Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/12. The contractual start date was in April 2011. The draft report began editorial review in November 2015 and was accepted for publication in April 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Thomas RE Barnes reports personal fees from Roche, Sunovion Pharmaceuticals Inc. and Otsuka Pharmaceutical/Lundbeck, outside the submitted work. Carol Paton reports personal fees from Sunovion Pharmaceuticals Inc. and Eli Lilly and Company, outside the submitted work. Linda Davies reports a grant from the National Institute for Health Research (NIHR) to the University of Manchester, during the conduct of the study. Patrick Keown reports personal fees from Otsuka Pharmaceutical/Lundbeck and other from Janssen-Cilag, outside the submitted work. Hemant Bagalkote reports personal fees from Janssen-Cilag, Lundbeck and Eli Lilly and Company, outside the submitted work. Peter M Haddad reports grants from the NIHR during the conduct of the study; personal fees for lecturing and/or consultancy (including attending advisory boards) from Allergan plc, Eli Lilly and Company, Galen Limited, Janssen-Cilag, Lundbeck, Otsuka Pharmaceutical, Quantum Pharmaceutical, Roche, Servier Laboratories, Sunovion Pharmaceuticals Inc., Takeda Pharmaceutical Company, Teva Pharmaceutical Industries Ltd; and received conference support from Janssen-Cilag, Lundbeck, Otsuka Pharmaceutical, Servier Laboratories and Sunovion Pharmaceuticals Inc., outside the submitted work. Mariwan Husni reports other from Otsuka Pharmaceutical, outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Barnes et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
In around one-third of people with schizophrenia, the illness shows a poor response to standard treatment with antipsychotic medication. Although a relatively small proportion will fail to achieve remission even after the first exposure to antipsychotic medication, with either first- or second-generation drugs,1 more commonly the illness becomes progressively more unresponsive to medication with subsequent relapses. 2,3 This treatment-resistant subgroup of patients represents a major clinical challenge in everyday psychiatry, and consumes a disproportionate amount of NHS funding. 4–6 Mangalore and Knapp7 estimated that the total societal cost of schizophrenia in the UK in 2004/5 was £6.7B. The direct cost of treatment and care, falling on the UK public purse, was around £2B, whereas the burden of indirect costs to society was approaching £4.7B. The cost of informal care and private expenditures by families and carers was around £615M, whereas the loss of productivity as a result of unemployment, absence from work and premature mortality of people with schizophrenia was estimated to be £3.4B and the lost productivity of their carers estimated to be around £32M. Furthermore, Mangalore and Knapp7 calculated that, in addition to costs to the criminal justice system, around £570M was being paid out in benefit payments, associated with about £14M administration costs. Treatment-resistant illness is considered to be more costly, usually requiring longer-term residential and intensive community treatments. There is clinical and economic need to evaluate treatments to improve outcomes in this group of patients.
Clozapine augmentation with a second antipsychotic
For treatment-resistant schizophrenia, a common therapeutic approach is to use more than one antipsychotic, although a robust evidence base to justify this is lacking. Recent surveys of prescribing patterns in the USA suggest that about 15% of outpatients and up to 50% of inpatients with schizophrenia receive two or more antipsychotics. 8 In the UK in 2012, national clinical audit data from the Prescribing Observatory for Mental Health (POMH-UK) on over 5000 acute inpatients and over 3000 forensic patients prescribed antipsychotics revealed regularly prescribed combined antipsychotics in 14% and 17%, respectively. 9 A common reason given by clinical teams for prescribing such a combination was failure of the illness to respond to treatment with a single antipsychotic. In the POMH-UK audit, just under 5% of prescriptions for combined antipsychotics in acute adult wards represented the augmentation of clozapine with another antipsychotic, whereas in rehabilitation/complex needs services and forensic services the respective figures were in excess of 20%. These figures are in line with other reports of the prevalence of clozapine augmentation, ranging from 18% to 44% depending on the clinical setting and country. 10–12 In summary, it seems that around one-third of all clozapine-treated patients receive augmentation with another antipsychotic. 13 This is because it is one of the few therapeutic strategies available to clinicians for those people with schizophrenia that has proved to be poorly responsive to clozapine.
Clozapine is the only antipsychotic for which there is convincing evidence of efficacy in strictly defined treatment-resistant schizophrenia, but in such cases it has limited effectiveness, with 30–40% of patients showing an inadequate response to the drug. 14 In some patients, a range of potentially serious side effects such as seizures, sedation and tachycardia may prevent the optimal dose being reached. In addition, weight gain and metabolic side effects that increase the risk of diabetes and cardiovascular disease become apparent in many.
In an attempt to improve efficacy and limit tolerability problems, clinicians commonly augment clozapine with another antipsychotic, despite limited evidence on the potential risks and benefits of this practice. 15 In 2005, Kontaxakis et al. 16 identified 15 case studies, with a total of 33 patients, of adjunctive agents in clozapine-resistant schizophrenia, 10 of which involved a second antipsychotic (eight using risperidone, one using sulpiride and one using olanzapine). They concluded that various methodological shortcomings limited the impact of the findings. These authors came to a similar conclusion after conducting a critical review of 11 randomised controlled trials (RCTs; with a total of 270 participants) of clozapine augmentation in treatment-resistant schizophrenia,17 in only one of which was an antipsychotic (sulpiride) used as the adjunctive medication. Methodological weaknesses included the fact that only one trial adequately reported the dose and duration of the clozapine monotherapy phase. Similarly, Remington et al. 15 noted that the current body of evidence for clozapine augmentation consisted of data from a limited number of small open trials and case series reports. But they suggested that systematic research was warranted and argued for detailed cost–benefit analysis. Buckley et al. 11 agreed, stating that there was ‘a dearth of double-blind studies’, and concluded that these adjunctive therapies were worthy of further testing in carefully controlled clinical trials.
The subsequent publication of several new open studies and small RCTs testing the therapeutic value of augmenting clozapine with another antipsychotic prompted us to conduct a meta-analysis of eligible RCTs. 18 A systematic literature search identified eight open studies and four eligible RCTs in which clozapine was augmented with a second antipsychotic, with a total of 166 participants. The two RCTs that had lasted 10 weeks or more gave an odds ratio (OR) of response to treatment of 4.41 [95% confidence interval (CI) 1.38 to 14.07]. We concluded that for clozapine-refractory schizophrenia, augmentation of clozapine with another antipsychotic drug is worthy of an individual clinical trial, but this may need to be longer than the 4–6 weeks usually recommended for acute antipsychotic monotherapy, a view supported by Correll et al. 19 Mouaffak et al. 13 noted that the discrepant results of the published studies of clozapine augmentation with another antipsychotic; they identified methodological shortcomings that related to the heterogeneity of definitions of resistance to clozapine, choice of outcome measures, and the dose and duration of the adjunctive drugs, which they considered ‘a major limitation for drawing conclusions’. Clinical response in such studies has generally been defined as a 20% reduction in total score on the Brief Psychiatric Rating Scale (BPRS) or the Positive and Negative Syndrome Scale (PANSS). Both the BPRS and the PANSS assess a broad range of symptoms, including both positive symptoms (e.g. delusions, hallucinations and thought disorder) and negative symptoms (e.g. blunted affect and emotion, poverty of speech, lack of motivation, and social and emotional withdrawal).
The updated National Institute for Health and Care Excellence (NICE) 2009 guideline for the treatment of schizophrenia20 supported the augmentation of clozapine with a second antipsychotic in patients with an inadequate response to clozapine alone; advice that was in accord with our own meta-analysis. 18 After the publication of our meta-analysis, data became available for four further, short-term, clozapine augmentation RCTs, one each for risperidone21 and haloperidol,22 and two using aripiprazole,23,24 a medicine that has a different pharmacology to other antipsychotic drugs in that it is a D2 partial agonist. All four studies were reported as negative, although the trial by Chang et al. 23 showed a statistically significant advantage for augmentation with aripiprazole in regard to a reduction in negative symptom score.
By 2012, Sommer et al. 25 were able to identify 10 RCTs of augmentation of clozapine with a second antipsychotic and found only modest or absent efficacy. Also in 2012, Taylor et al. 26 conducted a meta-analysis of 14 such studies. These investigators were more positive about this strategy for clozapine-refractory illness, concluding from their findings that augmentation of clozapine with a second antipsychotic was modestly superior to placebo and equally well tolerated. Looking at effect size by treatment duration, they found no significant relationship between duration of treatment and reduction in symptoms. In other words, there was no confirmation of the earlier suggestion that longer trials might produce relatively more robust outcomes for adding a second antipsychotic.
Risks of clozapine augmentation
When adding one drug to another it is important to consider any potential interactions that could lead to adverse consequences for the patient. Drug interactions can be either pharmacokinetic, where one drug interferes with the way the body handles the other, usually by increasing or decreasing metabolism or pharmacodynamic, where one drug enhances or opposes the pharmacological action of the other. Case reports have described clinically significant elevations in serum clozapine levels after augmentation with the second-generation antipsychotic, risperidone. 27 The potential clinical relevance of such a pharmacokinetic effect is, first, that it could cause clozapine plasma levels to reach an individual patient’s threshold level for response, a benefit that might be erroneously attributed to a pharmacodynamic synergy between clozapine and the augmenting drug. Second, the increased clozapine plasma levels could be associated with the development of serious dose-related side effects. However, clozapine levels had been systematically measured before and after antipsychotic augmentation in four23,28–30 of the clozapine-augmentation RCTs included in our meta-analysis,8 and in one of these RCTs30 the clozapine metabolite norclozapine was also measured and no significant changes in mean plasma clozapine levels were reported.
In terms of side effects, RCTs and open studies have found clozapine augmentation with a second antipsychotic to be relatively well tolerated. The main treatment-emergent side effects have been predictable from pharmacology of the augmenting drug, with extrapyramidal side effects (EPSs) and prolactin elevation being the most common problems. However, there are isolated case reports of more serious side effects. Published case reports of clozapine augmentation with risperidone have noted agranulocytosis, atrial ectopic beats and possible neuroleptic malignant syndrome,31–33 whereas case reports of clozapine augmentation with aripiprazole have mentioned nausea, vomiting, insomnia, headache and agitation in the first 2 weeks,34 tachycardia23 and also modest weight loss. 34,35 Clozapine itself is commonly associated with sedation, weight gain and postural hypotension. Any augmenting antipsychotic should ideally have a low propensity to compound these side effects.
In summary, the studies of augmentation of clozapine with a second antipsychotic for schizophrenic illness that has shown an insufficient response to clozapine have shown only modest improvements in overall symptom severity that may take 10 weeks to be evident. No particular augmenting antipsychotic has been shown to be consistently superior to any other. Any benefit seen is not obviously attributable to pharmacokinetic interaction: clozapine and norclozapine levels have not been found to be significantly raised in studies when measured. The augmenting antipsychotic drugs tested have generally not been systematically assessed for compounding clozapine side effects (e.g. sedation, weight gain, metabolic side effects) or problems such as akathisia or significant elevations in serum prolactin. Furthermore, any uncommon but severe side effects with this strategy are unlikely to have been detected in the relatively small studies conducted thus far.
Amisulpride augmentation of clozapine
Despite the lack of robust clinical evidence on the potential risks and benefits of clozapine augmentation with amisulpride, this is a strategy commonly used by clinicians in the NHS. The POMH-UK clinical data from 20129 revealed that amisulpride was the antipsychotic most commonly prescribed in association with clozapine.
Amisulpride has been tested in case reports and case series36–38 and open studies of clozapine augmentation. 39–41 Augmentation with amisulpride was found to be well tolerated, and clinical responses (again defined as a ≥ 20% reduction on PANSS total score) in the open studies by Munro et al. 40 and Ziegenbein et al. 39 occurred in around 70% of patients. Hotham et al. 42 augmented clozapine with amisulpride in six forensic (high-secure) patients and reported a reduction in aggression and violence. There has been one previous pilot, double-blind, placebo-controlled RCT of clozapine augmentation with amisulpride43 for 6 weeks in 16 patients, with established schizophrenia, who were partially responsive to clozapine. The primary outcome measures, such as reduction in BPRS total score, failed to show a significant improvement, which the investigators attributed to the study’s lack of power.
Study aims
The main aims of the study were:
-
to test the benefits, costs and risks of augmenting clozapine with amisulpride compared with placebo
-
to add to the clinical and economic evidence base for clozapine augmentation with a second-generation antipsychotic
-
to examine the potential benefits, costs and risks of clozapine augmentation in treatment-resistant schizophrenia.
The key aim of the economic analysis was to assess whether or not augmenting clozapine with amisulpride was likely to be cost-effective. To address this aim, two sets of analyses were conducted. The first set used the trial data set (within-trial analyses) and the 3-month, scheduled, follow-up period to:
-
estimate the use of health- and social-care services to manage participants who were treated with clozapine and placebo and those who had clozapine plus amisulpride
-
estimate the direct costs of health- and social-care services to manage participants who were treated with clozapine and placebo and those who had clozapine plus amisulpride
-
estimate the overall health status of participants who had clozapine and placebo and those who were treated with clozapine plus amisulpride
-
estimate the utility and quality-adjusted life-years (QALYs) of participants who were treated with clozapine and placebo and those who had clozapine plus amisulpride
-
estimate the net costs, QALYs and incremental cost per QALY gained associated with augmenting clozapine with amisulpride.
The second set of analyses used an economic model to explore the following questions over the longer term:
-
What are the likely net costs, QALYs and incremental cost per QALY gained, if symptom response of augmenting clozapine with amisulpride increases/decreases in the long term?
-
What are the likely net costs, QALYs and incremental cost per QALY gained, if augmenting clozapine with amisulpride is associated with increased/decreased side effects in the long term?
Chapter 2 Methods
Trial design
The study was a randomised, double-blind, placebo-controlled trial lasting 12 weeks. Eligible patients, all receiving continuing clozapine treatment, were randomised 1 : 1 to receive augmentation with either amisulpride or placebo. Researchers, participants and care providers were blinded to patients’ treatment randomisation until the end of their participation by the use of identically matched placebo medicine. The trial recruited from November 2011 to December 2014.
Sample size
In previous augmentation studies of clozapine with a second antipsychotic lasting ≥ 10 weeks, 35%28 and 50%44 of the actively augmented arm responded compared with 10% in those augmented with placebo. Response was defined as a > 20% improvement on the BPRS or PANSS. To detect a more conservative response of 30% in our amisulpride arm and 10% in the placebo arm, with 90% power and an alpha of 0.05, we required 92 participants per group (two-sided). Using existing continuous data, detecting an effect size of 0.5 BPRS score standard deviations (SDs; compared with the final placebo group mean BPRS score) would require 85 participants per arm. This held whether we used the results from Josiassen et al. 28 [mean BPRS: 44.7 (placebo) vs. 40.1 (active arm), SD 9.2] or Shiloh et al. 44 [mean BPRS: 41.2 (placebo) vs. 35.1 (active), SD 12.2], both using 90% power and a two-sided alpha of 0.05.
The plan was to randomise 230 patients (including 20% inflation for drop-outs) on clozapine 1 : 1 to receive augmentation with placebo or amisulpride. Clinical protocols for the use of clozapine involve regular attendance for haematological testing and most patients either are inpatients or attend clozapine clinics. Once stabilised on treatment, the attrition rate is low. Small studies of clozapine augmentation report completion rates of > 80%, so we estimated there would be 184 study completers (i.e. 92 completers in each treatment group).
Our sample size calculation was based on results from previous studies. 28,44 These studies were comparable with the proposed study in terms of length of follow-up, intervention used and proposed primary outcome. From these we based our sample size on 30% of participants in the amisulpride arm of the trial achieving a > 20% improvement on the BPRS or on the PANSS, which is slightly lower than those shown in the literature. 28,44 Therefore, it was expected that there would be a large difference in the percentage achieving such a threshold level of improvement on the BPRS or on the PANSS between the amisulpride and placebo groups at the end of follow-up, and so there was no statistical justification for increasing the sample size.
As part of the amisulpride augmentation in clozapine-unresponsive schizophrenia (AMICUS) study rescue plan submitted to the Health Technology Assessment (HTA) programme in March 2013, the sample size was recalculated with the same assumptions but with 80% power. This gave 72 participants in each arm (144 in total); accounting for 20% drop-out, the revised target was 90 participants in each group (180 participants in total). This was a pragmatic decision to determine the sample size needed while maintaining power at an acceptably high level.
Primary outcomes
The primary outcome measure was the proportion of patients with a criterion response threshold of a 20% reduction in total PANSS score. This is a commonly used criterion for therapeutic response in schizophrenia trials, which allows for comparison with other published trials18 and inclusion of the study results in any appropriate systematic review or meta-analysis. The PANSS45,46 is a 30-item rating scale designed to provide a comprehensive assessment of psychopathology in adult patients with schizophrenia.
Inter-rater reliability
Following initial training sessions on the PANSS for the study researchers, the reliability of PANSS ratings by researchers across the various research sites was formally tested. Sixteen researchers were asked to independently rate the same PANSS interview (video of an actor). The intraclass correlation for individual items was 0.63 (moderate agreement) and subscales at 0.86 (substantial agreement). Discrepancies on the ratings of scale items between the researchers who had taken part were then reviewed and discussed with them, in order to further improve the reliability of the ratings.
Secondary outcomes
Negative symptoms
The PANSS negative symptom subscale score was used to assess negative symptoms. The validity and reliability of this negative subscale have been established. 47,48
Social and occupational function
Impact on social and occupational function was measured using the Social and Occupational Functioning Assessment Scale (SOFAS),49 which was derived from the Global Assessment Scale but more focused on a patient’s social and occupational functioning; for an impairment to be rated, it must relate to psychological problems not lack of opportunity. Note that higher scores on the SOFAS indicate a better level of functioning.
Treatment-refractory, target symptoms or behaviours
As part of the final assessment of eligibility for each participant, the PANSS and SOFAS assessments were reviewed with members of the participant’s clinical team. The team was asked to identify up to three critical, target symptoms and/or behaviours that were refractory to treatment; these were phenomena that had proved to be persistent and were judged clinically to have made a major adverse impact on the participant’s social function and community reintegration, been a major cause of psychological distress and/or precluded discharge from hospital.
Service engagement
The level of engagement with clinical services was assessed using the Service Engagement Scale (SES),50 a 14-item measure consisting of statements that assess client engagement with services, rated on a 4-point Likert scale from ‘not at all or rarely’ to ‘most of the time’. Four subscales assess availability, collaboration, help-seeking and treatment adherence. High internal consistency and retest reliability, including discrimination between criterion groups, have been demonstrated for the SES in a community setting. 50
Depression
Depressive symptoms were assessed using the Calgary Depression Rating Scale for Schizophrenia (CDSS),51 a scale designed to minimise the potentially confounding symptom overlap between depressive features and both negative symptoms and EPSs.
Insight
Insight was assessed using the Schedule for the Assessment of Insight (SAI). 52 This scale comprises a semistructured interview that obtains measures of three dimensions of insight: (1) awareness of mental illness, scored 0–6; (2) the ability to correctly attribute psychotic experiences, scored 0–4; and (3) acceptance of the need for treatment, scored 0–4. The maximum total score on the three dimensions is 14, but the scale also includes a supplementary question on hypothetical contradiction, scored 0–4. Thus, the maximum total score for the scale is 18, which indicates full insight.
Side effects
The Antipsychotic Non-Neurological Side Effects Scale (ANNSERS)53 has 44 clinician-judged items with good inter-rater reliability. It was designed to systematically and comprehensively assess the full range of side effects (weight gain, sexual dysfunction, aversive subjective experiences, etc.), other than movement disorders, that are recognised as occurring with first- and/or second-generation antipsychotics. For this study, an enhanced version of the ANNSERS was generated (ANNSERS-E) by adding potential cardiac symptoms such as palpitations, dizziness and syncope.
Metabolic side effects were assessed at baseline, and at the 12-week follow-up only, using an obesity measure [body mass index (BMI) and waist circumference] and assessment of blood pressure, serum prolactin concentration, plasma glucose concentration (non-fasting sample) and lipid profile. In line with best practice safety monitoring,54 an electrocardiogram (ECG) was done and reported on at baseline, before the study medication was initiated, to establish a baseline for any subsequent cardiac monitoring and exclude cardiac contraindications to potentially high-dose antipsychotic medication, including long QT syndromes.
With regard to EPSs, drug-induced parkinsonism was assessed using the Simpson and Angus55 Extrapyramidal Side Effects Scale (EPSE). 56 The Barnes Akathisia Rating Scale (BARS)57 was used to assess akathisia, and the Abnormal Involuntary Movement Scale (AIMS)58,59 for rating tardive dyskinesia. Researchers received thorough training on the use of these scales.
Health economics measures
The costs and health benefits for a cost-effectiveness acceptability and net benefit analysis were also measured. The primary economic measure was the incremental cost-effectiveness ratio (ICER) of clozapine augmentation with amisulpride, estimated as net cost of such clozapine augmentation divided by net QALY of clozapine augmentation.
Protocol changes
In addition to wording changes to clarify procedures, a number of amendments were made to the protocol during the trial, as described in sections The addition of study sites to Changes to study medication procedures.
The addition of study sites
Additional sites were added as the trial progressed and, in total, took the number of study sites from 4 to 23.
The addition of screening electrocardiography
Electrocardiography to exclude cardiac contraindications and establish a baseline for any subsequent cardiac monitoring was added to the protocol prior to randomisation of the first participant.
Payment of participants for study assessments
In line with a number of contemporaneous studies that were remunerating participants for their time, a payment to participants of £20 for each assessment was introduced, in recognition of any expenses incurred (e.g. travel) and inconvenience. This was backdated for any participants who were already randomised.
Changes to study medication procedures
Several changes were made to allow clinicians and site pharmacies more flexibility to manage the study medication. The option of repackaging trial medication into dose administration boxes (1 week at a time) was introduced, and a standardised approach to the management of temperature excursions in pharmacies was added for recorded temperatures between 25 °C and 30 °C. Moreover, the addition of an option for the prescriber to provide a further 28-day supply of study medication after the final 12-week follow-up assessment was added. In combination with the direct unblinding of the referring psychiatrist, this allowed time for participants allocated to the amisulpride arm of the trial to be provided with a standard prescription of amisulpride and, therefore, allowed an uninterrupted supply of medication when the participant and prescribing clinician agreed that this was desirable.
Patient and public involvement
A service user group contributed to the design of the study at the proposal stage. In particular, feedback from qualitative interviews with service users highlighted the relevance of a systematic assessment of side effects, including subjective experience. Once funded, two experts by experience, were involved in the trial throughout: one a service user and the other a parent and carer of a service user. These individuals sat on the Trial Steering Committee and the Trial Management Group, respectively, and, as a result, contributed to both trial oversight and the strategic direction of the trial, including ways to improve the recruitment rate.
Both of the experts by experience who were members of the trial’s oversight committees made additional contributions. These included shaping the content of the website and newsletters, and communicating the importance of the study to health-care professionals through the Clinical Research Network. The last, in particular, was well received and influenced clinician engagement in the trial.
One further issue that was felt to particularly necessitate the perspective of service users was whether or not to introduce remuneration for completing assessments, and we sought the opinion of a wider network of service users through support from the Clinical Research Network on this issue before making a decision.
Participants
People aged 18–65 years with schizophrenia that had proved to be unresponsive, at a criterion level of persistent symptom severity (as used by Honer et al. 30) and impaired social function, to an adequate trial of clozapine monotherapy in terms of dosage, duration and adherence, and who were competent and willing to provide written, informed consent.
Inclusion criteria
To be eligible for enrolment, a patient’s illness had to meet a criterion level of persistent symptom severity (as used by Honer et al. 30) and the patient had to exhibit impaired social function, despite an adequate trial of clozapine monotherapy in terms of dosage, duration and adherence:
-
treatment for at least 12 weeks at a stable dose of 400 mg or more per day of clozapine, unless the size of the dose was limited by side effects
-
a total score of ≥ 80 at baseline on the PANSS;45,46 the range of possible scores is 30–210, with higher scores indicating more severe symptoms
-
a clinical global impression (CGI)58 score of ≥ 4 (range of possible scores 1 = not mentally ill to 7 = extremely ill)
-
a SOFAS49 score of ≤ 40; range of possible scores 1–100, with lower scores indicating impaired functioning
-
clinically stable for the last 3 months, with a consistent clozapine regimen.
Exclusion criteria
-
Clinically significant alcohol/substance use in the previous 3 months.
-
The presence of a developmental disability.
-
Indication for current treatment with clozapine was intolerance or movement disorder rather than a treatment-refractory schizophrenic illness.
-
A previous trial of clozapine augmentation with amisulpride.
-
Existing relevant physical health problems such as cardiovascular disease, previous problems with prolactin levels or impaired liver/renal function.
-
Any woman who was pregnant or planning a pregnancy, and any woman of child-bearing potential unless using adequate contraception.
Pharmacological intervention
The pharmacological intervention was the augmentation of clozapine treatment with another second-generation antipsychotic, amisulpride, or placebo: 400 mg of amisulpride or two placebo capsules for the first 4 weeks, with the option of titrating up to 800 mg of amisulpride or four placebo capsules for the remaining 8 weeks. The amisulpride and placebo tablets were encapsulated to look identical. A fully automated online (and telephone) randomisation service was provided by the Clinical Trials Research Unit, University of Sheffield, Sheffield, UK. In addition, a 24-hour unblinding service was provided by Emergency Scientific and Medical Services (ESMS Global; Medical Toxicology Information Service Ltd, London, UK).
Data analysis
Clinical data analysis
All the main analyses were performed on an intention-to-treat basis. Baseline summary statistics by randomised group were calculated. Group differences in the primary outcome (criterion response threshold of > 20% reduction in total PANSS score) and other binary outcome measures were evaluated through the use of logistic regression after allowing for stratification by baseline symptom severity. Differences in quantitative outcome measures were evaluated through corresponding analysis of covariance model, controlling for baseline symptom severity (the stratification variable) and baseline values of the outcome in question. The results of the trial are presented following the standard Consolidated Standards of Reporting Trials (CONSORT) recommendations.
There were three interim analyses carried out by the trial statistician for the Data Monitoring and Ethics Committee (DMEC) for the first three meetings. Baseline sex, age and PANSS score, by randomised group, were shown to check for imbalances between randomised groups. Analyses were limited to the primary outcome and adverse events. However, as fewer participants than projected had been recruited and completed the 12-week follow-up at these time points, it was not possible to analyse data in a similar way to the final analysis. For the second and third meetings, Fisher’s exact tests and chi-squared tests were carried out to assess randomised group comparisons. There were alpha spending plans in place for efficacy (> 20% reduction in PANSS score) and safety (adverse events). However, all interim analyses showed p-values greater than those set in the alpha spending plan. At subsequent meetings, overall statistics were presented (i.e. without disclosure of treatment allocation). Additionally, as the final study population was much smaller than the sample size calculation (approximately one-third recruited), it was decided to revert to the conventional threshold of p < 0.05 to signify statistical significance. With the data available, to show a significant difference between randomised groups would have required a very large difference in outcome, so it would have been futile setting the p-value lower than the conventional p-value (as would have been the case if we had used the final p-value from the alpha spending plan).
Given the tentative evidence in the published literature, suggesting that a significant clinical response to the amisulpride intervention being assessed may not be evident before 10 weeks of treatment, the 6-week data were used to determine whether or not there was earlier benefit from the intervention. The 6-week outcome data were also examined as a (tertiary) outcome, looking at the data longitudinally using mixed-effects modelling using both 6-and 12-week outcomes and controlling for baseline values of the given measure. Mixed-effects modelling does not assume that outcome data are independent of one another (in this case, outcome values at 6 weeks are related to outcome values at 12 weeks), as standard methods such as multiple linear regression do. If methods that assume independence were used with longitudinal data, then it is likely the standard errors would be too small, giving 95% CIs that were too narrow. This mixed-effects modelling included a dichotomous time variable indicating the time point (6 or 12 weeks) and a variable indicating randomised group. It was possible to undertake this analysis only on outcomes that were measured at all three time points (baseline, 6 weeks and 12 weeks); however, it is possible to include those participants in whom data from only one of the two follow-up time points were available. There was a random intercept for each participant.
Per-protocol sensitivity analyses were also conducted. Those participants taking mood stabilisers, antidepressants or antipsychotic medication (other than the randomised medication and clozapine) at either 6 or 12 weeks were excluded. Analyses were then carried out using the same methods as the primary analyses, although unadjusted (that is not adjusting for baseline symptom severity or baseline values of the outcome in question) because of the large decrease in power as a result of the large numbers of participants taking one of these classes of medication (small numbers who were only taking the study medications) as a result of the stricter inclusion criteria. Predictors of missingness of the primary outcome were assessed using means (SDs), medians [interquartile ranges (IQRs)] or frequencies (%) depending on the distribution of the data. However, statistical tests were not carried out as only a small absolute number of participants had missing values for the primary outcome. It was decided, a priori, that imputation methods would not be used (i.e. complete-case analysis was used).
Data were analysed using Stata version 13.0 for Windows (StataCorp, College Station, TX, USA).
Health economic analysis
Approach
The framework for the within-trial and economic model analyses was cost-effectiveness analysis, to estimate the net cost per unit of health benefit gained by amisulpride augmentation. A cost-effectiveness acceptability approach was used to estimate the likelihood that amisulpride augmentation is cost-effective.
The population for the economic evaluation (within trial and economic model) consists of people who are unresponsive to clozapine, require additional treatment and have no contraindications for amisulpride augmentation. The intervention is clozapine augmented by amisulpride compared with clozapine not augmented by amisulpride.
The measure of health benefit for the analyses is the QALY. The primary outcome measure for all the economic analyses is the ICER (e.g. cost per QALY gained). The time horizon for the within-trial analyses is the 12 weeks from entry into the trial (baseline) to the final scheduled follow-up assessment. The time horizon for the economic model is 1–10 years from initiating clozapine augmented by amisulpride. The perspective for all the economic analyses is the health- and social-care sector (that incur the costs of providing care) and the patient (for health benefits). Previous evaluations of clozapine related treatment suggest that this approximates a broadly societal perspective. 60,61
The within-trial analysis used an intent-to-treat approach and included all eligible participants randomised to start treatment in both trial groups.
Within-trial analyses
Direct costs
The range of costs included the costs of the following formal health and social care services:
-
inpatient psychiatric and non-psychiatric care (including intensive care, emergency and crisis admissions)
-
hospital outpatient and day hospital attendances
-
primary care contacts with the general practitioner (GP) and GP practice staff (including office and home visits)
-
medicines taken on a daily basis
-
community mental health-care contacts
-
social care contacts.
Most of the resource-use data were collected using an economic patient questionnaire (EPQ) for each patient to identify the services used. Participants who reported using hospital inpatient and outpatient services were asked for details of the hospitals they attended. Data on the number of admissions, length of stay, number of outpatient visits were collected from case records at each of the hospitals reported by the participants. The frequency of other service use was collected from each patient using the EPQ. The data on service use were collected at the baseline and 12-week assessments. Data on medicines (dose, frequency, duration) were collected at baseline and at 6 weeks and 12 weeks.
The direct costs were estimated from resource-use data combined with the most recent published national unit costs available at the time of data analysis. These were the Department of Health’s National Schedules of Reference Costs 2013–2014,62 the Unit Costs of Health and Social Care 2014 produced by the Personal Social Services Research Unit, University of Kent63 and the British National Formulary64 for the price year 2013–14. Each item of service use was costed by multiplying the quantity of service used with the average unit cost for that item.
The cost of amisulpride augmentation was estimated from the drug accountability records collected as part of the trial. This recorded the number of 400-mg capsules per day that were prescribed at baseline, at week 4 and at week 8 and whether or not the medication was received by the participant.
Quality-adjusted life-years
Quality-adjusted life-years gained from baseline to end of the scheduled 12-week follow-up were estimated as the number of weeks multiplied by the utility of observed survival. The utility values were estimated from the EuroQol EQ-5D health status questionnaire and the associated published societal utility tariffs. The EQ-5D three levels (EQ-5D-3L) version was used in the trial (no problems, some problems, severe problems/unable to do activity) and was included in the baseline and 12-week follow-up assessment interviews conducted with all the trial participants.
The EQ-5D is a generic and validated health status questionnaire shown to have acceptable validity in people with schizophrenia in European countries. 59,65,66 The EQ-5D has been used successfully in two recent UK trials of antipsychotics in schizophrenia. 60,67 Data from these trials demonstrated that the EQ-5D correlates with clinical quality of life and effectiveness measures, and is sensitive to change.
The EQ-5D-3L gives a profile of the individual’s health state at the time of assessment. Each possible health state has a published utility weight. Using the published tariff for the UK,68 the health profile for each individual over the duration of follow-up was converted to a single utility value. QALYs were then estimated as:
where Ui = utility value and ti = number of days between assessments for participant i.
Missing data
The primary outcome for the economic analysis combines a measure of cost and a measure of health benefit. This is in contrast to the analyses of the clinical outcomes, which is conducted for a single measure. One implication is that the impact of missing observations can be higher for the economic analyses than for the clinical analyses. From experience in previous clinical and economic trials, it was anticipated that there would be missing observations about use of services, for those people who completed the 12-week assessment. 60,67 This can then lead to a low proportion of participants with complete cost and QALY data, which can increase bias and reduce the robustness of the economic analysis. Accordingly, we specified the use of multiple imputation for the economic data in the protocol and analysis plan.
There were complete EQ-5D data for all the people who completed a 12-week assessment. However, there were missing observations for the cost data. These were treated as missing at random. Multiple imputation was used to impute values for the different cost categories. Data were imputed for missing observations at both baseline and at the 12-week assessment. Total costs were generated from these imputed data (using the Stata passive estimate command).
The imputations were conducted in Stata version 13.1, using predictive mean matching and sequential chained equations. The predictive mean-matching models included demographic and clinical variables. These were initially selected from measures previously reported to be statistically and conceptually associated with costs and or QALYs. The selection of variables was further informed by correlation analyses of the pooled baseline data. 69
Economic analyses
Descriptive analysis was used to summarise the EQ-5D data, QALYs, service use and cost. Regression analysis was used to estimate incremental (net) costs and QALYs accounting for the impact of key covariates. The key covariates were identified from the literature and analyses used to identify variables for the multiple imputation models (described in Missing data).
The primary measure for the economic analysis was the ICER. Accordingly, no statistical tests of differences in mean costs or outcomes were conducted. The ICER was estimated as the net cost divided by the net QALY estimates from the regression analyses.
The estimates of net costs and outcomes from the regression were bootstrapped to simulate 10,000 pairs of net cost and net outcomes for the amisulpride-augmentation group. These simulated pairs of net cost and net outcomes were used to generate cost-effectiveness acceptability curves (CEACs), as recommended by NICE for health technology appraisals. 70
The simulated data were also used to estimate the probability that clozapine plus amisulpride augmentation is cost-effective compared with clozapine alone. This takes a Bayesian approach to estimating the likelihood that the intervention is cost-effective and avoids hypothesis testing and risk of a type 1 error.
The approach described above to estimate CEACs and probability that amisulpride augmentation is cost-effective revalues the health benefits gained by the intervention in monetary terms. However, in the UK there is no universally agreed monetary value for the types of health benefit measures (such as QALYs or criterion response) typically used in cost-effectiveness analyses. An approach used in health care is to ask the question: what is the maximum amount decision-makers are willing to pay to gain one unit of health benefit? Accordingly, the simulated net QALY values from the bootstrap simulation were revalued using a range of maximum willingness-to-pay values from £1 to £30,000 to gain one QALY. This was based on the range of willingness-to-pay value thresholds (WTPTs) historically implied by NICE decisions. 71
The data for the CEAC were derived by first revaluing each of the 10,000 net health benefit estimates from the bootstrap simulation by a single WTPT. This was repeated for each WTPT within the range used. A net benefit (NB) statistic for each pair of simulated net costs and net outcomes for each WTPT can then be calculated. For example, if the measure of health benefit is the QALY, the NB of amisulpride augmentation is estimated as:
This calculation was repeated for each WTPT. The CEACs plotted the proportion of bootstrapped simulations where the net benefit of amisulpride augmentation is > 0 for each WTPT. 72–75
Sensitivity analysis
Sensitivity analyses were used to explore the impact of changing the methods used on the estimates of whether or not amisulpride augmentation was cost-effective. This included rerunning the analyses:
-
for complete-case analysis rather than multiple imputation of missing observations
-
using key measures of outcome for the clinical effectiveness analysis as the measure of health benefit to estimate the cost per person with a 20% criterion response gained and cost per person with no recorded adverse event gained
-
excluding inpatient costs from the total cost (there was an imbalance in inpatient admissions at baseline and participants with inpatient admissions in the follow-up period were also inpatients at the baseline assessment)
-
excluding medication costs from the total cost (there was a higher number of missing data for medication costs, which was also dominated by the cost of clozapine)
-
for participants who did and did not have a 20% criterion response and for participants who did and did not have an adverse event recorded.
Economic model
The economic within-trial sensitivity analyses explored aspects of the trial design and participants that could affect generalisability. A key issue for the within-trial economic evaluation is the short length of follow-up and whether or not any savings in health-care costs or gains in QALYs may be sustained over a longer 12-month period.
An economic model was constructed using the same target population, perspective, health benefit measure and costs as the within-trial analyses. The intervention arm of the model was defined as clozapine with amisulpride augmentation. The control arm of the model was defined as clozapine with placebo augmentation.
A focused literature search identified no full economic evaluations of amisulpride-augmentation treatment or any clozapine-augmentation treatment that could be used to inform the economic model design or provide data to estimate model parameters. The searches were conducted in September 2015 and were restricted to the previous 10 years (restricted because of relevance in terms of resource-use and health-care service changes, as well as modelling techniques). The databases searched were: NHS Economic Evaluation Database (NHS EED), the HTA database, EMBASE, MEDLINE, PsycINFO and EconLit. The search strategy included treatment terms, for example, ‘clozapine unresponsive’ and ‘clozapine augmentation’ and economic evaluation terms derived from the NHS EED search strategy. Two clinical effectiveness reviews were found in a search of The Cochrane Library for clozapine-augmented therapy. 76,77 These reviews revealed that there were insufficient data about the long-term effects of any augmentation on clinical outcomes or side effects. The reviews included a few studies with a follow-up of up to 1 year, which showed that some patients take longer to respond (responding between 6 months and 1 year into treatment).
Given the paucity of evidence for long-term use of clozapine augmentation, as well as the complexities of the disease area, it was concluded that there is little to gain from building a full economic model. The evidence collected in this clinical and economic trial is the most robust evidence available to inform the model structure and economic parameter estimates. Accordingly, to explore what happens after the 12-week trial period, a partial model was constructed. The model explored the impact of varying the probabilities that participants would experience improvement in symptoms (as measured by the PANSS) and that participants would experience side effects. The analyses were based on the assumption that these probabilities are key drivers of costs and QALYs.
The economic model used a simple Markov structure to model the impact of symptom inprovement and side effects over 12 months, split into four 3-month cycles (Figure 1). The initial cycle was assigned the probabilities, costs and QALYs for amisuplride and clozapine, to mirror the outcome of the trial. The subsequent cycles used pooled trial data to estimate the baseline values. For the initial cycle the mean costs/QALYs for clozapine were used. The net saving/QALYs gain from the primary analysis were applied to the clozapine values to estimate the mean costs and QALYs for the initial cycle of the amisulpride choice.
FIGURE 1.
The Markov model.

All data were entered into the model as distributions. Beta distributions were used for the probability data. Gamma distributions were used for the cost and QALY data. Monte Carlo simulation in TreeAge software (TreeAge Pro Health Care Module 2015, TreeAge Software Inc., Williamstown, MA, USA) (10,000 iterations) was used to estimate the net costs, QALYs and ICER of amisulpride augmentation. Probabilistic sensitivity analysis was used to estimate the likelihood that amisulpride augmentation was cost-effective compared with clozapine and placebo.
Chapter 3 Results
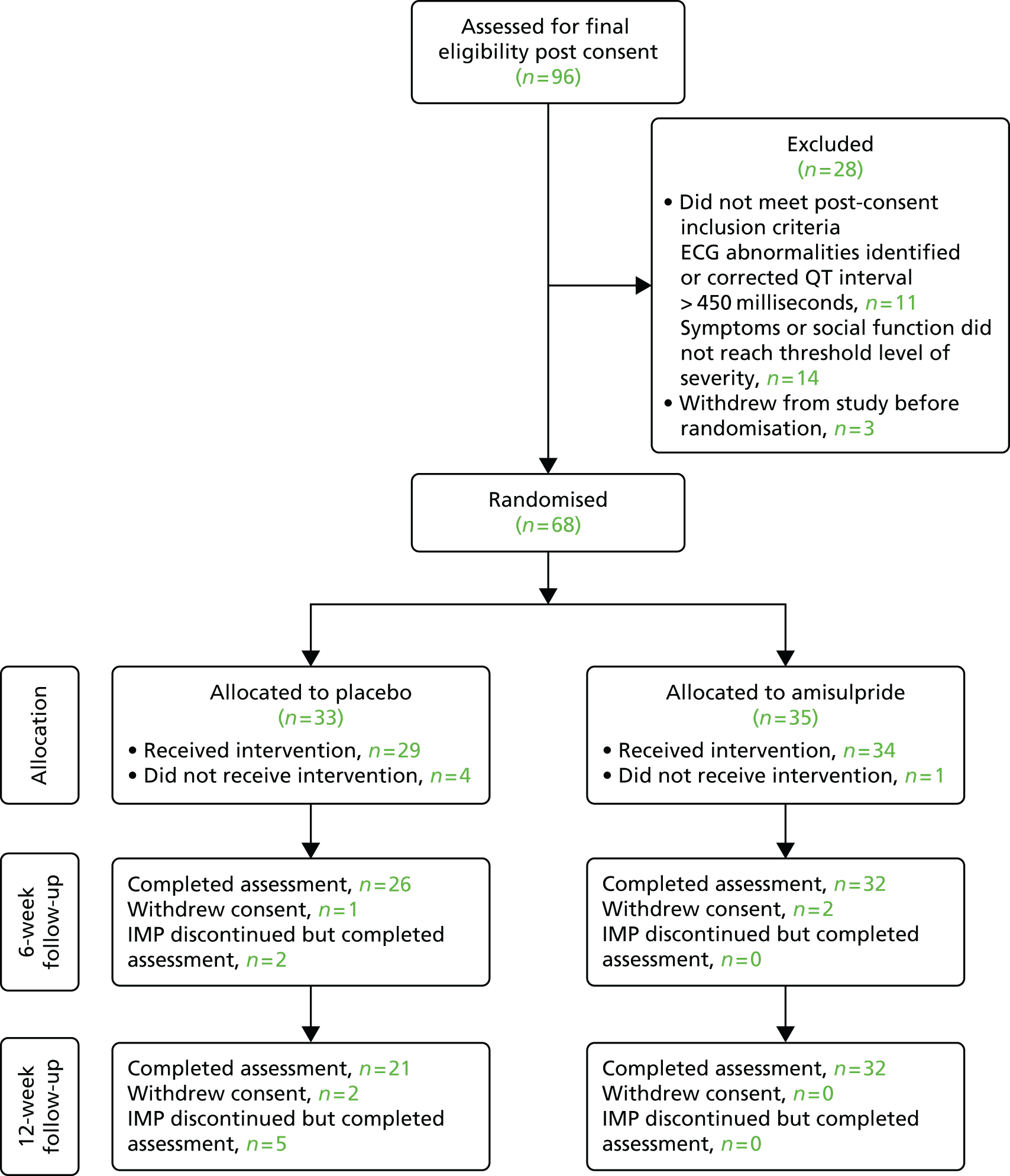
Ninety-six patients were recruited and 68 patients were randomised, with 52 patients completing assigned treatment and assessment at the 12-week follow-up (Figure 2). This number of randomised participants fell well short of the target sample size. Table 1a shows that the participants in both groups were predominantly male and white. The mean age was close to 40 years in both groups. Almost all participants were not in paid work. Most had attended primary care services in the 3 months before baseline. Table 1b shows that, at baseline, the mean PANSS total score was a little higher in the placebo group than in the amisulpride group [98 (SD 24) and 93 (SD 13), respectively], although there were similar proportions with a high PANSS score (stratification variable). Mean PANSS negative symptom subscale scores were similar between the two groups, as were scores on other standardised instruments. Mean body weight, systolic and diastolic blood pressure were a little higher at baseline assessment in the amisulpride treatment group.
FIGURE 2.
The CONSORT flow diagram. IMP, investigational medicinal product.
| Variable | Treatment group | |||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Male | 24/35 | 69 | 23/33 | 70 |
| Age (years) | 39 | (11) | 40 | (10) |
| White | 28/35 | 80 | 24/33 | 73 |
| Living alone | 12/32 | 38 | 11/28 | 39 |
| Living with parents | 5/32 | 16 | 8/28 | 29 |
| Living with others | 15/32 | 47 | 9/28 | 32 |
| Owner occupied flat or house | 0/34 | 0 | 0/29 | 0 |
| Flat or house rented | 19/34 | 56 | 21/29 | 72 |
| Other accommodation | 15/34 | 44 | 8/29 | 28 |
| Not in paid employment because of treatment | 24/25 | 96 | 23/25 | 92 |
| Currently an inpatient | 5/35 | 14 | 4/33 | 12 |
| Psychiatric inpatient in the last 3 months | 1/22 | 5 | 0/20 | 0 |
| Non-psychiatric inpatient in the last 3 months | 0/21 | 0 | 1/19 | 5 |
| Any inpatient in the last 3 months | 5/34 | 15 | 6/30 | 20 |
| Psychiatric outpatient in the last 3 months | 5/22 | 23 | 7/20 | 35 |
| Non-psychiatric outpatient in the last 3 months | 1/20 | 5 | 0/19 | 0 |
| Any outpatient in the last 3 months | 13/33 | 39 | 9/30 | 30 |
| Psychiatric emergency in the last 3 months | 0/21 | 0 | 0/20 | 0 |
| Attended non-psychiatric A&E department in the last 3 months | 0/21 | 0 | 0/19 | 0 |
| Community-based services used in the last 3 months | 14/33 | 42 | 16/29 | 55 |
| Attended NHS day hospital or day centre in the last 3 months | 0/22 | 0 | 1/20 | 5 |
| Other primary or community care contacts in the last 3 months | 28/33 | 85 | 26/29 | 90 |
| In contact with the criminal justice system in the last 3 months | 0/34 | 0 | 2/29 | 7 |
| Variable | Treatment group | |||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Primary psychiatric diagnosis | ||||
| Schizophrenia | 32/34 | 94 | 29/30 | 97 |
| Schizophreniform disorder | 1/34 | 3 | 0/30 | 0 |
| Schizoaffective disorder | 1/34 | 3 | 0/30 | 0 |
| Psychosis NOS | 0/34 | 0 | 1/30 | 3 |
| Medication | ||||
| Any antidepressant | 15/35 | 43 | 13/33 | 39 |
| Any antipsychotic (excluding clozapine and amisulpride) | 3/35 | 9 | 1/33 | 3 |
| Any mood stabiliser | 9/35 | 26 | 4/33 | 12 |
| Cardiac symptoms, checked 7–10 days after starting study medication | ||||
| Irregular heartbeat | 1/31 | 3 | 0/26 | 0 |
| Shortness of breath | 5/32 | 16 | 0/26 | 0 |
| Dizziness | 5/32 | 16 | 1/26 | 4 |
| Fainting | 0/31 | 0 | 0/26 | 0 |
| Hypotension | 0/30 | 0 | 0/26 | 0 |
| Physical health measures | ||||
| Weight (kg) | 96 | (24) | 91 | (19) |
| Height (m) | 1.72 | (0.08) | 1.73 | (0.11) |
| BMI (kg/m2) | 33 | (8) | 30 | (6) |
| Waist circumference (cm) | 112 | (13) | 103 | (13) |
| Systolic blood pressure (mmHg) | 126 | (17) | 122 | (11) |
| Diastolic blood pressure (mmHg) | 83 | (11) | 82 | (8) |
| Serum prolactin concentration (ng/ml), median (IQR) | 12 | (5–14) | 12 | (5–18) |
| Plasma glucose concentration (mmol/l), non-fasting sample | 6.8 | (2.7) | 6.2 | (2.9) |
| Total cholesterol concentration (mmol/l) | 5.1 | (1.3) | 5.4 | (1.2) |
| HDL cholesterol concentration (mmol/l) | 1.1 | (0.4) | 1.4 | (1.1) |
| LDL cholesterol concentration (mmol/l) | 3.0 | (1.4) | 3.2 | (0.9) |
| Triglyceride concentration (mmol/l), median (IQR) | 2.0 | (1.3–2.5) | 1.7 | (1.0–3.3) |
| Clinical assessment | ||||
| PANSS | 93 | (13) | 98 | (24) |
| PANSS high score (stratification variable) | 16/35 | 46 | 14/33 | 42 |
| PANSS negative symptom subscale score | 25 | (6) | 25 | (7) |
| CDSS, median (IQR) | 5 | (1–10) | 5 | (2–8) |
| SOFAS, median (IQR) | 35 | (32–39) | 35 | (30–40) |
| SES, median (IQR) | 8 | (4–13) | 10 | (4–18) |
| SAI, median (IQR) | 12 | (8–13) | 12 | (9–14) |
| Side effects | ||||
| ANNSERS-E, median (IQR) | 16 | (11–22) | 13 | (10–24) |
| BARS, median (IQR) | 0 | (0–2) | 2 | (0–2) |
| Akathisia present (global clinical assessment score of ≥ 2) | 3/33 | 9 | 4/31 | 13 |
| AIMS positive: tardive dyskinesia | 4/35 | 11 | 4/33 | 12 |
| EPSE, median (IQR) | 0.1 | (0–0.3) | 0.1 | (0–0.3) |
| Parkinsonism present | 10/29 | 34 | 6/24 | 25 |
For each participant, up to three critical, target symptoms and/or behaviours refractory to treatment were identified at baseline by the responsible clinical team. Positive symptoms were most commonly identified: hallucinations were cited for 51% of the participants, delusions for 43% of the participants and suspiciousness/persecutory or paranoid ideas cited for 33% of the participants. Difficulty in abstract thinking or conceptual disorganisation were cited for 8% of the participants. With regard to negative symptoms, 20% of participants were considered to have a lack of drive, motivation, volition and/or spontaneity, whereas general negative symptoms were mentioned for 12% of the participants. Reduced social interaction was identified as a problem for 37% of the participants, and emotional withdrawal for 4% of the participants. Anxiety was relatively common, being identified as a persistent issue for 35% of the participants, whereas depression was a key symptom in only 9% of the participants. Lack of judgement or insight was named as an issue for 8% of the participants and poor attention for 4% of the participants. Poor management of physical health problems or self-care was named for 8% of the participants. One participant had particular difficulty with obsessive behaviour and one with hostility.
Table 2 shows that, at the 6-week study assessment, the mean PANSS score was higher in the placebo group than in the amisulpride group [85 (SD 23) vs. 80 (SD 15)], although the proportion who showed a 20% drop in PANSS score from baseline was the same in both groups (25%). Median SES was lower in the placebo group [7 (IQR 4–14)] than in the amisulpride treatment group [10 (IQR 4–13)]. All other standardised scales show similar scores between treatment groups.
| Variable | Treatment group | |||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Clinical assessment | ||||
| PANSS | 80 | (15) | 85 | (23) |
| 20% reduction in PANSS from baseline | 8/32 | 25 | 7/28 | 25 |
| PANSS negative symptom subscale | 22 | (8) | 23 | (7) |
| CDSS: depression, median (IQR) | 4 | (1–7) | 3 | (1–6) |
| SOFAS, median (IQR) | 38 | (35–40) | 40 | (35–41) |
| SES, median (IQR) | 10 | (4–13) | 7 | (4–14) |
| SAI, median (IQR) | 12 | (8–15) | 12 | (10–13) |
| Side effects | ||||
| ANNSERS-E, median (IQR) | 13 | (9–20) | 15 | (6–22) |
| BARS, median (IQR) | 0 | (0–2) | 1 | (0–2) |
| Akathisia present (global clinical assessment score of ≥ 2) | 3/32 | 9 | 4/28 | 14 |
| AIMS positive: tardive dyskinesia | 1/32 | 3 | 1/28 | 4 |
| EPSE, median (IQR) | 0.2 | (0.1–0.4) | 0.1 | (0.0–0.3) |
| Parkinsonism present | 11/29 | 38 | 6/23 | 26 |
| Medication | ||||
| Any antidepressant | 11/32 | 34 | 13/28 | 46 |
| Any antipsychotic (excluding clozapine and amisulpride) | 4/32 | 13 | 1/28 | 4 |
| Any mood stabiliser | 7/32 | 22 | 5/28 | 18 |
Table 3a provides a comparison of the clinical characteristics and status of the amisulpride and placebo treatment groups at 12 weeks. Table 3b allows for comparison of primary and secondary outcomes at 12 weeks between the two treatment arms. The data show that the percentage of participants who showed a 20% reduction in PANSS score between baseline and 12 weeks was similar in both groups (44% of amisulpride participants and 40% of placebo participants). Mean weight, BMI, waist circumference and blood pressure were greater in the amisulpride group than in the placebo group. Median plasma prolactin concentration was higher in the amisulpride group than in the placebo group [43 ng/ml (IQR 9–87 ng/ml) vs. 11 ng/ml (IQR 7–12 ng/ml), respectively], as was mean plasma glucose concentration [6.9 mmol/l (SD 2.8 mmol/l) vs. 5.4 mmol/l (SD 0.7 mmol/l), respectively].
| Variable | Treatment group | |||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| Living alone | 12/31 | 39 | 11/25 | 44 |
| Living with parents | 5/31 | 16 | 5/25 | 20 |
| Living with others | 14/31 | 45 | 9/25 | 36 |
| Owner occupied flat or house | 0/32 | 0 | 0/25 | 0 |
| Flat or house rented | 18/32 | 56 | 17/25 | 68 |
| Other accommodation | 14/32 | 44 | 8/25 | 32 |
| Not in paid employment because of treatment | 22/25 | 88 | 19/20 | 95 |
| Psychiatric inpatient in the last 3 months | 3/22 | 14 | 0/14 | 0 |
| Non-psychiatric inpatient in the last 3 months | 1/22 | 5 | 0/15 | 0 |
| Any inpatient in the last 3 months | 5/32 | 16 | 3/25 | 12 |
| Psychiatric outpatient in the last 3 months | 5/19 | 26 | 3/14 | 21 |
| Non-psychiatric outpatient in the last 3 months | 2/19 | 11 | 1/14 | 7 |
| Any outpatient in the last 3 months | 12/32 | 38 | 6/32 | 24 |
| Psychiatric emergency in the last 3 months | 0/22 | 0 | 0/14 | 0 |
| Attended A&E department in the last 3 months | 0/22 | 0 | 0/15 | 0 |
| Community-based services used in the last 3 months | 10/30 | 33 | 14/25 | 56 |
| Attended NHS day hospital or day centre in the last 3 months | 0/21 | 0 | 0/14 | 0 |
| Other primary or community care contacts in the last 3 months | 23/32 | 72 | 19/25 | 76 |
| In contact with the criminal justice system in the last 3 months | 0/32 | 0 | 1/25 | 4 |
| Medication | ||||
| Any antidepressant | 11/32 | 34 | 12/25 | 48 |
| Any antipsychotic (excluding clozapine and amisulpride) | 2/32 | 6 | 2/25 | 8 |
| Any mood stabiliser | 6/32 | 19 | 5/25 | 20 |
| Incomplete participation in the study | 5/34 | 15 | 6/30 | 20 |
| Variable | Treatment group | |||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n/N or mean | % or (SD) | n/N or mean | % or (SD) | |
| PANSS | 76 | (16) | 80 | (24) |
| Primary outcome | ||||
| 20% reduction in total PANSS score from baseline | 14/32 | 44 | 10/25 | 40 |
| Secondary outcomes | ||||
| PANSS negative symptoms | 21 | (7) | 21 | (8) |
| SES, median (IQR) | 8 | (3–13) | 7 | (3–13) |
| CDSS, median (IQR) | 5 | (2–8) | 3 | (1–7) |
| SAI, median (IQR) | 12 | (10–13) | 14 | (9–15) |
| Physical health measures | ||||
| Weight (kg) | 100 | (25) | 93 | (22) |
| BMI (kg/m2) | 34 | (9) | 31 | (7) |
| Waist circumference (cm) | 112 | (15) | 103 | (14) |
| Systolic blood pressure (mmHg) | 124 | (14) | 119 | (11) |
| Diastolic blood pressure (mmHg) | 82 | (10) | 79 | (6) |
| Serum prolactin concentration (ng/ml), median (IQR) | 43 | (9–87) | 11 | (7–12) |
| Plasma glucose concentration (mmol/l), non-fasting sample | 6.9 | (2.8) | 5.4 | (0.7) |
| Total cholesterol concentration (mmol/l) | 5.1 | (1.4) | 4.7 | (1.3) |
| HDL cholesterol concentration (mmol/l) | 1.3 | (0.6) | 1.3 | (0.5) |
| LDL cholesterol concentration (mmol/l) | 2.8 | (1.6) | 2.9 | (1.1) |
| Triglyceride concentration (mmol/l), median (IQR) | 2.0 | (1.3–3.8) | 2.1 | (1.2–2.9) |
| Side effects | ||||
| ANNSERS-E, median (IQR) | 12 | (7–22) | 12 | (9–15) |
| BARS, median (IQR) | 1 | (0–2) | 1 | (0–2) |
| Akathisia present (global clinical assessment score of ≥ 2) | 2/32 | 6 | 4/25 | 16 |
| AIMS positive: tardive dyskinesia | 1/32 | 3 | 2/25 | 8 |
| EPSE, median (IQR) | 0.1 | (0.0–0.4) | 0.2 | (0.0–0.5) |
| Parkinsonism present | 8/28 | 29 | 7/18 | 39 |
Table 4 shows descriptive statistics about possible predictors of whether or not the primary outcome (20% reduction in PANSS score between baseline and 12-week follow-up) was missing (‘missingness’). These may be of limited use because of the small numbers in the missing primary outcome group, some numbers being very small (with a maximum of 11).
| Variable | Missingness of primary outcome | |||
|---|---|---|---|---|
| Present | Not present | |||
| n/N or median | % or (IQR) | n/N or median | % or (IQR) | |
| Male | 40/57 | 70 | 6/10 | 60 |
| Age (years) | 38 | (31–46) | 37 | (31–42) |
| White | 44/57 | 77 | 7/10 | 70 |
| Living alone | 21/54 | 39 | 2/6 | 33 |
| Living with parents | 12/54 | 22 | 1/6 | 17 |
| Living with others | 21/54 | 39 | 3/6 | 50 |
| Flat or house rented | 35/57 | 61 | 5/6 | 83 |
| Other accommodation | 22/57 | 39 | 1/6 | 17 |
| Not in paid employment because of treatment | 44/46 | 96 | 3/4 | 75 |
| Currently an inpatient | 9/57 | 16 | 0/10 | 0 |
| Psychiatric inpatient in the last 3 months | 1/38 | 3 | 0/4 | 0 |
| Non-psychiatric inpatient in the last 3 months | 0/36 | 0 | 1/4 | 25 |
| Any inpatient in the last 3 months | 10/57 | 18 | 1/7 | 14 |
| Psychiatric outpatient in the last 3 months | 11/38 | 29 | 1/4 | 25 |
| Non-psychiatric outpatient in the last 3 months | 1/35 | 3 | 0/4 | 0 |
| Any outpatient in the last 3 months | 19/56 | 34 | 3/7 | 43 |
| Psychiatric emergency in the last 3 months | 0/37 | 0 | 0/4 | 0 |
| Attended A&E in the last 3 months | 0/36 | 0 | 0/4 | 0 |
| Community-based services used in the last 3 months | 25/56 | 45 | 5/6 | 83 |
| Attended NHS day hospital or day centre in the last 3 months | 1/38 | 3 | 0/4 | 0 |
| Other primary or community care contacts in the last 3 months | 48/56 | 86 | 6/6 | 100 |
| In contact with the criminal justice system in the last 3 months | 2/57 | 4 | 0/6 | 0 |
| Primary psychiatric diagnosis | ||||
| Schizophrenia | 53/56 | 95 | 8/8 | 100 |
| Schizophreniform disorder | 1/56 | 2 | 0/8 | 0 |
| Schizoaffective disorder | 1/56 | 2 | 0/8 | 0 |
| Psychosis NOS | 1/56 | 2 | 0/8 | 0 |
| Clinical assessment | ||||
| PANSS high score (stratification variable) | 89 | (84–98) | 98 | (86–111) |
| PANSS negative symptoms | 25 | (21–29) | 28 | (24–30) |
| CDSS depression | 5 | (1–10) | 7 | (4–14) |
| SOFAS | 35 | (31–40) | 35 | (30–39) |
| SES | 9 | (4–15) | 9 | (4–19) |
| SAI | 12 | (9–14) | 13 | (9–14) |
| Side effects | ||||
| ANNSERS-E | 15 | (10–24) | 20 | (14–57) |
| BARS | 0 | (0–2) | 3 | (1–4) |
| Akathisia present (global clinical assessment score of ≥ 2) | 4/56 | 7 | 3/8 | 38 |
| AIMS positive: tardive dyskinesia | 5/57 | 9 | 3/11 | 27 |
| EPSE | 0.1 | (0.0–0.3) | 0.0 | (0.0–0.1) |
| Parkinsonism present | 16/48 | 33 | 0/5 | 0 |
| Other psychotropic medication | ||||
| Any antidepressant | 25/57 | 43 | 3/11 | 27 |
| Any antipsychotic (excluding clozapine and amisulpride) | 4/57 | 7 | 0/11 | 0 |
| Any mood stabiliser | 11/57 | 19 | 2/11 | 18 |
| Physical health measures | ||||
| Weight (kg) | 89 | (79–103) | 84 | (80–91) |
| Height (m) | 1.72 | (1.68–1.78) | 1.70 | (1.69–1.72) |
| BMI (kg/m2) | 30 | (27–34) | 29 | (25–29) |
| Waist circumference (cm) | 105 | (100–115) | 106 | (96–106) |
| Systolic blood pressure (mmHg) | 121 | (115–133) | 121 | (112–129) |
| Diastolic blood pressure (mmHg) | 80 | (77–87) | 83 | (76–93) |
| Serum prolactin concentration (ng/ml) | 12 | (5–15) | 11 | (5–14) |
| Plasma glucose concentration (mmol/l), non-fasting sample | 5.4 | (5.0–6.6) | 5.9 | (4.0–8.3) |
| Total cholesterol concentration (mmol/l) | 5.3 | (4.5–6.1) | 4.8 | (4.0–5.5) |
| HDL cholesterol concentration (mmol/l) | 1.1 | (0.9–1.4) | 1.7 | (1.1–1.9) |
| LDL cholesterol concentration (mmol/l) | 3.2 | (2.5–3.7) | 2.4 | (2.1–3.1) |
| Triglycerides (mmol/l) | 2.0 | (1.4–3.2) | 1.2 | (0.9–1.5) |
The data in Table 5 show that the patients in the amisulpride group had higher odds than those assigned to placebo of achieving the primary outcome response criterion of a 20% reduction in PANSS total score at 12 weeks [OR 1.17 (95% CI 0.40 to 3.42)]. Table 6 presents the unadjusted, per-protocol analysis in terms of the amisulpride intervention. This analysis excluded those participants who were prescribed an additional antipsychotic medication, a mood stabiliser or antidepressant at either 6 or 12 weeks, which was a substantial proportion. The findings are, therefore, hard to interpret: the estimates are close to zero and the 95% CIs are wide.
| Variable | OR or coefficient | 95% CI |
|---|---|---|
| Primary outcome | ||
| 20% reduction in PANSS from baseline | 1.17a | 0.40 to 3.42 |
| Secondary outcomes | ||
| PANSS negative symptoms | –0.71 | –3.22 to 1.81 |
| SES | 1.17 | –1.63 to 3.97 |
| CDSS | 0.23 | –1.54 to 2.00 |
| SAI | 0.02 | –1.33 to 1.37 |
| Side effects | ||
| Non-neurological | ||
| ANNSERS-E | 1.58 | –3.60 to 6.76 |
| Metabolic side effects | ||
| Weight (kg) | 0.79 | –1.40 to 2.99 |
| BMI (kg/m2) | –0.02 | –1.05 to 1.01 |
| Waist circumference (cm) | 1.05 | –2.33 to 4.42 |
| Systolic blood pressure (mmHg) | 3.49 | –3.66 to 10.63 |
| Diastolic blood pressure (mmHg) | 3.33 | –1.65 to 8.31 |
| Serum prolactin concentration (ng/ml) | 50.47 | –8.86 to 109.80 |
| Lnb serum prolactin concentration (ng/ml) | 1.43 | 0.71 to 2.14 |
| Plasma glucose concentration, non-fasting sample (mmol/l) | 0.66 | –0.22 to 1.54 |
| Total cholesterol concentration (mmol/l) | 0.48 | –0.11 to 1.07 |
| HDL cholesterol concentration (mmol/l) | 0.09 | –0.23 to 0.41 |
| LDL cholesterol concentration (mmol/l) | 0.11 | –0.62 to 0.85 |
| Triglycerides concentration (mmol/l) | 0.78 | –0.10 to 1.65 |
| Motor side effects | ||
| BARS | –0.23 | –0.73 to 0.27 |
| Akathisia present (global clinical assessment score of ≥ 2)c | 0.35a | 0.06 to 2.09 |
| AIMS positivec | 0.37a | 0.03 to 4.34 |
| EPSE | –0.04 | –0.22 to 0.14 |
| Extrapyramidal side effects presentc | 0.63a | 0.18 to 2.20 |
| Variable | OR or coefficient | 95% CI |
|---|---|---|
| Primary outcome | ||
| 20% reduction in PANSS from baseline | 3.00a | 0.57 to 15.77 |
| Secondary outcomes | ||
| PANSS negative symptom subscale | –2.70 | –9.53 to 4.13 |
| SES: service engagement | –0.73 | –6.44 to 4.97 |
| CDSS: depression | 0.06 | –3.41 to 3.53 |
| SAI: insight | 1.07 | –2.63 to 4.77 |
| Physical health measures | ||
| Weight (kg) | –1.86 | –23.03 to 19.32 |
| BMI (kg/m2) | 0.72 | –5.79 to 7.23 |
| Waist circumference (cm) | 11.27 | –3.60 to 26.14 |
| Systolic blood pressure (mmHg) | 0.81 | –11.72 to 13.34 |
| Diastolic blood pressure (mmHg) | 2.23 | –6.19 to 10.65 |
| Serum prolactin concentration (ng/ml) | 41.66 | –57.26 to 140.59 |
| Lnc serum prolactin concentration (ng/ml) | 1.54 | –0.13 to 3.20 |
| Plasma glucose concentration: non-fasting sample (mmol/l) | 0.72 | –1.13 to 2.57 |
| Total cholesterol concentration (mmol/l) | 0.06 | –1.42 to 1.53 |
| HDL cholesterol concentration (mmol/l) | 0.19 | –0.19 to 0.57 |
| LDL cholesterol concentration (mmol/l) | 0.40 | –1.35 to 2.16 |
| Triglyceride concentration (mmol/l) | –0.56 | –2.22 to 1.09 |
| Side effects | ||
| ANNSERS-Eb | –1.25 | –17.57 to 15.07 |
| BARS | –0.27 | –1.42 to 0.88 |
| Akathisia present (global clinical assessment score of ≥ 2)d | – | – |
| AIMS positive: tardive dyskinesiad | – | – |
| EPSE | 0.05 | –0.21 to 0.32 |
| Parkinsonism present | 1.56a | 0.21 to 11.37 |
Table 7 presents the results of mixed-effects modelling to take time into account in terms of the amisulpride intervention. These reveal a time effect associated with a 20% reduction on the PANSS; the odds of 20% reduction on the PANSS at 12 weeks is 4.19 times that of a 20% reduction of 6 weeks (95% CI 1.20 to 14.56), controlling for baseline PANSS score and including the randomised condition (OR 1.43, 95% CI 0.24 to 8.44). Likewise, the PANSS negative subscale score shows that there is a slight decrease in score at 12 weeks compared with 6 weeks (coefficient –1.32, 95% CI –2.20 to –0.44), controlling for baseline negative PANSS score and including the randomised condition (coefficient –0.60, 95% CI –2.58 to 1.39).
| Variable | OR or coefficient | 95% CI |
|---|---|---|
| Primary outcome | ||
| 20% reduction in PANSS from baseline | 1.43a | 0.24 to 8.44 |
| Secondary outcomes | ||
| PANSS negative symptom subscale | –0.60 | –2.58 to 1.39 |
| SES | 1.75 | –0.54 to 4.04 |
| CDSS: depression | 0.19 | –1.10 to 1.49 |
| SAI | –0.52 | –2.32 to 1.28 |
| Side effects | ||
| ANNSERS-E | 3.11 | –0.91 to 7.13 |
| BARS | –0.11 | –0.57 to 0.35 |
| Akathisia present (global clinical assessment score of ≥ 2) | 0.29a | 0.01 to 7.82 |
| AIMS positive: tardive dyskinesia | 0.18a | 0.00 to 32.67 |
| EPSE | 0.05 | –0.09 to 0.19 |
| Parkinsonism presentb | – | – |
Side effects
The data in Table 7 regarding side effect assessment using the ANNSERS-E show that, over the course of the study, the mean ANNSERS-E total score was 3 points higher in those participants assigned to amisulpride than in those in the placebo group. The information in Table 1b shows a greater frequency of cardiac symptoms in the amisulpride group, when checked 7–10 days after starting study medication. The data in Table 5 on plasma prolactin concentration (including following logarithmic transformation, i.e. ln prolactin) reveal that those participants in the amisulpride group had, on average, a much higher level during the study. Plasma prolactin concentration is 24% lower (95% CI 12% to 49%) in the placebo group than in the amisulpride group.
Table 8 shows the data relating to the nature and frequency of adverse events reported during the trial. There were 65 adverse events in 31 participants; more of these events were in the amisulpride intervention group than in the placebo group (47 events vs. 18 events, respectively). Almost half of adverse events in the amisulpride group were in the cardiac, general or endocrine systems. With regard to the nature of the cardiac adverse events in the amisulpride-treated group, dizziness and breathlessness were the most common symptoms, each reported by six participants, with postural dizziness, irregular heartbeat and tachycardia each reported by two participants.
| Treatment group | ||||
|---|---|---|---|---|
| Amisulpride | Placebo | |||
| n / N | % | n / N | % | |
| Adverse events as the denominator | ||||
| Body system classification | ||||
| Blood/bone marrow | 1/47 | 2 | 1/18 | 6 |
| Cardiac: general | 12/47 | 26 | 1/18 | 6 |
| Dermatological | 1/47 | 2 | 3/18 | 17 |
| Endocrine | 10/47 | 21 | 0/18 | 0 |
| Gastrointestinal | 5/47 | 11 | 4/18 | 22 |
| Infection | 3/47 | 6 | 0/18 | 0 |
| Metabolic/laboratory | 2/47 | 4 | 0/18 | 0 |
| Neurology | 1/47 | 2 | 1/18 | 6 |
| Pain | 1/47 | 2 | 0/18 | 0 |
| Pulmonary/upper respiratory | 4/47 | 9 | 0/18 | 0 |
| Renal/genitourinary | 0/47 | 0 | 1/18 | 6 |
| Vascular | 3/47 | 6 | 0/18 | 0 |
| Other | 4/47 | 9 | 7/18 | 39 |
| Serious adverse event | 1/45 | 2 | 2/18 | 11 |
| Adverse event severity | ||||
| Mild | 24/42 | 57 | 13/17 | 76 |
| Moderate | 15/42 | 36 | 2/17 | 12 |
| Severe | 3/42 | 7 | 2/17 | 12 |
| Adverse event resulted in unblinding | 1/44 | 2 | 2/18 | 11 |
| Trial drug related | ||||
| Unrelated | 17/47 | 36 | 1/18 | 6 |
| Unlikely to be related | 7/47 | 15 | 5/18 | 28 |
| Possibly | 7/47 | 15 | 10/18 | 56 |
| Probably | 13/47 | 28 | 2/18 | 11 |
| Definitely | 3/47 | 6 | 0/18 | 0 |
| Adverse event unexpected | 18/45 | 40 | 7/17 | 41 |
| Outcome | ||||
| Resolved | 24/44 | 55 | 12/14 | 86 |
| Persisting | 17/44 | 39 | 2/14 | 14 |
| Not assessable | 3/44 | 7 | 0/14 | 0 |
| Participants as the denominator | ||||
| Any adverse event | 21/35 | 60 | 10/33 | 30 |
| Serious adverse event | 1/35 | 3 | 2/33 | 6 |
| Most severe adverse event | ||||
| None | 14/35 | 40 | 23/32 | 72 |
| Mild | 9/35 | 26 | 5/32 | 16 |
| Moderate | 10/35 | 29 | 2/32 | 6 |
| Severe | 2/35 | 6 | 2/32 | 6 |
| No adverse event | 14/35 | 40 | 23/33 | 70 |
| Adverse event not resulting in unblinding | 20/35 | 57 | 8/33 | 24 |
| Adverse event resulted in unblinding | 1/35 | 3 | 2/33 | 6 |
Most of the adverse events reported were mild and resolved. Almost one-third of adverse events in the amisulpride group were either ‘probably’ or ‘definitely’ related to the study medication, compared with a little over one-tenth in the placebo group. Sixty per cent of participants in the amisulpride group had at least one adverse event, compared with 30% in the control group; however, serious adverse events were rare, with one person experiencing a serious adverse event in the amisulpride group and two people experiencing such an event in the placebo group.
Health economics
Within-trial analysis
Covariates for the analyses of cost and quality-adjusted life-years and imputation of missing data
The EQ-5D utility score at baseline was correlated with all the clinical measures except the SES and SOFAS (Pearson’s correlation coefficient, p ≤ 0.05; see Appendix 1, Table 22, for the Pearson correlation coefficients of each analysis). The following variables were selected as baseline covariates for the analyses to estimate nets costs and QALYs. The covariates used in the analysis of QALYs were:
-
PANSS
-
whether or not the participant was white British
-
whether or not the participant lived alone
-
age
-
baseline EQ-5D visual analogue scale (VAS) score.
The PANSS was included to take into account the relationship between utility and psychosis symptoms (Pearson’s correlation coefficient = –0.304; p = 0.014). In addition, a complete set of baseline data for the PANSS measure was available, and correlated with the clinical measures that were associated with the EQ-5D utility score. Age was included to reflect the fact that health and, therefore, utility typically declines with age and was identified as an important covariate in previous studies of antipsychotic treatment. 60,67 The binary variables to reflect whether or not the participants lived alone or were white British were included as proxies for social isolation. Additionally, whether or not a person is white British may act as a proxy for socioeconomic status, given that most of the trial participants were not in employment at the time of the trial.
The covariates used in the analysis of costs were:
-
the costs in the 3 months prior to baseline
-
study site
-
whether or not the participant was white British
-
whether or not the participant lived alone
-
EQ-5D utility measure.
Past studies indicate that previous cost is a strong predictor of future cost. 60 Study site was included to reflect the fact that the total cost of treatment also depends on the availability and mix of services in different localities, particularly the relative provision of hospital compared with primary and community services. Whether or not a person lives alone or is white British may indicate a greater need for formal health and social services because of a lack of support, lower access to health, and socioeconomic deprivation. The EQ-5D was included as an overall measure of health and health need.
In addition to the baseline measures described in the preceding paragraph, to impute missing costs the clinical measures with complete data at baseline and follow-up, for participants who completed a follow-up assessment, were included in the imputation models. The variables with missing observations that were imputed were inpatient, outpatient, community, primary care and medication costs and total costs for each assessment.
EQ-5D health status, utility and quality-adjusted life-years
There were no missing EQ-5D observations for participants who completed the 12-week follow-up assessment (n = 57). Tables 9 and 10 present the EQ-5D data for these participants. Baseline and follow-up data, where available, are reported in Appendix 1, Tables 20 and 21. Table 9 summarises the proportion of people with no problems on each of the EQ-5D health domains. Table 10 presents the mean VAS scores, which represent how participants rate their own health. Table 10 also includes the utility scores, estimated from the EQ-5D domains and published population weights. The utility scores are anchored at 0 (dead) and 1 (full health), with some states also valued as worse than death. There are no clear differences between the amisulpride and placebo groups at baseline or follow-up. As noted above, there were statistically significant correlations between the EQ-5D utility score and clinical measures of outcome, including measures of side effects. These indicated the utility score increased with decreased symptoms or lower levels of side effects. The results of the effectiveness analyses described above indicated that amisulpride may be associated with improvements in symptoms, but also increased side effect burden. This may explain the lack of difference in utility scores, which is a measure of overall health.
| EQ-5D health states | Treatment group | |||
|---|---|---|---|---|
| Amisulpride (n = 32/35) | Placebo (n = 25/33) | |||
| n/N | % | n/N | % | |
| Baseline | ||||
| No problem with mobility | 25/32 | 78 | 18/25 | 72 |
| No problem with self-care | 27/32 | 84 | 19/25 | 76 |
| No problem with usual activities | 24/32 | 75 | 14/25 | 56 |
| No problem with pain/discomfort | 17/32 | 53 | 17/25 | 68 |
| No problem with anxiety/depression | 12/32 | 38 | 10/25 | 40 |
| 12-week assessment | ||||
| No problem with mobility | 25/32 | 78 | 19/25 | 76 |
| No problem with self-care | 25/32 | 78 | 19/25 | 76 |
| No problem with usual activities | 23/32 | 72 | 13/25 | 52 |
| No problem with pain/discomfort | 18/32 | 56 | 16/25 | 64 |
| No problem with anxiety/depression | 11/32 | 34 | 11/25 | 44 |
| Assessment period | Treatment group | |||
|---|---|---|---|---|
| Amisulpride (n = 32/35) | Placebo (n = 25/33) | |||
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | |
| EQ-5D VAS scores | ||||
| Baseline | 58 (26) | 52 to 56 | 59 (22) | 53 to 66 |
| 12-week assessment | 58 (23) | 52 to 63 | 53 (23) | 46 to 59 |
| EQ-5D utility values | ||||
| Baseline | 0.64 (0.37) | 0.50 to 0.77 | 0.68 (0.38) | 0.53 to 0.83 |
| 12-week assessment | 0.64 (0.37) | 0.51 to 0.77 | 0.65 (0.36) | 0.50 to 0.79 |
Table 11 summarises the QALY measure for participants who completed the 12-week follow-up assessment and for the sample of participants for whom complete cost and QALY data at baseline and follow-up were available. Again, there is no evidence of differences in QALYs between the two groups.
| QALY | Treatment group | |||||
|---|---|---|---|---|---|---|
| Amisulpride | Placebo | |||||
| Sample size (n/N) | Mean (SD) | 95% CI | Sample size (n/N) | Mean (SD) | 95% CI | |
| Participants for whom complete QALY and cost data at baseline and follow-up were available | 18/35 | 0.178 (0.141) | 0.127 to 0.228 | 15/33 | 0.188 (0.131) | 0.141 to 0.235 |
| Participants who completed the 12-week follow-up assessments | 32/35 | 0.179 (0.106) | 0.142 to 0.217 | 25/33 | 0.176 (0.108) | 0.137 to 0.214 |
Service use and costs
Table 12 summarises the use of primary and community-based services and hospital-based services, for baseline and follow-up, for those participants with complete data about the services used at both baseline and follow-up.
| Type of service | Treatment group | |
|---|---|---|
| Amisulpride (n = 30) | Placebo (n = 22) | |
| Mean (SD) | Mean (SD) | |
| Number of visits to community services in 3 months prior to baseline | 3.20 (8.42) | 7.64 (16.48) |
| Number of visits to community services baseline to follow-up | 2.90 (8.05) | 7.18 (15.62) |
| Number of GP visits in 3 months prior to baseline | 0.93 (1.46) | 1.59 (2.30) |
| Number of GP visits baseline to follow-up | 0.67 (1.12) | 1.23 (1.54) |
| Number of other primary visits in 3 months prior to baseline | 5.57 (9.34) | 4.64 (5.04) |
| Number of other primary visits baseline to follow-up | 6.87 (9.66) | 4.32 (6.99) |
| Total mental health outpatient visits in 3 months prior to baseline | 0.27 (0.69) | 1.82 (3.70) |
| Total mental health outpatient visits baseline to follow-up | 0.63 (1.52) | 1.05 (2.80) |
| Total physical health outpatient visits in 3 months prior to baseline | 0.07 (0.25) | 0.00 (0.00) |
| Total physical health outpatient visits baseline to follow-up | 0.03 (0.18) | 0.00 (0.00) |
| Length of stay for physical health admission to hospital in 3 months prior to baseline, days | 0.00 (0.00) | 0.00 (0.00) |
| Length of stay for physical health admission to hospital baseline to follow-up, days | 0.03 (0.18) | 0.00 (0.00) |
| Length of stay for mental health admission to hospital in 3 months prior to baseline, days | 15.00 (34.11) | 12.27 (31.61) |
| Length of stay for mental health admission to hospital baseline to follow-up, days | 13.83 (36.24) | 17.95 (39.14) |
Table 13 reports the costs for the different types of services used. Overall, inpatient admissions for mental health care were the highest cost component, although a small proportion of participants had a mental health hospital admission (3 months to baseline – five participants in the amisulpride treatment group and three participants in the placebo group; baseline to follow-up – four participants in the amisulpride treatment group and five participants in the placebo group). Table 14 presents the total costs for participants with complete cost data, as well as the imputed cost for participants who completed the week 12 follow-up assessment. Overall, there are no clear differences in total cost between the amisulpride and placebo groups. The total costs are characterised by high levels of variance and overlap in the 95% CIs.
| Type of service | Treatment group | |
|---|---|---|
| Amisulpride (n = 18), mean (SD) | Placebo (n = 15), mean (SD) | |
| Cost of community services in 3 months prior to baseline | 373 (552) | 744 (2295) |
| Cost of community services baseline to follow-up | 251 (436) | 841 (2282) |
| Cost of primary care in 3 months prior to baseline | 492 (654) | 367 (436) |
| Cost of primary care baseline to follow-up | 568 (839) | 198 (274) |
| Cost of daily medications in 3 months prior to baseline | 246 (142) | 426 (716) |
| Cost of daily medications baseline to follow-up | 309 (204) | 232 (139) |
| Amisulpride baseline to follow-up | 27 (5) | 0 (0) |
| Cost of mental health outpatient visits in 3 months prior to baseline | 53 (126) | 278 (473) |
| Cost of mental health outpatient visits baseline to follow-up | 166 (283) | 191 (414) |
| Cost of physical health outpatient visits in 3 months prior to baseline | 0 (0) | 0 (0) |
| Cost of physical health outpatient visits baseline to follow-up | 0 (0) | 0 (0) |
| Cost of physical health admission to hospital in 3 months prior to baseline | 6 (26) | 0 (0) |
| Cost of physical health admission to hospital baseline to follow-up | 0 (0) | 0 (0) |
| Cost of mental health admission to hospital in 3 months prior to baseline | 3690 (8490) | 1476 (5717) |
| Cost of mental health admission to hospital baseline to follow-up | 2193 (6460) | 3378 (8916) |
| Total costs | Treatment group | |||||
|---|---|---|---|---|---|---|
| Amisulpride | Placebo | |||||
| Sample size (n/N) | Mean (SD) | 95% CI | Sample size (n/N) | Mean (SD) | 95% CI | |
| Participants with complete cost data at baseline and follow-up | ||||||
| 3 months prior to baseline | 18/35 | 4854 (10,844) | 950 to 8759 | 15/33 | 3291 (8425) | 258 to 6325 |
| Baseline to 12-week follow-up assessment | 18/35 | 3520 (8336) | 519 to 6522 | 15/33 | 4840 (12,874) | 205 to 9476 |
| Participants who completed 12-week follow-up assessments: missing costs imputed | ||||||
| 3 months prior to baseline | 32/35 | 5014 (9063) | 1801 to 8226 | 25/33 | 4616 (8329) | 1664 to 7568 |
| Baseline to 12-week follow-up assessment | 32/35 | 3988 (8103) | 1116 to 6860 | 25/33 | 5330 (10,252) | 1697 to 8964 |
Net costs, quality-adjusted life-years and cost-effectiveness of amisulpride augmentation
Table 15 reports the net costs and QALYs after adjusting for differences in baseline covariates. The data suggest a net saving for amisulpride and a small net gain in QALYs. It is important to note that the net cost and QALY data are associated with wide CIs that cross zero, which suggests that the differences are not statistically significant.
| Analysis | Net cost | Net QALY | ||
|---|---|---|---|---|
| Mean (SE) | 95% CI | Mean (SE) | 95% CI | |
| Participants who completed 12-week follow-up assessments: missing costs imputed | –£1788 (£1182) | –£4165 to £590 | 0.0009 (0.014) | –0.026 to 0.028 |
Figures 3 and 4 show the cost-effectiveness plane and CEAC. Table 16 reports the bootstrap simulations of net cost and QALYs, as well as the results of the cost-effectiveness acceptability analyses. These results show similar net costs and QALYs to the analysis adjusted for covariates. However, in this case, the 95th percentiles of cost indicate a net saving associated with augmentation of clozapine with amisulpride. The cost-effectiveness plane illustrates the scatter of the 10,000 simulated net costs, all of which indicate a net saving. Figure 3 also indicates the distribution of the 10,000 simulated net QALYs, which are evenly spread between net QALY gain and net QALY loss. Overall, there is a net saving with no clear difference in QALYs, with a mean net benefit of £1806 (SD £373, 95th percentiles £1088 to £2530). In this simulation, the probability that amisulpride augmentation is cost-effective is 1.
FIGURE 3.
Cost-effectiveness plane of net costs and QALYs: primary analysis.

FIGURE 4.
Cost-effectiveness acceptability curve, probability that amisulpride augmentation is cost-effective: primary analysis.
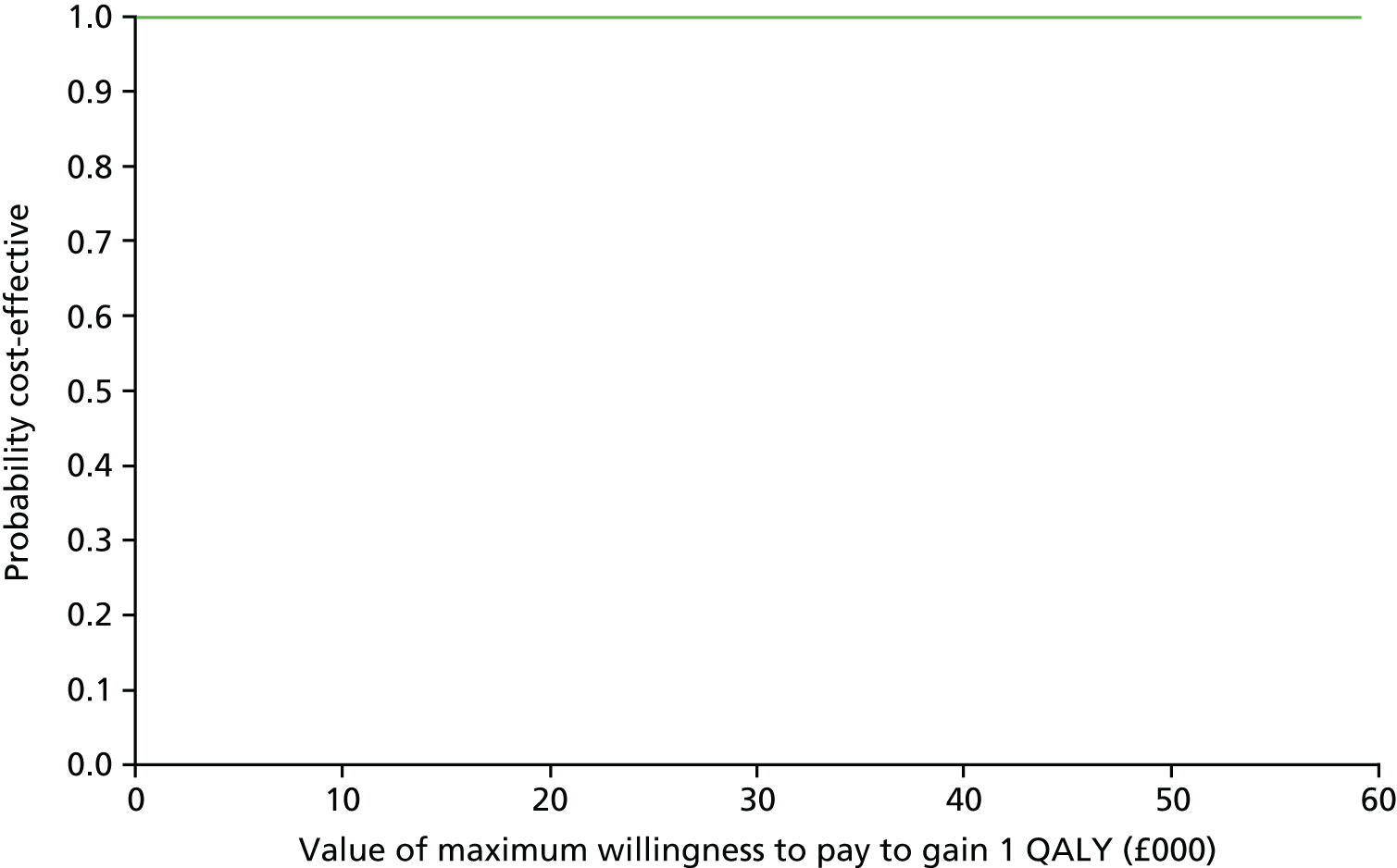
| Statistic | Participants completing the 12-week follow-up assessments: missing costs imputed |
|---|---|
| Net (2014) cost (SE) [95th percentiles], £ | –1816 (369) [–2540 to –1092] |
| Net QALY (SE) [95th percentiles] | 0.0009 (0.004) [–0.007 to 0.008] |
| Incremental cost/QALY gained | Amisulpride dominates (net cost saving and no difference in QALYs) |
| Net benefit (SE) [95th percentiles], £; WTPT = £15,000 | 1806 (£373) [1088 to 2530] |
| Probability amisulpride cost-effective if WTPT = £15,000 | 1.00 |
Table 17 presents the results of the sensitivity analyses. Overall, these are similar to the results of the primary analysis, which supports a conclusion that amisulpride augmentation of clozapine is likely to be cost-effective. However, this conclusion only applies to the short treatment and follow-up period for the 57 participants who completed a follow-up assessment. In addition, excluding inpatient costs reduces the potential savings associated with amisulpride. If the configuration of available services is an important driver of whether or not a person can access and use inpatient care, the savings estimated in the primary analysis are likely to be overestimated.
| Analysis | Net (2014) cost, £ (SE) | 95th percentiles, £ | Net QALY, (SE) | 95th percentiles | Net (2014) benefit, £ (SD) | 95th percentiles, £ | Probability that amisulpride is cost-effective (WTPT = £15,000) |
|---|---|---|---|---|---|---|---|
| Sensitivity analyses | |||||||
| Participants with complete cost data | –2011 (1761) | –5463 to 1441 | –0.003 (0.027) | –0.057 to 0.050 | 1671 (1863) | –1016 to 5793 | 0.82 |
| Excludes inpatient costs | –329 (132) | –587 to 71 | 0.0009 (0.004) | –0.007 to 0.008 | 327 (145) | 52 to 629 | 0.99 |
| Excludes costs of daily medications | –1864 (368) | –2586 to –1142 | 0.0009 (0.004) | –0.007 to 0.008 | 1855 (372) | 1129 to 2575 | 1.00 |
| Cost per person with a 20% response on the PANSS | –1816 (369) | –2540 to –1092 | 0.22 (0.19) people with a 20% response | 0.155 to 0.586; people with a 20% response | 3362 (2824); WTPT = £15,000 to gain one person with a response | –1842 to 882; WTPT = £15,000 to gain one person with a response | 0.89 |
| Cost per person with no serious side effects/adverse events | –1816 (369) | –2540 to –1092 | –0.39 (0.28) | –0.94 to 0.16 | –7563 (4058) | –15,422 to 522 | 0.04 |
Because amisulpride augmentation was associated with a higher rate of side effects and adverse events, there was no evidence that it was cost-effective when the cost per serious side effect (any severe non-neurological side effect, any EPS or any serious adverse event) was used as the measure of cost-effectiveness.
The economic model was used to explore the potential of amisulpride augmentation to be cost-effective over the longer time frame of 1 year. The results are discussed in the Economic model section below.
Economic model
Table 18 reports the baseline parameter values estimated from the trial data for cycles 2–4. The parameters were estimated from the pooled data for amisulpride augmentation and placebo. Regression of costs on whether or not a person had a 20% response on the PANSS, controlling for baseline total cost indicated a net saving associated with response. Regression of QALYs on whether or not a person had a 20% response on the PANSS, controlling for baseline total cost indicated a net increase in QALYs. Similarly, side effects were associated with an additional cost and a reduction in QALYs.
| Parameter | Mean (SD) | Distribution type | Source |
|---|---|---|---|
| Probability | |||
| Probability of 20% response on the PANSS | 0.40 (0.35) | Beta | Pooled AMICUS trial data |
| Probability of side effects | 0.89 (0.22) | Beta | Pooled AMICUS trial data |
| Alive, point estimate (range) | 0.99 (0.98–1) | Triangular | Healey et al., 201278 |
| Cost (£) | |||
| Reduction in cost per person with 20% response on the PANSS | –2411 (6356) | Gamma | Pooled AMICUS trial data |
| Cost per person without 20% response on the PANSS | 5904 (9218) | Gamma | Pooled AMICUS trial data |
| Additional cost per person with increased side effectsa | 757 (1293) | Gamma | Pooled AMICUS trial data |
| QALYs | |||
| Additional QALY per person with 20% response on the PANSS | 0.005 (0.111) | Gamma | Pooled AMICUS trial data |
| QALY per person without 20% response on the PANSS | 0.174 (0.069) | Gamma | Pooled AMICUS trial data |
| Decrement in QALY per person with increased side effectsb | –0.006 (0.011) | Gamma | Pooled AMICUS trial data |
Table 19 shows the net costs and QALYs associated with amisulpride augmentation for the base-case analysis and sensitivity analyses, along with the probability that amisulpride is cost-effective. Overall, modelling the expected costs and QALYs over a 1-year time frame increased the variability in the results. This contributed to the reduction in the probability that amisulpride augmentation is cost-effective, from the values of 0.82–1.00 found in the within-trial analyses to between 0.54 and 0.58 for the model-based analyses. The model analyses were not sensitive to the probability of response, side effects or the time horizon used. This suggests that the initial net saving and QALY gain is not offset in the future if the probability of response and side effects for amisulpride is the same as clozapine from the second cycle onwards
| Analysis | Net (2014) cost, £ (SE) | 95th percentiles, £ | Net QALY, (SE) | 95th percentiles | Net (2014) benefit, £ (SD) | 95th percentiles, £ | Probability amisulpride is cost-effective (WTPT = £15,000) |
|---|---|---|---|---|---|---|---|
| Base-case analysis | –1935 (13,735) | –33,908 to 25,968 | 0.000 (0.153) | –0.309 to 0.309 | 1936 (13,882) | –24,201 to 37,041 | 0.58 |
| Sensitivity analyses | |||||||
| Probability of response differs between amisulpride (p = 0.420) and clozapine (p = 0.37) over all cycles | –2151 (14,174) | –34,853 to 26,542 | 0.009 (0.522) | –0.314 to 0.325 | 2682 (37,113) | –29,093 to 37,158 | 0.59 |
| Probability of side effects differs between amisulpride (p = 0.903) and clozapine (p = 0.885) over all cycles | –1650 (13,755) | –32,483 to 27,595 | 0.003 (0.153) | –0.311 to 0.316 | 1813 (17,515) | –29,246 to 36,300 | 0.58 |
| Probability of response and side effects differs between amisulpride (p = 0.420; p = 0.903) and clozapine (p = 0.37; p = 0.885) over all cycles | –1856 (14,329) | –34,330 to 27,110 | –0.004 (0.588) | –0.326 to 0.314 | 1640 (18,162) | –32,499 to 39,282 | 0.58 |
| Probability of response in cycles 2–4 differs between amisulpride (p = 0) in and clozapine (p = 0.4) | –821 (14,274) | –32,264 to 29,410 | –0.004 (0.203) | –0.320 to 0.305 | 582 (22,453) | –35,696 to 32,454 | 0.54 |
| Time horizon: 10 years | –1749 (13,914) | –34,247 to 28,094 | 0.002 (0.153) | –0.313 to 0.315 | 1781 (13,925) | –25,265 to 34,292 | 0.58 |
Chapter 4 Discussion
Efficacy
This trial is only the second double-blind, placebo-controlled RCT testing amisulpride augmentation of clozapine in patients with schizophrenia that has shown an insufficient response to clozapine treatment. It has a much larger sample size than the first RCT43 but, nevertheless, it under-recruited, and the power of any analysis to detect significant differences between the active and placebo groups is necessarily limited. Looking at the primary outcome measure, the participants in the amisulpride group had higher odds of achieving the response criterion of a 20% or greater reduction in the total PANSS score by the end of the 12-week study, although this was not statistically significant. This advantage was not evident at 6 weeks, reinforcing the possibility that an adequate trial of clozapine augmentation with a second antipsychotic may be at least 10–12 weeks,18 that is, longer than the 4–6 weeks considered adequate for acute treatment of a psychotic episode. There was some evidence of a greater reduction in the PANSS negative subscale score by 12 weeks in those participants assigned to amisulpride than in those of the placebo group. This finding is in line with earlier reports of a greater improvement in negative symptoms than positive symptoms in randomised studies where clozapine augmentation with a second antipsychotic for treatment-refractory schizophrenia had proved to be beneficial,23,28 as well as some limited evidence for improvement in negative symptoms with amisulpride monotherapy. 79–83
The clinical relevance of a reduction in total PANSS score of 20% or more as a response threshold for people with treatment-refractory schizophrenia may have its limitations. First, it involves the interpretation of scores on a scale rarely used in clinical practice. Second, Leucht et al. 84 showed that even a 25% percentage reduction in the PANSS total score only reflected a reduction of the CGI scale score by one severity step. Thus, given the marked heterogeneity of the clinical presentation of treatment-refractory schizophrenia, future studies might consider adding an individualised response criterion, based on the change in severity of each participant’s critical target symptoms. This last point is reinforced by the finding of a heterogeneous clinical picture in relation to the key symptoms and behaviours presenting in this study sample. Although persistent positive symptoms were the most common symptoms at baseline judged to be of clinical significance by the mental health professionals providing care, some participants presented other such target symptoms, including anxiety and negative symptoms in the avolition/amotivation domain.
Side effects
Amisulpride was chosen for this study because of the robust evidence for tolerability benefits, particularly a low risk of compounding characteristic clozapine side effects such as sedation, weight gain and other metabolic problems and a low risk of EPSs. Amisulpride is more likely than most second-generation antipsychotics to cause hyperprolactinaemia,85 but causes little or no weight gain, has a similarly low risk of causing diabetes and lipid abnormalities and a relatively low liability for EPSs. 86,87 With regard to cardiac side effects, QT interval prolongation and the potentially fatal arrhythmia, torsade de pointes, are not uncommon with overdose,88 but the risk at therapeutic dosages is rather uncertain. 89,90
In our clinical trial, the results of the ANNSERS-E assessments revealed a greater side effect burden with amisulpride augmentation; that is, over time, the mean ANNSERS-E total score for those participants in the amisulpride-treated group was 3 points higher than that for the placebo group. However, the separate assessment of EPSs, such as akathisia and parkinsonism, revealed that these were not treatment-emergent problems with amisulpride augmentation of clozapine, despite previous studies reporting a high rate of tremor, bradykinesia and akathisia with this drug combination. 43,76
Among the participants in the amisulpride group, 60% experienced at least one adverse event over the course of the study, compared with 30% of participants in the placebo group. However, these adverse events were predominantly mild and the vast majority resolved. Serious adverse events were rare. Cardiac symptoms were a relatively common prompt for an adverse event report, occurring more commonly in the amisulpride group. Furthermore, an additional check for any emerging cardiac symptoms in the 7–10 days after starting study medication revealed that shortness of breath and dizziness were more common in the amisulpride treatment group. Amisulpride augmentation was also associated with endocrine effects; the most common was raised plasma prolactin concentration, an expected side effect that also provides some indirect but reassuring evidence of adherence to the study medication.
Mechanism of action
The rationale for the choice of an augmenting antipsychotic in patients on clozapine includes a complementary receptor profile, that is, potent D2 dopamine receptor blockade. 8,41,91 Amisulpride was chosen for this study because it fulfils this criterion in that it preferentially binds to dopamine D2 and D3 receptors in limbic rather than striatal brain structures92,93 and has low affinity for other dopamine receptor subtypes, although it also has affinity for a range of other receptors, including serotonergic, histaminergic and adrenergic receptors.
The limited benefit seen with amisulpride in this study supports the emerging notion that the rationale of potent D2 blockade for an augmenting antipsychotic to treat clozapine-unresponsive illness may be simplistic. Treatment-refractory schizophrenia may have a more complex pathophysiology than illness showing a good therapeutic response to standard antipsychotic therapy; the underlying pathophysiology may even be non-dopaminergic. For example, dopamine synthesis capacity is lower in those patients with a treatment-resistant illness (indeed, no different from healthy controls) than in those with a responsive illness. 94,95 Vayısoğlu and Anıl Yağcıoğlu96 speculated that treatment-resistant illness may benefit from a multisite receptor effect rather than a stronger antidopaminergic effect. 97
Economic analyses
Overall, the economic analyses indicate that amisulpride augmentation has the potential to be cost-effective in the short term and, possibly, the longer term. The within-trial analysis indicated that amisulpride augmentation is associated with a net saving of £1816 (SD £369, 95th percentiles –£2540 to £1092) and no clear difference in QALYs. Although the extent of any savings is uncertain, because of the wide variation in costs, the cost-effectiveness acceptability analysis indicated that amisulpride augmentation can be cost-effective. The probability that amisulpride was cost-effective estimated from the bootstrap simulation was high with p-values of 0.82–1.00. An economic model extrapolated the results over a longer time frame of 1 year. This found similar net savings, but increased the variance associated with both net costs and QALYs. For the base case, the net saving was –£1935 (SD £13,735, 95th percentiles –£33,908 to £25,968). The 95th percentiles cross zero, indicating less certainty that there is a net saving for amisulpride augmentation; the probability that amisulpride is cost-effective is reduced to 0.54–0.58. However, the extent to which amisulpride augmentation represents value for money from the patients’ perspective depends on their preferences and willingness to accept a potentially higher side effect burden for reduced symptoms.
Strengths and limitations of the health economics analyses
The within-trial economic evaluation was limited by the short 12-week follow-up period. This had several implications. First, the impact of service use at baseline, particularly inpatient stay may be relatively high compared with longer periods of follow-up. This was controlled for to some extent by including the costs for the 3 months prior to baseline as a covariate in the analyses of net cost. However, when combined with the small sample size, high level of variance and a high level of missing observations for service use data (n = 24/57 participants who completed a 12-week follow-up), the differences in costs may have been driven by small but important differences in the use of high-cost, low-volume services. Excluding the costs of inpatient care from the analysis reduced the net saving associated with amisulpride from £1816 to £329. Even so, the probability that amisulpride was cost-effective was still high (p = 0.99).
The second consequence of the short follow-up period was that there was insufficient time to observe the impact of the increased side effects of amisulpride on the longer-term costs and QALYs. If the side effects were transient, then the net saving and QALY gains may be underestimated. Conversely, if the side effects were permanent and/or the rate of side effects increased, then the net saving and QALY gain would be overestimated. Additionally, the short follow-up period may not be sufficient to capture the impact of improved symptoms in the amisulpride group on service use and on overall health and QALYs. If the level of symptom improvement is constant over the longer term or increases, then the net savings and QALYs may be underestimated. The economic model analyses indicate that the within-trial results may be robust to large reductions in symptom response after the initial 12-week follow-up period. This applies when higher rates of side effects over the longer term are assigned to amisulpride.
As noted above, observations about service use were missing for a large proportion of participants. This was partly as a result of incomplete EPQ data and partly because of inconsistencies between the EPQ records and hospital case notes. Multiple imputation was used to impute missing costs for those participants who completed a 12-week follow-up assessment. It was assumed that the missing observations were missing at random. Analysis of the baseline pooled trial data indicated that it was possible to identify key covariates that were associated with costs, to use in the imputation model. Even so, the robustness of the multiple imputation model was reduced by the proportion of participants with missing observations (42%). Analysis of the smaller sample of participants with complete cost and QALY data indicated that the net saving associated with amisulpride treatment was less certain, and the probability that it was cost-effective reduced the p-value to 0.82.
For 11 out of 68 participants, no service use or EQ-5D data, at baseline or follow-up, were available. It was not possible to impute cost or QALY values for these participants. This, combined with the small sample and high rate of missing cost data, reduces confidence that the results are representative of all people with schizophrenia who are unresponsive to clozapine and eligible for amisulpride augmentation.
The EQ-5D utility values correlated well with the clinical measures of symptoms and side effects, indicating that it is a relevant measure of health benefit for this evaluation. Analysis of the pooled data for the economic model indicated that QALY values were higher for people who had a 20% response on the PANSS and lower for people who had one or more severe non-neurological or EPSs. This is an indication that, for the sample of participants in the trial, the measure reflects the impact of both side effects and symptom response. However, the QALY may not be an appropriate measure for complex needs or complex interventions. 98 People with schizophrenia have complex needs, with antipsychotic treatment adding therapy-induced side effects to the complexity of decision-making. 99,100 In these situations the QALY may not adequately reflect the trade-off between side effects and symptom response for specific individuals requiring a change in treatment.
A focused literature search identified no full economic evaluations of amisulpride augmentation or any clozapine augmentation treatment that could be used to inform the economic model design or provide data to estimate model parameters. Two effectiveness reviews were found in a search of the Cochrane Library for clozapine augmentation. 76,77 These reviews indicated there were insufficient data about the long-term effects of any augmentation on clinical outcomes or side effects. Given the paucity of evidence for long-term use of clozapine augmentation, as well as the complexities of the disease area, it was concluded that there is little to gain from building a full economic model. The evidence collected in this clinical and economic trial is the most robust evidence available to inform the model structure and economic parameter estimates. This meant that a partial model was used to explore the impact of varying the probabilities of symptom response and side effects. The analyses were based on the assumption that these probabilities are key drivers of cost and QALYs. These analyses indicated that amisulpride was relatively robust to assumptions about the longer-term probability of response and side effects. However, the model was limited in scope, which reduces the generalisability and robustness of the results.
Implications of the findings of the health economics analyses
The results of the within-trial economic evaluation and economic model demonstrate there is uncertainty about the extent of savings as a result of amisulpride treatment over both the short and longer term. In addition, it is not clear whether or not the lack of evidence of differences in QALYs is because of a lack of effect or the small sample size and duration of this trial. Even so, the analyses do demonstrate that amisulpride augmentation is potentially cost-effective and that the analyses are relatively robust to different assumptions. These factors indicate a need for a longer-term, prospective evaluation of amisulpride augmentation. The costs of impatient care over the short term were an important driver of costs. It is important that attention is paid to collecting accurate data about the number of admissions and the length of stay of admissions. The high level of missing observations indicates a need to test service-use data collection methods in each of the study centres prior to the start of the full trial.
It is acknowledged that trade-offs between positive and negative symptoms, side effects and functional outcomes are important issues in managing schizophrenia. However, there is a paucity of qualitative and quantitative research to understand the relative importance of these different aspects of treatment. 99,100 Research that is codesigned with service users is required to explore the issues in more depth and to develop relevant quantitative surveys to measure and value preferences and priorities.
Recruitment
From early on, the AMICUS trial did not achieve the rate of recruitment that was anticipated and necessary in order to reach the original target of 230 participants. A rescue plan was agreed with the HTA programme, as funders, which included a lower recruitment target of 180 participants based on a reduced power of 80%. However, subsequently the recruitment rate decreased, partly as a result of loss of study momentum following a temporary closure of the study at the original end date. By the close of the extended recruitment period, we had achieved only 38% of the revised target. Negotiation of the hurdles of research governance, regulation, and NHS permissions, contracts and costs allocation meant that opening a single study site often took many months, undoubtedly impacting on our accrual. Nevertheless, even after sites were open to recruitment, referrals to the study were slow, despite feasibility work prior to this trial indicating that an adequate number of patients would fulfil the eligibility criteria.
The slow rate of participant recruitment was discussed by the Trial Management Group, during teleconferences with researchers at the study sites, and by the Trial Steering Committee. Particular consideration was given to barriers to recruitment and how these might be overcome. Some changes were made, such as the addition of a small remuneration for participants, following feedback that other clinical studies were offering this. Another amendment provided the option of an additional prescription of trial medication to allow time for unblinding at the end of the study follow-up period and, therefore, continuity of medication for the participant, an issue that had been raised by clinicians at sites.
Much of the discussion on the slow rate of recruitment revolved around the eligibility criteria and whether or not these were appropriate and pragmatic. Fifteen per cent of participants were excluded during final eligibility screening because their level of residual symptoms and/or impaired social function failed to reach the severity threshold necessary for inclusion. Given that these individuals had been identified by their clinical team as having shown a poor response to clozapine, we considered whether or not it would be pragmatic to include them in the trial. However, as the aim of the trial was to establish the risks and benefits of this augmentation strategy in patients with an indubitable treatment-refractory illness, we decided this was not appropriate. To modify these inclusion criteria would have resulted in a larger sample size and thus an increase in the power of the study, but it would have rendered any effectiveness findings more difficult to translate into recommendations for the management of treatment-refractory schizophrenia in clinical practice.
Another reason for the exclusion of participants was concern about ECG abnormalities or a prolonged corrected QT interval (> 450 milliseconds, following Medicines and Healthcare products Regulatory Agency guidance). Eleven per cent of participants were excluded on these criteria during final eligibility screening, but several further patients did not reach the consent stage as cardiac abnormalities had already been identified. We are aware that, subsequent to not being eligible to enter the trial, a number of these patients were prescribed amisulpride augmentation, raising the question of whether or not they should have been included in this trial as representative of routine clinical practice. However, the exclusion of patients with cardiac contraindications, including long QT syndromes, from exposure to potentially high-dose antipsychotic medication was in line with recommendations for best practice safety monitoring54 and, therefore, it was not acceptable for this exclusion criterion to be removed, despite our awareness that the same conservative approach was not systematically adopted in clinical practice. The greater number of cardiac adverse events seen in participants in the amisulpride arm during the trial reassured us that we had made the right decision.
Feedback from researchers at the sites indicated that many clinical staff felt unable to review their case load and identify potential participants, citing competing clinical priorities, concerns about how introducing a trial to the patient might impact on their therapeutic relationship, or demonstrating a lack understanding of the clinical equipoise of the research question. 101,102 On numerous occasions, clinicians expressed the view that it would be unethical for a patient to be given placebo rather than amisulpride. Furthermore, researchers found some clinical services commonly employed amisulpride augmentation as a pharmacological option for clozapine-refractory schizophrenia, limiting the number of eligible patients for the trial.
As referrals had to come from a member of a patient’s clinical team, recruitment tended to occur in the services in which we encountered sympathetic clinical staff. Thus, many eligible patients in other areas missed the opportunity to participate. We opened additional trial sites over time in order to access more eligible patients, and worked hard to promote the trial with talks to clinical teams, newsletters, and researcher recruitment training days that covered engagement of both patients and clinical staff. However, recruitment failed to achieve the necessary rate for us to attain our target sample size. Indeed, 7 of the 23 sites failed to randomise a single participant.
Conclusions
The risk–benefit balance of amisulpride augmentation of clozapine for schizophrenia that has shown an insufficient response to a trial of clozapine monotherapy is worthy of further investigation in larger studies. This is the first study to provide detailed cost and QALY data about amisulpride augmentation of clozapine treatment. The economic analysis demonstrated that amisulpride augmentation has the potential to be cost-effective in the short term and, possibly, the longer term, but larger prospective RCTs of amisulpride augmentation that were methodologically sound and of adequate duration would be necessary to establish this. Whether or not such trials are feasible remains uncertain, given the continuing challenge of recruitment in mental health studies in the NHS. Nevertheless, this trial provides important process, clinical and economic data to inform the design of future studies.
The analysis of the data in our relatively small trial found that, even among patients with a clozapine-refractory illness, the addition of amisulpride was associated with a greater chance of improvement to a criterion level of overall symptom reduction within 12 weeks and some suggestion of improvement in negative symptoms. However, despite amisulpride being chosen for its favourable tolerability and safety profile, when combined with clozapine treatment in this study it was associated with a greater side effect burden, including cardiac side effects. This may partly reflect the thorough assessment of side effects in this study, which was more systematic and comprehensive than is generally conducted in clinical trials of antipsychotics. 103 These findings have implications for the nature and frequency of safety and tolerability monitoring of clozapine augmentation with a second antipsychotic in both clinical and research settings.
Acknowledgements
We would like to thank all the patients who agreed to be interviewed as part of this trial and extend special thanks to all those who agreed to participate. We thank the AMICUS trial investigators and research staff at the study sites, many of whom worked extremely hard to support the study. The study sites and their principal investigator are listed below. We would also like to extend our gratitude to all the mental health professionals at the study sites who supported the study, finding time for us in their busy schedules. Our thanks also to other colleagues who cowrote the original grant application: Dr Andrew Proctor and Professor Michael King. Thanks also to the helpful and friendly staff at the University of Sheffield Clinical Trials Research Unit who provided the trial database and randomisation service. We also thank the staff at the Medical Toxicology and Information Services Ltd for providing a professional and reliable emergency unblinding service for the trial.
Trial sites and principal investigators
Avon and Wiltshire Mental Health Partnership NHS Trust (Dr Tim Amos).
Birmingham and Solihull Mental Health NHS Foundation Trust (Dr Domingo Gonzalez).
Bradford District Care Trust (Dr Khalid Iqbal).
Camden and Islington NHS Foundation Trust (Professor David Osborn).
Central and North West London NHS Foundation Trust (Dr Mariwan Husni).
Derbyshire Healthcare NHS Foundation Trust (Dr Vineet Singh).
Devon Partnership NHS Trust (Dr Christine Brown).
Greater Manchester West Mental Health NHS Foundation Trust (Professor Peter M Haddad).
Kent and Medway NHS and Social Care Partnership Trust (Dr Ahmed Ibrahim Ismail).
Leeds and York Partnership NHS Foundation Trust (Dr Ranga Rattahali).
Lincolnshire Partnership NHS Foundation Trust (Dr Rameez Zafar).
Manchester Mental Health and Social Care Trust (Dr Zachary Fitzgerald).
Northumberland, Tyne and Wear NHS Foundation Trust (Dr Patrick Keown).
North Essex Partnership University NHS Foundation Trust (Dr Pavel Fridrich).
Nottinghamshire Healthcare NHS Foundation Trust (Dr Hemant Bagalkote).
Oxleas NHS Foundation Trust (Ms Carol Paton).
Plymouth Teaching Primary Care Trust (Dr Jocelyn Dawe).
Somerset Partnership NHS Foundation Trust (Dr Fatin Hussein).
South Staffordshire and Shropshire Healthcare NHS Foundation Trust (Dr Manoj Kumar).
Southern Health NHS Foundation Trust (Dr Amanda Taylor).
Tees, Esk and Wear Valley NHS Foundation Trust (Dr Raj Kumar).
West London Mental Health NHS Trust (Professor Thomas RE Barnes).
Study oversight committees
We thank all the members of the study oversight committees for their valued contributions.
Trial Steering Committee: Professor Stefan Priebe (independent chairperson), Professor Eileen Joyce, Ms Jenny Trite (expert by experience), Professor Tom Jamieson-Craig and Professor David Osborn.
DMEC: Professor Mohammed Abou-Saleh (chairperson), Dr Stuart Cox, Dr Louise Marston and Ms Ulrike Naumann.
Trial Management Group: Professor Thomas RE Barnes (chairperson), Dr Verity C Leeson, Mrs Carol Paton, Professor David Osborn, Mr Kavi Gakhal, Dr Louise Marston, Ms Megan Lawrence, Mr Roger Oliver (expert by experience), Mr George Salaminios and Dr Dilveer Sually.
Contributions of authors
Thomas RE Barnes (Professor of Clinical Psychiatry, Centre for Mental Health, Imperial College London) was the principal grant applicant, contributed to the development of the trial protocol and was responsible for the overall conduct of the trial as chief investigator. He led the interpretation of the results and drafting of the final report.
Verity C Leeson (Clinical Trials Manager, Centre for Mental Health, Imperial College London) contributed to the development of the original trial protocol and its amendments, and was responsible for the day-to-day management of the trial. She contributed to the interpretation of the results and preparation of the final report.
Carol Paton (Chief Pharmacist, Oxleas NHS Foundation Trust) cowrote the original grant application, was a principal investigator, contributed to the trial protocol and its amendments, and to the interpretation of the results for the final report.
Louise Marston (Senior Trial Statistician, Department of Primary Care and Population Health and PRIMENT Clinical Trials Unit, University College) cowrote the original grant application, and the development and subsequent amendments of the trial protocol. She led the statistical analysis, and contributed to the interpretation and write-up of the results for the final report.
Linda Davies (Professor of Health Economics, Institute of Population Health, University of Manchester) contributed to the original grant application and the development of the trial protocol. She co-led the health economics analysis and write-up.
William Whittaker (Research Fellow, Institute of Population Health, University of Manchester) co-led the health economics analysis and write-up.
David Osborn (Professor of Psychiatric Epidemiology, Division of Psychiatry, University College London) cowrote the original grant application, was a principal investigator and commented on earlier drafts of the final report.
Raj Kumar (Consultant Psychiatrist, Tees, Esk and Wear Valley NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Patrick Keown (Consultant Psychiatrist, Northumberland, Tyne and Wear NHS Foundation Trust and Honorary Senior Clinical Lecturer, Newcastle University) was a principal investigator and commented on earlier drafts of the final report.
Rameez Zafar (Consultant Psychiatrist, Lincolnshire Partnership NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Khalid Iqbal (Consultant Psychiatrist, Bradford District Care Trust) was a principal investigator and commented on earlier drafts of the final report.
Vineet Singh (Consultant Psychiatrist, Derbyshire Healthcare NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Pavel Fridrich (Consultant Psychiatrist, North Essex Partnership University NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Zachary Fitzgerald (Consultant Psychiatrist, Manchester Mental Health and Social Care NHS Trust) was a principal investigator and commented on earlier drafts of the final report.
Hemant Bagalkote (Consultant Psychiatrist, Nottinghamshire Healthcare NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Peter M Haddad (Consultant Psychiatrist, Greater Manchester West Mental Health NHS Foundation Trust and Honorary Reader, University of Manchester) cowrote the original grant application, was a principal investigator and commented on earlier drafts of the final report.
Mariwan Husni (Consultant Psychiatrist, Central and North West London NHS Foundation Trust) was a principal investigator and commented on earlier drafts of the final report.
Tim Amos (Consultant Forensic Psychiatrist, Avon and Wiltshire Mental Health Partnership NHS Trust and Senior Lecturer in Forensic Psychiatry, University of Bristol) was a principal investigator and commented on earlier drafts of the final report.
Data sharing statement
All data are available on request from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Lambert M, Naber D, Schacht A, Wagner T, Hundemer HP, Karow A, et al. Rates and predictors of remission and recovery during 3 years in 392 never-treated patients with schizophrenia. Acta Psychiatr Scand 2008;118:220-9. http://dx.doi.org/10.1111/j.1600-0447.2008.01213.x.
- Wiersma D, Nienhuis FJ, Slooff CJ, Giel R. Natural course of schizophrenic disorders: a 15-year follow-up of a Dutch incidence cohort. Schizophr Bull 1998;24:75-8. http://dx.doi.org/10.1093/oxfordjournals.schbul.a033315.
- Barnes TRE, Buckley P, Schulz SC, Hirsch SR, Weinberger DR. Schizophrenia. Oxford: Blackwell Publishing; 2003.
- Davies LM, Drummond MF. Assessment of costs and benefits of drug therapy for treatment-resistant schizophrenia in the United Kingdom. Br J Psychiatry 1993;162:38-42. http://dx.doi.org/10.1192/bjp.162.1.38.
- Knapp M, Kavanagh S. Economic outcomes and costs in the treatment of schizophrenia. Clin Ther 1997;19:128-38. http://dx.doi.org/10.1016/S0149-2918(97)80080-X.
- Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry 2004;184:346-51. http://dx.doi.org/10.1192/bjp.184.4.346.
- Mangalore R, Knapp M. Equity in mental health. Epidemiol Psichiatr Soc 2006;15:260-6. http://dx.doi.org/10.1017/S1121189X00002141.
- Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand 2002;106:323-30. http://dx.doi.org/10.1034/j.1600-0447.2002.01331.x.
- Topic 1f & 3c. Prescribing High-Dose and Combination Antipsychotics: Acute/PICU, Rehabilitation/Complex Needs, and Forensic Psychiatric Services. London: POMH-UK, CCQI125; 2012.
- Potter WZ, Ko GN, Zhang LD, Yan WW. Clozapine in China: a review and preview of US/PRC collaboration. Psychopharmacology 1989;99:S87-91. http://dx.doi.org/10.1007/BF00442568.
- Buckley P, Miller A, Olsen J, Garver D, Miller DD, Csernansky J. When symptoms persist: clozapine augmentation strategies. Schizophr Bull 2001;27:615-28. http://dx.doi.org/10.1093/oxfordjournals.schbul.a006901.
- Taylor D, Mir S, Mace S, Whiskey E. Co-prescribing of atypical and typical antipsychotics – prescribing sequence and documented outcome. Psychiatr Bull 2002;26:170-2. http://dx.doi.org/10.1192/pb.26.5.170.
- Mouaffak F, Tranulis C, Gourevitch R, Poirier MF, Douki S, Olié JP, et al. Augmentation strategies of clozapine with antipsychotics in the treatment of ultraresistant schizophrenia. Clin Neuropharmacol 2006;29:28-33. http://dx.doi.org/10.1097/00002826-200601000-00009.
- Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry 2001;158:518-26. http://dx.doi.org/10.1176/appi.ajp.158.4.518.
- Remington G, Saha A, Chong SA, Shammi C. Augmentation strategies in clozapine-resistant schizophrenia. CNS Drugs 2005;19:843-72. http://dx.doi.org/10.2165/00023210-200519100-00004.
- Kontaxakis VP, Ferentinos PP, Havaki-Kontaxaki BJ, Paplos KG, Roukas DK, Christodoulou GN. Case studies of adjunctive agents in clozapine-resistant schizophrenic patients. Clin Neuropharmacol 2005;28:50-3. http://dx.doi.org/10.1097/01.wnf.0000154222.37887.a8.
- Kontaxakis VP, Ferentinos PP, Havaki-Kontaxaki BJ, Roukas DK. Randomized controlled augmentation trials in clozapine-resistant schizophrenic patients: a critical review. Eur Psychiatry 2005;20:409-15. http://dx.doi.org/10.1016/j.eurpsy.2004.12.007.
- Paton C, Whittington C, Barnes TR. Augmentation with a second antipsychotic in patients with schizophrenia who partially respond to clozapine: a meta-analysis. J Clin Psychopharmacol 2007;27:198-204. http://dx.doi.org/10.1097/JCP.0b013e318036bfbb.
- Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 2009;35:443-57. http://dx.doi.org/10.1093/schbul/sbn018.
- Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care. London: NICE; 2009.
- Freudenreich O, Henderson DC, Walsh JP, Culhane MA, Goff DC. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res 2007;92:90-4. http://dx.doi.org/10.1016/j.schres.2006.12.030.
- Mossaheb I, Sacher J, Wiesegger G, Klein O, Spindelegger CJ, Asenbaum S, et al. Haloperidol in combination with clozapine in treatment-refractory patients with schizophrenia. Eur Neuropsychopharmacol 2006;16. http://dx.doi.org/10.1016/S0924-977X(06)70524-7.
- Chang JS, Ahn YM, Park HJ, Lee KY, Kim SH, Kang UG, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week randomised, double-blind, placebo-controlled trial. J Clin Psychiatry 2008;69:720-31. http://dx.doi.org/10.4088/JCP.v69n0505.
- Fleischhacker WW, Heikkinen T, Olie JP, Landsberg W, Dewaele P, McQuade RD, et al. Weight change on aripiprazole-clozapine combination in schizophrenic patients with weight gain and suboptimal response on clozapine: 16-week double-blind study. Eur Psychiatry 2008;23:114-15. http://dx.doi.org/10.1016/j.eurpsy.2008.01.784.
- Sommer IE, Begemann MJ, Temmerman A, Leucht S. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull 2012;38:1003-11. http://dx.doi.org/10.1093/schbul/sbr004.
- Taylor DM, Smith L, Gee SH, Nielsen J. Augmentation of clozapine with a second antipsychotic – a meta-analysis. Acta Psychiatr Scand 2012;125:15-24. http://dx.doi.org/10.1111/j.1600-0447.2011.01792.x.
- Tyson SC, Devane CL, Risch SC. Pharmacokinetic interaction between risperidone and clozapine. Am J Psychiatry 1995;152:1401-2. http://dx.doi.org/10.1176/ajp.152.9.1401b.
- Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 2005;162:130-6. http://dx.doi.org/10.1176/appi.ajp.162.1.130.
- Yagcioglu AEA, Akdede BBK, Turgut TI, Tümüklü M, Yazici MK, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry 2005;66:63-72. http://dx.doi.org/10.4088/JCP.v66n0109.
- Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med 2006;354:472-82. http://dx.doi.org/10.1056/NEJMoa053222.
- Godleski LS, Sernyak MJ. Agranulocytosis after addition of risperidone to clozapine treatment. Am J Psychiatry 1996;153:735-6. http://dx.doi.org/10.1176/ajp.153.5.735b.
- Chong SA, Tan CH, Lee HS. Atrial ectopics with clozapine–risperidone combination. J Clin Psychopharmacol 1997;17:130-1. http://dx.doi.org/10.1097/00004714-199704000-00019.
- Kontaxakis VP, Havaki-Kontaxaki BJ, Stamouli SS, Christodoulou GN. Toxic interaction between risperidone and clozapine: a case report. Prog Neuropsychopharmacol Biol Psychiatry 2002;26:407-9. http://dx.doi.org/10.1016/S0278-5846(01)00257-3.
- Ziegenbein M, Wittmann G, Kropp S. Aripiprazole augmentation of clozapine in treatment-resistant schizophrenia: a clinical observation. Clin Drug Investig 2006;26:117-24. http://dx.doi.org/10.2165/00044011-200626030-00001.
- Karunakaran K, Tungaraza TE, Harborne GC. Is clozapine–aripiprazole combination a useful regime in the management of treatment-resistant schizophrenia?. J Psychopharmacol 2007;21:453-6. http://dx.doi.org/10.1177/0269881106068289.
- Croissant B, Hermann D, Olbrich R. Saving potential of clozapine due to combination with amisulpride. Psychopharmakotherapie 2001;8:128-30.
- Ziegenbein M, Rosenthal O, Garlipp P. Coadministration of clozapine and amisulpride in psychotic patients. Eur Psychiatry 2002;17. http://dx.doi.org/10.1016/S0924-9338(02)80449-7.
- Kämpf P, Agelink MW, Naber D. Augmentation of clozapine with amisulpride: a promising therapeutic approach to refractory schizophrenic symptoms. Pharmacopsychiatry 2005;38:39-40. http://dx.doi.org/10.1055/s-2005-837772.
- Ziegenbein M, Sieberer M, Kuenzel HE, Kropp S. Augmentation of clozapine with amisulpride in patients with treatment-resistant schizophrenia. An open clinical study. German J Psychiatry 2006;9:17-21.
- Munro J, Matthiasson P, Osborne S, Travis M, Purcell S, Cobb AM, et al. Amisulpride augmentation of clozapine: an open non-randomized study in patients with schizophrenia partially responsive to clozapine. Acta Psychiatr Scand 2004;110:292-8. http://dx.doi.org/10.1111/j.1600-0447.2004.00356.x.
- Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther 2007;24:1-13. http://dx.doi.org/10.1007/BF02849987.
- Hotham JE, Simpson PJ, Brooman-White RS, Basu A, Ross CC, Humphreys SA, et al. Augmentation of clozapine with amisulpride: an effective therapeutic strategy for violent treatment-resistant schizophrenia patients in a UK high-security hospital. CNS Spectr 2014;19:403-10. http://dx.doi.org/10.1017/S1092852913000874.
- Assion HJ, Reinbold H, Lemanski S, Basilowski M, Juckel G. Amisulpride augmentation in patients with schizophrenia partially responsive or unresponsive to clozapine. A randomized, double-blind, placebo-controlled trial. Pharmacopsychiatry 2008;41:24-8. http://dx.doi.org/10.1055/s-2007-993209.
- Shiloh R, Zemishlany Z, Aizenberg D, Radwan M, Schwartz B, Dorfman-Etrog P, et al. Sulpiride augmentation in people with schizophrenia partially responsive to clozapine. A double-blind, placebo-controlled study. Br J Psychiatry 1997;171:569-73. http://dx.doi.org/10.1192/bjp.171.6.569.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. http://dx.doi.org/10.1093/schbul/13.2.261.
- Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res 1988;23:99-110. http://dx.doi.org/10.1016/0165-1781(88)90038-8.
- Barnes TRE, Barnes TRE, Nelson H. The Assessment of Psychosis: A Practical Handbook. London: Chapman & Hall Medical; 1994.
- Gilbert EA, Liberman RP, Ventura J, Kern R, Robertson MJ, Hwang S, et al. Concurrent validity of negative symptom assessments in treatment refractory schizophrenia: relationship between interview-based ratings and inpatient ward observations. J Psychiatr Res 2000;34:443-7. http://dx.doi.org/10.1016/S0022-3956(00)00041-8.
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992;149:1148-56. http://dx.doi.org/10.1176/ajp.149.9.1148.
- Tait L, Birchwood M, Trower P. A new scale (SES) to measure engagement with community mental health services. J Ment Health 2002;11:191-8. http://dx.doi.org/10.1080/09638230020023570-2.
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry 1993;22:39-44.
- David AS. Insight and psychosis. Br J Psychiatry 1990;156:798-80. http://dx.doi.org/10.1192/bjp.156.6.798.
- Ohlsen RI, Williamson R, Yusufi B, Mullan J, Irving D, Mukherjee S, et al. Interrater reliability of the Antipsychotic Non-Neurological Side effects Rating Scale measured in patients treated with clozapine. J Psychopharmacol 2008;22:323-9. http://dx.doi.org/10.1177/0269881108091069.
- Consensus Statement on High-Dose Antipsychotic Medication. Council Report CR138. London: Royal College of Psychiatrists; 2006.
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970;212:11-9. http://dx.doi.org/10.1111/j.1600-0447.1970.tb02066.x.
- Janno S, Holi MM, Tuisku K, Wahlbeck K. Validity of Simpson–Angus Scale (SAS) in a naturalistic schizophrenia population. BMC Neurol 2005;5. http://dx.doi.org/10.1186/1471-2377-5-5.
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry 1989;154:672-6. http://dx.doi.org/10.1192/bjp.154.5.672.
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Washington, DC: US Department of Health, Education and Welfare; 1976.
- Mental Health Outcomes Compendium. London: Department of Health; 2008.
- Davies LM, Barnes TR, Jones PB, Lewis S, Gaughran F, Hayhurst K, et al. A randomized controlled trial of the cost-utility of second-generation antipsychotics in people with psychosis and eligible for clozapine. Value Health 2008;11:549-62. http://dx.doi.org/10.1111/j.1524-4733.2007.00280.x.
- Rosenheck RA, Leslie DL, Sindelar J, Miller EA, Lin H, Stroup TS, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9. http://dx.doi.org/10.1176/ajp.2006.163.12.2080.
- National Schedules of Reference Costs 2013–2014. London: Department of Health; 2014.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 22 March 2016).
- Bobes J, García-Portilla P, Sáiz PA, Bascarán T, Bousoño M. Quality of life measures in schizophrenia. Eur Psychiatry 2005;20:313-7. http://dx.doi.org/10.1016/S0924-9338(05)80182-8.
- Prieto L, Novick D, Sacristán JA, Edgell ET, Alonso J. SOHO Study Group . A Rasch model analysis to test the cross-cultural validity of the EuroQoL-5D in the Schizophrenia Outpatient Health Outcomes Study. Acta Psychiatr Scand 2003;107:24-9. http://dx.doi.org/10.1034/j.1600-0447.107.s416.6.x.
- Davies LM, Lewis S, Jones PB, Barnes TR, Gaughran F, Hayhurst K, et al. Cost-effectiveness of first- v. second-generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. Br J Psychiatry 2007;191:14-22. http://dx.doi.org/10.1192/bjp.bp.106.028654.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. http://dx.doi.org/10.1007/s40273-014-0193-3.
- Guide to Methods of Technology Appraisals. London: NICE; 2013.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. http://dx.doi.org/10.1136/bmj.329.7459.224.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. http://dx.doi.org/10.1002/hec.678.
- Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ 2001;10:675-80. http://dx.doi.org/10.1002/hec.639.
- Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol 2012;22:165-82. http://dx.doi.org/10.1016/j.euroneuro.2011.08.005.
- Muscatello MR, Bruno A, De Fazio P, Segura-Garcia C, Pandolfo G, Zoccali R. Augmentation strategies in partial responder and/or treatment-resistant schizophrenia patients treated with clozapine. Expert Opin Pharmacother 2014;15:2329-45. http://dx.doi.org/10.1517/14656566.2014.956082.
- Healey D, Le Noury J, Harris M, Butt M, Linden S, Whitaker C, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875–1924 and 1994–2010. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001810.
- Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F. Treatment of negative symptoms in schizophrenia with amisulpride. Br J Psychiatry 1995;166:68-72. http://dx.doi.org/10.1192/bjp.166.1.68.
- Danion JM, Rein W, Fleurot O. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. Amisulpride Study Group. Am J Psychiatry 1999;156:610-16.
- Loo H, Poirier-Littre MF, Theron M, Rein W, Fleurot O. Amisulpride versus placebo in the medium-term treatment of the negative symptoms of schizophrenia. Br J Psychiatry 1997;170:18-22. http://dx.doi.org/10.1192/bjp.170.1.18.
- Storosum JG, Elferink AJ, van Zwieten BJ, van Strik R, Hoogendijk WJ, Broekmans AW. Amisulpride: is there a treatment for negative symptoms in schizophrenia patients?. Schizophr Bull 2002;28:193-201. http://dx.doi.org/10.1093/oxfordjournals.schbul.a006931.
- Arango C, Garibaldi G, Marder SR. Pharmacological approaches to treating negative symptoms: a review of clinical trials. Schizophr Res 2013;150:346-52. http://dx.doi.org/10.1016/j.schres.2013.07.026.
- Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology 2006;31:2318-25. http://dx.doi.org/10.1038/sj.npp.1301147.
- Fric M, Laux G. Prolactin levels and symptoms of hyperprolactinaemia in patients treated with amisulpride, risperidone, olanzapine and quetiapine. Psychiatr Prax 2003;20:97-101.
- Tschoner A, Engl J, Laimer M, Kaser S, Rettenbacher M, Fleischhacker WW, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract 2007;61:1356-70. http://dx.doi.org/10.1111/j.1742-1241.2007.01416.x.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951-62. http://dx.doi.org/10.1016/S0140-6736(13)60733-3.
- Isbister GK, Balit CR, Macleod D, Duffull SB. Amisulpride overdose is frequently associated with QT prolongation and torsades de pointes. J Clin Psychopharmacol 2010;30:391-5. http://dx.doi.org/10.1097/JCP.0b013e3181e5c14c.
- McKeage K, Plosker GL. Amisulpride: a review of its use in the management of schizophrenia. CNS Drugs 2004;18:933-56. http://dx.doi.org/10.2165/00023210-200418130-00007.
- Chung AK, Chua SE. Torsade de pointes associated with low-dose amisulpride: a case report. J Psychopharmacol 2010;24:433-5. http://dx.doi.org/10.1177/0269881108098385.
- Kontaxakis VP, Ferentinos PP, Havaki-Kontaxaki BJ, Paplos KG, Pappa DA, Christodoulou GN. Risperidone augmentation of clozapine: a critical review. Eur Arch Psychiatry Clin Neurosci 2006;256:350-5. http://dx.doi.org/10.1007/s00406-006-0643-9.
- Möller HJ. Amisulpride: limbic specificity and the mechanism of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 2003;27:1101-11. http://dx.doi.org/10.1016/j.pnpbp.2003.09.006.
- Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 1997;280:73-82.
- Stone JM, Raffin M, Morrison P, McGuire PK. Review: The biological basis of antipsychotic response in schizophrenia. J Psychopharmacol 2010;24:953-64. http://dx.doi.org/10.1177/0269881109106959.
- Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry 2012;169:1203-10. http://dx.doi.org/10.1176/appi.ajp.2012.12010144.
- Vayısoğlu S, Anıl Yağcıoğlu E. Augmentation strategies in patients with schizophrenia who show partial response to clozapine treatment. Turk Psikiyatri Derg 2014;25:201-11.
- Giegling I, Drago A, Schäfer M, Möller HJ, Rujescu D, Serretti A. Interaction of haloperidol plasma level and antipsychotic effect in early phases of acute psychosis treatment. J Psychiatr Res 2010;44:487-92. http://dx.doi.org/10.1016/j.jpsychires.2009.11.004.
- Payne K, McAllister M, Davies LM. Valuing the economic benefits of complex interventions: when maximising health is not sufficient. Health Econ 2013;22:258-71. http://dx.doi.org/10.1002/hec.2795.
- Byrne R, Davies L, Morrison A. Priorities and preferences for the outcomes of treatment of psychosis: a service user perspective. Psychosis 2010;2:1-8. http://dx.doi.org/10.1080/17522430903456913.
- Eiring Ø, Landmark BF, Aas E, Salkeld G, Nylenna M, Nytrøen K. What matters to patients? A systematic review of preferences for medication-associated outcomes in mental disorders. BMJ Open 2015;5. http://dx.doi.org/10.1136/bmjopen-2015-007848.
- Leeson VC, Tyrer P. The advance of research governance in psychiatry: one step forward, two steps back. Epidemiol Psychiatr Sci 2013;22:313-20. http://dx.doi.org/10.1017/S2045796013000255.
- Rendell JM, Merritt RD, Geddes JR. Incentives and disincentives to participation by clinicians in randomised controlled trials. Cochrane Database Syst Rev 2007;2. http://dx.doi.org/10.1002/14651858.mr000021.pub3.
- Pope A, Adams C, Paton C, Weaver T, Barnes TR. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. Br J Psychiatry 2010;197:67-72. http://dx.doi.org/10.1192/bjp.bp.109.070961.
Appendix 1 EQ-5D health status and utility
| EQ-5D health states | Treatment group | |
|---|---|---|
| Amisulpride (n = 35) | Placebo (n = 33) | |
| n/N (%) | n/N (%) | |
| Baseline | ||
| No problem with mobility | 26/34 (77) | 14/31 (77) |
| No problem with self-care | 29/34 (85) | 23/31 (74) |
| No problem with usual activities | 25/34 (74) | 16/31 (52) |
| No problem with pain/discomfort | 18/34 (53) | 21/31 (68) |
| No problem with anxiety/depression | 12/34 (35) | 10/31 (32) |
| 12-week assessment | ||
| No problem with mobility | 25/32 (78) | 19/25 (76) |
| No problem with self-care | 25/32 (78) | 19/25 (76) |
| No problem with usual activities | 23/32 (72) | 13/25 (52) |
| No problem with pain/discomfort | 18/32 (56) | 16/25 (64) |
| No problem with anxiety/depression | 12/32 (34) | 10/25 (44) |
| Assessment | Treatment group | |||||
|---|---|---|---|---|---|---|
| Amisulpride | Placebo | |||||
| Sample size (n/N) | Mean (SD) | 95% CI | Sample size (n/N) | Mean (SD) | 95% CI | |
| EQ-5D VAS scores | ||||||
| Baseline | 34/35 | 58 (25) | 49 to 67 | 31/33 | 57 (22) | 49 to 65 |
| 12-week assessment | 32/35 | 58 (23) | 50 to 66 | 25/35 | 53 (23) | 44 to 62 |
| EQ-5D utility values | ||||||
| Baseline | 34/35 | 0.64 (0.36) | 0.52 to 0.76 | 31/33 | 0.64 (0.36) | 0.51 to 0.77 |
| 12-week assessment | 32/35 | 0.64 (0.37) | 0.51 to 0.77 | 25/35 | 0.65 (0.36) | 0.50 to 0.79 |
| Assessment | Correlation with baseline EQ-5D utility weight |
|---|---|
| BARS at baseline | |
| Pearson’s correlation | –0.292 |
| p-value (two-tailed) | 0.020* |
| n | 63 |
| Calgary at baseline | |
| Pearson’s correlation | –0.655 |
| p-value (two-tailed) | < 0.001** |
| n | 64 |
| PANSS at baseline | |
| Pearson’s correlation | –0.304 |
| p-value (two-tailed) | 0.014* |
| n | 65 |
| SAI at baseline | |
| Pearson’s correlation | –0.296 |
| p-value (two-tailed) | 0.017* |
| n | 64 |
| SES at baseline | |
| Pearson’s correlation | –0.060 |
| p-value (two-tailed) | 0.641 |
| n | 62 |
| SOFAS at baseline | |
| Pearson’s correlation | 0.028 |
| p-value (two-tailed) | 0.828 |
| n | 65 |
| ANNSERS-E at baseline | |
| Pearson’s correlation | –0.498 |
| p-value (two-tailed) | 0.001** |
| n | 41 |
| EPSE at baseline | |
| Pearson’s correlation | –0.315 |
| p-value (two-tailed) | 0.021* |
| n | 53 |
List of abbreviations
- AIMS
- Abnormal Involuntary Movement Scale
- AMICUS
- amisulpride augmentation in clozapine-unresponsive schizophrenia trial
- ANNSERS
- Antipsychotic Non-Neurological Side Effects Scale
- ANNSERS-E
- Antipsychotic Non-Neurological Side Effects Scale – Enhanced Version
- BARS
- Barnes Akathisia Rating Scale
- BMI
- body mass index
- BPRS
- Brief Psychiatric Rating Scale
- CDSS
- Calgary Depression Rating Scale for Schizophrenia
- CEAC
- cost-effectiveness acceptability curve
- CGI
- clinical global impression
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMEC
- Data Monitoring and Ethics Committee
- ECG
- electrocardiogram
- EPQ
- economic patient questionnaire
- EPS
- extrapyramidal side effect
- EPSE
- Extrapyramidal Side Effects Scale
- EQ-5D-3L
- EQ-5D three levels
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- NB
- net benefit
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National institute for Health Research
- OR
- odds ratio
- PANSS
- Positive and Negative Syndrome Scale
- POMH-UK
- Prescribing Observatory for Mental Health
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAI
- Schedule for the Assessment of Insight
- SD
- standard deviation
- SES
- Service Engagement Scale
- SOFAS
- Social and Occupational Functioning Assessment Scale
- VAS
- visual analogue scale
- WTPT
- willingness-to-pay value threshold