Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/17/06. The contractual start date was in February 2016. The draft report began editorial review in October 2016 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Westwood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Objective
The overall objective of this project is to summarise the evidence on the clinical effectiveness and cost-effectiveness of using quantitative faecal immunochemical tests (FITs) as a triage test for people presenting, in primary care settings, with lower abdominal symptoms, who are at low risk for colorectal cancer (CRC) according to the criteria defined in the 2015 National Institute for Health and Care Excellence (NICE) guideline (NG12). 1 Use of occult blood testing in the faeces has been recently recommended for this population; this assessment will consider the clinical effectiveness and cost-effectiveness of quantitative faecal immunochemical testing as a replacement for guaiac testing. The following research questions have been defined to address the review objective:
-
What is the clinical effectiveness of faecal immunochemical testing compared with guaiac faecal occult blood testing or no triage, for achieving appropriate referral for further investigation within the 2-week suspected cancer referral target?
-
What is the comparative accuracy of different quantitative FIT assays and guaiac faecal occult blood testing, for which CRC determined by colonoscopy (the reference standard method) is the target condition?
-
What is the diagnostic accuracy of different quantitative FIT assays, where CRC determined by colonoscopy (the reference standard method) is the target condition?
-
What is the cost-effectiveness of using faecal immunochemical testing for the presence of occult blood as a triage step in the investigation of symptomatic patients for suspected CRC compared with the recent recommendation of guaiac faecal occult blood testing and no triage (referral straight to colonoscopy)?
Note
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 2 Background and definition of the decision problem(s)
Population
The primary indication for this assessment is the use of tests for the presence of occult blood in the faeces as a triage step in the investigation of people presenting in primary care settings, with lower abdominal symptoms in whom investigation for possible CRC is being considered. NICE guidance on suspected cancer: recognition and referral (NG121) recommends the use of guaiac testing for occult blood in the faeces as a triage step before referral for secondary care investigations, in specified symptomatic patient groups – people aged:
-
≥ 50 years, who have unexplained abdominal pain or weight loss
-
< 60 years, who have changes in their bowel habit or iron-deficiency anaemia
-
≥ 60 years, who have anaemia in the absence of iron deficiency.
This assessment will consider the clinical effectiveness and cost-effectiveness of using quantitative faecal immunochemical testing for haemoglobin (Hb) as a triage test. The clinical effectiveness and cost-effectiveness of triage testing using faecal immunochemical testing will be considered for all people presenting, in primary care settings, with lower abdominal symptoms who require investigation for possible CRC, not limited to the specific groups detailed in NG12. 1
Colorectal cancer is the third most common cancer in the UK population overall and in people aged ≥ 50 years, after breast cancer and lung cancer for females and prostate cancer and lung cancer for males. The most common cancers differ for younger age groups. The Office for National Statistics (ONS) cancer registration data for 2013 showed approximately 35,000 new cases of CRC in England (18,839 males and 14,926 females). 2 The incidence of CRC was 87 cases per 100,000 males and 52 cases per 100,000 females; the age-standardised incidence rate was 54.4% higher in males than in females and has increased for both males and females over the last 10 years. 2 CRC accounted for approximately 11.5% of all new cancers diagnosed in 2013 (12.6% in males and 10.4% in females) and increasing with age to 14.2% of cancers in males aged ≥ 80 years and 15.2% in females aged ≥ 80 years. 2 The age-standardised 1-year survival rates for men and women who were diagnosed with CRC between 2009 and 2013, and followed up to 2014, were 77.5% and 75.8%, respectively. 3 The corresponding 5-year survival rates were 58.5% and 58.2%, respectively. 3 Survival rates for CRC have not changed substantively since the previous data collection period (2008–12).
The UK NHS Bowel Cancer Screening Programme in England currently utilises guaiac faecal occult blood testing, but faecal immunochemical testing has been recently recommended by the UK National Screening Committee, has been piloted for national roll-out and recommended by European Commission guidelines. 4,5 However, studies assessing the effectiveness of faecal immunochemical testing or comparing the performance of faecal immunochemical testing and guaiac faecal occult blood testing in asymptomatic population-based screening for CRC will not be included in this assessment. This is because the prevalence of CRC is likely to be higher in a population with even relatively low-risk symptoms than in the general population without symptoms that are eligible for screening, and faecal immunochemical testing used for screening applications will generally use higher cut-off faecal Hb concentrations than would be used for triage of people with symptoms. The cost-effectiveness modelling used to inform NG121 based its estimate of the prevalence of CRC in a low-risk population on the positive predictive value (PPV) of symptoms in 22 studies that were identified as relevant. 6 The PPV of altered bowel habit in men and women aged < 60 years ranged from 0.01 to 15.7, and the base-case analysis used a CRC prevalence estimate that was at the lower end of this range (1.5%). 6 By comparison, estimating the prevalence of CRC in the general population of England, based on ONS cancer registration and population data, gives approximately 0.065% for the whole population and 0.226% for the screening-eligible age group (60–74 years), that is, those who were most likely to match the population included in screening studies. Furthermore, it has been shown that differences in disease prevalence can affect estimates of test performance; data from 23 meta-analyses, which covered a wide range of clinical conditions, showed changes in sensitivity and specificity estimates of between 0% and 40% from the lowest to the highest prevalence. 7 In relation to faecal immunochemical testing, a recent meta-analysis of 19 studies conducted in average-risk, asymptomatic screening populations reported summary estimates of sensitivity and specificity for CRC of 79% [95% confidence interval (CI) 69% to 86%] and 94% (95% CI 92% to 95%), respectively; however, this analysis pooled data for eight different FIT assays. 8
The 2015 National Bowel Cancer Audit Report9 stated that, of all patients diagnosed with CRC in 2014, 55% were diagnosed following a general practitioner (GP) referral and 9% (20% of those in the eligible age range for screening, 60–74 years) were diagnosed through the NHS Bowel Cancer Screening Programme; however, 20% were diagnosed only following an emergency presentation (referral source data were missing for 16% of patients). 9 Treatment with curative intent was possible for more of those patients who were diagnosed through screening (90%) and following GP referral (70%) than those who were presenting as an emergency admission (52%). 9 Work to promote screening uptake and awareness of CRC symptoms is stated as a recommendation, with the aim of reducing the proportion of emergency presentations and improving outcomes. However, increased uptake of screening and increased awareness of, and presentation in, primary care of patients with low-risk symptoms could result in more invasive investigations, such as colonoscopy, being conducted. Estimates from the charity Bowel Cancer UK10 have suggested that there will be a 10–15% year-on-year increase in demand for colonoscopies, which impacts on the 2-week suspected cancer referral time and NHS capacity. 1 In addition, colonoscopy has associated risks, which include bowel perforation, bleeding, infection and abdominal pain. 11 A recent review reported that most colonoscopies performed in symptomatic patients do not find either CRC or other serious bowel disease, and do not yield changes to the therapeutic approach. 12 The identification of tests that can help to rule out CRC and select people who are more likely to benefit from further investigation is therefore an important goal. It has been suggested that using quantitative immunochemical measurement of faecal Hb concentration to select patients for referral has the potential to reduce unnecessary colonoscopies and provide more accurate classification of patients than traditional, symptoms-based guidelines. 13
This assessment provides a comprehensive summary of the evidence about the performance of faecal immunochemical testing as a triage test for people, presenting in primary care settings, with lower abdominal symptoms, who are at low risk for CRC according to the criteria defined in NG121 and for whom a referral for secondary care investigation for possible CRC is being considered.
Intervention technologies
There are two major types of test for the presence of small amounts of blood in faeces: these are guaiac based [guaiac faecal occult blood test (gFOBT)] or immunochemical based (FIT). Guaiac-based methods detect the haem complex, whereas immunochemical methods specifically detect the globin moiety of human Hb.
Guaiac faecal occult blood tests rely on the pseudo-peroxidase activity of haem. A faecal sample is placed on to a paper that is impregnated with guaiac, to which hydrogen peroxide is applied as developer of the test. In the presence of haem, a chemical reaction occurs, yielding a blue- or green-coloured product within seconds. Usually, two faecal samples from each of three separate bowel motions are required. 14 The test is not specific for human blood and will also respond to animal blood, muscle protein and iron supplements. In addition, certain vegetables contain constituents with peroxidase activity, which can lead to false-positive (FP) results, although this can be minimised by waiting for 72 hours before development of the test. Bleeding gums or medicines that can cause gastrointestinal (GI) irritation or bleeding, for example aspirin and non-steroidal anti-inflammatory drugs, can also result in a FP test result. 15 In addition, a high intake of vitamin C can cause a false-negative (FN) result. In consequence, dietary and medicine restrictions are often imposed prior to testing. 14 gFOBTs are not considered an intervention technology in this assessment.
Faecal immunochemical tests use antibodies that specifically recognise the globin of human Hb. Faecal immunochemical testing has the potential to reduce FPs caused by upper GI bleeding because globin is degraded in the upper GI tract and, therefore, is not present in faecal samples for faecal immunochemical testing to detect. However, haem is resistant to degradation in the upper GI tract and, therefore, this molecule remains in faecal samples and can be detected by guaiac faecal occult blood testing (FP). Usually, only one faecal sample (but sometimes two) is collected and no dietary or medicine restriction is required. 16 Faecal immunochemical testing can be either qualitative or quantitative, and both are available from many different manufacturers with variable designs. Qualitative tests have an end point that is read as positive or negative visually; usually they are of a lateral flow immunochromatographic design, similar to home pregnancy tests. Faecal samples can be collected on to cards, similar to the traditional guaiac faecal occult blood testing, or, more commonly, into specimen collection devices that use probes attached to the lid of the device to transfer a few milligrams of faeces into a few millilitres of stabilising buffer in the device. Each manufacturer sets their own cut-off faecal Hb concentration for a positive test and available qualitative FITs are very different. The need for visual interpretation of the results can introduce interobserver variation. Determination of the presence of a trace line in the test portion of the cassette is a subjective judgement, which can sometimes be difficult. It is difficult to introduce quality control and, if qualitative FITs are used outside laboratories, the stringent recommendations and guidelines for point-of-care tests must be followed. Quantitative faecal immunochemical testing often uses immunoturbidimetric methods to measure the actual concentration of faecal Hb. Analysis is usually automated, facilitating quality management procedures. Most quantitative FITs require ‘wet’ collection, whereby samples are collected with a probe attached to the lid of the specimen collection device and transferred into a small volume of buffer in the device. The sample may degrade between collection and analysis if not handled properly14,16 because faecal Hb is very unstable: indeed, faecal samples for faecal immunochemical testing must be collected into the specimen collection devices and cannot be collected by patients into traditional collection pots that are then returned to primary care for onward transport for FIT analysis.
A summary of the product properties of quantitative FIT assays that are available in the NHS in England and Wales, and included in this assessment, is provided in Table 1.
| Name | Manufacturer | Test system description | Measurement range | Limit of detection | Limit of quantitation | Cut-off point | Capacity |
|---|---|---|---|---|---|---|---|
| HM-JACKarc system | Kyowa Medex/Alpha Laboratories Ltd, Tokyo, Japan | mAb human Hb | 7–400 µg Hb/g faeces (7–400 ng Hb/ml buffer) | 0.6 µg Hb/g faeces (0.6 ng Hb/ml buffer) | 1.25 µg Hb/g faeces (1.25 ng Hb/ml buffer) | 10 µg Hb/g faeces (10 ng Hb/ml buffer) | 200 samples/hour (maximum capacity of 80 samples/run) |
| Automated detection using immunoturbidimetry | |||||||
| FOB Gold system | Sentinel/Sysmex, Sentinel Diagnostics, Milan, Italy | Automated detection using immunoturbidimetry | 10 ng Hb/ml buffer to highest calibrator concentration | 9.5 ng Hb/ml buffer | 13.9 ng Hb/ml buffer | To be determined by each laboratory | Dependent on analyser used |
| OC-Sensor | Eiken Chemical Co./MAST Diagnostics, Tokyo, Japan | pAb human Hb | 10–1000 ng Hb/ml buffer | IO analyser: 4 µg Hb/g faeces (20 ng Hb/ml buffer) | IO analyser: 6 µg Hb/g faeces (30 ng Hb/ml buffer) | 10 µg Hb/g faeces (50 ng Hb/ml buffer) | Dependent on analyser used |
| Automated detection using immunoturbidimetry | PLEDIA analyser: 2 µg Hb/g faeces (10 ng Hb/ml buffer) | PLEDIA analyser: 2 µg Hb/g faeces (10 ng Hb/ml buffer) | |||||
| Micro analyser: 4 µg Hb/g faeces (20 ng Hb/ml buffer) | Micro analyser: NI | ||||||
| RIDASCREEN Hb and Hb/Hp test | R-Biopharm, Darmstadt, Germany | pAb human Hp, mAb Hb | 0.65–50 µg Hb/g faeces | 0.42 µg Hb/g faeces | 0.65 µg Hb/g faeces | 2 µg Hb/g faeces | Manual processing: 91 samples in 150 minutes |
| ELISA | 0.38 µg Hb/Hp complex/g faeces | 2 µg Hb/Hp complex/g faeces | DYNEX agility®, DYNEX Technologies, Chantilly, VA, USA: 546 samples in 7 hours | ||||
| Manual or automated colour detection |
Units for faecal immunochemical tests for haemoglobin
Different FITs use a variety of sampling methods, with variation in the mass of faeces collected and the volume and characteristics of the buffer used in the sampling device. These differences make the comparisons of test performance between FIT assay types difficult. FIT results can be expressed as Hb concentration in the sampling device buffer (ng Hb/ml buffer) or as Hb concentration by mass of faeces (µg Hb/g faeces). Initiatives aimed at standardising the units of measurement have resulted in recommendations, from the World Endoscopy Organization Colorectal Cancer Screening Committee’s Expert Working Group on ‘FIT for Screening’17 and the Guildford Medical Device Evaluation Centre,18 that manufacturers should adopt the use of ‘µg Hb/g faeces’.
OC-Sensor
OC-Sensor (Eiken Chemical Co./MAST Diagnostics, Tokyo, Japan) is a quantitative faecal immunochemical test. A sample is collected on a probe and inserted immediately into a unique specimen collection device that contains buffer. Analysis is fully automated using the OC-PLEDIA analyser or the OC-Sensor IO analyser – both quantitatively determine the concentrating of Hb present in faecal samples using polyclonal antibodies for human Hb and latex agglutination turbidimetry. 19,20 The OC-PLEDIA can process up to 320 samples per hour, with a capacity of 200 samples per run. The OC-Sensor IO analyser can process up to 88 samples per hour, with a maximum capacity of 20 samples per run.
HM-JACKarc system
The HM-JACKarc system (Kyowa Medex/Alpha Laboratories Ltd, Tokyo, Japan) is a fully automated quantitative faecal immunochemical test. A sample is obtained using the insertion of a probe attached to the cap of the specimen collection device, which is then inserted into a specialised collection tube containing buffer. The system picks up a small volume from the specimen collection devices and adds reagents, including latex reagent pre-coated with antibodies that are specific to the globin moiety of human Hb. Binding of the latex reagent to globin that is present in the faecal sample creates a complex that can be detected using turbidimetry. The system comprises an analyser, faecal sample collection devices (the Extel Hemo-auto MC A device; Kyowa Medex/Alpha Laboratories Ltd, Tokyo, Japan), latex agglutination reagents (Extel Hemo-Auto HS; Kyowa Medex/Alpha Laboratories Ltd) and buffer (Extel Hemo-auto; Kyowa Medex/Alpha Laboratories Ltd). The test has a measuring range of 7–400 µg Hb/g faeces. The HM-JACKarc analyser can process up to 200 samples per hour, with a maximum capacity of 80 samples per run. 21
FOB Gold
The FOB Gold system (Sentinel/Sysmex, Sentinel Diagnostics, Milan, Italy) is an automated quantitative faecal immunochemical test. Faecal samples are collected on probes, which are immersed immediately into solution within the specimen collection device. This ensures sample stability (14 days at 2–8 °C or 7 days at 15–30 °C). The devices are then placed into an automated analyser. A latex agglutination assay is used which is detected via turbidimetry. 22 The FOB Gold kit has CE (Conformité Européene)-marked applications for a range of clinical chemistry analysers, including those supplied by Roche, Siemens, Beckman Coulter and Abbott. The test has a measuring range of 10 ng/ml to the highest calibrator concentration used, and the instructions for use state that laboratories should establish their own population specific cut-off points. Test throughput is dependent on the analyser that is used to process samples.
RIDASCREEN haemoglobin/haptoglobin complex
The RIDASCREEN Hb test (R-Biopharm, Darmstadt, Germany) is a quantitative human Hb/Hp complex immunochemical test. Detection alone is automated. Samples are collected and kept in chilled storage media. Before analysis, the samples are diluted with extraction buffer and mixed. This can be done manually or using the DSX (DSX®, DYNEX Technologies, Chantilly, VA, USA) automated enzyme-linked immunosorbent assay system. The test is run on a 96-well microtitre plate, which is pre-coated with polyclonal antibodies for human haptoglobin (Hp). The sample solution is applied, followed by a wash step and then application of monoclonal antibody for anti-Hb, which is conjugated to peroxidase. In the presence of a Hb/Hp complex, a sandwich complex forms between the polyclonal and monoclonal antibodies. After further washes, a substrate is added, which reacts with the peroxidase, creating a colour change that can be detected by a plate reader. The values produced by the plate reader are interpreted with the RIDA-SOFT Win.net software (R-Biopharm). The company recommends a cut-off value of > 2 µg/g to determine a positive sample. The test has a limit of detection of 0.42 µg/g. The company suggests that the determination of the Hb/Hp complexes has a diagnostic advantage: as the Hb/Hp complex is resistant to decomposition by acids or proteolytic enzymes, it will maintain in the faeces after long periods in the intestine. Thus, blood admixtures from larger intestinal polyps and colon carcinomas that are located higher up in the intestine can also be recorded with high sensitivity. 23 However, discussion with clinical experts at the scoping stage of this assessment has suggested that this method may also result in an increased number of FPs.
Potential advantages of faecal immunochemical testing
It has been suggested that faecal immunochemical testing may offer improved accuracy compared with guaiac faecal occult blood testing, particularly in relation to the rule-out of CRC. Although most studies do not provide evidence about the performance of the test in symptomatic populations, the idea that faecal immunochemical testing may be associated with improved diagnostic performance relative to guaiac faecal occult blood testing is supported by data from systematic reviews of studies that have been conducted in screening populations. 24,25 A meta-analysis25 of 18 studies demonstrated that faecal immunochemical testing (OC-Sensor) had a higher sensitivity (87% vs. 47%) with similar specificity (93% vs. 92%) to guaiac faecal occult blood testing (Hemoccult® test, Beckman Coulter Inc., Brea, CA, USA) for screening for CRC. More recent studies comparing faecal immunochemical testing to guaiac faecal occult blood testing in screening populations have also reported increased sensitivity of faecal immunochemical testing for the detection of CRC of between 31.7% and 61.5%, relative to guaiac faecal occult blood testing, with no change in associated specificity. 26–28 A recent study in symptomatic and asymptomatic patients scheduled for diagnostic colonoscopy reported a smaller difference in sensitivity (14.7%). 29 The results of these studies indicate that faecal immunochemical testing may be associated with a decrease in the number of FN results and potentially missed CRC, relative to guaiac faecal occult blood testing, but not a reduction in FP results (inappropriate referrals).
This assessment systematically reviews the evidence about the performance of faecal immunochemical testing as a triage test for people presenting in primary care settings, with lower abdominal symptoms, who are at low risk for CRC according to the criteria defined in NG12,1 and for whom a referral for secondary care investigation for possible CRC is being considered. We have preferentially sought direct comparisons of faecal immunochemical testing and guaiac faecal occult blood testing, to inform comparative cost-effectiveness modelling; however, our assessment also included studies of the diagnostic accuracy of quantitative FIT assays alone (no comparison with gFOBT or other FIT). Where available, data were collected on the use of different faecal Hb concentration cut-off points and/or multiple sampling strategies in order to determine the best way to operationalise FIT use.
A meta-analysis of studies comparing faecal immunochemical testing and guaiac faecal occult blood testing reported that faecal immunochemical testing was associated with a small increase in participation in asymptomatic population-based screening (relative risk 1.16; 95% CI 1.03 to 1.30). 30 Initial reports from the NHS Bowel Cancer Screening Programme in England pilot of faecal immunochemical testing also indicate that faecal immunochemical testing may be associated with increased uptake compared with guaiac faecal occult blood testing (63.9% vs. 54.4% in 60-year-olds who were invited for screening for the first time). 31 We are not aware of any similar studies on testing uptake (or compliance) in symptomatic populations, and the extent to which the acceptability of FIT sample collection would be an issue for people with symptoms is unclear. In order to inform this question, we have collected all of the available data on the return rates for FIT sample collection devices that were issued to participants in the studies that were included in our systematic review.
Comparator
The comparators for this technology are gFOBT1 and no faecal occult blood triage testing.
Care pathway
Testing for occult blood in faeces in patients presenting to primary care settings
The NHS Bowel Cancer Screening Programme in England offers screening every 2 years to all men and women aged between 60 and 74 years. People aged > 74 years can request a screening kit by contacting the Programme. Screening is currently based on guaiac faecal occult blood testing, but FITs have been recommended by the UK Screening Committee for this purpose and a pilot evaluation has already been completed. FITs are currently recommended as the best non-invasive screening modality in all national and international recommendations. 32
According to NG12,1 patients should be referred for an appointment within 2 weeks if they have suspected CRC, defined as:
-
aged ≥ 40 years with unexplained weight loss and abdominal pain, or
-
aged ≥ 50 years with unexplained rectal bleeding, or
-
aged ≥ 60 years with iron-deficiency anaemia or changes in their bowel habit
-
tests showing occult blood in their faeces
-
having a rectal or abdominal mass
-
adults aged < 50 years with rectal bleeding and abdominal pain or change in bowel habit or weight loss or iron-deficiency anaemia.
According to NG12,1 testing for occult blood in faeces should be offered to adult patients who present with initial symptoms without rectal bleeding who are:
-
aged ≥ 50 years with unexplained abdominal pain or weight loss
-
aged < 60 years with changes in their bowel habit or iron-deficiency anaemia
-
aged ≥ 60 years and having anaemia, even in the absence of iron deficiency.
Further testing following a positive test result for occult blood in faeces
Following a positive test result for occult blood in faeces, people in England are usually offered a colonoscopy within 2 weeks of referral to establish a diagnosis.
The 2011 NICE clinical guideline (CG131)33 states that patients should be advised that one or more investigations may be necessary to confirm or exclude a diagnosis of CRC. Colonoscopy is offered to patients without significant comorbidity to confirm a diagnosis of CRC; if a suspicious lesion is detected then a biopsy should be performed (unless contraindicated). For people with comorbidities, computed tomography colonography (CTC) can be offered as an alternative to colonoscopy.
The Scottish Intercollegiate Guidelines Network (SIGN) 201134 guidance for CRC (updated in 2016) states that patients aged > 40 years who present with new-onset, persistent or recurrent rectal bleeding should be referred for investigation. Review of the patient by a regional clinical genetics service is recommended for accurate risk assessment if family history of CRC is the principal indication for referral for investigation. GPs should perform an abdominal and rectal examination on all patients with symptoms that are indicative of CRC. A positive finding should expedite referral, but a negative rectal examination should not rule out the need to refer. All symptomatic patients should have a full blood count; in cases of anaemia, the presence of iron deficiency should be determined. When CRC is suspected clinically, the whole of the large bowel should be examined:
-
Colonoscopy is recommended as a very sensitive method of diagnosing CRC, enabling biopsy and polypectomy.
-
CTC can be used as a sensitive and safe alternative to colonoscopy.
Guidelines from clinical professional bodies follow a similar pattern: the Royal College of Radiologists recommends that symptomatic patients with suspected CRC should receive evaluation/diagnosis by imaging studies (colonoscopy, CTC or barium enema);35 the Association of Coloproctology of Great Britain and Ireland recommends that patients with higher-risk symptoms should be fast-tracked in special clinics or given urgent appointments in routine clinics. Patients so referred should be investigated with sigmoidoscopy (flexible or rigid) plus a high-quality, double-contrast barium enema, or colonoscopy or CTC. 36
Treatment of colorectal cancer
Following diagnosis and staging, CRC may be treated with surgery, chemotherapy and radiotherapy, or, in some cases, with biological agents such as cetuximab. Treatment is dependent on the stage of the cancer and is described in more detail in CG131. 33
Chapter 3 Assessment of clinical effectiveness
A systematic review was conducted to summarise the evidence on the clinical effectiveness of faecal immunochemical testing as a triage step in the investigation of people, presenting in primary care, with lower abdominal symptoms, who are at low risk for CRC according to the criteria defined in NG121 and for whom a referral for secondary care investigation for possible CRC is being considered. Systematic review methods followed the principles outlined in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care37 and the NICE Diagnostic Assessment Programme manual. 38
Systematic review methods
Search strategy
Search strategies were based on intervention (FIT assays) and target condition (CRC), as recommended in the CRD guidance for undertaking reviews in health care37 and the Cochrane Handbook for Diagnostic Test Accuracy Reviews. 39
Candidate search terms were identified from target references, browsing database thesauri [e.g. MEDLINE medical subject heading (MeSH) and EMBASE Emtree] and from existing reviews that were identified during initial scoping searches. Strategy development involved an iterative approach, testing candidate text and indexing terms across a sample of bibliographic databases, aiming to reach a satisfactory balance of sensitivity and specificity. Search strategies were developed specifically for each database and the keywords associated with faecal immunochemical tests for occult blood were adapted according to the configuration of each database.
No restrictions on language, publication status or date of publication were applied. Searches took into account generic and other product names for the intervention. The main EMBASE strategy for each search was independently peer reviewed by a second Information Specialist, using the Canadian Agency for Drugs and Technologies in Health (CADTH) Peer Review checklist. 40 Identified references were downloaded in EndNote X6 software (Thomson Reuters, CA, USA) for further assessment and handling. References in retrieved articles were checked for additional studies. The final list of included papers was also checked on PubMed for retractions, errata and related citations. 41–44
The following databases were searched for relevant studies from database inception date to the most recent date available:
-
MEDLINE (via Ovid): 1946 to March Week 3 2016
-
MEDLINE In-Process & Other Non-Indexed Citations, and Daily Update (via Ovid): to 29 March 2016
-
MEDLINE Epub Ahead of Print (via Ovid): 20 June 2016
-
EMBASE (via Ovid): 1974 to 29 March 2016
-
The Cochrane Library:
-
Cochrane Database of Systematic Reviews (CDSR) (via the internet): to Issue 3 of 12, March 2016
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via the internet): to Issue 2 of 12, February 2016
-
Database of Abstracts of Reviews of Effects (DARE) (via the internet): to Issue 2 of 4, April 2015
-
Health Technology Assessment (HTA) database (via the internet): to Issue 1 of 4, January 2016
-
NHS Economic Evaluation Database (NHS EED) (via the internet): to Issue 2 of 4, April 2015
-
-
International Network of Agencies for Health Technology Assessment (INAHTA) Publications (via the internet): to 30 March 2016: www.inahta.org/publications/
-
National Institute for Health Research (NIHR) HTA programme (via the internet): to 30 March 2016
-
Aggressive Research Intelligence Facility (ARIF) database (via the internet): to 30 March 2016: www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/index.aspx
-
PROSPERO (International Prospective Register of Systematic Reviews) (via the internet): to 30 March 2016: www.crd.york.ac.uk/prospero/.
Completed and ongoing trials were identified by searches of the following resources:
-
National Institutes of Health ClinicalTrials.gov: to 8 March 2016
-
EU Clinical Trials Register: to 8 March 2016
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP): to 8 March 2016
Electronic searches were undertaken for abstracts and poster presentations from the following conferences:
-
American Gastroenterological Association – Digestive Disease Week (DDW): 2011–15
-
Annual Meeting of the American Association for Clinical Chemistry and Laboratory Medicine (AACC): 2011–15
-
British Society of Gastroenterology (BSG) Annual Meeting: 2011–15
-
EuroMedLab: IFCC-EFLM (International Federation of Clinical Chemistry and Laboratory Medicine/European Federation of Clinical Chemistry and Laboratory Medicine) European Congress of Clinical Chemistry and Laboratory Medicine: 2011–15
-
United European Gastroenterology Week (UEGW): 2011–15.
Full search strategies are presented in Appendix 1.
Specialist members of the Assessment SubGroup and one additional clinical expert (CF) were contacted to seek additional studies.
Inclusion and exclusion criteria
Inclusion criteria for each of the clinical effectiveness questions are summarised in Table 2. Studies that fulfilled these criteria were eligible for inclusion in the review.
| Question | What is the accuracy of different quantitative FIT assays, when the target condition is CRC determined by colonoscopy (the reference standard method)? | What is the clinical effectiveness of faecal immunochemical testing, compared with guaiac faecal occult blood testing or no triage, for achieving appropriate referral for further investigation within the 2-week suspected cancer referral target? |
|---|---|---|
| Participants | People presenting with lower abdominal symptoms, who are being investigated for possible CRCa | |
| Setting | Primary care | |
| Interventions (index test) | Quantitative FIT assays listed in Table 1 | |
| Comparators | Any other FIT method (including different faecal Hb cut-off points or different numbers of samples) or gFOBT or no comparator | gFOBT or no triage |
| Reference standard | Colonoscopy | NA |
| Outcomes | Diagnostic accuracy (the numbers of TP, FN, FP and TN test results), where the target condition is CRC determined by colonoscopyb | Appropriate referral for secondary care investigations with 2 weeks from presentation (proportion of patients referred for secondary care investigation in whom CRC was confirmed and proportion of patients not referred for secondary care investigation in whom CRC was later diagnosedc), long-term CRC mortality,d any patient acceptability/satisfaction or HRQoL measures |
| Study design | Diagnostic cohort studies | RCTs (CCTs will be considered if no RCTs are identified) |
Inclusion screening and data extraction
Two reviewers (MW and SL) independently screened the titles and abstracts of all of the reports identified by searches and any discrepancies were discussed and resolved by consensus. Full copies of all of the studies that were deemed to be potentially relevant were obtained and the same two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus. Details of the studies excluded at the full-paper screening stage are presented in Appendix 4.
When studies reported insufficient information (e.g. FIT assay not specified or incomplete accuracy data), the authors were contacted to request additional information. The authors of studies that included a mixed population (e.g. screening, surveillance and symptomatic patients) were contacted to request subgroup data for symptomatic patients. Authors were initially contacted by e-mail, which was followed up with a reminder e-mail after 2 weeks and, subsequently (where possible), by personal contact from a clinical specialist member of the Assessment SubGroup (RL).
Studies cited in the materials provided by the manufacturers of FIT assays were first checked against the project reference database, in Endnote; any studies that were not already identified by our searches were screened for inclusion following the process described above.
Data were extracted on the following: study details, inclusion and exclusion criteria, participant characteristics (demographic characteristics, presenting symptoms, other CRC risk factors), target condition {CRC, advanced neoplasia [high-risk adenoma (HRA) or CRC], other significant bowel disease outcomes (as reported)}, details of the FIT (manufacturer, analyser used, definition of a cut-off point, sampling procedure, detection method, etc.), details of any comparator test(s) (manufacturer, antibody, limit of quantitation, definition of cut-off point, sampling procedure, detection method, etc.), details of the reference standard, definitions of the target conditions, test performance outcome measures [numbers of true-positive (TP), FP, FN and true-negative (TN) test results] and proportion of study participants who returned a FIT sample (extracted as an indicator of acceptability). Data were extracted by one reviewer, using a piloted, standard data extraction form and checked by a second reviewer (MW and SL); any disagreements were resolved by consensus. Full data extraction tables are provided in Appendix 2.
Quality assessment
The methodological quality of included studies was assessed using QUADAS-2. 45
Note: Protocol change Studies that reported results for risk prediction scores that included faecal immunochemical testing, as well as test accuracy data for faecal immunochemical testing, were assessed using PROBAST (Prediction model study Risk Of Bias Assessment Tool)46 in addition to QUADAS-2.
Quality assessment was undertaken by one reviewer and checked by a second reviewer (MW and SL); any disagreements were resolved by consensus or discussion with a third reviewer.
The results of the quality assessments are summarised and presented in tables and graphs in the results of the systematic review, and are presented in full, by study, in Appendix 3.
Methods of analysis/synthesis
Sensitivity and specificity were calculated for each set of 2 × 2 data. The bivariate/hierarchical summary receiver operating characteristic (HSROC) model was used to estimate summary sensitivity and specificity with 95% CIs and prediction regions around the summary points, and to derive HSROC curves for meta-analyses involving four or more studies. 47–49 This approach allows for between-study heterogeneity in sensitivity and specificity, and for the trade-off (negative correlation) between sensitivity and specificity that is commonly seen in diagnostic meta-analyses. For meta-analyses with fewer than four studies we estimated separate pooled estimates of sensitivity and specificity, using random-effects logistic regression. 50 Heterogeneity was assessed visually using summary receiver operating characteristic (SROC) plots and statistically using the variance of logit (sensitivity) and logit (specificity), for which ‘logit’ indicates the logistic function: the smaller these values, the less heterogeneity between studies. Analyses were performed in Stata 10 (StataCorp LP, College Station, TX, USA), using the metandi command. For analyses that would not run in Stata we used Meta-DiSc (version 1.4, free source). 51
Studies were grouped by FIT assay type, target condition and threshold. We compared the accuracy of different FIT assays by tabulating summary estimates from analyses for commonly used thresholds. Stratified results tables receiver operating characteristic (ROC) space plots were used to illustrate the variation of test performance by threshold.
We used SROC plots to display summary estimates from analyses that included a minimum of four data points.
Results of the assessment of clinical effectiveness assessment
After initial screening of titles and abstracts, 113 papers were considered to be potentially relevant and ordered for full-paper screening; of these, 21 papers13,52–71 were included in the review.
Additionally, four presentations72–75 were obtained through contact with a clinical expert (CF). One unpublished manuscript was provided, through NICE, by the manufacturer of FOB Gold [e-mail from Philippa Pinn, Sysmex UK Ltd, via Rebecca Albrow, NICE, to Marie Westwood, Kleijnen Systematic Reviews (KSR) Ltd, 21 June 2016, personal communication]. Additional unpublished work was provided by the clinical expert (CF) (e-mail from Callum Fraser, NHS Tayside, to Marie Westwood, KSR Ltd, 10 July 2016, personal communication, ahead of publication). All potentially relevant studies cited in other documents supplied by the test manufacturers had already been identified through other sources. Figure 1 shows the flow of studies through the review process, and Appendix 4 provides details, with reasons for exclusions, of all of the publications that were excluded at the full-paper screening stage. In total there were 10 included studies derived from 27 articles.
FIGURE 1.
Flow of studies through the review process. AGA DDW, American Gastroenterological Association Digestive Disease week; UEGW, United European Gastroenterology Federation Week.

Seventy-four articles were excluded after full-text screening. Twelve articles76–86 could not be obtained and one further article87 was published in Russian with no English abstract.
We contacted the authors of publications that reported data from studies with mixed populations (symptomatic, screening and surveillance patients) to request separate data for the symptomatic subgroup; when no additional data were obtained, these studies were excluded (see Appendix 4). The authors of two studies56,58 provided additional test accuracy data, which were included in our review.
Overview of included studies
Details of the 10 included studies and their associated references are provided in Table 3. Additional data were supplied by the authors of two studies. 56,58 In the case of Terhaar sive Droste et al.,58 the authors provided overall data for symptomatic study from the master database, which holds data for all of their publications (e-mail from Sietze van Turenhout, University Medical Center, Amsterdam, to Marie Westwood, KSR Ltd, 12 June 2016, personal communication). The results section of this report cites studies using the primary publication and, where this is different, the publication (shown in bold text in Table 3) in which the referenced data were reported.
| Details | Country | n | Reference standard |
|---|---|---|---|
| HM-JACKarc | |||
| Thomas 2016 75 | England | 450 | CT/colonoscopy |
| Godber 2016 56 | Scotland | 484 | Colonoscopy |
| Macdonald 201571 | |||
| Godber 201465 | |||
| Auge 2016 57 | Spain | 208 | Colonoscopy |
| Auge 201466 | |||
| Auge 201572 | |||
| FOB Gold system | |||
| Krivec 2011 54 | Slovenia | NR | Colonoscopy |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| OC-Sensor | |||
| McDonald 2012 13 | Scotland | 280 | Colonoscopy and flexible sigmoidoscopy |
| Mowat 2015 52 | Scotland | 755 | Colonoscopy |
| Steele 201474 | |||
| Rodríguez-Alonso 2015 53 | Spain | 1003 | Colonoscopy |
| Cubiella 2014 (COLONPREDICT) 55 | Spain | 787 | Colonoscopy |
| Diaz Ondina 201461 | |||
| Cubiella 201573 | |||
| Unpublished dataa | |||
| Terhaar sive Droste 2011 58 | The Netherlands | 2058 | Colonoscopy |
| Oort 201163 | |||
| van Turenhout 201069 | |||
| van Turenhout 201459 | |||
| van Turenhout 201262 | |||
| van Turenhout 201168 | |||
| Oort 201064 | |||
| van Turenhout 201260 | |||
| Larbi 201267 | |||
| van Turenhout 201070 | |||
Five studies2,13,53,55,58 reported accuracy data for the OC-Sensor assay; one study52 used the IO analyser (Eiken Chemical Co.), one study13 used the OC-Sensor Diana automated immunoturbidimetric analyser (Eiken Chemical Co.), two studies53,58 used the MICRO desktop analyser (Eiken Chemical Co.) and one study55 did not report the analyser used. Three studies56,57,75 reported accuracy data for the HM-JACKarc automated system (Kyowa Medex, Tokyo, Japan). The remaining two studies reported accuracy data for the FOB Gold assay; one used the Roche Modular P/917 analyser (Roche Diagnostics Ltd, West Sussex, UK),54 and the other used the SENTiFIT 270 analyser (Sentinel Diagnostics, Milan, Italy) (Philippa Pinn, personal communication). There were no studies, using the RIDASCREEN Hb or the RIDASCREEN Hb/Hp complex assays, which met the inclusion criteria for this assessment. None of the included studies reported data comparing different FIT assays, or comparing one or more FIT assays with a gFOBT method. All of the studies included in our systematic review were diagnostic cohort studies that reported data on the diagnostic accuracy of faecal immunochemical testing for which the target condition was CRC or advanced neoplasia (defined as CRC or HRA). Five studies13,52,56,58,75 reported additional accuracy data for various non-malignant and composite target conditions.
Six of the diagnostic accuracy studies13,52,53,55,56,75 included in our systematic review also reported uptake rates for participants who were invited to provide a sample for faecal immunochemical testing.
No randomised controlled trials (RCTs) or controlled clinical trials (CCTs) were identified; no studies provided data on patient-relevant outcomes following faecal immunochemical testing compared with guaiac faecal occult blood testing or no faecal occult blood testing.
All 10 of the included studies2,13,52–58,75 were conducted in Europe: one in England,75 three in Scotland,13,52,56 three published studies53,55,57 and one unpublished study (Philippa Pinn, personal communication) in Spain, and one each in the Netherlands58 and Slovenia. 54 Three studies52,53,55 were publicly funded, five studies13,55–57,75 reported receiving some funding from manufacturers (including supply of test kits, reagents and analysers), one study54 did not report details of funding and the unpublished study was conducted at the request of the test manufacturer.
Full details of the characteristics of study participants, study inclusion and exclusion criteria, and FIT assay used and reference standard are reported in the data extraction tables presented in Appendix 2 (see Tables 65 and 66).
Study quality
All studies included in this systematic review were diagnostic cohort studies. The methodological quality of these studies was assessed using the QUADAS-2 tool45 (summarised in Table 4 and Figure 2). One of these studies53 and an additional report73 and unpublished paper (Callum Fraser, personal communication) linked to a second study55 reported the development and validation of risk prediction scores that included faecal immunochemical testing, in addition to test accuracy results. These studies were assessed using PROBAST (Table 5), as well as QUADAS-2. The full QUADAS-2 assessments for each study are provided in Appendix 3a and PROBAST assessment results are provided in Appendix 3b.
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient | Index test | Reference standard | |
| Auge 201657 | + | + | + | + | – | + | + |
| Cubiella 2014 (COLONPREDICT)55 | + | + | + | + | – | + | + |
| Godber 201656 | + | + | ? | + | – | + | + |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Krivec 201154 | ? | – | ? | ? | – | + | – |
| McDonald 201213 | + | + | ? | – | – | + | ? |
| Mowat 201552 | + | + | + | – | – | + | + |
| Rodríguez-Alonso 201553 | ? | + | + | + | – | + | + |
| Terhaar sive Droste 201158 | + | + | + | ? | – | + | + |
| Thomas 201675 | ? | + | ? | – | – | + | + |
FIGURE 2.
Summary of QUADAS-2 results for studies of FIT assays.

| Study | Risk of bias | Applicability concerns | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant selection | Predictors | Outcome | Sample size and participant flow | Analysis | Overall judgement | Participant selection | Predictors | Outcome | Overall judgement | |||||||||
| Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | Development | Validation | |||
| aCubiella 2015 (COLONPREDICT)73 | + | + | – | – | + | – | ? | ? | ? | ? | – | – | – | – | – | + | + | – |
| Rodríguez-Alonso 201553 | + | + | ? | ? | + | + | ? | ? | – | – | – | – | – | – | – | + | + | – |
Two studies54,75 were reported only as conference abstracts, with limited descriptions of methods.
Two studies55,57 were rated as ‘low’ risk of bias for all domains. (Confidential information has been removed.) The main potential sources of bias, across the included studies, concerned flow and timing, and application of the index test. Three studies13,52,75 were rated as ‘high’ risk of bias on the flow and timing domain because some patients who returned a sample for faecal immunochemical testing or who agreed to participate in the study were subsequently excluded from the analysis: Mowat et al. 201552 excluded 11% of participants who returned a FIT sample because they were not subsequently referred to secondary care or because the referral was cancelled; Thomas et al. 201675 excluded 12.5% of participants who returned a FIT sample (no reasons for exclusion were reported); McDonald et al. 201213 excluded 41% of people who originally agreed to participate in the study (38% did not return a FIT sample before endoscopy and 3% completed faecal immunochemical testing but not endoscopy). (Confidential information has been removed.)
All of the included studies were rated as having ‘high’ concerns about applicability with respect to participants. This was because no study reported data that were specific to the population that was defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms and who are at low risk for CRC according to the criteria defined in NG121). Only one study52 was conducted in a primary care setting, reporting that faecal immunochemical testing was ordered by GPs at the point of referral to secondary care. All of the studies included some participants who had symptoms that may be considered to be associated with a higher probability of CRC, and which are components of the criteria for 2-week referral as defined in NG121 (e.g. rectal bleeding). In addition, although all of the included studies were conducted in Europe, only four studies13,52,56,75 were conducted in the UK (one in England75 and three in Scotland13,52,56). Given that population studies have shown variation in faecal Hb concentrations, and hence potential variation in optimal thresholds for faecal immunochemical testing across different geographic location,88,89 this may limit the applicability of our findings to UK settings.
Diagnostic performance of the OC-Sensor faecal immunochemical test assay
Details of OC-Sensor studies
Five diagnostic cohort studies,13,52,53,55,58 reported in 17 publications,13,52,53,55,58–64,67–70,73,74 provided data on the diagnostic performance of the OC-Sensor FIT assay. All five studies13,52,53,55,58 reported accuracy data, for which CRC was the specified target condition,13,52,53,55,58 and one of these studies55 reported further information about the sensitivity of faecal immunochemical testing for differentiating CRC stage and location. The prevalence of CRC, diagnosed at colonoscopy, was 2.1% in the McDonald et al. study,13 3.0% in the Rodríguez-Alonso et al. study,53 3.7% in the Mowat et al. study,52 5.4% in the symptomatic subgroup from Terhaar sive Droste et al. study58 and 12.3% in the Cubiella et al. study. 55 Four studies13,52,53,55 also reported data for the composite target condition of advanced neoplasia (CRC or HRA); where a definition was provided, HRA was defined as adenoma ≥ 10 mm in diameter, with villous architecture or high-grade dysplasia. 53,55 Three studies reported additional accuracy data various non-malignant and composite target conditions. 13,52,58
No study reported data that were specific to the population that was defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms and who are at low risk for CRC according to the criteria defined in NG121). Only one study52 was conducted in a primary care setting, reporting that faecal immunochemical testing was ordered by GPs at the point of referral to secondary care. In another study,53 faecal immunochemical testing was ordered either by GPs at the point of referral or at the initial secondary care appointment. Two studies reported that faecal immunochemical testing was requested after referral to secondary care and before colonoscopy13,55 but did not report whether the test was requested by GPs or secondary care clinicians. The remaining study stated that patients already scheduled for colonoscopy were asked to provide a sample for faecal immunochemical testing before bowel preparation. 58 Four52,53,55,58 of the five OC-Sensor studies13,52,53,55,58 explicitly reported that they included only symptomatic participants and provided details of the presenting symptoms, and the remaining study13 included patients referred for colonoscopy (no screening colonoscopies included). However, all of the studies included some participants who had symptoms that may be considered to be associated with a higher probability of CRC and that are components of the criteria for 2-week referral as defined in NG121 (e.g. rectal bleeding). Presenting symptoms included altered bowel habit, rectal bleeding, diarrhoea, constipation, abdominal pain, bloating, unspecified anaemia, iron-deficiency anaemia, weight loss and palpable mass. Where reported, the median age of study participants was 64 years,52 67 years55 and 60 years,58 and the overall age range was 16–91 years. There were no data linking presenting symptoms to age or sex. Full details of participant characteristics are provided in Appendix 2, Table 65.
All five studies13,52,53,55,58 reported data on the accuracy of OC-Sensor faecal immunochemical testing using a single faecal sample. No data were available on the effects of multiple sampling on test performance in symptomatic patients; one additional publication,63 associated with Terhaar sive Droste et al. ,58 reported no difference between various double sampling strategies and single sampling. However, this study was conducted in a mixed population and additional subgroup data provided by the authors were only for single sampling.
Four studies13,52,53,55 reported information about uptake rates in participants invited to provide a sample for faecal immunochemical testing.
Accuracy of OC-Sensor for the detection of colorectal cancer
Five studies13,52,53,55,58 reported data on the accuracy of OC-Sensor FIT using a single faecal sample, and thresholds ranging from any detectable Hb (the limit of detection for the assay is 452,53 to 4058 µg Hb/g faeces). Full test performance results for all thresholds evaluated in individual studies and summary estimates, where these were calculated, are provided in Table 6. The variation in test performance characteristics according to the threshold used is illustrated in a ROC space plot (Figure 3). Although test performance did not vary greatly with threshold, as might be expected, sensitivity estimates generally decreased and specificity estimates increased with increasing threshold. Specificity was low (< 50%) in both of the studies that used any detectable Hb to define a positive test. 52,53
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Any detectable Hb | |||||||||
| Mowat 201552 | 0a | 28 | 0 | 409 | 313 | 750 | Reported | 100 (87.7 to 100)b | 43.4 (39.7 to 47.1)b |
| Rodríguez-Alonso 201553 | 0a | 30 | 0 | 552 | 421 | 1003 | Calculated | 100 (88.4 to100) | 43.3 (40.1 to 46.4) |
| Summary estimate | 100 (93.8 to 100) | 43.3 (40.9 to 45.7) | |||||||
| 10 µg Hb/g faeces or equivalent | |||||||||
| McDonald 201213 | ≥ 10c | 6 | 0 | 17 | 257 | 280 | Calculated | 100 (54.1 to 100) | 93.8 (90.3 to 96.3) |
| Mowat 201552 | ≥ 10 | 25 | 3 | 151 | 571 | 750 | Reported | 89.3 (71.8 to 97.7)b | 79.1 (75.9 to 82)b |
| Rodríguez-Alonso 201553 | ≥ 10 | 29 | 1 | 196 | 777 | 1003 | Calculated | 96.7 (82.8 to 99.9) | 79.9 (77.2 to 82.3) |
| dTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Summary estimate | 92.1 (86.9 to 95.3) | 85.8 (78.3 to 91.0) | |||||||
| 15 µg Hb/g faeces or equivalent | |||||||||
| Rodríguez-Alonso 201553 | ≥ 15 | 29 | 1 | 164 | 809 | 1003 | Calculated | 96.7 (82.8 to 99.9) | 83.1 (80.6 to 85.4) |
| dTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Summary estimate | 92.3 (86.6 to 96.1) | 86.9 (85.6 to 88.1) | |||||||
| 20 µg Hb/g faeces or equivalent | |||||||||
| Cubiella 2014 (COLONPREDICT)55 | ≥ 20c | 85 | 12 | 156 | 534 | 787 | Calculated | 87.6 (79.0 to 93.2) | 77.4 (74.0 to 80.4) |
| Rodríguez-Alonso 201553 | ≥ 20 | 28 | 2 | 135 | 838 | 1003 | Calculated | 93.3 (77.9 to 99.2) | 86.1 (83.8 to 88.2) |
| dTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Summary estimate | 89.5 (84.9 to 93.1) | 86.6 (85.4 to 87.7) | |||||||
| Other thresholds | |||||||||
| dTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| dTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
FIGURE 3.
(Confidential information has been removed.)
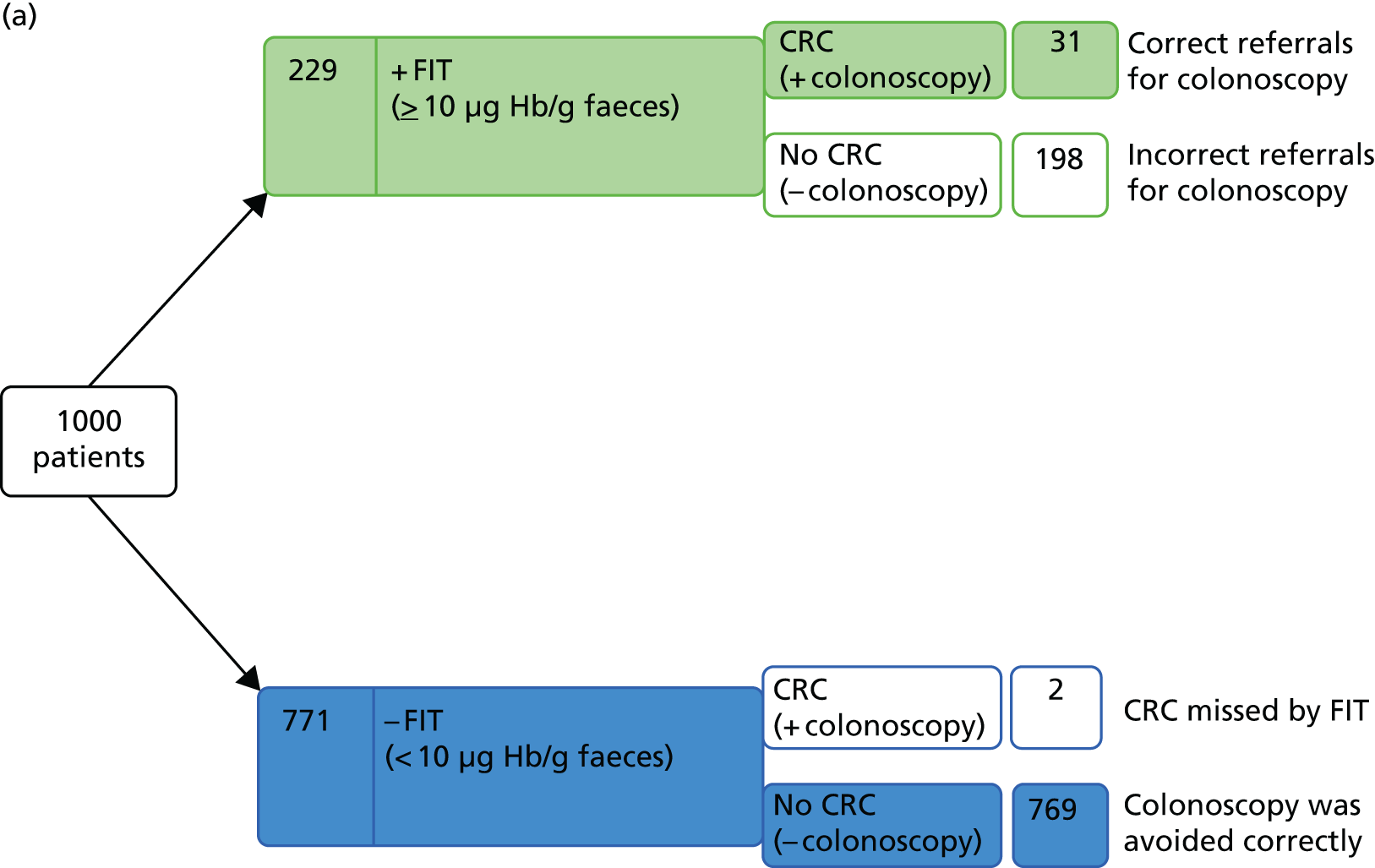
The optimal test performance (maximising both sensitivity and specificity) appeared to occur with thresholds of 10 or 15 µg Hb/g faeces, with most data being available for the 10 µg Hb/g faeces threshold. The summary estimates of sensitivity and specificity, using the 10 µg Hb/g faeces threshold, were 92.1% (95% CI 86.9% to 95.3%) and 85.8% (95% CI 78.3% to 91.0%), respectively, based on data from four studies,13,52,53,58 (see Table 6 and Figure 4). Mowat et al. 52 and Rodríguez-Alonso et al. 53 reported data for both the 10 µg Hb/g faeces threshold and the minimum threshold of any detectable Hb; the prevalence of CRC in these two studies52,53 was 3.3%. Using test performance data from these two studies,52,53 and a CRC prevalence estimate of 3.3%, to consider the outcome of testing for a hypothetical cohort of 1000 patients indicates that two CRCs would be missed using the 10 µg Hb/g faeces threshold and 198 unnecessary colonoscopies would be carried out (assuming that all patients with a positive FIT result receive colonoscopy and all of the colonoscopies that are conducted in patients without CRC are considered unnecessary); CRC would be correctly ruled out by faecal immunochemical testing, avoiding colonoscopy, in 769 people (Figure 5a). Alternatively, using a very low threshold (any detectable Hb) would result in no CRCs being missed, but would increase the number of ‘unnecessary’ colonoscopies to 548 and reduce the number of people in whom CRC would be correctly ruled out to 419 (Figure 5b). Please see subsequent sections for information on other significant bowel pathologies that may be detected by faecal immunochemical testing and hence may form part of the FP or ‘unnecessary colonoscopy’ population.
FIGURE 4.
(Confidential information has been removed.)
FIGURE 5.
Testing outcomes for a hypothetical cohort of 1000 patients using OC-Sensor at (a) the 10 µg Hb/g faeces threshold; and (b) any detectable Hb, for the target condition CRC.
Limited data from Cubiella et al. 55 indicated that the sensitivity of OC-Sensor (using a threshold of 20 µg Hb/g faeces) is higher for more advanced stages of CRC [American Joint Committee on Cancer (AJCC) stages II–IV] and is also higher for tumours of the distal colon than for tumours of the proximal colon or rectum (Table 7).
| Study | Target condition | Subgroup | Threshold (µg Hb/g faeces) | TP | FN | Total n | Sensitivity % (95% CI) |
|---|---|---|---|---|---|---|---|
| CRC location | |||||||
| Cubiella 2014 (COLONPREDICT)55 | Rectum | Participants with CRC | ≥ 20a | 26 | 4 | 30 | 86.7 (69.3 to 96.2) |
| Distal colon | Participants with CRC | ≥ 20a | 40 | 4 | 44 | 90.9 (78.3 to 97.5) | |
| Proximal colon | Participants with CRC | ≥ 20a | 19 | 4 | 23 | 82.6 (61.2 to 95.0) | |
| CRC stage (AJCC classification) | |||||||
| Cubiella 2014 (COLONPREDICT)55 | 0 | Participants with CRC | ≥ 20a | 4 | 1 | 5 | 80 (28.4 to 99.5) |
| I | Participants with CRC | ≥ 20a | 12 | 3 | 15 | 80 (51.9 to 95.7) | |
| II | Participants with CRC | ≥ 20a | 21 | 2 | 23 | 91.3 (72.0 to 98.9) | |
| III | Participants with CRC | ≥ 20a | 35 | 5 | 40 | 87.5 (73.2 to 95.8) | |
| IV | Participants with CRC | ≥ 20a | 12 | 1 | 13 | 92.3 (64.0 to 99.8) | |
Accuracy of OC-Sensor for the detection of advanced neoplasia (colorectal cancer or high-risk adenoma)
Four of the studies described in the previous section also reported data on the accuracy of OC-Sensor FIT using a single faecal sample, where the target condition was expanded to include CRC or HRA. 13,52,53,55 The thresholds assessed ranged from any detectable Hb (the limit of detection for the assay is 4 µg Hb/g faeces)52,53 to 20 µg Hb/g faeces. 53 Full test performance results for all thresholds evaluated in individual studies and summary estimates, where they were calculated, are provided in Table 8. The variation in test performance characteristics according to the threshold used is illustrated in a ROC space plot (Figure 6).
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Any detectable Hb | |||||||||
| Mowat 201552 | 0a | 61 | 7 | 376 | 306 | 750 | Calculated | 89.7 (79.9 to 95.8)b | 44.9 (41.1 to 48.7)b |
| Rodríguez-Alonso 201553 | 0a | 108 | 25 | 474 | 396 | 1003 | 81.2 (73.5 to 87.5) | 45.5 (42.2 to 48.9) | |
| Summary estimate | 84.1 (78.3 to 88.8) | 45.2 (42.7 to 47.7) | |||||||
| 10 µg Hb/g faeces | |||||||||
| McDonald 201213 | ≥ 10c | 17 | 12 | 6 | 245 | 280 | Calculated | 58.6 (38.9 to 76.5) | 97.6 (94.9 to 99.1) |
| Mowat 201552 | ≥ 10 | 45 | 23 | 131 | 551 | 750 | 66.2 (53.7 to 77.2)b | 80.8 (77.6 to 83.7)b | |
| Rodríguez-Alonso 201553 | ≥ 10 | 82 | 51 | 144 | 726 | 1003 | 61.7 (52.8 to 69.9) | 83.4 (80.8 to 85.9) | |
| Summary estimate | 62.6 (56.0 to 68.9) | 84.4 (82.7 to 86.1) | |||||||
| 20 µg Hb/g faeces or equivalent | |||||||||
| Cubiella 2014 (COLONPREDICT)55 | ≥ 20c | 127 | 50 | 114 | 496 | 787 | Calculated | 71.8 (64.4 to 78.1) | 81.3 (77.9 to 84.3) |
| Rodríguez-Alonso 201553 | ≥ 20 | 71 | 62 | 92 | 778 | 1003 | 53.4 (44.5 to 62.1) | 89.4 (87.2 to 91.4) | |
| Summary estimate | 63.9 (58.2 to 69.2) | 86.1 (84.2 to 87.8) | |||||||
| Other thresholds | |||||||||
| Rodríguez-Alonso 201553 | ≥ 15 | 76 | 57 | 117 | 753 | 1003 | Calculated | 57.1 (48.3 to 65.7) | 86.6 (84.1 to 88.7) |
FIGURE 6.
Receiver operating characteristic space plot for the OC-Sensor assay using different thresholds for the target condition of advanced neoplasia (CRC or HRA).

For this expanded target condition, sensitivity estimates were low for all thresholds except any detectable Hb; the summary sensitivity and specificity estimates for this threshold were 84.1% (95% CI 78.3% to 88.8%) and 45.2% (95% CI 42.7% to 47.7%), respectively, based on data from the same two studies52,53 that evaluated this threshold for the detection of CRC.
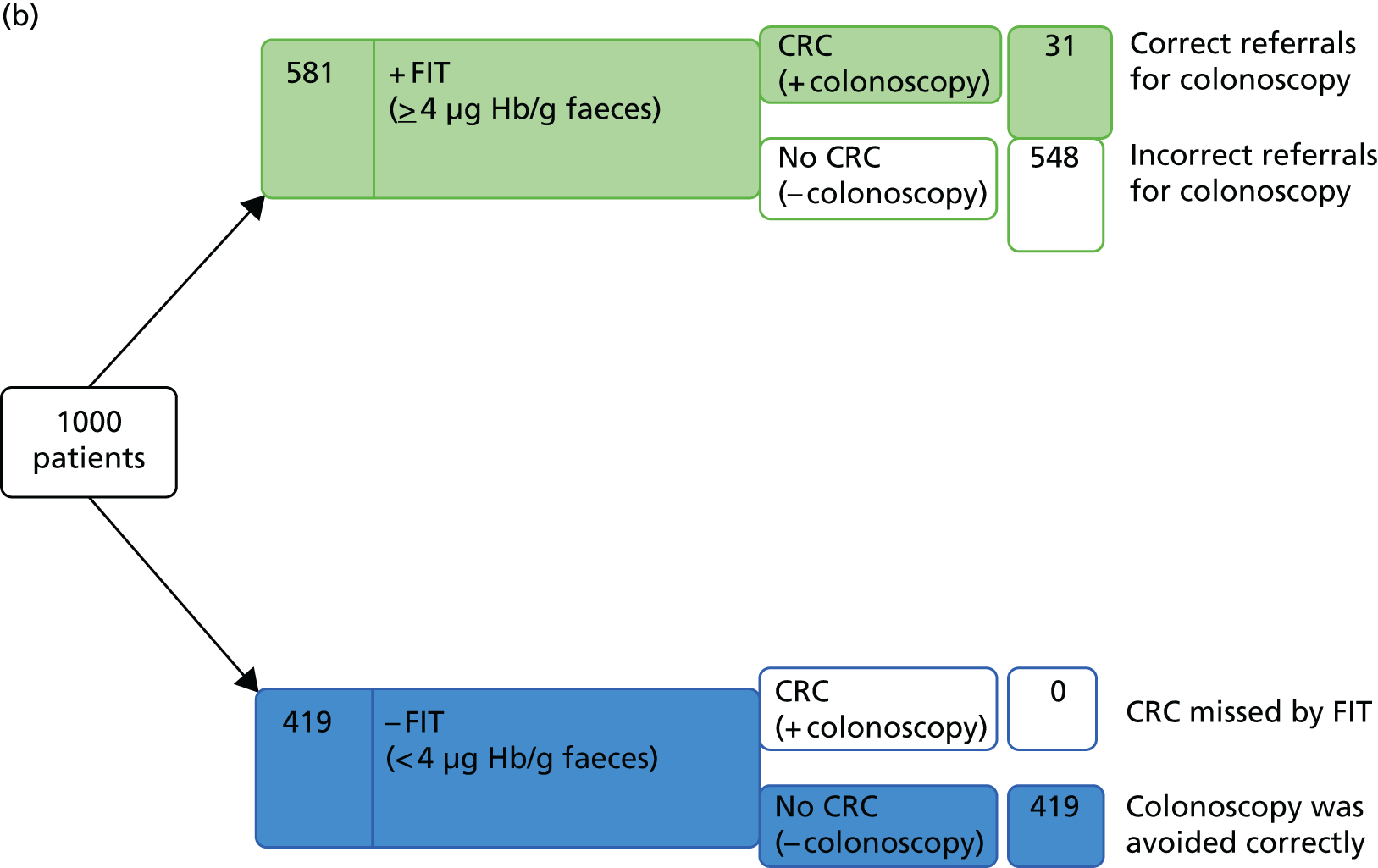
Expanding the target condition from CRC only, to include CRC or HRA, resulted in an increase in prevalence from 3.3% to 11.5%. 52,53 Using test performance data from these two studies,52,53 and an estimate for the prevalence of advanced neoplasia of 11.5%, to consider the outcome of testing for a hypothetical cohort of 1000 patients indicates that 18 advanced neoplasias would be missed, even when using the minimum threshold of any detectable Hb. As data indicate that no CRCs would be missed at this threshold (see previous section), it may be assumed that the missed cases would all be HRAs. Using the any detectable Hb threshold, 485 unnecessary colonoscopies would be carried out (assuming that all patients with a positive FIT result receive colonoscopy and all of the colonoscopies that are conducted in patients without at least HRA are considered unnecessary); CRC and HRA would be correctly ruled out in 401 people (Figure 7a). If the 10 µg Hb/g faeces threshold were applied to the expanded target condition, for hypothetical cohort of 1000 patients, the number of missed cases would increase from 2 to 42 (two CRCs and 40 HRAs); using this threshold, 157 unnecessary colonoscopies would be carried out and CRC and HRA would be correctly ruled out in 729 people (Figure 7b).
FIGURE 7.
Testing outcomes for a hypothetical cohort of 1000 patients using OC-Sensor at (a) any detectable Hb, for the target condition of advanced neoplasia (CRC or HRA); and (b) the 10 µg Hb/g faeces threshold.


Accuracy of OC-Sensor for the detection of other/composite target conditions
Three studies13,52,58 reported accuracy data for target conditions other than CRC or advanced neoplasia (CRC or HRA).
McDonald et al. 13 evaluated the diagnostic performance of OC-Sensor (10 µg Hb/g faeces threshold) for two composite target conditions: one including CRC and all adenomas and the other including CRC and all adenomas plus inflammatory bowel disease (IBD). The sensitivity estimates were low for both composite target conditions, 58.3% (95% CI 44.9% to 70.9%) and 57.0% (95% CI 45.8% to 67.6%), respectively, and the corresponding specificity estimates were high, 98.6% (95% CI 96.1% to 99.7%) and 99.0% (95% CI 96.3% to 99.9%). Mowat et al. 52 reported similar test performance characteristics for the 10 µg Hb/g faeces threshold and a composite target condition that included CRC, HRA or IBD; the reported sensitivity was 68.6% (95% CI 58.7% to 77.5%) and the specificity was 83.6% (95% CI 80.6% to 86.4%). It should be noted that an important consequence of this increased specificity estimate, relative to that reported for the target condition of CRC in the same cohort of patients, is that 45 of the 151 participants (29.8%) who were classified as having FP FIT results for CRC actually had other significant bowel pathologies (HRA or IBD) and may thus have benefited from secondary care investigation. 52 Mowat et al. 52 also reported test performance data for this composite target condition, using the minimum threshold of any detectable Hb. This lowering of the threshold resulted in an increased estimate of sensitivity, 88.2% (95% CI 80.4% to 93.8%), and lower specificity, 46.5% (95% CI 42.6% to 50.4%). 52
Mowat et al. 52 reported separate test performance estimates for the individual target conditions of HRA and IBD. However, these estimates are likely to be of limited clinical relevance, as they appear to have been calculated by classifying any patient who was not in the specified target condition category as disease negative: for HRA estimates, patients with CRC (as well as those with low-risk adenoma IBD or no significant findings) were classified as disease negative; for IBD, patients with CRC or HRA (as well as those with no significant findings) were classified as disease negative. One further study58 calculated test performance estimates for HRA using a range of thresholds from 10 to 40 µg Hb/g faeces; patients with CRC were excluded from these analyses. Full test performance results for all of the thresholds and target conditions that were evaluated in individual studies are provided in Table 9.
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All neoplasia (CRC, HRA or low-risk adenoma) | |||||||||
| McDonald 201213 | ≥ 10a | 35 | 25 | 3 | 217 | 280 | Calculated | 58.3 (44.9 to 70.9) | 98.6 (96.1 to 99.7) |
| HRA | |||||||||
| Mowat 201552 | 0b | 33 | 7 | 404 | 306 | 750c | Reported | 82.5 (67.2 to 92.7)d | 43.1 (39.4 to 46.8)d |
| Mowat 201552 | ≥ 10 | 20 | 20 | 156 | 554 | 750c | 50.0 (33.8 to 66.2)d | 78.0 (74.8 to 81.0)d | |
| e,fTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| e,fTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| e,fTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| e,fTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| e,fTerhaar sive Droste 201158 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| IBD | |||||||||
| Mowat 201552 | 0b | 29 | 5 | 408 | 308 | 750g | Reported | 85.3 (68.9 to 95.0)d | 43.0 (39.4 to 46.7)d |
| Mowat 201552 | ≥ 10 | 25 | 9 | 151 | 565 | 750g | 73.5 (55.6 to 87.1)d | 78.9 (75.7 to 81.8)d | |
| Significant bowel disease (CRC or HRA or IBD) | |||||||||
| Mowat 201552 | 0b | 90 | 12 | 347 | 301 | 750 | Reported | 88.2 (80.4 to 93.8)d | 46.5 (42.6 to 50.4)d |
| Mowat 201552 | ≥ 10 | 70 | 32 | 106 | 542 | 750 | 68.6 (58.7 to 77.5)d | 83.6 (80.6 to 86.4)d | |
| Significant bowel disease (CRC or HRA or low-risk adenoma or IBD) | |||||||||
| McDonald 201213 | ≥ 10a | 49 | 37 | 2 | 192 | 280 | Calculated | 57.0 (45.8 to 67.6) | 99.0 (96.3 to 99.9) |
Acceptability of faecal immunochemical testing using OC-Sensor
The proportion of people who are invited to participate in faecal immunochemical testing and who return a faecal sample can be regarded as a possible indicator of the acceptability of faecal immunochemical testing. Four13,52,53,55 of the five OC-Sensor studies reported faecal immunochemical testing uptake rates and these varied widely, ranging from 41% (in a study for which patients were sent an invitation to participate along with their referral letter)13 to 98% (in a study for which patients were given the specimen collection device at their initial consultation with a gastroenterologist). 53 Full results are provided in Table 10.
| Study | Point in the care pathway at which faecal immunochemical testing was requested | n | n returned | Uptake (%) |
|---|---|---|---|---|
| Mowat 201552 | Faecal immunochemical testing was requested by GPs at the point of referral to secondary care, and sampling devices and patient instruction sheets were distributed by practice nurses | 2173 | 1043 | 48 |
| Rodríguez-Alonso 201553 | Patients were given the specimen collection device at their initial consultation with a gastroenterologist | 1054 | 1035 | 98 |
| McDonald 201213 | Patients were sent an invitation to participate along with their referral letter. This was followed up by a telephone call from a research nurse. Sample collection devices and written and pictorial instructions were provided by post | 739 | 306 | 41 |
| Cubiella 2014 (COLONPREDICT)55 | Patients were invited to participate after they had been referred for colonoscopy | 825 | 799 | 97 |
Other outcome measures for faecal immunochemical testing using OC-Sensor
The primary care study by Mowat et al. 52 reported a number of additional outcomes relating to referral pathways for patients receiving faecal immunochemical testing. This study found that 11% (114/1031) of patients for whom a FIT sample was analysed were subsequently not referred to secondary care. 13 The study13 also reported that 69% (715/1031) of patients for whom a FIT sample was analysed were referred straight to endoscopy and 20% (202/1031) were referred to an outpatient clinic; however, decisions about the urgency of the referral appear to have been made prior to faecal immunochemical testing.
The Mowat et al. study52 was also the only OC-Sensor study to report information about the proportion of returned samples that were considered to be unsuitable for FIT analysis; this was found to be < 1% (7/1043). 13
Prediction modelling results from OC-Sensor faecal immunochemical testing studies
Rodríguez-Alonso et al. 53 also conducted multivariable analysis, using forward conditional logistic regression modelling, with the aim of identifying independent predictors of CRC and advanced neoplasia. The CRC analysis identified male gender (OR 2.39, 95% CI 1.039 to 5.519; p = 0.041), iron-deficiency anaemia (OR 2.99, 95% CI 1.27 to 7.03; p = 0.012) and faecal Hb ≥ 10 µg Hb/g faeces (OR 86.60, 95% CI 11.70 to 641.16; p < 0.001) as independent predictors. 53 The advanced neoplasia analysis identified male gender (OR 2.36, 95% CI 1.50 to 3.40; p < 0.001), age (OR 1.36, 95% CI 1.13 to 1.63; p < 0.001) and faecal Hb ≥ 10 µg Hb/g faeces (OR 7.54, 95% CI 5.03 to 11.28; p < 0.001) as independent predictors; age was treated a categorical variable in this model (≤ 40 years, 41–60 years, 51–60 years, 61–70 years, ≥ 70 years). 53 None of the NICE or SIGN 2-week referral criteria was identified as an independent predictor in either model, provided that faecal Hb measured by faecal immunochemical testing was included in the model. 53 The results of modelling were used to derive a risk score for advanced neoplasia; the scoring system assigned integer values to each independent predictor based on their coefficients from the logistic regression model. 53 The score ranged from 0 to 11 points, with points assigned as follows: age < 40 years = 0 points, age 41–50 years = 1 point, age 51–60 years = 2 points, age 61–70 years = 3 points, age > 70 years = 4 points; female gender = 0 points, male gender = 2 points; faecal Hb of < 10 µg Hb/g faeces = 0 points and faecal Hb ≥ 10 µg Hb/g faeces = 5 points. 53 The model was validated using a split sampling technique [data from 680 study participants (67.8%) were used to develop the model and data from 323 participants (32.2%) were used for validation]. 53 In the validation sample, a risk score of ≥ 5 had a sensitivity for advanced neoplasia of 88.1% (95% CI 74.3% to 96.0%) and a specificity of 63.3% (95% CI 57.4% to 69.0%). 53
As part of the COLONPREDICT study,55 Cubiella et al. have also developed a prediction model for CRC in symptomatic patients, based on FIT age and sex; this work has yet to be published in full, but was presented at the 2015 meeting of the World Endoscopy Association Colorectal Cancer Screening Committee. 73 A draft of the full paper reporting the development and validation of a risk score, the Faecal haemoglobin, Age and Sex Test (FAST) score, was provided ahead of publication (Callum Fraser, personal communication). The logistic regression model used to develop the FAST score included sex, age as a continuous variable and faecal Hb as a categorical variable (0, 0–20, 20–200, and ≥ 200 µg Hb/g faeces). 73 The validation cohort for this model used data from four studies13,52,53,56 that are included in this systematic review, and an additional cohort recruited to the COLONPREDICT study55 between March 2014 and March 2015; in this latter cohort, faecal immunochemical testing was measured using a variety of methods [OC-Sensor 202, OC-Auto 3 Latex (Eiken Chemical Co.) and FOB Gold]. The thresholds used to assess the performance of the FAST score in the validation cohort corresponded to the beta coefficients of the FAST score with 90% and 99% sensitivity in the development cohort (4.5 and 2.12, respectively). In the validation cohort, a FAST score of ≥ 4.5 had a sensitivity of 89.3% (95% CI 84.1% to 93.0%) and a specificity of 82.3% (95% CI 81.1% to 83.5%) for CRC. In order to avoid missing any CRCs, a lower FAST score threshold of ≥ 2.12 was required; the sensitivity and specificity estimates at this threshold were 100% (95% CI 97.7% to 100%) and 19.8% (95% CI 18.6% to 21.1%), respectively. Post hoc analysis indicated that there were no significant differences in FAST score performance, for the detection of CRC, when comparing patients referred for colonoscopy from primary care with those referred from secondary care; CRC prevalence in the validation cohort was similar in the two settings (5.6% in primary care and 4.9% in secondary care). The performance of the FAST score was also assessed for the detection of the expanded target conditions of advanced neoplasia (CRC or HRA) and significant colonic lesions; the definitions of these target conditions varied between the studies included in the validation cohort. Studies that were conducted in Scotland defined HRA as a lesion of ≥ 10 mm diameter or more than three lesions, and significant colonic lesions as CRC, HRA or IBD. 13,52,56 The published Spanish study53 and the additional, unpublished cohort from the COLONPREDICT study55 defined HRA as a lesion of ≥ 10 mm diameter or with villous histology or high-grade dysplasia; in these cohorts, the definition of significant colonic lesions included CRC, HRA, polyposis (> 10 polyps of any histology including serrated lesions), colitis, polyps of ≥ 10 mm, complicated diverticular disease, colonic ulcer or bleeding angiodysplasia). Sensitivity estimates for the lower FAST score threshold (2.12) remained high (≥ 95%) for all of the target conditions. For the target condition ‘advanced neoplasia’, the sensitivity estimates for the FAST score using the 4.5 and 2.12 thresholds were 60.7% (95% CI 56.6% to 64.7%) and 96.7% (95% CI 94.9% to 98.0%), respectively, and the corresponding specificity estimates were 85.4% (95% CI 84.1% to 86.5%) and 21.5% (95% CI 20.1% to 22.9%). For the target condition ‘significant colonic lesion’, the sensitivity estimates for the FAST score using the 4.5 and 2.12 thresholds were 57.8% (95% CI 54.3% to 61.3%) and 94.5% (95% CI 92.6% to 96.0%), respectively, and the corresponding specificity estimates were 87.4% (95% CI 86.2% to 88.5%) and 22.0% (95% CI 20.6% to 23.5%).
Diagnostic performance of the HM-JACKarc faecal immunochemical test assay
Details of HM-JACKarc studies
Three diagnostic cohort studies,56,57,75 reported in seven publications,56,57,65,66,71,72,75 provided data on the diagnostic performance of the HM-JACKarc FIT assay. Two studies56,75 reported accuracy data, for which CRC was the specified target condition. The prevalence of CRC, diagnosed at colonoscopy, was 2.2% in the Godber et al. study56 and 4.9% in the Thomas et al. study. 75 One of the CRC studies56 and one additional study57 also reported data for the composite target condition of advanced neoplasia (CRC or HRA). Each of the studies used a different definition of HRA; Auge et al. 57 defined HRA based on size, morphology or number of lesions (any lesion of ≥ 10 mm in diameter or with villous architecture or high-grade dysplasia, or three or more non-advanced adenomas) and Godber et al. 56 defined higher-risk adenoma, based on size or number of lesions, using a combination of the BSG categories ‘high risk’ (five or more adenomas or three or more adenomas, at least one of which is of ≥ 10 mm in diameter) and ‘intermediate risk’ (three or four small adenomas or at least one adenoma of ≥ 10 mm in diameter). 90 Two studies56,75 reported additional accuracy data for various non-malignant and composite target conditions.
No study reported data that were specific to the population that was defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms, who are at low risk for CRC according to the criteria defined in NG121). No studies were conducted in a primary care setting; all three of the studies56,57,75 were conducted in outpatient clinics. One study56 stated that faecal immunochemical testing was requested after referral for colonoscopy, and in the remaining two studies57,75 the timing of faecal immunochemical testing in relation to colonoscopy referral was unclear. One study,75 reported as a conference abstract, stated that only symptomatic patients were included, but did not report details of presenting symptoms. The remaining two studies56,57 included patients who had been referred for colonoscopy, and one study56 recorded presenting symptoms (reason for referral); both of the studies56,57 that reported presenting symptoms included some participants who had symptoms that may be considered to be associated with a higher probability of CRC and that are components of the criteria for 2-week referral as defined in NG121 (e.g. rectal bleeding). Presenting symptoms included rectal bleeding/haematochezia, abdominal pain, change in bowel habit, constipation or diarrhoea, anaemia and weight loss. The median age of study participants was 67 years,75 63 years57 and 59 years,56 and the overall age range was 16–93 years. There were no data linking presenting symptoms to age. One study57 reported data on the effects of the sex of participants on the accuracy of HM-JACKarc for the detection of advanced neoplasia. Full details of participant characteristics are provided in Appendix 2, Table 65.
All of the three studies56,57,75 reported data on the accuracy of HM-JACKarc FIT using a single faecal sample. One study57 also compared the accuracy of single versus double sampling faecal immunochemical testing for the detection of advanced neoplasia.
Two studies reported information about uptake rates in participants invited to provide a sample for faecal immunochemical testing. 56,75
Accuracy of HM-JACKarc for the detection of colorectal cancer
Two studies56,75 reported data on the accuracy of HM-JACKarc FIT using a single faecal sample and thresholds of 10 µg Hb/g faeces56 and 7 µg Hb/g faeces. 75 Full test performance results are provided in Table 11. There was little variation in test performance between the 7- and 10 µg Hb/g faeces thresholds; the sensitivity estimates were 91.3% and 100%, respectively, and the corresponding specificity estimates were 76.6% and 79.2%. 56,75 As with the OC-Sensor FIT assay, the optimal test performance (maximising both sensitivity and specificity) appeared to occur with thresholds of 7 or 10 µg Hb/g faeces; none of the HM-JACKarc studies reported test performance characteristics for any detectable Hb. Using test performance data from the Godber et al. study56 – and a CRC prevalence estimate of 2.2% taken from the same study – to consider the outcome of testing for a hypothetical cohort of 1000 patients indicates that no CRCs would be missed using the 10 µg Hb/g faeces threshold, but 229 unnecessary colonoscopies would be carried out (assuming that all of the patients with a positive FIT result receive colonoscopy and that all colonoscopies conducted in patients without CRC are considered unnecessary). CRC would be correctly ruled out in 750 people (Figure 8). Please see subsequent sections for information on other significant bowel pathologies that may be detected by faecal immunochemical testing and hence may form part of the FP or ‘unnecessary colonoscopy’ population.
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| aGodber 201656 | ≥ 10 | 11 | 0 | 116 | 380 | 507 | Reported | 100 (71.5 to 100)b | 76.6 (72.6 to 80.3)b |
| aThomas 201675 | ≥ 7 | 21 | 2 | 89 | 338 | 450 | Calculated | 91.3 (72.0 to 98.9) | 79.2 (75.3 to 83) |
FIGURE 8.
Testing outcomes for a hypothetical cohort of 1000 patients using HM-JACKarc: the 10 µg Hb/g faeces threshold, for the target condition of CRC.
Accuracy of HM-JACKarc for the detection of advanced neoplasia (colorectal cancer or high-risk adenoma)
Two studies56,57 reported data on the accuracy of HM-JACKarc FIT using a single faecal sample, for which the target condition was expanded to include CRC or HRA. Two studies56,57 assessed the diagnostic performance of the 10 µg Hb/g faeces threshold for different definitions of this expanded target condition (see Details of HM-JACKarc studies, above); these two studies56,57 reported very different estimates of sensitivity (70% and 34.5%, respectively). In addition to the difference in the definition of HRA, the Auge et al. study57 differed from Godber et al. study56 in that it included some patients who were undergoing colonoscopy for polyp surveillance and excluded people with GI bleeding or active rectal bleeding; the prevalence of CRC in the Auge et al. study57 was the lowest of any study included in this review (0.96%). 57 Auge et al. 57 reported test performance characteristics using a range of thresholds; sensitivity decreased and specificity increased with increasing threshold, and the ‘any detectable Hb’ threshold was associated with high sensitivity (96.6%) and very low specificity (10.6%). Full test performance results for all of the thresholds that were evaluated in individual studies are provided in Table 12. The variation in test performance characteristics according to the threshold used is illustrated in a ROC space plot (Figure 9).
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Any detectable Hb | |||||||||
| Auge 201657 | 0a | 28 | 1 | 160 | 19 | 208 | Calculated | 96.6 (82.8 to 93.4) | 10.6 (6.9 to 15.9) |
| 10 µg Hb/g faeces | |||||||||
| Auge 201657 | ≥ 10 | 10 | 19 | 23 | 156 | 208 | Calculated | 34.5 (19.9 to 52.7) | 87.2 (81.6 to 91.3) |
| bGodber 201656 | ≥ 10 | 21 | 9 | 106 | 371 | 507 | Reported | 70.0 (50.6 to 85.3)c | 77.8 (73.8 to 81.4)c |
| Other thresholds | |||||||||
| Auge 201657 | ≥ 20 | 9 | 20 | 13 | 166 | 208 | Calculated | 31 (17.3 to 49.2) | 92.8 (88 to 95.7) |
| ≥ 30 | 9 | 20 | 12 | 167 | 208 | 31 (17.3 to 49.2) | 93.3 (88.7 to 96.1) | ||
| ≥ 40 | 8 | 21 | 11 | 168 | 208 | 27.6 (14.7 to 45.7) | 93.9 (89.4 to 96.6) | ||
FIGURE 9.
Receiver operating characteristic space plot for the HM-JACKarc assay using different thresholds for the target condition of advanced neoplasia (CRC or HRA).

Based on the data from Godber et al. ,56 expanding the target condition from CRC only to include CRC or HRA resulted in an increase in prevalence from 2.2% to 5.9%. Using test performance data from this study – and an estimate for the prevalence of advanced neoplasia of 5.9% – to consider the outcome of testing for a hypothetical cohort of 1000 patients indicates that applying faecal immunochemical testing at the 10 µg Hb/g faeces threshold would result in 21 advanced neoplasias being missed. As data indicate that no CRCs would be missed at this threshold (see previous section), it may be assumed that the missed cases would all be higher-risk adenomas. Using the 10 µg Hb/g faeces threshold, 205 unnecessary colonoscopies would be carried out (assuming that all patients with a positive FIT result receive colonoscopy and that all of the colonoscopies that were conducted in patients without at least HRA are considered unnecessary); CRC and higher-risk adenoma would be correctly ruled out in 727 people (Figure 10).
FIGURE 10.
Testing outcomes for a hypothetical cohort of 1000 patients using HM-JACKarc: the 10 µg Hb/g faeces threshold for the target condition of advanced neoplasia (CRC or HRA).

Data from Auge et al. study57 indicated that, in this population (CRC prevalence < 1%), high sensitivity (good rule-out performance) could be achieved only by using the ‘any detectable Hb’ threshold; the sensitivity at this threshold was 96.6%. This study57 also compared the performance of double sampling with single sampling, and found that 100% sensitivity could be achieved by using the higher value from two consecutive samples and the ‘any detectable Hb’ threshold. The use of two consecutive samples increased sensitivity compared with single sampling, at all thresholds, but sensitivity remained low (< 50%) throughout. 57 Full results for single and double sampling at all of the thresholds that were evaluated are provided in Table 13.
| Study | Sampling strategy | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Auge 201657 | First of two consecutive samples | 0a | 28 | 1 | 160 | 19 | 208 | Calculated | 96.6 (82.8 to 93.4) | 10.6 (6.9 to 15.9) |
| Higher of two consecutive samples | 0a | 29 | 0 | 173 | 6 | 208 | 100 (88.3 to 100) | 3.3 (1.5 to 7.1) | ||
| First of two consecutive samples | ≥ 10 | 10 | 19 | 23 | 156 | 208 | 34.5 (19.9 to 52.7) | 87.2 (81.6 to 91.3) | ||
| Higher of two consecutive samples | ≥ 10 | 12 | 17 | 37 | 142 | 208 | 41.4 (25.5 to 59.3) | 79.4 (73 to 84.7) | ||
| First of two consecutive samples | ≥ 20 | 9 | 20 | 13 | 166 | 208 | 31 (17.3 to 49.2) | 92.8 (88 to 95.7) | ||
| Higher of two consecutive samples | ≥ 20 | 10 | 19 | 26 | 153 | 208 | 34.5 (19.9 to 52.7) | 85.6 (83.5 to 92.9) | ||
| First of two consecutive samples | ≥ 30 | 9 | 20 | 12 | 167 | 208 | 31 (17.3 to 49.2) | 93.3 (88.7 to 96.1) | ||
| Higher of two consecutive samples | ≥ 30 | 10 | 19 | 25 | 154 | 208 | 34.5 (19.9 to 52.7) | 86.1 (83.6 to 92.9) | ||
| First of two consecutive samples | ≥ 40 | 8 | 21 | 11 | 168 | 208 | 27.6 (14.7 to 45.7) | 93.9 (89.4 to 96.6) | ||
| Higher of two consecutive samples | ≥ 40 | 10 | 19 | 21 | 158 | 208 | 34.5 (19.9 to 52.7) | 88.3 (82.8 to 92.2) |
Auge et al. 57 also reported lower sensitivity estimates, at all thresholds, when HM-JACKarc FIT was used, in women than in men. 57 Full results for test performance in men and women are provided in Table 14.
| Study | Subgroup | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Auge 201657 | Men | 0a | 17 | 0 | 69 | 6 | 92 | Calculated | 100 (81.6 to 100) | 8 (3.7 to 16.4) |
| Women | 0a | 11 | 1 | 91 | 13 | 116 | 91.7 (64.6 to 98.5) | 12.4 (7.4 to 20) | ||
| Men | ≥ 10 | 8 | 9 | 10 | 65 | 92 | 47.1 (26.2 to 69) | 86.7 (77.2 to 92.6) | ||
| Women | ≥ 10 | 2 | 10 | 13 | 91 | 116 | 16.7 (4.7 to 44.8) | 87.6 (79.8 to 92.6) | ||
| Men | ≥ 20 | 7 | 10 | 6 | 69 | 92 | 41.2 (21.6 to 64) | 92 (83.6 to 96.3) | ||
| Women | ≥ 20 | 2 | 10 | 7 | 97 | 116 | 16.7 (4.7 to 44.8) | 93.3 (86.8 to 96.7) | ||
| Men | ≥ 30 | 7 | 10 | 6 | 69 | 92 | 41.2 (21.6 to 64) | 92 (83.6 to 96.3) | ||
| Women | ≥ 30 | 2 | 10 | 6 | 98 | 116 | 16.7 (4.7 to 44.8) | 94.3 (88 to 97.3) | ||
| Men | ≥ 40 | 7 | 10 | 6 | 69 | 92 | 41.2 (21.6 to 64) | 92 (83.6 to 96.3) | ||
| Women | ≥ 40 | 1 | 11 | 5 | 99 | 116 | 8.3 (1.5 to 35.4) | 95.2 (89.3 to 97.9) |
Accuracy of HM-JACKarc for the detection of other/composite target conditions
Two studies56,75 reported accuracy data for target conditions other than CRC or advanced neoplasia. Both of the studies evaluated the performance of HM-JACKarc for the target condition ‘significant bowel disease’; Thomas et al. 75 defined significant bowel disease as CRC, HRA or IBD, and Godber et al. 56 defined significant bowel disease as CRC, higher-risk adenoma, IBD or colitis.
Thomas et al. 75 reported sensitivity and specificity estimates of 72.1% and 80.6%, using a threshold of 7 µg Hb/g faeces, but did not provide sufficient information to extract 2 × 2 data,75 and Godber et al. 56 reported similar sensitivity and specificity estimates of 68.9% and 80.2% for the 10 µg Hb/g faeces threshold. 56 Because the Godber et al. study56 assessed both this composite target condition and CRC alone in the same patient cohort, we can see that the small increase in the specificity estimate, relative to that reported for CRC, means that 26 of the 116 participants (22.4%) who were classified as having FP FIT results for CRC actually had other significant bowel pathologies (HRA, IBD or colitis) and may thus have benefited from secondary care investigation.
Godber et al. 56 also reported test performance data using a range of thresholds from 10 to 40 µg Hb/g faeces; there was little variation in the sensitivity and specificity of HM-JACKarc for ‘significant bowel disease’ with increasing threshold. 56 Full results for the accuracy of HM-JACKarc for detecting ‘significant bowel disease’, at all thresholds investigated, are provided in Table 15.
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Significant bowel disease (CRC or HRA or IBD) | |||||||||
| Thomas 201675 | ≥ 7 µg Hb/g faeces | NC | NC | NC | NC | 450 | NA | 72.1 (58.7 to 85.5) | 80.6 (76.7 to 84.4) |
| Significant bowel disease (CRC or higher-risk adenoma or IBD or colitis) | |||||||||
| Godber 201656 | ≥ 10 µg Hb/g faeces | 32 | 13 | 90 | 349 | 484 | Calculated | 68.9 (53.2 to 81.4) | 80.2 (76.1 to 83.7) |
| ≥ 20 µg Hb/g faeces | 29 | 16 | 63 | 376 | 484 | 64.4 (48.7 to 77.7) | 85.7 (81.9 to 88.7) | ||
| ≥ 15 µg Hb/g faeces | 31 | 14 | 77 | 362 | 484 | 66.7 (50.9 to 79.6) | 83.1 (79.2 to 86.5) | ||
| ≥ 25 µg Hb/g faeces | 29 | 16 | 55 | 384 | 484 | 64.4 (48.7 to 77.7) | 87.5 (83.9 to 90.3) | ||
| ≥ 30 µg Hb/g faeces | 29 | 16 | 50 | 389 | 484 | 64.4 (48.7 to 77.7) | 88.6 (85.2 to 91.4) | ||
| ≥ 35 µg Hb/g faeces | 29 | 16 | 47 | 392 | 484 | 64.4 (48.7 to 77.7) | 89.2 (85.9 to 92) | ||
| ≥ 40 µg Hb/g faeces | 29 | 16 | 44 | 395 | 484 | 64.4 (48.7 to 77.7) | 90 (86.7 to 92.5) | ||
Acceptability of faecal immunochemical testing using HM-JACKarc
The proportion of people invited to participate in faecal immunochemical testing who return a faecal sample can be regarded as a possible indicator of the acceptability of faecal immunochemical testing. Two56,75 of the four HM-JACKarc studies reported FIT uptake rates. The proportion of samples returned was higher (66%) in the study for which information and collection devices were provided at an outpatient appointment75 than in the study that sent collection devices and information by post (56%). 56 Full results are provided in Table 16.
| Study | Point in the care pathway at which faecal immunochemical testing was requested | n | n returned | Uptake (%) |
|---|---|---|---|---|
| Thomas 201675 | Faecal immunochemical testing was requested after the first secondary care 2-week wait clinic. Participants were given a pack that included a sample collection device and pictorial instructions | 773 | 514 | 66 |
| Godber 201656 | Patients were invited to participate after they had been referred for colonoscopy. A letter of invitation to participate and a sample collection device were sent with the materials sent for bowel cleansing prior to colonoscopy | 909 | 507 | 56 |
Diagnostic performance of the FOB Gold faecal immunochemical test assay
Details of FOB Gold studies
Two diagnostic cohort studies provided data on the diagnostic performance of the FOB Gold FIT assay; the Krivec et al. study54 was published as an conference abstract and provided only limited data,54 and the other, Hospital Clinic de Barcelona 2015, was provided in confidence by the manufacturer (Philippa Pinn, personal communication). Krivec et al. 54 reported accuracy data for significant bowel disease (defined as cancer polyps or bleeding) and Hospital Clinic de Barcelona 2015 (confidential information has been removed). The prevalence of CRC was not reported in the Krivec et al. study54 (confidential information has been removed).
No study clearly reported data that were specific to the population that was defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms, who are at low risk for CRC according to the criteria defined in NG121). Both study populations were reported as symptomatic patients who had been referred for colonoscopy. Krivec et al. 54 did not report any information about the age of participants54 (confidential information has been removed). No details of presenting symptoms were reported in either study. Full details of participant characteristics are provided in Appendix 2, Table 65.
Neither study reported information about uptake rates in participants who were invited to provide a sample for faecal immunochemical testing, or any other outcome measure.
Accuracy of FOB Gold for the detection of colorectal cancer
(Confidential information has been removed.) Full test performance results for all thresholds evaluated are provided in Table 17. (Confidential information has been removed), to consider the outcome of testing for a hypothetical cohort of 1000 patients indicates that (confidential information has been removed). CRCs would be missed and (confidential information has been removed) unnecessary colonoscopies would be carried out (assuming that all patients with a positive FIT result receive colonoscopy and that all colonoscopies conducted in patients without CRC are considered unnecessary); CRC would be correctly ruled out by faecal immunochemical testing, avoiding colonoscopy, in (confidential information has been removed) people (Figure 11).
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
FIGURE 11.
(Confidential information has been removed.)
Accuracy of FOB Gold for the detection of advanced neoplasia (colorectal cancer or high-risk adenoma)
(Confidential information has been removed.) Full test performance results for all of the strategies evaluated are provided in Table 18.
| Study | Sampling strategy | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
Accuracy of FOB Gold for the detection of other/composite target conditions
Both Krivec et al. 54 and Hospital Clinic de Barcelona 2015 reported accuracy data for the composite target condition ‘significant bowel disease’. Krivec et al. 54 defined significant bowel disease as cancer, polyps or bleeding, and reported a sensitivity of 45.2% and a specificity of 92.3%, using a threshold of 9 µg Hb/g faeces. 54 (Confidential information has been removed.) Full test performance results for both studies and for all thresholds evaluated are provided in Table 19.
| Study | Threshold (µg Hb/g faeces) | TP | FN | FP | TN | Total n | 2 × 2 data | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All neoplasia (cancer or HRA or low-risk adenoma) | |||||||||
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| Significant bowel disease (CRC, HRA, low-risk adenoma, hyperplastic or inflammatory polyps, IBD, haemorrhoids, diverticulosis, angiodysplasia, other) | |||||||||
| Hospital Clinic de Barcelona 2015a | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | |
| Significant bowel disease (CRC polyps or bleeding) | |||||||||
| Krivec 201154 | ≥ 9b | NC | NC | NC | NC | 83 | NA | 45.2 (NC to NC) | 92.3 (NC to NC) |
(Confidential information has been removed.) Full test performance results for all thresholds evaluated are provided in Table 19.
Diagnostic performance of the RIDASCREEN faecal immunochemical test assay
No studies were identified that assessed the diagnostic performance of RIDASCREEN Hb or RIDASCREEN Hb/Hp complex in symptomatic patients.
Comparative diagnostic accuracy of different faecal immunochemical test assays and guaiac faecal occult blood testing
No studies were identified that directly compared the performance of different FIT assays, or that compared one or more FIT assays with a gFOBT method.
Selection of test strategies for inclusion in cost-effectiveness modelling
The selection of FIT accuracy data for use in cost-effectiveness modelling was based on the optimal threshold (maximum sensitivity and specificity) for each assay method and the threshold required to proved optimal rule-out performance (highest sensitivity and lowest number of cases missed). Because no studies were identified which directly compared the performance of one or more FIT assays with a gFOBT method, accuracy data for guaiac faecal occult blood testing used in the base case for cost-effectiveness modelling were those used in the NG126 model. The primary study from which these data were taken, Gilbert et al. ,91 was a retrospective study using a Swedish cancer registry, in which a diagnosis of CRC was classified as any diagnosed CRC within 2 years of guaiac faecal occult blood testing; it was unclear whether or not all of the patients who were included in the study had been symptomatic at the time of guaiac faecal occult blood testing, and the prevalence of CRC, in the subgroup of study participants used to provide data for the model was very low (0.36%). We therefore conducted scenario analyses using published estimates of gFOBT accuracy obtained from studies that were identified during the process of inclusion screening for our systematic review: Niv and Sperber92 and Bjerregaard et al. ;93 although guaiac faecal occult blood testing was not included in our systematic review, as an intervention, our searches included general terms for faecal occult blood testing and guaiac faecal occult blood testing. Both of these studies92,93 were conducted in symptomatic patients and the prevalence of CRC was 2.5% and 3.1%, respectively, making these studies closer to the population defined for this assessment.
Chapter 4 Assessment of cost-effectiveness
In this chapter we explore the cost-effectiveness of using quantitative faecal immunochemical tests for Hb (FIT) as a triage test for people presenting, in primary care settings, with lower abdominal symptoms who are at low risk for CRC according to the criteria defined in NG12. 1
Review of economic analyses of faecal immunochemical tests
Methods
Search strategy
Literature searches were performed to identify published economic evaluations and cost-effectiveness data. Additional searches were conducted to identify adverse event/mortality data and utility studies for diagnostic techniques and procedures for colorectal neoplasms that were not included within the scope of the clinical effectiveness searches. All of the searches aimed to identify studies that could be used to support the development of a health economic model to estimate the model input parameters and answer the research questions of the assessment, but not to perform a systematic review. Searches were therefore pragmatic in design and date limits were applied where appropriate.
Methodological study design filters were included in the search strategy where relevant. No restrictions on language or publication status were applied. The main EMBASE strategy for each search was independently peer reviewed by a second Information Specialist, using the CADTH Peer Review checklist. 40 Identified references were downloaded in EndNote X6 software for further assessment and handling. References in retrieved articles were checked for additional studies.
Full search strategies are presented in Appendix 1.
Full cost-effectiveness studies were summarised and appraised using the Drummond checklist. 94
Economic evaluations and cost studies
Economic evaluations and cost studies associated with colonoscopy, CTC, magnetic resonance imaging and computed tomography (CT) scans were searched for on the following databases and resources, from 2005 to the most recent date available:
-
MEDLINE (via Ovid): 2005 to March Week 4 2016
-
MEDLINE In-Process & Other Non-Indexed Citations, and Daily Update (via Ovid): 2005 to 1 April 2016
-
EMBASE (via Ovid): 2005 to 1 April 2016
-
NHS EED (via the internet): 2005 to Issue 2 of 4, April 2015
-
EconLit (via EBSCOhost): 2005 to 1 February 2016
-
Cost-effectiveness Analysis Registry (via the internet): 2005 to April 2016: www.cearegistry.org/
-
Research Papers in Economics (via the internet): 2005 to April 2016: http://repec.org/.
Adverse events and mortality data
Adverse events and mortality data that are associated with colonoscopy and CTC were searched for on the following databases and resources, from database inception date to the most recent date available:
-
MEDLINE (via Ovid): 1946 to March Week 4 2016
-
MEDLINE In-Process & Other Non-Indexed Citations, and Daily Update (via Ovid): to 4 April 2016
-
EMBASE (via Ovid): 1974 to 4 April 2016
-
CENTRAL (via the internet): to Issue 3 of 12, March 2016.
Utility data
Studies containing utility data that are associated with colonoscopy and CTC were searched for on the following databases and resources, from database inception date to the most recent date available:
-
MEDLINE (via Ovid): 1946 to March Week 4 2016
-
MEDLINE In-Process & Other Non-Indexed Citations Daily Update (via Ovid): to 4 April 2016
-
EMBASE (via Ovid): 1974 to 4 April 2016
-
CENTRAL (via the internet): to Issue 3 of 12, March 2016
-
NHS EED (via the internet): 2005 to Issue 2 of 4, April 2015
-
School of Health and Related Research (ScHARR) Health Utilities Database (ScHARRHUD) (via the internet): to 4 April 2016. www.scharrhud.org/.
Inclusion criteria and screening
Inclusion criteria as reported in Table 20 were applied. Relevant studies were identified in two stages. Two researchers (ML and ICR) screened, independently, for possible inclusion, the titles and abstracts that were returned by the search strategy. Disagreements were resolved by discussion. Full texts of the selected studies were obtained. Two researchers (ML and ICR) examined these independently for inclusion or exclusion, and disagreements were, again, resolved by discussion.
| Type of studies | Studies reporting cost-effectiveness/utility/benefit/minimisation analyses or economic evaluations in general. No study design restrictions were applied. All of the studies were included, apart from reviews, letters and comment articles |
| Type of participants | Symptomatic people presenting in primary care settings in whom investigation for possible CRC is being considered |
| Type of intervention | FIT |
| Type of outcomes | Costs or incremental costs and QALYs or other effectiveness units, such as LYs, reported together with costs |
Results
The total number of potentially relevant economic papers that were initially retrieved by searches was 923. In addition, 19 clinical guidelines relating to CRC were identified. Most of the economic studies that were identified were excluded because they related to screening for CRC in asymptomatic populations. None of the included studies directly assessed the decision problem of this diagnostic assessment.
The only included study was the one conducted to inform NG12. 1,6 This study1,6 is reviewed in detail below.
Summary of the National Institute for Health and Care Excellence guideline 12 cost-effectiveness study
The aim of the NG12 study1,6 was to estimate the cost-effectiveness of five different investigations for suspected CRC (FOBT, barium enema, flexible sigmoidoscopy, CTC or colonoscopy) ordered by primary care practitioners for patients aged ≥ 40 years with a change in bowel habit as main symptom. 6 The characteristics of this study are summarised in Table 21. The base-case results indicated that both guaiac faecal occult blood testing and barium enema were cost-effective compared with colonoscopy at a threshold of £20,000 per quality-adjusted life-year (QALY) gained. However, an analysis that included all of the comparators showed that barium enema would have been dominated by faecal immunochemical testing [the incremental cost-effectiveness ratio (ICER) for faecal immunochemical testing vs. barium enema being lower than barium enema vs. guaiac faecal occult blood testing] and that faecal immunochemical testing would be cost-effective at a threshold of £20,000 per QALY gained.
| Study, year, country | Summary of model | Investigationsa | Patient population | Costs (£) per 1000 patients (all investigations) | QALYs per 1000 patients (all investigations) | ICER (per QALY gained) | Sensitivity analyses |
|---|---|---|---|---|---|---|---|
| NG12 20151,6 |
|
Base case:
|
People aged ≥ 40 years with a change in bowel habit as a main symptom who have presented to their GP for the first time |
|
|
ICERs (all comparators included); FOBT = NA
Flexible sigmoidoscopy = dominated
Safety netting = £251,851 |
|
Scenarios:
|
Study quality
The results of the quality assessment of the NG12 study1,6 are presented in Table 22.
| Criteria | NG12,1,6 2015 |
|---|---|
| Study design | |
| 1. Was the research question stated? | Yes |
| 2. Was the economic importance of the research question stated? | Yes |
| 3. Was/were the viewpoint(s) of the analysis clearly stated and justified? | Yes |
| 4. Was a rationale reported for the choice of the alternative programmes or interventions compared? | Yes |
| 5. Were the alternatives being compared clearly described? | No. It is not mentioned which individual tests are considered to model faecal occult blood testing and faecal immunochemical testing. The other investigations considered in the analysis (i.e. barium enema, flexible sigmoidoscopy, CTC and colonoscopy) are not described in detail in the economic evaluation section, but discussed throughout the clinical guideline |
| 6. Was the form of economic evaluation stated? | Yes |
| 7. Was the choice of form of economic evaluation justified in relation to the questions addressed? | Yes |
| Data collection | |
| 8. Was/were the source(s) of effectiveness estimates used stated? | Yes |
| 9. Were details of the design and results of the effectiveness study given (if based on a single study)? | No. For some investigations considered (e.g. accuracy of colonoscopy or CTC), only the reference to a paper is given |
| 10. Were details of the methods of synthesis or meta-analysis of estimates given (if based on an overview of a number of effectiveness studies)? | NA |
| 11. Was/were the primary outcome measure(s) for the economic evaluation clearly stated? | Yes |
| 12. Were the methods used to value health states and other benefits stated? | Yes |
| 13. Were the details of the subjects, from whom valuations were obtained, given? | NA; utilities from literature |
| 14. Were productivity changes (if included) reported separately? | NA |
| 15. Was the relevance of productivity changes to the study question discussed? | NA |
| 16. Were quantities of resources reported separately from their unit cost? | No |
| 17. Were the methods for the estimation of quantities and unit costs described? | No |
| 18. Were currency and price data recorded? | Yes |
| 19. Were details of price adjustments for inflation or currency conversion given? | No. The only reference to price inflation is the following sentence: ‘Data on lifetime costs were taken from Tappenden et al. 2004 and inflated to 2014 prices’ |
| 20. Were details of any model used given? | Yes |
| 21. Was there a justification for the choice of model used and the key parameters on which it was based? | Yes |
| Analysis and interpretation of results | |
| 22. Was the time horizon of cost and benefits stated? | Yes |
| 23. Was the discount rate stated? | Yes |
| 24. Was the choice of rate justified? | Yes |
| 25. Was an explanation given if cost or benefits were not discounted? | NA |
| 26. Was/were the details of statistical test(s) and CIs given for stochastic data? | Not explicitly but CIs and/or standard errors reported (including reference) |
| 27. Was the approach to sensitivity analysis described? | Yes |
| 28. Was the choice of variables for sensitivity analysis justified? | Not explicitly but references reported |
| 29. Were the ranges over which the parameters were varied stated? | Yes |
| 30. Were relevant alternatives compared? (That is, were appropriate comparisons made when conducting the incremental analysis?) | Yes |
| 31. Was an incremental analysis reported? | Yes |
| 32. Were major outcomes presented in a disaggregated form as well as aggregated form? | No, only aggregated |
| 33. Was the answer to the study question given? | Yes |
| 34. Did conclusions follow from the data reported? | Yes |
| 35. Were conclusions accompanied by the appropriate caveats? | Yes |
| 36. Were generalisability issues addressed? | No |
Study design
The economic evaluation presented in NG126 was a modelling study to assess the cost-effectiveness of different investigations for CRC. The research question and the approach to economic evaluation were stated clearly. Results were presented as costs per QALY gained. In the base-case analysis, the investigations considered were faecal occult blood testing, barium enema and colonoscopy. In additional scenarios, flexible sigmoidoscopy, CTC, faecal immunochemical testing and safety netting were incorporated to the analysis. Results were presented in the form of full incremental analysis (i.e. all investigations compared at the same time) and not in terms of pairwise comparisons of intervention against comparator. When faecal occult blood testing and faecal immunochemical testing were considered, it was not mentioned which individual assays are used to determine the input parameters of the model.
Data
When possible, the data that were used to inform the model input parameters were based on the systematic literature review conducted in the clinical guideline. Otherwise, there was reliance on expert opinion. A summary of the data sources for the different groups of input parameters is given below.
Prevalence of colorectal cancer
No evidence was found to inform the prevalence of CRC in the study population. Thus, the PPV of various symptoms associated with CRC was used to inform this. The systematic review identified 22 studies as relevant, but the evidence could not be pooled due to large heterogeneity. Therefore, the prevalence of CRC was estimated based on the opinion of the guideline development group (GDG).
Natural history of disease
The initial distributions of cancer stages at diagnosis and disease-specific mortality were estimated using data from the National Cancer Intelligence Network (NCIN). 97 Age-related mortality probabilities were calculated using data from published interim life tables for the UK ONS 2013. 98 The study from Tappenden et al. 99 reports data on progression between cancer stages for people with undiagnosed CRC. As the GDG considered that obtaining progression probabilities in patients with CRC would be unlikely, the probabilities in Tappenden et al. 99 were used in the model.
Diagnostic accuracy
Diagnostic accuracy was modelled using data on sensitivity and specificity from the systematic review. Six papers were identified as relevant for faecal occult blood testing and three for barium enema. However, the sensitivity and specificity reported in those papers could not be pooled because of the large heterogeneity between the studies. Therefore, based on the GDG advice, the paper by Gillberg et al. 91 was used for the diagnostic accuracy of faecal occult blood testing, and the paper by Jensen et al. 100 was chosen for barium enema. For the remaining interventions that were considered in the decision problem, no relevant evidence was identified. Data for these investigations were then collected by removing the ‘primary care’ filter from the search. The following papers were considered to inform the accuracy of the remaining investigations: Oono et al. 101 for faecal immunochemical testing, Thompson et al. 102 for flexible sigmoidoscopy, Pickhardt et al. 103 (only sensitivity) and Halligan et al. 104 for CTC, and Pickhardt et al. 103 for colonoscopy.
Adverse events
It was assumed that only colonoscopy and flexible sigmoidoscopy would require treatment for adverse events. Data on adverse events that were associated with colonoscopy were obtained from the UK colonoscopy audit from Gavin et al. 105 As no data were found to inform the probability of adverse events for flexible sigmoidoscopy, this was assumed to be the same as for colonoscopy, based on clinical expert opinion.
Costs
Price of tests and consultations were informed based on NHS Reference Costs 2012–13106 and the Personal and Social Services Research Unit (PSSRU) 2013. 107 Costs on adverse events were also taken from NHS Reference Costs 2012–13. 106 The prices used for faecal occult blood testing and faecal immunochemical testing were estimated from the screening programme’s Southern Hub 2011. 108 Lifetime costs for patients with CRC were taken from the Tappenden et al. study99 and inflated to 2014 prices.
Utilities
The utilities that are associated with the different stages of cancer that were used in the model were obtained from Ness et al. ,95 although this paper assessed quality-of-life values in the USA using the standard gamble technique and its results were not valued in the UK. For healthy patients, utilities were taken from the Kind et al. study96 [a large UK-based study on population health using the EuroQol-5 Dimensions (EQ-5D) survey]. No adjustment was made for age.
Analysis and interpretation of results
The study was generally well conducted, although details of which specific assays were considered to model faecal occult blood testing and faecal immunochemical testing were not reported, and the generalisability of findings was not discussed. In addition, faecal immunochemical testing was considered only in an additional analysis and not in the base case. The results showed that faecal immunochemical testing was cost-effective compared with colonoscopy, and, when all of the investigations were included, it remained cost-effective at a threshold of £20,000 per QALY. Given its recent date and UK setting, any parameter estimates included in this study were considered to be relevant for this assessment (see Model structure and methodology, below).
Other cost-effectiveness studies
The cost-effectiveness analysis in NG126 was largely based on the study by Allen et al. 109 However, this paper does not meet our inclusion criteria because faecal occult blood tests (FOBTs) were not considered in the analysis (the study109 investigated people with visible rectal bleeding and thus FOBTs were not relevant to this population). The cost-effectiveness of four diagnostic strategies (watchful waiting, flexible sigmoidoscopy, flexible sigmoidoscopy followed by air contrast barium enema, and colonoscopy) for patients with rectal bleeding was evaluated in this modelling study. The study was performed from a US-modified societal perspective. The authors concluded that colonoscopy was cost-effective compared with flexible sigmoidoscopy, with an ICER of US$5480 per QALY. Watchful waiting, defined as bleeding for 1 year followed by colonoscopy, was the most expensive strategy.
A second study, by Rae and Cleator,110 was identified, which assessed the cost-effectiveness of the Hemoccult assay, the HO Sensa assay, the HemeSelect assay and a two-tier test combing HO Sensa and HemeSelect. Only HemeSelect is a FIT assay; this is a qualitative method and, therefore, does not meet our inclusion criteria. This study110 was observational and its results showed that the costs per cancer detected were significantly lower for the two-tier test. Results were also presented for subgroups of asymptomatic and symptomatic patients. Symptomatic patients were defined as those who have:
-
a personal history of ulcerative colitis, Crohn’s disease or other IBDs, CRC, adenomas or polyps, or
-
a family history of CRC or polyps, or
-
one or more of the following symptoms: haemorrhoids, anal fissures, upper GI disease, diverticular disease, miscellaneous lower GI disease, or non-specific symptoms such as diarrhoea, nausea, bloating, fatigue, incontinence, visible blood in the faeces, melaena, weight loss, abdominal pain, anaemia, rectal pain, change in faeces calibre or change in bowel habits. This definition does not match the population specified for this assessment. The inclusion of people with a history or family history of cancer and the use of a qualitative FIT method mean that the study by Rae and Cleator110 was considered to be of limited value for the current decision problem.
Although the studies by Allen et al. 109 and Rae and Cleator110 did not meet our inclusion criteria, their characteristics are summarised in Appendix 5.
Model structure and methodology
A de novo health economic model was developed to explore the cost-effectiveness of using a quantitative FIT for Hb (occult blood) as a triage step in the investigation of symptomatic people presenting in the primary care who are at low risk of CRC, as defined in NG12. 1 The cost-effectiveness of faecal immunochemical testing was compared with both gFOBTs and no triage (referral straight to colonoscopy). The model consists of three parts: (1) a decision model reflecting the diagnosis of CRC; (2) a Markov state-transition model to estimate the long-term costs and effects [life-years (LYs) and QALYs] that are associated with the treatment and progression of CRC; and (3) a Markov state-transition model to estimate the LYs and QALYs that are associated with those who do not have CRC. Note that this second Markov model was not used to estimate costs because any differences in costs between the tests in those without CRC are assumed to occur only in the first year. The structure of the economic model used in this diagnostic assessment is similar to that used in NG12. 6 However, for some of the input parameters of our model, we could not find clear data from the literature. Thus, expert opinion was of key importance to the success of the model implementation. Therefore, we sent a questionnaire to 10 specialists; five of whom returned it, although not all questions were completely answered. The full questionnaire is included in Appendix 6 (example questions included the percentage of patients who would eventually remain symptomatic after a positive FIT/gFOBT result and the plausible range for this percentage). The model used in the economic analyses of this diagnostic assessment is described, in detail, below.
Diagnostic model structure
The model begins with a cohort of symptomatic patients, presenting in primary care in whom referral to secondary care for investigation of possible CRC is being considered. A patient in the cohort is offered one of the following choices: faecal immunochemical testing, guaiac faecal occult blood testing or no triage testing at all (referral straight to colonoscopy). A positive FIT or gFOBT results in referral to colonoscopy, whereas a negative test results in a watchful waiting strategy, in which a repeated test or further investigation can be performed when symptoms persist. Patients who had a FN gFOBT or FIT, and whose symptoms persisted, were assumed to receive a colonoscopy and thus be diagnosed within 1 year should they survive. Given the delay in diagnosis, it was also assumed that they would have an increased probability of progressing to a worse cancer state (i.e. these patients enter the CRC Markov model in a worse health state). This logically implies that no patients whose symptoms do not persist will have CRC, that is, all FIT-/gFOBT-negative patients who become asymptomatic do not have the target condition. These patients plus those found not to have CRC by colonoscopy were further modelled with a simple alive or dead Markov model, as described below (see Healthy population Markov model). Colonoscopy is considered to be the gold standard investigation for the diagnosis of CRC owing to its ability to visualise the entire colon and allow biopsies to be performed. 6 CTC is an alternative investigation of choice for people who are unfit for colonoscopy, but does not include biopsy. 6 For patients who test positive for CRC after colonoscopy or CTC, it was assumed that contrast-enhanced CT of the chest, abdomen and pelvis was performed, to determine the stage of disease. A time frame of 1 year was assumed for the diagnostic model. A schematic representation of the decision-analytic model is provided in Figure 12.
FIGURE 12.
Diagnostic decision-analytic model.
Colorectal cancer Markov model structure
The disease natural history used in the model was consistent with the existing UK-based screening economic models and divided the disease states by Dukes’ grading (i.e. stage A – tumour confined to the mucosa; stage B – tumour infiltrating through muscle; stage C – lymph node metastases present; and stage D – distant metastases). 111 The structure of the Markov model was similar to the one used in NG12,6 where cancer stages were defined based on the Dukes’ grading system for CRC and then mapped into the health states of the Markov model. The initial distribution of patients with CRC through the model’s health states was determined by the probability of being in a certain Dukes’ stage. After the initial distribution of patients in the CRC model was determined, it was assumed that patients may stay in their current health state, progress to the health state representing the next worst stage of the condition or die (from CRC or another cause). A schematic representation of the CRC model is provided in Figure 13. A lifetime horizon with a 1-year cycle length captures the probability of progression for treated and untreated patients.
FIGURE 13.
Colorectal cancer Markov model.
Healthy population Markov model
Colonoscopy is the reference standard used to determine the accuracy of quantitative FIT assays and guaiac faecal occult blood testing, for which CRC is the target condition. Thus, it was assumed that patients with a negative colonoscopy result do not have the target condition. It was also assumed that patients with the target condition will remain symptomatic; it therefore follows that all patients with a FN FIT or gFOBT result will remain symptomatic. All patients with a negative test result who become asymptomatic were assumed not to have the target condition.
Patients who do not have the target condition were further modelled with a simple alive or dead Markov model, as depicted in Figure 14. Survival estimates for this model were based on UK life tables. 98 Hence, patients entering this model can either die of any cause (including CRC, as a negative test result or a negative colonoscopy does not imply that these patients will never develop CRC) or stay in the alive health state. LYs and QALYs are accounted, but costs were not calculated in this model. This approach essentially implies two main assumptions for the difference in outcomes between intervention and comparator for those without CRC:
-
for life expectancy, this is caused only by a difference in mortality due to colonoscopy/CTC
-
for cost, this is due only to the difference in cost of guaiac faecal occult blood testing and colonoscopy/CTC.
FIGURE 14.
Healthy population Markov model.
This approach assumes that testing has no long-term (after 1 year) effect on costs or QALYs in disease-negative people and is consistent with our approach taken in previous NICE diagnostic assessments. 112 We have assumed that, in patients without CRC, faecal occult blood testing would not significantly delay diagnosis of the underlying cause of presenting symptoms and hence would not incur any extra cost or effect on mortality.
Model parameters
This section describes the input parameters used in the diagnostic model and in the Markov models and how their values were estimated for the base case.
Diagnostic model
Probabilities for faecal immunochemical testing and guaiac faecal occult blood testing diagnostic accuracy
Diagnostic accuracy was captured in the model using data on sensitivity and specificity that were obtained from our systematic review. For the base-case scenario, we considered a threshold of 10 µg Hb/g faeces or equivalent for the detection of CRC using a single faecal sample. This choice was based on the optimal threshold (maximum sensitivity and specificity) for each assay method and the threshold required to prove optimal rule-out performance (highest sensitivity and lowest number of cases missed), as explained in Chapter 3 (see Selection of test strategies for inclusion in cost-effectiveness modelling). For this threshold and target condition, our systematic review obtained data only for OC-Sensor and HM-JACKarc. Therefore, the FOB Gold assay was not included in the base-case analysis. Pooled estimates for OC-Sensor (see Table 6) were obtained from the studies by McDonald et al.,13 Mowat et al. ,52 Rodríguez-Alonso et al. 53 and Terhaar sive Droste et al. 58 Sensitivity and specificity for HM-JACKarc were obtained from Godber et al. 56 and are presented in Table 11. Finally, for guaiac faecal occult blood testing in the base-case scenario, we considered the sensitivity and specificity estimates used in NG12. 6 These estimates were reported by Gillberg et al. 91 This study91 reports that the sensitivity of guaiac faecal occult blood testing is 75% and the specificity is 87%. However, in the NG12 economic model,6 reported gFOBT sensitivity is 50% and the specificity is 88%. These figures were sourced from a subgroup of patients aged 41–50 years, because it was thought to better reflect the population considered in the NG12 economic model6 (i.e. patients aged ≥ 40 years with a change in bowel habit as main symptom). Nevertheless, our systematic review identified two papers92,93 reporting data on gFOBT accuracy, which are thought to represent a better match for the population specified for this diagnostic assessment. Therefore, additional scenarios, for which gFOBT accuracy data were based on these two studies,92,93 were explored.
For the probabilistic sensitivity analysis (PSA), we assumed beta distributions for sensitivity and specificity. The parameters of the beta distributions used in the base-case scenario are summarised in Table 23. Technical details about the estimation of the parameters of the different probability distributions are given in Appendix 10. Several scenarios based on different assumptions for the accuracy of faecal immunochemical testing/guaiac faecal occult blood testing were explored. These are described below (see Scenario analyses).
| Accuracy measure | OC-Sensor | HM-JACKarc | gFOBT |
|---|---|---|---|
| Sensitivity (95% CI) | 92.1% (86.9% to 95.3%) | 100% (71.5% to 100%) | 50% (15.0% to 85.0%) |
| Probability distribution (parameters) | Beta (155.90, 13.45) | Betaa (1.16, 0.23) | Beta (2, 2) |
| Specificity (95% CI) | 85.8% (78.3% to 91.0%) | 76.6% (72.6% to 80.3%) | 88% (85.0% to 89.0%) |
| Probability distribution (parameters) | Beta (100.80, 16.65) | Beta (380, 116) | Beta (962, 137) |
Our model also requires the calculation of the probability of having the condition or not, contingent on the test performance. When the disease prevalence is known, sensitivity and specificity can be used to calculate the PPVs and negative predictive values (NPVs) using the formulae below:
and
The prevalence of CRC in the base-case population was assumed to be 1.5%, the same as in NG12. 6 For the PSA, we assumed a triangular distribution of between 1% and 2%. The results of our systematic review showed that, for a threshold 10 µg Hb/g faeces or equivalent, and CRC as target condition, prevalence ranges between 2.1% and 5.4%. Therefore, the impact of this assumption on the cost-effectiveness results was explored with additional scenario analyses, for which the most likely value for the prevalence was assumed to be 3% and 5.4%, and with an additional scenario for which we used a triangular distribution with, most likely, a value of 1.5% and limits of 1% and 5.4% in the PSA.
Assuming that the prevalence of CRC in the base-case population is 1.5%, PPV and NPV estimates were calculated. Whereas the PPV was directly used as a node probability in the model (the probability labelled ‘C’ in Figure 12), the NPV was included in the model in the calculation of the probability that CRC is present for symptomatic patients after a negative FIT/gFOBT (the probability labelled ‘G’ in Figure 12). Details for probability (‘G’) are given below. PPV and NPV estimates for the base-case scenario are summarised in Table 24.
| Accuracy measure | OC-Sensor (%) | HM-JACKarc (%) | gFOBT (%) |
|---|---|---|---|
| PPV | 8.9 | 6.1 | 5.7 |
| NPV | 99.8 | 100 | 99.1 |
Probability of remaining symptomatic after a negative test result
The model assumes that only those patients with a negative test result, and whose symptoms do not persist, do not receive a colonoscopy/CTC. Therefore, it is for this group of patients that most of the savings in costs are expected, when the test strategies are compared with no triage (referral straight to colonoscopy). We did not identify any data, from the literature, on the proportion of patients with a negative faecal occult blood test result in whom symptoms will persist and who will eventually receive a colonoscopy/CTC. This information was therefore derived from clinical expert opinion, using the questionnaire described previously. In the questionnaire, we asked what percentage of patients who test negative with faecal immunochemical testing/guaiac faecal occult blood testing would persist in their symptoms and eventually undergo colonoscopy, and the plausible range for this percentage. In addition, we asked for the percentage of patients who would receive a second FIT/gFOBT. The responses to these two questions are presented in Table 25. It is essential to note that a second FIT/gFOBT is not considered to be a replacement for a colonoscopy, given that only the latter is diagnostic. Ideally, we would have liked to estimate the percentage of patients who get a colonoscopy that is contingent on the result of the second FIT/gFOBT, but the data on accuracy of repeat test were unavailable and it was believed that accuracy could not be adequately estimated by clinical experts. Therefore, in our model, having a second FIT/gFOBT increases the costs of only 20% (see Table 25) of the patients who, after testing negative, will remain symptomatic. The impact of this assumption on the cost-effectiveness results was explored in an additional scenario.
| Expert | % of patients who remain symptomatic (undergo colonoscopy) | Lowest (%) | Highest (%) | % of patients who get second FIT/gFOBT | Lowest (%) | Highest (%) |
|---|---|---|---|---|---|---|
| 1 | 55 | 30 | 80 | 30 | 20 | 40 |
| 2 | 10 | 5 | 15 | 10 | 5 | 15 |
| Mode (pooled average) | 32.5% | – | – | 20 | – | – |
| SD | 7.3 | – | – | 3.2 | – | – |
| Parameters beta distribution | α = 13.05; β = 27.11 | – | – | α = 31.05; β = 124.20 | – | – |
We assumed that the percentages of test-negative patients who undergo colonoscopy and the percentages who get a repeat FIT/gFOBT follow a triangular distribution, with the point estimate given by the experts representing the mode of the distribution. After simulating from these triangular distributions, we were able to estimate the pooled means and standard deviations (SDs) of the aforementioned probabilities, which are further assumed to have beta distributions. For the probability of undergoing colonoscopy after a negative result we found a mean of 32.5% and a SD of 7.3%, whereas for the mean probability and SD of getting a second FOB test were 20% and 3.2%, respectively.
Probability that colorectal cancer is present for symptomatic patients after a negative faecal immunochemical test/guaiac faecal occult blood test
This is the probability labelled ‘G’ in Figure 12. This probability can be calculated as ‘(1 – NPV)’, divided by the probability of remaining symptomatic after a negative test result (the latter probability was estimated by clinical experts as explained above); details of calculations are provided in Appendix 7. Defining ‘G’ as a quotient and having the probability of remaining symptomatic after a negative test result in the denominator, which is assumed to follow a beta distribution, may cause numerical problems when performing a PSA (numerical division by 0). Therefore, for the PSA, we assumed that the probability of remaining symptomatic after a negative test result was fixed to its mean value (0.325). The estimated probability ‘G’ for the base-case scenario is summarised in Table 26.
| Accuracy measure | OC-Sensor | HM-JACKarc | gFOBT |
|---|---|---|---|
| Probability (%) that CRC present for symptomatic patients after negative FIT/gFOBT | 0.4 | 0 | 2.65 |
Table 27 shows a summary of the probabilities used at the decision nodes in the diagnostic model. These were labelled as ‘A’ to ‘J’ in Figure 12. Full derivations of the equations are provided in Appendix 7.
| Label | Definition | Formula | Base-case value (%) |
|---|---|---|---|
| A | Probability that FIT/gFOBT is positive | (sensitivity × prevalence) + [(1 – specificity) × (1 – prevalence)] | OC-Sensor = 15.35 |
| HM-JACKarc = 24.53 | |||
| gFOBT = 13.02 | |||
| B | Probability that FIT/gFOBT is negative | 1 – (A) | OC-Sensor = 84.65 |
| HM-JACKarc = 75.47 | |||
| gFOBT = 86.98 | |||
| C | Probability that CRC is present after a positive FIT/gFOBT | PPV | OC-Sensor = 8.99 |
| HM-JACKarc = 6.11 | |||
| gFOBT = 5.75 | |||
| D | Probability that CRC is not present after a positive FIT/gFOBT | 1 – PPV | OC-Sensor = 91.01 |
| HM-JACKarc = 93.89 | |||
| gFOBT = 94.25 | |||
| E | Probability that symptoms persist after a negative FIT/gFOBT | NA – based on expert opinion | 32.50 |
| F | Probability that symptoms do not persist after a negative FIT/gFOBT | 1 – (E) | 67.50 |
| G | Probability that CRC is present for symptomatic patients after a negative FIT/gFOBT | (1 – NPV)/(E) | OC-Sensor = 0.43 |
| HM-JACKarc = 0 | |||
| gFOBT = 2.65 | |||
| H | Probability that CRC is not present for symptomatic patients after a negative test | 1 – (G) | OC-Sensor = 99.57 |
| HM-JACKarc = 100 | |||
| gFOBT = 97.35 | |||
| I | Probability that CRC is present | Prevalence | 1.5 |
| J | Probability that CRC is not present | 1 – (I) | 98.5 |
Probability of being referred to colonoscopy or CTC after a positive test result:
If the gFOBT or FIT result is positive, patients are referred to either colonoscopy or CTC. To estimate the percentage of patients who are referred to each of these options in the UK, we also relied on expert opinion. The responses to this question are presented in Table 28.
| Expert | % of patients referred to colonoscopy | Lowest | Highest |
|---|---|---|---|
| 1 | 75 | NR | NR |
| 2 | 95 | 90 | 100 |
| 3 | 93 | NR | NR |
| 4 | 90 | 85 | 95 |
| Mean (pooled) | 88.3 | – | – |
| SD | 1.49 | – | – |
| Parameters beta distribution | α = 417.40; β = 55.81 | – | – |
We adopted the approach described above for estimating the probability of remaining symptomatic after having a negative test result. In this case, for the probability of being referred to colonoscopy versus CTC after a positive test result we calculated a mean of 88.3% and a SD of 1.49%. The impact of this assumption was explored in an additional scenario.
Probability of adverse events associated with colonoscopy
Our diagnostic model considers the probability of experiencing adverse events that are associated with colonoscopy. The economic model presented in the NG126 was used as a starting point for selecting the adverse events to be included. The economic model in NG126 includes two adverse events that are related to colonoscopy: bowel perforation and bleeding. Probability data for these adverse events were obtained from Gavin et al. 105 This study was a 2-week audit, performed in the UK, which provided data on colonoscopy completion rates and associated adverse events.
A literature search was performed to assess whether or not the adverse events and associated probabilities used in NG126 were complete and still appropriate for the current decision problem. Our search identified five potentially relevant studies. 113–117 These were four reviews113–116 and one population-based study. 117 Two of the reviews, by Manta et al. 115 and Church,116 were overviews that did not include a systematic approach to searching literature; neither did these reviews include further analysis. The two other reviews, by Reumkens et al. 113 and Day et al. ,114 were systematic literature reviews and both included a meta-analysis. The review by Day et al. 114 studied adverse events in elderly patients who were undergoing colonoscopy. Elderly patients were defined as those who were ≥ 65 years of age. Therefore, this study114 was of limited relevance to the population that was specified for this assessment. The review by Reumkens et al. 113 studied post-colonoscopy complications in population-based studies. It is a very recent systematic review; it provides estimates of adverse events rates, including CIs, and is not limited to a specific patient population. The estimates provided for bowel perforation and bleeding are very similar to those in Gavin et al. ,105 which were the ones used in the cost-effectiveness analysis performed in NG12. 6 The studies by Gavin et al. 105 and Saraste et al. 18 were population-based studies. These were deemed to be of less relevance, as systematic literature reviews are preferred over single studies. Therefore, the study by Reumkens et al. 113 was deemed to be the most appropriate study to populate the probability of adverse events due to colonoscopy in the diagnostic model. The adverse events included in the model are bowel bleeding, perforation and death. The corresponding probabilities and the parameters of a beta distribution used for the PSA are presented in Table 29.
| Adverse event | Rate % (95% CI) | Beta distribution parameters | Source |
|---|---|---|---|
| Bleeding | 0.26 (0.17 to 0.37) | α = 25.82; β = 9887.90 | Reumkens 2016113 |
| Perforation | 0.05 (0.04 to 0.07) | α = 49.52; β = 91,719.86 | |
| Death | 0.0029 (0.0011 to 0.0055) | α = 6.40; β = 222,513 |
The probabilities of experiencing adverse events due to CTC are scarce in the literature. The study of Burling et al. 118 found that the proportion of patients with symptomatic perforation that was attributable to CTC was 0.03% but none of these was classified as a serious adverse event. The same article states that perforation rates for colonoscopy are four times higher than for CTC. No death events were reported and the proportion of patients experiencing bleeding after examination was not described in this study. Owing to the uncertainty around these parameters and in order to keep the model simpler, we assumed that, in the base-case scenario, adverse event rates due to CTC were the same as those of colonoscopy. Give that this assumption will result in an overestimation, it can be interpreted as a worst-case scenario regarding the total number of adverse events. In addition, as, on average, only 11.7% of the patients receive CTC (as opposed to colonoscopy) the impact of this assumption is expected to be very small. We explored the impact of this assumption on the cost-effectiveness results by performing additional scenario analyses.
Costs
Direct costs included in the model are test costs, costs of colonoscopy/CTC, adverse event costs and CT costs. Indirect costs parameters were not included in the model, given the perspective of the NHS.
Test costs
Faecal immunochemical testing and guaiac faecal occult blood testing cost estimates reported in the literature vary quite significantly, and the assumptions made to derive those estimates are not clear in the majority of cases. Therefore, in this section we present the cost estimates used in this diagnostic assessment and the underlying assumptions leading to them. At the end of this section, several test costs from different sources are compared (see Table 33).
Faecal immunochemical test To estimate the total costs of a FIT, we assumed that these include the following subcategories: costs of the collection device, acquisition costs of the analyser, cost of materials to analyse the collected sample (i.e. reagents, buffer, reaction cells, bottles) and costs of maintenance of the analyser. The manufacturers were the only source of unit costs. Training costs and the costs of the laboratory staff for analysing the test results were not included in the total costs because it was assumed that these costs are the same for faecal immunochemical testing and guaiac faecal occult blood testing, and, therefore, these costs have no impact on the incremental cost-effectiveness results when these two alternatives are compared.
Costs of the collection device For both the FIT and gFOBT, a faecal sample from the patient is required. The patient has to collect the sample at home. The collection device is an instrument, designed for patient use, to collect the sample in a hygienic and easy way. Generally, the device consists of a vessel that contains a buffer and a probe to collect the faecal sample. 119
Acquisition costs of analyser Resource use data are needed to estimate the acquisition costs of the FIT analysers. The report from the Association of Coloproctologists of Great Britain and Ireland 2015120 suggests that, for a population of 500,000, up to 6000 colonoscopies and 4000 flexible sigmoidoscopies will be needed annually if international comparisons and trends are followed. These figures were also validated by expert opinion. Therefore, it was assumed that 10,000 colonoscopies would be required to deal with referrals from primary care per 500,000 people. Assuming that there is one laboratory per 500,000 people and that 10,000 requests for faecal immunochemical testing are made each year (at 253 ‘working days’ per annum) would imply that approximately 40 requests would be received each day. Thus, only one analytical system would be needed, which would be run for only a short period of time. In order to have the minimal turnaround time that is a prerequisite for such a service, the analyser would need to be run each day. For the acquisition costs of the analyser, we considered the prices provided by the manufacturers (list pricing based on the capital purchase of an instrument). As the time frame for the diagnostic part of the health economic model is 1 year, the total cost of the analyser was used. A summary of the estimated resource is presented in Table 30.
| Resource usea | |
|---|---|
| Average daily workload, UK | 40 |
| No. of samples analysed per year | 10,000 |
| No. of machines required to analyse daily workload (per year) | 1 |
Costs of reagents Material costs include costs of the reagents, buffer, reaction cells and analyser cups. For OC-Sensor, the manufacturer provided prices for only the reagents (£0.80). Therefore, it was assumed that this price also includes the costs of buffers and other consumables. The manufacturer of HM-JACKarc provided information on all of the materials. The costs of the buffers and reaction cells were given per package. These were converted to costs per test.
Costs for maintenance of the analyser Maintenance costs include costs of calibration, costs of daily controls, service costs and costs for cleaning the analyser. For OC-Sensor, only the costs of a calibration kit and costs of daily controls were provided by the manufacturer. The costs of the calibration kit were given per half-year for OC-Sensor. No information about calibration costs was provided for HM-JACKarc. The manufacturer of OC-Sensor recommends that, before the analysis of samples, a single high and a single low control should be analysed, that is, one low test and one high test per day. This information was not provided for HM-JACKarc, and hence we assumed that it was the same. Finally, the cost of washing material was provided only for HM-JACKarc.
Guaiac faecal occult blood test Costs of guaiac faecal occult blood testing were obtained from the literature and from clinical experts. Estimates found in the literature range from (confidential information has been removed) up to £9.57 per gFOBT. However, in most cases it is not clearly reported how the costs were estimated. For those reasons, the cost estimate provided by clinical experts was chosen for the base-case scenario. A summary of the different cost estimates for guaiac faecal occult blood testing considered in this diagnostic assessment is given in Table 31.
| Total cost per test (£) | Source |
|---|---|
| 0.7758a | Personal communication: e-mail from Callum Fraser, 19 July 2016 |
| (Confidential information has been removed) | Personal communication: e-mail from Jacqueline Murphy, 21 July 2016 |
| 9.57b | Sharp 2012122 |
| 1.46c | Whyte 2011123 |
| 4.86 | NG126 |
Table 32 presents the estimated costs for the OC-Sensor and the HM-JACKarc FIT assays. The information provided by the manufacturer did not allow the cost of the FOB Gold system to be estimated in detail, hence FOB Gold is not included in Table 32; total cost is provided in Table 33.
| Cost item | OC-Sensor (£) | HM-JACKarc (£) |
|---|---|---|
| Test costs | ||
| Reagents | 0.80 | 0.49 |
| Sample device | 1.30 | 2.10 |
| Total costs per test | 2.10 | 2.59 |
| Material costs | ||
| Measurement device (per year) | 19,061.00 | 22,458.00 |
| Measurement materials (per year) | ||
| Bottles | NR | 910.40a |
| Analyser control cups | 40.00b | NR |
| Maintenance (per year) | ||
| Service costs | NR | 0.00 |
| Auto washer | NR | 10.18c |
| Calibration kit | 1200.00d | NR |
| Daily controlse | 4048.00 | 11,134.53 |
| Total costs of device and maintenance (per year) | 24,349.00 | 34,513.11 |
| Material cost per test | 2.43 | 3.45 |
| Total costs | ||
| Total cost per test (including material costs) | 4.53 | 6.04 |
| FIT assay | Total cost per test (£) | Source |
|---|---|---|
| OC-Sensor | 4.53 | This diagnostic assessment |
| HM-JACKarc | 6.04 | This diagnostic assessment |
| FOB Gold | 1.96 | This diagnostic assessment |
| NR | 2.15a | Grazinni 2008121 |
| NR | 10.23b | Sharp 2012122 |
| NR | 9.42c | NG126 |
| NR | 3.63 | Whyte 2011123 |
| NR | (Confidential information has been removed) | Personal communication [e-mail from Jacqueline Murphy, Health Economics Research Centre (HERC), Nuffield Department of Population Health, University of Oxford, to Marie Westwood, KSR Ltd, 21 July 2016] |
A summary of the different cost estimates for faecal immunochemical testing that have been considered in this diagnostic assessment and compared with the literature/other sources can found in Table 33.
Other costs included in the diagnostic model
Estimates of the costs of colonoscopy, CTC and CT were obtained from the NHS Reference Costs 2014–15. 124 We estimated the costs of colonoscopy as the average cost of diagnostic colonoscopy (for adults aged ≥ 19 years) with and without biopsy, because, as stated in NG12,6 a biopsy may be taken during the colonoscopy investigation. For the CTC costs, we followed the approach by Halligan et al. 104 and, in the absence of a specific UK national cost for CTC, we used the cost of CT (more than three areas) as an approximation. Finally, to estimate the CT costs, we calculated the average of all of the CT procedures that were available for adults, that is, CT of one, two, three or more areas with or without (pre and/or post) contrast and complex CT [Healthcare Resource Group (HRG) codes RD20A, RD21A, RD22Z, RD23Z, RD24Z, RD25Z, RD26Z, RD27Z and RD28Z – Outpatient department].
Costs associated with GP and gastroenterology outpatient appointments were also considered in the model. In particular, we assumed that the cost of colonoscopy/CTC includes the costs of a follow-up appointment with a gastroenterologist. The definition of our patient population, as symptomatic people presenting in the primary care who are at low risk of CRC, implies that patients have already visited the GP (and therefore these costs have been incurred) before entering the model. Only for test-negative patients whose symptoms persist were additional GP appointment costs considered.
Costs of bleeding and perforation were estimated based on the average length of hospital stay that was associated with each of these adverse events. Average length of stay was obtained from the study of Gavin et al. 105 The average length of stay for bleeding due to colonoscopy was 1.7 days and for perforation 9.1 days. To estimate the total costs, these were multiplied by the NHS cost of a regular day or night admission.
All of these costs (with the input parameters used in the PSA) are summarised in Table 34.
| Type of cost | Mean cost (£) | Gamma distribution parameters | HRG codes | Source |
|---|---|---|---|---|
| Investigation | ||||
| Diagnostic colonoscopy | 372 | (4.12, 90.15) | Average of FZ51Z (Diagnostic Colonoscopy, 19 years and over) and FZ52Z (with biopsy) – Outpatient procedures (Gastroenterology) | NHS Reference Costs 2014–15 124 |
| CTC | 136 | (9.18, 14.80) | RD27Z (Computerised Tomography Scan of more than three areas) – Outpatient department | Halligan 2015104 and NHS Reference Costs 2014–15124 |
| CT | 116 | (7.56, 15.32) | Average of RD20A, RD21A, RD22Z, RD23Z, RD24Z, RD25Z, RD26Z, RD27Z and RD28Z – Outpatient department | NHS Reference Costs 2014–15 124 |
| Referral | ||||
| GP visit | 44 | NA | NA | PSSRU 2015 – table 10.8b125 |
| Gastroenterology outpatient appointment | 135 | (9.02, 14.97)a | Total outpatient attendances – Gastroenterology (Service code 301) | NHS Reference Costs 2014–15 124 |
| Adverse event | ||||
| Bleeding | 603 | (9.02, 66.78)a | RP (Regular Day or Night Admissions) – £354.67; average length of stay 1.7 days | Gavin 2012105 and NHS Reference Costs 2014–15124 |
| Perforation | 3228 | (9.02, 357.47)a | RP (Regular Day or Night Admissions) – £354.67; average length of stay 9.1 days | Gavin 2012105 and NHS Reference Costs 2014–15124 |
| Death | 0 | NA | Assumption | |
Utilities
No evidence was found on the effects of bleeding and perforation on quality of life. Thus, in line with the cost-effectiveness analysis performed in NG12,6 our diagnostic model did not include disutilities for adverse events. In addition, as these events are often of short duration, the effects on quality of life can be assumed to be negligible. Therefore, the only utilities that were considered in our diagnostic model were those associated with the different stages of cancer (from Ness et al. 95) and the general population sex- and age-related utilities for healthy patients (from Kind et al. 96). These utilities are described in detail below.
Colorectal cancer Markov model
Initial distribution of patients per Dukes’ stage
The initial distribution of patients with CRC over the model’s health states was determined by the probability of being in a certain Dukes’ stage at diagnosis. In NG12,6 this was estimated using data from the NCIN,98 which showed the percentage of patients in England diagnosed at each stage of CRC between 1996 and 2002. The reported percentages per Dukes’ stage were 13%, 37%, 36% and 14% for stages A–D, respectively, for 202,694 patients. The paper by Cubiella et al. ,55 included in our systematic review (see Chapter 3, Diagnostic performance of the OC-Sensor faecal immunochemical test assay), also reported the sensitivity of OC-Sensor for determining the stage and location of CRC using a single faecal sample when a threshold of ≥ 20 µg Hb/g faeces is considered. The total number of patients with CRC in this study was 91, and the percentages per stages A–D were 16%, 25%, 44% and 14%, respectively. We also consulted about this with our clinical experts and the percentages (average between four experts) per stages A–D were 19%, 35%, 32%, and 15%, respectively. All of these values are summarised in Table 35. For the base-case scenario, we considered the estimates from NCIN as in NG12. 6 The main reasons were that the sample size used to obtain these estimates is large in NCIN and data were obtained from UK population; therefore, the estimated percentages can be considered more reliable than those in Cubiella et al. 55 In addition, estimates derived from NCIN were similar to those provided by clinical experts who answered our questionnaire.
| Dukes’ stage | Source | |||
|---|---|---|---|---|
| A | B | C | D | |
| 26,727 (13%) | 74,784 (37%) | 72,806 (36%) | 28,377 (14%) | Patients diagnosed between 1996 and 2002, England (NICN)98 |
| 15 (16%) | 23 (25%) | 40 (44%) | 13 (14%) | Cubiella 2014 (COLONPREDICT)55 |
| 38,512 (19%) | 70,943 (35%) | 64,862 (32%) | 30,404 (15%) | Expert opinion and assuming total figure in NCIN |
Probability of progression
The probability of progression was modelled as in NG12. 6 In the clinical guideline, it was noted that obtaining observed probabilities of progression for the patient population considered was unlikely. Therefore, in the absence of such evidence, the probability of progression was estimated using progression probabilities for undiagnosed patients with CRC, which were obtained from Tappenden et al. 99 The uncertainty within the model results resulting from this assumption was explored in the sensitivity analysis. The progression probabilities used in the model are presented in Table 36.
| CRC Dukes’ stage transition | Annual progression probability – undiagnosed CRC (95% CI) | PSA distribution | Source |
|---|---|---|---|
| A to B | 0.58 (0.57 to 0.59) | Uniform | Tappenden 200799 |
| B to C | 0.66 (0.64 to 0.67) | ||
| C to D | 0.87 (0.85 to 0.88) |
Delayed diagnosis
Our model assumes that patients who had a FN gFOBT/FIT, and whose symptoms persisted, have an increased probability of progressing to a worse cancer state, given the delay in diagnosis. This was implemented in the model by shifting the initial distribution of patients per Dukes’ stages in Table 35 according to the progression probabilities shown in Table 36. Note that, for the base-case scenario, the initial proportions of patients per Dukes’ stage were 13% (A), 37% (B), 36% (C) and 14% (D). Using the annual progression probabilities for undiagnosed patients with CRC in Table 36, that is, assuming a delay of 1 year, the proportions of patients per Dukes’ stage were estimated as 6% (A), 20% (B), 29% (C) and 45% (D). However, it was assumed that FN patients would persist in their symptoms and they would be diagnosed within 1 year by colonoscopy or CTC. Therefore, it seems reasonable to assume that a delay of 1 year would overestimate the time to delayed diagnosis. For that reason, we assumed a delay of 6 months for the base-case scenario. The probabilities of progression between Dukes’ stages at 6 months were calculated by halving those in Table 36 (0.29 from A to B, 0.33 from B to C and 0.435 from C to D). This method implicitly assumes a uniform distribution of yearly progression probabilities. Assuming a delay of 6 months, the proportions of patients per Dukes’ stage were estimated as 9% (A), 29% (B), 32% (C) and 30% (D). A summary of the number of cases per stage, with and without delay in diagnosis, are presented in Table 37. In the PSA, this was modelled as a Dirichlet distribution, with the number of cases as the parameters of the probability distribution.
| Number of cases | Dukes’ stage | |||
|---|---|---|---|---|
| A | B | C | D | |
| Initial | 26,727 (13%) | 74,784 (37%) | 72,806 (36%) | 28,377 (14%) |
| Delayed 6 months | 18,976 (9%) | 57,856 (29%) | 65,814 (32%) | 60,048 (30%) |
| Delayed 1 year | 11,225 (6%) | 40,928 (20%) | 58,822 (29%) | 91,718 (45%) |
Survival probability per Dukes’ stage
Disease-specific mortality was also estimated using data from the NCIN, with the reported 5-year survival rates used as a starting point for extrapolation. 98 Tables 38 and 39 show the 5-year survival probabilities with 95% CIs at each Dukes’ stage, and the observed survival probabilities at each year per Dukes’ stage, from NCIN data, respectively. 98
| Stage at diagnosis | 5-year relative survival (%) | 95% CI |
|---|---|---|
| Dukes’ A | 93.20 | 92.5 to 93.9 |
| Dukes’ B | 77.00 | 76.4 to 77.5 |
| Dukes’ C | 47.70 | 47.1 to 48.3 |
| Dukes’ D | 6.60 | 6.1 to 7.0 |
| Year | Stage observed | |||
|---|---|---|---|---|
| Dukes’ A | Dukes’ B | Dukes’ C | Dukes’ D | |
| 0 | 100 | 100 | 100 | 100 |
| 1 | 96.68 | 91.17 | 80.67 | 37.39 |
| 2 | 96.11 | 86.74 | 67.06 | 18.55 |
| 3 | 95.22 | 82.50 | 57.56 | 11.03 |
| 4 | 94.17 | 78.94 | 51.12 | 8.07 |
| 5 | 92.64 | 76.29 | 46.87 | 6.43 |
It was not possible to properly extrapolate survival curves beyond year 5 using these data. We therefore used available 15-year predicted survival data from NG126 (e-mail from Matthew Prettyjohns, National Collaborating Centre for Cancer, Cardiff, to Marie Westwood, KSR Ltd, 7 July 2016, personal communication) to model CRC survival/mortality in the 15 first cycles of the CRC Markov model. These probabilities are shown in Appendix 8. Furthermore, we assumed that, after year 15, CRC-related mortality remains constant. However, overall mortality increases after year 15 owing to the inclusion of age-specific mortality based on UK life tables. 98 The annual mortality estimates and the parameters of beta distributions used in the PSA are also shown in Appendix 8. Finally, we used two different data sources for the transition probabilities: annual mortality per Dukes’ stage and annual progression (for undiagnosed patients) between Dukes’ stages separately. To accommodate these two data sets in the CRC model, we assumed that transitions between the states of the model occurred in two steps. First we calculated the number of patients dying per cycle and then, conditional on survival, we calculated progression between Dukes’ stages. The implications of these assumptions were explored in the sensitivity analysis, and further discussed in Chapter 5 of this report.
Costs
Estimated total costs were collected over the modelled lifetime horizon. The costs considered in the model reflect those that are relevant to the NHS and Personal Social Services. The total costs include initial and follow-up investigations, staging, treatment, drug costs and any other resource use that may be required (e.g. GP visit). All of the costs were taken from NG12. 6 Data on lifetime costs associated with CRC (based on the stage of cancer at diagnosis) were sourced from Tappenden et al. 99 and inflated to 2015 prices using the 2014/15 Hospital and Community Health Services index available from the PSSRU. 125 All of the costs applied in the model are shown in the Table 40. The costs associated with the health states of the CRC Markov model were estimated as lifetime costs (i.e. one-off cost). To properly account for discounting, it is necessary to calculate costs for each cycle of the Markov model (i.e. costs per year) in order to apply the corresponding discount rate per year. We assume that these lifetime costs are uniformly distributed over the model’s time horizon. It can be argued that this assumption may not reflect accurately the distribution per year of lifetime costs. However, given the low CRC prevalence in the patient population, this assumption is expected to have little impact on the model results.
| Cancer stage | Mean cost, £ (SE) | Gamma distribution parameters (α, β)a | Source |
|---|---|---|---|
| Dukes’ A | 10,681 (3959) | (7.28, 1467.43) | Tappenden 200799 (inflated to 2015) |
| Dukes’ B | 18,011 (6676) | (7.28, 2474.54) | |
| Dukes’ C | 29,139 (10,800) | (7.28, 4002.88) | |
| Dukes’ D | 19,391 (7187) | (7.28, 2663.76) |
Utilities
Health benefits were expressed in terms of LYs and QALYs (QALYs) gained. QALYs were estimated by combining the LY estimates, with utility values associated, with being in a particular health state of the CRC model. These utility values were sourced from the Ness et al. study95 and were also used in the cost-effectiveness model developed for NG12. 6 The study by Ness et al. 95 concluded that factors such as age and sex were not significant predictors for any utility value. Therefore, the utilities applied in the model are dependent only on the CRC stage and remain constant for all model cycles. The utilities included in the CRC Markov are presented in Table 41.
| Model state | QoL | Beta distribution (alpha, beta) | Source |
|---|---|---|---|
| Dukes’ A | 0.74 | (145.00, 51.69) | Ness 199995 |
| Dukes’ B | 0.70 | (56.60, 24.53) | |
| Dukes’ C | 0.50 | (33.78, 32.28) | |
| Dukes’ D | 0.25 | (1.03, 2.35) |
Healthy population Markov model
This model accounted only for LYs and QALYs accrued on a healthy health state over a lifetime. Age- and sex-specific all-cause mortality estimates were based on UK life tables. 98 In particular, we assumed, for the base-case scenario, patients aged ≥ 40 years, as in NG12. 6 For the proportion of female patients, we used the figures reported in the Gillberg et al. study,91 in which 715 females and 388 males were included. Mortality estimates, and the corresponding parameters of a beta distribution for the PSA, can be found in Appendix 9. Utilities were sourced from Kind et al. 96 Unlike in NG12,6 for which only a mean utility value equal to 0.79 was considered for all healthy patients, we included sex- and age-related utilities for every cycle of the Markov model. These were calculated as a weighted average, using the proportion of females reported in Gillberg et al. 91 (35% male, 65% female). These utilities are presented in Table 42.
| Age (years) | Weighted average | Males | Females | |
|---|---|---|---|---|
| 35–44 | Mean | 0.91 | 0.91 | 0.91 |
| SD | 0.11 | 0.17 | 0.15 | |
| Beta (α, β) | (5.24, 0.51) | – | – | |
| 45–54 | Mean | 0.85 | 0.84 | 0.85 |
| SD | 0.18 | 0.27 | 0.23 | |
| Beta (α, β) | (2.49, 0.44) | – | – | |
| 55–64 | Mean | 0.80 | 0.78 | 0.81 |
| SD | 0.20 | 0.28 | 0.26 | |
| Beta (α, β) | (2.40, 0.60) | – | – | |
| 65–74 | Mean | 0.78 | 0.78 | 0.78 |
| SD | 0.19 | 0.28 | 0.25 | |
| Beta (α, β) | (2.92, 0.82) | – | – | |
| ≥ 75 | Mean | 0.72 | 0.75 | 0.71 |
| SD | 0.20 | 0.28 | 0.27 | |
| Beta (α, β) | (2.90, 1.13) | – | – | |
Overview of main model assumptions
Table 43 summarises the main assumptions made in our economic model.
| General | Source |
|---|---|
| 1. It was assumed that a lifetime horizon with a 1-year cycle length captures the probability of progression for treated and untreated patients | Assumption/NG126 |
| 2. Any differences in costs between the tests in patients without CRC were assumed to occur only in the first year | Assumption |
| 3. Any differences in life expectancy between intervention and comparator for patients without CRC are due only to the difference in mortality due to colonoscopy/CTC | |
| 4. Any differences in costs between intervention and comparator for patients without CRC are only due to difference in cost of guaiac faecal occult blood testing and colonoscopy/CTC | |
| 5. Testing has no long-term (after 1 year) effect on costs or QALYs in disease-negative people. Thus, in patients without CRC, faecal occult blood testing would not significantly delay diagnosis of the underlying cause of presenting symptoms and hence would not incur any extra cost or effect on mortality | |
| Diagnostic model | |
| 6. A time frame of 1 year was assumed for the diagnostic model | NG126/expert opinion |
| 7. A positive FIT/gFOBT results in referral to colonoscopy | |
| 8. A negative FIT or gFOBT results in a watchful waiting strategy, in which a colonoscopy/CTC will be performed when symptoms persist. A repeat FIT/gFOBT might also be performed, but referral to colonoscopy/CTC is not modelled as being contingent on the results of the repeat test | |
| 9. The sensitivity and specificity of colonoscopy for detection of CRC is 100%. Thus, patients with a positive colonoscopy/CTC all have CRC and those with a negative colonoscopy result do not have the target condition | Assumption |
| 10. The symptoms of all those patients with CRC who are FNs will persist such that they will all receive a colonoscopy and thus be diagnosed (within 1 year) should they survive | NG126/expert opinion |
| 11. Patients who had FN gFOBT or FIT, and whose symptoms persisted, have an increased probability of progressing to a worse cancer state due to the delay to diagnosis | |
| 12. Probability of delayed diagnosis of CRC was assumed to be the probability of progression within Dukes’ states at 6 months | Assumption |
| 13. TN, FP and FN patients who become asymptomatic do not have the target condition | |
| 14. The model assumes that only those patients with a negative test result, and whose symptoms do not persist, do not receive a colonoscopy/CTC | |
| 15. A CT scan of the chest, abdomen and pelvis is performed for all of the patients testing positive for CRC after colonoscopy or CTC, to estimate the stage (Dukes’ A–D) of the disease | NG126 |
| 16. For the base-case scenario, we considered a threshold of 10 µg Hb/g faeces or equivalent for the detection of CRC using a single faecal sample. Other options were explored in sensitivity analyses | Assumption |
| 17. The prevalence of CRC in the base-case population was assumed to be 1.5% | NG126 |
| CRC Markov model | |
| 18. After the initial distribution of patients in the CRC model is determined, patients may stay in their current health state, progress to the health state representing the next worsening in the condition or die (from CRC or another cause) | NG126 |
| 19. Costs associated with the health states of the CRC Markov model were estimated as lifetime costs (i.e. one-off cost) | |
| Healthy population Markov model | |
| 20. Patients entering this model can either die of all of the causes or stay in the ‘alive’ health state | Assumption |
| 21. We assumed for the base-case scenario patients aged ≥ 40 years | NG126 |
| Adverse events | |
| 22. The adverse events included in the diagnostic model are bowel bleeding, perforation and death | Literature |
| 23. Reduction of quality of life due to adverse events is assumed to be negligible within a lifetime | Assumption |
| 24. No costs of patients who die due to adverse events of colonoscopy | |
| 25. Adverse events due to CTC were assumed to be the same as those of colonoscopy | |
| Test costs | |
| 26. Costs of laboratory staff to analyse the test were assumed to be the same for faecal immunochemical testing and guaiac faecal occult blood testing | Assumption |
| 27. Training costs and the costs of the laboratory staff for analysing the test results were not included in the total costs because it was assumed that these are the same for faecal immunochemical testing and guaiac faecal occult blood testing | |
| 28. Costs of the material needed to analyse a sample include costs of the reagents, buffer, reaction cells and analyser cups | |
| 29. Maintenance costs for HM-JACKarc and OC-Sensor were assumed to be equal | |
| Other costs | |
| 30. Costs of colonoscopy/CTC, adverse event costs and CT costs were included in the diagnostic model | NG126 |
| 31. We assumed that the cost of colonoscopy/CTC includes the costs of a follow-up appointment with a gastroenterologist | Assumption/expert opinion |
| 32. For test-negative patients whose symptoms persist, an additional GP appointment cost was considered | Assumption |
| 33. Indirect costs parameters were not included in the model, given the perspective of the NHS | |
Model analyses
Expected costs and effects, the latter expressed in LYs and QALYs, were estimated for all of the diagnostic tests considered in this assessment. Costs were estimated from the perspective of the NHS in England and Wales. All costs and effects were discounted by 3.5%. The model’s time horizon was set to lifetime. Incremental costs and incremental QALYs for each strategy compared with the next best alternative and compared with the comparators (colonoscopy or guaiac faecal occult blood testing) were calculated. By dividing the incremental costs by the incremental QALYs, the ICER was then calculated. The uncertainty about the model input parameters and the potential impact on the model results were explored by scenario, one-way deterministic analyses and PSAs. A series of one-way sensitivity analyses were conducted, whereby the value of one input parameter was changed and its effect on the overall outcome was recorded and assessed. The impact of statistical uncertainties regarding the model’s input parameters was explored through PSA. The results of 5000 iterations were presented in the cost-effectiveness plane for all of the interventions compared. Cost-effectiveness acceptability curves (CEACs) were used to describe the probability of an intervention being considered cost-effective, given a threshold ICER. The different probability distributions used in the PSA were described above (see Model structure and methodology).
Scenario analyses
Scenario analyses were performed to explore the impact on costs and QALYs of using different assumptions on diagnostic accuracy of faecal immunochemical testing/guaiac faecal occult blood testing, prevalence, test costs, initial and delayed CRC diagnosis, probability of CRC progression, probability of remaining symptomatic after a negative test result, adverse events (including mortality) attributable to colonoscopy, probability of being referred to colonoscopy (vs. CTC) and probability of receiving a second FIT/gFOBT.
Diagnostic accuracy of faecal immunochemical testing/guaiac faecal occult blood testing
In the base-case scenario, we assumed for faecal immunochemical testing a threshold of 10 µg Hb/g faeces or equivalent for the detection of CRC using a single faecal sample. For guaiac faecal occult blood testing we assumed the accuracy estimates in NG12. 6 In these additional scenarios, we considered other sources for the accuracy estimates for guaiac faecal occult blood testing, other thresholds for faecal immunochemical testing, and the inclusion of FOB Gold as an intervention in the analysis. A summary of the additional scenarios on the accuracy of faecal immunochemical testing/guaiac faecal occult blood testing can be seen in Table 44.
| Scenario | Threshold FIT | FIT accuracy estimates (sensitivity, specificity)a | gFOBT accuracy estimates: sensitivity, specificity |
|---|---|---|---|
| Base case | 10 µg Hb/g faeces | OC-Sensor (92.1%, 85.8%); HM-JACKarc (100%, 76.6%) | 50.0%, 88.0%1 |
| Accuracy–I | 10 µg Hb/g faeces | OC-Sensor (92.1%, 85.8%); HM-JACKarc (100%, 76.6%) | 69.2%, 73.2%92 |
| Accuracy–II | 10 µg Hb/g faeces | OC-Sensor (92.1%, 85.8%); HM-JACKarc (100%, 76.6%) | 75%, 79.4%93 |
| Accuracy–III | 0 µg Hb/g faeces | OC-Sensor (100%, 43.3%) | 50.0%, 88.0%1 |
| Accuracy–IV | OC-Sensor ≥ 20 µg Hb/g faeces; (confidential information has been removed) | OC-Sensor (89.5%, 86.6%); (confidential information has been removed) | 50.0%, 88.0%1 |
| Accuracy-V | OC-Sensor and HM-JACKarc 10 µg Hb/g faeces; (confidential information has been removed) | OC-Sensor (92.1%, 85.8%); HM-JACKarc (100%, 76.6%); (confidential information has been removed) | 50.0%, 88.0%1 |
Prevalence of colorectal cancer
In the base-case scenario, it was assumed that the prevalence of CRC was 1.5%, as in NG12. 6 We explored the scenarios in which prevalence was assumed to be 3% (also explored in NG126) and 5.4% (the highest value found in our systematic review). We considered an additional scenario with a wide uncertainty range (mean 1.5%, lower limit 1% and upper limit 5.4%) to investigate how this would impact on the PSA results.
Costs of faecal immunochemical testing/guaiac faecal occult blood testing
The cost estimates for faecal immunochemical testing and guaiac faecal occult blood testing that are reported in the literature vary significantly and the assumptions made to derive those estimates are not always presented in a transparent way. Whereas the cost estimates used in this diagnostic assessment are properly reported, we acknowledge that there is uncertainty around the underlying assumptions leading to them. For this reason, we considered additional scenarios on the test costs. In particular, we performed threshold analyses on the cost differences between tests, and between tests and colonoscopy, to estimate how large these cost differences should be so that the conclusions from the base-case scenario still hold.
Initial and delayed diagnosis
In the base-case scenario, the distribution of patients per Dukes’ stages was 13%, 37%, 36% and 14%, for stages A–D, respectively. 98 In these additional scenarios, we first considered the distribution of patients reported by Cubiella et al. :55 this was 16%, 25%, 44% and 14%, for stages A–D, respectively. Then, the distribution of patients estimated by the experts who filled in our questionnaire (19%, 35%, 32% and 15%, for stages A–D, respectively) was chosen. Finally, in the base-case scenario we assumed a delayed diagnosis of 6 months for those patients testing negative with faecal immunochemical testing/guaiac faecal occult blood testing who were persistent in their symptoms. This delay implied a distribution of patients per Dukes’ stages equal to 9%, 29%, 32% and 30%, for stages A–D, respectively. For a 1-year delay, this was 6%, 20%, 29% and 45%.
Colorectal cancer mortality and progression
In the base-case scenario, the probability of CRC progression was modelled as in NG12,6 using progression probabilities for undiagnosed patients with CRC, which were obtained from the Tappenden et al. study. 99 Mortality was modelled using observational data on mortality by CRC stage from the NCIN at the time when the initial diagnosis took place. 98 Thus, besides the limitation of using progression probabilities for undiagnosed patients in a model in which patients are considered, it is also questionable whether or not these mortality data included patients who might eventually progress, as the data looked at only the initial diagnosis and subsequent mortality. Therefore, it might be reasonable to consider that the effect of progression could be included in the mortality data. Hence, in this scenario, we considered the latter assumption and the CRC progression probabilities were set to zero in the model.
Probability of persisting in symptoms after having a negative test result
In the base-case scenario, this probability was estimated by the experts who filled in our questionnaire. This value was 32.5%. In this scenario we explored the consequences of doubling and halving this value.
Adverse events associated with colonoscopy
In the base-case scenario, we considered a mortality rate associated with colonoscopy of 0.0029%. This was based on the study by Reumkens et al. 113 To investigate the impact of this assumption on the cost-effectiveness results, we considered in this scenario the highest value that was found in the literature for the mortality due to colonoscopy; this was 0.0970% and it was reported by Day et al. 114 for elderly patients undergoing colonoscopy. Additionally, we explored a scenario for which no adverse events (including mortality) were considered.
Probability of being referred to colonoscopy versus computed tomography colonography
The probability of undergoing colonoscopy as opposed to CTC was estimated to be 83% by four experts. The study by Logan et al. 126 found that, for approximately 98% of the screening patients with an abnormal test result, the first investigation was colonoscopy. In this scenario, we assumed that all patients would be referred to colonoscopy. Note that, as it was also assumed that adverse event rates were equal for colonoscopy and CTC, differences between this and the base-case scenario are expected to be observed only on the costs side.
Probability of receiving a second faecal immunochemical test/guaiac faecal occult blood test
In our base-case scenario, we assumed that patients who after testing negative remain symptomatic were referred for a colonoscopy or CTC. However, the clinical experts consulted indicated that approximately 20% of these patients would have a second FIT/gFOBT.
Results of cost-effectiveness analyses
Base-case analysis
The base-case lifetime results, reported as cost per QALY gained (ICER) per patient and strategy, are summarised in Table 45. From these results, it is clear that the difference in QALYs between all of the strategies is minimal and that the no triage strategy (referral straight to colonoscopy) is the most expensive one.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | |||
| OC-Sensor | 18.6239 | 244.42 | 0.00198 | 12.14 | 6133 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarca | ||
| HM-JACKarc | 18.6242 | 274.75 | 0.0003 | 30.33 | 88,798 |
Note that, where the question is whether or not to recommend faecal immunochemical testing instead of guaiac faecal occult blood testing, then the pairwise comparisons of the different FIT assays against gFOBT assays may be more informative than the full incremental analysis shown above. The base-case ICERs for the two interventions against guaiac faecal occult blood testing are presented in Table 46. In both cases, the ICER was < £30,000. Therefore, both interventions are deemed cost-effective compared with guaiac faecal occult blood testing. Table 46 also shows the results obtained when the comparator is no triage (referral straight to colonoscopy). The HM-JACKarc strategy dominates no triage (referral straight to colonoscopy); it results in more QALYs and it is cost saving. It should be noted that, the ICER obtained when the OC-Sensor was compared with no triage (referral straight to colonoscopy) is extremely high (£4,133,559) but that this results from both negative incremental costs and incremental QALYs (i.e. the ICER is in the south-west quadrant of the cost-effectiveness plane); in this case, the cost savings outweigh the loss in QALYs and, therefore, the OC-Sensor strategy is more cost-effective than no triage (referral straight to colonoscopy).
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 42.47 | 18,296 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.14 | 6133 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259.25 | 4,133,559 |
Finally, Table 47 shows the breakdown of the total costs and total QALYs for all of the strategies that were included in the analysis. As some values were very small (e.g. number of adverse events or deaths), the number of patients per outcome of the diagnostic model is multiplied by 1000. Costs and QALYs are shown per patient, for consistency with Table 45 above. Note that number of patients with the disease might be lower than the prevalent CRC population. This is simply because in the model we assumed three possible outcomes of colonoscopy: positive, negative and death. Therefore, those dead, who eventually had CRC, were not counted as having the disease. The number of positive tests was the largest for HM-JACKarc (245.36), followed by OC-Sensor (153.50) and gFOBT (130.28). In the model, all of these patients were assumed to be referred for further investigation with colonoscopy or CTC. Following further investigation, the number of patients with CRC who had a positive FIT was 14.99 for HM-JACKarc, 13.80 for OC-Sensor and 7.49 for gFOBT. Thus, all patients with CRC tested positive with HM-JACKarc, compared with approximately 92% with OC-Sensor and 50% with gFOBT. The immediate consequence of this was that diagnosis of CRC was delayed in no patients tested with HM-JACKarc, in 8% of patients tested with OC-Sensor and in 50% of patients with with gFOBT. The apparent advantage of FIT with respect to gFOBT in detecting patients with CRC earlier comes at the cost of performing more colonoscopies, as the total number of colonoscopies performed was 490.62 for HM-JACKarc, 428.61 for OC-Sensor and 412.95 for gFOBT. This also implies that total costs and risks associated with colonoscopy are higher for FIT than for gFOBT. Because of this, and because performing a FIT was estimated to have a higher cost than gFOBT, the HM-JACKarc diagnostic strategy was more expensive (£255.27) than the OC-Sensor (£225.16) and gFOBT (£214.17) strategies. All testing strategies were clearly cheaper than no triage (£484.19).
| No triage (referral straight to colonoscopy) | gFOBT | OC-Sensor | HM-JACKarc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (× 1000) | Costs (£) | QALYs | Patients (× 1000) | Costs | QALYs | Patients (× 1000) | Costs (£) | QALYs | Patients (× 1000) | Costs (£) | QALYs | |
| Branch: test + | 130.2889 | 0.10 | 0.1160 | 153.5014 | 0.70 | 0.1350 | 245.3629 | 1.48 | 0.2182 | |||
| Colonoscopy + | 14.9996 | 7.19 | 0.0086 | 7.4998 | 3.59 | 0.0043 | 13.8082 | 6.62 | 0.0079 | 14.9996 | 7.19 | 0.0086 |
| AE bleeding | 0.0390 | 0.02 | – | 0.0195 | 0.01 | – | 0.0359 | 0.02 | – | 0.0390 | 0.02 | – |
| AE perforation | 0.0075 | 0.02 | – | 0.0037 | 0.01 | – | 0.0069 | 0.02 | – | 0.0075 | 0.02 | – |
| Dukes’ A | 1.9778 | 0.23 | 0.0015 | 0.9889 | 0.11 | 0.0007 | 1.8207 | 0.21 | 0.0013 | 1.9778 | 0.23 | 0.0015 |
| Dukes’ B | 5.5341 | 0.64 | 0.0039 | 2.7670 | 0.32 | 0.0019 | 5.0945 | 0.59 | 0.0036 | 5.5341 | 0.64 | 0.0039 |
| Dukes’ C | 5.3877 | 0.62 | 0.0027 | 2.6939 | 0.31 | 0.0013 | 4.9598 | 0.58 | 0.0025 | 5.3877 | 0.62 | 0.0027 |
| Dukes’ D | 2.0999 | 0.24 | 0.0005 | 1.0500 | 0.12 | 0.0003 | 1.9331 | 0.22 | 0.0005 | 2.0999 | 0.24 | 0.0005 |
| Colonoscopy – | 984.9714 | 472.07 | 0.8963 | 122.7853 | 58.85 | 0.1117 | 139.6887 | 66.95 | 0.1271 | 230.3562 | 110.40 | 0.2096 |
| AE bleeding | 2.5609 | 1.54 | – | 0.3192 | 0.19 | – | 0.3632 | 0.22 | – | 0.5989 | 0.36 | – |
| AE perforation | 0.4925 | 1.59 | – | 0.0614 | 0.20 | – | 0.0698 | 0.23 | – | 0.1152 | 0.37 | – |
| Death | 0.0290 | 0.01 | 0.0000 | 0.0038 | 0.00 | 0.0000 | 0.0045 | 0.00 | 0.0000 | 0.0071 | 0.00 | 0.0000 |
| Branch: test – | 869.7111 | 0.67 | 0.7884 | 846.4986 | 3.83 | 0.7698 | 754.6371 | 4.56 | 0.6867 | |||
| Symptoms | 282.6561 | – | – | 275.1120 | – | – | 245.2571 | – | – | |||
| Colonoscopy + | 7.4998 | 3.92 | 0.0038 | 1.1914 | 0.62 | 0.0006 | 0.0000 | – | 0.0000 | |||
| AE bleeding | 0.0195 | 0.01 | – | 0.0031 | 0.00 | – | 0.0000 | – | – | |||
| AE perforation | 0.0037 | 0.01 | – | 0.0006 | 0.00 | – | 0.0000 | – | – | |||
| Dukes’ A | 0.7021 | 0.08 | 0.0005 | 0.1115 | 0.01 | 0.0001 | 0.0000 | – | 0.0000 | |||
| Dukes’ B | 2.1407 | 0.25 | 0.0015 | 0.3401 | 0.04 | 0.0002 | 0.0000 | – | 0.0000 | |||
| Dukes’ C | 2.4352 | 0.28 | 0.0012 | 0.3868 | 0.04 | 0.0002 | 0.0000 | – | 0.0000 | |||
| Dukes’ D | 2.2218 | 0.26 | 0.0006 | 0.3529 | 0.04 | 0.0001 | 0.0000 | – | 0.0000 | |||
| Colonoscopy – | 275.1481 | 143.98 | 0.2504 | 273.9127 | 143.33 | 0.2493 | 245.2499 | 128.33 | 0.2232 | |||
| AE bleeding | 0.7154 | 0.43 | – | 0.7122 | 0.43 | – | 0.6376 | 0.38 | – | |||
| AE perforation | 0.1376 | 0.44 | – | 0.1370 | 0.44 | – | 0.1226 | 0.40 | – | |||
| Death | 0.0082 | 0.00 | 0.0000 | 0.0080 | 0.00 | 0.0000 | 0.0071 | 0.00 | 0.0000 | |||
| No symptoms | 587.0550 | – | 0.5342 | 571.3866 | – | 0.5200 | 509.3800 | – | 0.4635 | |||
| Total diagnostic | 484.19 | 0.9048 | 214.17 | 0.9044 | 225.16 | 0.9048 | 255.27 | 0.9048 | ||||
| Total healthy Markov | 17.6968 | 17.6971 | 17.6971 | 17.6970 | ||||||||
| CRC Markov | 19.48 | 0.0222 | 18.10 | 0.0203 | 19.26 | 0.0219 | 19.48 | 0.0222 | ||||
| Total lifetime | 503.67 | 18.6239 | 232.28 | 18.6218 | 244.42 | 18.6239 | 274.75 | 18.6242 | ||||
Differences in QALYs between the four strategies were minimal. For HM-JACKarc, OC-Sensor and no triage (referral straight to colonoscopy), the total QALYs gained were 0.9048 per patient for the first year (diagnostic phase). For gFOBT this was 0.9044. In fact, there was a difference in QALYS between HM-JACKarc, OC-Sensor and no triage (referral straight to colonoscopy) observed at the fifth decimal place. QALYs were the highest for HM-JACKarc, followed by no triage (referral straight to colonoscopy) and OC-Sensor. This was because for HM-JACKarc no delayed diagnosis was observed. Thus, the difference in QALYs between HM-JACKarc and no triage (referral straight to colonoscopy) was due only to the number of colonoscopies. This was smaller for HM-JACKarc and, therefore, fewer deaths occurred with this strategy. When compared with OC-Sensor, both no triage (referral straight to colonoscopy) and HM-JACKarc strategies, resulted in more colonoscopies being performed, implying more deaths with the last two strategies. However, for OC-Sensor, delayed diagnosis was observed, and this seemed to ‘outweigh’ the gain in QALYs due to fewer deaths.
Note, finally, that the differences in costs and QALYs observed in the two Markov models between the four strategies were also minimal. The healthy population Markov model was used to account for lifetime QALYs of patients who did not have the target condition. The number of QALYs accrued in this model was simply determined by the initial number of patients entering in the model. This was the highest for guaiac faecal occult blood testing, as this was the strategy leading to the fewest colonoscopies and, therefore, the fewest deaths. Hence, the total number of QALYs estimated by the healthy population Markov model was 17.6971 for gFOBT and OC-Sensor (higher than gFOBT in the fifth decimal place), 17.6970 for HM-JACKarc and 17.6968 for no triage (referral straight to colonoscopy). Likewise, the total costs and QALYs estimated by the CRC Markov model were determined only by the initial distribution of patients per Dukes’ stage entering in the model. Note that all of the four strategies correctly diagnosed all CRC alive patients. Thus, although the total number of patients entering the model was approximately the same (prevalence minus those who died) for all of the strategies, differences in staging were observed, due to delayed diagnosis, with the gFOBT and OC-Sensor strategies. Because of this, estimated costs (£19.48) and QALYs (0.0222) were higher for no triage (referral straight to colonoscopy) and HM-JACKarc, as for these two strategies no delayed diagnosis was observed. Compared with gFOBT and OC-Sensor, these patients with CRC were healthier and, therefore, lived longer, but also incurred more costs. For gFOBT, diagnosis of CRC was delayed in 50% of patients, the highest among the four strategies compared. Thus, costs and QALYs estimated by the CRC Markov model for gFOBT were the lowest (£18.10 and 0.0203, respectively). Finally, for OC-Sensor, 8% delayed diagnosis was observed. Therefore, costs (£19.26) and QALYs (0.0219) estimated by the CRC Markov model for this strategy were similar to (but slightly lower than) those estimated for HM-JACKarc and no triage (referral straight to colonoscopy).
Sensitivity analysis
The cost-effectiveness results from the PSA are very similar to the deterministic results except that, in this simulation, the ICERs calculated in the PSA were lower than those shown in Table 45. These results are presented in Table 48.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6415 | 230.49 | NA | NA | NA |
| OC-Sensor | 18.6439 | 242.51 | 0.0024 | 12.02 | 5039 |
| No triage (referral straight to colonoscopy) | 18.6440 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6444 | 272.50 | 0.0005 | 29.99 | 61,619 |
The probabilistic ICERs for the two interventions compared with every comparator are shown in Table 49. These results are also similar to those presented in Table 46 for the deterministic case, and so are the conclusions drawn.
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0029 | 42.01 | 14,626 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0004 | –228.10 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0024 | 12.02 | 5039 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –258.09 | 2,578,543 |
The scatterplot of the PSA outcomes in the cost-effectiveness plane in Figure 15 shows that the uncertainty around QALYs is similar for all of the strategies, but the uncertainty around costs is clearly larger (and with higher cost values) for the no triage (referral straight to colonoscopy) strategy. The CEACs for each strategy are shown in Figure 16. It can be observed that at low values of the ICER threshold, the probability of being cost-effective is higher for those strategies with lower costs (i.e. gFOBT and OC-Sensor). As the ICER threshold increases, the CEAC for HM-JACKarc increases, whereas those for gFOBT and OC-Sensor decrease. Both CEACs for HM-JACKarc and gFOBT seem to converge to 0.5, but for all values of the ICER threshold considered in this analysis the cost-effectiveness probability for gFOBT was the highest. In particular, at the ICER threshold equal £30,000, the cost-effectiveness probabilities for gFOBT, HM-JACKarc, OC-Sensor and no triage (referral straight to colonoscopy) were 0.47, 0.41, 0.11 and 0.01, respectively.
FIGURE 15.
Cost-effectiveness plane with PSA outcomes for all of the strategies in the base-case scenario.
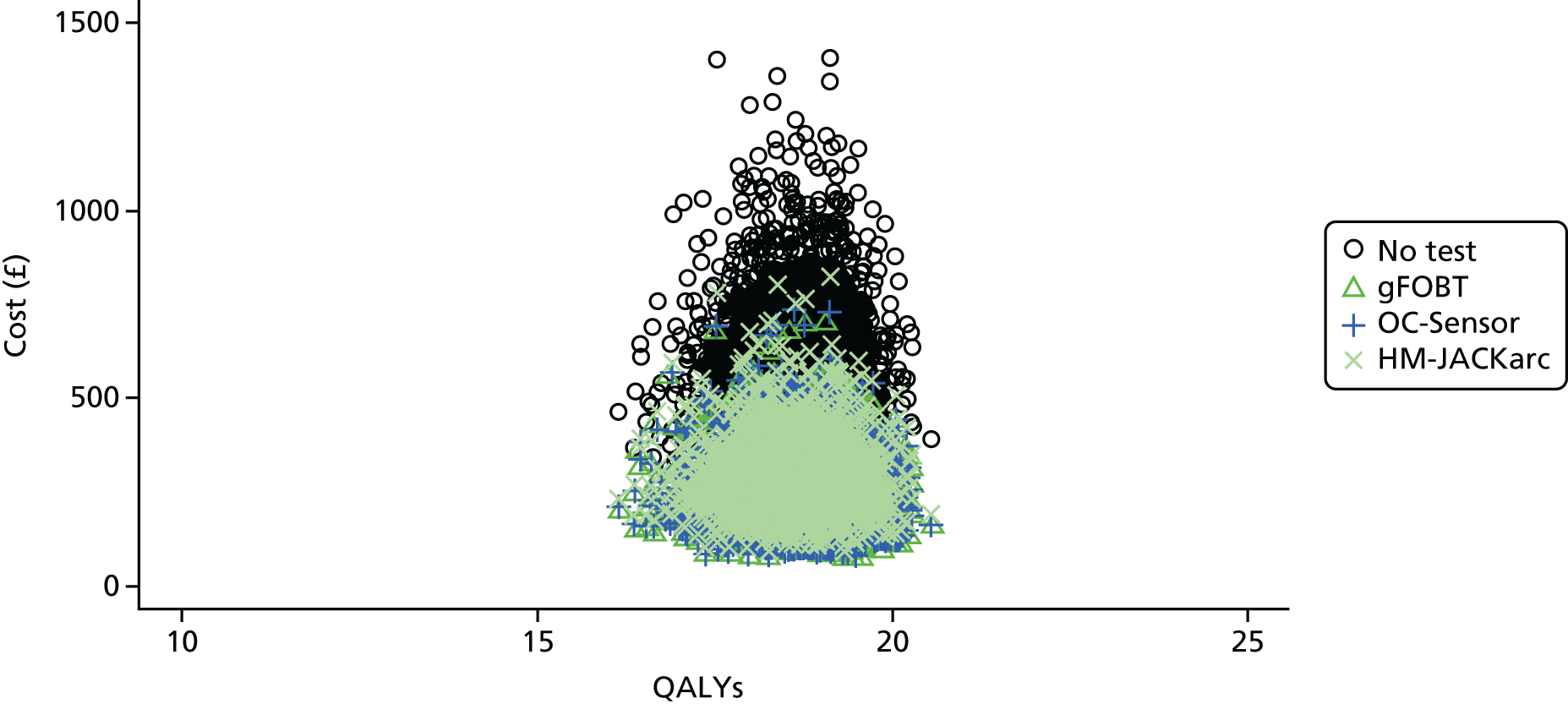
FIGURE 16.
Cost-effectiveness acceptability curves for all of the strategies in the base-case scenario.
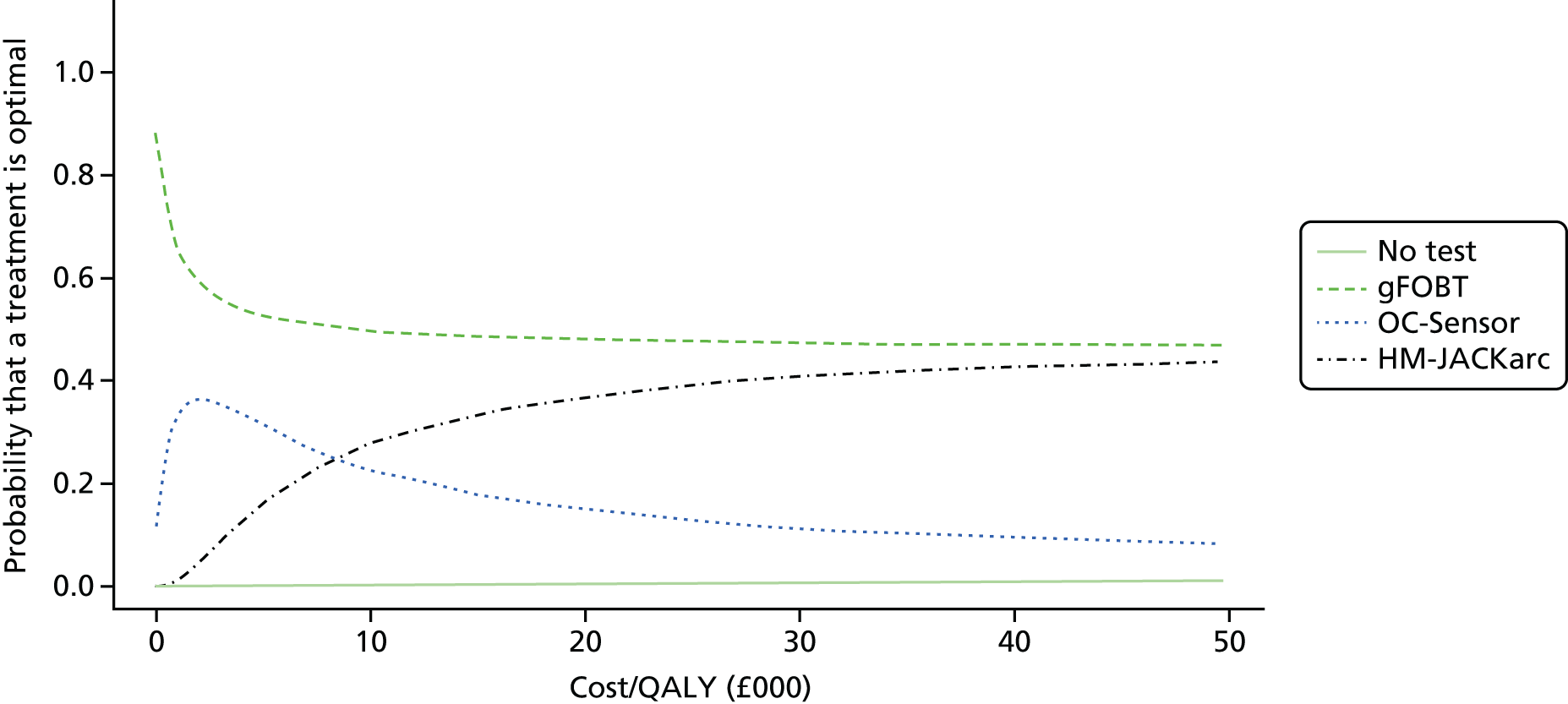
Pairwise comparisons of the two different FIT assays against the two comparators included in this diagnostic assessment (gFOBT and no triage) were also explored in the PSA. The results are presented in Figure 17 (cost-effectiveness planes) and Figure 18 (CEACs). When the comparator was no triage (referral straight to colonoscopy), all PSA outcomes were located in the southern quadrants of the cost-effectiveness plane, indicating cost savings. They seem to be evenly scattered over the two quadrants. Thus, for approximately half of the PSA outcomes the cost savings due to faecal immunochemical testing do not outweigh the loss in QALYs and, therefore, the FIT strategies are no more cost-effective than no triage (referral straight to colonoscopy). However, as most of the PSA outcomes are located very close to the y-axis (where incremental QALYs are zero), no triage (referral straight to colonoscopy) will be cost-effective compared with HM-JACKarc, or OC-Sensor, only for very large values of the ICER threshold. In fact, this was not observed for any of the values of the ICER threshold that were considered in the CEACs shown in Figure 18. When the comparator was gFOBT, the PSA outcomes were mostly located in the northern quadrants of the cost-effectiveness plane (given that faecal immunochemical testing is more expensive). When the intervention was the OC-Sensor, we observed PSA outcomes scattered over the four quadrants of the cost-effectiveness plane, indicating that there is large uncertainty about which strategy is the most cost-effective. Note, finally, that the probabilistic ICERs for both HM-JACKarc compared with gFOBT (£14,626) and OC-Sensor compared with gFOBT (£5039) were estimated to be in the north-east quadrant of the cost-effectiveness plane, with an ICER of < £30,000, meaning that on average the FIT strategies are cost-effective compared with gFOBT. However, this is not readily apparent when we observed the CEACs in Figure 18, as these cross each other at values of the ICER threshold that are much larger than the probabilistic ICERs. This can be explained by the asymmetry of the distribution of the PSA outcomes on the cost-effectiveness plane. Nevertheless, given the small difference in QALYs between all of the strategies observed in the results, the conclusions from the PSA regarding which strategy is the most cost-effective are expected to be sensitive to changes in the accuracy estimates. This is explored in several scenarios within the next section.
FIGURE 17.
Cost-effectiveness plane with PSA outcomes for intervention vs. comparator in the base-case scenario. (a) Cost-effectiveness plane – HM-JACKarc vs. no test; (b) cost-effectiveness plane – HM-JACKarc vs. gFOBT; (c) cost-effectiveness plane – OC-Sensor vs. no test; and (d) cost-effectiveness plane – OC-Sensor vs. gFOBT.

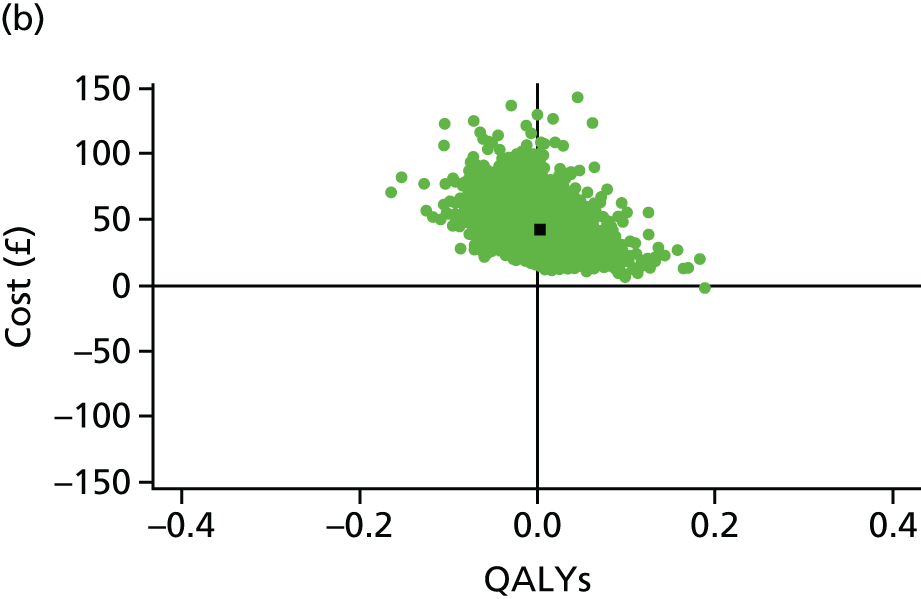
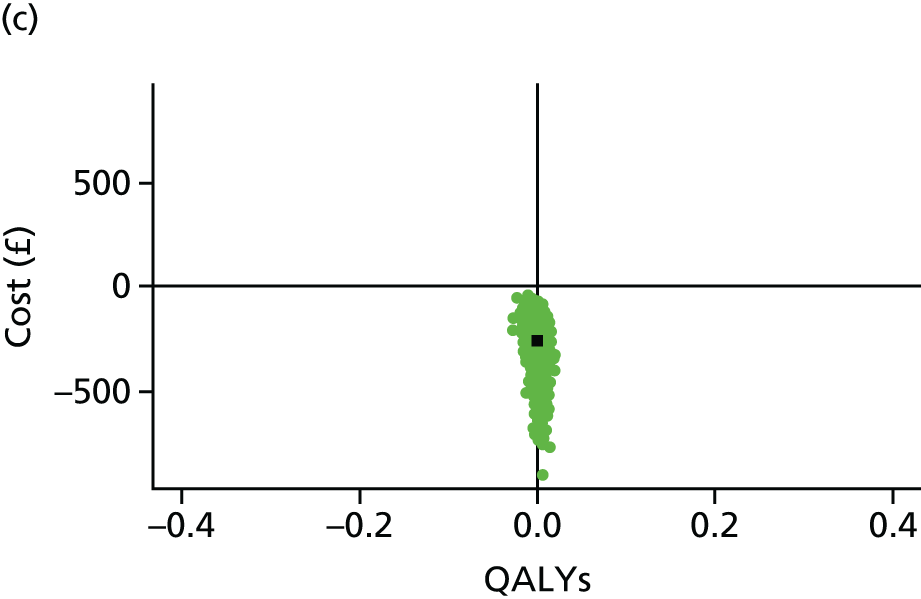
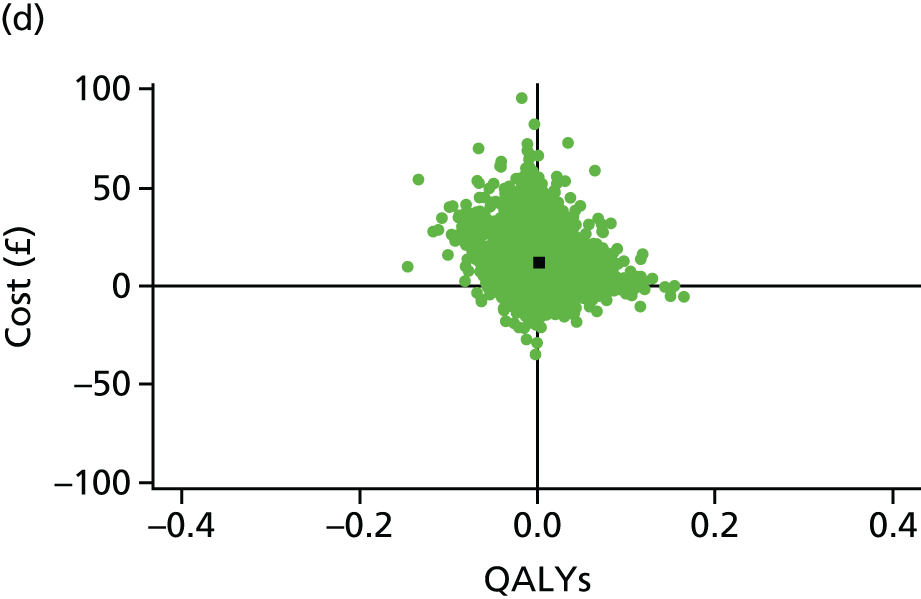
FIGURE 18.
Cost-effectiveness acceptability curves for intervention vs. comparator in the base-case scenario. (a) Acceptability curves (HM-JACKarc vs. no test); (b) acceptability curves (HM-JACKarc vs. gFOBT); (c) acceptability curves (OC-Sensor vs. no test); and (d) acceptability curves (OC-Sensor vs. gFOBT).

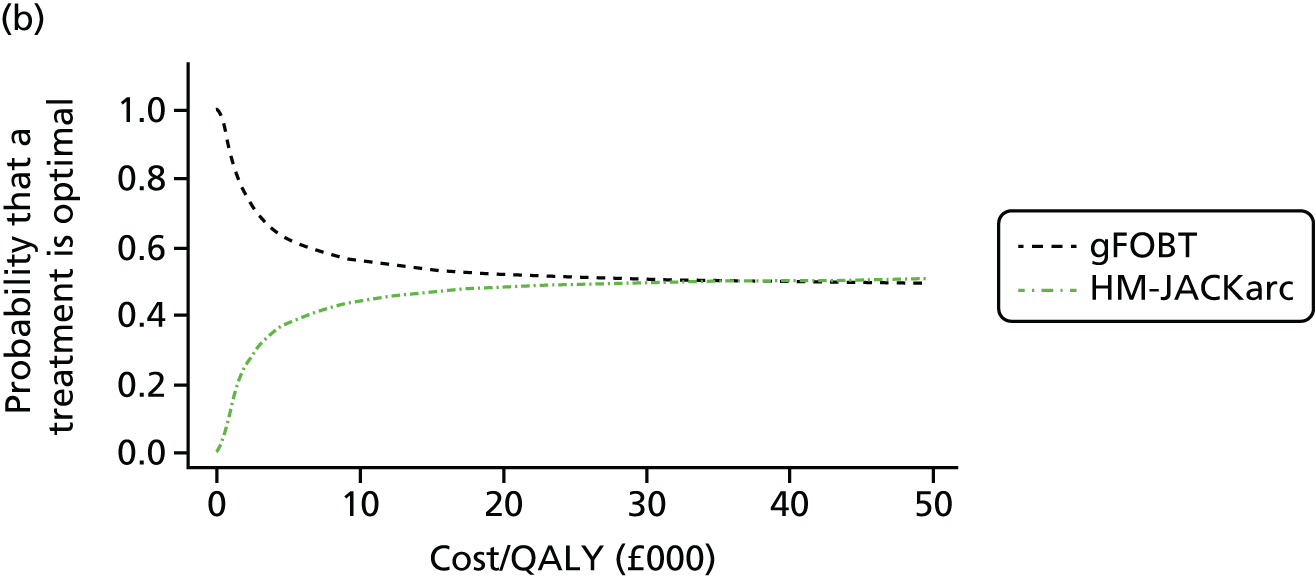
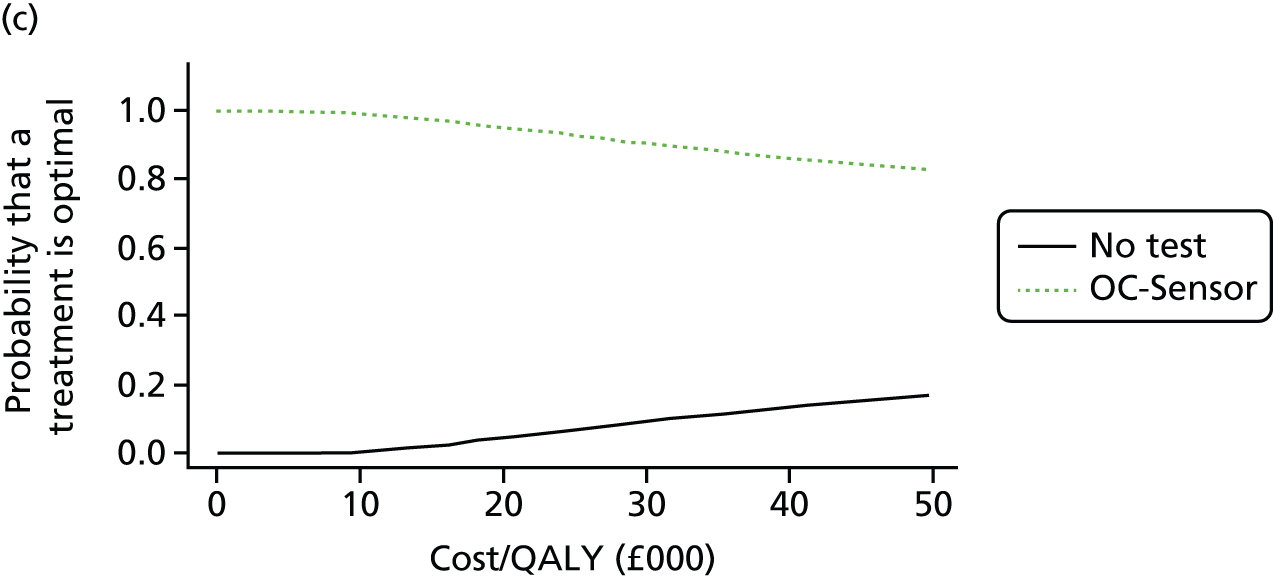

Scenario analysis
In the scenarios presented below, only the most significant differences with respect to the base-case scenario are discussed. Complete results are provided in Appendix 11.
Diagnostic accuracy of faecal immunochemical test/guaiac faecal occult blood test
Accuracy scenario I: guaiac faecal occult blood test accuracy estimates based on the Niv and Sperber study
In this scenario analysis, we considered the accuracy estimates for guaiac faecal occult blood testing reported by Niv and Sperber,92 as these are considered more representative of the population of this diagnostic assessment than those reported in NG12. 6 The only difference with respect to the base-case results can be observed in the gFOBT estimates. In this case, the total QALYs and total costs estimated per patient were 18.6227 and £277.54, respectively. Thus, in this scenario, guaiac faecal occult blood testing was dominated by both FIT strategies. Probabilistic results show that the main difference with respect to the base-case scenario was that guaiac faecal occult blood testing was now clearly less cost-effective. This can be seen in the CEACs plot for all of the strategies in Figure 19, where guaiac faecal occult blood testing is the most cost-effective strategy only when the ICER threshold ranges between (approximately) £12,750 and £24,500 (whereas in the base-case gFOBT was the most cost-effective strategy for the whole range of ceiling ratios that were considered in the analysis). The CEACs for each intervention compared with guaiac faecal occult blood testing separately are presented in Figure 20. These show that both interventions are more cost-effective than guaiac faecal occult blood testing for all of the ceiling ratio values that were considered in this analysis.
FIGURE 19.
Cost-effectiveness acceptability curves for all of the strategies: alternative gFOBT accuracy estimates.

FIGURE 20.
Cost-effectiveness acceptability curves for intervention vs. gFOBT, alternative gFOBT accuracy estimates. (a) acceptability curves – HM-JACKarc vs. gFOBT; and (b) acceptability curves – OC-Sensor vs. gFOBT.
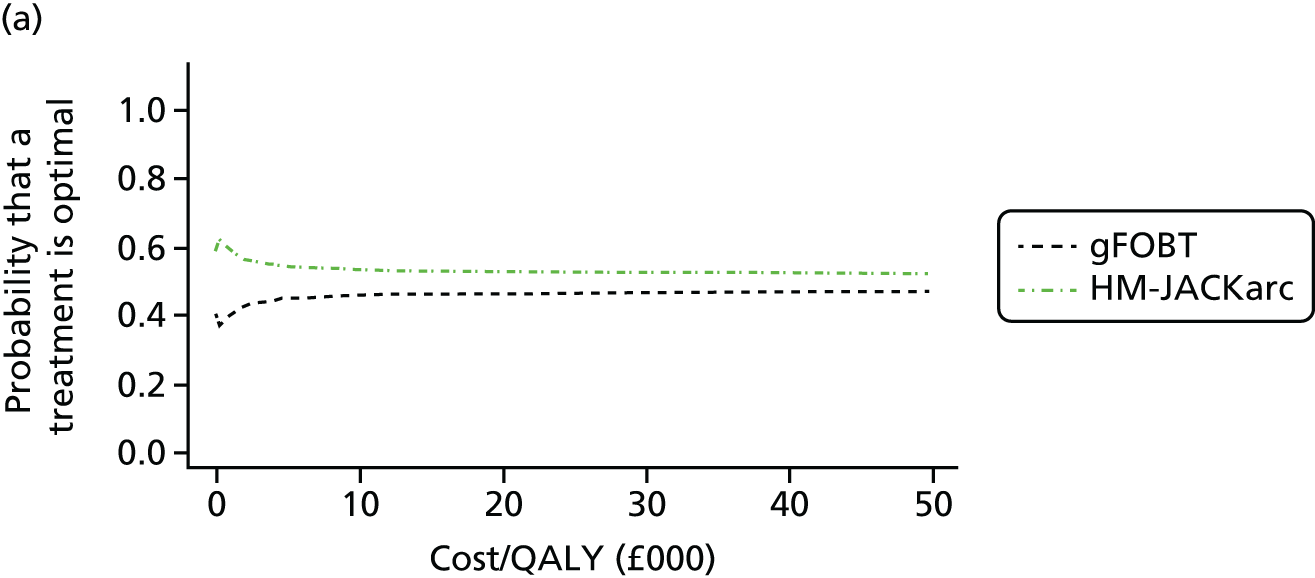
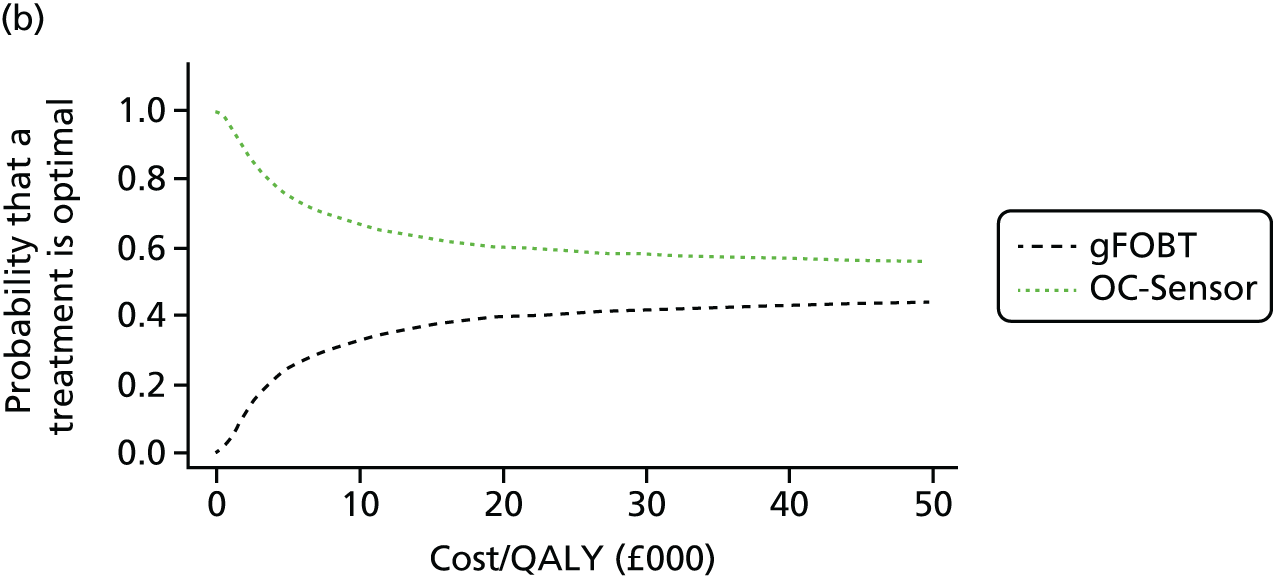
Accuracy scenario II: guaiac faecal occult blood test accuracy estimates based on the Bjerregaard et al. study
The accuracy estimates for guaiac faecal occult blood testing reported in Bjerregaard et al. 93 were considered for this scenario, as these are also regarded as more representative of the population of this diagnostic assessment than those in NG12. 6 In this scenario, the total QALYs and total costs estimated per gFOBT patient were 18.6230 and £258.97, respectively. Thus, guaiac faecal occult blood testing was dominated by the OC-Sensor. The ICER reported when the intervention was HM-JACKarc was £13,482. Therefore, at the common value of the ceiling ratio of £30,000, HM-JACKarc was deemed cost-effective compared with guaiac faecal occult blood testing. All of the CEACs were similar to those in the scenario for which the gFOBT accuracy estimates were obtained from Niv and Sperber92 and, therefore, they are not shown here. The main difference with respect to the previous scenario is that guaiac faecal occult blood testing is now the most cost-effective strategy when the ICER threshold ranges between (approximately) £12,250 and £23,500. The CEACs for each intervention compared with guaiac faecal occult blood testing are also similar, but the cost-effectiveness probability for guaiac faecal occult blood testing is now higher.
Accuracy scenario III: faecal immunochemical test threshold any detectable haemoglobin (OC-Sensor only)
In this scenario, a threshold of any detectable Hb was assumed for faecal immunochemical testing. The idea of this scenario is to look at the effects of using a threshold at the lowest level that faecal immunochemical testing is able to detect. For this threshold, our systematic review obtained data only for the OC-Sensor. The total QALYs and total costs estimated per patient for the OC-Sensor strategy were 18.6240 and £375.40, respectively. For gFOBT and no triage (referral straight to colonoscopy) these were as in the base-case scenario. In this case, no triage (referral straight to colonoscopy) was dominated by the OC-Sensor, and the ICER obtained when the OC-Sensor was compared with gFOBT was £65,192. Thus, at the common value of the ceiling ratio of £30,000, the OC-Sensor was not cost-effective compared with gFOBT. This is illustrated in the CEAC presented in Figure 21.
FIGURE 21.
Cost-effectiveness acceptability curves for OC-Sensor vs. gFOBT: scenario – any detectable Hb.
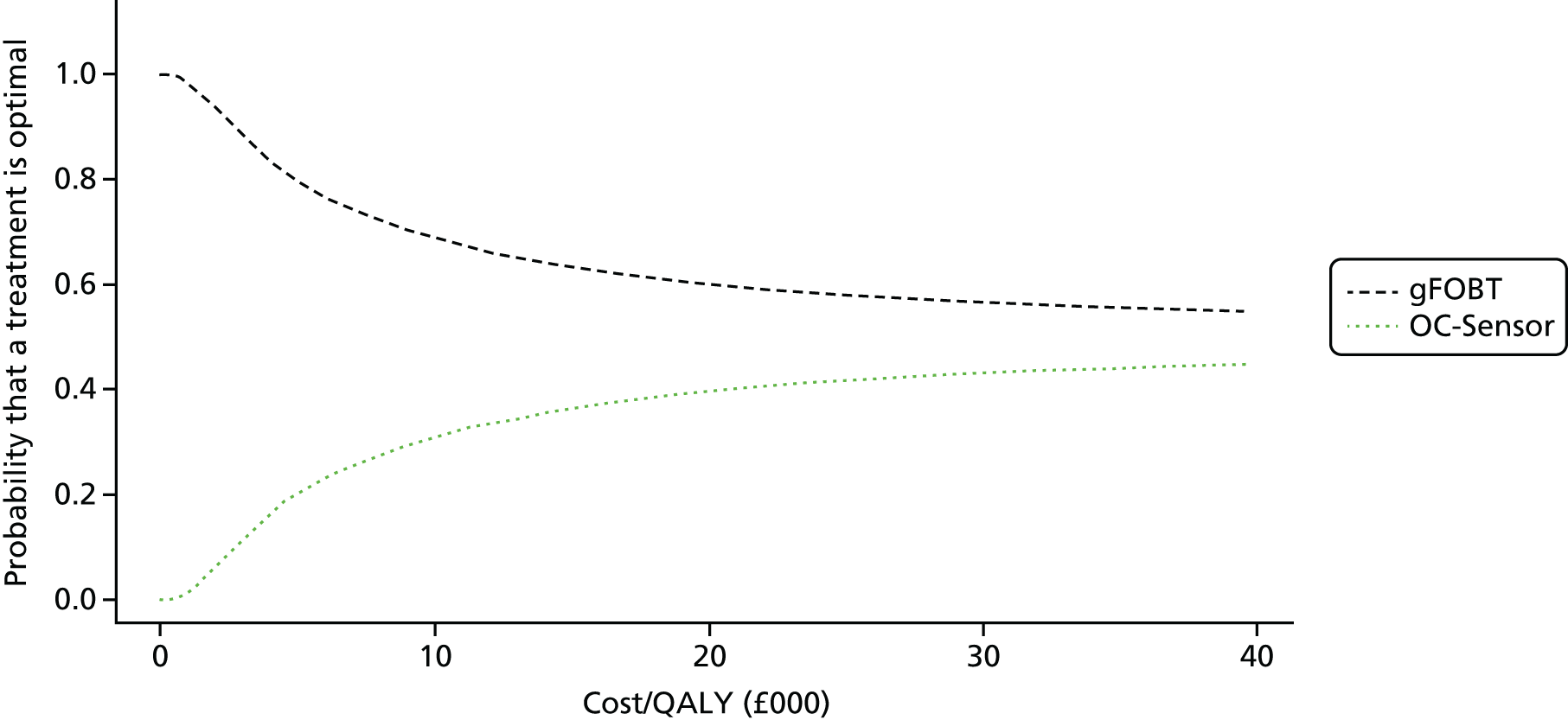
Accuracy scenario IV: faecal immunochemical test threshold of ≥ 20 µg Hb/g faeces (OC-Sensor and FOB Gold)
In this scenario, we assumed a threshold of ≥ 20 µg Hb/g faeces. For this threshold, our systematic review obtained data for the OC-Sensor assay and also for the FOB Gold assay. Although the reliability of the accuracy data for the latter was questionable, as explained in detail in Chapter 3 (see Diagnostic performance of the FOB Gold faecal immunochemical test assay), we decided to include this FIT assay in this scenario. Deterministic results are shown in Table 50. The no triage (referral straight to colonoscopy) strategy was the most expensive, but also the strategy with the most QALYs. However, as in previous scenarios, little difference in QALYs was observed between any of the strategies.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | |||
| FOB Gold | 18.6237 | 240.62 | 0.0018 | 8343 | 4725 |
| OC-Sensor | 18.6238 | 241.83 | 0.0001 | 1210 | 12,576 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | 0.0002 | 261,839 | 1,449,585 |
The ICERs for the two interventions in comparison with guaiac faecal occult blood testing are shown in Table 51. In both cases, the ICER was very low (around £5000). Therefore, the two interventions are cost-effective compared with gFOBT. Table 51 also shows the results obtained when the comparator is no triage (referral straight to colonoscopy). In both cases, the ICERs obtained were very high, but these result from both negative incremental costs and incremental QALYs. In this case, the cost savings outweigh the loss in QALYs and thus the FIT strategies are more cost-effective than no triage (referral straight to colonoscopy).
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| FOB Gold vs. gFOBT | 0.0018 | 8.34 | 4725 |
| FOB Gold vs. no triage (referral straight to colonoscopy) | –0.0003 | –263.05 | 950,102 |
| OC-Sensor vs. gFOBT | 0.0019 | 9.55 | 5131 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –261.84 | 1,449,585 |
The CEACs of all of the strategies presented in Figure 22 show that guaiac faecal occult blood testing is the strategy with the highest probability of being cost-effective for all of the values of the ceiling ratio considered in this analysis. In particular, at an ICER threshold equal to 30,000, this probability was approximately 0.5. The probability of being cost-effective was similar for both interventions, but slightly higher for the FOB Gold. Compared with no triage (referral straight to colonoscopy), both interventions have a higher probability of being cost-effective for all of the values of the ICER threshold considered in the analysis (CEACs not shown). CEACs for each intervention compared with gFOBT are shown in Figure 23. We can observe that, at lower values of the ICER threshold, the gFOBT has a higher probability of being cost-effective because the interventions are more expensive. However, as the ICER threshold increases, the probability of the two interventions being cost-effective also increases and becomes slightly higher than that for gFOBT, although in both cases the probability is close to 0.5.
FIGURE 22.
Cost-effectiveness acceptability curves for all of the strategies: FIT threshold of ≥ 20 µg Hb/g faeces.

FIGURE 23.
Cost-effectiveness acceptability curves for intervention vs. gFOBT: FIT threshold of ≥ 20 µg Hb/g faeces. (a) Acceptability curves – FOB Gold vs. gFOBT; and (b) acceptability curves – OC-Sensor vs. gFOBT.
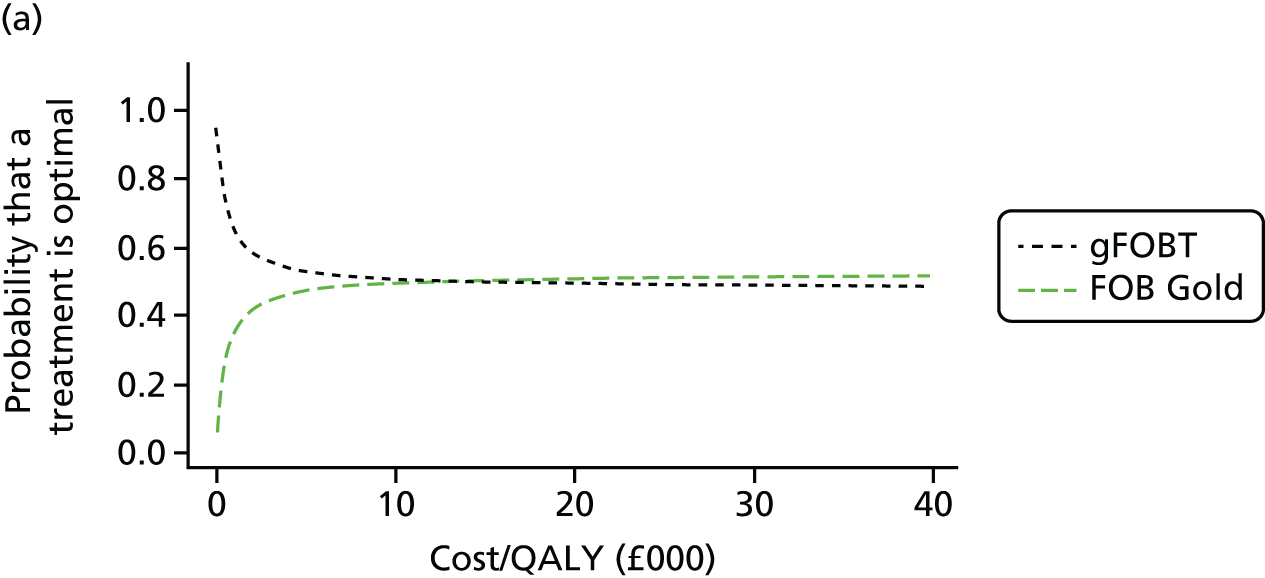

Accuracy scenario V: threshold of ≥ 10 µg Hb/g faeces (base case) including FOB Gold
In this scenario, we included the FOB Gold assay as well as the interventions and comparators that were considered in the base case. We assumed a threshold of 6.8 µg Hb/g faeces for the FOB Gold test, as this was the closest available value to the base-case threshold of ≥ 10 µg Hb/g faeces, and because for this threshold the FOB Gold test showed performance characteristics (sensitivity and specificity) similar to those for the other FITs at the base-case threshold. In the full incremental deterministic analysis, the FOB Gold assay was dominated by the OC-Sensor, and, as no triage (referral straight to colonoscopy) was dominated by HM-JACKarc, the results of this scenario reduce to those in the base case presented in Table 45. The ICER of FOB Gold compared with gFOBT was £15,720, and compared with no triage (referral straight to colonoscopy) was £2,273,829 (in the south-west quadrant of the cost-effectiveness plane). Thus, in both cases, FOB Gold is cost-effective given the common threshold ICER of £30,000. The CEACs for each strategy are shown in Figure 24. It can be observed that the FOB Gold strategy has a low probability of being cost-effective. This is not surprising, as the full incremental analysis results showed that FOB Gold was dominated by the OC-Sensor. The rest of the CEACs are similar to those obtained in the base-case scenario (see Figure 16).
FIGURE 24.
Cost-effectiveness acceptability curves for all of the strategies in the scenario including FOB Gold.
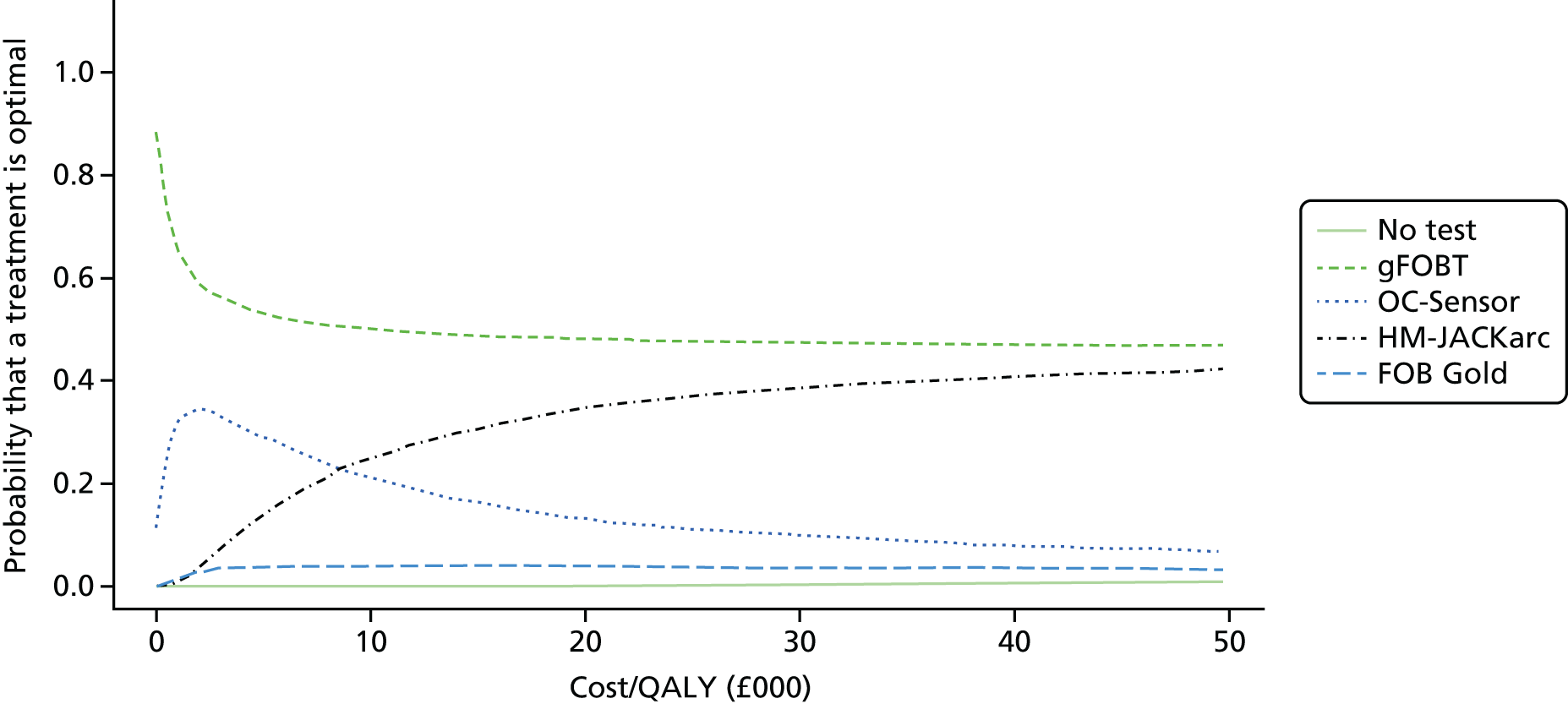
The CEACs in Figure 25 show that the probability of FOB Gold being cost-effective compared with no triage (referral straight to colonoscopy) is high even at large values of the ceiling ratio of the ICER threshold. However, gFOBT clearly more cost-effective than FOB Gold at lower values of the ICER threshold. As the ceiling ratio increases, the probability of being cost-effective for both strategies seems to converge to 0.5.
FIGURE 25.
Cost-effectiveness acceptability curves for FOB Gold vs. comparator in the scenario including FOB Gold. (a) Acceptability curves – FOB Gold vs. no test; and (b) acceptability curves – FOB Gold vs. gFOBT.

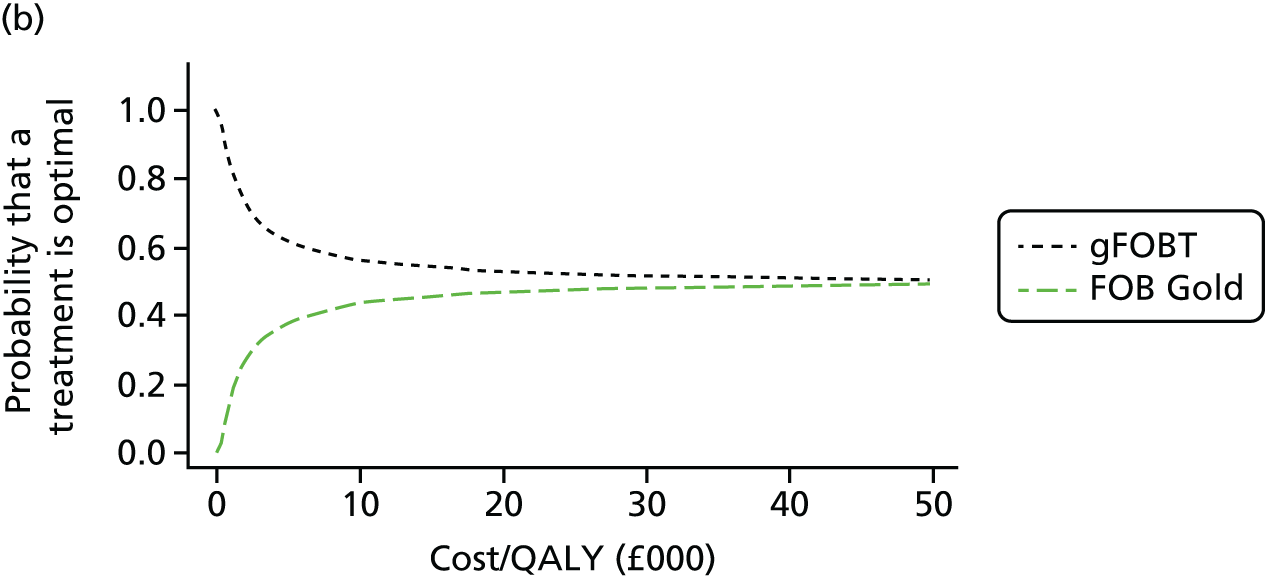
Prevalence of colorectal cancer
The results of the scenarios with prevalence 3% and 5.4% were similar to those in the base case (Tables 52 and 53). In particular, the ICERs obtained in the scenarios in which the two interventions are compared with gFOBT are lower than those ICERs obtained in the base-case scenario (< £10,000). Thus, in both cases, the two interventions are deemed cost-effective.
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0047 | 45.68 | 9754 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –225.34 | –820,612 |
| OC-Sensor vs. gFOBT | 0.0040 | 15.19 | 3829 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –255.83 | 578,092 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0085 | 50.80 | 6004 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –219.62 | –820,054 |
| OC-Sensor vs. gFOBT | 0.0071 | 20.06 | 2808 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0011 | –250.36 | 238,380 |
The scenario with an asymmetrical CI changed only the probabilistic results. For this scenario, we assumed a triangular distribution with the most likely value of 1.5% and limits of 1% and 5.4%. Note that, for this choice of parameters, the mean of the triangular distribution is 2.6%. The main consequence of this choice was that the PSA outcomes were more scattered over the cost-effectiveness plane (especially on the QALYs axis), but all CEACs were very similar to those obtained in the base-case scenario (results not shown).
Costs of faecal immunochemical test/guaiac faecal occult blood test (threshold analysis)
For this analysis, we first considered a hypothetical situation in which all test costs were £0. Then we calculated what the cost difference between HM-JACKarc and gFOBT, and between the OC-Sensor and gFOBT, should be in order to obtain an ICER of (approximately) £30,000. From this analysis, we concluded that HM-JACKarc could be up to £32 more expensive than gFOBT while still keeping the ICER < £30,000. OC-Sensor could be up to £51 more expensive than gFOBT. Compared with the no triage (referral straight to colonoscopy) strategy, both FIT strategies were already so much cheaper in the base case that the cost of the tests themselves could be increased by well over £200 and still be cost-effective. Thus, no threshold analysis was needed for this comparator.
Initial and delayed diagnosis
Changing the probability of being in a certain Dukes’ stage influenced only the outcomes calculated from the Markov models, as these probabilities are not input parameters for the diagnostic (decision tree) model. Note that, in the base-case scenario, the percentages of patients in each Dukes’ stage were 13%, 37%, 36% and 14% for stages A–D, respectively. 98 In these additional scenarios, we first considered the distribution of patients reported in a study included in our systematic review (Cubiella et al. 55); this was 16%, 25%, 44% and 14% for stages A–D, respectively, that is, more patients in stages A and C, but fewer in stage B. The results in Table 54 are similar to those in the base case, with a slight loss in QALYs for all of the strategies and also lower costs. Both interventions dominated no triage (referral straight to colonoscopy) and, when compared with gFOBT, both ICERs were < £30,000. The PSA results (not shown here) did not show any significant difference with respect to those in the base case.
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0019 | 42.22 | 22,319 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | –820,947 |
| OC-Sensor vs. gFOBT | 0.0016 | 11.93 | 7370 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0000 | –259.21 | –46,570,596 |
The distribution of patients (estimated by the experts) who filled in our questionnaire was 19%, 35%, 32% and 15%, for stages A–D, respectively. Thus, there would be more patients in stages A and D, but fewer in stages B and C. The results were also similar to those in the base case, but now with a slight gain in QALYs and an increase in costs for all of the strategies (Table 55). In this case, only HM-JACKarc dominated no triage (referral straight to colonoscopy). The ICER for OC-Sensor compared with no triage (referral straight to colonoscopy) was high, but in the south-west quadrant of the cost-effectiveness plane. Therefore, OC-Sensor was cost-effective. When compared with gFOBT both ICERs were < £30,000. The PSA results did not show any significant difference with respect to those in the base case either.
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0032 | 42.90 | 13,574 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | –820,947 |
| OC-Sensor vs. gFOBT | 0.0027 | 12.50 | 4655 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –259.32 | 1,322,872 |
Finally, note that in the base-case scenario we assumed a delayed diagnosis of 6 months for those patients who were testing negative with faecal immunochemical testing/gFOBT and were persistent in their symptoms. The 6-month delay implied a distribution of patients, per Dukes stage, equal to 9%, 29%, 32% and 30%, for stages A–D, respectively; for a 1-year delay, this was 6%, 20%, 29% and 45%, respectively, that is, most patients were in stage D. The results shown in Table 56 are similar to those in the base case, with a slight loss in QALYs for gFOBT and OC-Sensor, and also lower costs. Note that, in the base-case scenario, HM-JACKarc detected all patients with CRC. Therefore, the assumption on the delay of diagnosis had no impact on these results.
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0047 | 43.85 | 9360 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | –820,947 |
| OC-Sensor vs. gFOBT | 0.0040 | 13.30 | 3352 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –259.47 | 592,092 |
Colorectal cancer mortality and progression
The impact of not considering CRC progression explicitly in the model was explored in this scenario. The full incremental results in Table 57 were similar to those in the base case, with a slight gain in QALYs for all of the strategies and an increase in costs.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.701 | 263.92 | NA | NA | NA |
| OC-Sensor | 18.711 | 279.10 | 0.0101 | 15.18 | 1508 |
| No triage (referral straight to colonoscopy) | 18.713 | 538.93 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.713 | 310.01 | 0.0019 | 30.90 | 16,528 |
When the interventions were compared with gFOBT and no triage (referral straight to colonoscopy) separately (Table 58), we observed, as in previous scenarios, that HM-JACKarc dominated no triage (referral straight to colonoscopy); the ICER for OC-Sensor compared with no triage (referral straight to colonoscopy) was large but in the south-west quadrant of the cost-effectiveness plane (hence, OC-Sensor was cost-effective); and the ICERs obtained when the comparator was gFOBT were both < £30,000 (and, therefore, both were cost-effective).
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0119 | 46.09 | 3859 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | –820,947 |
| OC-Sensor vs. gFOBT | 0.0101 | 15.18 | 1508 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0016 | –259.83 | 163,305 |
The main difference with respect to the base-case PSA is that results are now more favourable to the interventions, as can be observed in the CEACs for all of the strategies in Figure 26 and the CEACs for the interventions against gFOBT in Figure 27.
FIGURE 26.
Cost-effectiveness acceptability curves for all of the strategies: scenario – without CRC progression.
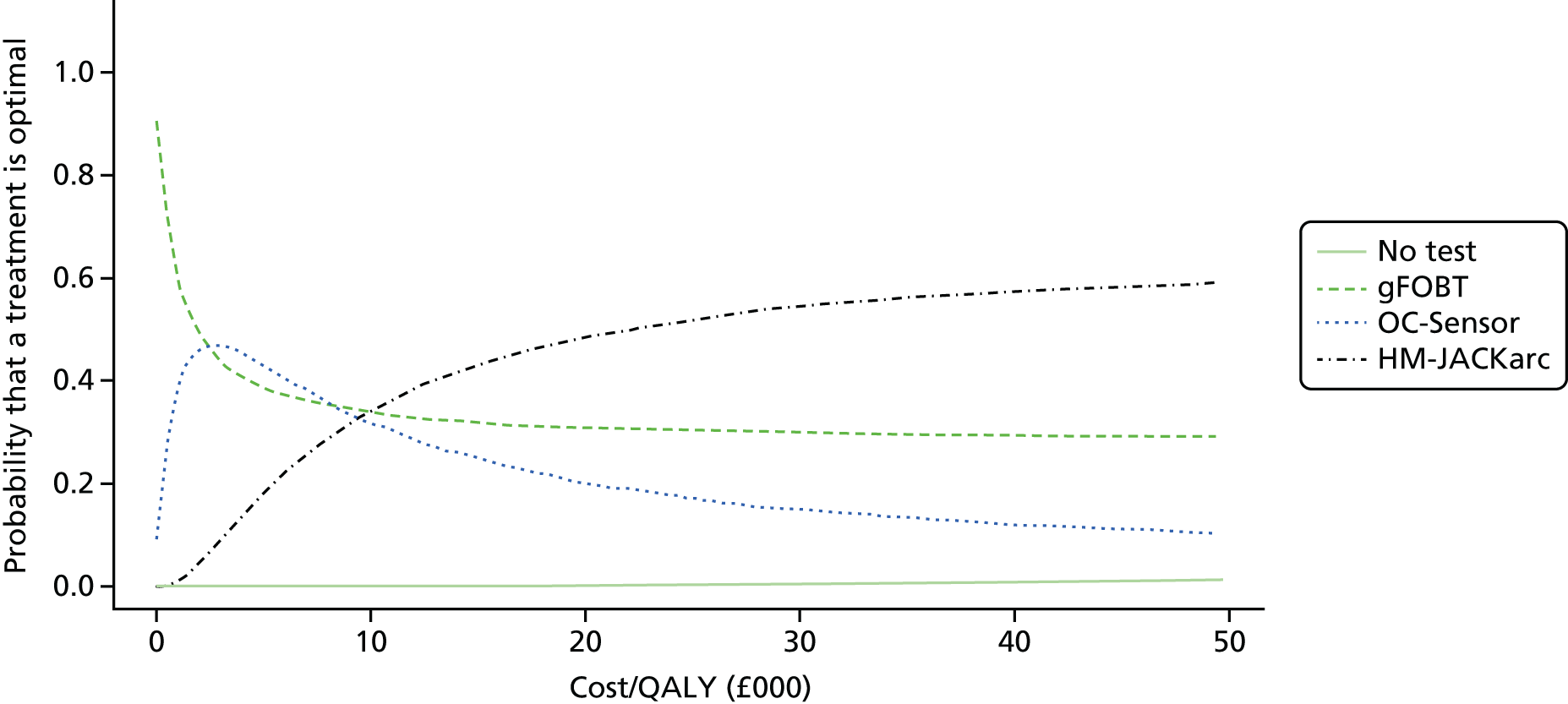
FIGURE 27.
Cost-effectiveness acceptability curves for intervention against gFOBT in the scenario without CRC progression. (a) Acceptability curves – HM-JACKarc vs. gFOBT; and (b) acceptability curves – OC-Sensor vs. gFOBT.


Probability of persisting in symptoms after having a negative test result
In the base-case scenario, this probability was estimated by the experts who filled in our questionnaire. This value was 32.5%. In this scenario we explored the consequences of doubling and halving this value. Doubling the probability of persisting in symptoms after having a negative result had a minor impact on the estimated QALYs, as can be seen in Table 59. This resulted in a slight loss in QALYs for all of the testing strategies owing to an increase in the number of patients with a delayed diagnosis. The impact on costs was large, as this implies a larger number of colonoscopies for all of the testing strategies. The ICERs for the interventions compared with gFOBT were low (both < £10,000). In addition, as in previous scenarios, no triage (referral straight to colonoscopy) was dominated by HM-JACKarc and the ICER of OC-Sensor compared with no triage was high, and it lay in the south-west quadrant of the cost-effectiveness plane. The PSA results (not shown) were similar to those in the base case, but in this scenario the interventions were more likely to be deemed cost-effective.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6217 | 381.09 | |||
| OC-Sensor | 18.6237 | 389.26 | 0.0020 | 8.17 | 4118 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6241 | 403.87 | 0.0004 | 14.61 | 36,534 |
When the probability of persistent symptoms after having a negative result was halved (Table 60), the opposite was observed. Nevertheless, in this case, no triage (referral straight to colonoscopy) was dominated by both interventions and the ICERs for both HM-JACKarc and OC-Sensor compared with gFOBT were < £30,000.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6220 | 157.88 | |||
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6239 | 172.01 | 0.0020 | 14.13 | 7143 |
| HM-JACKarc | 18.6243 | 210.19 | 0.0003 | 38.19 | 114,546 |
Adverse events associated with colonoscopy
We first considered the highest value found in the literature for the mortality due to colonoscopy. This was 0.0970%, and it was reported by Day et al. 114 for elderly patients. This can be seen as a worst-case scenario regarding mortality due to colonoscopy. As expected, the main differences with respect to the base-case scenario were observed for those strategies for which the number of colonoscopies was larger: no triage (referral straight to colonoscopy) and HM-JACKarc. The results are shown in Table 61; in particular, no triage (referral straight to colonoscopy) was dominated by all of the strategies. Whereas the ICER for OC-Sensor compared with gFOBT was < £10,000, the ICER for HM-JACKarc compared with gFOBT was £45,271. Thus, at the common value for the ICER threshold of £30,000, HM-JACKarc was not cost-effective compared with gFOBT.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| No triage (referral straight to colonoscopy) | 18.6064 | 503.65 | Dominated by gFOBT | ||
| gFOBT | 18.6148 | 232.26 | |||
| HM-JACKarc | 18.6157 | 274.73 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6165 | 244.40 | 0.0017 | 12.14 | 7144 |
When no adverse events were considered (Table 62), no triage (referral straight to colonoscopy) was dominated by HM-JACKarc. This was expected because the difference in QALYs between these two strategies observed in the base-case scenario was due to only the adverse events. In this case, as no adverse events were considered, the QALYs estimated were exactly the same. Likewise, differences in QALYs between faecal immunochemical testing and gFOBT are not present in this scenario. Therefore, the results of this scenario are slightly more favourable to faecal immunochemical testing than those in the base-case scenario.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6221 | 230.97 | |||
| OC-Sensor | 18.6241 | 243.06 | 0.0020 | 12.09 | 6081 |
| No triage (referral straight to colonoscopy) | 18.6245 | 500.49 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6245 | 273.19 | 0.0004 | 30.13 | 80,244 |
Probability of being referred to colonoscopy versus computed tomography colonography
In this scenario, we assumed that all patients would be referred to colonoscopy. The results are presented in Table 63.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 243.73 | |||
| OC-Sensor | 18.6239 | 256.31 | 0.0020 | 12.58 | 6352 |
| No triage (referral straight to colonoscopy) | 18.6239 | 531.40 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 288.36 | 0.0003 | 32.05 | 93,832 |
As expected, differences between this and the base-case scenario were minor and due to the increase in the costs of colonoscopy. For this reason, the results were slightly better for gFOBT, as this was the strategy that required fewer colonoscopies.
Probability of receiving a second faecal immunochemical test/guaiac faecal occult blood test
In this scenario, we assumed that (on average) 20% of the patients who remain symptomatic after testing negative on FIT/gFOBT had a second FIT/gFOBT. The results are shown in Table 64.
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.32 | |||
| OC-Sensor | 18.6239 | 244.67 | 0.0020 | 12.35 | 6237 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 275.05 | 0.0003 | 30.38 | 88,936 |
As expected, having a second FIT/gFOBT increased the costs of the patients who tested negative on FIT/gFOBT and remained symptomatic. This increase in costs had little impact on the cost-effectiveness results, as, on average, 32.5% of the patients testing negative would persist in their symptoms and 20% of these patients would get the second test. Thus, this increase would have an effect on only 6.5% of the patients who tested negative on FIT/gFOBT.
Chapter 5 Discussion
Statement of principal findings
Clinical effectiveness
All of the studies included in the systematic review were diagnostic cohort studies reporting accuracy data. Eight published studies13,52,53,55–58,75 and one unpublished study (Hospital Clinic de Barcelona 2015; Philippa Pinn, personal communication) assessed accuracy of a quantitative FIT method for the target condition of CRC or for the composite target condition of advanced neoplasia (CRC or HRA); the remaining study,54 which was published as a conference abstract, reported accuracy data for only the composite target condition ‘significant bowel disease’ (defined as CRC polyps or bleeding). Five studies13,52,53,55,58 reported accuracy data for the OC-Sensor assay. Three studies56,57,75 reported accuracy data for the HM-JACKarc automated system. One study,54 published as an abstract, and one unpublished study (Hospital Clinic de Barcelona 2015) reported accuracy data for the FOB Gold assay. No studies using the RIDASCREEN Hb or the RIDASCREEN Hb/Hp complex assays met the inclusion criteria for this assessment. Two studies of OC-Sensor53,55 also developed risk scores for CRC or advanced neoplasia that included faecal immunochemical testing; in the case of the COLONPREDICT study,55 risk score development was reported in a separate publication,73 namelya manuscript, which was provided ahead of publication (Callum Fraser, personal communication). None of the included studies reported data comparing different FIT assays, or comparing one or more FIT assays with a gFOBT method. No RCTs or CCTs were identified, and no studies provided data on patient-relevant outcomes following faecal immunochemical testing compared with gFOBT or no faecal occult blood testing.
No study reported data that were specific to the population defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms who are at low risk for CRC according to the criteria defined in NG121). Only one study52 was conducted in a primary care setting, and this study reported that faecal immunochemical testing was ordered by GPs at the point of referral to secondary care. In addition, all of the studies included some participants who had symptoms that may be considered to be associated with a higher probability of CRC and that are components of the criteria for 2-week referral as defined in NG121 (e.g. rectal bleeding).
When faecal immunochemical testing was based on a single faecal sample and a threshold of 10 µg Hb/g faeces was applied, sensitivity estimates indicated that a negative test using either OC-Sensor and HM-JACKarc may be considered adequate to rule out CRC; the summary estimate of sensitivity for C-Sensor was 92.1% (95% CI 86.9% to 95.3%), based on four studies, and the only study of HM-JACKarc to assess the 10 µg Hb/g faeces cut-off point reported a sensitivity of 100% (95% CI 71.5% to 100%). 56 The corresponding specificity estimates were 85.8% (95% CI 78.3% to 91.0%) and 76.6% (95% CI 72.6% to 80.3%), respectively. (Confidential information has been removed.) When a hypothetical cohort of 1000 patients is considered, assuming prevalence estimates derived from the included studies, the estimated number of CRC cases that would be missed using each of the three FIT assays (OC-Sensor and HM-JACKarc 10 µg Hb/g faeces threshold and (confidential information has been removed) is 2, 0 and (confidential information has been removed), respectively; under the same assumptions, the number of ‘unnecessary’ colonoscopies carried out on people without CRC would be 198, 229 and (confidential information has been removed), respectively (see Chapter 3, Results of the assessment of clinical effectiveness assessment, see Figures 5a, 9 and 11). Reducing the threshold to ‘any detectable Hb’ produced summary estimates of sensitivity and specificity, for OC-Sensor, of 100% (95% CI 87.7% to 100%) and 43.4% (95% CI 39.7% to 47.1%), based on two studies. For the hypothetical cohort of 1000 patients, this lowering of the threshold would result in no cases of CRC being missed, but would increase the number of ‘unnecessary’ colonoscopies from 198 to 548 (see Figure 5b). No study of HM-JACKarc assessed the any detectable Hb threshold for CRC. (Confidential information has been removed.)
No study reported data on the effects of patient age, sex or presenting symptoms, or on the effects of multiple sampling, on the accuracy of faecal immunochemical testing for the target condition of CRC. However, the results of multivariable regression analyses indicate that participant age73 and sex,53,73 but not presenting symptoms (NICE and SIGN 2-week referral criteria),53 as well as faecal Hb concentration,53,73 are independent predictors of CRC. Validation of the FAST score (Cubiella et al. 2016;127 Callum Fraser, personal communication), which incorporates age, sex and faecal Hb concentration, has suggested that a score threshold of 2.12 (range of score not reported) can be used to reliably rule out CRC (sensitivity 100%, 95% CI 97.7% to 100%); however, the specificity of the score is very low (19.8%, 95% CI 18.6 to 21.1%) at this threshold.
If a lower diagnostic threshold is applied, that is, the target condition includes HRA as well as CRC (advanced neoplasia), then the rule-out performance of all FIT methods is reduced. For faecal immunochemical testing based on a single faecal sample and a threshold of 10 µg Hb/g faeces, the sensitivity estimates indicated that neither a negative OC-Sensor nor a negative HM-JACKarc FIT would be likely to be considered adequate to rule out CRC; the summary estimate of sensitivity for OC-Sensor was 62.9% (95% CI 55.9% to 69.4%), based on three studies, and the estimate of sensitivity for HM-JACKarc was 70.0% (95% CI 50.6% to 85.3%), based on one study. 56 The corresponding specificity estimates were 84.6% (95% CI 82.8% to 86.2%) and 77.8% (95% CI 73.8% to 81.4%), respectively. One additional study57 reported sensitivity and specificity estimates for HM-JACKarc at the 10 µg Hb/g faeces threshold and any detectable Hb thresholds; however, this study57 differed from others in the systematic review in that it included some patients who were undergoing colonoscopy for polyp surveillance and excluded people with GI bleeding or active rectal bleeding; the prevalence of CRC in this study was the lowest of any included study (0.96%). 57 When a hypothetical cohort of 1000 patients is considered, assuming that prevalence estimates have been derived from the included studies, the estimated number of HRA cases that would be missed using either of the two FIT assays (OC-Sensor and HM-JACKarc at the 10 µg Hb/g faeces threshold) is 40 and 22, respectively; under the same assumptions the number of ‘unnecessary’ colonoscopies carried out on people without CRC would be 157 and 205, respectively (see Chapter 3, Results of the assessment of clinical effectiveness assessment, Figures 7a and 10). (Confidential information has been removed.) Using the OC-Sensor assay and a threshold of any detectable Hb increased the summary estimate of sensitivity to 84.1% (95% CI 78.3% to 88.8%), with a corresponding specificity estimate of 45.2% (95% CI 42.7% to 47.7%). For the hypothetical cohort of 1000 patients, this lower threshold would still result in 18 cases of HRA being missed and 485 ‘unnecessary’ colonoscopies being conducted in people without HRA or CRC (see Figure 7a).
Only the Auge et al. study57 reported any data on the effects of patient characteristics (sex) and number of faecal samples tested on the accuracy of faecal immunochemical testing for the target condition of advanced neoplasia. However, the results of multivariable regression analyses indicate that participant age53,73 and sex,53,73 but not presenting symptoms (NICE and SIGN 2-week referral criteria),53 as well as faecal Hb concentration,53,73 are independent predictors of advanced neoplasia. Validation of two risk scores based on the analyses (both incorporating age, sex Hb concentration) gave increased estimates of sensitivity and specificity. One study reported sensitivity and specificity estimates of 88.1% (95% CI 74.3% to 96.0%) and 63.3% (95% CI 57.4% to 69.0%), for a threshold of ‘5’ on a score with a scale of 0–11. 53 The FAST score (Cubiella et al. 2016;127 Callum Fraser, personal communication) threshold of 2.12 (range of score not reported) gave sensitivity and specificity estimates of 96.7% (95% CI 94.9% to 98.0%) and 21.5% (95% CI 20.1% to 22.9%). These data suggest that risk scores may have the potential to provide a more reliable rule-out method than faecal immunochemical testing alone at lower thresholds of disease.
No studies were identified which assessed the diagnostic performance of RIDASCREEN Hb or RIDASCREEN Hb/Hp complex in symptomatic patients, and no studies were identified which directly compared the performance of different FIT assays, or which compared one or more FIT assays with a gFOBT method.
The selection of FIT accuracy data for use in cost-effectiveness modelling was based on the optimal threshold (maximum sensitivity and specificity) for each assay method and the threshold required to provide optimal rule-out performance (highest sensitivity and lowest number of cases missed). Because no studies were identified which directly compared the performance of one or more FIT assays with a gFOBT method, accuracy data for gFOBT that were used in the base case for cost-effectiveness modelling were those used in the NG12 model. 6 The primary study from which these data were taken, Gillberg et al. ,91 was a retrospective study using a Swedish cancer registry, in which a diagnosis of CRC was classified as any diagnosed CRC within 2 years of guaiac faecal occult blood testing. It was unclear whether or not all of the patients included in the study had been symptomatic at the time of guaiac faecal occult blood testing, and the prevalence of CRC, in the subgroup of study participants used to provide accuracy data for the model, was very low (0.36%); the prevalence of CRC for the whole population of the Gillberg et al. study91 (1.8%) was closer to the prevalence estimate that was used in the NG12 model6 (1.5%). Because of these problems with the Gillberg et al. study91 data, we chose to conduct scenario analyses using published estimates of gFOBT accuracy in symptomatic patients obtained from studies that were identified during the process of inclusion screening for our systematic review: the Niv and Sperber92 and Bjerregaard et al. 93 studies. Both of these studies were conducted in symptomatic patients and the prevalence of CRC was 2.5% and 3.1%, respectively, making these studies closer to the population defined for this assessment.
Cost-effectiveness
We assessed the cost-effectiveness of using quantitative faecal immunochemical tests for Hb (faecal immunochemical testing) as a triage test for people presenting, in primary care settings, with lower abdominal symptoms who are at low risk for CRC according to the criteria defined in NG12. 1 Our review of economic analysis found no papers or reports assessing specifically this decision problem. The most relevant study was the one conducted in NG12,6 which estimated the cost-effectiveness of five different investigations (i.e. faecal occult blood testing, barium enema, flexible sigmoidoscopy, CTC or colonoscopy) for suspected CRC ordered in primary care for patients aged 40 years and over with a change in bowel habit as the main symptom. This study was used to guide a part of the development of our diagnostic assessment model.
Diagnostic accuracy of faecal immunochemical testing was captured in the model using data on sensitivity and specificity for the detection of CRC using a single faecal sample obtained from our systematic review. For the base-case scenario, we considered a threshold of 10 µg Hb/g faeces or equivalent. For this threshold, our systematic review obtained data only for OC-Sensor and HM-JACKarc. The comparators chosen for this diagnostic assessment were gFOBTs and no triage (referral straight to colonoscopy). For gFOBT, we considered the sensitivity and specificity estimates used in NG121 (base-case scenario) and in two papers identified in our systematic review (scenario analyses) that were thought to represent a better match for the population that was specified for this diagnostic assessment. Additionally, the FOB Gold FIT system was included as an intervention in scenario analyses, despite the limitations of the accuracy data found in our systematic review, as described in Chapter 3.
The base-case cost-effectiveness results suggested that the difference in QALYs between all of the strategies included in this assessment is minimal and that the ‘no triage’ strategy (referral to colonoscopy) is the most expensive. Overall, faecal immunochemical testing was cost-effective when compared with no triage. This was either because the latter was dominated (less effective and more costly) or because the ICERs obtained were high, but in the south-west quadrant of the cost-effectiveness plane, that is, faecal immunochemical testing was slightly less effective, but cheaper, than no triage. In this case the cost savings could be said to ‘outweigh’ the slight loss in QALYs. When the comparator was gFOBT, the cost-effectiveness results showed that faecal immunochemical testing was more effective and more costly than gFOBT, but the ICERs obtained were below the common threshold ICER of £30,000, and thus the interventions can also be considered cost-effective. However, it should be noted that the PSA results showed that this finding should be interpreted with caution. Because the PSA outcomes were scattered over the four quadrants of the cost-effectiveness plane in an asymmetrical fashion, gFOBT had in general a high probability of being cost-effective (especially at low values of the ICER threshold) and sometimes gFOBT was the strategy with the highest probability of being cost-effective.
The results of the different scenario analyses did not differ substantively from the base-case results. The scenarios for which the accuracy estimates for gFOBT were based on the studies by Niv and Sperber92 and Bjerregaard et al. 93 (those that are considered more representative of the population of this diagnostic assessment) were more favourable than the base-case scenario with regard to the interventions. In only two scenarios would FIT strategies be considered to be not cost-effective because the ICER exceeded the £30,000 threshold. The highest ICER was obtained when OC-Sensor was compared with gFOBT when a threshold of any detectable Hb was assumed for this FIT assay (£65,192). This was expected, as reducing the threshold for FIT results in the test being less effective in avoiding colonoscopies, that is, this threshold is associated with the highest number of FPs. When HM-JACKarc was compared with gFOBT in the scenario with high mortality due to colonoscopy, the ICER was £45,271.
Strengths and limitations of assessment
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying test accuracy studies using study design-related search terms,128 search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened, relatively few of which met the inclusion criteria of the review.
The possibility of publication bias remains a potential problem for all systematic reviews. Considerations may differ for systematic reviews of test accuracy studies. It is relatively simple to define a positive result for studies of treatment, for example a significant difference between the treatment and control groups, which favours treatment. This is not the case for test accuracy studies, which measure agreement between index test and reference standard. It would seem likely that studies finding greater agreement (high estimates of sensitivity and specificity) will be published more often; however, the relative priorities given to sensitivity and specificity estimates may vary depending on the intended application of the test. In addition, test accuracy data are often collected as part of routine clinical practice, or by retrospective review of records; test accuracy studies are not subject to the formal registration procedures applied to RCTs and are therefore more easily discarded when results appear unfavourable. The extent to which publication bias occurs in studies of test accuracy remains unclear; however, simulation studies have indicated that the effect of publication bias on meta-analytic estimates of test accuracy is minimal. 129 Formal assessment of publication bias in systematic reviews of test accuracy studies remains problematic and reliability is limited. 39 We did not undertake a statistical assessment of publication bias in this review. However, our search strategy included a variety of routes to identify unpublished studies and resulted in the inclusion of a number of conference abstracts.
Despite our extensive searches, no studies were identified that assessed the diagnostic performance of RIDASCREEN Hb or RIDASCREEN Hb/Hp complex in symptomatic patients. We identified one article that assessed the performance of Hb/Hp complex in faeces for detecting CRC; the reported sensitivity and specificity estimates were 77% and 95%, using a threshold of 2 µg/g faeces. 130 The test manufacturer stated, of this study, that: ‘My understanding is that all of the patients were symptomatic’ (e-mail from Andrea Lennerz, R-Biopharm AG, Germany, via Rebecca Albrow, NICE, to Marie Westwood, KSR Ltd, 27 May 2016, personal communication). However, this study did not meet the inclusion criteria for our systematic review, as it used an early developmental immunoluminometric assay for Hb/Hp complex and not an assay that is currently commercially available or in use in a NHS laboratory.
No studies were identified which directly compared the performance of different FIT assays, or which compared one or more FIT assays to a gFOBT method; therefore, all of the data included in this assessment refer to the clinical effectiveness of individual FIT methods and not to their comparative effectiveness.
Although gFOBT was not one of the interventions included in our systematic review, it was considered to be a comparator for cost-effectiveness modelling. In order to inform cost-effectiveness modelling, our searches therefore included general terms for faecal occult blood testing and guaiac faecal occult blood testing. Diagnostic accuracy studies of gFOBT conducted in symptomatic patients, retrieved by these searches, were identified during the systematic review process. However, we do not consider that we have conducted a full systematic review of guaiac faecal occult blood testing in symptomatic patients, as our searches did not include terms for gFOBT product names and our inclusion criteria specified only those studies of gFOBT that included a comparison to one or more FIT methods.
Clear inclusion criteria were specified in the protocol for this review, a copy of which is available online (www.nice.org.uk/guidance/GID-DG10005/documents/final-protocol). The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for exclusion for all of the studies which were considered potentially relevant at initial citation screening and were subsequently excluded on assessment of the full publication (see Appendix 4). The review process followed recommended methods to minimise the potential for error and/or bias;37 studies were independently screened for inclusion by two reviewers, and data extraction and quality assessment were undertaken by one reviewer and checked by a second (MW and SL). Any disagreements were resolved by consensus.
All of the studies included in this review were assessed for risk of bias and applicability using the QUADAS-2 tool,45 which is recommended by the Cochrane Collaboration. 39 QUADAS-2 is structured into four key domains: participant selection, index test, reference standard and the flow of patients through the study (including timing of tests). Each domain is rated for risk of bias (low, high or unclear); the participant selection, index test and reference standard domain are also separately rated for concerns regarding the applicability of the study to the review question (low, high or unclear). The results of the QUADAS-2 assessment are reported, in full, for all of the included studies in Appendix 3 and are summarised in Chapter 3 (see Study quality). Those studies that reported development of risk scores, in addition to test accuracy data, were also assessed using the PROBAST tool. 46 PROBAST has been designed to assess both the risk of bias and concerns regarding applicability of a study that evaluates (develops and/or validates) a multivariable diagnostic or prognostic prediction model. It has a domain-based structure, similar to that of QUADAS-2, and is intended to be used for the assessment of primary studies that are included in a systematic review. PROBAST is not yet published, but has been used with the consent of the steering group, of which the lead author of this assessment report is a member.
This assessment includes information on the development and validation of risk scores that incorporate faecal Hb levels measured by faecal immunochemical testing, as well as test accuracy data for faecal immunochemical testing alone. Available data on the performance characteristics of such scores have been included in order to inform the question of whether or not the performance characteristics of faecal immunochemical testing can be improved by using it as part of a simple scoring system that includes other information that is readily available to medical practitioners.
All of the studies included in our systematic review were conducted in Europe; however, only four studies13,52,56,75 were conducted in the UK (one in England75 and three in Scotland13,52,56). Given that population studies have shown variation in faecal Hb concentrations, and hence potential variation in optimal thresholds for faecal immunochemical testing, across different geographic location,88,89 this may limit the applicability of our findings to UK settings. In addition, the definitions of HRA used in UK studies are generally based on number and size of lesions, as per BSG guidelines,90 whereas those conducted in mainland Europe have used definitions that also include morphology. However, it should also be noted that validation data for the FAST score (Callum Fraser, personal communication) indicated that there was no significant difference in the sensitivity of the tool (for either CRC or advanced neoplasia) between Scotland and Spain, the two countries where the majority of studies in our systematic review were conducted.
Although the sample sizes of studies included in our systematic review were generally large for diagnostic accuracy studies (median n = 474, range 83–2058), it should be noted that study samples may not be representative of all of those people presenting in primary care with symptoms as specified in NG12. 1 Only three of the studies13,52,56 included in our systematic review explicitly reported the number of people who were invited to participate, and proportions agreeing were relatively low (64%,13 56%56 and 48%52), implying that study samples may be self-selecting for more motivated patients.
A further limitation of the study populations is that no study reported data that were specific to the population that was defined in the scope for this assessment (people presenting, in primary care settings, with lower abdominal symptoms who are at low risk for CRC according to the criteria defined in NG121). Only one study52 was conducted in a primary care setting, and this study reported that faecal immunochemical testing was ordered by GPs at the point of referral to secondary care. Although the primary care study might be considered the most applicable to this setting, because it is the only study to allow a change in referral decision based on FIT result, this design also gives rise to a risk of bias, as 11% of participants who returned a FIT sample were excluded from the analysis because they were not subsequently referred to secondary care or the referral was cancelled. 52 All of the studies included some participants who had symptoms that may be considered to be associated with a higher probability of CRC and which are components of the criteria for 2-week referral as defined in NG121 (e.g. rectal bleeding). The prevalence of CRC and advanced neoplasia is likely to differ with different presenting symptoms and with the health-care setting in which testing is undertaken (primary care vs. secondary care); the median prevalence of CRC reported in the studies included in our systematic review was 3.0% (range 1.0–12.3%) compared with the estimate of 1.5% for the relevant symptomatic group used in NG12. 6 Although it is known that the prevalence of the target condition can affect test performance characteristics,7 there are insufficient data to adequately assess these effects for the use of faecal immunochemical testing in symptomatic patients. The results of our systematic review do not suggest significant differences in test performance between the primary care study52 and other included studies that used the same FIT assay, threshold(s) and target condition (see Chapter 3, Diagnostic performance of the OC-Sensor faecal immunochemical test assay). Similarly, validation data for the FAST score (Callum Fraser, personal communication) indicated that there was no significant difference in the sensitivity or specificity estimates for the score between primary and secondary care settings.
Systematic reviews conducted previously have assessed the test characteristics of various FIT assays in screening settings8,131 and compared the performance of faecal immunochemical testing for CRC screening with that of gFOBT. 24,25 However, given the potential for target condition prevalence to affect test performance characteristics,7 it is important to determine the diagnostic accuracy of faecal immunochemical testing in the population of interest; we are not aware of any previous systematic review on this topic. We identified one large systematic review132 that assessed the value of symptoms and additional diagnostic tests for CRC, used in symptomatic patients in primary care. However, the searches for this review were completed in 2008 and identified only three studies of quantitative FIT assays. Our searches identified all of the three studies:133–135 one study133 was excluded from this assessment because it included a mixed population of symptomatic, screening and high-risk patients and no separate data for the symptomatic subgroup, and the remaining two studies134,135 were excluded because they used development stage Hb/Hp complex tests, which are not currently available in the NHS. Additionally, Williams et al. 136 have recently published a systematic review of risk prediction models for CRC or HRA in people with symptoms. This review136 included 15 risk models, none of which included faecal Hb measured by faecal immunochemical testing (or any other method) as a variable. Our systematic review is the first to assess the performance of faecal immunochemical testing, as a triage test for CRC, in people with symptoms and to consider the potential utility of applying faecal immunochemical testing as part of a simple risk score.
Cost-effectiveness
A major strength of our analysis is that it is the first study to assess the cost-effectiveness of faecal immunochemical testing as a triage test for symptomatic low-risk people presenting in primary care settings, based on a systematic review of the accuracy of faecal immunochemical testing. The input parameters used to model the accuracy of faecal immunochemical testing in the diagnostic part of the cost-effectiveness model (decision tree) were informed by a comprehensive high-quality systematic literature review on the clinical effectiveness of several FIT assays. For consistency, we sourced costs (other than those for faecal immunochemical testing and gFOBT) and CRC natural progression parameters from the current clinical guideline NG12. 6 These parameters were updated to their most recent values when deemed necessary. Although many of the input parameters of our cost-effectiveness model are the same as those described in NG12,6 the ones pertaining to faecal immunochemical testing differed because we performed cost-effectiveness analyses for specific FIT assays. In NG12,6 faecal immunochemical testing was included only as a single intervention and only in a scenario analysis.
A further strength of our study is that it includes detailed data on resource use and equipment costs for faecal immunochemical testing. Data on resource use were obtained from expert opinion (e-mail from Callum Fraser, NHS Tayside, to Marie Westwood, KSR Ltd, 19 July 2016, personal communication). This was important because figures on resource use for the population considered in this diagnostic assessment are scarce in the literature; we could not find any published study. Data on equipment costs were obtained from the relevant companies and are presented in a detailed and transparent way in this diagnostic assessment. This was also important because, in the papers found, which assessed the costs of faecal immunochemical testing, it was not always clear how the total costs were estimated from resource use and equipment costs.
Finally, we have considered a large variety of scenarios to explore the uncertainties around the assumptions that were made in the cost-effectiveness model and, in order to quantify these uncertainties in a statistical way, we performed PSAs for all of them.
The secondary care aspects of our model are a simplification of what actually occurs in practice. The ‘no triage’ comparator arm would be more accurately described as ‘no FIT/gFOBT’, as the secondary care consultation with a gastroenterologist clearly forms part of the triage before colonoscopy. The model structure follows a similar approach to that used in NG12,6 in that it assumes that all of the patients with a positive FIT/gFOBT and all of the patients in the ‘no FIT/gFOBT’ arm receive colonoscopy. However, the alternative would involve trying to estimate the proportion of patients whom the gastroenterologist would refer for colonoscopy without faecal immunochemical testing/gFOBT (this proportion would apply to the ‘no FIT/gFOBT’ arm). We would also need to estimate the effect that the positive FIT/gFOBT result has on the gastroenterologist’s probability of requesting colonoscopy (numbers referred from the FIT/gFOBT-positive pathway). We are not aware of any published data to inform these parameters, and asking clinicians for estimates of their own performance (the accuracy of their referral decisions) has a high risk of bias.
There is a potential problem with the population, which is based on NG12,6 in that it is likely to be heterogeneous, such that many patients might better be treated with a watchful waiting strategy rather than being referred immediately to secondary care. The model assumes that all of the patients who present with the symptoms specified in NG126 would be treated in the same way by the GP. Hence, we cannot capture the role of the GP’s clinical judgement in ruling out other possible explanations for the presenting symptoms for which faecal immunochemical testing/gFOBT would not be helpful. In practice, this is may be less of a problem than it appears, as the evidence from our systematic review is in a clinically appropriate population (those for whom the GP is considering referral to secondary care or for whom a referral decision has already been made). The prevalence of CRC in this population is also uncertain; NG126 relied on expert opinion to provide an estimate, which was 1.5%. In the absence of any data that were specific to this population, we also chose this value for the base case, although we tested the effect of using prevalence as observed in the accuracy studies from our systematic review.
There is a potential for patients with a FIT FP test for CRC to benefit from referral to secondary care/colonoscopy for diagnosis and treatment of conditions other than CRC (see Chapter 5, Clinical effectiveness). This is not captured in our model, which is, therefore, likely to underestimate the cost-effectiveness of faecal immunochemical testing.
The limitations described for clinical effectiveness regarding the lack, or the poor quality, of accuracy data for the different FIT assays are also applicable to the assessment of cost-effectiveness. Accuracy data were not available for all of the relevant FIT methods and there were no studies that compared more than one FIT method or that compared faecal immunochemical testing with gFOBT.
We lacked data to inform the parts of the diagnostic model that followed a FIT or gFOBT negative result. Therefore, we assumed that those who have CRC would remain symptomatic and would be diagnosed using colonoscopy or CTC. However, we did not know how many non-patients with CRC would also remain symptomatic; hence, we sought expert opinion. We also had to seek expert opinion on the number of patients who would get a second FIT/gFOBT, as well as the time to diagnosis of CRC. We did not seek expert opinion on the accuracy of a second FIT/gFOBT, even though we had no data, because we believe that this is something that cannot be reliably estimated by a clinician. We therefore sent out a questionnaire to 10 specialists, five of whom returned it, although not all of the questions were completely answered. We also conducted scenario analyses on the proportion who were symptomatic and time to diagnosis.
In the absence of data for this population, the probability of undergoing colonoscopy, as opposed to CTC, was estimated to be 83% by the experts (four answers). However, this value seems to be low compared with the screening population. Logan et al. 126 found that, for approximately 98% of the screening patients with an abnormal test result, the first investigation was colonoscopy. However, the ratio of colonoscopy to CTC seems to vary by centre and ranges from 0.3% to 9.1%;137 therefore, this parameter was varied in the scenario analysis.
In the base-case scenario, we assumed that adverse event rates for CTC were the same as those for colonoscopy. However, Burling et al. 19 reported that perforation rates for colonoscopy are four times higher than for CTC. No death events were reported and the proportion of patients experiencing bleeding after examination was not described. Thus, it is likely that our assumption would result in an overestimation of the total number of adverse events. Nevertheless, as there is uncertainty around the appropriate estimates for these adverse events, we decided to keep this assumption and interpret it as a worst-case scenario regarding the total number of adverse events. We explored the impact of this assumption on the cost-effectiveness results by performing additional scenario analyses.
Cost estimates for faecal immunochemical testing and gFOBT reported in the literature vary significantly. In particular, the costs of faecal immunochemical testing found in the literature ranged from £1.96 to £10.23 per test, and from (confidential information has been removed) to £9.57 per gFOBT. The estimated costs for the OC-Sensor and the HM-JACKarc FIT assays used in this diagnostic assessment were £4.53 and £6.04, respectively. Although the cost estimates used in this diagnostic assessment are properly reported, we acknowledge that there is uncertainty around the underlying assumptions leading to them. For example, it can be seen that, in Table 32, information for certain costs categories is missing. This may lead to an underestimation of the cost estimates for faecal immunochemical testing. On the other hand, the manufacturers indicated that the prices provided were also subject to discount, which could imply a lower cost in practice. Ideally, training and staff costs should have been included for faecal immunochemical testing and gFOBT. However, information about training and staff costs is scarce and, when available, relates to dedicated service provision for the national screening programme. For this reason, we decided not to include these costs in our health economic model. Nevertheless, as FIT assays use automated immunoassay platforms, which are standard in clinical laboratories, we believe that training costs would be minimal. To overcome these limitations, we performed threshold analyses on the cost differences between tests.
Our diagnostic model did not include reduction in quality of life (disutilities) for the adverse events that are associated with colonoscopy. Although no evidence was found on the effects of bleeding and perforation on quality of life, some effects on quality of life might be expected. Nevertheless, as these events are often of short duration, the effects on quality of life can be assumed negligible. Kapidzic et al. 138 also reported a slight reduction in quality of life for participants in a screening programme who had a positive result. This was due to the anxiety of knowing that they tested positive. However, these anxiety episodes can also be assumed to be of short duration, with minimal effects on overall quality of life.
As mentioned in Chapter 3 (see Methods of analysis/synthesis), the sensitivity and specificity point estimates for the OC-Sensor assay were derived using the bivariate model, which accounts for the correlation between sensitivity and specificity. However, in the PSA, sensitivity and specificity were sampled independently from the beta distributions presented in Table 23. Thus, the PSA does not account for the correlation between sensitivity and specificity. This was a pragmatic choice, as insufficient data were available to allow an analysis accounting for correlation for the HM-JACKarc or the FOB Gold assays.
The effect of a delayed CRC diagnosis was assumed to be mediated through the probability of progression within Dukes’ states, for undiagnosed patients, given a delay in the base case of 6 months. This was because the proportion of patients per Dukes’ stage for 6 months seemed to match reasonably well with the idea that FN patients would remain symptomatic and thus be picked up well within 1 year. Estimates for delayed diagnosis were also obtained from clinical experts, although only three of the experts who received our questionnaire answered this question; expert estimates were even lower, with two of them estimating a delay of approximately 2–3 months, whereas the third expert estimated this at between 1 and 2 months. A delay of 1 year also seems to be too long because it would result in 45% at Dukes’ stage D. This was deemed too high, given that the initial proportion of patients at stage D was 14%. The only published source that we found reporting delayed diagnosis was the Meester et al. study. 139 This study used a microsimulation model to calculate the effect of delay to colonoscopy on an average-risk population cohort in the USA who underwent annual FIT screening (from the age of 50 to 75 years), with follow-up colonoscopy examinations for individuals with positive results in the following 12 months. For the scenario of diagnostic follow-up at 12 months from a positive FIT, diagnosed cancers shifted towards more advanced stages, but still with only 8% in stage D. Regardless of the precise delay and its effect on progression, the cost-effectiveness results did not change significantly assuming 6 months’ or 1 year’s delay and so we decided not to run an additional scenario for a smaller delay. Note, however, that, as the delay in diagnosis diminishes, the differences in QALYs between the strategies decrease as well. In a hypothetical scenario with no delay, the difference in QALYs will be caused only by mortality. In this situation, the strategy with the least number of colonoscopies (gFOBT) is likely to dominate all of the others.
Colorectal cancer mortality was modelled using observational data by CRC stage at the time of diagnosis from the NCIN,98 as used in NG12. 6 Patients who survive might eventually progress, thus increasing mortality. It was not clear the extent to which the effect of progression was included. We chose to include a separate estimate of the rate of progression as in NG12. 6 However, the progression probabilities, which were obtained from Tappenden et al. ,99 were for undiagnosed patients with CRC. This is an obvious limitation, as diagnosed patients are considered in the model following colonoscopy. We also did not model separately the effect of treatment on patients with CRC mortality or cost, assuming that the estimates of mortality and cost were from all diagnosed patients with CRC, including those who had been treated and indeed ‘cured’. This does mean that the effect of newer treatments and thus the effect of delay to diagnosis cannot be taken into account.
Despite the limitations of the model for CRC progression described above, it should be emphasised that the lifetime cost-effectiveness results of this diagnostic assessment are completely determined by the results of the diagnostic part of the economic model. Thus, any difference between the strategies observed in the lifetime results was already captured in the diagnostic phase. Beyond the diagnostic model, the differences between the strategies were very small because these were caused only by the number of CRC-diagnosed patients and the distribution of these between Dukes’ stages. Given that death (due to colonoscopy) in the diagnostic phase is almost negligible, we could roughly estimate these as 15 patients with CRC from a hypothetical cohort of 1000 (as the estimated prevalence of the disease is 1.5%). Therefore, any difference in the lifetime results between the strategies will be caused by only these 15 patients. Hence, all of the limitations and uncertainties of the model for CRC progression will have a minimal impact on the model results of this diagnostic assessment. If the disease prevalence, the life expectancy or the costs and benefits of the treatments and surveillance of the population with the disease are likely to make a difference in the lifetime cost-effectiveness results then all of the limitations of the CRC model should be explored with extra caution.
Finally, we would like to emphasise the importance of specificity on the cost-effectiveness results. This can be partly explained, for example, with simple algebra. As described in Chapter 4 (see Model parameters), the probability that FIT/gFOBT is positive can be calculated as (sensitivity × prevalence) + [(1 – specificity) × (1 – prevalence)]. The first part of this equation (sensitivity × prevalence) is the probability of a TP, that is, of sending patients who will be diagnosed with CRC. The second part [(1 – specificity) × (1 – prevalence)] is the probability of a FP, that is, of wrongly sending healthy patients for colonoscopy. It is clear that in a situation for which the prevalence is low, the weight of specificity on this equation is larger than that of sensitivity, that is, any change in specificity will have a greater effect on the probability of FPs than any change in sensitivity will have on the probability of TPs. In this diagnostic assessment, the prevalence of CRC in the base-case population was assumed to be 1.5%. This means that the probability of a FP can vary from 0 to 0.985, whereas the probability of a TP can vary only from 0 to 0.015. The question then is what are the consequences, in terms of cost and QALYs, of either not missing TPs or avoiding FPs. People without CRC might be subjected to unnecessary colonoscopies, incur unnecessary cost and experience unnecessary adverse events (including death). On the other hand, patients with CRC might receive an earlier diagnosis, resulting in an increased number of QALYs. In fact, there was very little difference in QALYs between the FIT/gFOBT and the ‘no triage test’, which indicates little difference between avoiding FPs or not missing TPs in terms of health effects. Nevertheless, it appears that, in our model, improved specificity does outweigh improved sensitivity. This was clearly shown by the fact that OC-Sensor was almost identical to ‘no triage test’ in terms of QALYs, but cheaper, whereas HM-JACKarc, with its higher sensitivity and lower specificity, produced fewer QALYs and was more costly than OC-Sensor. This effect was also observed in the scenario analyses using the estimates of gFOBT accuracy from Niv and Sperber92 and Bjerregaard et al. ,93 where, with respect to the base-case scenario, sensitivity increased more than specificity decreased (approximately 20–25% vs. 9–15%). The net effect of these changes was that the cost-effectiveness results for gFOBT were worse than those obtained in the base-case scenario.
Uncertainties
Clinical effectiveness
There remain a number of areas of uncertainty in relation to the performance characteristics of faecal immunochemical testing in specific patient subgroups and test combinations.
Population data indicate that concentrations of faecal Hb vary with age and sex, being higher in men and the elderly. 88,140 Further, a recent study conducted in Scotland found that faecal Hb concentrations also increased with increasing levels of deprivation (measured using the Scottish Index of Multiple Deprivation) and that this trend remained after controlling for age and sex. 141 Thus, at any cut-off concentration, more men, more older people and more people in high deprivation groups are likely to have a positive result on faecal immunochemical testing. The performance of faecal immunochemical testing in these subgroups, within the symptomatic population, is therefore an important consideration for this assessment; optimal thresholds may differ between subgroups and may also differ depending on the geographic location in which the test is being applied. The baseline characteristics of our included studies (where reported) indicated that the average age of participants ranged from 59 to 67 years (total range was 16–94 years) and the percentage of males ranged from 40.4% to 56%. However, only one of the studies included in our systematic review compared the accuracy of a FIT assay (HM-JACKarc) in men and women,57 and no study compared different age groups. The study reporting separate data for men and women found that, at all thresholds, the observed sensitivity of HM-JACKarc for advanced neoplasia was higher in men than in women and the observed specificity was similar for men and women. 57 This indicates that, at any given threshold, more women than men with CRC or HRA may be missed by using a FIT as a triage test to determine referral to secondary care. However, it should be noted that this study differed from others in the systematic review in that it included some patients who were undergoing colonoscopy for polyp surveillance and excluded people with GI bleeding or active rectal bleeding; the prevalence of CRC in this study was the lowest of any included study (0.96%). 57 More data are needed to adequately assess whether or not there are clinically relevant differences in test performance between men and women, and these data are needed for all FIT assays. We did not identify any studies that compared the accuracy of faecal immunochemical testing in different age groups, or with varying levels of deprivation. Validation data for the FAST score (Callum Fraser, personal communication) indicated that there were no significant differences in the sensitivity of this tool between men and women and between patients aged < 50 years and those who were ≥ 50 years old.
The effects on FIT performance of using multiple faecal samples per patient remain unclear. One published study57 and one unpublished study, Hospital Clinic de Barcelona 2015 (Philippa Pinn, personal communication), included in our systematic review, compared single sampling with double sampling, and both asked participants to collect two consecutive faecal samples. The Auge et al. study57 used HM-JACKarc and reported that sensitivity for advanced neoplasia was increased (at all thresholds) when the highest value from two consecutive FIT samples was used, compared with using only the first sample; in this study, FIT results were discordant in 39.2% of participants. The Hospital Clinic de Barcelona 2015 study (confidential information has been removed). There is currently insufficient information about intra-individual variation in faecal Hb concentration over time to determine the clinical utility of multiple sampling.
The scope of this assessment did not include evaluation of the performance characteristics of faecal immunochemical testing when used in combination with other biomarkers. However, we identified one study142 that did not meet the inclusion criteria for our systematic review because it used an obsolete FIT assay, but that reported data on the performance characteristics of faecal immunochemical testing in combination with faecal calprotectin, M2-PK or both (for which a positive result was defined as at least one test being positive) for the target conditions of CRC and HRA, as well as data on the performance characteristics of faecal immunochemical testing alone. Faecal calprotectin is an inflammatory marker, whereas M2-PK is a key enzyme in tumour metabolism. 142 This study142 found that, in all cases, the addition of at least one further test to faecal immunochemical testing resulted in markedly increased sensitivity and decreased specificity. The sensitivity and specificity estimates for faecal immunochemical testing alone and CRC were 61.7% (95% CI 47.4% to 74.2%) and 88.8% (95% CI 84.1% to 92.3%), respectively; for the combination of faecal immunochemical testing and faecal calprotectin these estimates were 90.9% (95% CI 78.8% to 96.4%) and 35.9% (95% CI 29.7% to 42.6%), respectively; for faecal immunochemical testing and M2-PK, sensitivity and specificity were 91.5% (95% CI 80.1% to 96.6%) and 57.1% (95% CI 50.6% to 63.2%), respectively, and, for all of the three markers, they were 95.7% (85.7% to 98.8%) and 24.1% (18.8% to 30.2%), respectively. 142 Although all of the sensitivity estimates were generally lower, this pattern was repeated when the target condition was advanced neoplasia. 142 However, the FIT threshold in this study (20 µg Hb/g faeces) was higher than that which the results of our systematic review indicate is likely to be the optimal threshold (10 µg Hb/g faeces or a lower threshold). A second study143 of accuracy for the target condition of advanced neoplasia, which did not meet the inclusion criteria for this assessment because it used a qualitative FIT method, also found that combining faecal calprotectin with faecal immunochemical testing (where a positive result was defined as either or both tests positive) resulted in increased sensitivity and decreased specificity [92% (95% CI 82% to 97%) and 49% (95% CI 43% to 54%)] compared with faecal immunochemical testing alone [74% (95% CI 62% to 83%) and 82% (95% CI 78% to 86%)]. 143 The effectiveness of combining other biomarkers with quantitative faecal immunochemical testing (at the threshold at which faecal immunochemical testing is likely to be used in practice) remains unclear. We did not identify any data about the effects of adding faecal calprotectin (or any other biomarker) to risk scores that include faecal immunochemical testing. A study provided ahead of publication (e-mail from Karel Moons, University Medical Center, Utrecht, the Netherlands, to Marie Westwood, KSR Ltd, 20 June 2016, personal communication) reported the development of a multivariable diagnostic model for significant colorectal disease (defined as CRC, adenoma of > 10 mm, IBD or diverticulitis). This model included age, symptoms, digital rectal examination, and point-of-care FITs and faecal calprotectin tests. The authors concluded that this model may avoid approximately 30% of colonoscopy referrals from primary care at the cost of delaying around 6% of diagnoses (mostly HRA or diverticulitis); whether faecal calprotectin adds value, over and above point-of-care FIT was uncertain. Given the trade-off between ease of use/simplicity and diagnostic performance, the clinical value of including additional variables (e.g. symptoms and further diagnostic tests) in risk scores for CRC and/or other significant bowel disease is likely to require further investigation.
There is uncertainty about downstream consequences of using faecal immunochemical testing to triage symptomatic patients in primary care. It can be seen from the findings of our systematic review (see, in Chapter 3, the sections relating to the diagnostic performance of the OC-Sensor, HM-JACKarc, FOB Gold and RIDASCREEN FIT assays) that triage using faecal immunochemical testing at thresholds around 10 µg Hb/g faeces has the potential to correctly rule out CRC and avoid colonoscopy in approximately 75% of symptomatic patients and that this estimate does not appear to vary greatly between FIT assays. Further, the relatively high proportion of FIT FPs observed when the target condition is CRC may be mitigated by the detection of other bowel pathologies in these patients; we estimate that between 22.5% and 93% of patients with a positive FIT and no CRC will have other significant bowel pathologies, depending largely on how many, and which, diagnoses are included in the target condition.
The full potential benefits of faecal immunochemical testing in symptomatic patients, including those relating to diagnoses other than CRC, remain unclear. This issue may be particularly important in younger patients, in whom the prevalence of CRC is lowest and other diagnoses are more likely. Our systematic review identified a recently published protocol for a cluster randomised trial (NCT02308384) of a clinical education intervention (provision of a starting package of faecal immunochemical testing with clinical instructions, including guidance on interpretation of results) in general practices in Denmark, which may partially address these issues. 144 The stated aim of this trial is to assess the diagnostic value and clinical implications of using faecal immunochemical testing, in general practice, for patients presenting with non-alarm symptoms of CRC (‘low risk, but not no risk’). This population is defined as patients aged ≥ 30 years with symptoms and signs of CRC, but who do not meet the Danish Cancer Patient Pathway referral criteria; typical indications are given as change in bowel habit, abdominal pain, anaemia or decrease in Hb of > 10%, non-specific symptoms (weight loss, fatigue, loss of appetite). The specified outcome measures for clinical impact are age- and sex-standardised number and rate of urgent referrals for CRC; age- and sex-standardised number and rate of colonoscopies; all colonoscopy findings by ICD-10 code; age- and sex-standardised number of CRCs diagnosed; stage distribution of CRC diagnosed (I–IV). This trial has the potential to inform the clinical effectiveness component of this assessment, but will not address all potentially relevant applications of faecal immunochemical testing, as it is designed to assess faecal immunochemical testing as a rule-in test, with all patients who have a positive result (≥ 50 µg/l Hb, equivalent to 10 µg Hb/g faeces using OC-Sensor) receiving urgent colonoscopy referral; interpretation guidance in the protocol states that a negative FIT should not exclude CRC.
Finally, the acceptability of faecal immunochemical testing, as indicated by reported sample return rates, varied widely between the studies included in our systematic review. There is evidence that faecal immunochemical testing is more acceptable, when used as a screening test/method, than gFOBT; the use of faecal immunochemical testing in screening programmes results in increased uptake compared with gFOBT. 145 However, these data are unlikely to be transferable to symptomatic patients, for whom factors such as the setting in which the test is requested, and advice and information received directly from clinicians, will affect uptake rates. It therefore remains unclear whether or not the method of assessing faecal occult blood has any effect on uptake in patients who are symptomatic.
Cost-effectiveness
The main uncertainties described for the review of clinical effectiveness also apply to the assessment of cost-effectiveness. These are caused by a lack of clinical effectiveness data for the performance characteristics of faecal immunochemical testing in specific patient subgroups and for the effects of using multiple faecal samples. Once these uncertainties are resolved, the cost-effectiveness of faecal immunochemical testing could be assessed for different subgroups of patients (e.g. elderly) and faecal immunochemical testing using multiple faecal samples per patient could be included as an intervention in our cost-effectiveness model.
Chapter 6 Conclusions
Implications for service provision
There is evidence to suggest that triage using faecal immunochemical testing, when used at a threshold of 10 µg Hb/g faeces for OC-Sensor or HM-JACKarc, may be sufficient to rule out CRC in symptomatic patients. In addition, the relatively high proportion of FIT FPs observed when the target condition is CRC may be mitigated by the potential to diagnose other bowel pathologies in these patients. There was insufficient evidence to adequately assess the diagnostic performance of FOB Gold in symptomatic patients. No evidence about the diagnostic performance of RIDASCREEN Hb or RIDASCREEN Hb/Hp complex in symptomatic patients was identified. No studies were identified that directly compared the performance of different FIT assays, or that compared one or more FIT assays with a gFOBT method; therefore, the relative effectiveness of different methods of measuring faecal occult blood remains uncertain.
The base-case cost-effectiveness results suggested that the difference in QALYs between all of the strategies included in this assessment is minimal and that the ‘no triage’ strategy (referral to colonoscopy) is the most expensive. Overall, faecal immunochemical testing appeared to be cost-effective when compared with no triage or gFOBT. The results of the different scenario analyses did not differ substantively from the base-case results. However, the scenarios for which the accuracy estimates for gFOBT were based on studies that were considered to be more representative of the population of this diagnostic assessment were more favourable than the base-case scenario with regard to faecal immunochemical testing. The results of our analysis suggest that faecal immunochemical testing could provide a cost-effective (cost-saving) triage option for patients whose symptoms are not considered high risk for CRC.
Suggested research priorities
Further, large diagnostic cohort studies are needed to fully evaluate the performance of quantitative faecal immunochemical testing in the setting (primary care) and population (symptomatic patients who are at low risk of CRC, as defined in NG121) specified in the scope for this assessment. Studies comparing the performance of different FIT assays are also needed; such studies should be direct comparisons, involving patients providing multiple samples from a single bowel movement.
If adoption of the FOB Gold, RIDASCREEN Hb or RIDASCREEN Hb/Hp complex is to be considered, studies are needed to evaluate the diagnostic accuracy of these tests in symptomatic patients and to determine optimal thresholds.
If FIT is introduced into routine practice in primary care, a post-implementation audit would be valuable. For example, this could be used to investigate the proportion of FIT-negative patients who go on to have a colonoscopy as well as the delay to getting the colonoscopy. This would improve cost-effectiveness modelling, given that these two parameters had to be estimated using expert opinion. In addition, patients could be followed up to measure mortality and progression, particularly as, as in NG12,6 the cost-effectiveness analysis relied on data on progression from those who were undiagnosed.
Studies comparing the effectiveness of different approaches to safety netting in patients who have negative FIT results may be useful in optimising care pathways.
Multivariable prediction modelling studies may be useful to assess the independent predictive value of a positive FIT result, in the context of other clinical risk factors and tests. This approach may be considered to be the most efficient method of capturing the effects of clinical judgement. Prediction modelling studies should consider the trade-off between the potential for improved model performance and ease of use (the extent to which the components of any risk score developed are readily available to GPs).
Studies that are designed to capture the full potential benefits of faecal immunochemical testing in symptomatic patients, including those relating to diagnoses other than CRC, are likely to be informative. This issue may be particularly important in younger patients, among whom the prevalence of CRC is lowest and other diagnoses are more likely. An example of such a study might be a cluster RCT, in which primary care practices are randomised to use FIT triage in the relevant patient group or refer all patients in the relevant symptomatic group to secondary care; outcomes could include urgent referral rates, colonoscopy rates and all diagnoses at colonoscopy.
Further diagnostic cohort studies, or subgroup analyses of existing data sets, are needed to fully explore the possible variation in the accuracy of FIT assays and optimal thresholds in relevant subgroups, for example sex and age.
Studies exploring the diagnostic accuracy of faecal immunochemical testing using multiple samples and faecal immunochemical testing in combination with other biomarkers (e.g. faecal calprotectin or M2-PK), sequential testing using faecal immunochemical testing and other biomarkers, as well as comparisons between faecal immunochemical testing and other biomarkers, may also be of value.
Acknowledgements
The authors would like to thank Professor Callum Fraser, Honorary Consultant Clinical Biochemist, NHS Tayside, and Honorary Professor, Centre for Research into Cancer Prevention and Screening, for his ongoing expert advice and support, and for his assistance in obtaining access to unpublished data. The authors also acknowledge the clinical advice and expert opinion provided by specialist members of the assessment subgroup: Miss Farhat Din, Senior Lecturer and Honorary Consultant Surgeon, University of Edinburgh; Dr Ian Godber, Consultant Clinical Scientist, NHS Lanarkshire; Mrs Judith Strachan, Consultant Clinical Scientist, NHS Tayside; Dr Paul O’Toole, Consultant Gastroenterologist, Royal Liverpool University Hospital; Dr Robert Logan, Consultant Gastroenterologist, King’s College Hospital NHS Foundation Trust; and Dr Sophie Nelson, General Practitioner, Kenmore Medical Centre, Wilmslow.
We are grateful to the following researchers for providing unpublished data to support this assessment: Dr Sietze van Turenhout, VU University Medical Center, Amsterdam; Dr Joaquín Cubiella, Department of Gastroenterology, Complexo Hospitalario Universitario de Ourense; and Dr Ian Godber, Consultant Clinical Scientist, NHS Lanarkshire.
We would also like to thank the lay members of the NICE Diagnostics Advisory Committee and Assessment Sub-group for providing input on the patients’ perspective at key stages of the assessment process.
Contributions of authors
Marie Westwood planned and performed the systematic review and interpretation of evidence.
Isaac Corro Ramos planned and performed the cost-effectiveness analyses and interpreted results.
Shona Lang planned and performed the systematic review and interpretation of evidence.
Marianne Luyendijk planned and performed the cost-effectiveness analyses and interpreted results.
Remziye Zaim planned and performed the cost-effectiveness analyses and interpreted results.
Lisa Stirk devised and performed the literature searches and provided information support to the project.
Maiwenn Al provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
Nigel Armstrong contributed to planning and interpretation of the systematic review and cost-effectiveness analyses, and the acquisition of input data, and conducted the model peer review.
Jos Kleijnen provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Data sharing statement
This is a systematic review and, therefore, all extracted data are included in the report. Further information can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Suspected Cancer: Recognition and Referral. London: NCC-C; 2015.
- Cancer Registration Statistics, England, 2013. London: ONS; 2015.
- Cancer Survival in England: Adults Diagnosed in 2009 to 2013, followed up to 2014. London: ONS; 2015.
- Moss S, Mathews C, Day TJ, Smith S, Seaman HE, Snowball J, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England [published online ahead of print June 7 2016]. Gut 2016. http://dx.doi.org/10.1136/gutjnl-2015-310691.
- Halloran SP, Launoy G, Zappa M. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition: Faecal occult blood testing. Endoscopy 2012;44:65-87.
- Suspected Cancer: Recognition and Management of Suspected Cancer in Children, Young People and Adults. London: NCC-C; 2015.
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013;185:E537-44. http://dx.doi.org/10.1503/cmaj.121286.
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014;160. http://dx.doi.org/10.7326/M13-1484.
- National Bowel Cancer Audit Report 2015. Leeds: HSCIC; 2015.
- Bowel Cancer UK. Improving Capacity, Saving Lives: Endoscopy in the UK. London: Bowel Cancer UK; 2012.
- Rutter CM, Johnson E, Miglioretti DL, Mandelson MT, Inadomi J, Buist DS. Adverse events after screening and follow-up colonoscopy. Cancer Causes Control 2012;23:289-96. http://dx.doi.org/10.1007/s10552-011-9878-5.
- Vega P, Valentín F, Cubiella J. Colorectal cancer diagnosis: pitfalls and opportunities. World J Gastrointest Oncol 2015;7:422-33. http://dx.doi.org/10.4251/wjgo.v7.i12.422.
- McDonald PJ, Digby J, Innes C, Strachan JA, Carey FA, Steele RJ, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis 2013;15:e151-9. http://dx.doi.org/10.1111/codi.12087.
- Beg M, Singh M, Saraswat MK, Rewari BB. Occult gastro-intestinal bleeding: detection, interpretation, and evaluation. JIACM 2002;3:153-8.
- Rockey DC. Occult gastrointestinal bleeding. N Engl J Med 1999;341:38-46. http://dx.doi.org/10.1056/NEJM199907013410107.
- Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut 2015;64:1327-37. http://dx.doi.org/10.1136/gutjnl-2014-308074.
- Fraser CG, Allison JE, Halloran SP, Young GP. Expert Working Group on Fecal Immunochemical Tests for Hemoglobin, Colorectal Cancer Screening Committee, World Endoscopy Organization. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst 2012;104:810-14. http://dx.doi.org/10.1093/jnci/djs190.
- Evaluation of Quantitative Faecal Immunochemical Tests for Haemoglobin. Guildford: Guildford Medical Device Evaluation Centre; 2014.
- OC-SENSOR PLEDIA. FIT (iFOBT) Automation. Toyko: Eiken Chemical Co Ltd; 2016.
- OC-SENSOR IO. Toyko: Eiken Chemical Co Ltd; 2010.
- Diagnostics for Digestive Health Management. Eastleigh: Alpha Laboratories Ltd; 2015.
- FOB Gold®: The Universal System for Fecal Occult Blood Testing (FIT). Milan: Sentinel Diagnostics; n.d.
- R-Biopharm AG. RIDASCREEN® Haemo-Haptoglobin Complex. Darmstadt: R-Biopharm AG; 2015.
- Launois R, Le Moine JG, Uzzan B, Fiestas Navarrete LI, Benamouzig R. Systematic review and bivariate/HSROC random-effect meta-analysis of immunochemical and guaiac-based fecal occult blood tests for colorectal cancer screening. Eur J Gastroenterol Hepatol 2014;26:978-89. http://dx.doi.org/10.1097/MEG.0000000000000160.
- Basu A, Smartt P. Comparison of Diagnostic Accuracy between Immunochemical and Guaiac Based Faecal Occult Blood Tests for Colorectal Cancer Detection: A Systematic Review of the Literature. Christchurch: Health Services Assessment Collaboration (HSAC); 2009.
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155-9. http://dx.doi.org/10.1056/NEJM199601183340304.
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013;49:3049-54.
- Parra-Blanco A, Gimeno-García AZ, Quintero E, Nicolás D, Moreno SG, Jiménez A, et al. Diagnostic accuracy of immunochemical versus guaiac faecal occult blood tests for colorectal cancer screening. J Gastroenterol 2010;45:703-12. http://dx.doi.org/10.1007/s00535-010-0214-8.
- Vasilyev S, Smirnova E, Popov D, Semenov A, Eklund C, Hendolin P, et al. A new-generation fecal immunochemical test (FIT) is superior to Quaiac-based test in detecting colorectal neoplasia among colonoscopy referral patients. Anticancer Res 2015;35:2873-80.
- Hassan C, Giorgi Rossi P, Camilloni L, Rex DK, Jimenez-Cendales B, Ferroni E, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012;36:929-40. http://dx.doi.org/10.1111/apt.12071.
- Cancer Research UK . Major Increase in Bowel Cancer Screening Uptake Shown With New Screening Test 2015. www.cancerresearchuk.org/about-us/cancer-news/press-release/2015-03-27-major-increase-in-bowel-cancer-screening-uptake-shown-with-new-screening-test-0 (accessed 8 February 2016).
- Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, et al. Advances in Fecal Occult Blood Tests: the FIT revolution. Dig Dis Sci 2015;60:609-22. http://dx.doi.org/10.1007/s10620-014-3445-3.
- Colorectal Cancer: Diagnosis and Management. London: NICE; 2011.
- Diagnosis and Management of Colorectal Cancer: A National Clinical Guideline. Edinburgh: SIGN; 2011.
- iRefer: Making the Best Use of Clinical Radiology. London: The Royal College of Radiologists; 2012.
- Guidelines for the Management of Colorectal Cancer. London: The Association of Coloproctology of Great Britain and Ireland; 2007.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Diagnostics Assessment Programme Manual. Manchester: NICE; 2011.
- Cochrane Diagnostic Test Accuracy Working Group . Handbook for DTA Reviews 2009. http://srdta.cochrane.org/handbook-dta-reviews (accessed 23 March 2011).
- CADTH Peer Review Checklist for Search Strategies. Ottawa, ON: CADTH; 2013.
- Wright K, McDaid C. Is the Retraction of Journal Articles in Electronic Journals and Databases Consistent and Timely? A Case Study. Madrid: Cochrane Collaboration; 2011.
- Wright K, McDaid C. Reporting of article retractions in bibliographic databases and online journals. J Med Libr Assoc 2011;99:164-7. http://dx.doi.org/10.3163/1536-5050.99.2.010.
- Royle P, Waugh N. Should systematic reviews include searches for published errata?. Health Info Libr J 2004;21:14-20. http://dx.doi.org/10.1111/j.1471-1842.2004.00459.x.
- Waffenschmidt S. Assessing the Completeness of Systematic Reviews via the ‘Related Articles’ Function and/or a Simple Structured Boolean Search in PubMed – A Pilot Study (B202). Madrid: Cochrane Collaboration; 2011.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Wolff R, Whiting P, Mallett S, Riley R, Westwood M, Kleijnen J, et al. PROBAST: Prediction Model Risk of Bias Assessment Tool Evidence Synthesis Network n.d.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, et al. An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 2008;61:1095-103. http://dx.doi.org/10.1016/j.jclinepi.2007.09.013.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. https://doi.org/10.1093/biostatistics/kxl004.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Thompson JR. Bivariate random-effects meta-analysis and the estimation of between-study correlation. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-3.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-31.
- Mowat C, Digby J, Strachan JA, Wilson R, Carey FA, Fraser CG, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut 2016;65:1463-9. https://doi.org/10.1136/gutjnl-2015-309579.
- Rodríguez-Alonso L, Rodríguez-Moranta F, Ruiz-Cerulla A, Lobatón T, Arajol C, Binefa G, et al. An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis 2015;47:797-804. http://dx.doi.org/10.1016/j.dld.2015.05.004.
- Krivec S, Konda G, Sibli R, Marc J. Assessment of the diagnostic applicability of quantitative immunochemical faecal occult blood tests. Clin Chem Lab Med 2011;49.
- Cubiella J, Salve M, Díaz-Ondina M, Vega P, Alves MT, Iglesias F, et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis 2014;16:O273-82. http://dx.doi.org/10.1111/codi.12569.
- Godber IM, Todd LM, Fraser CG, MacDonald LR, Younes HB. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med 2016;54:595-602. http://dx.doi.org/10.1515/cclm-2015-0617.
- Auge JM, Fraser CG, Rodriguez C, Roset A, Lopez-Ceron M, Grau J, et al. Clinical utility of one versus two faecal immunochemical test samples in the detection of advanced colorectal neoplasia in symptomatic patients. Clin Chem Lab Med 2016;54:125-32. http://dx.doi.org/10.1515/cclm-2015-0388.
- Terhaar sive Droste JS, Oort FA, van der Hulst RW, van Heukelem HA, Loffeld RJ, van Turenhout ST, et al. Higher fecal immunochemical test cutoff levels: lower positivity rates but still acceptable detection rates for early-stage colorectal cancers. Cancer Epidemiol Biomarkers Prev 2011;20:272-80. http://dx.doi.org/10.1158/1055-9965.EPI-10-0848.
- van Turenhout ST, Oort FA, van der Hulst RW, Visscher AP, Terhaar sive Droste JS, Scholten P, et al. Prospective cross-sectional study on faecal immunochemical tests: sex specific cut-off values to obtain equal sensitivity for colorectal cancer?. BMC Gastroenterol 2014;14. http://dx.doi.org/10.1186/s12876-014-0217-7.
- van Turenhout ST, van Rossum LG, Oort FA, Laheij RJ, van Rijn AF, Terhaar sive Droste JS, et al. Similar fecal immunochemical test results in screening and referral colorectal cancer. World J Gastroenterol 2012;18:5397-403. http://dx.doi.org/10.3748/wjg.v18.i38.5397.
- Diaz Ondina M, Blanco Vila MI, Ceballos Ogando S, Salve Bouzo M, Macia Cortinas P, Cubiella Fernandez J. Clinical or analytical criteria for colorectal cancer (CRC) detection in symptomatic patients? A diagnostic tests study. Clin Chem Lab Med 2014;52.
- van Turenhout ST, Oort FA, Terhaar sive Droste JS, Coupé VM, van der Hulst RW, Loffeld RJ, et al. Hemorrhoids detected at colonoscopy: an infrequent cause of false-positive fecal immunochemical test results. Gastrointest Endosc 2012;76:136-43. http://dx.doi.org/10.1016/j.gie.2012.03.169.
- Oort FA, van Turenhout ST, Coupe VM, van der Hulst RW, Wesdorp EI, Terhaar sive Droste JS, et al. Double sampling of a faecal immunochemical test is not superior to single sampling for detection of colorectal neoplasia: a colonoscopy controlled prospective cohort study. BMC Cancer 2011;11. https://doi.org/10.1186/1471-2407-11-434.
- Oort FA, Terhaar sive Droste JS, van Der Hulst RW, Van Heukelem HA, Loffeld RJ, Wesdorp IC, et al. Colonoscopy-controlled intra-individual comparisons to screen relevant neoplasia: faecal immunochemical test vs. guaiac-based faecal occult blood test. Aliment Pharmacol Ther 2010;31:432-9. http://dx.doi.org/10.1111/j.1365-2036.2009.04184.x.
- Godber IM, Todd LM, Fraser CG, Robertson C, Smith L, McDonald L, et al. Can an automated faecal immunochemical test (FIT) determine whether faecal haemoglobin (f-Hb) concentrations can aid in stratifying symptomatic patients referred for colonoscopy. Clin Chem Lab Med 2014;52.
- Auge Fradera JM, Roset A, Escudero JM, Foj L, Filella X, Molina R. Clinical utility of HM-JACKarc for the detection of colorectal cancer and high-risk adenomas. Tumor Biol 2014;35.
- Larbi IB, van Turenhout ST, Oort FA, sive Droste JST, van Der Hulst RW, Scholten P, et al. FIT in the elderly: performance of a frequently used fecal immunochemical test in subjects 75 of age and older. Gastroenterology 2012;142. https://doi.org/10.1016/S0016-5085(12)62996-5.
- van Turenhout ST, Oort FA, Droste JSTS, Visscher AP, Coupe VM, van Der Hulst RW, et al. Gender disparities in performance of a fecal immunochemical test for detection of advanced neoplasia. Gastroenterology 2011;140:405-6.
- van Turenhout ST, Oort FA, Coupe VM, van Der Hulst RW, Wesdorp EC, Larbi IB, et al. Double versus single sampling of fecal immunochemical tests for colorectal cancer screening; added value or added costs?. Gastroenterology 2010;138. https://doi.org/10.1016/S0016-5085(10)60839-6.
- van Turenhout ST, Oort FA, Coupe VM, van Der Hulst RW, Wesdorp EC, Larbi IB, et al. Comparing three different strategies of double sampling by fecal immunochemical tests for detection of advanced colorectal neoplasm’s. Gastroenterology 2010;138. https://doi.org/10.1016/S0016-5085(10)60614-2.
- Macdonald LR, Smith L, Godber IM, Todd LM, Fraser CG, Downey M, et al. Faecal immunochemical testing for haemoglobin in symptomatic patients can help decide need for colonoscopy. Gut 2015;64. https://doi.org/10.1136/gutjnl-2015-309861.98.
- Auge Fradera JM. The Performance of FIT to Triage Symptomatic Patients (Clinical evaluation of ‘HM-JACKarc’ Analyser). Barcelona: Hospital Clinic – Barcelona, Biomedical Diagnostic Center, Biochemistry and Molecular Genetics Department; 2015.
- Cubiella J. Colorectal Cancer Prediction Model in Symptomatic Patients Based on FIT, Age and Sex. Barcelona: WEO Colorectal Cancer Screening Meeting; 2015.
- Steele R, Digby J, Strachan J, Mowat C, Lang J, McDonald P, et al. Quantitative FIT as Triage for Colonoscopy. Vienna, Austria: WEO Colorectal Cancer Screening Meeting; 2014.
- Thomas CL, Tomkins C, Widlak M, Smith S, Arasaradnam R. Can immunochemical tests for faecal haemoglobin and faecal calprotectin be used to risk stratify patients for referral to colonoscopy for suspected colorectal cancer?. Ann Clin Biochem 2016;53:38-9.
- Peacock O, Watts ES, Hanna NM, Kerr K, Goddard AF, Lund JN. Inappropriate use of the faecal occult blood test outside of the NHS colorectal cancer screening programme. Colorectal Dis 2012;14.
- Kaul A, Shah A, Magill FH, Hawkins SA, Skaife P. Immunological faecal occult blood testing: a discriminatory test to identify colorectal cancer in symptomatic patients. West Indian Med J 2012;61.
- Saccomanno S, Castiglione G, Aste H, Pacini F. Validity of the guaiac test (Hemoccult) in the diagnosis of neoplasms of the left colon in symptomatic patients. Minerva Dietol Gastroenterol 1985;31:635-9.
- Takeshita T, Chen PC. Fecal occult blood testing. Concentration of the accuracy on immunological fecal occult blood testing. Ther Res 1988;8:107-12.
- Osone T, Sudo I, Hori T, Sugita K, Taguti Y, Saito Y, et al. Clinical assessment of immunological latex agglutination fecal occult blood testing – 2nd Report. Ther Res 1988;8:201-5.
- Tanabe H, Shiraishi F, Isono T, Ueda O, Toyoda T, Tamura K, et al. Clinical evaluation of immunologic test (Latex agglutination) for fecal occult blood. Ther Res 1988;8:223-7.
- Lin HH, Huang LC, Yau JH, Chao CJ, Lin DY. Immunological test of induced fecal occult blood in colorectal cancer screening among high risk population: a preliminary study. Gastroenterol J Taiwan 1995;12:230-7.
- Winawer SJ, Fleisher M. Sensitivity and specificity of the fecal occult blood test for colorectal neoplasia. Gastroenterology 1982;82:986-91.
- Morini S, Manurita L, Stroppa I, Bassi O. Value of the study of occult fecal blood with Hemoccult II in 211 symptomatic patients controlled by total colonoscopy. Minerva Med 1984;75:963-6.
- Dvorák M, Kocna P, Vanícková Z. Occult fecal blood loss – comparison of immunochemical and biochemical tests. Cas Lek Cesk 2002;141:217-19.
- Van Rossum LG, van Rijn AF, van Munster IP, Jansen JB, Fockens P, Laheij RJ, et al. Earlier stages of colorectal cancer detected with immunochemical faecal occult blood tests. Neth J Med 2009;67:182-6.
- Mĭkhailova EI, Pimanov SI, Voropaev EV. Fecal oncomarkers in the diagnostics of colorectal cancer. Klin Med 2007;85:62-7.
- Fraser CG, Rubeca T, Rapi S, Chen LS, Chen HH. Faecal haemoglobin concentrations vary with sex and age, but data are not transferable across geography for colorectal cancer screening. Clin Chem Lab Med 2014;52:1211-16. http://dx.doi.org/10.1515/cclm-2014-0115.
- Fraser CG, Auge JM. PROCOLON Group . Faecal haemoglobin concentrations do vary across geography as well as with age and sex: ramifications for colorectal cancer screening. Clin Chem Lab Med 2015;53:e235-7. http://dx.doi.org/10.1515/cclm-2014-1172.
- Atkin WS, Saunders BP. British Society for Gastroenterology . Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002;51:6-9. https://doi.org/10.1136/gut.51.suppl_5.v6.
- Gillberg A, Ericsson E, Granstrom F, Olsson LI. A population-based audit of the clinical use of faecal occult blood testing in primary care for colorectal cancer. Colorectal Dis 2012;14:e539-46. http://dx.doi.org/10.1111/j.1463-1318.2012.03149.x.
- Niv Y, Sperber AD. Sensitivity, specificity, and predictive value of fecal occult blood testing (Hemoccult II) for colorectal neoplasia in symptomatic patients: a prospective study with total colonoscopy. Am J Gastroenterol 1995;90:1974-7.
- Bjerregaard NC, Tøttrup A, Sørensen HT, Laurberg S. Detection of colorectal cancer in symptomatic outpatients without visible rectal bleeding: Validity of the fecal occult blood test. Clin Epidemiol 2009;1:119-24. https://doi.org/10.2147/CLEP.S7097.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999;94:1650-7. https://doi.org/10.1111/j.1572-0241.1999.01157.x.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics; 1999.
- National Cancer Intelligence Network (NCIN) . National Cancer Intelligence Network n.d. www.ncin.org.uk/home (accessed 11 July 2016).
- Office for National Statistics (ONS) . National Life Tables, United Kingdom: 2011–2013 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/previousReleases (accessed 11 July 2016).
- Tappenden P, Chilcott J, Eggington S, Patnick J, Sakai H, Karnon J. Option appraisal of population-based colorectal cancer screening programmes in England. Gut 2007;56:677-84. https://doi.org/10.1136/gut.2006.095109.
- Jensen J, Kewenter J, Swedenborg J. The correlation of symptoms, occult blood tests, and neoplasms in patients referred for double-contrast barium enema. Scand J Gastroenterol 1993;28:911-14. https://doi.org/10.3109/00365529309103134.
- Oono Y, Iriguchi Y, Doi Y, Tomino Y, Kishi D, Oda J, et al. A retrospective study of immunochemical fecal occult blood testing for colorectal cancer detection. Clin Chim Acta 2010;411:802-5. http://dx.doi.org/10.1016/j.cca.2010.02.057.
- Thompson MR, Flashman KG, Wooldrage K, Rogers PA, Senapati A, O’Leary DP, et al. Flexible sigmoidoscopy and whole colonic imaging in the diagnosis of cancer in patients with colorectal symptoms. Br J Surg 2008;95:1140-6. https://doi.org/10.1002/bjs.6234.
- Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection – systematic review and meta-analysis. Radiology 2011;259:393-405. http://dx.doi.org/10.1148/radiol.11101887.
- Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1185-93. http://dx.doi.org/10.1016/S0140-6736(12)62124-2.
- Gavin D, Valori R, Anderson J, Donnelly M, Williams JG, Swarbrick E. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2012;61. https://doi.org/10.1136/gutjnl-2012-302514a.7.
- NHS Reference Costs 2012–2013. London: DH; 2013.
- Unit Costs of Health and Social Care 2013. Canterbury: University of Kent; 2013.
- Royal Surrey County Hospital NHS Foundation Trust . Bowel Cancer Screening Southern Programme Hub n.d. www.royalsurrey.nhs.uk/service-list/bowel-cancer-screening/bowel-cancer-screening-southern-programme-hub/ (accessed 18 July 2016).
- Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults. A cost-effectiveness analysis comparing four diagnostic strategies. J Gen Intern Med 2005;20:81-90. https://doi.org/10.1111/j.1525-1497.2005.40077.x.
- Rae AJ, Cleator IGM. The two-tier fecal occult blood test: cost effective screening. Can J Gastroenterol 1994;8:362-8. https://doi.org/10.1155/1994/659527.
- Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis. Br J Cancer 1958;12:309-20. https://doi.org/10.1038/bjc.1958.37.
- Westwood M, Al M, Burgers L, Redekop K, Lhachimi S, Armstrong N, et al. A systematic review and economic evaluation of new-generation computed tomography scanners for imaging in coronary artery disease and congenital heart disease: Somatom Definition Flash, Aquilion ONE, Brilliance iCT and Discovery CT750 HD. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17090.
- Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-colonoscopy complications: a systematic review, time trends, and meta-analysis of population-based studies. Am J Gastroenterol 2016;111:1092-101. http://dx.doi.org/10.1038/ajg.2016.234.
- Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc 2011;74:885-96. http://dx.doi.org/10.1016/j.gie.2011.06.023.
- Manta R, Tremolaterra F, Arezzo A, Verra M, Galloro G, Dioscoridi L, et al. Complications during colonoscopy: prevention, diagnosis, and management. Tech Coloproctol 2015;19:505-13. http://dx.doi.org/10.1007/s10151-015-1344-z.
- Church J. Complications of colonoscopy. Gastroenterol Clin North Am 2013;42:639-57. http://dx.doi.org/10.1016/j.gtc.2013.05.003.
- Saraste D, Martling A, Nilsson PJ, Blom J, Törnberg S, Hultcrantz R, et al. Complications after colonoscopy and surgery in a population-based colorectal cancer screening programme. J Med Screen 2016;23:135-40. http://dx.doi.org/10.1177/0969141315625701.
- Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology 2006;239:464-71. https://doi.org/10.1148/radiol.2392051101.
- NHS Bowel Cancer Screening Southern Programme Hub . Annual Report 2014 2015 2015. www.royalsurrey.nhs.uk/wp-content/uploads/2015/12/BCSP-Southern-Hub-Annual-Report-2014-2015.pdf (accessed 11 July 2016).
- Resources for Coloproctology 2015. London: Association of Coloproctology of Great Britain and Ireland; 2015.
- Grazzini G, Ciatto S, Cislaghi C, Castiglione G, Falcone M, Mantellini P, et al. Working Group of Regional Reference Centre for Oncological Screening of Tuscany . Cost evaluation in a colorectal cancer screening programme by faecal occult blood test in the District of Florence. J Med Screen 2008;15:175-81. http://dx.doi.org/10.1258/jms.2008.008032.
- Sharp L, Tilson L, Whyte S, O’Ceilleachair A, Walsh C, Usher C, et al. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer 2012;106:805-16. http://dx.doi.org/10.1038/bjc.2011.580.
- Whyte S, Chilcott J, Cooper K, Essat M, Stevens J, Wong R, et al. Re-appraisal of the Options for Colorectal Cancer Screening: Full Report. Sheffield: ScHARR; 2011.
- NHS Reference Costs 2014–2015. London: DH; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C. English Bowel Cancer Screening Evaluation Committee . Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. https://doi.org/10.1136/gutjnl-2011-300843.
- Cubiella J, Vega P, Salve M, Diaz-Ondina M, Alves MT, Quintero E, et al. Development and external validation of a faecal immunochemical test-based prediction model for colorectal cancer detection in symptomatic patients. BMC Med 2016;14. http://dx.doi.org/10.1186/s12916-016-0668-5.
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. J Clin Epidemiol 2011;64:602-7. http://dx.doi.org/10.1016/j.jclinepi.2010.07.006.
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882-93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
- Luthgens K, Maier A, Kampert I, Sieg A, Schmidt-Gayk H. Hemoglobin-haptoglobin-complex: a highly sensitive assay for the detection of fecal occult blood. Clin Lab 1998;44:543-51.
- Barrett P, Stump T, Monahan P, Imperiale T. Test characteristics of fecal immunochemical tests for colorectal cancer and advanced adenoma: systematic review and meta-analysis. Am J Gastroenterol 2014;109.
- Jellema P, van der Windt DA, Bruinvels DJ, Mallen CD, van Weyenberg SJ, Mulder CJ, et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c1269.
- Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 2007;146:244-55. https://doi.org/10.7326/0003-4819-146-4-200702200-00003.
- Sieg A, Scheida M, John MR, Hertel A, Schröter M, Lüthgens K, et al. Validity of new immunological human fecal hemoglobin and albumin tests in detecting colorectal neoplasms – an endoscopy-controlled study. Z Gastroenterol 1998;36:485-90.
- Sieg A, Thoms C, Lüthgens K, John MR, Schmidt-Gayk H. Detection of colorectal neoplasms by the highly sensitive hemoglobin-haptoglobin complex in feces. Int J Colorectal Dis 1999;14:267-71. https://doi.org/10.1007/s003840050226.
- Williams TG, Cubiella J, Griffin SJ, Walter FM, Usher-Smith JA. Risk prediction models for colorectal cancer in people with symptoms: a systematic review. BMC Gastroenterol 2016;16. http://dx.doi.org/10.1186/s12876-016-0475-7.
- Goddard A, Nickerson C, Blanks R, Burling D, Patnick J. PWE-072: Current role of radiology as the first investigation in the English bowel cancer screening programme (BCSP). Gut 2012;61:A326-7. https://doi.org/10.1136/gutjnl-2012-302514d.72.
- Kapidzic A, Korfage IJ, van Dam L, van Roon AH, Reijerink JC, Zauber AG, et al. Quality of life in participants of a CRC screening program. Br J Cancer 2012;107:1295-301. http://dx.doi.org/10.1038/bjc.2012.386.
- Meester RG, Zauber AG, Doubeni CA, Jensen CD, Quinn VP, Helfand M, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016;14:1445-51.e8. http://dx.doi.org/10.1016/j.cgh.2016.05.017.
- McDonald PJ, Strachan JA, Digby J, Steele RJ, Fraser CG. Faecal haemoglobin concentrations by gender and age: implications for population-based screening for colorectal cancer. Clin Chem Lab Med 2011;50:935-40. http://dx.doi.org/10.1515/CCLM.2011.815.
- Digby J, McDonald PJ, Strachan JA, Libby G, Steele RJ, Fraser CG. Deprivation and faecal haemoglobin: implications for bowel cancer screening. J Med Screen 2014;21:95-7. http://dx.doi.org/10.1177/0969141314535388.
- Parente F, Marino B, Ilardo A, Fracasso P, Zullo A, Hassan C, et al. A combination of faecal tests for the detection of colon cancer: a new strategy for an appropriate selection of referrals to colonoscopy? A prospective multicentre Italian study. Eur J Gastroenterol Hepatol 2012;24:1145-52. http://dx.doi.org/10.1097/MEG.0b013e328355cc79.
- Kok L, Elias SG, Witteman BJ, Goedhard JG, Muris JW, Moons KG, et al. Diagnostic accuracy of point-of-care fecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: the Cost-Effectiveness of a Decision Rule for Abdominal Complaints in Primary Care (CEDAR) study. Clin Chem 2012;58:989-98. http://dx.doi.org/10.1373/clinchem.2011.177980.
- Juul JS, Bro F, Hornung N, Andersen BS, Laurberg S, Olesen F, et al. Implementation of immunochemical faecal occult blood test in general practice: a study protocol using a cluster-randomised stepped-wedge design. BMC Cancer 2016;16. http://dx.doi.org/10.1186/s12885-016-2477-9.
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012;55:87-92. http://dx.doi.org/10.1016/j.ypmed.2012.05.006.
- Search Strategies: NHS EED EMBASE using OvidSP (Economics Filter). York: CRD; 2014.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- Papaioannou D, Brazier JE, Paisley S. Figure 4: Common Free-text Terms for Electronic Database Searching for HSUVs. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature 2011. www.nicedsu.org.uk (accessed 18 August 2011).
Appendix 1 Literature search strategies
Clinical effectiveness searches
MEDLINE (via Ovid): 1946 to March Week 3 2016
Date searched: 30 March 2016.
Records found: 3198.
-
((immunochem$ or immuno-chem$ or immunohistochem$ or immuno-histochem$ or immunol$ or immunochromatographic or immuno-chromatographic or immunoassay or immuno assay) adj4 (f?ecal or f?eces or stool or stools)).ti,ab,ot,hw. (910)
-
iFOBT.ti,ab,ot,hw. (83)
-
1 or 2 (932)
-
F?ecal h?emoglobin.ti,ab,ot,hw. (94)
-
H?emoccult.ti,ab,ot,hw. (669)
-
FOBT.ti,ab,ot,hw. (1013)
-
(guaiac$ or gFOBT).ti,ab,ot,hw. (3444)
-
Guaiac/ (319)
-
or/4-8 (4898)
-
(f?ecal or f?eces or stool or stools).ti,ab,ot,hw. (246,345)
-
occult blood/ or occult blood.ti,ab,ot,hw. (6307)
-
(test$ or measur$ or screen$ or exam$).ti,ab,ot,hw. (6,577,832)
-
10 and 11 and 12 (3172)
-
3 or 9 or 13 (7254)
-
exp colorectal neoplasms/ (162,552)
-
exp cecal neoplasms/ (4892)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (199,488)
-
CRC.ti,ab,ot. (13,848)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1846)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1675)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (26)
-
15 or 16 or 17 or 18 or 19 or 20 or 21 (208,172)
-
14 and 22 (3206)
-
(FOB gold$ or FOBgold$).ti,ab. (11)
-
(jack-arc$ or jackarc$ or HM-JACKarc$).ti,ab. (0)
-
(RIDASCREEN$ H?emo$ or RIDASCREEN$ Hapto$).ti,ab. (1)
-
(OC Sensor$ or OC-Sensor$ or OC Pledia$ or OC-Pledia$).ti,ab. (38)
-
or/24-27 (43)
-
23 or 28 (3208)
-
exp animals/ not (exp animals/ and humans/) (4,205,567)
-
29 not 30 (3198)
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via Ovid): March 29, 2016
Date searched: 30 March 2016.
Records found: 255.
-
((immunochem$ or immuno-chem$ or immunohistochem$ or immuno-histochem$ or immunol$ or immunochromatographic or immuno-chromatographic or immunoassay or immuno assay) adj4 (f?ecal or f?eces or stool or stools)).ti,ab,ot,hw. (134)
-
iFOBT.ti,ab,ot,hw. (9)
-
1 or 2 (138)
-
F?ecal h?emoglobin.ti,ab,ot,hw. (16)
-
H?emoccult.ti,ab,ot,hw. (23)
-
FOBT.ti,ab,ot,hw. (72)
-
(guaiac$ or gFOBT).ti,ab,ot,hw. (321)
-
Guaiac/ (0)
-
or/4-8 (418)
-
(f?ecal or f?eces or stool or stools).ti,ab,ot,hw. (18,710)
-
occult blood/ or occult blood.ti,ab,ot,hw. (302)
-
(test$ or measur$ or screen$ or exam$).ti,ab,ot,hw. (671,105)
-
10 and 11 and 12 (202)
-
3 or 9 or 13 (632)
-
exp colorectal neoplasms/ (220)
-
exp cecal neoplasms/ (1)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (14,744)
-
CRC.ti,ab,ot. (2522)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (197)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (40)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (0)
-
15 or 16 or 17 or 18 or 19 or 20 or 21 (15,079)
-
14 and 22 (254)
-
(FOB gold$ or FOBgold$).ti,ab. (2)
-
(jack-arc$ or jackarc$ or HM-JACKarc$).ti,ab. (3)
-
(RIDASCREEN$ H?emo$ or RIDASCREEN$ Hapto$).ti,ab. (0)
-
(OC Sensor$ or OC-Sensor$ or OC Pledia$ or OC-Pledia$).ti,ab. (3)
-
or/24-27 (6)
-
23 or 28 (255)
-
exp animals/ not (exp animals/ and humans/) (3648)
-
29 not 30 (255)
MEDLINE Epub Ahead of Print (via Ovid): June 20, 2016
Date searched: 21 June 2016.
Records found: 80.
-
((immunochem$ or immuno-chem$ or immunohistochem$ or immuno-histochem$ or immunol$ or immunochromatographic or immuno-chromatographic or immunoassay or immuno assay) adj4 (f?ecal or f?eces or stool or stools)).ti,ab,ot,hw. (36)
-
iFOBT.ti,ab,ot,hw. (3)
-
1 or 2 (37)
-
F?ecal h?emoglobin.ti,ab,ot,hw. (4)
-
H?emoccult.ti,ab,ot,hw. (1)
-
FOBT.ti,ab,ot,hw. (24)
-
(guaiac$ or gFOBT).ti,ab,ot,hw. (77)
-
Guaiac/ (0)
-
or/4-8 (102)
-
(f?ecal or f?eces or stool or stools).ti,ab,ot,hw. (1652)
-
occult blood/ or occult blood.ti,ab,ot,hw. (73)
-
(test$ or measur$ or screen$ or exam$).ti,ab,ot,hw. (131,690)
-
10 and 11 and 12 (65)
-
3 or 9 or 13 (156)
-
15 exp colorectal neoplasms/ (0)
-
exp cecal neoplasms/ (0)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (3630)
-
CRC.ti,ab,ot. (799)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (16)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (12)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (0)
-
15 or 16 or 17 or 18 or 19 or 20 or 21 (3677)
-
14 and 22 (80)
-
(FOB gold$ or FOBgold$).ti,ab. (1)
-
(jack-arc$ or jackarc$ or HM-JACKarc$).ti,ab. (0)
-
(RIDASCREEN$ H?emo$ or RIDASCREEN$ Hapto$).ti,ab. (0)
-
(OC Sensor$ or OC-Sensor$ or OC Pledia$ or OC-Pledia$).ti,ab. (5)
-
or/24-27 (5)
-
23 or 28 (80)
-
exp animals/ not (exp animals/ and humans/) (0)
-
29 not 30 (80)
EMBASE (via Ovid): 1974 to 2016 March 29
Date searched: 30 March 2016.
Records found: 5255.
-
Fecal Immunochemical Test/ [EMTREE candidate term 13.1.16] (135)
-
((immunochem$ or immuno-chem$ or immunohistochem$ or immuno-histochem$ or immunol$ or immunochromatographic or immuno-chromatographic or immunoassay or immuno assay) adj4 (f?ecal or f?eces or stool or stools)).ti,ab,ot,hw. (1630)
-
iFOBT.ti,ab,ot,hw. (164)
-
1 or 2 or 3 (1672)
-
F?ecal h?emoglobin.ti,ab,ot,hw. (155)
-
H?emoccult.ti,ab,ot,hw. (866)
-
FOBT.ti,ab,ot,hw. (1857)
-
(guaiac$ or gFOBT).ti,ab,ot,hw. (5159)
-
Guaiac/ (696)
-
or/5-9 (7538)
-
(f?ecal or f?eces or stool or stools).ti,ab,ot,hw. (347,542)
-
occult blood/ or occult blood.ti,ab,ot,hw. (11,184)
-
(test$ or measur$ or screen$ or exam$).ti,ab,ot,hw. (9,112,793)
-
11 and 12 and 13 (5121)
-
4 or 10 or 14 (11,582)
-
exp colon tumor/ (239,813)
-
exp rectum tumor/ (182,401)
-
exp colon cancer/ (191,557)
-
exp rectum cancer/ (148,462)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (318,031)
-
CRC.ti,ab,ot. (27,205)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (2649)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1929)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (29)
-
or/16-24 (329,839)
-
15 and 25 (5264)
-
(FOB gold$ or FOBgold$).ti,ab. (27)
-
(jack-arc$ or jackarc$ or HM-JACKarc$).ti,ab. (7)
-
(RIDASCREEN$ H?emo$ or RIDASCREEN$ Hapto$).ti,ab. (2)
-
(OC Sensor$ or OC-Sensor$ or OC Pledia$ or OC-Pledia$).ti,ab. (151)
-
or/27-30 (169)
-
26 or 31 (5274)
-
animal/ (1,733,374)
-
animal experiment/ (1,919,080)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,178,883)
-
or/33-35 (6,178,883)
-
exp human/ (16,972,709)
-
human experiment/ (350,478)
-
or/37-38 (16,974,155)
-
36 not (36 and 39) (4,862,933)
-
32 not 40 (5255)
The Cochrane Library (Wiley) (via the internet)
Date searched: 30 March 2016.
Records found:
-
CDSR: Issue 3 of 12, March 2016: 25 records
-
DARE: Issue 2 of 4, April 2015: 34 records
-
HTA Database: Issue 1 of 4, January 2016: 27 records
-
NHS EED: Issue 2 of 4, April 2015: 103 records
-
CENTRAL: Issue 2 of 12, February 2016: 446 records.
#1 (immunochem* or “immuno-chem*” or immunohistochem* or “immuno-histochem*” or immunol*) near/4 (f*ecal or f*eces) (237)
#2 iFOBT (14)
#3 “F*ecal h*emoglobin” (15)
#4 H*emoccult (143)
#5 FOBT (247)
#6 gFOBT or guaiac (132)
#7 [mh ^guaiac] (30)
#8 f*ecal or f*eces or stool or stools (9516)
#9 MeSH descriptor: [Occult Blood] this term only (480)
#10 “occult blood” (907)
#11 #9 or #10 (907)
#12 test* or measur* or screen* or exam* (430,115)
#13 #8 and #11 and #12 (683)
#14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #13 (931)
#15 MeSH descriptor: [Colorectal Neoplasms] explode all trees (6053)
#16 MeSH descriptor: [Cecal Neoplasms] explode all trees (9)
#17 (colorec* or rectal* or rectum* or colon* or sigma* or sigmo* or rectosigm* or bowel* or anal or anus) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumo*r* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (12,274)
#18 CRC (1334)
#19 (cecum or cecal or caecum or caecal or il*eoc*ecal or il*eoc*ecum) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumo*r* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (99)
#20 “large intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumo*r* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (54)
#21 “lower intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumo*r* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
#22 #15 or #16 or #17 or #18 or #19 or #20 or #21 (12627)
#23 #14 and #22 (638)
#24 “FOB gold*” or “FOBgold*” (2)
#25 “jack-arc*” or “jackarc*” or “HM-JACKarc*” (0)
#26 “RIDASCREEN* Haemo*” or “RIDASCREEN* Hemo*” or “RIDASCREEN* Hapto*” (0)
#27 “OC Sensor*” or “OC-Sensor*” or “OC Pledia*” or “OC-Pledia*” (16)
#28 #24 or #25 or #26 or #27 (16)
#29 #23 or #28 (638) (CDSR: 25; DARE: 34; HTA: 27; NHS EED: 103; CENTRAL: 446)
International Network of Agencies for Health Technology Assessment Publications
Date searched: 30 March 2016.
Records found: 8.
| Search terms | Records found |
|---|---|
| Faeces | 0 |
| Feces | 0/2 |
| Faecal | 1/2 |
| Fecal | 7/20 |
| Guaiac | 4/4 |
| Total (before deduplication) | 12 |
| Total (after deduplication) | 8 |
National Institute for Health Research Health Technology Assessment programme
www.nets.nihr.ac.uk/projects?collection=netscc&meta_P_sand=Project
Date searched: 30 March 2016.
Records found: 1.
| Search terms | Records found |
|---|---|
| Faeces | 0/3 |
| Feces | 0 |
| Faecal | 1/13 |
| Fecal | 0/3 |
| Guaiac | 0 |
| Total (before deduplication) | 1 |
| Total (after deduplication) | 1 |
Aggressive Research Intelligence Facility Reviews Database
www.birmingham.ac.uk/research/activity/mds/projects/HaPS/PHEB/ARIF/databases/index.aspx
Date searched: 30 March 2016.
Records found: 17.
| Search terms | Records found |
|---|---|
| Faeces | 0/2 |
| Feces | 0/1 |
| Faecal | 8/23 |
| Fecal | 12/20 |
| Guaiac | 3/3 |
| Total (before deduplication) | 23 |
| Total (after deduplication) | 17 |
PROSPERO (via internet)
Date searched: 30 March 2016.
Records found: 9.
| Search terms | Records found |
|---|---|
| Faeces | 1/9 |
| Feces | 2/14 |
| Faecal | 1/38 |
| Fecal | 8/50 |
| Guaiac | 4/4 |
| Total (before deduplication) | 16 |
| Total (after deduplication) | 9 |
ClinicalTrials.gov (via internet)
Date searched: 8 March 2016.
Records found: 51.
| Search terms | Records found |
|---|---|
| “faecal immunochemical” OR “fecal immunochemical” OR “faecal immuno-chemical” OR “fecal immuno-chemical” OR iFOBT OR “FOB Gold” OR FOBGold OR JACKarc OR RIDASCREEN OR “OC Sensor” OR “OC Pledia” | 51 |
European Union Clinical Trials Register (via internet)
www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 8 March 2016.
Records found: 1.
| Search terms | Records found |
|---|---|
| “faecal immunochemical” OR “fecal immunochemical” OR “faecal immuno-chemical” OR “fecal immuno-chemical” OR iFOBT OR FOBT OR “FOB Gold” OR FOBGold OR JACKarc OR RIDASCREEN OR “OC Sensor” OR “OC Pledia” | 1 |
International Clinical Trials Registry Platform (via internet)
http://apps.who.int/trialsearch/
Date searched: 8 March 2016.
Records found: 29.
| Search terms | Records found |
|---|---|
| faecal immunochemical OR fecal immunochemical OR faecal immuno-chemical OR fecal immuno-chemical OR iFOBT OR FOB Gold OR FOBGold OR JACKarc OR RIDASCREEN OR OC Sensor OR OC Pledia | 29 |
American Gastroenterological Association – Digestive Disease Week 2011–15
Date searched: 13 April 2016.
Records found: 92.
www.gastrojournal.org/content/ddw_abstracts
2011 DDW Abstract Supplement
www.gastrojournal.org/issue/S0016-5085%2811%29X6001-8
2012 DDW Abstract Supplement
www.gastrojournal.org/issue/S0016-5085%2812%29X6001-3
2013 DDW Abstract Supplement
www.gastrojournal.org/issue/S0016-5085%2813%29X6001-9
2014 DDW Abstract Supplement
www.gastrojournal.org/issue/S0016-5085%2814%29X6001-4
2015 DDW Abstract Supplement
www.gastrojournal.org/issue/S0016-5085%2815%29X6001-X
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 | TOTAL |
|---|---|---|---|---|---|---|
| “faecal immunochemical” | 1 | 5 | 0 | 2 | 3 | |
| “fecal immunochemical” | 9 | 7 | 14 | 16 | 15 | |
| “faecal immuno-chemical” | 0 | 0 | 0 | 0 | 0 | |
| “fecal immuno-chemical” | 0 | 0 | 0 | 0 | 0 | |
| iFOBT | 4 | 0 | 3 | 1 | 10 | |
| “FOB Gold” | 0 | 0 | 0 | 0 | 0 | |
| FOBGold | 0 | 0 | 0 | 0 | 0 | |
| JACKarc | 0 | 0 | 0 | 0 | 0 | |
| RIDASCREEN | 0 | 0 | 0 | 0 | 0 | |
| “OC Sensor” | 0 | 1 | 0 | 0 | 0 | |
| “OC Pledia” | 0 | 0 | 0 | 0 | 0 | |
| TOTAL (after deduplication) | 14 | 14 | 17 | 19 | 28 | 92 |
Annual Meeting of the American Association for Clinical Biochemistry and Laboratory Medicine 2011–15
Date searched: 13 April 2016.
Records found: 3.
www.aacc.org/science-and-research/annual-meeting-abstracts-archive
2011 AACC Annual Meeting
www.aacc.org/∼/media/files/annual-meeting/2011/aacc_11_fullabstract.pdf?la=en
2012 AACC Annual Meeting
www.aacc.org/∼/media/files/annual-meeting/2012/aacc_12_abstractbookfinalcomplete.pdf?la=en
2013 AACC Annual Meeting
www.aacc.org/∼/media/files/annual-meeting/2013/aacc_13_abstractbook_complete.pdf?la=en
2014 AACC Annual Meeting
www.aacc.org/∼/media/files/annual-meeting/2014/abstracts/aacc_14_abstractbook_1_combined.pdf?la=en
2015 AACC Annual Meeting
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 | TOTAL |
|---|---|---|---|---|---|---|
| faecal immunochemical | 0 | 0 | 0 | 0 | 0 | |
| fecal immunochemical | 1 | 0 | 0 | 1 | 0 | |
| faecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| fecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| FIT | 1 | 0 | 0 | 1 | 0 | |
| iFOBT | 0 | 0 | 0 | 0 | 0 | |
| FOB Gold | 0 | 0 | 1 | 1 | 0 | |
| FOBGold | 0 | 0 | 0 | 1 | 0 | |
| JACKarc | 0 | 0 | 0 | 0 | 0 | |
| RIDASCREEN | 0 | 0 | 0 | 0 | 0 | |
| OC Sensor | 0 | 0 | 0 | 0 | 0 | |
| OC Pledia | 0 | 0 | 0 | 0 | 0 | |
| TOTAL (after deduplication) | 1 | 0 | 1 | 1 | 0 | 3 |
British Society of Gastroenterology Annual Meeting 2011–15
Date searched: 13 April 2016.
Records found: 6.
www.bsg.org.uk/education/meeting/index.html
British Society of Gastroenterology Annual Meeting 2011
www.bsg.org.uk/images/stories/docs/education/2011_bsg_prog_text.pdf
British Society of Gastroenterology Annual Meeting 2012
www.bsg.org.uk/images/stories/docs/education/2012_ddf_prog_text.pdf
British Society of Gastroenterology Annual Meeting 2013
www.bsg.org.uk/images/stories/docs/education/2013_bsg_prog_text.pdf
British Society of Gastroenterology Annual Meeting 2014
www.bsg.org.uk/images/stories/docs/education/2014_bsg_prog_text.pdf
2015 – 2nd Digestive Disorders Federation Conference 22–25 June 2015 – Joint conference with The Association of Coloproctology of Great Britain & Ireland (ACPGBI), Association of Upper Gastrointestinal Surgeons (AUGIS), British Association for Parenteral and Enteral Nutrition (BAPEN), British Association for the Study of the Liver (BASL), British Society of Gastroenterology (BSG)
http://gut.bmj.com/content/64/Suppl_1.toc
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 | TOTAL |
|---|---|---|---|---|---|---|
| faecal immunochemical | 0 | 0 | 1 | 1 | 3 | |
| fecal immunochemical | 0 | 0 | 0 | 0 | 0 | |
| faecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| fecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| FIT | 0 | 1 | 1 | 0 | 3 | |
| iFOBT | 0 | 0 | 0 | 0 | 0 | |
| FOB Gold | 0 | 0 | 0 | 0 | 0 | |
| FOBGold | 0 | 0 | 0 | 0 | 0 | |
| JACKarc | 0 | 0 | 0 | 0 | 0 | |
| RIDASCREEN | 0 | 0 | 0 | 0 | 0 | |
| OC Sensor | 0 | 0 | 0 | 0 | 1 | |
| OC Pledia | 0 | 0 | 0 | 0 | 0 | |
| TOTAL (after deduplication) | 0 | 1 | 1 | 1 | 3 | 6 |
EuroMedLab: International Federation of Clinical Chemistry and Laboratory Medicine/European Federation of Clinical Chemistry and Laboratory Medicine, European Congress of Clinical Chemistry and Laboratory Medicine 2011–15 (every 2 years)
Date searched: 13 April 2016.
Records found: 5.
www.ifcc.org/ifcc-congresses-and-conferences/ifcc-eflm-euromedlab-congresses/
EuroMedLab 2011 – 19th IFCC-EFCC European Congress of Clinical Chemistry and Laboratory Medicine
http://ukb.lf1.cuni.cz/abstrakta/ifcc2011_abstr.pdf
EuroMedLab 2013 - 20th IFCC-EFLM European Congress of Clinical Chemistry and Laboratory Medicine
www.sibioc.it/bc/numero/bcnum/132
EuroMedLab 2015 – 21st IFCC-EFLM European Congress of Clinical Chemistry and Laboratory Medicine
www.degruyter.com/view/j/cclm.2015.53.issue-s1/issue-files/cclm.2015.53.issue-s1.xml
| Search terms | 2011 | 2013 | 2015 | TOTAL |
|---|---|---|---|---|
| faecal immunochemical | 0 | 1 | 1 | |
| fecal immunochemical | 0 | 2 | 0 | |
| faecal immuno-chemical | 0 | 0 | 0 | |
| fecal immuno-chemical | 0 | 0 | 0 | |
| FIT | 0 | 1 | 1 | |
| iFOBT | 0 | 0 | 0 | |
| FOB Gold | 1 | 1 | 0 | |
| FOBGold | 0 | 0 | 0 | |
| JACKarc | 0 | 0 | 0 | |
| RIDASCREEN | 0 | 0 | 0 | |
| OC Sensor | 0 | 0 | 1 | |
| OC Pledia | 0 | 0 | 0 | |
| TOTAL (after deduplication) | 1 | 2 | 2 | 5 |
United European Gastroenterology Week 2011–15
Date searched: 4 May 2016.
Records found: 47.
23rd United European Gastroenterology Week
Barcelona, Spain. October 24–28, 2015
www.ueg.eu/epaper/UEGWeek.2015.FinalProgramme/files/assets/common/downloads/publication.pdf
22nd United European Gastroenterology Week
Vienna, Austria. October 18–22, 2014
www.ueg.eu/fileadmin/user_upload/documents/week14/ueg%20week%202014_final_programme.pdf
21st United European Gastroenterology Week
Berlin, Germany. October 12–16, 2013
www.ueg.eu/fileadmin/user_upload/documents/Week13/ueg_week_2013_final_programme.pdf
20th United European Gastroenterology Week
Amsterdam, the Netherlands. October 20–24, 2012
www.ueg.eu/fileadmin/user_upload/documents/Week13/ueg_week_2012.final_programme.pdf
19th United European Gastroenterology Week 2011
Stockholm, Sweden. October 21–26, 2011
www.hungaronotes.hu/minden/UEGW2011.pdf
| Search terms | 2011 | 2012 | 2013 | 2014 | 2015 | TOTAL |
|---|---|---|---|---|---|---|
| faecal immunochemical | 2 | 3 | 1 | 1 | 2 | |
| fecal immunochemical | 0 | 6 | 2 | 4 | 6 | |
| faecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| fecal immuno-chemical | 0 | 0 | 0 | 0 | 0 | |
| FIT | 6 | 4 | 3 | 2 | 4 | |
| iFOBT | 0 | 0 | 1 | 0 | 0 | |
| FOB Gold | 0 | 0 | 0 | 0 | 0 | |
| FOBGold | 0 | 0 | 0 | 0 | 0 | |
| JACKarc | 0 | 0 | 0 | 0 | 0 | |
| RIDASCREEN | 0 | 0 | 0 | 0 | 0 | |
| OC Sensor | 0 | 0 | 0 | 0 | 0 | |
| OC Pledia | 0 | 0 | 0 | 0 | 0 | |
| TOTAL (after deduplication) | 8 | 13 | 7 | 7 | 12 | 47 |
Cost-effectiveness searches
Economic evaluations and cost studies
MEDLINE (via Ovid): 1946 to March Week 4 2016
Date searched: 4 April 2016.
Records found: 818.
-
exp Colonoscopy/ (23,858)
-
Colonoscop$.ti,ab,ot,hw. (28,519)
-
Coloscop$.ti,ab,ot,hw. (579)
-
Sigmoidoscop$.ti,ab,ot,hw. (6409)
-
or/1-4 (32,887)
-
Colonography, Computed Tomographic/ (1746)
-
virtual colonoscop$.ti,ab,ot,hw. (501)
-
CT colonograph$.ti,ab,ot,hw. (1107)
-
CT pneumocolon.ti,ab,ot,hw. (12)
-
computed tomographic colonograph$.ti,ab,ot,hw. (263)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/6-11 (2091)
-
Magnetic Resonance Imaging/ (309,510)
-
magnetic resonance imaging.ti,ab,ot,hw. (360,372)
-
(MRI or MRIs or NMRI).ti,ab,ot,hw. (148,957)
-
(magneti?ation transfer adj1 imaging).ti,ab,ot,hw. (413)
-
MR imaging.ti,ab,ot,hw. (32,911)
-
NMR imaging.ti,ab,ot,hw. (1135)
-
magnetic resonance tomography.ti,ab,ot,hw. (1668)
-
MR tomography.ti,ab,ot,hw. (342)
-
chemical shift imaging.ti,ab,ot,hw. (794)
-
proton spin tomography.ti,ab,ot,hw. (34)
-
zeugmatography.ti,ab,ot,hw. (22)
-
or/13-23 (393,772)
-
exp Tomography, X-Ray Computed/ (334,373)
-
(CAT scan$ or CT scan$ or CT x-ray$ or CT xray$).ti,ab,ot,hw. (66,895)
-
(comput$ tomograph$ or computed xray tomograph$ or computed x-ray tomograph$ or computeri?ed axial tomograph$).ti,ab,ot,hw. (196,347)
-
electron beam tomograph$.ti,ab,ot,hw. (375)
-
tomodensitometry.ti,ab,ot,hw. (584)
-
or/25-29 (424,513)
-
5 or 12 or 24 or 30 (763,480)
-
exp colorectal neoplasms/ (162,710)
-
exp cecal neoplasms/ (4892)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (199,696)
-
CRC.ti,ab,ot. (13,878)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1846)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1676)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (26)
-
or/32-38 (208,381)
-
31 and 39 (26,596)
-
economics/ (26,664)
-
exp “costs and cost analysis”/ (195,358)
-
economics, dental/ (1876)
-
exp “economics, hospital”/ (21,229)
-
economics, medical/ (8858)
-
economics, nursing/ (3933)
-
economics, pharmaceutical/ (2608)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (471,438)
-
(expenditure$ not energy).ti,ab. (18,999)
-
(value adj1 money).ti,ab. (24)
-
budget$.ti,ab. (18,713)
-
or/41-51 (601,712)
-
((energy or oxygen) adj cost).ti,ab. (2826)
-
(metabolic adj cost).ti,ab. (868)
-
((energy or oxygen) adj expenditure).ti,ab. (17,419)
-
or/53-55 (20,370)
-
52 not 56 (597,185)
-
letter.pt. (876,097)
-
editorial.pt. (373,604)
-
historical article.pt. (328,206)
-
or/58-60 (1,561,761)
-
57 not 61 (567,144)
-
40 and 62 (1448)
-
limit 63 to yr = “2005-Current” (818)
Economics terms based on Costs filter:
-
Centre for Reviews and Dissemination (CRD). Search strategies: NHS EED MEDLINE using OvidSP (Economics Filter). York: CRD; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 2 June 2014). 146
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via Ovid): 1 April 2016
Date searched: 4 April 2016.
Records found: 103.
-
exp Colonoscopy/ (28)
-
Colonoscop$.ti,ab,ot,hw. (2422)
-
Coloscop$.ti,ab,ot,hw. (7)
-
Sigmoidoscop$.ti,ab,ot,hw. (207)
-
or/1-4 (2536)
-
Colonography, Computed Tomographic/ (0)
-
virtual colonoscop$.ti,ab,ot,hw. (43)
-
CT colonograph$.ti,ab,ot,hw. (94)
-
CT pneumocolon.ti,ab,ot,hw. (0)
-
computed tomographic colonograph$.ti,ab,ot,hw. (26)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/6-11 (146)
-
Magnetic Resonance Imaging/ (448)
-
magnetic resonance imaging.ti,ab,ot,hw. (18,196)
-
(MRI or MRIs or NMRI).ti,ab,ot,hw. (18,665)
-
(magneti?ation transfer adj1 imaging).ti,ab,ot,hw. (41)
-
MR imaging.ti,ab,ot,hw. (1820)
-
NMR imaging.ti,ab,ot,hw. (73)
-
magnetic resonance tomography.ti,ab,ot,hw. (29)
-
MR tomography.ti,ab,ot,hw. (6)
-
chemical shift imaging.ti,ab,ot,hw. (49)
-
proton spin tomography.ti,ab,ot,hw. (0)
-
zeugmatography.ti,ab,ot,hw. (0)
-
or/13-23 (30,159)
-
exp Tomography, X-Ray Computed/ (392)
-
(CAT scan$ or CT scan$ or CT x-ray$ or CT xray$).ti,ab,ot,hw. (7114)
-
(comput$ tomograph$ or computed xray tomograph$ or computed x-ray tomograph$ or computeri?ed axial tomograph$).ti,ab,ot,hw. (23,966)
-
electron beam tomograph$.ti,ab,ot,hw. (8)
-
tomodensitometry.ti,ab,ot,hw. (14)
-
or/25-29 (28,315)
-
5 or 12 or 24 or 30 (56,276)
-
exp colorectal neoplasms/ (154)
-
exp cecal neoplasms/ (1)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (14,543)
-
CRC.ti,ab,ot. (2480)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (195)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (38)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (0)
-
or/32-38 (14,877)
-
31 and 39 (1940)
-
economics/ (3)
-
exp “costs and cost analysis”/ (205)
-
economics, dental/ (0)
-
exp “economics, hospital”/ (19)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (0)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (71,397)
-
(expenditure$ not energy).ti,ab. (2126)
-
(value adj1 money).ti,ab. (4)
-
budget$.ti,ab. (2907)
-
or/41-51 (74,321)
-
((energy or oxygen) adj cost).ti,ab. (377)
-
(metabolic adj cost).ti,ab. (116)
-
((energy or oxygen) adj expenditure).ti,ab. (1622)
-
or/53-55 (2060)
-
52 not 56 (73,745)
-
letter.pt. (32,301)
-
editorial.pt. (24,232)
-
historical article.pt. (183)
-
or/58-60 (56,696)
-
57 not 61 (73,074)
-
40 and 62 (114)
-
limit 63 to yr=“2005 -Current” (103)
Economics terms based on Costs filter:
-
Centre for Reviews and Dissemination (CRD). Search Strategies: NHS EED MEDLINE using OvidSP (Economics Filter). York: CRD; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 2 June 2014). 146
EMBASE (via Ovid): 1974 to 1 April 2016
Date searched: 4 April 2016.
Records found: 2333.
-
Colonoscopy/ (54,012)
-
Sigmoidoscopy/ (10,176)
-
Colonoscop$.ti,ab,ot,hw. (61,058)
-
Coloscop$.ti,ab,ot,hw. (987)
-
Sigmoidoscop$.ti,ab,ot,hw. (11,721)
-
or/1-5 (67,824)
-
computed tomographic colonography/ (3341)
-
virtual colonoscop$.ti,ab,ot,hw. (723)
-
CT colonograph$.ti,ab,ot,hw. (1574)
-
CT pneumocolon.ti,ab,ot,hw. (21)
-
computed tomographic colonograph$.ti,ab,ot,hw. (3369)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/7-12 (3740)
-
nuclear magnetic resonance imaging/ (556,588)
-
Magnetic resonance imaging.ti,ab,ot,hw. (629,542)
-
(MRI or MRIs or NMRI).ti,ab,ot,hw. (272,277)
-
(magneti?ation transfer adj1 imaging).ti,ab,ot,hw. (571)
-
MR imaging.ti,ab,ot,hw. (42,912)
-
NMR imaging.ti,ab,ot,hw. (1406)
-
magnetic resonance tomography.ti,ab,ot,hw. (1496)
-
MR tomography.ti,ab,ot,hw. (344)
-
chemical shift imaging.ti,ab,ot,hw. (982)
-
proton spin tomography.ti,ab,ot,hw. (11)
-
zeugmatography.ti,ab,ot,hw. (26)
-
or/14-24 (662,853)
-
exp computer assisted tomography/ (713,414)
-
(CAT scan$ or CT scan$ or CT x-ray$ or CT xray$).ti,ab,ot,hw. (117,999)
-
(comput$ tomograph$ or computed xray tomograph$ or computed x-ray tomograph$ or computeri?ed axial tomograph$).ti,ab,ot,hw. (351,851)
-
electron beam tomograph$.ti,ab,ot,hw. (2201)
-
tomodensitometry.ti,ab,ot,hw. (716)
-
or/26-30 (771,131)
-
6 or 13 or 25 or 31 (1,312,128)
-
exp colon tumor/ (240,252)
-
exp rectum tumor/ (182,819)
-
exp colon cancer/ (191,943)
-
exp rectum cancer/ (148,848)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (318,696)
-
CRC.ti,ab,ot. (27,373)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (2651)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1929)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (29)
-
or/33-41 (330,526)
-
32 and 42 (52,394)
-
health-economics/ (35,302)
-
exp economic-evaluation/ (240,103)
-
exp health-care-cost/ (231,067)
-
exp pharmacoeconomics/ (177,750)
-
or/44-47 (530,895)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (725,833)
-
(expenditure$ not energy).ti,ab. (28,158)
-
(value adj2 money).ti,ab. (1676)
-
budget$.ti,ab. (28,112)
-
or/49-52 (753,216)
-
48 or 53 (1,043,923)
-
letter.pt. (930,553)
-
editorial.pt. (504,001)
-
note.pt. (634,165)
-
or/55-57 (2,068,719)
-
54 not 58 (947,501)
-
(metabolic adj cost).ti,ab. (1063)
-
((energy or oxygen) adj cost).ti,ab. (3494)
-
((energy or oxygen) adj expenditure).ti,ab. (23,677)
-
or/60-62 (27,341)
-
59 not 63 (941,755)
-
exp animal/ (21,427,388)
-
exp animal-experiment/ (1,922,177)
-
nonhuman/ (4,721,186)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,295,240)
-
or/65-68 (23,039,014)
-
exp human/ (16,996,370)
-
exp human-experiment/ (350,705)
-
70 or 71 (16,997,816)
-
69 not (69 and 72) (6,042,168)
-
64 not 73 (867,075)
-
43 and 74 (3250)
-
limit 75 to yr = “2005-Current” (2333)
Economics terms based on Costs filter:
-
Centre for Reviews and Dissemination (CRD). Search Strategies: NHS EED EMBASE using OvidSP (Economics Filter). York: CRD; 2014. URL: www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 4 April 2016). 146
NHS Economic Evaluation Database (Wiley) (via the internet): Issue 2 of 4, April 2015
Date searched: 4 April 2016.
Records found: 126.
#1 MeSH descriptor: [Colonoscopy] explode all trees (1772)
#2 Colonoscop* or Coloscop* or Sigmoidoscop* (3631)
#3 #1 or #2 (3631)
#4 MeSH descriptor: [Colonography, Computed Tomographic] explode all trees (128)
#5 “virtual colonoscop*” or “CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon” (200)
#6 #4 or #5 (218)
#7 MeSH descriptor: [Magnetic Resonance Imaging] this term only (5990)
#8 “magnetic resonance imaging” or MRI or MRIs or NMRI (13,927)
#9 “magneti?ation transfer” near/1 imaging (18)
#10 “MR imaging” or “NMR imaging” or “magnetic resonance tomography” or “MR tomography” (854)
#11 “chemical shift imaging” or “proton spin tomography” or zeugmatography (20)
#12 #7 or #8 or #9 or #10 or #11 (14,073)
#13 [mh “Tomography, X-Ray Computed”] (4799)
#14 “CAT scan*” or “CT scan*” or “CT x-ray*” or “CT xray*” (2684)
#15 “comput* tomograph*” or “computed xray tomograph*” or “computed x-ray tomograph*” or “computeri?ed axial tomograph*” (8685)
#16 “electron beam tomograph*” or tomodensitometry (45)
#17 #13 or #14 or #15 or #16 (11,656)
#18 #3 or #6 or #12 or #17 (27,348)
#19 MeSH descriptor: [Colorectal Neoplasms] explode all trees (6056)
#20 MeSH descriptor: [Cecal Neoplasms] explode all trees (9)
#21 (colorect* or rectal* or rectum* or colon* or sigma* or sigmo* or rectosigm* or bowel* or anal or anus) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (12,310)
#22 CRC (1341)
#23 (cecum or cecal or caecum or caecal or ileocecal or ilaeocaecal or ileocecum or ilaeocaecum) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (98)
#24 “large intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (54)
#25 “lower intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
#26 #19 or #20 or #21 or #22 or #23 or #24 or #25 (12,665)
#27 #18 and #26 Publication Year from 2005 to 2016, in Economic Evaluations (126)
EconLit (via EBSCOhost): 2005 to 1 February 2016
Date searched: 5 April 2016.
Records found: 13.
S1 Colonoscop* or Coloscop* or Sigmoidoscop* (24)
S2 “virtual colonoscop*” or “CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon” (4)
S3 “magnetic resonance imaging” or MRI or MRIs or NMRI (115)
S4 “magneti?ation transfer” N1 imaging (0)
S5 “MR imaging” or “NMR imaging” or “magnetic resonance tomography” or “MR tomography” (1)
S6 “chemical shift imaging” or “proton spin tomography” or zeugmatography (0)
S7 “CAT scan*” or “CT scan*” or “CT x-ray*” or “CT xray*” (15)
S8 “comput* tomograph*” or “computed xray tomograph*” or “computed x-ray tomograph*” or “computeri?ed axial tomograph*” (28)
S9 “electron beam tomograph*” or tomodensitometry (0)
S10 S1 OR S2 OR S3 or S4 or S5 or S6 or S7 or S8 or S9 (173)
S11 (colorect* or rectal* or rectum* or colon* or sigma* or sigmo* or rectosigm* or bowel* or anal or anus) N3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (104)
S12 CRC (332)
S13 (cecum or cecal or caecum or caecal or ileocecal or ilaeocaecal or ileocecum or ilaeocaecum) N3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
S14 “large intestin*” N3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
S15 “lower intestin*” N3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
S16 S11 OR S12 OR S13 OR S14 OR S15 (410)
S17 S10 AND S16 (14)
S18 (ZR “2005”) or (ZR “2006”) or (ZR “2007”) or (ZR “2008”) or (ZR “2009”) or (ZR “2010”) or (ZR “2011”) or (ZR “2012”) or (ZR “2013”) or (ZR “2014”) or (ZR “2015”) or (ZR “2016”) (638,824)
S19 S17 AND S18 (13)
Cost-Effectiveness Analysis Registry (via the internet)
https://research.tufts-nemc.org/cear4/Home.aspx
Date searched: 5 April 2016.
Records found: 96.
| Search term (basic search) | Records found |
|---|---|
| colonoscopy | 24 |
| Computed tomographic colonography | 0 |
| CTC | 1 |
| coloscopy | 0 |
| sigmoidoscopy | 11 |
| magnetic resonance imaging | 21 |
| MRI | 28 |
| CT scan | 11 |
| CAT scan | 0 |
| TOTAL | 96 |
Research Papers in Economics IDEAS database (via internet)
Date searched: 5 April 2016.
Records found: 21.
(colonoscopy | “computed tomographic colonography” | “CT colonography” | coloscopy | sigmoidoscopy | “magnetic resonance imaging” | MRI | “CT scan” | “CAT scan”) + (colorectal | rectal | rectum | colon | bowel | intestine)
Adverse events and mortality data
MEDLINE (via Ovid): 1946 to March Week 4 2016
Date searched: 5 April 2016.
Records found: 759.
-
exp colorectal neoplasms/ (162,710)
-
exp cecal neoplasms/ (4892)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (199,696)
-
CRC.ti,ab,ot. (13,878)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1846)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1676)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (26)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (208,381)
-
exp Colonoscopy/ae, mo [Adverse Effects, Mortality] (1888)
-
Colonography, Computed Tomographic/ae, mo [Adverse Effects, Mortality] (82)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (185)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (39)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (118)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (11)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (4)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (3)
-
or/9-16 (2209)
-
8 and 17 (762)
-
exp animals/ not (exp animals/ and humans/) (4,208,134)
-
18 not 19 (759)
Adverse events filter based on:
-
Appendix 4: Searching for adverse events. In Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care: An Update of CRD’s Guidance. York: University of York; 2009. pp. 253–4. 147
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via Ovid): 4 April 2016
Date searched: 5 April 2016.
Records found: 20.
-
exp colorectal neoplasms/ (302)
-
exp cecal neoplasms/ (9)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (14,770)
-
CRC.ti,ab,ot. (2502)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (205)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (38)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (0)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 (15,116)
-
exp Colonoscopy/ae, mo [Adverse Effects, Mortality] (2)
-
Colonography, Computed Tomographic/ae, mo [Adverse Effects, Mortality] (0)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (25)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (10)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (12)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (1)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (0)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (0)
-
or/9-16 (47)
-
8 and 17 (20)
-
exp animals/ not (exp animals/ and humans/) (2786)
-
20 18 not 19 (20)
Adverse events filter based on:
-
Appendix 4: Searching for adverse events. In Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care: An Update of CRD’s Guidance. York: University of York; 2009. pp. 253–4. 147
EMBASE (via Ovid): 1974 to 4 April 2016
Date searched: 5 April 2016.
Records found: 539.
-
exp colon tumor/ (240,283)
-
exp rectum tumor/ (182,837)
-
exp colon cancer/ (191,971)
-
exp rectum cancer/ (148,865)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (31,732)
-
CRC.ti,ab,ot. (27,377)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (2651)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1929)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (29)
-
or/1-9 (330,564)
-
colonoscopy/ae, co [Adverse Drug Reaction] (346)
-
sigmoidoscopy/ae, co [Adverse Drug Reaction, Complication] (70)
-
computed tomographic colonography/ae, co [Adverse Drug Reaction] (10)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (398)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (101)
-
((colonoscop$ or coloscop$ or sigmoidoscop$) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (224)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (safe or safety or side effect$ or undesirable effect$ or treatment emergent or tolerability)).ti,ab. (14)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes))).ti,ab. (5)
-
((CT colonograph$ or CT pneumocolon or computed tomographic colonograph$ or computed tomographic pneumocolon) adj3 (mortalit$ or dead or death or deaths or died or fatal$)).ti,ab. (3)
-
or/11-19 (1117)
-
10 and 20 (543)
-
animal/ (1,738,058)
-
animal experiment/ (1,920,454)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,186,843)
-
or/22-24 (6,186,843)
-
exp human/ (16,998,345)
-
human experiment/ (350,771)
-
or/26-27 (16,999,791)
-
25 not (25 and 28) (4,867,829)
-
21 not 29 (539)
Adverse events filter based on:
-
Appendix 4: searching for adverse events. In Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care: An update of CRD’s Guidance. York: University of York; 2009. pp. 253–4. 147
Cochrane Central Register of Controlled Trials (Wiley) (via internet): issue 3 of 12, March 2016
Date searched: 5 April 2016.
Records found: 114.
#1 MeSH descriptor: [Colorectal Neoplasms] explode all trees (6056)
#2 MeSH descriptor: [Cecal Neoplasms] explode all trees (9)
#3 (colorect* or rectal* or rectum* or colon* or sigma* or sigmo* or rectosigm* or bowel* or anal or anus) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (12,310)
#4 CRC (1341)
#5 (cecum or cecal or caecum or caecal or ileocecal or ilaeocaecal or ileocecum or ilaeocaecum) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (98)
#6 “large intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (54)
#7 “lower intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7 (12,665)
#9 MeSH descriptor: [Colonoscopy] explode all trees and with qualifier(s): [Adverse effects - AE, Mortality - MO] (159)
#10 MeSH descriptor: [Colonography, Computed Tomographic] explode all trees and with qualifier(s): [Adverse effects - AE, Mortality - MO] (6)
#11 (colonoscop* or coloscop* or sigmoidoscop*) near/3 (safe or safety or “side effect*” or “undesirable effect*” or “treatment emergent” or tolerability) (143)
#12 (colonoscop* or coloscop* or sigmoidoscop*) near/3 (adverse near/2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)) (271)
#13 (colonoscop* or coloscop* or sigmoidoscop*) near/3 (mortalit* or dead or death or deaths or died or fatal*) (55)
#14 (“CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon”) near/3 (safe or safety or “side effect*” or “undesirable effect*” or “treatment emergent” or tolerability) (3)
#15 (“CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon”) near/3 (adverse near/2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)) (1)
#16 (“CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon”) near/3 (mortalit* or dead or death or deaths or died or fatal*) (3)
#17 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 (445)
#18 #8 and #17 in Trials (114)
Adverse events filter based on:
-
Appendix 4: searching for adverse events. In Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care: An upDate of CRD’s Guidance. York: University of York; 2009. pp. 253–4. 147
Utility data
MEDLINE (via Ovid): 1946 to March Week 4 2016
Date searched: 5 April 2016.
Records found: 142.
-
exp Colonoscopy/ (23,858)
-
Colonoscop$.ti,ab,ot,hw. (28,519)
-
Coloscop$.ti,ab,ot,hw. (579)
-
Sigmoidoscop$.ti,ab,ot,hw. (6409)
-
or/1-4 (32,887)
-
Colonography, Computed Tomographic/ (1746)
-
virtual colonoscop$.ti,ab,ot,hw. (501)
-
CT colonograph$.ti,ab,ot,hw. (1107)
-
CT pneumocolon.ti,ab,ot,hw. (12)
-
computed tomographic colonograph$.ti,ab,ot,hw. (263)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/6-11 (2091)
-
5 or 12 (33,666)
-
exp colorectal neoplasms/ (162,710)
-
exp cecal neoplasms/ (4892)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (199,696)
-
CRC.ti,ab,ot. (13,878)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1846)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1676)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (26)
-
or/14-20 (208,381)
-
13 and 21 (17,376)
-
quality-adjusted life years/ or quality of life/ (141,086)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (16,837)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (1063)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (3105)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (486)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (343)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (283)
-
“health related quality of life”.ti,ab,ot. (23,887)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (6861)
-
“assessment of quality of life”.ti,ab,ot. (1231)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (4671)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (11,290)
-
(hye or hyes).ti,ab,ot. (54)
-
health$ year$ equivalent$.ti,ab,ot. (38)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (947)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (630)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (1999)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (7724)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (4122)
-
15d.ti,ab,ot. (1230)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (247)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (7273)
-
(utilities or disutili$).ti,ab,ot. (4335)
-
(Functional Assessment of Cancer Therapy$ or FACT-G).ti,ab,ot. (1334)
-
(QLQ-C30 or QLQ-C-30 or EORTC QLQ$ or “European Organization for Research and Treatment of Cancer Quality of Life Questionnaire” or “EORTC Quality of Life Questionnaire”).ti,ab,ot. (2735)
-
or/23-47 (167,380)
-
22 and 48 (142)
-
animals/ not (animals/ and humans/) (4,175,932)
-
49 not 50 (142)
Health-related quality of life (HRQoL) free-text terms based on:
-
Figure 4: Common free-text terms for electronic database searching for HSUVs. In Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011). 147
MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE Daily Update (via Ovid): 4 April 2016
Date searched: 5 April 2016.
Records found: 15.
-
exp Colonoscopy/ (37)
-
Colonoscop$.ti,ab,ot,hw. (2467)
-
Coloscop$.ti,ab,ot,hw. (7)
-
Sigmoidoscop$.ti,ab,ot,hw. (208)
-
or/1-4 (2581)
-
Colonography, Computed Tomographic/ (1)
-
virtual colonoscop$.ti,ab,ot,hw. (43)
-
CT colonograph$.ti,ab,ot,hw. (95)
-
CT pneumocolon.ti,ab,ot,hw. (0)
-
computed tomographic colonograph$.ti,ab,ot,hw. (26)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/6-11 (147)
-
5 or 12 (2635)
-
exp colorectal neoplasms/ (302)
-
exp cecal neoplasms/ (9)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (14,770)
-
CRC.ti,ab,ot. (2502)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (205)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (38)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (0)
-
or/14-20 (15,116)
-
13 and 21 (1285)
-
quality-adjusted life years/ or quality of life/ (344)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (1882)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (480)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (462)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (68)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (14)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (48)
-
“health related quality of life”.ti,ab,ot. (3528)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (999)
-
“assessment of quality of life”.ti,ab,ot. (123)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (882)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (1608)
-
(hye or hyes).ti,ab,ot. (3)
-
health$ year$ equivalent$.ti,ab,ot. (2)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (136)
-
(quality time or qwb or quality of well being or “quality of wellbeing” or “index of wellbeing” or “index of well being”).ti,ab,ot,hw. (58)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (336)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (1165)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (628)
-
15d.ti,ab,ot. (118)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (32)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (951)
-
(utilities or disutili$).ti,ab,ot. (652)
-
(Functional Assessment of Cancer Therapy$ or FACT-G).ti,ab,ot. (168)
-
(QLQ-C30 or QLQ-C-30 or EORTC QLQ$ or “European Organization for Research and Treatment of Cancer Quality of Life Questionnaire” or “EORTC Quality of Life Questionnaire”).ti,ab,ot. (372)
-
or/23-47 (9820)
-
22 and 48 (12)
-
animals/ not (animals/ and humans/) (2780)
-
49 not 50 (12)
HRQoL free-text terms based on:
-
Figure 4: Common free-text terms for electronic database searching for HSUVs. In Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011). 148
EMBASE (via Ovid): 1974 to 4 April 2016
Date searched: 5 April 2016.
Records found: 208.
-
Colonoscopy/ (54,017)
-
Sigmoidoscopy/ (10,176)
-
Colonoscop$.ti,ab,ot,hw. (61,064)
-
Coloscop$.ti,ab,ot,hw. (987)
-
Sigmoidoscop$.ti,ab,ot,hw. (11,721)
-
or/1-5 (67,830)
-
computed tomographic colonography/ (3342)
-
virtual colonoscop$.ti,ab,ot,hw. (723)
-
CT colonograph$.ti,ab,ot,hw. (1574)
-
CT pneumocolon.ti,ab,ot,hw. (21)
-
computed tomographic colonograph$.ti,ab,ot,hw. (3370)
-
computed tomographic pneumocolon.ti,ab,ot,hw. (0)
-
or/7-12 (3741)
-
6 or 13 (69,101)
-
exp colon tumor/ (240,283)
-
exp rectum tumor/ (182,837)
-
exp colon cancer/ (191,971)
-
exp rectum cancer/ (148,865)
-
((colorect$ or rectal$ or rectum$ or colon$ or sigma$ or sigmo$ or rectosigm$ or bowel$ or anal or anus) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot,hw. (318,732)
-
CRC.ti,ab,ot. (27,377)
-
((cecum or cecal or caecum or caecal or il?eoc?ecal or il?eoc?ecum) adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (2651)
-
(large intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (1929)
-
(lower intestin$ adj3 (cancer$ or neoplas$ or oncolog$ or malignan$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or adenom$ or lesion$)).ti,ab,ot. (29)
-
or/15-23 (330,564)
-
14 and 24 (33,692)
-
quality adjusted life year/ or quality of life index/ (17,805)
-
Short Form 12/ or Short Form 20/ or Short Form 36/ or Short Form 8/ (18,197)
-
“International Classification of Functioning, Disability and Health”/ or “ferrans and powers quality of life index”/ or “gastrointestinal quality of life index”/ (2090)
-
(sf36 or sf 36 or sf-36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab,ot. (28,658)
-
(sf6 or sf 6 or sf-6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab,ot. (1719)
-
(sf12 or sf 12 or sf-12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,ot. (5712)
-
(sf6D or sf 6D or sf-6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab,ot. (945)
-
(sf20 or sf 20 or sf-20 or short form 20 or shortform 20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,ot. (373)
-
(sf8 or sf 8 or sf-8 or short form 8 or shortform 8 or sf eight or sfeight or shortform eight or short form eight).ti,ab,ot. (539)
-
“health related quality of life”.ti,ab,ot. (37,958)
-
(Quality adjusted life or Quality-adjusted-life).ti,ab,ot. (11,553)
-
“assessment of quality of life”.ti,ab,ot. (2056)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab,ot. (10,075)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab,ot. (19,883)
-
(hye or hyes).ti,ab,ot. (100)
-
health$ year$ equivalent$.ti,ab,ot. (40)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab,ot. (2395)
-
(quality time or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or index of well being).ti,ab,ot,hw. (886)
-
(Disability adjusted life or Disability-adjusted life or health adjusted life or health-adjusted life or “years of healthy life” or healthy years equivalent or “years of potential life lost” or “years of health life lost”).ti,ab,ot. (2768)
-
(QALY$ or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL or qald$ or qale$ or qtime$ or AQoL$).ti,ab,ot. (14,817)
-
(timetradeoff or time tradeoff or time trade-off or time trade off or TTO or Standard gamble$ or “willingness to pay”).ti,ab,ot. (6976)
-
15d.ti,ab,ot. (1920)
-
(HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab,ot. (377)
-
(utilit$ adj3 (“quality of life” or valu$ or scor$ or measur$ or health or life or estimat$ or elicit$ or disease$)).ti,ab,ot. (12,545)
-
(utilities or disutili$).ti,ab,ot. (7816)
-
(Functional Assessment of Cancer Therapy$ or FACT-G).ti,ab,ot. (2475)
-
(QLQ-C30 or QLQ-C-30 or EORTC QLQ$ or “European Organization for Research and Treatment of Cancer Quality of Life Questionnaire” or “EORTC Quality of Life Questionnaire”).ti,ab,ot. (5757)
-
or/26-52 (124,961)
-
25 and 53 (208)
-
animal/ (1,738,058)
-
animal experiment/ (1,920,454)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (6,186,843)
-
or/55-57 (6,186,843)
-
exp human/ (16,998,345)
-
human experiment/ (350,771)
-
or/59-60 (16,999,791)
-
58 not (58 and 61) (4,867,829)
-
54 not 62 (208)
HRQoL free-text terms based on:
-
Figure 4: Common free-text terms for electronic database searching for HSUVs. In Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011). 148
NHS Economic Evaluation Database (Wiley) (via the internet): Issue 2 of 4, April 2015; Cochrane Central Register of Controlled Trials (Wiley) (via the internet): Issue 3 of 12, March 2016
Date searched: 6 April 2016.
Records found: NHS EED 84, CENTRAL 22.
#1 MeSH descriptor: [Colonoscopy] explode all trees (1772)
#2 Colonoscop* or Coloscop* or Sigmoidoscop* (3631)
#3 #1 or #2 (3631)
#4 MeSH descriptor: [Colonography, Computed Tomographic] explode all trees (128)
#5 “virtual colonoscop*” or “CT colonograph*” or “CT pneumocolon” or “computed tomographic colonograph*” or “computed tomographic pneumocolon” (200)
#6 #4 or #5 (218)
#7 #3 or #6 (3684)
#8 MeSH descriptor: [Colorectal Neoplasms] explode all trees (6056)
#9 MeSH descriptor: [Cecal Neoplasms] explode all trees (9)
#10 (colorect* or rectal* or rectum* or colon* or sigma* or sigmo* or rectosigm* or bowel* or anal or anus) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (12,310)
#11 CRC (1341)
#12 (cecum or cecal or caecum or caecal or ileocecal or ilaeocaecal or ileocecum or ilaeocaecum) near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (98)
#13 “large intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (54)
#14 “lower intestin*” near/3 (cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or adenom* or lesion*) (0)
#15 #8 or #9 or #10 or #11 or #12 or #13 or #14 (12,665)
#16 #7 and #15 (1672)
#17 MeSH descriptor: [Quality-Adjusted Life Years] this term only (4067)
#18 MeSH descriptor: [Quality of Life] this term only (17,726)
#19 sf36 or “sf 36” or “sf-36” or “short form 36” or “shortform 36” or “sf thirtysix” or “sf thirty six” or “shortform thirtysix” or “shortform thirty six” or “short form thirty six” or “short form thirtysix” or “short form thirty six” (6863)
#20 sf6 or “sf 6” or “sf-6” or “short form 6” or “shortform 6” or “sf six” or sfsix or “shortform six” or “short form six” (154)
#21 sf12 or “sf 12” or “sf-12” or “short form 12” or “shortform 12” or “sf twelve” or sftwelve or “shortform twelve” or “short form twelve” (1132)
#22 sf6D or “sf 6D” or “sf-6D” or “short form 6D” or “shortform 6D” or “sf six D” or sfsixD or “shortform six D” or “short form six D” (206)
#23 sf20 or “sf 20” or “sf-20” or “short form 20” or “shortform 20” or “sf twenty” or sftwenty or “shortform twenty” or “short form twenty” (78)
#24 sf8 or “sf 8” or “sf-8” or “short form 8” or “shortform 8” or “sf eight” or sfeight or “shortform eight” or “short form eight” (66)
#25 “health related quality of life” (7949)
#26 “Quality adjusted life” or “Quality-adjusted-life” (6898)
#27 “assessment of quality of life” (348)
#28 euroqol or “euro qol” or eq5d or “eq 5d” (3194)
#29 hql or hrql or hqol or “h qol” or hrqol or “hr qol” (3007)
#30 hye or hyes (58)
#31 “health* year* equivalent*” (5)
#32 hui or hui1 or hui2 or hui3 or hui4 or “hui-4” or “hui-1” or “hui-2” or “hui-3” (2929)
#33 “quality time” or qwb or “quality of well being” or “quality of wellbeing” or “index of wellbeing” or “index of well being” (238)
#34 “Disability adjusted life” or “Disability-adjusted life” or “health adjusted life” or “health-adjusted life” or “years of healthy life” or “healthy years equivalent” or “years of potential life lost” or “years of health life lost” (370)
#35 QALY* or DALY* or HALY* or YHL or HYES or YPLL or YHLL or qald* or qale* or qtime* or AQoL* (5551)
#36 timetradeoff or “time tradeoff” or “time trade-off” or “time trade off” or TTO or “Standard gamble*” or “willingness to pay” (2030)
#37 15d (116)
#38 HSUV* or “health state* value*” or “health state* preference*” or HSPV* (89)
#39 utilit* near/3 (“quality of life” or valu* or scor* or measur* or health or life or estimat* or elicit* or disease*) (4790)
#40 utilities or disutili* (1711)
#41 “Functional Assessment of Cancer Therapy*” or “FACT-G” (588)
#42 “QLQ-C30” or “QLQ-C-30” or “EORTC QLQ*” or “European Organization for Research and Treatment of Cancer Quality of Life Questionnaire” or “EORTC Quality of Life Questionnaire” (1174)
#43 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 (39,531)
#44 #16 and #43 in Trials and Economic Evaluations (106)
HRQoL free-text terms based on:
-
Figure 4: Common free-text terms for electronic database searching for HSUVs. In Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the literaTure. 2011. URL: www.nicedsu.org.uk (accessed 18 August 2011). 147
School of Health and Related Research Health Utilities Database (via the internet)
Date searched: 6 April 2016.
Records found: 10.
colorectal or rectal or rectum or colon or bowel or intestine [Any field]
and
cancer or neoplasm or neoplasms or tumor or tumour [Any field].
Appendix 2 Data extraction tables
| Study Details | Selection criteria | Participant details | Test manufacturer |
|---|---|---|---|
| Auge 2016 57 |
|
|
Kyowa Medex, Japan |
| Related publications: Auge 2014;66 Auge 201572 | |||
| Country: Spain | |||
| Funding: Menarini Diagnósticos, S.A., provided instruments, reagents and technical support | |||
| Recruitment: December 2013 to March 2014 | |||
| Study design: Diagnostic cohort | |||
| Cubiella 2014 (COLONPREDICT) 55 |
|
|
Eiken Chemical Co., Tokyo, Japan |
| Related publications: Diaz Ondina 2014;61 Cubiella 201573 | |||
| Country: Spain | |||
| Funding: Instituto de Salud Carlos III, Madrid, Spain (PI11/00094) | |||
| Recruitment: April 2012 to November 2012 | |||
| Study design: Diagnostic cohort | |||
| Godber 2016 56 |
|
|
Kyowa Medex, Japan |
| Related publications: Macdonald 2015;71 Godber 201465 | |||
| Country: Scotland | |||
| Funding: NHS Lanarkshire; analyser, training and consumables were supplied by Kyowa Medex Co., Ltd, Tokyo, Japan | |||
| Recruitment: June 2013 to December 2013 | |||
| Study design: Diagnostic cohort | |||
| (Confidential information has been removed) | (Confidential information has been removed) |
|
(Confidential information has been removed) |
| Krivec 2011 54 |
|
|
Sentinel Diagnostics |
| Related publications: None | |||
| Country: Slovenia | |||
| Funding: NR | |||
| Recruitment: NR to NR | |||
| Study design: Diagnostic cohort | |||
| McDonald 2012 13 |
|
|
Eiken Chemical Co., Tokyo, Japan |
| Related publications: None | |||
| Country: Scotland | |||
| Funding: Chief Scientist Office; analyser, training and consumables supplied by Mast Diagnostics Division | |||
| Recruitment: February 2010 to March 2012 | |||
| Study design: Diagnostic cohort | |||
| Mowat 2015 52 |
|
|
Eiken Chemical Co., Tokyo, Japan |
| Related publications: Steele 201474 | |||
| Country: Scotland | |||
| Funding: Detect cancer early, Scottish Government | |||
| Recruitment: October 2013 to March 2014 | |||
| Study design: Diagnostic cohort | |||
| Rodríguez-Alonso 2015 53 |
|
|
Eiken Chemical Co., Tokyo, Japan |
| Related publications: None | |||
| Country: Spain | |||
| Funding: Societat Catalana de Digestologia, Catalonia, Spain; Instituto de Salud Carlos III; FIS grants | |||
| Recruitment: September 2011 to October 2012 | |||
| Study design: Diagnostic cohort | |||
| Terhaar sive Droste 2011 58 |
|
|
|
| Related publications: Oort 2011;63,69 van Turenhout 2014;59 van Turenhout 2012;62 van Turenhout 2011;68 Oort 2010;64 van Turenhout 2012;60 Larbi 2012;67 van Turenhout 201070 | |||
| Country: The Netherlands | |||
| Funding: Nycomed BV; Hoofddorp to ‘the Amsterdam Gutclub’; the OC-Sensor MICRO desktop analyser was provided by Eiken Chemical Co., Tokyo, Japan | |||
| Recruitment: May 2006 to May 2010 | |||
| Study design: Diagnostic cohort | |||
| Thomas 2016 75 |
|
|
Kyowa Medex, Tokyo, Japan |
| Related publications: None | |||
| Country: England | |||
| Funding: Reagents and FIT devices provided by industry | |||
| Recruitment: March 2015 to March 2016 | |||
| Study design: Diagnostic cohort |
| Study details | Index test details | Reference standard details | ||||
|---|---|---|---|---|---|---|
| Test analyser | Thresholds (µg Hb/g faeces) | Target conditions | Sample collection, storage and processing | Reference standard details and information recorder | Definition of the target condition | |
| Auge 201657 |
|
> 0, ≥ 10, ≥ 20, ≥ 30, ≥ 40 |
|
|
|
|
| Cubiella 2014 (COLONPREDICT)55 |
|
≥ 20 |
|
|
|
|
| Godber 201656 |
|
≥ 10, ≥ 15, ≥ 20, ≥ 25, ≥ 30, ≥ 35, ≥ 40 |
|
|
|
|
| Hospital Clinic de Barcelona 2015 | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) | (Confidential information has been removed) |
| Krivec 201154 |
|
≥ 9 |
|
|
|
|
| McDonald 201213 |
|
≥ 10 |
|
|
|
|
| Mowat 201552 |
|
≥ 10, ≥ 0 |
|
|
|
|
| Rodríguez-Alonso 201553 |
|
≥ 20, ≥ 15, ≥ 10, ≥ 0 |
|
|
|
|
| Terhaar sive Droste 201158 |
|
≥ 10, ≥ 15, ≥ 20, ≥ 30, ≥ 40 |
|
|
|
|
| Thomas 201675 |
|
≥ 7 |
|
|
|
|
Appendix 3 Assessments of study quality
A. QUADAS-2 assessments
Auge 201657
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients, requiring colonoscopy for lower abdominal symptoms or polyp surveillance | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Patients referred for colonoscopy, some polyp surveillance patients included, symptoms not specified | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected two consecutive faecal samples, which were stored at 4 °C until analysis. Faecal immunochemical testing was conducted before colonoscopy | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Colonoscopy was performed blind to the results of the symptoms questionnaire and faecal immunochemical testing | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| All participants appeared to have been included in the analysis. Colonoscopy was conducted 5 days after the index test | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | Yes |
| Could the patient flow have introduced bias? | RISK: Low |
Cubiella 2014 (COLONPREDICT)55
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Consecutive patients, with GI symptoms, referred from primary and secondary health care | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Patients referred from a mixture of primary and secondary care. Symptoms included some not specified in NG121 (e.g. rectal bleeding) | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| No details of sample collection and storage. Faecal immunochemical testing was performed the week before colonoscopy | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Colonoscopy was performed blind to the results of the symptoms questionnaire and faecal immunochemical testing | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Faecal immunochemical testing was performed 1 week before colonoscopy; 26 patients who did not return a faecal sample and 12 who did not attend for colonoscopy were excluded from the study | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: Low |
Godber 201656
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Patients who had been referred from primary care for endoscopic examination of the lower GI tract | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Patients who were referred for colonoscopy for reasons, which include the following: altered bowel habit, constipation or diarrhoea, abdominal pain, fresh rectal bleeding, follow-up of previous disease, anaemia, weight loss and abnormal CT findings | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected single faecal samples and returned by post. Sample stored at 4 °C. Samples were analysed using a HM-JACKarc automated immunoturbidimetric analyser. The index test was conducted before colonoscopy. ROC analysis was reported, but data were extracted for a range of specified thresholds | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Colonoscopy was performed by the endoscopy service | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| The time interval between index test and reference standard was not reported; 402 people declined to participate, 23 (4.5%) failed colonoscopy | |
| Was there an appropriate time interval between the index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | No |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: Low |
Hospital Clinic de Barcelona 2015
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| Could the selection of patients have introduced bias? | (Confidential information has been removed) |
| B. APPLICABILITY | |
| (Confidential information has been removed) | |
| Do the included patients match the question? | (Confidential information has been removed) |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| Could the conduct or interpretation of the index test have introduced bias? | (Confidential information has been removed) |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | (Confidential information has been removed) |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| Could the reference standard, its conduct or its interpretation have introduced bias? | (Confidential information has been removed) |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | (Confidential information has been removed) |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| (Confidential information has been removed) | |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| (Confidential information has been removed) | (Confidential information has been removed) |
| Could the patient flow have introduced bias? | (Confidential information has been removed) |
Krivec 201154
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Symptomatic patients undergoing endoscopic and/or histological examinations | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Symptomatic adults undergoing endoscopy and/or histology, patients already referred and symptoms not specified | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| No details of sample collection or storage/processing were reported. The FIT cut-off point was derived from ROC analysis | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear |
| If a threshold was used, was it prespecified? | No |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: High |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Endoscopy and/or histological examination | |
| Is the reference standard likely to correctly classify the target condition? | Unclear |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: High |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| The time interval between index test and reference standard was not reported. No exclusions were reported | |
| Was there an appropriate time interval between the index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | unclear |
| Were all patients included in the analysis? | Unclear |
| Could the patient flow have introduced bias? | RISK: Unclear |
McDonald 201213
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Patients who had been referred from primary care for endoscopic examination of the lower GI tract | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Patients who were referred for colonoscopy for the following reasons: rectal bleeding, change in bowel habit, iron-deficiency anaemia, abdominal pain, bloating, polyp/CRC surveillance, family history and assessment of IBD | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected single faecal samples and returned by post. No details of sample storage. Samples were analysed using a OC-Sensor. The index test was conducted before colonoscopy. ROC analysis was reported, but data were extracted for the prespecified 50-ng/ml threshold | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Colonoscopy and flexible sigmoidoscopy were performed in the Endoscopy Unit | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Unclear |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| The median time between faecal immunochemical testing and colonoscopy was 9 days (range 1–112 days); 265 declined to participate, 15 completed faecal immunochemical testing only, 138 completed endoscopy only, 30 did not complete faecal immunochemical testing/endoscopy, 11 excluded (faecal immunochemical testing completed after endoscopy/sample not dated/sample arrived after endoscopy) | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | No |
| Did patients receive the same reference standard? | No |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Mowat 201552
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| All adult patients referred for investigation of bowel symptoms over a 6-month period | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Adult patients referred (straight to endoscopy or outpatient clinic followed by referral to endoscopy) by GP for investigation of bowel symptoms, who returned faecal tests (1043/2173) and underwent lower endoscopy. Symptoms included some not specified in NG121 (e.g. rectal bleeding). The low rate of return may mean that included participants are not representative of those who would undergo the test in practice | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected samples from a single faeces, which were returned to the GP surgery at room temperature and stored at 4 °C (FHb) or –20 °C (FC) prior to analysis to ensure stability. FHb measurement was performed using a single OC-Sensor IO analyser. Any FHb sample that was reported by the analytical system as a positive numerical result of > 0 mg/g was considered as a ‘detectable FHb’. Samples with results above the upper analytical limits were not diluted and reassayed but reported as greater than that upper concentration limit. FHb results were converted from the instrument-generated ‘ng/ml’ to the internationally recommended unit of ‘mg/g’ by multiplying by 0.2. The laboratory has a total quality management system in place and is accredited to ISO 15189-based standards | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Patients referred to endoscopy were investigated within 6 weeks of referral. The endoscopy units participate in the accreditation scheme of the Joint Accreditation Group on GI Endoscopy. Participating clinicians and endoscopists were blind to the faecal test results. All findings were recorded on the endoscopy reporting system by the endoscopist. The diagnoses of CRC, HRA and IBD were confirmed following assessment by a GI pathologist | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Patients referred for endoscopy were investigated within 6 weeks of referral; 1003 patients not returning the faecal test, 114 patients not referred by GP, 18 patients with a cancelled referral, 60 patients cancelling or not attending endoscopy, 78 patients referred to outpatients but not referred for endoscopy | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | No |
| Did patients receive the same reference standard? | yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
Rodríguez-Alonso 201553
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Symptomatic, adult patients referred for diagnostic colonoscopy | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Symptomatic adult patients referred for colonoscopy. Symptoms included some not specified in NG121 (e.g. rectal bleeding); 66.3% of participants were referred from primary care | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected single faecal samples and were asked to store the sample at < 4 °C and to return it within 7 days. Samples were analysed using OC-Sensor and a MICRO desktop analyser (Eiken). The index test was conducted before colonoscopy | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Colonoscopies were performed by experienced endoscopists who were blind to FIT results | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Time to colonoscopy was determined by the level of Hb detected and NICE/SIGN guidelines. All colonoscopies were completed within 16 weeks; 19 patients not returning the faecal test were excluded from the study; 22 patients with incomplete colonoscopy, and 10 patients who did not attend for colonoscopy, were excluded from the analyses | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | No |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: Low |
Terhaar sive Droste 201158
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| All adult patients referred for colonoscopy to one of five hospitals in the Netherlands; subgroup data for symptomatic patients were provided by the lead author | |
| Was a consecutive or random sample of patients enrolled? | Yes |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Yes |
| Could the selection of patients have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Symptomatic adult patients referred for colonoscopy. Symptoms included some not specified in NG121 (e.g. rectal bleeding). Testing was conducted after referral for colonoscopy | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| Patients collected a sample on the day before colonoscopy and before the start of bowel preparation. Samples were stored at –5 °C and analysed within 1 week. Technicians performing FIT analyses were blind to clinical data | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| Endoscopists were blinded to the results of faecal immunochemical testing | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| Colonoscopy was conducted 1 day after faecal immunochemical testing. Patients with incomplete colonoscopy or inadequate bowel cleansing, as judged by the endoscopist (number not reported), were excluded from the analyses | |
| Was there an appropriate time interval between the index test and reference standard? | Yes |
| Did all patients receive a reference standard? | Yes |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: Unclear |
Thomas 201675
DOMAIN 1: PATIENT SELECTION
| A. RISK OF BIAS | |
|---|---|
| Symptomatic patients were recruited after their first 2-week wait clinic; 64 patients were excluded after faecal immunochemical testing (no reason reported) | |
| Was a consecutive or random sample of patients enrolled? | Unclear |
| Was a case–control design avoided? | Yes |
| Did the study avoid inappropriate exclusions? | Unclear |
| Could the selection of patients have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Symptomatic adults, tested after referral to secondary care | |
| Do the included patients match the question? | Concerns: High |
DOMAIN 2: INDEX TEST(S)
| A. RISK OF BIAS | |
|---|---|
| No details of sample collection or storage/processing were reported. The cut-off point was predefined as the lower limit of the assay. CT/colonoscopy was conducted after faecal immunochemical testing | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes |
| If a threshold was used, was it prespecified? | Yes |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: Low |
| B. APPLICABILITY | |
| Are there concerns that the index test, its conduct or interpretation differ from the review question? | Concerns: Low |
DOMAIN 3: REFERENCE STANDARD
| A. RISK OF BIAS | |
|---|---|
| CT/colonoscopy, no further details reported | |
| Is the reference standard likely to correctly classify the target condition? | Yes |
| Were the reference standard results interpreted without knowledge of the results of the index test? | Unclear |
| Could the reference standard, its conduct or its interpretation have introduced bias? | RISK: Unclear |
| B. APPLICABILITY | |
| Is there concern that the target condition, as defined by the reference standard, does not match the review question? | Concerns: Low |
DOMAIN 4: FLOW AND TIMING
| A. RISK OF BIAS | |
|---|---|
| The time between faecal immunochemical testing and colonoscopy was not reported; 259 patients not returning the faecal test were excluded from the study, 64 patients were excluded after faecal testing (no reason for exclusion was reported and it was unclear whether these patients underwent colonoscopy) | |
| Was there an appropriate time interval between the index test and reference standard? | Unclear |
| Did all patients receive a reference standard? | Unclear |
| Did patients receive the same reference standard? | Yes |
| Were all patients included in the analysis? | No |
| Could the patient flow have introduced bias? | RISK: High |
B. PROBAST assessments
Cubiella 2015 (COLONPREDICT)73 and personal communication,* ahead of publication
(*Callum Fraser, personal communication.)
| DOMAIN 1: Participant selection | |||
|---|---|---|---|
| A. Risk of bias | |||
| Describe the sources of data and criteria for participant selection: The development cohort consisted of consecutive, symptomatic patients included in a previous diagnostic accuracy study.55 The validation cohort consisted of patients included in four further published studies,13,52,53,56 plus an additional unpublished cohort. FIT assay methods differed between studies and within the unpublished cohort | |||
| Dev | Val | ||
| 1. Were participants defined and assessed in a similar way to participants in the model development study? | No | ||
| 2. Were appropriate data sources used, e.g. cohort, RCT or nested case–control study data? | Yes | Yes | |
| 3. Were all inclusions and exclusions of participants appropriate? | Yes | Yes | |
| Risk of bias introduced by selection of participants | RISK: (low/high/unclear) | Low | Low |
| Rationale of bias rating: FIT assay methods differed between studies and within the unpublished cohort used in validation. However, the effect of different assay methods on risk score performance was assessed | |||
| B. Applicability | |||
| Describe included participants, setting and dates: Symptomatic adult patients referred for colonoscopy. Symptoms included some not specified in NG121 (e.g. rectal bleeding). Not all participants were referred from primary care | |||
| Concern that the included participants and setting do not match the review question | CONCERN: (low/high/unclear) | High | High |
| Rationale of applicability rating: The study population is not a complete match for that specified in the scope for this assessment | |||
| DOMAIN 2: Predictors | |||
|---|---|---|---|
| A. Risk of bias | |||
| List and describe predictors included in the final model, e.g. definition and timing of assessment: Sex, age (continuous variable) and ‘µg Hb/g faeces’ (categorical: undetectable, 0–19, 20–199 and ≥ 200). No further details were reported. Colonoscopy was conducted after faecal immunochemical testing | |||
| Dev | Val | ||
| 1. Were predictors defined and assessed in a similar way for all participants? | Yes | No | |
| 2. Were predictors defined and assessed in a similar way to predictors in the development model? | No | ||
| 3. Were predictor assessments made without knowledge of outcome data? | Yes | Yes | |
| 4. Are all predictors available at the time the model is intended to be used? | Yes | Yes | |
| Risk of bias introduced by predictors or their assessment | RISK: (low/high/unclear) | High | High |
| Rationale of bias rating: FIT methods used in validation differed from those used in development | |||
| B. Applicability | |||
| Concern that the definition, assessment or timing of predictors in the model do not match the review question | CONCERN: (low/high/unclear) | High | High |
| Rationale of applicability rating: Faecal immunochemical testing used OC-Sensor, but was undertaken after patients had already been referred for colonoscopy | |||
| DOMAIN 3: Outcome | |||
|---|---|---|---|
| A. Risk of bias | |||
| Describe the outcome and how it was defined and determined: Models were reported for the outcomes CRC and advanced neoplasia, both determined by colonoscopy. The definition of advanced neoplasia varied between the studies used in the validation cohort | |||
| Dev | Val | ||
| 1. Was the outcome determined appropriately? | Yes | Yes | |
| 2. Was a prespecified or standard outcome definition used? | Yes | No | |
| 3. Were predictors excluded from the outcome definition? | Yes | Yes | |
| 4. Was the outcome defined and determined in a similar way for all participants? | Yes | No | |
| 5. Was the outcome defined and determined in a similar way to the outcome in the model development study? | No | ||
| 6. Was the outcome determined without knowledge of predictor information? | Unclear | Unclear | |
| Risk of bias introduced by the outcome or its determination | RISK: (low/high/unclear) | Low | High |
| Rationale of bias rating: The definition of HRA varied between the studies used in the validation cohort. | |||
| B. Applicability | |||
| At what time point was the outcome determined: The time point at which the outcome was determined varied between studies, but all colonoscopies were completed within 16 weeks | |||
| If a composite outcome was used, describe the relative frequency/distribution of each contributing outcome: NA | |||
| Concern that the outcome, its definition, timing or determination do not match the review question | CONCERN: (low/high/unclear) | Low | Low |
| Rationale of applicability rating: | |||
| DOMAIN 4: Sample size and participant flow | |||
|---|---|---|---|
| Risk of bias | |||
| Describe numbers of participants, number of candidate predictors, outcome events and events per candidate predictor: 1572 participants were included in the development sample and 3976 in the validation sample. The number of candidate variables and total number of participants with the outcome were not reported | |||
| Describe the time interval between predictor assessment and outcome determination: The time between predictor and outcome assessment varied between studies included in the validation cohort, but the maximum time between predictor assessment and outcome determination was 16 weeks | |||
| Describe any participants who were excluded from the analysis: Participants who did not complete faecal immunochemical testing or colonoscopy were excluded | |||
| Describe missing data on predictors and outcomes, as well as methods used for missing data: No details were reported | |||
| Dev | Val | ||
|
Unclear | Unclear | |
|
Yes | Yes | |
|
No | No | |
|
Unclear | Unclear | |
| Risk of bias introduced by sample size or participant flow | RISK: (low/high/unclear) | Unclear | Unclear |
| Rationale of bias rating: Modelling methods were not reported in sufficient detail to adequately assess risk of bias | |||
| DOMAIN 5: Analysis | |||
|---|---|---|---|
| Risk of bias | |||
| Describe how the model was developed (predictor selection, optimism, risk groups, model performance): No details reported | |||
| Describe whether and how the model was validated, either internally (e.g. bootstrapping, cross validation, random split sample) or externally (e.g. temporal validation, geographical validation, different setting, different type of participants): The external validation cohort comprised five different studies, which were separate from the study used as the development cohort | |||
| Describe the performance measures of the model, e.g. (re)calibration, discrimination, (re)classification, net benefit: R2 (measure of variation), Akaike information criterion and Bayesian information criterion. The study describes the diagnostic accuracy of a risk score based on the model results | |||
| Dev | Val | ||
| 1. Were continuous and categorical predictors handled appropriately? | Yes | ||
| 2. Was selection of predictors based on univariable analysis avoided? | Unclear | ||
| 3. Were any complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | Unclear | Unclear | |
| 4. Were relevant model performance measures evaluated, e.g. (re)calibration and discrimination? | Unclear | Unclear | |
| 5. Was model overfitting and optimism in model performance accounted for? | Unclear | ||
| 6. Do predictors and their assigned weights in the final model correspond with the results from multivariable analysis? | Unclear | ||
| Risk of bias introduced by the analysis | RISK: (low/high/unclear) | Unclear | Unclear |
| Rationale of bias rating: Modelling methods were not reported in sufficient detail to adequately assess the risk of bias | |||
| Overall judgement about risk of bias and applicability of the prediction model evaluation | ||
|---|---|---|
| Overall judgement of risk of bias | RISK: (low/high/unclear) | High |
| Summary of sources of potential bias: Modelling methods were not reported in sufficient detail to adequately assess the risk of bias; however, faecal immunochemical testing methods and advanced neoplasia definitions varied between the studies included in the validation cohort | ||
| Overall judgement of applicability | CONCERN: (low/high/unclear) | High |
| Summary of applicability concerns: The study population is not a complete match for that specified in the scope for this assessment | ||
Rodríguez-Alonso 201553
| DOMAIN 1: Participant selection | |||
|---|---|---|---|
| A. Risk of bias | |||
| Describe the sources of data and criteria for participant selection: Participants were patients included in prospective diagnostic accuracy study. The study included patients aged > 18 years, referred for diagnostic colonoscopy between September 2011 and October 2102. Patients referred for adenoma surveillance or post-surgical surveillance of CRC, and those with a history of previous colectomy, IBD or polyp syndromes were excluded. Diagnosis was established by colonoscopy. Validation of the model used a split sample procedure | |||
| Dev | Val | ||
| 1. Were participants defined and assessed in a similar way to participants in the model development study? | Yes | ||
| 2. Were appropriate data sources used, e.g. cohort, RCT or nested case–control study data? | Yes | Yes | |
| 3. Were all inclusions and exclusions of participants appropriate? | Yes | Yes | |
| Risk of bias introduced by selection of participants | RISK: (low/high/unclear) | Low | Low |
| Rationale of bias rating: | |||
| B. Applicability | |||
| Describe included participants, setting and dates: Symptomatic adult patients referred for colonoscopy. Symptoms included some not specified in NG121 (e.g. rectal bleeding); 66.3% of participants were referred from primary care | |||
| Concern that the included participants and setting do not match the review question | CONCERN: (low/high/unclear) | High | High |
| Rationale of applicability rating: The study population is not a complete match for that specified in the scope for this assessment | |||
| DOMAIN 2: Predictors | |||
|---|---|---|---|
| A. Risk of bias | |||
| List and describe predictors included in the final model, e.g. definition and timing of assessment: Male gender, iron-deficiency anaemia (not defined) and Hb level ≥ 10 µg/g faeces were included in the final model for CRC. No further details were reported. Colonoscopy was conducted after faecal immunochemical testing and other initial assessments, and endoscopists were blinded to test results | |||
| Dev | Val | ||
| 1. Were predictors defined and assessed in a similar way for all participants? | Unclear | Unclear | |
| 2. Were predictors defined and assessed in a similar way to predictors in the development model? | Unclear | ||
| 3. Were predictor assessments made without knowledge of outcome data? | Yes | Yes | |
| 4. Are all predictors available at the time the model is intended to be used? | Yes | Yes | |
| Risk of bias introduced by predictors or their assessment | RISK: (low/high/unclear) | Unclear | Unclear |
| Rationale of bias rating: Reporting of the model development and validation methods was limited | |||
| B. Applicability | |||
| Concern that the definition, assessment or timing of predictors in the model do not match the review question | CONCERN: (low/high/unclear) | High | High |
| Rationale of applicability rating: Faecal immunochemical testing used OC-Sensor, but was undertaken after patients had already been referred for colonoscopy and attended an assessment appointment | |||
| DOMAIN 3: Outcome | |||
|---|---|---|---|
| A. Risk of bias | |||
| Describe the outcome and how it was defined and determined: Models were reported for the outcomes CRC and advanced neoplasia, both determined by colonoscopy. Advanced neoplasia was defined as CRC or HRA (lesion ≥ 10-mm diameter, villous component or high-grade dysplasia). Endoscopists were blinded to test results | |||
| Dev | Val | ||
| 1. Was the outcome determined appropriately? | Yes | Yes | |
| 2. Was a prespecified or standard outcome definition used? | Yes | Yes | |
| 3. Were predictors excluded from the outcome definition? | Yes | Yes | |
| 4. Was the outcome defined and determined in a similar way for all participants? | Yes | Yes | |
| 5. Was the outcome defined and determined in a similar way to the outcome in the model development study? | Yes | ||
| 6. Was the outcome determined without knowledge of predictor information? | Yes | Yes | |
| Risk of bias introduced by the outcome or its determination | RISK: (low/high/unclear) | Low | Low |
| Rationale of bias rating: | |||
| B. Applicability | |||
| At what time point was the outcome determined: All colonoscopies were completed within 16 weeks | |||
| If a composite outcome was used, describe the relative frequency/distribution of each contributing outcome: NA | |||
| Concern that the outcome, its definition, timing or determination do not match the review question | CONCERN: (low/high/unclear) | Low | Low |
| Rationale of applicability rating: | |||
| DOMAIN 4: Sample size and participant flow | |||
|---|---|---|---|
| Risk of bias | |||
| Describe numbers of participants, number of candidate predictors, outcome events and events per candidate predictor: 680 participants were included in the development sample and 323 in the validation sample. There were 14 candidate variables in the initial analysis. A total of 30 study participants had CRC and 133 had advanced neoplasia | |||
| Describe the time interval between predictor assessment and outcome determination: The maximum time between predictor assessment and outcome determination was 16 weeks | |||
| Describe any participants who were excluded from the analysis: Participants who did not complete faecal immunochemical testing or colonoscopy were excluded | |||
| Describe missing data on predictors and outcomes, as well as methods used for missing data: No details were reported | |||
| Dev | Val | ||
| 1. Were there a reasonable number of participants with the outcome? | Unclear | Unclear | |
| 2. Was the time interval between predictor assessment and outcome determination appropriate? | Yes | Yes | |
| 3. Were all enrolled participants included in the analysis? | No | No | |
| 4. Were participants with missing data handled appropriately? | Unclear | Unclear | |
| Risk of bias introduced by sample size or participant flow | RISK: (low/high/unclear) | Unclear | Unclear |
| Rationale of bias rating: Modelling methods were not reported in sufficient detail to adequately assess risk of bias | |||
| DOMAIN 5: Analysis | |||
|---|---|---|---|
| Risk of bias | |||
| Describe how the model was developed (predictor selection, optimism, risk groups, model performance): A multivariate analysis based on forward conditional logistic regression was performed. Factors were included in the multivariate models, based on based on their univariate association with CRC or advanced neoplasia (p < 0.05). Factors not reaching statistical significance were also included if they were judged to be ‘clinically relevant or biologically plausible’ | |||
| Describe whether and how the model was validated, either internally (e.g. bootstrapping, cross validation, random split sample) or externally (e.g. temporal validation, geographical validation, different setting, different type of participants): The internal validity of the model was assessed using a random split sampling technique. No further details were reported | |||
| Describe the performance measures of the model, e.g. (re)calibration, discrimination, (re)classification, net benefit: Not reported; the study describes the diagnostic accuracy of a risk score based on the model results | |||
| Dev | Val | ||
| 1. Were continuous and categorical predictors handled appropriately? | Unclear | ||
| 2. Was selection of predictors based on univariable analysis avoided? | No | ||
| 3. Were any complexities in the data (e.g. competing risks, multiple events per individual) accounted for appropriately? | Unclear | Unclear | |
| 4. Were relevant model performance measures evaluated, e.g. (re)calibration and discrimination? | Unclear | Unclear | |
| 5. Was model overfitting and optimism in model performance accounted for? | Unclear | ||
| 6. Do predictors and their assigned weights in the final model correspond to the results from multivariable analysis? | Unclear | ||
| Risk of bias introduced by the analysis | RISK: (low/high/unclear) | High | High |
| Rationale of bias rating: Modelling methods were not reported in sufficient detail to adequately assess the risk of bias; however, selection of predictors based on univariate analysis was reported | |||
| Overall judgement about risk of bias and applicability of the prediction model evaluation | ||
|---|---|---|
| Overall judgement of risk of bias | RISK: (low/high/unclear) | High |
| Summary of sources of potential bias: Modelling methods were not reported in sufficient detail to adequately assess the risk of bias; however, selection of predictors based on univariate analysis was reported | ||
| Overall judgement of applicability | CONCERN: (low/high/unclear) | High |
| Summary of applicability concerns: The study population is not a complete match for that specified in the scope for this assessment | ||
Appendix 4 Excluded studies
To be included in the review, studies had to fulfil the following criteria:
-
Population Adults (≥ 18 years of age) presenting with symptoms suggestive of possible CRC.
-
Setting Primary care.
-
Index test Quantitative faecal immunochemical testing [OC-Sensor, HM-JACKarc, FOB Gold, RIDASCREEN Hb (R-Biopharm, Darmstadt, Germany), RIDASCREEN Hb/Hp complex].
-
Reference standard Colonoscopy.
-
Outcome Sufficient data to construct 2 × 2 table of test performance.
The table below summarises studies that were screened for inclusion, based on full-text publication, but which did not fulfil one or more of the above criteria. Studies were assessed sequentially against criteria; the first criterion failed is classified as the reason for exclusion. The table shows which of the criteria each study fulfilled (‘Yes’) and on which items it failed (‘No’), as well as any which were ‘Unclear’. Articles that did not report primary research were not assessed further. Any criteria that are not applicable to a study are marked ‘NA’. When population was the only reason for exclusion and studies reported a mixed population, authors were contacted to request subgroup data for symptomatic patients; this is noted in the comments column.
| Study details | Primary study | Population | Setting | Index test | Reference standard | Outcome | Reason for exclusion and comments |
|---|---|---|---|---|---|---|---|
| Abdullah M, Simadibrata M, Syam AF, Wijayadi T, Fauzi A, Santi A, et al. The accuracy of fecal immunochemical test in early detection of colorectal cancer. J Gastroenterol Hepatol 2010;25:A138 | Yes | Unclear | Unclear | No | Yes | Yes | No relevant index test |
| Qualitative FIT | |||||||
| Population unclear, participants undergoing colonoscopy for any indication | |||||||
| Allison JE, Fraser CG, Halloran SP, Young GP. Comparing fecal immunochemical tests: improved standardization is needed. Gastroenterology 2012;142:422–4 | No | Not primary research | |||||
| Banaszkiewicz Z, Jawien A, Jarmocik P, Tojek K, Jankowski M, Switonski M. [Evaluation of usefulness of faecal occult blood test. Prospective screening study in patients with colorectal neoplasia.] Pol Merkuriusz Lek 2004;17:579–82 | Yes | Unclear | Unclear | No | Unclear | Unclear | No relevant index test |
| Polish-language publication | |||||||
| Qualitative FIT | |||||||
| Bjerregaard NC, Tottrup A, Sorensen HT, Laurberg S. Detection of colorectal cancer in symptomatic outpatients without visible rectal bleeding: validity of the faecal occult blood test. Clin Epidemiol 2009;1:119–24 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| gFOBT only | |||||||
| Bonfrate L, Ruggiero V, Dambrosio P, Castorani L, De Bari O, Larizza M, et al. Double vs. standard fecal occult blood testing (FOBT) in patients with alarm symptoms of colorectal cancer (CRC). Eur J Clin Invest 2013;43:71 | Yes | Yes | Unclear | No | Yes | Yes | No relevant index test |
| Qualitative FIT | |||||||
| Carroll M, Piggott C, Pearson S, Seaman HE, Bruce H, Halloran SP. An evaluation of quantitative faecal immunochemical tests for haemoglobin. British Society of Gastroenterology Annual Meeting. Manchester, 2014:89 | Yes | No | Unclear | Yes | No | No | Not a symptomatic population |
| Spiked samples, technical performance only | |||||||
| Castro I, Estevez P, Cubiella J, Hernandez V, Gonzalez-Mao C, Rivera C, et al. Diagnostic performance of fecal immunochemical test and sigmoidoscopy for advanced right-sided colorectal neoplasms. Dig Dis Sci 2015;60:1424–32 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Average risk cohort of asymptomatic men | |||||||
| Chen JG, Cai J, Wu HL, Xu H, Zhang YX, Chen C, et al. Colorectal cancer screening: comparison of transferrin and immunofecal occult blood test. World J Gastroenterol 2012;18:2682–8 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic surveillance | |||||||
| Qualitative FIT | |||||||
| Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Immunochemical FOBT was accurate for detecting colorectal cancer but less so for other GI lesions. Ann Intern Med 2012;156:JC2–10 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Screening with qualitative FIT | |||||||
| Chiu HM, Wu MS, Lee YC, Liao WC, Wang HP, Lin JT. Fecal immunochemical test has lower performance in detecting early and proximal advanced colorectal neoplasm. Gastroenterology 2012;142(5 Suppl. 1):145 | Yes | No | No | No | Yes | Yes | Not a symptomatic population |
| Screening population | |||||||
| Cole SR, Upton J, Lane JM, Young GP. A faecal immunochemical test for haemoglobin using a single stool sample is effective for detecting significant colorectal neoplasia. J Gastroenterol Hepatol 2009;24:A239 | Yes | Unclear | Unclear | No | Yes | Yes | No relevant index test |
| Unclear population, all patients scheduled for colonoscopy | |||||||
| No relevant FIT | |||||||
| Crouse AL, De Koning L, Sadrzadeh SM, Naugler C. Sensitivity and specificity of community fecal immunotesting screening for colorectal carcinoma in a high-risk Canadian population. Arch Pathol Lab Med 2015;139:1441–5 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Screening population | |||||||
| Dutta AK, Alagammai P, Chowdhury SD, Chacko A. Preprocedure haemoglobin and fecal occult blood testing for optimising colonoscopy rates in a resource limited setting for diagnosing colonic malignancy. Gastrointest Endosc 2012;75(4 Suppl. 1):AB325–6 | Yes | No | Unclear | No | Yes | No | Not a symptomatic population |
| gFOBT only, test performance only reported for combined gFOBT and anaemia | |||||||
| Fenocchi E, Gaggero P, Rondan M, Lopez-Alvarenga JC, Sobrino-Cossio S, Lambert N, et al. Usefulness of the fecal immunochemical test in the detection of advanced adenomas in subjects at average risk for colorectal cancer. Endoscopia 2015;27:64–8 | Yes | Yes | Unclear | Yes | Yes | No | No relevant outcomes |
| Separate data for symptomatic and asymptomatic patients | |||||||
| Reported outcome was mean FIT level for different adenoma sizes | |||||||
| Gillberg A, Ericsson E, Granstrom F, Olsson LI. A population-based audit of the clinical use of faecal occult blood testing in primary care for colorectal cancer. Colorectal Dis 2012;14:e539–46 | Yes | Unclear | Unclear | No | No | Yes | No relevant index test |
| Retrospective study, | |||||||
| Symptoms data collected only for those patients with a diagnosis of CRC | |||||||
| Gopalswamy N, Stelling HP, Markert RJ, Maimon HN, Wahlen SD, Haddy RI. A comparative study of eight fecal occult blood tests and HemoQuant in patients in whom colonoscopy is indicated. Arch Fam Med 1994;3:1043–8 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic, asymptomatic high risk and surveillance | |||||||
| No relevant FIT (gFOBT only) | |||||||
| Greenberg PD, Bertario L, Gnauck R, Kronborg O, Hardcastle JD, Epstein MS, et al. A prospective multicenter evaluation of new fecal occult blood tests in patients undergoing colonoscopy. Am J Gastroenterol 2000;95:1331–8 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic screening, history of CRC or polyps, or family history (30% symptomatic) | |||||||
| No relevant FIT technology | |||||||
| Haug U, Kuntz KM, Knudsen AB, Hundt S, Brenner H. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer 2011;104:1779–85 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Average risk screening population | |||||||
| Hogberg C, Karling P, Rutegard J, Lilja M, Ljung T. Immunochemical faecal occult blood tests in primary care and the risk of delay in the diagnosis of colorectal cancer. Scand J Prim Health Care 2013;31:209–14 | Yes | No | Unclear | Unclear | NA | No | Patients with an established cancer diagnosis |
| Retrospective study of cancer patients who had received qualitative FIT | |||||||
| Reports sensitivity of FIT and delay to diagnosis following a negative FIT | |||||||
| Iwase N, Oya M, Yanagida T, Hirayasu Y, Ishii Y, Kubota T, et al. [Immunological fecal occult blood test in patients with anal diseases.] Nihon Daicho Komonbyo Gakkai Zasshi 1995;48:1065–9 | Yes | Yes | Unclear | No | Yes | No | No relevant index test |
| Japanese-language publication, gFOBT only | |||||||
| Only sensitivity data reported | |||||||
| Jin P, Wu Z, Meng M, Wang X, Gong L, Yu D, et al. Combined fecal transferrin test and immuno fecal occult blood test for detecting colorectal cancer and advanced adenoma in asymptomatic and symptomatic populations. J Cancer Sci Ther 2012;4:243–8 | Yes | Yes | Unclear | No | Yes | No | No relevant index test |
| Qualitative FIT, no accuracy data | |||||||
| Kalimutho M, Del Vecchio Blanco G, Cretella M, Mannisi E, Sileri P, Formosa A, et al. A simplified, non-invasive fecal-based DNA integrity assay and iFOBT for colorectal cancer detection. Int J Colorectal Dis 2011;26:583–92 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| Qualitative FIT | |||||||
| Kaul A, Shah A, Magill FH, Hawkins SA, Skaife P. Immunological faecal occult blood testing: a discriminatory test to identify colorectal cancer in symptomatic patients. Int J Surg 2013;11:329–31 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| Qualitative FIT | |||||||
| Kennell M, Antle S, Hammond M, Stone S, Mahar D, Randell E. Evaluation of the analytical and diagnostic performance of the iFOBT NS-Plus system for use in a province-wide colorectal cancer screening program. Clin Biochem 2012;45:1116 | Yes | Unclear | Unclear | No | Yes | Yes | No relevant index test |
| Qualitative FIT | |||||||
| Ko CW, Dominitz JA, Nguyen TD. Fecal occult blood testing in a general medical clinic: comparison between guaiac-based and immunochemical-based tests. Am J Med 2003;115:111–14 | Yes | No | Unclear | No | No | No | Not a symptomatic population |
| Screening with qualitative FIT | |||||||
| Kovarova JT, Zavoral M, Zima T, Zak A, Kocna P, Kohout P, et al. Improvements in colorectal cancer screening programmes – quantitative immunochemical faecal occult blood testing – how to set the cut-off for a particular population. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2012;156:143–50 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic, previous CRC, family history (32% symptomatic) – authors contacted, reply received stating that subgroup data are not available | |||||||
| Leicester RJ, Lightfoot A, Millar J, Colin-Jones DG, Hunt RH. Accuracy and value of the Hemoccult test in symptomatic patients. BMJ 1983;286:673–4 | Yes | Yes | Yes | No | No | Yes | No relevant index test |
| gFOBT only – reference standard proctosigmoidoscopy and double-contrast barium enema (colonoscopy in patients with normal findings on these tests who had positive FOBT results) | |||||||
| Leodolter A, Zielinski D, Vieth M, Labenz J. Comparison of different immunological FOBTs for colorectal cancer screening: wide range of sensitivity between different rapid tests. Gastroenterology 2010;138(5 Suppl. 1):S159 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic (approximately 66% symptomatic) – authors contacted, no reply received | |||||||
| Levi Z, Hazazi R, Rozen P, Vilkin A, Waked A, Niv Y. A quantitative immunochemical faecal occult blood test is more efficient for detecting significant colorectal neoplasia than a sensitive guaiac test. Aliment Pharmacol Ther 2006;23:1359–64 | Yes | No | No | Yes | Yes | Yes | Not a symptomatic population |
| Asymptomatic screening | |||||||
| Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med 2007;146:244–55 | Yes | No | Yes | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population symptomatic and asymptomatic screening or high risk (47% symptomatic) – authors contacted, no reply receiveda | |||||||
| Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. Sensitivity, but not specificity, of a quantitative immunochemical fecal occult blood test for neoplasia is slightly increased by the use of low-dose aspirin, NSAIDs, and anticoagulants. Am J Gastroenterol 2009;104:933–8 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, increased risk and mildly symptomatic, proportions not specified – authors contacted, no reply receiveda | |||||||
| Levy BT, Bay C, Xu Y, Daly JM, Bergus G, Dunkelberg J, et al. Test characteristics of faecal immunochemical tests (FIT) compared with optical colonoscopy. J Med Screen 2014;21:133–43 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population (symptomatic, screening or surveillance), proportion not reported | |||||||
| No relevant FIT technology | |||||||
| Luthgens K, Maier A, Kampert I, Sieg A, Schmidt-Gayk H. Hemoglobin-haptoglobin-complex: a highly sensitive assay for the detection of fecal occult blood. Clin Lab 1998;44:543–51 | Yes | Unclear | Unclear | No | Yes | Yes | No relevant index test |
| Participants from a gastroenterological practice; unclear if symptomatic | |||||||
| Study is of a development version of the test, not that which is currently marketed | |||||||
| McDonald R, Tomlins A, Smith S, Harmston C. Outcomes of faecal occult blood tests requested outside the UK National Bowel Cancer Screening Programme. J Clin Pathol 2013;66:330–4 | Yes | Unclear | Unclear | No | NA | No | No relevant index test |
| Unclear population, survey of testing requests before and after introduction of screening | |||||||
| Miyoshi H, Oka M, Sugi K, Saitoh O, Katsu K, Uchida K. Accuracy of detection of colorectal neoplasia using an immunochemical occult blood test in symptomatic referred patients: comparison of retrospective and prospective studies. Intern Med 2000;39:701–6 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Qualitative FIT | |||||||
| Narula N, Ulic D, Al-Dabbagh R, Ibrahim A, Mansour M, Balion C, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol 2014;28:421–6 | Yes | No | No | No | Yes | Yes | Not a symptomatic population |
| Hospital inpatients, not all symptomatic | |||||||
| Type of FOB test not reported | |||||||
| Niv Y, Sperber AD. Sensitivity, specificity, and predictive value of fecal occult blood testing (Hemoccult II) for colorectal neoplasia in symptomatic patients: a prospective study with total colonoscopy. Am J Gastroenterol 1995;90:1974–7 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| gFOBT only | |||||||
| Ogawa M, Iso A, Ootsuka H, Shimizu S, Aoki Y, Tada M, et al. [Clinical evaluation of a new immunological fecal occult blood test.] Ther Res 1989;10:275–82 | Yes | No | Unclear | No | No | Yes | No relevant index test |
| Japanese-language publication | |||||||
| Qualitative FIT | |||||||
| Case–control study | |||||||
| Oono Y, Iriguchi Y, Doi Y, Tomino Y, Kishi D, Oda J, et al. A retrospective study of immunochemical fecal occult blood testing for colorectal cancer detection. Clin Chim Acta 2010;411:802–5 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| No relevant FIT technology | |||||||
| Oort FA, Droste JSTS, van Der Hulst RW, Van Heukelem H, Loffeld RJ, Wesdorp EC, et al. Flat colonic neoplasia are left undetected by fecal immunochemical tests (FIT) and will be missed in colorectal cancer screening. Gastroenterology 2009;136(5 Suppl. 1):A113 | Yes | Unclear | Unclear | Yes | Yes | No | No relevant outcomes |
| Unclear population, all patients scheduled for colonoscopy | |||||||
| No accuracy data | |||||||
| Ou CH, Kuo FC, Hsu WH, Lu CY, Yu FJ, Kuo CH, et al. Comparison of the performance of guaiac-based and two immunochemical fecal occult blood tests for identifying advanced colorectal neoplasia in Taiwan. J Dig Dis 2013;14:474–83 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population (history of CRC or polyp, family history, symptomatic, asymptomatic screening) proportions not reported – authors contacted, no reply received | |||||||
| Parente FR, Marino B, Perna F, Saracino IM, Zullo A, Hassan C, et al. Multiple faecal tests (colon panel) for the detection of colon cancer: a new strategy for appropriate prioritization of screening referrals? Preliminary experience in Italy. Gastroenterology 2010;138(5 Suppl. 1):192 | Yes | Yes | Unclear | No | Yes | Yes | No relevant index test – HM-JACKARC (no longer available in Europe), not HM-JACKarc |
| Parente F, Marino B, Perna F, Saracino I, Zullo A, Hassan C, et al. Multiple faecal tests (colon panel) for the detection of colon cancer: a new strategy for appropriate prioritization of screening referrals? Preliminary experience in Italy. Dig Liver Dis 2010;42:S86 | Yes | Yes | Unclear | No | Yes | Yes | No relevant index test – HM-JACKARC (no longer available in Europe), not HM-JACKarc |
| Parente F, Marino B, Ilardo A, Fracasso P, Zullo A, Hassan C, et al. A combination of faecal tests for the detection of colon cancer: a new strategy for an appropriate selection of referrals to colonoscopy? A prospective multicentre Italian study. Eur J Gastroenterol Hepatol 2012;24:1145–52 | Yes | Yes | Unclear | No | Yes | Yes | No relevant index test – HM-JACKARC (no longer available in Europe), not HM-JACKarc |
| Piper MA. Immunochemical versus Guaiac Fecal Occult Blood Tests. Chicago, IL: Blue Cross and Blue Shield Association, Technology Evaluation Center; 2004 | No | Not primary research | |||||
| Provisional DARE abstract, with no publication details | |||||||
| Rae AJ, Cleator IGM. The two-tier fecal occult blood test: cost effective screening. Can J Gastroenterol 1994;8:362–8 | Yes | No | Unclear | No | No | No | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic screening and surveillance, gFOBT only | |||||||
| Rao S, Forbes G, Venugopal K. High yield for advanced colorectal neoplasia (carcinoma and advanced adenoma) detection with community based faecal immunochemical testing. J Gastroenterol Hepatol 2014;29:133–4 | Yes | No | Unclear | Unclear | Yes | No | Not a symptomatic population |
| Patients referred from primary care because of a positive FIT | |||||||
| FIT method not specified | |||||||
| Rodriguez-Moranta F, Ariza X, Berrozpe A, Vazquez X, Binefa G, Navarro M, et al. Comparative study of guaiac and quantitative immunochemical fecal occult blood test for colorectal neoplasia. Preliminary results. Gastroenterology 2009;136(5 Suppl. 1):A623 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic screening, or surveillance (66% symptomatic). Unspecified FIT – authors contacted, no reply received | |||||||
| Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, et al. Quantitative colonoscopic evaluation of relative efficiencies of a quantified immunochemical fecal occult blood test and a sensitive guaiac test for detecting significant colorectal neoplasms. Gastroenterology 2009;136(5 Suppl. 1):A113 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Unclear population, ‘consecutive ambulatory colonoscopy patients, some above average risk’– authors contacted, no reply receiveda | |||||||
| Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, et al. Identification of colorectal adenomas by a quantitative immunochemical faecal occult blood screening test depends on adenoma characteristics, development threshold used and number of tests performed. Aliment Pharmacol Ther 2009;29:906–17 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and high risk asymptomatic (59% symptomatic) – authors contacted, no reply receiveda | |||||||
| Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, et al. Colonoscopic evaluation of a quantitative immunochemical fecal occult blood test to determine its optimal clinical use. Gastroenterology 2009;136(5 Suppl. 1):A624 | Yes | No | Yes | Yes | Yes | Yes | Not a symptomatic population |
| Unclear population, ‘consecutive ambulatory colonoscopy patients, some above average risk’– authors contacted, no reply receiveda | |||||||
| Rozen P, Levi Z, Hazazi R, Waked A, Vilkin A, Maoz E, et al. Quantitative colonoscopic evaluation of relative efficiencies of an immunochemical faecal occult blood test and a sensitive guaiac test for detecting significant colorectal neoplasms. Aliment Pharmacol Ther 2009;29:450–7 | Yes | No | Yes | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic screening or high risk (23% symptomatic) – authors contacted, no reply receiveda | |||||||
| Rozen P, Comaneshter D, Levi Z, Hazazi R, Vilkin A, Maoz E, et al. Cumulative evaluation of a quantitative immunochemical fecal occult blood test to determine its optimal clinical use. Cancer 2010;116:2115–25 | Yes | No | No | Yes | Yes | Yes | Not a symptomatic population |
| Screening asymptomatic | |||||||
| Sailer M. [A quantitative immunological fecal occult blood test in colorectal neoplasia.] Coloproctology 2010;32:68–70 | No | Not primary research | |||||
| Journal club, screening | |||||||
| Sailer M. [The sensitivity and specificity of guaiac and immunochemical fecal occult blood tests for the detection of advanced colonic adenomas and cancer.] Coloproctology 2013;35:148–50 | No | Not primary research | |||||
| Journal club, screening | |||||||
| Sieg A, Scheida M, John MR, Hertel A, Schroter M, Luthgens K, et al. Validity of new immunological human fecal hemoglobin and albumin tests in detecting colorectal neoplasms – an endoscopy-controlled study. Z Gastroenterol 1998;36:485–90 | Yes | Yes | Yes | No | Yes | Yes | No relevant index test |
| Early development paper (not a commercially available test) | |||||||
| Shaw AG, Lund JN, Longman C, Tierney GM, Goddard AF. The misuse of the faecal occult blood test under the lower gastrointestinal two week wait rule. Colorectal Dis 2009;11:94–6 | Yes | Yes | Yes | No | NA | No | No relevant index test |
| Survey of guaiac faecal occult blood testing prior to referral (no test performance data) | |||||||
| Symonds EL, Young GP, Osborne JM, Cole SR, Gopalsamy G, Gaur S, et al. Detection of colorectal neoplasia: comparison of a methylated two-gene (BCAT1-IKZF1) blood test with a faecal immunochemical test. J Gastroenterol Hepatol 2015;30:83 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population: states ‘scheduled for colonoscopy for any reason’ but the objective describes test evaluation in ‘a patient population exhibiting the full spectrum of pathology encountered in the colon/rectum’– authors contacted, no reply received | |||||||
| Tate JJ, Northway J, Royle GT, Taylor I. Faecal occult blood testing in symptomatic patients: comparison of three tests. Br J Surg 1990;77:523–6 | Yes | Yes | Yes | No | No | Yes | No relevant index test |
| gFOBT only | |||||||
| Patients referred for double-contrast barium enema (reference standard), assumed by the authors to be symptomatic | |||||||
| Thomas WM, Hardcastle JD, Jackson J, Pye G. Chemical and immunological testing for faecal occult blood: a comparison of two tests in symptomatic patients. Br J Cancer 1992;65:618–20 | Yes | Yes | Yes | No | Yes | No | No relevant index test |
| Only sensitivity data reported | |||||||
| Tibble J, Sigthorsson G, Foster R, Sherwood R, Fagerhol M, Bjarnason I. Faecal calprotectin and faecal occult blood tests in the diagnosis of colorectal carcinoma and adenoma. Gut 2001;49:402–8 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population, healthy controls, known CRC and referred patients (symptomatic and polyp surveillance, proportions not specified) | |||||||
| No relevant FIT technology | |||||||
| Tsumuraya M, Noda A, Tsubura S, Sugimoto K, Minowa M, Seki T, et al. [Comparative clinical study of ‘Monohem’ and four reagents for faecal occult blood test.] Ther Res 1989;10(Suppl. 1):87–95 | Yes | No | Unclear | No | No | Yes | No relevant index test |
| Japanese-language publication | |||||||
| Qualitative FIT | |||||||
| Case–control study | |||||||
| University of Aarhus. A Trial of the Implementation of iFOBT in General Practice. NCT02308384. International Clinical Trials Registry Platform. Geneva: World Health Organization; 2014. URL: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02308384 (accessed 9 March 2016) | Yes | Yes | Yes | Unclear | NA | No | No relevant outcomes |
| Ongoing study, behavioural intervention for GPs | |||||||
| University of Malaya. Quantitative Versus Qualitative Fecal Immunochemical Tests (FIT) to Prioritize Urgency of Colonoscopy Referral. NCT02037646. International Clinical Trials Registry Platform. Geneva: World Health Organization; 2014. URL: http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02037646 (accessed 9 March 2016) | Yes | No | No | Unclear | Yes | Yes | Not a symptomatic population |
| Screening study, index test and comparator unclear | |||||||
| Uppsala University. Quantitative Immunochemical Fecal Occult Blood Test in Symptomatic Patients. NCT02491593. ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2015. URL: https://ClinicalTrials.gov/show/NCT02491593 (accessed 9 March 2016) | Yes | Yes | Yes | Unclear | Unclear | Yes | No data available |
| Ongoing study, potentially relevant | |||||||
| Unclear intervention, quantitative FIT | |||||||
| Unspecified reference standard | |||||||
| van Turenhout ST, Van Rossum LG, Oort FA, Laheij RJ, Van Rijn AF, Fockens P, et al. Differences in FIT results between screening and referred colorectal cancer patients are explained by differences in tissue tumor stage. Gastroenterology 2010;138(5 Suppl. 1):185 | Yes | No | Unclear | Yes | Yes | No | Not a symptomatic population |
| Comparison of stages of CRC between screening detected and symptomatic patients | |||||||
| Viana Freitas BR, Kibune Nagasako C, Pavan CR, Silva Lorena SL, Guerrazzi F, Saddy Rodrigues Coy C, et al. Immunochemical fecal occult blood test for detection of advanced colonic adenomas and colorectal cancer: comparison with colonoscopy results. Gastroenterol Res Pract 2013; 384561 | Yes | No | No | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic high risk, or surveillance | |||||||
| Qualitative FIT | |||||||
| Vilkin A, Rozen P, Levi Z, Waked A, Maoz E, Birkenfeld S, et al. Performance characteristics and evaluation of an automated-developed and quantitative, immunochemical, fecal occult blood screening test. Am J Gastroenterol 2005;100:2519–25 | Yes | No | Yes | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic high risk (approximately 73% symptomatic) – authors contacted, no reply receiveda | |||||||
| Vogel T, Driemel C, Hauser A, Hansmann A, Lange S, Jonas M, et al. [Comparison of different stool tests for the detection of cancer of the colon.] Dtsch Med Wochenschr 2005;130:872–7 | Yes | No | No | No | Yes | Yes | Not a symptomatic population |
| Includes health control participants | |||||||
| No relevant FIT technology | |||||||
| Wakamura K, Kudo SE, Ikehara N, Mori Y, Hayashi S, Takeda K, et al. A prospective evaluation using the colonoscope of the fecal occult blood test-negative colorectal neoplasms in a referral hospital. Gastrointest Endosc 2012;75(4 Suppl. 1):AB343–4 | Yes | No | Unclear | No | Yes | Yes | Not a symptomatic population |
| Mixed population (approximately 25% symptomatic). Unspecified FIT method – authors contacted, reply received | |||||||
| No relevant FIT technology | |||||||
| De Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol 2012;107:1570–8 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Asymptomatic screening | |||||||
| Williams JA, Hunter R, Coles ME, Thomas DW, Huber TW. An assessment of an immunochemical test for human haemoglobin in the detection of colonic polyps. Aust N Z J Surg 1985;55:485–8 | Yes | No | Unclear | Unclear | Yes | No | Not a symptomatic population |
| Mixed population symptomatic and asymptomatic, or history of CRC (proportions not reported) | |||||||
| FIT type not reported | |||||||
| Wong BC, Wong WM, Cheung KL, Tong TS, Rozen P, Young GP, et al. A sensitive guaiac faecal occult blood test is less useful than an immunochemical test for colorectal cancer screening in a Chinese population. Aliment Pharmacol Ther 2003;18:941–6 | Yes | No | Unclear | No | Yes | No | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic surveillance (46% symptomatic) | |||||||
| FlexSure OBT and Hemoccult SENSA, no relevant FIT technology | |||||||
| Only sensitivity data reported | |||||||
| Wong WM, Lam SK, Cheung KL, Tong TS, Rozen P, Young GP, et al. Evaluation of an automated immunochemical fecal occult blood test for colorectal neoplasia detection in a Chinese population. Cancer 2003;97:2420–4 | Yes | No | No | No | Yes | No | Not a symptomatic population |
| Mixed population, symptomatic, polyp surveillance, history of CRC, or family history (37% symptomatic) | |||||||
| No relevant FIT technology | |||||||
| Only sensitivity data reported | |||||||
| Woo HY, Mok RS, Park YN, Park DI, Sung IK, Sohn CI, et al. A prospective study of a new immunochemical fecal occult blood test in Korean patients referred for colonoscopy. Clin Biochem 2005;38:395–9 | Yes | No | Unclear | Yes | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic or history of CRC (60% symptomatic) – authors contacted, no reply received | |||||||
| Wu D, Luo HQ, Zhou WX, Qian JM, Li JN. The performance of three-sample qualitative immunochemical fecal test to detect colorectal adenoma and cancer in gastrointestinal outpatients: an observational study. PLOS ONE 2014;9:e106648 | Yes | No | No | No | Yes | Yes | Not a symptomatic population |
| Mixed population, symptomatic and asymptomatic history of CRC or polyp | |||||||
| Qualitative FIT | |||||||
| Yeasmin F, Ali MA, Rahman MA, Sultana T, Rahman MQ, Ahmed AN. A comparative study of chemical and immunological method of faecal occult blood test in the diagnosis of occult lower gastrointestinal bleeding. Bangladesh Med Res Counc Bull 2013;39:52–6 | Yes | Unclear | Unclear | Unclear | Yes | No | No relevant outcomes |
| Only patients who were positive on either FIT or gFOBT received colonoscopy; the sensitivity and specificity of FIT and gFOBT individually were then assessed against colonoscopy in this preselected sample | |||||||
| Unclear whether participants were symptomatic | |||||||
| FIT unspecified |
Appendix 5 Excluded studies (cost-effectiveness review)
| Author Year | Country | Summary of model | Intervention/comparator | Patient population | QALYs | Costs | ICER | Sensitivity analyses |
|---|---|---|---|---|---|---|---|---|
| Allen 2005109 | USA
|
Decision tree and Markov model; prevalence of serious disease in patients with rectal bleeding obtained from systematic review; estimates of specificity and sensitivity obtained from review of prospective diagnostic studies; utilities obtained from different studies; instruments CRC/HUI, univariate and multivariate sensitivity analysis; discount rate 3% |
|
Patients aged ≥ 40 years, with otherwise asymptomatic rectal bleeding |
|
|
|
The ICER was affected by different variables: age, prevalence of polyps, costs of colonoscopy, charges of procedures |
| Rae 1994110 | Canada/USA | No model | Different FOBTs:
|
Patients ≥ 40 years
|
No QALYs | Total costs (incl. tests and colonoscopy)
|
Costs per cancer detected:
|
NA |
Cancer detected:
|
Appendix 6 Questionnaire and information sent to clinical experts
One of the goals of our assessment is to explore the cost-effectiveness of using a quantitative faecal immunochemical test (FIT) for haemoglobin (occult blood) as a triage test in the investigation of symptomatic people presenting in the primary care setting who are at low risk of colorectal cancer (CRC). The cost-effectiveness of FIT will be compared with guaiac faecal occult blood tests (gFOBT) and with no triage (referral straight to colonoscopy).
A health economic model is currently being developed to address the question above. The model consists of two parts:
-
the diagnostic model – a decision tree reflecting the diagnosis of CRC, and
-
the CRC model – a Markov state-transition model to estimate the long-term costs and effects associated with the treatment and progression of CRC.
Information about the diagnostic performance of FIT will be derived from the systematic review component of our assessment; however, additional information about the patient pathway after FIT testing is needed in order to fully populate the model. Specific questions regarding modelling assumptions are listed below. Any information that you are able to provide (from publications, unpublished data or personal experience) would be very much appreciated.
The model begins with a cohort of symptomatic patients, presenting in primary care, who require investigation for possible CRC. A patient in the cohort can have one of the following tests: FIT, gFOBT or no triage testing at all (referral straight to colonoscopy). A positive test will result in referral to colonoscopy while a negative test will result in a watchful waiting strategy in which a repeated test or further investigation can be performed when symptoms persist. A schematic representation of the decision-analytic model is given below:

Patient compliance
1) What percentage of symptomatic patients (not yet referred for colonoscopy) would you expect to return a faecal sample for FIT or gFOBT?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate | |
|---|---|---|---|
| FIT | |||
| gFOBT |
2) What percentage of patients not returning a sample would then need to be referred to colonoscopy?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate |
|---|---|---|
3) What percentage of patients do not consent to colonoscopy?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate | |
|---|---|---|---|
| Of those testing positive with FIT/gFOBT? | |||
| In those referred without triage? |
Colonoscopy versus CT colonography
4) If the gFOBT or FIT result is positive, patients are referred to either colonoscopy or CT colonography (CTC). What would be the (estimated) percentage of patients that receive colonoscopy versus CTC in the UK?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate | |
|---|---|---|---|
| Colonoscopy | |||
| CTC | |||
| Other (please specify) |
Negative test result FIT/gFOBT
5) What percentage of those patients who test negative with FIT/gFOBT will eventually undergo colonoscopy?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate |
|---|---|---|
6) Could you also estimate the time interval to getting this colonoscopy?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate |
|---|---|---|
7) What percentage of those patients that test negative with FIT/gFOBT would get a second FIT/gFOBT?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate |
|---|---|---|
Positive test result FIT/gFOBT
8) What is the average time interval between presentation and colonoscopy?
No triage
9) What is the average time-interval between presentation and colonoscopy?
Positive test result colonoscopy
10) The study population is symptomatic patients at low risk, presenting in primary care, with lower abdominal symptoms, who require investigation for possible CRC. In those who eventually have a colonoscopy and are staged by CT what percentage would be found to be in each of the Dukes’ stages (I, II, III, IV)?
Please provide your answer here:
| Lowest estimate | Most likely estimate | Highest estimate | |
|---|---|---|---|
| Dukes’ stage I | |||
| Dukes’ stage II | |||
| Dukes’ stage III | |||
| Dukes’ stage IV |
Appendix 7 Equations used to estimates probabilities in the diagnostic (decision tree) model
The probabilities used at the decision nodes in the diagnostic model, labelled as (A)–(J) in Figure 12, were summarised in Table 27. Most of them are functions of the sensitivity and specificity of the tests and the prevalence of the disease in the patient population. In this appendix we provide full derivations of those equations.
(A) Probability that faecal immunochemical test/guaiac faecal occult blood test is positive
By the Law of Total Probability and the definitions of sensitivity, specificity and prevalence, the following holds: P[T+] = P[T+|CRC+] × P[CRC+] + P[T+|CRC–] × P[CRC–] = (sensitivity × prevalence) + [(1 – specificity) × (1 – prevalence)].
(B) Probability that faecal immunochemical test/guaiac faecal occult blood test is negative
This was calculated as 1 – (A).
(C) Probability that colorectal cancer is present after a positive faecal immunochemical test/guaiac faecal occult blood test
This is the definition of the PPV. For the PSA, we assumed a beta distribution on the PPV.
(D) Probability that colorectal cancer is not present after a positive faecal immunochemical test/guaiac faecal occult blood test
This was calculated as 1 – PPV.
(E) Probability that symptoms persist after a negative faecal immunochemical test/guaiac faecal occult blood test
This was estimated based on expert opinion (mean value 0.325). A beta distribution was assumed for the PSA.
(F) Probability that symptoms do not persist after a negative faecal immunochemical test/guaiac faecal occult blood test
This was calculated as 1 – (E).
(G) Probability that colorectal cancer is present for symptomatic patients after a negative faecal immunochemical test/guaiac faecal occult blood test
This was calculated as (1 – NPV)/(E). The full derivation is as follows.
Note that this probability cannot be directly estimated, as we do not have data on the ‘specificity’ of persisting in symptoms, that is, we have assumed that persisting in symptoms is perfectly sensitive (all patients with CRC with test negative will persist in their symptoms), but there will also be some ‘FPs’ (patients whose symptoms persist after testing negative that will not have CRC). With the data we have, we can estimate the joint probability of (E) and (G) as follows.
The probability of persisting in symptoms after testing negative was estimated based on expert opinion. This is (E) in the diagnostic model. We denote this probability as P[symp|T–] here. Note that this will include patients with and without CRC (denoted by CRC+ and CRC–, respectively). Therefore, we need to calculate the following probabilities:
-
P[symp and CRC+|T–]: probability of remaining symptomatic and CRC+ after testing negative.
-
P[symp and CRC–|T–]: probability of remaining symptomatic and CRC– after testing negative.
-
P[no symp and CRC+|T–]: probability of becoming asymptomatic and CRC+ after testing negative.
-
P[no symp and CRC–|T–]: probability of becoming asymptomatic and CRC– after testing negative.
By the Law of Total Probability, we have that:
As we are assuming that P[no symp and CRC+|T–] = 0 (i.e. all patients with CRC remain symptomatic), equation 4 reduces to:
Applying the Law of Total Probability again, we have that P[CRC + |T–] = P[symp and CRC+|T–] + P[no symp and CRC+|T–], which also reduces to:
Thus, the probability of having CRC given test negative is equal to the probability of being symptomatic and having CRC given test negative (as we had assumed). Rearranging equation 6 and substituting into equation 3, we have that:
As P[CRC+|T–] is defined as 1 – NPV, we can conclude that:
It should be emphasised that equation 8 is the joint probability of (E) and (G); equation 9 is the joint probability of (E) and (H); and equation 10 is the probability (F). Thus, (G) can be estimated as (1 – NPV)/(E).
There are two potential problems here:
As P[symp|T–] was estimated by experts independently of the accuracy estimates of faecal immunochemical testing/gFOBT and the prevalence, it might not be guaranteed that P[symp and CRC–|T–], equation 9, is always positive. Note that the experts estimated the mean P[symp|T–] as 0.325. For example, when the accuracy data from Mowat et al. 52 (threshold of 0 µg Hb/g faeces for the detection of CRC using a single faecal sample) was used, the NPV was high enough (0.9948 if based on prevalence in study or 0.9979 if based on 1.5% prevalence) to keep the equation positive, but this would be a problem if the NPV fell below 0.675 (i.e. 1 – 0.325). With the data we have, this is probably not a problem, but particular attention must be taken when the PSA is run.
The second potential problem is that, we have shown that, conditional on testing negative, the probability of remaining symptomatic and having CRC was 1 – NPV. However, as we previously mentioned, that is not the probability needed for the model, we need (G). We have shown above that (G) = (1 – NPV)/P[symp|T–]. This is not a problem when the mode is run deterministically, but having P[symp|T–] in the denominator, which is assumed to follow a beta distribution, causes numerical problems when performing the PSA (numerical division by 0). For that reason, for the PSA, we decided to consider (G) = (1 – NPV)/mean(P[symp|T–]), where mean(P[symp|T–]) = 0.325, given by the experts.
Note, finally, that there should be no uncertainty from being symptomatic in estimating the probability of having the disease given test negative. This is because in equation 8 we concluded that P[symp and CRC+|T–] = 1–NPV. This is the joint probability of (E) and (G), but, in the model, this joint probability is calculated as P[symp|T–] × (1 – NPV)/mean(P[symp|T–]), where P[symp|T–] and mean(P[symp|T–]) do not cancel each other out. Therefore, our model is likely to overestimate the uncertainty around this probability.
(H) Probability that colorectal cancer is not present for symptomatic patients after a negative test
This was calculated as 1 – (G).
(I) Probability that colorectal cancer is present
This was estimated as the prevalence of CRC in our population.
Note that this is equivalent to the probability of having a positive colonoscopy in the no triage (referral straight to colonoscopy) arm of the diagnostic model (thus colonoscopy only). As prevalence is defined as the proportion of the population with the condition and given that we are assuming that colonoscopy is a perfect diagnostic for CRC, the prevalence is indeed the probability that we need. In NG121 it was assumed that the prevalence of CRC in the base-case population was 1.5%. This value was obtained from experts. Further, a range of variation was assumed and then a uniform or triangular probability distribution can be used to include this parameter in the PSA.
(J) Probability that colorectal cancer is not present
This was calculated as 1 – (I).
Appendix 8 Annual survival/mortality rates included in the colorectal cancer Markov model
| Year | Dukes’ A predicted | Dukes’ B predicted | Dukes’ C predicted | Dukes’ D predicted |
|---|---|---|---|---|
| 0 | 100 | 100 | 100 | 100 |
| 1 | 97.211 | 92.233 | 82.357 | 38.112 |
| 2 | 95.541 | 85.403 | 65.111 | 17.669 |
| 3 | 94.577 | 81.644 | 56.749 | 11.270 |
| 4 | 93.900 | 79.078 | 51.476 | 8.192 |
| 5 | 93.377 | 77.144 | 47.725 | 6.396 |
| 6 | 92.953 | 75.598 | 44.865 | 5.225 |
| 7 | 92.595 | 74.316 | 42.581 | 4.404 |
| 8 | 92.286 | 73.222 | 40.696 | 3.798 |
| 9 | 92.015 | 72.271 | 39.103 | 3.333 |
| 10 | 91.773 | 71.431 | 37.731 | 2.965 |
| 11 | 91.555 | 70.679 | 36.532 | 2.668 |
| 12 | 91.356 | 70.000 | 35.470 | 2.422 |
| 13 | 91.173 | 69.381 | 34.520 | 2.217 |
| 14 | 91.004 | 68.812 | 33.664 | 2.042 |
| 15 | 90.848 | 68.287 | 32.886 | 1.891 |
| Cycle | Dukes’ A | Dukes’ B | Dukes’ C | Dukes’ D | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) | α | β | Mortality (%) | α | β | Mortality (%) | α | β | Mortality (%) | α | β | |
| 1 | 2.89 | 793 | 26610 | 7.87 | 5893 | 68982 | 17.75 | 12912 | 59847 | 61.99 | 17551 | 10761 |
| 2 | 1.83 | 497 | 26676 | 7.52 | 5198 | 63945 | 21.05 | 12621 | 47328 | 53.75 | 5808 | 4998 |
| 3 | 1.13 | 313 | 27371 | 4.52 | 2909 | 61428 | 12.96 | 6162 | 41374 | 36.34 | 1824 | 3196 |
| 4 | 0.85 | 241 | 28240 | 3.27 | 2024 | 59830 | 9.42 | 3916 | 37648 | 27.45 | 880 | 2327 |
| 5 | 0.69 | 204 | 29186 | 2.58 | 1558 | 58723 | 7.42 | 2808 | 35026 | 22.06 | 515 | 1820 |
| 6 | 0.60 | 183 | 30237 | 2.15 | 1273 | 57938 | 6.14 | 2162 | 33052 | 18.45 | 337 | 1489 |
| 7 | 0.54 | 170 | 31403 | 1.85 | 1083 | 57386 | 5.25 | 1745 | 31505 | 15.87 | 237 | 1257 |
| 8 | 0.50 | 163 | 32609 | 1.64 | 948 | 56994 | 4.59 | 1455 | 30250 | 13.93 | 176 | 1086 |
| 9 | 0.47 | 161 | 34026 | 1.48 | 850 | 56781 | 4.09 | 1246 | 29227 | 12.42 | 135 | 955 |
| 10 | 0.45 | 162 | 35556 | 1.35 | 777 | 56700 | 3.70 | 1090 | 28377 | 11.22 | 108 | 852 |
| 11 | 0.44 | 166 | 37266 | 1.26 | 723 | 56760 | 3.39 | 969 | 27674 | 10.24 | 88 | 769 |
| 12 | 0.44 | 174 | 39221 | 1.19 | 684 | 56977 | 3.13 | 876 | 27103 | 9.42 | 73 | 701 |
| 13 | 0.45 | 186 | 41372 | 1.13 | 657 | 57338 | 2.93 | 803 | 26646 | 8.74 | 62 | 644 |
| 14 | 0.46 | 202 | 43677 | 1.09 | 639 | 57828 | 2.76 | 745 | 26290 | 8.16 | 53 | 596 |
| 15 | 0.48 | 220 | 46085 | 1.07 | 629 | 58432 | 2.62 | 699 | 26025 | 7.67 | 46 | 555 |
| 16 | 0.51 | 243 | 47550 | 1.10 | 652 | 58670 | 2.65 | 698 | 25653 | 7.70 | 43 | 516 |
| 17 | 0.54 | 267 | 48963 | 1.13 | 675 | 58908 | 2.68 | 697 | 25294 | 7.74 | 40 | 480 |
| 18 | 0.58 | 294 | 50384 | 1.17 | 701 | 59173 | 2.72 | 698 | 24959 | 7.77 | 38 | 446 |
| 19 | 0.62 | 323 | 51802 | 1.21 | 729 | 59459 | 2.76 | 700 | 24646 | 7.82 | 35 | 415 |
| 20 | 0.66 | 355 | 53152 | 1.25 | 758 | 59740 | 2.80 | 702 | 24347 | 7.86 | 33 | 386 |
| 21 | 0.71 | 389 | 54452 | 1.30 | 790 | 60022 | 2.85 | 705 | 24064 | 7.90 | 31 | 360 |
| 22 | 0.76 | 424 | 55688 | 1.35 | 823 | 60297 | 2.90 | 710 | 23795 | 7.95 | 29 | 335 |
| 23 | 0.81 | 463 | 56872 | 1.40 | 859 | 60571 | 2.95 | 715 | 23544 | 8.00 | 27 | 312 |
| 24 | 0.86 | 503 | 57968 | 1.45 | 896 | 60826 | 3.00 | 721 | 23304 | 8.06 | 25 | 290 |
| 25 | 0.92 | 547 | 59016 | 1.51 | 936 | 61081 | 3.06 | 728 | 23084 | 8.12 | 24 | 271 |
| 26 | 0.98 | 595 | 60005 | 1.58 | 979 | 61329 | 3.12 | 737 | 22883 | 8.18 | 22 | 252 |
| 27 | 1.06 | 648 | 60973 | 1.65 | 1028 | 61593 | 3.19 | 749 | 22714 | 8.25 | 21 | 235 |
| 28 | 1.13 | 706 | 61881 | 1.72 | 1082 | 61852 | 3.27 | 763 | 22572 | 8.33 | 20 | 220 |
| 29 | 1.22 | 773 | 62754 | 1.81 | 1143 | 62121 | 3.36 | 780 | 22468 | 8.42 | 19 | 205 |
| 30 | 1.32 | 849 | 63587 | 1.92 | 1214 | 62401 | 3.46 | 803 | 22412 | 8.52 | 18 | 192 |
| 31 | 1.44 | 935 | 64323 | 2.03 | 1294 | 62655 | 3.58 | 830 | 22394 | 8.63 | 17 | 180 |
| 32 | 1.57 | 1033 | 64966 | 2.17 | 1386 | 62886 | 3.71 | 863 | 22426 | 8.77 | 16 | 169 |
| 33 | 1.72 | 1141 | 65447 | 2.31 | 1487 | 63044 | 3.86 | 901 | 22490 | 8.92 | 15 | 158 |
| 34 | 1.89 | 1260 | 65752 | 2.48 | 1599 | 63113 | 4.03 | 946 | 22589 | 9.09 | 15 | 149 |
| 35 | 2.08 | 1388 | 65849 | 2.67 | 1720 | 63062 | 4.22 | 997 | 22713 | 9.27 | 14 | 140 |
| 36 | 2.29 | 1526 | 65725 | 2.88 | 1851 | 62872 | 4.43 | 1054 | 22860 | 9.48 | 14 | 132 |
| 37 | 2.52 | 1671 | 65358 | 3.11 | 1989 | 62515 | 4.66 | 1118 | 23017 | 9.71 | 13 | 125 |
| 38 | 2.77 | 1827 | 64751 | 3.36 | 2138 | 61983 | 4.91 | 1191 | 23192 | 9.97 | 13 | 118 |
| 39 | 3.06 | 1994 | 63894 | 3.65 | 2299 | 61258 | 5.20 | 1274 | 23382 | 10.25 | 13 | 112 |
| 40 | 3.38 | 2171 | 62776 | 3.97 | 2470 | 60319 | 5.52 | 1368 | 23580 | 10.57 | 12 | 106 |
| 41 | 3.75 | 2362 | 61387 | 4.34 | 2655 | 59146 | 5.89 | 1477 | 23788 | 10.94 | 12 | 101 |
| 42 | 4.17 | 2565 | 59712 | 4.76 | 2853 | 57714 | 6.31 | 1602 | 23989 | 11.36 | 12 | 96 |
| 43 | 4.66 | 2782 | 57738 | 5.25 | 3064 | 56000 | 6.80 | 1746 | 24172 | 11.85 | 12 | 92 |
| 44 | 5.22 | 3010 | 55452 | 5.81 | 3287 | 53980 | 7.36 | 1911 | 24314 | 12.41 | 12 | 88 |
| 45 | 5.87 | 3244 | 52848 | 6.46 | 3515 | 51636 | 8.00 | 2097 | 24377 | 13.06 | 13 | 85 |
| 46 | 6.60 | 3475 | 49927 | 7.19 | 3741 | 48962 | 8.74 | 2302 | 24320 | 13.80 | 13 | 81 |
| 47 | 7.44 | 3694 | 46708 | 8.03 | 3954 | 45968 | 9.58 | 2520 | 24098 | 14.64 | 13 | 78 |
| 48 | 8.38 | 3886 | 43221 | 8.97 | 4138 | 42678 | 10.52 | 2743 | 23663 | 15.58 | 14 | 76 |
| 49 | 9.43 | 4036 | 39515 | 10.02 | 4280 | 39139 | 11.57 | 2958 | 22977 | 16.62 | 14 | 73 |
| 50 | 10.57 | 4131 | 35654 | 11.16 | 4365 | 35413 | 12.71 | 3151 | 22015 | 17.76 | 15 | 70 |
| 51 | 11.81 | 4159 | 31713 | 12.40 | 4381 | 31574 | 13.95 | 3307 | 20771 | 19.00 | 16 | 68 |
| 52 | 13.15 | 4114 | 27772 | 13.74 | 4322 | 27708 | 15.29 | 3410 | 19257 | 20.34 | 16 | 65 |
| 53 | 14.60 | 3997 | 23912 | 15.19 | 4188 | 23896 | 16.74 | 3450 | 17506 | 21.79 | 17 | 62 |
| 54 | 16.14 | 3805 | 20212 | 16.74 | 3977 | 20226 | 18.28 | 3413 | 15569 | 23.34 | 18 | 60 |
| 55 | 17.80 | 3545 | 16747 | 18.39 | 3697 | 16774 | 19.94 | 3297 | 13514 | 24.99 | 19 | 57 |
| 56 | 19.55 | 3228 | 13580 | 20.14 | 3359 | 13609 | 21.69 | 3100 | 11424 | 26.74 | 19 | 54 |
| 57 | 21.40 | 2865 | 10760 | 21.99 | 2974 | 10785 | 23.54 | 2830 | 9385 | 28.59 | 20 | 51 |
| 58 | 23.32 | 2472 | 8321 | 23.91 | 2561 | 8340 | 25.46 | 2500 | 7483 | 30.51 | 21 | 48 |
| 59 | 25.29 | 2071 | 6273 | 25.89 | 2142 | 6286 | 27.43 | 2135 | 5784 | 32.49 | 21 | 45 |
| 60 | 27.35 | 1687 | 4604 | 27.94 | 1740 | 4610 | 29.49 | 1764 | 4329 | 34.55 | 21 | 41 |
| 61 | 29.52 | 1334 | 3281 | 30.11 | 1374 | 3283 | 31.66 | 1410 | 3130 | 36.72 | 22 | 38 |
Appendix 9 Annual mortality rates included in the healthy population Markov model
| Age | Weighted average annual mortality | Beta distribution (α) | Beta distribution (β) |
|---|---|---|---|
| 40 | 0.0010 | 98,337 | 101 |
| 41 | 0.0011 | 98,227 | 111 |
| 42 | 0.0012 | 98,109 | 118 |
| 43 | 0.0013 | 97,981 | 127 |
| 44 | 0.0014 | 97,846 | 135 |
| 45 | 0.0015 | 97,704 | 143 |
| 46 | 0.0016 | 97,552 | 152 |
| 47 | 0.0016 | 97,392 | 160 |
| 48 | 0.0018 | 97,220 | 172 |
| 48 | 0.0018 | 97,220 | 172 |
| 49 | 0.0019 | 97,036 | 184 |
| 50 | 0.0021 | 96,836 | 199 |
| 51 | 0.0023 | 96,618 | 218 |
| 52 | 0.0025 | 96,379 | 240 |
| 53 | 0.0027 | 96,115 | 264 |
| 54 | 0.0030 | 95,823 | 291 |
| 55 | 0.0034 | 95,502 | 322 |
| 56 | 0.0037 | 95,148 | 353 |
| 57 | 0.0041 | 94,760 | 388 |
| 58 | 0.0045 | 94,336 | 425 |
| 59 | 0.0049 | 93,873 | 463 |
| 60 | 0.0054 | 93,369 | 503 |
| 61 | 0.0059 | 92,824 | 545 |
| 62 | 0.0064 | 92,235 | 590 |
| 63 | 0.0069 | 91,599 | 635 |
| 64 | 0.0075 | 90,915 | 684 |
| 65 | 0.0081 | 90,180 | 735 |
| 66 | 0.0088 | 89,387 | 793 |
| 67 | 0.0096 | 88,532 | 855 |
| 68 | 0.0105 | 87,608 | 924 |
| 69 | 0.0115 | 86,604 | 1004 |
| 70 | 0.0127 | 85,512 | 1092 |
| 71 | 0.0140 | 84,321 | 1191 |
| 72 | 0.0155 | 83,021 | 1299 |
| 73 | 0.0172 | 81,604 | 1417 |
| 74 | 0.0191 | 80,060 | 1544 |
| 75 | 0.0212 | 78,382 | 1678 |
| 76 | 0.0234 | 76,562 | 1819 |
| 77 | 0.0260 | 74,593 | 1969 |
| 78 | 0.0289 | 72,465 | 2129 |
| 79 | 0.0321 | 70,167 | 2298 |
| 80 | 0.0358 | 67,688 | 2479 |
| 81 | 0.0400 | 65,016 | 2672 |
| 82 | 0.0448 | 62,140 | 2877 |
| 83 | 0.0505 | 59,047 | 3092 |
| 84 | 0.0569 | 55,734 | 3313 |
| 85 | 0.0643 | 52,204 | 3531 |
| 86 | 0.0727 | 48,468 | 3736 |
| 87 | 0.0821 | 44,553 | 3914 |
| 88 | 0.0925 | 40,501 | 4052 |
| 89 | 0.1040 | 36,366 | 4135 |
| 90 | 0.1164 | 32,212 | 4154 |
| 91 | 0.1298 | 28,111 | 4101 |
| 92 | 0.1442 | 24,134 | 3977 |
| 93 | 0.1597 | 20,352 | 3782 |
| 94 | 0.1762 | 16,831 | 3521 |
| 95 | 0.1938 | 13,628 | 3203 |
| 96 | 0.2123 | 10,786 | 2842 |
| 97 | 0.2315 | 8334 | 2452 |
| 98 | 0.2512 | 6280 | 2054 |
| 99 | 0.2718 | 4607 | 1673 |
| 100 | 0.2935 | 3283 | 1324 |
Appendix 10 Technical details about parameter estimation
For all input parameters of the model included in the PSA, a certain measure of variation around the mean value is needed. This measure of variation is usually reported as a standard error (SE), 95% CI or 25% and 75% quartiles. With this information, the input parameters of the model can be fit to appropriate probability distributions (e.g. beta for sensitivity and specificity, gamma for costs, etc.) by estimating the parameters of the corresponding probability distributions. For some input parameters of the model (e.g. utilities for Dukes’ stages), the parameters of the probability distributions were reported in the literature and included directly in our model.
When the SE is reported, the parameters of a beta distribution can be obtained from the mean and SE as α = mean × [(mean × (1 – mean)/(SE)2) – 1] and β = (1 – mean) × [(mean × (1 – mean)/SE2) – 1]. This is the case for example of the sensitivity for the OC-Sensor. Similarly, the parameters of a gamma distribution can be obtained from the mean and SE as α = (mean/SE)2 and β = SE2/mean. This is done, for example, with the costs associated to Dukes’ stages.
When a CI or any other uncertainty range is reported, parameter estimation is not as straightforward as in the examples mentioned above, and more complex numerical procedures have to be applied. In those situations, the parameters of the probability distribution functions were estimated in R, version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria), using different methods. As probability distribution functions are usually multivariate functions (more than one parameter needs to be estimated), R methods to solve non-linear multivariate equations are preferred. In particular, the R function nleqslv (package nleqslv – https://cran.r-project.org/web/packages/nleqslv/nleqslv.pdf) was used. However, in some cases, this method did not converge and, therefore, it did not provide a solution. In those situations, after trying some other multivariate methods available in R, which also failed to find a solution, a univariate approach, the function uniroot (https://stat.ethz.ch/R-manual/R-devel/library/stats/html/uniroot.html) from the package stats, or a simulation approach were used. In this appendix, we describe, with the help of a few examples, the problems encountered and the solutions proposed. Complete results can be found in the file ‘Parameter calculator.R’.
Example 1: probability of bleeding
The mean reported was 2.6% and the 95% CI was (1.7% to 3.7%). In this case, the multivariate method nleqslv worked well. The idea behind this approach is to find the two parameters α and β such that the 2.5% percentile of a beta distribution is 1.7% and the 97.5% percentile of a beta distribution is 3.7%. This can be expressed in R as the two equations below:
These equations were solved with the nleqslv function and the solutions obtained were α = 25.82748 and β = 9887.901. The complete R code can be found in the file ‘Parameter calculator.R’.
Example 2: sensitivity for the HM-JACKarc system
The sensitivity for HM-JACKarc was obtained from a single study that reported a mean = 1 and 95% CI = (0.715 to 1.00). Because the mean is exactly 1, a beta distribution could not be properly fit. In this case, we used the uniroot function because it provided a better fit. However, the mean had to be set to 0.98, otherwise the method would not converge.
The idea behind this approach is to find the parameter α such that the mean of a beta distribution is 0.98 and (for example) the 2.5% percentile of a beta distribution is 0.715 (the 97.5% can also be used). The latter can be expressed in R as the following equation:
Note also that for the beta distribution the following equation always holds:
Thus, substitution in F1 above yields:
This equation was solved with the uniroot function and the solution obtained was α = 1.169235. With this value of α and the assumed mean, β = 0.02386193. The complete R code can be found in the file ‘Parameter calculator.R’.
Example 3: cost of colonoscopy
The mean reported was £372, the lower quartile £141 and the upper quartile £474. The multivariate methods we tried did not work for the gamma distribution. Therefore, the uniroot function was also used here.
In this case, the idea behind this method is to find the shape parameter such that the mean of a gamma distribution is £372 and (for example) the upper quartile of a gamma distribution is £474 (the lower quartile can also be used). The latter can be expressed in R as the following equation:
Note also that for the gamma distribution the following equation always holds:
Thus, substitution in F1 above yields:
This equation was solved with the uniroot function and the solution obtained was shape = 4.126228. With this value and the mean, scale = 90.15498. The complete R code can be found in the file ‘Parameter calculator.R’.
Appendix 11 Results (full incremental and intervention versus comparator) of base case and scenario analyses
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | NA | NA | NA |
| OC-Sensor | 18.6239 | 244.42 | 0.0020 | 12.14 | 6133 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 274.75 | –0.0003 | –30.33 | 88,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 42.47 | 18,296 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.14 | 6133 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259.25 | 4,133,559 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6415 | 230.49 | NA | NA | NA |
| OC-Sensor | 18.6439 | 242.51 | 0.0024 | 12.02 | 5039 |
| No triage (referral straight to colonoscopy) | 18.6440 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6444 | 272.50 | 0.0005 | 29.99 | 61,619 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0029 | 42.01 | 14,626 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0004 | –228.10 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0024 | 12.02 | 5039 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –258.09 | 2,578,543 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.32 | NA | NA | NA |
| OC-Sensor | 18.6239 | 244.67 | 0.002 | 12.35 | 6237 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 275.05 | 0.0003 | 30.38 | 88,936 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 43 | 18,405 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –229 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12 | 6237 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259 | 4,129,585 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6403 | 231 | NA | NA | NA |
| OC-Sensor | 18.6427 | 243 | 0.0024 | 12.22 | 5125 |
| No triage (referral straight to colonoscopy) | 18.6428 | 501 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6431 | 273 | 0.0005 | 30.04 | 61,322 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0029 | 42.26 | 14,706 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0004 | –227.80 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0024 | 12.22 | 5125 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –257.84 | 2,533,617 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232 | NA | NA | NA |
| OC-Sensor | 18.6239 | 244 | 0.0020 | 12.14 | 6133 |
| No triage (referral straight to colonoscopy) | 18.6239 | 504 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 275 | 0.0003 | 30.33 | 88,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 42 | 18,296 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –229 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12 | 6133 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259 | 4,133,559 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.4512 | 246.83 | NA | NA | NA |
| OC-Sensor | 18.4554 | 261.06 | 0.0042 | 14.24 | 3354 |
| No triage (referral straight to colonoscopy) | 18.4559 | 516.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.4564 | 291.04 | 0.0009 | 29.98 | 32,276 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0052 | 44.22 | 8545 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0005 | –225.56 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0042 | 14.24 | 3354 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –255.54 | 601,774 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | NA | NA | NA |
| FOB Gold | 18.6238 | 262.72 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6239 | 244.42 | 0.0020 | 12.14 | 6133 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 274.75 | 0.0003 | 30.33 | 88,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 42.47 | 18,296 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.14 | 6133 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259.25 | 4,133,559 |
| FOB Gold vs. gFOBT | 0.0019 | 30.44 | 15,720 |
| FOB Gold vs. no triage (referral straight to colonoscopy) | –0.0001 | –240.95 | 2,273,829 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6424 | 230.92 | NA | NA | NA |
| FOB Gold | 18.6444 | 261.07 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6444 | 242.92 | 0.0020 | 12.00 | 6081 |
| No triage (referral straight to colonoscopy) | 18.6445 | 500.69 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6446 | 272.94 | 0.0002 | 30.02 | 128,618 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0022 | 42.02 | 19,040 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0001 | –227.75 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.00 | 6081 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –257.76 | 2,830,605 |
| FOB Gold vs. gFOBT | 0.0020 | 30.14 | 15,456 |
| FOB Gold vs. no triage (referral straight to colonoscopy) | –0.0001 | –239.62 | 2,097,433 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| No triage (referral straight to colonoscopy) | 18.6064 | 503.65 | Dominated by gFOBT | ||
| gFOBT | 18.6148 | 232.26 | |||
| HM-JACKarc | 18.6157 | 274.73 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6165 | 244.40 | 0.0017 | 12.14 | 7144 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0009 | 42.47 | 45,271 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0093 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0017 | 12.14 | 7144 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0101 | –259.25 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| No triage (referral straight to colonoscopy) | 18.6258 | 500 | Dominated by gFOBT | ||
| gFOBT | 18.6343 | 230 | |||
| HM-JACKarc | 18.6350 | 272 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6359 | 243 | 0.0016 | 12.08 | 7535 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0007 | 42.05 | 58,563 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0092 | –227.77 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0016 | 12.08 | 7535 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0101 | –257.74 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6195 | 230.90 | NA | NA | NA |
| OC-Sensor | 18.6235 | 244.20 | 0.0040 | 13.30 | 3352 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 274.75 | 0.0007 | 30.55 | 43,642 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0047 | 43.85 | 9360 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0040 | 13.30 | 3352 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –259.47 | 592,092 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6316 | 229.27 | NA | NA | NA |
| OC-Sensor | 18.6357 | 242.63 | 0.0040 | 13.37 | 3306 |
| No triage (referral straight to colonoscopy) | 18.6361 | 500.61 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6363 | 272.76 | 0.0006 | 30.13 | 48,223 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0047 | 43.50 | 9318 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0001 | –227.85 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0040 | 13.37 | 3306 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0005 | –257.97 | 539,543 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | NA | NA | NA |
| FOB Gold | 18.6237 | 240.62 | 0.0018 | 8.34 | 4725 |
| OC-Sensor | 18.6238 | 241.83 | 0.0001 | 1.21 | 12,576 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | 0.0002 | 261.84 | 1,449,585 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| FOB Gold vs. gFOBT | 0.0018 | 8.34 | 4725 |
| FOB Gold vs. no triage (referral straight to colonoscopy) | –0.0003 | –263.05 | 950,102 |
| OC-Sensor vs. gFOBT | 0.0019 | 9.55 | 5131 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –261.84 | 1,449,585 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6386 | 231.69 | NA | NA | NA |
| OC-Sensor | 18.6406 | 241.21 | Dominated by FOB-Gold | ||
| FOB Gold | 18.6406 | 240.01 | 0.0019 | 8.33 | 4283 |
| No triage (referral straight to colonoscopy) | 18.6408 | 500.60 | 0.0002 | 260.59 | 1,365,199 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| FOB Gold vs. gFOBT | 0.0019 | 8.33 | 4283 |
| FOB Gold vs. no triage (referral straight to colonoscopy) | –0.0002 | –260.59 | 1,365,199 |
| OC-Sensor vs. gFOBT | 0.0019 | 9.53 | 4962 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –259.39 | 1,212,654 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6230 | 258.97 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6239 | 244.42 | |||
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 274.75 | 0.0003 | 30.33 | 88,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0012 | 15.78 | 13,482 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0008 | –14.55 | OC-Sensor dominates |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259.25 | 4,133,559 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6432 | 257.38 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6440 | 242.90 | |||
| No triage (referral straight to colonoscopy) | 18.6441 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6444 | 272.90 | 0.0003 | 30.00 | 88,544 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0012 | 15.52 | 13,019 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –227.70 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0009 | –14.48 | OC-Sensor dominates |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –257.70 | 3,119,555 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6227 | 277.55 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6239 | 244.42 | |||
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 274.75 | 0.0003 | 30.33 | 88,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0015 | –3 | HM-JACKarc dominates |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –229 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0011 | –33 | OC-Sensor dominates |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –259 | 4,133,559 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6425 | 275.67 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6436 | 242.63 | |||
| No triage (referral straight to colonoscopy) | 18.6436 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6439 | 272.69 | 0.0004 | 30.05 | 83,261 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0014 | –2.99 | HM-JACKarc dominates |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –227.91 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0010 | –33.04 | OC-Sensor dominates |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –257.96 | 3,117,361 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6215 | 232.02 | NA | NA | NA |
| No triage (referral straight to colonoscopy) | 18.6231 | 503.16 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6231 | 243.95 | 0.0016 | 11.93 | 7454 |
| HM-JACKarc | 18.6234 | 274.24 | 0.0003 | 30.29 | 110,838 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0019 | 42 | 22,319 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –229 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0016 | 12 | 7370 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0000 | –259 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| OC-Sensor | 18.6355 | 242.48 | 0.0018 | 11.94 | 6499 |
| No triage (referral straight to colonoscopy) | 18.6355 | 500.11 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6357 | 272.41 | 0.0002 | 29.93 | 183,714 |
| OC-Sensor | 18.6355 | 242.48 | 0.0018 | 11.94 | 6499 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0020 | 41.88 | 20,934 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0002 | –227.70 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0018 | 11.94 | 6499 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0000 | –257.64 | 64,414,134 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6227 | 232.71 | NA | NA | NA |
| OC-Sensor | 18.6254 | 245.21 | 0.0027 | 12.50 | 4655 |
| No triage (referral straight to colonoscopy) | 18.6256 | 504.53 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6259 | 275.61 | 0.0005 | 30.40 | 60,798 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0032 | 42.90 | 13,574 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0027 | 12.50 | OC-Sensor dominates |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –259.32 | 1,322,872 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6424 | 230.92 | NA | NA | NA |
| OC-Sensor | 18.6455 | 243.30 | 0.0031 | 12.38 | 3984 |
| No triage (referral straight to colonoscopy) | 18.6457 | 501.46 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6461 | 273.34 | 0.0006 | 30.04 | 50,961 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0037 | 42.42 | 11,476 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0004 | –228.12 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0031 | 12.38 | 3984 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –258.16 | 1,081,160 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6221 | 230.97 | |||
| OC-Sensor | 18.6241 | 243.06 | 0.002 | 12.09 | 6081 |
| No triage (referral straight to colonoscopy) | 18.6245 | 500.49 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6245 | 273.19 | 0.0004 | 30.13 | 80,244 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0024 | 42.23 | 17,862 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0000 | –227.30 | Dominated by HM-JACKarc |
| OC-Sensor vs. gFOBT | 0.0020 | 12.09 | 6081 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –257.43 | 685,532 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6431 | 229.66 | |||
| OC-Sensor | 18.6453 | 241.61 | 0.0022 | 11.96 | 5406 |
| HM-JACKarc | 18.6457 | 271.48 | 0.0004 | 29.87 | 81,573 |
| No triage (referral straight to colonoscopy) | 18.6457 | 497.00 | 0.0000 | 225.52 | 9,135,836 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0026 | 41.82 | 16,224.64 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | –0.00002 | –225.52 | 9,135,836.87 |
| OC-Sensor vs. gFOBT | 0.0022 | 11.96 | 5406.14 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –255.39 | 653,493.58 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.701 | 263.92 | |||
| OC-Sensor | 18.711 | 279.10 | 0.0101 | 15.18 | 1508 |
| No triage (referral straight to colonoscopy) | 18.713 | 538.93 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.713 | 310.01 | 0.0019 | 30.9054 | 16,528 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0119 | 46.09 | 3859 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –228.92 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0101 | 15.18 | 1508 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0016 | –259.83 | 163,305 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.7191 | 262.22 | |||
| OC-Sensor | 18.7291 | 277.18 | 0.0101 | 14.96 | 1485 |
| HM-JACKarc | 18.7307 | 307.57 | 0.0015 | 30.39 | 19,790 |
| No triage (referral straight to colonoscopy) | 18.7307 | 535.84 | 0.0001 | 228.27 | 4,171,882 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0116 | 45.36 | 3907 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | –0.0001 | –228.27 | 4,171,882 |
| OC-Sensor vs. gFOBT | 0.0101 | 14.96 | 1485 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0016 | –258.66 | 162,620 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 243.73 | |||
| OC-Sensor | 18.6239 | 256.31 | 0.002 | 12.58 | 6352 |
| No triage (referral straight to colonoscopy) | 18.6239 | 531.40 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6242 | 288.36 | 0.0003 | 32.05 | 93,832 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 44.63 | 19,224 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –243.05 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.58 | 6352 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –275.10 | 4,386,188 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6390 | 243.13 | |||
| OC-Sensor | 18.6410 | 255.60 | 0.0020 | 12.46 | 6158 |
| No triage (referral straight to colonoscopy) | 18.6411 | 527.98 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6412 | 286.99 | 0.00 | 31.39 | 156,950 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 43.86 | 19,406 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –243.05 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 12.46 | 6159 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –272.39 | 3,859,404 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6219 | 232.28 | |||
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6241 | 375.40 | 0.0022 | 143.12 | 65,055 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| OC-Sensor vs. gFOBT | 0.0022 | 143.12 | 65,191.91 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0002 | –128.27 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6384 | 232.10 | |||
| No triage (referral straight to colonoscopy) | 18.6404 | 500.63 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6406 | 373.75 | 0.0022 | 141.65 | 64,386 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| OC-Sensor vs. gFOBT | 0.0022 | 141.65 | 65,696 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.0002 | –126.88 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6217 | 381.09 | |||
| OC-Sensor | 18.6237 | 389.26 | 0.002 | 8.17 | 4118 |
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6241 | 403.87 | 0.0004 | 14.61 | 36,534 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 22.78 | 9729 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0001 | –99.80 | Dominated by HM-JACKarc |
| OC-Sensor vs. gFOBT | 0.0020 | 8.17 | 4118 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –114.42 | 536,359 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6416 | 379.39 | |||
| OC-Sensor | 18.6437 | 387.44 | 0.0021 | 8.05 | 3793 |
| No triage (referral straight to colonoscopy) | 18.6439 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6440 | 401.82 | 0.0003 | 14.38 | 47,933 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0024 | 22.43 | 9421 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0000 | –98.78 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0021 | 8.05 | 3794 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0002 | –113.16 | 508,628 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6220 | 157.88 | |||
| No triage (referral straight to colonoscopy) | 18.6239 | 503.67 | Dominated by OC-Sensor | ||
| OC-Sensor | 18.6239 | 172.01 | 0.002 | 14.13 | 7143 |
| HM-JACKarc | 18.6243 | 210.19 | 0.0003 | 38.19 | 114,546 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0023 | 52.32 | 22,637 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –293.48 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0020 | 14.13 | 7143 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | 0.00001 | –331.67 | OC-Sensor dominates |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.6427 | 156.42 | |||
| OC-Sensor | 18.6445 | 170.45 | 0.0018 | 14.04 | 7697 |
| No triage (referral straight to colonoscopy) | 18.6446 | 500.60 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.6449 | 208.24 | 0.0004 | 37.79 | 94,475 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0022 | 51.82 | 23,340 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –292.36 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0018 | 14.04 | 7697 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0001 | –330.15 | 5,370,520 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.3672 | 253.88 | |||
| OC-Sensor | 18.3712 | 269.06 | 0.0040 | 15.19 | 3829 |
| No triage (referral straight to colonoscopy) | 18.3716 | 524.89 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.3719 | 299.55 | 0.0007 | 30.49 | 42,517 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0047 | 45.68 | 9754 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –225.34 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.004 | 15.19 | 3829 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0004 | –255.83 | 578,092 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 18.3886 | 252.18 | |||
| OC-Sensor | 18.3934 | 267.17 | 0.0048 | 14.99 | 3137 |
| No triage (referral straight to colonoscopy) | 18.3939 | 521.86 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 18.3944 | 297.15 | 0.001 | 30 | 29,980 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0058 | 44.97 | 7773 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0005 | –224.71 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0048 | 14.99 | 3137 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0005 | –254.69 | 491,877 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 17.9597 | 288.43 | |||
| OC-Sensor | 17.9668 | 308.48 | 0.0071 | 20.06 | 2808 |
| No triage (referral straight to colonoscopy) | 17.9679 | 558.85 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 17.9681 | 339.23 | 0.0013 | 30.75 | 23,327 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0085 | 50.80 | 6004 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0003 | –219.62 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0071 | 20.06 | 2808 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0011 | –250.36 | 238,380 |
| QALYs | Cost (£) | Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|---|---|
| gFOBT | 17.9840 | 286.88 | |||
| OC-Sensor | 17.9926 | 306.63 | 0.0086 | 19.75 | 2304 |
| No triage (referral straight to colonoscopy) | 17.9937 | 555.87 | Dominated by HM-JACKarc | ||
| HM-JACKarc | 17.9943 | 336.60 | 0.0017 | 29.97 | 17,629 |
| Incremental QALYs | Incremental cost (£) | ICER (£) | |
|---|---|---|---|
| HM-JACKarc vs. gFOBT | 0.0104 | 49.72 | 4798 |
| HM-JACKarc vs. no triage (referral straight to colonoscopy) | 0.0006 | –219.28 | HM-JACKarc dominates |
| OC-Sensor vs. gFOBT | 0.0086 | 19.75 | 2304 |
| OC-Sensor vs. no triage (referral straight to colonoscopy) | –0.0012 | –249.25 | 210,923 |
Appendix 12 National Institute for Health and Care Excellence guidance relevant to colorectal cancer
Published National Institute for Health and Care Excellence guidance
Aflibercept in Combination with Irinotecan and Fluorouracil-based Therapy for Treating Metastatic Colorectal Cancer that has Progressed Following Prior Oxaliplatin-based Chemotherapy. NICE technology appraisal guidance TA307; March 2014. URL: www.nice.org.uk/guidance/ta307 (accessed 9 August 2016).
Bevacizumab and Cetuximab for the Treatment of Metastatic Colorectal Cancer. NICE technology appraisal guidance TA118; January 2007. URL: www.nice.org.uk/guidance/ta118 (accessed 9 August 2016).
Bevacizumab in Combination with Oxaliplatin and either Fluorouracil plus Folinic Acid or Capecitabine for the Treatment of Metastatic Colorectal Cancer. NICE technology appraisal guidance TA212; December 2010. URL: www.nice.org.uk/guidance/ta212 (accessed 9 August 2016).
Capecitabine and Oxaliplatin in the Adjuvant Treatment of Stage III (Dukes’s C) Colon Cancer. NICE technology appraisal guidance TA100; April 2006. URL: www.nice.org.uk/guidance/ta100 (accessed 9 August 2016).
Cetuximab and Panitumumab for Previously Untreated Metastatic Colorectal Cancer. NICE technology appraisal guidance TA439; March 2017. URL: www.nice.org.uk/guidance/ta439 (accessed 9 August 2016).
Cetuximab for the First-line Treatment of Metastatic Colorectal Cancer. NICE technology appraisal guidance TA176; August 2009. URL: www.nice.org.uk/guidance/ta176 (accessed 9 August 2016).
Cetuximab, Bevacizumab and Panitumumab for the Treatment of Metastatic Colorectal Cancer after First-line Chemotherapy: Cetuximab (monotherapy or combination chemotherapy), Bevacizumab (in combination with non-oxaliplatin chemotherapy) and Panitumumab (monotherapy) for the Treatment of Metastatic Colorectal Cancer after first-line Chemotherapy. Review of technology appraisal guidance TA150 and part review of technology appraisal guidance TA118. NICE technology appraisal guidance TA242; January 2012. URL: www.nice.org.uk/guidance/ta242 (accessed 9 August 2016).
Colorectal Cancer Prevention: Colonoscopic Surveillance in Adults with Ulcerative Colitis, Crohn’s Disease or Adenomas. NICE clinical guideline CG118; March 2011. URL: www.nice.org.uk/guidance/cg118 (accessed 9 August 2016). Date for review: February 2019.
Colorectal Cancer: Diagnosis and Management. NICE clinical guideline CG131; November 2011, updated December 2014. URL: www.nice.org.uk/guidance/cg131 (accessed 9 August 2016). Date for review: February 2016.
Combined Endoscopic and Laparoscopic Removal of Colonic Polyps. NICE interventional procedure guidance IPG503; September 2014. URL: www.nice.org.uk/guidance/ipg503 (accessed 9 August 2016).
Computed Tomographic Colonography (Virtual Colonoscopy). NICE interventional procedure guidance IPG129; June 2005. URL: www.nice.org.uk/guidance/ipg129 (accessed 9 August 2016).
Cytoreduction Surgery followed by Hyperthermic Intraoperative Peritoneal Chemotherapy for Peritoneal Carcinomatosis. NICE interventional procedure guidance IPG331; February 2010. URL: www.nice.org.uk/guidance/ipg331 (accessed 9 August 2016).
Endoscopic Submucosal Dissection of Lower Gastrointestinal Lesions. NICE interventional procedure guidance IPG335; March 2010. URL: www.nice.org.uk/guidance/ipg335 (accessed 9 August 2016).
Fluorouracil chemotherapy: The My5-FU Assay for Guiding Dose Adjustment. NICE diagnostic guidance DG16; December 2014. URL: www.nice.org.uk/guidance/dg16 (accessed 9 August 2016).
Guidance on the Use of Capecitabine and Tegafur with Uracil for Metastatic Colorectal Cancer. NICE technology appraisal guidance TA61; May 2003. URL: www.nice.org.uk/guidance/ta61 (accessed 9 August 2016).
Improving Outcomes in Colorectal Cancer. NICE guidance on cancer services CSG5; June 2004. URL: www.nice.org.uk/guidance/csg5 (accessed 9 August 2016). To be updated by the update of the guideline on colorectal cancer (NICE guideline CG131).
Laparoscopic Surgery for Colorectal Cancer. NICE technology appraisal guidance TA105; August 2006. URL: www.nice.org.uk/guidance/ta105 (accessed 9 August 2016).
Preoperative High Dose Rate Brachytherapy for Rectal Cancer. NICE interventional procedure guidance IPG531; August 2015. URL: www.nice.org.uk/guidance/ipg531 (accessed 9 August 2016).
Radiofrequency Ablation for Colorectal Liver Metastases. NICE interventional procedure guidance IPG327; December 2009. URL: www.nice.org.uk/guidance/ipg327 (accessed 9 August 2016).
Selective Internal Radiation Therapy for Non-resectable Colorectal Metastases in the Liver. NICE interventional procedure guidance IPG401; July 2011, updated May 2013. URL: www.nice.org.uk/guidance/ipg401 (accessed 9 August 2016).
Suspected Cancer: Recognition and Referral. NICE guideline NG12; June 2015. URL: www.nice.org.uk/guidance/ng12 (accessed 9 August 2016).
Transanal Total Mesorectal Excision of the Rectum. NICE interventional procedure guidance IPG514; March 2015. URL: www.nice.org.uk/guidance/ipg514 (accessed 9 August 2016).
Wireless Capsule Endoscopy for Investigation of the Small Bowel. NICE interventional procedure guidance IPG101; 2004. URL: www.nice.org.uk/guidance/ipg101 (accessed 9 August 2016).
National Institute for Health and Care Excellence guidance under development
Colon Cancer (adjuvant) – Irinotecan [ID379]. NICE technology appraisal guidance. URL: www.nice.org.uk/guidance/indevelopment/gid-tag380 (accessed 9 August 2016). Expected publication date: TBC.
Low-energy Contact X-ray Brachytherapy (the Papillon Technique) for Early-stage Rectal Cancer. NICE interventional procedure guidance IPG532; September 2015. URL: www.nice.org.uk/guidance/indevelopment/gid-ip1234 (accessed 9 August 2016).
Glossary
- Adenoma
- A benign tumour of the epithelial tissue that, over time, may transform to become malignant.
- Colonoscopy
- Endoscopic examination of the large intestine and distal small intestine with a charge-coupled device camera or a fibre-optic camera.
- Colorectal cancer
- Cancer of the colon or rectum (large intestine).
- Computed tomography colonography
- A medical imaging procedure using X-rays to produce two- and three-dimensional images of the colon and distal small intestine.
- Cost-effectiveness analysis
- An economic analysis that converts effects into health terms and describes the costs for additional health gain.
- Decision modelling
- A mathematical construct that allows the comparison of the relationship between costs and outcomes of alternative health-care interventions.
- Faecal immunochemical test
- An immunochemical method of detecting blood in the faeces, which specifically detects the globin moiety of human haemoglobin.
- Faecal occult blood
- Blood in the faeces that is not visibly apparent.
- False negative
- Incorrect negative test result – number of diseased persons with a negative test result.
- False positive
- Incorrect positive test result – number of non-diseased persons with a positive test result.
- Guaiac faecal occult blood test
- A chemical method of detecting blood in the faeces that utilises the pseudo-peroxidase activity of haem to detect the haem complex.
- Incremental cost-effectiveness ratio
- The difference in the mean costs of two interventions in the population of interest divided by the difference in the mean outcomes in the population of interest.
- Index test
- The test whose performance is being evaluated.
- Inflammatory bowel disease
- A group of inflammatory diseases of the colon and small intestine (e.g. Crohn’s disease, ulcerative colitis).
- Markov model
- An analytic method that is particularly suited to modelling repeated events or the progression of a chronic disease over time.
- Meta-analysis
- Statistical techniques used to combine the results of two or more studies and obtain a combined estimate of effect.
- Meta-regression
- Statistical technique used to explore the relationship between study characteristics and study results.
- Negative predictive value
- The probability of non-disease among persons with a negative test result.
- Opportunity costs
- The cost of forgone outcomes that could have been achieved through alternative investments.
- Positive predictive value
- The probability of disease among persons with a positive test result.
- Probabilistic sensitivity analysis
- A method of quantifying the uncertainty in a mathematical model, such as a cost-effectiveness model.
- Publication bias
- Bias arising from the preferential publication of studies with statistically significant results.
- Quality of life
- An individual’s emotional, social and physical well-being and their ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain, used in economic evaluations, in which survival duration is weighted or adjusted by the patient’s quality of life during the survival period.
- Receiver operating characteristic curve
- A graph that illustrates the trade-offs between sensitivity and specificity that result from varying the diagnostic threshold.
- Reference standard
- The best currently available method for diagnosing the target condition. The index test is compared against this to allow calculation of estimates of accuracy.
- Regression analysis
- A statistical method for estimating relationships among variables.
- Sensitivity
- Proportion of people with the target disorder who have a positive test result.
- Specificity
- Proportion of people without the target disorder who have a negative test result.
- True negative
- Correct negative test result – number of non-diseased persons with a negative test result.
- True positive
- Correct positive test result – number of diseased persons with a positive test result.
List of abbreviations
- AACC
- American Association for Clinical Chemistry
- AJCC
- American Joint Committee on Cancer
- ARIF
- Aggressive Research Intelligence Facility
- BSG
- British Society of Gastroenterology
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CCT
- controlled clinical trial
- CDSR
- Cochrane Database of Systematic Reviews
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CRC
- colorectal cancer
- CRD
- Centre for Reviews and Dissemination
- CT
- computed tomography
- CTC
- computed tomography colonography
- DARE
- Database of Abstracts of Reviews of Effects
- DDW
- Digestive Disease Week
- EQ-5D
- EuroQol-5 Dimensions
- FAST
- Faecal haemoglobin, Age and Sex Test
- FIT
- faecal immunochemical test
- FN
- false negative
- FOBT
- faecal occult blood test
- FP
- false positive
- GDG
- guideline development group
- gFOBT
- guaiac faecal occult blood test
- GI
- gastrointestinal
- GP
- general practitioner
- Hb
- haemoglobin
- Hp
- haptoglobin
- HRA
- high-risk adenoma
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSROC
- hierarchical summary receiver operating characteristic
- HTA
- Health Technology Assessment
- IBD
- inflammatory bowel disease
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- INAHTA
- International Network of Agencies for Health Technology Assessment
- LY
- life-year
- MeSH
- medical subject heading
- NCIN
- National Cancer Intelligence Network
- NHS EED
- NHS Economic Evaluations Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPV
- negative predictive value
- ONS
- Office for National Statistics
- PPV
- positive predictive value
- PROBAST
- Prediction model study Risk Of Bias Assessment Tool
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Service Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SE
- standard error
- SIGN
- Scottish Intercollegiate Guidelines Network
- SROC
- summary receiver operating characteristic
- TN
- true negative
- TP
- true positive
- UEGW
- European Gastroenterology Federation Week
- WHO
- World Health Organization
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.