Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/81/01. The contractual start date was in September 2011. The draft report began editorial review in October 2015 and was accepted for publication in April 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Karina Lovell, Sarah Byford and Shirley Reynolds report grants from the National Institute for Health Research during the conduct of the study. Michael Barkham reports that he was the lead investigator in the development of the Clinical Outcomes in Routine Evaluation – Outcome Measure, which is used in the trial. Simon Gilbody reports previous membership of the Health Technology Assessment Clinical Trials Board.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Lovell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Obsessive–compulsive disorder
Obsessive–compulsive disorder (OCD) is characterised by:
-
intrusive, unwanted, recurrent and distressing thoughts, images or impulses (i.e. obsessions)
-
repetitive actions/rituals (i.e. compulsions), which serve to reduce the distress and anxiety evoked by the obsessions.
Typical examples of obsessions include excessive doubts regarding the maintenance of safety and security (e.g. fears of failing to lock doors, or turning off electrical or gas appliances), intrusive thoughts, images or impulses of contaminating or harming others, or repugnant, sexual thoughts of abusing others.
Typical compulsions include repetitive washing or cleaning, and checking or repeating numbers, words or phrases. The majority of people with OCD understand that their thoughts and rituals are senseless.
Prior to the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition,1 OCD was grouped as an anxiety disorder, but it is now incorporated into a separate chapter on OCD and related disorders. For a definitive diagnosis, obsessions and compulsions (or both) must:
-
be distressing
-
impair functioning
-
be time-consuming (> 1 hour per day).
There are only a few minor differences in the criteria for OCD between the Diagnostic and Statistical Manual of Mental Disorders Editions III, III Revised and IV (DSM-IV),2 and the International Classification of Diseases, Tenth Edition. 3
Obsessive–compulsive disorder has an estimated lifetime prevalence of 2–3%4 and, in the absence of adequate treatment, will usually follow a chronic course. OCD is associated with reduced quality of life and substantial impairment of role, specifically work, social, home and family/relationship functioning. 5 In addition to the individual costs, the economic burden of OCD is high, with an estimated US$8.4M being attributed to the direct and indirect costs of the illness in the USA. 6 The World Health Organization rates OCD as one of the top 10 leading causes of disability worldwide. 7 High levels of comorbidity are associated with OCD, most notably other anxiety disorders, depression, impulse control disorders and substance misuse. 8 There is significant evidence to suggest that people with OCD are often left untreated or are inappropriately or inadequately treated, with consistent reports of a marked time delay between OCD onset and diagnosis. One study found a 10-year gap between onset and seeking professional help, and 17 years between onset and receipt of an effective intervention. 9
Context of the Obsessive–Compulsive Treatment Efficacy randomised controlled Trial
To understand the rationale for the Obsessive–Compulsive Treatment Efficacy randomised controlled Trial (OCTET), we first describe the following contextual issues:
-
cognitive–behavioural therapy (CBT) and low-intensity psychological interventions
-
National Institute for Health and Care Excellence (NICE) guidelines for OCD
-
service delivery models for psychological therapies in the UK
-
summary of the evidence for low-intensity interventions for OCD.
Cognitive–behavioural therapy and low-intensity interventions
Therapist-delivered CBT, including exposure and response prevention (ERP), is a mainstay of psychological treatment for OCD. 10 CBT is a ‘talking therapy’ that aims to change unhelpful thoughts and behaviour. ERP are specific CBT techniques used in the treatment of OCD. Exposure therapy involves confronting the feared stimuli until fear subsides, through a process known as extinction. Response prevention means resisting the need to ritualise (i.e. perform a compulsive reaction).
One of the major problems related to the management of OCD is access to CBT, with many patients traditionally facing significant delays and spending large amounts of time on waiting lists for this treatment. This reflects a lack of trained therapists and the significant amount of therapist time traditionally seen as necessary for each individual treatment.
There has been significant interest in ways of overcoming this issue that are sustainable for health-care systems. A key development has involved ‘low-intensity’ interventions. Although there is a lack of consensus over precise definitions, common features of low-intensity interventions include:11
-
typically CBT based
-
communicate CBT principles in accessible ways and delivered in flexible forms (e.g. telephone, e-mail)
-
involve a health technology (e.g. a computer package or book) to deliver key ‘active ingredients’ of therapy
-
usually delivered by a low-intensity worker [psychological wellbeing practitioner (PWP)]
-
use fewer resources (in terms of therapist time) than conventional CBT.
Low-intensity interventions can be used by patients unsupported (reducing therapist time to zero). However, they are often used in a ‘guided’ or ‘supported’ form, with some therapist time, often with a therapist who is fully trained but has less experience than a conventional CBT therapist. Two key forms of low-intensity interventions are ‘guided self-help’ (use of a book providing instruction in CBT principles), often supported via telephone by a professional; and computerised CBT (cCBT), using computer- or web-based systems to provide instruction in CBT principles, again supported by a professional.
An advantage of low-intensity interventions that use health technologies (cCBT) or are delivered by technology (telephone) is that they are more readily accessed by those who are unable to attend scheduled clinic appointments because of geographical, social, physical or psychological difficulties.
Low-intensity interventions complement, rather than replace, existing CBT provision. The way in which conventional therapist-delivered CBT and low-intensity treatments are delivered as part of a system of care is the subject of the next section.
National Institute for Health and Care Excellence guidelines
The NICE guidelines for OCD12 recommend management using a ‘stepped-care’ approach. 13 Stepped care is viewed as a potential solution to poor access to traditional treatments by increasing the efficiency of service provision and better-matching treatment to patient need.
In stepped-care models, different ‘steps’ or levels of treatment intensity exist. However, there are two ways in which patients are assigned to different steps.
-
Stepped care: a sequential approach in which all patients move through steps in a systematic way irrespective of illness severity, patient need or choice. Under this model all patients will initially receive low-intensity treatments and only ‘step up’ if and when these first-line interventions fail.
-
Stratified care: a targeted approach in which patients are assessed and referred to an appropriate step of treatment based on severity, complexity or other agreed criteria. Patients can thus access an appropriate level of treatment and, if needed, access a more intensive intervention without first having had to receive a lower-intensity treatment. 14
Although these can be considered as alternatives, models of care can include aspects of both models (i.e. the majority of patients use the stepped model, but patients with certain characteristics are stratified).
The NICE model uses stratification based on (1) severity of OCD symptoms and (2) functional impairment. The model consists of six steps divided into three stages: awareness (step 1), recognition and assessment (step 2) and treatment (steps 3–6). Our focus is on steps 3–6.
-
At step 3, adults with OCD with mild functional impairment, low-intensity psychological (CBT) treatments are recommended. Low-intensity treatments are delivered by a PWP and defined as < 10 hours of therapist time per patient. Treatments include (1) brief individual CBT using structured self-help materials, (2) individual CBT delivered by telephone or (3) group CBT.
-
At step 4, adults with OCD who have moderate functional impairment (or who have mild functional impairment, but have failed to engage or improve with lower-intensity treatments) will usually be offered a selective serotonin reuptake inhibitor and/or higher-intensity CBT delivered by a high-intensity therapist (HIT).
-
Step 5 is recommended for those with severe functional impairment and delivered within secondary care by mental health professionals with expertise in OCD. Treatment recommendations include a combination of a selective serotonin reuptake inhibitor and CBT.
-
Step 6 is reserved for people presenting with severe and chronic functional impairment, treatment refractory, and/or a risk to self or others. Specialist inpatient treatment and care may be offered at this step.
The NICE guidelines for OCD have been in existence since 2005. 12 An evidence update was set for February 2014, but no significant evidence was identified to change the recommendations. During the completion of the evidence update, the effectiveness of technology-enhanced treatment for OCD was identified as an evidence gap and, as a result, was registered on the UK Database of Uncertainties about the Effects of Treatments. 15
Service delivery models for psychological therapies in the UK
Psychological interventions for OCD are usually delivered in Improving Access to Psychological Therapies (IAPT) services. 16 IAPT commenced in 2007 with the aim of achieving a better balance between supply of psychological therapies and demand.
Since its inception, > 5000 new psychological therapists have been trained. IAPT training, which follows a national curriculum, produces two types of therapists:
-
low-intensity therapists, also known as PWPs
-
HITs.
Psychological wellbeing practitioners are tasked with facilitating a range of low-intensity interventions including guided self-help, cCBT and psychoeducational groups for mild to moderate depression and anxiety disorders. HITs provide predominantly CBT interventions for moderate to severe cases. The amount of time allocated to individuals varies but, on average, PWPs provide 3–4 hours of therapeutic support per person over 6–8 weeks, whereas HITs provide weekly 60-minute sessions over 8–16 weeks.
Given the NICE guidelines, it would be sensible to assume that low-intensity interventions for OCD (guided self-help or cCBT) would be supported by a PWP and provided in IAPT services for people with mild functional impairment.
However, the NICE model is not being implemented precisely, as designed in practice,17 for a number of reasons:
-
In most IAPT services, people with OCD are referred directly to, and treated by, HITs regardless of their illness severity. OCD is perceived as a complex mental health disorder and hence the PWP IAPT National Curriculum18 does not address OCD knowledge or treatment.
-
Although the NICE guidelines recommend low-intensity interventions for mild OCD, there is evidence that people with OCD with mild functional impairment do not present to services. Consequently, a substantial proportion of patients seen by IAPT practitioners will be experiencing OCD with at least a moderate level of functional impairment.
-
Discrepancies exist between the definitions of low-intensity treatments adopted by IAPT services and NICE. Although there is no formal consensus regarding the precise number of therapist hours required by low-intensity interventions, NHS and IAPT services typically presume no more than 3–6 hours of input. The NICE guidelines advocate up to 10 hours of support for low-intensity interventions for OCD, thereby positioning at least some of these treatments within the remit of higher-intensity services.
Summary of the evidence for low-intensity interventions for obsessive–compulsive disorder
Despite NICE’s recommendations for low-intensity interventions for OCD, evidence of the clinical effectiveness, cost-effectiveness and acceptability of these interventions is limited. Effective synthesis is hampered by confusion over the scope of low-intensity interventions.
Lovell and Bee19 completed a systematic review of 13 studies (n = 492 participants) of CBT-based treatments that used health or communication technology in adults with OCD, including self-help manuals and cCBT alongside telephone and videoconferencing. Heterogeneity of populations, interventions and outcomes across the studies prevented meta-analysis. Self-help manuals were assessed in five small, uncontrolled, quasi-experimental studies and found moderate to large improvements in OCD symptoms; however, the absence of controlled trials means that the generalisability of the findings is unclear. cCBT was assessed in five studies, four of which were of BT Steps [now called OCFighter version 1.0 (CCBT Ltd, Birmingham, UK)]. The results revealed significant moderate to large effects on OCD symptoms score in favour of BT Steps. However, all studies were undertaken by the developers of the software and no independent analysis was available. The authors concluded that preliminary data support the idea that technology holds promise in treatment for OCD. Nevertheless, definitive conclusions about the relative efficacy of using health technologies as a replacement for therapist contact needed stronger evidence from rigorous randomised controlled trials (RCTs).
Herbst et al. 20 similarly examined telemental health applications for adults and children with OCD and included studies delivered by computer, the internet, telephone or self-help literature either delivered alone or supported by a health professional. No meta-analysis was completed and individual effect sizes ranged from 0.46 to 2.5. Of the 24 studies (n = 839 participants) included in this review, seven used written self-help materials (guided self-help), 11 were delivered by telephone, three were computer assisted, one comprised an online self-help group and two used videoconferencing. The review suggested that telemental applications may have promise but, once again, clinical and methodological heterogeneity, and small sample sizes precluded definite conclusion.
A more recent review21 looked at the efficacy of all types of technology-delivered CBT for OCD versus control conditions and in comparison with high-intensity CBT. Eight RCTs (n = 420 participants) were included and results found that CBT delivered by technology was superior to the control intervention in reducing OCD symptoms but not on comorbid depression. There were no differences in reductions in OCD symptoms between CBT delivered by technology and therapist-delivered CBT. Similar to previous reviews, this review concluded that further RCTs are warranted to examine the efficacy of technology-delivered CBT.
Guided self-help
Although NICE recommended the use of brief CBT (guided self-help) supported by a therapist, there is only a very limited evidence base to support this. A limited number of small open/uncontrolled trials22–26 of self-help materials, with guidance from a therapist have demonstrated promising results. One RCT25 randomised 41 patients with OCD to a self-help book with two contacts with a therapist or to 15 sessions of face-to-face therapist-delivered CBT including self-administered or therapist-administered ERP. Patients in both treatment conditions showed statistically and clinically significant symptom reduction, but therapist-delivered CBT was superior in OCD symptom and functional impairment reduction.
Since commencing OCTET, two RCTs have been published,27,28 neither of which used ERP (both RCTs used different unsupported self-help interventions). The first27 focused on a self-help book based on metacognitive training for OCD. Patients with OCD (n = 87) were randomised to either metacognitive training for OCD or a waiting list control and results showed significant changes in OCD symptoms in the intervention group post treatment.
The second RCT28 randomised 70 patients with OCD to either an unsupported self-help manual focusing on meridian tapping (a body-oriented technique from the field of alternative medicine) or progressive relaxation. The study did not lead to improvement in OCD symptoms.
Computerised cognitive–behavioural therapy
As a treatment option, cCBT is not recommended by NICE. A systematic review29 of cCBT for OCD found only four studies, all using the software program OCFighter (previously known as BT Steps). The results showed significantly better outcomes and less attrition for scheduled than for unscheduled telephone support. The conclusion of the review found OCFighter to be as good as standard high-intensity CBT in reducing time spent in rituals and obsessions, and in improving work and social functioning. Overall, standard high-intensity CBT was more effective than OCFighter, but not for those who actually started the intervention as opposed to those who failed to begin self-exposure therapy.
A key limitation of this work is that all OCFighter evaluations have been conducted by the commercial company who developed the program. Furthermore, this program was originally delivered with an interactive voice response and workbook. A more recent version, which has not yet been evaluated in a RCT, comprises a web-based platform in conjunction with brief support via telephone, face-to-face or e-mail contact with a mental health worker. A cost-effectiveness analysis has been completed30 with the original BT Steps program, but this was not independent from the developers of the commercially produced package.
Since the beginning of our study a further RCT has been published with BT Steps. 31 Eighty-seven participants with OCD were randomised to cCBT with (1) no therapist support, (2) lay (non-therapist) support or (3) support from an experienced CBT therapist. The results showed a positive change post treatment compared with pretreatment, with no significant difference between the three treatment arms in OCD symptoms or sessions completed. A number of internet-delivered CBT studies have been published including open studies. 32,33 Andersson et al. 33 randomised 101 adults with OCD to either 10 weeks of internet CBT or to an attention control condition, with online supportive therapy. Results found that both interventions led to significant improvements in OCD symptoms, but internet CBT resulted in greater improvements in OCD symptoms.
Summary
The provision of high-intensity CBT is ‘current best practice’ according to NICE OCD clinical guidelines. Despite the introduction of IAPT, access to high-intensity CBT can still involve significant delay, and the requirement to visit a therapist for treatments is poorly suited to the needs of some patients (e.g. the housebound, those with caring responsibilities, those whose OCD makes it difficult to be with people or to attend NHS settings, or those who live in rural locations).
There is clearly a potential role for low-intensity interventions as part of a stepped-care model. However, current evidence concerning low-intensity interventions (such as cCBT or guided self-help) is insufficient to provide accurate estimates of clinical effectiveness and cost-effectiveness. No studies have compared different low-intensity interventions (such as cCBT vs. guided self-help), nor do we know the numbers of people who will not improve with low-intensity interventions and who will require more intensive CBT in the stepped-care model.
The core question for patients, clinicians and policy-makers is ‘what is the role of low-intensity interventions in relation to “usual care” (i.e. referral to a waiting list for high-intensity CBT)?’. Implicit in the stepped-care model is the idea that giving patients on a waiting list for high-intensity CBT access to low-intensity interventions, prior to high-intensity CBT, could potentially augment care by:
-
improving patient outcomes either through more rapid improvement in clinical outcomes prior to high-intensity CBT or by augmenting the effect of high-intensity CBT in the longer term
-
reducing costs either by reducing the numbers of patients who need to access high-intensity CBT or by reducing general health-care utilisation in the short and longer term.
The aims of the Obsessive–Compulsive Treatment Efficacy randomised controlled Trial
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial emerged from a research recommendation in the NICE OCD guidelines that specified the need to evaluate CBT treatment intensity formats among adults with OCD.
In response to this recommendation, the National Institute for Health Research (NIHR)’s Health Technology Assessment programme commissioned research on ‘self-managed therapy packages’ for OCD, with specific reference to cCBT and guided self-help compared with treatment as usual. In this case, treatment as usual was defined as being placed on a waiting list for high-intensity CBT.
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial was designed to address this commissioned call. Our aims were to determine:
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) versus a waiting list for high-intensity CBT in the management of OCD at 3 months
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) plus high-intensity CBT versus a waiting list for high-intensity CBT plus high-intensity CBT in the management of OCD at 12 months
-
the acceptability of low-intensity interventions (guided self-help and supported cCBT) among patients and professionals.
Chapter 2 Trial design and methods
Study design
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial was a pragmatic, three-arm, multicentre RCT and the primary outcome was OCD symptoms using the Yale–Brown Obsessive Compulsive Scale – Observer Rated (Y-BOCS-OR) that aimed to determine:
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) versus a waiting list for high-intensity CBT in the management of OCD at 3 months
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) plus high-intensity CBT versus a waiting list plus high-intensity CBT in the management of OCD at 12 months
-
the acceptability of low-intensity interventions (guided self-help and supported cCBT) among patients and professionals.
The trial protocol has been published. 34 No major changes to the protocol were made after its publication.
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial involved two phases: an internal pilot, moving seamlessly into the substantive trial. We describe the methods and outcomes of the internal pilot, with a focus on the impact on the recruitment strategies used for the substantive trial. We then describe the methods used for the substantive trial.
Internal pilot
An internal pilot study is defined by NIHR as:35
. . . a version of the main study that is run in miniature to test whether the components of the main study can all work together. It is focused on the processes of the main study, for example to ensure recruitment, retention, randomisation, treatment, and follow-up assessments all run smoothly.
The OCTET pilot study therefore resembled the substantive study in all respects, including an assessment of the feasibility of the proposed primary outcome time point.
The internal pilot was conducted over 9 months and was designed to assess three questions:
-
Is it feasible to recruit the number of participants required to meet the planned sample size?
-
Do participants remain on a high-intensity CBT waiting list for a sufficient length of time (i.e. at least 3 months) to conduct an evaluation of the short-term clinical effectiveness and cost-effectiveness of self-managed therapies, prior to access to high-intensity CBT?
-
Should the primary outcome point be 3 or 6 months? The commissioning call specified 6 months as the primary assessment point but given the pressure of IAPT sites to minimise waiting lists we were unsure if 6 months would be achievable.
Results of the internal pilot
In terms of question 1, the internal pilot proved that recruitment was feasible (with the addition of more sites) and that the planned sample size could be achieved in principle. The pilot recruitment graph is presented in Appendix 1.
However, evaluation of questions 2 (time patients spent on the high-intensity CBT waiting list) and 3 (timing of a 3- or 6-month outcome for the primary assessment point) demonstrated significant challenges. As highlighted in the introduction, one of the key reasons for conducting OCTET was that the need for psychological therapy services for OCD exceeds demand, meaning that patients often face long waiting lists for high-intensity CBT treatment. Low-intensity interventions are designed to allow more rapid access and better management of demand.
The OCTET recruitment method was to screen existing high-intensity CBT IAPT waiting lists for potentially eligible patients where OCD was known or noted in the referral letter. Similar methods (large-scale screening of existing lists of potentially eligible patients) have underpinned a number of our successful large multicentre mental health trials [e.g. the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT trial),36 cost-effectiveness of collaborative care for depression in UK primary care trial (CADET)37].
Extensive mapping of IAPT high-intensity CBT waiting lists at recruitment sites was conducted prior to the trial commencing, which identified waiting lists of between 60 and 800 patients and between 40 and 100 OCD referrals per year. Therefore, it was predicted that we would be able to achieve and maintain the required recruitment rate.
During the internal pilot, the Department of Health informed all IAPT sites in England that patients should be offered more rapid access to treatment as part of the IAPT initiative. This had two major effects:
-
A proportion of participants randomised to a low-intensity intervention were offered their high-intensity CBT appointment prior to commencing the intervention or before completing the low-intensity intervention. One waiting list reduced from in excess of 700 patients to < 50 patients in 4 weeks.
-
A proportion of participants were ‘removed’ from the high-intensity CBT waiting list and offered access to a form of low-intensity intervention. In many cases, these interventions were in line with current good practice (including written material focusing on understanding and managing anxiety, supported by a PWP), but were not OCD specific or recommended by the NICE OCD guidelines. However, having been removed from the waiting list, such patients were lost to the OCTET recruitment procedure, significantly reducing the numbers of potentially eligible patients who could be contacted.
These changes to the high-intensity CBT waiting list procedure had significant implications for OCTET. In response, we made the following changes:
-
As a proportion of participants were reaching the top of the CBT waiting list prior to 12 weeks, we proposed to services that regardless of the length of waiting list, patients would be asked to wait at least 12 weeks before high-intensity CBT was offered. This proposal was supported by the Health Technology Assessment programme, the Data Monitoring and Ethics Committee (DMEC) and Trial Steering Committee (TSC), and was approved by the National Research Ethics Service Committee North West Lancaster. Despite this, only one site agreed to implement this change because of concerns that such a strategy contradicted the requirements of the Department of Health.
-
We initiated a new strategy in some sites, whereby all patients on waiting lists for high-intensity CBT were mailed with an invite to OCTET rather than the original strategy that restricted mailing to patients with an indication of OCD on NHS records. The rationale was that OCD is often underdiagnosed and in consultation with clinical colleagues it transpired that many referrals from general practitioners (GPs) did not indicate OCD on the referral letter. The trial consent procedures and inclusion criteria remained the same. This strategy proved successful in increasing numbers, but was more time-consuming as a number of people who self-reported OCD did not meet our trial inclusion criteria.
-
We extended self-referral opportunities in two sites (as recruitment was low at these particular sites) by placing adverts in local newspapers, on social media [e.g. local OCD group Facebook page (Facebook, Inc., Menlo Park, CA, USA)], in GP surgeries, community centres, trust magazines and in other local publications in one site. The trial consent procedures and inclusion criteria remained the same.
-
We engaged additional IAPT services within 10 NHS trusts in addition to our existing sites. Despite increasing the number of potential participants, this raised logistical and resource challenges, as additional recruitment and training of researchers and PWPs was required.
-
Given the directive from the Department of Health regarding high-intensity CBT waiting lists, it was not feasible to use the proposed 6-month follow-up as the primary outcome assessment point, and hence it was agreed to retain the 3-month assessment as the primary outcome assessment point. Given that removing the 6-month assessment would result in a variety of ethical issues and could potentially reduce participant engagement at 12 months, it was decided that the 6-month assessment should be retained.
Main trial methods
Ethics and governance
Ethics approval for the study was granted by National Research Ethics Service Committee North West – Lancaster (reference number 11/NW/0276). Site-specific approvals were obtained from the relevant local research governance offices covering the trusts involved in the trial. The trial was registered with the International Standard Randomised Controlled Trial Number Register (ISRCTN73535163).
Low-intensity interventions
Experimental group: supported computerised cognitive–behavioural therapy
Supported cCBT was delivered using OCFighter, a commercially produced cCBT program for people with OCD. OCFighter consists of a nine-step CBT approach (focused on ERP) to help people with OCD to design, carry out and monitor their treatment and progress.
Participants randomised to OCFighter were given an access ID and password to log in to the system and advised to use the program at least six times over a 12-week period. OCFighter was available to patients for 12 months following activation.
Participants received six brief (10-minute) scheduled telephone calls from a PWP (total direct clinical input 60 minutes). The support offered consisted of a brief risk assessment, ensuring patients had been able to access OCFighter, reviewing progress and solving any difficulties that were impeding progress.
Experimental group: guided self-help
The guided self-help consisted of a self-help book, Obsessive Compulsive Disorder: a Self-Help Book, written by the chief investigator. 38 The self-help book focused on information about OCD, maintenance and provided guidance on how to implement the NICE-recommended treatment for OCD (i.e. CBT using ERP). The self-help book was a refined and expanded version of a previous free-to-use self-help manual by the trial team and a user and carer from an OCD self-help group. 39 The self-help book was sent to the participant immediately following randomisation.
Participants received weekly guidance from a PWP, with one initial session of up to 60 minutes (either face to face or by telephone, dependent on patient preference) followed by up to 10 30-minute sessions over a 12-week period (total direct clinical input 6 hours).
The role of the PWP was to conduct a semistructured interview, to explain the structure and content of the book and devise patient-centred goals. PWPs supported patients to use CBT (ERP) as described in the self-help book, reviewed progress, pre-empted difficulties as they arose and engaged the participants in collaborative problem-solving as required.
Comparator group: waiting list for high-intensity cognitive–behavioural therapy
The comparator group for the short-term outcomes (3-month follow-up) was a waiting list for high-intensity CBT. In the longer term (12-month follow-up), the comparator was a waiting list for high-intensity CBT plus high-intensity CBT. High-intensity CBT is typically 8–20 face-to-face, 45- to 60-minute weekly sessions and uses a combination of ERP and cognitive therapy.
Psychological wellbeing practitioner training and supervision
Psychological wellbeing practitioners were trained in both the guided self-help and supported cCBT interventions. Training was delivered in all IAPT services in the 15 NHS sites. Top-up training was provided to PWPs at sites where a large delay between training and participant recruitment occurred, and because of the high turnover of PWPs the training was repeated at a number of sites.
The standardised training consisted of 3 days, and included 1 day explaining the nature, features, treatment and NICE recommendations for the management of OCD. In addition, day 1 also explained OCTET in terms of study design, rationale and trial procedures.
Day 2 focused on guided self-help and involved small- and large-group work and skills practice of ERP with specific feedback using exemplar cases. Training was delivered by the chief investigator and two coapplicants. cCBT training for OCFighter was delivered at the participating sites for 1 day by CCBT Ltd (the commercial producers of OCFighter). The training was standardised, using the same trainers (OCTET chief investigator, coapplicants and CCBT Ltd), materials and intervention manuals.
Training manuals for PWPs were developed by the trial team for guided self-help and by CCBT Ltd for the supported cCBT arm. A reference manual for PWPs was also generated that included general information about OCTET, recruitment, randomisation and allocation procedures, recording and storage of session recordings and monitoring of allocated participants.
Psychological wellbeing practitioners delivering the interventions were provided with telephone supervision on a 2-weekly basis for between 10 and 30 minutes (dependent on the number of patients to be discussed). Supervision was delivered by OCTET applicants or CBT therapists within IAPT services. All supervisors (two senior clinicians within a service and coapplicants) were required to attend the 3-day training).
Adherence and fidelity
To ensure the adherence to the guided self-help and supported cCBT interventions, and potentially enhance the reliability and internal validity of the trial, a number of strategies were implemented during trial development and completion.
Treatment adherence was examined by requesting PWPs complete contact sheets detailing dates of all sessions attended, length of sessions and mode of contact (face to face, telephone or e-mail). CCBT Ltd provided automated recordings of frequency and duration of supported cCBT use.
Fidelity was examined by asking PWPs to record all face-to-face and telephone sessions (with participant consent), using a digital recorder and (when required) a telephone-recording device.
These recordings were used to examine fidelity to the low-intensity interventions. A rating scale was developed based on the low-intensity intervention PWP manuals, which defined specific tasks to be carried out in session 1 and in subsequent sessions for both supported cCBT and guided self-help. Criteria for the fidelity scale were established via discussions within the OCTET team. The components extracted for fidelity were rated as ‘implicit’, ‘explicit’ or ‘absent’, and an overall rating generated using a 5-point Likert scale from ‘unacceptable’ to ‘excellent’. Fidelity was evaluated by a rater (a PWP independent of OCTET), who was blind to the treatment outcome.
Site recruitment
Mental health trusts across four UK centres in England (Manchester, York, East Anglia and Sheffield) were involved in the trial. A dedicated site lead was allocated at each centre. Trusts were recruited throughout the trial and further support was sought from the Mental Health Research Network (MHRN) to engage with additional trusts.
Patient recruitment
Inclusion criteria
-
Adults aged ≥ 18 years.
-
On a waiting list for high-intensity CBT in either primary or secondary mental health-care settings.
-
Met DSM-IV criteria for OCD, assessed using six OCD questions from the Mini-International Neuropsychiatric Interview,40 module G.
-
Scored ≥ 16 on the Yale–Brown Obsessive Compulsive Scale – Self-Report (Y-BOCS-SR), indicating a moderate level of OCD. This is the cut-off score used in most trials. Previous studies suggest that only a minority of people are referred for treatment or excluded from trials with a Y-BOCS score of < 16 (e.g. 2.3%,41 0%,42 14%25).
-
Reported an ability to read English at a level of age ≥ 11 years.
Exclusion criteria
-
Actively suicidal.
-
Had organic brain disease.
-
Currently experiencing psychosis.
-
Had a diagnosis of alcohol or substance dependence using DSM-IV criteria (assessed using the Mini-International Neuropsychiatric Interview,40 modules I and J).
-
Currently receiving psychological treatment for OCD.
-
Had literacy or language difficulties to an extent that would preclude them from reading written or web-based materials.
Potential participants were identified using a variety of recruitment methods: waiting lists in primary and secondary care in our clinical sites were screened by administrative and clinical staff; PWPs screening patients entering services identified those who may be eligible; and self-referral options were used at one site (i.e. via adverts in local newspapers, GP surgeries, on social media sites and community centres).
Individuals who were waiting for high-intensity CBT or responded to an advert were provided with a participant information pack (including an invitation letter, patient information sheet and consent-to-contact form) in the post or in person. Those who returned a completed consent-to-contact form initially took part in a brief telephone eligibility screen to determine that they were aged > 18 years, not currently receiving a psychological therapy for their OCD symptoms or experiencing severe and distressing psychotic symptoms. Where participants met the initial eligibility screen they were offered a face-to-face eligibility appointment (either at the clinical site or in their own home). During the interview, individuals had the opportunity to ask any further questions prior to providing consent and completing the eligibility assessment.
Randomisation, concealment of allocation and blinding
Patients were randomised (in a ratio 1 : 1 : 1) into one of the three arms using a central randomisation service, via a secure web-based system administered by the York Trials Unit (YTU).
Allocation involved minimisation on the following factors:
-
OCD severity on the Y-BOCS-SR (16–23, moderate; 24+, severe/very severe)
-
current antidepressant medication use (yes/no)
-
depression on the Patient Health Questionnaire-9 (PHQ-9) (< 10, mild depression; 10–14, moderate depression; 15–19, moderate to severe depression; > 20, severe depression)
-
duration of OCD (0–5 years; 6–10 years; > 10 years).
Researcher blinding
We attempted to blind outcome assessors to allocation; by ensuring that outcome assessments by researchers were separate from days and locations in which treatment was delivered, asking OCTET participants to refrain from revealing allocations during assessments and restricting researcher access to the group allocation section of the trial database.
Blinding was monitored throughout the trial. Researchers were asked to complete an unblinding report form at all follow-up time points, indicating if they had been unblinded and, if so, at what point during the interview this occurred. Partial (treatment/no treatment) or full unblinding (trial arm) was recorded. Researchers were also asked to complete an unblinding form if they became unblinded at any other point during the trial (e.g. when arranging a follow-up interview with the participant).
Follow-up assessments
Follow-up assessments were conducted 3, 6 and 12 months following randomisation. Participants were contacted approximately 1 month to 1 fortnight prior to the follow-up time point to arrange a suitable time and location to meet (either at the clinical site or in their own home). To reduce the chance of the researcher becoming unblinded, the Adult Service Use Schedule (AD-SUS) self-complete, Client Satisfaction Questionnaire-8 (CSQ-8) and Pathway questionnaire were posted prior to the visit for return in a Freepost envelope to the YTU. Participants were given a £5 shopping voucher to thank them for their time for each follow-up they completed or partially completed.
Outcome assessments
As the primary outcome was the Y-BOCS-OR, the collection of data was face to face. However, we acknowledged that achieving this with all follow-ups could prove difficult. Therefore, we developed a highly structured standardised operating procedure (SOP) to assist researchers with participants who were difficult to contact, to ensure retention rates were maximised. These procedures included contacting participants using a scaling-down approach (i.e. if not willing/unable to attend face to face we offered telephone assessment using Y-BOCS-SR and if this failed, to post the primary outcome with a Freepost envelope for return to the YTU).
Measures
Primary outcome
The primary outcome was OCD symptoms as measured by the Y-BOCS-OR. 43 The Y-BOCS-OR is an interview-administered structured assessment that measures symptom severity in individuals with obsessive and compulsive symptoms. It consists of two comprehensive symptom checklists, exploring current (over the past week) and past symptoms, and a 10-item severity scale with obsession and compulsion subscales exploring current symptoms. The severity scale is designed to identify the impairment experienced by individuals over five clinical domains: time consumed, functional impairment, psychological distress, efforts to resist and perceived sense of control. Responses are rated on a 5-point Likert scale from 0 (none) to 4 (extreme). Responses to all items are added to generate subscale scores and a total Y-BOCS-OR score. Scores are indicative of OCD severity over five severity categories: 0–7 (subclinical), 8–15 (mild), 16–23 (moderate), 24–31 (severe) and 32–40 (extreme).
The interview took, on average, 30–40 minutes to complete. Individuals conducting the interview required prior training on OCD symptomatology and in how to rate respondents’ responses. The Y-BOCS-OR has good psychometric properties. 43,44
Secondary outcomes
Secondary outcomes are listed in Table 1, adapted with permission from Gellatly et al. 34
| Secondary outcome | Measured using/by |
|---|---|
| Self-reported OCD symptoms | Y-BOCS-SR |
| Self-reported health-related quality of life | SF-36 |
| Health-related quality of life | EQ-5D-3L |
| Resource use | AD-SUS |
| Generic mental health | CORE-OM |
| Depression | PHQ-9 |
| Anxiety | GAD-7 |
| Functioning | WSAS |
| Employment status | IAPT employment status questions A13–14 |
| Patient satisfaction | CSQ-8 |
| Patient progress through mental health services/proportion of patients not improved or partially improved and requiring more intensive CBT | Pathway questionnaire |
| Comorbiditiesa | CIS-R |
| Attachmenta,b | Relationship Styles Questionnaire |
| Perceived criticisma,b | Perceived Criticism Scale |
| Expressed emotiona,b | Family Emotional Involvement and Criticism Scale |
Yale–Brown Obsessive Compulsive Scale – Self-Report
The Y-BOCS-SR43 is a modified version of the Y-BOCS-OR scale for completion by individuals in the absence of an interviewer. Identical questions from the Y-BOCS-OR 10-item severity scale are presented along with responses for each for the individual to select.
As research has demonstrated a moderate relationship between the Y-BOCS-OR and Y-BOCS-SR in a clinical sample of OCD patients,45 it was agreed at the inception of the trial that, where it was not possible to complete the Y-BOCS-OR (primary outcome measure), the Y-BOCS-SR would be used as a proxy for the primary outcome.
Short Form questionnaire-36 items
The Short Form questionnaire-36 items (SF-36)46 is a widely used generic measure of health-related quality of life. It has eight dimensions of health: physical functioning; social functioning; physical role limitations; emotional role limitations; energy; pain; mental health; and general health perceptions. Individuals provide responses based on how they have felt over the previous week on Likert-type scales. Each question carries equal weight, and is transformed into a 0–100 scale. Lower scores denote more disability. Two summary scores are produced: the mental health component score and the physical health component score. The scale has good psychometric properties. 47
European Quality of Life-5 Dimensions-3 levels
The European Quality of Life-5 Dimensions-3 levels (EQ-5D-3L)48 is a self-complete instrument used to measure health-related quality of life, providing health utility scores capable of generating quality-adjusted life-years (QALYs). It consists of five questions addressing five dimensions of health: mobility; self-care; ability to undertake usual activities; pain and discomfort; and anxiety and depression. Respondents report difficulties in each area on three levels (none, some/moderate, extreme), generating individual health states that can be converted into a weighted health index score, based on values derived from general population samples. 49 The measure has been extensively used and its psychometric properties are adequate. 50
Adult Service Use Schedule
The AD-SUS was used to measure individual-level resource use over the period of the trial. The AD-SUS is used to collect service use and related data, and has been successfully applied in a range of adult mental health populations, including common mental disorders. 51–53
The AD-SUS was adapted for OCD on the basis of clinical expertise and refined using feedback from a stakeholder group of service users and carers to assess coverage, acceptability, ‘user friendliness’ and ease of completion. The AD-SUS was completed in an interview with participants and recorded all-cause hospital- and community-based health- and social-care services, use of psychotropic medication and out-of-pocket expenses and savings. Use of psychological therapies (‘talking therapies’) was recorded using a separate self-complete version of the AD-SUS to ensure that interviewers remained blinded to randomisation allocation status.
Productivity losses were also recorded, using the absenteeism questions from the World Health Organization’s Health and Work Performance Questionnaire (HPQ). 54,55
Clinical Outcomes in Routine Evaluation – Outcome Measure
Clinical Outcomes in Routine Evaluation – Outcome Measure (CORE-OM)56 is a self-complete measure designed to measure global distress. Thirty-four questions explore how individuals have been feeling over the last week, using a 5-point scale ranging from ‘not at all’ to ‘most or all of the time’. Four dimensions of global distress are addressed: subjective well-being; problems/symptoms; life functioning; and risk/harm. Eight items are positively framed and scoring for these is reversed. Higher scores indicate higher levels of distress. The CORE-OM has high internal and test–retest reliability, and demonstrates convergent validity with other measures. 57
Patient Health Questionnaire-9
The PHQ-958 is a 9-item self-report scale that facilitates the recognition and diagnosis of depression. Scores range from 0 to 27, with a score of ≥ 10 considered to be a clinically significant level of depression. The PHQ-9 has demonstrated good reliability and validity. 59
Generalised Anxiety Disorder Scale-7
The Generalised Anxiety Disorder Scale-7 (GAD-7)60 is a 7-item self-report scale used to identify and measure the severity of generalised anxiety disorder. Scores range from 0 to 21, with a cut-off score of ≥ 8 distinguishing between clinical and non-clinical populations. Good psychometric properties have been reported. 60
Work and Social Adjustment Scale
The Work and Social Adjustment Scale (WSAS)61 is a 5-item self-report measure that assesses functional impairment. Scores range from 0 to 40. The scale assesses the impact on work, home, social and private activities, and personal or family relationships. A score of > 20 provides indication of severe functional impairment, whereas scores between 10 and 20 suggest less severe but significant functional impairment. Scores of < 10 are considered subclinical. The scale is reported to display good reliability and validity. 61
Improving Access to Psychological Therapies employment status questions A13–14
This consists of two questions addressing the employment status of individuals and if statutory sick pay is being received. This measure is used as part of the IAPT minimum data set. 62
Pathway questionnaire
This is a self-complete measure developed specifically for use within OCTET to identify whether patients had or had not stayed on the waiting list for high-intensity CBT.
Clinical Interview Schedule – Revised
The Clinical Interview Schedule – Revised (CIS-R)63 is a self-administered computerised assessment of psychiatric disorder. The interview begins with general questions to establish an overall picture of health, appetite and physical activity. The main body of the CIS-R contains 14 sections labelled A–N. Each section scores a symptom, which may range in severity between 0 and 4. These symptoms are somatic symptoms, fatigue, concentration and forgetfulness, sleep problems, irritability, worry about physical health, depression, depressive ideas, worry, anxiety, phobias, panic, compulsions and obsessions.
The diagnostic output corresponds to the ICD-10 Classification of Mental and Behavioural Disorders64 diagnostic criteria for mild, moderate and severe depressive episodes. CIS-R has established reliability and validity in primary care, occupational and community studies. 63
Client Satisfaction Questionnaire-8
The CSQ-865 is a self-report scale that asks patients to assess their satisfaction with a service on a 4-point Likert scale. Scores range from 8 to 32, with higher values indicative of higher satisfaction. The measure has been tested with diverse client samples and demonstrates good retest reliability, internal consistency and sensitivity to treatment. 66
Table 2 provides detail about the time points at which each measure was completed.
| Outcome measures | Time point | ||||
|---|---|---|---|---|---|
| Eligibility | Baseline | 3 months | 6 months | 12 months | |
| Primary outcome | |||||
| Y-BOCS-OR | ✗ | ✗ | ✗ | ✗ | |
| Secondary outcomes | |||||
| Y-BOCS-SR | ✗ | ✗ | ✗ | ✗ | ✗ |
| SF-36 | ✗ | ✗ | ✗ | ✗ | |
| CORE-OM | ✗ | ✗ | ✗ | ✗ | |
| PHQ-9 | ✗ | ✗ | ✗ | ✗ | |
| GAD-7 | ✗ | ✗ | ✗ | ✗ | |
| WSAS | ✗ | ✗ | ✗ | ✗ | |
| EQ-5D-3L | ✗ | ✗ | ✗ | ✗ | |
| IAPT employment status questions A13–14 | ✗ | ✗ | ✗ | ✗ | |
| CSQ-8 | ✗ | ✗ | |||
| CBT uptake (Pathway questionnaire) | ✗ | ✗ | ✗ | ||
| AD-SUS – interview | ✗ | ✗ | ✗ | ✗ | |
| AD-SUS – self-complete | ✗ | ✗ | ✗ | ✗ | |
| CIS-R | ✗ | ||||
Researcher training
Researchers and clinical study officers (CSOs) from the MHRN involved in the recruitment of participants were provided with a 1-day training session covering trial procedures and completion of eligibility, baseline and follow-up interviews. The trial manager provided training. A proportion of the day was spent equipping individuals with the necessary skills to complete the Y-BOCS-OR. Inter-rater reliability among the researchers/CSOs was employed to measure agreement between ratings for the Y-BOCS-OR. Top-up training was provided throughout the trial.
Inter-rater reliability: Yale–Brown Obsessive Compulsive Scale – Observer Rated
All researchers and CSOs who undertook the 1-day training were trained on the purpose, content and conduct of the Y-BOCS-OR. During the training, a practice recording of a Y-BOCS-OR interview (conducted by an expert clinician) was played and ratings were collected and discussed in a group format. Researchers were informed that if they were experiencing difficulties making a decision about the score to any of the questions, they should allocate the higher of the two scores. All researchers had the opportunity to ask questions and any queries were addressed. The aim was to investigate the inter-rater reliability between researchers/CSOs and the expert rating of the practice sessions. Total scores for the measure were compared; a difference of 4 points between researcher/CSO ratings and expert ratings was tolerated. In addition, if differences in ratings crossed diagnostic boundaries a reassessment may be required. Where the tolerance criterion was not met, a discussion was held with the researcher/CSO and additional training and advice provided.
Following the training day an additional practice recording was provided and researchers/CSOs were asked to return their rating to the trial manager within 2 weeks. Ratings were again compared with the expert score. During the trial (6 months following commencement) researchers were asked to rate a third practice tape. A ‘cues and prompts’ sheet was prepared to assist with the conduct of the Y-BOCS-OR.
Sample size calculation
At each time point three pairwise comparisons were carried out between the three intervention options: supported cCBT, guided self-help and a waiting list for high-intensity CBT. The study was therefore powered using a 1.67% significance level.
The comparison of either supported cCBT or guided self-help is a partially nested design for which the sample size calculation needs to consider the intracluster correlation coefficient (ICC) for therapist. The comparison of supported cCBT with guided self-help is a crossed therapist design, as support for both treatments was delivered by the same therapists. Sample size for crossed therapist design depends on the ICC for therapist for treatment within therapist, which is smaller than the ICC for therapists. Formulae for this calculation are given in Walwyn and Roberts. 67 In the absence of estimates of the two ICCs required for the two calculations, sensitivity of study power to larger values was considered in the calculation below.
Assuming a standard deviation (SD) for the primary outcome (Y-BOCS-OR) at 6 months of 7.3 units, a correlation between baseline Y-BOCS-OR and 6-month Y-BOCS-OR of 0.43,41 a study with 366 service users followed up to the primary end point has a power > 80% to detect a difference of 3 Y-BOCS points for each comparison. We were unable to find evidence for a ‘clinically important difference’. A reduction of 3 points was agreed based on clinical consensus with the study team. This calculation assumed that supported cCBT and guided self-help were delivered by 24 therapists. It also assumes that the ICC for therapists was 0.06 and an ICC for treatment within therapist was 0.015, which implies that the correlation between the random effect for supported cCBT and guided self-help is 0.75. The design effects, sometimes called the sample size inflation factor, were 1.1225 and 1.06125 for the partially nested and crossed designs, respectively. We considered these values of the ICC to be plausible, but in the event that the ICC for therapist was as large as 0.1 and the ICC for treatment within therapist was 0.05, the power of the trial is still > 75% for all three comparisons.
Based on an 85% follow-up rate, the target sample size for the trial was set at 432 in the initial application. Monitoring during the trial suggested that the follow-up rate to the primary end point (3 months) was likely to be lower rather than the assumed 85%. Assuming a follow-up rate of 78%, total sample size was increased to 472.
Analysis
Low-intensity intervention uptake
Patient engagement with the treatment process was summarised and reported descriptively. There is no consensus for the level of uptake of low-intensity treatments that might define an appropriate ‘dose’.
Analysis of the primary outcome measure and secondary quantitative outcome measures
Cleaning of outcome and baseline data was conducted without the treatment group allocations in view. Summary statistics from these preliminary analyses were reviewed by the trial research team to identify data errors.
Preliminary analyses compared the characteristics of subjects with and without complete data at follow-up time points, by treatment group, using a logistic regression model. This was carried out for the primary outcome at the three follow-up assessment points. This analysis was used to develop an understanding of the missing data mechanism and to determine the appropriate methods for dealing with missing outcome data.
Statistical analyses of the primary outcome measure, Y-BOCS-OR, are based on a linear mixed model with random effects for supported cCBT and guided self-help therapist using restricted maximum likelihood. As therapist is crossed with treatment, separate random effects were included for each treatment enabling the estimation of the intracluster correlation for supported cCBT and guided self-help. In addition to treatment, the following fixed baseline or demographic covariates were included to improve statistical efficiency:
-
OCD duration (by categories 0–5, 5–10, > 10 years)
-
OCD severity (as measured by Y-BOCS-OR at baseline)
-
anxiety (as measured by GAD-7)
-
depression score (as measured by PHQ-9)
-
antidepressant drug use (yes/no)
-
sex.
A small number of baseline covariates were missing for those covariates not used in the minimisation. To maximise the number of subjects included in the model, these values were imputed by single imputation using other covariates in keeping with the method suggested by White and Thompson. 68 Using this procedure, statistical modelling can be carried out on all participants with outcome data. A logistic regression model was used, fitted to CBT uptake, with treatment allocation, sex, duration of OCD, baseline Y-BOCS-OR, GAD-7, PHQ-9 and antidepressant medication on entry into the trial as covariates.
The same analyses were carried out for quantitative secondary outcomes. These analyses assume that subjects are missing at random.
Uptake of high-intensity cognitive–behavioural therapy
Uptake of high-intensity CBT was recorded at the 3-, 6- and 12-month follow-ups. A logistic regression model was used to estimate the adjusted odds ratio (OR) for uptake (as a binary outcome of a patient attending at least one CBT appointment), comparing the two low-intensity interventions separately with a waiting list for high-intensity CBT. The models included treatment allocation, sex, duration of OCD, baseline Y-BOCS-OR, GAD-7, PHQ-9 and antidepressant medication on entry into the trial as covariates. Where presented, tables give the adjusted ORs with confidence intervals (CIs) and p-values and the exponent of the model constant.
Recovery and remission
Recently, expert consensus guidelines for defining treatment response and remission in OCD have been published based on YBOC-OR. 69 These are defined as ≥ 35% reduction on the Y-BOCS-OR for response and Y-BOCS-OR score of ≤ 12 for remission. A logistic model was fitted to responses at 12 months adjusting for sex, baseline GAD-7 and PHQ-9 scores, antidepressant medication use at randomisation and duration of OCD. The analysis is additional to the statistical analysis plan as this was signed-off prior to publication of the guidelines.
Moderator analyses
A subgroup analysis by severity specified in the trial protocol was conducted by adding a treatment severity interaction term to the analysis of the primary outcome at the primary end point (3 months). In addition, analysis of treatment effect moderation by both age and chronicity of OCD was carried out.
Two types of interactions between moderators and treatment can occur. If the moderator is just affecting the magnitude of the treatment effect it is called a quantitative interaction. If the moderation causes a reversal of the treatment effect it is called a qualitative interaction. Interactions of treatment with both chronicity and severity were hypothesised to be quantitative, whereas interactions of treatment with age were hypothesised to be qualitative (representing potential difficulties of older patients in engaging with supported cCBT relative to guided self-help). Thus, a significant treatment effect would be required before assessing treatment moderation via severity and chronicity of OCD.
The analyses were carried out by adding a treatment with moderator interaction terms to the primary analysis model. For severity and chronicity, an overall test of the interaction was carried out. The hypothesis related to age concerned only the low-intensity interventions, so the contrast between guided self-help and supported cCBT was estimated.
Data were analysed using Stata version 13 (StataCorp LP, College Station, TX, USA).
Economic evaluation
Perspective
The primary perspective of the economic evaluation was the NHS/Personal Social Services perspective preferred by NICE. Secondary analyses included all additional resources likely to be relevant to a societal perspective in this population: productivity losses (as a result of time off work resulting from illness), and out-of-pocket expenses and savings.
Data collection
An adapted version of the AD-SUS was used to measure individual-level resource use. The AD-SUS is used to collect service use and related data, and has been successfully used in a range of adult mental health populations. 51–53 The AD-SUS was adapted for OCD, as described above (see Secondary outcomes), and used to record all-cause hospital and community-based health- and social-care services, medication and out-of-pocket expenses and savings. Information on medications used including drug, dose and duration were collected for economic purposes only. Productivity losses were recorded using the World Health Organization’s HPQ. 54,55 The AD-SUS and HPQ were administered by interview at baseline, and the 3-, 6- and 12-month follow-ups, and covered the previous 6-month period at baseline interview and the time since last interview at each follow-up point. Data on the number and duration of supported cCBT and guided self-help contacts were recorded on a session-by-session basis by PWPs using an intervention proforma. Use of all other psychological therapies, including high-intensity CBT, was collected and self-reported by participants in a separate self-complete proforma kept separate from the AD-SUS in order to ensure that interviewers remained blinded to randomisation allocation status. Participants completed the form alone and placed it in a sealed envelope before handing it to the interviewers. Data on the number and duration of intervention contacts were recorded on a session-by-session basis by PWPs using an intervention proforma.
Costs
All costs are reported in pounds sterling at 2013/14 prices. Discounting was not relevant, as the follow-up did not exceed 12 months. Unit costs were applied to individual-level resource use data to calculate total costs per participant and are detailed in Appendix 2. In summary, unit costs for most hospital and primary care services were obtained from NHS Reference Costs,70 Unit Costs of Health and Social Care71 and the British National Formulary for medications. 72
Productivity losses because of OCD were calculated using the human capital approach by multiplying days off work attributable to illness by the individual’s salary and not accounting for early retirement resulting from illness. 73 Lost productivity costs were capped at 5 days per week (maximum of 130 days for the 6-month period and 65 days for the 3-month period).
Intervention costs
Psychological wellbeing practitioner sessions were costed using published data on the cost of low-intensity IAPT interventions,74 inflated to 2013/14 prices using the Hospital and Community Health Services Pay and Prices Index. 71 The approach is outlined in Appendix 3.
Training for PWPs to provide supported cCBT and guided self-help support was provided to all PWPs, and associated costs were calculated as outlined in Appendix 4.
For the supported cCBT arm, the cost to the trial of the OCFighter program was £10,000. This was divided by the 157 participants who were randomised to supported cCBT to give a cost per participant in this group of £63.69. In the guided self-help arm, participants were provided with a self-help manual (£1.87 per manual for printing costs only, excluding development and design costs as these are sunk costs), photocopied worksheets (£0.81 per set of photocopied sheets per participant) and a CD (£1.18 per CD, one per participant), plus £1.68 postage charge. Thus, the total cost of guided self-help materials per participant was £5.54.
Access to high-intensity CBT, for all groups, was recorded using the intervention proforma. Unit costs for CBT are reported in Appendix 2.
Analysis
Data were analysed using Stata. Participants were analysed on an intention-to-treat basis (i.e. according to the group to which they were randomised regardless of intervention compliance).
Costs and outcomes were compared at baseline, 3, 6 and 12 months and are presented as mean values by arm with SDs. Mean differences and 95% CIs were obtained by non-parametric bootstrap regressions (1000 repetitions) to account for the non-normal distribution commonly found in economic data, with adjustment for clustering at the therapist level, using the ‘cluster’ option in Stata. To provide more relevant treatment-effect estimates75 regressions to calculate mean differences in costs were repeated with the further inclusion of covariates for the baseline value of the relevant variable (costs or EQ-5D-3L utility) plus variables thought to influence costs and outcomes: antidepressant drug use; anxiety (GAD-7); depression score (PHQ-9); sex; OCD duration (0–5, 5–10 and > 10 years); and Y-BOCS-OR score.
The primary analysis was a complete-case analysis (i.e. excluding those lost to follow-up or with missing ADSUS and/or EQ-5D-3L data at a particular time point). To explore the potential impact of excluding non-responders, we examined the sociodemographic and clinical characteristics of those included in the analyses and those in the full sample. A secondary analysis was carried out with missing baseline, 3-month and 12-month total costs and outcomes imputed using the input imputation command in Stata (version 11) and including the baseline variables described above.
Cost-effectiveness was explored in terms of QALYs calculated using the EQ-5D-3L measure of health-related quality of life, assessed at baseline, 3, 6 and 12 months. Appropriate utility weights were attached to health states76 and QALYs were calculated using the total area-under-the-curve approach with linear interpolation between assessments. 77
Two sets of cost–utility analyses were conducted: one compared groups at 3 months and one compared groups at 12 months (primary end point). Incremental cost-effectiveness ratios (ICERs) were calculated, defined as the mean difference in cost between two groups divided by the mean difference in effect.
Uncertainty was explored using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs) based on the net-benefit approach. 78 Cost-effectiveness planes illustrate the uncertainty around the estimates of costs and effects by plotting the bootstrapped cost and effects, with points in each quadrant indicating a different implication for economic evaluation. CEACs are an alternative to CIs around ICERs and show the probability that one intervention is cost-effective compared with the other, for a range of values that a decision-maker would be willing to pay for an additional unit of an outcome. A series of net benefits were calculated for each individual for a range of values for willingness to pay for a QALY. After calculating net benefits for each participant for each value of willingness to pay, coefficients of differences in net benefits between the trial arms were obtained through a series of bootstrapped linear regressions (1000 repetitions) of group upon net benefit, which included the same covariates used for comparisons of outcomes in the primary economic analyses. The resulting coefficients were then examined to calculate the proportion of times that the intervention group had a greater net benefit than the control group for each value of willingness to pay. These proportions were then plotted to generate CEACs for all cost–outcome combinations.
This analysis takes a decision-making approach, ignoring statistical significance and focusing instead on the probability of one intervention being cost-effective compared with another intervention, given the data available. This is the recommended approach to economic evaluation, preferred over traditional reliance on arbitrary decision rules regarding statistical significance, which are being increasingly criticised as irrelevant in a decision-making context. 79,80 Instead, it is argued that the decision to adopt one intervention over another should be based on the expected cost-effectiveness of the intervention, or the probability of making the correct decision.
Patient and professional acceptability: qualitative methods
Successful implementation of research into NHS practice requires that new interventions are accepted by both patients and mental health professionals. Two qualitative acceptability studies were conducted. We conducted post-intervention interviews with a subgroup of patients in both low-intensity arms of the trial and with PWPs delivering the low-intensity interventions across clinical sites.
The methods and associated findings from these studies are presented in Chapter 5.
Trial administration
Trial monitoring
Independent committees
The TSC met twice per year, chaired by an academic GP. The TSC included an experienced PWP, a consultant child and adolescent psychiatrist with a special interest in OCD, a service user with lived experience of OCD, the chief investigator and the trial managers.
The DMEC was chaired by an academic GP, and included a mental health nurse with significant experience of working with OCD and an independent statistician, with the trial statistician also in attendance. The DMEC met once per year. Terms of reference for the TSC and DMEC committees were agreed at the commencement of the study. Members of these committees are named in the Acknowledgements.
Clinical trials unit
The YTU (UKCRC registration: 40) was responsible for facilitating the randomisation of participants, establishing a study database, handling of safety reporting and the management of data for OCTET. Data management included oversight of trial retention rates and monitoring of data entry and completeness. Where required, additional procedures were generated to ensure that follow-up was completed with all consenting participants and data entry was completed promptly.
The database established for use in OCTET was devised to facilitate study contacts, trial oversight and data management. This included facilities to:
-
record participant contact details
-
record contact attempts and completed visits
-
generate randomised allocation letters (for the participant and their GP)
-
facilitate the allocation of PWPs to participants
-
record documentation received at the YTU
-
enable entry of collected data for compilation for the end analysis.
The database also provided an opportunity to run routine reports on recruitment and retention rates, which facilitated the smooth administration of OCTET.
In addition, the YTU completed an independent quality control and verification process for the collected data, by checking that a random 10% sample of data (across each time point, stratified by centre) did not exceed a predefined error rate of 5%. This proportionate approach to data verification was deemed appropriate because of the nature of this trial. 81
The YTU was also available to provide trial management during periods of absence, thus ensuring continued support throughout the trial for OCTET researchers and PWPs.
Audits
To ensure that all data collected during OCTET were complete and stored correctly, internal audits of all data and documents collected at each research site were conducted. This included monitoring of the following:
-
consent-to-contact forms
-
consent forms
-
baseline/follow-up booklets
-
adverse event (AE) forms
-
risk forms
-
voucher receipts for follow-up interviews
-
sending of booklets to the YTU.
An audit procedure guidance document was prepared by the trial managers that included a data checklist for completion by each research team.
Trial-specific procedures
To standardise processes across all sites and to maximise data quality, trial-specific SOPs were generated for all individuals involved in the trial covering all procedures and frequently asked questions.
Researcher trial-specific SOPs covered booking eligibility; baseline and follow-up interviews; recruitment procedures; dealing with difficult to contact participants; retention procedures; conducting and reporting risk assessments; AEs recording and reporting; managing participants; and own distress and blinding.
Checklists and letter templates were included to assist with all aspects of the trial and the trial procedure document was updated and disseminated to all researchers as and when necessary throughout the trial.
Risk assessment
A trial-specific SOP was implemented for reporting and managing suicidal risk. Question 9 on the PHQ-9 (Have you had thoughts that you would be better off dead or hurting yourself in some way?) was used to identify potential suicide risk. If the patient indicated risk then a series of questions were asked to determine level of risk including ‘thoughts only but no intent’, ‘thoughts with some intent but not immediate’ and ‘thoughts with immediate intent’. Where risk was identified it was referred to a trial coapplicant with clinical experience. Appropriate action was then taken usually involving informing both the clinical site and the GP. All reported risk was documented on risk forms that were signed by the research site lead.
Safety reporting and disclosure
To fulfil requirements for safety reporting and disclosure, a trial-specific procedure for detecting and reporting AEs was implemented.
Initially in OCTET an AE was defined as any untoward medical occurrence in a participant that may or may not have a causal relationship with the treatment. Events could then be classified as ‘serious’ or ‘non-serious’ as per The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use good clinical practice guidance. 82
This resulted in a high level of AEs being reported that were unrelated to the condition or treatments of interest. Following discussions with the OCTET DMEC, non-serious AE reporting was amended to include events relating to (1) any mental health condition; (2) use of psychotropic medications; (3) dissatisfaction with trial procedures; and (4) unplanned hospital visits for any medical condition. Serious AE reporting remained unchanged.
Researchers and PWPs were requested to inform the trial manager at the YTU of any serious or non-serious AEs using the OCTET AE reporting form. All serious AEs were independently reviewed by two clinicians appointed by the YTU to ascribe relationship and expectedness. All serious and non-serious AEs were summarised for discussion with the DMEC and TSC at each scheduled meeting.
Any serious AEs deemed to be related and unexpected were required to be reviewed by the DMEC prior to subsequent reporting to the Research Ethics Committee and study sponsor within 7 days. No such events were experienced during OCTET.
Patient and public involvement
Patient and public involvement was incorporated throughout all stages of OCTET, with links being made during the early stages of the trial. The contributions made were extremely valuable, providing alternative views and suggestions to those of the trial team and strengthening ideas.
From the outset, involvement derived from the chief executive of Anxiety UK (a national, user-led charity run by sufferers and ex-sufferers of anxiety disorders), who was included as an applicant.
Members of an OCD self-help group agreed to be part of a consultation group at the inception of OCTET. The aim of the OCD consultation group was to determine the ‘user-friendliness’ and ease of completion of the guided self-help manual during the development stages. Comments provided during the focus group were fed back to the OCTET team and various refinements to wording and presentation were made.
The consultation group also contributed to the adaptation of the AD-SUS for use with an OCD population. Feedback was gathered in relation to the wording of questions, ease of completion and if additional questions should be included, and appropriate amendments were made.
In addition to the development and conduct of the research, service users were involved in the undertaking and monitoring of the research. As detailed previously (see Trial administration), a service user sat on the TSC and provided advice and guidance during the trial.
The patient acceptability study was supported by a service user, who attended bespoke qualitative interview training delivered by assisted experienced members of the OCTET team, to assist with the conduct of the interviews. Further details are provided in Chapter 5.
The trial team have additionally collaborated with a national service user-led OCD charity, OCD-UK, to support the dissemination of the research findings.
A consultancy fee, based on INVOLVE guidelines,83 was paid to service users involved.
Chapter 3 Results
In this chapter, we detail the results of OCTET.
Site recruitment
In our pilot phase we recruited five IAPT sites within five NHS trusts, each led by a coapplicant designated as site lead. Following the dissolution of waiting lists, a further 10 sites were recruited either via the existing site leads or through the MHRN (Table 3) to supplement recruitment activity.
| Trust | Number of PWPs attending OCTET training, n (%) |
|---|---|
| Bradford District Care Trust | 15 (7.4) |
| Camden & Islington NHS Foundation Trust | 18 (8.8) |
| Cheshire & Wirral Partnership NHS Foundation Trust | 20 (9.8) |
| Coventry & Warwickshire Partnership Trust | 5 (2.5) |
| Lancashire Care NHS Foundation Trust | 20 (9.8) |
| Manchester Mental Health and Social Care Trust | 7 (3.4) |
| NHS City Health Care Partnership Trust | 15 (7.4) |
| Norfolk and Suffolk Mental Health NHS Foundation Trust | 27 (13.2) |
| Nottingham County Health Partnership | 15 (7.4) |
| Pennine Care NHS Foundation Trust | 6 (2.9) |
| Rotherham, Doncaster & South Humber (RDaSH) | 8 (3.9) |
| Sheffield Health & Social Care Foundation Trust | 21 (10.3) |
| South Staffordshire and Shropshire Healthcare NHS Foundation Trust | 17 (8.3) |
| South West Yorkshire Partnership NHS Foundation Trust | 9 (4.4) |
| Worcestershire Health & Care NHS Trust | 1 (0.5) |
| Total | 204 (100.0) |
Psychological wellbeing practitioners: recruitment and characteristics
Recruitment
Psychological wellbeing practitioners were recruited from IAPT services within NHS trusts to deliver the trial interventions. In total, 204 PWPs from 15 NHS trusts attended the 3-day OCTET training. The number of PWPs trained from each trust ranged from 1 to 27, with an average of 13 per trust (see Table 3).
Psychological wellbeing practitioner characteristics
Of the 93 PWPs allocated OCTET patients, 68 returned a questionnaire detailing demographics, qualifications, OCD training and previous experience (Table 4).
| Characteristic | PWPs (N = 68) |
|---|---|
| Age (years) | |
| Range | 24–61 |
| Mean (SD) | 33.9 (10.8) |
| Sex (%) | |
| Female | 59 (87) |
| Male | 9 (13) |
| Highest educational qualification, n (%) | |
| Undergraduate degree | 12 (18) |
| Postgraduate certificate | 30 (44) |
| Higher education diploma | 1 (1) |
| Postgraduate diploma | 14 (21) |
| Master’s degree | 9 (13) |
| PhD | 1 (1) |
| Length of time in PWP role, n (%) | |
| 6 months to 1 year | 6 (9) |
| 1–2 years | 17 (25) |
| 2–5 years | 45 (66) |
| Length of time in mental health, n (%) | |
| Up to 1 year | 1 (1) |
| 1–5 years | 34 (50) |
| 5–10 years | 25 (37) |
| 10–20 years | 8 (11.8) |
| Received OCD training as part of IAPT training, n (%) | |
| Yes | 37 (54) |
| No | 31 (45) |
Most PWPs (87%) were female, aged between 24 and 61 years, with a mean age of 34 years. Their highest educational qualification was a postgraduate certificate/diploma (81%) and the majority (66%) were experienced in delivering psychological interventions with > 2 years in post.
As expected, most PWPs had experience of delivering guided self-help (94%), with a lower proportion reporting experience of delivering supported cCBT (69%). The types of low-intensity interventions most frequently delivered were behavioural activation (91%) and structured problem-solving (79%).
More than half of the PWPs had received some training in OCD as part of their IAPT training, but this had largely focused on identification rather than treatment.
Patient recruitment
Patients were recruited from IAPT services within 14 NHS trusts between February 2011 and May 2014, and all follow-up data collection was complete by May 2015. Participants were not recruited from one NHS trust as a result of waiting list reductions.
Revised sample size
Our original sample was 432; however, because of concerns about meeting the proposed retention rate (85%), we increased the target sample size to 472 (see Chapter 2, Sample size calculation). Subsequently, there was a small overshoot of recruitment and randomisation to 475, but there were also two post-randomisation exclusions because of a participant being aged < 18 years (n = 1, supported cCBT) and issues relating to participant suicide risk (n = 1, waiting list). Both exclusions were ratified with the DMEC, giving a trial sample of 473 participants. The Consolidated Standards of Reporting Trials (CONSORT) diagram for OCTET is shown in Figure 1. Of the 473 patients recruited, 158 were randomised to guided self-help, 157 to supported cCBT and 158 to a waiting list for high-intensity CBT. The number of patients recruited by site is shown in Table 5.
FIGURE 1.
The CONSORT flow chart illustrating recruitment participants into OCTET. a, Post-randomisation exclusion required because of a participant aged < 18 years (n = 1, cCBT) and risk issues relating to increased risk and not a change in supervisor decision (n = 1, waiting list). Source: Lovell et al. 84 This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

| Trust | Number of patients recruited |
|---|---|
| Bradford District Care Trust | 34 |
| Camden & Islington NHS Foundation Trust | 22 |
| Cheshire & Wirral Partnership NHS Foundation Trust | 62a |
| Coventry & Warwickshire Partnership Trust | 26a |
| Lancashire Care NHS Foundation Trust | 22 |
| Manchester Mental Health and Social Care Trust | 31 |
| NHS City Health Care Partnership Trust | 26 |
| Norfolk and Suffolk Mental Health NHS Foundation Trust | 87 |
| Nottingham County Health Partnership | 0 |
| Pennine Care NHS Foundation Trust | 28 |
| Rotherham, Doncaster & South Humber | 12 |
| Sheffield Health & Social Care Foundation Trust | 68 |
| South Staffordshire and Shropshire Healthcare NHS Foundation Trust | 18 |
| South West Yorkshire Partnership NHS Foundation Trust | 34 |
| Worcestershire Health & Care NHS Trust | 5 |
Retention
Retention rates were 81% at 3 months, 75% at 6 months and 71% at 12 months (Figure 2). Retention rates by group at 3 months were broadly similar: guided self-help was 82% (n = 130), supported cCBT was 77% (n = 121); and a waiting list for high-intensity CBT was 84% (n = 133). At 12 months, retention rates were: guided self-help at 72% (n = 114), supported cCBT at 67% (n = 105); and a waiting list for high-intensity CBT at 73% (n = 115). Participants who withdrew from the study are reported in the CONSORT flow chart (see Figure 2); more specific reasons for withdrawal are detailed in Appendix 5.
FIGURE 2.
The CONSORT flow chart illustrating retention of participants in OCTET.
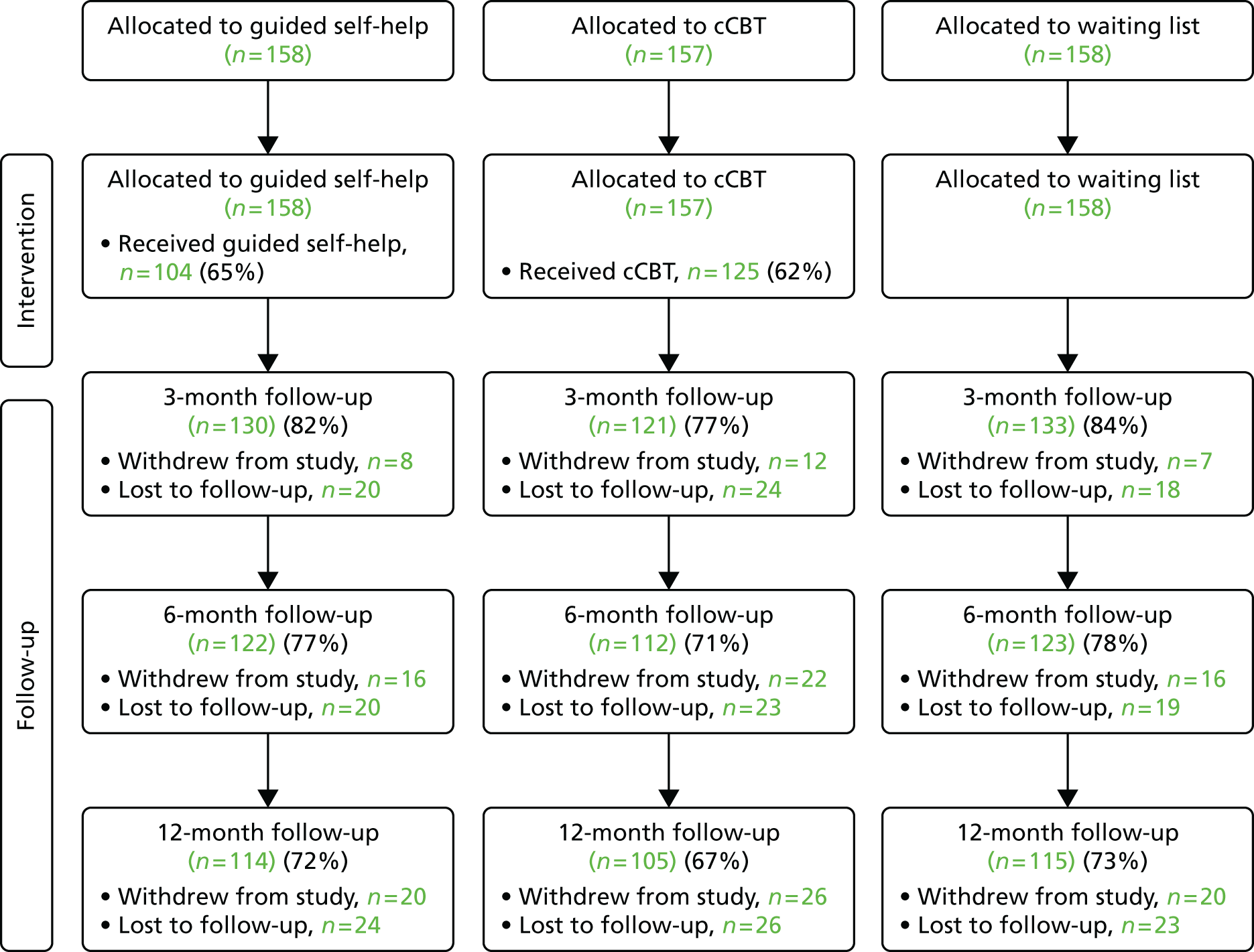
Patient baseline characteristics
Demographic characteristics
Baseline demographic characteristics are presented in Table 6 (variable counts are because of missing data items).
| Characteristic | Intervention | ||
|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | |
| Age (years), median (range) | 32.0 (18–77) | 32.8 (18–72) | 33.3 (19–66) |
| Sex, n (%) | |||
| Male | 66 (42.0) | 57 (36.1) | 65 (41.1) |
| Female | 91 (58.0) | 101 (63.9) | 93 (58.9) |
| Ethnicity, n (%) | |||
| White | 145 (92.4) | 154 (97.5) | 149 (94.3) |
| Non-white | 12 (7.6) | 4 (2.5) | 8 (5.1) |
| Missing | 0 (0) | 0 (0) | 1 (0.6) |
| Marital status, n (%) | |||
| Married/living with partner | 84 (53.5) | 81 (51.3) | 85 (53.8) |
| Other | 70 (44.6) | 75 (47.4) | 73 (46.2) |
| Missing | 3 (1.9) | 2 (1.3) | 0 (0) |
| Employment status,a n (%) | |||
| Employed | 86 (54.4) | 95 (60.1) | 97 (61.4) |
| Unemployed and seeking work | 10 (6.3) | 14 (8.9) | 9 (5.7) |
| Student | 17 (10.8) | 19 (12.0) | 17 (10.8) |
| Long-term sick/disabled receiving income support or incapacity benefit | 22 (14.0) | 23 (14.6) | 15 (9.5) |
| Homemaker, not actively seeking work | 15 (9.6) | 9 (5.7) | 11 (7.0) |
| Not receiving benefits and not actively seeking work | 1 (0.6) | 0 (0) | 1 (0.6) |
| Unpaid voluntary work and not actively seeking work | 1 (0.6) | 1 (0.6) | 0 (0) |
| Retired | 6 (3.8) | 5 (3.2) | 6 (3.8) |
| Missing | 2 (1.3) | 1 (0.6) | 5 (3.2) |
| Receiving statutory sick pay, n (%) | |||
| Yes | 8 (5.1) | 8 (5.1) | 11 (7.0) |
| No | 144 (91.7) | 146 (92.4) | 138 (87.3) |
| Missing | 5 (3.2) | 4 (2.5) | 9 (5.7) |
| Accessed previous OCD help, n (%) | |||
| Yes | 76 (48.4) | 86 (54.4) | 72 (45.6) |
| No | 80 (51.0) | 71 (44.9) | 85 (53.8) |
| Missing | 1 (0.6) | 1 (0.7) | 1 (0.6) |
| Education, n (%) | |||
| Below degree level | 107 (68.2) | 110 (69.6) | 112 (70.9) |
| Degree level or higher | 45 (28.6) | 43 (27.2) | 40 (25.3) |
| Missing | 5 (3.2) | 5 (3.2) | 6 (3.8) |
Of the 473 patients recruited, 95% were white, 60% were female and the median age was 33 years. Around three-quarters were employed, half were married or living with a partner, and 28% had been educated to degree level or above.
Just over 50% of participants reported previous professional help with OCD and around half were currently using antidepressant medication. Patients reported that the duration of their OCD was > 10 years (55%), 5–10 years (12%) or < 5 years (33%).
The groups were largely comparable in terms of employment status and receipt of statutory sick pay. Marginally fewer instances of long-term sickness/disability and being in receipt of associated benefits were found in the waiting list for high-intensity CBT group than in the intervention groups (9.5%, waiting list for high-intensity CBT; 14.0%, supported cCBT; 14.6%, guided self-help).
Patients detailed their treatment preferences prior to randomisation. They were asked whether they preferred to stay on the waiting list for high-intensity CBT, had no preference or preferred to receive a low-intensity intervention. Of the total sample, 42% preferred allocation to the intervention groups, 47% had no preference and 11% preferred to stay on the waiting list for high-intensity CBT.
Patients who were allocated to a low-intensity intervention were also asked if they would prefer supported cCBT or guided self-help. Preferences between the two low-intensity treatments were evenly balanced, with 50% not having a preference (Table 7).
| Characteristic | Intervention, n (%) | ||
|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | |
| Therapy preference | |||
| Wait for scheduled CBT | 15 (9.6) | 18 (11.4) | 17 (10.8) |
| Limited or no preference | 74 (47.1) | 78 (49.4) | 70 (44.3) |
| Low intensity | 68 (43.3) | 62 (39.2) | 70 (44.3) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Low-intensity preference | |||
| Supported cCBT | 32 (20.4) | 30 (19.0) | 47 (29.7) |
| No preference | 83 (52.9) | 83 (52.5) | 68 (43.0) |
| Guided self-help | 42 (26.7) | 44 (27.9) | 41 (26.0) |
| Missing | 0 (0.0) | 1 (0.6) | 2 (1.3) |
There were no striking differences in demographic characteristics at baseline.
Clinical characteristics
Baseline clinical characteristics are presented in Table 8 (variable counts are because of missing data). The mean baseline Y-BOCS-OR score was approximately 25 at baseline (indicating severe OCD). The mean PHQ-9 score was approximately 12 at baseline (indicative of mild to moderate depression), whereas GAD-7 scores were approximately 12.5 (indicating moderate anxiety).
| Characteristic | Intervention | ||
|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | |
| Current antidepressant medication, n (%) | |||
| Yes | 82 (52) | 81 (51) | 80 (51) |
| No | 75 (48) | 77 (49) | 78 (49) |
| OCD chronicity, n (%) | |||
| 0–5 years | 53 (34) | 52 (33) | 51 (32) |
| 6–9 years | 18 (11) | 18 (11) | 19 (12) |
| ≥ 10 years | 86 (55) | 88 (56) | 88 (56) |
| Y-BOCS-SR | |||
| Mean (SD) | 24.34 (5.1) | 24.18 (4.82) | 24.20 (4.99) |
| Median | 24 | 24 | 24 |
| Min., max. | 16, 36 | 16, 40 | 16, 38 |
| Baseline characteristics | |||
| Comorbidity (primary diagnosis), n (%) | |||
| Mixed anxiety and depressive disorder | 23 (15) | 23 (15) | 15 (10) |
| Mild depressive disorder | 18 (11) | 18 (11) | 20 (13) |
| Moderate depressive disorder | 28 (18) | 24 (15) | 26 (17) |
| Severe depressive disorder | 7 (5) | 13 (8) | 12 (8) |
| Generalised anxiety disorder | 18 (11) | 27 (17) | 18 (11) |
| Specific phobia | 10 (6) | 6 (4) | 6 (4) |
| Social phobia | 2 (1) | 1 (1) | 0 (0) |
| Agoraphobia | 0 (0) | 2 (1) | 3 (2) |
| Panic disorder | 2 (1) | 0 (0) | 5 (3) |
| Y-BOCS-ORa | |||
| Mean (SD) | 25.03 (5.45) | 25.01 (5.02) | 25.34 (5.44) |
| Median | 25 | 25 | 25 |
| Min., max. | 13, 38 | 14, 39 | 13, 38 |
| Missing | 0 | 0 | 0 |
| PHQ-9 | |||
| Mean (SD) | 11.90 (6.27) | 11.40 (6.56) | 11.93 (6.29) |
| Median | 12 | 11.5 | 12 |
| Min., max. | 0, 27 | 0, 26 | 0, 26 |
| GAD-7 | |||
| Mean (SD) | 12.90 (5.33) | 12.72 (5.56) | 12.52 (5.52) |
| Median | 13 | 14 | 13 |
| Min; max | 2, 21 | 1, 21 | 0, 21 |
| Missing | 2 | 4 | 4 |
| CORE-OM | |||
| Mean (SD) | 15.95 (6.27) | 15.23 (6.67) | 15.79 (6.63) |
| Median | 16 | 16 | 16 |
| Min., max. | 5, 35 | 1, 34 | 1, 33 |
| Missing | 3 | 3 | 5 |
| SF-36 – PCS | |||
| Mean (SD) | 54.39 (11.29) | 54.18 (9.57) | 54.09 (10.56) |
| Median | 57.36 | 56.01 | 57.14 |
| Min., max. | 18.04, 71.89 | 17.59, 70.35 | 22.21, 72.23 |
| Missing | 3 | 3 | 5 |
| SF-36 – MCS | |||
| Mean (SD) | 32.89 (9.87) | 33.86 (11.05) | 33.23 (11.71) |
| Median | 32.66 | 34.33 | 33.17 |
| Min., max. | 11.88, 59.52 | 7.30, 58.55 | 10.64, 65.08 |
| Missing | 3 | 3 | 5 |
| WSAS | |||
| Mean (SD) | 14.78 (9.85) | 15.05 (10.54) | 14.74 (9.66) |
| Median | 13 | 14 | 13 |
| Min., max. | 2, 21 | 1, 21 | 0, 21 |
| Missing | 2 | 4 | 4 |
Just over half of the patients were currently taking antidepressant medication and 69% of patients had a primary comorbid diagnosis, as derived from the CIS-R.
There were no striking differences in clinical characteristics at baseline.
Treatment delivery and fidelity
Allocation of patients to psychological wellbeing practitioners
Of the PWPs trained (n = 204), 93 (46%) were allocated an OCTET patient (Table 9). The number of PWPs allocated patients per trust ranged from 0 to 14, a range of 0 to 100% of those trained within each trust. PWPs were not allocated patients for various reasons: leaving the service, high clinical workload or insufficient patients compared with the number of therapists in each site. In total, 315 patients were allocated to the 93 PWPs. The number of patients allocated to PWPs ranged from 1 to 18. Nearly half the PWPs (46%, n = 42) were allocated patients in both supported cCBT and guided self-help; 33% (n = 30) were allocated only guided self-help patients, and 20.9% (n = 19) were allocated only supported cCBT patients.
| Trust | Number of PWPs (% of PWPs trained per trust) |
|---|---|
| Bradford District Care Trust | 10 (66.7) |
| Camden & Islington NHS Foundation Trust | 8 (44.4) |
| Cheshire & Wirral Partnership NHS Foundation Trust | 12 (60.0) |
| Coventry & Warwickshire Partnership Trust | 4 (80.0) |
| Lancashire Care NHS Foundation Trust | 4 (20.0) |
| Manchester Mental Health and Social Care Trust | 6 (85.7) |
| NHS City Health Care Partnership Trust | 5 (33.3) |
| Norfolk and Suffolk Mental Health NHS Foundation Trust | 11 (40.7) |
| Nottingham County Health Partnership | 0 (0.0) |
| Pennine Care NHS Foundation Trust | 4 (66.7) |
| Rotherham, Doncaster & South Humber (RDaSH) | 3 (37.5) |
| Sheffield Health & Social Care Foundation Trust | 15 (71.4) |
| South Staffordshire and Shropshire Healthcare NHS Foundation Trust | 4 (23.5) |
| South West Yorkshire Partnership NHS Foundation Trust | 6 (66.7) |
| Worcestershire Health & Care NHS Trust | 1 (100.0) |
| Total | 93 (45.6) |
Uptake of interventions
As detailed in Chapter 2, PWPs completed a contact sheet for each allocated participant and recorded session date, number and the length of the session. Of the 158 patients allocated to guided self-help, 155 (98%) contact sheets were received and of 157 patients allocated to supported cCBT, 154 (98%) contact sheets were available.
Uptake and adherence to guided self-help
Of the 158 patients randomised to guided self-help, 103 (65%) received at least one session of support from a PWP (Table 10). The mean number of guided self-help sessions provided was 4.11 (SD 4.29 sessions), the average length of session 1 was 56.49 minutes (as per protocol: 60 minutes) and the average length of sessions 2–11 was 30.79 minutes (as per protocol: 30 minutes). Nine patients were provided with more than the 11 sessions detailed in the protocol, eight received one extra session (session 12) and one received two extra sessions (sessions 12 and 13).
| Total number of guided self-help sessions attended | N (%) | Percentage receiving at least n sessions |
|---|---|---|
| 0 | 55 (34.8) | – |
| 1 | 9 (5.7) | 65.2 |
| 2 | 17 (10.8) | 59.5 |
| 3 | 7 (4.4) | 48.7 |
| 4 | 9 (5.7) | 44.3 |
| 5 | 6 (3.8) | 38.6 |
| 6 | 3 (1.9) | 34.8 |
| 7 | 10 (6.3) | 32.9 |
| 8 | 7 (4.4) | 26.6 |
| 9 | 6 (3.8) | 22.2 |
| 10 | 7 (4.4) | 18.4 |
| 11 | 13 (8.2) | 13.9 |
| 12 | 8 (5.1) | 5.7 |
| 13 | 1 (0.6) | 0.6 |
The mode of delivery of sessions was face to face only (n = 50, 49%), telephone only (n = 26, 25%) or mixed face to face/telephone (n = 23, 22%). For four patients the delivery mode was not recorded (4%).
Uptake and adherence to supported computerised cognitive–behavioural therapy
Of the 157 patients randomised to supported cCBT, 93 (59%) received at least one session of PWP support (Table 11). The mean number of supported cCBT sessions was 2.25 (SD 2.47) and the average session length was 13.42 minutes. Of the nine steps available on OCFighter, the mean number of steps completed was 3.64 (SD 3.19).
| Number of supported cCBT sessions attended | N (%) | Percentage receiving at least n sessions |
|---|---|---|
| 0 | 64 (40.8) | – |
| 1 | 17 (10.8) | 59.2 |
| 2 | 16 (10.2) | 48.4 |
| 3 | 13 (8.3) | 38.2 |
| 4 | 9 (5.7) | 29.9 |
| 5 | 7 (4.5) | 24.2 |
| 6 | 26 (16.6) | 19.7 |
| 7 | 3 (1.9) | 3.2 |
| 8 | 1 (0.6) | 1.3 |
| 9 | 1 (0.6) | 0.6 |
Delivery mode of sessions provided by PWPs to patients was telephone only (n = 90, 93%), mixed telephone and face to face (n = 3, 3%) and mixed telephone and e-mail (n = 1, 1%). For three patients delivery mode was not recorded.
Fidelity of delivery of guided self-help and supported computerised cognitive–behavioural therapy
Psychological wellbeing practitioners were asked to record all face-to-face and telephone sessions using a digital recorder and, when required, a telephone-recording device.
Owing to an ethics breach (a digital recorder was lost at one site), all recordings were stopped for 5 weeks. Even with this lapse, adherence to recording was low, with only 169 (26%) of a possible 648 guided self-help sessions recorded, and only 61 (17%) of a possible 350 supported cCBT sessions recorded.
All available session recordings were given a random number using an online random number generator and the independent rater randomly selected 25% of the guided self-help and supported cCBT recordings. The independent rater randomly selected 43 recordings from guided self-help sessions 1–12, and 17 recordings from supported cCBT sessions 1–6 to ensure an equal representation of each of the different stages of the interventions.
Of the guided self-help sessions, nine (21%) were rated as average, 24 (56%) were rated as good and 10 (23%) were rated as excellent. Of the supported cCBT session recordings, 11 (65%) were rated as good and six (35%) were rated as excellent.
The length of sessions on the digital audio files was examined to see if they were consistent with the self-report data available, as recorded on the contact sheets. For guided self-help the mean duration of session 1 (assessment) was 53 minutes (60 minutes was detailed in the PWP manual), the mean duration of session 2 (treatment rationale) was 35 minutes (30 minutes recommended), the mean duration of sessions 3–9 (setting weekly goals and support) was 26 minutes (30 minutes recommended) and the mean duration for sessions 10–12 (relapse prevention/staying well) was 19 minutes (recommended 30 minutes). The mean duration of sessions 1–6 for supported cCBT was 15 minutes (recommended 10 minutes).
In summary, PWP adherence was satisfactory, although the mean number of sessions for both guided self-help and supported cCBT was substantially less than recommended. A small number of patients receiving guided self-help had more sessions than in the protocol, although duration, as measured by self-report and digital audio files, was within the recommended limits. No extra sessions were delivered in the supported cCBT but both self-report and digital audio files revealed that the mean session length was 13 minutes, just over the recommended 10 minutes. Fidelity to the interventions was good, although only a limited number of digital recordings were available.
Uptake of cognitive–behavioural therapy prior to primary outcome assessment (3 months)
As designed, patients in OCTET were not expected to receive high-intensity CBT prior to the 3-month outcome assessment. However, because of changes in service delivery outside the control of OCTET, a number of patients started to receive high-intensity CBT prior to the 3-month assessment.
The Pathway questionnaire was used at each follow-up to collect data on high-intensity CBT uptake. Owing to the nature of the delivery and completion of this questionnaire (left with the participant to complete independently prior to postal return to the YTU), there were high levels of missing CBT uptake data when compared with the number of visits completed at each time point (28% missing at 3 months, 29% at 6 months and 30% at 12 months).
In addition, the CBT uptake data, as reported by the participant, did not always correspond to other patient-reported measures collecting treatment access data, nor did the data necessarily correspond to information provided by the IAPT services. As a result, to ensure validity and accuracy of the CBT uptake data, all sites were contacted to determine which participants had received a minimum of one session of high-intensity CBT prior to each follow-up time point.
In total, 67 (42%) of the patients allocated to the waiting list for high-intensity CBT group started full CBT prior to the primary outcome assessment, compared with 21% in supported cCBT and 23% in guided self-help (Table 12).
| Uptake | Intervention | Overall (n = 473) | ||
|---|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | ||
| No, n (%) | 123 (78.3) | 119 (75.3) | 92 (58.2) | 334 (70.6) |
| Yes, n (%) | 33 (21.0) | 37 (23.4) | 66 (41.8) | 136 (28.8) |
| Missing, n (%) | 1 (0.6) | 2 (1.3) | 0 (0.0) | 3 (0.6) |
Patients in waiting list for high-intensity CBT group had a significantly higher uptake of high-intensity CBT than supported cCBT (adjusted OR 0.36, 95% CI 0.19 to 0.68; p = 0.001) and guided self-help (adjusted OR 0.43, 95% CI 0.22 to 0.84; p = 0.014). There were no differences between supported cCBT and guided self-help (adjusted OR 0.84, 95% CI 0.47 to 1.51; p = 0.562). No other factors predicted uptake of full CBT (Table 13).
| Predictor | Adjusted OR | 95% CI | p-value |
|---|---|---|---|
| Exposure group | |||
| cCBT vs. WL | 0.36 | 0.19 to 0.68 | 0.001a |
| GSH vs. WL | 0.43 | 0.22 to 0.84 | 0.014 |
| cCBT vs. GSH | 0.84 | 0.47 to 1.51 | 0.562 |
| Baseline outcome measures | |||
| YBOC-OR | 1.02 | 0.97 to 1.07 | 0.462 |
| GAD-7 | 1.01 | 0.96 to 1.06 | 0.828 |
| PHQ-9 | 0.99 | 0.95 to 1.04 | 0.789 |
| Antidepressant medication | |||
| Yes | 1.02 | 0.66 to 1.57 | 0.931 |
| Duration of OCD (years) | |||
| 6–9 | 1.12 | 0.55 to 2.27 | 0.756 |
| ≥ 10 | 0.89 | 0.55 to 1.42 | 0.606 |
| Sex | |||
| Male | 1.21 | 0.78 to 1.88 | 0.395 |
| Exp (constant) | 0.44 | 0.14 to 1.36 | 0.157 |
Short-term clinical outcomes: primary
Aim 1: the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) versus waiting list for high-intensity CBT in the management of OCD at 3 months
As noted earlier, the 3-month follow-up point was taken as the primary assessment point following the internal pilot. As described in Chapter 1, Results of the internal pilot: (1) we retained the 6-month follow-up point and report these data for completeness and (2) valid values of Y-BOCS-OR where obtained for 346, 308 and 283 services users at 3, 6 and 12 months, respectively. Substitution of Y-BOCS-SR for missing values increased the sample available for analysis to 383 at 3 months, 356 at 6 months and 332 at 12 months.
Analysis of the primary outcome (Y-BOCS-OR) (Table 14) showed that the benefit of supported cCBT over waiting list for high-intensity CBT was less than 1 point on the Y-BOCS-OR (adjusted mean difference –0.71, 95% CI –2.12 to 0.70; p = 0.325) at 3 months.
| Primary outcome measure and time point | Intervention | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | Supported cCBT: waiting list | Guided self-help: waiting list | Supported cCBT: guided self-help | |||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | |
| Y-BOCS-OR | ||||||||||||||||||
| Baseline | 25.03 | 5.45 | 157 | 25.01 | 5.02 | 158 | 25.34 | 5.44 | 158 | |||||||||
| 3 months | 21.16 | 6.89 | 121 | 20.19 | 6.83 | 130 | 22.18 | 6.54 | 132 | –0.71 | –2.12 to 0.70 | 0.325 | –1.91 | –3.27 to –0.55 | 0.006b | 1.2 | –0.22 to 2.61 | 0.097 |
| 6 months | 18.96 | 7.26 | 112 | 18.7 | 7.7 | 122 | 20.29 | 7.27 | 122 | –1.13 | –2.84 to 0.58 | 0.195 | –1.32 | –3.00 to 0.35 | 0.121 | 0.19 | –1.51 to 1.90 | 0.824 |
| Y-BOCS-SR | ||||||||||||||||||
| Baseline | 24.34 | 5.1 | 157 | 24.18 | 4.82 | 158 | 24.2 | 4.99 | 158 | |||||||||
| 3 months | 20.46 | 7.06 | 119 | 19.8 | 6.9 | 128 | 20.88 | 6.48 | 127 | –0.43 | –1.79 to 0.93 | 0.531 | –1.31 | –2.65 to 0.04 | 0.056 | 0.87 | –0.49 to 2.23 | 0.209 |
| 6 months | 18.6 | 7.47 | 110 | 18.29 | 7.78 | 119 | 19.34 | 7.24 | 118 | –0.87 | –2.52 to 0.78 | 0.3 | –1.17 | –2.87 to 0.53 | 0.178 | 0.3 | –1.42 to 2.02 | 0.735 |
There was evidence of benefit of guided self-help over a waiting list for high-intensity CBT (adjusted mean difference –1.91, 95% CI –3.27 to –0.55; p = 0.006) at 3 months. This was statistically significant at the Bonferroni-corrected significance level (1.67%). This was < 3 points on the Y-BOCS-OR defined as a ‘clinically important difference’ in the sample size calculation, although the 95% CIs did include an effect of this magnitude.
Comparison of guided self-help with supported cCBT showed a reduction of around 1 point on the Y-BOCS-OR for guided self-help as compared with supported cCBT (adjusted mean difference 1.2, 95% CI –0.22 to 2.61; p = 0.097) at 3 months.
Similar trends were observed for Y-BOCS-SR, although no significant differences between treatments were observed at the 1.67% significance level used.
Short-term clinical outcomes: secondary
Analyses of the secondary outcomes at 3 months are shown in Table 15.
| Secondary outcome measure and time point | Intervention | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | Supported cCBT: waiting list | Guided self-help: waiting list | Supported cCBT: guided self-help | |||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | |
| SF-36 version 2 PCS | ||||||||||||||||||
| Baseline | 54.4 | 11.3 | 154 | 54.2 | 9.6 | 155 | 54.1 | 10.6 | 153 | |||||||||
| 3 months | 53.6 | 10.8 | 104 | 53.5 | 10.0 | 117 | 53.9 | 10.0 | 123 | 0.14 | –1.58 to 1.87 | 0.870 | –0.38 | –2.22 to 1.46 | 0.686 | 0.52 | –1.51 to 2.56 | 0.614 |
| 6 months | 53.4 | 10.8 | 91 | 53.9 | 10.0 | 107 | 51.8 | 10.7 | 107 | 0.64 | –1.46 to 2.75 | 0.550 | 1.36 | –0.43 to 3.15 | 0.140 | –0.71 | –2.74 to 1.31 | 0.490 |
| SF-36 version 2 MCS | ||||||||||||||||||
| Baseline | 32.9 | 9.9 | 154 | 33.9 | 11.1 | 155 | 33.2 | 11.7 | 153 | |||||||||
| 3 months | 37.0 | 11.7 | 104 | 36.3 | 12.1 | 117 | 35.6 | 11.5 | 123 | 1.48 | –1.12 to 4.09 | 0.264 | 0.46 | –1.82 to 2.73 | 0.694 | 1.03 | –1.57 to 3.62 | 0.438 |
| 6 months | 38.9 | 10.8 | 91 | 37.3 | 12.6 | 107 | 38.1 | 11.9 | 107 | 0.87 | –1.90 to 3.63 | 0.540 | –1.66 | –4.36 to 1.04 | 0.230 | 2.53 | –0.25 to 5.30 | 0.070 |
| PHQ-9 | ||||||||||||||||||
| Baseline | 11.9 | 6.3 | 157 | 11.4 | 6.6 | 158 | 11.9 | 6.3 | 158 | |||||||||
| 3 months | 9.3 | 6.5 | 105 | 9.1 | 6.0 | 118 | 9.6 | 6.0 | 124 | –0.48 | –1.66 to 0.70 | 0.427 | –0.34 | –1.48 to 0.81 | 0.565 | –0.14 | –1.33 to 1.05 | 0.815 |
| 6 months | 8.3 | 6.1 | 96 | 8.9 | 5.8 | 107 | 8.5 | 5.9 | 106 | –0.09 | –1.42 to 1.24 | 0.890 | 0.73 | –0.53 to 1.99 | 0.260 | –0.82 | –2.10 to 0.46 | 0.210 |
| GAD-7 | ||||||||||||||||||
| Baseline | 12.9 | 5.3 | 155 | 12.7 | 5.6 | 154 | 12.5 | 5.5 | 154 | |||||||||
| 3 months | 9.9 | 5.9 | 104 | 10.6 | 5.7 | 115 | 11.2 | 5.8 | 124 | –1.50 | –2.67 to –0.33 | 0.012b | –0.77 | –1.91 to 0.37) | 0.186 | –0.73 | –1.97 to 0.50 | 0.245 |
| 6 months | 9.4 | 5.9 | 94 | 9.4 | 5.6 | 107 | 9.4 | 5.9 | 107 | –0.24 | –1.63 to 1.15 | 0.730 | 0.15 | –1.19 to 1.49 | 0.830 | –0.39 | –1.78 to 0.99 | 0.580 |
| CORE-OM | ||||||||||||||||||
| Baseline | 16.0 | 6.3 | 154 | 15.2 | 6.7 | 155 | 15.8 | 6.6 | 153 | |||||||||
| 3 months | 12.9 | 6.9 | 104 | 13.0 | 6.6 | 116 | 13.5 | 6.7 | 124 | –0.38 | –1.61 to 0.85 | 0.550 | –0.20 | –1.38 to 0.98 | 0.734 | –0.17 | –1.48 to 1.14 | 0.797 |
| 6 months | 11.8 | 6.8 | 90 | 12.8 | 6.9 | 106 | 12.3 | 6.7 | 107 | –0.16 | –1.61 to 1.28 | 0.830 | 1.30 | –0.13 to 2.74 | 0.080 | –1.47 | –2.93 to –0.01 | 0.050 |
| WSAS | ||||||||||||||||||
| Baseline | 14.78 | 9.85 | 153 | 15.05 | 10.54 | 154 | 14.74 | 9.66 | 154 | |||||||||
| 3 months | 12.98 | 11.01 | 104 | 13.70 | 10.41 | 117 | 13.42 | 9.65 | 123 | –0.55 | –2.35 to 1.26 | 0.554 | 0.02 | –1.17 to 1.75 | 0.985 | –0.56 | –2.38 to 1.25 | 0.544 |
| 6 months | 12.46 | 11.09 | 94 | 13.11 | 10.53 | 106 | 12.48 | 9.83 | 107 | –0.06 | –2.24 to 2.12 | 0.956 | 0.60 | –1.56 to 2.76 | 0.587 | –0.66 | –2.87 to 1.55 | 0.557 |
There were no statistically significant effects at 3 months on physical and mental functioning of the SF-36, depression (PHQ-9) or distress (CORE-OM), but there was a statistically significant effect of supported cCBT versus a waiting list for high-intensity CBT at 3 months on anxiety (GAD-7) (adjusted mean difference –1.50, 95% CI –2.67 to –0.33; p = 0.012).
Patient satisfaction
Analysis of the satisfaction outcome (CSQ-8) showed no differences in the satisfaction of patients receiving supported cCBT compared with those allocated to the waiting list for high-intensity CBT (adjusted mean difference –0.31, 95% CI –2.07 to 1.45; p = 0.732) at 3 months (Table 16).
| Satisfaction outcome and time point | Intervention | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | Supported cCBT: waiting list | Guided self-help: waiting list | Supported cCBT: guided self-help | |||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | |
| 3 months | 22.4 | 5.9 | 92 | 24.4 | 5.5 | 101 | 22.8 | 6.1 | 83 | –0.31 | –2.07 to 1.45 | 0.732 | 1.69 | –0.04 to 3.42 | 0.055 | –2.00 | –3.63 to –0.37 | 0.016b |
| 6 months | 23.8 | 5.8 | 81 | 24.3 | 6.3 | 82 | 24.6 | 5.8 | 77 | –0.87 | –2.77 to 1.03 | 0.371 | –0.31 | –2.23 to 1.61 | 0.751 | –0.56 | –2.44 to 1.32 | 0.561 |
Analysis of the satisfaction outcome (CSQ-8) showed that patients receiving guided self-help were more satisfied than those allocated to a waiting list for high-intensity CBT (adjusted mean difference 1.69, 95% CI –0.04 to 3.42; p = 0.055) at 3 months. The effect estimate did not reach statistical significance according to the corrected significance level (1.67%).
Analysis of the satisfaction outcome (CSQ-8) showed that patients receiving supported cCBT were less satisfied than those receiving guided self-help (adjusted mean difference –2.00, 95% CI –3.63 to –0.37; p = 0.016) at 3 months. The effect estimate did reach statistical significance according to the corrected significance level (1.67%).
Longer-term clinical outcomes: primary
Aim 2: the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) plus high-intensity CBT versus waiting list for high-intensity CBT plus high-intensity CBT in the management of OCD at 12 months
Analysis of the primary outcome (Y-BOCS-OR) showed that the benefit of supported cCBT over a waiting list for high-intensity CBT was around 1.5 points (adjusted mean difference –1.37, 95% CI –3.59 to 0.84; p = 0.224) at 12 months (Table 17).
| Outcome measure and time point | Intervention | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supported cCBT | Guided self- help | Waiting list | Supported cCBT: waiting list | Guided self-help: waiting list | Supported cCBT: guided self-help | |||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | Adjusted mean differencea | 95% CI | p-valueb | |
| Y-BOCS-OR | ||||||||||||||||||
| Baseline | 25.03 | 5.45 | 157 | 25.01 | 5.02 | 158 | 25.34 | 5.44 | 158 | |||||||||
| 12 months | 16.14 | 8.69 | 105 | 15.19 | 8.35 | 113 | 17.93 | 8.07 | 114 | –1.37 | –3.59 to 0.84 | 0.224 | –2.37 | –4.37 to –0.38 | 0.020 | 1.00 | –1.19 to 3.19 | 0.371 |
| Y-BOCS-SR | ||||||||||||||||||
| Baseline | 24.34 | 5.1 | 157 | 24.18 | 4.82 | 158 | 24.2 | 4.99 | 158 | |||||||||
| 12 months | 15.61 | 8.7 | 101 | 15.72 | 8.11 | 109 | 17.38 | 8.24 | 107 | –1.45 | –3.67 to 0.76 | 0.198 | –1.52 | –3.54 to 0.49 | 0.137 | 0.07 | –2.01 to 2.16 | 0.946 |
| SF-36 version 2 PCS | ||||||||||||||||||
| Baseline | 54.4 | 11.3 | 154 | 54.2 | 9.6 | 155 | 54.1 | 10.6 | 153 | |||||||||
| 12 months | 54.7 | 9.7 | 84 | 53.4 | 10.0 | 97 | 53.1 | 10.9 | 98 | 0.69 | –1.20 to 2.57) | 0.474 | –0.37 | –2.18 to 1.43 | 0.685 | 1.06 | –0.82 to 2.94 | 0.269 |
| SF-36 version 2 MCS | ||||||||||||||||||
| Baseline | 32.9 | 9.9 | 154 | 33.9 | 11.1 | 155 | 33.2 | 11.7 | 153 | |||||||||
| 12 months | 43.0 | 11.6 | 84 | 40.7 | 12.7 | 97 | 40.0 | 11.4 | 98 | 2.29 | –0.94 to 5.51 | 0.165 | 0.13 | –2.79 to 3.05 | 0.932 | 2.16 | –1.11 to 5.43 | 0.195 |
| PHQ-9 | ||||||||||||||||||
| Baseline | 11.9 | 6.3 | 157 | 11.4 | 6.6 | 158 | 11.9 | 6.3 | 158 | |||||||||
| 12 months | 6.4 | 5.9 | 86 | 7.1 | 5.9 | 99 | 7.7 | 6.1 | 98 | –1.35 | –2.96 to 0.25 | 0.098 | –0.41 | –1.78 to 0.96 | 0.557 | –0.94 | –2.50 to 0.62 | 0.236 |
| GAD-7 | ||||||||||||||||||
| Baseline | 12.9 | 5.3 | 155 | 12.7 | 5.6 | 154 | 12.5 | 5.5 | 154 | |||||||||
| 12 months | 7.8 | 6.0 | 84 | 8.0 | 5.8 | 100 | 9.0 | 6.0 | 98 | –1.04 | –2.64 to 0.55 | 0.199 | –0.89 | –2.43 to 0.64 | 0.253 | –0.15 | –1.70 to 1.40 | 0.849 |
| CORE-OM | ||||||||||||||||||
| Baseline | 16.0 | 6.3 | 154 | 15.2 | 6.7 | 155 | 15.8 | 6.6 | 153 | |||||||||
| 12 months | 9.7 | 7.1 | 82 | 10.7 | 7.4 | 97 | 11.3 | 7.4 | 98 | –0.81 | –2.65 to 1.02 | 0.385 | –0.16 | –1.92 to 1.60 | 0.857 | –0.65 | –2.47 to 1.16 | 0.482 |
| WSAS | ||||||||||||||||||
| Baseline | 14.78 | 9.85 | 153 | 15.05 | 10.54 | 154 | 14.74 | 9.66 | 154 | |||||||||
| 12 Months | 10.65 | 10.34 | 84 | 11.94 | 11.11 | 99 | 12.37 | 10.69 | 98 | –0.39 | –3.16 to 2.38 | 0.782 | –0.31 | –2.67 to 2.06 | 0.798 | –0.08 | –2.82 to 2.65 | 0.952 |
Analysis of the primary outcome (Y-BOCS-OR) showed that the benefit of guided self-help over waiting list for high-intensity CBT was around 2.5 points (adjusted mean difference –2.37, 95% CI –4.37 to –0.38; p = 0.02) at 12 months, although the effect estimate did not reach statistical significance according to the Bonferroni-corrected significance level (1.67%).
The benefit of guided self-help over supported cCBT was 1 point on the Y-BOCS-OR, but this was not statistically significant (adjusted mean difference –1.00, 95% CI –1.19 to 3.19; p = 0.37).
Longer-term clinical outcomes: secondary
Analyses of the secondary outcomes at 12 months are also shown in Table 17.
There were no statistically significant effects at 12 months on physical and mental functioning (SF-36), depression (PHQ-9), distress (CORE-OM) or anxiety (GAD-7).
Intracluster correlation coefficients caused by therapist variation and design effects
Sample size calculation and statistical analysis allowed for ICC for therapist variation. Estimates of the ICC are given in Table 18 for the primary outcome and quantitative secondary outcome measures. These were calculated from the variance components of the linear mixed-model analyses. The estimation procedure (restricted maximum likelihood) constrains estimates of the random-effects variance to be positive. Twenty of the 52 estimates had a magnitude < 0.001. Estimates of the ICC were highly variable with a maximum of 0.225 for SF-36 mental functioning.
| Outcome measure | Time point | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | 12 months | ||||
| Supported cCBT | Guided self-help | Supported cCBT | Guided self-help | Supported cCBT | Guided self-help | |
| Y-BOCS-OR | 0.027 | < 0.001 | < 0.001 | < 0.001 | 0.109 | 0.001 |
| Y-BOCS-SR | 0.003 | 0.009 | < 0.001 | 0.078 | 0.098 | 0.004 |
| SF-36 version 2 PCS | 0.182 | 0.178 | 0.164 | 0.004 | 0.011 | 0.002 |
| SF-36 version 2 MCS | 0.225 | < 0.001 | 0.018 | 0.048 | 0.090 | 0.007 |
| PHQ-9 | < 0.001 | < 0.001 | 0.019 | < 0.001 | 0.160 | 0.011 |
| GAD-7 | 0.028 | 0.030 | < 0.001 | < 0.001 | 0.151 | 0.082 |
| WSAS | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.158 | 0.012 |
| CORE-OM | 0.026 | 0.003 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| CSQ-8 | < 0.001 | < 0.001 | 0.041 | 0.097 | – | – |
When designing the trial we assumed that the interventions would be delivered by 24 therapists, each with an average caseload of six supported cCBT and six guided self-help patients. The number of therapists was somewhat larger (see Table 9) making the study power less sensitive to larger values of the ICC.
The observed design effect can be calculated as the ratio of the variance of the treatment effect estimate when cluster is accounted for in a random-effects model to the same variance in an ordinary least squares model that takes no account of clustering. As there are three treatment comparisons there are three design effects for each end point. For the primary outcome measure, the largest value of the three design effects was 1.035 at 3 months when supported cCBT was compared with a waiting list. At 6 months, design effects were 1 as the ICC was negligible (< 0.001). At 12 months, the largest design effect was 1.129, again when supported cCBT was compared with a waiting list. The design effects of the sample size calculation (see Chapter 2, Sample size calculation) were 1.123 for the comparison of supported cCBT or guided self-help with a waiting list and 1.061 when supported cCBT was compared with guided self-help. The design effect of the study was similar to that hypothesised in the trial protocol.
A 98.33% CI corresponds to a 1.67% significance level that we have used for hypothesis testing. For the comparison of cCBT against a waiting list, the 98.33% CI is –4.07 to 1.33, and for guided self-help against a waiting list it is –4.81 to 0.06. Given that the upper limits are small, we can conclude that both active interventions are non-inferior to waiting list.
Longer-term employment status
At 12 months, employment levels had increased in the waiting list for high-intensity CBT and guided self-help groups (Table 19), compared with the 6-month follow-up.
| Employment status | Intervention, n (%) | ||
|---|---|---|---|
| Supported cCBT (n = 98) | Guided self-help (n = 109) | Waiting list (n = 109) | |
| Employed | 45 (45.9) | 63 (57.8) | 58 (53.2) |
| Unemployed and seeking work | 8 (8.2) | 3 (2.8) | 5 (4.6) |
| Student | 6 (6.1) | 11 (10.1) | 7 (6.4) |
| Long-term sick/disabled receiving income support or incapacity benefit | 8 (8.2) | 16 (14.7) | 14 (12.8) |
| Homemaker: not actively seeking work | 10 (10.2) | 5 (4.6) | 7 (6.4) |
| Not receiving benefits and not actively seeking work | 0 (0) | 1 (0.9) | 1 (0.9) |
| Unpaid voluntary work and not actively seeking work | 3 (3.1) | 0 (0) | 2 (1.8) |
| Retired | 5 (5.1) | 4 (3.7) | 6 (5.5) |
Unemployment levels had also decreased compared with baseline, except in the supported cCBT group, in which levels had increased since both the baseline and 6-month follow-up time points.
Receipt of statutory sick pay also reduced over the course of follow-up. The guided self-help group reported greater levels of sick pay receipt than the supported cCBT and waiting list for high-intensity CBT groups. Levels of receipt of sick pay gradually reduced during the trial. In the guided self-help group, sick pay initially increased but by the 12-month time point had fallen compared with baseline.
Recovery and remission
We used the recently published international expert consensus guidelines for defining treatment response and remission in OCD. 69
-
Responders are defined as those who achieved a ≥ 35% reduction on the Y-BOCS-OR.
-
Remitters are defined as those with a Y-BOCS-OR score of ≤ 12.
Logistic regression models were fitted to responses at 12 months adjusting for sex, baseline GAD-7 and PHQ-9 scores, antidepressant medication use at randomisation and duration of OCD.
Consistent with the statistical analysis plan for other outcome measures, we conducted an additional analysis of differences in rates of response. At 12 months, response to treatment was higher in the supported cCBT and guided self-help groups than in the waiting list for high-intensity CBT group, but neither analysis reached statistical significance (supported cCBT adjusted OR 1.76, 95% CI 0.99 to 3.15; p = 0.055; guided self-help adjusted OR 1.79, 95% CI 0.94 to 3.40; p = 0.077) (Table 20).
| Response and remission rates | Intervention | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||||||||
| Frequency | % | n | Frequency | % | n | Frequency | % | n | Frequency | % | n | |
| Response | ||||||||||||
| 3 months | 20 | 16.5 | 121 | 28 | 21.9 | 128 | 17 | 12.9 | 132 | 65 | 17.1 | 381 |
| 6 months | 39 | 34.8 | 112 | 45 | 37.2 | 121 | 30 | 24.6 | 122 | 114 | 32.1 | 355 |
| 12 months | 55 | 52.4 | 105 | 59 | 52.2 | 113 | 44 | 38.6 | 114 | 158 | 47.6 | 332 |
| Remission | ||||||||||||
| 3 months | 15 | 12.4 | 121 | 11 | 8.6 | 128 | 14 | 10.6 | 132 | 40 | 10.5 | 381 |
| 6 months | 22 | 19.6 | 112 | 30 | 24.8 | 121 | 19 | 15.6 | 122 | 71 | 20.0 | 355 |
| 12 months | 38 | 36.2 | 105 | 48 | 42.5 | 113 | 31 | 27.2 | 114 | 117 | 35.2 | 332 |
Longer-term cognitive–behavioural therapy uptake
In total, 136 (86%) of the patients allocated to the waiting list for high-intensity CBT started CBT by the end of the trial, compared with 98 (62%) in supported cCBT and 90 (57%) in guided self-help (Table 21).
| Uptake of CBT | Intervention, n (%) | Overall, n (%) (n = 473) | ||
|---|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | ||
| 6 months | ||||
| No | 68 (43.3) | 72 (45.6) | 38 (24.1) | 178 (37.6) |
| Yes | 76 (48.4) | 69 (43.7) | 113 (71.5) | 258 (54.6) |
| Missing | 13 (8.3) | 17 (10.8) | 7 (4.4) | 37 (7.8) |
| 12 months | ||||
| No | 44 (28.0) | 50 (31.7) | 17 (10.8) | 111 (23.5) |
| Yes | 98 (62.4) | 90 (56.9) | 136 (86.1) | 324 (68.5) |
| Missing | 15 (9.6) | 18 (11.4) | 5 (3.2) | 38 (8.0) |
A logistic regression model was fitted to uptake of high-intensity CBT at 6 and 12 months adjusting for sex, baseline GAD-7 and PHQ-9 scores, antidepressant medication use at randomisation and duration of OCD (Table 22).
| Uptake predictor | Adjusted OR | 95% CI | p-value |
|---|---|---|---|
| 6 months | |||
| Exposure group | |||
| cCBT vs. WL | 0.42 | 0.24 to 0.73 | 0.002b |
| GSH vs. WL | 0.48 | 0.22 to 1.03 | 0.06 |
| cCBT vs. GSH | 0.87 | 0.42 to 1.84 | 0.718 |
| Baseline outcome measures | |||
| Y-BOCS-OR | 1.02 | 0.97 to 1.06 | 0.514 |
| GAD-7 | 0.99 | 0.94 to 1.04 | 0.591 |
| PHQ-9 | 1.02 | 0.98 to 1.07 | 0.271 |
| Antidepressant medication | |||
| Yes | 0.71 | 0.46 to 1.09 | 0.117 |
| Duration of OCD (years) | |||
| 6–9 | 1.26 | 0.60 to 2.64 | 0.552 |
| ≥ 10 | 0.89 | 0.55 to 1.42 | 0.619 |
| Sex | |||
| Male | 1.12 | 0.73 to 1.73 | 0.606 |
| Exp (constant) | 2.14 | 0.67 to 6.82 | 0.201 |
| 12 months | |||
| Exposure group | |||
| cCBT vs. WL | 0.34 | 0.15 to 0.79 | 0.011b |
| GSH vs. WL | 0.27 | 0.12 to 0.60 | 0.001b |
| cCBT vs. GSH | 1.27 | 0.53 to 3.00 | 0.59 |
| Baseline outcome measures | |||
| Y-BOCS-OR | 1.03 | 0.97 to 1.08 | 0.36 |
| GAD-7 | 1.03 | 0.97 to 1.08 | 0.341 |
| PHQ-9 | 0.99 | 0.94 to 1.04 | 0.73 |
| Antidepressant medication | |||
| Yes | 1.02 | 0.63 to 1.67 | 0.933 |
| Duration of OCD (years) | |||
| 6–9 | 2.66 | 1.03 to 6.89 | 0.043 |
| ≥ 10 | 0.99 | 0.59 to 1.67 | 0.968 |
| Sex | |||
| Male | 1.25 | 0.76 to 2.03 | 0.395 |
| Exp (constant) | 2.86 | 0.76 to 10.81 | 0.121 |
At 12 months the adjusted OR was significantly lower in supported cCBT than in the waiting list for high-intensity CBT group (adjusted OR 0.34, 95% CI 0.15 to 0.79; p = 0.011). For guided self-help, the adjusted OR of starting high-intensity treatment as compared with the waiting list for high-intensity CBT group was also significantly lower (adjusted OR 0.27, 95% CI 0.12 to 0.60; p = 0.001).
As noted in Chapter 1, Summary, there was a number of potential hypotheses concerning the longer-term outcomes of patients in OCTET, and the pattern of findings with respect to clinical outcomes, high-intensity CBT uptake and overall costs.
The broad pattern of similar clinical outcomes, and significantly lower uptake of higher intensity CBT in the low-intensity treatments arms, is potentially a positive outcome for low-intensity interventions. However, it is important to explore whether or not patients who received low-intensity interventions and did not go on to access higher-intensity CBT demonstrated poor outcomes. We therefore present descriptive statistics comparing the use of low-intensity interventions among patients who did and did not access high-intensity CBT, and the clinical outcomes of those groups.
Table 23 shows the number of supported cCBT and guided self-help support sessions received by patients that did or did not go on to receive high-intensity CBT by 12 months.
| Low-intensity intervention | High-intensity CBT received? | Number of sessions | Number of patients | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min. | Max. | |||
| Supported cCBT | No | 3.33 | 2.55 | 2.5 | 0 | 8 | 30 |
| Yes | 3.95 | 2.44 | 4 | 0 | 9 | 65 | |
| Guided self-help | No | 5.41 | 4.63 | 5 | 0 | 12 | 41 |
| Yes | 5.1 | 3.96 | 4.5 | 0 | 13 | 70 | |
In Table 24, we present descriptive data on the mean scores of patients in the low-intensity arms according to their pattern of high-intensity CBT use.
| Y-BOCS-OR (proxy included) | Allocation | CBT received? | Number of sessions | Number of patients | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min. | Max. | ||||
| Baseline | cCBT | No | 24.05 | 5.66 | 22.5 | 16 | 36 | 44 |
| Yes | 25.16 | 5.25 | 25.5 | 13 | 38 | 98 | ||
| GSH | No | 24.7 | 5.21 | 25 | 14 | 35 | 50 | |
| Yes | 25.43 | 4.71 | 25 | 14 | 39 | 90 | ||
| WL | No | 23.82 | 4.65 | 23 | 15 | 33 | 17 | |
| Yes | 25.59 | 5.59 | 26 | 13 | 38 | 136 | ||
| 3 months | cCBT | No | 19.42 | 7.6 | 20 | 6 | 35 | 33 |
| Yes | 21.63 | 6.62 | 23 | 7 | 36 | 83 | ||
| GSH | No | 20.19 | 7.5 | 20.5 | 1 | 31 | 42 | |
| Yes | 20.59 | 6.26 | 20 | 0 | 36 | 79 | ||
| WL | No | 22.13 | 6.08 | 22 | 11 | 31 | 15 | |
| Yes | 22.17 | 6.66 | 23 | 7 | 36 | 114 | ||
| 6 months | cCBT | No | 18.34 | 7.29 | 19 | 5 | 33 | 29 |
| Yes | 19.00 | 7.36 | 19.5 | 0 | 36 | 78 | ||
| GSH | No | 18.51 | 8.26 | 19 | 0 | 32 | 41 | |
| Yes | 18.96 | 7.58 | 19 | 1 | 35 | 75 | ||
| WL | No | 21.67 | 6.36 | 21.5 | 12 | 33 | 12 | |
| Yes | 20.11 | 7.41 | 21 | 0 | 35 | 107 | ||
| 12 months | cCBT | No | 16.81 | 8.71 | 15 | 1 | 31 | 31 |
| Yes | 15.67 | 8.69 | 15 | 0 | 37 | 72 | ||
| GSH | No | 16.37 | 8.45 | 18 | 0 | 36 | 38 | |
| Yes | 14.59 | 8.29 | 13 | 0 | 36 | 75 | ||
| WL | No | 20.07 | 7.01 | 21 | 10 | 32 | 14 | |
| Yes | 17.59 | 8.23 | 17 | 0 | 35 | 99 | ||
Such a comparison does not have the benefits afforded by randomisation, but the data do not suggest that those who accessed only low-intensity treatments demonstrated markedly different outcomes than those who accessed both low- and high-intensity interventions.
Sensitivity and subgroup analyses
The trial protocol specified that a moderator analysis of severity would be conducted by adding a treatment severity interaction term to the analysis model of the primary outcome (YBOC-OR) at the primary end point (3 months), with severity measured by YBOC-OR at baseline. In addition, analysis of treatment effect moderation by both age and chronicity of OCD (< 5 or ≥ 5 years) was proposed and included in the statistical analysis plan.
The interactions of treatment with both chronicity and severity were hypothesised to be quantitative, whereas interactions of treatment with age were hypothesised to be qualitative, representing potential difficulties of older patients in engaging with supported cCBT relative to guided self-help. Thus, a significant treatment effect would be required before assessing treatment moderation via severity and chronicity of OCD. This was found and so all three analyses were carried out.
When an interaction of treatment with YBOC-OR at baseline was added to the model there was no evidence of an effect (p = 0.44; degrees of freedom 2). When an interaction between chronicity and treatment was added there was again no evidence of an effect (p = 0.37; degrees of freedom 2). When the interaction between treatment (supported cCBT vs. guided self-help) and age was fitted, the interaction with age was in the hypothesised direction with YBOC-OR scores increasing for supported cCBT relative to guided self-help in older patients, but this result was small and not statistically significant (0.027, 95% CI –0.084 to 0.138; p = 0.63). Note that this represents a 1.35-point increase in YBOC-OR scores for supported cCBT compared with guided self-help across the approximate 50-year age range observed in the OCTET cohort.
Unblinding
As described in Chapter 2, we collated data on unblinding. Information was collected regarding the point at which unblinding occurred and if the outcome was partial (treatment/no treatment) or full (trial arm) unblinding. Data were available for 278 3-month interviews, 255 6-month interviews and 264 12-month interviews. Unblinding was reported to have occurred in 30%, 22% and 26% of the 3-, 6- and 12-month interviews, respectively.
Where unblinding occurred, the majority of follow-up visits were conducted by another researcher at the site to limit the introduction of any bias to the outcome assessment.
Adverse events
During the trial, 13 serious AEs were reported. Of these, 10 were related to unplanned hospitalisation, one event related to significant disability and two events related to a potential risk to life (self-harm and suicidality). Twelve events were deemed to be unrelated to the trial treatment. One event was deemed to be possibly related – OCD and another comorbidity resulting in the participant leaving permanent employment. Eight events were deemed to be unexpected, whereas five were deemed to be expected. No events were deemed to be suspected, unexpected serious AEs.
During the trial, 173 non-serious AEs were reported. A total of 111 events were reported detailing any untoward medical occurrence experienced by the participant. Following changes to the AE reporting procedure (see Chapter 2), reporting became more targeted to the condition of interest. This resulted in a further 62 events being reported, of which 32 related to a change in mental health status, four related to dissatisfaction in trial procedures, 12 related to a change in psychotropic medication use and 14 related to unplanned hospital visits (not involving emergency inpatient admission).
Chapter 4 Economic evaluation: results
Response rates
The response rates for the AD-SUS, the EQ-5D-3L and the PWP intervention data are summarised in Table 25. One hundred per cent of data were available for intervention provision in all three arms, and > 60% of AD-SUS data and 50% of EQ-5D-3L data were available for the total sample at all four time points. AD-SUS and EQ-5D-3L data availability were similar across the groups at each follow-up, but were generally lower in the supported cCBT group.
| Data response rates | Intervention, n (%) | Total, n (%) (n = 473) | ||
|---|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | ||
| PWP intervention | ||||
| Baseline | 157 (100) | 158 (100) | 158 (100) | 473 (100) |
| 3 months | 157 (100) | 158 (100) | 158 (100) | 473 (100) |
| 6 months | 157 (100) | 158 (100) | 158 (100) | 473 (100) |
| 12 months | 157 (100) | 158 (100) | 158 (100) | 473 (100) |
| AD-SUS | ||||
| Baseline | 156 (99.4) | 156 (98.7) | 155 (98.1) | 467 (98.7) |
| 3 months | 118 (75.2) | 130 (82.3) | 129 (81.7) | 377 (79.7) |
| 6 months | 102 (65.0) | 115 (72.8) | 117 (74.1) | 334 (70.6) |
| 12 months | 88 (56.1) | 100 (63.3) | 100 (63.3) | 288 (60.9) |
| EQ-5D-3L | ||||
| Baseline | 155 (98.7) | 155 (98.1) | 154 (97.5) | 464 (98.1) |
| 3 months | 104 (66.2) | 117 (74.1) | 124 (78.5) | 345 (72.9) |
| 6 months | 94 (59.9) | 105 (66.5) | 106 (67.1) | 305 (64.5) |
| 12 months | 84 (53.5) | 100 (63.3) | 99 (62.7) | 283 (59.8) |
Table 26 summarises the percentage of participants with all intervention, AD-SUS and EQ-5D-3L data at the 3-month and 12-month analysis points. Just over 70% of participants (334/473) had both baseline and 3-month cost and outcome data, allowing them to be included in complete-case economic analyses at the 3-month analysis point. Slightly fewer than 50% of participants (231/473) had all cost and outcome data at baseline and all follow-up points, and could thus be included in complete-case economic analyses at the 12-month analysis point.
| Analysis point | Intervention, n (%) | Total, n (%) (n = 473) | ||
|---|---|---|---|---|
| Supported cCBT (n = 157) | Guided self-help (n = 158) | Waiting list (n = 158) | ||
| All data 3 months | 104 (66) | 113 (72) | 117 (74) | 334 (71) |
| All data 12 months | 71 (45) | 76 (48) | 84 (53) | 231 (49) |
Table 27 compares the baseline characteristics of the full sample with those participants with all economic data for the 3- and 12-month economic analyses. The samples with enough data to be included in complete-case economic analyses were very similar to the full sample in terms of sex, ethnicity, age, number of years with OCD, Y-BOCS-SR and EQ-5D-3L score and costs.
| Characteristics | Full sample (n = 473) | Full data for 3-month analyses (n = 334) | Full data for 12-month analyses (n = 231) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 188 (39.8) | 137 (41.0) | 100 (43.3) |
| Female | 285 (60.3) | 197 (59.0) | 131 (56.7) |
| Ethnicity, n (%) | |||
| White British | 431 (91.3) | 305 (91.6) | 215 (93.1) |
| Other | 41 (8.7) | 28 (8.4) | 16 (6.9) |
| Age (years), mean (SD) | 35.86 (12.4) | 36.22 (12.7) | 37.76 (13.3) |
| Years with OCD, mean (SD) | 13.93 (12.2) | 13.74 (12.4) | 15.03 (12.9) |
| Y-BOCS-SR, mean (SD) | 24.24 (5.0) | 24.24 (4.9) | 24.20 (4.8) |
| EQ-5D-3L utility, mean (SD) | 0.67 (0.27) | 0.67 (0.27) | 0.66 (0.27) |
| Costs (£), mean (SD) | 625 (1149) | 604 (1125) | 641 (1182) |
Resource use
Resource use by group is reported in Tables 28–30, for the 6 months prior to baseline, the 3-month period from baseline to first follow-up and the 12-month period from baseline to last follow-up. Individual point resource use in the 3–6 and 6–12 month periods are presented in Appendix 6. Hospital inpatient services varied and mostly involved admission to general wards. Hospital outpatient services used also varied and no one type of service was predominant. Outpatient mental health contacts were low.
| Resource | Unit | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Supported cCBT (n = 156) | Guided self-help (n = 156) | Waiting list (n = 155) | |||||
| n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | ||
| Hospital | |||||||
| Inpatient | Nights | 7 (4.5) | 5.17 (8.0) | 9 (5.8) | 2.58 (1.3) | 12 (7.7) | 3.20 (3.4) |
| Outpatient | Attendances | 42 (26.9) | 2.50 (1.7) | 45 (28.9) | 2.64 (3.6) | 48 (30.8) | 3.68 (7.8) |
| A&E | Attendances | 19 (12.2) | 1.16 (0.4) | 23 (14.7) | 1.13 (0.3) | 28 (18.1) | 1.96 (4.0) |
| Community | |||||||
| GP at surgery | Contacts | 149 (95.5) | 3.77 (3.1) | 142 (91.0) | 4.60 (4.2) | 144 (92.9) | 4.33 (4.1) |
| GP at home | Contacts | 2 (1.3) | 3.00 (2.8) | 0 (0.0) | 0.00 (0.00) | 3 (1.9) | 1.33 (0.6) |
| GP by telephone | Contacts | 20 (12.8) | 2.85 (4.2) | 24 (15.4) | 2.58 (3.0) | 33 (21.3) | 3.27 (4.1) |
| Practice nurse | Contacts | 39 (25.0) | 1.95 (1.3) | 63 (40.4) | 1.92 (1.6) | 49 (31.6) | 1.82 (1.4) |
| District nurse | Contacts | 6 (3.9) | 5.83 (7.3) | 6 (3.9) | 2.17 (1.9) | 9 (5.8) | 3.78 (2.9) |
| NHS walk-in clinic | Contacts | 9 (5.8) | 1.22 (0.7) | 9 (5.8) | 1.33 (0.7) | 13 (8.4) | 1.46 (0.9) |
| Community psychiatric nurse | Contacts | 7 (4.5) | 5.29 (9.2) | 2 (1.3) | 1.00 (–) | 9 (5.8) | 1.67 (1.7) |
| Psychiatrist in community | Contacts | 6 (3.9) | 1.67 (0.82) | 8 (5.1) | 3.00 (2.33) | 9 (5.8) | 1.33 (0.71) |
| Occupational therapist | Contacts | 6 (3.9) | 2.17 (1.94) | 5 (3.2) | 2.80 (2.49) | 6 (3.9) | 1.83 (1.17) |
| Marriage counselling | Contacts | 1 (0.6) | 1.00 (–) | 0 (0.0) | 0.00 (0.00) | 2 (1.3) | 3.00 (2.83) |
| Social worker | Contacts | 1 (0.6) | 12.00 (–) | 4 (2.6) | 6.25 (6.7) | 3 (1.9) | 5.67 (4.5) |
| Advice service | Contacts | 4 (2.6) | 3.50 (3.1) | 13 (8.3) | 1.92 (1.1) | 13 (8.4) | 4.00 (5.8) |
| Helpline | Contacts | 0 (0.0) | 0.00 (0.0) | 7 (4.5) | 2.29 (1.6) | 10 (6.4) | 1.30 (0.5) |
| Day centre/drop-in | Contacts | 0 (0.0) | 0.00 (0.0) | 1 (0.6) | 25.00 (–) | 1 (0.7) | 3.00 (–) |
| Complementary therapy | Contacts | 8 (5.1) | 3.13 (2.7) | 8 (5.1) | 4.75 (6.4) | 3 (1.9) | 5.00 (1.00) |
| Other psychological therapies | Contacts | 39 (25.0) | 5.37 (6.7) | 38 (24.4) | 5.61 (4.5) | 43 (27.7) | 6.27 (4.5) |
| Psychotropic medication | Yes/no | 81 (51.9) | – | 84 (53.9) | – | 79 (51.0) | – |
| Time off work | Days | 21 (13.5) | 24.3 (36.7) | 25 (16.0) | 21.0 (30.0) | 29 (18.7) | 20.7 (30.8) |
| Resource | Unit | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Supported cCBT (n = 105) | Guided self-help (n = 118) | Waiting list (n = 121) | |||||
| n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | ||
| Hospital | |||||||
| Inpatient | Nights | 3 (2.9) | 7.33 (11.0) | 3 (2.5) | 1.00 (0.00) | 3 (2.5) | 1.00 (0.00) |
| Outpatient | Attendances | 24 (22.9) | 1.96 (1.4) | 27 (22.9) | 2.07 (1.5) | 28 (23.1) | 3.14 (4.8) |
| A&E | Attendances | 5 (4.8) | 1.00 (0.00) | 6 (5.1) | 1.17 (0.4) | 11 (9.1) | 1.09 (0.30) |
| Community | |||||||
| GP at surgery | Contacts | 67 (63.8) | 2.43 (1.9) | 79 (67.0) | 2.35 (1.5) | 78 (64.5) | 2.73 (2.6) |
| GP by telephone | Contacts | 7 (6.7) | 1.57 (1.5) | 12 (10.2) | 1.75 (0.8) | 17 (14.1) | 2.00 (1.27) |
| GP at home | Contacts | 0 (0.0) | 0.00 (0.0) | 0 (0.0) | 0.00 (0.00) | 0 (0.0) | 0.00 (0.00) |
| Practice nurse | Contacts | 22 (21.0) | 1.23 (0.4) | 20 (17.0) | 1.60 (0.8) | 16 (13.2) | 1.69 (1.5) |
| District nurse | Contacts | 5 (4.8) | 1.40 (0.6) | 8 (6.8) | 1.13 (0.4) | 2 (1.7) | 3.50 (3.5) |
| NHS walk-in clinic | Contacts | 2 (1.9) | 1.00 (0.00) | 6 (5.1) | 1.33 (0.8) | 3 (2.5) | 1.00 (0.00) |
| Community psychiatric nurse | Contacts | 1 (1.0) | 1.00 (–) | 1 (0.9) | 1.00 (–) | 2 (1.7) | 2.50 (2.1) |
| Psychiatrist in community | Contacts | 1 (1.0) | 2.00 (–) | 2 (1.7) | 1.00 (0.00) | 3 (2.5) | 1.33 (0.6) |
| Occupational therapist | Contacts | 4 (3.8) | 1.75 (1.0) | 3 (2.5) | 4.67 (3.5) | 1 (0.8) | 6.00 (–) |
| Marriage counselling | Contacts | 1 (1.0) | 5.00 (–) | 1 (0.9) | 8.00 (–) | 2 (1.7) | 3.00 (2.8) |
| Social worker | Contacts | 0 (0.0) | 0.00 (0.00) | 3 (2.5) | 1.67 (0.6) | 1 (0.8) | 3.00 (–) |
| Advice service | Contacts | 3 (2.9) | 1.67 (0.58) | 5 (4.2) | 1.80 (1.8) | 5 (4.1) | 6.20 (10.6) |
| Helpline | Contacts | 0 (0.0) | 0.00 (0.00) | 0 (0.0) | 0.00 (0.0) | 5 (4.1) | 1.40 (0.6) |
| Day centre/drop-in | Contacts | 0 (0.0) | 0.00 (0.00) | 1 (0.9) | 24.00 (–) | 1 (0.8) | 8.00 (–) |
| Complementary therapy | Contacts | 2 (1.9) | 3.00 (2.83) | 2 (1.7) | 2.00 (1.4) | 2 (1.7) | 5.50 (3.5) |
| Other psychological therapies | Contacts | 30 (28.6) | 5.14 (4.13) | 37 (31.4) | 5.52 (2.9) | 62 (51.2) | 4.71 (3.8) |
| Psychotropic medication | Yes/no | 48 (45.7) | – | 54 (45.8) | – | 48 (39.7) | – |
| Time off work | Days | 5 (4.8) | 15.1 (25.4) | 5 (4.2) | 18.0 (26.7) | 6 (5.0) | 20.2 (21.3) |
| Resource | Unit | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Supported cCBT (n = 88) | Guided self-help (n = 100) | Waiting list (n = 100) | |||||
| n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | n (%) of users | Mean (SD) of those using | ||
| Hospital | |||||||
| Inpatient | Nights | 7 (8.0) | 3.9 (7.1) | 6 (6.0) | 2.3 (2.0) | 9 (9.0) | 2.8 (2.9) |
| Outpatient | Attendances | 27 (30.7) | 4.4 (5.1) | 36 (36.0) | 3.8 (3.6) | 42 (42.0) | 4.9 (5.5) |
| A&E | Attendances | 10 (11.4) | 1.4 (0.7) | 20 (20.0) | 1.3 (0.6) | 24 (24.0) | 1.5 (0.7) |
| Community | |||||||
| GP at surgery | Contacts | 74 (84.1) | 5.7 (4.3) | 90 (90.0) | 6.3 (3.8) | 89 (89.0) | 7.5 (10.1) |
| GP at home | Contacts | 1 (1.1) | 2.00 (–) | 1 (1.0) | 1.00 (–) | 0 (0.0) | 0.0 (0.0) |
| GP by telephone | Contacts | 13 (14.8) | 1.5 (1.1) | 19 (19.0) | 2.2 (1.4) | 24 (24.0) | 3.0 (2.5) |
| Practice nurse | Contacts | 31 (35.2) | 2.2 (1.6) | 43 (43.0) | 2.9 (5.2) | 34 (34.0) | 2.9 (3.6) |
| District nurse | Contacts | 6 (6.8) | 2.5 (2.74) | 9 (9.0) | 3.00 (4.2) | 10 (10.0) | 2.3 (1.6) |
| NHS walk-in clinic | Contacts | 5 (5.7) | 1.2 (0.5) | 11 (11.0) | 1.4 (0.7) | 11 (11.0) | 1.6 (0.9) |
| Community psychiatric nurse | Contacts | 3 (3.4) | 9.3 (14.4) | 3 (3.0) | 2.7 (1.5) | 4 (4.0) | 4.3 (5.9) |
| Psychiatrist in community | Contacts | 4 (4.6) | 2.3 (1.3) | 4 (4.0) | 2.3 (1.0) | 9 (9.0) | 3.3 (5.6) |
| Occupational therapist | Contacts | 4 (4.6) | 1.8 (1.0) | 4 (4.0) | 4.3 (3.0) | 3 (3.0) | 3.0 (2.0) |
| Marriage counselling | Contacts | 0 (0.0) | 0.00 (0.00) | 1 (1.0) | 1.00 (–) | 2 (2.0) | 3.0 (2.8) |
| Social worker | Contacts | 2 (2.3) | 3.5 (3.5) | 2 (2.0) | 4.5 (5.0) | 3 (3.0) | 4.0 (1.0) |
| Advice service | Contacts | 5 (5.7) | 3.8 (3.5) | 8 (8.0) | 3.6 (3.6) | 9 (9.0) | 8.9 (15.4) |
| Helpline | Contacts | 0 (0.0) | 0.0 (0.0) | 3 (3.0) | 1.3 (0.6) | 5 (5.0) | 4.4 (7.1) |
| Day centre/drop-in | Contacts | 0 (0.0) | 0.0 (0.0) | 4 (4.0) | 13.0 (12.2) | 1 (1.0) | 15.0 (–) |
| Complementary therapy | Contacts | 7 (8.0) | 4.3 (7.4) | 3 (3.0) | 7.0 (2.7) | 6 (6.0) | 3.8 (2.5) |
| Other psychological therapies | Contact | 71 (80.7) | 11.2 (8.2) | 76 (76.0) | 10.1 (6.4) | 94 (94.0) | 11.4 (6.5) |
| Psychotropic medication | Yes/no | 52 (59.1) | – | 61 (61.0) | – | 49 (49.0) | – |
| Time off work | Days | 9 (10.2) | 10.8 (7.6) | 10 (10.0) | 23.2 (37.7) | 12 (12.0) | 52.0 (64.4) |
Cognitive–behavioural therapy was the most commonly reported psychological therapy, with a much smaller number reporting the use of counselling and other psychotherapies. The pattern of access to psychological therapies, primarily CBT, is similar to the CBT data from clinical records reported in Chapter 3, with a larger proportion of the waiting list group accessing psychological therapies between baseline and the 3-month follow-up (51%) than either the supported cCBT group (29%) or the guided self-help group (31%), and between baseline and 12 months (94% compared with 81% for supported cCBT and 76% for the guided self-help group).
Use of other resources appears similar for all randomisation arms across all time points. Services most commonly used at baseline and over the follow-up period included contacts with GPs at the GP surgery or by telephone, contacts with GP practice nurses, outpatient attendances, accident and emergency attendances, use of psychological therapies and psychotropic medication. There was no obvious pattern to the differences between the three groups, with service use being similar between groups at each time point.
The percentage of participants taking time off work because of their OCD was relatively low at all time points and was highest in the 6 months before trial entry (14% in supported cCBT group, 16% in the guided self-help group and 19% in the CBT group). Over the follow-up periods, the proportion of participants reporting time off work in all groups was approximately 5% from baseline to 3-month follow-up and approximately 10% from baseline to 12-month follow-up. For those who reported time off work because of OCD, mean days off work were similar at baseline (mean days 24, 21 and 21, respectively) and 3-month follow-up (mean days 15, 18 and 20, respectively) but were substantially higher in the waiting list group for the full 12-month follow-up period (mean 52 days compared with 11 days for supported cCBT and 23 days for guided self-help).
Out-of-pocket expenses and savings were variable and some items were difficult for participants to value. As a result, some out-of-pocket items had to be excluded from the analysis, so the cost and savings calculations are, therefore, underestimated.
Participants attributed over 800 individual out-of-pocket expenses or savings to their OCD over the four time points. By far the most commonly reported items of expense were cleaning materials including (but not limited to) antibacterial gel, antibacterial wipes, hand wash, soap, toiletries, bleach and other household cleaners. Other items were highly variable, but examples included extra petrol for additional journeys required to complete checking, throwing away food as a result of contamination concerns and spending sprees to improve mood. Cost savings reported were mostly because of avoidance of, or inability to do, certain things, such as socialising or participating in leisure activities (e.g. attending the gym, eating out or going on holiday) or being very careful with money (e.g. avoiding spending money, careful control of finances, heating and electricity).
Tables 31 and 32 describe the number and proportion of participants who reported out-of-pocket expenses and savings at each time point by randomisation allocation. Out-of-pocket expenses were far more commonly reported than out-of-pocket savings. On the whole, the percentage of people reporting out-of-pocket expenses reduced over time in all three groups and a slightly lower percentage of the supported cCBT group reported such expenses, compared with guided self-help and the waiting list for high-intensity CBT groups. Out-of-pocket savings were reported so rarely that no conclusions can be drawn.
| Time point | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Number of participants reporting (n/N) | Percentage | Number of participants reporting (n/N) | Percentage | Number of participants reporting (n/N) | Percentage | |
| Baseline | 57/156 | 36.5 | 62/156 | 39.7 | 62/155 | 40.0 |
| 3 months | 31/105 | 29.5 | 39/118 | 33.1 | 42/121 | 34.7 |
| 6 months | 21/95 | 22.1 | 31/107 | 29.0 | 35/108 | 32.4 |
| 12 months | 20/88 | 22.7 | 33/100 | 33.0 | 28/100 | 28.0 |
| Time point | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Number of participants reporting (n/N) | Percentage | Number of participants reporting (n/N) | Percentage | Number of participants reporting (n/N) | Percentage | |
| Baseline | 0/156 | 0 | 0/156 | 0 | 3/155 | 1.9 |
| 3 months | 0/105 | 0 | 1/118 | 0.9 | 2/121 | 1.7 |
| 6 months | 3/95 | 3.2 | 1/107 | 0.9 | 2/108 | 1.9 |
| 12 months | 2/88 | 2.3 | 0/100 | 0 | 1/100 | 1.0 |
Costs
Supported computerised cognitive–behavioural therapy and guided self-help
Table 33 provides details of resources and costs in the two low-intensity interventions. The mean cost of guided self-help (£383) was over twice that of supported cCBT (£155) as a result of the greater number and length of the PWP contacts.
| Cost component | Intervention, mean (SD) | |
|---|---|---|
| Supported cCBT | Guided self-help | |
| Number of sessions attended | 2.3 (2.5) | 4.11 (4.3) |
| Total session minutes | 30.2 (38.6) | 142.9 (146.1) |
| Cost of materials (£) | 63.7 (0) | 5.5 (0) |
| Cost of training (£) | 18.8 (20.7) | 34.5 (35.9) |
| Cost of PWP contacts (£) | 72.4 (92.6) | 343.0 (350.7) |
| Total cost (£) | 154.9 (111.1) | 383.0 (385.0) |
Health- and social-care services
Cost components over the three follow-up periods (from baseline to 3 months, between 3 and 6 months and between 6 and 12 months) are summarised in Table 34 and cost comparisons between groups are presented in Table 35.
| Cost component | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Valid n | Mean (SD), £ | Valid n | Mean (SD), £ | Valid n | Mean (SD), £ | |
| Intervention | 157 | 155 (111) | 158 | 383 (385) | 158 | 0 (0) |
| Baseline to 3 months | ||||||
| Health- and social-care services | 118 | 366 (366) | 130 | 315 (402) | 129 | 400 (501) |
| Employment losses | 118 | 87 (799) | 130 | 103 (763) | 129 | 53 (346) |
| Out-of-pocket expenses | 118 | 138 (1104) | 130 | 71 (168) | 129 | 113 (339) |
| Out-of-pocket savings | 118 | 0 (0) | 130 | 0 (2) | 129 | –3 (24) |
| Between 3 and 6 months | ||||||
| Health- and social-care costs | 102 | 407 (556) | 115 | 368 (521) | 117 | 529 (626) |
| Employment losses | 102 | 100 (813) | 115 | 25 (212) | 117 | 28 (151) |
| Out-of-pocket expenses | 102 | 44 (173) | 115 | 48 (119) | 117 | 98 (249) |
| Out-of-pocket savings | 102 | –3 (19) | 115 | –1 (12) | 117 | –9 (67) |
| Between 6 and 12 months | ||||||
| Health- and social-care costs | 88 | 663 (934) | 100 | 546 (546) | 100 | 792 (828) |
| Employment losses | 88 | 10 (86) | 100 | 64 (455) | 100 | 199 (1875) |
| Out-of-pocket expenses | 88 | 131 (372) | 100 | 128 (295) | 100 | 200 (493) |
| Out-of-pocket savings | 88 | –62 (555) | 100 | 0 (0) | 100 | –13 (130) |
| Cost component | Intervention comparison, £ (95% CI; p-value) | |||||
|---|---|---|---|---|---|---|
| Supported cCBT vs. waiting list | Guided self-help vs. waiting list | Supported cCBT vs. guided self-help | ||||
| Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | |
| Intervention costs | 155 (134 to 175; < 0.001)c | 158 (137 to 180; < 0.001)c | 383 (316 to 449; < 0.001)c | 383 (313 to 450; < 0.001)c | –228 (–299 to –157; < 0.001)c | –223 (–298 to 149; < 0.001)c |
| Baseline to 3 months | ||||||
| Health- and social-care costs | –34 (–310 to 242; 0.808) | –25 (–276 to 227; 0.848) | –85 (–198 to 28; 0.140) | –76 (–184 to 32; 0.167) | 51 (–200 to 301; 0.691) | 51 (–184 to 287; 0.669) |
| Employment losses | 33 (–129 to 196; 0.686) | 39 (–117 to 196; 0.622) | 49 (–95 to 194; 0.505) | –16 (–95 to 62; 0.686) | –16 (–221 to 190; 0.881) | 56 (–99 to 210; 0.480) |
| Out-of-pocket expenses | 25 (–194 to 243; 0.825) | 28 (–191 to 247; 0.801) | –42 (–108 to 25; 0.220) | –35 (–101 to 31; 0.298) | 66 (–146 to 279; 0.540) | 63 (–146 to 272; 0.554) |
| Out-of-pocket savings | 3 (–1 to 7; 0.217) | 0 (–1 to 1; 0.948) | 2 (–2 to 6; 0.259) | 0 (–2 to 1; 0.704) | 0 (0 to 1; 0.311) | 0 (0 to 1; 0.325) |
| Between 3 and 6 months | ||||||
| Health- and social-care costs | –122 (–273 to 30; 0.115) | –102 (–262 to 59; 0.213) | –160 (–308 to –13; 0.034) | –130 (–291 to 32; 0.116) | 39 (–96 to 173; 0.572) | 28 (–95 to 150; 0.656) |
| Employment losses | 72 (–91 to 235; 0.387) | 71 (–88 to 230; 0.382) | –3 (–51 to 45; 0.909) | –13 (–71 to 44; 0.655) | 75 (–91 to 241; 0.378) | 84 (–75 to 243; 0.299) |
| Out-of-pocket expenses | –53 (–110 to 3; 0.062) | –47 (–105 to 12; 0.116) | –50 (–101 to 2; 0.060) | –40 (–88 to 8; 0.103) | –4 (–43 to 35; 0.847) | –7 (–47 to 33; 0.731) |
| Out-of-pocket savings | 6 (–7 to 19; 0.373) | 6 (–9 to 20; 0.451) | 8 (–5 to 20; 0.218) | 8 (–6 to 21; 0.267) | –2 (–7 to 2; 0.372) | –2 (–7 to 3; 0.406) |
| Between 6 and 12 months | ||||||
| Health- and social-care costs | –129 (–390 to 132; 0.333) | –108 (–356 to 140; 0.394) | –246 (–443 to –48; 0.015)c | –216 (–413 to –18; 0.033) | 117 (–101 to 335; 0.293) | 108 (–96 to 311; 0.300) |
| Employment losses | –189 (–567 to 189; 0.327) | –213 (–621 to 196; 0.307) | –135 (–526 to 257; 0.501) | –191 (–580 to 198; 0.336) | –54 (–150 to 41; 0.266) | –22 (–103 to 60; 0.599) |
| Out-of-pocket expenses | –69 (–199 to 61; 0.296) | –59 (–179 to 62; 0.339) | –72 (–190 to 45; 0.226) | –54 (–166 to 57; 0.342) | 3 (–88 to 94; 0.947) | –5 (–88 to 79; 0.915) |
| Out-of-pocket savings | –49 (–176 to 77; 0.447) | –44 (–167 to 80; 0.489) | 13 (–12 to 38; 0.316) | 17 (–22 to 55; 0.400) | –62 (–185 to 61; 0.324) | –60 (–179 to 58; 0.320) |
Health- and social-care services (the largest cost component in each arm) were lowest in the guided self-help group and highest in the waiting list for high-intensity CBT group over all three time periods. At the adjusted p-value threshold of 0.0167, only the intervention costs were significantly different between the groups, with the intervention costs in the supported cCBT group (unadjusted mean difference £155, 95% CI £134 to £175; p < 0.001; adjusted mean difference £158, 95% CI £137 to £180; p < 0.001) and the guided self-help group (unadjusted mean difference £383, 95% CI £316 to £449; p < 0.001; adjusted mean difference £383, 95% CI £313 to £450; p < 0.001) being higher in the adjusted and unadjusted analyses, and with the cCBT group having lower intervention costs than the guided self-help group (unadjusted mean difference –£228, 95% CI –£299 to –£157; p < 0.001; adjusted mean difference –£223, 95% CI –£298 to –£149; p < 0.001).
Productivity losses and out-of-pocket expenses and savings
Productivity losses were highest in the guided self-help group between baseline and 3 months, in the supported cCBT group between 3 and 6 months and in the waiting list for high-intensity CBT group between 6 and 12 months, but there were no significant differences between either supported cCBT or guided self-help compared with the waiting list for high-intensity CBT over any of the time periods. Out-of-pocket expenses also varied greatly between groups and over the three time periods, but there were no significant differences. Out-of-pockets savings were minimal over all time periods in all arms (see Tables 34 and 35).
Table 36 reports total health- and social-care costs and total societal costs (including productivity losses and out-of-pocket expenses and savings) for the two economic analysis periods: baseline to 3 months and baseline to 12 months.
| Costs | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Valid n | Mean cost, £ (SD) | Valid n | Mean cost, £ (SD) | Valid n | Mean cost, £ (SD) | |
| Baseline to 3 months | ||||||
| Health- and social-care costsa | 118 | 529 (1521) | 130 | 756 (557) | 129 | 400 (501) |
| Societal costsb | 118 | 754 (2435) | 130 | 930 (1059) | 129 | 564 (774) |
| Baseline to 12 months | ||||||
| Health- and social-care costsa | 88 | 1691 (2353) | 100 | 1696 (1155) | 100 | 1714 (1288) |
| Societal costsb | 88 | 2207 (3664) | 100 | 2141 (1786) | 100 | 2400 (2636) |
Table 37 details the results of the statistical comparisons between the two self-help interventions and between the waiting list for high-intensity CBT group and the supported cCBT and guided self-help groups.
| Cost component | Intervention comparison, £ (95% CI; p-value) | |||||
|---|---|---|---|---|---|---|
| Supported cCBT vs. waiting list | Guided self-help vs. waiting list | Supported cCBT vs. guided self-help | ||||
| Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | |
| Baseline to 3 months | ||||||
| Health- and social-care costsc | 129 (–157 to 415; 0.375) | 138 (–123 to 399; 0.300) | 356 (207 to 505; < 0.001)d | 364 (220 to 507; < 0.001)d | –227 (–508 to 54; 0.113) | –226 (–492 to 41; 0.097) |
| Societal costse | 190 (–266 to 646; 0.413) | 202 (–201 to 605; 0.327) | 366 (112 to 620; 0.005)d | 310 (123 to 498; 0.001)d | –176 (–637 to 285; 0.454) | –109 (–523 to 305; 0.606) |
| Baseline to 12 months | ||||||
| Health- and social-care costsc | –24 (–697 to 649; 0.945) | –9 (–602 to 583; 0.976) | –18 (–384 to 347; 0.921) | 24 (–334 to 381; 0.896) | –5 (–591 to 581; 0.986) | –33 (–540 to 475; 0.899) |
| Societal costse | –193 (–1242 to 856; 0.719) | –159 (–1066 to 748; 0.731) | –259 (–935 to 416; 0.452) | –355 (–951 to 242; 0.244) | 66 (–843 to 976; 0.886) | 196 (–555 to 946; 0.610) |
Over the baseline to 3-month period, guided self-help was the most expensive group and the waiting list for high-intensity CBT group was the least expensive at the 0.0167 p-value threshold. The guided self-help group was significantly more expensive in unadjusted and adjusted analyses at 3 months than the waiting list for high-intensity CBT group in terms of both health- and social-care costs and societal costs, but no other significant differences were detected.
Over the full baseline to 12-month period (primary end point), differences between the groups had disappeared for health- and social-care costs, which were almost identical between the three groups. In terms of societal costs, guided self-help was the cheapest group and the waiting list for high-intensity CBT group the most expensive, although these differences were not significant. Tables 38 and 39 report the same results but with missing data imputed, and show very similar results with no changes in terms of statistical significance.
| Costs | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Valid n | Mean cost, £ (SD) | Valid n | Mean cost, £ (SD) | Valid n | Mean cost, £ (SD) | |
| Baseline to 3 months | ||||||
| Health- and social-care costsa | 157 | 535 (1321) | 158 | 721 (518) | 158 | 443 (472) |
| Societal costsb | 157 | 739 (2115) | 158 | 894 (973) | 158 | 608 (721) |
| Baseline to 12 months | ||||||
| Health- and social-care costsa | 157 | 1665 (1798) | 158 | 1676 (976) | 158 | 1737 (1143) |
| Societal costsb | 157 | 2174 (2823) | 158 | 2154 (1534) | 158 | 2352 (2216) |
| Cost component | Intervention comparison, £ (95% CI; p-value) | |||||
|---|---|---|---|---|---|---|
| Supported cCBT vs. waiting list | Guided self-help vs. waiting list | Supported cCBT vs. guided self-help | ||||
| Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | Unadjusted mean differencea | Adjusted mean differenceb | |
| Baseline to 3 months | ||||||
| Health- and social-care costsc | 92 (–130 to 314; 0.418) | 115 (–99 to 328; 0.293) | 278 (153 to 402; < 0.001)d | 297 (182 to 411; < 0.001)d | –186 (–395 to 24; 0.082) | –182 (–389 to 24; 0.084) |
| Societal costse | 132 (–222 to 485; 0.465) | 161 (–478 to 469; 0.305) | 286 (74 to 499; 0.008)d | 307 (118 to 495; 0.001)d | –155 (–520 to 210; 0.406) | –145 (–472 to 181; 0.383) |
| Baseline to 12 months | ||||||
| Health- and social-care costsc | –71 (–471 to 328; 0.727) | –18 (–372 to 336; 0.920) | –60 (–330 to 209; 0.660) | 24 (–205 to 253; 0.836) | –11 (–336 to 314; 0.947) | –42 (–346 to 261; 0.785) |
| Societal costse | –178 (–796 to 440; 0.573) | –119 (–636 to 399; 0.654) | –198 (–665 to 269; 0.407) | –119 (–525 to 288; 0.567) | 20 (–489 to 529; 0.939) | 0 (–434 to 435; 0.999) |
Outcomes
Table 40 reports the EQ-5D-3L results at baseline, and at 3, 6 and 12 months, plus QALYs at the two analysis points – between baseline and 3 months and between baseline and 12 months – for the complete case sample and the full sample with missing data imputed.
| Time point | Intervention | |||||
|---|---|---|---|---|---|---|
| Supported cCBT | Guided self-help | Waiting list | ||||
| Valid n | Mean (SD) | Valid n | Mean (SD) | Valid n | Mean (SD) | |
| Baseline | ||||||
| EQ-5D-3L utility | 155 | 0.67 (0.29) | 155 | 0.68 (0.26) | 154 | 0.68 (0.26) |
| 3 months | ||||||
| EQ-5D-3L utility | 104 | 0.69 (0.31) | 117 | 0.73 (0.24) | 124 | 0.67 (0.28) |
| QALYs | 104 | 0.17 (0.07) | 115 | 0.18 (0.05) | 123 | 0.17 (0.06) |
| QALYs imputed | 157 | 0.17 (0.07) | 158 | 0.17 (0.05) | 158 | 0.17 (0.06) |
| 6 months | ||||||
| EQ-5D-3L utility | 94 | 0.71 (0.29) | 105 | 0.72 (0.25) | 106 | 0.71 (0.25) |
| 12 months | ||||||
| EQ-5D-3L utility | 84 | 0.79 (0.27) | 100 | 0.73 (0.26) | 99 | 0.70 (0.31) |
| QALYs | 71 | 0.72 (0.27) | 81 | 0.71 (0.22) | 86 | 0.70 (0.23) |
| QALYs imputed | 157 | 0.72 (0.24) | 158 | 0.72 (0.11) | 158 | 0.71 (0.21) |
Table 41 details the results of the statistical comparisons between the groups. In terms of the observed data, EQ-5D-3L scores were very similar between the groups at all time points. There were no statistically significant differences between the groups at the 0.0167 p-value threshold. Analyses using imputation for missing data were very similar.
| Outcome measure and time point | Intervention comparison, £ (95% CI; p-value)a | |||||
|---|---|---|---|---|---|---|
| Supported cCBT vs. waiting list | Guided self-help vs. waiting list | Supported cCBT vs. guided self-help | ||||
| Unadjusted mean differenceb | Adjusted mean differencec | Unadjusted mean differenceb | Adjusted mean differencec | Unadjusted mean differenceb | Adjusted mean differencec | |
| 3 months | ||||||
| EQ-5D-3L utility | 0.0223 (–0.0538 to 0.0984; 0.556) | 0.0333 (–0.0229 to 0.0895; 0.246) | 0.0573 (–0.0095 to 0.1241; 0.566) | 0.0525 (0.0018 to 0.1032; 0.042) | –0.0350 (–0.1008 to 0.0308; 0.297) | –0.0192 (–0.0729 to 0.0354; 0.491) |
| QALYs | 0.0004 (–0.0159 to 0.0166; 0.965) | 0.0042 (–0.0029 to 0.0112; 0.246) | 0.0069 (–0.0076 to 0.0214; 0.348) | 0.0066 (0.0002 to 0.0129; 0.042) | –0.0066 (–0.0202 to 0.0071; 0.347) | –0.0024 (–0.0092 to 0.0044; 0.491) |
| QALYs imputed | 0.0014 (–0.0127 to 0.0154; 0.850) | 0.0034 (–0.0019 to 0.0086; 0.206) | 0.0056 (–0.0070 to 0.0182; 0.385) | 0.0051 (–0.0000 to 0.0101; 0.049) | –0.0042 (–0.0159 to 0.0074; 0.478) | –0.0017 (–0.0066 to 0.0032; 0.500) |
| 6 months | ||||||
| EQ-5D-3L utility | 0.0077 (–0.0682 to 0.0838; 0.841) | 0.0015 (–0.0600 to 0.0631; 0.961) | 0.0165 (–0.0537 to 0.0867; 0.645) | 0.0071 (–0.0523 to 0.0664; 0.815) | –0.0087 (–0.0845 to 0.0671; 0.822) | –0.0055 (–0.0688 to 0.0577; 0.864) |
| 12 months | ||||||
| EQ-5D-3L utility | 0.0890 (0.0010 to 0.1771; 0.047) | 0.0741 (0.0029 to 0.1452; 0.041) | 0.0299 (–0.0512 to 0.1111; 0.469) | 0.0255 (–0.0405 to 0.0915; 0.449) | 0.0591 (–0.0231 to 0.1413; 0.159) | 0.0485 (–0.0223 to 0.1194; 0.179) |
| QALYs | 0.0202 (–0.0550 to 0.0955; 0.599) | 0.0151 (–0.0347 to 0.0649; 0.553) | 0.0100 (–0.0589 to 0.0890; 0.776) | 0.0061 (–0.0419 to 0.0541; 0.804) | 0.0102 (–0.0656 to 0.0859; 0.792) | 0.0090 (–0.0463 to 0.0643; 0.750) |
| QALYs imputed | 0.0032 (–0.0476 to 0.0541; 0.900) | 0.0075 (–0.0185 to 0.0335; 0.570) | 0.0079 (–0.0376 to 0.0534; 0.733) | 0.0037 (–0.0223 to 0.0298; 0.779) | –0.0047 (–0.0500 to 0.0407; 0.840) | 0.0038 (–0.0229 to 0.0305; 0.781) |
Cost-effectiveness analyses
Incremental cost-effectiveness ratios
Table 42 presents the ICERs for all three pairwise comparisons at 3 and 12 months. Results are reported for both the health- and social-care perspective, and the societal perspective.
| Perspective | Intervention comparison, £ (incremental cost per QALY) | ||
|---|---|---|---|
| Supported cCBT vs. waiting list | Guided self-help vs. waiting list | Supported cCBT vs. guided self-help | |
| 3 months | |||
| Health and social care | 138/0.0042 (32,857) | 364/0.0066 (55,152) | –226/–0.0024 (94,167) |
| Societal | 202/0.0042 (48,095) | 310/0.0066 (46,970) | –109/–0.0024 (45,417) |
| 12 months | |||
| Health and social care | –9/0.0151 (–596) | 24/0.0061 (3934) | –5/0.0090 (–556) |
| Societal | –159/0.0151 (–10,530) | –355/0.0061 (–58,197) | 196/0.0090 (21,778) |
At 3 months, cost-effectiveness ratios for both supported cCBT and guided self-help compared with waiting list for high-intensity CBT are greater than £30,000 per QALY for both the health- and social-care perspective and the societal perspective. At 12 months (primary end point), however, ratios for supported cCBT compared with the waiting list for high-intensity CBT are negative for both perspectives, indicating, on average, that the supported cCBT group is both cheaper and more effective than waiting list for high-intensity CBT.
Ratios for guided self-help compared with the waiting list for high-intensity CBT are negative from the societal perspective (on average guided self-help is cheaper and more effective than the waiting list for high-intensity CBT) and approximately £4000 per QALY from the health- and social-care perspective (on average guided self-help is more expensive but also more effective), which is below the NICE willingness-to-pay threshold level of £20,000–30,000 or less. However, as ICERs are based on point estimates, we must examine the cost-effectiveness planes and CEACs to account for the variability and uncertainty around these estimates.
Supported computerised cognitive–behavioural therapy versus the waiting list for high-intensity cognitive–behavioural therapy
Figures 3–6 show the bootstrapped replications for cost and effect pairs on the cost-effectiveness plane for supported cCBT versus a waiting list for high-intensity CBT at 3 and 12 months (health- and social-care perspective and societal perspective). At 3 months, a greater proportion of scatter points lie to the right of the vertical axis where the bootstrap replications represent points where cCBT is more effective than the waiting list. In addition, a greater proportion of scatter points lie above the horizontal axis where the bootstrap replications represent supported cCBT having higher costs than the waiting list. This is the same from both perspectives and generates a trade-off scenario – there are better outcomes but for a greater cost.
FIGURE 3.
Cost-effectiveness plane for supported cCBT vs. the waiting list for high-intensity CBT at 3 months from the health- and social-care perspective.
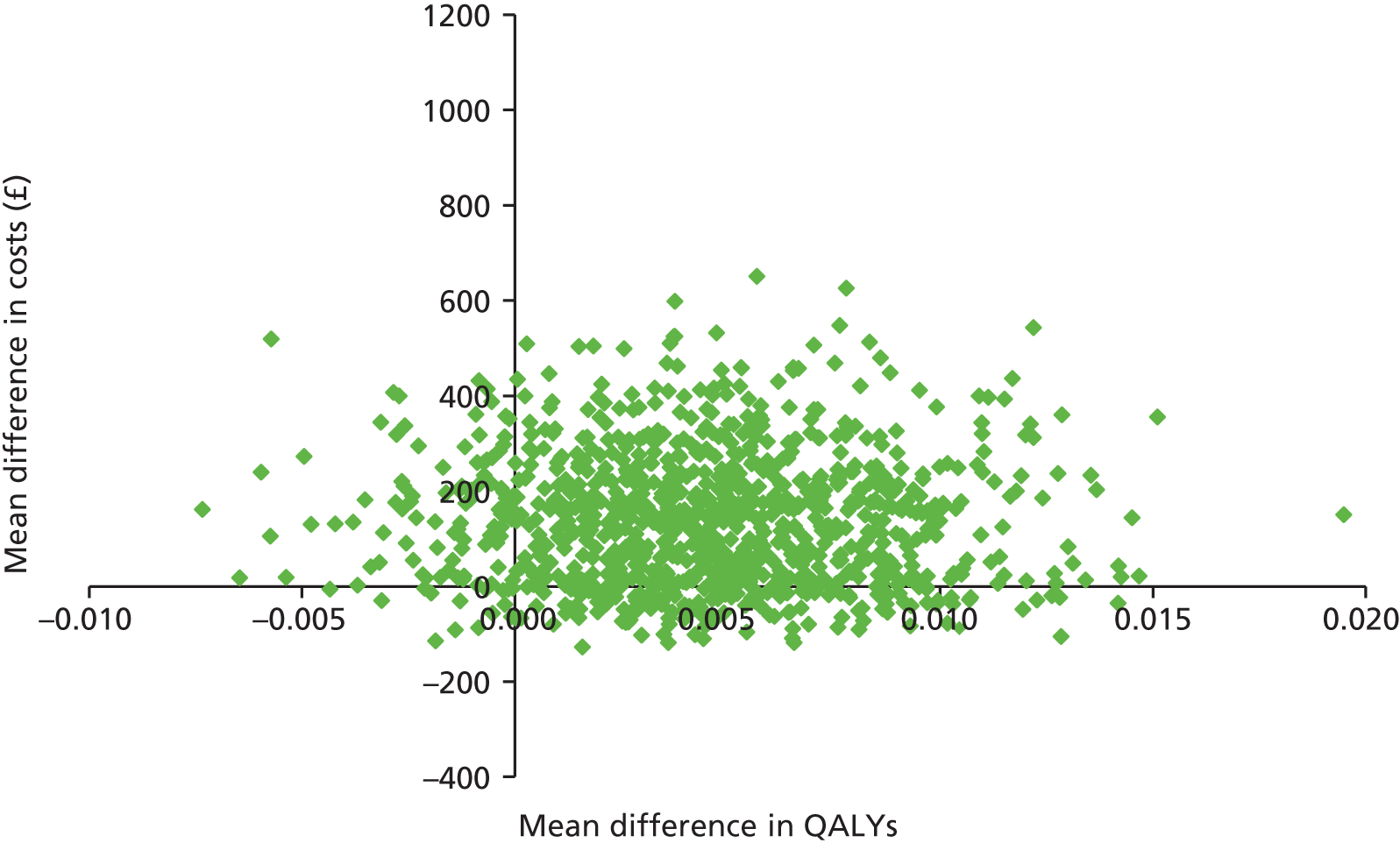
FIGURE 4.
Cost-effectiveness plane for supported cCBT vs. the waiting list for high-intensity CBT at 3 months from the societal perspective.
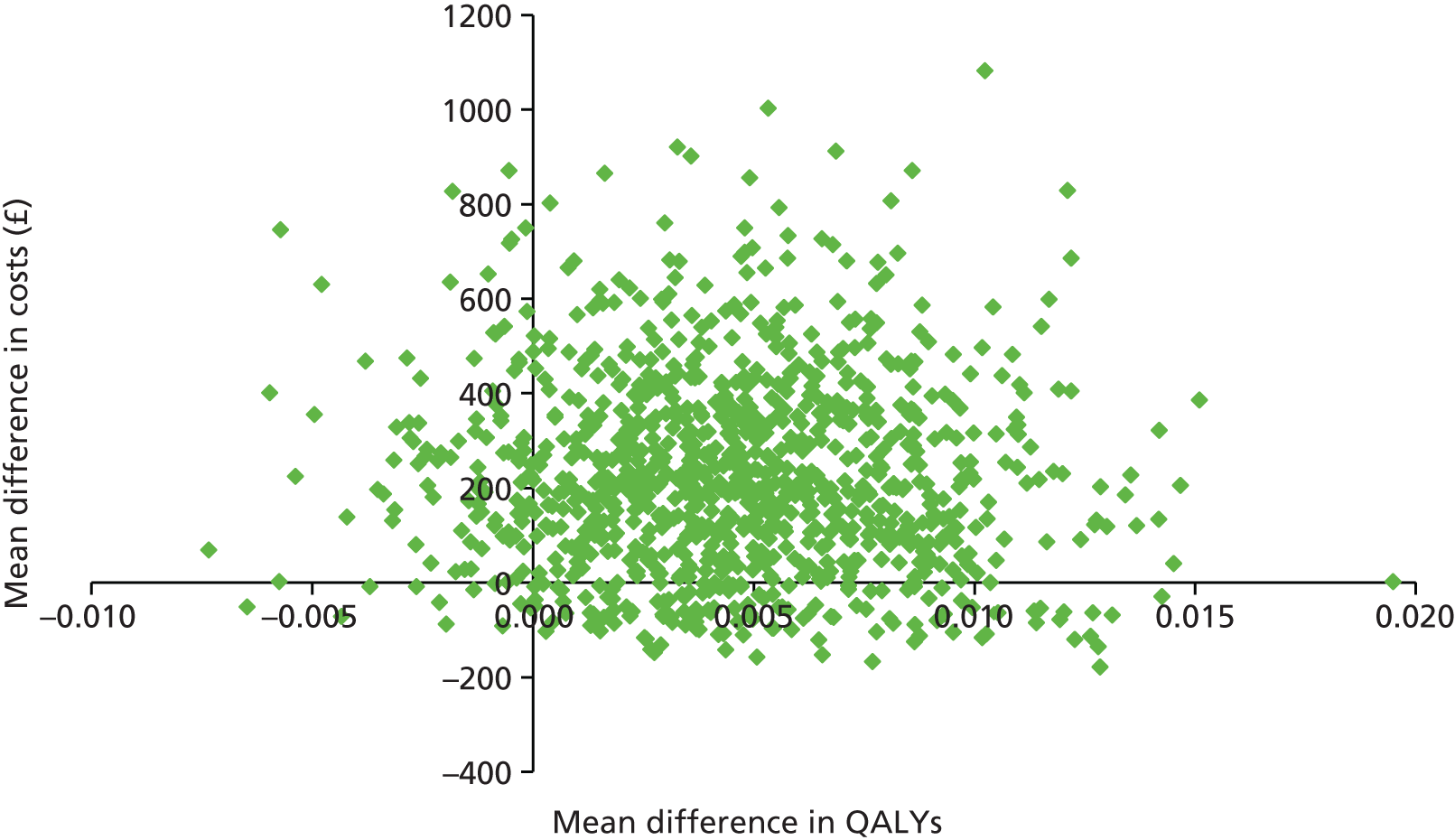
FIGURE 5.
Cost-effectiveness plane for supported cCBT vs. the waiting list for high-intensity CBT at 12 months from the health- and social-care perspective.
FIGURE 6.
Cost-effectiveness plane for supported cCBT vs. the waiting list for high-intensity CBT at 12 months from the societal perspective.
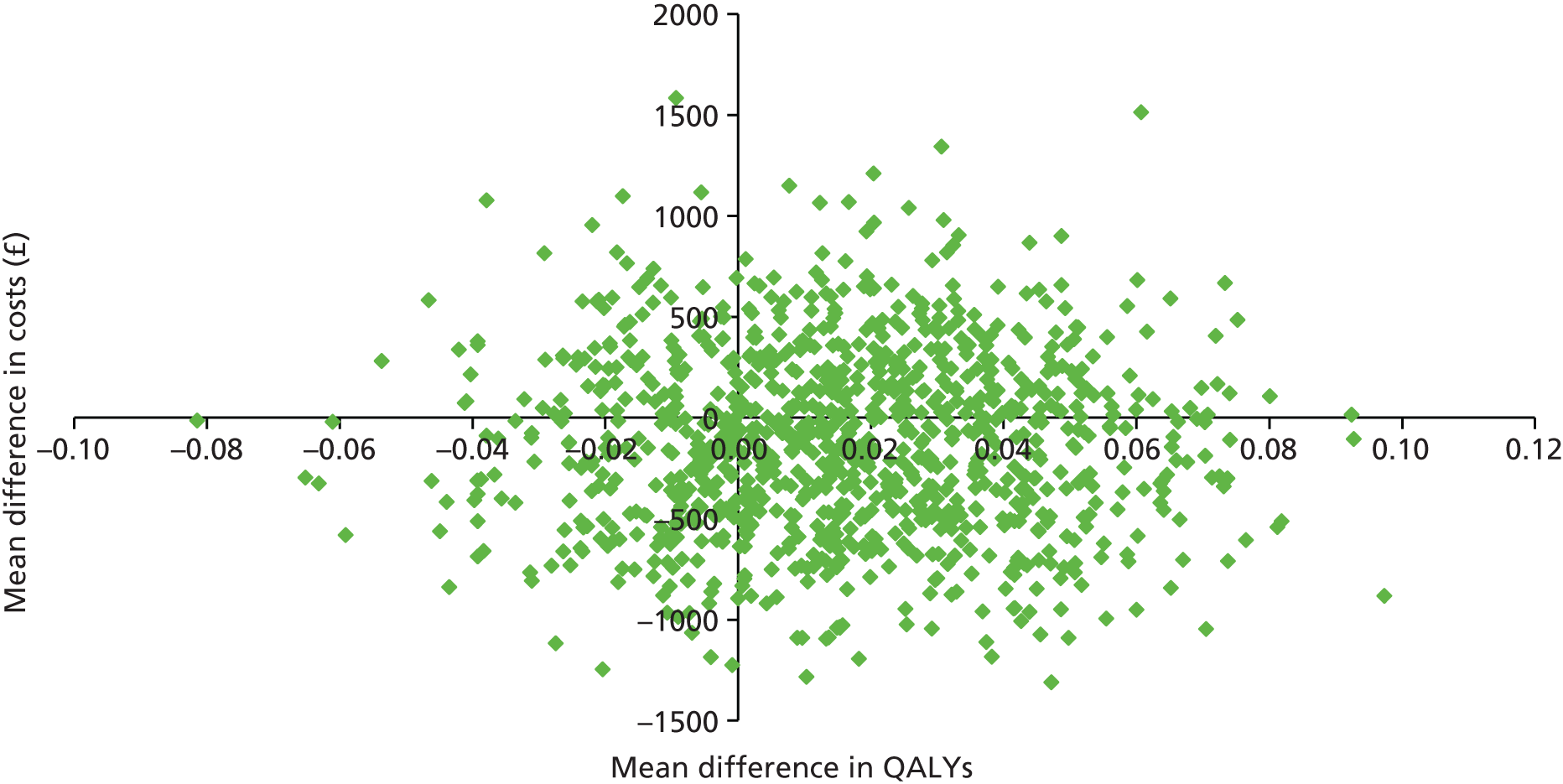
Figure 7 shows the CEACs for supported cCBT versus the waiting list for high-intensity CBT at 3 months from both perspectives. The probability of supported cCBT being cost-effective compared with the waiting list at the NICE willingness-to-pay level of £20,000–30,000 is around 40–50% from the NHS/Personal Social Services perspective and 25–35% from the societal perspective.
FIGURE 7.
Cost-effectiveness acceptability curves for supported cCBT vs. the waiting list for high-intensity CBT at 3 months from the health- and social-care perspective and societal perspective.

At 12 months, from both perspectives, a greater proportion of scatter points lie to the right of the vertical axis, where the bootstrap replications represent supported cCBT being more effective than the waiting list, while a slightly greater proportion of scatter points fall below the horizontal axis where the bootstrap replications represent cCBT being less costly than the waiting list.
Figure 8 shows the CEACs for supported cCBT versus the waiting list at 12 months (primary end point) from both perspectives. The probability of supported cCBT being cost-effective compared with the waiting list at the NICE willingness-to-pay level of £20,000–30,000 is around 70% from both perspectives.
FIGURE 8.
Cost-effectiveness acceptability curves for supported cCBT vs. the waiting list for high-intensity CBT at 12 months from the health- and social-care perspective and societal perspective.
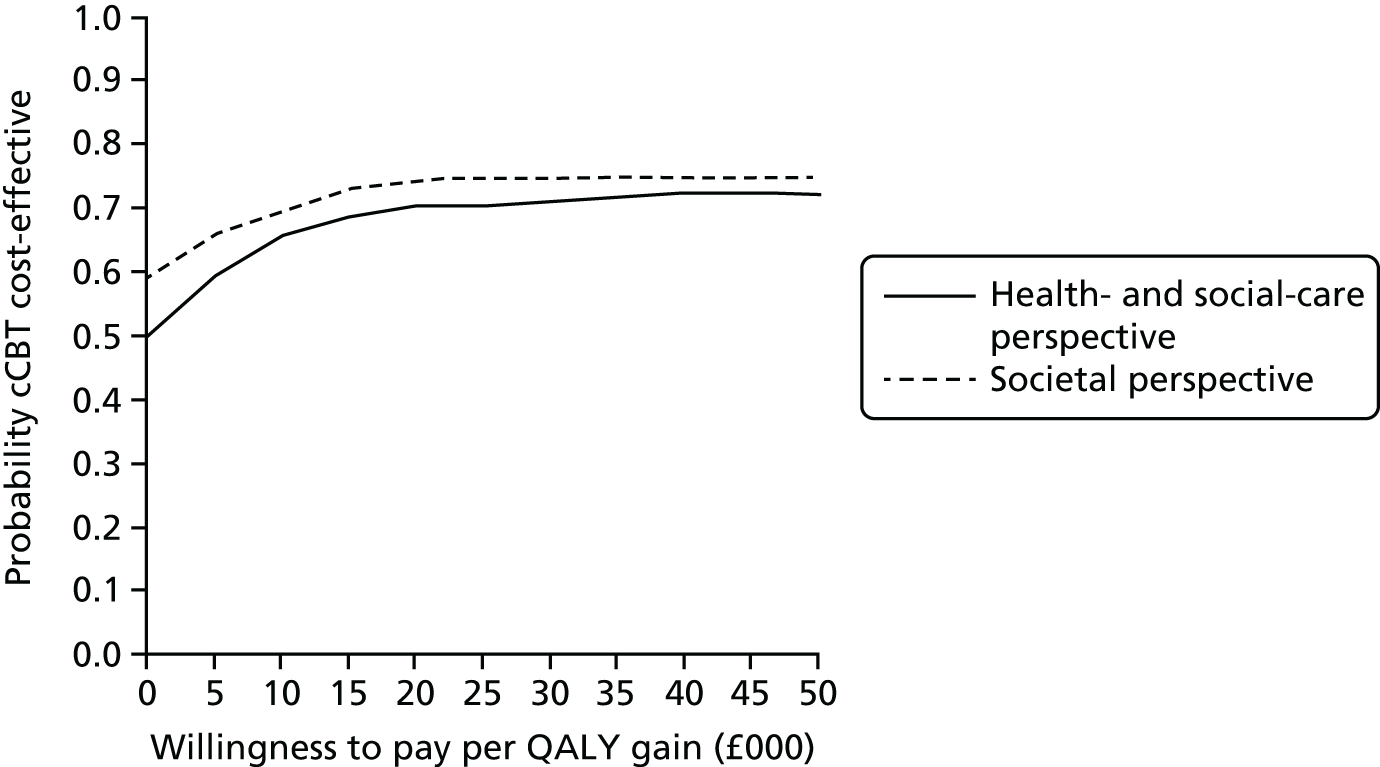
Guided self-help versus the waiting list for high-intensity cognitive–behavioural therapy
Figures 9–12 show the bootstrapped replications for cost and effect pairs on the cost-effectiveness plane for guided self-help versus the waiting list for high-intensity CBT at 3 and 12 months (health- and social-care perspective and societal perspective). At 3 months, the majority of the scatter points lie to the right of the vertical axis, where the bootstrap replications represent guided self-help being more effective than the waiting list, and the majority of the scatter points lie above the horizontal axis where the bootstrap replications represent guided self-help having higher costs from both perspectives, indicating a trade-off.
FIGURE 9.
Cost-effectiveness plane for guided self-help vs. the waiting list for high-intensity CBT at 3 months for the health- and social-care perspective.

FIGURE 10.
Cost-effectiveness plane for guided self-help vs. the waiting list for high-intensity CBT at 3 months for the societal perspective.

FIGURE 11.
Cost-effectiveness plane for guided self-help vs. the waiting list for high-intensity CBT at 12 months from the health- and social-care perspective.
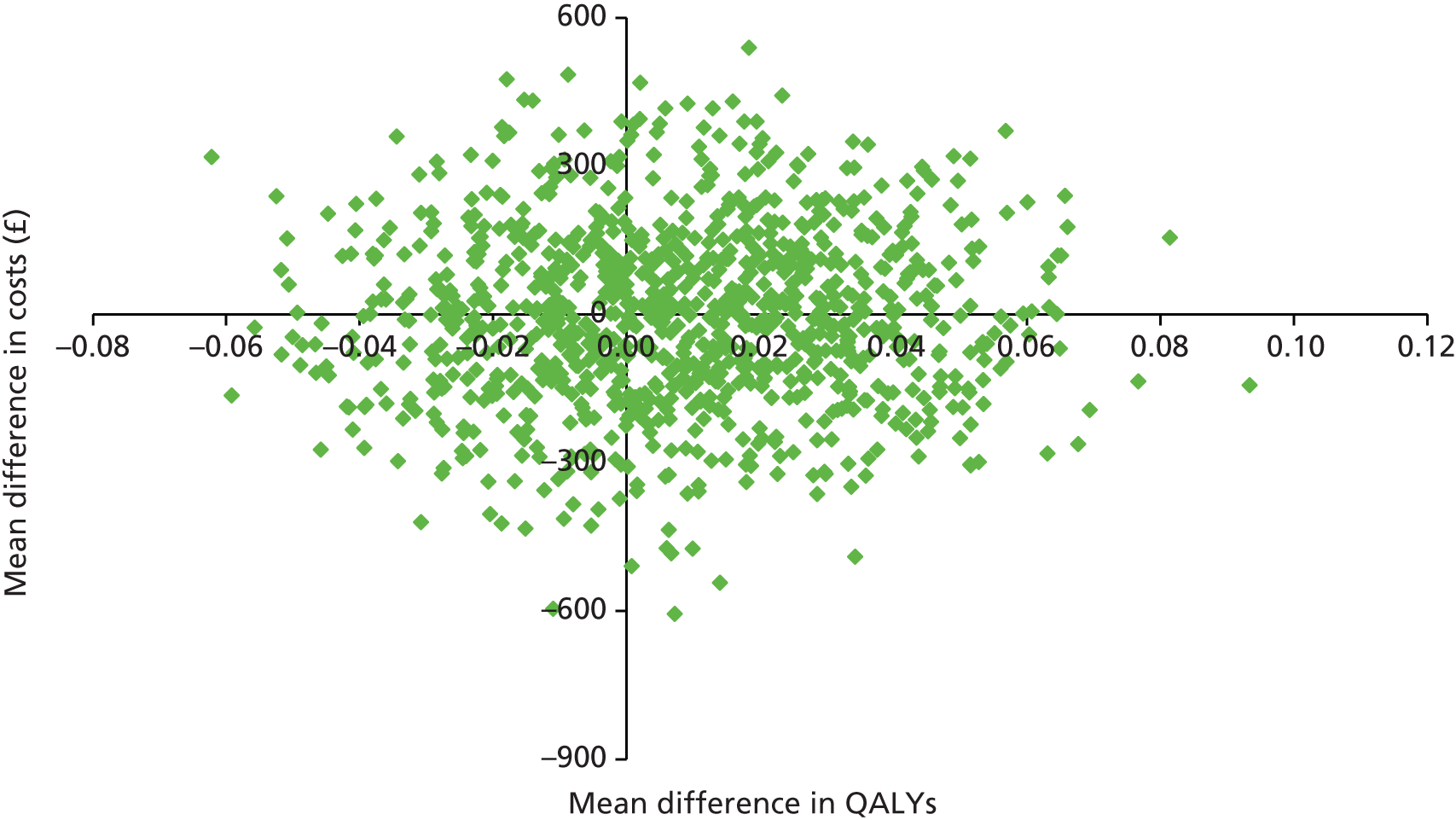
FIGURE 12.
Cost-effectiveness plane for guided self-help vs. the waiting list for high–intensity CBT at 12 months from the societal perspective.

The guided self-help versus the waiting list for high-intensity CBT CEACs in Figure 13 indicate that the probability of guided self-help being cost-effective compared with the waiting list at the NICE willingness-to-pay level of £20,000–30,000 is 0–5% from the health- and social-care perspective and 5–10% from the societal perspective.
FIGURE 13.
Cost-effectiveness acceptability curves for guided self-help vs. the waiting list for high-intensity CBT at 3 months from the health- and social-care perspective and societal perspective.
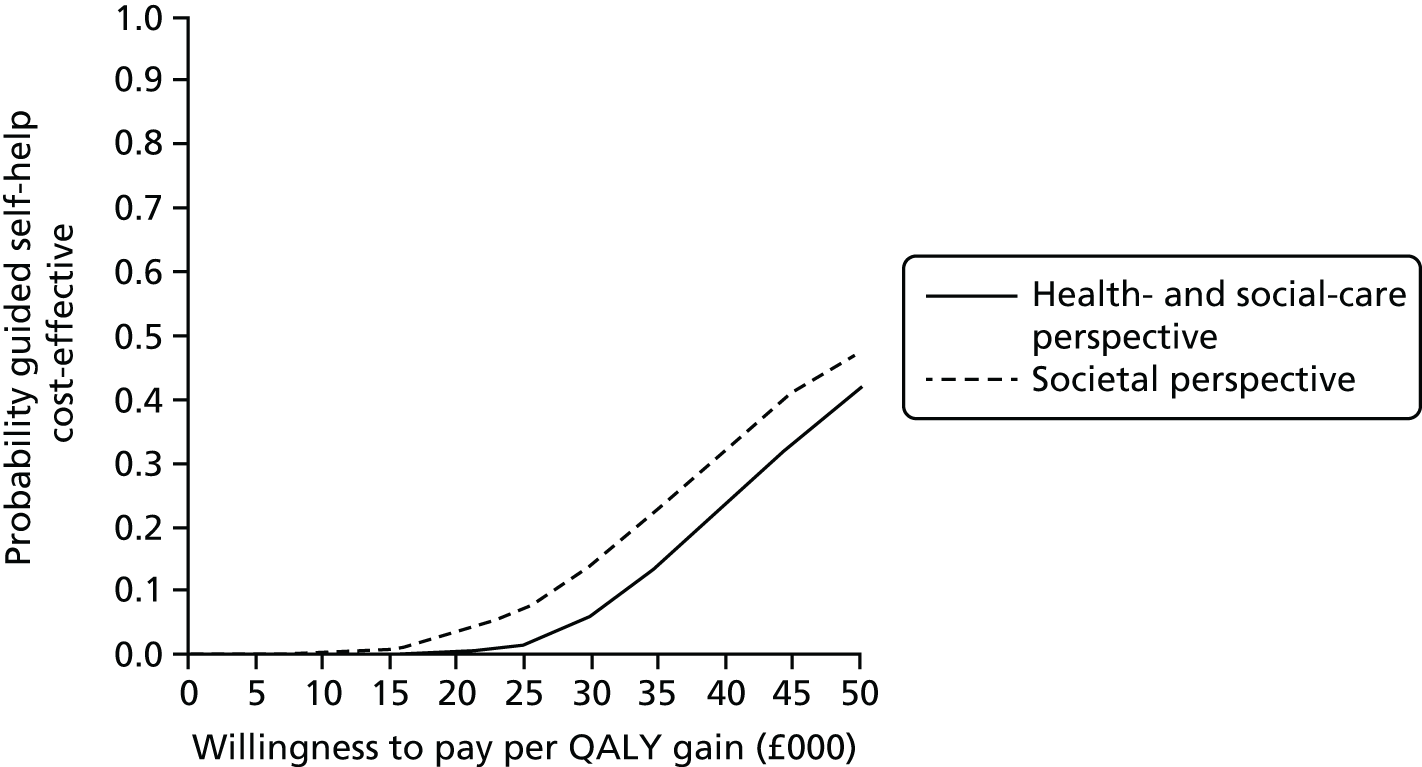
At 12 months (primary end point), from a health- and social-care perspective, slightly more of the scatter points lie to the right of the vertical axis where the bootstrap replications represent guided self-help being more effective than the waiting list. In addition, slightly more of the scatter points lie below the horizontal axis, where the bootstrap replications represent guided self-help having lower costs than the waiting list. From a societal perspective, more of the scatter points lie to the right of the vertical axis, where the bootstrap replications represent guided self-help being more effective than the waiting list, while the majority of the scatter points lie below the horizontal axis, where the bootstrap replications represent guided self-help having lower costs.
This is reflected in the guided self-help versus the waiting list CEACs in Figure 14, which show that the probability of guided self-help being cost-effective compared with the waiting list group at £20,000–30,000 per QALY is around 60% from the health- and social-care perspective and below 80% from the societal perspective.
FIGURE 14.
Cost-effectiveness acceptability curves for guided self-help vs. the waiting list for high-intensity CBT at 12 months from the health- and social-care perspective and societal perspective.

Supported computerised cognitive–behavioural therapy versus guided self-help
Figures 15–18 show the bootstrapped replications for cost and effect pairs on the cost-effectiveness plane for supported cCBT versus guided self-help at 3 and 12 months, and from the health- and social-care perspective and societal perspective.
FIGURE 15.
Cost-effectiveness plane for supported cCBT vs. guided self-help at 3 months from the health- and social-care perspective.
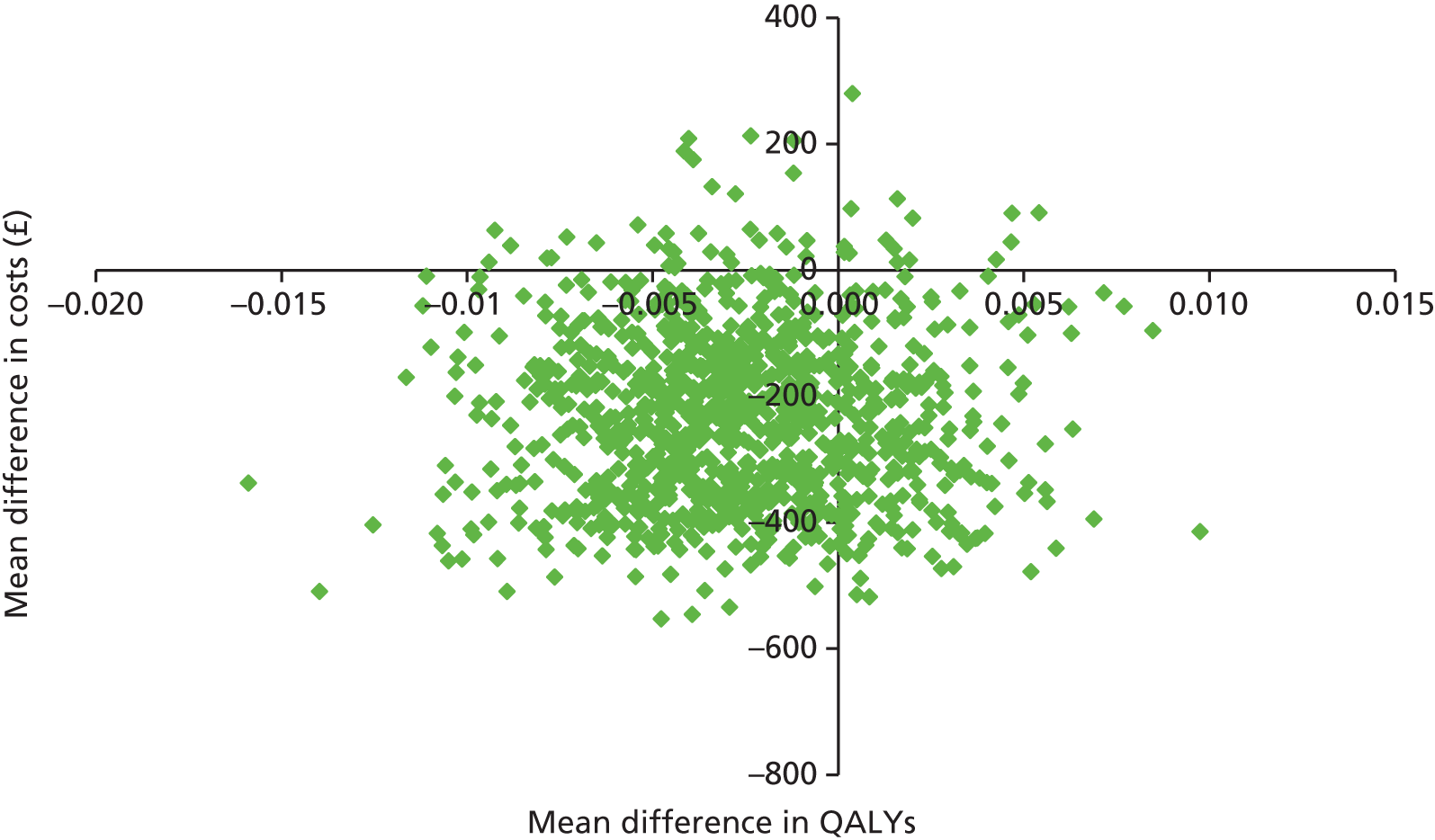
FIGURE 16.
Cost-effectiveness plane for supported cCBT vs. guided self-help at 3 months from the societal perspective.
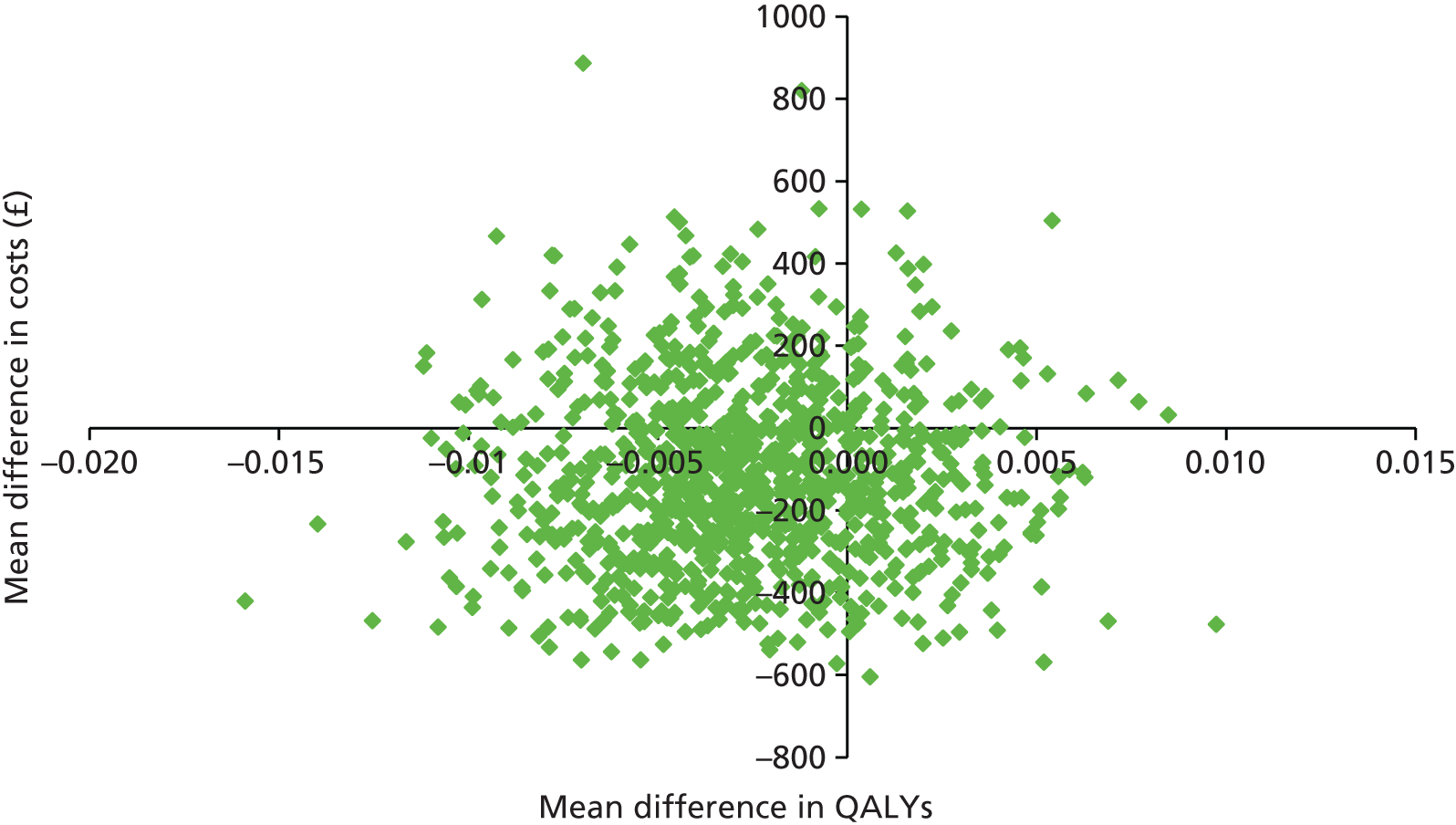
FIGURE 17.
Cost-effectiveness plane for supported cCBT vs. guided self-help at 12 months from the health- and social-care perspective.
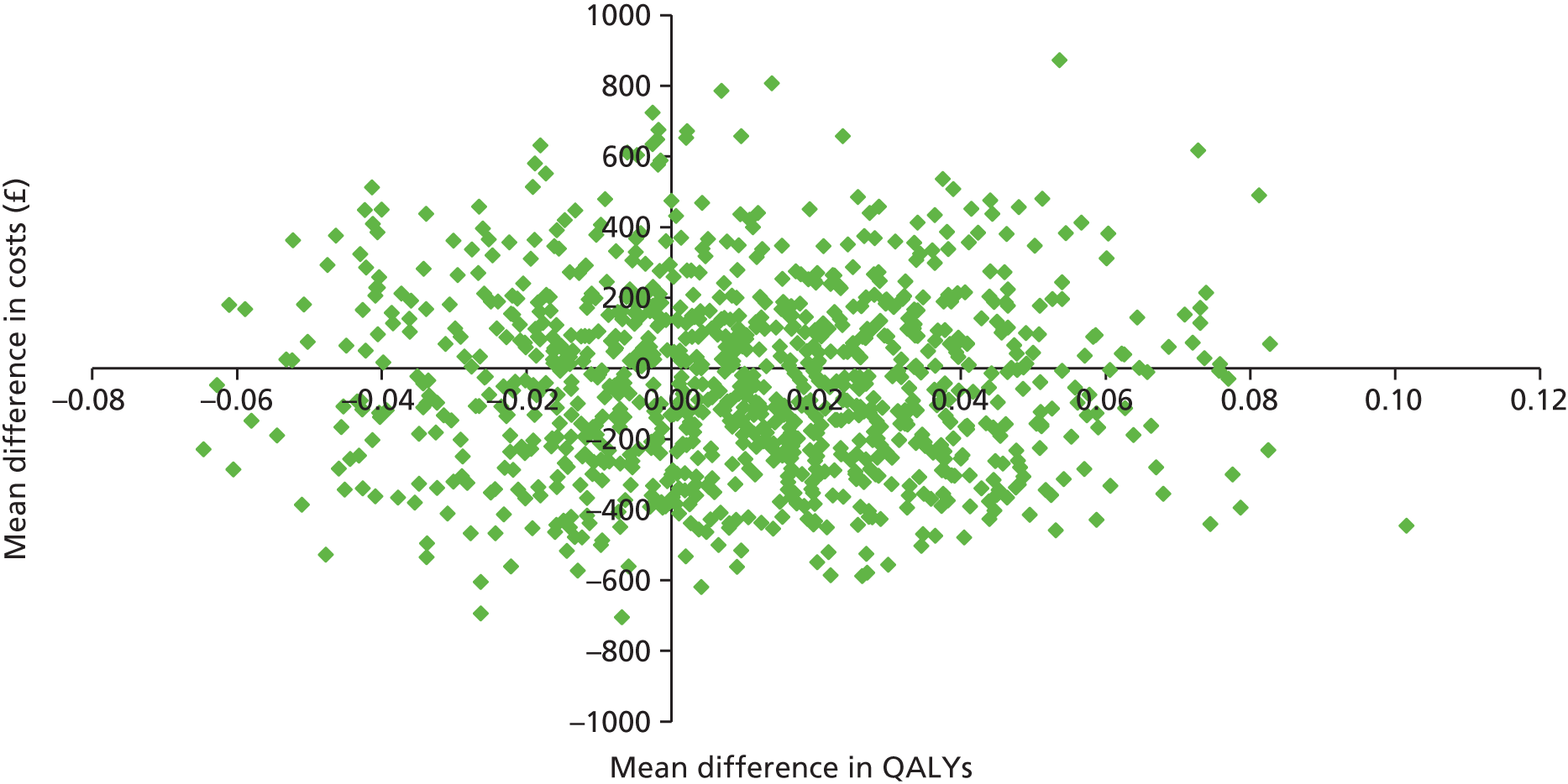
FIGURE 18.
Cost-effectiveness plane for supported cCBT vs. guided self-help at 12 months from the societal perspective.
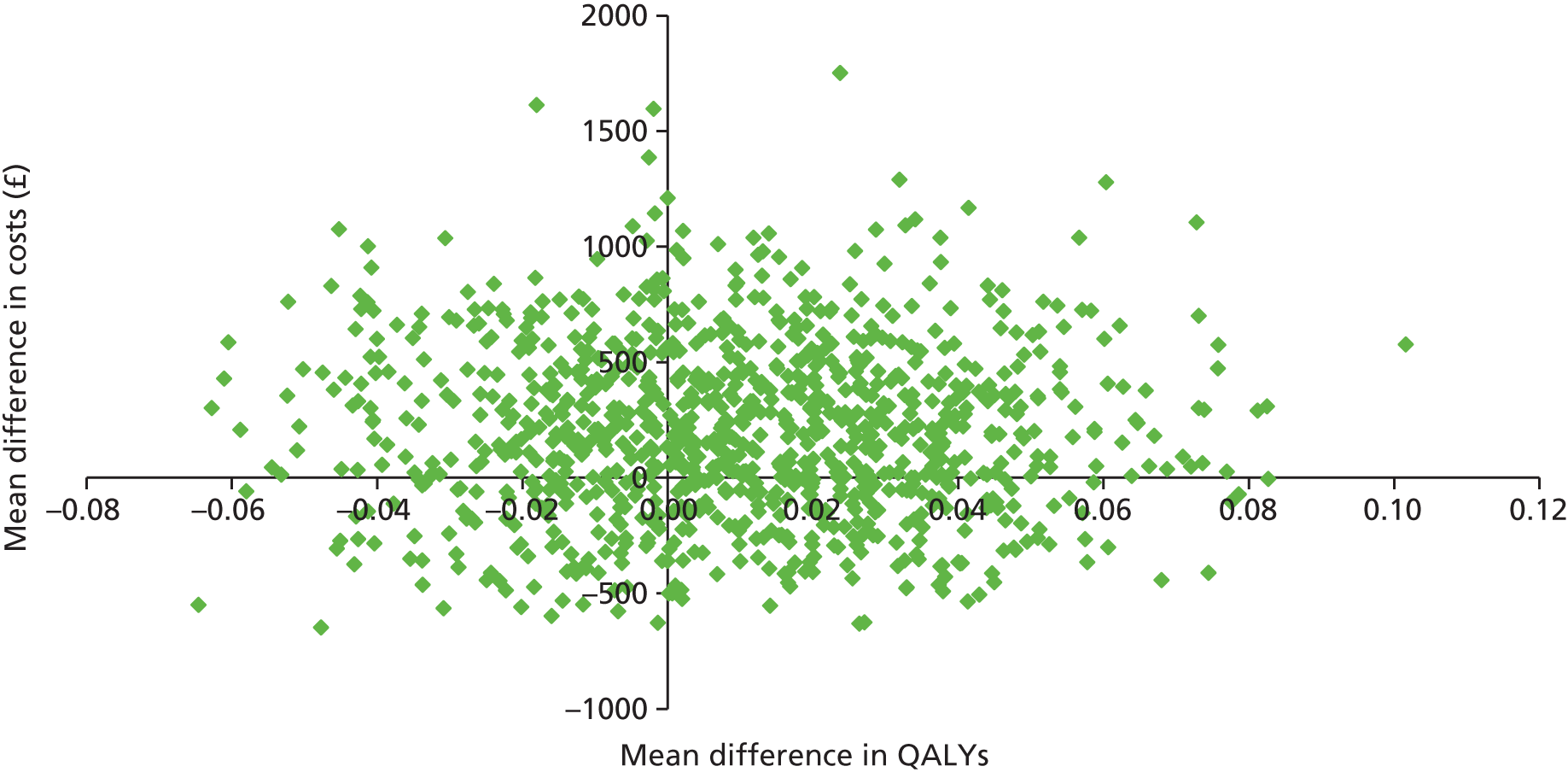
At 3 months, for both the health- and social-care perspective and societal perspective, more bootstrap replications lie below the horizontal axis, where the replications represent supported cCBT being less costly than guided self-help. In addition, more replications lie to the left of the vertical axis indicating lower effectiveness of supported cCBT indicating a trade-off scenario.
The supported cCBT versus guided self-help CEACs in Figure 19 indicate that supported cCBT has an 80% probability of being cost-effective compared with guided self-help at the NICE willingness-to-pay levels of £20,000–£30,000 from the health- and social-care perspective, and over a 60% probability from the societal perspective.
FIGURE 19.
Cost-effectiveness acceptability curves for supported cCBT vs. guided self-help at 3 months from the health- and social-care perspective and societal perspective.

At 12 months (primary end point), from both the health- and social-care perspective and the societal perspective, slightly more replications lie to the right of the vertical axis, indicating that the effectiveness of supported cCBT is higher than that of guided self-help. From a health- and social-care perspective, slightly more bootstrap replications lie below the horizontal axis, where the replications represent supported cCBT being less costly than guided self-help. In addition, from a societal perspective, slightly more replications lie above the horizontal axis, where the bootstraps indicate supported cCBT being more costly.
The supported cCBT versus guided self-help CEACs in Figure 20 suggest that the probability of supported cCBT being cost-effective compared with guided self-help is around 65% from the health- and social-care perspective and around 50% from the societal perspective.
FIGURE 20.
Cost-effectiveness acceptability curves for supported cCBT vs. guided self-help at 12 months from the health- and social-care perspective and societal perspective.
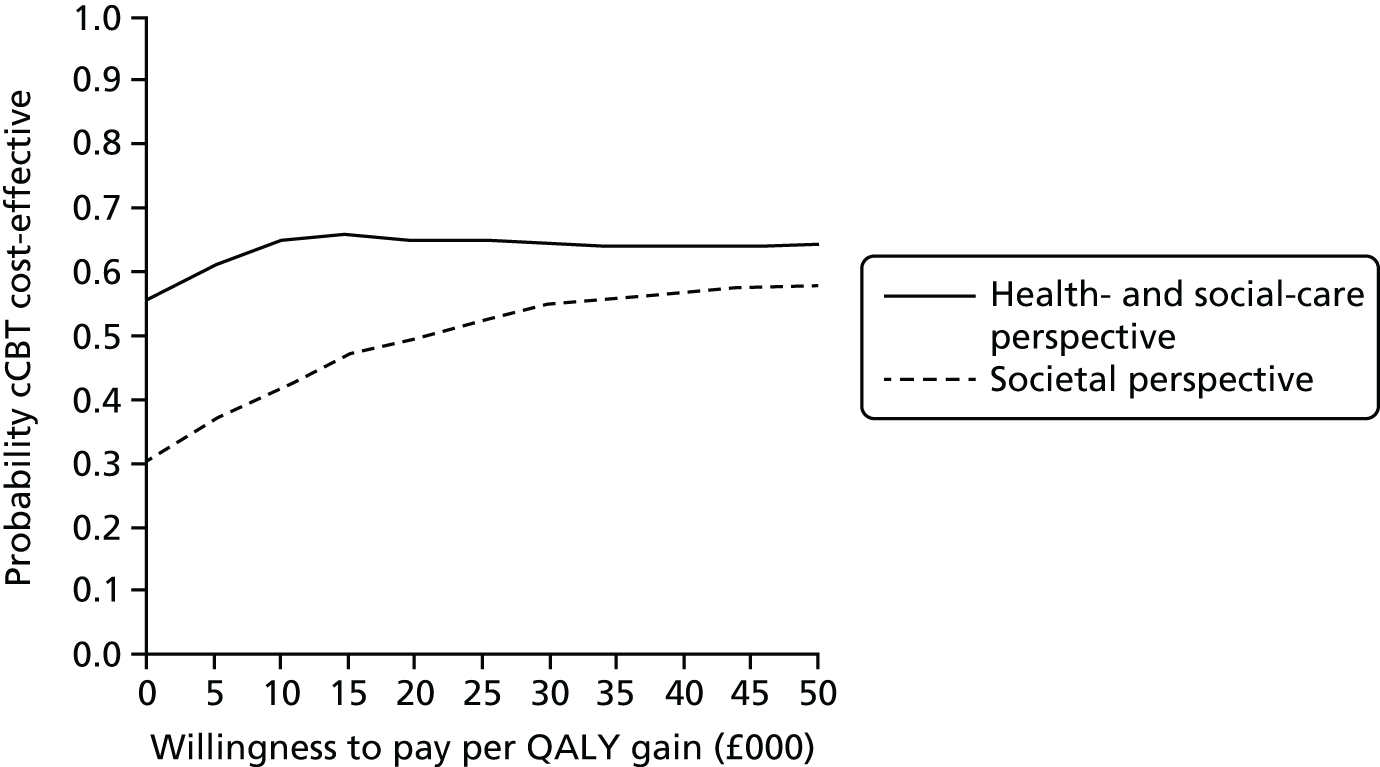
Supported computerised cognitive–behavioural therapy versus guided self-help versus the waiting list for high-intensity cognitive–behavioural therapy
The three interventions were compared head to head in a three-way comparison at 12 months (the primary end point). The CEAC in Figure 21 shows that the probability of supported cCBT being cost-effective at the NICE willingness-to-pay level of £20,000–30,000 is between 40% and 50% from the NHS/Personal Social Services perspective, compared with between 30% and 40% for guided self-help and around 20% for the waiting list for high-intensity CBT. The CEAC in Figure 22 shows that the probability of supported cCBT being cost-effective at the NICE willingness-to-pay threshold of £20,000–30,000 is between 35% and 40%, compared with 50–55% for guided self-help and around 10% for the waiting list for high-intensity CBT.
FIGURE 21.
Cost-effectiveness acceptability curves for supported cCBT vs. guided self-help vs. the waiting list for high-intensity CBT at 12 months from the health- and social-care perspective.
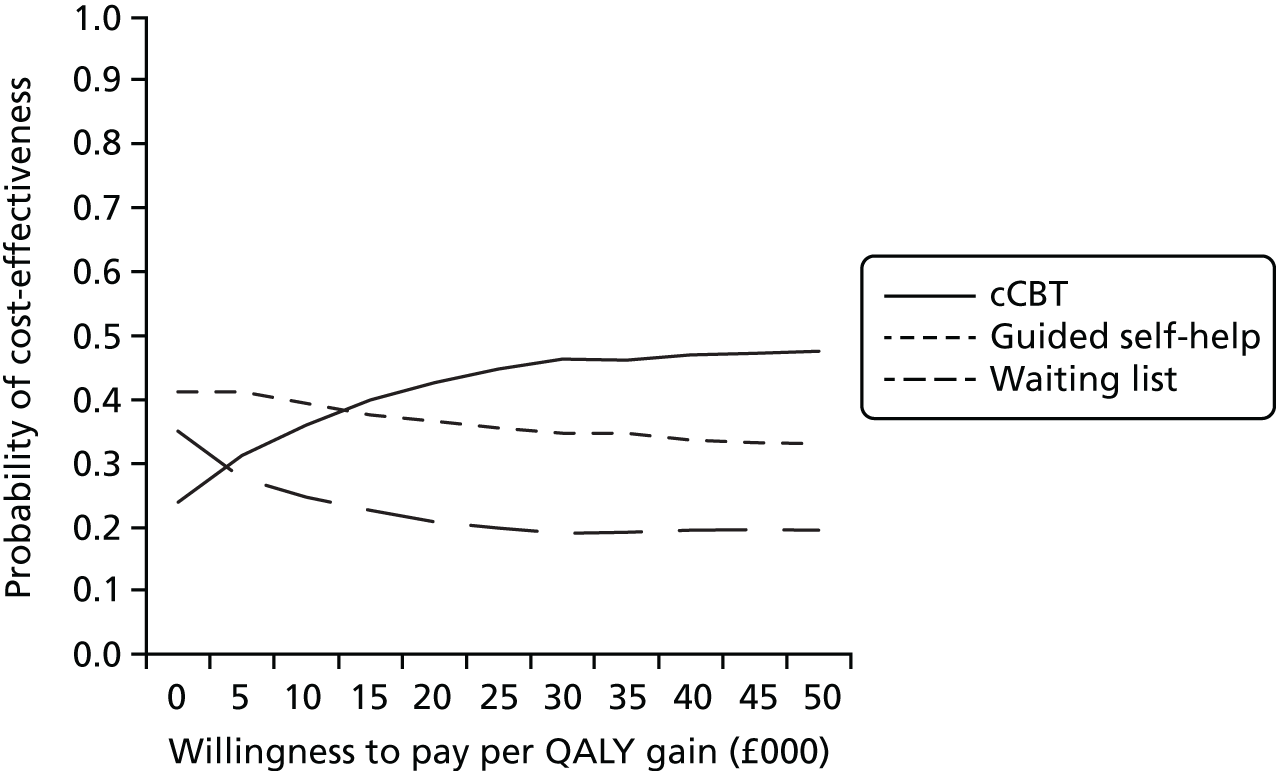
FIGURE 22.
Cost-effectiveness acceptability curves for supported cCBT vs. guided self-help vs. the waiting list for high-intensity CBT at 12 months from the societal perspective.
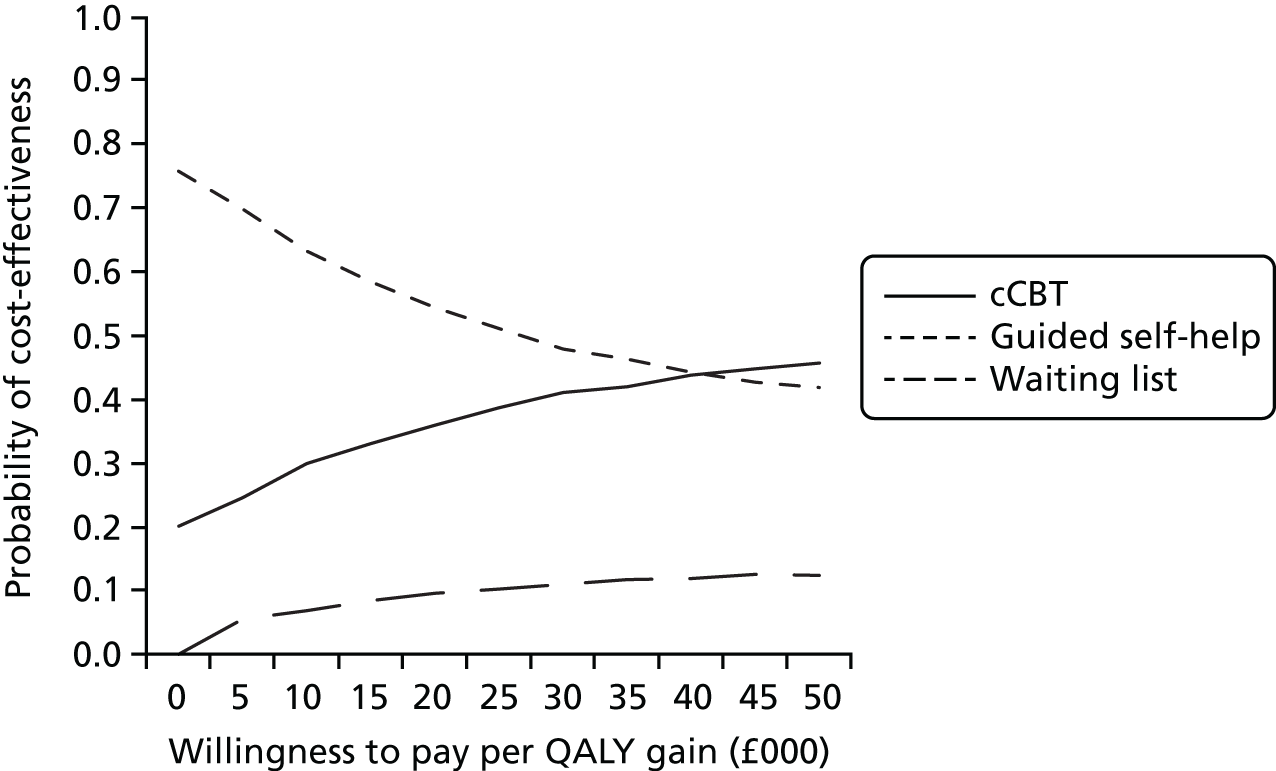
Chapter 5 Acceptability of guided self-help and supported computerised cognitive–behavioural therapy
Nested qualitative research is increasingly popular in RCTs to explore the acceptability of trial interventions from the perspectives of those delivering and receiving a treatment. Findings from qualitative research form an important part of a trial’s evaluation and can aid the understanding of quantitative findings.
Two qualitative studies were conducted as part of OCTET: the first explored the acceptability of supported cCBT and guided self-help from the perspective of trial participants, and the second explored the feasibility and acceptability of delivering these two interventions from the perspective of the PWPs. This chapter reports the findings of these two qualitative studies.
Quantitative data relating to trial participants’ baseline treatment preferences and treatment satisfaction (as measured using the CSQ-8) at 3- and 6-month follow-up are presented in Chapter 3.
Study 1: acceptability of guided self-help and supported computerised cognitive–behavioural therapy to trial participants
The aim of this study was to explore the acceptability of supported cCBT and guided self-help from the perspective of trial participants. Quantitative data demonstrated a 66% and 61% uptake of guided self-help and supported cCBT, respectively (defined as participants receiving one or more sessions of their trial allocated treatment). The overall mean number of sessions was 4.87 (SD 4.21 sessions) for guided self-help and 3.73 (SD 2.49 sessions) for supported cCBT. Although informative, these quantitative data are unable to elucidate important process factors. Therefore, an in-depth qualitative study was nested within OCTET.
Methods
Sampling and participant recruitment
Sampling was purposive, and only those allocated to an intervention arm were invited for interview. We deployed maximum variation sampling techniques to ensure a range of ages and geographical locations (trial sites) and a relatively even split between sexes and intervention groups. Data collection and analysis occurred in parallel and sampling continued until data saturation was achieved.
Between September 2012 and January 2014 invitation letters, consent-to-contact forms and study information sheets were posted to 125 participants who had consented at trial baseline to subsequent contact from the research team regarding additional research opportunities. To be eligible for inclusion in our qualitative study, participants had to have been allocated to one of the two trial intervention arms (guided self-help or supported cCBT) and had to have reached or passed the anticipated date for intervention completion (i.e. ≥ 12 weeks post randomisation). Following the return of the consent-to-contact forms, participants were contacted by telephone to arrange a suitable time and place for the interview. Written consent was obtained at the time of a face-to-face interview or in writing prior to telephone interviews. Participation was voluntary and no reimbursement was offered for taking part.
Data collection
Interviews were conducted face to face or over the telephone, depending on participant preference. A semistructured interview schedule was developed from prior knowledge of the research area and via discussion between the clinical and academic research team members and service users with OCD.
The schedule was structured to focus researcher attention while simultaneously enabling participants to raise salient issues (Table 43). To ease participants into the interview process and reduce anxiety, the opening section of the interview schedule covered general questions about OCD symptoms and their daily impact. Subsequent sections use open-ended questions to explore preferences and expectations of the trial interventions and their subsequent treatment experiences. Additional questions relating to potential influences on intervention uptake and engagement were included to accommodate the research questions of a Doctor of Philosophy (PhD) student.
| Topic | Subtopic |
|---|---|
| OCD |
|
| Treatment preferences and expectations |
|
| Trial treatment experiences |
|
The majority of the interviews (31 out of 36) were conducted by a female PhD student trained in qualitative methods, the remaining five interviews were conducted by a service user researcher trained by the research team. An interviewer manual was developed to guide both through the interview process; this manual included information relating to health and safety requirements, ethical conduct, informed consent, research governance procedures, transcription services, data management and support in dealing with challenges prior to, throughout, and following, the interviews. The research aims and interview schedule were regularly revisited and discussed between interviewers to ensure consistency between their interviews.
Data analysis
Interviews were audio-recorded and transcribed verbatim with written participant consent. Data collection and analysis occurred in parallel to allow early analysis to inform the focus and analysis of later interviews. Interview data were subjected to a thematic analysis,85 using the constant comparison method. 86 In line with current gold standard recommendations, data were analysed without prior knowledge of trial effects.
Transcripts were read and reread independently by three researchers (an OCTET team member, a research student and an independent student supervisor) all with qualitative expertise. Data coding was led by the research student who met the other researchers periodically to discuss their interpretations and establish an NVivo-hosted (version 10; QSR International, Warrington, UK) coding manual. As the constant comparison of new data occurred, and the researchers’ understandings of the themes under consideration developed, the coding manual was amended and reshaped to enable the introduction of new codes and/or the deletion of redundant, similar or otherwise compromised codes.
Coding was subsequently discussed with a service user representative to ensure that the analysis remained grounded in the original data and to ensure that any perceived omissions or ambiguities were resolved.
Results
Sample characteristics
A total of 315 of the 473 trial participants were allocated to a treatment arm, 125 were offered an acceptability interview and 44 returned a consent-to-contact form, expressing an interest in taking part. Eight participants (18%) who returned consent-to-contact forms did not complete an interview; three had competing commitments or time constraints, one failed to provide written consent and, despite repeated attempts, four could not be reached by a researcher.
Thirty-six individuals (11% of participants randomised to a trial treatment) participated in an interview. Eighteen had been randomised to supported cCBT and 18 to guided self-help. All interviews took place between October 2012 and January 2014, and between 4 and 13 months post randomisation. Interviews ranged in length from 27 to 129 minutes; 47% were conducted face to face in the participants’ homes or a university setting, and 53% were completed by telephone.
As per our study protocol, all participants were contacted after the anticipated date of trial treatment completion; however, because of unplanned delays in therapist allocation and bookings, two (6%) were still undergoing their trial-allocated treatments at the time of their interview. Half of the sample (50%) had reached the top of the waiting list for high-intensity CBT and were currently receiving, or had recently completed, high-intensity CBT.
Representativeness of the sample
Baseline characteristics for the 36 participants providing qualitative interview data are presented in Table 44. Data for the 473 OCTET participants are provided for comparative purposes.
| Characteristic | Sample | |
|---|---|---|
| Acceptability (n = 36) | Overall trial (n = 473) | |
| Age (years) | ||
| Range | 20.7–64.5 | 18.0–77.6 |
| Mean (SD) | 42.7, 13.4 | 35.9, 12.4 |
| Sex, n (%) | ||
| Female | 21 (58) | 285 (60) |
| Male | 15 (42) | 188 (40) |
| Treatment preference, n (%) | ||
| Wait for scheduled CBT | 1 (3) | 50 (10.6) |
| No preference | 16 (44) | 222 (46.9) |
| Try a self-help intervention | 19 (53) | 200 (42.3) |
| Missing | 0 (0) | 1 (0.2) |
| Self-management preference, n (%) | ||
| Try supported cCBT | 11 (30) | 109 (23.0) |
| No preference | 14 (39) | 234 (49.5) |
| Try guided self-help | 11 (30) | 127 (26.9) |
| Missing | 0 (0) | 3 (0.6) |
| Y-BOCS-OR score at baseline, mean (SD) | ||
| cCBT arm | 25.06 (6.47) | 25.03 (5.45) |
| Guided self-help arm | 24.11 (5.57) | 25.03 (5.02) |
| Y-BOCS-OR score at 3 months, mean (SD) | ||
| cCBT arm | 20.71 (6.47) | 20.92 (7.09) |
| Guided self-help arm | 17.59 (10.08) | 20.92 (6.9) |
| Number of support sessions received, mean (SD) | ||
| cCBT arm | 4.42 (3.00) | 3.73 (2.49) |
| Guided self-help arm | 5.94 (4.15) | 4.87 (4.21) |
Participant views
Findings are laid out to reflect the aim of this study [i.e. to report on the acceptability of two low-intensity interventions (supported cCBT and guided self-help) to adult trial participants with OCD].
Participants highlighted six characteristics of ‘good’ therapy, against which the acceptability of the trial interventions was judged. Individual narratives highlighted, to a greater or lesser extent, the need for psychological treatments that were:
-
accessible (capable of overcoming or accommodating traditional barriers to care)
-
enabling (able to reduce the fear, embarrassment or burden of OCD disclosure)
-
applicable (providing the type or components of therapy valued by service users)
-
relevant (tailored to individual symptoms and experiences)
-
responsive (capable of acknowledging and reacting to users’ real-time needs)
-
motivating (providing the incentive to attend or engage with treatment).
These characteristics provide an empirical structure through which to examine the acceptability of guided self-help and supported cCBT. Participants are assigned a number rather than a name or pseudonym within the text. Sex (male or female) and intervention allocation (guided self-help or supported cCBT) are provided.
Accessibility
At the time of study, NICE guidelines recommended low-intensity interventions for mild to moderate OCD, delivered within a stepped-care model designed to address a gap between psychological therapy demand and supply.
For participants in the current study, treatment accessibility was perceived to be a key advantage of low-intensity interventions, albeit in slightly different terms to that implied by the stepped-care model. Concepts of access, defined in terms of reduced waiting times for psychological treatment, were strikingly absent from service user narratives. Instead, their focus was on the operational flexibility of the service that they received, with high emphasis placed on the capacity of low-intensity interventions to overcome many user-centred barriers to care.
Perceived flexibility stemmed from two distinct, yet complementary, features of the low-intensity interventions evaluated in the trial: (1) the provision of an omnipresent health technology (i.e. the self-help manual or supported cCBT program); and (2) the delivery of brief PWP support, capable of being delivered remotely as well as face to face. Unbounded access to a health technology was advocated as a major strength of both supported cCBT and guided self-help, enabling participants to engage with professionally endorsed therapy materials at a time and place convenient to them. The provision of telephone support maximised this flexibility, facilitating access to PWP guidance and overcoming multiple geographical and practical barriers to care:
If you have a psychologist you have to make time to go. They give you specific days you’ve got to be there. If you’ve got a book or you’re doing it on the computer you can do it yourself when you want to do it, when you feel like doing it, not when the appointment’s due or something like that. It’s a more casual approach isn’t it?
P1446, male, supported cCBT
When you have a very little daughter, it’s quite hard, which means I usually read the booklet at night when she is sleeping. And I tried to do the sort of work after she was in bed. And more, I tried at the weekend, because obviously during weekdays, my husband is working. At the weekend he’s at home, this means . . . I can do it at the weekend.
P1276, female, guided self-help
Flexibility in the timing and delivery of scheduled PWP support emerged as a critical factor influencing the acceptability of low-intensity interventions, particularly within the guided self-help arm, in which trial and service delivery protocols permitted practitioners to provide this support either remotely or face to face. For some participants, face-to-face provision challenged the notion of accessible care, reintroducing treatment barriers and diluting the advantages of a portable health technology:
[The PWP support] was going to be in [site]; the appointment day, from what I can remember, I was working that day, so I had to cancel it.
P1334, female, guided self-help
The place I got offered, it was too far . . . when we found finally, a place that is not far away, and easy travel for me, there wasn’t an appointment in that centre that was suitable for both of us. Plus, the problem was, I have a daughter . . . I don’t mind, she is a very good girl, and all the time I’m talking to someone she’s usually drawing, but they didn’t let children in the building. The phone . . . this for me, was easier. I don’t need to go out, still I got the one to one attention . . . I can’t say it would work for everyone, but personally for me, it was really suitable.
P1276, female, guided self-help
Enablement
Help-seeking for OCD is often delayed, in part because of egodystonic symptoms and a low level of public awareness about the condition. Within the current study, stigma and embarrassment emerged as two key factors influencing participants’ OCD disclosures. Trial participants recognised that low-intensity service models had potential to negate the need for face-to-face contact and that, by doing so, were capable of preserving anonymity in care:
So, I thought, well, OK, that’s something I can do at home, I can do it in the privacy of my own home, because going, actually going to a counsellor, in the first place, I think, is quite daunting, that you’re expected to sit there and talk to somebody, you know, so, yes, so I plumped for the computer one.
P1006, female, supported cCBT
I do. I think on the phone’s quite good because you’re not here in the room are you, so you’re not seeing the reactions on the face, I’m not seeing your reactions if I tell you something bad that you’ve sort of looked disgusted at me or interpret anything, and I don’t know who you are either, and you’re miles away, so . . .
P1213, male, guided self-help
Anonymity and privacy were two features of treatment that, although important to most service users, were a specific priority for individuals experiencing intrusive thoughts of a sexual or a violent nature. Within the context of OCTET, ‘privacy’ was a construct that applied to both the characteristics of the environment in which treatment was received (often a participant’s own home) and the health technology platforms with which people interacted. A secure cCBT interface conveyed a sense of confidentiality that was unavailable in the guided self-help arm, which when combined with a lack of face-to-face contact, provided a novel opportunity to disclose important yet sensitive information:
Because it was passworded as well it became private and I knew that nobody would be looking at it. I mean I don’t know if that’s true. I don’t know if you can even see what I’ve done, but I think I felt like it was mine and mine only . . . There are some questions on there that I’ve answered really honestly, but I don’t think I would’ve answered them to a person and some of them I struggled answering with [the researcher]. I think from that you’d probably get a better result because it’s private . . . you’re not telling anyone anything really; you’re just doing it with yourself so you can be more honest.
P1198, female, supported cCBT
Competing participant narratives revealed somewhat divided opinions regarding the therapeutic benefits of non-face-to-face care. Although some participants clearly benefited from the privacy that low-intensity interventions conferred, others argued that face-to-face contact was essential to overcome the shame associated with OCD disclosure and ensure that an appropriate level of therapeutic progress was maintained. Such views were evident across both the supported cCBT and guided self-help modalities. Often informed by direct personal experience of face-to-face therapies, they hinted at a possible disjuncture between users’ initial preferences for treatment and subsequent service satisfaction:
Originally . . . I would rather have had something where I could just do it myself . . . but I’ve changed my mind now that I’ve started therapy sessions . . . I personally found that more helpful. Like I said originally I’d have probably said ‘Oh no I just want to deal with it myself’, but having started the therapy sessions, especially with the person I’ve just started with now, it’s like quite a bit of a weight off your mind as well when you can talk to somebody.
P1027, female, supported cCBT
Face to face, they’re asking you for answers and if you’re not giving the right answers, she’ll explain why, or saying you’re going off on a tangent and then pulls you back in and then you have to face what you’re saying and sometimes you don’t . . . I think you do hide from it, you do hide from what you want to say.
P1282, male, guided self-help
Applicability
The process of psychological therapy is typically conceptualised in terms of two key domains: specific and common (or non-specific) factors. Specific factors refer to the elements, principles or techniques that are clearly delineated by proponents of that therapy as the active causes of change. Common factors refer to elements that are present across multiple therapeutic models or are not specified in the theoretical or practical delineation of the therapy in question.
In the context of the interventions evaluated in OCTET, the specific factors comprised CBT principles delivered predominantly through an internet (cCBT) or bibliographic (self-help manual) platform. Common factors included the elements and characteristics of the interpersonal relationships forged between users and practitioners. Participants were able to distinguish clearly between these two intervention components, with conflicting discourse reflecting personal differences in the value and significance attributed to them.
For most participants, the interpersonal components of treatment remained a central feature of mental health care. Among these individuals, guided self-help attracted proportionally more support than supported cCBT, primarily because of its enhanced level of PWP input. Although the specific factors underpinning the two interventions remained similar, supported cCBT was more likely to be perceived as a suboptimal intervention, because of its inherent lack of richness in feedback and interaction.
I quite liked the fact that I had a face-to-face appointment once a week, I got on really, really, well with my therapist, [name]; she was lovely, and I think that was probably the bigger help to me than the book actually.
P1047, female, guided self-help
Really isolation, loneliness, cold, not very friendly, you know, it might be saying all these things to you but at the end of the day you’re talking to a machine you’re not talking to a human being, and things like that so, the reactions, the gestures, the smiles, and all the rest of it, and things like that, you can’t get that.
P1149, male, supported cCBT
In a small number of cases, neither of the two trial interventions was felt to provide adequate therapeutic support. Guided self-help was criticised, albeit infrequently, for time-bounded protocols that were perceived to limit a shared dialogue and negatively impact on the therapeutic alliance:
I’m not saying the guided self-help is not a good thing, I’m just saying I think talking is a better way really, you know, for somebody who has problems . . . I wouldn’t say she didn’t have the insight but I think she was working to the trial . . . she was working to trial guidelines . . . she had a set time to do it in. And so I thought it wasn’t, you know, a one on one situation, if you like, it was just me giving my opinion really . . . rather than a one on one, you know.
P1443, male, guided self-help
Comparatively few participants identified the acquisition of specific CBT techniques as the only driver of therapy engagement. At most, the prioritisation of self-management strategies appeared to diminish rather than negate the need for professional input, giving rise to two separate yet essential components of intervention delivery:
So yeah, I mean, the techniques are not going to work without the therapist, because you’re doing them for . . . well, you’re not doing it for the therapist, but you don’t want to let the therapist, you’ve got to be doing what he says. But equally, it’s the techniques as well, isn’t it, those are the techniques that make you understand it more, and enable you. So to me, the techniques win, but the therapist is equally, you know, as important, just slightly the techniques are more.
P1188, male, supported cCBT
With the vast majority of participants emphasising a need for practitioner contact, choice in the delivery mode of the PWP sessions became vital. Different communication methods provoked different responses in different individuals, leading to an additional layer of complexity in participants’ appraisals of intervention acceptability. Although criticisms of remote service provision were by no means universal, a key observation emerged – service user views of supported cCBT and guided self-help were both occasionally challenged by the practical difficulties encountered in non-face-to-face support and/or the socioemotional responses that these more innovative models engendered:
The only thing I didn’t like much, and this isn’t obviously your fault because it’s pragmatics, but, doing it over the phone was a little awkward somehow. It reminded me of a call centre. Not at all that the therapist had a call centre manner, it was just the act of being on the phone rather than being face to face, it just made it a tad more difficult, but not impossible.
P1083, male, guided self-help
Relevance
Obsessive–compulsive disorder is an often chronic mental health disorder comprising multiple symptoms and subtypes. Participants’ existing knowledge of OCD and condition management emerged as a significant influence on engagement with trial interventions. Many participants perceived the content of the resources to be too superficial, and/or restricted in scope, with potential relevance only to those with little therapy experience or knowledge of their condition:
I mean, there’s much more information out there than there was when I was young, you know, much more than even 10 years ago, that you can find out things on your own really on the internet and whatever. So really, maybe if that was somebody, like, newly diagnosed, it would be really good but I think I’m so, sort of, like an old hand with it that, you know, it probably didn’t have as much effect on me.
P1185, female, guided self-help
To this end, participants relied heavily on PWP support to adapt and personalise therapy materials, the success of which ultimately depended on the amount of professional contact available and individual practitioner expertise. For the most part, the availability of greater practitioner input into the guided self-help arm increased its capacity to deliver individualised care. On one occasion, however, a decision was reached to discontinue therapy because of the perceived irrelevance of the guided self-help material. Whether or not this decision was driven by the practitioner’s strict interpretation of trial protocols or reflected an underlying prejudice or lack of confidence in adapting therapy materials to a particular symptom profile was unclear:
. . . she [the PWP] said, you know, maybe you want to have a look through the booklet and see what you think. And I think because she was wondering whether it would actually be suitable for me . . . because it was something which was based on obsessive thoughts, and my issues were more about obsessive . . . about compulsive actions. I think she was, sort of, saying well, do you want to have a look through this and see if it applies to you, and having a little bit of a think about it, you know, it’s not really . . . I can’t use that tool to stop myself doing my OCD symptoms. And I think she said, you know, you should really only get assigned to this if you were more obsessive thoughts based.
P1364, female, guided self-help
User dissatisfaction with the supplied therapy materials was most evident in the supported cCBT arm, where the relatively fixed content of the internet program generated perceptions of a less tailored and, by implication, more superficial intervention model. Restricted contact between cCBT users and PWPs was perceived to exacerbate the weakness inherent in the computerised program, reducing its application to different disorder subtypes and lessening its potential reach:
I suppose it was probably the worst actual, you know, out of all of them. Because it had to be . . . the worst thing about it, you go to see an individual therapist, he analyses you and then he comes up with his strengths, but because that program has to be for everybody with OCD, it was too generic, it was too . . . and there’s no other way you can do it . . . it was too kind of [I] couldn’t hone down on it.
P1188, male, supported cCBT
Responsiveness
Intervention responsiveness is a concept that aligns with, but remains separate from, intervention relevancy. Whereas relevancy refers to the applicability of original therapeutic content, responsiveness refers to the provision of subsequent opportunities for clarification and information exchange.
Common expectations among participants were that low-intensity interventions, by virtue of their reliance on portable health technologies, would enhance the flexibility of OCD treatment and empower service users to progress through therapy at a self-determined rate. Yet, within the supported cCBT arm, technological and therapeutic design constraints slowed participants’ progression through the sessions, reducing the utility of the internet program and negating one of its principal benefits:
I mean I didn’t know how the computer program was going to be, but I find the time I have had to do it I’m going with it and then it stops and it says you’ve got to wait 24 hours. I thought oh, it’s a real bugger because I want to carry on. But we’ll get to . . . I mean I know that’s what they’re replicating. But it would be better I think if it was more flexible, otherwise you may as well go and see someone face to face.
P1198, female, supported cCBT
Rather than mediating practitioner contact, computerised therapies are designed to replace professional input; cCBT support was permitted within OCTET, but was constrained in both time and scope. More so than guided self-help, supported cCBT was perceived to lack the depth of contact necessary to facilitate a user’s understanding or respond dynamically to changing concerns:
No, I thought it would be good to have a one to one as well, because if you’re on the internet, you’re not conversing, you can’t ask questions can you? So, in the one to one, it was very handy because you could ask questions about this, and ask questions about that. He was setting me exercises, I know I do them on the computer program as well, but . . . If you’ve got any problems, you can’t ask the computer can you really?
P1008, male, supported cCBT
Motivation
Few participants had been exposed to low-intensity therapies for OCD prior to OCTET, although many had informally engaged in self-help by reading books, participating in internet forums and researching their condition on the web. Those consenting to interview regarded OCD as a chronic condition and very few expected a ‘cure’. Nevertheless, most seemed content to trial a new intervention, in the hope that it would alleviate the symptoms and burden of OCD via an enhanced capacity for self-management:
Yeah, definitely, I mean, I’ve lived with it (OCD) for years, and I know that it’s got to be . . . I need help to move on to try and . . . and any help that is offered, or any help that is available, I’d be prepared to take that.
P1207, male, supported cCBT
Maintaining the motivation to remain in treatment was more challenging. Central to participant discourse was the recognition that external support systems were critical to treatment progress, with proactive, repetitive and scheduled contact providing a much-needed impetus for intervention engagement:
Yeah, I think it was once a month. I can’t remember. That’s it. Mrs X I think they call her. They sort of encourage you to carry on I think. That’s what you miss, encouragement, when you’re doing it on your own on the computer or whether you’ve been left, you miss the encouragement and the support, and you feel lost.
P1446, male, supported cCBT
I think somebody phoning, that was helpful . . . You can always turn a computer off; you can’t turn something like that off!
P1158, female, guided self-help
Differences in the levels of PWP support permitted under the guided self-help and supported cCBT protocols emerged as a key influence on therapy continuation, and thus, by implication, on intervention acceptability. Less clear were participants’ views regarding the source of support that was required. For some, non-clinical contact, provided as part of the trial was sufficient to ensure ongoing treatment participation. Although small in number, these occurrences occurred across both guided self-help and supported cCBT arms, raising the possibility that the mere instigation of monitoring may ultimately be more important than the provision of clinical expertise:
I would think, well, I’ll do that, I’ll do it. And it was constantly trying to put it off. Whereas with you coming out, you know, coming in and doing the interviews and what have you, it was a lot better, because you know that you are there. So I am doing the questionnaires and what have you, and it’s carrying on with the treatment. I wouldn’t . . . if it had carried on with the computer, I don’t think I’d have . . . well, I know I couldn’t have carried on with it.
P1207, male, supported cCBT
Yeah, I’m really pleased, and thank you for ringing and speaking to me. I know it’s a long time on the phone, but it’s really helpful. I mean that’s the thing, speaking to you again, it reinforces my desire as it is to want to do something about it. So although you’ve not really given me any treatment as such, it is in a way treatment for me because it’s making me just more aware, keeping me aware of what’s going on around me and addressing it. So it’s good, so thank you.
P1198, female, supported cCBT
Summary of findings
Qualitative research was nested in the intervention arms of OCTET to explore participants’ views of two low-intensity interventions for OCD. Group consensus suggested that the provision of low-intensity psychological interventions may confer substantial benefits in terms of increasing the perceived accessibility of psychological treatments for this population. Both guided self-help and supported cCBT were professed to increase perceived service flexibility, overcome intervention access barriers and sustain, where desired, a sense of anonymity or privacy in care.
Both guided self-help and supported cCBT are based on CBT principles. Integral to both interventions is the concept of problem-solving, through which participants were encouraged to work in a time-limited process towards specific, agreed goals. Much of the rise in the popularity of low-intensity interventions has been attributed to their ability to convey these empirically grounded techniques (CBT) with limited therapist resource. Paradoxically, however, the findings of our current study suggest that a minimum amount of therapeutic contact may be necessary to optimise the acceptability of these interventions.
Intervention acceptability emerged as a complex construct that extended beyond a basic concept of accessible care. Key differences in the two interventions were observed in terms of their capacity to provide the type or components of therapy most valued by service users, the ability to tailor therapy to individual symptoms and experiences, their capability to respond dynamically to participant need and to sustain participant motivation for therapy engagement. Although not guaranteed, greater amounts of practitioner contact appeared to increase the likelihood that these requirements would be met, and thus that the intervention would be positively appraised by study participants. With the two low-intensity interventions differing in the amount of professional support available, guided self-help benefited from greater PWP support and personalisation and thus was more likely to be the subject of positive feedback.
As with any study determining the acceptability of a new health intervention, variation in patient preferences and values will be observed. For a minority of individuals in the current study, access to evidence-based techniques was a priority and was in itself sufficient to confer treatment satisfaction. cCBT, with proportionally less practitioner support, thus still attracted a level of positive participant feedback, albeit less frequently and among the most self-efficacious participants.
Increasingly, innovations in treatment delivery are being proposed by health-care providers to maximise the availability, accessibility and cost-effectiveness of mental health care. Differences in the underlying treatment experiences of different individuals raises the question of whether the acceptability of low-intensity interventions is determined more by the features of the delivery model itself or the characteristics and underlying treatment preferences of the patients being referred. The current study identified six different characteristics of ‘good’ therapy, against which the acceptability of the trial interventions was judged. Future generations of low-intensity treatments for OCD, whether computerised or manual based, should seek to encompass and optimise features to maximise therapeutic uptake and population reach. Greater recognition of each of these characteristics and of their potential loss in current incarnations of low-intensity interventions would ultimately enable service users to achieve their desire for personalised treatment with a more rapid, anonymised and less stigmatising pathway to care.
Strengths and limitations of qualitative study 1
Qualitative exploration of the views of trial participants provides critical insight into the experiences of low-intensity intervention users. However, it is nonetheless subject to many limitations inherent in qualitative research, particularly with respect to generalisability. Nesting a qualitative study within a trial inevitably raises the possibility of selection bias. All interviewees had consented to participate in a randomised trial of low-intensity interventions for OCD and thus may be argued to display a level of openness towards low-intensity interventions atypical of a broader service population. OCTET recruited participants from waiting lists for high-intensity CBT and it is, therefore, possible that satisfaction with the trial interventions was elevated because of the difficulties participants were experiencing in accessing usual care. Conversely, it may also be argued that participants’ existing treatment expectations could negatively bias attitudes towards alternative and less-intensive forms of care. OCTET offered low-intensity interventions as a precursor, rather than a replacement, to high-intensity CBT. This design reflected the ethos of the NICE-recommended stepped-care models for adults with OCD. Within stepped-care models, low-intensity interventions are advocated as front-line treatments for mild impairments, with the option of ‘stepping up’ to higher-intensity care if initial intervention is ineffective. Hence, our nested trial design offered an opportunity to explore important tensions between users’ treatment priorities and preferences, and the economic and political drivers behind the routine integration of low-intensity interventions into mental health care.
By employing a purposive sampling approach, we ensured maximum variation in the ages and geographical locations of study participants. Heterogeneity in ethnicity was more limited. Our sample was predominantly white British, reflecting an underlying bias in our trial population. Had it been possible to include the views of individuals from different ethnic backgrounds, this may have contributed substantially to informing culturally relevant and sensitive care.
The majority of the interviews were conducted by a single researcher, who had also conducted trial follow-up visits for the purposes of quantifying intervention outcomes. Although this may have hindered as much as facilitated participants’ willingness to discuss their treatment experiences, the broad array of views expressed in relation to supported cCBT and guided self-help go some way to negating this concern. Independent data coding, undertaken by three academic researchers in collaboration with a service user representative, raises confidence in the rigour of our analysis. The views of those allocated to a waiting list for high-intensity CBT were not explored qualitatively; as such, data were unable to illuminate treatment experiences. They may, however, have offered an objective insight into the treatment expectations and preferences of a clinical sample, not affected by recall bias or the post hoc reconstruction of events. Baseline treatment preferences were measured quantitatively in the trial to redress this knowledge gap.
Study 2: acceptability of guided self-help and supported computerised cognitive–behavioural therapy to health professionals
The aim of this study was to explore the acceptability of supported cCBT and guided self-help from the perspective of those delivering treatment, the PWPs.
Methods
Sampling and psychological wellbeing practitioner recruitment
Sampling for this study was purposive; invitations were sent to all PWPs who had delivered one or both of the trial interventions to at least one participant. Invites were not sent to one site at which recruitment had not commenced because of a lack of waiting lists and to one other site because of research governance delays. The final sample size was determined by the limits of the population and the numbers of PWPs consenting to take part.
Between October 2013 and March 2014, all eligible PWPs (n = 71) were e-mailed a personal invitation letter, information sheet and consent-to-contact form. Upon receipt of a completed consent-to-contact form, the OCTET trial manager made contact with the PWP to address any outstanding queries and arrange a convenient interview date. Participation in the study was voluntary and no reimbursement was offered for taking part. Verbal, recorded consent was taken prior to each interview.
Data collection
All interviews were conducted over the telephone. A semistructured interview schedule was developed from prior knowledge of the research area, via discussion between clinical and academic members of the trial team in conjunction with a PWP working at one of the trial sites. A preliminary interview schedule was piloted with an independent PWP employed by the NHS but not taking part in OCTET. Minor amendments to question length and flow were made on the basis of their feedback. The final interview schedule explored:
-
PWPs’ personal views of the low-intensity treatments they delivered (guided self-help and/or supported cCBT)
-
perceived influences on patient engagement and outcomes
-
any issues or challenges that PWPs experienced while delivering the intervention
-
potential barriers, and facilitators, to implementing guided self-help and supported cCBT in routine practice
-
PWPs’ views on their involvement in the trial as a whole.
All interviews were conducted by a qualified PWP, employed as a member of the trial team to deliver treatments as part of OCTET. Qualitative interview training, covering obtaining consent, conducting interviews and qualitative data analysis was provided by two experienced qualitative researchers, both of whom were part of the OCTET team. Interviews ranged in length between 18 and 125 minutes; all were completed by telephone.
Data analysis
Interviews were audio-recorded and transcribed verbatim. Anonymised transcripts were imported and managed in NVivo qualitative data analysis software.
Transcripts were read and reread independently by four researchers comprising the trial manager, an OCTET research team member and two PWPs, one of whom conducted interviews and delivered treatment as part of the trial. Qualitative analysis training was provided to the PWPs prior to the study commencing. The in-depth training comprised general aspects of collecting and analysing qualitative data, incorporating practical exercises on the different phases of thematic analysis.
Data coding was led by the trial manager, who met the other researchers periodically to discuss their interpretations and establish a shared coding manual. Interview data were subjected to a thematic analysis,85 using the constant comparison method. 86 Although traditionally associated with the generation of theory, the constant comparison method can be used in other forms of qualitative analysis. The method ensures that all data are systematically compared with all other data available in a data set. 87 As the constant comparison of new data occurred and the researchers’ understandings of the themes under consideration developed, the coding manual was amended and reshaped to enable the introduction of new codes and/or the deletion of redundant, similar or otherwise compromised codes. In line with current gold standard recommendations, data were analysed without prior knowledge of trial effects.
Results
Sample characteristics
A total of 20 PWPs responded to the initial invitation, all of whom subsequently participated in an interview. Table 45 provides details of the PWPs who took part. Data for the PWPs who had been allocated a patient and returned a PWP demographic questionnaire are provided for comparative purposes.
| Characteristic | Sample | |
|---|---|---|
| Acceptability (n = 20) | Trial (n = 68) | |
| Age (years) | ||
| Range | 24–59 | 24–61 |
| Mean (SD) | 34.1 (9.49) | 33.9 (10.8) |
| Sex, n (%) | ||
| Female | 18 (90) | 59 (87) |
| Male | 2 (10) | 9 (13) |
| Highest educational qualification, n (%) | ||
| Undergraduate degree | 7 (35) | 12 (18) |
| Postgraduate certificate | 8 (40) | 30 (44) |
| Postgraduate diploma | 3 (15) | 14 (21) |
| Master’s degree | 2 (10) | 9 (13) |
| PhD | 0 | 1 (2) |
| Length of time in PWP role, n (%) | ||
| 6 months to 1 year | 1 (5) | 6 (9) |
| 1–2 years | 4 (20) | 17 (25) |
| 2–5 years | 15 (75) | 44 (65) |
| Received OCD training as part of IAPT training, n (%) | ||
| Yes | 9 (45) | 37 (54) |
| No | 11 (56) | 31 (46) |
Representativeness of the sample
Practitioners involved in the qualitative study represented 11 of the 14 sites recruiting patients to OCTET. The mean number of patients treated per practitioner was four. The sample had a similar sex and age distribution to the overall sample of practitioners participating in OCTET. The vast majority of the acceptability sample (75%) had been in post for > 2 years. Compared with the overall sample delivering trial interventions, a smaller proportion of practitioners in the acceptability study had received OCD-specific training prior to starting OCTET (45% vs. 54%).
Practitioner views
From the perspective of PWPs, the acceptability of low-intensity psychological interventions for OCD was based upon their perceived fit with:
-
patient lifestyle
-
patient need
-
existing service delivery protocols
-
intervention objectives and purpose
-
practitioner
-
practitioner role.
These six issues emerged directly from the data and take into account matters that are both patient centred and professionally relevant. They thus provide a professionally centred platform through which to examine the relative strengths and weaknesses of guided self-help and supported cCBT. Participants are assigned a number rather than a name or pseudonym within the text. Sex (male or female) and length of professional experience (≤ 1, ≤ 2 or ≤ 5 years) are provided.
Fit with patient lifestyle
Increasingly, low-intensity psychological interventions are being advocated to maximise the availability and accessibility of effective mental health care. Among PWPs in the current study, the provision of any intervention that enhanced access to services was considered a major advantage for people living with OCD.
Reminiscent of the underlying philosophy of the stepped-care model, practitioners who had been exposed to the OCTET intervention conceived substantial benefit in moving towards a sequential service delivery model that offered lower-intensity interventions as a first-line treatment:
I think particularly with the client I was speaking with and seeing the benefits of that I think that it’s made me feel quite hopeful about the intervention being used at step 2 and the usefulness of a brief intervention rather than the person being on a longer waiting list to be seen at step 3. They could get a lot of benefits from the interventions available at step 2.
167, female, > 2 years
Many of the PWPs who were interviewed acknowledged the need not only to increase treatment accessibility on a population level but also to enhance patient access at an individual level. Akin to service user views of the low-intensity interventions, PWPs were consistent in emphasising the flexibility of the treatments they provided and their capacity to overcome different user-centred barriers to care. Across the PWP sample, advantages were conceived both in terms of the opportunity to provide patients with a readily accessible health technology (the guided self-help manual or supported cCBT program) and the choice of accessing brief support remotely as well as face to face:
We did a mixture [of phone and face-to-face sessions] because she had child-care issues because it wasn’t every session and it was intermittent, by alternating between phone sessions and face-to-face it actually worked quite well, because she wasn’t under the pressure of having to try and get to the sessions, it meant I knew where she was at . . . She liked the flexibility of having some telephone and face-to-face sessions as well, it created less stress for her because she didn’t want to miss any sessions, and knowing that the phone calls were there kept her on track.
53, female, 2–5 years
Although enhanced access was advocated as a strength of both supported cCBT and guided self-help, PWP discourse revealed some unique advantages to cCBT that could not be gained so easily from other delivery methods. In seeking to provide effective therapeutic intervention independently of practitioner support, supported cCBT appeared to hold more promise as a flexible and accessible intervention unconstrained by service norms:
. . . the thing is, that when you’ve got your sessions with somebody, you know, if they don’t remember what you’ve said, and they didn’t take any notes . . . they might look back at their guided self-help book and think, now [the PWP] said something, and that really triggered in my mind, now what was it? They might not remember it, but if they had access to OCFighter . . . they could replay it! And I just think, then, that’s . . . and they can replay it 24/7 as long as they’ve got access to the internet.
127, female, > 2 years
Fit with patient need
Obsessive–compulsive disorder is a diagnosable mental health disorder associated with reduced quality of life and substantial impairment of role, specifically work, social, home and family/relationship functioning. 5 Reflecting on their beliefs at the start of the trial, many PWPs acknowledged OCD as a complex condition with symptoms that were often ‘ingrained’ and requiring ‘deep-level work’. Initial expectations revealed a level of scepticism towards low-intensity interventions for OCD, a perception that practitioners themselves recognised was fuelled by a lack of prior exposure to such treatments and/or professional cynicism regarding their underpinning evidence base. Negative attitudes towards low-intensity psychological interventions for OCD were, for the most part, transient and once PWPs had undergone training and experiential learning via OCTET, more positive views emerged:
. . . once [guided self-help] is up and running, people have got a really good knowledge of what they need to do, it’s just continuing to implement it and managing with the challenges that are faced as a result of implementing it. But, I think it’s very, very clear and very easy to deliver, and I think that’s what was really good about it, and very fitting to a person’s problem as well, because the compulsions often take over their day, their time. And actually, it’s an intervention that allows a person to gain back some time, and some of their life in terms of engaging in other things as well.
09, female, 1–2 years [reproduced from Gellatly et al. 88 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made]
Some key implementation issues nonetheless remained. PWPs retained the view that OCD is a complex mental health disorder, characterised by an exceptionally heterogeneous symptom profile. PWPs therefore stressed the importance of tailoring any psychological intervention, whether high or low intensity, to service user preference and need.
I’d always adapt, and then try and pace it and deal with whatever the patient presents. So it’s prescriptive but flexible. It’s down to you as the therapist to meet the needs of the patient. [The interventions] gave us the steps and so on, so I’d got a clear plan on how to do it and deal with it [OCD], and then it sometimes is that flexibility, when to go back and repeat the early steps and motivate the patient and bits and pieces. So it’s prescriptive, but on the other hand you are using your own knowledge and experience to adapt to the patient’s needs.
158, male, 2–5 years [reproduced from Gellatly et al. 88 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made]
Notably, however, practitioner concepts of treatment personalisation rarely prioritised changes to therapy content over changes to treatment delivery protocols and access routes. Being able to offer different interventions that varied in presentational style and therapeutic contact was perceived to be particularly advantageous, enabling practitioners to adopt a more flexible and beneficial treatment approach. In this regard, both guided self-help and supported cCBT were perceived as viable and acceptable interventions, capable of enhancing service provision and extending patient choice:
I think it’s good to offer people a variety of options and interventions to meet their needs. And yeah, just to be flexible with the patient . . . Some people prefer the online programs than seeing somebody one-to-one.
06, female, > 2 years
Key to ensuring PWP acceptance of low-intensity interventions was the ability to offer flexibility in one-to-one support. The frequency of support sessions were discussed repeatedly and were subject to contrasting views across both interventions, specifically in relation to patients’ motivations for treatment engagement. Although some practitioners perceived that weekly contact with patients was necessary for them to maintain therapeutic focus, others alluded to the possibility that patient uptake of one-to-one support may, for some clients, be a consequence of its availability rather than necessity:
It [weekly sessions] keeps people’s focus, so I don’t tend to . . . and you know whether they’re not engaging, so you can help them to engage. If you leave it 2 weeks you can . . . well, if you left something with me for 2 weeks I’d forgotten the whole lot, you know what I mean, it’s gone!
40, female, > 2 years
. . . it’s funny actually because . . . with the cCBT obviously the . . . you have six sessions with . . . six sessions, 10 minutes each, but actually despite the much level of support people . . . clients really took to that. They didn’t complain about not having enough support whereas the guided self-help it does offer people more support and I feel that people take what they’re given, and I think it made me realise that actually some people are motivated enough, can get on with the program, and just have very little support from a clinician.
205, female, > 2 years
Ambiguity surrounding the impact of regular support on patient motivation was often superseded by concerns regarding the negative impact of excessive appointments. A number of practitioners discussed the difficulties that some patients may experience in fitting weekly sessions into busy and chaotic lifestyles. Others viewed less frequent sessions not only as more convenient but also vital for skill consolidation and optimal therapeutic pacing:
. . . all the documentation says weekly therapy is seen to be more effective. I’m not certain it is, especially if you’re talking in a CBT format, because patients have to learn set techniques and start practising them, which can often take, I think, longer to understand and interpret the information and nibble away into forming that practice of the techniques and repeating it.
158, male, > 2 years [reproduced from Gellatly et al. 88 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made]
Fit with existing service protocols
Although the notion of delivering effective psychological interventions for OCD was attractive to PWPs, the ease of integrating the OCTET interventions into routine service provision was deliberated. Some practitioners alluded to potential resistance from high-intensity, accredited therapists who they feared may question changes to long-standing, embedded working practices and assumptions:
. . . well, I don’t really know, I can’t speak for them, can I, I don’t really know. I think that some CBT therapists think that the self-help with OCD wouldn’t work, I think there would be resistance from CBT therapists, the high workers. I think . . . it seems that that’s a view they still hold about OCD, that maybe it’s not the type of condition you should be working with at step 2.
84, female, > 2 years
For others, operational support was seen as key. Ongoing effort was deemed necessary to enhance practitioner competencies, maintain organisational standards and ensure an equitable and high-quality patient experience. Specifically, the availability and standardisation of disorder-specific training for low-intensity workers was considered a prerequisite to a national roll-out of low-intensity interventions for OCD:
I hope so [that low-intensity interventions could be implemented for OCD at step 2] . . . I think hopefully showing them the effects of perhaps the trial and also making sure that all of us as step 2 practitioners have consistent training again because I think that’s the reason why it was kept to step 3 because some hadn’t had any training. Some had had some training so I think say for example it wouldn’t be fair if a client went to one step 2 practitioner to get treatment around OCD when, if they were assessed by another person, they’d be stepped up. So I think as long as the consistency of training across step 2 was there . . .
89, female, > 2 years
Central to participant discourse was the recognition that research trial conditions often remained distinct from those experienced in routine clinical practice. Thus, although the trial had demonstrated the feasibility of both PWP training and low-intensity interventions for OCD, some major challenges to implementation were likely to remain. Potential incompatibility between the intervention delivery protocols specified for the trial and the resources available to deliver them on the ground was a key concern:
It depends, because if I’m speaking in regards to my manager at the moment, there’s no way she’d allow us to do 12 sessions, so I’d say, no, to that, but if there was any way of cutting it down so it wasn’t 12 sessions, then I think she’d be a bit more open to that. So I really don’t know, if it was 12 sessions, I’d say, no, because I think would be the answer that she’d give me.
88, female, > 2 years
Fit with intervention objectives and purpose
Guided self-help and supported cCBT both fall under the umbrella of self-managed therapy packages. These packages, by definition, are supported by limited amounts of contact with a paraprofessional. Practitioners’ perspectives on the acceptability of these two interventions were thus frequently influenced by the extent to which the two interventions facilitated efficient therapeutic care. Contrasting the breadth and depth of the resources provided under trial conditions with those normally available to practitioners in routine services, PWPs acknowledged the value they placed on having a ready-made therapy manual and the assurance that this provided in terms of optimising the quality and safety of their work:
I think because it’s having a tailored manual and a tailored online program, it’s had a lot of thought and evidence, and work go into it, and I think what can happen at times is the techniques might be the same, or the method might be the same but you’re scrambling to find information and get things together that are appropriate. And, actually having a ready-made manual that can be used in a session, and is very self-explanatory, and it’s something that the client has access to, and the practitioner has access to, I think it just makes it a much more safe and effective way of working at step 2.
09, female, 1–2 years
From a patient’s perspective, practitioners perceived multiple benefits to having a well-structured therapy package. At a micro level, interesting materials were advocated to encourage patient engagement, while at the meso level, much emphasis was placed on the potential impact of presentational style on service credibility:
I think the structure of the book in particular, because that was her first comment when she came back, she read the whole thing and then came back and she was like oh, her excitement helped, she goes I’m looking forward to this . . . she likes the look of the book and she could see the therapy behind it.
53, female, > 2 years
. . . it helps towards them having . . . this looks like a professional, serious . . . is going to work, but . . . yeah. Even if you didn’t show them the book and you worked through and went through it section by section and they never saw the book, I think they’d still benefit. But this adds to the credibility of the delivery of this intervention at this level, and that it’s taken seriously and it’s being invested in and it’s not just a bit of paper.
40, female, > 2 years
Discourse focusing on the perceived functionality of the two interventions revealed some specific differences in the acceptability of guided self-help and supported cCBT. Although guided self-help was seen as directly fulfilling the remit of a self-managed therapy package, procedural delays in accessing the cCBT program reduced its usability from both patient and practitioner perspectives. Lock-out time points, automatically imposed after the completion of specific therapy steps, were viewed as a specific barrier to user engagement:
One guy pointed out, if I don’t finish one step until 9 o’clock at night, he said it’s not going to be available to me again until 9 o’clock the following night. He said it should be 24 hours from when he starts the program, because by the time nine o’clock the next night comes round, he said ‘I’m too tired to go on to it’. He’s then got to wait another day to get on to it, so he loses the flow, which I thought was a fair point really.
130, female, > 2 years [reproduced from Gellatly et al. 88 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made]
Although such lock-out points were deliberately sanctioned on the basis that they would facilitate client learning, other ad hoc difficulties in supported cCBT access were also reported. For some PWPs, these unanticipated complications were sufficient to render supported cCBT ineffectual, slowing patient progress and demanding more time-intensive support than delivery protocols allowed:
They, kind of, lost interest because they’d got so frustrated with it . . . I don’t think any of my cCBT people have actually stayed the course . . . a lot of them weren’t able to do anything. They weren’t able to move on from that first section.
130, female, > 2 years
Oh gosh . . . let’s see, it was that they couldn’t access it because they only had a tablet, they couldn’t access it because they were using the wrong . . . wrong Internet Explorer . . . sometimes the video would just stop, and then they’d have to watch it all over again. And I did have that happen to me a couple of times. But I think it was my internet connection. The most annoying thing was that I couldn’t access it at work.
127, female, > 2 years
Fit with practitioner skills
The PWP role predominantly focuses on supporting patients through low-intensity interventions, case-managing referrals and signposting to other relevant services, dependent on patient need. Minimum quality standards of training are in place within IAPT services to ensure that the needs are met to enable practitioners to perform their role competently and confidently. Although a small minority of PWPs believed that the knowledge and skills they had developed previously could be drawn upon to deliver low-intensity interventions for OCD, the vast majority initially lacked self-confidence in supporting patients with this diagnosis at step 2:
It’s that, kind of, the feeling of unknown, really. I hadn’t got any experience in it at all, but I think also perhaps a bit of the stigma that we have with regard to the step 2 and the step 3. We’ve always stepped it [OCD] up. It’s always been something that’s been out of our remit. So you just think, well, if that’s always been the case, then this might not work and you’re going to struggle with it.
130, female, > 2 years
Equipping professionals with the necessary skills, knowledge and awareness through additional training was vital. PWPs reflected upon the benefits of training they had received as part of OCTET and the positive impact that this had upon their confidence. The importance of having the opportunity to become familiar with the interventions and practise the associated techniques was consistently regarded as important:
I think it’s just time really, and once you’re familiar with the techniques, and have a really good understanding of why we’re delivering those techniques and how they can work for people. When you’ve also seen that it has been helpful to other people as well, I think confidence in delivering them grows, and even though I didn’t work with many people with OCD in the trial, I think that really understanding the techniques, and actually having that practice in delivering the techniques it helped quite a lot, then those expectations soon changed.
09, female, 1–2 years
Differences in the levels and nature of support permitted under guided self-help and supported cCBT protocols emerged as a key influence on practitioners’ readiness to deliver the two interventions in practice. Growth in practitioner confidence was most evident in the guided self-help intervention, where the availability of written resources (i.e. a self-help manual) generated a greater sense of safety and security in therapy delivery. With greater scope for therapeutic interaction, the written resources that accompanied the guided self-help intervention were viewed as particularly valuable, serving not only as a mechanism to keep patients ‘on track’ but also as a direct source of practitioner reassurance and skill development:
Yeah, definitely, that was always good to refer back to. But I mean I had it with me in the sessions anyway. It did help in case . . . because obviously the anxiety around treating a new condition, but having it there was reassuring . . . That really helped, because obviously I needed to know where I needed to be at each session. I was going at the right pace but it was nice to know that I had the cues there to fall back on. I mean if I had got to use it more than once then each time it would’ve been easier and easier.
53, female, > 2 years
Fit with the practitioner role
Psychological wellbeing practitioners play a vital role within psychological services. Under IAPT service and training models, practitioners are explicitly trained to work collaboratively with patients, developing and maintaining therapeutic alliances and being responsive to individual needs.
Entrenched professional roles were a key factor influencing practitioner support for the two interventions delivered within OCTET. Most practitioners agreed that increasing service choice and accessibility would ultimately be of benefit to patients, yet several also voiced concerns regarding the extent to which these new interventions may denigrate their own practice. The majority of practitioners perceived direct therapeutic contact with a patient as a central tenet of their professional remit and one that could not easily be reduced:
I have to say I found it quite difficult, because it was, it was quite a strange experience really where I found I was ringing people up to see how they were getting on, with really no indication or idea of their problems at all.
111, female, > 2 years
With less scope for practitioner input, supported cCBT was typically viewed with a higher level of suspicion than guided self-help and divergent discourses reflected disagreement regarding the true role of the practitioner in this intervention. Several PWPs described ‘being absent’ or ‘taking a back seat’ in the cCBT intervention, which left them feeling at worst devalued and at best unanchored in their professional role:
I think it takes the one-to-one therapist relationship out of therapy, because it’s a computer . . . we’re saying the therapeutic relationship is key to any kind of improvement patients make, isn’t it? That’s the thing they always write on the feedback form; patient . . . therapist listened to me, therapist was nice, therapist helped me.
53, female, > 2 years
Those who had the opportunity to support guided self-help patients face to face highlighted the benefits of the approach, indicating that they worked more effectively and suggesting that patients’ needs were often better met. Guided self-help was frequently perceived as a ‘practitioner-led’ model (173, female, > 2 years), with many interviewees emphasising a preference for this intervention from the outset:
Certainly there is a bit more of me invested in that [guided self-help]. And I think that’s the case even with the sessions being different lengths as well. 10 minutes means you only have . . . you don’t really have that opportunity to develop a deep level of a therapeutic relationship with somebody in 10 minutes really. It’s more of a, oh, how are you? Check in. Any problems? Anything that you’re stuck with? OK, and you take good care and that’s about it really. Whereas I could see if you’re seeing someone especially face to face there is something different about that.
111, female, > 2 years
With the vast majority of PWPs emphasising a need for client contact, choice in the delivery mode of low-intensity interventions became paramount. Practitioners’ views of both supported cCBT and guided self-help were often challenged by remote working, leading to an additional layer of complexity in their appraisals of intervention acceptability. Despite alternative delivery approaches, such as telephone or web-based support, being advocated with existing stepped-care models, PWPs repeatedly raised concerns regarding the normalisation of these media, often in terms of the personal fears or socioindividual responses that these new delivery models engendered:
I prefer working with human beings, not telephones, not machines. I’ve always been interactive with other people.
158, male, > 2 years
In a small number of cases, negativity towards remote working was found to reduce over the course of the trial. PWPs who initially expressed a preference for delivering face-to-face support later contradicted this view, emphasising the vital role that remote facilitation could play in maintaining patient engagement. Such data raise the possibility that professional resistance to new working methods may be partially fuelled by a lack of experience of alternative delivery models and hint at a potential incongruity between acceptability judged from the practitioner perspective and acceptability judged on behalf of the patient:
We did an initial assessment appointment face-to-face and then we did the first treatment face to face, and the rest were delivered via the phone, and it was also during a late appointment, so it was quarter to six when I contacted the client, which was fantastic for her because otherwise she wouldn’t have been able to attend the treatment because she couldn’t have got off work. She had work commitments, and especially the amount of sessions that we were offering, it would have been really difficult for her to be able to access that treatment if it was nine to five . . . There were no problems over the telephone at all. The client prefers it. It was more accessible for her, and delivering the treatment was no different to face to face really.
89, female, > 2 years
Summary of findings
This qualitative study aimed to explore PWP perceptions of two low-intensity interventions, guided self-help and supported cCBT, for OCD. Intervention acceptability was judged both from the perspective of the professionals concerned, and their personal interpretations of patient need. Practitioners were consistent in acknowledging the advantages of low-intensity interventions at a population level. Both guided self-help and supported cCBT were advocated to overcome long-standing barriers to the delivery and receipt of mental health care, improving accessibility via enhanced service flexibility and patient choice.
At an individual level, differences in practitioners’ perceptions of patient need emerged as a key factor influencing their intervention acceptability judgements. Group consensus identified OCD as a complex and chronic mental health disorder with variable symptoms profiles and presentations. Concerns were thus initially raised regarding the ability of low-intensity interventions to meet the needs of this client group, with different levels of professional confidence generating different levels of readiness to deliver these treatments. Dedicated training and the provision of essential therapist resources were regarded as vital precursors of a service roll-out, and a particularly advantageous element of OCTET. Practitioner training was perceived to be instrumental in changing practitioner attitudes, reducing fears and ensuring the fidelity and delivery of the trialled interventions.
Potential challenges to national implementation nonetheless remain. Practitioners in the current study often referred to their own professional role and responsibilities, contrasting these with the remit of higher-intensity, accredited cognitive–behavioural therapists. Differentiation between these two service levels gave rise to a distinction in the types of patients and disorders traditionally seen by each group and thus to a prospect of wider professional resistance. A shared fear among several PWPs was that OCD was a disorder that conventionally fell within the remit of higher-intensity psychological services and thus was unlikely to be sanctioned for treatment by paraprofessionals. Even within the context of entrenched models of PWP, existing service philosophies and protocols appeared to affirm the importance and centrality of the therapeutic relationship, and thus sometimes brought into question the legitimacy of reduced client contact achieved via the instigation of self-managed therapy packages. Trial protocols stipulated the amount and nature of contact permitted for the guided self-help and supported cCBT intervention. Longer-term integration into services demanding remote working practices or seeking additional resource efficiency savings may only exacerbate this problem.
Studies of remote psychiatric consultations suggest that the normalisation of any new health technology into statutory health care is likely to be mediated both by the properties of the technology itself and the sociological orientation of its users. 89 With the two low-intensity interventions differing in the amount of professional support available, guided self-help aligned more readily with practitioners’ own expectations of their role. Differences in the presentation and functioning of guided self-help and supported cCBT resources exacerbated this effect, influencing the perceived suitability of the two different intervention models for the tasks in hand. Although supported cCBT was initially perceived to be an innovative solution to service access, technical difficulties were found to limit its reach. The importance of providing high-quality intervention resources was a key theme emerging from practitioner discourse, and a key feature influenced the perceived credibility and acceptability of low-intensity interventions. Future generations of supported cCBT and guided self-help must, therefore, ensure that they are optimised in this regard.
Strengths and limitations of qualitative study 2
Qualitative exploration of the views of practitioners provides insight into the experiences of delivering low-intensity psychological intervention to adults with OCD. All practitioners taking part in this qualitative study had already consented to deliver these interventions as part of OCTET. It may be argued that these individuals are not representative of the broader professional population working in statutory psychological services. It is important to acknowledge, however, that not all practitioners participating in the trial did so voluntarily, and a substantial proportion was required to do so by their employing NHS trust.
By employing a purposive sampling approach, we ensured that we recruited only practitioners who had delivered a trial intervention to at least one service user. Three-quarters of the practitioners taking part in our qualitative study had already accumulated ≥ 2 years of professional experience as a PWP working within IAPT services. This proportion was slightly higher than that observed across the whole sample of practitioners trained to deliver OCTET interventions. It is therefore possible that this enhanced level of experience was reflected in the data obtained. Notably, fewer than half of interviewees had received OCD-specific training prior to joining OCTET. Their perceived self-confidence in treating adults with this diagnosis may thus not be too dissimilar to other practitioners working in a non-research context.
Treatment delivery protocols and training for the low-intensity interventions evaluated within OCTET were distinct from routine practice. Nesting a qualitative evaluation of practitioner views within this trial enabled us to gain a critical insight into to the potential challenges and tensions that may be encountered in statutory implementation.
The majority of the interviews were conducted by a single researcher who had prior experience both of working as a PWP and a practitioner delivering treatments within the trial. Although this may have risked a loss of objectivity, the a priori development of a semistructured interview schedule and independent data coding, undertaken by four academic researchers, goes some way to negating this concern. The researchers’ understanding of service provision and of trial procedures may equally have facilitated deeper examination of the salient issues.
Chapter 6 Discussion and conclusions
Principal outcomes
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial explored three core aims:
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) versus a waiting list for high-intensity CBT in the management of OCD at 3 months
-
the clinical effectiveness and cost-effectiveness of low-intensity interventions (guided self-help and supported cCBT) plus high-intensity CBT versus a waiting list plus high-intensity CBT in the management of OCD at 12 months
-
acceptability of supported cCBT and guided self-help among patients and professionals.
The main results are illustrated diagrammatically in Figure 23, which shows the standardised effect sizes at 3 (Y-BOCS and satisfaction) and 12 months (Y-BOCS, quality of life and costs) for each of the two low-intensity treatments compared with the waiting list for high-intensity CBT group.
FIGURE 23.
Summary of the main comparisons in OCTET. All adjusted outcomes (continuous and dichotomous) from OCTET have been translated onto a common ‘standardised mean difference’ metric. All outcomes have been coded so that a good outcome (lower symptoms, high quality of life, reduced utilisation and costs) is coded as a positive effect size.
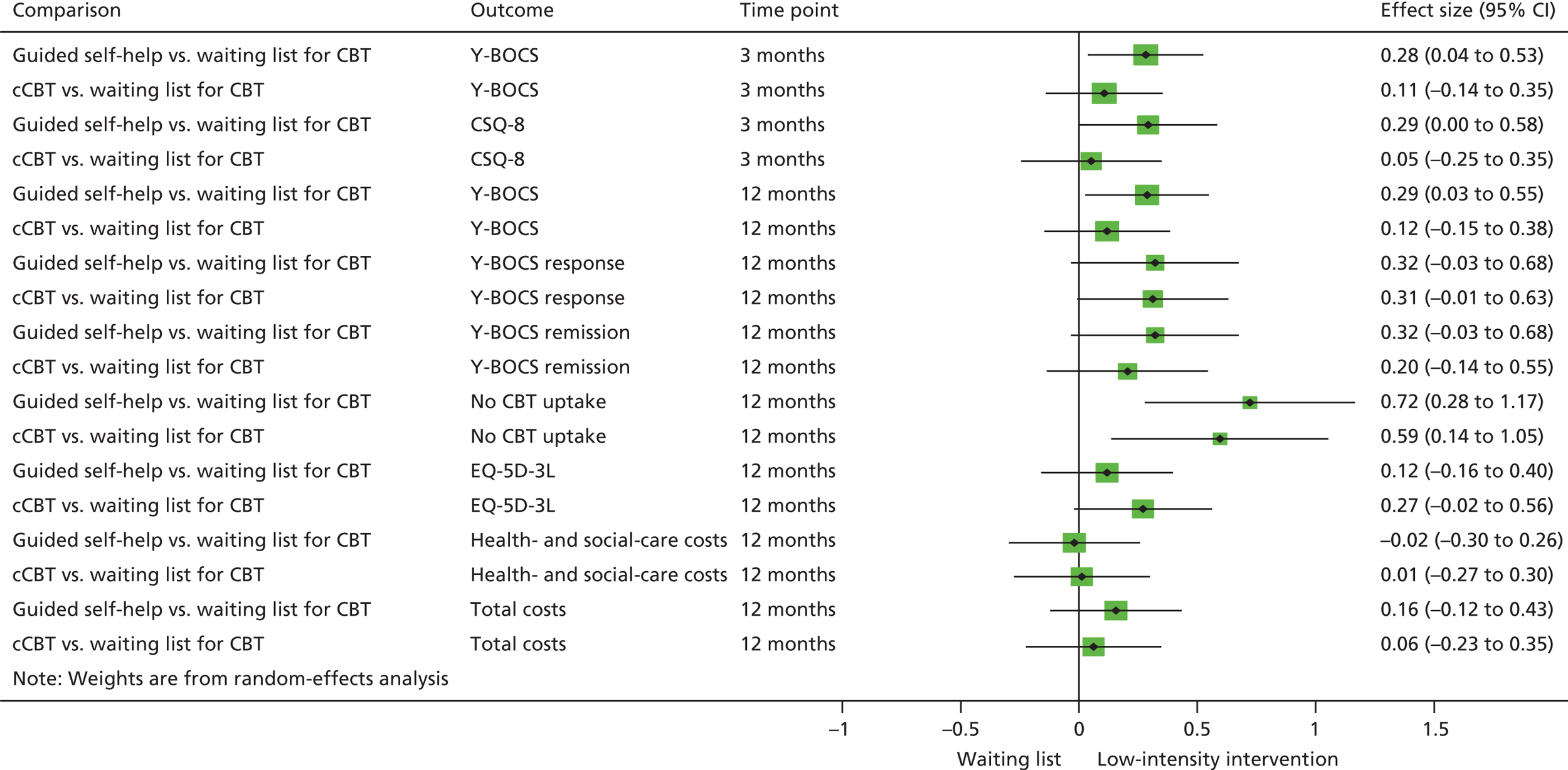
In the short term, prior to access to high-intensity CBT, guided self-help demonstrated statistically significant benefits over waiting list for high-intensity CBT. These differences did not meet the prespecified criterion for clinical significance. In contrast, supported cCBT did not demonstrate any significant benefit.
Over a 12-month period, access to guided self-help and supported cCBT prior to high-intensity CBT did not lead to differences in outcomes compared with access to high-intensity CBT alone. Access to either of the low-intensity interventions does not significantly augment the effect of high-intensity CBT in the longer term.
The effects of the low-intensity interventions did not generalise to other outcomes other than symptoms of OCD. The short-term benefit of supported cCBT on anxiety is noted, but does not seem plausible given the wider pattern of results.
Early access to either of the low-intensity interventions led to significant reductions in uptake of high-intensity CBT over the full 12 months of OCTET, with 86% of the patients allocated to a waiting list for high-intensity CBT starting CBT by the end of the trial, compared with 62% in supported cCBT and 57% in guided self-help. Access to either of the low-intensity interventions may reduce demand on psychological therapy services by reducing the number of patients who access high-intensity CBT.
These reductions in high-intensity therapy utilisation do not seem to compromise patient outcomes at 12 months. The modest effect of guided self-help plus high-intensity CBT compared with the waiting list for high-intensity CBT found at 3 months was broadly maintained at 12 months, although the effects again did not meet the prespecified criterion for clinical significance. This suggests that low-intensity interventions can reduce demand on high-intensity services, while still achieving similar outcomes over the longer term (as there were no statistically significant differences between group at 12 months). We note that the lack of statistically significant differences between arms at 12 months is not a formal test of their equivalence or non-inferiority, which OCTET was not designed to assess.
Of course, one concern is that these average group effects mask more variable outcomes. Of specific concern is the suggestion that early access to either of the low-intensity interventions might inappropriately deflect patients from access to high-intensity CBT, and that initial good outcomes from low-intensity interventions at 3 months might not be maintained in the subgroup that has those interventions alone.
We presented descriptive data on the mean scores of patients in the low-intensity arms according to their pattern of later CBT use. Comparisons do not have the benefit of randomisation, but the data do not suggest that those who accessed low-intensity treatments did not demonstrate markedly different outcomes than those who accessed both.
In terms of the economic analysis, guided self-help was a more expensive low-intensity treatment to deliver than supported cCBT. As noted above, both low-intensity interventions were associated with reductions in uptake of high-intensity CBT over 12 months. The net effect was that health- and social-care costs across the three groups did not differ significantly, despite the additional cost of the low-intensity interventions. Differences in EQ-5D-3L and associated QALY scores were minor in magnitude and mostly non-significant. Nevertheless, when costs and quality of life were jointly analysed, both low-intensity interventions dominated waiting list plus high-intensity CBT in the cost-effectiveness analysis, with lower costs and better outcomes.
Taking a decision-making approach, which focuses on which decision has a higher probability of being cost-effective, rather than statistical significance of the results, there was little evidence that supported cCBT and guided self-help were more cost-effective at the 3-month follow-up compared with the waiting list. However, by the 12-month follow-up (primary end point), the data suggest that the probability that guided self-help was more cost-effective than the waiting list was 60% from the health- and social-care perspective and 80% from the societal perspective and the probability of supported cCBT being more cost-effective than the waiting list was 70% from both perspectives.
The data suggested some small differences in satisfaction at 3 months, with patients most satisfied with guided self-help and least satisfied with supported cCBT. However, only the difference between guided self-help and supported cCBT was statistically significant.
The pattern of results in OCTET is complex and reflects, in part, differences in the analysis and interpretation of clinical, economic and patient experience outcomes.
To readers more interested in the assessment of clinical outcomes, the focus will be on the additional benefit provided to patients through access to low-intensity interventions prior to high-intensity CBT. OCTET demonstrated that neither low-intensity intervention was responsible for clinically significant improvements in OCD symptoms at 3 months prior to high-intensity CBT. In the absence of such clinical benefit over a passive waiting list strategy, such readers may have concerns about reductions in use of high-intensity CBT over 12 months, as it might reflect substitution of an evidence-based high-intensity treatment with low-intensity alternatives showing no evidence of clinically significant benefit.
The analyses of clinical effectiveness and cost-effectiveness in OCTET are not directly comparable. The cost-effectiveness analysis has as its primary focus the longer-term assessment over 12 months, assesses patient outcome through health-related quality of life rather than OCD symptoms and jointly evaluates the effects of the low-intensity interventions on quality of life and costs. The analytical method underlying the cost-effectiveness analysis is also not based on notions of clinical or statistical significance. Rather, the decision to adopt one intervention over another should be based on the expected cost-effectiveness of the intervention, or the probability of making the correct decision. 79,80 The analyses of OCTET suggest that, despite the lack of clinically significant improvement in OCD symptoms at 3 months, both low-intensity treatments are likely to be cost-effective over 12 months and thus reflect a better use of NHS resources.
Indeed, it should be noted that, in the analysis of cost-effectiveness, the supported cCBT intervention performed better than guided self-help. This occurred, despite supported cCBT failing to demonstrate statistical or clinically significant improvements on OCD symptoms at 3 months, and performing worse than guided self-help on patient satisfaction.
The presence of qualitative and quantitative data on patient experience adds additional complexity to the interpretation of OCTET. Many readers may feel that such patient experience data are important, but secondary to clinical and economic outcomes. However, patient experience may be more important where interventions demonstrate broadly similar clinical and economic outcomes. The NHS is also placing more importance on patient experience as a key measure of the quality of services. 90
Strengths and weaknesses
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial represents the largest trial of psychological treatments for OCD worldwide. The trial recruited to target and retention in the short term was high (81%), with acceptable levels of follow-up of clinical outcomes over the longer term.
Recruitment occurred across multiple sites and involved a very large number of PWPs, all of whom received standardised training. This was an advantage in terms of external validity, in that delivery was not restricted to either a small number of specialised sites or to a small number of highly selected therapists, highlighting the pragmatic nature of the study. The clinical effectiveness and cost-effectiveness demonstrated should be representative of the sorts of outcomes that might be found in routine services in the wider NHS.
However, the size of the recruitment and delivery platform for OCTET also has disadvantages. As with many mental health trials, there will always be concerns about the potential bias in the small proportion of patients who agree to participate in research. The nature of the recruitment process did mean it was difficult to get accurate estimates of the numbers of patients who were offered the trial across multiple, dispersed sites, in organisations without a primary focus on research.
Many PWPs saw few patients, which restricted the scope for formal analysis of therapist effects, and may have provided insufficient opportunity to practise the skills learned in the OCTET training sessions.
Uptake of the two self-managed therapies was reasonable (66% guided self-help and 61% supported cCBT), with some evidence of greater engagement with guided self-help. The analysis of fidelity provides some evidence that the delivery of the two trial interventions was in line with the protocols provided. However, the relatively low proportion of PWPs providing recordings means that we cannot be sure that all PWPs were delivering interventions according to protocol.
The geographical spread of OCTET did make data collection more logistically complex. These difficulties were most clearly demonstrated in the collection of the economic data, where levels of complete data were fairly low. As with many trials, we focused on primary outcomes when patients were resistant to fuller assessments, and detailed economic data are always more challenging to collect when patient resistance is high and time is short. Although there were a significant number of missing data, analyses using imputed data did suggest that the results were fairly robust and remain useful for decision-makers.
A significant issue in the interpretation of the results of OCTET concerns the high level of access to high-intensity CBT during the waiting list period. Around 40% of patients allocated to waiting list for high-intensity CBT started to receive some contact with their high-intensity CBT therapist prior to the 3-month assessment, compared with around 20% in the two low-intensity groups. As there is evidence that high-intensity CBT is effective, this would tend to reduce any positive effects of the two low-intensity interventions, meaning that the 3-month results of OCTET are conservative with respect of the likely effects of the interventions under test. This may be of importance, given that the 3-month outcomes of guided self-help were statistically significant, but under the criterion for clinical significance. It should be noted that the percentages quoted above regarding access to CBT relates to those who had any contact with a HIT prior to the 3-month assessment, which in many cases (given the timelines involved in OCTET) would have involved an initial session or two, rather than a full ‘dose’.
This threat to validity reflects the nature of provision in the UK at the time of delivery of OCTET, as many services were taking active steps to reduce their waiting lists for CBT. The problems were compounded by the fact that few services were willing to ask patients to actively delay their high-intensity CBT until they had completed the OCTET interventions, which reflects both service pressures in terms of managing demand and ethical concerns about delaying access to a proven treatment.
As noted above, these issues mean that the estimates of clinical effectiveness at 3 months will be conservative. However, the longer-term outcome and cost data are less affected, as all patients were expected to receive both their low-intensity interventions and high-intensity CBT over the timeline of OCTET (12 months). Therefore, decision-makers can have high confidence in the cost-effectiveness analyses, which provide the broadest assessment of the value of low-intensity interventions in a stepped-care system.
In terms of wider international relevance, the delivery of OCTET did rely on features of psychological therapy services (such as PWPs) that are fairly unique to the UK IAPT model. The economic results especially will reflect aspects of service delivery (such as waiting times and staff costs), which may be particular to the UK.
Nevertheless, interest in low-intensity interventions and stepped care is international,91–93 and the broad findings (that low-intensity interventions delivered prior to conventional treatments can reduce later service utilisation without compromising outcomes) remain important.
Possible mechanisms and explanations
In designing OCTET, we hypothesised that giving patients on the waiting list access to low-intensity interventions prior to high-intensity CBT could have two positive effects:
-
augmenting patient outcomes through either:
-
more rapid improvement in clinical outcomes prior to high-intensity CBT or
-
augmenting the effect of high-intensity CBT in the longer term
-
-
increasing efficiency of service delivery either by reducing the number of patients who need to access high-intensity CBT or by reducing general health-care utilisation in the short and longer term, without compromising patient outcomes. 94
We found limited evidence that low-intensity interventions led to more rapid improvements than a waiting list, prior to high-intensity CBT. Only guided self-help demonstrated statistically significant benefits on the primary outcome at 3 months, and the effect did not meet the prespecified threshold for clinically significant effects (3 points on the Y-BOCS).
We did not find strong evidence that low-intensity interventions augmented the effects of high-intensity CBT over the longer term, as the outcomes in the three groups did not differ significantly at 12 months on the primary outcome. The difference between guided self-help plus high-intensity CBT and the waiting list plus high-intensity CBT remained reasonably large, but not formally clinically or statistically significant according to the criteria adopted in OCTET. Analysis of ‘response’ and ‘remission’ also demonstrated potential augmentation, but this form of analysis of the Y-BOCS was not a primary outcome of OCTET.
In terms of service efficiency, both low-intensity interventions were also associated with a lower uptake of high-intensity CBT. Reductions in the uptake of high-intensity CBT would reduce any augmentation of clinical outcomes from the compound effects of multiple treatments.
The lack of differences in clinical outcomes over 12 months suggests that, on average, provision of low-intensity interventions is potentially efficient and does not lead to poor outcomes.
When outcomes and costs are considered jointly, evidence suggests that both low-intensity interventions have a higher probability of being cost-effective than the waiting list for high-intensity CBT, at conventional levels of willingness to pay for QALYs. Supported cCBT continues to dominate when the perspective is restricted to health- and social-care costs, and guided self-help is likely to be cost-effective.
It is important to consider the patient satisfaction evidence. It is noteworthy that neither low-intensity intervention demonstrated significantly greater satisfaction than the waiting list for high-intensity CBT at 3 months, although patients allocated to guided self-help reported significantly greater satisfaction than those allocated to supported cCBT. The qualitative data reported in Chapter 5 identified characteristics of ‘good’ therapy and identified some reasons why guided self-help may have been preferred to supported cCBT, although it should be noted that the comparison of the technologies (written self-help vs. web-based therapy) is confounded by differences in therapist time associated with their delivery.
Implications
The design of OCTET is an analogue of a stepped-care model, in which patients are offered low-intensity interventions in the first instance, with a smaller proportion going on to higher-intensity options. However, a conventional stepped care was not formally implemented in OCTET, as the offer of low-intensity interventions was made on the basis that high-intensity CBT would then be available for all, not as part of a ‘stepping’ mechanism.
The effects of low-intensity interventions demonstrated in OCTET may be improved in a formal stepped-care system, where patients might not be given an expectation of high-intensity CBT, and only those failing to benefit from the low-intensity interventions according to agreed criteria would continue on to the more-intensive intervention.
As noted previously, during the internal pilot, the Department of Health requested that sites improve access to treatment as part of their commitment to IAPT, which led to a reduction in waiting lists and subsequently increased pressure on recruitment. Given that long waiting lists were one of the core rationales for OCTET, it might be supposed that the relevance of OCTET has been lessened.
However, it is critical to note that a response of many services to the Department of Health request was that patients were ‘removed’ from the waiting list and offered access to interventions other than high-intensity CBT. In many cases, these interventions were in line with current ‘good clinical practice’, but were not evidence based (i.e. OCD specific or recommended by NICE guidelines). For example, patients may have been offered generic groups for managing anxiety. The evaluation of the low-intensity interventions in OCTET has provided exactly the sort of evidence-based intervention that would make such a waiting list management strategy robust. Thus, the results of OCTET remain highly relevant to demand management in NHS psychological therapy services and place current practice on a far firmer evidential base.
The OCTET results would support an important role for low-intensity interventions in the care pathway for OCD. Rapid access to guided self-help leads to modest improvements in clinical outcomes in the shorter term. Following receipt of either low-intensity intervention, a proportion of patients do not progress on the pathway to high-intensity CBT. There is no evidence that this leads to poor outcomes in this group of patients, and the overall health- and social-care costs associated with these patients do not differ.
Providing low-intensity interventions as part of a care pathway may reduce pressure on high-intensity CBT services, without any obvious disbenefit for patients. This may allow psychological therapy services to deliver a greater total amount of therapeutic benefit from the same resource, with low-intensity interventions providing effective outcomes for some patients and higher-intensity CBT more accessible for those who require this service. However, it should be noted that the overall costs to the NHS (including costs outside the psychological therapy service) would likely not be significantly different with the introduction of low-intensity interventions.
The two low-intensity interventions differ in the pattern of results they show. Guided self-help showed statistically significant reductions in OCD symptoms, and patients receiving guided self-help were more satisfied than those receiving supported cCBT. However, supported cCBT showed statistically significant longer-term benefits in quality of life and a slight advantage over guided self-help in terms of cost-effectiveness at 12 months.
From a service perspective, focusing on one low-intensity intervention would simplify delivery and the associated training and infrastructure needs, which might lead decision-makers to prefer the supported cCBT model. However, we would caution against a focus on supported cCBT alone. Supported cCBT did not perform well on the primary clinical outcome, and the quantitative data on satisfaction suggested that guided self-help was significantly more likely to satisfy patients. The qualitative data confirmed the broad findings from the quantitative satisfaction measure, and suggest that providing options may be important to meet the variation in patient preferences for low-intensity interventions that reflect individual differences in the ways patients interact with interventions. 95
Suggestions for further research
As noted above (see Implications), although the design was relevant to the evaluation of stepped care, OCTET did not introduce a formal stepped-care system. If services are to implement low-intensity interventions for OCD into routine practice as part of a stepped-care system, high-quality health services and delivery research is required to explore how best to introduce these interventions. 96
For example, it will be important to determine what information and preparation patients require concerning low-intensity interventions to ensure that they understand the role of these interventions. Effective use of digital technology (combined with relevant patient experience material) may provide an effective and efficient method of preparation for many patients.
As well as provision of low-intensity treatments, stepped care is based on effective flow of patients through the system depending on their outcomes. It will be necessary to ensure that patients understand the content and process of the decision-making that will determine access to high-intensity CBT. 97 Although OCTET did not suggest that access to low-intensity treatments alone was associated with poor outcomes on average, there will be the need for the development of appropriate procedures to monitor OCD outcomes and ensure safety for individual patients who do not access or respond to low-intensity interventions.
The Obsessive–Compulsive Treatment Efficacy randomised controlled Trial has also highlighted the importance of training and support for staff delivering low-intensity interventions, given their limited experience in managing OCD to date. There is a great deal of research on ‘complex interventions’ and their mechanisms of effect, but comparatively little research on effective ways of training staff to deliver such interventions and ways of maintaining and enhancing their skills. The training materials developed for OCTET provide a useful basis for further development and evaluation of training materials for the workforce delivering low-intensity interventions.
Although the high levels of uptake of high-intensity CBT would have lessened the comparative benefit of low-intensity interventions at 3 months, neither intervention showed clinically significant effects at 3 months, which suggests that further development of more effective low-intensity interventions is required.
We were unable to find methodologically rigorous research on clinically significant differences on the Y-BOCS to inform the sample size calculation and the interpretation of trial. Providing a robust threshold for clinical significance would improve future research on OCD.
Research into the ‘active ingredients’ of low-intensity interventions is at a relatively early stage, with most of the evidence relating to depression rather than OCD. As the evidence base for low-intensity interventions in OCD grows, appropriate use of techniques such as metaregression98 and mediational analysis99 may provide useful insights into key ‘active ingredients’ that might enhance their effectiveness. Equally, better understanding of the sorts of patient groups most likely to benefit100 may enable better targeting of interventions as part of a combined stepped- and stratified-care model. 96
The qualitative data in Chapter 5 may also provide useful insights into aspects of the interventions that might benefit from modification to enhance uptake. The next generation of low-intensity interventions might also be able to take advantages of improvements of technology to enhance outcomes. This might involve improved use of multimedia within cCBT or development of other aspects of low-intensity interventions (such as text reminders to enhance uptake and adherence, or access to appropriate social networking sites to develop social support). All such enhancements will require appropriate patient input and ongoing evaluation.
If more effective low-intensity interventions can be developed, there may be a case for trials to actively compare low-intensity interventions with high-intensity CBT head to head (rather than the sequential delivery tested within OCTET). This could evaluate whether or not enhanced low-intensity interventions can achieve equivalent (or at least non-inferior) outcomes to high-intensity CBT. This was the subject of a commissioning brief prior to OCTET, and could be informed by the ongoing Cost and Outcome of Behavioural Activation (COBRA) non-inferiority trial. 101
Acknowledgements
The authors would like to thank all the patients, health professionals and NHS trust staff who took part in and contributed to this piece of research. Particular thanks to all of the PWPs, clinical leads, clinical supervisors and to the NIHR Clinical Research Network (formerly the Mental Health Research Network) for providing substantial support.
We are very grateful to the members of our TSC [Professor Christopher Dowrick (chairperson), Dr Isobel Heyman, Claire McMyler and Dickon Allen] and DMEC [Professor David Kessler (chairperson), Christopher Sutton and David Ekers] for their invaluable advice and support during the project.
Thanks also to Neil O’Leary, who drafted the statistical analysis plan and set up the blinded interim analysis requested by Health Technology Assessment programme, and to Christine Molloy and Jennifer Butler for their assistance with the conduct and analysis of the PWP acceptability interviews and fidelity assessment.
Contributions of authors
All authors were involved in the conception and design of the study, the collection of data or analysis, data interpretation, drafting and/or revising of the final report.
Individual contributions were as follows:
Karina Lovell (Professor of Mental Health, Research Director) was the principal investigator and was involved in the conception and design of the study, day-to-day supervision of the project, developed the guided self-help intervention manual, trained and provided supervision to PWPs involved in the delivery of the trial interventions, and drafting and making revisions to the final report.
Peter Bower (Lead, Centre for Primary Care, Institute of Population Health) was involved in the conception and design of the study, interpretation of outcome data, and drafting and making revisions to the full report.
Judith Gellatly (Research Fellow) was the trial manager, supported all sites and staff on a day-to-day basis, providing training for the researchers and clinical studies officers, leading the PWP acceptability study, collated and analysed trial data, and drafting and making revisions to the full report.
Sarah Byford (Professor of Health Economics and Director) was involved in the conception and design of the study (particularly the economic component), development of the trial economic measure, providing day-to-day supervision of the economic analysis, drafting and revising the economic analysis, and commenting on the full report.
Penny Bee (Senior Lecturer) was involved in the conception and design of the study, co-ordinating one of the trial sites, leading the patient acceptability study, and drafting and making revisions to the full report.
Dean McMillan (Senior Lecturer) was involved in the conception and design of the study, co-ordinating one of the trial sites, trained and provided supervision to PWPs involved in the delivery of the trial interventions, and commenting on the full report.
Catherine Arundel (Research Fellow) managed the trial and co-ordinated the data at YTU, and collated and analysed trial data.
Simon Gilbody (Professor of Psychological Medicine) was involved in the conception and design of the study, co-ordinating one of the trial sites and commenting on the full report.
Lina Gega (Reader in Mental Health) was involved in the conception and design of the study, co-ordinating one of the trial sites, developed the guided self-help intervention manual, trained and provided supervision to PWPs involved in the delivery of the trial interventions, and commenting on the full report.
Gillian Hardy (Professor of Clinical Psychology) was involved in the conception and design of the study, co-ordinating one of the trial sites and commenting on the full report.
Shirley Reynolds (Professor of Evidence-Based Psychological Therapies) was involved in the conception and design of the study, co-ordinating one of the trial sites and commenting on the full report.
Michael Barkham (Professor of Clinical Psychology) was involved in the conception and design of the study, co-ordinating one of the trial sites and commenting on the full report.
Patricia Mottram (Research and Effectiveness Manager) provided research governance support and was local collaborator for one of the sites.
Nicola Lidbetter (Chief Executive, Anxiety UK) provided service user input and was local collaborator for one of the sites.
Rebecca Pedley (Research Associate) was responsible for data collection at one of the main trial sites, assisted with devising audit plans and the interpretation of the PWP acceptability interviews.
Jo Molle (Research Associate) was responsible for data collection at one of the main trial sites.
Emily Peckham (Research Fellow) was responsible for data collection at one of the main trial sites.
Jasmin Knopp-Hoffer (PhD student) was responsible for data collection at one of the main sites, and collection of the service user qualitative data and analysis.
Owen Price (Research Associate) was responsible for data collection at one of the main trial sites and assisted with the set-up of the study.
Janice Connell (Research Associate) was responsible for data collection at one of the main trial sites, and conducted and summarised the demographic PWP data.
Margaret Heslin (Health Economist) conducted the analysis of the economic data, and assisted with drafting and making revisions to the full report.
Christopher Foley (Research Methods Fellow) conducted the final statistical analyses, and assisted with drafting and making revisions to the full report.
Faye Plummer (Trial Support Officer) was responsible for data collection at one of the main trial sites.
Christopher Roberts (Professor of Biostatistics) participated in the design of the study, developed the statistical analysis plan, led the clinical outcome analysis, and assisted with drafting and making revisions to the full report.
Publications
Gellatly J, Bower P, McMillan D, Roberts C, Byford S, Bee P, et al. Obsessive Compulsive Treatment Efficacy Trial (OCTET) comparing the clinical and cost-effectiveness of self-managed therapies: study protocol for a randomised controlled trial. Trials 2014;15:278.
Knopp-Hoffer J, Knowles S, Bower P, Lovell K, Bee PE. ‘One man’s medicine is another man’s poison’: adult users’ perspectives on low intensity interventions for obsessive–compulsive disorder (OCD). BMC Health Serv Res 2016;16:188.
Gellatly J, Pedley R, Molloy C, Butler J, Lovell K, Bee P. Low intensity interventions for Obsessive-Compulsive Disorder (OCD): a qualitative study of mental health practitioner experiences. BMC Psychiatry 2017;17:77.
Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Low-intensity cognitive-behaviour therapy interventions for obsessive-compulsive disorder compared to waiting list for therapist-led cognitive-behaviour therapy: 3-arm randomised controlled trial of clinical effectiveness. PLOS Med 2017;14:e1002337.
Data sharing statement
All available data from OCTET can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Publishing; 2013.
- Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Arlington, VA: American Psychiatric Publishing; 1994.
- International Classification of Diseases. Geneva: World Health Organization; 2011.
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive–compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:53-6. http://dx.doi.org/10.1038/mp.2008.94.
- Jenike MA. Clinical practice. Obsessive–compulsive disorder. N Engl J Med 2004;350:259-65. http://dx.doi.org/10.1056/NEJMcp031002.
- DuPont RL, Rice DP, Shiraki S, Rowland CR. Economic costs of obsessive–compulsive disorder. Med Interface 1995;8:102-9.
- The Global Burden of Disease: 2004 Update. Geneva: World Health Organization Press; 2008.
- Pigott TA, L’Heureux F, Dubbert B, Bernstein S, Murphy DL. Obsessive compulsive disorder: comorbid conditions. J Clin Psychiatry 1994;55:15-27.
- Hollander E, Broatch J, Himelein C, Rowland C, Stein D, Kwon J. Psychosocial function and economic costs of obsessive–compulsive disorder. CNS Spect 1997;2:16-25.
- Stanley MA, Turner SM. Current status of pharmacological and behavioural treatment of obsessive–compulsive disorder. Behav Ther 1995;26:163-86. http://dx.doi.org/10.1016/S0005-7894(05)80089-9.
- Bennett-Levy J, Richards DA, Farrand P, Bennett-Levy J, Richards DA, Farrand P. Oxford Guide to Low Intensity CBT Interventions. Oxford: Oxford University Press; 2010.
- Obsessive–Compulsive Disorder: Core Interventions in the Treatment of Obsessive–Compulsive Disorder and Body Dysmorphic Disorder (Clinical Guideline 31). London: NICE; 2005.
- Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. Narrative literature review. Br J Psychiatry 2005;186:11-7. http://dx.doi.org/10.1192/bjp.186.1.11.
- Lovell K, Richards D. Multiple access points and levels of entry (MAPLE): ensuring choice, accessibility and equity for CBT services. Behav Cogn Psychother 2000;28:379-91.
- UK Database of Uncertainties about the Effects of Treatments . Technology-Enhanced Delivery of Treatment for Obsessive Compulsive Disorder n.d. www.library.nhs.uk/DUETs/viewResource.aspx?resid=415418 (accessed 27 August 2015).
- IAPT Three Year Report: The First Million Patients. London: Department of Health; 2012.
- Lovell K, Bee P. Implementing the NICE OCD/BDD guidelines. Psychol Psychother 2008;81:365-76. http://dx.doi.org/10.1348/147608308X320107.
- Richards D, Farrand P, Chellingsworth M. National Curriculum for the Education of Psychological Wellbeing Practitioners (PWPs) 2015. www.library.nhs.uk/DUETs/viewResource.aspx?resid=415418 (accessed 29 September 2015).
- Lovell K, Bee P. Optimising treatment resources for OCD: a review of the evidence base for technology-enhanced delivery. J Ment Health 2011;20:525-42. http://dx.doi.org/10.3109/09638237.2011.608745.
- Herbst N, Voderholzer U, Stelzer N, Knaevelsrud C, Hertenstein E, Schlegl S, et al. The potential of telemental health applications for obsessive–compulsive disorder. Clin Psychol Rev 2012;32:454-66. http://dx.doi.org/10.1016/j.cpr.2012.04.005.
- Dèttore D, Pozza A, Andersson G. Efficacy of technology-delivered cognitive behavioural therapy for OCD versus control conditions, and in comparison with therapist-administered CBT: meta-analysis of randomized controlled trials. Cogn Behav Ther 2015;44:190-211. http://dx.doi.org/10.1080/16506073.2015.1005660.
- Fritzler BK, Hecker JE, Losee MC. Self-directed treatment with minimal therapist contact: preliminary findings for obsessive–compulsive disorder. Behav Res Ther 1997;35:627-31. http://dx.doi.org/10.1016/S0005-7967(97)00024-7.
- Lovell K, Ekers D, Fullford J, Baguley C, Bradshaw T. A pilot study of a self-help manual with minimal therapist contact in the treatment of obsessive–compulsive disorder. Clin Eff Nurs 2005;8:122-7. http://dx.doi.org/10.1016/j.cein.2004.05.004.
- Tolin DF, Diefenbach GJ, Maltby N, Hannan SE. Stepped care for obsessive–compulsive disorder: a pilot study. Cogn Behav Pract 2005;12:403-14. http://dx.doi.org/10.1016/S1077-7229(05)80068-9.
- Tolin DF, Hannan S, Maltby N, Diefenbach GJ, Worhunsky P, Brady RE. A randomized controlled trial of self-directed versus therapist-directed cognitive-behavioral therapy for obsessive–compulsive disorder patients with prior medication trials. Behav Ther 2007;38:179-91. http://dx.doi.org/10.1016/j.beth.2006.07.001.
- Moritz S, Jelinek L, Klinge R, Naber D. Fight fire with fireflies! Association splitting: a novel cognitive technique to reduce obsessive thoughts. Behav Cogn Psychother 2007;35:631-5. http://dx.doi.org/10.1017/S1352465807003931.
- Moritz S, Jelinek L, Hauschildt M, Naber D. How to treat the untreated: effectiveness of a self-help metacognitive training program (myMCT) for obsessive–compulsive disorder. Dialogues Clin Neurosci 2010;12:209-20.
- Moritz S, Aravena SC, Guczka SR, Schilling L, Eichenberg C, Raubart G, et al. Knock, and it will be opened to you? An evaluation of meridian-tapping in obsessive–compulsive disorder (OCD). J Behav Ther Exp Psychiatry 2011;42:81-8. http://dx.doi.org/10.1016/j.jbtep.2010.07.002.
- Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10330.
- McCrone P, Marks IM, Greist JH, Baer L, Kobak KA, Wenzel KW, et al. Cost-effectiveness of computer-aided behaviour therapy for obsessive–compulsive disorder. Psychother Psychosom 2007;76:249-50.
- Kobak KA, Greist R, Jacobi DM, Levy-Mack H, Greist JH. Computer-assisted cognitive behavior therapy for obsessive–compulsive disorder: a randomized trial on the impact of lay vs. professional coaching. Ann Gen Psychiatry 2015;14. http://dx.doi.org/10.1186/s12991-015-0048-0.
- Wootton BM, Titov N, Dear BF, Spence J, Andrews G, Johnston L, et al. An internet administered treatment program for obsessive–compulsive disorder: a feasibility study. J Anxiety Disord 2011;25:1102-7. http://dx.doi.org/10.1016/j.janxdis.2011.07.009.
- Andersson E, Enander J, Andrén P, Hedman E, Ljótsson B, Hursti T, et al. Internet-based cognitive behaviour therapy for obsessive–compulsive disorder: a randomized controlled trial. Psychol Med 2012;42:2193-203. http://dx.doi.org/10.1017/S0033291712000244.
- Gellatly J, Bower P, McMillan D, Roberts C, Byford S, Bee P, et al. Obsessive Compulsive Treatment Efficacy Trial (OCTET) comparing the clinical and cost effectiveness of self-managed therapies: study protocol for a randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-278.
- National Institute for Health Research . Feasibility and Pilot Studies n.d. www.nihr.ac.uk/CCF/RfPB/FAQs/Feasibility_and_pilot_studies.pdf (accessed 15 July 2015).
- Littlewood E, Duarte A, Hewitt C, Knowles S, Palmer S, Walker S, et al. A randomised controlled trial of computerised cognitive behaviour therapy for the treatment of depression in primary care: the Randomised Evaluation of the Effectiveness and Acceptability of Computerised Therapy (REEACT) trial. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta191010.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Lovell K, Gega L. Obsessive Compulsive Disorder: A Self-Help Book. Manchester: University of Manchester; 2011.
- Lovell K. Overcoming Obsessive Compulsive Disorder: A Self-Help Manual. Manchester: University of Manchester; 1999.
- Sheehan DV, Lecrubier Y, Jheehan KH, Amorim P, Janavas J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Lovell K, Cox D, Haddock G, Jones C, Raines D, Garvey R. Telephone administered cognitive behaviour therapy for treatment of obsessive compulsive disorder: Randomised controlled non-inferiority trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38940.355602.80.
- Anderson RA, Rees CS. Group versus individual cognitive–behavioural treatment for obsessive–compulsive disorder: a controlled trial. Behav Res Ther 2007;45:123-37. http://dx.doi.org/10.1016/j.brat.2006.01.016.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46:1006-11. http://dx.doi.org/10.1001/archpsyc.1989.01810110048007.
- Woody SR, Steketee G, Chambless DL. Reliability and validity of the Yale-Brown Obsessive Compulsive Scale. Behav Res Ther 1995;33. http://dx.doi.org/10.1016/0005-7967(94)00076-V.
- Federici A, Summerfeldt LJ, Harrington JL, McCabe RE, Purdon CL, Rowa K, et al. Consistency between self-report and clinician-administered versions of the Yale-Brown obsessive–compulsive Scale. J Anxiety Disord 2010;24:729-33. http://dx.doi.org/10.1016/j.janxdis.2010.05.005.
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36® Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993.
- EuroQoL Group . EuroQoL: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Barrett B, Byford S, Crawford MJ, Patton R, Drummond C, Henry JA, et al. Cost-effectiveness of screening and referral to an alcohol health worker in alcohol misusing patients attending an accident and emergency department: a decision-making approach. Drug Alcohol Depend 2006;81:47-54. http://dx.doi.org/10.1016/j.drugalcdep.2005.05.015.
- Bower P, Byford S, Sibbald B, Ward W, King M, Lloyd M, et al. Randomised controlled trial of non-directive counselling, cognitive behaviour therapy, and usual general practitioner care for patients with depression. II: cost-effectiveness. BMJ 2000;321:1389-92. http://dx.doi.org/10.1136/bmj.321.7273.1389.
- Byford S, Knapp M, Greenshields J, Ukoumunne OC, Jones V, Thompson S, et al. Cost-effectiveness of brief cognitive behaviour therapy versus treatment as usual in recurrent deliberate self-harm: a rational decision making approach. Psychol Med 2003;33:977-86. http://dx.doi.org/10.1017/S0033291703008183.
- Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med 2003;45:156-74. http://dx.doi.org/10.1097/01.jom.0000052967.43131.51.
- Kessler RC, Ames M, Hymel PA, Loeppke R, McKenas DK, Richling DE, et al. Using the World Health Organization Health and Work Performance Questionnaire (HPQ) to evaluate the indirect workplace costs of illness. J Occup Environ Med 2004;46:23-37. http://dx.doi.org/10.1097/01.jom.0000126683.75201.c5.
- Evans C, Mellor-Clark J, Margison F, Barkham M, Audin K, Connell J, et al. CORE: Clinical Outcomes in Routine Evaluation. J Ment Health 2000;9:247-55. http://dx.doi.org/10.1080/713680250.
- Evans C, Connell J, Barkham M, Margison F, McGrath G, Mellor-Clark J, et al. Towards a standardised brief outcome measure: psychometric properties and utility of the CORE-OM. Br J Psychiatry 2002;180:51-60. http://dx.doi.org/10.1192/bjp.180.1.51.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737-44. http://dx.doi.org/10.1001/jama.282.18.1737.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. http://dx.doi.org/10.1192/bjp.180.5.461.
- The IAPT Data Handbook . Guidance on Recording and Monitoring Outcomes to Support Local Evidence-Based Practice 2011. www.iapt.nhs.uk/silo/files/the-iapt-data-handbook.pdf (accessed 3 November 2011).
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. http://dx.doi.org/10.1017/S0033291700030415.
- The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization; 1993.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. http://dx.doi.org/10.1016/0149-7189(79)90094-6.
- Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Eval Program Plann 1983;6:299-313. http://dx.doi.org/10.1016/0149-7189(83)90010-1.
- Walwyn R, Roberts C. Therapist variation within randomised trials of psychotherapy: implications for precision, internal and external validity. Stat Methods Med Res 2009;19:291-315. http://dx.doi.org/10.1177/0962280209105017.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. http://dx.doi.org/10.1002/sim.1981.
- Mataix-Cols D, Fernandez de la Cruz L, Nordsletten AE, Lenhard F, Isomura K, Simpson HB. Towards an international expert consensus for defining treatment response, remission, recovery, and relapse in obsessive–compulsive disorder: a Delphi survey. World Psychiatry 2016;15:80-1. http://dx.doi.org/10.1002/wps.20299.
- Department of Health . NHS Reference Costs 2013 14 2015. www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014 (accessed 7 May 2015).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. PharmacoEconomics 1996;10:460-6. http://dx.doi.org/10.2165/00019053-199610050-00003.
- Hammond GC, Croudace TJ, Radhakrishnan M, Lafortune L, Watson A, McMillan-Shields F, et al. Comparative effectiveness of cognitive therapies delivered face-to-face or over the telephone: an observational study using propensity methods. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0042916.
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064-9. http://dx.doi.org/10.1016/S0140-6736(00)02039-0.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK Population Survey. Discussion Paper 138. York: University of York; 1995.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Briggs AH. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ 1999;8:257-61. http://dx.doi.org/10.1002/(SICI)1099-1050(199905)8:3<257::AID-HEC427>3.0.CO;2-E.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. http://dx.doi.org/10.1016/S0167-6296(98)00039-3.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-15. http://dx.doi.org/10.1016/S0140-6736(02)09832-X.
- Good Clinical Practice Guide. London: Medicines and Healthcare products Regulatory Agency; 2012.
- Harmonised Tripartite Guidelines for Good Clinical Practice E6 (R1). Geneva: ICH Secretariat; 1996.
- Mental Health Research Network, INVOLVE . Budgeting for Involvement: Practical Advice on Budgeting for Actively Involving the Public in Research Sites 2013. www.invo.org.uk/posttypepublication/budgeting-for-involvement (accessed 21 September 2013).
- Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Low-intensity cognitive-behaviour therapy interventions for obsessive-compulsive disorder compared to waiting list for therapist-led cognitive-behaviour therapy: 3-arm randomised controlled trial of clinical effectiveness. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002337.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Pract 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Problem 1965;12:436-45. http://dx.doi.org/10.2307/798843.
- O’Connor MK, Netting FE, Thomas ML. Grounded theory: managing the challenge for those facing institutional review board oversight. Qual Inquiry 2008;14:28-45. http://dx.doi.org/10.1177/1077800407308907.
- Gellatly J, Pedley R, Molloy C, Butler J, Lovell K, Bee P. Low intensity interventions for Obsessive-Compulsive Disorder (OCD): a qualitative study of mental health practitioner experiences. BMC Psychiatry 2017;17. https://doi.org/10.1186/s12888-017-1238-x.
- May C. Agency and implementation: understanding the embedding of healthcare innovations in practice. Soc Sci Med 2013;78:26-33. http://dx.doi.org/10.1016/j.socscimed.2012.11.021.
- Transforming Patient Experience: The Essential Guide. Coventry: NHS Institute; 2012.
- Wilson GT, Vitousek KM, Loeb KL. Stepped care treatment for eating disorders. J Consult Clin Psychol 2000;68:564-72. http://dx.doi.org/10.1037/0022-006X.68.4.564.
- Haaga DA. Introduction to the special section on stepped care models in psychotherapy. J Consult Clin Psychol 2000;68:547-8. http://dx.doi.org/10.1037/0022-006X.68.4.547.
- Davison GC. Stepped care: doing more with less?. J Consult Clin Psychol 2000;68:580-5. http://dx.doi.org/10.1037/0022-006X.68.4.580.
- Panagioti M, Richardson G, Murray E, Rogers A, Kennedy A, Newman S, et al. Reducing care utilisation through self-management interventions (RECURSIVE): a systematic review and meta-analysis. Health Serv Deliv Res 2014;2.
- Knowles SE, Toms G, Sanders C, Bee P, Lovell K, Rennick-Egglestone S, et al. Qualitative meta-synthesis of user experience of computerised therapy for depression and anxiety. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0084323.
- Richards DA, Bower P, Pagel C, Weaver A, Utley M, Cape J, et al. Delivering stepped care: an analysis of implementation in routine practice. Implement Sci 2012;7. http://dx.doi.org/10.1186/1748-5908-7-3.
- Bower P, Gilbody S, Barkham M. Making decisions about patient progress: the application of routine outcome measurement in stepped care psychological therapy services. Prim Care Mental Health 2006;4:21-8.
- Gellatly J, Bower P, Hennessy S, Richards D, Gilbody S, Lovell K. What makes self-help interventions effective in the management of depressive symptoms? Meta-analysis and meta-regression. Psychol Med 2007;37:1217-28. http://dx.doi.org/10.1017/S0033291707000062.
- Dunn G, Emsley R, Liu H, Landau S, Green J, White I, et al. Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19930.
- Knopp J, Knowles S, Bee P, Lovell K, Bower P. A systematic review of predictors and moderators of response to psychological therapies in OCD: do we have enough empirical evidence to target treatment?. Clin Psychol Rev 2013;33:1067-81. http://dx.doi.org/10.1016/j.cpr.2013.08.008.
- Rhodes S, Richards DA, Ekers D, McMillan D, Byford S, Farrand PA, et al. Cost and outcome of behavioural activation versus cognitive behaviour therapy for depression (COBRA): study protocol for a randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-29.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: Personal Social Services Research Unit, University of Kent; 2009.
- Samaritans . Samaritans: Annual Report 2010–2011 2011. www.samaritans.org/sites/default/files/kcfinder/files/Annual%20Report%202011.pdf (accessed 16 April 2013).
- Cancer Research UK . Individual Therapies n.d. www.cancerresearchuk.org/about-cancer/cancers-in-general/treatment/complementary-alternative/therapies/ (accessed 2 July 2015).
- Nolan G. Developing a Local Tariff for IAPT Services in NHS East of England. Cambridge: NHS East of England; 2009.
- Health and Social Care Information Centre . Psychological Therapies, Annual Report on the Use of IAPT Services: England – 2013 14 2014. www.hscic.gov.uk/catalogue/PUB14899/psyc-ther-ann-rep-2013-14.pdf (accessed 24 August 2015).
- Ekers D, Godfrey C, Gilbody S, Parrott S, Richards DA, Hammond D, et al. Cost utility of behavioural activation delivered by the non-specialist. Br J Psychiatry 2011;199:510-11. http://dx.doi.org/10.1192/bjp.bp.110.090266.
Appendix 1 Pilot recruitment graph

Appendix 2 Unit costs
| Resource | Unit | Cost (£) | Source | Notes |
|---|---|---|---|---|
| Inpatient services | ||||
| ENT and dental | Per day | 531 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Respiratory system | Per day | 370 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Cardiac surgery and primary cardiac conditions | Per day | 533 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Digestive system | Per day | 482 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Hepatobiliary and pancreatic system | Per day | 434 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Musculoskeletal system | Per day | 523 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Urinary tract and male reproductive system | Per day | 389 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Female reproductive system and assisted reproduction | Per day | 644 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Obstetrics | Per day | 931 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Haematology, chemotherapy, radiotherapy and specialist palliative care | Per day | 496 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| General | Per day | 483 | NHS Reference Costs 2013/14 70 | Calculated from NHS reference costs non-elective long stay |
| Outpatient services | ||||
| Asthma clinic | Per contact | 150 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 340 |
| Clinical haematology | Per contact | 160 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 303 |
| Dental medicine | Per contact | 119 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 450 |
| ENT | Per contact | 92 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 120 |
| General medicine | Per contact | 157 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 300 |
| Mental health | Per contact | 233 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 710 |
| Obstetrics | Per contact | 120 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 501 |
| Orthopaedics | Per contact | 113 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 110 |
| Pain management | Per contact | 135 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 191 |
| Physiotherapy | Per contact | 46 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 650 |
| Plastic surgery | Per contact | 93 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 160 |
| Diagnostic imaging | Per contact | 93 | NHS Reference Costs 2013/14 70 | Index tab: IMAG |
| Phlebotomy | Per contact | 3 | NHS Reference Costs 2013/14 70 | DAPS tab: DAPS08 |
| Infectious diseases | Per contact | 219 | NHS Reference Costs 2013/14 70 | Total Outpatient Attendances tab: service code 350 |
| MRI | Per contact | 145 | NHS Reference Costs 2013/14 70 | IMAG tab: RA01A |
| Ultrasound | Per contact | 52 | NHS Reference Costs 2013/14 70 | IMAG tab: RA23Z |
| Psychology | Per contact | 177 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 656 |
| Interventional radiology | Per contact | 192 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 811 |
| Stroke medicine | Per contact | 212 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 328 |
| Vascular surgery | Per contact | 149 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 107 |
| Midwifery service | Per contact | 71 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 560 |
| ECG | Per contact | 167 | NHS Reference Costs 2013/14 70 | IMAG tab: RA60A |
| General surgery | Per contact | 125 | NHS Reference Costs 2013/14 70 | Total Outpatient Attendances tab: service code 100 |
| Rehabilitation service | Per contact | 138 | NHS Reference Costs 2013/14 70 | Total Outpatient Attendances tab: service code 314 |
| Colonoscopy | Per contact | 334 | NHS Reference Costs 2013/14 70 | OPROC tab: FZ51Z |
| Endoscopy | Per contact | 64 | NHS Reference Costs 2013/14 70 | OPROC tab: FZ42A |
| A&E | ||||
| A&E without ambulance | Per contact | 135 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab – service code 180 – accident & emergency |
| A&E with ambulance | Per contact | 366 | NHS Reference Costs 2013/14 70 | A&E plus ambulance (AMB tab – ASS02 – see and treat and convey – £231) |
| Community-based contacts | ||||
| GP in GP surgery | Per contact | 38 | PSSRU71 | Per-patient contact lasting 11.7 minutes; including direct care staff costs; without qualifications |
| GP at home | Per contact | 77 | PSSRU71 | Based on £3.30 per minute for 11.4 minutes per home visit plus 12 minutes travelling; including direct care staff costs; without qualifications |
| GP on telephone | Per contact | 23 | PSSRU71 | Per-patient contact lasting 7.1 minutes; including direct care staff costs; without qualifications |
| Practice nurse in GP surgery | Per contact | 11 | PSSRU71 | £44 per hour of face to face (excluding qualifications), based on a 15.5-minute contact |
| District nurse/health visitor/midwife | Per contact | 15 | PSSRU71 | £57 per hour of face to face (excluding qualifications), based on a 15.5-minute contact from practice nurse (location 10.6) |
| NHS walk-in clinic | Per contact | 56 | PSSRU71 | Per clinic contact lasting 17.2 minutes; including direct care staff costs; without qualifications |
| Community psychiatric nurse | Per contact | 33 | PSSRU71 | £66 per hour of face to face (excluding qualifications); based on a 30-minute contact from assertive outreach team (location 12.4) |
| Psychiatrist in the community | Per contact | 66 | PSSRU71 | £103 per hour (excluding qualifications) plus patient contact ratio taken from Curtis102 (£266); based on an assumption of a 15-minute contact |
| Occupational therapist | Per contact | 27 | PSSRU71 | £32 per hour (excluding qualifications) plus client contact ratio added from Curtis102 (£53); based on a 30-minute contact (based on data from Curtis103) |
| Marriage counselling (e.g. Relate) | Per contact | 46 | PSSRU71 | £50 per hour (excluding qualifications); based on a 55-minute contact |
| Art/drama/music therapy | Per contact | 27 | PSSRU71 | Occupational therapist |
| Social worker | Per contact | 28 | PSSRU71 | £55 per hour of patient-related work (excluding qualifications); based on the assumption of a 30-minute contact |
| Advice service (e.g. Citizen’s Advice Bureau/housing association) | Per contact | 27 | PSSRU71 | Occupational therapist |
| Helpline (e.g. Samaritans/Mind) | Per contact | 4 | Samaritans104 | Based on £3.88 cost per contact in 2010–11, inflated to 2013–14 prices using retail price index from Curtis71 |
| Day centre/drop-in centre | Per contact | 30 | PSSRU71 | £30 per client session |
| Complementary therapy (e.g. homeopathy/acupuncture) | Per contact | 58 | Cancer Research UK105 | Complementary therapies range from £15 to £100, so used the mid-point |
| Psychological therapies | ||||
| CBT | Per contact | 93 | PSSRU71 | Cost per session |
| Counselling | Per contact | 50 | PSSRU71 | Cost per hour, assume 1 hour |
| Psychotherapy | Per contact | 100 | NHS Reference Costs 2013/14 70 | Total Outpatient Attendances tab: service code 713 |
| IAPT | Per contact | 97 | NHS Reference Costs 2013/14 70 | MHSTIAPTA – mental health tab. Coded as general IAPT as unclear if level 2 or 3 |
| Community psychiatric nurse | Per contact | 33 | PSSRU71 | Assume 30-minute contact; £66 per hour of face-to-face contact |
| Group therapy | Per contact | 14 | PSSRU71 | Based on mindfulness group therapy |
| GP nurse | Per contact | 11.37 | PSSRU71 | Assume 15.5-minute contact, face to face |
| Mind | Per contact | 50 | PSSRU71 | Cost per hour, assume 1 hour |
| Clinical psychologist | Per contact | 138 | PSSRU71 | Per hour of face to face, assume 1 hour |
| Psychiatrist | Per contact | 51.5 | PSSRU71 | £103 per contact hour, assume 30 minutes |
| Behavioural therapy | Per contact | 93 | PSSRU71 | Cost per session |
| Occupational therapy | Per contact | 64 | NHS Reference Costs 2013/14 70 | Total outpatient attendances tab: service code 651 |
| Crisis team | Per contact | 38 | PSSRU71 | Per hour, per team member, assume one team member for 1 hour |
| GP | Per contact | 38 | PSSRU71 | Per-patient contact lasting 11.7 minutes, without qualifications |
| Mindfulness | Per contact | 14 | PSSRU71 | Based on mindfulness group therapy |
Appendix 3 Psychological wellbeing practitioner unit cost per minute
Appendix 4 Psychological wellbeing practitioner training costs
| Components | Details | Unit cost | Total cost (£) | Cost per therapist (£) | Cost per OCD-related session (£) | Source |
|---|---|---|---|---|---|---|
| Trainer’s time | One band 6 nurse for 3 days for 7.5 hours, divided by average of 7 trainees per training session | £ 50 per hour including qualifications | 1125.00 | 160.71 | – | PSSRU71 |
| Trainee’s time | One band 5 nurse for 3 days for 7.5 hours | £ 39 per hour including qualifications | – | 877.50 | – | PSSRU71 |
| Manual | One GSH manual plus CCBT therapist guide plus photocopied sheets per therapist | £ 1.87 + £ 5.20 + £ 0.23 | – | 7.30 | – | OCTET team |
| Total training cost per therapist | – | 1045.51 | – | |||
| Total training cost per OCD-related sessionsa | – | – | 8.38 | |||
| Assumptions | Details | Source |
|---|---|---|
| Working days per annum | 210 days | PSSRU71 |
| Average patient contacts per day | 9 contacts | Nolan106 |
| Percentage of caseload with OCD | 2.2 | Health and Social Care Information Centre107 |
| Training valid over years | 3 years | Ekers et al.108 |
| Number of OCD-related sessions | 124.74 OCD-related sessions (210 × 9 × 3 × 2.2%) |
Appendix 5 Full Consolidated Standards of Reporting Trials diagram
FIGURE 24.
The CONSORT diagram for OCTET. a, Did not continue with PWP support and unknown if intervention was accessed independently; b, post-randomisation exclusion required resulting from a participant aged < 18 years (n = 1; cCBT), and risk issues relating to increased risk and not a change in supervisor decision (n = 1; waiting list).
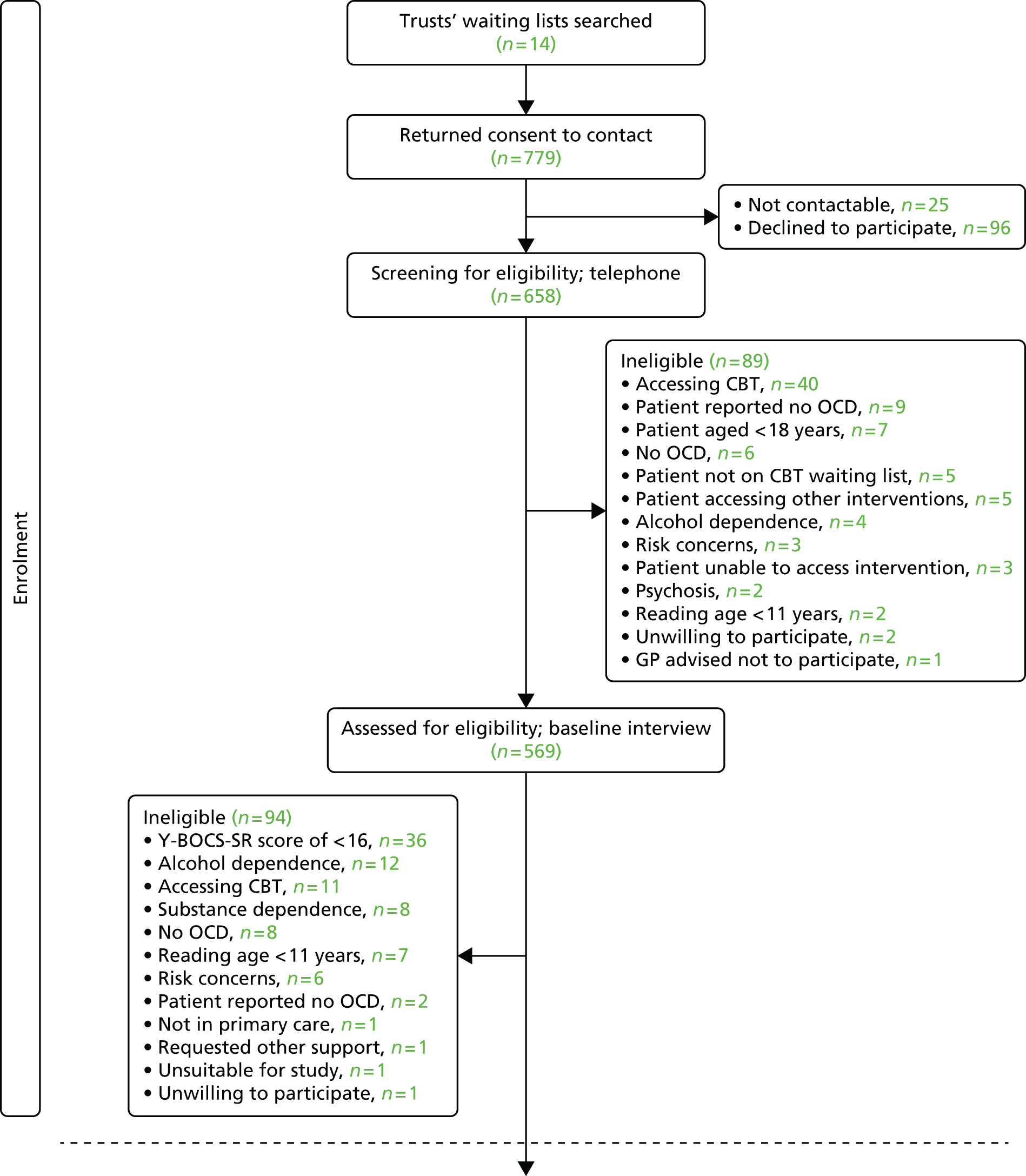
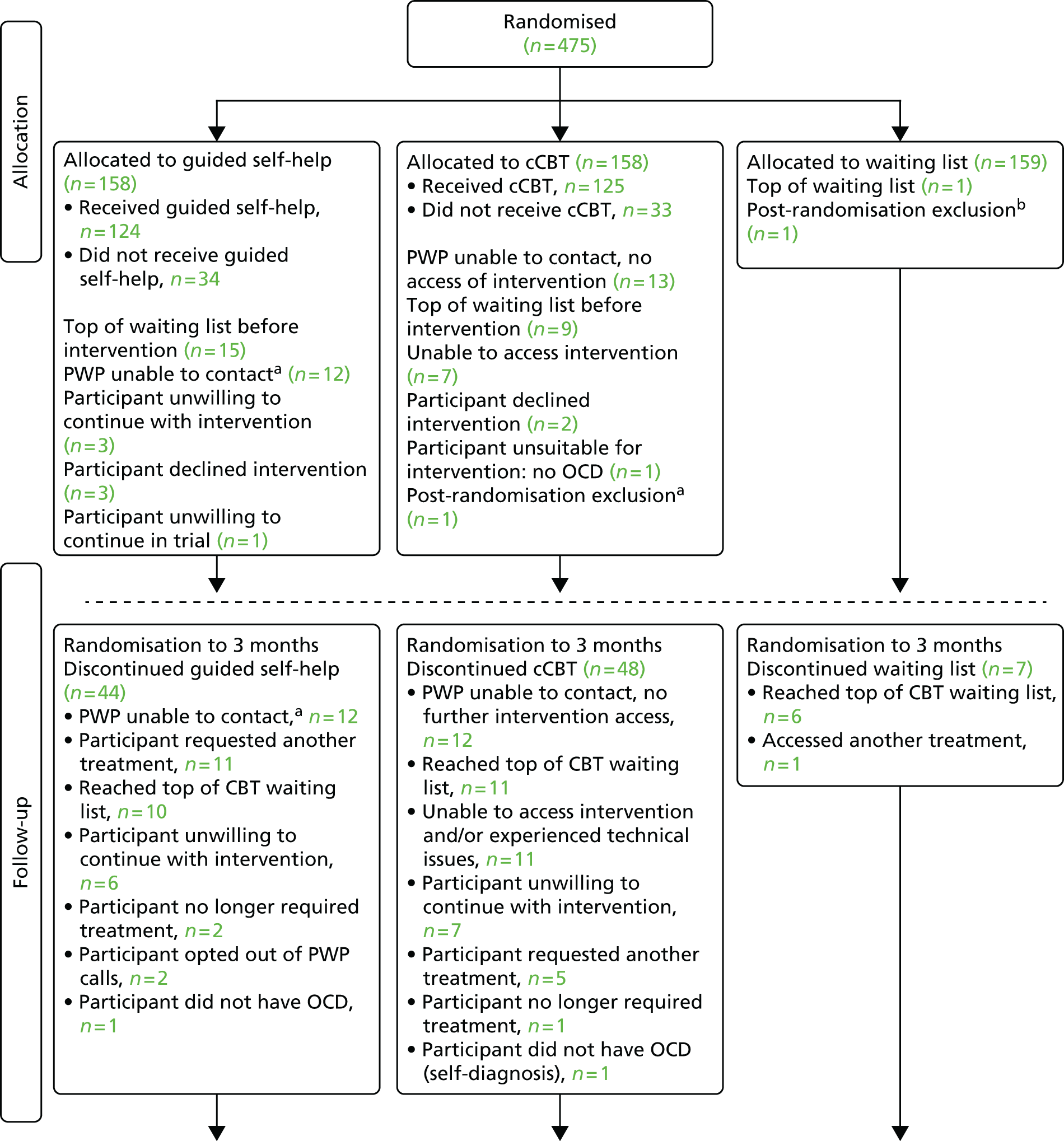
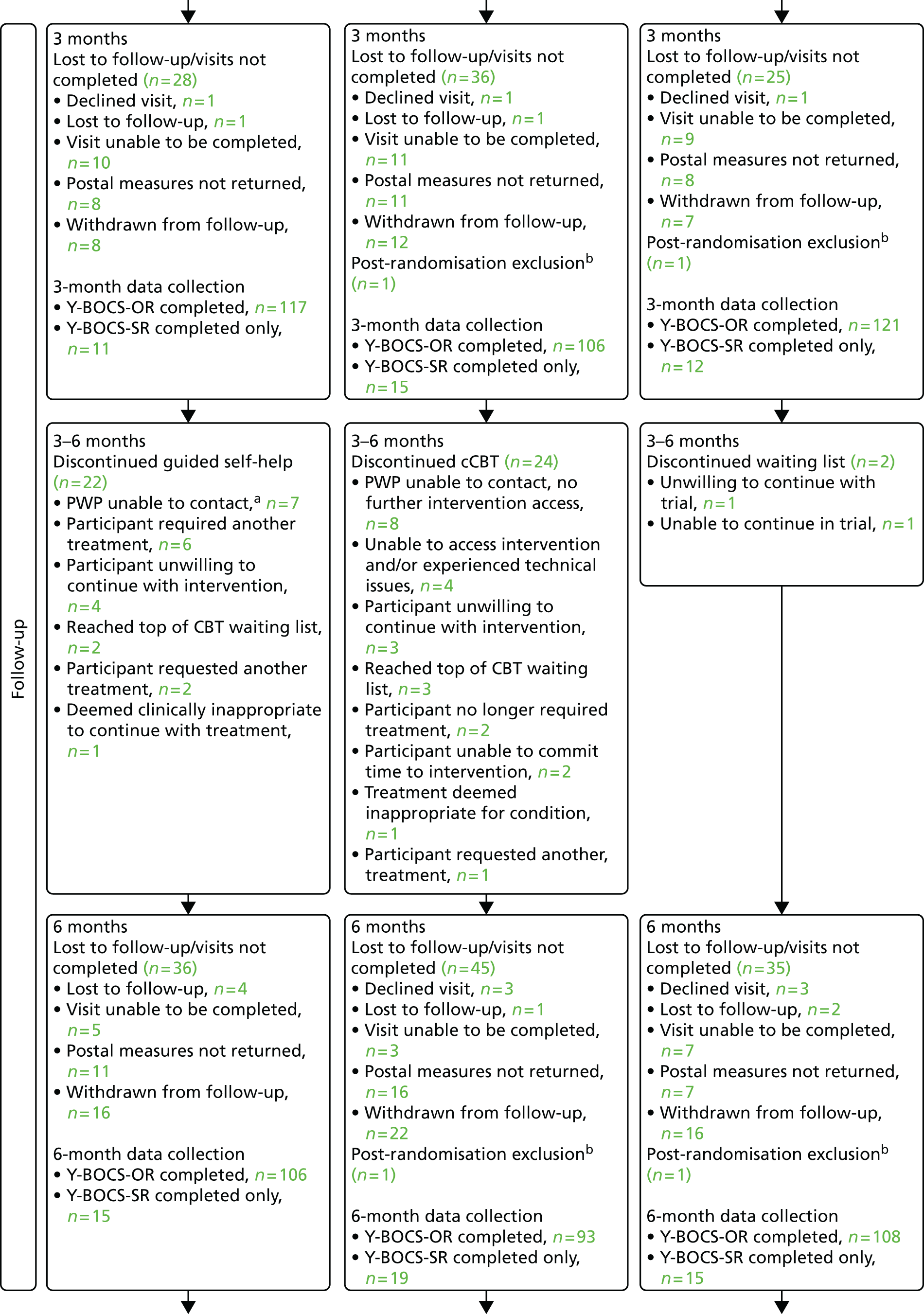
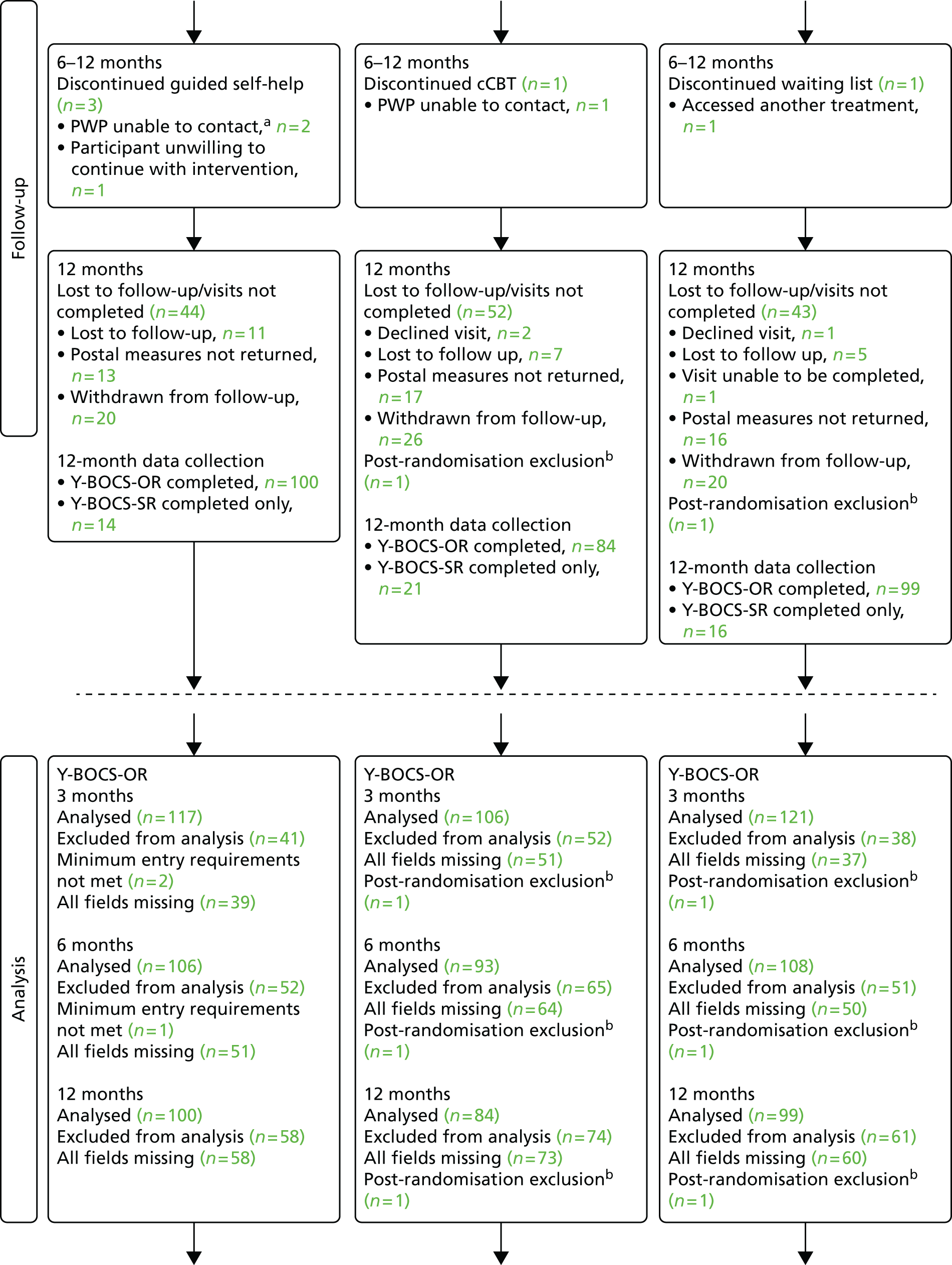
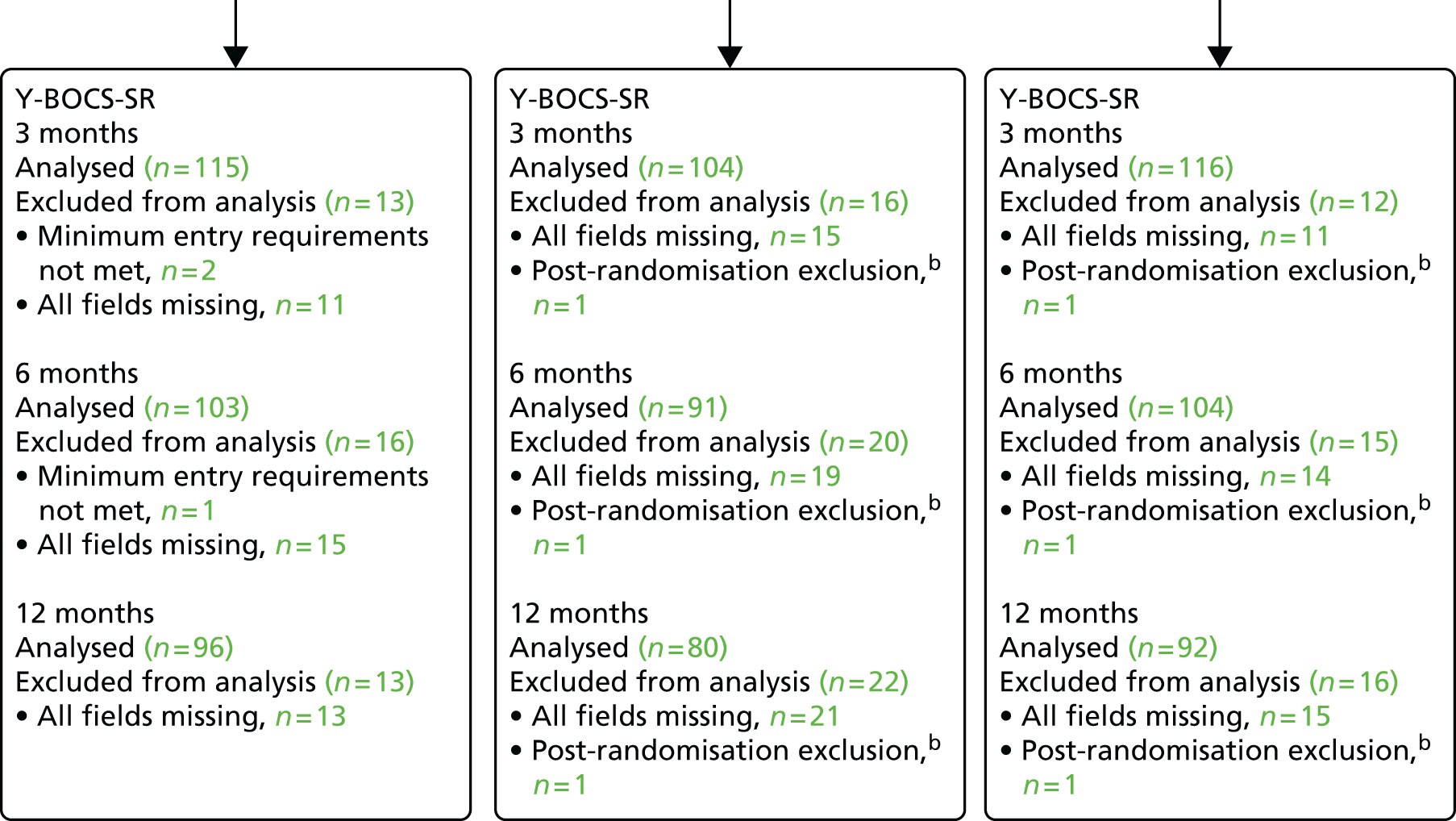
Appendix 6 Individual point resource use
| Resource use | Unit | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cCBT (n = 95) | Guided self-help (n = 107) | Waiting list (n = 108) | ||||||||
| n users | Mean use | SD | n users | Mean use | SD | n users | Mean use | SD | ||
| Hospital | ||||||||||
| Inpatient | Nights | 2 | 1.00 | 0.00 | 3 | 2.67 | 2.89 | 1 | 10.00 | – |
| Outpatient | Attendances | 15 | 2.13 | 2.07 | 17 | 2.65 | 3.16 | 25 | 2.29 | 2.38 |
| A&E | Attendances | 4 | 1.00 | 0.00 | 11 | 1.82 | 0.60 | 12 | 1.00 | 0.00 |
| Community | ||||||||||
| GP at surgery | Contacts | 64 | 2.08 | 1.16 | 76 | 2.37 | 1.85 | 71 | 2.61 | 2.93 |
| GP at home | Contacts | 1 | 2.00 | – | 0 | – | – | 0 | – | – |
| GP by telephone | Contacts | 5 | 1.60 | 0.89 | 4 | 1.75 | 0.96 | 11 | 1.27 | 0.47 |
| Practice nurse | Contacts | 13 | 1.54 | 0.66 | 18 | 3.39 | 7.92 | 13 | 1.62 | 1.39 |
| District nurse | Contacts | 5 | 1.20 | 0.45 | 3 | 1.00 | 0.00 | 4 | 1.75 | 0.96 |
| NHS walk-in clinic | Contacts | 4 | 1.25 | 0.50 | 3 | 1.00 | 0.00 | 4 | 2.50 | 1.73 |
| Community psychiatric nurse | Contacts | 2 | 13.50 | 17.68 | 2 | 2.50 | 0.71 | 1 | 6.00 | – |
| Psychiatrist in community | Contacts | 2 | 2.50 | 2.12 | 2 | 2.00 | 1.41 | 4 | 4.25 | 5.25 |
| Occupational therapist | Contacts | 0 | – | – | 0 | – | – | 1 | 5.00 | – |
| Marriage counselling | Contacts | 1 | 1.00 | – | 0 | – | – | 0 | – | – |
| Art/music/drama therapy | Contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker | Contacts | 1 | 3.00 | – | 1 | 2.00 | – | 2 | 3.00 | 1.41 |
| Advice service | Contacts | 3 | 4.33 | 4.93 | 2 | 5.50 | 6.36 | 6 | 2.83 | 2.71 |
| Helpline | Contacts | 0 | – | – | 1 | 1.00 | – | 3 | 4 | 4.20 |
| Day centre/drop-in | Contacts | 0 | – | – | 3 | 7.33 | 10.97 | 0 | – | – |
| Complementary therapy | Contacts | 4 | 1.75 | 0.96 | 2 | 2.00 | 1.41 | 1 | 7.00 | – |
| Self-help groups | Contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Other psychological therapies | 60 | – | – | 57 | – | – | 94 | – | – | |
| Psychotropic medication | Yes/no | 46 | – | – | 56 | – | – | 42 | – | – |
| Time off work | Days | 5 | 26 | 26.94 | 3 | 45 | 34.06 | 6 | 18 | 26.73 |
| Resource use | Unit | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cCBT (n = 88) | Guided self-help (n = 100) | Waiting list (n = 100) | ||||||||
| n users | Mean use | SD | n users | Mean use | SD | n users | Mean use | SD | ||
| Hospital | ||||||||||
| Inpatient | Nights | 3 | 1.00 | 0.00 | 3 | 1.00 | 0.00 | 5 | 2.45 | 1.12 |
| Outpatient | Attendances | 15 | 3.87 | 3.78 | 23 | 1.98 | 1.76 | 30 | 3.33 | 4.06 |
| A&E | Attendances | 3 | 1.67 | 0.58 | 9 | 1.00 | 0.00 | 12 | 1.25 | 0.62 |
| Community | ||||||||||
| GP at surgery | Contacts | 59 | 3.29 | 3.12 | 80 | 3.21 | 2.00 | 74 | 4.46 | 6.23 |
| GP at home | Contacts | 0 | – | – | 1 | 1.00 | – | 0 | – | – |
| GP by telephone | Contacts | 7 | 1.14 | 0.38 | 14 | 1.43 | 0.76 | 12 | 2.33 | 1.78 |
| Practice nurse | Contacts | 24 | 1.63 | 1.01 | 27 | 1.44 | 0.64 | 24 | 2.25 | 3.50 |
| District nurse | Contacts | 1 | 6.00 | – | 5 | 3.8 | 5.72 | 5 | 1.80 | 1.30 |
| NHS walk-in clinic | Contacts | 1 | 1.00 | – | 3 | 1.33 | 0.58 | 7 | 1.29 | 0.49 |
| Community psychiatric nurse | Contacts | 0 | – | – | 2 | 2.00 | 1.41 | 4 | 1.75 | 0.96 |
| Psychiatrist in community | Contacts | 2 | 1.00 | 0.00 | 3 | 1.00 | 0.00 | 5 | 2.40 | 2.19 |
| Occupational therapist | Contacts | 1 | 1.00 | – | 1 | 3.00 | – | 2 | 2.00 | 1.41 |
| Marriage counselling | Contacts | 0 | – | – | 1 | 1.00 | – | 0 | – | – |
| Art/music/drama therapy | Contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker | Contacts | 2 | 2.00 | 1.41 | 1 | 6.00 | – | 1 | 5.00 | – |
| Advice service | Contacts | 1 | 2.00 | – | 4 | 2.50 | 1.91 | 4 | 8.00 | 10.92 |
| Helpline | Contacts | 0 | – | – | 2 | 1.50 | 0.71 | 1 | 6.00 | – |
| Day centre/drop-in | Contacts | 0 | – | – | 2 | 3.00 | 0.00 | 1 | 7.00 | – |
| Complementary therapy | Contacts | 4 | 5.50 | 8.35 | 3 | 4.67 | 1.15 | 4 | 3.00 | 1.83 |
| Self-help groups | Contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Other psychological therapies | 64 | 62 | 76 | |||||||
| Psychotropic medication | Yes/no | 44 | 53 | 44 | ||||||
| Time off work | Days | 3 | 48 | 71.51 | 6 | 28 | 38.02 | 4 | 67 | 72.49 |
List of abbreviations
- AD-SUS
- Adult Service Use Schedule
- AE
- adverse event
- CBT
- cognitive–behavioural therapy
- cCBT
- computerised cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- CONSORT
- Consolidated Standards of Reporting Trials
- CORE-OM
- Clinical Outcomes in Routine Evaluation – Outcome Measure
- CSO
- clinical study officer
- CSQ-8
- Client Satisfaction Questionnaire-8
- DMEC
- Data Monitoring and Ethics Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 levels
- ERP
- exposure and response prevention
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HIT
- high-intensity therapist
- HPQ
- Health and Work Performance Questionnaire
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- MHRN
- Mental Health Research Network
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OCD
- obsessive–compulsive disorder
- OCTET
- Obsessive–Compulsive Treatment Efficacy randomised controlled Trial
- OR
- odds ratio
- PhD
- doctor of philosophy
- PHQ-9
- Patient Health Questionnaire-9
- PWP
- psychological wellbeing practitioner
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SOP
- standardised operating procedure
- TSC
- Trial Steering Committee
- WSAS
- Work and Social Adjustment Scale
- Y-BOCS
- Yale–Brown Obsessive Compulsive Scale
- Y-BOCS-OR
- Yale–Brown Obsessive Compulsive Scale – Observer Rated
- Y-BOCS-SR
- Yale–Brown Obsessive Compulsive Scale – Self-Report
- YTU
- York Trials Unit