Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/17/01. The contractual start date was in May 2011. The draft report began editorial review in November 2015 and was accepted for publication in November 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan Sterne was a member of the National Institute for Health Research (NIHR) Health Technology Assessment Clinical Evaluation and Trials Board while the study was being conducted.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Mangtani et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Tuberculosis epidemiology
Tuberculosis (TB) remains a significant and preventable cause of morbidity and mortality globally. Approximately 10% of infections with Mycobacterium tuberculosis progress to clinical disease. 1 The World Health Organization2 estimates that > 2 billion of the world’s population is infected. In 2014, 9.6 million people developed symptoms of TB disease and 1.5 million died from TB. 2 In the UK, after many decades during which both the risk of infection with M. tuberculosis and the incidence of TB decreased, the last decade of the 20th century and the first decade of the 21st century saw a steady rise in TB cases. 3 From 2005 to 2011, there were around 8000 cases of TB per year in England. This declined to 6520 cases in 2014, but England still has the highest rate of TB in Western Europe. 4 There has been no decline in TB rates among the UK-born population overall; however, the incidence of childhood TB in UK-born children, including miliary disease and meningitis, has started to fall. TB continues to be concentrated in urban areas, with much higher rates in the most deprived areas and in non-UK-born populations. 5 Drug-resistant TB has increased among culture-confirmed cases in the UK (the percentage resistant to any first-line drug increased from 5.6% in 1998 to 7.5% in 2005), mainly because of a rise in isoniazid resistance,6 and has remained stable. 5 However, the percentage of patients with multidrug resistance has started to fall and is < 1.4%, although complex long-term treatment requirements and poor completion rates make such an outcome an ongoing concern.
Bacillus Calmette–Guérin vaccine effectiveness and UK policies on bacillus Calmette–Guérin vaccination in relation to the changing epidemiology in the UK
Bacillus Calmette–Guérin (BCG) vaccination is widely used globally. In the UK, the vaccine has mainly been given either to infants or to adolescents at school. The protection against pulmonary TB in the UK is high when BCG is given to tuberculin-negative schoolchildren at around age 13 years. This was shown by a trial initiated by the Medical Research Council (MRC) in 19517 and in subsequent analyses of the effectiveness of the vaccine given in the routine school immunisation programme. 8 However, there have been variable findings with respect to the effectiveness of the vaccine against pulmonary disease in different countries or between different studies in the same country. 7,9,10 The effectiveness of the BCG vaccine given in infancy (to prevent pulmonary TB, miliary TB and tuberculous meningitis) has been found to be consistently high in all countries where it has been measured. 11,12 Although the World Health Organization13 recommends not to re-vaccinate, mostly because of lack of evidence of the efficacy of revaccination, many countries implement re-vaccination programmes. Trials in Malawi14 and more recently in Brazil15 found no increase in effectiveness or a modest increase in effectiveness associated with repeat BCG vaccination.
Although the MRC trial of adolescent vaccination with BCG demonstrated high levels of protection in the UK,7 there have been several subsequent policy changes in the UK with respect to BCG vaccination, prompted by changes in the epidemiology of TB. In brief, from 1953, BCG vaccine was given to tuberculin-negative [‘purified protein derivative (PPD)-negative’] schoolchildren at age 10–13 years, as part of the national vaccination programme. In 1972, as the proportion of cases of TB in ethnic minority groups increased, BCG vaccination in infancy was recommended for newborns of recent immigrants from countries with a high incidence of TB (e.g. Indian subcontinent and Africa) as well as all refugees and asylum seekers. It was also given to all newborns in some areas [health districts/primary care trusts (PCTs)] with a high TB incidence.
In 1991, a survey was conducted in the UK of how well the policies for BCG vaccination in the first year of life were implemented. 16 At that time, five districts offered the BCG vaccine to all newborn children, 31 districts offered it to none and 148 districts offered it to infants born to those in ethnic groups from the Indian subcontinent, Africa, the West Indies, China, the Middle East and South-East Asia. Of the 184 districts, 120 reported that they offered the vaccine to the newborn children of recent migrants from other countries with a high incidence of TB.
There was discussion over whether or not BCG vaccination of the general population should be discontinued when the risk of TB decreased, based primarily on the high number of vaccinations needed to prevent one case of TB in the UK and worldwide. 17 The International Union Against Tuberculosis and Lung Disease (IUATLD) developed a set of criteria for the discontinuation of mass BCG programmes in low-prevalence populations. 18 IUATLD recommends that BCG vaccination be discontinued if an efficient TB notification system is in place and:
-
the average annual notification rate of smear-positive pulmonary TB is < 5 per 100,000 or
-
the average annual notification rate of TB meningitis in children aged < 5 years has been < 1 per 10 million population over the previous 5 years or
-
the average annual risk of infection is < 0.1%.
The UK met all of these criteria and the BCG vaccination policy for the UK was changed by the Department of Health in 2005 to the current policy. 19 The school vaccination programme was stopped and BCG vaccination was recommended for infants using a risk-based approach, in line with the IUATLD guidelines. In the UK, infants are eligible for vaccination if they have a parent/grandparents originating from a high TB incidence country or if they are born in a part of the UK with a high incidence of TB (> 40 per 100,000). It is also recommended that some occupational groups, and uninfected contacts of TB cases, receive BCG vaccination. 20
Evidence for the duration of bacillus Calmette–Guérin protection
In the UK MRC trial,7 the efficacy of the BCG vaccine by time since vaccination of adolescents at school was estimated as 84% during the first 5 years after vaccination, 68% at between 5 and 10 years since vaccination and 63% at between 10 and 15 years since vaccination. Although all of these estimates were statistically significantly different from zero, the number of cases at 10–15 years post vaccination was small and the efficacy estimate had a wide 95% confidence interval (CI) (17% to 84%). There were too few cases between 15 and 20 years after vaccination to assess efficacy. A summary of protection by time since vaccination, with 95% CIs (calculated by us based on the trial data presented in the paper), is provided in Table 1. The level of protective effect in the first 10 years after vaccination was confirmed in a subsequent cohort analysis of data from the school-aged BCG vaccination programme in England. 8 There are no data on long-term protection post-infant BCG vaccination in high-risk groups.
| Trial group | Number of participants | Time since vaccination (years) | |||
|---|---|---|---|---|---|
| 0–5 | 5–10 | 10–15 | 15–20 | ||
| Negative reaction to tuberculin, unvaccinated | 12,867 | 160 | 67 | 16 | 5 |
| Negative reaction to tuberculin, BCG vaccinated | 13,598 | 27 | 22 | 7 | 6 |
| Negative reaction to tuberculin, vole bacillus vaccinated | 5817 | 12 | 11 | 2 | 1 |
| Total negative reaction, vaccinated with either vaccine | 19,415 | 39 | 33 | 9 | 7 |
| BCG vaccine effectiveness (95% CI) (%) | 84 (77 to 89) | 68 (51 to 79) | 63 (17 to 84) | 9 (–187 to 71) | |
The National Institute for Health Research stated, and we agree, that it is not known how long protection from the BCG vaccine lasts, particularly in different age and population groups, and this hinders the development of evidence-based policies. Until recently there was little evidence of protection lasting beyond 10 years after vaccination at any age. In a review of published studies conducted by two of the current authors, the pooled estimate of protection after 10 years was 14% (95% CI –9% to 32%). 21 Considerable heterogeneity was observed between studies in the annual change in BCG vaccine efficacy (VE) with time since vaccination. There was no relation between average annual change in efficacy and overall efficacy. As with most vaccines, immunological memory may wane with time, leading to a lower level of protection. Other explanations proposed include decreasing susceptibility among the unvaccinated because of continued exposure to environmental mycobacteria and an increase in the proportion of disease caused by reactivation or reinfection, against which BCG may not protect. 22
An update of this systematic review of the duration of protection conferred by the BCG vaccine against TB was conducted by our group. 23 The review included the recent additional follow-up of a BCG vaccine trial in Native Americans (who were, on average, aged 7 years when vaccinated in the 1930s), which has reported protection lasting for several decades,24 and a cohort study in the control arm of the Brazilian BCG re-vaccination trial, suggesting that protection lasted for 15–20 years. 25
However, there is evidence from some countries of poor protection of the BCG vaccine in adult life and much of the existing research is of uncertain relevance to the UK. The aims of this research project were to estimate the duration of protection of the BCG vaccine given to high-risk infants in the UK and to school-aged children in the general population. If the study provided evidence of a long duration of protection, beyond 10 years, this would have several implications, including changing the estimates of the cost-effectiveness of the BCG vaccine, the number of vaccinations needed to prevent a case, the possible characteristics of new BCG-like vaccines and the timing of vaccination for any new TB vaccine developed, that is, it would provide evidence for vaccination policies as well as inform the research and development of new TB vaccines.
Chapter 2 Research objectives
The aim of this study was to estimate the change in the effectiveness of the BCG vaccine in preventing TB with time since vaccination in the current UK population.
Primary objectives
-
To estimate the effectiveness of BCG vaccination in preventing TB when given in the first year of life to high-risk groups, at 5-year intervals since vaccination.
-
To estimate the effectiveness of BCG vaccination in preventing TB when given in adolescence to the general population, at 5-year intervals since vaccination, starting at 10 years since vaccination.
-
To explore whether or not protection wanes with time since vaccination in high-risk groups and in the general population.
Health technology assessed
The health technology assessed was BCG vaccination in the UK given to:
-
infants at higher risk of TB (referred to throughout as ‘infant BCG’)
-
schoolchildren in the general population (referred to throughout as ‘school-aged BCG’).
Chapter 3 Methods
Overview
Two main observational analytical studies aiming to estimate the effectiveness of the BCG vaccine against TB by time since vaccination were conducted, as well as three supporting studies. The two main studies were case–control studies aimed at estimating the effectiveness of (1) the BCG vaccine given to infants in high-risk groups (results generalisable to high-risk groups in the UK) and (2) the BCG vaccine given at school age to the general population (results generalisable to the UK population). The three supporting studies were (1) a survey of BCG vaccination policy in England, (2) a pilot study for the main observational studies and (3) a validation study of BCG scar reading.
In the two case–control studies, TB cases included in the study were sampled among those notified in the years 2003–12 to the UK national Enhanced Tuberculosis Surveillance (ETS) system. Control subjects (controls) were recruited from the community in the areas where sampled cases had arisen. BCG vaccination status was established based on BCG records when available, scar reading (inspection of both arms) and BCG history (recall of vaccination). Information on potential confounding variables (including demographic and social variables) was collected from cases and controls in a face-to-face computer-assisted interview conducted by trained staff from the National Centre for Social Research (NatCen), a leading centre for independent social research with > 40 years’ experience in nationwide surveys. Clinical and microbiological information, including type of disease for cases, was retrieved from the ETS system.
Ethics
The protocol for all studies, information leaflets and data collection tools were reviewed and approved by the NHS National Research Ethics Service Committee – London and South East (reference number 11/H1102/11) and the London School of Hygiene & Tropical Medicine Observational/Interventional Research Ethics Committee (reference number 5996). NHS research and development permission was obtained with Public Health England (formerly the Health Protection Agency) as the ‘NHS participating organisation’.
We report first on the policy survey (one of the supporting studies) and then on the two main observational studies.
Chapter 4 Survey of infant bacillus Calmette–Guérin vaccination policies in England
Background
There appeared to be widespread variation at the local level in the implementation of the recommendations for infant BCG vaccinations as well as in vaccine delivery pathways.
A survey of both past and current vaccination policies was conducted to support the main studies by assessing what infant BCG vaccination provision was in place prior to 2005 in local areas and to identify and engage with services or individuals on the delivery pathway who managed or had access to vaccination records.
Methods
We designed a standardised, mostly close-ended structured questionnaire covering both the historical and the current BCG vaccination policy in and outside infancy, eligibility criteria and their documentation, delivery pathways and constraints to service delivery. The questionnaire was tested by asking immunisation co-ordinators from four London PCTs to complete it and questions were then adjusted accordingly.
We surveyed all 152 PCTs, the local administrative areas for health-care services in England between November 2010 and March 2011. We also checked PCT websites and related NHS sources for publicly available documents to assess agreement with the questionnaire data received. Details of the survey are published as a peer-reviewed paper. 26
We also obtained the source data (original questionnaires/tables) from previous BCG policy surveys16,27 from the investigators to complement information on historical infant vaccination policies.
Results
Questionnaires were returned from 85% (129/152) of the PCTs in England. There were no differences in TB notification rates between responding and non-responding PCTs. We found publicly available current BCG policy documents for 114 of the 129 (88%) PCTs that responded. Two (2%) PCTs were excluded from the subsequent analysis because their BCG policies could not be determined clearly from their responses.
Current bacillus Calmette–Guérin vaccination policy
The agreement with publicly available BCG vaccination policy documents was high, with only three (2%) PCTs reporting policies that were different from the information in these documents. Details of the findings are provided elsewhere. 26 In summary, the new policy for the delivery of infant BCG in high-risk groups had been implemented in all PCTs, but with considerable heterogeneity with regard to the organisation of the delivery of the vaccine and some difficulties experienced in the identification of eligible children. Sixteen of the 127 (13%) PCTs reported universal infant vaccination and 111 (87%) reported selective infant vaccination. PCTs with a selective infant vaccination policy most frequently vaccinated on postnatal wards (51/102, 50%), whereas PCTs with a universal infant vaccination policy most frequently vaccinated in community clinics (9/13, 69%; p = 0.011). All (100%) PCTs that vaccinated primarily on postnatal wards did so during the infants’ first month of life, whereas only 13 out of 37 (35%) PCTs that mainly vaccinated in community clinics did so in the infants’ first month of life (p < 0.001).
Past (pre 2005) bacillus Calmette–Guérin vaccination policy
Before 2005, the national policy was to vaccinate all tuberculin skin test (TST)-negative schoolchildren aged 11–14 years. The TST was performed by school nurses, who also administered the vaccine in eligible children. However, the 1983 survey of BCG vaccination policies reported that five health authorities had stopped their routine BCG vaccination programme for schoolchildren (two in 1974 and one each in 1977, 1980 and 1983) and replaced it with selective infant BCG vaccination targeting immigrants. 27 By 1992, at least 15 health authorities in England and Wales had stopped their routine BCG vaccination programme for schoolchildren, mostly in areas with very low TB notification rates. 16 This later survey also found that 18 health authorities offered BCG vaccination to selected groups at school entry, mostly recent immigrants, although this was offered in only two of the 15 districts that had discontinued BCG vaccination for schoolchildren. 16
Infant bacillus Calmette–Guérin vaccination pre 2005
The 1983 survey of BCG vaccination policies found that six of the 201 health districts in England and Wales had universal infant BCG programmes, with a further 98 health districts already having some form of selective infant BCG programme, mostly targeting newborns of immigrants and/or neonatal TB contacts. 27 The 1992 survey reported that, of the 184 health districts surveyed, five had a universal infant BCG programme and 148 had a selective infant BCG programme (including 14 of the 15 districts where vaccination of schoolchildren had been discontinued). 16 The main groups targeted in areas with selective policies were infants from ethnic minority groups (all 148 districts) and recent immigrants from high TB burden countries (120 districts), as well as infants from families with a history of TB (40 districts). This survey also found that only one of the 31 health districts with no infant BCG vaccination programme had an estimated population of Indian subcontinent origin of > 10%.
Overall, therefore, these previous surveys suggested that about half of the health districts in England and Wales already offered the BCG vaccine to ethnic minority infants by 1983, with > 80% doing so by 1992, including nearly all areas with an estimated population of Indian subcontinent origin of > 10%.
Bacillus Calmette–Guérin vaccine administration
In 1983, 152 out of 201 (76%) health districts vaccinated using a syringe and needle exclusively, 14 used a jet injector and 25 used either of these methods at the discretion of the provider. Two districts used the multipuncture method. 27 By 1992, 163 out of 169 (96%) health districts still implemented routine vaccination of schoolchildren using a needle and syringe, with three using the multipuncture method and one using a jet injector. All districts offering infant BCG administered it using a syringe and needle. 16
Discussion
The data for this survey were collected during and after a major reorganisation of the NHS. This complicated access to key informants, as staff responsibilities at this time were not clear. The potential implications of the recent NHS reorganisation for BCG vaccination policies at the local level are also not now reflected in this report.
Implications of the findings for the main studies
One of the main features of BCG vaccination policies in England highlighted by this survey and the previous surveys is the substantial heterogeneity in infant BCG policies between health areas, as well as the changes over time. However, there are several consistent patterns that are relevant to the main studies:
-
Up to 1992, > 90% of health districts still had a routine universal vaccination programme for schoolchildren. Given that the birth cohorts eligible for the main study of BCG vaccination in the general population are subjects born from 1965 to 1989, it is reasonable to assume that most of the target population had similar opportunities for vaccination/exposure to the vaccination programme for schoolchildren.
-
From 1983, > 50% of health districts had an infant BCG programme, with ethnic minority groups being the prime population target; this had increased to > 80% by 1992. Furthermore, all but one district with > 10% of residents being of Indian subcontinent origin (the majority minority ethnic group in England and Wales) had an infant BCG programme.
Chapter 5 Observational studies of bacillus Calmette–Guérin vaccine effectiveness with time since vaccination in England
Objectives
The primary objectives of the observational studies were to:
-
estimate the effectiveness of BCG vaccination in preventing TB when given in the first year of life (‘infant BCG’) to high-risk groups, in 5-year intervals since vaccination
-
estimate the effectiveness of BCG vaccination in preventing TB when given at school age (‘school-age BCG’) to the general population, in 5-year intervals since vaccination, starting 10 years after vaccination.
For both exposures it was also explored whether or not protection wanes with time since vaccination.
Methods
Study design and study areas
Participants in both studies were recruited using a case–control design. For logistical efficiency, the recruitment for the infant BCG study was restricted to areas of England with a ≥ 30% resident black and Asian minority ethnic (BAME) population, based on the 2001 general census. These communities are geographically clustered. The school-age BCG study was carried out across England, reflecting the much larger geographical spread of TB cases in the target population. A pilot study to test the methods for recruiting controls was conducted first.
Pilot study
The main objectives for the pilot study were to:
-
estimate the response rate for cases to allow the sampling strategy to be refined
-
assess the feasibility of the control recruitment strategy (nominated controls)
-
field test the operating procedures and the questionnaire.
A total of 115 subjects with a previous TB diagnosis notified to the ETS system were selected and invited to take part, 58 for the infant BCG study and 57 for the school-age BCG study. Those successfully recruited were invited to nominate up to five unrelated acquaintances of a roughly similar age, sex and broad ethnic background residing in the fieldwork area to serve as controls.
Study main exposure and primary outcome
Infant bacillus Calmette–Guérin study
-
Main exposure: BCG vaccination given in infancy to subjects at higher risk of TB (study population restricted to BAME populations as they were the main target of the vaccination programme), as recommended by UK Department of Health guidelines. 20
-
Primary outcome: level and duration of BCG-derived protection against any notified TB disease up to 19 years after vaccination.
School-age bacillus Calmette–Guérin study
-
Main exposure: BCG vaccination given to TST-negative schoolchildren as part of the UK universal school BCG vaccination programme until 2005, when it was discontinued.
-
Primary outcome: level and duration of BCG-derived protection against any notified TB disease from 10 to 29 years after school-age vaccination.
Participants
Infant bacillus Calmette–Guérin study
Cases were UK-born subjects from a BAME background with a confirmed first TB episode diagnosed and notified to the Public Health England ETS system between 2003 and 2012, aged between 1 and 19 years at the time of diagnosis (i.e. born between 1984 and 2012) and residing in the study area at the time of diagnosis. The BAME populations included those from high TB burden settings and were any black or South Asian populations, including those from India, Pakistan, Bangladesh, Nepal, Bhutan, the Maldives and Sri Lanka (including those whose families were originally from these backgrounds but who migrated to Britain from other regions, e.g. the Caribbean or east or southern Africa).
Controls were population-based UK-born subjects from the same target BAME backgrounds, residing in the same study area as cases, from the same birth cohorts as cases (i.e. born between 1984 and 2012) and with no previous episode of TB. Controls were frequency matched to cases within 5-year birth cohorts.
Cases with known human immunodeficiency virus (HIV) infection were not included in the study. This criterion was not applied to controls because the prevalence of HIV infection in the general population from which they were sampled was small. Cases and controls from BAME backgrounds other than those targeted (e.g. Chinese, Japanese, Korean) were not included.
School-age bacillus Calmette–Guérin study
Cases were UK-born subjects from a white ethnic background with a confirmed first TB episode diagnosed and notified to the Public Health England ETS system, residing in England at the time of diagnosis between 2003 and 2012 and aged between 23 and 38 years at the time of diagnosis (i.e. born between 1965 and 1989). BCG vaccination was routinely offered to schoolchildren aged about 13 years (range 10–15 years) and so the age range of cases would allow measurement of the effect of the vaccine from about 10 to 29 years after vaccination.
Controls were UK-born subjects from a white ethnic background residing in England, from the same birth cohorts as cases (i.e. born between 1965 and 1989) and with no previous episode of TB. Controls were frequency matched to cases within 5-year birth cohorts.
Cases with known HIV infection were not included. This criterion was not applied to controls because the prevalence of HIV infection in the general population from which they were sampled was small.
Sample size
The sample size calculations for both studies were based on assumptions around expected BCG vaccine uptake and minimum level of VE for successive time bands since vaccination for the different populations. All calculations were carried out for a frequency-matched case–control study design, with 90% power and a 5% significance level. Numbers were inflated by 15% to allow for post-recruitment exclusions because of ineligibility and loss of power after controlling for confounding variables. For the infant BCG study, at a ratio of case to control of 1 : 1, we estimated the required sample size to be 627 cases and 627 controls. For the school-age BCG study, the number of eligible cases available to be invited was limited and, hence, the sample size was estimated for an average ratio of up to two controls per case, for a total of 665 cases and 1183 controls. The ratio of 2 : 1 was applied to mitigate the effect on statistical efficiency of an expected low case recruitment rate, based on the results of the pilot study; however, the intention was to recruit as many cases as possible. The detailed sample size estimates for each study are presented in Tables 2 and 3.
| Age at TB diagnosis (years) | Time since vaccination (years) | Assumed BCG uptake (%) | Minimum VE to be detected (%) | Frequency-matched design with ratio of one control per case | |
|---|---|---|---|---|---|
| Cases, n | Controls, n | ||||
| 0–5 | 0–5 | 90 | 60 | 252 | 252 |
| 6–12 | 6–12 | 80 | 60 | 158 | 158 |
| 13–17 | 13–17 | 60 | 50 | 217 | 217 |
| Total number in infant BCG sample | 627 | 627 | |||
| Age at TB diagnosis (years) | Time since vaccination (years) | Assumed BCG uptake (%) | Minimum VE to be detected (%) | Frequency-matched design with ratio of two controls per casea | |
|---|---|---|---|---|---|
| Cases, n | Controls, n | ||||
| 23–27 | 10–14 | 80 | 60 | 116 (145) | 232 (258) |
| 28–32 | 15–19 | 80 | 50 | 208 (260) | 416 (463) |
| 33–37 | 20–24 | 80 | 50 | 208 (260) | 416 (463) |
| Total number in school-age BCG sample | 532 (665) | 1064 (1184) | |||
Study sampling
Selection of cases
For both studies, eligible TB cases were identified from the ETS database based on the date of diagnosis and reported date of birth, residential address at the time of diagnosis and self-reported ethnic background. Cases with a reported previous TB episode or with a previous notification of TB in the database were not included. For the infant BCG study, cases were included if they resided in the study area (defined to include small areas where ≥ 30% of the population were from BAME groups). For the school-age BCG study, the study area included all of England.
Selection of controls
The procedure for selection of controls was amended after the pilot study. We required community-based controls who represented the population in which the cases occurred. We had two potential strategies for this: the first, which was lower cost but high risk, was nominated controls. We piloted this strategy to recruit individually matched controls among unrelated acquaintances nominated by cases. The pilot study indicated that recruiting nominated controls was not feasible: people either were reluctant to nominate friends or reported that they did not have friends of an eligible age or ethnicity. The control recruitment strategy was thus changed to our second-choice strategy (more resource intensive but lower risk): self-weighted multistage stratified sampling of the target populations across the area from which cases were recruited. Multistage sampling was preferred to straightforward simple random sampling (SRS) to ensure wider geographical coverage of the respective study areas while maintaining reasonable clustering of field data collection for optimal logistical efficiency.
For the infant BCG study we used a two-stage self-weighted sampling design. Based on previous work by the Health Survey for England28,29 and the 2001 census,30 we estimated that, on average, screening 12 residential addresses would result in one eligible (based on BAME group and birth cohort) control successfully recruited from the community. A total of 7750 addresses were selected probability proportional to size (PPS) of the eligible BAME population in the study area, which consisted of geographical areas with ≥ 30% BAME residents based on the 2001 census. Intercensus estimates were not thought to be as robust and were not available by small areas.
The first stage consisted of sampling the lower-level super output areas (LSOAs) with a ≥ 30% resident BAME population by PPS. LSOAs are designed to have a fairly socially homogeneous population of, on average, 1500 residents each. 31 A total of 2659 out of 32,482 (8.2%) LSOAs had ≥ 30% BAME residents, accounting for 50% (2,027,398/4,024,287) of the total BAME population in England. The study area also included 60% of all eligible TB cases in these groups notified to the ETS system.
The second stage involved SRS of seven residential addresses within each LSOA selected in the first stage. This was carried out using the small-user Postcode Address File. To ensure equal geographical spread of selected addresses within the LSOA, we randomly sampled seven distinct postcode units in each LSOA and then one address per postcode unit. Postcode units each include, on average, 15 residential addresses and LSOAs include, on average, 30 postcode units each.
Overall, 1107 LSOAs were sampled with PPS out of 2659 LSOAs with ≥ 30% BAME residents and, in each selected LSOA, seven residential addresses were selected using SRS.
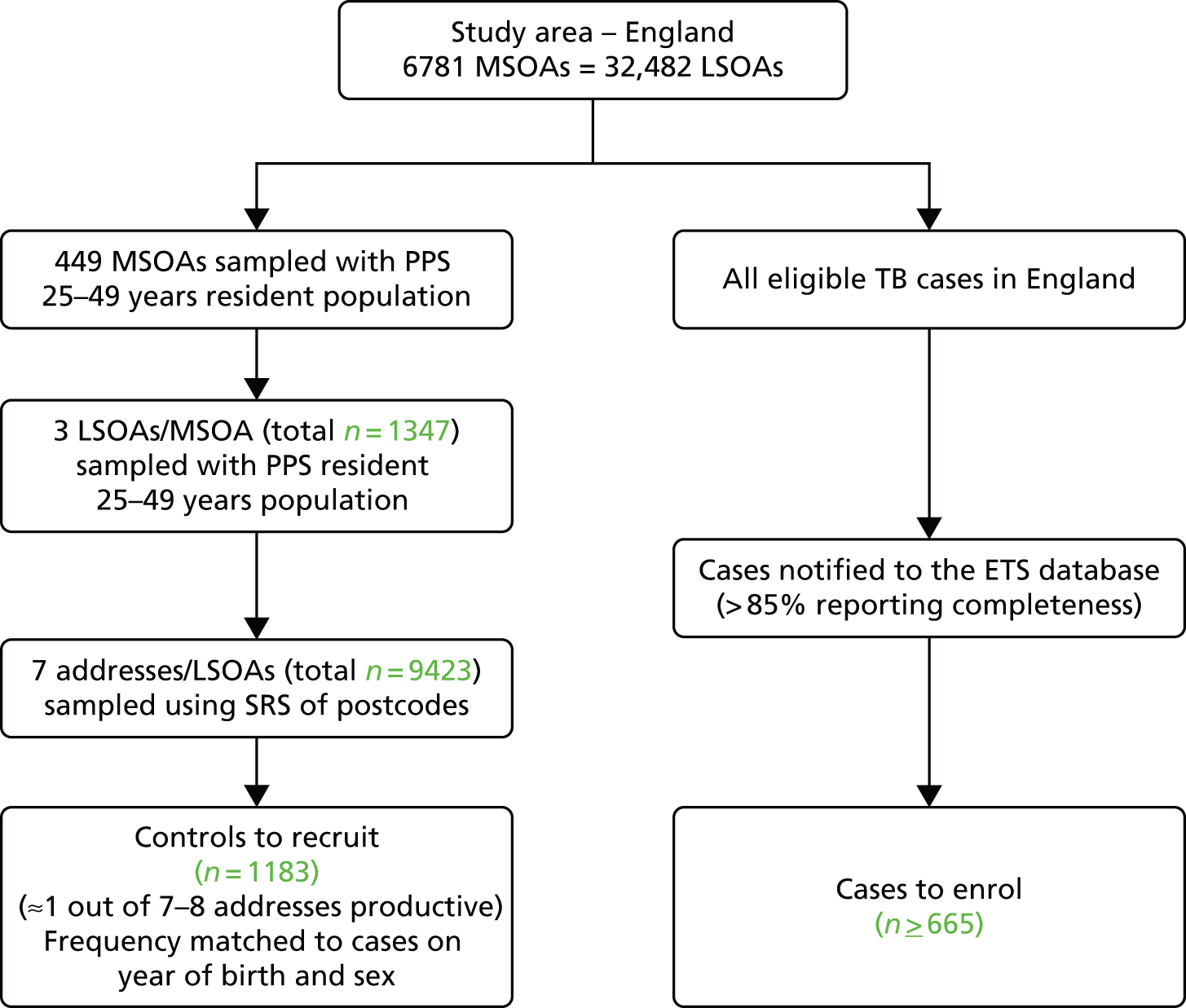
For the school-age BCG study we used a three-stage self-weighted sampling design to reflect the much wider study area (the whole of England). We estimated that a total of about 9500 residential addresses would have to be screened to meet our target, based on an average of one eligible control successfully recruited from every seven to eight addresses screened, based on the Health Survey for England28,29 and 2001 census30 data broken down by age and ethnicity.
The first stage was the selection of 449 mid-level super output areas (MSOAs) out of a total of 6781 in England, with PPS of their 2010 mid-year estimates of the population aged 25–49 years. 32 MSOAs consist of contiguous LSOAs, constrained by the 2003 local authority boundaries; each has a minimum population of 5000 and an average of 7200 inhabitants. 31
The second stage was the random selection of three LSOAs in each MSOA, by PPS.
The third stage was the selection of seven residential addresses from each LSOA by SRS using the same procedure as for the infant BCG study.
In total, we selected 449 out of 6781 MSOAs and a total of 1347 LSOAs across England (three per MSOA). Seven randomly sampled addresses were selected in each LSOA. A few addresses (n = 6) no longer existed, leaving a total of 9423 addresses screened for eligible controls.
Summaries of the sampling strategies for the infant BCG study and the school-age BCG study are provided in Figures 1 and 2, respectively.
FIGURE 1.
Summary of the sampling strategy for the infant BCG study. a, LSOA sampled at random using probability proportional to size of the number of residents from ethnic minority background in the LSOA.
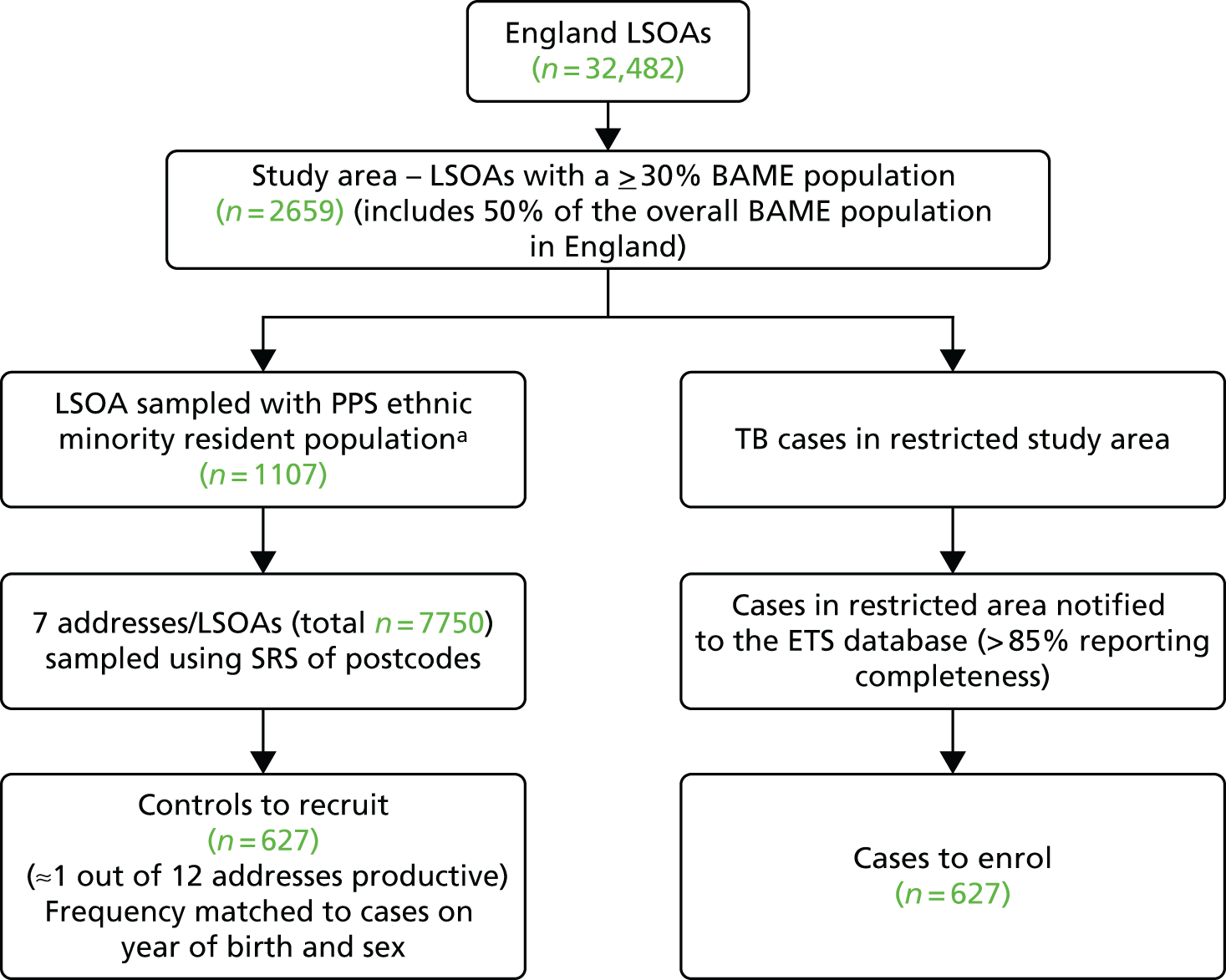
FIGURE 2.
Summary of the sampling strategy for the school-age BCG study.
Data sources
Data for the studies were obtained from four main sources.
Face-to-face interviews
The core study data, including indicators of BCG status (vaccination history, inspection of both arms for vaccination scar and personally held vaccination record or card, e.g. the ‘red book’), were collected in the community through computer-assisted personal interviews (CAPIs) carried out by trained and experienced field interviewers. These data are referred to as CAPI data in this report.
Surveillance register
Clinical information on TB cases was obtained from Public Health England’s ETS system. These data are referred to as ETS data in this report.
NHS vaccination records
Information on BCG vaccination held by the NHS was requested from, and manually searched for in, the Child Health Information Systems (CHISs) of NHS PCTs, as well as NHS community care trusts, in areas where participants resided at the time when eligible for vaccination. These data are referred to as NHS records in this report.
Other
Small area-level (LSOA) Indices of Multiple Deprivation (IMD) – a proxy measure for socioeconomic status – were obtained from the Office for National Statistics (ONS) English Indices of Deprivation 201033 by linkage, using study participants’ residential postcode. Local annual TB rates in the areas of residence (local authorities from 1988 to 1990 and PCTs from 2005 to 2007) when subjects were eligible for vaccination were obtained from Public Health England historical data.
Study variables
Indicators of vaccination status
Data were collected on four indicators of BCG vaccination status: history (recall from parent or respondent), the inspection of both arms for a BCG scar by the interviewer following a standardised procedure, personal vaccination record (red book) or card, and NHS vaccination record. All four indicators were incomplete and had strengths and limitations in the two different studies:
-
Vaccination history. For the infant BCG study, information was collected from parents or subjects (if older and living away from home, although they were encouraged to check information with their parents over the telephone). Subjects were, of course, not able to remember vaccination in the first year of life and parents’ reports were subject to considerable recall bias because BCG vaccination was confused with any of the several other vaccinations given in infancy. For the school-age BCG study, BCG vaccination history was less likely to be affected by recall error as the BCG vaccine was the sole vaccine routinely given at school in all children aged around 13 years (an age that they could recall).
-
Inspection for BCG scar. Interviewers were trained to recognise a BCG vaccination scar and inspected both arms after consent was obtained. Higher refusal rates were noted among young/teenage female cases with family origins in Africa and Pakistan.
-
Personal vaccination record. Study participants were encouraged to look for any vaccination record or card ahead of the interview. The most common personally held vaccination record (commonly known as the red book) was introduced in England in the mid-1990s; thus, these records were missing for older participants in the infant BCG study. No participants in the school-age BCG study had hand-held records.
-
NHS vaccination record. For the infant BCG study, vaccination was checked through the CHIS in local area health trusts. There were several practical challenges to this: the decentralisation of primary health care in the NHS, with heterogeneity in how data are stored and procedures to request authorisations between trusts; the major NHS reform that coincided with our study period, which meant that not every trust could be successfully contacted to check their records; and the fact that CHIS records are discarded or archived according to regulatory requirements when individuals reach a certain age, varying from 21 to 25 years depending on the area. For the school-age BCG study, there was no central database of school vaccination records and this information was inconsistently recorded in the CHIS. NHS vaccination records were therefore not available for nearly all participants.
Tuberculosis events
Information on the date of notification of TB events, as well as the reported date when symptoms started and the date of diagnosis for all cases, was retrieved from the ETS system. We also extracted data on the site of disease as well as checking that no previous TB episode had been reported. Controls were asked for any past TB diagnosis and their details were also checked against the ETS system for any notified TB events.
Other variables/potential confounders
-
Basic demographics, including date of birth, sex and ethnic background (CAPI). Ethnic background was self-assessed, choosing from the standard ONS categories in the latest UK general population census.
-
Education level (CAPI). For the infant BCG study we collected information on the education level of both parents and for the school-age BCG study we collected information on participants’ education level.
-
Small-area level deprivation index. The postcodes of cases at the time of diagnosis as well as those of controls were used to obtain the LSOA-level IMD scores and ranks, a proxy measure of socioeconomic status. The IMD, a composite measure of deprivation generated by the ONS, combines scores in seven deprivation domains (income, employment, health and disability, education, skills and training, crime and living environment). 33
-
Household crowding (CAPI). Information was collected on the number of residents in the household and the total number of rooms (excluding kitchen and bathroom) and the number of bedrooms. This was used to generate two commonly used measures of crowding, the number of persons per room and the number of persons per bedroom. 34,35
-
Background risk of TB. Data were collected on the broad world region of origin of parents and grandparents, as well as frequent travel and/or prolonged stays (≥ 3 months) to parts of the world with a high TB burden.
-
Lifestyle/behaviour risk factors (CAPI). For the school-age BCG study, participants were asked about potential risk behaviours, including their history of tobacco smoking, alcohol abuse, drug misuse, stays in prison and homelessness.
-
Other. Information was collected on areas of residence of participants between birth and age 14 years. Historical average TB notification rates in those areas at relevant time periods were retrieved as a proxy measure of the local TB epidemiology.
Ethics and consent
Eligible subjects were given detailed information on the relevant study by way of an information leaflet and a dedicated webpage; an opportunity was also provided for them to ask clarification questions. For those willing to take part in one of the studies, we obtained up to three separate informed consents: (1) for the face-to-face interview, (2) for the inspection of both arms for a BCG scar (as well as photographs for about 25% of participants) and (3) for permission to contact the NHS using their personal details to check their vaccination records for BCG vaccination. Subjects aged < 16 years provided assent, with formal consent obtained from their parent or legal guardian.
All subjects contacted about one of the studies were given the option to opt out following the initial invitation, in which case no further contact was attempted by the field worker. Subjects could also freely withdraw from the study at any stage, including during the interview, or afterwards by telephone, and their data would be deleted.
Participants in the study were given a £15 gift voucher as compensation for their time, irrespective of whether they completed the interview or withdrew their consent at any stage.
Field procedures and data collection
The pilot study for the recruitment of cases and nominated controls was carried out in September and October 2011.
The main change instigated after the pilot study was the approach to the recruitment of controls (see Selection of controls). After preparations for the new approach to the recruitment of controls were complete, data collection for the main studies was conducted from February 2012 to September 2014.
Cases were sent an information letter inviting them to take part in the study and were offered the opportunity to request further information or opt out either by telephone or by returning an opt-out slip. Controls at the residential addresses selected for screening were sent a similar advance information letter, which also included an opportunity to opt out, as for cases.
Trained interviewers attempted contact with both the invited cases and the selected control residential addresses using a standard visit schedule, including weekdays and weekend days and morning, afternoon and evening visits. For controls, the residents at sampled addresses were first screened for eligibility. If required at the door, a translation screening card with 17 of the most common other languages spoken in England was used to ask which language was their first language, together with a show card asking for their help with the study in their first language. Eligible controls were provided with information on the study. Informed consent was obtained from all those who were eligible and willing to participate in the study before face-to-face interviews were conducted.
Study participants aged ≥ 16 years were interviewed directly. Parents or those with parental responsibility were interviewed for participants aged 0–15 years. For the infant BCG study, in which some parents from BAME groups were not fluent in English, translation (from the 17 most commonly spoken foreign languages in England) was offered if requested.
All interviews were carried out by experienced staff from NatCen, a leading independent social research not-for-profit organisation that routinely conducts large-scale national surveys (e.g. Health Survey for England, National Survey of Sexual Attitudes and Lifestyles). All interviewers undertook a day’s training specifically for this study before taking part in any fieldwork, including carrying out homework and intensive practical training on the inspection of both arms to identify BCG vaccination scars. The training in scar reading included the use of photos and volunteers with and without scars.
Monitoring and quality control during field data collection included formal supervisory field visits to individual interviewers and blind telephone recall of at least a 10% random sample of study participants, checking for quality of the face-to-face interviews and compliance with protocols and procedures for interviewing, as well as other specific instructions.
The CAPI included a standardised pretested questionnaire with only close-ended questions and a preset standard script that interviewers had to read. Part of the questionnaire for the school BCG study collected sensitive data on tobacco, alcohol and controlled substance use, and previous stays in prison via a computer-assisted self-interview during which the interviewees entered the data themselves and then locked the data to be inaccessible to the interviewer before returning the laptop to him or her.
Information on a range of other potential confounding variables was collected during the interviews, including indicators of socioeconomic status, education level, household crowding and lifestyle behaviours.
Analysis
Data cleaning and descriptive analysis
For both studies, data from the different data sources were merged, cleaned and checked using consistency and range checks. The distribution of quantitative variables was examined and they were transformed into categorical variables as required (IMD scores, birth year). The distribution of covariates and any missing data on them were summarised by case/control status.
Definition of vaccination status
Infant bacillus Calmette–Guérin study
After exploration of completeness, agreements between the different indicators of vaccination status were assessed. Based on the assessment we judged that BCG vaccination status was best defined using combinations of observed vaccination records, as described in Table 4. Briefly, the recording of BCG vaccination in either the red book or NHS records was used as evidence of previous BCG vaccination. Subjects were classified as unvaccinated only if both records were available and BCG vaccination was not recorded in both records. The date of BCG vaccination was taken as that reported in the vaccination records.
| Red book | NHS records | BCG vaccination status (definition 1) |
|---|---|---|
| BCG vaccination recorded | BCG vaccination recorded | Vaccinated |
| BCG vaccination recorded | BCG vaccination not recorded | |
| BCG vaccination not recorded | BCG vaccination recorded | |
| BCG vaccination not recorded | BCG vaccination not recorded | Not vaccinated |
| BCG vaccination not recorded | Missing | Treated as missing |
| Missing | BCG vaccination not recorded | |
| Missing | Missing | Missing |
School-age bacillus Calmette–Guérin study
After checking for completeness, BCG vaccination status was based on the two indicators available, that is, self-reported history and scar inspection. Self-reported history was based on recall of the TST prior to vaccination and subsequent BCG vaccination; participants’ self-reported BCG vaccination history was, therefore, classified into three categories:
-
convincing history of BCG vaccination – subjects who recalled the TST and subsequent BCG vaccination 48–72 hours later or post-vaccination soreness, pustule and/or scar
-
probably BCG vaccinated – those who reported receiving the BCG vaccination at school but who did not recall the TST and/or post-vaccination soreness, pustule or scar
-
not vaccinated – subjects who reported never receiving the BCG vaccination.
Self-reported history was combined with the result of inspection of the arms for a scar to define BCG vaccination status, as detailed in Table 5. Briefly, subjects with a convincing self-reported history of BCG vaccination, or a probable history of BCG vaccination or a scar were classified as vaccinated. Those with no history of BCG vaccination and no scar at inspection, or either, were classified as not vaccinated.
| Self-reported historya | Scar inspection | BCG vaccination status |
|---|---|---|
| Convincing BCG vaccination history | Scar present | Vaccinated |
| Convincing BCG vaccination history | No scar | |
| Convincing BCG vaccination history | Not inspected | |
| Probable BCG vaccination history | Scar present | |
| Probable BCG vaccination history | No scar | |
| Probable BCG vaccination history | Not inspected | |
| No BCG vaccination history | Scar present | |
| Unsure | Scar present | |
| No BCG vaccination history | No scar | Not vaccinated |
| No BCG vaccination history | Not inspected | |
| Unsure | No scar | |
| Unsure | Not inspected | Missing |
Those with a history of vaccination reported the age when they were vaccinated, but the exact date of vaccination was not available and so a date of vaccination was assigned randomly by sampling dates within the year corresponding to the age at vaccination (excluding the school holiday months of July and August), using a uniform distribution. For those for whom the age of vaccination was not available (e.g. ‘possibly vaccinated’ based on scar), the age at vaccination was assigned to be age 12 years, which was the median age at vaccination among those for whom age at vaccination was available.
Association between time since bacillus Calmette–Guérin vaccination and risk of tuberculosis
We investigated the association between time since vaccination and risk of TB in two steps.
-
First, we estimated the association for successive time since vaccination intervals of approximately 5 years: 1–5, 5–10, 10–15 and 15–19 years after vaccination for the infant BCG study and 10–15, 15–20, 20–25 and 25–29 years after vaccination for the school-age BCG study.
-
Second, we modelled VE smoothly as a function of time since BCG vaccination.
Statistical methods
The data were analysed using the case–cohort approach,36,37 with controls forming the ‘subcohort’, that is, the set of potential controls for each case. This provided an efficient analytical approach to the data, which made best use of data for controls throughout the time that they were at risk and allowed flexible modelling of VE by time since vaccination. 38 This approach was appropriate because our controls were sampled at random from the underlying population within which cases arose (with frequency matching on year of birth) and because the outcome (TB) is rare in the underlying population (with annual TB notification rates in our study populations in the tens per 100,000); the controls can therefore be considered approximately as random samples from the underlying population within each of the frequency-matching strata.
Under our selected approach, each case was compared at its event time with all controls in the subcohort who were still at risk at that time and who were in the same stratum as the case. The statistical analyses assumed an underlying Cox proportional hazards model, the parameters for which were estimated using a pseudopartial likelihood analysis with robust standard errors; the latter is necessary in case–cohort analyses to account for the ‘shared’ control groups between cases.
Follow-up started for all participants from their date of birth (i.e. analyses were done on the age time scale). Time at risk was accrued from the age at which participants would have been eligible for BCG vaccination, corresponding to the first birthday for participants to the infant BCG study (when most would have had an opportunity to receive infant BCG vaccination). In both studies, the basic model for VE was stratified by year of birth and adjusted for sex. Allowing separate baseline hazards within each year of birth was used to account for frequency matching of controls on year of birth. For the infant BCG study, given that not only the risk of TB in each ethnic group is different but also the age distribution of cases, the baseline hazard was also allowed to vary by ethnic group. Other covariates were then added in turn in the basic model to assess the potential confounding effect by examining changes in the point estimates and the standard errors, and a final multivariable model was then built.
We present the results as hazard ratios (HRs) and corresponding 95% CIs. VE estimates can be computed as VE = 1 – HR).
We modelled VE smoothly as a function of time since vaccination using two methods. First, we used a restricted cubic spline with three knots, with the knots reflecting the time intervals of each study. Second, we fitted a simpler model in which the log HR associated with BCG vaccination was assumed to change linearly with time. The results were displayed graphically as smooth curves, including 95% confidence limits. The results from the linear model were also tabulated.
Handling of missing data
The results presented in this report are based on ‘complete-case’ analyses. Individuals with missing information with regard to vaccination status (according to our definitions) were excluded from the analyses. Individuals with missing data with regard to adjustment variables were excluded from models that included those variables. The results from the models, which included different adjustment variables, were compared by fitting the simpler models on both the maximum possible number of individuals for the model in question and on the subset of individuals on whom more fully adjusted models were fitted.
Given the presence of some missing data, especially vaccination records, further analyses including use of scar information and possibly multiple imputation will be explored. These analyses are not reported here.
Results: pilot study
Main results from the pilot study
The response rates in the pilot study were estimated as 59% for the infant BCG cases and 33% for the school-age BCG cases (Table 6). The refusal rates were similar between the two studies and were consistent with community-based studies (about 10%), with the difference in response rates predominantly the result of non-contact, mainly because of address changes. This was not unexpected as the target population for the school-age BCG study included more young and middle-age adults of working age and, hence, they were likely to be more mobile.
| Outcome | BCG sample | |
|---|---|---|
| Infant | School-age | |
| Total invited, n | 58 | 57 |
| Could not be contacted, n (%) | 17 (29) | 32 (56) |
| Contacted but refused to take part, n (%) | 7 (12) | 6 (11) |
| Successfully recruited, n (%) | 34 (59) | 19 (33) |
Fewer than one in four infant BCG study cases and fewer than one in three school-age BCG study cases were willing and/or able to nominate two or more potential controls and 29% and 26%, respectively, were unable to suggest any acquaintance (Table 7). Furthermore, of the total nominated acquaintances, we were able to recruit only 50% (17/34) for the infant BCG study and 35% (8/23) for the school-age BCG study. A further challenge was the time needed to recruit each control, as nominated acquaintances (when more than one) could not all be contacted concomitantly.
| Number of controls nominated per case recruited | BCG sample, n (%) | |
|---|---|---|
| Infant (N = 34) | School-age (N = 19) | |
| 0 | 10 (29) | 5 (26) |
| 1 | 16 (47) | 8 (42) |
| 2–5 | 8 (24) | 6 (32) |
The data collection tools and study procedures worked well and required only minor alterations.
Conclusions of the pilot study
The two main conclusions from the pilot study were as follows.
-
Nomination of acquaintances by cases was unlikely to be effective in recruiting controls in this context. The low recruitment rate for controls using this method was partly because of the paucity of friends of cases of a similar age as well as people not wanting to risk any potential disclosure of past health issues. We decided instead to use the alternative, already-planned, community-based controls.
-
The low recruitment rate of cases in the PILOT school BCG study (35% of those invited) suggested a reduction in the number of cases expected to be recruited even if all those eligible and notified to the ETS system were invited. This led us to adjust the sample size by increasing the ratio of controls to cases, from one control per case to two controls per case.
Results: concordance between different measures of bacillus Calmette–Guérin vaccination
These results are based on eligible participants successfully recruited to the respective studies, as detailed in the subsequent sections. For the infant BCG study, it was found that participants had difficulties distinguishing BCG vaccination in infancy from other childhood vaccines. As the self-reported vaccination information was clearly of poor quality, the three other BCG indicators were examined: scar inspection, personally held records (red book or vaccination card) and NHS records. For the school-age BCG study, the quality of recall was better, probably because vaccination was offered at an older age, that is, to schoolchildren aged 12–13 years on average.
Availability of information on indicators of bacillus Calmette–Guérin status
In the infant BCG study, 15% of cases and 10% of controls declined scar inspection. Red books and vaccination cards were available for 48% of cases and 57% of controls, and NHS records were found for 52% of cases and 40% of controls (Table 8).
| Variable | Group | |
|---|---|---|
| Cases (N = 744) | Controls (N = 694) | |
| Red book and vaccination card | ||
| Available: BCG vaccination not recorded | 156 (21.0) | 152 (21.9) |
| Available: BCG vaccination recorded | 203 (27.3) | 241 (34.7) |
| Not available | 385 (51.7) | 301 (43.4) |
| NHS records | ||
| Available: BCG vaccination not recorded | 100 (13.4) | 72 (10.4) |
| Available: BCG vaccination recorded | 286 (38.4) | 209 (30.1) |
| Not available | 358 (48.1) | 413 (59.5) |
| Scar inspection | ||
| BCG scar present | 207 (27.8) | 174 (25.1) |
| No BCG scar | 422 (56.7) | 451 (65.0) |
| Not inspected | 115 (15.5) | 69 (9.9) |
In the school-age BCG study, self-reported history was available in > 95% of participants and scar inspection was carried out in > 90% of participants. Vaccination records were unavailable for > 93% of participants and, thus, could not be used (Table 9).
| Variable | Group, n (%) | |
|---|---|---|
| Cases (N = 677) | Controls (N = 1170) | |
| Self-reported BCG history | ||
| History of BCG vaccination | 170 (25.1) | 169 (14.4) |
| No history of BCG vaccination | 476 (70.3) | 954 (81.5) |
| Do not remember | 31 (4.6) | 47 (4.0) |
| Scar inspection | ||
| No BCG scar | 204 (30.1) | 269 (23.0) |
| BCG scar | 424 (62.6) | 844 (72.1) |
| Not inspected | 49 (7.2) | 57 (4.9) |
| Red book and vaccination card | ||
| Available: BCG vaccination not recorded | 29 (4.3) | 59 (5.0) |
| Available: BCG vaccination recorded | 6 (0.9) | 12 (1.0) |
| Not available | 642 (94.8) | 1099 (93.9) |
| NHS records | ||
| Available: BCG vaccination not recorded | 20 (2.9) | 27 (2.3) |
| Available: BCG vaccination recorded | 16 (2.4) | 20 (1.7) |
| Not available | 641 (94.7) | 1123 (96.0) |
Agreement between NHS and red book records, and scar inspection and NHS/red book records in the infant bacillus Calmette–Guérin study
The agreement over BCG vaccination between NHS records and the red book was poor (Table 10), suggesting different patterns of incompleteness in the two types of records (i.e. it was unlikely that the absence of BCG vaccination in one record equated to no vaccination). We found that 40% (35/88) of subjects with no BCG vaccination recorded in their NHS records were vaccinated according to their red book and 67% (110/163) of those with no BCG vaccination recorded in their red book were vaccinated according to their NHS record.
| BCG vaccination in NHS records | BCG vaccination in red book | |||||
|---|---|---|---|---|---|---|
| Overall, n (%) | Cases | Controls | ||||
| No | Yes | No | Yes | No | Yes | |
| No | 53 (14.8) | 35 (9.8) | 35 (17.4) | 18 (9.0) | 18 (11.5) | 17 (10.8) |
| Yes | 110 (30.7) | 160 (44.7) | 61 (30.3) | 87 (43.3) | 49 (31.2) | 73 (46.5) |
| κ | 0.15 | 0.20 | 0.09 | |||
The data also suggested that, for infant vaccination, scar inspection was not very specific compared with vaccination records (Table 11). Of those with no BCG vaccination recorded in their red book, only 42% (111/262) had no scar on inspection and, of those with no BCG vaccination recorded in their NHS records, only 40% (58/146) had no scar on inspection. This proportion was slightly higher when we combined the records and considered no BCG vaccination recorded in both records as our best evidence of unvaccinated status: 53% (23/43) of those classified as unvaccinated had no scar on inspection.
| Scar inspection | Red book, n (%) | NHS records, n (%) | Combined records, n (%) | |||
|---|---|---|---|---|---|---|
| BCG vaccination recorded | BCG vaccination not recorded | BCG vaccination recorded | BCG vaccination not recorded | BCG vaccination recorded | No BCG vaccination recorded in both | |
| Cases | n = 182 | n = 130 | n = 248 | n = 80 | n = 351 | n = 28 |
| BCG scar | 128 (70.3) | 76 (58.5) | 183 (73.8) | 47 (58.7) | 252 (71.8) | 14 (50) |
| No BCG scar | 54 (29.7) | 54 (41.5) | 65 (26.2) | 33 (41.3) | 99 (28.2) | 14 (50) |
| Controls | n = 226 | n = 132 | n = 188 | n = 66 | n = 346 | n = 15 |
| BCG scar | 184 (81.4) | 75 (56.8) | 152 (80.8) | 41 (62.1) | 280 (80.9) | 6 (40) |
| No BCG scar | 42 (18.6) | 57 (43.2) | 36 (19.2) | 25 (37.9) | 66 (19.1) | 9 (60) |
| Overall | n = 408 | n = 262 | n = 436 | n = 146 | n = 697 | n = 43 |
| BCG scar | 312 (76.5) | 151 (57.6) | 335 (76.8) | 88 (60.3) | 532 (76.3) | 20 (46.5) |
| No BCG scar | 96 (23.5) | 111 (42.4) | 101 (23.2) | 58 (39.7) | 165 (23.7) | 23 (53.5) |
Agreement between bacillus Calmette–Guérin vaccination history and the presence of a scar in the school-age bacillus Calmette–Guérin study
There was good agreement between self-reported history of BCG vaccination and the presence of a scar among participants in the school-age BCG study (κ = 0.6; Table 12).
| Scar inspection | Self-reported history of BCG vaccination | |||||
|---|---|---|---|---|---|---|
| Overall, n (%) | Cases, n (%) | Controls, n (%) | ||||
| Yes | No | Yes | No | Yes | No | |
| BCG scar | 1181 (70.5) | 43 (2.6) | 394 (65.1) | 16 (2.6) | 787 (73.5) | 27 (2.5) |
| No BCG scar | 195 (11.6) | 257 (15.3) | 60 (9.9) | 135 (22.3) | 135 (12.6) | 122 (11.4) |
| κ | 0.60 | 0.69 | 0.52 | |||
Interpretation
Information on BCG vaccination was clearly better for school vaccination than for infant vaccination.
In the infant BCG study it appeared that no single indicator of BCG status was good enough to be used on its own to define BCG vaccination status, our main exposure. There was some suggestion that combining information from both types of vaccination record was helpful. In the school-age BCG study, data were available on two main indicators, self-reported history and scar inspection, and there was good agreement between both measures. This suggested that they could be combined to better define BCG vaccination status.
Results: infant bacillus Calmette–Guérin study
Overview of recruitment
Recruitment of cases
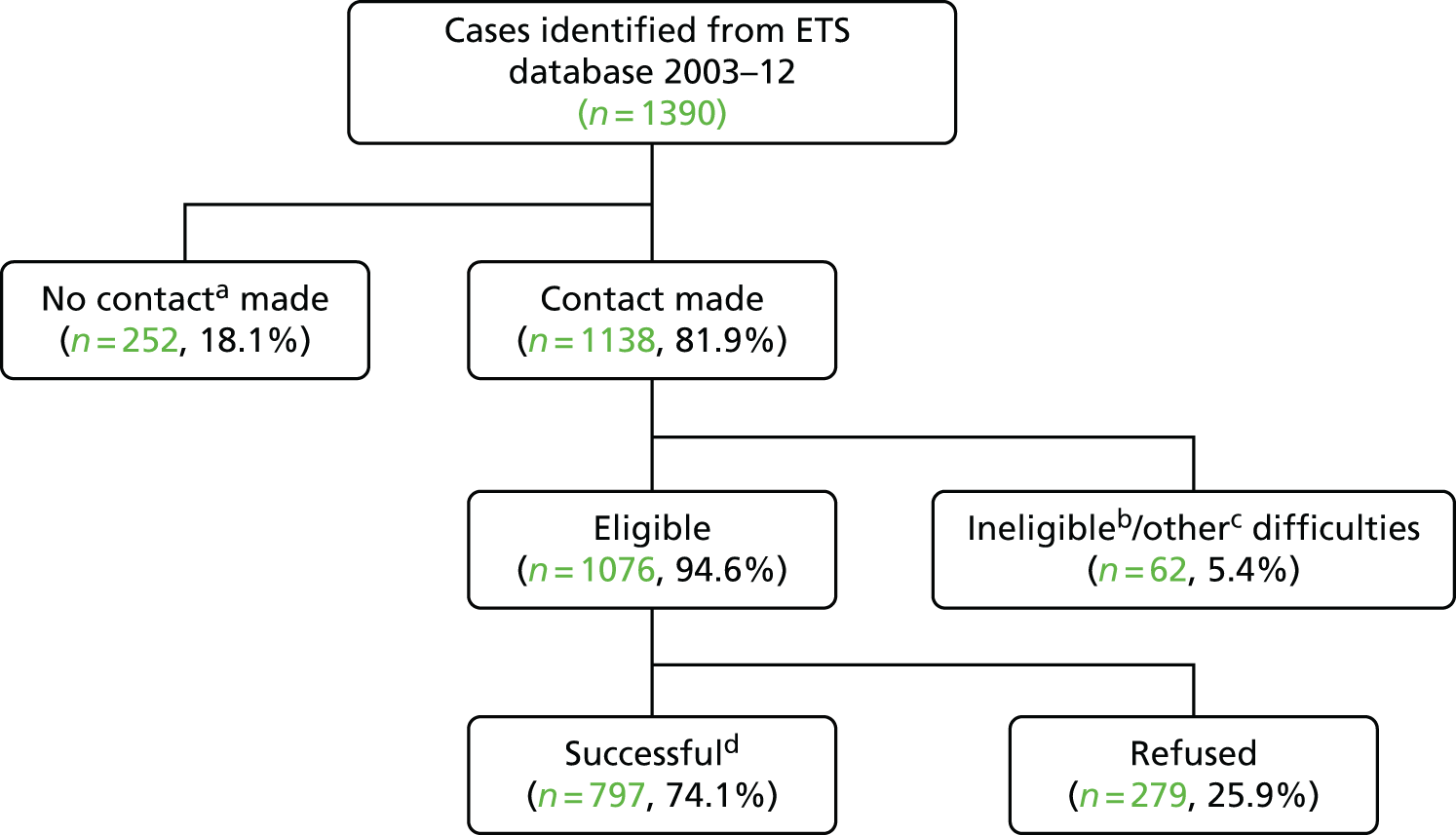
Of the 1390 potentially eligible cases from the ETS system who were invited to take part in the study, it was possible to contact 1138. Of these, 6% were excluded because they were not born in the UK, they were away, as reported by a household member, or, during interview, they were noted to have been vaccinated because of contact with a case of TB. Of the 1076 subjects contacted and eligible, 797 (74%) were enrolled (Figure 3).
FIGURE 3.
Overview of recruitment of infant BCG cases. a, No contact because changed address (n = 142), no contact with anybody at the address provided (n = 87), moved from England to another part of the UK (n = 10), moved abroad (n = 8) or unspecified (n = 5). b, Ineligible because not born in the UK (n = 20), not from a BAME group (n = 2), reported contact with a TB case in the red book and consistent with the date of TB diagnosis on the ETS system (n = 2) or unspecified (n = 5). c, Other difficulties include away or in hospital during the survey (n = 22), too frail to take part (n = 5) and various other difficulties (n = 6). d, In total, 797 cases were successfully recruited but 53 were not included in the analysis because they developed TB before they had a chance to receive the BCG vaccination (i.e. before their first birthday).
Recruitment of controls
We sampled 7755 residential addresses, of which 1089 (14%) could not be screened to establish eligibility for the study because they could not be located or were inaccessible, or because no contact could be established following the standard visit pattern of visiting more than once on different days and more than once at different times. Among the 6666 addresses that were screened, 1073 (16%) had at least one eligible resident, with 694 (65%) eligible subjects successfully recruited to the control group (Figure 4).
FIGURE 4.
Overview of recruitment of infant BCG controls. a, Not screened because no contact with anybody at the address provided after several visits following a preset visit pattern. b, Includes 4861 addresses screened with no eligible subjects and 720 addresses with non-residential, vacant, derelict or demolished buildings.

Comparison of recruitment between cases and controls
The proportion of cases who could be contacted was fairly comparable across quintiles of area-level IMD (Table 13). Among those contacted and eligible, the refusal rate did not vary much by quintile of IMD. Similarly, the proportion of addresses successfully screened for eligibility was similar across quintiles of IMD. Among subjects identified as being eligible to be controls, there was a similar trend for refusal as for cases, but the refusal rate was consistently higher for controls than for cases.
| Area-level deprivation quintiles | Group | |||
|---|---|---|---|---|
| Cases | Controls | |||
| Cases invited to participate | Addresses screened for eligibility | |||
| Total, n | Contacted, n (%) | Total, n | Screened, n (%) | |
| Least deprived | 159 | 134 (84) | 1556 | 1377 (88) |
| 2 | 219 | 174 (79) | 1559 | 1379 (88) |
| 3 | 273 | 217 (79) | 1544 | 1305 (85) |
| 4 | 330 | 283 (86) | 1547 | 1307 (84) |
| Most deprived | 407 | 328 (81) | 1549 | 1298 (84) |
| Eligible cases contacted | Eligible controls contacted | |||
| Eligible, n | Refused, n (%) | Eligible, n | Refused, n (%) | |
| Least deprived | 125 | 38 (30) | 217 | 82 (38) |
| 2 | 160 | 42 (26) | 223 | 87 (39) |
| 3 | 205 | 55 (27) | 186 | 65 (35) |
| 4 | 271 | 67 (25) | 197 | 71 (36) |
| Most deprived | 312 | 76 (24) | 250 | 74 (30) |
The distribution of interviewer visits by time of the day and day of the week was also comparable between cases and controls (Figure 5).
FIGURE 5.
Distribution of interviewer visits by (a) time of the day and (b) day of the week for invited cases and sampled control addresses in the infant BCG study.

Fifty-three subjects who developed TB in the first year of life were censored (i.e. exited the study) before the start of ‘follow-up’, as some may have developed TB before having the opportunity to receive the infant BCG vaccination (which could have been offered at any point before the age of 1 year); hence, these subjects were not included in the analysis. Table 14 shows the number of cases by age range at the time of TB diagnosis and birth cohort.
| Birth cohort | Group | Total | Time interval since vaccination (years) | |||
|---|---|---|---|---|---|---|
| 1–4 | 5–9 | 10–14 | 15–19 | |||
| 1985–9 | Cases | 54 | – | – | 9 | 45 |
| Controls | 67 | 0 | 0 | 33 | 67 | |
| 1990–4 | Cases | 188 | – | 2 | 72 | 114 |
| Controls | 130 | 0 | 55 | 130 | 130 | |
| 1995–9 | Cases | 202 | 3 | 59 | 109 | 31 |
| Controls | 151 | 61 | 151 | 151 | 90 | |
| 2000–4 | Cases | 189 | 93 | 73 | 17 | – |
| Controls | 168 | 168 | 168 | 56 | 0 | |
| 2005–11 | Cases | 117 | 103 | 14 | – | – |
| Controls | 178 | 178 | 67 | 0 | 0 | |
Descriptive statistics by case and control status
The distribution of cases and controls by sex and birth cohort was not substantially different; however, there were more cases than controls with a Pakistani or black African ethnic background (63% vs. 44%) (Table 15). The distribution of cases by area-level deprivation quintiles and parental education level was consistent with the known relationship between TB and deprivation, with increasing proportions of cases in the more deprived quintiles and a higher proportion of cases having parents with fewer educational qualifications. There were also more cases than controls from overcrowded households. Nearly 95% of the study participants had a place of residence in infancy or early childhood where infant BCG vaccination was offered, and this was similar between cases and controls. The distribution of the other variables was also fairly similar.
| Characteristic | Group, n (%) | |
|---|---|---|
| Casesa (N = 744) | Controls (N = 694) | |
| Birth cohort | ||
| 1985–9 | 54 (7.3) | 67 (9.7) |
| 1990–4 | 188 (25.3) | 130 (18.7) |
| 1995–9 | 202 (27.2) | 151 (21.8) |
| 2000–4 | 183 (24.6) | 168 (24.2) |
| 2005–11 | 117 (15.7) | 178 (25.6) |
| Sex | ||
| Female | 417 (56.0) | 350 (50.4) |
| Male | 327 (44.0) | 344 (49.6) |
| Ethnicity | ||
| Indian + mixed | 146 (19.6) | 172 (24.8) |
| Bangladeshi + mixed | 55 (7.4) | 79 (11.4) |
| Pakistani + mixed | 323 (43.4) | 206 (29.7) |
| Other Asian + mixed | 27 (3.6) | 51 (7.3) |
| Black African + mixed | 150 (20.2) | 97 (14.0) |
| Other black + mixed | 43 (5.8) | 89 (12.8) |
| Area-level deprivation quintiles | ||
| 1 (least deprived) | 86 (11.6) | 139 (20.0) |
| 2 | 105 (14.1) | 140 (20.2) |
| 3 | 182 (24.5) | 139 (20.0) |
| 4 | 199 (26.7) | 141 (20.3) |
| 5 (most deprived) | 172 (23.1) | 135 (19.5) |
| Parental highest educational (academic, professional and/or vocational) qualification | ||
| None | 263 (35.3) | 154 (22.2) |
| O levels or equivalentb | 214 (28.8) | 160 (23.1) |
| A levels or equivalentc | 88 (11.8) | 89 (12.8) |
| Degree level or equivalentd | 142 (19.1) | 270 (38.9) |
| Missing | 37 (5.0) | 21 (3.0) |
| Average number of people per room | ||
| ≤ 1 | 524 (70.4) | 568 (81.8) |
| > 1 | 191 (25.7) | 126 (18.2) |
| Missing | 29 (3.9) | 0 (0.0) |
| Average number of people per bedroom | ||
| ≤ 1 | 104 (14.0) | 168 (24.2) |
| > 1 | 610 (82.0) | 526 (75.8) |
| Missing | 30 (4.3) | 0 (0.0) |
| TB infection risk from regular travels abroad | ||
| Lowe | 453 (60.9) | 434 (62.5) |
| Highf | 289 (38.8) | 260 (37.5) |
| Missing | 2 (0.3) | 0 (0.0) |
| TB infection risk from long-term stays abroad | ||
| Lowe | 637 (85.6) | 618 (89.0) |
| Highf | 106 (14.2) | 76 (11.0) |
| Missing | 1 (0.1) | 0 (0.0) |
| Infant BCG vaccination policy in health district/PCT of residence up to age 4 years | ||
| None | 34 (4.6) | 39 (5.6) |
| Selective | 502 (67.5) | 420 (60.5) |
| Universal | 186 (25.0) | 206 (29.7) |
| Missing | 22 (3.0) | 29 (4.2) |
| 3-year average TB notification rate in local authority or PCT of residence in childhood (per 100,000) | ||
| < 20 | 172 (23.1) | 177 (25.5) |
| 20–39 | 394 (53.0) | 299 (43.1) |
| ≥ 40 | 178 (23.9) | 216 (31.1) |
| Missing | 0 (0.0) | 2 (0.3) |
Indicators of vaccination status
We examined our main definition of BCG vaccination status combining information from the red book and NHS records (see Table 4). The distribution of BCG vaccination status according to these definitions by case and control status is presented in Table 16.
| Red book | NHS record | BCG vaccination status (definition 1) | Group, n (%) | |
|---|---|---|---|---|
| Cases (N = 744) | Controls (N = 694) | |||
| BCG vaccination recorded | BCG vaccination recorded | Vaccinated | 402 (54.0) | 377 (54.3) |
| BCG vaccination recorded | BCG vaccination not recorded | |||
| BCG vaccination recorded | Missing | |||
| BCG vaccination not recorded | BCG vaccination recorded | |||
| Missing | BCG vaccination recorded | |||
| BCG vaccination not recorded | BCG vaccination not recorded | Not vaccinated | 35 (4.7) | 18 (2.6) |
| BCG vaccination not recorded | Missing | Treated as missing | 60 (8.1) | 85 (12.2) |
| Missing | BCG vaccination not recorded | 47 (6.3) | 37 (5.3) | |
| Missing | Missing | Missing | 200 (26.9) | 177 (25.5) |
Association between time since bacillus Calmette–Guérin vaccination and tuberculosis (all types, i.e. both pulmonary and non-pulmonary disease)
The detailed uptake of vaccination in cases and controls is presented in Table 17 for each interval of time since vaccination and by birth cohort stratum; in the analysis, we stratified by year of birth.
| Birth cohort | Group | Total, n/N (%) | Uptake by time since vaccination (years), n/N (%) | |||
|---|---|---|---|---|---|---|
| 1–5 | 5–10 | 10–15 | 15–19 | |||
| 1985–9 | Cases vaccinated | 16/17 (94) | 0 | 0 | 2/3 | 14/14 |
| Controls vaccinated | 15/16 (94) | 0 | 0 | 6/7 | 15/16 | |
| 1990–4 | Cases vaccinated | 78/85 (92) | 0 | 1/1 | 27/31 | 50/53 |
| Controls vaccinated | 46/48 (96) | 0 | 19/21 | 46/48 | 46/48 | |
| 1995–9 | Cases vaccinated | 118/125 (94) | 3/3 | 38/39 | 62/67 | 15/16 |
| Controls vaccinated | 86/90 (96) | 40/41 | 86/90 | 86/90 | 46/49 | |
| 2000–4 | Cases vaccinated | 107/121 (88) | 56/62 | 42/49 | 9/10 | 0 |
| Controls vaccinated | 97/102 (95) | 97/102 | 97/102 | 24/27 | 0 | |
| 2005–11 | Cases vaccinated | 83/89 (93) | 73/78 | 10/11 | 0 | 0 |
| Controls vaccinated | 133/139 (96) | 133/139 | 48/53 | 0 | 0 | |
| Total for each time period since BCG vaccination, across birth cohorts | Cases vaccinated | 132/143 (92.3) | 91/100 (91.0) | 100/111 (90.1) | 79/83 (95.2) | |
| Controls vaccinated | 270/282 (95.7) | 250/266 (94.0) | 162/172 (94.2) | 107/113 (94.7) | ||
Potential confounding variables
There was little evidence of variation in vaccine uptake with covariates in the study data set. There was some evidence of different uptake by ethnic background and of higher vaccine uptake in participants from crowded households (Table 18). We did not find a strong correlation between covariates.
| Covariates | Vaccinated, n (%) | OR | 95% CI | p-value |
|---|---|---|---|---|
| Sex | ||||
| Female (n = 193) | 182 (94.3) | 1 | 0.286 | |
| Male (n = 202) | 195 (96.5) | 1.68 | 0.64 to 4.44 | |
| Birth cohort | ||||
| 1985–9 (n = 16) | 15 (93.8) | 1 | 0.997 | |
| 1990–4 (n = 48) | 46 (95.8) | 1.53 | 0.13 to 18.1 | |
| 1995–9 (n = 90) | 86 (95.6) | 1.43 | 0.15 to 13.7 | |
| 2000–4 (n = 102) | 97 (95.1) | 1.29 | 0.14 to 11.8 | |
| 2005–11 (n = 139) | 133 (95.7) | 1.48 | 0.17 to 13.11 | |
| Ethnic group | ||||
| Indian (n = 96) | 93 (96.9) | 1 | ||
| Bangladeshi (n = 50) | 48 (96.0) | 0.77 | 0.13 to 4.8 | 0.007 |
| Pakistani (n = 133) | 131 (98.5) | 2.11 | 0.35 to 12.9 | |
| Other Asian (n = 30) | 30 (100.0) | – | – | |
| Black African (n = 56) | 50 (89.3) | 0.27 | 0.06 to 1.12 | |
| Other black (n = 30) | 25 (83.3) | 0.16 | 0.04 to 0.72 | |
| Area-level deprivation quintiles | ||||
| Least deprived (n = 78) | 74 (94.9) | 1 | 0.56 | |
| 2 (n = 79) | 77 (97.5) | 2.08 | 0.37 to 11.7 | |
| 3 (n = 73) | 68 (93.2) | 0.73 | 0.19 to 2.8 | |
| 4 (n = 80) | 78 (97.5) | 2.11 | 0.37 to 11.8 | |
| Most deprived (n = 85) | 80 (94.1) | 0.86 | 0.22 to 3.3 | |
| People per bedroom | ||||
| ≤ 1 (n = 75) | 67 (89.3) | 1 | 0.011 | |
| > 1 (n = 320) | 310 (96.9) | 3.70 | 1.41 to 9.73 | |
| Highest parental education level | ||||
| None (n = 90) | 87 (96.7) | 1 | 0.56 | |
| O level (n = 87) | 82 (94.3) | 0.56 | 0.13 to 2.4 | |
| A level (n = 52) | 51 (98.1) | 1.76 | 0.18 to 17.4 | |
| Degree (n = 160) | 151 (94.4) | 0.58 | 0.15 to 2.2 | |
The analyses were stratified by year of birth and ethnic background, thus allowing for the change in TB rates with age, as well as the known variation in TB rates between ethnic groups. 39 The latter also accounted for any differential uptake in vaccine between ethnic groups. In the multivariable analysis, we adjusted for those variables associated with vaccine uptake as well as other potential confounders, as detailed in Statistical methods.
The results of the complete-case analysis (i.e. using only those subjects with vaccine records) are presented in Table 19. Good evidence of a protective effect of the vaccine was noted in the < 5-year time period and in the 5- to 10-year period before and after adjusting for confounding (HRadj 0.34, 95% CI 0.15 to 0.78; p = 0.011; and HRadj 0.25, 95% CI 0.11 to 0.56; p = 0.001, respectively). The evidence for a protective effect of the vaccine in the time period 10–15 years was weaker, both in the baseline analysis and more so in the adjusted analysis (HRbaseline 0.50, 95% CI 0.22 to 1.13; p = 0.096; and HRadj 0.64, 95% CI 0.24 to 1.68; p = 0.361, respectively). Examination in a baseline analysis of the effect of vaccination on the smaller number of records used in the multivariable analysis suggested that this lack of evidence of an effect was the result of chance (sampling error) rather than the effect of controlling for confounding. Evidence was lacking for a protective effect 15–20 years after vaccination in both models based on the small numbers available. CIs were wide, ranging in the baseline analysis from 0.28 to 5.64.
| Time since vaccination (years) | Base modela (based on 417 cases and 382 controls) | Base modela restricted to subjects with no missing data on covariates (based on 379 cases and 378 controls) | Multivariable adjusted modelb (based on 379 cases and 378 controls) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Unvaccinated | 1 (reference) | 1 (reference) | ||||
| 1–5 | 0.38 (0.17 to 0.86) | 0.021 | 0.43 (0.18 to 1.03) | 0.058 | 0.34 (0.15 to 0.78) | 0.011 |
| 5–10 | 0.37 (0.15 to 0.88) | 0.025 | 0.32 (0.13 to 0.79) | 0.014 | 0.25 (0.11 to 0.56) | 0.001 |
| 10–15 | 0.50 (0.22 to 1.13) | 0.096 | 0.68 (0.28 to 1.66) | 0.396 | 0.64 (0.24 to 1.68) | 0.361 |
| 15–20 | 1.25 (0.28 to 5.64) | 0.775 | 2.06 (0.33 to 12.91) | 0.440 | 2.26 (0.34 to 15.10) | 0.400 |
Trends in the association between time since bacillus Calmette–Guérin vaccination and risk of tuberculosis
Table 20 shows the results from modelling of the association between BCG vaccination and the log-hazard of TB as a linear function of time since vaccination, centred at 5 years post vaccination. The results are displayed graphically in Figure 6. The model suggests a 12% increase (95% CI –1% to 26%) in the HR with each year post vaccination, although this was of only borderline statistical significance. We also explored the trend in time using restricted cubic splines with three knots, at 5, 10 and 15 years post vaccination. The results were similar to those found using the linear model and are not shown here. The results suggest a statistically significant protective effect of the vaccine up to about 10 years post vaccination, with a gradual reduction in the protective effect up to that time. After 10 years post vaccination, the confidence bounds are wide and there is insufficient information to draw conclusions about vaccine effectiveness.
| Time since BCG vaccination | HR (95% CI) | p-value |
|---|---|---|
| Unvaccinated | 1 (reference) | |
| HR at 5 years post vaccination | 0.32 (0.16 to 0.64) | 0.001 |
| Multiplying factor for each year increase in time since vaccination | 1.12 (0.99 to 1.26) | 0.062 |
FIGURE 6.
Results from modelling of the time-varying effect of the vaccine as a linear function of time (on the log-scale) in the infant BCG study. The left-hand vertical axis shows the HR and the right-hand vertical axis shows the VE, both on the log-scale. The results are based on the multivariable adjusted model. The dashed lines show the 95% confidence bounds.
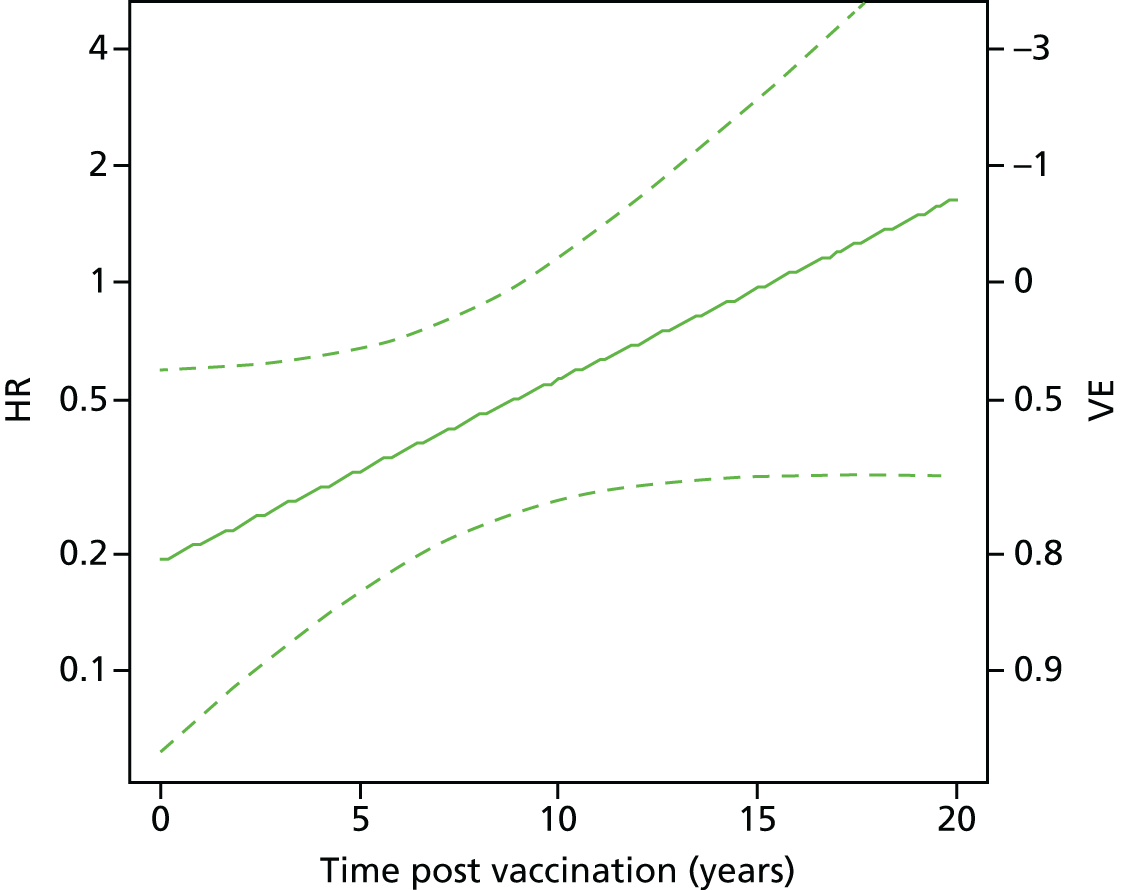
Results: school-age bacillus Calmette–Guérin study
Overview of recruitment
Recruitment of cases
A total of 1602 potentially eligible cases was identified from the ETS system and invited to take part in the study, of whom 1047 (65%) were successfully contacted. About 11% were not included either because they were ineligible or because of other difficulties (Figure 7). Of those contacted and eligible, 257 (28%) declined to participate and 677 (72%) were enrolled.
FIGURE 7.
Overview of recruitment of school-age BCG cases. a, No contact because changed addresses (n = 353), no contact with anybody at address provided (n = 165), moved from England to another part of the UK (n = 10), moved abroad (n = 12), inaccessible (n = 6) or unspecified (n = 9). b, Ineligible because not born in the UK (n = 13), not white (n = 23), reported vaccination at a later age and following contact with a TB case in the red book and consistent with the date of TB diagnosis on the ETS system (n = 4) or unspecified (n = 20). c, Other difficulties includes away or in hospital during the survey (n = 23), too frail to take part (n = 20), died (n = 6) and various other difficulties (n = 4).
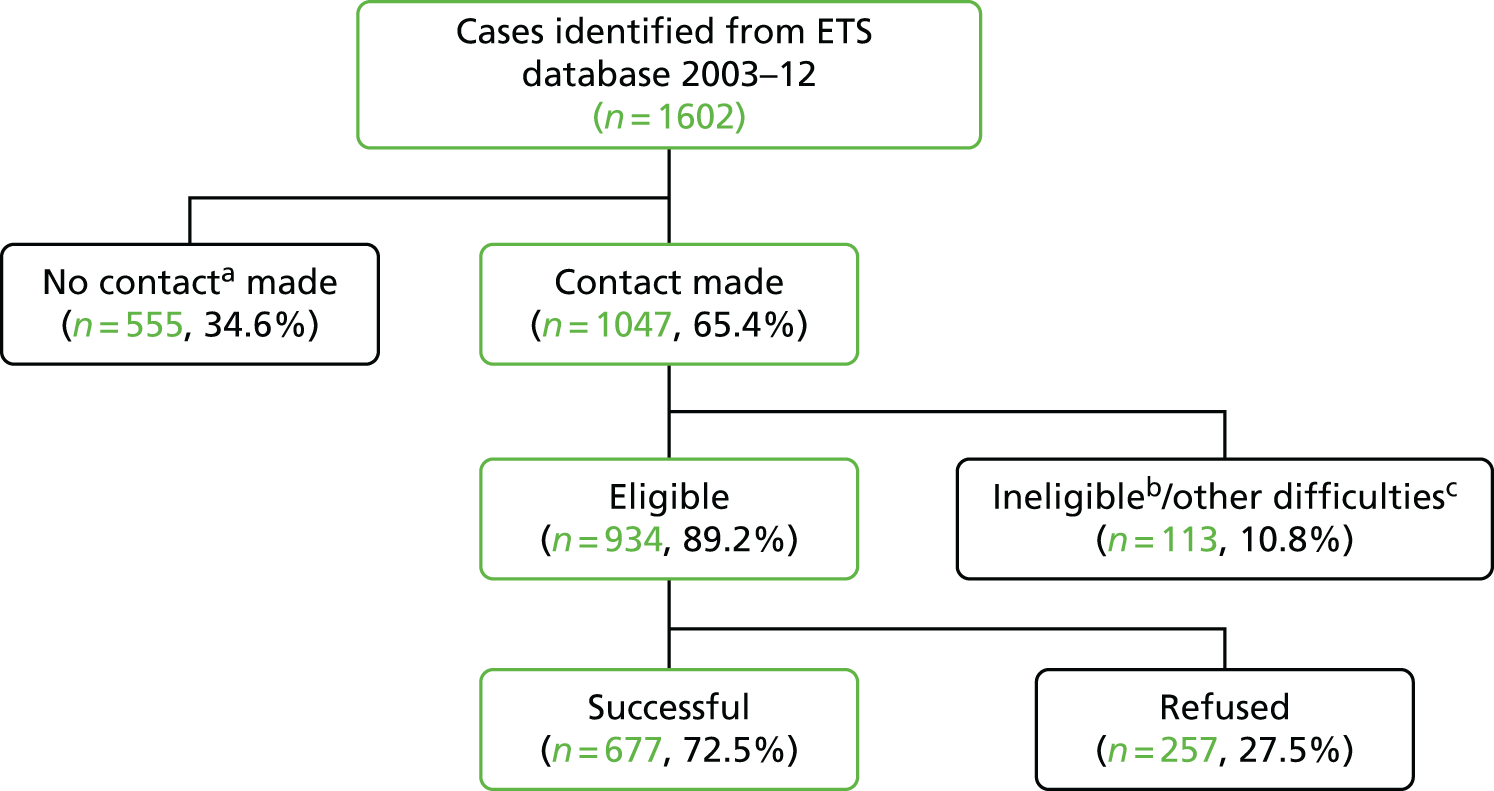
Recruitment of controls
We sampled 9424 residential addresses, of which 1248 (13%) could not be screened because the address no longer existed or no-one was contactable at the household after several visits to establish if any of the residents were eligible for the study (Figure 8). Among the 8176 addresses that were screened, 1790 (22%) had at least one eligible resident, with 1170 (65%) eligible subjects successfully recruited to the control group.
FIGURE 8.
Overview of recruitment of school-age BCG controls. a, Not screened because no contact with anybody at the address provided after several visits following the preset visit pattern. b, Includes 5692 addresses screened with no eligible subjects and 694 addresses with non-residential, vacant, derelict or demolished buildings.
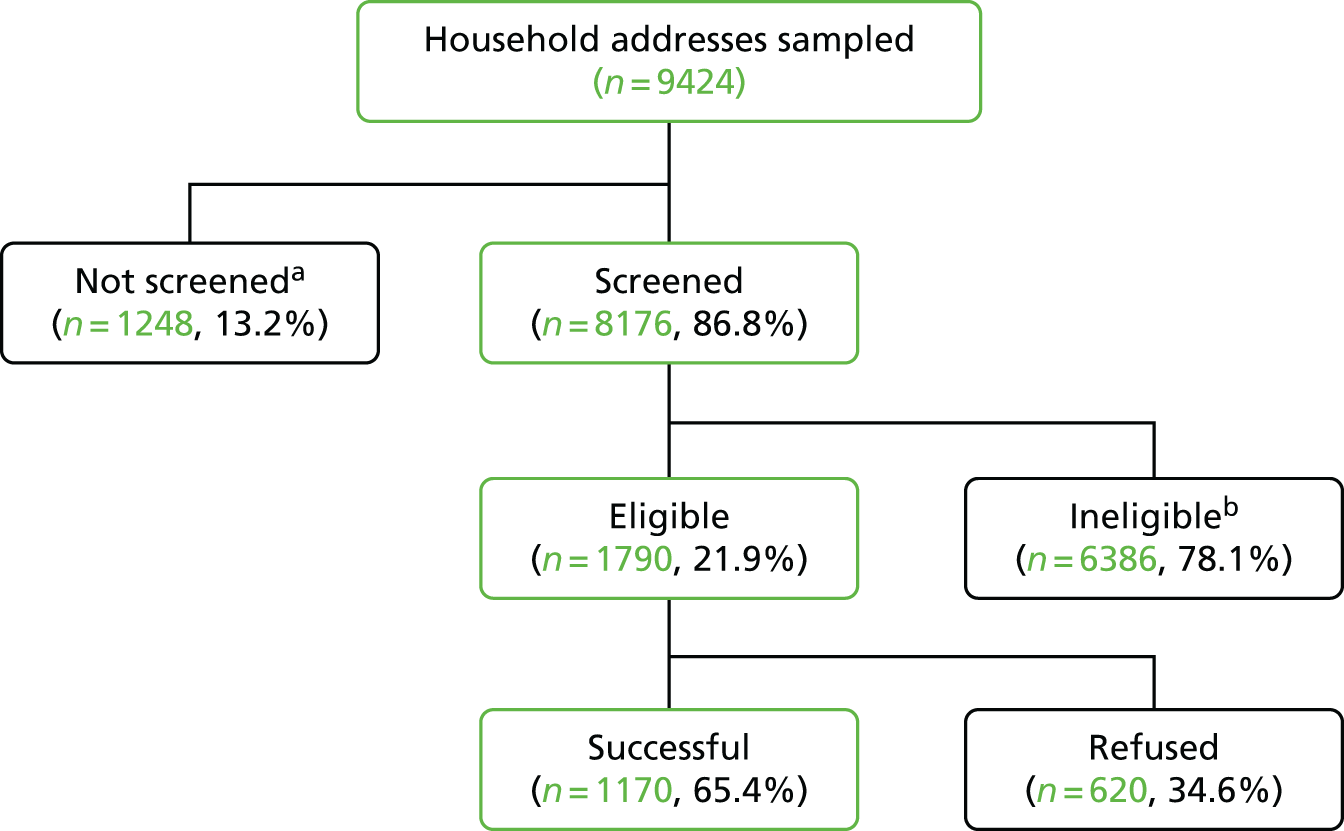
Comparison of recruitment between cases and controls
The proportion of cases who could be contacted was slightly lower in the more deprived quintiles of area-level IMD (Table 21). Among those contacted and eligible, the refusal rate was slightly lower in the lower quintiles of area-level IMD. The proportion of addresses successfully screened for eligible controls was similar across quintiles of IMD. Among subjects identified as eligible to be controls, the refusal rate was similar across quintiles. The refusal rate was slightly higher for controls than for cases.
| Area-level deprivation quintiles | Group | |||
|---|---|---|---|---|
| Cases | Controls | |||
| Cases invited to participatea | Addresses screened for eligibility | |||
| Total, n | Contacted, n (%) | Total, n | Contacted, n (%) | |
| Least deprived | 165 | 119 (72) | 1889 | 1634 (87) |
| 2 | 218 | 157 (72) | 1885 | 1599 (85) |
| 3 | 233 | 148 (64) | 1886 | 1631 (86) |
| 4 | 394 | 254 (64) | 1886 | 1659 (88) |
| Most deprived | 575 | 357 (62) | 1878 | 1653 (88) |
| Eligible cases contacted | Eligible controls contacted | |||
| Eligible, n | Refused, n (%) | Eligible, n | Refused, n (%) | |
| Least deprived | 107 | 35 (33) | 429 | 155 (36) |
| 2 | 146 | 36 (25) | 363 | 130 (36) |
| 3 | 137 | 35 (26) | 358 | 127 (35) |
| 4 | 217 | 63 (29) | 359 | 113 (31) |
| Most deprived | 316 | 86 (27) | 281 | 95 (34) |
The distribution of interviewer visits by time of the day and day of the week was comparable between cases and controls (Figure 9).
FIGURE 9.
Distribution of interviewer visits by (a) time of the day and (b) day of the week for invited cases and sampled control addresses in the school-age BCG study.

We found that retrospective ascertainment of the results of the TST was challenging in the school-aged BCG study, with very poor recall and no records to support or validate participants’ self-reports. Further investigation of the literature47 indicated that the initially high TB risk in those who were PPD positive dropped to be the same as that for those who were PPD negative and unvaccinated (see Figure 11). Thus, we included all those who were unvaccinated, irrespective of PPD status prior to the offer of school-aged BCG vaccination.
Descriptive statistics by case and control status
The cases were slightly younger than the controls and were more likely to be male (Table 22). The distribution of cases by area-level deprivation quintiles and parental education level was consistent with the known association of TB with deprivation, with a higher proportion of cases than controls in the most deprived quintile and a higher proportion of cases than controls with fewer educational qualifications. There were also slightly more cases than controls from overcrowded households. Cases were slightly more likely than controls to have regularly travelled to a high-risk area and were somewhat more likely than controls to have had a long-term stay in a high-risk area. A higher proportion of cases than controls reported drinking at a hazardous or harmful level and being a smoker. A much higher proportion of cases than controls reported having used class A drugs. Cases were also more likely than controls to report a history of homelessness for ≥ 1 week and a history of prison detention in the UK or abroad.
| Characteristic | Group, n (%) | |
|---|---|---|
| Cases (N = 677) | Controls (N = 1170) | |
| Birth cohort | ||
| 1965–9 | 65 (9.6) | 174 (14.9) |
| 1970–4 | 178 (26.3) | 312 (26.7) |
| 1975–9 | 215 (31.8) | 260 (22.2) |
| 1980–9 | 219 (32.3) | 424 (36.2) |
| Sex | ||
| Female | 341 (50.4) | 700 (59.8) |
| Male | 336 (49.6) | 470 (40.2) |
| Area-level deprivation quintiles | ||
| 1 (least deprived) | 63 (9.3) | 234 (20.0) |
| 2 | 99 (14.6) | 234 (20.0) |
| 3 | 109 (16.1) | 234 (20.0) |
| 4 | 130 (19.2) | 234 (20.0) |
| 5 (most deprived) | 276 (40.8) | 234 (20.0) |
| Highest educational (academic, professional and/or vocational) qualification | ||
| None | 132 (19.5) | 75 (6.4) |
| O level or equivalenta | 207 (30.6) | 363 (31.0) |
| A level or equivalentb | 91 (13.4) | 246 (21.0) |
| Degree level or equivalentc | 216 (31.9) | 455 (38.9) |
| Missing | 31 (4.6) | 31 (2.6) |
| Average number of people per room | ||
| ≤ 1 | 634 (93.6) | 1144 (97.8) |
| > 1 | 26 (3.8) | 24 (2.1) |
| Missing | 17 (2.5) | 2 (0.2) |
| Average number of people per bedroom | ||
| ≤ 1 | 385 (56.9) | 705 (60.3) |
| > 1 | 275 (40.6) | 463 (39.6) |
| Missing | 17 (2.5) | 2 (0.2) |
| TB infection risk from regular travels abroad | ||
| Lowd | 618 (91.3) | 1099 (93.9) |
| Highe | 58 (8.6) | 71 (6.1) |
| Missing | 1 (0.1) | 0 (0.0) |
| TB infection risk from long-term stays abroad | ||
| Lowd | 607 (89.7) | 1113 (95.1) |
| Highe | 70 (10.3) | 57 (4.9) |
| Alcohol consumptionf | ||
| Very low/no risk | 166 (24.5) | 329 (28.1) |
| Low risk | 346 (51.1) | 632 (54.0) |
| Hazardous risk | 36 (5.3) | 68 (5.8) |
| Harmful risk | 41 (6.1) | 25 (2.1) |
| Missing | 88 (13.0) | 116 (9.9) |
| Tobacco smoking | ||
| Never smoker | 188 (27.8) | 499 (42.6) |
| Ex-smoker | 62 (9.2) | 135 (11.5) |
| Smoker: < 20 pack-years | 308 (45.5) | 422 (36.1) |
| Smoker: ≥ 20 pack-years | 99 (14.6) | 85 (7.3) |
| Missing | 20 (3.0) | 29 (2.5) |
| Drug misuse/abuseg | ||
| No drug use | 379 (56.0) | 847 (72.4) |
| Class B and/or C use only | 69 (10.2) | 108 (9.2) |
| Class A use | 217 (32.1) | 188 (16.1) |
| Missing | 12 (1.8) | 27 (2.3) |
| History of homelessness | ||
| Never been homeless for ≥ 1 week | 553 (81.7) | 1091 (93.2) |
| Ever been homeless for ≥ 1 week | 117 (17.3) | 68 (5.8) |
| Missing | 7 (1.0) | 11 (0.9) |
| History of prison stayh | ||
| Never detained | 590 (87.1) | 1119 (95.6) |
| Ever detained in the UK or abroad | 82 (12.1) | 35 (3.0) |
| Missing | 5 (0.7) | 16 (1.4) |
Indicators of vaccination status
The availability of the various indicators of BCG status is presented in Table 23. As noted earlier (see Table 12), self-report of BCG vaccination at school was judged to be more accurate than self-report or parental report of infant BCG vaccination. Both personal and NHS records were missing for > 90% of study participants in the school-aged BCG study and insufficient information was available to allow any formal assessment of the validity of either of these measures. There was, however, a good level of agreement between self-reported history and scar inspection. These two indicators were therefore combined to define BCG status in this study (Table 24).
| Indicator | Group, n (%) | |
|---|---|---|
| Cases (N = 677) | Controls (N = 1170) | |
| BCG vaccination history | ||
| Vaccinated | 470 (69.4) | 922 (78.8) |
| Probably vaccinated | 6 (0.9) | 32 (2.7) |
| Not vaccinated | 170 (25.1) | 169 (14.4) |
| Missing | 31 (4.6) | 47 (4.0) |
| Scar inspection | ||
| BCG scar | 424 (62.6) | 844 (72.1) |
| No BCG scar | 204 (30.1) | 269 (23.0) |
| Not inspected | 49 (7.2) | 57 (4.9) |
| Personal vaccination record | ||
| BCG vaccination recorded | 6 (0.9) | 12 (1.0) |
| BCG vaccination not recorded | 29 (4.3) | 59 (5.0) |
| No personal record | 642 (94.8) | 1099 (93.9) |
| NHS vaccination records | ||
| BCG vaccination recorded | 16 (2.4) | 20 (1.7) |
| BCG vaccination not recorded | 20 (3.0) | 27 (2.3) |
| NHS record missing | 641 (94.7) | 1123 (96.0) |
| Self-reported history | Scar inspection | BCG vaccination status | Group, n (%) | |
|---|---|---|---|---|
| Cases (N = 677) | Controls (N = 1170) | |||
| Convincing BCG history | Scar present | Vaccinated | 473 (69.9) | 933 (79.7) |
| Convincing BCG history | No scar | |||
| Convincing BCG history | Not inspected | |||
| Probable BCG history | Scar present | |||
| Probable BCG history | No scar | Likely vaccinateda | 33 (4.9) | 78 (6.7) |
| Probable BCG history | Not inspected | |||
| No BCG history | Scar present | |||
| Unsure | Scar present | |||
| No BCG history | No scar | Not vaccinated | 163 (24.1) | 154 (13.2) |
| No BCG history | Not inspected | |||
| Unsure | No scar | |||
| Unsure | Not inspected | Missing | 8 (1.2) | 5 (0.4) |
Association between time since bacillus Calmette–Guérin vaccination and all cases of tuberculosis: complete-case analysis
The distribution of case and control subjects by vaccination status and time since vaccination is presented in Table 25. There was strong evidence of a protective effect of the vaccine in each of the 5-year periods from 10 to 20 years post vaccination (Table 26). Results from the model fully adjusted for confounders provided evidence of a protective effect 10–15 years (HRadj 0.49, 95% CI 0.31 to 0.79; p = 0.003) and 15–20 years (HRadj 0.43, 95% CI 0.28 to 0.67; p < 0.001) after vaccination compared with > 20 years after vaccination. The numbers were too small to assess the effect from 20 to 25 years but there was a suggestion of a protective effect.
| Birth cohort | Group | Total, n/N (%) | Uptake by time since vaccination (years), n/N (%) | |||
|---|---|---|---|---|---|---|
| 10–15 | 15–20 | 20–25 | 25–29 | |||
| 1965–9 | Cases vaccinated | 51/64 (79.7) | – | – | 32/39 | 19/25 |
| Controls vaccinated | 147/173 (85.0) | 0 | 0 | 124/144 | 147/173 | |
| 1970–4 | Cases vaccinated | 136/174 (78.2) | – | 28/34 | 93/120 | 15/20 |
| Controls vaccinated | 262/311 (84.2) | 0 | 208/259 | 262/311 | 262/311 | |
| 1975–9 | Cases vaccinated | 161/212 (75.9) | 33/46 | 66/81 | 61/84 | 1/1 |
| Controls vaccinated | 231/259 (89.2) | 231/259 | 231/259 | 231/259 | 55/64 | |
| 1980–9 | Cases vaccinated | 158/219 (72.1) | 121/159 | 37/59 | 0/1 | – |
| Controls vaccinated | 371/422 (87.9) | 371/422 | 236/259 | 48/55 | 0 | |
| Total for each time period since BCG vaccination, across birth cohorts | Cases vaccinated | 154/205 (75.1) | 131/174 (75.3) | 186/244 (76.2) | 35/46 (76.1) | |
| Time since vaccination (years) | Base modela (based on 669 cases and 1165 controls) | Partially adjusted modelb (based on 638 cases and 1134 controls) | Fully adjusted modelc (based on 532 cases and 993 controls) | Base modela fitted on same subset as in fully adjusted modelc (based on 532 cases and 993 controls) | Partially adjusted modelb fitted on same subset as in fully adjusted modelc (based on 532 cases and 993 controls) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Unvaccinated | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |||||
| 10–15 | 0.400 (0.274 to 0.584) | 0.000 | 0.419 (0.272 to 0.644) | 0.000 | 0.49 (0.31 to 0.79) | 0.003 | 0.43 (0.28 to 0.66) | < 0.001 | 0.49 (0.31 to 0.79) | 0.004 |
| 15–20 | 0.346 (0.246 to 0.487) | 0.000 | 0.390 (0.264 to 0.575) | 0.000 | 0.43 (0.28 to 0.67) | < 0.001 | 0.34 (0.23 to 0.50) | < 0.001 | 0.41 (0.27 to 0.63) | < 0.001 |
| 20–25 | 0.554 (0.404 to 0.759) | 0.000 | 0.640 (0.448 to 0.916) | 0.015 | 0.75 (0.49 to 1.14) | 0.174 | 0.56 (0.39 to 0.80) | 0.001 | 0.69 (0.46 to 1.02) | 0.065 |
| 25–29 | 0.565 (0.342 to 0.932) | 0.025 | 0.772 (0.453 to 1.315) | 0.341 | 0.99 (0.53 to 1.84) | 0.97 | 0.70 (0.40 to 1.24) | 0.225 | 0.88 (0.48 to 1.59) | 0.66 |
These results are based on a complete-case analysis (i.e. based on subjects with no missing data). Missing information may be explored in a future analysis.
Trends in the association between time since bacillus Calmette–Guérin vaccination and risk of tuberculosis
Table 27 shows the results from modelling of the association between BCG vaccination and log-hazard of TB as a linear function of time since vaccination, centred at 10 years post vaccination. The results are displayed graphically in Figure 10. The model suggests a 7% increase (95% CI 0.2% to 12%) in the HR with each year post vaccination and this was statistically significant. We also explored the trend in time using restricted cubic splines with three knots, at 15, 20 and 25 years post vaccination. The results were similar to those found using the linear model and are not shown here.
| Time since BCG vaccination | Adjusted model | |||
|---|---|---|---|---|
| Partiallya | Fullyb | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Unvaccinated | 1 (reference) | 1 (reference) | ||
| HR at 10 years post vaccination | 0.310 (0.188 to 0.511) | 0.000 | 0.374 (0.215 to 0.652) | 0.001 |
| Multiplying factor for each year increase in time since vaccination | 1.07 (1.00 to 1.12) | 0.015 | 1.057 (1.002 to 1.116) | 0.042 |
FIGURE 10.
Results from modelling of the time-varying effect of the vaccine as a linear function of time (on the log-scale) in the school-age study. The left-hand vertical axis shows the HR and the right-hand vertical axis shows the VE, both on the log-scale. The results are based on the multivariable adjusted model. The dashed lines show the 95% confidence bounds.

The results showed a statistically significant protective effect of the vaccine up to about 23 years post vaccination, with a gradual reduction in the protective effect up to that time. The protective effect of the vaccine appears to reduce more steeply after about 20 years post vaccination. However, we had only a relatively small number of cases with > 25 years post vaccination and, thus, have insufficient evidence to assess protection beyond that time.
Discussion
The results from our studies of the duration of protection of BCG vaccination indicate that protection persists for at least 10 years for infant vaccination in a population at high risk for TB and for at least 20 years for school-age vaccination in a low-risk population. A protective effect of 48% (95% CI 17% to 68%) was seen 10–15 years after school-age BCG vaccination and a protective effect of 55% (95% CI 30 to 71%) was seen 15–20 years after school-age vaccination, beyond which protection appeared to wane. For infant BCG vaccination, a protective effect was seen for up to 10 years post vaccination (< 5 years post vaccination: VE 66%, 95% CI 17% to 86%; 5–10 years post vaccination: VE 75%, 95% CI 43% to 89%), but there was less evidence of an effect 10–15 post vaccination (VE 36%, 95% CI negative to 77%; p = 0.396). In the infant BCG study, in the analysis adjusted for several confounders, including birth cohort and ethnicity, the results were based on subjects for whom vaccine records were available. By adjusting only for ethnicity and birth cohort, slightly more records with complete data could be included, giving weak evidence of 50% VE (95% CI negative to 78%; p = 0.096) 10–15 years post vaccination. The higher than expected infant BCG vaccine uptake rate in this high-risk ethnic minority study population and the sparsity of vaccine record data in the later time periods precluded further assessment.
The study findings are consistent with recent findings from Norway41 and Brazil,25 and provide additional evidence to that from the seminal MRC trial,7 in which protection of 63% was seen 10–15 years after vaccination, with wide CIs (95% CI 17% to 84%), with no evidence of protection 15–20 years after vaccination (VE 9%, 95% CI negative to 71%).
The BCG vaccine composition and changes in administration were not considered to be important sources of variation over the study period. Although the Danish liquid BCG vaccine was replaced in the UK in the 1960s by the Glaxo freeze-dried BCG vaccine (Glaxo 1077 vaccine strain developed by Glaxo from the Danish strain and produced in the UK by Evans-Medeva), a study comparing both showed non-inferiority. 42,43 From 2002, the UK has been able to source the BCG vaccine only from Denmark’s Statens Serum Institut (SSI vaccine, also based on the Danish strain 1331). 44 A recent systematic review of the BCG vaccine trials by our research group also suggested that protection did not vary by vaccine strain. 10 Multipuncture vaccination use was limited. In our analysis of policy we noted that the 1983 survey indicated that 76% of districts used intradermal BCG vaccination, which increased in the 1992 survey to 96% of districts. No data were available comparing protection using multipuncture with protection using intradermal vaccination, but sensitisation based on the TST was similar. 45,46
There was a good response rate in both studies and we were able to successfully recruit population-based controls to represent both the children born in the UK to populations from high TB burden settings and the general population. We were unable to link to vaccine records for most of the school-aged BCG subjects, despite their willingness to consent to such linkage. Self-reported history of BCG vaccination and scar inspection were used instead to measure BCG vaccine uptake, having been found to have good agreement with each other (this agreement was not observed, by contrast, in those offered the BCG vaccine in infancy). Although interviewers were not blind to case–control status or to knowledge of a history of BCG vaccination before scar reading, they were specifically trained to identify a BCG scar, which included examining volunteers with and without scars. In addition, 10% of interviews selected at random were checked by senior staff and standardised reporting was required. The interviewers were aware that the study was to assess the duration of protection from BCG. As there was little information on how long BCG protects, it is unlikely that the interviewers would have been biased in one direction with the presence or absence of a BCG scar. There was some confounding because of lower BCG vaccine uptake in poorer subjects, who had a higher risk of TB. We were, however, able to control for this.
We were able to evaluate the effectiveness of the BCG vaccine in BAME population groups despite initial concerns that this group would be difficult to assess. Consent was obtained to link to vaccine records, but records could be retrieved for only about half of the participants, from either NHS administrative areas or patient-held records. There was a considerable lack of agreement between the different sources of information on infant BCG vaccination. In the infant study, self-reported BCG vaccine uptake was also found to be a poor measure of vaccination and scar reading was poorly concordant with vaccine records, when available. Red book and NHS records also had poor concordance, indicating that BCG vaccination was being noted in one record but not necessarily the other. We also noted a higher refusal rate for reading a BCG scar in some BAME young adult female cases, who were not comfortable having their upper arms examined compared with controls. For reasons of poor concordance of the two sources of records and probably biased assessments of scars, we therefore used vaccine records when available, despite not having information on uptake for about 40% of subjects. Future work may include exploring different approaches to determine vaccination status, which could include scar reading, despite the relatively poor specificity of scar reading noted in this study, to reduce the level of missing information on vaccination status in the infant vaccination study.
Addressing the issue of prior infection as the reason for not receiving bacillus Calmette–Guérin vaccination
A theoretical limitation in the school-age BCG study was the inability to assess, and exclude, controls for a prior positive TST. Retrospective ascertainment of TST results based on recall was clearly not feasible and there were no records to validate participants’ memory recall. Under the routine school vaccination programme, prior infection by M. tuberculosis or sensitisation by environmental mycobacteria was investigated through the TST and such subjects were not offered the BCG vaccine. These subjects are usually not included in studies measuring the effect of the BCG vaccine in the short term, as there is evidence that the risk of TB is higher in TST-positive subjects during the first few years after infection. Their inclusion would, thus, act to overestimate the protective effect of BCG vaccination. It is also thought that the BCG vaccine does not confer protection in TST-positive subjects. However, follow-up data from the UK MRC BCG trial of adolescents7,47 showed that, at least in settings with a low transmission rate, the risk of TB in TST-positive participants declined over time and was similar to that of subjects who were TST negative at baseline by about 10 years after enrolment (Figure 11). Thus, the TST was thought unlikely to play a major role when measuring the association between BCG vaccination and TB beyond 10 years after vaccination, as in our study. Extrapolation of earlier modelling work by one of the authors of the paper (extrapolation not published)48 and this report also suggested that the prevalence of tuberculin positivity in the white population in general would have been around 4% at the time of screening for vaccination.
FIGURE 11.
Comparison of TB rates in vaccinated and unvaccinated subjects by TST status at the start of follow-up in the UK MRC BCG trial of adolescents. TU, tuberculin units. Figure based on data from the UK MRC trial published in the Fourth Report to the Medical Research Council by its Tuberculosis Vaccines Clinical Trials Committee. 47

Conclusions
In summary, our two observational studies suggest that BCG vaccination provides longer-lasting protection than previously described, particularly for BCG vaccination at school age, for which we were able to use scar identification and history as measures of BCG uptake. Protection was noted for more than two decades after vaccination, but waned 20–25 years after vaccination. The findings are consistent with the limited data emerging from other trials and observational studies,23 as well as from a more recent Norwegian historical cohort study of the duration of protection of the BCG vaccine. 41 BCG vaccination at school age may thus have helped in the control of TB, including reducing the potential for developing multidrug-resistant disease, as those vaccinated at about 13 years of age have moved into adulthood.
Our current analysis of the infant BCG study, based on just over 50% of the subjects with vaccine records and complete information on covariates, was able to confirm the known protective effect of infant BCG vaccination against TB for up to 10 years after vaccination, adjusting for confounders. However, numbers were too sparse after 10 years to provide robust evidence of VE for this time period. Vaccine coverage was higher than expected in those with records. Further exploratory analyses to deal with missing records could include multiple imputation of missing BCG vaccination status, among other sensitivity analyses. This assessment might inform a review of incidence levels when countries are recommended to suspend BCG vaccination, as well as estimates of the cost-effectiveness of the BCG vaccine in the prevention of TB, and might have implications for the testing and scheduling of new vaccines against TB, which will need to provide better protection than the BCG vaccine and be as durable. More systematic recording of BCG vaccine uptake at the population level by ethnic group using the CHIS and linkage to TB events could help to provide ongoing data for assessing protection and the duration of protection over the life course.
Acknowledgements
We would like to thank Reen Polonsky, who helped administer the project and supported many of the logistics at the London School of Hygiene & Tropical Medicine. We would particularly like to thank the several members of the research team at NatCen who made the fieldwork possible, including Elizabeth Fuller, Alison Moody and Soazig Clifton, among others. We also thank all of the interviewers for their careful work and commitment to the project, their supervisors and the operation team members, as well as all the individuals who allowed us to contact them and helped us by taking part in the study. Public Health England was a key partner in the research. We thank staff there, especially colleagues from the TB section. We also thank our steering group team members for their support and advice.
Contributions of authors
Punam Mangtani co-designed and cowrote the proposal with Laura Rodrigues, provided the managerial lead for the project with Patrick Nguipdop Djomo, provided academic leadership and cowrote a first draft of the report.
Patrick Nguipdop-Djomo provided the managerial lead for the project with Punam Mangtani, provided academic leadership, assisted with the analyses, carried out data cleaning and merging of data across sources, and cowrote a first draft of the report.
Ruth H Keogh devised and carried out the analyses, carried out data cleaning and merging of data across sources, provided valued academic expertise and advice at key points, and cowrote a first draft of the report.
Lucy Trinder assisted with the analyses, carried out data cleaning and merging of data across sources, and provided valued academic expertise and advice at key points.
Peter G Smith provided valued academic expertise and advice at key points, and provided additional expertise in BCG vaccine epidemiology and study design.
Paul EM Fine provided valued academic expertise and advice at key points, and provided additional expertise in BCG vaccine epidemiology and study design.
Jonathan Sterne provided valued academic expertise and advice at key points, and provided additional expertise in statistics.
Ibrahim Abubakar provided valued academic expertise and advice at key points, and provided additional expertise in TB epidemiology, the ETS system, BCG vaccine records, public health policy and clinical aspects.
Emilia Vynnycky provided valued academic expertise and advice at key points, and provided additional expertise in exploring PPD positivity levels in the general population.
John Watson provided valued academic expertise and advice at key points, and provided additional expertise in TB epidemiology, the ETS system, BCG vaccine records, public health policy and clinical aspects.
David Elliman provided valued academic expertise and advice at key points, and provided additional expertise in TB epidemiology, the ETS system, BCG vaccine records, public health policy and clinical aspects.
Marc Lipman provided valued academic expertise and advice at key points, and provided additional expertise in TB epidemiology, the ETS system, BCG vaccine records, public health policy and clinical aspects.
Laura C Rodrigues co-designed and cowrote the proposal with Punam Mangtani, and provided academic leadership.
All authors contributed to the final report.
Publications
Pilger D, Nguipdop-Djomo P, Abubakar I, Elliman D, Rodridgues LC, Watson JM, et al. BCG vaccination in England since 2005: a survey of policy and practice. BMJ Open 2012;2:e001303.
Nguipdop-Djomo P, Mangtani P, Pedrazzoli D, Rodrigues LC, Abubakar I. Uptake of neonatal BCG vaccination in England: performance of the current policy recommendations. Thorax 2014;69:87–9.
Data sharing statement
A copy of the final data will be kept on the London School of Hygiene & Tropical Medicine’s data archive [LSHTM Data Compass; see http://datacompass.lshtm.ac.uk/ (accessed 24 March 2017)] and will be available after the main results are published. At this time it can be obtained from the corresponding author through the London School of Hygiene & Tropical Medicine repository.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974;99:131-8. https://doi.org/10.1093/oxfordjournals.aje.a121593.
- World Health Organization . Global Tuberculosis Report 2015 2015.
- Crofts JP, Gelb D, Andrews N, Delpech V, Watson JM, Abubakar I. Investigating tuberculosis trends in England. Public Health 2008;122:1302-10. https://doi.org/10.1016/j.puhe.2008.04.011.
- European Centre for Disease Prevention and Control/World Health Organization Regional Office for Europe . Tuberculosis Surveillance and Monitoring in Europe 2015 2015.
- Public Health England . Tuberculosis in England 2016 (Presenting Data to End of 2015) n.d. www.gov.uk/government/uploads/system/uploads/Attachment_data/file/555343/TB_Annual_Report_2016_GTW2309.pdf (accessed November 2016).
- Kruijshaar ME, Watson JM, Drobniewski F, Anderson C, Brown TJ, Magee JG, et al. Increasing antituberculosis drug resistance in the United Kingdom: analysis of National Surveillance Data. BMJ 2008;336:1231-4. https://doi.org/10.1136/bmj.39546.573067.25.
- Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J 1977;2:293-5. https://doi.org/10.1136/bmj.2.6082.293.
- Sutherland I, Springett VH. Effectiveness of BCG vaccination in England and Wales in 1983. Tubercle 1987;68:81-92. https://doi.org/10.1016/0041-3879(87)90023-7.
- Anonymous . Trial of BCG vaccines in south India for tuberculosis prevention: first report – Tuberculosis Prevention Trial. Bull World Health Organ 1979;57:819-27.
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58:470-80. https://doi.org/10.1093/cid/cit790.
- Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette–Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 1995;96:29-35.
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol 1993;22:1154-8. https://doi.org/10.1093/ije/22.6.1154.
- World Health Organization . BCG vaccine. WHO position paper. Wkly Epidemiol Rec 2004;79:27-38.
- Karonga Prevention Trial Group . Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996;348:17-24. https://doi.org/10.1016/S0140-6736(96)02166-6.
- Barreto ML, Pereira SM, Pilger D, Cruz AA, Cunha SS, Sant’Anna C, et al. Evidence of an effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: second report of the BCG-REVAC cluster-randomised trial. Vaccine 2011;29:4875-7. https://doi.org/10.1016/j.vaccine.2011.05.023.
- Joseph CA, Watson JM, Fern KJ. BCG immunisation in England and Wales: a survey of policy and practice in schoolchildren and neonates. BMJ 1992;305:495-8. https://doi.org/10.1136/bmj.305.6852.495.
- Sutherland I, Springett VH. The effects of the scheme for BCG vaccination of schoolchildren in England and Wales and the consequences of discontinuing the scheme at various dates. J Epidemiol Community Health 1989;43:15-24. https://doi.org/10.1136/jech.43.1.15.
- Anonymous . Criteria for discontinuation of vaccination programmes using Bacille Calmette–Guérin (BCG) in countries with a low prevalence of tuberculosis. A statement of the International Union Against Tuberculosis and Lung Disease. Tuber Lung Dis 1994;75:179-80. https://doi.org/10.1016/0962-8479(94)90003-5.
- Department of Health . PL CMO (2005)3: Changes to the BCG Vaccination Programme 2005. www.dh.gov.uk/en/Publicationsandstatistics/Lettersandcirculars/Professionalletters/Chiefmedicalofficerletters/DH_4114993 (accessed 15 April 2012).
- Department of Health . Immunisation Against Infectious Disease – ‘The Green Book’ 2006. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_079917 (accessed 15 April 2012).
- Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination?. Int J Tuberc Lung Dis 1998;2:200-7.
- Fine PE, Vynnycky E. The effect of heterologous immunity upon the apparent efficacy of (e.g. BCG) vaccines. Vaccine 1998;16:1923-8. https://doi.org/10.1016/S0264-410X(98)00124-8.
- Abubakar I, Pimpin L, Ariti C, Beynon R, Mangtani P, Sterne JA, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette–Guérin vaccination against tuberculosis. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17370.
- Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA 2004;291:2086-91. https://doi.org/10.1001/jama.291.17.2086.
- Barreto ML, Cunha SS, Pereira SM, Genser B, Hijjar MA, Yury Ichihara M, et al. Neonatal BCG protection against tuberculosis lasts for 20 years in Brazil. Int J Tuberc Lung Dis 2005;9:1171-3.
- Pilger D, Nguipdop-Djomo P, Abubakar I, Elliman D, Rodrigues LC, Watson JM, et al. BCG vaccination in England since 2005: a survey of policy and practice. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2012-001303.
- Miller CL, Morris J, Pollock TM. PHLS inquiry into current BCG vaccination policy. Br Med J 1984;288. https://doi.org/10.1136/bmj.288.6416.564.
- Becker E, Boreham R, Chaudhury M, Craig R, Deverill C, Doyle M, et al. Health Survey for England 2004 Volume 2: Methodology and Documentation. Leeds: The Information Centre; 2006.
- Mindell JS, Tipping S, Pickering K, Hope S, Roth MA, Erens B. The effect of survey method on survey participation: analysis of data from the Health Survey for England 2006 and the Boost Survey for London. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-83.
- ONS . Population by Ethnic Group (2001 Census): Neighbourhood Statistics Geographies 2001 n.d. https://data.gov.uk/dataset/ethnic_group_2001_census (accessed 17 November 2011).
- ONS . A Beginner’s Guide to UK Geography: Census Geography 2011 n.d. www.ons.gov.uk/ons/guide-method/geography/beginner-s-guide/census/index.html (accessed 24 March 2017).
- ONS . Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2010 n.d. www.ons.gov.uk/ons/rel/pop-estimate/population-estimates-for-uk--england-and-wales--scotland-and-northern-ireland/mid-2010-population-estimates/index.html (accessed 17 November 2011).
- ONS . The English Indices of Deprivation 2010 n.d. www.gov.uk/government/statistics/english-indices-of-deprivation-2010 (accessed 24 March 2017).
- Blake KS, Kellerson RL, Simic A. Measuring Overcrowding in Housing. US Department of Housing and Urban Development, Office of Policy Development and Research; 2007.
- Office of the Deputy Prime Minister . The Impact of Overcrowding on Health and Education: A Review of the Evidence and Literature 2004.
- Keogh RH, Cox DR. Case–Control Studies. Cambridge: Cambridge University Press; 2014.
- Prentice RL. A case–cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1-11. https://doi.org/10.1093/biomet/73.1.1.
- Keogh RH, Mangtani P, Rodrigues L, Nguipdop-Djomo P. Estimating time-varying exposure–outcome associations using case–control data: logistic and case–cohort analyses. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-015-0104-0.
- Health Protection Agency . Tuberculosis in the UK: 2011 Report 2011. www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1317131784267 (accessed 15 April 2012).
- Rehm J, Greenfield TK, Walsh G, Xie X, Robson L, Single E. Assessment methods for alcohol consumption, prevalence of high risk drinking and harm: a sensitivity analysis. Int J Epidemiol 1999;28:219-24. https://doi.org/10.1093/ije/28.2.219.
- Nguipdop-Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis 2016;16:219-26. https://doi.org/10.1016/S1473-3099(15)00400-4.
- Springett VH, Sutherland I. Comparison of the efficacy of liquid and freeze-dried strains of BCG vaccine in preventing tuberculosis. Br Med J 1970;4:148-50. https://doi.org/10.1136/bmj.4.5728.148.
- Gorak-Stolinska P, Weir RE, Floyd S, Lalor MK, Stenson S, Branson K, et al. Immunogenicity of Danish-SSI 1331 BCG vaccine in the UK: comparison with Glaxo-Evans 1077 BCG vaccine. Vaccine 2006;24:5726-33. https://doi.org/10.1016/j.vaccine.2006.04.037.
- National Audit Office . Procurement of Vaccines by the Department of Health 2003. www.nao.org.uk/wp-content/uploads/2003/04/0203625.pdf (accessed 12 October 2016).
- A report from the Research Committee of the British Thoracic and Tuberculosis Association . BCG vaccination by multiple-puncture: fourth report. Tubercle 1971;52:19-30. https://doi.org/10.1016/0041-3879(71)90027-4.
- Smith DS. BCG vaccination by multiple-puncture: third report. Tubercle 1965;46:111-20. https://doi.org/10.1016/S0041-3879(65)80053-8.
- Fourth report to the Medical Research Council by its Tuberculosis Vaccines Clinical Trials Committee . BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Bull World Health Organ 1972;46:371-85.
- Vynnycky E, Fine PE. The annual risk of infection with Mycobacterium tuberculosis in England and Wales since 1901. Int J Tuberc Lung Dis 1997;1:389-96.
List of abbreviations
- BAME
- black and Asian minority ethnic
- BCG
- bacillus Calmette–Guérin
- CAPI
- computer-assisted personal interview
- CHIS
- Child Health Information System
- CI
- confidence interval
- ETS
- Enhanced Tuberculosis Surveillance
- HIV
- human immunodeficiency virus
- HR
- hazard ratio
- IMD
- Indices of Multiple Deprivation
- IUATLD
- International Union Against Tuberculosis and Lung Disease
- LSOA
- lower-level super output area
- MRC
- Medical Research Council
- MSOA
- mid-level super output area
- NatCen
- National Centre for Social Research
- ONS
- Office for National Statistics
- PCT
- primary care trust
- PPD
- purified protein derivative
- PPS
- probability proportional to size
- SRS
- simple random sampling
- TB
- tuberculosis
- TST
- tuberculin skin test
- VE
- vaccine effectiveness