Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/53. The contractual start date was in February 2012. The draft report began editorial review in March 2016 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Jeffcoate et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
The protocol has been previously published, and some sections of this earlier publication are replicated in Chapters 1 and 2 under the terms of the licence granted by BioMed Central Ltd. 1 © Jeffcoate et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Foot ulcers are a major source of suffering in people with diabetes mellitus. 2 They affect an estimated 15% of people with the disease, and those with foot ulcers are typically affected by other complications of diabetes mellitus including cardiovascular, renal and eye disease. Almost 28% of ulcers have been reported to lead to some form of amputation. 3 Overall life expectancy is only 50% at 5 years after presentation, which is lower than that for many cancers. 4,5 Diabetic foot ulcers are also enormously costly and are estimated to account for almost 0.7–0.8% of the total NHS budget in the UK. 6 Only two-thirds of all ulcers heal without amputation within 12 months, and the median time to healing for these is 78 days. 7,8 Forty per cent of patients whose ulcers heal will develop a recurrence within 12 months. 9
Ulcers of the heel present particular difficulties, reflected in the proverbial expression ‘heel ulcers don’t heal’. Although 7% of heel ulcers result in amputation of the limb in diabetes mellitus and 20% persist until death,10 a single-centre review of a consecutive series of 154 heel ulcers in 97 patients with diabetes mellitus managed in the UK revealed that the eventual incidence of healing without surgery was very similar to that of ulcers elsewhere on the foot. The median time to healing was, however, very much longer at 200 days (range 24–1225 days),10 almost three times longer than that of ulcers elsewhere on the foot. A more recent multicentre survey in 14 expert centres in Europe reported a very similar median time to healing of heel ulcers: 237 days. 11 Heel ulcers in diabetes mellitus also differ from ulcers elsewhere on the foot in that they are frequently painful.
Although the principles of care of foot ulcers in diabetes mellitus have been promoted by the National Institute for Health and Care Excellence (NICE), the Royal College of Nursing12 and the International Working Group on the Diabetic Foot (International Diabetes Federation),13 there are no specific interventions that have been shown to improve the outcome for patients with diabetes mellitus and foot ulcers. The use of a non-removable below-knee (total contact or variant) cast is known to hasten healing in ulcers caused by abnormal pressure loading on other parts of the foot,14 but this treatment has previously been reported to be ineffective when the ulceration is on the heel. 15
In the absence of specific treatment of proven effectiveness for heel ulcers, a small number of specialists in the UK have started to use lightweight, removable fibreglass heel casts. Based on uncontrolled observational evidence, it has been reported that these devices result in both a reduced time to healing and a prompt improvement in pain. Healing was observed in 42 (84%) of a consecutive series of 50 heel ulcers (in patients both with and without diabetes mellitus, but all with peripheral arterial disease), with a median (range) time to healing of 6 weeks (3–13 weeks). 16 The mechanism for any positive effect is not known, but it could relate to the reduction of shearing and stretching forces applied to the surface of the ulcer. Current strategies to reduce local forces in an area of ulceration (or an area at risk of ulceration) are largely concentrated attempts to reduce vertical forces, with minimal, if any, effect on shear and on stretching.
Lightweight fibreglass heel casts take approximately 15 minutes to mould to the heel and can easily be fashioned in a domiciliary setting. The casts are applied over the primary wound dressing and held in place with an outer dressing, and they are saved and reused each time the dressing is changed. They are replaced when stained, damaged or lost and can often be worn inside shoes. Health-care professionals can be trained in their use in approximately 30 minutes and the material cost of each cast is approximately £7. On average, casts need to be replaced every 3 weeks.
The purpose of the proposed study was to determine the clinical effectiveness and cost-effectiveness of this simple and apparently beneficial intervention, when compared with usual care.
Chapter 2 Methods
This was an observer-blind randomised controlled trial comparing the use of lightweight, fibreglass heel casts in addition to usual care with usual care alone in people with diabetes mellitus complicated by ulcers of the heel. The study was conducted in accordance with (1) independent ethics committee approval, (2) relevant informed consent regulations (Declaration of Helsinki),17 (3) ISO (International Organization for Standardization)-14155 guidelines,18 (4) the Data Protection Act 1998,19 (5) local regulatory requirements with particular reference to participant safety and (6) the principles of good clinical practice (GCP). An independent Trial Steering Committee and a Data Monitoring Committee were convened in accordance with Medical Research Council guidelines. The study was approved by Yorkshire and the Humber – Leeds West National Research Ethics Committee (reference 10/H1307/124) under the UK National Integrated Research Application System. All of the participants gave written informed consent.
Protocol amendment
A major amendment to the protocol was approved on 29 March 2012. Prior to that date, if there was a protocol violation from non-use of the heel cast by a participant allocated to the intervention arm, the participant was withdrawn and took no further part in the study. After the amendment took effect, such a protocol violation was noted but the participant was not withdrawn. On the same date, the protocol was amended such that adverse events (AEs) were no longer logged, and safety was documented only by the use of adverse device effects (ADEs) and serious adverse device effects (SADEs). The number of participants who were randomised before and after this protocol amendment was 133 and 396, respectively. The amended protocol has been published. 1
Participants
People with diabetes mellitus and ulcers of the heel attending specialist centres in the UK were screened for inclusion in the study. The ulcers of the heel were required to be of National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel (NPUAP/EPUAP) grades 2, 3 or 4 (Box 1); to be affecting the skin below the malleoli but overlying the calcaneum inferiorly, posteriorly, medially or laterally; and to have been present for at least 2 weeks. The eligibility criteria, shown in Box 2, were chosen to be as inclusive as possible; neither soft tissue infection nor mild or moderate degrees of limb ischaemia were contraindications to inclusion. Those who met the criteria for inclusion and who provided informed written consent were recruited and randomised to either the intervention arm, that is, to receive treatment with a lightweight, moulded, fibreglass heel cast together with continuing usual care, or to the control arm, that is, to continue with usual care alone (Box 3). If a person had more than one ulcer that fulfilled the selection criteria, only one ulcer (generally the largest and most clinically significant) was selected as the index ulcer for the purpose of the study. All data were entered into an electronic case report form (eCRF), which also incorporated the randomisation tool.
-
Non-blanchable erythema.
-
Partial thickness skin loss.
-
Full-thickness skin loss.
-
Full-thickness tissue loss.
-
Unstageable/unclassified: full-thickness tissue loss – depth unknown.
Note: the text of this box is reproduced from the previously published protocol,1 and is used here according to the terms of the licence granted by BioMed Central Ltd. © Jeffcoate et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
-
Type 1 or type 2 diabetes mellitus.
-
Aged ≥ 18 years.
-
An ulcer of the heel (below the malleoli and affecting the skin overlying the calcaneum) of NPUAP/EPUAP grade 2–4 that has been present for ≥ 2 weeks and that has a cross-sectional area of ≥ 25 mm2. If there is more than one heel ulcer, one – the largest or the most clinically significant – will be selected as the index ulcer.
-
Able and willing to give written informed consent.
-
Frailty or disability that would mean participation in the study might have an adverse effect on patient well-being and mood.
-
The need for any offloading device to be non-removable.
-
The likelihood of protocol violation because of planned travel.
-
Those who withhold consent.
-
Active participation in another study of a wound-care product.
-
The use of topical negative pressure or application of larvae to the index heel ulcer.
Note: the text of this box is reproduced from the previously published protocol,1 and is used here according to the terms of the licence granted by BioMed Central Ltd. © Jeffcoate et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
-
Provision of any necessary offloading.
-
Debridement:
-
Sharp.
-
Other as appropriate (excluding the use of larvae).
-
-
Appropriate dressing products.
-
Appropriate antibiotic therapy.
-
Nutrition and self-care.
-
Optimal glycaemic control.
-
Revascularisation if deemed necessary and possible.
-
Continued close observation.
Note: the text of this box is reproduced from the previously published protocol,1 and is used here according to the terms of the licence granted by BioMed Central Ltd. © Jeffcoate et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Baseline clinical assessment
Participants
The participants’ age, sex and type and duration of diabetes were recorded, as were details of their mobility. Mobility was graded on a 4-point scale: (1) able to walk unaided, (2) able to walk with assistance, (3) chairbound and (4) bedbound. A record was also kept of the type of offloading used to reduce pressure on the ulcerated area. For the purpose of sensitivity analysis of the primary outcome, types of offloading were divided into two categories: (1) ‘more effective’, that is, those more likely to achieve effective pressure relief (i.e. a removable below-knee offloading device or cast or a removable fibreglass slipper/whole foot device) and (2) ‘less effective’, that is, those less likely to achieve effective pressure relief (i.e. being bedbound or immobile, normal footwear, fitted footwear/orthoses, fitted insoles/inserts or a padded slipper or shoe).
Complications of diabetes were recorded simply as whether or not the patient had any history of cerebrovascular or cardiovascular disease and whether or not they had known nephropathy or retinopathy. The health status of the participants was documented using the EuroQol-5 Dimensions three-level version (EQ-5D-3L),20 the EuroQOL health score and the Cardiff Wound Impact Schedule (CWIS). 21 The CWIS is a condition-specific quality-of-life tool with questions grouped into sections on social life, physical symptoms, daily living and well-being, generating scores of 0–100 for each section. Higher scores indicate a better quality of life.
Affected foot
The side and the position of the ulcer on the heel were recorded. The arterial blood flow to the foot was defined by palpability of pedal pulses (posterior tibial and dorsalis pedis) and ankle brachial pressure index (ABPI). Neuropathy (loss of protective sensation) was assessed using a 10-g monofilament under the first metatarsal head, the fifth metatarsal head and under the pulp of the hallux.
Ulcer
The area of the chosen index ulcer was determined using Image J software (National Institutes of Health, Bethesda, MD, USA)22 from an acetate tracing made using a prespecified procedure. For the purposes of inclusion and stratification, the area was assessed at baseline by a non-blinded clinical researcher from a tracing made onto a sterile acetate sheet. These assessments were later checked (after randomisation) by a single, central, blinded observer who was not otherwise involved in the conduct of the study, and the area was determined using Image J software. This assessment of the area was taken as the definitive baseline area for use in data analyses. Images were also taken of acetate tracings of the ulcer (as well as of the ulcer itself) at each subsequent clinic visit. The non-blinded clinical observer also estimated the granulation percentage of the ulcer base, the amount of exudate and the condition of the surrounding skin. Pain in the region of the ulcer (if any) was quantified using a 10-cm visual analogue scale (VAS).
Intervention
Both groups received usual care delivered by a specialist service designed for the management of ulcers of the foot in diabetes mellitus. In addition to usual care, those in the intervention group had a lightweight, fibreglass cast moulded to their heel. Each cast was lined and flexible at the edges but reinforced over the ulcerated area and was intended to be thin enough to be worn inside footwear or another offloading device. It was applied over the primary dressing and a single protective layer of Softban® (BSN Medical, Hull, UK) or equivalent bandage. The cast was then held in place by bandaging. The choice of primary dressing and any other secondary dressing was at the discretion of the participating centre. The cast was removable and was reapplied over the primary dressing with each dressing change until it needed to be replaced when it was soiled, lost or otherwise unusable. The design of the cast was based on that reported by the group originally reporting a good outcome with the use of such casts,16 and all researchers involved in the clinical management of ulcers at each centre were trained by a single specialist podiatrist in how to make heel casts according to the study specific procedure. Researcher performance was assessed every 6 months by a designated competent researcher, whose own performance was also assessed twice over the course of the study.
Study conduct
Following randomisation, participants were asked to attend the specialist clinic for review every 2 weeks. The ulcer was cleaned with local sharp debridement if required. It was then dressed using the primary dressing of choice of the participant’s usual carer. Participants in the intervention group then had their heel cast repositioned (a new cast having been made if necessary) and held in place with a bandage as described above. Any intercurrent infection, deterioration or other clinical problem was treated in accordance with usual practice.
If the ulcer was thought to be healed, it was checked by an observer who was blind to randomisation group and it was reviewed after a further 2 and 4 weeks. If the ulcer broke down within 4 weeks, the person was asked to continue in the study, attending the clinic every 2 weeks. If healing was confirmed 4 weeks after initial healing, the person was asked to attend only the research visits at week 12 (assuming that this had not already passed) and week 24. If an ulcer was first judged to be healed at week 22 or 24, the participant remained in the study until healing had or had not been confirmed by a blinded observer after 4 weeks. A participant’s failure to attend two consecutive fortnightly visits or a total of three visits during the 24-week follow-up period was regarded as a protocol violation necessitating that person’s withdrawal from the study. Failure of those in the intervention group to use the heel cast for > 7 consecutive days or for a cumulative total of > 14 days was regarded as a protocol violation, but – following the protocol amendment of 29 March 2012 (see Protocol amendment) – did not necessitate their withdrawal from the study.
Withdrawal
Participants were removed from the study if they (1) withdrew consent to participate, (2) lost capacity, (3) were recruited in error, (4) did not attend the required number of follow-up visits or (5) were lost to follow-up. Prior to the protocol amendment in March 2012, participants randomised to the intervention arm were also withdrawn if they violated the protocol with regard to use of the cast (see Protocol amendment).
Objectives
The primary objective was to determine whether or not the use of a heel cast in addition to usual care was associated with a higher incidence of healing at or before 24 weeks than usual care alone.
Outcomes
Primary outcomes
The primary outcome was healing that was first identified on or before 24 weeks from randomisation. Healing was defined as epithelialisation maintained for 4 weeks and confirmed by an observer blind to randomisation group. When confirmed, the date of healing was taken as that on which it was first observed.
Secondary outcomes
Ulcer-related outcomes
-
Time to healing.
-
Change in ulcer area (measured from digital images made of acetate tracings with area calculated using Image J software).
-
Secondary infection.
-
Major and minor amputation.
-
Ulcer recurrence.
-
Secondary ulceration on either limb.
Patient-related outcomes
-
Local pain (VAS).
-
EQ-5D-3L.
-
CWIS.
-
Hospital admission (including both admissions that were primarily related and admissions that were unrelated to the heel ulcer).
-
Adverse effects.
-
Death.
Data on length of hospital stay were not recorded.
Sample size
The expected percentages of healed ulcers at or before 24 weeks in the control group and the treatment group were 40% and 55%, respectively. With two-sided significance level of 5% (α = 0.05), power = 80% and estimated non-collection of primary outcome data of 25%, a total of 496 patients to be randomised was required in order to achieve 186 in each group for the primary analysis.
Randomisation
Randomisation was stratified by ulcer grade (NPUAP/EPUAP grade 2, 3 or 4; see Box 1) and by ulcer area (25–100 mm2 or > 100 mm2), using randomly permuted blocks of randomly varying size. Randomisation was undertaken by Nottingham Clinical Trials Unit, using a secure web-based system to ensure allocation concealment. This system was embedded within the eCRF, and, once the clinical data were entered at the time of the randomisation visit, the allocation was revealed immediately to the researcher online.
Blinding
Owing to the nature of the intervention, it was not possible to blind either the participant or the clinical researcher. However, all of the confirmation assessments of healing and laboratory measures were undertaken by an observer who was blind to randomisation group. In addition, all images of ulcer tracings used to document change in ulcer area were measured by a single, central blinded observer.
Statistical analysis
The analysis and reporting of the trial was in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines. 23 Analyses were detailed in a statistical analysis plan (SAP), which was finalised prior to database lock but after publication of the trial protocol. Participants were analysed according to their randomised allocation regardless of their adherence to their allocated intervention and without imputation for missing data for all primary and secondary outcomes [in keeping with the intention-to-treat (ITT) principle]. All analyses were conducted using Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
Preliminary analyses
Descriptive statistics of demographic and clinical measures were used to examine the balance between the randomised arms at baseline.
Primary outcome
Ulcer healing (the primary outcome measure) was assessed independently by two statisticians based on data recorded at each participant visit by clinical researchers and an observer who was blinded to treatment allocation. Full details of the assessment of ulcer healing can be found in Appendix 1. A participant met the criteria for a healed ulcer if, 4 weeks after the clinical researcher and a blinded clinician at the site had initially judged the ulcer to have healed, the ulcer was judged to have remained healed by both the clinical researcher and the blinded observer. The time to healing was recorded as the number of days between randomisation and the date of the visit that the ulcer was first judged healed by the clinical researcher.
The primary analysis compared the proportion of participants with healed ulcers at or before 24 weeks in the intervention group versus the standard care group. The number and percentage of participants with a healed ulcer at or before 24 weeks were presented for each group, and relative [odds ratio (OR)] and absolute (risk difference) measures of effect, along with 95% confidence intervals (CIs) and p-values, were estimated using multivariable, generalised linear regression models for binary variables, adjusted for baseline ulcer size and grade as stratification factors.
Sensitivity analyses of the primary outcome
Sensitivity analyses of the primary outcome were performed with additional adjustment for any other prognostic variables that showed a marked imbalance at baseline. Missing data were imputed using the multiple imputation by chained equation procedure, implemented using the ‘mi’ command in Stata version 13.1. The number of imputations was set to 20, which ensured that the number of imputations was greater than the number of missing data. The imputation model followed recommended practice by including demographic variables without missing data alongside outcomes with missing data. The ORs for ulcer healing from the logistic regression analysis in each imputed data set were combined using Rubin’s rules. In addition to multiple imputation, simple imputation was used assuming that all participants with missing primary outcome data remained unhealed. The primary analysis was also repeated with the data restricted to participants who were deemed not to have violated the protocol.
Subgroup analyses of the primary outcome
Prespecified subgroup analyses of the primary outcome were performed according to the following variables: baseline ulcer area (≤ 100 vs. > 100 mm2), baseline ulcer grade (depth grade 2 vs. grade 3 vs. grade 4), baseline mobility (immobile vs. mobile), baseline ABPI (< 0.9 vs. 0.9–1.4 vs. > 1.4), neuropathy (yes vs. no) and age at randomisation (< 70 vs. ≥ 70 years). A further post hoc subgroup analysis was also performed according to offloading at baseline (‘more effective’ vs. ‘less effective’). These subgroup analyses were conducted by the inclusion of appropriate interaction terms in the regression model and were considered to be exploratory.
Secondary outcomes
Time to healing
The time to complete healing of the index ulcer was compared between the two randomised groups using survival analysis. Kaplan–Meier survival curves were produced for the two groups, and the adjusted hazard ratio and 95% CI were estimated using a Cox proportional hazards model including stratification factors (ulcer size and grade). Participants with heel ulcers that were not healed or for which healing data were not available were treated as censored, and the date of their exit from the trial, their last available assessment or their 24-week visit, as appropriate, was used to calculate the duration of time for which they participated in the trial.
Ulcer area
The ulcer area was estimated based on the measurements from digital images of an acetate tracing, or from the digital images of the ulcer itself if it was not possible to determine the area from the image of the acetate tracing. The mean ulcer area for those participants who remained unhealed was summarised at each time point by treatment arm. In a post hoc analysis, the association between ulcer area change from baseline to 4 weeks and final healing status was investigated using logistic regression. Extreme outliers, defined as those with a greater than twofold increase in ulcer area at 4 weeks compared with baseline, were excluded from this analysis. Using interaction terms in the model, we investigated whether or not any association differed according to baseline ulcer area or treatment arm. No evidence of interaction was found, and the estimated association is adjusted for baseline area and grade of ulcer and for treatment arm.
Other ulcer-related secondary outcomes
The binary outcomes of infection, major amputation, minor amputation, revascularisation of the limb with the target ulcer or a trip or fall leading to hospital admission were derived as outlined in Appendix 2. The binary secondary outcomes for any hospital admission, a new ulcer on the target foot or a new ulcer on the contralateral foot were derived as outlined in Appendix 3.
Data are described using the number and percentage of participants with each outcome by treatment arm, and compared using multivariable logistic regression, adjusted for baseline ulcer area and grade.
EuroQol-5 Dimensions-3 level version and Cardiff Wound Impact Schedule
Cardiff Wound Impact Schedule scores were derived for social life, physical symptoms and daily living and well-being on a scale of 0–100. Up to 50% of missing items could be imputed for each scale based on the within-person mean score of observed items in the scale. The experience and stressfulness items for the social life and physical symptoms and daily living scales were considered separately for this purpose.
The EQ-5D-3L utility score, EQ-5D-3L health status score, CWIS social life score, CWIS physical symptom and daily living score, CWIS well-being score, CWIS overall quality of life and satisfaction with quality of life over the past week were summarised by treatment groups at baseline, 12 weeks and 24 weeks. The scores were analysed using a linear mixed-effects model, with the participant as a random effect and baseline value of the outcome and the baseline ulcer area and grade as covariates. This model used all of the observed data and estimated the adjusted between-group difference ‘averaged’ across all follow-up occasions, in this case at 12 weeks and 24 weeks. We investigated whether or not any between-group differences changed over time using interaction terms in the model between treatment arm and time and found no evidence of any such interactions.
Ulcer-related local pain (visual analogue scale)
Ulcer-related pain for those who remained unhealed at the end of the study was summarised in the two groups and analysed using analysis of covariance, implemented using multivariable linear regression adjusting for baseline pain score, ulcer area and ulcer grade. Pain scores at baseline, 4 weeks and 24 weeks were positively skewed and were log-transformed for analysis, with adjusted ratio of geometric means as the between-group estimate of effect. Pain at follow-up was also analysed as a binary variable (any pain vs. no pain) using multivariable logistic regression. Additional post hoc analyses restricted to the subset of participants who reported any ulcer-related pain at baseline were performed and designed to compare the difference in pain scores (continuous variable) between groups at 2 weeks and 4 weeks, adjusted for baseline pain and baseline ulcer area and grade.
Health economic analysis
The health economic evaluation originally set out to estimate the cost-effectiveness of the use of lightweight heel casts versus usual care by developing a decision-analytic model across a series of time horizons that reflect the management of patients with diabetic ulcers of the heel. A health economic analysis plan was constructed in accordance with the trial SAP as follows:
-
Carry out a within-trial analysis of cost-effectiveness (including cost–utility) of the use of lightweight heel casts versus usual care.
-
Depending on the within-trial results, carry out an analysis of the longer-term cost-effectiveness over agreed time horizons with a budget impact analysis for 1-year and 5-year periods, which will compare the costs to the NHS of the use of heel casts plus usual care with usual care alone in the management of ulcers of the heel in diabetes mellitus.
In accordance with the NICE reference case,24 the perspective adopted was a UK NHS and Personal Social Services (PSS) one. The primary end point for the economic analysis was an incremental cost per quality-adjusted life-year (QALY), and the secondary end point was an estimation of incremental cost per percentage of additional healed ulcers at 24 weeks. Base case results were calculated using a series of sensitivity analyses, undertaken to assess the degree to which variations in parameter estimates would affect the relative cost-effectiveness ratios. Bootstrapping was undertaken to address the uncertainty associated with point estimates of costs and outcomes, and to produce CIs for the incremental cost-effectiveness ratio (ICER). Cost-effectiveness acceptability curves (CEACs) were generated to give a range of cost-effectiveness thresholds for decision-makers. A detailed description of the health economics methods is provided in Appendix 4, including the justification for the health economic analysis being constrained to a within-trial analysis.
Safety end points
The study population was one that was relatively older and that had a high prevalence of comorbidities, including renal, cardiac and cerebrovascular diseases. Moreover, ulcers of the foot may worsen, potentially resulting in hospital admission. Although no specific safety issues were foreseen with the use of the heel cast, significant events were listed among the secondary outcome measures. Other unexpected ADEs were recorded and, if considered serious (SADEs), were reported to the sponsor in accordance with the principles of GCP.
Adverse events
Adverse events were documented only in the period prior the protocol amendment dated 29 March 2012. Those AEs that were documented were mentioned either spontaneously or in response to questioning. The clinical researcher assessed the causal relationship of the AE to the use of the fibreglass heel cast (if used). In addition, AEs were rated according to severity – mild, moderate or severe – using predefined criteria.
Foreseeable adverse events
A number of AEs were likely to occur in the study population. These were recorded as secondary outcomes and included:
-
ulcer-related outcomes
-
increase in ulcer area
-
infection
-
major and minor amputation
-
recurrence after healing
-
new ulceration of either limb
-
-
patient-related outcomes
-
increase in pain
-
worsening mood or function
-
hospital admission or death from pre-existing medical conditions.
-
Adverse device effects
Following the change to the protocol on 29 March 2012, data were collected on ADEs and SADEs. An ADE was regarded as any untoward and unintended consequence of the use of the device, including any effect resulting from insufficiencies or inadequacies in the instructions for its use and any effect resulting from user error or accident. The researcher was required to grade the severity of any ADE as ‘mild’, ‘moderate’ or ‘severe’ and to assess the causal relationship of the ADE to the device as ‘none’, ‘possible’ or ‘probable’ using prespecified criteria. Foreseeable ADEs were those related to worsening of the clinical state of the ulcer and were reported as secondary outcomes. These included increase in ulcer area, infection, major and minor amputation, ulcer recurrence and secondary ulceration on either foot. Other foreseeable patient-related AEs were also recorded as secondary outcomes, including an increase in pain, worsening mood or function, hospital admission (relating primarily to the heel ulcer) and death from pre-existing medical conditions.
Serious adverse device effects
These were defined as any ADE that resulted in death, life-threatening illness or injury, hospitalisation or additional medical or surgical intervention. The causal relationship between the SADE and the use of the device was documented as ‘none’, ‘possible’ or ‘probable’ using prespecified criteria.
Patient-related AEs that were unrelated were also recorded as secondary outcomes. These included an increase in pain, worsening mood or function, hospital admission (relating primarily to the heel ulcer) and death from pre-existing medical conditions.
Chapter 3 Results
Participant flow
The details of participant flow are shown in Figure 1. From 1915 people who were screened at participating specialist units and who had diabetes mellitus complicated by heel ulcers, 509 participants were recruited. Two hundred and fifty-three were randomised to continue with usual care (control), and 256 were randomised to the intervention arm (although the actual use of a heel cast was later recorded in only 244). A total of 84 participants (16.5%) did not complete the study (44 in the intervention group and 40 in the control group), but a primary outcome was available for the remaining 425 (212 in the intervention group and 213 in the control group).
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.

Recruitment
Recruitment started in April 2011 and continued until September 2014. Follow-up was completed in March 2015. Specialist NHS centres routinely involved in the management of diabetic foot disease were included progressively during the active phase of the study. The total number of involved centres was 35 (Table 1).
| Site | Usual care (n = 253) | Intervention (n = 256) | Total (n = 509) |
|---|---|---|---|
| Royal Derby Hospital | 26 | 23 | 49 |
| Nottingham University Hospitals NHS Trust | 27 | 14 | 41 |
| Torbay Hospital – South Devon Healthcare | 20 | 20 | 40 |
| Norfolk and Norwich Hospitals NHS Trust | 13 | 18 | 31 |
| Central Essex Community Services | 15 | 13 | 28 |
| Royal Cornwall Hospitals NHS Trust | 13 | 12 | 25 |
| Bradford Teaching Hospitals | 12 | 12 | 24 |
| Sheffield Teaching Hospitals | 11 | 12 | 23 |
| Ipswich Hospital NHS Trust | 9 | 11 | 20 |
| Royal Devon and Exeter Foundation Trust | 11 | 9 | 20 |
| Royal Berkshire NHS Foundation Trust | 7 | 10 | 17 |
| NHS Gloucestershire Care Services | 7 | 10 | 17 |
| Cambridge University Hospitals | 5 | 10 | 15 |
| City Hospitals Sunderland | 7 | 7 | 14 |
| Northumbria Healthcare NHS Trust | 6 | 6 | 12 |
| Salford Royal NHS Foundation Trust | 5 | 7 | 12 |
| Mid Yorkshire Hospitals | 4 | 7 | 11 |
| Newcastle upon Tyne Hospitals | 9 | 2 | 11 |
| Morriston Hospital Swansea NHS Trust | 7 | 4 | 11 |
| Northampton General Hospital NHS Trust | 4 | 7 | 11 |
| The Royal Wolverhampton Hospitals | 5 | 5 | 10 |
| Barnsley Hospital NHS Foundation Trust | 2 | 8 | 10 |
| Royal Bournemouth and Christchurch Hospitals | 6 | 3 | 9 |
| South Tees Hospitals NHS Trust | 3 | 4 | 7 |
| University of Leicester Hospitals | 4 | 3 | 7 |
| Plymouth Hospitals NHS Trust | 3 | 3 | 6 |
| St George’s Healthcare NHS Trust | 0 | 5 | 5 |
| University Hospital of North Staffordshire | 2 | 2 | 4 |
| New Victoria Hospital – Glasgow and Clyde | 0 | 4 | 4 |
| The Pennine Acute Hospitals NHS Trust | 2 | 2 | 4 |
| Yeovil District Hospital NHS Foundation Trust | 1 | 2 | 3 |
| University Hospitals Coventry and Warwickshire | 1 | 1 | 2 |
| Greater Glasgow and Clyde Southern General | 2 | 0 | 2 |
| Royal United Hospital Bath NHS Trust | 2 | 0 | 2 |
| Imperial College Healthcare NHS Trust | 2 | 0 | 2 |
Baseline data
Demographics
Baseline details on age, sex, relative mobility, diabetes mellitus type and duration, and the occurrence of other complications for participating individuals are shown in Table 2. The mean age was as expected for this population, as was the male preponderance and the relative prevalence of type 1 and type 2 diabetes mellitus. There were slight imbalances between the intervention and control arms in age, sex and types of immobility, but the two groups were otherwise well matched.
| Baseline characteristic | Usual care (N = 253) | Intervention (N = 256) | Total (N = 509) |
|---|---|---|---|
| Age at inclusion (years) | |||
| Mean (SD) | 66.1 (12.6) | 68.9 (12.1) | 67.5 (12.4) |
| Median (25th centile, 75th centile) | 66.2 (58.1, 75) | 70.8 (60.3, 78.1) | 68.5 (59.5, 76.8) |
| Minimum, maximum | 25.9, 91.9 | 28.3, 94.3 | 25.9, 94.3 |
| Sex | |||
| Female | 88 (35) | 77 (30) | 165 (32) |
| Male | 165 (65) | 179 (70) | 344 (68) |
| Immobility | |||
| No | 117 (46) | 111 (43) | 228 (45) |
| Yes | 136 (54) | 145 (57) | 281 (55) |
| Type of immobility | |||
| Walking with aid of assistance | 92 (36) | 112 (44) | 204 (40) |
| Chairbound | 43 (17) | 31 (12) | 74 (14) |
| Bedbound | 1 (1) | 2 (1) | 3 (1) |
| Type of diabetes mellitus | |||
| Type 1 | 39 (15) | 37 (14) | 76 (15) |
| Type 2 | 214 (85) | 219 (86) | 433 (85) |
| Duration (years) | |||
| Mean (SD) | 16.9 (11.4) | 19.2 (13.0) | 18.1 (12.3) |
| Median (25th centile, 75th centile) | 15 (9, 24) | 18 (9, 27) | 16 (9, 25) |
| Minimum, maximum | 0.5, 61 | 0.5, 62 | 0.5, 62 |
| Diabetic complications | |||
| Cerebrovascular | |||
| Yes | 45 (18) | 46 (18) | 91 (18) |
| Cardiovascular | |||
| Yes | 128 (51) | 135 (53) | 263 (52) |
| Nephropathy | |||
| Yes | 89 (35) | 90 (35) | 179 (35) |
| Retinopathy | |||
| Yes | 128 (51) | 144 (56) | 272 (53) |
Baseline health status
Baseline health status was mostly well balanced between the intervention and control arms, with the exception of EQ-5D-3L health score, and the scores overall reflected the impact of chronic ulceration (Table 3).
| Measures of well-being and function | Usual care (N = 253) | Intervention (N = 256) | Total (N = 509) |
|---|---|---|---|
| EQ-5D-3L health score (0–100) | |||
| Mean (SD) | 50.9 (24.5) | 55.4 (23.9) | 53.2 (24.3) |
| Median (25th centile, 75th centile) | 50 (40, 70) | 55 (40, 73) | 50 (40, 70) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| n | 246 | 251 | 497 |
| EQ-5D-3L utility score | |||
| Mean (SD) | 0.43 (0.35) | 0.45 (0.31) | 0.44 (0.33) |
| Median (25th centile, 75th centile) | 0.5 (0.1, 0.7) | 0.6 (0.2, 0.7) | 0.5 (0.2, 0.7) |
| Minimum, maximum | –0.5, 1 | –0.5, 1 | –0.5, 1 |
| n | 241 | 249 | 490 |
| CWISa | |||
| Social life | |||
| Mean (SD) | 62.9 (25.6) | 62.8 (22.1) | 62.9 (23.9) |
| Median (25th centile, 75th centile) | 66.1 (42.9, 84.8) | 62.5 (48.2, 80.4) | 64.3 (46.4, 80.4) |
| Minimum, maximum | 3.6, 100 | 0, 100 | 0, 100 |
| n | 244 | 252 | 496 |
| Physical symptoms and daily living | |||
| Mean (SD) | 61.3 (21.1) | 61.5 (19.1) | 61.4 (20.1) |
| Median (25th centile, 75th centile) | 62.5 (45.8, 79.2) | 62.5 (49, 75) | 62.5 (46.9, 77.1) |
| Minimum, maximum | 8.3, 100 | 9.4, 100 | 8.3, 100 |
| n | 245 | 253 | 498 |
| Well-being | |||
| Mean (SD) | 45.9 (20.3) | 48.9 (19.2) | 47.4 [19.8] |
| Median (25th centile, 75th centile) | 42.9 (32.1, 57.1) | 46.4 (35.7, 60.7) | 46.4 (32.1, 60.7) |
| Minimum, maximum | 0, 100 | 3.6, 100 | 0, 100 |
| n | 244 | 253 | 497 |
| Overall quality of life during the past week | |||
| Mean (SD) | 5.8 (2.4) | 6.0 (2.3) | 5.9 (2.3) |
| Median (25th centile, 75th centile) | 6 (5, 8) | 6 (5, 8) | 6 (5, 8) |
| Minimum, maximum | 0, 10 | 0, 10 | 0, 10 |
| n | 243 | 250 | 493 |
| Satisfaction with overall quality of life during the past week | |||
| Mean (SD) | 5.7 (2.8) | 5.7 (2.7) | 5.7 (2.7) |
| Median (25th centile, 75th centile) | 6 (4, 8) | 6 (4, 8) | 6 (4, 8) |
| Minimum, maximum | 0, 10 | 0, 10 | 0, 10 |
| n | 243 | 251 | 494 |
Baseline details of the limb and foot
The details of the target ulcer and foot shown in Table 4 demonstrate that the intervention and control arms were mostly well balanced. The majority had demonstrable loss of sensation using a 10-g monofilament. The presence of neuropathy (reduced protective sensation) was defined as the inability to detect a 10-g monofilament at two or three of the three sites tested on the index foot (under the first metatarsal head, under the fifth metatarsal head and under the hallux pulp), and was documented in 64% of the participants. Only 34% had both the posterior tibial and dorsalis pedis pulses palpable, and mean ABPI lay in the accepted normal range of 0.9–1.4 in only 36%, although it should be noted that ABPI was not reported in 30% of the total population (see Appendix 5).
| Clinical details | Usual care (N = 253) | Intervention (N = 256) | Total (N = 509) |
|---|---|---|---|
| Target foot | |||
| Right | 121 (48) | 115 (45) | 236 (46) |
| Left | 132 (52) | 141 (55) | 273 (54) |
| Position of ulcer on heel | |||
| Plantar | 67 (27) | 73 (28) | 140 (27) |
| Tip | 111 (44) | 107 (42) | 218 (43) |
| Medial | 57 (22) | 71 (28) | 128 (25) |
| Lateral | 66 (26) | 57 (22) | 123 (24) |
| Dorsalis pedis palpable | |||
| Yes | 124 (49) | 119 (46) | 243 (48) |
| Posterior tibial palpable | |||
| Yes | 99 (39) | 93 (36) | 192 (38) |
| Dorsalis pedis palpable and posterior tibial palpable | 92 (36) | 83 (32) | 175 (34) |
| ABPI value | |||
| < 0.9 | 70 (28) | 68 (27) | 138 (27) |
| 0.9–1.4 | 88 (35) | 95 (37) | 183 (36) |
| > 1.4 | 16 (6) | 20 (8) | 36 (7) |
| ABPI not done | 79 (31) | 73 (28) | 152 (30) |
| Loss of sensation in the following locations | |||
| First metatarsal head alone | 3 (1) | 3 (1) | 6 (1) |
| Fifth metatarsal head alone | 6 (2) | 6 (2) | 12 (2) |
| Hallux alone | 9 (4) | 3 (1) | 12 (2) |
| Sensation lost at ≥ two of the above sites | 170 (67) | 156 (61) | 326 (64) |
| Area of the wound (as recorded at randomisation) | |||
| 25–100 mm2 | 94 (37) | 94 (37) | 188 (37) |
| > 100 mm2 | 159 (63) | 162 (63) | 321 (63) |
| Area of the wound (as measured using images of acetate tracings of ulcer), mm2 | |||
| Digital image of acetate tracing taken at baseline | 253 (100) | 256 (100) | 509 (100) |
| Wound area measured | 251 (99) | 256 (100) | 207 (99) |
| Mean (SD) | 470.5 (621.2) | 556.4 (737.9) | 513.9 (683.3) |
| Median (25th centile, 75th centile) | 206 (77, 649) | 275 (104, 683) | 245 (87, 677) |
| Minimum, maximum | 6, 4243 | 8, 5002 | 6, 5002 |
| 1–24 mm2 | 14 (5) | 10 (4) | 24 (5) |
| 25–100 mm2 | 66 (26) | 51 (20) | 117 (23) |
| > 100 mm2 | 171 (68) | 195 (76) | 366 (72) |
| Grade of ulcer (using NPUAP/EPUAP criteria) | |||
| 2 | 82 (32) | 83 (32) | 165 (32) |
| 3 | 156 (62) | 158 (62) | 314 (62) |
| 4 | 15 (6) | 15 (6) | 30 (6) |
| Local pain VAS (mm) | |||
| No pain | 56 (22) | 57 (22) | 113 (22) |
| With pain | 195 (78) | 197 (77) | 392 (78) |
| Mean (SD) | 32.2 (30.2) | 32.9 (28.5) | 32.5 (29.3) |
| Median (25th centile, 75th centile) | 24 (2, 56) | 28 (5, 56) | 26 (4, 56) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| n | 251 | 254 | 505 |
| Wound bed | |||
| Granulating (%) | |||
| Mean (SD) | 46.8 (42.1) | 44.7 (40.4) | 45.8 (41.2) |
| Median (25th centile, 75th centile) | 40 (0, 97) | 40 (0, 90) | 40 (0, 90) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| n | 253 | 256 | 509 |
| Slough (%) | |||
| Mean (SD) | 40.5 (40.3) | 38.7 (38.1) | 39.6 (39.2) |
| Median (25th centile, 75th centile) | 40 (0, 97) | 27.5 (0, 80) | 30 (0, 80) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| n | 253 | 256 | 509 |
| Necrosis (%) | |||
| Mean (SD) | 10.6 (27.8) | 15.6 (32.6) | 13.1 (39.2) |
| Median (25th centile, 75th centile) | 0 (0, 0) | 0 (0, 0) | 30 (0, 80) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| n | 253 | 256 | 509 |
| Exudate levels | |||
| None | 19 (7) | 22 (9) | 41 (8) |
| Light | 112 (44) | 114 (44) | 226 (44) |
| Moderate | 99 (39) | 102 (40) | 201 (39) |
| Heavy | 23 (9) | 18 (7) | 41 (8) |
| Condition of the surrounding skin | |||
| Healthy intact | 85 (34) | 88 (34) | 173 (34) |
| Callus | 138 (54) | 139 (54) | 277 (54) |
| Macerated | 89 (35) | 87 (34) | 176 (35) |
| Erythematous | 33 (13) | 28 (11) | 61 (12) |
| Oedematous | 36 (14) | 20 (8) | 56 (11) |
| Other | 20 (8) | 22 (9) | 42 (8) |
| Type of offloading used | |||
| Bedbound or immobile | 13 (5) | 13 (5) | 26 (5) |
| Normal footwear | 57 (22) | 57 (22) | 114 (22) |
| Fitted footwear/orthoses | 41 (16) | 36 (14) | 77 (15) |
| Fitted insoles/inserts | 16 (6) | 8 (3) | 24 (5) |
| Removable offloading device or cast | 57 (22) | 53 (21) | 110 (22) |
| Removable fibreglass slipper/whole foot device | 6 (2) | 26 (10) | 32 (6) |
| Padded slipper or shoe | 93 (37) | 86 (34) | 179 (35) |
Baseline details of the ulcer (see Table 4)
Area
The median baseline area of the index ulcer, determined retrospectively by the blinded observer, was 206 mm2 [25th centile, 75th centile: 77 mm2, 649 mm2] in the control group and 275 mm2 (25th centile, 75th centile: 104 mm2, 683 mm2) in the intervention group.
National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel grade (depth)
The distribution of ulcers was identical in the intervention and control groups: grade 2, 32%; grade 3, 62%; and grade 4, 6%.
Pain
Local pain was reported by 78% of participants overall: intervention group, 77%; and control group, 78%.
Wound bed
The base of each ulcer was scored for the granulating percentage (median 40%), the extent of slough coverage of the ulcer (median 30%) and of necrosis (median 30%), the level of exudate (none, light, moderate or heavy) and the condition of the surrounding skin (intact, callus, macerated, erythematous, oedematous or other) and was well balanced between the two arms.
Offloading
The participants used a range of offloading devices, and the proportion using these devices was similar in the two arms.
Study quality
Baseline ulcer area
Baseline ulcer area used for determining eligibility and for stratified randomisation, and ulcer area later judged by the central blinded observer, are shown in Table 5. In 24 participants, the ulcer area was assessed as < 25 mm2 by the blinded observer; categorisation of ulcer size was discrepant for a further 94 participants. As would be expected, the number of discrepancies was approximately the same in each arm.
| Size of heel ulcer based on measurements using images of acetate tracings of ulcer by blinded observer | Heel ulcer size selected at randomisation | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| 25–100 mm2 | > 100 mm2 | 25–100 mm2 | > 100 mm2 | |
| 1–24 mm2 | 14 | 0 | 10 | 0 |
| 25–100 mm2 | 53 | 13 | 40 | 11 |
| > 100 mm2 | 26 | 145 | 44 | 151 |
Completeness of follow-up at weeks 12 and 24 by randomisation group
Table 6 shows the numbers of participants in either group who completed visits at weeks 12 and 24. These figures include those who died or were withdrawn as well as those in whom healing had already been confirmed. Completeness of data is also shown in Table 6 for participants whose ulcer did not heal or for whom primary healing data are missing. The proportion attending the visit at week 24 was similar in the two groups.
| Follow-up visit | Usual care (N = 253) | Intervention (N = 256) | Total (N = 509) |
|---|---|---|---|
| Week 12 visit completed, n (%) | 211 (83) | 214 (84) | 425 (83) |
| Weeks from randomisation to week 12 visit | |||
| Median (25th centile, 75th centile) | 12 (12, 12.4) | 12 (12, 12.3) | 12 (12, 12.3) |
| Minimum, maximum | 11, 20.4 | 10, 18.0 | 10, 20.4 |
| Week 24 visit completed, n (%) | 198 (78) | 203 (79) | 401 (79) |
| Weeks from randomisation to week 24 visit | |||
| Median (25th centile, 75th centile) | 24 (24, 25) | 24 (24, 24.7) | 24 (24, 25) |
| Minimum, maximum | 20, 38.9 | 18, 38.3 | 18, 38.9 |
| Completeness of data for participants who did not heal or for whom primary healing data are missinga | |||
| Number of visits completed | |||
| ≤ 10 visits attended | 64 (37) | 62 (38) | 126 (38) |
| 11 visits attended | 9 (5) | 11 (7) | 20 (6) |
| 12 visits attended | 37 (21) | 21 (13) | 58 (17) |
| All 13 visits attended | 63 (36) | 68 (42) | 131 (39) |
| Median (25th centile, 75th centile) | 12 (7, 13) | 12 (6, 13) | 12 (6, 13) |
| Minimum, maximum | 1, 13 | 1, 13 | 1, 13 |
| Week 24 visit completed, n (%) | 124 (72) | 114 (70) | 238 (71) |
| Local pain VAS completed | 107 (62) | 92 (57) | 199 (59) |
| Digital image of acetate of trace of wound | |||
| Taken at week 24 | 92 (53) | 84 (52) | 176 (52) |
| Wound area measured | 89 (51) | 80 (49) | 169 (50) |
| Digital images of wound | |||
| Taken at week 24 | 104 (60) | 92 (57) | 196 (58) |
| Number of images in which the wound area is measurable | |||
| 0 | 15 (9) | 19 (12) | 34 (10) |
| 1 | 4 (2) | 3 (2) | 7 (2) |
| 2 | 154 (89) | 140 (86) | 294 (88) |
Baseline characteristics according to collection of primary outcome data
The primary outcome was collected for a total of 425 (83%) randomised participants. Tables 7–9 suggest that the baseline characteristics of participants who did and did not provide primary outcome data did not differ between the treatment arms.
| Baseline characteristics | Usual care (N = 253) | Intervention (N = 256) | ||
|---|---|---|---|---|
| Primary outcome data available (n = 213) | Primary outcome not known (n = 40) | Primary outcome data available (n = 212) | Primary outcome not known (n = 44) | |
| Age at inclusion (years) | ||||
| Mean (SD) | 65.4 (12.3) | 66.8 (13.8) | 67.8 (11.6) | 71.3 (14.5) |
| Median (25th centile, 75th centile) | 66 (58, 74) | 69.5 (55, 76.5) | 68.5 (60, 77) | 76 (61.5, 80) |
| Minimum, maximum | 25, 91 | 38, 91 | 39, 94 | 28, 90 |
| Sex | ||||
| Female | 70 (33) | 18 (45) | 63 (30) | 14 (32) |
| Male | 143 (67) | 22 (55) | 149 (70) | 30 (68) |
| Immobility | ||||
| No | 98 (46) | 19 (47) | 98 (46) | 13 (29) |
| Yes | 115 (54) | 21 (53) | 114 (54) | 31 (71) |
| Type of immobility | ||||
| Walking with aid of assistance | 78 (37) | 14 (35) | 89 (42) | 23 (52) |
| Chairbound | 36 (17) | 7 (17) | 23 (11) | 8 (18) |
| Bedbound | 1 (1) | 0 | 2 (1) | 0 |
| Diabetes mellitus | ||||
| Type of diabetes mellitus | ||||
| Type 1 | 32 (15) | 7 (17) | 30 (14) | 7 (16) |
| Type 2 | 181 (85) | 33 (83) | 182 (86) | 37 (84) |
| Duration (years) | ||||
| Mean (SD) | 17.0 (11.1) | 16.7 (13.0) | 18.7 (12.6) | 21.8 (14.7) |
| Median (25th centile, 75th centile) | 15 (9, 25) | 12.5 (8, 22) | 17 (9, 26.5) | 20 (11.5, 27.5) |
| Minimum, maximum | 0.5, 61 | 1, 61 | 0.5, 62 | 0.5, 61 |
| Diabetic complications | ||||
| Cerebrovascular | ||||
| Yes | 39 (18) | 6 (15) | 38 (18) | 8 (18) |
| Cardiovascular | ||||
| Yes | 108 (51) | 20 (50) | 112 (53) | 23 (52) |
| Nephropathy | ||||
| Yes | 76 (36) | 13 (32) | 75 (35) | 15 (34) |
| Retinopathy | ||||
| Yes | 112 (53) | 16 (40) | 125 (59) | 19 (43) |
| Measures of well-being and function | Usual care (N = 253) | Intervention (N = 256) | ||
|---|---|---|---|---|
| Primary outcome data available (n = 213) | Primary outcome not known (n = 40) | Primary outcome data available (n = 212) | Primary outcome not known (n = 44) | |
| EQ-5D-3L health score (0–100) | ||||
| Mean (SD) | 51.7 (24.9) | 46.9 (21.9) | 55.5 (24.5) | 54.8 (20.8) |
| Median (25th centile, 75th centile) | 50 (40, 70) | 49.5 (30, 60) | 56 (40, 75) | 50 (41, 69) |
| Minimum, maximum | 0, 100 | 4, 94 | 0, 100 | 1, 97 |
| n | 208 | 38 | 207 | 44 |
| EQ-5D-3L utility score | ||||
| Mean (SD) | 0.4 (0.3) | 0.5 (0.4) | 0.4 (0.3) | 0.4 (0.3) |
| Median (25th centile, 75th centile) | 0.5 (0.1, 0.7) | 0.5 (0.1, 0.7) | 0.6 (0.2, 0.7) | 0.6 (0.3, 0.6) |
| Minimum, maximum | –0.3, 1 | –0.5, 0.9 | –0.5, 1 | –0.4, 0.8 |
| n | 203 | 38 | 205 | 44 |
| CWIS social life | ||||
| Mean (SD) | 63.6 (25.5) | 59.3 (26.2) | 61.9 (22.4) | 67.5 (20.6) |
| Median (25th centile, 75th centile) | 66.1 (44.6, 85.7) | 59 (35.7, 78.6) | 60.7 (46.4, 78.6) | 67.9 (42.2, 78.6) |
| Minimum, maximum | 3.6, 100 | 16.1, 100 | 0, 100 | 21.4, 100 |
| n | 206 | 38 | 208 | 44 |
| Physical symptoms and daily living | ||||
| Mean (SD) | 61.6 (21.1) | 59.6 (21.5) | 61.9 (18.7) | 59.6 (20.6) |
| Median (25th centile, 75th centile) | 62.5 (46.9, 80.2) | 62.5 (38.5, 78.1) | 62.5 (51, 74) | 62.5 (42.2, 78.6) |
| Minimum, maximum | 8.3, 100 | 20.8, 100 | 9.4, 100 | 16.7, 91.7 |
| n | 207 | 38 | 209 | 44 |
| Well-being | ||||
| Mean (SD) | 46.1 (20.7) | 44.5 (18.4) | 49.3 (19.3) | 46.7 (18.8) |
| Median (25th centile, 75th centile) | 42.9 (32.1, 57.1) | 46.4 (32.1, 57.1) | 50 (35.7, 60.7) | 41.1 (32.1, 62.5) |
| Minimum, maximum | 0, 100 | 3.6, 82.1 | 7.1, 100 | 3.6, 89.3 |
| n | 205 | 39 | 209 | 44 |
| Overall quality of life during the past week | ||||
| Mean (SD) | 5.9 (2.4) | 5.4 (2.1) | 6.0 (2.3) | 6.1 (2.1) |
| Median (25th centile, 75th centile) | 6 (5, 8) | 5 (4, 7) | 6 (5, 8) | 6 (5, 8) |
| Minimum, maximum | 0, 10 | 0, 10 | 0, 10 | 0, 10 |
| n | 205 | 38 | 208 | 42 |
| Satisfaction with overall quality of life during the past week | ||||
| Mean (SD) | 6.0 (2.8) | 4.7 (2.7) | 5.6 (2.7) | 6.3 (2.7) |
| Median (25th centile, 75th centile) | 6 (4, 8) | 5 (3, 6) | 6 (4, 8) | 7 (5, 8) |
| Minimum, maximum | 0, 10 | 0, 10 | 0, 10 | 0, 10 |
| n | 205 | 38 | 208 | 43 |
| Baseline foot ulcer details | Usual care (N = 253) | Intervention (N = 256) | ||
|---|---|---|---|---|
| Primary outcome data available (n = 213) | Primary outcome not known (n = 40) | Primary outcome data available (n = 212) | Primary outcome not known (n = 44) | |
| Target foot | ||||
| Right | 107 (50) | 14 (35) | 98 (46) | 17 (39) |
| Left | 106 (50) | 26 (65) | 114 (54) | 27 (61) |
| Position of ulcer on heel | ||||
| Plantar | 61 (29) | 6 (15) | 62 (29) | 11 (25) |
| Tip | 91 (43) | 20 (50) | 85 (40) | 22 (50) |
| Medial | 46 (22) | 11 (28) | 62 (29) | 9 (20) |
| Lateral | 54 (25) | 12 (30) | 44 (21) | 13 (29) |
| Dorsalis pedis palpable | ||||
| Yes | 103 (48) | 21 (52) | 100 (57) | 19 (43) |
| Posterior tibial palpable | ||||
| Yes | 82 (35) | 17 (42) | 79 (37) | 14 (32) |
| Dorsalis pedis palpable and posterior tibial palpable | ||||
| Yes | 75 (35) | 17 (42) | 70 (33) | 13 (29) |
| ABPI value | ||||
| < 0.9 | 52 (24) | 18 (45) | 59 (28) | 9 (20) |
| 0.9–1.4 | 78 (37) | 10 (25) | 78 (37) | 17 (39) |
| > 1.4 | 14 (7) | 2 (5) | 15 (7) | 5 (11) |
| ABPI not done | 69 (32) | 10 (25) | 60 (28) | 13 (29) |
| Loss of sensation in the following locations | ||||
| First metatarsal head alone | 2 (1) | 1 (2) | 3 (1) | 0 |
| Fifth metatarsal head alone | 4 (2) | 2 (5) | 4 (2) | 2 (4) |
| Hallux alone | 8 (4) | 1 (2) | 1 (1) | 0 |
| Sensation lost at two or more of the above sites | 147 (69) | 23 (57) | 128 (60) | 28 (64) |
| Area of the wound (as recorded at randomisation) | ||||
| 25–100 mm2 | 73 (34) | 21 (52) | 74 (35) | 20 (45) |
| > 100 mm2 | 140 (66) | 19 (47) | 138 (65) | 24 (54) |
| Area of the wound (mm2) (as measured using images of acetate tracings of ulcer) | ||||
| Digital image of acetate tracing taken at baseline | 213 (100) | 40 (100) | 212 (100) | 44 (100) |
| Wound area measured | 213 (100) | 38 (95) | 212 (100) | 44 (100) |
| Mean (SD) | 480.6 (601.1) | 415.2 (730.3) | 542.6 (701.6) | 624.0 (901.4) |
| Median (25th centile, 75th centile) | 220 (80, 663) | 170.5 (46, 531) | 275.5 (113, 674) | 283.5 (83.5, 809) |
| Minimum, maximum | 6, 4007 | 9, 4243 | 8, 5002 | 15, 4553 |
| 1–24 mm2 | 10 (5) | 4 (10) | 9 (4) | 1 (2) |
| 25–100 mm2 | 55 (26) | 11 (27) | 39 (18) | 12 (27) |
| > 100 mm2 | 148 (69) | 23 (57) | 164 (77) | 31 (71) |
| Grade of ulcer (using NPUAP/EPUAP criteria) | ||||
| 2 | 69 (32) | 14 (35) | 68 (32) | 15 (34) |
| 3 | 131 (61) | 25 (62) | 130 (61) | 28 (64) |
| 4 | 14 (7) | 1 (2) | 14 (7) | 1 (2) |
| Local pain VAS (mm) | ||||
| Mean (SD) | 30.4 (29.7) | 41.9 (31.9) | 31.9 (28.6) | 37.7 (27.6) |
| Median (25th centile, 75th centile) | 22 (1.5, 55) | 44 (9, 75) | 26.5 (4, 53) | 35 (16, 59.5) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 | 0, 90 |
| n | 212 | 40 | 210 | 44 |
| Wound bed | ||||
| Granulating (%) | ||||
| Mean (SD) | 48 (42) | 42 (43) | 44 (41) | 50 (38) |
| Median (25th centile, 75th centile) | 40 (1, 95) | 25 (0, 100) | 35 (0, 87) | 50 (13, 95) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| n | 213 | 40 | 212 | 44 |
| Slough (%) | ||||
| Mean (SD) | 41 (40) | 38 (41) | 39 (38) | 38 (36) |
| Median (25th centile, 75th centile) | 30 (0, 80) | 20 (0, 85) | 20 (0, 80) | 30 (0, 72) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| n | 213 | 40 | 212 | 44 |
| Necrosis (%) | ||||
| Mean (SD) | 9 (26) | 17 (35) | 16 (33) | 12 (30) |
| Median (25th centile, 75th centile) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 | 0, 100 |
| n | 213 | 40 | 212 | 44 |
| Exudate levels | ||||
| None | 18 (8) | 1 (2) | 16 (7) | 6 (14) |
| Light | 90 (42) | 22 (55) | 96 (45) | 18 (41) |
| Moderate | 83 (39) | 16 (40) | 85 (40) | 17 (39) |
| Heavy | 22 (10) | 1 (2) | 15 (7) | 3 (7) |
| Condition of the surrounding skin | ||||
| Health intact | 74 (35) | 11 (27) | 73 (34) | 15 (34) |
| Callus | 114 (53) | 24 (60) | 116 (55) | 23 (52) |
| Macerated | 76 (36) | 13 (32) | 72 (34) | 15 (34) |
| Erythematous | 26 (12) | 7 (17) | 25 (12) | 3 (7) |
| Oedematous | 34 (16) | 2 (5) | 16 (7) | 4 (9) |
| Other | 16 (7) | 4 (10) | 16 (7) | 6 (14) |
| Type of offloading used | ||||
| Bedbound or immobile | 11 (5) | 2 (5) | 11 (5) | 2 (4) |
| Normal footwear | 43 (20) | 14 (35) | 50 (24) | 7 (16) |
| Fitted footwear/orthoses | 36 (17) | 5 (12) | 32 (15) | 4 (9) |
| Fitted insoles/inserts | 15 (7) | 1 (2) | 7 (3) | 1 (2) |
| Removable below-knee offloading device or cast | 47 (22) | 10 (25) | 40 (19) | 13 (29) |
| Removable fibreglass slipper/whole foot device | 6 (3) | 0 | 20 (9) | 6 (14) |
| Padded slipper or shoe | 80 (38) | 13 (32) | 69 (32) | 17 (39) |
Adherence to wearing the heel cast (in those randomised to the intervention group)
The median percentage of expected time that the heel cast was worn in each case was 100% (Table 10). Reported mean adherence to wearing the heel casts was higher among those with confirmed ulcer healing.
| Measures of adherence | Intervention (N = 256) |
|---|---|
| Participant attended at least one follow-up visit, n (%) | 244 (95) |
| Percentage of time that heel cast was worn during study | |
| Mean (SD) | 90 (23) |
| Median (25th centile, 75th centile) | 100 (90, 100) |
| Minimum, maximum | 0, 100 |
| n | 244 |
| Heel cast not worn for 7 consecutive days or > 14 days in total, n (%) | 44 (17) |
| Percentage of time that the heel cast was worn for different participant groups distinguished by clinical outcome | |
| With confirmed ulcer healing | |
| 100%, n | 77 (82%) |
| < 100%, n | 17 (18%) |
| Mean (SD) | 97% (8%) |
| Median (25th centile, 75th centile) | 100% (100%, 100%) |
| Minimum, maximum | 40%, 100% |
| n | 94 |
| Who did not heal and attended the week 24 visit | |
| 100%, n | 60 (58%) |
| < 100%, n | 43 (42%) |
| Mean (SD) | 87% (27%) |
| Median (25th centile, 75th centile) | 100% (90%, 100%) |
| Minimum, maximum | 0, 100% |
| n | 103 |
| Who did not heal and did not attend week 24 visit | |
| 100%, n | 6 (54%) |
| < 100%, n | 5 (46%) |
| Mean (SD) | 79% (34%) |
| Median (25th centile, 75th centile) | 100% (90%, 100%) |
| Minimum, maximum | 0, 100% |
| n | 11 |
| With healing status unavailable | |
| 100%, n | 16 (45%) |
| < 100%, n | 20 (55%) |
| Mean (SD) | 85% (27%) |
| Median (25th centile, 75th centile) | 100% (80%, 100%) |
| Minimum, maximum | 0, 100% |
| n | 36 |
Protocol violations
The numbers of protocol violations leading to failure to complete the study are given in the CONSORT diagram (see Figure 1). The details of all protocol violations are listed in Appendix 6.
Withdrawals
The total withdrawals are given in the CONSORT diagram (see Figure 1). The detailed reasons for withdrawal are listed in Appendix 7.
Primary outcome
Ninety-four out of 212 (44%) ulcers healed in the intervention group, compared with 80 out of 213 (37%) in the usual care group (OR 1.42, 95% CI 0.95 to 2.14; p = 0.088). The risk difference was 8% (95% CI –1% to 17%; p = 0.087) (Table 11). These data suggest that any effect of the intervention is less than that used in designing the trial (risk difference 15%). The possibility of no effect or even a very small harmful effect cannot be ruled out using conventional 95% CIs. Further adjustment for sex and baseline mobility type (due to baseline imbalance) had minimal impact and resulted in OR 1.42 (95% CI 0.94 to 2.15).
| Percentage healed within 24 weeks | Usual care (n = 253) | Intervention (n = 256) | OR for ulcer healing (95% CI); p-value | Risk difference (%) for ulcer healing (95% CI); p-value |
|---|---|---|---|---|
| Primary outcome data available | 213 | 212 | 1.42 (0.95 to 2.14); p = 0.088 | 8 (–1 to 17); p = 0.087 |
| Unhealed | 133 (62%) | 118 (56%) | ||
| Healed | 80 (38%) | 94 (44%) |
Imputation of missing primary outcome data
Simple and multiple imputation of missing primary outcome data slightly reduced the magnitude of the effect (Table 12).
| Arm | Percentage healed (%) | OR adjusted for stratification factorsa (95% CI) |
|---|---|---|
| Using multiple imputation assuming missing data are missing at random | ||
| Usual care | 38 | – |
| Intervention | 43 | 1.31 (0.89 to 1.92) |
| Using simple imputation assuming that all participants with missing data remain unhealed | ||
| Usual care | 32 | – |
| Intervention | 37 | 1.35 (0.93 to 1.98) |
Subgroup analysis of the primary outcome
Although the subgroup-specific ORs suggest a greater intervention effect among participants with larger ulcers, there was no strong statistical evidence of a differential treatment effect for this or any of the other subgroup analyses (Table 13).
| Baseline detail | Usual care (n = 213) | Intervention (n = 212) | Subgroup-specific crude OR (95%CI) | Adjusted interaction effect (95% CI)a | p-value (for interaction) |
|---|---|---|---|---|---|
| Baseline ulcer area | |||||
| ≤ 100 mm2 | 1.11 (0.45 to 2.72) | 0.817 | |||
| Unhealed | 32 | 20 | 0.86 (0.50 to 1.47) | ||
| Healed | 33 | 28 | |||
| > 100 mm2 | |||||
| Unhealed | 101 | 98 | 1.45 (0.91 to 2.31) | ||
| Healed | 47 | 66 | |||
| Baseline ulcer grade | |||||
| Grade 2 | 0.97 (0.46 to 2.03) | 0.939 | |||
| Unhealed | 36 | 30 | 1.42 (0.72 to 2.80) | ||
| Healed | 32 | 38 | |||
| Grade 3 | |||||
| Unhealed | 86 | 78 | 1.27 (0.77 to 2.11) | ||
| Healed | 45 | 52 | |||
| Grade 4 | |||||
| Unhealed | 11 | 10 | 1.47 (0.26 to 8.23) | ||
| Healed | 3 | 4 | |||
| Baseline mobility status | |||||
| Mobile | 1.00 (0.44 to 2.27) | 0.999 | |||
| Unhealed | 57 | 54 | 1.13 (0.64 to 1.99) | ||
| Healed | 41 | 44 | |||
| Immobile | |||||
| Unhealed | 76 | 64 | 1.52 (0.89 to 2.60) | ||
| Healed | 39 | 50 | |||
| Baseline ABPI | |||||
| < 0.9 | 0.90 (0.41 to 1.98) | 0.800 | |||
| Unhealed | 32 | 36 | 1.02 (0.47 to 2.20) | ||
| Healed | 20 | 23 | |||
| 0.9–1.4 | |||||
| Unhealed | 46 | 40 | 1.36 (0.72 to 2.57) | ||
| Healed | 32 | 38 | |||
| > 1.4 | |||||
| Unhealed | 9 | 10 | 0.90 (0.19 to 4.16) | ||
| Healed | 5 | 5 | |||
| Baseline nephropathy | |||||
| No | 1.60 (0.66 to 3.85) | 0.293 | |||
| Unhealed | 77 | 73 | 1.12 (0.70 to 1.81) | ||
| Healed | 60 | 64 | |||
| Yes | |||||
| Unhealed | 56 | 45 | 1.87 (0.94 to 3.72) | ||
| Healed | 20 | 30 | |||
| Baseline age (years) | |||||
| < 70 | 0.97 (0.42 to 2.22) | 0.945 | |||
| Unhealed | 81 | 60 | 1.32 (0.79 to 2.21) | ||
| Healed | 51 | 50 | |||
| ≥ 70 | |||||
| Unhealed | 52 | 58 | 1.36 (0.75 to 2.48) | ||
| Healed | 29 | 44 | |||
| Baseline offloading | |||||
| ‘More effective’ | 1.26 (0.50 to 3.18) | 0.621 | |||
| Unhealed | 34 | 30 | 1.79 (0.84 to 3.81) | ||
| Healed | 19 | 30 | |||
| ‘Less effective’ | |||||
| Unhealed | 99 | 88 | 1.18 (0.75 to 1.86) | ||
| Healed | 61 | 64 | |||
Per-protocol analysis
Of the 425 participants with primary outcome data, a total of 420 (99%, 210 in each treatment arm) were deemed to have completed the study as per protocol (PP). Comparison of the primary outcome in the PP sample was therefore very similar to that from the primary analysis (adjusted OR 1.41, 95% CI 0.94 to 2.12) (Table 14).
| Percentage healed within 24 weeks | Usual care (n = 210) | Intervention (n = 210) | OR for ulcer healing (95% CI); p-value |
|---|---|---|---|
| Primary outcome data available | 210 | 210 | 1.41 (0.94 to 2.12); p = 0.100 |
| Unhealed | 130 | 116 | |
| Healed | 80 (38%) | 94 (45%) |
Secondary outcomes
Time to healing
The Kaplan–Meier curve for time to healing in the two groups is illustrated in Figure 2. The Cox regression analysis suggested some evidence that participants in the intervention arm healed faster than those in the usual care arm (adjusted hazard ratio 1.30, 95% CI 0.97 to 1.75; p = 0.083).
FIGURE 2.
Kaplan–Meier plot of time to healing.

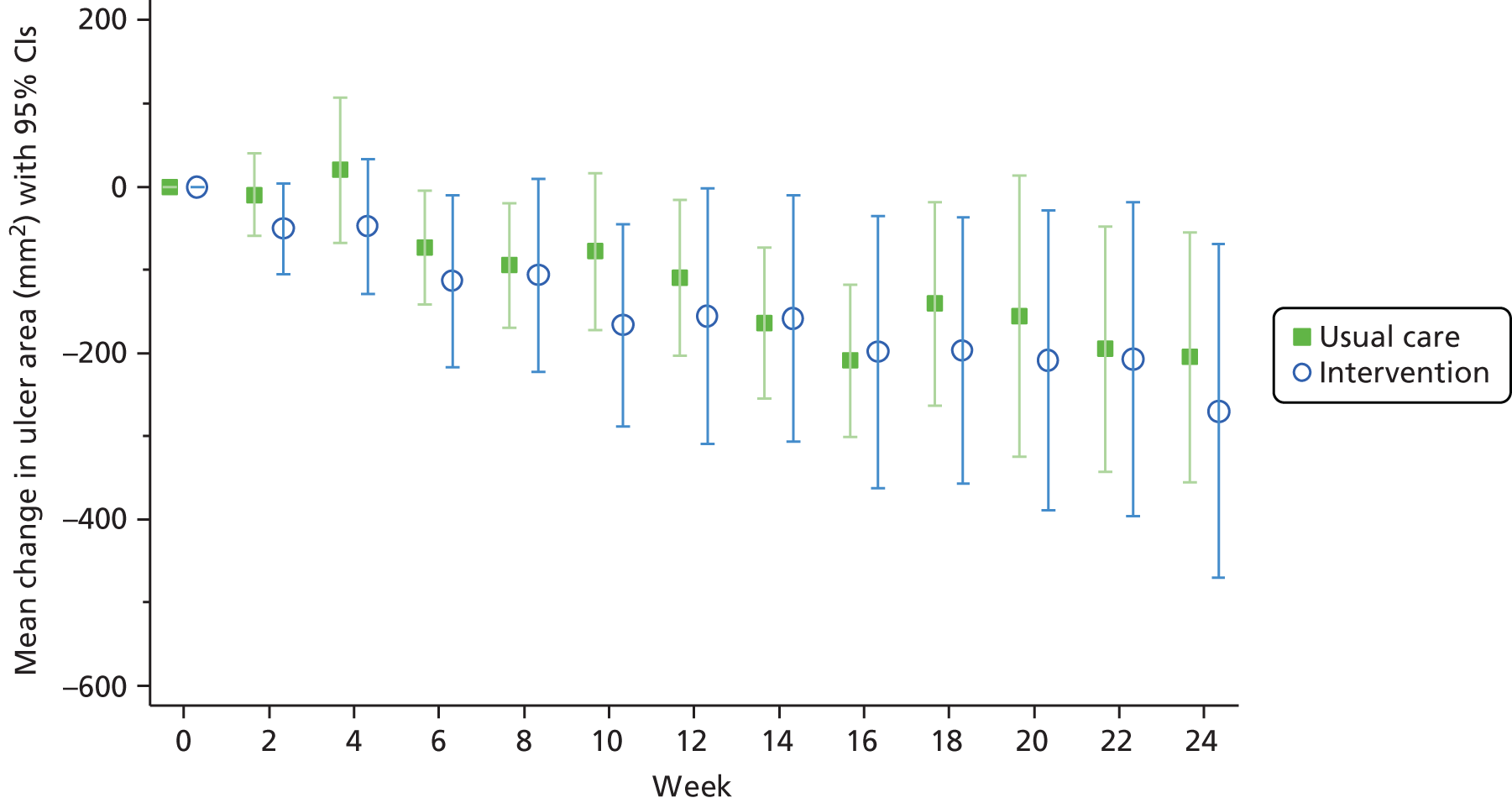
Change in ulcer area
The mean ulcer area in each of the two groups is plotted against time in Figure 3. The mean change from baseline is shown in Figure 4. It should be noted that these plots include all participants who remained unhealed at each time point.
FIGURE 3.
Mean (95% CI) ulcer area over time for unhealed ulcers.
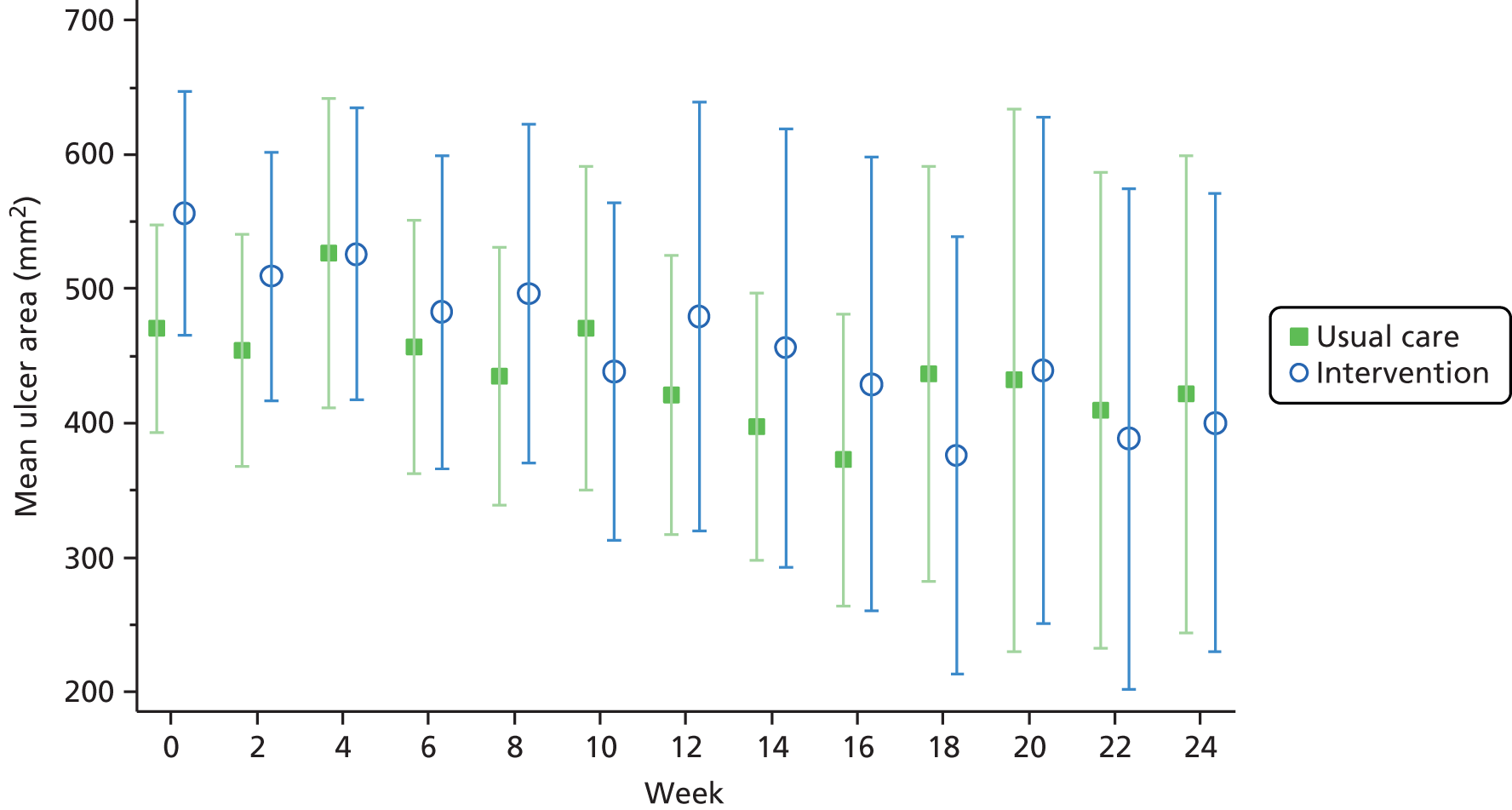
FIGURE 4.
Change of ulcer area from baseline for unhealed ulcers.
Ulcer recurrence after healing
Early recurrence may reflect the quality of healing. The incidence of recurrence was assessed in ulcers that healed at week 18 or earlier. This cut-off point was selected because an ulcer that recurs within 4 weeks would have been defined as unhealed, and a minimum follow-up of 6 weeks was therefore needed to assess recurrence. Seventy-six ulcers (30% of 256) healed in the intervention group by 18 weeks and recurrence was recorded in 5 (7%) by 24 weeks. In the control arm, 68 (27% of 253) ulcers healed by 18 weeks and recurrence was recorded in 3 (4%) by 24 weeks (Table 15).
| Number of participants | Usual care (N = 253) | Intervention (N = 256) |
|---|---|---|
| Number of participants confirmed healed | 80 | 94 |
| Number of participants with healing at week 18 or earlier | 68 | 76 |
| Ulcer recurrence, n (%) | ||
| Yes | 3 (4) | 5 (7) |
| No | 57 (84) | 66 (87) |
| Not known | 8 (12) | 5 (6) |
Health status at weeks 12 and 24
Health status was determined using both the EQ-5D-3L and the CWIS. All measures showed a tendency to increase between baseline and 12 weeks, indicating an improvement in health status. CWIS measures increased further at 24 weeks (Table 16). There was evidence of a difference in mean CWIS well-being score between the intervention and control groups, but there was no evidence of any other differences between the groups.
| Measures of well-being and function | Baseline mean (SD) | 12 weeks mean (SD) | 24 weeks mean (SD) | Adjusted difference in mean (95% CI) | p-value |
|---|---|---|---|---|---|
| EQ-5D-3L utility score | |||||
| Usual care | 0.43 (0.35) | 0.49 (0.34) | 0.54 (0.31) | – | 0.438 |
| Intervention | 0.45 (0.31) | 0.50 (0.32) | 0.52 (0.35) | 0.02 (–0.03 to 0.07) | |
| EQ-5D-3L health score (0–100) | |||||
| Usual care | 50.9 (24.5) | 57.9 (26.9) | 57.4 (26.8) | – | 0.175 |
| Intervention | 55.4 (23.9) | 58.6 (24.7) | 57.9 (27.9) | 2.4 (–1.1 to 5.8) | |
| CWIS social life score | |||||
| Usual care | 62.9 (25.6) | 67.3 (24.7) | 72.5 (25.2) | – | 0.820 |
| Intervention | 62.8 (22.1) | 68.6 (24.1) | 69.6 (23.9) | 0.4 (–3.2 to 4.0) | |
| CWIS physical symptoms and daily living score | |||||
| Usual care | 61.3 (21.1) | 65.6 (21.7) | 71.3 (21.6) | – | 0.385 |
| Intervention | 61.5 (19.1) | 67.8 (20.2) | 72.0 (19.9) | 1.6 (–1.4 to 4.7) | |
| CWIS well-being score | |||||
| Usual care | 45.9 (20.3) | 50.5 (19.3) | 57.0 (20.0) | – | 0.008 |
| Intervention | 48.9 (19.2) | 56.6 (21.6) | 58.9 (20.8) | 4.0 (1.1 to 7.0) | |
| CWIS overall quality of life during the past week | |||||
| Usual care | 5.8 (2.4) | 6.1 (2.4) | 6.3 (2.4) | – | 0.294 |
| Intervention | 6.0 (2.4) | 6.1 (2.3) | 6.5 (2.4) | 0.2 (–0.2 to 0.5) | |
| CWIS satisfaction with overall quality of life during the past week | |||||
| Usual care | 5.7 (2.8) | 5.9 (2.7) | 6.2 (2.8) | – | 0.323 |
| Intervention | 5.7 (2.7) | 6.1 (2.8) | 6.4 (2.7) | 0.2 (–0.2 to 0.6) | |
Other ulcer-related secondary outcomes
There was some evidence of increased risk of a new ulcer on the contralateral foot in the intervention group [intervention (17%) vs. control (11%): OR 1.65, 95% CI 0.98 to 2.80; p = 0.061], but no evidence of any difference for any of the other ulcer-related secondary outcomes (Table 17).
| Secondary outcome | Usual care (N = 245a) | Intervention (N = 244a) | OR (95% CI) | p-value |
|---|---|---|---|---|
| Minor amputation on limb with target ulcer | ||||
| Number of participants (%) | 4 (2) | 6 (2) | 1.63 (0.45 to 5.90) | 0.453 |
| Major amputation on limb with target ulcer | ||||
| Number of participants (%) | 8 (3) | 8 (3) | 0.93 (0.34 to 2.57) | 0.887 |
| Infection on limb with target ulcer | ||||
| Number of participants (%) | 93 (38) | 97 (40) | 0.99 (0.67 to1.46) | 0.978 |
| Total number of visits at which infection reported (total number of visits asked) | 269 (2156) | 235 (2167) | ||
| Hospital admission | ||||
| Number of participants (%) | 86 (35) | 96 (39) | 1.18 (0.81 to 1.71) | 0.379 |
| Total number of visits at which hospital admission reported (total number of visits asked) | 141 (2156) | 151 (2167) | ||
| Trip or fall on the limb with the target ulcer leading to a hospital admission | ||||
| Number of participants (%) | 2 (1) | 5 (2) | 2.60 (0.49 to 13.7) | 0.261 |
| Total number of visits resulting from a trip or fall reported (total number of visits asked) | 2 (1822) | 7 (1920) | ||
| New ulcer on target foot | ||||
| Number of participants (%) | 55 (22) | 57 (23) | 1.08 (0.71 to 1.65) | 0.719 |
| Total number of visits at which new ulcer reported (total number of visits asked) | 79 (2156) | 79 (2167) | ||
| New ulcer on contralateral foot | ||||
| Number of participants (%) | 27 (11) | 41 (17) | 1.65 (0.98 to 2.80) | 0.061 |
| Total number of visits at which new ulcer reported (total number of visits asked) | 33 (2156) | 54 (2167) | ||
| Revascularisation on limb with target ulcer | ||||
| Number of participants | 21 (9) | 28 (11) | 1.36 (0.75 to 2.48) | 0.313 |
| Total number of visits at which revascularisation reported (total number of visits asked) | 25 (2152) | 34 (2164) | ||
Ulcer-related pain
There was no evidence of any between-group differences at 4 or 24 weeks in the proportion of participants who reported pain or in mean pain scores (Table 18). When the population was restricted to those participants reporting pain at baseline, there was no evidence of any differences between the intervention and control groups in pain score at baseline or in the reduction of pain between baseline and either 2 or 4 weeks (Table 19).
| Baseline | 4 weeks | Change from baseline | Adjusted ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Number of participants with unhealed ulcers | 509 | 416 | |||
| Ulcer-related pain (VAS score), mean (SD) | |||||
| Usual care | 31.5 (29.4) | 25.6 (28.5) | –4.0 (22.9) | – | 0.351 |
| Intervention | 34.2 (28.8) | 23.2 (25.2) | –8.7 (26.8) | 0.88 (0.68 to 1.14)a | |
| Ulcer-related pain (any pain vs. no pain), n/N (%) | |||||
| Usual care | 195/244 (78%) | 137/200 (64%) | – | 0.853 | |
| Intervention | 197/245 (77%) | 137/195 (65%) | 0.95 (0.58 to 1.58)b | ||
| Baseline | 24 weeks | Change from baseline | Adjusted ratio (95% CI)c | p-value | |
| Number of participants with unhealed ulcers | 509 | 178 | |||
| Ulcer-related pain (VAS score), mean (SD) | |||||
| Usual care | 31.5 (29.4) | 12.7 (21.1) | –18.6 (32.0) | – | 0.282 |
| Intervention | 34.2 (28.8) | 11.1 (19.8) | –23.6 (28.6) | 0.81 (0.55 to 1.19)a | |
| Ulcer-related pain (any pain vs. no pain), n (%) | |||||
| Usual care | 195/244 (78%) | 59/104 (44%) | – | 0.83 (0.55 to 1.25)b | 0.378 |
| Intervention | 197/245 (77%) | 51/90 (43%) | |||
| Participants with ulcer-related pain | Usual care | Intervention | Adjusted difference in meansa (95% CI) | p-value |
|---|---|---|---|---|
| Baseline ulcer-related pain | ||||
| Mean (SD) | 41.4 (28.2) | 42.4 (25.3) | – | – |
| Median (IQR) | 42 (13–65) | 43 (21–61) | ||
| Minimum, maximum | 1, 100 | 2, 100 | ||
| n | 195 | 197 | ||
| Ulcer pain at 2 weeks | ||||
| Mean (SD) | 34.9 (29.5) | 33.8 (26) | –1.9 (–6.9 to 3.2) | 0.472 |
| Median (IQR) | 29 (10–56.5) | 27 (13–53) | ||
| Minimum, maximum | 0, 100 | 0, 92 | ||
| n | 180 | 183 | ||
| Ulcer pain at 4 weeks | ||||
| Mean (SD) | 32.6 (28.7) | 29.4 (25.9) | –3.8 (–8.9 to 1.3) | 0.140 |
| Median (IQR) | 28 (5–60) | 21 (7–50) | ||
| Minimum, maximum | 0, 100 | 0, 90 | ||
| n | 173 | 170 | ||
Death
Thirteen participants in the usual care group (5%) and 10 participants in the intervention group (4%) died before the week 24 visit.
Ancillary analyses
Association between change in ulcer area at 4 weeks and healing by 24 weeks
The median per cent reduction in area at 4 weeks was 53% [interquartile range (IQR) –75% to –34%] in those that went on to heal by 24 weeks, compared with 24% (IQR –48% to 2%) in those that remained unhealed (adjusted OR = 1.02, 95% CI 1.01 to 1.03; p < 0.001) (Table 20).There was no evidence that this association differed according to ulcer area at baseline (interaction OR = 1.00, 95% CI 0.99 to 1.01; p = 0.445) or treatment arm (interaction OR = 0.99, 95% CI 0.97 to 1.00; p = 0.205). Receiver operating characteristic (ROC) analysis gave an area under the curve (AUC) of 0.78. There was no clear threshold of change in ulcer area at 4 weeks that reliably differentiated between participants who healed and participants who remained unhealed at 24 weeks.
| Percentage change in ulcer area at 4 weeks | Unhealed (n = 197) | Healed (n = 139) | Adjusted ORa (95% CI) | p-value |
|---|---|---|---|---|
| Median (IQR) | –24% (–48% to 2%) | –53% (–75% to –34%) | 1.02 (1.01 to 1.03) | < 0.001 |
| Minimum, maximum | –96%, 87% | –98%, 48% |
Serious adverse events, adverse events, serious adverse device effects and adverse device effects
Data on serious adverse events (SAEs) and AEs were collected until the time of the protocol amendment (29 March 2012). There was no clear evidence of a difference in the incidence of AEs between the two groups, as shown in Table 21. Six AEs were, however, judged to be ‘probably related’, and five were judged to be ‘possibly related’ to the use of the device. Two AEs were ‘possibly’ or ‘probably’ attributed to the use of the device in the control group, in which the device was not used. The reason for this attribution by the clinical researchers is not clear. Similarly, there was no clear evidence of a difference between groups in the incidence of SAEs, and only one episode was judged ‘possibly related’ to the use of the device. Full details of AEs and SAEs are listed in Appendix 7.
| AEs and SAEs | Usual care arm | Intervention arm |
|---|---|---|
| Number of AEs | 53 | 38 |
| Relationship of AEs to the device | ||
| None | 51 | 27 |
| Possible | 1 | 5 |
| Probable | 1 | 6 |
| Number of SAEs | 21 | 16 |
| Relationship of SAEs to the device | ||
| None | 21 | 15 |
| Possible | 0 | 1 |
| Probable | 0 | 0 |
Data on ADEs were collected from 29 March 2012 and are summarised in Table 22. A total of 18 ADEs were recorded in the control group, in which the participants were not exposed to the device. There were 40 ADEs in the intervention group, of which only 25 were judged to be related to the use of the device. There were no SADEs in either the intervention or the control groups. The full details of ADEs that occurred during the study are listed in Appendix 8.
| ADEs and SADEs | Usual care arm | Intervention arm |
|---|---|---|
| Number of SADEs | 0 | 0 |
| Number of ADEs | 18 | 40 |
| Relationship of ADEs to the device | ||
| None | 14 | 15 |
| Related | 4 | 25 |
Health economic analysis
Quality-adjusted life-years gained
Health utilities
Although the clinical effectiveness analyses considered the impact of missing data on the primary clinical outcome, for the purposes of the health economic analysis, we further considered the impact of missing EQ-5D-3L data on the derivation of utilities. At baseline, 3.5% of the EQ-5D-3L data were missing across both groups. At 12 weeks, 22% of the data were missing (20% when those who died during this period were taken into account) and 24% of the data (21% when deaths were accounted for) were missing at 24 weeks, with similar results in both groups. Following the principles set out in the statistical methods for assessing missing data, multiple imputation was undertaken. Similar to the results of the primary clinical outcome, imputation slightly reduced the magnitude of effect. Table 23 presents the differences in utilities derived. The multiple imputed utilities were subsequently used in the cost–utility analysis.
| Assessment | Available cases | Cases after multiple imputation | ||
|---|---|---|---|---|
| Usual care, EQ-5D-3L utility score (95% CI) | Heels, EQ-5D-3L utility score (95% CI) | Usual care, EQ-5D-3L utility score (95% CI) (n = 253) | Heels, EQ-5D-3L utility score (95% CI) (n = 256) | |
| Baseline | 0.438 (0.395 to 0.481)a | 0.442 (0.402 to 0.482)b | 0.438 (0.396 to 0.479) | 0.442 (0.403 to 0.481) |
| 12 weeks | 0.480 (0.435 to 0.526)c | 0.489 (0.443 to 0.535)d | 0.482 (0.445 to 0.520) | 0.489 (0.453 to 0.526) |
| 24 weeks | 0.511 (0.467 to 0.555)e | 0.498 (0.447 to 0.549)f | 0.508 (0.473 to 0.543) | 0.497 (0.457 to 0.537) |
The estimation of QALYs was undertaken as per the same conditions as the clinical outcomes described earlier to take into account the impact of baseline covariates. When adjusted and unadjusted QALYs were compared, small differences were found, with unadjusted results showing a 0.0009 (95% CI –0.0210 to 0.00229) QALY gain in favour of intervention versus usual care, and the adjusted results showing a –0.0019 (–0.0043 to 0.0006) difference in favour of the usual care group. These results are presented in Table 24. No statistically significant differences were found. In accordance with the analysis plan, the adjusted QALYs were used in the base case analysis.
| Mean QALY | Usual care (n = 253), mean QALY (SD) | Intervention (n = 256), mean QALY (SD) | Mean QALY difference (between HEELs intervention and usual care) (95% CI) | p-value |
|---|---|---|---|---|
| Unadjusted | 0.2388 (0.1245) | 0.2397 (0.1273) | 0.0009 (–0.0210 to 0.0229) | 0.934 |
| Adjusted | 0.2413 (0.0143) | 0.2395 (0.0135) | –0.0019 (–0.0043 to 0.0006) | 0.133 |
Costs
The detailed analysis of resource utilisation and costs associated with the intervention compared with usual care is presented in Appendix 4.
Cost data are inherently skewed, as some participants report £0 resource utilisation while others have more serious health conditions requiring expensive inpatient admissions. This has the potential to affect the precision of the mean cost per patient estimates. Therefore, bootstrapping has been used to produce a sampling distribution of the mean cost from the original sample for the main ITT base case analysis. The bootstrap results are shown in Table 25 and are in line with the original sample. The overall costs of the intervention compared with those of usual care alone are also presented in Table 25. The mean per patient cost of the intervention was £286.49 (95% CI –£428.7 to £1001.70) and was higher than that of the usual care group, although the difference was not statistically significant. When the adjusted total costs were compared, this difference was smaller, with a mean cost difference of £67.41 (95% CI –£52.51 to £187.34), and this difference was also not statistically significant. Across the main health-care cost categories, the intervention was associated with higher costs of hospital admissions, which had a mean cost difference of £337.64 (95% CI –£336.96 to –£1012.23). This difference was, however, reduced to £9.90 (95% CI –£76.52 to £96.32) when costs were adjusted. The intervention group had lower medication costs (cost difference –£13.11, 95% CI £31.41 to £5.20) than the usual care alone group, and lower primary care/outpatient costs (mean cost difference –£99.22, 95% CI –£290.58 to £92.14), but, when adjusted costs were examined, costs were slightly higher in the intervention (mean cost difference £1.00 and £25.16 for medication and primary care/outpatient costs, respectively). No statistically significant differences were seen in either unadjusted or adjusted costs. As part of the assessment of costs, an alternative scenario was considered, in which patients with resource utilisation of £0 were excluded from the analysis; this had no effect on costs. Table 26 shows a similar pattern to the ITT base case results with no differences between the groups.
| Cost group | Usual care, £ (SD) (n = 253) | Intervention, £ (SD) (n = 256) | Mean cost difference, £ (95% CI) | p-value |
|---|---|---|---|---|
| Unadjusted costs associated with the heel cast intervention | 0 | 61.18 (69.96) | 61.18 (52.588 to 69.768) | – |
| Adjusted costs associated with the heel casts intervention | 0 | 31.35 (6.30) | 31.35 (30.58 to 32.13) | – |
| Unadjusted costs associated with primary and outpatient care | 995.36 (1193.36) | 896.14 (996.39) | –99.22 (–290.58 to 92.14) | 0.309 |
| Adjusted costs associated with primary and outpatient care | 932.80 (214.70) | 957.96 (205.09) | 25.16 (–11.40 to 61.72) | 0.177 |
| Unadjusted costs associated with hospital admissions | 1447.25 (3734.79) | 1784.89 (4005.41) | 337.64 (–336.96 to 1012.23) | 0.326 |
| Adjusted costs associated with hospital admissions | 1612.08 (496.55) | 1621.98 (495.79) | 9.90 (–76.52 to 96.32) | 0.822 |
| Unadjusted costs associated with medication | 30.06 (144.90) | 16.96 (34.90) | –13.11 (–31.41 to 5.20) | 0.160 |
| Adjusted costs associated with medication | 22.97 (8.97) | 23.97 (8.61) | 1.00 (–0.53 to 2.53) | 0.200 |
| Unadjusted total costs | 2472.67 (4068.71) | 2759.16 (4143.48) | 286.49 (–428.72 to 1001.70) | 0.432 |
| Adjusted total costs | 2567.85 (689.61) | 2635.26 (687.53) | 67.41 (–52.51 to 187.34) | 0.270 |
| Bootstrapped adjusted total costs | 2567.79 (43.85) | 2636.61 (41.24) |
| Cost group | Usual care, £ (SD) (n = 222) | Intervention, £ (SD) (n = 228) | Mean cost difference, £ (95% CI) | p-value |
|---|---|---|---|---|
| Unadjusted costs associated with the heel casts intervention | 0 | 65.49 (72.99) | 65.49 (55.86 to 75.11) | |
| Adjusted costs associated with the heel casts intervention | 31.47 (6.27) | 31.47 (30.64 to 32.30) | ||
| Unadjusted costs associated with primary and outpatient care | 1134.35 (1210.57) | 1006.19 (1002.01) | –128.16 (–333.81 to 77.49) | 0.221 |
| Adjusted costs associated with primary and outpatient care | 945.43 (206.45) | 962.92 (201.80) | 17.49 (–20.33 to 55.31) | 0.364 |
| Unadjusted costs associated with hospital admissions | 1649.34 (3945.94) | 2004.08 (4192.97) | 354.74 (–400.00 to 1109.48) | 0.356 |
| Adjusted costs associated with hospital admissions | 1623.51 (498.35) | 1632.62 (488.32) | 9.12 (–82.29 to 100.53) | 0.845 |
| Unadjusted costs associated with medication | 34.26 (154.26) | 19.04 (36.45) | –15.22 (–35.87 to 5.42) | 0.148 |
| Adjusted costs associated with medication | 23.48 (8.66) | 24.18 (8.47) | 0.70 (–0.89 to 2.28) | 0.388 |
| Unadjusted total costs | 2817.95 (4230.74) | 3094.80 (4272.19) | 276.84 (–511.03 to 1064.72) | 0.490 |
| Adjusted total costs | 2592.41 (686.25) | 2651.19 (676.03) | 58.78 (–67.43 to 184.98) | 0.361 |
Costs per quality-adjusted life-year
Table 27 reports the base case results (based on the adjusted analysis) of the cost–utility analysis. This indicated that the use of lightweight heel casts in addition to usual care was dominated by usual care alone (i.e. was more costly and less effective). The ICER was –£35,478.95; bootstrapping generated an adjusted ICER of –£36,645.04 (95% CI 2.5th –£280,800 to 97.5th £208,378). Similar results were found (Table 28) when a PP analysis was undertaken, in that the use of lightweight heel casts plus usual care was dominated by usual care alone, with an ICER of –£10,312.28.
| Total mean cost (£) | Incremental mean cost (£) | Total mean QALY | Incremental mean QALY | ICER (£) | |
|---|---|---|---|---|---|
| Usual care | 2567.85 | 0.2413 | Dominated | ||
| Heels intervention | 2635.26 | 67.41 | 0.2395 | –0.0019 | –35,478.95 |
| Sensitivity analysis | |||||
| Usual care mean estimate | 2567.85 | 0.2413 | |||
| Heels intervention, lower 95% CI estimate | 2550.64 | –17.21 | 0.2395 | –0.0019 | 9057.89 |
| Mean upper 95% CI estimate | 2719.89 | 152.04 | 0.2395 | –0.0019 | –80,021.05 |
| Total mean cost (£) | Incremental mean cost (£) | Total mean QALY | Incremental mean QALY | ICER (£) | |
|---|---|---|---|---|---|
| Usual care (n = 222) | 2592.41 | 0.2401 | Dominated | ||
| Heels intervention (n = 228) | 2651.19 | 58.78 | 0.2391 | –0.0057 | –10,312.28 |
| Sensitivity analysis | |||||
| Usual care, mean estimate | 2592.41 | 0.2401 | |||
| Heels intervention, lower 95% CI estimate | 2562.97 | –29.44 | 0.2391 | –0.0057 | 5164.91 |
| Mean upper 95% CI estimate | 2739.41 | 147.00 | 0.2391 | –0.0057 | –25,789.47 |
One-way sensitivity analysis
Incremental cost-effectiveness ratios were recalculated based on the lower and upper 95% CIs for the intervention mean cost differences reported. The only parameter change that estimated that the lightweight heel cast plus usual care was more cost-effective (£9057.89 per QALY gain) than usual care alone occurred when the lower-bound estimate of the total mean cost of the intervention was used. Within the PP analysis, the ICER was £5164.91 per QALY gain for the lightweight heel cast plus usual care versus usual care alone.
Figure 5 represents the cost-effectiveness plane for all patients of estimates resulting from bootstrapping. The vertical axis of the plane shows the incremental total costs, and the horizontal axis shows the QALY gains. This illustrates how the lightweight heel cast plus usual care is dominated by usual care alone, with the majority of results occurring in the north-west quadrant of the plane.
FIGURE 5.
Cost-effectiveness plane for all patients of estimates resulting from bootstrapping.
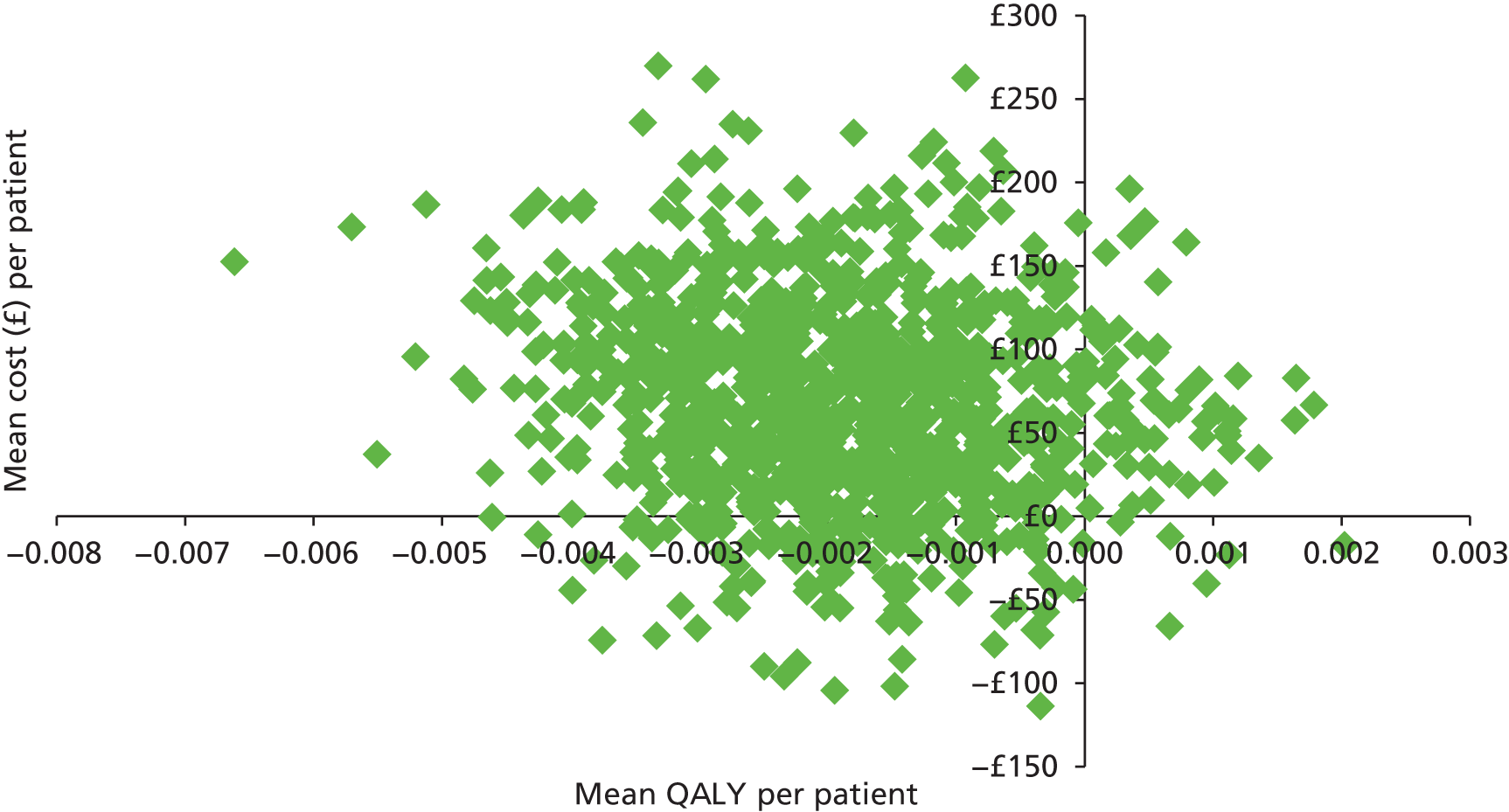
The CEAC for the base case is shown in Figure 6. The CEAC shows the probability (expressed as a percentage) of the use of lightweight heel cast plus usual care being a cost-effective intervention against different willingness-to-pay (WTP) values. Based on a NICE threshold of < £20,000 per QALY gain, the lightweight heel cast plus usual care would be 5.2%, with a negative net benefit of –£106 (95% CI –£241 to £19) when a QALY is valued at £20,000.
FIGURE 6.
Cost-effectiveness acceptability curve, sensitivity analysis: probability of the HEEL cast being cost-effective.
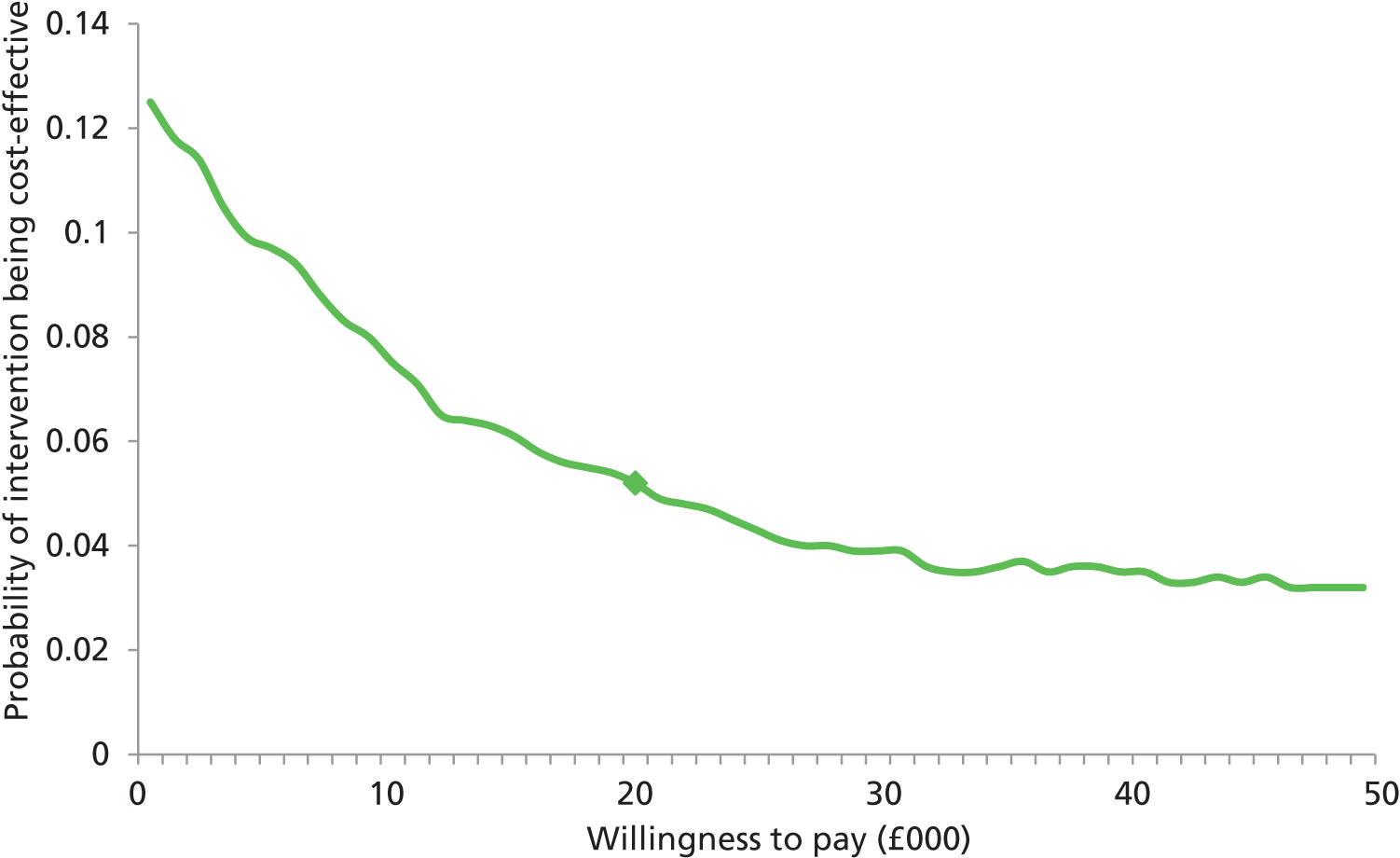
Cost-effectiveness results
The base case results of the analysis are shown in Table 29. The ICER provides an indication of the benefits of additional healed ulcers relative to the additional costs incurred. The unadjusted ICER is £40.93 per 1% likelihood increase in healed ulcers at 24 weeks as a result of the intervention. When the adjusted figures for both costs and outcomes were used, the costs incurred in securing a 1% likelihood increase in healed ulcers by using the heel cast was £9.63. The cost of generating an additional healed ulcer using a heel cast would be £963 (95% CI –£745.82 to £2671.82). A one-way sensitivity analysis using the lower-bound mean cost estimate of the heel cast intervention produced a cost saving of –£246 per additional healed ulcer, while the upper-bound mean cost estimate was £2172, showing wide variation.
| Total adjusted cost (£) | Incremental mean cost (£) | Total effect % probability of healing | Incremental difference in % effect/healing | ICER (£) | |
|---|---|---|---|---|---|
| Usual care | 2567.85 | 37 | |||
| Heels intervention | 2635.26 | 67.41 | 44 | 7 | 963 |
| Sensitivity analysis | |||||
| Usual care, mean estimate | 2567.85 | 37 | |||
| Heels intervention, lower 95% CI estimate | 2550.64 | –17.21 | 44 | 7 | –246 |
| Mean upper 95% CI estimate | 2719.89 | 152.04 | 44 | 7 | 2172 |
Chapter 4 Discussion
This trial was designed to investigate whether or not the use of lightweight fibreglass heel casts would increase the number of heel ulcers in patients with diabetes mellitus that healed by 24 weeks. The trial was undertaken in specialist foot care centres in the UK and was based on comparing the outcomes in those who were managed with the heel casts in addition to usual care with the outcomes in those managed with usual care alone. It was powered to detect a difference of 15 percentage points, assuming an incidence of healing of 40% in the usual care arm. The results of the trial suggest that the true effect may be smaller than expected, although there was insufficient precision to rule out the possibility that the intervention had no effect, or even that it had a very small harmful effect. In this respect, it should noted that there was the possibility of an increase in new ulcers on the contralateral foot of participants in the intervention group.
Reported adherence to the use of the cast in the intervention group was a median 100%, and compliance with the protocol was high. Therefore, as expected, a PP analysis did not alter the conclusions of the primary analysis. The primary analysis was also robust to assumptions about missing data, and there was no evidence that the intervention was differentially effective in any of a number of prespecified subgroups. It is possible that a larger sample may provide stronger evidence of a small but still clinically important effect. It is also possible that the effectiveness of the heel cast is limited to certain populations or types of ulcer; however, assessing this possibility would almost certainly require a much larger study.
The trial also investigated whether or not the use of a cast was associated with reduction in local pain or discomfort, as had been suggested by uncontrolled clinical observations. No such effect was demonstrable, and we found no difference between the intervention and control groups in either the number of patients reporting any local pain/discomfort or the median pain scores determined using a VAS.
The trial failed to identify any differences between the intervention arm and the control arm in terms of predefined secondary measures, with the exception of a possible trend towards an increase in the speed of healing in the intervention group. The isolated finding of a significant difference between groups for one component of the CWIS questionnaire (see Table 16) may be attributable to chance.
The trial was conducted to a high standard, with recruitment and completion of the required population being concluded on time. The demographic details of the recruited sample were as expected for a population with diabetic foot ulcers. The total population for whom a primary outcome was not available because of withdrawal or any other reason was 84 (16.5%) and lay within the limit set of 25%.
Limitations
A decision was made to base this pragmatic trial on a comparison between standard care alone and standard care plus the use of heel casts, because the intention was to determine whether or not the inclusion of heel casts in routine practice in expert centres resulted in clinical improvement. This meant that the comparator treatment was deliberately chosen as the usual standard of care in the centres selected on the basis of their specialist interest in the management of foot disease in diabetes mellitus. And even though the criteria of care were agreed (see Box 3), there was considerable variation documented in the use of offloading. 15 This was, however, assumed to be a reflection of current expert practice in UK. It should also be noted that there is currently no evidence to suggest that ulcers of the heel should be managed with non-removable offloading.
An error that was not detected during the course of the study was the failure of some of the clinical researchers to correctly report the results of the ABPI measurements. However, this had no impact on the conduct of the study or on the interpretation of the results. Another error was the failure to document the prevalence of infection in the two groups at the time of randomisation, even though it was recorded at every other visit, and the use of antibiotics was recorded when appropriate. There is no reason to suspect that the distribution of infection differed between the two groups.
There were minor problems relating to the stratification used at the time of randomisation; however, these were detected early and remedied. Very occasional discrepancies found between the area assessed by researchers at the time of randomisation and the area later determined by a central blinded observer may be attributable to the less precise methods of measurement used by the researchers. This resulted in a total of 24 participants being recruited in error because the ulcer area at baseline was ultimately judged to be < 25 mm2.
The results of the trial suggest that the incidence of AEs was greater in the intervention group than in the usual care group. It should, however, be noted that AEs (which were recorded only up until the protocol change in 29 March 2012) were recorded by researchers who were not blind to randomisation group; therefore, the data are susceptible to bias. Moreover, none of the observed differences was statistically significant.
Ancillary analysis
An ancillary analysis was also undertaken to determine whether or not the reduction in cross-sectional area (with ulcers from both the intervention and control arms combined) by 4 weeks was associated with eventual healing within 24 weeks, as has previously been reported for other types of ulcer of the foot in diabetes mellitus. 25 There was strong evidence of an association, but ROC analysis failed to identify any clear threshold value for change in ulcer area at 4 weeks that predicted eventual healing. This issue would benefit from further research that may inform patient counselling and influence management decisions.
Health economic analysis
There were small differences in costs between the intervention and usual care arms, but none was statistically significant. When costs were adjusted, the magnitude of differences in cost became smaller. The results of the incremental cost per QALY analysis did not provide evidence of cost-effectiveness. When the results of the incremental costs were expressed per percentage increase in likelihood of achieving an additional healed ulcer, there were differences between the adjusted and unadjusted analyses. With further examination of the impact on changes in parameter variation around the adjusted base case, there was wide variation in the results. This uncertainty should be clearly noted in any interpretation of these results, particularly in the light of the clinical effectiveness results.
Because of the complex nature of diabetes mellitus, it is possible that other factors played a part in the resources consumed, something that has been identified as an issue in accurately costing the inpatient management of diabetic foot ulcers. 26 Some of the challenges encountered in obtaining accurate data on resource utilisation must also be acknowledged. Although a patient diary allowed for timely data collection, this method is, inevitably, rather crude; however, any resultant errors in assigning costs will have applied to both study groups. It was not possible to explore the costs beyond those to the NHS (e.g. costs to the patient, loss of productivity) because of limitations of the data collected. One interesting finding is that the baseline utilities in both groups appeared to be much lower, and the utility gains smaller, than in other published estimates of diabetes mellitus models and cost–utility evaluations. 27 Overall, however, the results of the health economic analysis did not provide evidence to substantiate the cost-effectiveness of using lightweight heel casts in the management of heel ulcers in diabetes mellitus.
Acknowledgements
The authors thank the Health Technology Assessment programme of the National Institute for Health Research for funding the study and the Research and Innovation Department of Nottingham University Hospitals Trust for sponsoring it. They also thank others involved in the original application for funding (Sarah Pankhurst, Jim Thornton, Phil Wiles, Michelle Proudman, Umberto Saoncella and Louise Stuart) and express especial gratitude to the independent members of the Trial Steering Committee [Roger Gadsby, Richard Holt (chairperson), Fiona King and Peter Wilson] and the Data Monitoring Committee [Martin Bland, Simon Heller (chairperson) and Jane Nixon], as well as members of the Nottingham Clinical Trials Unit for enabling its conduct according to the principles of GCP. Special thanks go to Dan Simpkins for his help with the design, construction and maintenance of the eCRF and to Liz Mudge for documenting the area of all visual images.
Patient and public involvement
The authors endorse the principles of patient and public involvement, and users/members of the public have been involved at every stage of this project from the planning, the application for funding and the trial conduct to the review of the results.
Contributions of authors
The study was planned by William Jeffcoate (Diabetologist and Clinical Triallist) and Frances Game (Diabetologist and Clinical Triallist). Patricia Price (Pro-Vice Chancellor, University of Cardiff and Clinical Triallist) prepared the original SAP.
Ceri Phillips (Health Economist) prepared the original health economics analysis plan.
William Jeffcoate, Frances Game, Patricia Price, Alison Musgrove (Research Podiatrist) and Ceri Phillips were co-applicants for funding of the study by the Health Technology Assessment programme.
The SAP was later revised by Alan Montgomery (Professor of Medical Statistics and Clinical Trials), Lucy Bradshaw (Statistician) and Wei Tan (Statistician).
The conduct of the trial was managed by William Jeffcoate, Frances Game and Vivienne Turtle-Savage (Trial Manager).
Statistical analysis was undertaken by Wei Tan, Lucy Bradshaw and Alan Montgomery.
Health economic analysis was undertaken by Deborah Fitzsimmons (Professor of Health Outcome Research) and Angela Farr (Researcher in Health Economics), with the assistance of Thomas Winfield (Researcher in Health Economics).
The first draft of the manuscript was prepared by William Jeffcoate, and all authors reviewed and corrected this and subsequent drafts and approved the final version.
Publications
Trial protocol
The protocol of this trial has been previously published as:
Jeffcoate W, Game F, Price P, Phillips C, Turtle-Savage V. Evaluation of lightweight fibreglass heel casts in the management of ulcers of the heel in diabetes: study protocol for a randomised controlled trial. Trials 2014;15:462.
Other publications
Game F, Jeffcoate W, Musgrove A, Sprengel M, Turtle-Savage V, Whitham D. Bringing the evidence to heel: heel cups and the diabetic foot. Diabet Foot J 2012;15:100.
Jeffcoate W, Musgrove A, Lincoln N. Using Image J to document healing in ulcers of the foot in diabetes. Int Wound J 2017; in press.
Data sharing statement
The authors are keen to share anonymised data with scientific colleagues. Available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Jeffcoate W, Game F, Price P, Phillips C, Turtle-Savage V. Evaluation of lightweight fibreglass heel casts in the management of ulcers of the heel in diabetes: study protocol for a randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-462.
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719-24. https://doi.org/10.1016/S0140-6736(05)67698-2.
- Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855-9. https://doi.org/10.2337/diacare.21.5.855.
- Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer?. Int Wound J 2007;4:286-7. https://doi.org/10.1111/j.1742-481X.2007.00392.x.
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33:1493-8. http://dx.doi.org/10.1111/dme.13054.
- Kerr M. Improving footcare for people with diabetes and saving money: an economic study in England. n.d.
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB, et al. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med 2001;18:133-8. https://doi.org/10.1046/j.1464-5491.2001.00422.x.
- Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer-related and person-related measures. Diabetes Care 2006;29:1784-7. https://doi.org/10.2337/dc06-0306.
- Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med 2005;22:1306-9. https://doi.org/10.1111/j.1464-5491.2005.01640.x.
- Chipchase SY, Treece KA, Pound N, Game FL, Jeffcoate WJ. Heel ulcers don’t heal in diabetes. Or do they?. Diabet Med 2005;22:1258-62. https://doi.org/10.1111/j.1464-5491.2005.01665.x.
- Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC. Eurodiale consortium . Diabetic foot disease: impact of ulcer location on ulcer healing. Diabetes Metab Res Rev 2013;29:377-83. http://dx.doi.org/10.1002/dmrr.2400.
- The Management of Pressure Ulcers in Primary and Secondary Care: A Clinical Practice Guideline. London: Royal College of Nursing; 2005.
- Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Londahl M, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2015;32:154-68.
- Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care 2005;28:551-4. https://doi.org/10.2337/diacare.28.3.551.
- Nabuurs-Franssen MH, Sleegers R, Huijberts MS, Wijnen W, Sanders AP, Walenkamp G, et al. Total contact casting of the diabetic foot in daily practice: a prospective follow-up study. Diabetes Care 2005;28:243-7. https://doi.org/10.2337/diacare.28.2.243.
- Stuart L, Gordon H, Proudman M, Farrar S, Wiles PG. A revolution in heel ulcer management: a novel community project. Diabet Med 2009;26.
- Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire: WMA; 2013.
- ISO 14155:2011: Clinical Investigation of Medical Devices for Human Subjects – Good Clinical Practice. Geneva: ISO; 2011.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Chang AC, Dearman B, Greenwood JE. A comparison of wound area measurement techniques: visitrak versus photography. Eplasty 2011;11.
- EuroQol Research Foundation . EQ-5D-5L Self-Complete Version On Paper 2017. www.euroqol.org/eq-5d-products/eq-5d-5l/self-complete-version-on-paper.html (accessed 11 February 2016).
- Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10-7.
- The CONSORT Group . The CONSORT Statement 2010 Checklist 2010. www.consort-statement.org (accessed 11 February 2016).
- Guide to the Methods of Technology Appraisal, 2013. London: NICE; 2013.
- Lavery LA, Barnes SA, Keith MS, Seaman JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31:26-9. https://doi.org/10.2337/dc07-1300.
- Diabetic Foot Problems: Prevention and Management. London: NICE; 2015.
- Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab 2004;30:549-56. https://doi.org/10.1016/S1262-3636(07)70154-4.
- Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia 2001;44:2077-87. http://dx.doi.org/10.1007/s001250100013.
- Ortegon MM, Redekop WK, Niessen LW. Cost-effectiveness of prevention and treatment of the diabetic foot: a Markov analysis. Diabetes Care 2004;27:901-7. https://doi.org/10.2337/diacare.27.4.901.
- Marques E, Johnson EC, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Value Health 2013;16:195-201. http://dx.doi.org/10.1016/j.jval.2012.09.008.
- Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- NHS Reference Costs 2013–14. London: DH; 2015.
- British National Formulary (online). London: BMJ Group and Pharmaceutical Press; 2017.
Appendix 1 Assessment of ulcer healing in the definition of the primary outcome
A participant met the criteria for a healed ulcer if their ulcer was judged to have remained healed 4 weeks after it was initially judged to be healed by both the on-site research nurse and the blinded observer. The time to healing was calculated as the number of days between randomisation and the date of the visit at which the clinical researcher first judged the ulcer to be healed.
A participant’s ulcer was defined as unhealed if:
-
it was judged so by the clinical researcher (confirmed by a blinded observer) at 24 weeks and had not met the criteria for earlier healing defined above, or
-
there was a major amputation of the target limb, or
-
the participant died during the study prior to the visit at week 24 and had not met the criteria for healing defined above prior to their death.
The primary outcome was recorded as missing in cases where the participant did not complete the visit at week 24 (or week 26 or 28 if healing first noted at week 22 or 24, respectively) and was not known to have died, did not meet the criteria for healing described above and had not had a major amputation of the target limb.
If the recorded date of verification of ulcer healing by a blinded assessor was outside the required protocol window (+4 days), the blinded assessor verification was used in the analysis provided that any of the following criteria were met:
-
confirmation by the (blinded) chief investigator that the image was taken on the correct visit date and shows that the ulcer is healed
-
confirmation of healing by a blinded assessor at the site 5, 6 or 7 days after the ulcer was first assessed as healed by the on-site researcher (protocol violation)
-
confirmation of healing by a blinded assessor 4 weeks after initial healing, when the initial healing had not been confirmed by a blinded assessor
-
confirmation by the (blinded) chief investigator that an image taken 2 weeks after initial healing showed that the ulcer remained healed and that healing was likely to be maintained at 4 weeks following initial healing.
Appendix 2 Derivation of binary outcomes for infection, major amputation, minor amputation revascularisation of the limb with the target ulcer or trip or fall leading to hospital admission
-
Yes, coded as 1, if at any visit the outcome was reported and related to the target limb.
-
No, coded as 0, if at any visit the outcome was not reported or was unrelated to the target limb.
-
Coded as missing if the outcome was not reported at any visit and the participant did not attend the week 24 visit or the information was not completed at the week 24 visit in order to confirm that no events occurred.
Appendix 3 Derivation of binary outcomes for hospital admission, new ulcer on target foot, new ulcer on contralateral foot
-
Yes, coded as 1, if at any visit the outcome was reported.
-
No, coded as 0, if the outcome was not reported at any visit.
-
Coded as missing if the outcome was not reported at any visits and the participant did not attend the week 24 visit or the information was not completed at the week 24 visit in order to confirm that no events occurred.
Appendix 4 Health economic methods
Introduction
This appendix describes in detail the health economic objectives and methods used for the health economic evaluation. A summary of the health economic methods used to evaluate the cost-effectiveness and cost implications of lightweight fibreglass heel casts was provided in the published study protocol. 1 This was supplemented by the development of a health economic analysis plan, developed alongside the SAP. This appendix also provides the rationale for a major amendment to the published health economic protocol, in which longer-term evaluation of the cost-effectiveness of lightweight fibreglass heel casts and budget impact analysis were not undertaken. As this substantial amendment to the published protocol could be questioned, a full rationale for this is presented.
Summary of the decision problem
As discussed in Chapter 1, ulcers of the heel can cause substantial pain and suffering in people affected with diabetes mellitus. Diabetic foot ulcers are extremely costly and have been estimated to account for 0.7% of the total UK NHS budget. With the consequences of unhealed and/or recurrent ulcers leading to further health interventions (including amputation), establishing the cost-effectiveness and clinical effectiveness of new interventions is warranted. Although other health economic analyses have assessed the cost-effectiveness of a range of orthotic devices within the context of patient management strategies,28,29 the orthotic devices assessed have largely been intended for use as preventative strategies across the spectrum of risk categories for developing an ulcer of the foot, as opposed to being specifically intended for use in the management of an established ulcer of the heel.
A rapid review of the health economic evidence, completed as part of the preparatory stages of the health economic analysis, failed to locate specific studies of the cost-effectiveness of lightweight fibreglass heel casts specific to the patient population described within the trial protocol and setting (either the UK NHS or another health-care system). Thus, a health economic analysis, alongside a prospective randomised controlled trial, can provide evidence to support decision-makers in the assessment of whether or not the use of lightweight fibreglass heel casts is a beneficial intervention in the management and care of people with a diabetic ulcer of the heel within the UK NHS setting.
Original protocol intentions
In the published protocol,1 it was stated that cost-effectiveness would be assessed by developing a decision-analytic model to estimate costs and health outcomes, including the percentage of healed ulcers and QALYs gained. ICERs and cost–utility ratios would be generated for a series of time horizons (including a lifetime perspective) that reflect the management of people affected by diabetic ulcers of the heel. It was also stated that a budget impact analysis would be undertaken.
Summary of the major protocol change to the published protocol
Based on the clinical effectiveness results, the plan was to undertake further modelling to assess the longer-term costs and outcomes of the intervention compared with usual care. The proposed structure was based on a Markov model with a series of health states to represent healed and unhealed target ulcer, amputation and death, with the recently published NICE economic evaluation of the cost-effectiveness of providing custom orthotic footwear to patients at low, moderate and high risk of developing foot ulcers identified as a possible model for adaption. 26 The adaption proposed would focus on the trial population as the entry point, that is, those who are already affected, with the trial results being used as the primary source to estimate required inputs, such as healing time and number of unhealed ulcers. Longer-term impact would be considered on the basis of the trial results, published literature as part of a rapid review of the literature (including assessment of the evidence used in the recent NICE model26) and in discussion with the trial team. A sensitivity analysis was undertaken to account for the uncertainty in key parameters (one-way sensitivity analysis) and a probabilistic sensitivity analysis was undertaken to quantify the joint uncertainty in parameter estimates. The aim of undertaking this longer-term modelling was to capture a more accurate estimation of the cost-effectiveness based on a more realistic explanation of the clinical pathway and to capture health states (and associated costs/utilities) that may not have been experienced owing to the short duration of the trial.
Prior to the further development of the model and running of the analysis, the feasibility of undertaking this longer-term modelling was discussed with the Trial Steering Committee and trial team after initial consideration of the main clinical results. The evaluation of further long-term modelling was tested against the feasibility that (a) the intervention would be able to demonstrate clinical effectiveness in the short term, (b) the trial results would provide a realistic estimation of health states attributed to the intervention (different healing rates/amputation, etc. , and the additional costs and time that these would incur) and (c) the utilities derived as a result of the intervention could be confidently estimated over a longer period of time.
However, in the light of the clinical effectiveness findings, a decision was made to focus on reporting the within-trial analysis only. The specific reasons were:
-
On the basis of the trial, there was no evidence that the use of a heel cast could be seen as clinically effective.
-
The results of the within-trial health economic analysis showed no evidence of cost-effectiveness, based on the results of the incremental cost per QALY analysis.
-
With a lack of evidence of short-term benefits from using the heel cast, there was no clinical evidence or opinion that these would or could translate into longer-term benefits.
The justification for undertaking a budget impact analysis was also discussed. Based on the trial findings, and given the lack of support for the use of lightweight heel casts within the NHS, the Trial Steering Committee and the trial team decided that the budget impact analysis would be an arbitrary one.
Methods
Patient population
The target population for the health economic analysis was commensurate with the trial population (see Chapter 2, Participants). Although the SAP set out a subgroup analysis of key variables for the primary outcome, a subgroup analysis was not proposed in the health economics analysis plan.
Study perspective
The perspective adopted for the health economic analysis was that of the NHS/PSS, in accordance with the NICE reference case. 24 A separate analysis was planned to explore the costs to the patient and their family (as a result of travel time/time off work) of attending an appointment related to their heel ulcer.
Comparators
As the health economic evaluation was conducted alongside the main trial, the comparators have been described previously (see Chapter 2, Intervention).
Time horizon
The revised time horizon was based on the trial follow-up duration, that is, 26 weeks (0.5 years).
Discount rate
As the revised analysis was based on the trial duration only, and thus the duration was < 12 months, discounting of costs and outcomes was not undertaken.
Collection of resource utilisation and costs
Two main costs were assessed: first, the implementation costs associated with the manufacture and use of the lightweight heel cast device, and, second, the costs associated with health-care utilisation during the trial period. In addition, the assessment of costs associated with time away from home or work as a result of the management of the heel ulcer were included as part of the data collection.
All assumptions made in the calculation of costs were verified by the trial team to ensure that they were appropriate and commensurate with the trial protocol and/or standard clinical practice. Only resource usage and associated costs, as part of the management of the target diabetic heel ulcer, were considered.
Implementation costs for the intervention
The costs of training staff in the production and use of the heel cast device were derived from interviews with the research team and clinical staff. It was estimated that the cost of one heel cast device was £7.00. Podiatrists in each of the participating study centres were trained in the production of the heel cast, which involved fashioning of the heel cast device for individual patient requirements, as described in the section Intervention. All podiatrists were involved in the routine care of patients with diabetic foot ulcers, therefore the training and time required to mould the heel cast for each patient was included. It was estimated that it would take a specialist podiatrist 30 minutes to train two podiatrists per study centre in the production of heel casts. The production of each heel cast device was estimated to take a podiatrist 15 minutes each time a new product was required.
There is no specific clinical assessment required when considering the suitability of a patient for a heel cast beyond usual standard care in the assessment of a diabetic heel ulcer. No additional and/or different dressing or external orthotic footwear is required for the use of the heel cast device. In addition, all patients, whether or not they received the heel cast device, would receive standard written information, supplied at each centre, regarding the management and care of their heel ulcer. Although additional instructions on the use of the heel cast were prepared for the trial, these were estimated to be of minimal cost (£0.05 per patient for one A4 sheet), and therefore were not included.
Health and Personal Social Services resource utilisation
A bottom-up approach was taken to the collection of patient-level information for additional health and personal social costs as part of the trial data collection process. Two key data collection tools were used to collect resource utilisation across key categories:
-
A patient log was used to capture resource usage as a result of health-care contacts in primary care [such as general practitioner (GP) surgery visits] and hospital outpatient care associated with dressing changes and any further consultations with any health-care professionals as a result of the heel ulcer.
-
The trial eCRF was used to collect data on hospital admissions as a result of the diabetic foot ulcer or heel cast device and medications associated with the treatment of the diabetic ulcer on the target foot.
Patient log
The patient log was developed to capture information that was recorded at each fortnightly assessment by the researcher, and the participant or carer was asked to keep a simple diary/calendar of relevant intervening events in order to overcome some of the challenges caused by recall bias and missing data. 30 This was deemed particularly important for this patient population, who would probably receive numerous health-care contacts as a result of the diabetic ulcer and other comorbidities. These data were then uploaded into the electronic data capture system.
Information was collected across the following cost categories: primary and outpatient care contacts (associated with dressing changes/care of the target ulcer); details for all types of visits (e.g. face to face or telephone call) were collected by recording all resources used, duration of contact and location of contact. The patient log also contained questions on other resources used, such as travel mileage, public transport costs or other expenses incurred by the patient. Although the primary analysis would be from a NHS/PSS perspective, the inclusion of these wider costs would be presented separately to consider the wider costs to the individual and family.
Missing data considerations in the use of the patient log
Owing to the design of the patient log (reproduced in Appendix 9), which asked patients to report contacts with health professionals only, rather than tick boxes indicating whether or not a contact had taken place, it was assumed that the patient had completed the log section only if a contact had occurred (for interpretation rules of patient responses see Table 31). For logs in which there were blank sections, it was assumed that where the fields for dressing changes and additional consultations with health-care professionals were left blank, a dressing change or consultation had not taken place rather than that a response was missing. A particular problem arose with the capturing of transport detail (including time out of the house to attend the appointment and the cost associated with this). There were only limited responses to questions regarding transport costs, and the quality of responses was poor. As this was intended to be reported separately and not included in the cost calculations for the health economic analysis, these data were not analysed.
However, a minority of patients did not submit a patient log during their follow-up visit. It is not clear whether the patients did not return their resource logs because they did not receive any NHS service provision in the management of their ulcer (i.e. they self-managed their dressing changes) or because service provision was received but not reported. Further examination was undertaken to assess the potential impact of the missing patient logs. In total, 465 out of 509 (91%) patients returned a patient log across the follow-up assessment points, with no pattern of missing data, for example patients who repeatedly failed to provide a log. When assessed by trial arm, 233 patients assigned to the usual care group returned a log, while 20 (8%) did not, and 232 intervention patients returned a log, while 24 (9%) did not. By grade of ulcer, of the 44 not submitting a resource log, 16 (36%) patients had a grade 2 ulcer, 26 (59%) patients had a grade 3 ulcer and two (5%) patients had a grade 4 ulcer.
The pattern of missing data by group and ulcer grade can be summarised as follows.
-
In the usual care group, 7 (8%) out of 82 patients with a grade 2 ulcer and 13 (8%) out of 156 patients with a grade 3 ulcer did not return a resource log.
-
In the intervention group, 9 (11%) out of 83 patients with a grade 2 ulcer, 13 (8%) out of 158 patients with a grade 3 ulcer and 2 (13%) out of 15 patients with a grade 4 ulcer did not return a resource log.
It was also noted that 9 of the 44 patients had died (four in the usual care arm and five in the intervention group), leaving 35 (7%) patients without a single resource log entry. For the 44 patients who did not report NHS resource use via the patient log, a £0 cost was applied for the base case.
Collection of resources as a result of hospitalisation
Other health resource data were obtained from the trial eCRF. Hospital admission rates were taken from the eCRF secondary outcomes data and recorded as either yes or no, and included AEs such as minor and major amputations, revascularisation, falls and serious infection. Details regarding the nature, complexity and length of stay of each hospital admission for amputations, revascularisation, falls and ‘other’ reasons (primarily related to systemic infections) were sought from the SAE files. However, the SAE data collection was largely incomplete for patients and had not been routinely collected. Therefore, standard unit costs were applied (as shown in Table 30). This was also undertaken in the light of the protocol amendments on the collection of AE data reported in Chapter 2.
| Secondary care unit costs (from the secondary outcomes data) | |||
|---|---|---|---|
| Resource use | Variable | Unit cost (£) | Source |
| Hospital attendance since last visit – and mainly related to the target foot ulcer | Hosp (y/n) Hosptarg (y/n) |
3848 | Hospital admission – Foot ulcer was primary cause and cost driver Hospital Episode Statistics 2014, referenced in NICE Guideline 1926 |
| Patient has had a minor amputation since last visit on the foot with the target ulcer | Minor (y/n) Mintarg (y/n) |
6720 | Minor amputation is defined as the removal of any part of the foot below the ankle Hospital Episode Statistics 2014, NICE Guideline 1926 |
| Patient has had a major amputation since the last visit on the foot with the target ulcer | Major (y/n) Majtarg (y/n) |
10,907 | Major amputation is defined as removal of the foot above the ankle Hospital Episode Statistics 2014, NICE Guideline 1926 |
| Patient has had revascularisation surgery on the foot of the target ulcer (see Costing methodology) | Rvas (y/n) Rvastarg (y/n) |
7221 | Based on HRG codes YQ10A to YQ12D relating to multiple or single open procedures on blood vessels of the lower limb NHS Reference Costs, 2013/1432 |
| Patient admitted to hospital due to a trip or fall that affected the target foot (see Costing methodology) | Fallhosp (y/n) Falltarg (y/n) |
2326 | Based on HRG codes WA23 A, B, C – non-elective long-stay admissions relating to falls without specific cause. Owing to the nature and complexity of the trial patient population, only non-elective long-stay HRG costs were used to calculate a weighted average cost, as a hospital admission is assumed to be > 1 day (NEL) NHS Reference Costs 2013/1432 |
Collection of medication data
Details of medication prescribed to treat infections of the target foot ulcer were collected via the eCRF medicine log: drug name, reason for taking the medication, whether or not the patient had started taking the medication prior to participating in the trial (yes/no), and the dates that the patient had started and finished taking the medication were noted.
Assumption made in the attribution of costs to resources incurred
Owing to the broad categories within the patient log and the information recorded in the eCRF, challenges arose in making a precise interpretation of the health-care contacts from the data recorded. Thus, a range of assumptions were developed and verified by the trial team in order to attribute costs incurred from the use of resources for each patient. All assumptions were applied consistently across each arm of the trial to minimise the potential for costs being attributed for reasons unrelated to the intervention (i.e. our focus was on costs related to management and care according to standard clinical practice for both arms, and, in the case of the heel cast device arm, to the management of the heel cast itself). These assumptions are presented in Table 31.
| Resource category | Assumption made |
|---|---|
| Patient log: concurrent dressing change and consultation visits | When a dressing change and consultation with the same health-care professional were recorded on the same date for a patient, we applied one cost to the health-care professional’s time |
| If different NHS staff members were reported for the same record number for dressing changes and consultation, then both health professionals were assigned a unit cost and counted | |
| Patient log: hospital doctor | A hospital appointment was assumed to be at a consultant-led outpatient clinic and the appropriate unit cost was applied |
| Patient log: hospital nurse | For hospital-based NHS staff, an appointment with a hospital nurse was assumed to be a consultation at a nurse-led outpatient clinic and the appropriate cost was applied |
| Patient log: outpatient consultations | Not all patients specified who they saw when attending a hospital appointment; alongside the further consultation field are fields for reporting mode of transport for hospital visits. Some patients reported hospital visits that were actually admissions to hospital, and these were ignored. For hospital visits where NHS health-care professional was unspecified but the related transport field reported transport by car/public transport, it was assumed that this was an outpatient visit |
| Patient log: primary care contacts | When partial information was collected, for example location of consultation (home or surgery) and contact type (face to face or telephone consultation), but not with whom, we assumed this to be a district nurse visit (face to face at home) or a GP telephone consultation (surgery and telephone call). When a home visit was recorded as a consultation with no information on the health-care professional, this was assumed to be with a district nurse |
| eCRF secondary outcomes: hospital admissions | When a hospital admission was reported on two consecutive trial visits and the reason for admission was the same, this was assumed to be one hospital inpatient episode and a single cost was applied |
| When a hospital admission was reported for two consecutive trial visits, but the reason for admission was for two different reasons, these were assumed to be two different procedures and both procedures were costed | |
| When hospital admissions were reported on non-consecutive trial visits, these admissions were costed separately | |
| When a patient received an amputation during the trial period, we have not included costs of follow-up, for example measurement for a prosthetic. Information captured in the HEELs data set on the types of revascularisation procedures for patients was insufficient. There are a number of HRG codes associated with revascularisation procedures based on type, complexity and mode of procedure. We applied a weighted average cost of open procedures on blood vessels of the lower limb to calculate a cost associated with revascularisation | |
| The details of injuries sustained by falls were not clearly captured with the HEELS data set (within neither the hospital admissions nor the SAE data). The HEELs trial population was considered to be more susceptible to injury from a fall; thus, non-elective long-stay HRG codes were used to calculate a weighted average unit cost | |
| eCRF medicines: long-term medication | Only medications related to infection of the target foot ulcer or other related ulcer event such as cellulitis (following advice from the HEELS clinical team) have been included |
| Medication that had been prescribed before the patient’s entry into the trial was excluded from the analysis | |
| All medication lists and associated costs (BNF) were checked and verified by the chief investigator prior to being included in the costing analysis. We assumed that all medications incurred a cost to the NHS | |
| We excluded medication that was specifically prescribed for infection in the contralateral foot | |
| We excluded i.v. medications, as it was assumed that these would be costed as part of the relevant HRG for that hospital admission | |
| We excluded medication that was not related to an infection of the target ulcer, for example medication taken to treat a urinary tract infection or a chest infection | |
| All medications were related to those prescribed and taken while patients were treated in the community, that is, for hospital discharge, outpatient consultations and/or primary care. We have assumed that the lowest price generic brand medication as stated in the BNF unless a brand name is specifically stated in the medication data set | |
| When misspellings or abbreviations have been used we have costed to the lowest price generic band. When dosage was missing, the HEELS trial team advised on the correct dosage for costing purposes | |
| For some patients, it was noted that medication therapy was ongoing and an end date was not available or not collected before the end of the trial. In these instances, a median number of days prescribed was calculated for each drug and used as a substitute for prescriptions with missing end dates |
The resource log questions were divided into two sections. The first related to dressing changes (the patient was asked to report the NHS staff member) and the second related to other consultations with a doctor, nurse, podiatrist or other health-care professional). Entries into the resource log were interpreted as a reported contact taking place. If the field was left blank either for dressing change or other consultation it was assumed that the patient had no contact with any NHS health professional during that 2-week period. As such, missing data were viewed as having no contact with NHS professionals.
A range of assumptions were developed and verified by the trial team in order to attribute costs to the resources incurred for each patient. These are presented in Table 32.
| Primary and community care costs (from the patient log data) | |||
|---|---|---|---|
| Resource use | Variable | Unit cost (£) | Source |
| NHS staff carrying out dressing changes (Dress who); number of dressing changes (recno) | District nurse | 39 | Mean average cost for a one-to-one contact – PSSRU 201431 |
| Practice nurse | 10.25 | Average patient contact: 15.5 minutes – PSSRU 201431 | |
| Podiatrist (specialist) | 49 | Mean average cost for a one-to-one contact Grade 6 – PSSRU 201431 | |
| Hospital nurse – has been classified as referring to nurse-led outpatient consultations | 69 | As the specific reason for an outpatient consultation is unknown, only that it is related to the target ulcer, the weighted average cost by attendance activity has been used – NHS Reference Costs, 2013/1432 | |
| Research nurse/podiatrist | 49 | Included as the results suggest that the research nurse/podiatrist was utilised in place of usual care NHS staff Costed as Grade 6 specialist podiatrist – PSSRU 201431 |
|
| Consultations with health-care professionals: related to target foot; type of consultation; where face to face took place; (visit who); (foolrel); (contype); (ffloc) | GP consultation – in person | 46 | Average surgery contact: 11.7 minutes – PSSRU 201431 |
| GP consultation – by telephone | 28 | Average telephone consultation: 7.1 minutes – PSSRU 201431 | |
| GP consultation – home visit | 115 | Per out-of-surgery visit lasting 23.4 minutes – PSSRU 201431 | |
| Practice nurse appointment | 10.25 | Average patient contact: 15.5 minutes – PSSRU 201431 | |
| Clinic provided in GP practice | 67 | Average clinic contact: 17.2 minutes – PSSRU 201431 | |
| District nurse | 39 | Mean average cost for a one-to-one contact – PSSRU 201431 | |
| Podiatrist grade 6 (specialist) | 49 | Mean average cost for a one-to-one contact Grade 6 – PSSRU 201431 | |
| Outpatient | 111 | Weighted average by attendance activity – NHS Reference Costs, 2013/1432 | |
| Hospital consultant-led outpatient appointment | 128 | Weighted average by attendance activity – NHS Reference Costs, 2013/1432 | |
Valuation of costs
All data were valued in monetary terms and unit costs were reported in pounds sterling for the financial year 2014 (representing the nearest end point of the trial where costs were available). The unit costs were derived from published unit costs (Personal Social Services Research Unit 2014,31 NHS Reference Costs 2013/14,32 British National Formulary 201433) or from financial information supplied by the trial team.
Calculating the unit cost of revascularisation therapy
As the type, complexity and mode of hospital delivery of revascularisation treatment was unknown, the weighted average cost of open procedures on blood vessels of the lower leg was calculated as shown in Table 33.
| Currency code | Currency description (all HRGs combined) | Total HRG activity | National average unit cost (£) |
|---|---|---|---|
| YQ10A | Multiple open procedures on blood vessels of lower limbs with CC score 11+ | 283 | 14,366 |
| YQ10B | Multiple open procedures on blood vessels of lower limbs with CC score 7–10 | 560 | 10,719 |
| YQ10C | Multiple open procedures on blood vessels of lower limbs with CC score 4–6 | 929 | 8194 |
| YQ10D | Multiple open procedures on blood vessels of lower limbs with CC score 0–3 | 1028 | 6610 |
| YQ11A | Single open procedure on blood vessel of lower limb with imaging intervention, with CC score 7+ | 292 | 12,170 |
| YQ11B | Single open procedure on blood vessel of lower limb with imaging intervention, with CC score 4–6 | 417 | 8660 |
| YQ11C | Single open procedure on blood vessel of lower limb with imaging intervention, with CC score 0–3 | 421 | 6849 |
| YQ12A | Single open procedure on blood vessel of lower limb with CC score 11+ | 329 | 11,323 |
| YQ12B | Single open procedure on blood vessel of lower limb with CC score 7–10 | 786 | 7474 |
| YQ12C | Single open procedure on blood vessel of lower limb with CC score 4–6 | 1668 | 6211 |
| YQ12D | Single open procedure on blood vessel of lower limb with CC score 0–3 | 2636 | 4939 |
Calculating the unit cost of falls relating to the foot with the target ulcer that resulted in a hospital admission
As the details of injuries sustained due to falls were not clearly captured and the trial population was considered to be more susceptible to injury due to a fall, non-elective long-stay Healthcare Resource Group codes were used to calculate a weighted average unit cost as shown in Table 34.
| WA | Falls | Number of FCE | National average unit cost (£) |
|---|---|---|---|
| Non-elective long stay HRG codes and unit costs (length of stay > 1 day) | |||
| WA23A | Falls without specific cause, with CC score 4+ (mean LOS 13.34 days) | 259 | 4128 |
| WA23B | Falls without specific cause, with CC score 2–3 (mean LOS 7.74 days) | 898 | 2672 |
| WA23C | Falls without specific cause, with CC score 0–1 (mean LOS 3.57 days) | 931 | 1491 |
Costing methodology
Hospital admission rates were taken from the secondary outcomes data, and more details regarding the nature, complexity and length of stay of each hospital admission for amputations, revascularisation, falls and ‘other’ reasons (primarily related to systemic infections) was sought from the SAE files. However, the SAE data collection was largely incomplete for patients who had reported a hospital admission and had not been routinely collected. Little information was collected describing the type, the complexity or the mode of delivery (i.e. elective, non-elective long/short stay) of a revascularisation procedure. Therefore, the ‘total Healthcare Resource Group activity’, combining all procedures, was used to calculate the weighted average. It was assumed that falls were accidental injuries and that hospital admission was non-elective in nature. A weighted average cost of non-elective long stay admissions was calculated using full consultant episodes.
For this reason, already compiled unit costs from the literature and national reference costs were used to cost hospital admissions (see Table 32). For every trial participant, treatment costs were calculated by multiplying each individual resource use by an appropriate unit cost. These costs were added to produce a total cost for each participant during the trial. These totals were then summated for each trial arm.
Outcomes used in the economic analysis
Two outcomes were used in the evaluation of cost-effectiveness, (1) the percentage of healed ulcers at or before 24 weeks and (2) the EQ-5D-3L, in order to generate QALYs.
Clinical outcome
The primary clinical outcome (healing first identified at or before 24 weeks from randomisation) was used to describe the percentage of healed ulcers obtained in the intervention plus usual care) versus usual care alone. No further analysis was done, that is, to ensure consistency in reporting with the clinical end point, the percentage of healed ulcers was taken directly from the primary analysis undertaken.
Patient-reported (preference-based) outcome
The EQ-5D-3L is a generic, single-index, preference-based health-related quality of life instrument. It measures health-related quality of life on five dimensions with three levels for each (35 = 243). A tariff of utility values associated with each of the 243 possible combinations (health states), based on a social survey in the UK using the time trade-off method, is provided by the EuroQol Group.
As the EQ-5D-3L was included within the trial as one of the secondary patient-related outcomes, the main SAP reported the methods used to analyse the EQ-5D-3L in order to record patient-reported changes in health outcomes, using the EQ-5D-3L VAS and EQ-5D-3L descriptive system to report changes in health status and utilities, respectively. Thus, the economic analysis focused on utilising the utilities derived from the EQ-5D-3L in order to generate QALYs.
The utilities derived from the EQ-5D-3L were thoroughly investigated with the trial statisticians to ensure that, when required, (a) results obtained were commensurate, and (b) additional or extended interrogation of the EQ-5D-3L requiring deviation from the main statistical analysis or findings was fully discussed. These changes were predominantly as a result of the EQ-5D-3L being a secondary end point within the main trial and thus were not subjected to elements expected within a health economic analysis (e.g. assessment of the impact of missing data).
The following additional analyses were undertaken for the health economic analysis.
At baseline, the EQ-5D-3L sample contains < 5% of cases with missing values (3.5%), which can be considered to be missing at random. At the 12-week time point, 22% of the data were missing, and at the 24-week time point 24% of the data were missing. Patients who died during the study were given a value of 0 at the appropriate time point. This meant that 3.5% of the data were missing at baseline, 20% were missing at 12 weeks and 21% were missing at 24 weeks. To evaluate whether or not the missing EQ-5D-3L data across the time points was missing at random, Little’s chi-squared test was undertaken, with the null hypothesis that the data were missing completely at random and the p-value set at the 0.05 significance level. The variables used to examine whether or not associations between patient characteristics and missing EQ-5D-3L data existed were age, sex, immobility status, diabetes mellitus type and exudate category. The results of Little’s missing completely at random test show that missing data can be considered missing completely at random. Multiple imputation of missing EQ-5D-3L data was then undertaken. The following predictor variables were used: age, sex, immobility status, diabetes mellitus type and exudate type. The number of imputations performed was 20.
Generation of quality-adjusted life-years
Quality-adjusted life-years were calculated using AUC, with the area under a health state calculated from the duration of that health state multiplied by the weight for that health state to estimate the QALYs gained. As the total trial follow-up duration was 0.5 years, this produced a QALY gain for this time period. The QALY per patient was derived from the multiply imputed EQ-5D-3L data set. The QALY derived was adjusted for baseline covariates as per the statistical analysis (adjustment for grade of ulcer and size of ulcer area). Mean QALY differences were calculated using independent samples t-test with 95% CIs.
Health economic analysis
Cost–utility analysis
Analysis (incremental cost-effectiveness ratios)
Base case
As the trial was set up to be analysed under ITT principles, for consistency the base case for the health economic analysis was conducted following these principles: ICERs were computed by presenting incremental costs and effects and subsequent ICER in order to allow easily reproducible calculations to be made, with 95% CIs derived from bootstrapping. Given the impact that missing data could have on the health economics results, further analysis was undertaken to assess the impact of the ICER when missing cost data were taken into account. As part of the assessment of costs, an alternative scenario was considered, in which patients with resource utilisation of £0 were excluded from the analysis (PP sample). An ICER calculating the cost of generating an additional healed ulcer was also calculated.
The ICER is represented as:
The scenario could arise in a cost-effectiveness analysis in which one strategy is both more effective and less costly. In this situation, this strategy would be considered to dominate the other strategy. In such a situation, the ICER becomes negative and the numerical value would be uninformative. In these situations, the dominating strategy can then be seen as preferred regardless of any consideration of budget.
Sensitivity analyses
In order to assess uncertainty in the estimation of the ICERs, deterministic one-way sensitivity analyses of the total NHS resource use cost were undertaken using the lower- and upper-bound value of the 95% CI for the Heels intervention total NHS resource use cost and the usual care mean total NHS resource use cost, as shown in Table 35.
| Parameter varied | Result |
|---|---|
| ITT base case mean cost AND cost-effectiveness of healed ulcers – adjusted lower 95% CI limit | Heels lower estimate of £2550.64 vs. usual care mean estimate of £2567.85 cost per patient |
| ITT base case mean cost AND cost-effectiveness of healed ulcers – adjusted upper 95% CI limit | Heels upper estimate of £2719.89 vs. usual care mean estimate of £2567.85 cost per patient |
| PP complete case costs associated with reported NHS resource use (£0 cost excluded) – adjusted lower 95% CI limit | Heels lower estimate of £2562.97 vs. usual care mean estimate of £2592.41 cost per patient |
| PP complete case costs associated with reported NHS resource use (£0 cost excluded) – adjusted upper 95% CI limit | Heels upper estimate of £2739.41 vs. usual care mean estimate of £2592.41 cost per patient |
Bootstrapping and cost-effectiveness acceptability curve
Non-parametric bootstrapping using 1000 replications was undertaken to estimate the probability of the intervention being cost-effective across a range of cost-effectiveness threshold values, plotted as CEAC. For the cost–utility analysis, the probability of the intervention representing value for money using NICE threshold values of £20,000–30,000) would be presented.
In general, NICE considers an intervention cost-effective if one of the following applies.
-
Where an intervention is less costly and more clinically effective than all other relevant alternatives. In this case, the reporting of an ICER becomes meaningless, as the strategy in question dominates the alternative
-
Below a most plausible ICER of £20,000 per QALY gained, the decision to recommend the use of a technology is normally based on the cost-effectiveness estimate and the acceptability of a technology as an effective use of NHS resources
-
As the ICER of an intervention increases in the range of £2000 to £30,000 per QALY, judgement about the acceptability of the technology as an effective use of NHS resource will make explicit reference to other relevant factors, as set out in the guidelines. 24
Net monetary benefit
In addition, net-benefit analysis was used to determine whether or not the heel cast intervention can be considered cost-effective in terms of the decision-maker’s WTP threshold of £20,000. The net benefit statistic is based on the value of the WTP threshold, and if the net benefit produced is positive (> £0), then the intervention can be considered cost-effective. However, a negative net benefit (< £0) indicates that the intervention is not cost-effective, as any benefits of the intervention are outweighed by its costs.
Cost-effectiveness analysis
For the cost-effectiveness analysis, the primary clinical end point to derive the outcome was used, with costs estimated as described above. A series of one-way sensitivity analyses were undertaken based on the distribution (95% CIs) of the total NHS resource use cost parameters identified from the main analysis.
All analyses were conducted in IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA).
Appendix 5 Reasons documented by researchers for not recording ankle brachial pressure index in 152 participants
Sixty-five cases
In 65 cases, ABPI was not recorded but was likely to have been > 1.4 because the reading was said to be too high or the vessel non-compressible, calcified or equivalent.
Twenty-nine cases
In 29 cases, ABPI could not be measured because of pain, ulceration, fragile skin, wound dressing or oedema.
Fifty-eight cases
In 58 cases, no clear clinical reason was provided.
Appendix 6 Details of documented protocol violations
| Participant number | Randomisation date | Date of discontinuation from study (if available) | Group | Reason | Details of protocol violation | Further details (if available) |
|---|---|---|---|---|---|---|
| 1008 | 30 August 2011 | 16 December 2011 | Intervention | Patient withdrew consent | Loss of capacity | |
| 1012 | 18 November 2011 | 3 January 2012 | Intervention | Protocol violation | Failure to wear heel cast | |
| 2001 | 1 June 2011 | 1 December 2011 | Usual care | Lost to follow-up | ||
| 3004 | 20 June 2012 | 4 September 2012 | Intervention | Lost to follow-up | ||
| 3007 | 9 July 2013 | 28 January 2014 | Usual care | Protocol violation | Missed visits | |
| 3009 | 14 March 2014 | 11 June 2014 | Intervention | Patient withdrew consent | The patient had become terminally ill and was not expected to live much longer | |
| 4003 | 11 October 2011 | 7 November 2011 | Intervention | Protocol violation | Failure to wear heel cast | |
| 5002 | 29 July 2011 | 6 October 2011 | Intervention | Protocol violation | Eligibility criteria breach | The patient had to go into total contact cast as they developed a further ulcer on the same foot |
| 5003 | 8 September 2011 | 7 October 2011 | Intervention | Patient withdrew consent | ||
| 5004 | 16 September 2011 | 17 October 2011 | Intervention | Lost to follow-up | ||
| 5010 | 28 May 2012 | 11 June 2012 | Intervention | Patient withdrew consent | ||
| 5011 | 25 June 2012 | 6 September 2012 | Intervention | Patient withdrew consent | ||
| 5027 | 17 January 2014 | 13 March 2014 | Usual care | Patient withdrew consent | ||
| 6001 | 1 September 2011 | 19 November 2011 | Usual care | Patient withdrew consent | The patient was to ill to continue | |
| 6002 | 17 February 2012 | 22 February 2012 | Usual care | Other | Recruited in error | |
| 7002 | 2 August 2011 | 3 October 2011 | Intervention | Protocol violation | Failure to wear heel cast | |
| 7006 | 17 January 2012 | 19 June 2012 | Intervention | Patient withdrew consent | ||
| 8003 | 2 May 2012 | 19 September 2012 | Usual care | Other | A heel cast was no longer appropriate; the patient developed a new ulcer and thus needed a different kind of device | |
| 8004 | 10 May 2012 | 22 June 2012 | Intervention | Protocol violation | Missed visits | |
| 8005 | 23 July 2012 | Intervention | Lost to follow-up | |||
| 9010 | 20 January 2012 | 17 April 2012 | Usual care | Patient withdrew consent | ||
| 9011 | 22 February 2012 | 7 March 12 | Intervention | Other | Recruited in error – the patient was allocated in error to the wrong arm | |
| 9012 | 19 March 2012 | 18 May 12 | Usual care | Protocol violation | Missed visits | |
| 9020 | 21 September 2012 | 25 January 2013 | Usual care | Patient withdrew consent | The patient was deemed too unwell to attend by nursing home | |
| 9021 | 24 September 2012 | 2 January 2013 | Intervention | Protocol violation | Eligibility criteria breach | The patient started maggot therapy on 2 January 2013 |
| 9034 | 3 January 2014 | 4 April 2014 | Usual care | Patient withdrew consent | The patient preferred not to travel to hospital clinic | |
| 9036 | 6 March 2014 | 21 March 2014 | Usual care | Patient withdrew consent | ||
| 10003 | 10 August 2011 | 7 September 2011 | Intervention | Protocol violation | Failure to wear heel cast | |
| 10007 | 21 September 2011 | 14 December 2011 | Intervention | Protocol violation | Failure to wear heel cast | |
| 10010 | 18 January 2012 | Usual care | Lost to follow-up | |||
| 10012 | 14 March 2012 | 15 August 2012 | Intervention | Protocol violation | Eligibility criteria breach | The patient went into a total contact cast |
| 10021 | 5 December 2012 | Usual care | Lost to follow-up | |||
| 10025 | 27 February 2013 | 4 April 2013 | Usual care | Patient withdrew consent | Lost capacity | |
| 10033 | 19 June 2013 | 31 July 2013 | Usual care | Patient withdrew consent | Lacks capacity | |
| 11001 | 12 July 2011 | 11 November 2011 | Intervention | Other | Recruited in error – the patient was not randomised | |
| 11002 | 12 July 2011 | 11 November 2011 | Intervention | Other | Recruited in error – the patient was not treated | |
| 12009 | 5 September 2013 | 3 October 2013 | Intervention | Patient withdrew consent | ||
| 12010 | 26 September 2013 | 19 December 2013 | Usual care | Other | Recruited in error – the patient’s ulcer did not fulfil the criteria for inclusion into study of the wound overlying the calcaneum | |
| 13003 | 16 August 2011 | 7 December 2011 | Usual care | Lost to follow-up | Under the care of the vascular team at Freeman Hospital | |
| 13005 | 27 September 2011 | 7 November 2011 | Intervention | Protocol violation | Failure to wear heel cast | |
| 13012 | 11 February 2013 | 18 April 2013 | Intervention | Protocol violation | Missed visits | |
| 14002 | 1 May 2012 | 24 July 2012 | Intervention | Protocol violation | Eligibility criteria breach | Required larvae |
| 14006 | 26 June 2012 | 10 July 2012 | Intervention | Patient withdrew consent | Psychosis | |
| 14014 | 5 February 2013 | 10 May 2013 | Intervention | Protocol violation | Failure to wear heel cast | Failure to wear offloading device |
| 14015 | 19 February 2013 | 7 June 2013 | Intervention | Patient withdrew consent | The patient went into palliative care | |
| 14017 | 10 May 2013 | 2 August 2013 | Usual care | Lost to follow-up | ||
| 14019 | 31 May 2013 | 23 August 2013 | Usual care | Patient withdrew consent | ||
| 14020 | 14 June 2013 | Usual care | Lost to follow-up | |||
| 17001 | 2 September 2011 | 21 October 2011 | Usual care | Other | Recruited in error – error in enrolment | |
| 17002 | 7 October 2011 | 7 February 2012 | Usual care | Protocol violation | Missed visits | |
| 17007 | 10 July 2012 | 16 October 2012 | Usual care | Lost to follow-up | ||
| 17009 | 15 March 2013 | 7 August 2013 | Usual care | Lost to follow-up | ||
| 18005 | 26 April 2012 | 12 June 2012 | Intervention | Lost to follow-up | ||
| 18008 | 23 April 2013 | 12 August 2013 | Intervention | Protocol violation | Missed visits | |
| 18011 | 17 May 2013 | 1 August 2013 | Usual care | Protocol violation | Missed visits | |
| 18013 | 28 May 2013 | 11 April 2014 | Usual care | Protocol violation | Missed visits | |
| 20001 | 7 November 2011 | 19 December 2011 | Usual care | AE | ||
| 20002 | 5 December2011 | 13 January 2012 | Intervention | Protocol violation | Failure to wear heel cast | |
| 20003 | 15 February 2012 | 13 March 2012 | Usual care | Patient withdrew consent | ||
| 21015 | 11 September 2013 | 23 October 2013 | Intervention | Lost to follow-up | Admitted to another hospital | |
| 22006 | 10 July 2013 | 8 October 2013 | Usual care | Protocol violation | Eligibility criteria breach | The patient went into a total contact cast |
| 22007 | 9 December 2013 | 4 March 2014 | Usual care | Protocol violation | Eligibility criteria breach | 2 × surgical debridement and surgeons requested that nurses use TNP therapy |
| 23012 | 15 October 2013 | 19 February 2014 | Usual care | Protocol violation | Missed visits | |
| 24010 | 26 February 2014 | 29 May 2014 | Intervention | Protocol violation | Missed visits | |
| 24012 | 2 May 2014 | Intervention | Lost to follow up | |||
| 25004 | 10 December 2012 | 23 January 2013 | Intervention | Patient withdrew consent | ||
| 25009 | 14 August 2013 | 24 September 2013 | Intervention | Patient withdrew consent | The patient was too ill to continue | |
| 25012 | 3 March 2014 | 11 June 2014 | Usual care | Patient withdrew consent | ||
| 26001 | 15 March 2012 | 1 March 2013 | Usual care | Lost to follow-up | ||
| 26002 | 12 April 2012 | 15 September 2014 | Usual care | Lost to follow-up | ||
| 27006 | 25 May 2012 | 11 October 2012 | Intervention | Protocol violation | Missed visits | |
| 27019 | 29 May 2014 | 22 August 2014 | Usual care | Protocol violation | Missed visits | |
| 28004 | 15 November 2013 | 13 December 2013 | Intervention | Patient withdrew consent | Ill health and prolonged hospitalisation | |
| 30006 | 2 June 2014 | 11 September 2014 | Usual care | Protocol violation | Eligibility criteria breach | Application of larvae therapy |
| 31007 | 8 April 2013 | Usual care | Lost to follow-up | |||
| 31009 | 9 May 2013 | 21 June 2013 | Intervention | Lost to follow-up | ||
| 31014 | 11 September 2013 | Intervention | Lost to follow-up | |||
| 31018 | 3 October 2013 | Intervention | Lost to follow-up | |||
| 31021 | 28 November 2013 | 5 Mar 2014 | Intervention | Other | The patient was diagnosed with squamous cell carcinoma | |
| 31022 | 6 January 2014 | 4 February 2014 | Intervention | Protocol violation | Missed visits | |
| 31024 | 4 September 2014 | 30 October 2014 | Usual care | Lost to follow-up | The patient went abroad for 3 months due to the illness of a family member | |
| 33001 | 29 January 2013 | 19 April 2013 | Usual care | Lost to follow-up | ||
| 33013 | 13 August 2013 | 27 August 2013 | Usual care | ADE | ||
| 35004 | 1 July 2014 | Intervention | Lost to follow-up |
Appendix 7 Details of adverse events and serious adverse events in 113 participants recruited prior to the protocol change on 29 March 2012
| SAEs | Usual care (n = 57) | Intervention (n = 56) |
|---|---|---|
| Any SAE during study | 14 | 12 |
| Number of SAEs | 21 | 16 |
| SAE preferred term | ||
| Angioplasty | 2 | 0 |
| Cerebrovascular accident | 1 | 0 |
| Chest pain | 1 | 0 |
| Chronic obstructive pulmonary disease | 2 | 0 |
| Death | 1 | 2 |
| Debridement | 0 | 1 |
| Diabetic ketoacidosis | 0 | 1 |
| Femoral neck fracture | 0 | 1 |
| Gangrene | 0 | 1 |
| Gastrointestinal infection | 1 | 0 |
| Grand mal convulsion | 0 | 1 |
| Hypoglycaemia | 1 | 0 |
| Infected skin ulcer | 0 | 1 |
| Infection | 1 | 1 |
| Localised infection | 1 | 0 |
| Myocardial infarction | 1 | 2 |
| Opiates | 1 | 0 |
| Osteomyelitis | 0 | 1 |
| Paralysis | 0 | 1 |
| Pneumonia | 2 | 0 |
| Renal failure | 1 | 0 |
| Sepsis | 0 | 1 |
| Skull fracture | 1 | 0 |
| Soft tissue infection | 1 | 0 |
| Tibia fracture | 1 | 0 |
| Transient ischaemic attack | 0 | 2 |
| Urinary tract infection | 1 | 0 |
| Vascular test | 1 | 0 |
| SAE relationship to device | ||
| None | 21 | 15 |
| Possible | 0 | 1 |
| AEs | Usual care (n = 57) | Intervention (n = 56) |
| Any AE during study | 24 | 22 |
| Number of AEs | 53 | 38 |
| AE preferred term | ||
| Abdominal pain | 1 | 0 |
| Abscess drainage | 1 | 0 |
| Angioplasty | 2 | 0 |
| Application site erosion | 0 | 1 |
| Application site rash | 1 | 0 |
| Application reaction | 0 | 1 |
| Blister | 0 | 1 |
| Cellulitis | 2 | 0 |
| Cerebrovascular accident | 1 | 0 |
| Chest pain | 2 | 0 |
| Chronic obstructive pulmonary disease | 2 | 0 |
| Contusion | 1 | 0 |
| Coronary artery bypass | 0 | 1 |
| Death | 2 | 2 |
| Debridement | 1 | 5 |
| Decreased appetite | 1 | 0 |
| Diabetic ketoacidosis | 0 | 1 |
| Erythema | 0 | 2 |
| Fall | 1 | 0 |
| Femoral neck fracture | 0 | 1 |
| Foot operation | 1 | 0 |
| Gangrene | 0 | 1 |
| Gastrointestinal infection | 1 | 0 |
| Grand mal convulsion | 0 | 1 |
| Hypoglycaemia | 1 | 0 |
| Infected skin ulcer | 3 | 3 |
| Infection | 3 | 1 |
| Localised infection | 8 | 0 |
| Loss of consciousness | 0 | 1 |
| Mobility decreased | 1 | 0 |
| Muscular weakness | 1 | 0 |
| Myocardial infarction | 1 | 2 |
| Opiates | 1 | 0 |
| Osteomyelitis | 0 | 4 |
| Paralysis | 0 | 1 |
| Paronychia | 0 | 1 |
| Pneumonia | 2 | 0 |
| Postoperative wound | 1 | 1 |
| Renal failure | 1 | 0 |
| Respiratory tract infection | 1 | 0 |
| Sepsis | 0 | 1 |
| Skin injury | 0 | 2 |
| Skin ulcer | 1 | 2 |
| Skull fracture | 1 | 0 |
| Soft tissue infection | 2 | 0 |
| Tibia fracture | 1 | 0 |
| Transient ischaemic attack | 0 | 2 |
| Urinary tract infection | 1 | 0 |
| Vascular test | 1 | 0 |
| Wound complication | 1 | 0 |
| Wound necrosis | 1 | 0 |
| AE relationship to offloading device | ||
| None | 51 | 27 |
| Possible | 1 | 5 |
| Probable | 1 | 6 |
Appendix 8 Summary of adverse device effects (collected from 29 March 2012)
| ADEs | Usual care (n = 253) | Intervention (n = 256) |
|---|---|---|
| Any SADEs during study | 0 | 0 |
| Any ADE during study | 15 | 31 |
| Number of ADEs | 18 | 40 |
| ADE preferred term | ||
| Amputation | 0 | 1 |
| Application site erosion | 1 | 6 |
| Application site reaction | 0 | 1 |
| Application site ulcer | 0 | 2 |
| Debridement | 0 | 1 |
| Deep-vein thrombosis | 1 | 0 |
| Diarrhoea | 0 | 1 |
| Excoriation | 0 | 1 |
| Foot fracture | 0 | 1 |
| General symptom | 1 | 0 |
| Haematoma | 1 | 0 |
| Hospitalisation | 1 | 0 |
| Hypoglycaemia | 0 | 1 |
| Infected skin ulcer | 3 | 1 |
| Infection | 2 | 2 |
| Leg amputation | 1 | 0 |
| Limb injury | 0 | 1 |
| Lower respiratory tract infection | 0 | 1 |
| Necrosis | 1 | 0 |
| Onychomadesis | 1 | 0 |
| Postoperative wound infection | 0 | 1 |
| Skin ulcer | 4 | 9 |
| Skin wound | 0 | 1 |
| Ulcer | 0 | 1 |
| Urinary tract infection | 0 | 1 |
| Wound | 0 | 2 |
| Wound decomposition | 0 | 3 |
| Wound secretion | 0 | 1 |
| Wound sepsis | 1 | 0 |
| ADE relationship to offloading device | ||
| Not related | 14 | 15 |
| Related | 4 | 25 |
Appendix 9 Copy of patient resource log
| Date | Dressing changes (options) | Any other consultation with your doctor, nurse, podiatrist or other health-care professional (options) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Who did the dressing change? [(1) self, (2) family member, (3) friend or carer, (4) district nurse, (5) podiatrist, (6) practice nurse, (7) hospital nurse or (8) research nurse/podiatrist] | Was a new heel cast made? (1. yes; 2. no) | Who was the conversation/visit with? [(1) GP, (2) hospital doctor, (3) podiatrist, (4) district nurse, (5) practice nurse, (6) ward nurse or (7) research nurse/podiatrist] | Was it wholly or at least partly to do with the foot? | Was it (1) a telephone conversation or (2) a face-to-face visit? | If face to face, where did the visit take place? [(1) home, (2) doctor’s surgery, (3) community clinic or (4) hospital] | How long did the visit take in minutes? (i.e. how long were you inside the hospital?) | If seen somewhere other than at home, what was the means of travel? (e.g. taxi, bus, own car) | How long did it take? (i.e. total time out of the house altogether) | Roughly how much did it cost to get back home again? (e.g. taxi fare, bus, parking, miles in own car) | |
Appendix 10 Analysis of resources used and costs
This appendix describes in detail the analysis of the resources used and associated costs as a result of the intervention compared with usual care.
Implementation of the intervention and costs
The implementation of the intervention incurred initial costs of training podiatrists in each participating centre and of producing the lightweight heel cast device for each patient, in addition to the cost of the device itself. The training costs were estimated from discussion with the trial team, based on the estimation that 30 minutes of training delivered by a specialist podiatrist would be required at each of the 35 centres, with two podiatrists in each centre receiving the training. that 30 minutes of training delivered by a specialist podiatrist would be required at each of the 35 centres, with two podiatrists in each centre receiving the training. The initial cost of producing the first heel cast for each participant was calculated for all participants in the intervention group.
During the trial, participants in the intervention group may also have received a subsequent Heels device owing to damage or other reasons. The additional costs of subsequent Heels devices were captured from the patient diaries, and thus relied on participants self-reporting that they had received a new one. In the 115 participants who reported receiving at least one replacement device, a wide range in number of replacement devices was reported (median, 3; range 1–24), see Table 36. The total number of replacement devices (n = 513), with podiatrist time included, was used to estimate the costs of replacement.
| Mean (SD) | 4.46 (4.97) |
| Median (25th centile, 75th centile) | 3.00 (1.00, 5.00) |
| Minimum, maximum | 1, 24 |
The implementation costs for the intervention are summarised in Table 37. The mean implementation cost for the intervention was estimated to be £61.18.
| Resource use | Unit cost (£) | Source of unit cost | Cost per component (£) |
|---|---|---|---|
| Staff costs | |||
| Specialist podiatrist providing training for 30 minutes for 35 trusts | 21 | PSSRU 201431 | 735 |
| One-off training cost of two specialist podiatrists per trust (35 trusts in the trial) | 42 | PSSRU 201431 | 1470 |
| Heel cast costs: first cast | |||
| Cost of initial heel cast per patient in the trial (256 patients) | 7 | Trial team | 1792 |
| Staff time to produce the initial heel cast per patient (15 minutes) | 10.50 | PSSRU 201431 | 2688 |
| Heel cast costs: additional casts | |||
| Additional heel casts (n = 513) | 7 | Trial team | 3591 |
| Time taken by specialist podiatrist to produce a new heel cast (15 minutes per each of the 513 new heel casts) | 10.50 | PSSRU 201431 | 5386.50 |
| Total cost | 15,662.50 | ||
| Mean cost per patient (256 patients) | 61.18 | ||
Life span of the first heel cast applied at baseline/week 0
The number of days between the first heel cast being applied to a patient’s heel ulcer at baseline (week 0) and the first date a patient reported that a new heel cast had been made (alongside the date of a dressing change) was examined, and the results are displayed in Table 38, which shows the number of days before a new heel cast was reported by any patient with no differentiation between grade of ulcer. In Table 39, the number of days reported when a new heel cast was applied is examined by the grade of ulcer. The median number of days before a new heel cast was reported was 14 for all grades of ulcers. The mean number of days was 30 (mean days for grade 2 ulcers = 27, grade 3= 32.5, grade 4 = 26). After this initial reporting of receiving a new heel cast, it is difficult to tell if subsequent reports of new heel casts are a reflection of a patient continuing to receive necessary new heel casts, or if a patient is continuing to report they have a new heel cast – the same one. In the majority of cases, there is continued reporting of new heel casts every 2 weeks, which has likely led to an overestimation of the number of replacement heel casts required.
| Mean days (SD) | 30.46 (35.01) |
| Median days (25th centile, 75th centile) | 14 (11.25, 35.00) |
| Minimum, maximum days | 1, 194 |
| Grade 2 (n = 35)a | Mean days (SD) | 26.91 (25.22) |
| Median days (25th centile, 75th centile) | 14 (9.00, 35.00) | |
| Minimum, maximum days | 1, 115 | |
| Grade 3 (n = 73) | Mean days (SD) | 32.49 (39.03) |
| Median days (25th centile, 75th centile) | 14 (10.50, 37.50) | |
| Minimum, maximum days | 1, 194 | |
| Grade 4 (n = 6) | Mean days (SD) | 26.33 (35.56) |
| Median days [25th centile, 75th centile] | 14 [10.75, 37.25] | |
| Minimum, maximum days | 1, 98 |
Summary of resource utilisation and costs associated with HEELs in primary and outpatient care
Dressing changes
The usual care group reported more instances of dressing changes than the intervention group (Table 40), and made significantly more reports of self-managed ulcer care than the intervention group. The intervention group reported lower rates of dressing changes, but of those dressing changes there were slightly higher rates attributed to NHS practitioners than in the control group. None of these differences was statistically significant.
| Dressing changes | Usual care (n = 253) | Intervention (n = 256) | Total number of visits (n = 509) | Mean difference (95% CI); p-value |
|---|---|---|---|---|
| District nurse | ||||
| Mean (SD) | 9.45 (19.52) | 9.1 (17.82) | 2391 usual | 0.35 (–2.90 to 3.60); p = 0.463 |
| Median (25th centile, 75th centile) | 0 (0, 9) | 0 (0, 10) | 2330 HEELs | |
| Minimum, maximum | 0, 137 | 0, 129 | ||
| Practice nurse | ||||
| Mean (SD) | 4.43 (10.74) | 4.24 (12.60) | 1120 usual | 0.19 (–1.85 to 2.22); p = 0.941 |
| Median (25th centile, 75th centile) | 0 (0, 2) | 0 (0, 1) | 1086 HEELs | |
| Minimum, maximum | 0, 76 | 0, 115 | ||
| Community/hospital podiatrist | ||||
| Mean (SD) | 3.45 (7.07) | 3.11 (6.02) | 873 usual | 0.34 (–0.80 to 1.48); p = 0.558 |
| Median (25th centile, 75th centile) | 0 (0, 4) | 0 (0, 2) | 796 HEELs | |
| Minimum, maximum | 0, 57 | 0, 42 | ||
| Outpatient visit – nurse led | ||||
| Mean (SD) | 1.43 (5.25) | 0.98 (3.29) | 363 usual | 0.45 (–0.31 to 1.21); p = 0.246 |
| Median (25th centile, 75th centile) | 0 (0, 0) | 0 (0, 0) | 252 HEELs | |
| Minimum, maximum | 0, 47 | 0, 32 | ||
| Research nurse/podiatrist | ||||
| Mean (SD) | 2.84 (4.69) | 2.16 (4.06) | 719 usual | 0.68 (–0.09 to 1.44); p = 0.082 |
| Median (25th centile, 75th centile) | 0 (0, 4) | 0 (0, 2) | 554 HEELs | |
| Minimum, maximum | 0, 27 | 0, 28 | ||
| Family member | ||||
| Mean (SD) | 5.32 (17.75) | 5.16 (19.86) | 1346 usual | 0.16 (–3.12 to 3.44); p = 0.922 |
| Median (25th centile, 75th centile) | – | – | 1320 HEELs | |
| Minimum, maximum | 0, 165 | 0, 179 | ||
| Self | ||||
| Mean (SD) | 6.41 (24.13) | 2.62 (10.10) | 1622 usual | 3.79 (0.58 to 7.01); p = 0.021 |
| Median (25th centile, 75th centile) | – | – | 670 HEELs | |
| Minimum, maximum | 0, 193 | 0, 74 | ||
| Friend or carer | ||||
| Mean (SD) | 0.84 (8.02) | 0.98 (9.10) | 213 usual | –0.14 (–1.64 to 1.35); p = 0.852 |
| Median (25th centile, 75th centile) | – | – | 252 HEELs | |
| Minimum, maximum | 0, 123 | 0, 118 | ||
The mean cost of dressing changes was £821.28 in the usual care group and £724.76 in the intervention group. Overall costs associated with dressing changes were £96.52 lower in the intervention group than in the usual care group, but the difference was not statistically significant (Tables 41 and 42).
| Usual care (n = 253) | Intervention (n = 256) | Mean cost difference (95% CI) | p-value | |
|---|---|---|---|---|
| Mean (SD) | 821.28 (1037.67) | 724.76 (831.63) | 96.52 (–67.15 to 260.18) | 0.247 |
| Mode of dressing change | Usual care (n = 253) | Intervention (n = 256) | Total cost (n = 509) | Mean difference (95% CI); p-value |
|---|---|---|---|---|
| District nurse | ||||
| Mean (SD) | 368.57 (761.27) | 354.96 (694.88) | 93,249.00 usual | 13.61 (–113.29 to 140.52); p = 0.833 |
| Median (25th centile, 75th centile) | 0 (0, 351.0) | 0 (0, 390.0) | 90,870.00 HEELs | |
| Minimum, maximum | 0, 5343 | 0, 5031 | ||
| Practice nurse | ||||
| Mean (SD) | 45.38 (110.04) | 43.48 (129.13) | 11,480.00 usual | 1.89 (–19.01 to 22.80); p = 0.859 |
| Median (25th centile, 75th centile) | 0 (0, 20.5) | 0 (0, 10.25) | 11,132.00 HEELs | |
| Minimum, maximum | 0, 779 | 0, 1178.75 | ||
| Community/hospital podiatrist | ||||
| Mean (SD) | 169.08 (346.35) | 152.36 (294.97) | 42,777.00 usual | 16.72 (–39.28 to 72.72); p = 0.558 |
| Median (25th centile, 75th centile) | 0 (0, 147) | 24.50 (0, 196) | 39,004.00 HEELs | |
| Minimum, maximum | 0, 2793 | 0, 2058 | ||
| Outpatient visit – nurse led | ||||
| Mean (SD) | 99.0 (362.10) | 67.92 (227.17) | 25,047.00 usual | 31.08 (–21.50 to 83.65); p = 0.246 |
| Median (25th centile, 75th centile) | 0 (0, 0) | 0 (0, 51.75) | 17,388.00 HEELs | |
| Minimum, maximum | 0, 3243 | 0, 2208 | ||
| Research nurse/podiatrist | ||||
| Mean (SD) | 139.25 (229.82) | 106.03 (199.08) | 35,231.00 usual | 33.21 (–4.22 to 70.64); p = 0.082 |
| Median (25th centile, 75th centile) | 0 (0, 196) | 0 (0, 98) | 27,146.00 HEELs | |
| Minimum, maximum | 0, 1323 | 0, 1372 | ||
Health-care consultations (primary care and hospital outpatient visits)
Aside from reporting the mode of dressing change for the target heel ulcer, patients were asked if they had consulted any other health-care practitioners regarding the care of their heel ulcer. Table 43 summarises the health-care consultations reported by participants for a consultation regarding their target heel ulcer. These consultations may have occurred in the same week as a dressing change performed by another health-care practitioner (such as a district nurse or a podiatrist), or may have occurred without a dressing change being reported. Many patients reported a dressing change and a consultation with the same health-care professional during one week. It was assumed in such cases that the dressing change and the consultation (for example with a district nurse or a podiatrist) occurred at the same time. In such cases, a unit cost was applied only once to avoid double costing the same appointment. For outpatient appointments, if a patient reported seeing a ‘hospital doctor,’ this was assumed to be at a consultant-led outpatient clinic, and so the consultant-led outpatient clinic unit cost was applied. If a patient reported attending a hospital clinic without describing the health-care professional seen, then the generic cost for an outpatient appointment was applied. In total, 105 patients reported an outpatient visit, with 93 (89%) reporting seeing a ‘hospital doctor’ and 12 (11%) not specifying who they saw. Of the participants who reported attending a health-care consultation, participants receiving usual care reported more hospital outpatient consultations than those receiving the intervention, but this difference was not statistically significant. There were similar numbers of primary care contacts across the different categories, with no statistically significant differences.
| The number of reported additional consultations by health-care practitioner | Usual care (n = 253) | Intervention (n = 256) | Total number of visits (n = 509) | Mean difference (95% CI); p-value |
|---|---|---|---|---|
| Outpatient visit: consultant led | ||||
| Mean (SD) | 0.48 (1.52) | 0.34 (0.99) | 121 usual | 0.14 (–0.08 to 0.37); p = 0.211 |
| Median (25th centile, 75th centile) | – | – | 86 HEELs | |
| Minimum, maximum | 0, 14 | 0, 8 | ||
| Hospital outpatient visit (clinic lead unknown) | ||||
| Mean (SD) | 0.15 (1.78) | 0.02 (0.12) | 39 usual | 0.14 (–0.08 to 0.36); p = 0.215 |
| Median (25th centile, 75th centile) | – | – | 4 HEELs | |
| Minimum, maximum | 0, 28 | 0, 1 | ||
| GP: home visit | ||||
| Mean (SD) | 0.04 (0.29) | 0.03 (0.16) | 10 usual | 0.01 (–0.03 to 0.05); p = 0.562 |
| Median (25th centile, 75th centile) | – | – | 7 HEELs | |
| Minimum, maximum | 0, 4 | 0, 1 | ||
| GP: surgery appointment | ||||
| Mean (SD) | 0.21 (0.90) | 0.20 (0.72) | 53 usual | 0.01 (–0.13 to 0.15); p = 0.887 |
| Median (25th centile, 75th centile) | – | – | 51 HEELs | |
| Minimum, maximum | 0, 9 | 0, 5 | ||
| GP: telephone consultation | ||||
| Mean (SD) | 0.004 (0.063) | 0.008 (0.09) | 1 usual | –0.004 (–0.02 to 0.009); p = 0.570 |
| Median (25th centile, 75th centile) | – | – | 2 HEELs | |
| Minimum, maximum | 0, 1 | 0, 1 | ||
| Practice nurse | ||||
| Mean (SD) | 4.15 (10.11) | 3.82 (11.07) | 1049 usual | 0.33 (–1.52 to 2.18); p = 0.726 |
| Median (25th centile, 75th centile) | 0 (0, 1.5) | 0 (0, 1) | 977 HEELs | |
| Minimum, maximum | 0, 72 | 0, 86 | ||
The costs of primary care and outpatient consultations are reported in Table 44. The number of outpatient visits in which the clinic lead was unspecified was higher in the usual care group, which may have led us to underestimate usual care costs in this area. However, even so, costs related to outpatient consultations were higher in the usual care group than in the intervention group. Overall, mean cost per participant was slightly higher in the usual care group than in the intervention group (£1182.00 vs. £1072.00), but the difference was not a statistically significant; the mean cost difference was £110.26 (95% CI –£101.10 to £321.63; p = 0.306).
| Cost of reported additional consultations by health-care practitioner | Usual care (n = 253) | Intervention (n = 256) | Total number of visits (n = 509) | Mean difference (95% CI); p-value |
|---|---|---|---|---|
| Outpatient visit: consultant led | ||||
| Mean (SD) | 61.22 (194.71) | 43.00 (127.02) | 15,488 usual | 18.22 (–0.10.38 to 46.81); p = 0.211 |
| Median (25th centile, 75th centile) | – | – | 11,008 HEELs | |
| Minimum, maximum | 0, 1792 | 0, 1024 | ||
| Hospital outpatient visit (clinic lead unknown) | ||||
| Mean (SD) | 17.11 (197.65) | 1.73 (13.79) | 4329 usual | 15.38 (–8.95 to 39.71); p = 0.215 |
| Median (25th centile, 75th centile) | – | – | 444 HEELs | |
| Minimum, maximum | 0, 3108 | 0, 111 | ||
| GP: home visit | ||||
| Mean (SD) | 4.55 (33.67) | 3.15 (18.79) | 1150 usual | 1.40 (–3.34 to 6.14); p = 0.562 |
| Median (25th centile, 75th centile) | – | – | 805 HEELs | |
| Minimum, maximum | 0, 460 | 0, 115 | ||
| GP: surgery appointment | ||||
| Mean (SD) | 9.64 (41.58) | 9.16 (33.20) | 2438 usual | 0.47 (–6.08 to 7.02); p = 0.887 |
| Median (25th centile, 75th centile) | – | – | 2346 HEELs | |
| Minimum, maximum | 0, 414 | 0, 230 | ||
| GP: telephone consultation | ||||
| Mean (SD) | 0.11 (1.76) | 0.22 (2.47) | 28 usual | £0.11 (–0.48 to 0.27); p = 0.570 |
| Median (25th centile, 75th centile) | – | – | 56 HEELs | |
| Minimum, maximum | 0, 28 | 0, 28 | ||
| Practice nurse | ||||
| Mean (SD) | 42.50 (103.63) | 39.12 (113.47) | 10,752.25 usual | £3.38 (–15.55 to 22.31); p = 0.726 |
| Median (25th centile, 75th centile) | 0 (0, 15.38) | 0 (0, 10.25) | 10,014.25 HEELs | |
| Minimum, maximum | 0, 738 | 0, 881.50 | 10,752.25 usual | |
Hospital admissions and associated costs
Following our costing methodology (see Appendix 4), the total number of hospital admissions costed was 43 (17%) in the usual care group, compared with 56 (22%) in the HEELs group. An overall summary of the number and cost of hospital admissions is reported in Table 45. Reasons for admissions were amputation (minor/major), revascularisation, falls and other less defined reasons reported as specifically related to the target heel ulcer and were, in the majority of cases, accompanied by systemic infection. Many patients reporting hospital admissions spent more than one spell in hospital during the trial.
| Number of admissions | Usual care (n = 43) | Intervention (n = 56) | Mean difference (95% CI); p-value |
|---|---|---|---|
| One admission during the trial | n = 26 | n = 35 | |
| Mean (SD) | 6094.96 (2707.94) | 6154.20 (2267.10) | –59.24 (–1335.53 to 1217.06); p = 0.926 |
| Median (25th centile, 75th centile) | 3848, 7221 | 3848, 7221 | |
| Minimum, maximum | 2326, 10,907 | 2326, 10,907 | |
| Two admissions during the trial | n = 12 | n = 16 | |
| Mean (SD) | 11,493.67 (4490.71) | 10,093.94 (5295.50) | 1399.73 (–2502.30 to 5301.76); p = 0.468 |
| Median (25th centile, 75th centile) | 8142.50, 14,676.75 | 4691.25, 14,316.75 | |
| Minimum, maximum | 3848, 18,128 | 2326, 18,128 | |
| Three admissions during the trial | n = 4 | n = 4 | |
| Mean (SD) | 11,905.75 (3247.15) | 15,435.25 (4758.18) | –3529.50 (–10,577.32 to 3518.32); p = 0.266 |
| Median (25th centile, 75th centile) | 8539.25, 14,673 | 11,536.50, 20,170.75 | |
| Minimum, maximum | 7696, 14,917 | 10,568, 21,976 | |
| Four admissions during the triala | n = 1 | n = 1 | |
| Mean (SD) | 22,138 | 18,290 | 3848 |
| Median (25th centile, 75th centile) | |||
| Minimum, maximum |
Costs of medications (antibiotics)
Of the 509 patients, 209 patients (107 in the usual care group and 102 in the intervention grou) reported receiving prescribed medication for an infection related to the target heel ulcer. For those prescribed medication, the mean cost of antibiotics was £71.09 in the usual care group and £42.56 in the intervention group, but the difference was not statistically significant (£28.53, 95% CI –£14.63 to £71.68; p = 0.194. Table A9.10 below provides a descriptive analysis of the medications prescribed for infections related to the target foot ulcer (Table 46).
| Drug name | Usual care (n = 107) | Intervention (n = 102) |
|---|---|---|
| Amoxicillin | n = 13 | n = 10 |
| Mean (SD) | 0.12 (0.527) | 0.10 (0.330) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 4 | 0, 2 |
| Augmentin | n = 12 | n = 12 |
| Mean (SD) | 0.11 (0.346) | 0.12 (0.405) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 2 | 0, 2 |
| Co-fluampicil | n = 2 | n = 0 |
| Mean (SD) | 0.02 (0.193) | |
| Median (25th centile, 75th centile) | – | |
| Minimum, maximum | 0, 2 | |
| Ciprofloxacin | n = 9 | n = 5 |
| Mean (SD) | 0.08 (0.279) | 0.05 (0.217) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 1 | 0, 1 |
| Clarithromycin | n = 14 | n = 15 |
| Mean (SD) | 0.13 (0.391) | 0.15 (0.408) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 2 | 0, 2 |
| Clindamycin | n = 27 | n = 15 |
| Mean (SD) | 0.25 (0.551) | 0.15 (0.383) |
| Median (25th centile, 75th centile) | 0, 4 | 0, 2 |
| Minimum, maximum | ||
| Co-amoxiclav | n = 50 | n = 61 |
| Mean (SD) | 0.47 (0.828) | 0.60 (0.947) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 4 | 0, 5 |
| Co-trimoxazole | n = 2 | n = 0 |
| Mean (SD) | 0.02 (0.136) | |
| Median (25th centile, 75th centile) | – | |
| Minimum, maximum | 0, 1 | |
| Doxycycline | n = 17 | n = 18 |
| Mean (SD) | 0.16 (0.367) | 0.18 (0.432) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 1 | 0, 2 |
| Erythromycin | n = 2 | n = 7 |
| Mean (SD) | 0.02 (0.136) | 0.07 (0.254) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 1 | 0, 1 |
| Fluloxacillin | n = 62 | n = 56 |
| Mean (SD) | 0.58 (1.055) | 0.55 (0.897) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 8 | 0, 6 |
| Fusidic acid | n = 4 | n = 4 |
| Mean (SD) | 0.04 (0.305) | 0.04 (0.279) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 3 | 0, 2 |
| Metronidazole | n = 34 | n = 21 |
| Mean (SD) | 0.32 (0.722) | 0.21 (0.452) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 5 | 0, 2 |
| Penicillin | n = 4 | n = 5 |
| Mean (SD) | 0.04 (0.191) | 0.05 (0.294) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 1 | 0, 2 |
| Rifampicin | n = 7 | n = 7 |
| Mean (SD) | 0.07 (0.284) | 0.07 (0.290) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 2 | 0, 2 |
| Trimethoprim | n = 4 | n = 5 |
| Mean (SD) | 0.04 (0.305) | 0.05 (0.217) |
| Median (25th centile, 75th centile) | – | – |
| Minimum, maximum | 0, 3 | 0, 1 |
| Linezolid | n = 2 | n = 0 |
| Mean (SD) | 0.02 (0.136) | |
| Median (25th centile, 75th centile) | – | |
| Minimum, maximum | 0, 1 |
List of abbreviations
- ABPI
- ankle brachial pressure index
- ADE
- adverse device effect
- AE
- adverse event
- AUC
- area under the curve
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CWIS
- Cardiff Wound Impact Schedule
- eCRF
- electronic case report form
- EPUAP
- European Pressure Ulcer Advisory Panel
- EQ-5D-3L
- EuroQol-5 Dimensions three-level version
- GCP
- good clinical practice
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NPUAP
- National Pressure Ulcer Advisory Panel
- OR
- odds ratio
- PP
- per protocol
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- ROC
- receiver operating characteristic
- SADE
- serious adverse device effect
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- VAS
- visual analogue scale
- WTP
- willingness to pay