Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/65/01. The contractual start date was in February 2015. The draft report began editorial review in August 2015 and was accepted for publication in December 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Huxley et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Aetiology and pathology
Colorectal cancer (CRC), also referred to as bowel cancer, is any cancer that affects the colon (large bowel) and rectum. It usually develops slowly over a period of 10–15 years. The tumour typically begins as a non-cancerous polyp. A polyp is a growth of tissue that develops on the lining of the large intestine (colon or rectum) that can become cancerous. Metastatic colorectal cancer (mCRC) refers to disease that has spread beyond the large intestine and nearby lymph nodes. 1 This type of cancer most often spreads first to the liver, but metastases may also occur in other parts of the body including the lungs, brain and bones. 1
The pathology of the tumour is usually determined by analysis of tissue taken from a biopsy or surgery. The extent to which the cancer has spread is described as its stage. 2 Staging is essential in determining the choice of treatment and in assessing prognosis. 2 More than one system is used for the staging of cancer. CRC stage can be described using the modified Dukes’ staging system (based on postoperative findings – a pathological staging based on resection of the tumour and measuring the depth of invasion through the mucosa and bowel wall) or the more precise tumour invasion, nodal involvement and metastatic spread (TNM) staging system, which is based on the depth of tumour invasion (T), nodal involvement (N) and metastatic spread (M) assessed preoperatively by radiological examination (Table 1). 2 Metastatic disease is classified as stage IV or modified Dukes’ stage D.
| Staging group | TNM staging and sites involved | Modified Dukes’ stage |
|---|---|---|
| Stage 0 | Carcinoma in situ (Tis, N0, M0) | |
| Stage I | No nodal involvement, no distant metastases | A |
| Tumour is confined to submucosa (T1, N0, M0) | ||
| Tumour has grown into (but not through) muscularis propria (T2, N0, M0) | ||
| Stage II | No nodal involvement, no distant metastases | B |
| Tumour has grown into (but not through) the serosa (T3, N0, M0) | ||
| Tumour penetrates through the serosa and peritoneal surface, directly extends to other organs or body structures or perforates the bowel (T4a/b, N0, M0) | ||
| Stage III | Nodal involvement, no distant metastases (any T, N1/N2, M0) | C |
| Stage IV | Distant metastases (any T, any N, M1) | D |
Epidemiology
Incidence and prevalence
In terms of incidence, CRC is the fourth most common cancer in the UK behind breast, lung and prostate cancer, accounting for 13% of all new cancer cases. 3 It is the third most common cancer in both men (14% of the total for men) and women (11% of the total) separately. 3 Table 2 summarises the number of new cases and incidence rates in the UK.
| Variable | England | Wales | Scotland | Northern Ireland | UK |
|---|---|---|---|---|---|
| Male | |||||
| Cases, n | 18,971 | 1297 | 2239 | 664 | 23,171 |
| Crude rate | 72.6 | 86.2 | 87.9 | 74.7 | 74.6 |
| AS rate (95% CI) | 56.7 (55.9 to 57.5) | 60.2 (57.0 to 63.5) | 67.4 (64.6 to 70.2) | 66.4 (61.3 to 71.4) | 58.0 (57.3 to 58.8) |
| Female | |||||
| Cases, n | 15,073 | 1046 | 1756 | 535 | 18,410 |
| Crude rate | 55.9 | 67.1 | 64.9 | 57.8 | 57.2 |
| AS rate (95% CI) | 36.8 (36.2 to 37.4) | 40.6 (38.2 to 43.1) | 41.9 (39.9 to 43.9) | 42.9 (39.3 to 46.5) | 37.6 (37.1 to 38.2) |
| Total | |||||
| Cases, n | 34,044 | 2343 | 3995 | 1199 | 41,581 |
| Crude rate | 64.1 | 76.5 | 76.0 | 66.1 | 65.8 |
| AS rate (95% CI) | 46.0 (45.5 to 46.5) | 49.6 (47.6 to 51.6) | 53.3 (51.7 to 55.0) | 53.5 (50.5 to 56.5) | 47.0 (46.6 to 47.5) |
Approximately two-thirds (66%) of cancer cases affect the colon and one-third (34%) affect the rectum, although this distribution varies by sex. 3 The crude incidence rates show that there are 46 and 41 new colon cancer cases per year for every 100,000 men and women in the UK, respectively, and around 29 and 17 new rectal cancer cases per year for every 100,000 men and women in the UK, respectively. 3
Approximately 25% of people present with metastases at initial diagnosis and almost 50% of people with CRC will develop metastases. 4
Prevalence refers to the number of people who have previously received a diagnosis of cancer and who are still alive at a given time point. Some people will have been cured of their disease and others will not. In the UK, > 143,000 people were still alive at the end of 2006, up to 10 years after being diagnosed with CRC (Table 3). 3
| Group | Prevalence | ||
|---|---|---|---|
| 1 year | 5 years | 10 years | |
| Male | 14,635 | 51,183 | 78,483 |
| Female | 11,415 | 40,594 | 65,075 |
| Total | 26,050 | 91,777 | 143,558 |
Risk factors
Risk factors for CRC include age and family history. In the UK between 2009 and 2011, an average 43% of bowel cancer cases were diagnosed in people aged ≥ 75 years and 95% of cases were diagnosed in those aged ≥ 50 years. 3 The lifetime risk of developing bowel cancer in the UK is 1 in 14 for men and 1 in 19 for women. 3
Mortality
Colorectal cancer is the second most common cause of cancer death in the UK (2012 data), accounting for 10% of all deaths from cancer. 5 In 2012 there were 16,187 deaths from CRC in the UK (Table 4). The crude mortality rates show that there are 28 CRC deaths per year for every 100,000 men in the UK and 23 per year for every 100,000 women. 5
| Group | England | Wales | Scotland | Northern Ireland | UK |
|---|---|---|---|---|---|
| Male | |||||
| Cases, n | 7200 | 525 | 837 | 233 | 8795 |
| Crude rate | 27.3 | 34.8 | 32.5 | 26.0 | 28.1 |
| AS rate (95% CI) | 20.0 (19.5 to 20.4) | 23.0 (21.1 to 25.0) | 23.3 (21.7 to 24.8) | 22.2 (19.3 to 25.0) | 20.5 (20.1 to 20.9) |
| Female | |||||
| Cases, n | 6036 | 387 | 784 | 185 | 7392 |
| Crude rate | 22.2 | 24.7 | 28.7 | 19.9 | 22.8 |
| AS rate (95% CI) | 12.6 (12.3 to 12.9) | 13.1 (11.8 to 14.4) | 16.2 (15.1 to 17.4) | 12.8 (10.9 to 14.6) | 13.0 (12.7 to 13.3) |
| Total | |||||
| Cases, n | 13,236 | 912 | 1621 | 418 | 16,187 |
| Crude rate | 24.7 | 29.7 | 30.5 | 22.9 | 25.4 |
| AS rate (95% CI) | 15.9 (15.7 to 16.2) | 17.6 (16.5 to 18.7) | 19.2 (18.3 to 20.1) | 17.0 (15.3 to 18.6) | 16.3 (16.1 to 16.6) |
Around 6 in 10 (61%) CRC deaths are due to cancers of the colon and around 4 in 10 (39%) are due to cancers of the rectum. 5 Almost one-fifth (18%) of CRC deaths occur in people aged 60–69 years. 5
Survival and prognosis
Approximately 77% of men survive CRC for at least 1 year, which is predicted to fall to 59% at ≥ 5 years, as shown by age-standardised net survival for people diagnosed with CRC during 2010–11 in England and Wales. 6 Survival for women at 1 and 5 years is slightly lower, with 74% surviving for ≥ 1 year and 58% predicted to survive for at least 5 years. 6
Survival is, however, highly dependent on the stage of disease at diagnosis. Survival by stage is not yet routinely available for the UK because of inconsistencies in the collecting and recording of staging data in the past. However, published estimates suggest that approximately 90% of people diagnosed at the earliest stage will survive for > 5 years, whereas < 10% of people diagnosed with distant metastases will survive for > 5 years. 7 In general, the earlier the diagnosis the higher the chances of survival. 7
Impact of the health problem
Colorectal cancer is a significant cause of morbidity and mortality. 8 When treating people with mCRC, the main aims of treatment are to relieve symptoms and to improve health-related quality of life (HRQoL) and survival. 1
Measurement of disease
The outcome end points of CRC can be measured in a variety of ways.
-
Overall survival (OS): defined as the time from randomisation to death from any cause. 9
-
Progression-free survival (PFS): defined as time from randomisation until disease progression or death. 9
-
Objective response rate (ORR): defined as either a partial response (PR) or a complete response (CR). The numbers of CRs and PRs are important as the benefits from CRs tend to be greater:
-
CR – all detectable tumour has disappeared
-
PR – roughly corresponds to at least a 50% decrease in the total tumour volume but with evidence of some residual disease still remaining
-
Stable disease – includes either a small amount of growth (typically < 20% or < 25%) or a small amount of shrinkage
-
Progressive disease – means that the tumour has grown significantly or that new tumours have appeared. The appearance of new tumours is always progressive disease, regardless of the response of other tumours. Progressive disease normally means that the treatment has failed.
-
-
HRQoL: how a person’s well-being is affected by treatment.
Current service provision
National Institute for Health and Care Excellence (NICE) guidance is available on the diagnosis and management of mCRC1 and first-line chemotherapeutic treatments for mCRC10–12 [see Current National Institute for Health and Care Excellence guidelines: biological agents (first line)]. NICE guidance on the use of second-line or subsequent treatments is also available;13 however, it is not discussed in detail in this report as it is beyond the scope of this multiple technology appraisal (MTA). 14
Management of disease
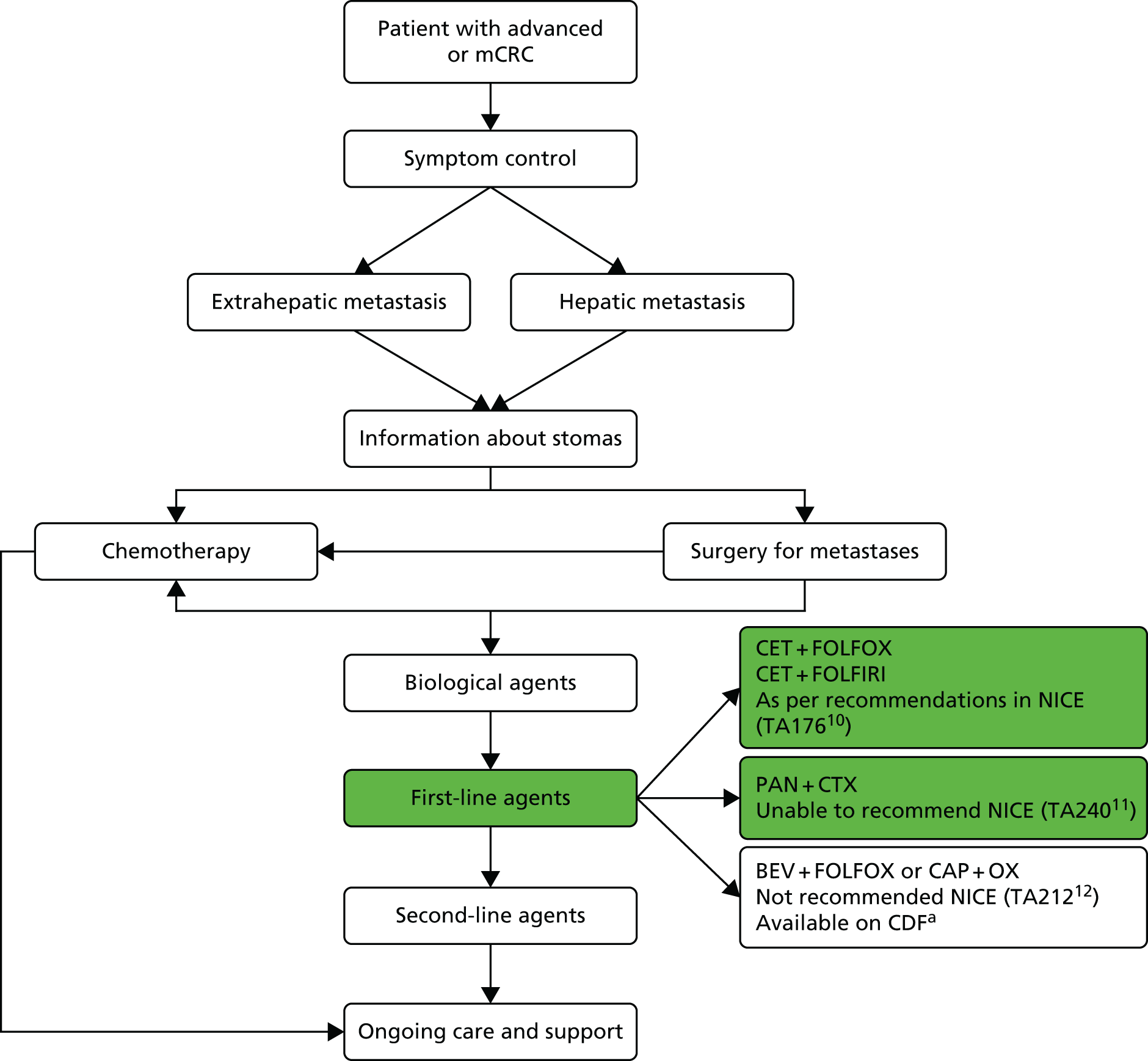
Treatment of mCRC may involve a combination of surgery, chemotherapy, radiotherapy and supportive care (Figure 1).
FIGURE 1.
Managing advanced and metastatic CRC (NICE pathways). BEV, bevacizumab; CAP, capecitabine; CDF, Cancer Drugs Fund; CET, cetuximab; CTX, chemotherapy; FOLFIRI, folinic acid + 5-fluorouracil + irinotecan; FOLFOX, folinic acid + 5-fluorouracil + oxaliplatin; OX, oxaliplatin; PAN, panitumumab; TA, technology appraisal. a, Bevacizumab is not recommended by NICE (TA21212). At the time of scoping bevacizumab was available (subject to satisfying criteria for access) via the CDF; however, this drug was delisted for the indication under review in this TA in March 2015. Adapted with permission from the National Institute for Health and Care Excellence’s publication entitled NICE Pathway: Managing Advanced and Metastatic Colorectal Cancer. Available from http://pathways.nice.org.uk/pathways/colorectal-cancer13 (accessed 13 March 2017). Content accurate at time of going to press.
The majority of people with metastatic disease are not initially suitable for potentially curative resection. 1,4 Up to 30% of people may be cured if metastases in the liver can be resected. For surgery to be considered, there must be no evidence of cancer outside the liver and there must be an adequate amount of normal liver left behind after the resection to sustain life. 1 Surgical skill is crucial to outcomes and there is evidence of wide variation in survival rates depending on the individual surgeon who operates. 15 Chemotherapy may be recommended before surgery in some cases, even if the metastatic disease appears to be confined to the liver. 1,4 This approach may help a person who is a borderline candidate for surgery (because of the size or location of the tumours) to become suitable for resection, after a response has been achieved with combination chemotherapy. 1,4
For the majority of people, however, surgery with curative intent is not an option because of the widespread nature of their disease and/or their poor suitability for surgery. 1 These people are treated with palliative intent using a combination of specialist treatments – palliative surgery (e.g. in cases in which the tumour is causing an obstruction), chemotherapy or radiotherapy – to improve both the duration and the quality of their remaining life. 1 NICE recommends chemotherapy options including 5-fluorouracil and folinic acid in combination with oxaliplatin (FOLFOX), tegafur (UFToral®, Merck Serono UK Ltd, Feltham, UK; no longer produced in the UK) in combination with 5-fluorouracil and folinic acid, capecitabine in combination with oxaliplatin (XELOX) and capecitabine alone. 1 In practice, 5-fluorouracil and folinic acid may also be used in combination with irinotecan (FOLFIRI) in some people for whom oxaliplatin is not suitable. 1 FOLFOX may be administered in different regimens, most commonly FOLFOX4 and FOLFOX6. The differences in drug acquisition and administration costs of all of these regimens are discussed in Chapter 6 (see Model parameters, Costs), but in effectiveness they are widely considered by the clinical community to be equal. Single-agent fluoropyrimidine regimens (tegafur, folinic acid and 5-fluorouracil and capecitabine monotherapy) are generally given to patients for whom combination therapy is not suitable (Dr Mark Napier, Royal Devon & Exeter NHS Foundation Trust, 2015, personal communication; Merck Serono submission version 2, 15 June 2015, Table 4, p. 22).
Folinic acid is also known as leucovorin (Sodiofolin®; medac GmbH, Stirling, UK) and is given alongside 5-fluorouracil to improve the response rate compared with 5-fluorouracil alone. It is given as calcium folinate (also known as leucovorin calcium) or less frequently as disodium folinate. 16 Folinic acid (and calcium folinate and disodium folinate), unless otherwise stated, are racemic mixtures (with equal amounts of left- and right-handed enantiomers), in which only the levoisomer (left-handed form) is pharmacologically active. 17 The levoisomer, levoleucovorin, has marketing authorisation in the UK [as calcium levofolinate (Isovorin®; Pfizer Limited, Sandwich, UK) and disodium levofolinate (levofolinic acid; medac GmbH, Stirling, UK)] and is administered at half the dose of standard (racemic) leucovorin. There appears to be no significant difference between levoleucovorin and leucovorin in terms of efficacy or adverse events (AEs), but levoleucovorin is significantly more expensive than leucovorin at present. 17
Chemotherapy may be combined with biological agents such as cetuximab (Erbitux®, Merck Serono) (currently recommended for people satisfying criteria specified in NICE technology appraisal (TA) no. 17610), panitumumab (Vectibix®, Amgen, Cambridge, UK) and bevacizumab (Avastin®, Roche Products Ltd) [see Current National Institute for Health and Care Excellence guidelines: biological agents (first line)]. Although bevacizumab is included in the final scope for this TA, it is not recommended by NICE (TA21212). It was available subject to satisfaction of criteria for access via the Cancer Drugs Fund (CDF), but has recently (March 2015) been delisted for the indication under review in this TA. 18 As of 17 July 2015, bevacizumab remains delisted for this indication.
Personalised treatment
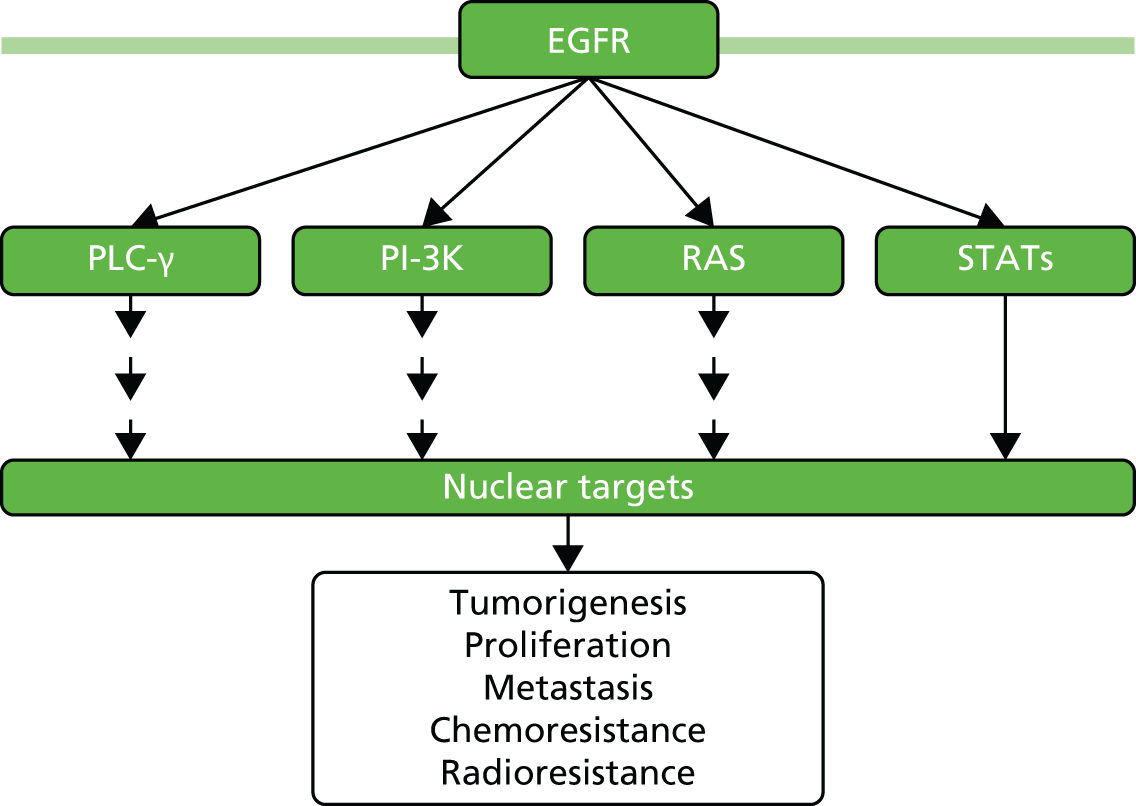
Normal cell behaviour in multicellular organisms is controlled by a complex network of signalling pathways that ensures that cells proliferate only when they are required to, for example in wound healing. 19 Cancer occurs when normal growth regulation breaks down, usually because of defects within these signalling mechanisms. 19 The rat sarcoma (RAS) genes play an important role in the epidermal growth factor receptor (EGFR) pathway, a complex signalling cascade that is involved in the development and progression of cancer (Figure 2). 20 Signals are passed from protein to protein along several different pathways. Disruption of the signals through mutation of the RAS gene is involved in many tumour types.
FIGURE 2.
Epidermal growth factor receptor signalling pathway. PI-3K, phosphoinositide 3-kinase; PLC-γ, phospholipase C gamma; STAT, signal transducer and activator of transcription. Adapted by permission from Macmillan Publishers Ltd on behalf of Cancer Research UK: British Journal of Cancer. Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. British Journal of Cancer 2006;94(2):184–8. Copyright 2006. 21
The three RAS genes – Kirsten rat sarcoma (KRAS), Harvey rat sarcoma (HRAS) and neuroblastoma rat sarcoma (NRAS) – are the most common oncogenes in human cancer. 19,20 All three are widely expressed, with KRAS expressed in almost all cell types. 19 Published research has demonstrated that mutations in codons 12 and 13 of exon 2 of the KRAS gene are predictive of a reduced response to anti-EGFR therapies in mCRC. 22–29 For this reason, only people with KRAS exon 2 wild-type (WT) tumours were initially approved for treatment with this class of agents. 30–32
More recently, it has been shown that other mutations in genes of the RAS family (mutations in codon 61 of exon 3 and codons 117 and 146 of exon 4 of KRAS, and mutations in exons 2, 3 and 4 of NRAS) are also associated with a reduced response to anti-EGFR therapy. 4,26,28,29,33,34 These developments led the European Medicines Agency (EMA) to update the marketing authorisations for cetuximab and panitumumab in 2013 by restricting the indication in mCRC to the treatment of people with RAS WT tumours (see Interventions considered in the scope of this assessment). 35–40
Exon 2 mutations in the KRAS gene occur in approximately 40% of mCRC cases and other KRAS and NRAS mutations occur in approximately 10% of mCRC cases (Figure 3). 22,26,33,41–44 Approximately 50% of people do not have RAS mutations and are classified as RAS WT.
FIGURE 3.
Grouping of molecular characteristics of tumours: research progress. ID, identification; MT, mutant.

RAS mutation testing
A biomarker test is a simple way of looking at the type and status of particular genes of interest in a cancer. Biomarkers have been found for many different types of cancer, such as colorectal, breast and lung cancer, and have an increasingly important role in helping physicians to tailor care and treatment on an individual basis, known as ‘personalised medicine’. RAS − a predictive biomarker − is a group of genes that includes KRAS and NRAS and can be used to help select the most appropriate therapy for each individual mCRC.
Methods for RAS mutation testing, whose use in the UK has been identified in a previous Health Technology Assessment report45 and by the Peninsula Technology Assessment Group (PenTAG), are summarised in Table 5. Additional techniques have also been developed and are in use internationally, including the Randox KRAS, BRAF, PIK3CA* Array (Randox Laboratories Ltd, Crumlin, UK) and the SNaPshot® Multiplex kit (Applied Biosystems, Foster City, CA, USA).
| Test | Limit of detection (%) | Source |
|---|---|---|
| KRAS and NRAS | ||
| Sanger sequencing | 10–20 | Wong et al.46 |
| Pyrosequencing | 5 | Wong et al.46 |
| High-resolution melt | 1–5 | Wong et al.46 |
| StripAssay® (ViennaLab Diagnostics, Vienna, Austria) | 1 | ViennaLab Diagnostics47 |
| Next-generation sequencing | ≈5 | Westwood et al.45 |
| KRAS | ||
| Cobas® (Roche Diagnostics Limited, Rotkreuz, Switzerland) | 5 | Wong et al.46 |
| Therascreen® (Qiagen, Venlo, the Netherlands) | 1–5 | Wong et al.46 |
| Peptide Nucleic Acid Clamp® (Panagene, Daejeon, Republic of Korea) | 1 | Panagene48 |
Many techniques and products reported are assays associated with the polymerase chain reaction (PCR) or that require use of PCR techniques prior to their implementation. Additionally, some laboratories offer their own in-house variant of real-time PCR. 45
Currently, there are no NICE recommendations for which mutation test should be used in the NHS. A NICE diagnostics review of KRAS mutation testing for identifying adults with mCRC was suspended in 2013, following notification of potential changes to clinical practice over who may benefit from first-line treatment with cetuximab or panitumumab. 49 A review by Westwood et al. 45 did demonstrate that evidence linking test accuracy with treatment effects is unavailable for most techniques currently in use. It concluded that there were ‘no clear differences in the treatment effects . . . regardless of which KRAS mutation test was used to select patients’. 45 Further discussion of the tests available and their impact on this review is reported in Appendix 1.
Current National Institute for Health and Care Excellence guidelines: biological agents (first line)
National Institute for Health and Care Excellence TA176: cetuximab for the first-line treatment of metastatic colorectal cancer
National Institute for Health and Care Excellence TA176 states that:
Cetuximab in combination with 5-fluorouracil (5-FU), folinic acid and oxaliplatin (FOLFOX), within its licensed indication, is recommended for the first-line treatment of mCRC only when all of the following criteria are met:
the primary colorectal tumour has been resected or is potentially operable
the metastatic disease is confined to the liver and is unresectable
the person is fit enough to undergo surgery to resect the primary colorectal tumour and to undergo liver surgery if the metastases become resectable after treatment with cetuximab
the manufacturer rebates 16% of the amount of cetuximab used on a per patient basis.
Reproduced with permission from National Institute for Health and Clinical Excellence (2009) TA176 Cetuximab for the first-line treatment of metastatic colorectal cancer. Available from: www.nice.org.uk/guidance/TA176. 10 Content accurate at time of going to press
Cetuximab in combination with 5-FU, folinic acid and irinotecan (FOLFIRI), within its licensed indication, is recommended for the first-line treatment of mCRC only when all of the following criteria are met:
the primary colorectal tumour has been resected or is potentially operable
the metastatic disease is confined to the liver and is unresectable
the patient is fit enough to undergo surgery to resect the primary colorectal tumour and to undergo liver surgery if the metastases become resectable after treatment with cetuximab
the patient is unable to tolerate or has contraindications to oxaliplatin.
Reproduced with permission from National Institute for Health and Clinical Excellence (2009) TA176 Cetuximab for the first-line treatment of metastatic colorectal cancer. Available from: www.nice.org.uk/guidance/TA176. 10 Content accurate at time of going to press
Patients who meet these criteria should receive treatment with cetuximab for no more than 16 weeks. 10 At 16 weeks, treatment with cetuximab should stop and patients should be assessed for resection of liver metastases. 10
National Institute for Health and Care Excellence TA240: panitumumab for the first-line treatment of metastatic colorectal cancer
The appraisal of panitumumab in combination with chemotherapy for the treatment of mCRC (TA240) was ended because no evidence submission was received from the manufacturer or sponsor of the technology. 11 Therefore, NICE was unable to make a recommendation about the use in the NHS of panitumumab in combination with chemotherapy for the treatment of mCRC. 11
National Institute for Health and Care Excellence TA212: bevacizumab in combination with oxaliplatin and either 5-fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer
Bevacizumab in combination with oxaliplatin and either 5-fluorouracil plus folinic acid or capecitabine is not recommended by NICE for the treatment of mCRC. 12
Current usage in the NHS
Currently, only cetuximab is recommended by NICE and is available for use on the NHS in England subject to satisfaction of criteria set out in TA17610 [see Current National Institute for Health and Care Excellence guidelines: biological agents (first line)]. For people with mCRC not meeting the criteria set out in TA176, cetuximab is available through the CDF. 50
The National Institute for Health and Care Excellence was unable to make a recommendation about the use in the NHS of panitumumab in combination with chemotherapy for the treatment of mCRC [TA240;11 see Current National Institute for Health and Care Excellence guidelines: biological agents (first line)]. Panitumumab is currently available for the first-line treatment of mCRC through the CDF. 51
Bevacizumab was not recommended by NICE for the treatment of mCRC [TA212;12 see Current National Institute for Health and Care Excellence guidelines: biological agents (first line)]. At the time of scoping, bevacizumab was available (subject to satisfaction of eligibility criteria) through the CDF; however, it was delisted in March 2015. 18
Almost one-third of patients in the UK receive cetuximab or panitumumab in combination with oxaliplatin- or irinotecan-based chemotherapy (Table 6).
| Regimen | Estimated current proportion of first-line mCRC patients in UK (%) | Estimated proportion of first-line mCRC patients in UK if CET/PAN/BEV no longer available through CDF and not recommended by NICE (%) |
|---|---|---|
| FOLFOXa | 30 | 60 |
| FOLFIRIb | 10 | 20 |
| Tegafur, FA + 5-FU, capecitabinec | 20 | 20 |
| BEV + OX- or IRIN-based CTX | 10 | NA |
| CET/PAN + OX- or IRIN-based CTX | 30 | NA |
Current service cost
Treatment costs can include the following: cost of first-line chemotherapy drugs (cetuximab, panitumumab, irinotecan or oxaliplatin, folinic acid, 5-fluorouracil), cost of administration in the first line, cost of curative intent liver surgery, cost of post-resection therapy in those who underwent curative resection of liver metastases, cost of management of AEs in the first line, cost of treatments in the second line, cost of treatment in the third line and cost of RAS screening.
Description of technology under assessment
Interventions considered in the scope of this assessment
The scope of this review was to ascertain the clinical effectiveness and cost-effectiveness of two interventions for previously untreated mCRC. These interventions were cetuximab and panitumumab.
Cetuximab
Cetuximab is a recombinant monoclonal antibody that blocks the human EGFR and therefore inhibits the proliferation of cells that depend on EGFR activation for growth. 35
Previously, cetuximab was indicated for use in people with EGFR-expressing KRAS WT mCRC. 30,31,52,53 In November 2013, in response to new biomarker data, the Committee for Medicinal Products for Human Use (CHMP) changed the indication to clarify the particular genetic make-up of the cancer that must be present before treatment with cetuximab is initiated. 37,39 Based on this recommendation, cetuximab is now indicated for the treatment of people with EGFR-expressing RAS WT mCRC:
-
in combination with irinotecan-based chemotherapy
-
in first line in combination with FOLFOX
-
as a single agent in people who have failed oxaliplatin- and irinotecan-based therapy and who are intolerant to irinotecan. 35
In this label change, the combination of cetuximab with oxaliplatin-containing chemotherapy is now contraindicated for people with RAS mutant mCRC or for whom the RAS status is unknown. 35
Premedication with an antihistamine and a corticosteroid at least 1 hour prior to the administration of cetuximab should be given. This premedication is recommended before the initial and subsequent infusions. Cetuximab is administered once a week; the initial dose is 400 mg/m2 of body surface area, with subsequent weekly doses of 250 mg/m2 of body surface area. 35
One common AE related to cetuximab treatment is the development of skin reactions, which occurs in > 80% of people and mainly presents as an acne-like rash or, less frequently, as pruritus, dry skin, desquamation, hypertrichosis or nail disorders (e.g. paronychia). 35 The majority of skin reactions develop within the first 3 weeks of treatment. 35 The Summary of Product Characteristics (SPC) notes that, if a person experiences a grade 3 or 4 skin reaction, cetuximab treatment must be stopped, with treatment being resumed only if the reaction resolves to grade 2. 35 Other common AEs of cetuximab include mild or moderate infusion-related reactions such as fever, chills, nausea, vomiting, headache, dizziness or dyspnoea that occur soon after the first cetuximab infusion. 35
Panitumumab
Panitumumab is a recombinant monoclonal antibody that targets the EGFR, thereby inhibiting the growth of EGFR-expressing tumours. 36
In June 2013, the CHMP changed the indication for the use of panitumumab for the treatment of mCRC,38,40 restricting use to the treatment of adults with RAS WT mCRC:
-
in first line in combination with FOLFOX or FOLFIRI
-
in second line in combination with FOLFIRI for people who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan)
-
as monotherapy after failure of fluoropyrimidine-, oxaliplatin- and irinotecan-containing chemotherapy regimens. 36
In this label change, the combination of panitumumab with oxaliplatin-containing chemotherapy is now contraindicated for people with RAS mutant mCRC or for whom the RAS mCRC status is unknown. 36
The recommended dose of panitumumab is 6 mg/kg of bodyweight given once every 2 weeks. 36 Before infusion, panitumumab should be diluted in 0.9% sodium chloride to a final concentration not exceeding 10 mg/ml. 36
Panitumumab is contraindicated in people with a history of severe or life-threatening hypersensitivity reactions to the active substance or to any of the excipients. 36 The most common AEs observed (incidence ≥ 20%) are skin toxicities (i.e. erythema, dermatitis acneiform, pruritus, exfoliation, rash and fissures), paronychia, hypomagnesaemia, fatigue, abdominal pain, nausea, diarrhoea and constipation. 36
As noted, recent research (see Personalised treatment) has resulted in the CHMP adopting a change to the licensed indication for both cetuximab and panitumumab, restricting use to people with RAS WT mCRC. These developments and changes to the licensed indications provide the rationale for this MTA.
Cetuximab and panitumumab for previously untreated metastatic colorectal cancer (review of TA176 and partial review of TA240) (ID794)
Although this MTA seeks to update previous guidance (TA17610 and TA24011), it is important to note the differences between the scope for the previous single technology appraisals (STAs) and the scope for this current MTA review (ID794). 14 The main difference is in the population criterion. The current scope specifies people with RAS WT mCRC, whereas previous STA reviews specified EGFR-expressing mCRC (TA176)54 and KRAS WT mCRC (TA240). 11 A summary of the differences between the scopes for the reviews and how the product licences have changed is provided in Table 7.
| Variable | CET | PAN | CET | PAN | CET + PAN | ||
|---|---|---|---|---|---|---|---|
| CHMP30,31,52,53 | TA17654 | CHMP32,55 | TA24011 | CHMP37,39 | CHMP38,40 | Current MTA (ID794)14 | |
| Year | 2008, 2011 | 2009 | 2011 | 2011 | 2013 | 2013 | 2014–16 |
| NICE appraisal method | NA | STA | NA | STA | NA | NA | MTA |
| NICE guidance | NA | TA176 | NA | TA240 (suspendeda) | NA | NA | Due 2017 |
| Population | KRAS WT mCRC | Untreated mCRC, first-line palliative | KRAS WT mCRC | NA | RAS WT-expressing mCRC | RAS WT-expressing mCRC | RAS WT-expressing mCRC |
| Metastases | Any location | Untreated, any location | Any location | NA | Any location | Any location | Untreated, any location (subgroup of interest liver metastases)14 |
| Intervention (first line) | CET + FOLFOX4 or IRIN-based CTX | CET + CTX54 | PAN + FOLFOX | NA | CET + FOLFOX or CET + FOLFIRI | PAN + FOLFOX | CET + FOLFOX or IRIN-based regimens; PAN + FOLFOX regimens |
| Comparators | NA | OX-based CTX; IRIN-based CTX54 | NA | NA | NA | NA | FOLFOX; XELOX; FOLFIRI; CAP; TEG + FA + 5-FU; BEV + OX- or IRIN-based CTXb |
| Supporting trials | CRYSTAL, OPUS, COIN, NORDIC VII | CRYSTAL, OPUS | KRAS WT subgroup from PRIME | NA | RAS WT subgroup from OPUS, CRYSTAL, FIRE-3 | RAS WT subgroup from PEAK, PRIME | RAS WT subgroup from CRYSTAL, OPUS, PRIME, PEAK, FIRE-3 |
Chapter 2 Definition of the decision problem
Decision problem
Cetuximab and panitumumab (interventions of interest to this appraisal) were evaluated separately in 2009 (TA17610) and 2011 (TA24011) [see Chapter 1, Current National Institute for Health and Care Excellence guidelines: biological agents (first line)].
At the time of TA176 (2009), RAS WT status was defined based on a single part (‘exon’) of the KRAS gene and testing typically focused on KRAS codons 12 and 13. 56 However, subsequent research has suggested that mutations in other KRAS codons and other genes downstream of the EGFR may also confer drug resistance, explaining why some individuals with KRAS codon 12 and 13 WT tumours did not respond to therapy. 56 The absence of mutations in the NRAS gene and in two further exons (3 and 4) of KRAS was found to improve the effectiveness of cetuximab and panitumumab. 56 These developments led the EMA to update the marketing authorisations for cetuximab39 and panitumumab40 in 2013 by restricting the indication in CRC to the treatment of people with RAS WT tumours. It is this change to the licensed indications for these products that provides the rationale for this appraisal. 14
Population, including subgroups
The population specified in the final scope issued by NICE was people with previously untreated RAS WT mCRC. 14
The subgroup of interest was based on the location of metastases, specifically liver- and non-liver-limited disease. 14
Interventions
This MTA considered two interventions.
-
Cetuximab is a recombinant monoclonal antibody that blocks the human EGFR, inhibiting the growth of tumours expressing EGFR. 35 Cetuximab has a UK marketing authorisation for the treatment of people with EGFR-expressing RAS WT mCRC, in combination with either FOLFOX or irinotecan-based chemotherapy. 10
-
Panitumumab is a recombinant, fully human immunoglobulin G2 monoclonal antibody that binds to the EGFR, blocking its signalling pathway and inhibiting the growth of tumours. 36 It has a UK marketing authorisation for use in combination with FOLFOX for treating previously untreated RAS WT mCRC. 36 Panitumumab is also licensed for use second line in combination with FOLFIRI for people who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan), although clinical trials have also measured the effectiveness of panitumumab in combination with FOLFIRI for previously untreated mCRC. 36
Comparators
The scope issued by NICE14 specified that the interventions should be compared with each other and with:
-
FOLFOX
-
XELOX
-
FOLFIRI
-
capecitabine
-
tegafur, folinic acid and 5-fluorouracil
-
bevacizumab, in combination with oxaliplatin- or irinotecan-based chemotherapy.
The Assessment Group noted that tegafur/uracil was discontinued in 2013 (Merck Serono submission version 2, 15 June 2015, section 1.2, p. 19). Capecitabine and folinic acid plus 5-fluorouracil are typically preferred for patients with poor performance status (Dr Mark Napier, 2015, personal communication, and Merck Serono submission version 2, 15 June 2015).
Outcomes
The outcomes of interest considered in this review included:
-
OS
-
PFS
-
response rate (including ORR, CR, PR, progressive disease, stable disease)
-
rate of resection of metastases
-
adverse effects of treatment
-
HRQoL. 14
Overall aims and objectives of the assessment
The aim of this project was to review the clinical effectiveness and cost-effectiveness of cetuximab and panitumumab in a MTA. This included a review of TA17610 (cetuximab) and a partial review of TA24011 (panitumumab) for adults with previously untreated mCRC with RAS WT status. The medical benefits and risks associated with these treatments were assessed and compared across the treatments and against available standard drug treatments. The review also assessed whether or not these drugs are likely to be considered good value for money for the NHS.
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Evidence for the clinical effectiveness of cetuximab and panitumumab for people with previously untreated RAS WT mCRC was assessed by conducting a systematic review of published research evidence. The review was undertaken following the general principles published by the Centre for Reviews and Dissemination (CRD). 57 The project was undertaken in accordance with a protocol (PROSPERO number CRD42015016111). There were no major departures from this protocol.
Individuals respond differently to some drugs. 58,59 Genotype is an important determinant of both the response to treatment and the susceptibility to adverse reactions for a wide range of drugs,60,61 for example response to EGFR inhibitors has been shown to be dependent on gene expression in colon cancer, with studies demonstrating a treatment interaction between RAS status and the effectiveness of EGFR inhibitors. 62–64 In line with research evaluating the negative impact of RAS mutations on the effectiveness of EGFR inhibitors, approval for the use of anti-EGFR antibodies has now been limited to people with mCRC with RAS WT tumours. 44,65 Tumour samples from trial populations supporting the original licensed indications were evaluated retrospectively for RAS status. Importantly, therefore, data supporting this recent licence change and this NICE assessment are not from the intention-to-treat (ITT) population for any of the included studies but from a subgroup of people contained within the original randomised controlled trials (RCTs), and the results are therefore subject to uncertainty. However, no RCTs with an ITT population by RAS WT status were identified.
Previously, NICE has appraised cetuximab (TA17610) for the treatment of people with EGFR-expressing mCRC, in line with the licensed indication at the time. Two of the identified cetuximab trials were included in the last appraisal; however, only data from the subgroup of people evaluated as RAS WT from those trials are relevant to the scope of this review, as set out in the final scope from NICE (see Chapter 2, Population, including subgroups). The appraisal of panitumumab in combination with chemotherapy for the treatment of mCRC (NICE TA24011) ended because no evidence submission was received from the manufacturer or sponsor of the technology. As such, NICE was unable to make recommendations relating to the use of panitumumab in the NHS. All data included in this update review for both cetuximab and panitumumab have been identified by the Assessment Group’s searches.
Identification of studies
The search strategy for clinical effectiveness studies included the following search methods:
-
searching of bibliographic and ongoing trials databases
-
searching of conference proceedings
-
contact with experts in the field
-
scrutiny of bibliographies of retrieved papers and company submissions.
The following bibliographic and ongoing trials databases were searched for clinical effectiveness studies: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid), The Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects and Health Technology Assessment database, Web of Science (Thomson Reuters), ClinicalTrials.gov, the UK Clinical Research Network’s (UKCRN) portfolio, the International Standard Randomised Controlled Trials Number (ISRCTN) registry and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
The bibliographic database searches were developed and run by an information specialist (SB) in January 2015. Search filters were used to limit the searches to RCTs, when appropriate, and all searches were limited to English-language studies when possible. No date limits were used. An update search was carried out on 27 April 2015. No papers or abstracts published after this date were included in the review. The ongoing trials databases were searched by a reviewer in March 2015. The search strategies for each database are detailed in Appendix 1.
In addition to the clinical effectiveness searches, the Health Management Information Consortium Ovid) database was searched for grey literature; this produced no new studies.
The following websites were searched for conference proceedings:
-
National Cancer Research Institute [http://conference.ncri.org.uk/ (accessed 7 March 2017)]
-
American Association for Cancer Research [http://aacrmeetingabstracts.org/ (accessed 7 March 2017)]
-
American Society of Clinical Oncology (ASCO) [http://meetinglibrary.asco.org/abstracts (accessed 7 March 2017)].
The bibliographic search results were exported to, and deduplicated using, EndNote X7 (Thomson Reuters, CA, USA). Deduplication was also performed using manual checking. Titles and abstracts returned by the search strategy were examined independently by two researchers (LC and MB) and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of potentially relevant studies were ordered. Full publications were assessed independently by two reviewers (LC and MB) for inclusion or exclusion against prespecified criteria, with disagreements resolved by discussion.
After the reviewers had completed the screening process, the bibliographies of included papers were scrutinised for further potentially relevant studies. The manufacturers’ submissions were assessed for unpublished data.
Eligibility criteria
Inclusion and exclusion criteria for the selection of clinical effectiveness and safety evidence were defined according to the decision problem outlined in the NICE scope;14 inclusion criteria are summarised in Table 8.
| Study characteristic | Inclusion criteria |
|---|---|
| Population | Adults with previously untreated, RAS WTa mCRC |
| Intervention | Cetuximab, in combination with FOLFOX or irinotecan-based chemotherapy |
| Panitumumab, in combination with 5-fluorouracil-containing regimens | |
| Comparator | FOLFOX |
| XELOX | |
| FOLFIRI | |
| Capecitabine | |
| Tegafur, folinic acid and 5-fluorouracil | |
| Bevacizumab, in combination with oxaliplatin- or irinotecan-based chemotherapy | |
| Outcomes | OS |
| PFS | |
| Response rate | |
| Rate of resection of metastases | |
| AEs | |
| HRQoL | |
| Study design | RCTs |
| Systematic reviews of RCTsb |
The systematic review of clinical effectiveness was based on RCT evidence. Studies published as abstracts or conference presentations were included only if sufficient details were presented to allow both an appraisal of the methodology and an assessment of the results to be undertaken. Systematic reviews of RCTs (although not formally included in the systematic review) were used as potential sources of additional efficacy evidence. A systematic review was defined as including:
-
a focused research question
-
explicit search criteria that were available to review, either in the document or on application, and explicit inclusion/exclusion criteria defining the population(s), intervention(s), comparator(s) and outcome(s) of interest
-
a critical appraisal of included studies, including consideration of internal and external validity of the research
-
a synthesis of the included evidence, whether narrative or quantitative.
The following study types were excluded: animal models, preclinical and biological studies, narrative reviews, editorials, opinions and non-English-language papers.
Data extraction and management
Included papers were split between two reviewers for the purposes of data extraction, which was carried out using a standardised data specification form. Data extraction was checked independently by another reviewer. Information extracted and tabulated included details of the study design and methodology, baseline characteristics of participants and results, including any AEs if reported. When information on key data was incomplete, we attempted to contact the study’s authors to gain further details. Discrepancies were resolved by discussion. When multiple publications of the same study were identified, data were extracted and reported as a single study. In addition, the companies (Merck Serono and Amgen) were approached through NICE to provide missing data for the RAS WT population; this information was provided as commercial-in-confidence (CiC).
Assessment of risk of bias
The methodological quality of each included study was assessed by one reviewer and checked by a second reviewer, using criteria based on those proposed by the NHS CRD for RCTs (Table 9). 57 The potential generalisability of the studies was also assessed, as well as the judged applicability to the current organisation, clinical pathways and practices of the NHS in England.
| Study characteristic | Assessment criteria |
|---|---|
| Treatment allocation | 1. Was the assignment to the treatment groups really random? |
| 2. Was treatment allocation concealed? | |
| Similarity of groups | 3. Were the groups similar at baseline in terms of prognostic factors? |
| Implementation of masking | 4. Were the care providers blinded to the treatment allocation? |
| 5. Were the outcome assessors blinded to the treatment allocation? | |
| 6. Were the participants blinded to the treatment allocation? | |
| Completeness of trial | 7. Were all a priori outcomes reported? |
| 8. Were complete data reported [e.g. was attrition and exclusion (including reasons) reported for all outcomes]? | |
| 9. Did the analyses include an ITT analysis? | |
| Generalisability | 10. Are there any specific limitations that might limit the applicability of this study’s findings to the current NHS in England? |
Methods of data analysis/synthesis
The results of the clinical effectiveness and quality assessment for each included study are presented in structured tables and as a narrative summary. The possible effects of study quality on the clinical effectiveness data and review findings are discussed.
Network meta-analysis
Network meta-analyses (NMAs) were undertaken within a Bayesian framework in WinBUGS (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK). When prior distributions were used these were defined to be as vague as possible. The NMAs could have been conducted outside of WinBUGS (especially because of the low number of RCTs); however, the approach taken here allowed calculation of the probability that each treatment was the most effective compared with all others within the network.
Two networks were analysed: those using FOLFOX regimens and those using FOLFIRI regimens. The treatment FOLFOX was the baseline treatment in the FOLFOX regimens network, whereas FOLFIRI was the baseline treatment in the FOLFIRI regimens network.
For the analysis of PFS, OS and ORR, models with a normal likelihood and identify link were used. 66 Analysis of AEs used a model with a binomial likelihood and logit link. 66 For the analysis of AEs, when no events were reported in a study arm, a continuity correction of 0.5 was added to every cell for that particular study to allow analysis to be conducted. 66
Analyses were run with three chains and an initial burn-in of 50,000 iterations, followed by an additional 100,000 iterations, on which the results were based. Because of the small number of RCTs contributing to each network, only fixed-effects models were used. Convergence of the models was assessed visually using the autocorrelation, density and trace plots for all monitored variables, and checking that each chain was sampling from the same posterior distribution. The posterior means and 95% credible intervals (CrIs) from these analyses are reported. The probability that each treatment in the network was ranked as the most effective (rank 1) down to the least effective (rank 4) was also calculated.
Results
The results of the included studies are discussed in the following sections. Results relating to the patient population of interest (RAS WT) are subgroup analyses of the original studies. Initially, a summary of the quantity and quality of the evidence is provided, together with a table presenting an overview of the included trials. This is followed by a more detailed narrative description, together with an overview of trial quality, for each included trial. A narrative description of population baseline characteristics and potential imbalances is provided for each trial. The clinical effectiveness results are reported by outcome (OS, PFS, ORR, resection rate, HRQoL and AEs). For the efficacy outcomes of OS, PFS and ORR, the results are presented separately for cetuximab and panitumumab.
Studies identified
We screened the titles and abstracts of 2636 unique references identified by the PenTAG searches and additional sources and retrieved 52 papers for detailed consideration. Of these, 45 were excluded (a list of these items with reasons for their exclusion can be found in Appendix 2). Of the excluded items, four abstracts were identified as relevant to the review33,34,67,68 (see Appendix 3) but were excluded as not enough information was available to adequately appraise their quality. The authors of the abstracts were contacted, which led to the identification of an additional two full papers. 43,65 In total, post hoc analyses from five RCTs28,29,43,44,65 met the inclusion criteria. In assessing titles and abstracts, agreement between the two reviewers was substantial (κ = 0.801). At the full-text stage, agreement was good (κ = 0.636). At both stages, initial disagreements were easily resolved by consensus.
Update searches were conducted on 27 April 2015 using the same methodology as described earlier. A total of 175 records were screened by two reviewers (LC and JVC) and four records were selected for full-text retrieval. Of these, none was formally included in the review; although three were considered to meet the eligibility criteria for the review,69–71 they were available only in abstract form and, as such, could not be quality appraised (see Appendix 3).
No studies comparing either cetuximab or panitumumab with the following comparators met the eligibility criteria for the review: XELOX, capecitabine monotherapy and tegafur plus folinic acid + 5-fluorouracil (specified in the NICE scope14). In addition, no studies evaluating panitumumab plus FOLFIRI met the eligibility criteria for the review.
The study selection process is outlined in Figure 4.
Cetuximab
Study characteristics
The 2009 STA (TA17610) identified two RCTs investigating the effectiveness of the addition of cetuximab to either oxaliplatin- (FOLFOX) or irinotecan-based (FOLFIRI) chemotherapy [CRYSTAL (Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer)24 and OPUS (Oxaliplatin and Cetuximab in First-line Treatment of Metastatic Colorectal Cancer)23]. As research into the impact of KRAS and NRAS tumour mutations on the effectiveness of EGFR inhibitors developed, the ITT populations from the pivotal trials were re-evaluated, forming the basis for the revision of the licensed population.
In total, three subgroup analyses from three randomised, open-label trials {OPUS,65 CRYSTAL43 and FIRE-3 [5-FU, Folinic Acid and Irinotecan (FOLFIRI) Plus Cetuximab versus FOLFIRI Plus Bevacizumab in First Line Treatment of Colorectal Cancer]28} were included in the update review. Of note, in the FIRE-328 trial a protocol amendment was made, restricting eligibility for the ITT population to those with KRAS WT exon 2 tumours, because of the emerging evidence on the negative predictive value of KRAS exon 2 mutations and the subsequent changes to the licence for cetuximab. However, in all of the included trials the extended RAS subgroup analysis of interest to this review was conducted retrospectively.
Of the included trials, two evaluated the addition of cetuximab to background chemotherapy (FOLFOX65 or FOLFIRI43) and one evaluated the addition of cetuximab or bevacizumab to background chemotherapy (FOLFIRI)28 (Table 10). All of the trials evaluated the same dose of cetuximab and administration method.
| First author (trial); study design | Included in TA176a | Included in update review | Inclusion criteria | ITT (n)b | RAS WT (n)/analysed (N)c | Randomisation stratification factors | Interventions evaluated and dose | Primary end point | Treatment duration (months), median (IQR) | Follow-up (months), median (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bokemeyer 201523,33,65 and data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (OPUS; NCT00125034); retrospective subgroup analysis | No | Yes | Aged ≥ 18 years; ECOG PS ≤ 2; first occurrence of metastatic disease | 337 | 87/118 | ECOG PS 0–1 or 2 | CET + FOLFOX4 vs. FOLFOX4 | ORR | CET + FOLFOX4 5.7 (NR) vs. FOLFOX4 4.7 (NR) | NR |
| CET: 400 mg/m2 on day 1, then 250 mg/m2 per week | ||||||||||
| FOLFOX: Q2W as 85 mg/m2 of IV OX on day 1 + 200 mg/m2 i.v. infusion of folinic acid (over 2 hours) on days 1 and 2 + 400 mg/m2 bolus i.v. infusion of 5-FU (2–4 minutes) then 600 mg/m2 infusion (during 22 hours) on days 1 and 2 | ||||||||||
| Van Cutsem 201543 and data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (CRYSTAL; NCT00154102); retrospective subgroup analysis | No | Yes | Aged ≥ 18 years; ECOG PS ≤ 2; first occurrence of metastatic disease | 1198 | 367/430 | ECOG PS 0–1 or 2; region (Western Europe vs. Eastern Europe vs. outside Europe) | CET + FOLFIRI vs. FOLFIRI | PFS | CET + FOLFIRI 7.41 (NR) vs. FOLFIRI 5.77 (NR) | NR |
| CET: 400 mg/m2 on day 1, then 250 mg/m2 per week | ||||||||||
| FOLFIRI: 30- to 90-minute infusion of 180 mg/m2 of IRIN + 120-minute infusion of 400 mg/m2 of racemic leucovorin or 200 mg/m2 of L-leucovorin + 5-FU bolus of 400 mg/m2 then continuous infusion for 46 hours at 2400 mg/m2 | ||||||||||
| Heinemann 201428 and data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (FIRE-3; NCT00433927); retrospective subgroup analysis | No | Yes | Aged ≥ 18 years; ECOG PS ≤ 2; first occurrence of metastatic disease | 592 | 342/542 | ECOG PS 0–1 or 2; number of metastatic sites (one or more than one); white blood cell count | CET + FOLFIRI vs. BEV + FOLFIRI | ORR | NR | CET + FOLFIRI 33.0 (19.0–55.4) vs. BEV + FOLFIRI 39.0 (22.5–56.9) |
| CET: 400 mg/m2 on day 1, then 250 mg/m2 per week | ||||||||||
| BEV: 90-minute infusion of 5 mg/kg on day 1, 60-minute infusion of 5 mg/kg 2 weeks later; 30-minute infusion of 5 mg/kg every 2 weeks thereafter | ||||||||||
| FOLFIRI: 60- to 90-minute infusion of 180 mg/m2 of IRIN + 120-minute infusion of 400 mg/m2 of racemic leucovorin + 5-FU bolus of 400 mg/m2 then continuous infusion for 46 hours at 2400 mg/m2 |
All of the included trials measured the following outcomes: ORR, PFS, OS, secondary resection of liver metastases with curative intent and safety and tolerability (including the incidence and type of AEs). 28,43,65
In two of the included trials the primary end point was the proportion of participants who had an ORR. 28,65 In the OPUS trial65 tumour response was assessed by an independent review committee according to modified WHO criteria, whereas in the FIRE-3 trial28 tumour response was measured according to the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0, as assessed by the study investigators. The independent review committee conducted a blinded review of images and clinical data. In the CRYSTAL trial,43 the primary end point, PFS time, was defined as the time from randomisation to disease progression or death from any cause (within 60 days following randomisation or the last tumour assessment). No data were identified for HRQoL for the RAS WT population from any of the included trials.
Median follow-up was not reported in the OPUS trial65 or the CRYSTAL trial. 43 In the FIRE-3 trial28 the median follow-up was 33.0 months [interquartile range (IQR) 19.0–55.4 months] in the cetuximab plus FOLFIRI arm and 39.0 months (IQR 22.5–56.9 months) in the bevacizumab plus FOLFIRI arm.
Population characteristics
The baseline demographic and disease characteristics for the RAS WT subgroup are reported in Table 11.
| First author (trial) | Intervention | n | Age (years), median (range) | Male, n/N (%) | ECOG PS, n/N (%) | Number of metastatic sites, n/N (%) | Primary tumour diagnosis, n/N (%) | LLD, n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Bokemeyer 201533,65 and data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (OPUS) | CET + FOLFOX4 | 38 | 60.5 (24–75) | 19/38 (50.0) | 0: 18/38 (47.4); 1: 19/38 (50.0); 2: 1/38 (2.6) | 1: 22/38 (57.9); 2: 12/38 (31.6); ≥ 3: 4/38 (10.5) | NR | 15/38 (39.5) |
| FOLFOX4 | 49 | 59.0 (36–79) | 29/49 (59.2) | 0: 16/49 (32.7); 1: 29/49 (59.2); 2: 4/49 (8.2) | 1: 18/49 (36.7); 2: 21/49 (42.9); ≥ 3: 10/38 (26.3) | NR | 12/49 (24.5) | |
| Van Cutsem 201543 (CRYSTAL) | CET + FOLFIRI | 178 | 60.0 (24.0–79.0) | 109/178 (61.2) | 0: 97/178 (54.5); 1: 76/178 (42.7); 2: 5/178 (2.8) | ≤ 2: 157/178 (88.2); ≥ 2: 17/178 (9.6); othera: 4/178 (2.2) | Colon: 106/178 (59.6); rectum: 68/178 (38.2); colon and rectum: 4/178 (2.2); missing: 0/178 (0) | 43/178 (24.2) |
| FOLFIRI | 189 | 59.0 (19.0–82.0) | 120/189 (63.5) | 0: 114/189 (60.3); 1: 68/189 (36.0); 2: 7/189 (3.7) | ≤ 2: 161/189 (85.2); ≥ 2: 25/189 (13.2); othera: 3/189 (1.6) | Colon: 117/189 (61.9); rectum: 70/189 (37.0); colon and rectum: 2/189 (1.1); missing: 0/189 (0) | 46/189 (24.3) | |
| Heinemann 201428 and data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (FIRE-3) | CET + FOLFIRI | 171 | 64.0 (41.0–76.0) | 125/171 (73.1) | 0: 87/171 (50.9); 1: 82/171 (48.0); 2: 2/171 (1.2) | 1: 75/171 (43.9); 2: 56/171 (32.7); ≥ 3: 38/171 (22.2) | Colon: 106/171 (62.0); rectum: 55/171 (32.2); colon and rectum: 7/171 (4.1); missing: 3/171 (1.8) | 62/171 (36.3) |
| BEV + FOLFIRI | 171 | 65.0 (33.0–76.0) | 114/171 (66.7) | 0: 87/171 (50.9); 1: 81/171 (47.4); 2: 3/171 (1.8) | 1: 76/171 (44.4); 2: 54/171 (31.6); ≥ 3: 41/171 (24.0) | Colon: 105/171 (61.4); rectum: 59/171 (34.5); colon and rectum: 7/171 (4.1); missing: 0/171 (0) | 58/171 (33.9) |
For the ITT population, for each of the included trials the baseline demographic and disease characteristics were well matched between the groups. In all studies, existing deoxyribonucleic acid samples from KRAS exon 2 WT tumours were reanalysed for other RAS mutations in four additional KRAS codons (exons 3 and 4) and six NRAS codons (exons 2, 3 and 4). Mutation status was evaluable in 796 (73.0%) of 1090 trial participants with KRAS exon 2 WT tumours (see Table 10). The proportions of study participants evaluated to be RAS WT in the different trials are summarised in Table 10. In all trials, the baseline and disease characteristics were comparable with those seen for the KRAS WT population (see Appendix 4 for baseline and disease characteristics for the KRASWT population).
Participants were similar in terms of age, sex distribution and site of primary cancer (see Table 11). However, as is usually the case with cancer trials, the study populations were significantly younger than the general population presenting with mCRC, with a median age of 59–65 years for the study populations (see Table 11) compared with a peak in number of cases in the UK at between 70 and 79 years of age for men and 75 and 85 years for women.
Panitumumab
Study characteristics
The appraisal of panitumumab in combination with chemotherapy for the treatment of mCRC (TA24011) was suspended as no evidence submission was received from the manufacturer or sponsor of the technology. As such, all data included in this update review for panitumumab were identified by the Assessment Group’s searches. It is also important to consider that, as for cetuximab, the ITT population from the pivotal trials for panitumumab were re-evaluated in line with research developments on the impact of RAS mutations on the effectiveness of EGFR inhibitors.
For this MTA review, a total of two subgroup analyses of the RAS WT population from two RCTs29,44 evaluating panitumumab were eligible for inclusion. In the PEAK [Panitumumab Plus mFOLFOX6 vs. Bevacizumab Plus mFOLFOX6 for First Line Treatment of Metastatic Colorectal Cancer (mCRC) Patients With Wild-Type Kirsten Rat Sarcoma-2 Virus (KRAS) Tumors] study29 the extended RAS subgroup analysis was prespecified. In the PRIME (Panitumumab Randomized trial In combination with chemotherapy for Metastatic colorectal cancer to determine Efficacy) study44 the extended RAS subgroup analysis was noted alongside a protocol amendment restricting the analysis of the ITT population to compare PFS and OS according to KRAS status.
Of the two included trials, one evaluated the addition of panitumumab to background chemotherapy (FOLFOX4)44 and one evaluated the addition of panitumumab or bevacizumab to background chemotherapy [modified FOLFOX6 (mFOLFOX6)]. 29 All trials evaluated the same dose of panitumumab and administration method (Table 12). No clinical evidence assessing the effectiveness of panitumumab in conjunction with FOLFIRI was identified.
| First author (trial); study design | Included in TA176a | Included in update review | Inclusion criteria | ITT (n)b | RAS WT (n)/analysed (n)c | Randomisation stratification factors | Interventions evaluated and dose | Primary end point | Treatment duration (months), median (IQR) | Follow-up (months), median (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Douillard 201344 and data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PRIME; NCT00364013); retrospective subgroup analysis | No | Yes | Aged ≥ 18 years; ECOG PS ≤ 2; first occurrence of metastatic disease | 1183 | 512/1060 | ECOG PS (0–1 vs. 2); region (Western Europe, Canada, and Australia vs. rest of the world) | PAN + FOLFOX4 vs. FOLFOX4 | PFS | PAN + FOLFOX 46.47 (3.68– 11.40) vs. FOLFOX4 (NR) | PAN + FOLFOX 22.31 (10.12–35.65) vs. FOLFOX4 17.71 (8.74–32.20) |
| PAN: 60-minute i.v. infusion of 6 mg/kg Q2W on day 1 | ||||||||||
| FOLFOX4: Q2W as 85 mg/m2 of i.v. OX on day 1 + 200 mg/m2 i.v. infusion of racemic leucovorin on days 1 and 2 + 400 mg/m2 i.v. bolus of 5-FU followed by a 600 mg/m2 infusion over 22 hours on days 1 and 2 | ||||||||||
| Schwartzberg 201429 and data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PEAK; NCT00819780); prospective subgroup analysis | No | Yes | Aged ≥ 18 years; ECOG PS ≤ 2; first occurrence of metastatic disease | 285 | 170/250 | Prior adjuvant OX therapy | PAN + mFOLFOX6 vs. BEV + mFOLFOX6 | PFS | PAN + mFOLFOX 67.45 (3.91–11.66) vs. BEV + mFOLFOX 65.86 (3.13–9.57) | PAN + mFOLFOX 14.97 (8.83–22.81) vs. BEV + mFOLFOX 14.93 (8.76–21.39) |
| PAN: 60-minute i.v. infusion of 6 mg/kg Q2W on day 1 | ||||||||||
| BEV: 90-minute infusion of 5 mg/kg on day 1, 60-minute infusion of 5 mg/kg 2 weeks later; 30-minute infusion of 5 mg/kg every 2 weeks thereafter | ||||||||||
| mFOLFOX6: Q2W as 85 mg/m2 i.v. infusion of OX (over 2 hours) on day 1 + 400 mg/m2 i.v. infusion of leucovorin (over 2 hours) on day 1 + 400 mg/m2 i.v. bolus of 5-FU (over 2–4 minutes) on day 1 followed by a 2400 mg/m2 ambulatory pump (46–48 hours) |
Both of the included trials measured the following outcomes: ORR, PFS, OS, secondary resection of liver metastases with curative intent, and safety and tolerability (including the incidence and type of AEs). 29,44 The primary end point in both trials was PFS, defined as the time from randomisation to disease progression or death from any cause within 60 days after the last tumour assessment or after randomisation. No data were identified for HRQoL for the RAS WT population from the included trials.
Median follow-up in the PRIME trial was 22.31 months (IQR 10.12–35.65 months) for the panitumumab plus FOLFOX4 treatment group and 17.71 months (IQR 8.74–32.20 months) for the FOLFOX4 alone treatment group. 44 In the PEAK trial, median follow-up was 14.97 months (IQR 8.83–22.81 months) in the cetuximab plus mFOLFOX6 treatment group and 14.93 months (IQR 8.76–21.39 months) in the bevacizumab plus mFOLFOX6 treatment group. 29
Population characteristics
Baseline demographic and disease characteristics for the RAS WT subgroup are reported in Table 13.
| First author (trial) | Intervention | n | Age (years), median (range) | Male, n/N (%) | ECOG PS, n/N (%) | Number of metastatic sites, n/N (%) | Primary tumour diagnosis, n/N (%) | LLD, n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Douillard 201344 and data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PRIME) | PAN + FOLFOX4 | 253a | 61 (27–81) | 170/253 (67) | 0: 150/253 (59); 1: 88/253 (35); 2: 15/253 (6) | 1: 56/253 (22); 2: 92/253 (36); ≥ 3: 104/253 (41) | Colon: 165/253 (65); rectum: 88/253 (35) | 48/253 (19.0) |
| FOLFOX4 | 252b | 61 (24–82) | 158/252 (63) | 0: 137/252 (54); 1: 98/252 (39); 2: 16/252 (6) | 1: 50/252 (20); 2: 93/252 (37); ≥ 3: 109/252 (43) | Colon: 164/252 (65); rectum: 88/252 (35) | 41/252 (16.3) | |
| Schwartzberg 201429 (PEAK) | PAN + mFOLFOX6 | 88 | 62 (23–82) | 58/88 (66) | 0: 53/88 (60); 1: 35/88 (40); other:b NA | 1: 32/88 (36); 2: 28/88 (32); ≥ 3: 28/88 (32); other:a 0/88 (0) | Colon: 64/88 (73); rectum: 24/88 (27) | 23/88 (26.1) |
| BEV + mFOLFOX6 | 82 | 60 (39–82) | 56/82 (68) | 0: 52/82 (63); 1: 29/82 (35); other:a 1/82 (1) | 1: 33/82 (40); 2: 29/82 (35); ≥ 3: 19/82 (23); other:a 1/82 (1) | Colon: 57/82 (70); rectum: 25/82 (30) | 22/82 (26.8) |
In all studies, existing deoxyribonucleic acid samples from KRAS exon 2 WT tumours were reanalysed for other RAS mutations in four additional KRAS codons (exons 3 and 4) and six NRAS codons (exons 2, 3 and 4). Mutation status was evaluable in 682 (65.6%) out of 1345 trial participants with KRAS exon 2 WT tumours (see Table 12). The proportions of study participants evaluated to be RAS WT for the different studies are summarised in Table 12. In all trials, the baseline demographic and disease characteristics were comparable to those seen for the KRAS WT population (see Appendix 4 for baseline and disease characteristics for the KRAS WT population).
Participants were similar between the arms in terms of age, sex distribution and site of primary cancer (see Table 13). However, as is usually the case with cancer trials, the study populations were significantly younger than the general population presenting with mCRC, with a median age of 60–62 years for the study populations (see Table 13) compared with a peak in the number of cases in the UK at between 70 and 79 years of age for men and 75 and 85 years for women.
Quality appraisal
We appraised the five identified subgroup analyses. On occasion, however, we referred to the original trials to clarify issues relating to study design or methods. The reason for this was to put identified limitations associated with subgroup analyses into context for this appraisal. Quality assessments of the included trials are presented in Table 14.
| First author (trial) | Random allocation | Allocation concealment | Baseline similarity | Care providers blinded | Outcome assessors blinded | Patients blinded | All a priori outcomes reported | Complete data reported | ITT analysis | Applicability |
|---|---|---|---|---|---|---|---|---|---|---|
| Van Cutsem 201543 (CRYSTAL) | Inadequatea | Unclearb | Adequate | Inadequatec | Inadequatec,d | Inadequatec | Uncleare | Inadequatef | Inadequateg | Inadequateh |
| Bokemeyer, 201523,65 (OPUS) | Inadequatea | Unclearb | Adequate | Inadequatec | Inadequatec,d | Inadequatec | Uncleare | Inadequatef | Inadequateg | Inadequateh |
| Heinemann 201428 (FIRE-3) | Inadequatea,i | Unclearb | Adequate | Inadequatec | Inadequatec | Inadequatec | Uncleare | Inadequatef | Inadequateg | Inadequateh |
| Douillard 201327,44 (PRIME) | Inadequatea,j | Unclearb | Adequate | Inadequatec | Inadequatec,d | Inadequatec | Uncleare | Inadequatef | Inadequateg | Inadequateh |
| Schwartzberg 201429 (PEAK) | Inadequatea,k | Unclearb | Adequate | Inadequatec | Inadequatec,d | Inadequatec | Uncleare | Inadequatef | Inadequateg | Inadequateh |
Overall, the risk of bias was similar between studies with regard to treatment allocation, allocation concealment, blinding, outcome reporting and loss to follow-up.
Treatment allocation
The method of random allocation, including the method of sequence generation, was clearly stated and adequate for all of the included trials. All trials used a stratified permuted block procedure. Stratification factors varied between the studies but were predominantly based on Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1 vs. 2) and region (Eastern or Western Europe vs. outside of Europe and Western Europe, Canada and Australia vs. rest of the world).
However, data for those with RAS WT mCRC were available only from subgroup analyses and not from the ITT trial population for any of the included trials. In response to research developments demonstrating a treatment interaction of RAS and EGFR inhibitors (specifically the negative impact of RAS mutations on the effectiveness of EGFR inhibitors), tumour samples from participants of the original RCTs were re-evaluated for RAS status. None of the included studies stratified randomisation by RAS status; this was because the impact of RAS mutations on the effectiveness of EGFR inhibitors was not known at the protocol development phase. For four of the trials (OPUS,65 CRYSTAL,43 FIRE-328 and PRIME44) the subgroup analyses were retrospective. However, for two of these trials (PRIME and FIRE-3) protocol amendments were made in line with research developments. The only trial in which the extended RAS WT subgroup analysis was prespecified was the PEAK trial. 29
Tumour samples from participants in the ITT population identified as KRAS exon 2 WT were re-evaluated for RAS mutations and allocated to either the RAS WT subgroup or the RAS mutant subgroup. The methods used to detect RAS mutations varied between studies, minimising the potential for ascertainment bias. The ascertainment rate for RAS testing of participants with KRAS WT status was 78% [excluding the PRIME trial in which all participants were tested (n = 512/1060)] and the missing data largely resulted from unavailable tumour samples or inconclusive RAS test results. Of note, none of the included subgroup analyses reported the results of a test for treatment interaction.
Similarity of the groups
Three of the included trials fully reported baseline characteristics for the RAS WT population (OPUS,65 CRYSTAL43 and PEAK29). Although the other two trials (PRIME44 and FIRE-328) did not report baseline characteristics for the subgroup of interest in the trial publication, we were able to confirm these through Merck Serono and Amgen [19 March 2015, personal communication (via NICE)]. Of note, however, baseline characteristics provided by the manufacturer for the PRIME study were for a total of 505 participants, whereas the study publication44 reports a total of 512 participants in the RAS WT subgroup.
Given the use of subgroup data, all comparisons were made without protection by stratification/randomisation, increasing the risk of selection bias. However, from the evidence provided (published and unpublished) we were able to confirm that the treatment groups were similar at baseline on a range of prognostic factors for the RAS WT population. Moreover, the characteristics were similar to those for both the ITT population and the KRAS WT population, suggesting a low risk of selection bias in the RAS-tested trial population.
Implementation of masking
The trials had an open-label design and, as such, participants and outcomes assessors were not blinded. However, a blinded retrospective review of radiological assessment and clinical data was carried out for progression and best ORR in two of the studies43,65 and for ORR in one study. 44 In addition, in one study44 an Independent Data Monitoring Committee reviewed interim analyses of safety and one descriptive interim analysis of PFS. No independent assessment was performed in either the PEAK trial29 or the FIRE-3 trial. 28
Completeness of trial data
With regard to the reporting of a priori outcomes, all included trials were rated as unclear. This was because the original trial reports for the ITT population failed to explicitly state if all outcomes defined in the study protocol were reported. Therefore, we were by default unable to assess if all a priori outcomes had been reported for the RAS WT population. Summary data, including event numbers and denominators, were reported for the majority of expected outcomes for the RAS WT population and, when not reported, we were able to confirm data (predominantly ORRs and resection rates) using secondary sources, for example EMA documents30–32,38–40,52,53 or through the manufacturers [Merck Serono and Amgen, 19 March 2015, personal communication (via NICE)].
Withdrawals and dropouts were adequately reported in all of the original trial publications (by providing numbers and reasons by treatment group in the form of a Consolidated Standards of Reporting Trials flow diagram) for the ITT population. Loss to follow-up was, however, unclear. With respect to the RAS WT population, missing data largely resulted from unavailable tumour samples or inconclusive RAS test results.
Currently, available data on the effectiveness of both cetuximab and panitumumab in the RAS WT population are from subgroup analyses and not from the ITT trial population and, as such, ITT analysis was not conducted. Because of the retrospective nature of the RAS analysis, a low number of samples was available for analysis, reducing the power of the studies to show statistical significance.
Applicability to the NHS in England
The population evaluated is in line with that specified in the licensed indication and the NICE final scope. 14 The study arm populations had median/mean ages of between 59 and 65 years and the majority of participants had an ECOG performance status of < 2, meaning that participants were younger and fitter than the UK population of people with mCRC. This is a recurrent problem, however, in the findings of trials of therapies for mCRC in the UK population. All of the included studies were multicentre studies (including European centres) and evaluated the study drugs in line with their licensed indications. Importantly, however, data for the RAS WT population were available only from subgroup analyses rather than ITT analyses and, as such, sample sizes were often small and the results are subject to a high degree of uncertainty.
The rationale for the use of subgroup data is based on research developments that demonstrated that genotype is an important determinant of both the response to treatment and the susceptibility to adverse reactions for a wide range of drugs. 60,61 In CRC response to EGFR inhibitors has been shown to be dependent on gene expression; studies have demonstrated a treatment interaction between RAS status and the effectiveness of EGFR inhibitors. 62–64 It was in line with these research developments evaluating the negative impact of RAS mutations on the effectiveness of EGFR inhibitors that tumour samples from trial populations supporting the original licensed indications were evaluated retrospectively for RAS status. Therefore, data are not from the ITT population for any of the included studies, but are from a subgroup of people contained within the original RCTs.
Although subject to the uncertainties outlined above, these subgroup data are currently the only available data for the RAS WT subpopulation. The Assessment Group did not identify any RCTs with an ITT population by RAS WT status and only one of the included trials29 prespecified the extended RAS analysis. Of note, the EMA’s recent change to the licensed indication was based on subgroup data from trials that inform this current assessment and, although subgroup analyses were defined post hoc, the rationale was based on research developments into tumour biology and results were in line with the expected direction of effect and consistent across included studies. Hence, the extent to which the results of the included trials can provide a reasonable basis for generalisation to the UK NHS population of people with mCRC is unclear.
Assessment of effectiveness
The following outcomes were assessed:
-
PFS
-
OS
-
ORR
-
resection rate.
We also sought HRQoL outcome data from included RCTs; however, no data on HRQoL were reported.
Because of an insufficient number of RCTs, meta-analysis was not undertaken and publication bias was not investigated using funnel plots.
Cetuximab
Progression-free survival
All of the included cetuximab trials reported PFS. 28,43,65 Of these, one trial reported PFS as a primary outcome. 43 The definition of disease progression was relatively consistent across the three trials. In each case PFS was defined as the interval from random assignment of treatment to radiological evidence of disease progression or death from any cause. Disease progression was radiologically assessed according to either RECIST criteria28 or modified WHO criteria. 43,65 The time-to-event data were summarised by stratified hazard ratio (HR). A HR of < 1 indicates an improvement in PFS for treatment (cetuximab) compared with control.
Bokemeyer et al. 65 reported median PFS as 12 months [95% confidence interval (CI) 5.8 months to not reported] and 5.8 months (95% CI 4.7 to 7.9 months) for the cetuximab plus FOLFOX4 and FOLFOX4 arms, respectively (Table 15). The addition of cetuximab to FOLFOX4 was associated with a 47% reduction in the risk of progression in people with RAS WT tumours (HR 0.53, 95% CI 0.27 to 1.04) (see Table 15).
| First author (trial) | Experimental (n/Na) | PFS (months), median (95% CI) | Control (n/Na) | PFS (months), median (95% CI) | HRb (95% CI) |
|---|---|---|---|---|---|
| Bokemeyer 201565 (OPUS) | CET + FOLFOX4 (13/38) | 12 (5.8 to NR) | FOLFOX4 (29/49) | 5.8 (4.7 to 7.9) | 0.53 (0.27 to 1.04) |
| Van Cutsem 201543 (CRYSTAL) | CET + FOLFIRI (73/178) | 11.4 (10 to 14.6) | FOLFIRI (99/189) | 8.4 (7.4 to 9.4) | 0.56 (0.41 to 0.76) |
| Heinemann 201428 (FIRE-3) | CET + FOLFIRI (144/171) | 10.4 (9.5 to 12.2) | BEV + FOLFIRI (143/171) | 10.2 (9.3 to 11.5) | 0.93 (0.74 to 1.17) |
Van Cutsem et al. 43 reported median PFS as 11.4 months (95% CI 10 to 14.6 months) and 8.4 months (95% CI 7.4 to 9.4 months) for the cetuximab plus FOLFIRI and FOLFIRI arms, respectively (see Table 15). 43 The addition of cetuximab to FOLFIRI was associated with a 44% reduction in the risk of progression in those with RAS WT tumours (HR 0.56, 95% CI 0.41 to 0.76).
In the FIRE-3 trial,28 median PFS was similar between the treatment groups: 10.4 months (95% CI 9.5 to 12.2 months) in the cetuximab plus FOLFIRI arm and 10.2 months (95% CI 9.3 to 11.5 months) in the bevacizumab plus FOLFIRI arm (HR 0.93, 95% CI 0.74 to 1.17) (see Table 15).
Overall survival
All of the included cetuximab trials reported OS. 28,43,65 In each of the trials OS was defined as the interval from random assignment of treatment to death. The time-to-event data were summarised by stratified HR. A HR of < 1 indicates an improvement in OS for treatment compared with control.
In the OPUS trial,65 median OS was 19.8 months (95% CI 16.6 to 25.4 months) in the cetuximab plus FOLFOX4 group compared with 17.8 months (95% CI 13.8 to 23.9 months) in the FOLFOX4 group (HR 0.94, 95% CI 0.56 to 1.56) (Table 16).
| First author (trial) | Experimental (n/Na) | OS (months), median (95% CI) | Control (n/Na) | OS (months), median (95% CI) | HRb (95% CI) |
|---|---|---|---|---|---|
| Bokemeyer 201565 (OPUS) | CET + FOLFOX4 (27/38) | 19.8 (16.6 to 25.4) | FOLFOX4 (36/49) | 17.8 (13.8 to 23.9) | 0.94 (0.56 to 1.56) |
| Van Cutsem 201543 (CRYSTAL) | CET + FOLFIRI (130/178) | 28.4 (24.7 to 31.6) | FOLFIRI (154/189) | 20.2 (17 to 24.5) | 0.69 (0.54 to 0.88) |
| Heinemann 201428 (FIRE-3) | CET + FOLFIRI (91/171) | 33.1 (24.5 to 39.4) | BEV + FOLFIRI (110/171) | 25.6 (22.7 to 28.7) | 0.7 (0.53 to 0.92) |
In the CRYSTAL trial,43 median OS was 28.4 months (95% CI 24.7 to 31.6 months) in the cetuximab plus FOLFIRI group compared with 20.2 months (95% CI 17 to 24.5 months) in the FOLFIRI group (HR 0.69, 95% CI 0.54 to 0.88) (see Table 16).
In the FIRE-3 trial,28 median OS was 33.1 months (95% CI 24.5 to 39.4 months) in the cetuximab plus FOLFIRI group compared with 25.6 months (95% CI 22.7 to 28.7 months) in the bevacizumab plus FOLFIRI group (HR 0.7, 95% CI 0.53 to 0.92) (see Table 16).
Objective response rate
Data for ORR were available from all three of the included cetuximab studies. 28,43,65 In all of the trials response rate was defined as the percentage of study participants who achieved a PR or CR as the best ORR according to radiological assessment.
In two of the analyses28,65 ORR was evaluated using RECIST; no independent review was performed. Tumour response evaluation was performed every 6 weeks (±7 days) in the OPUS trial65 and every 8 weeks (±7 days) in the FIRE-3 trial,28 and treatment was continued until disease progression, unacceptable toxicities, death, withdrawal of consent or investigator decision, whichever was earlier. In the CRYSTAL analysis,43 tumour response, including disease progression, was assessed by an independent review committee according to modified WHO criteria. The independent review committee conducted a blinded review of images and clinical data using a common set of prespecified criteria. 43
The WHO criteria for response rate72 are older than the current standard RECIST criteria. 73 It can be seen that the two sets of criteria do not fully match: WHO criteria are multidimensional, whereas the RECIST criteria are unidimensional. This is not necessarily important when considering a single trial but, when there are several trials and some use one set of criteria and some use another, the results cannot easily be compared.
The effect of treatment on response was measured as an odds ratio (OR) (i.e. odds of a response with cetuximab vs. odds of a response without cetuximab). The best-available response rate (i.e. CR, PR, stable disease, progressive disease) is reported in Appendix 5.
Bokemeyer et al. 65 reported confirmed CRs or PRs in 22 people (58%) receiving cetuximab plus FOLFOX4 and in 14 people (29%) receiving FOLFOX4 alone (Table 17). The adjusted OR for a tumour response with cetuximab plus FOLFOX4 compared with FOLFOX4 alone was 3.33 (95% CI 1.36 to 8.17), favouring the cetuximab plus FOLFOX4 arm.
| First author (trial) | Experimental | ORR, n/N [% (95% CI)] | Control | ORR, n/N [% (95% CI)] | ORa (95% CI) |
|---|---|---|---|---|---|
| Bokemeyer 201565 (OPUS)b | CET + FOLFOX4 | 22/38 [58 (41 to 74)] | FOLFOX4 | 14/49 [29 (17 to 43)] | 3.33 (1.36 to 8.17) |
| Van Cutsem 201543 (CRYSTAL)b | CET + FOLFIRI | 118/178 [66 (59 to 73)] | FOLFIRI | 73/189 [39 (32 to 46)] | 3.11 (2.03 to 4.78) |
| Heinemann 201428 (FIRE-3)c | CET + FOLFIRI | 112/171 [65.5 (58 to 73)] | BEV + FOLFIRI | 102/171 [60 (52 to 67)] | 1.28 (0.83 to 1.99) |
Van Cutsem et al. 43 reported confirmed CRs or PRs in 118 people (66%) receiving cetuximab plus FOLFIRI and in 73 people (39%) receiving FOLFIRI alone (see Table 17). The adjusted OR for a tumour response with cetuximab plus FOLFIRI compared with FOLFIRI alone was 3.11 (95% CI 2.03 to 4.78), favouring the cetuximab plus FOLFIRI arm (see Table 17).
Heinemann et al. 28 reported confirmed CRs or PRs in 112 people (65%) receiving cetuximab plus FOLFIRI and in 102 people (60%) receiving bevacizumab plus FOLFIRI (see Table 17). The adjusted OR for a tumour response with cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI was 1.28 (95% CI 0.83 to 1.99), favouring the cetuximab plus FOLFIRI arm.
Rate of complete resection
Data for rate of complete resection with curative intent before disease progression were available from one of the included cetuximab trials. 43
Rate of surgery with curative intent [with complete resection of all lesions (R0)] was defined as the number of subjects with any resection of metastasis of curative intent and all lesions completely resected to R0 divided by all subjects qualifying for the ITT population. The effect of treatment on the likelihood of complete resection was measured as an OR.
No data were reported from the OPUS trial65 for the rate of complete resection for this comparison for the RAS WT population.
No data were reported for the rate of complete resection in the CRYSTAL trial publication;43 however, data were provided by Merck Serono [19 March 2015, personal communication (via NICE)]. The rate of complete resection with curative intent before disease progression was higher in the cetuximab plus FOLFIRI group than in the FOLFIRI group (7.3% vs. 2.1%; OR 3.11, 95% CI 2.03 to 4.78; p-value not reported) (Table 18).
| First author (trial) | Experimental | n/N (%) | Control | n/N (%) | ORa (95% CI) |
|---|---|---|---|---|---|
| Bokemeyer 201565 (OPUS) | CET + FOLFOX4 | NR | FOLFOX4 | NR | NR |
| Data on file [Merck Serono, 19 March 2015, personal communication (via NICE)] (CRYSTAL)b | CET + FOLFIRI | 13/178 (7.3) | FOLFIRI | 4/189 (2.1) | 3.11 (2.03 to 4.78) |
| Heinemann 201428 (FIRE-3) | CET + FOLFIRI | NR | BEV + FOLFIRI | NR | NR |
No data were available from the FIRE-3 trial28 for the rate of complete resection for this comparison for the RAS WT population.
Subgroup analyses: liver metastasis at baseline
There were no planned subgroup analyses in the RAS WT population as the data for this population were obtained retrospectively. However, data for people with liver metastasis at baseline were available from two of the included cetuximab trials43,65 [provided by Merck Serono, 19 March 2015, personal communication (via NICE)].
In the OPUS trial, among the RAS WT subgroup a total of 27 (31.0%) participants had metastasis to the liver at baseline. The results for this subgroup are summarised in Table 19.
| Outcome | Trial | |||||
|---|---|---|---|---|---|---|
| OPUSa | CRYSTALa | FIRE-328 | ||||
| CET + FOLFOX4 (n = 15) | FOLFOX4 (n = 12) | CET + FOLFIRI (n = 43) | FOLFIRI (n = 46) | CET + FOLFIRI (n = NR) | BEV + FOLFIRI (n = NR) | |
| PFS | ||||||
| Progression/death events, n/N (%) | NR | NR | NR | NR | NR | NR |
| PFS (months), median (95% CI) | NR | 7.4 (NR) | 14.0 (NR) | 8.1 (NR) | NR | NR |
| Stratified HRb (95% CI) | 0.35 (0.06 to 1.91) | 0.21 (0.09 to 0.49) | NR | |||
| OS | ||||||
| Deaths, n/N (%) | NR | NR | NR | NR | NR | NR |
| OS (months), median (95% CI) | 23.9 (NR) | 24.8 (NR) | 29.8 (NR) | 29.5 (NR) | NR | NR |
| Stratified HRb (95% CI) | 0.90 (0.33 to 2.42) | 0.65 (0.38 to 1.10) | NR | |||
| ORR | ||||||
| n/N, (%) | 11/15c (73.3) | 5/12c (41.7) | 36/43c (83.7) | 17/46c (37.0) | NR | NR |
| Stratified ORb (95% CI) | 3.30 (0.63 to 17.16) | 8.99 (3.17 to 25.52) | NR | |||
| Resection rate | ||||||
| Surgical resection rate, n/N (%) | NR | NR | NR | NR | NR | NR |
| Stratified ORb (95% CI) | NR | NR | NR | |||
| Complete R0 resection rate, n/N (%) | 2/15 (13.3) | 0/12 (0) | 7/43 (16.3) | 3/46 (6.5) | NR | NR |
| Stratified ORb (95% CI) | NE | 2.68 (0.63 to 11.43) | NR | |||
Complete resection was performed in two of 15 (13.3%) participants in the cetuximab plus FOLFOX4 arm and no (0/12; 0%) participants in the FOLFOX4 alone arm.
Among the RAS WT subgroup a total of 89 (24.3%) participants in the CRYSTAL trial had metastasis to the liver at baseline. The results of this subgroup are summarised in Table 19.
Complete resection was performed in seven of 43 (16.3%) participants in the cetuximab plus FOLFOX arm and three of 46 (6.5%) participants in the FOLFOX alone arm (OR 2.68, 95% CI 0.63 to 11.43).
No data were available for people with liver metastasis at baseline from the FIRE-3 trial. 28
Panitumumab
Progression-free survival
Both of the included panitumumab trials reported PFS in the RAS WT subgroup. 29,44 The definition of disease progression was relatively consistent in both trials. In each case PFS was defined as the interval from random assignment of treatment to radiological evidence of disease progression or death from any cause. Disease progression was radiologically assessed according to RECIST criteria. The time-to-event data were summarised by stratified HR. A HR of < 1 indicates an improvement in PFS for treatment compared with control.
Douillard et al. 44 reported median PFS as 10.1 months (95% CI 9.3 to 12 months) and 7.9 months (95% CI 7.2 to 9.3 months) for the panitumumab plus FOLFOX4 and FOLFOX4 arms, respectively. The addition of panitumumab to FOLFOX4 was associated with a reduction in risk of progression of 28% (HR 0.72, 95% CI 0.58 to 0.9) (Table 20).
| First author (trial) | Experimental (n/N) | PFS (months), median (95% CI) | Control (n/N) | PFS (months), median (95% CI) | HRa (95% CI) |
|---|---|---|---|---|---|
| Douillard 201344 (PRIME)b | PAN + FOLFOX4 (156/259) | 10.1 (9.3 to 12) | FOLFOX4 (170/253) | 7.9 (7.2 to 9.3) | 0.72 (0.58 to 0.9) |
| Schwartzberg 201429 (PEAK) | PAN + mFOLFOX6 (50/88) | 13 (10.9 to 15.1) | BEV + mFOLFOX6 (60/82) | 9.5 (9 to 12.7) | 0.65 (0.44 to 0.96) |
Schwartzberg et al. 29 reported median PFS as 13 months (95% CI 10.9 to 15.1 months) and 9.5 months (95% CI 9 to 12.7 months) for the panitumumab plus mFOLFOX6 and bevacizumab plus FOLFOX4 arms, respectively. The addition of panitumumab to mFOLFOX6 was associated with a reduction in risk of progression of 35% (HR 0.65, 95% CI 0.44 to 0.96) (see Table 20).
Overall survival
Both of the included panitumumab trials reported OS for the RAS WT subgroup. 29,44 In each case OS was defined as the interval from random assignment of treatment to death. The time-to-event data were summarised by stratified HR. A HR of < 1 indicates an improvement in OS for treatment compared with control.
Douillard et al. 44 reported median OS of 25.8 months (95% CI 21.7 to 29.7 months) and 20.2 months (95% CI 17.6 to 23.6 months) for the panitumumab plus FOLFOX4 and FOLFOX4 arms, respectively (HR 0.77, 95% CI 0.64 to 0.94), favouring the panitumumab plus FOLFOX4 treatment group (Table 21).
| First author (trial) | Experimental (n/N) | OS (months), median (95% CI) | Control (n/N) | OS (months), median (95% CI) | HRa (95% CI) |
|---|---|---|---|---|---|
| Douillard 201344 (PRIME)b | PAN + FOLFOX4 (204/259) | 25.8 (21.7 to 29.7) | FOLFOX4 (218/253) | 20.2 (17.6 to 23.6) | 0.77 (0.64 to 0.94) |
| Schwartzberg 201429 (PEAK) | PAN + mFOLFOX6 (30/88) | 41.3 (28.8 to 41.3) | BEV + mFOLFOX6 (40/82) | 28.9 (23.9 to 31.3) | 0.63 (0.39 to 1.02) |
Schwartzberg et al. 29 reported median OS of 41.3 months (95% CI 28.8 to 41.3 months) and 28.9 months (95% CI 23.9 to 13.1 months) for the panitumumab plus mFOLFOX6 and bevacizumab plus mFOLFOX6 arms, respectively (HR 0.63, 95% CI 0.39 to 1.02), favouring the panitumumab plus mFOLFOX6 treatment group (see Table 21).
Objective response rate
Data for ORR were available from both included panitumumab studies. 29,44
Overall response rate was defined as the percentage of participants who achieved a PR or CR as the best overall response according to radiological assessments. In both trials29,44 ORR was evaluated using RECIST; no independent review was performed. Tumour response evaluation was performed every 8 weeks (±7 days) and treatment was continued until disease progression, unacceptable toxicities, death, withdrawal of consent or investigator decision, whichever was earlier.
The effect of treatment on response was measured as an OR.
The best-available response rate (i.e. CR, PR, stable disease, progressive disease) is reported in Appendix 5.
Douillard et al. 44 reported confirmed CRs or PRs, and Amgen also reported results in the company submission as academic-in-confidence (AiC) [19 March 2015, personal communication (via NICE)].
Schwartzberg et al. 29 reported confirmed CRs and PRs in 56 people (64%) receiving panitumumab plus mFOLFOX6 and in 49 people (61%) receiving FOLFOX alone (Table 22). The adjusted OR for a tumour response with panitumumab plus FOLFOX compared with mFOLFOX6 alone was 1.08 (95% CI 0.55 to 2.12).
| First author (trial) | Experimental | n/N [% (95% CI)] | Control | n/N [% (95% CI)] | ORa (95% CI) |
|---|---|---|---|---|---|
| Data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PRIME)b | PAN + FOLFOX4 | (Confidential information has been removed)c | FOLFOX4 | (Confidential information has been removed)c | (Confidential information has been removed)c |
| Schwartzberg 201429 (PEAK)b | PAN + mFOLFOX6 | 56/88 [64 (53 to 74)] | BEV + mFOLFOX6 | 49/81 [60 (49 to 71)] | 1.08 (0.55 to 2.12) |
Rate of complete resection
Data for rate of complete resection with curative intent before disease progression were available from both of the included panitumumab trials. 29,44
The rate of surgery with curative intent [with complete resection of all lesions (R0)] was defined as the number of subjects with any resection of metastasis of curative intent and all lesions completely resected to R0 divided by all subjects qualifying for the ITT population.
The effect of treatment on the likelihood of complete resection was measured as an OR.
No data were reported for the rate of complete resection in the PRIME trial publication;44 however, data were provided as AiC by the manufacturer [19 March 2015, personal communication (via NICE)] (Table 23). The rate of R0 resection with curative intent before disease progression for metastases was higher in the panitumumab plus FOLFOX4 group than in the FOLFOX4 group.
| First author (trial) | Experimental | n/N [% (95% CI)] | Control | n/N [% (95% CI)] | ORa (95% CI) |
|---|---|---|---|---|---|
| Data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PRIME)b | PAN + FOLFOX4 | (Confidential information has been removed)c | FOLFOX4 | (Confidential information has been removed)c | (Confidential information has been removed)c |
| Data on file [Amgen, 19 March 2015, personal communication (via NICE)] (PEAK)]b | PAN + mFOLFOX6 | 11/88 [13 (6 to 21)] | BEV + mFOLFOX6 | 9/82 [11 (5 to 20)] | 1.16 (0.45 to 2.96) |
No data were reported for the rate of complete resection in the PEAK trial publication;29 however, data were provided by the manufacturer [19 March 2015, personal communication (via NICE)] (see Table 23). The rate of R0 resection with curative intent before disease progression for metastases was higher in the panitumumab plus mFOLFOX6 group (13%) than in the mFOLFOX6 group (11%) (OR 1.61, 95% CI 0.45 to 2.96; p-value not reported).
Subgroup analyses: liver metastases at baseline
There were no planned subgroup analyses in the RAS WT population as the data for this population were obtained retrospectively. However, data for people with liver metastases at baseline were available from both of the included panitumumab trials [from Peeters et al. 67 and data provided by Amgen, 19 March 2015, personal communication (via NICE)].
Among the RAS WT subgroup a total of 89 (17.6%) participants in the PRIME trial44 had metastasis to the liver at baseline. The results for this subgroup are summarised in Table 24. Complete resection was performed in 15 out of 48 (31%) participants in the panitumumab plus FOLFOX4 arm and seven out of 41 (17%) participants in the FOLFOX4 alone arm, with an OR for panitumumab plus FOLFOX4 compared with FOLFOX4 of 2.2 (95% CI 0.80 to 6.10), favouring panitumumab plus FOLFOX4.
| Outcome | Trial | |||
|---|---|---|---|---|
| aPRIME67 | PEAKa,b | |||
| PAN + FOLFOX4 (n = 48) | FOLFOX4 (n = 41) | PAN + mFOLFOX6 (Confidential information has been removed) | BEV + mFOLFOX6 (Confidential information has been removed) | |
| PFS | ||||
| Progression/death events, n/N (%) | 38/48 (79) | 37/41 (90) | (Confidential information has been removed) | (Confidential information has been removed) |
| PFS (months), median (95% CI) | 11.3 (9.4 to 21.3) | 9.9 (7.2 to 12.9) | (Confidential information has been removed) | (Confidential information has been removed) |
| Stratified HR (95% CI)c,d | 0.75 (0.48 to 1.19) | (Confidential information has been removed) | ||
| OS | ||||
| Deaths, n/N (%) | 32/48 (67) | 31/41 (76) | (Confidential information has been removed) | (Confidential information has been removed) |
| OS (months), median (95% CI) | 40.7 (26.6 to 51.7) | 33.4 (19.4 to 46.8) | (Confidential information has been removed) | (Confidential information has been removed) |
| Stratified HR (95% CI)c,d | 0.71 (0.43 to 1.16) | (Confidential information has been removed) | ||
| ORR | ||||
| n/N (%) | 38/47 (81) | 27/41 (66) | (Confidential information has been removed) | (Confidential information has been removed) |
| Stratified OR (95% CI)c,d | 2.18 (0.75 to 6.41) | (Confidential information has been removed) | ||
| Resection rate | ||||
| Surgical resection rate, n/N (%) | 16/48 (33) | 10/41 (24) | (Confidential information has been removed) | (Confidential information has been removed) |
| Stratified OR (95% CI)c,d | 1.55 (0.61 to 3.94) | (Confidential information has been removed) | ||
| Complete resection rate, n/N (%) | 15/48 (31) | 7/41 (17) | (Confidential information has been removed) | (Confidential information has been removed) |
| Stratified OR (95% CI)c,d | 2.2 (0.80 to 6.10) | (Confidential information has been removed) | ||
Adverse events
Data for AEs from the RAS WT subgroup from the individual trials are reported in the following sections. Within each trial, the safety population consisted of study participants who had received at least one dose of the study drug. The most frequently reported AEs were as expected for the individual treatments based on the SPCs for the interventions of interest for this review (cetuximab and panitumumab).
Adverse events in the included trials were coded using versions of the Medical Dictionary for Regulatory Activities (MedDRA). For all of the included cetuximab and panitumumab trials, the National Cancer Institute Common Terminology Criteria (NCI-CTC) version 374 (Table 25), frequently used by trials to report drug toxicities, were used to grade AE severity. For each AE, a grade was assigned using a scale from 0 to 5. Grade 0 is defined as absence of an AE or values within normal limits and grade 5 is defined as death associated with an AE.
| Grade | Description |
|---|---|
| 0 | No AE or within normal limits |
| 1 | Mild AE |
| 2 | Moderate AE |
| 3 | Severe AE |
| 4 | Life-threatening or disabling AE |
| 5 | Death related to an AE |
Cetuximab
All of the included cetuximab trials reported AEs. 28,43,65 All trials reported grade 3 or 4 events,28,43,65 with one trial also reporting grade 1 or 2 events. 43 Two trials also reported summary AE information (any AEs or any serious AEs). 43,65
As RAS mutation status refers to the tumour only, the EMA concluded in its report that there were no good reasons to postulate differences in safety profiles related to RAS status other than from the perspective that people with RAS WT tumours would be treated for longer periods of time. Taking small sample sizes into account, the assumption that safety is independent of tumour RAS status was considered to be in line with reported data. 39
Cetuximab plus FOLFOX4 compared with FOLFOX4
In the OPUS trial,65 all AEs were recorded between the onset of or after the first day of study medication up to 6 weeks after the end of the last administration of study treatment. AEs were coded using the MedDRA version 10.0 and summarised by worst severity per patient according to the NCI-CTC for AEs version 3.0. Only AEs with a frequency of ≥ 5% in either treatment group were reported.
The incidence of any AE was the same in both treatment arms (100% in each arm) (Table 26). However, both grade 3 or 4 AEs and serious AEs were more commonly reported in the cetuximab plus FOLFOX4 arm (78.9% and 39.5%, respectively) than in the FOLFOX4 arm (63.3% and 16.3%, respectively). More specifically, commonly reported grade 3 and 4 AEs included diarrhoea, leukopenia, neutropenia, paraesthesia, peripheral sensory neuropathy, rash, any skin reactions and acne-like rash skin reaction. The incidences of these AEs were similar between treatment arms, except for the skin reactions (any and acne-like), for which the incidence was higher in the cetuximab plus FOLFOX4 arm (skin reaction any: 13% vs. 0%; skin reaction acne-like: 8% vs. 0%), and paraesthesia, for which the incidence was higher in the FOLFOX4 arm (0% vs. 6%) (Table 27).
| AE | Trial | |||||
|---|---|---|---|---|---|---|
| a,b,cOPUS,65 n/N (%) | a,b,dCRYSTAL,43 n/N (%) | e,fFIRE-3,28 n/N (%) | ||||
| CET + FOLFOX4 (n = 38) | FOLFOX4 (n = 49) | CET + FOLFIRI (n = 178) | FOLFIRI (n = 189) | CET + FOLFIRI (n = 171) | BEV + FOLFIRI (n = 171) | |
| Any AE | 38/38 (100) | 49/49 (100) | 178/178 (100) | 187/189 (98.9) | NR | NR |
| Worst grade of 3 | NR | NR | NR | NR | NR | NR |
| Worst grade of 4 | NR | NR | NR | NR | NR | NR |
| Worst grade of 5 | NR | NR | NR | NR | NR | NR |
| Any grade 1 or grade 2 event | NR | NR | 34/178 (19.1) | 79/189 (41.8) | NR | NR |
| Any grade 3 or grade 4 event | 30/38 (79) | 31/49 (63) | 144/178 (80.9) | 110/189 (58.2) | 118/171 (69.0) | 115/171 (67.3) |
| Any serious AE | 15/38 (40) | 8/49 (16) | 69/178 (38.8) | 62/189 (32.8) | NR | NR |
| AE | Trial | |||||
|---|---|---|---|---|---|---|
| b,cOPUS65 n/N (%) | b,dCRYSTAL,43 n/N (%) | b,eFIRE-3,28 n/N (%) | ||||
| CET + FOLFOX4 (n = 38) | FOLFOX4 (n = 49) | CET + FOLFIRI (n = 178) | FOLFIRI (n = 189) | CET + FOLFIRI (n = 171) | BEV + FOLFIRI (n = 171) | |
| Acneiform/exanthema | NR | NR | NR | NR | 33/171 (19.3) | 0/171 (0) |
| Deep-vein thrombosis | NR | NR | 11/178 (6.2) | 1/189 (0.5) | NR | NR |
| Dermatitis acneiform | NR | NR | 9/178 (5.1) | 0/189 (0) | NR | NR |
| Desquamation | NR | NR | NR | NR | 12/171 (7.0) | 1/171 (0.6) |
| Diarrhoea | 1/38 (3) | 2/49 (4) | 26/178 (14.6) | 18/189 (9.5) | 18/171 (10.5) | 24/171 (14.0) |
| Fatigue | NR | NR | 12/178 (6.7) | 9/189 (4.8) | NR | NR |
| Haematotoxicity | NR | NR | NR | NR | 47/171 (27.5) | 37/171 (21.6) |
| Hepatotoxicity | NR | NR | NR | NR | 9/171 (5.3) | 9/171 (5.3) |
| Hypertension | NR | NR | NR | NR | 11/171(6.4) | 12/171 (7.0) |
| Hypokalaemia | NR | NR | NR | NR | 17/171 (9.9) | 7/171 (4.1) |
| Infection | NR | NR | NR | NR | 16/171 (9.4) | 15/171 (8.8) |
| Leukopenia | 1/38 (3) | 3/49 (6) | 15/178 (8.4) | 7/189 (3.7) | NR | NR |
| Mucositis/stomatitis | NR | NR | NR | NR | 8/171 (4.7) | 6/171 (3.5) |
| Nail changes/paronychia | NR | NR | NR | NR | 12/171 (7.0) | 0/171 (0) |
| Nausea | NR | NR | NR | NR | 6/171 (3.5) | 9/171 (5.3) |
| Neurotoxicity | 2/38 (5) | 5/49 (10) | NR | NR | NR | NR |
| Neutropenia | 12/38 (32) | 14/49 (29) | 55/178 (30.9) | 38/189 (20.1) | NR | NR |
| Pain | NR | NR | NR | NR | 6/171 (3.5) | 10/171 (5.8) |
| Paraesthesia | 0/38 (0) | 3/49 (6.1) | NR | NR | NR | NR |
| Rash | 2/38 (5) | 0/49 (0) | 16/178 (9.0) | 0/189 (0) | NR | NR |
| Skin reactions | NR | NR | NR | NR | 49/171 (28.7) | 5/171 (2.9) |
| Thromboembolic event | NR | NR | NR | NR | 8/171 (4.7) | 12/171 (7.0) |
| Thrombosis (any) | NR | NR | NR | NR | 10/171 (5.8) | 13/171 (7.6) |
| Composite categories | ||||||
| Infusion-related reaction | NR | NR | 4/178 (2.2) | 0/189 (0) | NR | NR |
| Skin reactions | ||||||
| Any | 5/38 (13) | 0/49 (0) | 37/178 (20.8) | 1/189 (0.5) | NR | NR |
| Acne-like rash | 3/38 (8) | 0/49 (0) | 30/178 (16.9) | 0/189 (0) | NR | NR |
All AEs reported were noted as likely to occur in the SPC and were consistent with the known safety profile of cetuximab.
Cetuximab plus FOLFIRI compared with FOLFIRI
In the CRYSTAL trial,43 AEs were recorded continuously and categorised according to the MedDRA version 12.0, except for composite categories, which were categorised according to the MeDRA version 10.0. The severity of AEs was assessed according to the NCI-CTC for AEs (version 3.0). Only AEs with a frequency of ≥ 5% in either treatment group were reported.
The incidence of any AE was slightly higher in the cetuximab plus FOLFIRI arm (100%) than in the FOLFIRI arm (98.9%; see Table 26). Any grade 1 or 2 AEs were more frequently reported in the FOLFIRI arm (41.8%) than in the cetuximab plus FOLFIRI arm (19.1%), whereas both grade 3 or 4 AEs and serious AEs were more commonly reported in the cetuximab plus FOLFIRI arm (80.9% and 38.8%, respectively) than in the FOLFIRI arm (58.2% and 32.8%, respectively). More specifically, commonly reported grade 3 and 4 AEs included deep-vein thrombosis, dermatitis acneiform, diarrhoea, fatigue, leukopenia, neutropenia, infusion-related reaction, any skin reactions and acne-like rash skin reaction (see Table 27), which all occurred with a greater incidence in the cetuximab plus FOLFIRI arm than in the FOLFIRI arm. Incidences were notably higher in the cetuximab plus FOLFIRI arm for any skin reactions (20.8% vs. 0.5%), acne-like skin reactions (16.9% vs. 0%), neutropenia (30.9% vs. 20.1%) and rash (9.0% vs. 0%).
All AEs reported were noted as likely to occur in the SPC and were consistent with the known safety profile of cetuximab.
Cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI
In the FIRE-3 trial,28 AEs were recorded continuously from enrolment to the end of the final study visit and were coded by the MedDRA version 13.1 and classified and graded according to the NCI-CTC for AEs version 3.0. Only AEs with a frequency of ≥ 5% in either treatment group were reported. Information on the safety population definition was not available.
The incidence of any grade 3 or 4 AE was similar between the cetuximab plus FOLFIRI arm (69.0%) and the bevacizumab plus FOLFIRI arm (67.3%); other subcategories of AEs were not reported (see Table 26). More specifically, commonly reported grade 3 and 4 AEs included acneiform/exanthema, desquamation, diarrhoea, haematotoxicity, hepatotoxicity, hypertension, hypokalaemia, infection, mucositis/stomatitis, nail changes/paronychia, nausea, pain, skin reactions, thromboembolic events and thrombosis (any) (see Table 27). The incidences of these AEs were all comparable between the two arms except for the following, which occurred with a greater incidence in the cetuximab plus FOLFIRI arm than in the bevacizumab plus FOLFIRI arm: skin reactions (28.7% vs. 2.9%), nail changes/paronychia (7.0% vs. 0%), desquamation (7% vs. 0.6%) and acneiform/exanthema (19.3% vs. 0%).
Data on specific AEs that were classified as grade 1 or 2 in severity were also reported by Heinemann et al. ,28 a summary of which is provided in Appendix 5.
Panitumumab
Data were available for AEs from both the PRIME trial44 and the PEAK29 trial. Both trials reported any AEs, AEs with a worst grade of 3, AEs with a worst grade of 4, AEs with a worst grade of 5, any grade 1 or 2 AEs, any grade 3 or 4 AEs and any serious AEs. AEs with a worst grade of 1 or 2 and AEs with a worst grade of 3 or 4 were available from the PEAK trial29 but not the PRIME trial. 44
The EMA concluded that no new safety concerns were identified for the safety profile of panitumumab in patients with RAS WT tumour status as these patients were indistinguishable from patients with KRAS WT tumour status.
Panitumumab plus FOLFOX4 compared with FOLFOX4
In the PRIME trial44 patients were followed for safety 30 days after the last study drug administration. AEs were coded using the MedDRA (version 15.0) and were graded for severity using the NCI-CTC for AEs version 3.0, with modifications for specific skin- and nail-related toxicities. The safety population consisted of those who received at least one dose of the protocol therapy. Only AEs with a frequency of ≥ 5% in either treatment group were reported.
Similar incidences were found in the panitumumab plus FOLFOX4 arm and the FOLFOX4 arm for any AE (100% vs. 99%), AEs with a worst grade of 3 (57% vs. 50%), AEs with a worst grade of 4 (28% vs. 20%), AEs with a worst grade of 5 (5% vs. 6%), any grade 1 or 2 AE (10% vs. 22%), any grade 3 or 4 AE (85% vs. 70%) and any serious AE (43% vs. 37%) (Table 28). More specifically, commonly reported grade 3 and 4 AEs included abdominal pain, anaemia, asthenia, dermatitis acneiform, diarrhoea, fatigue, hypokalaemia, hypomagnesaemia, mucosal inflammation, peripheral neuropathy, neutropenia, paraesthesia, rash and stomatitis (Table 29). The incidences of these AEs were similar between the treatment arms except for the following AEs, which occurred with a greater incidence in the panitumumab plus FOLFOX4 arm than in the FOLFOX4 arm: dermatitis acneiform (10% vs. 0%), diarrhoea (19% vs. 9%) and rash (17% vs. 0%).
| AE | Trial | |||
|---|---|---|---|---|
| a,b,cPRIME,26,44 n/N (%) | a,bPEAK,29 n/N (%) | |||
| PAN + FOLFOX4 (n = 250) | FOLFOX4 (n = 249) | PAN + mFOLFOX6 (n = 86) | BEV + mFOLFOX6 (n = 80) | |
| Any AE | 250/250 (100) | 247/249 (99) | 86/86 (100) | 80/80 (100) |
| Worst grade of 3 | 142/250 (57) | 125/249 (50) | 60/86 (70) | 43/80 (54) |
| Worst grade of 4 | 70/250 (28) | 50/249 (20) | 17/86 (20) | 15/80 (19) |
| Worst grade of 5 | 13/250 (5) | 16/249 (6) | 4/86 (5) | 7/80 (9) |
| Worst grade 1 or grade 2 event | NR | NR | 5/86 (6) | 15/80 (19) |
| Worst grade 3 or grade 4 event | NR | NR | 77/86 (90) | 58/80 (73) |
| Any grade 1 or grade 2 event | 25/250 (10) | 56/249 (22) | 86/86 (100) | 80/80 (100) |
| Any grade 3 or grade 4 event | 212/250 (85) | 175/249 (70) | 80/86 (93) | 65/80 (81) |
| Any serious AE | 108/250 (43) | 92/249 (37) | 37/86 (43) | 31/80 (39) |
| AE | Trial | |||
|---|---|---|---|---|
| a,b,cPRIME,26 n/N (%) | a,b,cPEAK,29 n/N (%) | |||
| PAN + FOLFOX4 (n = 250) | FOLFOX4 (n = 249) | PAN + FOLFOX6 (n = 86) | BEV + FOLFOX6 (n = 80) | |
| Abdominal pain | 12/250 (5) | 13/249 (5) | NR | NR |
| Anaemia | 13/250 (5) | 6/249 (2) | NR | NR |
| Asthenia | 13/250 (5) | 9/249 (4) | 3/86 (3) | 4/80 (5) |
| Decreased appetite | NR | NR | 5/86 (6) | 0/80 (0) |
| Deep-vein thrombosis | NR | NR | 2/86 (2) | 6/80 (8) |
| Dehydration | NR | NR | 5/86 (6) | 1/80 (1) |
| Dermatitis acneiform | 26/250 (10) | 0/249 (0) | NR | NR |
| Diarrhoea | 47/250 (19) | 22/249 (9) | 7/86 (8) | 8/80 (10) |
| Fatigue | 27/250 (11) | 7/249 (3) | 10/86 (12) | 8/80 (10) |
| Hypertension | NR | NR | 0/86 (0) | 6/80 (8) |
| Hypokalaemia | 25/250 (10) | 12/249 (5) | 7/86 (8) | 6/80 (8) |
| Hypomagnesaemia | 19/250 (8) | 0/249 (0) | 7/86 (8) | 0/80 (0) |
| Mucosal inflammation | 13/250 (5) | 1/249 (0) | 6/86 (7) | 2/80 (3) |
| Neuropathy peripheral | 16/250 (6) | 17/249 (7) | 8/86 (9) | 8/80 (10) |
| Neutropenia | 103/250 (41) | 98/249 (39) | 27/86 (31) | 25/80 (31) |
| Paraesthesia | 21/250 (8) | 15/249 (6) | 5/86 (6) | 4/80 (5) |
| Peripheral sensory neuropathy | NR | NR | 5/86(6) | 3/80 (4) |
| Polyneuropathy | NR | NR | 9/86 (10) | 7/80 (9) |
| Pulmonary embolism | NR | NR | 8/86 (9) | 7/80 (9) |
| Rash | 43/250 (17) | 1/249 (0) | 12/86 (14) | 0/80 (0) |
| Skin disordersd | NR | NR | 29/86 (34) | 1/80 (1) |
| Stomatitis | 14/250 (6) | 1/249 (0) | 6/86 (7) | 0/80 (0) |
Data on specific grade 1 or 2 AEs were also provided by Douillard et al. ,44 a summary of which is provided in Appendix 5.
Panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6
In the PEAK trial29 patients were followed for safety 30 days after the last study drug administration. AEs were coded using the MedDRA (version 15.0) and were graded for severity using the NCI-CTC for AEs (version 3.0), with modifications for specific skin- and nail-related toxicities. The safety population consisted of those who received at least one dose of the protocol therapy. Only AEs with a frequency of ≥ 5% in either treatment group were reported. 29
The incidences of any AE and any grade 1 and 2 AE were the same in both treatment arms (100% in each). Similar incidences were also found between the arms for AEs with a worst grade of 3 (70% vs. 54%), AEs with a worst grade of 4 (20% vs. 19%), AEs with a worst grade of 5 (5% vs. 9%), the worst grade 1 or 2 AEs (6% vs. 19%), the worst grade 3 or 4 AEs (90% vs. 73%), any grade 3 or 4 AE (93% vs. 81%) and any serious AE (43% vs. 39%) (see Table 28). More specifically, commonly reported grade 3 and 4 AEs included asthenia, decreased appetite, deep-vein thrombosis, dehydration, diarrhoea, fatigue, hypertension, hypokalaemia, hypomagnesaemia, mucosal inflammation, peripheral neuropathy, neutropenia, paraesthesia, peripheral sensory neuropathy, polyneuropathy, pulmonary embolism, rash, skin disorders and stomatitis (see Table 29). The incidences of these AEs were similar between the treatment arms, except for the following, which occurred with a greater incidence in the panitumumab plus mFOLFOX6 arm than in the bevacizumab plus mFOLFOX6 arm: rash (14% vs. 0%) and skin disorders (34% vs. 1%).
Data on specific grade 1 or 2 AEs were also provided by Schwartzberg et al. ,29 a summary of which is provided in Appendix 5.
Network meta-analysis
To inform the decision problem, a NMA was carried out. Based on the trials identified, it was not possible to construct a complete network. Two discrete networks were generated, one evaluating FOLFOX-containing chemotherapy regimens and the second comparing FOLFIRI-containing chemotherapy regimens. It should be stressed that the results from the two discrete networks are not directly comparable.
Convergence of analyses was confirmed using diagnostic plots and by checking that all chains were sampling from the same posterior distribution.
FOLFOX regimens
Three RCTs26,29,33 contributed to estimating the effectiveness of four treatments: FOLFOX, bevacizumab plus FOLFOX, panitumumab plus FOLFOX and cetuximab plus FOLFOX. As there was no direct evidence for cetuximab plus FOLFOX compared with panitumumab plus FOLFOX, the NMA allowed indirect estimation of this comparison. The network diagram, including which trials informed the NMA for each outcome of interest, is shown in Figure 5.
FIGURE 5.
Network diagram for the FOLFOX network. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab; SAE, serious adverse event. a, AEs based on incidence rates reported in the trials (occurring in ≥ 5% participants in either treatment arm). b, All trials informed the NMA for grade 3/4 neutropenia, paraesthesia, rash and skin conditions occurring in ≥ 5% participants in either treatment arm and two trials26,29 informed the NMA for grade 3/4 diarrhoea, hypokalaemia, hypomagnesaemia, mucositis/stomatitis, mucosal inflammation, fatigue, neuropathy and asthenia occurring in ≥ 5% participants in either treatment arm. c, Data were available to inform the NMA for both surgical resection rate (partial and complete resection) and complete resection rate. Notes: for the purposes of the NMA, skin conditions included acneiform exanthema, dermatitis acneiform, desquamation, nail changes/paronychia, skin reactions and skin disorders; rash was treated separately. As composite reactions appeared to include conditions also reported by specialist preferred terms, these were excluded from the analysis. Incidence rates are reported in Adverse events. Figure based on information from the available from the OPUS,33 PRIME26 and PEAK29 trials.

Progression-free survival
All three RCTs contributed to the estimation of PFS. The NMA found no evidence to suggest that cetuximab plus FOLFOX is any more effective than panitumumab plus FOLFOX at increasing the time to progression (TTP) or death (HR 0.74, 95% Crl 0.36 to 1.49) (Table 30). Nevertheless, as the upper 95% CrI for cetuximab plus FOLFOX compared with all of the other treatments is > 1, it is possible that cetuximab plus FOLFOX could be associated with greater progression or death than FOLFOX, bevacizumab plus FOLFOX or panitumumab plus FOLFOX.
| Intervention treatment | Comparator treatmenta | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | < 1 | 2 | 66 | 32 | |||
| BEV + FOLFOX | 1.11 (0.71 to 1.73) | < 1 | 4 | 29 | 67 | ||
| PAN + FOLFOX | 0.72 (0.58 to 0.90)b | 0.65 (0.44 to 0.96)c | 20 | 79 | 1 | < 1 | |
| CET + FOLFOX | 0.53 (0.27 to 1.04)d | 0.48 (0.21 to 1.07) | 0.74 (0.36 to 1.49) | 80 | 15 | 3 | 2 |
The direct evidence from the PRIME26 and PEAK29 trials suggests that panitumumab plus FOLFOX is more effective than FOLFOX (HR 0.72, 95% CrI 0.58 to 0.90) and bevacizumab plus FOLFOX (HR 0.65, 95% CrI 0.44 to 0.96).
Overall survival
All three RCTs contributed to the estimation of OS. The analysis suggests that there is no evidence that panitumumab plus FOLFOX is more effective than cetuximab plus FOLFOX (HR 1.22, 95% CrI 0.71 to 2.11), as the upper 95% CrI is > 1 (Table 31).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | < 1 | 32 | 55 | 13 | |||
| BEV + FOLFOX | 1.22 (0.73 to 2.05) | 2 | 12 | 18 | 67 | ||
| PAN + FOLFOX | 0.77 (0.64 to 0.93)b | 0.63 (0.39 to 1.02)c | 74 | 25 | < 1 | < 1 | |
| CET + FOLFOX | 0.94 (0.56 to 1.57)d | 0.77 (0.37 to 1.59) | 1.22 (0.71 to 2.11) | 24 | 31 | 26 | 19 |
The direct evidence from the PRIME trial26 suggests that panitumumab plus FOLFOX is more effective than FOLFOX (HR 0.77, 95% CrI 0.64 to 0.93).
Objective response rate
All three RCTs contributed to the estimation of ORR. The ORR was measured at either 6- or 8-week intervals (according to methods reported in the primary publications). However, because of differences in the reporting of the timing of the ORR in each study, it is unclear whether or not the timings are entirely comparable across studies. Given this uncertainty, results reported for the RAS WT population for this outcome should be treated with caution.
The NMA suggests that there is little evidence that cetuximab plus FOLFOX is any more effective than panitumumab plus FOLFOX for ORR (OR 1.90, 95% CrI 0.72 to 5.02) (Table 32).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | < 1 | < 1 | 11 | 88 | |||
| BEV + FOLFOX | 1.62 (0.75 to 3.51) | 9 | 34 | 46 | 11 | ||
| PAN + FOLFOX | (Confidential information has been removed)b | 1.08 (0.55 to 2.12)c | 6 | 57 | 37 | < 1 | |
| CET + FOLFOX | 3.33 (1.36 to 8.12)d | 2.05 (0.63 to 6.70) | 1.90 (0.72 to 5.02) | 85 | 9 | 6 | < 1 |
Resection rates
Only data from the PRIME and PEAK trials were available to analyse resection rates; therefore, a comparison with cetuximab plus FOLFOX could not be made. The data suggest that there is little difference in resection rates between the treatments, as the 95% CrIs all include a value of 1 (Table 33).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 18 | 35 | 46 | ||
| BEV + FOLFOX | 1.04 (0.35 to 3.10) | 35 | 21 | 44 | |
| PAN + FOLFOX | (Confidential information has been removed)b | 1.61 (0.45 to 2.98)c | 47 | 44 | 9 |
Adverse events
The indirect evidence suggests that there is no difference in the ORs for any grade 3/4 AEs or any serious AEs between cetuximab plus FOLFOX and panitumumab plus FOLFOX (Tables 34 and 35). However, panitumumab plus FOLFOX is estimated (from direct evidence) to be associated with more grade 3/4 AEs than FOLFOX or bevacizumab plus FOLFOX. The evidence is less clear for cetuximab plus FOLFOX compared with FOLFOX or bevacizumab plus FOLFOX as the 95% CrIs include a value of 1 (see Table 34).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 34 | 63 | 3 | 0 | |||
| BEV + FOLFOX | 0.81 (0.24 to 2.43) | 64 | 28 | 8 | < 1 | ||
| PAN + FOLFOX | 2.58 (1.59 to 4.30)c | 3.20 (1.21 to 9.56)d | 0 | < 1 | 40 | 60 | |
| CET + FOLFOX | 2.24 (0.85 to 6.24)e | 2.80 (0.64 to 13.34) | 0.86 (0.29 to 2.69) | 2 | 9 | 49 | 40 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 57 | 37 | 6 | < 1 | |||
| BEV + FOLFOX | 1.09 (0.53 to 2.23) | 40 | 31 | 26 | 2 | ||
| PAN + FOLFOX | 1.30 (0.91 to 1.86)c | 1.19 (0.64 to 2.24)d | 2 | 31 | 64 | 2 | |
| CET + FOLFOX | 3.45 (1.28 to 9.88)e | 3.18 (0.94 to 11.33) | 2.66 (0.93 to 8.05) | < 1 | 1 | 3 | 95 |
The results of analyses of specific grade 3/4 AEs are shown in Tables 36–39. The available information allows estimation of the ORs for cetuximab plus FOLFOX compared with panitumumab plus FOLFOX for neutropenia (see Table 36), paraesthesia (see Table 37), rash (see Table 38) and skin conditions (see Table 39). The estimated ORs (and 95% CrIs) suggest that there is little difference in the number of individuals experiencing these AEs between cetuximab plus FOLFOX and panitumumab plus FOLFOX. Note that for the outcomes of rash and skin conditions, the 95% CrIs are very wide because of the low number of events reported in all three RCTs.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 28 | 38 | 26 | 8 | |||
| BEV + FOLFOX | 1.07 (0.50 to 2.26) | 31 | 17 | 22 | 30 | ||
| PAN + FOLFOX | 1.08 (0.75 to 1.54)c | 1.01 (0.52 to 1.95)d | 12 | 32 | 38 | 18 | |
| CET + FOLFOX | 1.15 (0.45 to 2.94)e | 1.08 (0.32 to 3.57) | 1.07 (0.39 to 2.90) | 30 | 13 | 14 | 44 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 3 | 54 | 34 | 10 | |||
| BEV + FOLFOX | 1.21 (0.24 to 5.76) | 5 | 35 | 22 | 38 | ||
| PAN + FOLFOX | 1.44 (0.73 to 2.94)c | 1.19 (0.29 to 5.21)d | < 1 | 7 | 43 | 50 | |
| CET + FOLFOX | 0.09 (0.01 to 1.45)e | 0.07 (0.01 to 1.92) | 0.06 (0.01 to 1.10) | 92 | 4 | 2 | 2 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 53 | 45 | 2 | 0 | |||
| BEV + FOLFOX | 1.34 (0.01 to 82.99) | 44 | 38 | 18 | < 1 | ||
| PAN + FOLFOX | 74.61 (13.2 to 1958)c | 56.33 (4.71 to 16,540)d | 0 | < 1 | 24 | 76 | |
| CET + FOLFOX | 13.06 (0.67 to 5480)e | 13.12 (0.06 to 36,870) | 0.17 (0.01 to 86.72) | 3 | 17 | 56 | 24 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 54 | 44 | 2 | 0 | |||
| BEV + FOLFOX | 1.32 (0.03 to 43.18) | 43 | 42 | 15 | 0 | ||
| PAN + FOLFOX | 135.90 (24.97 to 2660)d | 103.1 (18.17 to 2906)e | 0 | 0 | 18 | 82 | |
| CET + FOLFOX | 13.22 (0.66 to 69.02)f | 11.93 (0.10 to 13,540) | 0.09 (0.01 to 60.23) | 3 | 14 | 64 | 18 |
For the remaining AEs, the OPUS trial33 did not provide the required information and so no comparisons could be made between cetuximab plus FOLFOX and panitumumab plus FOLFOX for diarrhoea, hypokalaemia, hypomagnesaemia, mucositis/stomatitis, mucosal inflammation, fatigue, peripheral neuropathy or asthenia. Instead, analyses are reported to allow an indirect comparison to be made between bevacizumab plus FOLFOX and FOLFOX (see Appendix 5). Note that, because of the small numbers of events for hypomagnesaemia, mucositis/stomatitis and mucosal inflammation, the 95% CrIs are wide.
Subgroup analysis by liver metastases at baseline
Restricting the evidence to the subgroup of people with liver metastases at baseline has little impact on the overall conclusions: there is limited evidence to suggest any difference between cetuximab plus FOLFOX and panitumumab plus FOLFOX for PFS (Table 40), OS (Table 41) and ORR (Table 42) as the 95% CrIs all include a value of 1.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 2 | 17 | 42 | 39 | |||
| BEV + FOLFOX | 1.04 (0.42 to 2.59) | 6 | 21 | 24 | 49 | ||
| PAN + FOLFOX | 0.79 (0.49 to 1.27)b | (Confidential information has been removed)c | 13 | 56 | 28 | 4 | |
| CET + FOLFOX | 0.35 (0.06 to 1.96)d | 0.34 (0.05 to 2.37) | 0.44 (0.07 to 2.66) | 79 | 6 | 6 | 8 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | 3 | 41 | 53 | 2 | |||
| BEV + FOLFOX | 1.95 (0.35 to 10.79) | < 1 | 2 | 10 | 88 | ||
| PAN + FOLFOX | 0.69 (0.42 to 1.15)b | (Confidential information has been removed)c | 65 | 30 | 5 | 0 | |
| CET + FOLFOX | 0.90 (0.33 to 2.43)d | 0.46 (0.06 to 3.39) | 1.29 (0.42 to 3.94) | 32 | 27 | 31 | 10 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | < 1 | 10 | 45 | 45 | |||
| BEV + FOLFOX | 0.98 (0.16 to 5.80) | 6 | 15 | 29 | 49 | ||
| PAN + FOLFOX | 2.18 (0.74 to 6.36)b | (Confidential information has been removed)c | 29 | 55 | 14 | < 1 | |
| CET + FOLFOX | 3.30 (0.63 to 17.10)d | 3.35 (0.30 to 38.24) | 1.51 (0.21 to 10.80) | 64 | 19 | 12 | 5 |
Only data from two RCTs were available for the analysis of surgical resection rates for the liver metastases subgroup (Table 43). As the OPUS trial did not report this outcome, no comparison could be made between cetuximab plus FOLFOX and panitumumab plus FOLFOX. The available data suggest that there is little evidence of a difference in surgical resection rates between FOLFOX, bevacizumab plus FOLFOX and panitumumab plus FOLFOX.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 8 | 19 | 72 | ||
| BEV + FOLFOX | 2.18 (0.42 to 11.43) | 66 | 18 | 36 | |
| PAN + FOLFOX | 1.55 (0.61 to 3.93)b | (Confidential information has been removed)c | 26 | 62 | 33 |
For complete resection, all three RCTs reported relevant evidence and so a comparison could be made between panitumumab plus FOLFOX and cetuximab plus FOLFOX (Table 44). However, there was very little evidence to say that any one treatment was associated with a greater number of complete resections than any other, although these analyses were based on a small number of participants.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||||
|---|---|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | PAN + FOLFOX | First | Second | Third | Fourth | |
| FOLFOX | < 1 | 3 | 23 | 73 | |||
| BEV + FOLFOX | 4.22 (0.58 to 30.68) | 43 | 39 | 12 | 6 | ||
| PAN + FOLFOX | 2.20 (0.80 to 6.07)b | (Confidential information has been removed)c | 7 | 39 | 49 | 4 | |
| CET + FOLFOX | 4.63 (0.20 to 104.60)d | 1.09 (0.03 to 44.34) | 2.09 (0.08 to 56.28) | 50 | 19 | 15 | 16 |
FOLFIRI regimens
Two RCTs28,43 contributed to the estimation of the effectiveness of three treatments: FOLFIRI, bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI. Even though there was no evidence on the effectiveness of panitumumab plus FOLFIRI in this network, the NMA was conducted for the evidence that was available, that is, to inform the indirect comparison between bevacizumab plus FOLFIRI and FOLFIRI. The network diagram, including which trials informed the NMA for each outcome of interest, is shown in Figure 6.
FIGURE 6.
Network diagram for the FOLFIRI network. BEV, bevacizumab; CET, cetuximab; SAE, serious adverse event. a, AEs based on incidence rates reported in the trials (occurring in ≥ 5% participants in either treatment arm). b, The CRYSTAL trial used WHO criteria and the FIRE-3 trial used RECIST criteria to assess response. c, Grade 3/4 skin conditions occurring in ≥ 5% participants in either treatment arm and grade 3/4 diarrhoea occurring in ≥ 5% participants in either treatment arm. d, Surgical resection rate (partial and complete resection). Notes: for the purposes of the NMA, skin conditions included acneiform exanthema, dermatitis acneiform, desquamation, nail changes/paronychia, skin reactions and skin disorders based on rates reported in the included trials. Rash was treated separately. As composite reactions appeared to include conditions also reported by specialist preferred terms, these were excluded from the analysis. Incidence rates are reported in Adverse events. Figure based on information available from the CRYSTAL43 and FIRE-328 trials.

Progression-free survival
The NMA suggests that bevacizumab plus FOLFIRI is more effective than FOLFIRI at increasing TTP or death (HR 0.60, 0.41 to 0.88) and cetuximab plus FOLFIRI is more effective than FOLFIRI at increasing TTP or death (HR 0.56, 0.41 to 0.76) (Table 45). Evidence from the FIRE-3 RCT suggests that cetuximab plus FOLFIRI is no more effective than bevacizumab plus FOLFIRI at increasing TTP or death (see Table 45).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | < 1 | < 1 | 99 | ||
| BEV + FOLFIRI | 0.60 (0.41 to 0.88) | 27 | 73 | < 1 | |
| CET + FOLFIRI | 0.56 (0.41 to 0.76)b | 0.93 (0.74 to 1.17)c | 73 | 27 | < 1 |
Overall survival
The NMA suggests that there is no evidence that bevacizumab plus FOLFIRI is more effective than FOLFIRI at increasing OS; however, the evidence indicates that cetuximab plus FOLFIRI is more effective than both FOLFIRI and bevacizumab plus FOLFIRI at increasing OS (Table 46).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | < 1 | 47 | 53 | ||
| BEV + FOLFIRI | 0.99 (0.68 to 1.42) | < 1 | 53 | 47 | |
| CET + FOLFIRI | 0.69 (0.54 to 0.88)b | 0.70 (0.53 to 0.92)c | 99 | < 1 | < 1 |
Objective response rate
Two RCTs contributed to the estimation of ORR in the FOLFIRI network. 28,43 However, because of differences in the reporting of the timing of ORR in each study, it is unclear whether or not the timings are entirely comparable across studies. Given this uncertainty, the results reported for the RAS WT population for this outcome should be treated with caution.
The NMA suggests that bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI are both more effective than FOLFIRI for the outcome of ORR; however, the evidence that cetuximab plus FOLFIRI is any more effective than bevacizumab plus FOLFIRI for ORR is uncertain because of the wide 95% CrI (OR 1.28, 95% CrI 0.83 to 1.99) (Table 47).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | 0 | 13 | 87 | ||
| BEV + FOLFIRI | 2.43 (1.32 to 4.48) | < 1 | 87 | 13 | |
| CET + FOLFIRI | 3.11 (2.03 to 4.77)b | 1.28 (0.83 to 1.99)c | 100 | < 1 | 0 |
Adverse events
The NMA suggests that bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI are associated with more grade 3/4 AEs than FOLFIRI (Table 48) and that cetuximab plus FOLFIRI is associated with more skin conditions than FOLFIRI or bevacizumab plus FOLFIRI (Table 49). For diarrhoea, the evidence is unclear regarding whether or not one treatment is associated with more cases than any other treatment (Table 50).
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | 99 | < 1 | 0 | ||
| BEV + FOLFIRI | 2.82 (1.46 to 5.49) | < 1 | 64 | 36 | |
| CET + FOLFIRI | 3.06 (1.91 to 4.95)c | 1.09 (0.69 to 1.72)d | 0 | 36 | 64 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | 72 | 28 | 0 | ||
| BEV + FOLFIRI | 2.67 (0.18 to 1177) | 28 | 72 | 0 | |
| CET + FOLFIRI | 127.60 (11.12 to 53,970)d | 47.60 (21.30 to 129.40)e | 0 | 0 | 100 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | 85 | 11 | 4 | ||
| BEV + FOLFIRI | 2.04 (0.82 to 5.20) | 4 | 13 | 82 | |
| CET + FOLFIRI | 1.46 (0.77 to 2.82)c | 0.72 (0.37 to 1.38)d | 10 | 76 | 14 |
Sensitivity analyses
Addition of FIRE-3 data from the manufacturer’s submission (15 June 2015, version 2; see also Appendix 5) to the analysis of HRs for progression or death (Table 51), HRs for OS (Table 52) and ORs for ORR (Table 53) had very little effect on the overall conclusions for the FOLFIRI network.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | < 1 | < 1 | 100 | ||
| BEV + FOLFIRI | 0.58 (0.40 to 0.84) | 39 | 61 | < 1 | |
| CET + FOLFIRI | 0.56 (0.41 to 0.76)b | 0.97 (0.78 to 1.20)c | 61 | 39 | < 1 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | < 1 | 47 | 53 | ||
| BEV + FOLFIRI | 0.99 (0.69 to 1.40) | < 1 | 53 | 47 | |
| CET + FOLFIRI | 0.69 (0.54 to 0.88)b | 0.70 (0.54 to 0.90)c | 100 | < 1 | < 1 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFIRI | BEV + FOLFIRI | First | Second | Third | |
| FOLFIRI | 0 | 8 | 92 | ||
| BEV + FOLFIRI | 2.34 (1.29 to 4.22) | < 1 | 91 | 8 | |
| CET + FOLFIRI | 3.11 (2.03 to 4.76)b | 1.33 (0.89 to 2.00)c | > 99 | < 1 | 0 |
Summary
Summary of the clinical effectiveness systematic review
-
Of 2811 titles/abstracts screened, five RAS WT subgroup analyses from RCTs met the inclusion criteria for the clinical effectiveness systematic review.
-
Research has demonstrated a treatment interaction between RAS and EGFR inhibitors. Tumour samples from trial populations were therefore re-evaluated for RAS status. In response to these research developments, the EMA has recently amended the licence for cetuximab and panitumumab to restrict use to people with RAS WT mCRC. Importantly, currently available data for the effectiveness of EGFR inhibitors in people with RAS WT mCRC are from a subgroup of the ITT trial populations for both cetuximab and panitumumab. Reported data were in line with the expected direction of effect across all of the include studies. No RCTs with an ITT population by RAS status were identified in the Assessment Group’s searches.
-
The risk of bias was high but generally similar between studies with regard to randomisation, allocation concealment, blinding, outcome reporting and loss to follow-up. The main limitation in terms of interpretation and validity was that all of the included studies were subgroup analyses of ITT trial populations. Allocation to subgroups was based on re-evaluating tumour samples from the KRAS WT exon 2 population for RAS status. Although this minimised the potential for ascertainment bias, there were missing data for some of the trials (either tumours were not evaluable for RAS status or the results were inconclusive). No significant imbalances between the trial populations were observed, minimising the potential for selection bias. Because of the retrospective nature of the RAS analysis, for some studies a low number of samples was available for analysis, reducing the power of the studies to show statistical significance. Despite these limitations, these are currently the only data available that have evaluated the effectiveness of cetuximab and panitumumab in people with mCRC with RAS WT tumour status in line with the recently revised licensed indication and the NICE final scope.
Cetuximab
-
Two trials provided evidence for the effectiveness of cetuximab in combination with chemotherapy (FOLFOX or FOLFIRI) compared with chemotherapy alone (FOLFOX or FOLFIRI). 43,65 The evidence consistently suggested a treatment effect in favour of the addition of cetuximab to chemotherapy compared with chemotherapy alone.
-
Median PFS ranged from 11.4 months in the CRYSTAL study to 12 months in the OPUS study for the experimental arms, and from 5.8 months in the CRYSTAL study to 8.4 months in the OPUS study for the control arms.
-
Median OS ranged from 19.8 months in the CRYSTAL study to 20.4 months in the OPUS study for the experimental arms, and from 17.8 months in the CRYSTAL study to 20.2 months in the OPUS study for the control arms.
-
Tumour response rates ranged from 58% in the OPUS study to 66% in the CRYSTAL study in the experimental arms, and from 29% in the OPUS study to 60% in the CRYSTAL study in the control arms.
-
In people with liver metastases at baseline, the results in terms of improvement in OS and PFS were consistent with the results for the overall RAS WT population. Of people with liver metastases at baseline, 13.3% in the OPUS study and 16.3% in the CRYSTAL study had complete resection in the experimental arms.
-
Overall, clinical safety data for the RAS WT population were consistent with the results for the KRAS WT population in both of the trials. The most common AEs were diarrhoea, haematotoxicity, neutropenia and skin reactions.
-
-
One trial provided evidence for the effectiveness of cetuximab in combination with chemotherapy (FOLFIRI) compared with bevacizumab in combination with chemotherapy (FOLFIRI). 28
-
The proportion of people who achieved an objective response was similar between the cetuximab plus FOLFIRI arm and the bevacizumab plus FOLFIRI arm. However, the association with longer OS suggests a benefit for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI (HR 0.70, 95% CI 0.53 to 0.92).
-
Panitumumab
-
One trial provided evidence for the effectiveness of panitumumab in combination with chemotherapy (FOLFOX4) compared with chemotherapy alone (FOLFOX4). No evidence was identified comparing panitumumab plus FOLFIRI with FOLFIRI. The evidence consistently suggested a treatment effect in favour of the addition of panitumumab to FOLFOX4 compared with FOLFOX4.
-
Median PFS was 10.1 months in the experimental arm and 7.9 months in the control arm.
-
Median OS was 25.8 months in the experimental arm and 20.2 months in the control arm.
-
In people with liver metastases at baseline, the results in terms of improvement in OS and PFS were consistent with the results for the overall RAS WT population.
-
Overall, clinical safety data for the RAS WT population were consistent with results for the KRAS WT population in all of the trials. The most common AEs were diarrhoea, haematotoxicity, neutropenia and skin reactions.
-
-
One trial provided evidence for the effectiveness of panitumumab in combination with chemotherapy (mFOLFOX6) compared with bevacizumab in combination with chemotherapy (mFOLFOX6).
-
The proportion of people who achieved an objective response was similar between the panitumumab plus mFOLFOX6 arm and the bevacizumab plus mFOLFOX6 arm. For PFS, the addition of panitumumab to mFOLFOX6 was associated with a 35% reduction in the risk of progression compared with bevacizumab plus mFOLFOX6. In addition, a trend towards an OS benefit with panitumumab plus mFOLFOX6 was observed (HR 0.63, 95% CI 0.39 to 1.02).
-
Summary of the network meta-analysis
-
A NMA was also conducted based on the trials identified. It was not possible to construct a complete network and so two discrete networks were generated, one evaluating FOLFOX-containing chemotherapy regimens and the other comparing FOLFIRI-containing chemotherapy regimens.
FOLFOX network
-
Three RCTs contributed to the estimation of the effectiveness of four treatments (FOLFOX, bevacizumab plus FOLFOX, panitumumab plus FOLFOX and cetuximab plus FOLFOX).
-
There was no evidence to suggest that cetuximab plus FOLFOX was any more effective than FOLFOX, bevacizumab plus FOLFOX or panitumumab plus FOLFOX in terms of increasing the time to death or increasing the TTP or death.
-
Direct evidence suggested that panitumumab plus FOLFOX was more effective at increasing the TTP or death than FOLFOX and bevacizumab plus FOLFOX. Panitumumab plus FOLFOX was also estimated to be more effective at increasing the time to death than FOLFOX.
-
There was limited evidence to suggest that cetuximab plus FOLFOX was more effective at improving the ORR than panitumumab plus FOLFOX.
-
There was little evidence that cetuximab plus FOLFOX was associated with fewer AEs than panitumumab plus FOLFOX; however, some of these analyses were limited by the small number of events recorded in the treatment arms.
FOLFIRI network
-
No evidence was identified comparing panitumumab plus FOLFIRI with FOLFIRI.
-
Two RCTs contributed to the estimation of the effectiveness of three treatments (FOLFIRI, bevacizumab plus FOLFIRI and cetuximab plus FOLFIRI).
-
The evidence suggested that cetuximab plus FOLFIRI and bevacizumab plus FOLFIRI were more effective than FOLFIRI at increasing the TTP or death and the ORR.
-
Direct evidence suggested that cetuximab plus FOLFIRI was more effective than FOLFIRI and bevacizumab plus FOLFIRI at increasing the time to death.
Summary results tables: clinical effectiveness
Summaries of the results (direct and indirect evidence) for cetuximab plus FOLFOX, cetuximab plus FOLFIRI and panitumumab plus FOLFOX compared with the interventions of interest are provided for efficacy (PFS, OS, ORR and complete resection rate) and safety outcomes in Tables 54 and 55, respectively. Note that for grade 3 or 4 AEs by type (reported in ≥ 5% of participants in either treatment arm), only those analyses in the NMA are included in the summary results tables. A more complete summary of grade 3 or 4 AEs by type is provided in Adverse events.
| Comparison | RAS WT | RAS WT with liver metastases at baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| PFS, HR (95% CrI) | OS, HR (95% CrI) | ORR, OR (95% CrI) | Complete resection rate, OR (95% CrI) | PFS, HR (95% CrI) | OS, HR (95% CrI) | ORR, OR (95% CrI) | Complete resection rate,d OR (95% CrI) | |
| Intervention: CET + FOLFOX vs. | ||||||||
| FOLFOX | 0.53 (0.27 to 1.04) e | 0.94 (0.56 to 1.57) e | 3.33 (1.36 to 8.12) e | NE | 0.35 (0.06 to 1.96) e | 0.90 (0.33 to 2.43) e | 3.30 (0.63 to 17.10) e | 4.63 (0.20 to 104.60) e |
| PAN + FOLFOX | 0.74 (0.36 to 1.49) | 1.22 (0.71 to 2.11) | 1.90 (0.72 to 5.02) | NE | 0.44 (0.07 to 2.66) | 1.29 (0.42 to 3.94) | 1.51 (0.21 to 10.80) | 2.09 (0.08 to 56.28) |
| BEV + FOLFOX | 0.48 (0.21 to 1.07) | 0.77 (0.37 to 1.59) | 2.05 (0.63 to 6.70) | NE | 0.34 (0.05 to 2.37) | 0.46 (0.06 to 3.39) | 3.35 (0.30 to 38.24) | 1.09 (0.03 to 44.34) |
| Intervention: PAN + FOLFOX vs. | ||||||||
| FOLFOX | 0.72 (0.58 to 0.90) f | 0.77 (0.64 to 0.93) f | (Confidential information has been removed)f | (Confidential information has been removed)f | 0.79 (0.49 to 1.27) f | 0.69 (0.42 to 1.15) f | 2.18 (0.74 to 6.36) f | 2.20 (0.80 to 6.07) f |
| BEV + FOLFOX | 0.65 (0.44 to 0.96) g | 0.63 (0.39 to 1.02) g | 1.08 (0.55 to 2.12) g | 1.16 (0.45 to 2.96) g | (Confidential information has been removed)g | (Confidential information has been removed)g | (Confidential information has been removed)g | (Confidential information has been removed)g |
| Intervention: CET + FOLFIRI vs. | ||||||||
| FOLFIRI | 0.56 (0.41 to 0.76) h | 0.69 (0.54 to 0.88) h | 3.11 (2.03 to 4.77) i | NE | NE | NE | NE | NE |
| PAN + FOLFIRI | NE | NE | NE | NE | NE | NE | NE | NE |
| BEV + FOLFIRI | 0.93 (0.74 to 1.17) i , j | 0.70 (0.53 to 0.92) i , k | 1.28 (0.83 to 1.99) l | NE | NE | NE | NE | NE |
| Intervention: PAN + FOLFIRI vs. | ||||||||
| FOLFIRI | NE | NE | NE | NE | NE | NE | NE | NE |
| BEV + FOLFIRI | NE | NE | NE | NE | NE | NE | NE | NE |
| Comparison | Any grade 3/4 AEs,d OR (95% CrI) | Any SAEs,d OR (95% CrI) | Grade 3/4 neutropenia,d OR (95% CrI) | Grade 3/4 paresthesia,d OR (95% CrI) | Grade 3/4 rash,d OR (95% CrI) | Grade 3/4 skin conditions,d OR (95% CrI) | Grade 3/4 diarrhoea,d OR (95% CrI) |
|---|---|---|---|---|---|---|---|
| Intervention: CET + FOLFOX vs. | |||||||
| FOLFOX | 2.24 (0.85 to 6.24) e | 3.45 (1.28 to 9.88) e | 1.15 (0.45 to 2.94) e | 0.09 (0.01 to 1.45) e | 13.06 (0.67 to 5480) e | 13.22 (0.66 to 69.02) e | NE |
| PAN + FOLFOX | 0.86 (0.29 to 2.69) | 2.66 (0.93 to 8.05) | 1.07 (0.39 to 2.90) | 0.06 (0.01 to 1.10) | 0.17 (0.01 to 86.72) | 11.93 (0.10 to 13,540) | NE |
| BEV + FOLFOX | 2.80 (0.64 to 13.34) | 3.18 (0.94 to 11.33) | 1.08 (0.32 to 3.57) | 0.07 (0.01 to 1.92) | 13.12 (0.06 to 36,870) | 0.09 (0.01 to 60.23) | NE |
| Intervention: PAN + FOLFOX vs. | |||||||
| FOLFOX | 2.58 (1.59 to 4.30) f | 1.30 (0.91 to 1.86) f | 1.08 (0.75 to 1.54) f | 1.44 (0.73 to 2.94) f | 74.61 (13.2 to 1958) f | 135.90 (24.97 to 2660) f | NE |
| BEV + FOLFOX | 3.20 (1.21 to 9.56) g | 1.19 (0.64 to 2.24) g | 1.01 (0.52 to 1.96) g | 1.19 (0.29 to 5.21) g | 56.33 (4.71 to 16,540) g | 103.1 (18.17 to 2906) g | NE |
| Intervention: CET + FOLFIRI vs. | |||||||
| FOLFIRI | 3.06 (1.91 to 4.95)h | NE | NE | NE | NE | 127.60 (11.12 to 53,970)h | 1.46 (0.77 to 2.82)h |
| PAN + FOLFIRI | NE | NE | NE | NE | NE | NE | NE |
| BEV + FOLFIRI | 1.09 (0.69 to 1.72)i | NE | NE | NE | NE | 47.60 (21.30 to 129.40)i | 0.72 (0.37 to 1.38)i |
| Intervention: PAN + FOLFIRI vs. | |||||||
| FOLFIRI | NE | NE | NE | NE | NE | NE | NE |
| PAN + FOLFIRI | NE | NE | NE | NE | NE | NE | NE |
Ongoing trials
Searches of ClinicalTrials.gov, the WHO ICTRP, the UKCRN and the ISRCTN registry were conducted (see Appendix 1 for the search strategy used) in March 2015. Ten trials were considered as being relevant to this review (see Appendix 6 for trial details) and were investigated further. Seven trials were identified as ongoing (ongoing, n = 2; ongoing not recruiting, n = 2; active, not recruiting, n = 1; recruiting, n = 2). Three trials were completed and included in this review (OPUS,65 CRYSTAL43 and PRIME44).
Manufacturers’ reviews of clinical effectiveness
Both manufacturers, Amgen (panitumumab; 30 April 2015) and Merck Serono (cetuximab; 15 June 2015, version 2), submitted clinical evidence for consideration for this MTA.
Amgen
Amgen carried out literature searches for clinical evidence in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations and EMBASE through Ovid and CENTRAL through The Cochrane Library (Amgen submission, section 1.2, pp. 11–12). It also carried out a rapid appraisal search in The Cochrane Library to identify existing systematic reviews and protocols in the topic area. The search strategies combine free-text and index terms for relevant cancers with free-text and index terms for relevant interventions (Amgen submission, appendix 2, pp. 86–114). The Cochrane RCT publication filter was used to limit the search results to RCTs. No language or date limits were applied.
Amgen also searched grey literature resources, including trials registries, online conference proceedings and the websites of national guideline and regulatory agencies (Amgen submission, section 1.2, pp. 12–13).
The Amgen literature searches used an appropriate range of databases and grey literature resources for the topic. The choice of free-text and index terms was also appropriate and the searches had an appropriate balance of sensitivity and specificity. The search strategies were reproduced in appendix 2, including the number of hits retrieved per search and the dates that the searches were carried out (Amgen submission, appendix 2, pp. 86–114).
The submission set out to identify the evidence available from RCTs evaluating the efficacy and safety of panitumumab and other therapies for the treatment of people with previously untreated mCRC. The review identified two panitumumab trials (PRIME44 and PEAK29), of which one44 was considered to meet the criteria set out in the decision problem specified in the final scope14 (Table 56). The PRIME trial44 was also included in the PenTAG systematic review. In addition, the PenTAG review included the PEAK trial,29 which evaluated the efficacy of panitumumab in combination with mFOLFOX6 compared with bevacizumab in combination with mFOLFOX6. This trial was excluded from the Amgen submission as bevacizumab is no longer available through the CDF, but information from the trial was provided as supporting evidence (Amgen submission, section 4.6, p. 44).
| Trial | First author, year | Included in PenTAG review | Reason for exclusion from PenTAG review |
|---|---|---|---|
| PRIME (PAN + FOLFOX4 vs. FOLFOX4) | Douillard 201344 | Yes | NA |
| Reference also made in section 4.4 of the Amgen submission to Siena et al.69 and Wang et al.70 | No | Identified and listed in Appendix 3. Both available only in abstract format; not enough information to quality appraise |
Health-related quality-of-life data from the PRIME trial69 [EuroQol 5-Dimensions (EQ-5D) health state index and overall health rating] were included in the Amgen submission (section 4.4, p. 31). An analysis of quality-adjusted survival in participants with RAS WT tumours using the quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) method was also completed (Amgen submission, section 4.4, p. 31). No HRQoL data were identified for inclusion in the PenTAG review; however, two abstracts were identified69,70 (listed in Appendix 3; not formally included as not enough information was provided to conduct a quality appraisal). Amgen reported a summary of AEs, patient incidence of AEs of interest, AEs occurring in ≥ 10% of participants in either treatment arm and AEs with a > 5% difference in incidence between treatment arms (Amgen submission, section 4.7, pp. 49–51; appendix 6, tables 1 and 2). For AEs, the Assessment Group reported a summary of AEs and grade 3/4 AEs occurring in ≥ 5% of participants in either treatment arm.
In section 4.6 of the Amgen submission (pp. 44–5), the company presented supporting evidence for panitumumab in combination with FOLFIRI and noted the data used to obtain regulatory approval. These are included for information in Table 57.
| Trial | First author, year | Included in PenTAG review | Reason for exclusion from PenTAG review |
|---|---|---|---|
| PLANET (PAN + FOLFIRI vs. FOLFIRI) | Abad 201475 | No | Published as abstract only (see Appendix 3; not enough information to conduct a quality appraisal); reports data predominantly for the KRAS WT population (response rate only is reported for the RAS WT population) |
| Study 20060314 (PAN + FOLFIRI) | Data on file from Amgen (CSR RAS analysis), October 2014 | No | Not identified in searches as unpublished information; study design single arm |
| Study 20050181 (PAN + FOLFIRI vs. FOLFIRI) | Peeters 201476 | No | Population previously treated; not first line |
| Study 20080763 (ASPECCT) (PAN vs. CET) | Price 201477 | No | Population previously treated; not first line; not RAS WT. Intervention PAN or CET as monotherapy |
Network meta-analysis
Amgen performed a NMA to compare panitumumab in combination with FOLFOX with other identified comparators in the scope (Amgen submission, section 2.1, p. 51).
The company conducted a systematic review: the search strategy combined ‘drug names’ with ‘disease terms’ and ‘study design terms’ (the search strategy was provided as an appendix). Inclusion criteria for the NMA were in line with the PICO (population, intervention, comparator, outcome) criteria specified in the NICE scope14 (Amgen submission, section 2.1, p. 51).
Evidence informing the NMA came from a total of 21 RCTs [reported in 22 publications28,29,33,34,44,78–94 and one unpublished article (Amgen Ltd. Data on File: Supplemental CSR 20050203 RAS/BRAF Analysis. 15 April 2013)]. Four trials84,87,88,92 were excluded from the primary analysis because of population differences or differences in the treatment regimen administered. Based on the 17 RCTs, Amgen built one network. Studies excluded from the company’s primary analysis were included in a sensitivity analysis. The sensitivity analysis included clinically similar chemotherapy regimens [FOLFOX/XELOX and FOLFIRI/XELIRI (capecitabine + irinotecan)] and relevant comparators (FOLFOX, XELOX, XELIRI and cetuximab plus FOLFOX/FOLFIRI). There were too few data to perform a NMA comparing panitumumab plus FOLFOX or FOLFIRI with the comparators of interest in the subgroup of people with liver metastases.
The study designs of the included studies were comparable; however, not all studies reported all outcomes of interest (OS, PFS or ORR) and, hence, not all studies contributed to the analysis for each outcome (see Amgen submission, appendix 8, pp. 27–35). In addition, disease progression and response rate were not assessed using the same method in all of the included studies; however, it was assumed that this had no impact on the comparative treatment effect on the PFS or ORR end points. Population characteristics were assumed to be the same between the studies; however, the studies evaluating a non-EGFR inhibitor included those with mixed or unknown RAS status.
The company used meta-analysis techniques (random effects with fixed effects examined in sensitivity analysis) to pool direct comparisons using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA). For indirect comparisons, the company used the method of Bucher et al. 95 The indirect estimate of panitumumab compared with the comparator was calculated using the results of the direct comparisons with a common control. Both fixed- and random-effects meta-analyses were used. Each indirect comparison was estimated separately within the indirect comparison framework. Within the indirect comparison, the underlying assumptions of homogeneity, similarity and consistency were reviewed according to guidelines by Song et al. 96 Details of the implementation of the meta-analysis and indirect comparison were provided in the Amgen submission (appendix 8).
For the NMA, a Bayesian framework with Markov chain Monte Carlo simulation was used, using methodology outlined by Ades et al. 97 Analyses were performed using SAS version 9.3. Non-informative priors were used. Analyses were run with an initial burn-in of 10,000 iterations followed by an additional 50,000 iterations. To address the potential for autocorrelation, it was necessary to thin the samples generated through SAS (a thinning factor of 40 was used). The posterior mean/median and 95% CrI were reported together with the probability that each treatment was better (more effective) than the others. Within the indirect comparison, the underlying assumptions of homogeneity, similarity and consistency were reviewed according to guidelines by Song et al. 96 Convergence of the models was examined and Amgen noted that, in some cases, the models for the treatment arm-level analyses did not converge to a stationary distribution, showing a high level of autocorrelation between draws of the Markov chain, even with thinning factors of ≥ 100 and a burn-in period of > 1,000,000 iterations attempted. The results for these models were not shown; the company note that this was because of their unsuitability. Details of the implementation of the mixed-treatment comparison are provided in the Amgen submission (appendix 8).
Point estimates for relative effectiveness (including 95% CrIs and the probability of each treatment being the better treatment) were reported in full in the Amgen submission (appendix 8, pp. 41–2 and pp. 87–97) and full results (including the results of the sensitivity analyses conducted) were reported (appendix 8, pp. 87–97). Amgen’s NMA was not used to analyse liver resection rates or AEs.
The following limitations of the NMA were acknowledged: (1) data for non-EGFR inhibitors were from populations with mixed or unspecified RAS status and (2) data for the RAS WT population were not from the protocol-defined population for any of the EGFR inhibitor studies and the results were not for the ITT population but a retrospective subgroup.
Comparison with the Assessment Group’s network-meta-analysis
Of the studies included in Amgen’s NMA [n = 21, reported in 22 publications28,29,33,34,44,78–94 and one unpublished article (Amgen Ltd. Data on File: Supplemental CSR 20050203 RAS/BRAF Analysis. 15 April 2013)], 18 (including the unpublished article) were not included in the Assessment Group’s NMA. 78–94 The reason for their exclusion was that they did not evaluate the effectiveness of the interventions in the RAS WT population. In addition to the abstracts for the OPUS33 and CRYSTAL34 trials included in the Amgen NMA, the Assessment Group identified the full publications. 43,65
Evidence from the included studies enabled the company to construct a complete network. The study by Badulescu et al. 78 compared FOLFOX and FOLFIRI and enabled the complete network approach based on the assumption that there was little difference between FOLFOX and FOLFIRI in terms of effectiveness. The NMA conducted by the Assessment Group included two separate networks (FOLFOX and FOLFIRI) as none of the included studies provided evidence to link the two networks for the RAS WT population.
Assumptions regarding the similarity between the included trials in terms of study design were considered by the Assessment Group to be appropriate. However, in terms of population characteristics, although data included in the NMA for panitumumab and cetuximab were restricted to the RAS WT population in line with the population specified in the NICE scope,14 data for non-EGFR inhibitors were not available for the RAS WT population, given that efficacy is not contingent on the expression of the RAS genotype. Although the Assessment Group consider this to be a logical approach, it should be noted that data included in the NMA for non-EGFR inhibitor treatments came from study populations with mixed or unspecified RAS status, the likely impact of which would be to increase the uncertainty surrounding the effect estimates.
Analyses were conducted for the following outcomes: PFS, OS, ORR, CR and PR. Time-to-event data were analysed using study-level data (HR) and response rate data were analysed using study-level data (relative risk). The company noted that there were too few data to perform a NMA for panitumumab plus FOLFOX compared with cetuximab plus FOLFOX or cetuximab plus FOLFIRI in the subgroup of people with liver metastases.
The methods used in Amgen’s NMA were in line with guidance set out in the publication by Ades et al. 97
Despite the broader approach taken, the results for panitumumab plus FOLFOX compared with FOLFOX were similar to those of the Assessment Group’s NMA for OS and PFS. The effect estimates for this comparison for all outcomes showed a greater effect of panitumumab plus FOLFOX than FOLFOX, but the 95% CrIs were wider in the Assessment Group’s results. There was no evidence to suggest that panitumumab plus FOLFOX was any more or less effective than cetuximab plus FOLFOX in terms of TTP or death or time to death. All of the results, however, are subject to uncertainty as a result of the acknowledged limitations. As the Assessment Group’s NMA focused entirely on the RAS WT population, no comparison could be made with Amgen’s analysis of panitumumab plus FOLFOX compared with XELOX. Also, given that the Assessment Group’s approach to the NMA resulted in two networks, the results could not be compared with Amgen’s NMA for panitumumab plus FOLFOX compared with either FOLFIRI or cetuximab plus FOLFIRI.
Merck Serono
Merck Serono also carried out literature searches for clinical evidence in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations and EMBASE through Ovid, and CENTRAL through The Cochrane Library (Merck Serono submission, section 3.1.2.1, p. 11). As per the Amgen review, the searches combined free-text and index terms for relevant cancers with free-text and index terms for relevant interventions; however, unlike the Amgen review, the cancer search terms were combined with RAS search terms to further refine the results (Merck Serono submission, appendix A, pp. 44–9). A publication filter was used to limit the results to RCTs and observational studies. No language or date limits were applied.
Merck Serono also searched grey literature resources, including an online trials registry (ClinicalTrials.gov) and several online conference proceedings (Merck Serono submission, section 3.1.2.1, p. 12).
The Merck Serono literature searches used an appropriate range of bibliographic databases and grey literature resources for the topic, albeit fewer grey literature resources were searched than in the Amgen review. The choice of free-text and index terms was also appropriate and there was no evidence that the balance of sensitivity and specificity was compromised by the inclusion of RAS search terms. The database search strategies are reproduced in appendix A, including the number of hits retrieved per search (Merck Serono submission, appendix A, pp. 44–9). The dates that the searches were carried out are reported elsewhere in the submission (Merck Serono submission, section 3.1.2.1, p. 11). The grey literature search strategies are not reproduced in the appendices, but the numbers of articles retrieved are reported in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagrams (Merck Serono submission, section 4.1, pp. 22–5).
The submission set out to identify the relevant efficacy and safety evidence for the interventions of interest in first-line treatment of people with RAS WT mCRC. Seven studies were identified that evaluated cetuximab. Of these, four were included in the systematic review presented by Merck Serono (Table 58). Three of the studies were included in the PenTAG systematic review; however, only the studies reporting results for the RAS WT population were considered relevant to the scope for this review and, as such, the other related publications were excluded on population. The CALGB-80405 study101 was not identified in the PenTAG searches. This was because we did not search the European Society of Medical Oncology (ESMO) conference database, instead checking the American Society of Clinical Oncology database, in line with published recommendations on searching for health technology assessment (HTA) reviews. 102 This study would have been excluded from our review because, although the CALGB-80405 trial randomised participants to cetuximab or bevacizumab, participants were not randomised to the background chemotherapy (FOLFOX or FOLFIRI), which could introduce bias into the analysis. In addition, the data are published only in abstract form and not in a full paper and, as such, not enough information is provided to conduct a quality appraisal.
| Trial acronym | First author, year | Included in PenTAG’s review | Reason for exclusion from PenTAG’s review |
|---|---|---|---|
| CRYSTAL (CET + FOLFIRI vs. FOLFIRI) | Van Cutsem 200924 (primary study reference); Van Cutsem 2011;25 Ciardiello 2014;34 Van Cutsem 201543 | Yes (only data for the RAS WT population43) | Van Cutsem 2009:24 no data for the RAS WT population; Van Cutsem 2011:25 no data for the RAS WT population; Ciardiello 2014:34 abstract |
| OPUS (CET + FOLFOX4 vs. FOLFOX4) | Bokemeyer 200923 (primary study reference); Tejpar 201498 | Yes (only data for the RAS WT population65) | Bokemeyer 2009:23 no data for the RAS WT population |
| FIRE-3 (CET + mFOLFOX6 vs. BEV + mFOLFOX6) | Heinemann 201399 (primary study reference); Stintzing 2014;100 Heinemann 201428 | Yes (only data for the RAS WT population28) | Heinemann 2013:99 abstract of Heinemann 2014;28 Stintzing 2014:100 no data for the RAS WT population, abstract |
| CALGB-80405 (CET + CTXa vs. BEV + CTXa) | Lenz 2014101 | No | Study not identified in searches (not indexed in EMBASE or MEDLINE). Participants were randomised only to CET or BEV and not to the background CTX. Study published in abstract format (presented at ESMO 2014) and not enough information provided to quality appraise |
Health-related quality-of-life data from the OPUS trial [European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 global health status; unpublished data] and the CALGB-80405 trial101 (EORTC QLQ-C30 and Dermatology-Specific Quality of Life Questionnaire) were also included in the Merck Serono submission (section 2.1.3.3, pp. 34–5). No HRQoL data were identified for inclusion in the Assessment Group’s review. Merck Serono reported a summary of AEs, grade 3/4 AEs by special AE category and a comparison of the frequency of grade 3/4 AEs (number of subjects) known for cetuximab (section 2.1.4, pp. 36–40). For AEs, the Assessment Group reported a summary of AEs and grade 3/4 AEs occurring in ≥ 5% participants in either treatment arm.
Data reported for the FIRE-3 trial in the Merck Serono submission are different from those in the analysis conducted by the Assessment Group (values as reported by Heinemann et al. 28). It is possible that the data reported in the Merck Serono submission are from a more recent data cut-off point, as the number of participants evaluated as RAS WT in the Merck Serono submission was 199 in the cetuximab plus FOLFIRI treatment group and 201 in the bevacizumab plus FOLFIRI treatment group; this compares with 171 participants in each treatment group in the published paper. 28 These unpublished data were subjected to sensitivity analysis in the NMA (Merck Serono submission, p. 118). Although the results change slightly, this difference does not impact on the direction of effect.
Network meta-analysis
Merck Serono performed a NMA to compare cetuximab plus chemotherapy (FOLFOX or FOLFIRI) for the treatment of RAS WT mCRC with other comparators specified in the NICE scope14 (Merck Serono submission, section 2, p. 51).
The company conducted a systematic review, with the search strategy combining ‘drug names’ with ‘disease terms’ and ‘study design terms’ (the search strategy was provided as an appendix). Inclusion criteria for the NMA were in line with the PICO criteria specified in the NICE scope14 (see section 2.1, p. 51).
Six trials were included in the NMA. 28,29,43,44,101 Evidence from these trials enabled one complete network to be established for the outcomes of OS and PFS. This was possible as the CALGB-80405 trial compared cetuximab plus FOLFOX or FOLFIRI with bevacizumab plus FOLFOX or FOLFIRI, reporting separate Kaplan–Meier curves for each of the possible combination therapies. 101 Within the global network, a sensitivity analysis was also conducted with FOLFOX and FOLFIRI grouped as generic chemotherapy. The complete network approach was not possible for ORR as neither the PEAK trial29 nor the CALGB-80405 trial101 reported ORR and, as a result, only a FOLFIRI network was possible for this outcome. It was also not possible to include the CALGB-80405 trial101 in any safety outcome network because of lack of reporting. Therefore, two separate networks, one for FOLFOX and one for FOLFIRI, were created to allow an indirect treatment comparison for safety outcomes.
The study designs of the included studies were comparable. Although Merck Serono noted that disease progression was not assessed using the same method in all of the included studies, it was assumed that this had no impact on the comparative treatment effect for the PFS end point. For the safety outcomes, in the absence of reported data for the RAS WT population in the PRIME trial,44 Merck Serono used data reported for the KRAS WT population. Although the company pre specified safety outcomes of interest, not all could be analysed because of limited reporting in several trials.
Population characteristics were assumed to be the same, although for some trials the baseline characteristics for the RAS WT population were not reported44 or very little published information was available101 and data from the KRAS WT population were used as a proxy. Merck Serono highlighted differences with respect to disease progression (ECOG performance status ≤ 2 in four of the trials28,43,44 vs. 0 or 1 in two of the trials29,101). However, the proportion of participants with an ECOG performance status of 2 in the OPUS and PRIME44 studies was low and, as such, was not considered to have an impact on the comparative treatment effect. It was assumed that both FOLFOX regimens (FOLFOX4 and mFOLFOX6) have a comparable effect.
Network meta-analyses were undertaken using a Bayesian approach with Markov chain Monte Carlo simulation in WinBUGS. Non-informative prior distributions were used. For the analysis of PFS, OS and ORR, models with a normal likelihood and identify link were used. In addition, survival data extracted from the Kaplan–Meier curves were also analysed using a binomial or log-likelihood and log-link using a fractional polynomial model. Analysis of AEs used a model with a binomial likelihood and logit link. Analyses were run with an initial burn-in of 10,000 iterations (30,000 for the fractional polynomial models), followed by an additional 80,000 iterations (30,000 for fractional polynomials) and convergence of the samples was examined visually. Monte Carlo error was checked to ensure that it was ≤ 5% of the posterior standard deviation (SD) for the parameters examined. Both fixed- and random-effects models were used. The deviance information criterion was used to compare the fixed- and random-effects models to determine goodness of fit (deviance information criterion values were reported for both models); when a difference of < 5 was observed, a fixed-effects model was reported and the results of the random-effects model were reported in appendix B (Merck Serono submission). The posterior mean/median and 95% CrI were reported together with the probability that each treatment was better (more effective) than the others.
Point estimates for relative effectiveness (including the 95% CrI and the probability of each treatment being the better treatment) were reported in full in the Merck Serono submission (pp. 51–82). Table 59 summarises the results for OS, PFS and ORR for cetuximab plus FOLFOX and cetuximab plus FOLFIRI compared with relevant comparators. In terms of AEs (not shown here), cetuximab plus FOLFIRI was associated with more events than FOLFIRI alone for grade 3–4 venous thromboembolism, skin reactions, acne-like rash, mucositis, neutropenia, hypokalaemia, hypomagnesaemia and paronychia. Compared with bevacizumab plus FOLFIRI, cetuximab plus FOLFIRI was worse for skin reactions, acne-like rash, hypokalaemia, hypomagnesaemia and paronychia. However, cetuximab plus FOLFIRI was better than bevacizumab plus FOLFIRI for nausea (all grades) and vomiting (all grades). For the FOLFOX network, cetuximab plus FOLFOX was worse than FOLFOX alone for grade 3–4 pulmonary embolism and skin reactions. Compared with panitumumab plus FOLFOX, cetuximab plus FOLFOX was worse for grade 3–4 skin reactions.
| Comparison | OS,b HR [95% CrI; Probability(better)] | PFS,b HR [95% CrI; Probability(better)] | ORR, OR [95% CrI; Probability(better)] |
|---|---|---|---|
| CET + FOLFIRI vs. | |||
| FOLFIRI | 0.69 (0.54 to 0.88; > 99%) | 0.56 (0.41 to 0.76; > 99%) | 3.14 (2.07 to 4.85; > 99%) |
| BEV + FOLFIRI | 0.80 (0.64 to 1.01; 97%) | 0.98 (0.81 to 1.19; 58%) | 1.29 (0.83 to 2.00; 87%) |
| FOLFOX | 0.96 (0.61 to 1.52; 56%) | 0.95 (0.61 to 1.47; 60%) | NAc |
| CET + FOLFOX | 0.98 (0.73 to 1.31; 56%) | 1.04 (0.81 to 1.35; 37%) | NAc |
| PAN + FOLFOX | 1.26 (0.80 to 1.99; 16%) | 1.39 (0.92 to 2.11; 6%) | NAc |
| BEV + FOLFOX | 0.83 (0.60 to 1.13; 88%) | 1.08 (0.85 to 1.39; 26%) | NAc |
| CET + FOLFOX vs. | |||
| FOLFOX | 0.99 (0.67 to 1.45; 53%) | 0.91 (0.61 to 1.36; 68%) | NAc |
| PAN + FOLFOX | 1.29 (0.87 to 1.91; 10%) | 1.33 (0.91 to 1.95; 7%) | NAc |
| BEV + FOLFOX | 0.85 (0.64 to 1.12; 88%) | 1.04 (0.84 to 1.259; 37%) | NAc |
| FOLFIRI | 0.71 (0.48 to 1.04; 96%) | 0.54 (0.36 to 0.80; > 99%) | NAc |
| BEV + FOLFIRI | 0.82 (0.61 to 1.11; 90%) | 0.94 (0.72 to 1.22; 68%) | NAc |
| CET + chemotherapyd vs. | |||
| Chemotherapyd | 0.76 (0.62 to 0.94; > 99%) | 0.67 (0.53 to 0.85; > 99%) | – |
| PAN + chemotherapyd | 1.02 (0.79 to 1.32; 43%) | 1.05 (0.80 to 1.37; 38%) | – |
| BEV + chemotherapyd | 0.79 (0.67 to 0.94; > 99%) | 0.98 (0.85 to 1.13; 61%) | – |
The following limitations of the NMA were noted: (1) because of the retrospective nature of the RAS analysis, for some studies there was a low number of samples available for analysis, reducing the power of the studies to show statistical significance and (2) limited data were available on safety for the CALGB-80405 trial,101 resulting in many of the indirect comparison analyses having very wide CIs and making interpretation of the indirect comparison difficult.
Comparison with the Assessment Group’s network meta-analysis
Of the studies included in the NMA, only the CALGB-80405 trial101 was not included in the NMA conducted by the Assessment Group. The CALGB-80405 trial compared cetuximab plus FOLFOX or FOLFIRI with bevacizumab plus FOLFOX or FOLFIRI; however, participants were randomised only to the cetuximab or bevacizumab component of the treatment, with the background chemotherapy (FOLFOX or FOLFIRI) chosen at the discretion of physicians. In addition, data from the CALGB-80405 trial are currently available only in abstract form. For these reasons this study was excluded from the Assessment Group’s systematic review and NMA. No trials were identified analysing the effectiveness of panitumumab plus FOLFIRI compared with any of the comparators specified in the NICE scope. 14
Using the CALGB-80405 trial101 enabled Merck Serono to construct a complete network for the outcomes of PFS and OS. The company conducted two analyses. One analysis used data from participants in the trial according to chemotherapy received; however, in this approach randomisation is broken and this could introduce bias into the analysis. The second analysis was a sensitivity analysis in which the results for FOLFOX and FOLFIRI were pooled as generic chemotherapy, based on the assumption that there was little difference between FOLFOX and FOLFIRI in terms of effectiveness, as reported in the study by Colucci et al. 103 The analysis of ORR and safety outcomes required two separate networks (one for FOLFOX and one for FOLFIRI). The Assessment Group’s NMA used two separate networks (FOLFOX and FOLFIRI) for the analysis of all outcomes in the RAS WT population, as none of the included studies provided evidence to link the two networks.
Assumptions regarding the similarity of the trials in terms of study and population characteristics were considered by the Assessment Group to be appropriate.
The absence of reported data for the PRIME44 and PEAK29 trials meant that analysis of ORR and analysis of all-grade AEs could not be conducted for the FOLFOX network. The Assessment Group, however, had access to unpublished data from the PRIME and PEAK trials and was able to analyse safety outcomes for any grade 3/4 AEs and serious AEs as well as grade 3–4 AEs by type, occurring in ≥ 5% participants in either treatment arm. The Assessment Group also conducted NMA for the outcome of resection rates and also for the subgroup of patients with liver metastases at baseline.
The methods used in Merck Serono’s NMA were in line with guidance from the NICE Decision Support Unit. 66
Despite the slight differences in approach between the Merck Serono NMA and the Assessment Group’s NMA, the overall results were similar, with both analyses subject to significant uncertainty.
Chapter 4 Assessment of cost-effectiveness
The cost-effectiveness of cetuximab and panitumumab for people with previously untreated RAS WT mCRC was assessed by conducting a systematic review of published research evidence.
Objectives
The objectives of this systematic review were to:
-
gain insights into the key drivers of cost-effectiveness in this disease area
-
obtain an overview of the alternative modelling approaches that have been adopted in this disease and treatment area
-
provide a summary of the findings of previous relevant cost–utility, cost-effectiveness and cost–benefit studies generalisable to the UK.
Methods
Study identification
The search strategy for the economic review included the following search methods:
-
searching of bibliographic and ongoing trials databases
-
searching of conference proceedings
-
scrutiny of the bibliographies of retrieved papers and company submissions.
The following databases were searched for economic studies: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid), NHS Economic Evaluation Database (NHS EED) (via The Cochrane Library), EconLit (EBSCOhost) and Web of Science (Thomson Reuters).
A supplementary search for health utilities was run in the following databases: MEDLINE (Ovid), MEDLINE In-Process & Other Non-Indexed Citations (Ovid), EMBASE (Ovid), PsycINFO (Ovid), Web of Science (Thomson Reuters) and School of Health and Related Research Health Utilities Database.
The searches were developed and run by an information specialist (SB) in January 2015. Search filters were used to limit the searches to economic or health utility studies as appropriate, and searches were limited to English-language studies when possible. No date limits were used. An updated search was carried out on 27 April 2015. No papers or abstracts published after this date were included in the review. Ongoing trials databases were searched by a reviewer in March 2015. The search strategies for each database are detailed in Appendix 1.
The database search results were exported to and deduplicated using EndNote X7. Deduplication was also performed using manual checking. After the reviewer had completed the screening process, the bibliographies of included papers were scrutinised for further potentially relevant studies. The manufacturers’ submissions were assessed for unpublished data.
Titles and abstracts returned by the search strategy were examined by one researcher (NH) and screened for possible inclusion. Full texts of potentially relevant studies were ordered. Full publications were assessed by the same reviewer (NH) for inclusion or exclusion against prespecified criteria.
Eligibility criteria
Inclusion and exclusion criteria were identical to those in the clinical effectiveness systematic review (see Chapter 3, Eligibility criteria), with the following exceptions (as specified in the appraisal protocol):
-
non-randomised studies were included (e.g. decision model-based analyses or analyses of patient-level cost and effectiveness data alongside observational studies)
-
full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost–consequence analyses were included (economic evaluations that reported only average cost-effectiveness ratios were included only if the incremental ratios could be easily calculated from the published data)
-
studies that measured costs but not health benefits were excluded, except for stand-alone cost analyses from the perspective of the UK NHS.
Data extraction
Study characteristics and results were abstracted by one reviewer (NH). In addition, parameters that could be used in the construction of an independent economic model were identified and noted.
The evidence base was assessed using narrative synthesis supported by summary data extraction tables.
Critical appraisal
Selected studies were quality assessed by one reviewer (NH) using the checklist developed by Evers et al. 104 Studies were marked as ‘no’ when they did not meet a criterion or when there was insufficient evidence to assess the criterion.
Studies based on decision models were further quality assessed using the checklist developed by Philips et al. 105
Results
Figure 7 shows the study flow diagram for this update review. The electronic database search for cost-effectiveness evidence identified 1979 records after deduplication. After screening by title and abstract, 1955 studies were excluded and 24 studies were identified for full-text screening; five of these were conference abstracts and one full-text article could not be retrieved. 106 In total, therefore, 19 full texts were retrieved and assessed for eligibility. Of the five conference abstracts, one was a duplicate and one was a duplicate of a full paper.
FIGURE 7.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for cost-effectiveness studies.

Of the 19 full texts assessed for eligibility, one was deemed to meet the eligibility criteria. 107 This study and the two abstracts for which posters were available108,109 were assessed in full. The poster for the remaining abstract could not be identified. 110 This study could therefore not be assessed in full, but the summary information is presented here.
Characteristics of identified cost-effectiveness studies
Details of the included studies are provided in Tables 60 and 61. Theses tables show that none of the included studies compared both cetuximab and panitumumab. The comparator arms were either bevacizumab in combination chemotherapy agents or chemotherapy alone. The range of chemotherapies differed across studies. One study108 was based in the UK but used a perspective of the Scottish NHS. This study considered only cetuximab in combination with chemotherapy (FOLFOX and FOLFIRI).
| First author, year | Setting, perspective | Population | Study purpose | Study approach | Comparators |
|---|---|---|---|---|---|
| Graham 2014107 | French health collective perspective | Adults aged ≥ 18 years with RAS WT mCRC | Cost-effectiveness of first-line PAN + FOLFOX compared with BEV + FOLFOX | Semi-Markov decision model; lifetime horizon (≤ 20 years), 2-week cycle length | PAN + FOLFOX; BEV + FOLFOX |
| Jarrett 2014108 | Scottish NHS | RAS WT mCRC patients | Cost-effectiveness of first-line CET in combination with chemotherapy vs. currently available treatments | Markov cohort decision model; lifetime horizon (10 years), 1-month cycles | CET + FOLFOX/FOLFIRI; FOLFOX/FOLFIRI alone |
| Kourlaba 2014110 | Greek health-care perspective | RAS WT mCRC patients | Cost-effectiveness of first-line PAN + FOLFOX vs. BEV + FOLFOX | Semi-Markov decision model | PAN + FOLFOX; BEV + FOLFOX |
| Ortendahl 2014109 | US payer | US adults with previously untreated RAS WT mCRC | Cost-effectiveness of first-line CET + FOLFIRI vs. BEV + FOLFIRI | Markov cohort decision model; lifetime horizon | CET+FOLFIRI; BEV+FOLFIRI |
| First author, year | Outcomes measured | Discount rate | Base-case results | Sensitivity analysis approach | Main sensitivity analysis results |
|---|---|---|---|---|---|
| Graham 2014107 | Costs, LYs, QALYs; ICERs: €/LYG, €/QALY gained | 4.0% costs and benefits | PAN + FOLFOX: 3.58 LYs, 2.68 QALYs, €97,203; BEV + FOLFOX: 2.73 LYs, 2.05 QALYs, €74,440 ICERs vs. BEV + FOLFOX: €26,918/LYG, €36,577/QALY gained |
Scenario analysis, one-way sensitivity analysis and PSA | Most notable scenario: all patients receive best supportive care after first-line treatment (ICER €50,390/QALY gained). One-way sensitivity analysis: model most sensitive to drug acquisition costs, best supportive care costs and costs of subsequent treatments. PSA: PAN + FOLFOX most likely to be cost-effective at WTP threshold of €40,000 |
| Jarrett 2014108 | Costs, LYs, QALYs; ICERs: £/LYG, £/QALY gained | NR | CET + FOLFIRI: 1.79 LYs, 1.30 QALYs, £41,015; FOLFIRI: 1.45 LYs, 1.05 QALYs, £28,301 | Scenario analysis, one-way sensitivity analysis | Scenario analysis: no vial sharing increased ICERs to £58,220 (FOLFIRI) and £56,520 (FOLFOX) per QALY gained. One-way sensitivity analysis: model sensitive to treatment duration, body surface area, progression HR and proportion referred for curative resection |
| ICERs vs. FOLFIRI: £39,631/LYG, £52,802/QALY gained | |||||
| CET + FOLFOX: 1.81 LYs, 1.32 QALYs, £39,612; FOLFOX: 1.50 LYs, 1.08 QALYs, £27,685 | |||||
| ICERs vs. FOLFOX: £38,936/LYG, £50,894/QALY gained | |||||
| Kourlaba 2014110 | Costs, LYs, QALYs; ICERs: €/QALY gained | NR | PAN + FOLFOX vs. BEV + FOLFOX: incremental LYs 0.87, QALYs 0.65, costs €22,464 | PSA | PSA: PAN + FOLFOX 81.5% likely to be cost-effective at WTP threshold of €51,000 per QALY gained |
| ICER vs. BEV + FOLFOX: €34,644/QALY gained | |||||
| Ortendahl 2014109 | Costs, LYs, QALYs; ICERs: $/LYG, $/QALY gained | NR | CET + FOLFIRI: 4.04 LYs, 3.11 QALYs, $305,727; BEV + FOLFIRI: 3.17 LYs, 2.43 QALYs, $238,255 | NR for RAS WT subgroup | NR for RAS WT subgroup |
| ICERs vs. BEV + FOLFIRI: $77,380/LYG, $99,636/QALY gained |
All studies used Markov or semi-Markov models and included resection and subsequent lines of treatment as health states, although the overall number of health states varied.
Jarrett et al. 108 reported the smallest estimate of life-years gained, which may be a consequence of the shorter time horizon of 10 years used in the model, compared with 20 years in the study by Graham et al. 107 and non-specified ‘lifetimes’ in the other studies.
The following sections report the methods and results for the four included studies. As bevacizumab is no longer available through the CDF, the focus is on those studies that report other comparator treatments.
Jarrett et al.108
In this study the authors based the model population on the RAS WT subset of patients who were retrospectively identified in the CRYSTAL and OPUS trials of cetuximab in combination with FOLFOX4 (or FOLFIRI) compared with FOLFOX4 (or FOLFIRI) alone. Further details of these studies can be found in Chapter 3. The authors used a Markov cohort model with five states to conduct a cost–utility analysis of cetuximab plus FOLFOX4 compared with FOLFOX4 alone and cetuximab plus FOLFIRI compared with FOLFIRI alone, from the Scottish NHS perspective.
The model included states such as first line (progression free), second- and third-line progressed disease states, post-curative resection and death. PFS was based on parametric survival curves estimated using the CRYSTAL trial data, using Weibull distributions. Resection transition probabilities were based on the CRYSTAL trial and death post resection was based on trial OS data. Transition probabilities for subsequent treatment were based on a study by Tournigand et al. 111 Transition to death following third-line therapy was based on a study by Jonker et al. 112
Unit cost data were based on Scottish sources or UK national sources when Scottish-specific sources were not available. Resource use for post resection was taken from Adam et al. 113 and validated by a clinical expert in Scotland. The full reference for the study by Adam et al. 113 was not reported is not reported. Other resource use was based on a systematic literature review.
Utilities were based on a systematic literature review. The sources were identified through the Scottish Medicines Consortium (SMC) report114 of this study as Bennett et al. ,115 Wang et al. 116 (both also identified by our review) and Petrou and Hockley117 [not included in our review as this was a validation study of the EQ-5D and Short Form questionnaire-6 Dimensions (SF-6D) for the general population].
In this study, cetuximab plus FOLFOX4 resulted in 1.81 life-years [1.32 quality-adjusted life-years (QALYs)], whereas FOLFOX alone resulted in 1.50 life-years (1.08 QALYs). Similarly, cetuximab plus FOLFIRI resulted in 1.79 life-years (1.30 QALYs), whereas FOLFIRI alone resulted in 1.45 life-years (1.05 QALYs). Cetuximab in combination with chemotherapy was approximately £12,000 more expensive than chemotherapy alone. This led to an incremental cost-effectiveness ratio (ICER) of > £50,000 per QALY gained for cetuximab plus chemotherapy compared with chemotherapy alone.
A scenario analysis in which full vial wastage was assumed, which may be closer to the situation in general practice, increased the ICER by > £5000. Sensitivity analyses showed that the model was sensitive to the cost and effect of treatment with cetuximab; duration of treatment, body surface area, progression HR and the proportion of the cohort referred for curative resection had large impacts on the ICER.
The poster claims that this analysis shows that cetuximab plus chemotherapy is a cost-effective treatment, especially in light of meeting the SMC’s end-of-life criteria. According to the SMC report,114 cetuximab was accepted for this patient population, but only after a Patient Access Scheme (PAS) was applied to demonstrate cost-effectiveness. A further analysis of cetuximab plus FOLFOX4 compared with CAPOX (XELOX) was requested by the SMC, assuming that XELOX and FOLFOX had similar efficacy, which resulted in an ICER of > £70,000 per QALY gained (without the PAS). 118
This study is the most relevant to our review as it is UK based and compares the intervention with chemotherapy agents available on the NHS. It does not include bevacizumab as a comparator but, with bevacizumab no longer available through the CDF for this indication, this analysis may still be relevant. However, it does not assess panitumumab in a similar context and therefore does not address the entire scope of our review.
Graham et al.107
In this study the authors based their model population on the RAS WT subset of patients who were retrospectively identified in the PEAK trial. 29 In summary, these were patients aged at least 18 years who were diagnosed with previously untreated RAS WT mCRC. Further details of the PEAK population can be found in the clinical effectiveness review (see Chapter 3). The authors used a semi-Markov model with seven states to conduct a cost–utility analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6, from the perspective of the French health collective.
The model included states such as progression-free and progressive disease with subsequent therapy or best supportive care (BSC), as well as separate disease states for attempted resection and post resection. PFS and OS were based on parametric survival curves estimated using the PEAK trial patient-level data, using Weibull distributions. These were converted to transition probabilities for disease progression and death. Resection transition probabilities were based on the PEAK trial and a study by Adam et al. 113 Transition probabilities for subsequent treatment were also based on the PEAK trial.
Drug acquisition costs were estimated using French Health National Insurance unit costs, and dose intensity and frequency were calculated from PEAK trial data. Other costs, including the costs of AEs, RAS mutation testing, drug administration, chemotherapy, physician visits, diagnostic tests, resection, subsequent treatment and BSC, were taken from the literature and French health-care cost sources. Costs were reported in 2013 euros.
Utilities were based on the EQ-5D responses from the RAS WT patients in the PRIME trial. For subsequent lines of treatment, utilities for the RAS WT population were assumed to be similar to those for the KRAS WT population. Therefore, EQ-5D responses from trials of KRAS WT mCRC patients on subsequent lines of treatment were used to estimate utilities for subsequent lines of treatment. The EQ-5D responses were converted to utilities using the Dolan algorithm,119 which were valued using UK responses.
Costs and benefits were discounted at 4% per annum, the suggested discount rate in France.
In this study panitumumab plus mFOLFOX6 resulted in 3.58 life-years (2.68 QALYs), whereas bevacizumab plus mFOLFOX6 resulted in 2.73 life-years (2.05 QALYs). Costs were also higher for panitumumab plus mFOLFOX6 at €97,203, compared with €74,440 for bevacizumab plus mFOLFOX6. This was because of the higher drug costs associated with panitumumab. This resulted in an ICER of €36,577 per QALY gained for panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6.
The authors conducted multiple scenario analyses, univariate sensitivity analyses and a probabilistic sensitivity analysis. The most notable scenario analysis, in which no active subsequent treatments were assumed (all patients received BSC), raised the ICER to > €50,000 per QALY gained. The probabilistic sensitivity analysis showed that panitumumab plus mFOLFOX6 was more likely to be cost-effective than bevacizumab plus mFOLFOX6 at a willingness-to-pay threshold of €40,000.
Kourlaba et al.110
The results of this study were available only as a conference abstract. In this study the authors based their model population on the RAS WT subset of patients who were retrospectively identified in the PEAK trial29 and used a previously existing model consisting of seven health states. The authors used this Markov model to conduct a cost–utility analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6, from the perspective of the Greek health-care setting. Given the description, we believe this model to be the same as that reported in Graham et al. 107
Panitumumab plus mFOLFOX6 led to an increase in QALYs of 0.65 compared with bevacizumab plus mFOLFOX6, and a cost increase of €22,464. This gave an ICER of €34,644 per QALY gained compared with bevacizumab plus mFOLFOX6.
Ortendahl et al.109
This study was published as a poster in 2014. In this study the authors based their model population on the KRAS WT subset of patients who were retrospectively identified in the FIRE-3 trial of cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI. 28 However, as a scenario analysis, the RAS WT subset was identified and assessed. The authors used a Markov cohort model with four states to conduct a cost–utility analysis of cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI from a US perspective.
The model included states such as first line (progression free), second-line progressed disease states, post-curative resection and death. OS was based on FIRE-3 trial data, using Weibull distributions. Resection transition probabilities and transition probabilities for subsequent treatment were also based on FIRE-3 trial data.
Unit costs were reported in 2013 US dollars, but sources were not given. Utilities were based on published literature.
In this study, cetuximab plus FOLFIRI resulted in 4.04 life-years (3.11 QALYs), whereas bevacizumab plus FOLFIRI resulted in 3.17 life-years (2.43 QALYs). Cetuximab plus FOLFIRI was calculated to cost > $67,000 more than bevacizumab plus FOLFIRI. This led to an ICER of > $99,000 per QALY gained for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI.
As this was only a scenario analysis, the sensitivity analyses were applied to the base case and therefore the exact results are not applicable. However, OS and treatment costs appeared to be the most influential parameters in the base case and this is likely to carry over into the scenario analysis.
Quality of identified cost–utility studies
The study by Jarrett et al. 108 is currently available only as a poster presentation, with further information available through the SMC report on this assessment. 114 As such, it lacks some details, primarily justification for modelling techniques, which may have been presented in a full paper. It was also funded by Merck Serono and so was not an independent assessment. The assessment does not include all comparators relevant to our review and this was a criticism raised by the SMC when it requested an additional comparison be carried out between cetuximab plus FOLFOX4 and XELOX (referred to as CAPOX), as this was believed to be in regular use in the Scottish NHS. However, this is the only study that was conducted in the UK and it does include two relevant comparators, FOLFOX and FOLFIRI.
The study by Graham et al. 107 is currently the only study that is published in full that assesses the cost-effectiveness of panitumumab. However, the only comparator is bevacizumab in combination with chemotherapy, which has not been recommended by NICE and is no longer available through the CDF for this indication. Furthermore, it is not a UK-based study, making the results less generalisable to the NHS. This means that the cost-effectiveness estimates provide limited information for this appraisal. The study was sponsored by Amgen and so this study was not an independent assessment. However, the model is generally well reported and relevant to answering the objective set in the study. The level of reporting of methods for validating the model (e.g. sensitivity analyses) was low, as demonstrated in Tables 62 and 63.
| Criteria | Study | ||
|---|---|---|---|
| Graham 2014107 | Jarrett 2014108 | Ortendahl 2014109 | |
| 1. Is the study population clearly described? | Yes | Yes | Yes |
| 2. Are competing alternatives clearly described? | Yes | Yes | Yes |
| 3. Is a well-defined research question posed in answerable form? | Yes | Yes | Yes |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Yes | Yes |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes | Yes |
| 6. Is the actual perspective chosen appropriate? | Yes | Yes | Yes |
| 7. Are all important and relevant costs for each alternative identified? | Yes | No | No |
| 8. Are all costs measured appropriately in physical units? | Yes | Yes | Yes |
| 9. Are costs valued appropriately? | Yes | Unclear | Unclear |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | Yes | Yes |
| 11. Are all outcomes measured appropriately? | Yes | Yes | Yes |
| 12. Are outcomes valued appropriately? | Yes | Yes | Yes |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Yes | Yes |
| 14. Are all future costs and outcomes discounted appropriately? | Yes | NR | NR |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | No | No | No |
| 16. Do the conclusions follow from the data reported? | Yes | Yes | Yes |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | No | Yes |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | No | No | No |
| 19. Are ethical and distributional issues discussed appropriately? | No | Yes | No |
| Criteria | Study | ||
|---|---|---|---|
| Graham 2014107 | Jarrett 2014108 | Ortendahl 2014109 | |
| Structure (S) | |||
| S1: Statement of decision problem/objective | Yes | Yes | Yes |
| S2: Statement of scope/perspective | Yes | Yes | Yes |
| S3: Rationale for structure | Yes | No | No |
| S4: Structural assumptions | No | No | No |
| S5: Strategies/comparators | Yes | Yes | Yes |
| S6: Model type | Yes | Yes | Yes |
| S7: Time horizon | Yes | Yes | Yes |
| S8: Disease states/pathways | Yes | Yes | Yes |
| S9: Cycle length | Yes | Yes | Yes |
| Data (D) | |||
| D1: Data identification | No | No | No |
| D2: Pre-model data analysis | No | No | No |
| D2a: Baseline data | No | No | No |
| D2b: Treatment effects | No | No | No |
| D2c: Quality-of-life weights (utilities) | Yes | No | No |
| D3: Data incorporation | No | No | No |
| D4: Assessment of uncertainty | No | No | No |
| D4a: Methodological | Yes | No | No |
| D4b: Structural | Yes | No | No |
| D4c: Heterogeneity | No | No | No |
| D4d: Parameter | No | No | No |
| Consistency (C) | |||
| C1: Internal consistency | No | No | No |
| C2: External consistency | Yes | No | No |
The RAS WT analysis by Ortendahl et al. 109 was conducted only as a scenario analysis and so the quality assessment is based on the reporting of the base-case model. As this study is currently presented only in poster form, there are limits to the reporting, including cost sources and justification of modelling methods. Given this limitation, and the fact that the analysis of interest is reported only as a scenario analysis, the quality assessment is of limited use.
As the study by Kourlaba et al. 110 is currently reported only in abstract form and no further details could be found, we did not quality assess this study.
All of the studies appeared to feature contributions from or were funded by manufacturers and so they all have the potential for bias.
Discussion
There is limited knowledge to be gained from the studies identified in this review. None of the studies included all of the comparators relevant to the NHS and only one is relevant to a UK setting. 108 Further details of this study were identified by accessing the SMC-associated documents,114,118 but this study is still limited in its reporting and does not include panitumumab as a comparator.
The quality of the reporting was mixed, primarily because most studies have been published only in abstract form and presented at conferences. This also suggests the potential for these results to change before a full journal publication. Although posters were sought for those abstracts presented at conferences, it is important to remember that the posters themselves are not subject to peer review and so they have not been through a level of quality assessment prior to this review. The only study that has been fully peer reviewed and published is that by Graham et al. ,107 which is not a UK-based study and whose main comparator, bevacizumab in combination with chemotherapy, is no longer funded by the CDF and is therefore not the focus of our research.
Strengths and limitations
This review was conducted by an independent group using a systematic approach to identify and review studies. Update searching also allowed for the most recent evidence to be identified. Strict review criteria meant that only papers relevant to the decision problem were identified, giving a clear demonstration of the limited evidence currently available.
The review also identified relevant posters associated with the abstracts identified at the title and abstract stage, which aided in informing this review in greater detail.
As only one reviewer was involved at both the title and abstract screening stage, there is the potential for studies to have been missed that may have been identified by a second reviewer. Furthermore, the full text of one study could not be retrieved and assessed at a full-text level. 106 However, given the clear inclusion/exclusion criteria we do not believe that any relevant studies were missed at the title and abstract screening stage and comparison with similar reviews, such as that provided in the Merck Serono submission, do not indicate that any studies were missed, nor that the irretrievable study would have been included at the full-text stage.
Conclusions
It was not stated in the abstract by Jarrett et al. ,108 but the associated SMC documents report that a PAS was required for cetuximab to be considered a cost-effective treatment in Scotland. However, this may not be indicative of the NHS in England and Wales and, given the limited reporting of all studies, the evidence is not conclusive enough at this stage to state whether or not cetuximab and/or panitumumab are cost-effective first-line treatments for RAS WT mCRC patients. Therefore, we believe that our development of a de novo model is both justified and necessary to answer the decision problem described in this report.
Chapter 5 Economic evaluations submitted by manufacturers
In this chapter we present and critique the economic evidence submitted by Merck Serono (15 June 2015, version 2). No economic evidence was submitted by Amgen.
Economic evaluation submitted by Merck Serono
Merck Serono submitted both a systematic review of economic evidence and an economic model.
Cost-effectiveness review
Merck Serono carried out literature searches for cost-effectiveness evidence in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and EconLit (through Ovid), and NHS EED and Health Economic Evaluations Database (HEED) (via The Cochrane Library) (Merck Serono submission, section 3.2.1, p. 16). The searches combined free-text and index terms for relevant cancers, free-text terms for cetuximab and free-text and index terms for relevant cost-effectiveness measurements and study types (Merck Serono submission, appendix F, pp. 52–63). No language or date limits were applied.
The literature searches used an appropriate range of databases for the topic. The choice of free-text and index terms was also appropriate and the searches had an appropriate balance of sensitivity and specificity. The search strategies were reproduced in the appendices, including the number of hits retrieved per search (Merck Serono submission, appendix F, pp. 58–63). The dates searched were reported elsewhere in the submission (Merck Serono submission, section 3.2.1, p. 16).
There is a small discrepancy between the list of databases in section 3.2.1 of the Merck Serono submission and the search strategies reproduced in appendix F. Section 3.2.1 reports that the databases HEED and NHS EED were searched, but no HEED search strategy is provided in the appendix; two NHS EED search strategies are provided, which is probably a typographical error rather than a methodological error. There is also an error in the EMBASE search strategy: line 8 reads ‘6 AND 7’ but should read ‘5 AND 7’. This error means that the search terms for cetuximab on line 5 are not included in the final results. However, the search is not adversely affected as the results consist of records related to mCRC and cost-effectiveness; this is a broader set of records than would have been retrieved by combining the results with terms for cetuximab using the AND Boolean operator.
Merck Serono also searched for literature containing HRQoL utility values related to mCRC and cetuximab (Merck Serono submission, section 3.2.1, pp. 18–19). These searches were carried out in MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations and EMBASE (through Ovid). The choice of databases and search terms is appropriate for the topic, as is the balance of sensitivity and specificity. The search strategies are reproduced in the appendices with appropriate detail and without errors (Merck Serono submission, appendix G, pp. 64–7).
Merck Serono states that its review had two aims: (1) to identify cost-effectiveness evaluations of cetuximab in KRAS/RAS WT populations and (2) to identify UK-based costs and resource use. In general, its PICOS (population, intervention, comparator, outcome, study design) inclusion/exclusion criteria were appropriate and corresponded to the scope of the project. Detailed comments are presented in Table 64.
| Criteria | Review stage | Inclusion | Exclusion | PenTAG comments |
|---|---|---|---|---|
| Population | Abstract/full text | Cost-effectiveness evaluations on cetuximab in (K)RAS WT mCRC in all countries of interest; patients with KRAS WT mCRC receiving first-line therapy for their metastatic disease in the UK; patients with RAS WT mCRC receiving first-line therapy for their metastatic disease in the UK; patients with mCRC in the UK | Studies conducted outside the UK [except for cost-effectiveness studies in (K)RAS WT mCRC with cetuximab]; non-mCRC studies | These inclusion criteria do not restrict to first-line treatment, so cost-effectiveness results and resource identification will be of limited use in this scenario. These inclusion criteria also excluded panitumumab studies when panitumumab is not compared with cetuximab. This fits Merck Serono’s aims but not those of the NICE scope. It is appropriate to limit studies identified for cost and resource use to the UK only |
| Intervention/treatments | Abstract/full text | Cetuximab in combination with FOLFOX or irinotecan-based chemotherapy; panitumumab in combination with FOLFOX | All other therapies that are not relevant to cetuximab | In line with the NICE scope |
| Comparator | Abstract/full text | No limitations | No limitations | This could include comparators not relevant to the NICE scope |
| Outcomes | Abstract selection | No selection on outcomes | Appropriate | |
| Full-text selection | Utilities/health states; costs (UK); resource use (UK); cost–utility, cost-effectiveness and budget impact outcomes; model structure and sources; cost-effectiveness results (cost per LY; cost per QALY) in the target population cetuximab in (K)RAS WT mCRC (not limited to UK) | Costs other than UK costs | Appropriate for the aim of the review | |
| Study design | Abstract/full text | Economic evaluations (cost-effectiveness, cost–utility and budget impact analyses); HTA submissions and reports including economic data; cost-of-illness studies; utility studies | Pharmacokinetic studies, genomic studies, methodology/protocols, case reports/studies, editorials/letters etc., conference proceedings published before 2013, studies lasting for < 2 weeks | Appropriate for the aim of the review |
Our review had stricter population inclusion criteria, in line with the NICE scope. 14 Of the studies included by Merck Serono, we also identified two. 108,109 The remaining studies identified by Merck Serono were excluded from our review on the basis of population (either not first line or not RAS WT). Merck Serono’s restriction to cetuximab studies also contradicts the NICE scope, which includes panitumumab plus chemotherapy as an intervention of interest.
Although we chose a narrower population for our economic review, we agree with the broader population criteria that Merck Serono used for the HRQoL search. However, it appears that these wider population criteria were not necessarily implemented in practice, as 10 studies were excluded as not being ‘not specific to RAS WT mCRC type patients’ (Merck Serono submission, section 3.4.1, p. 59). The utilities studies that Merck Serono used to inform its model seem, in general, to be appropriate.
De novo economic evaluation
As well as a review of economic studies, Merck provided an executable economic model. We received several iterations of this model, which we have summarised below.
History of submission
We received Merck Serono’s original submission on 6 May 2015. We requested an explanation of the discrepancies between the model and the report, as well as how to reproduce the liver metastases subgroup analysis within the model.
Merck Serono submitted a new executable model and report on 15 June 2015, which had one significant change. Merck Serono claimed that it had detected another error of its own in the cost of cetuximab and it adjusted this value accordingly. Some other discrepancies between this model and the previous version were identified, but checks revealed that these were unlikely to have a big impact on the cost-effectiveness results: implementing the changes that we could identify into the original model gave very similar results to the new model (ICERs differed by < £3 per QALY). This also suggested that no major wiring errors had been introduced into this new model. As such, the model methods and results described in this section refer to the version of the model that we received on 15 June 2015.
Merck Serono also submitted an additional executable model for the liver metastases subgroup on 16 June 2015. On request, Merck Serono submitted on 26 June 2015 a list of the parameters that had been altered in the ‘overall population model’ to create this subgroup analysis. The ICERs for this subgroup had again been updated.
Even with the list of parameters, we were unable to reconcile the overall population model and the liver-limited disease subgroup model. We also noted that OS had been hard-coded into this subgroup model, which we believe was in error, as this meant that survival did not alter when different interventions and comparators were selected.
As we could not reconcile this subgroup model with the model for the overall population, and as Merck Serono submitted its independent model for the liver metastases subgroup at a late stage in this HTA assessment, we have not critiqued the liver-limited disease subgroup model. We therefore present the results for this subgroup without comment.
Description of methods
Comparator treatments
Merck Serono considered the following three independent comparisons in its economic evaluation:
-
cetuximab plus FOLFOX compared with FOLFOX
-
cetuximab plus FOLFIRI compared with FOLFIRI
-
cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI.
Merck Serono stated (section 2.2.2, p. 44) that, ‘As there was significant uncertainty surrounding the results of the NMA, head-to-head trial data was preferred for use in the health economic model’. Although we believe that it is possible to perform a three-way comparison between cetuximab plus FOLFIRI, FOLFIRI and bevacizumab plus FOLFIRI, we believe that Merck Serono’s approach of performing the three independent comparisons is reasonable because:
-
bevacizumab plus FOLFIRI has been delisted from the CDF18 and, hence, is no longer a main comparator
-
we agree that there are no clinical data that allow the comparison of FOLFOX- and FOLFIRI-based treatments.
However, we note that Merck Serono did not include panitumumab plus FOLFOX as a comparator, even though the relevant RCT data are publicly available. 29,44
In its economic model, Merck Serono considered XELOX (also referred to as CAPOX) as a treatment in a scenario analysis, despite the lack of head-to-head data specific to RAS WT mCRC patients. Merck Serono assumed:
-
that the clinical effectiveness of XELOX, that is, the percentage of patients resected, PFS rates, mortality from PFS, incidences of AEs, is exactly the same as for FOLFOX
-
a higher mean per patient total cost of acquisition of XELOX than of FOLFOX (£8093 vs. £6416)
-
a slightly lower mean per patient total cost of administration of XELOX than of FOLFOX (£2296 vs. £2803).
Merck Serono justified the first assumption as follows:
In a Phase III trial by Cassidy et al. (Cassidy et al. , 2006,121 Cassidy et al. , 2007122) CAPOX was shown to be non-inferior to FOLFOX-4 as a first-line treatment for mCRC. Therefore the two regimens are expected to be equivalent in terms of efficacy and can thus be treated as equal in terms of outcomes. In addition, this assumption was validated by clinical experts (Merck Serono, 2015) who stated that the combinations of different forms of 5FU (differing infusion regimens and oral analogues) along with both FOLFIRI and FOLFOX have equivalent efficacy.
Merck Serono submission (section 3.7.3.1, p. 66)
We agree with Merck Serono that there are no trials that directly compare cetuximab-based treatment with XELOX. Our systematic review of the literature (see Chapter 3) also found no such trials comparing panitumumab-based treatment with XELOX.
Given the time constraints, we did not perform a full systematic search of the literature for clinical effectiveness evidence for XELOX compared with any other treatment in our base-case analysis. Instead, we report the findings of a review of XELOX compared with FOLFOX by Douillard et al. 123 This study found that several RCTs have compared continuous-infusion 5-fluorouracil/oxaliplatin with the oral fluoropyrimidine capecitabine plus oxaliplatin. In all of these trials, non-inferiority was demonstrated for the use of oral fluoropyrimidines for the predefined end points such as PFS, OS and response rate. However, the HRs and median TTP/PFS were almost always in favour of FOLFOX (Table 65).
| Trial | Number of patients | Median TTP/PFS (months) | PFS/TTP HR | |
|---|---|---|---|---|
| Continuous-infusion 5-fluorouracil-based treatment | Oral fluoropyrimidine-based treatment | |||
| NO16966 trial121 | 634 | FOLFOX4 7.7 | XELOX 7.3 | 0.96 (97.5% CI 0.8 to 1.16) |
| TREE-1 trial124 | 106 | mFOLFOX6 6.4 | CAPEOX 4.4 | NR |
| Ducreux et al.125 | 306 | FOLFOX6 9.3 | XELOX 8.8 | 1.00 (90% CI 0.82 to 1.22) |
| Díaz-Rubio et al.126 | 348 | FUOX 9.5 | XELOX 8.9 | 1.18 (95% CI 0.9 to 1.5) |
| Porschen et al.87 | NR | FUFOX 8.0 | CAPOX 7.1 | 1.17 (95% CI 0.96 to 1.43) |
| COFFEE trial127 | 322 | OXAFAFU 6.3 | OXXEL 6.2 | 1.06 (95% CI 0.81 to 1.35) |
These data give us an idea of the likely relative clinical effectiveness of CAPOX/XELOX and FOLFOX. However, it should be noted that these data do not relate specifically to patients with RAS WT mCRC but rather to both RAS WT and mutant patients.
Of course, there are several other parameters that could differ between CAPOX/XELOX and FOLFOX:
-
mean treatment duration
-
resection rates; however, it seems plausible that resection rates are correlated with PFS
-
incidences of AEs; however, given that we find that incidences of AEs have little impact on cost-effectiveness, we consider this to be a minor issue.
Given all these uncertainties, we believe that it is reasonable for Merck Serono to model XELOX as a comparator treatment in a scenario analysis, assuming differences in treatment acquisition and administration costs but equal clinical effectiveness to that of FOLFOX.
Merck Serono did not include tegafur/uracil as a comparator treatment, even though it is a comparator in the NICE scope. 14 Merck Serono stated that it withdrew this product from the market in the UK in 2013 and no other equivalent preparations are available in the UK (Merck Serono submission, p. 19). We agree that tegafur/uracil has been discontinued and our clinical advisor believes that it is unlikely to be used in the UK.
Merck Serono did not include capecitabine monotherapy as a comparator, even though it is a comparator in the NICE scope,14 as its expert advice indicated that it is typically used in elderly patients with poor performance status, as these patients would not generally be fit enough to receive biological agents in combination with chemotherapy (Merck Serono submission, p. 19). It also did not identify any studies that compare cetuximab plus chemotherapy with capecitabine in a RAS WT population (Merck Serono submission, section 3.2.3, table 22, p. 52).
Our clinical advisor agreed that capecitabine monotherapy and 5-fluorouracil plus folinic acid are not the preferred first-line treatments in mCRC patients. In general, single-agent fluoropyrimidine regimens (capecitabine or 5-fluorouracil plus folinic acid) would be used for patients who were unfit for combination therapy or who have overlapping comorbidities that make other agents problematic. We also did not identify any studies comparing cetuximab plus chemotherapy with capecitabine in a RAS WT population
Patient population and liver metastases subgroup
Merck Serono considered two patient populations, with a separate model for each group:
-
all first-line patients with RAS wild-type mCRC
-
a subgroup of these patients with metastases confined to the liver (liver metastases subgroup).
As discussed previously, we did not critique the liver metastases subgroup model.
Merck Serono claimed that the following parameters were unique to the liver metastases subgroup:
-
resection rates
-
PFS for unresected patients.
All other parameters were stated to be unchanged from those in the total population analysis.
Model structure
In common with us, in the base case Merck Serono did not use OS data from the RCTs of first-line drug treatments. Instead, the RCTs were used to estimate resection rates and PFS only for first-line treatment. OS was instead estimated as the sum of times on first-, second- and third-line treatments, allowing for mortality from each line.
Merck Serono’s model included five health states: first-line progression free, second-line progressive disease, third-line progressive disease, post resection and dead. Patients remain in first line until they move to either post resection or to further lines of treatment. Patients can die in any state.
The model used tunnel states to apply time-dependent transition probabilities to move patients between states.
Differences in clinical effectiveness between first-line drug treatments were represented by the differences between:
-
first-line PFS
-
resection rates
-
incidences of AEs.
The model cycle length was 1 month, which is appropriate. A model half-cycle correction was applied.
The model time horizon was 10 years, which we believe is far too short. The model time horizon should be sufficiently long that the vast majority of deaths are modelled. However, 10 years after resection, Merck Serono estimate that 12% of patients are still alive. Merck Serono’s model can deal with a time horizon of up to 20 years, at which time it estimates that 4% of patients are still alive. When we change the time horizon from 10 to 20 years, the ICERs for cetuximab plus FOLFOX compared with FOLFOX and cetuximab plus FOLFIRI compared with FOLFIRI both decrease because we now model more QALYs post resection and more patients receive a resection under the cetuximab plus FOLFOX regime than under the FOLFOX regime and under the cetuximab plus FOLFIRI regime than under the FOLFIRI regime.
However, as explained later (see Model parameters, Post-liver resection: progression-free survival and overall survival), we believe that their estimates of PFS and OS post resection are logically impossible after about 11 years, as they then estimate that PFS is greater than OS.
In our model, we used a time horizon of 30 years.
Future costs and benefits were discounted at 3.5% per annum and the perspective taken was that of the NHS and personal social services, in accordance with the NICE reference case. 128
Overall survival
As in our model, Merck Serono did not use OS data from the RCTs. Instead, life expectancy for all randomised patients was calculated separately for each treatment arm as:
The last quantity, life expectancy for unresected patients for each treatment arm was calculated as the sum of life expectancy on first, second and third lines of treatment, allowing for mortality from each line.
Model parameters
Resection of liver metastases is an important component of both our model and Merck Serono’s model, as cost-effectiveness is sensitive to it.
Merck Serono used the resection rates from the RCTs to estimate the rates for use in its model (Table 66).
| Treatment | All RAS WT patients |
|---|---|
| FOLFIRI network | |
| CET + FOLFIRI | 7.3% (Merck Serono data from the CRYSTAL trial129) |
| FOLFIRI | 2.1% (Merck Serono data from the CRYSTAL trial111) |
| BEV + FOLFIRI | 7.3% (no justification given) |
| FOLFOX network | |
| CET + FOLFOX | 7.3% (derivation explained in accompanying text) |
| FOLFOX | 2.1%111 |
Merck Serono did not discuss the derivation of their estimate of the rate of resection for cetuximab plus FOLFOX, 7.3%. We assumed that Merck Serono set the resection rate as equal to its rate for cetuximab plus FOLFIRI, which we believe is unreasonable. It estimated the rate of resection for FOLFOX (2.1%) from Tournigand et al. 111 This is substantially lower than our estimate (see Chapter 6, Model parameters, Resection rates). The study by Tournigand et al. 111 was of second-line treatment not restricted to RAS WT, whereas our estimate was taken from the first-line treatment of RAS WT patients; therefore, we prefer our value.
Merck Serono simulated liver resection at cycle 3 in its model. Notably, the timing of liver resection was not clearly stated in the submission. As detailed in table 20 (Merck Serono submission, section 3.2.2, p. 49), resection was modelled at cycle/month 4; however, in table 21 the submission states that at 3 months in its model some patients can be referred for curative-intent resection of liver metastases.
Merck Serono’s assumption regarding the timing of liver resection surgery was based on the study by Adam et al. ,113 as indicated in table 20 of the submission (section 3.2.2, p. 49). This assumption seems reasonable, based on advice from our clinical experts and the values used in TA176. 10
In its submission, Merck Serono stated that it assumed that all patients who underwent curative liver resection for initially unresectable colorectal liver metastases, turned resectable by systematic chemotherapy, and who are cured of the disease ‘remain in a progression free state until death and do not require second-line treatment’ (Merck Serono submission, section 3.2.2, p. 47). However, elsewhere in the submission and in the executable model there exists a progressive disease state, including treatment, for patients post liver resection.
Merck Serono model PFS and OS after liver resection surgery according to data from Adam et al. 113 We also used these data as we understand that they are the most appropriate data available. Further discussion of this study can be found in Chapter 6 (see Model parameters, Post liver resection: progression-free survival and overall survival).
Merck Serono fitted a log-logistic distribution to both PFS and OS post resection (Figure 8). Technically, these data are taken from rows 95 and 96 of Merck Serono’s worksheet ‘Survival models’. Importantly, Merck Serono did not explain its choice of distribution, or indeed how it estimated the curve fits.
FIGURE 8.
Merck Serono’s PFS and OS post-resection fit to the empirical data. Source: figure produced from data available in the Merck Serono submission (May 2015).
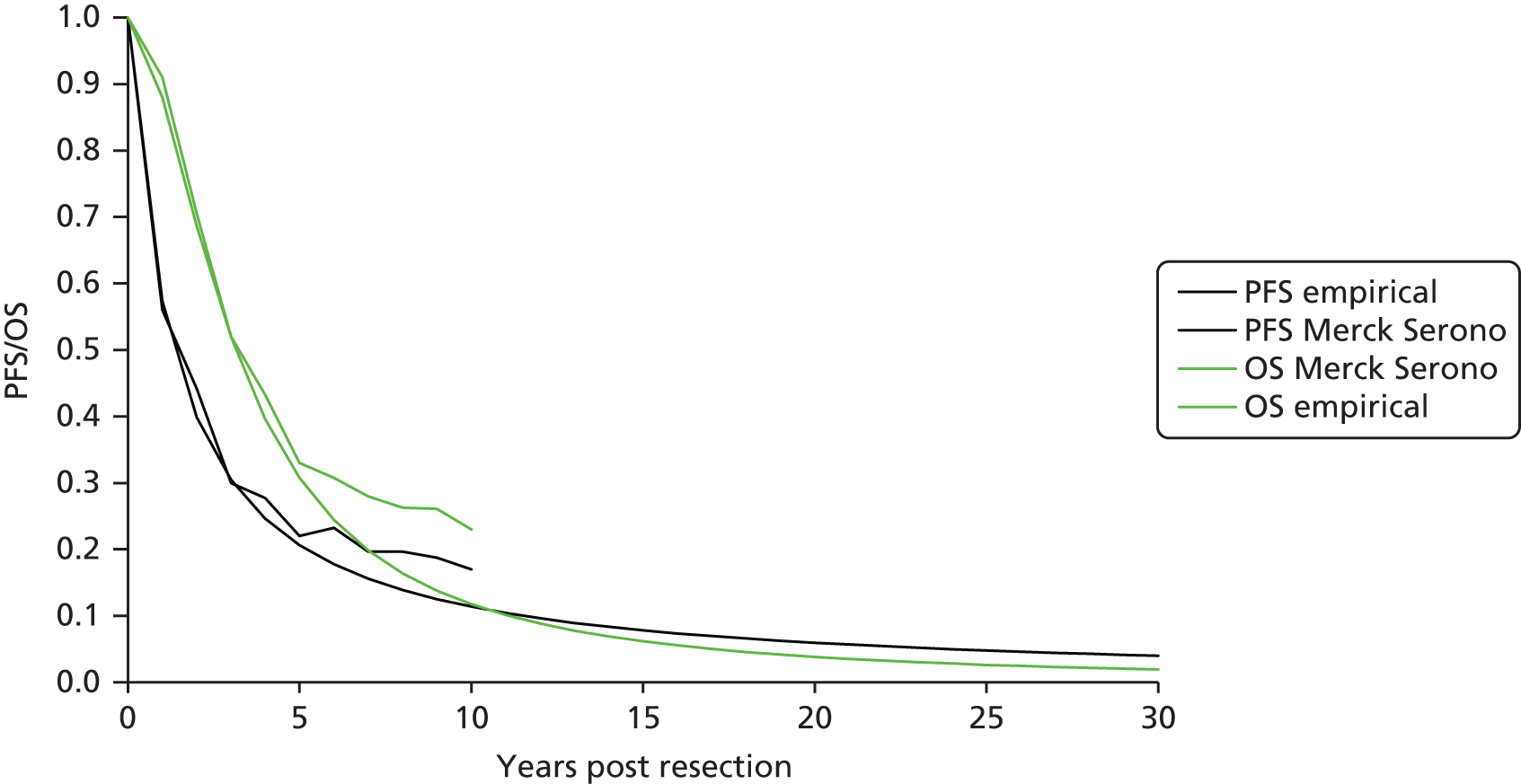
The fits appear reasonable up to end of study follow-up at 10 years, which is also the time horizon of Merck Serono’s model. However, after about 11 years, PFS in the Merck Serono model is greater than OS, which is clearly impossible. We believe that this renders the results from Merck Serono’s model for time horizons > 11 years incorrect.
In common with us, for those patients who had a successful resection, Merck Serono assumed that PFS and OS were independent of first-line treatment.
Based on its 10-year time horizon, which we believe is far too short, we calculate that Merck Serono estimates a mean PFS of 2.8 years and mean OS of 4.1 years.
Merck estimated first-line PFS for unresected patients directly from the pivotal RCTs: CRYSTAL,24 FIRE-328 and OPUS. 23 It compared pairs of treatments independently and did not perform simultaneous comparisons of multiple treatments. Therefore, unlike us, it did not perform indirect comparisons for first-line PFS for unresected patients.
Merck Serono estimated PFS for unresected patients from data for all patients (resected and unresected) in the RCTs. We believe that this is an important mistake. Given that it modelled PFS for resected patients separately, as described in the previous section, it effectively double counted PFS for resected patients. It overestimated PFS for unresected patients because PFS for resected patients (our estimate 4.5 years) is far greater than that for unresected patients (e.g. our estimate for cetuximab plus FOLFIRI 1.0 years).
In our analysis, as explained in the previous section, we also estimated PFS for resected patients from the study by Adam et al. 113 However, we estimated PFS for unresected patients using the RCT data for PFS for all patients and then subtracting PFS for resected patients (see Chapter 6, Model parameters, First-line progression-free survival: unresected patients).
Merck Serono’s choices of statistical distributions and estimates for mean PFS for first-line unresected patients are provided in Table 67.
| Treatment | Distribution | Mean PFS (months)a |
|---|---|---|
| CET + FOLFOX | Log-normal | 13.4 |
| FOLFOX | Log-normal | 9.0 |
| CET + FOLFIRI (vs. FOLFIRI) | Weibull | 12.5 |
| CET + FOLFIRI (vs. BEV + FOLFIRI) | Weibull | 12.8 |
| FOLFIRI | Weibull | 8.9 |
| BEV + FOLFIRI | Weibull | 10.8 |
We believe that the PFS curve fits; however, as already stated, we believe that these are overestimates of PFS for unresected patients.
All other things being equal, Merck Serono’s approach results in cetuximab plus FOLFOX/FOLFIRI appearing to be better value for money than we believe, given that a greater proportion of patients in the cetuximab plus FOLFOX/FOLFIRI arms compared with the FOLFOX/FOLFIRI arms are resected and that PFS for resected patients is substantially greater than that for unresected patients.
Merck Serono states in table 21 of the submission (section 3.2.2, p. 50) that the rate of postoperative death was set to 0%, based on data from the CRYSTAL trial. However, in the executable model Merck Serono assumes a probability of postoperative death of 1% for all treatment regimens. As Merck Serono used data from the study by Adam et al. 113 to model the cohort post resection, we think that it would be more appropriate to use the value of 0.7% reported by Adam et al. 113 for operative mortality within 2 months.
The mean times on first-line drug treatment are extremely important quantities because, in Merck Serono’s model, they affect the total mean cost of drug acquisition and administration per person. In Merck Serono’s model, the former, in particular, is a critical driver of cost-effectiveness. Therefore, treatment duration is worthy of close scrutiny.
Despite its importance, Merck Serono mentioned treatment duration only very briefly.
Merck Serono estimated the mean duration of cetuximab use in England as 24–25 weeks, ‘depending on chemotherapy backbone and disease progression’, citing the source as ‘data on file’ (Merck Serono submission, table 3, p. 17). The submission stated that ‘The period of treatment with cetuximab plus chemotherapy used in the model were obtained from the relevant clinical trials. As stated in the clinical evidence section, the period of treatment in the clinical trial represents clinical practice as Merck Serono research indicates that the period of cetuximab treatment is 25 weeks on average’ (section 3.7.2, p. 64).
In its model, Merck Serono assumed that all patients take first-line drug treatment while in PFS, up to a certain cut-off time, which varies slightly by treatment arm. After the cut-off time, patients take no first-line drug. The cut-off times were:
-
cetuximab plus FOLFOX – 5.5 months
-
FOLFOX – 5.5 months
-
cetuximab plus FOLFIRI (vs. FOLFIRI) – 5.8 months
-
cetuximab plus FOLFIRI (vs. bevacizumab plus FOLFIRI) – 4.8 months
-
FOLFIRI – 5.9 months
-
bevacizumab plus FOLFIRI – 5.3 months.
Under its method of modelling treatment duration, we calculate that Merck Serono estimates the following mean treatment durations:
-
cetuximab plus FOLFOX – 4.9 months
-
FOLFOX – 4.6 months
-
cetuximab plus FOLFIRI (vs. FOLFIRI) – 5.3 months
-
cetuximab plus FOLFIRI (vs. bevacizumab plus FOLFIRI) – 4.5 months
-
FOLFIRI – 5.2 months
-
bevacizumab plus FOLFIRI – 5.1 months.
Below, we argue that these are underestimates (see Critique of the Merck Serono model, Model parameters, Time on treatment).
Both we and Merck Serono assumed that all patients have second-line FOLFIRI after first-line FOLFOX-based treatment and second-line FOLFOX after first-line FOLFIRI-based treatment.
Merck Serono modelled second-line PFS using data from the study by Tournigand et al. 111 Inspection of its model revealed that it assumed a log-logistic distribution, and we calculated a mean of 0.31 years in second-line PFS for patients who start on second-line treatment. Merck Serono assumed that this value was independent of first-line treatment (whether FOLFOX or FOLFIRI based).
Given the lack of data to the contrary, we also assume that PFS on second-line FOLFOX or FOLFIRI is independent of first-line treatment.
Although not stated in its report, and in common with us, inspection of its model revealed that Merck Serono assumed that patients take FOLFOX or FOLFIRI for the entire duration of second-line PFS.
In common with us, Merck Serono modelled third-line survival using data from the study by Jonker et al. 112 Inspection of its model revealed that it assumed a Weibull distribution, and we calculated a mean of 0.74 years’ survival for patients who start on third-line treatment. Merck Serono also assumed that this value was independent of first- or second-line treatment.
Merck Serono assumed that most patients receive BSC in third line, with 17% receiving capecitabine or cetuximab. It further assumed that patients would not be retreated with cetuximab.
The utilities used in Merck Serono’s model are reported in Table 68. We noted that there were differences between the utilities in the main report and those reported in appendix B. The values in the appendix correspond to those used in the model.
| Health state | Merck Serono main report | Merck Serono model (and appendix B) | Source |
|---|---|---|---|
| First line | 0.77 | 0.778 | Bennett et al.115 |
| Second line | 0.73 | 0.769 | Bennett et al.115 |
| Third line | 0.68 | 0.663 | Wang et al.116 |
| PFS post resection | NR | 0.789 | Petrou and Hockley 2005117 |
| Progressive disease post resection | NR | 0.682 | Average of second- and third-line utilities, weighted by time spent in second and third line |
No RAS WT utility data were identified by Merck Serono or reported by the included trials. Merck Serono used the study by Bennett et al. 115 to obtain estimates of utilities in first- and second-line treatment. Bennett et al. 115 report utilities for first- and second-line KRAS WT mCRC populations. Further discussion of this source can be found in Chapter 6 (see Model parameters, Utilities). For first-line utility, Merck Serono used the estimate of first-line utility reported at baseline for the panitumumab plus FOLFOX population (0.778). For second-line utility, Merck Serono used the second-line baseline results for panitumumab plus FOLFIRI (0.769).
Merck Serono used an estimate of 0.663 from Wang et al. 116 for third-line treatment. This utility was for a previously treated KRAS WT mCRC population who were receiving BSC. This source is also discussed further in Chapter 6 (see Model parameters, Utilities).
Merck Serono used a general population estimate for the utility of PFS post resection. The source of this value was the study by Petrou and Hockley,117 which used Health Survey for England data from 1996. More recent data and approaches for using these data are available. 130,131
For post-resection progressive disease states, the utility was assumed to be a weighted average of second-line and third-line health states, adjusted for time in each state.
Merck Serono reported a cost of £200 for RAS mutation testing from the All Wales Genetic Laboratory (Merck Serono submission, appendix B, table 2), which was applied to all arms of the model, regardless of treatment.
Merck Serono assumed costs for drug acquisition per month as shown in Table 69.
| Regimen | Cost per month of drug acquisition (£) |
|---|---|
| CET + FOLFOX4 | 5083 |
| FOLFOX4 | 1546 |
| FOLFOX6 (second line only) | 1616 |
| XELOX | 1950 |
| CET + FOLFIRI | 4876 |
| BEV + FOLFIRI | 3345 |
| FOLFIRI | 1339 |
These monthly costs were calculated based on the pharmaceutical costs shown in Table 70, all of which are list prices and do not include any discounts that may be obtained by the NHS.
| Agent | Cost (£) | Source |
|---|---|---|
| Cetuximab | 20-ml vial (5 mg/ml): 178.10 | Merck Serono |
| 100-ml vial (5 mg/ml): 890.50 | ||
| Bevacizumab | 4-ml vial (25 mg/ml): 242.66 | BNF132 |
| 16-ml vial (25 mg/ml): 924.40 | ||
| Oxaliplatin | 10-ml vial (5 mg/ml): 155.00 | BNF132 |
| 40-ml vial (5 mg/ml): 622.38 | ||
| Fluorouracil | 10-ml vial (50 mg/ml): 6.40 | BNF132 |
| 50-ml vial (50 mg/ml): 32.00 | ||
| Leucovorin | 10-tablet (15-mg) pack: 19.41 | BNF132 |
| Irinotecan | 2-ml vial: 46.50 | BNF132 |
| 5-ml vial: 114.00 | ||
| 25-ml vial: 601.25 | ||
| Capecitabine | 60-tablet (150-mg) pack: 40.00 | BNF132 |
| 120-tablet (500-mg) pack: 295.65 | ||
| Doxycycline | 8-tablet (100-mg) pack: 1.11 | BNFa |
| Ondansetron | 30-tablet (4-mg) pack: 5.37 | BNFa |
| Dexamethasone | 50-tablet (2-mg) pack: 7.05 | BNFa |
For each agent in each regimen, the target dosage was calculated based on an assumed constant body surface area or body mass (Table 71) and then wastage was considered by using the minimum number of vials to achieve the minimum wastage; for example, for a target cetuximab dose of 895 mg, two 500-mg vials would lead to wastage of 105 mg, whereas one 500-mg vial and four 100-mg vials would lead to wastage of 5 mg (in which case the latter was assumed). Wastage was not minimised based on cost, but if the average cost per milligram was the same across vial sizes (or very similar), this method will minimise cost. It was assumed that for all regimens there would be 2.17 cycles per month, which is accurate for 14-day cycles.
| Regimen | Agent | Cycles per month | Dosage per cycle | Cost per cycle (£) | Monthly cost (£) |
|---|---|---|---|---|---|
| CET + FOLFOX4 | CET | 2.17 | 500 mg/m2 | 1616.37 | 3507.52 |
| FOLFOX4 | See below | 1546.45 | |||
| Doxycycline | 2.17 | 200 mg | 1.11 | 2.41 | |
| Ondansetron | 2.17 | 8 mg | 7.05 | 15.30 | |
| Dexamethasone | 2.17 | 8 mg | 5.37 | 11.65 | |
| Total | 5083.33 | ||||
| FOLFOX4 | Oxaliplatin | 2.17 | 85 mg/m2 | 622.38 | 1350.56 |
| Leucovorin | 2.17 | 200 mg/m2 | 58.23 | 126.36 | |
| 5-fluorouracil | 2.17 | 1600 mg/m2 | 32.04 | 69.53 | |
| Total | 1546.45 | ||||
| FOLFOX6 | Oxaliplatin | 2.17 | 100 mg/m2 | 622.38 | 1350.56 |
| Leucovorin | 2.17 | 200 mg/m2 | 58.23 | 126.36 | |
| 5-fluorouracil | 2.17 | 2800 mg/m2 | 64.02 | 138.92 | |
| Total | 1615.85 | ||||
| XELOX | Capecitabine | 2.17 | 28,000 mg/m2 | 245.94 | 533.69 |
| Oxaliplatin | 2.17 | 130 mg/m2 | 652.90 | 1416.79 | |
| Total | 1950.50 | ||||
| CET + FOLFIRI | CET | 2.17 | 500 mg/m2 | 1616.37 | 3507.52 |
| FOLFIRI | See below | 1339.04 | |||
| Doxycycline | 2.17 | 200 mg | 1.11 | 2.41 | |
| Ondansetron | 2.17 | 8 mg | 7.05 | 15.30 | |
| Dexamethasone | 2.17 | 8 mg | 5.37 | 11.65 | |
| Total | 4875.92 | ||||
| BEV + FOLFIRI | BEV | 2.17 | 5 mg/kg | 924.40 | 2005.95 |
| FOLFIRI | See below | 1339.04 | |||
| Total | 3344.99 | ||||
| FOLFIRI | Irinotecan | 2.17 | 180 mg/m2 | 456.00 | 989.52 |
| Leucovorin | 2.17 | 400 mg/m2 | 97.05 | 210.60 | |
| 5-fluorouracil | 2.17 | 2800 mg/m2 | 64.02 | 138.92 | |
| Total | 1339.04 | ||||
Merck Serono’s model allowed for both weekly and fortnightly administration of cetuximab, but we present only the parameter values for fortnightly administration because we believe that this is a more appropriate base case as it reflects current clinical practice more closely.
Merck Serono assumed premedication with doxycycline, ondansetron and dexamethasone prior to cetuximab administration, but these did not significantly contribute to overall costs.
Merck Serono did not include any adjustments for mean dose intensity; in practice, some patients would likely require reductions in their target dose (often because of side effects).
The drug administration unit costs used in Merck Serono’s economic model are provided in Table 72. The report differed from the model in that appendix B appeared to report inpatient and outpatient costs the other way around.
| Administration setting | Visit number | Unit cost (£) | Source |
|---|---|---|---|
| Inpatient chemotherapy administration | First visit | 287 | NHS Reference Costs 2012–2013:133 SB14Z [OP] |
| Subsequent visits | 255 | NHS Reference Costs 2012–2013:133 SB15Z [OP] | |
| Outpatient chemotherapy administration | First visit | 226 | NHS Reference Costs 2013–2014:133 SB14Z [OP] |
| Subsequent visits | 314 | NHS Reference Costs 2013–2014:133 SB15Z [OP] |
It was not stated in Merck Serono’s report how these unit costs were used and so it was necessary to determine this from the executable model.
Merck Serono assumed that the ‘first visit’ cost applied to the whole of the first cycle and that the ‘subsequent visits’ cost applied to all subsequent cycles, that is, even if a patient had multiple attendances per cycle, only one attendance was costed. Drug administration costs were consistent across all regimens per cycle and all regimens were assumed to have 2.17 treatment cycles per month (including XELOX).
Merck Serono also assumed that drug administration for first-line treatment was carried out 100% of the time in the outpatient setting and for second-line treatment was carried out 100% of the time in the inpatient/day-case setting.
In summary, total drug administration costs per month in Merck Serono’s model were £633.38 (first month) or £681.38 (subsequent months) for first-line treatments, and £585.35 (first month, except XELOX) or £553.35 (subsequent months, all months for XELOX) for second-line treatments.
The executable model submitted by Merck Serono used resource use and unit costs for medical management as shown in Table 73. As can be seen, Merck Serono assumed no medical management costs in three health states (first-line progression free, second-line and post-resection progression free), a cost of £315 per month for post-resection progressive disease and a cost of £1040 per month for third-line treatment (mainly BSC).
| Health state | Item | Unit cost (£) | Resource use (per month) | Monthly cost (£) |
|---|---|---|---|---|
| First-line progression free | 0 | |||
| Second line | 0 | |||
| Third line | BSC costs | 997 | ||
| Capecitabine monotherapy | Per month per patient receiving: 246 | 17.5% of patients | 43 | |
| Total | 1040 | |||
| Post-resection progression free | 0 | |||
| Post-resection progressive disease | Evaluation of tumour markers: CEA | 60 | 1a | 60 |
| Evaluation of tumour markers: CA 19–9 | 60 | 1a | 60 | |
| Liver function tests | 28 | 1a | 28 | |
| Hepatic ultrasonography | 51 | 1a | 51 | |
| Oncology outpatient attendance | 333 | 0.25a | 83 | |
| Abdominal CT scan | 90 | 0.125a | 11 | |
| Lung CT scan | 90 | 0.125a | 11 | |
| Large bowel CT scan | 90 | 0.125a | 11 | |
| Total | 315 |
Merck Serono specified in the submission (appendix B, table 2) that the average cost of liver resection surgery assumed in the model was £2707. Merck Serono stated that this cost was derived from NHS reference costs 2013/14134 [Healthcare Resource Group (HRG) unit costs for hepatobiliary and pancreatic surgery in malignant gastrointestinal disorders]. It represents the average of the HRG unit costs weighted by the number of finished consulting episodes (Merck Serono submission, appendix B, table 2). The relevant HRG unit costs are detailed in table 3 of appendix B.
Notably, national average unit costs for the HRGs used to estimate the average cost of liver resection in Merck Serono’s model (Merck Serono submission, appendix B, table 3) are not consistent with the NHS reference costs 2013/2014. 134 The average cost of liver resection based on the actual average unit costs reported for these HRG codes is £2467.
Merck Serono assumed a cost of £333 per oncological outpatient attendance. In the executable model it reported the source of this cost as NHS Reference Costs 2012–2013,133 but we could not confirm this cost.
The frequency of follow-up consultations in Merck Serono’s model was one visit per 4 months, as in the study by Adam et al. 113 We agree that this is appropriate.
Merck Serono modelled the following blood tests in patients post resection: liver function test and the tests for the tumour markers carcinoembryonic antigen and carbohydrate antigen 19–9 (appendix B, table 2).
The submission states that the cost of the liver function test was £28.76 (in 2013 UK pounds). However, in the executable model Merck Serono used a cost of £27.60 per test (in 2013 UK pounds). This cost was based on the TA17610 (appendix B, table 2) and we believe that this source is appropriate.
Merck Serono assumed that each tumour marker test cost £59.87, based on information from the Information Services Division (ISD) Scotland. We were unable to identify this source and so cannot comment on its relevance.
In the manufacturer’s model, the blood tests are conducted during the first month after resection and then every 4 months, based on the study by Adam et al. 113 Based on advice from our clinical expert (Dr Mark Napier, 2015, personal communication), we believe that this cost should be incurred every 3 months.
Despite the difference in cost and frequency of blood tests between our estimates and those of Merck Serono, we found that using our values in place of Merck Serono’s resulted in a minimal change to the cost-effectiveness results.
Merck Serono modelled hepatic ultrasonography and computerised tomography (CT) scans in patients post resection. The cost of the hepatic ultrasonography test was assumed to be £51 (Merck Serono submission, table 2, appendix B) and it was assumed that this test was conducted during the first month after the surgery and then every 8 months. Merck Serono modelled abdominal, lung and large bowel CT scans separately, at a cost of £90 per test (Merck Serono submission, table 2, appendix B). The tests were assumed to be performed every 8 months.
Merck Serono stated that the above cost estimates were based on NHS reference costs 2012/13;133 however, we could not confirm these estimates.
We note that, although Merck Serono calculated different costs for the first month after resection and for subsequent months, based on changes in resource use, it did not implement these correctly in the model and instead use the first month costs throughout.
Merck Serono modelled costs and disutilities of grade 3/4 AEs. The probability of an AE was taken directly from each of the relevant trials and for some AEs these came from a KRAS WT rather than a RAS WT population. Merck Serono assumed that all AEs lasted for 1 month.
The costs and disutilities associated with each AE are reported in Table 74. Periphery sensory neuropathy and vomiting have disutilities but no costs.
| AE | Cost (£) | Source provided | Utility decrement | Source |
|---|---|---|---|---|
| Hypertension | 622 | NHS Reference Costs 2013–2014:134 non-elective inpatient stay – EB04Z – hypertension | –0.069 | Doyle et al.135 |
| Gastrointestinal perforation | 2693 | NHS Reference Costs 2013–2014:134 FZ38K – gastrointestinal bleed with single intervention with a CC of score 5–7 | –0.195 | Tolley et al.136 |
| Arterial thromboembolism | 777 | NHS Reference Costs 2013–2014:134 deep-vein thrombosis with a CC score of 3–5 – QZ20D | –0.195 | Tolley et al.136 |
| Venous thromboembolism | 777 | NHS Reference Costs 2013–2014:134 deep-vein thrombosis with a CC score of 3–5 – QZ20D | –0.195 | Tolley et al.136 |
| Skin reactions | 13.09 | BNF132 | –0.03248 | Nafees et al.137 |
| Neutropenia | 877 | NHS Reference Costs 2013–2014:134 non-elective inpatient stay – PA45Z – medical oncology | –0.09 | Nafees et al.137 |
| Diarrhoea | 153 | NHS Reference Costs 2013–2014:134 general medicine outpatient visit – service code 300 | –0.103 | Lloyd et al.138 |
| Leukopenia | 153 | NHS Reference Costs 2013–2014:134 general medicine outpatient visit – service code 300 | –0.03248 | Assumption: equal to disutility for neutropenia |
| Periphery sensory neuropathy | –0.116 | Lloyd et al.138 | ||
| Fatigue | 153 | NHS Reference Costs 2013–2014:134 general medicine outpatient visit – service code 300 | –0.115 | Lloyd et al.138 |
| Vomiting | –0.103 | Lloyd et al.138 | ||
| Neurological toxicities | 1400 | NHS Reference Costs 2013–2014:134 WA17A medical oncology neoplasm-related admission with a CC score of 3+ | –0.116 | Assumption: equal to disutility for peripheral sensory neuropathy |
| Hypokalaemia | 153 | NHS Reference Costs 2013–2014:134 general medicine outpatient visit – service code 300 | –0.115 | Assumption: equal to disutility for fatigue |
The cost sources for AEs were poorly reported. We were unable to confirm the source of the costs for hypertension, arterial thromboembolism, venous thromboembolism, neutropenia or neurological toxicities.
The disutility estimates for AEs were better reported and came from a range of published literature. 135–138 All of these sources were UK-based studies, using EQ-5D vignettes, but none was conducted on a CRC population, and some studies reported on the EQ-5D visual analogue scale and some reported on the EQ-5D time trade-off scale.
Merck Serono results
Base case
Merck Serono reported six base cases, three pairwise comparisons based on cetuximab provided in a weekly dose and three pairwise comparisons in which cetuximab was given fortnightly. The three pairwise comparisons were:
-
cetuximab plus FOLFOX compared with FOLFOX alone
-
cetuximab plus FOLFIRI compared with FOLFIRI alone
-
cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI (bevacizumab and FOLFIRI)
It is unclear whether weekly or fortnightly administration of cetuximab is Merck Serono’s preferred base case (Merck submission, section 3.5, p. 59 vs. section 3.9, p. 68). However, we believe that the results of fortnightly dosing are most relevant and these are the results that we focus on here. We also focus on the results for the pairwise comparisons between cetuximab plus FOLFOX and FOLFOX, and between cetuximab plus FOLFIRI and FOLFIRI, and present only summary results for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI. These base-case deterministic results are presented in Tables 75–79.
| Treatment | Costs (£) | LYs | QALYs | ICER (£ per LY) | ICER (£ per QALY) |
|---|---|---|---|---|---|
| CET + FOLFOX | 41,301 | 2.22 | 1.64 | ||
| FOLFOX | 26,408 | 1.81 | 1.32 | ||
| Increment (CET + FOLFOX vs. FOLFOX) | 14,894 | 0.41 | 0.32 | 36,048 | 46,503 |
| Outcomes | CET + FOLFOX | FOLFOX | Increment (CET + FOLFOX vs. FOLFOX) |
|---|---|---|---|
| Costs (£) | |||
| PF (first line) | 25,741 | 9888 | 15,853 |
| Post resection (PD) | 364 | 153 | 211 |
| Post resection (PF) | 0.00 | 0.00 | 0.00 |
| PD (second line) | 7289 | 7968 | –679 |
| PD (third line) | 7907 | 8398 | –491 |
| Total | 41,302 | 26,408 | 14,894 |
| LYs | |||
| PF (first line) | 1.02 | 0.73 | 0.29 |
| Post resection (PD) | 0.08 | 0.02 | 0.06 |
| Post resection (PF) | 0.19 | 0.05 | 0.13 |
| PD (second line) | 0.30 | 0.33 | –0.03 |
| PD (third line) | 0.63 | 0.67 | –0.04 |
| Total | 2.22 | 1.81 | 0.41 |
| QALYs | |||
| PF (first line) | 0.79 | 0.56 | 0.22 |
| Post resection (PD) | 0.06 | 0.02 | 0.04 |
| Post resection (PF) | 0.15 | 0.04 | 0.10 |
| PD (second line) | 0.23 | 0.25 | –0.02 |
| PD (third line) | 0.42 | 0.45 | –0.03 |
| Total | 1.64 | 1.32 | 0.32 |
| Treatment | Costs (£) | LYs | QALYs | ICER (£ per LY) | ICER (£ per QALY) |
|---|---|---|---|---|---|
| CET + FOLFIRI | 43,592 | 2.19 | 1.61 | ||
| FOLFIRI | 27,139 | 1.81 | 1.32 | ||
| Increment (CET + FOLFIRI vs. FOLFIRI) | 16,453 | 0.38 | 0.29 | 42,990 | 55,971 |
| Outcomes | CET + FOLFIRI | FOLFIRI | Increment (CET + FOLFIRI vs. FOLFIRI) |
|---|---|---|---|
| Costs (£) | |||
| PF (first line) | 27,193 | 10,000 | 17,193 |
| Post resection (PD) | 385 | 160 | 224 |
| Post resection (PF) | 0.00 | 0.00 | 0.00 |
| PD (second line) | 7927 | 8492 | –565 |
| PD (third line) | 8087 | 8487 | –400 |
| Total | 43,592 | 27,139 | 16,453 |
| LYs | |||
| PF (first line) | 0.97 | 0.73 | 0.25 |
| Post resection (PD) | 0.08 | 0.02 | 0.06 |
| Post resection (PF) | 0.19 | 0.05 | 0.13 |
| PD (second line) | 0.30 | 0.33 | –0.02 |
| PD (third line) | 0.65 | 0.68 | –0.03 |
| Total | 2.19 | 1.81 | 0.38 |
| QALYs | |||
| PF (first line) | 0.75 | 0.56 | 0.19 |
| Post resection (PD) | 0.06 | 0.02 | 0.04 |
| Post resection (PF) | 0.15 | 0.04 | 0.10 |
| PD (second line) | 0.23 | 0.25 | –0.02 |
| PD (third line) | 0.43 | 0.45 | –0.02 |
| Total | 1.61 | 1.32 | 0.29 |
| Treatment | Costs (£) | LYs | QALYs | ICER (£ per LY) | ICER (£ per QALY) |
|---|---|---|---|---|---|
| CET + FOLFIRI | 37,978 | 2.16 | 1.60 | ||
| BEV + FOLFIRI | 34,605 | 2.03 | 1.49 | ||
| Increment (CET + FOLFIRI vs. BEV + FOLFIRI) | 3373 | 0.14 | 0.10 | 24,191 | 32,726 |
Cetuximab plus FOLFOX has an ICER of £46,503 per QALY gained compared with FOLFOX alone and cetuximab plus FOLFIRI has an ICER of £55,971 per QALY gained compared with FOLFIRI alone.
For all comparisons the health state with the highest costs and QALYs is first-line PFS. This is because of the length of time spent in this state, the cost of treatment and the higher utilities of this state.
The cetuximab plus FOLFIRI results differ for the two different pairwise comparisons (vs. FOLFIRI or vs. bevacizumab plus FOLFIRI) because they are based on different trials (CRYSTAL24 for the FOLFIRI comparison; FIRE-399 for the bevacizumab plus FOLFIRI comparison). The difference between these results seems to be primarily driven by costs: the cetuximab plus FOLFIRI arm has similar QALYs for both comparisons (1.61 for CRYSTAL and 1.60 for FIRE-3).
Probabilistic sensitivity analysis
Merck Serono performed a probabilistic sensitivity analysis for all of its base-case comparisons. According to the results, cetuximab plus FOLFOX was the most likely cost-effective treatment compared with FOLFOX at a willingness-to-pay threshold of >£50,000 per QALY and cetuximab plus FOLFIRI was the most likely cost-effective treatment compared with FOLFIRI at a willingness-to-pay threshold of ≈£60,000 per QALY. The results of the cetuximab plus FOLFOX compared with FOLFOX probabilistic sensitivity analysis demonstrated the highest uncertainty in terms of QALYs and, in a small proportion of simulations, cetuximab plus FOLFOX was dominated by FOLFOX, having larger costs and fewer QALYs. In neither probabilistic sensitivity analysis did cetuximab plus chemotherapy dominate chemotherapy alone.
Univariate sensitivity analysis
Merck Serono also conducted univariate sensitivity analyses to find the most influential parameters in the model. For both the FOLFOX and the FOLFIRI comparisons, the parameters used to estimate the costs of treatment (number of months of treatment, average body surface area), time in PFS, utility in PFS and the proportion of patients who underwent liver resection were the parameters that had the largest effects on the ICERs.
Scenario analysis
Merck Serono conducted a scenario analysis in which cetuximab plus FOLFOX was compared with an alternative chemotherapy strategy, XELOX (also referred to as CAPOX). It assumed the same effectiveness of XELOX as FOLFOX but a higher cost. As the cost of XELOX was calculated to be higher than that of FOLFOX, the ICER for cetuximab plus FOLFOX compared with XELOX was slightly lower than the ICER for cetuximab plus FOLFOX compared with FOLFOX (£42,853 per QALY gained vs. £46,503 per QALY gained). The results are presented in Table 80.
| Treatment | Costs (£) | LYs | QALYs | ICER (£ per LY) | ICER (£ per QALY) |
|---|---|---|---|---|---|
| CET + FOLFOX | 41,302 | 2.22 | 1.64 | ||
| XELOX | 27,577 | 1.81 | 1.32 | ||
| Increment (CET + FOLFOX vs. XELOX) | 13,725 | 0.41 | 0.32 | 33,219 | 42,853 |
Subgroup analysis
Merck Serono conducted a subgroup analysis for a population with metastases confined to the liver. As we were unable to reconcile this analysis against the overall population model, we present the results here without comment (Table 81).
| Treatment | Costs (£) | LYs | QALYs | ICER (£ per LY) | ICER (£ per QALY) |
|---|---|---|---|---|---|
| CET + FOLFIRI vs. FOLFIRI | |||||
| CET + FOLFIRI | 45,422 | 2.76 | 2.04 | ||
| FOLFIRI | 27,790 | 2.18 | 1.60 | ||
| Increment (CET + FOLFIRI vs. FOLFIRI) | 17,632 | 0.59 | 0.45 | 29,955 | 39,545 |
| CET + FOLFOX vs. FOLFOX | |||||
| CET + FOLFOX | 43,692 | 2.30 | 1.69 | ||
| FOLFOX | 26,199 | 1.49 | 1.07 | ||
| Increment (CET + FOLFOX vs. FOLFOX) | 17,494 | 0.81 | 0.62 | 21,465 | 28,230 |
Critique of the Merck Serono model
In this section we use our critique of the executable model provided by Merck Serono to assess the impact of parameters that we believe to be inappropriate on the cost-effectiveness results. These help form the basis of the comparison between Merck Serono’s results and our cost-effectiveness results.
Model structure
No major wiring errors were discovered in the Merck Serono model. Several small errors and inconsistencies were discovered in the Markov trace sheets, but these had a minimal impact on the ICERs, for example the ICER for cetuximab plus FOLFOX compared with FOLFOX changed from £46,503 per QALY gained to £47,185 per QALY gained once these errors were resolved.
Model parameters
Time on treatment. As stated earlier, Merck Serono assumed that no first-line drugs were given after a certain cut-off time, which varied slightly by treatment arm. It provided no justification for the cut-off times. In NICE TA242,139 which considered the cost-effectiveness of cetuximab for post-first-line treatment of mCRC, Merck Serono assumed cut-off times for cetuximab that were comparable to those in the current analysis: 13 weeks for cetuximab plus BSC and 24 weeks for cetuximab plus irinotecan plus BSC. As the Assessment Group, we, PenTAG, disagreed with the use of a cut-off time and argued for far longer treatment durations. We estimated a mean treatment duration of 4.8 months for cetuximab compared with 2.6 months estimated by Merck Serono (section 4.3.13139) and 8.8 months for cetuximab plus irinotecan compared with 4.4 months estimated by Merck Serono (section 4.3.14139).
The NICE committee preferred our estimates of treatment duration, as detailed below:
The Committee therefore concluded that it did not accept the assumption in the manufacturer’s model that a fixed treatment period for cetuximab represented UK clinical practice
Reproduced with permission from National Institute for Health and Clinical Excellence (2012). 139 Section 4.4.11. Available from www.nice.org.uk/guidance/ta242 (accessed 13 March 2017). Content accurate at time of going to press
The Committee also noted that because the manufacturer did not provide an estimate of the average length of cetuximab treatment in the CO.17 trial, the Assessment Group contacted Dr Mittman to obtain this estimate after the assessment report had been submitted to the Committee. This estimate was provided to the Committee as an addendum, and is not given in this document because it is considered academic-in-confidence. The Committee agreed that this estimate of time on treatment was more appropriate because it was derived from trial data rather than from an assumption.
Reproduced with permission from National Institute for Health and Clinical Excellence (2012). 139 Section 4.4.14. Available from www.nice.org.uk/guidance/ta242 (accessed 13 March 2017). Content accurate at time of going to press
In the course of this appraisal, Merck Serono provided us with mean treatment durations (CiC) [24 August 2015, personal communication (via NICE)]. We have used these to produce our estimates of treatment duration and they are discussed in Chapter 6 (see Model parameters, First line time on treatment). Importantly, we did not apply a cut-off time and the estimates of treatment duration are greater than those used in the Merck Serono model. We adjusted these values to ensure that we did not model first-line drug treatment after progression, as both we and Merck Serono assumed no clinical benefit of any first-line treatment after progression (as our models use only PFS, not OS, from the first-line RCTs) (see Chapter 6, Structure of the Peninsula Technology Assessment Group model).
The result is that we assumed far longer treatment durations than Merck Serono. This has the important effect that we estimated higher drug acquisition and drug administration costs.
In general, we agree with the sources and approach used by Merck Serono to identify and implement utilities.
Merck Serono used the study by Bennett et al. 115 to obtain estimates of utilities for first- and second-line treatment. As no RAS WT utility data have been identified, we agree that this is the most relevant source currently available. We also agree that there is no significant evidence of a difference between treatment arms (or over time) based on published results of quality of life for first- and second-line KRAS WT mCRC populations (Table 82).
| Health state | Merck Serono | PenTAG |
|---|---|---|
| First line | 0.778 | 0.767 |
| Second line | 0.769 | 0.762 |
| Third line | 0.663 | 0.641 |
| PFS post resection | 0.789 | < 0.831 (age related) |
| Disutility PD post resection | 0.107 | 0.142 |
Merck Serono used an estimate of utility from the study by Wang et al. 116 for third-line treatment. Again, this source is appropriate as it is for a previously treated KRAS WT mCRC population who are receiving BSC (see Table 82).
Although we agree with these sources, the PenTAG base case used alternative values based on these sources (see Table 82). Further information on the values and the sources themselves can be found in Chapter 6 (see Model parameters, Utilities).
Merck Serono used the higher estimates of utility reported at baseline for the panitumumab plus chemotherapy populations. 115 We believe that a better estimate for first-line treatment would be to take a weighted average of the treatment arms (0.767), under the assumption that any difference in utility between them is the result of random chance. This is discussed in detail in Chapter 6 (see Model parameters, Utilities). Applying this value results in only a slight increase in the ICERs.
In second line, as patients are expected to receive only chemotherapy alone in practice, we believe that it would be more appropriate to use the utility estimate for the FOLFIRI-only population (0.762). Again, Merck Serono’s ICERs change only very slightly when this value is applied.
Merck Serono’s estimate of utility in third-line BSC is for patients without symptoms of disease or toxicity. We believe that it would be more appropriate to use an utility estimate for those in the progressive disease state (reduced utility of 0.641). This leads to a marginal increase in the ICERs compared with Merck Serono’s base case.
As the utilities for Merck Serono’s base case and our base case are quite similar, the impact of altering these values is minimal. Even altering first-, second- and third-line utilities to be in line with those in the PenTAG model results in ICER changes of < £1000.
Merck used general population estimates for utility for PFS post resection, which is the same approach used in the PenTAG model. However, we recommend using the approach of Ara and Brazier130 to calculate this utility, adjusted for more recent Health Survey for England data. 131 The value used in the PenTAG submission was also adjusted for age throughout the model and therefore has a maximum value of 0.831 for the starting age of 63 years in the base case. For post-resection progressive disease states in the Merck Serono model, the utility was assumed to be a weighted average of the second- and third-line health states, adjusted for time in each state. Again, this seems a reasonable assumption and is an approach that we also used; however, as our post-resection PFS utility altered according to age, we instead calculated a disutility to apply in this state (0.142).
Once again, adjusting for these parameters resulted in very little change to the ICERs in the Merck Serono model.
The cost of RAS mutation testing used in Merck Serono’s model (£200) seems appropriate and information from other genetics laboratories in the UK (discussed in Chapter 6, see Costs, Cost of RAS testing) has reinforced the suitability of this cost. However, in the model, this cost is applied to both arms with cetuximab and arms without cetuximab. If all patients were treated with FOLFOX or FOLFIRI alone, a test for RAS mutation status would not be performed. RAS mutation testing can be used as a prognostic tool, but this does not occur in UK practice and for some hospitals RAS mutation testing is available only through the CDF as a prerequisite for cetuximab or panitumumab treatment (Dr Mark Napier, 2015, personal communication). Removing this cost from the FOLFOX and FOLFIRI arms has a minimal impact on cost-effectiveness.
After allowing for drug wastage, but not dose intensity, similar acquisition costs per month for cetuximab and bevacizumab were estimated by Merck Serono and us. However, Merck Serono estimated far higher costs for FOLFOX and FOLFIRI (Figure 9). This is because it used list prices, whereas we obtained discounted drug acquisition costs from the Commercial Medicines Unit Electronic Market Information Tool (CMU eMit) database140 in our base case. Merck Serono did not consider panitumumab.
FIGURE 9.
Mean first-line drug acquisition costs: PenTAG vs. Merck Serono. BEV, bevacizumab; CET, cetuximab.

Merck Serono estimated the mean total cost of drug acquisition as the product of the mean time on first-line treatment and the cost of treatment per unit time, with no allowance for dose intensity. We also estimated the mean total cost of drug acquisition as the product of the mean time on first-line treatment and the cost of treatment per unit time, but we also allowed for dose intensity.
Although we used a similar method of calculation and although our estimate of the mean cost per unit time for cetuximab is similar, Merck Serono’s estimates of the mean total cost of drug acquisition are far lower than ours for cetuximab plus FOLFOX and cetuximab plus FOLFIRI. This is because we assumed a greater time on treatment than Merck Serono, as discussed earlier.
Although we estimated longer treatment durations than Merck Serono for FOLFOX and FOLFIRI, we estimated lower mean total costs for these treatments. This is because we estimated lower costs per unit time than Merck Serono for FOLFOX and FOLFIRI, as discussed earlier. However, this large difference in the mean total cost of acquisition of FOLFOX and FOLFIRI between us and Merck Serono had little impact on cost-effectiveness, as FOLFOX and FOLFIRI are used in both treatment arms in any comparison.
Our estimate of the total cost of acquisition of bevacizumab plus FOLFIRI is coincidentally similar to that of Merck Serono. One the one hand, we estimate a far greater treatment duration, whereas on the other hand we estimate a far lower cost per unit time. These two effects cancel each other out to a large extent.
In the rest of this section we critique Merck Serono’s estimates of drug prices.
We believe that some of the drug acquisition costs used by Merck Serono were not appropriate for the following reasons:
-
the costs of certain agents, and particularly those for oxaliplatin, irinotecan and capecitabine, did not include very significant discounts that are reliably obtained by the NHS
-
the drug acquisition costs for XELOX were overestimated because a 14-day cycle was assumed instead of the actual 21-day cycle
-
the dosages for some agents in some regimens appear to be incorrect
-
leucovorin tablets were assumed instead of leucovorin vials for infusion
-
the premedication assumed for cetuximab does not appear to match the premedication recommended in the SPC. 35
The combined effect of replacing the drug acquisition costs used by Merck Serono with values preferred by PenTAG is to reduce the total discounted costs of all regimens, but most significantly the cost of XELOX. Cetuximab becomes slightly less cost-effective than comparators.
The NICE Guide to the Methods of Technology Appraisal128 states that ‘When there are nationally available price reductions . . ., the reduced price should be used in the reference-case analysis to best reflect the price relevant to the NHS’ and makes reference to the CMU eMit database for medicines in the National Generics Programme Framework for England [reproduced with permission from National Institute for Health and Care Excellence (2013). Guide to the Methods of Technology Appraisal. Available from www.nice.org.uk/process/pmg9 (accessed 13 March 2017)]. The CMU eMit database140 includes average acquisition costs for oxaliplatin, irinotecan, capecitabine, 5-fluorouracil, leucovorin and suitable premedications for cetuximab. Table 83 indicates that substantial price reductions are achieved of, on average, 87–98% of the list price.
| Agent | Unit cost based on list price (BNF16) (£ per mg) | Unit cost based on average acquisition cost (CMU eMit140) (£ per mg) | Average discount (%) |
|---|---|---|---|
| Oxaliplatin | 3.10 | 0.0630 | 98 |
| Irinotecan | 1.14 | 0.0742 | 93 |
| Fluorouracil | 0.0128 | 0.0012 | 91 |
| Leucovorin | 0.2249 | 0.0276 | 88 |
| Capecitabine | 0.0047 | 0.0006 | 87 |
The drug acquisition costs for XELOX were further overestimated because the model submitted by Merck Serono assumed a 14-day cycle, whereas XELOX is administered on a 21-day cycle (with 7 rest days).
Merck Serono assumed that, for FOLFOX4, the dosages for each cycle are as follows: oxaliplatin 85 mg/m2, leucovorin 200 mg/m2 and 5-fluorouracil 1600 mg/m2. We believe that the correct dosage for leucovorin is 400 mg/m2 (200 mg/m2 infusions on days 1 and 2) and for 5-fluorouracil is 2000 mg/m2 (400 mg/m2 bolus and 600 mg/m2 prolonged infusion on days 1 and 2). 23,27 Merck Serono assumed that, for FOLFOX6, the dosages for each cycle are as follows: oxaliplatin 100 mg/m2, leucovorin 200 mg/m2 and 5-fluorouracil 2800 mg/m2. We believe that the correct dosage for leucovorin is 400 mg/m2 (or 200 mg/m2 levoleucovorin, which is equivalent). 24,28 When the price for leucovorin is estimated based on the average acquisition cost in the NHS (see Table 83), this does not have a significant impact on overall costs or cost-effectiveness.
It was assumed that leucovorin tablets were used for infusion instead of vials. Leucovorin is administered intravenously over 1 hour in all regimens (except for XELOX), so tablets are not appropriate. The NHS, on average, acquires leucovorin tablets at a cost of £0.083 per mg, whereas vials cost £0.0276 per mg. 140
The SPC for cetuximab states that premedication with an antihistamine and a corticosteroid is mandatory prior to the first cetuximab infusion and is recommended prior to subsequent infusions. 35 Merck Serono assumed that doxycycline (an antibiotic), ondansetron (an antiemetic) and dexamethasone (a corticosteroid) [Auden Mckenzie (Pharma Division) Ltd, Ruislip, UK] would be used as premedication and therefore seems to have included an antibiotic and an antiemetic that are not indicated in the SPC (although they may be used in practice, they may also be used in practice across regimens) and has not included an antihistamine. PenTAG estimated that the overall impact of this is small as all of these premedication drugs are inexpensive, particularly considering the reliably obtained discounts.
Finally, Merck Serono calculated wastage based on average patient characteristics, including an average patient body surface area of 1.79 m2 and body mass of 80 kg. We believe more appropriate values are 1.84 m2 and 74.7 kg, respectively, which in the absence of drug wastage would increase the acquisition costs of all drugs except bevacizumab, which uses weight-based dosing. However, these increases are unlikely to have a significant impact given wastage. We are also satisfied that calculating wastage based on mean patient characteristics (rather than calculating average wastage based on a distribution of patient characteristics) is unlikely to significantly impact on cost-effectiveness in this case. This is because, as the Assessment Group, we found this to be the case for the NICE HTA of cetuximab, panitumumab and bevacizumab for subsequent lines of treatment for mCRC in 2011. 141 We note that accounting for the distribution of patient characteristics can, in general, impact on cost-effectiveness in other situations. 142
The combined effect of replacing the drug acquisition costs used by Merck Serono with values preferred by PenTAG is that the total discounted costs of all regimens are reduced, with the costs of XELOX reduced the most. The ICER for cetuximab plus FOLFOX compared with FOLFOX increases slightly, from approximately £46,500 to £51,900 per QALY, and the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increases from £56,000 to £62,900 per QALY, which is likely to be because of the reduced costs of second-line treatment (meaning that extending the time before second-line treatment has less of a beneficial impact on cost-effectiveness).
We believe that the drug administration costs used by Merck Serono were not appropriate for the following reasons.
-
NHS reference costs were used inappropriately for all regimens.
-
The drug administration costs for XELOX were particularly poorly estimated.
-
Drug administration activity on the second day each cycle for FOLFOX4 was not costed.
-
An outpatient setting was assumed for all patients in first-line treatment.
-
Other cost items were not included.
The combined effect of replacing the drug administration costs used in Merck Serono’s model with values preferred by PenTAG is to increase the total discounted costs for all regimens, with the costs of regimens containing FOLFOX4 increasing the most and the costs of regimens including XELOX increasing the least.
NHS reference costs were used inappropriately in the following ways.
-
Inpatient drug administration costs were estimated using outpatient drug administration reference costs from 2012/13133 (with no justification provided). The NHS reference costs do not include the costs of chemotherapy delivery in an inpatient setting, but, given that inpatient and ‘day case’ seem to have been used interchangeably, the more appropriate costs to use are those in the ‘Daycase and Regular Day/Night’ setting, and from the most recent reference costs (2013/14134).
-
The HRG code SB15Z (Deliver Subsequent Elements of a Chemotherapy Cycle) was used inappropriately for administration costs for complete cycles after the first chemotherapy cycle rather than for activity not on the first day of a chemotherapy cycle. The first attendance in every cycle should use the HRG code SB14Z (or another delivery code except for SB15Z) and then any subsequent attendances within each cycle should use SB15Z.
The drug administration costs for XELOX were poorly estimated because Merck Serono did not account for the longer duration of XELOX cycles (3 weeks rather than 2 weeks), which results in a 33% reduction in administration costs, and because Merck Serono continued to use HRG code SB14Z (Deliver Complex Chemotherapy, including Prolonged Infusional Treatment, at First Attendance) for XELOX even though the duration of infusion is significantly shorter. We believe that SB13Z (Deliver More Complex Parenteral Chemotherapy at First Attendance) is more appropriate and also results in a cost reduction.
The drug administration costs for FOLFOX4 were poorly estimated because no account was taken of the necessity for an attendance or health-care professional visit to deliver the bolus and prolonged infusion on the second day of each cycle. We believe that this should generate an additional cost estimated by HRG code SB15Z each cycle.
Merck Serono also assumed that first-line chemotherapy is always delivered in the outpatient setting, whereas second-line chemotherapy is always delivered in an inpatient/day-case setting. The NHS reference costs and clinical expert opinion suggest that, in fact, the day-case setting is more common overall. This has a significant impact as the costs in the day-case setting are often more expensive.
When we used our unit costs of drug administration in place of Merck Serono’s unit costs, Merck Serono’s base-case ICERs for cetuximab plus chemotherapy compared with chemotherapy only altered marginally.
We believe that some of the medical management costs used by Merck Serono are inappropriate for the following reasons.
-
No medical management was assumed in the progression-free health states or in the second-line progressive disease state.
-
The cost of oncology outpatient attendances was estimated from an inappropriate NHS reference cost and should be roughly half the price.
Merck Serono assumed no medical management in the progression-free health states or in the second-line progressive disease state. This is not appropriate because patients in these states will receive medical management in the form of regular consultant outpatient appointments and imaging (CT) to monitor response to treatment.
The cost of oncology outpatient attendances was estimated from HRG code SB01Z (Procure Chemotherapy Drugs for Regimens in Band 1) in the outpatient setting, which is incorrect. Instead, the cost of outpatient attendances should have been estimated from service code 370 (medical oncology), which would have resulted in a cost of £144 (consultant led; 2012/13 prices) as opposed to £333 (2012/13 prices).
The executable model submitted by Merck Serono did not allow for medical management costs to be added to the states in which they are not currently modelled, but it is not considered likely that incorporating values preferred by PenTAG would significantly affect cost-effectiveness as medical management costs are significantly smaller than the costs associated with chemotherapy and do not vary between regimens. Indeed, using our model, we find that cost-effectiveness is insensitive to these costs.
However, we estimate a higher cost per unit time for treatment post progression for resected patients. We assumed a cost of £1254 per month, whereas Merck Serono assumed a cost of £315 per month. When we used our estimate, Merck Serono’s base-case ICERs increased slightly:
-
cetuximab plus FOLFOX compared with FOLFOX – from £47,000 to £49,000 per QALY
-
cetuximab plus FOLFIRI compared with FOLFIRI – from £56,000 to £59,000 per QALY.
We believe that Merck Serono’s estimate of the cost of liver resection (£2707) is too low. In TA176,10 the NICE committee agreed that an average cost of £8900 for liver resection was an accurate reflection of current UK clinical practice. Furthermore, the HRG codes selected by Merck Serono refer to malignant gastrointestinal tract disorder, which, although relevant to CRC, do not appear to be entirely relevant for liver surgery. More appropriate codes are those associated with very complex liver resection surgery, which we use in our base case.
Using our estimate of liver surgery (£17,582), which includes repeat operations and the chance of operation failure, Merck’s base-case ICERs increase slightly:
-
cetuximab plus FOLFOX vs. FOLFOX – from £47,000 to £49,000 per QALY
-
cetuximab plus FOLFIRI vs. FOLFIRI – from £56,000 to £59,000 per QALY.
In Merck Serono’s executable model, the disutility for leukopenia was reported to be the same as that for neutropenia, but the value used was for the disutility for skin reactions. However, correcting this does not alter the ICERs.
The length of time that the AEs occur for in Merck Serono’s model (a 1-month cycle) seems quite long. Previous estimates of the duration of AEs suggest that this should be much shorter, as described in study by Freeman et al. 143 Reducing the length of time that AEs occur for primarily reduces the disutility of these AEs, but also affects some costs. Reducing the duration of AEs to 7 days, as in the PenTAG model, changes the ICERs only marginally.
The main driver for the costs and QALYs associated with the AEs is the type and incidence of each AE. The Merck Serono model appears to use AE data for the KRAS WT population rather than the RAS WT population, as the incidences reported for the CRYSTAL trial24,43 are different in Merck Serono’s model from what is reported in our clinical effectiveness results. As the PenTAG and Merck Serono models include very different sets of AEs and PenTAG includes comparisons of more than two technologies, it is difficult to adjust individual parameters in the Merck Serono model to values that we believe are more accurate. Instead, we present the total costs and QALYs associated with AEs in the PenTAG and Merck Serono base cases (Table 84). Despite these being different, the AE costs and QALYs have little impact on the overall results, increasing the ICERs by < £1500 when the PenTAG values are used.
| Arm of model | Total AE costs (£) | Total AE QALYs | ||
|---|---|---|---|---|
| Merck Serono | PenTAG | Merck Serono | PenTAG | |
| CET + FOLFOX | 458 | 1472 | –0.0075 | –0.0018 |
| FOLFOX | 469 | 1039 | –0.0058 | –0.0012 |
| CET + FOLFIRI | 567 | 803 | –0.0111 | –0.0009 |
| FOLFIRI | 418 | 780 | –0.0077 | –0.0005 |
Conclusions
As no economic evaluation was submitted by Amgen, and Merck Serono did not report results for panitumumab, we are unable to draw conclusions about panitumumab based on the industry submissions.
The cost-effectiveness review submitted by Merck Serono did not raise any additional analyses relevant to the decision problem. The model structure seems to be generally appropriate and fit for purpose. Merck Serono concluded that its de novo analysis demonstrated that cetuximab was cost-effective, but we believe that important parameter estimates such as treatment duration have been underestimated.
Chapter 6 Independent economic assessment
Methods
Comparator treatments
In our base-case analysis we simultaneously compared treatments separately within the following two groups. All treatments were in the NICE scope. 14
-
FOLFOX network:
-
cetuximab plus FOLFOX
-
panitumumab plus FOLFOX
-
FOLFOX
-
-
FOLFIRI network:
-
cetuximab plus FOLFIRI
-
FOLFIRI.
-
Two networks were considered as we found no RCT evidence that connects the networks (see Chapter 3, Results).
These treatments are all widely used on the NHS (Table 85).
| Scope comparatora | Merck Serono | PenTAGb |
|---|---|---|
| Cetuximab/panitumumab + oxaliplatin- or irinotecan-based chemotherapy | Important | 30% of all patients |
| Bevacizumab + oxaliplatin or irinotecan-based chemotherapy | Not reflected in clinical practice as bevacizumab is no longer funded by NHS England or the CDF for the treatment of CRC. Therefore, these comparisons are not meaningful (Merck Serono submission, p. 69) | 10% of all patients |
| FOLFOX/XELOX | Important | 30% of all patients |
| FOLFIRI/XELIRI | Important | 10% of all patients |
| Capecitabine | Not comparators | 20% of all patients |
| Tegafur, folinic acid and 5-fluorouracil |
Bevacizumab-based treatments
Bevacizumab plus FOLFOX and bevacizumab plus FOLFIRI are both listed as treatments in the NICE scope. 14 However, NICE has not recommended these treatments for first-line mCRC. Furthermore, as discussed in Chapter 1, since the NICE scope was issued, bevacizumab-containing treatment for first-line mCRC has been delisted from the CDF. 18 For this reason, we did not consider this as a comparator in our base-case analysis.
However, in a sensitivity analysis, we considered bevacizumab plus FOLFOX in the FOLFOX network and bevacizumab plus FOLFIRI in the FOLFIRI network, as these treatments have recently accounted for approximately 10% of all eligible patients (see Table 85).
XELOX
In common with Merck Serono we modelled CAPOX/XELOX as a comparator treatment in a scenario analysis, assuming equal clinical effectiveness for CAPOX/XELOX and FOLFOX. As in the Merck Serono model, we assumed that the only difference between CAPOX/XELOX and FOLFOX was in the treatment acquisition and administration costs (see Chapter 5, Comparator treatments, XELOX).
Capecitabine monotherapy and tegafur, folinic acid and 5-fluorouracil
Although our estimates suggest that they account for 20% of all first-line treatments in patients with metastatic cancer treated on the NHS, capecitabine monotherapy and 5-fluorouracil plus folinic acid are not included as comparators in our model. On advice from our clinical advisor, we believe that these single fluoropyrimidine regimens are used only in patients for whom combination therapies are not suitable, for example when patients have comorbidities such as diabetes or liver dysfunction for which oxaliplatin or irinotecan would not be appropriate. Merck Serono stated that capecitabine is ‘typically used in elderly patients with poor performance status’ (Merck Serono submission, table 4, p. 20), which broadly agrees with our clinical advisor.
If the subgroup of first-line mCRC patients for whom combination chemotherapies are not recommended were to be modelled, it should be included as a separate subgroup in every treatment arm. As such, this subgroup would apply to all arms equally and it would therefore have no impact on the cost-effectiveness results.
To model these treatments as a separate arm seems clinically implausible (our estimates suggest that 80% of patients receive combination chemotherapy in clinical practice and that single fluoropyrimidine regimens are not the preferred first-line treatment). Furthermore, no evidence of single fluoropyrimidine regimens in comparison to cetuximab or panitumumab was identified in our clinical effectiveness review. The trials that inform the treatment effect of panitumumab and cetuximab are restricted to patients who can receive combination chemotherapies and therefore the patients who receive single fluoropyrimidine regimens are not accounted for in these effectiveness estimates.
We also did not model tegafur because, as well as being used in single fluoropyrimidine regimens, tegafur/uracil (the combination most appropriate to this assessment) has been discontinued in the UK and no relevant alternatives are available (Merck Serono submission, table 4, p. 20).
Patient population and liver metastases subgroup
In common with Merck Serono and the NICE scope,14 we considered two patient populations:
-
all first-line patients with RAS WT mCRC
-
a subgroup of these patients with liver metastases confined to the liver (liver metastases subgroup).
We estimated that the liver metastases subgroup included approximately 26% of all patients, based on the patients in the five pivotal RCTs [based on data submitted by Amgen and Merck Serono, 19 March 2015, personal communication (via NICE)].
The following parameters were unique for the liver metastases subgroup:
-
resection rates
-
PFS for unresected patients
-
treatment duration.
All other parameters were unchanged from those in the total population analysis.
In contrast, Merck Serono claimed that it changed only the resection rates and PFS for unresected patients for the liver metastases population.
Model structure
Structure of relevant published models
Key aspects of the structure of relevant published models of the cost-effectiveness of drugs for first-line mCRC are provided in Table 86. This table includes all models that we included in our systematic review, plus the Merck Serono model from TA176. 10 Although the Merck Serono model was included in our review, as it was based on KRAS WT patients, we have included this model in the table as the current HTA is a review of TA176. For comparison, we also include our current model in the table.
| Model component | TA176 Merck Serono model, ERG report144 and Westwood et al.45 | Graham et al.107 | Jarrett et al.108/SMC 2014 submission118 | Ortendahl et al.109 | PenTAG: this HTA |
|---|---|---|---|---|---|
| Patients | First-line mCRC KRAS WT | First-line mCRC RAS WT | First-line mCRC RAS WT | First-line mCRC RAS WT | First-line mCRC RAS WT |
| Treatments | CET + FOLFIRI vs. FOLFIRI; CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. BEV + FOLFOX | CET + FOLFIRI vs. FOLFIRI; CET + FOLFOX vs. FOLFOX | CET + FOLFIRI vs. BEV + FOLFIRI | CET + FOLFIRI vs. BEV + FOLFIRI vs. FOLFIRI; CET + FOLFOX vs. PAN + FOLFOX vs. BEV + FOLFOX vs. FOLFOX |
| Health states | |||||
| PFS and drug costs | First-line treatment assumed up to progression or until curative resection | Number of cycles of treatment from the PEAK trial29 | Not stated | Not stated | First-line treatment assumed up to progression |
| PD treatments second line | FOLFOX or FOLFIRI (FAD section 3.1810); split between patients with no resection and patients with unsuccessful resection. PFS in second line was derived from the PFS curves published in Tournigand et al.,111 regardless of the time of progression from the first line | Distribution of treatments from the PEAK trial:29 anti-EGFR + FOLFIRI or BEV + FOLFIRI or BSC. Treatment duration was estimated from published PFS rates in second-line treatment145,146 (see table 1 in Graham et al.107), as this was not collected in the PEAK trial. Transition probabilities to third-line treatment were calculated from the weighted PFS for each second-line treatment | FOLFOX or FOLFIRI. PFS in second-line treatment was derived from the PFS curves published in Tournigand et al.,111 regardless of the time of progression from the first line | Based on treatments in the FIRE-3 trial28 | FOLFOX or FOLFIRI, independent of treatment arm |
| Treatments third line | BSC (FAD section 3.1810). The probability of death was derived from the study by Jonker et al.,112 which compared CET + BSC with BSC alone. Similar to second-line therapy, the risk of death was independent of the treatment arm | BSC | BSC. The probability of death was derived from the study by Jonker et al.,112 which compared CET + BSC with BSC alone | Not stated | BSC. The probability of death was derived from the study by Jonker et al.,112 with the risk of death independent of treatment arm |
| After successful curative resection | One health state only; CET not given | Two health states: PFS and PD | One health state only | One health state only | Two health states: PFS and PD |
| After unsuccessful curative resection | As if no resection attempted | As if no resection attempted | Not stated | Not stated | As if no resection attempted |
| Method of estimating OS | Not clear but appears to be a combination of survival in the first-, second- and third-line trials and survival post resection. It appears that survival from the first-line trials was not extrapolated because of the immaturity of the data | From extrapolation of OS data from the PEAK trial29 | Not clear but stated that ‘the PFS benefit translates into a direct overall survival benefit’ (p. 9)118 | Not stated | Base case: combination of survival in the first-, second- and third-line trials and survival post resection. Sensitivity analysis: as Graham et al.107 (i.e. extrapolation of OS from the PEAK trial29) |
| Model basic variables | |||||
| Patient age at model entry (years) | 60 | Not stated | Not stated | 63 | |
| Cycle length (weeks) | 1 | 2 | 4.3 (1 month) | 2 | 4.3 (1 month) |
| Time horizon (years) | 23 | 20 | 10 | 30 | |
The model for the cost-effectiveness of KRAS testing by Westwood et al. 45 was based on the Merck Serono model for TA176. 10 Indeed, the key model structures are identical (see Table 86).
Structure of the Peninsula Technology Assessment Group model
We identified two candidate model structures: structure 1 and structure 2 (Table 87). Ordinarily, we would choose structure 2 because of the consistency between the costs and health outcomes. However, this is arguably inappropriate because the RCTs of the first-line drugs included second-line drugs that are not commonly used in the NHS (Table 88). Also, subsequent lines, for example second-line treatment, may have a very strong effect on OS. For example, in the FIRE-3 trial28 there was no significant difference in median PFS, but there was a significant difference in median OS (see Chapter 3, Results, Cetuximab) and very different subsequent treatments between treatment arms (see Table 88).
| Model component | Structure 1: PenTAG base case | Structure 2: scenario analysis |
|---|---|---|
| Summary of clinical data | Based on RCTs of first-line drugs up to first-line progression;43,44,65 time on second-line treatment based on second-line trial of FOLFOX and FOLFIRI;111 time in third-line BSC based on published data112 | Based completely on RCTs of first-line drugs43,44,65 |
| Similarity to previous included economic evaluations | Appears to be similar to the Merck Serono TA17610 model | Graham et al.107 |
| OS | For unresected patients, the sum of times on first, second and third lines of treatment, allowing for mortality from each line, and affected by survival for resected patients (see end of this section for details) | Estimated by extrapolation from RCTs of first-line drugs43,44,65 |
| Subsequent treatments | Second-line FOLFOX for patients on first-line FOLFIRI-based treatments; second-line FOLFIRI for patients on first-line FOLFOX-based treatments | Percentage of patients taking each subsequent treatment, as in the first-line RCTs43,44,65 |
| Advantages and disadvantages of methods | ||
| Simplicity | Less complex | More complex |
| Consistency between costs and outcomes in RCTs | Mostly consistent, except we do not have access to IPD for mortality on first-line treatment only from the first-line RCTs. Also, assumed that progression and survival on second-line treatment do not depend on first-line treatment | Consistent |
| Use of first-line RCT data | Uses data up to progression only | Uses all relevant data, including OS data |
| Effect of first-line treatment post progression | Assumed either no effect or assumed equal for all treatment arms | Captured (but confounded with effect of subsequent lines of treatment) |
| Consistency with subsequent lines of treatments on the NHS | Consistent, as FOLFOX and FOLFIRI are most likely second-line treatments on NHS | Less consistent, as not all treatments (e.g. cetuximab, panitumumab, bevacizumab) after progression are available on the NHS |
| Suitability for indirect comparisons between multiple treatment arms | Suitable | Less suitable because the relative numbers of patients taking the various second-line treatments vary between treatments in the evidence networks |
| RCT | Treatment strategy | Population | n | Anti-EGFR (CET/PAN) (%) | Anti-VEGF (BEV) | Oxaliplatin or irinotecan | Reference |
|---|---|---|---|---|---|---|---|
| FOLFOX network | |||||||
| PRIME44 | PAN + FOLFOX | KRAS WT | 325 | 13 | NR | 59% chemotherapy | Douillard et al.26 (p. 1350) |
| FOLFOX | 331 | 25 | NR | 65% chemotherapy | |||
| PEAK29 | PAN + FOLFOX | RAS WT | 88 | 22 (presumably CET) | 40% | Irinotecan based 50%, oxaliplatin based 13% | Schwarzberg et al.29 (table 3 and appendix A2) |
| BEV + FOLFOX | 82 | 37 (presumably mixture of CET/PAN) | 33% | Irinotecan based 51%, oxaliplatin based 23% | |||
| OPUS65 | FOLFOX | KRAS WT | 97 | 18 | 19% | Irinotecan based 48%, oxaliplatin based 9% | Bokemeyer et al.22 (table 2) |
| CET + FOLFOX | 82 | 10 | 16% | Irinotecan based 45%, oxaliplatin based 18% | |||
| FOLFIRI network | |||||||
| FIRE-328 | CET + FOLFIRI | KRAS WT | 260 | 13 | 46% | Oxaliplatin based 34.3%a | Ortendahl et al.109 cost-effectiveness analysis |
| BEV + FOLFIRI | 250 | 39 | 17% | Oxaliplatin based 38.3%a | |||
| CRYSTAL43 | CET + FOLFIRI | KRAS WT | 316 | NR | NR | NR | Van Cutsem et al.25 |
| FOLFIRI | 350 | NR | NR | NR | |||
Structure 1 assumed that the PFS benefits of the first-line drugs translate into OS benefits if the subsequent lines of treatment are balanced between treatment arms. Expressed differently, we assumed that survival after first-line progression was independent of first-line treatment, which seems plausible, given a lack of evidence to the contrary. We used structure 1 in our base-case analysis.
Conversely, structure 2 assumed that OS is a product of responses to both first and subsequent lines of treatment, as experienced in the RCTs. We considered structure 2 in a scenario analysis. Given the limited data on subsequent treatments, we were forced to make approximations for the costs of these.
In our experience, both structures have been used in many previous NICE appraisals. For example, structure 1 was used in the recent NICE assessment of obinutuzumab in combination with chlorambucil for previously untreated chronic lymphocytic leukaemia (TA343147) and was endorsed by the NICE committee. Furthermore, we are not aware of any guidance concerning which structure is the most appropriate. We note that Merck Serono also used structure 1 in its analysis (see Chapter 5, De novo economic evaluation).
The PenTAG cost-effectiveness model, implemented in Microsoft Excel® (2010; Microsoft Corporation, Redmond, WA, USA), simulated a cohort of people with RAS WT mCRC starting on first-line line treatment. The structure of the model was informed by a review of the literature (see Structure of relevant published models) and the opinions of our clinical expert, Dr Mark Napier (2015, personal communication) (Figure 10). The structure of our model is very similar to that of Merck Serono’s model (see Chapter 5, De novo economic evaluation).
FIGURE 10.
Structure of the PenTAG cost-effectiveness model. PD, progressive disease. a, For cetuximab plus FOLFIRI and FOLFIRI only.
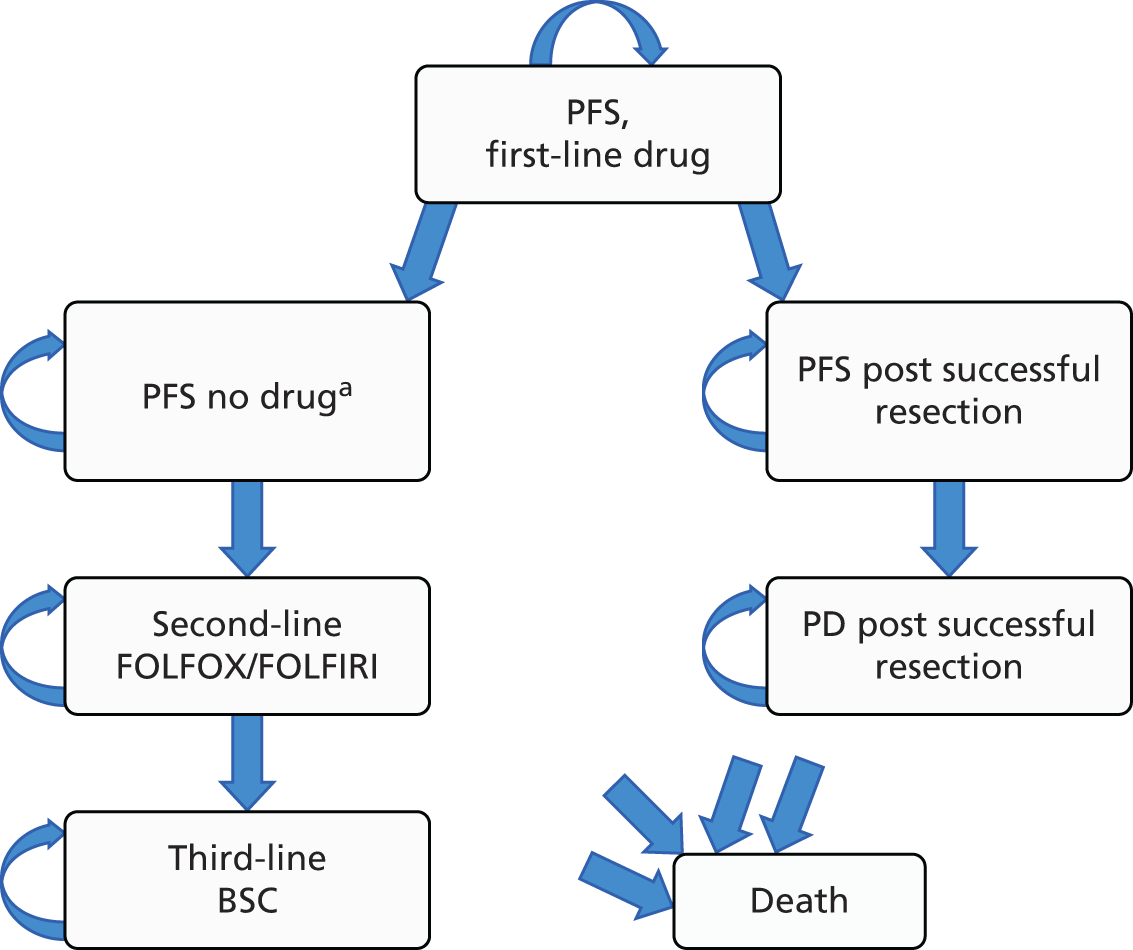
In Figure 10, straight arrows represent the possible transitions between health states. Circular arrows denote that patients can remain in a state at the end of each model cycle. During each cycle, a patient is assumed to be in one of the states. Patients are assumed to move between states once at the end of each cycle. Patients can die while in any state.
As with Merck Serono’s model, differences in clinical effectiveness between first-line drug treatments are represented by the differences between:
-
first-line PFS
-
resection rates
-
incidences of AEs.
Estimates of cost and utility per cycle were assigned to each health state. These were aggregated over the modelled time horizon to estimate the total per-patient costs and QALYs for each treatment. The main economic outcome was the ICER, the incremental cost per QALY gained.
The model cycle length was 1 month and the model time horizon was 30 years, after which time virtually all people in all cohorts have died. This is substantially longer than the 10-year time horizon assumed by Merck Serono [we have criticised their assumption in Chapter 5 (see De novo economic evaluation)]. A model half-cycle correction was applied.
Future costs and benefits were discounted at 3.5% per annum and the perspective was that of the NHS and personal social services, in accordance with the NICE reference case. 128
We assumed that all patients were aged 63 years at the start of first-line treatment and that 66% were male, to be consistent with the clinical effectiveness data from the RCTs. In the model, this affects only the age-related utilities and background mortality.
Baseline randomised controlled trials
For the FOLFIRI network, the CRYSTAL RCT43 was chosen as the baseline trial because this contains the only two treatments in our base-case analysis, CET plus FOLFIRI and FOLFIRI. The other RCT, FIRE-3,28 includes BEV plus FOLFIRI, which we consider only in a sensitivity analysis.
For the FOLFOX network, the PRIME RCT44 was selected as the baseline trial as it included two of the three treatments, PAN plus FOLFOX and FOLFOX, in our base-case analysis. The PEAK trial29 was not selected as it included one treatment, BEV plus FOLFOX, not in our base case. Although the OPUS trial65 also included two of the three treatments in our base-case analysis (CET plus FOLFOX and FOLFOX), we did not select this trial as it was far smaller than the PRIME trial (87 vs. 512 RAS WT patients).
However, we used the OPUS trial as the baseline RCT for the FOLFOX network in a scenario analysis (see Scenario analyses, OPUS trial as the baseline randomised controlled trial in the FOLFOX network). In this case, the following parameters change in the FOLFOX network:
-
resection rates
-
PFS for unresected patients
-
treatment durations (see Model parameters).
Modelled patients resected
Drug treatment can reduce the sizes of tumours to allow resection surgery to remove metastases. Our clinical advisor, Dr Mark Napier, suggested that, generally, resection is offered only to patients with metastases confined to the liver.
As in the Merck Serono model, and in all other previous models of treatments in this indication, we assumed that a proportion of patients randomised to each treatment arm have liver metastases resected (see Figure 10). This proportion varies by treatment arm and according to whether the cohort represents all patients or only patients with liver metastases confined to the liver (liver metastases subgroup).
Life expectancy after successful resection is substantially greater than for patients without successful resection. Survival after resection was split into PFS and progressive disease (see Figure 10, ‘PFS post successful resection’ and ‘PD post successful resection’), and patients could die from PFS and progressive disease.
Modelled first-line progression-free survival: unresected patients
In the RCTs relevant to this HTA, the mean time on first-line treatment was less than the mean time in PFS for the CET + FOLFIRI and FOLFIRI treatments. Given that we also assumed that patients start second-line treatment at the time of progression, for these two treatments there is therefore a period in first-line PFS during which patients are on no active drug treatment (see Figure 10, ‘PFS, no drug’ state). In this way, for unresected patients, first-line PFS was split into two states: on drug and not on drug. Merck Serono also made this assumption, although it was not stated in its report. For all other treatments, patients were assumed to receive first-line treatment for the complete duration of first-line PFS.
Time in the ‘PFS, no drug’ state was calculated as the difference between time in PFS first line and first-line treatment duration, using the simple area under the curve method, that is, transition probabilities from ‘PFS, first-line drug’ to ‘PFS, no drug’ were not calculated explicitly.
As explained in Model parameters (see First-line progression-free survival: unresected patients), first-line PFS for unresected patients was calculated using PFS from the five pivotal RCTs,28,29,43,44,65 with an adjustment for indirect comparisons and an adjustment to subtract PFS for resected patients. First-line PFS for unresected patients was calculated separately for all patients and for the liver metastases subgroup.
Patients could die from first-line PFS, that is, before progressing (see Figure 10).
Modelled second-line treatments: unresected patients
We assumed that all unresected patients have second-line FOLFIRI after first-line FOLFOX-based treatment and second-line FOLFOX after first-line FOLFIRI-based treatment (see Figure 10).
Merck Serono also made these assumptions (see Chapter 5, De novo economic evaluation).
Our clinical expert, Dr Mark Napier, advised us that this is the standard treatment for UK patients. In addition, our assumptions are consistent with NICE clinical guideline 131,1 which recommends second-line FOLFIRI or irinotecan treatment after first-line FOLFOX. After first-line FOLFIRI, there is no recommendation for second-line treatment.
Even though second-line panitumumab, cetuximab and bevacizumab were used extensively in the relevant RCTs (see Table 88), we did not model these because:
-
NICE has recommended none of these treatments (Table 89)
-
the CDF has recommended only second-line bevacizumab plus FOLFOX; it has recommended neither panitumumab nor cetuximab
-
our clinical expert, Dr Mark Napier, advised us that these treatments are used little in UK practice.
| Source | Second-line drug | ||
|---|---|---|---|
| Panitumumab | Cetuximab | Bevacizumab | |
| NICE recommendations | Monotherapy not recommended139 | Monotherapy or with chemotherapy not recommended139 | Bevacizumab in combination with fluoropyrimidine-based chemotherapy not recommended139 |
| CDF50 | Not recommended | Not recommended | Bevacizumab + FOLFIRI not recommended; bevacizumab + FOLFOX recommended |
Modelled third-line treatment: unresected patients
Based on clinical advice, we assumed that all unresected patients have third-line BSC after progression on second-line treatment. This consists of palliative care, with no active drug treatment.
Merck Serono assumed similarly that most patients (83%) receive third-line BSC, with only 17% receiving capecitabine or cetuximab (see Chapter 5, De novo economic evaluation).
Overall survival
In our base-case analysis, we modelled only PFS from the RCTs. Life expectancy for all randomised patients was calculated separately for each treatment arm as:
The last quantity, life expectancy for unresected patients for each treatment arm, was calculated as the sum of expected survival times on first, second and third lines of treatment, allowing for mortality from each line (see Model parameters, Overall survival: unresected patients).
Model parameters
Most parameter values in the model are the same as or very similar to those assumed by Merck Serono. The parameters that differ and that strongly affect cost-effectiveness are described in Comparison of the results with those in the Merck Serono submission.
Resection rates
Resection of liver metastases is an important component of both our model and Merck Serono’s model (see Figure 10) as we find that cost-effectiveness is sensitive to the rates of resection.
In TA176 (Final Appraisal Determination, p. 12),10 Merck Serono judged the rates of resection from the RCTs to be low compared with clinical practice. Therefore, it considered a resection rate of 43% for the KRAS WT population for CET plus FOLFIRI and CET plus FOLFOX, taken from the CELIM (Cetuximab in Neoadjuvant Treatment of Non-Resectable Colorectal Liver Metastases) trial (cited as Folprecht et al. 148 by Merck Serono). This value is substantially greater than the resection rates in the RCTs. The NICE clinical experts and committee instead preferred a lower value of 35% (Final Appraisal Determination, p. 20, p. 22),10 still greater than in the RCTs.
Conversely, our clinical expert, Dr Mark Napier, believes that the rates of liver resection in normal practice will be similar to or lower than those rates seen in the PEAK and CRYSTAL trials [2–13% for all patients, Table 90; data on file from Amgen and Merck, 19 March 2015, personal communication (via NICE)]. He believes that the CELIM trial data are not comparable as these represented carefully selected patients with liver-only low-volume metastases and ‘nearly’ operable patients.
| RCT | Type of resection | Treatment | Liver-limited subgroup, % (n/N) | All patients, % (n/N) | ||
|---|---|---|---|---|---|---|
| RAS WT | KRAS WT | RAS WT | KRAS WT | |||
| FOLFIRI network | ||||||
| CRYSTAL | Surgical resection – attempted resection | CET + FOLFIRI | 16.3 (7/43) (Merck Serono) | NR | 7.3 (13/178) (Merck Serono) | NR |
| FOLFIRI | 6.5 (3/46) (Merck Serono) | 2.1 (4/189) (Merck Serono) | ||||
| FIRE-3 | Secondary resection of liver metastases with curative intent | CET + FOLFIRI | Not reported | NR | NR | 12.1 (36/297)28 |
| BEV + FOLFIRI | 13.6 (40/295)28 | |||||
| FOLFOX network | ||||||
| OPUS | Rate of curative metastatic surgery (complete resection to R0) | CET + FOLFOX | 13.3 (2/15) (Merck Serono) | NR | NR | 9.8 (6/61)23 |
| FOLFOX | 0 (0/12) (Merck Serono) | 4.1 (3/73)23 | ||||
| PEAK | Rate of curative metastatic surgery (complete resection to R0) | PAN + FOLFOX | (Confidential information has been removeda) (Amgen) | NR | 12.5 (11/88) (Amgen) | 9.9 (14/142)29 |
| BEV + FOLFOX | (Confidential information has been removeda) (Amgen) | 11.0 (9/82) (Amgen) | 8.4 (12/143)29 | |||
| PRIME | Results reported in the KRAS trials as complete resection (R0) but end-point definition was ‘reported as complete or partial [status of surgical margins not required to be captured]’ (p. 1347)26 | PAN + FOLFOX | 31 (15/48) (Amgen) | 27.9 (17/61)26 | (Confidential information has been removeda) (Amgen) | 9.5 (31/325)26 |
| FOLFOX | 17 (7/41) (Amgen) | 17.5 (10/57)26 | (Confidential information has been removeda) (Amgen) | 7.6 (25/331)26 | ||
Given this, and in common with Merck Serono’s model, we used the resection rates from the RCTs (see Table 90) to estimate the rates for use in our model (Table 91).
| Treatment | Liver-limited metastases RAS WT subgroup (%) | All RAS WT patients (%) | ||
|---|---|---|---|---|
| PenTAG | Merck Serono | PenTAG | Merck Serono | |
| FOLFIRI network | ||||
| CET + FOLFIRI | 16.3a | 16.3a | 7.3a | 7.3a |
| FOLFIRI | 6.5a | 6.5a | 2.1a | 2.1a |
| BEV + FOLFIRI | 20.9 (derivation explained in text) | 9.0 (derivation explained in text) | 7.3 (no justification given) | |
| FOLFOX network | ||||
| CET + FOLFOX | (Confidential information has been removedb)c (derivation explained in text) | 13.3d | 20.7 (derivation explained in text) | 7.3 (derivation explained in text) |
| FOLFOX | 17.1c | 0d | (Confidential information has been removedb)c | 2.1111 |
| PAN + FOLFOX | 31.3c | NA, as not modelled | (Confidential information has been removedb)c | NA, as not modelled |
| BEV + FOLFOX | (Confidential information has been removedb)c (derivation explained in text) | NA, as not modelled | (Confidential information has been removedb)c (derivation explained in text) | NA, as not modelled |
FOLFIRI network
In the FOLFIRI network, resection rates for cetuximab plus FOLFIRI and FOLFIRI were taken directly from the CRYSTAL trial (Figure 11 and see Table 91). This was also the approach taken by Merck Serono.
FIGURE 11.
Peninsula Technology Assessment Group- vs. Merck Serono-modelled resection rates: FOLFIRI network – (a) all patients; and (b) liver metastases subgroup. BEV, bevacizumab; CET, cetuximab.
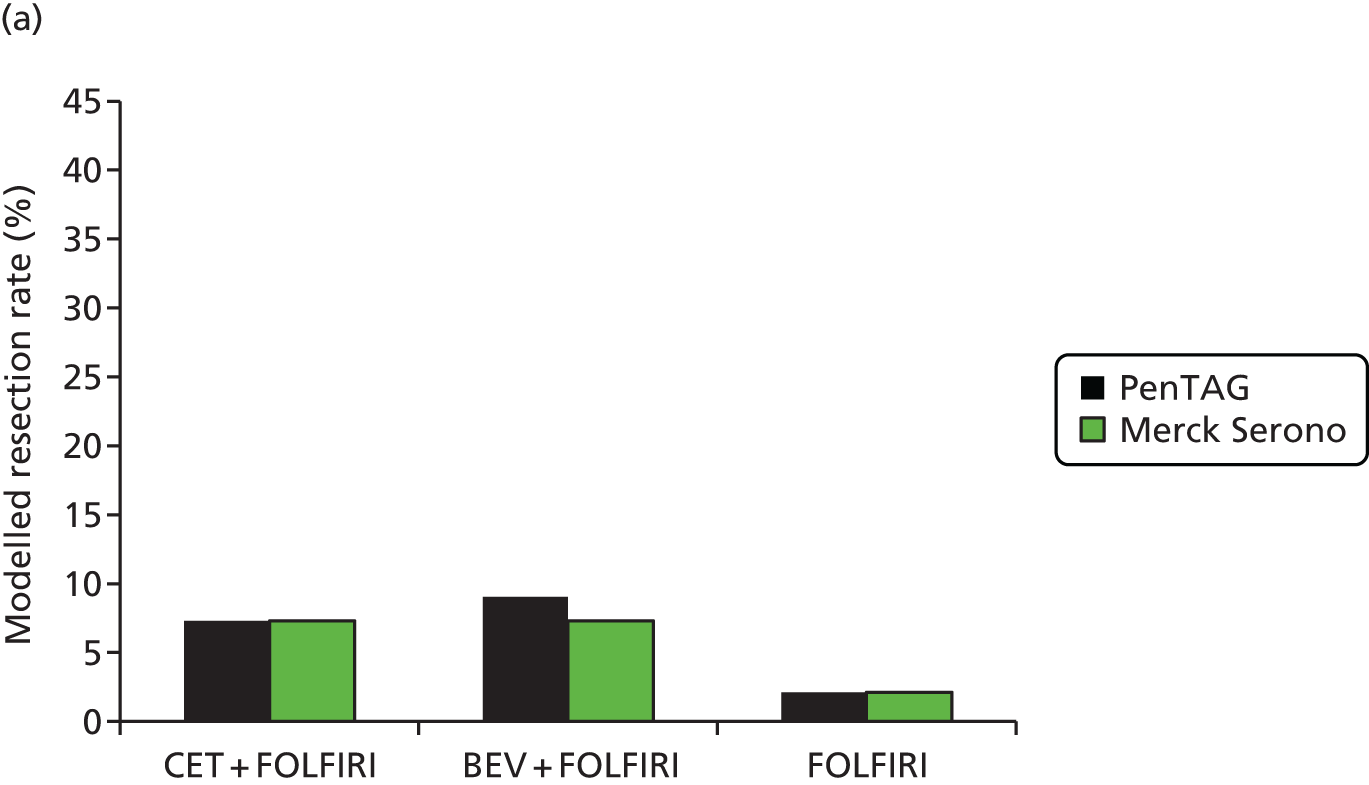
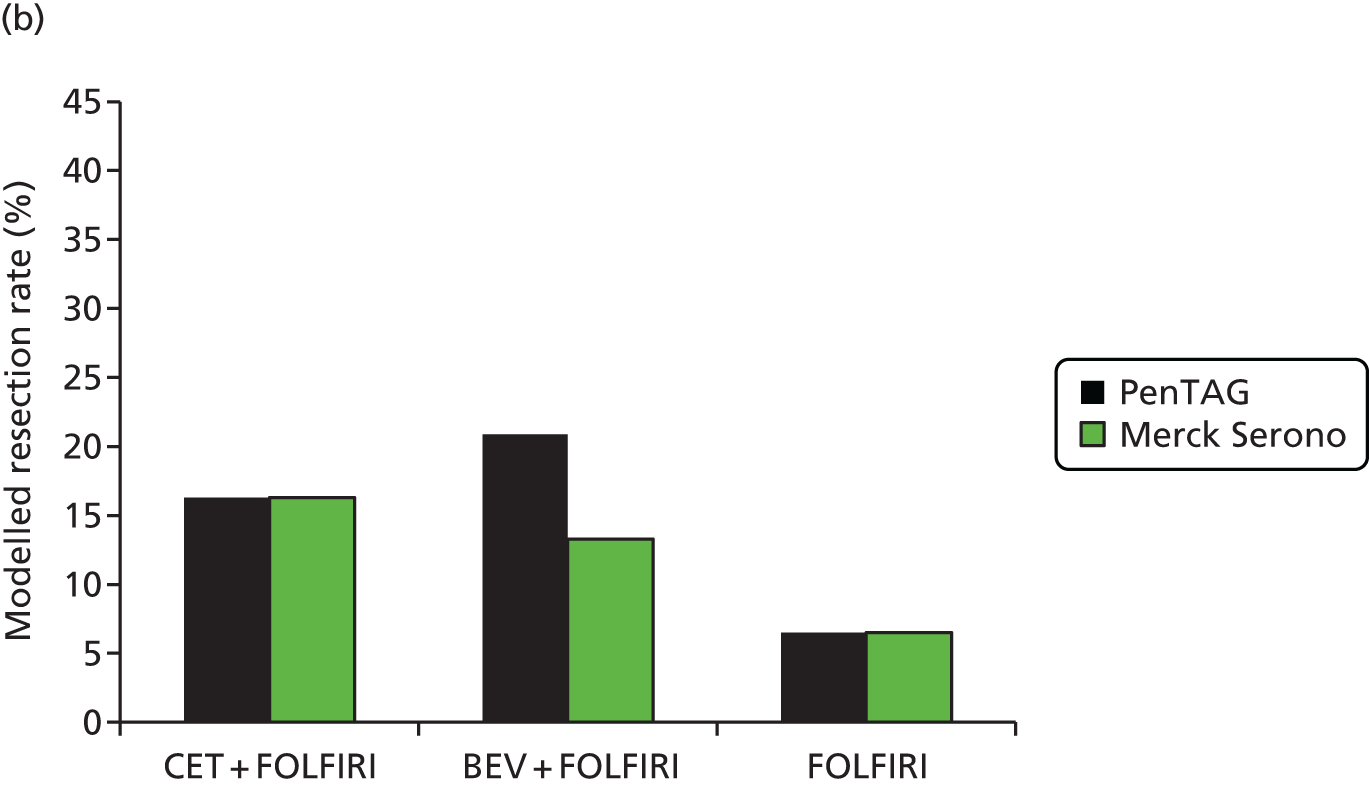
For bevacizumab plus FOLFIRI, some assumptions were necessary. The ‘all patients’ resection rate for bevacizumab plus FOLFIRI in the FIRE-3 trial28 for RAS WT patients was estimated as 17.7% [13.6% × (11.0%/8.4%); value for KRAS WT patients was 13.6% (see Table 90) and we adjust from the KRAS WT population to the RAS WT population using a ratio of 11.0% : 8.4%, as in the PEAK trial29 for bevacizumab plus FOLFOX].
Next, the ‘all patients’ value in the FIRE-3 trial28 for RAS WT patients for cetuximab plus FOLFIRI was estimated as 14.6% [12.1%/83%; value for KRAS WT patients was 12.1% (see Table 90) and we assume that 83% of KRAS WT patients are also RAS WT]. It was also assumed that only participants with RAS WT tumours were resected, given that cetuximab plus FOLFIRI has been shown to be more effective, and is licensed, for this population.
Finally, the logit of the value of 9.0% for bevacizumab plus FOLFIRI (see Table 91) was calculated on the logit scale as logit(7.3%) + (logit(17.7%) – logit(14.6%)), in the manner of an adjusted indirect comparison, in which 7.3% is the chosen value for cetuximab plus FOLFIRI and 17.7% and 14.6% are derived as explained above. We worked on the logit transformation, as this ensured that the resulting resection rates would lie between 0% and 100%.
This is slightly different from the value of 7.3% estimated by Merck Serono. Merck Serono did not justify its value for the resection rate for bevacizumab plus FOLFIRI (7.3%), but it appears that this was set equal to the resection rate for cetuximab plus FOLFIRI (7.3%).
In the liver metastases subgroup, the resection rates for cetuximab plus FOLFIRI and FOLFIRI were taken directly from the CRYSTAL trial (see Table 91 and Figure 11). This was also the approach taken by Merck Serono.
For bevacizumab plus FOLFIRI in the liver metastases subgroup, some assumptions were necessary. First, we estimated the resection rate for the RAS WT population in the FIRE-3 trial28 for cetuximab plus FOLFIRI as 32.6% [14.6% × (16.3%/7.3%), in which 14.6% is the estimated resection rate for all patients and 16.3% and 7.3% are the values reported for the RAS WT population for cetuximab plus FOLFIRI in the subgroup and all patients populations, respectively (see Table 90)].
Next, we estimated the resection rate for the RAS WT population in the FIRE-3 trial28 for bevacizumab plus FOLFIRI as 39.6% [17.7% × (16.3%/7.3%), in which 17.7% is the estimated resection rate for all patients and 16.3% and 7.3% are as described in the previous paragraph].
Finally, the value of 19.8% for bevacizumab plus FOLFIRI (see Table 91) was calculated as 16.3% × (39.6%/32.6%), in the manner of an adjusted indirect comparison, in which 16.3% is the chosen value for cetuximab plus FOLFIRI and 39.6% and 32.6% are derived as explained above.
Finally, the value of the logit of 20.9% for bevacizumab plus FOLFIRI (see Table 91) was calculated as logit(16.3%) + (logit(39.6%) – logit (32.6%)), in the manner of an adjusted indirect comparison, in which 16.3% is the chosen value for cetuximab plus FOLFIRI and 39.6% and 32.6% are derived as explained above.
FOLFOX network
In the FOLFOX network, resection rates for all patients for panitumumab plus FOLFOX and FOLFOX were taken directly from the PRIME trial (see Table 91), as this is the baseline RCT in our model for the FOLFOX network. Merck Serono did not consider panitumumab plus FOLFOX. It estimated the resection rate for FOLFOX as 2.1%, which it reports was taken from the study by Tournigand et al. 111 This is substantially lower than our estimate. The study by Tournigand et al. 111 concerns second-line treatment not restricted to RAS WT patients, whereas our estimate is taken from first-line treatment for RAS WT patients. Therefore, we prefer our value.
The value for bevacizumab plus FOLFOX was calculated as an adjusted indirect comparison, using the chosen value for panitumumab plus FOLFOX and 11.0% and 12.5% as the resection rates for bevacizumab plus FOLFOX and panitumumab plus FOLFOX from the PEAK trial29 (see Table 90). Merck Serono did not model this treatment.
The value of the logit of 20.7% for cetuximab plus FOLFOX (see Table 91) was calculated by first estimating the resection rates for cetuximab plus FOLFOX and FOLFOX for RAS WT patients using the corresponding values for KRAS WT patients from the OPUS and PRIME trials, respectively. Specifically, the estimated rate for RAS WT patients for cetuximab plus FOLFOX = 9.8%/83% = 11.9% (as for the FOLFIRI network, we assumed that 83% of KRAS WT patients are also RAS WT). The estimated rates for RAS and KRAS patients for FOLFOX were taken directly from the PRIME trial.
Finally, the value of the logit of 20.7% for cetuximab plus FOLFOX was calculated as an adjusted indirect comparison, using the 11.9% rate for RAS patients for cetuximab plus FOLFOX in the OPUS trial and the rate for FOLFOX in the PRIME trial and the estimated rate for FOLFOX just calculated.
By comparison, Merck Serono estimated the resection rate for cetuximab plus FOLFOX as 7.3%, substantially lower than our value of 20.7%. Merck Serono did not discuss the derivation of its estimate. However, we assume that it was set equal to their rate for cetuximab plus FOLFIRI. If so, we believe that our estimate, although apparently high, is methodologically more sound, as Merck Serono’s assumption seems unreasonable.
Now, we turn to the derivation of the resection rates for the liver metastases subgroup.
The rates of 17.1% and 31.3% for FOLFOX and panitumumab plus FOLFOX, respectively, were taken directly from the PRIME trial, the base-case RCT in the FOLFOX network.
The rate for bevacizumab plus FOLFOX was estimated using an indirect comparison, using the estimated rate of 31.3% for panitumumab plus FOLFOX and the rates for bevacizumab plus FOLFOX and panitumumab plus FOLFOX from the PEAK trial. 29
Finally, the rate for cetuximab plus FOLFOX was estimated as follows. Ordinarily, we would estimate the rate as logit(17.1%) × (logit(13.3%)/logit(0%)), in which 17.1% is the chosen rate for FOLFOX and 13.3% and 0% are the rates for cetuximab plus FOLFOX and FOLFOX in the OPUS trial. However, estimating the rate in this way gives an estimate of infinity, which is clearly impossible. The extreme value of 0% in the OPUS trial is partly a result of the very small sample size of 12 patients (see Table 90), the result of considering a small subgroup in a small RCT.
Instead, we estimated the rate for cetuximab plus FOLFOX using 17.1% for FOLFOX, as before, and the other estimated rates for all patients in the OPUS trial.
For the probabilistic sensitivity analysis, the resection rates were assumed to follow gamma distributions, with means from the RCTs and variances of the mean calculated by p(1 – p)/n, in which p is the deterministic resection rate and n is the number of patients (see Table 90).
In a scenario analysis we considered the OPUS trial rather than the PRIME trial as the baseline RCT for the FOLFOX network (see Structure of the Peninsula Technology Assessment Group model). As many of the resulting resection rates are based on data we received as AiC, we do not report them here.
Time of resection
In the previous assessment TA176,10 Merck Serono assumed in its revised analysis that the time point at which patients were assessed for curative resection was 16 weeks after the start of treatment (Table 92).
| Time to resection | Source |
|---|---|
| Normally assess after 8 weeks, but others might assess at 16 weeks | Dr Mark Napier (clinical advisor to PenTAG, 2015, personal communication) |
| Of people whose disease responds sufficiently to cetuximab to enable resection of liver metastases, approximately 90% would do so within 12 weeks of treatment with cetuximab | NICE TA176,10 clinical specialists’ opinion |
| All patients would normally stop receiving treatment with cetuximab at the time of the assessment for possible liver resection (i.e. after approximately 12–16 weeks) | NICE TA176,10 clinical specialists’ opinion |
| 16 weeks after the start of treatment | Merck Serono’s revised analysis in TA17610 (section 3.31) |
| Unresected patients were routinely reassessed every four courses of chemotherapy. Surgery was reconsidered every time a documented response to chemotherapy was observed | Adam et al.113 |
| At cycle/month 4 based on Adam et al.,113 who found that most resections occur before 4 months | Merck Serono’s submission, current HTA (section 3.2.2, table 20, p. 49) |
| At 3 months in the model some patients can be referred for curative-intent resection of liver metastases | Merck Serono’s submission, current HTA (section 3.2.2, table 21, p. 50) |
In its submission for the current HTA, Merck Serono’s assumption on the timing of liver resection surgery was based on the study by Adam et al. ,113 as indicated in table 20 (section 3.2.2, p. 49) of the submission, and was 3 months after the start of treatment.
We believe that it is reasonable to assume that liver resection is performed approximately 12 weeks after the start of treatment. This is based on expert opinion (Dr Mark Napier, 2015, personal communication) and TA176,10 and also agrees with the value of 3 months used in the submission from Merck Serono. Given that this is so soon after randomisation, in our model, in common with Merck Serono, and for simplicity, we assume that resection occurs at time zero. The only loss of accuracy is due to a slight overestimation of discounted costs and QALYs for resected patients of just 1%.
Post liver resection: progression-free survival and overall survival
We found that the cost-effectiveness of cetuximab plus FOLFOX/FOLFIRI and panitumumab plus FOLFOX/FOLFIRI was sensitive to mean PFS and OS post resection. Therefore, estimation of these quantities is worthy of close scrutiny.
In the previous assessment TA176,10 OS after liver resection with curative intent was based on the study by Adam et al. 113 This was also the source used by Merck Serono in its submission.
Given sufficient time, we would have performed a systematic review of the literature for PFS and OS after resection. However, because of time constraints, we searched the literature as follows. We performed a forward reference search on Adam et al. 113 in PubMed to identify all relevant studies relating to survival after liver resection for mCRC. This yielded two other candidate studies:
A comparative analysis of these publications is provided in Table 93.
| Variable | Adam et al. 2004113 | Adam et al. 2009149 | Adam et al. 2012150 |
|---|---|---|---|
| Patient characteristics and treatment | |||
| Patients from | Centre Hepato-Biliaire and INSERM E0354 ‘Cancer Chronotherapeutics’, Hôpital Paul-Brousse, Assistance Publique–Hopitaux de Paris Université Paris, Sud Villejuif, France | The AP-HP Hôpital Paul-Brousse, Centre Hepato-Biliaire and Department of Medical Oncology; INSERM, Unité 785; INSERM, Laboratoire ‘Rythmes biologiques et cancers’, Unité 776; Université Paris-Sud, Villejuif, France; and Department of Surgery, University Medical Center Utrecht, Utrecht, the Netherlands | 330 centres in 58 countries, including the UK, with the majority from Western Europe. Data from LiverMetSurvey (www.livermetsurgery.org), accessed 23 November 2011 |
| Patient population | Patients whose metastases were significantly downstaged by chemotherapy | Patients with unresectable colorectal liver metastases at the time of diagnosis who underwent rescue surgery after downsizing chemotherapy and had a minimum follow-up of 5 years from surgery | Patients who underwent conversion chemotherapy and resection for colorectal liver metastases |
| Number of patients initially unresectable | 138 | 184 | 1999 |
| Lines of treatment | One line, 77%; two lines,14%; three lines, 9% | One line, 74%; more lines, 26% | Not reported |
| Stage of disease | Patients with initially unresectable colorectal liver metastases | Patients with initially unresectable colorectal liver metastases | Patients with initially unresectable colorectal liver metastases |
| Site of metastases | 62% of patients with metastases confined to the liver | 73% of patients with metastases confined to the liver | Not reported |
| RAS status | Not determined | Not determined | Not determined |
| Year | 1988–99 | 1988–2002 | 2004–11 |
| Mean age (years) | 57 | 56.9 | Not reported |
| Sex | 56% male, 44% female | 58% male, 42% female | Not reported |
| Total number of resections, including repeat resections | 223 [i.e. 223/138 = 1.6 per patient (p. 650)] | Not reported | Not reported |
| Treatment after resection | Systemic chemotherapy continued for six to eight courses after resection because of the high risk of recurrence (p. 646) | Postoperative chemotherapy in 93% of patients for six to eight cycles | |
| Type of resection | 93% first hepatectomies; 75% major, 25% limited hepatectomies (p. 647) | Major resections in 48% of patients; 26% anatomical, 25% non-anatomical, 49% both | |
| Outcomes | |||
| Postoperative mortality (%) | 0.7 | 0 | Not reported |
| Postoperative morbidity (%) | 28 | 25 | Not reported |
| 5-year disease-free survival, % (number of patients exposed) | 22 (28) | 19 (31) | Not reported |
| 10-year disease-free survival, % (number of patients exposed) | 17 (12) | 15 (12) | Not reported |
| 5-year survival, % (number of patients exposed) | 33 (37) | 33 (41) | 33 (131) |
| 10-year survival, % (number of patients exposed) | 23 (12) | 27 (14) | 20 (23) |
Key information concerning the patient population (such as age and sex composition) was reported in Adam et al. 113 but not in the other two studies. 149,150 OS was reported in all three studies113,149,150 and PFS was detailed in two of the studies. 113,149 Frequencies of surgeries were provided only in Adam et al. 113 Therefore, in common with Merck Serono, we estimated PFS and OS post resection from the study by Adam et al. 113
However, the choice of study had little impact on cost-effectiveness, as OS was similar across the three studies and PFS was similar in the studies by Adam et al. 113,149 (see Table 93).
Modelled progression-free survival post resection
Given the lack of data to the contrary, and in common with Merck Serono, for those patients who had a successful resection we assumed that PFS and OS were independent of first-line treatment.
Progression-free survival was modelled as follows. A progression event is assumed to occur if either a patient dies as a result of general background non-CRC mortality or there is progression because of any other cause. General background non-CRC mortality was modelled explicitly because the PFS tail in Adam et al. 113 is long. Two functional forms were chosen for progression from any other cause: Weibull and log-logistic (Figure 12). The choice of parameters for these distributions was assessed pragmatically by minimising the sums of squares of differences between Kaplan–Meier PFS and modelled PFS. Under this method, the Akaike information criterion and Bayesian information criterion are not obtained. We acknowledge that it would have been preferable to estimate the underlying individual patient data by using the method of Hoyle and Henley151 [as we did for first-line PFS (see Model parameters, First-line progression-free survival: unresected patients)] or the method of Guyot et al. 152 However, given the time constraints, we did not do this, in part because the adjustment for background mortality would have required additional analysis.
Given a 30-year time horizon, mean PFS was estimated as 4.5 years assuming the Weibull distribution and 4.8 years assuming the log-logistic distribution, substantially greater than the mean PFS for unresected patients (see Model parameters, First-line progression-free survival: unresected patients).
For our base-case analysis we chose the Weibull distribution as it is possible that the long tail in the study by Adam et al. 113 is heavily influenced by the small numbers of patients at risk in the tail (e.g. 17 patients at 8 years) and the tail of the log-logistic distribution is longer than the tail of the Weibull distribution (Figure 13).
FIGURE 13.
Peninsula Technology Assessment Group-modelled PFS and OS post resection.
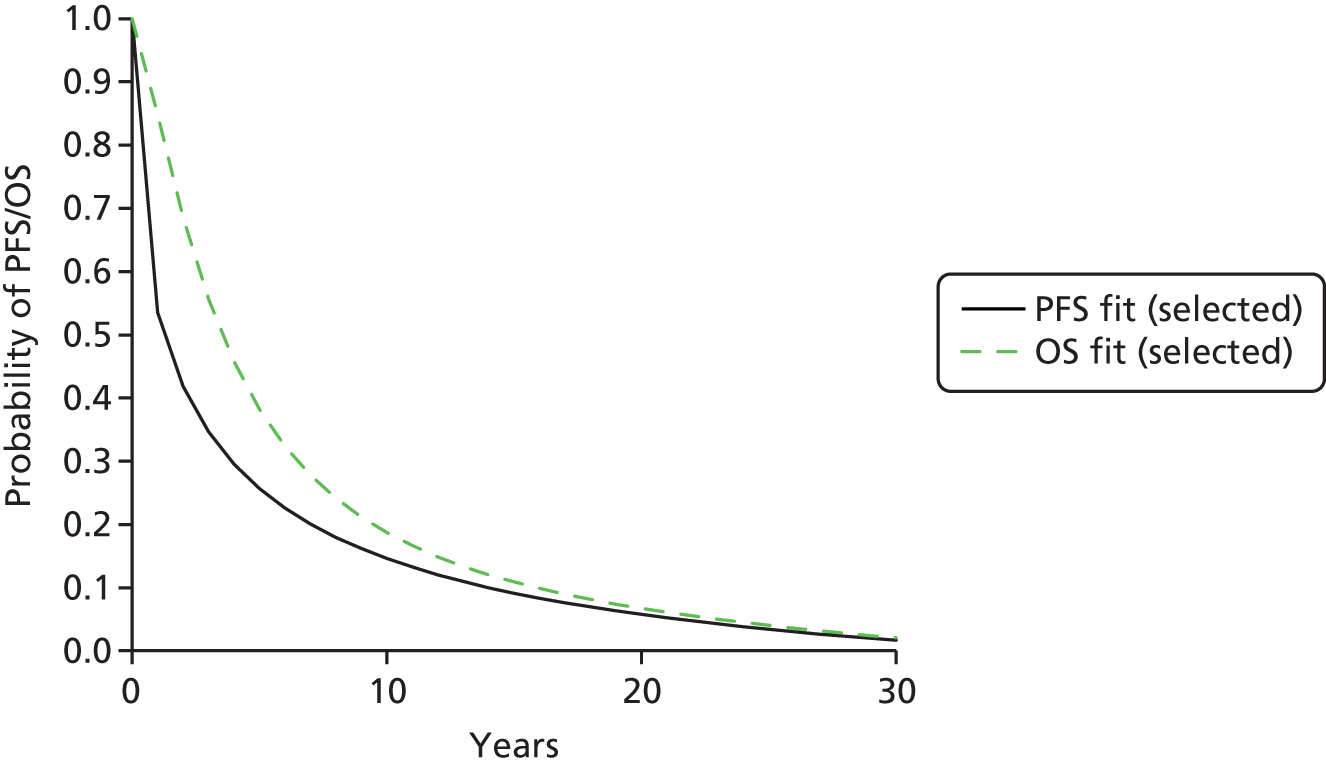
For the probabilistic sensitivity analysis, parameter gamma (shape) of the Weibull distribution was held constant and parameter lambda (scale) was varied in such a way to give the required mean PFS. Mean PFS was modelled as a gamma distribution with the mean equal to the deterministic mean and the standard error (SE) of the mean given by the SD of the Weibull distribution divided by the square root of the number of patients in Adam et al. 113 (n = 138).
Modelled overall survival post resection
Overall survival post resection was modelled as for PFS (Figure 14).
Given a 30-year time horizon, mean OS was estimated as 5.6 years assuming the Weibull distribution and 6.2 years assuming the log-logistic distribution.
We rejected the Weibull distribution as, for time periods of > 13 years, OS was predicted to be lower than PFS. For our base-case analysis we chose the log-logistic distribution as OS was predicted always to be greater than PFS (see Figure 13).
Based on their overly short time horizon of 10 years, Merck Serono predicted substantially shorter mean PFS and OS than us:
-
PFS – 4.5 years in our model compared with 2.8 years in Merck Serono’s model
-
OS – 6.2 years in our model compared with 4.1 years in Merck Serono’s model.
This difference in itself acts to improve the cost-effectiveness of cetuximab plus FOLFOX/FOLFIRI and panitumumab plus FOLFOX/FOLFIRI in our model compared with Merck Serono’s model, given that these treatments have relatively high resection rates.
For the probabilistic sensitivity analysis of OS, similar to the calculations for PFS, one parameter of the log-logistic distribution was held constant and the other parameter was varied in such a way to give the required mean OS. Mean OS was modelled as a gamma distribution, with the mean equal to the deterministic mean and the SE of the mean given by the SD of the log-logistic distribution divided by the square root of the number of patients in Adam et al. 113 (n = 138).
First-line progression-free survival: unresected patients
In common with Merck Serono, we based our estimates of first-line PFS for unresected patients on the data from the pivotal RCTs.
However, Merck Serono (see Chapter 5, De novo economic evaluation) and, as far as we are aware, all previous economic analyses of first-line treatments for mCRC, estimated PFS for unresected patients directly from the RCTs of all patients (resected and unresected). We believe that this overestimates PFS for unresected patients, given that some patients in the RCTs are resected and that PFS for these patients is substantially longer than that for unresected patients (see Model parameters, Post liver resection: progression-free survival and overall survival).
In summary, we estimated PFS for unresected patients as follows.
-
Extrapolate PFS for all patients (resected and unresected) separately for each treatment arm from the five RCTs28,29,43,44,65 relevant to the current HTA. We found that the Weibull distribution was most appropriate in all cases.
-
Calculate the mean PFS and SE of the mean from each extrapolated PFS curve.
-
Perform a mixed-treatment comparison of the mean PFS.
-
Estimate the mean PFS for patients post resection based on data from Adam et al. ,113 which are likely to be available at the maximum follow-up time of 3 years in the RCTs. This was assumed to apply in all modelled treatment arms.
-
Estimate PFS for unresected patients. The mean PFS for unresected patients was estimated from the mean PFS for all patients (step 3), the mean PFS for resected patients (step 4) and the proportion of patients in each treatment arm who undergo resection (see Chapter 1, Measurement of disease). It was assumed that PFS for unresected patients follows the same type of distribution as for all patients (step 1) (Weibull in all cases). The shape parameter for the Weibull distribution was estimated from step 2 and the scale parameter was estimated from the mean PFS for unresected patients (step 2) and the shape parameter.
These steps are provided in more detail in the following sections.
1. Extrapolate progression-free survival for all patients (resected and unresected)
First, the Kaplan–Meier data were extracted from the RCTs28,29,43,44,65 using DigitizeIt software [see www.digitizeit.de/ (accessed 14 March 2017)]. The published numbers of patients at risk at each of several time points were recorded. Next, the underlying individual patient data were estimated using these data and the method of Hoyle and Henley,151 using the online spreadsheet. 151 This method has been shown to be accurate. 153
The fits of the exponential, Weibull, log-logistic, log-normal and logistic distributions were estimated using the maximum likelihood method, using the R code (The R Foundation for Statistical Computing, Vienna, Austria) in the spreadsheet of Hoyle and Henley (version 1.2). 151 In every case, we chose the Weibull distribution because:
-
The Weibull usually gave the lowest Akaike information criterion and Bayesian information criterion values. If it did not, the values were nearly the lowest of all distributions.
-
It seemed desirable to choose the same type of distribution for each treatment within the FOLFOX network and, separately, for each treatment within the FOLFIRI network, because the choice of distribution affects mean PFS and we believe that substantial evidence would be required to choose different distributions.
We note that Merck Serono chose the Weibull distribution for all treatments in the FOLFIRI network and the log-logistic distribution for both treatments in the FOLFOX network.
Our chosen curve fits are provided in Figure 15. In each case, the mean and variance–covariance matrix of the parameters of the Weibull distribution were recorded.
FIGURE 15.
First-line PFS (unresected patients) in the PenTAG model: (a) CET + FOLFOX vs. FOLFOX from the OPUS trial;65 (b) PAN + FOLFOX vs. FOLFOX from the PRIME trial;44 (c) PAN + FOLFOX vs. BEV + FOLFOX from the PEAK trial;29 (d) CET + FOLFIRI vs. FOLFIRI from the CRYSTAL trial;43 and (e) CET + FOLFIRI vs. BEV + FOLFIRI from the FIRE-3 trial. 28 BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.



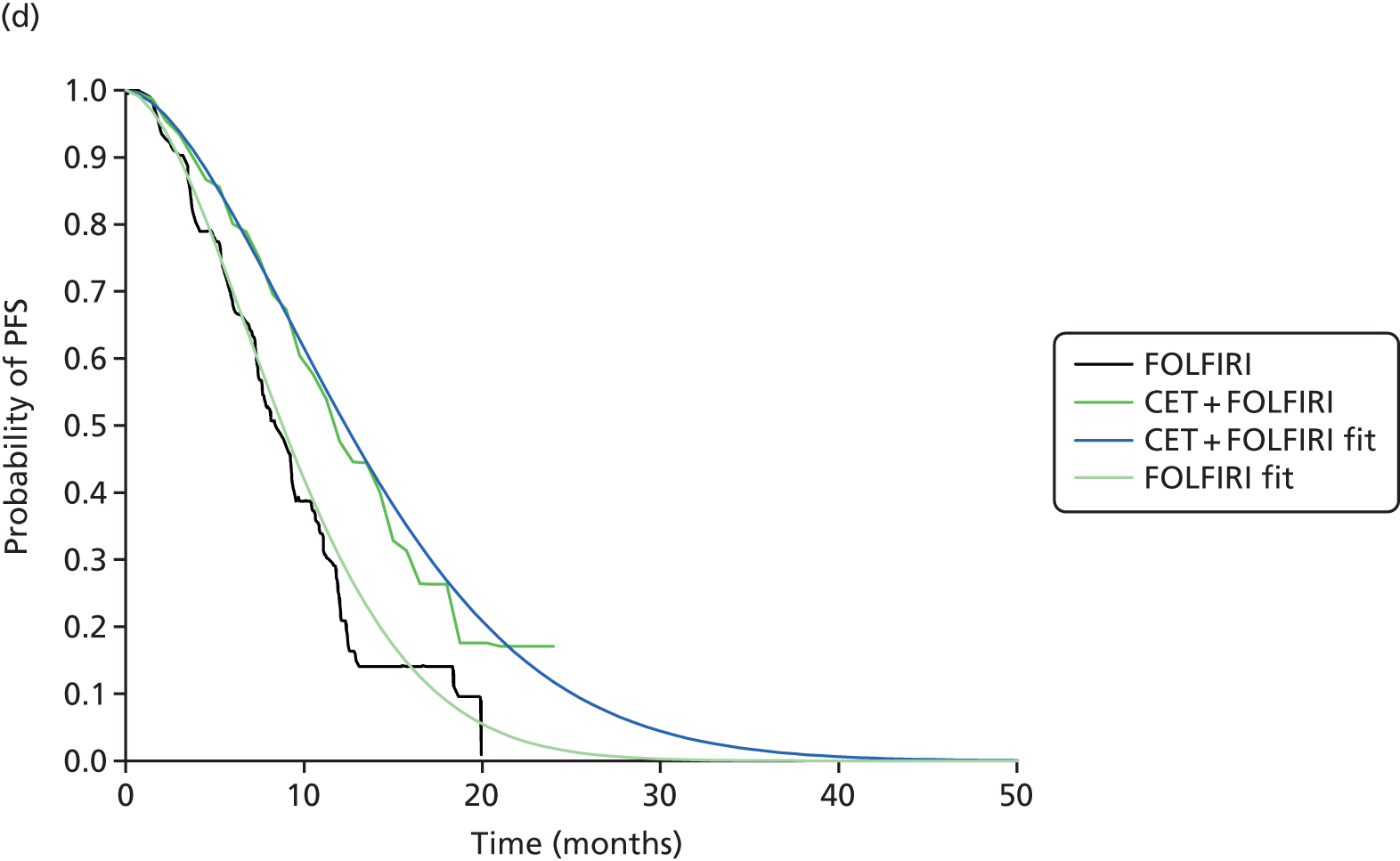
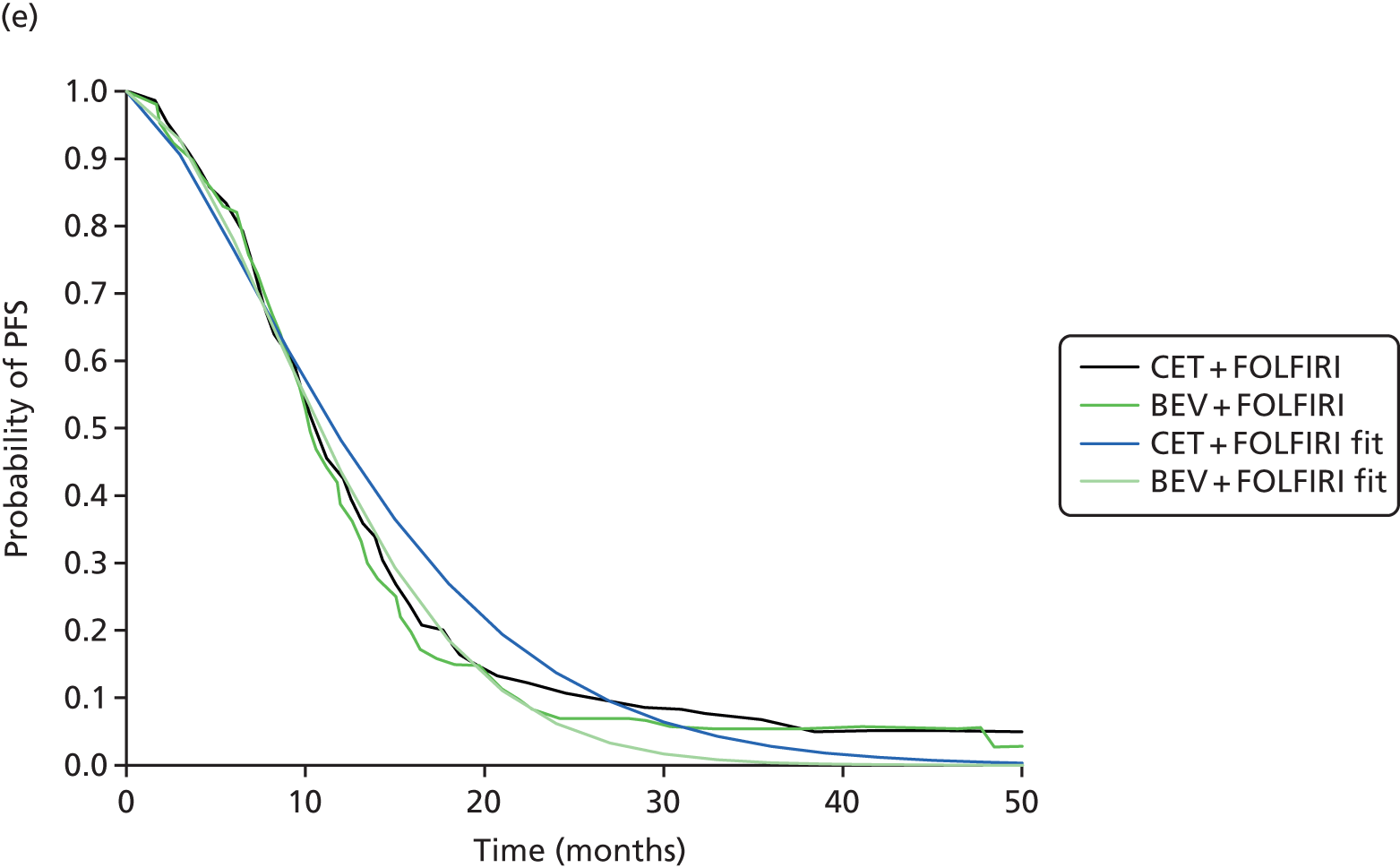
2. Calculate mean progression-free survival and standard error of the mean
The means and SEs of the mean were then calculated from the mean and variance–covariance matrices of the Weibull parameters (Table 94).
| RCT | Analysis | CET + FOLFIRI | FOLFIRI | BEV + FOLFIRI |
|---|---|---|---|---|
| FOLFIRI network | ||||
| CRYSTAL (baseline)43 | Mean | 13.68 | 9.67 | |
| SE | 1.09 | 0.59 | ||
| Gamma of Weibull | 1.69 | 1.74 | ||
| FIRE-328 | Mean | 13.53 | 11.88 | |
| SE | 0.8 | 0.58 | ||
| Gamma of Weibull | 1.45 | 1.74 | ||
| RCT | PFS | CET + FOLFOX | FOLFOX | PAN + FOLFOX |
| FOLFOX network | ||||
| PRIME (baseline)44 | Mean | 9.46 | 11.55 | |
| SE | 0.45 | 0.57 | ||
| Gamma of Weibull | 1.67 | 1.68 | ||
| OPUS65 | Mean | 9.38 | 6.72 | |
| SE | 1.63 | 0.64 | ||
| Gamma of Weibull | 1.7 | 1.74 | ||
| PEAK29 | Mean | 15.14 | ||
| SE | 1.28 | |||
| Gamma of Weibull | 1.59 | |||
3. Mixed-treatment comparison of mean progression-free survival
The CRYSTAL trial43 was chosen as the baseline trial for the FOLFIRI network and the PRIME trial44 was chosen as the baseline trial for the FOLFOX network (see Structure of the Peninsula Technology Assessment Group model).
For the purposes of the economic model, we performed a mixed-treatment comparison for PFS on mean survival, not the HR. Indeed, this was our approach in our role as the Assessment Group in 2011 for the NICE MTA of cetuximab, panitumumab and bevacizumab for subsequent lines of treatment for CRC. 141 Our approach was endorsed by the NICE appraisal committee.
Furthermore, there is growing awareness that the HR cannot be recommended as a general measure of the treatment effect in RCTs. 154 It has recently been argued that, for a HR to make scientific sense, we must assume that the proportional hazards of the treatment effect holds, at least approximately, and that when the proportional hazards assumption fails it is misleading to report the treatment effect through the estimated HR, as it depends on follow-up time. 154 Instead, the ‘restricted mean’ has recently been advocated as a superior method of assessment of the treatment effect in trials, with the restricted mean for a trial arm defined as survival up to some agreed time point. 154 For our purposes, as in the previous assessment of cetuximab, panitumumab and bevacizumab for subsequent lines of treatment for CRC,141 we performed a mixed-treatment comparison of mean survival, which is in the spirit of the ‘restricted mean’, but with the time point set to infinity and survival extrapolated to infinity. We argue that the full, not restricted, life expectancy is a preferable clinical outcome as (1) cost-effectiveness is driven by the overall mean and (2) for the purposes of the mixed-treatment comparison it would be difficult to choose a time point relevant to all trials.
The NMAs were undertaken within a Bayesian framework using WinBUGS. Prior distributions, when used, were defined as vaguely as possible.
The FOLFOX and FOLFIRI networks were analysed independently. FOLFOX was the baseline treatment in the FOLFOX network and FOLFIRI was the baseline treatment in the FOLFIRI network. The absolute treatment effects were obtained from the NMA models, with the FOLFOX analysis based on the PRIME trial and the FOLFIRI analysis based on the CRYSTAL trial.
Models with a normal likelihood and identity link were used. 66 Analyses were run with three chains and an initial burn-in of 50,000 iterations, followed by an additional 20,000 iterations on which the results were based. Further implementation in WinBUGS was as described in Chapter 3 (see Network meta-analysis). Because of the small number of RCTs contributing to each network, only fixed-effects models were used.
4. Estimate mean progression-free survival for patients post resection
We estimated mean PFS for patients post resection using data from the study by Adam et al. ,113 which are likely to be available at the maximum follow-up times in the RCTs. Expressed differently, we estimated the likely PFS from data for resected patients in the RCTs.
We judge that it is reasonable to assume that PFS from Adam et al. 113 up to 3 years is likely to be fully reflected in PFS from the RCTs, as this appears to be the latest time at which there are few censorships in the OS data from the RCTs in our base-case analysis: CRYSTAL,43 PRIME44 and OPUS. 65
Specifically, for the CRYSTAL trial, inspection of figure 3B in the study by Van Cutsem et al. 43 reveals that there were very few censorships for OS for follow-up up to 3 years. In detail, in the chemotherapy arm, at 3 years the probability of OS is approximately 0.23, which, given that 189 patients were randomised to this arm, gives an estimated 43 patients at risk at 3 years if there are no censorships. Given that this is close to the 38 patients at risk, this implies that follow-up is largely complete up to 3 years. By 4 years, at 3 years the probability of OS is approx. 0.18, which, given that 189 patients are randomised to this arm, gives an estimated 34 patients at risk at 3 years if there are no censorships. Given that this is substantially greater than the actual 10 patients at risk, follow-up is incomplete up to 4 years.
Similarly, inspection of the OS Kaplan–Meier graphs from the PRIME and OPUS trials revealed a similar follow-up time.
Given that the probability of PFS for resected patients at 3 years is 0.30 from Adam et al. ,113 we estimated the mean PFS for resected patients given data up to 3 years as 2.5 years, assuming a constant hazard.
5. Estimate progression-free survival for unresected patients
Next, we estimated mean PFS for unresected patients using the following equation:
We assumed that PFS for unresected patients follows the same distribution as for all patients (Weibull in all cases). The shape parameter for the Weibull distribution was estimated from step 1 and the scale parameter was estimated from the mean PFS for unresected patients and the shape parameter.
Modelled PFS for all patients from the RCTs, resected patients and unresected patients is provided in Figure 16 for the FOLFOX network and in Figure 17 for the FOLFIRI network.
FIGURE 16.
First-line PFS for the FOLFOX network in the PenTAG model: (a) all patients; (b) resected patientsa; and (c) unresected patients. a, PFS post resection is assumed to be the same for all arms. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.


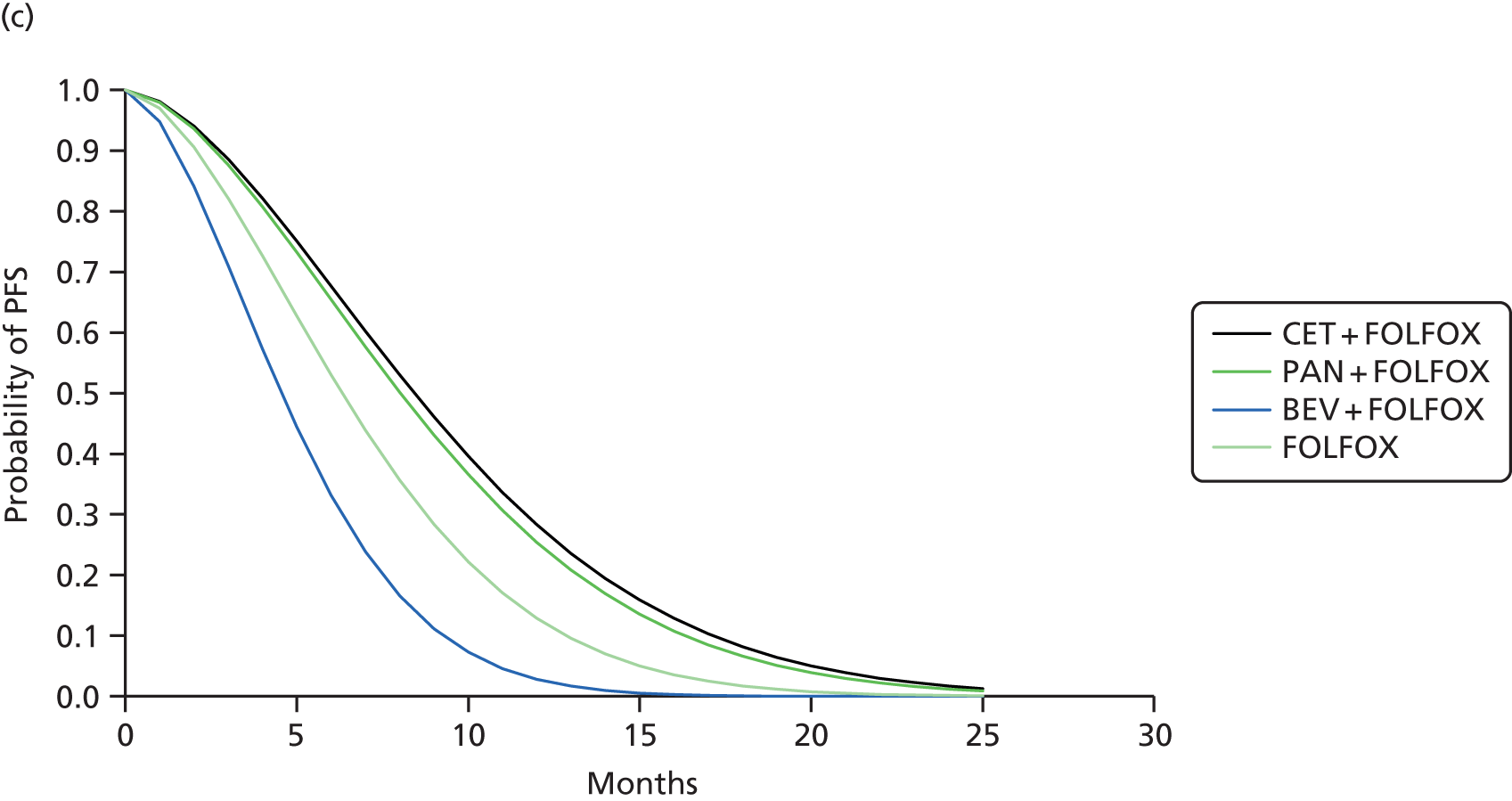
FIGURE 17.
First-line PFS for the FOLFIRI network in the PenTAG model: (a) all patients; (b) resected patients; and (c) unresected patients. PFS post resection is assumed to be the same for all arms. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
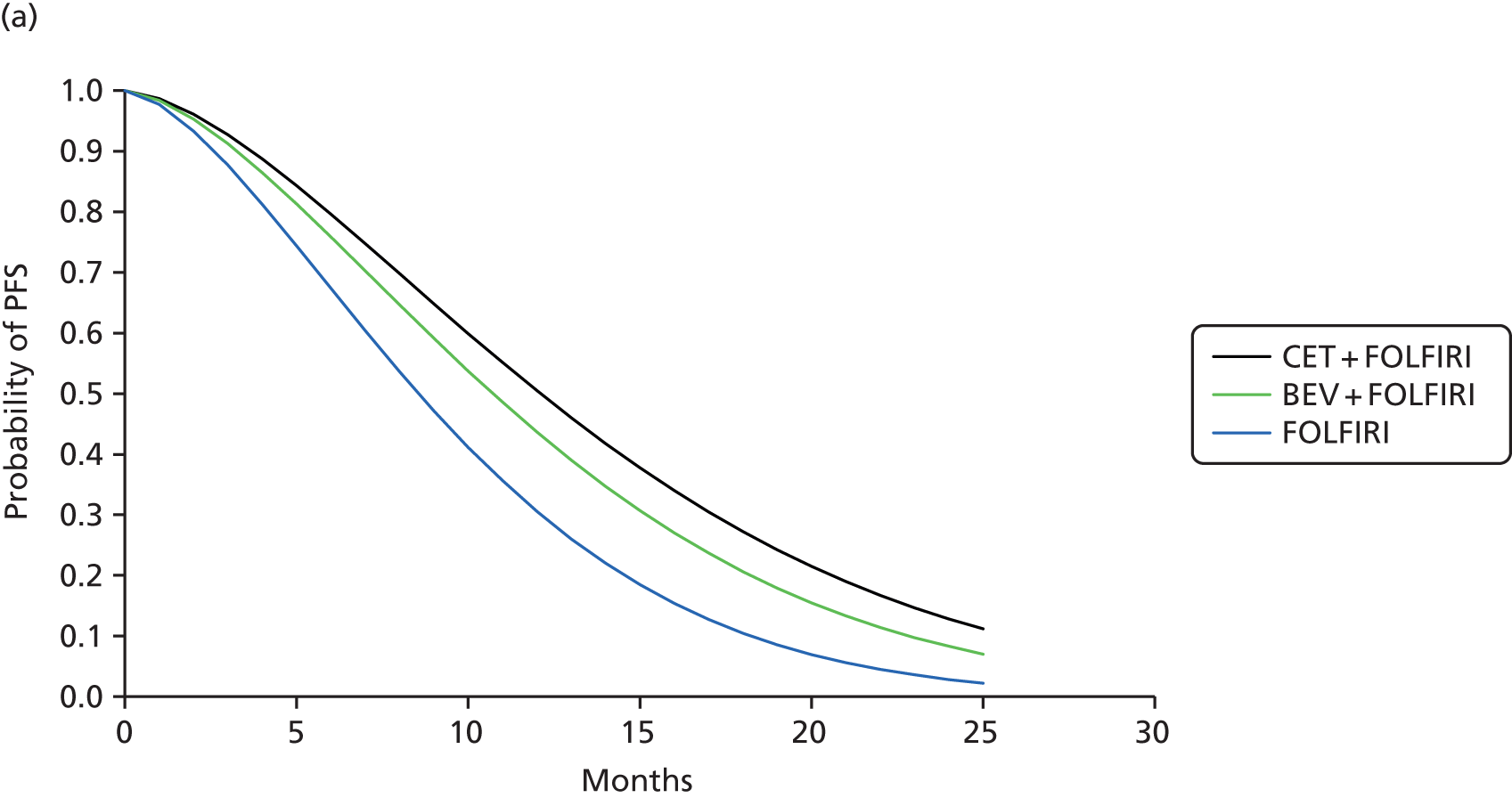
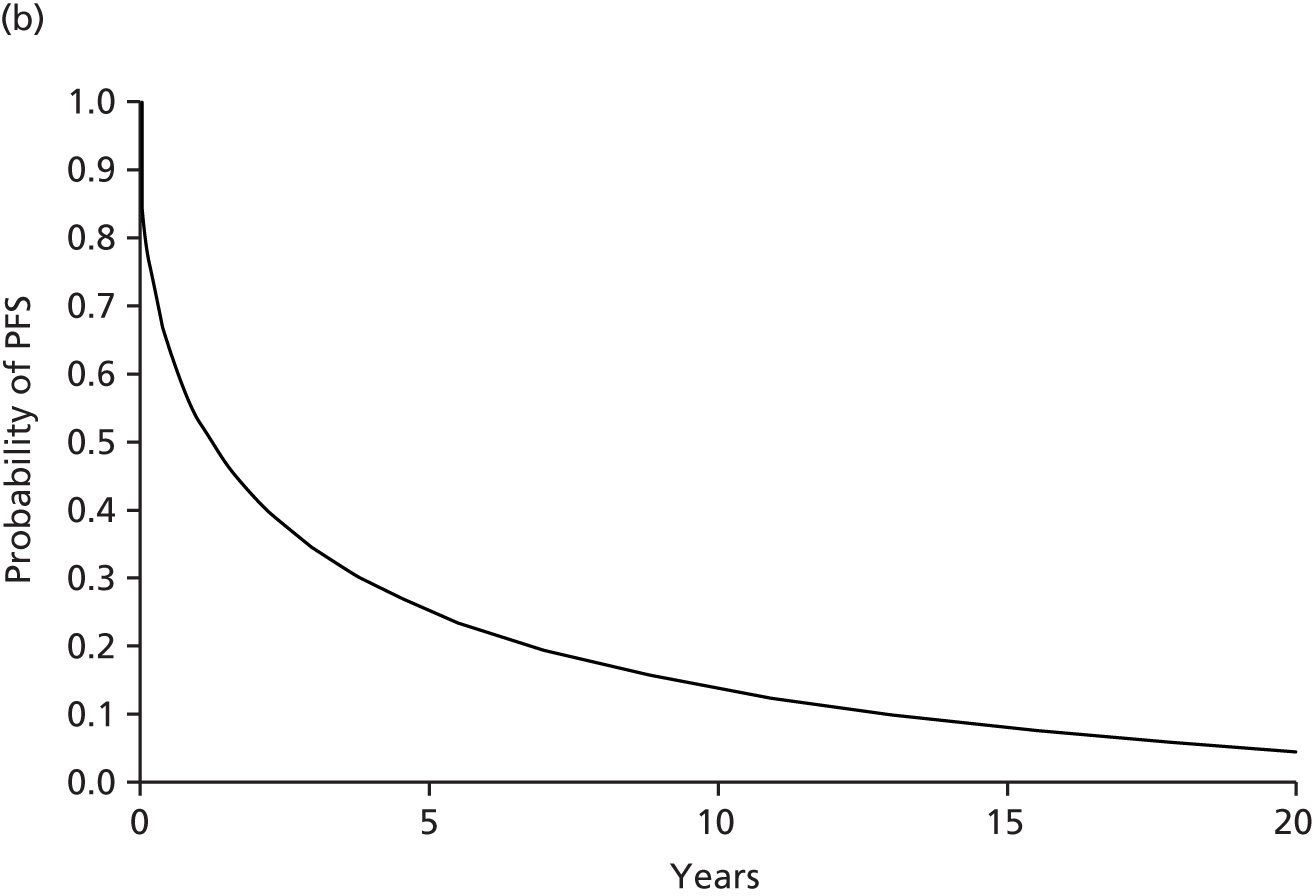

Notice that PFS for unresected patients is shorter than that for all patients, as PFS for resected patients is substantially greater than that for unresected patients (noting the difference in the scale of the time axis).
Comparison with the Merck Serono model
For the FOLFOX network, our estimates of mean PFS for resected and unresected patients are very similar to those of Merck Serono (see Chapter 5, De novo economic evaluation) for unresected patients only (Figure 18a). However, our estimates of mean PFS for unresected patients only are substantially lower than those of Merck Serono as we subtracted PFS for resected patients, as described earlier.
FIGURE 18.
First-line mean PFS in the PenTAG model and the Merck Serono model: (a) FOLFOX network; and (b) FOLFIRI network. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
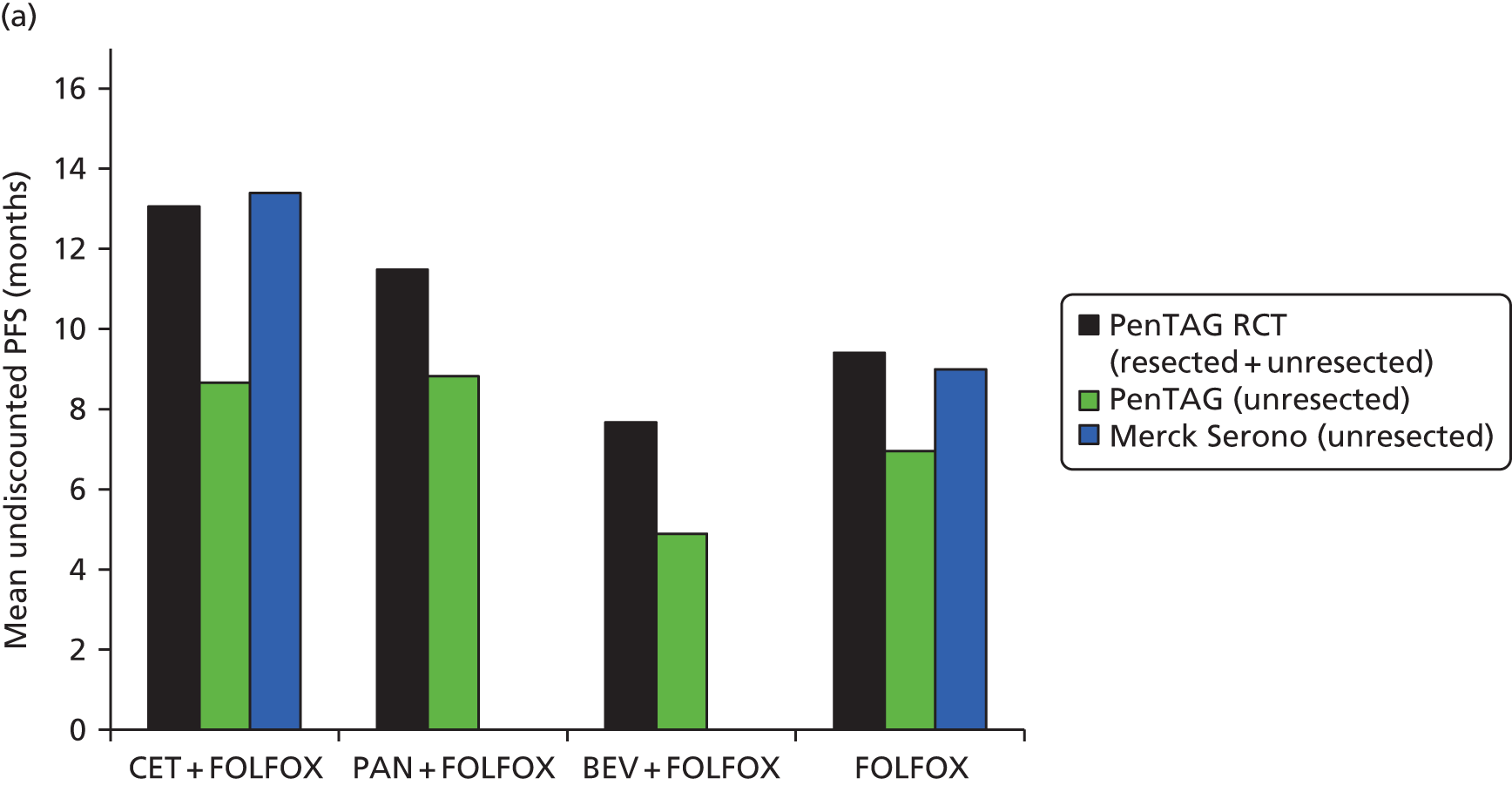
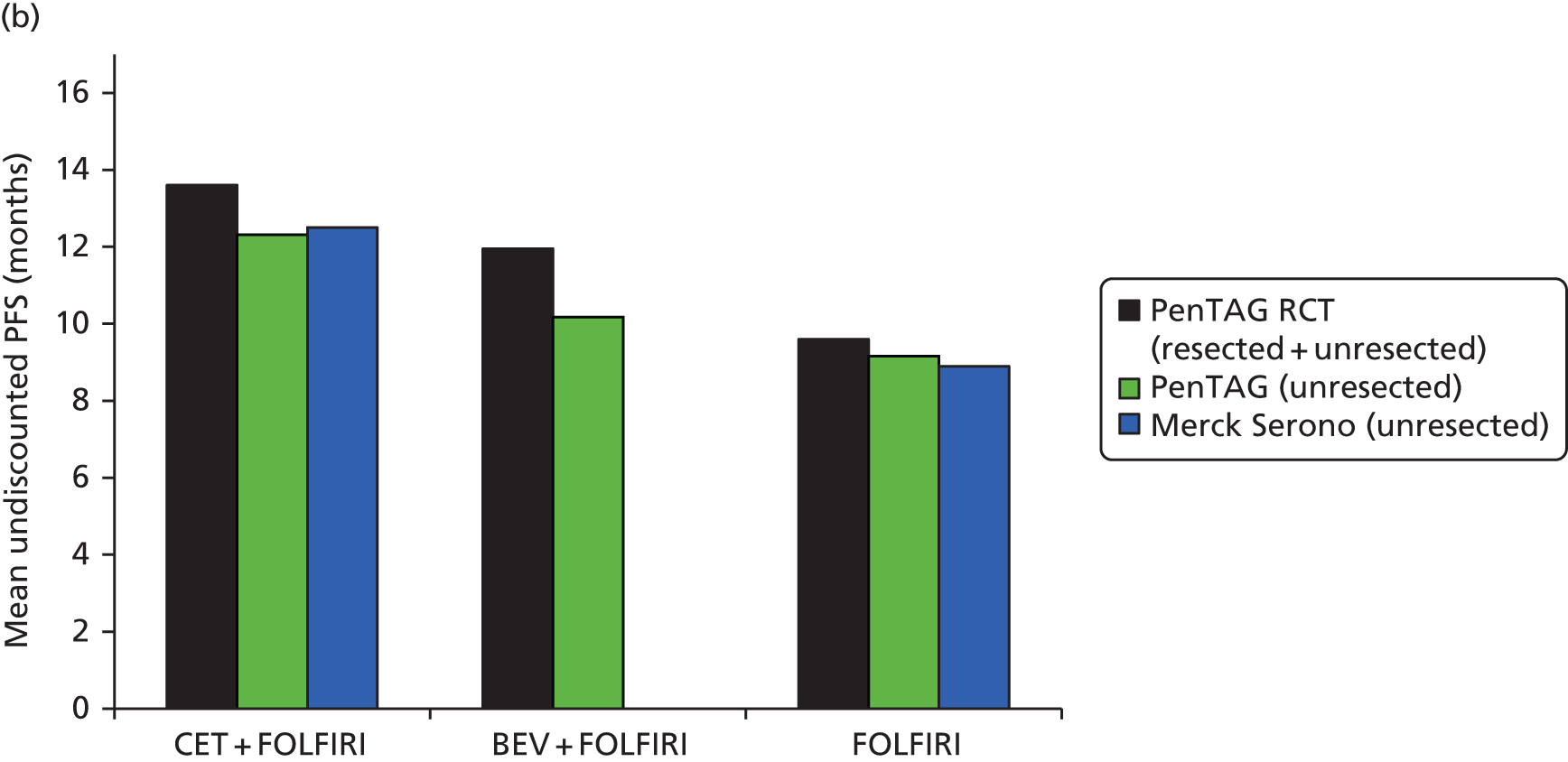
For the FOLFIRI network, our estimates of mean PFS for resected and unresected patients are slightly higher than those of Merck Serono for unresected patients only (see Figure 18b). Coincidently, even though we subtracted PFS for resected patients, our estimates of mean PFS for unresected patients only are very similar to those of Merck Serono.
OPUS trial as the baseline randomised controlled trial in the FOLFOX network
In a scenario analysis we used the OPUS trial65 rather than the PRIME trial44 as the baseline RCT for the FOLFOX network (see Structure of the Peninsula Technology Assessment Group model).
In this case, we estimated the following mean PFS for unresected patients with and without liver-limited disease (‘all patients’):
-
cetuximab plus FOLFOX – 9.4 months (OPUS trial;65 see Table 94).
-
panitumumab plus FOLFOX – 8.2 months {estimated as 6.7 months FOLFOX [OPUS trial65] × [11.55 months panitumumab plus FOLFOX (PRIME trial44)/9.46 months FOLFOX (PRIME trial44)]}
-
bevacizumab plus FOLFOX – 5.5 months {estimated as 8.2 months panitumumab plus FOLFOX [estimate] × [10.12 months bevacizumab plus FOLFOX (PEAK trial29)/15.14 panitumumab plus FOLFOX (PEAK trial29)]}
First-line progression-free survival liver metastases subgroup: unresected patients
Data on first-line PFS for the liver metastases subgroup of the RAS WT population are rather limited (Table 95).
| RCT | Treatment | HR (95% CI) | Median PFS (months) (95% CI) |
|---|---|---|---|
| FOLFIRI network | |||
| CRYSTAL | CET + FOLFIRI | 0.21 (0.09 to 0.49) | 14 (NR) |
| FOLFIRI | 8.1 (NR) | ||
| FIRE-3 | CET + FOLFIRI | NR (Merck Serono) | NR (Merck Serono) |
| BEV + FOLFIRI | |||
| FOLFOX network | |||
| OPUS | CET + FOLFOX | 0.35 (0.06 to 1.91) | NR |
| FOLFOX | 7.4 (NR) | ||
| PEAK | PAN + FOLFOX | (Confidential information has been removeda) | (Confidential information has been removedb) |
| BEV + FOLFOX | (Confidential information has been removedb) | ||
| PRIME | PAN + FOLFOX | 0.75 (0.48 to 1.19) | |
| FOLFOX | |||
Progression-free survival for the liver metastases subgroup for resected and unresected patients combined was estimated as follows. When the median PFS for a particular treatment A for the subgroup was available, the mean PFS for the subgroup was estimated as:
The assumption is that, for each treatment, the shape of the PFS curve for the subgroup is the same as the shape of the PFS curve for all patients.
For cetuximab plus FOLFOX, no estimates of median PFS for the liver metastases subgroup were available. Instead, we estimated the ratio in Equation 4 for cetuximab plus FOLFOX as equal to the corresponding ratio for cetuximab plus FOLFIRI.
Similarly, for bevacizumab plus FOLFIRI, no estimates of median PFS for the liver metastases subgroup were available. Instead, we estimated the ratio in Equation 4 for bevacizumab plus FOLFIRI as equal to the corresponding ratio for bevacizumab plus FOLFOX.
This approach yielded the estimates of mean PFS for all patients (resected and unresected) for the liver metastases subgroup in Figure 19.
FIGURE 19.
First-line mean PFS for the liver metastases subgroup: (a) FOLFOX network; and (b) FOLFIRI network. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
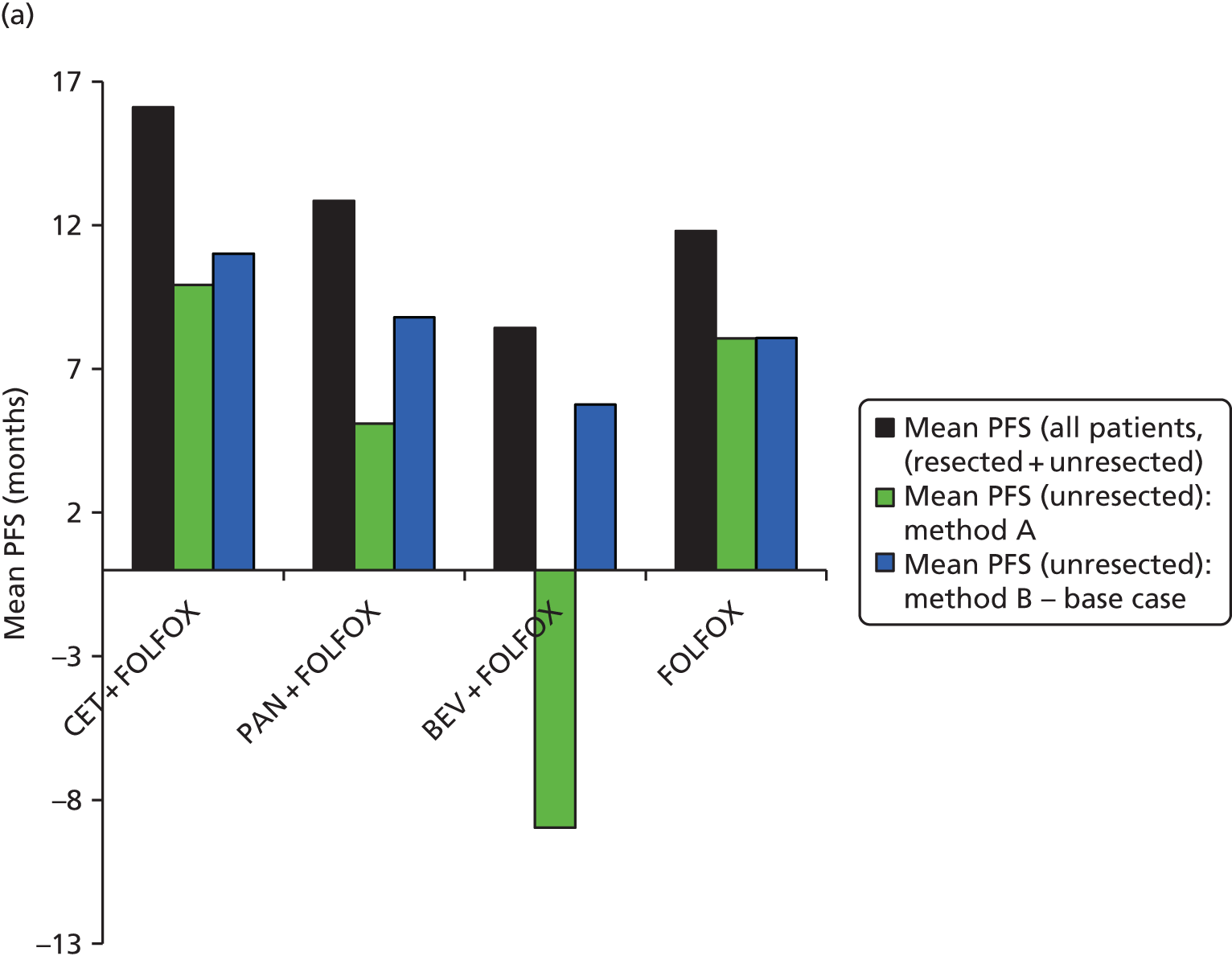
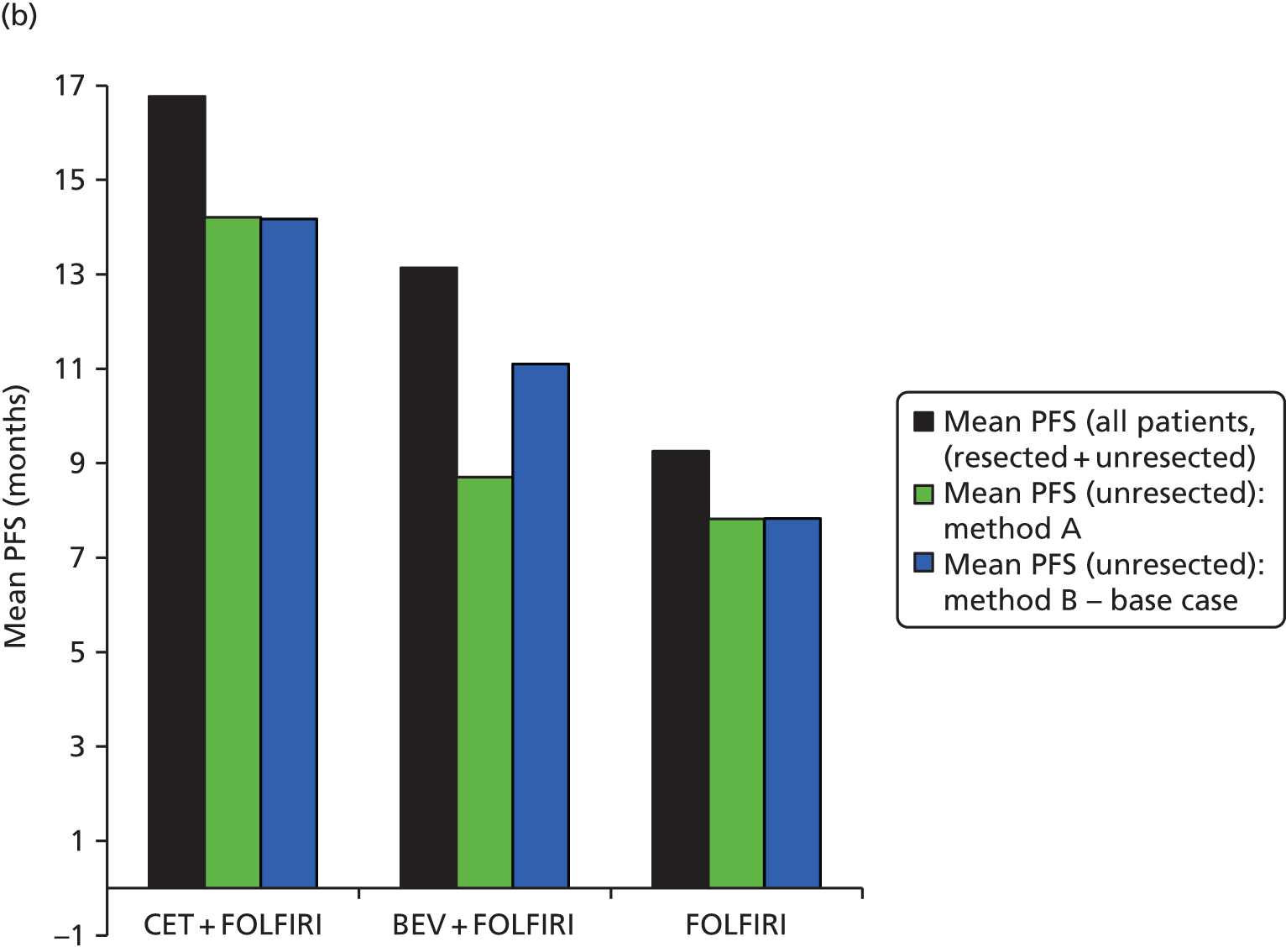
Next, mean PFS for the unresected patients in the liver metastases subgroup was first estimated using step 1, as described earlier for all patients, by subtracting the mean PFS for resected patients and using the resection rates specific to the subgroup. This yielded the estimates of mean PFS for unresected patients in the liver metastases subgroup in Figure 19.
However, this method is clearly inappropriate because it yields a negative estimated mean PFS for unresected patients for bevacizumab plus FOLFOX (see Figure 19a).
We stress that the mean PFS for unresected patients for the liver metastases subgroup is highly uncertain for all treatments given the number of assumptions. Given that cost-effectiveness is sensitive to PFS, then cost-effectiveness is also highly uncertain for all treatments for the liver metastases subgroup.
For the probabilistic sensitivity analysis, PFS for unresected patients was calculated as for the deterministic analysis, but, in addition, we allowed for uncertainty in the following variables as these variables are all used to calculate PFS for unresected patients:
-
PFS (resected and unresected patients) (discussed below)
-
resection rates (see Model parameters, Resection rates)
-
post resection PFS (see Model parameters, Post liver resection: progression-free survival and overall survival).
Mean PFS for resected and unresected patients was calculated using a mixed-treatment comparison, as described in step 3 (see Mixed-treatment comparison of mean progression-free survival). For the FOLFOX network, this yielded the following covariance matrix on the log scale, with columns and rows corresponding to FOLFOX, cetuximab plus FOLFOX and panitumumab plus FOLFOX, in that order:
The log of the mean PFS for resected and unresected patients was then estimated as a multivariate normal distribution with deterministic means and the covariance matrix given above.
The covariance matrix for the FOLFIRI network, with columns and rows corresponding to FOLFIRI and cetuximab plus FOLFIRI, in that order, is:
Similarly, the log of the mean PFS for resected and unresected patients was then estimated as a multivariate normal distribution with deterministic means and the covariance matrix given above.
Mortality from first-line progression-free survival
Some of the progression events will be related to deaths. Unfortunately, we could find no information on the number of deaths in the first-line PFS health state in either the RAS or the KRAS populations in the five pivotal RCTs. 28,29,43,44,65 However, Merck Serono provided some useful data in its model. We estimated mortality in the first-line PFS health state as follows.
Merck Serono provided the survival curve for progressions not related to death for the treatment arms in Table 96. We calculated the means in Table 96.
| Progression parameter | CET + FOLFOX | FOLFOX | CET + FOLFIRI | FOLFIRI |
|---|---|---|---|---|
| Mean progression (years) not related to death | 1.15 | 0.77 | 1.07 | 0.76 |
| Mean PFS in unresected patients (years) (Merck Serono model) | 1.04 | 0.74 | 0.98 | 0.73 |
| Estimated number of deaths as a percentage of all progressions | 10 | 4 | 8 | 4 |
First:
-
mean progression not related to death was set equal to 1/(rate of progression not related to death).
-
mean progression all causes was set equal to 1/(rate of progression not related to death + rate of progression related to death).
From these simultaneous equations, we can calculate each component rate.
Then, the proportion of all progressions relating to death was estimated as:
Because of the paucity of data, we pragmatically estimated the proportion of all progressions related to death as the average of the proportions in Table 96 (6%).
This figure was used for all seven treatment arms of our model to calculate the number of deaths in each model cycle from the first-line PFS health state.
Furthermore, given the lack of alternative data, the same proportion was used to calculate the number of deaths in each model cycle from the second-line health state.
In the results section, we show that cost-effectiveness is very insensitive to the proportion used.
First-line time on treatment
The mean times on first-line drug treatment are extremely important quantities because they affect the total mean costs of drug acquisition and administration per person, which are critical drivers of cost-effectiveness.
We estimated the mean treatment duration for each first-line treatment as follows.
-
We either used the actual mean first-line treatment duration in each of the pivotal RCTs provided by the relevant company or estimated this quantity based on the median treatment duration in each RCT (Tables 97 and 98).
-
We estimated the mean treatment duration for each first-line treatment by simple indirect comparison. Given that the CRYSTAL and PRIME trials were baseline RCTs, the modelled mean treatment durations for FOLFIRI and cetuximab plus FOLFIRI were taken directly from the CRYSTAL trial and for FOLFOX and cetuximab plus FOLFOX were taken directly from the PRIME trial (see Table 97).
-
For each treatment, we compared the estimated mean treatment duration with the estimated mean first-line PFS for unresected patients (see Model parameters, First-line progression-free survival: unresected patients). We would expect the mean treatment duration to be lower, because in all RCTs treatment was supposed to stop on progression. However, we show below that this was generally not the case; usually, mean treatment duration was greater than mean first-line PFS for unresected patients.
| Treatment | From RCTs: median treatment duration (months) | Step 1: estimated or actual mean treatment duration (months) | Step 2: modelled mean treatment duration (months) |
|---|---|---|---|
| FOLFOX network | |||
| CET + FOLFOX | 5.6 (OPUS65) | (Confidential information has been removeda)b (OPUS65) | (Confidential information has been removeda) (indirect comparison) |
| FOLFOX | 4.6 (OPUS65), 6.2 (PRIME44) | (Confidential information has been removeda)b (OPUS65), 7.2b (PRIME44) | 7.2 (PRIME44) |
| PAN + FOLFOX | 6.5 (PRIME44), 7.5 (PEAK29) | 8.2b (PRIME44), 10.7c (PEAK29) | 8.2 (PRIME44) |
| BEV + FOLFOX | 5.9 (PEAK29) | 8.5c (PEAK29) | 6.4 (indirect comparison) |
| FOLFIRI network | |||
| CET + FOLFIRI | 7.4 (CRYSTAL43), 4.8 (FIRE-328) | (Confidential information has been removeda)b (CRYSTAL43), 6.9c (FIRE-328) | (Confidential information has been removeda) (CRYSTAL43) |
| FOLFIRI | 5.8 (CRYSTAL43) | (Confidential information has been removeda)b (CRYSTAL43) | (Confidential information has been removeda) (CRYSTAL43) |
| BEV + FOLFIRI | 5.3 (FIRE-328) | 7.6c (FIRE-328) | (Confidential information has been removeda) (indirect comparison) |
| Treatment | Step 1: estimated or actual mean treatment duration (months) | Step 2: modelled mean treatment duration (months) |
|---|---|---|
| FOLFOX network | ||
| CET + FOLFOX | (Confidential information has been removeda)b (OPUS65) | (Confidential information has been removeda) (indirect comparison) |
| FOLFOX | (Confidential information has been removeda)b (OPUS6), 8.6b (PRIME44) | 8.6 (PRIME44) |
| PAN + FOLFOX | 9.3b (PRIME44), NR (PEAK29) | 9.3 (PRIME44) |
| BEV + FOLFOX | NR (PEAK29) | 7.1c |
| FOLFIRI network | ||
| CET + FOLFIRI | (Confidential information has been removeda)b (CRYSTAL43), NR (FIRE-328) | (Confidential information has been removeda) (CRYSTAL43) |
| FOLFIRI | (Confidential information has been removeda)b (CRYSTAL43) | (Confidential information has been removeda) (CRYSTAL43) |
| BEV + FOLFIRI | NR (FIRE-328) | 11.1c |
Given that we used only PFS and not OS data from the RCTs, we assumed no or equal treatment effects across treatment arms post progression. Therefore, we should not model first-line treatment after first-line PFS for unresected patients. If we did, we would incur the costs of first-line drug treatment after progression, but gain no clinical benefit from this, which is clearly inappropriate. Therefore:
-
if mean treatment duration was estimated to be less than mean first-line PFS for unresected patients, our estimate of mean treatment duration was left unaltered
-
otherwise, mean treatment duration was capped at mean first-line PFS for unresected patients.
In our base case we used the resulting mean treatment durations for the calculation of drug administration and drug acquisition costs. In particular, the mean total cost of drug acquisition per patient was estimated as the product of the drug price per unit time, the mean treatment duration and the mean dose intensity (see Model parameters, Drug acquisition costs).
For the purposes of discounting of costs only, we assumed that treatment duration follows an exponential distribution. Cost-effectiveness was almost completely independent of this assumption.
In a sensitivity analysis we used OS data in addition to PFS data from the RCTs. In this case we used the mean treatment duration in step 3 above, but without the cap for mean first-line PFS, because any first-line treatment after progression could affect OS.
Data were available for treatment duration from the five RCTs for all patients only; there were no data for the liver metastases subgroup.
For the probabilistic sensitivity analysis, uncertainty in treatment duration was reflected in the uncertainty in PFS for unresected patients and the uncertainty in treatment duration from the RCTs. The treatment durations from the trials were estimated as gamma distributions (hence the minimum value is zero), with the same deterministic mean and the SE given by the SD from the trial divided by the square root of the number of patients.
OPUS trial as the baseline randomised controlled trial for the FOLFOX network
In a scenario analysis we used the OPUS trial65 not the PRIME trial44 as the baseline RCT for the FOLFOX network (see Structure of the Peninsula Technology Assessment Group model).
In this case we estimated the following mean treatment durations for unresected patients (considering all patients), using the same methodology as discussed for the base case, in which we used the PRIME trial44 as the baseline RCT:
-
cetuximab plus FOLFOX – 6.6 months
-
panitumumab plus FOLFOX – 5.2 months
-
bevacizumab plus FOLFOX – 3.9 months
-
FOLFOX – 5.0 months.
Overall survival: unresected patients
In our base-case analysis, we modelled only PFS from the RCTs. As mentioned in Structure of the Peninsula Technology Assessment Group model, in a sensitivity analysis we modelled OS for unresected patients from the RCTs in addition to PFS for unresected patients. In particular, our method of estimating OS for unresected patients was the same as that for estimating PFS for unresected patients, using steps 1–5 (see Model parameters, First-line progression-free survival: unresected patients).
For the same reasons as for PFS, we found the Weibull distribution to be the most appropriate distribution.
Our chosen curve fits are provided in Figure 20.
FIGURE 20.
First-line OS (unresected patients) in the PenTAG model: (a) CET + FOLFOX vs. FOLFOX from the OPUS trial; (b) PAN + FOLFOX vs. FOLFOX from the PRIME trial; (c) PAN + FOLFOX vs. BEV + FOLFOX from the PEAK trial; (d) CET + FOLFIRI vs. FOLFIRI from the CRYSTAL trial; and (e) CET + FOLFIRI vs. BEV + FOLFIRI from the FIRE-3 trial. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
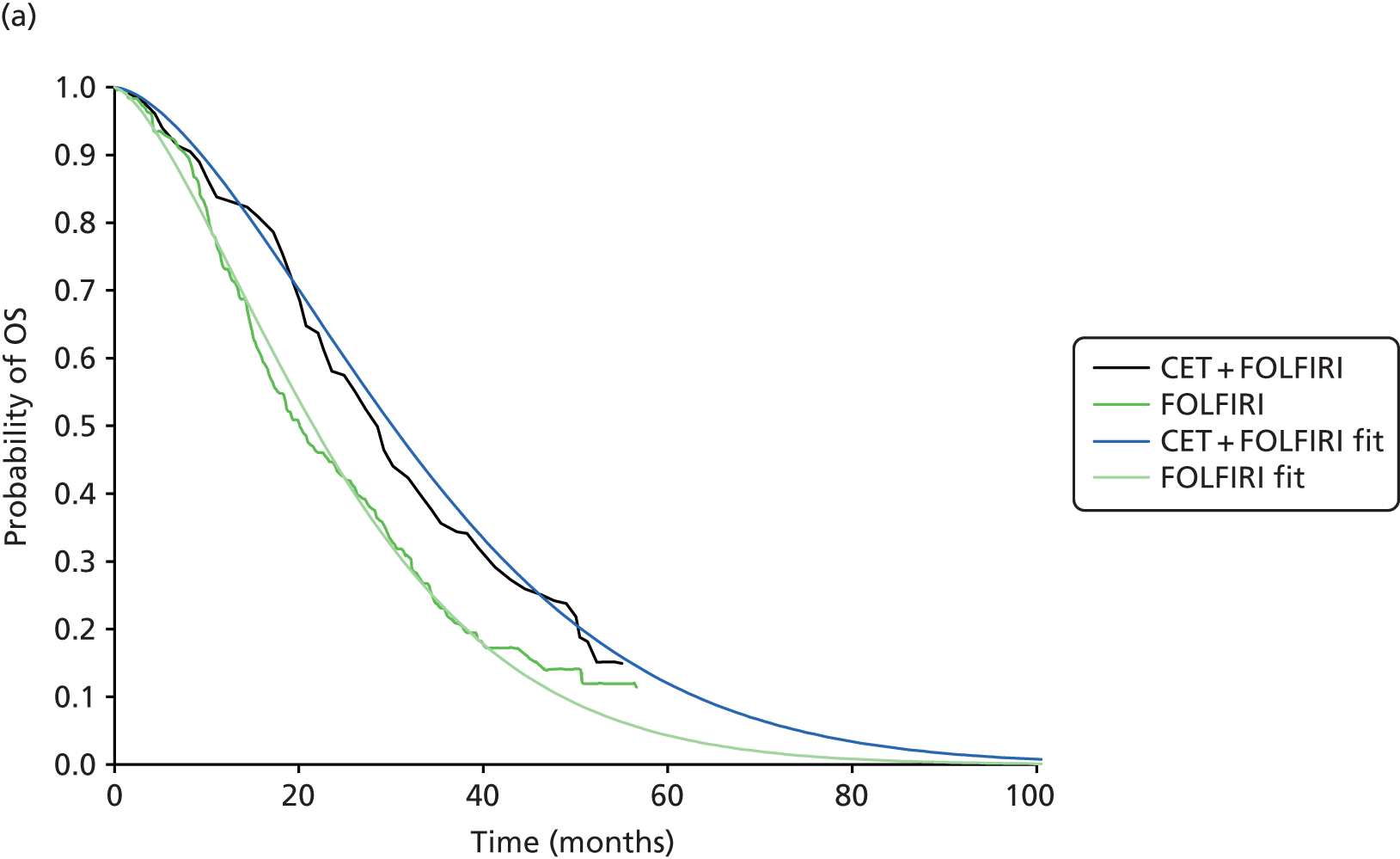
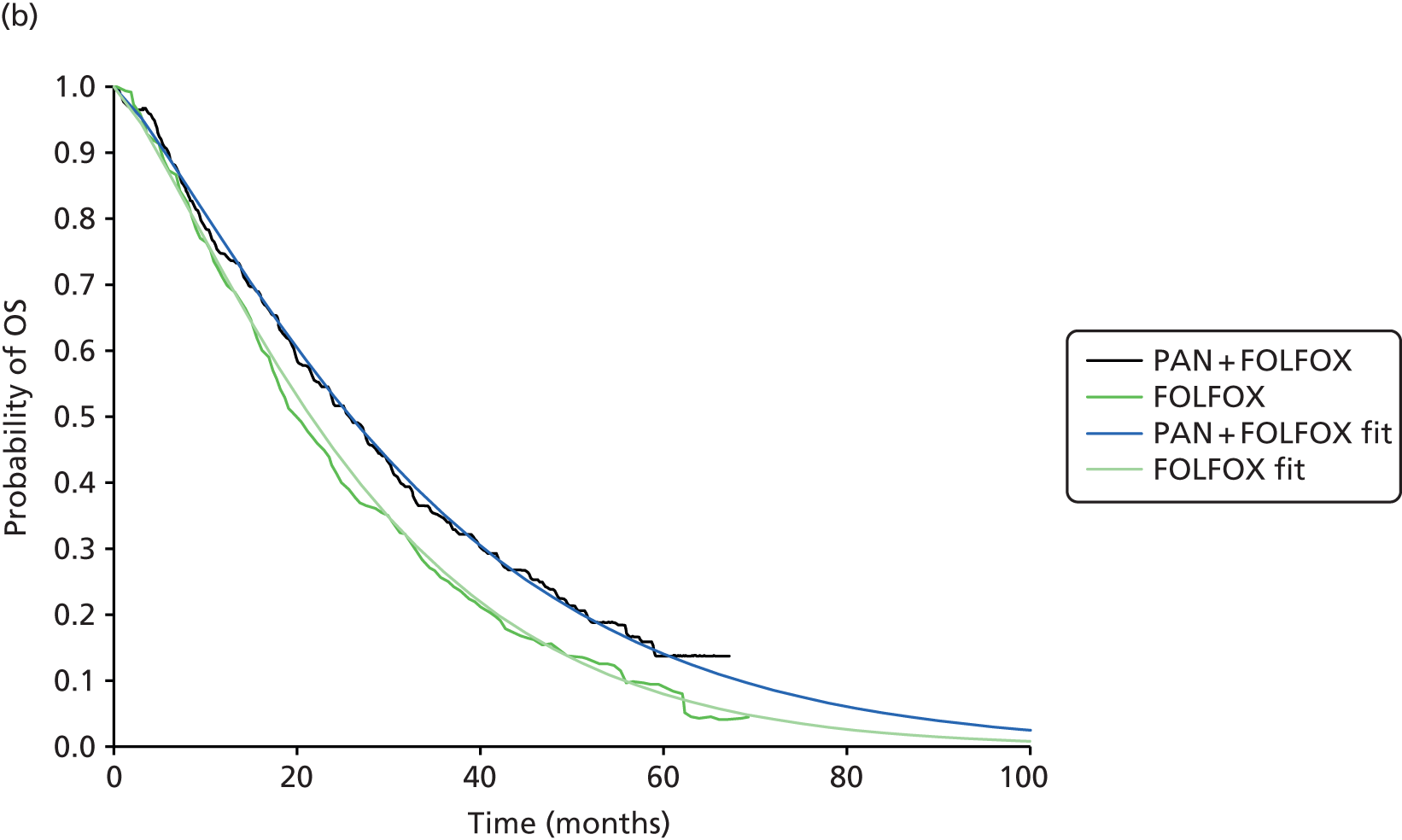
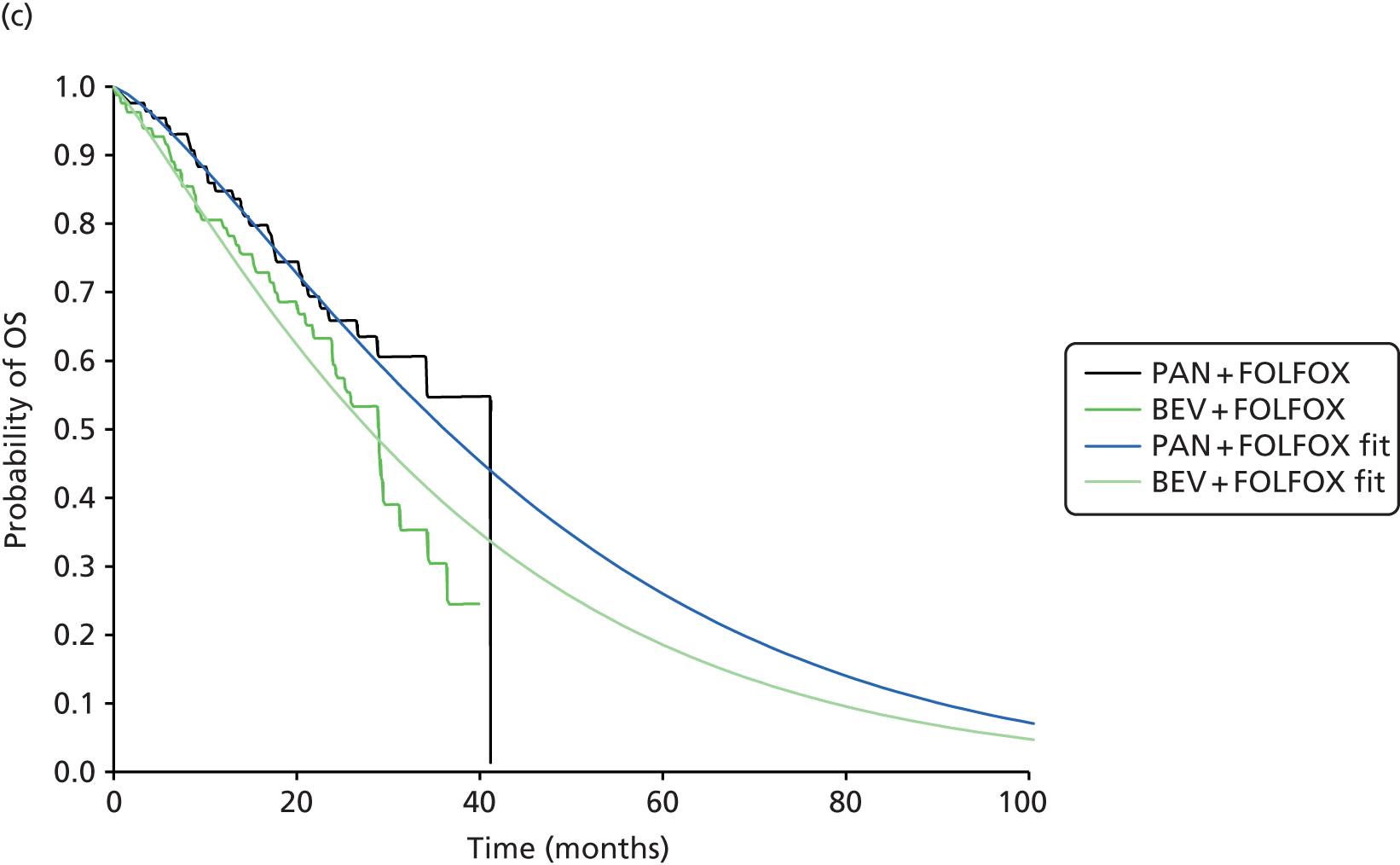
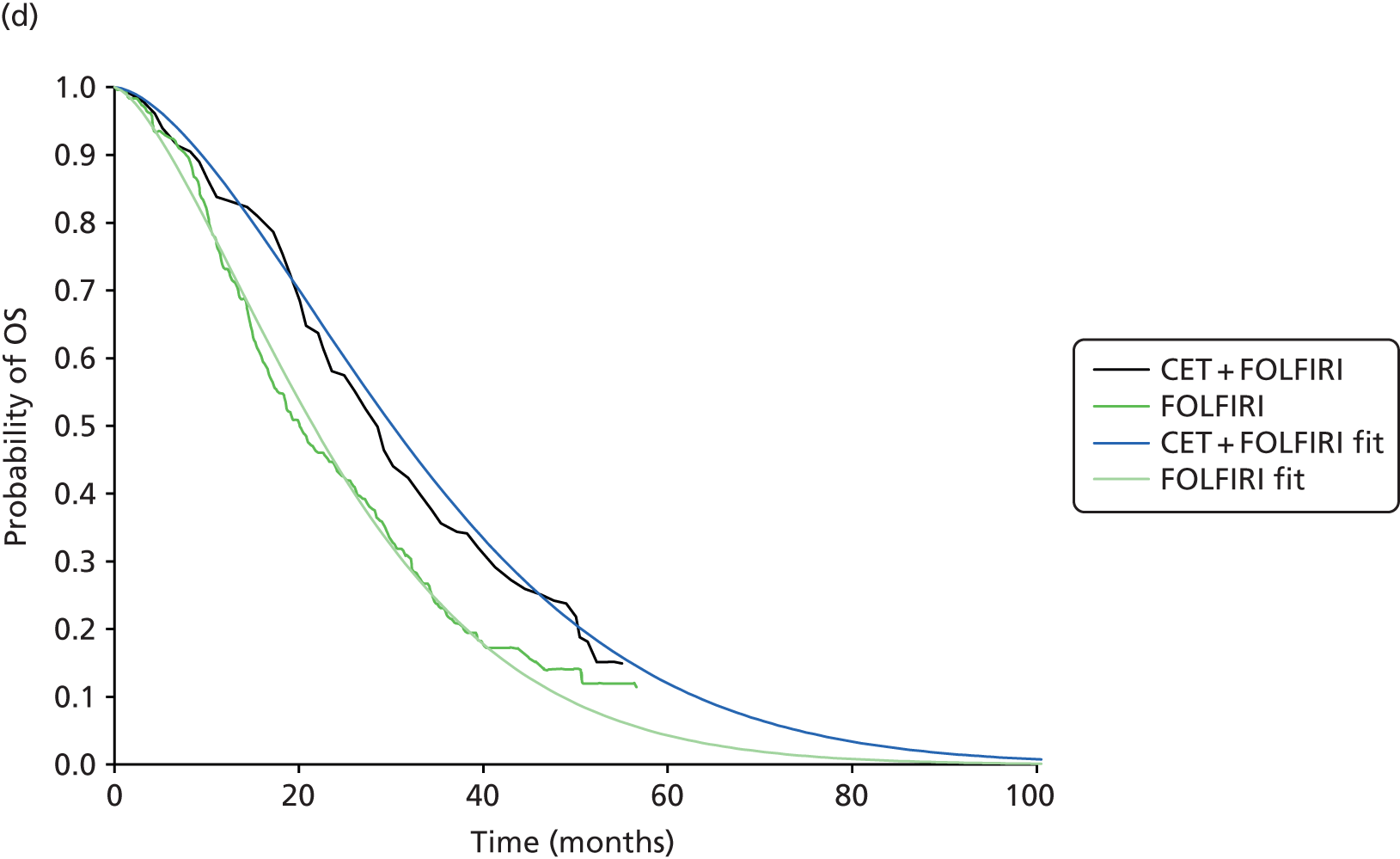
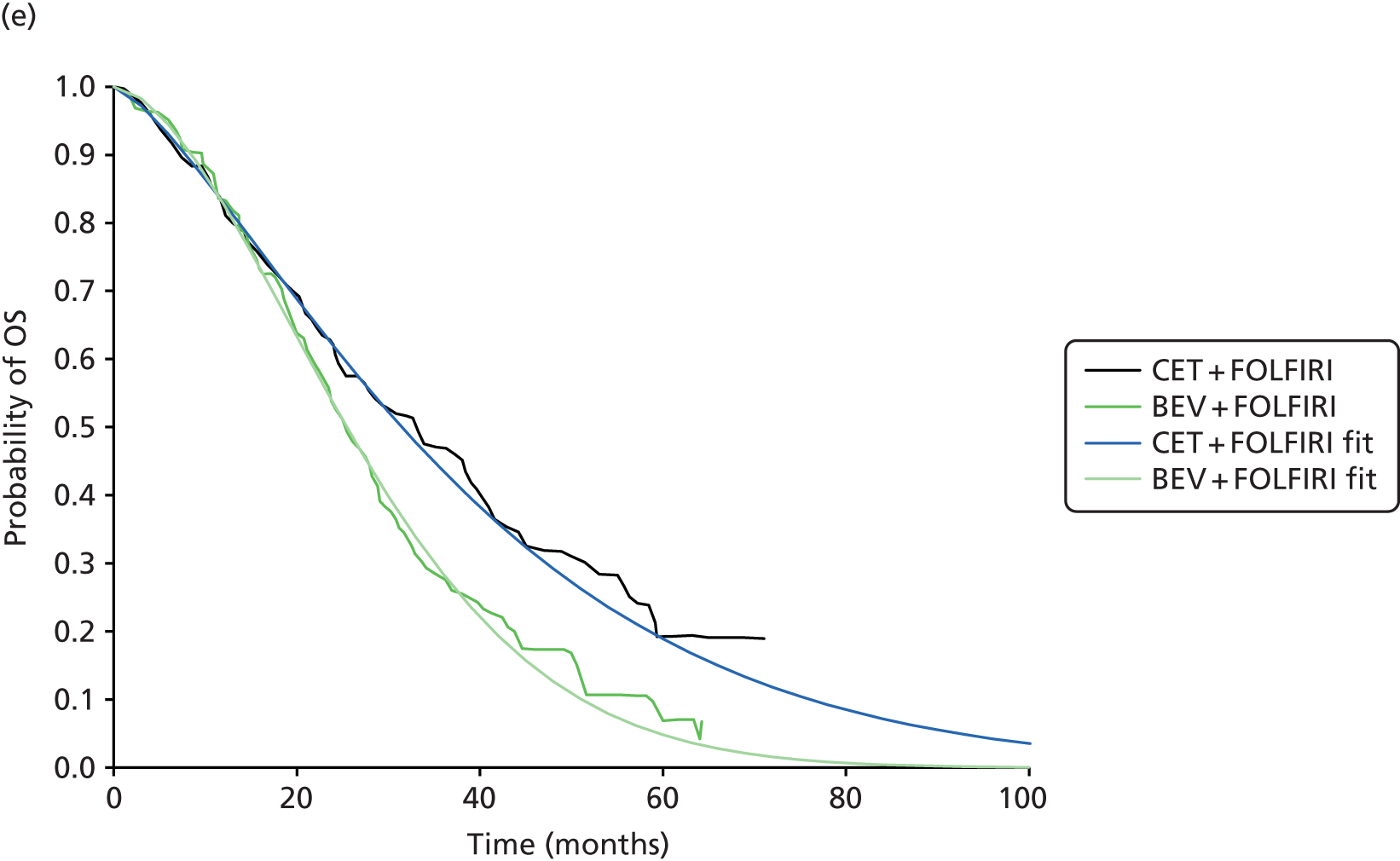
As for PFS, OS data from the RCTs were adjusted using data from Adam et al. 113 to allow for the fact that these data reflected some patients after resection (Figure 21).
FIGURE 21.
Peninsula Technology Assessment Group-modelled mean OS from first-line RCTs: (a) FOLFOX network; and (b) FOLFIRI network. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
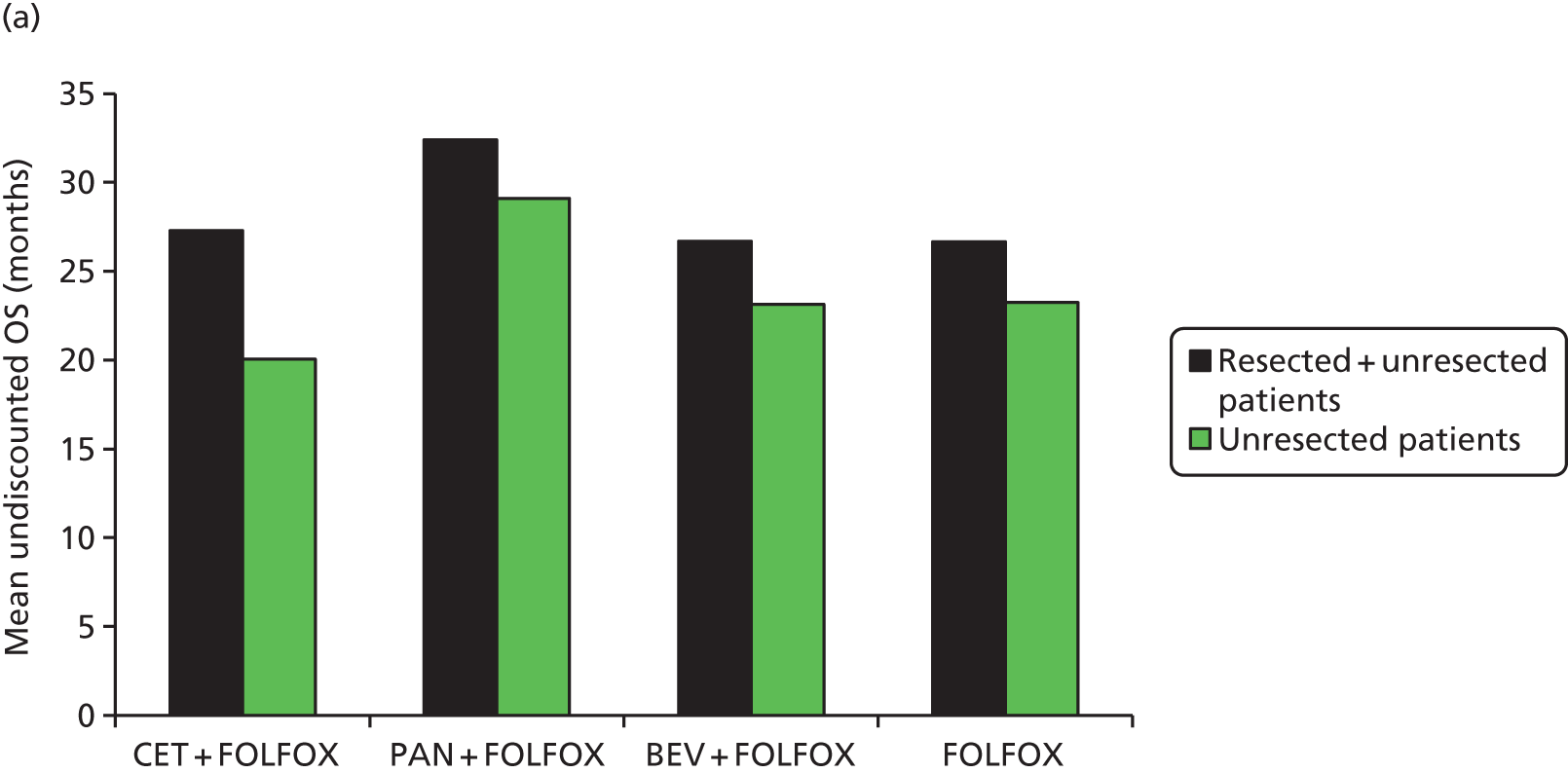

The mean OS for unresected patients when estimated directly from the RCTs of first-line drugs was substantially greater than the mean OS estimated in our base case (Figure 22). Differences are to be expected as the subsequent treatments in the RCTs (see Table 88) were different from those assumed in our model. Indeed, this is the key reason that we chose our model structure (see Structure of the Peninsula Technology Assessment Group model).
FIGURE 22.
Mean OS for unresected patients: PenTAG’s base case vs. first-line RCTs. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.

Second-line progression-free survival: unresected patients
Both we and Merck Serono assumed that all patients have second-line FOLFIRI after first-line FOLFOX-based treatment and second-line FOLFOX after first-line FOLFIRI-based treatment (see Structure of the Peninsula Technology Assessment Group model and Chapter 5, De novo economic evaluation, Description of methods).
We found that the cost-effectiveness of cetuximab and panitumumab was insensitive to our assumption about the duration of second-line PFS, because we also assumed that this is equal in all treatment arms. Therefore, this parameter does not merit close scrutiny.
In common with Merck Serono, we also modelled second-line PFS from Tournigand et al. 111 In particular, we modelled separately PFS on second-line FOLFOX and PFS on second-line FOLFIRI.
Given the lack of data to the contrary, both we and Merck Serono assumed that PFS on second-line FOLFOX or second-line FOLFIRI was independent of first-line treatment.
First, we digitised the Kaplan–Meier data from Tournigand et al. 111 We then fitted Weibull distributions to each of the two curves (Figure 23). Given that cost-effectiveness is only weakly affected by second-line PFS, we used a simple pragmatic fitting method by minimising the weighted sums of squares of differences between empirical and fitted PFS at each month up to 11 months. The weights pragmatically decreased linearly over time, from 1 at 0 months to 0 at 11 months, to reflect the reduction in the numbers of patients at risk over time.
FIGURE 23.
Weibull curves fit to PFS data from the study by Tournigand et al.:111 (a) second-line FOLFOX; and(b) second-line FOLFIRI.
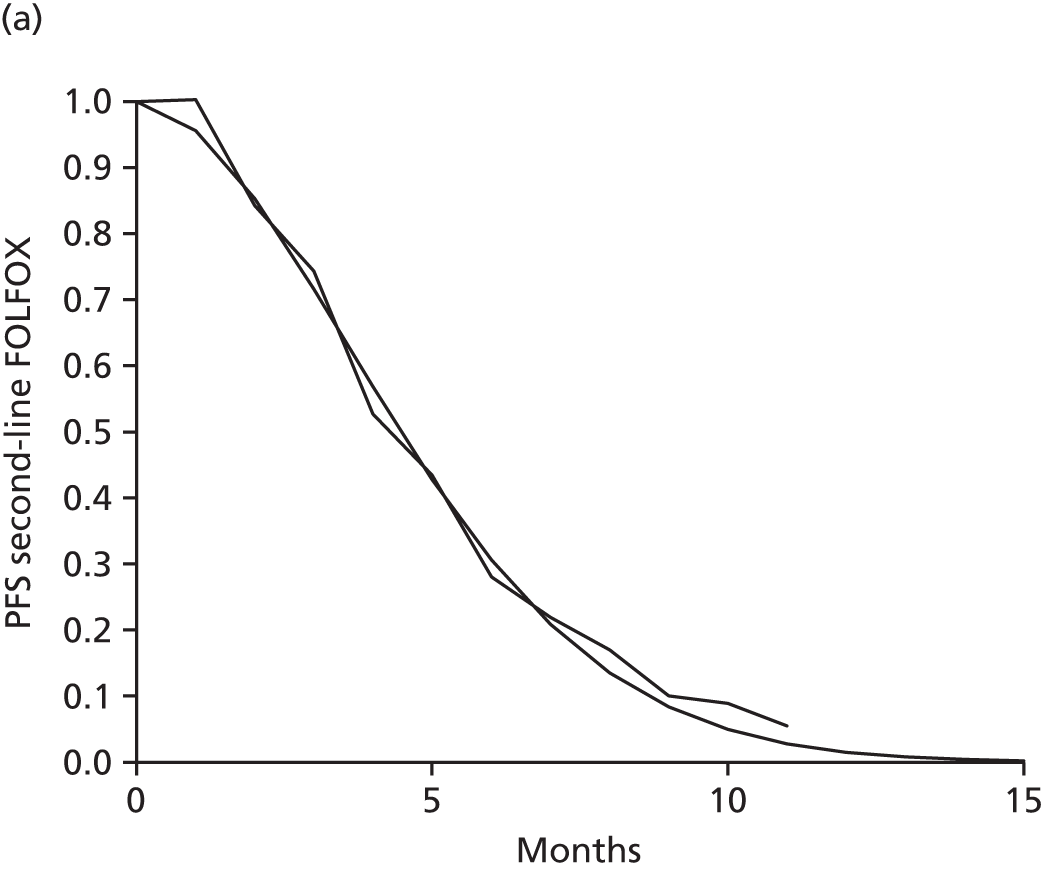
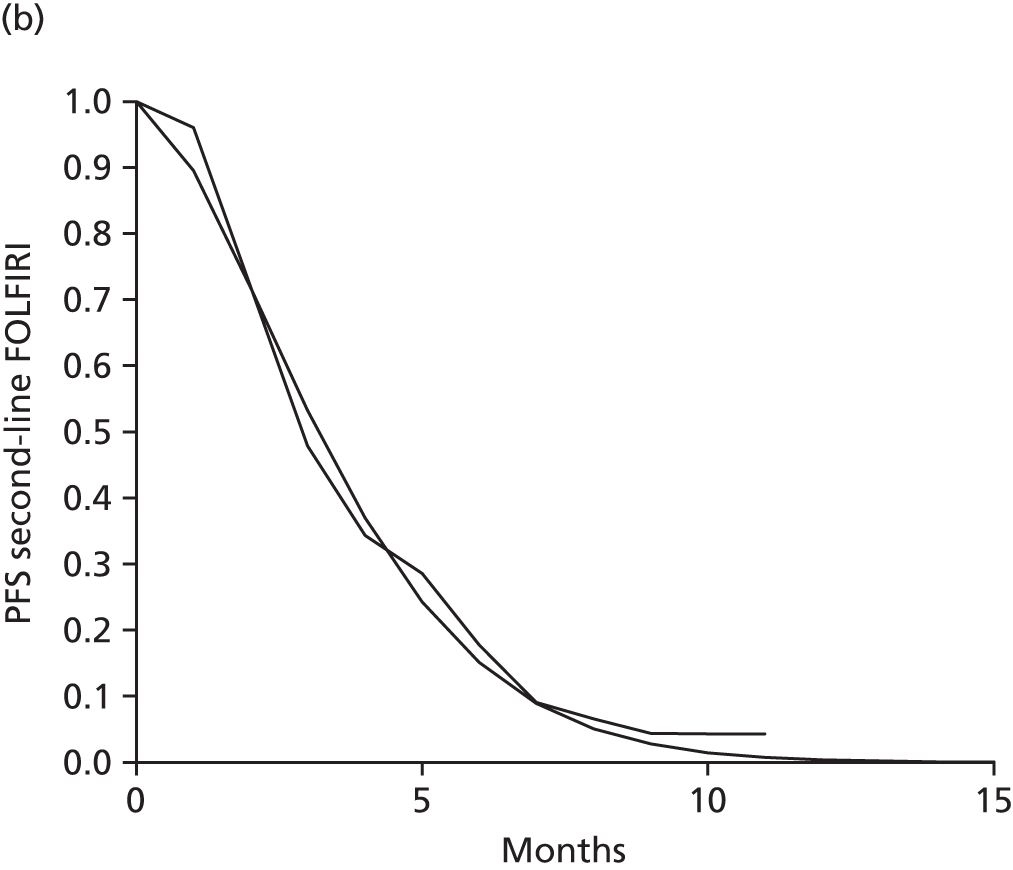
This yielded an estimated mean PFS for FOLFOX of 0.41 years and for FOLFIRI of 0.30 years.
Ideally, we would then model second-line PFS corresponding to the fitted Weibull distributions. However, this would substantially complicate the model, as it would demand time-in-state specific transition probabilities. Therefore, we pragmatically assumed that second-line PFS follows an exponential distribution, with the lambda parameter set to 0.186 and 0.242 (time measured in months) for FOLFOX and FOLFIRI, respectively, giving means equal to those above. This then renders the second-line transition probabilities independent of time. This assumption affects cost-effectiveness only incrementally.
Mortality from second-line progression-free survival
Given the lack of data to the contrary, we estimated the proportion of progressions from second-line treatment that were related to death as 6%, the corresponding value for first-line treatment (see Model parameters, First-line progression-free survival: unresected patients). Cost-effectiveness was almost completely unaffected by this estimate.
Second-line time on treatment: unresected patients
It is appropriate to base time on second-line treatment on data from Tournigand et al. 111 as this study informs second-line PFS.
In this study there was a median of eight cycles of second-line FOLFOX and six cycles of second-line FOLFIRI. 111 Given that one cycle lasted for 2 weeks in this study, this equates to a median time on treatment of 16 and 12 weeks for FOLFOX and FOLFIRI, respectively. Given the lack of data to the contrary, we assumed that treatment duration follows an exponential distribution. Therefore, the mean time on treatment is 0.44 and 0.33 years for FOLFOX and FOLFIRI, respectively. These values are very similar to the estimated mean PFS in the previous section for FOLFOX and FOLFIRI (0.41 and 0.30 years, respectively). Therefore, we pragmatically assumed that second-line treatments are taken for the entire duration of PFS.
Although not stated in its report, inspection of Merck Serono’s model revealed that it also assumed that patients take FOLFOX or FOLFIRI for the entire duration of second-line PFS.
Third-line survival: unresected patients
We found that the cost-effectiveness of cetuximab and panitumumab is insensitive to our assumption about the duration of third-line PFS, because we also assumed that this is equal in all treatment arms. Therefore, this parameter does not merit close scrutiny.
We estimated the mean time in third-line treatment as 0.51 years, which was our estimated value for third-line treatments for mCRC in the KRAS WT population in our model from TA242139 and that was endorsed by the NICE committee. This estimate itself was derived from the study by Jonker et al. ,112 which compared treatment with cetuximab plus BSC with treatment with BSC alone.
Merck Serono also modelled third-line survival using data from Jonker et al. 112 Inspection of its model revealed that Merck Serono assumed a Weibull distribution; we calculated a mean of 0.74 years’ survival for patients who start on third-line treatment. Merck Serono also assumed that this value was independent of first- or second-line treatment.
Test accuracy
The Evidence Review Group (ERG) report for TA176 raised concerns that the model did not account for patients who were incorrectly diagnosed. 144 Some time was spent determining the relative accuracy of RAS testing in clinical practice compared with RAS testing in the trials described in the clinical effectiveness section. This is described in detail in Appendix 7. This was necessary to assess whether or not some adjustment was necessary to account for differences in patients being incorrectly diagnosed between the trials and clinical practice.
However, the relationship between a test’s ability to diagnose mutation status and the test’s ability to predict the outcome of this diagnosis (which treatment patients receive and how effective this is) is a complex one. In their assessment of diagnostic tests for detecting KRAS mutations, Westwood et al. 45 adjusted the meaning of accuracy from ‘test accuracy’ (as discussed in our previous sections) to include ‘accuracy for predicting response to treatment with cetuximab in combination with standard chemotherapy, or variation in clinical outcomes following treatment with cetuximab in combination with standard chemotherapy depending on which method is used to classify patients as having KRAS wild-type tumours’.
The study by Westwood et al. 45 explains that, because of the nature of companion diagnostics, the only conclusions that could reasonably be drawn regarding the diagnostic tests used in trials were that they appeared to result in a benefit for patients and that there is no evidence to show that different tests used in practice would lead to significantly different outcomes. Unfortunately, this was difficult to assess, as not all tests used in practice have been used in trials of this nature.
Given the paucity of significant accuracy data to say otherwise and the apparent similarity in test accuracy between KRAS and RAS WT testing, we agree with the conclusion provided in Westwood et al. ’s45 assessment that there is no evidence of a difference between testing techniques. As such, the true proportion of incorrect diagnoses in trials or clinical practice is not considered in our model and we do not adjust the test accuracy in the trials to reflect what is carried out in practice.
Similarly, our clinical advisors (Dr Mark Napier and Christopher Bowles, based at the Royal Devon and Exeter Hospital) advised that testing for EGFR expression is rarely, if ever, carried out in practice, as it is not believed to be indicative of the effectiveness of treatment (2015, personal communication). Therefore, we did not include EGFR testing in the model in either a cost or effectiveness capacity.
Utilities
In this section we follow the principles for the identification, review and synthesis of health-state utility values from the literature, as recommended by the NICE DSU in the UK. 155 There are no agreed reporting standards for studies of utilities, but the following information is key to understanding the nature and the quantity and quality of evidence:155
-
the population describing the health state (e.g. age, sex, disease severity)
-
the approach used to describe the health state
-
utility value elicitation technique, for example time trade-off, standard gamble and visual analogue scale
-
sample size
-
respondent selection and recruitment, and inclusion and exclusion criteria
-
survey response rates, numbers lost to follow-up (and reasons) and methods of handling missing data.
Clearly, the relevance of the data to the decision model, and to the agency to which the model will be submitted, is important. In the current project, the NICE reference case was used. 128 Modification of utility values from the literature for use in economic models, and sensitivity analyses using less relevant utility values, should be considered. 155 A systematic search for studies reporting utilities should be undertaken. 155 For the current project, the search method is provided in Appendix 1. The results of this search were combined with the cost-effectiveness search results and the identified studies were screened simultaneously. We expanded the population to all cases of mCRC, rather than just RAS WT mCRC, as we believed that little evidence would be available on the utility of the RAS WT population. In addition, sources of utility values were obtained from published models on the cost-effectiveness of panitumumab and cetuximab in combination with chemotherapy. We also considered any sources presented in the manufacturers’ submissions.
We also compare the results of our utility review with the results of studies reported by a recent diagnostic appraisal report,143 which included a complete mCRC population (both KRAS mutant and WT).
We report the findings of the quality of life search in Table 99 and the utilities from the cost-effectiveness papers in Table 100. Only sources of KRAS WT utilities were identified as credible. When RAS WT utilities were reported, the sources were unclear107 or important details were not provided and the values could not be used. 69 However, we believe that the KRAS WT population would not differ greatly from the RAS WT population in terms of utility.
| Study | Study population | Preference elicitation | Results | Criticisms of study |
|---|---|---|---|---|
| First line | ||||
| Bennett 2011115 | PRIME trial: 576 previously untreated KRAS WT mCRC patients receiving either PAN + FOLFOX or FOLFOX alone | EQ-5D questionnaire, UK value set | Baseline EQ-5D: PAN + FOLFOX 0.778 (SD 0.247), FOLFOX 0.756 (SD 0.244); LSM change from baseline: PAN + FOLFOX 0.022 (95% CI 0.003 to 0.041), FOLFOX 0.027 (95% CI 0.008 to 0.046), difference –0.005 (95% CI –0.032 to 0.022) | RAS WT results not currently published; reports only on PAN + FOLFOX and FOLFOX |
| Láng 2013156 | CRYSTAL trial: 627 previously untreated KRAS WT mCRC patients receiving either CET + FOLFIRI or FOLFIRI alone | EORTC QLQ-C30 questionnaire | Values using EORTC QLQ-C30 global health scale: baseline ≈60 both CET + FOLFIRI and FOLFIRI, end of follow-up ≈65 CET + FOLFIRI, ≈63 FOLFIRI; values converted to EQ-5D scores all lie within the range 0.62–0.63 | RAS WT results not currently published; EQ-5D preferred to EORTC QLQ-C30 |
| Post first line | ||||
| Bennett 2011115 | NCT00339183 trial: 597 previously treated KRAS WT mCRC patients receiving either PAN + FOLFIRI or FOLFIRI alone | EQ-5D questionnaire, UK value set | Baseline EQ-5D: PAN + FOLFIRI 0.769 (SD 0.230), FOLFOX 0.762 (SD 0.252); LSM change from baseline: PAN + FOLFIRI –0.024 (95% CI –0.045 to –0.003), FOLFIRI 0.000 (95% CI –0.021 to 0.022), difference –0.024 (95% CI –0.054 to 0.006) | RAS WT results not currently published; reports only on PAN + FOLFIRI and FOLFIRI |
| Wang 2011116 | Previously treated KRAS WT mCRC patients receiving PAN + BSC or BSC alone | EQ-5D questionnairea | BSC only: toxicity 0.4409; without disease or toxicity (progression free) 0.6630; relapse/disease progression 0.6407 | KRAS WT not RAS WT population; small population size (n = 13 informed toxicity utility) |
| Study | Utility | Stated source | Notes |
|---|---|---|---|
| Graham 2014107 | Progression free 0.821 | PRIME trial, RAS WT results | Not reported elsewhere; most recent values from Siena et al.69 appear much lower (≈0.75) |
| Subsequent treatment 0.782 | Second-line panitumumab trial, KRAS WT results | This trial is also reported in Bennett et al.,115 in which second-line utility is reported as 0.762–0.769 depending on the arm | |
| BSC 0.681 | Third-line trial, KRAS WT results | This trial is also reported in Odom et al.,157 in which post first-line utility is reported as 0.68 | |
| Post resection 0.821 | Assumed the same as progression free | ||
| Ortendahl 2014109 (KRAS WT) | First line 0.77 | Meads et al. (2010)a | Source not confirmed, but Ewara et al.159 report the same value; their source is also unconfirmed |
| Second line 0.75 | Meads et al. (2010), Mittman et al. (2009)a | Source not confirmed | |
| Post successful resection 0.84 | Fryback et al. (1993)a | Source not confirmed; study is > 20 years old |
As well as our included cost-effectiveness studies, we identified the studies by Lawrence et al. 158 and Ewara et al. 159 as potential utility sources, as these were cost-effectiveness studies of KRAS WT mCRC populations. Ewara et al. 159 did not highlight any sources of utilities that we had not already found through other sources and the main utility study used by Lawrence et al. ,158 by Petrou and Campbell,160 was irretrievable. However, this study is nearly 20 years old and was conducted on UK oncology nurses and so we do not believe it to be relevant.
Sources of progression-free utilities
From the searches we identified two full papers reporting progression-free utilities in KRAS WT populations from the PRIME115 and CRYSTAL trials. 156
The utilities from the CRYSTAL trial were valued using the EORTC QLQ-C30, a cancer-specific quality-of-life questionnaire, and reported by Láng et al. 156 The difference in utilities between cetuximab plus FOLFIRI and FOLFIRI alone did not appear to be significant and neither was the change in utility over time. This supports the conclusions of other utility sources. 69,115 EQ-5D-based utilities are preferred in the NICE reference case. 128 There are methods to convert EORTC QLQ-C30 values to EQ-5D values, including that provided by Kim et al. 161 This transformation was calculated for a population that included multiple cancers, but was validated on a CRC population and therefore is the most relevant transformation to our results. It includes several covariates but can be used as a simple linear transformation using the global health score reported by the EORTC QLQ-C30. We manually extracted data points from the study by Láng et al. 156 and used the Kim et al. 161 transformation to calculate utility values between 0.62 and 0.63 for the KRAS WT population receiving cetuximab plus FOLFIRI, across the follow-up time reported in Láng et al. 156 This seems quite low compared with other utilities reported for the KRAS WT population,69,107,115 which are preferred as they do not require transformation to EQ-5D scores.
Graham et al. ,107 Siena et al. 69 and Bennett et al. 115 all report utilities from the PRIME trial for either KRAS WT or RAS WT populations. However, the estimates are quite different across these studies. Bennett et al. 115 is the only full paper that reports utility data collected for the KRAS WT population from the PRIME trial and it also includes utility results for a second-line panitumumab trial. It includes the results of EQ-5D questionnaires valued on the UK value set calculated by Dolan. 119 Bennett et al. 115 also report that the utility change from baseline until disease progression for both arms is not clinically significant and that the difference between arms is not statistically or clinically significant. This group included both patients who completed treatment and those who had to withdraw early. The weighted average of baseline utility from Bennett et al. 115 is 0.767 (to three significant figures). This is similar to the utility used in the study by Ortendahl et al. 109 (0.77), also for a KRAS WT mCRC population.
The publication by Siena et al. 69 is an abstract reporting utility values for the RAS WT subpopulation of the PRIME trial. The abstract does not specify at what time point the reported utilities were measured, but it does state that the change in utility from baseline and the difference between arms were not found to be statistically significant for this subgroup. In this abstract, the weighted average of the utility of the panitumumab plus FOLFOX and FOLFOX arms is 0.750, which is below, but not dissimilar to, the utility of the KRAS WT population reported in the study by Bennett et al. 115
The utility estimate reported by Graham et al. 107 is noticeably higher (0.821) than either the baseline or the end-point utilities reported in Bennett et al. 115 and Siena et al. 69 It is unclear why this is the case as the authors report that these are EQ-5D utility data for the RAS WT population, valued from the UK valuation set, as in Siena et al. 69 and Bennett et al. 115 Both Graham et al. 107 and Siena et al. 69 report utilities for a RAS WT population rather than a KRAS WT population, but are still markedly different, suggesting that the difference in population between Graham et al. 107 and Bennett et al. 115 is not responsible for this higher utility. It is possible that an increase in utility at an earlier time point in the follow-up could result in a higher overall utility. However, this was not described in any of the PRIME trial reports and the results from the CRYSTAL trial156 suggest a fairly linear relationship between utility and time.
Sources of post first-line utilities
The study by Bennett et al. 115 also contains information on utilities for a second-line KRAS WT mCRC population, comparing panitumumab plus FOLFIRI with FOLFIRI. No significant difference between arms was reported in this study. The most relevant of the reported utilities to a UK setting is that for FOLFIRI, as chemotherapy alone is recommended as second-line treatment. To be consistent with our approach to estimating first-line utility, we took the baseline utility for FOLFIRI post first line (0.762). This is not significantly different from the first-line utility (0.767), but does indicate that progression to second-line treatment is associated with a reduction in quality of life, which seems clinically plausible.
Graham et al. 107 report a higher utility (0.782), but quote the source as the same trial reported in Bennett et al. 115 (NCT00339183). As with the first-line utility, it is unclear why this value is higher than that reported by Bennett et al. 115 Merck Serono also used the study by Bennett et al. 115 as the source for second-line utility, but used the value for the panitumumab plus FOLFIRI arm, which is only marginally higher at 0.769.
Ortendahl et al. 109 report a utility value from Meads et al. (2010) and Mittmann et al. (2009) of 0.75 (full details for these two publications were not provided by Ortendahl et al. 109). We could not confirm the source of this value nor how this value was elicited.
The utility of progressive disease on BSC is reported by Graham et al. 107 as 0.681. This is based on the trial reported by Odom et al. ,157 in which the KRAS WT population was in a progressive disease state receiving either panitumumab plus BSC or BSC alone. This trial also forms the basis for the analyses conducted by Wang et al. ,116 which aimed to estimate utilities for patients in a post-first-line health state based on their disease progression or AE status. Merck Serono used the study by Wang et al. 116 to inform third-line utility in its submitted model, choosing a utility for BSC without symptoms or AEs.
Post-resection progression-free utilities are generally high in the models. Both Graham et al. 107 and Ortendahl et al. 109 report utilities of > 0.8 (0.821 and 0.84, respectively). However, the utility reported by Graham et al. 107 corresponds to the first-line progression-free state and that reported by Ortendahl et al. 109 relates to a study by Fryback et al. (1993) (full details for this publication details were not provided by Ortendahl et al. 109). Neither of these sources has been confirmed and, furthermore, the Fryback et al. study is > 20 years old.
Merck Serono suggested that the utility of this progression-free post-resection population should be equal to the population utility for the mean age of the cohort. Although this is likely to be an upper limit for this utility, it is a reasonable approach to take because of the curative intent of the resection.
The utility of progressive disease post resection was assumed to be an average of the second- and third-line utility weights in both the study by Graham et al. 107 and the Merck Serono submission. These are the only studies that we identified that reported utility for progressive disease post resection and the approach seems to be a reasonable compromise to include second- and third-line information while keeping progressive disease post resection as one health state.
One additional utility source that was identified was the study by Färkkilä et al. ,162 which assessed 508 CRC patients in Finland, with EQ-5D data valued on the UK valuation set. In total, 151 patients had metastatic disease; the average age of this cohort was 66 years and 58% of the cohort were men. For those with metastatic disease receiving treatment (n = 108) the utility was 0.820 (95% CI 0.783 to 0.858) and for those with metastatic disease receiving palliative care (n = 41) the utility was 0.643 (95% CI 0.546 to 0.747). The mean time since diagnosis was 18 months. The utility for metastatic disease with treatment is higher than that reported in Bennett et al. 115 and indeed seems high compared with estimates of general population utility for this cohort (≈0.0821 using the PenTAG model methods). The utility for those receiving palliative care is similar to that reported in Wang et al. 116 The study by Färkkilä et al. 162 included patients who underwent resection as well as those who were unresectable and may also reflect differences between different countries’ values of HRQoL. However, in general, this study supports the findings of Bennett et al. 115 and Wang et al. ,116 and does not supersede their relevance to this analysis.
Summaries of the findings of the quality-of-life search and the utilities from the cost-effectiveness papers are reported in Tables 99 and 100, respectively.
Utilities in the Peninsula Technology Assessment Group model
The health-state utilities used in the PenTAG base case are presented in Table 101.
| Parameter | Base case | SE | Distribution | Source |
|---|---|---|---|---|
| First line (PFS) | 0.767 | 0.0110 | Beta | Bennett et al.115 |
| Second line | 0.762 | 0.0155 | Beta | Bennett et al.115 |
| Third line (PD) | 0.6407 | 0.0155 | Beta | Wang et al.116 |
| PFS post successful resection | 0.831 at age 63 years | NA | Age-related general population utility130,131 | |
| PD post successful resection disutility | 0.142 | NA | Average of second- and third-line utilities weighted by time spent in second or third line |
We concluded that utility in first-line PFS would be the same for all treatments and that the most relevant results are those reported by Bennett et al. 115 Therefore, these form the basis of the PenTAG base case. We used the value of 0.767, the average of the panitumumab plus FOLFOX and FOLFOX arms of the trial, weighted by the number of patients.
For consistency, and because it is a recent study in a relevant population and provides EQ-5D data valued on a UK data set, we also used the study by Bennett et al. 115 for the second-line utility estimate.
For third-line utility we concluded that the most sensible value to use is that for people receiving BSC who are in disease progression. 116
For post-resection progression-free utility we used the same approach as Merck Serono. However, instead of the approach in the study by Petrou and Hockley,117 which uses Health Survey for England data from 1996, we used the well-established methodology published by Ara and Brazier,130 updated with Health Survey for England data from 2012:131
As with Graham et al. 107 and the Merck Serono submission, we also estimated the utility in disease progression post successful resection by averaging the second- and third-line utilities. We used the same approach as Merck Serono and weighted the average utility by the time spent in each line of treatment, which gives us a disutility value in this health state of 0.142.
In the probabilistic sensitivity analysis, utilities for unresected patients were varied with beta distributions based on their means and SEs.
The utilities post resection are driven by other parameters (e.g. PFS post resection is driven by the mean age of the cohort). Although strictly these parameters should have additional uncertainty assigned to them, the lack of information on this uncertainty would lead to estimates of SEs that would overshadow the influence of the primary drivers of these parameters. Therefore, to ensure that the impact of these parameters is recognised in our results, we do not assign additional uncertainty to the post-resection utilities.
Costs
Inflation to 2015/16 prices
Unit costs were inflated to 2015/16 prices by inflating to 2013/14 prices using the Hospital and Community Health Services pay and prices index163 and then to 2015/16 prices at a rate of 1.64% per annum.
The rate of growth of the pay and prices index appears to have slowed in recent years (Figure 24) and so the inclusion of historical values could lead to an overestimate of the likely inflation between 2013/14 and 2015/16. We therefore adopted the approach of taking the average increase in the index for the previous 3 years (i.e. from 2010/11 to 2013/14), that is, a rate of 1.64% per annum.
Table 102 provides the inflation factor used in the model for each year for inflation to 2015/16 prices.
| From calendar year | From financial year | Inflation factor |
|---|---|---|
| 2000 | 2000/1 | 1.527 |
| 2001 | 2001/2 | 1.453 |
| 2002 | 2002/3 | 1.404 |
| 2003 | 2003/4 | 1.335 |
| 2004 | 2004/5 | 1.292 |
| 2005 | 2005/6 | 1.246 |
| 2006 | 2006/7 | 1.201 |
| 2007 | 2007/8 | 1.168 |
| 2008 | 2008/9 | 1.124 |
| 2009 | 2009/10 | 1.117 |
| 2010 | 2010/11 | 1.084 |
| 2011 | 2011/12 | 1.062 |
| 2012 | 2012/13 | 1.044 |
| 2013 | 2013/14 | 1.033 |
| 2014 | 2014/15 | 1.016 |
| 2015 | 2015/16 | 1 |
Conversion to UK pounds
When conversion from other currencies to UK pounds was required, International Monetary Fund purchasing power parity was used to convert within the year (e.g. from 2010 euros to 2010 UK pounds), after which inflation was applied. The Campbell and Cochrane Economics Methods Group (CCEMG) – Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) Cost Converter version 1.5165 was used for the purchasing power parity conversion.
Cost of RAS testing
As detailed in Appendix 7, personal communication with the All Wales Medical Genetics Service (9 April 2015) and the Genetics Laboratory at Royal Devon and Exeter Hospital (11 March 2015) suggested a cost of £200 for joint KRAS and NRAS mutation testing. This was despite differences in the number of codons assessed and possible differences in the type of test used.
As such, we assumed a unit cost of £200 for RAS mutation testing in our model. We also allowed for the cost of patients who were tested as RAS mutant. We did this by setting the cost as follows: £200/50% = £400, in which 50% of patients are assumed to be RAS WT.
Drug acquisition costs
We estimated the mean drug acquisition cost per patient as:
As discussed previously (see Model parameters, First-line time on treatment), in the base case we used the mean treatment duration from the RCTs capped by the mean time in first-line PFS for unresected patients.
Table 103 summarises the cost per month of the chemotherapy regimens in the PenTAG model.
| Regimen | Cost per month of drug acquisition (£) |
|---|---|
| CET + FOLFOX4 | 3955 |
| CET + FOLFOX6 | 3961 |
| PAN + FOLFOX4 | 4195 |
| PAN + FOLFOX6 | 4200 |
| BEV + FOLFOX4 | 2089 |
| BEV + FOLFOX6 | 2094 |
| FOLFOX4 | 86 |
| FOLFOX6 | 91 |
| XELOX | 76 |
| CET + FOLFIRI | 3987 |
| BEV + FOLFIRI | 2131 |
| FOLFIRI | 128 |
Unit costs for each agent were drawn from the CMU eMit database140 when possible or from the British National Formulary (BNF)16 when an agent was not present in the CMU eMit database (Table 104). When the CMU eMit database price was used, the average unit cost was derived using a weighted average (weighted by the market share in milligrams sold of each preparation). The unit cost for bevacizumab was calculated assuming usage of 16-mg vials, as this resulted in slightly lower costs and did not increase wastage, thereby slightly lowering total costs. The company submissions from Merck Serono and Amgen included details of an alternative pricing strategy for cetuximab and a PAS for panitumumab; we were advised by NICE to use the list prices in the base case and the PAS prices in scenario analyses. These were presented to NICE in a confidential appendix and are not presented here.
| Agent | Cost (£) | Source |
|---|---|---|
| Cetuximab | 20-ml vial (5 mg/ml): 178.10 | BNF16 |
| 100-ml vial (5 mg/ml): 890.50 | ||
| Panitumumab | 5-ml vial (20 mg/ml): 379.29 | BNF16 |
| 20-ml vial (20 mg/ml): 1517.16 | ||
| Bevacizumab | 4-ml vial (25 mg/ml): 242.66 | BNF16 |
| 16-ml vial (25 mg/ml): 924.40 | ||
| Oxaliplatin | 20-ml vial (5 mg/ml): 6.14 | CMU eMit140 |
| 10-ml vial (5 mg/ml): 3.65 | ||
| Fluorouracil | 20-ml vial (50 mg/ml): 1.33 | CMU eMit140 |
| 100-ml vial (25 mg/ml): 6.14 | ||
| 50-ml vial (50 mg/ml): 2.04 | ||
| 5 × 10-ml vial (25 mg/ml): 17.63 | ||
| 10-ml vial (50 mg/ml): 0.87 | ||
| 10 × 20-ml vial (25 mg/ml): 47.50 | ||
| 100-ml vial (50 mg/ml): 3.71 | ||
| Leucovorin | 10-ml vial (10 mg/ml): 2.41 | CMU eMit140 |
| 5 × 2-ml vial (7.5 mg/ml): 32.39 | ||
| 30-ml vial (10 mg/ml): 3.98 | ||
| 5 × 10-ml vial (3 mg/ml): 23.42 | ||
| 5 × 1-ml vial (3 mg/ml): 25.33 | ||
| 5-ml vial (10 mg/ml): 1.86 | ||
| Irinotecan | 5-ml vial (20 mg/ml): 7.38 | CMU eMit140 |
| 15-ml vial (20 mg/ml): 20.11 | ||
| 2-ml vial (20 mg/ml): 5.43 | ||
| 25-mg vial (20 mg/ml): 48.53 | ||
| Capecitabine | 60-tablet (150-mg) pack: 5.63 | CMU eMit140 |
| 120-tablet (500-mg) pack: 39.04 | ||
| Chlorphenamine | 5 × 1-ml vial (10 mg/ml): 14.47 | CMU eMit140 |
| Dexamethasone | 28-tablet (0.5-mg) pack: 45.10 | CMU eMit140 |
| 50-tablet (2-mg) pack: 21.50 | ||
| 100-tablet (2-mg) pack: 33.96 | ||
| 150-ml oral solution (60 mg): 19.13 | ||
| 75-ml oral solution (30 mg): 17.00 |
Target dosages per cycle were drawn from the literature (i.e. from RCTs) (Table 105). Cetuximab was assumed to be administered on a biweekly schedule to coincide with FOLFOX/FOLFIRI administration, as this is common clinical practice within the NHS. In addition, Merck Serono argued, on the basis of an open-label RCT by Brodowicz et al. 166 and a literature review,167 that biweekly administration of 500 mg/m2 of cetuximab is equivalent to induction with 400 mg/m2 followed by weekly administration of 250 mg/m2. Biweekly administration is not included in the SPC for cetuximab. We consider the RCT by Brodowicz et al. 166 to be of sufficient quality to make this claim and believe the claim of equivalence to be reasonable.
| Regimen | Agent | Cycles per month | Dosage per cycle | Cost per cycle (£) | Monthly cost (£) |
|---|---|---|---|---|---|
| CET + FOLFOX4 | CET | 2.17 | 500 mg/m2 | 1781 | 3859 |
| FOLFOX4 | See below | 86 | |||
| Chlorphenamine | 2.17 | 10 mg | 2.89 | 6 | |
| Dexamethasone | 2.17 | 8 mg | 2.08 | 5 | |
| Total | 3955 | ||||
| CET + FOLFOX6 | CET | 2.17 | 500 mg/m2 | 1781 | 3859 |
| FOLFOX6 | See below | 91 | |||
| Chlorphenamine | 2.17 | 10 mg | 2.89 | 6 | |
| Dexamethasone | 2.17 | 8 mg | 2.08 | 5 | |
| Total | 3961 | ||||
| PAN + FOLFOX4 | PAN | 2.17 | 6 mg/kg | 1896.45 | 4109 |
| FOLFOX4 | See below | 86 | |||
| Total | 4195 | ||||
| PAN + FOLFOX6 | PAN | 2.17 | 6 mg/kg | 1896.45 | 4109 |
| FOLFOX6 | See below | 91 | |||
| Total | 4200 | ||||
| BEV + FOLFOX4 | BEV | 2.17 | 5 mg/kg | 924.40 | 2003 |
| FOLFOX4 | See below | 86 | |||
| Total | 2089 | ||||
| BEV + FOLFOX6 | BEV | 2.17 | 5 mg/kg | 924.40 | 2003 |
| FOLFOX6 | See below | 91 | |||
| Total | 2094 | ||||
| FOLFOX4 | Oxaliplatin | 2.17 | 85 mg/m2 | 12.59 | 27 |
| Leucovorin | 2.17 | 400 mg/m2 | 22.07 | 48 | |
| Fluorouracil | 2.17 | 2000 mg/m2 | 4.92 | 11 | |
| Total | 86 | ||||
| FOLFOX6 | Oxaliplatin | 2.17 | 100 mg/m2 | 12.59 | 27 |
| Leucovorin | 2.17 | 400 mg/m2 | 11.03 | 48 | |
| Fluorouracil | 2.17 | 2800 mg/m2 | 7.38 | 16 | |
| Total | 91 | ||||
| XELOX | Capecitabine | 1.45 | 28,000 mg/m2 | 33.55 | 49 |
| Oxaliplatin | 1.45 | 130 mg/m2 | 18.89 | 27 | |
| Total | 76 | ||||
| CET + FOLFIRI | CET | 2.17 | 500 mg/m2 | 1781 | 3859 |
| FOLFIRI | See below | 128 | |||
| Chlorphenamine | 2.17 | 10 mg | 2.89 | 6 | |
| Dexamethasone | 2.17 | 8 mg | 2.08 | 5 | |
| Total | 3987a | ||||
| BEV + FOLFIRI | BEV | 2.17 | 5 mg/kg | 924.40 | 2003 |
| FOLFIRI | See below | 128 | |||
| Total | 2131 | ||||
| FOLFIRI | Irinotecan | 2.17 | 180 mg/m2 | 29.68 | 64 |
| Leucovorin | 2.17 | 400 mg/m2 | 11.03 | 48 | |
| Fluorouracil | 2.17 | 2800 mg/m2 | 7.38 | 16 | |
| Total | 128 | ||||
The cost-effectiveness of weekly dosing of cetuximab was evaluated in a scenario analysis. In this analysis the cost per month of drug acquisition for cetuximab (alone) was £4393 for the first month and £3859 thereafter.
Target dosages and unit costs were not varied in the probabilistic sensitivity analysis.
Target dosage and wastage were calculated based on an assumed body surface area of 1.85 m2 and a body weight of 74.7 kg.
Next, we discuss our estimates of mean dose intensity, the last term in the calculation of mean drug acquisition costs (see Equation 7). Mean dose intensities were assumed to be equal to the following median dose intensities from the RCTs, provided to us by Merck Serono and Amgen:
-
cetuximab plus FOLFOX – 89% (OPUS trial65)
-
FOLFOX – 79% (OPUS trial65)
-
panitumumab plus FOLFOX – 80% (PRIME trial27)
-
bevacizumab plus FOLFOX – 85% (PEAK trial29)
-
cetuximab plus FOLFIRI – 92% (CRYSTAL trial43)
-
bevacizumab plus FOLFIRI – 85% (from the PEAK trial29 as not provided in the FIRE-3 trial28)
-
FOLFIRI – 91% (CRYSTAL43).
The resulting mean drug acquisition costs per patient are provided in Figure 25.
FIGURE 25.
Mean drug acquisition costs per patient for all patients combined in the PenTAG model. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
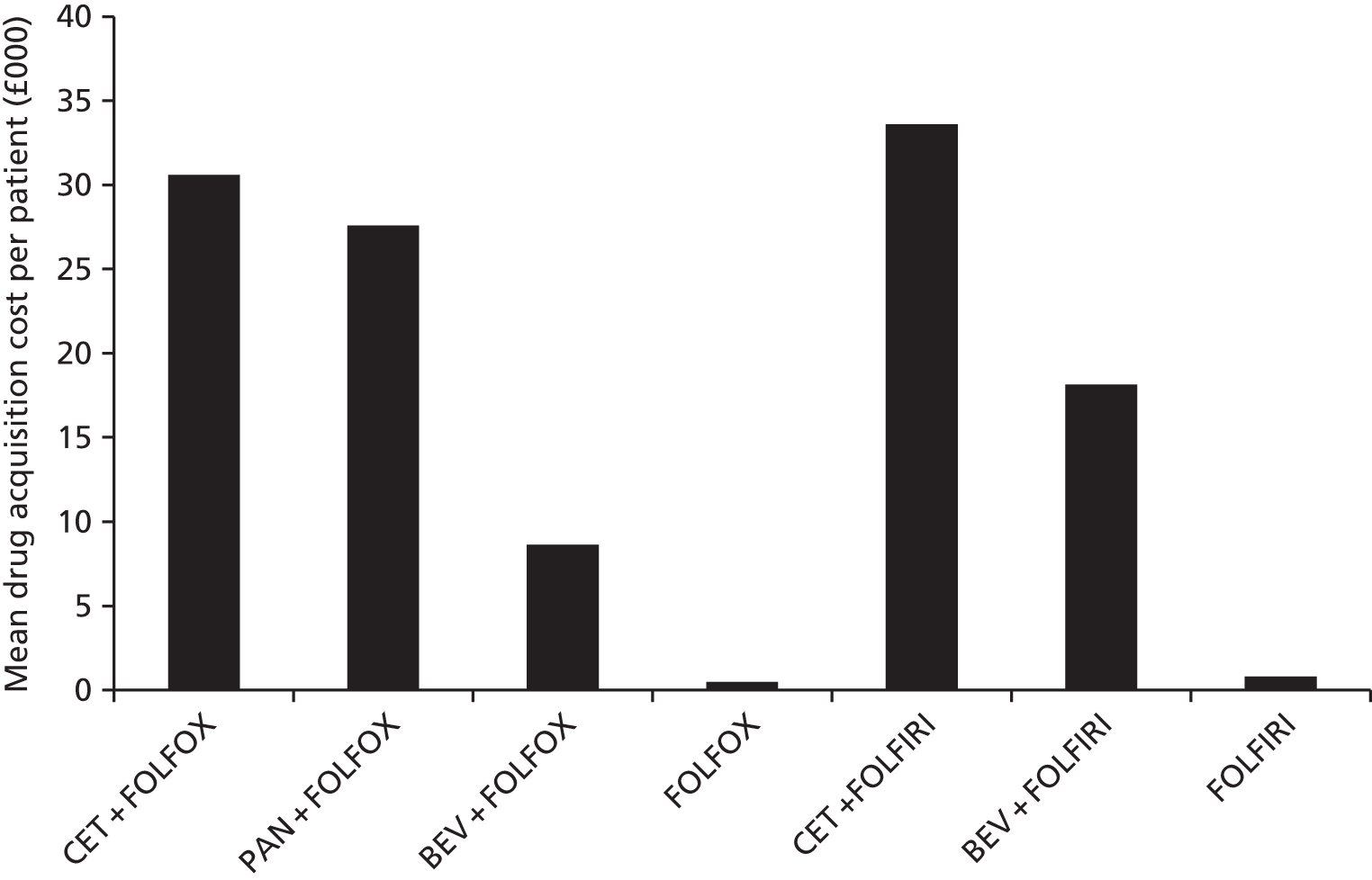
For the scenario analysis in which we modelled OS from the RCTs, we assumed that some patients in the FOLFOX network take cetuximab- or panitumumab-based treatments (see Scenario analyses, Overall survival from randomised controlled trials).
Drug administration costs
Drug administration costs are all costs borne by the NHS and personal social services of administering chemotherapy to a patient, excluding the direct cost of drug acquisition (i.e. payments to drug manufacturers or distributors).
Following a similar approach to that in previous NICE appraisals relating to mCRC,143,168 we included the following cost components in drug administration:
-
delivery costs
-
pharmacy costs
-
infusion pump costs
-
line maintenance costs.
The greatest of these cost components is delivery costs; this is followed by pharmacy costs.
According to NHS reference costs guidance,169 chemotherapy ‘patients receive a core HRG [relating to the purpose of their attendance (which is SB97Z if no other significant procedure takes place besides chemotherapy delivery)] and one or more unbundled chemotherapy HRGs split into two categories’. The first category is procurement HRGs, one of which is generated per chemotherapy cycle and includes the cost of the entire procurement service, including pharmacy costs. The procurement HRGs are divided according to setting and cost bands. The second category is delivery HRGs, which are generated for each attendance (not just at the start of each cycle). The delivery HRGs are divided according to setting and complexity (for the first day only, subsequent elements have a single unit cost per day in each setting).
It was not possible to use the procurement HRGs to estimate non-delivery administration costs because they would include the cost of drug acquisition and because the mapping from chemotherapy regimens to cost bands is not publicly available.
Evidence from the Department of Health, provided to us by Merck Serono [data on file, personal communication, 24 August 2015 (via NICE)], shows that pharmacy, infusion pump and line maintenance costs are already included in the delivery HRGs.
The total drug administration costs for each chemotherapy regimen are given in Table 106.
| Regimen | Drug administration costs per cycle (£) |
|---|---|
| CET + FOLFOX4 | 721 |
| PAN + FOLFOX4 | 721 |
| BEV + FOLFOX4 | 721 |
| CET + FOLFOX6 | 392 |
| PAN + FOLFOX6 | 392 |
| BEV + FOLFOX6 | 392 |
| FOLFOX4 | 713 |
| FOLFOX6 | 383 |
| CET + FOLFIRI | 392 |
| BEV + FOLFIRI | 392 |
| FOLFIRI | 383 |
| XELOX | 303 |
The interventions (cetuximab and panitumumab) are delivered as intravenous infusions prior to the initiation of the other component of chemotherapy (FOLFOX or FOLFIRI). 35,36 The comparator bevacizumab is administered similarly. FOLFOX6 and FOLFIRI are provided as a 2-hour infusion of leucovorin plus oxaliplatin or irinotecan followed by bolus 5-fluorouracil and then prolonged infusional 5-fluorouracil (46 hours). FOLFOX4 is provided as a 2-hour infusion of leucovorin plus oxaliplatin followed by bolus 5-fluorouracil and prolonged infusional 5-fluorouracil (22 hours), which is repeated on the subsequent day of the cycle.
Based on guidance for NHS reference costs for 2013–14169 (Table 107), we believe that the appropriate unit cost for one cycle of FOLFOX4 will consist of the unit cost of HRG code SB14Z (Deliver Complex Chemotherapy, including Prolonged Infusional Treatment) for day 1 and the unit cost of HRG code SB15Z (Deliver Subsequent Elements of a Chemotherapy Cycle) for day 2 of the cycle. FOLFOX6 and FOLFIRI will incur only the unit cost of HRG code SB14Z. This results in significantly increased costs for FOLFOX4 compared with FOLFOX6 and FOLFIRI, but these are justified by the necessity of removing the infusion pump, flushing the line, delivering a 2-hour infusion and initiating the next 22-hour infusion, which must be carried out either in hospital or by a nurse visitor.
| Definition | Explanation |
|---|---|
| Deliver simple parenteral chemotherapy | Overall time of 30 minutes nurse time and 30–60 minutes chair time for the delivery of a complete cycle |
| Deliver more complex parenteral chemotherapy | Overall time of 60 minutes nurse time and up to 120 minutes chair time for the delivery of a complete cycle |
| Deliver complex chemotherapy, including prolonged infusional treatment | Overall time of 60 minutes nurse time and > 2 hours chair time for the delivery of a complete cycle |
| Deliver subsequent elements of a chemotherapy cycle | Delivery of any part of an outpatient chemotherapy regimen, other than the first attendance (i.e. day 8 of a day 1 and 8 regimen, or days 8 and 15 of a day 1, 8 and 15 regimen) |
The setting of chemotherapy delivery is also important, as the unit costs vary considerably according to setting (Table 108). It can be seen that, although the day-case and regular day/night settings accounts for the majority of activity, they also produce the highest unit costs. Delivery in an outpatient or ‘other’ setting significantly reduces the unit cost of the first attendance in a cycle, and delivery in an ‘other’ setting significantly reduces the unit cost of delivery of subsequent elements of a chemotherapy cycle. The ‘other’ setting refers to community chemotherapy, in which patients receive their chemotherapy treatment in a facility nearer to home than their cancer centre (e.g. a GP surgery) or in their own home.
| Setting | SB14Z (Deliver Complex Chemotherapy, including Prolonged Infusional Treatment) | SB15Z (Deliver Subsequent Elements of a Chemotherapy Cycle) | ||
|---|---|---|---|---|
| Total activity count | Unit cost (£) | Total activity count | Unit cost (£) | |
| Day case and regular day/night | 151,689 | 401 | 167,850 | 328 |
| Outpatient | 37,146 | 266 | 40,880 | 314 |
| Other | 8577 | 284 | 7313 | 187 |
The estimated SE for each unit cost was calculated from the underlying reference cost data, which provide the unit cost and activity supplied by each submitting organisation.
First, the weighted SD was calculated for each unit cost, with the weight for each organisation equal to its activity. Then, the SE was estimated by dividing by the square root of the number of organisations (Table 109).
| HRG code | Setting | Number of organisations | Total activity count | Unit cost (£) | SD (£) | SE (£) |
|---|---|---|---|---|---|---|
| SB13Z | DCRDN | 128 | 132,260 | 316.95 | 248.46 | 21.96 |
| OP | 49 | 25,223 | 218.60 | 96.55 | 13.79 | |
| Other | 10 | 5468 | 189.91 | 107.72 | 34.06 | |
| SB14Z | DCRDN | 127 | 151,689 | 401.48 | 307.37 | 27.27 |
| OP | 41 | 37,146 | 265.85 | 113.46 | 17.72 | |
| Other | 11 | 8577 | 283.81 | 175.79 | 53.00 | |
| SB15Z | DCRDN | 117 | 167,850 | 327.75 | 258.29 | 23.88 |
| OP | 36 | 40,880 | 313.80 | 156.91 | 26.15 | |
| Other | 11 | 7313 | 187.00 | 106.79 | 32.20 |
A gamma distribution was used for each unit cost, with parameters derived using the method of moments. 170
The drug delivery cost per cycle of FOLFOX6 and FOLFIRI was therefore £383, whereas the cost per cycle of FOLFOX4 was £713.
It was further deemed important to reflect on the additional nursing time required to deliver monoclonal antibody therapy (cetuximab, panitumumab or bevacizumab) at the start of each cycle, even though this would not result in a different HRG currency being generated for the attendance. It is acknowledged (e.g. paragraph 5.5.6 of the NICE methods guide128) that in such circumstances other sources of evidence may be appropriate. As such, it was considered appropriate to estimate the additional nursing time and cost required. Our clinical expert advised that 15 minutes of additional nursing time would be required to administer monoclonal antibody therapy, which was costed at £34 (£35.12) per hour in 2013/14 (2015/16) prices,163 resulting in an additional cost per cycle of £8.78 for chemotherapy regimens including monoclonal antibodies. A gamma distribution was used for the duration of nursing time (independently drawn for each monoclonal antibody), with a SE of 20% of the mean. Likewise, a gamma distribution was used for the cost per hour of nursing time, with a SE of 20% of the mean.
Finally, the drug delivery cost per cycle of XELOX was estimated using HRG code SB13Z (Deliver More Complex Parenteral Chemotherapy at First Attendance) as £303 per cycle. It was assumed that there would be no additional cost for the delivery of oral capecitabine.
In the scenario analysis of weekly cetuximab administration (see Scenario analyses, Weekly administration of cetuximab), the delivery cost per cycle for cetuximab regimens was increased by £303 to reflect the extra attendance for drug delivery.
Cost of liver resection
We found the following sources of data for the failure rate of resection of liver metastases (Table 110).
| Rate (%) | Source |
|---|---|
| < 10 | Dr Mark Napier (clinical advisor to PenTAG, 2015, personal communication) |
| 27.8 | NICE TA176,10 manufacturer’s initial submission |
| 5 | NICE TA176,10 clinical specialists’ opinion (section 4.7) |
| 5 | NICE TA176,10 manufacturer’s revised economic analysis |
| 0 | Merck submission, current HTA |
| 33.3 | PAN + FOLFOX (PEAK trial29) (used in Graham et al.,107 p. 2795) |
| 22.2 | BEV + FOLFOX (PEAK trial29) (used in Graham et al.,107 p. 2795) |
In Merck Serono’s revised analysis in TA176,10 the failure rate was assumed to be 5%. Higher liver surgery failure rates of 33% for panitumumab plus FOLFOX and 22% for bevacizumab plus FOLFOX were observed in the PEAK trial. 29
In our model we assumed a liver resection failure rate of 5% (based on TA17610 and expert advice from Dr Mark Napier; see Table 110).
In its current submission, Merck Serono modelled a cost of £2707 per liver resection operation.
In the study by Graham et al. ,107 liver resection surgery and hospitalisation were assumed to cost €14,428 (£11,356 as of 21 May 2015) (Table 111).
| Cost (£, 2015) | Source |
|---|---|
| 11,356a | Graham et al.107 |
In TA176, in its original submission to NICE, Merck Serono estimated a cost of £2271 for liver resection. This was later revised to £8929 and approved by the NICE committee (NICE Final Appraisal Determination10). In the revised submission, Merck Serono used a weighted average cost per liver resection surgery calculated from two liver HRG codes: G02 (Liver – Complex Procedures) and G03 (Liver – Very Major Procedures) (Table 112).
| OPCS | HRG v3.5 codes | Description | HRG v4+ codes |
|---|---|---|---|
| J021 | G02 | Right hemihepatectomy NEC | GA03, GA04 |
| J022 | G02 | Left hemihepatectomy NEC | GA03, GA04 |
| J023 | G02 | Resection of segment of liver | GA03, GA04, GA05 |
| J028 | G02 | Other specified partial excision of liver | GA03, GA04, GA05 |
| J029 | G02 | Unspecified partial excision of liver | GA05, GA06, GA07 |
| J024 | G03 | Wedge excision of liver | GA03, GA04, GA05 |
| J031 | G03 | Excision of lesion of liver NEC | GA05, GA06, GA07 |
| J032 | G03 | Destruction of lesion of liver NEC | GA06, GA07, GA13 |
We could not map from HRG v3.5171 to HRG v4+,172 so instead we identified which OPCS Classification of Interventions and Procedures version 4.6 mapped to HRG v3.5 codes G02 and G03. Of these, the codes shown in Table 112 seem potentially relevant to resection of liver metastases.
Based on clinical advice we understand that all liver resection surgeries for mCRC are very complex: 80% of them are open operations and the remaining 20% are laparoscopic surgeries. Based on this assumption, the HRG v4+ code GA03 (Very Complex Open Hepatobiliary or Pancreatic Procedures) is likely to be a suitable candidate for calculating the cost of liver surgery.
We estimated the unit cost of very complex open liver resection surgery as a weighted average of the costs for the HRGs GA03C, GA03D and GA03E (Table 113). They were derived including:
-
elective inpatients
-
elective inpatient excess bed-days
-
non-elective inpatients (long stay)
-
non-elective inpatient (long stay) excess bed-days
-
non-elective inpatients (short stay).
| Currency | Currency description | Total activity count | Unit cost (£) | Total cost, (£) |
|---|---|---|---|---|
| GA03C | Very Complex Open Hepatobiliary or Pancreatic Procedures, with CC Score 4+ | 627 | 13,433 | 8,422,455 |
| GA03D | Very Complex Open Hepatobiliary or Pancreatic Procedures, with CC Score 2–3 | 596 | 10,258 | 6,113,911 |
| GA03E | Very Complex Open Hepatobiliary or Pancreatic Procedures, with CC Score 0–1 | 940 | 8659 | 8,139,070 |
| Weighted average | 2163 | 10,483 | 22,675,436 | |
In the previous section we estimated the unit cost of open liver resection as £10,483 in 2013/14 prices (£10,829 in 2015/16 prices). We were not able to identify appropriate HRGs in the NHS reference costs for laparoscopic liver resection, but we identified a cost study reported by Polignano et al. 173 in which the costs of elective laparoscopic and open liver segmentectomy, performed with an intention to treat the disease, were compared (Table 114). Twenty-five laparoscopic liver resections carried out at Ninewells Hospital and Medical School, Dundee, UK, between 2005 and 2007 were compared with 25 matching open resections conducted at the same institution between 2004 and 2007. The two groups were homogeneous by age, sex, coexistent morbidity and magnitude of resection. Hospital costs were obtained from the Scottish Health Service Costs (ISD Scotland) and average costs were calculated. Laparoscopic surgery was associated with a reduction in total costs of 18.0%, from which we estimated the cost of laparoscopic liver resection to be £8598 in 2013/14 prices.
| Laparoscopic surgery (£) | Open surgery (£) | |
|---|---|---|
| Total (mean ± SD) | 11,727 ± 3288 | 14,298 ± 3817 |
Based on expert opinion that 80% of liver resections for metastases are open and 20% are laparoscopic, we estimated an average cost for liver resection (weighted for the proportion of resections that are open and the proportion that are laparoscopic) of £10,106 in 2013/14 prices, which was inflated to £10,440 in 2015/16 prices.
In TA176 (p. 13),10 the cost of liver resection was assumed to occur only once. This was despite the fact that the NICE appraisal committee believed that some patients may undergo more than one operation to achieve complete resection of metastases (NICE Final Appraisal Determination, p. 22). 10 In its current submission, Merck Serono also assumed one liver resection per patient.
Adam et al. 113 reported that 223 hepatectomies (out of 342 surgical procedures) were performed on 138 patients, that is, 1.6 per patient. The frequencies of repeat hepatectomies for recurring CRC in patients with initially unresectable metastases, observed between January 1990 and January 2010 in a French hospital, were reported by Wicherts et al. 174 (Table 115). In total, 1.4 operations were carried out per patient.
| Number of hepatectomies | Number of patients |
|---|---|
| 2 | 42/114 |
| 3 | 8/114 |
In conclusion, we assumed a mean of 1.6 operations per patient, based on the study by Adam et al. ,113 as our estimates for OS and PFS post resection were based on this source.
Medical management costs
In this section we describe medical management costs not covered in other cost categories, including the costs of:
-
oncology outpatient attendances
-
blood tests
-
imaging tests [magnetic resonance imaging (MRI), CT]
-
colonoscopy
-
palliative care.
Resource use is different pre and post progression, as well as depending on whether or not liver metastases have been successfully resected.
Resource use parameters are presented per month unless otherwise stated.
Individuals receiving first- or second-line chemotherapy who have not undergone successful liver resection are estimated to have a consultant outpatient appointment every 2 weeks regardless of their chemotherapy regimen, according to expert opinion (Dr Mark Napier, 2015, personal communication). This assumption was also made in TA242. 139 One appointment every 2 weeks corresponds to 2.17 appointments on average per month.
Simple blood tests are performed every 2 weeks, but are low cost and are therefore not included. More involved blood tests (tumour markers and liver function tests) are estimated to be performed at 1 month and then every 4 months. 113 For simplicity it was assumed that these tests would be performed on average 0.25 times per month.
During staging, all patients are offered (and are very likely to receive) contrast-enhanced CT of the chest, abdomen and pelvis. 1 This was not included as it is common to all regimens and occurs before chemotherapy commences.
Rectal cancer patients are also offered MRI to assess the risk of local recurrence during staging;1 this was also not included.
Magnetic resonance imaging, contrast-enhanced CT and positron emission tomography-CT may be offered to patients with metastatic disease to determine the locations of disease and inform multidisciplinary teams. 1 These are not included since they are common to all regimens and are likely to occur before chemotherapy commences.
It was estimated that CT scans are conducted every 3 months to monitor response to chemotherapy. 175 Ultrasound imaging and MRI are not believed to be conducted routinely to monitor response, but it was considered plausible that patients may receive one or two MRI scans per course (Dr Mark Napier, 2015, personal communication). Based on mean time on first-line FOLFOX in unresected patients of 0.58 years and assuming two MRI scans over this period, we estimated that patients would receive 0.288 MRI scans per month.
It was assumed, on the basis of expert opinion, that these patients would not undergo routine surveillance for local recurrence (i.e. colonoscopy).
Resource use parameters were assumed to follow a gamma distribution in the probabilistic sensitivity analysis, with SEs of 20% of the mean.
Post-progression patients are expected to receive BSC, with their management largely being transferred from secondary care to a palliative care team and/or their GP.
Rather than estimate resource use across a large number of cost components, we instead estimated the cost of BSC per month (see Best supportive care).
Given that patients post-successful resection pre progression have a good prognosis (compared with patients unsuitable for liver resection or in whom liver resection is incomplete), less intensive medical management is expected to be required.
Oncology outpatient attendances are expected every 4 months, that is, 0.25 appointments per month on average. 113 Blood tests (tumour markers and liver function) are conducted every 3 months (Dr Mark Napier, 2015, personal communication). It was assumed that CT scans are conducted every 3 months (Dr Mark Napier, 2015, personal communication). MRI scans may be conducted but, given the limited size of this population and the low number of tests that would be expected to be conducted, these were not included.
Colonoscopy may be recommended for surveillance of local recurrence in these patients. It is recommended that the first surveillance colonoscopy be offered at 1 year after initial treatment,1 with subsequent surveillance dictated by the risk of further malignancy, which may be 1- to 3-yearly if adenomas are found (Dr Mark Napier, 2015, personal communication) or at 5 years if there are no abnormal findings. We assumed that there would be one colonoscopy at 12 months, plus one colonoscopy every 3 years thereafter (giving an average of 0.028 colonoscopies per month).
Resource use parameters were assumed to follow a gamma distribution in the probabilistic sensitivity analysis, with SEs of 20% of the mean.
Patients post-successful resection post progression were assumed to receive the same treatment as third-line post-progression patients who were not resected, that is, BSC.
Unless otherwise stated, unit costs for medical management were drawn from gamma distributions in the probabilistic sensitivity analysis, with SEs of 20% of the mean.
A cost of £155 (2015/16 prices) was assumed per oncology outpatient attendance, based on a cost of £150 for consultant-led outpatient attendances in medical oncology (service code 370) from NHS reference costs 2013–14. 134
We used the same unit cost for blood tests for medical management as we did for blood tests post resection, namely £13 per tumour marker test and £27 per liver function test (identified from NICE 2005 interventional procedures guidance176 and inflated to 2015/16 prices).
Imaging tests were assumed to be carried out in the outpatient setting and costs were estimated from NHS reference costs 2013–14. 134 CT scans were assumed to be carried out in three areas, with contrast, with an estimated cost of £132 in 2013/2014 prices (£137 in 2015/16 prices). 134 MRI scans were assumed to be carried out in two to three areas, with contrast, with an estimated cost of £193 in 2013/2014 prices (£200 in 2015/16 prices). 134
Colonoscopy was assumed to be carried out as either a day-case or an outpatient procedure (and was weighted according to the activity recorded for each setting). The cost of colonoscopy was estimated from NHS reference costs 2013–14134 as £519 (2015/16 prices).
In previous assessments the cost of BSC was estimated based on a cost-of-illness study in stage IV breast cancer by Remák and Brazil. 177 The cost per month of BSC was estimated as £675 (£1031) in 2000 (2015/16) prices, whereas the total cost of end-of-life care was estimated as £1316 (£2010) in 2000 (2015/16) prices.
We performed a pragmatic literature search for cost-of-illness studies in mCRC and identified the following two studies of interest.
-
A Finnish study by Färkkilä et al. 178 estimated direct health-care costs per month of €1667 in 2010 prices (£1254 in 2015/16 prices) in the ‘palliative state’, with over half of this accounted for by ‘primary/hospice care’.
-
A US study by Song et al. 179 estimated average medical expenditure per month of US$26,649 in 2008 prices (£17,402 in 2015/16 prices) in the ‘death phase’ (which covered up to 3 months prior to death) based on commercial and Medicare claims data, although this might include time on active treatment.
Given the significant differences between the US and UK health-care systems, it was decided that the estimate from Song et al. 179 was not generalisable to the NHS.
It was judged that the estimate from Färkkilä et al. 178 was more recent than the estimate from Remák and Brazil,177 and was determined using the correct patient population. In addition, although this study was carried out in Finland, Finland has ‘fairly comprehensive provision of public health care’ (p. 456). 178 On this basis we used a cost per month of BSC of £1254. This is substantially greater than Merck Serono’s estimate of £315 per month (see Chapter 5, De novo economic evaluation, Description of methods).
No separate cost for end-of-life care was included, as these costs should be included in the palliative state in the analysis by Färkkilä et al. 178
The 95% CI for direct medical costs ranged from 54.5% to 145.5% of the mean cost. This suggests a SE of approximately 23.2% of the mean. To further acknowledge the uncertainty resulting from the generalisation of the BSC cost from a different country, a SE of 40% of the mean was used in the probabilistic sensitivity analysis.
Adverse events
The NMAs for AEs reported in Chapter 3 (see Results, Adverse events) provide limited results for types of grade 3 or 4 AEs. The FOLFOX network reports incidences for all comparators for neutropenia, paraesthesia, rash and skin conditions, and the FOLFIRI network reports incidences for all comparators for skin conditions and diarrhoea.
On advice from our clinical expert (Dr Mark Napier, 2015, personal communication) we believe that not all clinically important AEs are likely to have been picked up by these NMAs.
As such, we used an alternative approach to estimate costs and QALYs associated with AEs that was not reliant on every trial. Instead, we chose two trials as the bases for our two cost-effectiveness networks, calculated total AE costs and QALYs for FOLFOX and FOLFIRI for these trials using the incidence of each type of AE that was reported and then calculated costs and QALYs for the other arms of those trials by adjusting for the relative risk of any grade 3/4 AE.
The two trials chosen as our bases were the PRIME trial27 for the FOLFOX network and the CRYSTAL trial43 for the FOLFIRI network. These were the largest trials with the most relevant comparators and were chosen for consistency with the rest of the model.
The relative risk of any grade 3/4 AE was calculated by adjusting the ORs reported in Chapter 3 (see Network meta-analysis) using the following formula:180
For the purposes of our analysis, we grouped together different types of AEs that were thought to have similar costs and utilities.
Disutilities for adverse events
The only cost-effectiveness study to report AE disutility was that by Ortendahl et al. ,109 which used a value of –0.07 from Jonker et al. (2007) (full reference not provided by Ortendahl). This is a simplistic approach as it assumes the same disutility for all AEs. No studies on disutilities for AEs were identified from our literature review of quality of life. However, we identified a recent NICE diagnostic appraisal report143 that included a review on AEs in CRC, including UK data. We also consulted the sources provided by Merck Serono in its submission as potential sources for our model. Our chosen base-case utilities for AEs are presented in Table 116.
| AE | Base case | SE | Source |
|---|---|---|---|
| Anaemia | –0.08500 | 0.17 | Harrow et al.,181 scaled to the EQ-5D, as reported in Crathorne et al.182 |
| Asthenia | –0.08000 | 0.0615 | Assumed to be the same as fatigue |
| Diarrhoea | –0.09000 | 0.0379 | Freeman et al.143 (SCOT trial data183) |
| Fatigue | –0.08000 | 0.0615 | Freeman et al.143 (SCOT trial data183) |
| Hypokalaemia | –0.08000 | 0.0615 | Assumed to be the same as fatigue |
| Infection | –0.19500 | 0.012 | Tolley et al.136 |
| Leukopenia | –0.06070 | 0.0457 | Assumed to be the same as neutropenia |
| Mucosal inflammation | –0.03750 | 0.1438 | Assumed to be the same as mucositis |
| Mucositis/stomatitis | –0.03750 | 0.1438 | Freeman et al.143 (SCOT trial data183) |
| Neuropathy | –0.19700 | 0.091 | Freeman et al.143 (SCOT trial data183) |
| Neutropenia | –0.06070 | 0.0457 | Freeman et al.143 (SCOT trial data183) |
| Pain | –0.06900 | 0.012 | Doyle et al.135 (chest pain) |
| Paraesthesia | –0.06900 | 0.012 | Assumed to be equal to pain |
| Skin conditions | –0.03248 | 0.01171 | Nafees et al.137 |
| Thrombosis | –0.19000 | 0.038 | Hogg et al.184 |
Freeman et al. 143 identified the SCOT (Short Course Oncology Treatment) trial,183 which reported UK-based EQ-5D data for CRC patients. They also received a personal communication related to this trial, which included additional information. 143 Although the Freeman et al. 143 study has not yet been published, it has been reviewed as part of the NICE process and as such we believe it to be of relevance to our report. However, the EQ-5D data are limited to a few AEs and, as such, we were required to use the studies identified by Freeman et al. ,143 the Merck Serono submission and some additional searching to find disutility estimates for all AEs reported in our identified trials.
Many of the utility studies identified by Freeman et al. 143 and the Merck Serono submission were not specific to CRC patients. Neither of these studies reported the disutility associated with anaemia or thromboembolic events. We used a recent NICE technology assessment of cancer treatment-induced anaemia (TA323)182 to estimate the utility difference for anaemia. This assessment used estimates from Harrow et al. ,181 scaled from the SF-6D to the EQ-5D, and was based on a cancer population.
We did not identify any UK-based studies that reported disutility for thrombosis or any studies specific to a CRC population. Instead, we used the value of –0.190 reported by Hogg et al. 184 This study was conducted with 215 people who underwent treatment for thromboembolic events at the Ottawa Hospital Thrombosis Clinic in Canada. In total, 23% of patients had cancer-related thrombosis. A standard gamble approach was used to elicit quality-of-life data from patients, but the measure used was not reported. The value of –0.190 is similar to the value of –0.195 used by Merck Serono in its model (Merck Serono submission, appendix B, table 1), although Merck Serono based its value on the disutility associated with infection.
A length of 1 week was applied to disutilities, in line with the approach used in the study by Freeman et al. ,143 in which expert opinion indicated durations of a maximum of 7 days for grade 3/4 AEs. Freeman et al. 143 state that this was broadly similar to the length of stay associated with AEs reported in Twelves et al. 185 Some AEs may persist for > 7 days but with reduced severity and, in this analysis, grade 1/2 AEs were assumed to have no disutility.
It is probable that some of the disutility of AEs is already captured in the first-line utility reported by Bennett et al. ,115 as the PRIME trial also recorded AEs and utilities. However, it is unclear what crossover there is between the cohort who reported utility estimates and the cohort who reported AE data. To arbitrarily reduce the disutility of AEs related to the PRIME trial would likely underestimate the impact of these events. As such, we calculated the disutilities independently from the utility estimates in the base case and set them equal to 0 in a sensitivity analysis. As the values are small for all arms (ranging from –0.0018 to –0.0005) and the PRIME health state utilities are applied for all treatment arms, any double counting is also applied in all arms and therefore does not impact greatly on the results.
Unit costs for adverse events
Unit costs were again based on the submission by Merck Serono and the study by Freeman et al. 143 These are detailed in Table 117; most are NHS reference costs relating to specific events. As these are AE costs, the duration of the AE is not applied to these values.
| AE | Base-case cost (£) | SE (£) | Source |
|---|---|---|---|
| Anaemia | 799 | 159.80 | Crathorne et al.182 |
| Asthenia | 157 | 31.40 | Same as fatigue |
| Diarrhoea | 157 | 31.40 | General medicine outpatient visit service code 300134 |
| Fatigue | 157 | 31.40 | General medicine outpatient visit service code 300134 |
| Hypokalaemia | 157 | 31.40 | Same as fatigue |
| Infection | 2160 | 432.00 | Spell-based average inpatient stay134 |
| Leukopenia | 157 | 31.40 | General medicine outpatient visit service code 300134 |
| Mucosal inflammation | 941 | 188.20 | Assumed to be the same as mucositis |
| Mucositis/stomatitis | 941 | 188.20 | Based on Freeman et al.143: non-malignant, ear, nose, mouth, throat or neck disorders (CB02A, CB02B, CB02C, CB02D, CB02E, CB02F)134 |
| Neuropathy | 1736 | 347.20 | Based on Merck submission: neoplasm-related admission (WA17A, WA17B, WA17C, WA17D)134 |
| Neutropenia | 2160 | 432.00 | Spell-based average inpatient stay134 |
| Pain | 135 | 27.00 | Outpatient pain management code 191134 |
| Paraesthesia | 0 | – | Assumed no cost |
| Skin conditions | 6 | 1.20 | Diprobase® (Bayer plc, Newbury, Berkshire, UK) 500-mg pump16 (as used in Freeman et al.143) |
| Thrombosis | 712 | 142.40 | Deep-vein thrombosis (YQ51A, YQ51B, YQ51C, YQ51D)134 |
Checking the Peninsula Technology Assessment Group’s model for wiring errors
The PenTAG model was checked for wiring errors in the following ways.
-
All model formulae that were written were checked by members of the team who did not build the model (NH, IT, TS).
-
The reasonableness of outputs given extreme input values was checked, for example the model estimated QALYs were calculated to be equal to estimated life-years when the utility estimates were set to 1.
-
A simplified model was built that did not rely on model cycles and the results were compared with the results of the full model to quickly identify errors.
-
The base-case model results were checked for reasonableness using numerous graphs.
-
The model results were checked for reasonableness through numerous univariate sensitivity analyses and a probabilistic sensitivity analysis.
Peninsula Technology Assessment Group’s results
The base-case results are presented first, followed by the results of the sensitivity analyses.
Base-case results
All patients: base-case results
Our base-case results for the FOLFOX and FOLFIRI networks are provided in Tables 118–121.
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.41 | 2.08 | 1.86 | 0.55 | 0.22 |
| QALYs (mean, discounted) | 1.61 | 1.41 | 1.26 | 0.35 | 0.15 |
| Total costs (mean, discounted) (£) | 67,057 | 61,225 | 30,585 | 36,471 | 30,640 |
| ICER (£ per QALY) vs. FOLFOX (£) | 104,205 | 204,103 | |||
| ICER (£ per QALY) on efficiency frontier (£) | 104,205 | Extendedly dominated | Reference |
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET+FOLFOX vs. | PAN + FOLFOX vs. FOLFOX | |
|---|---|---|---|---|---|---|
| PAN + FOLFOX | FOLFOX | |||||
| Life-years (mean, undiscounted) | ||||||
| First-line drug (resected + unresected) | 0.72 | 0.68 | 0.58 | 0.04 | 0.14 | 0.10 |
| PFS unresected | 0.57 | 0.64 | 0.52 | –0.07 | 0.06 | 0.12 |
| PFS post resection | 0.85 | 0.52 | 0.44 | 0.33 | 0.41 | 0.08 |
| PFS first line | 1.42 | 1.16 | 0.96 | 0.26 | 0.46 | 0.2 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.26 | 0.28 | 0.29 | –0.03 | –0.03 | –0.01 |
| Third-line BSC (unresected) | 0.38 | 0.42 | 0.43 | –0.04 | –0.05 | –0.01 |
| PD post resection | 0.35 | 0.21 | 0.18 | 0.14 | 0.17 | 0.03 |
| OS (mean) | 2.41 | 2.08 | 1.86 | 0.33 | 0.55 | 0.22 |
| Cohort split | ||||||
| % starting second-line FOLFOX/FOLFIRI (unresected) | 93.50 | 93.50 | 93.50 | 0.00 | 0.00 | 0.00 |
| % starting third-line BSC (unresected) | 87.50 | 87.50 | 87.50 | 0.00 | 0.00 | 0.00 |
| Life-years (mean) (undiscounted for patients who spend at least some time in given health state) | ||||||
| PFS unresected | 0.72 | 0.73 | 0.58 | –0.01 | 0.14 | 0.16 |
| PFS post resection | 4.09 | 4.09 | 4.09 | 0 | 0 | 0 |
| PFS first line | 4.81 | 4.82 | 4.67 | –0.01 | 0.14 | 0.16 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.34 | 0.34 | 0.34 | 0 | 0 | 0 |
| Third-line BSC (unresected) | 0.55 | 0.55 | 0.55 | 0 | 0 | 0 |
| PD post resection | 1.69 | 1.69 | 1.69 | 0 | 0 | 0 |
| OS unresected | 1.53 | 1.54 | 1.38 | –0.01 | 0.14 | 0.16 |
| QALYs (discounted) | ||||||
| PFS unresected | 0.43 | 0.48 | 0.39 | –0.05 | 0.04 | 0.09 |
| PFS post resection | 0.56 | 0.34 | 0.29 | 0.22 | 0.27 | 0.05 |
| AEs first line | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PFS first line | 0.99 | 0.82 | 0.68 | 0.16 | 0.31 | 0.14 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.19 | 0.21 | 0.21 | –0.02 | –0.02 | –0.01 |
| Third-line BSC (unresected) | 0.23 | 0.26 | 0.26 | –0.02 | –0.03 | –0.01 |
| PD post resection | 0.20 | 0.12 | 0.10 | 0.08 | 0.10 | 0.02 |
| Total | 1.61 | 1.41 | 1.26 | 0.20 | 0.35 | 0.15 |
| Costs (discounted) (£) | ||||||
| RAS test | 400 | 400 | 0 | 0 | 400 | 400 |
| First-line drug acquisition | 29,850 | 26,969 | 461 | 2881 | 29,389 | 26,508 |
| First-line drug administration | 13,212 | 12,508 | 10,527 | 704 | 2685 | 1981 |
| First-line AEs | 1512 | 1582 | 1068 | –70 | 444 | 514 |
| First-line medical management (unresected) | 3029 | 3394 | 2746 | –365 | 283 | 648 |
| Second-line FOLFOX or FOLFIRI acquisition (unresected) | 379 | 417 | 429 | –38 | –50 | –12 |
| Second-line FOLFOX or FOLFIRI administration (unresected) | 2456 | 2704 | 2778 | –247 | –322 | –75 |
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1325 | 1458 | 1499 | –133 | –174 | –40 |
| Third-line BSC (unresected) | 5481 | 6033 | 6199 | –552 | –718 | –166 |
| Resection operation | 3635 | 2224 | 1884 | 1411 | 1751 | 340 |
| PFS post resection | 1014 | 620 | 526 | 394 | 488 | 95 |
| PD post resection | 4763 | 2914 | 2469 | 1849 | 2294 | 446 |
| Total | 67,057 | 61,225 | 30,585 | 5832 | 36,471 | 30,640 |
| ICER (£ per QALY) | 29,176 | 104,205 | 204,103 | |||
| Outcome | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|
| Life-years (mean, undiscounted) | 2.21 | 1.75 | 0.46 |
| QALYs (mean, discounted) | 1.53 | 1.23 | 0.30 |
| Total costs (mean, discounted) (£) | 65,380 | 28,250 | 37,130 |
| ICER (£ per QALY) (£) | 122,554 |
| Outcome | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| First-line drug (resected + unresected) | 0.76 | 0.56 | 0.20 |
| PFS unresected | 0.95 | 0.75 | 0.20 |
| PFS post resection | 0.30 | 0.09 | 0.21 |
| PFS first line | 1.25 | 0.83 | 0.42 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.39 | 0.41 | –0.02 |
| Third-line BSC (unresected) | 0.45 | 0.47 | –0.03 |
| PD post resection | 0.12 | 0.04 | 0.09 |
| OS (mean) | 2.21 | 1.75 | 0.46 |
| Cohort split | |||
| % unresected | 92.7 | 97.9 | –5.2 |
| % starting second-line FOLFOX/FOLFIRI (unresected) | 93.5 | 93.5 | 0.0 |
| % starting third-line BSC (unresected) | 87.5 | 87.5 | 0.0 |
| % resected | 7.3 | 2.1 | 5.2 |
| Life-years (mean) (undiscounted for patients who spend at least some time in given health state) | |||
| PFS unresected | 1.03 | 0.76 | 0.26 |
| PFS post resection | 4.09 | 4.09 | 0.00 |
| PFS first line | 5.12 | 4.85 | 0.26 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.45 | 0.45 | 0.00 |
| Third-line BSC (unresected) | 0.55 | 0.55 | 0.00 |
| PD post resection | 1.69 | 1.69 | 0.00 |
| OS unresected | 1.93 | 1.67 | 0.26 |
| QALYs (discounted) | |||
| PFS unresected | 0.71 | 0.56 | 0.15 |
| PFS post resection | 0.20 | 0.06 | 0.14 |
| AEs first line | –0.00 | –0.00 | –0.00 |
| PFS first line | 0.91 | 0.62 | 0.29 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.28 | 0.30 | –0.02 |
| Third-line BSC (unresected) | 0.27 | 0.29 | –0.02 |
| PD post resection | 0.07 | 0.02 | 0.05 |
| Total | 1.53 | 1.23 | 0.30 |
| Costs (discounted) (£) | |||
| RAS test | 400 | 0 | 400 |
| First-line drug acquisition | 32,742 | 776 | 31,965 |
| First-line drug administration | 7543 | 5506 | 2037 |
| First-line AEs | 821 | 482 | 339 |
| First-line medical management (unresected) | 4993 | 3948 | 1045 |
| Second-line FOLFOX or FOLFIRI acquisition (unresected) | 382 | 407 | –25 |
| Second-line FOLFOX or FOLFIRI administration (unresected) | 6868 | 7317 | –449 |
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1991 | 2122 | –130 |
| Third-line BSC (unresected) | 6316 | 6730 | –413 |
| Resection operation | 1284 | 372 | 912 |
| PFS post resection | 358 | 104 | 254 |
| PD post resection | 1683 | 488 | 1195 |
| Total | 65,380 | 28,250 | 37,130 |
| ICER (£ per QALY) | 122,554 | ||
Survival results
The relative proportions of patients in each health state for each treatment throughout the time horizon of the model are displayed in Figure 26. The mean duration in each health state for each treatment (see Tables 119 and 121) is represented in these graphs by the area under each curve. Virtually all patients are predicted to have died 20 years from start of treatment, which is less than the model time horizon of 30 years.
FIGURE 26.
Cohort composition over time by treatment: (a) cetuximab plus FOLFOX; (b) panitumumab plus FOLFOX; (c) FOLFOX; (d) cetuximab plus FOLFIRI; and (e) FOLFIRI. PD, progressive disease. (t), time.

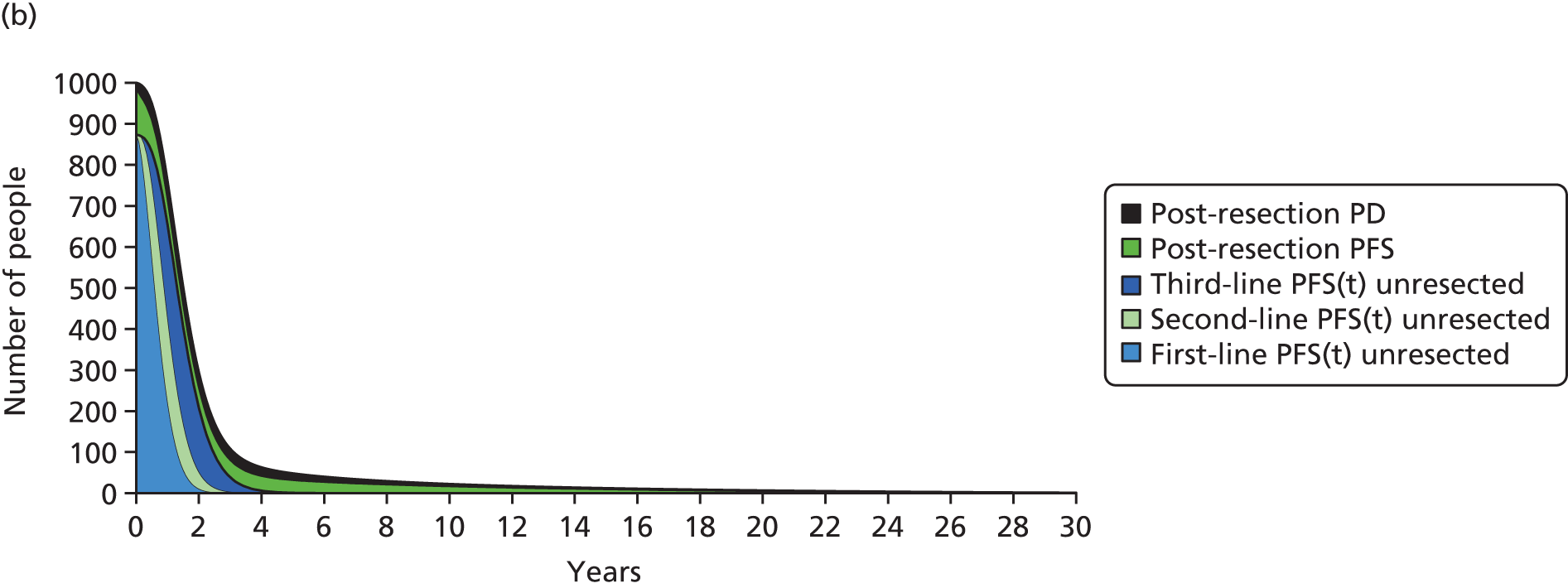

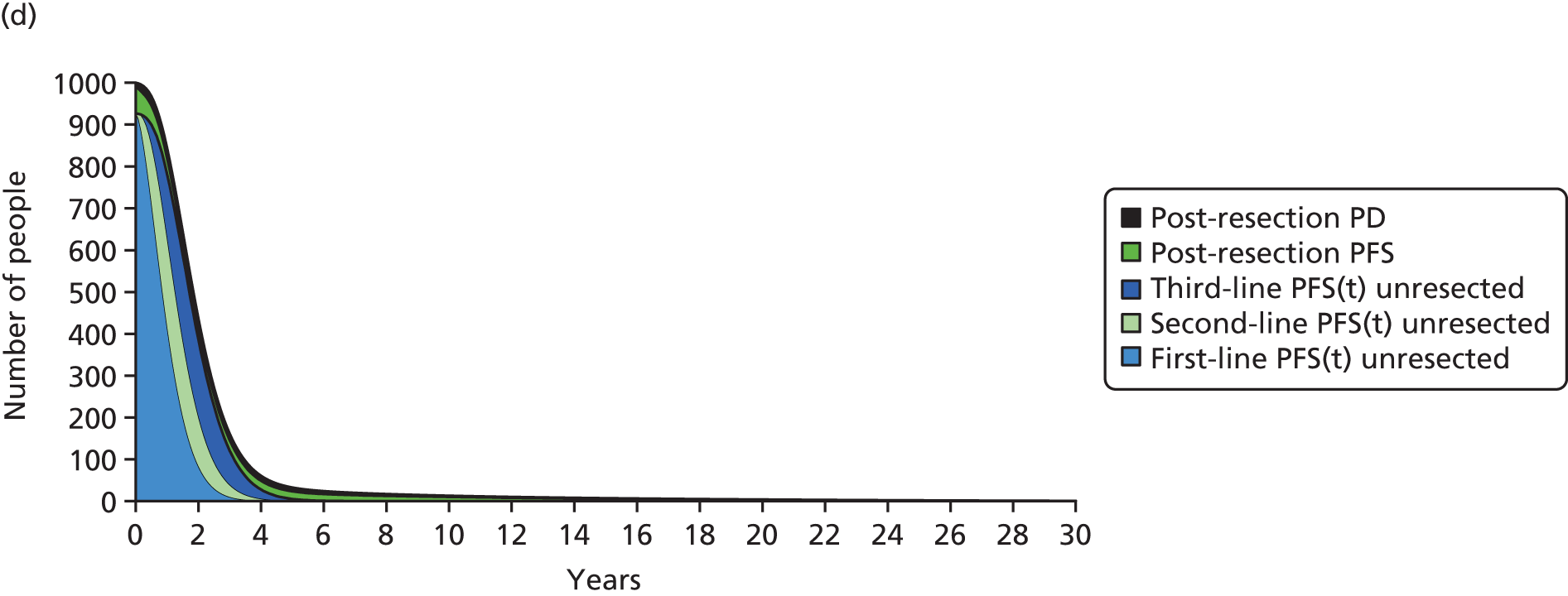

The graphs show two distinct features. The times on first-, second- and third-line treatment for unresected patients are short and last in total up to about 4 years. The times in PFS and progressive disease post resection are much longer. This reflects the substantial improvement in survival that we predict for patients post resection.
It can be clearly seen that we predict higher rates of resection in the FOLFOX network than in the FOLFIRI network. However, it is important to note that comparisons between the two networks should be made with caution, as they represent different cohorts of patients, as the data are not randomised between networks.
Figure 26 demonstrates that we expect slightly longer times in first-line PFS for unresected patients for cetuximab plus FOLFOX and panitumumab plus FOLFOX compared with FOLFOX, and for cetuximab plus FOLFIRI compared with FOLFIRI.
We predict similar mean times across the treatment arms in second-line PFS and third-line treatment for unresected patients. Any differences are the result of the slightly different expected proportions of patients who reach these lines of treatment (see cohort split data in Tables 119 and 121).
The relative magnitudes of the QALYs are similar to the relative magnitudes of the life-years, as the QALYs are simply the life-years discounted and then multiplied by the utilities appropriate for each health state.
Reductions in QALYs as a result of AEs are very small in all cases. Incremental QALYs with respect to times in second- and third-line treatment for unresected patients are small in all cases, because patients are expected to spend similar times in second- and third-line treatment for all comparator arms.
We predict that, for the comparison between cetuximab plus FOLFOX and FOLFOX, most incremental QALYs come from PFS post resection (Figure 27a). This is largely because of the high expected resection rate for cetuximab plus FOLFOX compared with FOLFOX. Total incremental QALYs for panitumumab plus FOLFOX compared with FOLFOX are far lower than for cetuximab plus FOLFOX compared with FOLFOX. This is mostly because we predict a lower resection rate for panitumumab plus FOLFOX than for cetuximab plus FOLFOX.
FIGURE 27.
Incremental QALYs: PenTAG’s base case, all patients: (a) FOLFOX network; and (b) FOLFIRI network. CET, cetuximab; PAN, panitumumab; PD, progressive disease.
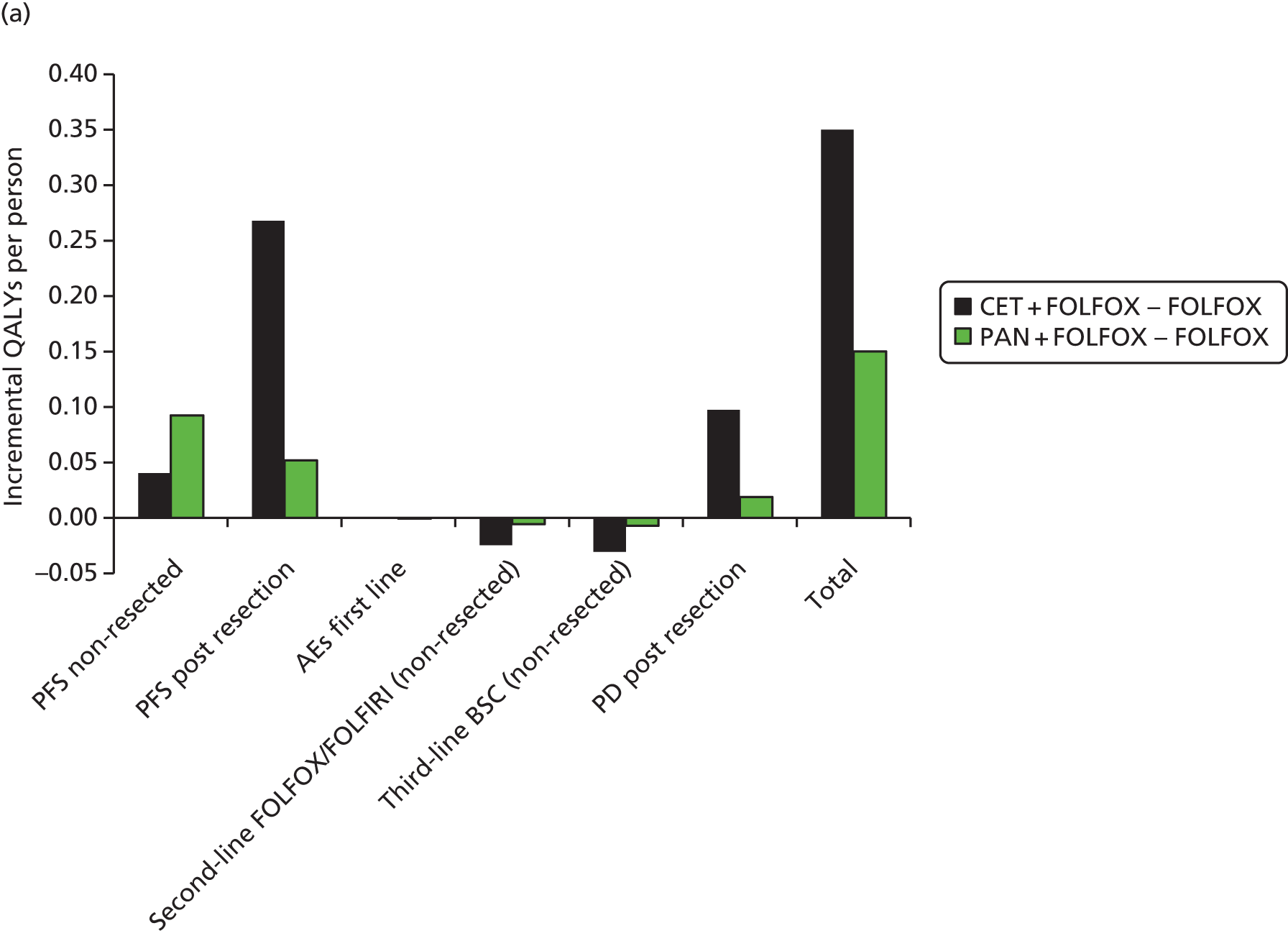
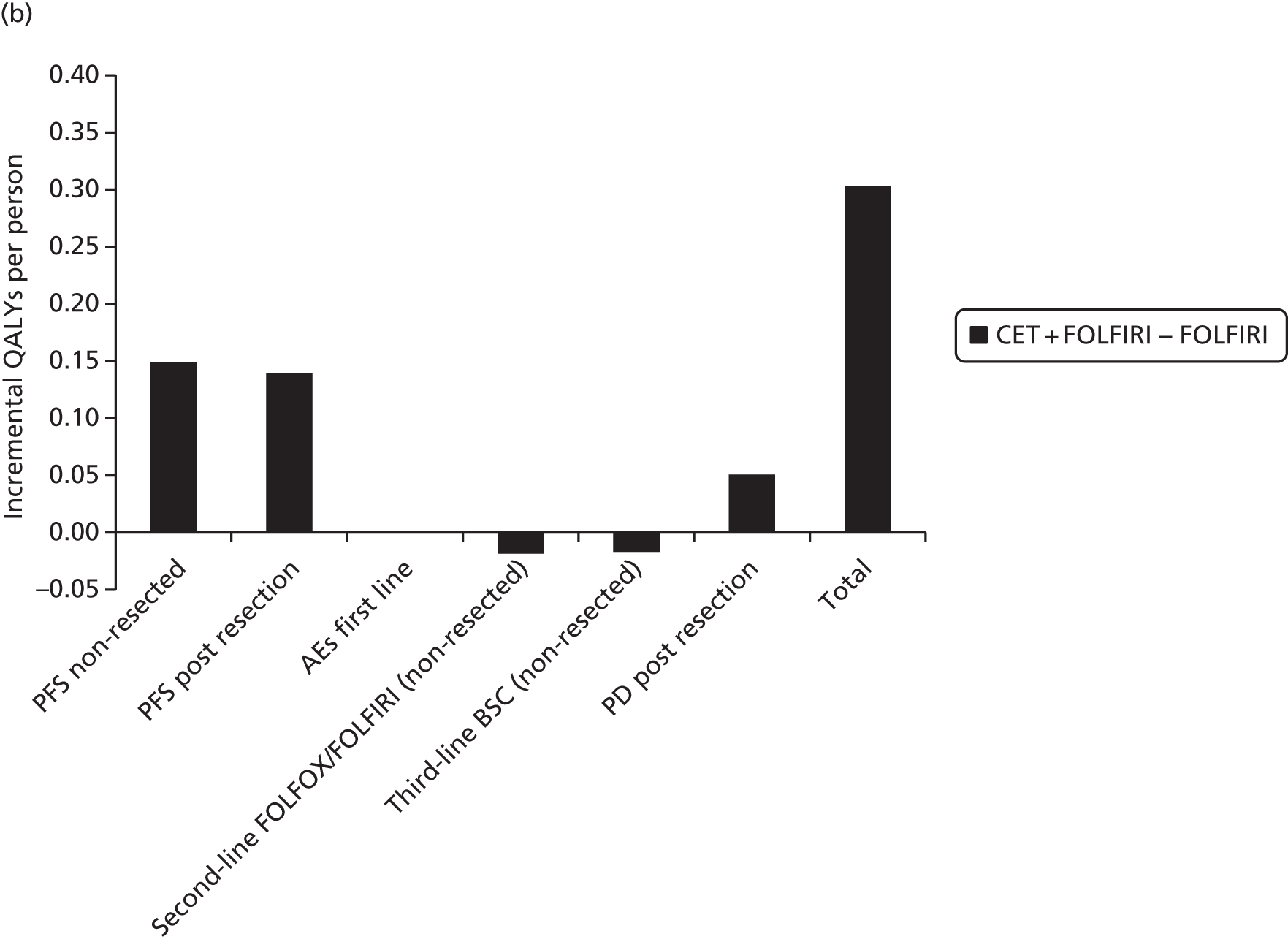
For the comparison between cetuximab plus FOLFIRI and FOLFIRI, most incremental QALYs come from the PFS unresected and PFS post-resection health states (see Figure 27b). Post-resection QALYs are less important than for cetuximab plus FOLFOX compared with FOLFOX, as we predict low rates of resection for cetuximab plus FOLFIRI (7.3%) and FOLFIRI (2.1%).
Cost results
The expected absolute first-line drug acquisition costs and first-line drug administration costs are by far the largest cost items in the FOLFOX network (see Table 119). In the FOLFIRI network, the largest cost items are the first-line drug acquisition costs and first-line drug administration costs, but also the second-line drug administration costs (see Table 121). The second-line drug administration costs are large because we predict that a larger proportion of patients in the FOLFIRI network are unresected and because we predict that patients spend longer on second-line FOLFOX than second-line FOLFIRI.
Turning to incremental costs, we predict that first-line drug acquisition costs dominate (Figure 28). The incremental costs of drug acquisition for cetuximab plus FOLFOX and panitumumab plus FOLFOX are similar because cetuximab and panitumumab cost a similar amount per month and because we predict that these two treatments are taken for a similar duration (8.7 and 8.2 months, respectively). First-line drug administration costs also make an important contribution to total incremental costs.
FIGURE 28.
Incremental costs: PenTAG’s base case, all patients: (a) FOLFOX network; and (b) FOLFIRI network. CET, cetuximab; PAN, panitumumab; PD, progressive disease.
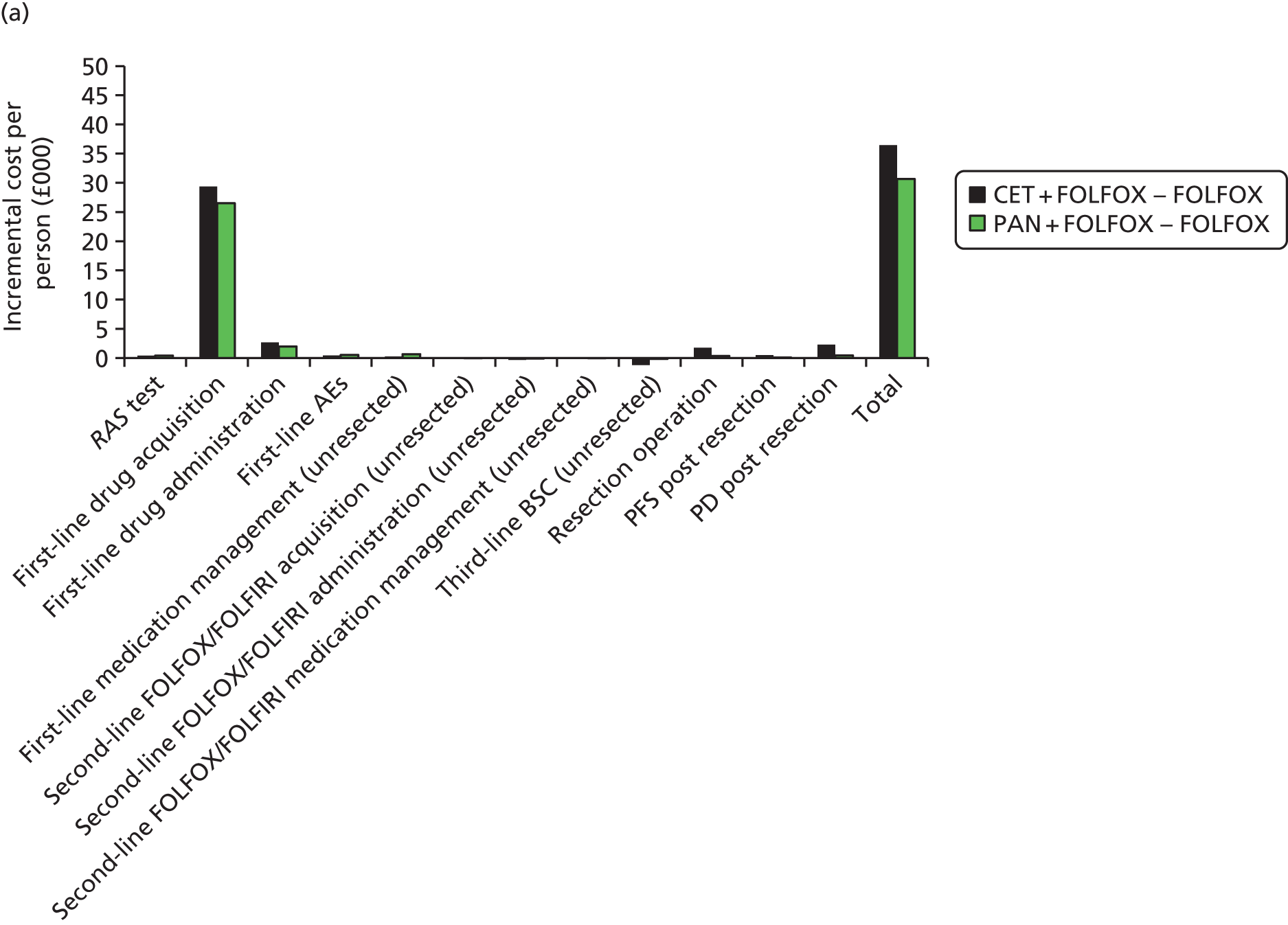
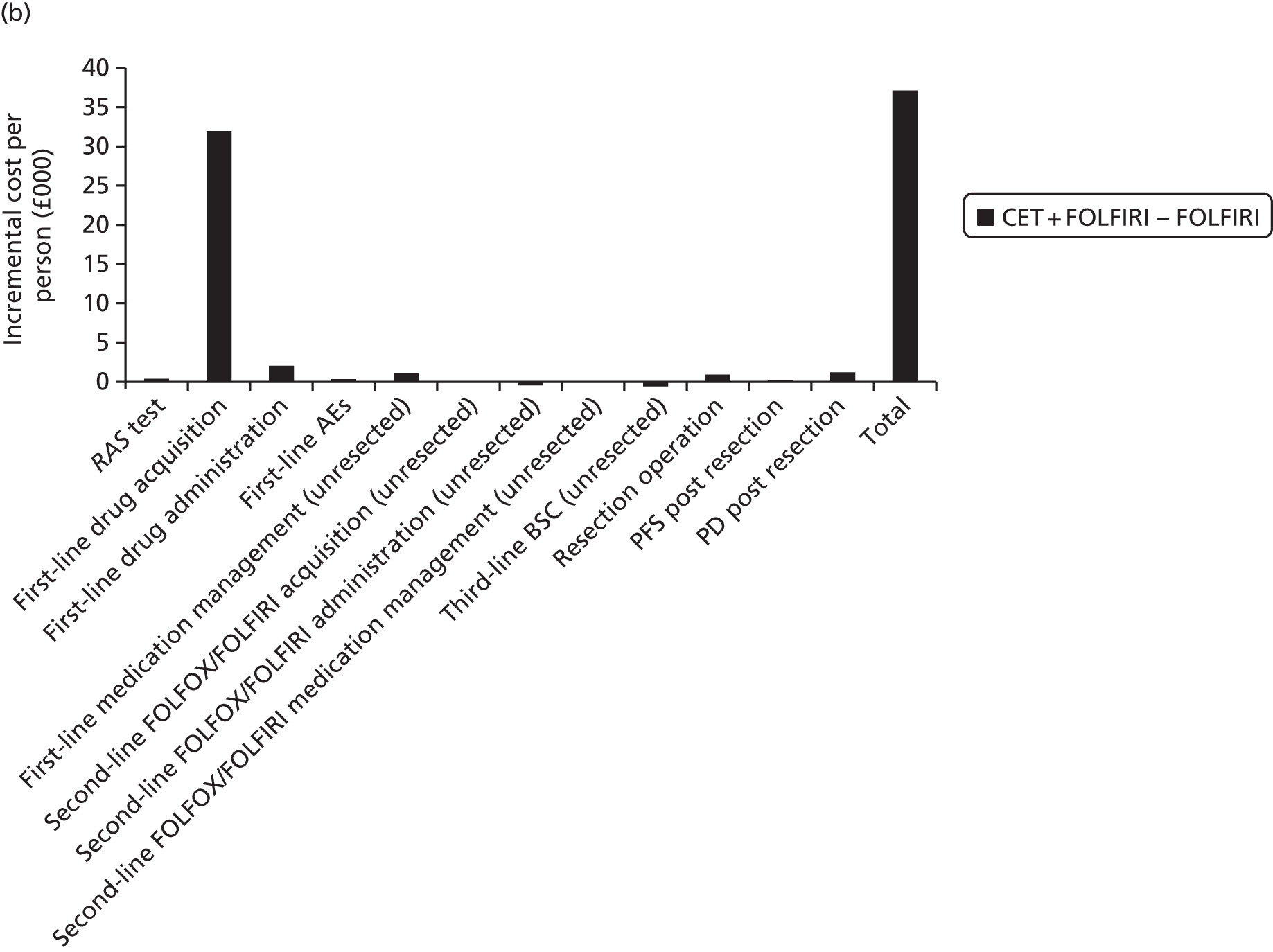
The incremental costs of RAS testing and treating AEs are very small. As for incremental QALYs, incremental costs in respect of second- and third-line states are also very small, as we predict that patients spend very similar times in these states between treatment arms.
Cost-effectiveness results and associated uncertainty
Combining all of the information on expected costs and QALYs per person (Figure 29), we estimate the following ICERs:
-
cetuximab plus FOLFOX compared with FOLFOX – £104,000 per QALY
-
panitumumab plus FOLFOX compared with FOLFOX – £204,000 per QALY (extendedly dominated by cetuximab plus FOLFOX and FOLFOX)
-
cetuximab plus FOLFIRI compared with FOLFIRI – £123,000 per QALY.
FIGURE 29.
Results on the cost-effectiveness plane: PenTAG’s base case, all patients: (a) FOLFOX network; and (b) FOLFIRI network. Note: straight lines represent the £20,000 and £30,000 per QALY willingness-to-pay thresholds.
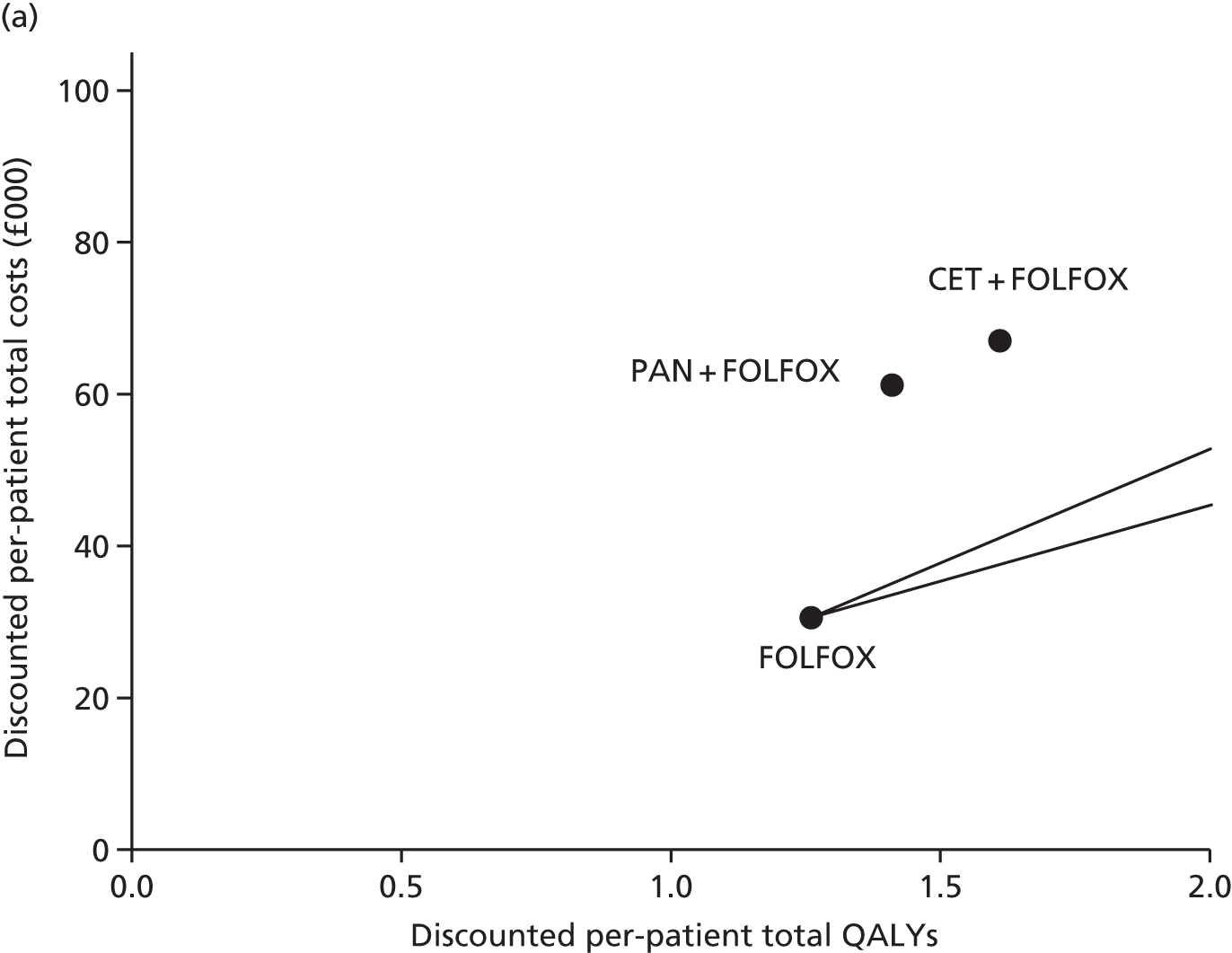
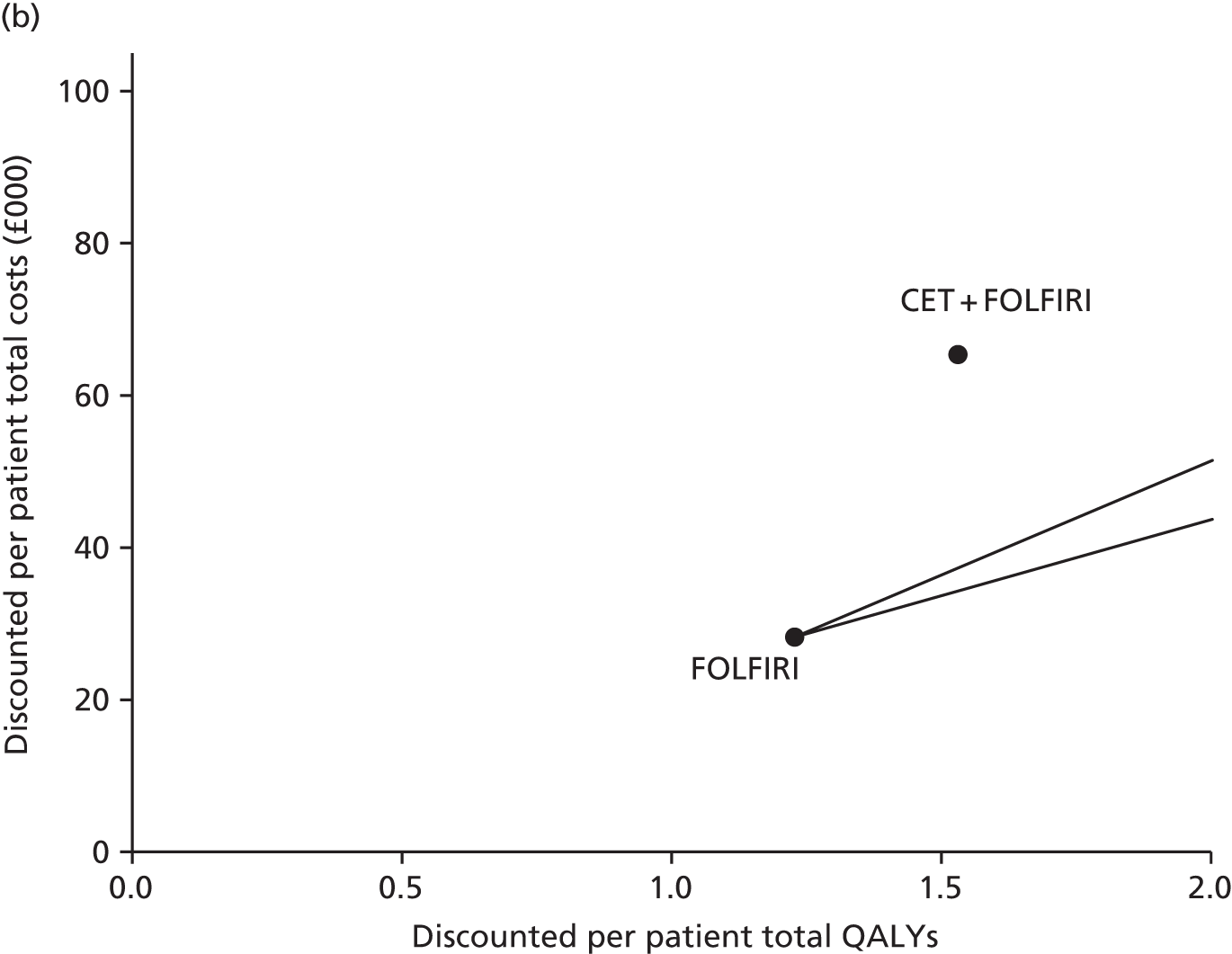
All ICERs throughout are rounded to the nearest thousand pounds, given the limited confidence in the accuracy of any further significant figures.
Overall, we believe that these estimates are subject to substantial uncertainty, only some of which is captured in the probabilistic sensitivity analysis (see Probabilistic sensitivity analysis).
In favour of our approach, the PFS data for first-line treatment is of high quality, as it comes directly from RCTs. However, we note that the evidence for cetuximab plus FOLFOX is not as strong as that for panitumumab plus FOLFOX, as the OPUS trial of cetuximab plus FOLFOX compared with FOLFOX had far fewer RAS WT patients (n = 87)65 than the PRIME trial of panitumumab plus FOLFOX compared with FOLFOX (n = 512). 44
Furthermore, we adjusted the PFS from the RCTs of first-line drugs by subtracting those patients who were resected (see Model parameters, First-line progression-free survival: unresected patients). Without access to the underlying individual patient data from the RCTs, we acknowledge that our method is only approximate.
We estimated survival post resection from a study that is now several years old. 113 Also, none of the patients in this study took either cetuximab or panitumumab. It is therefore possible that survival post resection for patients initially treated with these drugs could differ from that reported in the study by Adam et al. 113
We assumed that any treatment effect from first-line drugs stops on progression. This is because we did not model OS from the RCTs, but instead only PFS. We explored the use of OS data from the RCTs in a scenario analysis (see Scenario analyses, Overall survival from randomised controlled trials).
Given the lack of data to suggest otherwise, we assumed the same accuracy of the RAS test in clinical practice as in the first-line RCTs (see Model parameters, Test accuracy). Any differences are likely to result in worse estimates of cost-effectiveness for cetuximab and panitumumab.
For FOLFOX, our clinical effectiveness results were based on the PRIME trial. 44 This was investigated in scenario analysis (see Scenario analyses, OPUS trial as the baseline randomised controlled trial in the FOLFOX network). Also, we assumed that cetuximab was given fortnightly, whereas in the RCTs of cetuximab it was given weekly. 43,65 We therefore assumed that the frequency of administration does not affect the effectiveness of cetuximab. We modelled the weekly administration of cetuximab in a scenario analysis (see Scenario analyses, Weekly administration of cetuximab).
We have confidence in our estimated rates of resection for the FOLFIRI network (cetuximab plus FOLFIRI 7.3%, FOLFIRI 2.1%). Also, our estimated rates of resection for panitumumab plus FOLFOX and FOLFOX in the FOLFOX network are reliable as they are taken directly from the PRIME trial. 44 However, our estimate for cetuximab plus FOLFOX (20.7%) is subject to a good deal of uncertainty as it was obtained by an indirect comparison (see Model parameters, Resection rates).
Liver metastases subgroup: base-case results
Our base-case results for the FOLFOX and FOLFIRI networks for the liver metastases subgroup are provided in Tables 122–125.
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.98 | 2.86 | 2.21 | 0.76 | 0.65 |
| QALYs (mean, discounted) | 1.97 | 1.89 | 1.49 | 0.49 | 0.40 |
| Total costs (mean, discounted) (£) | 80,931 | 69,515 | 34,598 | 46,333 | 34,917 |
| ICER (£ per QALY) vs. FOLFOX (£) | 95,514 | 86,875 | |||
| ICER (£ per QALY) on efficiency frontier (£) | 137,270 (vs. PAN + FOLFOX) | 86,875 (vs. FOLFOX) | Reference |
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. | PAN + FOLFOX vs. FOLFOX | |
|---|---|---|---|---|---|---|
| PAN + FOLFOX | FOLFOX | |||||
| Life-years (mean, undiscounted) | ||||||
| First-line drug (resected + unresected) | 0.90 | 0.73 | 0.67 | 0.17 | 0.23 | 0.06 |
| PFS unresected | 0.63 | 0.50 | 0.56 | 0.13 | 0.08 | –0.05 |
| PFS post resection | 1.26 | 1.28 | 0.70 | –0.01 | 0.57 | 0.58 |
| PFS first line | 1.90 | 1.78 | 1.26 | 0.12 | 0.64 | 0.53 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.22 | 0.22 | 0.27 | 0 | –0.04 | –0.05 |
| Third-line BSC (unresected) | 0.33 | 0.33 | 0.40 | 0 | –0.07 | –0.07 |
| PD post resection | 0.52 | 0.53 | 0.29 | –0.01 | 0.23 | 0.24 |
| OS (mean) | 2.98 | 2.86 | 2.21 | 0.11 | 0.76 | 0.65 |
| Cohort split | ||||||
| % starting second-line FOLFOX/FOLFIRI (unresected) | 93.5 | 93.5 | 93.5 | 0.0 | 0.0 | 0.0 |
| % starting third-line BSC (unresected) | 87.5 | 87.5 | 87.5 | 0.0 | 0.0 | 0.0 |
| Life-years (mean) (undiscounted for patients who spend at least some time in given health state) | ||||||
| PFS unresected | 0.92 | 0.73 | 0.67 | 0.19 | 0.25 | 0.06 |
| PFS post resection | 4.09 | 4.09 | 4.09 | 0 | 0 | 0 |
| PFS first line | 5.01 | 4.82 | 4.76 | 0.19 | 0.25 | 0.06 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.34 | 0.34 | 0.34 | 0 | 0 | 0 |
| Third-line BSC (unresected) | 0.55 | 0.55 | 0.55 | 0 | 0 | 0 |
| PD post resection | 1.69 | 1.69 | 1.69 | 0 | 0 | 0 |
| OS unresected | 1.72 | 1.54 | 1.48 | 0.19 | 0.25 | 0.06 |
| QALYs (discounted) | ||||||
| PFS unresected | 0.48 | 0.38 | 0.42 | 0.10 | 0.06 | –0.04 |
| PFS post resection | 0.83 | 0.84 | 0.46 | –0.01 | 0.37 | 0.38 |
| AEs first line | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PFS first line | 1.31 | 1.22 | 0.88 | 0.09 | 0.43 | 0.34 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.16 | 0.16 | 0.20 | 0.00 | –0.03 | –0.03 |
| Third-line BSC (unresected) | 0.20 | 0.20 | 0.24 | 0.00 | –0.04 | –0.04 |
| PD post resection | 0.30 | 0.31 | 0.17 | 0.00 | 0.14 | 0.14 |
| Total | 1.97 | 1.89 | 1.49 | 0.08 | 0.49 | 0.40 |
| Costs (discounted) (£) | ||||||
| RAS test | 400 | 400 | 0 | 0 | 400 | 400 |
| First-line drug acquisition | 36,927 | 28,891 | 533 | 8036 | 36,393 | 28,357 |
| First-line drug administration | 16,344 | 13,399 | 12,175 | 2945 | 4169 | 1224 |
| First-line AEs | 1512 | 1582 | 1068 | –70 | 444 | 514 |
| First-line medical management (unresected) | 3339 | 2663 | 2952 | 676 | 386 | –290 |
| Second-line FOLFOX or FOLFIRI acquisition (unresected) | 328 | 329 | 397 | 0 | –69 | –69 |
| Second-line FOLFOX or FOLFIRI administration (unresected) | 2126 | 2128 | 2572 | –2 | –447 | –444 |
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1147 | 1148 | 1387 | –1 | –241 | –240 |
| Third-line BSC (unresected) | 4743 | 4748 | 5739 | –6 | –996 | –991 |
| Resection operation | 5432 | 5495 | 3002 | –62 | 2430 | 2493 |
| PFS post resection | 1515 | 1533 | 837 | –17 | 678 | 695 |
| PD post resection | 7119 | 7200 | 3934 | –84 | 3273 | 3357 |
| Total | 80,931 | 69,515 | 34,598 | 11,416 | 46,333 | 34,917 |
| ICER (£ per QALY) | 137,270 | 95,514 | 86,875 | |||
| Outcome | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|
| Life-years (mean, undiscounted) | 2.69 | 1.83 | 0.86 |
| QALYs (mean, discounted) | 1.83 | 1.26 | 0.57 |
| Total costs (mean, discounted) (£) | 73,153 | 29,809 | 43,345 |
| ICER (£ per QALY) (£) | 76,298 |
| Outcome | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|
| Life-years (mean, undiscounted) | |||
| First-line drug (resected + unresected) | 0.86 | 0.65 | 0.21 |
| PFS unresected | 0.99 | 0.61 | 0.38 |
| PFS post resection | 0.67 | 0.27 | 0.40 |
| PFS first line | 1.66 | 0.88 | 0.78 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.35 | 0.39 | –0.04 |
| Third-line BSC (unresected) | 0.40 | 0.45 | –0.05 |
| PD post resection | 0.28 | 0.11 | 0.17 |
| OS (mean) | 2.69 | 1.83 | 0.86 |
| Cohort split | |||
| % unresected | 83.7 | 93.5 | –9.8 |
| % starting second-line FOLFOX/FOLFIRI (unresected) | 93.5 | 93.5 | 0.0 |
| % starting third-line BSC (unresected) | 87.5 | 87.5 | 0.0 |
| % resected | 16.3 | 6.5 | 9.8 |
| Life-years (mean) (undiscounted for patients who spend at least some time in given health state) | |||
| PFS unresected | 1.18 | 0.65 | 0.53 |
| PFS post resection | 4.09 | 4.09 | 0.00 |
| PFS first line | 5.27 | 4.74 | 0.53 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.45 | 0.45 | 0.00 |
| Third-line BSC (unresected) | 0.55 | 0.55 | 0.00 |
| PD post resection | 1.69 | 1.69 | 0.00 |
| OS unresected | 2.08 | 1.56 | 0.53 |
| QALYs (discounted) | |||
| PFS unresected | 0.74 | 0.46 | 0.28 |
| PFS post resection | 0.44 | 0.17 | 0.26 |
| AEs first line | 0.00 | 0.00 | 0.00 |
| PFS first line | 1.18 | 0.64 | 0.54 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.25 | 0.29 | –0.03 |
| Third-line BSC (unresected) | 0.24 | 0.27 | –0.03 |
| PD post resection | 0.16 | 0.06 | 0.10 |
| Total | 1.83 | 1.26 | 0.57 |
| Costs (discounted) (£) | |||
| RAS test | 400 | 0 | 400 |
| First-line drug acquisition | 36,874 | 896 | 35,979 |
| First-line drug administration | 8495 | 6351 | 2143 |
| First-line AEs | 821 | 482 | 339 |
| First-line medical management (unresected) | 5169 | 3228 | 1941 |
| Second-line FOLFOX or FOLFIRI acquisition (unresected) | 343 | 390 | –47 |
| Second-line FOLFOX or FOLFIRI administration (unresected) | 6169 | 7016 | –847 |
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1789 | 2034 | –246 |
| Third-line BSC (unresected) | 5674 | 6453 | –779 |
| Resection operation | 2866 | 1143 | 1723 |
| PFS post resection | 799 | 319 | 481 |
| PD post resection | 3756 | 1498 | 2258 |
| Total | 73,153 | 29,809 | 43,345 |
| ICER (£ per QALY) | 76,298 | ||
Survival results
Much of the discussion relating to all patients is also relevant to the liver metastases subgroup. In this section we discuss features that are unique to the liver metastases subgroup.
We predict slightly longer life expectancy for the liver metastases subgroup (1.8–3.0 years) than for all patients (1.8–2.4 years). This is because we also predict greater resection rates for the liver metastases subgroup than for all patients and life expectancy is substantially greater for patients after resection than for patients without resection.
We predict that, for both cetuximab plus FOLFOX compared with FOLFOX and panitumumab plus FOLFOX compared with FOLFOX, most incremental QALYs come from PFS and progressive disease post resection (Figure 30a). This is largely because of the high expected resection rates for cetuximab plus FOLFOX and panitumumab plus FOLFOX (31.3%) compared with FOLFOX (17.1%).
FIGURE 30.
Incremental QALYs: PenTAG’s base case, liver metastases subgroup: (a) FOLFOX network; and (b) FOLFIRI network. CET, cetuximab; PAN, panitumumab; PD, progressive disease.
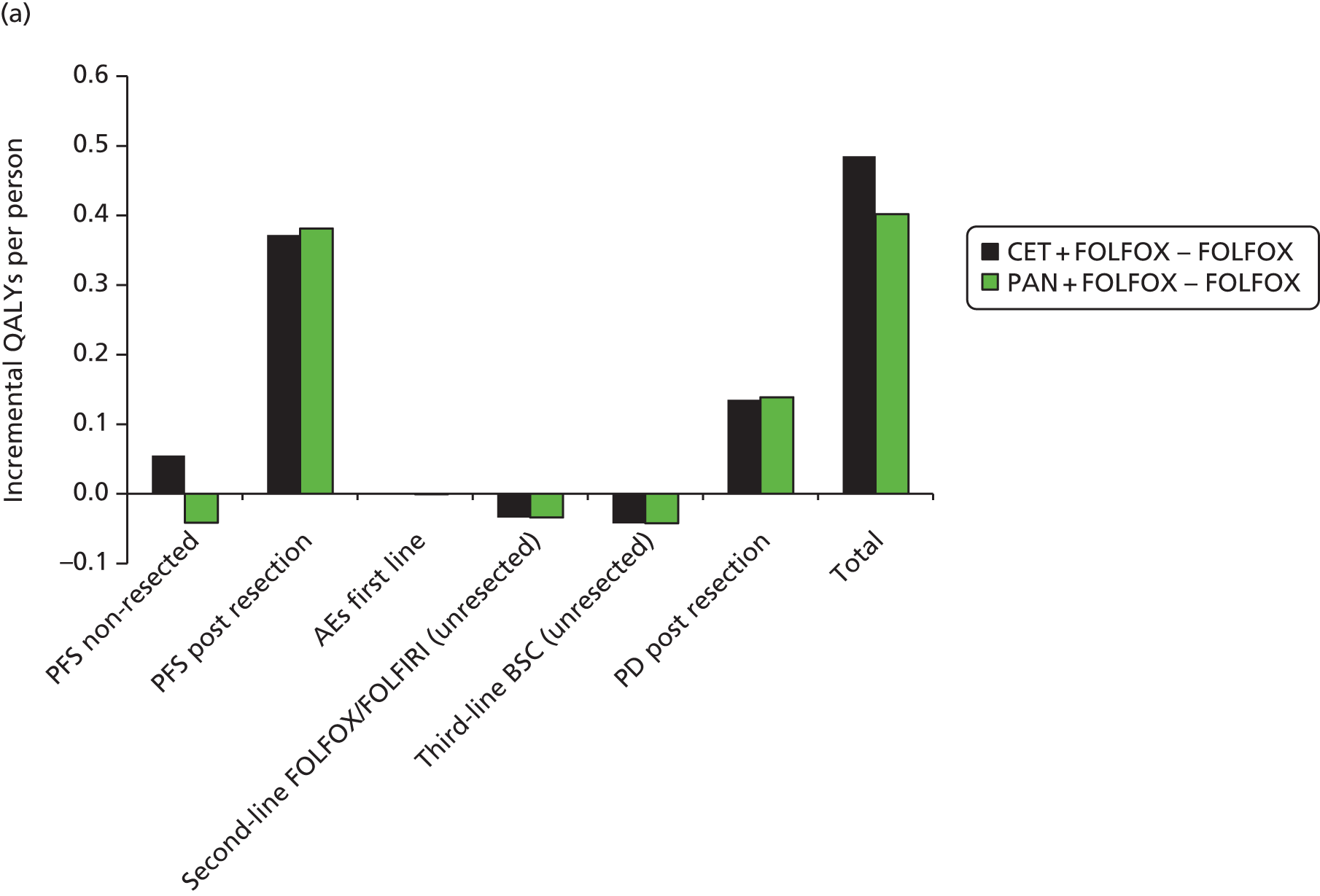

For the comparison between cetuximab plus FOLFIRI and FOLFIRI, most incremental QALYs come from PFS unresected and PFS post resection (see Figure 30b). Post-resection QALYs are less important than for cetuximab plus FOLFOX compared with FOLFOX, as we predict low rates of resection for cetuximab plus FOLFIRI (16.3%) and FOLFIRI (6.5%).
Cost results
The expected incremental first-line drug acquisition costs and, to a lesser extent, the first-line drug administration costs are the largest items in both networks (Figure 31).
FIGURE 31.
Incremental costs: PenTAG’s base case, liver metastases subgroup: (a) FOLFOX network; and (b) FOLFIRI network. CET, cetuximab; PAN, panitumumab; PD, progressive disease.
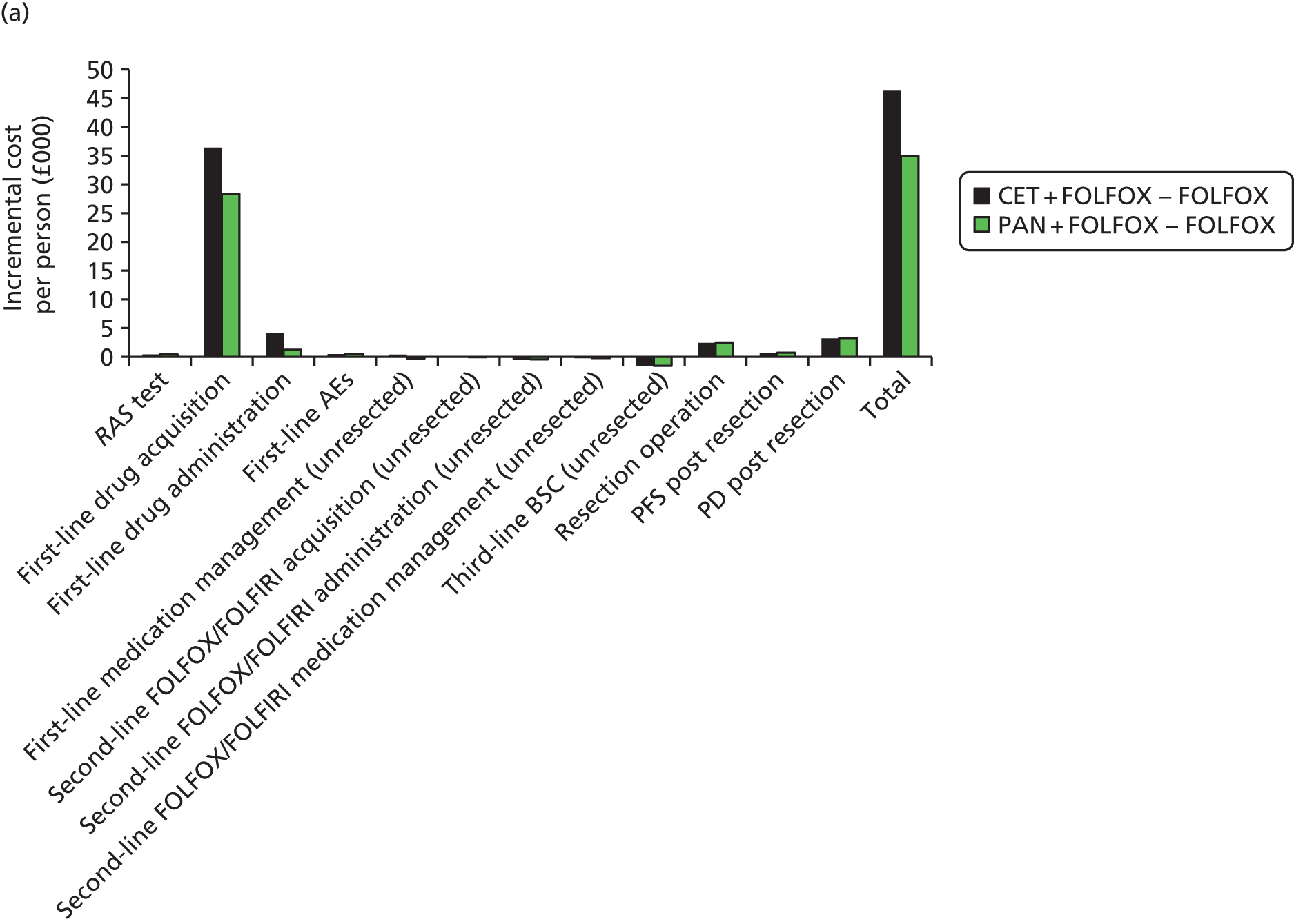

The incremental cost of drug acquisition for cetuximab plus FOLFOX compared with FOLFOX is greater than that for panitumumab plus FOLFOX compared with FOLFOX, even though the monthly acquisition costs of cetuximab plus FOLFOX and panitumumab plus FOLFOX are similar. This is because we predict that patients take cetuximab plus FOLFOX for longer than panitumumab plus FOLFOX (11.0 vs. 8.8 months).
Cost-effectiveness results and associated uncertainty
Combining all of the information on expected costs and QALYs per person (Figure 32), we estimate the following ICERs for the liver metastases subgroup:
-
cetuximab plus FOLFOX compared with FOLFOX – £96,000 per QALY
-
panitumumab plus FOLFOX compared with FOLFOX – £87,000 per QALY
-
cetuximab plus FOLFIRI compared with FOLFIRI – £76,000 per QALY.
FIGURE 32.
Results on the cost-effectiveness plane: PenTAG’s base case, liver metastases subgroup: (a) FOLFOX network; and (b) FOLFIRI network. CET, cetuximab; PAN, panitumumab. Note: straight lines represent the £20,000 and £30,000 per QALY willingness-to-pay thresholds.
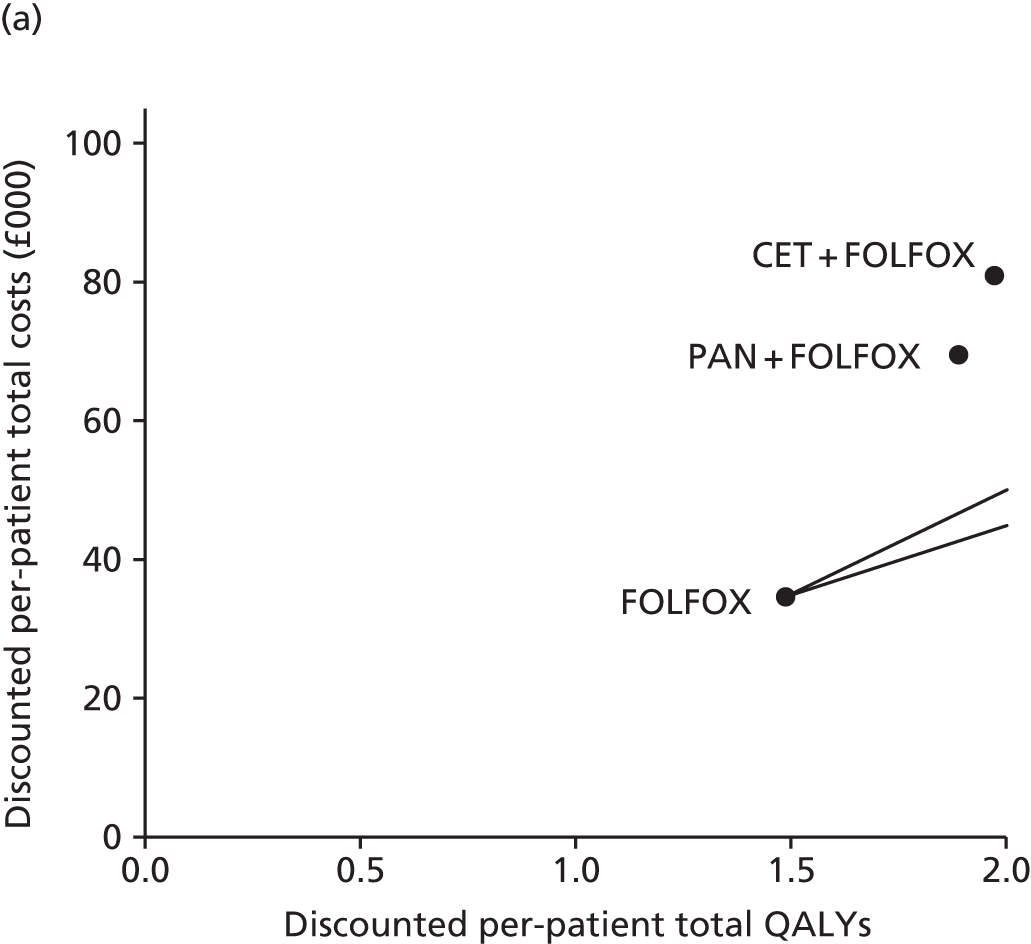

We believe that these estimates are highly uncertain, indeed, more uncertain than for all patients combined, for the reasons give below. Only some of the uncertainty is captured in the probabilistic sensitivity analysis (see Probabilistic sensitivity analysis).
-
All the uncertainties described for all patients in the previous section still apply.
-
PFS for unresected patients is more uncertain than that for all patients for the following two reasons:
-
PFS for resected plus unresected patients, which was used to estimate PFS for unresected patients, is more uncertain for the liver metastases subgroup than for all patients because it was estimated from the corresponding PFS for all patients, adjusted by the ratio of the median PFS for the liver metastases subgroup/median PFS for all patients (see Model parameters, First-line progression-free survival: unresected patients). Furthermore, given that the median PFS for cetuximab plus FOLFOX was not reported in the OPUS trial, we based our estimate for this treatment on the ratio corresponding to cetuximab plus FOLFIRI (see Model parameters, First-line progression-free survival: unresected patients), thus adding further uncertainty.
-
We were forced to estimate PFS for unresected patients from PFS for resected plus unresected patients for the liver metastases subgroup using a different, and arguably less rigorous, method from the method used for all patients (see Model parameters, First-line progression-free survival: unresected patients).
-
Probabilistic sensitivity analyses
All patients
The scatterplots shown in Figures 33–35 depict the results for all patients of the 1000 simulations of the probabilistic sensitivity analysis in terms of the incremental cost–utility of cetuximab plus FOLFOX compared with FOLFOX, panitumumab plus FOLFOX compared with FOLFOX and cetuximab plus FOLFIRI compared with FOLFIRI, respectively. The plots show that there is substantial uncertainty in the cost-effectiveness of cetuximab plus FOLFOX compared with FOLFOX, but less uncertainty for the other two comparisons. This is not surprising as there were relatively few patients in the OPUS RCT of cetuximab plus FOLFOX compared with FOLFOX. 65
FIGURE 33.
Peninsula Technology Assessment Group’s probabilistic sensitivity analysis results: incremental cost–utility per person of CET + FOLFOX vs. FOLFOX, all patients. CET, cetuximab. Dashed line, willingness-to-pay threshold of £20,000 per QALY gained; solid line, willingness-to-pay threshold of £30,000 per QALY.
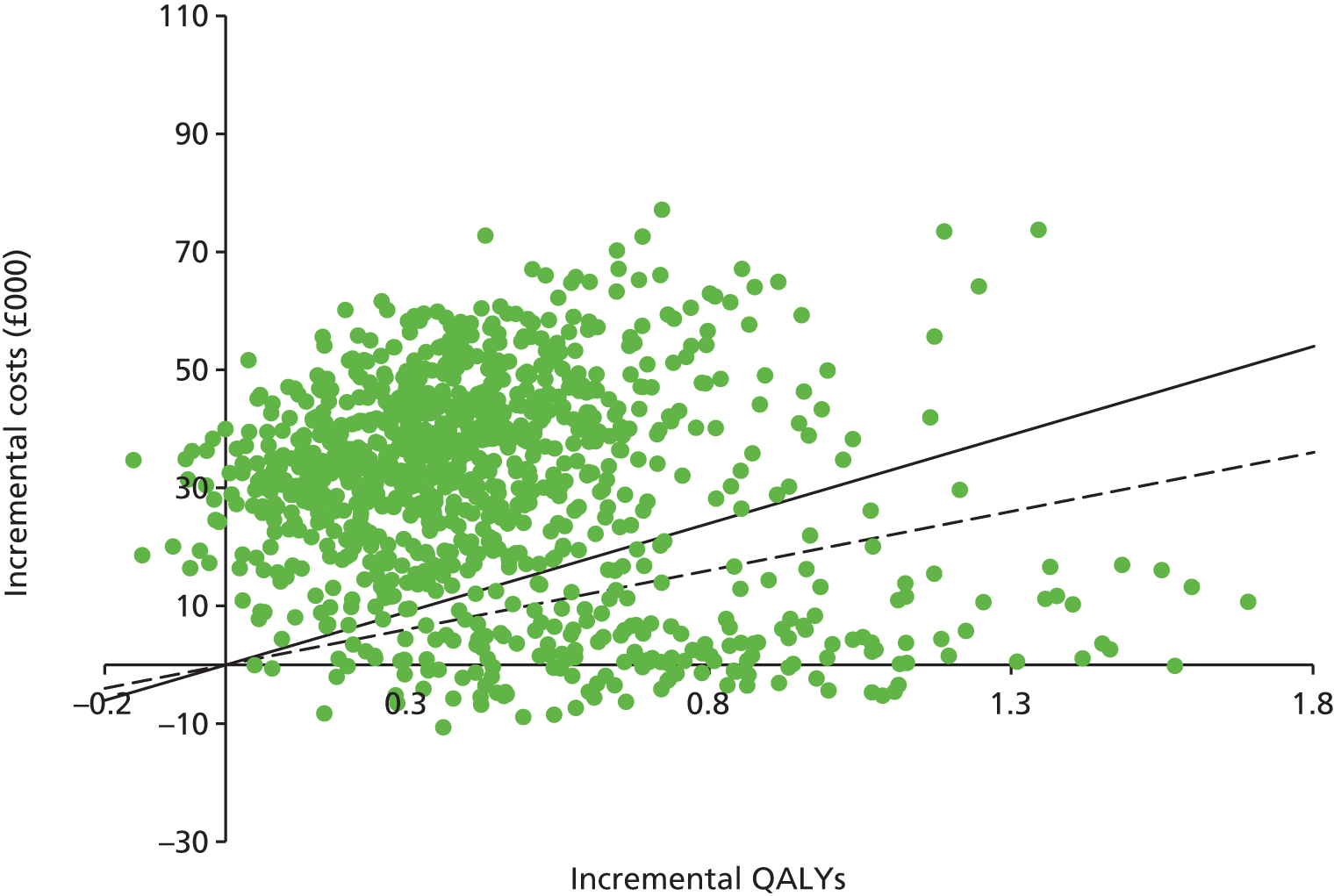
FIGURE 34.
Peninsula Technology Assessment Group’s probabilistic sensitivity analysis results: incremental cost–utility per person of PAN + FOLFOX vs. FOLFOX, all patients. PAN, panitumumab. Dashed line, willingness-to-pay threshold of £20,000 per QALY gained; solid line, willingness-to-pay threshold of £30,000 per QALY.
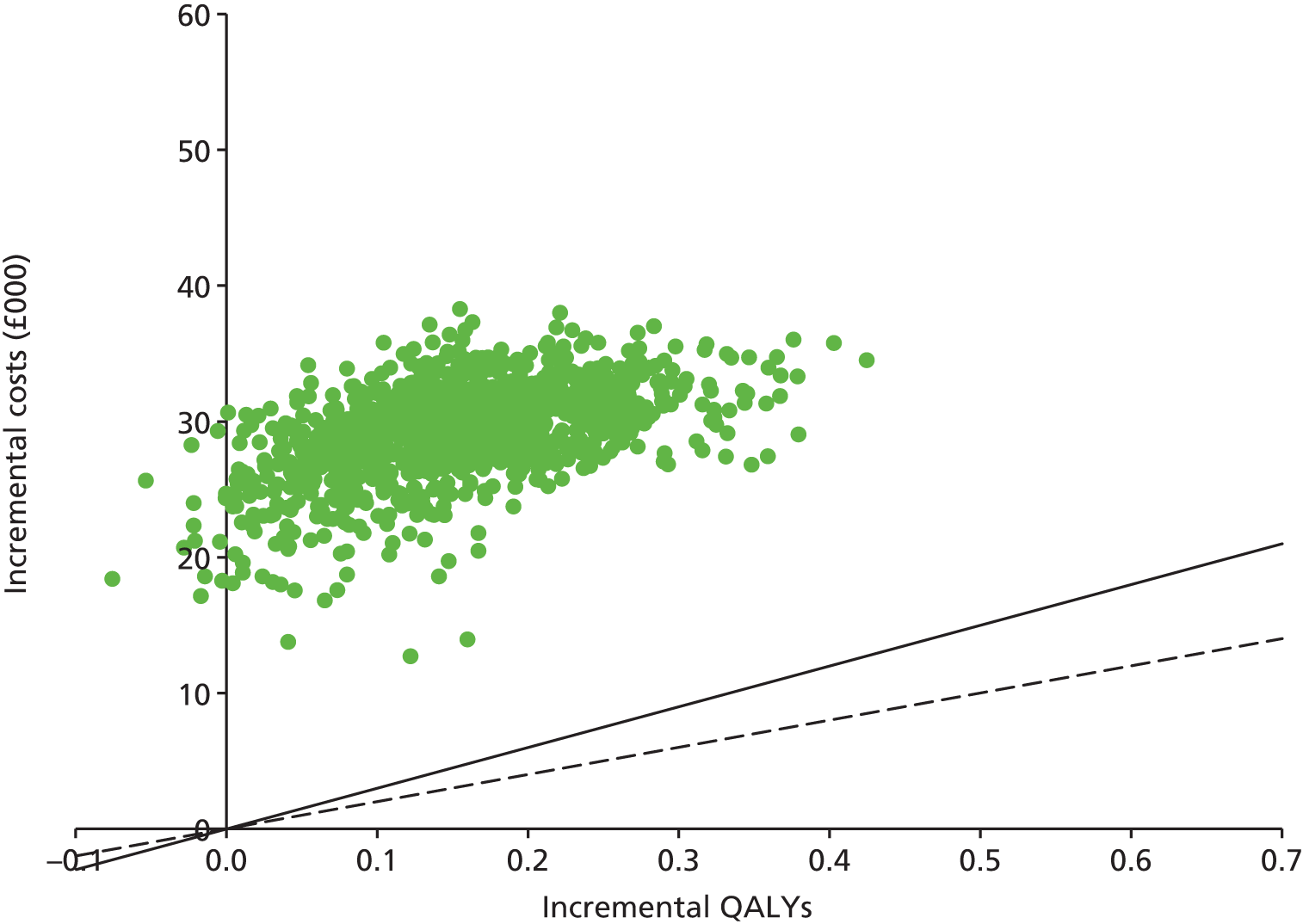
FIGURE 35.
Peninsula Technology Assessment Group’s probabilistic sensitivity analysis results: incremental cost–utility per person of CET + FOLFIRI vs. FOLFIRI, all patients. CET, cetuximab. Dashed line, willingness-to-pay threshold of £20,000 per QALY gained; solid line, willingness-to-pay threshold of £30,000 per QALY.
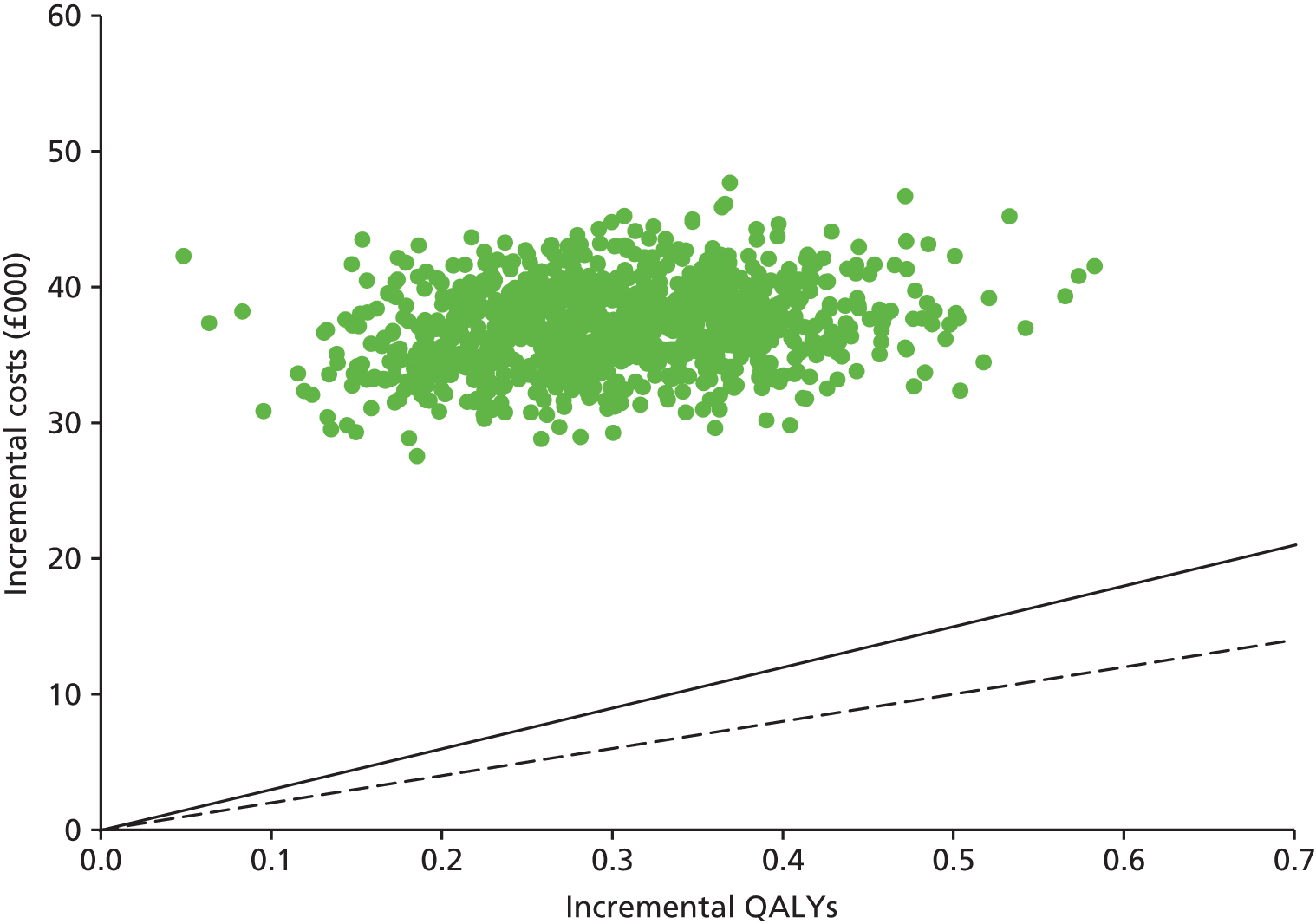
Figures 36 and 37 provide the cost-effectiveness acceptability curves for the treatments in the FOLFOX and FOLFIRI networks, respectively, showing the probability that each treatment provides the best value for money given a range of willingness-to-pay thresholds.
FIGURE 36.
Peninsula Technology Assessment Group’s probabilistic sensitivity analysis results: cost-effectiveness acceptability curves – FOLFOX network, all patients. CET, cetuximab; PAN, panitumumab.

FIGURE 37.
Peninsula Technology Assessment Group’s probabilistic sensitivity analysis results: cost-effectiveness acceptability curves – FOLFIRI network, all patients. CET, cetuximab.

In the FOLFOX network, we predict that the probability is zero that panitumumab plus FOLFOX provides the best value for money at any willingness-to-pay threshold investigated (£0–150,000 per QALY). The probability that cetuximab plus FOLFOX provides the best value for money exceeds 50% only at a willingness to pay of about £90,000 per QALY, which is similar to the deterministic ICER for cetuximab plus FOLFOX compared with FOLFOX of £104,000 per QALY.
We predict that the probability that cetuximab plus FOLFIRI provides the best value for money exceeds 50% only at a willingness to pay of about £120,000 per QALY, which is consistent with the deterministic ICER for cetuximab plus FOLFIRI compared with FOLFIRI of £123,000 per QALY.
The probabilities that the following treatments are most cost-effective at a willingness-to-pay threshold of £30,000 per QALY are:
-
cetuximab plus FOLFOX – 20%
-
panitumumab plus FOLFOX – 0%
-
cetuximab plus FOLFIRI – 0%.
Liver metastases subgroup
In the FOLFOX network, we again predict that the probability is zero that panitumumab plus FOLFOX provides the best value for money at any willingness-to-pay threshold investigated (£0–150,000 per QALY). The probability that cetuximab plus FOLFOX provides the best value for money tends to be about 40% above willingness-to-pay thresholds of £100,000 per QALY, which is consistent with the deterministic ICER for cetuximab plus FOLFOX compared with FOLFOX of £96,000 per QALY.
We predict that the probability that cetuximab plus FOLFIRI provides the best value for money exceeds 50% only at a willingness to pay of about £80,000 per QALY, which is consistent with the deterministic ICER for cetuximab plus FOLFIRI compared with FOLFIRI of £76,000 per QALY.
The probabilities that the following treatments are most cost-effective at a willingness-to-pay threshold of £30,000 per QALY are:
-
cetuximab plus FOLFOX – 2%
-
panitumumab plus FOLFOX – 0%
-
cetuximab plus FOLFIRI – 0%.
Scenario analyses
In this section we provide the cost-effectiveness results for the different scenario analyses.
Bevacizumab plus FOLFOX and bevacizumab plus FOLFIRI as comparators
For all patients, in the FOLFOX network we predict that bevacizumab plus FOLFOX is dominated by FOLFOX (Table 126), partly because the resection rate for bevacizumab plus FOLFOX is similar to that for FOLFOX (see Model parameters, Resection rates) and because the estimated PFS is rather low (see Model parameters, First-line progression-free survival: unresected patients). Therefore, it does not affect the conclusions of the cost-effectiveness analysis of cetuximab plus FOLFOX and panitumumab plus FOLFOX in the base case, in which bevacizumab plus FOLFOX was not a comparator (see Base-case results, All patients: base-case results).
| Outcome | CET + FOLFOX | PAN + FOLFOX | BEV + FOLFOX | FOLFOX | CET + FOLFOX vs. BEV + FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. BEV + FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.41 | 2.08 | 1.72 | 1.86 | 0.69 | 0.55 | 0.36 | 0.22 |
| QALYs (mean, discounted) | 1.61 | 1.41 | 1.16 | 1.26 | 0.45 | 0.35 | 0.25 | 0.15 |
| Total costs (mean, discounted) (£) | 67,057 | 61,225 | 34,916 | 30,585 | 32,141 | 36,471 | 26,309 | 30,640 |
| ICER (£ per QALY) vs. BEV + FOLFOX or FOLFOX (£) | 71,239 | 104,205 | 104,697 | 204,103 | ||||
| ICER (£ per QALY) on the efficiency frontier (£) | 104,205 | Extendedly dominated by FOLFOX and CET + FOLFOX | Dominated by FOLFOX | Reference |
In the FOLFIRI network, under our base case, in which we did not include bevacizumab plus FOLFIRI, the ICER for cetuximab plus FOLFIRI compared with FOLFIRI for all patients was approximately £123,000 (see Base-case results, All patients: base-case results). When we now include bevacizumab plus FOLFIRI, the ICER for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI is £199,000 (Table 127), that is, cetuximab plus FOLFIRI becomes even worse value for money compared with the most cost-effective comparator.
| Outcome | CET + FOLFIRI | BEV + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. BEV + FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.21 | 2.11 | 1.75 | 0.10 | 0.46 |
| QALYs (mean, discounted) | 1.53 | 1.45 | 1.23 | 0.08 | 0.30 |
| Total costs (mean, discounted) (£) | 65,380 | 50,278 | 28,250 | 15,102 | 37,130 |
| ICER (£ per QALY) vs. FOLFOX (£) | 198,573 | 122,554 | |||
| ICER (£ per QALY) on the efficiency frontier (£) | 198,573 (vs. BEV + FOLFIRI) | 97,075 vs. FOLFIRI | Reference |
In the liver metastases subgroup, in the FOLFOX network, we predict an ICER for bevacizumab plus FOLFOX compared with FOLFOX of £21,000 and that bevacizumab plus FOLFOX dominates both cetuximab plus FOLFOX and panitumumab plus FOLFOX (Table 128). Although PFS for bevacizumab plus FOLFOX is the lowest of the four treatments, it is the most cost-effective treatment because it has the highest estimated resection rate (see Model parameters, Resection rates).
| Outcome | CET + FOLFOX | PAN + FOLFOX | BEV + FOLFOX | FOLFOX | CET + FOLFOX vs. BEV + FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. BEV+FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.98 | 2.86 | 3.30 | 2.21 | –0.32 | 0.76 | –0.43 | 0.65 |
| QALYs (mean, discounted) | 1.97 | 1.89 | 2.14 | 1.49 | –0.16 | 0.49 | –0.25 | 0.40 |
| Total costs (mean, discounted) (£) | 80,931 | 69,515 | 48,386 | 34,598 | 32,545 | 46,333 | 21,130 | 34,917 |
| ICER (£ per QALY) vs. BEV + FOLFOX or FOLFOX (£) | –197,438 | 95,514 | –85,200 | 86,875 | ||||
| ICER (£ per QALY) on the efficiency frontier (£) | Dominated by BEV + FOLFOX | Dominated by BEV + FOLFOX | £21,214 (vs. FOLFOX) | Reference |
In the FOLFIRI network, under our base case, in which we did not include bevacizumab plus FOLFIRI, the ICER for cetuximab plus FOLFIRI compared with FOLFIRI for the liver metastases subgroup was approximately £76,000 (see Base-case results, All patients: base-case results). When we now include bevacizumab plus FOLFIRI, the ICER for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI is £399,000 (Table 129), that is, cetuximab plus FOLFIRI becomes even worse value for money compared with the most cost-effective comparator.
| Outcome | CET + FOLFIRI | BEV + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. BEV+FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.69 | 2.65 | 1.83 | 0.03 | 0.86 |
| QALYs (mean, discounted) | 1.83 | 1.79 | 1.26 | 0.04 | 0.57 |
| Total costs (mean, discounted) (£) | 73,153 | 55,911 | 29,809 | 17,242 | 43,345 |
| ICER (£ per QALY) vs. FOLFOX (£) | 398,854 | 76,298 | |||
| ICER (£ per QALY) on the efficiency frontier (£) | 398,854 (vs. BEV + FOLFIRI) | 49,731 (vs. FOLFIRI) | Reference |
XELOX as a comparator
In this scenario analysis, we used XELOX in place of FOLFOX as a comparator in the FOLFOX network. Only the drug acquisition and administration costs were changed from the base case; all effectiveness parameters were unchanged. In particular, we assumed that the drug acquisition costs for both XELOX and FOLFOX are similar and very low, and that the administration cost of XELOX is clearly lower than for FOLFOX. This explains why the ICERs compared with XELOX are higher than those compared with FOLFOX:
-
the ICER for all patients for cetuximab plus FOLFOX compared with FOLFOX is £104,000 per QALY, whereas the ICER for cetuximab plus FOLFOX compared with XELOX is £126,000 per QALY
-
the ICER for all patients for panitumumab plus FOLFOX compared with FOLFOX is £204,000 per QALY, whereas the ICER for panitumumab plus FOLFOX compared with XELOX is £255,000 per QALY
-
the ICER for liver metastases patients for cetuximab plus FOLFOX compared with FOLFOX is £96,000 per QALY, whereas the ICER for cetuximab plus FOLFOX compared with XELOX is £114,000 per QALY
-
the ICER for liver metastases patients for panitumumab plus FOLFOX compared with FOLFOX is £87,000 per QALY, whereas the ICER for panitumumab plus FOLFOX compared with XELOX is £109,000 per QALY.
Overall survival from randomised controlled trials
In our base-case analysis, we modelled only PFS from the RCTs. OS was estimated from the times on first-, second- and third-line treatment for unresected patients and for OS for resected patients. In a sensitivity analysis, we modelled OS in addition to PFS from the RCTs (see Structure of the Peninsula Technology Assessment Group model). The two differences in the models were:
-
The modelled mean treatment duration for each treatment arm was set equal to the treatment duration from the RCTs. Unlike in the base case, we did not cap treatment duration as the mean time in first-line PFS for unresected patients. The rationale for removing the cap is that OS from the RCTs is likely to be affected (probably lengthened) by first-line drugs taken post progression.
-
The time on third-line BSC for unresected patients was changed in such a way as to yield the OS curves from the RCTs (after subtracting patients post resection and after the indirect comparisons). The times in all other health states were unaltered.
We estimated the proportions of patients taking cetuximab- and panitumumab-based treatments second line from the limited data from the RCTs (see Table 88) and we estimated the mean treatment durations of the second-line treatments as the averages of the durations on first-line treatment (from the current model) and third-line treatment (from our 2011 mCRC model for the relevant NICE HTA139,141). From this, and the estimated monthly costs of drug acquisition and administration for the current model, we estimated the total costs of drug acquisition and administration for second-line cetuximab plus FOLFIRI and panitumumab plus FOLFIRI (Table 130).
| Second-line treatment | Estimated treatment duration (months) | First-line treatment: estimated % of patients on second-line treatment | ||||
|---|---|---|---|---|---|---|
| First line | Second line | Third line | CET + FOLFOX | PAN + FOLFOX | FOLFOX | |
| CET + FOLFIRI | (Confidential information has been removed)a | 8.9 | 8.8 | 0 | 12.9 | 12.7 |
| PAN + FOLFIRI | 8.2 | 8.5 | 8.8 | 14.1 | 0 | 12.7 |
| Estimated total cost of second-line treatment (£) | 7158 | 6625 | 12,965 | |||
Overall survival for unresected patients was greater in this sensitivity analysis for all treatment arms (Figure 38). This may be because a large proportion of patients in the RCTs took monoclonal antibodies after progression (see Table 88), whereas we assumed no such treatment in the base-case analysis.
FIGURE 38.
Overall survival estimated using the base-case method or estimated from RCTs. BEV, bevacizumab; CET, cetuximab; PAN, panitumumab.
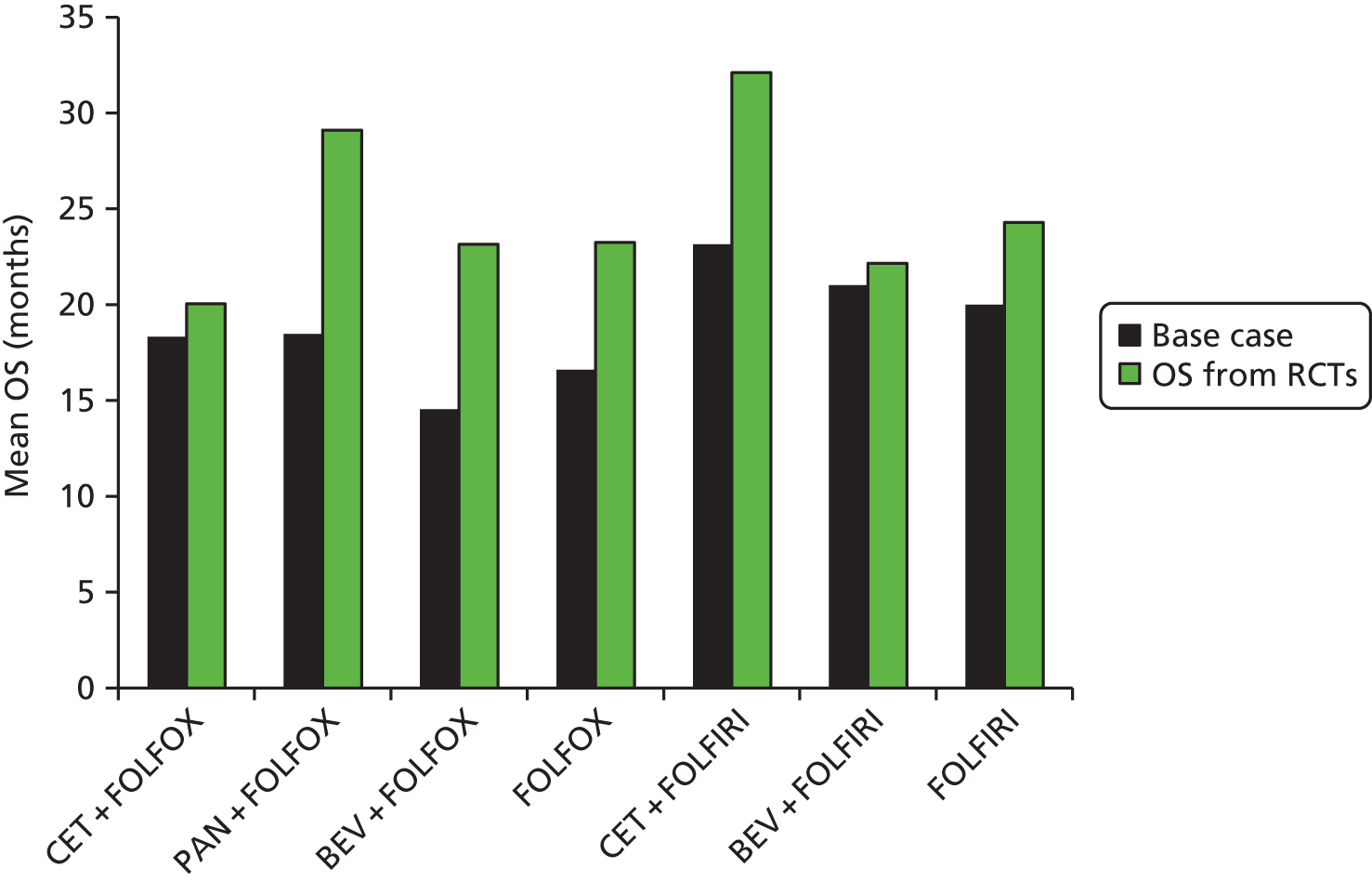
The cost-effectiveness of cetuximab plus FOLFOX compared with FOLFOX is substantially worse in this analysis so that cetuximab plus FOLFOX is now extendedly dominated by panitumumab plus FOLFOX compared with FOLFOX (Table 131). This is because, compared with baseline, OS increases less for cetuximab plus FOLFOX than for FOLFOX (see Figure 38).
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.52 | 2.85 | 2.35 | 0.17 | 0.50 |
| QALYs (mean, discounted) | 1.67 | 1.86 | 1.55 | 0.12 | 0.31 |
| Total costs (mean, discounted) (£) | 75,585 | 77,375 | 48,803 | 26,781 | 28,572 |
| ICER (£ per QALY) vs. FOLFOX (£) | 219,952 | 92,585 | |||
| ICER (£ per QALY) on the efficiency frontier (£) | Extendedly dominated | 92,585 | Reference |
The ICER for panitumumab plus FOLFOX compared with FOLFOX reduces substantially, from £204,103 to £92,585 per QALY, because, compared with baseline, OS increases more for panitumumab plus FOLFOX than for FOLFOX (see Figure 38) and because mean treatment duration increases less for panitumumab plus FOLFOX than for FOLFOX (see Model parameters, First-line time on treatment).
The ICER for cetuximab plus FOLFIRI compared with FOLFIRI reduces from £122,554 to £84,523 per QALY because, compared with baseline, OS increases more for cetuximab plus FOLFIRI than for FOLFIRI (Table 132) and mean treatment durations for both treatments are unchanged (see Figure 29).
| Outcome | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI |
|---|---|---|---|
| Life-years (mean, undiscounted) | 2.90 | 2.10 | 0.80 |
| QALYs (mean, discounted) | 1.92 | 1.43 | 0.49 |
| Total costs (mean, discounted) (£) | 74,587 | 32,973 | 41,614 |
| ICER (£ per QALY) (£) | 84,523 |
Merck Serono also presented a scenario analysis in which OS was taken directly from the RCTs. In this case, the base-case ICERs changed as follows:
-
cetuximab plus FOLFOX compared with FOLFOX – from £47,000 to £133,000 per QALY (a substantial increase)
-
cetuximab plus FOLFIRI compared with FOLFIRI – from £56,000 to £55,000 per QALY (virtually unchanged).
OPUS trial as the baseline randomised controlled trial in the FOLFOX network
For the FOLFOX network, the PRIME trial44 was selected as the baseline trial as it included two of the three treatments in our base-case analysis: panitumumab plus FOLFOX and FOLFOX. Although the OPUS trial65 also included two of the three treatments in our base-case analysis, cetuximab plus FOLFOX and FOLFOX, we did not select this trial as it is far smaller than the PRIME trial (n = 87 vs. 512 RAS WT patients) (see Structure of the Peninsula Technology Assessment Group model).
In a scenario analysis we used the OPUS trial as the baseline RCT for the FOLFOX network. In this case, the following parameters changed in the FOLFOX network:
-
resection rates (see Model parameters, Resection rates)
-
PFS unresected patients (see Model parameters, First-line progression-free survival: unresected patients)
-
treatment durations (see Model parameters, First-line time on treatment).
For all patients (Table 133):
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX increased slightly, from £104,000 to £111,000 per QALY gained
-
the ICER for panitumumab plus FOLFOX compared with FOLFOX increased slightly, from £204,000 to £211,000 per QALY gained.
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 1.88 | 1.66 | 1.51 | 0.37 | 0.15 |
| QALYs (mean, discounted) | 1.27 | 1.14 | 1.03 | 0.24 | 0.10 |
| Total costs (mean, discounted) | 52,195 | 47,507 | 25,661 | 26,534 | 21,846 |
| ICER (£ per QALY) vs. FOLFOX | 110,676 | 210,895 | |||
| ICER (£ per QALY) on efficiency frontier | 110,676 (vs. FOLFOX) | Extendedly dominated | Reference |
For liver metastases patients (Table 134):
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX reduced slightly, from £96,000 to £90,000 per QALY gained
-
the ICER for panitumumab plus FOLFOX compared with FOLFOX increased slightly, from £87,000 to £89,000.
| Outcome | CET + FOLFOX | PAN + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | PAN + FOLFOX vs. FOLFOX |
|---|---|---|---|---|---|
| Life-years (mean, undiscounted) | 2.30 | 2.17 | 1.51 | 0.80 | 0.66 |
| QALYs (mean, discounted) | 1.57 | 1.47 | 1.06 | 0.51 | 0.41 |
| Total costs (mean, discounted) | 76,095 | 66,446 | 30,198 | 45,897 | 36,249 |
| ICER (£ per QALY) vs. FOLFOX | 89,856 | 88,801 | |||
| ICER (£ per QALY) on the efficiency frontier | 94,053 vs. PAN + FOLFOX | 88,801 vs. FOLFOX | Reference |
Weekly administration of cetuximab
We carried out a scenario analysis in which cetuximab was administered weekly. In all cases, the ICERs for cetuximab increased, because the monthly cost of administration of cetuximab increased substantially:
-
cetuximab plus FOLFOX – £1563 to £1910
-
cetuximab plus FOLFIRI – from £849 to £1197.
For all patients:
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX increased from £104,000 to £123,000 per QALY
-
the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increased from £123,000 to £145,000 per QALY.
For the liver metastases subgroup:
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX increased from £96,000 to £112,000 per QALY
-
the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increased from £76,000 to £90,000 per QALY.
FOLFOX6
In this scenario analysis we used FOLFOX6 instead of FOLFOX4 as a comparator in the FOLFOX network. Only the drug acquisition and administration costs for cetuximab plus FOLFOX, panitumumab plus FOLFOX and FOLFOX were changed in this analysis; all effectiveness parameters were unchanged. We found that the drug acquisition costs were similar to those in the base case but the administration costs of all treatments fell substantially and by a similar amount, for example from £2348 to £1634 per month for FOLFOX. This explains why all of ICERs changed very little:
-
the ICER for all patients for cetuximab plus FOLFOX compared with FOLFOX decreased from £104,000 to £101,000 per QALY
-
the ICER for all patients for panitumumab plus FOLFOX compared with FOLFOX decreased from £204,000 to £199,000 per QALY
-
the ICER for all patients for cetuximab plus FOLFIRI compared with FOLFIRI increased very slightly, such that it remained £123,000 per QALY to three significant figures
-
the ICER for liver metastases patients for cetuximab plus FOLFOX compared with FOLFOX decreased from £96,000 to £92,000 per QALY
-
the ICER for liver metastases patients for panitumumab plus FOLFOX compared with FOLFOX decreased very slightly from £87,000 to £86,000 per QALY
-
the ICER for all patients for cetuximab plus FOLFIRI compared with FOLFIRI increased very slightly from £76,000 to £77,000 per QALY.
It should be noted that the ICERs for cetuximab plus FOLFIRI compared with FOLFIRI changed very slightly because of the change in the costs of acquisition and administration of second-line FOLFOX.
List prices for FOLFOX and FOLFIRI
In our base case we assumed that CMU eMit discounted prices were used for FOLFOX and FOLFIRI.
All ICERs increased when we used list prices for FOLFOX and FOLFIRI because the prices of these treatments increased and because we assumed a longer treatment duration for cetuximab plus FOLFOX and panitumumab plus FOLFOX than for FOLFOX, and a longer treatment duration for cetuximab plus FOLFIRI than for FOLFIRI (see Model parameters, First-line time on treatment).
For all patients:
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX increased from £104,000 to £116,000 per QALY
-
the ICER for panitumumab plus FOLFOX compared with FOLFOX increased from £204,000 to £217,000 per QALY
-
the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increased from £123,000 to £133,000 per QALY.
For the liver metastases subgroup:
-
the ICER for cetuximab plus FOLFOX compared with FOLFOX increased from £96,000 to £108,000 per QALY
-
the ICER for panitumumab plus FOLFOX compared with FOLFOX increased from £87,000 to £89,000 per QALY
-
the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increased from £76,000 to £82,000 per QALY.
Deterministic sensitivity analyses
Sensitivity analyses were chosen to demonstrate the drivers of cost-effectiveness by setting parameters to extreme values, for example setting the price of cetuximab equal to the price of panitumumab = £0. We do not suggest these parameter values as plausible alternatives to our base-case values. We investigate the choice of values for key parameters when we compare our model with Merck Serono’s model (see Comparison of the cost-effectiveness results with those of Merck Serono).
Cetuximab plus FOLFOX compared with FOLFOX
One-way deterministic sensitivity analyses for cetuximab plus FOLFOX compared with FOLFOX are reported in Figure 39, which shows the impact on the deterministic ICER of various alterations in model parameters.
FIGURE 39.
Sensitivity analyses: CET + FOLFOX vs. FOLFOX. CET, cetuximab; PD, progressive disease.

None of these sensitivity analyses resulted in an ICER below the £20,000 per QALY usual maximum accepted willingness-to-pay threshold for treatments that do not qualify for end of life.
Cost-effectiveness was very sensitive to the resection rates. In particular, if we set the resection rate for cetuximab plus FOLFOX equal to that for FOLFOX or if we set both rates equal to 0%, the ICER increased substantially.
Cost-effectiveness was sensitive to the assumed PFS and OS post resection. If we set these to zero, cetuximab plus FOLFOX was dominated by FOLFOX.
Cost-effectiveness was sensitive to the estimated PFS for unresected patients. Setting the PFS for cetuximab plus FOLFOX equal to that for FOLFOX when holding the treatment duration for cetuximab plus FOLFOX constant (as this is capped at PFS for unresected patients) resulted in a marked increase in the ICER.
As expected, the ICER fell substantially to £21,000 when we set the price of cetuximab to £0. However, even then, it was above the £20,000 per QALY threshold.
Cost-effectiveness was sensitive to the treatment durations. When we reduced the treatment duration for cetuximab plus FOLFOX from 8.7 to 7.0 months, the treatment duration for FOLFOX, the ICER fell substantially.
Cost-effectiveness was quite sensitive to discounting and the cost of administration of first-line drugs. If we set these independently to zero, the ICER fell noticeably.
Cost-effectiveness was also insensitive to changes in the remaining parameters:
-
mean starting age (affecting only utilities and general UK mortality, not treatment effectiveness)
-
dose intensity
-
PFS (unresected)
-
time on second-line treatment
-
time on third-line treatment
-
proportion of progressions that are deaths, that is, mortality from PFS – first line, second line and third line
-
price of FOLFOX
-
price of a RAS test
-
first-line medical management (unresected) costs
-
first-line AE costs
-
second-line costs
-
third-line costs
-
resection operation costs
-
PFS and progressive disease post resection costs
-
disutilities as a result of AEs
-
disutility resulting from health state – all utilities set equal to general UK population (age related).
Panitumumab plus FOLFOX compared with FOLFOX
One-way deterministic sensitivity analyses for panitumumab plus FOLFOX compared with FOLFOX are reported in Figure 40. Again, none of these sensitivity analyses brought the ICER below usually accepted willingness-to-pay thresholds. There are many similarities with the cetuximab plus FOLFOX compared with FOLFOX sensitivity analyses. Here, we discuss the differences.
FIGURE 40.
Sensitivity analyses: PAN + FOLFOX vs. FOLFOX. PAN, panitumumab; PD, progressive disease.
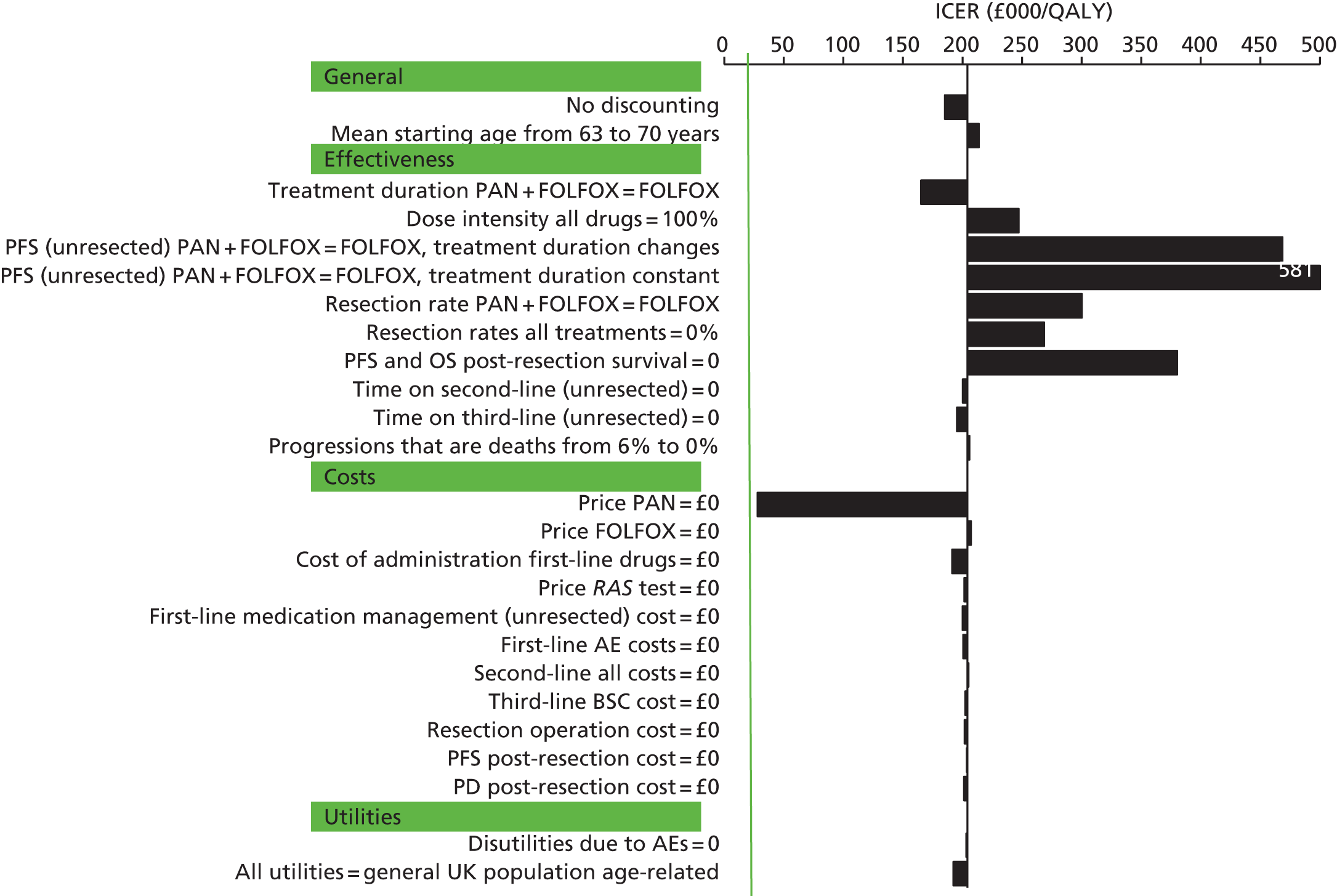
Cost-effectiveness was less sensitive to changes in resection rates because the rate for panitumumab plus FOLFOX is only slightly greater than that for FOLFOX, whereas the rate for cetuximab plus FOLFOX (20.7%) is far greater than that for FOLFOX.
Cost-effectiveness worsened substantially when PFS for unresected patients for panitumumab plus FOLFOX was set equal to that for FOLFOX when holding the treatment duration for panitumumab plus FOLFOX constant. At first sight it appears counterintuitive that the ICER changes proportionally far more than for the cetuximab plus FOLFOX comparison. However, this is explained because incremental QALYs in respect of PFS for unresected patients account for proportionally more of the total incremental QALYs for panitumumab plus FOLFOX compared with FOLFOX than for cetuximab plus FOLFOX compared with FOLFOX. This, in turn, is because we assumed a far lower resection rate for panitumumab plus FOLFOX than for cetuximab plus FOLFOX.
As expected, the ICER fell substantially, to £28,000 per QALY, when we set the price of panitumumab to £0. However, even then, as for cetuximab plus FOLFOX compared with FOLFOX, it lies above the £20,000 per QALY threshold.
Cetuximab plus FOLFIRI compared with FOLFIRI
One-way deterministic sensitivity analyses for cetuximab plus FOLFIRI compared with FOLFIRI are reported in Figure 41.
FIGURE 41.
Sensitivity analyses: CET + FOLFIRI vs. FOLFIRI. CET, cetuximab; PD, progressive disease.
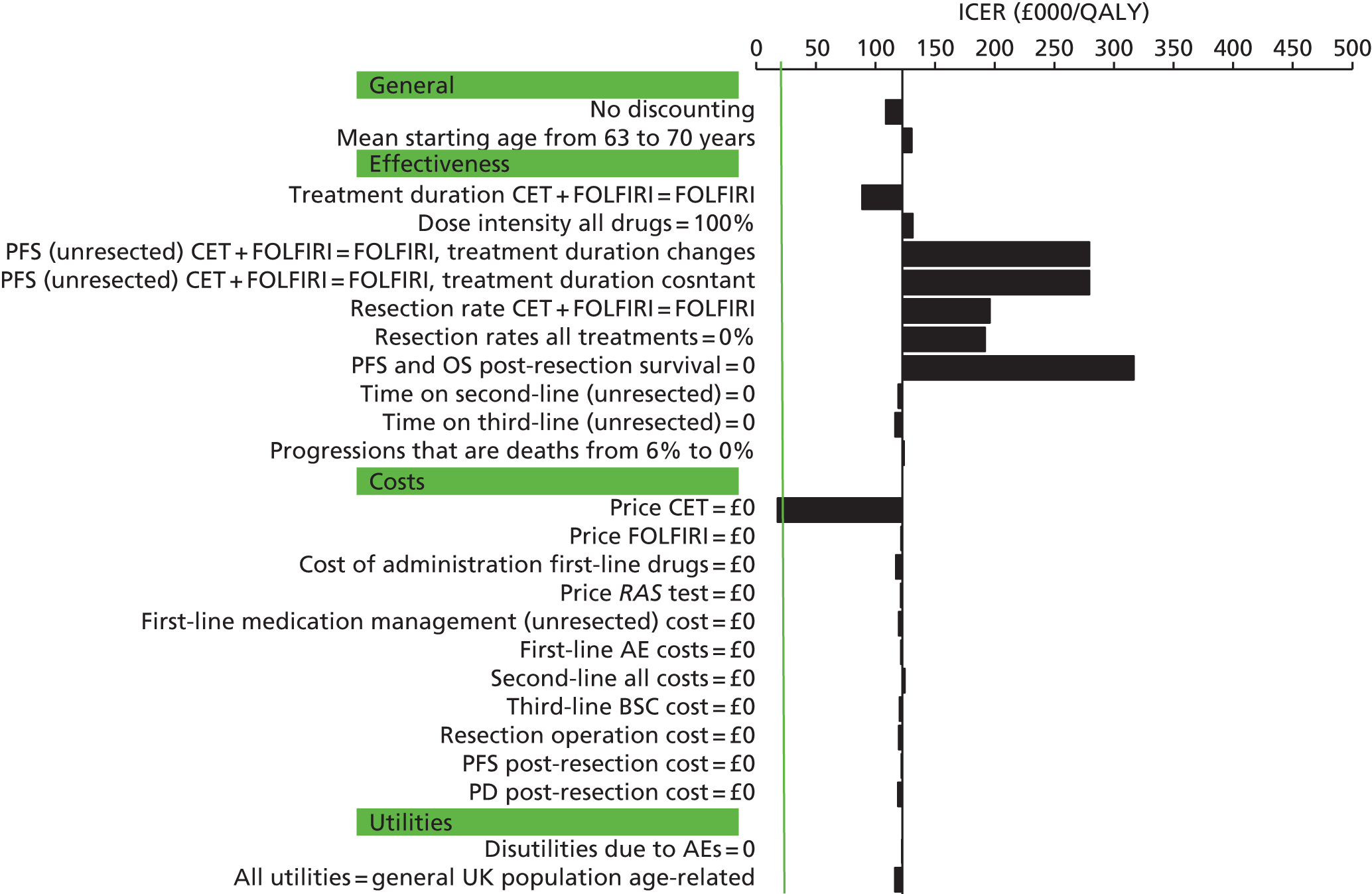
Again, there are many similarities with the cetuximab plus FOLFOX compared with FOLFOX sensitivity analyses. Here, we discuss the differences.
Cost-effectiveness was less sensitive to changes in resection rates because the estimated rate for cetuximab plus FOLFIRI is only slightly greater than that for FOLFIRI (7.3% vs. 2.1%), whereas the estimate for cetuximab plus FOLFOX (20.7%) is far greater than that for FOLFOX.
Cost-effectiveness worsened substantially when PFS for unresected patients for cetuximab plus FOLFIRI was set equal to that for FOLFIRI when holding the treatment duration for cetuximab plus FOLFIRI constant. The reason for this is the same as that for panitumumab plus FOLFOX.
As expected, the ICER fell substantially, to £18,000, when we set the price of cetuximab to £0, slightly below the £20,000 per QALY threshold.
Comparison of the cost-effectiveness results with those of Merck Serono
Merck Serono, but not Amgen, performed a cost-effectiveness analysis. Therefore, in this section we compare our cost-effectiveness results with those of Merck Serono. We have not critiqued the liver metastases model from Merck Serono for the reasons given in Chapter 5 (see De novo economic evaluation). Therefore, the comparison of results is confined to the ‘all patients’ group (Tables 135 and 136).
| Outcome | PenTAG | Merck Serono | ||||
|---|---|---|---|---|---|---|
| CET + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | CET + FOLFOX | FOLFOX | CET + FOLFOX vs. FOLFOX | |
| Life-years (mean, undiscounted) | ||||||
| First-line drug (resected + unresected) | 0.72 | 0.58 | 0.14 | 0.41 | 0.39 | 0.02 |
| PFS unresected | 0.57 | 0.52 | 0.06 | 1.04 | 0.74 | 0.30 |
| PFS post resection | 0.85 | 0.44 | 0.41 | 0.20 | 0.06 | 0.14 |
| PFS first line | 1.42 | 0.96 | 0.46 | 1.24 | 0.80 | 0.44 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.26 | 0.29 | –0.03 | 0.31 | 0.33 | –0.02 |
| Third-line BSC (unresected) | 0.38 | 0.43 | –0.05 | 0.67 | 0.70 | –0.03 |
| PD post resection | 0.35 | 0.18 | 0.17 | 0.09 | 0.03 | 0.06 |
| OS (mean) | 2.41 | 1.86 | 0.55 | 2.32 | 1.86 | 0.46 |
| QALYs (discounted) | ||||||
| PFS unresected | 0.43 | 0.39 | 0.04 | 0.79 | 0.57 | 0.22 |
| PFS post resection | 0.56 | 0.29 | 0.27 | 0.15 | 0.04 | 0.11 |
| AEs first line | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| PFS first-line | 0.99 | 0.68 | 0.31 | 0.94 | 0.60 | 0.34 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.19 | 0.21 | –0.02 | 0.23 | 0.25 | –0.02 |
| Third-line BSC (unresected) | 0.23 | 0.26 | –0.03 | 0.42 | 0.45 | –0.03 |
| PD post resection | 0.20 | 0.10 | 0.10 | 0.06 | 0.02 | 0.04 |
| Total | 1.61 | 1.26 | 0.35 | 1.65 | 1.32 | 0.33 |
| Costs (discounted) (£) | ||||||
| RAS test | 400 | 0 | 400 | 200 | 200 | 0 |
| First-line drug acquisition | 29,850 | 461 | 29,389 | 22,113 | 6416 | 15,697 |
| First-line drug administration | 13,212 | 10,527 | 2685 | 2971 | 2803 | 168 |
| First-line AEs | 1512 | 1068 | 444 | 458 | 469 | –11 |
| First-line medical management (unresected) | 3029 | 2746 | 283 | 0 | 0 | 0 |
| Second-line drug acquisition, drug administration and medical management | 4161 | 4706 | –545 | 7289 | 7968 | –679 |
| Third-line BSC (unresected) | 5481 | 6199 | –718 | 7907 | 8398 | –491 |
| Resection operation | 3635 | 1884 | 1751 | 196 | 56 | 140 |
| PFS post resection | 1014 | 526 | 488 | 0 | 0 | 0 |
| PD post resection | 4763 | 2469 | 2294 | 169 | 97 | 72 |
| Total | 67,057 | 30,585 | 36,471 | 41,303 | 26,407 | 14,896 |
| ICER (£ per QALY) | 104,205 | 46,503 | ||||
| Outcome | PenTAG | Merck Serono | ||||
|---|---|---|---|---|---|---|
| CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI vs. FOLFIRI | |
| Life-years (mean, undiscounted) | ||||||
| First-line drug (resected + unresected) | 0.76 | 0.56 | 0.20 | 0.44 | 0.43 | 0.01 |
| PFS unresected | 0.95 | 0.75 | 0.20 | 0.98 | 0.73 | 0.25 |
| PFS post resection | 0.30 | 0.09 | 0.21 | 0.20 | 0.06 | 0.14 |
| PFS first line | 1.25 | 0.83 | 0.42 | 1.18 | 0.79 | 0.39 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.39 | 0.41 | –0.02 | 0.31 | 0.33 | –0.02 |
| Third-line BSC (unresected) | 0.45 | 0.47 | –0.03 | 0.68 | 0.71 | –0.03 |
| PD post resection | 0.12 | 0.04 | 0.09 | 0.09 | 0.03 | 0.06 |
| OS (mean) | 2.21 | 1.75 | 0.46 | 2.27 | 1.86 | 0.41 |
| QALYs (discounted) | ||||||
| PFS unresected | 0.71 | 0.56 | 0.15 | 0.76 | 0.57 | 0.19 |
| PFS post resection | 0.20 | 0.06 | 0.14 | 0.15 | 0.04 | 0.11 |
| AEs first line | 0.00 | 0.00 | 0.00 | –0.01 | –0.01 | 0.00 |
| PFS first line | 0.91 | 0.62 | 0.29 | 0.91 | 0.61 | 0.30 |
| Second-line FOLFOX or FOLFIRI (unresected) | 0.28 | 0.30 | –0.02 | 0.23 | 0.25 | –0.02 |
| Third-line BSC (unresected) | 0.27 | 0.29 | –0.02 | 0.43 | 0.45 | –0.02 |
| PD post resection | 0.07 | 0.02 | 0.05 | 0.06 | 0.02 | 0.04 |
| Total | 1.53 | 1.23 | 0.30 | 1.63 | 1.33 | 0.30 |
| Costs (discounted) (£) | ||||||
| RAS test | 400 | 0 | 400 | 200 | 200 | 0 |
| First-line drug acquisition | 32,742 | 776 | 31,965 | 23,176 | 6234 | 16,942 |
| First-line drug administration | 7543 | 5506 | 2037 | 3250 | 3148 | 102 |
| First-line AEs | 821 | 482 | 339 | 567 | 418 | 149 |
| First-line medical management (unresected) | 4993 | 3948 | 1045 | 0 | 0 | 0 |
| Second-line drug acquisition, drug administration and medical management | 9241 | 9845 | –604 | 7927 | 8492 | –565 |
| Third-line BSC (unresected) | 6316 | 6730 | –413 | 8087 | 8487 | –400 |
| Resection operation | 1284 | 372 | 912 | 196 | 56 | 140 |
| PFS post resection | 358 | 104 | 254 | 0 | 0 | 0 |
| PD post resection | 1683 | 488 | 1195 | 189 | 104 | 85 |
| Total | 65,380 | 28,250 | 37,130 | |||
| ICER (£ per QALY) | 122,554 | 55,971 | ||||
First, there are many similarities between our model and Merck Serono’s model. For example, we assumed:
-
The same overall model structure (structure 1; see Structure of the Peninsula Technology Assessment Group model), that is, we both used only resection rates and PFS data and not OS data from the trials of first-line drugs. In scenario analyses, we both also modelled OS from the RCTs.
-
Similar utilities (see Model parameters, Utilities).
-
The same source for estimation of PFS and OS after resection (see Model parameters, Post liver resection: progression-free survival and overall survival).
-
The same prices of cetuximab, panitumumab and bevacizumab (see Model parameters, Costs) (we assumed far lower prices for FOLFOX and FOLFIRI, but this had little effect on cost-effectiveness).
-
Similar treatment durations for second-line FOLFOX and FOLFIRI (see Structure of the Peninsula Technology Assessment Group model; Model parameters, Second-line time on treatment: unresected patients; and Chapter 5, Description of methods, Model parameters).
However, there are several important differences between the models, which result in very different estimates of the cost-effectiveness of cetuximab.
The PenTAG ICERs are much higher than the Merck Serono ICERs (see Tables 135 and 136):
-
cetuximab plus FOLFOX compared with FOLFOX – £104,000 per QALY for the PenTAG model and £47,000 per QALY for the Merck Serono model
-
cetuximab plus FOLFIRI compared with FOLFIRI – £123,000 per QALY for the PenTAG model and £56,000 per QALY for the Merck Serono model.
In total, we identified eight items that differed between our model and Merck Serono’s model that have an important impact on cost-effectiveness.
For the FOLFOX network, treatment duration and PFS for unresected patients are the most important items. The ICER from Merck Serono’s model increases substantially when both are independently changed to our estimate, because we assumed substantially greater treatment durations than Merck Serono (see Model parameters, First-line time on treatment) and because we assumed a substantially smaller difference in mean PFS for unresected patients between cetuximab plus FOLFOX and FOLFOX than Merck Serono. This is because we estimated PFS for unresected patients by subtracting PFS for resected patients from the PFS data for resected plus unresected patients from the RCTs, whereas Merck Serono did not (see Model parameters, First-line progression-free survival: unresected patients).
For the FOLFIRI network, treatment duration is clearly the most important item. The ICER from Merck Serono’s model increases substantially when treatment durations are changed to our estimates. Unlike for the FOLFOX network, the ICER for cetuximab plus FOLFIRI compared with FOLFIRI increases only slightly when we use our estimates of PFS for unresected patients, even though we again subtracted PFS for resected patients from PFS for resected plus unresected patients from the RCTs. This is because we estimated substantially lower resection rates for the FOLFIRI network than for the FOLFOX network (see Model parameters, First-line progression-free survival: unresected patients).
Above all, treatment duration is the most critical issue in the current HTA with regard to explaining the difference in cost-effectiveness between our model and Merck Serono’s model.
We now turn to the two important differences between the models, with cost-effectiveness improving under our assumptions.
We assumed a far longer duration in PFS and progressive disease post resection than did Merck Serono (see Model parameters, Post liver resection: progression-free survival and overall survival). This substantially improves the cost-effectiveness of cetuximab plus FOLFOX compared with FOLFOX, and cetuximab plus FOLFIRI compared with FOLFIRI.
For the FOLFOX network, we assumed far higher resection rates than Merck Serono (see Model parameters, Resection rates). This also substantially improves the cost-effectiveness of cetuximab plus FOLFOX compared with FOLFOX (see Table 135). We assumed the same resection rates as Merck Serono for cetuximab plus FOLFIRI and FOLFIRI.
We have already described how our treatment duration estimates for both the FOLFOX network and the FOLFIRI network, and our estimates of PFS for unresected patients for the FOLFOX network both substantially worsen cost-effectiveness. There are four other differences under which cost-effectiveness worsens in both networks, although only slightly, under our assumptions:
-
We assumed higher unit costs of drug administration than Merck Serono (see Model parameters, Costs). Our values yield slightly worse cost-effectiveness results because we assumed that patients take cetuximab plus FOLFOX for longer than FOLFOX and take longer on cetuximab plus FOLFIRI for longer than FOLFIRI.
-
We assumed a higher cost for resection than Merck Serono (see Model parameters, Costs, Cost of liver resection). This acts to worsen cost-effectiveness as the resection rate is higher for cetuximab plus FOLFOX than FOLFOX and for cetuximab plus FOLFIRI than FOLFIRI (see Table 136).
-
We assumed a higher cost per month for treating patients in progressive disease post resection (see Model parameters, Costs). This acts to worsen cost-effectiveness, again as the resection rate is higher for cetuximab plus FOLFOX than FOLFOX and for cetuximab plus FOLFIRI than FOLFIRI.
-
We assumed different costs of drug acquisition per month (see Model parameters, Costs). We assumed a slightly higher cost of acquisition of cetuximab per month than Merck Serono (£3859 vs. £3478), which acts to worsen cost-effectiveness. Our estimates of the monthly cost of acquisition of FOLFOX and FOLFIRI were much lower than those of Merck Serono. However, cost-effectiveness was insensitive to these differences because they affect both arms similarly in treatment comparison pairs. We assumed a higher monthly acquisition cost of cetuximab than Merck Serono because we assumed a slightly larger body surface area than Merck Serono (1.85 m2 vs. 1.79 m2) and the dose of cetuximab depends on body surface area. In 2011, Merck Serono also estimated body surface area as 1.79 m2 and we estimated it as 1.85 m2. 141 Merck Serono did not give the source of their estimate in the current model. Furthermore, as we explained for the previous HTA, we prefer our estimate as it was taken from a database of people receiving palliative chemotherapy for CRC, with 66% males and 34% females, the typical sex mix in the RCTs of mCRC. 142
When we amended Merck Serono’s model for all eight changes simultaneously, the resulting ICERs were similar to the base-case ICERs in our model.
Of course, this does not in itself prove that there are no important differences between Merck Serono’s amended model and our model. However, we found no remaining large differences in incremental mean life-years, QALYs and costs between Merck Serono’s amended model and our model. We conclude that there are no further differences between our model and Merck Serono’s model that have a large impact on cost-effectiveness.
Chapter 7 Comparison of the current multiple technology appraisal with previous single technology appraisals
Although this MTA sought to update previous guidance from two STAs (TA17610 and TA24011), there are some important differences between the scope for the previous STA reviews and the scope for this current MTA (ID794). The main difference is in the patient population. The current scope specifies people with RAS WT mCRC, whereas the previous STAs specified people with EGFR-expressing mCRC (TA17610) and those with KRAS WT mCRC (TA 24011). A summary of all the differences between the scopes for the reviews alongside a summary of how the product licences have changed is provided in Chapter 1 [see Cetuximab and panitumumab for previously untreated metastatic colorectal cancer (review of TA176 and partial review of TA240) (ID794)].
The single technology appraisal TA176 (2009) (cetuximab) compared with the multiple technology appraisal ID794 (2015)
Assessment of clinical effectiveness
The appraisal of cetuximab in combination with chemotherapy for the treatment of mCRC (TA17610) included two studies, CRYSTAL24 and OPUS,23 whereas this MTA review included three studies. Although two of the three studies were included in the last HTA (CRYSTAL and OPUS), only data from the subgroup of people evaluated as RAS WT from these trials were relevant to this review,43 as set out in the final scope from NICE. The additional study identified by the Assessment Group’s searches for this MTA review was the FIRE-3 RCT. 28
The results from the previous STA of cetuximab (TA176) are summarised and compared with the results of the current MTA in Table 137. Comparisons can be made between TA176 and the current MTA only for the OPUS and CRYSTAL trials, as the FIRE-3 trial is new to the current appraisal. In line with research developments, effect estimates (when reported) for OS, PFS and ORR were either similar or point estimates were slightly decreased in the RAS WT subgroup compared with the KRAS WT population, suggesting a reduced risk of progression or death in the RAS WT population. However, these results should be interpreted with caution as the analyses are based on subgroup analyses and sample sizes (for some studies) were small, reducing the power of the studies to show statistical significance. No comparison could be made with respect to HRQoL data as the current MTA did not identify any data for HRQoL among the RAS WT population. Variability in the reporting of AEs between TA176 and the current MTA, for example summary AEs, AEs in ≥ 5% of participants or AEs with a > 5% difference between treatment arms, made it difficult to draw comparisons when data were reported. All results are subject to uncertainty (see Chapter 8, Strengths and limitations).
| Outcome | TA176 (2009),10 EGFR-expressing mCRCa | TA176 (2009),10 KRAS WT mCRC | PenTAG (2015), RAS WT mCRC |
|---|---|---|---|
| OPUS: CET + FOLFOX4 vs. FOLFOX4 | |||
| n | 336 | 134 | 87 |
| PFS, HR (95% CI) | NR | 0.570 (0.358 to 0.907) | 0.53 (0.27 to 1.04) |
| OS, HR (95% CI) | NR | NR | 0.94 (0.56 to 1.56) |
| ORR, % (95% CI) | 45.6 vs. 36.0b | 60.7 (47.3 to 72.9) vs. 37.0 (26.0 to 49.1)*b | 58 (41 to 74) vs. 29 (17 to 43)b |
| Resection rate (%) | NR | 11.5 vs. 4.1b | NR |
| HRQoL | NR | NR | NR |
| Safety | |||
| Any grade 3/4 events (%) | (Confidential information has been removedc) | NR | 79 vs. 63b |
| Most commonly reported grade 3/4 AEsd | NR | NR | Leukopenia, neutropenia, paraesthesia, rash, any skin reactions and acne-like rash skin reaction |
| CRYSTAL: CET + FOLFIRI vs. FOLFIRI | |||
| n | 1198 | 348 | 367 |
| PFS, HR (95% CI) | 0.85 (0.726 to 0.998) | 0.684 (0.501 to 0.934) | 0.56 (0.41 to 0.76) |
| OS, HR (95% CI) | 0.93 (0.81 to 1.07) | 0.84 (0.64 to 1.11) | 0.69 (0.54 to 0.88) |
| ORR, % (95% CI) | 45.6 vs. 36.0e | 59.3 (51.6 to 66.7) vs. 43.2 (35.8 to 58.9)**e | 66 (59 to 73) vs. 39 (32 to 46)e |
| Resection rate | NR | 3.5% vs. 2.3%e | OR 3.11 (95% CI 2.03 to 4.78) |
| HRQoL: EORTC QLQ-C30; EQ-5D | NR | Statistically significant differences between the two treatment groups in favour of the FOLFIRI-only group were reportedf | NR |
| Safety | |||
| Any grade 3/4 events (%) | (Confidential information has been removedc) | NR | 80.9 vs. 58.2e |
| Most commonly reported grade 3/4 AEsd | NR | Neutropenia, constipation, dyspepsia, dyspnoea, dysgeusia, injection site reaction, erythema, hypotension, hypertrichosis and cheilitisg | Deep-vein thrombosis, dermatitis acneiform, diarrhoea, fatigue, leukopenia, neutropenia, rash, any skin reactions and acne-like rash skin reaction |
| FIRE-3: CET + FOLFIRI vs. BEV + FOLFIRI | |||
| n | NA | NA | 342 |
| PFS, HR (95% CI) | NA | NA | 0.93 (0.74 to 1.17) |
| OS, HR (95% CI) | NA | NA | 0.7 (0.53 to 0.92) |
| ORR, % (95% CI) | NA | NA | 65.5 (58 to 73) vs. 60 (52 to 67)h |
| Resection rate | NA | NA | NR |
| HRQoL | NA | NA | NR |
| Safety | |||
| Any grade 3/4 events (%) | NA | NA | 69 vs. 67.3h |
| Most commonly reported grade 3/4 AEd | NA | NA | Acneiform/exanthema, desquamation, diarrhoea, haematotoxicity, hepatotoxicity, hypertension, hypokalaemia, infection, nail changes/paronychia, nausea, pain, skin reactions, thromboembolic events and thrombosis (any) |
Assessment of cost-effectiveness
As TA176 was a STA, only economic evidence submitted by the manufacturer (Merck Serono) was available, critiqued by an ERG. In this assessment, economic evidence was available both from the manufacturer and from us, the Assessment Group.
No studies were identified in the cost-effectiveness review carried out for TA176. In its recent submission, Merck Serono identified 15 studies, which included an economic analysis of cetuximab. Two of these studies were specific to the RAS WT population and were also identified by the Assessment Group. 108,109 Our review excluded the remaining 13 papers on the basis of population; the two included papers were both abstracts with associated posters. This indicates that some economic evidence is currently available compared with when TA176 was completed, but still not enough to adequately answer the decision problem.
Both TA176 and this assessment included a de novo economic analysis submitted by Merck Serono. Merck Serono appears to have updated its model from TA176, as both Merck Serono models (TA176 and the 2015 submission) appear to be very similar in structure. Furthermore, both Merck Serono models appear very similar in structure. In particular, the health states were generally similar: three lines of treatment plus post-resection states. For both models, modelling of first-line treatment was based on trial evidence, and subsequent lines of treatment and post resection were informed by the literature. 111–113 In both TA176 and the 2015 submission, Merck Serono presented the cost-effectiveness results as head-to-head comparisons based on trials. The main differences between TA176 and the cost-effectiveness analyses in this assessment are described in Table 138.
| Characteristic | TA176 (2009)10 | Merck Serono (2015) | PenTAG (2015) | |||
|---|---|---|---|---|---|---|
| Programme used to build model | TreeAge Pro 2006/7 software (TreeAge Software, Inc., Williamstown, MAUSA) | Microsoft Excel | Microsoft Excel | |||
| Population | EGFR-expressing, KRAS WT mCRC. Also required good performance status, suitable for irinotecan or oxaliplatin chemotherapy, initially unresectable liver metastases | RAS WT mCRC, unresectable metastases at any site | RAS WT mCRC, unresectable metastases at any site | |||
| Intervention(s) | CET + FOLFOX | CET + FOLFIRI | CET + FOLFOX | CET + FOLFIRI | CET + FOLFOX, PAN + FOLFOX | CET + FOLFIRI |
| Comparators including scenario analysis | FOLFOX | FOLFIRI | FOLFOX, XELOX | FOLFIRI, BEV + FOLFIRI | FOLFOX, BEV + FOLFOX, XELOX | FOLFIRI, BEV + FOLFIRI |
| Time horizon | Lifetime (mean 23 years in model) | 10 years | Lifetime (30 years) | |||
| Cycle length | 1 week | 1 month | 1 month | |||
Given the similarities in the models and the absence of the TA176 executable model, we present only summary results in Table 139 and narratively compare the results. We focus on the comparisons with FOLFOX and FOLFIRI, as in our comparison with Merck Serono’s submission (version 2; 15 June 2015).
| Outcome | TA176 (2009)10 | Merck Serono (2015) | PenTAG (2015) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FOLFOX | CET + FOLFOX | CET + FOLFOX vs. FOLFOX | FOLFOX | CET + FOLFOX | CET + FOLFOX vs. FOLFOX | FOLFOX | CET + FOLFOX | CET + FOLFOX vs. FOLFOX | |
| LYs | 1.48 | 1.89 | 0.41 | 1.81 | 2.22 | 0.41 | 1.86 | 2.41 | 0.55 |
| Costs (discounted) (£) | 21,842 | 42,084 | 20,242 | 26,408 | 41,301 | 14,894 | 30,585 | 67,057 | 36,471 |
| QALYs (discounted) | 1.09 | 1.41 | 0.32 | 1.32 | 1.64 | 0.32 | 1.26 | 1.61 | 0.35 |
| ICER (£ per QALY) | 63,245 | 46,503 | 104,205 | ||||||
| FOLFIRI | CET + FOLFIRI | CET + FOLFIRI vs. FOLFIRI | FOLFIRI | CET + FOLFIRI | CET + FOLFIRI vs. FOLFIRI | FOLFIRI | CET + FOLFIRI | CET + FOLFIRI vs. FOLFIRI | |
| LYs | 1.92 | 2.28 | 0.36 | 1.81 | 2.19 | 0.38 | 1.75 | 2.21 | 0.46 |
| Costs (discounted) (£) | 26,103 | 45,576 | 19,473 | 27,139 | 43,592 | 16,453 | 28,250 | 65,380 | 37,130 |
| QALYs (discounted) | 1.43 | 1.71 | 0.28 | 1.32 | 1.61 | 0.29 | 1.23 | 1.53 | 0.30 |
| ICER (£ per QALY) | 69,287 | 55,971 | 122,554 | ||||||
The life-years and QALYs for the FOLFOX network appear to have increased from TA176 to the Merck Serono submission (2015), but the life-years and QALYs for the FOLFIRI networks have decreased. These differences are presumably driven by the changes in population and time horizon. However, the incremental life-years and QALYs of the two analyses are virtually identical.
The main differences between the models are in the costs. The costs in TA176 and the Merck Serono submission are broadly similar; however, small changes in the costs are amplified in the cost-effectiveness results to give quite different ICERs, with reductions in the ICERs of between £13,000 and £17,000 per QALY depending on the network. These reductions result from higher costs for the FOLFOX/FOLFIRI arms in the most recent Merck Serono submission than in TA176 and lower costs for the cetuximab plus FOLFOX/FOLFIRI arms. The PenTAG model reports the highest costs of all.
Table 140 provides the disaggregated costs for the three analyses. The reporting of these costs varies across analyses, but overall the results suggest that the differences in costs between the PenTAG model and TA176 are driven by the same differences as those between the PenTAG model and the Merck Serono submission: costs relating to first-line treatment, including cheaper acquisition costs for FOLFOX and FOLFIRI (from use of the CMU eMit rather than the BNF), more expensive drug administration costs for FOLFOX and FOLFIRI and longer treatment durations. Neither the original submission for TA17610 nor the ERG report144 provides disaggregated life-years and so the effects of treatment duration cannot be confirmed, but as treatment duration is a driver of the cost of administration (and is a major driver of the differences between the Merck Serono model and the PenTAG model in this assessment) it seems plausible that differences in treatment duration will impact on costs. Other discrepancies in costs result from higher costs in second- and third-line treatment, higher costs of resection and the addition of medical management costs to first-line treatment.
| Cost item | TA176 (2009)10 | Merck Serono (2015) | PenTAG (2015) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CET + FOLFOX | FOLFOX | CET + FOLFOX – FOLFOX | CET + FOLFOX | FOLFOX | CET + FOLFOX – FOLFOX | CET + FOLFOX | FOLFOX | CET + FOLFOX – FOLFOX | |
| (K)RAS test | 462 | – | 462 | 200 | 200 | – | 400 | – | 400 |
| First-line drug acquisition | 27,332 | 9021 | 18,311 | 22,113 | 6416 | 15,697 | 29,850 | 461 | 29,389 |
| First-line drug administration | 3551 | 3202 | 349 | 2971 | 2803 | 168 | 13,212 | 10,527 | 2685 |
| First-line AEs | 820 | 467 | 353 | 458 | 469 | –11 | 1512 | 1068 | 444 |
| First-line medical management (unresected) | 3029 | 2746 | 283 | ||||||
| Total first line | 32,165 | 12,690 | 19,475 | 25,741 | 9888 | 15,853 | 48,003 | 14,802 | 33,201 |
| Second-line FOLFOX/FOLFIRI acquisition (unresected) | 379 | 429 | –50 | ||||||
| Second-line FOLFOX/FOLFIRI administration (unresected) | 2456 | 2778 | –322 | ||||||
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1325 | 1499 | –174 | ||||||
| Total second line (unresected) | 4856 | 5190 | –334 | 7289 | 7968 | –679 | 4161 | 4706 | –545 |
| Third-line BSC (unresected) | 2708 | 2863 | –155 | 7907 | 8398 | –491 | 5481 | 6199 | –718 |
| Resection operation | 351 | 164 | 187 | 196 | 56 | 139 | 3635 | 1884 | 1751 |
| PFS post resection | 1014 | 526 | 488 | ||||||
| PD post resection | 169 | 97 | 71 | 4763 | 2469 | 2294 | |||
| Total | 42,084 | 21,842 | 20,242 | 41,302 | 26,408 | 14,894 | 67,057 | 30,585 | 36,471 |
| CET + FOLFIRI | FOLFIRI | CET + FOLFIRI – FOLFIRI | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI – FOLFIRI | CET + FOLFIRI | FOLFIRI | CET + FOLFIRI – FOLFIRI | |
| (K)RAS test | 462 | 0 | 462 | 200 | 200 | 0 | 400 | – | 400 |
| First-line drug acquisition | 27,465 | 9887 | 17,578 | 23,176 | 6234 | 16,942 | 32,742 | 776 | 31,965 |
| First-line drug administration | 3467 | 3438 | 29 | 3250 | 3148 | 102 | 7543 | 5506 | 2037 |
| First-line AEs | 1147 | 491 | 656 | 567 | 418 | 150 | 821 | 482 | 339 |
| First-line medical management (unresected) | 4993 | 3948 | 1045 | ||||||
| Total first line | 32,541 | 13,816 | 18,725 | 27,193 | 10,000 | 17,193 | 46,498 | 10,712 | 35,786 |
| Second-line FOLFOX/FOLFIRI acquisition (unresected) | 382 | 407 | –25 | ||||||
| Second-line FOLFOX/FOLFIRI administration (unresected) | 6868 | 7317 | –449 | ||||||
| Second-line FOLFOX or FOLFIRI medical management (unresected) | 1991 | 2122 | –130 | ||||||
| Total second line (unresected) | 6088 | 6833 | –745 | 7927 | 8492 | –565 | 9241 | 9845 | –604 |
| Third-line BSC (unresected) | 2962 | 3288 | –326 | 8087 | 8487 | –400 | 6316 | 6730 | –413 |
| Resection operation | 511 | 278 | 233 | 196 | 56 | 139 | 1284 | 372 | 912 |
| PFS post resection | – | – | – | 358 | 104 | 254 | |||
| PD post resection | 189 | 104 | 85 | 1683 | 488 | 1195 | |||
| Total | 45,576 | 26,103 | 19,473 | 43,592 | 27,139 | 16,453 | 65,380 | 28,250 | 122,554 |
The single technology appraisal TA240 (2013) (panitumumab) compared with the multiple technology appraisal ID794 (2015)
The appraisal of panitumumab in combination with chemotherapy for the treatment of mCRC (TA24011) was ended because no evidence submission was received from the manufacturer or sponsor of the technology. Therefore, NICE was unable to make a recommendation about the use in the NHS of panitumumab in combination with chemotherapy for the treatment of mCRC. 11
Two studies of clinical effectiveness were identified in the current MTA review, the PEAK trial29 and the PRIME trial,44 both of which contained data from the RAS WT population.
Similarly, no economic evidence was submitted in TA240,11 but two published cost-effectiveness studies107,110 were identified in the current MTA review, which include economic assessments of panitumumab in combination with FOLFOX compared with relevant comparators. No de novo economic analysis was submitted by Amgen for either either TA24011 or this current MTA.
Chapter 8 Discussion
Statement of principal findings
Aim
The remit of this report was to review and update the evidence used to inform the current NICE guidance (TA17610 and TA24011) on the clinical effectiveness and cost-effectiveness of two EGFR inhibitors, cetuximab and panitumumab, for the treatment of first-line mCRC.
In this section we will not restate the previous evidence, but assume that the discussion will be read in the context of the previous evidence summaries and the decisions that flowed from them. The conclusions will focus on the implications of the new effectiveness and cost-effectiveness evidence for service provision.
Clinical effectiveness systematic review
Of 2811 titles/abstracts screened, five RAS WT subgroup analyses from RCTs met the inclusion criteria for the clinical effectiveness systematic review. Given the differences in the eligible population between this current MTA review and the previous STA reviews, the evidence included in this submission was all identified by the Assessment Group’s searches. Three subgroup analyses provided data for the effectiveness of cetuximab and two provided evidence for the effectiveness of panitumumab. Efficacy and safety outcomes were tabulated and discussed in a narrative review. All included studies provided evidence for the NMA when data were available for the outcome of interest. It was not possible to construct a complete network. Two discrete networks were generated, one evaluating FOLFOX-containing chemotherapy regimens and the other comparing FOLFIRI-containing chemotherapy regimens.
The risk of bias was generally similar between studies with respect to randomisation, allocation concealment, blinding, outcome reporting and loss to follow-up. The main consideration with respect to quality was that currently available data for both cetuximab and panitumumab are taken from only a subgroup of the ITT population. To set this in context, the rationale for this is based on tumour biology: research has shown a treatment interaction for RAS and EGFR inhibitors. In response to this, the EMA recently revised the licensed indications for these products based on the subgroup data from the ITT populations of the trials. Currently, the only available data demonstrating efficacy in people with RAS WT mCRC is from subgroup analyses; the Assessment Group did not identify any RCT evidence for which there was an ITT RAS WT population.
Despite this, the limitations associated with the interpretation of subgroup data still apply. Given the use of subgroup data all comparisons were made without protection by stratification/randomisation. Instead, allocation to subgroups was based on RAS analysis of tumour samples from the KRAS WT exon 2 trial participants; the RAS ascertainment rate was 61%, minimising the potential for significant ascertainment bias (missing data largely resulted from unavailable tumour samples or inconclusive RAS test results). In addition, although imbalances in baseline characteristics between groups were expected, no major differences were observed, minimising the potential for selection bias. Because of the retrospective nature of the RAS analysis, a low number of samples was available for analysis, reducing the power of the studies to show statistical significance.
Summary of benefits and risks
Individuals respond differently to some drugs. 58,59 Genotype is an important determinant of both the response to treatment and the susceptibility to adverse reactions for a wide range of drugs,60,61 for example response to EGFR inhibitors has been shown to be dependent on gene expression in colon cancer, with studies demonstrating a treatment interaction between RAS status and the effectiveness of EGFR inhibitors. 62–64 In line with research developments evaluating the negative impact of RAS mutations on the effectiveness of EGFR inhibitors, approval for the use of anti-EGFR antibodies has now been limited to people with mCRC with RAS WT tumours. Tumour samples from trial populations supporting the original licensed indications were evaluated retrospectively for RAS status. Importantly, therefore, data supporting this recent licence change and this NICE assessment are not from the ITT trial population for any of the included studies but from a subgroup of people contained within the original RCTs and the results are therefore subject to uncertainty. However, no RCTs with an ITT population by RAS WT status were identified.
Previously, NICE has appraised cetuximab (TA17610) for the treatment of people with EGFR-expressing mCRC, in line with the licensed indication at the time. Although two of the identified cetuximab trials in this review were included in the last appraisal, only data from the subgroup of people evaluated as RAS WT from those trials are relevant to the scope of this review, as set out in the final scope from NICE (see Chapter 3, Studies identified). The appraisal of panitumumab in combination with chemotherapy for the treatment of mCRC (TA24011) was ended because no evidence submission was received from the manufacturer or sponsor of the technology. As such, NICE was unable to make recommendations relating to the use of panitumumab in the NHS. All data included in this update review for both cetuximab and panitumumab have been identified by the PenTAG searches.
Cetuximab
Two trials provided evidence for the effectiveness of cetuximab in combination with chemotherapy (FOLFOX or FOLFIRI) compared with chemotherapy alone (FOLFOX or FOLFIRI). The evidence consistently suggested a treatment effect in favour of the addition of cetuximab to chemotherapy (FOLFOX or FOLFIRI) compared with chemotherapy alone (FOLFOX or FOLFIRI) for the outcomes of interest (PFS, OS, ORR and complete resection rate). Overall, clinical safety was consistent with the results for the KRAS WT population in the trials. The most common AEs were diarrhoea, haematotoxicity, neutropenia and skin reactions.
One trial provided evidence for the effectiveness of cetuximab in combination with chemotherapy (FOLFIRI) compared with bevacizumab in combination with chemotherapy (FOLFIRI). The proportion of people who achieved an objective response was similar between the cetuximab plus FOLFIRI arm and the bevacizumab plus FOLFIRI arm. However, the association with longer OS suggests a benefit for cetuximab plus FOLFIRI (HR 0.70, 95% CI 0.53 to 0.92).
Panitumumab
One trial provided evidence for the effectiveness of panitumumab in combination with chemotherapy (FOLFOX) compared with chemotherapy alone (FOLFOX). No evidence was identified comparing panitumumab plus FOLFIRI with FOLFIRI. The evidence consistently suggested a treatment effect in favour of the addition of cetuximab to FOLFOX compared with FOLFOX. Overall, clinical safety was consistent with the results for the KRAS WT population in the trial. The most common AEs were diarrhoea, haematotoxicity, neutropenia and skin reactions.
One trial provided evidence for the effectiveness of panitumumab in combination with chemotherapy (mFOLFOX6) compared with bevacizumab in combination with chemotherapy (mFOLFOX6). The proportion of people who achieved an ORR was similar between the panitumumab plus FOLFIRI arm and the bevacizumab plus FOLFIRI arm. For PFS, the addition of panitumumab to mFOLFOX6 was associated with a 35% reduction in the risk of progression compared with bevacizumab plus FOLFOX. In addition, a trend towards an OS benefit with panitumumab plus FOLFOX was observed (HR 0.63, 95% CI 0.39 to 1.02).
Network meta-analysis: FOLFOX network
There was no evidence to suggest that cetuximab plus FOLFOX is any more effective than FOLFOX, bevacizumab plus FOLFOX or panitumumab plus FOLFOX in terms of increasing the time to death or the TTP or death.
Direct evidence suggests that panitumumab plus FOLFOX is more effective at increasing the TTP or death than FOLFOX and bevacizumab plus FOLFOX. Panitumumab plus FOLFOX was also estimated to be more effective at increasing the time to death than FOLFOX.
There was limited evidence to suggest that cetuximab plus FOLFOX is more effective than panitumumab plus FOLFOX at improving the ORR.
There was little evidence that cetuximab plus FOLFOX is associated with fewer AEs than panitumumab plus FOLFOX; however, some of these analyses were limited by the small number of events recorded in the treatment arms.
Network meta-analysis: FOLFIRI network
The evidence suggests that cetuximab plus FOLFIRI and bevacizumab plus FOLFIRI are more effective than FOLFIRI at increasing the TTP or death and the ORR.
Direct evidence suggests that cetuximab plus FOLFIRI is more effective than FOLFIRI and bevacizumab plus FOLFIRI at increasing the time to death.
Cost-effectiveness
Published economic evaluations
Of the 1979 references identified in the searches, four studies were included in the review: one full paper,107 two conference abstracts with accompanying posters108,109 and one conference abstract in which the accompanying poster could not be retrieved. 110
One study was UK based but compared only cetuximab plus chemotherapy with chemotherapy alone. 108 This study was reported only as a conference abstract and poster. As this study was related to a SMC appraisal, additional details were sought from the SMC report. 114
The full paper compared panitumumab in combination with FOLFOX with bevacizumab in combination with FOLFOX and was conducted in France107 and so the results are of limited generalisability to the UK. One other conference abstract also looked at this comparison, from the Greek health-care perspective. 110
The final abstract with accompanying poster looked at the RAS WT population only as a scenario analysis and was conducted from a health-care perspective. 109
As the majority of included studies were not published as full papers, the quality of the reporting was limited. One important note relating to the quality assessment was that all studies included at least one author who was employed by a manufacturer.
No studies completely answered the decision problem and, as such, highlighted the need for a de novo cost-effectiveness model.
Critique of the company submission
Amgen did not submit an economic evaluation.
Merck Serono conducted a cost-effectiveness review and two executable models: one for the overall RAS WT population and one for a liver-limited disease subgroup. As Merck Serono sent us their liver subgroup model very late in the review period, and as we were unable to reconcile the subgroup analysis with the overall population model, we did not critique this subgroup analysis.
The model was generally poorly reported. There were several discrepancies between the parameters in the report and the parameters in the model, and the sources of some parameters could not be identified. A second iteration of the overall population model and report were received to solve discrepancies in the results reported in the first submission.
Merck Serono estimated the ICERs for the two key comparisons:
-
cetuximab plus FOLFOX compared with FOLFOX – £47,000 per QALY
-
cetuximab plus FOLFIRI compared with FOLFIRI – £56,000 per QALY.
The model itself contained some minor errors and inconsistencies, but no major wiring errors were identified.
We are satisfied with the general structure of and the great majority of the parameter values in Merck Serono’s model. However, we disagree with several of the parameters used, which is discussed elsewhere.
Independent economic assessment
In the independent economic assessment the ICERs for anti-EGFR therapy compared with chemotherapy alone were all > £100,000 per QALY gained. In the FOLFOX network, panitumumab plus FOLFOX was extendedly dominated by cetuximab plus FOLFOX compared with FOLFOX as it had fewer QALY gains compared with FOLFOX and higher ICERs. In general, there was a survival gain for patients on anti-EGFR therapy, of 0.46 undiscounted life-years gained in the FOLFIRI arm and ranging from 0.22 to 0.55 undiscounted life-years gained in the FOLFOX arm. This benefit remained in the QALY results: 0.15–0.35 QALYs gained in the FOLFOX network and 0.30 QALYs gained in the FOLFIRI network for anti-EGFR therapies. However, the additional costs were substantial: >£35,000 for all anti-EGFR therapies compared with FOLFOX or FOLFIRI.
The probabilistic sensitivity analyses suggested that anti-EGFR therapies are unlikely to be cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained. In the FOLFOX network, FOLFOX was 78% likely to be the most cost-effective treatment, cetuximab plus FOLFOX was 22% likely to be the most cost-effective treatment and panitumumab plus FOLFOX was 0% likely to be the most cost-effective treatment. Similarly, in the FOLOFIRI network, FOLFIRI was 100% likely to be the most cost-effective treatment and cetuximab plus FOLFIRI was 0% likely to be the most cost-effective treatment.
Deterministic sensitivity analyses showed that cost-effectiveness was very sensitive to resection rates, PFS and OS post resection, PFS for unresected patients and treatment duration. Cost-effectiveness was quite sensitive to discounting and the cost of administering first-line therapies. Other parameters had little impact on cost-effectiveness.
In subgroup analyses, the ICERs for patients with liver metastases only for anti-EGFR therapies compared with chemotherapy alone improved to £90,000–£104,000 per QALY gained in the FOLFOX network and £107,000 per QALY gained in the FOLFIRI network. However, because of the higher uncertainty in this subgroup (effectiveness estimates based on smaller sample sizes), the probabilistic sensitivity analyses demonstrated that anti-EGFR therapy is unlikely to be cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained: in the FOLFOX network, FOLFOX was 98% likely to be the most cost-effective treatment and in the FOLFIRI network FOLFIRI was 100% likely to be the most cost-effective treatment.
When bevacizumab was considered as a comparator it was found to not be cost-effective at a willingness-to-pay threshold of £30,000 per QALY: bevacizumab plus FOLFOX was dominated by FOLFOX (fewer QALYs and higher costs) and the ICER for cetuximab plus FOLFIRI compared with bevacizumab plus FOLFIRI was much higher than the ICER for cetuximab plus FOLFIRI compared with FOLFIRI.
When XELOX was considered as a comparator, the ICERs for panitumumab plus FOLFOX and cetuximab plus FOLFOX increased. This was because of the lower cost of XELOX compared with FOLFOX.
Strengths and limitations
Systematic review of effectiveness studies
A strength of this report is that a systematic review of RCTs for cetuximab and panitumumab in people with mCRC with RAS WT tumours and a NMA were conducted to evaluate relative efficacy. In the absence of head-to-head RCTs, a NMA was conducted to assess the relative efficacy of panitumumab in combination with chemotherapy and cetuximab in combination with chemotherapy.
However, there are some important sources of uncertainty that may have an impact on the conclusions.
-
Currently available data providing evidence for the effectiveness of cetuximab and panitumumab are taken from subgroups of protocol-defined trial populations. The rationale for this is based on developments in tumour biology research [i.e. research demonstrating an interaction between RAS and EGFR inhibitors (specifically the negative implications of RAS mutations on the effectiveness of EGFR inhibitors)]. Of note, the recent changes by the EMA to the licensed indications for cetuximab and panitumumab are based on these same subgroup data, and treatment effect estimates for both cetuximab and panitumumab are in the expected direction and consistent across trial populations.
-
Given the use of subgroup data all comparisons were made without protection by stratification/randomisation. Instead, allocation to subgroups was based on re-evaluating tumour samples from the KRAS WT exon 2 population for RAS status. Although this minimised the potential for ascertainment bias, there were missing data for some of the trials (either tumour samples were not evaluable for RAS status or the results were inconclusive). No significant imbalances between the trial populations was observed, minimising the potential for selection bias. Of note, none of the included subgroup analyses reported the results of a test for treatment interaction. Because of the retrospective nature of the RAS analysis, for some studies there was a low number of samples available for analysis, reducing the power of the studies to show statistical significance.
-
No evidence was identified to estimate the effectiveness of panitumumab plus FOLFIRI (licence approved for panitumumab plus FOLFIRI for the first-line treatment of adults with RAS WT mCRC in quarter 1, 2015).
-
The subgroup analyses all contributed to the NMA. However, it was not possible to construct a complete network and so two discrete networks were generated, one evaluating FOLFOX-containing chemotherapy regimens and the other comparing FOLFIRI-containing chemotherapy regimens. It was therefore not possible to make a comparison between FOLFOX-containing regimens and FOLFIRI-containing regimens.
-
Although there were some reporting omissions in the publications of the subgroup analyses, we were able to confirm estimates through other sources, for example through EMA reports or through the companies.
-
The time point at which the ORR was measured was unclear for all of the trials. The ORR was measured at either 6- or 8-week intervals (according to methods reported in the primary publications). Given this uncertainty, the results reported for the RAS WT population for this outcome should be treated with caution.
-
Sample sizes for the subgroup of the RAS WT population with liver metastases at baseline were small, increasing the level of uncertainty, lack of statistical power and limitations with regard to precision and validity. However, subgroup data were the only available data for this population. The effect estimates were consistent across all studies, although one trial, FIRE-3 (which contributed evidence for the effectiveness of cetuximab plus FOLFIRI compared with FOLFIRI), did not report data for all outcomes for this subgroup.
-
None of the included publications reported HRQoL estimates for the RAS WT population.
-
We are aware of other cetuximab trials, for example the COIN (Phase III trial comparing either COntinuous chemotherapy plus cetuximab or INtermittent chemotherapy with standard continuous palliative combination chemotherapy with oxaliplatin and a fluoropyrimidine in first line treatment of metastatic colorectal cancer) and NORDIC VII [FLOX (5-fluorouracil/folinic acid/oxaliplatin) in Combination with Cetuximab in First-line Treatment of Colorectal Cancer] trials, for which there are currently no RAS WT subgroup data available.
-
Data comparing cetuximab plus FOLFOX with panitumumab plus FOLFOX were available only from the NMA. The limitations regarding the data for the RAS WT population (see above) also apply to the NMA and, as such, the results should also be interpreted with caution.
-
The extent to which the results of included trials can provide a reasonable basis for generalisation to the population of people with mCRC in the UK NHS is unclear.
Economic model (Peninsula Technology Assessment Group)
Strengths
The PenTAG model is an independent model that was not sponsored by any of the manufacturers producing cetuximab or panitumumab. We used up-to-date clinical effectiveness data to populate the model, which were acquired through a systemic review of the current evidence.
Drug acquisition costs were obtained, when possible, from the CMU eMit database, which reflects the true cost to the NHS of acquiring these drugs, as it includes discounts obtained by hospital pharmacies. For other drugs the list price from the BNF was used, as in the NICE reference case. 128
We explored areas of uncertainty through scenario analyses and sensitivity analyses (deterministic and probabilistic). Although the ICERs for anti-EGFR therapies compared with chemotherapy alone altered quite substantially in some analyses, none fell below a willingness-to-pay threshold of £20,000 per QALY gained.
Limitations
The model is subject to the same limitations as the clinical effectiveness review as these limitations were carried through into the modelling
Similarly, when data were unavailable directly from trials, assumptions were made to inform the model, leading to areas of uncertainty, which are discussed in the following section.
Areas of uncertainty
The evidence was poor for estimating the accuracy (correct diagnoses) and effectiveness (how the diagnosis influences the treatment effectiveness) of companion diagnostic testing for testing RAS mutation status, with no trials presenting the effectiveness of treatment following diagnosis for all tests used in clinical practice. We assumed, from the evidence available, that this is the same in practice as it is in the trials, but this may not be true and would likely result in lower effectiveness for cetuximab and panitumumab in practice.
Some drugs (those for which the BNF price was used) may be obtained at lower costs than were assumed in the model because of locally procured discounts. There is no indication what these costs might be and the NICE reference case has been adhered to in this regard.
It was assumed that fortnightly cetuximab is used in the NHS as this is believed to be current clinical practice and is less costly and burdensome for patients. It was assumed that clinical effectiveness would be unchanged between weekly and fortnightly cetuximab on the basis of a single non-inferiority trial. It remains possible that there is, in fact, a difference in effectiveness between the schedules, although on the basis of the current evidence there is unlikely to be a substantial difference. This also adds complexity to the decision process, as to achieve the ICER reported in the PenTAG base case might require NICE to issue guidance outside the current marketing authorisation
The PFS data for first-line treatment are of high quality as they are taken directly from RCTs, but it should be noted that the evidence for cetuximab plus FOLFOX is not as strong as that for panitumumab plus FOLFOX, as the OPUS trial of cetuximab plus FOLFOX compared with FOLFOX had far fewer RAS WT patients (n = 87) than the PRIME trial of panitumumab plus FOLFOX compared with FOLFOX (n = 512). This is demonstrated in the probabilistic sensitivity analysis, in which the cetuximab plus FOLFOX compared with FOLFOX results are much more uncertain than the panitumumab plus FOLFOX compared with FOLFOX results.
As there were two trials on which to base the effectiveness of FOLFOX, one had to be chosen for the base case. Because of its larger size, we based our effectiveness estimates for FOLFOX on the PRIME trial. In the scenario analysis, in which the effectiveness estimates for FOLFOX were based on the OPUS trial, the ICERs for panitumumab plus FOLFOX compared with FOLFOX decreased substantially, particularly for the liver metastases subgroup.
We adjusted the PFS from the RCTs of first-line drugs by subtracting PFS for patients who were resected (see Chapter 6, Model parameters, First-line progression-free survival: unresected patients) to calculate PFS for unresected patients. As the underlying individual patient data from the RCTs were not available, this method is only approximate.
We estimated survival post resection from a study that is now several years old, in which no patients received either cetuximab or panitumumab. 113 It is therefore possible that survival post resection for patients initially treated with these drugs could differ from that reported by Adam et al. 113
Treatment effect from first-line drugs was assumed to stop following disease progression. This is because we did not model OS from the RCTs, only PFS. We explored the use of OS data from the RCTs in a scenario analysis. In this case, the ICER for cetuximab plus FOLFOX compared with FOLFOX significantly worsened, that for panitumumab plus FOLFOX compared with FOLFOX significantly improved and that for cetuximab plus FOLFIRI compared with FOLFIRI improved. These changes were driven by the treatment duration, which was now calculated directly from the RCTs.
For the liver metastases subgroup PFS was even more uncertain, as direct evidence was unavailable and so adjustments were made to PFS for all patients. Furthermore, we estimated PFS for unresected patients from PFS for resected plus unresected patients for the liver metastases subgroup using a different, and arguably less rigorous, method than was used for all patients.
Chapter 9 Conclusions
The clinical effectiveness evidence in this review suggests that there is some clinical benefit from anti-EGFR therapies in comparison with standard chemotherapy treatments and mixed clinical benefit from anti-EGFR therapies in comparison with anti-VEGF therapies. For example, direct evidence suggests that panitumumab plus FOLFOX is more effective at increasing TTP or death than FOLFOX and bevacizumab plus FOLFOX. Panitumumab plus FOLFOX is also estimated to be more effective at increasing time to death than FOLFOX. Evidence suggests that cetuximab plus FOLFIRI and bevacizumab plus FOLFIRI are more effective than FOLFIRI at increasing TTP or death and the ORR.
There is limited evidence to draw conclusions about which anti-EGFR therapy has most clinical benefit. There is no evidence to suggest that cetuximab plus FOLFOX is any more effective than panitumumab plus FOLFOX at increasing the time to death or the TTP or death and there is little evidence to suggest that cetuximab plus FOLFOX is more effective than panitumumab plus FOLFOX at improving the ORR or reducing the incidence of AEs.
Estimates of cost-effectiveness currently suggest poor value for money of anti-EGFR therapies at willingness-to-pay thresholds of £20,000–30,000. Our results indicate that the cost of drug acquisition and, to a lesser extent, the cost of drug administration drive this poor value for money. Probabilistic sensitivity analyses further demonstrate that anti-EGFR therapies are unlikely to be cost-effective at a willingness-to-pay threshold of £30,000 per QALY gained: for the FOLFOX network, FOLFOX has an 80% likelihood of being the most cost-effective treatment and, for the FOLFIRI network, FOLFIRI has a 100% likelihood of being the most cost-effective treatment.
In summary, there is the potential for clinical benefit from anti-EGFR therapies, but the cost of administering these therapies is substantial.
Implications for service provision
During this appraisal, both panitumumab and cetuximab were available on the CDF for first-line mCRC (correct as of the CDF updates, September 201550,186,187). As RAS WT status is a prerequisite for using cetuximab and panitumumab in this indication, RAS mutation testing was also funded through the CDF for many hospitals (Dr Mark Napier, 2015, personal communication). Therefore, both RAS mutation testing and cetuximab and panitumumab treatment were previously supported by the CDF. Were anti-EGFR therapies to be approved in NICE guidance, the implications for RAS mutation testing would have to be considered.
Bevacizumab, one of the named comparators in this analysis, became unavailable on the CDF during this appraisal and is not recommended by NICE for the first-line treatment of mCRC patients. As this is a relatively recent change, the proportion of patients who would have previously been considered for bevacizumab will now receive alternative treatment, which may have some impact on the proportion of patients tested for cetuximab and panitumumab treatment.
Suggested research priorities
-
There is uncertainty associated with drug administration costs for chemotherapy regimens. Given the significant number of technology appraisals in which parenteral chemotherapy is administered, a study to identify the most appropriate methods for costing drug administration in chemotherapy, considering microcosting and the use of NHS reference costs, could be justified.
-
We recommend that the economic analysis should be repeated when the PFS and OS data from the RCTs are more mature. Given sufficiently mature data, we would no longer need to use PFS and OS related to patients post resection, with all the associated uncertainty, as we do currently.
-
The RCTs of first-line drugs included subsequent treatments that are not widely used in the UK NHS. Therefore, the economic analysis would benefit from RCTs that use subsequent treatments that are in line with those widely used in the NHS. However, given the substantial costs of conducting trials, we appreciate that this is unlikely to happen.
-
Given the lack of data to suggest otherwise, we assumed the same accuracy of the RAS test in clinical practice as in the first-line RCTs. Any differences are likely to result in worse estimates of cost-effectiveness for cetuximab and panitumumab, as accuracy is more likely to be worse in practice than in the RCTs. Therefore, we would welcome further research into the relative accuracies of the tests as used in the trials and in clinical practice.
-
Our economic analysis was designed for the NHS in England and Wales; however, it could easily be adapted for the health-care systems of other countries.
-
Cetuximab plus FOLFOX, cetuximab plus FOLFIRI and panitumumab plus FOLFOX are all given intravenously. Our economic analysis suggests that the administration of these treatments is expensive and highlights that there is a strong economic incentive to develop oral treatments for mCRC. However, consideration would have to be given to adherence and safety when transferring the responsibility of delivering the regimen to the patient population.
-
The cost-effectiveness of treatments for the liver metastases subgroup is very uncertain, partly because of the small numbers of patients in the trials. Therefore, if there is further interest in providing these treatments to this subgroup of patients, a larger quantity of better-quality evidence is needed.
Acknowledgements
The authors are pleased to acknowledge Mrs Sue Whiffin and Ms Jenny Lowe, who provided administrative support; Mr Christopher Bowles (Royal Devon and Exeter Hospital), Mr Neil Atkey (Sheffield Children’s NHS Foundation Trust), Dr Michelle Wood (Institute of Medical Genetics, University Hospital of Wales) and Dr Marie Westwood (Kleijnen Systematic Reviews Ltd), who provided information about RAS mutation testing; Dr Sandi Deans [UK National External Quality Assessment Service (NEQAS)], who contacted UK genetics laboratories on our behalf; Dr Paul Tappenden (University of Sheffield), who commented on the draft report; and Professor Chris Hyde, who commented on the draft report and provided senior management support.
The Peninsula Technology Assessment Group is part of the Evidence Synthesis and Modelling for Health Improvement group based at the University of Exeter Medical School. PenTAG was established in 2000 and carries out independent HTAs for the National Institute for Health Research (NIHR) HTA programme, systematic reviews and economic analyses for other local and national decision-makers. The group is multidisciplinary and draws on individuals’ backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics and health economics. The Institute of Health Research (University of Exeter) is made up of discrete but methodologically related research groups, among which HTA is a strong and recurring theme.
Health technology assessment projects in 2014/15 included:
-
immunosuppressive therapy for kidney transplantation in children (review of TA99)
-
immunosuppressive therapy for kidney transplantation in adults (review of TA85)
-
ofatumumab in combination with chlorambucil or bendamustine for previously untreated chronic lymphocytic leukaemia
-
obinutuzumab for previously untreated chronic lymphocytic leukaemia
-
the effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of TA142): a systematic review and economic model
For a full list of previous projects please see http://medicine.exeter.ac.uk/esmi/workstreams/pentaghealthtechnologyassessment/ (accessed 19 March 2017).
Contributions of authors
Nicola Huxley (Research Fellow, Health Economic Modelling) provided project management (February 2015 to August 2015), led the review of published cost-effectiveness studies and the critique of the submission by Merck Serono, contributed to the parameterisation and checking of the PenTAG independent economic assessment, and contributed to the writing and editing of the report.
Louise Crathorne (Research Fellow, Systematic Review) provided project management (November 2014 to February 2015), led the systematic review of clinical effectiveness, including the assessment of all abstracts and titles for possible inclusion, wrote the background, decision problem and clinical effectiveness sections, and contributed to the writing and editing of other sections of the report.
Jo Varley-Campbell (Research Fellow, Systematic Review) screened titles, abstracts and papers for inclusion in the systematic review of clinical effectiveness, contributed to the writing of the clinical effectiveness section and corresponding sections within the executive summary, discussion and appendices, and contributed to the editing of the report.
Irina Tikhonova (Associate Research Fellow, Health Economic Modelling) contributed to the critique of the submission by Merck Serono, the parameterisation and checking of the PenTAG independent economic assessment, and the writing and editing of the report.
Tristan Snowsill (Research Fellow, Health Economic Modelling) contributed to the critique of the submission by Merck Serono, the parameterisation and checking of the PenTAG independent economic assessment, and the writing and editing of the report.
Simon Briscoe (Information Specialist, Information Science) designed and carried out the literature searches for the systematic reviews and identification of model parameters, and contributed to the writing and editing of the report.
Jaime Peters (Senior Research Fellow, Evidence Synthesis) carried out the NMA and contributed to the writing of the clinical effectiveness section.
Mary Bond (Senior Research Fellow, Systematic Review) screened titles, abstracts and papers for inclusion in the systematic review and commented on the draft report.
Mark Napier (Clinical Advisor, Oncology) provided clinical input into the design of the model and advised on clinical matters.
Martin Hoyle (Associate Professor, Health Economics) led the design and parameterisation of the PenTAG economic model and implemented the model in Microsoft Excel, wrote the sections on the design, parameterisation and results of the economic model, contributed to the critique of the submission by Merck Serono, contributed to the writing and editing of the report, and was overall director and guarantor of the report.
Data sharing statement
The trial data reported in this review were not produced by the Assessment Group and therefore we do not have ownership of these data. We are happy to consider releasing copies of our economic models of health technologies to not-for-profit organisations within the UK or overseas, such as other universities or government agencies.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Colorectal Cancer: Diagnosis and Management. London: NICE; 2011.
- NICE Pathways: Staging Colorectal Cancer. London: NICE; 2015.
- Bowel Cancer Incidence Statistics. London: Cancer Research UK; 2011.
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines Working Group . Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii1-9. https://doi.org/10.1093/annonc/mdu260.
- Bowel Cancer Mortality Statistics. London: Cancer Research UK; 2012.
- Bowel Cancer Survival Statistics. London: Cancer Research UK; 2011.
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7. https://doi.org/10.1055/s-0029-1242458.
- Stewart BW, Wild CP. World Cancer Report. Geneva: WHO; 2014.
- Gill S, Berry S, Biagi J, Butts C, Buyse M, Chen E, et al. Progression-free survival as a primary endpoint in clinical trials of metastatic colorectal cancer. Curr Oncol 2011;18:5-10. https://doi.org/10.3747/co.v18iS2.958.
- Cetuximab for the First-line Treatment of Metastatic Colorectal Cancer. London: NICE; 2009.
- Panitumumab in Combination with Chemotherapy for the Treatment of Metastatic Colorectal Cancer (Terminated Appraisal). London: NICE; 2011.
- Bevacizumab in Combination with Oxaliplatin and Either Fluorouracil Plus Folinic Acid or Capecitabine for the Treatment of Metastatic Colorectal Cancer. London: NICE; 2010.
- Colorectal Cancer Overview: Managing Advanced and Metastatic Colorectal Cancer. London: NICE; 2015.
- Final Scope: Cetuximab (Review of TA176) and Panitumumab (Partial Review of TA240) for the First Line Treatment of Metastatic Colorectal Cancer. London: NICE; 2014.
- Guidance on Cancer Services. Improving Outcomes in Colorectal Cancers. Manual Update. London: NICE; 2004.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Chuang VT, Suno M. Levoleucovorin as replacement for leucovorin in cancer treatment. Ann Pharmacother 2012;46:1349-57. http://dx.doi.org/10.1345/aph.1Q677.
- Cancer Drugs Fund Decision Summary: Bevacizumab in Combination with 1st Line Single Agent Fluoropyrimidine-based Chemotherapy for Metastatic Colorectal Cancer. London: NHS England; 2015.
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003;3:11-22. http://dx.doi.org/10.1038/nrc969.
- Goodsell DS. The molecular perspective: the ras oncogene. Oncologist 1999;4:263-4.
- Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer 2006;94:184-8. https://doi.org/10.1038/sj.bjc.6602941.
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535-46. http://dx.doi.org/10.1093/annonc/mdq632.
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. http://dx.doi.org/10.1200/JCO.2008.20.8397.
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. http://dx.doi.org/10.1056/NEJMoa0805019.
- Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-19. http://dx.doi.org/10.1200/JCO.2010.33.5091.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346-55. http://dx.doi.org/10.1093/annonc/mdu141.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. http://dx.doi.org/10.1200/JCO.2009.27.4860.
- Heinemann V, Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. https://doi.org/10.1016/S1470-2045(14)70330-4.
- Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240-7. http://dx.doi.org/10.1200/JCO.2013.53.2473.
- Cetuximab (Erbitux): Summary of Opinion (Post Authorisation). London: EMA; 2008.
- Cetuximab (Erbitux): Summary of Opinion (Post Authorisation). London: EMA; 2011.
- Panitumumab (Vectibix): Summary of Opinion (Post Authorisation). London: EMA; 2011.
- Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. J Clin Oncol 2014;32.
- Ciardiello F, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Treatment outcome according to tumor RAS mutation status in CRYSTAL study patients with metastatic colorectal cancer (mCRC) randomized to FOLFIRI with/without cetuximab. J Clin Oncol 2014;32.
- EMA . Summary of Product Characteristics: Erbitux (Cetuximab) 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000558/human_med_000769.jsp&mid=WC0b01ac058001d124 (accessed 8 May 2017).
- Summary of Product Characteristics: Vectibix (Panitumumab). London: EMA; 2014.
- Cetuximab (Erbitux): Summary of Opinion (Post Authorisation). London: EMA; 2013.
- Panitumumab (Vectibix). Summary of Opinion (Post Authorisation). London: EMA; 2013.
- Cetuximab (Erbitux) Assessment Report (Variation Assessment Report; EMEA/h/C/000558/II/0062). London: EMA; 2013.
- Panitumumab (Vectibix) Assessment Report (Variation Assessment Report; EMEA/H/C/000741/II/0050). London: EMA; 2013.
- Parsons BL, Marchant-Miros KE, Delongchamp RR, Verkler TL, Patterson TA, McKinzie PB, et al. ACB-PCR quantification of K-RAS codon 12 GAT and GTT mutant fraction in colon tumor and non-tumor tissue. Cancer Invest 2010;28:364-75. http://dx.doi.org/10.3109/07357901003630975.
- Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol 2015;26:13-21. http://dx.doi.org/10.1093/annonc/mdu378.
- Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692-700. http://dx.doi.org/10.1200/JCO.2014.59.4812.
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. http://dx.doi.org/10.1056/NEJMoa1305275.
- Westwood M, van Asselt T, Ramaekers B, Whiting P, Joore M, Armstrong N, et al. KRAS mutation testing of tumours in adults with metastatic colorectal cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18620.
- Wong NA, Gonzalez D, Salto-Tellez M, Butler R, Diaz-Cano SJ, Ilyas M, et al. RAS testing of colorectal carcinoma – a guidance document from the Association of Clinical Pathologists Molecular Pathology and Diagnostics Group. J Clin Pathol 2014;67:751-7. https://doi.org/10.1136/jclinpath-2014-202467.
- KRAS and NRAS StripAssays®. Vienna: ViennaLab; 2014.
- PNAClamp™ KRAS Mutation Detection Kit. Daejeon: Panagene; 2014.
- KRAS Mutation Testing of Tumours in Adults with Metastatic Colorectal Cancer (Discontinued). London: NICE; 2014.
- Cancer Drugs Fund Decision Summary: Cetuximab in Combination with 1st Line Irinotecan-based Chemotherapy for Metastatic Colorectal Cancer in Patients with RAS Wild Type (Nonmutated) Tumours. London: NHS England; 2015.
- Cancer Drugs Fund Decision Summary: Panitumumab – Treatment of Adult Patients with Wild-type RAS (KRAS and NRAS) Metastatic Colorectal Cancer (mCRC) in First-line in Combination with FOLFOX. London: NHS England; 2014.
- Cetuximab (Erbitux) Assessment Report (Variation Assessment Report; EMEA/H/C/000558/II/0020). London: EMA; 2008.
- Cetuximab (Erbitux) Assessment Report (Variation Assessment Report; EMEA/H/C/000558/II/0042). London: EMA; 2011.
- Final Scope: Cetuximab for the First-line Treatment of Metastatic Colorectal Cancer. London: NICE; 2007.
- Panitumumab (Vectibix) Assessment Report (Variation Assessment Report; EMEA/H/C/000741/II/0017). London: EMA; 2011.
- Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS. Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 2011;50:307-12. http://dx.doi.org/10.1002/gcc.20854.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. York: CRD, University of York; 2009.
- Kalow W, Gunn DR. Some statistical data on atypical cholinesterase of human serum. Ann Hum Genet 1959;23:239-50. https://doi.org/10.1111/j.1469-1809.1959.tb01467.x.
- Evans DA, Manley KA, McKusick VA. Genetic control of isoniazid metabolism in man. Br Med J 1960;2:485-91. https://doi.org/10.1136/bmj.2.5197.485.
- Weinshilboum R. Inheritance and drug response. N Engl J Med 2003;348:529-37. http://dx.doi.org/10.1056/NEJMra020021.
- Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 2001;286:2270-9. https://doi.org/10.1001/jama.286.18.2270.
- Shankaran V, Obel J, Benson AB. Predicting response to EGFR inhibitors in metastatic colorectal cancer: current practice and future directions. Oncologist 2010;15:157-67. http://dx.doi.org/10.1634/theoncologist.2009-0221.
- Shaib W, Mahajan R, El-Rayes B. Markers of resistance to anti-EGFR therapy in colorectal cancer. J Gastrointest Oncol 2013;4:308-18. http://dx.doi.org/10.3978/j.issn.2078-6891.2013.029.
- Er TK, Chen CC, Bujanda L, Herreros-Villanueva M. Current approaches for predicting a lack of response to anti-EGFR therapy in KRAS wild-type patients. Biomed Res Int 2014;2014. http://dx.doi.org/10.1155/2014/591867.
- Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015;51:1243-52. http://dx.doi.org/10.1016/j.ejca.2015.04.007.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. Sheffield: NICE Decision Support Unit; 2014.
- Peeters M, Tabernero J, Douillard JY, Siena S, Davison C, Braun S, et al. Resection rates and survival in patients with wild-type KRAS/NRAS metastatic colorectal cancer and liver metastases: data from the PRIME study. Eur J Cancer 2013;49:S17-18.
- Douillard JY, Tabernero J, Siena S, Peeters M, Koukakis R, Terwey JH, et al. Survival outcomes in patients (pts) with KRAS/NRAS (RAS) wild-type (WT) metastatic colorectal cancer (mCRC) and non-liver-limited disease (non-LLD): data from the PRIME study. J Clin Oncol 2014;32.
- Siena S, Tabernero J, Bodoky G, Cunningham D, Rivera F, Ruff P, et al. Quality of life (QoL) during first-line treatment with FOLFOX4 with or without panitumumab (pmab) in RAS wild-type (WT) metastatic colorectal carcinoma (mCRC). J Clin Oncol 2015;33. https://doi.org/10.1200/jco.2015.33.3_suppl.693.
- Wang J, Dong J, Johnson P, Maglinte GA, Rong A, Barber BL, et al. Quality-adjusted survival in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC) receiving first-line therapy with panitumumab plus FOLFOX versus FOLFOX alone in the PRIME trial. J Clin Oncol 2015;33. https://doi.org/10.1200/jco.2015.33.3_suppl.537.
- Rivera F, Karthaus M, Hecht JR, Fasola G, Re Canon J-L, Koukakis R, et al. First-line treatment with modified FOLFOX6 (mFOLFOX6) + panitumumab (pmab) or bevacizumab (bev) in wild-type (WT) RAS metastatic colorectal carcinoma (mCRC): tumor response outcomes beyond RECIST. J Clin Oncol 2015;33. https://doi.org/10.1200/jco.2015.33.3_suppl.660.
- WHO Handbook for Reporting Results of Cancer Treatment. Geneva: WHO; 1979.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
- Cancer Therapy Evaluation Program . Common Terminology Criteria for Adverse Events, Version 3.0 2003. http://ctep.cancer.gov (accessed 31 May 2015).
- Abad A, Massuti B, Escudero P, Guillen-Ponce C, Layos L, Safont MJ, et al. Panitumumab plus FOLFOX-4 or pantiumumab plus FOLFIRI in subjects with wild-type KRAS (exon 2) colorectal cancer and multiple or unresectable liver-limited metastases: data from the randomized, phase II PLANET study. Ann Oncol 2014;25:ii5-13.
- Peeters M, Oliner K, Price TJ, Cervantes A, Sobrero A, Ducreux M, et al. Analysis of KRAS/NRAS mutations in phase 3 study 20050181 of panitumumab (pmab) plus FOLFIRI versus FOLFIRI for second-line treatment (tx) of metastatic colorectal cancer (mCRC). J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.3_suppl.lba387.
- Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569-79. http://dx.doi.org/10.1016/S1470-2045(14)70118-4.
- Badulescu F, Badulescu A, Schenker M, Ionescu M, Ninulescu C, Crisan A, et al. FOLFOX-4 versus FOLFIRI in the treatment of metastatic colorectal cancer – a prospective randomised study. Joint ECCO 15-34th ESMO Multidisciplinary Congress; 2009; Berlin, Germany. Eur J Cancer 2009;7. https://doi.org/10.1016/S1359-6349(09)71184-X.
- Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer 2011;105:58-64. http://dx.doi.org/10.1038/bjc.2011.201.
- Comella P, Massidda B, Filippelli G, Farris A, Natale D, Barberis G, et al. Randomised trial comparing biweekly oxaliplatin plus oral capecitabine versus oxaliplatin plus i.v. bolus fluorouracil/leucovorin in metastatic colorectal cancer patients: results of the Southern Italy Cooperative Oncology study 0401. J Cancer Res Clin Oncol 2009;135:217-26. http://dx.doi.org/10.1007/s00432-008-0454-7.
- Ducreux M, Adenis A, Pignon JP, François E, Chauffert B, Ichanté JL, et al. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer 2013;49:1236-45. http://dx.doi.org/10.1016/j.ejca.2012.12.011.
- Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer 2011;128:682-90. http://dx.doi.org/10.1002/ijc.25369.
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 2008;26:3523-9. http://dx.doi.org/10.1200/JCO.2007.15.4138.
- Hong YS, Jung KH, Kim HJ, Kim KP, Kim SY, Lee JL, et al. Randomized phase II study of capecitabine with or without oxaliplatin as first-line treatment for elderly or fragile patients with metastatic colorectal cancer: a prospective, multicenter trial of the Korean Cancer Study Group CO06-01. Am J Clin Oncol 2013;36:565-71. http://dx.doi.org/10.1097/COC.0b013e31825d52d5.
- Karthaus M, Hecht J, Douillard J, Schwartzberg L, Siena S, Tabernero J, et al. An extended RAS analysis in patients with untreated metastatic colorectal cancer from the PRIME and PEAK studies. Virchows Archiv 2014;465:S228-9.
- Pectasides D, Papaxoinis G, Kalogeras KT, Eleftheraki AG, Xanthakis I, Makatsoris T, et al. XELIRI–bevacizumab versus FOLFIRI–bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis. BMC Cancer 2012;12. http://dx.doi.org/10.1186/1471-2407-12-271.
- Porschen R, Arkenau HT, Kubicka S, Greil R, Seufferlein T, Freier W, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol 2007;25:4217-23. https://doi.org/10.1200/JCO.2006.09.2684.
- Rosati G, Cordio S, Bordonaro R, Caputo G, Novello G, Reggiardo G, et al. Capecitabine in combination with oxaliplatin or irinotecan in elderly patients with advanced colorectal cancer: results of a randomized phase II study. Ann Oncol 2010;21:781-6. https://doi.org/10.1093/annonc/mdp359.
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-19. http://dx.doi.org/10.1200/JCO.2007.14.9930.
- Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol 2013;24:1580-7. https://doi.org/10.1093/annonc/mdt028.
- Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143-52. https://doi.org/10.1016/S0140-6736(07)61087-3.
- Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749-59. http://dx.doi.org/10.1016/S0140-6736(11)60399-1.
- Souglakos J, Ziras N, Kakolyris S, Boukovinas I, Kentepozidis N, Makrantonakis P, et al. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br J Cancer 2012;106:453-9. http://dx.doi.org/10.1038/bjc.2011.594.
- Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. A randomized phase III trial of mFOLFOX6 plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment for metastatic colorectal cancer: West Japan Oncology Group study 4407G (WJOG4407G). J Clin Oncol 2014;32.
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. https://doi.org/10.1016/S0895-4356(97)00049-8.
- Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1147.
- Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1-19. https://doi.org/10.2165/00019053-200624010-00001.
- Tejpar S, Lenz H-J, Kohne C-H, Heinemann V, Ciardiello F, Esser R, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX-4: new results from the OPUS study. J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.3_suppl.lba444.
- Heinemann V, Fischer Von Weikersthal L, Decker T, Kiani A, Verhling-Kaiser U, Al Batran S, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS-wildtype metastatic colorectal cancer: the FIRE-3 trial (AIO KRK 0307). Onkologie 2013;36.
- Stintzing S, Jung A, Rossius L, Modest DP, von Weikersthal LF, Decker T, et al. Mutations within the EGFR signaling pathway: influence on efficacy in FIRE-3 – a randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. J Clin Oncol 2014;32. https://doi.org/10.1200/jco.2014.32.3_suppl.445.
- Lenz HJ, Niedzwiecki D, Innocenti F. CALGB/SWOg 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (mCRC): expanded RAS analysis. Ann Oncol 2014;25. https://doi.org/10.1093/annonc/mdu438.13.
- Royle P, Waugh N. Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7340.
- Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866-75. https://doi.org/10.1200/JCO.2005.07.113.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer 2009;115:2081-91. http://dx.doi.org/10.1002/cncr.24246.
- Graham CN, Hechmati G, Hjelmgren J, de Liège F, Lanier J, Knox H, et al. Cost-effectiveness analysis of panitumumab plus mFOLFOX6 compared with bevacizumab plus mFOLFOX6 for first-line treatment of patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer 2014;50:2791-801. http://dx.doi.org/10.1016/j.ejca.2014.08.016.
- Jarrett J, Ovcinnikova O, Hnoosh A, Harty G, Byrne B, von Hohnhorst P. Cost effectiveness of cetuximab in first-line treatment of RAS wild-type metastatic colorectal cancer in Scotland: a summary of the submission to the Scottish Medicines Consortium. Value Health 2014;17. http://dx.doi.org/10.1016/j.jval.2014.08.2296.
- Ortendahl JD, Bentley TG, Anene AM, Purdum AG, Bolinder B. Cost-effectiveness of cetuximab as first-line treatment for metastatic colorectal cancer in the United States. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.03.500.
- Kourlaba G, Boukovinas I, Saridaki Z, Papagiannopoulou V, Tritaki G, Maniadakis N. Cost-effectiveness analysis of panitumumab + mFOLFOX over bevacizumab + mFOLFOX as a first-line treatment for metastatic colorectal cancer patients with wild-type RAS in Greece. Value Health 2014;17. http://dx.doi.org/10.1016/j.jval.2014.08.2268.
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. http://dx.doi.org/10.1200/JCO.2004.05.113.
- Jonker DJ, Karapetis C, Harbison C, O’Callaghan CJ, Tu D, Simes RJ, et al. High epiregulin (EREG) gene expression plus K-ras wild-type (WT) status as predictors of cetuximab benefit in the treatment of advanced colorectal cancer (ACRC): results from NCIC CTG CO.17 – A phase III trial of cetuximab versus best supportive care (BSC). J Clin Oncol 2009;27.
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57. https://doi.org/10.1097/01.sla.0000141198.92114.f6.
- Cetuximab, 100mg/20mL and 500mg/100mL Solution for Intravenous Infusion (Erbitux®). Glasgow: SMC; 2010.
- Bennett L, Zhao Z, Barber B, Zhou X, Peeters M, Zhang J, et al. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. J Clin Oncol 2011;29. https://doi.org/10.1200/jco.2011.29.15_suppl.e19500.
- Wang J, Zhao Z, Sherrill B, Peeters M, Wiezorek J, Barber B. A Q-twist analysis comparing panitumumab plus best supportive care (BSC) with BSC alone in patients with wild-type KRAS metastatic colorectal cancer. Value Health 2011;14. https://doi.org/10.1016/j.jval.2011.02.941.
- Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ 2005;14:1169-89. http://dx.doi.org/10.1002/hec.1006.
- Cetuximab, 100mg/20mL and 500mg/100mL Solution for Infusion (Erbitux®). SMC No. (1012/14). Glasgow: SMC; 2015.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Cassidy J, Clarke S, Rubio ED, Scheithauer W, Figer A, Wong R, et al. First efficacy and safety results from XELOX-1/NO16966, a randomised 2 × 2 factorial phase III trial of XELOX vs. FOLFOX4+bevacizumab or placebo in first-line metastatic colorectal cancer (MCRC). Ann Oncol 2006;17.
- Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. XELOX compared to FOLFOX4: survival and response results from XELOX-1/ NO16966, a randomized phase III trial of first-line treatment for patients with metastatic colorectal cancer (MCRC). J Clin Oncol 2007;25.
- Douillard JY, Bennouna J, Senellart H. Is XELOX equivalent to FOLFOX or other continuous-infusion 5-fluorouracil chemotherapy in metastatic colorectal cancer?. Clin Colorectal Cancer 2008;7:206-11. http://dx.doi.org/10.3816/CCC.2008.n.029.
- Hochster H, Hart L, Ramanathan R, Childs BH, Hainsworth JD, Cohn AL, et al. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): final results of the TREE-study. J Clin Oncol 2006;24.
- Ducreux M, Bennouna J, Hebbar M, . Efficacy and safety findings from a randomized Phase III study of capecitabine + oxaliplatin vs. infusional 5-FU/acid folinic + oxaliplatin (FOLFOX6) as first-line treatment for metastatic colorectal cancer (MCRC). J Clin Oncol 2007;25.
- Díaz-Rubio E, Tabernero J, Gómez-España A, Massutí B, Sastre J, Chaves M, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumor trial. J Clin Oncol 2007;25:4224-30. https://doi.org/10.1200/JCO.2006.09.8467.
- Comella P, Massidda B, Filippelli G, Farris A, Natale D, Maiorino L, et al. Capecitabine or Fluorouracil/Folinic Acid IV Bolus plus Eloxatin Evaluation (COFFEE trial) in metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Group (SICOG) Phase III trial 0401. Eur J Cancer 2007;5:248-9. https://doi.org/10.1016/S1359-6349(07)70972-2.
- NICE . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9 (accessed 13 March 2017).
- Köhne C-H, Ciardiello F, Ronga P, Beier F, Van Cutsem E. FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic disease: a subgroup analysis of the CRYSTAL study. Ann Oncol 2014;25.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. http://dx.doi.org/10.1016/j.jval.2010.10.029.
- Health Survey for England 2012. London: NHS Digital; 2013.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Department of Health . NHS Reference Costs 2012–2013 2013. www.gov.uk/government/publications/nhs-reference-costs-2012-to-2013 (accessed 10 May 2017).
- NHS Reference Costs 2013 to 2014. London: Department of Health; 2014.
- Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer 2008;62:374-80. http://dx.doi.org/10.1016/j.lungcan.2008.03.019.
- Tolley K, Goad C, Yi Y, Maroudas P, Haiderali A, Thompson G. Utility elicitation study in the UK general public for late-stage chronic lymphocytic leukaemia. Eur J Health Econ 2013;14:749-59. http://dx.doi.org/10.1007/s10198-012-0419-2.
- Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-84.
- Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer 2006;95:683-90. https://doi.org/10.1038/sj.bjc.6603326.
- NICE . Cetuximab, Bevacizumab and Panitumumab for the Treatment of Metastatic Colorectal Cancer After First-Line Chemotherapy: Cetuximab (Monotherapy or Combination Chemotherapy), Bevacizumab (in Combination With Non-Oxaliplatin Chemotherapy) and Panitumumab (Monotherapy) for the Treatment of Metastatic Colorectal Cancer After First-Line Chemotherapy n.d. www.nice.org.uk/guidance/ta242 (accessed 20 March 2017).
- Department of Health Commercial Medicines Unit . Drugs and Pharmaceutical Electronic Market Information (eMit) n.d. www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit (accessed 20 May 2015).
- Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, et al. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal no.150 and part review of technology appraisal no. 118): a systematic review and economic model. Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17140.
- Sacco JJ, Botten J, Macbeth F, Bagust A, Clark P. The average body surface area of adult cancer patients in the UK: a multicentre retrospective study. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0008933.
- Freeman K, Connock M, Cummins E, Gurung T, Taylor-Phillips S, Court R, et al. Fluorouracil Plasma Monitoring: the My5-FU Assay for Guiding Dose Adjustment in Patients Receiving Fluorouracil Chemotherapy by Continuous Infusion. Coventry: Warwick Evidence; 2014.
- Meads C, Round J, Tubeuf S, Moore D, Pennant M, Bayliss S. Cetuximab for the first-line treatment of metastatic colorectal cancer. Health Technol Assess 2010;14:1-8. https://doi.org/10.3310/hta14suppl1/01.
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. https://doi.org/10.1200/JCO.2009.27.6055.
- Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. https://doi.org/10.1200/JCO.2006.09.6305.
- Obinutuzumab in Combination with Chlorambucil for Untreated Chronic Lymphocytic Leukaemia. London: NICE; 2015.
- Folprecht G, Gruenberger T, Bechstein WO, Raab H-R, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47. https://doi.org/10.1016/S1470-2045(09)70330-4.
- Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure?. J Clin Oncol 2009;27:1829-35. http://dx.doi.org/10.1200/JCO.2008.19.9273.
- Adam R, De Gramont A, Figueras J, Guthrie A, Kokudo N, Kunstlinger F, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012;17:1225-39.
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-139.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-9.
- Wan X, Peng L, Li Y. A review and comparison of methods for recreating individual patient data from published Kaplan–Meier survival curves for economic evaluations: a simulation study. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0121353.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. http://dx.doi.org/10.1002/sim.1203.
- Papaioannou D, Brazier JE, Paisley S. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature 2011. www.nicedsu.org.uk (accessed 20 June 2015).
- Láng I, Köhne CH, Folprecht G, Rougier P, Curran D, Hitre E, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013;49:439-48. http://dx.doi.org/10.1016/j.ejca.2012.08.023.
- Odom D, Barber B, Bennett L, Peeters M, Zhao Z, Kaye J, et al. Health-related quality of life and colorectal cancer-specific symptoms in patients with chemotherapy-refractory metastatic disease treated with panitumumab. Int J Colorectal Dis 2011;26:173-81. https://doi.org/10.1007/s00384-010-1112-5.
- Lawrence D, Maschio M, Leahy KJ, Yunger S, Easaw JC, Weinstein MC. Economic analysis of bevacizumab, cetuximab, and panitumumab with fluoropyrimidine-based chemotherapy in the first-line treatment of KRAS wild-type metastatic colorectal cancer (mCRC). J Med Econ 2013;16:1387-98. http://dx.doi.org/10.3111/13696998.2013.852097.
- Ewara EM, Zaric GS, Welch S, Sarma S. Cost-effectiveness of first-line treatments for patients with KRAS wild-type metastatic colorectal cancer. Curr Oncol 2014;21:e541-50. http://dx.doi.org/10.3747/co.21.1837.
- Petrou S, Campbell N. Stabilisation in colorectal cancer. Int J Palliative Nurs 1997;3:275-80.
- Kim SH, Jo MW, Kim HJ, Ahn JH. Mapping EORTC QLQ-C30 onto EQ-5D for the assessment of cancer patients. Health Qual Life Outcomes 2012;10. http://dx.doi.org/10.1186/1477-7525-10-151.
- Färkkilä N, Sintonen H, Saarto T, Järvinen H, Hänninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Dis 2013;15:e215-22. http://dx.doi.org/10.1111/codi.12143.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- CCEMG and EPPI-Centre . CCEMG – EPPI-Centre Cost Converter Version 1.5 2014. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed 29 June 2015).
- Brodowicz T, Ciuleanu TE, Radosavljevic D, Shacham-Shmueli E, Vrbanec D, Plate S, et al. FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol 2013;24:1769-77. http://dx.doi.org/10.1093/annonc/mdt116.
- Hubbard JM, Alberts SR. Alternate dosing of cetuximab for patients with metastatic colorectal cancer. Gastrointest Cancer Res 2013;6:47-55.
- Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11120.
- Department of Health . NHS Reference Costs Collection Guidance for 2013 to 2014 n.d. www.gov.uk/government/publications/nhs-reference-costs-collection-guidance-for-2013-to-2014 (accessed 20 March 2017).
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- NHS Information Authority . HRG v3.5 Toolkit n.d. http://content.digital.nhs.uk/article/2310/HRG-v35-Toolkit (accessed 3 May 2017).
- National Casemix Office . HRG4+ 2013 14 Reference Costs Grouper n.d. http://content.digital.nhs.uk/article/6227/Costing (accessed 3 May 2017).
- Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 2008;22:2564-70. http://dx.doi.org/10.1007/s00464-008-0110-y.
- Wicherts DA, de Haas RJ, Salloum C, Andreani P, Pascal G, Sotirov D, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 2013;100:808-18. http://dx.doi.org/10.1002/bjs.9088.
- Kerr D, O’Connor K. An economic comparison of the net clinical benefit and treatment costs of raltitrexed and 5-fluorouracil + leucovorin (Mayo regimen) in advanced colorectal cancer. J Med Econ 1999;2:123-32. https://doi.org/10.3111/199902123132.
- Laparoscopic Liver Resection. London: NICE; 2005.
- Remák E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 2004;91:77-83. http://dx.doi.org/10.1038/sj.bjc.6601890.
- Färkkilä N, Torvinen S, Sintonen H, Saarto T, Järvinen H, Hänninen J, et al. Costs of colorectal cancer in different states of the disease. Acta Oncol 2015;54:454-62. http://dx.doi.org/10.3109/0284186X.2014.985797.
- Song X, Zhao Z, Barber B, Gregory C, Cao Z, Gao S. Cost of illness in patients with metastatic colorectal cancer. J Med Econ 2011;14:1-9. http://dx.doi.org/10.3111/13696998.2010.536870.
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. https://doi.org/10.1001/jama.280.19.1690.
- Harrow BS, Eaton CB, Roberts MB, Assaf AR, Luo X, Chen Z. Health utilities associated with hemoglobin levels and blood loss in postmenopausal women: the Women’s Health Initiative. Value Health 2011;14:555-63. http://dx.doi.org/10.1016/j.jval.2010.11.008.
- Crathorne L, Huxley N, Haasova M, Snowsill T, Jones-Hughes T, Hoyle M, et al. The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20130.
- Boyd KA, Briggs AH, Paul J, Iveson T, Midgely R, Harkin A, et al. Analysis of adverse events and quality of life data for an economic evaluation of adjuvant chemotherapy in colorectal cancer: when can we stop collecting?. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-S1-A41.
- Hogg K, Kimpton M, Carrier M, Coyle D, Forgie M, Wells P. Estimating quality of life in acute venous thrombosis. JAMA Intern Med 2013;173:1067-72. https://doi.org/10.1001/jamainternmed.2013.563.
- Twelves C, Boyer M, Findlay M, Cassidy J, Weitzel C, Barker C, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a Phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597-604. https://doi.org/10.1016/S0959-8049(00)00444-5.
- Cancer Drugs Fund Decision Summary: Cetuximab in Combination with 1st Line Oxaliplatin-Based Chemotherapy for Metastatic Colorectal Cancer in Patients with RAS Wild Type (Non-Mutated) Tumours. London: NHS England; 2015.
- Cancer Drugs Fund Decision Summary: Panitumumab in Combination with 1st Line Oxaliplatin-Based Chemotherapy for Metastatic Colorectal Cancer in Patients with RAS Wild Type (Non-Mutated) Tumours. London: NHS England; 2015.
- Salisbury NHS Foundation Trust . Wessex Regional Genetics Laboratory n.d. www.salisbury.nhs.uk/InformationForPatients/Departments/WRGL/Ourservices/Pages/Ourservices.aspx (accessed 26 June 2015).
- Blons H, Rouleau E, Charrier N, Chatellier G, Côté JF, Pages JC, et al. Performance and cost efficiency of KRAS mutation testing for metastatic colorectal cancer in routine diagnosis: the MOKAECM study, a nationwide experience. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0068945.
- Tack V, Ligtenberg MJ, Tembuyser L, Normanno N, Vander Borght S, Han van Krieken J, et al. External quality assessment unravels interlaboratory differences in quality of RAS testing for anti-EGFR therapy in colorectal cancer. Oncologist 2015;20:257-62. http://dx.doi.org/10.1634/theoncologist.2014-0382.
Appendix 1 Literature search strategies
Clinical effectiveness review
MEDLINE
Host: Ovid.
Data parameters: 1946 to November Week 3 2014.
Date searched: 5 January 2015.
Searcher: SB.
Hits: 447.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intestin*).tw.
-
(CRC or mCRC).tw.
-
exp Colorectal Neoplasms/
-
colon/
-
rectum/
-
or/4-8
-
(random* or rct* or ‘controlled trial*’ or ‘clinical trial*’).tw.
-
randomized controlled trial.pt.
-
10 or 11
-
3 and 9 and 12
-
limit 13 to english language
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 31 December 2014.
Date searched: 5 January 2015.
Searcher: SB.
Hits: 66.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
4 or 5
-
(random* or rct* or ‘controlled trial*’ or ‘clinical trial*’).tw.
-
3 and 6 and 7
EMBASE
Host: Ovid.
Data parameters: 1974 to 5 January 2015.
Date searched: 6 January 2015.
Searcher: SB.
Hits: 1948.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
cetuximab/
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
panitumumab/
-
or/1-4
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
exp colon/
-
exp colon tumor/
-
exp rectum/
-
exp rectum tumor/
-
or/6-11
-
(random* or rct* or ‘controlled trial*’ or ‘clinical trial*’).tw.
-
5 and 12 and 13
-
limit 14 to english language
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and Conference Proceedings Citation Index –Science (CPCI-S).
Date searched: 6 January 2015.
Searcher: SB.
Hits: 1093.
Search strategy
-
TITLE: (cetuximab or erbitux or C225 or ‘IMC C225’) OR TOPIC: (cetuximab or erbitux or C225 or ‘IMC C225’)
-
TITLE: (panitumumab or vectibix or ‘ABX-EGF’) OR TOPIC: (panitumumab or vectibix or ‘ABX-EGF’)
-
#1 OR #2
-
TITLE: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*) OR TOPIC: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*)
-
TITLE: (CRC or mCRC) OR TOPIC: (CRC or mCRC)
-
#4 OR #5
-
TITLE: (random* or rct* or ‘controlled trial*’ or ‘clinical trial*’) OR TOPIC: (random* or rct* or ‘controlled trial*’ or ‘clinical trial*’)
-
#7 AND #6 AND #3
Cochrane Central Register of Controlled Trials (CENTRAL)
Host: The Cochrane Collaboration.
Data parameters: Issue 12 of 12, December 2014.
Date searched: 6 January 2015.
Searcher: SB.
Hits: 255.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’):ti or (cetuximab or erbitux or C225 or ‘IMC C225’):ab
-
(panitumumab or vectibix or ‘ABX-EGF’):ti or (panitumumab or vectibix or ‘ABX-EGF’):ab
-
#1 or #2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*):ti or (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*):ab
-
(CRC or mCRC):ti or (CRC or mCRC):ab
-
MeSH descriptor: [Colorectal Neoplasms] explode all trees
-
MeSH descriptor: [Colon] explode all trees
-
MeSH descriptor: [Rectum] explode all trees
-
#4 or #5 or #6 or #7 or #8
-
#3 and #9 in Technology Assessments
Cochrane Database of Systematic Reviews (CDSR)
Host: The Cochrane Collaboration.
Data parameters: Issue 1 of 12, January 2015.
Date searched: 6 January 2015.
Searcher: SB.
Hits: none.
Search strategy
See CENTRAL strategy.
Database of Abstracts of Reviews of Effects (DARE)
Host: The Cochrane Collaboration.
Data parameters: Issue 4 of 4, October 2014.
Date searched: 6 January 2015.
Searcher: SB.
Hits: 14.
Search strategy
See CENTRAL strategy.
Health Techology Assessment database
Host: The Cochrane Collaboration.
Data parameters: Issue 4 of 4, October 2014.
Date searched: 6 January 2015.
Searcher: SB.
Hits: 18.
Search strategy
See CENTRAL strategy.
Summary of results: clinical effectiveness review
| Database | Hits |
|---|---|
| MEDLINE | 447 |
| MEDLINE In-Process & Other Non-Indexed Citations | 66 |
| EMBASE | 1948 |
| Web of Science | 1093 |
| CENTRAL | 255 |
| CDSR | 0 |
| DARE | 14 |
| HTA database | 18 |
| Total | 3841 |
| Duplicate records | 1205 |
| Total records to screen | 2636 |
Cost-effectiveness review
MEDLINE
Host: Ovid.
Data parameters: 1946 to November Week 3 2014.
Date searched: 8 January 2015.
Searcher: SB.
Hits: 126.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
exp Colorectal Neoplasms/
-
colon/
-
rectum/
-
or/4-8
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or ‘health utilit*’ or ‘value for money’).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(‘resource* alloca*’ or ‘resource* use’).tw.
-
exp Economics/
-
exp models, economic/
-
exp ‘Costs and Cost Analysis’/
-
Cost of illness/
-
ec.fs.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
decision trees/
-
or/10-20
-
3 and 9 and 21
-
limit 22 to english language
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 7 January 2015.
Date searched: 8 January 2015.
Searcher: SB.
Hits: 24.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
4 or 5
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or ‘health utilit*’ or ‘value for money’).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(‘resource* alloca*’ or ‘resource* use’).tw.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
or/7-11
-
3 and 6 and 12
EMBASE
Host: Ovid.
Data parameters: 1974 to 7 January 2015.
Date searched: 8 January 2015.
Searcher: SB.
Hits: 1314.
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
cetuximab/
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
panitumumab/
-
or/1-4
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
exp colon/
-
exp colon tumor/
-
exp rectum/
-
exp rectum tumor/
-
or/6-11
-
(pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or ‘health utilit*’ or ‘value for money’).tw.
-
(fiscal or funding or financial or finance* or expenditure* or budget*).tw.
-
(‘resource* alloca*’ or ‘resource* use’).tw.
-
exp Economics/
-
models, economic/
-
exp health economics/
-
exp ‘Costs and Cost Analysis’/
-
Cost of illness/
-
resource allocation/
-
pe.fs.
-
(decision adj2 (model* or tree* or analy*)).tw.
-
markov.tw.
-
decision trees/
-
or/13-25
-
5 and 12 and 26
-
limit 27 to english language
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and CPCI-S.
Date searched: 8 January 2015.
Searcher: SB.
Hits: 231.
Search strategy
-
TITLE: (cetuximab or erbitux or C225 or ‘IMC C225’) OR TOPIC: (cetuximab or erbitux or C225 or ‘IMC C225’)
-
TITLE: (panitumumab or vectibix or ‘ABX-EGF’) OR TOPIC: (panitumumab or vectibix or ‘ABX-EGF’)
-
#2 OR #1
-
TITLE: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*) OR TOPIC: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*)
-
TITLE: (CRC or mCRC) OR TOPIC: (CRC or mCRC)
-
#5 OR #4
-
TITLE: (pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or ‘health utilit*’ or ‘value for money’) OR TOPIC: (pharmacoeconomic* or economic* or price* or pricing* or cost* or cba or cea or cua or ‘health utilit*’ or ‘value for money’)
-
TITLE: (fiscal or funding or financial or finance* or expenditure* or budget*) OR TOPIC: (fiscal or funding or financial or finance* or expenditure* or budget*)
-
TITLE: (‘resource* alloca*’ or ‘resource* use’) OR TOPIC: (‘resource* alloca*’ or ‘resource* use’)
-
TITLE: (decision near/1 (model* or tree* or analy*)) OR TOPIC: (decision near/1 (model* or tree* or analy*))
-
TITLE: (markov) OR TOPIC: (markov)
-
#11 OR #10 OR #9 OR #8 OR #7
-
#12 AND #6 AND #3
NHS Economic Evaluation Database (NHS EED)
Host: The Cochrane Collaboration.
Data parameters: Issue 4 of 4, October 2014.
Date searched: 8 January 2015.
Searcher: SB.
Hits: 10.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’):ti or (cetuximab or erbitux or C225 or ‘IMC C225’):ab
-
(panitumumab or vectibix or ‘ABX-EGF’):ti or (panitumumab or vectibix or ‘ABX-EGF’):ab
-
#1 or #2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*):ti or (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*):ab
-
(CRC or mCRC):ti or (CRC or mCRC):ab
-
MeSH descriptor: [Colorectal Neoplasms] explode all trees
-
MeSH descriptor: [Colon] explode all trees
-
MeSH descriptor: [Rectum] explode all trees
-
#4 or #5 or #6 or #7 or #8
-
#3 and #9 in Economic Evaluations
EconLit
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 8 January 2015.
Searcher: SB.
Hits: none.
Search strategy
-
TI ( cetuximab or erbitux or C225 or ‘IMC C225’ ) OR AB ( cetuximab or erbitux or C225 or ‘IMC C225’ )
-
TI ( panitumumab or vectibix or ‘ABX-EGF’ ) OR AB ( panitumumab or vectibix or ‘ABX-EGF’ )
-
S1 OR S2
-
TI ( colorectal or colon or colonic or rectal or rectum or bowel or intenstin* ) OR AB ( colorectal or colon or colonic or rectal or rectum or bowel or intenstin* )
-
TI ( CRC or mCRC ) OR AB ( CRC or mCRC )
-
S4 OR S5
-
(S3 AND S6)
Summary of cost-effectiveness review
| Database | Hits |
|---|---|
| MEDLINE | 126 |
| MEDLINE In-Process & Other Non-Indexed Citations | 24 |
| EMBASE | 1314 |
| Web of Science | 231 |
| NHS EED | 10 |
| EconLit | 0 |
| Total | 1705 |
Quality-of-life review
MEDLINE
Host: Ovid.
Data parameters: 1946 to January Week 1 2015.
Date searched: 13 January 2015.
Searcher: SB.
Hits: 67.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
exp Colorectal Neoplasms/
-
colon/
-
rectum/
-
or/4-8
-
(‘quality of life’ or QoL or HRQL or HRQoL or AQoL).tw.
-
quality of life/
-
(‘quality adjusted life year*’ or QALY*).tw.
-
quality-adjusted life years/
-
(‘quality of wellbeing’ or QWB).tw.
-
(‘health* year* equivalent*’ or HYE*).tw.
-
‘health status’.tw.
-
health status/
-
health status indicators/
-
(‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’).tw.
-
(‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’).tw.
-
(‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’).tw.
-
(‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’).tw.
-
(‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’).tw.
-
(‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’).tw.
-
(Euroqol or ‘EQ-5D’).tw.
-
Health Surveys/
-
questionnaire*.tw.
-
exp Questionnaires/
-
‘willingness to pay’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
(‘visual analog* scale’ or VAS).tw.
-
(health adj2 (utilit*3 or value* or preference*)).tw.
-
(‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’).tw.
-
disutil*.tw.
-
‘standard gamble*’.tw.
-
‘discrete choice’.tw.
-
or/10-36
-
3 and 9 and 37
-
limit 38 to english language
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 12 January 2015.
Date searched: 13 January 2015.
Searcher: SB.
Hits: 13.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
4 or 5
-
(‘quality of life’ or QoL or HRQL or HRQoL or AQoL).tw.
-
(‘quality adjusted life year*’ or QALY*).tw.
-
(‘quality of wellbeing’ or QWB).tw.
-
(‘health* year* equivalent*’ or HYE*).tw.
-
‘health status’.tw.
-
(‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’).tw.
-
(‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’).tw.
-
(‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’).tw.
-
(‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’).tw.
-
(‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’).tw.
-
(‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’).tw.
-
(Euroqol or ‘EQ-5D’).tw.
-
questionnaire*.tw.
-
‘willingness to pay’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
(‘visual analog* scale’ or VAS).tw.
-
(health adj2 (utilit*3 or value* or preference*)).tw.
-
(‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’).tw.
-
disutil*.tw.
-
‘standard gamble*’.tw.
-
‘discrete choice’.tw.
-
or/7-27
-
3 and 6 and 28
EMBASE
Host: Ovid.
Data parameters: 1974 to 12 January 2015.
Date searched: 13 January 2015.
Searcher: SB.
Hits: 734.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
cetuximab/
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
panitumumab/
-
or/1-4
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
exp colon/
-
exp colon tumor/
-
exp rectum/
-
exp rectum tumor/
-
or/6-11
-
(‘quality of life’ or QoL or HRQL or HRQoL or AQoL).tw.
-
exp quality of life/
-
(‘quality adjusted life year*’ or QALY*).tw.
-
quality-adjusted life years/
-
(‘quality of wellbeing’ or QWB).tw.
-
(‘health* year* equivalent*’ or HYE*).tw.
-
‘health status’.tw.
-
health status/
-
health status indicators/
-
(‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’).tw.
-
(‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’).tw.
-
(‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’).tw.
-
(‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’).tw.
-
(‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’).tw.
-
(‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’).tw.
-
(Euroqol or ‘EQ-5D’).tw.
-
health survey/
-
questionnaire*.tw.
-
exp questionnaire/
-
‘willingness to pay’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
(‘visual analog* scale’ or VAS).tw.
-
(health adj2 (utilit*3 or value* or preference*)).tw.
-
(‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’).tw.
-
disutil*.tw.
-
‘standard gamble*’.tw.
-
‘discrete choice’.tw.
-
or/13-39
-
5 and 12 and 40
-
limit 41 to english language
PsycINFO
Host: Ovid.
Data parameters: 1806 to January Week 1 2015.
Date searched: 13 January 2015.
Searcher: SB.
Hits: two.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’).tw.
-
(panitumumab or vectibix or ‘ABX-EGF’).tw.
-
1 or 2
-
(colorectal or colon or colonic or rectal or rectum or bowel or intenstin*).tw.
-
(CRC or mCRC).tw.
-
4 or 5
-
(‘quality of life’ or QoL or HRQL or HRQoL or AQoL).tw.
-
quality of life/
-
(‘quality adjusted life year*’ or QALY*).tw.
-
(‘quality of wellbeing’ or QWB).tw.
-
(‘health* year* equivalent*’ or HYE*).tw.
-
‘health status’.tw.
-
(‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’).tw.
-
(‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’).tw.
-
(‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’).tw.
-
(‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’).tw.
-
(‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’).tw.
-
(‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’).tw.
-
(Euroqol or ‘EQ-5D’).tw.
-
questionnaire*.tw.
-
exp Questionnaires/
-
‘willingness to pay’.tw.
-
(‘time trade off’ or ‘time tradeoff’ or tto).tw.
-
(‘visual analog* scale’ or VAS).tw.
-
(health adj2 (utilit*3 or value* or preference*)).tw.
-
(‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’).tw.
-
disutil*.tw.
-
‘standard gamble*’.tw.
-
‘discrete choice’.tw.
-
or/7-29
-
3 and 6 and 30
-
limit 31 to english language
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and CPCI-S.
Date searched: 13 January 2015.
Searcher: SB.
Hits: 171.
Search strategy
-
TITLE: (cetuximab or erbitux or C225 or ‘IMC C225’) OR TOPIC: (cetuximab or erbitux or C225 or ‘IMC C225’)
-
TITLE: (panitumumab or vectibix or ‘ABX-EGF’) OR TOPIC: (panitumumab or vectibix or ‘ABX-EGF’)
-
#2 OR #1
-
TITLE: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*) OR TOPIC: (colorectal or colon or colonic or rectal or rectum or bowel or intenstin*)
-
TITLE: (CRC or mCRC) OR TOPIC: (CRC or mCRC)
-
#5 OR #4
-
TITLE: (‘quality of life’ or QoL or HRQL or HRQoL or AQoL) OR TOPIC: (‘quality of life’ or QoL or HRQL or HRQoL or AQoL)
-
TITLE: (‘quality adjusted life year*’ or QALY*) OR TOPIC: (‘quality adjusted life year*’ or QALY*)
-
TITLE: (‘quality of wellbeing’ or QWB) OR TOPIC: (‘quality of wellbeing’ or QWB)
-
TITLE: (‘health* year* equivalent*’ or HYE*) OR TOPIC: (‘health* year* equivalent*’ or HYE*)
-
TITLE: (health status) OR TOPIC: (health status)
-
TITLE: (‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’) OR TOPIC: (‘short form 36’ or ‘shortform 36’ or ‘short form thirty six’ or ‘shortform thirty six’ or ‘SF 36’ or SF36 or ‘SF thirty six’)
-
TITLE: (‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’) OR TOPIC: (‘short form 20’ or ‘shortform 20’ or ‘short form twenty’ or ‘shortform twenty’ or ‘SF 20’ or SF20 or ‘SF twenty’)
-
TITLE: (‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’) OR TOPIC: (‘short form 16’ or ‘shortform 16’ or ‘short form sixteen’ or ‘shortform sixteen’ or ‘SF 16’ or SF16 or ‘SF sixteen’)
-
TITLE: (‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’) OR TOPIC: (‘short form 12’ or ‘shortform 12’ or ‘short form twelve’ or ‘shortform twelve’ or ‘SF 12’ or ‘SF12 or ‘SF twelve’)
-
TITLE: (‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’) OR TOPIC: (‘short form 10’ or ‘shortform 10’ or ‘short form ten’ or ‘shortform ten’ or SF10 or ‘SF 10’ or ‘SF ten’)
-
TITLE: (‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’) OR TOPIC: (‘short form 6’ or ‘shortform 6’ or ‘short form six’ or ‘shortform six’ or SF6 or ‘SF 6’ or ‘SF six’)
-
TITLE: (Euroqol or ‘EQ-5D’) OR TOPIC: (Euroqol or ‘EQ-5D’)
-
TITLE: (questionnaire*) OR TOPIC: (questionnaire*)
-
TITLE: (‘willingness to pay’) OR TOPIC: (‘willingness to pay’)
-
TITLE: (‘visual analog* scale’ or VAS) OR TOPIC: (‘visual analog* scale’ or VAS)
-
TITLE: (‘time trade off’ or ‘time tradeoff’ or tto) OR TOPIC: (‘time trade off’ or ‘time tradeoff’ or tto)
-
TITLE: (health near/1 (utilit*3 or value* or preference*)) OR TOPIC: (health near/1 (utilit*3 or value* or preference*))
-
TITLE: (‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’) OR TOPIC: (‘health utilities index*’ or hui or hui1 or hui2 or hui3 or hui4 or ‘hui 1’ or ‘hui 2’ or ‘hui 3’ or ‘hui 4’)
-
TITLE: (disutil*) OR TOPIC: (disutil*)
-
TITLE: (‘standard gamble*’) OR TOPIC: (‘standard gamble*’)
-
TITLE: (‘discrete choice’) OR TOPIC: (‘discrete choice’)
-
#27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7
-
#28 AND #6 AND #3
School of Health and Related Research Health Utilities Database (ScHARRHUD)
Host: School of Health and Related Research (ScHARR).
Data parameters: not applicable.
Date searched: 13 January 2015.
Searcher: SB.
Hits: one.
Search strategy
-
(cetuximab or erbitux or C225 or ‘IMC C225’) in Title
-
(cetuximab or erbitux or C225 or ‘IMC C225’) in Abstract
-
(panitumumab or vectibix or ‘ABX-EGF’) in Title
-
(panitumumab or vectibix or ‘ABX-EGF’) in Abstract
-
#1 OR #2 OR #3 OR #4
Summary of quality-of-life review
| Database | Hits |
|---|---|
| MEDLINE | 67 |
| MEDLINE In-Process & Other Non-Indexed Citations | 13 |
| EMBASE | 734 |
| PsycINFO | 2 |
| Web of Science | 171 |
| ScHARRHUD | 1 |
| Total | 988 |
Total number of cost-effectiveness and quality-of-life titles/abstracts identified
| Database | Hits |
|---|---|
| Cost-effectiveness | 1705 |
| Quality of life | 988 |
| Total | 2693 |
| Duplicate records | 714 |
| Total records to screen | 1979 |
Update searches
Clinical effectiveness review
MEDLINE
Host: Ovid.
Data parameters: 1946 to April Week 3 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 48.
Search strategy
See main strategy (date limited 2014 to current).
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 66.
Search strategy
See main strategy (no date limit used).
EMBASE
Host: Ovid.
Data parameters: 1974 to 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 48.
Search strategy
See main strategy (date limited 2015 to current).
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and CPCI-S.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 42.
Search strategy
See main strategy (date limited 2015 to current).
Cochrane Central Register of Controlled Trials (CENTRAL)
Host: The Cochrane Collaboration.
Data parameters: Issue 3 of 12, March 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: one.
Search strategy
See main strategy (date limited 2015 to current).
Cochrane Database of Systematic Reviews (CDSR)
Host: The Cochrane Collaboration.
Data parameters: Issue 4 of 12, April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Database of Abstracts of Reviews of Effects (DARE)
Host: The Cochrane Collaboration.
Data parameters: Issue 1 of 4, January 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Notes: funding for DARE ended in March 2015 and no new records have been added since January 2015.
Health Technology Assessment database
Host: The Cochrane Collaboration.
Data parameters: Issue 1 of 4, January 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Summary of clinical effectiveness update searches
| Database | Hits |
|---|---|
| MEDLINE | 48 |
| MEDLINE In-Process & Other Non-Indexed Citations | 66 |
| EMBASE | 48 |
| Web of Science | 42 |
| CENTRAL | 1 |
| CDSR | 0 |
| DARE | 0 |
| HTA | 0 |
| Total | 205 |
| Duplicate records | 30 |
| Total records to screen | 175 |
Cost-effectiveness review
MEDLINE
Host: Ovid.
Data parameters: 1946 to April Week 3 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 12.
Search strategy
See main strategy (date limited 2014 to current).
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 20.
Search strategy
See main strategy (no date limit used).
EMBASE
Host: Ovid.
Data parameters: 1974 to 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 26.
Search strategy
See main strategy (date limited 2015 to current).
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and CPCI-S.
Date searched: 27 April 2015.
Searcher: SB.
Hits: nine.
Search strategy
See main strategy (date limited 2015 to current).
NHS Economic Evaluation Database (NHS EED)
Host: The Cochrane Collaboration.
Data parameters: Issue 4 of 4, October 2014.
Date searched: 8 January 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Notes: funding for NHS EED ended in March 2015 and no new records have been added since January 2015.
EconLit
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 8 January 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Summary of cost-effectiveness update searches
| Database | Hits |
|---|---|
| MEDLINE | 12 |
| MEDLINE In-Process & Other Non-Indexed Citations | 20 |
| EMBASE | 26 |
| Web of Science | 9 |
| NHS EED | 0 |
| EconLit | 0 |
| Total | 67 |
Quality of life
MEDLINE
Host: Ovid.
Data parameters: 1946 to April Week 3 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 0.
Search strategy
See main strategy (date limited 2015 to current).
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 14.
Search strategy
See main strategy (no date limit used).
EMBASE
Host: Ovid.
Data parameters: 1974 to 24 April 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: 14.
Search strategy
See main strategy (date limited 2015 to current).
PsycINFO
Host: Ovid.
Data parameters: 1806 to April Week 3 2015.
Date searched: 27 April 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Web of Science
Host: Thomson Reuters.
Data parameters: SCI-EXPANDED and CPCI-S.
Date searched: 27 April 2015.
Searcher: SB.
Hits: three.
Search strategy
See main strategy (date limited 2015 to current).
School of Health and Related Research Health Utilities Database (ScHARRHUD)
Host: School of Health and Related Research (ScHARR).
Data parameters:
Date searched: 27 April 2015.
Searcher: SB.
Hits: none.
Search strategy
See main strategy (date limited 2015 to current).
Summary of quality-of-life update searches
| Database | Hits |
|---|---|
| MEDLINE | 0 |
| MEDLINE In-Process & Other Non-Indexed Citations | 14 |
| EMBASE | 14 |
| PsycINFO | 0 |
| Web of Science | 3 |
| ScHARRHUD | 0 |
| Total | 31 |
Total number of cost-effectiveness and quality-of-life titles/abstracts identified in the update searches
| Database | Hits |
|---|---|
| Cost-effectiveness | 67 |
| Quality of life | 31 |
| Total | 98 |
| Duplicate records | 18 |
| Total records to screen | 80 |
Clinical trials registries
The following terms were used to search the ClinicalTrials.gov register for condition and interventions:
Condition: colorectal OR colon OR colonic OR rectal OR rectum OR bowel or intenstin* OR CRC OR mCRC
Intervention: cetuximab OR erbitux OR C225 OR ‘IMC C225’ OR panitumumab OR vectibix OR ‘ABX-EGF’
The following terms were used to search the WHO ICTRP for condition and interventions:
Condition: colorectal OR colon OR colonic OR rectal OR rectum OR bowel or intenstin* OR CRC OR mCRC
Intervention: cetuximab OR erbitux OR C225 OR ‘IMC C225’ OR panitumumab OR vectibix OR ‘ABX-EGF’
The following terms were used to search the UKCRN portfolio:
cetuximab erbitux C225 ‘IMC C225’ panitumumab vectibix ‘ABX-EGF’
The following terms were used to search the ISRCTN registry:
cetuximab OR erbitux OR C225 OR ‘IMC C225’ OR panitumumab OR vectibix OR ‘ABX-EGF’
Appendix 2 List of studies excluded from the clinical effectiveness review
| Study | Reason for exclusion |
|---|---|
| Berlin J, Posey J, Tchekmedyian S, Hu E, Chan D, Malik I, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer 2007;6:427–32 | Comparator |
| Douillard JY, Zemelka T, Fountzilas G, Barone C, Schlichting M, Heighway J, et al. FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: the randomized phase II FUTURE study. Clin Colorectal Cancer 2014;13:14–26.e1 | Comparator |
| Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 2011;12:642–53 | Population |
| Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383–93 | Population |
| Blons H, Emile JF, Le Malicot K, Julie C, Zaanan A, Tabernero J, et al. Prognostic value of KRAS mutations in stage III colon cancer: post hoc analysis of the PETACC8 phase III trial dataset. Ann Oncol 2014;25:2378–85 | Population |
| Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663–71 | Population |
| Bokemeyer C, Bondarenko I, Hartmann JT, Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011;22:1535–46 | Population |
| Bokemeyer C, Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466–75 | Population |
| Cervantes-Ruiperez A, Markman B, Siena S, Pericay C, Aprile G, Bridgewater JA, et al. The GAIN-C study (BP25438): randomized phase II trial of RG7160 (GA201) plus FOLFIRI, compared to cetuximab plus FOLFIRI or FOLFIRI alone in second-line KRAS wild type (WT) or mutant metastatic colorectal cancer (mCRC). J Clin Oncol 2012;30(Suppl.):TPS3637 | Population |
| Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17 | Population |
| Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–19 | Population |
| Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705 | Population |
| Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55 | Population |
| Lang I, Kohne CH, Folprecht G, Rougier P, Curran D, Hitre E, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013;49:439–48 | Population |
| Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14 | Population |
| Mitchell EP, Lacouture M, Shearer H, Iannotti N, Piperdi B, Pillai M, et al. Final STEPP results of prophylacatic versus reactive skin toxicity (ST) treatment (tx) for panitumumab (pmab)-related ST in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2009;27:RA4027 | Populationa |
| Mitchell EP, Piperdi B, Lacouture ME, Shearer H, Iannotti N, Pillai MV, et al. The efficacy and safety of panitumumab administered concomitantly with FOLFIRI or irinotecan in second-line therapy for metastatic colorectal cancer: the secondary analysis from STEPP (Skin Toxicity Evaluation Protocol With Panitumumab) by KRAS status. Clin Colorectal Cancer 2011;10:333–9 | Population |
| Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol 2010;16:3133–43 | Population |
| Polikoff J, Mitchell EP, Badarinath S, Graham CD, Jennis A, Chen TT, et al. Cetuximab plus FOLFOX for colorectal cancer (EXPLORE): preliminary efficacy analysis of a randomized phase III trial. J Clin Oncol 2005;23:264S | Populationa |
| Poulin-Costello M, Azoulay L, Van Cutsem E, Peeters M, Siena S, Wolf M. An analysis of the treatment effect of panitumumab on overall survival from a phase 3, randomized, controlled, multicenter trial (20020408) in patients with chemotherapy refractory metastatic colorectal cancer. Targeted Oncol 2013;8:127–36 | Population |
| Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol 2014;15:569–79 | Population |
| Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol 2014;15:601–11. [Erratum published in Lancet Oncol 2014;15:e253] | Population |
| Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 2013;14:749–59 | Population |
| Siena S, Glynne-Jones R, Adenis A, Thaler J, Preusser P, Aguilar EA, et al. Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer 2010;116:1827–37 | Population |
| Stintzing S, Fischer von Weikersthal L, Decker T, Vehling-Kaiser U, Jager E, Heintges T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer – subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol 2012;23:1693–9 | Population |
| Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862–73 | Population |
| Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755–62 | Population |
| Wasan H, Meade AM, Adams R, Wilson R, Pugh C, Fisher D, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol 2014;15:631–9 | Population |
| Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 2013;31:1931–8 | Population |
| Heinemann V, Fischer Von Weikersthal L, Decker T, Kiani A, Verhling-Kaiser U, Al Batran S, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS-wildtype metastatic colorectal cancer: the FIRE-3 trial (AIO KRK 0307). Onkologie 2013;36:105 | Populationa |
| Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Yu H, et al. Analysis of KRAS/NRAS mutations in PEAK: a randomized phase II study of FOLFOX6 plus panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC). J Clin Oncol 2013;31(Suppl.):3631 | Populationa |
| Seymour MT, Brown SR, Richman S, Middleton GW, Maughan TS, Maisey N, et al. Panitumumab in combination with irinotecan for chemoresistant advanced colorectal cancer: results of PICCOLO, a large randomised trial with prospective molecular stratification. Eur J Cancer 2011;47:S393 | Populationa |
| Siena S, Douillard JY, Tabernero J, Cassidy J, Burkes R, Barugel M, et al. Panitumumab with FOLFOX4 versus FOLFOX4 alone as first-line treatment for metastatic colorectal cancer (mCRC): results from the randomised phase III PRIME study. Onkologie 2010;33:68–9 | Populationa |
| Siena S, Tabernero J, Cunningham D, Koralewski P, Ruff P, Rother M, et al. Randomized phase III study of panitumumab (pmab) with FOLFOX4 compared to FOLFOX4 alone as fist-line treatment (tx) for metastatic colorectal cancer (mCRC): PRIME trial analysis by epidermal growth factor receptor (EGFR) tumor staining. J Clin Oncol 2010;28(Suppl.):3566 | Populationa |
| Siena S, Cassidy J, Tabernero J, Burkes RL, Barugel ME, Humblet Y, et al. Randomized phase III study of panitumumab (pmab) with FOLFOX4 compared with FOLFOX4 alone as first line treatment (tx) for metastatic colorectal cancer (mCRC): results by Eastern Cooperative Oncology Group (ECOG) performance status (PS). J Clin Oncol 2011;29(Suppl. 1):3567 | Populationa |
| Stein A, Duex M, Kickuth R, Petrovitch A, Pluntke S, Ricke J, et al. A randomized phase II trial of irinotecan drug-eluting beads administered by hepatic chemoembolization with intravenous cetuximab (DEBIRITUX) versus systemic treatment with intravenous cetuximab and irinotecan in patients with refractory colorectal liver metastases and Kras wild-type tumors. Cardiovascular and Interventional Radiological Society of Europe, CIRSE 2011 Munich Germany. Cardiovasc Intervent Radiol 2011;34:617 | Populationa |
| Stintzing S, Neumann J, Jung A, Fischer Von Weikersthal L, Decker T, Vehling-Kaiser U, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (mCRC): analysis of patients with KRAS-mutated tumors in the randomized German AIO study KRK-0306. J Clin Oncol 2011;29(Suppl. 1):3575 | Populationa |
| Wasan H, Adams RA, Wilson RH, Pugh C, Fisher D, Madi A, et al. Intermittent chemotherapy (CT) plus continuous or intermittent cetuximab (C) in the first-line treatment of advanced colorectal cancer (aCRC): results of the two-arm phase II randomized MRC COIN-B trial. J Clin Oncol 2012;30(Suppl. 1):536 | Populationa |
| Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672–80 | Intervention |
| Punt CJ, Tol J, Rodenburg CJ, Cats A, Creemers G, Schrama JG, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). J Clin Oncol 2008;26(Suppl.):LBA4011a | Intervention |
| Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 2007;14:1860–9 | Intervention |
| Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 2006;5:553–66 | Intervention |
| Tol J, Koopman M, Rodenburg CJ, Cats A, Creemers GJ, Schrama JG, et al. A randomised phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734–8 | Intervention |
| Pietrantonio F, Garassino MC, Torri V, de Braud F. Reply to FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer – subgroup analysis of patients with KRAS-mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol 2012;23:2771–2 | Study design |
| Saif MW, Kim R. Incidence and management of cutaneous toxicities associated with cetuximab. Expert Opin Drug Saf 2007;6:175–82 | Study design |
Appendix 3 Abstracts
We screened the abstracts identified by the clinical effectiveness searches. A total of 90 abstracts were screened, of which four met the eligibility criteria for the review. Authors of the abstracts were contacted, which led to the identification of an additional two full papers. 43,65 A further three abstracts were identified in the update searches conducted on 27 April 2015. The relevant abstracts are summarised in the following table.
| Abstract | Summary information |
|---|---|
| Abstracts identified in the clinical effectiveness review searches | |
| Bokemeyer C, Kohne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. Treatment outcome according to tumor RAS mutation status in OPUS study patients with metastatic colorectal cancer (mCRC) randomized to FOLFOX4 with/without cetuximab. J Clin Oncol 2014;32(Suppl. 1):3505 | OPUS trial – RAS WT analysis; author provided full paper65 |
| Ciardiello F, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Treatment outcome according to tumor RAS mutation status in CRYSTAL study patients with metastatic colorectal cancer (mCRC) randomized to FOLFIRI with/without cetuximab. J Clin Oncol 2014;32(Suppl. 1):3506 | CRYSTAL trial – RAS WT analysis; author provided full paper43 |
| Douillard JY, Tabernero J, Siena S, Peeters M, Koukakis R, Terwey JH, et al. Survival outcomes in patients (pts) with KRAS/NRAS (RAS) wild-type (WT) metastatic colorectal cancer (mCRC) and non-liver-limited disease (non-LLD): data from the PRIME study. J Clin Oncol 2014;32(Suppl. 1):3550 | PRIME trial – post hoc subgroup analysis by liver-limited and non-liver-limited disease (same as following abstract but reports different outcomes); author approached for more information on 13 February 2014 but none received |
| Peeters M, Tabernero J, Douillard JY, Siena S, Davison C, Braun S, et al. Resection rates and survival in patients with wild-type KRAS/NRAS metastatic colorectal cancer and liver metastases: data from the PRIME study. Eur J Cancer 2013;49(Suppl. 4):S17–18 | PRIME trial – post hoc subgroup analysis by liver-limited and non-liver-limited disease (same as abstract above by Douillard et al. but reports different outcomes); author approached for more information on 13 February 2014 but none received |
| Abstracts identified in the update searches (27 April 2015) | |
| Rivera F, Karthaus M, Hecht JR, Fasola G, Canon J-LR, Koukakis R, et al. First-line treatment with modified FOLFOX6 (mFOLFOX6) + panitumumab (pmab) or bevacizumab (bev) in wild-type (WT) RAS metastatic colorectal carcinoma (mCRC): tumor response outcomes beyond RECIST. J Clin Oncol 2015;33(Suppl. 3):660 | PEAK trial – post hoc subgroup analysis reporting tumour response outcomes beyond RECIST (e.g. early tumour shrinkage) |
| Siena S, Tabernero J, Bodoky G, Cunningham D, Rivera F, Ruff P, et al. Quality of life (QoL) during first-line treatment with FOLFOX4 with or without panitumumab (pmab) in RAS wild-type (WT) metastatic colorectal carcinoma (mCRC). J Clin Oncol 2015;33(Suppl. 3):693 | PRIME trial – post hoc subgroup analysis of quality of life in the RAS WT population (uses EQ-5D health-state index and overall health rating) |
| Wang J, Dong J, Johnson P, Maglinte GA, Rong A, Barber BL, et al. Quality-adjusted survival in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC) receiving first-line therapy with panitumumab plus FOLFOX versus FOLFOX alone in the PRIME trial. J Clin Oncol 2015;33(Suppl. 3):537 | PRIME trial – post hoc subgroup analysis of quality-adjusted survival in the RAS WT population |
| Abstracts identified by Amgen (excluded from the Assessment Group review) | |
| Abad et al. Panitumumab plus FOLFOX4 or panitumumab plus FOLFIRI in subjects with wild-type KRAS (exon 2) colorectal cancer and multiple or unresectable liver-limited metastases: data from the randomized, phase II PLANET study. Annual Meeting of the American Society of Clinical Oncology, ASCO Chicago, IL United States. 2014;32(Suppl. 1)a | PLANET trial – results predominantly reported for the KRAS WT population; RAS analysis reports data for ORR |
Appendix 4 KRAS wild-type subgroup
| First author (trial) | Intervention | n | Age (years), median (range) | Male, n/N (%) | ECOG PS, n/N (%) | Number of metastatic sites, n/N (%) | Primary tumour diagnosis, n/N (%) | LLD, n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Bokemeyer 201122 (OPUS) | CET + FOLFOX4 | 82 | 62 (24–75) | 42/82 (51) | 0: 62/82 (39); 1: 44/82 (54); 2: 6/82 (7) | 1: 41/82 (50); 2: 26/82 (32); ≥ 3: 15/82 (18) | NR | 25/82 (30) |
| FOLFOX4 | 97 | 59 (36–82) | 55/97 (57) | 0: 38/97 (39); 1: 49/97 (51); 2: 10/97 (10) | 1: 38/97 (39); 2: 37/97 (38); ≥ 3: 22/97 (23) | NR | 23/97 (24) | |
| Van Cutsem 201125 (CRYSTAL) | CET + FOLFIRI | 316 | 61 (24–79) | 196/316 (62.0) | 0: 183/316 (57.9); 1: 120/316 (38.0); 2: 13/316 (4.1) | ≤ 2: 277/316 (87.7) | NR | 68/316 (21.5) |
| FOLFIRI | 350 | 59 (19–84) | 211/350 (60.3) | 0: 200/350 (57.1); 1: 136/350 (38.9); 2: 14/350 (4.0) | ≤ 2: 295/350 (84.3) | NR | 72/350 (20.6) | |
| Heinemann 201428 (FIRE-3) | CET + FOLFIRI | 297 | 64 (38–79) | 214/297 (72) | 0: 154/297 (52); 1: 136/297 (46); 2: 7/297 (2) | 1: 119/297 (40); ≥ 2: 174/297 (59); unknown: 4/297 (1) | Colon: 168/297 (57); rectum: 115/297 (39); colon and rectum: 9/297 (3); unknown: 5/297 (2) | 93/297 (31) |
| BEV + FOLFIRI | 295 | 65.0 (27–76) | 196/295 (66) | 0: 158/295 (54); 1: 133/295 (45); 2: 4/295 (1) | 1: 123/295 (42); ≥ 2: 171/295 (58); unknown: 1/295 (< 1) | Colon: 177/295 (60); rectum: 106/295 (36); colon and rectum: 12/295 (4); unknown: 0/295 (0) | 94/295 (32) |
| First author (trial) | Intervention | n | Age (years), median (range) | Male, n/N (%) | ECOG PS, n/N (%) | Number of metastatic sites, n/N (%) | Primary tumour diagnosis, n/N (%) | LLD, n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Douillard 201426 (PRIME) | PAN + FOLFOX4 | 325 | 62 (27–85) | 217 (67) | 0–1: 305/325 (94); ≥ 2: 20/325 (6) | 1: 69/325 (21); 2: 114/325 (35); ≥ 3: 140/325 (43); not reported: 2/325 (1) | Colon: 214/325 (66); rectum: 111/325 (34) | 61/325 (19) |
| FOLFOX4 | 331 | 61 (24–82) | 204 (62) | 0–1: 312/331 (94); ≥ 2: 18/331 (5); unknown: 1/331 (< 1) | 1: 68/331 (21); 2: 118/331 (36); ≥ 3: 145/331 (44) | Colon: 216/331 (65); rectum: 115/331 (35) | 57/331 (17) | |
| Schwartzberg 201429 (PEAK) | PAN + mFOLFOX6 | 142 | 63 (23–82) | 89/142 (61) | 0: 89/142 (63); 1: 53/142 (37); other:a 0/142 (0) | 1: 53/142 (37); 2: 50/142 (35); ≥ 3: 39/142 (27); other:a 0/142 (0) | Colon: 96/142 (68); rectum: 46/142 (32) | 37/142 (26) |
| BEV + mFOLFOX6 | 143 | 61 (28–82) | 96/143 (67) | 0: 91/143 (64); 1: 51/143 (36); other:a 1/143 (< 1) | 1: 56/143 (39); 2: 49/143 (34); ≥ 3: 37/143 (26); other:a 1/143 (< 1) | Colon: 92/143 (64); rectum: 51/143 (36) | 39/143 (27) |
| Trial | Experimental (n/N), median months/% (95% CI) | Control (n/N), median months/% (95% CI) | HR/OR (95% CI) |
|---|---|---|---|
| PFS | |||
| OPUS22 | CET + FOLFOX4 (NR), 8.3 (7.2 to 12.0) | FOLFOX4 (NR), 7.2 (5.6 to 7.4) | HR 0.567 (0.375 to 0.856) |
| CRYSTAL25 | CET + FOLFIRI (146/316), 9.9 (9.0 to 11.3) | FOLFIRI (189/350), 8.4 (7.4 to 9.2) | HR 0.696 (0.558 to 0.867) |
| FIRE-328 | CET + FOLFIRI (250/297), 10.0 (8.8 to 10.8) | BEV + FOLFIRI (242/295), 10.3 (9.8 to 11.3) | NR |
| OS | |||
| OPUS22 | CET + FOLFOX4 (NR), 22.8 (19.3 to 25.9) | FOLFOX4 (NR), 18.5 (16.4 to 22.6) | HR 0.855 (0.599 to 1.219) |
| CRYSTAL25 | CET + FOLFIRI (242/316), 23.5 (21.2 to 26.3) | FOLFIRI (288/350), 20.0 (17.4 to 21.7) | HR 0.796 (0.670 to 0.946) |
| FIRE-328 | CET + FOLFIRI (158/297), 28.7 (24.0 to 36.6) | BEV + FOLFIRI (185/295), 25.0 (22.7 to 27.6) | NR |
| ORR | |||
| OPUS22 | CET + FOLFOX4 (43/82), 57% (46% to 68%) | FOLFOX4 (33/97), 34% (25% to 44%) | OR 2.551 (1.380 to 4.717) |
| CRYSTAL25 | CET + FOLFIRI (181/316), 57.3% (51.6% to 62.8%) | FOLFIRI (139/350), 39.7% (34.6% to 45.1%) | OR 2.069 (1.515 to 2.826) |
| FIRE-328 | CET + FOLFIRI (184/297), 62% (56.2% to 67.5%) | BEV + FOLFIRI (171/295), 58% (52.1% to 63.7%) | NR |
| Complete resection rate (R0) | |||
| OPUS22 | NR | NR | NR |
| CRYSTAL25 | CET + FOLFIRI (16/316), 5.1% | FOLFIRI (7/350), 2.0% | OR 2.650 (1.083 to 6.490) |
| FIRE-328 | NR | NR | NR |
| Trial | Experimental (n/N), median months/% (95% CI) | Control (n/N), median months/% (95% CI) | HR/OR (95% CI) |
|---|---|---|---|
| PFS | |||
| PRIME26 | PAN + FOLFOX4 (270/325), 10.0 (9.3 to 11.4) | FOLFOX4 (280/331), 8.6 (7.5 to 9.5) | HR 0.80 (0.67 to 0.95) |
| PEAK29 | PAN + mFOLFOX6 (90/142), 10.9 (9.4 to 13.0) | BEV + mFOLFOX6 (94/143), 10.1 (9.0 to 12.6) | HR 0.87 (0.65 to 1.17) |
| OS | |||
| PRIME26 | PAN + FOLFOX4 (214/325), 23.9 (20.3 to 27.7) | FOLFOX4 (231/331), 19.4 (17.6 to 22.7) | HR 0.88 (0.73 to 1.06) |
| PEAK29 | PAN + mFOLFOX6 (52/142), 34.2 (26.6 to NR) | BEV + mFOLFOX6 (78/143), 24.3 (21.0 to 29.2) | HR 0.62 (0.44 to 0.89) |
| ORR | |||
| PRIME26 | PAN + FOLFOX4 (181/317), 57% (51.5% to 62.6%) | FOLFOX4 (154/324), 48% (42.0% to 53.1%) | NR |
| PEAK29 | PAN + mFOLFOX6 (82/142), 57.8% (49.2% to 66.0%) | BEV + mFOLFOX6 (75/142), 53.5% (45.0% to 61.9%) | NR |
| Complete resection rate (R0) | |||
| PRIME26 | PAN + FOLFOX4 (31/325), 10% (NR) | FOLFOX4 (25/331), 8% (NR) | NR |
| PEAK29 | PAN + mFOLFOX6 (14/142), 10% (NR) | BEV + mFOLFOX6 (12/143), 8% | NR |
| AE | OPUS,22 n/N (%) | CRYSTAL,25 n/N (%) | FIRE-3,28 n/N (%) | |||
|---|---|---|---|---|---|---|
| CET + FOLFOX4 (n = 82) | FOLFOX4 (n = 97) | CET + FOLFIRI (n = 317) | FOLFIRI (n = 350) | CET + FOLFIRI (n = 297) | BEV + FOLFIRI (n = 295) | |
| Any AE | NR | NR | NR | NR | NR | NR |
| Any grade 1 or grade 2 event | NR | NR | NR | NR | NR | NR |
| Any grade 3 or grade 4 event | 67/82 (82) | 62/97 (64) | 257/317 (81.1) | 211/350 (60.3) | 211/297 (71) | 188/295 (64) |
| Any serious AE | NR | NR | NR | NR | NR | NR |
| AE | PRIME,26 n/N (%) | PEAK,29 n/N (%) | ||
|---|---|---|---|---|
| PAN + FOLFOX4 (n = 322) | FOLFOX4 (n = 327) | PAN + mFOLFOX6 (n = 139) | BEV + mFOLFOX6 (n = 139) | |
| Any AE | NR | NR | 139/139 (100) | 139/139 (100) |
| Any grade 1 or grade 2 event | NR | NR | NR | NR |
| Any grade 3 or grade 4 event | 270/322 (84) | 227/327 (69) | NR | NR |
| Any serious AE | NR | NR | 61/139 (44) | 53/139 (38) |
Appendix 5 Clinical effectiveness supplementary information
Best-available response rate
The best-available response rates for the cetuximab trials are reported in Table 147.
| First author and year (trial) | Experimental | n | N | % | Control | n | N | % |
|---|---|---|---|---|---|---|---|---|
| CR | ||||||||
| Bokemeyer 201565 (OPUS)a | CET + FOLFOX4 | 2 | 38 | 5 | FOLFOX4 | 0 | 49 | 0 |
| Van Cutsem 201543 (CRYSTAL)a | CET + FOLFIRI | 2 | 178 | 1.1 | FOLFIRI | 0 | 189 | 0 |
| Heinemann 201428 (FIRE-3)b | CET + FOLFIRI | 9 | 171 | 5 | BEV + FOLFIRI | 2 | 171 | 1 |
| PR | ||||||||
| Bokemeyer 201565 (OPUS)a | CET + FOLFOX4 | 20 | 38 | 53 | FOLFOX4 | 14 | 49 | 29 |
| Van Cutsem 201543 (CRYSTAL)a | CET + FOLFIRI | 116 | 178 | 65.2 | FOLFIRI | 73 | 189 | 38.6 |
| Heinemann 201428 (FIRE-3)b | CET + FOLFIRI | 103 | 171 | 60 | BEV + FOLFIRI | 100 | 171 | 59 |
| Stable disease | ||||||||
| Bokemeyer 201565 (OPUS)a | CET + FOLFOX4 | 10 | 38 | 26 | FOLFOX4 | 21 | 49 | 43 |
| Van Cutsem 201543 (CRYSTAL)a | CET + FOLFIRI | 48 | 178 | 27.0 | FOLFIRI | 90 | 189 | 47.6 |
| Heinemann 201428 (FIRE-3)b | CET + FOLFIRI | 26 | 171 | 15 | BEV + FOLFIRI | 50 | 171 | 29 |
| Progressive disease | ||||||||
| Bokemeyer 201565 (OPUS)a | CET + FOLFOX4 | 4 | 38 | 11 | FOLFOX4 | 8 | 49 | 16 |
| Van Cutsem 201543 (CRYSTAL)a | CET + FOLFIRI | 7 | 178 | 3.9 | FOLFIRI | 17 | 189 | 9.0 |
| Heinemann 201428 (FIRE-3)b | CET + FOLFIRI | 10 | 171 | 6 | BEV + FOLFIRI | 8 | 171 | 5 |
| Not evaluable | ||||||||
| Bokemeyer 201565 (OPUS)a | CET + FOLFOX4 | 2 | 38 | 5 | FOLFOX4 | 6 | 49 | 12 |
| Van Cutsem 201543 (CRYSTAL)a | CET + FOLFIRI | 5 | 178 | 2.8 | FOLFIRI | 9 | 189 | 4.8 |
| Heinemann 201428 (FIRE-3)b | CET + FOLFIRI | 23 | 171 | 13 | BEV + FOLFIRI | 11 | 171 | 6 |
The best-available response rates for the panitumumab trials are reported in Table 148.
| First author and year (trial) | Experimental | n | N | % | Control | n | N | % |
|---|---|---|---|---|---|---|---|---|
| CR | ||||||||
| Douillard 201426 (PRIME)a | PAN + FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b | FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b |
| Schwarzberg 201429 (PEAK)a | PAN + mFOLFOX6 | 2 | 88 | 2 | BEV + mFOLFOX6 | 1 | 82 | 1 |
| PR | ||||||||
| Douillard 201426 (PRIME)a | PAN + FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b | FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b |
| Schwarzberg 201429 (PEAK)a | PAN + mFOLFOX6 | 54 | 88 | 61 | BEV + mFOLFOX6 | 48 | 82 | 59 |
| Stable disease | ||||||||
| Douillard 201426 (PRIME)a | PAN + FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b | FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b |
| Schwarzberg 201429 (PEAK)a | PAN + mFOLFOX6 | 23 | 88 | 26 | BEV + mFOLFOX6 | 22 | 82 | 27 |
| Progressive disease | ||||||||
| Douillard 201426 (PRIME)a | PAN + FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b | FOLFOX4 | (Confidential information has been removed)b | (Confidential information has been removed)b | (Confidential information has been removed)b |
| Schwarzberg 201429 (PEAK)a | PAN + mFOLFOX6 | 1 | 88 | 1 | BEV + mFOLFOX6 | 4 | 82 | 5 |
| Not evaluable | ||||||||
| Douillard 201426 (PRIME)a | PAN + FOLFOX4 | NR | NR | NR | FOLFOX4 | NR | NR | NR |
| Schwarzberg 201429 (PEAK)a | PAN + mFOLFOX6 | 8 | 88 | 9 | BEV + mFOLFOX6 | 6 | 82 | 7 |
Safety
The incidence of grade 1 or grade 2 AEs for the cetuximab trial is reported in Table 149 and for the panitumumab trials is reported in Table 150.
| AE | FIRE-3,b,c,d n/N (%) | |
|---|---|---|
| CET + FOLFIRI (n = 171) | BEV + FOLFIRI (n = 171) | |
| Acneiform exanthema/rash | 99/171 (58)e | 14/171 (8)e |
| Desquamation | 51/171 (30)e | 18/171 (11)e |
| Diarrhoea | 85/171 (50) | 89/171 (52) |
| Haemotoxicity | 102/171 (60)e | 119/171 (70)e |
| Hepatotoxicity | 105/171 (61) | 89/171 (52) |
| Hypertension | 32/171 (19)e | 46/171 (27)e |
| Hypokalaemia | 56/171 (33)e | 27/171 (16) |
| Infection | 64/171 (37) | 69/171 (40)e |
| Mucositis/stomatitis | 61/171 (36) | 68/171 (40) |
| Nail changes/paronychia | 47/171 (28)e | 17/171 (10)e |
| Nausea | 74/171 (43)e | 97/171 (57)e |
| Pain | 75/171 (44)e | 87/171 (51)e |
| Skin reaction | 98/171 (57) | 72/171 (42) |
| Thromboembolic event | 3/171 (2) | 2/171 (1) |
| Thrombosis (any) | 3/171 (2) | 7/171 (4) |
| AE | Trial | |||
|---|---|---|---|---|
| PRIME,a,b,c n/N (%) | PEAK,a,b,d n/N (%) | |||
| PAN + FOLFOX4 (n = 250) | FOLFOX4 (n = 249) | PAN + mFOLFOX6 (n = 86) | BEV + mFOLFOX6 (n = 80) | |
| Abdominal pain | 67/250 (27) | 55/249 (22) | 17/86 (20) | 18/80 (23) |
| Abdominal pain (upper) | 18/250 (7) | 17/249 (7) | 7/86 (8) | 6/80 (8) |
| Acne | 37/250 (15)e | 1/249 (0)e | 19/86 (22) | 1/80 (1) |
| Alopecia | 42/250 (17)e | 22/249 (9)e | 16/86 (19) | 13/80 (16) |
| Anaemia | 32/250 (13) | 32/249 (13) | 16/86 (19)e | 11/80 (14)e |
| Anorexia | 87/250 (35)e | 64/249 (26)e | – | – |
| Anxiety | 20/250 (8) | 20/249 (8) | 6/86 (7) | 4/80 (5) |
| Arthralgia | – | – | 7/86 (8) | 8/80 (10) |
| Ascites | – | – | 0/86 (0) | 4/80 (5) |
| Asthenia | 54/250 (22) | 42/249 (17) | 28/86 (33)e | 22/80 (28)e |
| Back pain | 26/250 (10) | 30/249 (12) | 9/86 (10) | 9/80 (11) |
| Blood creatinine increased | – | – | 1/86 (1) | 6/80 (8) |
| Bronchitis | – | – | 4/86 (5) | 7/80 (9) |
| Cheilitis | – | – | 6/86 (7) | 0/80 (0) |
| Chills | 13/250 (5) | 14/249 (6) | 7/86 (8) | 9/80 (11) |
| Confusional state | – | – | 1/86 (1) | 4/80 (5) |
| Conjunctivitis | 49/250 (20)e | 9/249 (4)e | 12/86 (14) | 3/80 (4) |
| Constipation | 74/250 (30) | 78/249 (31) | 31/86 (36) | 30/80 (38) |
| Cough | 27/250 (11)e | 47/249 (19)e | 12/86 (14) | 5/80 (6) |
| Decreased appetite | – | – | 40/86 (47)e | 26/80 (33)e |
| Decreased weight | 46/250 (18)e | 15/249 (6)e | 21/86 (24)e | 7/80 (9)e |
| Dehydration | – | – | 12/86 (14)e | 5/80 (6)e |
| Depression | 13/250 (5) | 11/249 (4) | – | – |
| Dermatitis acneiform | 82/250 (33)e | 0/249 (0)e | 14/86 (16)e | 0/80 (0)e |
| Diarrhoea | 159/250 (64)e | 127/249 (51)e | 54/86 (63)e | 46/80 (58)e |
| Dizziness | 15/250 (6) | 22/249 (9) | 9/86 (10) | 9/80 (11) |
| Dry mouth | 13/250 (5) | 10/249 (4) | – | – |
| Dry skin | 56/250 (22)e | 13/249 (5)e | 31/86 (36)e | 6/80 (8)e |
| Dysaesthesia | 15/250 (6) | 19/249 (8) | 11/86 (13) | 12/80 (15) |
| Dysguesia | 38/250 (15) | 37/249 (15) | 20/86 (23)e | 13/80 (16)e |
| Dyspepsia | 32/250 (13) | 32/249 (13) | 5/86 (6) | 8/80 (10) |
| Dysphagia | – | – | 4/86 (5) | 4/80 (5) |
| Dysphonia | – | – | 3/86 (3) | 6/80 (8) |
| Dyspnoea | 20/250 (8) | 24/249 (10) | 10/86 (12) | 11/80 (14) |
| Dyspnoea exertional | – | – | 5/86 (6) | 4/80 (5) |
| Epistaxis | 40/250 (16)e | 29/249 (12)e | 20/86 (23) | 17/80 (21) |
| Erythema | 40/250 (916)e | 9/249 (4)e | 8/86 (9)e | 0/80 (0)e |
| Exfoliative rash | – | – | 9/86 (10)e | 2/80 (3)e |
| Fall | – | – | 1/86 (1) | 4/80 (5) |
| Fatigue | 93/250 (37) | 85/249 (34) | 35/46 (41)e | 39/80 (49)e |
| Flatulence | – | – | 8//86 (9) | 5/80 (6) |
| Haematoma | – | – | 1/86 (1)e | 5/80 (6)e |
| Haemoglobin decreased | – | – | 0/86 (0)e | 4/80 (5)e |
| Headache | 17/250 (7)e | 32/249 (13)e | 10/86 (12) | 11/80 (14) |
| Hypersensitivity | 7/250 (3) | 17/249 (7) | – | – |
| Hypertension | 14/250 (6) | 9/249 (4) | 2/86 (2)e | 18/80 (23)e |
| Hypertrichosis | – | – | 5/86 (6)e | 0/80 (0)e |
| Hypoaesthesia | – | – | 2/86 (2) | 5/80 (6) |
| Hypoalbuminaemia | – | – | 2/86 (2) | 5/80 (6) |
| Hypocalcaemia | 15/250 (6) | 5/249 (2) | 9/86 (10) | 4/80 (5) |
| Hypokalaemia | 40/250 (16) | 29/249 (12) | 21/86 (24)e | 6/80 (8)e |
| Hypomagnesaemia | 74/250 (30)e | 16/249 (6)e | 37/86 (43)e | 5/80 (6)e |
| Hypotension | – | – | 7/86 (8) | 2/80 (3) |
| Influenza | 2/250 (1)e | 15/249 (6)e | – | – |
| Infusion-related reaction | – | – | 5/86 (6) | 5/80 (6) |
| Insomnia | 34/250 (14) | 37/249 (15) | 10/86 (12)e | 14/80 (18)e |
| Lacrimation increased | 18/250 (7) | 21/249 (8) | 5/86 (6) | 2/80 (3) |
| Lethargy | 9/250 (4) | 15/249 (6) | – | – |
| Leukopenia | 19/250 (8) | 19/249 (8) | 6/86 (7) | 4/80 (5) |
| Mucosal inflammation | 58/250 (23)e | 40/249 (16)e | 28/86 (33)e | 12/80 (15)e |
| Muscular weakness | – | – | 2/86 (2) | 5/80 (6) |
| Musculoskeletal chest pain | – | – | 2/86 (2)e | 6/80 (8)e |
| Musculoskeletal pain | – | – | 6/86 (7) | 5/80 (6) |
| Nail disorder | 32/250 (13)e | 3/249 (1)e | 7/86 (8) | 3/80 (4) |
| Nasopharyngitis | 17/250 (7) | 13/249 (5) | – | – |
| Nausea | 111/250 (44) | 122/249 (49) | 50/86 (58)e | 50/80 (63)e |
| Neck pain | – | – | 1/86 (1)e | 7/80 (9)e |
| Neurotoxicity | 15/250 (6) | 12/249 (5) | 7/86 (8) | 6/80 (8%) |
| Neuropathy peripheral | 48/250 (19)e | 67/249 (27)e | 35/86 (41)e | 23/80 (29)e |
| Neutropenia | 114/250 (46) | 113/249 (45) | 23/86 (27) | 19/80 (24) |
| Oedema peripheral | 28/250 (11) | 26/249 (10) | 11/86 (13) | 8/80 (10) |
| Oropharyngeal pain | NR | NR | 4/86 (5)e | 9/80 (11)e |
| Pain in extremity | 18/250 (7) | 17/250 (7) | 10/86 (12) | 10/80 (13) |
| Pain in jaw | NR | NR | 2/86 (2) | 4/80 (5) |
| Palmar-plantar erythrodysaesthesia | 26/250 (10)e | 7/249 (3)e | 16/86 (19)e | 7/80 (9)e |
| Paraesthesia | 77/250 (31) | 75/249 (30) | 17/86 (20)e | 21/80 (26)e |
| Paronychia | 58/250 (23)e | 0/249 (0)e | 17/86 (20)e | 0/80 (0)e |
| Peripheral sensory neuropathy | 38/250 (15) | 44/249 (18) | 14/86 (16)e | 18/80 (23)e |
| Platelet count decreased | NR | NR | 6/86 (7) | 5/80 (6) |
| Pollakiuria | NR | NR | 5/86 (6) | 0/80 (0) |
| Polyneuropathy | 9/86 (10) | 7/80 (9) | ||
| Productive cough | NR | NR | 2/86 (2) | 4/80 (5) |
| Proteinuria | NR | NR | 12/86 (14)e | 5/80 (6)e |
| Pruritus | 62/250 (25)e | 11/249 (4)e | 12/86 (14)e | 3/80 (4)e |
| Pyrexia | 79/250 (32) | 68/249 (27) | 13/86 (15)e | 18/80 (23)e |
| Rash | 138/250 (55)e | 20/249 (8)e | 54/86 (63)e | 7/80 (9)e |
| Rectal haemorrhage | 13/250 (5) | 5/249 (2) | 7/86 (8) | 3/80 (4) |
| Rhinitis | 7/250 (3) | 13/249 (5) | 5/86 (6) | 2/80 (3) |
| Rhinorrhea | NR | NR | 1/86 (1)e | 7/80 (9)e |
| Skin disorders | NR | NR | 85/86 (99)e | 36/80 (45)e |
| Skin fissures | 42/250 (17) | 1/249 (0) | 17/86 (20)e | 1/80 (1)e |
| Skin hyperpigmentation | NR | NR | 1/86 (1) | 4/80 (5) |
| Skin toxicity | NR | NR | 5/86 (6)e | 0/80 (0)e |
| Stomatitis | 76/250 (30)e | 35/249 (14)e | 35/86 (41)e | 19/80 (24)e |
| Temperature intolerance | NR | NR | 6/86 (7) | 7/80 (9) |
| Thrombocytopenia | 47/250 (19)e | 64/249 (26)e | 22/86 (26)e | 6/80 (8)e |
| Upper respiratory tract infection | 13/250 (5) | 12/249 (5) | 3/86 (3)e | 9/80 (11)e |
| Urinary tract infection | 22/250 (9) | 17/249 (7) | 7/86 (8) | 7/80 (9) |
| Vision blurred | NR | NR | 8/86 (9)e | 1/80 (1)e |
| Vomiting | 75/250 (30) | 78/249 (31) | 31/86 (36)e | 24/80 (30)e |
| Weight increased | NR | NR | 8/86 (9)e | 2/80 (3)e |
Network meta-analysis: additional analyses (safety FOLFOX network)
For many AEs, the OPUS study did not provide the required information and so no comparison could be made between cetuximab plus FOLFOX and panitumumab plus FOLFOX for diarrhoea, hypokalaemia, hypomagnesaemia, mucositis/stomatitis, mucosal inflammation, fatigue, peripheral neuropathy or asthenia. Instead, analyses are reported here to allow the indirect comparison between bevacizumab plus FOLFOX and FOLFOX (Tables 151–158). Note that, because of the small numbers of events for hypomagnesaemia, mucositis/stomatitis and mucosal inflammation, the 95% CrIs are wide.
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 96 | 4 | < 1 | ||
| BEV + FOLFOX | 3.04 (0.90 to 10.49) | 4 | 30 | 66 | |
| PAN + FOLFOX | 2.40 (1.41 to 4.19)b | 0.79 (0.26 to 2.36)c | < 1 | 66 | 34 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 84 | 15 | < 1 | ||
| BEV + FOLFOX | 2.02 (0.50 to 8.03) | 16 | 41 | 43 | |
| PAN + FOLFOX | 2.23 (1.11 to 4.70)b | 1.10 (0.35 to 3.67)c | < 1 | 44 | 56 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 65 | 35 | 0 | ||
| BEV + FOLFOX | 2.80 (0.01 to 2176) | 35 | 65 | < 1 | |
| PAN + FOLFOX | 87.93 (7.55 to 50,590)b | 33.07 (2.42 to 22,180)c | 0 | < 1 | 100 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 45 | 55 | < 1 | ||
| BEV + FOLFOX | 0.75 (0.01 to 44.47) | 55 | 44 | < 1 | |
| PAN + FOLFOX | 20.07 (3.14 to 483.4)b | 27.01 (2.00 to 13,950)c | 0 | < 1 | 100 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 89 | 11 | < 1 | ||
| BEV + FOLFOX | 5.77 (0.36 to 186.4) | 11 | 82 | 7 | |
| PAN + FOLFOX | 18.59 (3.11 to 463.5)b | 3.32 (0.69 to 27.16)c | < 1 | 7 | 93 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 97 | 3 | < 1 | ||
| BEV + FOLFOX | 3.65 (0.98 to 14.15) | 3 | 61 | 36 | |
| PAN + FOLFOX | 4.34 (1.92 to 11.12)b | 1.20 (0.44 to 3.35)c | < 1 | 36 | 64 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 30 | 32 | 38 | ||
| BEV + FOLFOX | 1.00 (0.28 to 3.64) | 37 | 19 | 43 | |
| PAN + FOLFOX | 0.93 (0.45 to 1.90)b | 0.92 (0.32 to 2.68)c | 32 | 49 | 19 |
| Intervention treatment | Comparator treatment | Probability (%) ranked | |||
|---|---|---|---|---|---|
| FOLFOX | BEV + FOLFOX | First | Second | Third | |
| FOLFOX | 70 | 22 | 8 | ||
| BEV + FOLFOX | 2.22 (0.36 to 15.22) | 17 | 16 | 67 | |
| PAN + FOLFOX | 1.47 (0.62 to 3.67)b | 0.67 (0.12 to 3.32)c | 13 | 62 | 25 |
Appendix 6 Ongoing trials
Searches of ClinicalTrials.gov, WHO ICTRP, UKCRN and ISRCTN were conducted (see Appendix 1 for the search strategies used). All searches were carried out in March 2015. Seven trials were considered as being relevant to this review (Table 159).
| Register/identifier number | Sponsor/collaborators | Trial name | Study location | Established or anticipated sample size | Status (correct as of June 2017) | Included in PenTAG Review |
|---|---|---|---|---|---|---|
| NCT00819780 | Amgen | PEAK | USA, Canada, Belgium, Germany, Italy, Spain | 285 | Completeda | Yes |
| NCT00125034 | Merck KGaA | OPUS | Austria, Belgium, France, Germany, Greece, Israel, Italy, Poland, Portugal, Romania, Russia, Spain, Ukraine | 344 | Completed | Yes |
| NCT01228734 | Merck KGaA | TAILORb | China | 481 | Completedc | No |
| NCT00364013 | Amgen | PRIME | Multinational | 1183 | Completed | Yes |
| NCT00154102 | Merck KGaA | CRYSTAL | Multinational | 1221 | Completed | Yes |
| NCT00433927 | Merck KGaA | FIRE-3 | Germany | 568 | Ongoing not recruitingd | Yes |
| EUCTR2014–000543–33-BE | Amgen | PANIBe | Belgium | NR | Ongoingf | No |
Appendix 7 RAS mutation testing
Panitumumab and cetuximab are licensed only for EGFR-expressing, RAS WT populations, as their clinical effectiveness is associated with this. We therefore assessed whether or not the identification of people as having EGFR-expressing RAS WT tumours was significantly different between the trials and clinical practice, as this could impact on the effectiveness of panitumumab and cetuximab in practice.
Epidermal growth factor receptor expression
The clinical trials included in our review assessed patients for EGFR expression, in line with the technologies’ licensed indications. Our clinical advisors (Dr Mark Napier and Christopher Bowles, both based at the Royal Devon and Exeter Hospital) advised that testing for EGFR expression is rarely, if ever, carried out in practice, as it is believed to not be indicative of the effectiveness of treatment (2015, personal communication). They believe that not testing for EGFR expression is unlikely to alter the population included such that the treatment effect changes. Therefore, although this is different from normal practice in the trials, in which EGFR status was confirmed, it is believed that it is unlikely to affect the comparison of effectiveness between trials and clinical practice.
As it is not routinely carried out in practice and is not believed to affect the treatment effect, we did not include EGFR testing in our model.
RAS mutation testing in trials
We compared the testing techniques used across our included trials to see how similar they were to each other. In our included trials, people with mCRC who were RAS WT were identified retrospectively, using a range of techniques for testing. This information is summarised in Table 160.
| Variable | Trial | ||||
|---|---|---|---|---|---|
| CRYSTAL24,43 | FIRE-328 | OPUS23,65 | PEAK29 | PRIME27,44 | |
| Initial KRAS exon 2 test | Sample type: biopsy; testing technique: LNA-mediated qPCR clamping and melting curve analysis | Pyrosequencing (PyroMark Gold kit, Qiagen) | Melting curve analysis | NR | TheraScreen KRAS, Qiagen (Germantown, MD, USA) |
| Codons | 12, 13 | 12, 13 | 12, 13 | NR | NR |
| Size of cohort to test | 1198 | NR | 337 | NR | 1183 |
| Size of cohort actually tested | 1063 | NR | 233 | NR | 1096 |
| % of cohort tested | 89 | NR | 69 | NR | 93 |
| Reason for tests not conducted | Sample inadequate or unavailable | NR | NR | NR | NR |
| Failure rate of test | NR | NR | NR | NR | NR |
| RAS test | OncoBEAM RAS CRC test by Sysmex (Hamburg, Germany) | Pyrosequencing (PyroMark Gold kit, Qiagen) | OncoBEAM RAS CRC test by Sysmex (Hamburg, Germany) | Sanger sequencing, WAVE-based Surveyor CRC RAScan™ kit (Transgenomic, Omaha, NE, USA) | Sanger sequencing, WAVE-based Surveyor CRC RAScan™ kit (Transgenomic, Omaha, NE, USA) |
| Additional KRAS codons | 59, 61, 117, 146 | 61, 146 | 59, 61, 117, 146 | 12, 13, 59, 61, 117, 146 | 61, 117, 146 |
| NRAS codons | 12, 13, 59, 61, 117, 146 | 12, 13, 59, 61, 117, 146 | 12, 13, 59, 61, 117, 146 | 12, 13, 59, 61, 117, 146 | 12, 13, (59a), 61, 117, 146 |
| Size of cohort to test | 666 | 592 | 179 | 285 | 1183 |
| Size of cohort actually tested | 430 | 542 | 118 | 250 | 1060 |
| % of cohort tested | 65 | 92 | 66 | 88 | 90 |
| Reason for tests not being conducted | NR | NR | NR | Inadequate samples | NR |
| Failure rate of test (%) | NR | NR | NR | 2.60 | 3.28 |
In general, the methods for preparing tissue samples seemed similar across the trials, with PCR methods used. However, the actual tests used on the samples seemed to differ. For many studies looking at a RAS WT population, the population was already identified as KRAS WT and the reporting of these methods was variable, but indicated that, again, several testing techniques were used.
All trials looked at exons 2, 3 and 4 for both NRAS and KRAS testing and nearly all trials looked at the full range of identified codons for both NRAS and KRAS mutations.
One way that we can compare tests across trials and with tests in clinical practice is to compare the failure rates of the tests. This is when tests are not completed and therefore are unable to provide a diagnosis, rather than when they give an incorrect diagnosis. We attempted to ascertain the percentage of inadequate samples for each test and the failure rate of the tests on samples that were adequate. The trials were not always specific about why some of the cohort was not tested. In the PEAK trial29 it was specified that the intended testing cohort was those patients from whom samples were collected, so any tests that were not run were the result of inadequate samples. For the other trials, the number of patient samples collected was not reported and so the failure to run a test could have been the result of both inadequate and unavailable samples. Most trials did not report the reasons why people could not be tested and so it was not possible to adequately estimate the trial failure rate for sample collection. Even for the populations for whom a successful KRAS test was conducted, the reasons for samples being unable to be tested for RAS WT either were not reported or were unclear.
Similarly, few trials reported the failure rates of the actual tests, that is, tests that were unable to provide a diagnosis even with an adequate sample. The PEAK29 and PRIME44 trials reported a failure rate of ≈3% for Sanger sequencing, with the test failing on at least one of the codons.
Given the limited reporting of the testing carried out in the trials, it is difficult to compare them directly, which in turn makes it difficult to compare them with clinical practice. The one important similarity in terms of trial testing was that the trials generally looked for mutations in all identified codons for both the KRAS gene and the NRAS gene.
RAS mutation testing in the UK
A request was sent out for information on RAS mutation tests currently used in the UK through the UK National External Quality Assessment Service. The data that we received are summarised in Table 161. For laboratories that reported the cost of testing, this was generally £200 for joint KRAS and NRAS testing, regardless of the technique used or codons assessed. There is some variability in the tests used, but pyrosequencing appears to be generally well established. We received little information on the accuracy of the tests available, although personal communication from Dr Michelle Wood of the Cardiff and Vale University Health Board (8 April 2015) suggested that NRAS mutation testing may currently be less sensitive than KRAS mutation testing.
| Variable | Location | ||
|---|---|---|---|
| Cardiff | Exeter | Salisbury | |
| Type of test | Pyrosequencing; for tumour samples of < 10%, COLD-PCR reduces limit of detection | Next-generation sequencing | |
| KRAS codons tested | 12, 13, 61, 146 | 12, 13, 59, 61, 117, 146 | 12, 13, 61 |
| NRAS codons tested | 12, 13, 59, 61 | 12, 13, 59, 61 | 12, 13, 61 |
| Reported accuracy | Sensitivity: KRAS 99%, NRAS 88% | NR | NR |
| Cost | KRAS or NRAS £120, KRAS and NRAS £200 | KRAS and NRAS £200 | Contact |
| Notes | 20% tumour tissue required | ||
| Source | Dr Michelle Wood (8 April 2015, personal communication) | Christopher Bowles (18 April 2015, personal communication) | Wessex Regional Genetics Laboratory web page188 |
Comparing testing in clinical practice with that carried out in the trials, in general, fewer codons are assessed. The PRIME trial44 demonstrated that, by adding one additional codon to the tests (codon 59), an additional seven people who were RAS mutant were discovered. This suggests that in clinical practice there is the potential for people diagnosed as RAS WT to be diagnosed as RAS mutant if the techniques used were more similar to those used in the trials.
Published evidence of RAS mutation testing in practice
As well as contacting UK genetics laboratories directly and examining their websites, we searched for literature that described comparisons of tests to ascertain the accuracy data available. There is limited published evidence on the accuracy of RAS mutation tests. One study, by Blons et al. ,189 conducted in France on KRAS testing, compared several testing techniques; the results for tests used in more than one laboratory are reproduced in Table 162. The results show that, even with high levels of dilution, the sensitivity and specificity remain quite high, in accordance with the tests’ limits of detection.
| Test | Laboratories (n) | Samples (n) | Analytical failures (n) | Analytical failures (%) | Success rate in true negative (%) | Success rate in true positive (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dilutions | ||||||||||
| All | 100% | 50% | 25% | 5% | ||||||
| Direct sequencing | 15 | 1260 | 4 | 0.32 | 98.90 | 76.00 | 99.00 | 99.00 | 87.00 | 38.00 |
| Taqman | 8 | 672 | 11 | 1.64 | 99.00 | 92.30 | 95.80 | 100.00 | 99.30 | 76.40 |
| Snapshot | 7 | 588 | 4 | 0.68 | 98.80 | 89.70 | 95.20 | 100.00 | 93.70 | 73.80 |
| Pyrosequencing | 5 | 420 | 6 | 1.43 | 95.00 | 96.60 | 100.00 | 100.00 | 100.00 | 89.70 |
| HRM and sequencing | 5 | 420 | 0 | 0.00 | 100.00 | 78.00 | 100.00 | 98.90 | 88.90 | 40.00 |
A further study by Tack et al. 190 was identified that reported combined false-negative rates of 5.0% (sensitivity 95%) and false-positive rates of 1.5% (specificity 98.5%) for RAS mutation testing across 131 laboratories (10 samples). This included several different testing techniques in terms of the number of exons tested and the types of tests used. 190 This study summarises part of the work conducted by the European Society of Pathology Colon External Quality Assessment scheme and includes data from the UK. The results were similar to those from Blons et al. ,189 suggesting that the accuracy of testing for RAS mutations may not differ significantly from the accuracy of testing for KRAS mutations. It also suggests that testing may be fairly consistent, despite the wide range of techniques used.
A recent diagnostic assessment by Westwood et al. 45 compared diagnostic tests for detecting KRAS mutations. They found that the relationship between what the tests predicts (mutation status) and the outcome of this diagnosis (which treatment patients receive) is a complex one. As such, they adjusted the meaning of accuracy from ‘test accuracy’ to include ‘accuracy for predicting response to treatment with cetuximab in combination with standard chemotherapy, or variation in clinical outcomes following treatment with cetuximab in combination with standard chemotherapy depending on which method is used to classify patients as having KRAS wild-type tumours’.
The authors concluded that the diagnostic tests used in trials seem to result in a benefit for patients. Unfortunately, as not all tests used in practice have been used in trials, there is also little evidence to draw conclusions on the effectiveness of these tests. Westwood et al. 45 concluded that there was no significant evidence to suggest that different tests would result in different outcomes for patients, with the caveat that lack of evidence to show a difference is not equal to proving that the effectiveness of the tests is equivalent.
Given the paucity of significant accuracy data to contradict the findings of Westwood et al. ,45 and because it is outside the scope of this review, we currently agree with Westwood et al. ’s45 assessment. Therefore, in our model we assume that the accuracy of the tests in the trials are equal to the accuracy of those used in practice and make no adjustments for this.
Appendix 8 WinBUGs code for the network meta-analyses
Fixed-effects model with normal likelihood and identity link used for overall survival, progression-free survival and objective response rate outcomes
Fixed-effects model with binomial likelihood and logit link used for adverse event outcomes
Glossary
- Epidermal growth factor receptor (EGFR)
- The protein encoded by the EGFR gene is a transmembrane glycoprotein that is a member of the protein kinase superfamily. This protein is a receptor for members of the epidermal growth factor family. The epidermal growth factor receptor is a cell surface protein that binds to epidermal growth factor. Binding of the protein to a ligand induces receptor dimerisation and tyrosine autophosphorylation and leads to cell proliferation. Mutations in this gene are associated with lung cancer. Multiple alternatively spliced transcript variants that encode different protein isoforms have been found for this gene.
- Kirsten rat sarcoma (KRAS)
- The KRAS gene belongs to a class of genes known as oncogenes. When mutated, oncogenes have the potential to cause normal cells to become cancerous. The proteins encoded by these genes play important roles in cell division, cell differentiation and the self-destruction of cells (apoptosis).
- Neuroblastoma rat sarcoma (NRAS)
- The NRAS gene belongs to a class of genes known as oncogenes. When mutated, oncogenes have the potential to cause normal cells to become cancerous. The proteins encoded by these genes play important roles in cell division, cell differentiation and the self-destruction of cells (apoptosis).
- Rat sarcoma (RAS)
- This gene family consists of Harvey rat sarcoma (HRAS), neuroblastoma rat sarcoma (NRAS) and Kirsten rat sarcoma (KRAS).
- Wild type
- The normal, non-mutated version of a gene that is common in nature.
List of abbreviations
- AE
- adverse event
- AiC
- academic-in-confidence
- ASCO
- American Society of Clinical Oncology
- BNF
- British National Formulary
- BSC
- best supportive care
- CCEMG
- Campbell and Cochrane Economics Methods Group
- CDF
- Cancer Drugs Fund
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHMP
- Committee for Medicinal Products for Human Use
- CI
- confidence interval
- CiC
- commercial-in-confidence
- CMU eMit
- Commercial Medicines Unit Electronic Market Information Tool
- CPCI-S
- Conference Proceedings Citation Index –Science
- CR
- complete response
- CRC
- colorectal cancer
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CRYSTAL
- Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer
- CT
- computerised tomography
- ECOG
- Eastern Cooperative Oncology Group
- EGFR
- epidermal growth factor receptor
- EMA
- European Medicines Agency
- EORTC
- European Organisation for Research and Treatment of Cancer
- EPPI-Centre
- Evidence for Policy and Practice Information and Coordinating Centre
- EQ-5D
- EuroQol 5-Dimensions
- ERG
- Evidence Review Group
- ESMO
- European Society of Medical Oncology
- FIRE-3
- 5-FU, Folinic Acid and Irinotecan (FOLFIRI) Plus Cetuximab versus FOLFIRI Plus Bevacizumab in First Line Treatment of Colorectal Cancer
- FOLFIRI
- folinic acid + 5-fluorouracil + irinotecan
- FOLFOX
- folinic acid + 5-fluorouracil + oxaliplatin
- HEED
- Health Economic Evaluations Database
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- health technology assessment
- ICER
- incremental cost-effectiveness ratio
- ICTRP
- International Clinical Trials Registry Platform
- IQR
- interquartile range
- ISD
- Information Services Division
- ISRCTN
- International Standard Randomised Controlled Trials Number
- ITT
- intention to treat
- KRAS
- Kirsten rat sarcoma
- mCRC
- metastatic colorectal cancer
- MedDRA
- Medical Dictionary for Regulatory Activities
- mFOLFOX6
- modified FOLFOX6
- MRI
- magnetic resonance imaging
- MTA
- multiple technology appraisal
- NCI-CTC
- National Cancer Institute Common Terminology Criteria
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NRAS
- neuroblastoma rat sarcoma
- OPUS
- Oxaliplatin and Cetuximab in First-line Treatment of Metastatic Colorectal Cancer
- OR
- odds ratio
- ORR
- objective response rate
- OS
- overall survival
- PAS
- Patient Access Scheme
- PCR
- polymerase chain reaction
- PEAK
- Panitumumab Plus mFOLFOX6 vs. Bevacizumab Plus mFOLFOX6 for First Line Treatment of Metastatic Colorectal Cancer (mCRC) Patients With Wild-Type Kirsten Rat Sarcoma-2 Virus (KRAS) Tumors
- PenTAG
- Peninsula Technology Assessment Group
- PFS
- progression-free survival
- PICO
- population, intervention, comparator, outcome
- PICOS
- population, intervention, comparator, outcome, study design
- PR
- partial response
- PRIME
- Panitumumab Randomized trial In combination with chemotherapy for Metastatic colorectal cancer to determine Efficacy
- QALY
- quality-adjusted life-year
- RAS
- rat sarcoma
- RCT
- randomised controlled trial
- RECIST
- Response Evaluation Criteria in Solid Tumours
- SD
- standard deviation
- SE
- standard error
- SF-6D
- Short Form questionnaire-6 Dimensions
- SMC
- Scottish Medicines Consortium
- SPC
- Summary of Product Characteristics
- STA
- single technology appraisal
- TA
- technology appraisal
- TNM
- tumour invasion, nodal involvement and metastatic spread
- TTP
- time to progression
- UKCRN
- UK Clinical Research Network
- WHO
- World Health Organization
- WT
- wild type
- XELIRI
- capecitabine + irinotecan
- XELOX
- capecitabine + oxaliplatin
This monograph is based on the Technology Assessment Report produced for the National Institute for Health and Care Excellence (NICE). The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.