149.44) | 1672.28 (436.85) |
| Year 4 | 336.31 (43.47) | 623.87 (144.29) | 756.35 (156.58) | 698.35 (130.26) |
| Year 5 | 370.16 (34.57) | 536.06 (85.40) | 884.82 (136.60) | 888.14 (98.30) |
| Mean total 5-year discounted costs at 5 years | 3138.15 (333.72) | 4613.19 (438.35) | 6048.37 (393.22) | 6796.10 (617.78) |
The mean total undiscounted 5-year cost per patient in the minimum follow-up arm was lower than in the other three arms. Setting aside the costs of implementing the different intensive follow-up policies, Table 8 shows that much of this cost difference arose because fewer recurrences were diagnosed and treated with minimum follow-up. The CT policy was on average £1516 (95% CI £292 to £2740; p = 0.02) per patient more costly than the CEA testing policy, with much of this additional cost attributable to the CT scans and associated hospital outpatient visits. The combined CEA testing and CT policy was the most costly alternative, being on average £798 (95% CI –£674 to £2270; p = 0.29) per patient more costly than the CT arm. Much of this difference arose because of higher costs of (non-surgery related) hospital readmissions.
The final row in Table 8 shows mean (SE) total discounted 5-year costs of £3138 (£334), £4613 (£438), £6048 (£393) and £6796 (£618) for the minimum, CEA testing, CT and combined CEA testing and CT follow-up arms, respectively.
Survival
At 5 years, 48 (16.0%) patients in the minimum follow-up arm had died, as had 53 (17.7%) in the CEA testing arm, 57 (19.1%) in the CT arm and 46 (15.2%) in the CEA testing and CT arm. The mean (SE) total number of discounted life-years in each of these arms at 5 years was 4.37 (0.05), 4.32 (0.05), 4.33 (0.05) and 4.37 (0.05), respectively.
Health-related quality of life
Table 9 shows the mean (SE) self-reported HRQoL of survivors by arm and year of follow-up. HRQoL appeared lower from year 2 onwards with both minimum and combined CEA testing and CT follow-up. However, only in the year 5 EQ-5D scores was any significant difference observed (and then only between minimum and CEA testing follow-up). Appendix 3, Table 17 shows additional EQ-5D-3L data by domain and indicates that a slightly higher proportion of patients in the minimum follow-up and combined CEA testing and CT arms reported problems with pain and discomfort.
| Description | Minimum follow-up (N = 301) | CEA testing follow-up (N = 300) | CT follow-up (N = 299) | CEA testing and CT follow-up (N = 302) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | n | Mean | SE | n | Mean | SE | |
| Year 1 | 294 | 0.84 | 0.01 | 296 | 0.86 | 0.01 | 295 | 0.85 | 0.01 | 296 | 0.85 | 0.01 |
| Year 2 | 290 | 0.82 | 0.01 | 282 | 0.84 | 0.01 | 282 | 0.85 | 0.01 | 290 | 0.81 | 0.02 |
| Year 3 | 276 | 0.82 | 0.02 | 274 | 0.84 | 0.01 | 270 | 0.84 | 0.01 | 279 | 0.81 | 0.02 |
| Year 4 | 265 | 0.83 | 0.01 | 258 | 0.85 | 0.01 | 260 | 0.82 | 0.02 | 269 | 0.81 | 0.02 |
| Year 5 | 253 | 0.81 | 0.02 | 247 | 0.87a | 0.01 | 242 | 0.85 | 0.02 | 256 | 0.81 | 0.02 |
| Mean total 5-year QALYs, adjusted for survival | 3.85 | 0.07 | 3.91 | 0.07 | 3.86 | 0.07 | 3.83 | 0.08 | ||||
| Mean total 5-year discounted QALYs, adjusted for survival | 3.61 | 0.07 | 3.66 | 0.06 | 3.62 | 0.07 | 3.59 | 0.07 | ||||
Figure 4 shows the mean EQ-5D scores and 95% CIs across all study patients. The mean scores in all trial arms declined over time as the number of deaths increased.
FIGURE 4.
Mean (95% CI) EQ-5D-3L single-index scores for all patients in each trial arm at each year.

Quality-adjusted life-years
The mean (SE) estimated discounted QALYs for the minimum follow-up arm, the CEA testing arm, the CT arm and the combined CEA and CT arm were 3.61 (0.07), 3.66 (0.06), 3.62 (0.07) and 3.59 (0.07), respectively (see Table 9).
Cost-effectiveness
Table 10 shows the cost-effectiveness results based on a treatable recurrence end point. The ICERs are shown in the final row of the table and reveal that the lowest cost of detecting an additional treatable recurrence is achieved when moving from minimum follow-up to CEA testing-only follow-up (£40,131). The cost of detecting an additional treatable recurrence with CT over minimum follow-up, however, was only slightly higher, at £43,392. When compared with CT, follow-up with CEA testing and CT was, on average, more costly but detected fewer treatable recurrences.
| Description | Minimum follow-up (n = 301) | CEA testing follow-up (n = 300) | CT follow-up (n = 299) | CEA testing and CT follow-up (n = 302) | |
|---|---|---|---|---|---|
| Cost per patient, mean (SE) | £3138.15 (£333.72) | £4613.19 (£438.35) | £6048.37 (£393.22) | £6796.10 (£617.78) | |
| Number of recurrences treated surgically with curative intent, per patient, mean (SE) | 0.027 (0.009) | 0.063 (0.014) | 0.094 (0.017) | 0.070 (0.015) | |
| Incremental results | CEA testing vs. minimum | CT vs. minimum | CEA testing vs. CT | CEA testing and CT vs. minimum | CEA testing and CT vs. CT |
| Difference in mean costs (95% CI) | £1475 (£405 to £2545)* | £2910 (£1894 to £3926)** | £1435 (£265 to £2605)*** | £3658 (£2258 to £5058)** | £748 (–£650 to £2145) |
| Difference in mean number of recurrences treated surgically with curative intent, per patient (95% CI) | 0.037 (0.004 to 0.070)*** | 0.067 (0.029 to 0.105)* | 0.030 (–0.013 to 0.073) | 0.043 (0.009 to 0.077)*** | –0.024 (–0.068 to 0.020) |
| Incremental cost per recurrence treated surgically with curative intent detected | £40,131 | £43,392 | £47,347 | £85,151 | –£31,014 (dominated) |
The first row of Table 11 shows the mean total cost and QALY differences and associated ICERs for each of the imaging policies compared with minimum follow-up. A move from minimum follow-up to CT or to combined CEA testing and CT follow-up was, on average, more costly and produced fewer QALYs than a move from minimum follow-up to CEA testing follow-up. This is reflected in the ICERs, with the additional cost per QALY gained by moving from minimum follow-up to CEA (£25,951), lower than the ICER for CT (£246,107). When compared with minimum follow-up, combined CEA testing and CT was more costly and generated fewer QALYs, resulting in a negative ICER (–£208,347) and a dominated policy.
| Description | Minimum follow-up (n = 301) | CEA testing-only follow-up (n = 300) | CT-only follow-up (n = 299) | CEA testing and CT follow-up (n = 302) |
|---|---|---|---|---|
| Base-case analysis | ||||
| Mean total costs (£) | 3138 (334) | 4613 (438) | 6048 (393) | 6796 (618) |
| Mean total QALYs | 3.61 (0.07) | 3.66 (0.06) | 3.62 (0.07) | 3.59 (0.07) |
| CEA test cost £5 | ||||
| Mean total costs (£) | 3136 (334) | 4586 (439) | 6048 (393) | 6770 (618) |
| Mean total QALYs | 3.61 (0.07) | 3.66 (0.06) | 3.62 (0.07) | 3.59 (0.07) |
| CEA test cost £10 | ||||
| Mean total costs (£) | 3140 (334) | 4641 (438) | 6049 (393) | 6822 (618) |
| Mean total QALYs | 3.61 (0.07) | 3.66 (0.06) | 3.62 (0.07) | 3.59 (0.07) |
| Outliers removed arm 4 | ||||
| Mean total costs (£) | 3138 (334) | 4613 (438) | 6048 (393) | 6064 (391) |
| Mean total QALYs | 3.61 (0.07) | 3.66 (0.06) | 3.62 (0.07) | 3.59 (0.07) |
| Complete-case analysis | (n = 129) | (n = 151) | (n = 147) | (n = 146) |
| Mean total costs (£) | 2771 (434) | 3354 (505) | 5413 (489) | 5463 (722) |
| Mean total QALYs | 3.73 (0.10) | 3.78 (0.09) | 3.72 (0.10) | 3.89 (0.09) |
| Incremental results | CEA testing vs. minimum follow-up | CT vs.minimum follow-up | CEA testing and CT vs. minimum follow-up | |
| Base-case analysis | ||||
| Mean cost difference (95% CI) | £1475 (£405 to £2545)* | £2910 (£1894 to £3926)** | £3658 (£2258 to £5058)** | |
| Mean QALY difference (95% CI) | 0.06 (–0.12 to 0.24) | 0.01 (–0.17 to 0.19) | –0.02 (–0.20 to 0.17) | |
| ICER (probability cost-effective at £20,000 per QALY) | £25,951 (41.95%) | £246,107 (3.70%) | –£208,347 (dominated) (0.15%) | |
| CEA test cost £5.00 | ||||
| Mean cost difference (95% CI) | £1449 (£379 to £2520)* | £2912 (£1896 to £3928)** | £3633 (£2233 to £5034)** | |
| Mean QALY difference (95% CI) | 0.06 (–0.12 to 0.24) | 0.01 (–0.17 to 0.19) | –0.02 (–0.20 to 0.17) | |
| ICER (probability cost-effective at £20,000 per QALY) | £25,499 (42.45%) | £246,225 (3.65%) | –£206,952 (dominated) (0.15%) | |
| CEA test cost £10.00 | ||||
| Mean cost difference (95% CI) | £1501 (£431 to £2570)* | £2909 (£1893 to £3925)** | £3682 (£2283 to £5082)** | |
| Mean QALY difference (95% CI) | 0.06 (–0.12 to 0.24) | 0.01 (–0.17 to 0.19) | –0.02 (–0.20 to 0.17) | |
| ICER (probability cost-effective at £20,000 per QALY) | £26,404 (41.60%) | £245,990 (3.75%) | –£209,742 (dominated) (0.15%) | |
| Outliers removed arm 4 | ||||
| Mean cost difference (95% CI) | £1475 (£405 to £2545)* | £2910 (£1894 to £3926)** | £2926 (£1913 to £3938)** | |
| Mean QALY difference (95% CI) | 0.06 (–0.12 to 0.24) | 0.01 (–0.17 to 0.19) | –0.02 (–0.21 to 0.17) | |
| ICER (probability cost-effective at £20,000 per QALY) | £25,591 (41.15%) | £246,107 (3.15%) | –£151,716 (dominated) (1.55%) | |
| Complete-case analysis | ||||
| Mean cost difference (95% CI) | £583 (–£751 to £1918) | £2642 (£1339 to £3945)** | £2692 (£980 to £4404)* | |
| Mean QALY difference (95% CI) | 0.05 (–0.21 to 0.32) | –0.01 (–0.30 to 0.27) | 0.16 (–0.10 to 0.42) | |
| ICER (probability cost-effective at £20,000 per QALY) | £10,730 (35.15%) | –£232,771 (Dominated) (3.05%) | £17,034 (34.60%) | |
Figure 5 plots 2000 bootstrapped estimates of the mean discounted 5-year cost and QALY differences for each imaging policy versus minimum follow-up. Figure 6 plots the associated cost-effectiveness acceptability curves. CEA testing appears to offer the greatest potential for being cost-effective when compared with minimum follow-up; however, uncertainty surrounds these 5-year results. At a threshold of £20,000 per QALY, the probability of CEA testing being cost-effective is 41.95%, increasing to 49.85% at a threshold of £30,000 per QALY.
FIGURE 5.
Incremental cost-effectiveness plane showing 2000 bootstrapped mean total discounted 5-year costs and QALY differences for each intensive follow-up arm vs. minimum follow-up.

FIGURE 6.
Cost-effectiveness acceptability curves showing the probability that each intensive follow-up policy is cost-effective compared with minimum follow-up for different levels of the threshold ICER.

Sensitivity analyses
Table 11 also shows results for the sensitivity analyses. The findings were not sensitive to changes in the cost of the CEA test. However, a complete-case analysis showed combined CEA testing and CT to be more cost-effective than in the base-case analysis. In particular, the mean (SE) discounted 5-year QALYs for combined CEA testing and CT at 3.89 (0.09) were higher than for the other three arms [mean 3.74 (0.06)]. This was because, for patients with complete data, 5-year mortality was lower in the combined CEA testing and CT arm (7.5%) than in the other three arms (mean 16.4%).
Correspondingly, then, the larger reduction in QALYs seen in the combined CEA testing and CT arm following imputation was attributable to higher levels of mortality in patients with incomplete data in that arm (26% compared with just 17% with minimum follow-up, 18% with CT and 22% with CEA testing) and also to lower levels of HRQoL (e.g. in year 2 mean EQ-5D scores for surviving patients with incomplete data in the minimum, CEA testing and CT follow-up arms were 0.78, 083 and 0.83, respectively, but in the combined CEA testing and CT arm the level was 0.76).
Finally, two patients receiving combined CEA testing and CT follow-up reported substantially higher inpatient bed-days (120 days and 200 days) than other patients being admitted to hospital, and this resulted in unexpectedly high inpatient costs. After removing both patients and re-estimating the results, the cost in the combined CEA testing and CT arm fell from £6796 to £6064, but the mean QALYs were also reduced by 0.0017 and the policy remained dominated, with a low probability of being cost-effective (1.55%).
Discussion
The follow-up tests assessed in this trial – CEA testing and CT imaging – have been identified in meta-analyses as the most promising current methods of detecting curatively treatable metastatic recurrence in patients with colorectal cancer. 14,53 However, the economic evidence assembled to date is quite meagre. Secco et al. 54 reported some costs for patients in a randomised trial of risk-adapted FACS, but the analysis was not comprehensive in economic methods or costs included and related to only a subset of patients.
The one detailed published cost-effectiveness analysis of intensive versus conventional follow-up after curative resection for colorectal cancer derived its effectiveness estimates from a meta-analysis of five randomised trials, and reported that the number of life-years gained through intensive surveillance over standard care was between 0.73 and 0.82 per patient depending on the trials included, with a resulting ICER of £3402 per life-year gained. 55 However, there was considerable heterogeneity in the follow-up regimens across the trials included, an absence of patient-level data on resource use and costs and no HRQoL information. This study is therefore the first comprehensive analysis able to draw on patient-level cost and HRQoL data and with a reasonable sample size and 5-year follow-up.
Although this study has clear advantages over previous evaluations, a number of issues make it difficult to draw definitive conclusions on the cost-effectiveness of intensive follow-up. First, there were no clear differences in HRQoL between the trial arms. This is of interest in itself, as it does not support the perception that more intensive follow-up may be associated with increased anxiety and hence lower quality of life. The results from this trial show no evidence that patients in the CEA testing, CT or combined arms were more likely to report problems on the anxiety domain of the EQ-5D (see Table 17, Appendix 3), with some suggestion that, despite the slightly higher number of detected recurrences in the CEA testing and CT arms, quality of life was slightly, albeit non-significantly, higher in these arms than in the minimum follow-up arm (see Table 9) at most follow-up points. However, the absence of significant differences in HRQoL also meant that quality-adjusted survival was primarily influenced by the observed survival differences, which then directly influenced the cost-effectiveness analysis. As detailed above, however, the trial was not powered for survival, and a first analysis of the data when all patients had completed a minimum of 3 years’ scheduled follow-up showed not only no significant differences in the number of deaths between the intensive follow-up arms and the minimum follow-up arm [18.2% (164/901) vs. 15.9% (48/301), respectively; difference 2.3%, 95% CI –2.6% to 7.1%], but an unexpected 2% aggregate survival advantage with minimum follow-up. 56 Although longer-term follow-up (median 8.7 years) has shown that survival in the minimum follow-up arm has now fallen below that of the intensive follow-up arms (albeit not statistically so), the short-term survival advantage associated with minimum follow-up has influenced the cost-effectiveness results presented in this paper. The increase in recurrences treated with curative intent observed in the FACS trial would have been expected to translate into a small survival advantage and, as shown above and in Table 11, the CIs around total mortality and QALYs are still consistent with a survival advantage being possible with intensive follow-up.
In an attempt to provide a somewhat fairer representation of the cost-effectiveness of intensive follow-up, results were also presented using the trial’s primary end point of treatable recurrences detected. When compared with minimum follow-up, CEA testing detected additional treatable recurrences (mean 0.037 recurrences per patient) at the lowest additional cost (mean £1475 per patient), giving an ICER of £40,131. Whether or not this represents good value for money, however, can really be determined only by extrapolating the associated patient benefit. For example, if detection of a recurrence at a stage at which it could be managed surgically with curative intent was known to afford a patient an additional 2.5 QALYs, then the equivalent cost per QALY gained from moving from minimum follow-up to using CEA testing would be around £15,950 [£1475/(0.037 × 2.5)]. Threshold analyses (not shown) suggest that a benefit of at least an additional two discounted QALYs over the course of a patient’s lifetime would be needed for an ICER < £20,000. As noted above, however, there are few data available on life-years gained through early identification of recurrence.
A second difficulty encountered is that an assessment of cost-effectiveness at 5 years is almost certainly premature. Data in Table 6 showed fewer recurrences with minimum follow-up and suggest that, in that arm, there may be a number of undetected recurrences that are still to present. With no new curatively treatable recurrences presenting in that arm beyond 2 years, the majority of these as yet undiagnosed recurrences will probably be incurable. 56 In the longer term, therefore, mortality might be expected to increase in the minimum follow-up arm and a survival advantage from intensive follow-up could manifest with no additional intensive surveillance costs. Although data from this study show that it is unlikely that CT or combined CEA testing and CT will be cost-effective, it is still possible that follow-up with CEA testing could be proven to be cost-effective in the longer term.
This economic analysis has some limitations. For example, 11% of data were missing on resource use and HRQoL. A complete-case analysis suggested combined CEA testing and CT to be substantially more cost-effective than in the base-case analysis; however, patients with complete data in that arm were not representative of all patients in the combined CEA testing and CT arm. By imputing what may be considered a relatively small number of missing data, it was possible to include all patients in the analysis and, thus, to generate more reliable estimates.
A further limitation is that, in the case of patients with recurrence, data were not collected on therapies given beyond those used to treat the initial recurrence. This should be mitigated to some extent, however, by the inclusion of costs for health-care contacts with hospitals (outpatient clinics and inpatient admissions).
Conclusion
This study has generated detailed data on the costs and HRQoL of patients undergoing minimum and intensive follow-up regimes for the detection of recurrence following colorectal cancer. However, drawing definitive conclusions on cost-effectiveness using the FACS trial is impossible because the FACS data lack the necessary statistical power to determine whether or not the benefit in detecting treatable recurrence translates into a survival benefit. Analyses using both treatable recurrences and QALYs as end points suggest CEA testing to be the most promising of the intensive follow-up regimes, but, with the former approach, the longer-term data required to extrapolate from treatable recurrence to a final end point (life-years or QALYs) so as to facilitate an assessment of value for money are difficult to find, while the latter approach is influenced by overall survival in the FACS trial, which the trial was not powered to detect. The longer-term follow-up of patients and the monitoring of new recurrences and survival should help to ascertain whether or not CEA testing represents an efficient use of scarce health-care resources. Meanwhile, it is recommended that an economic evaluation of follow-up in colorectal cancer should follow good practice guidelines in viewing data from the FACS trial as being complementary to and used alongside information from all other relevant data sources. 57
Chapter 5 Observational analysis of participants with recurrence in the Follow-up After Colorectal Surgery study
Introduction
The FACS trial provides an opportunity to evaluate the characteristics of those who develop recurrence in a population of well-staged prospectively followed-up patients treated for colorectal cancer. Knowledge of those patients most likely to relapse is informative with respect to follow-up and planning adjuvant treatment strategies. For example, rectal cancer has a well-established tendency to recur locally; however, the combination of total mesorectal excision and optimal chemoradiotherapy has reduced rates to < 10% in modern series. 58–61 Existing evidence on the pattern of recurrence after curative resection of colorectal cancer is limited to retrospective audits,62–64 and data from high-quality randomised controlled trials are lacking. Trials of adjuvant therapies for colon and rectal cancer reveal certain information on patterns of recurrence, but these are by definition limited to more advanced-stage cancers requiring such treatments. 65
The present lack of evidence has resulted in conflicting guidance with respect to whether or not the same surveillance strategy should be offered to patients irrespective of the site and stage of the primary tumour. Some guidance suggests additional follow-up strategies for rectal cancer,66,67 whereas others recommend much less intensive follow-up for rectal cancer than for colon cancer. 68,69 Furthermore, much uncertainty exists with respect to whether or not early-stage cancers should be followed up at all, owing to the low likelihood of recurrence. Consequently, there is a need to advance understanding of the pattern of recurrence and benefit of follow-up in the era of modern management of both primary colorectal cancer and recurrence.
Methods
This retrospective cohort analysis aggregates data from all four trial arms and is restricted to 1132 patients (94.2%) for whom complete detailed data are available on initial stage and site of recurrence. The cut-off date used for this analysis is 31 August 2012, by which time all patients had reached a minimum of 3 years of follow-up, with a median follow-up of 4.4 years since randomisation.
Categorical variables are presented as frequencies (percentages) and the chi-squared test used for comparisons. The Kaplan–Meier method was utilised for survival analyses and the log-rank test used to compare survival between groups; p < 0.05 was considered significant. Tables were produced using SPSS version 22 and survival analyses were carried out using the ‘survival’ package in R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Incidence of recurrence
A total of 189 participants (17%) developed recurrence during the median follow-up period of 4.4 years (interquartile range 3.1–5.0 years). The mean age of participants with recurrence was 67.8 years, and 63% were male (Table 12). Predictably, recurrence was more frequent in those with a more advanced-stage primary tumour [Dukes’ A primary, 10% (26/249); Dukes’ B, 15% (81/537); Dukes’ C, 24% (82/346); p < 0.0001] (Table 13). The incidence of recurrence also varied according to the site of the original tumour [right-colonic primary, 14% (51/379); left-colonic primary, 16% (68/421); rectal primary, 21% (70/332); p = 0.023] (see Table 13).
| Characteristic | All (N = 189) | Right colon (N = 51) |
Left colon (N = 68) |
Rectum (N = 70) |
|---|---|---|---|---|
| Age (years), mean (SD) | 67.8 (8.9) | 69.3 (9) | 65.8 (9.6) | 68.6 (7.9) |
| Male, n (%) | 119 (63) | 29 (56.9) | 41 (60.3) | 49 (70) |
| Concurrent treatment for other illness, n (%) | 47 (24.9) | 12 (23.5) | 16 (23.5) | 19 (27.1) |
| Chemotherapy prior to trial entry, n (%) | 93 (49.2) | 25 (49) | 32 (47.1) | 36 (51.4) |
| Radiotherapy prior to trial entry, n (%) | 27 (14.3) | 0 | 3 (4.4) | 24 (34.3) |
| Dukes’ stage, n (%) | ||||
| A | 26 (13.8) | 3 (5.9) | 9 (13.2) | 14 (20) |
| B | 81 (42.9) | 24 (47.1) | 29 (42.6) | 28 (40) |
| C | 82 (43.4) | 24 (47.1) | 30 (44.1) | 28 (40) |
| Smoking status, n (%) | ||||
| Never smoked | 74 (41.8) | 21 (45.7) | 27 (41.5) | 26 (39.4) |
| Ex-smoker | 87 (49.2) | 22 (47.8) | 32 (49.2) | 33 (50) |
| Current smoker | 16 (9) | 3 (6.5) | 6 (9.2) | 7 (10.6) |
| Unknown | 12 (6.3) | 5 (9.8) | 3 (4.4) | 4 (5.7) |
| Description | Site of primary tumour | |||
|---|---|---|---|---|
| Total | Right colon | Left colon | Rectum | |
| All patients in the trial, n | 1132 | 379 | 421 | 332 |
| Recurrences, n (%) | 189 | 51 | 68 | 70 |
| Liver only | 50 | 9 (18.0) | 23 (46.0) | 18 (36.0) |
| Lung only | 33 | 3 (9.1) | 8 (24.2) | 22 (66.7) |
| Liver and lung only | 11 | 3 (27.3) | 6 (54.5) | 2 (18.2) |
| Locoregional only | 41 | 14 (34.1) | 14 (34.1) | 13 (31.7) |
| Multisite/other | 54 | 22 (40.7) | 17 (31.5) | 15 (27.8) |
| Description | Stage of cancer (Dukes’) | |||
| Total | A | B | C | |
| All patients in the trial, n | 1132 | 249 | 537 | 346 |
| Recurrences, n (%) | 189 | 26 | 81 | 82 |
| Liver only | 50 | 10 (20.0) | 25 (50.0) | 15 (30.0) |
| Lung only | 33 | 8 (24.2) | 14 (42.4) | 11 (33.3) |
| Liver and lung only | 11 | 1 (9.1) | 4 (36.4) | 6 (54.4) |
| Locoregional only | 41 | 4 (9.8) | 22 (53.7) | 15 (36.6) |
| Multisite/other | 54 | 3 (5.6) | 16 (29.6) | 35 (64.8) |
Site of recurrence
Two-thirds of the recurrences detected (124/189) were at a single site (liver, 50; lung, 33; and locoregional, 41), with the remainder having recurrence at other or multiple sites. Overall, the liver was the most frequent site of recurrence, with 42% (79/189) of all recurrences involving the liver. Interestingly, the distribution of recurrent disease varied according to the stage and location of the primary tumour. Both locoregional recurrence and recurrence at multiple/other sites were most frequently associated with more advanced-stage primary tumours (see Table 13). Recurrence involving just the lung was most frequently associated with rectal primary tumours (right colon, 3/33, 9%; left colon, 8/33, 24%; rectum, 22/33, 67%; p < 0.0001). In addition, recurrence at sites other than the lung, liver or locoregionally or at more than one of those sites varied according to the site of primary tumour (right colon, 25/65, 38%; left colon, 23/65, 35%; rectum, 17/65, 26%; p = 0.018).
Incidence of recurrent disease treatable surgically with curative intent
The primary outcome of the FACS trial was treatment of recurrence surgically with curative intent. When the analysis is restricted to the 189 participants with complete data on site and stage of the primary cancer, a total of 65 (34%) are known to have undergone treatment with curative intent (Table 14). Those participants with recurrence from a lower-stage primary tumour were more likely to be resectable (Dukes’ A, 13/26, 50%; Dukes’ B, 32/81, 40%; Dukes’ C, 20/82, 24%; p = 0.08). Although there was no significant difference in the likelihood of recurrent disease being amenable to curative resection according to the site of original tumour, a trend was apparent (right colon, 12/51, 24%; left colon, 23/68, 34%; rectum, 30/70, 43%; p = 0.086).
| Description | Site of primary tumour | ||||
|---|---|---|---|---|---|
| Total | Right colon | Left colon | Rectum | p-valuea | |
| Recurrences, n | 189 | 51 | 68 | 70 | |
| Treatable with curative intent, n (%) | 65 | 12 (23.5) | 23 (33.8) | 30 (42.9) | 0.086 |
| Description | Stage of cancer (Dukes’) | ||||
| Total | A | B | C | p-valuea | |
| Recurrences, n | 189 | 26 | 81 | 82 | |
| Treatable with curative Intent, n (%) | 65 | 13 (50) | 32 (39.5) | 20 (24.4) | 0.03 |
Benefit of follow-up
The proportion of participants with recurrence surgically treated with curative intent taken as a proportion of the whole trial cohort was similar for each Dukes’ stage (A, 13/249, 5%; B, 32/537, 6%; C, 20/346, 6%; p = 0.80). As such, a key finding of the FACS trial was that the benefit of follow-up is independent of stage; that is, although recurrence is less frequent in those with Dukes’ A primary tumours, it is more likely to be treatable. 56 By contrast, the benefit of follow-up did vary according to the site of the primary tumour. Those participants with a rectal primary tumour were more likely to have a treatable recurrence detected during the follow-up period (right colon, 12/379, 3%; left colon, 23/421, 6%; rectum, 30/332, 9%; p = 0.003).
Survival post recurrence
Of the 189 diagnosed with recurrent disease, 113 (60%, 106 specifically from recurrence and seven from other causes) died during the follow-up period. Survival post recurrence differed according to both the site (log-rank p = 0.01) and stage (log-rank p = 0.005) of the primary tumour (Figure 7a and b).
FIGURE 7.
Kaplan–Meier curves for post-recurrence survival for all participants with disease recurrence. (a) Dukes’ stage; (b) site of primary cancer; and (c) site of recurrence. Reprinted with permission, copyright © 2015 Wolters Kluwer Health. From Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg 2016;263:1143–7. 70


Survival post recurrence also differed according to the site of recurrent disease. Those with multisite recurrence or metastatic recurrence at other sites had an inferior survival to those with single-site recurrence in the liver, lung or locoregionally (see Figure 7c; log-rank p < 0.0001), consistent with the high proportion of patients with these single-site recurrences undergoing surgical treatment with curative intent (liver only, 30/50, 60%; lung only, 13/33, 40%; locoregionally only, 16/41, 40%; multisite/other recurrence, 6/65, 9%).
Of those amenable to treatment with curative intent, around three-quarters (44/65, 67.7%) were still alive at the end of the follow-up period. Neither the site nor the stage of the primary tumour influenced the survival of those with recurrent disease treated with curative intent, although there was a trend towards worse survival in those with a higher-stage primary tumour (Figure 8).
FIGURE 8.
Kaplan–Meier curves for post-recurrence survival for just those participants who underwent surgical treatment of recurrence with curative intent. (a) Stage of primary tumour; and (b) site of primary tumour. Reprinted with permission, copyright © 2015 Wolters Kluwer Health. From Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg 2016;263:1143–7. 70

Discussion
This observational analysis reports on patterns of recurrence and post-recurrence survival in the prospectively followed-up large cohort of patients curatively treated for Dukes’ A to C colorectal cancer in the FACS trial. The rigour of investigative procedures undertaken to ensure that patients were free of disease prior to recruitment affords an accurately staged population in which to assess the true incidence of disease recurrence.
The pattern of recurrence observed in the FACS cohort is similar to that reported by other studies, with the liver being the most common site of disease recurrence. The results also demonstrate a preponderance of isolated lung recurrence from rectal tumours, supporting the contention that haematogenous spread occurs from the rectum to the lungs via the iliac veins. The relatively low incidence of locoregional recurrence from rectal primary tumours is consistent with modern management combining total mesorectal excision with selective use of chemoradiotherapy. Indeed, locoregional recurrence was no more frequent in rectal tumours than in colonic tumours. This is at odds with certain guidance that recommends the use of regular proctosigmoidoscopy in the follow-up of rectal cancer patients. 67
This analysis demonstrates clear differences between right-colonic, left-colonic and rectal primary tumours. Right-colonic tumours resulted in fewer isolated recurrences in the liver, in the lung or locally than recurrences from left-colonic or rectal tumours. This is in agreement with data demonstrating more peritoneal and distant lymph node metastases in BRAF mutated and microsatellite unstable tumours,71 two features most commonly associated with right-sided cancers. The less favourable pattern of relapse from right-colonic tumours plausibly extends to an inferior survival post relapse for these patients in the FACS cohort. Indeed, in a recent consensus subtyping of colorectal cancer, the group with the highest proportion of right-colonic tumours had the worse survival after relapse, whereas those with the lowest proportion had the most favourable survival. 72
Recurrence was, predictably, more frequent in those who were followed up with a higher-stage primary tumour. However, as already noted,56 the benefit of follow-up was independent of the stage of the primary tumour. That is, although participants with a higher-stage primary tumour were more likely to have a recurrence detected during the follow-up period, it was less likely to be treatable. Indeed, it may be that a higher-stage tumour at original diagnosis reflects not simply a later stage in the developmental pathway of colorectal cancer, but rather a more aggressive tumour type, the prognostic implications of which remain if recurrence occurs. These results support the use of equivalent follow-up strategies in patients with early-stage cancers, as they derive equal benefit. This is a key finding, given that some guidance currently states that there is a lack of evidence for follow-up after resection of stage I/Dukes’ A colorectal cancer. 66
In contrast to the observation for Dukes’ stage, the benefit of follow-up was dependent on the site of the primary tumour. Patients with rectal tumours were three times as likely as those with right-colonic tumour to have a treatable recurrence detected during the follow-up period. This supports the practice of undertaking intensive follow-up for patients with rectal tumours similar to that for those with colonic tumours, which is contrary to certain guidance. 68,69
In conclusion, the FACS cohort demonstrates that characteristics of the primary colorectal tumour, specifically site and stage, influence not only the likelihood of recurrence, but the distribution of recurrent disease and post-recurrence survival. The influence of stage on outcome, even post recurrence, suggests that the stages of primary colorectal cancer represent different disease biology rather than simply points on the timeline of disease progression.
The post-recurrence survival of participants with recurrence is shown according to stage of primary tumour (see Figure 7a), site of primary tumour (see Figure 7b) and site of recurrent disease (see Figure 7c). All include the number of patients at risk and p-value calculated using the log-rank method.
The post-recurrence survival of participants with recurrence treated surgically with curative intent is shown according to stage of primary tumour (see Figure 8a) and site of primary tumour (see Figure 8b). All include the number of patients at risk and the p-value calculated using the log-rank method.
Chapter 6 Discussions and conclusions
Principal findings
This trial investigated two different strategies in the follow-up of patients having treatment for primary colorectal cancer with curative intent: hospital-based imaging and CEA measurement in primary care in a 2 × 2 trial design. The two follow-up strategies were chosen because meta-analyses suggest that CEA testing and CT imaging are the only practical and available modalities with significant potential to detect curatively treatable recurrence. Endoscopy is well established in follow-up and its use is evidence based73 in the detection of metachronous polyps and rarely luminal recurrence. However, it is commonly only performed 5 years after the initial resection, assuming that the colon is free of polyps at the time of treatment of primary disease. The hospital-based imaging arms therefore had an additional 2-year endoscopic examination scheduled in order to determine whether or not any additional recurrence may be detected.
Other follow-up modalities were considered and rejected. Liver ultrasound lacks sensitivity and specificity, as does clinical examination, and although MRI imaging is very sensitive in the detection of liver metastases, it has limited utility elsewhere. 14,53 CT–positron emission tomography (PET) was not widely available when this trial was designed, is complex and expensive, and would be used only if it were shown to be superior to the results obtained from modern CT scanners. No such evidence exists even from the perspective of 2016 and, thus, the modalities chosen in the FACS trial remain the only practical and useful tests.
Our results show that the proportion of patients treated with curative intent was around 6%, with little difference according to stage. Intensive follow-up by either scheduled CEA testing, imaging or both increased the likelihood of detecting such recurrences by around two to three times. The absolute difference in the proportion of participants treated with curative intent was 3.6–6.7% in the ITT analysis and 6.4–8.6% in the per-protocol analysis, indicating that between 12 and 20 patients need to be followed up to identify one potentially curable recurrence. Over 50% of the patients treated surgically with curative intent were still alive at a median of 8.7 years from trial entry and 4.4 years post recurrence, somewhat higher than would be expected from prior reports. 2,3
Although the proportion of recurrences treated with curative intent (and the success of such treatment) is high compared with earlier reports, the absolute number of treatable recurrences detected is lower. 14 This is not explicable by differences in stage-specific case mix (detection of recurrences treatable with curative intent was similar irrespective of stage) and neither is there any evidence that FACS trial participants were at low risk of recurrence within stage (84.5% of stage C participants had received adjuvant chemotherapy). Stage-specific overall survival of FACS trial participants is comparable with that reported in trials of adjuvant chemotherapy such as MOSAIC. 74
The probable explanation for the lower detection of treatable recurrence is the rigour of the investigative procedures undertaken to ensure that no residual cancer was present at trial entry. It suggests that the high rate of early recurrence reported from routine cancer statistics in England and Scandinavia75 reflects residual disease that would have been detected with fastidious imaging. Before entry to the trial, all patients had to have a CT scan of the chest, abdomen and pelvis and also a CEA test that was within normal limits. Although this would now be the standard of care for most patients with colorectal cancer, it certainly was not at the time that the trial was set up. It is, therefore, inevitable that many patients who were classed by superficial staging as having Dukes’ stage A to C colorectal cancer would actually have been in stage D and that these ‘occult’ metastases declared themselves relatively early after the patients were managed for the primary cancer. Fastidious staging to detect metastatic disease will inevitably reduce the number of patients with recurrent disease detected by follow-up, hence reducing its cost-effectiveness. It is one of the most important findings of the study that full staging of patients with primary colorectal cancer is essential if they are to be managed optimally.
The comparison between intervention arms suggests that monitoring with CEA testing combined with a single CT scan at 12–18 months is equally as effective as undertaking regular CT scanning. As CEA testing can be done in primary care, it is likely to be more cost-effective than regular CT imaging, and this is confirmed by the health economic analysis discussed in summary below. However, imaging is still necessary to confirm recurrence, and, in the combined CEA testing and CT arm, two-thirds of recurrences were first detected by CT. Indeed, in the factorial analysis, CT significantly outperformed CEA testing in the detection of recurrence. The diagnostic performance of CEA as a monitoring test depends on the frequency of testing and the algorithm used to interpret the result. The algorithm applied in the FACS trial (refer for imaging if blood CEA level is ≥ 7 µg/l above baseline) achieves good specificity, but at the cost of modest sensitivity. 76 We are currently now investigating whether or not a higher sensitivity can be achieved at an acceptable level of specificity by applying a diagnostic algorithm that takes account of change over time and that has been applied successfully in interpreting CA125 levels when screening for ovarian cancer. 77
The decision on whether or not the absolute benefit of follow-up is sufficient to justify its opportunity cost will differ between health economies. The benefits of follow-up appear to be independent of diagnostic stage because although there are fewer recurrences with earlier-stage tumours, they are more likely to be curable, and so it makes no sense to apply stage-specific follow-up strategies. 66 Because of the meticulous investigation carried out before trial entry to exclude residual disease, our results also provide useful new data on the timing of recurrence, which can strengthen the evidence base for choosing the optimal frequency of testing. Duplication of monitoring tests does not appear to add value; the participants in our CEA testing arm had a single CT scan at 12–18 months, when three recurrences were detected, but otherwise there was no suggestion of benefit from monitoring with both CEA testing and CT.
Despite the size of the trial, it provides very limited precision in estimating survival. The observed 2–3% apparent survival advantage in the minimum and maximal (CT plus CEA testing) follow-up arm is certainly correct and it is based on ONS data. However, our sample size means that the survival estimates for each arm are also imprecise. Even if 50% of the additional treatable recurrences detected in the more intensive intervention arms were cured, the overall survival advantage would be very small (and the 95% CIs would include the results we have actually observed). Even the possibility of harm cannot be excluded, although, bearing in mind the excellent survival in the patients treated for single-site metastatic disease, this seems unlikely. Furthermore, owing to the efficacy of modern treatments for advanced colorectal cancer, differences in overall survival at 5 years may not be evident in any case.
Health economic and quality-of-life analysis
The analysis was principally based on the trial end point, which is surgical treatment of recurrent disease with curative intent. The incremental cost per patient, compared with the minimum arm, ranged from £40,131 with CEA testing through £43,392 with hospital-based imaging to £85,151 with CEA testing and CT combined. As expected, CEA testing (combined with a single CT scan) is the most economical option, and imaging costs more per patient by around 50%. The cost of the most intensive schedule, however, is around four times that of CEA testing and, as there is no current clear advantage to using both modalities, it is obvious that, in economic terms, CEA testing or imaging appears preferable. The trial was not powered to detect a difference in overall survival. No difference was observed and by inference it could not be more than 3%. Mean quality of life was also found to differ little between the arms of the study, but, as expected, it did deteriorate over 5 years. Mean reported quality of life appeared to be significantly better in the CEA testing arm only in year 5. The consequence of these results was that there was little difference between arms in quality-adjusted survival. Combining cost and QALY differences, the incremental cost per QALY gained for CEA testing compared with minimum follow-up was £25,951, for CT compared with minimum follow-up was £246,107, and for the combined CEA testing and CT arm was more expensive and less effective, resulting in a negative cost per QALY gained of –£208,347. A high degree of uncertainty accompanied these results, with our analysis indicating a 42% probability that the cost per QALY gained for CEA testing versus minimum follow-up was below a £20,000 threshold.
Observational analysis
The analysis of just those patients with recurrence at a median follow-up of 4.4 years demonstrates that the characteristics of the primary tumour influence not only the likelihood of recurrence but also the distribution of recurrent disease and survival post recurrence. These data suggest that disease biology present at the time of diagnosis of primary disease remains relevant even after the diagnosis of recurrence.
Interestingly, whereas a key finding of the FACS trial is that all stages of primary tumour benefit equally from follow-up, this was not the case for site of primary tumour. Patients with rectal cancers were approximately three times as likely to have a treatable recurrence identified during follow-up as those with right-colonic tumours. This directly supports undertaking equivalent follow-up in these patients, contrary to certain guidance. 68 Overall, this analysis demonstrates favourable outcomes for patients treated surgically with curative intent for recurrence, supporting the use of this as a surrogate end point for the trial.
Strengths and weaknesses of the trial
The FACS trial is the largest follow-up trial in colorectal cancer that has been performed to date that utilises modern diagnostic facilities and applies modern surgical and other management to those with detected recurrence. Importantly, the trial was undertaken in a large number of hospital settings in England, including small district general hospitals as well as large teaching centres. This aspect has ensured that the results are generalisable throughout the NHS. Although many of the centres involved in the study did not locally undertake surgery for recurrent disease, such as liver resection, it was mandated that all of the patients managed in the trial had access through the multidisciplinary teams to surgical services that would include liver and lung resection as well specialist surgery to deal with local recurrence when this was not available locally. This type of service is now routine in the UK, although when the study began it was not universal.
As well as examining the treatment modalities involved in follow-up, this trial contained economic and quality-of-life evaluations in order to be able to determine the cost-effectiveness of follow-up. The trial also enabled us to obtain tissue from the resected primary tumours that allow biomarker studies to be undertaken. The combination of the clinical trial data with the availability of tissue for biomarkers is a major advantage and one that will allow further research. In half of the study cohort (those in whom CEA measurement was performed), serum was retained. This allows for the investigation of additional markers without the need to repeat the study.
The major weakness in the study is that, although it is the largest well-designed follow-up study performed to date, it is underpowered to look for differences in mortality between the treatment arms. The original study design did envisage an overall survival end point. This, however, was based on the relapse rates available at the time and commonly reported within colorectal cancer patients in the UK. This, for instance, envisaged a relapse rate of approximately 70% at 5 years for patients in Dukes’ stage C and approximately 50% relapse overall. 78 However, it was clear from the run-in phase of the study that the relapse rate was not nearly as high as had been predicted from national data. Indeed, the overall survival in the study was approximately 80% at 5 years and approximately 70% at 10 years (although the numbers reaching 10 years are very small). As detailed above, these data are entirely compatible with studies on the adjuvant treatment of resected colorectal cancer74 and almost certainly result from the fastidious pre-trial entry staging.
Owing to the much lower recurrence rate observed during the run-in phase of the study, the primary end point was changed to the treatment of recurrence surgically with curative intent. This was felt to be a clinically meaningful end point that would still allow the benefit of follow-up to be assessed. It is accepted that this end point is subject to ascertainment bias in so much as the outcome is not independent of the method of follow-up. It is, however, generally acknowledged that the majority of recurrences will present within 5 years79–81 and, therefore, by completion of 5 years of follow-up this should not be a major issue.
The study also suffers from a relatively high level of withdrawals and protocol deviations. With respect to withdrawals, this is perhaps to be expected in a study that lasts for many years in patients with cancer. It is noteworthy that there were more withdrawals from some arms than from others. The highest number of withdrawals was from the minimum follow-up arm, and in some cases this may represent a level of concern over the lack of continuing follow-up. The most intensive of the arms also suffered from more withdrawals, and in some cases this was documented to be because of the relatively onerous nature of the follow-up schedule, requiring both regular blood tests and CT imaging. Reassuringly, however, there is little difference in the outcome measures in the patients who were managed and analysed by the ITT method as opposed to the per-protocol analysis. All of the patients in the study were flagged with the ONS, and hence the death data, which include all patients in the study whether or not they withdrew, are known to be accurate.
The study had a pragmatic design, so most aspects of patient management, including the pathological examination of the primary tumour and the subsequent radiology, were done by local protocol. This has the advantage of ensuring that the results from the trial are applicable across the NHS, with the obvious unknown variations in practice. However, in respect of the pathology it may cause understaging (e.g. whether a patient is in Dukes’ stage A or C depends on a fastidious examination for lymph nodes in the mesentery of the resected specimen). If this is not undertaken diligently, a patient who is diagnosed as being in Dukes’ stage A may actually be at stage C; however, as we have demonstrated that benefit of follow-up is independent of disease stage, this appears to have made little difference to the results. There was also no central review of CT scans or, indeed, any set protocol at all for conducting the examination of these. It is commonly observed that different protocols and different scanners will have different sensitivity for picking up metastatic disease. Again, this is likely to introduce a degree of variability into the results. In addition, the multidisciplinary team process that patients go through once recurrent disease has been detected varies considerably. 82
Findings in context
Although other studies have been performed assessing the utility of follow-up in this setting,7–10,14,53,54,83,84 they are generally of historic significance only and, therefore, meta-analysis with the FACS trial is inappropriate. The authors of these studies did not have available modern imaging techniques and, in addition, procedures such as resection of colorectal liver metastases were usually not available. We know from this present study that examinations that bring to light recurrent or metachronous luminal cancer (five patients in total) can make only a very small contribution to the long-term outcome.
The Schoemaker study10 compared clinical follow-up with clinical follow-up plus CT and colonoscopy. The conclusion was that, although liver metastasis was detected at an earlier stage in the intensive arm, this did not change greatly the number of liver metastases resections performed. However, as the study consisted of only 325 patients, it was underpowered to detect any difference between the two arms.
The MRC-funded UK CEA trial12 recruited a large number of patients (n = 1447). It was conducted between 1982 and 1993, when patients with recurrent cancer were not managed as they are now (i.e. with detailed imaging to determine the site of recurrence and subsequent appropriate management). In the trial, patients who developed a CEA rise were randomised to either aggressive treatment that included a second-look laparotomy or a conservative approach. It was presumed that the second-look surgery would be able to treat some patients with local recurrence, but, with hindsight, this was a flawed strategy. It would not now be accepted that second-look surgery, on the basis of a rise in CEA level, is an appropriate or adequate treatment.
Three other studies that commenced at or around the time of the FACS trial have, so far, not reported in full. The Italian GILDA study85 examined the addition of ultrasound to CEA-based follow-up of colorectal cancer patients, although, confusingly, rectal cancer patients were also permitted a CT scan. As ultrasound is an insensitive method of examining for recurrence, and as all patients had regular CEA measurement, it is unsurprising that the experimental arm in this study did not demonstrate benefit. 85 A French study registered on ClinicalTrials.gov (NCT00199654) examines the use of PET compared with conventional imaging and CEA testing. The end point is time to diagnosis of recurrence, and the study authors planned to recruit 376 patients. However, this study started in 2005 and has yet to report. The technology of PET has changed radically since 2005 (now only CT–PET is performed); it is expensive, and with the end point chosen the investigators will be unable to determine the effectiveness or cost-effectiveness of the intervention in improving the outcome of patients with relapsed colorectal cancer. The mainly Scandinavian COLOFOL study initiated in 2005 (NCT00225641) examines the use of CT/MRI and CEA testing either twice (12 and 36 months) or more frequently (6-monthly for 2 years and then at 36 months). 86 This is a potentially large trial, with a recruitment target of 2500, so the results may be relevant to NHS practice and some meta-analysis with the FACS trial may be possible.
Since 2005, significant changes in the way in which advanced colorectal cancer is managed have increased the importance of detecting early metastatic disease. Chemotherapy, often with the addition of biological agents, has increased the survival of patients with advanced colorectal cancer up to a median of 24 months without surgical treatment. 87 Perhaps a bigger advantage of chemotherapy is that it is possible to bring patients who are found to have inoperable colorectal liver metastasis to operability; this is known to be associated with long-term survival in many cases. 88 Other treatments, including radioembolisation, are now available to treat colorectal liver metastasis that were not available when this study began. 89 Last, it has been shown that a combination of liver resection and ablation of unresectable disease, using radiofrequency or other techniques, can result in increased survival as demonstrated in a randomised trial. 90 However, all of these later treatments, which increase the length of survival even in the absence of cure, do make it more difficult to use the overall survival end point in follow-up studies. Indeed, although this study originally set out to examine outcomes at 5 years, it is clear that any survival analysis will have to be extended to 10 years or possibly beyond.
Implications for health care
This study has significant implications for the way in which patients with primary colorectal cancer are managed. It is evident that fully staging patients with primary colorectal cancer is paramount. In the case of patients presenting with synchronous metastatic disease, the management plans are likely to be radically different and may, for instance, include neoadjuvant chemotherapy prior to any surgical intervention. In relation to the methods used in this trial, pre-operative CT imaging and post-operative CEA measurement may seem to be appropriate in routine practice. Although detecting metastatic disease at presentation using CT imaging before trial entry was the most common reason for exclusion from recruitment, it was also noted that some patients were discovered not to have had full imaging of the colon prior to their colorectal resection. Endoscopic imaging mandated by trial entry picked up a number of previously unknown second primary tumours that required further surgery.
UK guidance on follow-up has suggested that patients should have at least one CT scan at 12–18 months as a matter of routine, and this was adopted in the minimum arm of the FACS trial. 41 It is the case, however, that many centres perform more CT scans than the minimum recommended in the guidelines. In this trial, we have been able to show that, with the end point of surgical treatment of recurrence with curative intent, CEA testing in combination with a single scan at 18 months performs equally well in this respect. We have also been able to show that doubling up (i.e. conducting regular CT scans as well as CEA blood tests) does not detect significantly more treatable recurrences.
National Institute for Health and Care Excellence guidance91 currently suggests that two CT scans should be performed in appropriate patients in the first 3 years in addition to regular CEA measurement. These guidelines were produced prior to the first publication from this trial. We are currently conducting a secondary analysis of the diagnostic performance of CEA testing in the trial, which will help to inform future guidance on the optimal testing interval, the threshold for initiating further investigation and the need for adjuvant CT scans.
These results should impact on how patients in the UK are managed; they also have implications overseas. We have been able to provide compelling evidence about which modalities are best at detecting treatable recurrence, thereby allowing health-care professionals to make evidence-based judgements on what is best in their particular setting. Our health economic modelling suggests that a single CT scan combined with regular CEA measurement is the most cost-effective approach.
Recommendations for research
In terms of the future, we are now in the era of genomics and biomarkers. A great deal of work has been carried out on the whole-genome sequencing of colorectal cancer, and different subtypes have been identified that have a different prognosis both in the short term and when the cancer recurs. 72 This study suffers somewhat from the context of the clinical trials from which the material for analysis was derived, and further useful insights may come from the UK GEL 100K initiative (www.genomicsengland.co.uk) and other sequencing programmes. In addition, the immune response to colorectal cancer has emerged as a key prognostic indicator. Specifically, the infiltration of T cells within the primary tumour strongly correlates with outcome. Thus, patients with tumours demonstrating a high T-cell infiltrate have a far better prognosis than those with very little, and certain data suggest that this is superior to traditional Dukes’ or TNM staging. 92,93
It seems likely in the future that follow-up of patients with colorectal cancer will be based on a combination of genetic analysis, biomarkers and traditional follow-up methodologies, as demonstrated by the FACS trial. It may be that it will be possible to identify a group of patients in whom the risk of relapse is so low that follow-up is not required. By contrast, it is also likely that patients will be identified who have a very high chance of relapse, and in whom a targeted trial of follow-up might be undertaken.
One of the major advantages of the FACS trial is the translational studies that it will permit. The trial provides a large cohort of well-staged patients with almost complete follow-up data and complete mortality data. Tissue from the primary resection has been collected, and we will go on to acquire tissue from the resection of metastases. The first translational study, funded by the HTA programme, will determine whether or not the immune infiltrate of the primary tumours can identify a cohort of patients who do not benefit from follow-up. This is of practical importance, as the techniques required to assess the immune infiltrate of tumours can be carried out in any routine histopathological laboratory in the NHS.
Conclusion
Among patients who had undergone curative surgery for primary colorectal cancer, follow-up with intensive imaging or CEA screening both provided an improved rate of recurrence treated with curative intent compared with minimal follow-up. The trial is underpowered to determine the effect of follow-up on either cancer-specific or overall survival, and no trend in favour of any strategy is evident from the perspective of 5 years of follow-up. It may be that a trend will be detected when follow-up for mortality is continued to 10 years, as we propose to do. Any survival advantage is, however, likely to be small, taking into account the whole cohort, the large majority of whom did not relapse.
Acknowledgements
The authors would like to acknowledge the key roles played by the following people and institutions.
Funding
The authors acknowledge funding from the UK National Institute for Health Research (NIHR) HTA Board and the NIHR Cancer Research Network.
Sponsor
This trial predated sponsorship.
Data Monitoring and Ethics Committee members
Professor Jack Hardcastle, Professor Michael Campbell and Professor David Whynes.
Current Trial Steering Committee members 2011–present
Dr Stephen Falk, Professor Andrew Protheroe, Professor Sally Stenning, Professor Roger James and Dr Mark Jeffery.
Original Trial Steering Committee members
Professor Roger James, Dr Mark Jeffery, Professor David Kerr and Maxine Stead.
Consumer representative
Lynn Faulds Wood.
Follow-up After Colorectal Surgery trial investigators
Birmingham Heartlands Hospital, Birmingham (Mr Gamal Barsoum); Castle Hill Hospital, Hull (Mr John Hartley); Charing Cross Hospital, London (Mr Peter Dawson); Cumberland Infirmary, Carlisle (Dr Jonathan Nicoll); Darent Valley Hospital, Dartford (Mr Mike Parker); Derriford Hospital, Plymouth (Mr Mark Coleman); Grantham and District Hospital, Grantham (Mr Dilip Mathur); Harrogate District Hospital, Harrogate (Mr Jon Harrison); Hillingdon Hospital, Uxbridge (Mr Yasser Mohsen); Hinchingbrooke Hospital, Huntingdon (Dr Litee Tan); King’s Mill Hospital, Sutton-in-Ashfield (Mr Mukul Dube); Leeds St James, Leeds (Mr Simon Ambrose); Leeds General Infirmary, Leeds (Mr Paul Finan); Leighton General Hospital, Crewe (Mr Arif Khan); Maidstone Hospital, Maidstone (Dr Mark Hill); Mayday Hospital, Croydon (Croydon University Hospital) (Mr Muti Abulafi); Newham University Hospital, London (Mr Roger Le Fur); Oxford Radcliffe Hospitals, Oxford (Professor Neil Mortensen); Queen Alexandra Hospital, Portsmouth (Mr Daniel O’Leary); Queen Elizabeth Hospital, Birmingham (Dr Neil Steven); Queen’s Hospital, Burton upon Trent (Mr Stelios Vakis); Queen’s Medical Centre, Nottingham (Professor John Scholefield); Royal Cornwall Hospital, Truro (Mr Ponnandai Arumugam); Royal Derby Hospital, Derby (Mr Jonathan Lund); Royal Shrewsbury Hospital, Shrewsbury (Mr Trevor Hunt); Russells Hall Hospital, Dudley (Professor David Ferry); Scarborough Hospital, Scarborough (Dr Ian Renwick); Southampton General Hospital, Southampton (Mr Paul Nichols); St Mark’s Hospital, Harrow (Professor John Northover and Dr Arun Gupta); St Peter’s Hospital, Chertsey (Mr Philip Bearn); St Richard’s Hospital, Chichester (Mr Neil Cripps); Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust (Dr Mary Tighe); Torbay Hospital, Torquay (Mr Rupert Pullan); Manor Hospital, Walsall (Mr Jonathan Stewart); Warrington Hospital, Warrington (Mr Barry Taylor); West Middlesex Hospital, Isleworth (Mr Subramanian Ramesh); Wexham Park Hospital, Slough (Dr Harpreet Wasan); Worcester Royal Hospital, Worcester (Mr Stephen Lake); and Wycombe General Hospital, High Wycombe (Dr Andrew Weaver).
Contributions of authors
David Mant (Professor of General Practice) was the co-chief investigator of the FACS trial.
Alastair Gray (Professor of Health Economics) was the FACS trial health economist.
Siân Pugh (MRC Clinical Research Training Fellow) was responsible for the data analysis and observational study.
Helen Campbell (Senior Researcher, Health Economics) was the FACS trial health economist.
Stephen George (Emeritus Fellow) was the FACS trial public health medicine specialist and methodologist.
Alice Fuller (FACS Data Manager) was responsible for trial data management.
Bethany Shinkins (Lecturer in Decision Anaytic Modelling) was responsible for statistical analyses.
Andrea Corkhill [FACS Trial Manager, Southampton Clinical Trials Unit (SCTU)] was responsible for site monitoring and set-up visits.
Jane Mellor (FACS Trial Co-ordinator, SCTU) was responsible for site monitoring and set-up visits.
Elizabeth Dixon (FACS Trial Co-ordinator and Trial Manager, SCTU) was responsible for site monitoring and set-up visits.
Louisa Little (FACS Trial Manager, SCTU) was responsible for trial management.
Rafael Perera-Salazar (Director of Medical Statistics) was the FACS trial statistician.
John Primrose (Professor of Surgery) was the co-chief investigator of the FACS trial.
Publications
Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomised clinical trial. JAMA 2014;311:263–70.
Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg 2016;263:1143–7.
Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James D, Mallett S, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev 2015;12:CD011134.
Shinkins B, Nicholson BD, James TJ, Primrose JN, Mant D. Carcinoembryonic antigen monitoring to detect recurrence of colorectal cancer: how should we interpret the test results? Clin Chem 2014;60:1572–4.
Data sharing statement
Data included in this report can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Statistics Report: Cancer Incidence and Mortality in the UK. Oxford: Cancer Research UK; 2014.
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283-301. http://dx.doi.org/10.2147/CLEP.S34285.
- Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. http://dx.doi.org/10.1245/s10434-012-2726-3.
- Colibaseanu DT, Mathis KL, Abdelsattar ZM, Larson DW, Haddock MG, Dozois EJ. Is curative resection and long-term survival possible for locally re-recurrent colorectal cancer in the pelvis?. Dis Colon Rectum 2013;56:14-9. http://dx.doi.org/10.1097/DCR.0b013e3182741929.
- Nordic Gastrointestinal Tumor Adjuvant Therapy Group . Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: a randomized trial. J Clin Oncol 1992;10:904-11. https://dx.doi.org/10.1200/jco.1992.10.6.904.
- Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509-20. https://doi.org/10.1097/00000658-199610000-00009.
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg 1997;84:666-9. https://doi.org/10.1002/bjs.1800840523.
- Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg 1995;130:1062-7. https://doi.org/10.1001/archsurg.1995.01430100040009.
- Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum 1995;38:619-26. https://doi.org/10.1007/BF02054122.
- Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology 1998;114:7-14. https://doi.org/10.1016/S0016-5085(98)70626-2.
- Northover JSW. A randomised trial of CEA prompted second look surgery in recurrent colorectal cancer. A preliminary report. Dis Col Rectum 1984;27.
- Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004385.
- Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg 1994;219:174-82. https://doi.org/10.1097/00000658-199402000-00009.
- Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.CD002200.pub2.
- Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. http://dx.doi.org/10.1038/sj.bjc.6603033.
- Treasure T, Utley M, Hunt I. When professional opinion is not enough. BMJ 2007;334:831-2. https://doi.org/10.1136/bmj.39161.403218.AD.
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995;19:59-71. https://doi.org/10.1007/BF00316981.
- Giacchetti S, Itzhaki M, Gruia G, Adam R, Zidani R, Kunstlinger F, et al. Long-term survival of patients with unresectable colorectal cancer liver metastases following infusional chemotherapy with 5-fluorouracil, leucovorin, oxaliplatin and surgery. Ann Oncol 1999;10:663-9. https://doi.org/10.1023/A:1008347829017.
- Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. http://dx.doi.org/10.1016/S0140-6736(08)60455-9.
- Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993;328:901-6. http://dx.doi.org/10.1056/NEJM199304013281301.
- Khoury DA, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB. Colon surveillance after colorectal cancer surgery. Dis Colon Rectum 1996;39:252-6. https://doi.org/10.1007/BF02049461.
- Safi F, Link KH, Beger HG. Is follow-up of colorectal cancer patients worthwhile?. Dis Colon Rectum 1993;36:636-43. https://doi.org/10.1007/BF02238589.
- Kjeldsen BJ, Thorsen H, Whalley D, Kronborg O. Influence of follow-up on health-related quality of life after radical surgery for colorectal cancer. Scand J Gastroenterol 1999;34:509-15. https://doi.org/10.1080/003655299750026254.
- Grunfeld E, Gray A, Mant D, Yudkin P, Adewuyi-Dalton R, Coyle D, et al. Follow-up of breast cancer in primary care vs specialist care: results of an economic evaluation. Br J Cancer 1999;79:1227-33. http://dx.doi.org/10.1038/sj.bjc.6690197.
- Smith JA, King PM, Lane RH, Thompson MR. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 2003;90:583-92. http://dx.doi.org/10.1002/bjs.4085.
- Neugut AI, Lautenbach E, Abi-Rached B, Forde KA. Incidence of adenomas after curative resection for colorectal cancer. Am J Gastroenterol 1996;91:2096-8.
- Ward J, Naik KS, Guthrie JA, Wilson D, Robinson PJ. Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative-free response receiver operating characteristic analysis. Radiology 1999;210:459-66. http://dx.doi.org/10.1148/radiology.210.2.r99fe05459.
- Carter R, Hemingway D, Cooke TG, Pickard R, Poon FW, McKillop JA, et al. A prospective study of six methods for detection of hepatic colorectal metastases. Ann R Coll Surg Engl 1996;78:27-30.
- Department of Health . Ionising Radiation (Medical Exposure) Regulations 2000 (IRMER) 2012. www.gov.uk/government/publications/the-ionising-radiation-medical-exposure-regulations-2000 (accessed 3 May 2017).
- Wall BF. Radiation protection dosimetry for diagnostic radiology patients. Radiat Prot Dosimetry 2004;109:409-19. http://dx.doi.org/10.1093/rpd/nch317.
- International Commission on Radiological Protection . Radiological Protection in Biomedical Research 1992.
- Macdonald JS. Carcinoembryonic antigen screening: pros and cons. Semin Oncol 1999;26:556-60.
- Ward U, Primrose JN, Finan PJ, Perren TJ, Selby P, Purves DA, et al. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer 1993;67:1132-5. https://doi.org/10.1038/bjc.1993.208.
- Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev 2015;12. http://dx.doi.org/10.1002/14651858.CD011134.pub2.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi-Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract 1999;49:705-10.
- Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238-47. https://doi.org/10.1016/S0959-8049(98)00357-8.
- Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 1999;8:181-95. https://doi.org/10.1023/A:1008821826499.
- ACPGBI Guidelines for the Management of Colorectal Cancer . 2007 CC Management Guidelines. 2007. www.acpgbi.org.uk/resources/guidelines/guidelines-for-the-management-of-colorectal-cancer (accessed 14 November 2016).
- British National Formulary No. 66. London: BMJ Group and RPS Publishing; 2013.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal and Social Services Research Unit, University of Kent; 2013.
- NHS National Schedule of Reference Costs 2012–13. London: Department of Health; 2013.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey (Discussion Paper No. 138. York: Centre for Health Economics, University of York; 1995.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Little R, Rubin D. Statistical Analysis with Missing Data. John Wiley and Sons: New York, NY; 1987.
- Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. http://dx.doi.org/10.1002/hec.903.
- Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7341.813.
- Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol 2002;28:418-23. https://doi.org/10.1053/ejso.2001.1250.
- Renehan AG, O’Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 2004;328. http://dx.doi.org/10.1136/bmj.328.7431.81.
- Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263-70. http://dx.doi.org/10.1001/jama.2013.285718.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72. http://dx.doi.org/10.1016/j.jval.2015.02.001.
- Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. http://dx.doi.org/10.1097/01.sla.0000257358.56863.ce.
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. http://dx.doi.org/10.1056/NEJMoa040694.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. https://doi.org/10.1056/NEJMoa060829.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. http://dx.doi.org/10.1056/NEJMoa010580.
- Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997;40:15-24. https://doi.org/10.1007/BF02055676.
- Ding P, Liska D, Tang P, Shia J, Saltz L, Goodman K, et al. Pulmonary recurrence predominates after combined modality therapy for rectal cancer: an original retrospective study. Ann Surg 2012;256:111-16. http://dx.doi.org/10.1097/SLA.0b013e31825b3a2b.
- Roth ES, Fetzer DT, Barron BJ, Joseph UA, Gayed IW, Wan DQ. Does colon cancer ever metastasize to bone first? a temporal analysis of colorectal cancer progression. BMC Cancer 2009;9. http://dx.doi.org/10.1186/1471-2407-9-274.
- Kornmann M, Staib L, Wiegel T, Kron M, Henne-Bruns D, Link KH, et al. Long-term results of 2 adjuvant trials reveal differences in chemosensitivity and the pattern of metastases between colon cancer and rectal cancer. Clin Colorectal Cancer 2013;12:54-61. http://dx.doi.org/10.1016/j.clcc.2012.07.005.
- Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013;31:4465-70. http://dx.doi.org/10.1200/JCO.2013.50.7442.
- Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, et al. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum 2015;58:713-25. http://dx.doi.org/10.1097/DCR.0000000000000410.
- Glimelius B, Tiret E, Cervantes A, Arnold D. ESMO Guidelines Working Group . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:81-8. http://dx.doi.org/10.1093/annonc/mdt240.
- Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:64-72. http://dx.doi.org/10.1093/annonc/mdt354.
- Pugh SA, Shinkins B, Fuller A, Mellor J, Mant D, Primrose JN. Site and stage of colorectal cancer influence the likelihood and distribution of disease recurrence and postrecurrence survival: data from the FACS randomized controlled trial. Ann Surg 2016;263:1143-7. https://doi.org/10.1097/SLA.0000000000001351.
- Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011;117:4623-32. http://dx.doi.org/10.1002/cncr.26086.
- Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. http://dx.doi.org/10.1038/nm.3967.
- Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006;56:160-7. https://doi.org/10.3322/canjclin.56.3.160.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. http://dx.doi.org/10.1200/JCO.2008.20.6771.
- Morris EJ, Sandin F, Lambert PC, Bray F, Klint A, Linklater K, et al. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut 2011;60:1087-93. http://dx.doi.org/10.1136/gut.2010.229575.
- Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol 2009;18:15-24. http://dx.doi.org/10.1016/j.suronc.2008.05.008.
- Drescher CW, Shah C, Thorpe J, O’Briant K, Anderson GL, Berg CD, et al. Longitudinal screening algorithm that incorporates change over time in CA125 levels identifies ovarian cancer earlier than a single-threshold rule. J Clin Oncol 2013;31:387-92. http://dx.doi.org/10.1200/JCO.2012.43.6691.
- Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-18. http://dx.doi.org/10.1002/bjs.7032.
- Cho YB, Chun HK, Yun HR, Lee WS, Yun SH, Lee WY. Clinical and pathologic evaluation of patients with recurrence of colorectal cancer five or more years after curative resection. Dis Colon Rectum 2007;50:1204-10. http://dx.doi.org/10.1007/s10350-007-0247-0.
- Broadbridge VT, Karapetis CS, Beeke C, Woodman RJ, Padbury R, Maddern G, et al. Do metastatic colorectal cancer patients who present with late relapse after curative surgery have a better survival?. Br J Cancer 2013;109:1338-43. http://dx.doi.org/10.1038/bjc.2013.388.
- Bouvier AM, Launoy G, Bouvier V, Rollot F, Manfredi S, Faivre J, et al. Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer 2015;137:2133-8. http://dx.doi.org/10.1002/ijc.29578.
- Jones RP, Vauthey JN, Adam R, Rees M, Berry D, Jackson R, et al. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg 2012;99:1263-9. http://dx.doi.org/10.1002/bjs.8835.
- Rodríguez-Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006;24:386-93. https://doi.org/10.1200/JCO.2005.02.0826.
- Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum 1998;41:1127-33. https://doi.org/10.1007/BF02239434.
- Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol 2016;27:247-80. http://dx.doi.org/10.1093/annonc/mdv541.
- Hansdotter Andersson P, Wille-Jørgensen P, Horváth-Puhó E, Petersen SH, Martling A, Sørensen HT, et al. The COLOFOL trial: study design and comparison of the study population with the source cancer population. Clin Epidemiol 2016;8:15-21. https://doi.org/10.2147/CLEP.S92661.
- Venook AP, Niedzwiecki D, Lenz H-J, Innocenti F, Mahoney MR, O’Neil BH, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.18_suppl.lba3.
- Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol 2014;26:702-8. http://dx.doi.org/10.1093/annonc/mdu580.
- van Hazel GA, Heinemann V, Sharma NK, Findlay MPN, Ricke J, Peeters M, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016;34:1723-31. https://doi.org/10.1200/JCO.2015.66.1181.
- Ruers T, Punt CJA, von Coevorden F, Pierie J-P, Rinkes IB, Ledermann JA, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): long-term survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). J Clin Oncol 2015;26:iv114-15. https://doi.org/10.1093/annonc/mdv235.17.
- Colorectal Cancer: Diagnosis and Management. Clinical Guideline. London: National Institute for Health and Care Excellence; 2011.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. http://dx.doi.org/10.1126/science.1129139.
- Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med 2012;10. http://dx.doi.org/10.1186/1479-5876-10-1.
- Department of Health . Electronic Market Information Tool 2011. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 12 April 2014).
- NHS Digital . HRG4 + 2012/13/Reference/Costs/Grouper 2013. http://content.digital.nhs.uk/article/4698/HRG4-201213-Reference-Costs-Grouper-and-Documentation (accessed 28 October 2016).
Appendix 1 Pre-trial modelling
Introduction
A pre-trial economic model was devised to assess the probable costs, effects and cost-effectiveness of each of the trial options and to identify areas in which it was likely to be particularly important to collect information during the trial.
Methods
The model took recurrence rates over the 5 years from the primary episode, stage-specific survival and initial stage distribution rates from the Wessex Colorectal Cancer Audit (based on 4466 audited cases). As the trial protocol proposed to recruit patients in Dukes’ stages A to C only, patients whose initial tumour was classified as stage D were excluded from the calculations. The overall survival rate of patients with initial colorectal cancer in Dukes’ stages A to C in the Wessex data was around 50%. The cumulative recurrence rate at 5 years was around 28%. The model assumed that, in routine care, 6% of patients with recurrences would be successfully treated and survive for 5 years.
The model included initial estimates of the frequency and cost of investigative procedures (CEA tests, ultrasound and CT scans, GP consultations) in normal care and by following the proposed trial protocol. Unit costs were taken from national sources. To calculate investigative costs, the predicted number of CEA tests, GP consultations, ultrasound and CT scans according to the protocol were adjusted by expected survival, taking into account cancer- and non-cancer-related deaths. To estimate treatment costs, the model assumed that all patients who were successfully treated and approximately 5% of patients who were not successfully treated would undergo resection. It was also assumed that all patients with a recurrence who were not successfully treated would have a period of palliative care.
The model estimated the effect of improving the colorectal cancer death rate and thus increasing the proportion of patients surviving recurrence by means of each of the three active intervention groups of the proposed study: CEA testing in primary care, hospital-based follow-up and combined follow-up. In the combined follow-up group, the improvement was set to give a survival gain of 8% over no follow-up, in line with the trial hypothesis. CEA testing in primary care was set to give a survival gain of approximately 4%, as was hospital-based follow-up. To obtain a 4% gain in overall survival, it was necessary for the CEA testing or hospital-based follow-up interventions to increase the proportion of patients surviving recurrence from 6% to 20%. To obtain an 8% gain in overall survival, it was necessary for the combined interventions to increase the proportion of patients surviving recurrence from 6% to 39%.
Cost-effectiveness was calculated as the incremental cost per life-year gained of each option compared with normal practice. Different discount rates were examined for costs and effects, ranging from 6% to 1.5%. The following table gives all main model parameter values and results. Figure 9 shows how the cost per life-year gained changes in each arm as the percentage improvement in colorectal cancer death rate alters. Figure 10 shows the model’s baseline estimate of cost-effectiveness of the three colorectal cancer follow-up strategies relative to no follow-up.
FIGURE 9.
Cost per life-year gained as percentage improvement in colorectal cancer death rate alters.

FIGURE 10.
Cost-effectiveness of three colorectal cancer follow-up strategies relative to no follow-up.

Model parameter values and results
| Parameter values | Protocol | Survival adjusted | ||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | 5 years | |
| Number of CEA tests | ||||||
| Group 1 (normal care) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Group 2 (CEA testing in primary care) | 4.00 | 4.00 | 2.00 | 2.00 | 2.00 | 10.40 |
| Group 3 (hospital follow-up) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Group 4 (CEA testing and hospital care) | 4.00 | 4.00 | 2.00 | 2.00 | 2.00 | 10.83 |
| Number of GP consultations related to recurrence | ||||||
| Group 1 (normal care) (assumed to be four times the recurrence rate) | 0.33 | 0.33 | 0.33 | 0.07 | 0.07 | 0.81 |
| Group 2 (CEA testing in primary care) | 4.00 | 4.00 | 2.00 | 2.00 | 2.00 | 10.40 |
| Group 3 (hospital follow-up) | 0.33 | 0.33 | 0.33 | 0.07 | 0.07 | 0.85 |
| Group 4 (CEA testing and hospital care) | 4.33 | 4.33 | 2.33 | 2.07 | 2.07 | 11.71 |
| Number of ultrasound and radiography scans | ||||||
| Group 1 (normal care; assumed that referrals get CT) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Group 2 (CEA testing in primary care) | 0.08 | 0.08 | 0.08 | 0.02 | 0.02 | 0.21 |
| Group 3 (hospital follow-up) | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 5.20 |
| Group 4 (CEA testingand hospital care) | 2.08 | 2.08 | 1.08 | 1.02 | 1.02 | 5.64 |
| Number of CT scans | ||||||
| Group 1 (normal care; assumed that 50% of consultations are referred) | 0.00 | 0.00 | 0.00 | 0.20 | 0.20 | 0.23 |
| Group 2 (CEA testing in primary care; assumed to be twice the actual recurrence rate) | 0.16 | 0.16 | 0.16 | 0.03 | 0.03 | 0.42 |
| Group 3 (hospital follow-up) | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 5.20 |
| Group 4 (CEA testing and hospital care) | 2.16 | 2.16 | 1.16 | 1.03 | 1.03 | 5.86 |
| Number of colonoscopies | ||||||
| Group 1 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.393 |
| Group 2 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.452 |
| Group 3 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.452 |
| Group 4 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.511 |
| Recurrence rate from Dukes’ stage A, B and C tumours (Wessex registry) | ||||||
| All | 0.10 | 0.19 | 0.25 | 0.26 | 0.28 | |
| Successful treatment rates | ||||||
| Assumed Improvement in colorectal cancer death rate | Proportion of recurrences surviving | Proportion of recurrences not surviving | Proportion of recurrences operated on | |||
| Group 1 (normal care; data from Wessex Colorectal Cancer Audit) | 0 | 0.06 | 0.94 | 0.11 | ||
| Group 2 (CEA testing in primary care) | 0.20 | 0.26 | 0.74 | 0.30 | ||
| Group 3 (hospital follow-up) | 0.20 | 0.26 | 0.74 | 0.30 | ||
| Group 4 (CEA testing and hospital care) | 0.39 | 0.45 | 0.55 | 0.48 | ||
| Survival probability based on above | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | (Gain) |
| Group 1 (normal care; Wessex registry) | 0.856 | 0.744 | 0.657 | 0.585 | 0.54 | |
| Group 2 (CEA testing in primary care) | 0.876 | 0.775 | 0.695 | 0.625 | 0.58 | 3.96% |
| Group 3 (hospital follow-up) | 0.876 | 0.775 | 0.695 | 0.625 | 0.58 | 3.96% |
| Group 4 (CEA testing and hospital care) | 0.895 | 0.806 | 0.732 | 0.666 | 0.62 | 7.92% |
| Unit costs (£) | ||||||
| Cost of radiography | 71 | |||||
| Cost of ultrasound scan | 35 | |||||
| Cost of CT scan | 185 | |||||
| Cost of colonoscopy | 175 | |||||
| Cost of outpatient appointment | 63 | |||||
| Cost of resection | 6000 | |||||
| Cost of CEA testing (£6.50 + £1.50 postage/packing) | 8 | |||||
| Cost of palliative care | 10,000 | |||||
| Cost of GP consultation | 14 | |||||
| Five-year results: cost (£) per life-year gained compared with normal practice | ||||||
| Group 2 (CEA testing in primary care) | 209 | |||||
| Group 3 (hospital follow-up) | 9911 | |||||
| Group 4 (CEA testing in primary care and hospital care) | 5647 | |||||
Appendix 2 Colorectal cancer-specific survival
FIGURE 11.
Cumulative survival by intervention arm: colorectal cancer deaths only.
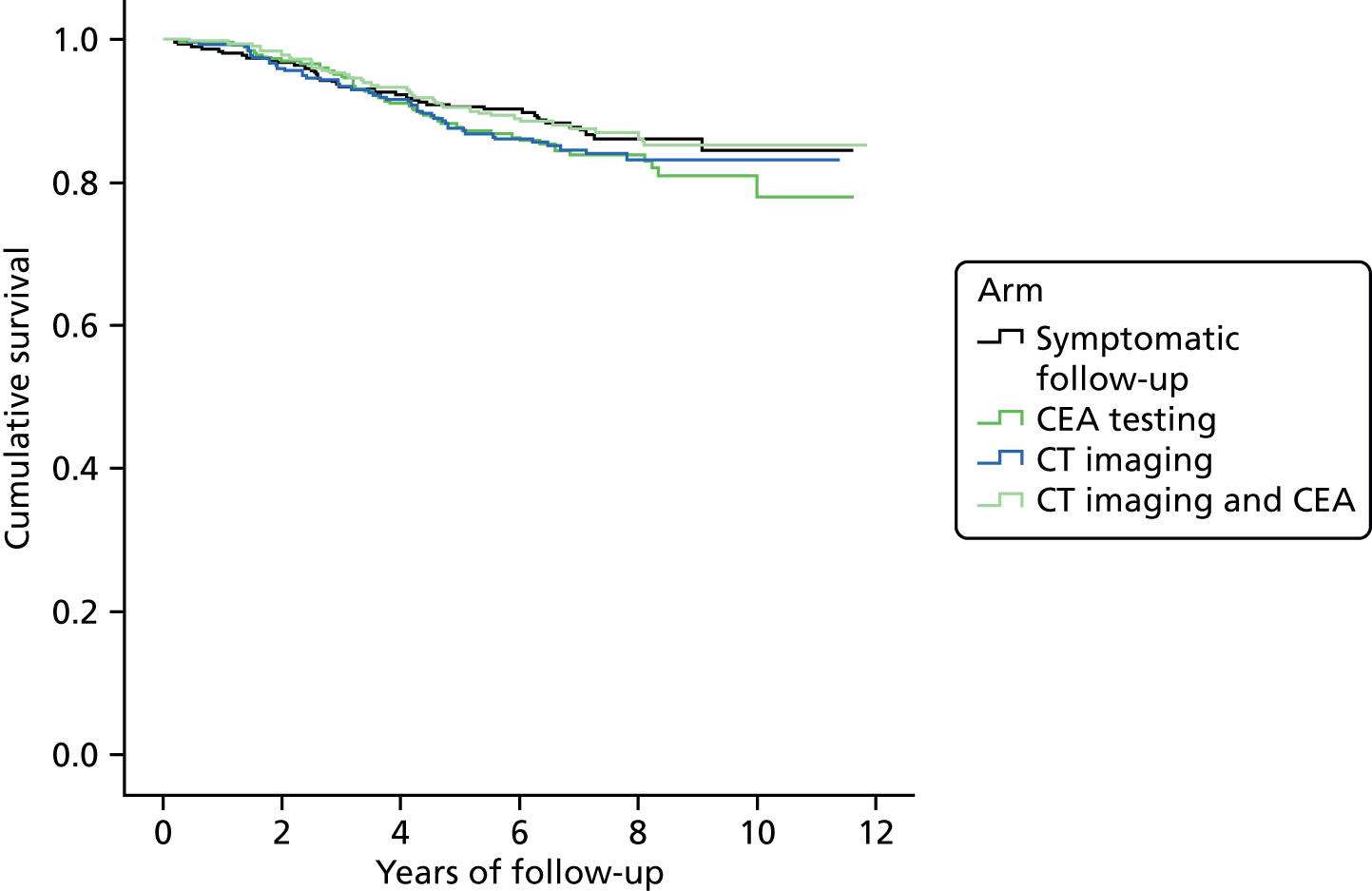
Appendix 3 Follow-up After Colorectal Surgery health economics
Costing methods
The unit cost for each CEA test recorded included consumables and laboratory processing costs. CEA tests were generally conducted by practice nurses but, because patients reported visits to health-care professionals separately, a practice nurse visit was not included as part of the CEA test unit cost to avoid double-counting. Cross-checks of the annual numbers of CEA tests and patient-reported practice nurse visits were performed, and when the number of practice nurse visits reported was lower than the number of CEA tests an upwards adjustment to the number of practice nurse visits was made so that that numbers for both variables were equivalent.
Computerised tomography scans, colonoscopies, radiography and MRI scans were costed using the sources listed in Table 15; these investigations were conducted at hospital outpatient clinics, but as with CEA tests, the associated clinic visit was counted separately and not included as part of these investigations. Again, cross-checks were performed and when the number of outpatient clinic visits reported was lower than the sum of the number of CT scans, colonoscopies, radiography and MRI scans, an upwards adjustment to the number of visits was made so that numbers for both variables were equivalent.
| Resource-use item | Unit cost (£) | Source |
|---|---|---|
| Tests/investigations | ||
| CEA test | 7.50 | £6.00 for laboratory analysis plus £1.50 for postage and packing – trial-participating hospital |
| CT scan | 130.72 | NHS reference costs 2012/1344 – average of codes RA08A, RA09A, RA10Z–RA14Z, RA50Z. Diagnostic imaging outpatients, medical oncology |
| Chest radiography | 40 | Finance department, trial participating hospital |
| MRI | 191.05 | NHS reference costs 2012/1344 – average of codes RA01A, RA02A, RA03Z–RA07Z. Diagnostic imaging outpatients, medical oncology |
| Colonoscopy | 514.00 | NHS reference costs 2012/1344 – code FZ51Z – diagnostic colonoscopy, aged ≥ 19 years, colorectal surgery day case |
| Treatment for recurrence | ||
| Surgery | Individual procedures were mapped to one or more HRG codes and the weighted average of 2012/13 NHS reference costs44 for each mapped HRG code (elective inpatients) was calculated | |
| 6980.80 | Code AA12 – intermediate intracranial procedures except trauma with brain tumours or cerebral cysts | |
| 8945.16 | Code AA06 – major intracranial procedures except trauma with brain tumours or cerebral cysts | |
| 7361.79 | Code DZ02 – complex thoracic procedures | |
| 3719.05 | Code DZ53 – major or intermediate thoracic procedures | |
| 5158.88 | Code FZ66/FZ67 – very major/major small intestine procedures, aged ≥ 19 years | |
| 6936.06 | Code FZ66 – very major small intestine procedures, aged ≥ 19 years | |
| 7471.52 | Code FZ73/FZ74/FZ75 – very complex/complex large intestine procedures/proximal colon procedures | |
| 8155.33 | Code FZ73/FZ74 – very complex/complex large intestine procedures | |
| 10,500.74 | Code GA03 – very complex open hepatobiliary or pancreatic procedures | |
| 9450.05 | GA03/GA04 – very complex/complex open hepatobiliary or pancreatic procedures | |
| 8517.09 | Code GA03/GA04/GA05 – very complex/complex/very major open hepatobiliary or pancreatic procedures | |
| 4242.29 | Code GA06/GA07/GA13 – major/intermediate open hepatobiliary or pancreatic procedures/laparoscopic hepatobiliary or pancreatic procedures | |
| 2741.87 | Code GA07/GA10 – intermediate open hepatobiliary or pancreatic procedures/laparoscopic/open cholecystectomy | |
| 4068.17 | Code GB01/GB08 – very major/complex endoscopic or percutaneous, hepatobiliary or pancreatic procedures | |
| 795.11 | Code GB04 – minor endoscopic or percutaneous, hepatobiliary or pancreatic procedures, aged ≥ 19 years | |
| 1098.63 | Code GB07 – minor diagnostic endoscopic retrograde cholangiopancreatography | |
| 3865 | Code HC04 – extradural spine intermediate | |
| 5476.86 | Code LB60/LB61/LB62 – complex/major open or percutaneous, kidney or ureter procedures, aged ≥ 19 years/major laparoscopic kidney or ureter procedures, aged ≥ 19 years | |
| 16,358.63 | Code LB71 – total pelvic exenteration | |
| 4289.45 | Code – MA06/MA26 complex/major open or laparoscopic, upper or lower genital tract procedures for malignancy | |
| Chemotherapy | ||
| First-line therapy drugs per 21-day cycle | ||
| Oxaliplatin 130 mg/m2, once | 18.25 | 100 mg/20 ml (£6.32) and 50 mg/10 ml (£5.61) from NHS eMIT.94 Body surface area of 1.79 m2 assumed |
| Capecitabine 1000 mg/m2, twice daily, days 1–14 | 41.16 | 500-mg tablets, 120 per pack (£47.84) and 150 mg tablets, 60 per pack (£8.34) from NHS eMIT.94 Body surface area of 1.79 m2 assumed |
| Bevacizumab (Avastin, Roche) 7.5 mg/kg, once | 1409.72 | 100 mg/4 ml (£242.66) and 400 mg/16 ml (£924.40) from the BNF 2015.42 Average weight is assumed to be 76.5 kg |
| Second-line therapy drugs per 21-day cycle | ||
| Irinotecan 180 mg/m2, once per cycle | 31.20 | 300 mg/15 ml (£25.58) and 40 mg/2 ml (£5.62) from NHS eMIT.94 Body surface area of 1.79 m2 assumed |
| Fluoroacil 2400 mg/m2 infusion, once | 3.47 | 5 g/100 ml (£3.47) from NHS eMIT.94 Body surface area of 1.79 m2 assumed |
| Fluoroacil 400 mg/m2 bolus, once | 4.46 | 500 mg/10 ml (£0.96) and 250 mg/10 ml (£3.50) from NHS eMIT.94 Body surface area of 1.79 m2 assumed |
| Folinic acid 300 mg, once | 89.95 | 300 mg/30 ml (£89.95) from the BNF 201542 |
| Aflibercept (Zaltrap, Sanofi-Aventis) 4 mg/kg, once | 1182.60 | 200 mg vial (£591.30) and 100 mg vial (£295.65) from the BNF 2015.42 Average weight is assumed to be 76.5 kg |
| Radiotherapy | ||
| With curative intent | ||
| Radical radiotherapy planning session | 733.92 | NHS reference costs 2012/1344 – average of codes SC40Z, SC41Z, SC51Z, SC52Z – preparation for conformal/IM radiation therapy (RAD worksheet) |
| Radical radiotherapy fraction cost | 121 | NHS reference costs 2012/1344 – code SC23Z – deliver a fraction of complex radiotherapy (RAD worksheet) |
| With palliative intent | ||
| Palliative radiotherapy planning session | 281.53 | NHS reference costs 2012/1344 – average of codes SC45Z–SC50Z – preparation for simple/superficial radiotherapy (RAD worksheet) |
| Palliative radiotherapy fraction cost | 91.00 | NHS reference costs 2012/1344 – code SC22Z, deliver a fraction of radiotherapy (RAD worksheet) |
| Longer-term follow-up | ||
| GP clinic attendance | 45 | PSSRU 201343 (assuming attendance duration of 11.7 minutes), section 10.8b GP unit costs |
| Visits to practice nurse | 13.43 | PSSRU 201343 (assuming attendance duration of 15.5 minutes), section 10.6 |
| District nurse at home | 39.00 | PSSRU 2013,43 section 10.1 community nurse |
| Stoma care nurse | 43.00 | NHS reference costs 2012/1344 – code N24AF – specialist nursing – stoma care services, adult, face to face |
| Oncology outpatient clinic attendance | 144 | NHS reference costs 2012/1344 – code 370 – consultant-led medical oncology clinic attendance |
| Excess inpatient bed-day on oncology ward | 369.79 | NHS reference costs 2012/1344 – weighted average of elective inpatient excess bed-day costs for medical oncology |
For patients with recurrence who underwent one or more surgeries, the type of surgical procedure performed was recorded along with hospital admission and discharge dates. Using the HRG4 + 2012/13 Reference Costs Grouping programme from the National Casemix Office, the Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures code for each type of surgery was identified and mapped to a corresponding HRG code(s). 95 HRG codes were then located in the NHS Reference Cost database, a weighted average was taken of the cost (and length of stay) across all specialties (excluding paediatrics) and subcategories within that code, and then the resulting costs were applied to the appropriate patients. 44 When a patient’s hospital stay was known to exceed the average length of stay for their particular procedure, additional days were costed using an excess bed-day cost for an oncology ward. 44
Detailed information was not collected on types of radiotherapy and/or chemotherapy received by patients with recurrence; instead, expert clinical opinion provided information on standard regimens, doses, frequency and duration of treatments, and Table 15 shows the resulting estimated radiotherapy and chemotherapy unit costs. Radiotherapy treatment schedules varied depending upon whether a recurrence was treated with curative intent or palliatively. Radical radiotherapy was assumed to involve a complex planning session and 25 sessions of treatment. Palliative radiotherapy was assumed to consist of a simple planning session followed by five sessions of treatment. For patients recorded as having received radiotherapy, treatment was assumed to have been radical if the patient also underwent surgery with curative intent following recurrence. Radiotherapy was assumed to have been palliative if the patient underwent surgery without curative intent or if the patient was not treated surgically for their recurrence. Patients receiving chemotherapy for recurrence and who survived at least 1 year from recurrence were assumed to have completed eight cycles of the first-line regimen shown in Table 15. Patients who died within 1 year of recurrence were assumed to have progressed mid-way through their remaining survival time and to have spent half of the time receiving the first-line chemotherapy regimen and half receiving the second-line regimen.
Additional practice nurse and outpatient clinic visits, together with visits to GPs and stoma nurses and home visits from district nurses, were also costed for each patient using appropriate unit costs from a variety of sources including Personal Social Services Research Unit and NHS Reference Costs (see Table 15). 43,44 When costing inpatient hospital admissions reported by patients, and to avoid double-counting for those patients who had undergone surgery for recurrence, one hospital admission and the appropriate number of surgery-related inpatient hospital days recorded by staff were subtracted from patients’ annual self-reported data. The remaining hospital inpatient days were costed using an average oncology bed-day cost taken from the NHS Reference Costs. 44
Multiple imputation
The MI approach uses predictions from regression models specified for each individual variable with missing data to fill in the missing data points. The form of each regression equation is dependent upon the type of data to be imputed, for example when imputing whether or not radiotherapy was given post recurrence (yes/no), a logistic regression was specified. When imputing the EQ-5D single-index score, a linear regression with prediction mean matching was used to ensure values were not predicted outside the permissible range (–0.594 to 1). Variables with complete data were used as predictors in each regression model (e.g. age and gender) along with all other variables to be imputed.
Table 16 gives a detailed breakdown, by each resource use category, of mean resource use and undiscounted costs per patient by trial arm and year of follow-up. Table 17 shows the proportion of surviving patients reporting some or severe problems on each domain of the EQ-5D, by year and arm.
| Resource item | Mean (SE) resource use or n (%) receiving resource | Mean cost [SE (£)] | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum follow-up arm (N = 301) | CEA testing-only follow-up arm (N = 300) | CT-only follow-up arm (N = 299) | CEA testing and CT follow-up (N = 302) | Minimum follow-up arm (N = 301) | CEA testing-only follow-up arm (N = 300) | CT-only follow-up arm (N = 299) | CEA testing and CT follow-up (N = 302) | |
| Year 1 | ||||||||
| CEA tests | 0.20 (0.04) | 3.22 (0.05) | 0.04 (0.02) | 3.10 (0.06) | 1.50 (0.28) | 24.18 (0.41) | 0.28 (0.12) | 23.22 (0.41) |
| CT tests | 0.30 (0.03) | 0.28 (0.03) | 1.37 (0.03) | 1.40 (0.03) | 38.65 (3.61) | 37.04 (3.78) | 179.25 (4.33) | 183.09 (4.56) |
| Colonoscopy | 0.08 (0.02) | 0.06 (0.01) | 0.12 (0.02) | 0.14 (0.02) | 42.69 (8.54) | 30.84 (7.46) | 63.61 (10.10) | 73.19 (10.35) |
| Radiography | 0.00 (0.00) | 0.00 (0.00) | 0.09 (0.02) | 0.10 (0.02) | 0.13 (0.13) | 0.13 (0.13) | 3.61 (0.76) | 3.84 (0.84) |
| MRI scans | 0.01 (0.00) | 0.00 (0.00) | 0.01 (0.01) | 0.01 (0.00) | 1.27 (0.90) | 0.00 (0.00) | 2.56 (1.27) | 1.27 (0.89) |
| Surgery | 6 (1.99%) | 5 (1.67%) | 7 (2.34%) | 7 (2.32%) | 177.09 (73.93) | 142.52 (63.65) | 250.79 (99.68) | 184.28 (71.08) |
| Chemotherapy | 4 (1.33%) | 12 (4.00%) | 15 (5.02%) | 15 (4.97%) | 160.01 (79.67) | 470.12 (133.19) | 555.22 (143.85) | 549.71 (142.46) |
| Radiotherapy | 3 (1.00%) | 0 (0.00%) | 4 (1.34%) | 4 (1.32%) | 17.38 (12.94) | 0.00 (0.00) | 17.50 (13.03) | 17.32 (12.90) |
| GP visits | 0.97 (0.12) | 1.06 (0.12) | 0.95 (0.13) | 1.08 (0.14) | 43.42 (5.42) | 47.52 (5.43) | 42.77 (5.92) | 48.73 (6.51) |
| PN visits | 1.38 (0.76) | 4.27 (0.63) | 0.59 (0.19) | 3.26 (0.06) | 18.48 (9.54) | 57.41 (8.44) | 7.91 (2.54) | 43.81 (0.80) |
| DN visits | 0.55 (0.17) | 1.62 (0.70) | 1.30 (0.36) | 0.85 (0.60) | 21.40 (6.53) | 63.36 (27.17) | 50.63 (14.15) | 33.24 (23.44) |
| SN visits | 0.34 (0.11) | 0.23 (0.07) | 0.36 (0.08) | 0.40 (0.10) | 14.49 (4.79) | 9.83 (3.01) | 15.42 (3.33) | 17.34 (4.43) |
| Outpatient visits | 1.42 (0.11) | 1.44 (0.17) | 3.17 (0.31) | 3.24 (0.27) | 204.66 (16.52) | 207.07 (24.05) | 456.75 (44.48) | 466.81 (39.10) |
| Inpatient stays | 0.13 (0.03) | 0.15 (0.03) | 0.19 (0.04) | 0.14 (0.03) | 321.63 (104.52) | 468.15 (117.28) | 278.76 (56.95) | 372.24 (163.00) |
| Year 2 | ||||||||
| CEA tests | 0.16 (0.03) | 3.44 (0.09) | 0.02 (0.01) | 3.29 (0.09) | 1.17 (0.22) | 25.80 (0.65) | 0.18 (0.10) | 24.69 (0.65) |
| CT tests | 0.49 (0.03) | 0.48 (0.03) | 1.62 (0.05) | 1.50 (0.05) | 64.27 (4.25) | 62.31 (4.30) | 212.04 (6.67) | 195.65 (6.68) |
| Colonoscopy | 0.07 (0.02) | 0.08 (0.02) | 0.54 (0.03) | 0.51 (0.03) | 37.57 (8.09) | 39.41 (8.27) | 275.05 (14.85) | 263.81 (14.81) |
| Radiography | 0.03 (0.01) | 0.02 (0.01) | 0.13 (0.03) | 0.10 (0.02) | 1.20 (0.39) | 0.93 (0.35) | 5.08 (1.17) | 4.11 (0.92) |
| MRI scans | 0.00 (0.00) | 0.01 (0.01) | 0.01 (0.01) | 0.00 (0.00) | 0.63 (0.63) | 1.91 (1.10) | 1.92 (1.92) | 0.63 (0.63) |
| Surgery | 7 (2.33%) | 10 (3.33%) | 14 (4.68%) | 5 (1.66%) | 192.91 (80.35) | 293.49 (91.96) | 457.69 (126.68) | 149.77 (67.02) |
| Chemotherapy | 8 (2.66%) | 9 (3.00%) | 7 (2.34%) | 9 (2.98%) | 312.37 (109.14) | 326.32 (110.14) | 283.92 (106.54) | 345.66 (114.77) |
| Radiotherapy | 3 (1.00%) | 1 (0.33%) | 0 (0.00%) | 2 (0.66%) | 17.38 (12.94) | 12.53 (–) | 0.00 (0.00) | 14.89 (12.68) |
| GP visits | 0.70 (0.10) | 0.94 (0.18) | 0.50 (0.10) | 0.89 (0.21) | 31.37 (4.61) | 42.24 (8.12) | 22.67 (4.65) | 40.02 (9.52) |
| PN visits | 0.26 (0.04) | 3.64 (0.09) | 0.14 (0.05) | 3.48 (0.09) | 3.44 (0.57) | 48.86 (1.26) | 1.94 (0.70) | 46.74 (1.25) |
| DN visits | 0.13 (0.06) | 1.98 (0.69) | 0.32 (0.14) | 0.87 (0.36) | 5.23 (2.26) | 77.40 (26.86) | 12.44 (5.42) | 33.89 (13.86) |
| SN visits | 0.08 (0.04) | 0.08 (0.04) | 0.11 (0.04) | 0.07 (0.02) | 3.23 (1.87) | 3.24 (1.68) | 4.80 (1.54) | 2.93 (0.97) |
| Outpatient visits | 1.64 (0.18) | 1.54 (0.14) | 3.41 (0.16) | 3.68 (0.37) | 236.43 (26.25) | 222.53 (20.47) | 491.24 (23.41) | 529.94 (53.94) |
| Inpatient stays | 0.09 (0.03) | 0.07 (0.02) | 0.23 (0.08) | 0.11 (0.03) | 131.45 (51.42) | 206.59 (70.45) | 255.76 (97.24) | 223.83 (70.26) |
| Year 3 | ||||||||
| CEA tests | 0.14 (0.03) | 1.61 (0.06) | 0.02 (0.01) | 1.54 (0.06) | 1.07 (0.21) | 12.08 (0.46) | 0.18 (0.09) | 11.52 (0.43) |
| CT tests | 0.11 (0.02) | 0.11 (0.02) | 0.76 (0.04) | 0.79 (0.04) | 14.33 (2.73) | 14.38 (2.44) | 99.68 (4.92) | 103.02 (5.14) |
| Colonoscopy | 0.07 (0.01) | 0.04 (0.01) | 0.11 (0.02) | 0.11 (0.02) | 35.86 (7.56) | 20.56 (5.82) | 55.01 (9.20) | 57.87 (9.97) |
| Radiography | 0.00 (0.00) | 0.00 (0.00) | 0.04 (0.01) | 0.06 (0.02) | 0.00 (0.00) | 0.00 (0.00) | 1.61 (0.56) | 2.25 (0.68) |
| MRI scans | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.63 (0.63) |
| Surgery | 2 (0.66%) | 5 (1.67%) | 1 (0.33%) | 5 (1.66%) | 53.96 (38.09) | 135.71 (63.59) | 47.46 (–) | 142.52 (64.04) |
| Chemotherapy | 1 (0.33%) | 4 (1.33%) | 4 (1.34%) | 4 (1.32%) | 15.01 (–) | 156.71 (77.96) | 150.00 (74.88) | 155.67 (77.45) |
| Radiotherapy | 0 (0.00%) | 2 (0.67%) | 3 (1.00%) | 1 (0.33%) | 0.00 (0.00) | 4.91 (3.47) | 4.93 (3.48) | 12.45 (–) |
| GP visits | 0.41 (0.07) | 0.53 (0.11) | 0.42 (0.08) | 0.68 (0.21) | 18.63 (3.24) | 23.67 (5.14) | 18.90 (3.51) | 30.55 (9.63) |
| PN visits | 0.19 (0.04) | 1.77 (0.07) | 0.20 (0.08) | 1.71 (0.07) | 2.61 (0.47) | 23.71 (0.97) | 2.73 (1.02) | 22.96 (0.87) |
| DN visits | 0.05 (0.03) | 0.07 (0.04) | 0.03 (0.02) | 0.69 (0.43) | 2.00 (1.36) | 2.70 (1.53) | 1.10 (0.62) | 26.76 (16.70) |
| SN visits | 0.09 (0.03) | 0.01 (0.01) | 0.06 (0.03) | 0.21 (0.10) | 3.97 (1.25) | 0.34 (0.34) | 2.67 (1.35) | 9.00 (4.37) |
| Outpatient visits | 1.30 (0.31) | 1.04 (0.17) | 1.68 (0.15) | 1.91 (0.16) | 186.48 (44.04) | 149.38 (25.19) | 241.86 (22.29) | 274.74 (23.09) |
| Inpatient stays | 0.08 (0.02) | 0.16 (0.06) | 0.17 (0.17) | 0.12 (0.05) | 143.74 (46.68) | 213.99 (73.13) | 138.52 (128.89) | 822.35 (414.00) |
| Year 4 | ||||||||
| CEA tests | 0.13 (0.03) | 1.50 (0.06) | 0.03 (0.01) | 1.42 (0.06) | 0.97 (0.20) | 11.25 (0.46) | 0.20 (0.09) | 10.68 (0.46) |
| CT tests | 0.09 (0.02) | 0.08 (0.02) | 0.70 (0.04) | 0.70 (0.04) | 11.73 (2.24) | 10.46 (2.39) | 91.81 (5.04) | 90.90 (4.83) |
| Colonoscopy | 0.06 (0.01) | 0.06 (0.01) | 0.03 (0.01) | 0.04 (0.01) | 32.45 (7.22) | 30.84 (7.06) | 13.75 (4.80) | 22.13 (6.01) |
| Radiography | 0.00 (0.00) | 0.00 (0.00) | 0.04 (0.01) | 0.04 (0.01) | 0.00 (0.00) | 0.00 (0.00) | 1.74 (0.54) | 1.59 (0.49) |
| MRI scans | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.64 (0.64) | 0.00 (0.00) |
| Surgery | 0 (0.00%) | 3 (1.00%) | 4 (1.34%) | 4 (1.32%) | 0.00 (0.00) | 118.84 (74.29) | 110.52 (55.24) | 102.27 (51.02) |
| Chemotherapy | 0 (0.00%) | 5 (1.67%) | 4 (1.34%) | 2 (0.66%) | 0.00 (0.00) | 191.12 (85.01) | 157.23 (78.22) | 77.83 (54.95) |
| Radiotherapy | 1 (0.33%) | 1 (0.33%) | 1 (0.33%) | 2 (0.66%) | 2.45 (–) | 12.53 (–) | 12.57 (–) | 4.88 (3.44) |
| GP visits | 0.45 (0.10) | 0.31 (0.06) | 1.07 (0.61) | 0.56 (0.14) | 20.03 (4.45) | 14.13 (2.91) | 47.95 (27.63) | 25.09 (6.44) |
| PN visits | 0.20 (0.04) | 1.76 (0.08) | 0.87 (0.60) | 1.76 (0.11) | 2.66 (0.57) | 23.59 (1.09) | 11.67 (8.11) | 23.62 (1.42) |
| DN visits | 0.02 (0.02) | 0.17 (0.11) | 0.22 (0.12) | 0.04 (0.03) | 0.65 (0.65) | 6.50 (4.38) | 8.43 (4.53) | 1.68 (1.16) |
| SN visits | 0.09 (0.03) | 0.04 (0.02) | 0.05 (0.02) | 0.12 (0.03) | 3.77 (1.27) | 1.58 (0.88) | 1.98 (0.71) | 5.21 (1.42) |
| Outpatient visits | 1.15 (0.19) | 1.17 (0.28) | 1.74 (0.18) | 1.31 (0.09) | 165.05 (27.93) | 168.77 (40.61) | 251.11 (26.63) | 189.20 (12.67) |
| Inpatient stays | 0.10 (0.02) | 0.03 (0.01) | 0.04 (0.02) | 0.06 (0.03) | 96.56 (27.87) | 34.27 (21.73) | 46.75 (20.84) | 143.26 (95.65) |
| Year 5 | ||||||||
| CEA tests | 0.08 (0.02) | 1.78 (0.08) | 0.01 (0.00) | 1.68 (0.07) | 0.57 (0.14) | 13.35 (0.57) | 0.05 (0.04) | 12.62 (0.54) |
| CT tests | 0.11 (0.02) | 0.06 (0.01) | 0.98 (0.05) | 1.09 (0.05) | 13.90 (2.63) | 7.41 (1.85) | 128.53 (6.39) | 141.97 (6.82) |
| Colonoscopy | 0.29 (0.03) | 0.27 (0.03) | 0.31 (0.03) | 0.34 (0.03) | 146.86 (13.41) | 137.07 (13.15) | 159.87 (13.78) | 173.60 (14.22) |
| Radiography | 0.00 (0.00) | 0.00 (0.00) | 0.05 (0.01) | 0.04 (0.02) | 0.13 (0.13) | 0.00 (0.00) | 1.87 (0.56) | 1.46 (0.60) |
| MRI scans | 0.00 (0.00) | 0.00 (0.00) | 0.01 (0.01) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 1.28 (1.28) | 0.00 (0.00) |
| Surgery | 0 (0.00%) | 2 (0.67%) | 4 (1.34%) | 1 (0.33%) | 0.00 (0.00) | 70.24 (51.80) | 176.03 (89.03) | 34.77 (–) |
| Chemotherapy | 1 (0.33%) | 2 (0.67%) | 2 (0.67%) | 2 (0.66%) | 4.88 (–) | 55.09 (42.24) | 78.62 (55.50) | 77.83 (54.95) |
| Radiotherapy | 0 (0.00%) | 0 (0.00%) | 1 (0.33%) | 0 (0.00%) | 0.00 (0.00) | 0.00 (0.00) | 2.46 (–) | 0.00 (0.00) |
| GP visits | 0.43 (0.14) | 0.31 (0.07) | 0.45 (0.14) | 0.53 (0.16) | 19.23 (6.34) | 14.16 (3.08) | 20.26 (6.18) | 23.78 (7.20) |
| PN visits | 0.17 (0.03) | 2.05 (0.09) | 0.14 (0.07) | 1.80 (0.08) | 2.22 (0.42) | 27.52 (1.18) | 1.89 (0.95) | 24.15 (1.03) |
| DN visits | 0.00 (0.00) | 0.05 (0.03) | 0.00 (0.00) | 0.26 (0.18) | 0.00 (0.00) | 1.92 (1.04) | 0.00 (0.00) | 10.28 (7.11) |
| SN visits | 0.06 (0.02) | 0.04 (0.02) | 0.10 (0.02) | 0.05 (0.02) | 2.46 (1.04) | 1.86 (0.69) | 4.37 (1.06) | 2.25 (0.73) |
| Outpatient visits | 0.99 (0.14) | 0.98 (0.14) | 1.64 (0.08) | 1.92 (0.11) | 142.56 (20.38) | 141.12 (19.62) | 236.37 (10.93) | 276.94 (16.27) |
| Inpatient stays | 0.02 (0.01) | 0.05 (0.02) | 0.08 (0.02) | 0.05 (0.02) | 37.35 (18.34) | 66.32 (33.93) | 73.22 (26.80) | 108.49 (41.40) |
| EQ-5D domain | Minimum follow-up | CEA testing only | CT only | CEA testing and CT |
|---|---|---|---|---|
| Mobility | ||||
| Baseline | 0.24 | 0.23 | 0.23 | 0.28 |
| 1 | 0.26 | 0.22 | 0.27 | 0.26 |
| 2 | 0.30 | 0.27 | 0.25 | 0.24 |
| 3 | 0.27 | 0.28 | 0.23 | 0.27 |
| 4 | 0.27 | 0.30 | 0.25 | 0.27 |
| 5 | 0.27 | 0.27 | 0.24 | 0.35 |
| Self-care | ||||
| Baseline | 0.07 | 0.05 | 0.06 | 0.07 |
| 1 | 0.06 | 0.05 | 0.07 | 0.05 |
| 2 | 0.07 | 0.06 | 0.06 | 0.06 |
| 3 | 0.05 | 0.08 | 0.06 | 0.09 |
| 4 | 0.05 | 0.04 | 0.05 | 0.07 |
| 5 | 0.06 | 0.07 | 0.06 | 0.10 |
| Usual activities | ||||
| Baseline | 0.32 | 0.33 | 0.33 | 0.39 |
| 1 | 0.29 | 0.24 | 0.28 | 0.24 |
| 2 | 0.33 | 0.24 | 0.26 | 0.25 |
| 3 | 0.29 | 0.33 | 0.22 | 0.28 |
| 4 | 0.24 | 0.30 | 0.29 | 0.27 |
| 5 | 0.30 | 0.26 | 0.24 | 0.32 |
| Pain/discomfort | ||||
| Baseline | 0.38 | 0.39 | 0.35 | 0.38 |
| 1 | 0.29 | 0.31 | 0.28 | 0.33 |
| 2 | 0.36 | 0.29 | 0.23 | 0.34 |
| 3 | 0.35 | 0.35 | 0.26 | 0.31 |
| 4 | 0.35 | 0.34 | 0.30 | 0.35 |
| 5 | 0.34 | 0.26 | 0.23 | 0.35 |
| Anxiety/depression | ||||
| Baseline | 0.25 | 0.25 | 0.25 | 0.24 |
| 1 | 0.24 | 0.26 | 0.25 | 0.23 |
| 2 | 0.29 | 0.23 | 0.29 | 0.26 |
| 3 | 0.31 | 0.23 | 0.28 | 0.25 |
| 4 | 0.26 | 0.22 | 0.25 | 0.26 |
| 5 | 0.28 | 0.21 | 0.25 | 0.27 |
Appendix 5 Patient and public involvement
The FACS trial had a patient and public involvement (PPI) member actively engaged in the development and planning of the trial. Having strong involvement in bowel cancer campaigns and patient groups, the PPI member was ideally placed to offer a patient voice to the trial and was a full member of the trial management group. Alongside this, the FACS trial was in the National Cancer Research Institute Colorectal Clinical Studies Group portfolio and was regularly discussed by the group, which included PPI representation.
List of abbreviations
- CEA
- carcinoembryonic antigen
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CT
- computerised tomography
- DMEC
- Data Monitoring and Ethics Committee
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- FACS
- Follow-up After Colorectal Surgery
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- MI
- multiple imputation
- MRC
- Medical Research Council
- MRI
- magnetic resonance imaging
- NCRN
- National Cancer Research Network
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OR
- odds ratio
- PET
- positron emission tomography
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- R0 resection
- ≥ 1-mm clearance at lateral margins
- SE
- standard error
- SPSS
- Statistical Product and Service Solutions
- TNM
- tumour, node, metastasis