Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/01/50. The contractual start date was in February 2013. The draft report began editorial review in November 2015 and was accepted for publication in August 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Hans Hauner reports grants from the German Ministry of Education and Research, the Bavarian Ministry of Agriculture and Nutrition, the Bavarian Ministry of Health, the Helmholtz Center Munich, the Else Kröner-Fresenius Foundation, AOK Bavaria (health insurance fund), Amway and the German Research Foundation outside the submitted work. Ben Willem Mol reports other grants from ObsEva during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Rogozińska et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Updated data can be found in the meta-analysis by the International Weight Management in Pregnancy (i-WIP) Collaborative Group. 1
Obesity is an epidemic. In the UK, every other woman of childbearing age is either overweight [body mass index (BMI) of 24.9–29.9 kg/m2] or obese (BMI of 30 kg/m2 or more), and one-fifth start pregnancy as obese. 2 The confidential enquiry into maternal and child health identified maternal obesity as a threat to the childbearing population in the UK. 3 The risks for the infant include stillbirth and neonatal death, macrosomia, neonatal unit admission, preterm birth and congenital abnormalities. In the longer term, maternal obesity is associated with an increased risk of childhood obesity and associated complications. 4 A significant proportion of women gain more than the recommended weight during pregnancy,5 with increased risk of maternal and fetal/neonatal complications. 6 Women who gain excess weight in pregnancy are at increased risk of postpartum weight retention. This predisposes normal weight and overweight women in index pregnancy into entering subsequent pregnancies as overweight or obese. Effective interventions that reduce maternal obesity and excess weight gain in pregnancy could derive significant advantages for the NHS and society.
Clearly defined, effective interventions that target those women at the highest risk in pregnancy are needed. Diet- and physical activity-based interventions have been widely evaluated for their effect on gestational weight gain (GWG) and clinical outcomes. There is limited information on their effects on specific groups of pregnant women known to be at increased risk of complications.
Aggregate meta-analysis of randomised trials on diet- and physical activity-based interventions [Health Technology Assessment (HTA) programme reference number 09/27/06] showed a significant reduction in GWG, with benefit for some clinical outcomes. 7 However, aggregate data meta-analysis was limited because of the inability to explain heterogeneity of effects for important maternal and fetal/neonatal outcomes. This heterogeneity might be a result of variation in maternal characteristics, such as BMI, age, ethnicity and parity with varied weight gain.
Pregnancy during adolescence alters normal growth processes and increases the risk of becoming overweight or obese. 8 Adolescent mothers retain more weight post partum than mature control subjects. 8 Inclusion of a large number of pregnant adolescents may overestimate postpartum weight changes or the risk of becoming overweight, and thus bias estimates for adult women. Migrant groups exhibit less GWG than the local population but similar rates of complications. 9 These aspects need investigation.
The National Institute for Health and Care Excellence (NICE) public health guidance Weight Management Before, During and After Pregnancy10 has prioritised the following areas for research: the clinical effectiveness and cost-effectiveness of weight management interventions in pregnancy for specific groups, such as teenagers, with differing needs and social circumstances; ethnic minorities, such as Asians, in whom comorbidity risk at any particular BMI value is relatively higher than in other ethnic groups; women who enter pregnancy obese; and the effect of adherence to the Institute of Medicine (IOM)’s weight-gain recommendations on pregnancy outcomes.
The paucity of published detail in research on the effects of interventions in particular subgroups of women based on BMI, ethnicity and other relevant factors restricts aggregate data meta-analyses. 11,12 Subgroup effects are rarely reported in sufficient detail, especially to derive differences in intervention effect between subgroups (‘treatment–covariate interactions’). Meta-regression examining the across-trial association between overall treatment effect and average patient characteristics (e.g. mean age) has low power to detect genuine subgroup effects and is also prone to study-level confounding. 13,14 Furthermore, the available data could not assess the impact of baseline prognostic factors on the effectiveness of the interventions. Meta-analysis of individual participant data (IPD),15 in which the raw patient-level data are obtained and synthesised across trials, overcomes the above limitations. Availability of the raw data substantially increases the power to detect baseline factors that truly modify intervention effect13 and enables intervention effects to be quantified for clinically relevant groups. 16 It will also allow the magnitude of benefit, due to weight change in pregnancy, to be quantified for both the women and their infants.
We undertook an IPD meta-analysis of randomised trials on diet- and physical activity-based interventions to assess differential effects of interventions in various subgroups of pregnant women.
Chapter 2 Objectives
Primary
-
To assess if the effects of diet- and physical activity-based interventions on (1) GWG, (2) composite maternal and (3) composite fetal/neonatal outcomes vary in subgroups of women based on BMI at booking, age, parity, ethnicity and underlying medical conditions.
Secondary
-
To quantify the relationship between the amount of weight gained in pregnancy and the risk of adverse maternal and fetal/neonatal outcomes for (1) women of normal weight, (2) overweight women and (3) obese women.
-
To assess the relationship between adherence to IOM’s guidelines and maternal and fetal complications in normal weight, overweight and obese pregnant women.
-
To identify the predictors of GWG in pregnancy based on patient characteristics such as parity, pre-pregnancy BMI, ethnicity, smoking, diet and lifestyle, and socioeconomic status.
-
To assess the cost-effectiveness of the interventions in pregnancy using model-based full economic evaluation with value of information analysis.
-
To undertake network meta-analysis to determine the rank order of interventions for clinical effectiveness, if appropriate.
Chapter 3 Methods
Our IPD meta-analysis followed existing guidelines and used a prospective protocol registered with PROSPERO (CRD42013003804). 17 Our output complied with the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) reporting guidelines for IPD meta-analysis. 18
Eligibility criteria
Criteria for including studies in individual participant data
We included studies that addressed the components of the structured question presented in Table 1.
| Component | Description |
|---|---|
| Population | Pregnant women with a BMI of ≥ 18.5 kg/m2 |
| Interventions | Diet-based, physical activity-based and mixed-approach intervention |
| Comparison | No intervention or routine antenatal care |
| Main outcomes | GWG, maternal composite outcome, fetal and neonatal composite outcome |
| Other outcomes | Maternal: gestational diabetes mellitus, pre-eclampsia or pregnancy-induced hypertension, preterm delivery (< 37 weeks), Caesarean section |
| Fetal/neonatal: intrauterine death, small for gestational age fetus, large for gestational age fetus, admission to the NICU | |
| Study design | Randomised controlled trial |
Randomised trials, with or without clustering, that evaluated the effects of diet- and physical activity-based interventions in pregnancy on maternal, fetal and neonatal outcomes were eligible for inclusion. We included studies on normal, overweight and obese pregnant women. Interventions that addressed mainly diet or mainly physical activity and interventions adopting a mixed approach that combined the two, with or without behavioural modification techniques, were eligible. The control arms included women without any intervention or with routine antenatal care, as defined by local health-care practices. The primary outcomes were maternal weight gain in pregnancy, and composite maternal and composite fetal/neonatal events complications. Studies should have assessed both maternal weight gain and clinical outcomes.
Maternal weight gain was defined as the difference between the weights recorded (kg or lb) at first clinic visit and last weight measured before birth. If weight at first clinic visit was not available, we used pre-pregnancy weight.
The maternal composite outcome included gestational diabetes mellitus (GDM), pre-eclampsia (PE) or pregnancy-induced hypertension (PIH), preterm delivery and Caesarean section. The fetal and neonatal composite outcome comprised intrauterine death (IUD), small for gestational age (SGA), large for gestational age (LGA) and admission to the neonatal intensive care unit (NICU). The components of the composite outcome were identified by a two-round Delphi survey. The final scores of the components are provided in Appendix 1. The details of the development of the composite outcomes are published elsewhere. 19
We excluded studies published before 1990, animal studies and those that evaluated the effects of intervention only on non-clinical outcomes (behaviour change and consumption of particular food groups) or aimed to increase weight gain in pregnancy.
Criteria for including participants in individual participant data
We excluded underweight women (BMI of < 18.5 kg/m2) and women with multiple pregnancies.
Literature search and study identification
We updated our previous literature search (October 2013 to March 2015) to identify new trials published since the completion of our systematic review (HTA number 09/27/067) on effects of diet- and physical activity-based interventions in pregnancy. The following databases were searched from October 2013 to March 2015 to update the search: MEDLINE, EMBASE, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects and HTA database without any language restrictions. We used additional sources, such as the internet [general search engines such as Google (Google Inc., Mountain View, CA, USA)], and directly contacted researchers to identify relevant trials. We did not contact authors of trials that were published or identified too close to the analysis stage because of lack of sufficient time to clean and format the data before analysis. The details of the search strategy are provided in Appendix 2.
We established the International Weight Management in Pregnancy (i-WIP) IPD Collaborative Network by contacting researchers who had published trials on diet and lifestyle interventions in pregnancy. 20 The network is a global effort in bringing together researchers, clinicians and epidemiologists involved, supported by the World Health Organization, from 16 countries (https://kamolo.org.ar/iwipipd, accessed 1 March 2016).
Study and participant selection
Study selection
We undertook a two-stage study selection process. In the first stage, the abstracts of all citations were evaluated for their eligibility. In the second stage, we studied the identified studies in detail before their inclusion. Two independent reviewers (ER and EM) evaluated all papers. In case of disagreement, an opinion of the third reviewer (ST) was sought. We applied the eligibility criteria provided above for inclusion of studies.
Data collection and storage
We set up a bespoke database and requested authors of the i-WIP Collaborative Network to supply data in any format [Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), IBM SPSS Statistics versions 22 and 23 (IBM Corporation, Armonk, NY, USA), Stata (StataCorp LP, College Station, TX, USA) and SAS (SAS Institute Inc., Cary, NC, USA)] convenient to them. We sent, on average, three reminders when there was no response. For studies that refused to provide IPD, and for those with which contact could not be established, we extracted the published aggregate data.
We obtained and uploaded the original anonymised data sets using the secure web-based server at Centro Rosarino de Estudios Perinatales, Rosario, Argentina, a World Health Organization Collaborative Centre in Child and Maternal Health. Data manipulations were performed and documented within this environment. The final meta-data set was securely transferred to the Pragmatic Clinical Trials Unit at Queen Mary University of London for final data checks and analysis. An independent data access committee and data access process were established for use of the data in future research.
Data items
We considered all recorded variables for inclusion when appropriate, including those not reported in the published studies. Data were extracted on the study and data set levels. At the study level, we collected information regarding study settings, intervention type, components, format and provider. At the participant level we requested information on individual characteristics including BMI, age, parity, ethnicity, socioeconomic status, pre-existing medical conditions, adherence to intervention and outcome data. The list of final variables collected during the project is available in Appendix 3.
Definition and standardisation of variables
Participant characteristics and other measurements were recorded in various different formats within the individual data sets. We chose the meta-data set format by including the variables that were most commonly reported. The standardisation process followed a predefined procedure (Figure 1).
FIGURE 1.
Flow diagram of standardisation of variables within the IPD sets. Interim DS refers to a temporary work-in-progress data set that will not be stored onto the database. DS, data set.
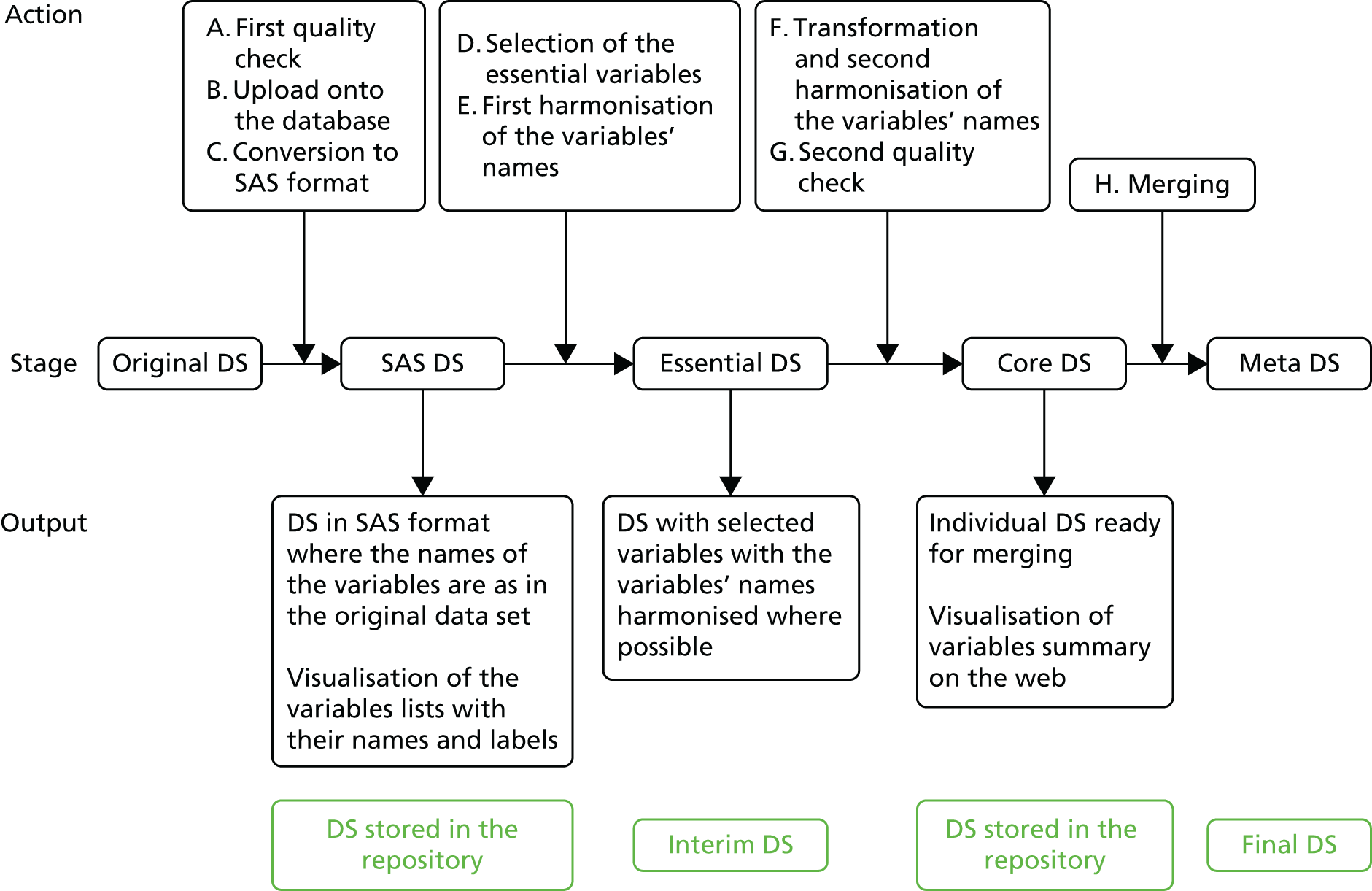
Standardisation of baseline variables
Maternal age (years) at baseline was recorded as a continuous variable in most studies except one, in which age at baseline was calculated from the date of first visit and the date of birth. In addition to continuous data, we used the cut-off point of 20 years for age, to dichotomise participants into teenagers and those over 19 years. Race/ethnicity was recorded in a variety of ways and standardisation required a larger number of assumptions to be made. The details of ethnicity coding are available in Appendix 4. BMI was recorded both as a continuous measure and categorised into clinically relevant categories as normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥ 30 kg/m2).
We used the woman’s educational status to represent the socioeconomic status by using local standards. After feedback from the study team we defined educational status as ‘low’ (secondary education completed before A-levels), ‘medium’ (secondary education to A-level equivalent) or ‘high’ (any further/higher education) (see Appendix 4). Smoking was generally recorded as yes/no, with some studies recording previous habits. If the woman had stopped smoking because of pregnancy or for other reasons at any time point, this was combined into the variable for ‘ex-smoker (yes/no)’.
We defined participants as adherent if they completed around 70–80% of the intervention protocol, if the data set provided adherence information in a yes/no format or if non-adherent women were excluded as per study protocol. Parity was defined as the number of times participants had given birth before the index pregnancy and was recorded consistently across the data set. We combined information from physical activity questionnaires, gym attendance, type of work and accelerometer data to standardise the approach for baseline physical activity (for details see Appendix 4). Previous macrosomia and GDM were defined as per individual study authors and were recorded in all data sets as ‘yes/no’.
Standardisation of outcome variables
Weight was standardised to kilograms and height to centimetres. BMI was defined as weight/height squared (kg/m2) and was consistently reported across all data sets. Baseline obesity was defined as a BMI of ≥ 30 kg/m2. Adherence to the IOM recommendations for GWG was as follows: 11–16 kg for normal weight women, 7–11 kg for overweight women and 5–9 kg for obese women. 9 We classified women as not reaching the recommendation (i.e. GWG less than the lower limit), adherent (i.e. GWG within limits) or exceeding the recommendation (i.e. GWG more than the upper limit).
Gestational diabetes mellitus, diabetes mellitus (DM), PIH, PE, chronic hypertension and Caesarean section were defined and reported in the data sets in accordance with local standards. IUD and admission to the NICU were analysed as defined in the data set. Outcomes SGA (< 10th centile) and LGA (≥ 90th centile) were generated for all data sets using a bulk birthweight centile calculator [Gestation Related Optimal Weight (GROW) customised centiles (CC) software, version 6.7; Gestation Network, Birmingham, UK, 2013] incorporating data on women’s height and baseline weight, parity, gestational age at birth and fetal birthweight.
Data quality (individual participant data integrity)
Data sets included in the i-WIP analysis were expected to be clean on receipt from the original trial team. We performed range checks on the variables used during the analysis and produced summary tables. We focused on checking the randomisation ratio, baseline characteristics and the method of analysis in the IPD data set with the published information. Any major discrepancies were discussed with the trial team.
Risk-of-bias assessment in individual studies
We evaluated the risk of bias in individual studies by considering six items used in the Cochrane risk-of-bias tool: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. 21 When required, we obtained the full trial protocol and directly contacted the primary investigators to obtain relevant details to assess the study quality. A study was classified as having a high risk of bias if it was assessed as high risk in at least one of the following domains: randomisation, allocation concealment, blinding of outcome assessment, incomplete outcome data and when no single item was assessed as being at low risk of bias.
Handling of trials without individual participant data availability
We explored the potential for publication bias, and the possible impact of unavailable non-IPD data, in accordance with recent guidelines. 22 For each analysis containing 10 or more studies, the potential for publication bias was investigated through contour-enhanced funnel plots, and appropriate statistical tests for ‘small-study effects’ (i.e. the tendency for small studies to provide more positive findings than large ones).
For all studies in which IPD were not available, we extracted suitable aggregate data from the study publications. When possible, we then incorporated these aggregate data into the second stage of the two-step meta-analysis framework, to combine the IPD trials with the aggregate data from other trials for the outcome of GWG. This allowed us to examine whether or not conclusions (on summary results and potential publication bias) were changed by the inclusion of additional non-IPD trials. 14,23 If the inclusion of studies that did not provide IPD seemed to have an important statistical or clinical impact, we compared the characteristics of the studies with IPD with those without to see if there were any key differences (such as in their quality, follow-up length and statistical methods). This was achievable only when examining the overall treatment effect, as aggregate data for subgroup effects were rarely provided by the non-IPD studies. For individual maternal and fetal/neonatal complications, we compared meta-analyses findings of only aggregate published data with IPD.
Sample size considerations
Although no formal sample size requirements are necessary for the meta-analysis, we have considered the potential power of our IPD meta-analysis in comparison with single trials in this field to detect clinically important effects in each subgroup separately. All calculations relate to a type I error of 5%, a power of 80% and a loss to follow-up of 5%. We chose a reduction of 2.5 kg in GWG as the minimally important difference. We expected the available sample size to be > 9000 women. For maternal weight gain, the sample size required for all subgroups is ≤ 300. For the composite outcome of adverse maternal and fetal/neonatal outcomes, we calculated the sample size needed to detect an intervention effect of a 30% reduction in adverse pregnancy outcomes. Our estimates of the standard deviation (SD) of the control group and the risk of composite pregnancy outcome were obtained from the data of primary studies included in our systematic review. 24
Given the large sample size available, it is highly likely that the study was powered to detect important differences between subgroups (i.e. to identify genuine factors that modify treatment effect). This allowed us to detect interaction terms as small as about 30% of the size of the overall treatment effect. If the overall intervention effect is a reduction in weight gain of approximately 2.5 kg, then our IPD meta-analysis would have 80% power to detect an interaction term of about 2.5 × 0.3 = 0.75 or above (e.g. a difference in intervention effect of 0.75 kg between obese and normal weight women) (Table 2).
| Subgroups | Control group SD | Sample size required to detect a 2.5-kg reduction in GWG | Control group probability of adverse pregnancy outcome | Sample size required to detect a 30% reduction in adverse pregnancy outcome |
|---|---|---|---|---|
| BMI category | ||||
| Obese | 7.5 | 300 | 0.30 | 770 |
| Overweight | 7.5 | 300 | 0.20 | 1290 |
| Normal weight | 5.1 | 140 | 0.12 | 2330 |
| Age (years) | ||||
| < 20 | 7.12 | 270 | ||
| ≥ 20 | 5.87 | 184 | ||
| Ethnicity | ||||
| Caucasian | 3.4 | 64 | ||
| Asian | 3.8 | 78 | ||
| African | 5.1 | 140 | ||
| Parity | ||||
| < 1 | 6.28 | 212 | ||
| ≥ 1 | 6.68 | 238 | ||
| Risk factors such as diabetes | ||||
| High risk | 6.81 | 248 | ||
| Low risk | 6.67 | 236 | ||
Data analysis
All analyses were carried out using Stata, version 12.1. Aggregate meta-analyses for components of maternal and fetal/neonatal composites were done using Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Primary analyses of studies providing individual participant data
For each outcome (i.e. GWG, composite maternal, composite fetal/neonatal) separately, we performed a two-stage IPD meta-analysis to obtain summary estimates and 95% confidence intervals (CIs) for the intervention effects [mean difference (MD) or odds ratios (ORs)] and the interactions (subgroup effects) of interest. All participants were analysed according to the group they were randomised to. We used a two-stage random-effects meta-analysis approach, which allows for between-study heterogeneity in intervention effect (and interaction effect). In any two-stage meta-analysis, the clustering of participants within trials is accounted for by analysing each trial separately in the first stage. Women with confirmed glucose intolerance or hypertensive disorder at baseline, as defined by the primary authors, were excluded in the analysis of composite adverse pregnancy outcomes.
First stage of individual participant data meta-analysis
Continuous outcome measures were checked for normality and log transformed if applicable. Variables (covariates) were kept as continuous as well as defining subgroups for BMI and maternal age. All analyses were performed on complete cases, that is individuals who provided the outcome and (if relevant for the analysis) baseline adjustment factors. When analysing cluster randomised trials, we included a random intercept for the unit of randomisation to account for clustering. For the continuous outcome of weight gain, we used analysis of covariance in each trial to regress the final weight value against the intervention, while adjusting for baseline weight. For the binary outcome of adverse fetal/neonatal or maternal outcome, the binomial nature was modelled using a logistic regression in each trial separately, with intervention as a covariate. Stratification or minimisation factors used in the randomisation of each study were not adjusted for in any analyses. The Sweeting et al. 25 approach was applied to include studies into the analysis of composite outcomes that had no information on outcome for one treatment group. This was only done for the primary analysis without interaction terms.
When examining intervention effect modifiers, we extended the models to include interaction terms between participant-level covariates and the intervention. For the interactions, continuous covariates (BMI and age) were analysed on continuous scales and as clinically defined categorical values. In addition, effects were presented within the subgroups defined by the interactions.
All primary analyses were performed on the combined intervention and any multiple treatment arms were combined into one intervention arm. For the secondary analysis of individual intervention types, multiple treatment arms were combined if they belonged to the same type, for example brochure arm and active counselling were grouped as mixed-approach intervention) or analysed separately if the treatment arms were categorised as different types (e.g. exercise and exercise plus dietary counselling).
Second stage of individual participant data meta-analysis
We pooled effect estimates (e.g. relating to treatment effects or treatment–covariate interactions) using a random-effects model using restricted maximum likelihood (REML) to produce a summary effect estimate for the mean (or average) effect across studies. The Knapp–Hartung correction was applied when deriving 95% CIs for each summary effect, to account for the uncertainty of the estimate of between-study heterogeneity (τ2). Forest plots were generated to display the study-specific and pooled results.
Heterogeneity was summarised using the I2 statistic and the estimated between-study variance (τ2) was obtained using REML. To reveal the impact of heterogeneity more clearly, we also calculated approximate 95% prediction intervals (PIs) for the intervention (or interaction) effect in a new study using the formula suggested by Higgins et al. 26
Sensitivity analyses
Small-study effects (and the potential for publication bias) were investigated by using contour-enhanced funnel plots and tests for asymmetry (using either the Egger’s test for continuous outcomes or Peter’s test for binary outcomes). In order to examine whether or not there may be availability bias in the obtained IPD, we compared summary results when including non-IPD studies with those in our IPD studies. When possible, we then incorporated this aggregate data into the second stage of the two-step meta-analysis framework (see below), to combine the IPD trials with the aggregate data from other trials, to ascertain if conclusions were robust.
We investigated the following sources of bias for all or a subset of the primary outcomes by performing the following sensitivity analyses.
Study quality
We excluded studies at high risk of bias in at least one of the following domains: randomisation, allocation concealment, blinding for outcomes assessment or completeness of outcome data, and not a single item of low risk.
Intervention
We analysed the primary outcomes separately for each intervention type (diet, physical activity and mixed) to ensure that the analysis of the combined intervention was valid.
Adherence
We excluded any participants not adherent to their intervention.
Outcome measurement
We analysed BMI change instead of weight change to assess the impact of those studies that reported only on BMI and not weight. The effect of timing of gestational weight measurement on the effects was addressed by excluding weights measured before 37 completed weeks of gestation to exclude systematic differences. We analysed each component separately to ensure validity of the composite outcome.
Secondary analyses
All secondary analyses were performed only on participants in control arms to exclude the effect of treatment.
Quantification of the relationship between gestational weight gain and risk of outcome
For each composite outcome separately, we fitted two-stage meta-analysis models (logistic regression in stage 1, followed by a random-effects meta-analysis in stage 2) to obtain a pooled estimate of how each 1-unit increase in weight gain changed the risk of a poor outcome depending on baseline BMI. Baseline BMI remained a continuous variable.
We assessed if adherence in pregnancy to IOM weight-gain recommendations was associated with a reduced risk of adverse pregnancy outcomes in normal weight, overweight and obese women. We used the two-stage logistic framework as described above, with a covariate for adherence to IOM. Baseline BMI was included as categorical (normal weight/overweight/obese) using the same cut-off points as the definition for IOM adherence. Adherence was defined in three categories as below IOM, adherent to IOM and exceeding IOM recommendations for weight gain.
Evaluation of factors associated with weight change in pregnancy
We evaluated those variables that may be associated with GWG including age, ethnicity, underlying medical conditions like DM, parity and socioeconomic status. To obtain adjusted factor results, a multivariable model was fitted including all variables reported in at least 10 studies to identify those that were independently associated.
Network meta-analysis
We refrained from undertaking network meta-analysis, as there were no differences in estimates of effect for GWG between diet, physical activity and mixed-approach interventions.
Chapter 4 Characteristics and quality of studies included in the individual participant data meta-analysis
Study selection and individual participant data acquisition
Our previous search (until 2013) had identified 44 randomised trials. 7 We identified 3551 potentially relevant citations (Figure 2). We also identified 57 potential papers from references of included studies and four from oral communications. Detailed evaluation of the 167 articles led to the final identification of 74 trials (n = 17,623) on diet- and physical activity-based interventions in pregnancy (see Figure 2).
FIGURE 2.
Flow diagram of studies in the IPD systematic review, showing the studies included in the review and meta-analysis. 27
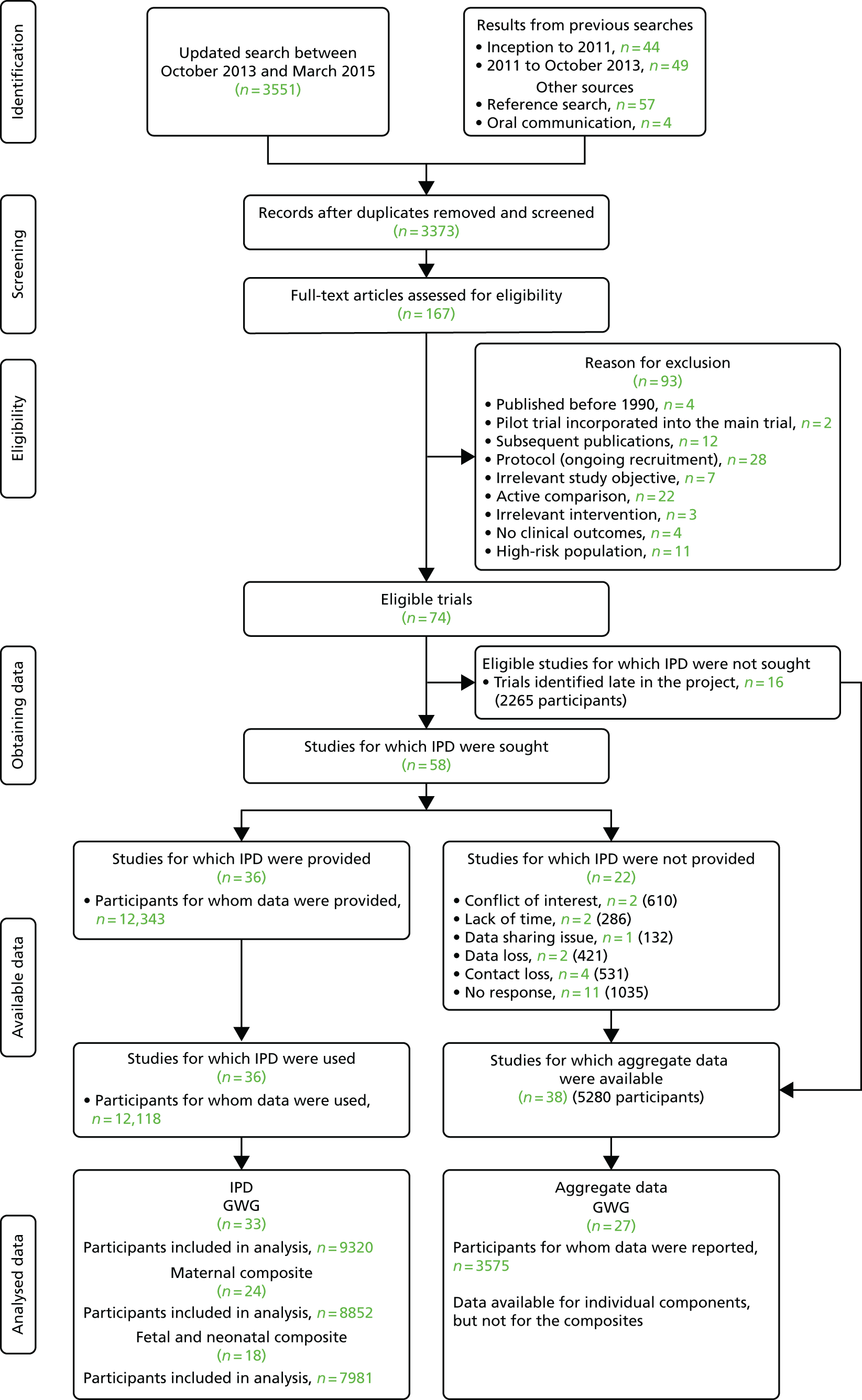
We invited the authors of 58 trials to join the project and share the IPD. Forty-one researchers from 29 teams, in 16 countries, joined the i-WIP Collaborative Network (until October 2015) and provided access to anonymised individual data on 12,343 women. The collaborators included obstetricians, academics, dietitians, nutritionists, physiotherapists, exercise physiologists, psychologists and clinical epidemiologists.
The most common combined reason for not being able to obtain IPD was difficulty in contacting the authors and contact loss (11/58). 28–38 Reasons for refusal to provide IPD included lack of time,39,40 problems with data sharing41 and conflicts of interest. 42,43 Data were lost in two trials. 44,45 Sixteen randomised controlled trials (RCTs) (including 2265 women) were identified too late in the project to be analysed, and thus we refrained from approaching the authors. 46–61 Most of these studies evaluated physical activity-based interventions (10 trials) and/or were conducted in developed countries (10 trials). Details of all 16 studies are provided in Appendix 5.
Characteristics of the studies included in the individual participant data analysis
Thirty-six RCTs contributed IPD to this project. Thirty-four trials were randomised trials with individual participant allocation and two were cluster RCTs. 62,63 Twenty-two trials were conducted in Europe, four in each of North America (three in the USA and one in Canada), Australia and South America (Brazil) and one each in Egypt and Iran. The size of the studies ranged from 12 to 2212 women. Eight studies included only obese women,64–71 three included obese and overweight women,72–74 one included overweight women75 and 24 included women of any BMI. Four trials assessed diet-based interventions,71,76–78 16 evaluated physical activity,27,42,67,75,79–89 and 15 trials adopted a mixed approach (diet, physical activity, behaviour-modifying techniques, etc.). 62–66,68,70,72–74,90–94 Four trials had a three-arm design (two interventions and routine care arm). 27,64,66,69 Of these, in three, interventions belonged to the same type (different type of counselling or different exercise routine). 27,64,66 In one trial, one arm of the intervention comprised exercise only and the other a combination of exercise and diet (mixed approach). 69 GWG was reported as an outcome in 57 studies; 33 provided IPD and in 27 studies only aggregate data were available. The numbers of studies reporting rates of individual maternal and fetal/neonatal outcomes are given in Table 3.
| Characteristics | Availability of IPD (number of studies) | |
|---|---|---|
| Available (n = 36 studies, n = 12,526 women) | Unavailable (n = 38 studies, n = 5280 womena) | |
| Population | ||
| Any BMI category | 24 | 27b |
| Obese or overweight | 12 | 11 |
| Intervention type | ||
| Diet based | 4 | 9 |
| Exercise based | 16 | 19 |
| Mixed approach | 16c | 10 |
| Outcomesa | ||
| GWG | 31 | 27 |
| GDM | 20 | 11 |
| PE or PIH | 15 | 6 |
| Preterm delivery | 16 | 8 |
| Caesarean section | 22 | 16 |
| IUD | 4 | 0 |
| SGA | 6 | 4 |
| LGA | 11 | 4 |
| Admission to the NICU | 4 | 2 |
| Country of conduct | ||
| Developed | 30 | 26 |
| Developing | 6 | 12 |
Overall, 38 eligible studies (38/74, 51.4%) comprising 5280 women did not contribute IPD. Table 3 compares the characteristics of studies that did and did not share IPD for the meta-analysis. The detailed descriptions of all trials are provided in Appendix 6.
Characteristics of the individual participants in the individual participant data meta-analysis
The average age of participants was 30 years in both arms of the trials. More than 80% of participants were of Caucasian ethnicity. About half of the participants had obtained a higher degree, were nulliparous and were not physically active. Table 4 shows a detailed comparison of baseline characteristics in both arms of the studies that contributed to the IPD.
| Baseline characteristics | Number of studies | Number of women | Study arm, mean (SD) or n (%)a | |
|---|---|---|---|---|
| Control | Intervention | |||
| Age (years) | 35 | 12,006 | 30.1 (5.2) | 30.0 (5.1) |
| Height (cm) | 31 | 11,689 | 165.0 (7.0) | 165.4 (6.7) |
| Race/ethnicity | 27 | 10,020 | ||
| Caucasian (including Russia and Australia) | 4217 (87.2%) | 4562 (88%) | ||
| Asian | 156 (3.2%) | 157 (3%) | ||
| Afro-Caribbean | 292 (6%) | 292 (5.6%) | ||
| Central/South American | 64 (1.3%) | 67 (1.3%) | ||
| Middle Eastern (including Iran and Turkey) | 37 (0.8%) | 37 (0.7%) | ||
| Other | 68 (1.4%) | 71 (1.4%) | ||
| Educational status of mother | 29 | 8914 | ||
| Low | 724 (16.9%) | 722 (15.6%) | ||
| Medium | 1292 (30.2%) | 1372 (29.6%) | ||
| High | 2268 (52.9%) | 2536 (54.8%) | ||
| Current smoker | 29 | 10,958 | 865 (16.4%) | 875 (15.4%) |
| Ex-smoker (pre-pregnancy) | 13 | 4099 | 456 (23.8%) | 523 (24%) |
| Adherence to intervention | 18 | 3321 | N/A | 2022 (60.9%) |
| Parity | 33 | 11,805 | ||
| 0 | 2692 (47.3%) | 3027 (49.5%) | ||
| 1 | 2083 (36.6%) | 2136 (34.9%) | ||
| 2 | 634 (11.1%) | 647 (10.6%) | ||
| 3 | 165 (2.9%) | 179 (2.9%) | ||
| ≥ 4 | 113 (2%) | 129 (2.1%) | ||
| No exercise or sedentary | 27 | 7583 | 1731 (47.6%) | 1761 (44.6%) |
| Obesity (BMI of ≥ 30 kg/m2) | 34 | 12,031 | 2434 (42.0%) | 2680 (43.0%) |
| Previous macrosomia | 8 | 2906 | 400 (29.1%) | 390 (25.5%) |
| Previous GDM | 11 | 4297 | 49 (2.4%) | 60 (2.9%) |
| GDM | 20 | 8256 | 14 (0.4%) | 23 (0.6%) |
| DM | 25 | 9589 | 9 (0.2%) | 6 (0.1%) |
| Hypertension in pregnancy | 20 | 5695 | 37 (1.3%) | 47 (1.6%) |
| Hypertension | 23 | 5494 | 54 (2.1%) | 73 (2.5%) |
The most common outcomes available in studies that contributed IPD were preterm delivery (11,731 women, 34 studies), Caesarean section (11,585 women, 34 studies) and SGA (11,682 women, 34 studies) and LGA (12,078 women, 36 studies) fetuses. This was followed by GWG (9320 women, 33 studies), PE (8350 women, 20 studies), PIH (9065 women, 25 studies) and GDM (9882 women, 30 studies). We were able to obtain maternal and fetal/neonatal outcome based on available individual data of 8852 (24 studies) and 8239 (19 studies) participants, respectively (Table 5).
| Outcomes | Number of studies | Number of women |
|---|---|---|
| Baseline weight (kg) | 33 | 11,748 |
| Follow-up weight (kg) | 33 | 9326 |
| Change in weight (kg) | 33 | 9320 |
| Baseline BMI | 34 | 12,031 |
| Follow-up BMI | 31 | 9240 |
| Change in BMI | 31 | 9238 |
| PE | 20 | 8350 |
| PIH | 25 | 9065 |
| PE or PIHa | 27 | 9915 |
| GDMa | 30 | 9882 |
| Preterm delivery (< 37 weeks’ gestational age)a | 34 | 11,731 |
| All Caesarean sectiona | 33 | 11,585 |
| Emergency Caesarean section | 16 | 7226 |
| Elective Caesarean section | 16 | 7226 |
| Caesarean section unspecified | 17 | 4423 |
| Maternal composite outcome | 24 | 8852 |
| IUDa | 22 | 9354 |
| SGAa | 34 | 11,682 |
| LGAa | 36 | 12,078 |
| Admission to the NICUa | 21 | 8749 |
| Fetal/neonatal composite outcome | 19 | 8239 |
Risk of bias within eligible studies
Two-thirds (52/74, 70.3%) of eligible trials were rated as having a low risk of bias for random sequence generation and selective reporting of outcomes. More than half of the studies (47/74, 63.5%) had complete outcome data, with 18% of the remaining trials (13 studies) being rated as being at high risk of bias. Allocation concealment was adequate in 45% (33/74) of included trials. In all studies the risk of bias for blinding of participants and personnel was rated as either unclear (45/74, 60.8%) or high (29/74, 39.2%). In 27 studies (36.5%) there were no concerns over the rating of risk of bias for blinding of outcome assessment, while 15 studies (20.3%) were assessed as being at high risk of bias. For the remaining studies there was not enough information to assess the risk of bias (32/74, 43.2%). Figure 3 presents a summary of the risk of bias rating by domain for all eligible RCTs. The detailed assessment and a global risk of bias are presented in Appendix 7.
FIGURE 3.
Summary of the risk of bias rating for all eligible studies (n = 74).

Quality assessment of studies that contributed data to individual participant data meta-analysis
Studies that contributed IPD were rated as being at low risk of bias for random sequence generation (94% vs. 47% among studies with unavailable IPD), allocation concealment (64% vs. 26%) and completeness of outcome data (78% vs. 50%) compared with non-IPD studies. The risk-of-bias rating was similar in both groups for selective reporting of outcomes (Table 6).
| Items | Risk-of-bias rating, n (%) | |||||
|---|---|---|---|---|---|---|
| Low | Unclear | High | ||||
| IPD | Non-IPD | IPD | Non-IPD | IPD | Non-IPD | |
| Random sequence generation | 34 (94) | 18 (47) | 2 (6) | 18 (47) | 0 (0) | 2 (5) |
| Allocation concealment | 23 (64) | 10 (26) | 11 (31) | 27 (71) | 2 (6) | 1 (3) |
| Blinding of participants and personnel | 0 (0) | 0 (0) | 17 (47) | 28 (74) | 19 (53) | 10 (26) |
| Blinding of outcome assessment | 16 (44) | 11 (29) | 6 (17) | 26 (68) | 14 (39) | 1 (3) |
| Incomplete outcome data | 28 (78) | 19 (50) | 3 (8) | 11 (29) | 5 (14) | 8 (21) |
| Selective reporting of outcomes | 23 (64) | 29 (76) | 6 (17) | 4 (11) | 7 (19) | 5 (13) |
| Total number of studies | 36 | 38 | 36 | 38 | 36 | 38 |
Chapter 5 Effect of diet- and physical activity-based interventions in pregnancy on maternal and fetal outcomes
Gestational weight gain
Overall effect
Overall, diet- and physical activity-based interventions (33 studies, 9320 women) reduced GWG by an average of –0.70 kg (95% CI –0.92 to –0.48 kg; I2 = 14.1%) (Figure 4), after accounting for baseline weight and clustering effect.
FIGURE 4.
Effects of diet- and physical activity-based interventions on GWG (kg). Trt, treatment.

All three individual interventions (diet, physical activity and mixed) had a similar effect on reducing GWG by an average of 0.7 kg (Table 7).
| Intervention | Number of studies | Number of women | Mean change of weight, mean (SD) | Summary-adjusted MDa of weight (95% CI) | 95% PI | |
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| Diet | 4 | 1168 | 11.0 (4.8) | 10.2 (4.4) | –0.72 (–1.48 to 0.04) | –1.75 to 0.30 |
| Physical activity | 15 | 2915 | 10.8 (5.3) | 9.8 (4.4) | –0.73 (–1.11 to –0.34) | –1.50 to 0.05 |
| Mixed approach | 15 | 5369 | 10.4 (5.7) | 10.0 (5.8) | –0.71 (–1.10 to –0.31) | –1.42 to 0.01 |
| Overallb | 33 | 9320 | 10.8 (5.4) | 10.1 (5.4) | –0.70 (–0.92 to –0.48) | –1.24 to –0.16 |
Sensitivity analysis
The beneficial effect on GWG was consistent after including all available aggregate data from an additional 27 non-IPD studies (MD –1.13 kg, 95% CI –1.58 to –0.68 kg) by including only IPD studies that were rated as being at low risk of bias (MD –0.67 kg, 95% CI –0.95 to –0.38 kg), excluding women non-adherent to the intervention (MD –0.76 kg, 95% CI –1.00 to –0.52 kg), restricting the IPD analysis to women who were followed up until 37 weeks of gestation (MD –0.91 kg, 95% CI –1.17 to –0.66 kg) and using BMI instead of maternal weight as a measure of weight change in pregnancy (MD –0.30 kg/m2, 95% CI –0.39 to –0.21 kg/m2) (see Appendix 8).
Differential effect of the intervention on gestational weight gain in various subgroups
Thirty-one studies (9285 women) provided data to evaluate the differential effect of interventions on GWG for women with varied BMI at booking. There was no significant treatment–covariate interaction for baseline BMI (–0.02 kg change in effect per 1 kg/m2 increase in BMI, 95% CI –0.08 to 0.04 kg change). We did not observe any interaction effect for other effect modifiers such as age (–0.03 kg change in effect per 1-year increase in age, 95% CI –0.08 to 0.02 kg), parity (0.10 kg change in effect for multiparous vs. nulliparous, 95% CI –0.39 to 0.60 kg change), ethnicity (0.05 kg change in effect for non-Caucasian vs. Caucasian, 95% CI –1.27 to 1.37 kg change) and underlying medical condition (1.51 kg change in effect for women with at least one condition vs. none, 95% CI –2.01 to 5.02 kg) (Table 8). The findings were consistent when we analysed the continuous covariates as dichotomised measures.
| Item | Number of studies | Number of women | Summary-adjusted MDa of weight (95% CI) | Summary treatment–covariate interaction (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|---|
| Baseline BMI category | ||||||
| Normal weight | 21 | 3376 | –0.77 (–1.15 to –0.39) | –1.68 to 0.14 | 33.9 | |
| Overweight | 28 | 2574 | –0.75 (–1.22 to –0.27) | –2.07 to 0.58 | 32.7 | |
| Obese | 31 | 3335 | –0.85 (–1.41 to –0.29) | –2.73 to 1.03 | 43.9 | |
| Per unit of BMI | 31 | 9285 | –0.02 (–0.08 to 0.04) | –0.21 to 0.17 | 39.8 | |
| Overweight vs. normal weight | 21 | 6023 | –0.11 (–0.77 to 0.55) | –1.48 to 1.25 | 32.0 | |
| Obese vs. normal weight | 21 | 6023 | 0.06 (–0.90 to 1.01) | –2.23 to 2.34 | 32.7 | |
| Obese vs. overweight | 28 | 8802 | –0.09 (–1.05 to 0.86) | –3.2 to 3.01 | 46.9 | |
| Age | ||||||
| ≥ 20 years | 32 | 9045 | –0.72 (–0.95 to –0.50) | –1.29 to –0.15 | 17.0 | |
| < 20 years | 13 | 232 | 0.05 (–1.34 to 1.44) | –2.11 to 2.21 | 1.0 | |
| Per year of age | 32 | 9277 | –0.03 (–0.08 to 0.02) | –0.14 to 0.09 | 25.9 | |
| < 20 vs. ≥ 20 years | 13 | 5012 | 0.65 (–1.11 to 2.41) | –2.66 to 3.97 | 10.8 | |
| Ethnicity | ||||||
| Caucasian | 21 | 6814 | –0.74 (–1.07 to –0.42) | –1.52 to 0.04 | 41.4 | |
| Non-Caucasian | 15 | 621 | –0.42 (–1.12 to 0.28) | –1.13 to 0.29 | 0.0 | |
| Non-Caucasian vs. Caucasian | 12 | 4439 | 0.05 (–1.27 to 1.37) | –1.28 to 1.39 | 26.1 | |
| Parity | ||||||
| Nulliparous | 27 | 4513 | –0.80 (–1.17 to –0.43) | –1.84 to 0.24 | 38.3 | |
| Multiparous | 27 | 4548 | –0.62 (–0.88 to –0.37) | –0.88 to –0.37 | 0.0 | |
| Multiparous vs. nulliparous | 24 | 7247 | 0.10 (–0.39 to 0.60) | –0.83 to 1.04 | 4.8 | |
| Pre-existing medical conditionb | ||||||
| No medical condition | 18 | 4335 | –0.62 (–0.90 to –0.34) | –1.07 to –0.17 | 0.0 | |
| At least one medical condition | 6 | 128 | 0.40 (–1.92 to 2.71) | –2.10 to 2.90 | 14.1 | |
| At least one medical condition vs. none | 5 | 1196 | 1.51 (–2.01 to 5.02) | –4.13 to 7.15 | 28.4 | |
Maternal outcomes
Overall effect
Diet- and physical activity-based interventions (24 studies, 8852 women) reduced the odds of adverse maternal outcomes by 10% (summary OR 0.90, 95% CI 0.79 to 1.03; I2 = 26.7%). The effect was not statistically significant at the 5% level (Figure 5).
FIGURE 5.
Effects of diet- and physical activity-based interventions on composite maternal outcome. Trt, treatment.

The effects on composite maternal outcomes were evaluated in two-thirds of participants in studies of mixed interventions, compared with 4% of participants in studies of diet-based interventions. The effects of physical activity, diet and mixed approaches were not statistically significant (Table 9).
| Intervention | Number of studies | Number of women | Study arm (number of events/total number of women) | Summary ORa (95% CI) | 95% PI | |
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| Diet | 3 | 397 | 84/218 | 42/179 | 0.60 (0.20 to 1.75) | 0.02 to 14.27 |
| Physical activity | 9 | 2311 | 367/1115 | 346/1196 | 0.81 (0.61 to 1.09) | 0.48 to 1.37 |
| Mixed approach | 13 | 6259 | 1438/3009 | 1508/3250 | 0.97 (0.84 to 1.12) | 0.82 to 1.13 |
| Overallb | 24 | 8852 | 1838/4226 | 1895/4624 | 0.90 (0.79 to 1.03) | 0.68 to 1.20 |
Sensitivity analysis by excluding studies rated as having a high risk of bias (summary OR 0.91, 95% CI 0.77 to 1.08) and women non-adherent to the intervention (summary OR 0.92, 95% CI 0.80 to 1.06) did not affect the findings. The results of all sensitivity analyses are provided in Appendix 8.
Effect of interventions on individual maternal outcomes
The odds of Caesarean section were reduced by 9%, which bordered on statistical significance (summary OR 0.91, 95% CI 0.83 to 0.99). For other maternal outcomes, such as GDM (OR 0.89, 95% CI 0.72 to 1.10), PE or PIH (OR 0.95, 95% CI 0.78 to 1.16), and preterm birth (OR 0.94, 95% CI 0.78 to 1.13), there was a trend towards reduction that was not statistically significant. Meta-analysis based on published aggregate data only showed a significant reduction in GDM (OR 0.78, 95% CI 0.64 to 0.95) and Caesarean section (OR 0.90, 95% CI 0.82 to 0.99) compared with the control group. There were no significant reductions in preterm birth (OR 0.80, 95% CI 0.63 to 1.01), PE or PIH (OR 0.89, 95% CI 0.75 to 1.05) (for details see Appendix 9). More participants were included in the IPD meta-analysis than in the meta-analysis based on published data for outcomes preterm birth and PE or PIH; the participant numbers were similar for GDM and Caesarean section (Table 10).
| Maternal outcome | Data | ||||||
|---|---|---|---|---|---|---|---|
| IPD (n = 36) | Aggregate (n = 74) | ||||||
| Number of studies | Number of women | Summary ORa (95% CI) | Number of studies | Number of women | ORb (95% CI) | I2 (%) | |
| GDMc | 27 | 9427 | 0.89 (0.72 to 1.10) | 29 | 11,118 | 0.77 (0.63 to 0.94) | 38 |
| PE or PIH | 22 | 9618 | 0.95 (0.78 to 1.16) | 20 | 9198 | 0.89 (0.75 to 1.05) | 0 |
| Preterm birth | 32 | 11676 | 0.94 (0.78 to 1.13) | 23 | 7480 | 0.80 (0.63 to 1.01) | 30 |
| Caesarean section | 32 | 11410 | 0.91 (0.83 to 0.99) | 37 | 11,340 | 0.90 (0.82 to 0.99) | 2 |
Differential effects of the intervention for composite maternal outcome in various subgroups
Twenty-four studies (8848 women) contributed IPD to assess the differential effects of interventions on the composite maternal outcome according to maternal BMI category. There was no significant treatment–covariate interaction for baseline BMI (no change in effect for every 1-kg/m2 increase in BMI, OR 95% CI 0.98 to 1.02). The effects of the interventions were not significantly modified by other relevant covariates such as age (1% increase in effect for every 1-year increase in age, OR 95% CI 0.99 to 1.03), parity (3% increase in effect for multiparity vs. nulliparity, OR 95% CI 0.75 to 1.39), ethnicity (7% decrease in effect for non-Caucasian vs. Caucasian, OR 95% CI 0.63 to 1.37) and underlying medical condition (44% increase in effect for women with none vs. at least one condition, OR 95% CI 0.15 to 13.74). The findings were consistent when continuous covariates were further analysed as categorical values (Table 11).
| Item | Number of studies | Number of women | Summary ORa (95% CI) | Summary treatment–covariate interaction (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|---|
| Baseline BMI category | ||||||
| Normal weight | 12 | 2445 | 0.91 (0.65 to 1.28) | 0.42 to 1.96 | 48.5 | |
| Overweight | 19 | 2222 | 1.04 (0.86 to 1.26) | 0.86 to 1.26 | 0.0 | |
| Obese | 20 | 4181 | 0.92 (0.80 to 1.05) | 0.8 to 1.05 | 0.0 | |
| Per unit BMI | 24 | 8848 | 1.00 (0.98 to 1.02) | 0.98 to 1.02 | 0.0 | |
| Overweight vs. normal weight | 12 | 4040 | 1.02 (0.67 to 1.55) | 0.52 to 1.99 | 20.2 | |
| Obese vs. normal weight | 12 | 4040 | 0.95 (0.57 to 1.59) | 0.57 to 1.60 | 0.0 | |
| Obese vs. overweight | 20 | 7400 | 0.95 (0.71 to 1.26) | 0.71 to 1.26 | 0.0 | |
| Age | ||||||
| ≥ 20 years | 24 | 8656 | 0.91 (0.81 to 1.02) | 0.73 to 1.13 | 20.5 | |
| < 20 years | 9 | 172 | 1.57 (0.66 to 3.71) | 0.65 to 3.80 | 0.0 | |
| Per year of age | 24 | 8828 | 1.01 (0.99 to 1.03) | 0.99 to 1.03 | 0.0 | |
| < 20 years vs. ≥ 20 years | 8 | 4720 | 1.84 (0.74 to 4.57) | 0.72 to 4.72 | 0.0 | |
| Ethnicity | ||||||
| Caucasian | 15 | 6510 | 0.92 (0.79 to 1.07) | 0.67 to 1.25 | 26.8 | |
| Non-Caucasian | 11 | 917 | 0.86 (0.63 to 1.17) | 0.62 to 1.17 | 0.0 | |
| Non-Caucasian vs. Caucasian | 9 | 4851 | 0.93 (0.63 to 1.37) | 0.62 to 1.38 | 0.0 | |
| Parity | ||||||
| Nulliparous | 21 | 4613 | 0.87 (0.71 to 1.07) | 0.54 to 1.41 | 39.8 | |
| Multiparous | 22 | 4186 | 0.92 (0.78 to 1.07) | 0.78 to 1.07 | 21.9 | |
| Multiparous vs. nulliparous | 20 | 8053 | 1.03 (0.75 to 1.39) | 0.53 to 2.00 | 34.0 | |
| Pre-existing medical conditionb | ||||||
| No medical condition | 15 | 3135 | 0.85 (0.66 to 1.09) | 0.46 to 1.57 | 42.5 | |
| At least one medical condition | 5 | 89 | 1.65 (0.36 to 7.51) | 0.29 to 9.37 | 0.0 | |
| None vs. at least one medical condition | 4 | 916 | 1.44 (0.15 to 13.74) | 0.03 to 76.75 | 24.9 | |
Fetal/neonatal outcomes
Overall effect
Diet- and physical activity-based interventions (18 studies, 7981 women) did not reduce the odds of the composite adverse fetal/neonatal outcome (summary OR 0.94, 95% CI 0.83 to 1.08) (Figure 6) after adjusting for clustering.
FIGURE 6.
Effect of diet- and physical activity-based interventions on composite fetal/neonatal outcome.
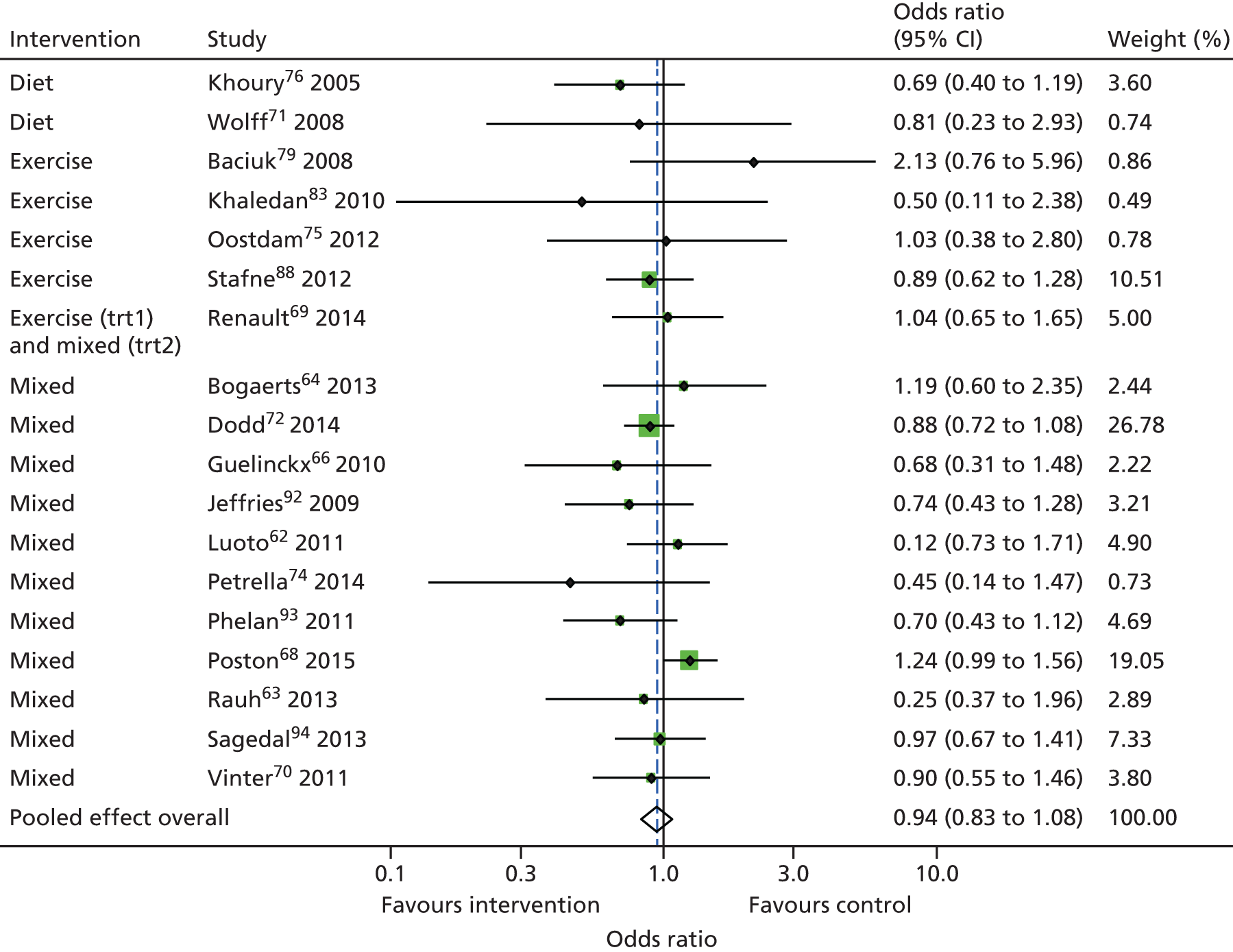
Two studies (346 women) evaluated diet-based interventions, five (1274 women) assessed physical activity-based interventions and 12 (6494 women) studied mixed interventions on composite fetal/neonatal outcomes. None of the three interventions reduced composite adverse fetal/neonatal outcome (Table 12).
| Intervention | Number of studies | Number of women | Study arm (number of events/total number of women) | Summary-adjusted ORa (95% CI) | 95% PI | |
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| Diet | 2 | 346 | 47/181 | 31/167 | 0.64 (0.02 to 18.06) | – |
| Physical activity | 5 | 1274 | 156/641 | 170/634 | 1.23 (0.72 to 2.10) | 0.45 to 3.32 |
| Mixed | 12 | 6494 | 875/3338 | 953/3626 | 1.02 (0.90 to 1.15) | 0.87 to 1.19 |
| Overallb | 18 | 7981 | 951/3802 | 1007/4179 | 0.94 (0.83 to 1.08) | 0.74 to 1.21 |
Differential effect of the intervention for composite fetal/neonatal outcome in various subgroups
Eighteen studies (7981 women) provided IPD to assess the differential effects of interventions by maternal baseline BMI on composite fetal/neonatal outcome. There was a 2% decrease in the treatment effect of borderline significance for every 1 kg/m2 increase in booking BMI for composite fetal/neonatal outcomes (OR 0.98, 95% CI 0.95 to 1.00). There was no treatment–covariate interaction for other variables, such as maternal age of < 20 years (OR 1.05, 95% CI 0.33 to 3.35), ethnicity (12% decrease in effect for non-Caucasian vs. Caucasian, 95% CI 0.75 to 1.68), parity (6% reduction in effect for multiparous vs. nulliparous, 95% CI 0.64 to 1.47), baseline medical conditions (42% increase in effect for women with at least one medical condition vs. none, 95% CI 0.00 to 2440.15) (Table 13).
| Item | Number of studies | Number of women | Summary ORa (95% CI) | Summary treatment–covariate interaction (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|---|
| Baseline BMI category | ||||||
| Normal weight | 7 | 1843 | 0.93 (0.60 to 1.43) | 0.40 to 2.16 | 31.6 | |
| Overweight | 12 | 2065 | 0.83 (0.61 to 1.13) | 0.49 to 1.39 | 0.0 | |
| Obese | 13 | 4327 | 0.92 (0.72 to 1.19) | 0.55 to 1.54 | 29.7 | |
| Per unit BMI | 18 | 7978 | 0.98 (0.95 to 1.00) | 0.94 to 1.02 | 18.5 | |
| Overweight vs. normal weight | 8 | 2827 | 0.87 (0.40 to 1.92) | 0.15 to 4.91 | 44.0 | |
| Obese vs. normal weight | 8 | 2827 | 0.84 (0.42 to 1.66) | 0.41 to 1.70 | 0.0 | |
| Obese vs. overweight | 14 | 6272 | 0.94 (0.60 to 1.45) | 0.51 to 1.71 | 0.0 | |
| Age | ||||||
| ≥ 20 years | 16 | 8061 | 0.95 (0.82 to 1.09) | 0.72 to 1.24 | 0.0 | |
| < 20 years | 7 | 162 | 1.01 (0.34 to 2.98) | 0.32 to 3.14 | 0.0 | |
| Per year of age | 18 | 7965 | 1.01 (0.98 to 1.04) | 0.97 to 1.05 | 4.1 | |
| < 20 vs. ≥ 20 years | 6 | 4941 | 1.05 (0.33 to 3.35) | 0.30 to 3.67 | 0.0 | |
| Ethnicity | ||||||
| Caucasian | 11 | 6018 | 0.93 (0.79 to 1.08) | 0.75 to 1.14 | 3.5 | |
| Non-Caucasian | 9 | 939 | 1.10 (0.78 to 1.54) | 0.78 to 1.55 | 0.0 | |
| Non-Caucasian vs. Caucasian | 9 | 5146 | 1.12 (0.75 to 1.68) | 0.74 to 1.69 | 0.0 | |
| Parity | ||||||
| Nulliparous | 16 | 4152 | 0.97 (0.80 to 1.17) | 0.69 to 1.35 | 21.1 | |
| Multiparous | 15 | 4048 | 0.91 (0.72 to 1.15) | 0.56 to 1.48 | 23.2 | |
| Multiparous vs. nulliparous | 15 | 7295 | 0.94 (0.64 to 1.37) | 0.39 to 2.28 | 35.5 | |
| Pre-existing medical conditionb | ||||||
| No medical condition | 12 | 3407 | 0.89 (0.74 to 1.08) | 0.74 to 1.08 | 0.0 | |
| At least one medical condition | 3 | 63 | 0.54 (0.04 to 7.52) | 0.00 to 1285.09 | 0.0 | |
| At least one medical condition vs. none | 3 | 925 | 0.58 (0.03 to 9.81) | 0.00 to 2440.15 | 0.0 | |
None of the sensitivity analyses performed showed a significant impact on the intervention effect (see Appendix 8).
Effect of interventions on individual fetal/neonatal outcomes
Compared with published aggregate data, IPD were available for more participants for fetal/neonatal outcomes, such as SG, LGA and admission to the NICU. IPD meta-analysis did not show a significant effect on SGA infants (summary OR 1.06, 95% CI 0.94 to 1.20), LGA infants (summary OR 0.90, 95% CI 0.76 to 1.07) or admission to the NICU (summary OR 1.01, 95% CI 0.84 to 1.23). Aggregate meta-analysis of published data showed similar results of no effect for all fetal outcomes (Table 14).
| Fetal/neonatal outcome | Data | ||||||
|---|---|---|---|---|---|---|---|
| IPD (n = 35 studies) | Aggregate (n = 74 studies) | ||||||
| Number of studies | Number of women | Summary ORa (95% CI) | Number of studies | Number of women | Summary ORb (95% CI) | I2 (%) | |
| IUDc | – | – | – | 4 | 4857 | 1.95 (0.55 to 6.90) | 0 |
| SGA | 33 | 11,666 | 1.06 (0.94 to 1.20) | 8 | 2835 | 1.27 (0.91 to 1.77) | 0 |
| LGA | 34 | 12,047 | 0.90 (0.76 to 1.07) | 13 | 5827 | 0.88 (0.68 to 1.15) | 37 |
| Admission to the NICU | 16 | 8140 | 1.01 (0.84 to 1.23) | 6 | 5200 | 0.95 (0.77 to 1.19) | 22 |
Publication bias
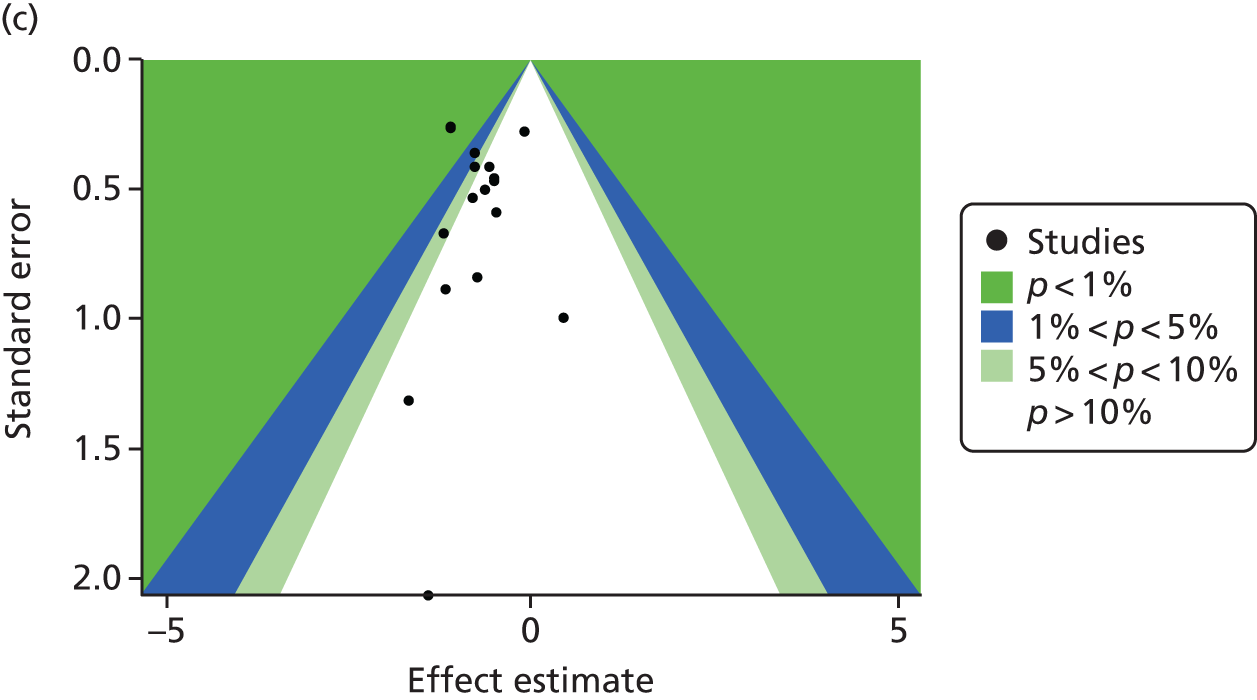
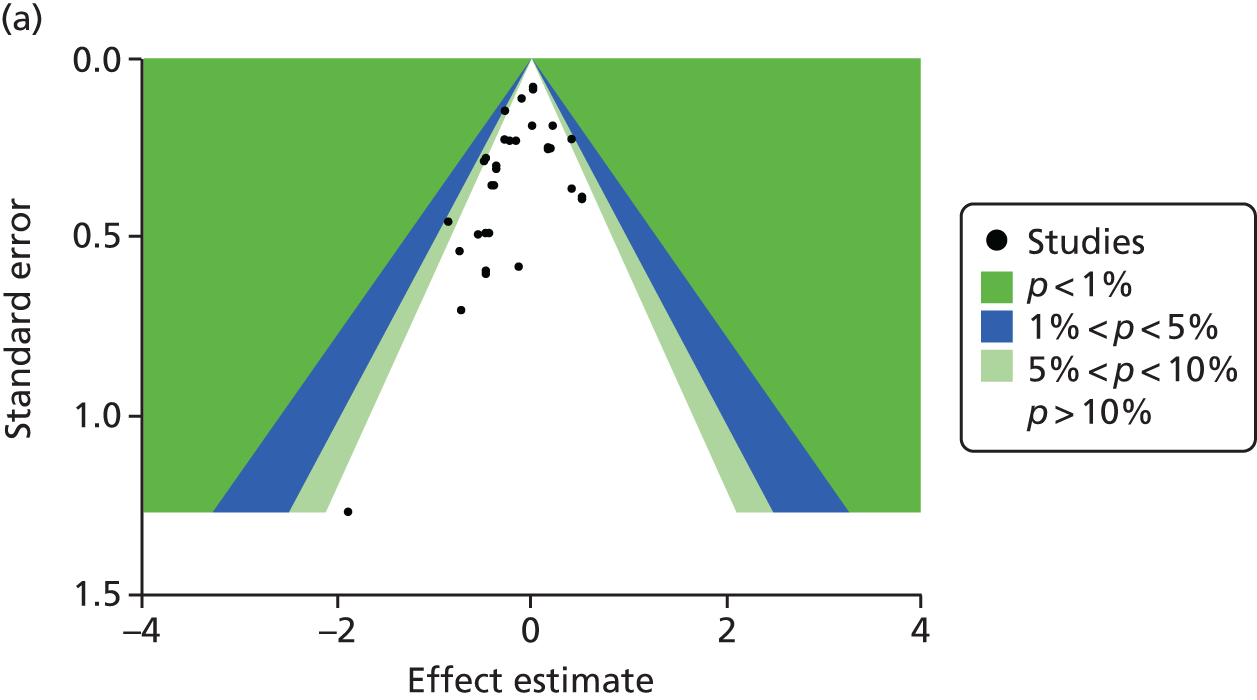
The contour-enhanced funnel plots on small-study effects (potential publication bias) for GWG (Figure 7) showed visual and statistical evidence (Egger’s test, p = 0.038) of asymmetry, indicating that smaller studies were more likely to have large intervention effects. Addition of aggregate data from non-IPD studies to the meta-analysis worsened the symmetry, suggesting that the asymmetry was not caused by availability bias. Exclusion of studies rated as being at high risk of bias to the analysis improved symmetry substantially (Egger’s test, p = 0.608).
FIGURE 7.
Contour-enhanced funnel plot for GWG. (a) IPD data only; (b) IPD and aggregate data; and (c) IPD studies classified as being at low risk of bias.


There was significant evidence of small-study effects for the composite maternal (Peter’s test, p = 0.036), but not for the fetal/neonatal composite outcome (p = 0.398) (Figure 8). Heterogeneity, which was present in all these meta-analyses, might be the cause (rather than publication bias) of asymmetry in the funnel plots.
FIGURE 8.
Contour-enhanced funnel plot for (a) composite maternal; and (b) fetal/neonatal outcomes.

Chapter 6 Association of maternal weight and weight gain in pregnancy and pregnancy complications
Gestational weight gain, maternal weight at booking and adverse maternal and fetal/neonatal outcomes
Twenty-three trials evaluated the association of GWG, BMI at booking and composite maternal outcomes (3367 women), and 19 trials provided data for composite fetal/neonatal outcomes (3030 women) from women in control groups. There was no association between GWG, booking BMI and risk of adverse maternal (OR 1.03, 95% CI 0.93 to 1.15) or fetal/neonatal complications (OR 1.02, 95% CI 0.91 to 1.15), and this effect does not differ by baseline BMI (Table 15).
| Outcomes | Number of studies | Number of women | Effect of GWG, ORa (95% CI) | Modifying effect of baseline BMI, OR (95% CI) | I2 (%) |
|---|---|---|---|---|---|
| Composite maternal outcome | 23 | 3367 | 1.03 (0.93 to 1.15) | N/Ab | 0 |
| Composite fetal and neonatal outcome | 19 | 3030 | 1.02 (0.91 to 1.15) | 1.00 (1.00 to 1.00) | 0 |
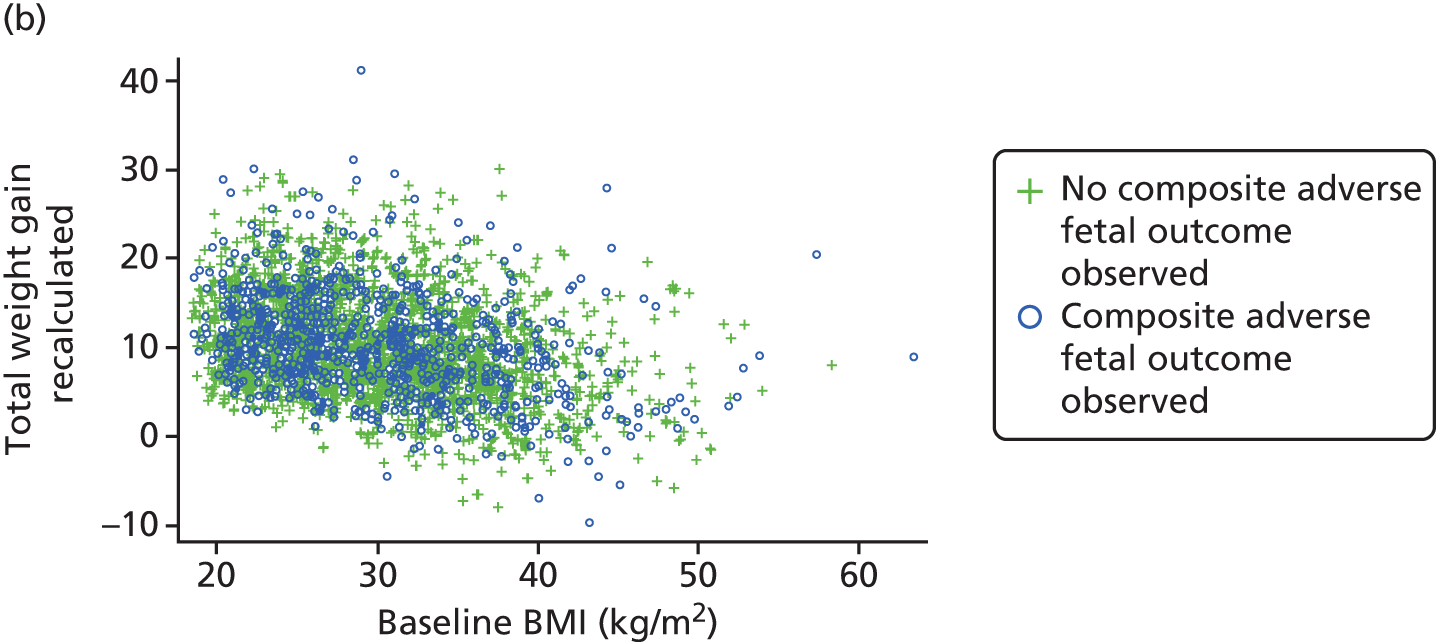
Figure 9 presents the relationship between presence or absence of adverse maternal and fetal complications for women entering pregnancy with varied BMI, for different values of GWG.
FIGURE 9.
Scatterplot of GWG against baseline BMI for adverse (a) maternal; and (b) fetal/neonatal composite outcomes.

Adherence to the Institute of Medicine’s recommendations and risk of adverse pregnancy outcomes
In women who were normal weight at booking, about 40% adhered to the IOM’s recommendations and gained up to 16 kg in pregnancy; another 40% gained less than the recommended range, and less than one-fifth exceeded the recommendations. One-third (29%) of overweight and obese (30%) women complied with the recommendations. About half of overweight women and 44% of obese women exceeded the recommended ranges (Table 16).
| GWG | Normal weight, n (%) | Overweight, n (%) | Obese, n (%) |
|---|---|---|---|
| Below IOM | 649 (40) | 242 (19) | 400 (26) |
| Adherent to IOM | 663 (41) | 362 (29) | 467 (30) |
| Exceeds IOM | 310 (19) | 641 (51) | 695 (44) |
| Total | 1622 | 1245 | 1562 |
The odds of adverse composite outcome were not significant when normal weight women gained above (summary OR 1.05, 95% CI 0.60, 1.82) and below (summary OR 0.99, 95% CI 0.67, 1.47) the recommended targets. We did not observe any significant additional increase in maternal risks when obese and overweight women did not comply with the recommended targets (Table 17). There was no significant increase in the odds of adverse maternal outcomes in overweight and obese women who gained below or above the recommendations.
| Adherence to IOM’s targets in pregnancy | Number of studies | Number of women | Summary ORa (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|
| Normal weight | |||||
| Below vs. adherent | 12 | 1092 | 0.99 (0.67 to 1.46) | 0.67 to 1.46 | 0.0 |
| Exceeds vs. adherent | 11 | 1083 | 1.05 (0.61 to 1.80) | 0.41 to 2.65 | 0.0 |
| Overweight | |||||
| Below vs. adherent | 16 | 889 | 1.28 (0.79 to 2.08) | 0.79 to 2.08 | 0.0 |
| Exceeds vs. adherent | 18 | 904 | 0.78 (0.49 to 1.26) | 0.34 to 1.80 | 0.0 |
| Obese | |||||
| Below vs. adherent | 17 | 1261 | 1.38 (0.95 to 2.01) | 0.88 to 2.18 | 0.0 |
| Exceeds vs. adherent | 19 | 1324 | 1.15 (0.85 to 1.56) | 0.85 to 1.56 | 0.0 |
Non-adherence to IOM’s targets for weight gain in pregnancy did not pose additional risks of fetal complications in normal weight, overweight and obese pregnant women (Table 18). The odds of composite adverse fetal outcomes were not significantly increased in normal weight, overweight and obese women who gained more or less than the recommended targets.
| Adherence to the IOM-recommended weight-gain targets in pregnancy | Number of studies | Number of women | Summary ORa (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|
| Normal weight | |||||
| Below vs. adherent | 9 | 821 | 0.87 (0.40 to 1.90) | 0.16 to 4.84 | 38.4 |
| Exceeds vs. adherent | 9 | 821 | 1.26 (0.60 to 2.65) | 0.35 to 4.57 | 29.0 |
| Overweight | |||||
| Below vs. adherent | 10 | 830 | 1.07 (0.51 to 2.22) | 0.38 to 2.99 | 0.0 |
| Exceeds vs. adherent | 10 | 830 | 1.09 (0.68 to 1.74) | 0.67 to 1.76 | 0.0 |
| Obese | |||||
| Below vs. adherent | 16 | 1285 | 1.57 (1.05 to 2.32) | 1.05 to 2.33 | 0.0 |
| Exceeds vs. adherent | 15 | 1271 | 1.36 (0.89 to 2.06) | 0.67 to 2.75 | 0.0 |
Chapter 7 Predictors of gestational weight gain
Maternal characteristics, such as increase in age and parity, showed a significant association with reduced GWG, on average, by 0.09 kg (95% CI –0.12 to –0.06 kg) and 0.51 kg (95% CI –0.78 to –0.24 kg), respectively, in univariate (crude) meta-analyses. Non-Caucasian ethnicity was a significant predictor of decreased weight gain (summary-adjusted difference –0.89 kg, 95% CI –1.76 to –0.02 kg). Other maternal characteristics (such as smoking, pre-existing medical conditions, baseline physical activity and maternal education) were not associated with weight gain in pregnancy (Table 19).
| Baseline characteristic | Number of studies | Sample size | Crude summary-adjusted differencea in GWG (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|
| Age (years) | 32 | 4424 | –0.09 (–0.12 to –0.06) | –0.12 to –0.06 | 14.7 |
| Non-Caucasian vs. Caucasian | 13 | 2101 | –0.89 (–1.76 to –0.02) | –1.83 to 0.05 | 18.7 |
| Pooled effect ethnicity (reference category: Caucasian) | |||||
| Asian | 7 | 1758 | –0.53 (–2.24 to 1.18) | –3.42 to 2.36 | 21.4 |
| Afro-Caribbean | 9 | 1822 | –1.17 (–2.65 to 0.30) | –2.69 to 0.34 | 0.0 |
| Central/South American | 1 | 110 | – | – | – |
| Middle Eastern | 4 | 289 | –1.35 (–7.12 to 4.42) | –9.16 to 6.46 | 0.0 |
| GDM | 2 | 532 | –1.43 (–16.58 to 13.72) | – | 0.0 |
| DM | 3 | 305 | –1.70 (–8.25 to 4.84) | –21.02 to 17.62 | 0.0 |
| PIH | 3 | 539 | –2.08 (–13.71 to 9.55) | –65.15 to 61.00 | 80.4 |
| Chronic hypertension | 5 | 546 | –1.43 (–4.80 to 1.95) | –8.81 to 5.96 | 52.8 |
| Current smoker | 21 | 3572 | –0.07 (–0.98 to 0.84) | –2.39 to 2.25 | 47.4 |
| Parity (number) | 27 | 3673 | –0.51 (–0.78 to –0.24) | –1.42 to 0.40 | 56.8 |
| Multiparous vs. nulliparous | 25b | 3427 | –1.12 (–1.55 to –0.69) | –2.30 to 0.07 | 32.4 |
| Maternal education (reference category: low) | |||||
| Medium | 23 | 3030 | 0.16 (–0.35 to 0.68) | –0.35 to 0.68 | 0.0 |
| High | 23 | 3030 | –0.09 (–0.71 to 0.53) | –0.71 to 0.53 | 23.8 |
| Some physical activity vs. physically inactive | 22 | 2697 | –0.30 (–0.70 to 0.10) | –0.71 to 0.53 | 23.8 |
Multivariable analysis showed that increase in maternal age (–0.1 kg, 95% CI –0.14 to –0.06 kg) and multiparity (–0.73 kg, 95% CI –1.24 to –0.23 kg) were associated with significantly reduced GWG. The details of the multivariable analysis for the association between baseline characteristics and GWG are provided in Table 20.
| Baseline characteristic | Number of studies | Sample size | Crude summary-adjusted differencea in GWG (95% CI) | 95% PI | I2 (%) |
|---|---|---|---|---|---|
| Age (years) | 17 | 2414 | –0.10 (–0.14 to –0.06) | –0.14 to –0.06 | 0.0 |
| Ethnicity: non-Caucasian vs. Caucasian | 10 | 1105 | –0.11 (–1.53 to 1.32) | –3.12 to 2.91 | 34.6 |
| Current smoker | 13 | 2075 | –0.06 (–1.65 to 1.52) | –3.93 to 3.81 | 57.1 |
| Multiparous vs. nulliparous | 15 | 2120 | –0.73 (–1.24 to –0.23) | –1.83 to 0.36 | 15.3 |
| Maternal education (reference category: low) | |||||
| Medium | 15 | 2307 | –0.07 (–0.91 to 0.77) | –1.88 to 1.74 | 27.6 |
| High | 15 | 2307 | –0.18 (–1.18 to 0.81) | –2.57 to 2.21 | 36.3 |
| Some physical activity vs. inactive | 17 | 2414 | –0.26 (–0.63 to 0.11) | –0.63 to 0.11 | 0.0 |
Chapter 8 Economic evaluation and decision-analytic modelling
Objectives
The main objective of this model-based economic evaluation was to determine the cost-effectiveness of diet- and exercise-based interventions in pregnancy to improve maternal and fetal clinical outcomes compared with usual care, using the results of the IPD meta-analysis for all women. A secondary objective was to compare the cost-effectiveness of the intervention for women whose pre-pregnancy weight was classed as normal, overweight or obese. The success of any intervention in supporting women to achieve optimum weight gain during pregnancy needs to be balanced against the resources required to achieve this outcome, and additional costs must be assessed in terms of any additional benefits that can be attributed to them. 95 Identification of specific subgroups of women in whom the intervention is cost-effective has the potential to target interventions to particular groups.
Methods
In the economic analysis, diet- and physical activity-based interventions in pregnancy were compared with care as usual (control). The principal clinical data used to populate the model were drawn from the IPD meta-analyses (see Chapter 5); this was supplemented with data from other published sources. Resource use was estimated from the published evidence and unit costs were based on published sources such as the Unit Costs of Health and Social Care 201496 and the National Schedule of Reference Costs: The Main Schedule. 97
Model structure
The appropriate model for this study was a decision tree because of the short-term nature of the decision problem. 98 The model was developed using TreeAge Pro 2014 software (TreeAge Software, Inc., Williamstown, MA, USA). The structure was informed by the objectives of the study, the pathways indicated by the data and trials included in the IPD meta-analysis, clinical input, NICE guidelines on the management of women in pregnancy10,99,100 and the approaches adopted in previously published model-based economic evaluations in relevant clinical areas. 101–105 For completeness, the model included all the potential pathways that could be followed by the women. Women entered the model at the point of randomisation to receive the intervention or care as usual (control). All women were assumed to follow one of six clinical pathways based on whether or not they developed pregnancy-related complications or experienced miscarriage or maternal death. These pathways were (1) PE, (2) GDM, (3) PIH, (4) no complication, (5) second-trimester miscarriage and (6) maternal death (Figure 10). Each pathway included appropriate maternal and fetal outcomes as detailed below.
FIGURE 10.
Patient pathways incorporated in the model.

For the base case, outcomes were considered until the point of discharge from hospital. Once women entered the model, it was assumed that they followed one of the clinical pathways defined in the model, based on whether or not they developed a pregnancy-related condition/complication. Complications were defined in accordance with the definitions used in studies included in the IPD meta-analysis. To illustrate the approach used for each patient pathway, a subset of the model is presented for the PE pathway (Figure 11). Women who developed more than one complication were allocated to the most resource-intensive pathway based on an analysis of NICE clinical pathways and clinical opinion. 106 The intensity of pathways was defined as follows (in decreasing order of intensity): (1) PE, (2) GDM99 and (3) PIH. 100 For the purposes of the model it was assumed that once women developed a complication they were treated in accordance with NICE guidelines and incurred associated antenatal health-care costs. Women who did not develop any of the specified conditions were assumed to receive routine antenatal care only. It was assumed that routine antenatal care would be received by all women, irrespective of whether or not they developed a pregnancy-related condition. 107 As the purpose of economic evaluation is to examine the differences in costs and outcomes between alternative courses of action,95 the costs of routine antenatal care were not included in the model as they would be identical for each arm.
FIGURE 11.
Detail for the PE pathway. C-section, Caesarean section.
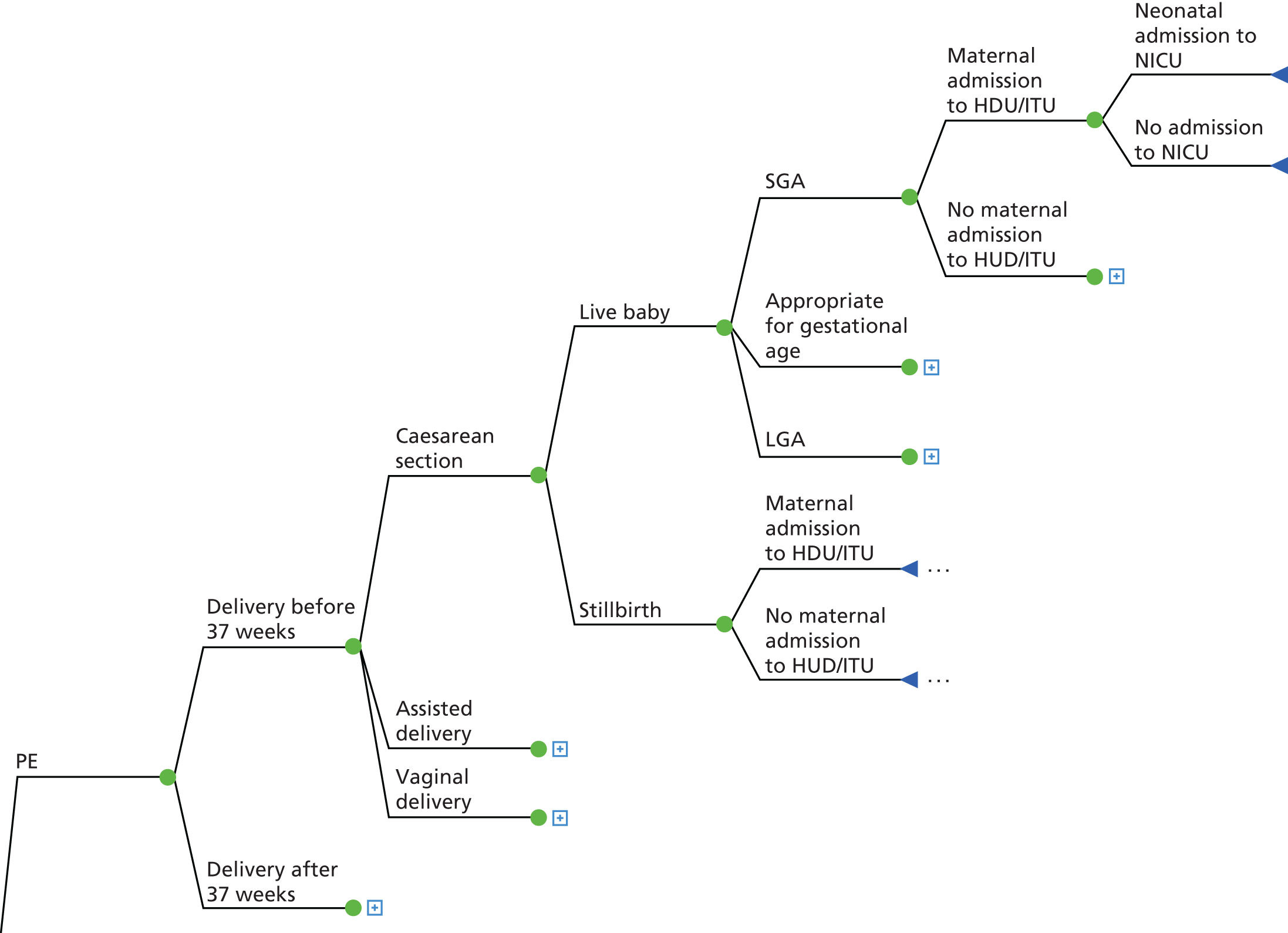
For all clinical pathways, women could either experience a preterm delivery or a delivery at term; preterm delivery was defined as delivery before 37 gestational weeks. 108 Three types of delivery were included in the model: Caesarean section, assisted delivery and vaginal delivery. The outcome of the delivery was either a stillbirth (or IUD) or live baby. Stillbirth was defined as a baby born with no signs of life after 24 weeks of completed pregnancy and IUD as a baby with no signs of life in utero. 109 Women who experienced stillbirth and IUD were assumed to have received appropriate antenatal care for any condition they were recorded as developing during the trial. Additional costs associated with investigations and counselling were included in the total costs for these women. 110
The model also included pathways for second-trimester miscarriage and maternal death in order to reflect all possible pathways for the women. Second-trimester miscarriage was defined as the spontaneous loss of pregnancy after the 14th week of pregnancy and before the 24 completed weeks. 111 The risk of second-trimester miscarriage and maternal death was based on secondary sources and applied to both arms equally to preserve the face validity of the model. 112 As the purpose of the economic evaluation was to examine the differences in costs and outcomes between the intervention and control arms, costs were not included for second-trimester miscarriage and maternal death, as they would be identical for each arm.
Model assumptions
To carry out the model-based analysis some further assumptions were required. These are presented below.
Pre-eclampsia pathway
A number of assumptions were made for the PE pathway based on NICE guidelines100 and the findings of a systematic review that was carried out for this report (see Appendix 10 for further details). NICE guidance states that before 37 weeks delivery should not be recommended for women with PE unless severe or refractory hypertension is present. In women with PE with mild and moderate hypertension, the offer of delivery will depend on maternal and fetal condition, risk factors and the availability of neonatal intensive care. A systematic review was conducted to identify studies that had considered the costs associated with hypertensive disorders in pregnancy (see Appendix 10 for further details). Five studies were identified that included primary data on the costs associated with PE in pregnancy. 113–117 Only one study collected primary data on resource use for women who were primarily diagnosed with PE and who were undergoing expectant monitoring. 113 This study was a RCT that compared expectant monitoring and immediate delivery for women with hypertensive disorders between 34 and 37 weeks of gestation [the Hypertension and Pre-eclampsia Intervention Trial At near Term (HYPITAT-II)]. The majority of the women included in the trial had PE (47%) or superimposed PE (13%). The findings of this study were used to inform the modelling of the PE pathway and the following assumptions were made:
-
Women develop PE and give birth between 34 and 37 weeks of gestation to reflect the findings of the HYPITAT II study. Other evidence suggests that only a minority of women would give birth before this period; for example, a large clinical trial in this area118 found that 82% of women with PE gave birth after 34 weeks.
-
All women receive expectant monitoring for their condition, in line with NICE guidance. 114
-
Antenatal care was as reported in the HYPITAT-II study. This included maternal admissions, cardiotocography and ultrasounds, outpatient visits, laboratory tests and medication. 113
-
Data from the IPD were used to estimate the timing and type of delivery. The cost of care in the intrapartum phase was estimated using nationally reported average costs. It was assumed that because the women were diagnosed with PE and hypertension that all types of delivery would have a ‘complications and comorbidities’ score of 2. 119
-
Women receive the postnatal care that was reported in the HYPITAT-II study. This included maternal admissions, neonatal admission, extra care and transfers. 113
-
The costs included in the analysis were a conservative estimate of the costs associated with PE during pregnancy, and the uncertainty around this estimate was explored in the sensitivity analysis (particularly around the inclusion of costs associated with early-onset PE, that is disease occurring before 34 weeks).
Gestational diabetes mellitus pathway
The following assumptions were made for the GDM pathway based on NICE guidelines99 and a systematic review of the literature conducted for this report. The systematic review identified 10 studies that were concerned with costs and resource use for women with GDM. Only one RCT that recorded primary health-care resource use for women who were diagnosed with GDM was identified. 120 This study included all costs incurred from the start of pregnancy until the final discharge of the mother and her child(ren). As routine antenatal care was included in the costs reported by the economic evaluation of the RCT, the estimate for the cost of antenatal care was based on the results of a modelling study produced to inform the development of for NICE guidelines on DM in pregnancy. 99 This cost was estimated from guideline recommendations for the treatment of women with GDM. The costs associated with delivery were based on national average costs reported in the UK. The costs associated with postnatal care of the mother and infant were based on the health-care costs reported in the economic analysis conducted alongside the RCT. 120 The following assumptions were made for women with GDM:
-
All women were initially treated with diet. After 10 days, if this treatment was not successful, women received insulin treatment (64% patients).
-
Health-care professionals instructed women how to undertake self-monitoring of blood glucose levels, provided dietary advice, assessed the success of diet treatment and instructed women who required insulin treatment on this treatment.
-
Estimates of the timing and type of delivery were based on data from the IPD. The cost of care in the intrapartum phase reflects nationally reported costs. As women had a diagnosis of GDM, all types of delivery had a complications and comorbidities score of 1. 119
-
The costs of postnatal care were as reported in an economic evaluation undertaken along a RCT. 120 This included NICU costs and costs associated with hospital care following delivery (all of the neonatal care costs and half of the non-delivery hospitalisation costs were incurred after the birth).
Pregnancy-induced hypertension pathway
A number of assumptions were made for the gestational hypertension pathway based on NICE guidelines and the results of a systematic review conducted to inform this report (see Appendix 10). For women with gestational hypertension, delivery of the infant should not be offered before 37 weeks and after this point the timing of, and maternal and fetal indications for, birth should be agreed between the woman and senior obstetrician. 114 The systematic review identified very few studies that were concerned with the resource use associated with the expectant monitoring of women with gestational hypertension (without PE) in line with this guidance. An economic evaluation was identified that included costs associated with expectant monitoring for women with hypertensive disorders (the HYPITAT-I117). This trial involved a majority of women with gestational hypertension only (65%). 121 The systematic review also identified an economic model developed to support NICE guidelines that was concerned with immediate birth compared with expectant management in women with mild to moderate gestational hypertension after 37 weeks of gestation. 100 However, the model used secondary data and did not involve primary cost collection.
-
For the purposes of simplification, it was assumed that women developed gestational hypertension after 37 weeks, as most hypertensive disorders present after 36 weeks of gestation. 121
-
Antenatal care was assumed to follow that recorded in the HYPITAT-I study and included maternal admission, home care, cardiotocography, ultrasound, outpatient visits, assessments, laboratory tests and medication. 117
-
The findings of the IPD meta-analysis were used to estimate the timing and mode of delivery for women with PIH. National data on costs were used to estimate the cost of intrapartum care. Because all women had a diagnosis of hypertension, it was assumed that all types of birth would have a complications and comorbidities score of 1. 119
-
In the postnatal period, just 2% of women were assumed to receive intensive care, while 90% were admitted to the maternity ward for a mean period of 3.4 days. In addition, 3% of infants were assumed to be admitted to the NICU, and 19% to receive medium-level care on the maternal ward, in line with findings from the HYPITAT–I trial. 117
No complications
A series of assumptions were made for the pathway followed by women who did not develop complications during pregnancy, based on an analysis of NICE guidance:107
-
Women who did not develop any complications received routine antenatal care only.
-
The estimate of the timing and mode of delivery was based on the findings of the IPD meta-analysis. For the purpose of simplification, this means that women would not have any complications and that their delivery would have a complications and comorbidities score of 0. 119
-
Women with no complications would not receive any additional postnatal care.
-
The findings of the IPD meta-analysis were used to estimate the probability that infants in this arm would be admitted to the NICU. The findings of a study that reported average length of stay in the NICU by gestational age were used to estimate the length of stay for infants who were born preterm and at term. 119
Clinical data
The primary focus of the economic evaluation was the effect of the intervention on adverse maternal and fetal outcomes. The maternal outcomes included the development of three pregnancy-related conditions (PE, GDM and PIH), as well as outcomes relating to the timing and mode of delivery (preterm delivery and Caesarean section). Fetal outcomes included IUD, SGA, LGA and admission to the NICU.
Maternal outcomes
The estimates of baseline risk and the effect of the intervention on the development of pregnancy-related conditions were drawn from the IPD meta-analysis. This is shown in Table 21. For the intervention effect, data from the IPD meta-analysis were used to estimate pooled effect ORs for the development of PE, GDM and PIH. A two-stage process was used using a REML approach, and CIs were corrected using the Knapp–Hartung correction. The baseline risk for the usual-care group was based on pooled data for the control groups included in the trials. Maternal outcomes were not considered when they were already observed at baseline, that is, we did not count the presence of GDM in women who had DM or GDM at baseline and we did not count PIH in women who had PIH at baseline. The estimate of the risk of second semester miscarriage was based on a review article that summarised evidence on the epidemiology of miscarriage. 122 Data on the risk of maternal death were drawn from the findings of the confidential enquiry into maternal mortality and morbidity in the UK and Ireland 2009–12. 123
| Description | Base-case value | Distribution and values | Source |
|---|---|---|---|
| Baseline risk of PE | 0.05 | Beta(199, 3786) | IPD |
| Baseline risk of GDM | 0.114 | Beta(542, 4205) | IPD |
| Baseline risk of PIH | 0.043 | Beta(187, 4133) | IPD |
| Baseline risk of no complications | 0.793 | Remainder from above | IPD |
| OR for PE | 0.99 | Log-normal(0.79 to 1.24) | IPD |
| OR for GDM | 0.89 | Log-normal(0.72 to 1.10) | IPD |
| OR for PIH | 0.93 | Log-normal(0.69 to 1.25) | IPD |
| Risk of second-trimester miscarriage | 0.01 | Beta(1, 99) | Regan and Rai122 |
| Risk of maternal death | 0.00010 | Beta(10, 99,990) | Knight et al.123 |
The IPD meta-analysis also considered maternal outcomes that related to the timing and type of delivery (Table 22). This included preterm delivery, normal delivery, assisted delivery and Caesarean section. Delivery-related outcomes were estimated for all women with a pregnancy-related condition and for women with no condition (irrespective of whether they received the intervention or care as usual). Women with no complications were defined as those not reported as having PE, GDM or PIH, with at least one of these conditions being reported in the trial. Women were excluded from the analysis if missing data for all of these three outcomes were missing.
| Description | Base-case value | Distribution and values | Source |
|---|---|---|---|
| Preterm delivery in women with PE | 0.234 | Beta(92, 302) | IPD |
| Preterm delivery in women with GDM | 0.076 | Beta(82, 1003) | IPD |
| Preterm delivery in women with PIH | 0.049 | Beta(19, 366) | IPD |
| Preterm delivery in women with no complications | 0.104 | Beta(193, 1671) | IPD |
| Caesarean section in women with PE | 0.46 | Beta(180, 211) | IPD |
| Assisted delivery in women with PE | 0.11 | Beta(43, 348) | IPD |
| Normal delivery in women with PE | 0.43 | Remainder from above | IPD |
| Caesarean section in women with GDM | 0.344 | Beta(373, 711) | IPD |
| Assisted delivery in women with GDM | 0.111 | Beta(120, 964) | IPD |
| Normal delivery in women with GDM | 0.545 | Remainder from above | IPD |
| Caesarean section in women with PIH | 0.279 | Beta(107, 276) | IPD |
| Assisted delivery in women with PIH | 0.154 | Beta(59, 324) | IPD |
| Normal delivery in women with PIH | 0.567 | Remainder from above | IPD |
| Caesarean section in women with no complications | 0.355 | Beta(660, 1198) | IPD |
| Assisted delivery in women with no complications | 0.119 | Beta(222, 1636) | IPD |
| Normal delivery in women with no complications | 0.525 | Remainder from above | IPD |
Fetal outcomes
Data from the IPD were used to estimate fetal and infant outcomes for all women with a pregnancy-related condition and for women with no condition recorded (irrespective of whether they received the intervention or care as usual). Women were defined as having no pregnancy-related condition if they were not reported as having PE, GDM or PIH, with at least one of these conditions being reported in the trial. Women were excluded from the analysis if data were missing for all of these three outcomes. Table 23 shows fetal outcomes for women by pregnancy-related condition/no condition.
| Description | Base-case value | Distribution and values | Source |
|---|---|---|---|
| Intrauterine infant death: women with PE | 0.03 | Beta(1, 358) | IPD |
| Intrauterine infant death: women with GDM | 0.02 | Beta(2, 888) | IPD |
| Intrauterine infant death: women with PIH | 0.03 | Beta(1, 332) | IPD |
| Intrauterine infant death: women with no complications | 0.03 | Beta(4, 1578) | IPD |
| LGA: women with PE | 0.096 | Beta(38, 356) | IPD |
| LGA: women with GDM | 0.156 | Beta(171, 928) | IPD |
| LGA: women with PIH | 0.109 | Beta(42, 344) | IPD |
| LGA: women with no complications | 0.134 | Beta(251, 1628) | IPD |
| SGA: women with PE | 0.193 | Beta(76, 318) | IPD |
| SGA: women with GDM | 0.091 | Beta(98, 984) | IPD |
| SGA: women with PIH | 0.123 | Beta(47, 336) | IPD |
| SGA: women with no complications | 0.119 | Beta(221, 1638) | IPD |
| Infant admission to the NICU: women with PE | 0.195 | Beta(71, 294) | IPD |
| Infant admission to the NICU: women with GDM | 0.115 | Beta(106, 814) | IPD |
| Infant admission to the NICU: women with PIH | 0.057 | Beta(19, 312) | IPD |
| Infant admission to the NICU: women with no complications | 0.121 | Beta(196, 1420) | IPD |
Cost data
The cost components of the decision model were parameterised with data from NHS reference costs 2013/1497 and other secondary sources. Costs were calculated in 2013–14 GBP. Costs from secondary sources were inflated to 2013–14 prices using the hospital and community health services pay and prices index. 96 When necessary, costs were converted to GBP using historical annual average rates124 and then inflated to 2013–14 prices.
The estimate of the cost of the weight management intervention was based on the results of a systematic review of economic evaluations of weight management interventions in pregnancy that was conducted for this report (see Appendix 10). The review identified 11 studies that were concerned with the cost-effectiveness of weight management interventions during pregnancy. 68,101,125–133 Four of these involved women who already had DM or GDM and hence the intervention costs included additional monitoring and medication. 125,130,131,133 Two did not report a cost for an intervention. 101,126 Data on the cost of the intervention were thus extracted for five studies68,127–129,132 and the median value was used to estimate the cost of the intervention in the model (Table 24).
| Description | Base-case value (£) | Distribution and values | Source |
|---|---|---|---|
| Intervention to manage weight gain in pregnancy | 217 | Gamma(1, 413) | Calculated valuea |
| Cost of antenatal care: women with PE | 2054 | Gamma(1, 2054) | van Baaren et al.113 |
| Cost of antenatal care: women with GDM | 1022 | Gamma(1, 1022) | NICE99 |
| Cost of antenatal care: women with PIH | 974 | Gamma(1, 974) | Vijgen et al.117 |
| Cost of preterm delivery, no complications (NZ17B, CC score of 0–1) | 642 | Gamma(1, 642) | Department of Health97 |
| Cost of preterm delivery, with complications (NZ17A, CC score of ≥ 2) | 946 | Gamma(1, 946) | Department of Health97 |
| Cost of normal delivery, with no complications (NZ30C, CC score of 0) | 1461 | Gamma(1, 1461) | Department of Health97 |
| Cost of normal delivery, with minor complications (NZ30B, CC score of 1) | 1623 | Gamma(1, 1623) | Department of Health97 |
| Cost of normal delivery, with major complications (NZ30A, CC score of ≥ 2) | 1892 | Gamma(1, 1892) | Department of Health97 |
| Cost of assisted delivery, without complications (NZ40C, CC score of 0) | 1860 | Gamma(1, 1860) | Department of Health97 |
| Cost of assisted delivery, with minor complications (NZ40C, CC score of 1 or 2) | 2153 | Gamma(1, 2153) | Department of Health97 |
| Cost of assisted delivery, with major complications (NZ40A, CC score of 2) | 2625 | Gamma(1, 2625) | Department of Health97 |
| Cost of Caesarean section, without complications (NZ40C, CC score of 0 or 1) | 3363 | Gamma(1, 3363) | Department of Health97 |
| Cost of Caesarean section, with minor complications (NZ40C, CC score of 2 or 3) | 4059 | Gamma(1, 4059) | Department of Health97 |
| Intensive care (XC04Z, adult critical care, three organs supported) | 789 | Gamma(1, 789) | Department of Health97 |
| Cost of neonatal critical care: intensive care | 1118 | Gamma(1, 1118) | Department of Health97 |
| Cost of postnatal care: women with PE | 4987 | Gamma(1, 4987) | van Baaren et al.113 |
| Cost of postnatal care: women with GDM | 1899 | Gamma(1, 1899) | Kolu et al.120 |
| Cost of postnatal care: women with PIH | 1814 | Gamma(1, 1814) | Vijgen et al.117 |
| Additional cost of IUD (core-recommended investigations and care immediately following miscarriage) | 1242 | Gamma(1, 1242) | Mistry et al.110 |
Estimates of antenatal and postnatal care costs were drawn from systematic reviews of the literature (as described in the previous section on assumptions). Delivery costs were based on national average costs in the UK. 97
Analysis
The decision-analytic model was constructed to compare the cost-effectiveness of diet- and physical activity-based interventions in pregnancy with usual care. Two separate economic analyses were conducted. The main analysis compared costs and outcomes for a hypothetical cohort of 10,000 pregnant women, based on the results of the IPD meta-analysis for all women. The secondary analysis compared costs and outcomes for three subgroups of women based on their pre-pregnancy BMI classification. Women were classified as normal weight (pre-pregnancy BMI of < 25 kg/m2), overweight (pre-pregnancy BMI 25–29.9 kg/m2) or obese (pre-pregnancy BMI of ≥ 30 kg/m2). This allowed an exploration of whether or not a weight management intervention in selective subgroups of women is a more cost-effective strategy than care as usual.
For both the primary and secondary analyses, the relative cost-effectiveness of the intervention was evaluated using effect size estimates from the IPD meta-analysis. An incremental approach was adopted, with a focus on the additional costs and benefits associated with a move from care as usual to diet and lifestyle interventions to manage weight gain in pregnancy. The results were reported in terms of an incremental cost-effectiveness ratio (ICER) of cost per unit benefit gained, measured in clinical outcomes. The IPD meta-analysis was based on a composite outcome, which included maternal and fetal complications. An economic evaluation based on such a composite outcome would not be meaningful, as the full extent of the cost implications based on such an outcome would be lost. The economic analysis therefore examined cost-effectiveness for each of the primary outcomes separately; for example, results are presented in terms of cost per case of PE avoided, cost per case of GDM avoided, etc. Results are also presented in terms of cost per major outcome avoided. The major outcome was predefined to include one or more of the fetal and maternal outcomes included in the composite measure that was used in the IPD meta-analysis.
The analysis was conducted from the perspective of the health service (NHS) and only direct health service costs were included. The time horizon adopted for both the primary and secondary analyses was from the start of pregnancy until the mother and infant were discharged from hospital following the birth. This was considered appropriate to reflect all key differences in terms of costs and benefits for the options compared, given the time horizon adopted in the trials included in the IPD meta-analysis. The effect of considering a longer time horizon was explored as part of the sensitivity analysis.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were conducted to explore the effects of the inherent uncertainty in the parameter estimates on the results produced by the model. 98 Deterministic sensitivity analysis involves varying one or more parameters while keeping the others at their baseline value. Such analysis can help to identify which model inputs are important in leading to a particular decision from the model, and can help to identify threshold values. A probabilistic sensitivity analysis (PSA) was also undertaken to allow uncertainty to be represented more comprehensively. A PSA involves varying all parameters simultaneously, and multiple sets of parameter values are sampled from defined probability distributions. 134 Monte Carlo simulation was used to sample from these distributions to allow the effect of parameter uncertainty to be evaluated. This involved 1000 repeated random draws from the distributions to indicate how variation in the model parameters would affect the results and hence illustrate the decision uncertainty. 135 Beta distributions were used for binomial data, log-normal distributions for ORs and gamma distributions for costs. 98 Ideally, when there are more than two possibilities at a chance node, a Dirichlet distribution would be applied. But to populate a Dirichlet distribution it is necessary that all included studies have reported data for all the branches from the appropriate chance node. This could be done if all data sources reported results for all possible branches. However, in this case, some studies did not report numbers for all branches in the model. For example, the number of LGA infants born to mothers with GDM was reported in 27 studies, but the numbers of SGA infants was reported in only 26 studies. Therefore, it is feasible to assign separate beta distributions only for the probabilities of each of these two outcomes. For consistency, we considered it appropriate to apply beta distributions throughout. The potential limitation of applying a beta distribution is that some correlation between parameters could be lost. However, in this study, this is unlikely to have had an impact on results.
Using the net monetary benefit for each of the 1000 simulations, the proportion of times the intervention had the highest net monetary benefit was calculated for a range of threshold values for the maximum willingness to pay for a major outcome averted. These values were summarised graphically using a cost-effectiveness acceptability curve (CEAC) to show the uncertainty surrounding the cost-effectiveness of the intervention, for a range of thresholds for cost-effectiveness. A value of information analysis was also conducted to estimate the expected costs of uncertainty. The expected cost of uncertainty is calculated by estimating the probability of making a wrong decision based on existing evidence and the consequences of this wrong decision. The expected value of perfect information (EVPI) estimates the difference between the expected value of the decision made with perfect information and the decision made on the basis of existing evidence. 98 EVPI was calculated based on the methods described in Claxton and Posnett. 136
Deterministic sensitivity analysis
A range of deterministic sensitivity analyses was carried out for the primary and secondary analyses. Both univariate and multivariate analyses were undertaken to assess how uncertainty around the parameters used in the model would impact on the results achieved. Univariate sensitivity analysis involves varying input values one at a time across a plausible range while holding the remaining values at their baseline values, while multivariate analysis involves varying two or more input values simultaneously. 136 Six univariate and two multivariate analyses were undertaken based on the following justifications.
Univariate analyses
-
The cost of the intervention was varied to reflect the maximum and minimum costs identified in the literature review (£136 and £1023, respectively). The base-case value was estimated based on the median cost reported in the identified studies and these costs were varied to examine the impact on results.
-
The effect of the intervention in increasing or reducing the odds of developing pregnancy-related conditions was varied using 95% CIs for pregnancy-related conditions: PE, 95% CI 0.79 to 1.24; GDM, 95% CI 0.72 to 110; and PIH, 95% CI 0.69 to 1.25.
-
The rate of preterm delivery and Caesarean section varied for each condition separately using 95% CIs calculated using an exact method. 137,138 The rate of preterm delivery varied for women with PE from 19.3% to 27.8%, for those with GDM from 6.1% to 9.3% and for women with PIH from 3% to 7.6%. The rate of Caesarean section varied from 41% to 51.1% for women with PE, from 31.6% to 37.3% for those with GDM and from 23.5% to 32.7% for those with PIH.
-
The costs associated with health care were increased and decreased for each condition. The costs of care before and after the birth were varied drawing on the results of the literature review. The total costs of care for women with PE varied from £4476104 to £12,052 (this was based on the highest estimate of the costs of PE identified, with a 15% increase102). The costs of delivery and postnatal care for women with PIH varied from £2988104 to £5530 (the higher cost included follow-up care117). The total costs associated with care for women with GDM varied between £3105130 (estimate for women with mild GDM) and £8753. 131
-
The costs of delivery were varied using the upper- and lower-quartile costs reported for elective and non-elective deliveries reported in the National Schedule of Reference Costs: The Main Schedule. 97 Thus, the costs of Caesarean section with a complications and comorbidities score of 0 or 1 varied from £1818 (planned elective delivery, lower-quartile cost) to £4289 (emergency delivery, non-elective patient, upper-quartile cost). The cost of a Caesarean section with a complications and comorbidities score of ≥ 2 varied from £2085 (planned elective delivery, lower-quartile cost) to £4289 (emergency delivery, non-elective patient, upper-quartile cost). For assisted deliveries, the costs of delivery varied for deliveries with no complications (£960–2721, reflecting non-elective short-stay lower-quartile costs and long-stay upper-quartile costs, respectively), a complications score of 1 (£1033–3050, for non-elective short-stay and long-stay costs, respectively) and a complications and comorbidities score of 2 (£1132–3604, non-elective short-stay and long-stay costs, respectively). For normal deliveries, similar variations in costs were explored; costs were varied for deliveries with no complications (£854–2688, reflecting non-elective short-stay lower-quartile costs and long-stay upper-quartile costs, respectively), deliveries with a complications and comorbidities score of 1 (£898–2968, for non-elective short-stay and long-stay costs, respectively) and develieries with a complication score of 2 (£957–3349, non-elective short-stay and long-stay costs, respectively).
-
Increasing the costs of IUD. The costs associated with IUD were increased using estimates provided in a published review of the literature. 110 The costs of IUD were increased to £1804 to reflect the increased costs associated with comprehensive investigations.
Multivariate analyses
Multivariate analyses were undertaken to reflect the fact that a change in the clinical effectiveness of the intervention is likely to affect more than one outcome measure. Hence, multiple parameters were varied simultaneously to examine the impact on the results:
-
Varying the effectiveness for all pregnancy-related conditions. For this analysis, CIs were used to examine the impact on cost-effectiveness using the highest and lowest estimates of effect.
-
Increasing and decreasing the cost of pregnancy-related conditions. All costs associated with pregnancy-related conditions were increased and decreased simultaneously using the estimates from the literature.
Results
Primary analysis
The results of the primary analysis are reported in Tables 25 and 26. Care as usual was the least costly arm, with the average cost estimated at £3242 (excluding routine antenatal care). However, the intervention arm was only slightly more expensive, with average costs estimated at £3390 (excluding routine antenatal care). In the base-case model (using point estimates) there was a reduction in pregnancy-related complications in the intervention arm (see Table 26). Overall, there were 55 fewer women who experienced any major outcome in the intervention arm, five fewer cases of PE, 113 fewer cases of GDM and 29 fewer women who developed PIH. No reduction was found in other outcomes such as preterm delivery, Caesarean section, IUD, LGA and NICU admission for the intervention arm. The ICERs indicated that there was an additional cost of around £306,000 for each case of PE avoided, £13,000 for each case of GDM avoided and £27,000 for each woman with a major outcome avoided. This means that, if for example, the outcome of interest is the reduction in cases of PE, an additional £306,000 is required for each additional woman who avoids the development of PE compared with usual care. These results taken in isolation would suggest that the intervention would be preferred at any willingness to pay for a case of PE avoided above £306,000.
| Group allocation | Total cost (point estimate)a | Difference in costs |
|---|---|---|
| Intervention | 3390 | 147 |
| No intervention | 3242 |
| Outcome | Number of women experiencing complications | Number of complications avoided per 10,000 women: outcome averted (point estimate) | Cost (£) per complication avoideda | |
|---|---|---|---|---|
| Intervention (point estimate) | No intervention (point estimate) | |||
| Any major outcome | 6981 | 7036 | 55 | 27,000 |
| PE | 495 | 499 | 5 | 306,000 |
| GDM | 1029 | 1142 | 113 | 13,000 |
| PIH | 398 | 426 | 29 | 51,000 |
| Preterm delivery | 1037 | 1033 | –4 | b |
| Caesarean section | 3526 | 3523 | –3 | b |
| IUD | 25 | 25 | 0 | b |
| LGA | 1608 | 1605 | –4 | b |
| SGA | 888 | 891 | 3 | |
| NICU admission | 1202 | 1200 | –2 | b |
These results need to be viewed together with those from the PSA (Tables 27 and 28). The PSA results show the modelled uncertainty in the cost and effectiveness between the intervention arm and the care-as-usual arm, from 1000 Monte Carlo simulations. These results show that it is uncertain whether the intervention is more effective than usual care or less effective (with respect to all the outcome measures) and whether the intervention is more costly or less expensive than the alternative. Hence, the most reasonable interpretation is that there is no significant difference between the intervention and control arm results for either cost or clinical effectiveness. This is further illustrated by Figure 12. The graph plots the result of each simulation on the cost-effectiveness plane, which gives information about the joint density of differences in cost and effectiveness between the two model arms. It is evident that the data in the scatterplot go into all quadrants of the incremental cost-effectiveness plane and, therefore, it should be assumed that there is no significant difference between the intervention and control arms for either cost or clinical effectiveness. This was the case for all of the outcome measures (for more details see Appendix 11). The CEAC (Figure 13) shows that the probability that the intervention is cost-effective increases as the willingness to pay for a major outcome averted increases. Thus, if the maximum willingness to pay for a major outcome averted for all pregnant women was £30,000, then the probability that the intervention was cost-effective would be 0.55.
| Group allocation | Cost (95% CI) | |
|---|---|---|
| Mean | Difference | |
| Intervention | 3457 (1651 to 6408) | 151 (–247 to 754) |
| No interventionb | 3306 (1432 to 6088) | |
| Outcome | Number of women experiencing complications, mean (95% CI) | Number of complications avoided per 10,000 women (95% CI) | |
|---|---|---|---|
| Intervention | No intervention | ||
| Any major outcome | 6984 (6758 to 7202) | 7036 (6875 to 7209) | 52 (–88 to 172) |
| PE | 496 (381 to 635) | 498 (432 to 567) | 3 (–116 to 103) |
| GDM | 1032 (825 to 1278) | 1142 (1050 to 1230) | 109 (–117 to 307) |
| PIH | 401 (289 to 547) | 426 (369 to 491) | 25 (–100 to 134) |
| Preterm delivery | 1036 (921 to 1153) | 1032 (923 to 1150) | –4 (–15 to 7) |
| Caesarean section | 3529 (3329 to 3718) | 3527 (3336 to 3714) | –3 (–17 to 13) |
| IUD | 25 (9 to 52) | 25 (9 to 51) | 0 (–1 to 1) |
| LGA | 1608 (1474 to 1742) | 1605 (1471 to 1739) | –3 (–18 to 12) |
| SGA | 889 (800 to 975) | 891 (808 to 975) | 2 (–14 to 18) |
| NICU admission | 1201 (1073 to 1333) | 1199 (1074 to 1328) | –2 (–13 to 10) |
FIGURE 12.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for pregnant women: major outcome averted. The mean of the distribution is highlighted.

FIGURE 13.
Incremental CEAC of intervention for pregnant women: major outcome averted.

The analysis of the EVPI demonstrates that at a willingness-to-pay threshold of £30,000 to avert a major outcome, the expected value of resolving the uncertainty around the decision is £142 per patient to whom the decision would apply (Figure 14). Thus, for a cohort of 10,000 women, the EVPI would be £1,420,000.
FIGURE 14.
Expected value of perfect information: major outcome averted.

Secondary analysis
Obese women
The results of the secondary analysis for obese women are reported in Tables 29 and 30. Care as usual was the least costly arm for obese women, with an average cost estimated at £3428 (excluding routine antenatal care). However, the intervention arm was only slightly more expensive, with average costs estimated at £3613 (excluding routine antenatal care). In the base-case model (using point estimates) there were 21 fewer obese women who experienced any major outcome in the intervention arm and 125 fewer cases of GDM. No reduction was found in other outcomes such as the development of PE, PIH and rates of preterm delivery, Caesarean section, IUD, LGA and NICU admission. The ICERs indicated that there was an additional cost of around £88,000 for each major outcome avoided and £15,000 for each case of GDM avoided. The PSA results show the modelled uncertainty in the cost and clinical effectiveness between the intervention arm and the care-as-usual arm and demonstrate that it is uncertain whether the intervention is more or less effective than usual care and whether the intervention is more or less costly than the alternative (Tables 31 and 32). These results suggest that there is no significant difference between the intervention and the control arm results for either cost or clinical effectiveness for obese women. This is further illustrated by Figure 15, which plots the result of each simulation on the cost-effectiveness plane and shows that there is no significant difference between the intervention and control arms for either cost or clinical effectiveness for all outcome measures (for more details see Appendix 11). The CEAC (Figure 16) shows if the maximum willingness to pay for a major outcome averted for obese pregnant women was £120,000, then the probability that the intervention was cost-effective would be 0.52.
| Group allocation | Cost | |
|---|---|---|
| Mean (point estimate) | Difference | |
| Intervention | 3613 | 185 |
| No interventiona | 3428 | |
| Outcome | Number of women experiencing complications (point estimate) | Number of complications avoided per 10,000 women: outcome averted (point estimate) | Cost (£) per complication avoideda | |
|---|---|---|---|---|
| Intervention | No intervention | |||
| Any major outcome | 7248 | 7269 | 21 | 88,000 |
| PE | 550 | 530 | –20 | b |
| GDM | 1512 | 1637 | 125 | 15,000 |
| PIH | 562 | 514 | –48 | b |
| Preterm delivery | 1021 | 1018 | –3 | b |
| Caesarean section | 3514 | 3514 | 0 | b |
| IUD | 25 | 25 | 0 | b |
| LGA | 1587 | 1589 | 3 | 712,000 |
| SGA | 905 | 902 | –3 | b |
| NICU admission | 1192 | 1193 | 1 | 2,057,000 |
| Group allocation | Cost (95% CI) | |
|---|---|---|
| Mean | Difference | |
| Intervention | 3675 (1794 to 6321) | 191 (–247 to 809) |
| No interventionb | 3484 (1608 to 6129) | |
| Outcome | Number of women experiencing complications, mean (95% CI) | Number of complications avoided per 10,000 women (95% CI) | |
|---|---|---|---|
| Intervention | No intervention | ||
| Any major outcome | 7252 (6992 to 7524) | 7268 (7108 to 7444) | 16 (–194 to 197) |
| PE | 553 (396 to 750) | 529 (437 to 630) | –24 (–189 to 108) |
| GDM | 1517 (1164 to 1940) | 1636 (1482 to 1786) | 119 (–262 to 445) |
| PIH | 564 (403 to 778) | 513 (420 to 615) | –51 (–223 to 95) |
| Preterm delivery | 1021 (912 to 1130) | 1018 (911 to 1125) | –3 (–17 to 10) |
| Caesarean section | 3518 (3329 to 3700) | 3518 (3336 to 3699) | 1 (–19 to 21) |
| IUD | 25 (10 to 50) | 25 (10 to 49) | 0 (–1 to 1) |
| LGA | 1587 (1463 to 1716) | 1590 (1463 to 1720) | 3 (–17 to 25) |
| SGA | 906 (820 to 993) | 903 (822 to 985) | –4 (–27 to 16) |
| NICU admission | 1192 (1070 to 1316) | 1193 (1076 to 1319) | 1 (–15 to 16) |
FIGURE 15.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for obese pregnant women: major outcome averted. The mean of the distribution is highlighted.

FIGURE 16.
Incremental CEAC of intervention for obese pregnant women: major outcome averted.
Overweight women
The results of the secondary analysis for overweight women are reported in Tables 33 and 34. As for obese women, care as usual had the lowest cost, with the average cost estimated at £3114 (excluding routine antenatal care). The intervention arm was slightly more expensive, with average costs estimated at £3326 (excluding routine antenatal care). In the base-case model (using point estimates) there were 115 fewer overweight women who developed PIH (see Table 34). However, for all other clinical outcomes, the intervention did not lead to a reduction in adverse outcomes. The PSA results allow an assessment of the uncertainty in the estimates of cost and clinical effectiveness between the intervention arm and the care-as-usual arm (Tables 35 and 36). It is evident that it is uncertain whether the intervention is more or less effective than usual care and whether the costs associated with the intervention arm are greater or lower than the alternative. Hence, there is no significant difference between the intervention and control arm results for either cost or effectiveness for overweight women. This is further illustrated by Figure 17, which plots the result of each simulation on the cost-effectiveness plane and shows that no significant differences were found between the intervention arm and control arm for either cost or effectiveness (for more details see Appendix 11). The CEAC (Figure 18) shows that, even if the maximum willingness to pay for a major outcome averted for overweight pregnant women was £500,000, then the probability that the intervention is cost-effective would be < 0.5.
| Group allocation | Cost | |
|---|---|---|
| Total (point estimate) | Difference | |
| Intervention | 3326 | 211 |
| No interventiona | 3114 | |
| Outcome | Number of women experiencing complications (point estimate) | Number of complications avoided per 10,000 women (point estimate) | Cost (£) per complication avoided | |
|---|---|---|---|---|
| Intervention | No intervention | |||
| Any major outcome | 6915 | 6903 | –11 | a |
| PE | 459 | 439 | –21 | a |
| GDM | 996 | 871 | –126 | a |
| PIH | 293 | 407 | 115 | 18,000 |
| Preterm delivery | 1039 | 1034 | –5 | a |
| Caesarean section | 3531 | 3521 | –10 | a |
| IUD | 25 | 25 | 0 | a |
| LGA | 1618 | 1614 | –4 | a |
| SGA | 879 | 881 | 1 | 1,621,000 |
| NICU admission | 1206 | 1198 | –8 | a |
| Group allocation | Cost (95% CI) | |
|---|---|---|
| Mean | Difference | |
| Intervention | 3399 (1546 to 6419) | 231 (–279 to 980) |
| No interventionb | 3169 (1334 to 6002) | |
| Outcome | Number of women experiencing complications, mean (95% CI) | Number of complications avoided per 10,000 women (95% CI) | |
|---|---|---|---|
| Intervention | No intervention | ||
| Any major outcome | 6930 (6613 to 7318) | 6902 (6720 to 7095) | –28 (–351 to 206) |
| PE | 472 (248 to 800) | 438 (324 to 569) | –34 (–325 to 158) |
| GDM | 1019 (581 to 1624) | 871 (712 to 1031) | –148 (–703 to 257) |
| PIH | 299 (159 to 530) | 405 (299 to 530) | 107 (–72 to 265) |
| Preterm delivery | 1039 (921 to 1159) | 1033 (915 to 1150) | –6 (–31 to 12) |
| Caesarean section | 3535 (3328 to 3729) | 3525 (3328 to 3714) | –10 (–43 to 14) |
| IUD | 25 (9 to 52) | 26 (9 to 53) | 0 (–2 to 2) |
| LGA | 1616 (1482 to 1759) | 1614 (1475 to 1754) | –2 (–25 to 32) |
| SGA | 882 (785 to 974) | 882 (796 to 970) | 0 (–40 to 27) |
| NICU admission | 1206 (1073 to 1341) | 1197 (1068 to 1332) | –9 (–33 to 9) |
FIGURE 17.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for overweight pregnant women: major outcome averted. The mean of the distribution is highlighted.
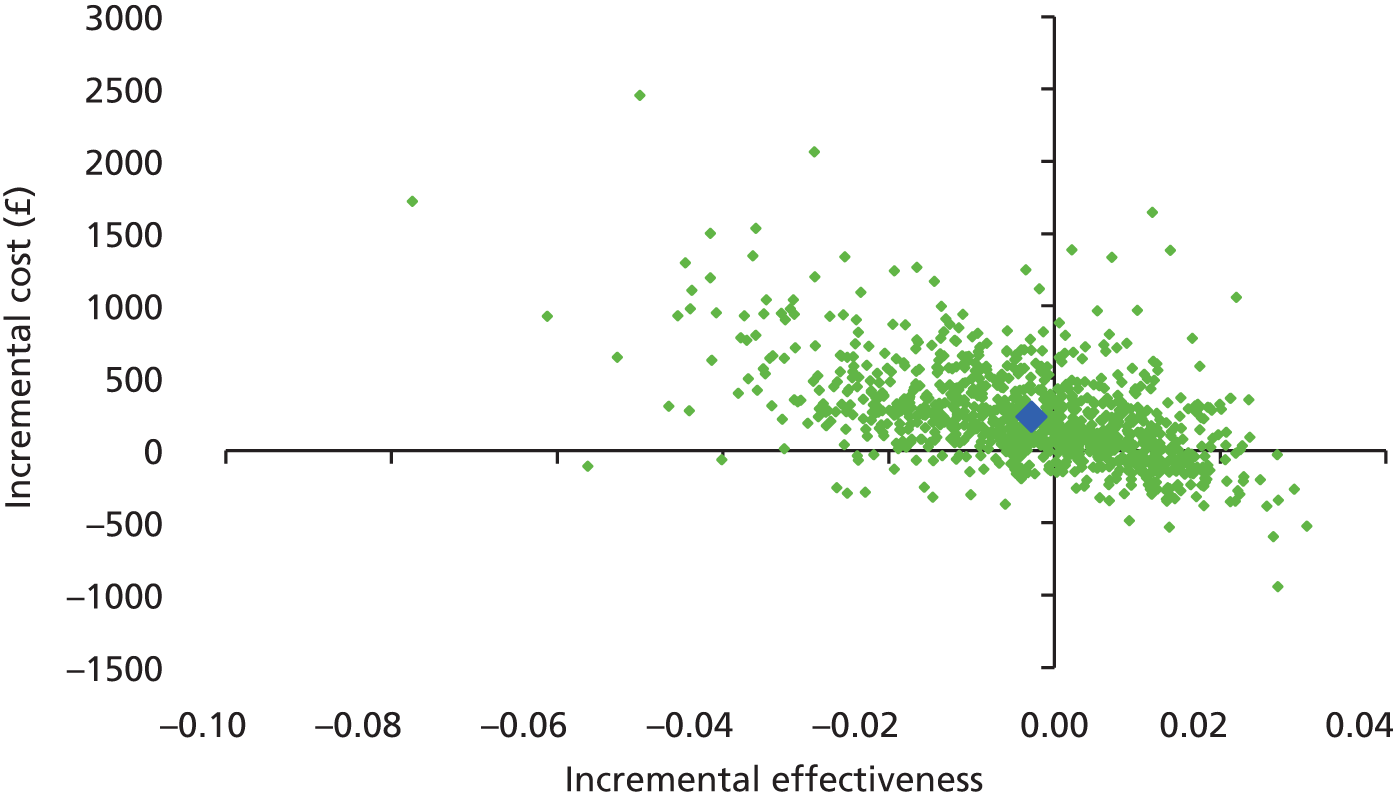
FIGURE 18.
Incremental CEAC of intervention for overweight pregnant women: major outcome averted.

Normal weight women
The results of the secondary analysis for normal weight women are reported in Tables 37 and 38. The intervention arm had the lowest costs, with an average cost estimated at £3056 (excluding routine antenatal care). This was because the additional costs associated with delivering the intervention were outweighed by savings associated with reduced health-care use overall. However, the care-as-usual arm was only slightly more expensive, with average costs estimated at £3063 (excluding routine antenatal care). In the base-case model (using point estimates) there were 108 fewer women who experienced any major outcome in the intervention arm, 231 fewer cases of PE and 53 fewer women who developed GDM. There were also reductions in the rate of preterm delivery, Caesarean section, IUD, SGA, and NICU admission.
| Group allocation | Cost | |
|---|---|---|
| Mean (point estimate) | Difference | |
| Intervention | 3056 | –7 |
| No interventiona | 3063 | |
| Outcome | Number of women experiencing complications (point estimate) | Number of complications avoided per 10,000 women (point estimate) | Cost (£) per complication avoided | |
|---|---|---|---|---|
| Intervention | No interventiona | |||
| Any major outcome | 6677 | 6785 | 108 | b |
| PE | 275 | 506 | 231 | b |
| GDM | 573 | 626 | 53 | b |
| PIH | 274 | 274 | 0 | b |
| Preterm delivery | 1029 | 1056 | 28 | b |
| Caesarean section | 3518 | 3541 | 24 | b |
| IUD | 25 | 25 | 0 | b |
| LGA | 1639 | 1621 | –18 | c |
| SGA | 854 | 880 | 26 | b |
| NICU admission | 1197 | 1213 | 17 | b |
The results suggested that, for most clinical outcome measures reported, the intervention was less costly and more effective than care as usual. These results need to be considered alongside the results of the PSA (Tables 39 and 40). The PSA results show the modelled uncertainty in terms of the cost and the effectiveness for the intervention arm and the care-as-usual arms. The findings suggest that it is uncertain whether the intervention is more or less effective than usual care and whether the intervention costs are higher or lower than the costs associated with usual care. Hence, there is no significant difference between the intervention and the control arm results for either cost or effectiveness for women of normal weight. This is further illustrated by Figure 19, which plots the result of each simulation on the cost-effectiveness plane and shows that, although there is a trend towards lower cost and increased effectiveness for the intervention arm, there is no statistically significant difference between the intervention and the control arms for either cost or effectiveness (see more details in Appendix 11). The CEAC (Figure 20) shows that as the willingness to pay for a major outcome averted for normal weight women increases, the probability that the intervention is cost-effective also increases. Thus, if the threshold for cost-effectiveness was £30,000 to avert a major outcome for normal weight women, then the probability that the intervention was cost-effective would be 0.67.
| Outcome | Cost (95% CI) | |
|---|---|---|
| Mean | Difference | |
| Intervention | 3140 (1244 to 6264) | 22 (–563 to 741) |
| No interventionb | 3118 (1265 to 6041) | |
| Outcome | Number of women experiencing complications, mean (95% CI) | Number of complications avoided per 10,000 women (95% CI) | |
|---|---|---|---|
| Intervention | No intervention | ||
| Any major outcome | 6704 (6403 to 7068) | 6784 (6599 to 6972) | 80 (–240 to 286) |
| PE | 290 (121 to 580) | 505 (376 to 662) | 214 (–44 to 394) |
| GDM | 603 (275 to 1125) | 627 (504 to 757) | 24 (–466 to 340) |
| PIH | 299 (96 to 725) | 272 (186 to 379) | –27 (–421 to 195) |
| Preterm delivery | 1028 (908 to 1155) | 1056 (933 to 1179) | 28 (4 to 59) |
| Caesarean section | 3520 (3302 to 3722) | 3545 (3345 to 3736) | 24 (–5 to 62) |
| IUD | 26 (8 to 54) | 26 (9 to 53) | 0 (–2 to 2) |
| LGA | 1636 (1493 to 1780) | 1621 (1481 to 1760) | –15 (–43 to 24) |
| SGA | 858 (761 to 954) | 882 (793 to 972) | 23 (–13 to 54) |
| NICU admission | 1195 (1055 to 1337) | 1212 (1080 to 1352) | 17 (–6 to 50) |
FIGURE 19.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for normal weight pregnant women: major outcome averted. The mean of the distribution is highlighted.

FIGURE 20.
Incremental CEAC of intervention for normal weight pregnant women: major outcome averted.

Deterministic sensitivity analysis
As demonstrated in Table 41, the results of the deterministic sensitivity analysis were as follows: (1) varying the cost of the intervention affected the average cost of the intervention arm and the cost per major outcome avoided, with this rising to £170,000 per major outcome avoided for the highest intervention cost; (2) improving the effect of the intervention meant that the intervention arm was more cost-effective than the control arm, the model was particularly sensitive to the estimate of the effect of the intervention on the odds of developing of PE; (3) varying the timing and mode of delivery did not change the overall result for the development of pregnancy-related conditions; (4) increasing and decreasing the costs for each condition had some impact on the overall results; (5) varying the costs associated with various types of delivery had a negligible effect on the overall results; and (6) increasing the costs of IUD had a negligible impact on overall results. As expected, the multivariate analyses demonstrated that using the highest estimates of effectiveness meant that the intervention arm dominated the control arm (it was less costly and more effective), as the additional costs of the intervention were outweighed by lower health-care resource use.
| Scenarios | Value | Result | ||
|---|---|---|---|---|
| Original | Revised | Original | Revised | |
| Univariate analyses | ||||
| (1) Varying the costs of the intervention | £217 | £136–1023 | Cost of intervention arm: £3390 | Cost of intervention arm: £3309–4187 |
| Cost per major outcome avoided: £26,000 | Cost per major outcome avoided: £12,000–170,000 | |||
| (2) Changing the intervention effect [development of pregnancy-related conditions (OR)] | PE: 0.99 | PE: 0.79–1.24 | Cases of PE avoided: 5 | Cases of PE avoided: 101 to –112 |
| GDM: 0.89 | GDM: 0.72–1.10 | Cost per case of PE avoided: £306,000 | Cost per case of PE avoided: £7000a | |
| PIH: 0.93 | PIH: 0.37–2.73 | Cases of GDM avoided: 113 | Cases of GDM avoided: 293 to –100 | |
| Cost per case of GDM avoided: £13,000 | Cost per case of GDM avoided: £3000a | |||
| Cases of PIH avoided: 29 | Cases of PIH avoided: 128 to –101 | |||
| Cost per case of PIH avoided: £51,000 | Cost per case of PIH avoided: £9000a | |||
| (3) Varying the timing and mode of delivery | Preterm delivery:
|
Preterm delivery:
|
Cost per case of PE avoided: £306,000 | Unchanged |
| Cost per case of GDM avoided: £13,000 | ||||
Caesarean section:
|
Caesarean section:
|
Cost per case of PIH avoided £51,000 | ||
| (4) Increasing and decreasing the costs for each condition | Varies according to pathway | PE: £4476–12,052 | Cost per case of PE avoided: £306,000 | Cost per case of PE avoided: £312,000–305,000 |
| GDM: £3105–8753 | Cost per case of GDM avoided: £13,000 | Cost per case of GDM avoided: £15,000–10,000 | ||
| PIH: £2988–5530 | Cost per case of PIH avoided £51,000 | Cost per case of PIH avoided £53,000–51,000 | ||
| (5) Varying the costs associated with various types of delivery | Caesarean section (CC score of 0 or 1): £3363 | Caesarean section (CC score of 0–1): £1818–4289 | Cost per major outcome avoided: £27,000 | Cost per major outcome avoided: £26,000–27,000 |
| Caesarean section (CC score of 2 or 3): £4059 | Caesarean section (CC score of 2–3): £2085–4289 | |||
| Normal delivery (CC score of 0): 1461 | Normal delivery (CC score of 0): 854–2688 | |||
| Normal delivery (CC score of 1): 1623 | Normal delivery (CC score of 1): 898–2968 | |||
| Normal delivery (CC score of 2): 1892 | Normal delivery (CC score of 2): 957–3349 | |||
| (6) Increasing the costs of IUD | IUD cost: £1242 | IUD cost: £1804 | Cost per major outcome avoided: £27,000 | Unchanged |
| Multivariate analyses | ||||
| (1) Varying estimates of effect simultaneously | PE: 0.99 | PE: 0.79–1.24 | Cost per case of PE avoided: £306,000 | Intervention is cost saving with highest estimates of effect and dominated using lowest estimates of effect |
| GDM: 0.89 | GDM: 0.72–1.10 | Cost per case of GDM avoided: £13,000 | ||
| PIH: 0.93 | PIH: 0.37–2.73 | Cost per case of PIH avoided: £51,000 | ||
| (2) Varying estimates of cost simultaneously | Varies according to pathway | PE: £4476–12,052 | Cost per case of PE avoided: £306,000 | Cost per case of PE avoided: £219,000–374,000 |
| GDM: £3105–8753 | Cost per case of GDM avoided: £13,000 | Cost per case of GDM avoided: £9000–16,000 | ||
| PIH: £2988–5530 | Cost per case of PIH avoided: £51,000 | Cost per case of PIH avoided: £37,000–63,000 | ||
Discussion
Principal findings
The results of this analysis suggest that there is no evidence that mixed interventions in pregnancy are cost-effective compared with care as usual. Although the primary base-case analysis indicated a small reduction in pregnancy-related complications, the PSA demonstrated that there is no evidence of a significant difference between the intervention and the control arms for either cost or clinical effectiveness. Similarly, the results of the secondary analysis suggested that for obese, overweight and normal weight women it is uncertain whether or not the intervention is more clinically effective than usual care (with respect to all the outcome measures) and whether or not the intervention is more costly than the alternative. The results of the deterministic sensitivity analyses demonstrated that the results were particularly sensitive to the estimates of the treatment effect in terms of the odds of developing PE.
Strengths and limitations of the economic study
The strength of this model-based economic evaluation is that the effect of interventions to manage weight gain in pregnancy was estimated via an IPD meta-analysis, involving data relating to 17,727 women and 30 studies. Furthermore, the resource use data were collected via a series of systematic reviews to identify studies that collected primary cost data. These reviews involved wide and detailed search and inclusion strategies. In addition, the study benefited from significant clinical input throughout its design and development. This study contributes to an area in which there is a paucity of economic studies. 101 The current public health emphasis on obesity and healthy lifestyles139 highlights the importance of contributions to understanding the costs and benefits of interventions in this area.
There were also some weaknesses. First, limited evidence was available about the resource use associated with some conditions in pregnancy. Although resource use data from high-quality RCTs were used to inform the model, the paucity of studies available limited the comparisons that could be undertaken to examine variations in costs for different groups of patients. A second limitation was that the study included a wide range of studies with different kinds of intervention models. This meant that it was difficult to estimate the costs associated with the intervention precisely; instead a median value was used, based on the findings of a systematic review. The cost of the intervention varied widely in the sensitivity analysis to account for the diversity of intervention types included in the IPD meta-analysis. In addition, for some outcomes, such as admission to the NICU of an infant whose mother did not have a pregnancy-related complication, it was difficult to obtain robust estimates of resource use. To address this limitation, the sensitivity analysis explored a wide range of plausible values for the costs associated with pregnancy-related conditions. A further limitation of the study is that outcomes were expressed in terms of clinical effectiveness rather than in terms that would allow comparison across programme areas, such as quality-adjusted life-years (QALYs). The absence of robust QALY data for women who are experiencing pregnancy-related conditions101,136 meant that a full cost–utility analysis incorporating QALYs was not possible. This means that some of the results are difficult to interpret as no willingness-to-pay threshold exists for individual clinical outcomes. As the results of the sensitivity analysis demonstrated that there was no significant difference between the intervention and the control arms results for either costs or clinical effectiveness, longer-term economic modelling was not undertaken. Finally, the economic evaluation was based on the results of the IPD meta-analysis. The findings may have been different if the evaluation had been based on the results of the meta-analysis using aggregated data (as these showed a statistically significant reduction for some outcomes). Further work could be undertaken to explore the cost-effectiveness of diet- and physical activity-based interventions using data from the aggregate meta-analyses, as this was beyond the scope of the current project.
Comparison with other studies
A limited number of studies were identified that were concerned with the costs and benefits of interventions to promote weight management during pregnancy. Only one study was identified that concluded that such an intervention was cost-effective. Dodd et al. 128 concluded that an intervention involving a lifestyle advice service was likely to be cost-effective using a monetary value of AUD 20,000 as a threshold for avoiding additional infants with a birthweight above 4 kg. However, several other studies have found no evidence of statistically significant differences in outcome measures and concluded that the intervention to manage weight gain in pregnancy was not cost-effective. For example, Oostdam et al. 132 examined the cost-effectiveness of an exercise intervention and concluded that the intervention was not cost-effective based on a range of outcome measures including infant birthweight and QALYs. Similarly, Kolu et al. found that a mixed intervention to prevent GDM was not cost-effective based on improvements in birthweight or 15D instrument scores. 129 A large-scale study concluded that a health training intervention was not cost-effective compared with usual care, based on comparison of QALY gains and costs for women. 68 Finally, a cost-comparison study for a weight-gain restriction programme for obese women found that that the weight-gain restriction programme was effective but had higher costs. 127
Meaning of the study
The results of the economic evaluation suggest that there is no evidence of cost-effectiveness for mixed interventions to manage weight gain in pregnancy. However, the lack of robust data on the quality of life of women and infants in the perinatal period means that further research is needed to fully understand the benefits of such programmes.
Unanswered questions and future research
The results of this economic evaluation highlight the need for accurate data on the quality of life of mothers and infants in the perinatal period, particularly around the impacts on quality of life for women with pregnancy-related conditions. This would enable a fuller analysis of the impact of interventions to manage weight gain in pregnancy on women’s health and that of their children. There is also a need for further work exploring the longer-term costs of weight gain in pregnancy for the mother and infant. This would need to include consideration of the wider societal costs of weight gain during pregnancy, as these are likely to be broader than health alone.
Chapter 9 Discussion
Summary of findings
Diet- and lifestyle-based interventions have a similar effect in all pregnant women for GWG, composite maternal and fetal/neonatal outcomes, irrespective of the woman’s characteristics such as BMI at booking, age, parity, Caucasian ethnicity and underlying medical condition(s). The interventions are effective in achieving a modest reduction in GWG, but there are no effects on composite maternal and fetal outcomes. There is no evidence of additional harm to the fetus. Adherence to the IOM-recommended GWG targets does not significantly reduce the risk of composite maternal or fetal/neonatal outcomes.
Strengths and limitations
Our IPD meta-analysis is the largest to date, and has greater power to detect any differential treatment effect across groups than single trials or aggregate meta-analysis. We modelled individual risk status (prognostic factor values) across participants within trials, to assess for variability in patient outcome. This is in contrast to aggregate data meta-analysis, which can model only average risk status values across studies, and thus only explain between-study variation. Our findings are more homogeneous, and are less likely to be affected by selective and biased reporting observed in aggregate meta-analysis. We have included more participants, and more outcomes than those that are currently available in the published literature by including available but unpublished data, particularly for outcomes such as preterm birth and SGA fetuses.
Although individual trials identified in our systematic review were powered to detect an overall treatment effect, the individual trial sample sizes were not sufficient to evaluate an effect in relevant subgroups of women. The sample size needed to be increased fourfold to have sufficient power to detect an interaction with the same magnitude as the overall treatment effect, and a 20-fold increase for an interaction term that is half the size of the overall treatment effect. 140 The costs and time to undertake a new trial for this purpose would be immense. By obtaining IPD from the multiple trials that have already been conducted, we have increased the sample size beyond a single trial, with substantially increased power to detect genuine interactions.
We focused on assessing the effect of the intervention on women across the BMI spectrum, including traditional categories of normal weight, overweight and obese. Ours is the first work to assess the effects of prognostic factors such as parity, ethnicity and underlying medical condition on the effectiveness of the intervention. The information about the components of the intervention was obtained in detail, including the adherence to the intervention by directly contacting the primary researchers. As experiencing more than one outcome out of GDM, preterm birth and PE was considered to be equally important for clinical management, we used composite outcome measures. We identified the components of the composite through a robust and transparent Delphi process. 19 The effects of the intervention on the individual components of the composite showed very similar effect sizes, confirming the valid use of the composite. We assessed the risk of bias in studies that contributed IPD and compared this with the risk of bias overall in all published studies. The relationship between GWG and pregnancy outcomes was evaluated using good-quality randomised data.
We were not able to explore the effects of ethnicity in detailed subcategories because of the wide variation in the definitions of race and ethnicity in individual studies. Furthermore, our assessment of differential effects for various individual characteristics was limited to those studies that only included women from all the subgroups. The trials varied in the type of intervention, duration, intensity, setting, provider and compliance. We were unable to fully disentangle the components of the intervention, and thus to identify those features that are effective in improving outcomes. The variation in criteria for the diagnosis or definition of GDM and PE may also have influenced the results. All studies that contributed to IPD were included in the analysis of the composite outcome, as the individual components of the outcome were not reported in all studies.
Very few studies that evaluated diet provided IPD, affecting the precision of the estimates for diet-based intervention. We approached all relevant authors within the time frame of the IPD meta-analysis. Although we identified more studies in the updated search, it was too late within the project to obtain data from these groups. However, the proportion of individual data not shared was lower than the proportion of studies not included in the IPD. Availability of additional non-IPD studies to our work may have improved the precision in our estimates. For validation of weight change as an outcome, we used the data only from control women, which reduced the available sample size for analysis. Inclusion of both intervention and control groups may help to improve the precision of the estimates, although precision may also be affected by the effects of intervention.
Comparison to existing evidence
Current national and international recommendations provide advice on diet and physical activity to manage weight in pregnancy. 10,141,142 These do not quantify the expected benefit to the woman or her infant from lifestyle-based interventions. They vary in their advice on compliance with weight-gain targets in pregnancy. Our findings are consistent with the previous systematic review that found a reduction in GWG. 24 Based on the findings of IPD meta-analysis, we were able to provide robust estimates for composite and individual maternal and fetal/neonatal outcomes, with minimal heterogeneity that limited previously published reviews.
Our findings on the effect of interventions for fetal/neonatal outcomes are similar to those of the recently published large studies that found no significant benefit. 68,72 Meta-analysis of published aggregate data showed significant benefit for GDM, preterm birth and Caesarean section compared with the observed trend in reduction of maternal composite and individual outcomes in our IPD meta-analysis. This is probably because of the inclusion of additional participants, although there were no large differences in sample sizes for individual maternal outcomes in both meta-analyses. However, unlike IPD, aggregate meta-analysis did not account for baseline prognostic factors in assessing the effects of intervention.
In the USA, the IOM guidance recommends weight-gain ranges in pregnancy for normal weight, overweight and obese women based on observational data. 143 In the absence of validation of these data in large interventional trials, the benefits of adhering to these targets in pregnancy are unclear. We have not found an association between GWG, including adherence to IOM targets and improvement in pregnancy outcomes.
Relevance to clinical practice
Currently, only obese pregnant women are offered diet and lifestyle interventions, which are often delivered by dietitians in many hospitals in the UK. Our work has shown that the effects of the interventions do not vary according to the maternal BMI for reduction in GWG. Existing strategy needs to be broadened to include normal weight and overweight women, as reduced GWG has the potential to minimise postpartum weight retention,144 thereby preventing these women from entering subsequent pregnancies as overweight or obese, respectively. Women should be encouraged to follow any intervention that is convenient and available, as the effects on GWG were similar for diet, physical activity and mixed-approach interventions. Similarly, it is not essential to configure services to target pregnant women based on age, ethnicity or underlying medical risk factors to deliver the intervention.
Health-care professionals should reassure women that lifestyle interventions do not increase the risk of adverse outcomes in their unborn child. The potential for benefit in improving maternal outcomes needs discussion. Pregnant women should be informed of the absence of relationship between prespecified weight-gain targets and composite adverse outcomes.
Research recommendations
The i-WIP Collaborative Network has provided a platform for lead researchers in this field to prioritise outcomes, standardise variables, plan future studies and influence public policies. 19,20 Further evaluation is needed to assess the differential effects of the interventions for individual maternal and fetal/neonatal outcomes. Addition of data from non-IPD studies to IPD meta-analysis for individual outcomes would be more informative than the comparison of IPD with only published aggregate data. Compliance with the IOM-recommended weight-gain targets and individual outcomes would add to the current evidence base.
Although interventions in pregnancy have been widely studied, very few have focused on the optimisation of the health of the mother in early pregnancy. Future studies will need to focus on interventions that optimise the health of women in the pre-pregnancy period. Such a strategy should also target women in the postpartum period, to ensure that their health status is optimal on entering a subsequent pregnancy. Long-term follow-up of women and their children exposed to the interventions should be prioritised as a research topic by funders and researchers. Health service delivery research is required to identify the ideal way to effectively deliver diet- and lifestyle-based interventions.
Acknowledgements
We acknowledge all researchers, research nurses and staff of the participating centres in the trials contributing to this IPD meta-analysis.
Contributions of authors
Anneloes E Ruifrok, Christianne JM de Groot, Sally Kerry, Richard D Riley and Shakila Thangaratinam developed the protocol.
Louise Jackson, Pelham Barton and Tracy Roberts wrote the health economic part of the protocol.
Julie Dodds oversaw the project and drafted the manuscript.
Ewelina Rogozińska, Nadine Marlin and Shakila Thangaratinam conducted the review, drafted the manuscript and led the project.
Ben Willem Mol and Khalid S Khan provided input into the protocol development and the drafting of the initial manuscript.
Ewelina Rogozińska and Emma Molyneaux undertook the literature searches and study selection.
Ewelina Rogozińska, Girish Rayanagoudar, Anneloes E Ruifrok, Emma Molyneaux and Shakila Thangaratinam acquired IPD.
Mireille NM van Poppel, Lucilla Poston, Christina A Vinter, Fionnuala McAuliffe, Jodie M Dodd, Julie Owens, Ruben Barakat, Maria Perales, Jose G Cecatti, Fernanda Surita, SeonAe Yeo, Annick Bogaerts, Roland Devlieger, Helena Teede, Cheryce Harrison, Lene Haakstad, Garry X Shen, Alexis Shub, Nermeen El Beltagy, Narges Motahari, Janette Khoury, Serena Tonstad, Riitta Luoto, Tarja I Kinnunen, Kym Guelfi, Fabio Facchinetti, Elisabetta Petrella, Suzanne Phelan, Tânia T Scudeller, Kathrin Rauh, Hans Hauner, Kristina Renault, Linda R Sagedal, Ingvild Vistad, Signe Nilssen Stafne, Siv Mørkved, Kjell Å Salvesen, Dorte M Jensen, Márcia Vitolo, Arne Astrup and Nina RW Geiker contributed data to the project and provided input at all stages of the project.
Ewelina Rogozińska, Nadine Marlin and Girish Rayanagoudar mapped the variables in the available data sets.
Ewelina Rogozińska and Nadine Marlin cleaned and quality-checked data.
Nadine Marlin harmonised the data.
Nadine Marlin, Sally Kerry and Richard D Riley conducted the data analysis.
Louise Jackson, Pelham Barton and Tracy Roberts performed the health economic simulations, with input from Shakila Thangaratinam, and wrote the health economic part of the report.
Arri Coomarasamy, Ben Willem Mol and Khalid S Khan were involved in project development and provided input at all stages.
All authors critically appraised the final draft of the report.
Publication
The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group . Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358. http://dx.doi.org/10.1136/bmj.j3119.
- Kanagalingam MG, Forouhi NG, Greer IA, Sattar N. Changes in booking body mass index over a decade: retrospective analysis from a Glasgow maternity hospital. BJOG 2005;112:1431-3. http://dx.doi.org/10.1111/j.1471-0528.2005.00685.x.
- Lewis G. Saving Mothers of Lives: Reviewing Maternal Deaths to Make Motherhood Safer. London: The Confidential Enquiry into Maternal and Child Health; 2007.
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010;140:387-98. http://dx.doi.org/10.1530/REP-10-0077.
- Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet 2006;93:269-74. http://dx.doi.org/10.1016/j.ijgo.2006.03.002.
- Thangaratinam S, Jolly K. Obesity in pregnancy: a review of reviews on the effectiveness of interventions. BJOG 2010;117:1309-12. http://dx.doi.org/10.1111/j.1471-0528.2010.02670.x.
- Thangaratinam S, Rogozińska E, Jolly K, Glinkowski S, Duda W, Borowiack E, et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16310.
- Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev 1999;21:261-75. https://doi.org/10.1093/oxfordjournals.epirev.a018001.
- Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (USA); 2009.
- Weight Management Before, During and After Pregnancy. London: National Institute for Health and Care Excellence; 2010.
- Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.cd007145.pub3.
- Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med 2012;10. http://dx.doi.org/10.1186/1741-7015-10-47.
- Lambert PC, Sutton AJ, Abrams KR, Jones DR. A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis. J Clin Epidemiol 2002;55:86-94. https://doi.org/10.1016/S0895-4356(01)00414-0.
- Riley RD, Lambert PC, Staessen JA, Wang J, Gueyffier F, Thijs L, et al. Meta-analysis of continuous outcomes combining individual patient data and aggregate data. Stat Med 2008;27:1870-93. https://doi.org/10.1002/sim.3165.
- Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c221.
- Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit?. Lancet 2005;365:341-6. http://dx.doi.org/10.1016/S0140-6736(05)17790-3.
- Ruifrok AE, Rogozinska E, van Poppel MN, Rayanagoudar G, Kerry S, de Groot CJ, et al. Study protocol: differential effects of diet and physical activity based interventions in pregnancy on maternal and fetal outcomes: individual patient data (IPD) meta-analysis and health economic evaluation. Syst Rev 2014;3. https://doi.org/10.1186/2046-4053-3-131.
- Tierney JF, Vale C, Riley R, Smith CT, Stewart L, Clarke M, et al. Individual participant data (ipd) meta-analyses of randomised controlled trials: guidance on their use. PLOS Med 2015;12. http://dx.doi.org/10.1371/journal.pmed.1001855.
- Rogozinska E, D’Amico MI, Khan KS, Cecatti JG, Teede H, Yeo S, et al. Development of composite outcomes for individual patient data (IPD) meta-analysis on the effects of diet and lifestyle in pregnancy: a Delphi survey. BJOG 2016;123:190-8. http://dx.doi.org/10.1111/1471-0528.13764.
- Dodd J, Thangaratinam S. i–WIP collaborative network . Researchers’ position statement on tackling obesity in pregnancy: the International Weight Management in Pregnancy (i-WIP) collaboration pleads for public health intervention. BJOG 2016;123:163-4. http://dx.doi.org/10.1111/1471-0528.13766.
- Higgins JP, Altman DG, Stern JAC, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane Collaboration; 2011.
- Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.d7762.
- Riley RD, Steyerberg EW. Meta-analysis of a binary outcome using individual participant data and aggregate data. Res Synth Methods 2010;1:2-19. http://dx.doi.org/10.1002/jrsm.4.
- Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2088.
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004;23:1351-75. http://dx.doi.org/10.1002/sim.1761.
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009;172:137-59. https://doi.org/10.1111/j.1467-985X.2008.00552.x.
- SeonAe Yeo. University of North Carolina at Chapel Hill, School of Nursing, Personal Communication 2013.
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol 2009;113:305-12. http://dx.doi.org/10.1097/AOG.0b013e318195baef.
- Badrawi H, Hassanein MK, Badraoui MHH, Wafa YA, Shawky HA, Badrawi N. Pregnancy outcome in obese pregnant mothers. J Perinat Med 1992;20.
- Bechtel-Blackwell DA. Computer-assisted self-interview and nutrition education in pregnant teens. Clin Nurs Res 2002;11:450-62. https://doi.org/10.1177/105477302237456.
- Briley C, Flanagan NL, Lewis N. In-home prenatal nutrition intervention increased dietary iron intakes and reduced low birthweight in low-income African-American women. J Am Diet Assoc 2002;102:984-7. https://doi.org/10.1016/S0002-8223(02)90225-7.
- Deveer R, Deveer M, Akbaba E, Engin-Üstün Y, Aydoğan P, Celikkaya H, et al. The effect of diet on pregnancy outcomes among pregnant with abnormal glucose challenge test. Eur Rev Med Pharmacol Sci 2013;17:1258-61.
- Garshasbi A, Faghih Zadeh S. The effect of exercise on the intensity of low back pain in pregnant women. Int J Gynaecol Obstet 2005;88:271-5. http://dx.doi.org/10.1016/j.ijgo.2004.12.001.
- Gomez-Tabarez G, Delgado JG, Agudelo AA, Hurtado H. Diet effects on the perinatal result of obese pregnant patient. Rev Colomb De Obstet Ginecol 1994;45:313-16.
- Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery 2011;27:257-64. http://dx.doi.org/10.1016/j.midw.2009.06.009.
- Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord 2002;26:1494-502. http://dx.doi.org/10.1038/sj.ijo.0802130.
- Santos IA, Stein R, Fuchs SC, Duncan BB, Ribeiro JP, Kroeff LR, et al. Aerobic exercise and submaximal functional capacity in overweight pregnant women: a randomized trial. Obstet Gynecol 2005;106:243-9. http://dx.doi.org/10.1097/01.AOG.0000171113.36624.86.
- Sedaghati P, Ziaee V, Ardjmand A. The effect of an ergometric training program on pregnants weight gain and low back pain. Gazz Med Ital Arch Sci Med 2007;166:209-13.
- Korpi-Hyövälti E, Schwab U, Laaksonen DE, Linjama H, Heinonen S, Niskanen L. Effect of intensive counselling on the quality of dietary fats in pregnant women at high risk of gestational diabetes mellitus. Br J Nutr 2012;108:910-17. http://dx.doi.org/10.1017/S0007114511006118.
- Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J Natl Med Assoc 2009;101:569-77. https://doi.org/10.1016/S0027-9684(15)30942-1.
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol 2011;51:141-6. http://dx.doi.org/10.1111/j.1479-828X.2010.01268.x.
- Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med 2012;46:656-61. http://dx.doi.org/10.1136/bjsports-2011-090009.
- Barakat R, Pelaez M, Lopez C, Lucia A, Ruiz JR. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomised controlled trial. Br J Sports Med 2013;47:630-6. http://dx.doi.org/10.1136/bjsports-2012-091788.
- Clapp JF, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 2000;183:1484-8. https://doi.org/10.1067/mob.2000.107096.
- Lee G, Challenger S, McNabb M, Sheridan M. Exercise in pregnancy. Modern Midwife 1996;6:28-33.
- Bisson M, Alméras N, Dufresne S, Robitaille J, Rhéaume C, Bujold E, et al. A 12-week exercise program for pregnant women with obesity to improve physical activity levels: an open randomised preliminary study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0137742.
- Cordero Y, Mottola MF, Vargas J, Blanco M, Barakat R. Exercise Is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc 2015;47:1328-33. http://dx.doi.org/10.1249/MSS.0000000000000547.
- de Oliveria Melo AS, Silva JL, Tavares JS, Barros VO, Leite DF, Amorim MM. Effect of a physical exercise program during pregnancy on uteroplacental and fetal blood flow and fetal growth: a randomized controlled trial. Obstet Gynecol 2012;120:302-10. http://dx.doi.org/10.1097/AOG.0b013e31825de592.
- Dekker Nitert M, Barrett HL, Denny KJ, McIntyre HD, Callaway LK. BAMBINO group . Exercise in pregnancy does not alter gestational weight gain, MCP-1 or leptin in obese women. Aust N Z J Obstet Gynaecol 2015;55:27-33. http://dx.doi.org/10.1111/ajo.12300.
- Di Carlo C, Iannotti G, Sparice S, Chiacchio MP, Greco E, Tommaselli GA, et al. The role of a personalized dietary intervention in managing gestational weight gain: a prospective, controlled study in a low-risk antenatal population. Arch Gynecol Obstet 2014;289:765-70. https://doi.org/10.1007/s00404-013-3054-y.
- Hawkins M, Hosker M, Marcus BH, Rosal MC, Braun B, Stanek EJ, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: a feasibility randomized controlled trial. Diabet Med 2015;32:108-15. http://dx.doi.org/10.1111/dme.12601.
- Hui AL, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean HJ, et al. Effects of lifestyle intervention on dietary intake, physical activity level, and gestational weight gain in pregnant women with different pre-pregnancy Body Mass Index in a randomized control trial. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-331.
- Jing W, Huang Y, Liu X, Luo B, Yang Y, Liao S. The effect of a personalized intervention on weight gain and physical activity among pregnant women in China. Int J Gynaecol Obstet 2015;129:138-41. http://dx.doi.org/10.1016/j.ijgo.2014.11.014.
- Kong KL, Campbell CG, Foster RC, Peterson AD, Lanningham-Foster L. A pilot walking program promotes moderate-intensity physical activity during pregnancy. Med Sci Sports Exerc 2014;46:462-71. http://dx.doi.org/10.1249/MSS.0000000000000141.
- Li Q, Cui H, Zheng D, Li N, Chang L, Liu C. Effects of walking exercise during late trimester on pregnancy outcome of low-risk primipara. Zhonghua Yi Xue Za Zhi 2014;94:1722-5.
- Mujsindi W, Habash D, Childs G. Impact of nutrition education on gestational weight gain in obese pregnant women. Am J Obstet Gynecol 2014;210. https://doi.org/10.1016/j.ajog.2013.10.402.
- Murtezani A, Paçarada M, Ibraimi Z, Nevzati A, Abazi N. The impact of exercise during pregnancy on neonatal outcomes: a randomized controlled trial. J Sports Med Phys Fitness 2014;54:802-8.
- Price BB, Amini SB, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes-a randomized trial. Med Sci Sports Exerc 2012;44:2263-9. http://dx.doi.org/10.1249/MSS.0b013e318267ad67.
- Ramírez-Vélez R, Aguilar de Plata AC, Escudero MM, Echeverry I, Ortega JG, Salazar B, et al. Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. J Obstet Gynaecol Res 2011;37:1601-8. http://dx.doi.org/10.1111/j.1447-0756.2011.01582.x.
- Ramírez-Vélez R. Effect of recommended physical activity dose on obstetrical, neonatal and maternal metabolic outcomes in pregnant Latina women. Ann Nutr Metab 2013;63.
- Ronnberg AK, Ostlund I, Fadl H, Gottvall T, Nilsson K. Intervention during pregnancy to reduce excessive gestational weight gain – a randomised controlled trial. BJOG 2015;122:537-44. http://dx.doi.org/10.1111/1471-0528.13131.
- Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001036.
- Rauh K, Gabriel E, Kerschbaum E, Schuster T, von Kries R, Amann-Gassner U, et al. Safety and efficacy of a lifestyle intervention for pregnant women to prevent excessive maternal weight gain: a cluster-randomized controlled trial. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-151.
- Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes 2013;37:814-21. http://dx.doi.org/10.1038/ijo.2012.162.
- El Beltagy N, Saad El Deen S, Mohamed R. Does physical activity and diet control reduce the risk of developing gestational diabetes mellitus in Egypt? A randomized controlled trial. J Perinat Med 2013;41.
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373-80. https://doi.org/10.3945/ajcn.2009.28166.
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009;35:418-21. http://dx.doi.org/10.1016/j.diabet.2009.04.008.
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767-77. http://dx.doi.org/10.1016/S2213-8587(15)00227-2.
- Renault KM, Norgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol 2014;210:134-9. https://doi.org/10.1016/j.ajog.2013.09.029.
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011;34:2502-7. http://dx.doi.org/10.2337/dc11-1150.
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes 2008;32:495-501. http://dx.doi.org/10.1038/sj.ijo.0803710.
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1285.
- Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity 2013;21:904-9. https://doi.org/10.1002/oby.20163.
- Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med 2014;27:1348-52. https://doi.org/10.3109/14767058.2013.858318.
- Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012;119:1098-107. http://dx.doi.org/10.1111/j.1471-0528.2012.03366.x.
- Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol 2005;193:1292-301. http://dx.doi.org/10.1016/j.ajog.2005.05.016.
- Vítolo MR, Bueno MS, Gama CM. Impact of a dietary counseling program on the gain weight speed of pregnant women attended in a primary care service. Rev Bras Ginecol Obstet 2011;33:13-9. https://doi.org/10.1590/S0100-72032011000100009.
- Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5605.
- Baciuk EP, Pereira RI, Cecatti JG, Braga AF, Cavalcante SR. Water aerobics in pregnancy: cardiovascular response, labor and neonatal outcomes. Reprod Health 2008;5. http://dx.doi.org/10.1186/1742-4755-5-10.
- Barakat R, Stirling JR, Lucia A. Does exercise training during pregnancy affect gestational age? A randomised controlled trial. Br J Sports Med 2008;42:674-8. http://dx.doi.org/10.1136/bjsm.2008.047837.
- Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol 2011;204. http://dx.doi.org/10.1016/j.ajog.2011.01.043.
- Haakstad L, Bo K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Health Care 2011;16:116-25. https://doi.org/10.3109/13625187.2011.560307.
- Khaledan A, Motahari Sh Tabari NS, Ahmad Shirvani M. Effect of an aerobic exercise program on fetal growth in pregnant women. HAYAT 2010;16.
- Nascimento SL, Surita FG, Parpinelli MÂ, Siani S, Pinto e Silva JL. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: a randomised clinical trial. BJOG 2011;118:1455-63. http://dx.doi.org/10.1111/j.1471-0528.2011.03084.x.
- Perales M, Refoyo I, Coteron J, Bacchi M, Barakat R. Exercise during pregnancy attenuates prenatal depression: a randomized controlled trial. Eval Health Prof 2015;38:59-72. https://doi.org/10.1177/0163278714533566.
- Prevedel T, Calderon I, De Conti MH, Consonni EB, Arudge MVC. Maternal and perinatal effects of hydrotherapy in pregnancy. Rev Bras Ginecol Obstet 2003;25:53-9.
- Ruiz JR, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc 2013;88:1388-97. http://dx.doi.org/10.1016/j.mayocp.2013.07.020.
- Stafne SN, Salvesen KÅ, Romundstad PR, Torjusen IH, Mørkved S. Does regular exercise including pelvic floor muscle training prevent urinary and anal incontinence during pregnancy? A randomised controlled trial. BJOG 2012;119:1270-80. http://dx.doi.org/10.1111/j.1471-0528.2012.03426.x.
- Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R. Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. J Reprod Med 2000;45:293-8.
- Althuizen E, van der Wijden CL, van Mechelen W, Seidell JC, van Poppel MN. The effect of a counselling intervention on weight changes during and after pregnancy: a randomised trial. BJOG 2013;120:92-9. http://dx.doi.org/10.1111/1471-0528.12014.
- Hui A, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean H, et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG Int J Obstet Gynaecol 2012;119:70-7. https://doi.org/10.1111/j.1471-0528.2011.03184.x.
- Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust 2009;191:429-33.
- Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr 2011;93:772-9. http://dx.doi.org/10.3945/ajcn.110.005306.
- Sagedal LR, Øverby NC, Lohne-Seiler H, Bere E, Torstveit MK, Henriksen T, et al. Study protocol: fit for delivery - can a lifestyle intervention in pregnancy result in measurable health benefits for mothers and newborns? A randomized controlled trial. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-132.
- Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- National Schedule of Reference Costs: The Main Schedule. London: Department of Health; 2014.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence; 2015.
- Hypertension in Pregnancy (Modified Version). London: National Institute for Health and Care Excellence; 2011.
- Madan J, Chilcott J. Weight Management in Pregnancy: Economic Modelling. Sheffield: ScHARR Public Health Collaborating Centre; 2012.
- Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2008;12. https://doi.org/10.3310/hta12060.
- Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period: NICE Guideline 3 – Methods, Evidence and Recommendations. London: National Institute for Health and Care Excellence; 2015.
- Hypertension in Pregnancy: the Management of Hypertensive Disorders during Pregnancy. London: National Institute for Health and Care Excellence; 2010.
- Round JA, Jacklin P, Fraser RB, Hughes RG, Mugglestone MA, Holt RI. Screening for gestational diabetes mellitus: cost-utility of different screening strategies based on a woman’s individual risk of disease. Diabetologia 2011;54:256-63. http://dx.doi.org/10.1007/s00125-010-1881-y.
- Antenatal Care Overview and Pathways. London: National Institute for Health and Care Excellence; n.d.
- Antenatal Care. London: National Institute for Health and Care Excellence; 2008.
- Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikström AK, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309:2362-70. http://dx.doi.org/10.1001/jama.2013.6295.
- Green-Top Guideline No. 55. Late Intrauterine Fetal Death and Stillbirth. London: Royal College of Obstetricians and Gynaecologists; 2010.
- Mistry H, Heazell AE, Vincent O, Roberts T. A structured review and exploration of the healthcare costs associated with stillbirth and a subsequent pregnancy in England and Wales. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-236.
- Edlow AG, Srinivas SK, Elovitz MA. Second-trimester loss and subsequent pregnancy outcomes: what is the real risk?. Am J Obstet Gynecol 2007;197:581.e1-6. http://dx.doi.org/10.1016/j.ajog.2007.09.016.
- Roberts M, Russell LB, Paltiel AD, Chambers M, McEwan P, Krahn M. Conceptualizing a model a report of the ISPOR-SMDM modeling good research practices task force – 2. Med Decis Mak 2012;32:678-89.
- van Baaren GJ, Broekhuijsen K, van Pampus MG, Ganzevoort W, Sikkema JM, Woiski MD, et al. An economic analysis of immediate delivery and expectant monitoring in women with hypertensive disorders of pregnancy, between 34 and 37 weeks of gestation (HYPITAT-II). BJOG 2017;124:453-61. https://doi.org/10.1111/1471-0528.13957.
- Hypertension in Pregnancy Pathway. London: National Institute for Health and Care Excellence; 2015.
- Shmueli A, Meiri H, Gonen R. Economic assessment of screening for pre-eclampsia. Prenat Diagn 2012;32:29-38. http://dx.doi.org/10.1002/pd.2871.
- Simon J, Gray A, Duley L. Magpie Trial Collaborative Group . Cost-effectiveness of prophylactic magnesium sulphate for 9996 women with pre-eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG 2006;113:144-51. http://dx.doi.org/10.1111/j.1471-0528.2005.00785.x.
- Vijgen S, Koopmans C, Opmeer B, Groen H, Bijlenga D, Aarnoudse J, et al. An economic analysis of induction of labour and expectant monitoring in women with gestational hypertension or pre-eclampsia at term (HYPITAT trial). BJOG 2010;117:1577-85. https://doi.org/10.1111/j.1471-0528.2010.02710.x.
- The Magpie Trial Collaborative Group M . Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie trial: a randomised placebo-controlled trial. Lancet 2002;359:1877-90. https://doi.org/10.1016/S0140-6736(02)08778-0.
- Health and Social Care Information Centre . HRG4+ 2013 14 Reference Costs Grouper HRG4+ Chapter Summaries 2014. http://digital.nhs.uk/media/13827/HRG4-201314-RC-Chapter-Summaries (accessed 28 June 2016).
- Kolu P, Raitanen J, Rissanen P, Luoto R. Health care costs associated with gestational diabetes mellitus among high-risk women – results from a randomised trial. BMC Pregnancy Childbirth 2012;12. http://dx.doi.org/10.1186/1471-2393-12-71.
- Koopmans CM, Bijlenga D, Groen H, Vijgen SM, Aarnoudse JG, Bekedam DJ, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet 2009;374:979-88. https://doi.org/10.1016/S0140-6736(09)60736-4.
- Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol 2000;14:839-54. http://dx.doi.org/10.1053/beog.2000.0123.
- Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk J. Saving Lives, Improving Mothers’ Care: Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
- UKForex . UKForex – Foreign Exchange Services n.d. www.ukforex.co.uk (accessed 28 June 2016).
- Cavassini ACM, Lima SAM, Calderon IMP, Rudge MVC. Cost-benefit of hospitalization compared with outpatient care for pregnant women with pregestational and gestational diabetes or with mild hyperglycemia, in Brazil. São Paulo Med J 2012;130. https://doi.org/10.1590/s1516-31802012000100004.
- Cleary J, Casey S, Hofsteede C, Moses RG, Milosavljevic M, Brand-Miller J. Does a low glycaemic index (GI) diet cost more during pregnancy?. Nutrients 2012;4:1759-66. http://dx.doi.org/10.3390/nu4111759.
- de Keyser N, Josefsson A, Monfils WG, Claesson IM, Carlsson P, Sydsjö A, et al. Total cost comparison of standard antenatal care with a weight gain restriction programme for obese pregnant women. Public Health 2011;125:311-17. http://dx.doi.org/10.1016/j.puhe.2011.02.004.
- Dodd JM, Ahmed S, Karnon J, Umberger W, Deussen AR, Tran T, et al. The cost-effectiveness of providing antenatal lifestyle advice for women who are overweight or obese: the LIMIT randomised trial. BMC Obes 2015;2. http://dx.doi.org/10.1186/s40608-015-0046-4.
- Kolu P, Raitanen J, Rissanen P, Luoto R. Cost-effectiveness of lifestyle counselling as primary prevention of gestational diabetes mellitus: findings from a cluster-randomised trial. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0056392.
- Moss JR, Crowther CA, Hiller JE, Willson KJ, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women Group . Costs and consequences of treatment for mild gestational diabetes mellitus – evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth 2007;7. http://dx.doi.org/10.1186/1471-2393-7-27.
- Ohno MS, Sparks TN, Cheng YW, Caughey AB. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011;205:282.e1-7. http://dx.doi.org/10.1016/j.ajog.2011.06.051.
- Oostdam N, Bosmans J, Wouters MG, Eekhoff EM, van Mechelen W, van Poppel MN. Cost-effectiveness of an exercise program during pregnancy to prevent gestational diabetes: results of an economic evaluation alongside a randomised controlled trial. BMC Pregnancy Childbirth 2012;12. http://dx.doi.org/10.1186/1471-2393-12-64.
- Todorova K, Palaveev O, Petkova VB, Stefanova M, Dimitrova Z. A pharmacoeconomical model for choice of a treatment for pregnant women with gestational diabetes. Acta Diabetol 2007;44:144-8.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 6. Value Health 2012;15:835-42. http://dx.doi.org/10.1016/j.jval.2012.04.014.
- Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14:339-47.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. https://doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- Armitage P, Berry G, Matthews JN. Statistical Methods in Medical Research. Hoboken, NJ: John Wiley & Sons; 2008.
- Miettinen OS. Estimation of relative risk from individually matched series. Biometrics 1970;26:75-86.
- Healthy People: A Call to Action on Obesity in England. London: Department of Health; 2011.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5.
- American College of Obstetricians and Gynecologists . Good Health Before Pregnancy: Preconception Care 2015. www.acog.org (accessed 8 July 2016).
- CMASE/RCOG Joint Guideline. Management of Women with Obesity in Pregnancy. London: Centre for Maternal and Child Enquiries/Royal College of Obstetricians and Gynaecologists; 2010.
- Rasmussen K, Yaktine A. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US); 2009.
- Rauh K, Günther J, Kunath J, Stecher L, Hauner H. Lifestyle intervention to prevent excessive maternal weight gain: mother and infant follow-up at 12 months postpartum. BMC Pregnancy Childbirth 2015;15. http://dx.doi.org/10.1186/s12884-015-0701-2.
- Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. for the BAMBINO Group . Prevention of gestational diabetes. Diabetes Care 2010;33:1457-9.
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab 2010;95:2080-8.
- Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns 2011;83:203-9.
- Marquez-Sterling S, Perry AC, Kaplan TA, Halberstein RA, Signorile JF. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med Sci Sports Exerc 2000;32:58-62.
- Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: a randomized trial. Obesity 2014;22:1989-96. https://doi.org/10.1002/oby.20831.
- Johnson SS, Paiva AL, Cummins CO, Johnson JL, Dyment SJ, Wright JA, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med 2008;46:238-46. https://doi.org/10.1016/j.ypmed.2007.09.010.
- Institute of Medicine . Dietary Reference Intakes: The Essential Guide to Nutrient Requirements 2006. www.iom.edu/Reports/2006/Dietary-Reference-Intakes-Essential-Guide- Nutrient-Requirements.aspx (accessed July 2010).
- Health Canada . Prenatal Nutrition Guidelines for Health Professionals – Background on Canada’s Food Guide 2009. www.hc-sc.gc.ca/fn-an/pubs/nutrition/guide-prenatal-eng.php (accessed 1 July 2010).
- Sagedal LR, Øverby NC, Bere E, Torstveit MK, Lohne-Seiler H, Smâstuen M, et al. Lifestyle intervention to limit gestational weight gain: the Norwegian Fit for Delivery randomised controlled trial. BJOG 2017;124:97-109. https://doi.org/10.1111/1471-0528.13862.
- Barakat R, Pelaez M, Lopez C, Montejo R, Coteron J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: results of a randomized controlled trial. J Matern Neonatal Med 2012;25:2372-6. http://dx.doi.org/10.3109/14767058.2012.696165.
- Gesell SB, Katula JA, Strickland C, Vitolins MZ. Feasibility and initial efficacy evaluation of a community-based cognitive-behavioral lifestyle intervention to prevent excessive weight gain during pregnancy in Latina women. Matern Child Health J 2015;19:1842-52. http://dx.doi.org/10.1007/s10995-015-1698-x.
- Perales M, Calabria I, Lopez C, Franco E, Coteron J, Barakat R. Regular exercise throughout pregnancy is associated with a shorter first stage of labor. Am J Heart Promot 2016;30:149-54. http://dx.doi.org/10.4278/ajhp.140221-QUAN-79.
Appendix 1 Outcomes prioritised in Delphi survey
| Outcomes | |
|---|---|
| Maternal | Fetal |
| Weight gain in pregnancy | SGA |
| Postpartum weight retention | LGA |
| Interpregnancy weight gain | Skinfold thickness |
| GDM | Fetal fat mass (%) |
| PE/PIH | Abdominal circumference |
| Postpartum haemorrhage | Head circumference |
| Prolonged labour | Ponderal index (g/cm3 × 100) |
| Preterm delivery | Neonate length/crown–heel length |
| Induction of labour | Head-to-abdomen ratio |
| Prelabour rupture of membranes | Birthweight-related outcomes, such as BMI |
| Caesarean section | Hypoglycaemia |
| Instrumental delivery | Hyperbilirubinaemia |
| Perineal trauma | IUD |
| Puerperal pyrexia (≥ 38 °C) | Respiratory distress syndrome |
| Miscarriage | Admission to the NICU |
| Need for resuscitation at delivery | Shoulder dystocia |
| Antepartum haemorrhage | ≥ 1 perinatal complication |
| Thromboembolism | Birth trauma number |
| Admission to high-dependency unit/intensive therapy unit | Neural tube defect |
| Anaemia | Cleft lip or palate or both |
| Infections | Other congenital abnormalities |
| Postnatal infections | Apgar score |
| Postnatal depression | Cardiotocograph abnormalities |
| Anxiety | Abnormal cord pH |
| Quality of life | Long-term neurological sequelae |
| Physical activity | Cord abnormalities |
| Dietary behaviour | Long-term metabolic sequelae |
| Body fat (%) | |
| Back pain | |
| Breastfeeding | |
| Threatened abortion | |
| Failed instrumental delivery | |
| Coronary artery disease | |
| Non-infective respiratory distress | |
| Items | Round | |||
|---|---|---|---|---|
| First | Second | |||
| Median | Interquartile range | Median | Interquartile range | |
| Maternal outcomes | ||||
| PEa | 8.5 | 1 | 9 | 0 |
| PIHa | 8.5 | 1 | 9 | 1 |
| GDM | 8.5 | 1 | 9 | 0 |
| Preterm delivery | 7.5 | 1 | 8 | 2 |
| Caesarean section: electivea | 8 | 2 | 8 | 1 |
| Caesarean section: emergencya | 8 | 2 | 8 | 0 |
| Fetal outcome | ||||
| IUD | 9 | 1.25 | 9 | 0 |
| SGA | 8 | 1 | 8 | 1 |
| LGA | 8 | 1 | 8 | 1 |
| Admission to the NICU | 8 | 1 | 8 | 0 |
Appendix 2 Search strategies
| Item | Term |
|---|---|
| 1 | Pregnancy/ |
| 2 | pregnan*.tw. |
| 3 | Gravidity/ |
| 4 | gravid*.tw. |
| 5 | gestation*.tw. |
| 6 | Pregnant Women/ |
| 7 | pregnant wom#n.tw. |
| 8 | (child adj3 bearing).tw. |
| 9 | childbearing.tw. |
| 10 | matern*.tw. |
| 11 | or/1-10 |
| 12 | Weight Gain/ph [Physiology] |
| 13 | weight gain*.tw. |
| 14 | Weight Loss/ph [Physiology] |
| 15 | weight loss*.tw. |
| 16 | weight change*.tw. |
| 17 | Obesity/dh, me, ph, pc, px, th [Diet Therapy, Metabolism, Physiology, Prevention & Control, Psychology, Therapy] |
| 18 | obes*.tw. |
| 19 | Adiposity/ph [Physiology] |
| 20 | adipos*.tw. |
| 21 | Overweight/dh, me, ph, pc, px, th [Diet Therapy, Metabolism, Physiology, Prevention & Control, Psychology, Therapy] |
| 22 | overweight*.tw. |
| 23 | Body Mass Index/ |
| 24 | bmi.tw. |
| 25 | or/12-24 |
| 26 | exp Randomized Controlled Trial/ |
| 27 | “randomized controlled trial”.pt. |
| 28 | “controlled clinical trial”.pt. |
| 29 | (random$ or placebo$).tw,sh. |
| 30 | ((singl$ or double$ or triple$ or treble$) and (blind$ or mask$)).tw,sh. |
| 31 | single-blind method/ |
| 32 | double-blind method/ |
| 33 | or/26-32 |
| 34 | 11 and 25 and 33 |
| 35 | exp Animals/ |
| 36 | (rat$ or mouse or mice or hamster$ or animal$ or dog$ or cat$ or bovine or sheep or lamb$).af. |
| 37 | 35 or 36 |
| 38 | Humans/ |
| 39 | human$.tw,ot,kf. |
| 40 | 37 or 38 |
| 41 | 37 not (37 and 40) |
| 42 | 34 not 41 |
| Item | Terms |
|---|---|
| #1 | (Pregnancy):ti,ab,kw |
| #2 | pregnan* |
| #3 | Gravidity |
| #4 | gravid* |
| #5 | gestation* |
| #6 | “Pregnant Women” |
| #7 | childbearing |
| #8 | matern* |
| #9 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) |
| #10 | (Weight Gain):ti,ab,kw |
| #11 | (Weight Loss):ti,ab,kw |
| #12 | weight loss* |
| #13 | weight change* |
| #14 | (Obesity):ti,ab,kw |
| #15 | obes* |
| #16 | Adiposity:ti,ab,kw |
| #17 | adipos* |
| #18 | Overweight:ti,ab,kw |
| #19 | overweight* |
| #20 | “Body Mass Index” |
| #21 | BMI |
| #22 | (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) |
| #23 | (#9 AND #22) |
Appendix 3 International Weight Management in Pregnancy individual participant data meta-analysis project variables
| Variable label | Variable type | Variable format | Variable range |
|---|---|---|---|
| Height (cm) | Numeric | 999 | 999 = missing |
| Pre-pregnancy weight (kg) | Numeric | 999.9 | 999 = missing |
| Pre-pregnancy BMI (kg/m2) | Numeric | 99.99 | 99 = missing |
| Early pregnancy weight (kg) | Numeric | 999.9 | 999 = missing |
| Early pregnancy BMI (kg/m2) | Numeric | 99.99 | 99 = missing |
| Gestational age at baseline (weeks) | Numeric | 99 | 0–50, 99 = missing |
| Gestational age at baseline (days) | Numeric | 9 | 0–6, 9 = missing |
| Gestational age at follow-up (weeks) | Numeric | 99 | 0–50, 99 = missing |
| Gestational age at follow-up (days) | Numeric | 9 | 0–6, 9 = missing |
| Follow-up weight (kg) | Numeric | 999.9 | 999 = missing |
| Follow-up BMI (kg/m2) | Numeric | 99.99 | 99 = missing |
| Total weight gain (kg) | Numeric | 999.9 | 999 = missing |
| Weight post delivery (kg) | Numeric | 999.9 | 999 = missing |
| Babies birthweight (g) | Numeric | 9999 | 9999 = missing |
| Age (years) | Numeric | 99.9 | 99 = missing |
| Land of birth | String | ||
| Race/ethnicity | Numeric categorical | 9 | 1 = Caucasian, 2 = Asian, 3 = Afro-Caribbean, 4 = Central/South American, 5 = Middle Eastern, 6 = other, 9 = nk |
| Education mother, detail | String | ||
| Education mother, low/medium/high | Numeric categorical | 9 | 1 = low, 2 = middle, 3 = high, 9 = nk |
| Current smoker | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Ex-smoker (pre pregnancy) | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Allocation | Numeric categorical | 9 | 0 = control, 1 = intervention1, 2 = intervention2, 9 = nk |
| Unit of randomisation | String | For example participantid, centre id . . . | |
| Adherence | Numeric categorical | 9 | 2 = control group, 1 = yes, 0 = no, 9 = nk |
| Number of fetuses | Numeric categorical | 9 | 1 = singleton, 2 = twin or more, 9 = missing |
| Gestational age at delivery (weeks) | Numeric | 99 | 99 = missing |
| Mode of delivery | Numeric categorical | 9 | 1 = nvd, 2 = instrumental/vacuum/forceps, 3 = cs, 9 = nk |
| Number of times giving birth before this pregnancy | Numeric | 99 | 99 = missing |
| Previous miscarriages | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Gravidity (number of times pregnant) | Numeric | 99 | 99 = missing |
| Obesity (BMI of ≥ 30 kg/m2) | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Previous large baby (≥ 4.5 kg) | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Previous GDM | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Family history of DM | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Baseline GDM | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Baseline DM | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Baseline PIH | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Baseline hypertension | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Exercise detail | String | ||
| GDM test value | Numeric | 999.9 | 999 = missing |
| GDM test unit | Text | ||
| Type of GDM test | Text | ||
| GDM | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| PIH | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| PE | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Preterm delivery | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Caesarean section | Numeric categorical | 9 | 0 = no, 1 = cs unspecified, 2 = elective, 3 = emergency, 9 = nk |
| IUD | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| SGA | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| LGA | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
| Admission to the NICU | Numeric categorical | 9 | 0 = no, 1 = yes, 9 = nk |
Appendix 4 Variables recoding
| Caucasian (including Russia and Australia) | Asian | Afro-Caribbean | Central/South American | Middle Eastern (including Iran and Turkey) | Other |
|---|---|---|---|---|---|
| Afro-Caribbean | Malaysia | Tunisia | Argentina | Iran | Aboriginal |
| Australia | Nepal | Uganda | Brazil | Iraq | Australia/Aboriginal |
| Australian – Aboriginal | Pakistan | Unclassified (other) | Brazil Black | Israel | Fiji |
| Austria | Pakistani | Zimbabwe | Brazil Pardo | Lebanon | New Zealand |
| Belgian/Dutch | Philippines | Maghreb | Brazil White | Middle Eastern | Non-Caucasian |
| Belgium | South East Asian | Chile | Turkey | Other | |
| Bosnia | Sri Lanka | Colombia | Turkish | ||
| Bosnia-Herzegovina | Sri-Lanka | Columbia | |||
| Bulgaria | Taiwan | El Salvador | |||
| Caucasian | Thailand | Mexico | |||
| Caucasian, excluding Turkey and Morocco | Unclassified (other) | ||||
| Croatia | Uzbekistan | ||||
| Czech | Vietnam | ||||
| Denmark | Japan | ||||
| East European | |||||
| England | |||||
| European | |||||
| Finland/England/Sweden/Russia | |||||
| France | |||||
| Germany | |||||
| Greece | |||||
| Hungary | |||||
| Iceland | |||||
| Iraq | |||||
| Italian | |||||
| Italy | |||||
| Kosovo | |||||
| Latvia | |||||
| Lebanon | |||||
| North American White | |||||
| Norway | |||||
| Other White | |||||
| Pakistan | |||||
| Poland | |||||
| Romania | |||||
| Russia | |||||
| Serbia | |||||
| Slovakia | |||||
| Spain | |||||
| Sweden | |||||
| The Faroes | |||||
| Turkey | |||||
| Ukraine | |||||
| Unclassified (other) | |||||
| White Irish | |||||
| Yugoslavia | |||||
| Caucasian |
We assumed IPD data to be clean and, therefore, individual items may seem to be in the wrong category. However, if the study already had categories that matched our structure, then we used those rather than the additional details provided.
| Low | Medium | High |
|---|---|---|
| < 12 years (preparatory school or occupational school) | 12 years (high school) | Vocational training school |
| < 4 years of study | 4–8 years of study | < 4 years additional education |
| First degree | A-level (or equivalent) | > 12 years (university or equivalent to it) |
| Grammar school ≤ 10 years | GCE (or equivalent) | > 8 years of study |
| LBO | General secondary school | ≥ 4 years additional education |
| Less than high school | General upper secondary education | College/university < 4 years |
| Low | HAVO/VWO | Further education 1–2 years |
| Low (basic or secondary education) | High school | Graduate degree |
| None | High school/grammar school | Graduated, 14 years |
| Preliminary, 5 years | High school diploma | Graduated, 16 years |
| Preliminary, 9 years | High school, 12 years | HBO |
| Primary | Intermediate secondary school | High (university degree) |
| Primary and secondary school | MBO | Higher degree |
| Primary education | Medium (polytechnic education) | Postgraduate education |
| Primary or less | Secondary | Postgraduate |
| Primary school | Secondary school 12 years | Tertiary |
| VMBO | Upper secondary school | Tertiary education 3–4 years (Bachelor level) |
| Year 10 or below | Vocational upper secondary education | Undergraduate |
| Year 11 or equivalent | Year 12 or equivalent | University |
| Elementary school | Complete secondary | University degree |
| Grade school (< 6 years) | High school | University/university college < 4 years |
| Junior high school (7–9 years) | High school (13 years) | University/university college > 4 years |
| Less than primary school | High school (10–12 years) | Vocational qualification |
| Less than primary school | Medium-length education | WO |
| Middle | School maximum 10 years, additional education | Year 12 or equivalent |
| Middle school (8 years) | Technical, additional education | Bachelors level |
| Primary school | Until 18 year, possible a speciality of 1/2 year | College (university) |
| School maximum 10 years, education unfinished | Vocational training | College/university degree |
| Some secondary | College/university 4+ years | |
| Technical/high school, education unfinished | Complete third level | |
| Graduate or professional education | ||
| Graduated | ||
| High school, additional education | ||
| Masters level or higher | ||
| Post graduation level | ||
| Same college (< 4 years) | ||
| Some third-level university |
| Level of activity | |
|---|---|
| No exercise/sedentary | At least some activity |
| < 600 MET minutes/week | ≥ 10,000 steps/day |
| < 600 MET hours/week | ≥ 600 MET minutes/week |
| Accelerometer < 2.5 hours/week | ≥ 600 MET hours/week |
| Does not attend gym | Accelerometer ≥ 2.5 hours/week |
| Does not exercise regularly at inclusion | Does attend gym |
| Fewer than 10,000 steps/day | Exercise regularly at inclusion |
| Low | Handiwork |
| PPAQ < 1000 cal | Hard |
| Sedentary | High |
| Sedentary work | Light–moderate |
| Work mainly sedentary | Moderate |
| Completely inactive | Moderate–hard |
| Completely sedentary | PPAQ ≥ 1000 cal |
| Lying | Physically active |
| Sedentary | Work in movement |
| Sedentary work | Work standing |
| Sitting | Work standing and in movement |
| Some activity occasionally | Active |
| Active (PPAQ) | |
| Active (exercise two or three times a week) | |
| Active work | |
| High-performance athlete | |
| Housewife | |
| Professional athlete | |
| Something active | |
| Standing | |
| Very active | |
| Very active (regular exercise four or five times a week) | |
| Walking | |
Appendix 5 Details of trials with unavailable individual participant data
| Study (first author and reference number) | Reason | Intervention group | Country | Sample size |
|---|---|---|---|---|
| Asbee et al.28 | No response | Mixed | USA | 100 |
| Barakat et al.42 | Conflict of interest | Exercise | Spain | 100 |
| Barakat et al.43 | Conflict of interest | Exercise | Spain | 510 |
| Bechtel-Blackwell30 | No response | Diet | USA | 46 |
| Briley et al.31 | No response | Diet | USA | 20 |
| aCallaway et al.145 | Not approached | Exercise | Australia | 50 |
| Clapp et al.44 | Data loss | Exercise | USA | 51 |
| Deveer et al.32 | No response | Diet | Turkey | 100 |
| Garshasbi et al.33 | No response | Exercise | Iran | 212 |
| Gomez-Tabarez et al.34 | No response | Diet | Colombia | 60 |
| Hopkins et al.146 | Contact loss | Exercise | New Zealand | 81 |
| Huang et al.35 | No response | Mixed | Taiwan | 125 |
| Jackson et al.147 | Contact loss | Mixed | USA | 321 |
| Korpi-Hyövälti et al.39 | Lack of time | Diet | Finland | 54 |
| Lee et al.45 | Data loss | Exercise | UK | 370 |
| Marquez-Sterling et al.148 | Contact loss | Exercise | USA | 15 |
| Polley et al.36 | No response | Mixed | USA | 110 |
| Quinlivan et al.41 | Data sharing issues | Diet | Australia | 132 |
| Santos et al.37 | No response | Exercise | Australia | 72 |
| Sedaghati et al.38 | No response | Exercise | Iran | 90 |
| Thornton et al.40 | Lack of time | Diet | USA | 232 |
| Vesco et al.149 | Contact loss | Mixed | USA | 114 |
| Ramírez-Vélez et al.59 | Not approached | Exercise | Colombia | 64 |
| Ramírez-Vélez60 | Not approached | Exercise | Colombia | 20 |
| Badrawi et al.29 | No response | Diet | Egypt | 100 |
| Cordero et al.47 | Not approached | Exercise | Spain | 342 |
| de Oliveria Melo et al.47 | Not approached | Exercise | Brazil | 187 |
| Di Carlo et al.50 | Not approached | Diet | Italy | 154 |
| Hawkins et al.51 | Not approached | Mixed | USA | 68 |
| Hui et al.52 | Not approached | Mixed | Canada | 113 |
| Jing et al.53 | Not approached | Mixed | China | 262 |
| Kong et al.54 | Not approached | Exercise | USA | 42 |
| Murtezani et al.57 | Not approached | Exercise | Republic of Kosovo | 72 |
| Price et al.58 | Not approached | Exercise | USA | 91 |
| Li et al.57 | Not approached | Mixed | China | 239 |
| Ronnberg et al.61 | Not approached | Exercise | Sweden | 445 |
| Bisson et al.46 | Not approached | Exercise | Canada | 37 |
| Mujsindi et al.47 | Not approached | Mixed | USA | 79 |
Appendix 6 Clinical characteristics of the randomised controlled trials
| Study, year and language | Methods | Participants | Interventions | Control | Outcomes |
|---|---|---|---|---|---|
| Althuizen et al.,90 2013; English | Method of randomisation: computerised random number generator Allocation concealment: prestratified allocation schedule for each practice with group allocation at random Blinding: examiners who assessed anthropometric outcome measures were unaware of group allocation. Participants could not be blinded but were requested not to reveal information about their treatment to the examiners. The coding key for group assignment was only known to the central database programmer |
Inclusion criteria
|
Two personal counsellors with a background in physical activity or remedial education provided five counselling sessions at 18, 22, 30 and 36 weeks of gestation and at 8 weeks post partum. Principles of a psychological intervention method called ‘problem-solving treatment for primary care’ were used. Sessions lasted for 15 minutes, except the first that lasted 30 minutes. A general information brochure was provided after the first session. The sessions were aimed at making the participants aware of issues related to weight gain in pregnancy, including IOM guidelines. Weight gain charts specific to BMI categories with markings to show recommended weight gain (IOM guidelines) were provided. Dietary advice was provided as per Dutch nutrition centre guidelines, with emphasis on healthy eating, adjusting energy intake to activity levels and decreasing intake of high-fat food. Physical activity was assessed by questionnaires and general information provided. Specific individualised activities were discussed in those not meeting physical activity guidelines. The American Centre for Disease Control and Prevention guidelines formed the basis for physical activity counselling. The last counselling session (via telephone) focused on delivery, breastfeeding, care of the newborn infant along with physical activity and diet The counsellors were trained for the study by recording conversations with 10 pregnant women, followed by feedback on performance by other members of the research team |
Standard care | Primary:
|
| Baciuk et al.,79 2008; English | Method of randomisation: computer-generated randomisation list of numbers. Volunteers were enrolled sequentially and randomised to one of the two study groups Allocation concealment: each sequential number corresponded to a sealed opaque envelope containing the information on the randomisation group Blinding: outcomes assessors |
Pregnant women < 20 weeks of gestation, carrying single fetus, with no gestational risk factors Inclusion criteria:
|
Physical activity (water aerobics): the intervention was the regular, moderate practice of water aerobics for 50 minutes three times a week in an indoor swimming pool with water warmed at 28–30 °C. Water aerobics was initiated following the first physical evaluation and continued up to delivery. The moderate intensity of exercises during the sessions was assured by monitoring patients’ heart rate using a heart rate monitor and kept around 70% of one’s predicted maximum heart rate | No intervention |
|
| Barakat et al.,80 2008; English | Method of randomisation: not reported Allocation concealment: the investigator responsible for randomly assigning participants did not know in advance which group the next person would be allocated to, and was not part of assessment Blinding: outcomes assessors |
Inclusion criteria:
|
The programme consisted of 35- to 40-minute sessions thrice weekly from 12–13 weeks of gestation to end of pregnancy (38–39 weeks), with an estimated average of 80 sessions per participant. They were supervised by a trained fitness specialist with each group consisting of 10–12 women. The venue was spacious and well lit with favourable conditions (altitude 600 m, temperature 19–21 °C and humidity 50–60%). The sessions were accompanied by music. The exercise activity was of light to moderate intensity with a target heart rate of ≤ 80% of maximum predicted heart rate for age (220 – age). All participants were provided with heart rate monitors. Each session included a warm-up (8 minutes), a core session (20 minutes) and a cool-down period (8 minutes). Warm-up and cool-down components involved light stretching exercises for limbs, neck and trunk. In addition, the cool-down period included relaxation exercises The core portion involved toning and very mild resistance exercises. Toning included shoulder shrugs and rotations, arm elevations and leg lateral elevations, pelvic rocks and tilts. The resistance exercises included one set of 10–12 repetitions of each of (1) abdominal curls and (2) the below exercises using barbells (3 kg/exercise) or low to medium resistance bands: bicep curls, arm side lifts and extensions, shoulder elevations, bench press, seated lateral row, leg circles and lateral leg elevations, knee (hamstring) curls and extensions, ankle flexions and extensions Exercises such as jumping, ballistics, extreme stretching and joint overextension were avoided |
The women were asked to maintain their level of activity |
|
| Barakat et al.,81 2011; English | Method of randomisation: use of a random number table Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
The programme consisted of 35- to 45-minute sessions thrice weekly from 6–9 weeks of gestation to the end of pregnancy (38–39 weeks), with an estimated average of 85 sessions per participant. The participants were supervised by a trained fitness specialist with each group consisting of 10–12 women. The venue was spacious and well lit with favourable conditions (altitude 600 m, temperature 19–21 °C and humidity 50–60%). High room temperatures and humid environment were avoided. The sessions were accompanied by music. The exercise activity was of light to moderate intensity with a target heart rate of ≤ 70% of maximum predicted heart rate for age (220 – age). All participants were provided with heart rate monitors Each session included a warm-up (7–8 minutes), a core session (25 minutes) and a cool-down period (7–8 minutes). Warm-up and cool-down components involved light stretching exercises for limbs, neck and trunk. In addition, the cool-down period included relaxation and pelvic floor exercises The core portion involved toning and very mild resistance exercises. Toning included shoulder shrugs and rotations, arm elevations and leg lateral elevations, pelvic rocks and tilts. The resistance exercises included one set of 10–12 repetitions of each of (1) abdominal curls and (2) the below exercises using barbells (3 kg/exercise) or low- to medium- resistance bands: bicep curls, arm side lifts and extensions, shoulder elevations, bench press, seated lateral row, leg circles and lateral leg elevations, knee (hamstring) curls and extensions, ankle flexions and extensions Exercises such as jumping, ballistics, extreme stretching and joint overextension were avoided. Supine exercises were limited to 2 minutes and exercises involving the Valsalva manoeuvre were avoided. Care was taken to ensure adequate nutrition prior to exercise sessions |
Usual care |
|
| Barakat et al.,42 2012; English | Method of randomisation: computer-generated list of random numbers Allocation concealment: not reported Blinding: randomisation procedure including sequence generation, allocation concealment, and implementation was made for three different authors to facilitate blinding |
Inclusion criteria
|
The programme consisted of 40- to 45-minute sessions thrice weekly from 6 to 9 weeks of gestation to end of pregnancy (38–39 weeks), with an estimated average of 85 sessions per participant. The participants were supervised by a trained fitness specialist with each group consisting of 10–12 women. The venue was spacious and well lit with favourable conditions (altitude 600 m, temperature 19–21 °C and humidity 50–60%). The sessions were accompanied by music The exercise activity was of light to moderate intensity with a target heart rate of ≤ 70% of maximum predicted heart rate for age (220 – age). All participants were provided with heart rate monitors. Each session included a warm-up (7–8 minutes), a core session (25 minutes) and a cool-down period (7–8 minutes). Warm-up and cool-down components involved light stretching exercises for limbs, neck and trunk The core portion included exercises for arms and abdomen, and aerobic dance to improve posture, strengthen muscles of labour and pelvic floor and prevent lower back pain Exercises such as jumping, ballistics, extreme stretching and joint overextension were avoided. Supine exercises were limited to a maximum of 2 minutes and exercises involving the Valsalva manoeuvre were avoided. Care was taken to ensure adequate nutrition prior to exercise sessions |
Usual care |
|
| Bogaerts et al.,64 2013; English | Method of randomisation: not reported Allocation concealment: opaque envelopes Blinding: not reported |
Inclusion criteria:
|
Brochure group: a study-specific brochure containing information on diet and physical activity during pregnancy including tips to limit excessive GWG was provided Lifestyle intervention group: this group received the same brochure but additionally had four antenatal lifestyle intervention sessions. The sessions included a group of up to three women led by a midwife trained in motivational intervention techniques. Each session lasted 1.5 to 2 hours and occurred:
|
Routine antenatal care as per national guideline ‘prenatal care’ |
|
| Dodd et al.,72 (LIMIT) 2011; English | Method of randomisation: central telephone randomisation with computer-generated schedule, balanced variable blocks prepared by an independent investigator not involved in recruitment and clinical care, with stratification for BMI category (overweight vs. obese) parity (parity zero vs. one or more) and participating centre Allocation concealment: central randomisation service Blinding: only assessor blinded to outcomes |
Inclusion criteria:
|
Intervention: a combination of dietary, exercise and behavioural strategies, delivered by a research dietitian and trained research assistants. Balanced diet containing carbohydrates, fat and protein was encouraged. They were asked to reduce refined carbohydrates and saturated fats, increase intake of fibre, and consume two servings of fruit and five servings of vegetables each day. Women were encouraged to adopt a more active lifestyle, mainly by increasing the amount of walking. Interventions were tailored by stage theories of health decision-making that suggests individuals’ progress through a series of cognitive phases when undertaking behavioural change. Initially, as part of a planning session with a research dietitian, women were given written dietary and activity information, a tailored diet and physical activity plan, a diary and recipe book. Women were encouraged to set their own goals for lifestyle changes and monitor their progress with support from the research team They were also asked to identify the barriers to achieving their goals. They were supported at regular intervals throughout their pregnancy, by the research dietitian (at 28 weeks’ gestation) and trained research assistants (telephone calls at 22, 24 and 32 weeks’ gestation and a face-to-face interview at 36 weeks’ gestation) |
Usual hospital guidelines, with no routine provision of dietary, lifestyle and behavioural recommendations | Primary:
|
| El Beltagy et al.,65 2013; abstract, English | Method of randomisation: information not available Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
Mild physical activity programme and diet modification for 12 weeks | No details |
|
| Guelinckx et al.,66 2010; English | Method of randomisation: random allocation through block randomisation Allocation concealment: not reported Blinding: none |
Inclusion criteria:
|
Lifestyle intervention based on a brochure or on active education Passive group: provided with a brochure containing information on diet, physical activity and tips to limit GWG at the first antenatal consultation Active group: received same brochure and also actively counselled by a trained nutritionist in three group sessions at 15, 20 and 32 weeks of gestation. The sessions had up to five women and lasted 1 hour each. Counselling on balanced diet was based on the official national dietary recommendations (energy intake: 9–11%, proteins; 30–35%, fat; 50–55%, carbohydrates). Aim was to limit intake of energy-dense foods, replacing with healthier alternatives such as fruits, increasing wholewheat grains and low-fat dairy products, and reducing saturated fatty acids. General topics such as energy balance, body composition, food labels, and physical activity were discussed. Tips for behavioural modification to reduce emotional eating and binge eating were provided. Total energy intake was not restricted in any group but aimed to do so indirectly by limiting the intake of energy-dense foods. Nutritional data were obtained from 7-day dietary records. A physical activity score was calculated for each trimester of the pregnancy by using the Baecke questionnaire |
No intervention |
|
| Haakstad and Bo,82 2011; English | Method of randomisation: simple computerised randomisation without stratification by a secretary not involved in exercise classes or assessment Allocation concealment: not reported Blinding: assessor blind. Participants were asked not to reveal their allocation to the principal investigator involved in outcomes assessment |
Inclusion criteria:
|
Physical activity: the exercise programme followed the ACOG exercise prescription. Participants were encouraged to participate in at least two out of three possible 1-hour classes per week, for a minimum of 12 weeks. Each session had a maximum of 25 participants and was supervised by certified aerobic instructor. Each session included 5 minutes of warm up, 35 minutes of aerobic dance, 15 minutes of strength training focusing on deep abdominal, pelvic floor and back muscles and 5 minutes stretching and relaxation The aerobic activities were low-impact and of moderate intensity with ratings of perceived exertion at 12–14 (somewhat hard) on the 6–20 Borg’s rating scale. Sudden movements were avoided and activities such as jumping, running and rotations were restricted Women were also asked to include 30 minutes of self-imposed moderate exercise at home on no-exercise days |
Participants were neither encouraged nor discouraged from exercising |
|
| Harrison et al.,73 2013; English | Method of randomisation: computer-generated randomisation Allocation concealment: sealed opaque envelopes Blinding: care providers, researchers and outcome assessors were blinded to group allocation |
Inclusion criteria:
|
Individual four sessions behaviour change lifestyle intervention in antenatal clinic setting at 14–16, 20, 24 and 28 weeks of gestation. The intervention was based on the social cognitive theory, adapted from the study group’s earlier lifestyle intervention programme (HeLP-her) The sessions were delivered by a health coach (exercise physiologist). Healthy eating and physical activity were encouraged along with specific dietary advice in pregnancy. Behavioural change strategies were aimed at identifying short-term goals and promoting self-efficacy and self-monitoring Goals included lifestyle changes such as reducing high-fat or convenience foods, increasing fruit/vegetable intake and increasing frequency of physical activity. Participants themselves set goals Pedometers and weight-gain charts based on IOM recommendations were provided to monitor the progress. Written Australian dietary and physical activity guidelines and other resources to encourage optimal health, GWG and lifestyle were provided |
A single brief education session based on Australian Dietary and Physical Activity Guidelines was provided along with written versions of guidelines. GWG was not discussed | Primary:
|
| Hui et al.,91 2012; English | Method of randomisation: computer-generated randomisation allocation table performed by a staff member without involvement in the study design Allocation concealment: sealed, labelled envelope Blinding: participants and study staff were not blinded |
Inclusion criteria:
|
Exercise component: a community-based exercise programme – recommended exercise included walking, mild to moderate aerobic, stretching and strength exercises (3–5 times per week for 30–45 minutes/session). The programme started around 20–26 weeks of gestation and finished at 36 weeks. The group exercise sessions were held in air-conditioned gymnasia in community centres. Group floor aerobic, stretching and strength exercises were led by licensed fitness trainers. Participants were instructed to record daily physical activities in activity logs Dietary component: interviews and counselling were provided twice in pregnancy by registered dietitians (at enrolment and 2 months after enrolment). Dietitians provided personalised dietary counselling to participants based on their food choice map interview results, pregnancy week, weight gain and the Health Canada guidelines for food intake in pregnancy151,152 |
Standard care: standard prenatal care recommended according to the Society of Obstetricians and Gynaecologists of Canada. Exercise instruction and dietary intervention were not provided to participants in the control group |
|
| Jeffries et al.,92 2009; English | Method of randomisation: computer random number generator Allocation concealment: number cards allocating women to the two groups were placed in opaque, sequentially numbered envelopes Blinding: patients |
Inclusion criteria:
|
Women allocated to the intervention group were given personalised weight measurement card including information on optimal GWG (based on their BMI at the time of recruitment and the US IOM guidelines) and were asked to record their weight at 16, 20, 24, 28, 30, 32 and 34 weeks of gestation The patient was allowed to choose to measure weight at hospital or at home |
No intervention |
|
| Khaledan et al.,83 2010; Persian/English | Method of randomisation: not reported Allocation concealment: not reported Blinding: none |
Inclusion criteria:
Number of participants
|
Three exercise sessions per week of 30–45 minutes each for 8 weeks. The first 15 minutes included stretching exercises. The aerobic component started as 5-minute session and progressively increased by 1 minute per session. The intensity was maintained by heart rate within 60% of maximum heart rate. This was followed by cooling down in sitting position for 10 to 15 minutes. Maximal heart rate was calculated through the formula 220 – age × 60/100 | No intervention |
|
| Khoury et al.,76 2005; English | Method of randomisation: the randomisation list was generated from a table of random numbers drawn up by the investigator who had no contact with the participants Allocation concealment: consecutively numbered, sealed, opaque envelopes provided to dietitian delivering intervention Blinding: investigators/clinicians and outcomes assessors |
Inclusion criteria:
|
Diet/dietary advice: cholesterol-lowering diet from gestational week 17–20 to birth Dietitian visits were arranged at inclusion, and at 24, 30 and 36 weeks of gestation Aims of dietary intervention were to:
Cooking lessons were arranged for special foods. Coffee was limited to two cups/day |
Control group was advised to consume their usual diet, not to introduce more oils, low-fat meat and dairy products than usual. Target weight gain was 8–14 kg and energy intake breakdown of fats, carbohydrate and proteins was same as intervention group |
|
| Luoto et al.,62 2011; English | Method of randomisation: cluster randomisation of municipalities that were paired on the basis of number of births, population size, socioeconomic status and type (rural/urban). The municipalities, not participants, were randomised into intervention and study groups Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
Five counselling sessions at 8–12 weeks, 16–18 weeks, 22–24 weeks, 32–34 weeks and 36–37 weeks. One primary session each for physical activity and diet followed by booster sessions. The primary session was 20- to 30-minutes long, but the booster sessions lasted for 10–15 minutes. The interventions were based on PRECEDE–PROCEED and stages of change models. GWG: IOM recommendations were discussed. A BMI-specific weight-gain chart was included Physical activity: the aim was to increase leisure time physical activity to meet recommendations or maintain it if they had already reached it. The weekly action plan was agreed with each participant and the recommended minimum weekly leisure time physical activity dose was 800 MET minutes. Monthly 2-hour thematic meetings including group exercises were offered and these were led by physiotherapists Diet: advised as per Finnish dietary recommendations – saturated fat ≤ 10%, polyunsaturated fat 5–10% and total fat 25–30% (includes saturated, monounsaturated, polyunsaturated and trans-fatty acids) of total energy intake and fibre 25 to 35 g/day. They were encouraged to include high-fibre bread, five portions of fruits/vegetables, low-fat dairy products, fish twice weekly, only moderate amounts of spread/oil and restrict sugar containing snacks/drink. The nurse checked if the written objectives were met at each booster visit |
Routine care including usual dietary and physical activity counselling | Primary:
|
| Nascimento et al.,84 2011; English | Method of randomisation: list of random numbers generated by SAS version 9.1 statistical program (SAS Institute Inc., Cary, NC, USA) Allocation concealment: sealed sequentially numbered opaque envelopes Blinding: not blinded |
Inclusion criteria:
|
Exercise protocol: women performed exercise weekly under the guidanceof a trained physical therapist. The exercises were light to moderate intensity exercises, with heart rates not exceeding 140 beats per minute. (ACOG recommendations). Standardised research protocol consisting of a 22-exercise sequence was followed. Group or individual exercises lasted 40 minutes with 10 minutes of general stretching, 22 minutes of exercises to strengthen the limb muscles, and 10 minutes of guided relaxation. Home exercise counselling. Women were counselled on home exercise to be done five times/week, with exercises from the protocol or walking. They were required to note the details of daily exercise in a monthly exercise book | Routine antenatal advice and standard nutritional counselling. They were not provided physical activity counselling | Primary:
|
| Ong et al.,67 2009; English | Method of randomisation: none reported Allocation concealment: none reported Blinding: not reported |
Inclusion criteria:
|
Physical activity: home-based exercise programme beginning at week 18 of gestation; three sessions per week of stationary cycling (home-based) supervised exercise. Exercise training was performed at home on an upright stationary cycle ergometer provided to each participant for the study period. Each session consisted of a 10-minute warm-up followed by one or two 15-minute bouts of cycling (with rest periods if necessary). Exercise intensity was controlled by heart rate initially aimed at 50–60% of maximum heart rate and later increased to 60–70% of maximum heart rate. The duration was later increased to 40–45 minutes. Sessions ended with a 10-minute cool-down period of slow pedalling | No intervention |
|
| Oostdam et al.,75 2012; English | Method of randomisation: computer generated randomised allocation schedule prestratified to the centre where participant will be followed up. Within each centre, participants randomly allocated to study or control group. Block randomisation in blocks of four performed Allocation concealment: only the programmer of central database knew key of coding related to group assignment Blinding: independent examiners assessing outcomes blinded but participants and researchers could not be blinded |
Inclusion criteria:
|
Exercise programme of aerobic and strength training twice weekly under supervision of a trained physiotherapist from recruitment through to remainder of pregnancy. Each session lasted for 60 minutes. Aerobic training provided using cycle ergometers, treadmills, cross-trainers and rowing machines. Strength and aerobic training tailored to individual participants, taking into consideration predicted maximum muscle strength, aerobic capacity and target heart rate. ACOG recommendations were used as a guidance | Usual care by midwives and obstetricians | Primary:
|
| Perales et al.,85 2015; English | Method of randomisation: computer-generated list of random numbers was used Allocation concealment: not reported Blinding: researchers and outcome assessors were blinded. Randomisation procedure including sequence generation, allocation concealment, and implementation was made for three different authors to facilitate blinding. Blinding of participants was not possible |
Inclusion criteria:
|
The programme consisted of three 55- to 60-minute sessions thrice weekly from 9–12 weeks of gestation to the end of pregnancy (39–40 weeks of gestation). Each session consisted of warm-up (5–8 minutes), aerobic dance and resistance exercises for muscle groups of legs, buttocks and abdomen to stabilise the lower back (25 minutes), balancing exercises (10 minutes), pelvic floor muscle training (10 minutes) and a cool-down period (5–8 minutes). Exercises in supine position were limited to 2 minutes and extreme stretching, jumping, ballistic movements, overextension of joints and exercises involving Valsalva manoeuvre were specifically avoided The exercise intensity was light to moderate and was guided by the target heart rate (55–60% of maximum heart rate) for each participant displayed on a poster. All participants wore heart rate monitors during exercise sessions. Karvonen’s formula based on trimester, physical condition and age was used to calculate maximum heart rate. Borg scale ratings were also used to adjust the intensity of exercise. Sessions had groups of 10–12 women and were supervised by a qualified fitness specialist and assisted by an obstetrician. The venue was a spacious well-lit room in a hospital (altitude 600 m, temperature 19–21 °C, and humidity 50–60%) and sessions were accompanied by music. Care was taken to ensure adequate nutrition prior to exercise sessions |
Usual care |
|
| Perales et al.,85 2015; English | Method of randomisation: computer-generated list of random numbers Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
The programme consisted of three 55- to 60-minute sessions thrice weekly from 9–12 weeks of gestation to end of pregnancy (39–40 weeks’ gestation). Each session consisted of warm-up (5–8 minutes), aerobic dance and resistance exercises for muscle groups of legs, buttocks and abdomen to stabilise the lower back (25 minutes), balancing exercises (10 minutes), pelvic floor muscle training (10 minutes) and a cool-down (5–8 minutes). Exercises in supine position were limited to 2 minutes and extreme stretching, jumping, ballistic movements, overextension of joints and exercises involving Valsalva manoeuvre were specifically avoided The exercise intensity was light to moderate and was guided by the target heart rate (55–60% of maximum heart rate) for each participant displayed on a poster. All participants wore heart rate monitors during exercise sessions. Karvonen’s formula based on trimester, physical condition and age was used to calculate maximum heart rate. Borg scale ratings were also used to adjust the intensity of exercise. Sessions had groups of 10–12 women and were supervised by a qualified fitness specialist and assisted by an obstetrician. The venue was a spacious well-lit room in a hospital (altitude 600 m, temperature 19–21 °C, and humidity 50–60%) and sessions were accompanied by music. Care was taken to ensure adequate nutrition prior to exercise sessions |
Usual care |
|
| Petrella et al.,74 2014; English | Method of randomisation: computer program that generated random allocation in blocks of three Allocation concealment: sealed numbered white envelopes Blinding: both gynaecologist and dietitian delivering the interventions knew allocation of the patient |
Inclusion criteria:
|
Diet: the intervention group diet was initiated at randomisation by a gynaecologist and a dietitian who provided a further 1-hour counselling on recommended weight gain in pregnancy for each BMI category. The calorie allowance was 1500 kcal/day with an extra 200 kcal/day for obese women and 300 kcal/day for overweight women to account for physical activity programme. The target diet composition was 55% carbohydrate (80% complex low glycaemic index), 20% protein (50% animal and 50% vegetable) and 25% fat (12% monounsaturated, 7% polyunsaturated and 6% saturated fat) given as three main meals and three snacks. The last snack was 2 hours after dinner to prevent overnight hypoglycaemia The minimum recommended intake of carbohydrates was 225 g/day. Urine was examined for ketonuria thrice during pregnancy Exercise: the exercise intervention was in line with recommendations for the general population. Women were advised 30 minutes of moderate intensity activity for a minimum of 3 days a week. Adherence was checked by a pedometer. Women were advised that the exercise intensity should allow them to maintain a conversation (‘talk test’) |
The control group received a simple nutritional booklet based on Italian guidelines for a healthy diet during pregnancy | Primary:
|
| Phelan et al.,93 2011; English | Method of randomisation: computerised, randomly changing block sizes, stratified as per clinic and BMI category Allocation concealment: opaque envelopes Blinding: no blinding |
Inclusion criteria:
|
Standard care plus a behavioural lifestyle intervention (‘Fit for Delivery’) to avoid excessive weight gain during pregnancy. The intervention was based on the 1990 IOM guidelines for weight and nutrition during pregnancy and used established principles of learning theory to encourage changes in eating and physical activity. The intervention included a face-to-face interview with an interventionist at the start of treatment. Discussion focused on appropriate GWG targets, physical activity (30 minutes of walking, most days), and calorie intake (20 kcal/kg). Daily self-monitoring of weight, diet and physical activity was recommended along with emphasis on limiting high-fat foods Weight scales, food diaries and pedometers were given to facilitate self-monitoring Postcards promoting healthy lifestyle were mailed weekly. Personalised weight gain graphs with feedback were provided following each visit Telephone support of dietitian was offered thrice during intervention to all women in intervention group. Women failing to meet targets received up to two additional telephone calls/month with personalised advice including structured meal plans until targets were met |
Standard scheduled visits, monthly until 28 weeks of gestation, fortnightly between 28 and 36 weeks of gestation, weekly until delivery and at 6 weeks after delivery. Participants were provided standard nutrition counselling by physicians, nutritionists, nurses, and counsellors A brief (15-minute) face-to-face visit with the study interventionist was arranged at recruitment. Women were provided with study newsletters containing general information related to pregnancy such as vitamins, at 2-monthly intervals and post partum. Women were weighed regularly but were not given weight graphs |
Primary:
|
| Poston et al.,68 (UPBEAT trial) 2015; English | Method of randomisation: online, computer-generated program. The randomisation schedule was minimised according to ethnicity, parity, age, BMI and centre Allocation concealment: sequential study numbers allocated, irrespective of allocation to the intervention or control group Blinding: not reported |
Inclusion criteria:
|
One-to-one interview at baseline with a health trainer specifically trained for the study, followed by eight weekly sessions of 1 to 1.5 hours each. Women are encouraged to attend all and strongly recommended to attend a minimum of five sessions with other sessions covered by telephone or e-mail. Health trainers cover specific goal-setting, self-monitoring and feedback on performance, problem-solving and use of social support. Women were provided with handbook, DVD of recommended exercise regime, pedometer, logbook for recording weekly goals and steps achieved through pedometer Exercise advice: to increase pedometer steps and daily activity incrementally; moderate activity in the form of walking encouraged in line with UK RCOG recommendations, with more options depending on baseline activity Diet: to promote healthier eating with no restriction of calories, substitute low glycaemic index for medium/high glycaemic index food, restrict sugar-sweetened beverages but not fruits and reduce saturated fatty acid intake |
Routine antenatal care, explaining the risks of obesity, advising on healthy diet and safe levels of physical activity | Primary:
|
| Prevedel et al.,86 2003; Portuguese (Brazilian) | Method of randomisation: women were randomly selected (model randomised) Allocation concealment: not reported Blinding: no blinding used |
Inclusion criteria:
|
Physical activity (moderate intensity hydrotherapy): the hydrotherapy programme was delivered by the physiotherapist accompanied by the obstetrician in subgroups of up to 10 pregnant women. The exercises were of moderate intensity and carried out at a heated and covered swimming pool (temperature between 28 °C and 32 °C) thrice a week and duration of 1 hour. The sessions were based on ACOG recommendations and comprised stretching, heating, resistance, localised exercises and relaxation with breathing exercises Exercise intensity was controlled by heart rate monitoring by frequency–grip |
No intervention |
|
| Rauh et al.,63 2013; English | Method of randomisation: computer-generated cluster randomisation of gynaecological practices into intervention or control groups Allocation concealment: randomisation performed by a researcher not involved in study design Blinding: study design did not permit blinding |
Inclusion criteria:
|
The intervention group received two individual counselling modules at 20 and 30 weeks of gestation, the first session lasting 60 minutes and the second 30 minutes. General lifestyle advice including nutrition, physical activity and appropriate GWG was provided. Healthy nutrition and energy balance as per German Nutrition Society were explained. The dietary goals were to reduce the intake of high-fat and energy-dense foods and increase the intake of low-fat foods and fruits, wholegrain foods and vegetables. Women were encouraged to consume more fish and advised regarding appropriate fat/cooking oil/spreads Physical activity equivalent to 30 minutes of moderate-intensity exercises on most days was recommended. Non-weight bearing endurance exercises such as walking, swimming, aquatic exercises and cycling were suggested. Women were also provided with information on local antenatal exercise programmes and encouraged to join them. The exercise recommendations were based on the guidelines of ACOG and Society of Obstetricians and Gynecologists of Canada Women were provided with personalised weight charts as per BMI category including IOM recommendations for that category. They were asked to monitor their weights on a weekly basis The individual counselling sessions also provided personalised feedback on diet and physical activity based on the 7-day records of diet and physical activity questionnaires |
Routine antenatal care including an information leaflet consisting of 10 general statements on a healthy lifestyle during pregnancy not including advice on diet or gaining weight | Primary:
|
| Renault et al.,69 2014; English | Method of randomisation: randomisation was stratified by parity to ensure equal distribution of primiparous women in all groups Allocation concealment: a web allocation by an independent agency allowed allocation concealment Blinding: not reported |
Inclusion criteria
|
All participants (before enrolment) received one consultation with a dietitian after the initial ultrasound scan at 11–14 weeks of gestation. A low-fat low-calorie (1200–1675 kcal/day) Mediterranean-style diet, with preference to fish and oils was recommended. Dietary advice was as per Danish national guidelines for healthy eating. Only oral advice was given and women were asked to aim for a GWG of < 5 kg Physical activity: a dietitian advised to increase physical activity aiming for a daily step count of 11,000/day, a validated pedometer was provided to the participants. Pedometer data were recorded for a consecutive 7-day period every 4 weeks. Women were reminded through text messages when a recording period started and encouraged to achieve the target. If 11,000 steps were not achievable, they were asked to set their own targets. They were asked to enter the pedometer data and weight into a chart and return it Diet: the women in the physical activity plus diet group also had alternate face-to-face or telephone consultations with an experienced dietitian every 2 weeks (11–13 consultations) during pregnancy. They received feedback, encouragement and specific dietary advice if diet was incorrect or if weight targets were not being achieved |
Standard care including one consultation with a dietitian after the initial ultrasound scan at 11–14 weeks’ gestation. Dietary advice was as per Danish national guidelines for healthy eating. Only oral advice was given and women were asked to aim for a GWG of < 5 kg | Primary:
|
| Ruiz et al.,87 2013; English | Method of randomisation: computer generated Allocation concealment: not reported Blinding: not reported |
Inclusion criteria
|
The programme consisted of supervised 50- to 55-minute physical activity sessions thrice weekly from week 9 to weeks 38–39, with an estimated average of 85 sessions per participant. Each group consisted of 10–12 women. The exercise activity was of light to moderate intensity with a target heart rate of ≤ 60% of maximum predicted heart rate for age [208 – (0.7 × age in years)]. All participants were provided with heart rate monitors. Intensity was also guided by Borg’s conventional (6–20 point) scale, with the rate of perceived exertion ranging from 10 to 12 (‘fairly light’ to ‘somewhat hard’) Each session included a warm-up period (10 minutes), a core session (25–30 minutes) and a cool-down period (10 minutes). Warm-up and cool-down components involved walking and light stretching exercises for limbs, neck and trunk. In addition, the cool-down period included relaxation and pelvic floor exercises The core portion involved moderate-intensity aerobic exercises once weekly and resistance exercises twice a week. Aerobic dance took place for periods of 3–4 minutes with 1-minute breaks and included stretching and relaxation. Resistance exercises for pectoral muscles, back, shoulder, upper and lower limb muscles aimed to improve posture, strengthen muscles of labour and pelvic floor and prevent lower back pain. They involved exercises using barbells (3 kg/exercise) or low to medium resistance elastic and included biceps curls, arm side lifts and extensions, shoulder elevations, bench press, seated lateral row, leg circles and lateral leg elevations, knee (hamstring) curls and extensions and ankle flexions and extensions Exercises such as jumping, ballistics, extreme stretching and joint overextension were avoided. Supine exercises were limited to a maximum of 2 minutes |
Usual care with regular scheduled visits to obstetricians and midwives. Information health-care professionals provided nutrition and physical activity counselling and they were not discouraged from exercising | Primary:
|
| Sagedal et al.,153 2016; English | Method of randomisation: computer-generated randomisation list with groups of 20 women. Consecutive randomisation based on the time of completion of consent form, questionnaires and blood tests required prior to enrolment Allocation concealment: staff providing intervention and checking outcomes were not involved in randomisation Blinding: not reported |
Inclusion criteria:
|
Diet: an initial telephone consultation with a physician, nutritionist or graduate student of public health, followed by another follow-up session 4–6 weeks later. Recommendations based on Norwegian directorate of health guidance. Focus on 10 key recommendations including intake of fruits and vegetables, drinking water instead of energy drinks, having regular meals and reducing intake of drinks and snacks containing added sugar. Pamphlets containing the key recommendations provided to the intervention group along with password-protected access to an interactive website containing information on healthy eating and exercise in pregnancy. They were also invited to two evening meetings where further information on the trial was provided along with a hands-on cooking class to reinforce their dietary recommendations Physical activity: two exercise sessions each week lasting 1 hour at local fitness centres where attendance was registered. The sessions were supervised by physiotherapists or graduates of sports science. Uniform exercise plan for all the women in intervention group, consisting of 40 minutes of strength training and moderate cardiovascular exercises, and 20 minutes warm-up and stretching. Pelvic floor exercises were included in each session. The women were also encouraged to have at least one additional unsupervised exercise session weekly with the eventual goal of achieving a total of 30 minutes of moderate activity 5 days a week. Information on safe physical activity in pregnancy provided in pamphlets and on the website |
Standard prenatal care |
|
| Stafne et al.,88 2012; English | Method of randomisation: concealed randomisation in blocks of 30 by web-based computerised procedure Allocation concealment: staff involved with training/assessment not involved in randomisation Blinding: unblinded except glucose and insulin measurements blinded to group allocation |
Inclusion criteria:
|
Standardised exercise programme including aerobic activity, strength training and balance exercises supervised by a physiotherapist. Training sessions in groups of 8–15 women offered once weekly for 12 weeks (between 20 and 36 weeks of gestation). Each session lasted 60 minutes A written 45-minute home exercise programme (30 minutes of endurance training and 15 minutes of strength/balance exercises) was recommended twice weekly and women were asked to record the exercise activities in personal training diaries. Physical activity was also assessed by questionnaires |
Usual care, not discouraged from exercising. Written recommendations on diet, pelvic floor exercises and pregnancy-related lumbopelvic pain | Primary:
|
| Vinter et al,70 2011; English | Method of randomisation: computerised 1 : 1 randomisation with stratification by smoking status Allocation concealment: closed envelopes were used Blinding: not reported |
Inclusion criteria:
|
Intervention type: dietary counselling and exercise Diet: trained dietitians provided counselling based on official Danish recommendations at 15, 20, 28 and 35 weeks’ gestation. The goal was to limit GWG in pregnancy to 5 kg. Individualised calorie goals based on weight and activity level were provided Physical activity: moderate physical activity lasting 30–60 minutes was encouraged and a pedometer was provided to motivate and improve physical activity. A free full-time membership to local fitness centre was provided for 6 months. This included a 1-hour weekly closed training session with a physiotherapist. The exercises included aerobic activities with elastic bands and light weights, and balance exercises. The women were grouped 4–6 times with the physiotherapist after physical training |
Information on purpose and content of the study. Access to a website with advice on diet and physical activity in pregnancy | Primary:
|
| Vítolo et al,77 2011; Portuguese | Method of randomisation: not reported Allocation concealment: not reported Blinding: not reported |
Inclusion criteria
|
Dietary counselling according to nutritional status. For pregnant women with low birthweight, this was adopted as a priority to increase the energy density of the diet with the addition of a tablespoon of oil in the main meals, eat two snacks per day of high energy (with sample portions) 100 g once a week and fruit daily. Well-nourished pregnant women received vegetables, legumes, fruits and water six times per day and restricted the consumption of foods rich in fat and cooking oils. For pregnant women with excess weight, between meals (3–4 hours) were prioritised; not repeat the food portions of meals and snacks; restrict daily consumption of soft drinks and sweets, processed foods high in fat and also oil preparations. They were determined daily servings of vegetables, vegetables and fruit. All guidance provided values and portion sizes | The control group did not receive the dietary guidelines but were informed about their nutritional status and were asked to carry on with their prenatal care |
|
| Walsh et al.,78 (ROLO trial) 2012; English | Method of randomisation: not reported Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
One 2-hour dietary education session with the research dietitian in groups of two to six women. The diet was in line with current recommendations for pregnant women. General advice on healthy eating in pregnancy and following the food pyramid was provided. Women were taught about the rationale for having low glycaemic index food and encouraged to replace high glycaemic index carbohydrates with low glycaemic index alternatives. Written resources were provided after the education session. Women were not advised to reduce their total caloric intake. The research dietitian met women again at 28 and 34 weeks of gestation to reinforce the advice and clarify any doubts All women completed three food diaries of 3 days each: before dietary intervention and in the second and third trimesters of pregnancy A questionnaire was provided at 34 weeks visit to assess adherence to the diet. It was based on a five-point Likert-type scale (1 = ‘I followed the recommended diet all of the time’, 5 = ‘I followed the recommended diet none of the time’) |
Routine antenatal care with no specific dietary recommendation or advice about GWG | Primary:
|
| Wolff et al.,71 2008; English | Method of randomisation: computerised randomisation Allocation concealment: not reported Blinding: investigators/clinicians |
Inclusion criteria:
|
Ten 1-hour dietary consultations (healthy diet, restriction of energy intake): the intervention group received 10 consultations of 1 hour each with a trained dietitian during the pregnancy. Women were asked to eat a healthy diet according to the official Danish dietary recommendations (fat intake, maximum 30 energy per cent; protein intake, 15–20 energy per cent; carbohydrate intake, 50–55 energy per cent). Energy intake was restricted on the basis of individually estimated energy requirements and estimated energy requirements of fetal growth [energy requirement = basal metabolic rate × 1.4 (physical activity level factor of 1.2 + 0.2 added to cover energetic cost of fetal growth)] | No intervention |
|
| Yeo et al.,89 2000; English | Method of randomisation: not reported Allocation concealment: not reported Blinding: not reported |
Inclusion criteria:
|
Exercise of moderate intensity Exercise sessions of 30 minutes each were held in a laboratory three times a week A motorised treadmill and bicycle ergometer were alternated. Exercise consisted of a 5-minute warm-up using the Branching protocol, followed by a 30-minute steady state, and ended with a 10-minute cool down. Steady state was defined as RPE 13, which was considered a moderate level of exercise |
No intervention |
|
| Yeo,27 2013; protocol, English | Method of randomisation: predetermined block randomisation Allocation concealment: information not available Blinding: not reported |
Inclusion criteria
any of the following conditions:
Number of participants: data unpublished |
There are two intervention groups, walking exercise and stretching and the intervention runs for 10 weeks and involves 30-minute activity three times a week. The participants are free to choose the days of exercise provided they have a rest day between two exercise days. Research staff will train both groups for the first 2 weeks. Subsequently, one session per week will be supervised and the remaining two unsupervised. Childcare facilities are arranged either onsite or by arranging exercise venues with child care arrangements The walking group: walking exercise consists of 30 minutes of moderate intensity walking in an environment (home, gym, workplace and neighbourhood) agreed with the research staff. The exercise intensity is guided by a heart rate monitor and the RPE. Women are advised to maintain the heart rate to 55–69% of age determined maximum heart rate and are guided by the digital screen on their wrists that senses information from the chest belts they wear. The suggested RPE is 12 or 13. If there is a discrepancy between heart rate and RPE, they are advised to keep both within/below the recommended limits Stretching group: this consists of 30 minutes of stretching exercise thrice weekly without increasing the heart rate by more than 10% of the resting heart rate. The exercise involves slow muscle movements without aerobic or muscle resistance components, and participants are guided by a videotape showing recommended movements |
Research nurse visits for 30 minutes every other week to take measurements and is allowed to answer any queries related to healthy pregnancy and lifestyle |
|
Appendix 7 Risk-of-bias assessment
FIGURE 21.
Detailed assessment of risk of bias: all eligible trials (n = 74). a, Data from the secondary publication of Dekker Nitert et al. 49

FIGURE 22.
Detailed assessment of risk of bias: all eligible trials (n = 74) (continuation).
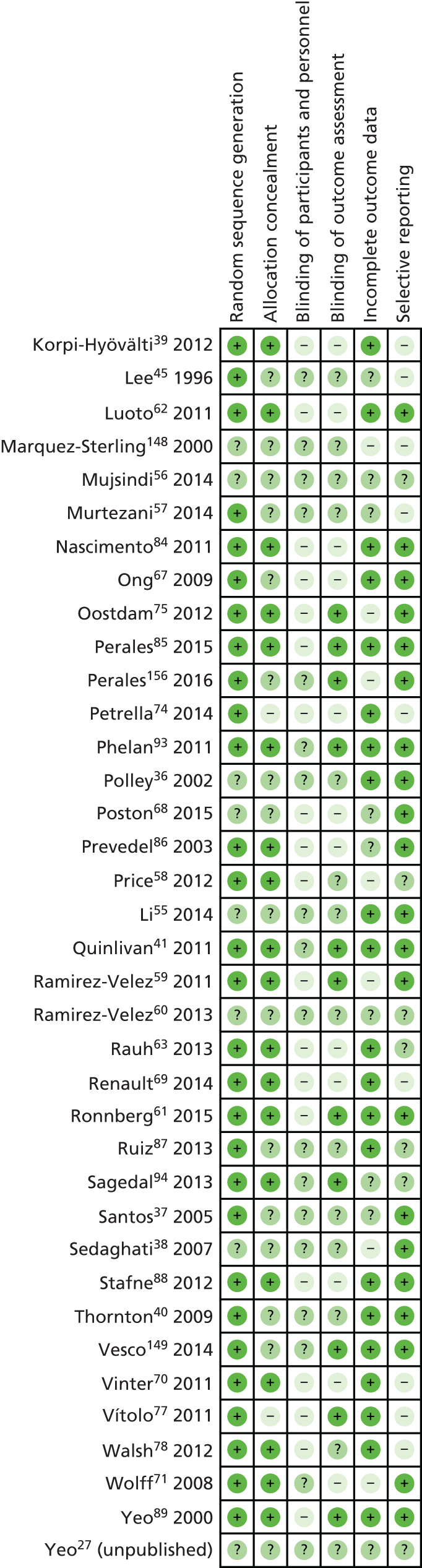
| Study (first author and reference number) | Item | Global risk of bias | |||||
|---|---|---|---|---|---|---|---|
| 1: randomisation | 2: allocation concealment | 3: blinding of participants | 4: blinding of outcome assessment | 5: incomplete outcome data | 6: selective reporting | ||
| Baciuk et al.79 | Low | Low | High | Low | Low | Low | Low/medium |
| Barakat et al.80 | Unclear | Unclear | Unclear | Low | Low | Low | Low/medium |
| Barakat et al.81 | Low | Unclear | Unclear | Unclear | Low | Low | Low/medium |
| Barakat et al.42 | Low | Unclear | Unclear | Unclear | High | Low | High |
| Bogaerts et al.64 | Low | Unclear | High | High | Low | Low | High |
| Dodd et al.72 | Low | Low | Unclear | Low | Low | Low | Low/medium |
| El Beltagy et al.65 | Low | Low | Unclear | Low | Low | Unclear | Low/medium |
| Guelinckx et al.66 | Low | Unclear | Unclear | High | High | High | High |
| Haakstad and Bo82 | Low | Low | Unclear | Low | Low | High | Low/medium |
| Harrison et al.73 | Low | Low | Unclear | Low | Low | Low | Low/medium |
| Hui et al.91 | Low | Unclear | High | Unclear | Low | Low | Low/medium |
| Jeffries et al.92 | Low | Low | Unclear | Low | Low | Low | Low/medium |
| Khaledan et al.83 | Low | Unclear | High | High | Low | Low | High |
| Khoury et al.76 | Low | Low | Unclear | Low | Low | Low | Low/medium |
| Luoto et al.62 | Low | Low | High | High | Low | Low | High |
| Nascimento et al.84 | Low | Low | High | High | Low | Low | High |
| Ong et al.67 | Low | Unclear | High | High | Low | Low | High |
| Oostdam et al.75 | Low | Low | High | Low | High | Low | High |
| Perales et al.85 | Low | Low | High | Low | Low | Low | Low/medium |
| Perales et al.156 | Low | Unclear | Unclear | Low | High | Low | High |
| Petrella et al.74 | Low | High | High | High | Low | High | High |
| Phelan et al.93 | Low | Low | Unclear | Low | Low | Low | Low/medium |
| Poston et al.68 | Unclear | Unclear | High | High | Unclear | Unclear | High |
| Prevedel et al.86 | Low | Low | High | High | Unclear | Low | High |
| Rauh et al.63 | Low | Low | High | High | Low | Unclear | High |
| Renault et al.69 | Low | Low | High | High | Low | High | High |
| Ruiz et al.87 | Low | Unclear | Unclear | Unclear | Low | Unclear | Low/medium |
| Sagedal et al.153 | Low | Low | Unclear | Low | Unclear | Unclear | Low/medium |
| Stafne et al.88 | Low | Low | High | High | Low | Low | High |
| Vinter et al.70 | Low | Low | High | High | Low | High | High |
| Vítolo et al.77 | Low | High | High | Low | Low | High | Low/medium |
| Walsh et al.78 | Low | Low | High | Unclear | Low | High | Low/medium |
| Wolff et al.71 | Low | Low | Unclear | High | High | Low | High |
| Yeo et al.89 | Low | Low | High | Low | Low | Low | Low/medium |
| Yeo (unpublished)27 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High |
Appendix 8 Sensitivity analysis for the main outcomes
| Sample size (number of studies) | Group, mean GWG (SD) | Adjusted differencea (95% CI) | 95% PI | |
|---|---|---|---|---|
| Control | Intervention | |||
| Primary analysis for GWG | ||||
| 9320 (33) | 10.8 (5.4) | 10.1 (5.4) | –0.70 (–0.92 to –0.48) | –1.24 to –0.16 |
| Analysis including aggregate data | ||||
| 12,895 (60) | 11.5b | 10.5b | –1.13 (–1.58 to –0.68) | –4.10 to 1.83 |
| Analysis excluding studies rated as being at a high risk of bias | ||||
| 5585 (15) | 11.5 (5.3) | 10.9 (5.2) | –0.67 (–0.95 to –0.38) | –1.14 to –0.19 |
| Analysis excluding participants with gestational age at follow-up < 37 weeks | ||||
| 5324 (28) | 12.2 (5.3) | 11.4 (5.4) | –0.91 (–1.17 to –0.66) | –1.17 to –0.66 |
| Analysis excluding women not adherent to intervention | ||||
| 8565 (33) | 10.8 (5.4) | 10.3 (5.4) | –0.76 (–1.00 to –0.52) | –1.31 to –0.21 |
| Analysis using change in BMI (kg/m2) | ||||
| 9238 (31) | 3.9 (2.0) | 3.6 (2.0) | –0.3 (–0.39 to –0.21) | –0.60 to 0.00 |
| Intervention groups | ||||
| Analysis including only studies with diet-based interventions | ||||
| 1168 (4) | 11.0 (4.8) | 10.2 (4.4) | –0.72 (–1.48 to 0.04) | –1.75 to 0.30 |
| Analysis including only studies with physical activity-based interventions | ||||
| 2915 (15) | 10.8 (5.3) | 9.8 (4.4) | –0.73 (–1.11 to –0.34) | –1.50 to 0.05 |
| Analysis including only studies with mixed approach | ||||
| 5369 (15) | 10.6 (5.9) | 10.2 (6.0) | –0.71 (–1.10 to –0.31) | –1.42 to 0.01 |
| Sample size (number of studies) | Group, n (%) | Summary ORa (95% CI) | 95% PI | |
|---|---|---|---|---|
| Control | Intervention group | |||
| Primary analysis for composite maternal outcome | ||||
| 8852 (24) | 1837/4227 (43.5) | 1896/4624 (41.0) | 0.90 (0.79 to 1.03) | 0.68 to 1.20 |
| Analysis excluding studies rated as being at a high risk of bias | ||||
| 4873 (10) | 1009/2421 (41.7) | 979/2452 (39.9) | 0.91 (0.77 to 1.08) | 0.70 to 1.19 |
| Analysis excluding women not adherent to intervention | ||||
| 7949 (24) | 1837/4227 (43.5) | 1527/3722 (41.0) | 0.92 (0.80 to 1.06) | 0.66 to 1.30 |
| Analysis of the intervention effects on the individual components of composite maternal outcome | ||||
| PE or PIH | ||||
| 9618 (22) | 423/4600 (9.2) | 432/5018 (8.6) | 0.95 (0.78 to 1.16) | 0.69 to 1.31 |
| Pooled-effect GDM | ||||
| 9427 (27) | 571/4510 (12.7) | 584/4917 (11.9) | 0.89 (0.72 to 1.10) | 0.49 to 1.60 |
| Preterm delivery | ||||
| 11,676 (32) | 345/5631 (6.1) | 332/6045 (5.5) | 0.94 (0.78 to 1.13) | 0.78 to 1.13 |
| Caesarean section | ||||
| 11,410 (32) | 1506/5500 (27.4) | 1527/5910 (25.8) | 0.91 (0.83 to 0.99) | 0.83 to 1.99 |
| Intervention groups | ||||
| Analysis including only studies with diet-based interventions | ||||
| 397 (3) | 84/218 (38.5) | 42/179 (23.5) | 0.60 (0.20 to 1.75) | 0.02 to 14.27 |
| Analysis including only studies with physical activity-based interventions | ||||
| 2311 (9) | 367/1115 (32.9) | 346/1196 (28.9) | 0.81 (0.61 to 1.09) | 0.48 to 1.37 |
| Analysis including only studies with mixed approach | ||||
| 6259 (13) | 1438/3009 (47.8) | 1508/3250 (46.4) | 0.97 (0.84 to 1.12) | 0.82 to 1.13 |
| Sample size (number of studies) | Group, n (%) | Summary ORa (95% CI) | 95% PI | |
|---|---|---|---|---|
| Control | Intervention group | |||
| Primary analysis for fetal and neonatal composite outcome | ||||
| 7981 (18) | 951/3802 (25.0) | 1007/4179 (24.1) | 0.94 (0.83 to 1.08) | 0.74 to 1.21 |
| Two-stage meta-analysis fetal composite excluding studies rated as being at a high risk of bias | ||||
| 3708 (6) | 467/1855 (25.2) | 417/1853 (22.5) | 0.86 (0.71 to 1.06) | 0.69 to 1.07 |
| Two-stage meta-analysis fetal composite excluding non-adherent participants | ||||
| 6875 (18) | 951/3802 (25.0) | 720/3073 (23.4) | 0.94 (0.83 to 1.06) | 0.83 to 1.06 |
| Analysis of the intervention effects on the individual components of fetal and neonatal composite outcome | ||||
| IUD | ||||
| Insufficient data | – | – | – | – |
| SGA | ||||
| 11,666 (33) | 632/5633 (11.2) | 709/6033 (11.8) | 1.06 (0.94 to 1.20) | 0.94 to 1.20 |
| LGA | ||||
| 12,047 (34) | 759/5811 (13.1) | 744/6236 (11.9) | 0.90 (0.76 to 1.07) | 0.63 to 1.30 |
| Admissions to the NICU | ||||
| 8140 (16) | 279/3865 (7.2) | 302/4275 (7.1) | 1.01 (0.84 to 1.23) | 0.84 to 1.23 |
| Intervention groups | ||||
| Analysis including only studies with diet-based interventions | ||||
| 346 (2) | 48/180 (26.7) | 34/166 (20.5) | 0.71 (0.03 to 18.23) | – |
| Analysis including only studies with physical activity-based interventions | ||||
| 1274 (5) | 143/641 (22.3) | 138/633 (21.8) | 0.99 (0.67 to 1.46) | 0.64 to 1.54 |
| Analysis including only studies with mixed approach | ||||
| 6494 (12) | 797/3114 (25.6) | 835/3380 (24.7) | 0.95 (0.81 to 1.11) | 0.71 to 1.27 |
Appendix 9 Results of aggregate meta-analyses
Maternal outcomes
FIGURE 23.
Outcome: GDM. a, Data from the secondary publication Dekker Nitert et al. ;49 b, combined active and passive; c, combined active and passive; d, cluster RCT; e, cluster RCT. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 24.
Outcome: PE or PIH. a, PE: combined active and passive; b, PIH: combined active and passive; c, PIH; d, PE; e, PE; f, PIH: combined active and passive; g, hypertension in exercise group, PE in control; h, PE; i, PIH; j, PE; k, cluster RCT; l, maternal hypertension; m, two intervention arms combined; n, PE; o, PIH. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 25.
Outcome: preterm birth. a, Two intervention arms combined. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 26.
Outcome: any Caesarean section. a, Combined active and passive; b, data from the secondary publication Dekker Nitert et al. ;49 c, combined active and passive; d, cluster RCT; and e, combined two intervention arms. df, degrees of freedom; M–H, Mantel–Haenszel.
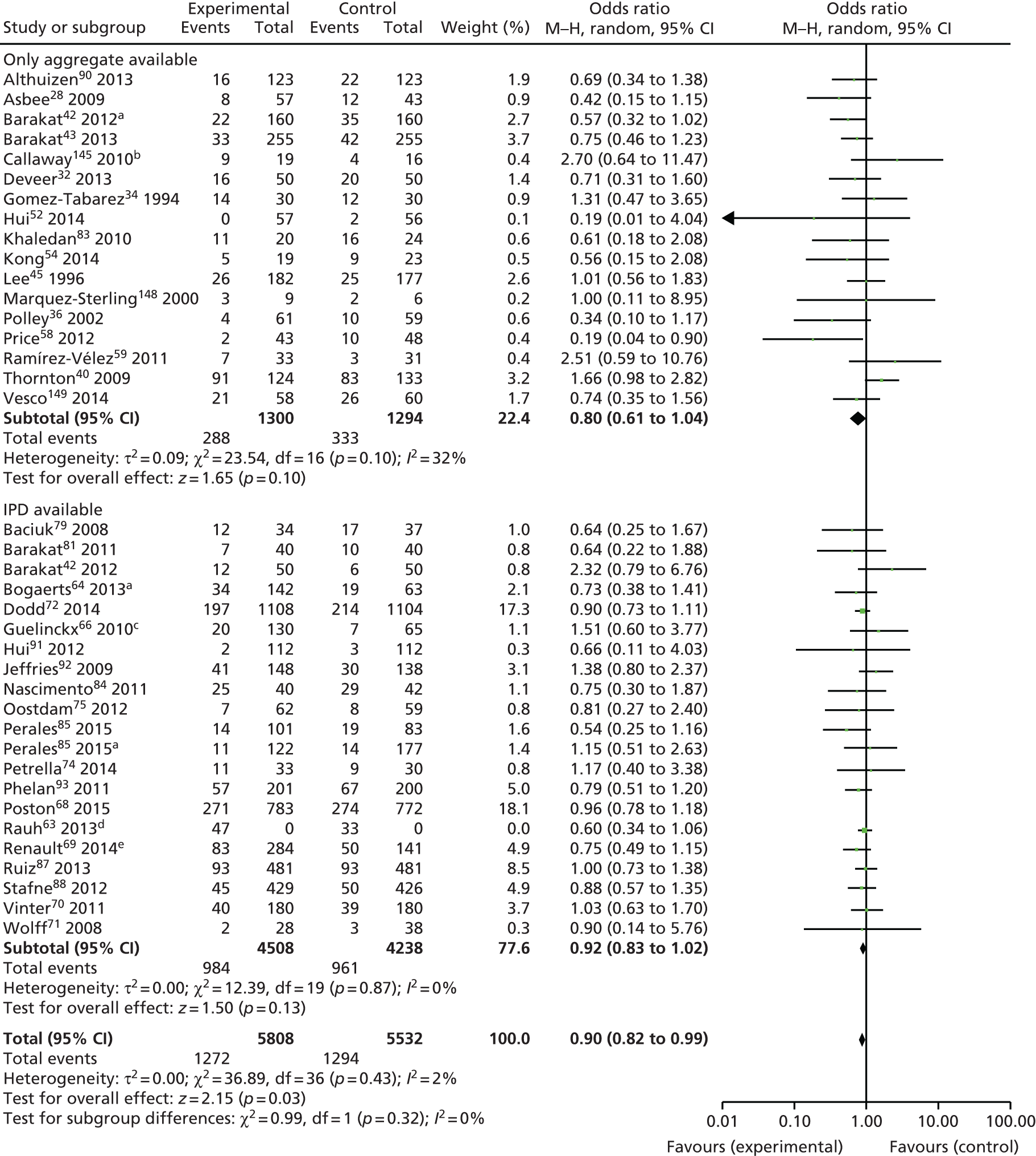
FIGURE 27.
Outcome: IUD. df, degrees of freedom; M–H, Mantel–Haenszel.

FIGURE 28.
Outcome: SGA infant. a, Combined two intervention arms. df, degrees of freedom; M–H, Mantel–Haenszel.
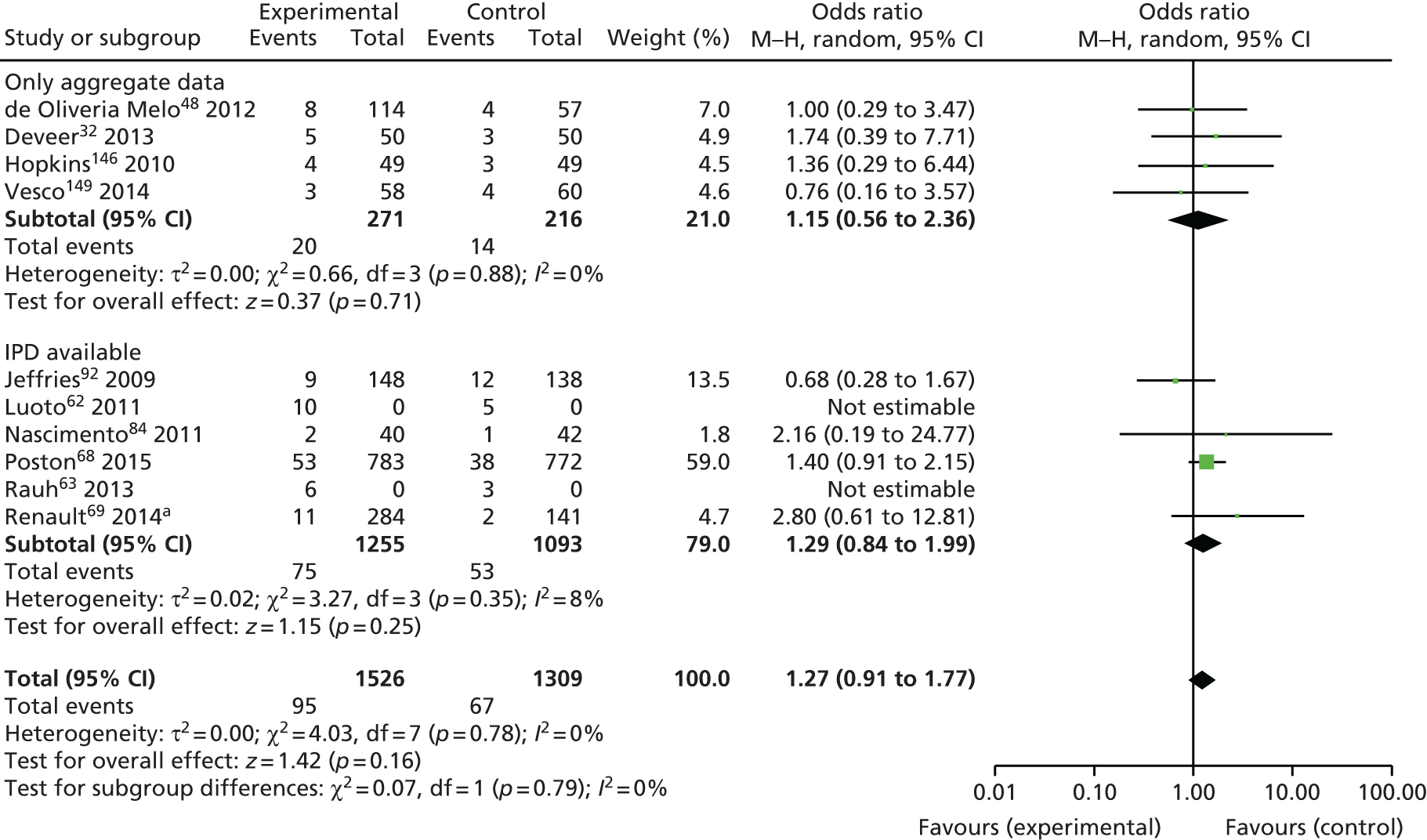
FIGURE 29.
Outcome: LGA infant. a, > 90th centile population birthweight; and b, combined two intervention arms. df, degrees of freedom; M–H, Mantel–Haenszel.
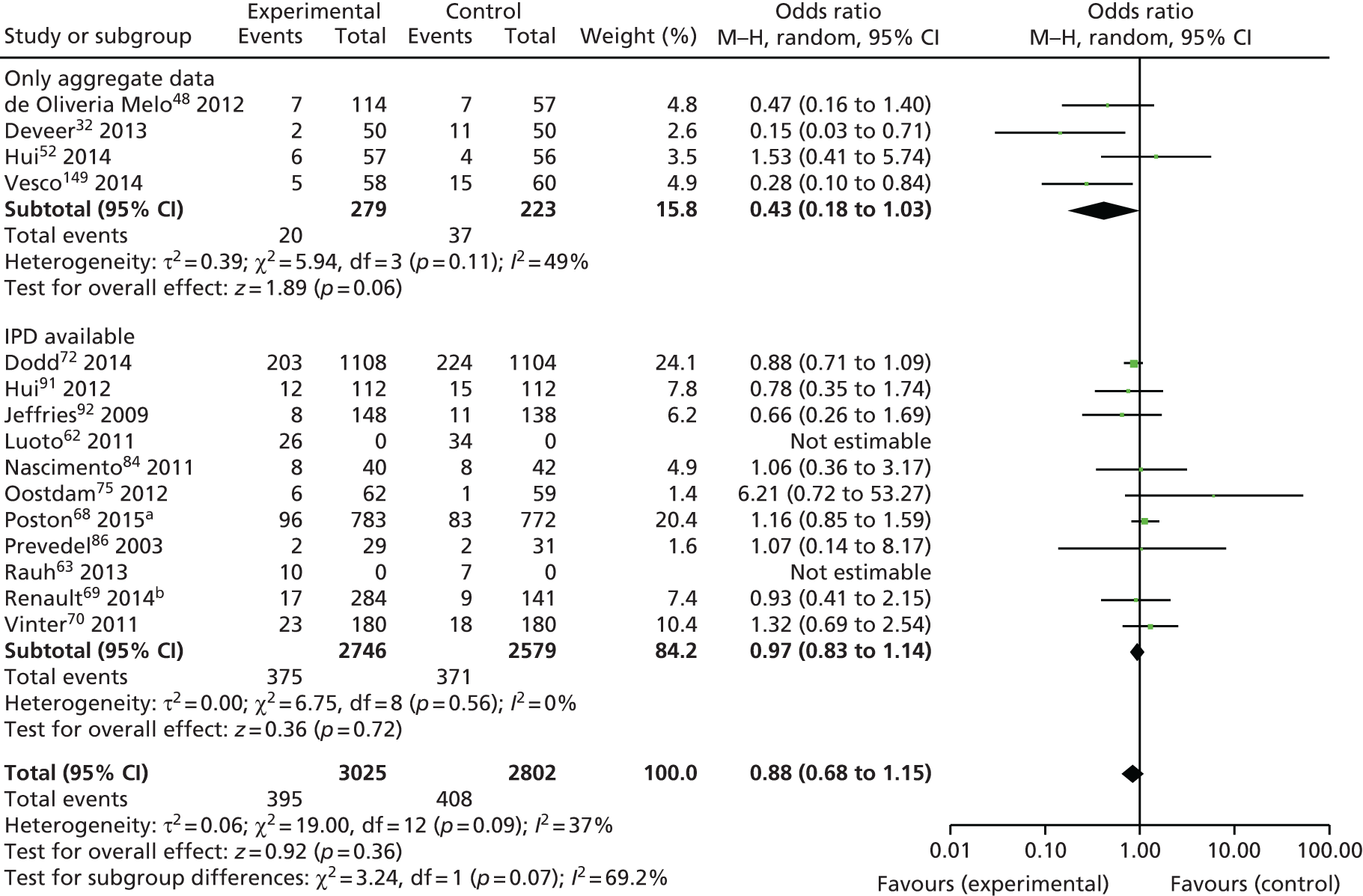
FIGURE 30.
Outcome: admissions to the NICU. df, degrees of freedom; M–H, Mantel–Haenszel.

Appendix 10 Search strategies for systematic reviews undertaken to inform economic modelling
| # | Term |
|---|---|
| 1 | Pre-Eclampsia/ or preeclamp*.mp. |
| 2 | pre-eclamp*.mp. |
| 3 | (pre and eclamp*).mp. |
| 4 | (pregnan* and hypertens*).mp. |
| 5 | Eclampsia/ or eclampsia.mp. |
| 6 | (EPH-gestosis or gestosis).mp. |
| 7 | (hypertension and pregnancy).mp. |
| 8 | Hypertension, Pregnancy-Induced/ |
| 9 | Hypertension/ |
| 10 | Pregnancy/ |
| 11 | 9 and 10 |
| 12 | cost benefit analysis.mp. or exp Cost-Benefit Analysis/ |
| 13 | (cost$ adj2 (effective$ or utili$ or benefit$ or consequence$ or minimi$)).ti,ab,kw. |
| 14 | (decision adj (analy$ or model$ or tree$)).ti,ab,kw. |
| 15 | (cost$ or economic$ or pharmacoeconomic$).ti. |
| 16 | quality-adjusted life year$.ti,ab,kw. or exp Quality-adjusted Life Years/ |
| 17 | exp “costs and cost analysis”/ or exp Health Care Costs/ |
| 18 | exp Economics, Pharmaceutical/ or exp Economics, Medical/ or Economics/ or exp Economics, hospital/ |
| 19 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 11 |
| 20 | 12 or 13 or 14 or 15 or 16 or 17 or 18 |
| 21 | 19 and 20 |
| # | Term |
|---|---|
| 1 | Diabetes, Gestational/ |
| 2 | (diabet$ adj3 (“pregnancy induced” or gestat$ or gravid$)).ti,ab. |
| 3 | GDM.ti,ab. |
| 4 | exp DIABETES MELLITUS/ |
| 5 | diabet$.ti. |
| 6 | PREDIABETIC STATE/ |
| 7 | prediabet$.ti,ab. |
| 8 | impaired glucose tolerance.ti,ab. |
| 9 | IGT.ti,ab. |
| 10 | Impaired fasting glucose.ti,ab. |
| 11 | IFG.ti,ab. |
| 12 | Impaired glucose regulation.ti,ab. |
| 13 | IGR.ti,ab. |
| 14 | GLUCOSE INTOLERANCE/ |
| 15 | PREGNANCY/ |
| 16 | (pregnan$ or gestation$).ti,ab. |
| 17 | PREGNANT WOMEN/ |
| 18 | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 |
| 19 | 15 or 16 or 17 |
| 20 | 18 and 19 |
| 21 | 1 or 2 or 3 or 20 |
| 22 | cost benefit analysis.mp. or exp Cost-Benefit Analysis/ |
| 23 | (cost$ adj2 (effective$ or utili$ or benefit$ or consequence$ or minimi$)).ti,ab,kw. |
| 24 | (decision adj (analy$ or model$ or tree$)).ti,ab,kw. |
| 25 | (cost$ or economic$ or pharmacoeconomic$).ti. |
| 26 | quality-adjusted life year$.ti,ab,kw. or exp Quality-adjusted Life Years/ |
| 27 | exp “costs and cost analysis”/ or exp Health Care Costs/ |
| 28 | exp Economics, Pharmaceutical/ or exp Economics, Medical/ or Economics/ or exp Economics, hospital/ |
| 29 | 22 or 23 or 24 or 25 or 26 or 27 or 28 |
| 30 | 21 and 29 |
| # | Term |
|---|---|
| 1 | Pregnant Women/ |
| 2 | Gravidity/ |
| 3 | gravid*.tw. |
| 4 | pregnan*.tw. |
| 5 | childbearing.tw. |
| 6 | matern*.tw. |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 8 | Weight Gain/ph [Physiology] |
| 9 | obes*.tw. |
| 10 | overweight*.tw. |
| 11 | bmi.tw. |
| 12 | Body Mass Index/ |
| 13 | weight los*.tw. |
| 14 | Weight Loss/ph [Physiology] |
| 15 | weight change*.tw. |
| 16 | weight control.mp. |
| 17 | weight management.mp. |
| 18 | weight reduction.mp. |
| 19 | diet*.tw. |
| 20 | exp Diet/ |
| 21 | nutritional therapy.mp. |
| 22 | food restriction.mp. or Caloric Restriction/ |
| 23 | fast$.mp. |
| 24 | Energy Intake/ph [Physiology] |
| 25 | Exercise/ or Exercise Therapy/ or exercise$.mp. |
| 26 | exercis*.tw. |
| 27 | aerobics.mp. |
| 28 | physical activit*.tw. |
| 29 | calisthenics.mp. or Gymnastics/ |
| 30 | Diabetes, Gestational/ |
| 31 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 |
| 32 | economic evaluation$.tw. |
| 33 | Cost-Benefit Analysis/ec, mt, og, sn, ut [Economics, Methods, Organization & Administration, Statistics & Numerical Data, Utilization] |
| 34 | cost effectiv*.tw. |
| 35 | cost utility.tw. |
| 36 | cost consequence*.tw. |
| 37 | health care cost*.tw. |
| 38 | cost*.tw. |
| 39 | Economics, Medical/ec, sn [Economics, Statistics & Numerical Data] |
| 40 | economic$.mp. |
| 41 | decision model*.tw. |
| 42 | markov model*.tw. |
| 43 | Decision Trees/ |
| 44 | 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 |
| 45 | 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 |
| 46 | 8 and 32 and 45 |
| 47 | limit 46 to humans |
| 48 | limit 47 to English language |
| 49 | limit 48 to last 15 years |
Appendix 11 Probabilistic sensitivity analysis additional results
Figures 31–54 show additional results for the PSAs that were conducted. Details of the methods and discussion of results are available in Chapter 8.
Additional results of probabilistic sensitivity analysis: primary analysis for a cohort of 10,000 pregnant women
FIGURE 31.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for all women: cases of PE averted. The mean of the distribution is shown by the blue diamond.
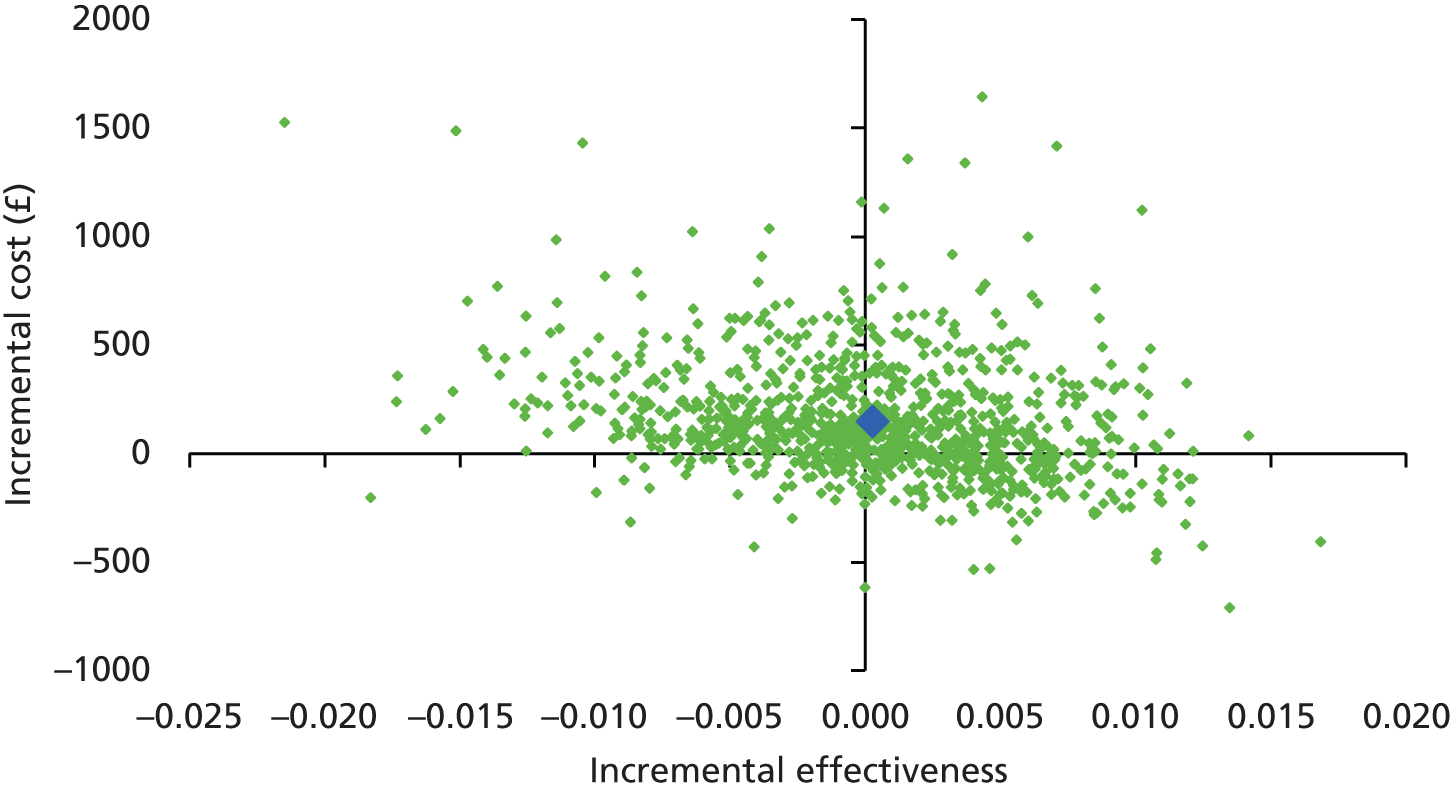
FIGURE 32.
Incremental CEAC of the intervention for all pregnant women: cases of PE averted.
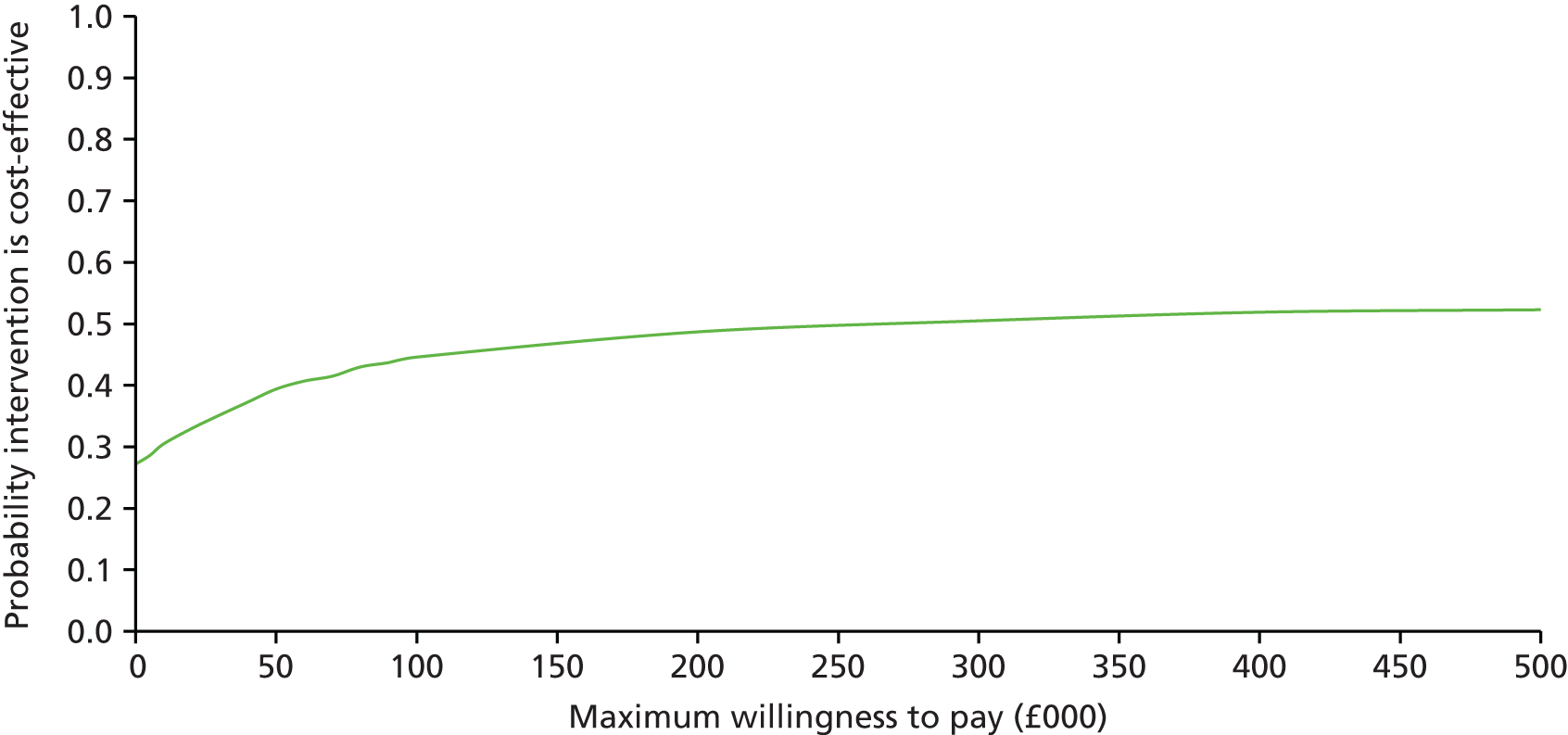
FIGURE 33.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for all pregnant women: cases of GDM averted. The mean of the distribution is shown by the blue diamond.

FIGURE 34.
Incremental CEAC of the intervention for all pregnant women: cases of GDM averted.

FIGURE 35.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for all pregnant women: cases of PIH averted. The mean of the distribution is shown by the blue diamond.
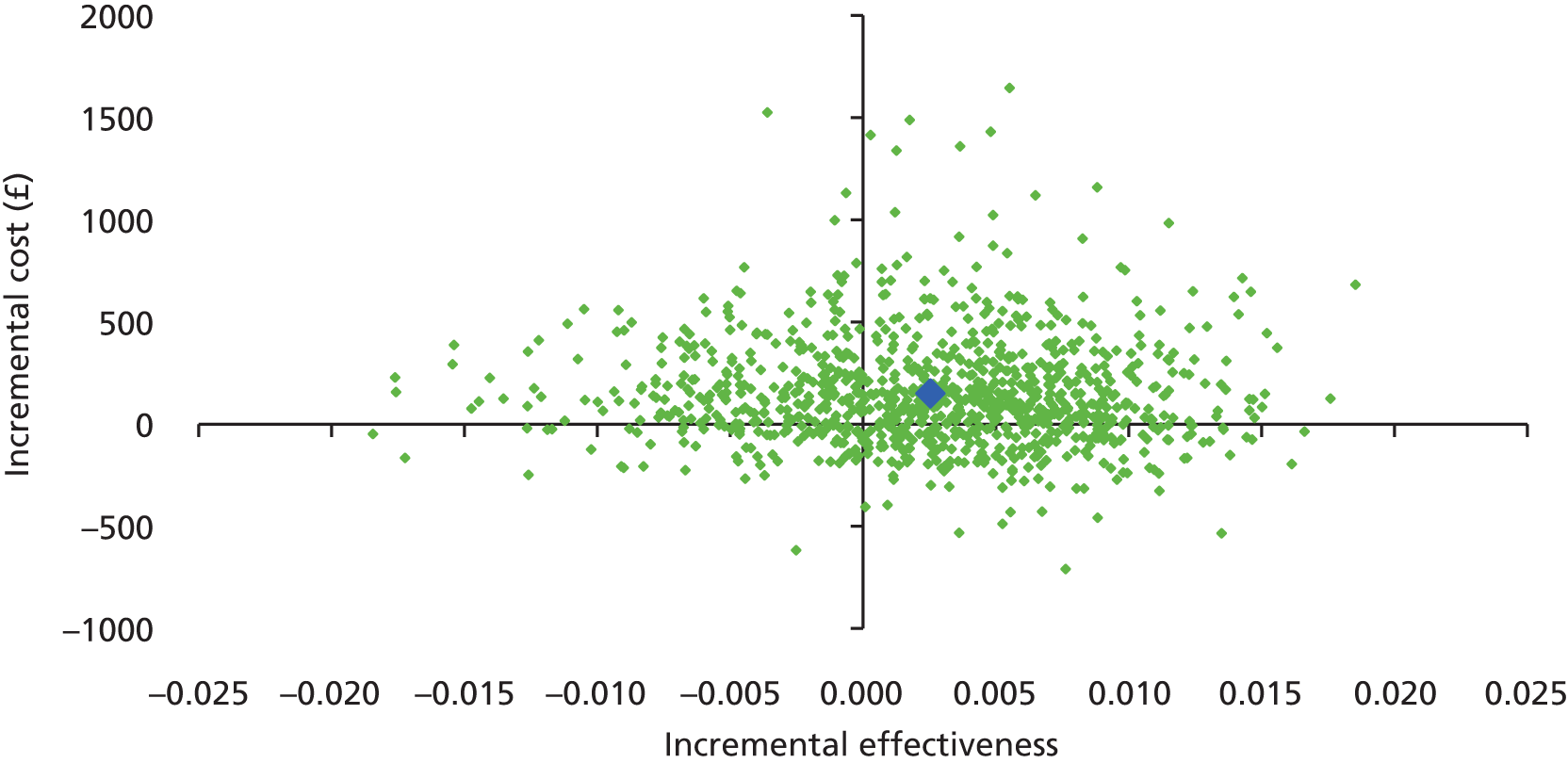
FIGURE 36.
Incremental CEAC of the intervention for all pregnant women: cases of PIH averted.
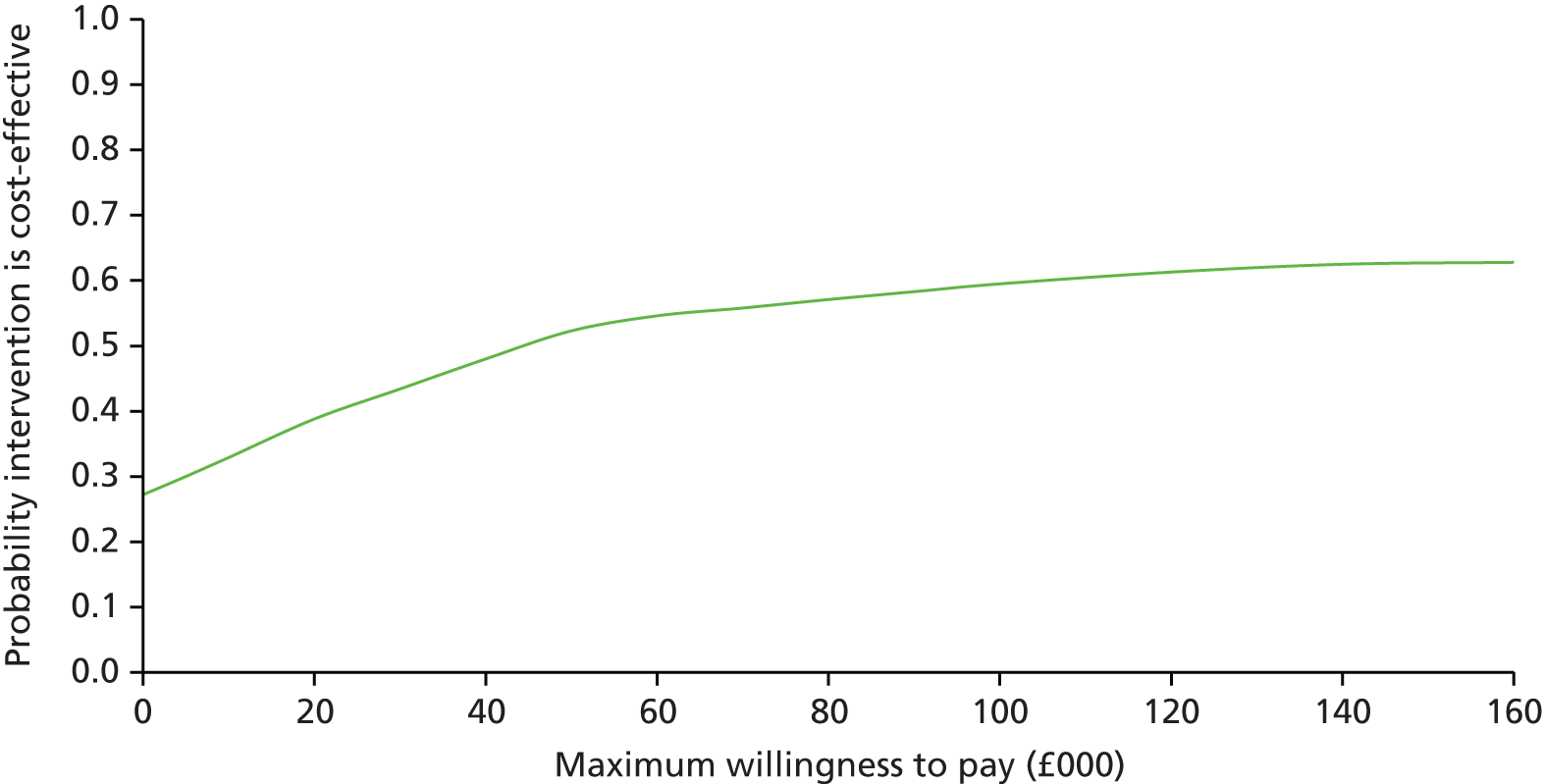
Additional results of probabilistic sensitivity analysis: secondary analysis for a cohort of 10,000 obese pregnant women
FIGURE 37.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for obese pregnant women: cases of PE averted. The mean of the distribution is shown by the blue diamond.

FIGURE 38.
Incremental CEAC of the intervention for obese pregnant women: cases of PE averted.

FIGURE 39.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for obese women: cases of GDM averted. The mean of the distribution is shown by the blue diamond.

FIGURE 40.
Incremental CEAC of the intervention for obese pregnant women: cases of GDM averted.
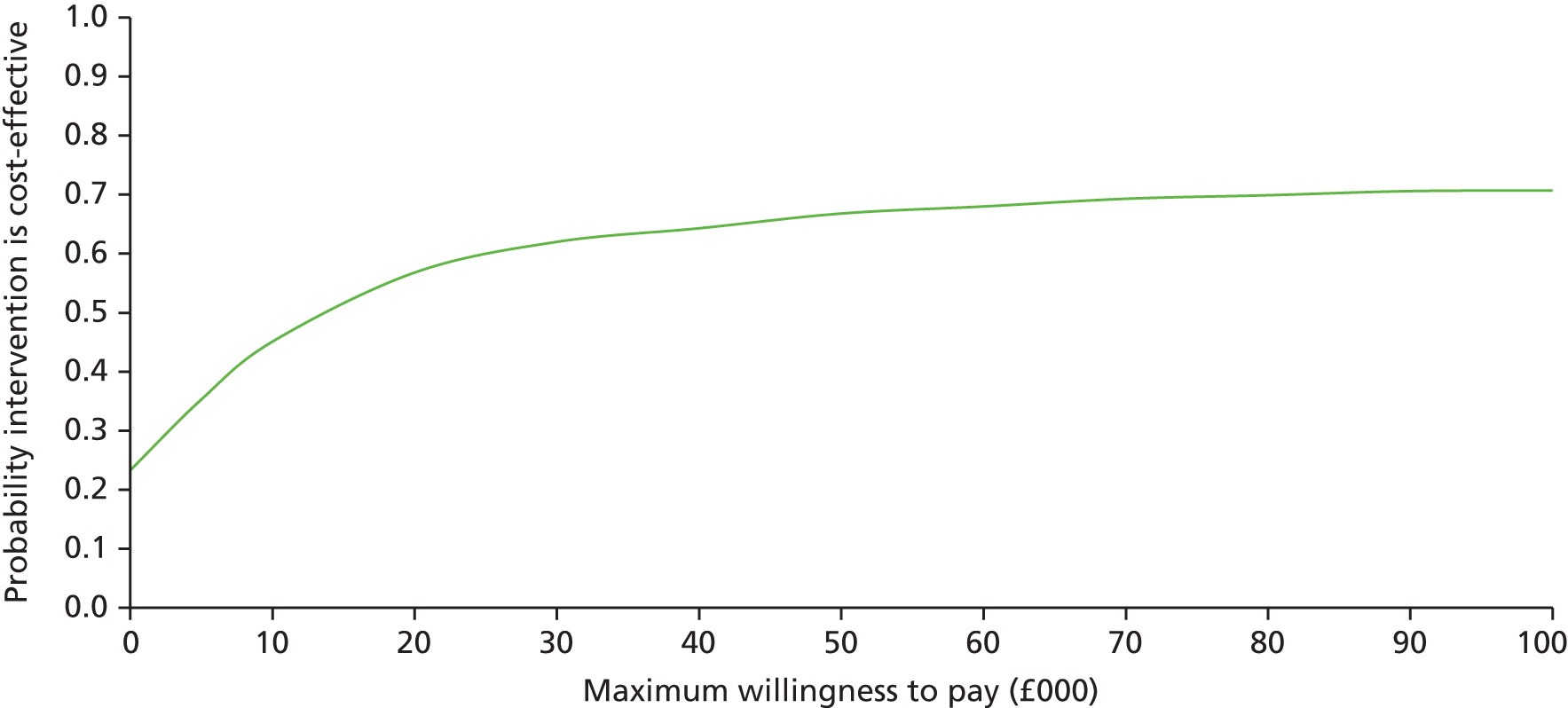
FIGURE 41.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for obese women: cases of PIH averted. The mean of the distribution is shown by the blue diamond.
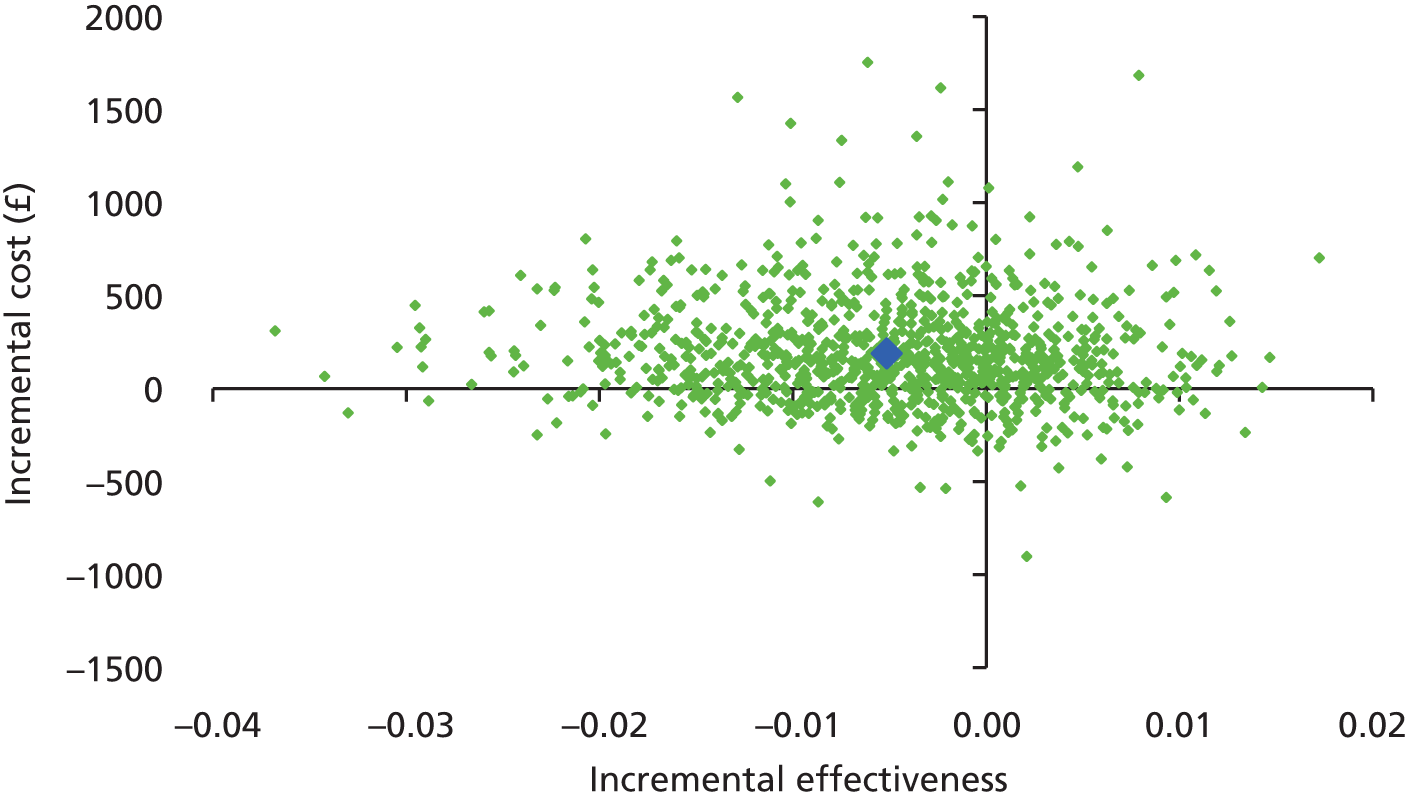
FIGURE 42.
Incremental CEAC of the intervention for obese pregnant women: cases of PIH averted.
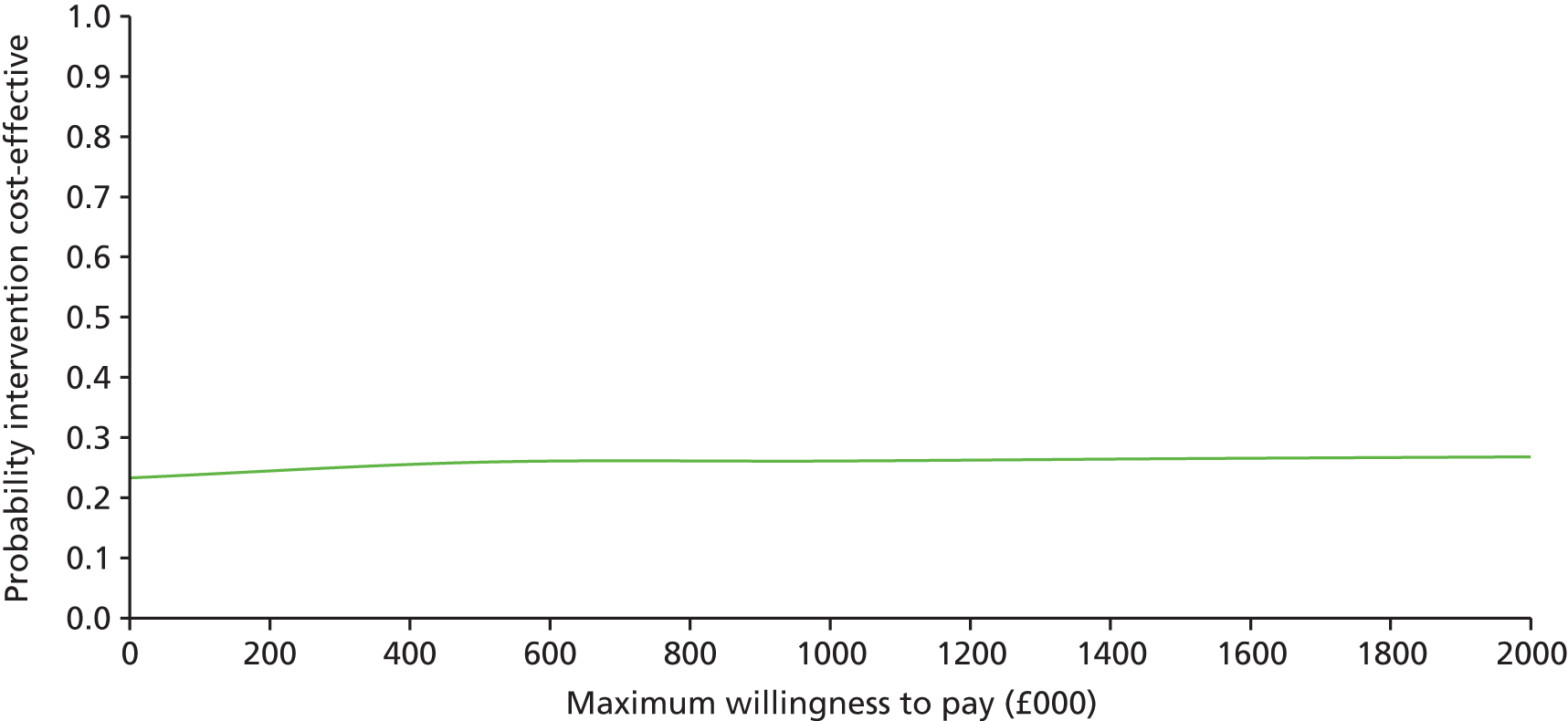
Additional results of probabilistic sensitivity analysis: secondary analysis for a cohort of 10,000 overweight pregnant women
FIGURE 43.
Incremental cost-effectiveness scatterplot of the intervention compared with care as usual for overweight women: cases of PE averted. The mean of the distribution is shown by the blue diamond.

FIGURE 44.
Incremental CEAC of the intervention for overweight pregnant women: cases of PE averted.
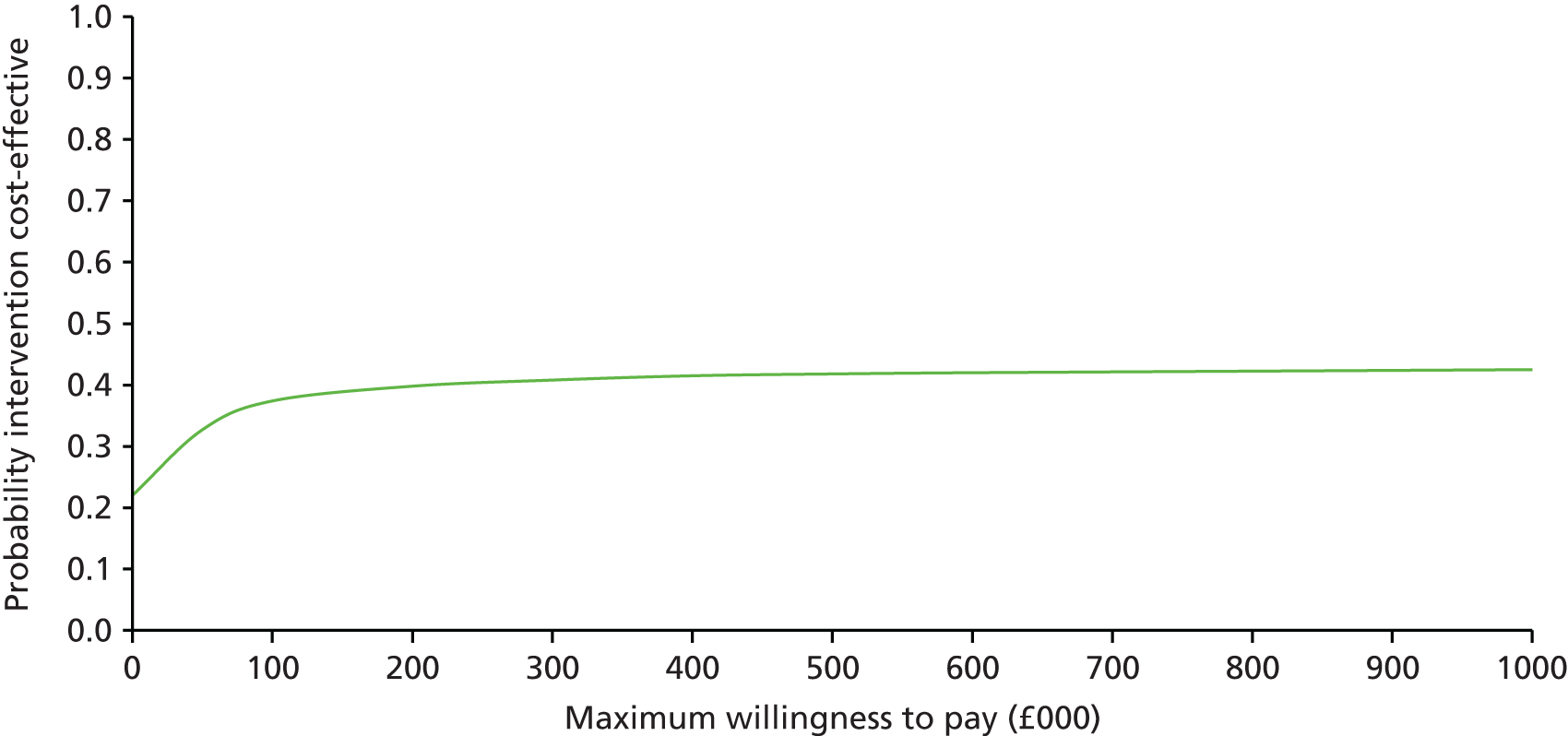
FIGURE 45.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for overweight women: case of GDM averted. The mean of the distribution is shown by the blue diamond.
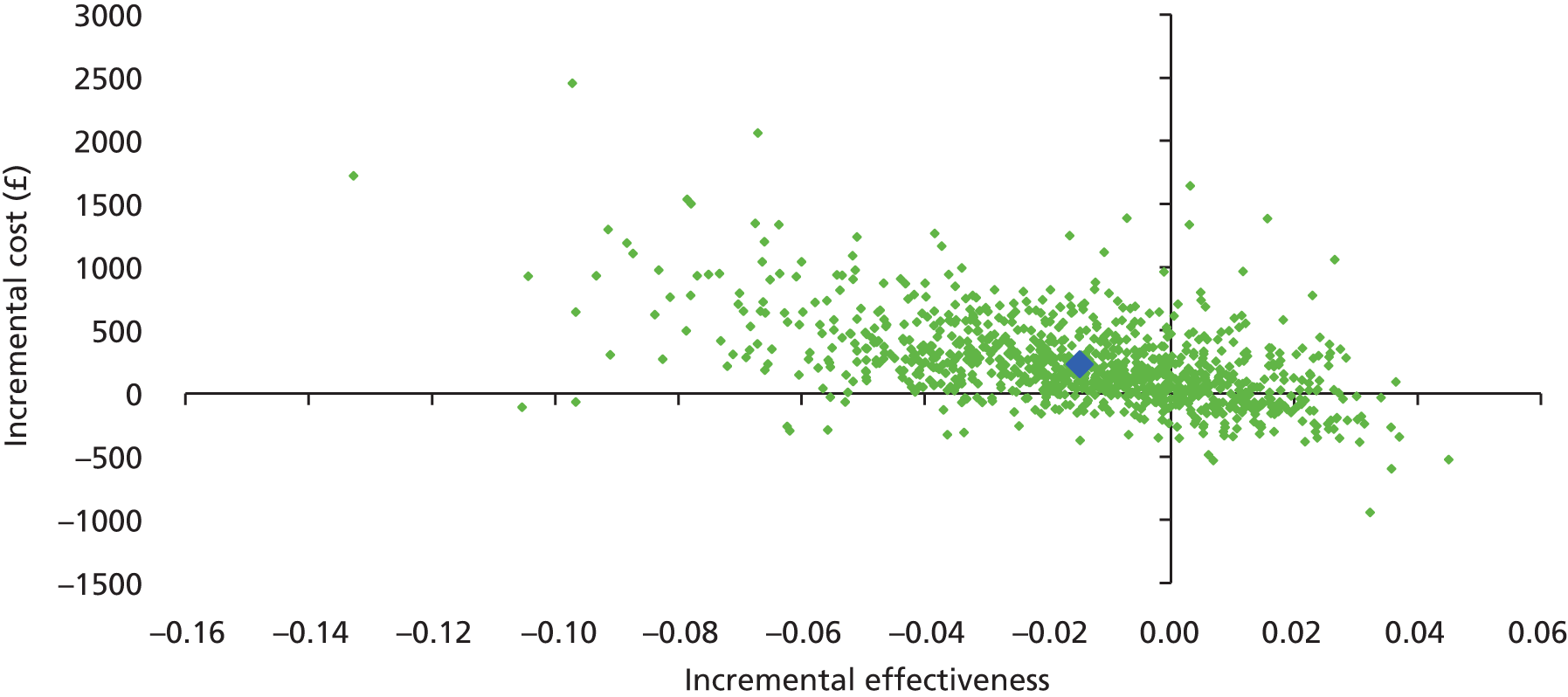
FIGURE 46.
Incremental CEAC of intervention for overweight pregnant women: case of GDM averted.

FIGURE 47.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for overweight pregnant women: case of PIH averted. The mean of the distribution is shown by the blue diamond.

FIGURE 48.
Incremental CEAC of intervention for overweight pregnant women: case of PIH averted.
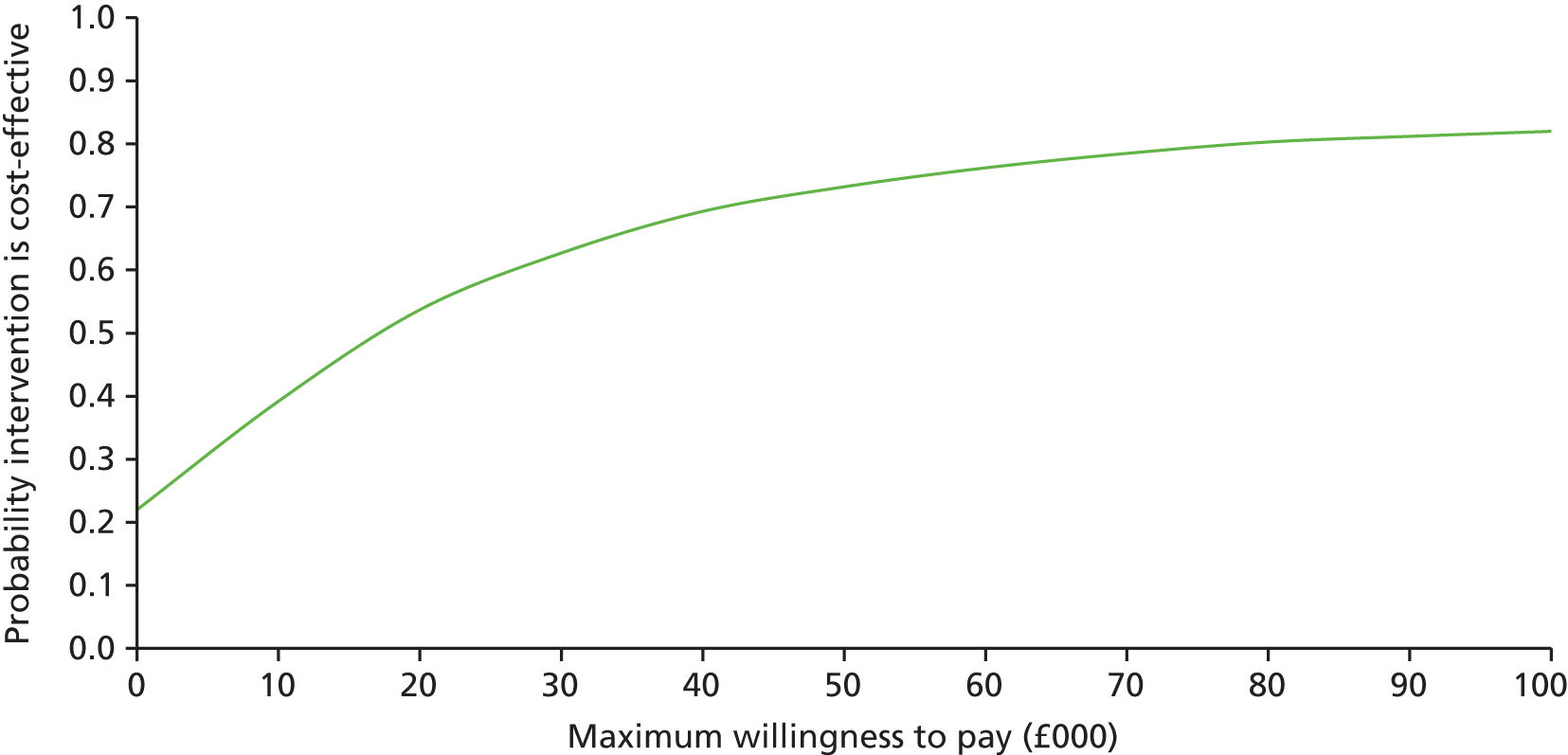
Additional results of probabilistic sensitivity analysis: secondary analysis for a cohort of 10,000 normal weight pregnant women
FIGURE 49.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for normal weight pregnant women: case of PE averted. The mean of the distribution is shown by the blue diamond.

FIGURE 50.
Incremental CEAC of intervention for normal weight pregnant women: case of PE averted.

FIGURE 51.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for normal weight pregnant women: case of GDM averted. The mean of the distribution is shown by the blue diamond.

FIGURE 52.
Incremental CEAC of intervention for normal weight pregnant women: case of GDM averted.

FIGURE 53.
Incremental cost-effectiveness scatterplot of intervention compared with care as usual for normal weight pregnant women: case of PIH averted. The mean of the distribution is shown by the blue diamond.

FIGURE 54.
Incremental CEAC of intervention for normal weight pregnant women: case of PIH averted.

List of abbreviations
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DM
- diabetes mellitus
- EVPI
- expected value of perfect information
- GDM
- gestational diabetes mellitus
- GWG
- gestational weight gain
- HTA
- Health Technology Assessment
- HYPITAT
- Hypertension and Pre-eclampsia Intervention Trial At near Term
- i-WIP
- International Weight Management in Pregnancy
- ICER
- incremental cost-effectiveness ratio
- IOM
- Institute of Medicine
- IPD
- individual participant data
- IUD
- intrauterine death
- LGA
- large for gestational age
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- OR
- odds ratio
- PE
- pre-eclampsia
- PI
- prediction interval
- PIH
- pregnancy-induced hypertension
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REML
- restricted maximum likelihood
- SD
- standard deviation
- SGA
- small for gestational age