Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/28/02. The contractual start date was in August 2014. The draft report began editorial review in September 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Tyrer and Irwin Nazareth were members of the National Institute for Health Research Health Technology Assessment commissioning board that commissioned this research.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Buszewicz et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Clinical background
Generalised anxiety disorder (GAD) is an anxiety disorder characterised by excessive, uncontrollable and often irrational worry that interferes with daily functioning and can cause physical symptoms. People with GAD often anticipate the worst and are very preoccupied with matters such as health issues, money, death, interpersonal problems or work difficulties. Typical physical symptoms include fatigue, nausea, muscle tension, palpitations or shortness of breath, difficulties concentrating and sleep difficulties, which can lead to a misdiagnosis. These symptoms must be consistent and ongoing, persisting at least 6 months, for a formal diagnosis of GAD to be made. The disorder often begins at an early age, and signs and symptoms may develop more slowly than in other anxiety disorders.
Generalised anxiety disorder is common, with a prevalence of 4.7% in the 2007 English National Psychiatric Morbidity Survey,1 but with lower rates of identification in primary care than expected. 2 As symptoms have to have been present at least 6 months before the diagnosis can be made, it is often a chronic disorder by the time it is identified. 3 It is often comorbid with depression or other anxiety or physical health disorders, worsening the prognosis. 4 In community samples, GAD is more common than depression,1 with higher associated health and societal costs,5 but it has received much less attention, so establishing the most effective treatments is crucial.
Generalised anxiety disorder is often associated with significant morbidity in terms of distressing psychological and physical symptoms, and significant functional impairment. 4 The degree of disability has been described as similar to that in major depression or chronic physical illness. 6 Rates of unemployment and social isolation are high,4 as is concomitant alcohol and substance misuse in an attempt to alleviate symptoms. 7 People with GAD have high numbers of general practitioner (GP) visits and secondary care contacts, both because of associated physical/somatic symptoms and because GAD is often comorbid with chronic physical health problems. 8
The 2011 National Institute for Health and Care Excellence (NICE) guidelines entitled Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults: Management in Primary, Secondary and Community Care9 established good evidence for the effectiveness of low-intensity psychological interventions in GAD. Step 1 interventions are usually delivered within primary care, involving identification, assessment, education and active GP monitoring. If symptoms persist, referral to a step 2 low-intensity psychological intervention is recommended [e.g. self-help interventions or psychoeducation groups, usually facilitated by a low-intensity Improving Access to Psychological Therapies (IAPT) psychological worker]. 10 However, a significant number of patients will not respond to these interventions and require ‘stepping up’ to more intensive step 3 interventions (Figure 1).
FIGURE 1.
Stepped-care model for GAD. a, Pure self-help is defined as a self-administered intervention intended to treat GAD and involves self-help materials (usually a book or workbook). It is similar to guided self-help but without any contact with a health-care professional. Red font shows the step or choice of treatments that are assessed in this trial.
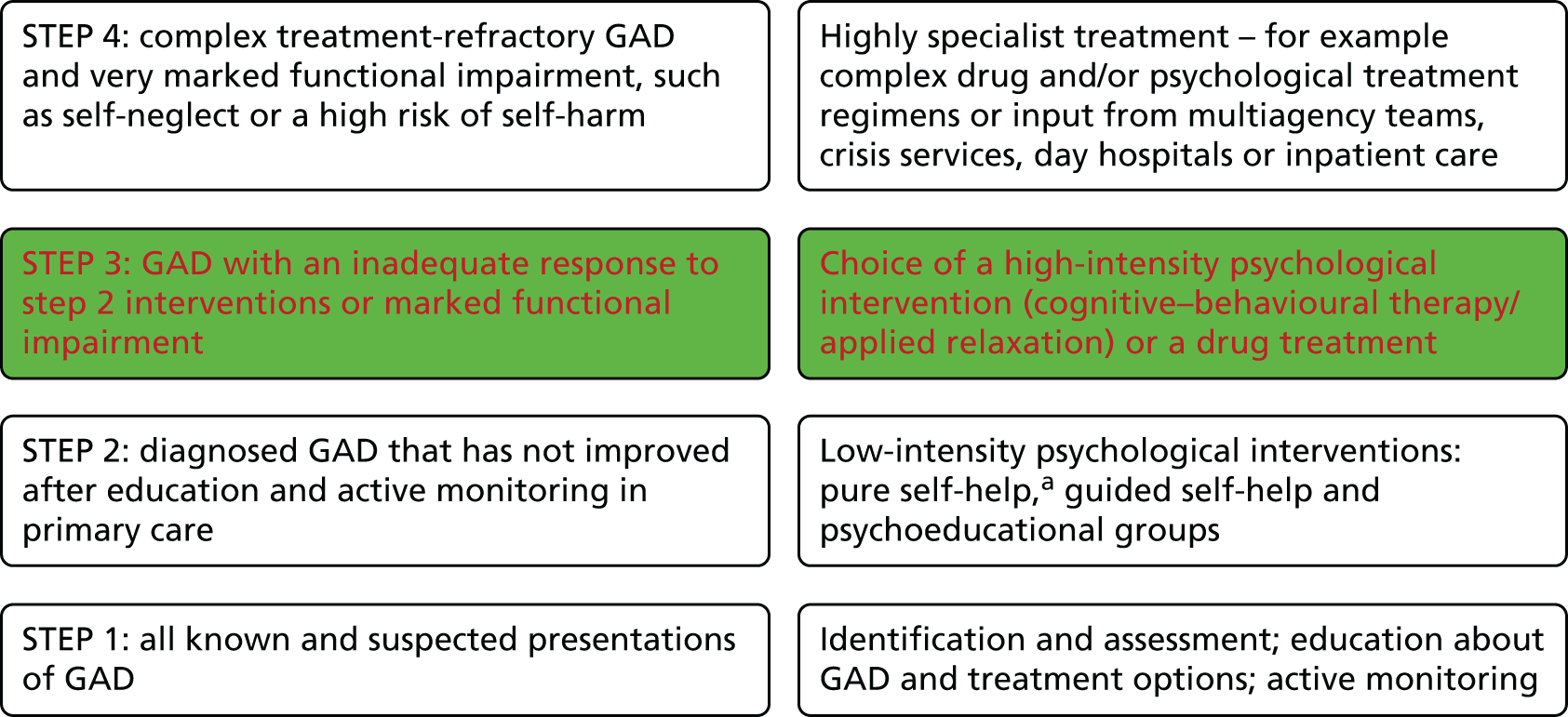
A substantial percentage of people with anxiety and/or depression referred to IAPT low-intensity workers still have high Generalised Anxiety Disorder-7 (GAD-7) questionnaire scores after receiving a step 2 intervention, indicating that they have not yet been effectively treated. 11 GAD is also potentially a long-term, relapsing condition. Thus, providing the most effective treatment, with a reduced likelihood of relapse, should provide significant benefits in terms of both individual morbidity and accompanying health and social costs to society, and was the focus of this study.
Existing research
The National Institute for Health and Care Excellence conducted a systematic review of placebo-controlled antidepressant studies in GAD. 9 Thirty-four studies were identified that were generally rated as being of high quality, although relatively short in duration (8–12 weeks). Of these trials, 17 involved selective serotonin reuptake inhibitors (SSRIs), whereas 16 involved the serotonin–noradrenaline reuptake inhibitors (SNRIs) venlafaxine and duloxetine. Both of the SNRIs, as well as the SSRIs paroxetine and escitalopram, have marketing authorisations for the treatment of GAD. The NICE summary concluded that, relative to placebo, SNRI and SSRI treatments were efficacious in the treatment of GAD in that they produced greater reductions in Hamilton Anxiety Rating Scale (HAM-A) scores12 and increased the probability of patients responding to treatment.
Generally, the effect sizes of antidepressants relative to placebo were in the low to moderate range and did not apparently vary between the different antidepressants to a clinically meaningful extent. 9 There was no clear evidence of a dose–response relationship for any particular antidepressant, and the most commonly experienced side effects were nausea and insomnia. These placebo-controlled studies were generally not more than 12 weeks in duration, although GAD is considered to be a chronic disorder and guidelines recommend the continuation of treatment in responders. A meta-analysis of available relapse prevention studies suggested an important effect of continuing effective pharmacological treatment for up to 1 year in patients with GAD who have responded to pharmacological therapy,13 although there is currently no evidence that sertraline is effective in preventing relapse. 14
Although there is evidence of the clinical effectiveness and cost-effectiveness of sertraline for GAD compared with placebo, and also of cognitive behavioural therapy (CBT) compared with waiting list controls,1 there have been no head-to-head comparisons of sertraline (or any other SSRI) versus CBT to evaluate which treatment is the most clinically effective and cost-effective. Currently NICE guidelines suggest that choice of treatment between a pharmacological or psychological treatment at step 3 should be based mainly on patient preference, although availability of CBT may determine if patients have such a choice in some areas.
In assessing the effectiveness of CBT or SSRIs for GAD, it is necessary to consider both clinical symptoms and functional impairment. It is also important to assess outcomes of more than a few months, given that most pharmacological studies do not have follow-ups of > 12 weeks9 and there is some limited evidence that CBT may have a protective effect against future episodes. 15 Longer follow-up is also crucial in making future recommendations, as the longer-term costs of prescriptions and use of health-care resources associated with each type of treatment are required to evaluate their relative cost-effectiveness.
Following the updated NICE guidelines, there has been increased interest in GAD in the primary care community, but uncertainty remains regarding whether pharmacological or psychological treatment is indicated in more persistent cases. 16 Clinicians can be reluctant to prescribe the SSRI sertraline, which, although found by NICE to be the most cost-effective acute drug therapy for GAD, does not currently have marketing authorisation for this use. Clear data regarding whether the SSRI sertraline or CBT is most effective longer term in treatment of GAD, as well as further information about the safety and efficacy of sertraline in this patient group, is of direct relevance to the NHS.
The NICE committee conducted a search for relevant updates to the 2010 guideline in 2012, but no relevant trials adding to the literature were identified. 17 The authors of this report have also conducted a further rapid search of the literature to see if they could identify any relevant trials published between 2013 and early 2016, but none was found. Most of the studies continue to evaluate the effect of either CBT or medication for patients with GAD with no head-to-head comparison of a recommended psychological treatment versus pharmacotherapy. One study by Crits-Christoph et al. 18 combined treatment with venlafaxine with 12 sessions of CBT for patients who wished to have this and found no added benefit from the CBT at the 24-week follow-up, although the numbers in both groups were relatively small and those in the venlafaxine-only group showed quite a marked benefit from this treatment.
The National Institute for Health and Care Excellence guidelines research recommendation
The research recommendation from the NICE GAD Guidelines Group9 suggested the following: ‘A comparison of the clinical and cost-effectiveness of sertraline and CBT in people with GAD that have not responded to guided self-help and psycho-education’.
This recommendation suggested using a randomised controlled design in which people who had not responded to low-intensity step 2 interventions for GAD would be allocated openly to one of three groups: sertraline, CBT or waiting-list control for 12–16 weeks, with the control group being important to assess whether or not the two active treatments would produce effects greater than that of natural remission. It was suggested that follow-up assessments should be continued over the next 2 years to establish whether or not any short-term benefits were maintained and whether or not either active treatment produces a better long-term outcome.
Health Technology Assessment-commissioned research proposal
The research proposal commissioning brief published subsequently by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme panel in 2013 was a little different, in that it gave the following brief for the research question, omitting the third control arm to the trial and not stipulating assessment after 12–16 weeks:
Is a selective serotonin reuptake inhibitor (SSRI) or cognitive behavioural therapy (CBT) more clinically and cost-effective for patients with generalised anxiety disorder (GAD) who have not responded to low intensity psychological interventions recommended in a stepped-care model?
Following from this, our final approved proposal was for a ‘Randomised controlled trial of the selective serotonin reuptake inhibitor sertraline versus cognitive behavioural therapy (CBT) for anxiety symptoms in people with Generalised Anxiety Disorder (GAD) who have failed to respond to low intensity psychological interventions as defined by the NICE GAD guidelines’, with the primary outcome being assessed at 12 months (see Chapter 2, Methods, for further details).
Background to the choice of interventions to be assessed
Sertraline
The NICE Guidelines Advisory Group proposed sertraline as a first-choice pharmacological treatment, although this agent does not have a marketing authorisation for GAD and there are relatively few randomised trials (only two trials with 706 patients in total19,20). 9 Nevertheless, in terms of risk of discontinuation because of adverse effects, sertraline was the best tolerated antidepressant and was available as a generic brand, making it the most cost-effective choice. Duloxetine (a SNRI) had a greater probability of producing clinical response in a network meta-analysis, but this is not commonly prescribed in UK primary care. The SSRIs paroxetine and escitalopram both have marketing authorisation for GAD, and there is little pharmacological difference between them and the SSRI sertraline. However, paroxetine has a more marked withdrawal syndrome than sertraline21 and escitalopram was still on patent and significantly more expensive at the time of submitting the proposal. There are also more concerns about it extending the QT interval, although it is recognised that sertraline can do this in vulnerable cases. 22 In the two sertraline studies in GAD, sertraline was dosed flexibly between 50 and 150 mg daily (mean dose at the end of treatment about 90 mg). In one study sertraline was started at 25 mg daily for 1 week to improve tolerance early in therapy,23 and this acclimatisation period is also recommended by the manufacturer in the licensed use of sertraline in post-traumatic stress disorder, social anxiety disorder and panic disorder. 24
Cognitive behavioural therapy
There are a number of cognitive behavioural models of GAD. Examples include the cognitive avoidance model,25 the metacognitive model26 and the emotion dysregulation model. 27 Dugas and Koerner28 have also developed a model of GAD known as the intolerance of uncertainty model. Stated simply, the model proposes that negative beliefs about uncertainty (or intolerance of uncertainty) lead to difficulty dealing with real or imagined uncertainty-inducing situations, which can then lead to excessive worry and GAD. Research has shown a consistent and robust relationship between intolerance of uncertainty and GAD; for example, their relationship is not accounted for by shared variance with other anxiety disorders, mood disorders or negative affect. 29,30 Data also suggest that intolerance of uncertainty is a causal risk factor for high levels of worry and GAD; for example, changes in intolerance of uncertainty precede changes in worry over the course of treatment,31 and the experimental manipulation of intolerance of uncertainty leads to corresponding changes in worry and monitoring behaviour. 32,33 Thus, data from correlational, longitudinal and experimental studies suggest that intolerance of uncertainty plays a key role in GAD. The Dugas and Koerner model28 is one of three CBT protocols for GAD, which guides IAPT services in how to carry out CBT effectively and in line with best practice. The treatment aims to help affected individuals develop beliefs about uncertainty that are less negative, rigid and pervasive. This is accomplished with the use of treatment strategies (such as behavioural exposure to uncertainty, problem-solving training and imaginal exposure) that aim to help patients confront uncertainty-inducing thoughts and situations. The treatment has been tested in four published randomised clinical trials, with results showing that it is more efficacious than a waiting list control,34,35 supportive therapy36 and applied relaxation. 37 The findings also show that 60–77% of patients attain GAD remission and that 50–55% achieve high-end state functioning following the treatment. The CBT protocol developed by Dugas and Robichaud (i.e. based on the intolerance of uncertainty model of GAD) was therefore used in this trial. 38
Time course of therapeutic effect and longer-term benefits
The planned comparison was between SSRI medication and CBT, which are both active treatments. However, we considered that in a pragmatic trial the time course of benefit is likely to differ. The SSRI medication might have a benefit earlier on, but it is likely that this effect could reduce over time, largely because many of the participants may stop taking their medication. In contrast, CBT is an educational approach that should be providing the participants with skills that they may use in the future. We would therefore expect that CBT would continue to have benefit for the 12-month duration of the trial. As a result, our hypothesis was that CBT would lead to a better outcome than SSRIs at the 12-month follow-up point.
Chapter 2 Trial design: aims, objectives and methods
This chapter details the aims, objectives and methods of the trial as originally planned and approved by the funder, and subsequent modifications made as a result of discussions among the Trial Management Group (TMG) and with the sponsor.
Summary of proposed research
The trial was entitled the ‘Randomised controlled trial of the SSRI sertraline versus CBT for anxiety symptoms in people with GAD who have failed to respond to low-intensity psychological treatments as defined by the NICE guidelines’. The trial acronym decided was ToSCA (Trial of Sertraline versus Cognitive behaviour therapy for generalised Anxiety).
Overall aim
To conduct a randomised controlled trial (RCT) to compare the clinical effectiveness and cost-effectiveness in terms of symptoms and function of a pharmacological treatment (the SSRI sertraline) prescribed at therapeutic doses, with a manualised psychological intervention (CBT) delivered by trained psychological therapists, to patients with persistent GAD that has not improved with low-intensity psychological interventions as defined by NICE.
Hypothesis
Our hypothesis was that in people with GAD who had not responded to low-intensity psychological interventions, as recommended by NICE, CBT would lead to a greater improvement in their GAD symptoms as measured by the Hospital Anxiety and Depression Scale – Anxiety component (HADS-A)39 at the 12-month follow-up than prescription of the SSRI sertraline by their GP in accordance with recommended clinical guidelines.
Primary aim
To assess the clinical effectiveness at 12 months of treatment with the SSRI sertraline compared with CBT for patients with persistent GAD that has not improved with low-intensity psychological interventions.
Secondary aim
To calculate the cost-effectiveness at 12 months with the SSRI sertraline compared with CBT for patients with persistent GAD that has not improved with low-intensity psychological interventions.
Detailed objectives
Internal pilot (first 12 months of the recruitment period)
-
To test and refine the recruitment methods for the main trial.
-
To ascertain recruitment rates across sites and the acceptability of the overall recruitment process.
-
To examine the extent of comorbidity between GAD, depression and other anxiety disorders in the population referred into the study.
-
To ensure that the intervention can be delivered in accordance with the protocol in both arms, with satisfactory delivery of training and monitoring procedures.
-
To monitor and assess follow-up rates of the completed primary outcome measure (HADS-A) at 3 and 6 months within the pilot trial.
Overall trial (whole 24 months of the recruitment period including the internal pilot)
-
To recruit sufficient eligible patients with a Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)40 diagnosis of GAD willing to participate.
-
To compare the effect of high-quality, reproducible pharmacological and psychological interventions delivered in accordance with clear criteria and evidence-based guidelines. We asked participating GPs to follow established clinical guidelines for delivery of the pharmacological intervention, and the psychological intervention was manualised and quality controlled.
-
To obtain high rates of follow-up data on a minimum of 80% of those recruited into this trial at 12 months in order to provide a definitive answer to the research question and assess the longer-term outcomes of both interventions.
-
To analyse the results in accordance with the Consolidated Standards of Reporting Trials (CONSORT) and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines.
-
To disseminate the outcomes to the NHS, academic colleagues, relevant service user groups and the wider community.
Methods
Setting
The study was community based and linked with local IAPT services. 10 We aimed to work with five recruitment sites in southern England during the pilot phase and up to 15 sites across the whole of England in the full trial.
Recruitment of participants
People who had not responded to step 2 low-intensity psychological interventions for anxiety or depression who were being considered for step 3 interventions within their local IAPT services were considered eligible (Box 1 contains a list of step 2 low-intensity interventions meeting the inclusion criteria). Identification was by low-intensity IAPT workers, who routinely administer the GAD-7 anxiety measure41 and Patient Health Questionnaire-9 (PHQ-9) depression measure,42 reviewing patients. Those patients scoring ≥ 10 on the GAD-7 were given brief details about the trial and if they were interested in finding out more, and possibly taking part, their permission was sought for contact by the research team. If they were unsure about their interest at this stage they were given a brief flyer about the aims of the trial and invited to contact the research team for more information or to discuss things further.
A key inclusion criterion for the study is that patients should have received an initial low-intensity intervention but still have above-threshold symptoms (GAD-7 score of ≥ 10). Set out below are the types of low-intensity intervention (and the minimum number of sessions for each) that meet this inclusion criterion and also some excluded interventions.
Included interventions-
Guided self-help carried out by a PWP or equivalent low-intensity worker: patient needs to have had at least two treatment sessions after initial assessment session.
-
Pure self-help (non-facilitated self-help): patient needs to have had at least one follow-up session after the self-help resource was recommended.
-
cCBT: patient needs to have logged on and completed at least two cCBT sessions.
-
Psychoeducational or similar group facilitated by a PWP or equivalent low-intensity worker: patient needs to have attended at least two sessions of the group.
-
Exercise intervention if the exercise is for mood/anxiety: patient needs to have tried the recommended exercise intervention at least twice.
-
One-off workshop lasting at least half a day, facilitated by a PWP or equivalent low-intensity worker.
-
Signposting interventions (signposting to other services).
-
Guided self-help carried out by a qualified (or trainee) CBT therapist or other high-intensity therapist.
-
Psychoeducational or similar group, or one-off workshop facilitated by a qualified (or trainee) CBT therapist or other high-intensity therapist.
cCBT, computerised cognitive behavioural therapy; PWP, psychological well-being practitioner.
The central research team (trial manager/research assistant) were faxed the details of those who had agreed to be contacted by the research team and aimed to respond within 1 week (preferably by telephone or e-mail or, if not, by letter) offering them an appointment at the IAPT premises or their own home, whichever was preferred. A full study information sheet was sent to potential participants at this stage (see Appendix 1) and the patient’s GP was contacted, with their permission, and asked to complete a Medical Suitability Review form to check that there were no known medical contraindications to them being prescribed sertraline if they were randomised to that intervention arm in the trial (see Appendix 2).
Recruitment appointment/baseline assessment
The baseline interviews and assessments were conducted by a member of the central or local research team (research assistant or clinical studies officer). They checked, at the outset, that potential participants had read and understood the study information leaflet, and answered any queries. They also checked that the patient understood the reasons for the study and confirmed at that point that they were interested in taking part on the understanding that, if randomised to the drug arm, they would receive the SSRI sertraline that, although proposed for use outwith a marketing authorisation for GAD, was recommended by NICE on the basis of its clinical effectiveness and cost-effectiveness in randomised trials. They also ensured prior to the appointment that there were no medical contraindications to receiving the medication sertraline recorded on the Medical Suitability Review form as returned by the GP (see Recruitment of participants, above).
Those agreeing to take part were asked to give fully informed consent before undergoing the eligibility check and baseline assessment. It was expected that most patients would be willing to consent to the study at the baseline visit, but if they were unsure they could be rescheduled for consent and baseline assessment at a later date. The assessment was conducted at the IAPT site or GP surgery in accordance with patient preference. Written consent was obtained by a delegated and appropriately trained member of staff. No clinical trial procedures, including confirmation of eligibility, were conducted prior to taking consent. A copy of the consent form was given to the patient, the original retained in the investigator site file and a further copy sent to the patient’s GP for their medical notes (see Appendix 3).
If potential participants were happy to proceed, then the inclusion and exclusion criteria were checked (see Inclusion criteria and Exclusion criteria), which included administering a pregnancy test to females of child-bearing potential. In assessing whether or not the potential participant fulfilled the DSM-IV criteria for GAD, the relevant sections of the Mini International Neuropsychiatric Interview (MINI) questionnaire43 were administered by the research team member (depression, panic, social anxiety, alcohol and substance misuse, and GAD). If the DSM-IV criteria for GAD were fulfilled, potential participants were asked to confirm whether their GAD or worry symptoms were more severe and of more concern to them than any symptoms that they might have associated with psychological comorbidities, such as depression and other anxiety disorders, and that this was an important problem for them that they wanted to address.
If they fulfilled this criterion and all the other eligibility criteria, the researcher then administered the HAM-A questionnaire12 and asked the participant to complete the primary and secondary outcome measures at baseline, aiming for 100% completion of measures at baseline (see Outcome measures).
Inclusion criteria
-
Age ≥ 18 years.
-
Positive score of ≥ 10 on the GAD-7.
-
Primary diagnosis of GAD as diagnosed on the MINI.
-
Failure to respond to NICE-defined low-intensity interventions.
Exclusion criteria
Exclusion criteria were expanded from those in the initial approved protocol after discussion with the sponsor [University College London (UCL) Joint Research Office (JRO)].
-
Inability to complete questionnaires because of insufficient English or cognitive impairment.
-
Current major depression.
-
Other comorbid anxiety disorder(s) of more severity or distress to the participant than their GAD.
-
Significant dependence on alcohol or illicit drugs.
-
Comorbid psychotic disorder, bipolar disorder.
-
Treatment with antidepressants in past 8 weeks or any high-intensity psychological therapy within past 6 months.
-
Currently on contraindicated medication: monoamine oxidase inhibitors within the past 14 days or pimozide.
-
Patients with poorly controlled epilepsy.
-
Known allergies to the investigational medicinal product or excipients.
-
Concurrent enrolment in another investigational medicinal product trial.
-
Severe hepatic impairment.
-
Women who are currently pregnant or planning pregnancy, or lactating.
-
Patients on anticoagulants.
-
History of bleeding disorders.
Randomisation and notification of general practitioner and cognitive behavioural therapists
A copy of the completed baseline assessment form was forwarded by the researcher to the chief investigator (CI) or delegated clinician in order to confirm participant eligibility. After confirming eligibility, the CI or delegated clinician accessed a web-based interface using a unique username and password, and entered the unique study identification number for the participant in order to randomise them to one of the two intervention arms via an independent computerised service (Sealed Envelope Ltd, London, UK) provided by the PRIMENT Clinical Trials Unit (CTU). There were no stratification variables. The randomisation outcome was transmitted electronically to the trial manager who then contacted the individual participants within 2 working days of their baseline assessment to inform them of which treatment group they were in.
The research team (trial manager or research assistant) ensured that the patient’s GP was notified about their patient being enrolled in the trial and which treatment arm they were in. They were also notified if the patient was not eligible for inclusion in the trial (see Appendix 4).
If they had been randomised to the medication/sertraline arm, the patient was asked to make an appointment within the next 2 weeks to see their GP to discuss starting the treatment, and the research team let their GP know that they would be doing this (see Appendix 5).
The research team also gave the relevant local IAPT services the details of participants randomised to the CBT arm – the IAPT team then contacted the patient to arrange a course of treatment.
Interventions
Pharmacological intervention: sertraline
The medication sertraline was prescribed by the patient’s GP in accordance with recognised clinical guidelines. The GPs were asked to review these patients regularly (at least six times in 12 months) and patients were to take the medication for 1 year unless they had significant adverse effects. The GPs were given details of the suggested timing and content of each of these appointments with the trial participants (see Appendix 6).
The GP was to act in the best interests of the participant at all times, so was free to refer them to secondary care services or psychological treatments if indicated. We explained that we would prefer patients in the SSRI arm not to be referred to or receive CBT while in the trial, but appreciated this might occasionally happen and should be documented in their GP notes.
If the patient made any interim visits to the surgery to discuss their treatment for GAD or issues to do with the medication being received, we asked for these to be clearly documented in their notes (we costed for up to four additional visits per GP to cover this possibility).
The GP was asked to record any adverse events and both the participants and their GPs were asked to report any serious adverse events (SAEs) or suspected unexpected serious adverse reactions (SUSARs) to the trial team (see Appendix 7).
Psychological intervention: cognitive behavioural therapy
This was delivered by high-intensity therapists from local IAPT services who were trained to deliver 14 (± 2) 50-minute sessions of a manualised treatment developed for use in GAD.
This covered six treatment modules:
-
Psychoeducation and worry awareness training – the first few sessions of treatment are devoted to psychoeducation in which patients begin to monitor their worrying on a day-to-day basis, and learn to distinguish between worries about current problems and worries about hypothetical situations.
-
Re-evaluation of the usefulness of worry – patients identify and re-evaluate their positive beliefs about worry using strategies such as role-play and hypothesis testing. Patients are helped recognise that their beliefs about the usefulness of worry are interpretations and not facts, and begin the process of ‘imagining a life without worry’.
-
Uncertainty recognition and behavioural exposure – participants learn that intolerance of uncertainty contributes to worry and anxiety, and that uncertainty-inducing situations are largely unavoidable. They then learn to seek out and experience uncertainty-inducing situations.
-
Problem-solving training – for worries about current problems, participants learn to use a problem-solving procedure targeting problem orientation, problem definition and goal formulation, generation of alternative solutions, decision-making, and solution implementation and verification. 44
-
Written exposure – in the field of health psychology, a method known as written emotional disclosure has been shown to lead to positive health outcomes. 45 Written exposure sessions are continued until writing about the feared outcome no longer provokes anxiety (typically 8–10 exposure sessions).
-
Relapse prevention – the final component is relapse prevention, the aim of which is to consolidate the attitudes, beliefs and skills acquired during therapy. Patients are encouraged to continue practising their new skills and prepare for stressors that may arise.
Sessions were to be digitally recorded and a random 10% to be assessed for quality (fidelity to the manual and therapist competence) by an independent external assessor according to prespecified criteria (see Chapter 6 for further details).
Usual care by general practitioner
Randomisation was between sertraline prescribed by the participant’s GP and CBT provided within an IAPT service, and both interventions were to be in addition to any other usual care provided by the GP. As always, the patients could be offered other medication or psychotherapy as part of their usual care, although we encouraged the GPs not to change the patient’s medication unless clinically indicated or requested by the patient, and not to refer them for CBT while in the sertraline arm if possible. Usual practice would be to allow the patient with GAD to choose, with the help of their GP, between a SSRI and CBT if they met the criteria for a step 3 intervention and if neither was contraindicated.
Patients in the CBT arm were likely to receive their CBT treatment more quickly than is usual in most NHS settings, and it was a psychological intervention specifically developed for people with GAD that is not current UK practice. NHS waiting lists mean that patients in the SSRI arm who then asked to be referred for CBT were likely to experience significant delays in receiving this. We proposed to record and measure all use of antidepressants and other forms of counselling or psychotherapy, whether NHS or private, and to take account of these in the analysis.
Outcome measures
The outcome measures to be collected during the trial are summarised in Table 1, according to the time at which each would be collected.
| Assessment/measure | Time point (months) | ||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | |
| Informed consent | ✓ | ||||
| Establishing eligibility | ✓ | ||||
| Randomisation | ✓ | ||||
| Urine pregnancy test | ✓ | ||||
| MINI (relevant sections) | ✓ | ✓ | |||
| HADS-A | ✓ | ✓ | ✓ | ✓ | ✓ |
| GAD-7 | ✓ | ✓ | ✓ | ||
| HAM-A | ✓ | ✓ | |||
| PHQ-9 | ✓ | ✓ | ✓ | ✓ | ✓ |
| WSAS | ✓ | ✓ | |||
| EuroQol-5 Dimensions | ✓ | ✓ | ✓ | ✓ | ✓ |
| Health economics questionnaire: ESC questionnaire | ✓ | ✓ | ✓ | ||
| Patient preference rating scale | ✓ | ✓ | |||
| Treatment acceptability scale: Client Satisfaction Questionnaire | ✓ | ✓ | |||
| Health service outcomes | ✓ | ✓ | |||
Primary outcome
The primary outcome was the HADS-A measured at 12 months. This is the 7-item anxiety component of the Hospital Anxiety and Depression Scale, a very widely used 14-item scale that can be self-administered. It has high validity and reliability, and the anxiety and depression components have been assessed separately as primary outcomes. 39
The primary outcome measure was initially the GAD-7,41 but this was changed to the HADS-A about 6 months after the study had started and before the recruitment of any trial participants had begun. This was done because we had originally understood, when selecting the GAD-7 as our primary outcome measure for both intervention arms, that it would be possible to ask participants seeing IAPT high-intensity therapists for treatment in the CBT intervention arm not to complete the GAD-7 questionnaire at every CBT session, which is IAPT’s current usual practice. Unfortunately, it was not possible to negotiate this in all the pilot study areas. As a result of this we were concerned that using the GAD-7 in the trial as the primary variable when this is associated with treatment in one of the groups (i.e. the CBT group) was a source of potential bias, and we therefore decided to change the primary outcome measure to the HADS-A.
Secondary outcomes
These were all self-completed measures to be collected by postal questionnaire, apart from the researcher-administered MINI and health service outcomes collected from patient notes.
-
HADS-A: the HADS-A was collected at baseline and then as a secondary outcome measure at 3, 6 and 9 months. 39
-
HAM-A: this is a 14-item observer-rated anxiety scale, which has been widely used, particularly in pharmacological studies. 12 It was to be administered by a member of the research team at baseline and at the 12-month follow-up.
-
GAD-7: a 7-item self-completion questionnaire with very good sensitivity (89%) and specificity (82%) for GAD. 41 It is one of the core measures regularly administered by the IAPT services. 10 It was to be collected at baseline, and at 6 and 12 months.
-
PHQ-9: this is a 9-item self-rate scale widely used to monitor the severity of depression. 41 It was to be collected every 3 months for the 12-month duration of the study, along with the HADS-A and EuroQol-5 Dimensions, three-level version (EQ-5D-3L).
-
Work and Social Adjustment Scale (WSAS): this is a 5-item self-completion questionnaire that we planned to use to assess participants’ difficulties with physical and social functioning. 46 It was to be collected at baseline and at the 12-month follow-up.
-
EQ-5D-3L: a 5-item self-completion measure used to assess quality of life and calculate utility scores for quality-adjusted life-years (QALYs). 47 It was to be collected every 3 months for the 12-month duration of the study, along with the HADS-A and PHQ-9.
-
Employment and Social Care questionnaire (ESC): relevant data on services used and productivity losses were to be collected using this modified version of the Client Service Receipt Inventory48 at baseline, and at the 6- and 12-month follow-up.
-
Patient acceptability measure: we planned to use the Client Satisfaction Questionnaire (CSQ), a brief 8-item self-completion questionnaire administered at 3 and 12 months. 49
-
Patient preference rating scale: we planned to use a Likert scale used by our team in other studies, also administered at baseline and at the 12-month follow-up.
-
MINI:43 it was an addition to the original protocol to also administer the MINI questionnaire at the 12-month follow-up to assess the depression, panic, social anxiety and GAD components, with the intention of establishing whether or not the participant met the criteria at follow-up for DSM-IV caseness for GAD or any of the common psychological comorbidities.
-
Health service outcomes: we planned to collect health service use data from both intervention arms at baseline, capturing health service use for the preceding 6 months, and again at 12-month follow-up for the following items of health service use during the preceding 12 months: total GP consultations as well as those coded for GAD, psychotropic drug prescriptions from the GP surgeries, and secondary care attendances including mental health and psychological services. The IAPT sites agreed to inform the research team of the attendance rates for CBT sessions attended by trial participants in the CBT intervention arm.
-
Serious Adverse Events Monitoring Form: we planned to use the standard SAE template provided by the PRIMENT CTU, modified for use in this study.
Final assessment at 12 months
A member of the research team would have administered the HAM-A face to face to all participants at 12 months and encouraged them to complete the 12-month outcome measures at this point to ensure optimum data collection. This would have involved a different member of the research team from the original assessor, who would have been blind to the participant’s trial allocation. As a check on this, they would have been asked to say which trial arm each participant they assessed had been randomised into.
Procedures for reporting and recording serious adverse events
Patients were asked at each GP visit about side effects or health issues occurring while on medication, as is normal clinical practice, and both the patients and GPs were asked to let the study team know about any serious medical problems that may have occurred between consultations or be reported at the time of the medication review. All patients recruited to the study were given a card with details of how to contact the central research team about any serious medical problems occurring while they were in the trial at the time that they were informed about the outcome of the randomisation procedure. The same applied to both the GPs and IAPT CBT intervention therapists, who were given a brief explanatory sheet indicating when they should be informing the research team about any serious medical problem affecting a participant in the trial (see Appendix 7).
Both the GPs and the IAPT therapists were asked to notify the CI about any serious medical problems (to cover SAEs, SUSARs or important medical events) that might affect any participant in the trial as soon as they were aware of this. The CI or an appropriate delegated member of staff would then be asked to complete the sponsor’s SAE form and to e-mail this to the PRIMENT CTU on behalf of the sponsor within 24 hours of his/her becoming aware of the event. The CI was expected to respond to any SAE queries raised by PRIMENT CTU as soon as possible. All SAEs were to be recorded in the relevant case report form and the sponsor’s adverse event log, which was to be reportable to the sponsor once per year.
The CI or delegate might also contact the patient’s GP, depending on the nature of the SAE, to obtain more information regarding the adverse event. All SUSARs were to be notified to the sponsor within 24 hours according to the sponsor’s written standard operating procedure.
Sample size calculation
The principal outcome variable is the change from baseline to 12 months in the HADS-A score which we planned to compare between treatment groups in a regression model that accounted for baseline scores. Tests and confidence intervals for treatment effects would be based on the normal distribution – an assumption justified by the central limit theorem. Estimates for the standard deviation (SD) of HADS-A scores are available from Tyrer et al. 50 In this study, SDs between 4 and 5 were found for the change of score between baseline and 12 months for both randomised conditions. Therefore, we used an estimate of 5 for the SD of our outcome measure, overlaid with an additional component of variance (essentially attributable to the therapist) sufficient to give an intracluster correlation coefficient of 0.02. Then, making the conservative assumption of a cluster size of 7 and an allowance of 20% for dropout and other challenges, this meant we would require a total sample size of 360 patients to detect a (‘true’) average difference of 2 between treatments with 90% power at p < 0.05 (two-sided). Furthermore, the expected half-width of the 95% confidence interval for the treatment difference is then 1.2.
With this sample size, we would have retained ≥ 80% power should the intraclass correlation coefficient turn out to be 0.05 rather than 0.02. Alternatively, we would retain 80% power should the SD of our outcome measure turn out to be 5.8 rather than 5. This design had the advantage of providing robust interpretation in a number of circumstances as a result of the planned precision. Thus, with no impact on alpha spending, it would be possible to interpret a significant difference between the groups of > 2 points as also being clinically relevant and, additionally, a non-significant result that excludes a difference of 2 points (i.e. the upper confidence interval range < 2 points) as demonstrating non-inferiority. Furthermore, a non-significant difference in which the outer bands of the confidence interval are < 2 points on the HADS-A would indicate equivalence. The planned precision of the trial is such that it should have provided a firm basis for decision-making even if opinions as to the correct size of the minimally important difference were to have altered somewhat before the results became available.
Statistical analysis plan
Summary of baseline data and flow of patients
We planned to follow the CONSORT guidelines in reporting and analysing our data. This included presenting a table of summary statistics for those secondary outcome variables collected at baseline showing clinical characteristics for each group along with (baseline) demographic characteristics. We also planned to create a flow chart that would provide the number of potential participants who were screened, eligible, randomised and followed up at each time point.
Primary outcome analysis
The principal outcome variable was the change from baseline to 12 months in the HADS-A score, which we aimed to compare between treatment groups in a regression model that accounted for baseline scores. Tests and confidence intervals for treatment effects would be based on the normal distribution – an assumption justified by the central limit theorem. Given that there is a single primary outcome, no corrections for multiple comparisons would be required for the statistical inference. The principal analyses would have been conducted according to a prespecified statistical analysis plan to be finalised before database lock. The principal analyses would have been conducted according to the intention-to-treat principle using generalised mixed models. The primary analysis would have used a generalised mixed model accounting for clustering of therapist effects, investigational sites (both as random effects) and a limited number of prespecified patient-level factors, including baseline HADS-A score. The principal analyses would have been based on available data, and supportive analyses would examine the extent to which the principal analyses are robust to the challenge presented by the observed loss to follow-up. Exploratory analyses would have been carried out to describe how patient preferences along with a limited number of other prespecified characteristics of participants may modify treatment effects. 50 Any subgroup analyses conducted would also have been regarded as exploratory.
Secondary outcome analysis
The secondary outcome variables would have been analysed using the same (generalised mixed model) framework as for the primary outcome variable. However, the presentation of the results would have been restricted to the confidence intervals that come out of the analysis, rather than the p-values.
Economic evaluation
We planned to calculate the net monetary benefit (NMB) of CBT compared with sertraline for patients with persistent GAD who had not improved with step 2 low-intensity psychological interventions. A higher NMB indicates greater relative cost-effectiveness. Health- and social-care resource use would have been collected for both interventions over the 12-month duration of the trial using patient GP records, and patients asked to complete a significantly reduced version of the Client Service Receipt Inventory (the Employment and Social Care questionnaire) at baseline and 12 months. The health service resource use data collected would have focused mostly on primary care and psychological therapies. Details of secondary care and mental health resource use would also have been collected. Health-care resource use for the preceding 6 months would have been collected at baseline for adjustment purposes only. Resource use would be multiplied by costs from nationally published sources and summed to calculate the total cost per patient. The health-care resource use associated with the interventions would have been captured in each arm as follows: the cost of sertraline and any follow-up, training or monitoring costs; the cost of CBT based on the number of sessions attended per patient, session duration, the staff type and grade delivering the CBT; and training and any overhead costs.
The mean cost per patient for patients in the sertraline and CBT groups would have been calculated and confidence intervals reported, calculated using non-parametric bootstrapping with replacement and adjusting for baseline service use. The mean QALYs per patient would have been calculated from the EQ-5D-3L51 and the UK algorithm for calculating utility scores. 52 The EQ-5D-3L would have been collected at baseline, 3, 6, 9 and 12 months to allow calculation of the area under the curve over the 12-month trial duration for the SSRI and CBT groups, adjusting for baseline differences. The NMB of both interventions would have been calculated for a range of values of willingness to pay for a QALY. Confidence intervals would have been constructed using non-parametric bootstrapping. A cost-effectiveness acceptability curve would have been used to report the probability that each intervention has the higher NMB for a range of values of willingness to pay for a QALY. One-, two- and multiway sensitivity analyses would have been conducted for any assumptions made. Missing data and clustering would have been handled as specified in the statistical analysis plan.
Sensitivity and other planned analyses
The principal analyses would have been conducted according to a prespecified statistical analysis plan, to be finalised before database lock. The principal analyses would have been based on available data and supportive analyses would have examined the extent to which the principal analyses were robust to the challenge presented by the observed loss to follow-up. Exploratory analyses would have been carried out to describe how patient preferences, along with a limited number of other prespecified characteristics of participants, might modify treatment effects.
Chapter 3 Obtaining ethics and research governance approvals
Timetable: sponsor and Medicines and Healthcare products Regulatory Agency approvals
The official start date of the trial as agreed with the NIHR’s HTA programme was 1 August 2014.
This was a clinical trial of an investigational medicinal product, and we started working with the sponsor (the UCL JRO) before the official start date on the sponsorship procedures required. After several iterations the JRO approved the first version of the trial protocol on 5 November 2014 (protocol number 14/0249).
Application for clinical trials authorisation was made to the Medicines and Healthcare products Regulatory Agency (MHRA) on 7 November 2014 and their approval was received on 13 November 2014.
Ethics approval and major amendments
Our local Research Ethics Committee (REC) was Brent National Research Ethics Service Committee London, and the relevant Integrated Research Application System application was submitted to them on 7 November 2014 and presented at a meeting of the REC on 24 November 2014.
We received a favourable ethics opinion, with conditions, on 3 December 2014 and gained full approval on 9 December (REC reference number 14/LO/2105).
-
Major amendment 1: at the TMG on 21 January 2015 a major protocol amendment altering the primary outcome measure from the GAD-7 to the HADS-A was suggested (see Chapter 2, Methods). This was developed and agreed with the NIHR’s HTA programme and Trial Steering Committee (TSC) before being submitted to the REC and MHRA on 9 March 2015. There were also some minor study document changes submitted with this major amendment request. Approvals were obtained from the REC on 17 March 2015 and from the MHRA on 10 April 2015.
-
Major amendment 2: a further major amendment was submitted on 13 April 2015 listing the four pilot sites for the trial and giving details of their principal investigators (PIs). This was approved by the REC on 23 April 2015.
-
Major amendment 3: a third major amendment was submitted on 23 July 2015 applying to change the named PI at the Bristol site and was approved on 30 July 2015.
-
Major amendment 4: the final major amendment was for approval for a 6-month replacement of the CI Dr Marta Buszewicz by Professor Irwin Nazareth, this amendment was submitted on 16 September 2015 and approved on 1 October 2015.
Research governance
Obtaining research governance permission proved more complicated and led to a significant delay in being able to start the trial. It was confirmed that we had submitted a full set of documents for study-wide approval on 27 January 2015, but significant delays followed while our lead Clinical Research Network (CRN) queried the governance arrangements for participating GP practices and whether or not they should be registered as individual research sites, which would have been very administratively burdensome, given that most of the planned 360 trial participants were likely to be registered at different practices. We worked with the CRN and the Health Research Authority to develop the documents required for a generic site-specific information form, which allowed us to obtain NHS approval for the collection of health service usage data for trial participants from their GP notes without registering each general practice as a research site. This was approved by the national NIHR Coordinated System for gaining NHS Permissions (NIHR CSP) co-ordinating centre on 14 April 2015 and we finally received study-wide research and development (R&D) approval on 27 April 2015 – 3 months after initial submission of the full set of documents (see Appendix 8).
Having received national approval on 14 April 2015, it then took several further months to receive local assurances for all the pilot sites – the last of these was the South London assurance for Kingston and Greenwich received on 3 July 2015.
Sponsor documentation prior to site set-up visits
From February to May 2015 the trial team worked with the PRIMENT CTU on the final documentation required for the trial management file and site files prior to the site initiation visits. This included the trial monitoring plan, trial pharmacovigilance documents and training procedures, and a formal agreement between the PRIMENT CTU and the CI because a large number of sponsor responsibilities were being delegated to the CTU.
Trial database and electronic case report form development
The trial team worked over the same period with the CTU data manager and database developer to finalise the data management plan, trial database and electronic case report forms for the study. Rigorous database testing was carried out and sign-off achieved on 25 June 2015.
Monitoring processes
The PRIMENT CTU led on developing the monitoring plan. The trial monitor was John Codington of Cod Clinical Ltd, an external monitor with whom the CTU had previously worked.
The plan was for the central research site at UCL to receive a monitoring visit 3 months after being opened and for the first monitoring visit following initiation for the IAPT pilot sites to take place after five patients had been randomised at each site. Further on-site monitoring visits were to take place annually at each site.
In the event, because of very poor recruitment no monitoring visits took place.
University College London data safe haven
We worked with the UCL Information and Services Division to set up a secure storage system for any confidential data on the UCL Data Safe Haven system, although in the event we did not need to store any data there given the lack of participant recruitment and early termination of the trial.
This service provides a technical solution for storing, handling and analysing identifiable data. It has been certified to the ISO27001 information security standard53 and conforms to the NHS Information Governance Toolkit. 54 Built using a walled garden approach, in which the data are stored, processed and managed within the security of the system, it avoids the complexity of assured end-point encryption. A file transfer mechanism enables information to be transferred into the walled garden simply and securely.
Chapter 4 Conduct of the trial: anticipated recruitment rate, Improving Access to Psychological Therapies and research staff training, and pilot site openings
Anticipated recruitment rate
To date, there have been few large-scale trials recruiting from IAPT services, and none to our knowledge that have recruited from the caseloads of low-intensity IAPT staff or that have compared psychological interventions with medication. Three of the co-applicants (JC, MS and RS) have experience of working with IAPT services as researchers, trainers and service leads. They arranged contacts between the research team and IAPT study sites to ensure local understanding of recruitment procedures and assist in troubleshooting problems, as well as arranging training and supervision of the high-intensity CBT therapists involved in delivering the intervention (see Chapter 6 for details of the training and supervision of the high-intensity CBT therapists).
Prior to writing the proposal we examined the IAPT data for the year 2011–12 for two local London boroughs with a combined population of 426,000 during the year (Camden and Islington), among which 6569 people had an initial appointment within their IAPT services. Of these, 694 people (11%) were initially seen by an IAPT low-intensity therapist and were stepped up from a low- to high-intensity intervention with a GAD-7 score of ≥ 10. Eighty-nine (13%) of these 694 people were given a provisional primary diagnosis of GAD by the low-intensity worker and would have been potential candidates for our trial. Sixty-nine per cent were given other provisional primary diagnoses, and for 18% no diagnostic coding was made. We assumed that 67% of those with a GAD-7 score of ≥ 10 and suitable to be stepped up would be on antidepressants already or decline to be randomised. Extrapolating from the 89 people with a provisional primary diagnosis of GAD, this would have excluded 60, leaving 29 suitable and potentially willing to be randomised for the study.
As low-intensity IAPT staff have minimal training in making psychiatric diagnoses, we were conscious that a recruitment strategy that relied on them identifying people with GAD would be likely to miss many suitable people with GAD. This would include people with GAD comorbid with other anxiety disorders and with depression, pure GAD being rare compared with comorbid GAD. Accordingly, our recruitment strategy would need to encourage low-intensity IAPT staff to identify people as suitable for the study if there was a possibility they might have GAD, including comorbid with depression and other types of anxiety. So, in terms of the numbers identified in our two local London boroughs above, we wanted not just the 89 people with a GAD-7 score of ≥ 10 for whom they gave a provisional diagnosis of GAD, but all 694 people with a GAD-7 score of ≥ 10 to be considered if there was a possibility they might have GAD. We assumed that if this broader identification approach was adopted, a much larger pool of potential participants would be identified and referred for baseline assessment by the low-intensity workers, although up to half of the patients assessed for the study might then be ineligible because of a comorbid major depressive disorder or because the patient identified another anxiety disorder as being more significant than their GAD.
In order to recruit sufficient people for this trial, we needed to recruit a total of 360 people (see Chapter 2, Sample size calculation), which equated to 24 participants per study site if there were 15 sites. This would have meant recruiting one participant per site per month over the full 24 months of the trial period, or two participants per month over a 12-month period.
During our internal pilot phase we worked on forming relationships with the local low-intensity IAPT workers during the initial 3 months and ensuring that they were committed and clear as to what was required. We then aimed to recruit two participants per month from each of the five pilot sites for the succeeding 9 months, resulting in a total planned recruitment of 90 participants during this pilot phase (i.e. 25% of our planned total recruitment of 360 participants). We also planned to consolidate our relationships with a further 10–15 sites throughout England during this time, so that we were in a good position to recruit the remaining 170 participants over the following 12 months of the main trial recruitment period.
Our assumptions regarding the number of patients to be screened and recruited across the 24 months of the whole trial are outlined in Figure 2 and the number of patients we planned to recruit at each of the pilot sites during the 12 months of the internal pilot is given in Table 2. The pilot sites would continue recruiting and treating patients until the end of the full-trial recruitment period (Figure 3).
FIGURE 2.
Planned screening and recruitment across the 2 years of ToSCA. GAD trial recruitment includes pilot phase.

| ToSCA IAPT pilot sites | Pilot recruitment target, n |
|---|---|
| Camden and Islington with Kingston | 24–36 |
| Coventry and Warwickshire | 24–36 |
| Bristol | 12–18 |
| Greenwich | 12–18 |
| Total | 72–108 |
FIGURE 3.
Proposed timelines for ToSCA, with a start date of 1 August 2014. a, We will offer treatment to completion to all participants recruited to the internal pilot even if not proceeding to the full trial; b, Start training therapist at the other sites in preparation for the full trial. D, Data Monitoring and Ethics Committee meeting; DMEC, Data Monitoring and Ethics Committee; IMP, investigational medicinal product; SOP, standard operating procedure; TSC, Trial Steering Committee.

In the event, we had four pilot IAPT sites in London, Central and South West England that agreed to take part at this stage and would have provided a range of populations; the London boroughs of Camden and Islington together with Kingston (all managed by the same IAPT service), Greenwich, Bristol, and Coventry and Warwickshire. Because of their relatively larger populations and IAPT service throughput, Camden and Islington with Kingston and Coventry and Warwickshire had target recruitment rates during the period of the internal pilot that were double those of Greenwich and Bristol.
The IAPT sites agreed to provide high-intensity CBT for 50% of the participants recruited. The number of therapists and supervisors trained was proportional to their target.
Because of the delays described in the previous chapter with obtaining research governance approvals as well as the sponsorship and site initiation processes (see Chapter 3), the first pilot site to open to recruitment was Camden and Islington with Kingston on 1 July 2015 (see Site initiation visits, opening to recruitment and standard operating procedures below), which was 5 months later than originally planned and meant that our 12-month internal pilot phase would have been due to end on 30 June 2016.
Trial preparation: staff training
Training of high-intensity cognitive behavioural therapists
A 2-day training session in London on 22 and 23 January 2015 was arranged for the IAPT high-intensity CBT therapists and their supervisors from each of the pilot sites, and was delivered by Professor Michel Dugas who came over from Canada to deliver the training (see Appendix 9 and Chapter 6).
Liaison with and training of low-intensity psychological well-being practitioners
In preparation for each pilot site opening for recruitment, training sessions were held with the IAPT psychological well-being practitioners (PWPs) at each site. Their purpose was to prepare the PWPs for their part in identifying suitable participants for the study (see Appendix 10).
Training of research staff to conduct informed consent, eligibility assessment and baseline measures
Two half-day training sessions were conducted for any member of the research staff who might be involved in the baseline recruitment process – this included a combination of the research staff based at the UCL central trial office and lead PWPs or clinical studies officers involved in these procedures at the pilot sites. The first session was held on 5 May 2015 in London and included the trial co-ordinator, Dr Anastasia Kalpakidou, the Bristol Clinical Studies Officer, Joy Farrimond, and lead PWPs from Camden, Islington and Kingston (Tarun Limbachaya, Annie Ormond, Elliott Rose, Rachel Lawrence and Natalie Gunn).
The second training session was held on 16 July 2015 in Nuneaton near Coventry and included the trial co-ordinator, Dr Anastasia Kalpakidou, research assistant Sally Gascoine, ToSCA intern Alessandro Bosco and the Coventry and Warwickshire lead PWP and clinical studies officers (Helen Fletcher, James Tucker, Abayomi Shomoyei and Emily Benson).
Current good clinical practice accreditation was a condition of having a research staff role on the trial (see Appendix 11).
The central research team produced a full training/recruitment manual for participating sites with all the relevant questionnaires and outcome measures as appendices for reference. This was distributed in draft version to participants at the training sessions and the full electronic version sent to the pilot sites when they were open to recruitment (manual available on request).
General practitioner recruitment processes
As recruitment of patients to the trial was via the IAPT PWPs, the GPs who would potentially be taking part in the study were identified only once their patient(s) had expressed an interest in being assessed for the trial. At this point they were contacted with their patient’s consent and asked to complete a Medical Suitability Review form to check that there were no known medical contraindications to them being prescribed sertraline, should they be randomised to that intervention arm in the trial (see Appendix 2).
In order to forewarn the local GPs and hopefully gain their co-operation with the study and a speedy response to any request for completion of the Medical Suitability Review form once requested, all the GPs in the areas where the pilot IAPT sites were situated were informed about the study in advance via their local primary care leads within their CRN structure. The procedures for this varied slightly between areas according to the procedures that they normally followed, but consisted, in essence, of an e-mail notifying the practices about ToSCA with an attached flyer (see Appendix 12) that informed them about the three potential procedures that they might be asked to assist with if one of their patients was recruited to the study, and the relevant rates of reimbursement per patient:
-
completion of the GP Medication Suitability Review – reimbursed at £35
-
prescribing sertraline for a period of 12 months or as appropriate – reimbursed at £140 in accordance with clinical guidelines (only applied to patients in the sertraline arm of the trial)
-
facilitating collection of health services data at the end of the trial – reimbursed at £20.
The primary care leads of the two London CRNs involved (North Thames and South London) asked their local GPs to respond to this initial notification by sending an expression of interest in taking part in the trial, which would be the normal process for recruiting interested general practices. Very few practices responded in this way in either area, but this was not a major issue as the chances of patients being recruited to the trial from any individual practice were small.
ToSCA video
The North Central London Research Consortium, which supports primary care and mental health research in north central London where the central research site at UCL is located, also funded the production of a promotional video about ToSCA for GPs to watch. This included some educational material about GAD as well as a brief description of the background to the trial and details of the reimbursement rates for GP practices with patients involved in the trial. It was the first time this methodology had been tried.
General practices in the local area (i.e. Camden and Islington with Kingston) were reimbursed £70 if they arranged to view this video within a practice clinical meeting and could give evidence of the GPs in the practice having watched it. Ten practices were reimbursed for watching the video: seven in Camden (out of a total of 40 practices) and three in Islington (out of a total of 38 practices). Of these, all but one practice in Islington expressed an interest in taking part in the study if any of their patients were potentially eligible.
Interest was also expressed in the video by the other pilot sites where the North Central London Research Consortium was not in a position to reimburse the practices, and they were given the link to the video to watch if they wished. This has now entered the public arena via YouTube (YouTube, LLC, San Bruno, CA, USA). 55
Site initiation visits, opening to recruitment and standard operating procedures
As described in Chapter 3, significant delays in obtaining research governance approval for the study as well as delays in some of the sponsorship processes meant that we were able only to start recruiting participants to the trial 5 months later than anticipated (i.e. at the beginning of July 2015 rather than 1 February 2015 as initially planned).
The central co-ordinating site based at the UCL Research Department of Primary Care and Population Health had its site initiation visit conducted by the UCL JRO on 29 May 2015 and the trial was declared open to recruitment from 1 July 2015.
The first pilot site initiation visit was at Camden and Islington with Kingston on 5 June 2015, and the site was also declared open to recruitment from 1 July 2015.
The Greenwich site initiation visit took place on 28 July 2015 and the site was declared open to recruitment on 17 August 2015.
The Coventry and Warwickshire site initiation visit took place on 29 June 2015 and the site was declared open to recruitment on 3 September 2015 (the delay between site initiation and opening to recruitment was because of discussions regarding whether or not the PI at this site might change; this was then decided against and an updating teleconference to ensure that the site was up to date with all the required procedures was held on 3 September 2015).
The Bristol site initiation visit took place on 27 August 2015 and the site was declared open to recruitment on 8 September 2015.
The research team worked with the PRIMENT CTU and the UCL JRO to identify the relevant standard operating procedures for the trial, and ensured that all relevant staff, centrally and at the pilot sites, were trained in their use and had signed the relevant registers confirming this.
Chapter 5 Participant recruitment: actual recruitment rates versus planned recruitment rate and strategies used to try and improve this
Participant recruitment to the trial
Once the sites were open to recruitment, both the screening and recruitment of trial participants was very slow. The results of screening and recruitment are summarised in Figure 4, the CONSORT diagram covering the time period from 1 July 2015 until 18 February 2016 following a monitoring meeting with the NIHR HTA programme, which took place on 14 January 2016 (see Table 3 for further details).
FIGURE 4.
The CONSORT diagram showing participant recruitment and allocation.
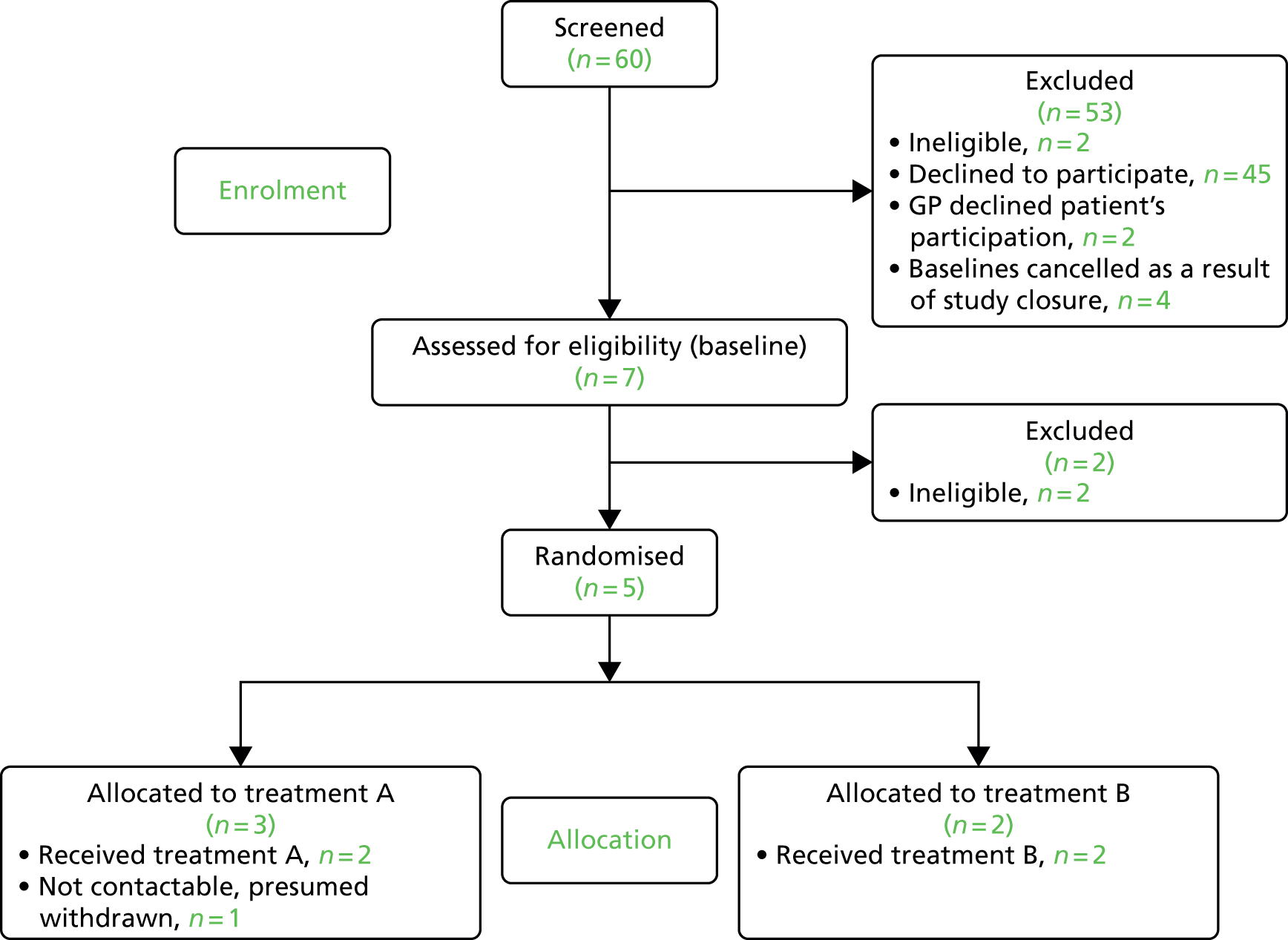
Details of recruitment are described in Table 3. The first participant recruited to the trial was from Camden and Islington with Kingston, and was assessed on 29 September 2015 and randomised on 1 October 2015.
| ToSCA pilot site | Open to recruitment date | Number of identified patients | Number of dropouts | Reasons for dropouts/uncertainty | Number of expressions of interest | Number of further dropouts/withdrawn patients | Number of completed baseline assessments | Number of scheduled baseline assessments |
|---|---|---|---|---|---|---|---|---|
| Camden and Islington (with Kingston) | 1 July 2015 | 32 | 25 | 7 | 4/1 | 2 eligible | 0 | |
| Camden | 11 | 9 |
|
2 | 2/0
|
0 | 0 | |
| Islington | 16 | 12 |
|
4 | 2/1a
|
1 eligible | 0 | |
| Kingston | 5 | 4 |
|
1 | 0 | 1 eligible | 0 | |
| Greenwich | 17 August 2015 | 4 | 3 |
|
1 | 0/0 | 1 eligible | 0 |
| Coventry and Warwickshire | 3 September 2015 | 12 | 6 |
|
6 | 0/1b | 3 (2 eligible and 1 ineligible) | Two pending baselines cancelled because of study closure |
| Bristol | 8 September 2015 | 12 | 6 |
|
6 | 3/0
|
1 ineligible | Two pending baselines cancelled because of study closure |
| Total | n/a | 60 | 40 | n/a | 20 | 7/2 | 7 | Four pending baselines cancelled because of study closure |
The second participant was from Greenwich, assessed on 18 November 2015 and randomised on 20 November 2015. The next three participants recruited came from Camden and Islington with Kingston (one participant), and Coventry and Warwickshire (two participants), and were randomised on 19 November 2015 and 4 and 6 January 2016. All were assessed no more than 2 working days previously – the participant randomised on 4 January 2016 had been assessed on 30 December 2015 just prior to the New Year holiday.
In addition, two potential participants were found ineligible for the trial at the baseline assessment – one from Coventry and Warwickshire assessed on 29 October 2015, who was uncertain whether or not GAD was the most important issue affecting their mental health, and one from Bristol assessed on 7 January 2016, who was excluded because they had current major depression as assessed on the MINI questionnaire. 43
In a further two cases (both in Camden and Islington with Kingston), their GPs were not prepared to agree to have their patients in the trial or to prescribe sertraline for their GAD should they be randomised to the medication arm, so it was not possible to proceed with the baseline assessment despite the patients expressing an interest in taking part in the trial.
A little ironically, a further four potential participants identified in January 2016 (two from Coventry and Warwickshire and two from Bristol) had to have their baseline assessments cancelled following the NIHR HTA programme decision to withdraw funding from the trial (see Chapter 8).
Actual recruitment rates versus planned recruitment rate
We had anticipated that recruitment would be slow in the first 3 months of the internal pilot while we were testing our recruitment methods, but had expected it to improve after this as the pilot sites became familiar with participant identification and recruitment processes. In our submitted key progress figures we indicated that we expected to recruit two participants in the first month, three in the second, four in the third, five in the fourth and then 10 participants in each of the following 8 months – resulting in an anticipated total recruitment of 90 participants over the 12-month period of the internal pilot. We had an internal pilot target to achieve at least 70% of this (i.e. 63 participants recruited at 1 year).
The very slow rate of recruitment to the trial unfortunately meant that at the end of January 2016, 7 months into the internal pilot, we had recruited only seven participants, as opposed to the projected 40 anticipated (Figure 5). This was despite trying a variety of strategies to improve the recruitment rates, as described in following sections.
FIGURE 5.
Graph showing ToSCA actual recruitment against anticipated recruitment.

Reasons for difficulties with recruitment
The most noticeable factor was that we had fewer potential participants identified by the PWPs than we had anticipated from our earlier data, and of the 60 potential participants identified at screening, 45 declined to participate – the majority (n = 30) because of their reluctance to be randomised to medication, with a further person not wishing to take medication for > 6 months. Four people only wanted CBT, one wanted a combination of antidepressants and CBT, and one went to their GP to obtain this. One person was already on medication (SSRIs), one had found it hard to engage with CBT, two did not think that their GAD was their main clinical priority, two did not want to take part in research, one did not give any reason for not taking part and one travelled extensively and, hence, could not keep regular clinical appointments as would have been expected with participation in the trial (see Chapter 8).
The research team worked hard to try and address all the possible factors contributing to this lower than anticipated identification rate, detailed in the next section.
Methods aiming to improve recruitment via psychological well-being practitioners
As soon as the research team became aware of the difficulties with participant recruitment, a number of methods were used to attempt to improve recruitment of participants by PWPs. These included:
-
materials to help the PWPs keep in mind the study and recruitment
-
funding of lead PWPs to facilitate recruitment
-
reminders to PWPs about the study
-
meetings with PWPs about the study
-
database searches to identify possible cases.
Various materials were distributed to PWPs to serve as reminders of the study and recruitment processes. A flow chart on a single sheet set out the recruitment criteria, key points to discuss with possible eligible participants, what to do if participants agreed to be contacted by the research team or were unsure, and the logging of the outcome of these discussions. Copies of this were posted at PWPs’ work stations (Figure 6) and in the materials PWPs took with them to clinics.
FIGURE 6.
Example of reminder for PWPs attached to their monitors in Camden and Islington. BOP, books on prescription; cCBT, computerised cognitive behavioural therapy; GSH, guided self-help; HI, high intensity; LI, low intensity.

In addition, each site had one or more PWPs who were part-funded from local R&D funds to help with recruitment and baseline assessments. These PWPs were embedded in their local IAPT services as they worked clinically as PWPs in their service as well as having dedicated funded sessions to facilitate recruitment. On a day-to-day basis they liaised with their PWP colleagues, reminding them of the study both individually and in team meetings, and giving advice regarding recruitment. Several sites used the PWP case management supervision sessions to consider suggesting assessment for the trial to patients likely to have GAD at their 4-week review.
To monitor recruitment, the lead PWPs completed log sheets of potentially eligible participants and the outcome of the PWP discussions with potential participants about the study. These were to be returned fortnightly to the research team. The routine of requesting these to be returned each fortnight and prompting if log sheets had not been returned served as a repeated reminder about the study and also gave them the opportunity to enquire about any issues regarding recruitment.
In some sites, the local PI and/or members of the study research team attended routine PWP team meetings to remind them about the study and problem-solve issues regarding recruitment. As the study progressed, and it became evident that there were greater than anticipated barriers to recruitment, the focus of these discussions was on identifying barriers that the PWPs might have in raising the study with potentially eligible participants or in pursuing these discussions in a sufficiently facilitative manner.
There were some common themes that emerged that may have contributed to the relatively low levels of identification of potential participants by the PWPs and that may be worth considering when planning future studies involving IAPT services.
Reasons given by psychological well-being practitioners for the difficulty recruiting participants to ToSCA
-
We explained to the PWPs in both the written materials and face-to-face training the importance of having equipoise in explaining the trial to potential participants, and that it was an important unanswered clinical question whether CBT or medication would be the most effective treatment for these patients. However, the fact that they had been trained to deliver a low-intensity CBT intervention is likely to have given them both an overt and also less conscious bias towards psychological therapy treatments.
-
A number of PWPs said that they thought many patients they saw were against trying psychotropic medication. This was backed up by many of the documented patient responses to being told about the trial, but it was unclear if the patients may have been influenced in this by the PWPs. We gave the PWPs examples of answers to give to patients who might have been concerned about the side effects of medication (Box 2).
-
Several PWPs were concerned that patients would not be offered CBT after trying medication if the medication proved unhelpful, possibly indicating a greater enthusiasm for psychological therapy.
-
Some PWPs said they thought GAD was not as prevalent in the clinical population as generally believed and that, in their experience, GAD presentations are often not the focus of psychological treatment.
-
Quite a few PWPs wanted to be certain that people they discussed the trial with had GAD, although we had stated that this was not possible at their stage in the process and that we would like them to offer the possibility of the trial to anyone with a reasonable likelihood of GAD, as they would then need to be assessed using the gold standard MINI questionnaire. The PWPs said they were concerned about wasting clients’ time if they did not have GAD.
-
Time pressures were a virtually universal problem – all the PWPs had significant clinical caseloads and found it difficult to keep the trial in mind.
-
Many PWPs reported that their clients were very anxious about the uncertainty of being referred and allocated to a random treatment. Given that their key problem is likely to have been GAD, with a core component being worry about uncertainty, this illustrates the potential difficulty of recruiting participants to a RCT in GAD. When this barrier was identified, PWPs were encouraged to suggest to potential participants that they meet with the researcher and address their questions to the researcher, rather than the PWP attempting to answer them.
-
This problem with worry about uncertainty also made the PWPs concerned about making their patients more anxious when discussing the trial, with implications for their workload, as well as concern for the well-being of their patients.
If the potential participant is not keen to take part in the trial because of concerns about being randomised/allocated to the medication arm, it’s suggested that you ask them if they could say what it is about the medication that concerns them and see if this can be briefly discussed. The following are some common preconceptions about antidepressant medication such as the SSRI sertraline.
-
That they will be addictive: there is no evidence of any physical addiction with a medication like sertraline, although it is better to stop it gradually rather than suddenly.
-
That they will make people feel like ‘zombies’ and not themselves: again, there is no evidence of this. People do sometimes experience mild side effects when starting sertraline, such as nausea, dizziness and tiredness, but these are usually mild and short lasting, and sertraline has been prescribed in millions of doses worldwide with a very good safety profile.
-
Sertraline has been shown in trials to be effective for GAD; what we do not know is whether it’s more or less effective than the psychological CBT treatment also being offered.
You could then see if the patient feels their concerns have been addressed and they are happy to consider being in the trial, if eligible, or if they still feel clear that they do not want to do this.
A further unanticipated barrier identified through these discussions was that the supervisors of the PWPs often considered that a comorbid problem which the patient had, either diagnostic (e.g. social anxiety) or psychosocial (e.g. debt problems or relationship difficulties), was the key clinical issue to address and would direct the PWP to refer the patient for an intervention addressing this problem which meant they were not considered for the study. When this barrier was identified, local PIs and the study team encouraged PWPs and supervisors to refer such patients for a ToSCA baseline assessment where the suitability of treatment for GAD would be addressed rather than pre-empting this.
IAPTUS database searches
Two months into recruitment at the first site in Camden and Islington with Kingston, there was a pilot of using the routine service clinical database (IAPTUS; adult version, Mayden, Bath) to identify potential cases. Two types of case identification method were piloted: a retrospective and prospective method.
For both methods it was staff at the pilot site (the lead PWPs) who conducted the searches.
Retrospective database searches
The retrospective method was initially used to identify cases potentially meeting study criteria, who had been discharged from low-intensity PWP treatment and stepped up to be awaiting high-intensity CBT within the service. It did not prove to be very useful in terms of identifying potential participants for the trial as, of the 18 patients who had been referred for stepping up who still had a GAD score of ≥ 10, nine had depression as their main problem, two had other clinical problems they wanted to address, one had moved, one dropped out, two declined as they did not want medication and three did not respond. There were also 14 patients who had been discharged from the IAPT service despite still having a GAD score of ≥ 10, of whom six reported having other clinical presentations as their main problem, one declined when contacted about the study and seven did not respond.
Following on from this, we examined the participant flow for the whole trial recruitment period retrospectively and this indicated a significant number of people who might have been suitable to approach to discuss assessment for participation in the trial who had not been identified by the PWPs. Using the IAPTUS database, the flow of people with GAD-7 scores of ≥ 10 during the active study recruitment period was analysed at the IAPT pilot site covering the same two local London boroughs used to estimate likely recruitment flows in 2011–12 before the trial (i.e. Camden and Islington with Kingston). Approximately 1 in 4 people assessed for treatment with a GAD-7 score of ≥ 10 were seen for at least three sessions of a low-intensity treatment (the other three out of four people either went straight to high-intensity CBT or were discharged, referred on or dropped out before having two low-intensity treatment sessions). Of those who completed at least three sessions of a low-intensity intervention, 37% were being a prescribed a psychotropic medication (data missing on a further 2%), and 50% of those who were not prescribed medication improved and had GAD-7 scores of < 10 at the end of their low-intensity treatment. All of these therefore did not meet criteria for inclusion in the study.
Of those patients who were not on medication and had not recovered, a proportion dropped out of treatment before the end of their low-intensity intervention and would not have been able to be approached by their low-intensity worker about the study. However, of those who remained potentially approachable to discuss the trial, approximately only 1 in 6 had been identified to the study team as having been approached about the study by their low-intensity worker/PWP. Unfortunately, following a change in the clinical database used by the IAPT service, the completion of diagnostic coding during the recruitment period was poor (only 28% of people with GAD-7 scores of ≥ 10 had primary diagnoses coded on the system) and so we were unable to use this to calculate how many of the potentially eligible and approachable patients were considered by their IAPT low-intensity worker to have a primary diagnosis of GAD rather than other diagnoses.
Prospective database searches
The prospective identification method was used to identify cases potentially meeting the study criteria who had already had three or more sessions of PWP treatment and were still in treatment with a PWP. It was instituted on a regular basis at the Camden and Islington with Kingston site, and the algorithms and methods were disseminated to the other pilot sites. On a regular fortnightly basis, cases were extracted from the IAPTUS database that met the following criteria:
-
most recent GAD-7 score of ≥ 10
-
seen three or more times in a step 2 treatment (could include the PWP assessment session)
-
still in a step 2 treatment
-
not on a psychotropic medication.
An e-mail was sent to the PWP treating each patient identified through these searches, giving the patient’s identification details, noting that the patient was potentially eligible for ToSCA and asking the PWP to e-mail, by return, if there was some reason that they were not eligible for the study (e.g. that the patient had an agreed diagnosis or diagnoses other than GAD).
In addition, a note was placed on the front page of the patient’s electronic records in IAPTUS (on the page that first comes up when the patient’s records are entered) that the patient was potentially eligible for ToSCA.
Using the standard IAPTUS search to identify people at step 2 who had a diagnosis of GAD highlighted a problem that people had not always been given an accurate provisional diagnosis. In order to identify potentially eligible participants, this involved checking clinical records for GAD scores, session number and medication use.
General practitioner factors affecting participant recruitment
As the trial participants were being recruited via the IAPT service and not their GPs, we were relying on their GPs agreeing to support the trial in terms of checking their patient’s medical suitability for the trial and agreeing to prescribe sertraline for those randomised to the medication arm once the patients had expressed an interest in taking part. We sent information to all the local GPs about the potential benefits of the trial for their patients, and the trial procedures and rates of reimbursement that would affect them via the local primary care research networks before the trial opened (see Chapter 2, Methods).
Thirteen GPs were approached with patient consent to complete Medication Suitability Review forms (i.e. for the five randomised participants, the two participants found to be ineligible at the baseline assessment and four participants whose baseline assessments were cancelled because the trial was terminated). Two GPs refused to complete the form for their patients.
Eleven GPs therefore completed the form and returned it. Of the two GPs who were not happy to do so, one considered that their patient had current physical problems that would make it inappropriate for them to take part in the trial despite the patient wanting to participate and the other GP simply refused to have any involvement. The research team attempted to contact both GPs to discuss the issues with them but without success.
Given the design of the study we did not consider there were any other strategies that might have allowed these two patients to participate, although they were disappointed to be excluded.
Feedback from study participants
As the trial was terminated prematurely we had only collected data at the 3-month time point from one participant and did not have a meaningful number of data to analyse. The five participants who had been recruited to the trial were all sent written notification that the study was finishing early but that we had made arrangements with their GPs or IAPT therapists to continue providing them with the intervention to which they had been randomised. The research team also contacted the participants in person to check that they had understood the implications of the trial finishing early and to get their feedback about participating in the trial in lieu of any formal results.
Out of the five randomised participants, four were spoken to via either telephone or e-mail.
-
Sertraline 1 (by telephone): was glad to have taken part. Had a positive response in terms of mood, but switched to citalopram the week of being contacted as a result of side effects.
-
Sertraline 2 (by e-mail): reported that everything is going well with the treatment.
-
Sertraline 3 (unable to contact): participant was presumed to have withdrawn/dropped out.
-
CBT 1 (by e-mail): travelling so unaware of trial closure. Was sorry that the trial was closing – reported that it had been a ‘really positive experience’ and offered to provide more specific feedback if necessary.
-
CBT 2 (by telephone): treatment ongoing. Felt very disappointed when the trial closed, which had a negative effect on mood. Still very anxious in general and unsure when the CBT treatment will end. Appreciated the follow-up call as they wanted to be able to give their views about the study closure.
Chapter 6 Cognitive behavioural therapy subgroup, cognitive behavioural therapy measures of competence and adherence, training and supervision of high-intensity therapists, and validation of tapes
Cognitive behavioural therapy subgroup
A CBT subgroup comprising Michel Dugas, Roz Shafran, Marc Serfaty, John Cape and Marta Buszewicz met by teleconference every 2 months during the study to discuss and confirm the procedures regarding delivery of the CBT intervention, as well as the processes for the assessment of therapist competence and adherence. This was also an opportunity for the group to discuss any issues that had come up in the expert supervision of the pilot site supervisors by Michel Dugas and Roz Shafran, and to keep the group informed about any general trial issues of relevance.
Background to cognitive behavioural therapy measures of competence and adherence
Lichstein et al. 56 described three important elements when evaluating CBT: first, whether or not a treatment was delivered by the therapist in accordance with the intervention model; second, whether or not there was ‘receipt’ by the patient (i.e. had it been understood); and, third, whether or not enactment had occurred (i.e. had the patient carried out the prescribed treatment). The parallel elements in the drug arm of ToSCA would be whether or not sertraline had been properly prescribed, whether or not the patient had understood how to take the medication (collected the prescription, understood the timing, dose, etc.) and whether or not they had actually taken the medication.
There are two main issues that were considered when deciding how to rate the CBT therapy delivered in this trial: first, did the therapist adhere to the Dugas Therapists’ Manual (see Appendix 13) by delivering interventions specified for the treatment of GAD (adherence); and, second, was the approach to delivering the therapy of a sufficiently high standard to be considered competently delivered (competence)?
Assessment of adherence and competence is required to make sure that the trial is testing the intervention as it was designed to be delivered. In effectiveness studies, such as the current one, it can be more difficult to ensure adherence and competence because of the challenges inherent in delivering the treatment in routine clinical services. To maximise adherence and competence to the protocol, therapists must be trained and closely supervised. Within IAPT services all qualified therapists are accredited by the professional organisation, the British Association of Behavioural and Cognitive Psychotherapy. This accreditation is not in itself sufficient assurance that the specific protocol for the treatment of GAD selected for this trial will be delivered with high levels of adherence and competence. Some of the therapists and supervisors may not have been trained to deliver the Dugas model37 but others might, and ensuring that all the therapists were able to deliver the protocol competently, and that the supervisors were able to supervise the delivery of the protocol within the clinical service, were key components of the study. The Dugas model was selected as the CBT protocol of choice because of the data supporting its efficacy as well as availability of training materials. 57
Assessment of competence
A scale for measuring therapist competence in cognitive therapy, based on the original Cognitive Therapy Scale,58 is the 12-item Cognitive Therapy Scale-Revised (CTS-R). 59 This revised version improves on the original Cognitive Therapy Scale by eliminating the overlap between items, improves on the scaling system and defines items more clearly. In this trial we planned to have an independent rater listening to audio-recordings of therapy sessions using the CTS-R.
Scoring
The CTS-R consists of 12 items: agenda-setting and adherence; feedback; collaboration; pacing and efficient use of time; interpersonal effectiveness; eliciting of appropriate emotional expression; eliciting key cognitions; eliciting and planning behaviours; guided discovery; conceptual integration; application of change methods; and homework setting. The CTS-R is more specific than the original Cognitive Therapy Scale in that therapist competence is defined very precisely. Each item is rated from 0 to 6 on a visual analogue scale ranging from incompetent, through to novice, advanced beginner, competent, proficient and expert. The total score ranges between 0 and 72, with a minimum score of 36 taken as competency for the delivery of therapy.
Assessment of adherence
In this context we defined therapist adherence as the extent to which the therapist stuck to the essential elements described within the treatment manual.
We aimed to collect detailed information about the content of the intervention using a Therapy Components Checklist (TCC), which was developed for use in the trial. The TCC summarises five main interventions described in the Dugas treatment manual (see Appendix 13) and deemed essential for successful treatment. Each of these areas is made up of a number of elements:
-
psychoeducation and worry work (three elements)
-
evaluation of the usefulness of worry (two elements)
-
uncertainty recognition/exposure (three elements)
-
problem-solving (three elements)
-
written exposure (five elements).
The TCC also includes sections about general therapy procedures (10 elements) and the specific materials used (nine elements). We also aimed to collect information about a number of interventions that are not permitted and, if used, to assess deviations from the protocol (e.g. use of controlled worry periods). There is one TCC completed per patient and the therapist reports which components were addressed after each therapy session. A more detailed description of these can be seen by referring to the TCC (see Appendix 14). The TCC was used in supervision, but not shared with the patient.
At the very end of a course of therapy, up to 16 sessions, the main elements of the treatment delivered are summarised in the brief End of Therapy Checklist (EoTC) (see Appendix 15) completed by the therapist. This consists of the same five main areas/interventions detailed above. The EoTC was not used in supervision or therapy, but solely for the purpose of assessing adherence. This was also developed by the CBT group for use in the trial.
Defining ‘sufficient’ adherence
The degree of adherence was rated using the EoTC as follows:
-
0 – non-adherent (between zero and two out of the five components delivered on the EoTC, i.e. < 40% of the session was spent using methods from the protocol; the rest of the time was spent on methods not in the protocol or generic techniques, such as empathic listening)
-
1 – somewhat adherent (two or three out of the five components delivered on the EoTC, i.e. 40–59% of the session was spent using methods from the protocol; the rest of the time was spent on methods not in the protocol or generic techniques)
-
2 – mostly adherent (three or four out of the five components delivered on the EoTC, i.e. 60–79% of the session was spent using methods from the protocol; the rest of the time was spent on methods not in the protocol or generic techniques)
-
3 – fully adherent (at least four out of the five components delivered on by the EoTC, i.e. ≥ 80% of the session was spent using methods from the protocol; the rest of the time was spent on methods not in the protocol or generic techniques).
Data collection
Therapists’ ratings of adherence
The therapists would have been asked to complete (1) the TCC at the end of every therapy session as well as (2) summarising the treatment delivered during a course of therapy using the EoTC.
Independent ratings of adherence
An independent rater would have been asked to listen to all of the audio-recordings available on a patient who had completed therapy and objectively rate adherence using the EoTC, giving a score generated by adding all of the items rated as adhered to on a scale. A representative sample through random selection of 10% of all the patients can be selected and an EoTC completed for each patient. An average score can then be generated to see if the study complied with the goal of ensuring that treatment delivery was at least mostly adherent to the protocol using the method set out above.
Training and supervision arrangements for ToSCA
Training workshop
Training for ToSCA consisted of a 2-day workshop, on 22 and 23 January 2015, led by Professor Michel Dugas, who developed the CBT intervention being used in this trial and has conducted previous trials in GAD.
Attendees included the CI (MB), co-applicants from the project who are experienced CBT therapists (RS, MS and JC) and staff from the IAPT pilot centres located in south-east England – London (Camden, Islington, Greenwich), Surrey (Kingston), the Midlands (Coventry, Warwickshire) and the west of England (Bristol). The IAPT staff at each of these sites were very enthusiastic about the opportunity to receive training from Michel Dugas and places had to be limited to two therapists and one supervisor for each single pilot site (i.e. Greenwich and Bristol) and four therapists and two supervisors for the sites committed to a double recruitment figure (i.e. Camden and Islington with Kingston, and Coventry and Warwickshire) (see Appendix 9).
The training was delivered as a workshop, with scenarios in which attendees were encouraged to get into groups and practise using role play. It was based on the manual provided by Michel Dugas, and each therapist and supervisor was given a copy for use in the trial (see Appendix 13).
Plan to ensure therapist competence
All attendees met at the end of training on the final day to discuss implementation issues at their local sites. The plan was for each therapist who was going to be involved in ToSCA to treat two patients who had already been identified as having GAD within the IAPT service, using the manualised intervention that they had been trained in using by Michel Dugas. These patients would be used to help therapists and supervisors become familiar with the protocol, and optimise adherence and competence for the main trial. As these patients had not been recruited or consented to take part in the trial, it was not possible for the tapes or transcripts of their therapy sessions to be made available to the external supervisors. The local supervisors brought any particular issues arising from the treatment of these patients to their ‘supervision of supervision’ sessions provided by Michel Dugas and Roz Shafran. We have termed the patients treated in this way ‘practice patients’, in order to distinguish them from patients who were recruited for the internal pilot who underwent a rigorous eligibility and consent process.
It was agreed that in the practice patient stage the therapists would need to treat at least two patients and the supervisors would rate the therapist on at least one session using the CTS-R. 60 It was agreed that the therapists would need to have achieved a score of ≥ 36 to be considered competent and be permitted to treat patients in the trial. A minimum score of 36 is the standard criterion for competence within IAPT services. It was intended that competence in the delivery of CBT for the main trial would be assessed in the same way (i.e. a score of ≥ 36 according to the CTS-R). In the main trial, it was intended that an expert rater (Melissa Robichaud) would independently rate 10% of all therapy sessions using the CTS-R to establish that the protocol had been delivered competently. In the unlikely event that the therapy had not been delivered with competence or adherence to the protocol, this would have been reported.
Plan to ensure therapist adherence
Adherence to the protocol is a critical part of any trial. We therefore developed a measure of adherence to the protocol for use in the main trial. The practice patient stage enabled us to test the feasibility of this measure. As described in Assessment of adherence, the TCC is intended to be completed after every session by the therapist to record the main content of the session, and the EoTC is designed to be completed at the end of the course of therapy by the therapist. The therapists were asked to bring the TCC completed on their ‘practice patients’ to their supervision sessions so that the local supervisors could help them maintain adherence to the protocol.
We planned to also use the TCC in the main trial to assess adherence, in addition to external assessment of adherence to the protocol by an expert rater (Melissa Robichaud), who had agreed to rate 10% of the sessions selected at random to ensure that the therapists had adhered to the protocol. The intention was for the external rater to listen to 10% of the sessions selected at random and rate them for adherence and competence simultaneously. We planned to examine the relationship between therapist self-ratings of adherence and external expert ratings of adherence. If there was a strong and positive relationship between the ratings, we could conclude that therapist ratings of adherence were accurate reflections of the content of therapy, which would allow future research to dispense with the need for costly independent ratings.
Supervision structures
The IAPT therapists were supervised by local IAPT leads from the participating centres – both the supervisors and therapists were all British Association of Behavioural and Cognitive Psychotherapy-accredited therapists. The IAPT supervisors from Kingston, Camden and Islington and Bristol were externally supervised by Roz Shafran and those from Greenwich, Coventry and Warwickshire by Michel Dugas. Both external supervisors were ToSCA co-applicants.
Routine supervision of therapy in IAPT takes place at least monthly, but is flexible within this period. However, in this trial, we recommended flexibility so that if any immediate issues needed attention, the therapists could consult their IAPT supervisors. The external ToSCA supervisors (RS and MD) provided additional monthly supervision sessions (‘supervision of supervision’) by teleconference link to the IAPT supervisors, which was part of the trial process and was in addition to usual clinical practice. The external ToSCA supervisors were also accessible to the local IAPT supervisors by e-mail to answer any additional queries that arose between supervision sessions.
Flexibility in the practice patient stage was used to learn about how clarification and modifications to the CBT intervention might be required. This was done by keeping rigorous notes during supervision to help inform the project, as well as e-mail discussions between the supervisors and the trial team. It was agreed that once the ‘practice phase’ had finished, there would be no modifications to the protocol allowed.
Findings and recommendations following the practice patient stage
Supervision
Direct supervision of IAPT therapists in the services took place, on average, monthly for between 60 and 90 minutes individually, in pairs or in groups of three or four, depending on the service. The expert ‘supervision of supervision’ (i.e. supervision of the IAPT supervisors by RS and MD) also took place monthly between July 2015 and January 2016 for 60 minutes. This was done over a dial-in telephone service, with times and dates agreed in advance. Michel Dugas supervised four IAPT supervisors directly and Roz Shafran supervised three. A total of 12 supervision sessions took place, six for each of the supervisors, monthly, with the exception of August 2015. Detailed notes were kept with any queries about protocols minuted and addressed at the next supervision session after consultation with the research team.
Patients treated with cognitive behavioural therapy
Of the team supervised by Roz Shafran, 7 out of the 11 therapists had cases. A total of 16 patients were seen, with a mean of 2.3 patients per therapist; the mean number of sessions was 8.8 (SD 8.5). Of these, three completed ≥ 14 sessions. In 8 out of the 16 cases the GAD checklists (TCC and EoTC) were completed. Reasons for ending therapy in the 16 patients were as follows: two clients completed therapy (one had received 16 sessions and the other was well after 10 sessions), three clients completed prematurely as they did not want to do ‘exposure’, two were unwilling to allow the recording of sessions, five had not yet completed treatment and four withdrew (one moved, one was too busy to attend and two withdrew without giving a specific reason).
Ease of supervision
Telephone supervision proved to be a useful way for the ToSCA expert supervisors to supervise the IAPT supervisors. The IAPT supervisors were able to represent the views and queries that their local therapists had raised. These are included in the summary of questions on the manual and treatment manual (see Clarification and modification to treatment protocol as a result of the practice patient stage below).
Adherence and competence
The TCC and EoTC checklist were completed or partially completed for 8 of the 16 participants seen. The supervisors considered the therapists to be adherent according to the checklist, but noticed that the most common component of the protocol that was omitted was the written exposure.
The supervisors considered the therapists to be competent on the basis of listening to excerpts from their sessions, but none had rated a full session using the CTS-R because of the time required to do so and the fact that not all of the ‘practice patients’ were being recorded as consent had not been provided for recording.
Clarification and modification to treatment protocol as a result of the practice patient stage
Improving Access to Psychological Therapies protocols
-
Patient attendance: the aim in the trial would have been to encourage the therapist to be proactive about following up patients and not to discharge participants unless they had failed to attend at least two sessions without notice.
-
Clarification of what constituted a ‘step 2 IAPT intervention’: the protocol required patients to have received a step 2 IAPT intervention prior to being referred into the study. This was clarified and confirmed early on in the CBT group discussions (see Figure 1).
-
Spacing of sessions: the Dugas treatment manual (see Appendix 13) indicated that patients should attend 14 sessions if possible, to be delivered within 16 weeks. For some, however, they might only complete treatment by 26 weeks. In the practice patient stage, some supervisors reported patients recovering within 10 sessions. It was agreed that if the therapists wanted to discharge patients after fewer than 14 sessions then they should make sure that patients were truly asymptomatic by asking them to complete the Penn State Worry Questionnaire,60 which is the problem-specific measure of GAD within IAPT services as well as the GAD-7. If their responses to the questionnaire indicated that the patient was within the normal range and had recovered fully, then they could be discharged with the reasons for this being clearly recorded. It was also agreed that such patients should have received at least two sessions dedicated to relapse prevention.
Study protocol
-
Adherence to the manual: the agenda should be set with the patient at the beginning of treatment, consistent with the collaborative approach of CBT, and included in this should be a full clinical assessment of the patient and also a risk assessment, to be conducted during the first 20 minutes of the session. It was agreed that all of the modules in the manual should be covered, but that modules 4 (problem-solving) and 5 (written exposure) could be swapped around. In some clients there was a concern that problem-solving may be used as a way of reducing their tolerance of uncertainty and this could be addressed by delaying the problem-solving module.
-
Consent: a prerequisite of participating in the trial is that the patients have agreed to their session being audio-taped.
-
Specific therapeutic interventions: it was accepted that some exposure tasks may be more effective than others and the therapists were encouraged to be flexible in their approach. They requested a relapse prevention sheet, which was provided by Michel Dugas (see Appendix 16). The therapists were encouraged to undertake exposure to uncertainty experiments in a graded way with their patients, in order to make them more manageable and facilitate engagement.
ToSCA participants (internal pilot proceeding to full trial)
Clear progression criteria were developed and stated to allow progression from the internal pilot to the full trial. One of these criteria was that the therapy could be delivered competently and that the therapists could adhere consistently to the protocol. This was operationalised as stating (1) that the therapists, during the internal pilot, should score a minimum of 36 on the CTS-R within 10% of randomly selected tapes independently rated by the external assessor, and (2) that a minimum of 60% of the components of the protocol had been delivered according to external assessor ratings on the EoTC. The random selection of 10% of audio-tapes taken from the total number of audio-recorded sessions delivered to all patients is commonplace in trials as it ensures that the quality of therapy is representative and the interpretation likely to be generalisable.
Confidentiality
The project aimed to use Data Safe Haven to transfer and store audio-recordings of therapy. This service provides a technical solution for storing, handling and analysing identifiable data. It has been certified to the ISO27001 information security standard and conforms to the NHS Information Governance Toolkit. This is built using a walled garden approach, in which the data are stored, processed and managed within the security of the system, avoiding the complexity of assured end point encryption. A file transfer mechanism enables information to be transferred into the walled garden simply and securely (see Chapter 3, Research governance).
Chapter 7 Public and patient involvement
Introduction
Public and patient involvement (PPI) for ToSCA was arranged by the McPin Foundation. The McPin Foundation is a mental health research charity with a particular specialism in the involvement of those with experience of mental illness in research. 61
The McPin Foundation additionally supports service users to carry out research in their own right in collaboration with others. PPI in ToSCA took two forms: first, from Thomas Kabir, one of the study co-applicants; and second, from a group of four service users known as the ToSCA Clinical Advisory Group (CAG).
As the research was the result of a commissioning brief, PPI perhaps had less of an impact at the design stage of the study than would otherwise have been the case. Nevertheless, the study had PPI input in the application for funding and at all subsequent stages of the research.
Methods
Clinical Advisory Group
The CAG was made up of three service users plus the PPI co-applicant. The CAG met roughly three times per year. Ad hoc meetings were held as needed. The responsibility for organising CAG meetings was held by the PPI co-applicant, Thomas Kabir, who is employed by the McPin Foundation.
The CAG members were recruited via an open advertisement. The three people that were subsequently recruited all had personal experience of anxiety. Meetings were chaired by Thomas Kabir. Members of the wider research team were invited to attend meetings, and all meetings were minuted. The minutes of CAG meetings were shared with the research team.
A total of six CAG meetings were held between August 2014 and June 2016. As per the NIHR’s INVOLVE Briefing Notes for Researchers,62 CAG members were reimbursed for any out-of-pocket expenses. Members were offered payment for attendance at meetings.
Thomas Kabir was the PPI co-applicant for the study. He provided input into the application for funding to the HTA programme as well as the application for ethical approval. He later:
-
sat on the Trial Management Group (TMG)
-
attended TSC meetings as an observer
-
recruited and organised CAG meetings
-
acted as a link between the CAG and the wider study team
-
advised on the recruitment materials for the study
-
provided ad hoc advice to the study team as needed.
Reflections
A major initial focus of the CAG was on measuring the acceptability from a patient perspective of the two interventions being tested against each other: sertraline and CBT. Developing ideas about how to measure the acceptability of both a psychological therapy and a medication proved to be a particular challenge. In the end, the decision was taken to use the Client Satisfaction Questionnaire (CSQ). The CAG disagreed with this decision, as the CSQ appeared to focus on satisfaction with services rather than treatments per se. However, the TMG decided on the CSQ because of the perceived need to use one questionnaire to get participant feedback about both interventions in order to be able to use this in the analysis, and no other questionnaires were found that were able to be used to do this.
A related issue arose when dealing with the issue of what outcome measures to use in the study. CAG members felt that the adverse effects of both CBT and sertraline should be measured. This posed a problem, as it proved that there were no measures that were truly applicable to both a medication and a psychological therapy. The CAG suggested using a modified form of the Toronto Side Effects Scale, but the wider study team did not see this as an adequate solution as it was designed with drug-related side effects in mind. The CAG felt that a modified version, taking out some of the terms, could still work, but the wider study team felt it was not possible within the trial timelines to proceed with this. Additionally, there was some debate within the wider study team as to whether or not adverse events that would not be classified as serious in nature should be measured at all, and the TMG noted that the side effects of sertraline have been fully documented in other studies. CAG members held the view that although this was true, the adverse effects experienced by people actually taking part in the study needed to be considered. The idea was that both the adverse and beneficial effects experienced by study participants needed to be weighed against each other to get a true notion of the net worth of the treatments being considered.
As the study progressed, more attention was given over to recruitment issues by CAG members and the PPI co-applicant. CAG members felt that the responses made by the TMG to the recruitment issues that the trial faced were appropriate. Indeed, it was felt that the study team had done all that they could to address the recruitment problems that the trial faced. It was noted from an early stage that recruitment to the study would be challenging. The following extract from the minutes of a CAG meeting held in November 2015 summarised the CAG’s position:
A possible downside to taking part in the study is that participants will have no choice about whether they take medication or CBT. Outside of the study people can potentially access both treatments. This is clearly a barrier to participation.
Specific suggestions were made regarding how low-intensity IAPT workers could introduce the study to potential participants. One CAG member wrote a script for IAPT workers to use, which was then shared with other study team members and used in the revised version. More practical issues, such as how best to ask female participants to take a pregnancy test as part of the baseline eligibility interview, were also discussed. The recruitment strategies that were identified by the study team were fully discussed by the CAG.
Summary and recommendations
After the decision had been taken to close the study, a final meeting of the CAG was arranged. This was at the suggestion of both the PPI co-applicant and CAG members.
-
Two CAG members felt that communication with them about the study could have been more regular and ongoing. It is recognised that this was as a result of staffing issues within the study team that were beyond anyone’s control.
-
The CAG patient voice could have been a bit stronger in the study. It was recommended that at least two lay people sit on both the TSC and TMG. There was only one lay member formally on each group.
-
It should have been made clearer what could have been measured as part of the study and what could not (i.e. looking at side effects and adverse events of sertraline and CBT).
-
CAG members said that they had enjoyed their experiences. One member said that her involvement with the McPin Foundation had been a positive experience. This involvement had helped her become involved in other research studies.
-
Everyone felt that the size of the CAG was appropriate. The relatively small size of the CAG enabled the group to discuss quite complex matters in depth. CAG members thought that this might not have always been possible with a larger group.
-
All members of the CAG fully recognised the challenges inherent in the study attributable to comparing two distinctly different treatment modalities in which patients are likely to have a preference of one over the other, thus leading to huge challenges in recruitment.
Questions from the Clinical Advisory Group as a result of the study
-
The CAG asked if the funder had sufficiently foreseen the potential recruitment problems with the study. The CAG felt that there would be significant numbers of people who would prefer to receive CBT rather than sertraline from the outset. This may have meant that a different study design may have been more beneficial.
-
CAG members asked if the commissioning brief for the study was reviewed by people with experience of mental health problems specifically. If so, did these people identify the potential recruitment issues that may arise from the original commissioning brief?
Recommendations for future research
-
It seemed that there are relatively few treatment acceptability measures in routine use. It was felt that it would be useful for the NIHR or another funder to fund research into developing a treatment acceptability measure that would be of broad use within a mental health research setting. Within the context of ToSCA, a measure that would work across different treatment modalities (a drug and a psychological therapy) was needed.
-
Likewise, there are few (if any) measures that can be used to measure the adverse effects of both a drug and a psychological therapy. Again, it was felt that it would be useful to develop a measure that could be used across different kinds of interventions.
Chapter 8 National Institute for Health Research Health Technology Assessment monitoring meeting
Recruitment was always the main concern of this study. Over the first 2 months of recruitment, six potential participants were identified in the only pilot site at that time open to recruitment. Although this met our anticipated recruitment rate of two participants per pilot site per month, the majority of those identified (4/6) declined to participate in the trial, largely because they were reluctant to be randomised to the medication arm. This trend persisted in the other sites and raised concerns about the study achieving the required numbers over the internal pilot recruitment period. In view of this, the focus of the TSC meeting that was held on 29 September 2015 was to develop an advance plan for an appropriate recruitment strategy. An analysis of the reasons for low recruitment was conducted and discussed at this meeting. However, the main reasons for poor recruitment did not change over time and some of the main reasons for non-participation in the trial by eligible patients, based on the data obtained until the closure of the study, are listed in this chapter.
Review of reasons for poor recruitment
-
Patients’ preferences were by far the most important reason for the non-participation of 45 of the 60 potentially eligible patients (see Table 3). Of these 45 participants, 30 did not want to take any medication and one for not more than 6 months; four wanted only CBT; one wanted a combination of antidepressants and CBT and one went to their GP to obtain this; one was already on medication (SSRIs); one found it hard to engage with CBT; two did not think that their GAD was their main clinical priority; two did not want to take part in research; one did not give any reason for not taking part; and one travelled extensively and, hence, could not keep regular clinical appointments as would have been expected with participation in the trial.
-
GP factors contributed to the non-participation of four of the remaining potentially eligible patients. In two such instances the GP was reluctant to prescribe medication and take part in the study, and in two cases the GP decided to start sertraline even though they were aware of the trial.
-
IAPT and the PWPs: informal discussions with the PWPs suggested that participants who were potentially eligible to take part but also had concomitant mental health comorbidities (i.e. depression, health anxiety and social phobia) were excluded as possible participants in the study by the PWPs as they perceived that these comorbidities would need to be treated first over and above any GAD. This was compounded by the fact that patients with GAD, by virtue of their illness, were likely to express considerable uncertainty about participation in a trial when approached to participate. This was then also perceived by the PWPs as a reason for excluding them from the study. In addition, potential participants often slipped through the recruitment net in the face of conflicting clinical pressures. Finally, and most importantly, recruitment via a psychological therapy service (i.e. IAPT), as per the HTA programme brief, was biased towards a pool of people who were expecting to receive psychological (CBT) therapies. This meant that presenting such patients with a randomised option of drug or psychological therapy was poorly received, especially considering that they were expecting to receive the latter.
Trial Steering Committee response
These issues were fully explored at the TSC meeting on 29 September 2015, and the chair of the TSC wrote to the HTA programme informing them of the recruitment figures and of the various strategies (please see the following paragraph for further details) that the study team had been working on to address this issue. In response to this letter, the HTA programme arranged a monitoring meeting for 14 January 2016 to discuss both the reasons for the poor recruitment levels and to explore possible alternative methods that could address this issue. At the meeting, a presentation of the various strategies that were adopted by the trial team to enhance recruitment was summarised.
Health Technology Assessment monitoring meeting: strategies adopted to enhance recruitment
-
PPI input to enhance recruitment: we had worked closely with our PPI co-applicant, Thomas Kabir, and the PIs at each clinical site to enhance recruitment by offering eligible participants a balanced view of both drug and psychological treatments for GAD. We refined, with help from the PPI CAG, a user-accessible script that the PWPs would adopt to offer an unbiased account of both treatments while also tackling concerns that people had about antidepressant therapy. This was intended to be discussed with all people with a GAD-7 score of ≥ 10 on completion of step 2 IAPT treatment.
-
Engaging with PWPs at the sites to ensure that they were actively recruiting to the study. This was done by:
-
the trial team regularly attending PWP site meetings, in which they reminded PWPs about the trial and were available to address any queries
-
weekly telephone contacts with sites by ToSCA to field any queries on participant identification and baseline assessments
-
2-monthly teleconferences at which all pilot sites discussed and shared information about effective recruitment strategies.
-
-
Other methods used to identify eligible patients were:
-
assisting the identification of potentially eligible people through the IAPTUS database (IAPT’s electronic clinical system), as an additional mechanism of identifying potential participants. We initially worked with the lead PWPs in Camden, Islington and Kingston on running the following searches:
-
– retrospective searches to identify potentially eligible people missed by the PWPs during their clinical reviews at the end of their step 2 treatment
-
– prospective searches to identify potential participants at their entry to the step 2 treatment (i.e. those with high GAD-7 scores not on antidepressants) and alerted the PWPs in advance about their possible eligibility
-
-
IAPTUS prompts to PWPs of potentially eligible people: we were working with the IAPT data managers on generating a computer prompt on the IAPTUS database in order to flag potential participants, as described in the paragraph above. This, however, was not finalised and, hence, not implemented
-
approaching people on the waiting list for step 3 (high-intensity therapy): we explored the possibility of getting eligible people to take part in the trial but, as they were on the waiting list for CBT, they were expecting to receive psychological treatments and, hence, this approach did not yield any participants
-
opening new pilot recruitment sites: on 13 November 2015 we proposed the recruitment of more clinical sites to boost our study numbers. We had received recent expressions of interest from six sites through our contact with the CRNs and NHS trusts, as well as several over the past year that the study had been advertised on the web. When approached, four sites expressed a definite interest in taking part in the study, but after further discussion only two of these were willing to actively recruit.
-
These strategies were discussed at the HTA programme monitoring meeting on 14 January 2016. It was made clear that despite all the efforts that had been made by the trial team, recruitment to this study was unlikely to achieve our final target number of 360 participants and we suggested that we alter our recruitment strategy through a protocol change. Prior to the HTA programme monitoring meeting, in discussion with the TSC, we had considered two possible future protocol changes: options A and B. We requested that one of these two, or both used in succession, be adopted following our meeting with the HTA programme monitoring committee. These two options are detailed below:
-
Option A – this would have involved a drive to identify more people in primary care through a retrospective search of GP databases to identify all adult patients aged ≥ 18 years with Read codes63 for anxiety/depression and/or GAD-7 scores of ≥ 10. Letters would then be sent to those so identified containing a reply slip, consent form for screening questionnaires, GAD-7 to complete, exclusion criteria assessment checklist and a brief questionnaire asking them to express interest in participation in the study. This approach aimed to maximise the identification of patients with GAD in primary care, and to refer them on to the local IAPT services. The TSC were supportive of this change of protocol as it was consistent with the HTA programme commissioning brief. They were concerned, however, that such a strategy could overwhelm the local IAPT services, which were likely to already be under pressure and may not have been prepared to deal with more referrals. Furthermore, there could be a significant delay while people were receiving step 2 treatment before they became eligible for participation in the trial. This could then lead to high levels of dropout. Nevertheless, the advantage of such an approach was that it was tied in with the changes we had already implemented to enhance recruitment from within IAPT services.
-
Option B – the process of identifying suitable patients in option B would have been similar to that of option A. We would have run a retrospective search of GP databases to identify all adult patients aged ≥ 18 years with Read codes for anxiety/depression and/or GAD-7 scores of ≥ 10. Letters would then be sent to patients containing a reply slip, consent form for screening questionnaires, GAD-7 to complete, checklist of exclusion criteria and a brief questionnaire asking them to express an interest in taking part in the study. Suitable patients would then be assessed for their eligibility to take part in the trial in general practice and then be randomised to either the sertraline medication arm or high-intensity CBT without having to engage with the step 2 treatment as delivered by the PWPs.
Option B would have been essentially a move from the original question proposed in the HTA programme brief as it involved a change in research population. Rather than pointing people towards IAPT teams for step 2 treatment (as in option A), this option would have recruited to the trial without a step 2 intervention. This in itself is an important clinical question, but would have constituted a move from the HTA programme brief as the study would not recruit merely those who have failed to respond to a step 2 intervention as specified in the brief. The TSC initially felt it premature to consider such a departure without (1) testing the effectiveness of the planned current changes to recruitment that would adhere to the HTA programme brief, and (2) seeking the opinion of the funder, the HTA programme, as to whether or not this amendment of the protocol (and thus research question) should be held in reserve if other changes, as described above, had failed to improve recruitment.
Finally, a radical suggestion, bearing in mind that patient preferences are a major hurdle to recruitment, was to design a patient preference trial in the form of a comprehensive cohort preference trial.
The HTA programme monitoring committee was not supportive of option A as this was an approach that would not be adopted within the NHS in the future, even though the proposed protocol change would have adhered to the HTA programme brief. The committee did consider option B to be a viable strategy but, as it represented a significant deviation from the original commissioning brief, they wanted to consult with the HTA programme director in order to arrive at a final decision. At the conclusion of the HTA programme monitoring meeting the trial team was advised to persist with efforts to recruit until further notice was given by the HTA programme regarding whether or not they would allow a deviation from the original commissioning brief. If this was not considered acceptable, we were advised that the trial would be expected to close down, hence bringing the study to an end.
Final decision to close down the trial
On 29 January 2016, following discussions with the HTA programme director, we were informed that a deviation from the original commissioning brief was not considered acceptable and the study should be brought to an end. The principal reasons for this decision were as follows.
-
Fairness, in that any research team that would have submitted a proposal if the commissioning brief had included option B, but chose not to because they felt the commissioning brief as it stood was not feasible or appropriate, would have legitimate cause for complaint if we subsequently proceeded to follow option B.
-
Option B involved a substantial change to the study population and would therefore represent a different research question to that originally commissioned.
-
Option B would appear to deviate from NICE guidance; it was not clear if this would be acceptable to the clinical community.
Following this decision we stopped recruiting to the trial and plans to close the trial down were developed and implemented from 1 March 2016.
Chapter 9 Conclusions and recommendations: implications for practice and research
Main conclusions
Low rates of identification of potential participants
It was unfortunately not possible to recruit sufficient participants to the trial of CBT versus sertraline following the recommended brief. The main barrier appears to have been at the level of identification of potential trial participants by the IAPT PWPs. There are a number of possible reasons for this, which can be summarised as follows:
-
The PWPs potentially having a bias towards psychological therapy as the treatment of choice and finding it difficult to maintain clinical equipoise when talking to the patients about the trial.
-
The PWPs being aware that having GAD meant that these patients found uncertainty very difficult and that the prospect of being randomised to a RCT for their treatment with the associated uncertainty of this was particularly difficult for them to manage; the PWPs felt uncomfortable raising something that might make their patients more anxious.
-
Some PWPs considered that the prevalence of GAD, or having this as the main clinical problem that patients wanted to address, was lower than suggested by the trial team and they were very keen to ensure that the patients they suggested the trial to definitely had GAD, despite the research team explaining that a definitive diagnosis could not be made at the routine PWP level.
-
Several PWPs raised the issue to their clinical supervisors, suggesting that a patient’s comorbid clinical or psychosocial problem was more important to address than their GAD. In such cases, the research team encouraged both the PWPs and their supervisors to consider suggesting to patients that they might be assessed for ToSCA if they had significant GAD.
-
Most PWPs had significant clinical workloads and it was difficult for them to also include the time needed to raise the possibility of being assessed for the trial with suitable patients.
Patient factors affecting recruitment once they had been identified
The reasons given by many of those patients who had been identified as not wanting to be recruited into the trial largely mirrored what the PWPs had suggested, in that by far the most significant reason given was that the patient did not want to risk being randomised to the sertraline arm of the trial, and several expressed a clear preference for wanting CBT treatment. Not wanting to be given medication appeared to be the predominant factor, although finding the uncertainty of the randomisation process difficult may also have been a factor. In retrospect, the nature of GAD – worry when there is uncertainty – should have alerted us to the likelihood that discussions about the study and the uncertainty raised by randomisation would prove challenging.
The reasons people gave for declining to participate were predominantly that they were unwilling to try antidepressant medication or that they were clear that they wanted psychological treatment. Although surveys indicate that people commonly report a preference for psychological over pharmacological treatment for common mental health problems, the number of people unwilling to consider antidepressant medication in our study was in excess of what would be expected from these surveys. One possibility is that the context of recruitment within an IAPT service may be significant. The service is focused on psychological treatments (in its name as well as in its treatment provision), and this sets a context for both patients and staff. The IAPT staff approaching patients about the study, however much the need for equipoise was discussed with them, are likely to have had an allegiance to psychological interventions and may have subtly communicated this to potential participants. Even if this did not happen, people opting to be seen in an IAPT service in the first place are likely to be those interested in psychological treatment and they may well have had less favourable views towards pharmacological treatments.
Another factor that was less of an issue than we had expected and made assumptions for was comorbid major depression – only one patient of the seven who had a baseline assessment was found to be ineligible because of major depression on the MINI questionnaire, which was much lower than we had expected with our 50% estimate, although the number of patients assessed was small. It may have been that by being very selective in the potential patients they identified, the PWPs had excluded those who were clearly significantly depressed. The other patient found to be ineligible at the baseline assessment had found it difficult to be sure whether or not GAD was the main psychological difficulty for which they wanted treatment – after discussion with the TMG we clarified the wording of this screening question to make it less potentially ambiguous.
In advance we had assumed that two-thirds of people meeting criteria for the study in other respects would either be on antidepressant medication or decline to participate. In the event, a little over one-third of potentially eligible people were already on antidepressant medication, and one in five participants approached ended up agreeing to participate in the study.
The fact that two GPs declined to support their patients in their wish to be assessed for the trial was disappointing, but it is difficult to know how we could have avoided this, given the trial design that recruited participants from IAPT who could be registered with any of the GPs linked to that service. The only way to have avoided this would have been to consider only patients whose GPs had agreed in advance to be involved in study, but the response rate to a request from the local research networks for interested GPs to state this was very low, which was not very surprising given that the chances of them having a patient selected to take part in the trial were not high. The only other way to deal with this issue would be to recruit directly from primary care, which is a strategy we considered (please see the following section) and suggested to the HTA programme at the monitoring meeting, but this was turned down by the funders because it would have been a major alteration to the commissioning brief.
Systemic factors affecting participant identification and recruitment
Linked with this is the fact that the advent of the national IAPT programme in England has meant that CBT and related evidence-based psychological treatments are now readily available and accessible as standard treatments. This means that if people have a preference for CBT they can access this relatively easily, in the same way that people have for much longer been able to easily access antidepressant medication via their GP. When access was more difficult, people might have been more prepared to consider a trial, but now if people have a preference they may well exercise this through opting for their preference. Two potential participants demonstrated this by stating a wish to have both CBT and antidepressant medication when the study was discussed with them.
An alternative treatment strategy would have been to recruit from primary care. In primary care, the full range of people with GAD would have been available to recruit, and primary care is where initial discussions naturally take place about treatment options between drug and psychological treatments. People are more likely to be in equipoise between drug and psychological treatments at this point than focused towards psychological treatments, as they are later in an IAPT service and, accordingly, are likely to be more open to accepting randomisation. They would also be less likely to already be on medication. This recruitment option was not available to the study team as the commissioned brief was, following the NICE stepped-care model, specifically to target people who had not improved following a low-intensity psychological intervention. Arguably, the restriction in the total GAD population from the brief as given would have limited the generalisability and utility of the findings even if recruitment had been successful, and recruiting from the wider primary care population would have been more useful and generalisable in the clinical sense. However, it is possible that, even if recruiting in primary care, people would have clear preferences between medication and psychological treatment and, as both are now relatively easy to access in England, they might well exercise that choice and be unwilling to accept randomisation to one or other intervention.
Future research and training recommendations
-
Running a RCT of medication versus psychological therapy involving two interventions that are already readily available to patients through the NHS is likely to have significant recruitment problems because many people will prefer not to be randomised if they are able to choose the treatment they would prefer. This was likely to have been further compounded in this trial by recruiting participants from a psychological therapy service and the difficulty of dealing with the uncertainty of randomisation for people with GAD. However, the unanswered question of whether psychological therapy or medication is more effective for people with GAD remains.
We would suggest that potential funding bodies consider a call for a randomised trial of medication versus psychological therapy for patients recruited from primary care, as this is where the discussion about treatment choice is likely to occur. Those randomised to the psychological therapy arm could be offered a low-intensity intervention initially and, if they do not improve sufficiently, proceed to high-intensity CBT, with the treatment paths in both arms of the trial clearly documented over the 12-month follow-up. The number of people accepting or declining treatment at each step would provide useful additional information.
Alternatively, a design that is more likely to reproduce the way in which patients are offered the choice between pharmacological and psychological therapy in practice would be to recruit participants with GAD from primary care to a randomised trial of a pharmacological therapy versus high-intensity CBT without them being referred for a step 2 intervention. This would not be following the NICE stepped-care model, but many patients are likely to be offered the choice between medication and psychological therapy at this stage by their GP and may be more willing to accept being randomised to one of the two interventions, allowing a direct comparison to be made.
-
Given the reluctance of patients to be randomised in this trial (both because of a reluctance to consider randomisation to the medication arm if recruited from the IAPT service, but also because of the uncertainty associated with randomisation, which people with GAD are likely to find particularly difficult), we would suggest that a patient preference design is considered. This could be a comprehensive cohort preference trial design, with patients allowed to choose whether they wish to opt for their preference of treatment or would be happy to be randomised to either intervention, with both cohorts being followed up for the duration of the study and the outcomes in the preference arms compared with the outcomes in randomised arms. However, it is possible that even recruiting via primary care, too few people may agree to be randomised to either intervention.
The recommendation would, therefore, be that if randomisation is not possible (either with recruitment via IAPT or primary care) then a naturalistic cohort design should be considered, following people up in accordance with their choice of treatment over a 12-month duration. 64,65 It would be important to accurately document all psychological comorbidities that might have an impact on the outcome.
A further possibility would be to use a Zelen’s preference design,66 with participants being randomised before their informed consent to participate has been obtained, and then being offered the treatment to which they were randomised. This would remove the intolerance of uncertainty as a potential barrier to patient involvement but is likely to also result in significant numbers not wanting to take part, thus reducing external validity. Ethics concerns may also be raised about using this design.
-
GAD appears underdiagnosed in primary care, for a variety of reasons, including imprecise diagnostic classification by GPs, which may have a negative impact on the outcome of people with this disorder. It would be helpful to conduct a study assessing patients diagnosed by their GPs as having depression or other anxiety disorders to see to what extent they may have been misdiagnosed, and conditions such as GAD or other psychological disorders underidentified.
The recommendation would be for a study examining the prevalence of GAD in a primary care population, possibly by reassessing patients diagnosed with a variety of depressive and anxiety disorders with a more detailed gold standard psychiatric instrument. This would also be helpful in establishing the numbers of people with ‘primary’ GAD, in which their GAD was their predominant concern, and those with comorbid depression or other anxiety disorders that were more of a concern to them.
A further recommendation would be to include semistructured interviews to establish if patients identified as having GAD saw this as a priority for treatment or if depression or other comorbidities were likely to be higher priorities for them.
-
Identification and recruitment of potential participants from our pilot IAPT services proved difficult, despite the enthusiasm of the IAPT leads at the various sites and the fact that we had expressions of interest from a significant number of other IAPT sites around England if the trial continued to the next stage. This was, however, a difficult role for the PWPs to undertake as it was clear that they had a heavy clinical commitment and little experience of research apart from the lead PWPs at each site, who were very energetic in their attempts to improve the recruitment rates.
We would suggest that training in research methods becomes a more formal part of PWP training if they are to be involved in recruiting for trials on a regular basis.
-
The PPI CAG identified a lack of suitable patient acceptability and adverse event measures that could be applicable to both the medication and psychological therapy arms of randomised trials.
Funding bodies may wish to commission the design of such an instrument for use in future RCTs.
Acknowledgements
We would like to acknowledge and thank the following for their contributions to this study.
-
The trial co-ordinator Dr Anastasia Kalpakidou and research assistant Sally Gascoine for their hard work in preparing all the paperwork for the trial, as well as interfacing with the participants, the pilot research sites, REC, PRIMENT CTU and sponsor. Bereni Oruitemeka provided administrative assistance.
-
The PIs and lead PWPs at the four pilot sites who were involved in the site initiation processes and participant recruitment, in particular John Cape, Tarun Limbachaya, Melanie O’Brien and Kathryn Chubb at Camden and Islington; Kay Wright and Helen Fletcher at Coventry and Warwickshire; Katie Egan and Joy Farrimond at Bristol; and Katy Grazebrook, Adam Cann and Natalie Mills at Greenwich, as well as all the PWPs and CBT therapists involved in participant recruitment and potential delivery of the CBT intervention.
-
Staff from the local UK Primary Care Research Networks and CRNs who assisted with service support costs, and local R&D leads who helped to facilitate the research permissions for each area, in particular Lynis Lewis, Selena Foroghi and Nicholas Portman at the North Central London Research Consortium.
-
The GPs and practice managers at participating practices.
-
The patients who agreed to be assessed for eligibility/participation in the trial.
-
Members of the TSC for all their advice and support: Professor Chris Williams, chairperson (University of Glasgow); Professor Carolyn Chew-Graham (Keele University); Dr Nicola Wiles (University of Bristol); and Mr Paul Lanham (lay representative).
-
Members of the Data Safety and Management Board (DSMB): Dr Victoria Cornelius, chairperson (Imperial College London); Professor Michael Moore (University of Southampton); and Dr Charlotte Connor (University of Birmingham).
-
Service user members of the PPI CAG.
-
Members of the PRIMENT CTU for their expert advice.
-
We would also like to thank all the co-applicant members of the ToSCA team who provided advice and assistance with the writing of the proposal and during the running of the study. They have all seen and agreed with the final report. In particular, we would like to thank Professor Michel Dugas for providing the CBT training manual and training the site therapists and supervisors who were going to deliver the CBT intervention; Professor Glyn Lewis for his methodological expertise; Professor Phil Cowen for all his advice about the pharmacological intervention arm; John Wood and Professor Nick Freemantle for their statistical expertise; Rachael Hunter for leading on the health economics; and Dr Helen Tyrer for her advice on recruitment in general practice.
Contributions of authors
Marta Buszewicz (Reader in Primary Care) was the CI, involved in the design of the study, writing and submission of the proposal, and had overall responsibility for the co-ordination and delivery of the study. She drafted and edited the final report.
John Cape (Director of Psychological Therapies Programme) was a co-applicant and assisted in the design of the study, as well as playing an active role in liaising with the local sites and training the PWPs during the study. He contributed to drafting the final report, revising it and providing detailed feedback.
Marc Serfaty (Clinical Reader in Psychiatry) was a co-applicant and assisted in the design of the study, and played an active role in refining the processes to be used for rating and validation of the tapes obtained in the CBT intervention arm. He contributed to drafting the final report, revising it and providing detailed feedback.
Roz Shafran (Professor of Translational Psychology) was a co-applicant and assisted in the design of the study, and played a leading role in delivering the research supervision of the CBT supervisors at the pilot sites. She contributed to drafting the final report, revising it and providing detailed feedback.
Thomas Kabir (Public Involvement in Research Lead) was a co-applicant and PPI lead for the study. He assisted in the study design and led the PPI CAG, which reviewed and gave feedback on the study protocol and materials. He contributed to drafting the final report, revising it and providing detailed feedback.
Peter Tyrer (Emeritus Professor of Community Psychiatry) was a co-applicant and assisted in the design of the study and ongoing discussions about amendments to the protocol and attempts to improve participant recruitment throughout the duration of the grant. He gave feedback on the writing of the report.
Caroline S Clarke (Research Associate in Health Economics) was actively involved in updating the various drafts of the study protocol and development of the health economic assessments for the trial. She contributed to revising the final report and providing detailed feedback.
Irwin Nazareth (Professor of Primary Care and Population Health and Joint Director of the PRIMENT CTU) was a co-applicant, assisted in the design of the study and gave ongoing methodological expertise throughout the grant as well as liaising with the staff of the PRIMENT CTU. He covered as CI for Marta Buszewicz for 6 months between October 2015 and March 2016. He contributed to drafting the final report, revising it and providing detailed feedback.
Data sharing statement
As only seven participants were recruited and the trial was closed prematurely with very limited follow-up data obtained, there are no useful data to be shared.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- McManus S, Meltzer H, Brugha T, Bebbington P, Jenkins R. Adult Psychiatric Morbidity in England, 2007. Results of a Household Survey 2009. Leeds: The NHS Information Centre for Health and Social Care; 2009.
- Walters K, Rait G, Griffin M, Buszewicz M, Nazareth I. Recent trends in the incidence of anxiety diagnoses and symptoms in primary care. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0041670.
- Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet 2006;368:2156-66. https://doi.org/10.1016/S0140-6736(06)69865-6.
- Sareen J, Jacobi F, Cox BJ, Belik SL, Clara I, Stein MB. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med 2006;166:2109-16. https://doi.org/10.1001/archinte.166.19.2109.
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care to 2026. London: The King’s Fund; 2008.
- Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety 2002;16:162-71. https://doi.org/10.1002/da.10065.
- Smith JP, Book SW. Comorbidity of generalized anxiety disorder and alcohol use disorders among individuals seeking outpatient substance abuse treatment. Addict Behav 2010;35:42-5. https://doi.org/10.1016/j.addbeh.2009.07.002.
- Culpepper L. Generalized anxiety disorder and medical illness. J Clin Psychiatry 2009;70:20-4. https://doi.org/10.4088/JCP.s.7002.04.
- Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults: Management in Primary, Secondary and Community Care. Clinical Guideline 113. London: NICE; 2011.
- NHS England . Children and Young People’s Improving Access to Psychological Therapies Programme n.d. www.england.nhs.uk/mental-health/adults/iap (accessed 26 February 2017).
- Gyani A, Shafran R, Layard R, Clark DM. Enhancing recovery rates: lessons from year one of IAPT. Behav Res Ther 2013;51:597-606. https://doi.org/10.1016/j.brat.2013.06.004.
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord 1988;14:61-8. https://doi.org/10.1016/0165-0327(88)90072-9.
- Donovan MR, Glue P, Kolluri S, Emir B. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders – a meta-analysis. J Affect Disord 2010;123:9-16. https://doi.org/10.1016/j.jad.2009.06.021.
- Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ 2011;342. https://doi.org/10.1136/bmj.d1199.
- Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. J Clin Psychiatry 2001;62:37-42.
- Buszewicz M, Chew-Graham C. Improving the detection and management of anxiety disorders in primary care. Br J Gen Pract 2012;61:489-50. https://doi.org/10.3399/bjgp11X588259.
- Generalised Anxiety Disorder in Adults: Evidence Update September 2012. London: NICE; 2012.
- Crits-Christoph P, Newman MG, Rickels K, Gallop R, Gibbons MB, Hamilton JL, et al. Combined medication and cognitive therapy for generalized anxiety disorder. J Anxiety Disord 2011;25:1087-94. https://doi.org/10.1016/j.janxdis.2011.07.007.
- Allgulander C, Dahl AA, Austin C, Morris PL, Sogaard JA, Fayyad R, et al. Efficacy of sertraline in a 12-week trial for generalised anxiety disorder. Am J Psychiatry 2004;161:1624-49. https://doi.org/10.1176/appi.ajp.161.9.1642.
- Brawman-Mintzner O, Knapp RG, Rynn M, Carter RE, Rickels K. Sertraline treatment for generalised anxiety disorder: a randomised, double-blind, placebo-controlled study. J Clin Psychiatry 2006;67:874-81. https://doi.org/10.4088/JCP.v67n0603.
- Haddad PM, Anderson IN. Recognising and managing antidepressant discontinuation symptoms. Adv Psychiatr Treat 2007;13:447-57. https://doi.org/10.1192/apt.bp.105.001966.
- Funk KA, Bostwick JR. A comparison of the risk of QT prolongation among SSRIs. Ann Pharmacother 2013;47:1330-41. https://doi.org/10.1177/1060028013501994.
- Schuurmans J, Comijs H, Emmelkamp PM, Gundy CM, Weijnen I, van den Hout M, et al. A randomized, controlled trial of the effectiveness of cognitive–behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. Am J Geriatr Psychiatry 2006;14:255-63. https://doi.org/10.1097/01.JGP.0000196629.19634.00.
- Aurobindo Pharma – Milpharm Ltd . Sertraline 50 mg or 100 mg Tablets 2013. www.medicines.org.uk/emc/medicine/23062 (accessed 26 February 2017).
- Borkovec TD, Newman MG, Bellack AS, Hersen M, Salkovskis P. Comprehensive Clinical Psychology: Vol. 4. Adults: Clinical Formulation and Treatment. Oxford: Elsevier Science; 1999.
- Wells A, Carter K. Further tests of a cognitive model of generalized anxiety disorder: metacognitions and worry in GAD, panic disorder, social phobia, depression, and non-patients. Behav Ther 2001;32:85-102. https://doi.org/10.1016/S0005-7894(01)80045-9.
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clin Psych Sci Prac 2002;9:85-90. https://doi.org/10.1093/clipsy.9.1.85.
- Dugas MJ, Koerner N. The cognitive–behavioural treatment for generalized anxiety disorder: current status and future directions. J Cogn Psychother 2005;19:61-8. https://doi.org/10.1891/jcop.19.1.61.66326.
- Norton PJ, Mehta PD. Hierarchical model of vulnerabilities for emotional disorders. Cogn Behav Ther 2007;36:240-54. https://doi.org/10.1080/16506070701628065.
- Norton PJ, Sexton KA, Walker JR, Norton GR. Hierarchical model of vulnerabilities for anxiety: replication and extension with a clinical sample. Cogn Behav Ther 2005;34:50-63. https://doi.org/10.1080/16506070410005401.
- Dugas MJ, Ladouceur R. Treatment of GAD. Targeting intolerance of uncertainty in two types of worry. Behav Modif 2000;24:635-57. https://doi.org/10.1177/0145445500245002.
- Rosen NO, Knäuper B. A little uncertainty goes a long way: state and trait differences in uncertainty interact to increase information seeking but also increase worry. Health Commun 2009;24:228-38. https://doi.org/10.1080/10410230902804125.
- Rosen NO, Knäuper B, Sammut J. Do individual differences in intolerance of uncertainty affect health monitoring?. Psychol Health 2007;22:413-30. https://doi.org/10.1080/14768320600941038.
- Dugas MJ, Ladouceur R, Léger E, Freeston MH, Langlois F, Provencher MD, et al. Group cognitive–behavioural therapy for generalized anxiety disorder: treatment outcome and long-term follow-up. J Consult Clin Psychol 2003;71:821-5. https://doi.org/10.1037/0022-006X.71.4.821.
- Ladouceur R, Dugas MJ, Freeston MH, Léger E, Gagnon F, Thibodeau N. Efficacy of a cognitive–behavioural treatment for generalized anxiety disorder: evaluation in a controlled clinical trial. J Consult Clin Psychol 2000;68:957-64. https://doi.org/10.1037/0022-006X.68.6.957.
- Gosselin P, Ladouceur R, Morin M, Dugas M, Baillargeon L. Benzodiazepine discontinuation among adults with GAD: a randomized trial of cognitive-behavioural therapy. J Consult Clin Psychol 2006;74:908-19. https://doi.org/10.1037/0022-006X.74.5.908.
- Dugas MJ, Brillon P, Savard P, Turcotte J, Gaudet A, Ladouceur R, et al. A randomized clinical trial of cognitive–behavioural therapy and applied relaxation for adults with generalized anxiety disorder. Behav Ther 2010;41:46-58. https://doi.org/10.1016/j.beth.2008.12.004.
- Dugas MJ, Robichaud M. Cognitive–Behavioural Treatment for Generalized Anxiety Disorder: From Science to Practice. New York, NY: Routledge; 2007.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;57:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Diagnostic and Statistical Manual of Mental Disorders 4th Edition. Washington, DC: American Psychiatric Association; 2000.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- D’Zurilla TJ, Dobson KS. Handbook of Cognitive–Behavioural Therapies. New York, NY: Guilford Press; 1986.
- Pennebaker JW, Colder M, Sharp LK. Accelerating the coping process. J Pers Soc Psychol 1990;58:528-37. https://doi.org/10.1037/0022-3514.58.3.528.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. https://doi.org/10.1192/bjp.180.5.461.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-20. https://doi.org/10.1016/0149-7189(79)90094-6.
- Tyrer P, Cooper S, Salkovskis P, Tyrer H, Crawford M, Byford S, et al. Clinical and cost-effectiveness of cognitive behaviour therapy for health anxiety in medical patients: a multicentre randomised controlled trial. Lancet 2014;383:219-25. https://doi.org/10.1016/S0140-6736(13)61905-4.
- EQ-5D . User Guide 2015. www.euroqol.org/eq-5d-publications/user-guides.html (accessed 26 February 2017).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- BSI . Certificate of Registration 2017. www.ucl.ac.uk/isd/itforslms/services/handling-sens-data/ig-documentation/IS_612909.pdf (accessed 21 August 2017).
- Department of Health . Information Governance Toolkit 2010. www.igt.hscic.gov.uk/ (accessed 21 August 2017).
- Selina Foroghi . TOSCA – Dr Marta Buszewicz 2015. www.youtube.com/watch?v=oN7DFQ7BOfE (accessed 26 February 2017).
- Lichstein K, Riedel BW, Grieve R. Fair test of clinical trials: a treatment implementation model. Adv Behav Res Ther 1994;16:1-29. https://doi.org/10.1016/0146-6402(94)90001-9.
- Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: a meta-analysis. Clin Psychol Rev 2014;34:130-40. https://doi.org/10.1016/j.cpr.2014.01.002.
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Philadelphia, PA: Centre for Cognitive Therapy; 1990.
- Blackburn IM, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The Revised Cognitive Therapy Scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990;28:487-95. https://doi.org/10.1016/0005-7967(90)90135-6.
- McPin Foundation . Home n.d. www.mcpin.org (accessed 26 February 2017).
- NIHR . INVOLVE Briefing Notes for Researchers 2012. www.invo.org.uk/posttypepublication/involve-briefing-notes-for-researchers/ (accessed 15 June 2017).
- NHS Digital . Terminology and Classifications n.d. http://systems.digital.nhs.uk/data/uktc/readcodes (accessed 26 February 2017).
- King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al. Impact of participant and physician intervention preferences on randomized trials: a systematic review. JAMA 2005;293:1089-99. https://doi.org/10.1001/jama.293.9.1089.
- King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al. Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9350.
- Torgerson DJ, Roland M. What is Zelen’s design?. BMJ 1998;316. https://doi.org/10.1136/bmj.316.7131.606.
Appendix 1 Patient information sheet
Appendix 2 Medication Suitability Review form
Appendix 3 Informed consent form
Appendix 4 General practitioner notification of patient not being eligible
Appendix 5 General practitioner notification of randomisation outcome
Appendix 6 General practitioner guidelines for prescribing sertraline
Appendix 7 Safety reporting information for participants, general practitioners and Improving Access to Psychological Therapies services
Appendix 8 Generic site-specific information justification
Appendix 9 Training workshop for high-intensity cognitive behavioural therapy therapists
The workshop covered background information, diagnostic criteria of GAD, the clinical presentation of GAD, the cognitive behavioural model, and treatment and conclusions. Some specific questions about the trial were raised during the training and were addressed by the CI and co-applicants.
The training consisted of providing information about the design of the trial, followed by taking trainees through the six typical phases of the treatment protocol:
-
psychoeducation and worry awareness training
-
re-evaluation of the usefulness of worry
-
uncertainty recognition and behavioural exposure
-
problem-solving training
-
written exposure
-
relapse prevention.
The training was delivered as a workshop, with scenarios in which attendees were encouraged to get into groups and practise using role play. It was based on the manual provided by Michel Dugas, and each therapist and supervisor was given a copy for use in the trial (see Appendix 13).
Appendix 10 Training for psychological well-being practitioners
The training sessions followed a common presentation format covering:
-
rationale and design of the study
-
PWPs’ role in the study
-
GAD identification and diagnosis.
Presenting the rationale and design of the study included the context of the study in terms of the evidence base for CBT and SSRIs as treatments for GAD, and the current lack of any head-to-head study determining which may be the more effective treatment. The NICE guideline stepped-care model for GAD and the associated clinical recommendations were presented in order to explain the choice needing to be made by the patient and their clinician between CBT and SSRIs as treatment options at step 3 in the NICE stepped-care pathway (see Figure 1). This provided the background for the study design as involving the recruitment of patients who had not improved in a step 2 low-intensity psychological intervention, which is why PWPs were critical to the identification and recruitment of study participants as they are responsible for delivering low-intensity psychological interventions within the IAPT services.
Discussion of the criteria for identifying participants who might be suitable for the study and how best to approach potential participants was the central part of the training.
Four key study criteria were set out for PWPs to use in considering patients they were treating:
-
that they were coming to the end of a low-intensity PWP treatment (having had at least three treatment sessions)
-
that they still had a GAD-7 score of ≥ 10
-
that they might have GAD either as the sole diagnosis or comorbid with other diagnoses
-
that they were not currently, and had not for the past 8 weeks been, on antidepressant medication (whether or not a patient was currently on a psychotropic medication was a data item that PWPs are asked to routinely record at each appointment as part of national IAPT data requirements).
Questions from the PWPs and discussion around these criteria during the training sessions mostly focused on:
-
whether or not patients in all types of PWP treatment should be considered (the answer to this was yes, with the exception of signposting interventions)
-
whether or not to approach patients who, it was decided early in their low-intensity treatment, were unlikely to progress (the answer was yes, as long as they had three treatment sessions)
-
how sure they needed to be that someone had GAD (the PWPs were told that they did not need to make a definitive diagnosis of GAD and were encouraged to be inclusive and refer to the study if they thought there was a possibility the patient might have GAD).
Following the discussion around participant identification criteria, PWPs were given a script of what they might say to a potential participant about the study (Box 3). The script was developed with suggestions from a PPI member of the CAG (see Chapter 7).
There are two treatments recommended for the kind of worry and anxiety you have, when initial treatment of the kind we have been working on together has not helped sufficiently. One is a type of medication, the other is a psychological therapy and, although both have been found in many research studies to be effective they have never been directly compared to see whether one is more effective than the other.
Our service is participating in a national study comparing these two kinds of treatment and we are therefore suggesting that suitable people take part, as there is evidence that people involved in medical research often do better, whichever treatment group they are included in.
You might be interested to join this study and, if so, I can pass your details to a member of the research team who would be happy to discuss it in more detail with you, or if you would prefer I can give you a leaflet that explains more about the study and gives the details of how to contact the research team yourself.
Might you be interested? However, if you are certain that you would definitely like to have one of these treatments rather than the other it might be better that you didn’t volunteer for this study, as it will involve people being randomised or selected by chance to be in one treatment group or the other.
The PWPs were encouraged to ask questions about this script during the training session and the advantages for patients in taking part in research studies, as well as any potential barriers, were explored.
Linked to this, the importance in randomised studies of discussing the two study arms from a position of equipoise was emphasised. It was stressed that equipoise was the current state of evidence for this important clinical question, but that they should be aware that they individually might well have a greater investment in psychological than pharmaceutical treatments given that they were working as PWPs and that this could potentially influence how they discussed the study with patients, including subconsciously. How to guard against this was rehearsed.
The final element of the training was about the identification of GAD. GAD DSM-IV diagnostic criteria were outlined and the differential diagnosis from other anxiety disorders and depression. Specific questions PWPs might ask the patients when screening for GAD were described.
The length of the training sessions varied from 1 to 2.5 hours depending on the site.
The longer training sessions included more about the background to the study and longer sessions on GAD diagnosis and identification (these sites had expressed an interest in using the study and the training sessions as an opportunity to train their PWPs in the identification of GAD). One training session was held for each pilot site, with the exception of Camden and Islington with Kingston, which had three training sessions, one for each Borough service.
All the training sessions were delivered by one of the co-investigators (JC), who had established and been the clinical lead of an IAPT service, so had a good understanding of the services and the role of PWPs within this.
Appendix 11 Research staff training
Participants were given an introduction to the trial and its aims and objectives. The research staff had all had some training in obtaining informed consent but were given an update and taken through the ToSCA consent form as well as the structure and format of the eligibility check and baseline assessment procedures. The procedures required asking female participants of child-bearing potential about the need to have a pregnancy test as part of the eligibility criteria, and how to do this sensitively and interpret the results was discussed and practised within the group.
The research staff then took part in interactive training in how to complete the relevant sections of the MINI. 43 This involved working in pairs, taking it in turns to role play scripted vignettes of potential patients with pure GAD (eligible) and also GAD with comorbid major depression and alcohol dependence (not eligible). The group then discussed its results and any queries regarding results that differed from the agreed consensus.
Training was also given in completing the HAM-A. 12 This involved one of the clinical co-applicants (JC or MB) role playing a patient with GAD, and each trainee rating their symptoms and presentation for the 14 items of the questionnaire. At the end the results were once more discussed within the group to ensure a consensus of within two points on each item.
The central research team produced a full training/recruitment manual for participating sites, with all the relevant questionnaires and outcome measures as appendices for reference.
This was distributed in draft version to participants at the training sessions and the full electronic version sent to the pilot sites when they were open to recruitment (manual available on request).
Appendix 12 ToSCA general practitioner flyer
Appendix 13 Cognitive behavioural treatment manual for generalised anxiety disorder
Appendix 14 Therapy Components Checklist
Appendix 15 End of Therapy Checklist
Appendix 16 Relapse prevention sheet
List of abbreviations
- CAG
- clinical advisory group
- CBT
- cognitive behavioural therapy
- CI
- chief investigator
- CONSORT
- Consolidated Standards of Reporting Trials
- CRN
- Clinical Research Network
- CSQ
- Client Satisfaction Questionnaire
- CTS-R
- Cognitive Therapy Scale-Revised
- CTU
- Clinical Trials Unit
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EoTC
- End of Therapy Checklist
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GAD
- generalised anxiety disorder
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- HADS-A
- Hospital Anxiety and Depression Scale – Anxiety component
- HAM-A
- Hamilton Anxiety Rating Scale
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- JRO
- Joint Research Office
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MINI
- Mini International Neuropsychiatric Interview
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PHQ-9
- Patient Health Questionnaire-9
- PI
- principal investigator
- PPI
- public and patient involvement
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SNRI
- serotonin–noradrenaline reuptake inhibitor
- SSRI
- selective serotonin reuptake inhibitor
- SUSAR
- suspected unexpected serious adverse reaction
- TCC
- Therapy Components Checklist
- TMG
- Trial Management Group
- ToSCA
- Trial of Sertraline versus Cognitive behaviour therapy for generalised Anxiety
- TSC
- Trial Steering Committee
- UCL
- University College London
- WSAS
- Work and Social Adjustment Schedule