Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/146/01. The contractual start date was in March 2013. The draft report began editorial review in January 2016 and was accepted for publication in November 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Saul N Faust was UK chief investigator and University Hospital Southampton NHS Foundation Trust principal investigator for a Cubist-sponsored clinical trial of daptomycin against standard of care antibiotic therapy in paediatric osteomyelitis. All funds were paid into accounts within the NHS trust or university and not paid as personal fees. He reports consultancy fees for advisory board participation paid into accounts within the NHS trust or university (not personal fees) from vaccine manufacturers and antimicrobial agent manufacturers, including AstraZeneca, Cubist, Merck, GlaxoSmithKline (GSK) and Pfizer, outside the submitted work. He acts as principal investigator for clinical trials and other studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers and antimicrobial agents, and has participated in advisory boards for vaccine manufacturers. Adam Finn reports grants and personal fees from Sanofi Pasteur MSD Ltd (SPMSD), and grants and personal fees from GSK, outside the submitted work. In addition, prior to October 2014, the University of Bristol and University Hospitals Bristol NHS Foundation Trust received funding for research conducted by Adam Finn and for consultancy and lectures from Pfizer, GSK, SPMSD and Novartis, who manufacture licensed and developmental meningococcal vaccines. Stuart C Clarke acts as principal investigator for clinical trials and other studies conducted on behalf of University Hospital Southampton NHS Foundation Trust/University of Southampton that are sponsored by vaccine manufacturers but receive no personal payments from them. He has participated in advisory boards for vaccine manufacturers and has received financial assistance from vaccine manufacturers to attend conferences, but receives no personal payments for this work. All grants and honoraria are paid into accounts within the respective NHS trusts or universities. Jethro Herberg reports that he was an investigator for a Cubist-sponsored clinical trial of daptomycin against standard of care antibiotic therapy in paediatric osteomyelitis; he received no personal payments. Andrew Riordan reports that the trust, but not he, has received funding for research sponsored by vaccine manufacturers and antimicrobial agents. He was lead editor for an e-learning package on meningitis produced by the Royal College of Paediatric and Child Health, funded by an unrestricted grant from Novartis Vaccines. Marieke Emonts was Newcastle upon Tyne Hospitals Foundation Trust’s principal investigator for a Cubist-sponsored clinical trial of daptomycin against standard of care antibiotic therapy in paediatric osteomyelitis. All grants and honoraria are paid into accounts within the NHS trust or university; she received no personal payment of any kind. Marieke Emonts reports acting as site principle investigator on behalf of the Newcastle upon Tyne Hospitals Foundation Trust/Newcastle University for clinical trials sponsored by Merck Sharp & Dohme Corp. and SPMSD outside the submitted work. Claire Ballinger is currently a member of the Health Technology Assessment (HTA) Primary Care, Community and Preventive Interventions (PCCPI) Panel and the HTA PCCPI Methods group. She was a former member of the Research for Patient Benefit London committee. Claire Ballinger is supported by the National Institute for Health Research (NIHR) Collaboration for Applied Health Research and Care Wessex. Catherine Spowart reports grants from NIHR during the conduct of the study. Philip Henman reports working as an investigator in a commercial clinical trial sponsored by Merck Sharpe & Dohme, on behalf of his employer, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by de Graaf et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Sections of text from this chapter were written for the Health Technology Assessment (HTA) programme application, but were published separately as Faust SN, Clark J, Pallett A, Clarke NM. Managing bone and joint infection in children. Arch Dis Child 2012;97:545–531 and are reproduced with permission from the British Medical Journal.
Osteomyelitis and septic arthritis in children
Osteomyelitis (OM) is inflammation of the bone accompanied by bone destruction,2 usually due to bacterial infection. It is an acute process, but if not treated effectively the inflammation can become chronic, leading to the development of sequestra and fistulae. 3 OM and septic arthritis (SA) can both be divided into three types according to the source of the infection: haematogenous; secondary to contiguous infection; and secondary to direct inoculation. Haematogenous OM can present acutely or subacutely as a more indolent, progressive process, with symptoms present for > 2 weeks. 4 In children, OM most often affects the long bones (femur 36%, tibia 33%, humerus 10%, pelvis 2.8%). 5 Single-site infection is most common, but 5–20% of children have multifocal OM. 6 SA is acute infection of synovial joints,7,8 usually secondary to bacteraemia. The infection affects the synovial membrane and the joint space. In younger children, the capsule of the joint often extends to the metaphysis, and damage to the cortex can lead to SA secondary to OM and vice versa. The epiphyseal growth plate can also be affected, causing growth discrepancies and long-term disability or permanent joint destruction if the acute infection is not treated promptly. 3
The estimated incidence for both OM and SA arthritis in Western populations is between 5 and 12 cases per 100,000 children per year. 3 Half of children with acute haematogenous OM are aged < 5 years. 3,8 Boys are 1.2–3.7 times more likely to be affected by osteoarticular infection (OAI) than girls. 3 The incidence in Southampton from 1979 to 1997 was between 1.4 and 10.5 cases per 100,000 per year,9 and in Newcastle from 1991 to 1999 was 7 cases per 100,000 for SA and 11 cases per 100,000 for OM (J Clark, Great North Children’s Hospital, 2011, unpublished data). Recent unpublished national data from England show that the admission rate for OM in children aged 0–18 years has varied between 4.8 and 7.0 per 100,000 child-years (M Sharland, St George’s Hospital London, 2011, personal communication). Subacute OM appears to be have increased in recent years,10 and is reported to be 5 per 100,000 children in Norway. 11 Neonatal infection can occur in preterm or term-born babies and is associated with a wider range of causative organisms (see below)12 and potential complications. Neonatal vascular anatomy allows infection within the bone to reach the growth plate or joint in 76% of neonatal osteomyelitis cases. 13
The pathogens implicated in paediatric bone and joint infections commonly include meticillin-sensitive Staphylococcus aureus (MSSA) (44–80%)8,14,15 and Kingella kingae (14–50%; higher in children aged < 36 months)8,15–19 and more rarely meticillin-resistant S. aureus (MRSA) (rare in the UK but found in 40–50% of cases in the USA),20,21 Panton–Valentine leukocidin (PVL) MSSA,22,23 group A Streptococcus (GAS), group B Streptococcus (GBS) (neonates),12,24 non-typeable Haemophilus spp. (incidence unknown), Haemophilus influenzae type b (in non-immunised or immunodeficient children), Escherichia coli (neonates),12,24 Streptococcus pneumoniae25 and coagulase-negative Staphylococcus (subacute). Very rarely (mostly in immunocompromised individuals) implicated are Pseudomonas aeruginosa (usually associated with inoculation injuries; therefore, in children aged > 1 year), Neisseria gonorrhoeae, Neisseria meningitidis (neonates, adolescents), Mycobacterium tuberculosis (older children as OAI develops 2 years after primary infection), Salmonella spp. (children with sickle cell disease),26 Bartonella henselae, N. gonorrhoeae, non-tuberculous mycobacteria (associated with defects of the interferon gamma pathway), Klebsiella spp., Bartonella henselae, Fusobacterium (often multifocal), Aspergillus and Candida albicans (neonates, children with damaged bone).
The pathogens most frequently seen according to age are:
-
neonates: GBS, MSSA, E. coli and other Gram-negative bacteria, and C. albicans
-
children aged < 2 years: MSSA, K. kingae, S. pneumoniae, H. influenzae type b, non-typeable Haemophilus spp., E. coli and MSSA PVL
-
children aged 2–5 years: MSSA, K. kingae, GAS, S. pneumoniae, H. influenzae type b, non-typeable Haemophilus spp., Pseudomonas spp., coagulase-negative Staphylococcus (subacute) and MSSA PVL
-
children aged > 5 years. MSSA and MSSA PVL.
Clinical features
The clinical features of OM and SA are dependent on age, site of infection and type of disease. The diagnosis and management of OAI in children should ideally be multidisciplinary, including paediatricians and orthopaedic surgeons with radiologists and microbiologists. The diagnosis of OM or SA is made on the basis of clinical presentation, laboratory tests, imaging and, where available, microbiology results. 1
White blood cell count, C-reactive protein and erythrocyte sedimentation rate
The white blood cell (WBC) count is an unreliable indicator of an OAI as, in many cases, it remains normal throughout the infection. 27 The inflammatory markers erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are more reliable, although normal values also do not exclude OM. 28 CRP levels are most sensitive (elevated in up to 98% of cases)7,8 but are not specific for bone or joint infection. Two studies have shown that CRP increases and also decreases faster than ESR, predicting recovery with more sensitivity than the ESR or the WBC count. 28,29 Differences in the causative organism may also cause differences in the acute-phase markers. Patients with OM caused by PVL-expressing S. aureus isolates have been found to have significantly higher mean values for ESR at admission, and higher maximum CRP, ESR and absolute neutrophil counts at presentation, than patients whose isolates were PVL negative. 23 Other markers remain unproven. In a small study, procalcitonin has not shown benefit over CRP. 30
Imaging
Imaging is of great importance in the diagnosis of acute OM. Where available, magnetic resonance imaging (MRI) with enhancement shows the best results regarding sensitivity and specificity of diagnosis of both OM and SA (sensitivity 97% and specificity 92% in SA;31,32 sensitivity 97–100% in OM). 7 However, as young children often require an anaesthetic to undergo MRI, and MRI is not immediately available in all UK centres, MRI is not widely used in the UK in the initial diagnosis.
A technetium (99mTc) radionuclide bone scan also has high sensitivity and specificity in the diagnosis of OM,33 but, as a result of the radiation burden, is now used less often except in difficult cases, and is not useful in discitis. In SA, a bone scan may be used to exclude underlying OM following aspiration and commencement of empirical therapy. A bone scan is especially useful where there is a suspicion of multifocal disease, but may give false-negative results in infancy, and sensitivity is reduced for the first 48 hours. New nuclear medicine technologies are available in some centres to combine computerised tomography (CT) with a low-dose radioactive substance, single-photon emission CT, which may be useful in increasing the resolution of nuclear medical images. 34
Plain radiography is less helpful than other imaging techniques as osteolytic changes or periosteal elevation occur most often 10–21 days after the onset of symptoms. 2,8,35 However, once apparent, the extent of bony change provides a good correlate to the severity of the disease. Plain radiographs also provide a baseline for comparison of subsequent change. Radiographic changes are frequently seen in subacute OM, but can be confused with malignancies such as Ewing’s sarcoma or osteoid osteoma. 13 In SA, plain radiographs are of limited use. In discitis, lateral radiographs of the spine 2–3 weeks into the illness often will reveal disc space narrowing with erosion of the vertebral end plates of the contiguous vertebrae. In vertebral OM, radiographs initially show localised rarefaction of a single vertebral body then anterior bone destruction.
Ultrasonography is useful in SA for identifying the presence of deep effusions and in OM for subperiosteal collections, but cannot differentiate between purulent and non-purulent material. 7,36 Ultrasonography may also be used to distinguish infection from other causes of similar symptoms or to direct fine-needle aspiration. 37
Computerised tomography is most valuable for guided procedures, such as aspiration or drainage of the infected bone or joint. 38 It effectively demonstrates air, sequestra and cortical destruction in chronic OM,36 but gives non-specific results in discitis.
Microbiological investigation
Identification of the pathogenic organism by culture should be attempted, with samples preferably taken prior to starting antibiotic therapy, as positive identification of the causative organisms allows targeted antibiotic therapy. Blood cultures, joint fluid (from aspiration), periosteal pus or bone biopsy can all be used. Samples from the infected bone or joint require an invasive procedure but are more likely to be positive (40–50% positive) than blood cultures (9–22% positive). 15,27 Yield is generally not high for identification of a bacteria in children with OM,27 as, unless therapeutic operative intervention is required, bone biopsy is infrequently necessary for diagnostic reasons alone.
New molecular techniques including polymerase chain reaction (PCR) and broad-range 16s ribosomal deoxyribonucleic acid PCR39,40 have established the basis for more rapid and sensitive microbiological diagnosis,18 although these methods currently do not provide information on specific organism antibiotic resistance profiles.
Blood cultures (a minimum 4-ml aerobic culture sample in older children, 2 ml in specific neonatal aerobic bottle)41 should therefore be taken and, where available, samples from infected bones or joints placed in a sterile universal container and sent for culture and sensitivity testing. Older reports suggesting an increase in K. kingae recovery is gained from inoculating synovial fluid or bony exudates directly into blood culture bottles have not been replicated in UK practice. 17 K. kingae is detectable using new PCR techniques from cultures where conventional direct plating of specimens on solid media has been used. 18,19
Surgical management
There is little current high-quality evidence on which to base current surgical practice.
Osteomyelitis
Surgical drainage in acute OM is indicated if the patient is not responding to antibiotics after 48–72 hours (although this may be because of resistance) or if there is radiological evidence of a substantial pus collection. 7 Best practice is to immobilise any surgically treated limb or focus of infection. Occasionally, where a soft tissue or subperiosteal collection is clearly demonstrated by ultrasonography or MRI, needle aspiration can be performed prior to starting intravenous (i.v.) antibiotics. When performed, the procedure should be carried out under sterile conditions. If there is bony destruction or pus aspirated, surgical debridement is usually required. With only early radiographic signs, conservative i.v. antibiotic therapy may suffice.
Historically, the role of surgery is poorly defined. Cole et al. 42 identified three groups of patients. In the group of patients aged > 1 year who presented within 48 hours, antibiotic therapy alone was sufficient. In the group aged > 1 year, 5 days after the onset of illness, patients usually required surgery and possibly multiple procedures. In infants aged < 1 year, in whom the exact diagnosis was difficult to make, a single operation and antibiotic therapy usually sufficed. In current practice, the relative roles of bacterial virulence and host age and immunity are unclear. More invasive surgery appears more common when bacteria have specific virulence genes, for example PVL. 22 Although most children recover rapidly with simple medical management, others require repeated debridement.
Septic arthritis
In SA, prompt drainage and washout of the affected joint (either arthroscopic or open) is advocated by some for both diagnostic and therapeutic purposes as the articular cartilage is damaged early. 7 The role of surgery in the treatment of SA is, in fact, poorly defined except in relation to the hip, where prompt surgical drainage is absolutely necessary. Open capsulotomy to allow continuing drainage of septic material is advocated, and, if the arthrotomy does not provide turbid material, drilling the femoral neck may decompress a proximal femoral OM. The anterior approach is preferred as this also allows open reduction of any displacement of the femoral head.
The indications for surgical drainage of septic joints other than the hip remain controversial. Where there is a large effusion, drainage is usually advocated, although in some joints arthroscopic irrigation may be appropriate, such as the knee or ankle. However, with arthroscopic treatment joint visualisation is less complete. Overall, for joints other than the hip, aspiration, irrigation and i.v. antibiotic therapy is the preferred first line of treatment. If the patient fails to respond, then the joint should be surgically drained, usually by formal open arthrotomy rather than arthroscopic drainage.
Medical management and antibiotics
Current evidence for how to initiate treatment
Intravenous antibiotics are started empirically as soon as the clinical diagnosis of acute OM or SA is made, as delaying therapy until the bacterium is identified increases the risk of complications. In SA, where urgent surgery is indicated, a widespread pragmatic approach has been to start antibiotics following surgery unless it will take > 4 hours to get to theatre. As soon as organisms are isolated, antimicrobial treatment should be adjusted and optimised. In subacute OM with no systemic reaction, oral antibiotics can be used from the start.
Although there has not been a definitive randomised controlled trial (RCT), a number of observational and retrospective studies in the literature show that several different antibiotic regimes have been effective in treating acute haematogenous OM in children, including the use of beta-lactam and macrolide antibiotics. 9
The initial antibiotics should always include potent cover against MSSA and GAS, and in younger children against K. kingae, although the choice will vary according to the age of the child, route of infection and local resistance patterns. 8 Recent data from the USA suggest increases in resistant S. aureus (MRSA), but these data have not been replicated in UK cohorts. 43 Activity against H. influenzae type b is essential in children who have not been fully immunised against it.
Switch to oral antibiotics and total duration of treatment
Currently there is no international and little UK consensus regarding the route or duration for antibiotic treatment of acute OAI in children.
Oral switch
Sequential i.v. and oral therapy has become usual as it is less inconvenient and painful for the patient, is associated with fewer complications and is cheaper than longer courses of i.v. therapy alone. 3,7,8 There is no current evidence to aid the clinical decision of when to switch from i.v. to oral therapy, although this practice is widely accepted and usually occurs when the patient has shown a marked clinical improvement. 9 A Canadian systematic review of short- (≤ 7 days) versus long-course (> 7 days) parenteral antibiotic treatment for acute haematogenous OM in children primarily due to S. aureus found no difference in the overall cure rate after 6 months between short- and long-course parenteral antibiotic therapy. 44 A recent retrospective cohort study of 1969 children in the USA found that early switch to oral therapy (median 4 days) was as effective as prolonged i.v. treatment,45 a finding also suggested in a smaller retrospective study of 186 children with SA. 46 The laboratory or clinical parameters that would determine the decision to switch to oral therapy remain undefined.
Most clinicians continue i.v. antibiotics until the child shows clinical improvement, if afebrile and oral fluids and medication can be established. Additionally, observing a decrease in inflammatory markers such as WBC, CRP and ESR is thought to be of value. 3 Studies have shown that serum CRP level decreases more rapidly than ESR in children recovering from acute OM, and that children with a raised CRP level are more likely to have symptoms or extensive radiographic abnormalities. 28,47,48 A recent Finnish clinical trial47,49 reported apparently good long-term results and apparently no failure rates using CRP as the biological marker of infection.
Failure to improve necessitates repeat blood culture, additional imaging for metastatic infection, assessment for deep-vein thrombosis and consideration of unusual pathogens such as PVL S. aureus or Fusobacterium.
No UK consensus currently exists to guide the criteria for oral switch for use in clinical practice or a clinical trial, which will be determined as part of this feasibility study.
Total duration of antibiotic therapy
The suggested duration for parenteral antibiotic treatment ranges from 3 days up to 6 weeks, resulting from several, mainly observational, studies with a relatively poor level of evidence. 9,50 In the past, the overall duration of antibiotic treatment has been considered an important factor to improve outcome and reduce relapse. Several paediatric textbooks recommend at least 4–6 weeks of treatment. 3,51
Although there are encouraging data from a recent clinical trial in Finland47,49 and from other review papers and case series, no recent formal RCT has been conducted to show good evidence for shorter courses of parenteral antibiotic treatment. There are a number of reasons why the recent Finnish data may not be directly applicable to practice in the UK or other countries. 52 Some historical observational studies showed an association between a short duration of antibiotic therapy and 15–19% poor outcome or relapse with courses of ≤ 3 weeks. 53–55
Currently there is no consensus about the route or duration for antibiotic treatment of acute OM in children.
Oral antibiotic choice and dose
Many different regimens are used as oral therapy following switch from oral antibiotics, including co-amoxiclav, flucloxacillin and clindamycin. Although flucloxacillin and clindamycin have good oral bioavailability and excellent tissue penetration, both drugs have to be given orally four times per day and both have poor taste and therefore poor drug adherence of the suspension in small children. 56 Although clindamycin rarely leads to C. difficile disease in children, there is no current evidence or consensus regarding oral antibiotic choice that will be acceptable to children and parents in terms of both palatability and dose frequency.
Continuation of intravenous antibiotics for more than 2 weeks
Complex disease requiring continuing i.v. therapy poses problems of vascular access, hospitalisation and schooling. Most children will require central or peripherally inserted central venous long line [peripherally inserted central catheter (PICC)] insertion for long-term antibiotic treatment. Delivery of subsequent care is either in hospital or at home, dependent on local services and the ability to provide outpatient parenteral antibiotic therapy (OPAT), although OPAT services for children are not yet well developed in the UK. Central venous lines or PICCs and OPAT has attendant risks, with 3–11% of central venous line-associated infection noted in the USA. 57,58
Additional or second-line antibiotics for complex disease or where resistant pathogens are identified
When cases are complex, additional antibiotics may be advised by local microbiologists, clinical infectious diseases specialists or national guidelines, for example PVL-positive S. aureus infection. 59
Complications
Deep-venous thrombosis and thromboembolism have been seen in up to 30% of children with OM and are associated with a higher risk of disseminated infection. 60 In addition, joint stiffness, limb shortening, dislocation (acutely neonates) and avascular necrosis of affected epiphysis may occur.
Routine follow-up allows most children with simple disease to be discharged without the need for long-term care or further assessment of growth or function.
In the context of clinical audit or clinical trials, outcome measures may include length of stay in hospital, total length of therapy, operative procedures required, as well as formal assessment of growth and function.
Currently accepted clinical equipoise
At this time, all clinical co-investigators have agreed in writing that they agree there is no international and little UK consensus regarding the route or duration for antibiotic treatment of acute bone and joint infections in children, and that there is also no clear evidence to aid the clinical decision of when to switch from i.v. to oral therapy.
Commissioning brief and objectives
Health Technology Assessment-provided background to commissioning brief
Usually treatment with an IV [intravenous] antibiotic is started as soon as possible. This may be continued for several weeks, with comorbidity caused by the need for prolonged IV access.
Empirically 3–6 weeks has been the usual regimen but there is little hard data upon which to base this opinion. Evidence is lacking for shorter duration IV antibiotics, but this could avoid the adverse events associated with protracted intravenous therapy, and still be an effective strategy to adopt in children.
In children with acute osteomyelitis or septic arthritis, can antibiotics be switched to the oral route after a short IV course to reduce the need for prolonged IV access.
HTA programme background document (provided with commissioning brief)
The HTA programme brief was to assess the feasibility of performing a hospital-based trial of short duration (3–5 days) i.v. antibiotic therapy followed by oral therapy for the usually recommended duration compared with a minimum of 14 days’ i.v. therapy prior to oral switch in children who have a clinical diagnosis of acute OM or SA (these two diagnostic groups should be considered separately) after any primary diagnostic or therapeutic surgical procedure. The feasibility study was required to identify appropriate non-inferiority margin and microbiological outcomes through surveys of different communities, and to assess if data will allow for subgroup analysis by culture status. The expectation was not for children to be actively recruited at this stage but for researchers to identify willingness of clinicians and families to participate.
Research objectives
In order to assess the feasibility and inform the potential design of a RCT to determine the safety of early oral switch from i.v. to oral antibiotic therapy we aimed:
-
to understand the current case load, disease spectrum and clinical practice in the diagnosis and treatment of OM/SA in secondary and tertiary UK care by conducting a service evaluation of OM/SA in children aged 1 month (the lower limit of the clindamycin licence) to 16 years
-
to assess if a new molecular test is an appropriate tool to assess the molecular epidemiology of children’s bone and joint infections by conducting a substudy at six of the service evaluation centres
-
to understand parents’ and children’s views and experiences of bone and joint infection, and gather their views and perceptions of both participating in a clinical trial and potential trial outcomes by conducting a qualitative study
-
to develop a core outcome set for use in a future RCT using a systematic literature review of previously used clinical trial outcomes in children’s bone and joint infections, a web-based clinician survey and results of the qualitative study to inform a stakeholder consensus meeting.
Patient and public involvement in this project
Patient and public involvement (PPI) was integrated into the design of this project via the National Institute for Health Research (NIHR) Medicines for Children Research Network (MCRN) PPI representation in the study initiation (via involvement in the NIHR MCRN Allergy, Immunology and Infectious Diseases Clinical Studies Group). The PPI representative reviewed the study protocols and all patient and parent facing materials, and reviewed the qualitative study protocol and study guide in detail. In addition, there was PPI representation on the study steering committee and consumer involvement at the study consensus meeting (PPI representative and member of NIHR Comprehensive Research Network: Paediatric Theme London Young Persons Advisory Group).
Chapter 2 Service evaluation
Aims and objectives
We aimed to conduct a service evaluation captured via an electronic web-based database to record every case of paediatric bone and joint infection in a 6-month period in participating centres, with 3 months’ clinical follow-up. The research objective was to define the current case load, disease spectrum and clinical practice in the diagnosis and treatment of OM/SA in secondary and tertiary UK care.
Methods
Service evaluation case record forms
A co-investigator meeting in November 2012 discussed the key items to include and exclude in the service evaluation, which were then further refined and discussed by the study team and investigators. These included demographic details and details of hospitalisation(s) including transfers between hospitals; type and site of disease; routine haematology, biochemistry and microbiology; radiological procedures; surgical procedures; length of i.v. therapy; antimicrobials used, route and duration; reason/criteria used for oral switch (if any); and clinical outcomes at 3 months. As per our application, we did not identify adverse outcomes occurring > 3 months after treatment (e.g. physical injury), which will be evaluated in a later RCT. The case record forms and service evaluation protocol are included in Service evaluation documents (see Report supplementary material 1).
Ethics approval was not required
We confirmed with the NHS Research Ethics Committee (REC) that formal REC approval was not required for the service evaluation data collection, in line with the House of Lords Select Committee report on ‘Fighting infection’,61 which recommended making infection surveillance a clear evidence-based priority, establishing collaboration between scientists (microbiologists), clinicians and epidemiologists, and encouraging the use of electronic capture and dissemination of information about infection.
Electronic records
A bespoke web-based data entry system was developed by the Medicines for Children Clinical Trials Unit, a division of the Clinical Trials Research Centre, a UK Clinical Research Collaboration fully registered clinical trials unit. The system, which stored and managed the data, was used as part of a national service evaluation. Data were collected using a custom web-based data entry system written in C#.Net, using jQuery (https://jquery.com). The data collection system allowed data to be validated on input, providing help/additional information as required for questions and to allow for the hiding of questions that did not need to be answered by the clinician.
Study sites
We recruited NHS tertiary and secondary care centres to participate in the service evaluation. Sites nominated a local principal investigator (paediatric infectious diseases, general paediatrics or paediatric orthopaedics) and achieved the support of local leads for all clinical stakeholders (paediatric medicine, orthopaedics, microbiology and radiology). All had the support of NIHR-funded research nurses [NIHR MCRN local research networks, paediatric clinical research facility or nurses funded by the local NIHR Comprehensive Local Research Network (from April 2014, the NIHR Comprehensive Research Network)].
Statistical analysis
A formal descriptive statistical analysis plan (v1.6, November 2014; see Report supplementary material 2) was developed and used to analyse the data. There were some major additions: notably that statistics have been generated for complex cases, that many statistics have been further split by diagnosis category (SA or OM) and some additional analyses have also been carried out (see Report supplementary material 3).
Important definitions appearing in service evaluation tables
Simple cases
Children presenting with SA or OM for the first time, and with no chronic comorbidities. Specifically:
-
no orthopaedic surgery 6 months prior to presentation on same limb/joint
-
no chronic comorbidities (sickle cell disease, known immunocompromise, indwelling central venous catheter or PICC, cystic fibrosis or cerebral palsy).
Complex cases
Children presenting with SA or OM who:
-
have had any orthopaedic surgery in the 6 months prior to presentation on same limb/joint
-
have chronic comorbidities (sickle cell disease, known immunocompromise, indwelling central venous catheter or PICC, cystic fibrosis or cerebral palsy)
-
are presenting because of an infected implant from previous orthopaedic surgery.
Misdiagnosed
Some children captured by the database were treated for SA/OM but were later found to have a different diagnosis.
Other
Some children had discitis. This is a form of SA, but it is treated differently and so is not of interest to this study.
Results
Sample descriptive
Participating centres
Fifty-one centres (49 hospital trusts) took part in the study. Eighteen of these were classed as tertiary centres and 33 as secondary centres.
Together, centres provided data for 356 children. In addition to these, three children were withdrawn as consent was withdrawn and five were excluded from analysis on the basis of a major lack of data. One tertiary centre (Chelsea and Westminster) and six secondary centres did not record any cases. For one of the secondary centres this was because the centre withdrew from the study.
Tertiary centres contributed 68% of the 356 cases (66% of all 218 simple cases). There was wide variation in the proportion of cases presenting with symptoms of OM/SA that were complex. In tertiary centres this varied from 0% to 75% (Table 1) and in secondary centres from 0% to 66.7% (Table 2).
| Site name | Total | By case type | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Recruitment period (months) | Simple cases | Complex cases | Misdiagnosed/discitis | ||||
| n (%) | Monthly rate | n (%) | Monthly rate | n (%) | Monthly rate | |||
| Alder Hey Children’s NHS Foundation Trust | 33 | 9.6 | 29 (87.9) | 3 | 2 (6.1) | 0.2 | 2 (6.1) | 0.2 |
| Barts Health NHS Trust (Newham and Royal London Hospitals) | 7 | 9.1 | 5 (71.4) | 0.5 | 2 (28.6) | 0.2 | 0 (0) | 0 |
| Cardiff and Vale University Health Board | 13 | 9.6 | 6 (46.2) | 0.6 | 7 (53.8) | 0.7 | 0 (0) | 0 |
| Central Manchester University Hospitals NHS Foundation Trust (Royal Manchester) | 15 | 6.8 | 14 (93.3) | 2.1 | 1 (6.7) | 0.1 | 0 (0) | 0 |
| Chelsea and Westminster Hospital NHS Foundation Trust | 0 | 9.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Derriford Hospital, Plymouth | 2 | 4.4 | 1 (50) | 0.2 | 1 (50) | 0.2 | 0 (0) | 0 |
| Evelina Children’s Hospital, London | 4 | 6.8 | 0 (0) | 0 | 3 (75) | 0.4 | 1 (25) | 0.1 |
| Great Ormond Street Hospital for Children NHS Foundation Trust | 4 | 9.1 | 1 (25) | 0.1 | 3 (75) | 0.3 | 0 (0) | 0 |
| Leeds Teaching Hospital | 21 | 9.3 | 16 (76.2) | 1.7 | 3 (14.3) | 0.3 | 2 (9.5) | 0.2 |
| Leicester Royal Infirmary | 4 | 8.5 | 2 (50) | 0.2 | 1 (25) | 0.1 | 1 (25) | 0.1 |
| Newcastle upon Tyne Hospitals NHS Foundation Trust | 24 | 9.6 | 11 (45.8) | 1.1 | 8 (33.3) | 0.8 | 5 (20.8) | 0.5 |
| Oxford University Hospitals NHS Trust | 16 | 6 | 11 (68.8) | 1.8 | 3 (18.8) | 0.5 | 2 (12.5) | 0.3 |
| Royal Alexandra (Brighton & Sussex Trust) | 8 | 8.9 | 4 (50) | 0.4 | 4 (50) | 0.4 | 0 (0) | 0 |
| Sheffield Children’s Hospital NHS Foundation Trust | 17 | 8.6 | 6 (35.3) | 0.7 | 11 (64.7) | 1.3 | 0 (0) | 0 |
| St George’s Healthcare NHS Trust | 6 | 6 | 6 (100) | 1 | 0 (0) | 0 | 0 (0) | 0 |
| St Mary’s Hospital, Imperial College London | 8 | 6 | 3 (37.5) | 0.5 | 3 (37.5) | 0.5 | 2 (25) | 0.3 |
| University Hospital Southampton NHS Foundation Trust | 42 | 9.6 | 20 (47.6) | 2.1 | 13 (31) | 1.4 | 9 (21.4) | 0.9 |
| University Hospitals Bristol NHS Foundation Trust | 18 | 6 | 9 (50) | 1.5 | 6 (33.3) | 1 | 3 (16.7) | 0.5 |
| Total | 242 | 144 (59.5) | 71 (29.3) | 27 (11.2) | ||||
| Median (IQR) | 8.8 (6–9.4) | 0.7 (0.2–1.7) | 0.4 (0.2–0.7) | 0.1 (0–0.3) | ||||
| Site name | Total | By case type | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Recruitment period (months) | Simple cases | Complex cases | Misdiagnosed/discitis | ||||
| n (%) | Monthly rate | n (%) | Monthly rate | n (%) | Monthly rate | |||
| Ashford & St Peters Hospitals NHS Foundation Trust | 3 | 6.8 | 0 (0) | 0 | 2 (66.7) | 0.3 | 1 (33.3) | 0.1 |
| Birmingham Heartlands Hospital | 2 | 6.0 | 2 (100) | 0.3 | 0 (0) | 0 | 0 (0) | 0 |
| Bradford Teaching Hospitals NHS Foundation Trust | 3 | 9.4 | 2 (66.7) | 0.2 | 1 (33.3) | 0.1 | 0 (0) | 0 |
| Burton Hospitals NHS Foundation Trust (Queens Hospital, Burton-on-Trent) | 7 | 9.6 | 4 (57.1) | 0.4 | 1 (14.3) | 0.1 | 2 (28.6) | 0.2 |
| County Durham and Darlington NHS Foundation Trust | 0 | 6.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dorset County Hospital, Dorchester | 0 | 5.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Furness General Hospital, Barrow-in-Furness | 0 | 5.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hampshire Hospitals NHS Foundation Trust (Basingstoke) | 1 | 8.5 | 0 (0) | 0 | 0 (0) | 0 | 1 (100) | 0.1 |
| Kingston Hospital NHS Trust | 2 | 6.6 | 1 (50) | 0.2 | 0 (0) | 0 | 1 (50) | 0.2 |
| Leighton Hospital (Mid Cheshire Hospitals NHS Foundation Trust) | 2 | 9.6 | 2 (100) | 0.2 | 0 (0) | 0 | 0 (0) | 0 |
| Luton and Dunstable NHS Foundation Trust | 1 | 6.6 | 1 (100) | 0.2 | 0 (0) | 0 | 0 (0) | 0 |
| Mid Yorkshire NHS Trust (Pinderfields, Wakefield) | 4 | 9.6 | 3 (75) | 0.3 | 1 (25) | 0.1 | 0 (0) | 0 |
| Norfolk & Norwich University Hospitals NHS Foundation Trust | 4 | 8.9 | 2 (50) | 0.2 | 2 (50) | 0.2 | 0 (0) | 0 |
| Manchester General Hospital | 0 | 6.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| North West London Hospital NHS Trust | 9 | 9.3 | 6 (66.7) | 0.6 | 1 (11.1) | 0.1 | 2 (22.2) | 0.2 |
| Pennine Acute Trust (Royal Oldham Hospital, Manchester) | 7 | 6.6 | 4 (57.1) | 0.6 | 2 (28.6) | 0.3 | 1 (14.3) | 0.2 |
| Portsmouth Hospitals NHS Trust | 1 | 7.5 | 1 (100) | 0.1 | 0 (0) | 0 | 0 (0) | 0 |
| Princess Alexandra Hospital NHS Trust | 5 | 8.7 | 4 (80) | 0.5 | 1 (20) | 0.1 | 0 (0) | 0 |
| Royal Berkshire Hospital, Reading | 4 | 9.4 | 3 (75) | 0.3 | 0 (0) | 0 | 1 (25) | 0.1 |
| Royal Blackburn Hospital | 12 | 9.1 | 2 (16.7) | 0.2 | 7 (58.3) | 0.8 | 3 (25) | 0.3 |
| Royal Devon and Exeter NHS Foundation Trust | 6 | 9.6 | 5 (83.3) | 0.5 | 1 (16.7) | 0.1 | 0 (0) | 0 |
| Royal Lancaster Infirmary | 0 | 5.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Royal Shrewsbury Hospital | 7 | 8.9 | 5 (71.4) | 0.6 | 1 (14.3) | 0.1 | 1 (14.3) | 0.1 |
| Royal Wolverhampton Hospitals NHS Trust (New Cross Hospital, Wolverhampton) | 7 | 5.6 | 6 (85.7) | 1.1 | 1 (14.3) | 0.2 | 0 (0) | 0 |
| Sandwell & West Birmingham NHS Trust | 2 | 7.5 | 2 (100) | 0.3 | 0 (0) | 0 | 0 (0) | 0 |
| Southend Hospital Trust | 4 | 7.5 | 3 (75) | 0.4 | 1 (25) | 0.1 | 0 (0) | 0 |
| Stockport NHS Foundation Trust | 1 | 8.7 | 1 (100) | 0.1 | 0 (0) | 0 | 0 (0) | 0 |
| University Hospitals of North Midlands NHS Trust | 2 | 9.2 | 1 (50) | 0.1 | 1 (50) | 0.1 | 0 (0) | 0 |
| University Hospital of North Staffordshire, Stoke-on-Trent | 10 | 9.3 | 6 (60) | 0.6 | 1 (10) | 0.1 | 3 (30) | 0.3 |
| University Hospitals Coventry and Warwickshire NHS Trust | 6 | 8.9 | 6 (100) | 0.7 | 0 (0) | 0 | 0 (0) | 0 |
| Westmoreland General Hospital, Kendal | 0 | 5.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wye Valley Hospitals NHS Trust | 1 | 5.6 | 1 (100) | 0.2 | 0 (0) | 0 | 0 (0) | 0 |
| Yeovil District Hospital NHS Foundation Trust | 1 | 9.6 | 1 (100) | 0.1 | 0 (0) | 0 | 0 (0) | 0 |
| Total | 114 | 74 (64.9) | 24 (21.1) | 16 (14) | ||||
| Median (IQR) | 8.5 (6.3–9.3) | 0.2 (0.1–0.5) | 0 (0–0.1) | 0 (0–0.1) | ||||
Recruitment
The study opened to recruitment in June 2013 and closed 1 year later. During this time frame, sites recorded all children presenting with SA/OM. Sites did not all open for recruitment at the same time but most achieved a minimum of 6 months (with the exception of one tertiary centre and six secondary centres). The median [interquartile range (IQR)] recruitment time was 8.8 (6–9.4) months for tertiary centres and 8.5 (6.3–9.3) months for secondary care centres (see Tables 1 and 2).
Accrual rates for simple and complex cases
The monthly rates of case accrual are given in Tables 1 and 2. The median recruitment rate in tertiary centres was 0.7 (IQR 0.2–1.7) cases per month for simple cases and 0.4 (IQR 0.2–0.7) cases per month for complex cases. For secondary centres the median recruitment rate was 0.2 (IQR 0.1–0.5) simple cases per month and 0 (IQR 0–0.1) complex cases per month.
Initial categorisation of types of cases and sample characteristics
A total of 356 children were recorded in the database as presenting with symptoms of SA or OM. Two hundred and eighteen (61.2%) children were classified as simple cases of OM or SA, 95 (26.7%) were classified as complex cases, five (1.4%) had discitis and 38 (10.7%) presented with the symptoms of OM or SA but were found to have a different diagnosis. Discitis is a special case of SA, but it is treated in a different way and so is not of interest to this study.
Table 3 gives the breakdown of how many children had OM or SA in each case-type group and the types of diagnosis of misdiagnosed children. Note that, for six children, either there was evidence of both SA and OM, or the diagnosis was ambiguous in the database, meaning that it could have been either SA or OM, or both. These children are not included in the analyses split by diagnosis group.
| Case type | n (%) (N = 356) |
|---|---|
| Simple casesa | 218 (61.2) |
| OM | 109 (50) |
| SA | 107 (49.1) |
| OM/SA or bothb | 2 (0.9) |
| Complex casesc | 95 (26.7) |
| OM | 56 (58.9) |
| SA | 35 (36.8) |
| OM/SA or bothb | 4 (4.2) |
| Misdiagnosed/discitis | 43 (12.1) |
| Misdiagnosed | 38 (88.4) |
| Cellulitis | 7 (16.3) |
| Reactive arthritis | 3 (7) |
| Joint effusiond | 2 (4.7) |
| Soft-tissue abscess | 2 (4.7) |
| Other | 22 (51.2) |
| Unknown/undecided | 2 (4.7) |
| Discitis | 5 (11.6) |
A total of 53.9% of participants were male, the median age was 3.4 years and most were of north European, east European or mid-European origin (73.3%). Table 4 shows the sex, age group and ethnicity for all 356 children, and also for the 313 children who were diagnosed with SA or OM. When misdiagnosed children and children with discitis are excluded, the demography of the sample remains largely unchanged.
| Demographics | All | Misdiagnoses/discitis excluded (n = 43) |
|---|---|---|
| Total number of children in database with data available for analysisa | 356 | 313 |
| Age (years) at presentation, median (IQR) | 3.4 (1.4–9) | 3.3 (1.4–8.7) |
| < 1, n (%) | 46 (12.9) | 40 (12.8) |
| 1–6, n (%) | 182 (51.1) | 161 (51.4) |
| 6–16, n (%) | 128 (36) | 112 (35.8) |
| Male, n (%) | 192 (53.9) | 173 (55.3) |
| Ethnicity, n (%) | ||
| European north/east/mid | 261 (73.3) | 228 (72.8) |
| European south (Mediterranean) | 2 (0.6) | 2 (0.6) |
| European Roma | 1 (0.3) | 1 (0.3) |
| African/Caribbean (North African) | 2 (0.6) | 2 (0.6) |
| African/Caribbean (sub-Saharan) | 8 (2.2) | 7 (2.2) |
| African/Caribbean (Afro-Caribbean) | 3 (0.8) | 2 (0.6) |
| Asian (Indian subcontinent) | 30 (8.4) | 26 (8.3) |
| Asian south-east Asia (Vietnam, Thailand, Indonesia, Malaysia, Philippines) | 3 (0.8) | 3 (1) |
| Asian east Asia (China, Japan, Korea) | 2 (0.6) | 2 (0.6) |
| Asian west Asia (Afghanistan, Iranian) | 1 (0.3) | 0 |
| Middle eastern (Turkish) | 0 | 0 |
| Middle eastern (Arab peninsula) | 1 (0.3) | 0 |
| Other/mixed | 37 (10.4) | 35 (11.2) |
| Missing | 5 (1.4) | 5 (1.6) |
Completeness of follow-up
Children were followed up for approximately 3 months post discharge. A total of 72% of simple cases and 80% of complex cases were contactable and followed up. In most cases, failure to follow up was because children were not contactable (Table 5). In 2.8% and 2.1% of simple and complex cases, respectively, follow-up data were missing for unknown reasons.
| Case type | Total, n (%) | Diagnosis, n (%)a | |
|---|---|---|---|
| SA | OM | ||
| Simple cases | n = 218 | n = 107 | n = 109 |
| Followed up | 157 (72) | 78 (72.9) | 79 (72.5) |
| Not contactable | 55 (25.2) | 26 (24.3) | 28 (25.7) |
| Unknown/missing | 6 (2.8) | 3 (2.8) | 2 (1.8) |
| Complex cases | n = 95 | n = 35 | n = 56 |
| Followed up | 76 (80) | 27 (77.1) | 45 (80.4) |
| Not contactable | 17 (17.9) | 7 (20) | 10 (17.9) |
| Unknown/missing | 2 (2.1) | 1 (2.9) | 1 (1.8) |
| Misdiagnosed/discitis | n = 43 | ||
| Followed up | 30 (69.8) | – | – |
| Not contactable | 10 (23.2) | – | – |
| Unknown/missing | 3 (7.0) | – | – |
Presentation and diagnosis
For the rest of this report, data are presented for the 313 children who were either simple or complex cases. The 43 misdiagnosed/discitis children are excluded from further analyses.
Medical history
Simple cases
Six simple cases had a history of orthopaedic surgery > 6 months prior to presentation (Table 6 shows details of bones/joints affected). Other medical histories of interest were that 31 (14.2%) children had at least one pre-existing medical condition not listed in Table 7, 43 (19.7%) children had at least one history of a severe infection in the 12 months preceding presentation and 29 (13.3%) children had a history of trauma in the month preceding presentation (Tables 8–10). A total of 18.8% of simple cases had had at least one antibiotic in the month prior to presentation (Table 11 shows further statistics by route type and for specific antibiotics).
| Bone/joint affected | Within 6 months prior to presentation, n (%)a | Any time prior to presentation, n (%) | |
|---|---|---|---|
| Simple cases (N = 218) | Complex cases (N = 95) | ||
| Cases | 6 | 6 (3.2) | 11 (16.8) |
| Bone/joint types affectedb | |||
| Knee | – | – | – |
| Clavicle | – | – | 1 |
| Radius | 1 | – | 1 |
| Ulna | – | – | – |
| Humerus | – | 3 | – |
| Tibia | 1 | 1 | 2 |
| Femur | – | 1 | 1 |
| Ankle | – | 1 | – |
| Hip | 3 | – | 4 |
| Spine | – | – | 1 |
| Toe | – | – | 1 |
| Elbow | – | – | 1 |
| Skull | 1 | – | 1 |
| Side of body | |||
| Right | – | 4 | 2 |
| Left | 2 | 2 | 4 |
| Both | 3 | – | 3 |
| N/A | 1 | – | 2 |
| Comorbidities indicating complex case | Yes (n) | No (n) |
|---|---|---|
| Sickle cell diseasea | 5 | 210 |
| Presentation as a result of an infected implant | 4 | 309 |
| Known immunocompromised | 2 | 311 |
| Indwelling central venous catheter or PICC | 31 | 282 |
| Cystic fibrosis | 1 | 306 |
| Cerebral palsy | 1 | 312 |
| Medical history | Simple cases (N = 218), n (%) | Complex cases (N = 95), n (%) |
|---|---|---|
| Other medical conditions | ||
| At least one | 31 (14.2) | 25 (26.3) |
| Congenital cardiac disease | 1 (0.5) | 2 (2.1) |
| Diabetes mellitus | – | 1 (1.1) |
| Purpura fulminans | – | – |
| Malnutrition | – | – |
| Othera | 30 (13.8) | 23 (24.2) |
| Immune-modulating treatment in last 6 calendar months? | ||
| Steroid | – | 1 (1.1) |
| Radiotherapy | – | – |
| Chemotherapy | – | – |
| Azathioprine | – | – |
| Ciclosporina | – | – |
| Cyclophosphamide | – | – |
| Rituximab | – | – |
| Leflunomide | – | – |
| Tacrolimus | – | – |
| Sirolimus | – | – |
| Other | – | 1 (1.1) |
| Infection history in the last 12 calendar months? | ||
| At least one | 43 (19.7) | 26 (27.4) |
| Pneumonia | 1 (0.5) | – |
| Sepsis | – | 2 (2.1) |
| Pyelonephritis | – | – |
| Cellulitis/soft-tissue infection | 5 (2.3) | 1 (1.1) |
| Meningitis | 1 (0.5) | – |
| OAI | – | 6 (6.3) |
| Abdominal sepsis | – | – |
| Varicella | 4 (1.8) | 3 (3.2) |
| Otherb | 32 (14.7) | 18 (18.9) |
| History of trauma in the last month? | 29 (13.3) | 15 (15.8) |
| Other surgery in previous 12 months? | 2 (0.9) | 4 (4.2) |
| Simple cases (N = 30) | Complex cases (N = 23) |
|---|---|
| Asthma (n = 7) | Abscess over the site of OM in her humerus drained July 2012 |
| Asthma, treated for 1 week with antibiotics for a 3-week dental abscess | ADHD, asthma, growth deficiency, insomnia |
| Atopic eczema | Asthma |
| Baker’s cyst (summer 2013) | Asthma, epilepsy, bilateral disease, death |
| Bilateral talipes valgus/internal tibial torsion | Bilateral AVN, acute chest syndrome |
| Capillary haemangioma currently undergoing laser treatment | Club foot |
| Chronic ITP | Congenital hip dislocation |
| Cleft lip/palate | Eczema (n = 3) |
| Coeliac disease | Fish odour syndrome, microcephaly, postural scoliosis, clawing of toe |
| Congenital dislocation of hips | G6PD deficiency |
| Duplication of chromosome 8p11–21 and severe learning difficultiesa | Gastro-oesophageal reflux |
| Eczema (n = 3) | Leucocyte adhesion deficiency type 1 |
| Haemophiliac | Mild asthma |
| Hydronephrosis (left kidney) | MRSA abscess (left neck) |
| Jaundice, oedema, lymphadenopathy | Normocytic normochromic anaemia (possibly secondary to infection) |
| Klippel–Trénaunay–Weber syndrome | Osteosarcoma |
| Leucocyte adhesion deficiency type 1b | Patient has a small PDA. Born at 32 weeks, patient was in the NICU and treated with fluconazole for possible fungal infection (patient born in August 2013). Patient home 1 week before starting symptoms |
| MRSA | Patient is being investigated for autism |
| Osteogenesis imperfecta type 1a | Right MCA abnormality, gastrostomy, sigmoid colostomy |
| Patient born with neonatal thrombocytopenia, now resolved | Spina bifida |
| Psychotic depression | Vitamin D deficiency |
| Under weather for 4 weeks prior with ear and chest infections |
| Simple cases (N = 32) | Complex cases (N = 18) |
|---|---|
| 2 × dental abscess, viral infection starting 19 August 2013 | Chest infection |
| Bronchiolitis (n = 2) | Ear infection |
| Diarrhoea | Ear/throat infection |
| Ear and chest infections | Fungal sepsis, patient grew C. albicans sensitive to fluconazole, premature neonate |
| Ear infection and tonsillitis | Left leg swelling/patient went to theatre for necrotising fasciitis. Endocarditis. All initially presented to Royal Berkshire Hospital, Reading |
| Ear infection, cold | Multiple bacterial URTI |
| Hand, foot and mouth disease | OM/Brodie abscess |
| MRSA | Patient initially presented on the 30 June with severe pain in the right distal tibia. Patient was placed in a back slab and had 2 weeks of antibiotics |
| MRSA sepsis and RSV positive | Pleural effusion |
| Non-specific viral illness week before | Possible impetigo (nose) |
| Probable viral URTI | Recurrent tonsillitis requiring tonsillectomy |
| Recurrent bouts of tonsillitis | Sinusitis and left frontoparietal subdural empyema |
| Recurrent ear infections | Tonsillitis |
| Scarlet fever (n = 2) | Tonsillitis and inflamed ear |
| Severe eczema exacerbation | Urinary tract infection |
| Sinusitis | Viral tonsillitis/URTI |
| Sore throat | Chronic infected tibia |
| Tick bite, Lyme serology negative | Vomiting illness |
| Tonsillitis (n = 3) | |
| URTI | |
| URTI – coryzal, sore throat, decreased appetite and a temperature of 38.3 °C | |
| Urinary tract infection | |
| Viral diarrhoea | |
| Viral illness (n = 2) | |
| Viral illness? migraine | |
| Viral infection | |
| Vomiting and diarrhoea illness |
| Antibiotic medical history prior to presentation | All simple cases (N = 218), n (%) | Route, n (%) | Duration > 1 week, n (%) | |
|---|---|---|---|---|
| i.v. | Oral | |||
| Penicillin allergy | 7 (3.2) | – | – | – |
| 1 month pre presentation | ||||
| At least one antibiotic takena | 41 (18.8) | 18 (8.3) | 26 (11.9) | 14 (6.4) |
| Antibiotics taken | ||||
| Flucloxacillin | 18 (8.3) | 9 | 9 | 4 |
| Clindamycin | 5 (2.3) | 2 | 3 | 2 |
| Cefuroxime | 3 (1.4) | 3 | 0 | 0 |
| Ceftriaxone | 10 (4.6) | 9 | 1 | 5 |
| Amoxicillin | 9 (4.1) | 0 | 9 | 3 |
| Co-amoxiclav | 8 (3.7) | 0 | 8 | 4 |
| Rifampicin | 2 (0.9) | 0 | 2 | 1 |
| Vancomycin | 2 (0.9) | 1 | 1 | 1 |
| Fusidic acid | 1 (0.5) | 0 | 1 | 1 |
| Teicoplanin | 3 (1.4) | 2 | 1 | 1 |
| Other | 1 (0.5) | 1 | 0 | 1 |
Complex cases
Eleven complex cases had a history of orthopaedic surgery; six of these were within 6 months of presentation (see Table 6 for details of bones/joints affected). Thirty-one (33.7%) children had an indwelling central venous catheter or PICC, four children presented with an infected implant, five children had sickle cell disease, one child had cystic fibrosis, one child had cerebral palsy and two children were known to be immunocompromised (see Table 7).
Other medical histories of interest were that 25 (26.3%) children had other pre-existing medical conditions not listed in Table 7, 26 (27.4%) children had a history of infection in the 12 months preceding presentation and 15 (15.8%) children had a history of trauma in the month preceding presentation (see Tables 8–10).
Presentation
Where children presented
Most children presented at emergency departments (65% of simple cases and 70% of complex cases). The next most likely location was at a general practitioner (GP) surgery (28% of simple cases and 14.7% of complex cases). Outpatient clinics, inpatient clinics and walk-in centres were much less likely places for children to present (Table 12).
| Presentation | All (N = 313), n (%) | Simple cases (N = 218), n (%) | Complex cases (N = 95), n (%) |
|---|---|---|---|
| Presenting to | |||
| Outpatient clinic | 13 (4.2) | 8 (3.7) | 5 (5.3) |
| Inpatient | 9 (2.9) | 5 (2.3) | 4 (4.2) |
| GP surgery | 75 (24) | 61 (28) | 14 (14.7) |
| Emergency department | 207 (66.1) | 141 (64.7) | 66 (69.5) |
| Walk-in centre | 9 (2.9) | 3 (1.4) | 6 (6.3) |
| Bones/joints affecteda | |||
| Hip | 68 (21.7) | 46 (21.1) | 22 (23.2) |
| Shoulder | 23 (7.3) | 16 (7.3) | 7 (7.4) |
| Knee | 73 (23.3) | 54 (24.8) | 19 (20) |
| Ankle | 46 (14.7) | 29 (13.3) | 17 (17.9) |
| Wrist | 9 (2.9) | 8 (3.7) | 1 (1.1) |
| Skull | 5 (1.6) | 2 (0.9) | 3 (3.2) |
| Mandible | 0 | 0 | 0 |
| Humerus | 14 (4.5) | 5 (2.3) | 9 (9.5) |
| Clavicle | 1 (0.3) | 0 | 1 (1.1) |
| Radius | 6 (1.9) | 3 (1.4) | 3 (3.2) |
| Ulna | 4 (1.3) | 2 (0.9) | 2 (2.1) |
| Pelvis | 11 (3.5) | 4 (1.8) | 7 (7.4) |
| Rib | 0 | 0 | 0 |
| Femur | 26 (8.3) | 16 (7.3) | 10 (10.5) |
| Tibia | 14 (4.5) | 6 (2.8) | 8 (8.4) |
| Fibula | 9 (2.9) | 4 (1.8) | 5 (5.3) |
| Sternum | 3 (1) | 0 | 3 (3.2) |
| Elbow | 29 (9.3) | 15 (6.9) | 14 (14.7) |
| Foot | 11 (3.5) | 4 (1.8) | 7 (7.4) |
| Calcaneus | 6 (1.9) | 5 (2.3) | 1 (1.1) |
| Lumbar vertebrae | 4 (1.3) | 2 (0.9) | 2 (2.1) |
| Cervical vertebrae | 0 | 0 | 0 |
| Thoracic vertebrae | 0 | 0 | 0 |
| Sacrum vertebrae | 4 (1.3) | 1 (0.5) | 3 (3.2) |
| Other | 2 (0.6) | 2 (0.9) | 0 (0) |
Joints and limbs affected
The most likely joints/limbs affected were the hip (21.7%), knee (23.3%) and ankle (14.7%). There are similarities between joints affected in simple and complex cases, although many joints/limbs were more affected in complex cases (see Table 12).
Septic arthritis was more likely than OM to occur in hips and knees (both simple and complex cases), whereas OM affected a much larger range of joints/limbs (Table 13).
| Limb/joint affecteda | Simple cases, n (%) | Complex cases, n (%) | ||
|---|---|---|---|---|
| SA (N = 107) | OM (N = 109) | SA (N = 35) | OM (N = 56) | |
| Hip | 31 (29) | 15 (13.8) | 11 (31.4) | 9 (16.1) |
| Shoulder | 7 (6.5) | 8 (7.3) | 3 (8.6) | 4 (7.1) |
| Knee | 41 (38.3) | 13 (11.9) | 9 (25.7) | 10 (17.9) |
| Ankle | 14 (13.1) | 14 (12.8) | 6 (17.1) | 10 (17.9) |
| Wrist | 4 (3.7) | 4 (3.7) | 1 (2.9) | 0 |
| Skull | 0 | 2 (1.8) | 0 | 3 (5.4) |
| Humerus | 1 (0.9) | 4 (3.7) | 4 (11.4) | 5 (8.9) |
| Clavicle | 0 | 0 | 0 | 1 (1.8) |
| Radius | 0 | 3 (2.8) | 2 (5.7) | 1 (1.8) |
| Ulna | 0 | 2 (1.8) | 1 (2.9) | 1 (1.8) |
| Pelvis | 0 | 4 (3.7) | 1 (2.9) | 5 (8.9) |
| Femur | 0 | 10 (9.2) | 3 (8.6) | 12 (21.4) |
| Tibia | 0 | 6 (5.5) | 1 (2.9) | 5 (8.9) |
| Fibula | 0 | 4 (3.7) | 1 (2.9) | 4 (7.1) |
| Sternum | 0 | 0 | 1 (2.9) | 2 (3.6) |
| Elbow | 7 (6.5) | 8 (7.3) | 9 (25.7) | 5 (8.9) |
| Foot | 0 | 4 (3.7) | 2 (5.7) | 4 (7.1) |
| Calcaneus | 0 | 5 (4.6) | 0 | 0 |
| Lumbar vertebrae | 1 (0.9) | 1 (0.9) | 0 | 2 (3.6) |
| Sacrum vertebrae | 0 | 1 (0.9) | 1 (2.9) | 2 (3.6) |
| Other | 1 (0.9) | 1 (0.9) | 0 | 0 |
Blood tests at presentation
Simple cases
A total of 105 (48.2%) children had blood tests taken at presentation. Of those tested, haemoglobin, WBC count, neutrophils, platelets and CRP were routinely measured in > 84% of cases. ESR was tested (53.3%) less often. Fifty-three (50.5%) children had both CRP and ESR tested. Of the 99 children with a CRP test result, 65 (65.7%) had their highest result at presentation (Table 14).
| Blood test at presentation | All, n (%) | Presentation centre type, n (%) | |
|---|---|---|---|
| Tertiary | Secondary | ||
| Simple cases | (N = 218) | (N = 106) | (N = 112) |
| At least one blood test carried out at presentation | |||
| Yes | 105 (48.2) | 51 (48.1) | 54 (48.2) |
| No | 113 (51.8) | 55 (51.9) | 58 (51.8) |
| Percentage of children who had a test with | |||
| Haemoglobin | 89 (84.8) | 43 (84.3) | 46 (85.2) |
| WBC | 96 (91.4) | 44 (86.3) | 52 (96.3) |
| Neutrophils | 91 (86.7) | 44 (86.3) | 47 (87) |
| Platelets | 89 (84.8) | 42 (82.4) | 47 (87) |
| CRP | 99 (94.3) | 47 (92.2) | 52 (96.3) |
| ESR | 56 (53.3) | 25 (49) | 31 (57.4) |
| CRP and ESR | 53 (50.5) | 23 (45.1) | 30 (55.6) |
| CRP and not ESR | 46 (43.8) | 24 (47.1) | 22 (40.7) |
| CRPmax at presentation | 65 (65.7)a | 33 (70.2)a | 32 (61.5)a |
| Complex cases | (N = 95) | (N = 51) | (N = 44) |
| At least one blood test carried out at presentation | |||
| Yes | 32 (33.7) | 18 (35.3) | 14 (31.8) |
| No | 63 (66.3) | 33 (64.7) | 30 (68.2) |
| Type of test | |||
| Haemoglobin | 26 (81.3) | 15 (83.3) | 11 (78.6) |
| WBC | 28 (87.5) | 16 (88.9) | 12 (85.7) |
| Neutrophils | 25 (78.1) | 14 (77.8) | 11 (78.6) |
| Platelets | 23 (71.9) | 14 (77.8) | 9 (64.3) |
| CRP | 31 (96.9) | 17 (94.4) | 14 (100) |
| ESR | 11 (34.4) | 3 (16.7) | 8 (57.1) |
| CRP and ESR | 11 (34.4) | 3 (16.7) | 8 (57.1) |
| CRP and not ESR | 20 (62.5) | 14 (77.8) | 6 (42.9) |
| CRPmax at presentation | 15 (48.4)a | 6 (35.3)a | 9 (64.3)a |
The prevalence of types of tests carried out was similar in tertiary and secondary centres (see Table 14 for more details). Table 15 presents median and IQRs for blood test results at presentation. Table 16 splits these results by diagnosis of SA or OM. Blood test results were similar in these two groups.
| Blood test at presentation | Simple cases | Complex cases | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Haemoglobin (g/dl) | 89 | 117 (109–126) | 26 | 124 (112–134) |
| WBC (109/l) | 96 | 12.14 (9.6–15.8) | 28 | 12.68 (11–14.5) |
| Neutrophils (109/l) | 91 | 7.3 (4.7–10.7) | 25 | 7.1 (5.47–8.71) |
| Platelets (109/l) | 89 | 342 (256–441) | 23 | 333 (280–419) |
| CRP (mg/l) | 99 | 32 (11.8–66.7) | 31 | 54 (24–141) |
| ESR (mm/hour) | 56 | 32 (20.5–70) | 9a | 28 (15–55) |
| Blood tests at presentation | SA | OM | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Simple cases | ||||
| Haemoglobin (g/dl) | 47 | 116 (108–128) | 42 | 120 (111–124) |
| WBC (109/l) | 48 | 12.66 (9.8–16.5) | 48 | 11.62 (9.5–15.2) |
| Neutrophils (109/l) | 48 | 7.89 (5.4–11.0) | 43 | 6.2 (4.68–10.7) |
| Platelets (109/l) | 45 | 341 (256–431) | 44 | 349.5 (258.5–482.5) |
| CRP (mg/l) | 49 | 32 (16.2–65) | 50 | 32.7 (10–68) |
| ESR (mm/hour) | 28 | 29 (14–70.5) | 28 | 35.5 (26–68.5) |
| Complex casesa | ||||
| Haemoglobin (g/dl) | 12 | 119.5 (109.5–127) | 13 | 127 (114–137) |
| WBC (109/l) | 13 | 12.9 (11.2–14.2) | 14 | 11.96 (10.65–14.8) |
| Neutrophils (109/l) | 13 | 7 (5.55–9.01) | 11 | 7.1 (4.3–8.4) |
| Platelets (109/l) | 12 | 331.5 (305.5–351.5) | 10 | 413 (269–523) |
| CRP (mg/l) | 15 | 53 (24–156) | 15 | 54 (16–141) |
| ESR (mm/hour) | 4 | 57.5 (41.5–91) | 4 | 15.5 (9.5–33.5) |
Complex cases
Thirty-two (33.7%) children had blood tests taken at presentation. Of those tested, haemoglobin, WBC count, neutrophils, platelets and CRP were routinely measured in > 71% of cases. All but one had a CRP measurement (96.9%) and ESR was less tested (34.4%). Eleven (34.4%) children had both CRP and ESR tested. Of the 31 children with a CRP test result, 15 (48.4%) had their highest result at presentation (see Table 14).
The prevalence of types of tests carried out were similar in tertiary and secondary centres, although ESR was over three times as likely in a secondary centre (see Table 14). Blood tests results were similar in the SA and OM groups (see Tables 15 and 16).
Clinical timeline
All children in the study spent time in hospital. On average, children went straight to hospital (median time from presentation to hospital referral was 0 days), and most children presented at accident and emergency (A&E) (see Table 12).
Not all children received i.v. antibiotics (see Table 31). Fifteen (6.9%) simple cases and two (2.1%) complex cases had either no i.v. treatment or missing data. Of those who did receive i.v. antibiotics, we can use the date of initiation of i.v. therapy as a proxy for date of diagnosis.
The clinical timeline for first diagnostic X-ray, radiological diagnosis, first diagnostic surgery and the initiation of i.v. antibiotics are given in Table 17. A total of 61% of simple cases and 59% of complex cases had at least one X-ray post presentation and prior to initiation of i.v. antibiotics (i.e. had a diagnostic X-ray). These were usually on the day of or day after presentation (see Table 17).
| Diagnostic and hospital stay timeline | n (%) | Median (IQR), days | Min., max. (days) |
|---|---|---|---|
| Simple cases (N = 218) | |||
| From first presentation to | |||
| Hospital referral | 218 (100) | 0 (0–3) | 0, 957 |
| First X-raya | 133 (61) | 0 (0–1) | 0, 957 |
| Radiological diagnosisb | 113 (51.8) | 1 (0–3) | 0, 957 |
| First surgeryc | 61 (28) | 1 (1–4) | 0, 56 |
| Initiation of i.v. antibiotics | 203 (93.1) | 1 (1–4) | 0, 961 |
| CRPmax | 202 (92.7) | 0 (0–6) | 0, 84 |
| Complex cases (N = 95) | |||
| From first presentation to | |||
| Hospital referral | 95 (100) | 1 (0–4) | 0, 1867 |
| First X-raya | 56 (58.9) | 0 (0–1) | 0, 2345 |
| Radiological diagnosisb | 50 (52.6) | 1 (0–4) | 0, 2345 |
| First surgeryc | 29 (30.5) | 2 (1–5) | 0, 223 |
| Initiation of i.v. antibiotics | 91 (95.8) | 2 (0–5) | 0, 2345 |
| CRPmax | 86 (90.5) | 4 (1–8) | 9, 2345 |
Diagnostic surgery was carried out in 28% of simple cases at a median of 1 day post presentation (25% carried out at ≥ 4 days post presentation). For complex cases, the surgery was slightly more likely (30.5%), at a median of 1 day post presentation (25% carried out ≥ 5 days post presentation).
A radiological diagnosis [an abnormal result via any imaging method (X-ray, ultrasonography, MRI, bone scan or CT) post presentation and prior to initiation of i.v. antibiotics] was obtained for 51.8% of simple cases (at a median of 1 day post presentation) and 52.6% of complex cases (at a median of 1 day post presentation).
Differences in clinical timeline between septic arthritis and osteomyelitis
Hospital referral times were more varied for OM (75% referred in 4 days for simple cases and 6 days for complex cases) than for SA (75% referred in 2 days for simple cases and 3 days for complex cases). The proportion of children with diagnostic X-rays was similar between SA and OM, as were the times at which these occurred. Radiological diagnosis was more likely in SA and at an earlier time than in OM. Diagnostic surgery was also more likely in SA and at an earlier time than in OM (Table 18).
| Diagnostic and hospital stay timeline | SA | OM | ||
|---|---|---|---|---|
| n (%) | Median (IQR), days | n (%) | Median (IQR), days | |
| Simple cases (N = 218) | (N = 107) | (N = 109) | ||
| From first presentation to | ||||
| Hospital referral | 107 (100) | 0 (0–2) | 109 (100) | 0 (0–4) |
| First X-raya | 66 (61.7) | 0 (0–1) | 66 (60.6) | 0 (0–1) |
| Radiological diagnosisb | 58 (54.2) | 1 (0–3) | 54 (49.5) | 1 (0–5) |
| First surgeryc | 39 (36.4) | 1 (1–2) | 21 (19.3) | 2 (1–10) |
| Initiation of i.v. antibiotics | 104 (97.2) | 1 (0–3) | 97 (89) | 2 (1–5) |
| CRPmax | 101 (46.3) | 2 (0–5) | 99 (90.8) | 3 (0–7) |
| Complex cases (N = 95) | (N = 35) | (N = 56) | ||
| From first presentation to | ||||
| Hospital referral | 35 (100) | 0 (0–3) | 56 (100) | 1 (0–6) |
| First X-raya | 21 (60) | 0 (0–2) | 33 (58.9) | 0 (0–1) |
| Radiological diagnosisb | 16 (45.7) | 1 (0–2) | 32 (57.1) | 1 (0–4) |
| First surgeryc | 15 (42.9) | 2 (1–5) | 14 (25) | 3 (1–8) |
| Initiation of i.v. antibiotics | 33 (94.3) | 2 (0–4) | 54 (96.4) | 2 (0–6) |
| CRPmax | 33 (94.3) | 3 (1–6) | 49 (87.5) | 4 (1–14) |
Diagnosis
Day of diagnosis was defined as the day when i.v. antibiotics were initiated as the standard of care has always been for i.v. antibiotics to be started as soon as possible in paediatric OM/SA. This can be pinpointed for 203 (93.1%) simple cases and 91 (94.6%) complex cases (see Table 31 for more information on children who did not receive i.v. antibiotics).
Blood tests at diagnosis
Simple cases
A total of 148 (72.9%) children who had i.v. antibiotics had blood tests taken on either the day before or the day of diagnosis. Of these, ≥ 85% had haemoglobin, WBC count, neutrophils, platelets and CRP measured. ESR was less frequently measured (57.4%), and 80 (54.1%) children had both CRP and ESR measured. Of the 137 children with a CRP test result, the result was highest at diagnosis in 89 (65%) (Table 19).
| Blood tests at diagnosis | All, n (%) | Centre type where i.v. antibiotics initiated, n (%) | |
|---|---|---|---|
| Tertiary | Secondary | ||
| Simple cases | (N = 203) | (N = 130) | (N = 73) |
| At least one blood test carried out the day of or day preceding initiation of i.v. antibiotics | |||
| Yes | 148 (72.9) | 82 (63.1) | 66 (90.4) |
| No | 55 (27.1) | 48 (36.9) | 7 (9.6) |
| Children who had at least one blood test had | |||
| Haemoglobin | 128 (86.5) | 72 (87.8) | 56 (84.8) |
| WBC count | 133 (89.9) | 72 (87.8) | 61 (92.4) |
| Neutrophils | 129 (87.2) | 72 (87.8) | 57 (86.4) |
| Platelets | 127 (85.8) | 71 (86.6) | 56 (84.8) |
| CRP | 137 (92.6) | 75 (91.5) | 62 (93.9) |
| ESR | 84 (56.8) | 46 (56.1) | 38 (57.6) |
| CRP and ESR | 80 (54.1) | 43 (52.4) | 37 (56.1) |
| CRP and not ESR | 57 (38.5) | 32 (39) | 25 (37.9) |
| CRPmax at diagnosis | 89 (65)a | 52 (69.3)a | 37 (59.7)a |
| Complex cases | (N = 91) | (N = 56) | (N = 35) |
| At least one blood test carried out the day of or day preceding initiation of i.v. antibiotics | |||
| Yes | 54 (59.3) | 34 (60.7) | 20 (57.1) |
| No | 37 (40.7) | 22 (39.3) | 15 (42.9) |
| Children who had at least one blood test had | |||
| Haemoglobin | 46 (85.2) | 28 (82.4) | 18 (90) |
| WBC count | 50 (92.6) | 31 (91.2) | 19 (95) |
| Neutrophils | 46 (85.2) | 28 (82.4) | 18 (90) |
| Platelets | 42 (77.8) | 28 (82.4) | 14 (70) |
| CRP | 48 (88.9) | 29 (85.3) | 19 (95) |
| ESR | 18 (33.3) | 9 (26.5) | 9 (45) |
| CRP and ESR | 17 (31.5) | 7 (20.6) | 10 (50) |
| CRP and not ESR | 31 (57.4) | 22 (64.7) | 9 (45) |
| CRPmax at diagnosis | 29 (60.4)a | 18 (62.1)a | 11 (57.9)a |
The prevalence of testing was similar in tertiary and secondary centres (see Table 19).
Table 20 presents the medians and IQRs for blood test results at diagnosis.
| Blood test at diagnostic | Simple cases | Complex cases | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Haemoglobin (g/dl) | 128 | 114 (106.5–122) | 46 | 115.5 (110–125) |
| WBC count (109/l) | 133 | 12.18 (9.5–16.4) | 50 | 12.83 (10–16.2) |
| Neutrophils (109/l) | 129 | 6.6 (4.7–10.7) | 46 | 7.365 (4.6–11.3) |
| Platelets (109/l) | 127 | 361 (272–471) | 42 | 331.5 (269–407) |
| CRP (mg/l) | 137 | 38.3 (20–74) | 48 | 86.7 (29–161) |
| ESR (mm/hour) | 83a | 46 (25–78) | 17a | 40 (21–60) |
Complex cases
In 54 (59.3%) children who received i.v. antibiotics, blood tests were carried out either on the day before or on the day of diagnosis. Among those tested, haemoglobin, WBC count, neutrophils, platelets and CRP were routinely measured in > 77% of cases. Most had a CRP measurement (88.7%). ESR was less often measured (33.3%), and in 17 (31.5%) children both CRP and ESR were measured. Of the 48 children with a CRP test result, (60.4%) the result was highest at diagnosis in 29 (see Table 19).
The prevalence of testing was similar in tertiary and secondary centres, although ESR was more likely to be carried out in a secondary centre (see Table 19).
Blood results of complex cases at diagnosis are summarised in Table 20.
Differences between septic arthritis and osteomyelitis
The proportion of children receiving blood tests was similar between SA and OM. Blood cell parameters were similar between the groups but median CRP and ESR were higher in the SA group of complex cases than in the OM group (107 mg/l and 60 mm/hour vs. 46 mg/l and 31.5 mm/hour, respectively). Within the simples cases this difference could not be found (Table 21).
| Blood tests at presentation | SA | OM | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Simple casesa | ||||
| Haemoglobin (g/dl) | 66 | 115 (107–123) | 60 | 114 (106.5–121.5) |
| WBC count (109/l) | 68 | 13.58 (9.95–17.6) | 64 | 11.2 (8.6–15.2) |
| Neutrophils (109/l) | 65 | 7.52 (4.9–11.1) | 62 | 6.0 (4.5–9.5) |
| Platelets (109/l) | 64 | 344 (257.5–449) | 61 | 368 (274–485) |
| CRP (mg/l) | 70 | 35.5 (21.2–72) | 66 | 39.5 (14–81) |
| ESR (mm/hour) | 41 | 46 (23–81) | 42 | 47 (26–76) |
| Complex casesa | ||||
| Haemoglobin (g/dl) | 19 | 111 (103–122) | 25 | 119 (113–130) |
| WBC count (109/l) | 22 | 12.4 (8.9–17.2) | 25 | 13.2 (10.7–16.2) |
| Neutrophils (109/l) | 20 | 5.8 (3.6–10) | 23 | 8.23 (5.5–12.1) |
| Platelets (109/l) | 19 | 312 (197–354) | 20 | 365 (298.5–459.5) |
| CRP (mg/l) | 22 | 107.0 (31–168) | 23 | 46 (13.9–110) |
| ESR (mm/hour) | 5 | 60 (40–103) | 10 | 31.5 (18–48) |
Diagnostic surgery
The types of surgery carried out post presentation and prior to start of i.v. antibiotics are given in Table 22. In simple cases, the types of surgery carried out most frequently were incision and drainage, and aspiration (60.7% and 55.7%, respectively), with arthroscopy third (9.8%). In complex cases, incision and drainage was the most common type of surgery (62.1%), followed by aspiration (24.1%) and debridement (17.2%) (see Table 22).
| Surgery types | Simple cases (N = 203), n (%)a | Complex cases (N = 91), n (%)a |
|---|---|---|
| At least one of | 61 (30) | 29 (31.9) |
| Aspiration | 34 (55.7) | 7 (24.1) |
| Incision and drainage | 37 (60.7) | 18 (62.1) |
| Drill decompression | 2 (3.3) | 1 (3.4) |
| Curettage/excision | 1 (1.6) | – |
| Arthrotomy | 2 (3.3) | 3 (10.3) |
| Arthroscopy | 6 (9.8) | 1 (3.4) |
| Amputation | – | – |
| Debridement | 6 (9.8) | 5 (17.2) |
| Fasciotomy | – | 1 (3.4) |
| Compartment decompression | – | – |
| Secondary closure | – | – |
| Skin graft | – | – |
| Other plastic surgery | – | – |
| Other | 1 (1.6) | 4 (13.8) |
Some types of surgery were not carried out prior to i.v. treatment: amputation, compartment decompression, secondary closure, skin graft and other plastic surgery.
Differences between septic arthritis and osteomyelitis
Surgery was carried out more often in children with OM than in those with SA (in 63.6% vs. 37.5% in the simple cases and in 25.9% vs. 15.5% in the complex cases). Incision and drainage was performed with the same frequency in both groups, but debridement was performed more often in the OM group (Table 23).
| Surgery types | Simple cases, n (%)a | Complex cases, n (%)a | ||
|---|---|---|---|---|
| SA (N = 104) | OM (N = 97) | SA (N = 33) | OM (N = 54) | |
| At least one of | 39 (37.5) | 21 (63.6) | 15 (15.5) | 14 (25.9) |
| Aspiration | 24 (23.1) | 10 (30.3) | 5 (5.2) | 2 (3.7) |
| Incision and drainage | 26 (25) | 11 (33.3) | 13 (13.4) | 5 (9.3) |
| Drill decompression | – | 2 (6.1) | – | 1 (1.9) |
| Curettage/excision | 1 (1) | – | – | – |
| Arthrotomy | 2 (1.9) | – | 3 (3.1) | – |
| Arthroscopy | 6 (5.8) | – | 1 (1) | – |
| Debridement | 1 (1) | 5 (5.2) | 1 (3) | 4 (7.4) |
| Fasciotomy | – | – | 1 (1) | – |
| Other | – | 1 (3) | – | 4 (7.4) |
Diagnostic imaging
Radiography was widely used as a diagnostic tool (64.5% of children who received i.v. antibiotics). The proportion of radiographs that were abnormal was 38% for simple cases and 48% for complex cases.
The next most commonly used imaging method was ultrasonography (41% of simple cases and 31% of complex cases). Of those imaged, 74.1% detected an abnormality.
Magnetic resonance imaging was used in 19% of simple cases, with an abnormality being detected in 89%, and in 23% of complex cases, with a detection rate of 95%. Table 24 gives the numbers of children presenting with each bone/joint that had a diagnostic MRI. CT and bone scanning were rarely carried out, and in only OM cases. In all four cases, CT revealed abnormalities (Table 25).
| Limb/joint affecteda | Simple cases, n (%)b | Complex cases, n (%)b | ||
|---|---|---|---|---|
| SA (N = 9) | OM (N = 29) | SA (N = 4) | OM (N = 16) | |
| Hip | 2 (22) | 4 (14) | 1 (25) | 3 (19) |
| Shoulder | 1 (11) | 4 (14) | – | 1 (6) |
| Knee | 4 (44) | 1 (3) | 2 (50) | 2 (13) |
| Ankle | – | 3 (10) | 2 (50) | 4 (25) |
| Wrist | 1 (11) | 1 (3) | – | – |
| Skull | – | – | – | 1 (6) |
| Humerus | – | 3 (10) | – | 1 (6) |
| Clavicle | – | – | – | 1 (6) |
| Pelvis | – | 2 (7) | – | 2 (13) |
| Femur | – | 3 (10) | – | 1 (6) |
| Tibia | – | 1 (3) | – | 2 (13) |
| Fibula | – | – | 1 (25) | 3 (19) |
| Elbow | 1 (11) | 2 (7) | – | – |
| Calcaneus | – | 2 (7) | – | – |
| Lumbar vertebrae | – | 1 (3) | – | 2 (13) |
| Sacral vertebrae | – | 1 (3) | – | 1 (6) |
| Other | – | 1 (3) | – | – |
| Imaging type | Simple cases (N = 203),a n (%)b | Complex cases (N = 91),a n (%)b | ||
|---|---|---|---|---|
| Abnormal | Abnormal | |||
| X-ray | 133 (66) | 51 (38) | 56 (62) | 27 (48) |
| CT | 3 (1) | 3 (100) | 1 (1) | 1 (100) |
| Ultrasonography | 84 (41) | 62 (74) | 28 (31) | 21 (75) |
| MRI | 38 (19) | 34 (89) | 21 (23) | 20 (95) |
| Bone scan | 4 (2) | 3 (75) | 0 | – |
Differences between septic arthritis and osteomyelitis
X-ray and ultrasonography were equally used for OM and SA cases, and MRI was used more often in OM cases than in SA cases (30% vs. 9%, respectively) (Table 26). If MRI was performed in OM, the ankle and hip were most affected (16%), followed by the shoulder (11%) and humerus, pelvis and femur (9%). In SA, the knee and the hip were most affected (46% and 23%, respectively) (see Table 24). X-rays found similar percentages of abnormalities for simple cases, but found higher percentages in OM for complex cases. Ultrasonography detected a higher percentage of abnormalities in SA than OM in both simple and complex cases (see Table 26).
| Imaging type | SA, n (%)a | OM, n (%)a | ||
|---|---|---|---|---|
| Abnormal | Abnormal | |||
| Simple cases | N = 104b | N = 97b | ||
| X-ray | 66 (63) | 24 (36) | 66 (68) | 26 (39) |
| CT | 0 | – | 3 (3) | 3 (100) |
| Ultrasonography | 53 (51) | 43 (81) | 31 (32) | 19 (61) |
| MRI | 9 (9) | 8 (89) | 29 (30) | 26 (90) |
| Bone scan | 1 (1) | 1 (100) | 3 (3) | 2 (67) |
| Complex cases | N = 33b | N = 54b | ||
| X-ray | 21 (64) | 8 (38) | 33 (61) | 18 (55) |
| CT | 0 | – | 1 (2) | 1 (100) |
| Ultrasonography | 11 (33) | 9 (82) | 16 (30) | 11 (69) |
| MRI | 4 (12) | 3 (75) | 16 (30) | 16 (100) |
| Bone scan | 0 | – | 0 | – |
Microbiology
Conventional tests: micro-organisms
A total of 244 children (simple and complex combined) are known to have had conventional microbiology tests, in whom least one test on bone, joint aspirate, blood or periosteal pus was carried out. The results for these 203 children are summarised in Table 27. Among the simple cases, 38.2% of children tested positive for at least one micro-organism, compared with 52.5% of complex cases. Of the children with OM, 58.7% underwent microbiology tests, with at least one micro-organism detected in 43.8%. Of the children with SA, 73.8% were tested and a micro-organism was found in 34.2% of these.
| Tested | Simple cases, n (%) | Complex cases, n (%) | ||
|---|---|---|---|---|
| Total | Testeda | Total | Testeda | |
| Children | 218 | 144 (66.1) | 95 | 59 (62.1) |
| SA | 107 | 79 (73.8) | 35 | 24 (68.6) |
| OM | 109 | 64 (58.7) | 56 | 32 (57.1) |
| Detected | Tested | Yes | Tested | Yes |
| At least one micro-organism detected? | 144 | 55 (38.2) | 59 | 31 (52.5) |
| SA | 79 | 27 (34.2) | 24 | 10 (41.7) |
| OM | 64 | 28 (43.8) | 32 | 19 (59.4) |
| Specification of micro-organisms | Tested | Tested | ||
| S. aureus | 29 (20.1) | 19 (32.2) | ||
| PVL | 2 (6.9) | 1 (5.3) | ||
| MRSA | 1 (3.4) | 1 (5.3) | ||
| Erythromycin resistant | 0 | 1 (5.3) | ||
| Not tested/missing | 26 (89.7) | 16 (84.2) | ||
| K. kingae | 3 (2.1) | 1 (1.7) | ||
| Streptococcus pyogenes | 5 (3.5) | 5 (8.5) | ||
| Pseudomonas | 1 (0.7) | 0 | ||
| GBS | 2 (1.4) | 2 (3.4) | ||
| Salmonella | – | – | ||
| Candida | – | – | ||
| Mycobacterium tuberculosis | – | – | ||
| Otherb | 17 (11.8) | 7 (11.9) | ||
Among those tested, 20.1% of simple cases were found to have S. aureus, compared with 32.2% of complex cases. This was the most prevalent micro-organism detected; other microbes were rare (Table 28 shows further details on other microbes that were detected).
| Simple cases (n = 17) | Complex cases (n = 7) |
|---|---|
| Diphtheroids (n = 2) | Unspecified (n = 7) |
| Gram-positive cocci | |
| Staphylococcus epidermidis | |
| Unspecified (n = 13) |
Polymerase chain reaction tests (substudy): organisms
Presented fully in Chapter 3, the summarised results of the microbiology substudy are given in Table 29. Fifty children were tested out of 63 who consented to being part of the substudy. Of the 13 children who were not tested, five attended a centre that failed to carry out tests, six did not have a complete set of samples taken, one gave partial consent (but not for PCR) and one had no data in the database. Of the 50 children in whom tests were performed, six had no final diagnosis of OM or SA and were excluded from further analyses. Results are therefore shown for 44 tested cases of OM or SA.
| Cases/micro-organisms | Simple cases (N = 218), n (%) | Complex cases (N = 92), n (%) | ||
|---|---|---|---|---|
| Total | Tested | Total | Tested | |
| Consented to the microbiology substudy | 48 (22.3) | 15 (16.3) | ||
| Tested | ||||
| Children | 48 | 32 (67) | 15 | 12a (53) |
| SA | 18 | 6 | ||
| OM | 14 | 5 | ||
| Centre failed to test | 4 (8.3) | 1 (6.7) | ||
| Other reason not tested | 4 (8.3) | 2 (13.3) | ||
| Detected cases | Tested | Yes | Tested | Yes |
| At least one micro-organism detected | 32 | 24 (75) | 12a | 8 (67) |
| SA | 18 | 16 (89) | 6 | 4 (67) |
| OM | 14 | 8 (57) | 5 | 3 (60) |
| Specification of micro-organisms | Tested | Tested | ||
| S. aureus | 11 (34.4) | 5 (41.7) | ||
| K. kingae | 7 (21.9) | 0 | ||
| GAS | 2 (6.3) | 2 (16.7) | ||
| Streptococcus pneumoniae lytA | 1 (3.1) | 0 | ||
| Streptococcus pneumoniae ply | 0 (0) | 1 (8.3) | ||
| Staphylococcus epidermidis | 3 (9.4) | 0 | ||
In 75% of the simple cases tested, at least one micro-organism was detected: this is a higher detection rate than conventional testing. Micro-organisms were detected in 67% of the complex cases tested: this was a similar detection rate to conventional tests. PCR detection rates were similar between SA and OM in complex cases, but in simple cases the detection rate for SA was higher than for OM (89% vs. 57%, respectively).
As in the conventional testing, the most prevalent micro-organism was S. aureus (34.4% in simple cases and 41.7% in complex cases). However, the prevalence rate of K. kingae in simple cases was higher when using PCR (21.9%) than when using conventional testing (2.1%).
Conventional tests: types of sample
Blood samples were most commonly used for conventional microbiology tests (blood cultures, 62% of children tested). Joint aspirates were the next most common type of samples (43.6%). Bone biopsies were rare. There were some differences in the proportions of children in whom each type of sample was taken. However, although tertiary centres took more of all sample types except blood, the relative proportions of different sample types were the same in both types of centres.
We have microbiology test results for 46 centres treating simple cases: 16 tertiary and 30 secondary. Blood samples were similarly very likely in both types of centre. Of the tertiary centres, 14 took blood samples (87.5%) and 14 took joint aspirate samples; 56.3% of the tertiary centres took pus/wound swabs. Secondary centres were just as likely to take blood samples (83.3%) but not as likely to use joint aspirate (46.7%) or pus/wound swabs (30%) (Table 30).
| Sample types | Children with at least one sample of each type, n (%) | Centres with at least one example of each type of sample, n (%) | ||||
|---|---|---|---|---|---|---|
| All | Tertiary | Secondary | All | Tertiary | Secondary | |
| Simple cases | n = 163 | n = 98 | n = 67 | n = 46 | n = 16 | n = 30 |
| Blood | 101 (62) | 58 (59.2) | 43 (64.2) | 39 (84.8) | 14 (87.5) | 25 (83.3) |
| Bone biopsy | 6 (3.7) | 4 (4.1) | 2 (3) | 5 (10.9) | 3 (18.8) | 2 (6.7) |
| Joint aspirate | 71 (43.6) | 49 (50) | 23 (34.3) | 28 (60.9) | 14 (87.5) | 14 (46.7) |
| Pus (periosteal) | 25 (15.3) | 17 (17.3) | 8 (11.9) | 8 (17.4) | 6 (37.5) | 2 (6.7) |
| Pus/wound swab | 15 (9.2) | 12 (12.2) | 3 (4.5) | 18 (39.1) | 9 (56.3) | 9 (30) |
| Other | 35 (21.5) | 26 (26.5) | 9 (13.4) | 14 (30.4) | 7 (43.8) | 7 (23.3) |
| Missing/unknown | 6 (3.7) | 1 (1) | 5 (7.5) | 5 (10.9) | 1 (6.3) | 4 (13.3) |
| Complex cases | n = 70 | n = 48 | n = 23 | n = 32 | n = 16 | n = 16 |
| Blood | 38 (54.3) | 27 (56.3) | 13 (56.5) | 20 (62.5) | 10 (62.5) | 10 (62.5) |
| Bone biopsy | 5 (7.1) | 5 (10.4) | 0 | 5 (15.6) | 5 (31.3) | 0 |
| Joint aspirate | 17 (24.3) | 12 (25) | 5 (21.7) | 12 (37.5) | 8 (50) | 4 (25) |
| Pus (periosteal) | 14 (20) | 13 (27.1) | 1 (4.3) | 9 (28.1) | 8 (50) | 1 (6.3) |
| Pus/wound swab | 18 (25.7) | 14 (29.2) | 4 (17.4) | 10 (31.3) | 7 (43.8) | 3 (18.8) |
| Other | 16 (22.9) | 12 (25) | 4 (17.4) | 12 (37.5) | 8 (50) | 4 (25) |
| Missing/unknown/not applicable | 4 (5.7) | 3 (6.3) | 1 (4.3) | 4 (12.5) | 3 (18.8) | 1 (6.3) |
Conventional microbiology tests were most commonly carried out on blood samples (54.3% of children tested), followed by pus/wound swabs (25.7%). The frequency of use of different sample types varied between tertiary and secondary centres. As for simple cases, tertiary centres took more of all sample types except blood, but, in contrast to simple cases, the relative proportions of different samples types were different in secondary and tertiary centres.
We have microbiology test results for 32 centres treating complex cases: 16 tertiary and 16 secondary. Blood samples were equally likely in both types of centre. Of the tertiary centres, 10 (62.5%) took at least one blood sample and other sample types were used with similar frequency. Secondary centres were less likely to use non-blood samples (see Table 30).
Treatment
Antibiotic treatment
Clinical timeline
Almost all children received antibiotics both intravenously and orally. Table 31 gives timelines for antibiotic use when data for start and end dates are available.
| Antibiotic treatment timeline | n (%) | Median (IQR), days | Min., max. (days) |
|---|---|---|---|
| Simple cases (n = 218) | |||
| Length of time on | |||
| i.v. antibioticsa | 202b (92.7) | 8 (5–15) | 0, 220c |
| Oral antibioticsa | 176 (80.7) | 28 (16–36.5) | 0, 197c |
| Totald | 210 (96.3) | 34 (20–45) | 0, 220c |
| Complex cases (n = 95) | |||
| Length of time on | |||
| i.v. antibioticsa | 91e (95.8) | 17 (8–38) | 0, 112c |
| Oral antibioticsa | 63 (66.3) | 28 (16–42) | 1, 226 |
| Totald | 92 (96.8) | 39 (23–53) | 1, 236 |
A total of 202 (93%) simple cases received i.v. antibiotics, compared with 91 (96%) complex cases. However, for one simple case discharge antibiotic data were missing, so no end date was known, and for two complex cases dates for antibiotics were missing. The median time on i.v. antibiotics for simple cases was 8 days (IQR 5–15 days). For complex cases, time on i.v. antibiotics was, on average, longer: a median of 17 days (IQR 8–3 days) (see Table 31 for more information on children who were not treated with i.v. antibiotics).
Not all children who were treated with i.v. antibiotics were switched to oral antibiotics. In some cases, oral antibiotics were given concurrently with i.v. treatment. A total of 81% of simple cases received some form of oral antibiotic for a median of 28 days, compared with 66.3% of complex cases, also for a median of 28 days.
The total time from first antibiotic to last antibiotic was a median of 34 days in simple cases and 39 days in complex cases. Note that a small number of children had breaks in treatment; this measure included these breaks in the total.
Differences between septic arthritis and osteomyelitis
Duration of i.v. antibiotics is similar for SA and OM, both in complex as well as in simple cases. The median total antibiotic treatment time is 40 days for OM, compared with 30 days for SA, in simple cases. This difference is also present in complex cases (42 days vs. 28.5 days, respectively) (Table 32).
| Antibiotic treatment timeline | SA | OM | ||
|---|---|---|---|---|
| n (%) | Median (IQR), days | n (%) | Median (IQR), days | |
| Simple cases | N = 107 | N = 109 | ||
| Length of time on | ||||
| i.v. antibioticsa | 103 (96.3) | 7 (4–13) | 97 (89) | 9 (6–16) |
| Oral antibioticsa | 85 (79.4) | 28 (14–34) | 90 (82.6) | 30.5 (21–42) |
| Totalb | 103 (96.3) | 30 (18–41) | 105 (96.3) | 40 (21–48) |
| Complex cases | N = 35 | N = 56 | ||
| Length of time on | ||||
| i.v. antibioticsa | 33 (94.3) | 14 (7–24) | 54 (96.4) | 18.5 (8–42) |
| Oral antibioticsa | 24 (68.6) | 25.5 (14–35) | 35 (62.5) | 28 (23–42) |
| Totalb | 34 (97.1) | 28.5 (15–46) | 54 (96.4) | 42 (26–57) |
Antibiotic regimes: overall
At initiation of i.v. treatment, over half of children (both types of case combined) were given one antibiotic (57.7%).
The timing of the switch from i.v. to oral antibiotics was different in simple cases and complex cases. In simple cases, switch occurred a median of 7 days after the start of i.v. therapy, whereas in complex cases this was a median of 13.5 days. Table 33 shows the percentages of children in whom switch dates were known who switched < 1 week, 2 weeks and > 2 weeks after commencement of i.v. treatment. Figure 1 shows a profile of time-to-switch data in the form of a Kaplan–Meier plot. This clearly shows that in complex cases the switch to oral antibiotics took place later, and that the time taken for i.v. therapy to be stopped or switched to oral therapy in half of children was 9 days in simple cases, compared with 21 days in complex cases (time point at which the curve reaches 0.5 on the y-axis).
| Antibiotic regime | Simple cases (n = 218) | Complex cases (n = 95) | ||
|---|---|---|---|---|
| n (%) | Median (IQR), days | n (%) | Median (IQR), days | |
| Number of different antibiotics given at initiation of i.v. treatment | ||||
| 1 | 131 (60.1) | 53 (55.8) | ||
| ≥ 2 | 72 (33) | 38 (40) | ||
| No i.v. antibiotics given | 12 (5.5) | 3 (3.2) | ||
| Unknown | 3 (1.4) | 1 (1.1) | ||
| Timing of oral switch | ||||
| Switch date known (weeks) | 168 (77.1) | 7 (5–13) | 62 (65.3) | 13 (7–24) |
| < 1 | 73 (43.5) | 4 (3–5) | 12 (19.4) | 3.5 (2.5–5.5) |
| 1–2 | 62 (36.9) | 10 (8–13) | 21 (33.9) | 9 (8–10.5) |
| > 2 | 33 (19.6) | 21 (16–30) | 29 (46.8) | 26 (20–41) |
| No switch date | 50 (22.9) | 33 (34.7) | ||
| Child stayed on i.v. antibiotics | 35 (70) | 15 (7–30) | 29 (87.9) | 25 (11–44) |
| No i.v. antibiotics | 15 (30) | 4 (12.1) | ||
| Total course of antibiotics (weeks) | ||||
| < 6 | 139 (63.8) | 49 (51.6) | ||
| ≥ 6 | 71 (32.6) | 43 (45.3) | ||
| Had no antibiotics/unknown | 8 (3.7) | 3 (3.2) | ||
| Dose | ||||
| First dose HFa | 88 (40.4) | 28 (29.5) | ||
| HFa throughout | 30 (13.8) | 9 (9.5) | ||
| No HFa doses given | 53 (24.3) | 23 (24.2) | ||
| Combination | 124 (56.9) | 59 (62.1) | ||
| Unknown | 11 (5) | 4 (4.2) | ||
| Total number of antibiotics prescribed | ||||
| i.v. | ||||
| 0 | 15 (6.9) | 4 (4.2) | ||
| 1 | 88 (40.4) | 26 (27.4) | ||
| 2 | 81 (37.2) | 31 (32.6) | ||
| > 2 | 34 (15.6) | 34 (35.8) | ||
| Oral | ||||
| 0 | 41 (18.8) | 28 (29.5) | ||
| 1 | 129 (59.2) | 41 (43.2) | ||
| 2 | 43 (19.7) | 20 (21.1) | ||
| > 2 | 5 (2.3) | 6 (6.3) | ||
FIGURE 1.
Kaplan–Meier plot showing time to switch.

In the majority of cases with no known switch date, there was no record of any oral antibiotics having been prescribed following treatment with i.v. antibiotics.
The proportion of children who received antibiotic treatment for < 6 weeks in total was 63.8% for complex cases, compared with 51.6% for complex cases. Figure 2 shows that, among simple cases, there was little difference in time to switch to oral antibiotics between those with OM and those with SA. Among complex cases, switch timing was similar in the OM and SA subgroups when switch took place before 2 weeks, but among those who received i.v. antibiotics for at least 2 weeks, children with OM received i.v. antibiotics for longer, and were switched to oral antibiotics later, than children with SA.
FIGURE 2.
Kaplan–Meier plot showing time to switch (by SA/OM).

For each antibiotic prescribed, a daily dose per kilogram body weight was calculated. Each dose was classified as ‘higher formulary’ (recommended intensive dosing as recommended by the current bone and joint infection guidelines on the treatment of severe infections) or ‘not higher formulary’ [following standard British National Formulary for Children (BNFC) dosing guidelines for antibiotics62]. The proportion of children who received a higher formulary initial dose of antibiotics was 40.4% in simple cases, compared with 29.5% in complex cases. Most children received both higher formulary doses and not higher formulary doses during their treatment (57% for simple cases and 62% for complex cases). A total of 13.8% of simple cases received only higher formulary doses throughout their treatment, compared with 9.5% of complex cases (see Table 33).
The total number of antibiotics prescribed for each route was calculated for each child. So, for example, in the case of a child for whom there were two records of oral flucloxacillin and one record of co-amoxiclav, the total number of oral antibiotics recorded would be two. Note that this calculation is limited to instances of the antibiotics flucloxacillin, clindamycin, cefuroxime, ceftriaxone, amoxicillin, co-amoxiclav, rifampicin, vancomycin, fusidic acid, benzylpenicillin, teicoplanin, and ‘other’. All ‘other’ antibiotics were combined as one type for the sake of parsimony. In simple cases, a single i.v. antibiotic and/or a single oral antibiotic was the most common treatment strategy employed, whereas for complex cases there were cases of one, two or more than two i.v. antibiotics being used together with a single oral antibiotic type (see Table 33).
Antibiotic regimes in hospital
Tables 33 and 34 summarise the antibiotics that were prescribed during hospital stay.
| Antibiotic regime | SA | OM | ||
|---|---|---|---|---|
| n (%) | Median (IQR), days | n (%) | Median (IQR), days | |
| Simple cases | N = 107 | N = 109 | ||
| Timing of oral switch | ||||
| Switch date known (weeks) | 85 (79.4) | 6 (4–11) | 82 (75.2) | 8.5 (5–14) |
| < 1 | 47 (55.3) | 4 (3–5) | 26 (31.7) | 5 (3–5) |
| 1–2 | 26 (30.6) | 10 (8–12) | 35 (42.7) | 9.5 (8–13) |
| > 2 | 12 (14.1) | 26 (18–34) | 21 (25.6) | 18.5 (15.5–25.5) |
| No switch date | 22 (20.6) | 27 (24.8) | ||
| Child stayed on i.v. antibiotics | 18 (81.8) | 14.5 (7–30) | 15 (55.6) | 17 (7–27) |
| No i.v. antibiotics | 4 (18.2) | 12 (44.4) | ||
| Total course of antibiotics (weeks) | ||||
| < 6 | 79 (73.8) | 58 (53.2) | ||
| ≥ 6 | 24 (22.4) | 47 (43.1) | ||
| Had no antibiotics/unknown | 4 (3.7) | 4 (3.7) | ||
| Complex cases | (N = 35) | (N = 56) | ||
| Timing of oral switch | ||||
| Switch date known (weeks) | 23 (65.7) | 13.5 (7–21) | 35 (62.5) | 10.5 (7.5–26) |
| < 1 | 5 (21.7) | 2 (2–5) | 7 (20) | 4 (3–6) |
| 1–2 | 7 (30.4) | 9 (7–13) | 12 (34.3) | 8.5 (8–10.5) |
| > 2 | 11 (47.8) | 21.5 (18–47) | 16 (45.7) | 29 (23–41) |
| No switch date | 12 (34.3) | 21 (37.5) | ||
| Child stayed on i.v. antibiotics | 10 (83.3) | 38 (11–52) | 19 (90.5) | 14.5 (8–25) |
| No i.v. antibiotics | 2 (16.7) | 2 (9.5) | ||
| Total course of antibiotics (weeks) | ||||
| < 6 | 22 (62.9) | 26 (46.4) | ||
| ≥ 6 | 12 (34.3) | 28 (50) | ||
| Had no antibiotics/unknown | 1 (2.9) | 2 (3.6) | ||
Of the 199 children who received i.v. antibiotics during their hospital stay, flucloxacillin (47.2%), ceftriaxone (36.7%) and cefuroxime (33.2%) were the antibiotics most frequently prescribed. These three antibiotics were also the most commonly prescribed as part of the initial treatment (Table 35).
In 78 (35.8%) simple cases at least one oral antibiotic was prescribed. In these cases, flucloxacillin was the most commonly prescribed antibiotic (43.6%). Oral antibiotics were rarely used in conjunction with the initial i.v. treatment (see Table 33).
Average doses in mg/kg weight of child, statistics on frequency of dosing and proportion of doses prescribed for more than a week are given in Table 35.
| Antibiotic | Given at least once, n (%)a | Given as part of initialb treatment, n (%)a | Median daily dose per kg body weight (mg/kg) (IQR)c | Number of prescriptionsc given | Prescriptions of duration > 1 week,c n (%)d | ||||
|---|---|---|---|---|---|---|---|---|---|
| 6-hourly | 8-hourly | 12-hourly | Once-daily | Other or not known | |||||
| i.v. | 199e | ||||||||
| Flucloxacillin | 94 (47.2) | 85 (42.7) | 188.1 (100.8–200) | 106 | 4 | 0 | 2 | 3 | 32 (26.7) |
| Clindamycin | 22 (11.1) | 13 (6.5) | 39.9 (18.5–40.6) | 23 | 0 | 0 | 0 | 1 | 7 (28) |
| Cefuroxime | 66 (33.2) | 60 (30.2) | 141.5 (83.7–150.6) | 14 | 52 | 4 | 4 | 4 | 7 (8.4) |
| Ceftriaxone | 73 (36.7) | 51 (25.6) | 50.3 (50–79.3) | 1 | 1 | 1 | 82 | 1 | 38 (43.2) |
| Benzylpenicillin | 20 (10.1) | 16 (8) | 160 (100.3–191.2) | 17 | 4 | 1 | 1 | 0 | 5 (20.8) |
| Co-amoxiclav | 16 (8) | 13 (6.5) | 89.5 (87.2–89.95) | 0 | 16 | 0 | 0 | 1 | 2 (11.8) |
| Other | 44 (22.1) | 24 (12.1) | 36.8 (11.25–77.7) | 11 | 28 | 10 | 11 | 2 | 16 (24.2) |
| Oral | 78 | ||||||||
| Flucloxacillin | 34 (43.6) | 2 (2.6) | 52.6 (38.5–73.5) | 37 | 0 | 0 | 0 | 0 | 11 (28.9) |
| Clindamycin | 17 (21.8) | 3 (3.8) | 22.7 (20.6–37.3) | 15 | 2 | 0 | 0 | 0 | 8 (44.4) |
| Amoxicillin | 4 (5.1) | 1 (1.3) | 96.4 (40.5–167.1) | 0 | 3 | 0 | 0 | 0 | 1 (25) |
| Co-amoxiclav | 18 (23.1) | 0 | 89.5 (87.2–90.0) | 0 | 14 | 3 | 0 | 2 | 6 (31.6) |
| Rifampicin | 10 (12.8) | 3 (3.8) | 20 (14.7–20.3) | 0 | 0 | 8 | 2 | 0 | 5 (45.5) |
| Other | 18 (23.1) | 3 (3.8) | 35 (21.6–56.5) | 5 | 8 | 3 | 4 | 1 | 8 (40) |
Among the 91 children who received i.v. antibiotics during their hospital stay, the most frequently prescribed antibiotics were flucloxacillin (52.7%), ceftriaxone (40.7%), clindamycin (27.5%) and cefuroxime (24.2%). Flucloxacillin was most likely to be prescribed as part of the initial treatment (see Table 36).
Thirty-six (37.9%) complex cases were prescribed at least one oral antibiotic, most frequently clindamycin (33.3%). Oral antibiotics were not frequently used in conjunction with the initial i.v. treatment (see Table 36).
Average doses in mg/kg weight of child, statistics on frequency of dosing and proportion of doses prescribed for > 1 week are given in Table 36.
| Antibiotic | Given at least once, n (%)a | Given as part of initial treatment,b n (%)a | Median daily dose per kg body weight (IQR)c | Number of prescriptionsc given | Prescriptions of duration > 1 week,c n (%)d | ||||
|---|---|---|---|---|---|---|---|---|---|
| 6-hourly | 8-hourly | 12-hourly | Once-daily | Other or not known | |||||
| i.v. | 91e | ||||||||
| Flucloxacillin | 48 (52.7) | 38 (41.8) | 142.6 (98–198.5) | 46 | 2 | 1 | 0 | 4 | 20 (35.1) |
| Clindamycin | 25 (27.5) | 10 (11) | 33.3 (24.1–39.2) | 21 | 0 | 0 | 0 | 4 | 9 (30) |
| Cefuroxime | 22 (24.2) | 17 (18.7) | 128 (83.3–150) | 7 | 13 | 2 | 0 | 3 | 4 (14.3) |
| Ceftriaxone | 37 (40.7) | 19 (20.9) | 77.7 (49–80) | 0 | 0 | 0 | 43 | 0 | 13 (28.9) |
| Benzylpenicillin | 16 (17.6) | 10 (11) | 100 (87.3–173.2) | 11 | 1 | 0 | 1 | 3 | 7 (41.2) |
| Co-amoxiclav | 10 (11) | 10 (11) | 90 (74.2–91.5) | 0 | 11 | 0 | 0 | 0 | 3 (25) |
| Other | 30 (33) | 19 (20.9) | 30.6 (17.1–142.9) | 17 | 28 | 15 | 15 | 7 | 33 (35.5) |
| Oral | 36 | ||||||||
| Flucloxacillin | 6 (16.7) | 0 | 65.2 (42.9–88.2) | 6 | 0 | 0 | 0 | 0 | 3 (42.9) |
| Clindamycin | 12 (33.3) | 1 (2.8) | 23.8 (12–24.4) | 10 | 1 | 0 | 0 | 2 | 6 (46.2) |
| Amoxicillin | 5 (13.9) | 0 | 33.1 (3.1–52.1) | 0 | 3 | 0 | 0 | 1 | 1 (20) |
| Co-amoxiclav | 9 (25) | 1 (2.8) | 43 (31.8–49.7) | 0 | 8 | 1 | 0 | 1 | 3 (33.3) |
| Rifampicin | 4 (11.1) | 0 | 18.6 (17.8–20) | 0 | 0 | 6 | 0 | 0 | 13 (28.9) |
| Other | 13 (36.1) | 4 (11.1) | 30.2 (18.5–46.9) | 0 | 12 | 5 | 4 | 0 | 9 (39.1) |
Children who did not have intravenous antibiotics
Fifteen simple cases did not receive any i.v. antibiotics: 13 were given oral antibiotics and two had no record of receiving any antibiotic treatment either in hospital or at discharge.
Four complex cases had no i.v. antibiotics. In two of these, antibiotic data are missing and in two cases there was no record of any antibiotic treatment either in hospital or at discharge.
Table 37 gives information about the characteristics of these 19 children.
| Presentation | Simple cases (n = 15) | Complex cases (n = 4) |
|---|---|---|
| Age (years) | ||
| < 1 | 0 | 2 |
| 1–6 | 9 | 0 |
| 6–16 | 6 | 2 |
| Presenting to | ||
| GP | 6 | 3 |
| Emergency department | 9 | 1 |
| Limbs/joints affected | ||
| Hip | 3 | 1 |
| Shoulder | – | 1 |
| Knee | 3 | – |
| Ankle | 2 | 1 |
| Humerus | – | 1 |
| Femur | – | 1 |
| Foot | 1 | – |
| Tibia | 1 | – |
| Fibula | 1 | – |
| Elbow | 1 | – |
| Calcaneus | 2 | – |
| Not specified | 1 | – |
| Diagnosis | ||
| OM | 11 | 2 |
| SA | 4 | 2 |
| Given oral antibiotics | ||
| Yes | 13 | 0 |
| No | 2 | 2 |
| Missing antibiotics data | 0 | 2 |
Use of blood tests to determine switch
This section investigates which blood tests were carried out at switch from i.v. to oral antibiotics. Table 38 provides statistics for blood tests on the day of switch or the day before, for children in whom a switch date could be ascertained. When blood tests were available for both days, the tests on the day of switch were used.
| Blood testsa at switchb | Cases with test results at switch,b n (%) | Median (IQR) test results | Median (IQR) percentage of diagnosis results | Median (IQR) percentage of CRPmax | ||
|---|---|---|---|---|---|---|
| All | Tertiary | Secondary | ||||
| Simple cases | n = 161c | n = 42d | n = 56e | |||
| At least one test | 61 (37.9) | 44 | 17 | – | ||
| Haemoglobin | 48 (78.7) | 32 (72.7) | 16 (94.1) | 111 (106–120) | 99.55 (92.75–105.1) | – |
| WBC count | 47 (77) | 32 (72.7) | 15 (88.2) | 8.9 (7.47–11.3) | 70.6 (61.7–85) | – |
| Neutrophils | 48 (78.7) | 32 (72.7) | 16 (94.1) | 3.345 (2.5–4.635) | 48.95 (29.7–74.5) | – |
| Platelets | 46 (75.4) | 31 (70.5) | 15 (88.2) | 470.5 (397–576) | 126.5 (106.05–152.05) | – |
| CRP | 56 (91.8) | 40 (90.9) | 16 (94.1) | 9.15 (4.55–21.5) | 32.6 (12.5–74.1) | 25 (10.2–63.4) |
| ESR | 19 (31.1) | 10 (22.7) | 9 (52.9) | 23 (13–43) | 107.2 (46.2–200) | – |
| CRP and ESR | 17 (27.9) | 9 (20.5) | 8 (47.1) | – | – | – |
| CRP and not ESR | 39 (63.9) | 31 (70.5) | 8 (47.1) | – | – | – |
| Complex cases | n = 57c | n = 9d | n = 11e | |||
| At least one test | 13 (22.8) | 10 | 3 | – | – | – |
| Haemoglobin | 9 (69.2) | 7 (70) | 2 (66.7) | 108 (96–111) | 100 (88.1–100) | – |
| WBC count | 9 (69.2) | 7 (70) | 2 (66.7) | 8.7 (7.9–10.1) | 92.9 (46.9–100) | – |
| Neutrophils | 8 (61.5) | 6 (60) | 2 (66.7) | 2.25 (1.715–3.2) | 51.3 (23.3–80) | – |
| Platelets | 11 (84.6) | 9 (90) | 2 (66.7) | 383 (311–569) | 108.05 (102.4–177.7) | – |
| CRP | 11 (84.6) | 8 (80) | 3 (100) | 13 (4–26) | 28.8 (14.5–100) | 25 (9.1–100) |
| ESR | 4 (30.8) | 3 (30) | 1 (33.3) | 35 (15.5–63) | 94.6 (57.5–131.7) | – |
| CRP and ESR | 3 (23.1) | 2 (20) | 1 (33.3) | – | – | – |
| CRP and not ESR | 8 (61.5) | 6 (60) | 2 (66.7) | – | – | – |
Among the children with a known switch date, 37.9% of simple cases had a blood test at switch and 22.8% of complex cases had a blood test at switch. Most of these had the full range of measurements, with the exception of ESR. When blood tests were taken, CRP was measured in 91.8% of simple cases and 84.6% of complex cases.
Median and IQRs for blood tests are given in Table 38. Forty-two simple cases and nine complex cases had blood tests both at presentation and at switch. For each of these children, switch results were expressed as a percentage of diagnosis results. These percentages are summarised in Table 38. Median percentages close to 100 indicate no change on average from diagnosis. For most children’s measurements, haemoglobin stayed the same, WBC count, neutrophils and CRP dropped to greater or lesser degrees, ESR varied (halving for some and doubling for others) and platelets rose. The measurement that showed the greatest decline in median value between diagnosis and switching was CRP. CRP as a proportion of its maximal value prior to switch was a median of 25% in both simple and complex cases, although the IQRs show that children varied considerably in this. Table 39 shows a summary of blood test results at switch, split by SA/OM.
| Blood tests at switch | SA | OM | ||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Simple cases | ||||
| Haemoglobin (g/dl) | 29 | 111 (107–123) | 19 | 109 (101–117) |
| WBC count (109/l) | 28 | 8.85 (7.035–11.065) | 19 | 9.1 (7.98–12.2) |
| Neutrophils (109/l) | 29 | 3.12 (2.5–3.91) | 19 | 3.5 (2.5–5.1) |
| Platelets (109/l) | 28 | 495 (390.5–583.5) | 18 | 466 (426–563) |
| CRP (mg/l) | 33 | 12.4 (6–22) | 23 | 7 (4–16) |
| ESR (mm/hour) | 12 | 30.5 (18.5–48) | 7 | 13 (12–37) |
| Complex cases | ||||
| Haemoglobin (g/dl) | 3 | 100 (89–111) | 5 | 111 (108–111) |
| WBC count (109/l) | 3 | 4.9 (4.24–7.9) | 5 | 10.1 (9–10.36) |
| Neutrophils (109/l) | 3 | 1.7 (1.44–3.4) | 4 | 2.75 (2.25–4.645) |
| Platelets (109/l) | 3 | 312 (202–375) | 6 | 492.5 (311–569) |
| CRP (mg/l) | 4 | 24.3 (7.8–76) | 6 | 15.35 (4–25) |
| ESR (mm/hour) | 0 | – | 2 | 15.5 (8–23) |
Post-diagnosis surgery
A total of 21.6% of simple cases went on to have surgery after the start of i.v. antibiotics, compared with 41.1% of complex cases. In both groups OM and SA cases were equally represented.
The most common procedure among the simple cases was aspiration (7.3%), for complex cases it was incision and drainage (16.8%), and this was often a wash-out in conjunction with another procedure. A total of 11.6% of complex cases had at least one debridement (Table 40).
| Surgery post initiation of i.v. antibiotics | Simple cases (N = 218), n (%) | Complex cases (N = 95), n (%) |
|---|---|---|
| At least one procedure | 47 (21.6) | 39 (41.1) |
| SA | 24 | 17 |
| OM | 23 | 20 |
| Number of procedures | ||
| 1 | 19 (8.7) | 16 (16.8) |
| 2 | 21 (9.6) | 11 (11.6) |
| > 2 | 7 (3.2) | 12 (12.6) |
| Type of procedure: children with at least one | ||
| Aspiration | 16 (7.3) | 8 (8.4) |
| Incision and drainage | 14 (6.4) | 16 (16.8) |
| Drill decompression | 2 (0.9) | 0 |
| Curettage/excision | 2 (0.9) | 2 (2.1) |
| Arthrotomy | 3 (1.4) | 1 (1.1) |
| Arthroscopy | 3 (1.4) | 5 (5.3) |
| Amputation | 0 | 1 (1.1) |
| Debridement | 5 (2.3) | 11 (11.6) |
| Fasciotomy | 0 | 0 |
| Compartment decompression | ||
| Secondary closure | 2 (0.9) | 1 (1.1) |
| Skin graft | 0 | 1 (1.1) |
| Other plastic surgery | 7 (3.2) | 2 (2.1) |
| Other | 2 (0.9) | 0 |
When only one post-diagnosis procedure was carried out, this was most likely to be incision and drainage as a single procedure (carried out in 52.6% of simple cases and 43.8% of complex cases) (Table 41).
| Surgery post initiation of i.v. antibiotics | Simple cases (N = 19), n (%) | Complex cases (N = 16), n (%) |
|---|---|---|
| Type of procedure where child had just one post-i.v. surgery | ||
| Aspiration | 4 (21.1) | 3 (18.8) |
| Incision and drainage | 10 (52.6) | 7 (43.8) |
| Drill decompression | 1 (5.3) | – |
| Curettage/excision | 1 (5.3) | 1 (6.3) |
| Arthrotomy | – | 1 (6.3) |
| Arthroscopy | – | 1 (6.3) |
| Amputation | – | – |
| Debridement | 2 (10.5) | 2 (12.5) |
| Secondary closure | – | 1 (6.3) |
| Other | 1 (5.3) | – |
Post-diagnosis imaging
Imaging was carried out post diagnosis, both during hospital stay and after discharge. MRI was the most used imaging technique in hospital, followed by X-rays and ultrasonography. This was the case in both simple and complex cases.
Of those undergoing MRI, a high proportion had abnormalities detected both prior to and post discharge (Table 42).
| Imaging type | Cases imaged at least once post diagnosis, n (%) | Of those imaged, those with at least one abnormal result, n (%) | ||
|---|---|---|---|---|
| Prior to discharge | Post discharge | Prior to discharge | Post discharge | |
| Simple cases (N = 215) | ||||
| X-ray | 28 (12.8) | 45 (20.6) | 14 (50) | 18 (40) |
| CT | 3 (1.4) | 0 | 2 (66.7) | – |
| Ultrasonography | 16 (7.3) | 6 (2.8) | 11 (68.8) | 5 (83.3) |
| MRI | 49 (22.5) | 14 (6.4) | 42 (85.7) | 13 (92.9) |
| Bone scan | 5 (2.3) | 1 (0.5) | 3 (60) | 1 (100) |
| Complex cases (N = 92) | ||||
| X-ray | 24 (25.3) | 28 (29.5) | 15 (62.5) | 14 (50) |
| CT | 3 (3.2) | 1 (1.1) | 3 (100) | 0 |
| Ultrasonography | 21 (22.1) | 2 (2.1) | 18 (85.7) | 1 (50) |
| MRI | 36 (37.9) | 10 (10.5) | 35 (97.2) | 9 (90) |
| Bone scan | 2 (2.1) | 0 | 2 (100) | – |
Length of hospital stay
The median length of stay in hospital was 7 days, with 50% of children staying for ≥ 6 days but no longer than 12 days. Children who presented at a tertiary centre stayed in hospital, on average, 3 days less than those presenting to a secondary centre. Older children (aged > 6 years) had longer stays in hospital than younger children, with a median stay of 10 days (Table 43).
| Presenting Centre Type | n | Age group, median (IQR) (days) | |||
|---|---|---|---|---|---|
| All | < 1 year | 1–6 years | 6–16 years | ||
| Simple cases | N = 27 | N = 116a | N = 74 | ||
| All | 217a | 7 (4–12) | 7 (5–12) | 6 (3.5–10.5) | 10 (6–15) |
| Tertiary | 115 | 7 (4–11) | 7 (5–10) | 6 (3–9) | 8.5 (5–13) |
| Secondary | 102 | 9 (4–14) | 9.5 (5–19.5) | 7 (4–12.5) | 10.5 (7–17) |
| Complex cases | N = 13 | N = 44 | N = 38 | ||
| All | 95 | 9 (6–18) | 16 (9–27) | 8 (5–14) | 10 (6–16) |
| Tertiary | 54 | 9 (7–19) | 16 (9–29) | 8 (6–19) | 12 (7–16) |
| Secondary | 41 | 8 (6–14) | 18 (8–27) | 6 (3–12) | 8.5 (6–15.5) |
The median length of stay in hospital was 9 days, with 50% of children staying for ≥ 6 days but no longer than 18 days. Children who presented at a tertiary centre stayed in hospital, on average, 1 day longer than those presenting to a secondary centre. The children who had the longest stays were aged < 1 year, staying in hospital, on average, for > 2 weeks.
Patients with simple SA tend to have a shorter hospital stay than the simple OM group (median 6 days vs. 9 days, respectively). In the complex group this difference is less clear (median 8 days vs. 9 days, respectively) (Table 44).
| Length of stay (days) | All | Diagnosisa | |
|---|---|---|---|
| SA | OM | ||
| Simple cases | n = 217b | n = 106 | n = 109 |
| Median (IQR) | 7 (4–12) | 6 (4–11) | 9 (5–13) |
| Complex cases | n = 95 | n = 35 | n = 56 |
| Median (IQR) | 9 (6–18) | 8 (6–15) | 9 (6–18.5) |
Discharge antibiotics
A large proportion of children were known to have gone home on i.v. antibiotics (27.1% of simple cases and 42.1% of complex cases). The proportion of children on i.v. treatment at discharge was not different in the SA and OM subgroups (Table 45). Among these children, the most frequently prescribed i.v. antibiotic was ceftriaxone (69.5% for simple cases and 77.5% for complex cases) (Table 46 gives further details, including a summary of the dose in mg per kg body weight and a median prescribed duration for each antibiotic).
| Antibiotics at discharge | All, n (%) | Diagnosis, n (%) | |
|---|---|---|---|
| SA | OM | ||
| Simple cases | N = 218 | N = 107 | N = 109 |
| Went home on | |||
| i.v. antibioticsa | 59 (27.1) | 30 (28) | 28 (25.7) |
| Oral alone | 130 (59.6) | 64 (59.8) | 65 (59.6) |
| None/unknown | 29 (13.3) | 13 (12.1) | 16 (14.7) |
| Complex cases | N = 95 | N = 35 | N = 56 |
| Went home on | |||
| i.v. antibioticsa | 40 (42.1) | 16 (45.7) | 23 (41.1) |
| Oral alone | 42 (44.2) | 15 (42.9) | 24 (42.9) |
| None/unknown | 13 (13.7) | 4 (11.4) | 9 (16.1) |
| Antibiotica | n (%) | Median (IQR) daily dose per kg weight of child | Median (IQR) days planned duration |
|---|---|---|---|
| Simple cases (N = 218) | |||
| i.v. antibiotics (N = 59) | |||
| Ceftriaxone | 41 (69.5) | 50.7 (49.3–79.4) | 7 (3–14) |
| Flucloxacillin | 4 (6.8) | 98.05 (43.5–155.7) | 28 (14–42) |
| Clindamycin | 2 (3.4) | 21.6 (5.3–37.9) | 21 (14–28) |
| Cefuroxime | 1 (1.7) | 46.7 (46.7–46.7) | 14 (14–14) |
| Other | 9 (15.3) | 9.7 (9.7–46.7) | 17 (8–42) |
| Unknown | 6 (10) | – | – |
| Oral antibiotics (N = 156)b | |||
| Co-amoxiclav | 58 (36.5) | 30.4 (13.8–41.8) | 28 (21–31) |
| Flucloxacillin | 46 (28.9) | 37.45 (14.15–82.3) | 28 (14–38.5) |
| Clindamycin | 17 (10.7) | 18.1 (6.1–23.6) | 34 (25–42) |
| Cefalexin | 20 (12.6) | 35 (24.5–91.1) | 26.5 (16.5–35) |
| Other | 62 (39) | 20.55 (9.3–40.5) | 28 (14–42) |
| Complex cases (N = 95) | |||
| i.v. antibiotics (N = 40) | |||
| Ceftriaxone | 31 (77.5) | 65.35 (49–80.3) | 14 (10–33) |
| Flucloxacillin | 2 (5) | 35.8 (0.2–71.4) | 31.5 (14–49) |
| Clindamycin | 3 (7.5) | 4 (0.2–8) | 28 (13–42) |
| Cefuroxime | 0 | – | – |
| Other | 5 (12.5) | 12 (10.2–55.3) | 42 (42–42) |
| Unknown | 4 (10) | – | – |
| Oral antibiotics (N = 58)b | |||
| Co-amoxiclav | 17 (28.8) | 23.1 (12.1–47.6) | 28 (14–39) |
| Flucloxacillin | 15 (25.4) | 41.7 (36.4–54.1) | 28 (21–42) |
| Clindamycin | 17 (28.8) | 15.1 (6–23.8) | 28 (14–38.5) |
| Cefalexin | 2 (3.4) | 62 (28.8–95.2) | 14 (7–21) |
| Other | 23 (39) | 18.7 (6.9–28.7) | 24.5 (14–42) |
Oral antibiotics were quite varied. The key antibiotics for both case types were co-amoxiclav, flucloxacillin, clindamycin and cefalexin (see Table 46).
Follow-up
Adherence to discharge antibiotics
Almost three-quarters of children were followed up (71.6% of simple cases and 80.4% of complex cases, no difference between SA and OM). Of these, almost all reported that antibiotics were completed as planned (92.4% of simple cases and 92.1% of complex cases) (Tables 47 and 48 give further details of the reasons for non-adherence in the nine children who did not complete the course of prescribed antibiotics).
| Adherence to prescribed antibiotic regimes | Simple cases (N = 218), n (%) | Complex cases (N = 95), n (%) |
|---|---|---|
| Number of children contacted for follow-up | 157 (72) | 76 (80) |
| SA | 78 (72.9) | 27 (77.1) |
| OM | 79 (72.5) | 45 (80.4) |
| Antibiotics completed as planned | ||
| Yes | 145 (92.4) | 70 (92.1) |
| No | 8a (5.1) | 3a (3.9) |
| Stopped early | 2 | 1 |
| Changed | 5 | 3 |
| Unknown | 2 | 0 |
| Stopped early by | ||
| Parent | 1 | 0 |
| Hospital | 0 | 1 |
| GP | 1 | 0 |
| Changed by | ||
| Hospital | 5 | 3 |
| GP | 0 | 0 |
| Reason for shortening/change of regime | ||
| Unpalatable antibiotic | 1 | 0 |
| Diarrhoea | 0 | 1 |
| Rash | 1 | 0 |
| Resolution of symptoms | 1 | 0 |
| Recurrence of infection | 2 | 1 |
| Otherb | 2 | 2 |
| Simple cases (n = 2) | Complex cases (n = 2) |
|---|---|
| Patient refused to take medication | Parent refusal for ongoing i.v. treatment |
| PICC line stopped working so swapped to oral | Hickman line infection |
Complications
A total of 8.3% of simple cases and 16.8% of complex cases experienced at least one complication. Table 49 lists the numbers of types of complication by action taken. The types listed were options given for data entry; as Table 49 shows, many were not experienced (dislocation, subluxation, pathological complication, fracture and sinus). The most selected category was ‘other’: text entered to give more detail about these complications is given in Tables 50 and 51.
| Complication | n (%) | Action taken, n | ||||
|---|---|---|---|---|---|---|
| None | Surgery | Change of antibiotic regime | Re-admission to hospital | Other actiona | ||
| Simple cases (N = 218) | ||||||
| At least one complication | 18 (8.3) | |||||
| SA | 8/107 | |||||
| OM | 9/109 | |||||
| Complication type | ||||||
| Fever | 1 | – | – | – | 1 | – |
| Recurrence of infection | 3 | – | 1 | 1 | 2 | 1 |
| Dislocation | 0 | – | – | – | – | – |
| Subluxation | 0 | – | – | – | – | – |
| Pathological | 0 | – | – | – | – | – |
| Fracture | 0 | – | – | – | – | – |
| Sinus | 0 | – | – | – | – | – |
| Other (N = 16)b | 16 | 3 | 2 | 4 | 1 | 7 |
| Complex cases (N = 95) | ||||||
| At least one complication | 16 (16.8) | |||||
| SA | 8/35 | |||||
| OM | 7/56 | |||||
| Complication type | ||||||
| Fever | 0 | – | – | – | – | – |
| Recurrence of infection | 4 | – | 3 | 2 | 1 | 1 |
| Dislocation | 0 | – | – | – | – | – |
| Subluxation | 0 | – | – | – | – | – |
| Pathological | 0 | – | – | – | – | – |
| Fracture | 0 | – | – | – | – | – |
| Sinus | 0 | – | – | – | – | – |
| Other (N = 13)b | 19 | 0 | 1 | 4 | 2 | 12 |
| Simple cases (n = 16)a | Complex cases (n = 13)a |
|---|---|
| Possible infectious endocarditis | Acute kidney Injury |
| Allergic reaction – rash | Blocked PICC line, replaced under general anaesthesia |
| Post-traumatic arthritis ankle | Cardiac tamponade |
| Possible development of Brodie abscess | Conjunctivitis and dermatitis |
| Coccydynia pain around scar | Fever, abdominal pain and diarrhoea |
| Diagnosis inconclusive. Patient underwent drilling and washout of right femur | Forearm rotation reduced |
| Diarrhoea | Hickman line sepsis |
| Femoral vein thrombus | Long line blocked |
| Increased swelling of knee | Long line leaking |
| Patient experiencing increased pain of the left knee | Major haemorrhage post renal biopsy |
| Phlebitis | Needs insoles to stop foot going into varus position as a result of removal of navicular |
| Reaction to teicoplanin | Numbness to toes |
| Self-limiting pain episodes | Pain, night sweats |
| Stiffness | Pneumococcal meningitis |
| Urticarial rash on lower abdomen, upper back, left side of back, behind both knees, right wrist and antecubital fossa and right leg around patella region | Post-operation complications: S. aureus sepsis/OM |
| Worsening pain in sacrum | Respiratory distress |
| RSV bronchiolitis | |
| Skin rash | |
| Tracheostomy |
| Simple cases (n = 8)a | Complex cases (n = 13)a |
|---|---|
| Blood cultures, electrocardiography | 48 hours high-frequency oscillation ventilation then conventional ventilation |
| Blood tests and ultrasonography | Embolisation of left kidney |
| Cold press | Long line removed |
| Enoxaparin on haematology advice | On haemofiltration |
| Follow-up, ankle fusion required in medium term | Pericardial drain in situ |
| Hand therapist mobilisation | Physiotherapy |
| Bloods and MRI, referral to tertiary hospital | Podiatry: insoles |
| PICC line removed and replaced under general anaesthesia | |
| Repeat MRI | |
| Not specified (n = 4) |
Ongoing symptoms
Of the children who were followed up, 27 (17.2%) simple cases had at least one ongoing symptom; 18 of these were still in pain and other symptoms were less common. Eighteen (24%) complex cases had at least one ongoing symptom. Types of symptoms were more diverse in this group: eight children were still in pain, eight had joint stiffness and five had a deformity (Table 52).
| Symptom | Simple cases (N = 157), n (%) | Complex cases (N = 76), n (%) |
|---|---|---|
| At least one ongoing symptom at follow-up | 27 (17.2) | 18 (23.7) |
| SA | 9/78 | 6/27 |
| OM | 18/79 | 10/45 |
| Types of symptom | ||
| Pain | 18 (11.5) | 8 (10.5) |
| Fever > 38 °C | 3 (1.9) | 2 (2.6) |
| Joint stiffness | 6 (3.8) | 8 (10.5) |
| Immobility | 4 (2.5) | 3 (3.9) |
| Fracture | 0 | 0 |
| Sinus | 2 (1.3) | 0 |
| Deformity | 2 (1.3) | 5 (6.6) |
Treatment failure
Treatment failure is defined as any of the following complications or ongoing symptoms at follow-up:
-
fever
-
recurrence of infection
-
dislocation
-
subluxation
-
pathological fracture
-
development of a draining sinus
-
other (pain, stiffness, mobility problems, swelling, numbness, increase in CRP compared with discharge).
And/or any of the following actions arising from a complication:
-
readmission to hospital
-
surgery
-
change of antibiotics.
Under this definition, 32 (14.7%) simple cases experienced a treatment failure, compared with 24 (25%) complex cases (see Tables 53–56 for associations between treatment failure and diagnosis, switch timing, length of time on antibiotics and surgery during hospital stay). Observed associations do not necessarily imply causal links that explain likelihood of treatment failure.
Diagnosis group
Among simple cases, treatment failure was half as likely in children with SA as in children with OM (9.3% vs. 19.3%, respectively), whereas among complex cases treatment failure was more likely in children with SA than in those with OM (28.6% vs. 21.4%, respectively) (Table 53).
| Treatment failure | All, n (%) | Diagnosis, n (%) | |
|---|---|---|---|
| SA | OM | ||
| Simple cases | n = 218 | n = 107 | n = 109 |
| Treatment failed | 32 (14.7) | 10 (9.3) | 21 (19.3) |
| No treatment failure | 186 (85.3) | 97 (90.7) | 88 (80.7) |
| Odds ratioa | – | 0.43 | 1.00 |
| Complex cases | n = 95 | n = 35 | n = 56 |
| Treatment failed | 24 (25.3) | 10 (28.6) | 12 (21.4) |
| No treatment failure | 71 (74.7) | 25 (71.4) | 44 (78.6) |
| Odds ratioa | – | 1.47 | 1.00 |
Switch timing
In simple cases, treatment failure was less likely the earlier switch occurred. Of the 73 simple cases in whom oral switch was instituted very early (< 1 week), seven (9.6%) experienced a treatment failure, compared with 10/62 (16.1%) of those in whom oral switch was early (between 1 and 2 weeks) and 6/33 (18.2%) of those in whom switch was standard (after 2 weeks of i.v. antibiotics). The risk of treatment failure can also be presented as odds ratios, which show that the risk of treatment failure following very early switch is half that of associated with switch after 2 weeks. Among complex cases, the risk of complications was highest in those in whom oral switch occurred after 2 weeks, at 37.9%, and lower in those undergoing switch very early (25%) or after 1–2 weeks of i.v. antibiotics (19%) (Table 54).
| Treatment failure | All, n (%) | Switch, n (%) | |||
|---|---|---|---|---|---|
| < 1 week | 1–2 weeks | > 2 weeks | No switch/unknown | ||
| Simple cases (N = 218) | |||||
| Treatment failed | 32 (14.7) | 7 (9.6) | 10 (16.1) | 6 (18.2) | 9 (18) |
| No treatment failure | 186 (85.3) | 66 (90.4) | 52 (83.9) | 27 (81.8) | 41 (82) |
| Odds ratioa | – | 0.48 | 0.87 | 1.00 | – |
| Complex cases (N = 95) | |||||
| Treatment failed | 24 (25.3) | 3 (25) | 4 (19) | 11 (37.9) | 6 (18.2) |
| No treatment failure | 71 (74.7) | 9 (75) | 17 (81) | 18 (62.1) | 27 (81.8) |
| Odds ratioa | – | 0.55 | 0.39 | 1.00 | – |
Length of time on antibiotics
In simple cases, treatment failure was equally likely for children who had antibiotics for < 6 weeks as for those who had courses for > 6 weeks. Among complex cases, however, children given antibiotics for < 6 weeks were much less likely to experience treatment failure than those given antibiotics for > 6 weeks (14.3% vs. 37%, respectively) (Table 55).
| Treatment failure | All, n (%) | Total course of antibiotics, n (%) | ||
|---|---|---|---|---|
| < 6 weeks | ≥ 6 weeks | Unknown | ||
| Simple cases (N = 218) | ||||
| Treatment failed | 32 (14.7) | 20 (14.4) | 10 (14.1) | 2 (25) |
| No treatment failure | 186 (85.3) | 119 (85.6) | 61 (85.9) | 6 (75) |
| Odds ratioa | – | 1.03 | 1.00 | – |
| Complex cases (N = 95) | ||||
| Treatment failed | 24 (25.3) | 7 (14.3) | 16 (37.2) | 1 (33.3) |
| No treatment failure | 71 (74.7) | 42 (85.7) | 27 (62.8) | 2 (66.7) |
| Odds ratioa | – | 0.28 | 1.00 | – |
Surgery during hospital stay
Table 56 shows the numbers of children who experienced treatment failure grouped according to whether or not surgery was carried out during their hospital stay. There was no indication of an association between treatment failure and surgery in either simple or complex cases.
| Treatment failure | All, n (%) | Had any surgery prior to discharge? n (%) | ||
|---|---|---|---|---|
| Yes | No | Unknown | ||
| Simple cases (N = 218) | ||||
| Treatment failed | 32 (14.7) | 15 (15.6) | 17 (14.5) | 0 |
| No treatment failure | 186 (85.3) | 81 (84.4) | 100 (85.5) | 5 (100) |
| Odds ratioa | – | 1.09 | 1.00 | – |
| Complex cases (N = 95) | ||||
| Treatment failed | 24 (25.3) | 16 (28.1) | 8 (22.2) | 0 (0) |
| No treatment failure | 71 (74.7) | 41 (71.9) | 28 (77.8) | 2 (100) |
| Odds ratioa | – | 1.37 | 1.00 | – |
Discussion
This multicentre national UK service evaluation has defined the current case load, disease spectrum and clinical practice in the diagnosis and treatment of OM/SA in secondary and tertiary UK care to provide information regarding the feasibility of a future RCT design.
Number of cases who may be offered participation in a future randomised controlled trial
Any future RCT will need to enrol cases defined as simple, that is, in which there is no underlying chronic disease, no orthopaedic implants and no history of recent (within 6 months) orthopaedic surgery. Overall, 313 cases had OM and/or SA. Of these, 109 had simple OM and 107 had simple SA. Two cases were defined as having simple disease but both or undifferentiated OM or SA. The monthly accrual was 0–3 simple cases per month in tertiary centres and 0–0.7 per month in secondary centres. The overall sample characteristics were as expected from previous literature. Of the 313 children, 55.3% were male, 12.8% were aged < 1 year, 51.4% were between their 1st and 6th birthday, and 35.8% were aged 6–16 years. The joints/limbs most frequently affected in simple disease were the hip (21.1%), the knee (24.8%) and the ankle (13.3%). Complex cases showed similar proportions of joints/bones affected (23.2%, 20% and 17.9%, respectively), although often with multiple rather than single joint/bone infections. SA was more common in the hips and knees than in other joints in both simple and complex cases, whereas OM affected a much larger range of joints/limbs.
In considering the health-care environment to which children first presented, most attended emergency departments (65% of simple cases and 70% of complex cases). Only one-third presented to primary care (28% simple and 14.7% complex) and very few to hospital outpatient clinics (3.7% of simple cases, 5.3% of complex cases).
Although not likely to impact on RCT choices, it was interesting to note that, of simple cases, nearly 20% were described as having had a severe infection in the 12 months preceding the presentation, 13.3% had a history of trauma and 18.8% had at least one antibiotic in the month before presentation.
The diagnosis of osteomyelitis and septic arthritis in children
As described in Chapter 1, making a diagnosis of bone or joint infection in children involves history, clinical examination and non-specific and specific investigations. Our data suggested inconsistency between treating UK centres that appears unrelated to whether the patients presented to, or were treated by, secondary or tertiary centres.
Blood tests
In order to inform diagnostic decisions, we organised our data collection to record the details of blood tests both at presentation and on the day of or the day preceding the diagnosis. It was perhaps surprising that, in our cohort, only 105 (48.2%) of the children with simple disease were recorded as having blood tests taken at presentation (see Table 14). The fact that these data also show only 33.7% of complex case children to have had a blood test at presentation is surprising, as this figure might have been expected to be higher than for simple cases. At first glance this suggests that our database question may not have been worded with enough clarity for the research staff to extract the correct information from the clinical records, or that not all blood tests carried out were recorded. However, we also collected information on the blood tests performed on the day of or the day immediately preceding the initiation of i.v. antibiotics (see Table 19), the time point at which, for most cases, there is an assumption that the diagnosis was made. It is also likely that clinicians performed blood tests at any point from presentation (with or without definitive symptoms suggestive of the diagnosis) or at any point up to when the diagnosis was actually made. As clinicians try to avoid repeated blood tests in children unless there is clinical change or clinical uncertainty, it is probable that the blood test data are valid but that in some cases only the results of blood tests carried out at presentation are available, whereas, in others, only the results of blood tests just before initiation of antibiotics (i.e. just before diagnosis) are available and in yet other cases results at both time points are available.
We analysed whether WBC/neutrophil counts, CRP and ESR were measured at presentation or before diagnosis as well as the values of these parameters to inform decisions regarding potential entry to a clinical trial (i.e. the usefulness in making the diagnosis of bone and/or joint infection). We also tried to establish at which point the CRP reached the maximum (CRPmax) recorded value to inform both diagnostic and subsequent monitoring decisions. These data need to be interpreted in light of the fact that the CRPmax recorded may have occurred before or after the initiation of antibiotics.
Of the currently available and routinely requested non-specific blood test biomarkers, the diagnosis of OM or SA was usually based on the CRP measurement alone (although a low CRP does not exclude OM or SA). At presentation, CRP was higher in complex disease than simple disease (simple cases: median 32 mg/l, IQR 11.8–66.7 mg/l; complex cases: median 54 mg/l, IQR 24–141 mg/l), which was not true for ESR (simple cases: median 32 mm/hour, IQR 20.5–70 mm/hour; complex cases: median 28 mm/hour, IQR 15–55 mm/hour) (see Table 15). At this time point, neither CRP nor ESR was able to distinguish between SA and OM (CRP: SA median 32 mg/l, IQR 16.2–65 mg/l; OM median 32.7 mg/l, IQR 10–68 mg/l; ESR: SA median 29 mm/hour, IQR 14–70.5 mm/hour; OM median 35.5 mm/hour, IQR 26–68.5 mm/hour) (see Table 16). As expected, the number of ESR results available for analysis was only around half of the number of CRP results (an ESR result was available in 56/218 simple cases and 9/95 complex cases, whereas a CRP measurement was available in 99/218 simple cases and 31/95 complex case, (see Table 15). This was probably due to both the perceived lack of need (many paediatric clinicians are less likely to request ESR in current practice) and the related fact that ESR is a technically harder test to perform than CRP because of the blood volume required and laboratory processes. A similar analysis is available for the blood tests performed on the day of or preceding the day of diagnosis. At this time point, CRP was also higher in complex disease than in simple disease (simple cases: median 38.3 mg/l, IQR 20–74 mg/l; complex cases: median 86.7 mg/l, IQR 29–161 mg/l), which was, again, not so for ESR (simple cases: median 46 mm/hour, IQR 25–78 mm/hour; complex cases: median 40 mm/hour, IQR 21–60) mm/hour) (see Table 20). Neither CRP nor ESR was able to distinguish between SA and OM (CRP: SA median 35.5 mg/l, IQR 21.2–72 mg/l; OM median 39.5 mg/l, IQR 14–81 mg/l; ESR: SA median 46 mm/hour, IQR 23–81 mm/hour; OM median 47 mm/hour, IQR 26–76 mm/hour) (see Table 21). Again, the number of ESR results available for analysis was half the number of CRP results (an ESR result was available in 83/218 simple cases and 17/95 complex cases, whereas a CRP result was available in 137/218 simple cases and 48/95 complex cases) (see Table 20).
Overall, CRP reached its highest recorded value at the start of the illness in simple disease and a few days after presentation in complex disease [in simple disease CRPmax occurred on median day 0 (IQR day 0–6) and in complex disease on median day 4 (IQR day 1–8)] (see Table 17).
The total WBC or specific neutrophil counts at both presentation or just prior to initiation antibiotics were not shown to be of clinical use in determining whether to suspect or confirm SA or OM. Although the normal ranges of total WBC and neutrophil counts vary at different ages, there was overlap for both parameters between the normal range and WBC/neutrophil values recorded for children with simple or complex OM and SA (see Tables 15, 16, 20 and 21). As for many other serious infections in children, a raised WBC and/or neutrophil count can indicate bacterial infection, but normal results do not preclude such a diagnosis and must not be used to rule out OM or SA.
Imaging
The use of imaging performed prior to i.v. antibiotics (prior to diagnosis of bone or joint infection) was determined. Overall, a radiological diagnosis was obtained for 51.8% of simple cases (at a median of 1 day post presentation, IQR 0–3) and 52.6% of complex cases (median of 1 day post presentation, IQR 0–4 days) (see Table 17), although, as can be seen from the detailed discussion in the next paragraph, these data may be skewed by soft-tissue abnormalities reported in formal X-ray reports. Regardless of the caution needed in interpreting this result overall, MRI has been shown to offer very high pick-up rates of abnormalities compared with X-ray or ultrasonography, and has almost completely replaced technetium bone scanning in current UK practice (see Tables 25 and 26). In detail, X-rays were commonly used (66% of simple cases of OM or SA combined, 62% of complex cases), ultrasonography was used in 41% of simple cases and 31% of complex cases and MRI was used in 19% of simple and 21% of complex cases. CT and technetium bone scanning were rarely used (1% of children with simple or complex disease underwent CT, whereas 2% of children with simple disease but none with complex disease underwent bone scanning) (see Table 25).
X-ray and ultrasonography were used with equal frequency in simple cases of OM and SA (see Table 26). Around two-thirds of children with simple or complex SA and OM underwent radiography before initiation of i.v. antibiotics (see Table 18). Of the children with simple SA, 66 (63%) had an X-ray, of which 24 (36%) were abnormal. Of the children with simple OM, 66 (68%) had an X-ray, of which 26 (39%) were reported as abnormal. Among those with complex disease, X-rays taken prior to antibiotics were abnormal in 8 of 21 (38%) of those with SA, compared with 18 of the 33 (55%) of those with OM. Because of the nature of the data collection in the study, these data must be interpreted with caution as it is likely that abnormalities recorded in the database included soft tissue changes visible (and reported by radiologists) and not just bony abnormalities that would not be expected to be present early in the OM disease process. Ultrasonography was carried out in 53 (51%) simple SA cases and was reported abnormal to be abnormal in 43 of these (81%). Eleven children with complex SA underwent ultrasonography (33% cases), and was reported to be abnormal in nine (82%). In complex OM there were 16 scans (30% cases), of which 11 (69%) were reported as abnormal.
Magnetic resonance imaging was used more often in children with OM cases than in those with SA and picked up a large number of abnormalities (see Table 26). Twenty-nine (30%) children with simple OM underwent MRI, in 26 (90%) of whom the results were reported as abnormal, and 16 (30%) children with complex OM underwent MRI, in all of whom the results were reported to be abnormal. MRI was also very successful in the diagnosis of SA. MRI was carried out in nine (9%) children with simple SA, with abnormal results reported in eight (89%), and in four (12%) children with complex SA. with abnormal results reported in three (75%).
Among children with OM, whether simple or complex, MRI revealed abnormalities in many different bones, whereas in children with SA abnormalities on MIR were seen only in large joints (see Table 24). The ability of MRI to detect a wide range of abnormalities explains the low frequency with which technetium bone scanning was performed over the course of the evaluation period. Bone scanning was performed in no children with complex SA or OM, in only one child with simple SA (in whom the result was abnormal) and only in three children (3%) with simple OM, of whom two (67%) showed an abnormality. CT was not used at all in children with SA, and in only three children (3%) with simple OM (100% abnormal) and one child (2%) with complex OM, in whom the results were was also abnormal (see Table 26).
Surgery
Surgery is carried out before starting i.v. antibiotics in children with clinically severe SA or OM either to prevent destructive outcomes or to obtain surgical specimens to aid a microbiological diagnosis, or where there is diagnostic dilemma.
Diagnostic surgery prior to i.v. antibiotic therapy was carried out in a higher proportion of SA cases than of OM cases [39/107 (36.4%) simple and 15/35 (42.9%) complex cases of SA, compared with 21/109 (19.3%) simple and 14/56 (25%) complex cases of OM]. On average, surgery was carried out earlier in children with SA than in those with OM. Among those with SA, the first surgery was carried out a median of 1 day after presentation, with 75% of first surgical procedures being carried by day 2 in simple cases and by day 5 in complex cases. Among children with OM, the first surgery was carried out a median of 1 day after presentation in simple cases, with 75% of first surgical procedures performed by day 10, and a median of 3 days after presentation in complex cases, with 75% of first surgical procedures performed by day 8 (see Table 18).
The types of surgery most often carried out before i.v. antibiotics were started were, in simple cases (both OM and SA), ‘incision and drainage’ (performed in 60.7%) and ‘aspiration’ (in 55.7%), followed by arthroscopy (9.8%), and, in complex cases, incision and drainage (62.1%), then aspiration (24.1%) and, third, debridement (17.2%) (see Table 22).
Surgery was carried out more often in children with OM than in those with SA (63.6% vs. 37.5%, respectively, in simple cases and 25.9% and 15.5%, respectively, in complex cases). From discussion at the investigator meeting (see Chapter 5), there remains the possibility that, although the database has been populated directly from terms used in the clinical records, there may be overlap between the different technical terms used by orthopaedic surgeons for ‘incision and drainage’ and ‘aspiration’. At database design we had intended ‘aspiration’ to record needle aspiration (expected to be mainly of infected joints in suspected SA) and ‘incision and drainage’ to represent a more formal, open surgical procedure (expected to be carried out in cases of both OM and SA). We found that incision and drainage was performed with approximately equal frequency in both groups (simple SA 25%, simple OM 33%, complex SA 13.4%, complex OM 9.3%) but there were also no reported differences in aspiration rates between SA and OM (simple cases SA 23.1%, OM 30.3%; complex cases SA 5.2%, OM 3.7%).
Surgical treatment after the initiation of antibiotics
A total of 21.6% of children with simple disease went on to have surgery after the start of i.v. antibiotics, compared with 41.1% of children with complex disease. In both groups, OM and SA cases were equally represented (see Table 40).
The most common procedure carried out in simple cases was aspiration (7.3%); in complex cases it was incision and drainage (16.8%), and this was often a wash-out in conjunction with another procedure. In 11.6% of all complex cases, debridement was performed at least once (see Table 40).
Conventional microbiological tests
As for any invasive infections in children or adults, clinical best practice in OM and SA is to take samples for microbiological diagnosis prior to starting empirical antibiotics. The aim of tests is to make a microbiological diagnosis in order to determine further, more specific antibiotic therapy and, in the case of infection with some specific pathogens such as PVL-positive Staphylococcus, to consider more complex or prolonged treatment regimens. Routinely available NHS microbiological tests are reported here, with a summary of the molecular data from a new targeted PCR test reported in Chapter 3.
In only 144 out of 218 (66.1%) children with simple OM or SA, and 59 out of 95 (62.1%) children with complex OM or SA, was at least one diagnostic test performed on blood or tissue samples (bone, joint aspirate or periosteal pus) (see Table 27). Blood culture was performed in only 62% of simple cases and 54.3% of complex cases. Blood culture reporting rates did not differ between secondary and tertiary centres (see Table 30).
A blood, tissue or synovial fluid sample tested positive for at least one micro-organism in 38.2% of children with simple disease, compared with 52.5% of complex cases. Among those children with OM, microbiology tests were carried out in 58%, and at least one micro-organism was detected in 49% of cases tested. The proportion of children with SA tested was 73%, and a micro-organism was found in 36% (see Table 27).
S. aureus was found in 20.1% of blood, tissue or synovial fluid samples taken from simple cases and in 32.2% of samples from complex cases; other pathogens were far less commonly detected by routinely available tests (see Table 27). PVL-positive S. aureus and MRSA were detected with similar frequency in simple and complex cases regardless of sample type [PVL-positive S. aureus: 2/29 (6.9%) simple cases and 1/19 (5.3%) complex cases; MRSA: 1/29 (3.4%) simple cases and 1/19 (5.3%) complex cases]. In only one case (complex) was erythromycin-resistant S. aureus reported (see Table 27). GAS, K. kingae and GBS were not commonly detected by conventional microbiology (see Table 27).
Medical treatment: intravenous course length and time before oral switch
All children in the study spent time in hospital. Most children with SA or OM went straight to hospital [median time from presentation to hospital referral 0 days (IQR 0–3 days) in simple cases and 1 day (IQR 0–4 days) in complex cases] (see Tables 17 and 18).
Not all children received i.v. antibiotics, although most did [104/107 (97.2%) simple SA cases, 33/95 (94.3%) complex SA cases, 97/109 (97%) simple OM cases and 54/56 (96.4%) complex OM cases]. In 15 (6.9%) simple cases and four (4.2%) complex cases, either no i.v. antibiotic treatment was given or the data are missing (see Tables 17 and 18). Among those who were treated with i.v. antibiotics, treatment was initiated, on average, a little earlier in children with SA [simple cases, median 1 day (IQR 0–3 days); complex cases, median 2 days (IQR 0–4 days)] than in those with OM [simple cases, median 2 days (IQR 1–5 days); complex cases, median 2 days (IQR 1–5 days)] (see Table 18).
Although there was no difference in overall duration of antibiotic treatment between simple and complex SA cases or between simple and complex OM cases, duration of i.v. therapy was longer in children with complex disease, whether SA or OM, than in those with simple disease (see Table 32):
-
Among the 103 children with simple SA treated with antibiotics, the median duration of total treatment was 30 (IQR 18–41) days, with a median duration of i.v. therapy of 7 (IQR 4–13) days.
-
Among the 34 children with complex SA treated with antibiotics, the median duration of total treatment was 28.5 (IQR 15–46) days, with a median duration of i.v. therapy of 14 (IQR 7–24) days.
-
Among the 105 children with simple OM treated with antibiotics, the median duration of total treatment was 40 (IQR 21–48) days, with a median duration of i.v. therapy of 9 (IQR 6–16) days.
-
Among the 54 children with complex OM treated with antibiotics, the median duration of total treatment was 42 (IQR 26–57) days, with a median duration of i.v. therapy of 18.5 (IQR 8–42) days.
These data suggest that in any potential clinical trial of antibiotics in simple OM or SA, although the duration of total may need to differ between OM and SA, it may be possible to plan for a similar length of initial i.v. therapy for both conditions.
The timing of switch from i.v. to oral antibiotics was related to disease complexity and type of disease: SA or OM. Intravenous antibiotics treatment was of shorter duration in children with simple disease than in those with complex disease (see Figures 1 and 2 and Tables 32 and 33). Among children in whom the switch date is known, a blood test at the time of the switch was carried out in only 37.9% of those with simple disease and 22.8% of those with complex disease (see Table 38).
When the switch date was recorded in the database, the median duration of i.v. therapy could be calculated:
-
Among 107 simple cases of SA, the switch date was known in 85 (79.4%). Switch occurred after a median of 6 (IQR 4–11) days of i.v. antibiotic treatment.
-
Among 109 simple cases of OM, the switch date was known in 82 (75.2%). Switch occurred after a median of 8.5 (IQR 5–14) days of i.v. antibiotic treatment.
-
Among 35 complex cases of SA, the switch date was known in 23 (65.7%). Switch occurred after a median of 13.5 (IQR 7–21) days of i.v. antibiotic treatment.
-
Among 56 complex cases of OM, the switch date was known in 35 (62.5%). Switch occurred after a median of 10.5 (IQR 7.5–26) days of i.v. antibiotic treatment.
As described above, the Kaplan–Meier plot of time to switch (by SA/OM; see Figure 2) shows that time to switch to oral antibiotics differed little between those with OM and SA in the simple cases group. Among complex cases, switch timing was similar in those with OM and SA when the switch took place before 2 weeks, but after this time point switch to oral antibiotics occurred later in those with OM than in those with SA.
Overall, 63.8% of those with simple disease (OM/SA) received antibiotic treatment for < 6 weeks in total, compared with 51.6% of those with complex disease (see Table 33). Simple OM and SA were most commonly treated with a single i.v. antibiotic and/or a single oral antibiotic, whereas in complex SA/OM cases more than two i.v. antibiotics were used, most often together with a single oral antibiotic type (see Table 33).
Medical treatment: initial empirical antibiotics
In order to design a randomised clinical trial, consensus needs to be reached regarding the initial empirical antibiotic to be started intravenously, and also regarding the post-switch oral antibiotic(s) to be used for the cases in which there is or is not a formal microbiology diagnosis. Although not investigated in this study, data from Finland suggest that children with culture-negative signs and symptoms of SA or OM can be treated in a similar fashion to children in whom the organism is identified in respect of type and duration of antimicrobial therapy. 63 The aim of the microbiology component of this study was to establish the most appropriate initial empirical therapies.
Beta-lactam antibiotics, either ‘simpler’ penicillin or second- or third-generation cephalosporins, are widely used in UK practice for the treatment of SA and OM. Among the 199 children with simple SA or OM who received i.v. antibiotics at any point during their hospital stay, the initial i.v. treatment was flucloxacillin in 85 (42.7%), clindamycin in 13 (6.5%), cefuroxime in 60 (30.2%), ceftriaxone in 51 (25.6%), benzylpenicillin in 16 (8%) and co-amoxiclav in 13 (6.5%) [24 (12.1%) children received other antibiotics] (see Table 35). Of the 91 children with complex SA or OM who received i.v. antibiotics at any point during their hospital stay, 38 (41.8%) received flucloxacillin, 10 (11%) received clindamycin, 17 (18.7%) received cefuroxime, 19 (20.9%) received ceftriaxone, 10 (11%) received benzylpenicillin and 10 (11%) received co-amoxiclav [with 19 (20.9%) children receiving other antibiotics] as part of their initial treatment (see Table 36).
Oral antibiotics were uncommon as part of initial therapy. Seventy-eight (35.8%) children with simple disease were prescribed at least one oral antibiotic. Flucloxacillin (34 cases, 43.6%), clindamycin (17 cases, 21.8%) and co-amoxiclav (18 cases, 23.1%) were all prescribed prior to discharge from hospital (see Table 35). Thirty-six (37.9%) children with complex disease were prescribed at least one oral antibiotic while in hospital, with the range of antimicrobials prescribed similar to those prescribed for simple disease, although ‘other’ antibiotics were used more often in children with complex disease than in those with simple disease (Tables 35 and 36).
Medical treatment: antibiotic at hospital discharge
Children with OM or SA were discharged on either i.v. or oral antibiotics. At discharge, 30 (28%) of 107 children with simple SA and 28 (25.7%) of 109 children simple OM remained on i.v. antibiotics. Around 60% [SA, 64 cases, (59.8%); OM, 65 cases (59.6%)] of children were taking only an oral antibiotic, and similar numbers of children with simple SA and OM either were on no antibiotic or the data are missing [SA, 13 children (12.1%); OM, 16 children (14.7%)]. Children with complex disease were more likely to remain on i.v. medication at discharge [of 95 children with complex SA/OM, 40 (42.1%) were on i.v. antibiotics, 42 (44.2%) were on oral antibiotics and 13 (13.7%) children were on none or this information was unknown] (see Table 45).
As expected, owing to the formulations available for outpatient or home-based therapy, the range of drugs prescribed was narrower than those used in hospital. Most children with simple disease were prescribed ceftriaxone for post-discharge i.v. therapy (41/59; 69.5%), although a few remained on flucloxacillin (4/59; 6.8%), clindamycin (2/59; 3.4%), cefuroxime (1/59; 1.7%) or other drugs (9/59, 15.3%). Children with complex disease were also treated after discharge mostly with ceftriaxone (31/40; 77.5%), but a few were treated with flucloxacillin (2/40; 5%), clindamycin (3/40; 7.5%) or other drugs (5/40; 12.5%) (see Table 46). Note: for 10% of cases known to have been discharged on i.v. antibiotics, the antibiotic type was not recorded, and children could be discharged on more than one antibiotic.
Of the oral drugs prescribed at discharge, co-amoxiclav was used in approximately one-third of cases [58/156 (36.5%) simple cases, 17/58 (28.8%) complex cases] and flucloxacillin in about one-quarter of cases [46/156 (28.9%) simple cases, 15/58 (25.4%) complex cases]. Oral clindamycin was used more frequently in complex than in simple cases [17/156 (10.7%) simple cases, 17/58 (28.8%) complex cases]. Cefalexin was used in simple SA/OM but infrequently in complex disease [20/156 (12.6%) simple cases, 2/58 (3.4%) complex cases] (see Table 46).
Medical treatment: dose of intravenous and oral antibiotics prescribed
Adequate dosing is a major concern of modern antimicrobial stewardship to ensure clinical cure with minimal risk of intrapatient and in-hospital interpatient antimicrobial resistance. 64 Recent legislative changes to clinical trial legislation65 ensure that newly available drugs are properly tested in children to establish dosing regimens and efficacy. However, almost all the widely used paediatric doses of antibiotics have been included in paediatric formularies, having been established by opinion and practice rather than formal clinical trial. With much variation in international practice, the UK BNFC62 has recently begun to revise the recommended doses of commonly used antibiotics, for example in 2014 doubling the recommended age band doses of oral amoxicillin. In addition, the UK BNFC is set out so that minimum and maximum doses for weight or age are specified. Many specialists in paediatric infection and paediatric microbiology, and National Institute for Health and Care Excellence guidelines where they exist,66–68 consider the high quoted doses to be the correct doses for serious (in-hospital) infections and that the low quoted doses are suboptimal. In order to inform the dose strategy for a future clinical trial, it was important to assess the antibiotic doses used in current practice in the context of overall reported clinical outcomes.
Overall, only 40.4% of simple cases received a higher formulary initial dose of antibiotics, compared with 29.5% of complex cases. Only 30 (13.8%) simple cases and nine (9.5%) complex cases received the higher formulary dose of antibiotic treatment throughout (see Table 33).
Specifically, for the most commonly prescribed i.v. empirical antimicrobials in hospital:
-
For simple disease (see Table 35), flucloxacillin, clindamycin and cefuroxime were prescribed at or near to the recommended formulary higher doses [flucloxacillin: median 188.1 (IQR 100.8–200) mg/kg/24 hours, formulary higher dose 200 mg/kg/24 hours; clindamycin: median 39.9 (IQR 18.5–40.6) mg/kg/24 hours, formulary higher dose 40 mg/kg/24 hours; cefuroxime: median 151.5 (IQR 83.7–150.) mg/kg/24 hours, formulary higher dose 150 mg/kg/24 hours]. However, ceftriaxone was generally prescribed at the lower formulary dose [median 50.3 (IQR 50–79.3) mg/kg/24 hours, formulary higher dose 80 mg/kg/24 hours].
-
For complex disease (see Table 36), however, prescribing trends suggest that inadequate dosing (less than the higher formulary dose) was more common. Although ceftriaxone was generally prescribed at the higher formulary dose when it was used (as initial treatment in 20.9% of cases, and in 40.7% of complex cases overall) [median 77.7 (IQR 49–8) mg/kg/24 hours, formulary higher dose 80 mg/kg/24 hours, EMA-approved maximum dose 100 mg/kg/24 hours], flucloxacillin, clindamycin and cefuroxime were all prescribed at less than the formulary higher doses [flucloxacillin: median 142.6 (IQR 98–198.5) mg/kg/24 hours, formulary higher dose 200 mg/kg/24 hours; clindamycin: median 33.3 (IQR 24.1–39.2) mg/kg/24 hours, formulary higher dose 40 mg/kg/24 hours; cefuroxime: median 128 (IQR 83.3–150) mg/kg/24 hours, formulary higher dose 150 mg/kg/24 hours].
Doses of i.v. and oral antimicrobials prescribed following discharge were also generally lower than higher formulary doses. The i.v. empirical antimicrobial most commonly prescribed at discharge (for use out of hospital) (see Table 46), in both simple and complex cases, was ceftriaxone, because of its once-daily regimen (prescribed in 69.5% of children with simple disease receiving i.v. home-based therapy and 77.5% of children with complex disease). At discharge, ceftriaxone was generally prescribed at the lower formulary dose in simple cases [median 50.7 (IQR 49.3–79.4) mg/kg/24 hours, formulary higher dose 80 mg/kg/24 hours], although in complex disease the dose was slightly higher but still not at the higher formulary dose [median 65.4 (IQR 49–80.3) mg/kg/24 hours]. When used out of hospital, i.v. flucloxacillin and clindamycin for both simple and complex disease were generally prescribed at below the lower formulary recommended doses (flucloxacillin: simple disease, median 98.1 mg/kg/24 hours; complex disease, median 35.8 mg/kg/24 hours; clindamycin: simple disease, median 21.6 mg/kg/24 hours; complex disease, median 4 mg/kg/24 hours) (see Table 46).
In the case of orally prescribed out-of-hospital antibiotic treatments, comparison with formulary doses is more complicated as a result of the age band variations in the current BNFC. 62 For reference, the recommended dose ranges for co-amoxiclav (expressed as mg/kg/24 hours of amoxicillin component) are as follows:
-
age 1 month to 6 years: low dose 18.75 mg/kg/24 hours; high dose 37.5 mg/kg/24 hours
-
age 6–12 years: low dose 22.5 mg/kg/24 hours; high dose 45 mg/kg/24 hours
-
age 12–18 years: low dose 15 mg/kg/24 hours; high dose 30 mg/kg/24 hours.
For flucloxacillin, the recommended dose ranges are as follows:
-
age 1 month to 2 years: low dose 31 mg/kg/24 hours; high dose 62 mg/kg/24 hours
-
age 2–10 years: low dose 43.3 mg/kg/24 hours; high dose 46.5 mg/kg/24 hours
-
age 2–18 years: low dose 25 mg/kg/24 hours; high dose 50 mg/kg/24 hours.
For all age groups, the recommended oral dose of clindamycin is 12 mg/kg/24 hours (low dose) to 24 mg/kg/24 hours (high dose) and of cephalexin is 25 mg/kg/24 hours (low dose) to 50 mg/kg/24 hours (high dose).
In most children with simple disease, the prescribed doses of the four most common oral antibiotics were nearer to the lower end of the dose range (see Table 46):
-
co-amoxiclav: median 30.4 mg/kg/24 hours (IQR 13.8–41.8 mg/kg/24 hours)
-
flucloxacillin: median 37.5 mg/kg/24 hours (IQR 14.2–82.3 mg/kg/24 hours)
-
clindamycin: median 18.1 mg/kg/24 hours (IQR 6.1–23.6 mg/kg/24 hours)
-
cefalexin: median 35 mg/kg/24 hours (IQR 24.5–91.1 mg/kg/24 hours).
Perhaps worryingly, children with complex disease who were prescribed co-amoxiclav or clindamycin (respectively 28.8% and 28.8% of children with complex disease given oral antibiotics) appear to have received lower doses of these drugs than children with simple OM or SA. Among children with complex disease prescribed oral antibiotics at discharge, the median dose of co-amoxiclav was 23.1 mg/kg/24 hours (IQR 12.1–47.6 mg/kg/24 hours), the median dose of flucloxacillin was 41.7 mg/kg/24 hours (IQR 36.4–54.1 mg/kg/24 hours) and the median dose of clindamycin was 15.1 mg/kg/24 hours (IQR 6–23.8 mg/kg/24 hours) (see Table 46).
Follow-up (3 months): completion of antimicrobial courses as planned at discharge
One of the key concerns in any clinical trial of oral therapies, and of antibiotics in particular, is the course completion rate. Although not all children with OM or SA were followed up (the reported follow-up rates were 72.9% of children with simple SA, 72.5% of children with simple OM, 77.1% of children with complex SA and 80.4% of children with complex OM), course completion rates among those who were followed up were very high (92.4% among those with simple disease and 92.1% among those with complex disease) (see Table 47). The reported reasons for non-completion of therapy were reassuring for future trial planning, when, to study clinical outcomes in relation to length of therapy, it will be important to know that prescribed medication is being given as planned. Among children with simple disease, there were only four for whom failure to complete treatment was reported, with the reported reasons being unpalatable antibiotic, rash, symptom resolution and refusal to take the medication.
Hospital stay
Overall, the median length of hospital stay among children with simple disease, whether OM or SA, was 7 days (IQR 4–12 days), with 50% of children staying for ≥ 6 days but no longer than 12 days. In complex cases the median length of stay in hospital overall was 9 days (IQR 6–18 days), with 50% of children staying for ≥ 6 days but no longer than 18 days (see Table 43).
Children with OM spent longer in hospital than those with SA, and this was true for both those with simple disease and those with complex disease (see Table 44). The median length of stay for simple SA was 6 days (IQR 4–11 days), compared with 9 days (IQR 5–13 days) for simple OM. The median length of stay for complex SA was 8 days (IQR 6–15 days), compared with 9 days (IQR 6–18.5 days) for complex OM. In the case of children with simple SA or OM, the median length of hospital stay was similar to the time on i.v. therapy prior to oral switch (oral switch occurring after a median of 6 and 8.5 days, respectively), but children with complex SA or OM were more likely to still be on i.v. therapy at the time of hospital discharge (oral switch occurring after a median of 13.5 and 10.5 days, respectively).
Younger (aged < 1 year) and older (aged 6–16 years) children were more likely to stay in hospital longer than the 1–6 years age group, which is probably a reflection of the difficulties of imposing bed rest on the younger mobile children than on babies and older children. Among those with simple disease, the median length of stay was 7 days (IQR 5–12 days) for those aged < 1 year and 10 days (IQR 6–15 days) for those aged 6–16 years but only 6 days (IQR 3.5–10.5) for those aged 1–6 years. Among those with complex disease, the median length of stay was 16 days (IQR 9–27 days) for those aged < 1 year and 10 days (IQR 6–16 days) for those aged 6–16 years, but only 8 days (IQR 5–14 days) for those aged 1–6 years (see Table 43).
The finding that children treated in secondary care centres spent slightly longer in hospital than those treated in tertiary centres (see Table 43) is difficult to explain fully, but may be a reflection of the fact that some children are transferred back to their secondary care centre prior to final discharge from hospital and this may incur delays overall prior to discharge home.
Follow-up (3 months): complications of the disease and therapies, and treatment failure
Complications and ongoing symptoms were recorded as separate items during data collection to try to capture all post-treatment events and problems that may need to be included as future clinical trial outcomes.
A total of 8.3% of children with simple disease and 16.8% of those with complex disease experienced at least one complication (see Table 49). In only three (of 218) children with simple disease and four (of 95) children with complex disease was recurrence of infection requiring medical or surgical intervention reported. Other complications were reported only once and were related to the disease (clinical outcomes, e.g. stiffness or pain), antibiotic therapy (e.g. possible allergy or drug reaction) or mode of giving the drug (e.g. phlebitis) (see Table 50). The complications of complex disease were generally more severe than those reported for simple disease, including acute kidney injury, Hickman line sepsis, pneumococcal meningitis and tracheostomy (see Table 50).
Twenty-seven (17.2%) children with simple disease and 18 (24%) with complex disease had at least one ongoing symptom. Eighteen of 157 (11.5%) children with simple SA or OM and 8 of 76 (10.5%) children with complex SA or OM followed up were still in pain. Six (3.8%) children with simple disease and eight (10.5%) with complex disease reported joint stiffness, and four (2.5%) children with simple disease and three (3.9%) with complex disease reported immobility of some kind. Other symptoms were less common and no fractures were reported (see Table 52).
To capture all possible cases of failure of initial empirical antibiotic and/or surgical therapy, necessitating further treatment, we used a broad definition of treatment failure (including fever, recurrence of infection including increase in CRP or development of draining sinus), dislocation, subluxation, pathological fracture, pain, mobility problems, swelling, numbness, hospital readmission, surgery and change of antibiotics. Using these criteria, 32 of 218 (14.7%) children with simple disease experienced a treatment failure, compared with 24 of 95 (25%) children with complex disease (see Table 53). Among those with simple disease, treatment failure was half as likely in those with SA (9.3%) as in those with OM (19.3%), whereas among those with complex disease treatment failure was more likely in those with SA (28.6%) than in those with OM (21.4%) (see Table 53).
These data demonstrate that treatment failure is less likely in children with simple disease in whom switch to oral antibiotics occurs early, suggesting that those with complex disease are more likely to fail initial therapy or require additional interventions, which is very important for the design of a RCT of reduced duration of i.v. and/or total antibiotic therapy. Of the 73 children with simple disease in whom oral switch occurred very early (< 1 week), 9.6% experienced a treatment failure, compared with 16.1% of those in whom switch occurred early (between 1 and 2 weeks) and 18.2% of those in whom switch took place after 2 weeks or more of i.v. antibiotics. Among children with complex disease, switching to oral antibiotics after 2 weeks or more was also associated with a higher risk of complications (37.9%) than very early switch (25%) or switch between 1 and 2 weeks (19%) (see Table 54).
Regarding the total length of therapy, among those with simple SA or OM, treatment failure was equally likely among those treated with antibiotics for < 6 weeks and those treated for > 6 weeks. Among those with complex disease, children given antibiotics for < 6 weeks were much less likely to experience treatment failure than those given antibiotics for > 6 weeks (14.3% compared with 37%), although this is likely to be a reflection of disease severity itself and not the treatments given (see Table 55).
Chapter 8 presents a detailed discussion of how the service evaluation data impact, with other study components, on potential RCT design.
Chapter 3 Microbiology substudy
Background
The current ‘gold standard’ for detection of bacteria associated with OM/SA is bacterial culture, although in the UK the pathogen detection rate from culture in children’s bone and joint infections is low. 1 This means that the true spectrum of organisms causing bone and joint infections in the UK is not known, and treatment is usually empirical.
New molecular techniques can enhance diagnostic detection rates. The use of 16S real-time PCR has become available in some specialist centres; however, in the UK it is available at one centre with a turnaround time of days to weeks. 16S real-time PCR is expensive and, although capable of detecting many pathogenic and non-pathogenic organisms, it has low sensitivity because the load of bacteria in the primary sample needs to be sufficiently high to allow detection.
Species-specific reverse transcription polymerase chain reaction (RT-PCR) to identify bacteria associated with OM/SA has been used in specialist centres to enhance the detection of certain organisms, in particular K. kingae, which is difficult to grow in routine culture. However, although it is much cheaper than 16S PCR, species-specific PCR is not routinely available in most UK or European Union laboratories.
Aims and objectives
This study evaluated the potential for the species-specific RT-PCR to be used both to establish the baseline UK microbiology of paediatric bone and joint infections more rigorously than would have been possible by culture alone and to assess the use of the test in a larger RCT.
We also aimed to understand the pathophysiology of K. kingae by using PCR to test throat swabs (taken from patients who have provided consent) for K. kingae to determine how many had concurrent pharyngeal colonisation with this organism.
Methods
Clinical cases
Children presenting with bone and joint infection at one of the six participating centres were approached by the Dinosaur study research team and recruited after informed consent was obtained. Clinical data were collected as part of the standard Dinosaur service evaluation.
Inclusion criteria
-
Children between the ages of 1 month and 16 years with a clinical diagnosis of OM or SA.
-
Written informed consent of participant or parent/legal guardian, and assent where appropriate for:
-
PCR to be done on routine samples taken for culture and microscopy
-
throat swab to be taken for PCR and culture and microscopy
-
additional 5-ml blood sample to be taken and stored for future analysis.
-
Exclusion criteria
Patients for whom informed consent is not obtained (parents/patients could decline consent for certain procedures, e.g. additional blood aliquot, but still give consent for others, such as PCR on routine samples).
The culture and preparation of control organisms
Sample handling and preparation
Samples from routinely collected blood and tissue cultures were taken in the local microbiology laboratory and processed in accordance with the study standard operating procedure (see Report supplementary material 4) before storage and transfer to the central laboratory (Southampton Public Health England South East Regional Laboratory) for PCR analysis.
All samples were taken for PCR after initial bacterial culture had been carried out. For blood cultures, an aliquot of sample was removed after at least 19 hours’ incubation (unless the sample had already been identified as positive) using a sterile needle and syringe, from which 1 ml was placed into a sterile nuclease-free skirted 2-ml tube before being capped and labelled and placed in storage at –80 °C until extraction and PCR was to be carried out. For liquid samples such as synovial fluid, pus and blood or joint washouts, 500 µl was taken from the original sample (where limited sample volume remained, 200 µl was used, thus allowing for repeat culture or further analysis using another method). Solid samples were divided and, where sample quantity allowed, a minimum of two aliquots of tissue or bone weighing around 10 mg each were taken and placed in to a sterile nuclease-free skirted 2-ml tube and stored at –80 °C. All samples were handled in a class 1 cabinet to prevent contamination.
For throat swabs, after swabbing, the cotton end was snipped off using sterile scissors into a flat-bottom 2-ml tube containing 1 ml of skimmed milk, tryptone, glucose and glycerine media, used to preserve bacteria while being frozen at –80 °C.
Polymerase chain reaction analysis
Control bacteria used in this study were sourced from Pro-Lab Diagnostics (Birkenhead, UK) and the UK National Culture Collection (Salisbury, UK). Bacterial deoxyribonucleic acid was extracted by standard methods and multiplex PCR used for analysis. Table 57 shows the multiplex panels used for all samples.
| Multiplex 1 | Multiplex 2 | Multiplex 3 |
|---|---|---|
| K. k. for | Sal. spp. for | GBS for |
| K. k. rev | Sal. spp. rev | GBS rev |
| GAS for | S. a. for | H. inf. for |
| GAS rev | S. a. rev | H. inf. rev |
| S. pn. ply for | S. epi. for | S. pn. lytA for |
| S. pn. ply rev | S. epi. rev | S. pn. lytA rev |
| K. k. | Sal. spp. | GBS |
| GAS | S. a. | H. inf. |
| S. pn. ply | S. epi. | S. pn. lytA |
Table 57 shows the constituent elements to each of the multiplexes. Two separate targets were used to detect S. pneumoniae as the pneumolysin (ply) gene is also common to other closely related streptococcal species including Streptococcus mitis, Streptococcus oralis and Streptococcus pseudopneumoniae. The N-acetylmuramoyl-L-alanine amidase (lytA) gene was later chosen as it occurs only in S. pneumoniae.
Results
Although seven sites took part in the study, samples from only six of the sites were processed because of incorrect sampling at one site, an error subsequently acknowledged by the local principal investigator. The total number of samples and patients consented to the study from each site is shown in Table 58.
| Geographical site (site number) | Number of throat swabs | Number of samples excluding throat swabs | Total number of samples | Total number of patients |
|---|---|---|---|---|
| Oxford (1) | 9 | 25 | 34 | 11 |
| Birmingham (2) | 1 | 4 | 5 | 1 |
| Imperial (3) | 0 | 22 | 22 | 4 |
| Bristol (4) | 11 | 28 | 39 | 12 |
| Liverpool (5) | 9 | 18 | 27 | 10 |
| Southampton (6) | 8 | 16 | 24 | 12 |
| Total | 38 | 113 | 151 | 50 |
Table 58 shows the number of patients consented to the study at each site and the number of samples (both throat swabs and native bone and joint samples). Site number refers to tables below.
A total of 151 samples were received from 50 patients. Of these, 113 were bone, fluid and blood culture samples, which were collected from 44 patients across six geographical sites. The remaining 38 samples were from throat and wound swabs. For five participants only throat swab samples were obtained, and for one participant only skin swab samples were obtained.
Of the 113 native tissue samples collected, 39 were blood cultures, in 16 of which pathogenic bacteria were identified using the RT-PCR panel. Sixty of the total native samples collected were joint fluids including pus, of which 46 were positive using the RT-PCR panel. Eleven tissue samples and three bone samples were collected, of which 10 and 1, respectively, were positive using the RT-PCR panel.
Of the 16 blood culture samples that were found to be positive, nine were identified as S. aureus whereas no K. kingae was detected in this sample type. Other notable results identified in blood cultures were three positives for Staphylococcus epidermidis, two positive for GAS, one positive for H. influenzae and one for S. pneumoniae (ply).
Of the 46 positive joint fluid samples that included aspirates and pus, 19 were identified as positive for S aureus. K. kingae was identified in 10 and GAS in 11 different samples. S. epidermidis and S. pneumoniae (lytA) were also identified in four and one sample, respectively. In three samples of this type that were positive for S. epidermidis, another more pathogenic organism was actually identified, and in these cases S. epidermidis was assumed to be a contaminant.
Of the 11 tissue samples received as part of this study, 10 were positive by RT-PCR, of which eight were positive for S. aureus with one sample each positive for K. kingae and GAS. Three bone samples were also received, of which one was positive for S. aureus.
Overall, excluding any data from skin or throat swabs, 32 of the 44 patients (72.7%) were PCR positive for one or more of the target organisms included in the RT-PCR panel (see Tables 59–67 for detailed results).
Table 59 shows the number of positive samples detected for each target organism from all of the six sites for all sample types (including skin and throat swabs), expressed as a percentage of the total sample number from each location.
| Site number (n) | Organism, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| 1 (34) | 6 (17.6) | 0 (0) | 9 (26.4) | 1 (2.9) | 0 (0) | 14 (41.1) | 8 (23.0) | 1 (2.9) | 4 (8.8) |
| 2 (5) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 3 (60.0) | 1 (20.0) | 0 (0) | 1 (20.0) |
| 3 (22) | 0 (0) | 11 (50.0) | 0 (0.0) | 0 (0) | 0 (0) | 8 (36.3) | 1 (4.5) | 0 (0) | 0 (0) |
| 4 (39) | 3 (10.3) | 1 (3.44) | 6 (20.6) | 0 (0) | 0 (0) | 7 (24.1) | 2 (6.8) | 0 (0) | 4 (13.7) |
| 5 (27) | 1 (3.7) | 4 (14.1) | 8 (29.6) | 0 (0) | 0 (0) | 8 (29.6) | 5 (18.5) | 0 (0) | 2 (7.4) |
| 6 (24) | 1 (4.1) | 5 (20.8) | 7 (29.1) | 1 (4.16) | 0 (0) | 11 (45.8) | 4 (16.6) | 0 (0) | 2 (8.3) |
| Total (151) | 11 (7.2) | 21 (13.9) | 31 (20.5) | 2 (13.2) | 0 (0) | 51 (33.7) | 21 (13.9) | 1 (0.6) | 13 (8.6) |
Table 60 identifies the number of positives for each PCR target detected based on all sample types across all geographical sites.
| Sample type | Organism, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| Throat swab | 0 | 6 | 30 | 1 | 0 | 14 | 14 | 1 | 12 |
| Blood culture | 0 | 2 | 1 | 0 | 0 | 9 | 3 | 0 | 1 |
| Joint fluid/aspirates/pus | 10 | 11 | 0 | 1 | 0 | 19 | 4 | 0 | 0 |
| Tissue | 1 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| Bone | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Swabs | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (n = 151) | 11 | 21 | 31 | 2 | 0 | 51 | 21 | 1 | 13 |
Table 61 shows the number of positive organisms in samples taken from usually sterile sites (excluding skin and throat swabs) from each geographical site expressed as a percentage of the total number of fluid, tissue, bone and blood samples collected at each location.
| Site number (n) | Organism, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| 1 (25) | 6 (24.0) | 0 (0) | 1 (3.8) | 0 (0) | 0 (0) | 9 (36.0) | 3 (12.0) | 0 (0) | 1 (4.0) |
| 2 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50.0) | 0 (0) | 0 (0) | 0 (0) |
| 3 (22) | 0 (0) | 11 (50.0) | 0 (0) | 0 (0) | 0 (0) | 8 (36.3) | 1 (4.5) | 0 (0) | 0 (0) |
| 4 (28) | 3 (10.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (21.4) | 1 (3.5) | 0 (0) | 0 (0) |
| 5 (18) | 1 (5.6) | 1 (5.6) | 0 (0) | 0 (0) | 0 (0) | 4 (22.2) | 1 (5.6) | 0 (0) | 0 (0) |
| 6 (16) | 1 (6.2) | 2 (12.5) | 0 (0) | 1 (6.2) | 0 (0) | 8 (50.0) | 1 (6.2) | 0 (0) | 0 (0) |
| Total (113) | 11 (9.7) | 14 (12.3) | 1 (0.8) | 1 (0.8) | 0 (0) | 37 (32.7) | 7 (6.1) | 0 (0) | 1 (0.8) |
Table 62 shows that a small number of tissue and fluid samples were positive for more than one of the target organisms in the bone and joint PCR panel.
| Patient number | Site number | Specimen type | Organisms identified |
|---|---|---|---|
| 1 | 1 | Blood culture | S. pn. (ply); S. a.; S. epi.; H. inf. |
| 2 | 1 | Aspirate | K. k.; S. epi. |
| 3 | 4 | Tissue | S. a.; S. epi. |
| 4 | 5 | Synovial fluid | K. k.; S. a. |
| 5 | 6 | Tissue | GAS; S. a. |
| 6 | 3 | Pus | GAS; S. epi |
Table 63 shows the number of positives for each PCR target detected based on blood, tissue and fluid sample types only across all geographical sites.
| Sample type | Organism, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| Blood culture | 0 | 2 | 1 | 0 | 0 | 9 | 3 | 0 | 1 |
| Joint fluid/aspirates/pus | 10 | 11 | 0 | 1 | 0 | 19 | 4 | 0 | 0 |
| Tissue | 1 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| Bone | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total | 11 | 14 | 1 | 1 | 0 | 37 | 7 | 0 | 1 |
Table 64 shows the total number of samples positive for any of the target organisms by sample type; these data exclude throat swabs and skin/wound swabs.
| Sample type | Total number of each sample type | Total PCR positive for any target organism included in the PCR panel, n (%) |
|---|---|---|
| Blood culture | 39 | 16 (41) |
| Joint fluid/aspirates/pus | 60 | 46 (76) |
| Tissue | 11 | 10 (91) |
| Bone | 3 | 1 (33) |
| Total | 113 | 73 (64.6) |
Table 65 shows the number of positives detected for each of the multiplex targets based on patient number rather than the actual number of samples from each geographical location (i.e. combining results from duplicate samples from individual patients). These data exclude throat and skin swabs.
| Site number | Organism | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| 1 | 3 | 0 | 1 | 0 | 0 | 4 | 1 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 3 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 |
| 5 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| 6 | 1 | 2 | 0 | 1 | 0 | 4 | 0 | 0 | 0 |
| Total (n = 32) | 7 | 4 | 1 | 1 | 0 | 16 | 3 | 0 | 0 |
PCR results according to simple and complex cases are summarised in Table 66.
| Cases detected | Simple cases (N = 218)a | Complex cases (N = 95)b | ||||||
|---|---|---|---|---|---|---|---|---|
| Tested, n | Yes, n (%) | Tested, n | Yes, n (%) | |||||
| Samples | Cases | Samples | Cases | Samples | Cases | Samples | Cases | |
| At least one micro-organism detected? | 32 | 24 (75) | 12 | 8 (67) | ||||
| Blood | 31 | 22 | 12 (39) | 8 (36) | 9 | 8 | 3 (33) | 3 (38) |
| Synovial fluid | 30 | 20 | 25 (83) | 18 (90) | 3 | 3 | 3 (100) | 3 (100) |
| Pus | 7 | 2 | 6 (86) | 2 (100) | 14 | 6 | 12 (86) | 4 (67) |
| Tissue | 5 | 5 | 5 (100) | 5 (100) | 5 | 4 | 5 (100) | 4 (100) |
| Bone | 1 | 1 | 1 (100) | 1 (100) | 0 | 0 | 0 | 0 |
Table 67 shows the number of positive throat swabs for each of the target organisms taken from the six geographical sites, also expressed as a percentage of the total of throat swabs collected at each location.
| Site number (n) | Organism, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. k. | GAS | S. pn. (ply) | S. pn. (lytA) | Sal. spp. | S. a. | S. epi. | GBS | H. inf. | |
| 1 (9) | 0 (0.0) | 0 (0) | 8 (88.8) | 1 (11.1) | 0 (0) | 5 (55.5) | 5 (55.5) | 1 (11.1) | 3 (33.3) |
| 2 (1) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 1 (100) |
| 3 (0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0) |
| 4 (11) | 0 (0) | 0 (0) | 5 (45.4) | 0 (0) | 0 (0) | 1 (9.0) | 1 (9.0) | 0 (0) | 4 (36.3) |
| 5 (9) | 0 (0) | 3 (33.3) | 8 (88.8) | 0 (0) | 0 (0) | 4 (44.4) | 4 (44.4) | 0 (0) | 2 (22.2) |
| 6 (8) | 0 (0) | 3 (37.5) | 8 (100) | 0 (0.0) | 0 (0) | 3 (37.5) | 3 (37.5) | 0 (0) | 2 (25.0) |
| Total (38) | 0 (0.0) | 6 (15.7) | 30 (78.9) | 1 (2.63) | 0 (0.0) | 14 (36.8) | 14 (36.8) | 1 (2.6) | 12 (31.5) |
Discussion
The microbiology substudy has demonstrated the diagnostic yield of the relatively cheap species-specific RT-PCR compared with routinely used microbiology culture based techniques in children’s bone and joint infections, achieving a definitive bacterial diagnosis in 32 of 44 patients [24/32 simple cases (75%) and 8/12 complex cases (67%)] in whom the test was used (see Table 66). Although the substudy was not designed to estimate formal sensitivity and specificity, a positive diagnosis in around 65–70% overall is higher than the reported yields of 40–50% (where tissue samples are achieved at surgical intervention) or 9–22% (where only blood cultures are achieved). 15,27
The study has also demonstrated the feasibility of the test being performed centrally or locally/regionally in a future randomised clinical trial. The protocol of the easy-to-perform, species-specific PCR panel is such that routine NHS molecular microbiology laboratories could adopt the test, or it could be performed on a regional or national basis for UK practice. A future trial could use results from locally or nationally performed tests depending on availability, as long as results were obtained in NHS/Public Health England-accredited laboratories performing routine molecular microbiological investigations.
The microbiology substudy has also provided important data on the current UK molecular epidemiology of children’s bone and joint infections. S. aureus remains the most commonly detected pathogen. Although pilot data (and anecdotal use of the species-specific panel on an ongoing basis at the Southampton site in routine clinical practice) suggested that K. kingae is almost as common in young children as S. aureus, these data also suggest that overall, during the period of this study, GAS (four cases detected by PCR) was as common cause of OM/SA as K. kingae (seven cases detected by PCR). In this study, GAS was identified by PCR in children aged between 2.3 and 12.4 years, whereas K. kingae was identified only in children aged 0.8–2.3 years. GAS caused simple (two cases) and complex (two cases) disease, whereas K. kingae caused only simple OM or SA. Although numbers are not high, our data confirm previous reports that K. kingae bone and joint infection primarily affects very young children. As expected, other pathogens were found more rarely.
The S. aureus data correlate with the existing literature and clinical experience as, historically, S. aureus has been known to be the most common cause of infection in both native and prosthetic bone and joint infections. 69 Thirty-seven native tissue samples from 16 patients (11 simple disease, five complex disease; age range 2.5–15.9 years, median 11.4 years) were positive for S. aureus using PCR, including eight tissue samples, nine blood cultures, one bone sample, one wound swab and 19 fluid samples (this includes joint fluids, pus and aspirates). S. aureus is a known coloniser of the upper respiratory tract, so to identify it in throat swabs is not uncommon. One patient (site 1) had significant levels of S. aureus in eight out of nine samples that had been taken, including pus and blood culture samples. Another patient (site 4) had high levels of S. aureus in both blood culture and pus, whereas in another (site 5) S. aureus was identified in three out of six samples tested.
A total of 11 samples taken from seven patients (all simple disease, age range 0.8 months to 2.3 years, median 1.15 years) were positive for K. kingae. These included 10 samples of joint fluids and aspirates and one other tissue sample. Although one group in Israel has reported that culture of tissue in blood culture bottle systems can increase the culture yield of this fastidious organism,17 this study has demonstrated again that species-specific PCR on routine blood culture samples or directly on tissue samples can formally diagnose K. kingae. 19
Four children (two with simple disease and two with complex disease; age range 2.3–12.4 years, median 6.7 years) had OM/SA caused by GAS detected by PCR from a total of 14 native tissue samples. Eleven samples were fluids including pus, two were blood cultures and one was tissue. Although a higher frequency than previously reported,1 this study was conducted during the time of a reported outbreak of GAS infections nationwide in the UK. 70
Two patients (ages 5.2 years and 0.8 months) were found to have OM/SA due to S. pneumoniae. One blood culture sample from one patient was positive for the ply gene of S. pneumoniae. One other joint sample from a different patient was positive for the more specific target for the lytA gene. The species-specific panel uses two targets to identify the presence of S. pneumoniae (the ply gene and the lytA gene). This is because the ply gene shows a degree of cross-reactivity between S. pneumoniae and S. mitis, S. oralis and S. pseudopneumoniae. The lytA target was chosen71 as it is common only in S. pneumoniae.
Haemophilus influenzae was identified in one blood culture from one patient aged 5.2 years but not from any PCR test. As expected, this was an unusual organism but potentially an important factor in the potential antibiotic choice in the ≥ 5 years age group1 in a future RCT.
No native tissue samples in this study were positive for GBS, included in the panel because of their frequency in neonatal OM/SA. 1 As no children aged ≤ 2 months were included in this microbiology substudy, this finding is not unexpected as GBS infections are rare in older infants and children.
No samples were found to be positive for Salmonella spp., an organism included in the PCR panel because of the reported frequency in OM/SA in children with sickle cell anaemia. 72
Staphylococcus epidermidis is generally considered a contaminant in skin and surface swabs, and in OM/SA infections overall in the absence of prosthetic material. 73 However, in the absence of other pathogens detected from usually sterile sites, and when S. epidermidis is detected in samples from those previously sterile sites, the role of this organism in the pathophysiology of bone and joint infections is undergoing more detailed investigation. In this study three blood cultures and four fluid samples were positive for S. epidermidis. One patient positive for this organism was also positive for GAS in 12 different samples, whereas another patient was positive for S. aureus but at low levels. Our data concur with the overall concept that in children without prosthetic joints or artificial implants of any kind, S. epidermidis is an unlikely pathogenic cause of SA/OM even when detected in sterile samples.
Our study also investigated K. kingae and other pathogens detected by PCR in throat (oropharyngeal) swabs74 to further investigate the relationship between OM/SA and respiratory tract carriage reported by a French group during the conduct of this study. 75 Thirty-eight throat swabs were received in total, among which K. kingae was identified in none. Thirty swabs were positive for S.pneumoniae (ply), of which only one was positive for the lytA gene, indicating that in 29 throat swabs the RT-PCR was detecting S. mitis, S. oralis or S. pseudopneumoniae as a result of ply gene cross-reactivity. Fourteen swabs were positive for S. aureus, 14 were positive for S. epidermidis, 12 were positive for H. influenzae, six were positive for GAS and one was positive for GBS.
The ability to perform species-specific RT-PCR in a local laboratory setting can benefit both patient and health-care system. By enabling antibiotic therapy targeted to a known organism, the switch from i.v. to oral therapy is facilitated, resulting in a shorter hospital stay. Although this study was not big enough for us to conduct a formal health-economic analysis, it is intuitive that being able to use directed therapy will result in shorter hospital stays as a result of earlier oral switch, as well as a reduction in treatment failure, cost, time and use of unnecessary antibiotics, and minimising the risk of antimicrobial resistance and nosocomial infections (the acquisition of which is proportional to length of stay, among other things).
Rapid identification of a bacterial organism causing OAI is important for a number of reasons. The use of conventional culture and microscopy can take a minimum of 48 hours, of which 24 hours is needed to establish organism growth (providing that it is a non-fastidious species) and 24 hours for identification and sensitivity profiling in order to correctly target antibiotic therapy. This process can be speeded up slightly by the use of matrix-assisted laser desorption/ionisation time of flight spectrometry, which can identify bacteria to a species level, providing that a pure culture can be grown initially. 76 The use of species-specific RT-PCR on bone and joint samples can speed up the identification process to a matter of hours. 77
Conclusions
This study evaluated the potential for the species-specific RT-PCR to be used both to establish the baseline UK microbiology of paediatric bone and joint infections better than would have been obtained by culture alone and to assess the use of the test in a larger RCT. We have established that the test identifies a pathogenic organism more frequently than conventional culture methods alone. The multiplex assay used has provided additional information on the UK molecular epidemiology of OM/SA in children in addition to the conventional culture results collected as part of the service evaluation (see Chapter 2). The test is feasible to use in a larger RCT and would be cheap and useful to implement in local microbiology laboratories for routine clinical care in the UK.
Data gained in the service evaluation and PCR substudy showed that the predominant pathogens in bone and joint infections in children in the UK are antibiotic-sensitive S. aureus in simple and complex disease in children of all ages outside the neonatal period; K. kingae in simple disease in young children (aged < 4 years in this study but < 6 years if currently unpublished data collected outside the time frame of the service evaluation and substudy is included); and GAS in simple and complex disease in children of all ages.
Chapter 4 Qualitative interviews with families and children treated for bone and joint infections
Background
There has been little previous qualitative research exploring the views of previously healthy children, or their parents, about treatment for, or the impact of, acute bone and joint infections on family life during and after admission to hospital with infection. Patient and parent perceptions and understanding of risks of complications, disability or adverse effects of treatment have also been previously underexplored.
In exploring the feasibility of a future RCT to identify the optimum point of switch from i.v. to oral antibiotics in the treatment of OM or SA, it is critical that the views of affected children and their parents are included in the data used to inform decision-making. We were also interested in understanding what information might help children and parents to make an informed decision about participating in such a trial, and in understanding what both children and parents view as important and meaningful outcomes that indicate that treatment has been successful. These data could potentially help to optimise meaning, acceptability and recruitment within a future RCT.
Aims
The overall purpose of the interviews was to explore parents’ and children’s views, understanding and experiences of both bone and joint infections and treatment, and willingness to participate in a future RCT. Our aims were:
-
to explore the experiences of parents and children treated with i.v. and/or oral antibiotic therapy for bone and joint infection about their condition and understanding about treatment
-
to identify which clinical outcomes are most important to parents and young people with bone and joint infections, in order to contribute to the wider stakeholder Delphi and stakeholder consultation process
-
to explore the views of parents and children admitted to hospital with bone and joint infections about participating in a RCT, in particular focusing on:
-
information required in order to provide informed consent
-
views about and acceptability of potential interventions
-
willingness to be randomised to either arm (i.e. treatment or control)
-
influences on the above factors.
-
Methods
Research approach
In line with the service evaluation and Delphi study included as part of the overall feasibility work, this qualitative study acknowledged the external reality of the experiences of both parent and child participants, drawing on a realist research tradition. However, in line with a critical or subtle realist research position,78 we argue that in recounting these experiences accounts will be mediated by subjective views and perspectives of those being interviewed. 79 This subtle realist approach has a number of implications for the conduct of this qualitative research, which we have identified and addressed in Table 68.
| Implication | Strategy |
|---|---|
| Interview guides are designed not to uncover objective facts, but to explore subjective reflections of events | Open questions are designed to orientate to the interviewees’ agendas, allowing them space to explore and describe subjective impressions associated with experiences When possible, children were interviewed in addition to parents, acknowledging the potential for different perspectives |
| The researcher’s role in making sense of and interpreting interview accounts is acknowledged | Field notes and a research diary were kept to promote reflection by the researcher about how she made sense of the data These reflections were further surfaced during supervision with the senior researcher. Interpretation is partial, acknowledging the potential for multiple readings |
| The proposed quality criteria against which it is suggested that this research should be evaluated acknowledge the subjective and co-constructed nature of the findings (see Rigour) | The researcher kept reflective notes to highlight her own role in interpretation Interviewees were offered the opportunity to revisit the synthesised findings and to comment if they felt that their own views/experiences were insufficiently represented The junior and senior qualitative researchers carried out independent analysis of a transcript to facilitate discussion about how meaning was created from the interviews |
Semistructured interviews
Semistructured interviews were employed to explore the views and experiences of parents and children treated for bone and joint infections within the previous year. Semistructured interviews are characterised by:
-
an interactional exchange
-
a topic-centred dialogue, but with a flexible structure
-
an acknowledgement that, within an interview context, meaning and knowledge are co-produced. 80
As recommended by Mason,81 the interview guides for parents and children were developed with reference to existing literature (e.g. qualitative work in this field, factors impacting on participation in RCTs, qualitative research interviews with children), researchers with expertise in this subject area and in working with children, and in consultation with our PPI contributors.
Interviews took the form of a conversation in which respondents initially told the story of their child’s (or their own) experience of bone and joint infection. Throughout the narrative, respondents were encouraged to describe both the events happening and the feelings and responses associated with them. Interviews covered a number of topics, with prompt materials used at various points as described in the following sections.
Experiences of becoming ill and diagnosis
-
Experiences of becoming ill with bone and joint infection, symptoms experienced.
-
Experiences of seeking treatment and diagnosis.
-
Treatments/interventions given, both in hospital and at home, including i.v. and oral antibiotic (antibiotic) therapy.
-
Experience of both i.v. and oral antibiotics.
-
Emotional responses to events happening.
A ‘timeline’ was used during the early section of the interview. The timeline consisted of three parallel, horizontal lines. The top horizontal line was labelled ‘becoming ill’, the second ‘hospital’ and the lowest ‘at home/getting better’. As respondents talked about their experiences, the researcher scribed key points/events on to the timeline in relevant places, with depiction of frequency, duration etc. Initially we had intended that respondents should scribe the timelines themselves as they spoke. However, during early pilot interviews it became apparent that this was distracting for respondents and interrupted the flow of their narratives. On the other hand, once the researcher began scribing, the timeline became a useful visual depiction of events. It also served as a reference point at later points during the interview.
Participants’ understandings and perceptions about aspects of antibiotic therapy
-
Perceptions about clinical reasons, advantages and disadvantages of both forms of antibiotic therapy (i.v. and oral).
-
Perceptions about length of treatment arms (i.v. and oral) and about point of switch from i.v. to oral.
Perspectives about the success of the treatment overall and important outcomes of recovery
-
Signs that indicated child was getting better.
-
Judgement of overall success of treatment and factors important when assessing level of success.
Views about participation in a hypothetical future randomised controlled trial
(Prompt material: RCT process diagram and information – see Report supplementary material 5.)
-
Initial reactions.
-
Information required (content and delivery) to assist decision-making.
-
Feelings about being randomised.
-
Views on alternative treatment arms.
-
Level of willingness to participate and why.
The prompt material describing the trial was altered during the course of fieldwork to reflect the finding that most patients were now receiving shorter courses of i.v. antibiotics rather than the traditional ‘standard’, and that the switch was being determined by a blood test. Initially, the adult prompt description of the trial showed the following process diagram (Figure 3).
FIGURE 3.
Initial process diagram.

This was replaced by Figure 4 to show that the difference between trial arms would relate to length of overall antibiotic treatment and that the point of switch would be guided by blood tests.
FIGURE 4.
Amended process diagram.
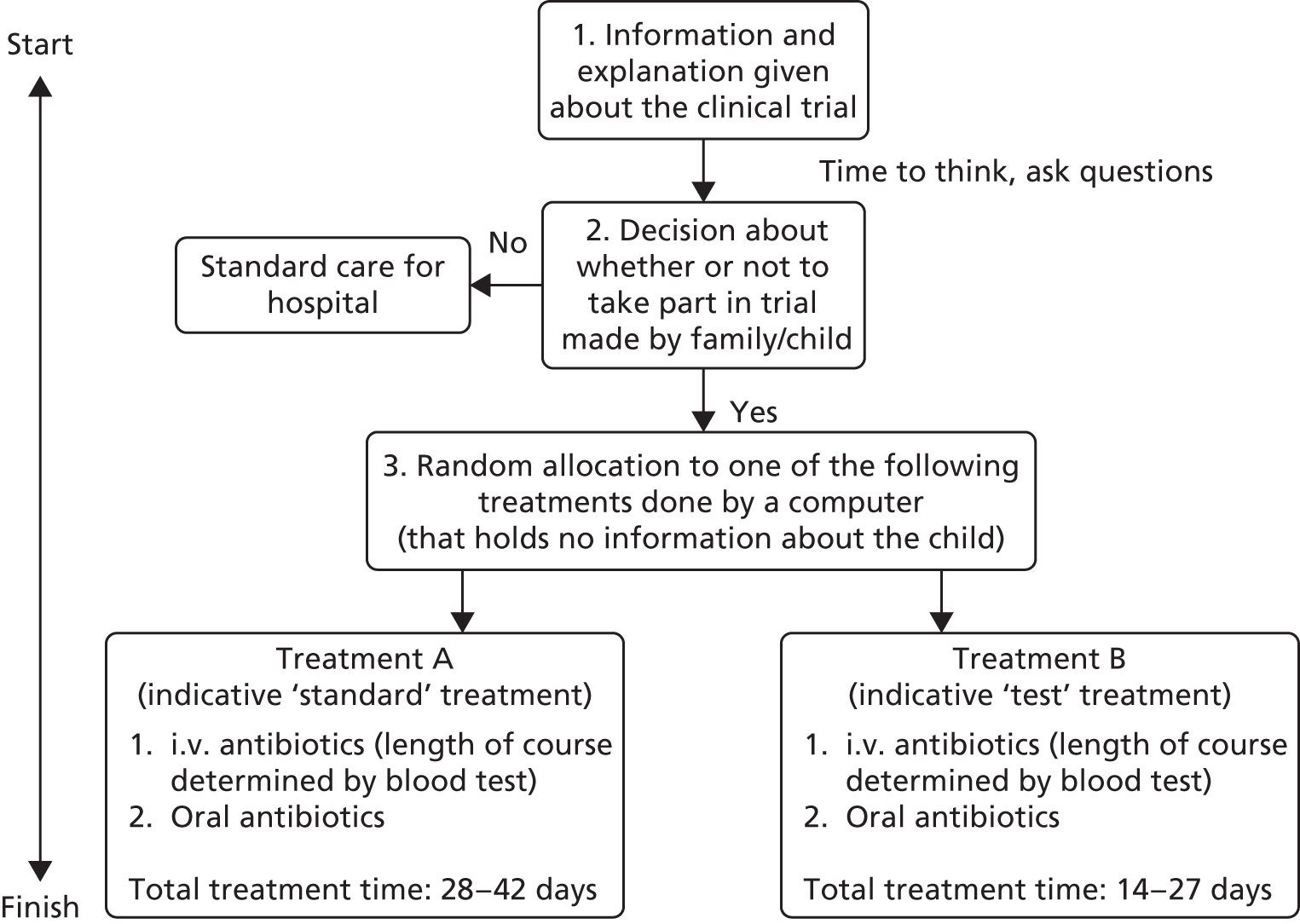
Because of the broad age span of children involved, three age-appropriate versions of interview guides and prompt material were developed:
-
Young children up to the age of 7 years – this interview guide (see Report supplementary material 5) consisted of simple questions about children’s experiences and understandings, involving the use of a doctor glove puppet and a dinosaur toy. Because of the complex issues involved, this interview guide did not include a section on the proposed RCT. As detailed in Findings, however, no children in this age group took part in interviews. This was primarily because parents did not wish to involve younger children in talking about an event that had often been upsetting and traumatic for them.
-
Children aged 8–12 years – this interview guide (see Report supplementary material 5) involved simplified language, but did cover all topics identified above, including seeking views about proposed trial (‘test’).
-
Young people aged ≥ 13 years and adults – the same guide (see Report supplementary material 5) was used for both young people and adults.
Participants
A purposive sample of children aged 0–16 years was identified from four of the seven centres in the south of England that had participated in the main Dinosaur service evaluation and microbiology substudy: Bristol Children’s Hospital, Oxford Children’s Hospital (University Hospitals Oxford NHS Trust), University Hospital Southampton NHS Foundation Trust and St George’s Hospital London. The purposive sampling strategy addressed variation with regard to age, sex and ethnicity.
The inclusion criteria identified were:
-
parents and children aged from 3 months to 16 years, treated for OM and/or SA
-
children who had been treated for bone and joint infections at one of the seven sites participating in the main service evaluation and microbiology substudy
-
parents/children who had taken part in the main Dinosaur study and also consented to take part in this qualitative study.
Procedure
At the point of initial consent for participation in the larger study, participants (i.e. children and their families) were asked if they would be willing to be contacted later about possible participation in a smaller qualitative study. Only families who had already participated in the larger study, and who had expressed willingness to be recontacted, were invited to participate in the interviews.
Once ethical and site-specific approvals were received, the qualitative researcher made contact with each site to arrange the procedure to be followed to contact potential respondents. In one site, a member of nursing staff at the hospital telephoned pre-consented families to check that they were still happy to be contacted by the qualitative researcher. The qualitative researcher then contacted those agreeing, by telephone.
In the remaining three sites, the qualitative researcher made the initial contact with families by telephone. During this initial telephone call the qualitative researcher described the interview process to families and asked if they would be interested in taking part. Provisional appointments were made, and a letter confirming arrangements was sent, with a participant information sheet, by e-mail or post. Contact details of the researcher were included in case families needed to change or cancel the appointment, or if they had any subsequent questions. The researcher also telephoned respondents on the day of (or the day before) the agreed appointment to check that it was still convenient for the family. If not, the appointment was rescheduled or, on one occasion, cancelled. Signed consent was provided by families interviewed in person just prior to the interview, and those interviewed by telephone were asked to sign and return the consent forms by post.
Ethics
Ethics approval for the qualitative interviews with parents and children was obtained from the National Research Ethics Committee (NRES Committee Yorkshire & The Humber – Leeds West, reference 14/YH/1166). All parents gave informed written consent to their participation and the use of quotations from their interviews for dissemination purposes. Assent was obtained from children. Because of the age of the children involved and the fact that families were being asked to talk about experiences that had been difficult and upsetting, the interview format and appointment times were kept flexible according to the needs and wishes of individual families and children. Parents of children who were aged ≤ 7 years were, in general, not willing to involve their children directly in interviews revisiting experiences that they had found upsetting or traumatic. These parents therefore opted to take part in an interview while their child was at school. Very young children (babies or toddlers) were often present in the room, although too young to be directly involved with answering questions.
Older children were more likely to be involved, and interview appointments were therefore arranged for times when they would be at home. Children chose whether they wished to be interviewed along with their parents or separately. Eight children were interviewed along with (in seven cases) or separately from (one case) their parents, according to the preferences of the child and their family. Children could participate in interviews as they wished – for example, one child joined in answering questions early in the interview but then drew her own (separate) timeline later as she became tired. A range of family members contributed to interviews including mothers, fathers, child treated, siblings and (on one occasion) grandparents.
Analysis
Interviews mainly took place at the respondents’ homes between January and March 2015. Three interviews were conducted over the telephone because of issues of location and availability. The interviews were digitally recorded, with permission from the families and children. The recordings were transcribed by a professional transcribing agency and returned to the qualitative researcher for analysis.
Thematic analysis82 was employed in this study. Thematic analysis is a method for identifying, analysing and reporting patterns (themes) across a data set. The following stages were followed:
-
familiarisation with the data
-
generating initial codes
-
searching for themes
-
reviewing themes
-
defining and naming themes
-
producing the report.
Data were stored and managed through the use of NVivo 10 (QSR International, Warrington, UK). Using this software package, transcripts were read in detail and coded, with codes being renamed, regrouped, merged or disregarded as issues emerged and re-emerged in subsequent transcripts. The coding frame was increasingly refined, discussed among members of the research team, and used as a basis for analysis and writing. Coding was both deductive (themes identified in interview guide/research questions were looked for within the text) and inductive (codes that have not been pre-conceived were allowed to ‘emerge’ from within the data).
Rigour
Based on guidelines for producing good-quality qualitative research, we employed the following strategies:
-
Reflexive notes were made during data collection by AL, in which she recorded contextual information and emerging ideas relating to analysis.
-
Data were sought from a diverse group of respondents in terms of factors thought to be pertinent to experiences of SA or OM (e.g. age of child, infection, location of treatment, ethnicity, sex).
-
CB and AL conducted some ‘double’ coding of transcripts initially; the broader research team (with a variety of experience and expertise) were also involved in discussions about analysis and coding.
-
Member checking – a summary of the study’s findings was circulated to all participants, who were contacted to seek their views about the study, to ask if findings ‘rang true’ and if there was anything they would like to add.
-
We invited all interview participants to attend a stakeholder day at which findings from the qualitative study were presented to the wider clinical team involved in the service evaluation and Delphi study.
Findings
Demographic data
Twenty-six families were recruited to the study. Families recruited represented children ranging in age from 0 to 16 years at the time of treatment. Seventeen children were male and nine were female. The majority of families were white British, with two families being European, one Pakistani and one Chinese/Caucasian. In terms of specific condition treated, 11 were treated for OM, 11 for SA and four children had both SA and OM. The parent of one of these four OM/SA children reported that the infection had been caused by GAS and the parent of another reported the cause as PVL-positive S. aureus. Children had been treated in Southampton (n = 11), Bristol (n = 4), Oxford (n = 9) or London (n = 2).
Themes
The key themes that emerged from the data are discussed below. After each theme a short discussion of its implications for the feasibility of a future trial, and more broadly, is presented.
Theme 1: experiences of diagnosis and seeking treatment
One of the most striking issues to emerge during the course of interviews was the length of time reported to have elapsed between children first presenting with symptoms and a diagnosis being given/treatment starting. Although this information was not explicitly sought as part of the interview guide, this point was repeatedly mentioned by interviewees. It also appears to have some relevance to parents’ subsequent thinking about the design of the potential RCT (discussed further in Theme 4: views on proposed randomised controlled trial).
Respondents often described a process of to-ing and fro-ing between GPs and A&E (or equivalent departments) at local hospitals over a period of days or weeks before a diagnosis was made. In a number of cases, children were admitted to local hospitals for investigations during the pre-diagnosis period.
Children presenting at GP surgeries were often referred immediately to a local hospital, but once their symptoms could be attributed to a range of factors other than bone or joint infection (such as injury, irritable hip, synovitis or Erb’s palsy). This period of delay in diagnosis, at a time when parents were convinced that there was something more serious wrong with their child, was described as frustrating and anxiety provoking:
I took him to the GP the following day and he was just complaining of a pain in his knee. Basically for the next 2 weeks we kept going backwards and forwards to the GP. I was told to take him to hospital to get his knee X-rayed, which they did, and that all came back fine and I was told he had some soft tissue damage. I felt that there was something wrong with him. I was very worried, but then I suppose I was beginning to think am I just being neurotic because five different doctors have told me that he’s fine, so are we just being neurotic?
Mother, patient 365
A number of families also spoke of how much their child deteriorated during this period:
We didn’t go to his GP, we bypassed it to (local) hospital . . . They said, ‘Look, the best thing is come back in a day or so, come back and we’ll put his leg in plaster. It may improve in that period of time’. So we go, no painkillers, no anything. We then went for this other appointment and they turned around and said, ‘Look, we’ve made an appointment with a bone specialist’. So we go to the bone specialist, this appointment, which was another week or so on again. In the process, he turned around and said, ‘We can’t really find any particular damage or crack or anything’, this is what this bone specialist said. He said, ‘Give it another week, then come back’. Within that last week he then was in dire straits with pain. He was in terrible pain . . . he’s to the point he’s literally got his head on my shoulder, he can’t go on any longer. We’re in the waiting room, he was literally slumped over me and I’ve got him in my arms.
Grandfather, patient 349
Issues including an apparent lack of pain, absence of fever, referred pain and pursuing alternative diagnoses were all highlighted as factors contributing to delay in diagnosis. Rarity of OM/SA was seen also seen as contributing to a slower diagnosis, as was the lack of experience of a number of health professionals, in particular within A&E departments where staff may be more junior.
Consultants with relevant expertise (or other professionals recognising symptoms, wherever they were located in the system) were praised for their expert knowledge. Contact with these experts after a period of delay in which parents felt untrusting of advice they were given was experienced as very reassuring and, at times, even life-saving:
If it hadn’t have been for him I think we were in big trouble. I’ll tell you that now. What his name was, who he was, I don’t know but if I could shake his hand I would do because he was the one who got it under way. He knew there was something . . . wrong with [name of child], it was him who done it . . . Being fair with you, when we went for the X-ray, they didn’t come up with the right answers so weeks and weeks were going past. That was the problem but he sussed it. He knew straightaway. He said, ‘And what sort of pain?’ and that was it, he’d already come to me, he said, ‘I’m not happy with this at all’, then it kicked off. Thank heavens it was for him . . . I don’t know where we’d be today if they hadn’t have got behind it and that fellow. If it had been left another few days I don’t think we’d have had [name of child] back here, that’s how bad it was.
Grandfather, patient 349
For these families, experience of treatment was often described as being in two halves: an unsatisfactory experience up to diagnosis, characterised by period of delay and frustration, followed by the treatment period with antibiotic and operation as necessary, often perceived by parents to be much more successful, in the hands of trusted experts.
A smaller number of families, however, did report positive experiences of diagnosis and swift commencement to treatment. In these cases, alignment of expert professionals occurred such that referral between different treatment locations went smoothly and symptoms were recognised without delay. Positive experiences at the start of the illness offered a number of benefits for families:
-
Early experience of treatment/seeking diagnosis was less anxiety provoking and frustrating overall.
-
Families felt that their concerns were being listened to and acted on.
-
The child’s condition was less likely to deteriorate because of early commencement of treatment.
-
Overall experience was less negatively impactful, with potential ramifications for thinking about potential clinical trial.
Respondents recognised that a number of factors about the condition itself could confound an early diagnosis (e.g. symptoms like that of an injury, absence of temperature or visible swelling/red patch on skin). However, it did often appear that the level of awareness and expertise of the professional with whom they came into contact directly affected how quickly symptoms were recognised to indicate bone or joint infections. This was evidenced by a number of instances in which children with unchanged symptoms were quickly diagnosed when a second opinion was sought from a practitioner with more experience in this field. Such experiences suggest that increased awareness among health professionals, in general, of the possibility of bone/joint infection may enhance trajectories of care:
I understood all of that but I guess after 2 weeks when it wasn’t better I just wonder whether there are better guidelines that GPs or A&E practitioners could have in terms of – there must be decision trees and it could have been irritable hip but it’s not getting better as quickly as it should have done with irritable hip, so what other things could it be? Instead of being fixated on irritable hip and carrying on X-raying my daughter’s hip and not her knee, what else could we look for and check for? She had a slightly hot knee and she had one knee slightly bigger than the other and I could see it and the private consultant could see it but nobody in A&E or the GP could really see it.
Mother, patient 281
Theme 2: experiences and understanding of antibiotic therapy
This theme reflects the experiences, knowledge and views of parents and children about both i.v. and oral antibiotic treatment. All participants understood the need for the use of i.v. antibiotics and identified the positive benefits of making them/their child better in the fastest, most effective, way possible:
It seemed to be really, really important that the antibiotics were administered in that way and the explanation I was given seemed to make sense to me as somebody without any medical knowledge at all. I understood that it was really important that the antibiotics really got to where they needed to go as quickly as possible and that time was really important at this stage and that that was the best way of treating. So, I was totally in favour of having that treatment, if they’d said, ‘Look, let’s not bother with the cannula, let’s just go to oral’, having had that explanation before, I would’ve been really uneasy with that. I wanted him to have the i.v. treatment . . . I just felt that it would just get rid of the bug that was making him ill and get rid of it as quickly as possible and make him better and also to reduce the possibility of long-term complications and any damage to the joint.
Mother, patient 204
Most participants understood i.v. antibiotics to be an intensive, strong form of treatment that, because injected straight into the bloodstream, would reach the site of infection in the shortest possible time. As alluded to in the quotation below, a smaller number of respondents also pointed out the benefit of the guaranteed delivery of antibiotic:
I mean it was horrible just to get the cannula for the whole week, but also it was reassuring to know that he was getting all the antibiotic because you never know. Sometimes when he was in there with the oral one sometimes he spit a little bit so you were worried, you’re not allowed to waste any of this because it might be really necessary for you.
Yes, that’s true. The most reassuring thing is that you know that everything that should go in, the treatment, is going in.
Everything was going through.
Parents, patient 178
Notwithstanding the perceived need for i.v. antibiotic treatment, a number of negative experiences related to the i.v. delivery was reported. Of particular significance was pain associated with insertion of the cannula, which was often described as a difficult and stressful process. Parents used emotive language to convey the distress this caused their children, and therefore also to them, using descriptors such as ‘traumatic’, ‘grim’, ‘horrible’ and even ‘barbaric’:
[T]hey could not cannulate him, it was just this horrific scenario of two or three doctors in the room with me trying to hold this baby who’d been through so, he was only 13 months at the time, he’d been through so much I felt. He was just being prodded and poked and he was, as you would, trying to wriggle away and I was trying to hold him down so they could do this and it just felt really, really wrong and horrible, because I was the person that he trusted and that I was helping this to happen and he didn’t understand. I knew that it was being done for the right reasons, but it just, from his point of view, I could just feel this must be awful for him that the people he trusts are basically contributing to making this really, really traumatic situation. So, it still makes me emotional, as you can see, talking about it.
Mother, patient 204
There was a suggestion by some parents that health professionals did not always show sufficient empathy of the level of distress children and parents were experiencing at this time. Other negative experiences reported were allergic reactions to antibiotics administered intravenously (resulting in rashes and abdominal pains), pain when the drug was being administered and need to re-insert the cannula (or replace with PICC line) if it was pulled out or veins collapsed. A number of children also expressed their discomfort at the administration of i.v. antibiotics:
. . . it felt like something biting me with only one tooth.
Patient 233
Yes, which I didn’t like it. It really hurt.
Patient 276
It is also important to note that not all patients experienced distress at the administration of i.v. antibiotics.
He was amazing with it really. I don’t know if it was his age, he just didn’t let it bother him too much.
Mother, patient 285
In particular, parents spoke highly of the skill of community nurses who came to administer i.v. antibiotics in the child’s home in putting children at ease and making delivery of antibiotics as easy as possible.
In contrast, parents often described oral antibiotics as a less intensive treatment, used to finish off the course, more as a backup or precaution than a first-line treatment. Oral antibiotics were also perceived as a more convenient way of taking medicine that fitted better with the family’s routine and enabled the child to be at home. Often parents reported that their child took the medicine with no problems. For others, though, administering oral antibiotics to children who really disliked the taste was challenging and in a number of cases resulted in tummy upsets and sickness. One child completely refused oral antibiotics, resulting in a longer course of i.v. antibiotics. Two parents reported that their children’s teeth had suffered brown staining from the use of oral antibiotics.
The patient quoted below made her distaste for the medicine clear:
It was cream and [makes a disgusted-sounding noise]. Oh, I don’t even know, it just smelt gross . . . and then when mum had to mix it up a few days later there was loads of bits in it, and I was like [makes a disgusted-sounding noise], ‘I’m not drinking that’, but I had to . . . So I literally went [demonstrates swallowing fast].
Did you have to have some chocolate or something afterwards?
No, just water [makes a disgusted-sounding noise].
In line with this, a small number of parents (and children) expressed that they would prefer a longer course of i.v. treatment rather than a combined course including oral antibiotics. Despite the reported challenges, parents and children persevered with the oral course of treatment, coming off these medicines only when the course was finished, or on specific medical advice.
Participants were specifically asked about their views about the point during treatment when children were ‘switched’ from i.v. to oral antibiotics. Respondents were in general happy about the timing of the switch, feeling that their child had made sufficient progress by this time. Often this was perceived to have been guided by the results of blood tests and respondents were happy to follow the doctors’ lead as to the timing of the switch.
Respondents also indicated that they felt that the medical team had listened to their own particular concerns regarding a desire to leave hospital and try oral antibiotics:
He wanted to come home, I mean we’d been in there that long but they were still . . . They were a bit, like, whether to let him home but as long as he took the antibiotics orally but if anything happened we were to go straight back. Well there was a specialist from [another hospital], a lady specialist . . . She said, ‘that’s where I am. If there’s any indication you’re not happy, either bring him or ring me and then we’ll get things under way, we’ll bring him back in’. She was just like, ‘Ring our line straightaway and one of us will be here waiting for you’. They were brilliant, they were really good.
Grandfather and mother, patient 349
In a small number of cases the parents had experienced some anxiety that their children were being switched to oral antibiotics too early:
My only concern was that we’d been told that you normally treat the osteo, they do three weeks of i.v. antibiotics and I suppose I was probably over-thinking it. I was thinking that, although he’s had 3-and-a-half weeks, he had 1 week of an antibiotic that effectively didn’t do anything because it wasn’t for the right bacteria, so in terms of the right antibiotic he’d only had that for 2 weeks 4 days I think so I felt a little bit concerned about that and I asked about that and was just told that you don’t know for sure what exactly is the right number of days. Is it 2 weeks or is it 3 weeks? I think based on his progress and bloods and X-rays they decided that that was all right, so I did feel slightly anxious about that.
Mother, patient 365
In two cases, parents expressed concerns that their children had not been ready to leave hospital, resulting in them being re-admitted at a later stage.
Theme 3: signs and outcomes of recovery
This theme reflects participants’ views about factors, such as activity levels, that indicated to them personally that children were recovering from OM/SA, and also their longer-term evaluation of treatment. Parents were asked how they first recognised that their children were getting better. In order of frequency, comments were made in relation to resumption of movement/walking/weight bearing, being ‘better in themselves’ and improved appetite (with associated weight gain in some cases). In terms of speed of recovery, responses were varied, with a number of parents commenting that their children seemed to ‘bounce back’ very quickly, but others reflecting that recovery was a slow and gradual process.
When asked which outcomes were important to them in judging the success of the treatment, the following were offered: no ill effects on bone/joint/growth, activity levels resumed, infection gone and no recurrence of infection. In the majority of cases parents reported that there were no long-term impacts of the condition:
Yes, successful, because then we had a follow-up appointment at the end of February of that year where he had an X-ray and a consultation with the orthopaedic consultant and she was really happy with how it looked and then we had another follow-up in the August, so 6 months later where they said, ‘This is totally fine, there’s been absolutely no impact on the growth or anything. Please just forget about it. Don’t worry about it’.
Parent, patient 204
There were a small number of exceptions to this. The parents of one child who was newborn at the time of infection reported that the infection had affected her growth plate, but that the impacts of this were as yet unknown.
They say it has definitely affected the growing.
And then it will just catch up or it won’t catch up they said . . . They said that the top of her humerus isn’t as visible as it should be.
Yes, so it was definite that the infection has affected it.
Mother and father, patient 343
In another instance, doctors had been unable to resolve the infection despite repeated courses of antibiotics and the child continued to experience painful symptoms, with ongoing visits to the hospital to try to resolve the situation. This appeared to be due to a longstanding infection present since an earlier abscess. The longevity of the condition was upsetting and wearing for the child and her family:
One of the doctors, the surgeons, she said maybe she will need more surgery, because the infection hasn’t gone yet, it’s still there, because it went chronic. It was there for a long time . . . they’re not sure if it was something to do with the first operation when they took the abscess out and they left the wound open, just to heal from inside out. So they don’t know if it was from there or not . . . She keeps on complaining. Every day she takes tablets for the pain. Every day. Yes, she has temperatures, she has night sweats every day. She can’t sleep when she’s in pain. She has to wake up 6 o’clock in the morning to go to school and she feels really tired and it’s hard sometimes when she comes, ‘Mummy, I’m so tired, can I stay at home?’.
Mother, patient 202
Two other children, although recovered from their bone or joint infections, had ongoing bone problems and therefore continued to experience discomfort and undergo further medical treatment and potentially further operations.
Despite physical recovery of their children, a number of parents continued to experience a level of anxiety and heightened vigilance with regards to the possibility of a re-emergence of the condition. Parents often described the whole episode of their child being unwell as highly anxiety provoking and distressing:
We have had one bit where we got a bit scared thinking perhaps was he getting it again, but it didn’t. So I guess what I’m trying to say is we’re very aware of it.
Father, patient 250
He didn’t have any significant cuts that had obviously been infected, so if it was through a cut it was one small one that I hadn’t really noticed. I’ve now got a little thing of Dettol spray [Reckitt Benckiser, Slough UK], so I drive him mad by every time he gets a little scratch I’m chasing him round spraying this stuff on him to clean him.
Mother, patient 365
Theme 4: views on proposed randomised controlled trial
In response to the question ‘Would you be willing for your child to take part in a (hypothetical) trial?’ supported with the process diagram and additional description of trial (see Report supplementary material 5), parents were a little more likely to respond positively than negatively. Those who expressed positive views about the trial gave the following reasons for being supportive in principle: personal interest and belief in medical research; belief that enrolment in a trial would ensure good care and better provision of information about treatment; and being supportive of the aim to reduce antibiotic therapy (and shorten treatment time), illustrated in the following excerpt:
Although personally, I normally say yes to trials because it often means we get better care – or at least more follow-up and stuff. But yes, so I suppose that’s just a thought that crossed my mind. I think it’s a good idea to want to not overtreat, basically, isn’t it, not give antibiotics longer than you have to. You need to have evidence about whether that’s an effective thing to do, to cut down the length of antibiotics.
Mother, patient 214
Those who gave negative opinions about the trial presented two main concerns: fears about the risks of a potentially shorter course of treatment and that a ‘set’ or ‘scheduled’ trial treatment would not be sufficiently flexible to respond sufficiently to the individual needs of patients, putting them at unnecessary risk. There appeared to be some link (although not in every case) between families’ views about participation in a potential trial and how seriously ill their child had been, including the length of time taken for diagnosis and, thus, how much deterioration (and fears of long-term damage) had taken place during this time.
The two quotations below illustrate this. In the case of patient 349, the delay in diagnosis led to the patient becoming seriously ill, whereas a less prolonged delay in the case of patient 350 led to parental fears about possibilities of long-term damage:
Reading through your reasons for proposing the study, which is to reduce the length of time and hopefully not reduce the risk, sorry not increase the risk. If the situation was different and we had a diagnosis on the first day, then my decision might have been different, but, no, I was glad it was of the length that it was and I wouldn’t have questioned, as a precaution, if they wanted it to be longer still. I would have had concerns if it was shortened, but that was because of our situation that we were in, that the 8 days, I think it was 9 days from when we first noticed the symptoms to seeing him and actually having the first [signal breaks up] . . . so my concern was that the longer that the infection had a hold, the greater the risk that there might be a long-term problem, either [signal breaks up] eradicate the infection totally or having any deterioration in his joint in the long term.
Father, patient 350 (expressing that a desire for a longer treatment period would make him unwilling for his son to join the trial)
If it had been left another few days I don’t think we’d have had [child] back here, that’s how bad it was. I would recommend no set pieces. Let the doctors decide what they’re going to do, let them have the say, no set guidelines on that one, that’s crazy. Let them do what they’ve got to. That’s what they did with him, they didn’t hesitate. There was no set pieces. They put this into motion. Whatever he needed, they gave him, that was it.
Grandfather, patient 349
Such findings suggest that heightened concerns about the risks of harm to children may reduce potential willingness to participate in a trial. This was indicated in other ways by respondents. Families positively disposed to participation in such a trial nevertheless required reassurances about safety in information presented. Information required included details of how their child would be monitored to ensure that infection was being treated successfully, the trajectory of care in case of failure to recover on a shorter course of antibiotics, data to support the safety of shorter arm and equipoise:
I guess it would be in the way it’s presented and the information that patients have, as in the outcomes maybe of those other areas where they have used a shorter course and they still had positive outcomes. I guess also would there be those caveats that, say you had been allocated the treatment B and at the end of day 25 or 26, that there was a noticeably, that wasn’t working or there was still an issue, surely there’d be a duty of care or something to put in that you wouldn’t just take them off anyway and see what happened? Or would there be? Do you know what I mean?
Mother, patient 350
Some parents suggested that children with more severe or difficult to treat OM/SA would be less suited to enrolment in a trial:
And I suppose it depends where it goes, where you get it as well. Because her feet are like, the blood supply is not very good and the chances are that they couldn’t do it there because it wouldn’t get through and it’s so deep in the bottom of her foot. Whereas if you have it in your knee, the antibiotics might work within 2 days, mightn’t they? That would depend how poorly your child is, wouldn’t it? It depends how ill you were wouldn’t it? It would depend how bad it is.
Mother, patient 248
Other factors that may dissuade parents from participating would be the need for additional invasive interventions or significant disruption of families’ routines.
The way in which information about the trial is presented was recognised as critical by respondents – who delivered it appeared less important – other than it should be done by someone knowledgeable and that consistent messages about the trial should be delivered by all those involved in a child’s care. Above all, parents would be looking for reassurance that participation in the trial would not be putting their child at increased risk:
I guess I’d have wanted to. I mean I work in a university where research is really important, so I would have wanted to. If I was given the right information and I thought that it was safe, then I would have done. If I was in any doubt that it might not have been safe, then I would have said no.
Mother, patient 247
Theme 5: timings, parental anxiety and capacity to absorb information
The final theme emerging from data analysis concerns the level of anxiety and distress experienced by parents at the time of their child’s illness and treatment. Key or ‘pinch’ points of anxiety for parents included the lead-up to diagnosis (especially in case of delays), occasions on which the child experienced distress (e.g. during insertion of a cannula), fears about their child receiving a general anaesthetic and some follow-up anxiety around the potential for future impacts or recurrence of infection.
Reflecting on these feelings, respondents reported that their capacity to think about, and remember, important information (about treatment as well as potentially about the trial) was reduced during this time – exacerbated by fear, heightened emotions, the strangeness/unexpected nature of the situation and input from many different professionals:
Because it’s quite an awkward time, when your child’s poorly and you’re not quite sure what’s wrong with them and you’re all a bit all over the place, your headspace is not really right and you don’t really know what’s going on and you’re just being led by what doctors and nurses are telling you, you’re quite terrified.
Mother, patient 174
So I think there were three, and that was why it was a little bit confusing because obviously one doctor is trying to co-ordinate these other two deciding what antibiotics and how much and this and that and then the other person looking after her joint. Yes, it was very confusing, and a lot of the time I think you are just so tired you don’t really take it all on and you are on your own, and then your husband comes along and says, ‘Oh, what happened this morning?’. I can’t really remember all of it.
Mother, patient 267
Bearing these responses in mind, participants suggested that the timing of the invitation to participate in the trial, along with associated information and the manner in which it is delivered, is likely to be key. Once antibiotics have been started and parents feel reassured that their child is receiving treatment, capacity to consider trial information is likely to be enhanced:
I personally think, yes, in order to make any sort of rational decisions about anything you have to know your child is safe. Particularly as, yes, I don’t know if your research would – I don’t think there’d be any reasons not to wait (until treatment had started), would there? Maybe there are, but I can see obviously if you’re testing something in the emergency period, you have to approach people before the thing happens. But if you don’t have to then I think you would be more likely to engage parents.
Mother, patient 214
A need for sensitivity and awareness of parental vulnerability at this time (in inviting participation in a trial, but also throughout the care trajectory) was also stressed:
. . . just remembering that sensitivity, that the person that you’re talking to, it’s the first time they’re in this situation and it’s very stressful and emotional. It’s just that giving a little bit of time I guess to process that information.
Mother, patient 350
If I’d been approached in a not very positive way it probably would have put me off to be honest.
Mother, patient 174
Discussion
In this section, we discuss findings arising from the thematic analysis of the interview data, particularly with reference to a future RCT. Some limitations of this qualitative study are also highlighted. We conclude with some brief recommendations for practice and for research.
Many families described the early stages of their child’s illness and seeking/commencement of treatment as a highly distressing and an anxiety-provoking time. However, once a working diagnosis had been obtained, expert advice given and antibiotic treatment commenced, parents began to feel reassured and less anxious, especially when signs of improvement were noted in their children. In the main, despite its challenges, families were satisfied with treatment for OM/SA and the outcomes achieved, most importantly, those of ‘no ill effects on bone/joint/growth’, ‘activity levels resumed’, ‘infection gone’ and no ‘recurrence of infection’.
One of the major issues highlighted by this empirical work was the delay experienced by many of the families in obtaining a diagnosis, and the consequent stress and anxiety experienced. Although not the direct focus of this qualitative study, the obvious concern experienced by families around the issue of diagnosis has led us to identify some recommendations both for future research and for practice. In terms of implications for participation in a future clinical trial, it is important to recognise that some children and families approached for recruitment may have experienced a long period of uncertainty and interaction with primary and secondary care prior to the definitive diagnosis. The child may have become seriously ill prior to a diagnosis being reached. These circumstances might increase family anxiety about long-term harm to their child and negatively impact on willingness to expose their child to any further uncertainty or ‘risk’. It is therefore important that research staff recruiting for a trial understand what the child and family have experienced prior to admission/recruitment and explore any continuing concerns.
Similarly, with regard to prior experiences of both i.v. and oral antibiotic therapy for OM/SA, although understanding the rationale for the different modes of delivery, parents and children in our study had both positive and negative experiences of both forms. It will therefore be important to provide an opportunity to discuss this, prior to inviting participation in the trial. Potential randomisation to either standard care or the intervention arm could therefore lead to anxiety about ‘more of the same’, and it may be important to provide reassurance in either eventuality.
From interviews, it is apparent that both children and parents had clear views about both how they knew they/their children were recovering and also on what they based their judgements about the success or otherwise of treatment for OM/SA. Some of these measures reflect clinical tools used both in everyday practice and in research, but others are less well reflected in commonly used measures in this specialism. Our recommendations for the future RCT, for practice and for general research reflect children/parental perspectives about indications of recovery and outcomes.
Although we recognise that participants in our study are probably more enthusiastic about research participation within a general sample of children with OM/SA and their parents (by virtue of having accepted the invitation to participate in this qualitative study), it was encouraging to note that more were positive than not about participation in a trial, at least in theory. The concerns of those who were potentially unwilling or not sure reflected anxiety about reduced courses of treatment and that rigid trial protocols might put their own child at risk. The previous section (see Theme 4: views on proposed randomised controlled trial) highlights a potential (albeit subjectively observed) correlation between length of time to diagnosis (and seriousness of child’s condition) and willingness to participate in a trial, which might perhaps suggest either an inclusion criterion or, at a minimum, the need to sensitively explore prior experiences and provide tailored reassurance and data. Families positively disposed to participation in such a trial would also require reassurances about safety in information presented. Information required included details of how a child would be monitored to ensure that infection was being dealt with, trajectory of care in case of failure to recover on shorter-course antibiotics, data to suggest safety of the shorter arm and equivalence/equipoise about treatment in both arms.
As highlighted previously (see Theme 1: experiences of diagnosis and seeking treatment), levels of anxiety and distress experienced by parents at the time of their child’s illness and treatment were at times high. Key or ‘pinch’ points of anxiety appeared to be during the lead-up to diagnosis, periods of child distress, anxiety about general anaesthetic and some follow-up anxiety around the potential for future impacts or recurrence of infection. Parents reported that they had diminished capacity during these times to attend to, absorb and reflect on information, relating to both treatment and potentially an invitation to participate in a trial. Awareness and empathy at these ‘pinch points’, plus avoidance of these where possible in presenting information, would be helpful and useful.
Limitations
-
Participants in this study had initially responded positively to an invitation to take part in the service evaluation phase of the study, and also to being approached with regard to inclusion in the qualitative interview phase. They are therefore unlikely to be representative of the general population of children/parents treated for OM/SA.
-
Via purposive sampling, invitations to participate in this phase were extended to those treated within four centres in the south of England, and findings may not therefore represent those receiving different treatment regimens at different centres.
-
Although most of our interviews were carried out in person, a few were conducted over the telephone. Although there did not appear to be any difference in terms of the quality of data generated, it is possible that as a result of the difficulty in responding to non-verbal cues, these interviews were slightly less responsive to participants’ emotions/agendas.
-
For practical reasons of time/convenience, interviews with children were usually carried out in the presence of parents, reducing the opportunity to explore differences in perspective.
Recommendations
For a future randomised controlled trial
-
There is an opportunity for parents and children to share their experiences with research staff prior to invitation to participate in the trial, and reassurance that inclusion in the RCT will not further compromise the child’s health.
-
This is also an opportunity for the child and their family to discuss previous experiences (including negative) of both modes of antibiotic delivery, and provide reassurance about randomisation to either arm.
-
Pre-trial information given should provide:
-
clear information about the oral to i.v. antibiotic switch criteria and reassurance that the trial protocol would not over-ride the individual needs of the child in terms of when the switch from i.v. to oral antibiotics is made
-
data to support the switch point in both standard care and the experimental intervention
-
details of how the child would be monitored to ensure that infection was being dealt with
-
trajectory of care in case of failure to recover in shorter treatment arm
-
data to demonstrate safety of the shorter arm
-
information regarding the equivalence/equipoise about treatment in both arms.
-
-
Consideration should be given to short-term outcomes that accord with children and parents’ observations (i.e. appetite and weight, temperature, pain, weight bearing on affected limb, functional movement in activities of daily living, X-ray findings).
-
Longer outcome measures that reflect children’s and parents’ perspectives and priorities (i.e. functional activity and mobility levels, pain, end of infection with no recurrence, impact on bone/joint/growth) should be included.
-
Consideration should be given to whether or not an exclusion criterion relating to the seriousness of child’s condition and/or previous trajectory leading to diagnosis should be included.
-
If no exclusions are made based on severity, particular care and attention to explanations about treatment and risk in a trial will be needed for those with difficult prior experiences, attending to the children’s and parents’ specific concerns about risk.
-
Where possible, researchers should avoid ‘pinch points’ of anxiety/distress when inviting trial participation. If such timing is unavoidable, parents and children will need to be offered clear, written information, supported by verbal explanation, with time to reflect and several opportunities to revisit/ask questions.
-
Discussion about perceptions of risks, precautions and safety within the context of a trial will be required to check understanding.
For practice
-
Information about signs, symptoms and diagnosis of OM and SA could be more readily available to frontline clinicians (e.g. GPs, junior doctors in A&E), with access to more experienced colleagues for a second opinion.
-
Clinicians could provide more clarity around:
-
orientation to parents’/families’ concern about the child and trust in their judgement as a stimulus to further clinical tests, in the absence of any clinical diagnoses
-
understanding and empathy with child/family during the course of both i.v. and oral antibiotic treatment, recognising that previous experience might lead to anxiety
-
discussion with parents about strategies for addressing problems with administration of oral antibiotics (e.g. objection to taste, teeth staining, upset stomach and vomiting)
-
exploration of potential of health-care providers to safely administer i.v. antibiotics at home, in order to facilitate faster discharge
-
sharing switch criteria (e.g. blood test results) and decision-making from i.v. to oral antibiotics with child and parents
-
reassurance and explanation about routes to infection in OM/SA
-
discussion and explanation about how children- and parent-focused outcomes are reflected/used in clinical decision-making
-
discussion about understandings of risks and outcomes in relation to OM/SA both during treatment and at follow-up appointments.
-
-
The provision of written resources to support information provision at particularly stressful times during the care trajectory may be useful, for example about treatment, risk and outcomes.
-
Signposting to reliable and trustworthy information on a website might also prove reassuring and helpful.
For future research more generally
-
It may be useful to:
-
explore and evaluate strategies to improve the speed and accuracy of diagnosis among non-specialist primary and secondary care providers at initial point of contact
-
research improved delivery mechanism of oral antibiotics for children (e.g. taste, consistency)
-
develop and test sensitive and robust patient-reported outcome measures for children recovering from OM/SA, including quality-of-life measures
-
investigate the possible correlation between prior negative OM/SA illness experiences (e.g. serious illness, long time to diagnosis) and willingness to participate in RCT, plus effectiveness of strategies to address specific concerns (e.g. child/parent-focused information provision)
-
explore mitigating strategies as times of maximum stress during the child’s illness trajectory (e.g. optimum level of information provision, whether or not critical information provided through more than one medium is more easily retained).
-
Chapter 5 Systematic literature review
Aims and objectives
The aim of this component of the study was to use a systematic review to inform development of a core outcome set to use in a future RCT of shortened duration of antibiotic therapy for paediatric bone and joint infections.
Methods
Types of study included in the review, types of intervention and types of participant
The research group recognised that there is a lack of high-quality data in this clinical area and that previous RCTs investigating the duration of antibiotic treatment have generally been small. As no large RCTs were expected to be found, the review aimed to include:
-
large or small RCTs, retrospective cohort studies and case series
-
any study investigating duration of antibiotic therapy used for bone and joint infection, regardless of what antibiotic agent was used, or the duration of i.v. versus oral antibiotic therapy
-
all children from birth to 16 years treated with systemic antibiotics for bone and joint infections, regardless of surgical intervention, comorbidities or site affected.
Exclusion criteria
Studies investigating the use of surgically placed antibiotic beads or other local antibiotic treatment were excluded. In addition, predominantly adult studies with small numbers of children were also excluded because the criteria for adult treatment are not the same as for paediatric cases, and there are different outcome risks.
Search strategy
EMBASE and MEDLINE databases were searched using the search strategy shown in Appendix 1. All publications from 1946 to the present were included in the search. The search was carried out in May 2012 and updated in July 2014.
Results
Eligibility of studies
Of 1321 papers identified, 218 were reviewed in detail. The remainder were excluded based on reviews of the title and, where necessary, the abstract.
Of the 218 papers that were reviewed in detail, 208 were excluded. Many were review articles discussing the existing literature, with no clear focus on intervention or outcome. All of the papers used in these articles were included in the original search. Articles comparing imaging techniques and surgical techniques were also excluded as the outcomes were not relevant to our study investigating antibiotic duration.
Two systematic reviews were selected, and the papers used analysed in detail. Le Saux et al. 44 found 12 prospective studies including one RCT comparing duration of antibiotics for acute haematogenous OM. The numbers of patients included in these studies were small, in some cases fewer than 10. However, overall, the conclusion was that there is no difference in outcome between long and short courses of antibiotic treatment. Of the papers used in this systematic review, three were selected for this analysis. The remainder did not have clearly stated outcomes or follow-up for patients.
Howard-Jones and Isaacs83 also published a systematic review of treatment of subacute and chronic pyogenic OM in 2010. This review looked at both studies comparing duration of antibiotic treatment and studies comparing different antibiotic agents. Of the papers used in this analysis, five were selected by the reviewers. Two of these had also been included in Le Saux et al. 44 One study was excluded because it predominantly included children with cellulitis and soft-tissue infection, and therefore outcomes would not be comparable to a group with only bone and joint infections. The other excluded papers did not clearly state the outcome measures used to define ‘cure’.
All of the studies reviewed in detail and selected for use in the stakeholder survey are detailed below. We used the following criteria for assessing papers and identifying outcomes:
-
Is the primary outcome clearly stated?
-
Is the primary outcome clearly defined so that another researcher would be able to reproduce its measurement?
-
Are any secondary outcomes clearly stated and defined?
-
Do the authors explain the use of the outcomes they have selected?
Extraction of data
Data were extracted from each of the papers by a single reviewer (PS) and checked by a second reviewer (SNF). These outcomes were then discussed with study clinical co-investigators, who also had the opportunity to review the papers, until there was consensus that all of the available outcomes had been extracted.
All of the papers are exclusively paediatric. The following data were extracted in the first instance: authors, journal and year of publication, type of study, number of cases included in the study and exclusion criteria. These are detailed in Table 69.
| Paper | Interventions | Study design and number of participants | Outcome measures |
|---|---|---|---|
| Pääkkönen et al. (2012)84 Scand J Inf Dis Shortened hospital stay for childhood bone and joint infections: analysis of 265 prospectively collected culture-positive cases in 1983–2005 |
2–4 days of i.v. antibiotics and up to 20 or 30 days of oral antibiotics for OM, up to 10 or 30 days of oral antibiotics for SA | RCT (culture positive only, culture negative excluded) 265 |
|
| Peltola et al. (2010)49 Pediatr Infect Dis J Short- versus long-term antimicrobial treatment for acute haematogenous osteomyelitis of childhood: prospective, randomised trial on 131 culture-positive cases |
2–4 days i.v. antibiotics; 9–108 days oral antibiotics | RCT (culture positive only, culture negative excluded) 131 |
|
| Bachur and Pagon (2007)85 Clin Pediatr Success of short-course parenteral antibiotic therapy for acute osteomyelitis of childhood |
≤ 7 days i.v. antibiotics vs. > 7 days i.v. antibiotics Median 4 days i.v. antibiotics |
Retrospective cohort 29, all uncomplicated, acute haematogenous OM |
|
| Zaoutis et al. (2009)58 Pediatrics Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children |
Prolonged i.v. antibiotics vs. early oral switch | Retrospective cohort 1969 |
|
| Messina et al. (2011)86 Pediatr Infect Dis J Trimethoprim-sulfamethoxazole therapy for children with acute osteomyelitis |
Trimethoprim-sulfamethoxazole, median duration 31 days | Prospective cohort 20 |
|
| Tetzlaff et al. (1978)87 J Pediatr Oral antibiotic therapy for skeletal infections of children. II. Therapy of osteomyelitis and suppurative arthritis |
7 days’ i.v. antibiotics then up to 6 weeks’ oral antibiotics Blood antibiotic concentrations monitored and dose adjusted |
Prospective cohort 35 |
|
| Ballock et al. (2009)46 J Paediatr Orthop A comparison of early versus late conversion from intravenous to oral therapy in the treatment of septic arthritis |
Prolonged i.v. antibiotics vs. early oral switch, prolonged total duration (median 7.4 days vs. median 18.6 days) Two different hospitals compared |
Retrospective cohort 186 |
|
| Kolyvas et al. (1980)88 Pediatrics Oral antibiotic therapy of skeletal infections in children |
72 hours of i.v. antibiotics then total of 4 weeks’ oral antibiotics vs. 4 weeks. i.v. antibiotics | RCT 15 |
|
| Jaberi et al. (2002)89 J Paediatr Orthop Short-term intravenous antibiotic treatment of acute hematogenous bone and joint infection in children: a prospective randomized trial |
All had surgical drainage prior to antibiotic treatment 7 days’ i.v. antibiotics vs. 14 days’ i.v. antibiotics for SA 10 days’ i.v. antibiotics vs. 21 days’ i.v. antibiotics for OM |
RCT 33 |
|
| Jagodzinski et al. (2009)90 J Paediatr Orthop Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children |
Assessed after 3 days’ i.v. antibiotics for oral conversion 86% converted to oral after 5 days All had 3 weeks’ oral antibiotics |
Prospective cohort 70 |
|
Conclusion
The key outcomes were combined and summarised, all of which are shown in Table 70. These were then discussed with the clinical co-investigator group, including paediatric infectious disease consultants, orthopaedic surgeons, general paediatricians and radiologists. Following this discussion, the important outcomes for a potential future RCT were identified (see Chapter 7).
| Outcome category | Specific outcome |
|---|---|
| Infection-related | Number of days to CRP of < 5 mg/l |
| Duration to resolution of fever | |
| Duration of hospital stay | |
| Duration to resolution of pain | |
| Need for one or more non-diagnostic surgical procedures | |
| Chronic OM | |
| Chronic arthritis | |
| Chronic myositis | |
| Amputation or fasciotomy | |
| Duration to CRP of < 20 mg/l | |
| Duration to CRP of less than one-third of highest value | |
| Duration to ESR of < 20 mm/hour | |
| Line- or antibiotic-related complication | Vomiting/diarrhoea/gastrointestinal |
| Agranulocytosis or neutropenia | |
| C. difficile infection | |
| Rash/adverse drug reaction | |
| Line infection | |
| Need for line removal or replacement | |
| Long-term outcome | Pain at 3 months |
| Pain at 12 months | |
| Limp/disability at 3 months | |
| Limp/disability at 12 months | |
| Number of cases symptom free at 12 months | |
| Treatment failure/recurrence of infection within 18 months | |
| Growth plate involvement/damage | |
| Rehospitalisation within 6 months | |
| Limb shortening or deformity (time frame hard to decide in future RCT, as could be 3–5 years’ follow-up) |
Chapter 6 Delphi survey to identify oral switch criteria, develop core outcomes and assess the overall feasibility of a future randomised controlled trial in children with bone and joint infections
Aims and objectives
Through a Delphi process we aimed to:
-
identify the oral switch criteria for use in a future RCT
-
develop a core outcome set for use in clinical trials of children with bone and joint infections
-
assess the overall feasibility of a future randomised clinical trial in children with bone and joint infections.
Methods
Delphi clinical stakeholder survey
Two rounds of Delphi process were performed. Programming for the online system was carried out by the Liverpool Medicines for Children Clinical Trials Unit (director, co-investigator PW). 91
Participants
Prior to the study, the national societies of the clinical stakeholder groups agreed that the Delphi survey could be sent to members via the society e-mail lists [those of the Royal College of Paediatrics and Child Health (RCPCH) Speciality Group for General Paediatrics, the British Paediatric Allergy, Immunology and Infection Group, British Society for Children’s Orthopaedic Surgery, the British Society or Paediatric Radiology and the British Society for Antimicrobial Chemotherapy]. In addition, as a result of the general rarity of the condition and the study requested, the study also made use of the wider RCPCH regional mailing lists to try to be able to invite a larger group of general paediatricians.
Clinical stakeholders were asked to participate if they had personally treated a child with SA or OM in the previous 12 months or had been part of a wider multidisciplinary team providing that clinical care. Although some of the participants were also principal investigators for the service evaluation, the survey was not limited to sites participating in the service evaluation in order to obtain as wide a range as possible of clinical opinion and views. In the invitation e-mail, all participants were also reminded of the importance of completing both rounds of the Delphi process.
Delphi round 1
The survey was presented in an online format (see Report supplementary material 6), with participants allocated a unique identifier (via name and e-mail address) to allow identification of individuals completing all rounds of the Delphi exercise. All participants were asked to record their grade and clinical role. At the beginning of the survey, participants were presented with selected information from the study service evaluation.
Questions that were asked:
-
What outcomes influence your decision to switch from i.v. to oral antibiotics when treating a child with OAI?
-
What outcomes influence the total duration of antibiotics when treating a child with OAI?
-
What line or treatment-related complications are important when using prolonged courses of antibiotics?
Participants were invited to score each of the outcomes listed using the Grading of Recommendations, Assessment, Development and Evaluations scale of 1 to 9, with 1–3 labelled ‘not important’, 4–6 labelled ‘important but not critical’ and 7–9 labelled ‘critical’. Participants were provided with an option to add additional outcomes that they think are relevant, together with a score for each outcome added.
Outcomes were listed alphabetically to avoid potential weighting of outcomes caused by the order in which they were displayed. For each outcome, the number of participants who scored the outcome and the distribution of scores (percentages of who had scored each outcome) were summarised by the stakeholder group. All outcomes were carried forward to Delphi round 2.
Predefined Delphi survey outcomes were the total number of registrations, a breakdown of respondents who had completed the survey and their inclusion in the initial e-mail invitation, the total number of respondents who completed the round, the total number of respondents in each stakeholder group, the percentage of respondents compared with potential respondents as identified from the information provided by clinical leads and the percentage of respondents from other sources (not included in the original e-mail invitation).
Following initial screening by two senior investigators (SNF and PW), the clinical co-investigators reviewed the additional outcomes added during round 1 and assessed if these differed significantly from those already on the list. Additional outcomes were added prior to round 2 for additional scoring.
Delphi round 2
In the second round of the Delphi process, each participant was presented with their individual scores from round 1 together with the number of respondents and distribution of scores for each outcome for their particular stakeholder group. As a result of the low numbers of paediatric microbiology respondents, the microbiology and clinical paediatric infection data were presented together.
Participants in round 2 were asked if they would be willing to participate in a future RCT.
Results
Participants
As a result of the national society e-mail distribution lists used to send out the invitation to as many clinical stakeholders as possible, it was not possible to accurately assess the denominator number of clinicians sent invitations for the general paediatrics and paediatric radiology clinical stakeholder groups. In addition, the number of orthopaedic surgeons actually in clinical paediatric practice was not accurately represented by the mailing list of the national society.
Engagement reflected both the expected uncommonness of the condition in general paediatric practice and that the most interested groups are paediatric orthopaedic specialists and paediatric infection specialists. Of note, only around 80 consultants are members of the RCPCH general paediatric special interest group. The ‘denominator’ of the number of paediatricians sent the invitation e-mail was impossible to establish with accuracy as we also attempted to send the e-mail to all RCPCH active members, but this was not reflected in co-investigator RCPCH members receiving the e-mail apparently ‘sent’. The responses of paediatric radiologists reflected the general literature opinion that treatment outcomes are clinical rather than radiological. Approximately half of British paediatric infection specialists participated, and approximately half of the known number of paediatric orthopaedic surgeons. Approximately 15 microbiologists were invited via the specialty’s e-mail list. Further accuracy of these data is unobtainable.
In round 1, 37 general paediatricians, 38 paediatric orthopaedic specialists, 34 paediatric infection specialists, four paediatric microbiologists and 13 paediatric radiologists took part. Of the 13 paediatric radiologists, seven answered ‘unable to score’ for every outcome.
The response rates in round 2 are shown in Table 71.
| Specialty | R2 response rate (number/R1 response) | % of R1 response |
|---|---|---|
| General paediatricians | 31/36a | 86.1 |
| Orthopaedic surgeons | 21/36b | 58.3 |
| Radiologists | 8/9c | 88.9 |
| Microbiologists | 4/4 | 100 |
| Paediatric infectious disease specialists | 33/33d | 100 |
Response bias was assessed between rounds 1 and 2 by comparing the average round 1 score from those responding in both rounds to those responding in round 1 only. There is no evidence of attrition bias.
Figure 5 shows the participant distribution of percentage of outcomes with changed scores between rounds 1 and 2 for the group as a whole. Three participants did not change their score for any outcome. These results suggest that including a second round in the Delphi was a useful exercise.
FIGURE 5.
Change in scores between rounds 1 and 2.
Summary of the answers from the Delphi process
A summary of the results is shown in Tables 72–76. Detailed tables on voting results are included in Appendix 2.
| Participants | Delphi survey round 1 | Delphi survey round 2 | Consensus meeting |
|---|---|---|---|
| Number of participants | 126 | 97 | 17 |
| Number of respondents who gave a rating for at least one CRP outcome | 116 | 92 | 17 |
| Number who scored at least one CRP outcome ≥ 7 (A) | 72 | 55 | 12 |
| Percentage (A/n) | 62.1 | 59.8 | 70.6 |
| Outcome: health professionals | Outcome: parents and young person | General paediatrics (%) | Orthopaedic surgeons (%) | Radiologists (%) | Microbiologists and paediatric infectious disease specialists (%) |
|---|---|---|---|---|---|
| CRP < 20 mg/l | Blood test showing level of inflammation in body | 39 | 38 | 0 | 38 |
| CRP less than one-third of the presenting value | 10 | 19 | 67 | 28 | |
| CRP less than two-thirds of the presenting value | 13 | 0 | 33 | 17 | |
| ESR < 20 mm/hour | Blood test showing level of inflammation in the body (not used as often in children as it needs more blood for technical reasons) | 3 | 10 | 0 | 0 |
| Resolution of fever for ≥ 48 hours | Childs’ body temperature has been normal for past 48 hours | 90a | 95a | 100a | 86a |
| Weight bearing/return of function to limb | Child able to put weight on affected limb/use affected body part as normal | 55 | 71a | 67 | 65 |
| Resolution of pain | Child no longer feels any pain | 48 | 52 | 0 | 43 |
| Tolerating oral input | Child able to drink and eat | 83a | 9 | 0 | 97a |
| Parental opinion regarding improvement or worsening of condition | Parents opinion about wellness of their child | 16 | 5 | 75a | 25 |
| Pain improvement (rather than resolution) as oral switch criteria | Less pain felt by child | 13 | 33 | 100a | 33 |
| Outcome: health professionals | Outcome: parents and young person | General paediatrics (%) | Orthopaedic surgeons (%) | Radiologists (%) | Microbiologists and paediatric infectious disease specialists (%) |
|---|---|---|---|---|---|
| Need for non-diagnostic surgical procedure (e.g. decompression, debridement) | Child has had to have surgery in order to treat the infection | 48 | 38 | 60 | 61 |
| Rehospitalisation or recurrence of symptoms while on oral antibiotics | Child has returned to the hospital or symptoms have returned while taking antibiotics by mouth | 97a | 95a | 100a | 97a |
| Treatment failure: recurrence of infection (either at original site or at new site) | Child has a new infection in the same area or a different area | 100a | 100a | 100a | 100a |
| Pain while on antimicrobial treatment | Pain experienced by the child while being treated for the infection | 0 | 10 | 0 | 0 |
| Ongoing pain at follow-up | Pain still experienced at outpatient appointments | 19 | 38 | 0 | 16 |
| Disability at follow-up | Child has continuing movement problems at outpatient appointments | 81a | 67 | 67 | 78a |
| Symptom free at 1 year | Child has not experienced any symptoms after 1 year | 61 | 90a | 83a | 68 |
| Limb shortening or deformity | Child’s limbs have become shorter or changed shape/appearance | 87a | 80a | 83a | 76a |
| Duration to CRP < 5 mg/l | Time taken for inflammation level to return to normal in the body | 0 | 19 | 25 | 5 |
| Duration to resolution of fever | Time taken for child’s temperature to return to normal | 16 | 19 | 0 | 43 |
| Duration to resolution of pain | Time taken for pain to go away | 16 | 5 | 60 | 8 |
| Duration of hospital stay | Time child has had to stay in hospital | 10 | 5 | 20 | 27 |
| Fracture at or near site of infection | Bone broken in the same location as the infection or near to the infection | 34 | 40 | 67 | 44 |
| Prolonged antibiotic therapy | Antibiotics needed for longer than expected | 27 | 24 | 50 | 56 |
| Quality of life | Child’s ability to enjoy normal activities | 71a | 50 | 33 | 44 |
| Outcome: health professionals | Outcome: parents and young person | General paediatrics (%) | Orthopaedic surgeons (%) | Radiologists (%) | Microbiologists and paediatric infectious disease specialists (%) |
|---|---|---|---|---|---|
| Chronic OM | Infection of the bone that does not get better with treatment | 94a | 100a | 100a | 95a |
| Chronic arthritis | Joint inflammation that does not get better with treatment | 84a | 100a | 83a | 76a |
| Chronic myositis | Muscle inflammation that does not get better with treatment | 68 | 48 | 100a | 39 |
| Amputation or fasciotomy | Removal of body part by surgery or skin and muscle have to be cut by a surgeon to relieve pressure between the skin and bone | 97a | 86a | 100a | 94a |
| Death | Death | 97a | 100a | 100a | 94a |
| PICU requirement (e.g. PICU-free days at day 30, if admitted) | Admission into a ward (PICU) specialising in the care of critically ill children | 77a | 52 | 40 | 81a |
| Outcome: health professionals | Outcome: parents and young person | General paediatrics (%) | Orthopaedic surgeons (%) | Radiologists (%) | Microbiologists and paediatric infectious disease specialists (%) |
|---|---|---|---|---|---|
| Agranulocytosis/neutropenia | Child more prone to infection because of low white cell count in body as a result of infection or treatments | 32 | 43 | 75a | 64 |
| Deranged liver function | Detection of inflammation/damage to the liver | 3 | 14 | 0 | 47 |
| C. difficile infection | An infection affecting the digestive system caused by antibiotics (very rare in children under 2 years old) | 10 | 33 | 33 | 28 |
| Rash or allergic reaction | Rash or allergic reaction | 23 | 10 | 17 | 67 |
| Suspected drug-related fever | Child’s temperature increases in line with taking treatment | 19 | 5 | 20 | 44 |
| Line infection | Infection of the drip that is giving treatment to the patient | 45 | 33 | 33 | 83a |
| Line occlusion | Blockage of the drip that is giving treatment to the patient | 6 | 10 | 33 | 50 |
| Vomiting/diarrhoea | Sickness and diarrhoea | 3 | 5 | 17 | 17 |
| Insertion of PICC or other central line because of a need for prolonged antibiotics at home or in hospital | The need to provide the child with a drip that can be used for a longer period | 32 | 24 | 60 | 75a |
| Infiltration or extravasation event | Medicine being given into the veins is accidently delivered to the surrounding area as a result of the drip coming out of place | 29 | 10 | 17 | 50 |
| Any complication of vascular access | Any complication of the drip itself | 23 | 24 | 60 | 58 |
Chapter 7 Consensus meeting to assess the feasibility of a future randomised clinical trial in children with bone and joint infections
Aims and objectives
In the consensus meeting we aimed to:
-
identify the oral switch criteria for use in a future RCT
-
develop a core outcome set for use in clinical trials of children with bone and joint infections
-
assess the overall feasibility of a future randomised clinical trial in children with bone and joint infections.
Methods
A consensus meeting was held on 27 April 2015 and an invitation to attend the meeting was sent to:
-
health professionals who had completed both rounds of the online Delphi survey and expressed an interest in attending future meetings
-
principal investigators who had taken part in the service evaluation component of the study (although individuals were advised that, if they had not completed both rounds of the Delphi survey, they would be ineligible to formally vote but could be involved in discussions)
-
members of the Comprehensive Research Network – Paediatrics London Young Persons Advisory Group
-
all parents and children who had taken part in a qualitative interview (although none of the parents participating in the qualitative study was able to attend, the meeting was attended by the study PPI representative and a member of the Young Persons Advisory Group).
A separate session was scheduled immediately before the main consensus meeting for 30 minutes to allow the qualitative investigators to meet with the parents and young people who attended the meeting. This meeting allowed any questions to be answered about the structure of the day, expectations and for additional information to be given with relation to the switch and core outcomes.
The meeting was chaired by Professor Adam Finn (University of Bristol), a co-investigator who had not been part of the core study management group and who was a non-voting participant, to avoid the possibility of Saul Faust introducing bias into the discussion when voting, and Carrol Gamble, who monitored from a statistics perspective to ensure an unbiased session. The meeting was structured according to the Dinosaur consensus meeting agenda (see Report supplementary material 6). Preliminary results from the service evaluation (see Chapter 2) and qualitative study (see Chapter 4) were used to inform the consensus meeting. The definition of consensus is shown in Tables 77 and 78.
| Consensus classification | Description | Definition |
|---|---|---|
| Consensus in | Consensus that criterion is important when deciding when or whether or not to switch from i.v. to oral antibiotics | ≥ 70% participants scoring as 7–9 AND < 15% participants scoring as 1–3 |
| Consensus out | Consensus that criterion is not important when deciding when or whether or not to switch from i.v. to oral antibiotics | ≥ 70% participants scoring as 1–3 AND < 15% of participants scoring as 7–9 |
| No consensus | Uncertainty about importance of criterion | Anything else |
| Consensus classification | Description | Definition |
|---|---|---|
| Consensus in | Consensus that outcome should be included in the core outcome set | ≥ 70% participants scoring as 7–9 AND < 15% participants scoring as 1–3 |
| Consensus out | Consensus that outcome should not be included in the core outcome set | ≥ 70% participants scoring as 1–3 AND < 15% of participants scoring as 7–9 |
| No consensus | Uncertainty about importance of outcome | Anything else |
Results
Participants
Meeting attendees and their representation are shown in Tables 79 and 80. In the items below, co-investigators are referred to by initials (see Table 79) but other meeting participants by stakeholder group.
| Attendee | Stakeholder group/role | Initials |
|---|---|---|
| Professor Saul Fausta | Chief investigator, paediatric infectious diseases | SF |
| Professor Adam Finna | Meeting facilitator, paediatric infectious diseases | AF |
| Professor Nick Clarkea | Orthopaedic surgeon | NC |
| Priya Sukhtanker | Clinical research fellow | PS |
| Eileen Baildamb | Paediatric rheumatology specialist | EB |
| Deborah Eastwoodb | Orthopaedic surgeon | DE |
| Marieke Emontsb | Paediatric infectious disease specialist | ME |
| Katy Fidlerb | Paediatric infectious disease specialist | KF |
| Vana Gandhib | General paediatrician | VG |
| Adam Irwinb | Paediatric infectious disease specialist | AI |
| Dominic Kellyb | Paediatric infectious disease specialist | DK |
| Nick Makwanab | General paediatrician | NM |
| Nuria Martinez-Alierb | Paediatric infectious disease specialist | NMA |
| Piers Mitchellb | Orthopaedic surgeon | PM |
| Fergal Monsellb | Orthopaedic surgeon | FM |
| Karyn Moshalb | Paediatric infectious disease specialist | KM |
| Ann Pallettb | Microbiologist with an interest in paediatrics | AP |
| Colin Powella,b | General paediatrician | CP |
| Fiona Shackleyb | Paediatric infectious disease specialist | FS |
| Stefania Vergnanob | Paediatric infectious disease specialist | SV |
| Andy Wainwrightb | Orthopaedic surgeon | AW |
| Benjamin Jacobsc | General paediatrician | BJ |
| Kadri Ayoc | General paediatrician | KA |
| Kuldeep Stohrc | General Paediatrician | KS |
| Catherine Prichardc | Parent representative | CaP |
| Esme Lynchc | Young persons advice group representative | EL |
| Professor Carrol Gamblea | Statistician | CG |
| Claire Ballingera | Qualitative researcher | CB |
| Amanda Lees | Qualitative researcher | AL |
| Nicola Harman | Meeting co-ordinator | NH |
| Cath Spowart | Meeting co-ordinator | CS |
| Abigail Bennett | Meeting co-ordinator | AB |
| Chloe Middleton | Meeting co-ordinator | CM |
| Stakeholder group | Number of voting members attending the consensus meeting | Percentage representation at consensus meeting |
|---|---|---|
| General paediatrician | 3 | 18 |
| Microbiologist with an interest in paediatrics | 1 | 6 |
| Orthopaedic surgeon | 4 | 23 |
| Paediatric infectious disease specialist | 9 | 53 |
| Radiologist | 0 | 0 |
| Participants in qualitative studya | 0 | 0 |
Formal voting was performed via Turning Point software (www.turningtechnologies.co.uk/). Only those attendees who had previously completed rounds 1 and 2 of the Delphi survey were eligible to vote, but involvement in discussion of the switch criteria and outcome set was encouraged from all attendees. Two public contributors who were present voted separately using a paper-based voting system (as they had not been involved in the qualitative interviews). One was the patient and public representative from the study steering committee and had direct experience of a child suffering from the condition and the other was a ‘trials-experienced’ member of the Medicines for Children Young Persons Advice Group. Three clinicians attended the meeting who had not completed the online survey, votes again were collected using a paper-based voting system (two complete voting sets and one partial were produced). These votes have not been included in the results presented in this report.
Presentation of the service evaluation data
-
The definitions of complex and simple cases of SA or OM were discussed, as well as the numbers of each case type. Forty-nine cases of misdiagnosis were recorded and the meeting queried if the patients were treated appropriately in each of the cases. The data captured did not cover this information.
-
A query was raised regarding whether or not some infection sites were more common than others. Among the study population, the most commonly affected joints were hip, knee and ankle in simple cases and femur and elbow in complex cases (and that this reflects the historical literature).
-
The potential issue of missing data was raised, as 7% of children with simple disease and 3% with complex disease did not appear to have received i.v. antibiotic treatment. Although concerning, there are reports of oral therapy alone being used in recent European cohorts to treat bone and joint infections (oral data from Spain presented at European Society for Paediatric Infectious Diseases, Leipzig, May 2015; J Saavedra-Lozano, 2015, personal communication).
-
There seems to be a lack of detailed information about the date that the child/parents first noticed symptoms. The date of first presentation at the health-care service provider is recorded, but the meeting participants agreed that this is often days, if not weeks, after first symptoms appear.
-
The definition of diagnostic surgery was discussed as many of the surgeries listed as diagnostic would normally be seen as therapeutic. For the service evaluation, diagnosis was defined as the start of i.v. antibiotic therapy and therefore any surgery completed before this was included in the diagnostic category.
-
Surprise was expressed at the number of aspiration surgeries. Research nurses reported these based on surgeons’ notes and/or cross-checking discussions with surgeons.
-
The participants discussed and concluded that lavage should fall into arthrotomy and that they would expect the numbers of arthrotomy procedures to be much higher as this is standard practice for simple SA. The lower numbers of arthrotomy procedures is very likely a result of their inclusion in the ‘Incision and drainage’ category.
-
There were concerns over the low numbers of X-rays of abnormal bone, and it was hypothesised that this can be attributed to failure to follow the norms of standard practice in a few centres lying, thus skewing the results. This discrepancy could be caused by the department/specialist that the child is first referred to, or junior doctors who lack experience.
-
The number of blood cultures taken prior to initiation of antibiotic therapy is not available in the existing data set.
-
The preliminary results show that in only 42.3% of the 75.8% of children who underwent microbiology tests was at least one micro-organism detected, a figure that may have been expected to be higher.
-
Results of the service evaluation suggest that standard practice in the UK is short-course i.v. antibiotic therapy (see Chapter 2). The data also imply that average timing of i.v./oral switch is between 1 and 2 weeks. The data set does not provide information about if location of infection impacted the timing of switch or length of overall antibiotic treatment.
-
Concerns were shared regarding discrepancies in dosing of i.v. ceftriaxone. There was general agreement that in clinical practice a high (80 mg/kg) dose should be used for bone and joint infections (the manufacturer, Roche, in the European summary of product characteristics states up to 1000 mg/kg/day), but one participant noted that the BNFC allows a lower dose of 50 mg/kg.
-
It was noted that only 40% of children underwent a blood test at the time of switching. It was queried whether or not the blood tests could have been done a few days before, which would be standard practice but not captured in the database. All agreed that this was an important question in relation to future discussions for the trial.
-
The results found for length of stay in hospital were discussed; hospital stay could be influenced (1) as a result of patients being transferred to a district general hospital in the case of tertiary centres and (2) by the age of the patient.
-
The group noted that there were not as many complications as might be expected, but that this could be due to the short length of follow-up.
-
Discussed was whether or not pain at follow-up should be classified as a treatment failure.
Presentation of the qualitative study
-
Interviews provided a reminder that both i.v. and oral antibiotic therapy can be traumatic for children; some may tolerate tablets/liquid well and find the i.v. insertion stressful, while others may not be bothered by an i.v. line but find tablets/liquid hard to take/keep down. Parents need to feel that therapy for their child is tailored to their individual case and this may make recruiting to a randomised trial difficult as it removes the element of personalised care.
-
Parents expressed their concern that complex conditions might not be suited to a randomised trial and would remove any parental choice regarding the methods of treatment. The data indicated that the period before diagnosis causes a high level of anxiety and a longer safer standard treatment arm would be preferred.
-
The qualitative researchers had thought carefully about how to explain a RCT to the children interviewed. They concluded that it is important that all information (patient information leaflet/consent and assent) is tailored to young persons’ needs and that PPI is important at this stage. Parents may be more concerned with safety specifics, but a child/young person may be more focused on what is required of them (e.g. blood test). It is sometimes important to discuss the trial with the child without the parent/guardian present and it was pointed out that there appeared to be a lack of children’s opinions in the qualitative research presentation.
-
Timing of consent is important, as parents are unlikely to consent to a trial while waiting for their child to go in for surgery or come round from anaesthetic, for example. It was suggested that discussion of the trial should wait until treatment has started. Once the patient is settled, parents are more likely to be receptive to a trial.
-
It was also pointed out that bone and joint infection is perhaps not on a junior doctor’s ‘radar’. Parents felt the that speed of diagnosis was often down to the treating medic, and therefore luck. It was pointed out that a trial would increase awareness of bone and joint infection.
-
The group should consider any subgroups of patients that clinicians would not be comfortable randomising.
Discussion and voting results
Oral to intravenous antibiotic switch criteria
Results from voting on oral to i.v. antibiotic switch criteria are presented in Table 81. Overall, resolution of fever for ≥ 48 hours, tolerating oral input (medicines and food/drink) and pain improvement (rather than resolution) were considered important criteria by consensus following the meeting voting.
| Switch criterion | Percentage of meeting participants scoring 7–9 | Percentage of meeting participants scoring 1–3 | Consensus |
|---|---|---|---|
| CRP < 20 mg/l | 35.29 | 11.76 | No consensusa |
| CRP less than one-third of the presenting value | 23.53 | 17.65 | No consensusa |
| CRP less than two-thirds of the presenting value | 25.53 | 29.41 | No consensusa |
| ESR < 20 mm/hour | 11.76 | 47.06 | No consensus |
| Resolution of fever for ≥ 48 hours | 100 | 0 | Consensus in |
| Weight bearing/return of function to the limb | 64.71 | 5.88 | No consensus |
| Resolution of pain | 29.41 | 11.76 | No consensus |
| Tolerating oral input | 100 | 0 | Consensus in |
| Parental opinion regarding improvement or worsening of condition | 35.29 | 0 | No consensus |
| Pain improvement (rather than resolution) | 70.59 | 0 | Consensus in |
Although there was no clear consensus regarding the absolute or relative (to presentation) of the CRP, there was agreement in both rounds 1 and 2 of the Delphi survey that CRP is an important parameter in around 60% of respondents, and among approximately 70% of those at the consensus meeting. In addition, the patient representative felt that a CRP < 20 mg/l was important but not critical.
Discussion about switching criteria:
-
CRP levels are an important test for recognising improvement, but picking an arbitrary absolute CRP level may not be appropriate.
-
A two-thirds reduction in CRP might not be sufficient reduction if the baseline CRP was very high.
-
A very high CRP level could also indicate a complex case as opposed to simple and should therefore not be ignored.
-
If CRP was used as a switch criterion, parents could find conflicting evidence online and not wish to consent and/or withdraw their child from the study.
-
The overall trend should be more important than any particular fixed value.
Conclusion
Monitoring CRP in terms of absolute value and trend would be important despite consensus not being reached.
Core outcomes voting
A summary of core outcome consensus is presented in Table 82. Overall, the outcomes assessed as ‘consensus in’ voting include rehospitalisation or recurrence of symptoms while on oral antibiotics, treatment failure or recurrence of infection, disability at follow-up, being symptom free at 1 year, limb shortening or deformity, chronic OM or chronic arthritis, amputation or fasciotomy, death, need for paediatric intensive care and line infection.
| Outcome | Percentage of meeting participants scoring 7–9 | Percentage of meeting participants scoring 1–3 | Consensus |
|---|---|---|---|
| Need for non-diagnostic surgical procedure (e.g. decompression, debridement) | 25 | 25 | No consensus |
| Rehospitalisation or recurrence of symptoms while on oral antibiotics | 100 | 0 | Consensus in |
| Treatment failure or recurrence of infection | 100 | 0 | Consensus in |
| Pain while on antimicrobial treatment | 6.25 | 37.5 | No consensus |
| Ongoing pain at follow-up | 31.25 | 6.25 | No consensus |
| Disability at follow-up | 81.25 | 0 | Consensus in |
| Symptom free at 1 year | 100 | 0 | Consensus in |
| Limb shortening or deformity | 100 | 0 | Consensus in |
| Duration to CRP of < 5 mg/l | 18.75 | 43.75 | No consensus |
| Duration to resolution of fever | 31.25 | 18.75 | No consensus |
| Duration to resolution of pain | 18.75 | 6.25 | No consensus |
| Duration of hospital stay | 18.75 | 18.75 | No consensus |
| Fracture at or near site of infection | 56.25 | 0 | No consensus |
| Prolonged antibiotic therapy | 50 | 0 | No consensus |
| Quality of life | 62.5 | 0 | No consensus |
| Chronic OM | 100 | 0 | Consensus in |
| Chronic arthritis | 100 | 0 | Consensus in |
| Chronic myositis | 50 | 6.25 | No consensus |
| Amputation or fasciotomy | 100 | 0 | Consensus in |
| Death | 100 | 0 | Consensus in |
| PICU requirement (e.g. PICU-free days at day 30, if admitted) | 75 | 0 | Consensus in |
| Agranulocytosis/neutropenia | 56.25 | 0 | No consensus |
| Deranged liver function | 25 | 6.25 | No consensus |
| C. difficile infection | 18.75 | 12.5 | No consensus |
| Rash or allergic reaction | 37.5 | 0 | No consensus |
| Suspected drug-related fever | 6.25 | 25 | No consensus |
| Line infection | 81.25 | 0 | Consensus in |
| Line occlusion | 50 | 6.25 | No consensus |
| Vomiting/diarrhoea | 6.25 | 49.75 | No consensus |
| Intravenous antibiotic necessitated insertion of PICC or other central line | 31.25 | 18.75 | No consensus |
| Infiltration or extravasation event | 31.25 | 6.25 | No consensus |
| Any complication of vascular access | 50 | 0 | No consensus |
Discussion about core trial outcomes
-
All would be uncomfortable taking part in a study that included only outcomes that had been voted as critical.
-
Critical outcomes would form a core outcome set and other important outcomes would be included.
-
Twelve out of 16 participants felt that an outcome should be included for both parental and child stress/anxiety.
-
It was agreed that a barrier to collecting quality-of-life data would be that often in these cases the negative impacts may not be seen for several decades after recruitment.
Overall feasibility of future trial
It was noted that the number of cases reported in the service evaluation was very promising in terms of a future trial.
Standard care
Concerns about standard care in a future RCT:
-
The absence of any agreement on diagnosis, antibiotic dosing or defined standard care procedures as identified in the service evaluation is a concern for a future RCT. A study aiming to standardise care is a different research question for which it might be hard to attract funding as there is no specific clinically related research question. Part of the rationale for this Dinosaur study was to review the current UK practice and establish the parameters for current standard of care that can be tested against.
-
Delay in diagnosing simple bone and joint infections overall in UK settings. The process of running a study could itself help to define the approach to these cases and improve diagnosis and treatment by raising awareness in participating centres. There is therefore a need to ensure that a study is accessible to all and does not take place only in tertiary centres.
-
Is the service evaluation coherent with personal experience of clinicians? This is the largest prospective global study to date on childhood bone and joint infections, and part of the problem in this condition is that treatment has been based on personal experience and mostly retrospective small cohort studies.
-
Should primary care trusts be included in the future study to ensure that standard care is consistent across the board? Running the future study would be the best way to increase awareness of these conditions and improve normal care.
-
Is 3 months’ follow-up, as that used in service evaluation, long enough to capture long-term complications like growth problems? A relatively short follow-up is often standard practice (in routine clinical care of the simple cases being considered for a RCT) but that perhaps conducting this study could improve follow-up times for normal care.
-
A future trial needs to consider the cost to the NHS within research costings.
Adherence to potential trial treatments
Adherence to oral antibiotics regimens potentially can influence/disrupt results for trial treatments involving oral antibiotic therapy at home. The items discussed were:
-
Parents are often better than doctors or nurses at complying with antibiotic regimens.
-
Parents in the qualitative research had reported high levels of adherence to antibiotic therapy as parents had a strong understanding of the consequences of not completing the course. This finding could be influenced by a study effect and selection bias.
-
Clinical experience shows concerns about adherence.
-
Older children and young adults are often given responsibility for their own treatment by parents and may report a higher level of compliance than is the case.
-
Adult RCTs successfully used an oral treatment strategy.
Recruitment and inclusion criteria issues
-
Parents often self-inform and have preferences for their child’s treatment (length of i.v. therapy, PICC line, liquid/tablet form).
-
Clinician preference for one type of therapy over another has made it harder than anticipated to recruit to some paediatric clinical trials, for example the TORPEDO (Trial of Optimal Therapy for Pseudomonas Eradication in Cystic Fibrosis) trial. 92 Despite these issues the trial has proceeded and achieves a consent rate of approximately 40%.
-
Service evaluation data suggest that short-course i.v. therapy is now more common. Randomising patients to longer than normal treatment may reduce participation.
-
Parents are sometimes less willing to take part in randomised trials because of the perceived lack of personalised care. The design and patient/parent information would have to clearly explain this issue, as apparently shorter therapy duration may be off-putting to parents. Parents interviewed in the qualitative research were generally happy with the timing of their child’s switch from i.v. to oral antibiotics as they felt they were well enough to be switched.
-
Children with bony (rather than soft tissue) abnormalities on X-ray imaging would need to be excluded from participation in a clinical trial of simple OM or SA. The final feasibility assessment needs to take into account cases from the service evaluation in which no bony abnormalities were found.
-
The study population will remain heterogeneous despite aiming for clear definitions because patients take different amounts of time to present at health-care service providers, doctors take differing amounts of time to diagnose the infection and infections progress at differing rates.
Developing the research question
A future study is thought to be important to reduce the use of antibiotics, reduce length of hospital stay and to avoid complications of i.v. therapy.
Possible research questions:
-
For how long should bone and joint infections be treated with antibiotics in total?
-
When should the switch from i.v. to oral therapy occur?
-
Should the study compare early with late switch rather than type of antibiotic treatment?
-
Early switch point of 48 hours compared with longer, such as 9 days (as the latter appeared to be the median time for switch in the data from the service evaluation).
Concerns noted:
-
Assessing more than one research question in one study may impact on results, creating smaller groups of patients with more compounding factors and reducing the power of the study.
-
The umbrella title of bone and joint infections covers two very different conditions: acute OM is perceived to have a lower risk of long-term problems due to treatment failure than SA, despite the fact that, historically, duration of antibiotic therapy has been shorter in OM than in SA. Differences in standard care procedures need to be examined from the service evaluation to decide if OM and SA need to be evaluated in different study groups.
-
Setting an arbitrary switch date would not take different infecting organisms into account. Any defined switch timings should be within a range that is comfortable for clinicians and parents.
-
Many clinicians would be uncomfortable switching as early as 48 hours. This would be possible only in a subgroup with mild disease, affecting the recruitment and power of the study.
Conclusion
The meeting concluded that a future study looking at switch timing should be prioritised over one looking at the effect of reducing the overall duration of antibiotic therapy.
Chapter 8 Summary discussion and conclusions for further research
Any future RCT will need to enrol children with simple disease in whom there is no underlying chronic disease, no orthopaedic implants and no recent history (within 6 months) of orthopaedic surgery. This study set out to establish the feasibility of a study in predefined simple or less complex bone and joint infection in children, and the qualitative component of the study confirmed that parents would also be less likely to consent to their children participating in such a trial if there was concern regarding the seriousness of their child’s condition and/or previous trajectory leading to diagnosis.
Number of cases
A future RCT would need to recruit from all tertiary and most secondary hospitals in the UK NIHR Comprehensive Research Network, where there are resources for recruiting children to clinical trials. The number of cases seen in UK clinical practice is currently less than previously reported. There were 109 simple OM and 107 simple SA cases seen in 49 trusts over the 6-month study period. Given the lower than expected monthly accrual (0–3 simple cases per month in tertiary centres, 0–0.7 per month in secondary centres), in tertiary centres there would be approximately 24–36 cases of eligible simple disease per year and in secondary care centres 8–12 potential eligible cases.
Logistics of a clinical trial including criteria for oral switch
From the service evaluation data, it appears that most children present to the emergency department and not primary care. These data do not align directly with the findings of the qualitative study as regards parents’ reported experience of the length of time before a formal diagnosis was made. From the observations in the qualitative study, pre-trial information provided to and gathered from parents will need to specifically address this issue by acknowledging that parents might have perceived a delay prior to diagnosis, and researchers will need to give parents a chance to express their concerns. If parents have experienced a delay in diagnosis, the service evaluation data can also be used to explain to parents of potential participants that the clinical severity at presentation to hospital, rather than the length of non-specific symptoms prior to presentation at hospital, is the key determinant of whether or not an individual child should be considered for recruitment to a clinical trial.
Although in some centres home-based antibiotic delivery is possible (paediatric outpatient antimicrobial therapy, paediatric OPAT), the overall length of hospital stay in our cohort of children with simple SA and OM was very similar to the length of i.v. antibiotic course (median 6 days for simple SA, 9 days for simple OM).
The service evaluation suggested that the at-home or in-hospital completion of an antibiotic course will not be of any concern in a RCT. Parents of children with bone and joint infection understand the need to complete antibiotic courses and report that they do so as per clinicians’ instructions. This is also reflected in the experience of many past and current paediatric trials in which there are few reports of problems with adherence to trial medications where adequate in-trial support is provided.
From a trial operation and logistical perspective, the qualitative study has shown the importance of offering parents the opportunity to discuss their immediate experiences of the condition and its presentation with the research team prior to being considered for a study. Embedding a future RCT in the routine NHS care of children with OM or SA would be a way of achieving this, and would also provide reassurance that both potential arms of a study would not be expected in advance to negatively impact on an individual child’s quality of care. Enrolment as part of routine care is also supported by a recent survey showing that NHS patients are more likely to participate in research if the topic is introduced by the potential research participant’s own doctor. 93
The qualitative study also provided clear advice regarding the need to approach parents at times other than ‘pinch points’ of anxiety/distress if at all possible, and regarding the need for a clear explanation of potential risk and safety aspects.
Oral switch criteria for use in a clinical trial
In preparing the design and materials for a future trial, it will be important to clearly state that a research protocol would not over-ride the individual need of the child in terms of when an oral switch was made. This is feasible as a RCT would not be testing the oral switch criteria that will be according to an agreed standard current practice. From the service evaluation, current clinical practice clearly is to switch with no consistent evidence base used to make this decision. By ensuring that switch criteria were strict, clinicians would be able to support following the trial protocol using predefined criteria such a modification of that previously proposed. 1 The Delphi survey and consensus meeting showed that resolution of fever for ≥ 48 hours, tolerating oral input (medicines and food/drink) and pain improvement (rather than resolution) were considered important criteria. Although there was much variability in the Delphi survey and consensus meeting regarding what the CRP criteria should be, around 60% of respondents in both rounds 1 and 2 of the Delphi survey agreed that CRP is an important parameter, as did approximately 70% of those at the consensus meeting. These criteria resonate with the views parents expressed during interviews. Parents were confident in the switch point when they had perceived an improvement in their child’s condition and felt that doctors were basing decisions on clinical indicators. Where this was not perceived to have been the case, parents expressed dissatisfaction and anxiety.
Recruitment following a child meeting specific clinical criteria in acute paediatric care has been used successfully in the recently published NIHR HTA-funded clinical trial of the management of infants admitted with bronchiolitis, who were randomised following admission requiring oxygen therapy to maintain saturations. 94 Other information that will be important to include in the pre-trial information includes data to support the switch point in both standard care and the experimental intervention, details of how a child would be monitored to ensure infection was being dealt with, trajectory of care in case of failure to recover in the shorter treatment arm, and data to demonstrate safety of the shorter arm and regarding the equivalence/equipoise about treatment in both arms.
Defining the core outcomes of a future randomised controlled trial in bone and joint infections in children
The qualitative study demonstrated the importance to parents and children of consideration of short- and long-term outcomes meaningful to the families themselves. Short-term examples that accord with children’s and parents’ observations include appetite and weight, temperature, pain, weight bearing on affected limb and functional movement in activities of daily living. Longer-term outcome examples reflecting children’s and parents’ perspectives and priorities include functional activity and mobility levels, pain, end of infection with no recurrence and the impact on bone/joint/growth.
Overall, the outcomes assessed as ‘consensus in’ from the Delphi and consensus meeting voting include rehospitalisation or recurrence of symptoms while on oral antibiotics, treatment failure or recurrence of infection, disability at follow-up, being symptom free at 1 year, limb shortening or deformity, chronic OM or chronic arthritis, amputation or fasciotomy, death, need for paediatric intensive care and line infection.
Consideration of possible trial designs in relation to the current standard of care for length of intravenous and oral therapy courses, and in relation to treatment failure reported in the service evaluation cohort
The most striking aspect of our data is that in the case of simple, non-complex disease, clinicians currently initiate oral switch early, at a median of 7 days in children with simple SA and 9 days in those with simple OM. What is very important for the design of a RCT of reduced duration of i.v. and/or total antibiotic therapy is that these data also demonstrate less treatment failure in simple disease in which there was an early oral switch, suggesting also that ‘more severe simple’ disease is more likely to fail initial therapy or require additional interventions. Of the 73 children with simple disease in whom oral switch occurred very early (< 1 week), 9.6% experienced a treatment failure, compared with 16.1% of those in whom switch occurred early (between 1 and 2 weeks) and 18.2% of those in whom switch took place after 2 weeks of i.v. antibiotic treatment.
Although there was no difference in the duration of i.v. antibiotic therapy between children with simple SA and those with OM, total antibiotic treatment duration is around 10 days shorter simple SA than in simple OM [median 30 (IQR 18–41) days compared with 40 (IQR 21–48) days]. These data suggest that, although there has been a move to short-course i.v. antibiotic therapy for simple disease in current NHS practice, overall course lengths have remained similar to ‘textbook’ longer overall courses of treatment. As a result of differences in duration of treatment between SA and OM, children with diseases would need to be enrolled in different groups in a future RCT.
To be able to conduct a RCT of even shorter courses of i.v. therapy (e.g. to randomise children with simple OM or SA to very short-course i.v. therapy of 3–4 days compared with 7 days), clinicians, parents and researchers would have to be convinced regarding the equipoise of potential treatment failure/safety, and agree on clear oral switch criteria.
The service evaluation showed that, although 8.3% of children with simple disease experienced at least one complication, only three such children (of 218) reported recurrence of infection requiring medical or surgical intervention. However, in order to capture all possible cases in which the initial empirical antibiotic and/or surgical therapy failed, necessitating further treatment, we instead used a broad definition of treatment failure including fever, recurrence of infection including increase in CRP or development of draining sinus, dislocation, subluxation, pathological fracture, pain, mobility problems, swelling, numbness, hospital readmission, surgery and change of antibiotics. Using these criteria, 32 of 218 children with simple disease (14.7%) experienced treatment failure and treatment failure was half as likely in SA (9.3%) as in OM (19.3%). In summary, (1) treatment failure was less likely the earlier switch occurred (9.6% in switch at < 1 week, 16.1% when between 1 and 2 weeks, 18.2% when after 2 weeks of i.v. therapy); (2) the likelihood of treatment failure in children with simple SA and OM was not different in those who received antibiotics for < 6 weeks as for who received antibiotics for > 6 weeks; and (3) differences in the rates of treatment failure among children with simple SA and those with simple OM suggest that children with OM and SA should be enrolled in separate groups in any future RCT.
Regarding the feasibility of a RCT to compare early with late oral switch, or of a trial to compare overall short-course with longer-course therapy in simple OM or SA, a series of statements can be made on the basis of the service evaluation data:
-
Clinicians and researchers would feel comfortable enrolling children to a trial of reduced total duration of antibiotic treatment, and parents would consent to their children taking part, only if there is genuine equipoise regarding whether or not reduced i.v. course length and/or reduced total length of therapy would be likely to increase the risk of treatment failure.
-
Because of the current practice of early oral switch apparent in the service evaluation data, there appears to be a group of children with simple disease who get better rapidly even though they receive less i.v. antibiotic. It is a reasonable clinical assumption (although not proved by these data) that such children are less likely to experience treatment failure because they have less severe disease than children in whom oral switch occurs later. Together with data from this study and from other countries (data from Spain presented at ESPID 2015 Congress, Leipzig, Germany, May 2015), and with strict oral switch criteria, a RCT in which those with very mild are treated with very short-course (1–3 days) i.v. antibiotic therapy prior to oral switch could be considered. However, the Delphi survey and consensus meeting showed that, although CRP is considered important in determining the timing of oral switch (with no consensus regarding level), other criteria should include resolution of fever for ≥ 48 hours, tolerating oral input (medicines and food/drink) and pain improvement (rather than resolution). This finding, together with the fact that the number of children likely to meet oral switch criteria in the first week of treatment is small, probably means such a study will not be feasible as a single-country (UK) trial.
-
An alternative strategy would be to consider a trial to randomise children whom a clinician has decided should be switched to oral antibiotics early to reduce overall length of antibiotic therapy compared with the current standard of care. This trial design would avoid the potential problem of parental anxiety about stopping i.v. therapy early, described in the qualitative study, as children currently treated with i.v. antibiotics for < 1 week experience fewer complications than those in whom oral switch occurs later. From the service evaluation data, it would be possible to plan that the duration of initial i.v. therapy prior to oral switch would be the same for children with SA and OM, but to vary the duration of oral antibiotic therapy in different study arms, for example in children with simple SA comparing 20 days’ treatment with 30 days’ treatment and in those with simple OM comparing 30 days’ treatment with 40 days’ treatment. However, although this study design (a comparison of reduced duration of oral antibiotic treatment with standard duration of treatment) appears to make sense based on current practice data, the expressed preference of the consensus meeting was for prioritising investigation of early oral switch rather than overall duration of therapy.
In addition, given the invasive nature of the initial diagnosis and the relatively low cost of home-based antibiotic therapy given at times around school attendance, it is debatable if the cost benefit to the NHS is great enough in such an uncommon disease to justify the large costs of a national multicentre clinical trial of duration of oral antibiotic treatment after oral switch. Although a positive outcome of such a trial would indeed reduce overall antibiotic use and consumption, as the condition affects so few children the overall impact on antimicrobial resistance in the community or in individual children will be very small. The cost saving of such a reduction in the duration of home-based oral therapy would also be small in terms of the costs of antibiotics saved and clinician time, as well as economically as children with disease mild enough to be randomised in this study would almost all be back at nursery or school during their period of oral therapy (parents will be able to return to work).
-
A third study design would be a trial of reduced duration of antibiotics in children who have simple disease but do not meet the criteria for oral switch in the first week of treatment, a group that appears to represent most children with simple OM and SA. The clinical course of these patients is less predictable, and it is not currently possible to predict clinically, or from biomarkers, which patients would potentially do well with a shorter duration of i.v. or total antibiotic therapy. In a RCT, the oral switch criteria defined in this study, together with a strict CRP cut-off level, could be key enrolment criteria, with participants then randomised to immediate or delayed oral therapy once the oral switch criteria were met after 7 days of i.v. therapy. Although there was no consensus on what the CRP cut-off should be (reflecting wide variation in current clinical practice, not based on evidence), recent experience in a commercial clinical trial suggests that a ‘cautious’ level of CRP change would be accepted by investigators in the context of a RCT. In the recent commercial study that has completed recruitment but not yet reported (clinicaltrials.gov NCT01922011), the CRP criterion was ‘CRP decreased by at least 30% from baseline (if available)’, but we would anticipate that in a UK study this would need to be ‘CRP reduced by at least 50% from baseline’ or perhaps ‘CRP reduced by at least two-thirds from baseline’ to make sure that there was wide participation. Children would be excluded from the trial if they met the criteria for oral switch in the first 7 days of i.v. therapy as the service evaluation suggests that this group experiences less treatment failure than those in whom oral switch occurs later. This study would compare shorter with longer i.v. therapy (e.g. 12–14 days’ total i.v. therapy compared with 21–28 days’ i.v. therapy). It would therefore establish the safety and treatment failure equivalence of shorter or longer courses of i.v. antibiotic therapy, keeping the total duration of antibiotic treatment (i.v. and oral combined) the same. In this design, which meets the preference of clinicians at the consensus meeting, there would be clear differences in duration of i.v. therapy and a potentially large impact on clinical and cost (length of stay) outcomes in the NHS.
-
Although this study has established three potentially feasible RCT designs (very short-course i.v. antibiotics in children with simple SA or OM compared with current standard practice; reduced overall duration of therapy compared with current standard practice following clinician-determined oral switch; shorter or longer duration of i.v. therapy after defined oral switch are met, with no difference in total i.v. or oral antibiotic combined total course), key questions remain. There are currently no biomarkers (currently used or researched) that would provide certainty of potential study eligibility to a trial of very early i.v. to oral switch, or aid decision-making in other trial designs. New biomarkers are required in order to better stratify the severity of disease in children with simple OM or SA.
Do the microbiological conventional and molecular data provide information on which empirical antimicrobial agent(s) could be used in a randomised clinical trial?
The available conventional tests and results of the targeted PCR substudy suggest that although S. aureus remains the most common single identified pathogen in children of all ages, K. kingae occurs in a significant proportion of younger children (aged 0.8–2.3 years in the molecular data from this study, but more common in children aged < 6 years in other series) and GAS (Streptococcus pyogenes) is an important pathogen in children of all ages. Other pathogens are much rarer, but using data from both conventional and molecular tests include pneumococcus (Streptococcus pneumoniae), GBS, H. influenzae (in one child) and Pseudomonas spp. (in one child). Importantly, where S. aureus was the identified pathogen, only two cases (one simple, one complex) of resistant bacteria (MRSA) were identified. The incidence of the more virulent PVL-positive S. aureus disease was also very low (two simple cases, one complex case) in our data.
Regarding dose, although it is impossible from the service evaluation data to determine whether or not any treatment failures are a result of non-maximum dose prescribing, in the case of the major in-hospital i.v. antibiotics used to treat simple disease (flucloxacillin, clindamycin and cefuroxime) clinicians mostly prescribed the maximum (high-dose) BNFC dose recommendations. When ceftriaxone was prescribed, however, the lower dose band was prescribed more often than the higher, severe infection, dose. Dosing strategies for orally prescribed antibiotics were more variable and often much lower than the formulary maximum recommended for age or weight.
For a clinical trial, these data overall suggest that a broad-spectrum i.v. antibiotic (such as cefuroxime 50 mg/kg three times per day) should be used in younger children (possibly aged < 6 years) to cover less common pathogens not completely sensitive to high-dose flucloxacillin. A narrower-spectrum antibiotic such as high-dose flucloxacillin (50 mg/kg four times per day) could be used in older children and teenagers in whom S. aureus and GAS predominate. To enable discharge from hospital while still on i.v. antibiotic therapy, once-daily ceftriaxone at maximum dose (80 mg/kg once per day to a maximum of 4 g daily) would provide empirical antibiotic cover for all common and less common organisms, as antimicrobial resistance is still very uncommon. For children with definite penicillin allergy, clindamycin is an acceptable in-hospital alternative (10 mg/kg four times per day), but there is no clear out-of-hospital once-daily alternative.
To complete the total course length after oral switch, in order to maximise the risk of treatment failure a RCT would need to carefully define doses of antibiotics to use at the higher BNFC dose recommendations that have both an acceptable palatability (taste) and achievable treatment regime. Although not shown in our data, it is established paediatric practice that parents and children are more likely to adhere accurately to three times per day dose regimens (such as midnight, 08.00 hours and 16.00 hours) than to four times a day regimens (such as midnight, 06.00 hours, 12.00 hours and 18.00 hours), which means both two night-time (usually when asleep) doses for children and a dose during school or nursery hours.
Summary
A core outcome set for future clinical trials in paediatric bone and joint infections was established by a Delphi survey and consensus meeting.
A service evaluation, qualitative study and consensus meeting discussion provided information to inform the feasibility of a RCT, and of potential RCT designs.
-
A RCT of very early oral switch would be supported by the service evaluation data. However, the Delphi survey and consensus meeting showed that, although CRP is considered important in determining the timing of oral switch (with no consensus regarding level), other criteria should include resolution of fever for ≥ 48 hours, tolerating oral input (medicines and food/drink) and pain improvement (rather than resolution). Such strict criteria, together the fact that the number of children with simple OM and SA likely to meet switch criteria in the first week of i.v. treatment is small, means that such a study will likely not be feasible as a single-country (UK) trial.
-
A RCT of reduced duration of total antibiotic therapy after clinician-directed oral switch based on the criteria determined in this study would be feasible, even though it was not the first choice study of the consensus meeting participants. It would be possible to explain the study to parents and children, and feasible to recruit in terms of numbers of cases and clinician willingness. It is unlikely that such a study design will make an enormous impact on the overall cost to the NHS of treating bone and joint infections in children or on the overall antibiotic burden of children in the community.
-
A RCT in children with simple OM and SA comparing shorter or longer i.v. therapy with no reduction in the duration of total (i.v. and oral combined) antibiotic therapy would be feasible with children randomised after the strict oral switch criteria had been met after 7 days of i.v. therapy. Children would be excluded from the trial if they met the criteria for oral switch in the first week of i.v. therapy as the service evaluation suggests that this group experiences less treatment failure than those in whom oral switch occurs later. This study design meets clinician preference and addresses parental concerns not to randomise prior to currently used oral switch criteria. It would also provide important information on whether or not i.v. therapy can be reduced without increasing treatment failure compared with longer-course i.v. therapy, or whether or not outcomes are worse in those treated with more i.v. antibiotics as a result of treatment failures being caused by the treatment modality itself.
Regardless of RCT design, the qualitative study has demonstrated gaps remaining in the understanding of parental and family decisions to participate in clinical trials. In order to explore prospectively the issues identified in the qualitative study (e.g. an understanding of the condition, actual circumstances around making a choice to join a study etc.), a longitudinal qualitative study could take place alongside any trial, including semistructured interviews with parents and children, focus groups with staff and ethnographic observation.
Acknowledgements
The study steering committee: chairperson Professor Gareth Morgan, Mrs Catherine Pritchard, Professor Mel Calvert and Dr James Robb.
The study management group: Saul Faust, Nicholas Clarke, Paula Williamson, Carrol Gamble, Priya Sukhtankar, Stuart Clarke, Helen Hickey, Abigail Bennett, Catherine Spowart, Jo Eatock, Barbara Arch and Duncan Applebe.
All service evaluation and microbiology substudy site principal investigators, consensus meeting participants, enrolling clinicians and research staff.
All supporting National Institute for Health Research Clinical Research Network Paediatric Theme regional research networks and research nurses and staff funded by Health and Care Research Wales, including:
-
Airedale General Hospital – Daniel Tang, Pronab Bala, Avijeet Ghosh, Paul Godwin, Guy Porter, Clive Orgles, Mandy Swanepoel, Mechele Couch-Upite and Lucy Sootheran.
-
Alder Hey Children’s Hospital – Andrew Riordan, Naomi Wallin, Jane England-Smith, Richard Drew, Colin Bruce, Caren Landes, Matthew Peak and Dot Lambert.
-
Royal Alexandra Children’s Hospital – Katy Fidler, Hannah Butler, Alex Smith, Ellie Alexander and Scott Harfield.
-
St Peters Hospital – Gillian Baksh, Arshad Khaleel, Angela Shaw, Lorna Walding and Freda Gomes.
-
Barts Health NHS Trust – Manoj Ramachandran, Anna Riddell, Susan Liebeschuetz, Mark Paterson, Mike Millar, Rosy Jalan, Nicki Plaatjies, Philip Good, Bahadur Anjum and Inayet Baz.
-
Basingstoke & North Hampshire – Ayo Kadri, Nigel Rossiter, Jorge Cepeda, Becci Petch and Hazel Griffith.
-
Royal Berkshire Hospital – Balaji Suryanarayan, Nev Davies, Shabnam Iyer, Rob Robertson, Morag Zelisko and Sue Hallett.
-
Royal Blackburn Hospital – Betty Wamola, Robin Paton and Chloe Rishton.
-
Birmingham Childrens Hospital – Nick Makwana, Siten Roy, Natasha Ratnaraja, Claire Keaney, Indy Birak, Sinead Baxter, Juliet Bradley-Hopkins, Fran Lloyd and Julie Colley.
-
Heartlands Hospital – Scott Hackett, Jacqui Daglish, June De La Rue and Lesley Horton.
-
Bradford Royal Infirmary – Kirsty Haslam, Khulood Khawaja and Trudy Booth.
-
Bristol Royal Hospital for Children – Jolanta Bernatoniene, Phoebe Moulsdale, Jo Jenkins, Smee Heather, Natalie Fineman and Helen Dunderdale.
-
University Hospital of Wales – Colin Powell, Robin Howe, Claire Carpenter, Pauline Jones and Linda Phillips.
-
Chelsea and Westminster – Saji Alexander.
-
University Hospitals Coventry & Warwickshire – Giles Pattison, Heather Stirling, Nigel Coad, Matthew Rogers, Emma Helm, Sue Dale, Natasha Wileman and Ceri Jones.
-
Derriford Hospital – Alan Cade, Robert Jeffery, James Greig, Joanne Gormally, Judith Foster, Sarah-Jane Sharman and Corinna Mossop.
-
Royal Devon & Exeter NHS Foundation Trust – Corinne Hayes, Peter Cox, Cressida Auckland, Suzanne Wilkins, Ruth Nicholson, Sue Ward, Mcmillan Christine, Rebecca Gumm, Nicola Jones and Caroline Renton.
-
Ealing Hospital – Colin Michie.
-
Royal Hospital for Sick Children Edinburgh – Laura Jones.
-
Evelina Childrens Hospital – Esse Menson, Fabian Norman-Taylor, John Klein, Dipalee Durve, Lauren Hardcastle, Shaun Larkin and Lorraine Hodsdon.
-
Royal Hospital for Sick Children Glasgow – Conor Doherty.
-
Great Ormond Street Hospital – Delane Shingadia, Deborah Eastwood, Garth Dixon, Cathy Owens and Evelyn Howley.
-
John Radcliffe Hospital – Rebecca Beckley, Dominic Kelly, Andy Wainwright, Sharon Westcar, Tim Sell and Louise Willis.
-
Kingston Hospital – Suzanne Luck, Dwight Lindo, Neel Mohan, Jill Leach, Caroline Ward and Liz Money.
-
Leeds General Infirmary – Sean O’Riordan, Brian Scott and Deborah Burton.
-
Leicester Royal Infirmary – Alwyn Abraham, Debbie Modah and Amit Maniyar.
-
Leighton Hospital – Simon Burns, Simon Dowson, Karen Luscombe, Samantha Tapscott and Charis Emmett.
-
Luton and Dunstable NHS Foundation Trust – Vanda Gandhi.
-
Royal Manchester Children’s Hospital – Naomi Davis, Ian Doughty, Janet Grundy, Neville Wright, Gemma Donohoe, Garine O’Connor, Joanne Reed, June Green, Sophia Lockwood and Alison Robinson.
-
Newcastle General Hospital – Marieke Emonts, Phillip Henman, Vicky Stevenson, Lynsey Rae, Rosy Lampitt and Sean Scott.
-
Royal Wolverhampton Hospitals NHS Trust – Sharon Kempson.
-
Norfolk and Norwich University Hospital – Kate Armon, Rachael Hutchinson, Catherine Tremlett, Duncan MacIver, Vanja Briggs, Louisa Fear and Clare Collum.
-
Pennine Acute Hospitals NHS Trust – Paddy McMaster, Manzoor Sheik, Ivor Cartmill, Suraj Amonkar and Sarah Rickard.
-
North Tees and Hartlepool – Jonathan Page, Tushar Banerjee and Deepa Nayar.
-
University Hospital of North Staffordshire – David Emery, Warren Lenney, Krithika Subramaniam, Krys Castro-Fockett and Ruth Jones.
-
Central Middlesex North West London – Michele Afif.
-
Royal Oldham Hospital – Abdul Rehman and Sarah Rickard.
-
Mid Yorkshire NHS Trust – Nirmal Tulwa, Aneel Sohal, Richard Robinson, Aimee Hayton, Tabitha Kavoi, Natasha DeVere and Kathryn Davison.
-
Princess Alexandra Harlow Essex – Sanjay Raina, Keel Carol, Shpuza Ervin and Rees Cait.
-
Queen Alexandra Hospital – Catherine Tuffrey, Rob Richards, Robert Porter, Rachel Harrison, Sharon McCready, Amanda Freeman and Kate Greenwood.
-
Queen’s Hospital Burton – Manisha Gowan-Gopal, Stephanie Boswell, Claire Lawrie and Katherine Lewney.
-
Royal National Orthopaedic Hospital, Middlesex – Benjamin Jacobs, Peter Calder and Damien Mack.
-
Sheffield Children’s Hospital – Patricia Fenton, Fiona Shacklady, Sanjeev Madhan, Isla Lang, Melanie Fox, Penny Broadley, Susan Lenthall and Wendy Swan.
-
Royal Shrewsbury Hospital – Sanjeev Deshpande, Richard Brough, Robert Freeman, Graham Harvey, Helen Watson, Charlotte Owen, Hilary Shepley and Adams Marion.
-
Southampton General Hospital – Rachel Fowler and Danny Pratt.
-
Southend Hospital – Frances Borg, Ravi Chetan, Anupam Shrivastava, Ravi Kuppuswamy, Sarah Mapplebeck, Saman Perara, Vicky Goater and Susan Bowman.
-
Staffordshire General Hospital – Olumuyiwa Oso, Ishan Bhoora, Kelly Amor, Julie Hollins, Marie Phipps and Dawn Sirdefield.
-
St Georges Healthcare NHS Trust – Mike Sharland, Karen Daly, Tim Planche, Rosemary Allan, Hana Tabusa, Tatiana Munera, Helen Bird and Paul Heath.
-
St Mary’s Imperial College – Jethro Herberg, Marianne Nolan, Sally Tennant, Jo Danin, Stuart Gormley, Selvy Raju, Akinkugbe Olugbenga, Mustafa Sobia and Natalie McCarthy.
-
Stepping Hill Hospital – Chris Cooper, Sarah Maxwell, Thomas Anna, Sara Bennett, Gary Cook, Deborah Kenyon and Jan Smith.
-
Worcester Royal Infirmary – John Scanlon.
-
Wye Valley Hospital – Simon Meyrick.
-
Yeovil District Hospital – Alex Stannett, Cen Thomas, Ben Lankester, Meredith Kane, Nicky Marks and Barbara Williams-Yesson.
Professor Geoffrey Meads, University of Winchester.
Professor Bridget Young and Dr Nicola Harman, University of Liverpool.
All children and adults who participated in the service evaluation and qualitative study.
Contributions of authors
All authors listed contributed to study design in addition to the tasks listed (apart from Hans De Graaf, who joined after design stage).
Hans de Graaf contributed to the analyses of the service evaluation and microbiological data, and writing of the report.
Priya Sukhtankar contributed to setting up the service evaluation and microbiology substudy protocol and NHS REC applications, literature review for Delphi survey and outcomes, input to design of qualitative study and NHS REC application, and support to Clinical Trials Research Centre in delivery of service evaluation.
Barbara Arch was involved in the statistical planning and analysis.
Nusreen Ahmad contributed to the laboratory work and analyses of the microbiology substudy.
Amanda Lees was involved with the co-ordination of the Delphi process and consensus meeting. She also contributed to the design of the qualitative study, conducted the interviews and drafted Chapter 4.
Abigail Bennett was involved with the co-ordination of the Delphi process and consensus meeting.
Catherine Spowart was involved with the co-ordination of the Delphi process and consensus meeting.
Helen Hickey Clinical Trials Research Centre project manager, was involved with the co-ordination of the Delphi process and consensus meeting.
Annmarie Jeanes was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Kate Armon was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Andrew Riordan was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Jethro Herberg was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Scott Hackett was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Carrol Gamble was involved in the statistical planning and analysis, and with the co-ordination of the Delphi process and consensus meeting.
Delane Shingadia was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Ann Pallett oversaw the laboratory work and analyses of the microbiology substudy.
Stuart C Clarke oversaw the laboratory work and analyses of the microbiology substudy.
Philip Henman was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Marieke Emonts was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Mike Sharland was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Adam Finn was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Andrew J Pollard was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Colin Powell was involved in patient recruitment, and enrolling collaborators and their colleagues at other NHS sites.
Peter Marsh supervised the laboratory work and analyses of the microbiology substudy.
Claire Ballinger was involved in the design of the qualitative study and provided input into Chapter 4.
Paula R Williamson was involved in the statistical planning and analysis, and the co-ordination of the Delphi process and consensus meeting.
Nicholas MP Clarke (lead orthopaedic surgeon) was involved in the initiation of the study, development of protocols and study components, analyses and report writing.
Saul N Faust (chief investigator) initiated the study, and oversaw set-up and delivery of all study components. He was involved in data collection, analyses and report writing. He also contributed to the design of the qualitative study.
Data sharing statement
Data will be available from the corresponding author, and via a data sharing repository.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Faust SN, Clark J, Pallett A, Clarke NM. Managing bone and joint infection in children. Arch Dis Child 2012;97:545-53. http://dx.doi.org/10.1136/archdischild-2011-301089.
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369-79. http://dx.doi.org/10.1016/S0140-6736(04)16727-5.
- Krogstad P, Feigin RD, Cherry JD, Demmler GJ, Kaplan SL. Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders; 2004.
- Rasool MN. Hematogenous osteomyelitis of the calcaneus in children. J Pediatr Orthop 2001;21:738-43. https://doi.org/10.1097/01241398-200111000-00007.
- Jaffe HL. Metabolic, Degenerative and Inflammatory Diseases of the Bones and Joints. Philadelphia, PA: Lea & Feibiger; 1972.
- Dahl LB, Høyland AL, Dramsdahl H, Kaaresen PI. Acute osteomyelitis in children: a population-based retrospective study 1965 to 1994. Scand J Infect Dis 1998;30:573-7. https://doi.org/10.1080/00365549850161124.
- Stott NS. Review article: paediatric bone and joint infection. J Orthop Surg 2001;9:83-90. https://doi.org/10.1177/230949900100900116.
- Gutierrez K. Bone and joint infections in children. Pediatr Clin North Am 2005;52:779-94. https://doi.org/10.1016/j.pcl.2005.02.005.
- Weichert S, Sharland M, Clarke NM, Faust SN. Acute haematogenous osteomyelitis in children: is there any evidence for how long we should treat?. Curr Opin Infect Dis 2008;21:258-62. http://dx.doi.org/10.1097/QCO.0b013e3283005441.
- Macnicol MF. Patterns of musculoskeletal infection in childhood. J Bone Joint Surg Br 2001;83:1-2. https://doi.org/10.1302/0301-620X.83B1.11892.
- Riise OR, Kirkhus E, Handeland KS, Flato B, Reiseter T, Cvancarova M, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr 2008;8. https://doi.org/10.1186/1471-2431-8-45.
- Wong M, Isaacs D, Howman-Giles R, Uren R. Clinical and diagnostic features of osteomyelitis occurring in the first three months of life. Pediatr Infect Dis J 1995;14:1047-53. https://doi.org/10.1097/00006454-199512000-00004.
- Baker ADL. Haematogenous osteomyelitis in children: epidemiology, classification, aetiology and treatment. Paediatr Child Health 2007;18. https://doi.org/10.1016/j.paed.2007.11.002.
- Nade S. Acute haematogenous osteomyelitis in infancy and childhood. J Bone Joint Surg Br 1983;65:109-19.
- Moumile K, Merckx J, Glorion C, Pouliquen JC, Berche P, Ferroni A. Bacterial aetiology of acute osteoarticular infections in children. Acta Paediatr 2005;94:419-22. https://doi.org/10.1080/08035250410023278.
- Moylett EH, Rossmann SN, Epps HR, Demmler GJ. Importance of Kingella kingae as a pediatric pathogen in the United States. Pediatr Infect Dis J 2000;19:263-5. https://doi.org/10.1097/00006454-200003000-00023.
- Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis 2004;4:358-67. http://dx.doi.org/10.1016/S1473-3099(04)01046-1.
- Monk A, Ahmad N, Woodsford M, Marsh P, Clarke SC, Saeed K, et al. Detection of uncultured organisms in paediatric bone and joint infections by a multiplex real-time PCR panel. Arch Dis Child 2009;94.
- Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop 2010;30:301-4. http://dx.doi.org/10.1097/BPO.0b013e3181d4732f.
- Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop 2006;26:703-8. http://dx.doi.org/10.1097/01.bpo.0000242431.91489.b4.
- Bocchini CE, Hulten KG, Mason EO, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics 2006;117:433-40. https://doi.org/10.1542/peds.2005-0566.
- Dohin B, Gillet Y, Kohler R, Lina G, Vandenesch F, Vanhems P, et al. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J 2007;26:1042-8. http://dx.doi.org/10.1097/INF.0b013e318133a85e.
- McCaskill ML, Mason EO, Kaplan SL, Hammerman W, Lamberth LB, Hultén KG. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr Infect Dis J 2007;26:1122-7. http://dx.doi.org/10.1097/INF.0b013e31814536e0.
- Isaacs D, Moxen ER. Handbook of Neonatal Infections: A Practical Guide. London: WB Saunders; 1999.
- Ibia EO, Imoisili M, Pikis A. Group A beta-hemolytic streptococcal osteomyelitis in children. Pediatrics 2003;112:e22-6. https://doi.org/10.1542/peds.112.1.e22.
- Chambers JB, Forsythe DA, Bertrand SL, Iwinski HJ, Steflik DE. Retrospective review of osteoarticular infections in a pediatric sickle cell age group. J Pediatr Orthop 2000;20:682-5. https://doi.org/10.1097/01241398-200009000-00025.
- Goergens ED, McEvoy A, Watson M, Barrett IR. Acute osteomyelitis and septic arthritis in children. J Paediatr Child Health 2005;41:59-62. https://doi.org/10.1111/j.1440-1754.2005.00538.x.
- Unkila-Kallio L, Kallio MJ, Eskola J, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics 1994;93:59-62.
- Pääkkönen M, Kallio MJ, Kallio PE, Peltola H. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res 2010;468:861-6. http://dx.doi.org/10.1007/s11999-009-0936-1.
- Faesch S, Cojocaru B, Hennequin C, Pannier S, Glorion C, Lacour B, et al. Can procalcitonin measurement help the diagnosis of osteomyelitis and septic arthritis? A prospective trial. Ital J Pediatr 2009;35. http://dx.doi.org/10.1186/1824-7288-35-33.
- Mazur JM, Ross G, Cummings J, Hahn GA, McCluskey WP. Usefulness of magnetic resonance imaging for the diagnosis of acute musculoskeletal infections in children. J Pediatr Orthop 1995;15:144-7. https://doi.org/10.1097/01241398-199503000-00002.
- Tuson CE, Hoffman EB, Mann MD. Isotope bone scanning for acute osteomyelitis and septic arthritis in children. J Bone Joint Surg Br 1994;76:306-10.
- Blickman JG, van Die CE, de Rooy JW. Current imaging concepts in pediatric osteomyelitis. Eur Radiol 2004;14:55-64. http://dx.doi.org/10.1007/s00330-003-2032-3.
- Shammas A. Nuclear medicine imaging of the pediatric musculoskeletal system. Semin Musculoskelet Radiol 2009;13:159-80. https://doi.org/10.1055/s-0029-1237687.
- Capitanio MA, Kirkpatrick JA. Early roentgen observations in acute osteomyelitis. Am J Roentgenol Radium Ther Nucl Med 1970;108:488-96. https://doi.org/10.2214/ajr.108.3.488.
- Offiah AC. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 2006;60:221-32. https://doi.org/10.1016/j.ejrad.2006.07.016.
- Howard CB, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br 1994;76:311-14.
- Restrepo S, Vargas D, Riascos R, Cuellar H. Musculoskeletal infection imaging: past, present, and future. Curr Infect Dis Rep 2005;7:365-72. https://doi.org/10.1007/s11908-005-0011-3.
- Rosey AL, Abachin E, Quesnes G, Cadilhac C, Pejin Z, Glorion C, et al. Development of a broad-range 16S rDNA real-time PCR for the diagnosis of septic arthritis in children. J Microbiol Methods 2007;68:88-93. https://doi.org/10.1016/j.mimet.2006.06.010.
- Verdier I, Gayet-Ageron A, Ploton C, Taylor P, Benito Y, Freydiere AM, et al. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty-four recent pediatric diagnoses. Pediatr Infect Dis J 2005;24:692-6. https://doi.org/10.1097/01.inf.0000172153.10569.dc.
- Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 2007;119:891-6. https://doi.org/10.1542/peds.2006-0440.
- Cole WG, Dalziel RE, Leitl S. Treatment of acute osteomyelitis in childhood. J Bone Joint Surg Br 1982;64:218-23. https://doi.org/10.1097/01241398-198208000-00074.
- Ratnayake K, Davis AJ, Brown L, Young TP. Pediatric acute osteomyelitis in the postvaccine, methicillin-resistant Staphylococcus aureus era. Am J Emerg Med 2015;33:1420-4. http://dx.doi.org/10.1016/j.ajem.2015.07.011.
- Le Saux N, Howard A, Barrowman NJ, Gaboury I, Sampson M, Moher D. Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review. BMC Infect Dis 2002;2. https://doi.org/10.1186/1471-2334-2-16.
- Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis 2009;49:65-71. https://doi.org/10.1086/599348.
- Ballock RT, Newton PO, Evans SJ, Estabrook M, Farnsworth CL, Bradley JS. A comparison of early versus late conversion from intravenous to oral therapy in the treatment of septic arthritis. J Pediatr Orthop 2009;29:636-42. http://dx.doi.org/10.1097/BPO.0b013e3181b2b860.
- Peltola H, Unkila-Kallio L, Kallio MJ. Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics 1997;99:846-50. https://doi.org/10.1542/peds.99.6.846.
- Roine I, Faingezicht I, Arguedas A, Herrera JF, Rodríguez F. Serial serum C-reactive protein to monitor recovery from acute hematogenous osteomyelitis in children. Pediatr Infect Dis J 1995;14:40-4. https://doi.org/10.1097/00006454-199501000-00008.
- Peltola H, Pääkkönen M, Kallio P, Kallio MJ. Osteomyelitis-Septic Arthritis Study Group . Short-versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010;29:1123-8. http://dx.doi.org/10.1097/INF.0b013e3181f55a89.
- Milcent K, Guitton C, Koné-Paut I. French nationwide survey about management of acute osteomyelitis in children. Arch Pediatr 2009;16:7-13. http://dx.doi.org/10.1016/j.arcped.2008.10.016.
- Behrman RE, Kliegman RM, Jenson HB. Nelson Textbook of Pediatrics. London: Saunders; 2003.
- Kaplan SL. Acute hematogenous osteomyelitis in children: differences in clinical manifestations and management. Pediatr Infect Dis J 2010;29:1128-9. http://dx.doi.org/10.1097/INF.0b013e3181f55a75.
- Dich VQ, Nelson JD, Haltalin KC. Osteomyelitis in infants and children. A review of 163 cases. Am J Dis Child 1975;129:1273-8. https://doi.org/10.1001/archpedi.1975.02120480007004.
- Blockey NJ, Watson JT. Acute osteomyelitis in children. J Bone Joint Surg Br 1970;52:77-8.
- Ring D, Johnston CE, Wenger DR. Pyogenic infectious spondylitis in children: the convergence of discitis and vertebral osteomyelitis. J Pediatr Orthop 1995;15:652-60. https://doi.org/10.1097/01241398-199509000-00021.
- Baguley D, Lim E, Bevan A, Pallet A, Faust SN. Prescribing for children – taste and palatability affect adherence to antibiotics: a review. Arch Dis Child 2012;97:293-7. http://dx.doi.org/10.1136/archdischild-2011-300909.
- Ruebner R, Keren R, Coffin S, Chu J, Horn D, Zaoutis TE. Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics 2006;117:1210-15. https://doi.org/10.1542/peds.2005-1465.
- Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics 2009;123:636-42. http://dx.doi.org/10.1542/peds.2008-0596.
- HPA . Guidance on the Diagnosis and Management of PVL-Associated Staphylococcus Aureus Infections (PVL-SA) in England 2008. www.gov.uk/government/uploads/system/uploads/attachment_data/file/391168/PVL-guidance_in_primary_care_quick_reference_guide.pdf (accessed 12 April 2017).
- Crary SE, Buchanan GR, Drake CE, Journeycake JM. Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr 2006;149:537-41. https://doi.org/10.1016/j.jpeds.2006.06.067.
- House of Lords . The Government Response to ‘Fighting Infection’, The 4th Report of The House of Lords Select Committee on Science and Technology n.d. www.publications.parliament.uk/pa/ld200203/ldselect/ldsctech/138/138.pdf (accessed 12 April 2017).
- BNF For Children 2012. London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2012.
- Pääkkönen M, Kallio MJ, Kallio PE, Peltola H. Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Pediatric Infect Dis Soc 2013;2:119-25. http://dx.doi.org/10.1093/jpids/pis108.
- Bielicki JA, Barker CI, Saxena S, Wong IC, Long PF, Sharland M. Not too little, not too much: problems of selecting oral antibiotic dose for children. BMJ 2015;351. http://dx.doi.org/10.1136/bmj.h5447.
- European Medicines Agency . Paediatric Regulation n.d. www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000068.jsp&mid=WCOb01ac0580925c45.
- Manual of Childhood Infections. Oxford: Oxford University Press; 2011.
- National Institute for Health and Care Excellence . Fever in Under 5s: Assessment and Initial Management 2013. www.nice.org.uk/guidance/cg160 (accessed 15 August 2016).
- National Institute for Health and Care Excellence . Meningitis (Bacterial) and Menigococcal Septicaemia in Children and Young People 2012. www.nice.org.uk/guidance/qs19 (accessed 15 August 2016).
- Cunningham R, Cockayne A, Humphreys H. Clinical and molecular aspects of the pathogenesis of Staphylococcus aureus bone and joint infections. J Med Microbiol 1996;44:157-64. http://dx.doi.org/10.1099/00222615-44-3-157.
- Public Health England . Group A Streptococcal Infection 7th Update on Seasonal Activity 2013–2014 2014.
- Neeleman C, Klaassen CH, Klomberg DM, de Valk HA, Mouton JW. Pneumolysin is a key factor in misidentification of macrolide-resistant Streptococcus pneumoniae and is a putative virulence factor of S. mitis and other streptococci. J Clin Microbiol 2004;42:4355-7. https://doi.org/10.1128/JCM.42.9.4355-4357.2004.
- Ortiz-Neu C, Marr JS, Cherubin CE, Neu HC. Bone and joint infections due to Salmonella. J Infect Dis 1978;138:820-8. https://doi.org/10.1093/infdis/138.6.820.
- Inman RD, Gallegos KV, Brause BD, Redecha PB, Christian CL. Clinical and microbial features of prosthetic joint infection. Am J Med 1984;77:47-53. https://doi.org/10.1016/0002-9343(84)90434-0.
- Basmaci R, Yagupsky P, Ilharreborde B, Guyot K, Porat N, Chomton M, et al. Multilocus sequence typing and rtxA toxin gene sequencing analysis of Kingella kingae isolates demonstrates genetic diversity and international clones. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0038078.
- Ceroni D, Dubois-Ferriere V, Cherkaoui A, Gesuele R, Combescure C, Lamah L, et al. Detection of Kingella kingae osteoarticular infections in children by oropharyngeal swab PCR. Pediatrics 2013;131:e230-5. http://dx.doi.org/10.1542/peds.2012-0810.
- Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, Rolain JM, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 2009;49:543-51. http://dx.doi.org/10.1086/600885.
- Kim H, Kim J, Ihm C. The usefulness of multiplex PCR for the identification of bacteria in joint infection. J Clin Lab Anal 2010;24:175-81. http://dx.doi.org/10.1002/jcla.20384.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Ballinger C. Writing up rigour: representing and evaluating good scholarship in qualitative research. Br J Occup Ther 2004;67:540-6. https://doi.org/10.1177/030802260406701204.
- Edwards R, Holland J. What is Qualitative Interviewing?. London: Bloomsbury Academic; 2013.
- Mason J. Qualitative Researching. Thousand Oaks, CA: Sage Publications Ltd; 2002.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Howard-Jones AR, Isaacs D. Systematic review of systemic antibiotic treatment for children with chronic and sub-acute pyogenic osteomyelitis. J Paediatr Child Health 2010;46:736-41. http://dx.doi.org/10.1111/j.1440-1754.2010.01831.x.
- Pääkkönen M, Kallio MJ, Kallio PE, Peltola H. Shortened hospital stay for childhood bone and joint infections: analysis of 265 prospectively collected culture-positive cases in 1983-2005. Scand J Infect Dis 2012;44:683-8. http://dx.doi.org/10.3109/00365548.2012.673729.
- Bachur R, Pagon Z. Success of short-course parenteral antibiotic therapy for acute osteomyelitis of childhood. Clin Pediatr 2007;46:30-5. https://doi.org/10.1177/0009922806289081.
- Messina AF, Namtu K, Guild M, Dumois JA, Berman DM. Trimethoprim-sulfamethoxazole therapy for children with acute osteomyelitis. Pediatr Infect Dis J 2011;30:1019-21. http://dx.doi.org/10.1097/INF.0b013e31822db658.
- Tetzlaff TR, McCracken GH, Nelson JD. Oral antibiotic therapy for skeletal infections of children. II. Therapy of osteomyelitis and suppurative arthritis. J Pediatr 1978;92:485-90. https://doi.org/10.1016/S0022-3476(78)80455-7.
- Kolyvas E, Ahronheim G, Marks MI, Gledhill R, Owen H, Rosenthall L. Oral antibiotic therapy of skeletal infections in children. Pediatrics 1980;65:867-71.
- Jaberi FM, Shahcheraghi GH, Ahadzadeh M. Short-term intravenous antibiotic treatment of acute hematogenous bone and joint infection in children: a prospective randomized trial. J Pediatr Orthop 2002;22:317-20. https://doi.org/10.1097/01241398-200205000-00009.
- Jagodzinski NA, Kanwar R, Graham K, Bache CE. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop 2009;29:518-25. http://dx.doi.org/10.1097/BPO.0b013e3181ab472d.
- Bruce I, Harman N, Williamson P, Tierney S, Callery P, Mohiuddin S, et al. The management of Otitis Media with Effusion in children with cleft palate (mOMEnt): a feasibility study and economic evaluation. Health Technol Assess 2015;19. http://dx.doi.org/10.3310/hta19680.
- TORPEDO-CF . TORPEDO-CF Homepage 2015. www.torpedo-cf.org.uk (accessed 11 June 2017).
- Stock CJ, Carley N, Hickman B, Primrose J, Ayres A, McGrath C, et al. Clinician engagement is critical to public engagement with clinical trials. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h3140.
- Cunningham S, Rodriguez A, Adams T, Boyd KA, Butcher I, Enderby B, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet 2015;386:1041-8. http://dx.doi.org/10.1016/S0140-6736(15)00163-4.
Appendix 1 Core outcome literature review
Search strategy used to identify trials investigating duration of antibiotics in paediatric bone and joint infection
The search was executed using MEDLINE and EMBASE databases. All publications from 1946 to the present were included in the search. The search was carried out in May 2012 and updated in July 2014.
| # | Search term | Number of hits |
|---|---|---|
| 1 | osteomyelitis.mp. | 39,394 |
| 2 | bone infection.mp. | 3979 |
| 3 | osteitis.mp. | 9113 |
| 4 | septic arthritis.mp. | 7340 |
| 5 | bacterial arthritis.mp | 6369 |
| 6 | joint infection.mp | 2450 |
| 7 | Staphylococc*.mp. | 322,123 |
| 8 | Streptococc*.mp. | 230,550 |
| 9 | Skeletal infection.mp | 109 |
| 10 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | 565,232 |
| 11 | child$.mp | 2,991,039 |
| 12 | paediatric or pediatric.mp. | 432,403 |
| 13 | neonat$.mp | 367,776 |
| 14 | infan$.mp. | 1,244,482 |
| 15 | (baby or babies).mp. | 119,622 |
| 16 | 11 or 12 or 13 or 14 or 15 | 4,882,434 |
| 17 | 10 and 16 | 98,071 |
| 18 | antibiotic.mp. | 549,340 |
| 19 | treatment.mp. | 6,513,851 |
| 20 | 18 or 19 | 6,866,563 |
| 21 | duration.mp. | 914,091 |
| 22 | course.mp. | 1,010,339 |
| 23 | length.mp | 831,145 |
| 24 | shortened.mp. | 56,581 |
| 25 | 21 or 22 or 23 or 24 | 2,641,076 |
| 26 | 17 and 25 | 7342 |
| 27 | remove duplicates from 26 | 1321 |
Appendix 2 Tables of the consensus meeting
Criteria for switching
| Switch criteria | Discussion | Number voting |
|---|---|---|
| What criteria are important to you when deciding when or whether or not to switch from i.v. to oral antibiotic therapy? | 17 | |
| Switch criterion | CRP (non-specific blood test done at diagnosis and for monitoring) of ≤ 20 mg/l |
|---|---|
| Number of participants scoring 1–9 | 13 |
| Number of participants unable to score | 4 |
FIGURE 6.
Results of scoring: CRP of < 20 mg/l.

| Switch criterion | CRP less than one-third of the presenting value |
|---|---|
| Number of participants scoring 1–9 | 13 |
| Number of participants unable to score | 4 |
FIGURE 7.
Results of scoring: CRP less than one-third of the presenting value.

| Switch criterion | CRP less than two-thirds of the presenting value |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 8.
Results of scoring: CRP less than two-thirds of the presenting value.
| Switch criterion | ESR of ≤ 20 mm/hour |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 9.
Results of scoring: ESR of < 20 mm/hour.
| Switch criterion | Resolution of fever for ≥ 48 hours (body temperature normal) |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 10.
Results of scoring: resolution of fever for ≥ 48 hours.
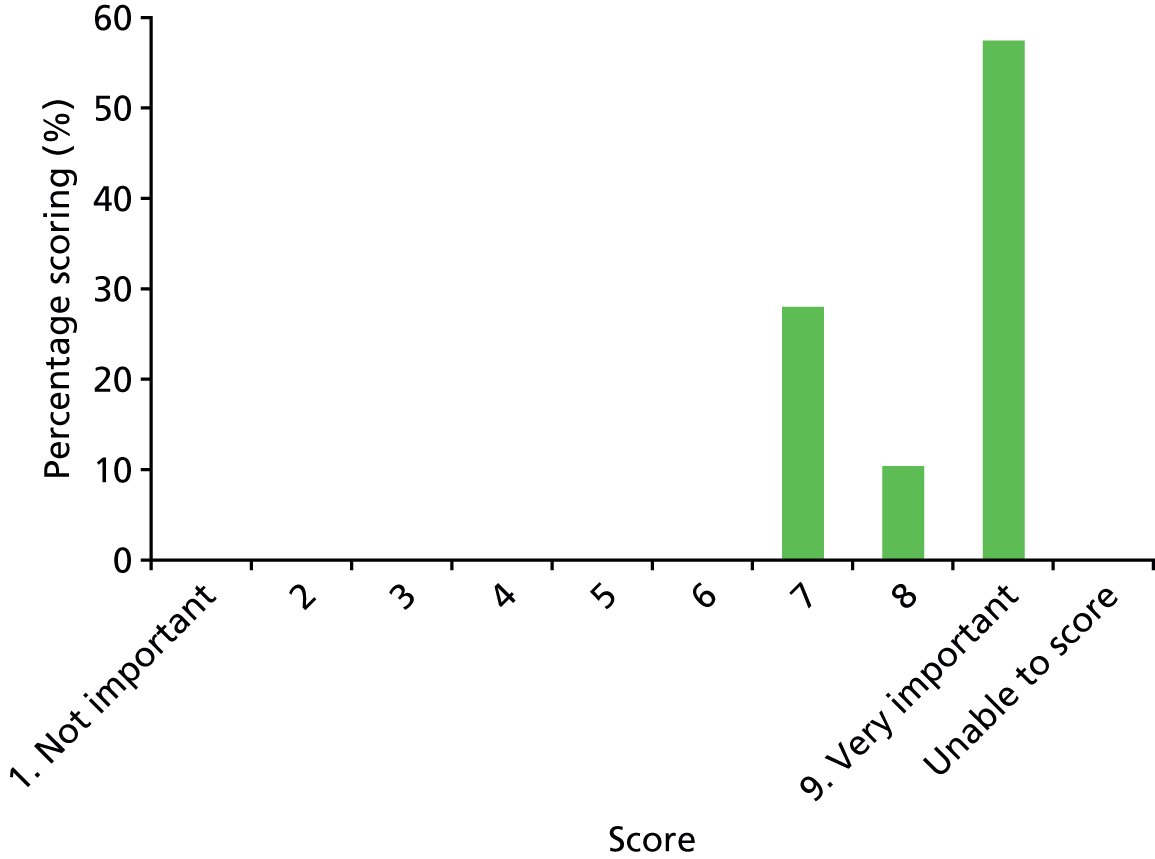
| Switch criterion | Weight bearing/return of function to limb |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 11.
Results of scoring: weight bearing/return of function to limb.
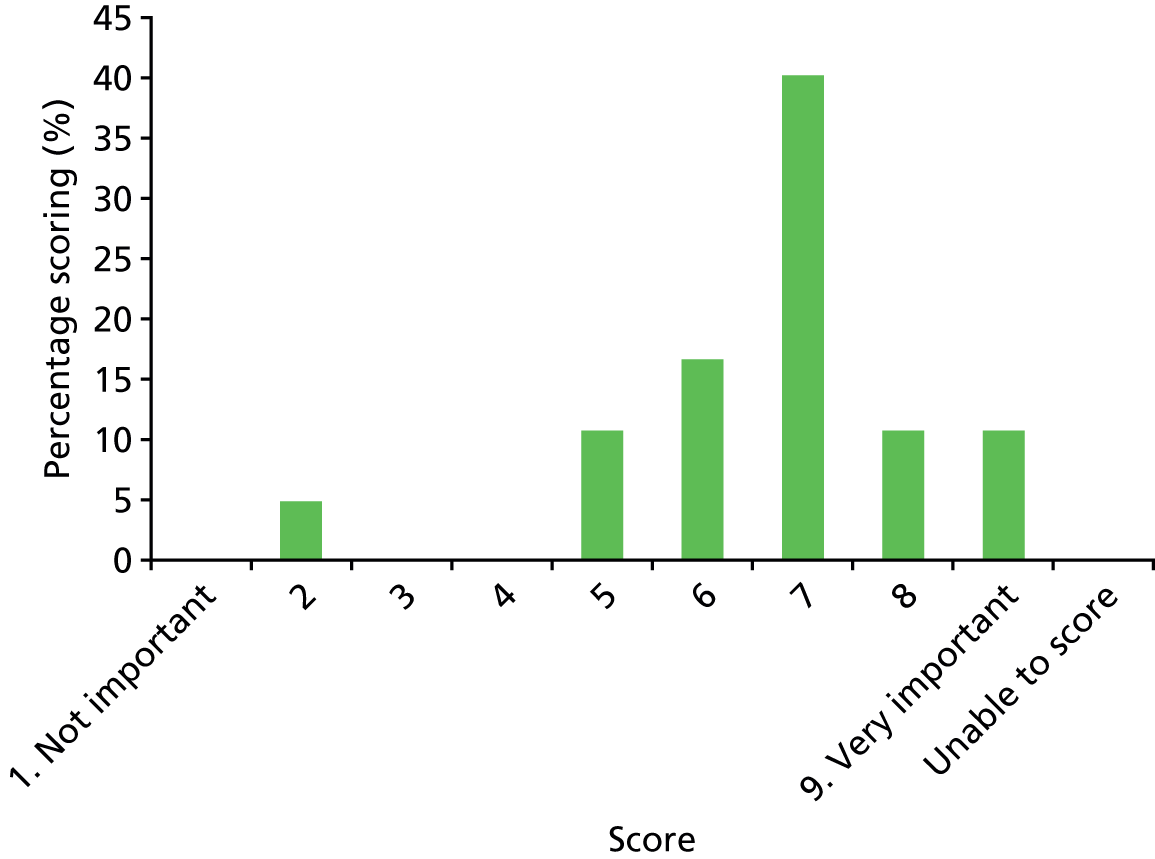
| Switch criterion | Resolution of pain (pain completely better) |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 12.
Results of scoring: resolution of pain.

| Switch criterion | Tolerating oral input (can eat and drink) |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 13.
Results of scoring: tolerating oral input.

| Switch criterion | Parental opinion regarding improvement or worsening of condition |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 14.
Results of scoring: parental opinion regarding improvement or worsening of condition.

| Switch criterion | Pain improvement (rather than resolution) |
|---|---|
| Number of participants scoring 1–9 | 17 |
| Number of participants unable to score | 0 |
FIGURE 15.
Results of scoring: pain improvement.

Outcomes
| Outcomes | Discussion | Number voting |
|---|---|---|
| What outcomes should be measured to assess whether or not treatment has been effective? | 16 | |
| Outcome name | Need for non-diagnostic surgical procedure (surgery for treatment of the infection itself, e.g. decompression, debridement) |
|---|---|
| Number of participants scoring 1–9 | 15 |
| Number of participants unable to score | 1 |
FIGURE 16.
Results of scoring: need for non-diagnostic surgical procedure.

| Outcome name | Rehospitalisation or recurrence of symptoms while on oral (by mouth) antibiotics |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 17.
Results of scoring: rehospitalisation or recurrence of symptoms while on oral antibiotics.
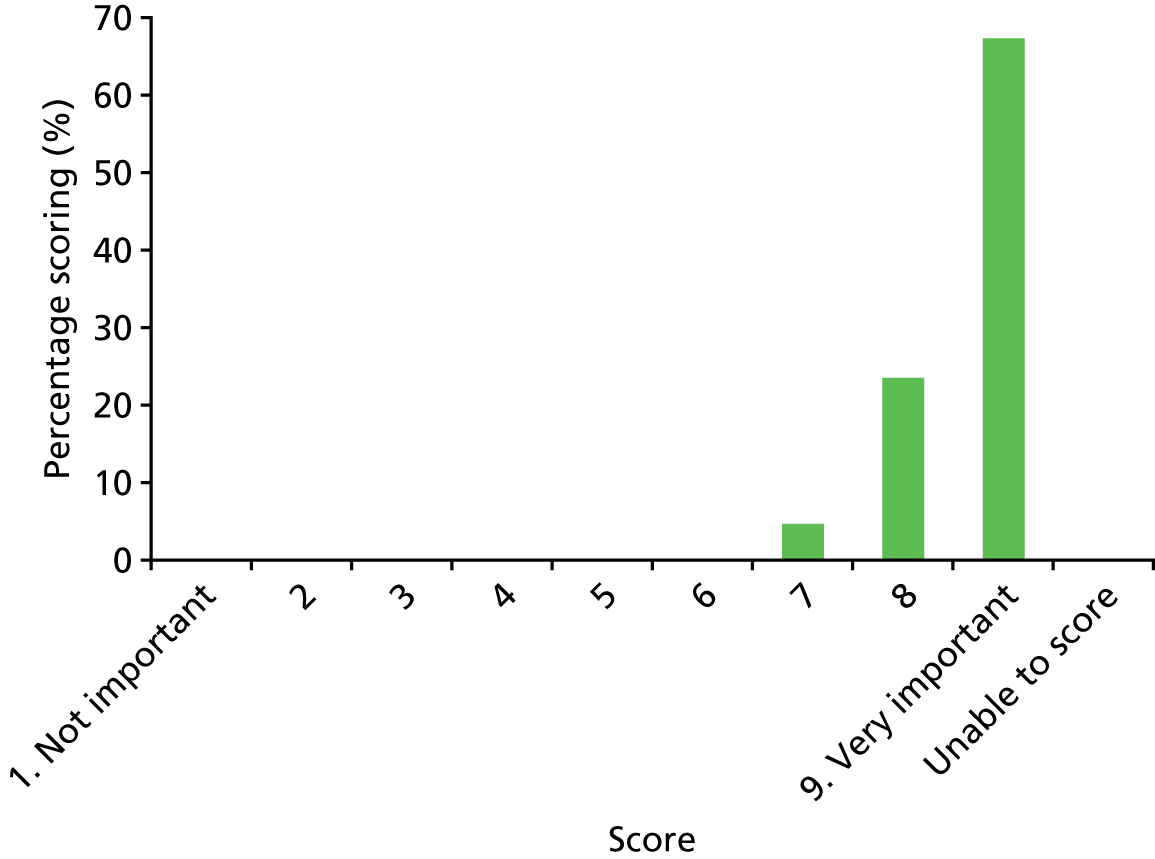
| Outcome name | Treatment failure: recurrence of infection (either at original site or at new site) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 18.
Results of scoring: treatment failure – recurrence of infection.
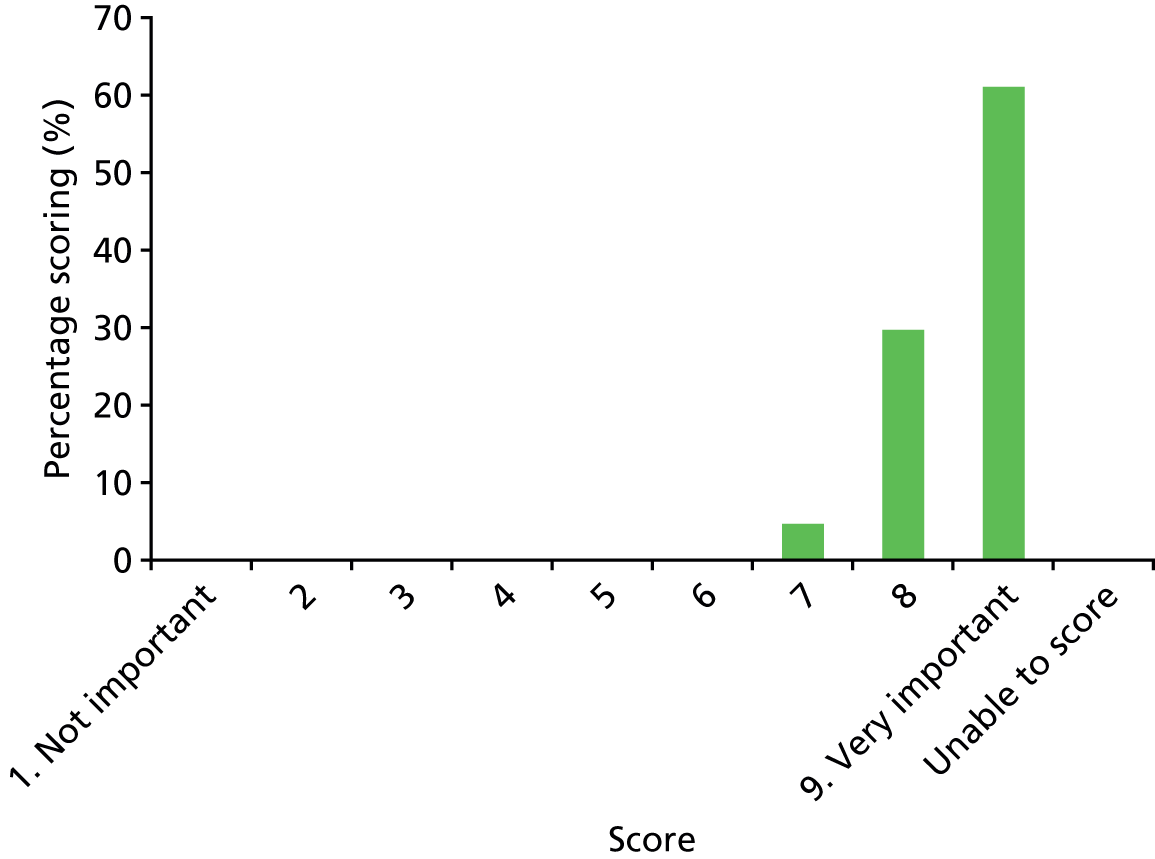
| Outcome name | Pain while on antimicrobial treatment |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 19.
Results of scoring: pain while on antimicrobial treatment.
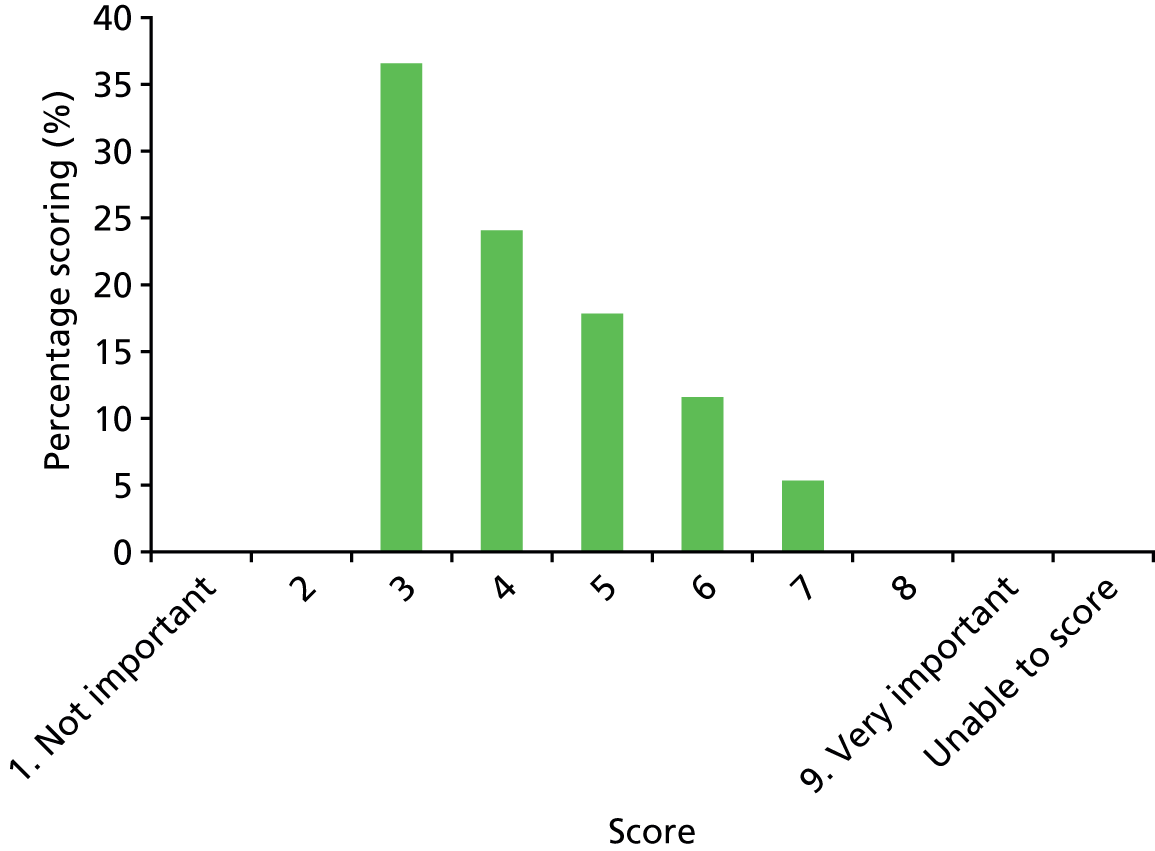
| Outcome name | Ongoing pain at follow-up |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 20.
Results of scoring: ongoing pain at follow-up.

| Outcome name | Disability at follow-up (any functional movement problems due to the original infection) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 21.
Results of scoring: disability at follow-up.
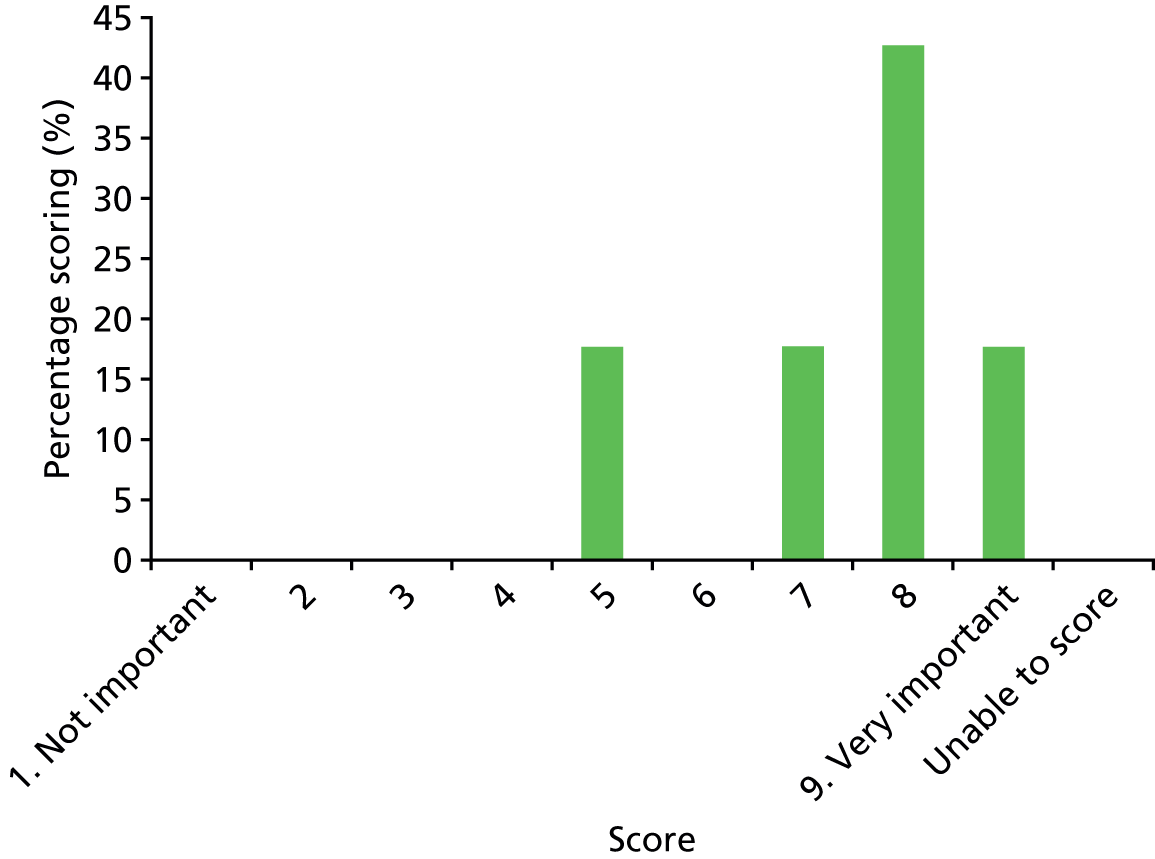
| Outcome name | Symptom free at 1 year |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 22.
Results of scoring: symptom free at 1 year.

| Outcome name | Limb shortening or deformity |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 23.
Results of scoring: limb shortening or deformity.

| Outcome name | Duration to CRP of ≤ 5 mg/l |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 24.
Results of scoring: duration to CRP of < 5 mg/l.

| Outcome name | Duration to resolution of fever (number of days for fever to get better) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 25.
Results of scoring: duration to resolution of fever.

| Outcome name | Duration to resolution of pain (number of days for pain to get better) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 26.
Results of scoring: duration to resolution of pain.
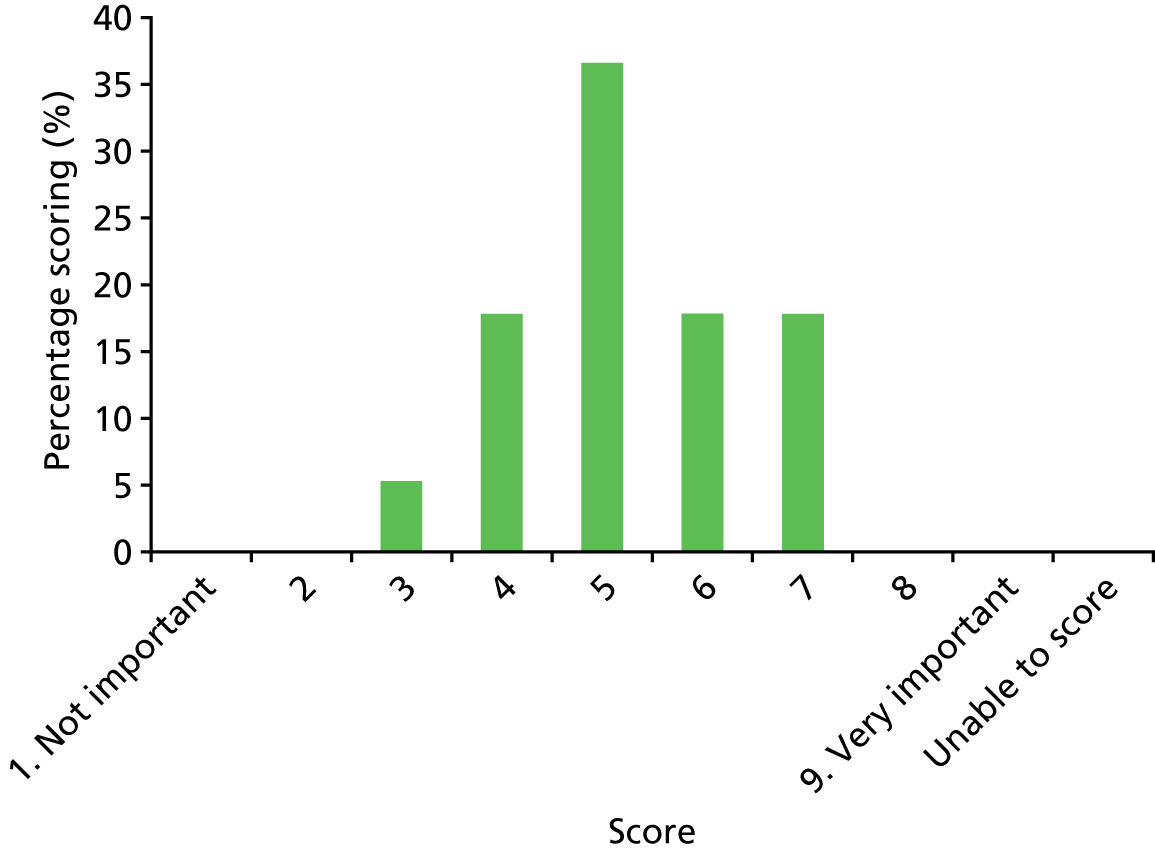
| Outcome name | Duration of hospital stay |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 27.
Results of scoring: duration of hospital stay.
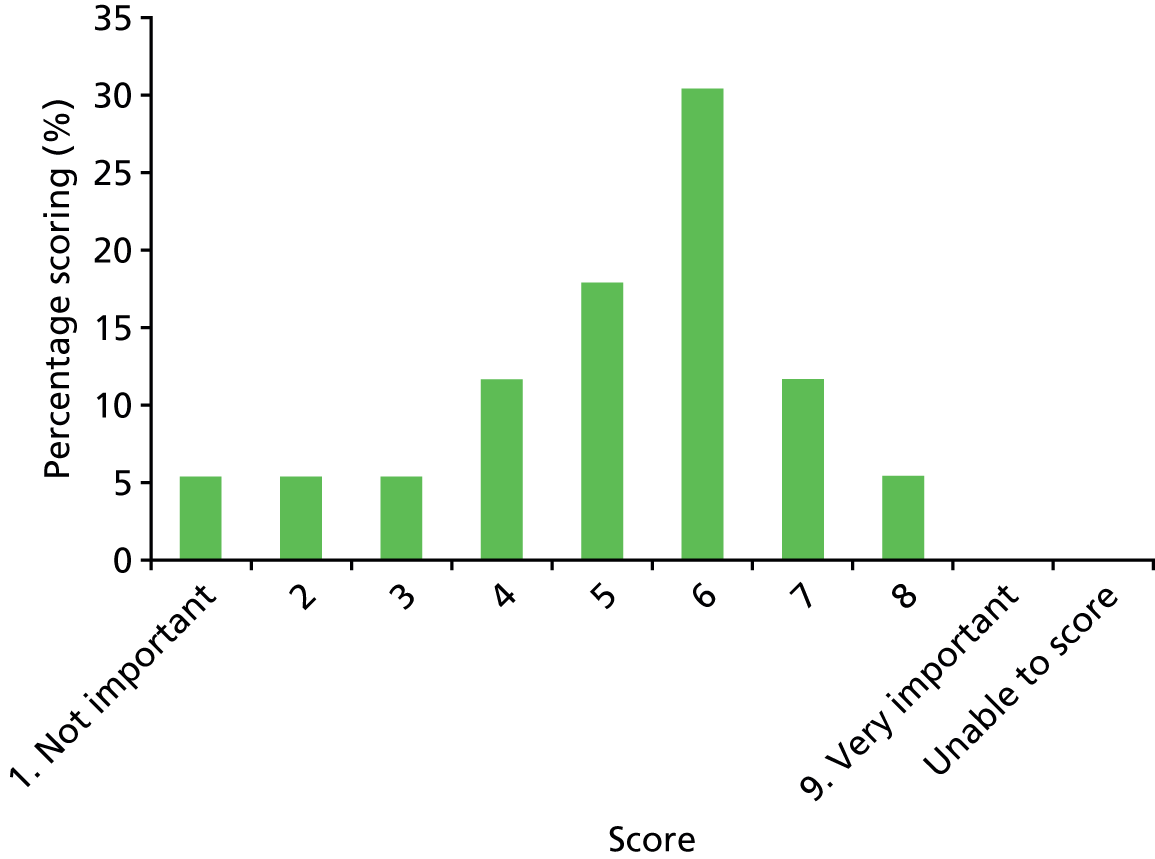
| Outcome name | Fracture at or near site of infection |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 28.
Results of scoring: fracture at or near site of infection.

| Outcome name | Prolonged antibiotic therapy |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 29.
Results of scoring: prolonged antibiotic therapy.
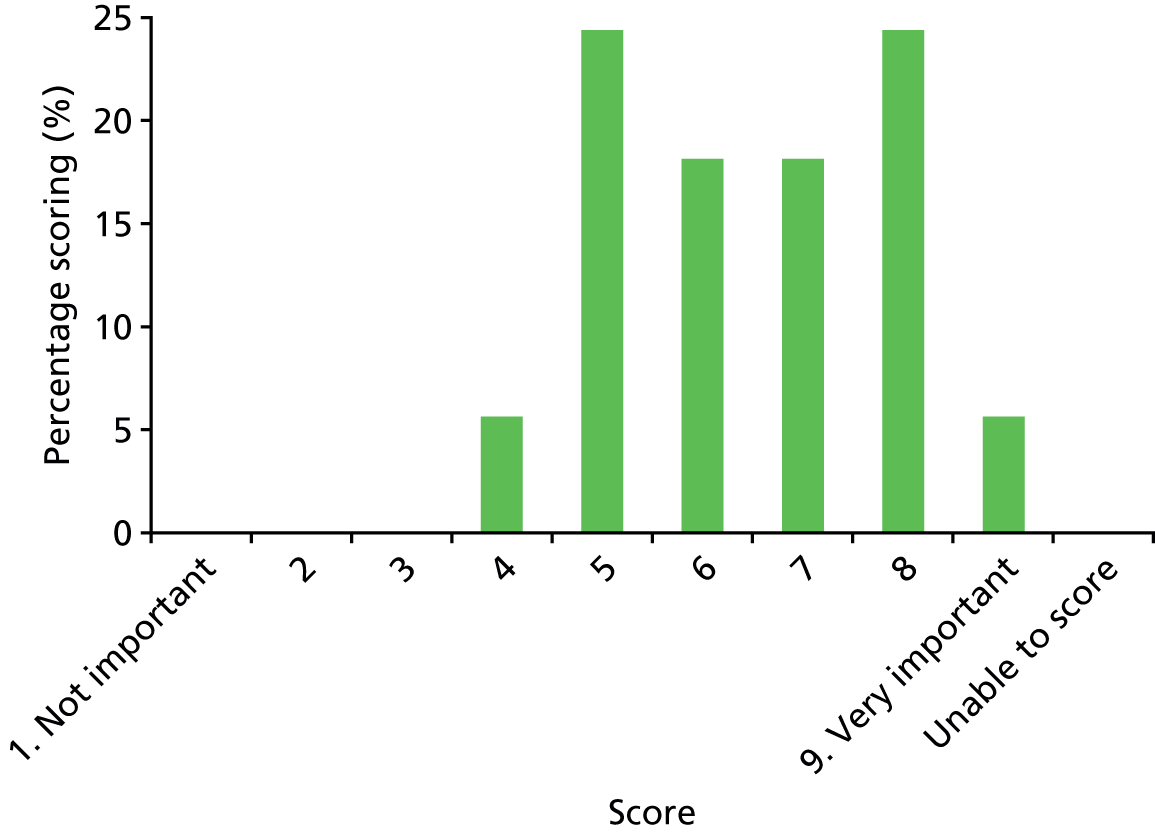
| Outcome name | Quality of life |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 30.
Results of scoring: quality of life.

| Outcome name | Chronic OM (infection that does not get better with treatment) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 31.
Results of scoring: chronic OM.
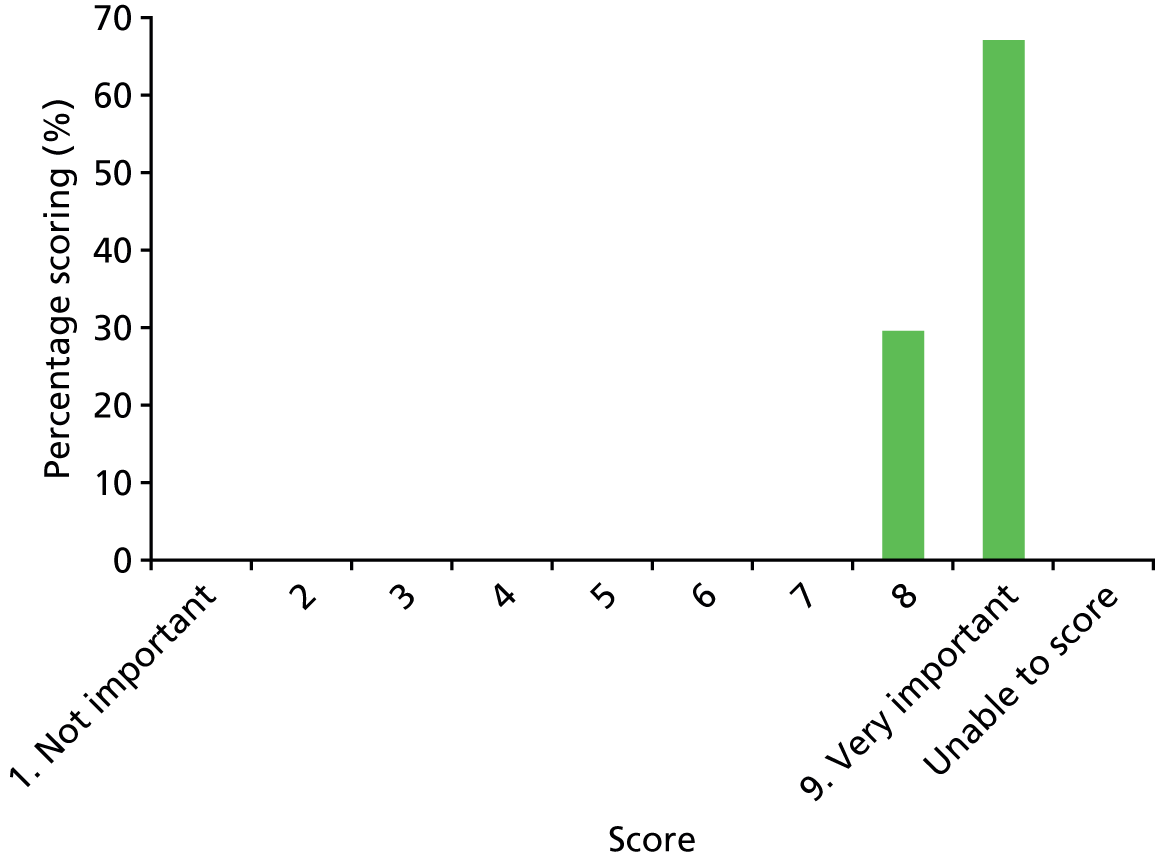
| Outcome name | Chronic arthritis (joint inflammation that does not get better with treatment) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 32.
Results of scoring: chronic arthritis.
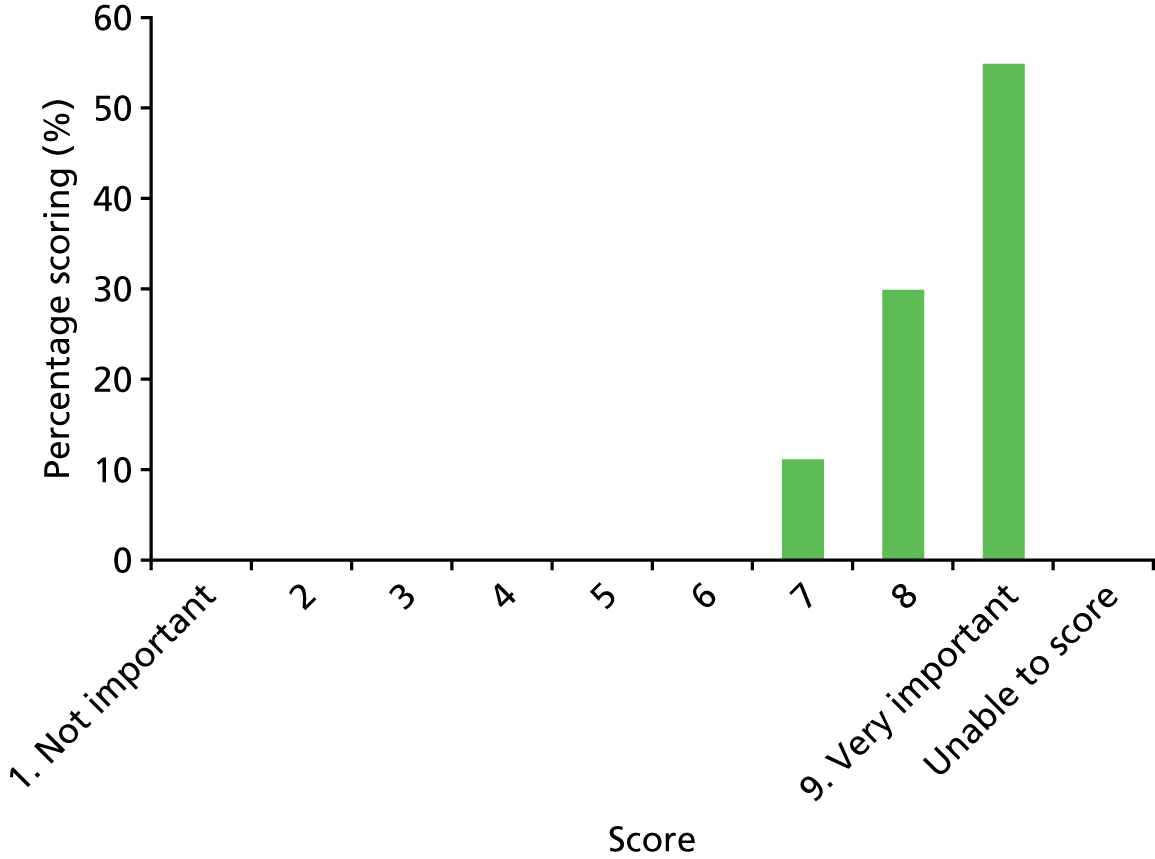
| Outcome name | Chronic myositis (muscle inflammation that does not get better with treatment) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 33.
Results of scoring: chronic myositis.
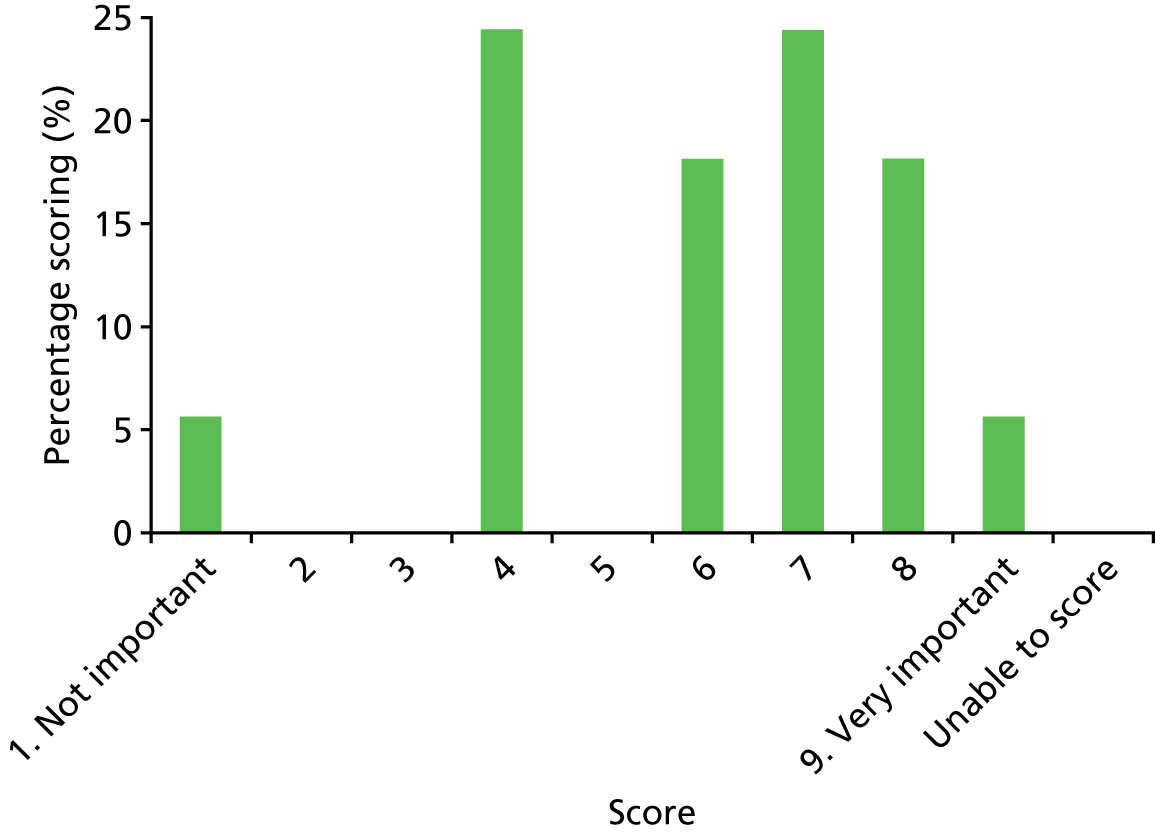
| Outcome name | Amputation or fasciotomy (when the skin and muscle have to be cut by a surgeon to relieve pressure between the skin and bone) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 34.
Results of scoring: amputation or fasciotomy.
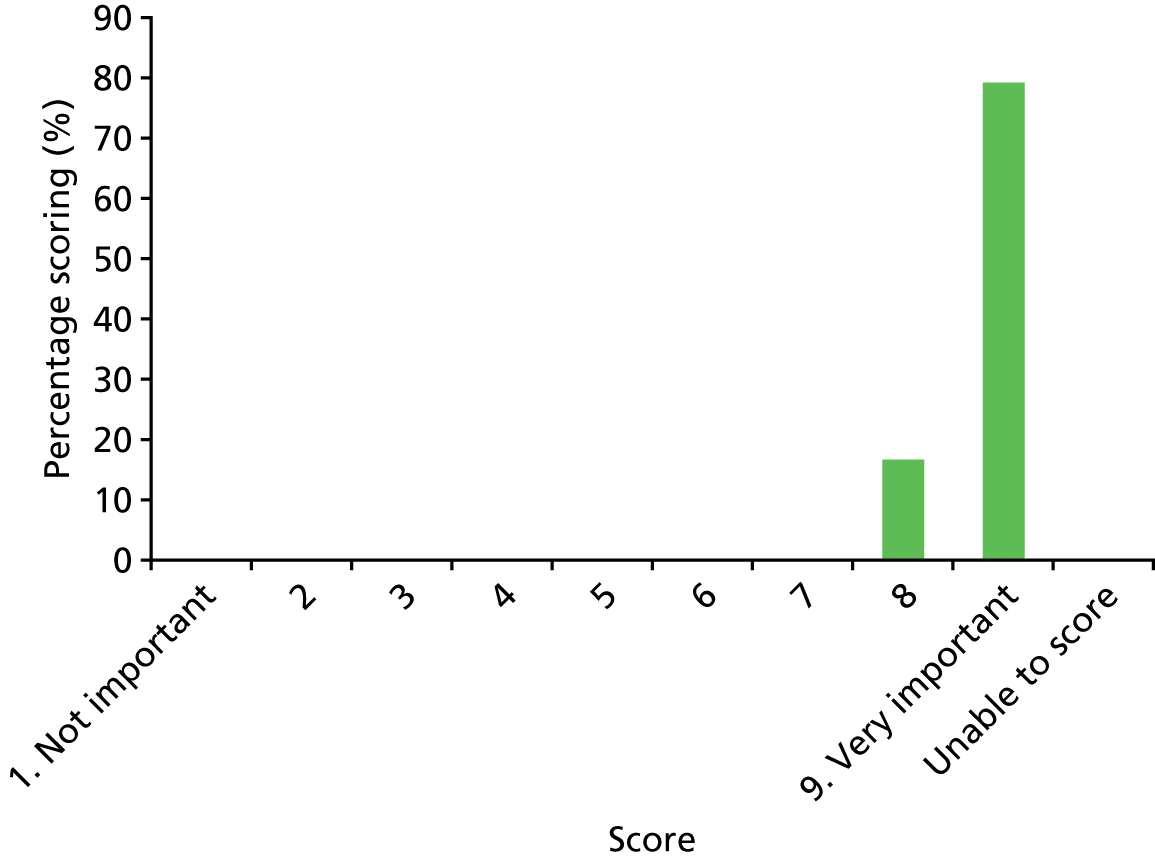
| Outcome name | Death |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 35.
Results of scoring: death.

| Outcome name | PICU requirement (e.g. PICU-free days at day 30, if admitted) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 36.
Results of scoring: paediatric intensive care unit requirement.

Treatment/line related
| Outcome name | Agranulocytosis/neutropaenia (abnormal white cell count) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 37.
Results of scoring: agranulocytosis/neutropaenia.
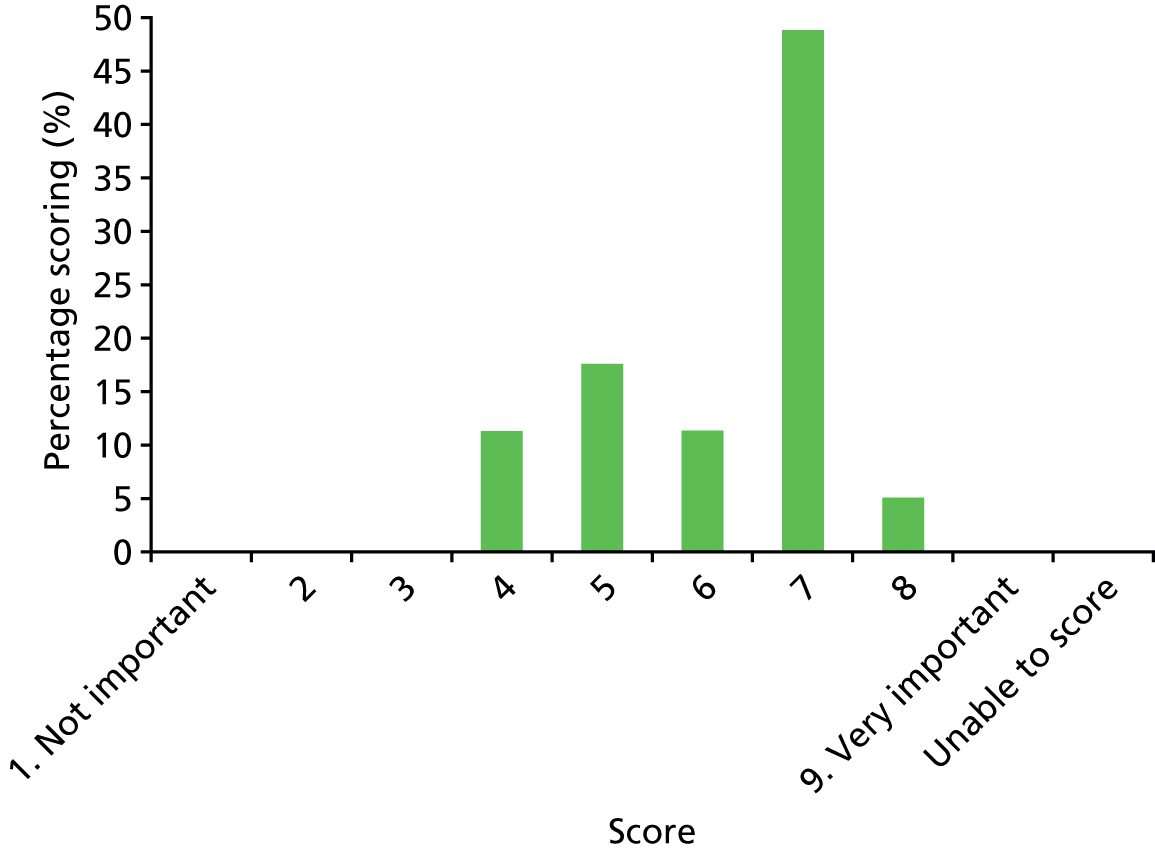
| Outcome name | Deranged (abnormal) liver function tests |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 38.
Results of scoring: deranged liver function tests.
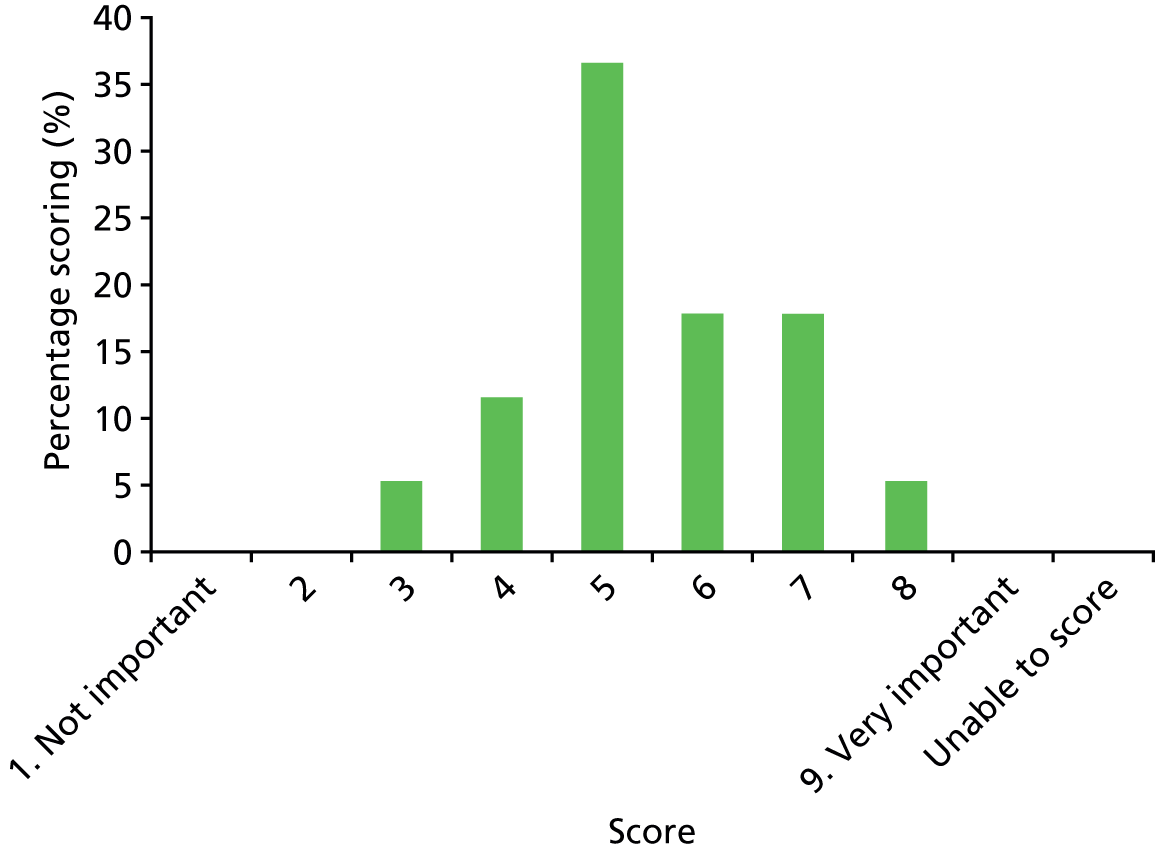
| Outcome name | C. difficile infection (infectious complication of prolonged antibiotic treatment, unusual in children and not able to be diagnosed in children aged < 2 years as the infection is carried by young children as a normal finding without causing problems) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 39.
Results of scoring: C. difficile infection.
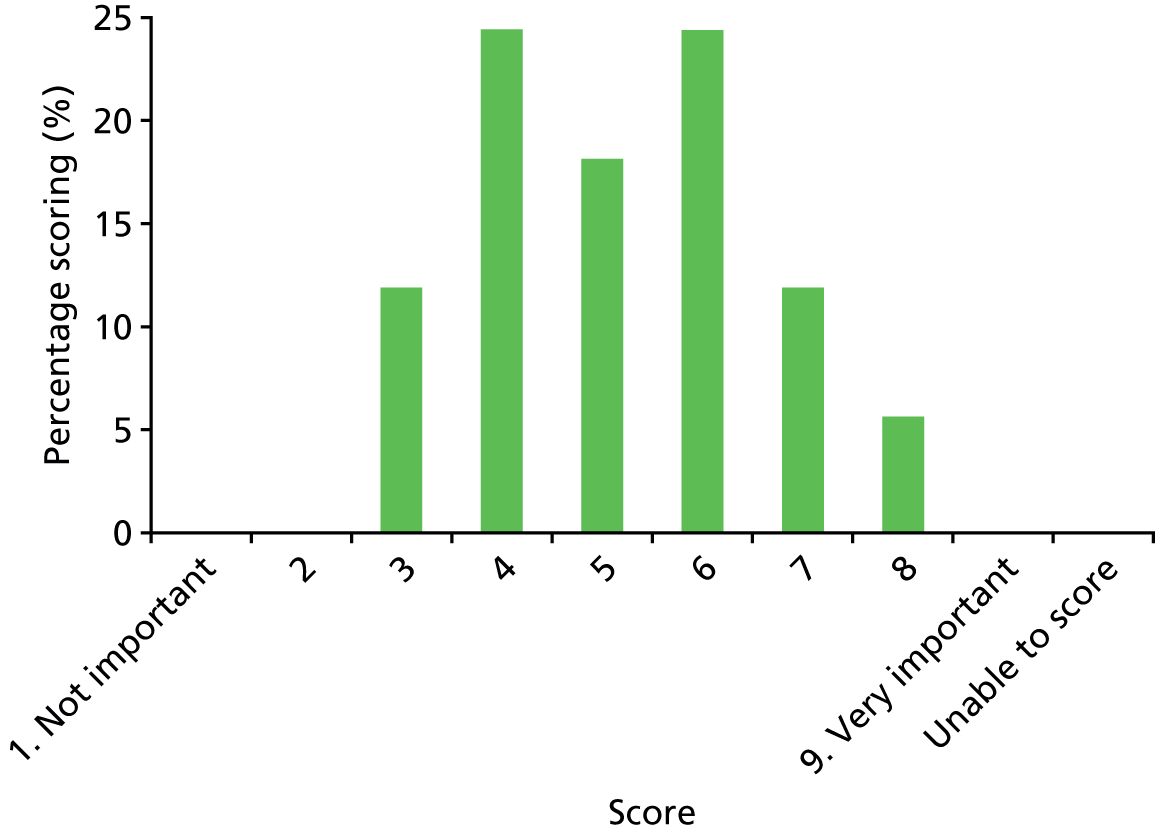
| Outcome name | Rash or allergic reaction |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 40.
Results of scoring: rash or allergic reaction.
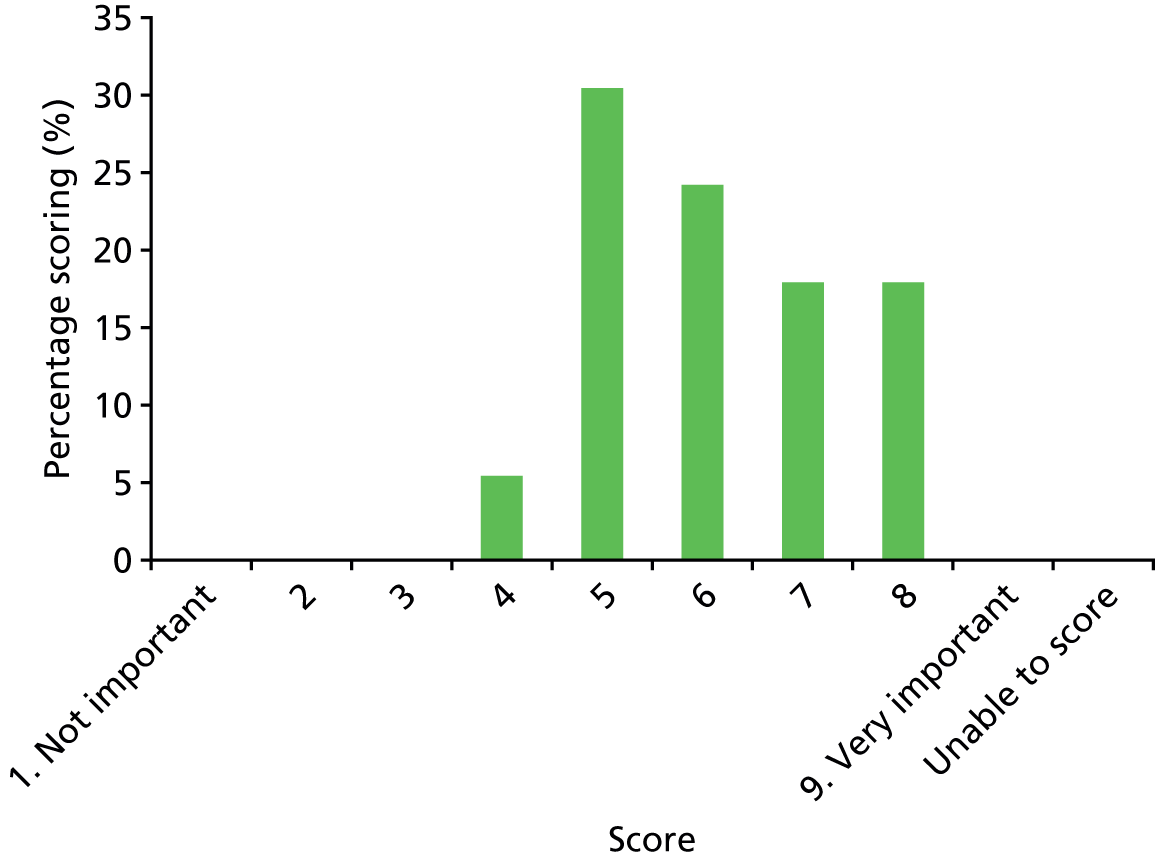
| Outcome name | Suspected drug-related fever |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 41.
Results of scoring: suspected drug-related fever.
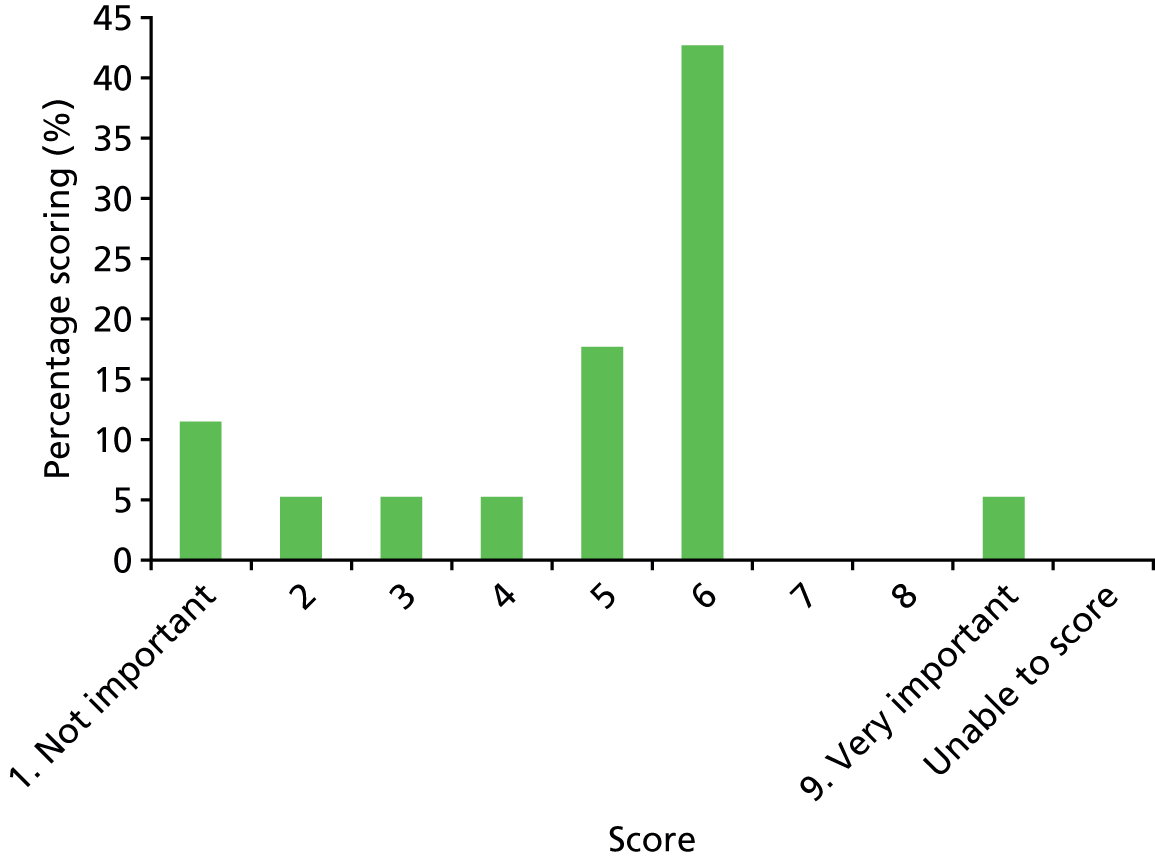
| Outcome name | Line infection (infection of the drip-line itself) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 42.
Results of scoring: line infection.

| Outcome name | Line occlusion (block of the drip line) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 43.
Results of scoring: line occlusion.
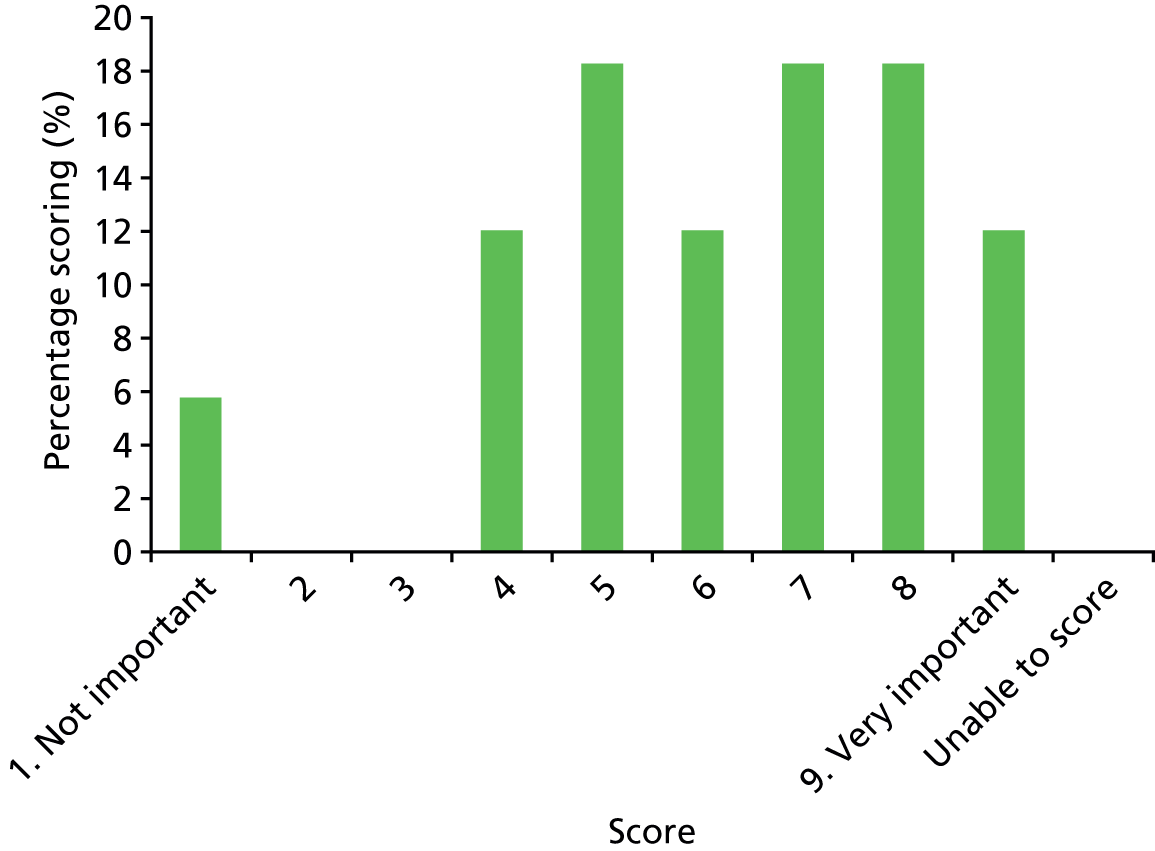
| Outcome name | Vomiting/diarrhoea |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 44.
Results of scoring: vomiting/diarrhoea.

| Outcome name | i.v. antibiotic necessitated insertion of PICC or other central line (need to insert another longer-term i.v. drip) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 45.
Results of scoring: antibiotic necessitated insertion of PICC or other central line.

| Outcome name | Infiltration or extravasation event (medicine being given into the veins accidently is delivered to the tissues due to the drip coming out of the blood vessel) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 46.
Results of scoring: infiltration or extravasation event.
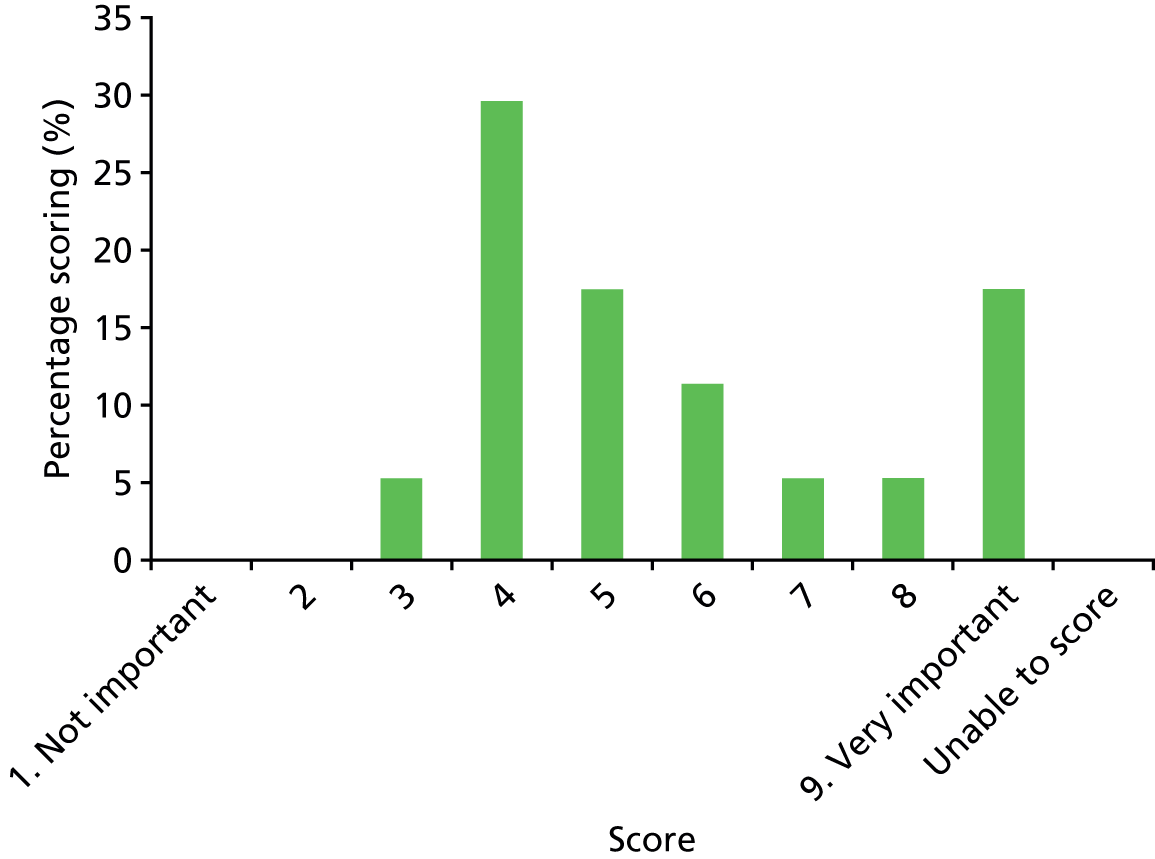
| Outcome name | Any complication of vascular access (any complication of the drip itself) |
|---|---|
| Number of participants scoring 1–9 | 16 |
| Number of participants unable to score | 0 |
FIGURE 47.
Results of scoring: any complication of vascular access.
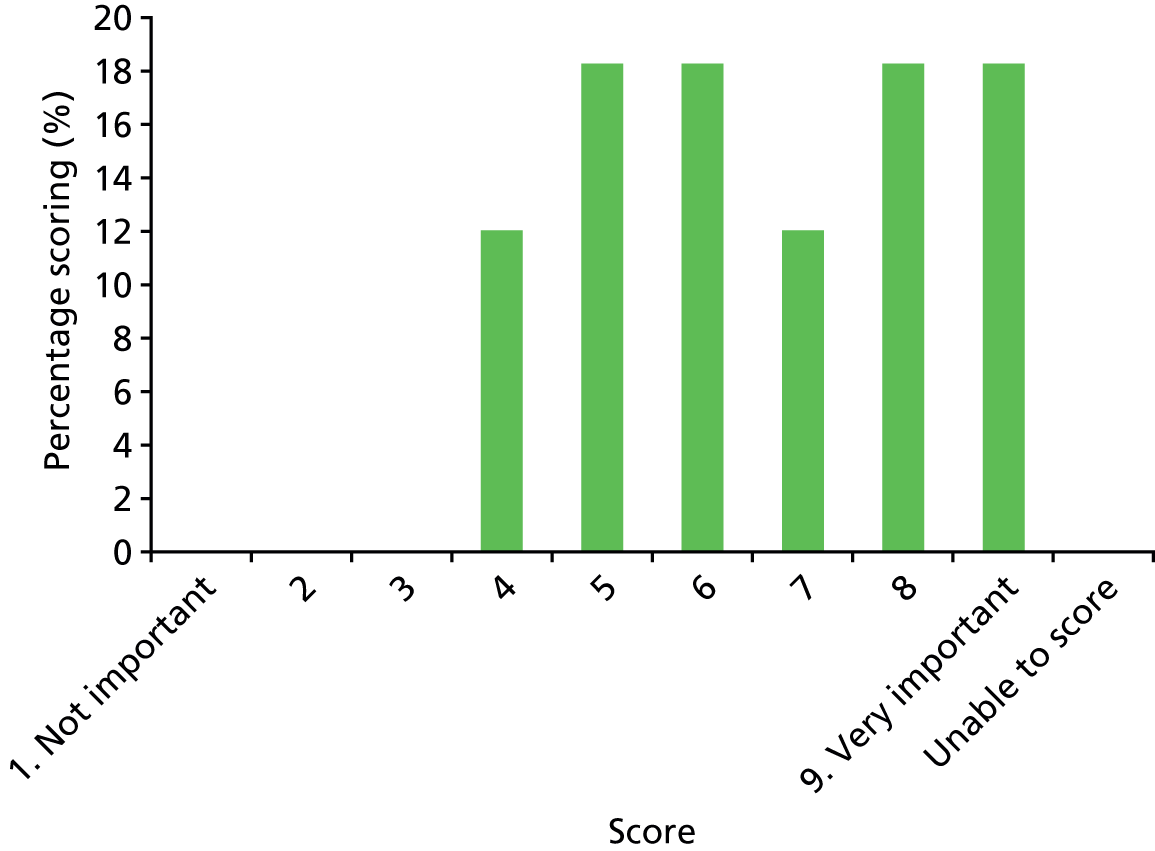
List of abbreviations
- A&E
- accident and emergency
- BNFC
- British National Formulary for Children
- CRP
- C-reactive protein
- CT
- computerised tomography
- ESR
- erythrocyte sedimentation rate
- GAS
- group A Streptococcus
- GBS
- group B Streptococcus
- GP
- general practitioner
- HTA
- Health Technology Assessment
- IQR
- interquartile range
- i.v.
- intravenous
- lytA
- N-acetylmuramoyl-L-alanine amidase
- MCRN
- Medicines for Children Research Network
- MRI
- magnetic resonance imaging
- MRSA
- meticillin-resistant Staphylococcus aureus
- MSSA
- meticillin-sensitive Staphylococcus aureus
- NIHR
- National Institute for Health Research
- OAI
- osteoarticular infection
- OM
- osteomyelitis
- OPAT
- outpatient parenteral antibiotic therapy
- PCR
- polymerase chain reaction
- PICC
- peripherally inserted central catheter
- ply
- pneumolysin
- PPI
- patient and public involvement
- PVL
- Panton–Valentine leukocidin
- RCPCH
- Royal College of Paediatrics and Child Health
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RT-PCR
- reverse-transcription polymerase chain reaction
- SA
- septic arthritis
- WBC
- white blood cell